Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - HESKA CORP | Financial_Report.xls |
| EX-31.1 - EX-31.1 - HESKA CORP | d305286dex311.htm |
| EX-23.1 - EX-23.1 - HESKA CORP | d305286dex231.htm |
| EX-10.8 - EX-10.8 - HESKA CORP | d305286dex108.htm |
| EX-31.2 - EX-31.2 - HESKA CORP | d305286dex312.htm |
| EX-32.1 - EX-32.1 - HESKA CORP | d305286dex321.htm |
| EX-21.1 - EX-21.1 - HESKA CORP | d305286dex211.htm |
| EX-10.37 - EX-10.37 - HESKA CORP | d305286dex1037.htm |
| EX-10.27 - EX-10.27 - HESKA CORP | d305286dex1027.htm |
| EX-10.36 - EX-10.36 - HESKA CORP | d305286dex1036.htm |
| EX-10.47 - EX-10.47 - HESKA CORP | d305286dex1047.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 0-22427
HESKA CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware | 77-0192527 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) |
| 3760 Rocky Mountain Avenue Loveland, Colorado |
80538 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (970) 493-7272
Securities registered pursuant to Section 12(b) of the Act:
| Public Common Stock, $.01 par value |
The Nasdaq Stock Market LLC | |
| (Title of Class) | (Name of Each Exchange on Which Registered) |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company as defined in Rule 12b-2 of the Exchange Act. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a small reporting company) | Smaller Reporting Company | x | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
The aggregate market value of voting common stock held by non-affiliates of the Registrant was approximately $49,179,493 as of June 30, 2011 based upon the closing price on the Nasdaq Capital Market reported for such date. This calculation does not reflect a determination that certain persons are affiliates of the Registrant for any other purpose.
5,257,840 shares of the Registrant’s Common Stock, $.01 par value, were outstanding at February 27, 2012.
DOCUMENTS INCORPORATED BY REFERENCE
Items 10 (as to directors), 11, 12, 13 and 14 of Part III incorporate by reference information from the Registrant’s Proxy Statement to be filed with the Securities and Exchange Commission in connection with the solicitation of proxies for the Registrant’s 2012 Annual Meeting of Stockholders.
TABLE OF CONTENTS
HESKA, ALLERCEPT, AVERT, E-SCREEN, FELINE ULTRANASAL, HEMATRUE, SOLO STEP, THYROMED, VET/OX and VITALPATH are registered trademarks and CBC-DIFF, G2 DIGITAL and VET/IV are trademarks of Heska Corporation. TRI-HEART is a registered trademark of Schering-Plough Animal Health Corporation (“SPAH”) in the United States and is a registered trademark of Heska Corporation in other countries. ACCUTREND is a registered trademark of Roche Diagnostics GmbH LLC. DRI-CHEM is a registered trademark of FUJIFILM Corporation. SPOTCHEM is a trademark of Arkray, Inc. This Form 10-K also refers to trademarks and trade names of other organizations.
Statement Regarding Forward Looking Statements
This Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. For this purpose, any statements contained herein that are not statements of current or historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, words such as “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” variations of such words and similar expressions are intended to identify such forward-looking statements. These statements are not guarantees of future performance and are subject to certain risks, uncertainties and assumptions that are difficult to predict. Therefore, actual results could differ materially from those expressed or forecasted in any such forward-looking statements as a result of certain factors, including those set forth in “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business” and elsewhere in this Form 10-K. Readers are cautioned not to place undue reliance on these forward-looking statements.
Although we believe that expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. These forward-looking statements apply only as of the date of this Form 10-K or for statements incorporated by reference from the 2012 definitive proxy statement on Schedule 14A, as of the date of the Schedule 14A.
Internet Site
Our Internet address is www.heska.com. Because we believe it provides useful information in a cost-effective manner to interested investors, via a link on our website our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 are publicly available free of charge and we believe are available as soon as reasonably practical after we electronically file such material with, or furnish it to, the Securities Exchange Commission. Information contained on our website is not a part of this annual report on Form 10-K.
We develop, manufacture, market, sell and support veterinary products. Our core focus is on the canine and feline companion animal health markets where we strive to provide high value products.
Our business is composed of two reportable segments, Core Companion Animal Health and Other Vaccines, Pharmaceuticals and Products. The Core Companion Animal Health segment (“CCA”) includes diagnostic instruments and supplies as well as single use diagnostic and other tests, vaccines and pharmaceuticals, primarily for canine and feline use. These products are sold directly to veterinarians by us as well as through distribution relationships. The Other Vaccines, Pharmaceuticals and Products segment (“OVP”) includes private label vaccine and pharmaceutical production, primarily for cattle but also for other animals including small mammals and fish. All OVP products are sold by third parties under third party labels. Please refer to Note 9 to our audited consolidated financial statements filed herewith for financial information about each of our segments.
1
Our principal executive offices are located at 3760 Rocky Mountain Avenue, Loveland, Colorado 80538, our telephone number is (970) 493-7272 and our internet address is www.heska.com. We originally incorporated in California in 1988, and we subsequently incorporated in Delaware in 1997.
Background
We were founded as Paravax, Inc. in 1988 and conducted research on vaccines to prevent infections by parasites. We changed our name to Heska Corporation in 1995, completed our initial public offering in 1997 and continued to be a research and development-focused company, devoting substantial resources to the research and development of innovative products for the companion animal health market. In 2001 and 2002, we took steps to lower our expense base, largely in internal research and development but also in other areas, and to rationalize and further focus our business. We have continued to concentrate our efforts on operating improvements, such as enhancing the effectiveness of our sales and marketing efforts and pursuing cost efficiencies, and seeking new product opportunities with third parties. In 2008, we underwent a restructuring primarily to reduce our operating costs.
Core Companion Animal Health Segment
We presently sell a variety of companion animal health products and services, among the most significant of which are the following:
Veterinary Instruments
We offer a line of veterinary diagnostic and other instruments which are described below. We also market and sell consumable supplies for these instruments. Our line of veterinary instruments includes the following:
| • | Blood Chemistry. The DRI-CHEM 4000 Veterinary Chemistry Analyzer (the “DRI-CHEM 4000”) is a robust system that uses dry slide technology for blood chemistry and electrolyte analysis and has the ability to run 22 tests at a time with a single blood sample. Test slides are available as both pre-packaged panels as well as individual slides. The instrument has an additional feature allowing simple, fully automated sample dilution and results calculations. We are supplied this instrument and affiliated test slides and supplies under a contractual agreement with FUJIFILM Corporation (“FUJIFILM”). The DRI-CHEM 7000 Veterinary Chemistry Analyzer (the “DRI-CHEM 7000”) is a line extension of our chemistry offering with higher throughput, multiple patient staging and a “STAT” feature which provides emergency sample flexibility in critical cases. The DRI-CHEM 7000 utilizes the same test slides as the DRI-CHEM 4000 and is manufactured by FUJIFILM. In addition, we continue to service and support our previous chemistry instrument for which we are supplied affiliated test strips and supplies under a contractual agreement with Arkray Global Business, Inc. (“Arkray”). |
| • | Hematology. The HEMATRUE Veterinary Hematology Analyzer is an easy-to-use blood analyzer that measures such key parameters as white blood cell count, red blood cell count, platelet count and hemoglobin levels in animals. In addition, we continue to service and support our previous hematology instrument, the HESKA CBC-DIFF Veterinary Hematology System. We are supplied new instruments and affiliated reagents and supplies of these products under a contractual agreement with Boule Medical AB (“Boule”). |
| • | Blood Gases. We have historically sold handheld instruments to fulfill our customers’ needs in this area. In 2009, our supplier of these instruments and affiliated cartridges and supplies informed us that they were cancelling our contractual agreement as of November 1, 2009 and that they would no longer supply us with these products after that date. In 2009, we signed an OEM |
2
| contractual agreement with Roche Diagnostics Corporation (“Roche”) to supply us with the VitalPath Blood Gas and Electrolyte Analyzer (“VitalPath”) and affiliated consumables. VitalPath delivers accurate results for blood gases, electrolytes, hematocrit and 27 additional calculated parameters in 50 seconds. We began to ship and install VitalPath units at customer locations in May 2010. |
| • | Lactate. The Accutrend Plus Lactate analyzer is a handheld, portable analyzer used to measure lactate. We are supplied this instrument and affiliated consumables for veterinary use under a contractual agreement with Roche. We announced the launch of this instrument in the first quarter of 2011. |
| • | IV Pumps. The VET/IV 2.2 infusion pump is a compact, affordable IV pump that allows veterinarians to easily provide regulated infusion of fluids, drugs or nutritional products for their patients. |
Point-of-Care Diagnostic Tests
Heartworm Diagnostic Products. Heartworm infections of dogs and cats are caused by the parasite Dirofilaria immitis. This parasitic worm is transmitted in larval form to dogs and cats through the bite of an infected mosquito. Larvae develop into adult worms that live in the pulmonary arteries and heart of the host, where they can cause serious cardiovascular, pulmonary, liver and kidney disease. Our canine and feline heartworm diagnostic tests use monoclonal antibodies or a recombinant heartworm antigen, respectively, to detect heartworm antigens or antibodies circulating in the blood of an infected animal.
We currently market and sell heartworm diagnostic tests for both dogs and cats. SOLO STEP CH for dogs and SOLO STEP FH for cats are available in point-of-care, single use formats that can be used by veterinarians on site. We also offer SOLO STEP CH Batch Test Strips, a rapid and simple point-of-care antigen detection test for dogs that allows veterinarians in larger practices to run multiple samples at the same time. We obtain SOLO STEP CH, SOLO STEP FH and SOLO STEP Batch Test Strips under a contractual agreement with Quidel Corporation (“Quidel”).
Veterinary Diagnostic Laboratory Products and Services
Allergy Diagnostic Products and Services. Allergy is common in companion animals, and it has been estimated to affect approximately 10% to 15% of dogs. Clinical symptoms of allergy are variable, but are often manifested as persistent and serious skin disease in dogs and cats. Clinical management of allergic disease is problematic, as there are a large number of allergens that may give rise to these conditions. Although skin testing is often regarded as the most accurate diagnostic procedure, such tests can be painful, subjective and inconvenient. The effectiveness of the immunotherapy that is prescribed to treat allergic disease is inherently limited by inaccuracies in the diagnostic process.
Our ALLERCEPT Definitive Allergen Panels provide the most accurate determination of which we are aware of the specific allergens to which an animal, such as a dog, cat or horse, is reacting. The panels use a highly specific recombinant version of the natural IgE receptor to test the serum of potentially allergic animals for IgE directed against a panel of known allergens. A typical test panel consists primarily of various pollen, grass, mold, insect and mite allergens. The test results serve as the basis for prescription ALLERCEPT Allergy Treatment Sets, discussed later in this document.
We sell kits to conduct blood testing using our ALLERCEPT Definitive Allergen Panels to third-party veterinary diagnostic laboratories outside of the United States. We also sell products to screen for the presence of allergen-specific IgE to these customers – we sell kits to conduct preliminary blood testing using products based on our ALLERCEPT Definitive Allergen Panels as well as a similar test requiring less technical sophistication, our E-SCREEN Test. Animals testing positive for allergen-specific IgE using these screening tests are candidates for further evaluation using our ALLERCEPT Definitive Allergen Panels.
3
We have veterinary diagnostic laboratories in Loveland, Colorado and Fribourg, Switzerland which both offer blood testing using our ALLERCEPT Definitive Allergen Panels.
Other Products and Services. We sell E.R.D. Reagent Packs used to detect microalbuminuria, the most sensitive indicator of renal damage, to VCA Antech, Inc. for use in its veterinary diagnostic laboratories.
Our Loveland veterinary diagnostic laboratory currently also offers testing using our canine and feline heartworm, renal damage, immune status and flea bite allergy assays as well as other diagnostic services including polymerase chain reaction, or PCR, based tests for certain infectious diseases. Our Loveland diagnostic laboratory is currently staffed by medical technologists experienced in animal disease and several additional technical staff. We intend to continue to use our Loveland veterinary diagnostic laboratory both as a stand-alone service center for our customers and as an adjunct to our product development efforts.
Pharmaceuticals and Supplements
Heartworm Prevention. We have an agreement with Schering-Plough Animal Health Corporation (“SPAH”), a unit of Merck & Co., Inc. (“Merck”), granting SPAH the distribution and marketing rights in the United States for TRI-HEART Plus Chewable Tablets, our canine heartworm prevention product. TRI-HEART Plus Chewable Tablets (ivermectin/pyrantel) are indicated for use as a monthly preventive treatment of canine heartworm infection and for treatment and control of ascarid and hookworm infections. We manufacture TRI-HEART Plus Chewable Tablets at our Des Moines, Iowa production facility.
Nutritional Supplements. We sell a novel fatty acid supplement, HESKA F.A. Granules. The source of the fatty acids in this product, flaxseed oil, leads to high omega-3:omega-6 ratios of fatty acids. Diets high in omega-3 fatty acids are believed to lead to lower levels of inflammatory mediators. The HESKA F.A. Granules include vitamins and are formulated in a palatable flavor base that makes the product convenient and easy to administer.
Hypothyroid Treatment. We sell a chewable thyroid supplement, THYROMED Chewable Tablets, for treatment of hypothyroidism in dogs. Hypothyroidism is one of the most common endocrine disorders diagnosed in older dogs, treatment of which requires a daily hormone supplement for the lifetime of the animal. THYROMED Chewable Tablets contain the active ingredient Levothyroxine Sodium, which is a clinically proven replacement for the naturally occurring hormone secreted by the thyroid gland. The chewable formulation makes this daily supplement convenient and easy to administer.
Vaccines and other Biologicals
Allergy Treatment. Veterinarians who use our ALLERCEPT Definitive Allergen Panels often purchase ALLERCEPT Allergy Treatment Sets for those animals with positive test results. These prescription immunotherapy treatment sets are formulated specifically for each allergic animal and contain only the allergens to which the animal has significant levels of IgE antibodies. The prescription formulations are administered in a series of injections, with doses increasing over several months, to ameliorate the allergic condition of the animal. Immunotherapy is generally continued for an extended time. Immunotherapy delivered by injection is referred to as subcutaneous immunotherapy. We offer canine, feline and equine subcutaneous immunotherapy treatment products. In February 2012, we announced we had licensed intellectual property to be utilized toward the ongoing development of a proprietary, sublingual (administered under the tongue) therapy treatment for pets suffering with allergies. We believe sublingual therapy offers a convenient alternative to subcutaneous injection, thereby enhancing the likelihood of pet owner compliance.
4
Feline Respiratory Disease. The use of injectable vaccines in cats has become controversial due to the frequency of injection site-associated side effects. The most serious of these side effects are injection site sarcomas, tumors which, if untreated, are nearly always fatal. While there is one competitive non-injectable two-way vaccine, all other competitive products are injectable formulations.
We sell the FELINE ULTRANASAL FVRCP Vaccine, a three-way modified live vaccine combination to prevent disease caused by the three most common respiratory viruses of cats: calicivirus, rhinotracheitis virus and panleukopenia virus. Our two-way modified live vaccine combination, FELINE ULTRANASAL FVRC, prevents disease caused by calicivirus and rhinotracheitis. These vaccines are administered without needle injection by dropping the liquid preparation into the nostrils of cats. Our vaccines avoid injection site side effects, and we believe they are very efficacious.
Other Vaccines, Pharmaceuticals and Products Segment
We have developed our own line of bovine vaccines that are licensed by the United States Department of Agriculture (“USDA”). We have a long-term agreement with a distributor, Agri Laboratories, Ltd., (“AgriLabs”), for the marketing and sale of certain of these vaccines which are sold primarily under the Titanium® and MasterGuard® brands – registered trademarks of AgriLabs. AgriLabs has non-exclusive rights to sell these bovine vaccines in the United States, Africa and Mexico into December 2015. We also manufacture other bovine products not covered under the agreement with AgriLabs.
We manufacture biological and pharmaceutical products for a number of other animal health companies. We manufacture products for animals including small mammals. Our offerings range from providing complete turnkey services which include research, licensing, production, labeling and packaging of products to providing any one of these services as needed by our customers as well as validation support and distribution services.
Marketing, Sales and Customer Support
We estimate that there are approximately 53,000 veterinarians in the United States whose practices are devoted principally to small animal medicine. Those veterinarians practice in approximately 24,000 clinics in the United States. In 2011, our products were sold to approximately 12,800 such clinics in the United States. Veterinarians may obtain our products directly from us or indirectly through others. All our Core Companion Animal Health products ultimately are sold primarily to or through veterinarians. In many cases, veterinarians will markup their costs to the end user. The acceptance of our products by veterinarians is critical to our success.
We currently market our Core Companion Animal Health products in the United States to veterinarians through an outside field organization, a telephone sales force, independent third-party distributors, as well as through trade shows and print advertising and through other distribution relationships, such as SPAH in the case of our heartworm preventive. Our outside field organization currently consists of 41 individuals in various parts of the United States. Our inside sales force consists of 19 persons.
We have a staff dedicated to customer and product support in our Core Companion Animal Health segment including veterinarians, technical support specialists and service technicians. Individuals from our product development group may also be used as a resource in responding to certain product inquiries.
Internationally, we market our Core Companion Animal Health products to veterinarians primarily through third-party veterinary diagnostic laboratories, independent third-party distributors and Novartis Agro K.K., Tokyo (“Novartis Japan”). These entities typically provide customer support. Novartis Japan exclusively markets and distributes SOLO STEP CH in Japan.
5
All OVP products are marketed and sold by third parties under third party labels.
We grant third parties rights to our intellectual property as well as our products, with our compensation often taking the form of royalties and/or milestone payments.
Manufacturing
The majority of our revenue is from proprietary products manufactured by third parties. Third parties manufacture our veterinary instruments, including affiliated consumables and supplies, as well as other products including key components of our heartworm point-of-care diagnostic tests. Our current chemistry instruments and affiliated supplies are manufactured under contract with FUJIFILM. Our hematology instruments and affiliated supplies are manufactured under contract with Boule. Key components of our heartworm point-of-care diagnostic tests are manufactured under a contract with Quidel. We manufacture and supply Quidel with certain critical raw materials and perform the final packaging operations for these products. Our facility in Des Moines, Iowa is a USDA, Food and Drug Administration (“FDA”), and Drug Enforcement Agency (“DEA”) licensed biological and pharmaceutical manufacturing facility. This facility currently has the capacity to manufacture more than 50 million doses of vaccine each year. We expect that we will manufacture most or all of our biological and pharmaceutical products at this facility, as well as most or all of our recombinant proteins and other proprietary reagents for our diagnostic tests. We currently manufacture our canine heartworm prevention product, our allergy treatment products, our FELINE ULTRANASAL Vaccines and all our OVP segment products at this facility. Our OVP segment’s customers purchase products in both finished and bulk format, and we perform all phases of manufacturing, including growth of the active bacterial and viral agents, sterile filling, lyophilization and packaging at this facility. We manufacture our various allergy diagnostic products at our Des Moines facility, our Loveland facility and our Fribourg facility. We believe the raw materials for products we manufacture are available from several sources.
Product Development
We are committed to providing innovative products to address health needs of companion animals. We may obtain such products from external sources, external collaboration or internal research and development.
We are committed to identifying external product opportunities and creating business and technical collaborations that lead to high value veterinary products. We believe that our active participation in scientific networks and our reputation for investing in research enhances our ability to acquire external product opportunities. We have collaborated, and intend to continue to do so, with a number of companies and universities. Examples of such collaborations include:
| • | Quidel for the development of SOLO STEP CH Cassettes, SOLO STEP CH Batch Test Strips and SOLO STEP FH Cassettes; |
| • | Boule for the development of veterinary applications for the HEMATRUE Veterinary Hematology Analyzer and associated reagents; and |
| • | FUJIFILM for the development of veterinary applications for the DRI-CHEM 7000 Veterinary Chemistry Analyzer and associated slides and supplies. |
Internal research and development is managed on a case-by-case basis. We employ individuals with microbiology, immunology, genetics, biochemistry, molecular biology, parasitology as well as veterinary expertise and will form multidisciplinary product-associated teams as appropriate. We incurred expenses of $1.7 million, $1.6 million and $1.7 million in the years ended December 31, 2009, 2010 and 2011, respectively, in support of our research and development activities.
6
Intellectual Property
We believe that patents, trademarks, copyrights and other proprietary rights are important to our business. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position. The proprietary technologies of our OVP segment are primarily protected through trade secret protection of, for example, our manufacturing processes in this area.
We actively seek patent protection both in the United States and abroad. Our issued and pending patent portfolios primarily relate to heartworm control, flea control, allergy, infectious disease vaccines, diagnostic and detection tests, immunomodulators, instrumentation, nutrition, pain control and vaccine delivery technologies. As of December 31, 2011, we owned, co-owned or had rights to 183 issued U.S. patents and 4 pending U.S. patent applications expiring at various dates from February 2013 to May 2028. Applications corresponding to pending U.S. applications have been or will be filed in other countries. Our corresponding foreign patent portfolio as of December 31, 2011 included 131 issued patents and 14 pending applications in various foreign countries expiring at various dates from June 2012 to January 2027.
We also have obtained exclusive and non-exclusive licenses for numerous other patents held by academic institutions and biotechnology and pharmaceutical companies.
Seasonality
We expect to experience less seasonality than we have in the past due to factors including increased instrument consumable revenue, which does not tend to be seasonal, and changes in the timing of certain product promotions. We anticipate the second half of 2012 will be significantly more profitable than the first half of 2012.
Government Regulation
Although the majority of our revenue is from the sale of unregulated items, many of our products or products that we may develop are, or may be, subject to extensive regulation by governmental authorities in the United States, including the USDA and the FDA, and by similar agencies in other countries. These regulations govern, among other things, the development, testing, manufacturing, labeling, storage, pre-market approval, advertising, promotion, sale and distribution of our products. Satisfaction of these requirements can take several years to achieve and the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the product. Any product that we develop must receive all relevant regulatory approval or clearances, if required, before it may be marketed in a particular country. The following summarizes the major U.S. government agencies that regulate animal health products:
| • | USDA. Vaccines and certain single use, point-of-care diagnostics are considered veterinary biologics and are therefore regulated by the Center for Veterinary Biologics, or CVB, of the USDA. Industry data indicate that it takes approximately four years and in excess of $1.0 million to license a conventional vaccine for animals from basic research through licensing. In contrast to vaccines, single use, point-of-care diagnostics can typically be licensed by the USDA in about two years, at considerably less cost. However, vaccines or diagnostics that use innovative materials, such as those resulting from recombinant DNA technology, usually require additional time to license. The USDA licensing process involves the submission of several data packages. These packages include information on how the product will be manufactured, information on the efficacy and safety of the product in laboratory and target animal studies and information on performance of the product in field conditions. |
7
| • | FDA. Pharmaceutical products, which typically include synthetic compounds, are approved and monitored by the Center for Veterinary Medicine of the FDA. Industry data indicate that developing a new drug for animals requires approximately 11 years from commencement of research to market introduction and costs approximately $5.5 million. Of this time, approximately three years is spent in animal studies and the regulatory review process. However, unlike human drugs, neither preclinical studies nor a sequential phase system of studies are required. Rather, for animal drugs, studies for safety and efficacy may be conducted immediately in the species for which the drug is intended. Thus, there is no required phased evaluation of drug performance, and the Center for Veterinary Medicine will review data at appropriate times in the drug development process. In addition, the time and cost for developing companion animal drugs may be significantly less than for drugs for livestock animals, as food safety issues relating to tissue residue levels are not applicable. |
| • | EPA. Products that are applied topically to animals or to premises to control external parasites are regulated by the Environmental Protection Agency, or EPA. |
After we have received regulatory licensing or approval for our products, numerous regulatory requirements typically apply. Among the conditions for certain regulatory approvals is the requirement that our manufacturing facilities or those of our third-party manufacturers conform to current Good Manufacturing Practices or other manufacturing regulations, which include requirements relating to quality control and quality assurance as well as maintenance of records and documentation. The USDA, FDA and foreign regulatory authorities strictly enforce manufacturing regulatory requirements through periodic inspections and/or reports.
A number of our animal health products are not regulated. For example, certain products such as our ALLERCEPT panels, as well as other reference lab tests, are not regulated by either the USDA or FDA. Similarly, none of our veterinary instruments requires regulatory approval to be marketed and sold in the United States.
We have pursued regulatory approval outside the United States based on market demographics of foreign countries. For marketing outside the United States, we are subject to foreign regulatory requirements governing regulatory licensing and approval for many of our products. Licensing and approval by comparable regulatory authorities of foreign countries must be obtained before we can market products in those countries. Product licensing approval processes and requirements vary from country to country and the time required for such approvals may differ substantially from that required in the United States. We cannot be certain that approval of any of our products in one country will result in approvals in any other country. To date, we or our distributors have sought regulatory approval for certain of our products in Canada, which is governed by the Canadian Food Inspection Agency, or CFIA; in Japan, which is governed by the Japanese Ministry of Agriculture, Forestry and Fisheries, or MAFF; in Australia, which is governed by the Australian Department of Agriculture, Fisheries and Forestry, or ADAFF; South Africa, which is governed by the Republic of South Africa Department of Agriculture, or RSADA; and in certain other countries requiring such approval.
Core Companion Animal Health products previously discussed which have received regulatory approval in the United States and/or elsewhere are summarized below.
8
| Products |
Country |
Regulated | Agency | Status | ||||
| ALLERCEPT Allergy Treatment Sets |
United States | Yes | USDA | Licensed | ||||
| FELINE ULTRANASAL FVRC Vaccine |
United States Canada South Africa |
Yes Yes Yes |
USDA CFIA RSADA |
Licensed Licensed Licensed | ||||
| FELINE ULTRANASAL FVRCP Vaccine |
United States Canada South Africa |
Yes Yes Yes |
USDA CFIA RSADA |
Licensed Licensed Licensed | ||||
| SOLO STEP CH |
United States | Yes | USDA | Licensed | ||||
| EU Canada Japan Australia |
No-in most countries Yes Yes Yes |
CFIA MAFF ADAFF |
Licensed Licensed Licensed | |||||
| SOLO STEP CH Batch Test Strips |
United States Canada |
Yes Yes |
USDA CFIA |
Licensed Licensed | ||||
| SOLO STEP FH |
United States Australia |
Yes Yes |
USDA ADAFF |
Licensed Licensed | ||||
| TRI-HEART Plus Heartworm Preventive |
United States Japan South Korea |
Yes Yes Yes |
FDA MAFF NVRQS |
Licensed Licensed Licensed | ||||
Competition
Our market is intensely competitive. Our competitors include independent animal health companies and major pharmaceutical companies that have animal health divisions. We also compete with independent, third-party distributors, including distributors who sell products under their own private labels. In the point-of-care diagnostic testing market, our major competitors include IDEXX Laboratories, Inc. (“IDEXX”), Abaxis, Inc. (“Abaxis”) and Synbiotics Corporation (“Synbiotics”), a company acquired by Pfizer Inc. (“Pfizer”) in January 2011. The products manufactured by our OVP segment for sale by third parties compete with similar products offered by a number of other companies, some of which have substantially greater financial, technical, research and other resources than us and may have more established marketing, sales, distribution and service organizations than our OVP segment’s customers. Companies with a significant presence in the animal health market such as Bayer AG, CEVA Santé Animale, Eli Lilly and Company, Merck, Novartis AG, Pfizer, sanofi-aventis, Vétoquinol S.A. and Virbac S.A. may be marketing or developing products that compete with our products or would compete with them if successfully developed. These and other competitors and potential competitors may have substantially greater financial, technical, research and other resources and larger, more established marketing, sales, distribution and service organizations than we do. Our competitors may offer broader product lines and have greater name recognition than we do.
Environmental Regulation
In connection with our product development activities and manufacturing of our biological, pharmaceutical and diagnostic and detection products, we are subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, handling and disposal of certain materials, biological specimens and wastes. Although we believe that we have complied with these laws, regulations and policies in all material respects and have not been required to take any significant action to correct any noncompliance, we may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. In the event of such an accident, we could be held liable for any damages that result and any such liability could exceed our resources.
9
Employees
As of December 31, 2011, we and our subsidiaries employed 277 people, of whom 134 were focused in production and technical and logistical services, including instrumentation service, 94 in sales, marketing and customer support, 43 in general administrative services, such as accounting, and 6 in product development. We believe that our ability to attract and retain skilled personnel is critical to our success. None of our employees is covered by a collective bargaining agreement, and we believe our employee relations are good.
Where You Can Find Additional Information
You may review a copy of this annual report on Form 10-K, including exhibits and any schedule filed therewith, and obtain copies of such materials at prescribed rates, at the Securities and Exchange Commission’s Public Reference Room in Room 1580, 100 F Street, NE, Washington, D.C. 20549-0102. You may obtain information on the operation of the Public Reference Room by calling the Securities and Exchange Commission at 1-800-SEC-0330. The Securities and Exchange Commission maintains a website (http://www.sec.gov) that contains reports, proxy and information statements and other information regarding registrants, such as Heska Corporation, that file electronically with the Securities and Exchange Commission.
Executive Officers of the Registrant
Our executive officers and their ages as of February 28, 2012 are as follows:
| Name |
Age | Position | ||||
| Robert B. Grieve, Ph.D. |
60 | Chairman of the Board and Chief Executive Officer | ||||
| Michael J. McGinley, Ph.D. |
51 | President and Chief Operating Officer | ||||
| Jason A. Napolitano |
43 | Executive Vice President, Chief Financial Officer and Secretary | ||||
| Joseph P. Aperfine, Jr. |
50 | Executive Vice President, Sales and Marketing | ||||
| Nancy Wisnewski, Ph.D. |
49 | Executive Vice President, Product Development and Customer Support | ||||
| Michael A. Bent |
57 | Vice President, Principal Accounting Officer and Controller | ||||
| Claudine M. Zachara |
44 | Vice President, Marketing and Communications | ||||
Robert B. Grieve, Ph.D., one of our founders, currently serves as Chief Executive Officer and Chairman of the Board. Dr. Grieve was named Chief Executive Officer effective January 1, 1999, Vice Chairman effective March 1992 and Chairman of the Board effective May 2000. Dr. Grieve also served as Chief Scientific Officer from December 1994 to January 1999 and Vice President, Research and Development, from March 1992 to December 1994. He has been a member of our Board of Directors since 1990. He holds a Ph.D. degree from the University of Florida and M.S. and B.S. degrees from the University of Wyoming.
Michael J. McGinley, Ph.D. was appointed President and Chief Operating Officer effective January 1, 2009. He previously served as Vice President, Global Operations from April through December 2008, Vice President, Operations and Technical Affairs and General Manager, Heska Des Moines from January 2002 to April 2008 and in other positions beginning in June 1997. Prior to joining Heska, Dr. McGinley held positions with Bayer Animal Health and Fort Dodge Laboratories. He holds Doctorate and M.S. degrees in Immunobiology from Iowa State University and successfully completed the Advanced Management Program at the Harvard Business School in 2008.
10
Jason A. Napolitano was appointed Executive Vice President and Chief Financial Officer in May 2002. He was appointed our Secretary in February 2009. He also served as our Secretary from May 2002 to December 2006. Prior to joining us formally, he was a financial consultant. From 1990 to 2001, Mr. Napolitano held various positions at Credit Suisse First Boston, an investment bank, including Vice President in health care investment banking and Director in mergers and acquisitions. He holds a B.S. degree from Yale University.
Joseph P. Aperfine, Jr. was appointed Executive Vice President, Sales and Marketing effective May 1, 2011. Prior to joining Heska, Mr. Aperfine held positions with Banfield The Pet Hospital (“Banfield”), most recently as Chief Learning Officer. Mr. Aperfine was an officer of Banfield since September 2004. Mr. Aperfine was Chief Operating Officer at Merlin Digital Technology, a diagnostic imaging and telemedicine business which shared management and ownership with Banfield, from September 2004 until Merlin’s sale in March 2009. He was Vice President of Sales for Novartis Animal Health from May 2003 to September 2004. Mr. Aperfine was employed by IDEXX Laboratories, Inc. in various positions from March 1996 to May 2003. He holds a B.S. in Engineering from the United States Military Academy and is a former U.S. Army Ranger.
Nancy Wisnewski, Ph.D. was appointed Executive Vice President, Product Development and Customer Support in April 2011. She served as Vice President, Product Development and Technical Customer Service from December 2006 to April 2011. From January 2006 to November 2006, Dr. Wisnewski was Vice President, Research and Development. Dr. Wisnewski held various positions in Heska’s Research and Development organization between 1993 and 2005. She holds a Doctorate in Parasitology/Biochemistry from the University of Notre Dame and a B.S. in Biology from Lafayette College.
Michael A. Bent was appointed Vice President, Principal Accounting Officer and Controller in May 2002. From September 1999 until April 2002, he was Corporate Controller. From November 1993 until September 1999, Mr. Bent was Director, Accounting Operations at Coors Brewing Company. Mr. Bent holds a B.S. in accounting from the University of Wyoming. Mr. Bent is a CPA in Colorado and Wyoming.
Claudine M. Zachara was appointed Vice President, Marketing and Communications in October 2009. She previously served as Senior Director, Marketing and Communications from April 2009 to October 2009 and Director of Sales Operations and Communications from January 2007 to April 2009. Ms. Zachara also served in various positions in Heska’s Sales organization between May 2000 and December 2006. Prior to joining Heska, Ms. Zachara held positions with L’Oreal USA. She holds a B.A. degree from Arizona State University.
11
Our future operating results may vary substantially from period to period due to a number of factors, many of which are beyond our control. The following discussion highlights some of these factors and the possible impact of these factors on future results of operations. The risks and uncertainties described below are not the only ones we face. Additional risks or uncertainties not presently known to us or that we deem to be currently immaterial also may impair our business operations. If any of the following factors actually occur, our business, financial condition or results of operations could be harmed. In that case, the price of our common stock could decline and you could experience losses on your investment.
If the third parties to whom we granted substantial marketing rights for certain of our existing products or future products under development are not successful in marketing those products, then our sales and financial position may suffer.
Our agreements with our corporate marketing partners generally contain no or small minimum purchase requirements in order for them to maintain their exclusive marketing rights. We are party to an agreement with SPAH, a unit of Merck, which grants SPAH exclusive distribution and marketing rights in the U.S. for our canine heartworm preventive product, TRI-HEART Plus Chewable Tablets. Novartis Japan markets and distributes our SOLO STEP CH heartworm test in Japan under an exclusive arrangement. AgriLabs has the non-exclusive right to sell certain of our bovine vaccines in the United States, Africa and Mexico and currently generates the majority of our sales of those vaccines in those territories. One or more of these marketing partners may not devote sufficient resources to marketing our products and our sales and financial position could suffer significantly as a result. Revenue from Merck entities, including SPAH, represented 13% of our LTM revenue. If SPAH personnel fail to market, sell and support our heartworm preventive sufficiently, our sales could decline significantly. Furthermore, there may be nothing to prevent these partners from pursuing alternative technologies or products that may compete with our products in current or future agreements. For example, we believe a unit of Merck has obtained FDA approval for a canine heartworm preventive product with additional claims compared with our TRI-HEART Plus Chewable Tablets which we believe is not currently being marketed actively. Should SPAH or a unit of Merck decide to emphasize sales and marketing efforts of this product rather than our TRI-HEART Plus Chewable Tablets or cancel our agreement regarding canine heartworm preventive distribution and marketing, our sales could decline significantly. In the future, third-party marketing assistance may not be available on reasonable terms, if at all. If any of these events occur, we may not be able to maintain our current market share or commercialize our products and our sales will decline accordingly.
We may be unable to market and sell our products successfully.
We may not develop and maintain marketing and/or sales capabilities successfully, and we may not be able to make arrangements with third parties to perform these activities on satisfactory terms. If our marketing and sales strategy is unsuccessful, our ability to sell our products will be negatively impacted and our revenues will decrease. This could result in the loss of distribution rights for products or failure to gain access to new products and could cause damage to our reputation and adversely affect our business and future prospects.
We believe the recent worldwide economic weakness has had a negative effect on our business, and this may continue in the future. This is particularly notable in the sale of new instruments, which is a capital expenditure many, if not most, veterinarians may choose to defer in times of perceived economic weakness. Even if the overall economy begins to grow in the future, there may be a lag before veterinarians display confidence such growth will continue and return to historical capital expenditure purchasing patterns. As the vast majority of cash flow to veterinarians ultimately is funded by pet owners without private insurance or government support, our business may be more susceptible to severe economic downturns than other health care businesses which rely less on individual consumers.
12
The market for companion animal healthcare products is highly fragmented. Because our CCA proprietary products are generally available only to veterinarians or by prescription and our medical instruments require technical training to operate, we ultimately sell all our CCA products primarily to or through veterinarians. The acceptance of our products by veterinarians is critical to our success. Changes in our ability to obtain or maintain such acceptance or changes in veterinary medical practice could significantly decrease our anticipated sales.
We believe that one of our largest competitors, IDEXX, in effect prohibits its distributors from selling competitive products, including our diagnostic instruments and heartworm diagnostic tests, which may hinder our ability to sell and market our products if these distributors are increasingly successful.
The loss of significant customers could harm our operating results.
Revenue from Merck entities, including SPAH, represented approximately 13%, 13% and 11% of our total revenue for the twelve months ended December 31, 2011, 2010 and 2009, respectively. Sales to no other single customer accounted for more than 10% of our consolidated revenue for those periods. No single customer accounted for more than 10% of our consolidated accounts receivable at December 31, 2011 and 2010.
The loss of significant customers who, for example, are historically large purchasers or who are considered leaders in their field could damage our business and financial results.
We operate in a highly competitive industry, which could render our products obsolete or substantially limit the volume of products that we sell. This would limit our ability to compete and maintain sustained profitability.
The market in which we compete is intensely competitive. Our competitors include independent animal health companies and major pharmaceutical companies that have animal health divisions. We also compete with independent, third-party distributors, including distributors who sell products under their own private labels. In the point-of-care diagnostic testing market, our major competitors include IDEXX, Abaxis and Synbiotics, a company acquired by Pfizer in January 2011. The products manufactured by our OVP segment for sale by third parties compete with similar products offered by a number of other companies, some of which have substantially greater financial, technical, research and other resources than us and may have more established marketing, sales, distribution and service organizations than those of our OVP segment’s customers. Competitors may have facilities with similar capabilities to our OVP segment, which they may operate and sell at a lower unit price to customers than our OVP segment does, which could cause us to lose customers. Companies with a significant presence in the companion animal health market, such as Bayer AG, CEVA Santé Animale, Eli Lilly and Company, Merck, Novartis AG, Pfizer, sanofi-aventis, Vétoquinol S.A. and Virbac S.A. may be marketing or developing products that compete with our products or would compete with them if developed. These and other competitors and potential competitors may have substantially greater financial, technical, research and other resources and larger, more established marketing, sales and service organizations than we do. Our competitors may offer broader product lines and have greater name recognition than we do. For example, if Pfizer is successful in integrating Synbiotics or devotes its significant commercial and financial resources to growing Synbiotics’ market share, our sales could suffer significantly. Our competitors may also develop or market technologies or products that are more effective or commercially attractive than our current or future products or that would render our technologies and products obsolete. Further, additional competition could come from new entrants to the animal health care market. Moreover, we may not have the financial resources, technical expertise or marketing, sales or support capabilities to compete successfully. We believe that one of our largest competitors, IDEXX, in effect prohibits its
13
distributors from selling competitive products, including our diagnostic instruments and heartworm diagnostic tests. Another of our competitors, Abaxis, recently launched a veterinary diagnostic laboratory offering which may serve to intensify competition and lower our margins.
If we fail to compete successfully, our ability to achieve sustained profitability will be limited and sustained profitability, or profitability at all, may not be possible.
We rely substantially on third-party suppliers. The loss of products or delays in product availability from one or more third-party suppliers could substantially harm our business.
To be successful, we must contract for the supply of, or manufacture ourselves, current and future products of appropriate quantity, quality and cost. Such products must be available on a timely basis and be in compliance with any regulatory requirements. Failure to do so could substantially harm our business.
We rely on third-party suppliers to manufacture those products we do not manufacture ourselves. Proprietary products provided by these suppliers represent a majority of our revenue. We currently rely on these suppliers for our veterinary instruments and consumable supplies for these instruments, for key components of our point-of-care diagnostic and other tests as well as for the manufacture of other products.
The loss of access to products from one or more suppliers could have a significant, negative impact on our business. Major suppliers who sell us proprietary products which are responsible for more than 5% of our 2011 revenue are Boule, FUJIFILM and Quidel. None of these suppliers sold us proprietary products which were responsible for more than 20% of our 2011 revenue, although the proprietary products of one of these suppliers was responsible for more than 15% of our 2011 revenue and one was responsible for more than 10% of our 2011 revenue. We often purchase products from our suppliers under agreements that are of limited duration or potentially can be terminated on an annual basis. In the case of our veterinary diagnostic instruments other than for our lactate instrument, we are typically entitled to non-exclusive access to consumable supplies for a defined period upon expiration of exclusive rights, which could subject us to competitive pressures in the period of non-exclusive access. Although we believe we will be able to maintain supply of our major product offerings in the near future, there can be no assurance that our suppliers will meet their obligations under any agreements we may have in place with them or that we will be able to compel them to do so. Risks of relying on suppliers include:
| • | Inability to meet minimum obligations. Current agreements, or agreements we may negotiate in the future, may commit us to certain minimum purchase or other spending obligations. It is possible we will not be able to create the market demand to meet such obligations, which could create a drain on our financial resources and liquidity. Some such agreements may require minimum purchases and/or sales to maintain product rights and we may be significantly harmed if we are unable to meet such requirements and lose product rights. |
| • | Changes in economics. An underlying change in the economics with a supplier, such as a large price increase or new requirement of large minimum purchase amounts, could have a significant, adverse effect on our business, particularly if we are unable to identify and implement an alternative source of supply in a timely manner. |
| • | The loss of product rights upon expiration or termination of an existing agreement. Unless we are able to find an alternate supply of a similar product, we would not be able to continue to offer our customers the same breadth of products and our sales and operating results would likely suffer. In the case of an instrument supplier, we could also potentially suffer the loss of sales of consumable supplies, which would be significant in cases where we have built a significant installed base, further harming our sales prospects and opportunities. Even if we were able to find an alternate supply for a product to which we lost rights, we would likely face increased |
14
| competition from the product whose rights we lost being marketed by a third party or the former supplier and it may take us additional time and expense to gain the necessary approvals and launch an alternative product. |
| • | Loss of exclusivity. In the case of our veterinary diagnostic instruments, if we are entitled to non-exclusive access to consumable supplies for a defined period upon expiration of exclusive rights, we may face increased competition from a third party with similar non-exclusive access or our former supplier, which could cause us to lose customers and/or significantly decrease our margins and could significantly affect our financial results. For example, a third party has gained access to chemistry instrument test strips and supplies for our previous chemistry instrument which are provided to us by Arkray, has increased competition for these products with our customers and such competition may cause us to lose customers and/or significantly decrease our margins in the future. In addition, current agreements, or agreements we may negotiate in the future, with suppliers may require us to meet minimum annual sales levels to maintain our position as the exclusive distributor of these products. We may not meet these minimum sales levels and maintain exclusivity over the distribution and sale of these products. If we are not the exclusive distributor of these products, competition may increase significantly, reducing our revenues and/or decreasing our margins. |
| • | High switching costs. In our diagnostic instrument products we could face significant competition and lose all or some of the consumable revenues from the installed base of those instruments if we were to switch to a competitive instrument. If we need to change to other commercial manufacturing contractors for certain of our regulated products, additional regulatory licenses or approvals generally must be obtained for these contractors prior to our use. This would require new testing and compliance inspections prior to sale thus resulting in potential delays. Any new manufacturer would have to be educated in, or develop, substantially equivalent processes necessary for the production of our products. We likely would have to train our sales force, distribution network employees and customer support organization on the new product and spend significant funds marketing the new product to our customer base. |
| • | The involuntary or voluntary discontinuation of a product line. Unless we are able to find an alternate supply of a similar product in this or similar circumstances with any product, we would not be able to continue to offer our customers the same breadth of products and our sales would likely suffer. Even if we are able to identify an alternate supply, it may take us additional time and expense to gain the necessary approvals and launch an alternative product, especially if the product is discontinued unexpectedly. |
| • | Inconsistent or inadequate quality control. We may not be able to control or adequately monitor the quality of products we receive from our suppliers. Poor quality items could damage our reputation with our customers. |
| • | Limited capacity or ability to scale capacity. If market demand for our products increases suddenly, our current suppliers might not be able to fulfill our commercial needs, which would require us to seek new manufacturing arrangements and may result in substantial delays in meeting market demand. If we consistently generate more demand for a product than a given supplier is capable of handling, it could lead to large backorders and potentially lost sales to competitive products that are readily available. This could require us to seek or fund new sources of supply, which may be difficult to find or under terms that are less advantageous if available. |
| • | Regulatory risk. Our manufacturing facility and those of some of our third-party suppliers are subject to ongoing periodic unannounced inspection by regulatory authorities, including the FDA, USDA and other federal, state and foreign agencies for compliance with strictly enforced Good |
15
| Manufacturing Practices, regulations and similar foreign standards. We do not have control over our suppliers’ compliance with these regulations and standards. Regulatory violations could potentially lead to interruptions in supply that could cause us to lose sales to readily available competitive products. |
| • | Developmental delays. We may experience delays in the scale-up quantities needed for product development that could delay regulatory submissions and commercialization of our products in development, causing us to miss key opportunities. |
| • | Limited intellectual property rights. We typically do not have intellectual property rights, or may have to share intellectual property rights, to the products supplied by third parties and any improvements to the manufacturing processes or new manufacturing processes for these products. |
Potential problems with suppliers such as those discussed above could substantially decrease sales, lead to higher costs and/or damage our reputation with our customers due to factors such as poor quality goods or delays in order fulfillment, resulting in our being unable to sell our products effectively and substantially harm our business.
Our future revenues depend on successful product development, commercialization and/or market acceptance, any of which can be slower than we expect or may not occur.
The product development and regulatory approval process for many of our potential products is extensive and may take substantially longer than we anticipate. Research projects may fail. New products that we may be developing for the veterinary marketplace may not perform consistent with our expectations. Because we have limited resources to devote to product development and commercialization, any delay in the development of one product or reallocation of resources to product development efforts that prove unsuccessful may delay or jeopardize the development of other product candidates. If we fail to successfully develop new products and bring them to market in a timely manner, our ability to generate additional revenue will decrease.
Even if we are successful in the development of a product or obtain rights to a product from a third-party supplier, we may experience delays or shortfalls in commercialization and/or market acceptance of the product. For example, veterinarians may be slow to adopt a product or there may be delays in producing large volumes of a product. The former is particularly likely where there is no comparable product available or historical use of such a product. The ultimate adoption of a new product by veterinarians, the rate of such adoption and the extent veterinarians choose to integrate such a product into their practice are all important factors in the economic success of one of our new products and are factors that we do not control to a large extent. If our products do not achieve a significant level of market acceptance, demand for our products will not develop as expected and our revenues will be lower than we anticipate. For example, our VitalPath Blood Gas and Electrolyte Analyzer generated less revenue than we anticipated following its launch in May 2010 as placements of this product with customers have not occurred as we expected.
We may face costly legal disputes, including related to our intellectual property or technology or that of our suppliers or collaborators.
We may face legal disputes related to our business. Even if meritless, these disputes may require significant expenditures on our part and could entail a significant distraction to members of our management team or other key employees. We may have to use legal means to collect payment for goods shipped to third parties. For example, we are currently involved in arbitration with a former distributor to whom we gave notice of contract termination in January 2010 regarding matters including amounts past due, for which we have recorded no specific reserves and counterclaims made by the former distributor. A legal dispute leading to an unfavorable ruling or settlement could have significant material adverse consequences on our business.
16
We may become subject to patent infringement claims and litigation in the United States or other countries or interference proceedings conducted in the United States Patent and Trademark Office, or USPTO, to determine the priority of inventions. The defense and prosecution of intellectual property suits, USPTO interference proceedings and related legal and administrative proceedings are likely to be costly, time-consuming and distracting. As is typical in our industry, from time to time we and our collaborators and suppliers have received, and may in the future receive, notices from third parties claiming infringement and invitations to take licenses under third-party patents. Any legal action against us or our collaborators or suppliers may require us or our collaborators or suppliers to obtain one or more licenses in order to market or manufacture affected products or services. However, we or our collaborators or suppliers may not be able to obtain licenses for technology patented by others on commercially reasonable terms, or at all, may not be able to develop alternative approaches if unable to obtain licenses or current and future licenses may not be adequate, any of which could substantially harm our business.
We may also need to pursue litigation to enforce any patents issued to us or our collaborative partners, to protect trade secrets or know-how owned by us or our collaborative partners, or to determine the enforceability, scope and validity of the proprietary rights of others. Any litigation or interference proceeding will likely result in substantial expense to us and significant diversion of the efforts of our technical and management personnel. Any adverse determination in litigation or interference proceedings could subject us to significant liabilities to third parties. Further, as a result of litigation or other proceedings, we may be required to seek licenses from third parties which may not be available on commercially reasonable terms, if at all.
We may not be able to continue to achieve sustained profitability or increase profitability on a quarterly or annual basis.
Prior to 2005, we incurred net losses on an annual basis since our inception in 1988 and, as of December 31, 2011, we had an accumulated deficit of $169.6 million. We have achieved only two quarters with income before income taxes greater than $1.5 million. Accordingly, relatively small differences in our performance metrics may cause us to generate an operating or net loss in future periods. Our ability to continue to be profitable in future periods will depend, in part, on our ability to increase sales in our CCA segment, including maintaining and growing our installed base of instruments and related consumables, to maintain or increase gross margins and to limit the increase in our operating expenses to a reasonable level as well as avoid or effectively manage any unanticipated issues. We may not be able to generate, sustain or increase profitability on a quarterly or annual basis. If we cannot achieve or sustain profitability for an extended period, we may not be able to fund our expected cash needs, including the repayment of debt as it comes due, or continue our operations.
Obtaining and maintaining regulatory approvals in order to market our products may be costly and delay the marketing and sales of our products. Failure to meet all regulatory requirements could cause significant losses from affected inventory and the loss of market share.
Many of the products we develop, market or manufacture may subject us to extensive regulation by one or more of the USDA, the FDA, the EPA and foreign and other regulatory authorities. These regulations govern, among other things, the development, testing, manufacturing, labeling, storage, pre-market approval, advertising, promotion and sale of some of our products. Satisfaction of these requirements can take several years and time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the product. The decision by a regulatory authority to regulate a currently non-regulated product or product area could significantly impact our revenue and have a corresponding adverse impact on our financial performance and position while we attempt to comply with the new regulation, if such compliance is possible at all.
17
The effect of government regulation may be to delay or to prevent marketing of our products for a considerable period of time and to impose costly procedures upon our activities. We have experienced in the past, and may experience in the future, difficulties that could delay or prevent us from obtaining the regulatory approval or license necessary to introduce or market our products. Such delays in approval may cause us to forego a significant portion of a new product’s sales in its first year due to seasonality and advanced booking periods associated with certain products. Regulatory approval of our products may also impose limitations on the indicated or intended uses for which our products may be marketed. Difficulties in making established products to all regulatory specifications may lead to significant losses related to affected inventory as well as market share. For instance, in 2010 we discovered we had produced a significant level of cattle vaccine product in our OVP segment which conformed to regulatory specifications for safety, potency and efficacy but not purity. We did not ship any related cattle vaccine product in the three months ended June 30, 2010 as we investigated and worked to resolve the situation. There can be no assurance that our efforts at remediation to ensure this or similar problems will not recur in the future will be successful or that the USDA will not suspend our ability to produce these, similar or other products for an extended time at some point in the future.
Among the conditions for certain regulatory approvals is the requirement that our facilities and/or the facilities of our third-party manufacturers conform to current Good Manufacturing Practices and other requirements. If any regulatory authority determines that our manufacturing facilities or those of our third-party manufacturers do not conform to appropriate manufacturing requirements, we or the manufacturers of our products may be subject to sanctions, including, but not limited to, warning letters, manufacturing suspensions, product recalls or seizures, injunctions, refusal to permit products to be imported into or exported out of the United States, refusals of regulatory authorities to grant approval or to allow us to enter into government supply contracts, withdrawals of previously approved marketing applications, civil fines and criminal prosecutions. In addition, certain of our agreements may require us to pay penalties if we are unable to supply products, including for failure to maintain regulatory approvals. Any of these events, alone or in unison, could damage our business.
We often depend on third parties for products we intend to introduce in the future. If our current relationships and collaborations are not successful, we may not be able to introduce the products we intend to in the future.
We are often dependent on third parties and collaborative partners to successfully and timely perform research and development activities to successfully develop new products. For example, we jointly developed point-of-care diagnostic products with Quidel. In other cases, we have discussed Heska marketing in the veterinary market an instrument being developed by a third party for use in the human health care market. In the future, one or more of these third parties or collaborative partners may not complete research and development activities in a timely fashion, or at all. Even if these third parties are successful in their research and development activities, we may not be able to come to an economic agreement with them. If these third parties or collaborative partners fail to complete research and development activities, fail to complete them in a timely fashion, or if we are unable to negotiate economic agreements with such third parties or collaborative partners, our ability to introduce new products will be impacted negatively and our revenues may decline.
18
Many of our expenses are fixed and if factors beyond our control cause our revenue to fluctuate, this fluctuation could cause greater than expected losses, cash flow and liquidity shortfalls.
We believe that our future operating results will fluctuate on a quarterly basis due to a variety of factors which are generally beyond our control, including:
| • | supply of products from third-party suppliers or termination, cancelation or expiration of such relationships; |
| • | competition and pricing pressures from competitive products; |
| • | the introduction of new products or services by our competitors or by us; |
| • | large customers failing to purchase at historical levels; |
| • | fundamental shifts in market demand; |
| • | manufacturing delays; |
| • | shipment problems; |
| • | information technology problems, which may prevent us from conducting our business effectively, or at all, and may also raise our costs; |
| • | regulatory and other delays in product development; |
| • | product recalls or other issues which may raise our costs; |
| • | changes in our reputation and/or market acceptance of our current or new products; and |
| • | changes in the mix of products sold. |
We have high operating expenses, including those related to personnel. Many of these expenses are fixed in the short term and may increase over the course of the coming year. If any of the factors listed above cause our revenues to decline, our operating results could be substantially harmed.
Our stock price has historically experienced high volatility, and could do so in the future, including experiencing a material price decline resulting from a large sale in a short period of time. In addition, our Public Common Stock has certain transfer restrictions which could reduce trading liquidity from what it otherwise would have been and have other undesired effects. Our recently completed 1-for-10 reverse stock split could also reduce liquidity in our stock.
According to the latest available filings with the SEC, we have one shareholder who holds approximately 15% of our shares outstanding and another who holds approximately 8% of our shares outstanding. Should either of these shareholders or another relatively large shareholder decide to sell a large number of shares in a short period of time, it could lead to an excess supply of our shares available for sale and correspondingly result in a significant decline in our stock price. For example, we had a shareholder who held over 16% of our shares outstanding as of September 30, 2011 sell all of its holdings in our stock on or before December 7, 2011 – and we believe this contributed to a corresponding decline in our stock price during this period.
The securities markets have experienced significant price and volume fluctuations and the market prices of securities of many microcap and smallcap companies have in the past been, and can in the future be expected to be, especially volatile. During the twelve months ended December 31, 2011, our closing stock price has ranged from a low of $4.66 to a high of $10.19. Fluctuations in the trading price or liquidity of our Public Common Stock may adversely affect our ability to raise capital through future equity financings. Factors that may have a significant impact on the market price and marketability of our Public Common Stock include:
| • | stock sales by large stockholders or by insiders; |
| • | changes in the outlook for our business, including any changes in our earnings guidance; |
| • | our quarterly operating results, including as compared to our revenue, earnings or other guidance and in comparison to historical results; |
19
| • | termination, cancellation or expiration of our third-party supplier relationships; |
| • | announcements of technological innovations or new products by our competitors or by us; |
| • | litigation; |
| • | regulatory developments, including delays in product introductions; |
| • | developments or disputes concerning patents or proprietary rights; |
| • | availability of our revolving line of credit and compliance with debt covenants; |
| • | releases of reports by securities analysts; |
| • | economic and other external factors; and |
| • | general market conditions. |
In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted. If a securities class action suit is filed against us, it is likely we would incur substantial legal fees and our management’s attention and resources would be diverted from operating our business in order to respond to the litigation.
On May 4, 2010, our shareholders approved an amendment (the “Amendment”) to our Restated Certificate of Incorporation. The Amendment places restrictions on the transfer of our stock that could adversely affect our ability to use our domestic Federal Net Operating Loss carryforward (“NOL”). In particular, the Amendment prevents the transfer of shares without the approval of our Board of Directors if, as a consequence, an individual, entity or groups of individuals or entities would become a 5-percent holder under Section 382 of the Internal Revenue Code of 1986, as amended, and the related Treasury regulations, and also prevents any existing 5-percent holder from increasing his or her ownership position in the Company without the approval of our Board of Directors. This may cause certain individuals or entities who may have otherwise been willing and able to bid on our stock to not do so, reducing the class of potential acquirers and trading liquidity from what it otherwise might have been. The Amendment could also have an adverse impact on the value of our stock if certain buyers who would otherwise have purchased our stock, including buyers who may not be comfortable owning stock with transfer restrictions, do not purchase our stock as a result of the Amendment. In addition, because some corporate takeovers occur through the acquirer’s purchase, in the public market or otherwise, of sufficient shares to give it control of a company, any provision that restricts the transfer of shares can have the effect of preventing a takeover. The Amendment could discourage or otherwise prevent accumulations of substantial blocks of shares in which our common stockholders might receive a substantial premium above market value and might tend to insulate management and the Board of Directors against the possibility of removal to a greater degree than had the Amendment not passed.
We completed a 1-for-10 reverse stock split effective December 30, 2010. The liquidity of our Public Common Stock could be adversely affected by the reduced number of shares resulting from the reverse stock split. Our reverse stock split may have left certain stockholders with one or more “odd lots”, which are stock holdings in fewer than 100 shares of Public Common Stock. These odd lots may be more difficult to sell and may incur higher brokerage commissions when sold than shares of our Public Common Stock in multiples of 100, reducing liquidity. Furthermore, due to the increased price per share following our 1-for-10 reverse stock split, certain smaller investors may be unwilling or unable to purchase shares of our Public Common Stock, also reducing liquidity.
Changes to, differing interpretations of, or changing circumstances under which we apply financial accounting standards may affect our results of operations, cause us to change our business practices or have a negative impact on us if we fail to track such changes.
We prepare our financial statements in conformance with United States generally accepted accounting principles, or GAAP. These accounting principles are established by and are subject to interpretation by the SEC, the Financial Accounting Standards Board (“FASB”) and others who interpret and create accounting policies. A change in those policies can have a significant effect on our reported results and
20
may affect our reporting of transactions completed before a change is made effective. Such changes may adversely affect our reported financial results, the way we conduct our business or have a negative impact on us if we fail to track such changes. For example, we have found FASB’s recent decision to codify the accounting standards has made it more difficult to research complex accounting matters, increasing the risk we will fail to account consistent with FASB rules in the future. Similarly, changes in the underlying circumstances which we apply given accounting standards and principles may affect our results of operations and have a negative impact on us. For example, in the twelve months ended December 31, 2011, we recognized approximately $1 million in revenue related to large upfront payments received over five years ago from three third parties for certain product rights. In all three cases, we have deferred the revenue with a related deferred revenue liability on our balance sheet and recognized the revenue over the estimated life of the related agreements, products, patents or technology. If we are to conclude any or all of the estimated lives of the related agreements, products, patents or technology are to be extended, it could lead to a significant reduction in related revenue recognized in corresponding future periods.
Our Public Common Stock is listed on the Nasdaq Capital Market and we may not be able to maintain that listing, which may make it more difficult for you to sell your shares.
Our Public Common Stock is listed on the Nasdaq Capital Market. The Nasdaq has several quantitative and qualitative requirements companies must comply with to maintain this listing, including a $1.00 minimum bid price. We completed a 1-for-10 reverse stock split effective December 30, 2010 in order to resolve an ongoing minimum bid price deficiency. While we believe we are currently in compliance with all Nasdaq requirements, there can be no assurance we will continue to meet Nasdaq listing requirements including the minimum bid price, that Nasdaq will interpret these requirements in the same manner we do if we believe we meet the requirements, or that Nasdaq will not change such requirements or add new requirements to include requirements we do not meet in the future. If we are delisted from the Nasdaq Capital Market, our Public Common Stock may be considered a penny stock under the regulations of the SEC and would therefore be subject to rules that impose additional sales practice requirements on broker-dealers who sell our securities. The additional burdens imposed upon broker-dealers may discourage broker-dealers from effecting transactions in our Public Common Stock, which could severely limit market liquidity of the Public Common Stock and any stockholder’s ability to sell our securities in the secondary market. This lack of liquidity would also likely make it more difficult for us to raise capital in the future.
We depend on key personnel for our future success. If we lose our key personnel or are unable to attract and retain additional personnel, we may be unable to achieve our goals.
Our future success is substantially dependent on the efforts of our senior management and other key personnel. The loss of the services of members of our senior management or other key personnel may significantly delay or prevent the achievement of our business objectives. Although we have an employment agreement with many of these individuals, all are at-will employees, which means that either the employee or Heska may terminate employment at any time without prior notice. If we lose the services of, or fail to recruit, key personnel, the growth of our business could be substantially impaired. We do not maintain key person life insurance for any of our senior management or key personnel.
We have historically not consistently generated positive cash flow from operations, may need additional capital and any required capital may not be available on reasonable terms or at all.
If our actual performance deviates from our operating plan, we may be required to raise additional capital in the future. If necessary, we expect to raise these additional funds by borrowing under our revolving line of credit, the sale of equity securities or the issuance of new term debt secured by the same assets as the term loans which we fully repaid in 2010. There is no guarantee that additional capital will be available from these sources on reasonable terms, if at all, and certain of these sources may require approval by existing lenders. Funds we expect to be available under our existing revolving line of credit may not be available and
21
other lenders could refuse to provide us with additional debt financing. The public markets may be unreceptive to equity financings and we may not be able to obtain additional private equity or debt financing. Any equity financing would likely be dilutive to stockholders and additional debt financing, if available, may include restrictive covenants and increased interest rates that would limit our currently planned operations and strategies. We believe the credit markets are particularly restrictive and difficult to obtain funding in versus recent history. Furthermore, even if additional capital is available, it may not be of the magnitude required to meet our needs under these or other scenarios. If additional funds are required and are not available, it would likely have a material adverse effect on our business, financial condition and our ability to continue as a going concern.
If we are unable to maintain various financial and other covenants required by our credit facility agreement we will be unable to borrow any funds under the agreement and fund our operations.
Under our credit and security agreement with Wells Fargo Bank, National Association (“Wells Fargo”), we are required to comply with various financial and non-financial covenants in order to borrow under the agreement. The availability of borrowings under this agreement may be important to continue to fund our operations. Among the financial covenants is a requirement to maintain minimum liquidity (cash plus excess borrowing base) of $1.5 million. Additional requirements include covenants for minimum capital monthly and minimum net income quarterly. Although we believe we will be able to maintain compliance with all these covenants and any covenants we may negotiate in the future, there can be no assurance thereof. We have not always been able to maintain compliance with all covenants under our credit and security agreement with Wells Fargo in the past. Although Wells Fargo granted us a waiver of non-compliance in each case, there can be no assurance we will be able to obtain similar waivers or other modifications if needed in the future on economic terms, if at all. Failure to comply with any of the covenants, representations or warranties, or failure to modify them to allow future compliance, could result in our being in default and could cause all outstanding borrowings under our credit and security agreement to become immediately due and payable, or impact our ability to borrow under the agreement. In addition, Wells Fargo has discretion in setting the advance rates which we may borrow against eligible assets. We may need to rely on available borrowings under the credit and security agreement to fund our operations in the future. If we are unable to borrow funds under this agreement, we will need to raise additional capital from other sources to continue our operations, which capital may not be available on acceptable terms, or at all.
Interpretation of existing legislation, regulations and rules or implementation of future legislation, regulations and rules could cause our costs to increase or could harm us in other ways.
The Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley”) has increased our required administrative actions and expenses as a public company since its enactment. The general and administrative costs of complying with Sarbanes-Oxley will depend on how it is interpreted over time. Of particular concern are the level of standards for internal control evaluation and reporting adopted under Section 404 of Sarbanes-Oxley. If our regulators and/or auditors adopt or interpret more stringent standards than we anticipate, we and/or our auditors may be unable to conclude that our internal controls over financial reporting are designed and operating effectively, which could adversely affect investor confidence in our financial statements. Even if we and our auditors are able to conclude that our internal controls over financial reporting are designed and operating effectively in such a circumstance, our general and administrative costs are likely to increase. Similarly, we are required to comply with the SEC’s mandate to provide interactive data using the eXtensible Business Reporting Language as an exhibit to certain SEC filings in 2012. Compliance with this mandate has required a significant time investment, which may have and may in the future preclude some of our employees from spending time on more productive matters. In addition, actions by other entities, such as enhanced rules to maintain our listing on the Nasdaq Capital Market, could also increase our general and administrative costs or have other adverse effects on us, as could further legislative, regulatory or rule-making action or more stringent interpretations of existing legislation, regulations and rules.
22
We may face product returns and product liability litigation in excess of or not covered by our insurance coverage or indemnities and/or warranties from our suppliers. If we become subject to product liability claims resulting from defects in our products, we may fail to achieve market acceptance of our products and our sales could substantially decline.
The testing, manufacturing and marketing of our current products as well as those currently under development entail an inherent risk of product liability claims and associated adverse publicity. Following the introduction of a product, adverse side effects may be discovered. Adverse publicity regarding such effects could affect sales of our other products for an indeterminate time period. To date, we have not experienced any material product liability claims, but any claim arising in the future could substantially harm our business. Potential product liability claims may exceed the amount of our insurance coverage or may be excluded from coverage under the terms of the policy. We may not be able to continue to obtain adequate insurance at a reasonable cost, if at all. In the event that we are held liable for a claim against which we are not indemnified or for damages exceeding the $10 million limit of our insurance coverage or which results in significant adverse publicity against us, we may lose revenue, be required to make substantial payments which could exceed our financial capacity and/or lose or fail to achieve market acceptance.
We may be held liable for the release of hazardous materials, which could result in extensive clean up costs or otherwise harm our business.
Certain of our products and development programs produced at our Des Moines, Iowa facility involve the controlled use of hazardous and biohazardous materials, including chemicals and infectious disease agents. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by applicable local, state and federal regulations, we cannot eliminate the risk of accidental contamination or injury from these materials. In the event of such an accident, we could be held liable for any fines, penalties, remediation costs or other damages that result. Our liability for the release of hazardous materials could exceed our resources, which could lead to a shutdown of our operations, significant remediation costs and potential legal liability. In addition, we may incur substantial costs to comply with environmental regulations if we choose to expand our manufacturing capacity.
Item 1B. Unresolved Staff Comments.
Not applicable.
Our principal administrative and research and development activities are located in Loveland, Colorado. We currently lease approximately 60,000 square feet at a facility in Loveland, Colorado under an agreement which expires in 2023. Our principal production facility located in Des Moines, Iowa, consists of 168,000 square feet of buildings on 34 acres of land, which we own. We also own a 175-acre farm used principally for testing products, located in Carlisle, Iowa. Our European facility in Fribourg, Switzerland is leased under an agreement which expires in 2015.
From time to time, we may be involved in litigation related to claims arising out of our operations. At December 31, 2011, we were not a party to any legal proceedings that are expected, individually or in the aggregate, to have a material adverse effect on our business, financial condition or operating results.
23
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our common stock is quoted on the Nasdaq Capital Market under the symbol “HSKA.” The following table sets forth the high and low sales prices for our Public Common Stock as reported by the Nasdaq Capital Market, adjusted for our 1-for-10 reverse stock split effective December 30, 2010, for the periods indicated below:
| High | Low | |||||||
| 2010 | ||||||||
| First Quarter |
$ | 10.80 | $ | 5.20 | ||||
| Second Quarter |
9.30 | 5.50 | ||||||
| Third Quarter |
6.80 | 4.10 | ||||||
| Fourth Quarter |
5.30 | 4.00 | ||||||
| 2011 |
||||||||
| First Quarter |
7.23 | 4.61 | ||||||
| Second Quarter |
10.04 | 6.12 | ||||||
| Third Quarter |
10.28 | 8.11 | ||||||
| Fourth Quarter |
8.64 | 6.53 | ||||||
| 2012 |
||||||||
| First Quarter (through February 27) |
9.50 | 6.83 | ||||||
As of February 27, 2012, there were approximately 250 holders of record of our Public Common Stock and approximately 3,700 beneficial stockholders. Historically, we have not declared or paid cash dividends on our capital stock. In the first quarter of 2012, however, our Board of Directors declared a cash dividend of $0.10 per share to the holders of record of our Public Common Stock on March 30, 2012, payable on April 10, 2012. We intend to pay a regular quarterly dividend of $0.10 per share of outstanding Public Common Stock for the foreseeable future at the discretion of our Board of Directors considering available cash, anticipated cash needs, overall financial condition, loan agreement restrictions, future prospects for earnings and cash flows, anticipated tax treatment as well as other relevant factors.
24
STOCK PRICE PERFORMANCE GRAPH
The following graph provides a comparison over the five-year period ended December 31, 2011 of the cumulative total stockholder return from a $100 investment in the Company’s common stock with the Center for Research in Securities Prices Total Return Index for Nasdaq Medical Devices, Instruments and Supplies, Manufacturers and Distributors Stocks (the “Nasdaq Medical Devices Index”), the CRSP Total Return Index for Nasdaq Pharmaceutical Stocks (the “Nasdaq Pharmaceutical Index”) and the CRSP Total Return Index for the Nasdaq Stock Market (U.S. and Foreign) (the “Nasdaq U.S. & Foreign Index”).
Comparison of Cumulative Total Return Among Heska Corporation,
the Nasdaq Medical Devices Index, the Nasdaq Pharmaceutical Index and the Nasdaq U.S. and Foreign Index
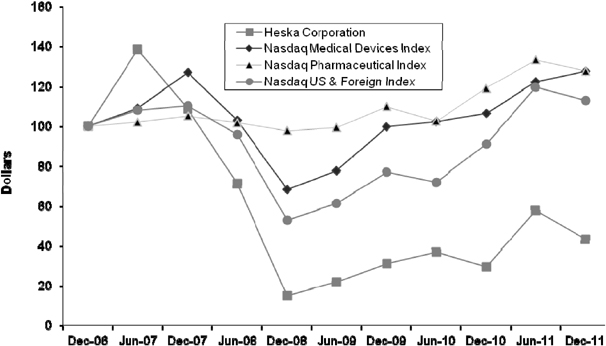
25
| Item 6. | Selected Financial Data. |
The following consolidated statement of operations and consolidated balance sheet data have been derived from our consolidated financial statements. The information set forth below is not necessarily indicative of the results of future operations and should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Consolidated Financial Statements and related Notes included as Items 7 and 8 in this Form 10-K. We completed a 1-for-10 reverse stock split effective December 30, 2010. Except as otherwise indicated, all related amounts reported below have been retroactively adjusted for the effect of this reverse stock split.
| Year Ended December 31, | ||||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | ||||||||||||||||
| (in thousands, except per share amounts) | ||||||||||||||||||||
| Consolidated Statement of Operations Data: |
||||||||||||||||||||
| Revenue: |
||||||||||||||||||||
| Core companion animal health |
$ | 67,279 | $ | 68,140 | $ | 66,449 | $ | 55,655 | $ | 57,481 | ||||||||||
| Other vaccines, pharmaceuticals and products |
15,056 | 13,513 | 9,229 | 9,796 | 12,584 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenue, net |
82,335 | 81,653 | 75,678 | 65,451 | 70,065 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cost of revenue |
49,148 | 52,809 | 47,219 | 40,659 | 40,878 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
33,187 | 28,844 | 28,459 | 24,792 | 29,187 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating expenses: |
||||||||||||||||||||
| Selling and marketing |
16,109 | 17,640 | 14,524 | 14,726 | 15,167 | |||||||||||||||
| Research and development |
2,679 | 1,951 | 1,718 | 1,597 | 1,650 | |||||||||||||||
| General and administrative |
8,925 | 8,917 | 8,173 | 8,111 | 9,121 | |||||||||||||||
| Restructuring expenses |
— | 785 | — | — | — | |||||||||||||||
| Other |
(47 | ) | 232 | — | — | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
27,666 | 29,525 | 24,415 | 24,434 | 25,938 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating income (loss) |
5,521 | (681 | ) | 4,044 | 358 | 3,249 | ||||||||||||||
| Interest and other (income) expense, net |
588 | 640 | 306 | 289 | (117 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) before income taxes |
4,933 | (1,321 | ) | 3,738 | 69 | 3,366 | ||||||||||||||
| Income tax expense (benefit) |
(29,875 | ) | (471 | ) | 1,496 | 51 | 1,221 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
$ | 34,808 | $ | (850 | ) | $ | 2,242 | $ | 18 | $ | 2,145 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic net income (loss) per share |
$ | 6.81 | $ | (0.17 | ) | $ | 0.43 | $ | 0.00 | $ | 0.41 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted net income (loss) per share |
$ | 6.27 | $ | (0.17 | ) | $ | 0.43 | $ | 0.00 | $ | 0.40 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Shares used for basic net income (loss) per share |
5,110 | 5,167 | 5,207 | 5,220 | 5,237 | |||||||||||||||
| Shares used for diluted net income (loss) per share |
5,551 | 5,167 | 5,212 | 5,254 | 5,338 | |||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 5,524 | $ | 4,705 | $ | 5,400 | $ | 5,492 | $ | 6,332 | ||||||||||
| Total current assets |
35,127 | 31,290 | 28,493 | 27,279 | 28,891 | |||||||||||||||
| Property and equipment, net |
10,669 | 8,509 | 6,349 | 5,486 | 4,869 | |||||||||||||||
| Total assets |
75,591 | 70,438 | 64,134 | 63,048 | 61,894 | |||||||||||||||
| Line of credit |
12,614 | 11,042 | 4,201 | 3,079 | — | |||||||||||||||
| Current portion of long-term debt and capital leases |
776 | 770 | 381 | — | — | |||||||||||||||
| Total current liabilities |
25,195 | 22,228 | 14,107 | 12,660 | 9,289 | |||||||||||||||
| Long-term debt and capital leases |
1,151 | 381 | — | — | — | |||||||||||||||
| Long-term deferred revenue and other |
6,362 | 5,306 | 4,972 | 4,590 | 4,166 | |||||||||||||||
| Total stockholders’ equity |
42,883 | 42,523 | 45,055 | 45,798 | 48,439 | |||||||||||||||
26
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with “Selected Consolidated Financial Data” and the Consolidated Financial Statements and related Notes included in Items 6 and 8 of this Form 10-K.
This discussion contains forward-looking statements that involve risks and uncertainties. Such statements, which include statements concerning future revenue sources and concentration, gross profit margins, selling and marketing expenses, research and development expenses, general and administrative expenses, capital resources, additional financings or borrowings and additional losses, are subject to risks and uncertainties, including, but not limited to, those discussed below and elsewhere in this Form 10-K, particularly in Item 1A “Risk Factors,” that could cause actual results to differ materially from those projected. The forward-looking statements set forth in this Form 10-K are as of the close of business on February 27, 2012, and we undertake no duty and do not intend to update this information.
Overview
We develop, manufacture, market, sell and support veterinary products. Our business is comprised of two reportable segments, Core Companion Animal Health, which represented 82% of our 2011 revenue, and Other Vaccines, Pharmaceuticals and Products which represented 18% of our 2011 revenue.
The Core Companion Animal Health segment (“CCA”) includes diagnostic and other instruments and supplies as well as single use diagnostic and other tests, pharmaceuticals and vaccines, primarily for canine and feline use.
Diagnostic and other instruments and supplies represented approximately 40% of our 2011 revenue. Many products in this area involve placing an instrument in the field and generating future revenue from consumables, including items such as supplies and service, as that instrument is used. Approximately 30% of our 2011 revenue resulted from the sale of such consumables to an installed base of instruments and approximately 10% of our revenue was from new hardware. A loss of or disruption in supply of consumables we are selling to an installed base of instruments could substantially harm our business. All of our diagnostic instruments and supplies are furnished to us by third parties, who typically own the product rights and sell the product to us under marketing and/or distribution agreements. In many cases, we have collaborated with a third party to adapt a human instrument for veterinary use. Major products in this area include our chemistry instruments, our hematology instruments and our blood gas instruments and their affiliated operating consumables. Revenue from products in these three areas, including revenue from consumables, represented approximately 36% of our 2011 revenue.
Other CCA revenue, including single use diagnostic and other tests, pharmaceuticals and vaccines as well as research and development, licensing and royalty revenue, represented approximately 42% of our 2011 revenue. Since items in this area are single use by their nature, our aim is to build customer satisfaction and loyalty for each product, generate repeat annual sales from existing customers and expand our customer base in the future. Products in this area are both supplied by third parties and provided by us. Major products in this area include our heartworm diagnostic tests, our heartworm preventive, our allergy test kits, our allergy immunotherapy and our allergy diagnostic tests. Combined revenue from heartworm-related products and allergy-related products represented approximately 37% of our 2011 revenue.
We consider the CCA segment to be our core business and devote most of our management time and other resources to improving the prospects for this segment. Maintaining a continuing, reliable and economic supply of products we currently obtain from third parties is critical to our success in this area. Virtually all of our sales and marketing expenses are in the Core Companion Animal Health segment. The majority of our research and development spending is dedicated to this segment, as well. We strive to provide high value products and advance the state of veterinary medicine.
27
All our CCA products ultimately are sold primarily to or through veterinarians. In many cases, veterinarians will mark up their costs to the end user. The acceptance of our products by veterinarians is critical to our success. CCA products are sold directly by us as well as through distribution relationships, such as our corporate agreement with SPAH, the sale of kits to conduct blood testing to third-party veterinary diagnostic laboratories and independent third-party distributors. Revenue from direct sales and distribution relationships represented approximately 67% and 33% of Core Companion Animal Health 2011 revenue, respectively.
We intend to increase profitability through a combination of revenue growth, gross margin improvement and expense control. Accordingly, we closely monitor revenue growth trends in our CCA segment. Revenue in this segment increased by $1.8 million, or 3%, in 2011 as compared to 2010. We believe poor economic conditions over the past several years have impacted our revenue as, for example, veterinarians have continued to delay or defer capital expenditures on new diagnostic instrumentation.
The Other Vaccines, Pharmaceuticals and Products segment (“OVP”) includes our 168,000 square foot USDA- and FDA-licensed production facility in Des Moines, Iowa. We view this facility as an asset which will allow us to control our cost of goods on any vaccines and pharmaceuticals that we may commercialize in the future. Virtually all our U.S. inventory is now stored at this facility and most fulfillment logistics are managed there. CCA segment products manufactured at this facility are transferred at cost and are not recorded as revenue for our OVP segment. We view OVP reported revenue as revenue primarily to cover the overhead costs of the facility and to generate incremental cash flow to fund our CCA segment.
Our OVP segment includes private label vaccine and pharmaceutical production, primarily for cattle but also for other animals such as small mammals. All OVP products are sold by third parties under third party labels.
We have developed our own line of bovine vaccines that are licensed by the USDA. We have a long-term, non-exclusive agreement with a distributor, Agri Laboratories, Ltd., (“AgriLabs”), for the marketing and sale of certain of these vaccines which are sold primarily under the Titanium® and MasterGuard® brands which are registered trademarks of AgriLabs. This agreement generates a significant portion of our OVP segment’s revenue. Our OVP segment also produces vaccines and pharmaceuticals for other third parties.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon the consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities as of the date of the financial statements, and the reported amounts of revenue and expense during the periods. These estimates are based on historical experience and various other assumptions that we believe to be reasonable under the circumstances. We have identified those critical accounting policies used in reporting our financial position and results of operations based upon a consideration of those accounting policies that involve the most complex or subjective decisions or assessment. We consider the following to be our critical policies.
Revenue Recognition
We generate our revenue through the sale of products, as well as through licensing of technology product rights, royalties and sponsored research and development. Our policy is to recognize revenue when the applicable revenue recognition criteria have been met, which generally include the following:
28
| • | Persuasive evidence of an arrangement exists; |
| • | Delivery has occurred or services rendered; |
| • | Price is fixed or determinable; and |
| • | Collectability is reasonably assured. |
Revenue from the sale of products is recognized after both the goods are shipped to the customer and acceptance has been received, if required, with an appropriate provision for estimated returns and allowances. We do not permit general returns of products sold. Certain of our products have expiration dates. Our policy is to exchange certain outdated, expired product with the same product. We record an accrual for the estimated cost of replacing the expired product expected to be returned in the future, based on our historical experience, adjusted for any known factors that reasonably could be expected to change historical patterns, such as regulatory actions which allow us to extend the shelf life of our products. Revenue from both direct sales to veterinarians and sales to independent third-party distributors are generally recognized when goods are shipped. Our products are shipped complete and ready to use by the customer. The terms of the customer arrangements generally pass title and risk of ownership to the customer at the time of shipment. Certain customer arrangements provide for acceptance provisions. Revenue for these arrangements is not recognized until the acceptance has been received or the acceptance period has lapsed. We reduce our revenue by the estimated cost of any rebates, allowances or similar programs, which are used as promotional programs.
Recording revenue from the sale of products involves the use of estimates and management judgment. We must make a determination at the time of sale whether the customer has the ability to make payments in accordance with arrangements. While we do utilize past payment history, and, to the extent available for new customers, public credit information in making our assessment, the determination of whether collectability is reasonably assured is ultimately a judgment decision that must be made by management. We must also make estimates regarding our future obligation relating to returns, rebates, allowances and similar other programs.
License revenue under arrangements to sell or license product rights or technology rights is recognized as obligations under the agreement are satisfied, which generally occurs over a period of time. Generally, licensing revenue is deferred and recognized over the estimated life of the related agreements, products, patents or technology. Nonrefundable licensing fees, marketing rights and milestone payments received under contractual arrangements are deferred and recognized over the remaining contractual term using the straight-line method.
Recording revenue from license arrangements involves the use of estimates. The primary estimate made by management is determining the useful life of the related agreement, product, patent or technology. We evaluate all of our licensing arrangements by estimating the useful life of either the product or the technology, the length of the agreement or the legal patent life and defer the revenue for recognition over the appropriate period.
Occasionally we enter into arrangements that include multiple elements. Such arrangements may include the licensing of technology and manufacturing of product. In these situations we must determine whether the various elements meet the criteria to be accounted for as separate elements. If the elements cannot be separated, revenue is recognized once revenue recognition criteria for the entire arrangement have been met or over the period that the Company’s obligations to the customer are fulfilled, as appropriate. If the elements are determined to be separable, the revenue is allocated to the separate elements based on relative fair value and
29
recognized separately for each element when the applicable revenue recognition criteria have been met. In accounting for these multiple element arrangements, we must make determinations about whether elements can be accounted for separately and make estimates regarding their relative fair values.
Allowance for Doubtful Accounts
We maintain an allowance for doubtful accounts receivable based on client-specific allowances, as well as a general allowance. Specific allowances are maintained for clients which are determined to have a high degree of collectability risk based on such factors, among others, as: (i) the aging of the accounts receivable balance; (ii) the client’s past payment experience; (iii) a deterioration in the client’s financial condition, evidenced by weak financial condition and/or continued poor operating results, reduced credit ratings, and/or a bankruptcy filing. In addition to the specific allowance, the Company maintains a general allowance for credit risk in its accounts receivable which is not covered by a specific allowance. The general allowance is established based on such factors, among others, as: (i) the total balance of the outstanding accounts receivable, including considerations of the aging categories of those accounts receivable; (ii) past history of uncollectable accounts receivable write-offs; and (iii) the overall creditworthiness of the client base. A considerable amount of judgment is required in assessing the realizability of accounts receivable. Should any of the factors considered in determining the adequacy of the overall allowance change, an adjustment to the provision for doubtful accounts receivable may be necessary.
Inventories
Inventories are stated at the lower of cost or market, cost being determined on the first-in, first-out method. Inventories are written down if the estimated net realizable value of an inventory item is less than its recorded value. We review the carrying cost of our inventories by product each quarter to determine the adequacy of our reserves for obsolescence. In accounting for inventories we must make estimates regarding the estimated net realizable value of our inventory. This estimate is based, in part, on our forecasts of future sales and shelf life of product.
Deferred Tax Assets – Valuation Allowance
Our deferred tax assets, such as an NOL, are reduced by an offsetting valuation allowance based on judgmental assessment of available evidence if we are unable to conclude that it is more likely than not that some or all of the related deferred tax assets will be realized. If we are able to conclude it is more likely than not that we will realize a future benefit from a deferred tax asset, we will reduce the related valuation allowance by an amount equal to the estimated quantity of income taxes we would pay in cash if we were not to utilize the deferred tax asset in the future. The first time this occurs in a given jurisdiction, it will result in a net deferred tax asset on our balance sheet and an income tax benefit of equal magnitude in our statement of operations in the period we make the determination. In future periods, we will then recognize as income tax expense the estimated quantity of income taxes we would have paid in cash had we not utilized the related deferred tax asset. The corresponding journal entry will be a reduction of our deferred tax asset. If there is a change regarding our tax position in the future, we will make a corresponding adjustment to the related valuation allowance.
30
Results of Operations
The following table summarizes our results of operations for the three most recent fiscal years:
| Year Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
| (in thousands except per share amounts) | ||||||||||||
| Consolidated Statement of Income Data: |
||||||||||||
| Revenue: |
||||||||||||
| Core companion animal health |
$ | 66,449 | $ | 55,655 | $ | 57,481 | ||||||
| Other vaccines, pharmaceuticals and products |
9,229 | 9,796 | 12,584 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue, net |
75,678 | 65,451 | 70,065 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cost of revenue |
47,219 | 40,659 | 40,878 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit |
28,459 | 24,792 | 29,187 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Selling and marketing |
14,524 | 14,726 | 15,167 | |||||||||
| Research and development |
1,718 | 1,597 | 1,650 | |||||||||
| General and administrative |
8,173 | 8,111 | 9,121 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
24,415 | 24,434 | 25,938 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating income |
4,044 | 358 | 3,249 | |||||||||
| Interest and other (income) expense, net |
306 | 289 | (117 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Income before income taxes |
3,738 | 69 | 3,366 | |||||||||
| Income tax expense |
1,496 | 51 | 1,221 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net income |
$ | 2,242 | $ | 18 | $ | 2,145 | ||||||
|
|
|
|
|
|
|
|||||||
| Basic net income per share |
$ | 0.43 | $ | 0.00 | $ | 0.41 | ||||||
|
|
|
|
|
|
|
|||||||
| Diluted net income per share |
$ | 0.43 | $ | 0.00 | $ | 0.40 | ||||||
|
|
|
|
|
|
|
|||||||
Revenue
Total revenue increased 7% to $70.1 million in 2011 compared to $65.5 million in 2010. Total revenue decreased 14% to $65.5 million in 2010 compared to $75.7 million in 2009.
CCA segment revenue increased 3% to $57.5 million in 2011 compared to $55.7 million in 2010. Key factors in the increase were greater sales of our instrument consumables, our canine heartworm preventive and international sales of our canine heartworm diagnostic tests, somewhat offset by lower revenue from our hematology instruments and our chemistry instruments. CCA segment revenue decreased 16% to $55.7 million in 2010 compared to $66.4 million in 2009. The largest factor in this decline was lower sales of consumables for our handheld blood analysis instruments which declined by $9.3 million in 2010 compared to 2009, primarily due to the loss of supply following cancellation of the underlying contract by our supplier. Other factors in the decline were lower sales of our heartworm diagnostic tests internationally and lower sales of our IV pumps.
OVP segment revenue increased 28% to $12.6 million in 2011 compared to $9.8 million in 2010. Greater sales of cattle vaccines to new customers, greater sales of cattle vaccines under our contract with AgriLabs and greater sales of other cattle vaccines, somewhat offset by lower sales of bulk bovine biologicals, were key factors in the increase. OVP segment revenue increased 6% to $9.8 million in 2010 compared to $9.2 million in 2009. Greater sales of bulk bovine and other biologicals were key factors in the increase. This was somewhat offset by lower sales of cattle vaccines under our contract with AgriLabs. We had issues producing cattle vaccines to appropriate specifications and, as a result, did not ship any related cattle vaccine products in the three months ended June 30, 2010 and replaced certain cattle vaccine inventory with new cattle vaccine inventory in the three months ended December 31, 2010.
31
We expect 2012 total revenue to increase as compared with 2011.
Cost of Revenue
2011 Cost of revenue was $40.9 million, an increase of 1% compared to $40.7 million in 2010. Gross profit increased 18% to $29.2 million in 2011 from $24.8 million in 2010. Gross Margin, i.e. gross profit divided by total revenue, increased to 41.7% in 2011 from 37.9% in 2010. The largest factor was approximately $1.4 million in net costs for destroyed product, replacement product and related reserves in our OVP segment regarding regulatory issues with certain of our cattle vaccines which was recognized in 2010, but not 2011.
2010 Cost of revenue was $40.7 million, a decrease of 14% compared to $47.2 million in 2009. Gross profit decreased 13% to $24.8 million in 2010 from $28.5 million in 2009. Gross Margin, i.e. gross profit divided by total revenue, increased to 37.9% in 2010 from 37.6% in 2009. A key factor in the increase was product mix, where the overall sales shift was toward higher margin products. This was somewhat offset by approximately $1.4 million in net costs for destroyed product, replacement product and related reserves in our OVP segment regarding regulatory issues with certain of our cattle vaccines.
We expect Gross Margin to increase in 2012 as compared to 2011.
Operating Expenses
Selling and marketing expenses increased by 3% to $15.2 million in 2011 compared to $14.7 million in 2010. Greater recruiting and relocation costs related to the expansion of our sales force and increased spending related to product marketing programs were factors in the increase. Selling and marketing expenses increased by 1% to $14.7 million in 2010 compared to $14.5 million in 2009. Spending related to the full launch of our new blood gas analyzer in 2010 was a factor in the increase.
Research and development expenses increased by $53 thousand to $1.7 million in 2011 from $1.6 million in 2010. A factor in the increase was a payment to a third party related to a product line we are collaborating to develop with that company. Research and development expenses decreased by $121 thousand to $1.6 million in 2010 from $1.7 million in 2009. A factor in the decline was lower spending on research and development resources, such as laboratory supplies.
General and administrative expenses were $9.1 million in 2011, a 12% increase as compared to $8.1 million in 2010. The largest factor in the increase was a Management Incentive Plan (“MIP”) expense in 2011 related to the achievement of certain objectives, which did not occur in 2010. General and administrative expenses were $8.1 million in 2010, a 1% decline as compared to $8.2 million in 2009. A factor in the decline was no MIP payouts were earned in 2010 while there was an MIP payout earned in 2009.
We expect 2012 operating expenses will be higher than in 2011.
Interest and Other Expense, Net
Interest and other expense, net was income of $117 thousand in 2011, as compared to an expense of $289 thousand in 2010 and an expense of $306 thousand in 2009. This line item can be broken into two components: net interest expense and net foreign currency gains and losses. Net interest was income of $144 thousand in 2011, as compared to expense of $131 thousand in 2010 and expense of $343 thousand in 2009. The largest factor in the improvement from 2010 to 2011 was $207 thousand in interest income from an arbitration judgment which is described in greater detail below in “Liquidity, Capital Resources and Financial Condition”. The largest factor in the decrease in net interest expenses between 2010 as compared to 2009 was lower loan balances and lower market interest rates, somewhat offset by an increased interest rate spread negotiated with Wells Fargo in December 2008. Net foreign currency losses were $27 thousand in 2011 and $158 thousand in 2010 and net foreign currency gains were $37 thousand in 2009.
32
We expect interest and other expense, net to be a net expense in 2012 as we have a $100 thousand minimum interest expense under our agreement with Wells Fargo and we do not anticipate we will experience interest income at the same level as in 2011, which resulted primarily from interest awarded in an arbitration judgment.
Income Tax Expense (Benefit)
In 2011, we had total income tax expense of $1.2 million, including $1.1 million in domestic deferred income tax expense, a non-cash expense, and $165 thousand in current income tax expense, the majority of which related to state income taxes. In 2010, we had $61 thousand of current tax expense and $10 thousand in deferred tax benefit, resulting in total income tax expense of $51 thousand. The largest component of 2010 current tax expense relates to the profitable operating performance of our Swiss subsidiary. Domestically, the effect of permanent differences between tax and GAAP accounting, such as incentive stock option amortization, at low profitability levels tends to raise the implied tax rate and contributed to our unusually high 74% tax rate. In 2009, domestic deferred income tax expense, a non-cash expense, represented $1.3 million of our $1.5 million total income tax expense and current tax expense of $205 thousand represented the balance.
In 2012, we expect higher income tax expense as compared to 2011 as we expect higher pre-tax income in 2012 as compared to 2011.
Net Income (Loss)
Our 2011 net income was $2.1 million as compared to 2010 net income of $18 thousand and net income of $2.2 million in 2009. Increased revenue and improved Gross Margin, somewhat offset by higher operating expenses, were key factors in the improvement from 2010 to 2011. Lower year-over-year revenue was the key factor in the decline in net income between 2010 and 2009.
We expect net income will be higher in 2012 than in 2011, primarily as a result of increased revenue and improved Gross Margin, somewhat offset by higher operating expenses.
Liquidity, Capital Resources and Financial Condition
We have incurred net cumulative negative cash flow from operations since our inception in 1988. For the year ended December 31, 2011, we had net income of $2.1 million. In 2011, net cash provided by operations was $4.9 million. At December 31, 2011, we had $6.3 million of cash and cash equivalents, working capital of $19.6 million and no outstanding borrowings under our revolving line of credit, discussed below.
In the fourth quarter of 2011, we received an arbitration judgment in a dispute with one of our former distributors to whom we gave notice of contract termination in January 2010. The dispute was regarding matters including amounts past due and counterclaims by our former distributor. We were awarded a net judgment in excess of $1 million, which was received prior to year end 2011. Included in the amount ordered by the arbiter were over $628 thousand in principal, which was credited against the affiliated receivable, $207 thousand in interest, which we recognized as interest income in the fourth quarter of 2011 and $306 thousand in reimbursed legal and other expenses, which were credited against our general and administrative expenses in the fourth quarter of 2011.
33
Net cash flows from operating activities provided cash of $4.9 million in 2011 as compared to $1.9 million in 2010. Key factors in the change were an improvement of $2.1 million in net income, a $1.1 million increase in cash provided by deferred tax expense, which is a non-cash charge, and a $573 thousand improvement in cash provided by accounts receivable, with the payment resulting from arbitration in the fourth quarter of 2011 as a key factor in this change. These were somewhat offset by $373 thousand greater cash used by accounts payable and accrued liabilities and other, related to the timing of payments, and $246 thousand in lower depreciation and amortization expense, primarily related to lower depreciation on instrument units available for customer rental. Net cash flows from operating activities provided cash of $1.9 million in 2010 as compared to providing cash of $8.6 million in 2009. The largest factor in the change was a $3.8 million decrease in cash provided from inventory as we did not lower our inventory level at year end 2010 compared to year end 2009 as much as in 2009 compared to 2008, and we had a greater non-cash transfer of inventory to property and equipment in 2010 as compared to 2009. Other major factors in the change were a $2.2 million decrease in cash provided by net income resulting from our operating performance and a $1.3 million decrease in cash provided by deferred tax benefit resulting from our lower level of profitability in 2010. This was somewhat offset by a $1.4 million decline in cash used by deferred revenue and other, primarily relating to lower contractual prepayments near year end and lower upfront payment amortization scheduled for 2010 versus 2009.
Net cash flows from investing activities used cash of $1.1 million in 2011 as compared to using cash of $620 thousand in 2010 and using cash of $276 thousand in 2009. Purchases of property and equipment increased $465 thousand in 2011 as compared to 2010 and $344 thousand in 2010 as compared to 2009, primarily due to greater property and equipment purchases in our OVP segment.
Net cash flows from financing activities used cash of $3.0 million in 2011, used cash of $1.4 million in 2010 and used cash of $7.6 million in 2009. In 2011, we used cash to fully repay our remaining $3.1 million in line of credit borrowings, which was partially offset by proceeds from the issuance of common stock under our Employee Stock Purchase Plan and upon option exercises. In 2010, we used cash to reduce our borrowings under our line of credit by $1.1 million and repay the remaining principal on term debt of $381 thousand, which was partially offset by proceeds from the issuance of common stock under our Employee Stock Purchase Plan and upon option exercises. In 2009, we used cash to reduce our borrowings under our line of credit by $6.8 million and repay principal on term debt of $770 thousand, which was partially offset by proceeds from the issuance of common stock under our Employee Stock Purchase Plan. We repaid more debt under our revolving line of credit in 2011 and 2009 as compared to 2010 primarily because we had greater cash provided by operating activities in 2011 and 2009 as compared to 2010.
At December 31, 2011, we had a $15.0 million asset-based revolving line of credit with Wells Fargo which has a maturity date of December 31, 2013. At December 31, 2011, there were no borrowings outstanding on this line of credit. Our ability to borrow under this line of credit varies based upon available cash, eligible accounts receivable and eligible inventory. As a result of an amendment to our agreement with Wells Fargo signed in December 2011, on December 31, 2011, any interest on borrowings due was to be charged at a stated rate of three month LIBOR plus 3.75% and was to be payable monthly. We expect any interest on borrowings due after February 2012 to be charged at a stated rate of three month LIBOR plus 2.75% based on the terms of our agreement with Wells Fargo. We are required to comply with various financial and non-financial covenants, and we have made various representations and warranties under our agreement with Wells Fargo. Among the financial covenants is a requirement to maintain a minimum liquidity (cash plus excess borrowing base) of $1.5 million. Additional requirements include covenants for minimum capital monthly and minimum net income quarterly. Failure to comply with any of the covenants, representations or warranties could result in our being in default on the loan and could cause all outstanding amounts payable to Wells Fargo to become immediately due and payable or impact our ability to borrow under the agreement. We were in compliance with all financial covenants as of December 31, 2011. At December 31, 2011, our available borrowing capacity based upon eligible accounts receivable and eligible inventory under our revolving line of credit was approximately $8.7 million.
In the first quarter of 2012, our Board of Directors declared a cash dividend of $0.10 per share to the holders of record of our Public Common Stock on March 30, 2012, payable on April 10, 2012. We intend to pay a regular quarterly dividend of $0.10 per share of outstanding Public Common Stock for the foreseeable future at the discretion of our Board of Directors considering available cash, anticipated cash needs, overall financial condition, loan agreement restrictions, future prospects for earnings and cash flows, anticipated tax treatment as well as other relevant factors.
34
We would consider strategic acquisitions if we felt they were consistent with our strategic direction. Our Board of Directors has considered, and may consider in the future, a stock buyback as an alternative use of our cash. Our primary short-term need for capital, which is subject to change, is to fund our operations, which consist of continued sales and marketing, general and administrative and research and development efforts, working capital associated with increased product sales and capital expenditures relating to maintaining and developing our manufacturing operations. Our future liquidity and capital requirements will depend on numerous factors, including the extent to which our marketing and selling efforts, as well as those of third parties who market, sell and distribute our products, are successful in increasing our revenue, competition, the extent to which currently planned products and/or technologies under development are successfully developed, launched and sold, any changes required by regulatory bodies to maintain our operations and other factors.
Our financial plan for 2012 indicates that our available cash and cash equivalents, together with cash from operations and borrowings expected to be available under our revolving line of credit, will be sufficient to fund our operations through 2012 and into 2013. However, our actual results may differ from this plan, and we may be required to consider alternative strategies. We may be required to raise additional capital in the future. If necessary, we expect to raise these additional funds through the sale of equity securities or the issuance of new term debt secured by the same assets as the term loans which were fully repaid in 2010. There is no guarantee that additional capital will be available from these sources on acceptable terms, if at all, and certain of these sources may require approval by existing lenders. If we cannot raise the additional funds through these options on acceptable terms or with the necessary timing, management could also reduce discretionary spending to decrease our cash burn rate through actions such as delaying or canceling budgeted hiring activities or marketing plans. These actions would likely extend the then available cash and cash equivalents, and then available borrowings to some degree. See “Risk Factors” in Item 1A of this Form 10-K for a discussion of some of the factors that affect our capital raising alternatives.
35
A summary of our contractual obligations at December 31, 2011 is shown below:
| Payments Due by Period (in thousands) | ||||||||||||||||||||
| Total | Less Than 1 Year |
1-3 Years |
4-5 Years |
After 5 Years |
||||||||||||||||
| Contractual Obligations |
||||||||||||||||||||
| Operating leases |
17,334 | 1,891 | 3,144 | 2,835 | 9,464 | |||||||||||||||
| Unconditional purchase obligations |
13,849 | 2,399 | 5,550 | 5,900 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual cash obligations |
$ | 31,183 | $ | 4,290 | $ | 8,694 | $ | 8,735 | $ | 9,464 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
In addition to those agreements considered above where our contractual obligation is fixed, we are party to commercial agreements which may require us to make milestone payments under certain circumstances. Any milestone obligations which we believe are likely to be triggered but are not yet paid are included in “Unconditional Purchase Obligations” in the table above. We do not believe other potential milestone obligations, some of which we consider to be of remote likelihood of ever being triggered, will have a material impact on our liquidity, capital resources or financial condition in the foreseeable future.
Net Operating Loss Carryforwards
As of December 31, 2011, we had a net domestic operating loss carryforward, or NOL, of approximately $145.6 million, a domestic alternative minimum tax credit carryforward of approximately $228 thousand and a domestic research and development tax credit carryforward of approximately $553 thousand for federal tax purposes. Our federal NOL is expected to expire as follows if unused: $32.2 million at the end of 2012, $107.6 million in 2018 through 2022, $5.5 million in 2024 and 2025 and $385 thousand in 2027. The NOL and tax credit carryforwards are subject to alternative minimum tax limitations and to examination by the tax authorities. In addition, we had a “change of ownership” as defined under the provisions of Section 382 of the Internal Revenue Code of 1986, as amended (an “Ownership Change”). We believe the latest Ownership Change occurred at the time of our initial public offering in July 1997. We do not believe this Ownership Change will place a significant restriction on our ability to utilize our NOLs in the future.
Recent Accounting Pronouncements
In 2011 the FASB amended the provisions of the Fair Value Measurement topic of the FASB Codification. This amendment provides a consistent definition of fair value and ensures that the fair value measurement and disclosure requirements are similar between GAAP and International Financial Reporting Standards (IFRS). This topic changes certain fair value measurement principles and enhances the disclosure requirements, particularly for Level 3 fair value measurements. These provisions are effective for reporting periods beginning on or after December 15, 2011, applied prospectively. The adoption of this amendment will not have a material effect on our Consolidated Financial Statements.
In 2011 the FASB amended the provisions of the Comprehensive Income topic of the FASB Codification. The amended provisions were issued to enhance comparability between entities that report under GAAP and IFRS and to provide a more consistent method of presenting non-owner transactions that affect an entity’s equity. This topic eliminates the option to report other comprehensive income and its components in the statement of changes in shareholders’ equity and requires an entity to present the total of comprehensive income, the components of net income and the components of other comprehensive income either in a single continuous statement or in two separate but consecutive statements. These amended provisions are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. Early adoption of the new guidance is permitted and full retrospective application is required. The adoption of this amendment will not have a material effect on our Consolidated Financial Statements as the amendment impacts presentation only.
36
In 2011 the FASB amended the provisions of the Intangibles-Goodwill and Other topic of the FASB Codification. The provision will allow companies to assess qualitative factors to determine if it is more-likely-than-not that goodwill might be impaired and whether it is necessary to perform the two-step goodwill impairment test required under current accounting standards. These amended provisions are effective for fiscal years beginning after December 15, 2011, with early adoption permitted. We have elected to enact early adoption of this amendment, which did not affect our 2011 Consolidated Financial Statements.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk. |
Market risk represents the risk of loss that may impact the financial position, results of operations or cash flows due to adverse changes in financial and commodity market prices and rates. We are exposed to market risk in the areas of changes in United States and foreign interest rates and changes in foreign currency exchange rates as measured against the United States dollar. These exposures are directly related to our normal operating and funding activities.
Interest Rate Risk
At December 31, 2011, there was no debt outstanding on our line of credit with Wells Fargo. At December 31, 2011, we had no borrowings on our line of credit. We also had approximately $6.3 million of cash and cash equivalents at December 31, 2011, the majority of which was invested in liquid interest bearing accounts. We had no interest rate hedge transactions in place on December 31, 2011. We completed an interest rate risk sensitivity analysis based on the above and an assumed one-percentage point increase/decrease in interest rates. If market rates increase/decrease by one percentage point, we would experience a decrease/increase in annual net interest expense of approximately $63 thousand based on our outstanding balances as of December 31, 2011.
Foreign Currency Risk
Our investment in foreign assets consists primarily of our investment in our Swiss subsidiary. Foreign currency risk may impact our results of operations. In cases where we purchase inventory in one currency and sell corresponding products in another, our gross margin percentage is typically at risk based on foreign currency exchange rates. In addition, in cases where we may be generating operating income in foreign currencies, the magnitude of such operating income when translated into U.S. dollars will be at risk based on foreign currency exchange rates. Our agreements with suppliers and customers vary significantly in regard to the existence and extent of currency adjustment and other currency risk sharing provisions. We had no foreign currency hedge transactions in place on December 31, 2011.
We have a wholly-owned subsidiary in Switzerland which uses the Swiss Franc as its functional currency. We purchase inventory in foreign currencies, primarily Euros and Japanese Yen, and sell corresponding products in U.S. dollars. We also sell products in foreign currencies, primarily Euros and Japanese Yen, where our inventory costs are largely in U.S. dollars. Based on our 2011 results of operations, if foreign currency exchange rates were to strengthen/weaken by 25% against the dollar, we would expect a resulting pre-tax loss/gain of approximately $310 thousand.
37
| Item 8. | Financial Statements and Supplementary Data. |
HESKA CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
38
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Heska Corporation
Loveland, Colorado
We have audited the accompanying consolidated balance sheets of Heska Corporation and subsidiaries (the “Company”) as of December 31, 2010 and 2011, and the related consolidated statements of income, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2011. Our audit also included the financial statement schedule listed in the Index at Item 15(a)(2). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Heska Corporation and its subsidiaries as of December 31, 2010 and 2011, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2011, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
Ehrhardt Keefe Steiner & Hottman PC
February 28, 2012
Denver, Colorado
39
HESKA CORPORATION AND SUBSIDIARIES
(dollars in thousands, except per share amounts)
| December 31, | ||||||||
| 2010 | 2011 | |||||||
| ASSETS |
| |||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 5,492 | $ | 6,332 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $136 and $174, respectively |
8,866 | 7,938 | ||||||
| Inventories, net |
11,901 | 12,401 | ||||||
| Deferred tax asset, current |
53 | 1,170 | ||||||
| Other current assets |
967 | 1,050 | ||||||
|
|
|
|
|
|||||
| Total current assets |
27,279 | 28,891 | ||||||
| Property and equipment, net |
5,486 | 4,869 | ||||||
| Goodwill |
999 | 1,000 | ||||||
| Deferred tax asset, net of current portion |
29,284 | 27,134 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 63,048 | $ | 61,894 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
| |||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 4,162 | $ | 3,999 | ||||
| Accrued liabilities |
3,087 | 2,311 | ||||||
| Accrued compensation |
521 | 1,077 | ||||||
| Current portion of deferred revenue |
1,811 | 1,902 | ||||||
| Line of credit |
3,079 | — | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
12,660 | 9,289 | ||||||
| Deferred revenue, net of current portion, and other |
4,590 | 4,166 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
17,250 | 13,455 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $.01 par value, 2,500,000 shares authorized; none issued or outstanding |
— | — | ||||||
| Common stock, $.01 par value, 7,500,000 shares authorized, none issued or outstanding |
— | — | ||||||
| Public common stock, $.01 par value, 7,500,000 shares authorized, 5,231,245 and 5,250,328 shares issued and outstanding, respectively |
52 | 52 | ||||||
| Additional paid-in capital |
217,240 | 217,778 | ||||||
| Accumulated other comprehensive income |
284 | 242 | ||||||
| Accumulated deficit |
(171,778 | ) | (169,633 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
45,798 | 48,439 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 63,048 | $ | 61,894 | ||||
|
|
|
|
|
|||||
See accompanying notes to consolidated financial statements.
40
HESKA CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
(in thousands, except per share amounts)
| Year Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
| Revenue: |
||||||||||||
| Core companion animal health |
$ | 66,449 | $ | 55,655 | $ | 57,481 | ||||||
| Other vaccines, pharmaceuticals and products |
9,229 | 9,796 | 12,584 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue, net |
75,678 | 65,451 | 70,065 | |||||||||
| Cost of revenue |
47,219 | 40,659 | 40,878 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit |
28,459 | 24,792 | 29,187 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Selling and marketing |
14,524 | 14,726 | 15,167 | |||||||||
| Research and development |
1,718 | 1,597 | 1,650 | |||||||||
| General and administrative |
8,173 | 8,111 | 9,121 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
24,415 | 24,434 | 25,938 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating income |
4,044 | 358 | 3,249 | |||||||||
| Interest and other (income) expense, net |
306 | 289 | (117 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Income before income taxes |
3,738 | 69 | 3,366 | |||||||||
| Income tax expense: |
||||||||||||
| Current income tax expense |
205 | 61 | 165 | |||||||||
| Deferred income tax expense (benefit) |
1,291 | (10 | ) | 1,056 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total income tax expense |
1,496 | 51 | 1,221 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net income |
$ | 2,242 | $ | 18 | $ | 2,145 | ||||||
|
|
|
|
|
|
|
|||||||
| Basic net income per share |
$ | 0.43 | $ | 0.00 | $ | 0.41 | ||||||
|
|
|
|
|
|
|
|||||||
| Diluted net income per share |
$ | 0.43 | $ | 0.00 | $ | 0.40 | ||||||
|
|
|
|
|
|
|
|||||||
| Weighted average outstanding shares used to compute basic net income per share |
5,207 | 5,220 | 5,237 | |||||||||
|
|
|
|
|
|
|
|||||||
| Weighted average outstanding shares used to compute diluted net income per share |
5,212 | 5,254 | 5,338 | |||||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes to consolidated financial statements.
41
HESKA CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands)
| Common Stock | Additional Paid-in |
Accumulated Other Comprehensive |
Accumulated | Total Stockholders’ |
||||||||||||||||||||
| Shares | Amount | Capital | Income (Loss) | Deficit | Equity | |||||||||||||||||||
| Balances, January 1, 2009 |
5,201 | $ | 52 | $ | 216,463 | $ | 46 | $ | (174,038 | ) | $ | 42,523 | ||||||||||||
| Issuance of common stock related to options, ESPP and other |
15 | — | 53 | — | — | 53 | ||||||||||||||||||
| Recognition of stock based compensation |
— | — | 313 | — | — | 313 | ||||||||||||||||||
| Comprehensive net income: |
||||||||||||||||||||||||
| Net income |
— | — | — | — | 2,242 | 2,242 | ||||||||||||||||||
| Minimum pension liability adjustments |
— | — | — | (132 | ) | — | (132 | ) | ||||||||||||||||
| Unrealized (loss) on available for sale investments |
— | — | — | (1 | ) | — | (1 | ) | ||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | 57 | — | 57 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Comprehensive net income |
— | — | — | — | — | 2,166 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balances, December 31, 2009 |
5,216 | 52 | 216,829 | (30 | ) | (171,796 | ) | 45,055 | ||||||||||||||||
| Issuance of common stock related to options, ESPP and other |
15 | — | 75 | — | — | 75 | ||||||||||||||||||
| Recognition of stock based compensation |
— | — | 336 | — | — | 336 | ||||||||||||||||||
| Comprehensive net income: |
||||||||||||||||||||||||
| Net income |
— | — | — | — | 18 | 18 | ||||||||||||||||||
| Minimum pension liability adjustments |
— | — | — | 22 | — | 22 | ||||||||||||||||||
| Unrealized gain on available for sale investments |
— | — | — | 4 | — | 4 | ||||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | 288 | — | 288 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Comprehensive net income |
— | — | — | — | — | 332 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balances, December 31, 2010 |
5,231 | 52 | 217,240 | 284 | (171,778 | ) | 45,798 | |||||||||||||||||
| Issuance of common stock related to options, ESPP and other |
19 | — | 124 | — | — | 124 | ||||||||||||||||||
| Recognition of stock based compensation |
— | — | 414 | — | — | 414 | ||||||||||||||||||
| Comprehensive net income: |
||||||||||||||||||||||||
| Net income |
— | — | — | — | 2,145 | 2,145 | ||||||||||||||||||
| Minimum pension liability adjustments |
— | — | — | (8 | ) | — | (8 | ) | ||||||||||||||||
| Unrealized (loss) on available for sale investments |
— | — | — | (20 | ) | — | (20 | ) | ||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | (14 | ) | — | (14 | ) | ||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Comprehensive net income |
— | — | — | — | — | 2,103 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balances, December 31, 2011 |
5,250 | $ | 52 | $ | 217,778 | $ | 242 | $ | (169,633 | ) | $ | 48,439 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
See accompanying notes to consolidated financial statements.
42
HESKA CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| Year Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
| CASH FLOWS PROVIDED BY (USED IN) OPERATING ACTIVITIES: |
||||||||||||
| Net income |
$ | 2,242 | $ | 18 | $ | 2,145 | ||||||
| Adjustments to reconcile net income to cash provided by (used in) operating activities: |
||||||||||||
| Depreciation and amortization |
2,565 | 2,298 | 2,052 | |||||||||
| Deferred tax (benefit) expense |
1,291 | (10 | ) | 1,056 | ||||||||
| Stock based compensation |
313 | 336 | 414 | |||||||||
| Unrealized (gain) loss on foreign currency translation |
126 | (12 | ) | 10 | ||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
292 | 356 | 929 | |||||||||
| Inventories |
3,103 | (699 | ) | (850 | ) | |||||||
| Other current assets |
40 | (44 | ) | (93 | ) | |||||||
| Accounts payable |
268 | (10 | ) | (164 | ) | |||||||
| Accrued liabilities and other |
(55 | ) | (40 | ) | (259 | ) | ||||||
| Income taxes payable |
38 | (38 | ) | — | ||||||||
| Deferred revenue and other |
(1,608 | ) | (214 | ) | (352 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) operating activities |
8,615 | 1,941 | 4,888 | |||||||||
|
|
|
|
|
|
|
|||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: |
||||||||||||
| Purchases of property and equipment |
(276 | ) | (620 | ) | (1,084 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
(276 | ) | (620 | ) | (1,084 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: |
||||||||||||
| Proceeds from issuance of common stock |
53 | 75 | 124 | |||||||||
| Proceeds from (repayments of) line of credit borrowings, net |
(6,841 | ) | (1,123 | ) | (3,079 | ) | ||||||
| Repayments of debt and capital lease obligations |
(770 | ) | (381 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) financing activities |
(7,558 | ) | (1,429 | ) | (2,955 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| EFFECT OF EXCHANGE RATE CHANGES ON CASH |
(86 | ) | 200 | (9 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| INCREASE IN CASH AND CASH EQUIVALENTS |
695 | 92 | 840 | |||||||||
| CASH AND CASH EQUIVALENTS, BEGINNING OF YEAR |
4,705 | 5,400 | 5,492 | |||||||||
|
|
|
|
|
|
|
|||||||
| CASH AND CASH EQUIVALENTS, END OF YEAR |
$ | 5,400 | $ | 5,492 | $ | 6,332 | ||||||
|
|
|
|
|
|
|
|||||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: |
||||||||||||
| Cash paid for interest |
$ | 409 | $ | 162 | $ | 28 | ||||||
|
|
|
|
|
|
|
|||||||
| Cash paid for income taxes |
$ | 87 | $ | 107 | $ | 214 | ||||||
|
|
|
|
|
|
|
|||||||
| Non-cash transfer of inventory to property and equipment |
$ | 128 | $ | 815 | $ | 351 | ||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes to consolidated financial statements.
43
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. ORGANIZATION AND BUSINESS
Heska Corporation (“Heska” or the “Company”) develops, manufactures, markets, sells and supports veterinary products. Heska’s core focus is on the canine and feline companion animal health markets.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements include the accounts of the Company and of its wholly-owned subsidiaries since their respective dates of acquisitions. All material intercompany transactions and balances have been eliminated in consolidation.
Reverse Stock Split
The Company completed a 1-for-10 reverse stock split which was effective on December 30, 2010. Except as otherwise indicated, all related amounts reported in the consolidated financial statements, including common share quantities, earnings per share amounts and exercise prices of options, have been retroactively adjusted for the effect of this reverse stock split.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates are required when establishing the allowance for doubtful accounts and the provision for excess/obsolete inventory, in determining the period over which the Company’s obligations are fulfilled under agreements to license product rights and/or technology rights, evaluating long-lived assets for impairment, estimating the expense associated with the granting of stock options and in determining the need for, and the amount of, a valuation allowance on deferred tax assets.
Trade Accounts Receivable
Trade accounts receivable are recorded at the invoiced amount. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses in the Company’s existing accounts receivable. The Company determines the allowance based on historical write-off experience. The Company reviews its allowance for doubtful accounts monthly. Past due balances over 90 days and over a specified amount are reviewed individually for collectibility. Account balances are charged against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company does not have any off-balance-sheet credit exposure related to its customers.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist of cash and cash equivalents and accounts receivable. The Company maintains the majority of its cash and cash equivalents with financial institutions that management believes are creditworthy in the form of demand deposits. The Company has no significant off-balance-sheet concentrations of credit risk such as foreign exchange contracts, options contracts or other foreign currency hedging arrangements. Its accounts receivable balances are due primarily from domestic veterinary clinics and individual veterinarians, and both domestic and international corporations.
44
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Cash and Cash Equivalents
Cash and cash equivalents are stated at cost, which approximates market, and include short-term, highly liquid investments with original maturities of less than three months. The Company valued its Euro and Japanese Yen cash accounts at the spot market foreign exchange rate as of each balance sheet date, with changes due to foreign exchange fluctuations recorded in current earnings. The Company held 506,016 and 556,173 Euros at December 31, 2010 and 2011, respectively. The Company held 38,539,410 and 9,685,521 Yen at December 31, 2010 and 2011, respectively. The Company held 217,356 and 330,533 Swiss Francs at December 31, 2010 and 2011, respectively. The majority of the Company’s cash and cash equivalents are held at U.S.-based or Swiss-based financial institutions in accounts not insured by governmental entities.
Fair Value of Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, short-term trade receivables and payables and the Company’s revolving line of credit. The carrying values of cash and cash equivalents and short-term trade receivables and payables approximate fair value. The fair value of the Company’s line of credit balance is estimated based on current rates available for similar debt with similar maturities and collateral, and at December 31, 2010 and 2011, approximates the carrying value due primarily to the floating rate of interest on such debt instruments.
Inventories
Inventories are stated at the lower of cost or market using the first-in, first-out method. Inventory manufactured by the Company includes the cost of material, labor and overhead. If the cost of inventories exceeds estimated fair value, provisions are made to reduce the carrying value to estimated fair value.
Inventories, net consist of the following (in thousands):
| December 31, | ||||||||
| 2010 | 2011 | |||||||
| Raw materials |
$ | 4,203 | $ | 5,580 | ||||
| Work in process |
3,483 | 2,505 | ||||||
| Finished goods |
5,388 | 5,043 | ||||||
| Allowance for excess or obsolete inventory |
(1,173 | ) | (727 | ) | ||||
|
|
|
|
|
|||||
| $ | 11,901 | $ | 12,401 | |||||
|
|
|
|
|
|||||
Property and Equipment
Property and equipment are recorded at cost and depreciated on a straight-line basis over the estimated useful lives of the related assets. Leasehold improvements are amortized over the applicable lease period or their estimated useful lives, whichever is shorter. Maintenance and repairs are charged to expense when incurred, and major renewals and improvements are capitalized.
45
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Property and equipment consist of the following (in thousands):
| Estimated | December 31, | |||||||||
| Useful Life |
2010 | 2011 | ||||||||
| Land |
N/A | $ | 377 | $ | 377 | |||||
| Building |
10 to 20 years | 2,678 | 2,678 | |||||||
| Machinery and equipment |
3 to 15 years | 27,302 | 28,617 | |||||||
| Leasehold and building improvements |
7 to 15 years | 5,322 | 5,322 | |||||||
| Construction in progress |
385 | 463 | ||||||||
|
|
|
|
|
|||||||
| 36,064 | 37,457 | |||||||||
| Less accumulated depreciation and amortization |
(30,578 | ) | (32,588 | ) | ||||||
|
|
|
|
|
|||||||
| $ | 5,486 | $ | 4,869 | |||||||
|
|
|
|
|
|||||||
From time to time, the Company utilizes marketing programs whereby its instruments in inventory may be placed in a customer’s location on a rental basis. The cost of these instruments is transferred to machinery and equipment and depreciated, typically over a four year period. During 2009, 2010 and 2011, total costs transferred from inventory were approximately $128 thousand, $815 thousand and $351 thousand, respectively.
Depreciation and amortization expense for property and equipment was $2.6 million, $2.3 million and $2.1 million for the years ended December 31, 2009, 2010 and 2011, respectively.
Realizability of Long-Lived Assets
The Company continually evaluates whether events and circumstances have occurred that indicate the remaining estimated useful life of long-lived assets may warrant revision, or that the remaining balance of these assets may not be recoverable. When deemed necessary, the Company completes this evaluation by comparing the carrying amount of the assets with the estimated undiscounted future cash flows associated with them. If such evaluations indicate that the future undiscounted cash flows of amortizable long-lived assets are not sufficient to recover the carrying value of such assets, the assets are adjusted to their estimated fair values.
Goodwill
Goodwill is subject to an annual assessment for impairment. Impairment is indicated when the carrying amount of the related reporting unit is greater than its estimated fair value.
The Company’s recorded goodwill relates to the 1997 acquisition of Heska AG, the Company’s Swiss subsidiary. This goodwill is reviewed at least annually for impairment. This impairment assessment is completed at the Heska AG reporting unit level. The Company completed its annual analysis estimating that the fair value of the reporting unit exceeds the carrying value of the goodwill at December 31, 2011 and determined there was no indicated impairment of its goodwill. The key inputs to the estimated fair value included historical and 2012 budgeted operating income, net income and cash flows for the reporting unit. At December 31, 2010 and 2011, goodwill was approximately $1.0 million, and was included in the assets of the Core Companion Animal Health segment. The change in carrying value of the goodwill between years was solely due to foreign currency rate changes. There can be no assurance that future goodwill impairments will not occur.
Revenue Recognition
The Company generates its revenues through sale of products and services, licensing of product and technology rights, and research and development services. Revenue is accounted for in accordance with the guidelines provided by SEC Codification of Staff Accounting Bulletins, Topic 13: Revenue Recognition. The Company’s policy is to recognize revenue when the applicable revenue recognition criteria have been met, which generally include the following:
46
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| • | Persuasive evidence of an arrangement exists; |
| • | Delivery has occurred or services rendered; |
| • | Price is fixed or determinable; and |
| • | Collectibility is reasonably assured. |
Revenue from the sale of products is generally recognized after both the goods are shipped to the customer and acceptance has been received, if required, with an appropriate provision for estimated returns and other allowances. The terms of the customer arrangements generally pass title and risk of ownership to the customer at the time of shipment. Certain customer arrangements provide for acceptance provisions. Revenue for these arrangements is not recognized until the acceptance has been received or the acceptance period has lapsed. The Company maintains an allowance for sales returns based upon its customer policies and historical experience. Shipping and handling costs charged to customers are included as revenue, and the related costs are recorded as a component of cost of products sold.
In addition to its direct sales force, the Company utilizes distributors to sell its products. Distributors purchase goods from the Company, take title to those goods and resell them to their customers in the distributors’ territory.
Upfront payments received by the Company under arrangements for product, patent or technology rights in which the Company retains an interest in the underlying product, patent or technology are initially deferred, and revenue is subsequently recognized over the estimated life of the agreement, product, patent or technology. The Company has not received any significant up-front payments in 2009, 2010 or 2011. Revenue from royalties is recognized based upon historical experience or as the Company is informed of sales on which it is entitled to royalties.
For multiple-element arrangements that are not subject to a higher level of authoritative literature, the Company follows the authoritative guidance for accounting for revenue arrangements with multiple deliverables in determining the separate units of accounting. For those arrangements subject to appropriate separation criteria, the Company must determine whether the various elements meet the criteria to be accounted for as separate elements. If the elements cannot be separated, revenue is recognized once revenue recognition criteria for the entire arrangement have been met or over the period that the Company’s obligations to the customer are fulfilled, as appropriate. If the elements are determined to be separable, the revenue is allocated to the separate elements based on relative fair value and recognized separately for each element when the applicable revenue recognition criteria have been met. In accounting for these multiple element arrangements, the Company must make determinations about whether elements can be accounted for separately and make estimates regarding their relative fair values.
Cost of Products Sold
Royalties payable in connection with certain licensing agreements (see Note 8) are reflected in cost of products sold as incurred.
Stock-Based Compensation
During the years ended December 31, 2009, 2010 and 2011, the Company’s income from operations and income before income taxes were reduced by $313 thousand, $336 thousand and $414 thousand, respectively, and net income was reduced by $233 thousand, $287 thousand and $348 thousand, respectively, for compensation related to stock options issued. Basic and diluted earnings per share were reduced by $0.04 and $0.04 in 2009, $0.05 and $0.05 in 2010 and $0.07 and $0.07 in 2011. For all years presented, there was no material impact on cash flow from operations and cash flow from financing activities. At December 31, 2011, the Company had two stock-based compensation plans. See Note 6 for a description of these plans and additional disclosures regarding the plans.
47
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Advertising Costs
The Company expenses advertising costs as incurred. Advertising expenses were $471 thousand, $735 thousand and $621 thousand for the years ended December 31, 2009, 2010 and 2011, respectively.
Income Taxes
The Company records a current provision for income taxes based on estimated amounts payable or refundable on tax returns filed or to be filed each year. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates, in each tax jurisdiction, expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date. The overall change in deferred tax assets and liabilities for the period measures the deferred tax expense or benefit for the period. Deferred tax assets are reduced by a valuation allowance based on a judgmental assessment of available evidence if the Company is unable to conclude that it is more likely than not that some or all of the deferred tax assets will be realized.
Basic and Diluted Net Income (Loss) Per Share
Basic net income (loss) per common share is computed using the weighted average number of common shares outstanding during the period. Diluted net income per share is computed using the sum of the weighted average number of shares of common stock outstanding, and, if not anti-dilutive, the effect of outstanding common stock equivalents (such as stock options and warrants) determined using the treasury stock method. At December 31, 2009, 2010 and 2011, securities that have been excluded from diluted net income per share because they would be anti-dilutive are outstanding options to purchase 1,259,721, 1,121,264 and 1,029,151 shares, respectively, of the Company’s common stock. Securities included in the diluted net income per share calculation at December 31, 2009, 2010, and 2011 using the treasury stock method, were outstanding options to purchase approximately 3 thousand, 34 thousand and 101 thousand shares of the Company’s common stock, respectively.
Comprehensive Income (Loss)
Comprehensive income (loss), as shown in the Consolidated Statements of Stockholders’ Equity, includes net income adjusted for the results of certain stockholders’ equity changes. Such changes include foreign currency items and minimum pension liability adjustments. At December 31, 2011, Accumulated Other Comprehensive Income (Loss) consists of $838 thousand gain for cumulative translation adjustments, $609 thousand loss for unrealized pension liability and $13 thousand of unrealized gain on available for sale investments. At December 31, 2010, Accumulated Other Comprehensive Income (Loss) consists of $851 thousand gain for cumulative translation adjustments, $589 thousand loss for unrealized pension liability and $22 thousand of unrealized gain on available for sale investments. At December 31, 2009, Accumulated Other Comprehensive Income (Loss) consists of $564 thousand gain for cumulative translation adjustments, $611 thousand loss for unrealized pension liability and $17 thousand of unrealized gain on available for sale investments.
Foreign Currency Translation
The functional currency of the Company’s Swiss subsidiary is the Swiss Franc. Assets and liabilities of the Company’s Swiss subsidiary are translated using the exchange rate in effect at the balance sheet date. Revenue and expense accounts and cash flows are translated using an average of exchange rates in effect during the period. Cumulative translation gains and losses are shown in the consolidated balance sheets as a
48
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
separate component of stockholders’ equity. Exchange gains and losses arising from transactions denominated in foreign currencies (i.e., transaction gains and losses) are recognized as a component of other income (expense) in current operations, as are exchange gains and losses on intercompany transactions expected to be settled in the near term.
Recent Accounting Pronouncements
In 2011 the FASB amended the provisions of the Fair Value Measurement topic of the FASB Codification. This amendment provides a consistent definition of fair value and ensures that the fair value measurement and disclosure requirements are similar between GAAP and International Financial Reporting Standards (IFRS). This topic changes certain fair value measurement principles and enhances the disclosure requirements, particularly for Level 3 fair value measurements. These provisions are effective for reporting periods beginning on or after December 15, 2011, applied prospectively. The adoption of this amendment will not have a material effect on the Company’s Consolidated Financial Statements.
In 2011 the FASB amended the provisions of the Comprehensive Income topic of the FASB Codification. The amended provisions were issued to enhance comparability between entities that report under GAAP and IFRS and to provide a more consistent method of presenting non-owner transactions that affect an entity’s equity. This topic eliminates the option to report other comprehensive income and its components in the statement of changes in shareholders’ equity and requires an entity to present the total of comprehensive income, the components of net income and the components of other comprehensive income either in a single continuous statement or in two separate but consecutive statements. These amended provisions are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. Early adoption of the new guidance is permitted and full retrospective application is required. The adoption of this amendment will not have a material effect on the Company’s Consolidated Financial Statements as the amendment impacts presentation only.
In 2011 the FASB amended the provisions of the Intangibles-Goodwill and Other topic of the FASB Codification. The provision will allow companies to assess qualitative factors to determine if it is more-likely-than-not that goodwill might be impaired and whether it is necessary to perform the two-step goodwill impairment test required under current accounting standards. These amended provisions are effective for fiscal years beginning after December 15, 2011, with early adoption permitted. We have elected to enact early adoption of this amendment, which did not affect the Company’s 2011 Consolidated Financial Statements.
3. CREDIT FACILITY
The Company has a credit and security agreement with Wells Fargo Bank, National Association which expires December 31, 2013. The agreement includes a $15.0 million asset-based revolving line of credit with a stated interest rate at December 31, 2011 of LIBOR plus 3.75% (4.05%). There is an annual minimum interest charge of $100 thousand under the agreement. Amounts due under the credit facility are secured by a first security interest in essentially all of the Company’s assets. Under the agreement, the Company is required to comply with certain financial and non-financial covenants. Among the financial covenants are requirements for monthly minimum capital, quarterly minimum net income and monthly minimum liquidity. The amount available for borrowings under the line of credit varies based upon available cash, eligible accounts receivable and eligible inventory. As of December 31, 2011, there were no borrowings outstanding and there was approximately $8.7 million available capacity for borrowings under the line of credit agreement.
49
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
4. SUPPLEMENTAL DISCLOSURE OF INTEREST AND OTHER EXPENSE (INCOME) INFORMATION
| Year Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
| (in thousands) | ||||||||||||
| Interest and other expense (income): |
||||||||||||
| Interest income |
$ | (64 | ) | $ | (58 | ) | $ | (268 | ) | |||
| Interest expense |
407 | 189 | 124 | |||||||||
| Other, net |
(37 | ) | 158 | 27 | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 306 | $ | 289 | $ | (117 | ) | ||||||
|
|
|
|
|
|
|
|||||||
5. INCOME TAXES
As of December 31, 2011, the Company had a domestic net operating loss carryforward (“NOL”), of approximately $145.6 million, a domestic alternative minimum tax credit carryforward of approximately $228 thousand and domestic research and development tax credit carryforward of approximately $553 thousand for federal tax purposes. The Company’s federal NOL is expected to expire as follows if unused: $32.2 million at the end of 2012, $107.6 million in 2018 through 2022, $5.5 million in 2024 and 2025 and $385 thousand in 2027. The NOL and tax credit carryforwards are subject to alternative minimum tax limitations and to examination by the tax authorities. In addition, the Company had a “change of ownership” as defined under the provisions of Section 382 of the Internal Revenue Code of 1986, as amended (an “Ownership Change”). The Company does not believe this Ownership Change will place a significant restriction on its ability to utilize its NOL in the future.
The Company is subject to income taxes in the U.S. federal jurisdiction, and various foreign, state and local jurisdictions. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment to apply. In the United States, the tax years 2008 – 2010 remain open to examination by the federal Internal Revenue Service and the tax years 2007 – 2010 remain open for various state taxing authorities.
The components of income (loss) before income taxes were as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
| Domestic |
$ | 3,576 | $ | (101 | ) | $ | 3,189 | |||||
| Foreign |
162 | 170 | 177 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 3,738 | $ | 69 | $ | 3,366 | |||||||
|
|
|
|
|
|
|
|||||||
50
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Temporary differences that give rise to the components of deferred tax assets are as follows (in thousands):
| December 31, | ||||||||
| 2010 | 2011 | |||||||
| Current deferred tax assets: |
||||||||
| Inventory |
$ | 453 | $ | 289 | ||||
| Accrued compensation |
226 | 237 | ||||||
| Net operating loss carryforwards – domestic |
618 | 1,151 | ||||||
| Other |
795 | 862 | ||||||
|
|
|
|
|
|||||
| 2,092 | 2,539 | |||||||
| Valuation allowance |
(2,039 | ) | (1,369 | ) | ||||
|
|
|
|
|
|||||
| Total current deferred tax assets |
$ | 53 | $ | 1,170 | ||||
|
|
|
|
|
|||||
| Noncurrent deferred tax assets: |
||||||||
| Research and development tax credit |
$ | 352 | $ | 553 | ||||
| Alternative minimum tax credit |
257 | 228 | ||||||
| Deferred revenue |
2,198 | 1,840 | ||||||
| Property and equipment |
1,873 | 1,932 | ||||||
| Net operating loss carryforwards – domestic |
58,419 | 52,456 | ||||||
|
|
|
|
|
|||||
| 63,099 | 57,009 | |||||||
| Valuation allowance |
(33,815 | ) | (29,875 | ) | ||||
|
|
|
|
|
|||||
| Total noncurrent deferred tax assets (liabilities) |
$ | 29,284 | $ | 27,134 | ||||
|
|
|
|
|
|||||
The components of the income tax expense (benefit) are as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
| Current income tax expense (benefit): |
||||||||||||
| Federal |
$ | 69 | $ | 7 | $ | 37 | ||||||
| State |
91 | 16 | 90 | |||||||||
| Foreign |
45 | 38 | 38 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current expense (benefit) |
205 | 61 | 165 | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred income tax expense (benefit): |
||||||||||||
| Federal |
1,148 | (9 | ) | 977 | ||||||||
| State |
143 | (1 | ) | 79 | ||||||||
| Foreign |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total deferred expense (benefit) |
1,291 | (10 | ) | 1,056 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total income tax expense (benefit) |
$ | 1,496 | $ | 51 | $ | 1,221 | ||||||
|
|
|
|
|
|
|
|||||||
51
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Company’s income tax expense (benefit) relating to income (loss) for the periods presented differs from the amounts that would result from applying the federal statutory rate to that income (loss) as follows:
| Year Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
| Statutory federal tax rate |
34 | % | 34 | % | 34 | % | ||||||
| State income taxes, net of federal benefit |
3 | % | 52 | % | 3 | % | ||||||
| Other permanent differences |
2 | % | 121 | % | 3 | % | ||||||
| Domestic NOL utilization |
— | — | — | |||||||||
| Change in tax rate |
31 | % | 40 | % | (4 | )% | ||||||
| Foreign rate difference |
— | (29 | )% | (1 | )% | |||||||
| Change in valuation allowance |
(29 | )% | (472 | )% | (137 | )% | ||||||
| Other |
(1 | )% | 328 | % | 138 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Effective income tax rate |
40 | % | 74 | % | 36 | % | ||||||
|
|
|
|
|
|
|
|||||||
6. CAPITAL STOCK
Common Stock
The Company completed a 1-for-10 reverse stock split which was effective on December 30, 2010. Except as otherwise indicated, all related amounts reported in the consolidated financial statements, including common share quantities, earnings per share amounts and exercise prices of options, have been retroactively adjusted for the effect of this reverse stock split.
Stock Option Plans
The Company has two stock option plans which authorize granting of stock options and stock purchase rights to employees, officers, directors and consultants of the Company to purchase shares of common stock. In 1997, the board of directors adopted the 1997 Stock Incentive Plan (the “1997 Plan”) and terminated two prior option plans. All shares that remained available for grant under the terminated plans were incorporated into the 1997 Plan. In addition, all shares subsequently cancelled under the prior plans are added back to the 1997 Plan on a quarterly basis as additional options available to grant. In May 2009, the stockholders approved an amendment to the 1997 Plan allowing for the continued issuance of incentive stock options and a 25,000 reduction in shares which may be issued under the 1997 Plan. In May 2003, the stockholders approved a new plan, the 2003 Stock Incentive Plan, which allows for the granting of options for up to 239,050 shares of the Company’s common stock. The number of shares reserved for issuance under all plans as of January 1, 2012 was 105,386.
The stock options granted by the board of directors may be either incentive stock options (“ISOs”) or non-qualified stock options (“NQs”). The exercise price for options under all of the plans may be no less than 100% of the fair value of the underlying common stock for ISOs or 85% of fair value for NQs. Options granted will expire no later than the tenth anniversary subsequent to the date of grant or three months following termination of employment, except in cases of death or disability, in which case the options will remain exercisable for up to twelve months. Under the terms of the 1997 Plan, in the event the Company is sold or merged, outstanding options will either be assumed by the surviving corporation or vest immediately.
There are four key inputs to the Black-Scholes model which the Company uses to estimate fair value for options which it issues: expected term, expected volatility, risk-free interest rate and expected dividends, all of which require the Company to make estimates. The Company’s estimates for these inputs may not be indicative of actual future performance and changes to any of these inputs can have a material impact on the resulting estimated fair value calculated for the option. The Company’s expected term input was estimated
52
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
based on the Company’s historical experience for time from option grant to option exercise for all employees in 2011, 2010 and 2009; the Company treated all employees in one grouping in all three years. The Company’s expected volatility input was estimated based on the Company’s historical stock price volatility in 2011, 2010 and 2009. The Company’s risk-free interest rate input was determined based on the U.S. Treasury yield curve at the time of option issuance in 2011, 2010 and 2009. The Company’s expected dividends input was zero in 2011, 2010 and 2009. Weighted average assumptions used in 2011, 2010 and 2009 for each of these four key inputs are listed in the following table:
| 2009 |
2010 |
2011 | ||||
| Risk-free interest rate |
1.41% | 1.10% | 0.64% | |||
| Expected lives |
3.0 years | 3.0 years | 3.0 years | |||
| Expected volatility |
64% | 66% | 70% | |||
| Expected dividend yield |
0% | 0% | 0% |
A summary of the Company’s stock option plans, with options to purchase fractional shares resulting from the Company’s December 2010 1-for-10 reverse stock split included in the “cancelled” row in 2010, is as follows:
| Year Ended December 31, | ||||||||||||||||||||||||
| 2009 | 2010 | 2011 | ||||||||||||||||||||||
| Options | Weighted Average Exercise Price |
Options | Weighted Average Exercise Price |
Options | Weighted Average Exercise Price |
|||||||||||||||||||
| Outstanding at beginning of period |
1,283,382 | $ | 12.835 | 1,291,634 | $ | 11.846 | 1,341,876 | $ | 11.003 | |||||||||||||||
| Granted at Market |
107,499 | $ | 4.529 | 104,900 | $ | 5.945 | 187,750 | $ | 6.962 | |||||||||||||||
| Cancelled |
(99,247 | ) | $ | 16.713 | (53,459 | ) | $ | 21.572 | (73,871 | ) | $ | 12.684 | ||||||||||||
| Exercised |
— | $ | — | (1,199 | ) | $ | 5.834 | (7,080 | ) | $ | 4.564 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Outstanding at end of period |
1,291,634 | $ | 11.846 | 1,341,876 | $ | 11.003 | 1,448,675 | $ | 10.425 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Exercisable at end of period |
1,098,560 | $ | 12.648 | 1,142,209 | $ | 11.871 | 1,175,731 | $ | 11.427 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
The total estimated fair value of stock options granted during the years ended December 31, 2011, 2010 and 2009 were computed to be approximately $602 thousand, $274 thousand and $205 thousand, respectively. The amounts are amortized ratably over the vesting periods of the options. The weighted average estimated fair value of options granted during the years ended December 31, 2011, 2010 and 2009 was computed to be approximately $3.21, $2.57 and $1.91, respectively. The total intrinsic value of options exercised during the years ended December 31, 2011, 2010 and 2009 was $10 thousand, $2 thousand and $0, respectively. The cash proceeds from options exercised during the years ended December 31, 2011, 2010 and 2009 was $32 thousand, $7 thousand and $0.
The following table summarizes information about stock options outstanding and exercisable at December 31, 2011, excluding outstanding options to purchase an aggregate of 121.0 fractional shares resulting from the Company’s December 2010 1-for-10 reverse stock split with a weighted average remaining contractual life of 2.69 years, a weighted average exercise price of $13.21 and exercise prices ranging from $3.40 to $31.50. The Company intends to issue whole shares only from option exercises.
53
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Exercise Prices |
Number of Options Outstanding at December 31, 2011 |
Weighted Average Remaining Contractual Life in Years |
Weighted Average Exercise Price |
Number of Options Exercisable at December 31, 2011 |
Weighted Average Exercise Price |
|||||||||||||||
| $ 2.70—$ 4.96 |
301,019 | 6.92 | $ | 4.4736 | 188,949 | $ | 4.3349 | |||||||||||||
| $ 5.00—$ 7.00 |
296,272 | 6.25 | $ | 6.8599 | 148,023 | $ | 6.8846 | |||||||||||||
| $ 7.01—$ 10.60 |
305,539 | 2.96 | $ | 9.1850 | 294,296 | $ | 9.1882 | |||||||||||||
| $ 10.61—$ 15.90 |
294,864 | 3.14 | $ | 13.3321 | 294,614 | $ | 13.3316 | |||||||||||||
| $ 15.91—$ 31.50 |
250,981 | 4.02 | $ | 19.8659 | 249,849 | $ | 19.8730 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| $ 2.70—$ 31.50 |
1,448,675 | 4.67 | $ | 10.4251 | 1,175,731 | $ | 11.4270 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
As of December 31, 2011, there was $716 thousand of total unrecognized compensation expense related to outstanding stock options. That cost is expected to be recognized over a weighted-average period of 2.1 years with all cost to be recognized by the end of December 2015, assuming all options vest according to the vesting schedules in place at December 31, 2011. As of December 31, 2011, the aggregate intrinsic value of outstanding options was $981 thousand and the aggregate intrinsic value of exercisable options was $621 thousand.
Employee Stock Purchase Plan (the “ESPP”)
Under the 1997 Employee Stock Purchase Plan, the Company is authorized to issue up to 325,000 shares of common stock to its employees, of which 300,087 had been issued as of December 31, 2011. Employees of the Company and its U.S. subsidiaries who are expected to work at least 20 hours per week and five months per year are eligible to participate. Under the terms of the plan, employees can choose to have up to 10% of their annual base earnings withheld to purchase the Company’s common stock. Each offering period is five years, with six-month accumulation periods ending June 30 and December 31. The purchase price of the stock for June 30 and December 31 was 85% of the end-of-measurement-period market price.
For the years ended December 31, 2009, 2010 and 2011, the weighted-average fair value of the purchase rights granted was $0.51, $0.95 and $1.09 per share, respectively.
7. MAJOR CUSTOMERS
The Company had one customer to whom sales represented approximately 13% of total revenue for 2011. The Company had one customer to whom sales represented approximately 13% of total revenue for 2010. The Company had one customer in 2009 to whom sales represented 11% of total revenue. No customer represented 10% or more of total accounts receivable at December 31, 2010 or 2011.
8. COMMITMENTS AND CONTINGENCIES
The Company holds certain rights to market and manufacture all products developed or created under certain research, development and licensing agreements with various entities. In connection with such agreements, the Company has agreed to pay the entities royalties on net product sales. In the years ended December 31, 2011, 2010 and 2009, royalties of $513 thousand, $515 thousand and $600 thousand became payable under these agreements, respectively.
The Company has contracts with suppliers for unconditional annual minimum inventory purchases and milestone obligations to third parties the Company believes are likely to be triggered currently totaling approximately $2.4 million in fiscal 2012, $2.3 million for fiscal 2013, $3.3 million for fiscal 2014, $3.0 million for fiscal 2015 and $2.9 million for fiscal 2016.
54
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Company has entered into operating leases for its office and research facilities and certain equipment with future minimum payments as of December 31, 2011 as follows (in thousands):
| Year Ending December 31, |
||||
| 2012 |
$ | 1,891 | ||
| 2013 |
1,643 | |||
| 2014 |
1,501 | |||
| 2015 |
1,474 | |||
| 2016 |
1,361 | |||
| Thereafter |
9,464 | |||
|
|
|
|||
| $ | 17,334 | |||
|
|
|
|||
The Company had rent expense of $2.0 million, $1.8 million and $1.8 million in 2009, 2010 and 2011, respectively.
From time to time, the Company may be involved in litigation relating to claims arising out of its operations. At December 31, 2011, the Company was not a party to any legal proceedings that were expected, individually or in the aggregate, to have a material adverse effect on our business, financial condition or operating results.
The Company’s current terms and conditions of sale include a limited warranty that its products and services will conform to published specifications at the time of shipment and a more extensive warranty related to certain of its products. The typical remedy for breach of warranty is to correct or replace any defective product, and if not possible or practical, the Company will accept the return of the defective product and refund the amount paid. Historically, the Company has incurred minimal warranty costs. The Company’s warranty reserve on December 31, 2011 was $432 thousand.
9. SEGMENT REPORTING
The Company is comprised of two reportable segments, Core Companion Animal Health (“CCA”) and Other Vaccines, Pharmaceuticals and Products (“OVP”). The Core Companion Animal Health segment includes diagnostic instruments and supplies, as well as single use diagnostic and other tests, pharmaceuticals and vaccines, primarily for canine and feline use. These products are sold directly by the Company as well as through independent third-party distributors and through other distribution relationships. CCA segment products manufactured at the Des Moines, Iowa production facility included in the OVP segment’s assets are transferred at cost and are not recorded as revenue for the OVP segment. The Other Vaccines, Pharmaceuticals and Products segment includes private label vaccine and pharmaceutical production, primarily for cattle, but also for other animals including small mammals and fish. All OVP products are sold by third parties under third-party labels.
Additionally, the Company generates non-product revenue from research and development projects for third parties, licensing of technology and royalties. The Company performs these research and development projects for both companion animal and livestock purposes.
55
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Summarized financial information concerning the Company’s reportable segments is shown in the following table (in thousands):
| Core Companion Animal Health |
Other
Vaccines, Pharmaceuticals and Products |
Total | ||||||||||
| 2009: |
||||||||||||
| Total revenue |
$ | 66,449 | $ | 9,229 | $ | 75,678 | ||||||
| Operating income |
3,156 | 888 | 4,044 | |||||||||
| Interest expense |
319 | 88 | 407 | |||||||||
| Total assets |
52,146 | 11,988 | 64,134 | |||||||||
| Net assets |
36,924 | 8,131 | 45,055 | |||||||||
| Capital expenditures |
254 | 22 | 276 | |||||||||
| Depreciation and amortization |
1,631 | 934 | 2,565 | |||||||||
| Core Companion Animal Health |
Other
Vaccines, Pharmaceuticals and Products |
Total | ||||||||||
| 2010: |
||||||||||||
| Total revenue |
$ | 55,655 | $ | 9,796 | $ | 65,451 | ||||||
| Operating income (loss) |
1,073 | (715 | ) | 358 | ||||||||
| Interest expense |
128 | 61 | 189 | |||||||||
| Total assets |
53,720 | 9,328 | 63,048 | |||||||||
| Net assets |
39,016 | 6,782 | 45,798 | |||||||||
| Capital expenditures |
366 | 254 | 620 | |||||||||
| Depreciation and amortization |
1,413 | 885 | 2,298 | |||||||||
| Core Companion Animal Health |
Other
Vaccines, Pharmaceuticals and Products |
Total | ||||||||||
| 2011: |
||||||||||||
| Total revenue |
$ | 57,481 | $ | 12,584 | $ | 70,065 | ||||||
| Operating income |
1,564 | 1,685 | 3,249 | |||||||||
| Interest expense |
107 | 17 | 124 | |||||||||
| Total assets |
51,172 | 10,722 | 61,894 | |||||||||
| Net assets |
40,435 | 8,004 | 48,439 | |||||||||
| Capital expenditures |
495 | 589 | 1,084 | |||||||||
| Depreciation and amortization |
1,192 | 860 | 2,052 | |||||||||
Total revenue by principal geographic area was as follows (in thousands):
| For the Years Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
| United States |
$ | 65,249 | $ | 57,927 | $ | 60,383 | ||||||
| Europe |
3,984 | 3,025 | 3,408 | |||||||||
| Other International |
6,445 | 4,499 | 6,274 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 75,678 | $ | 65,451 | $ | 70,065 | ||||||
|
|
|
|
|
|
|
|||||||
56
HESKA CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Total assets by principal geographic areas were as follows (in thousands):
| December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
| United States |
$ | 60,059 | $ | 59,155 | $ | 58,984 | ||||||
| Europe |
4,075 | 3,893 | 2,910 | |||||||||
| Other International |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 64,134 | $ | 63,048 | $ | 61,894 | ||||||
|
|
|
|
|
|
|
|||||||
10. QUARTERLY FINANCIAL INFORMATION (unaudited)
The following summarizes selected quarterly financial information for each of the two years in the periods ended December 31, 2010 and 2011 (amounts in thousands, except per share data).
| Q1 | Q2 | Q3 | Q4 | Total | ||||||||||||||||
| 2010: |
||||||||||||||||||||
| Total revenue |
$ | 17,694 | $ | 15,107 | $ | 17,635 | $ | 15,015 | $ | 65,451 | ||||||||||
| Gross profit |
6,205 | 5,847 | 6,593 | 6,147 | 24,792 | |||||||||||||||
| Operating income (loss) |
(488 | ) | (207 | ) | 365 | 688 | 358 | |||||||||||||
| Net income (loss) |
(331 | ) | (164 | ) | 241 | 272 | 18 | |||||||||||||
| Net income (loss) per share – basic |
(0.06 | ) | (0.03 | ) | 0.05 | 0.05 | 0.00 | |||||||||||||
| Net income (loss) per share – diluted |
(0.06 | ) | (0.03 | ) | 0.05 | 0.05 | 0.00 | |||||||||||||
| 2011: |
||||||||||||||||||||
| Total revenue |
$ | 19,505 | $ | 17,447 | $ | 17,608 | $ | 15,505 | $ | 70,065 | ||||||||||
| Gross profit |
8,298 | 7,469 | 6,827 | 6,593 | 29,187 | |||||||||||||||
| Operating income |
1,540 | 887 | 408 | 414 | 3,249 | |||||||||||||||
| Net income |
916 | 457 | 288 | 484 | 2,145 | |||||||||||||||
| Net income per share – basic |
0.18 | 0.09 | 0.06 | 0.09 | 0.41 | |||||||||||||||
| Net income per share – diluted |
0.17 | 0.09 | 0.05 | 0.09 | 0.40 | |||||||||||||||
57
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
None.
| Item 9A. | Controls and Procedures. |
Evaluation of Disclosure Controls and Procedures.
Our management, with the participation of our chief executive officer and our chief financial officer, evaluated the effectiveness of our disclosure controls and procedures, as defined by Rule 13a-15 of the Exchange Act, as of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our chief executive officer and chief financial officer have concluded that our disclosure controls and procedures are effective to provide reasonable assurance that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding disclosure.
Management’s Report on Internal Control Over Financial Reporting.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, the Company conducted an evaluation of the effectiveness of its internal control over financial reporting based on criteria outlined in the COSO Internal Control over Financial Reporting – Guidance for Smaller Public Companies, a supplemental implementation guide issued in 2007 which modified criteria established in the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, the Company’s management has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2011.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Accordingly, even an effective system of internal control will provide only reasonable assurance that the objectives of the internal control system are met.
This annual report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our independent registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permit us to provide only management’s report in this annual report.
Changes in Internal Control over Financial Reporting.
There has been no change in our internal control over financial reporting during the fourth fiscal quarter covered by this Form 10-K that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information. |
On February 22, 2012, the Compensation Committee of our Board of Directors approved the terms of our 2012 management incentive plan for our executive officers and director level employees. The plan establishes the eligible percentages of base pay for each of the participants, the percentage allocation between company and individual performance and the key parameters and payout structure terms under the plan. The specific terms adopted for the 2012 plan are filed herewith as Exhibit 10.8 and incorporated herein by reference.
58
Certain information required by Part III is incorporated by reference to our definitive Proxy Statement filed with the Securities and Exchange Commission in connection with the solicitation of proxies for our 2011 Annual Meeting of Stockholders.
| Item 10. | Directors, Executive Officers and Corporate Governance. |
Executive Officers
The information required by this item with respect to executive officers is incorporated by reference to Item 1 of this report and can be found under the caption “Executive Officers of the Registrant.”
Directors
The information required by this section with respect to our directors will be incorporated by reference to the information in the sections entitled “Election of Directors” and “Section 16(a) Beneficial Ownership Reporting Compliance” in the Proxy Statement.
Code of Ethics
Our Board of Directors has adopted a code of ethics for our senior executive and financial officers (including our principal executive officer, principal financial officer and principal accounting officer). The code of ethics is available on our website at www.heska.com. We intend to disclose any amendments to or waivers from the code of ethics at that location.
Audit Committee
The information required by this section with respect to our Audit Committee will be incorporated by reference to the information in the section entitled “Board Structure and Committees” in the Proxy Statement.
Section 16(a) Beneficial Ownership Reporting Compliance
The information required by this item is incorporated by reference to the information in the section entitled “Section 16(a) Beneficial Ownership Reporting Compliance” in the Proxy Statement.
| Item 11. | Executive Compensation. |
The information required by this section will be incorporated by reference to the information in the sections entitled “Director Compensation, “Executive Compensation”, “Compensation Committee Report” and “Compensation Committe Interlocks and Insider Participation” in the Proxy Statement.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The other information required by this section will be incorporated by reference to the information in the section entitled “Common Stock Ownership of Certain Beneficial Owners and Management” in the Proxy Statement.
59
Equity Compensation Plan Information
The following table sets forth information about our common stock that may be issued upon exercise of options and rights under all of our equity compensation plans as of December 31, 2011, including the 1988 Stock Option Plan, the 1997 Stock Incentive Plan, the 2003 Stock Incentive Plan and the 1997 Employee Stock Purchase Plan. Our stockholders have approved all of these plans.
| Plan Category |
(a) Number of Securities to be Issued Upon Exercise of Outstanding Options and Rights (1) |
(b) Weighted-Average Exercise Price of Outstanding Options and Rights (1) |
(c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) |
|||||||||
| Equity Compensation Plans Approved by Stockholders |
1,448,675 | $ | 10.43 | 129,020 | ||||||||
| Equity Compensation Plans Not Approved by Stockholders |
None | None | None | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
1,448,675 | $ | 10.43 | 129,020 | ||||||||
|
|
|
|
|
|
|
|||||||
| (1) | Excluding outstanding options to purchase an aggregate of 121.0 fractional shares resulting from our December 2010 reverse stock split. |
| Item 13. | Certain Relationships and Related Transactions and Director Independence. |
The information required by this section will be incorporated by reference to the information in the sections entitled “Board Structures and Committees” and “Significant Relationships and Transactions with Directors, Officers or Principal Stockholders” in the Proxy Statement.
| Item 14. | Principal Accountant Fees and Services. |
The information required by this section will be incorporated by reference to the information in the section entitled “Auditor Fees and Services” in the Proxy Statement.
The information required by Part III to the extent not set forth herein, will be incorporated herein by reference to our definitive Proxy Statement for the 2012 Annual Meeting of Stockholders.
60
| Item 15. | Exhibits and Financial Statement Schedules. |
(a) The following documents are filed as a part of this Form 10-K.
(1) Financial Statements:
Reference is made to the Index to Consolidated Financial Statements under Item 8 in Part II of this Form 10-K.
(2) Financial Statement Schedules:
Schedule II – Valuation and Qualifying Accounts.
SCHEDULE II
HESKA CORPORATION AND SUBSIDIARIES
VALUATION AND QUALIFYING ACCOUNTS
(amounts in thousands)
| Balance at Beginning of Year |
Additions Charged to Costs and Expenses |
Other Additions |
Deductions | Balance at End of Year |
||||||||||||||||
| Allowance for doubtful accounts |
||||||||||||||||||||
| Year ended: |
||||||||||||||||||||
| December 31, 2009 |
$ | 209 | $ | 89 | — | $ | (121 | ) (a) | $ | 177 | ||||||||||
| December 31, 2010 |
$ | 177 | $ | 57 | — | $ | (98 | ) (a) | $ | 136 | ||||||||||
| December 31, 2011 |
$ | 136 | $ | 109 | — | $ | (71 | ) (a) | $ | 174 | ||||||||||
| (a) | Write-offs of uncollectible accounts. |
61
(3) Exhibits:
The exhibits listed below are required by Item 601 of Regulation S-K. Each management contract or compensatory plan or arrangement required to be filed as an exhibit to this Form 10-K has been identified.
| Exhibit |
Notes |
Description of Document | ||
| 3(i) | (15) | Restated Certificate of Incorporation of the Registrant. | ||
| 3(ii) | (15) | Certificate of Amendment to Restated Certificate of Incorporation of Registrant. | ||
| 3(iii) | (15) | Certificate of Amendment to the Restated Certificate of Incorporation, as amended, of Registrant. | ||
| 3(iv) | (14) | Bylaws of the Registrant. | ||
| 10.1* | (15) | 1997 Incentive Stock Plan of Registrant, as amended. | ||
| 10.2* | (10) | 1997 Incentive Stock Plan Employees and Consultants Option Agreement. | ||
| 10.3* | (10) | 1997 Incentive Stock Plan Outside Directors Option Agreement. | ||
| 10.4* | (13) | 2003 Equity Incentive Plan, as amended and restated. | ||
| 10.5* | (13) | 2003 Equity Incentive Plan Option Agreement. | ||
| 10.6* | (15) | 1997 Employee Stock Purchase Plan of Registrant, as amended. | ||
| 10.7* | (9) | Management Incentive Plan Master Document. | ||
| 10.8* | 2012 Management Incentive Plan. | |||
| 10.9* | (16) | Director Compensation Policy. | ||
| 10.10* | (11) | Form of Indemnification Agreement entered into between Registrant and its directors and certain officers. | ||
| 10.11* | (8) | Amended and Restated Employment Agreement with Robert B. Grieve, dated March 29, 2006. | ||
| 10.12* | (11) | Amendment to Employment Agreement between Registrant and Robert B. Grieve, dated effective as of January 1, 2008. | ||
| 10.13* | (10) | Employment Agreement between Diamond Animal Health, Inc. and Michael McGinley, dated May 1, 2000. | ||
| 10.14* | (11) | Amendment to Employment Agreement between Diamond Animal Health, Inc. and Michael McGinley, dated effective as of January 1, 2008. | ||
| 10.15* | (17) | Assignment and Second Amendment to Employment Agreement between Registrant and Michael J. McGinley, dated effective as of August 4, 2011. | ||
| 10.16* | (4) | Employment Agreement between Registrant and Jason Napolitano, dated May 6, 2002. | ||
| 10.17* | (11) | Amendment to Employment Agreement between Registrant and Jason Napolitano, dated effective as of January 1, 2008. | ||
| 10.18* | (17) | Employment Agreement between Registrant and Joseph P. Aperfine, dated effective as of August 4, 2011. | ||
| 10.19* | (10) | Employment Agreement between Registrant and Nancy Wisnewski, dated April 15, 2002. | ||
| 10.20* | (11) | Amendment to Employment Agreement between Registrant and Nancy Wisnewski, dated effective as of January 1, 2008. | ||
| 10.21* | (4) | Employment Agreement between Registrant and Michael Bent, dated May 1, 2000. | ||
| 10.22* | (11) | Amendment to Employment Agreement between Registrant and Michael Bent, dated effective as of January 1, 2008. | ||
| 10.23* | (17) | Employment Agreement between Registrant and Claudine M. Zachara, dated effective as of August 4, 2011. | ||
| 10.24 | (6) | Net Lease Agreement between Registrant and CCMRED 40, LLC, dated May 24, 2004. | ||
| 10.25 | (7) | First Amendment to Net Lease Agreement and Development Agreement between Registrant and CCMRED 40, LLC, dated February 11, 2005. | ||
62
| Exhibit |
Notes |
Description of Document | ||
| 10.26 | (7) | Second Amendment to Net Lease Agreement between Registrant and CCMRED 40, LLC, dated July 14, 2005. | ||
| 10.27 | Third Amendment to Net Lease Agreement between Registrant and Millbrae Square Company, effective as of January 1, 2010. | |||
| 10.28+ | (10) | Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Business Credit, Inc., dated December 30, 2005. | ||
| 10.29+ | (11) | First Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated December 5, 2006. | ||
| 10.30+ | (11) | Second Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated July 20, 2007. | ||
| 10.31 | (11) | Third Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated December 21, 2007. | ||
| 10.32+ | (12) | Fourth and Fifth Amendments to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated October 16, 2008. | ||
| 10.33+ | (13) | Sixth Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated December 30, 2008. | ||
| 10.34+ | (18) | Seventh Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated November 30, 2009. | ||
| 10.35+ | (18) | Eighth Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated December 15, 2010. | ||
| 10.36+ | Ninth Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated December 21, 2011. | |||
| 10.37+ | Tenth Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated February 9, 2012. | |||
| 10.38+ | (1) | Product Supply Agreement between Registrant and Quidel Corporation, dated July 3, 1997. | ||
| 10.39+ | (2) | First Amendment to Product Supply Agreement between Registrant and Quidel Corporation, dated March 15, 1999. | ||
| 10.40 | (13) | Letter Amendment to Product Supply Agreement between Registrant and Quidel Corporation dated July 7, 2004. | ||
| 10.41+ | (3) | Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated September 30, 2002. | ||
| 10.42+ | (5) | First Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated September 20, 2004. | ||
| 10.43+ | (10) | Second Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated December 10, 2004. | ||
| 10.44+ | (10) | Third Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated May 26, 2006. | ||
63
| Exhibit |
Notes |
Description of Document | ||
| 10.45+ | (11) | Fourth Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated as of November 16, 2007. | ||
| 10.46+ | (15) | Fifth Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated as of December 23, 2010. | ||
| 10.47+ | Sixth Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated as of July 25, 2011. | |||
| 10.48+ | (10) | Supply and Distribution Agreement between Registrant and Boule Medical AB, dated June 17, 2003, Letter Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated June 1, 2004 and Letter Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated December 31, 2004. | ||
| 10.49+ | (12) | Letter Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated July 12, 2005; Letter Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated March 20, 2007; Letter Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated January 23, 2008; and Sixth Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated October 1, 2008. | ||
| 10.50 | (17) | Seventh Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated June 1, 2011. | ||
| 10.51+ | (10) | Supply and License Agreement between Registrant and Schering-Plough Animal Health Corporation, dated as of August 1, 2003. | ||
| 10.52+ | (13) | Amendment No. 1 to Supply and License Agreement between Registrant and Schering-Plough Animal Health Corporation, dated August 31, 2005. | ||
| 10.53+ | (11) | Clinical Chemistry Analyzer Agreement between Registrant and FUJIFILM Corporation, dated as of January 30, 2007. | ||
| 21.1 | Subsidiaries of the Company. | |||
| 23.1 | Consent of Ehrhardt Keefe Steiner & Hottman PC, Independent Registered Public Accounting Firm. | |||
| 24.1 | Power of Attorney (See Signature Page of this Form 10-K). | |||
| 31.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as amended. | |||
| 31.2 | Certification of Chief Financial Officer Pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as amended. | |||
| 32.1** | Certification of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |||
| 101.INS** | XBRL Instance Document. | |||
| 101.SCH** | XBRL Taxonomy Extension Schema Document. | |||
| 101.CAL** | XBRL Taxonomy Extension Calculation Linkbase Document. | |||
| 101.DEF** | XBRL Taxonomy Extension Definition Linkbase Document. | |||
| 101.PRE** | XBRL Taxonomy Extension Presentation Linkbase Document. | |||
| 101.LAB** | XBRL Taxonomy Extension Label Linkbase Document. | |||
64
Notes
| * | Indicates management contract or compensatory plan or arrangement. |
| + | Portions of the exhibit have been omitted pursuant to a request for confidential treatment. |
| ** | Furnished herewith. |
| (1) | Filed with the Registrant’s Form 10-Q for the quarter ended September 30, 1997. |
| (2) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2001. |
| (3) | Filed with the Registrant’s Form 10-Q for the quarter ended September 30, 2002. |
| (4) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2002. |
| (5) | Filed with the Registrant’s Form 10-Q for the quarter ended September 30, 2004. |
| (6) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2004. |
| (7) | Filed with the Registrant’s Form 10-Q for the quarter ended June 30, 2005. |
| (8) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2005. |
| (9) | Filed with the Registrant’s Form 10-Q for the quarter ended March 31, 2006. |
| (10) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2006. |
| (11) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2007. |
| (12) | Filed with the Registrant’s Form 10-Q for the quarter ended September 30, 2008. |
| (13) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2008. |
| (14) | Filed with the Registrant’s Form 10-Q for the quarter ended March 31, 2010. |
| (15) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2010. |
| (16) | Filed with the Registrant’s Form 10-Q for the quarter ended March 31, 2011. |
| (17) | Filed with the Registrant’s Form 10-Q for the quarter ended June 30, 2011. |
| (18) | Filed with the Registrant’s Form 10-Q for the quarter ended September 30, 2011. |
65
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on February 28, 2012.
| HESKA CORPORATION | ||
| By: | /s/ ROBERT B. GRIEVE | |
| Robert B. Grieve | ||
| Chairman of the Board and Chief Executive Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Robert B. Grieve, Jason A. Napolitano and Michael A. Bent, and each of them, his or her true and lawful attorneys-in-fact, each with full power of substitution, for him or her in any and all capacities, to sign any amendments to this report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact or their substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities and Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
| Signature |
Title |
Date | ||
| /s/ ROBERT B. GRIEVE |
Chairman of the Board and Chief Executive | February 28, 2012 | ||
| Robert B. Grieve |
Officer (Principal Executive Officer) and Director |
|||
| /s/ JASON A. NAPOLITANO |
Executive Vice President, Chief Financial | February 28, 2012 | ||
| Jason A. Napolitano |
Officer and Secretary (Principal Financial Officer) |
|||
| /s/ MICHAEL A. BENT |
Vice President, Controller | February 28, 2012 | ||
| Michael A. Bent |
(Principal Accounting Officer) | |||
| /s/ WILLIAM A. AYLESWORTH |
Lead Director | February 28, 2012 | ||
| William A. Aylesworth |
||||
| /s/ PETER EIO |
Director | February 28, 2012 | ||
| Peter Eio |
||||
| /s/ G. IRWIN GORDON |
Director | February 28, 2012 | ||
| Irwin Gordon |
||||
| /s/ LOUISE L. McCORMICK |
Director | February 28, 2012 | ||
| Louise L. McCormick |
||||
| /s/ SHARON J. RILEY |
Director | February 28, 2012 | ||
| Sharon J. Riley |
||||
| /s/ JOHN F. SASEN, Sr. |
Director | February 28, 2012 | ||
| John F. Sasen, Sr. |
||||
66
| Exhibit Number |
Notes |
Description of Document | ||
| 3(i) | (15) | Restated Certificate of Incorporation of the Registrant. | ||
| 3(ii) | (15) | Certificate of Amendment to Restated Certificate of Incorporation of Registrant. | ||
| 3(iii) | (15) | Certificate of Amendment to the Restated Certificate of Incorporation, as amended, of Registrant. | ||
| 3(iv) | (14) | Bylaws of the Registrant. | ||
| 10.1* | (15) | 1997 Incentive Stock Plan of Registrant, as amended. | ||
| 10.2* | (10) | 1997 Incentive Stock Plan Employees and Consultants Option Agreement. | ||
| 10.3* | (10) | 1997 Incentive Stock Plan Outside Directors Option Agreement. | ||
| 10.4* | (13) | 2003 Equity Incentive Plan, as amended and restated. | ||
| 10.5* | (13) | 2003 Equity Incentive Plan Option Agreement. | ||
| 10.6* | (15) | 1997 Employee Stock Purchase Plan of Registrant, as amended. | ||
| 10.7* | (9) | Management Incentive Plan Master Document. | ||
| 10.8* | 2012 Management Incentive Plan. | |||
| 10.9* | (16) | Director Compensation Policy. | ||
| 10.10* | (11) | Form of Indemnification Agreement entered into between Registrant and its directors and certain officers. | ||
| 10.11* | (8) | Amended and Restated Employment Agreement with Robert B. Grieve, dated March 29, 2006. | ||
| 10.12* | (11) | Amendment to Employment Agreement between Registrant and Robert B. Grieve, dated effective as of January 1, 2008. | ||
| 10.13* | (10) | Employment Agreement between Diamond Animal Health, Inc. and Michael McGinley, dated May 1, 2000. | ||
| 10.14* | (11) | Amendment to Employment Agreement between Diamond Animal Health, Inc. and Michael McGinley, dated effective as of January 1, 2008. | ||
| 10.15* | (17) | Assignment and Second Amendment to Employment Agreement between Registrant and Michael J. McGinley, dated effective as of August 4, 2011. | ||
| 10.16* | (4) | Employment Agreement between Registrant and Jason Napolitano, dated May 6, 2002. | ||
| 10.17* | (11) | Amendment to Employment Agreement between Registrant and Jason Napolitano, dated effective as of January 1, 2008. | ||
| 10.18* | (17) | Employment Agreement between Registrant and Joseph P. Aperfine, dated effective as of August 4, 2011. | ||
| 10.19* | (10) | Employment Agreement between Registrant and Nancy Wisnewski, dated April 15, 2002. | ||
| 10.20* | (11) | Amendment to Employment Agreement between Registrant and Nancy Wisnewski, dated effective as of January 1, 2008. | ||
| 10.21* | (4) | Employment Agreement between Registrant and Michael Bent, dated May 1, 2000. | ||
| 10.22* | (11) | Amendment to Employment Agreement between Registrant and Michael Bent, dated effective as of January 1, 2008. | ||
| 10.23* | (17) | Employment Agreement between Registrant and Claudine M. Zachara, dated effective as of August 4, 2011. | ||
| 10.24 | (6) | Net Lease Agreement between Registrant and CCMRED 40, LLC, dated May 24, 2004. | ||
| 10.25 | (7) | First Amendment to Net Lease Agreement and Development Agreement between Registrant and CCMRED 40, LLC, dated February 11, 2005. | ||
| 10.26 | (7) | Second Amendment to Net Lease Agreement between Registrant and CCMRED 40, LLC, dated July 14, 2005. | ||
67
| Exhibit Number |
Notes |
Description of Document | ||
| 10.27 | Third Amendment to Net Lease Agreement between Registrant and Millbrae Square Company, effective as of January 1, 2010. | |||
| 10.28+ | (10) | Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Business Credit, Inc., dated December 30, 2005. | ||
| 10.29+ | (11) | First Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated December 5, 2006. | ||
| 10.30+ | (11) | Second Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated July 20, 2007. | ||
| 10.31 | (11) | Third Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated December 21, 2007. | ||
| 10.32+ | (12) | Fourth and Fifth Amendments to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated October 16, 2008. | ||
| 10.33+ | (13) | Sixth Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated December 30, 2008. | ||
| 10.34+ | (18) | Seventh Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated November 30, 2009. | ||
| 10.35+ | (18) | Eighth Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated December 15, 2010. | ||
| 10.36+ | Ninth Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated December 21, 2011. | |||
| 10.37+ | Tenth Amendment to Third Amended and Restated Credit and Security Agreement between Registrant, Diamond Animal Health, Inc. and Wells Fargo Bank, National Association, dated February 9, 2012. | |||
| 10.38+ | (1) | Product Supply Agreement between Registrant and Quidel Corporation, dated July 3, 1997. | ||
| 10.39+ | (2) | First Amendment to Product Supply Agreement between Registrant and Quidel Corporation, dated March 15, 1999. | ||
| 10.40 | (13) | Letter Amendment to Product Supply Agreement between Registrant and Quidel Corporation dated July 7, 2004. | ||
| 10.41+ | (3) | Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated September 30, 2002. | ||
| 10.42+ | (5) | First Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated September 20, 2004. | ||
| 10.43+ | (10) | Second Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated December 10, 2004. | ||
| 10.44+ | (10) | Third Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated May 26, 2006. | ||
| 10.45+ | (11) | Fourth Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated as of November 16, 2007. | ||
68
| Exhibit Number |
Notes |
Description of Document | ||
| 10.46+ | (15) | Fifth Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated as of December 23, 2010. | ||
| 10.47+ | Sixth Amendment to Amended and Restated Bovine Vaccine Distribution Agreement between Diamond Animal Health, Inc. and Agri Laboratories, Ltd., dated as of July 25, 2011. | |||
| 10.48+ | (10) | Supply and Distribution Agreement between Registrant and Boule Medical AB, dated June 17, 2003, Letter Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated June 1, 2004 and Letter Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated December 31, 2004. | ||
| 10.49+ | (12) | Letter Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated July 12, 2005; Letter Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated March 20, 2007; Letter Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated January 23, 2008; and Sixth Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated October 1, 2008. | ||
| 10.50 | (17) | Seventh Amendment to Supply and Distribution Agreement between Registrant and Boule Medical AB, dated June 1, 2011. | ||
| 10.51+ | (10) | Supply and License Agreement between Registrant and Schering-Plough Animal Health Corporation, dated as of August 1, 2003. | ||
| 10.52+ | (13) | Amendment No. 1 to Supply and License Agreement between Registrant and Schering-Plough Animal Health Corporation, dated August 31, 2005. | ||
| 10.53+ | (11) | Clinical Chemistry Analyzer Agreement between Registrant and FUJIFILM Corporation, dated as of January 30, 2007. | ||
| 21.1 | Subsidiaries of the Company. | |||
| 23.1 | Consent of Ehrhardt Keefe Steiner & Hottman PC, Independent Registered Public Accounting Firm. | |||
| 24.1 | Power of Attorney (See Signature Page of this Form 10-K). | |||
| 31.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as amended. | |||
| 31.2 | Certification of Chief Financial Officer Pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as amended. | |||
| 32.1** | Certification of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |||
| 101.INS** | XBRL Instance Document. | |||
| 101.SCH** | XBRL Taxonomy Extension Schema Document. | |||
| 101.CAL** | XBRL Taxonomy Extension Calculation Linkbase Document. | |||
| 101.DEF** | XBRL Taxonomy Extension Definition Linkbase Document. | |||
| 101.PRE** | XBRL Taxonomy Extension Presentation Linkbase Document. | |||
| 101.LAB** | XBRL Taxonomy Extension Label Linkbase Document. | |||
69
Notes
| * | Indicates management contract or compensatory plan or arrangement. |
| + | Portions of the exhibit have been omitted pursuant to a request for confidential treatment. |
| ** | Furnished herewith. |
| (1) | Filed with the Registrant’s Form 10-Q for the quarter ended September 30, 1997. |
| (2) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2001. |
| (3) | Filed with the Registrant’s Form 10-Q for the quarter ended September 30, 2002. |
| (4) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2002. |
| (5) | Filed with the Registrant’s Form 10-Q for the quarter ended September 30, 2004. |
| (6) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2004. |
| (7) | Filed with the Registrant’s Form 10-Q for the quarter ended June 30, 2005. |
| (8) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2005. |
| (9) | Filed with the Registrant’s Form 10-Q for the quarter ended March 31, 2006. |
| (10) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2006. |
| (11) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2007. |
| (12) | Filed with the Registrant’s Form 10-Q for the quarter ended September 30, 2008. |
| (13) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2008. |
| (14) | Filed with the Registrant’s Form 10-Q for the quarter ended March 31, 2010. |
| (15) | Filed with the Registrant’s Form 10-K for the year ended December 31, 2010. |
| (16) | Filed with the Registrant’s Form 10-Q for the quarter ended March 31, 2011. |
| (17) | Filed with the Registrant’s Form 10-Q for the quarter ended June 30, 2011. |
| (18) | Filed with the Registrant’s Form 10-Q for the quarter ended September 30, 2011. |
70
