Attached files
| file | filename |
|---|---|
| 8-K - OPEXA THERAPEUTICS, INC. 8-K - Acer Therapeutics Inc. | a50134461.htm |
Exhibit 99.1

January 2012 Neil Warma
President & CEO Opexa Therapeutics, Inc.

Forward-Looking
Statements This presentation contains forward-looking statements which
are made pursuant to the safe harbor provisions of Section 27A of the
Securities Act of 1933, as amended, and Section 21E of the Securities
Exchange Act of 1934, as amended. The forward-looking statements in this
presentation do not constitute guarantees of future performance.
Investors are cautioned that statements in this presentation which are
not strictly historical statements, including, without limitation,
statements regarding the Company’s clinical development plans for
Tovaxin, constitute forward-looking statements. Such forward-looking
statements are subject to a number of risks and uncertainties that could
cause actual results to differ materially from those anticipated,
including, without limitation, risks associated with the Company’s
capital position, the ability of the Company to enter into and benefit
from a partnering arrangement for the Company’s product candidate,
Tovaxin, on reasonably satisfactory terms (if at all), and our
dependence (if partnered) on the resources and abilities of any partner
for the further development of Tovaxin, our ability to compete with
larger, better financed pharmaceutical and biotechnology companies, new
approaches to the treatment of our targeted diseases, our expectation of
incurring continued losses, our uncertainty of developing a marketable
product, our ability to raise additional capital to continue our
treatment development program and to undertake and complete any further
clinical studies for Tovaxin, the success of our clinical trials, the
efficacy of Tovaxin for any particular indication, such as for relapsing
remitting MS or secondary progressive MS, our ability to develop and
commercialize products, our ability to obtain required regulatory
approvals, our compliance with all Food and Drug Administration
regulations, our ability to obtain, maintain and protect intellectual
property rights (including for Tovaxin), the risk of litigation
regarding our intellectual property rights, our limited manufacturing
capabilities, our dependence on third-party manufacturers, our ability
to hire and retain skilled personnel, our volatile stock price, and
other risks detailed in our filings with the Securities and Exchange
Commission. These forward-looking statements speak only as of the date
of this presentation. We assume no obligation or undertaking to update
or revise any forward-looking statements contained herein to reflect any
changes in our expectations with regard thereto or any change in events,
conditions or circumstances on which any such statement is based. You
should, however, review additional disclosures we make in our Annual
Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports
on Form 8-K filed with the SEC. 2
•Proprietary T-cell
technology platform allows for the production of patient-specific T-cell
therapies fora variety of autoimmune diseases •Lead program, Tovaxin®, a
personalized cellular immunotherapy for the first-line treatment of
multiple sclerosis (MS) •Fast Track designation granted November 2011 by
FDA for Tovaxin for treatment of Secondary Progressive Multiple
Sclerosis (SPMS) •In-house cGMP manufacturing enables close control of
process and COGS 3
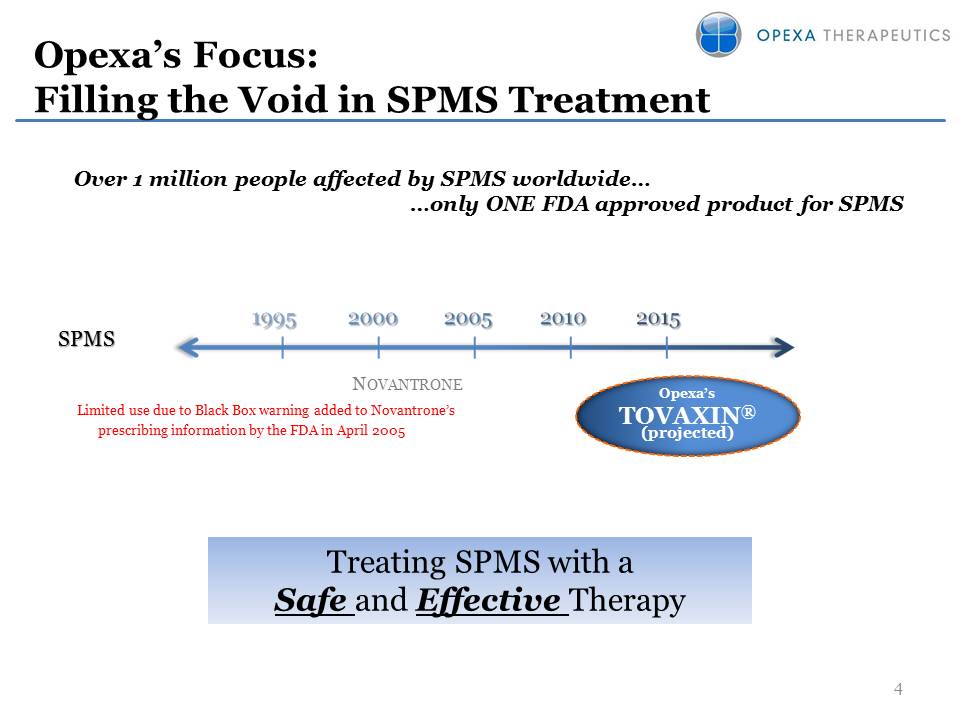
Opexa’s Focus: Filling the
Void in SPMS Treatment 4 Over 1 million people affected by SPMS
worldwide… …only ONE FDA approved product for SPMS S PMS Opexa’s
TOVAXIN®(projectedNOVANTRONE Limited use due to Black Box warning added
to Novantrone’s prescribing information by the FDA in April 2005
Treating SPMS with a Safe and Effective Therapy
Tovaxin® 5 A personalized
autologous T-cell immunotherapy, consisting of attenuated,
patient-specific myelin reactive T-cells (MRTCs) against peptides of the
three primary myelin proteins Proposed Mechanism The subcutaneous
injection of a therapeutic dose (30-45 million cells) of Tovaxin
stimulates the body’s immune system to recognize the bolus of injected
cells as a peripheral source of ‘over represented’ MRTC , resulting in
the induction of an opposing dominant negative ‘regulatory T-cell’
response: •Selective targeting by immune cells to down-regulate and
eliminate similar myelin reactive T-cells within the CNS •An
up-regulation of important regulatory cells (Foxp3+ and Tr1 cells) to
reduce inflammation and provide possible neuroprotection
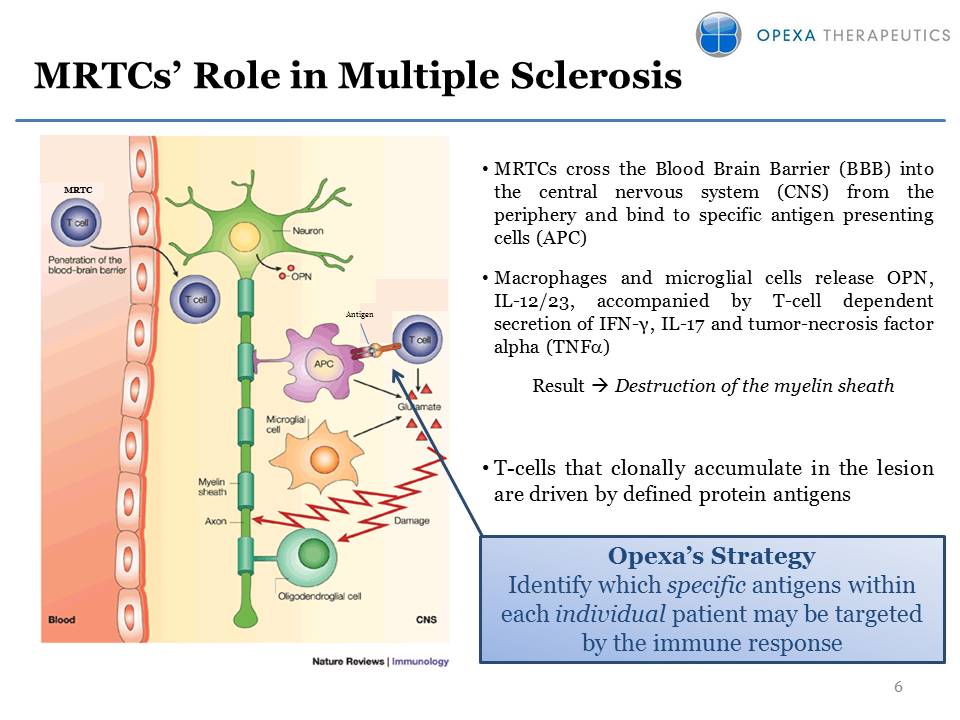
MRTCs’ Role in Multiple
Sclerosis 6 •MRTCs cross the Blood Brain Barrier (BBB) into the central
nervous system (CNS) from the periphery and bind to specific antigen
presenting cells (APC) •Macrophages and microglial cells release OPN,
IL-12/23, accompanied by T-cell dependent secretion of IFN-γ, IL-17 and
tumor-necrosis factor alpha (TNFa) Result Destruction of the myelin
sheath •T-cells that clonally accumulate in the lesion are driven by
defined protein antigens Antigen MRTC Opexa’s Strategy Identify which
specific antigens within each individual patient may be targeted by the
immune response
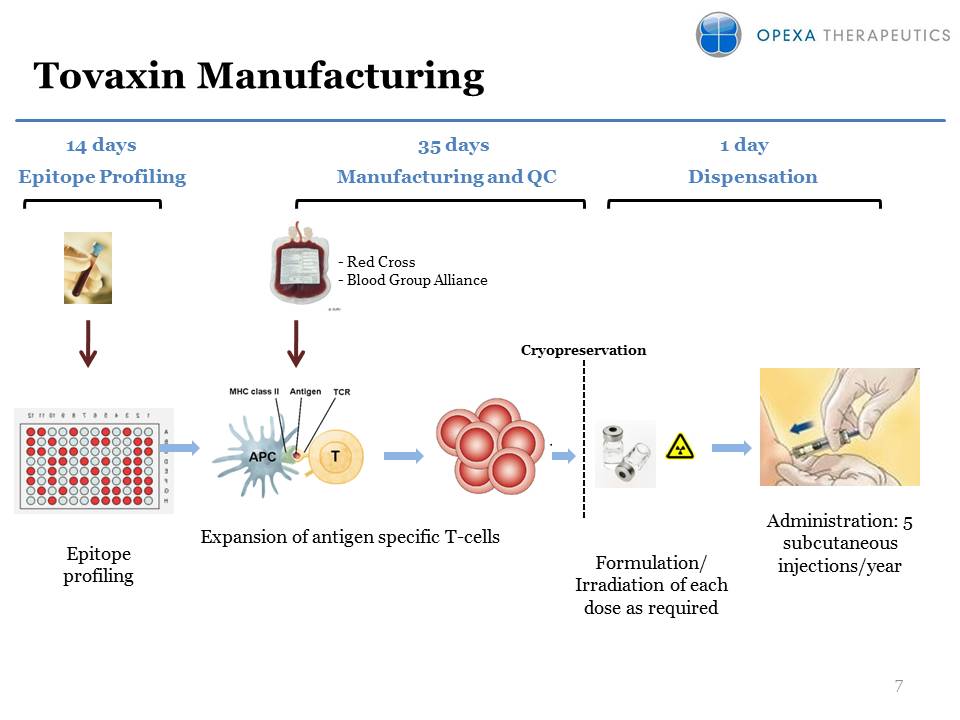
Tovaxin Manufacturing 7
Expansion of antigen specific T-cells Cryopreservation Formulation/
Irradiation of each dose as required Epitope profiling Administration: 5
subcutaneous injections/year Manufacturing and QC Dispensation 35 days
Epitope Profiling 1 day 14 days - Red Cross - Blood Group Alliance
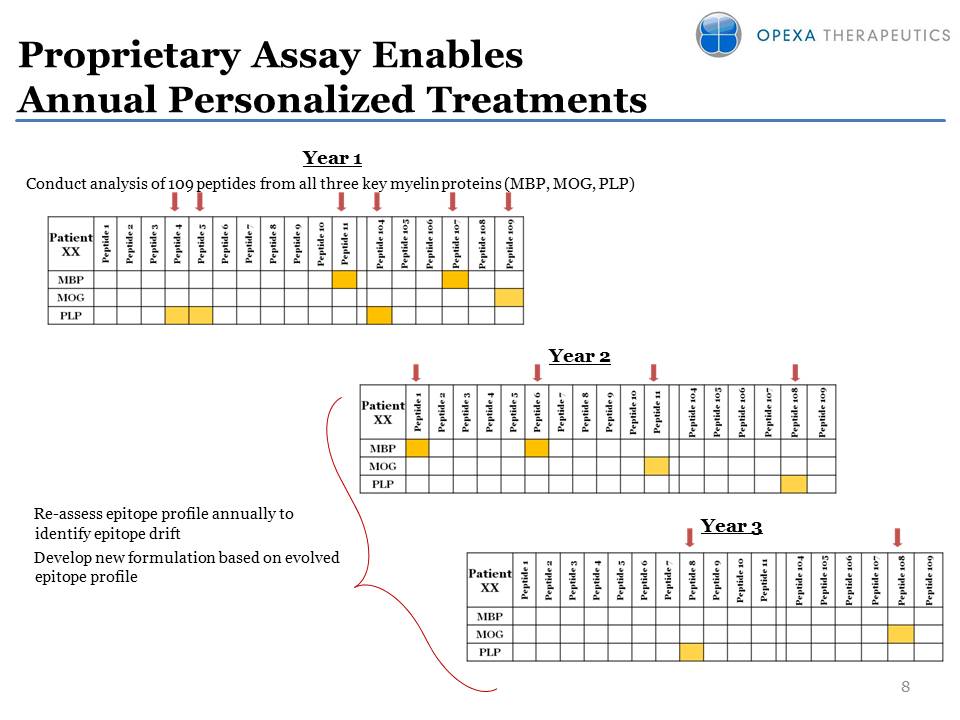
8 Year Year 3 Proprietary
Assay Enables Annual Personalized Treatments Year 1 Conduct analysis of
109 peptides from all three key myelin proteins (MBP, MOG, PLP)
Re-assess epitope profile annually to identify epitope drift Develop new
formulation based on evolved epitope profile
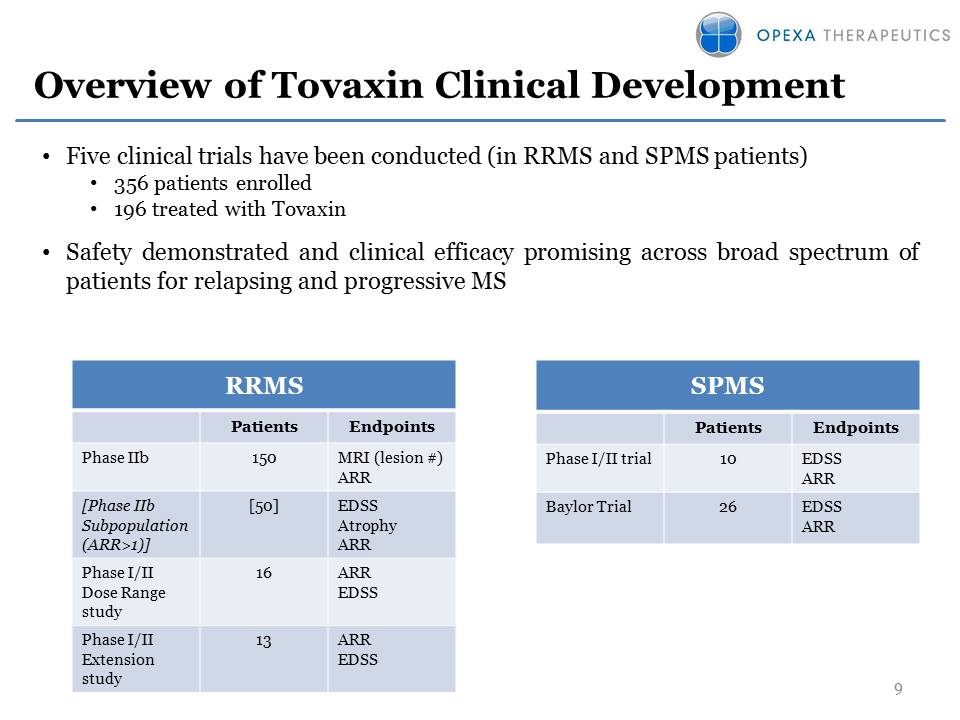
Overview of Tovaxin
Clinical Development SPMS Patients Endpoints Phase I/II trial10 EDSS ARR
Baylor Trial26 EDSS ARR 9 RRMS Patients Endpoints Phase IIb150 MRI
(lesion #) ARR[Phase IIb Subpopulation (ARR>1)] [50] EDSS Atrophy ARR
Phase I/II Dose Range study16 ARR EDSS Phase I/II Extension study13 ARR
EDSS•Five clinical trials have been conducted (in RRMS and SPMS
patients) •356 patients enrolled •196 treated with Tovaxin •Safety
demonstrated and clinical efficacy promising across broad spectrum of
patients for relapsing and progressive MS
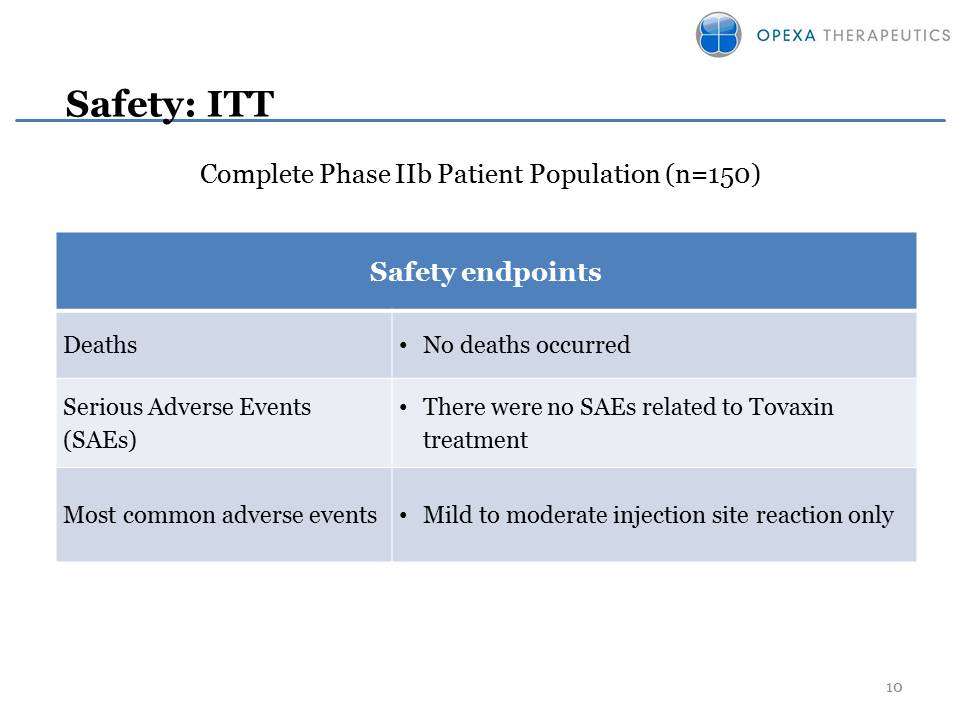
Safety endpoints Deaths No
deaths occurred Serious Adverse Events (SAEs)•There were no SAEs related
to Tovaxin treatment Most common adverse events•Mild to moderate
injection site reaction only Complete Phase IIb Patient Population
(n=150) Safety: ITT 10
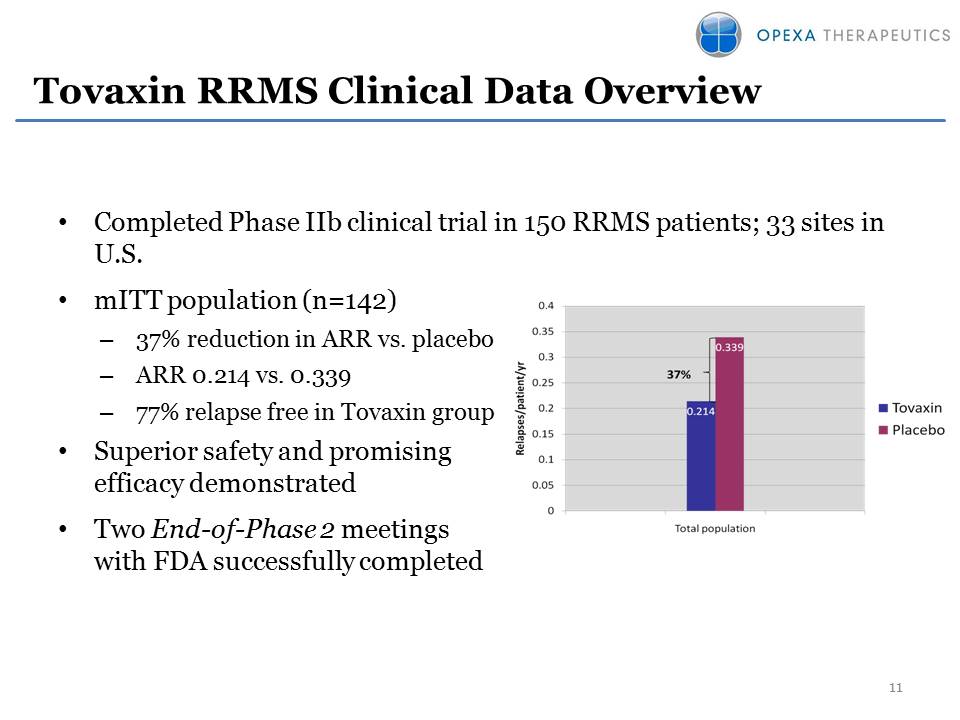
Tovaxin RRMS Clinical Data
Overview •Completed Phase IIb clinical trial in 150 RRMS patients; 33
sites in U.S. •mITT population (n=142) –37% reduction in ARR vs. placebo
–ARR 0.214 vs. 0.339 –77% relapse free in Tovaxin group •Superior safety
and promising efficacy demonstrated •Two End-of-Phase 2 meetings with
FDA successfully completed 11
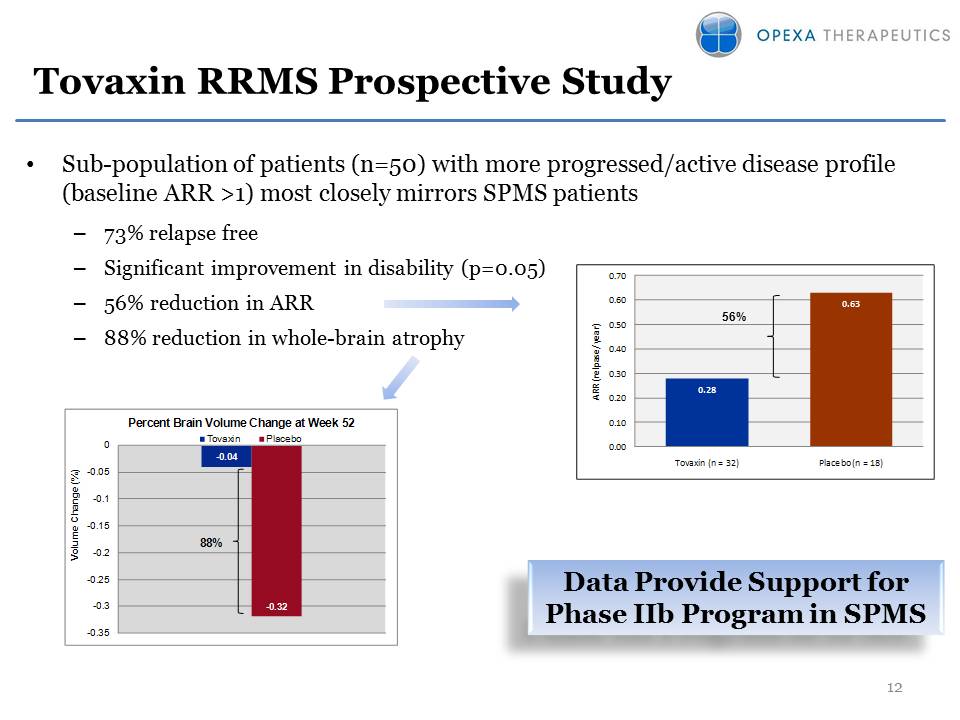
Tovaxin RRMS Prospective
Study •Sub-population of patients (n=50) with more progressed/active
disease profile (baseline ARR >1) most closely mirrors SPMS patients
–73% relapse free –Significant improvement in disability (p=0.05) –56%
reduction in ARR –88% reduction in whole-brain atrophy 12 56% Data
Provide Support for Phase IIb Program in SPMS

•36 patients treated in
three clinical trials •Promising efficacy observed •Disease
stabilization in 80% of patients at two years •Significant reduction in
relapse rates •Well-tolerated, no SAEs 13 Secondary Progressive MS:
Clinical Overview
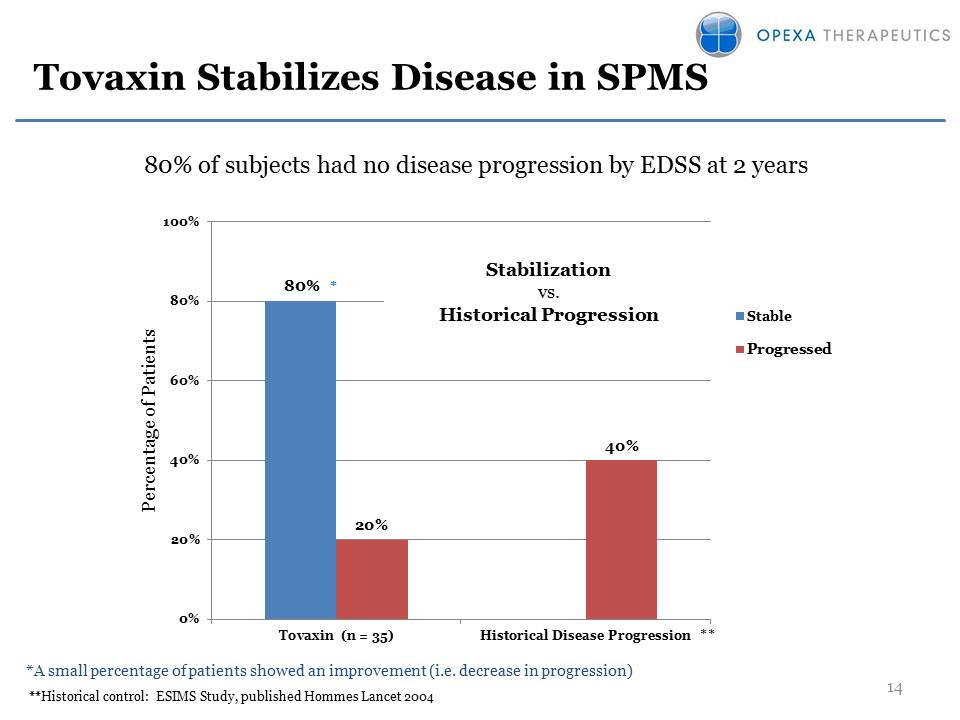
Tovaxin Stabilizes Disease
in SPMS *A small percentage of patients showed an improvement (i.e.
decrease in progression) **Historical control: ESIMS Study, published
Hommes Lancet 2004 80% 20% 40% 0%20%40%60%80%100%Tovaxin (n = 35)
Historical Disease ProgressionPercentage of Patients
StableProgressedStabilization vs. Historical Progression * ** 14 80% of
subjects had no disease progression by EDSS at 2 years
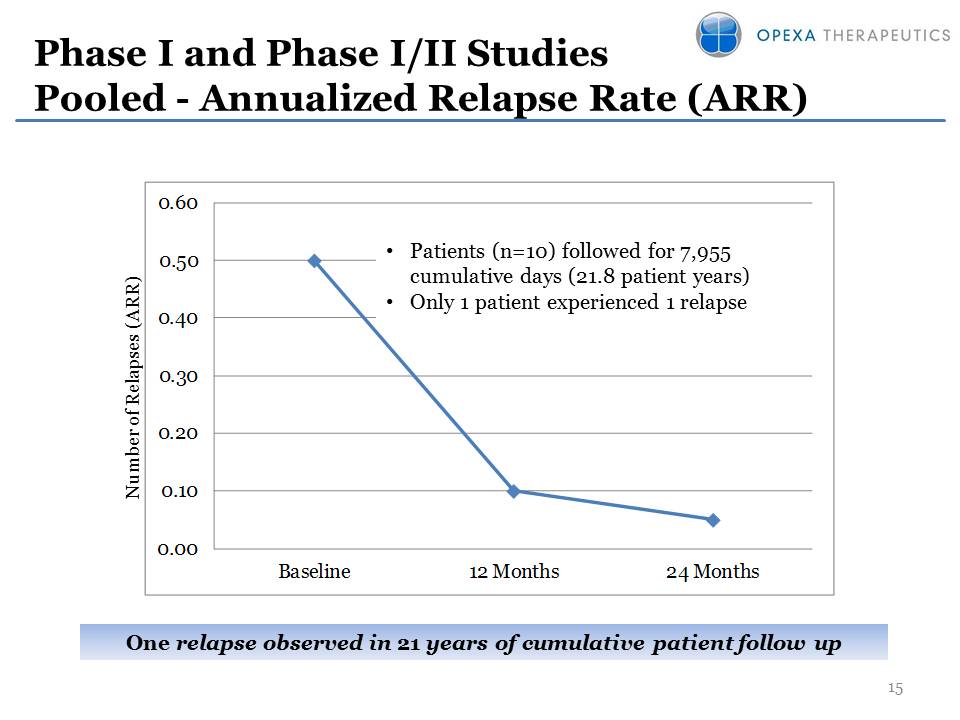
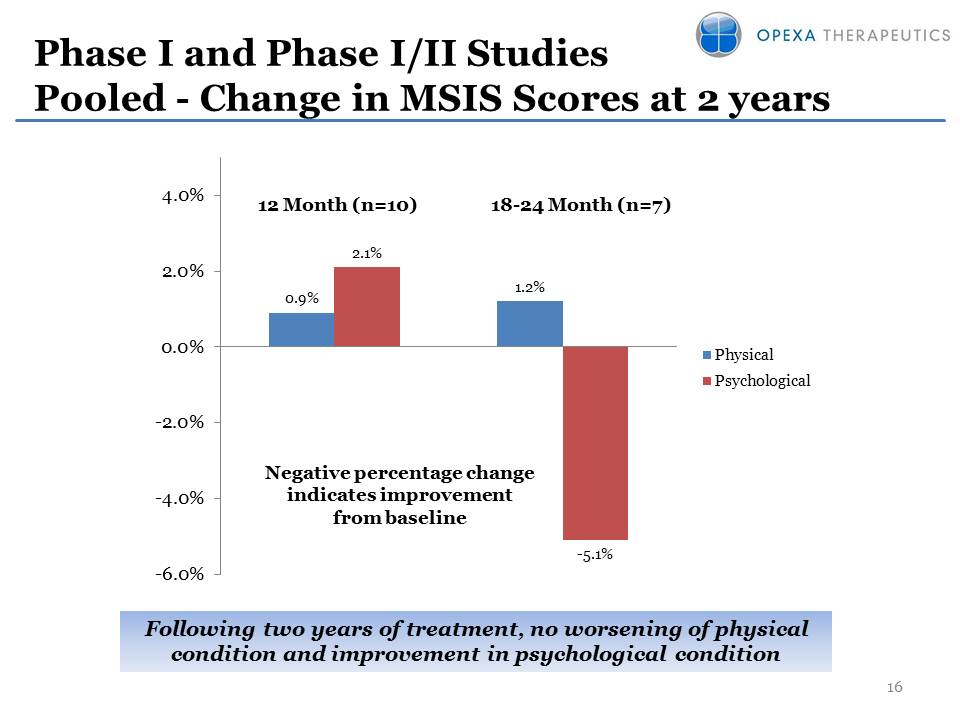
Phase I and Phase I/II
Studies Pooled - Annualized Relapse Rate (ARR) 15 Number of Relapses
(ARR) •Patients (n=10) followed for 7,955 cumulative days (21.8 patient
years) •Only 1 patient experienced 1 relapse One relapse observed in 21
years of cumulative patient follow up
Phase I and Phase I/II
Studies Pooled - Change in MSIS Scores at 2 years 16 0.9% 1.2% 2.1%
-5.1% -6.0%-4.0%-2.0%0.0%2.0%4.0%PhysicalPsychological12 Month (n=10)
18-24 Month (n=7) Negative percentage change indicates improvement from
baseline Following two years of treatment, no worsening of physical
condition and improvement in psychological condition
Overview of Tovaxin
Clinical Development 17 Clinical Status: Five clinical trials completed
with Tovaxin in 196 patients, many with multiple years of treatment
Efficacy: Data shows reduction in Annualized Relapse Rate (ARR), slowing
disease progression Safety & Tolerability: Appears superior to all
marketed and developmental MS drugs
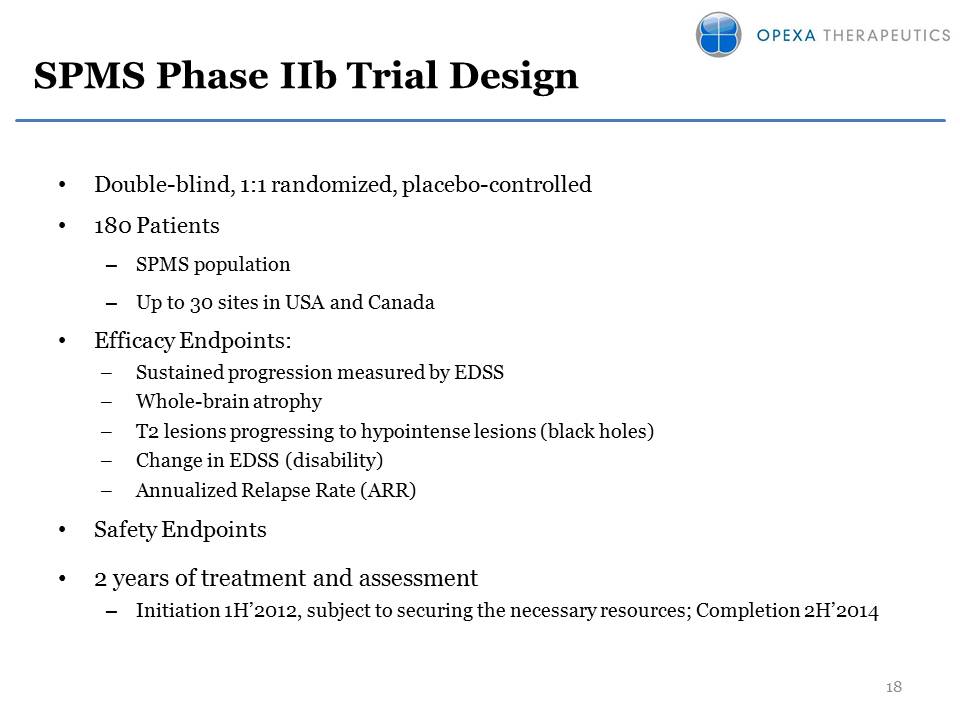
SPMS Phase IIb Trial
Design •Double-blind, 1:1 randomized, placebo-controlled •180 Patients
–SPMS population –Up to 30 sites in USA and Canada •Efficacy Endpoints:
–Sustained progression measured by EDSS –Whole-brain atrophy –T2 lesions
progressing to hypointense lesions (black holes) –Change in EDSS
(disability) –Annualized Relapse Rate (ARR) •Safety Endpoints •2 years
of treatment and assessment –Initiation 1H’2012, subject to securing the
necessary resources; Completion 2H’201418

Scientific Advisory
Board 19 Dawn McGuire, M.D., FAAN (Chair) •Advisory Council of the Gill
Heart Institute •American Academy of Neurology •National Institute of
Neurological Disorders and Stroke of the National Institutes of Health
Hans-Peter Hartung, M.D. •Chair of Neurology at Heinrich-Heine
University •President ECTRIMS •European Neurological Society
•International Society for Neuroimmunology •International Federation of
Multiple Sclerosis Societies •World Health Organization Advisory Board
on Multiple Sclerosis Mark S. Freedman, M.D., FRCP, FAAN •Director of
the Multiple Sclerosis Research Unit at Ottawa Hospital •Multiple
Sclerosis Society of Canada, National MS Society(USA) •Americas
Committee for Treatment and Research in MS •Consortium of MS Centres
Paul O’Connor, M.D., FRCP •MS Clinic Director at St. Michael’s Hospital,
University of Toronto •National Scientific and Clinical Advisor for the
Multiple Sclerosis Society of Canada

Scientific Advisory
Board Clyde Markowitz, M.D. •Director of the Multiple Sclerosis Center
at the University of Pennsylvania •Professor of Neurology at the
University of Pennsylvania School of Medicine in Philadelphia •Chairman
of the Clinical Advisory Committee for the Delaware Valley National MS
Society •American Academy of Neurology Doug Arnold, M.D. •James McGill
Professor Neurology and Neurosurgery at the Montreal Neurological
Institute of McGill University Arthur Vandenbark, Ph.D. •Co-Director of
the Neuroimmunology Research Laboratory at the Portland Veterans Affairs
Medical Center, Portland, Oregon •Director of the Tykeson Multiple
Sclerosis Research Laboratory at Oregon Health And Science University
•Professor of Neurology and Molecular Microbiology and Immunology Edward
Fox, M.D., Ph.D. •Director of Multiple Sclerosis Clinic of Central Texas
•Advisory Committee, Lone Star Chapter of the National Multiple
Sclerosis Society •Consortium of Multiple Sclerosis Centers •Clinical
Assistant Professor of Neurology, University of Texas Medical Branch 20

Milestones and Goals
Secured $8.5 million financing to advance clinical trials (Q1’11 )
Presented Tovaxin Phase IIb data at the American Academy of Neurology
(AAN) Meeting (Q2’11 ) Executed strategic agreements with the American
Red Cross and the Blood Group Alliance, Inc. (Q2’11 ) Initiated the
design and development of a proprietary Web-based system to manage
patient and product flow throughout future clinical trials (Q2’11 )
Furthered discussions with Health Canada’s Biologics and Genetics
Therapies Directorate to secure approval for future clinical trial
development in Canada (Q3’11 ) FDA Fast Track approval for Tovaxin in
SPMS (Q4’11 ) Secure resources to advance clinical development and
initiate 24-month Phase IIb SPMS clinical trial in North America
Initiate discussions with European Medicines Agency (EMA) for future
pivotal studies Evaluate expansion of platform to other autoimmune
indications and geographical territories 21
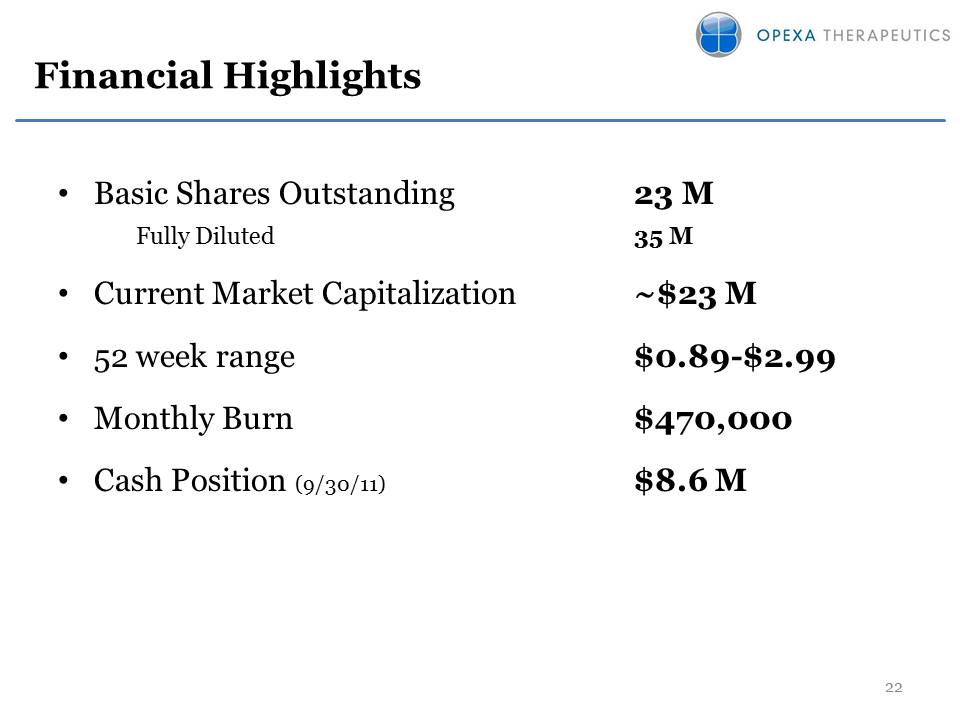
Financial Highlights •Basic Shares Outstanding 23 M Fully Diluted 35 M •Current Market Capitalization $23 M •52 week range $0.89-$2.99 •Monthly Burn $470,000 •Cash Position (9/30/11) $8.6 M 22

A Revolution in Cell
Therapy Thank you
