Attached files
| file | filename |
|---|---|
| 8-K - 8-K - HOSPIRA INC | a12-2376_18k.htm |
Exhibit 99.1
|
|
J.P. Morgan 30th Annual Healthcare Conference January 10, 2012 |
|
|
2 Safe Harbor This material contains forward-looking statements within the meaning of the federal securities laws. Hospira intends these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the federal securities laws. In some cases, you can identify these statements by our use of forward-looking words such as “may,” “will,” “should,” “anticipate,” “estimate,” “expect,” “plan,” “believe,” “predict,” “potential,” “project,” “intend,” “could” or similar expressions. In particular, statements regarding our plans, strategies, prospects, and goals; expectations regarding our business and the industries and markets in which we operate, and statements related to the progress of our quality initiatives or product development /portfolio expansion programs are forward-looking statements. You should be aware that these statements and any other forward-looking statements in this material only reflect our expectations and are not guarantees of performance. These statements involve risks, uncertainties and assumptions. Many of these risks, uncertainties and assumptions are beyond our control and may cause actual results and performance to differ materially from our expectations. Important factors that could cause our actual results to be materially different from our expectations include progress on our quality initiatives and portfolio expansion initiatives, and the factors, risks and uncertainties described under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Hospira’s latest Annual Report on Form 10-K and subsequent Forms 10-Q filed with the Securities and Exchange Commission. Accordingly, you should not place undue reliance on the forward-looking statements contained in this material. These forward-looking statements speak only as of the date on which the statements were made. We undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise. |
|
|
3 Agenda Hospira overview Reinforcing the foundation Growth opportunities Summary |
|
|
4 Hospira At a Glance Global specialty pharmaceutical / medication management company Market leadership positions in: generic injectable pharmaceuticals globally biosimilars in Europe and Australia medication management systems globally 70+ years experience; public since 2004 2010 annual revenues of ~$4B Hospira is the World’s Leading Provider of Injectable Drugs and Infusion Technologies |
|
|
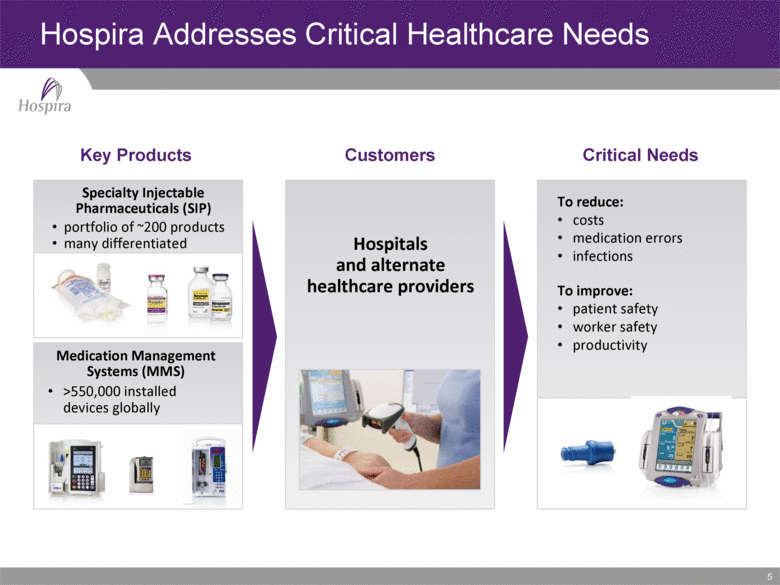
5 Hospira Addresses Critical Healthcare Needs Key Products Customers Critical Needs Specialty Injectable Pharmaceuticals (SIP) portfolio of ~200 products many differentiated Medication Management Systems (MMS) Hospitals and alternate healthcare providers To reduce: costs medication errors infections To improve: patient safety worker safety productivity >550,000 installed devices globally |
|
|
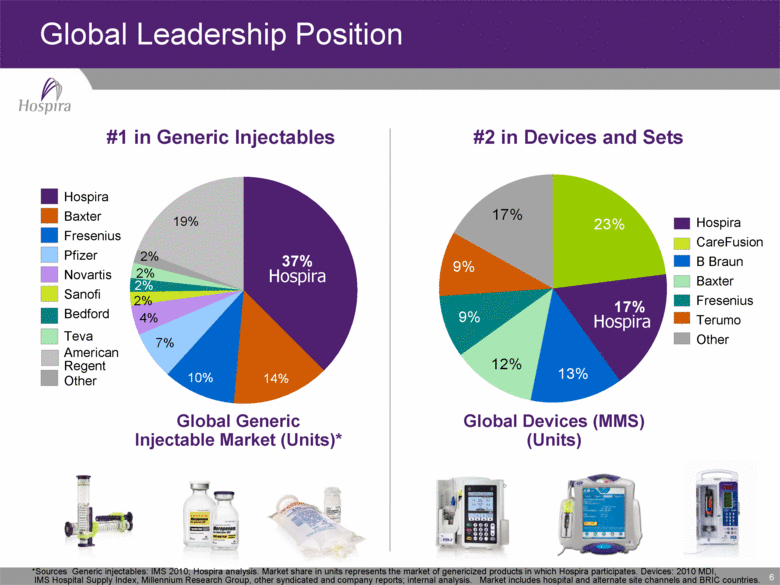
6 Global Leadership Position #1 in Generic Injectables #2 in Devices and Sets Hospira Hospira *Sources Generic injectables: IMS 2010; Hospira analysis. Market share in units represents the market of genericized products in which Hospira participates. Devices: 2010 MDI, IMS Hospital Supply Index, Millennium Research Group, other syndicated and company reports; internal analysis. Market includes hospital and alternate site channels and BRIC countries. Global Generic Injectable Market (Units)* Hospira Baxter Fresenius Pfizer Novartis Sanofi Bedford Teva American Regent Other Global Devices (MMS) (Units) Hospira CareFusion B Braun Baxter Fresenius Terumo Other Hospira 17% 13% 12% 9% 9% 17% 23% 19% 2% 2% 2% 4% 7% 10% 14% 37% 2% |
|
|
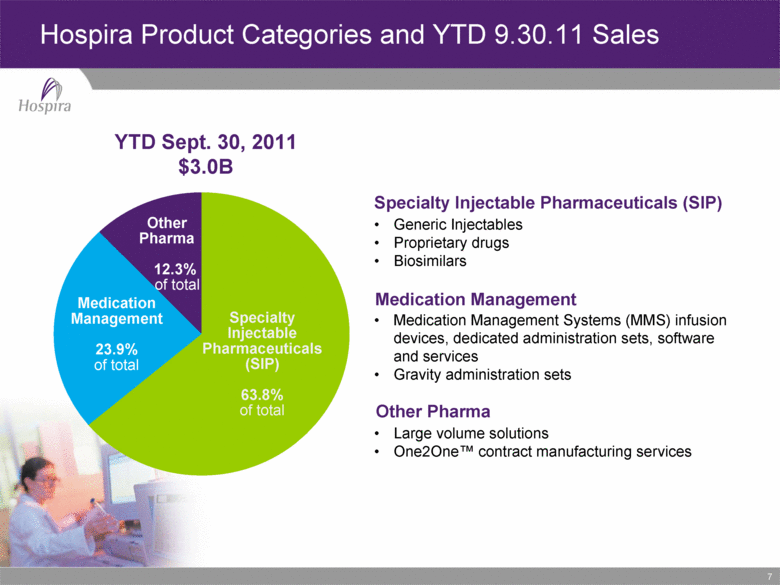
7 Hospira Product Categories and YTD 9.30.11 Sales Medication Management 23.9% of total Specialty Injectable Pharmaceuticals (SIP) 63.8% of total Other Pharma 12.3% of total YTD Sept. 30, 2011 $3.0B Generic Injectables Proprietary drugs Biosimilars Medication Management Systems (MMS) infusion devices, dedicated administration sets, software and services Gravity administration sets Large volume solutions One2One™ contract manufacturing services Specialty Injectable Pharmaceuticals (SIP) Medication Management Other Pharma |
|
|
8 Significant Competitive Advantages One of industry’s broadest injectable portfolios Specialized manufacturing expertise and facilities costly to duplicate Strong client and GPO relationships and preferences Expertise in complex Paragraph IV patent challenges Differentiated product portfolio with proprietary formats |
|
|
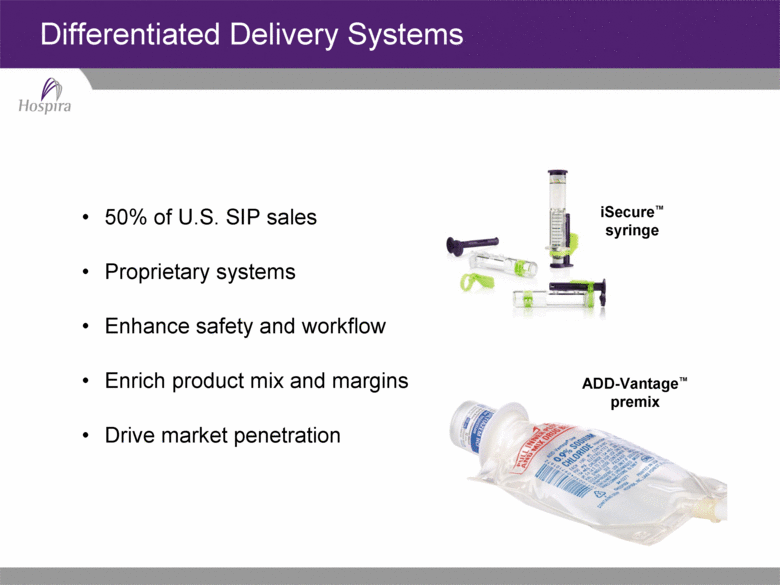
ADD-Vantage™ premix Differentiated Delivery Systems 50% of U.S. SIP sales Proprietary systems Enhance safety and workflow Enrich product mix and margins Drive market penetration iSecure™ syringe |
|
|
10 Agenda Hospira overview Reinforcing the foundation Growth opportunities Summary |
|
|
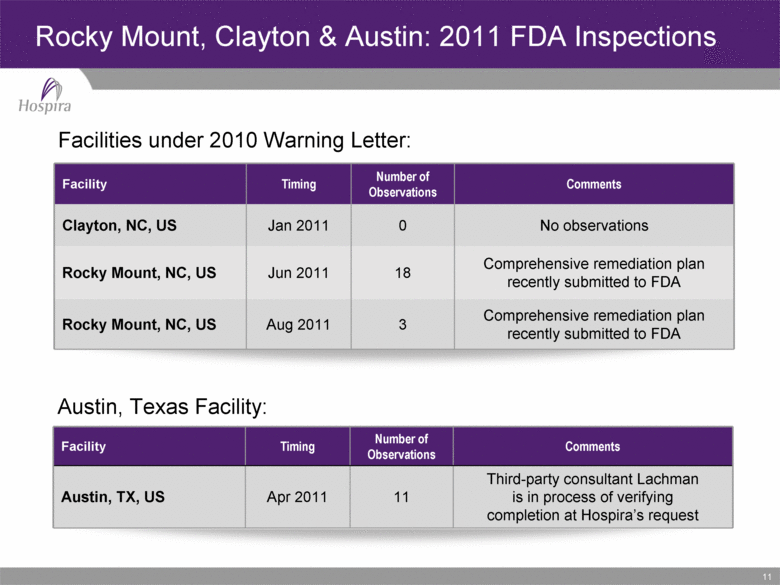
11 Rocky Mount, Clayton & Austin: 2011 FDA Inspections Facility Timing Number of Observations Comments Clayton, NC, US Jan 2011 0 No observations Rocky Mount, NC, US Jun 2011 18 Comprehensive remediation plan recently submitted to FDA Rocky Mount, NC, US Aug 2011 3 Comprehensive remediation plan recently submitted to FDA Facility Timing Number of Observations Comments Austin, TX, US Apr 2011 11 Third-party consultant Lachman is in process of verifying completion at Hospira’s request Facilities under 2010 Warning Letter: Austin, Texas Facility: |
|
|
12 Large, complex manufacturing facility produces generic injectables and large-volume solutions supports One2One™, Hospira’s contracting manufacturing services 1.3 million sq. ft. ~2,400 employees accounts for approximately 25% of Hospira’s net sales Hospira’s Rocky Mount, NC Facility |
|
|
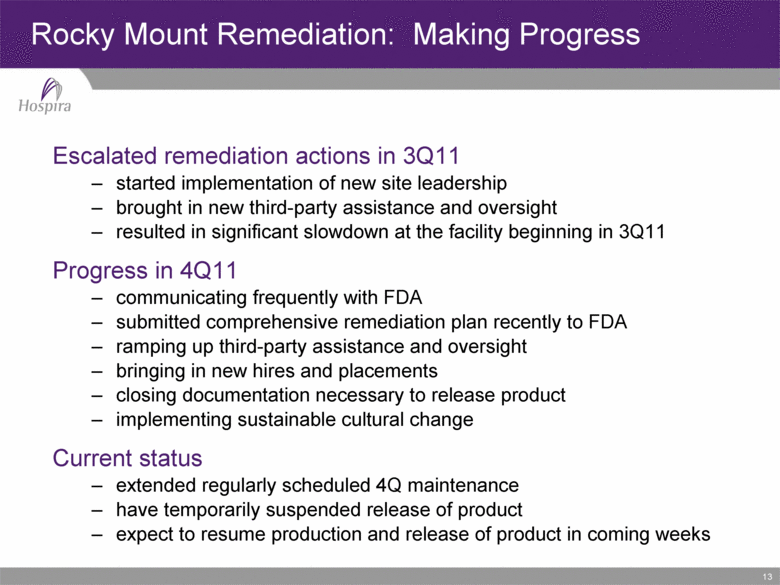
13 Escalated remediation actions in 3Q11 started implementation of new site leadership brought in new third-party assistance and oversight resulted in significant slowdown at the facility beginning in 3Q11 Progress in 4Q11 communicating frequently with FDA submitted comprehensive remediation plan recently to FDA ramping up third-party assistance and oversight bringing in new hires and placements closing documentation necessary to release product implementing sustainable cultural change Current status extended regularly scheduled 4Q maintenance have temporarily suspended release of product expect to resume production and release of product in coming weeks Rocky Mount Remediation: Making Progress |
|
|
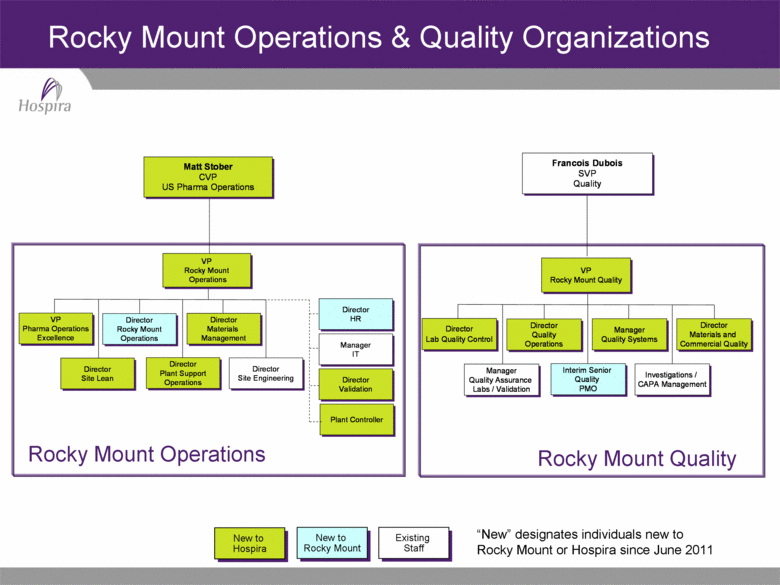
Rocky Mount Operations & Quality Organizations New to Hospira Existing Staff New to Rocky Mount Rocky Mount Operations Rocky Mount Quality “New” designates individuals new to Rocky Mount or Hospira since June 2011 Director Lab Quality Control Francois Dubois SVP Quality Director Quality Operations Manager Quality Systems Investigations / CAPA Management Interim Senior Quality PMO Manager Quality Assurance Labs / Validation VP Rocky Mount Quality Director Materials and Commercial Quality Matt Stober CVP US Pharma Operations Matt Stober CVP US Pharma Operations Mount Operations VP Rocky Mount Operations VP Rocky Mount Operations Pharma Operations Excellence VP Pharma Operations Director Rocky Mount Operations Director Rocky Mount Operations Director Materials Management Director Materials Management Director Site Lean Director Site Lean Director Plant Support Operations Director Plant Support Operations Director Site Engineering Director Site Engineering Director HR Director Manager Plant ControllerDirector Validation Director Validation IT Manager HR |
|
|
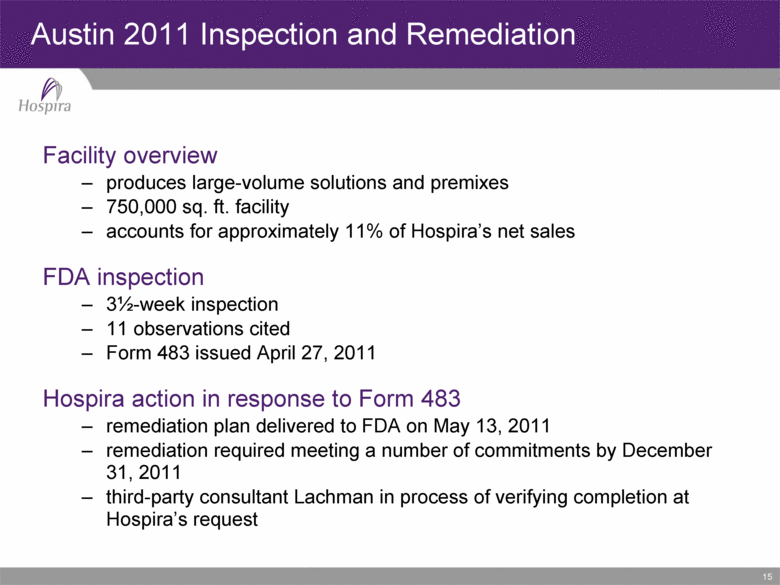
15 Facility overview produces large-volume solutions and premixes 750,000 sq. ft. facility accounts for approximately 11% of Hospira’s net sales FDA inspection 3½-week inspection 11 observations cited Form 483 issued April 27, 2011 Hospira action in response to Form 483 remediation plan delivered to FDA on May 13, 2011 remediation required meeting a number of commitments by December 31, 2011 third-party consultant Lachman in process of verifying completion at Hospira’s request Austin 2011 Inspection and Remediation |
|
|
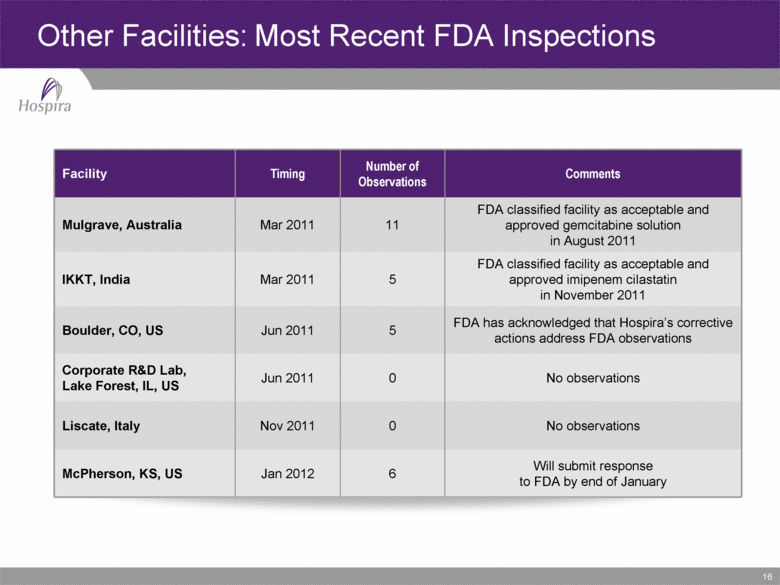
16 Facility Timing Number of Observations Comments Mulgrave, Australia Mar 2011 11 FDA classified facility as acceptable and approved gemcitabine solution in August 2011 IKKT, India Mar 2011 5 FDA classified facility as acceptable and approved imipenem cilastatin in November 2011 Boulder, CO, US Jun 2011 5 FDA has acknowledged that Hospira’s corrective actions address FDA observations Corporate R&D Lab, Lake Forest, IL, US Jun 2011 0 No observations Liscate, Italy Nov 2011 0 No observations McPherson, KS, US Jan 2012 6 Will submit response to FDA by end of January Other Facilities: Most Recent FDA Inspections |
|
|
17 Facility overview produces generic injectables, differentiated delivery systems, supports One2One™, Hospira’s contract manufacturing services 500,000 sq. ft. facility accounts for approximately 12% of Hospira’s net sales FDA inspection 3½ week inspection; 32 man-days 6 observations cited 483 issued January 4, 2012 Observations 6 FDA observations raised during inspection relate to the following areas: visual inspection, gowning, component testing, humidity monitoring, air circulation studies and reserve sample inspection Hospira action in response to Form 483 working on remediation plan to be delivered to FDA by end of January believe these observations can be addressed with minimal or no disruption McPherson 2011 Inspection and Remediation |
|
|
18 Driving Operational and Quality Excellence: Reinforcing the Foundation We are fixing the foundation and preparing the organization to support our growth plans Device and pharma remediation activities expected to cost $300-$375 million between 2011 and 2013 We are making sustainable change in our process and quality procedures across our global manufacturing footprint We are investing in the future with strategic capacity, best-in-class processes and people Quality improvement and remediation efforts will position us favorably for long-term success |
|
|
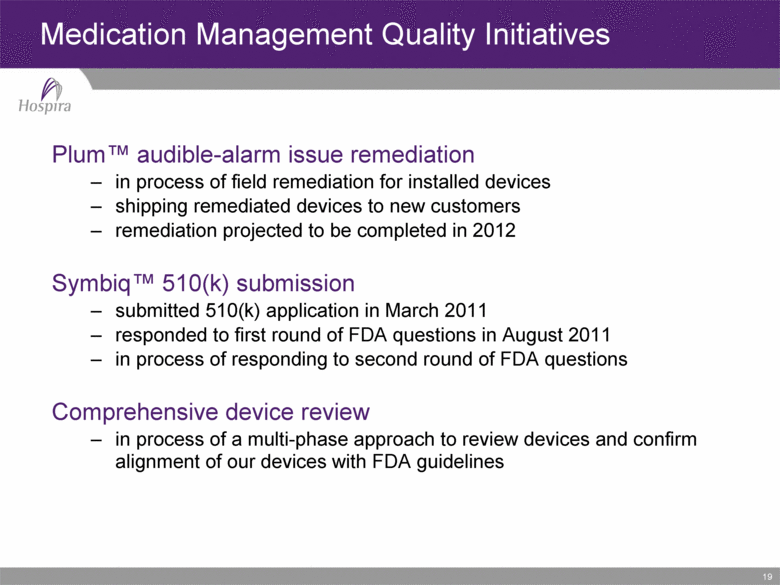
19 Plum™ audible-alarm issue remediation in process of field remediation for installed devices shipping remediated devices to new customers remediation projected to be completed in 2012 Symbiq™ 510(k) submission submitted 510(k) application in March 2011 responded to first round of FDA questions in August 2011 in process of responding to second round of FDA questions Comprehensive device review in process of a multi-phase approach to review devices and confirm alignment of our devices with FDA guidelines Medication Management Quality Initiatives |
|
|
20 Agenda Hospira overview Reinforcing the foundation Growth opportunities Summary |
|
|
21 Long Growth Runway for Hospira; Multiple Growth Drivers Patent expiry products Existing SIP market growth Medication Management growth Biosimilars Global portfolio expansion 21 |
|
|
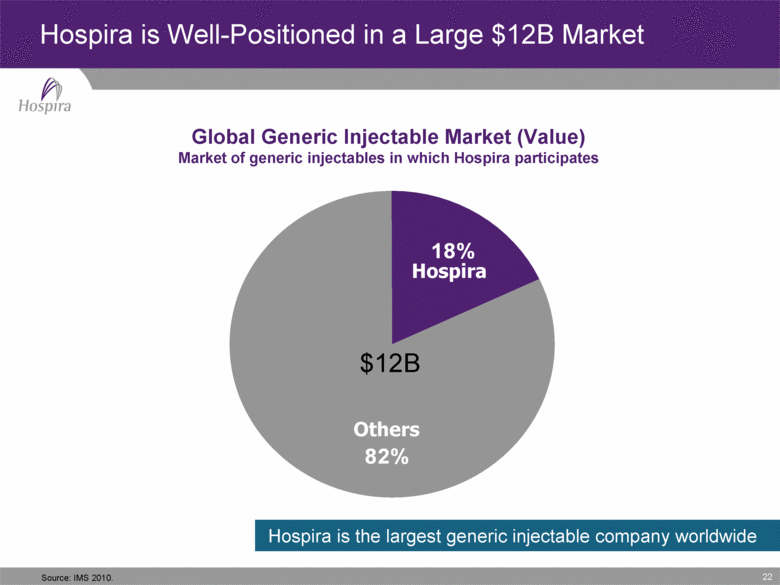
22 Global Generic Injectable Market (Value) Market of generic injectables in which Hospira participates Hospira is Well-Positioned in a Large $12B Market Hospira is the largest generic injectable company worldwide 22 Hospira Others $12B Source: IMS 2010. 82% 18% |
|
|
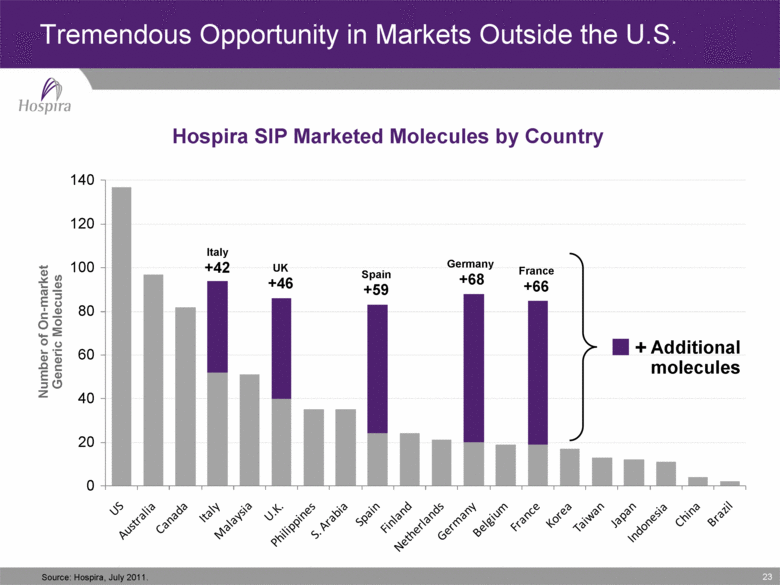
23 Tremendous Opportunity in Markets Outside the U.S. Source: Hospira, July 2011. Hospira SIP Marketed Molecules by Country Number of On-market Generic Molecules 0 140 20 40 60 80 100 120 Spain +59 Germany +68 Italy +42 UK +46 France +66 Additional molecules + |
|
|
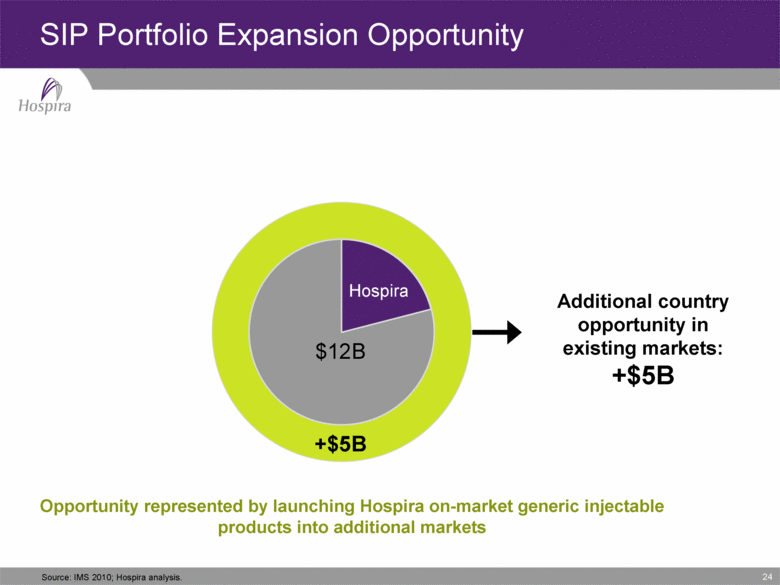
24 SIP Portfolio Expansion Opportunity Hospira +$5B $12B Additional country opportunity in existing markets: +$5B Source: IMS 2010; Hospira analysis. Opportunity represented by launching Hospira on-market generic injectable products into additional markets |
|
|
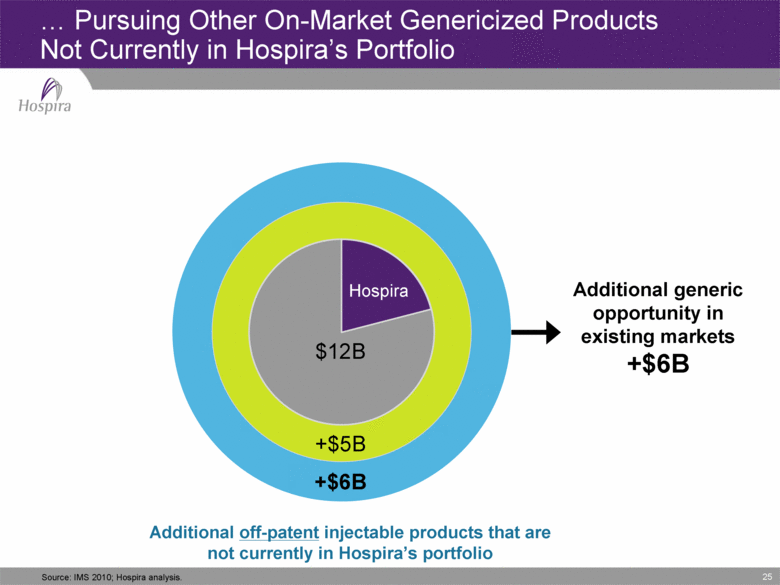
25 ...Pursuing Other On-Market Genericized Products Not Currently in Hospira’s Portfolio Hospira +$5B +$6B $12B Additional generic opportunity in existing markets +$6B Source: IMS 2010; Hospira analysis. Additional off-patent injectable products that are not currently in Hospira’s portfolio |
|
|
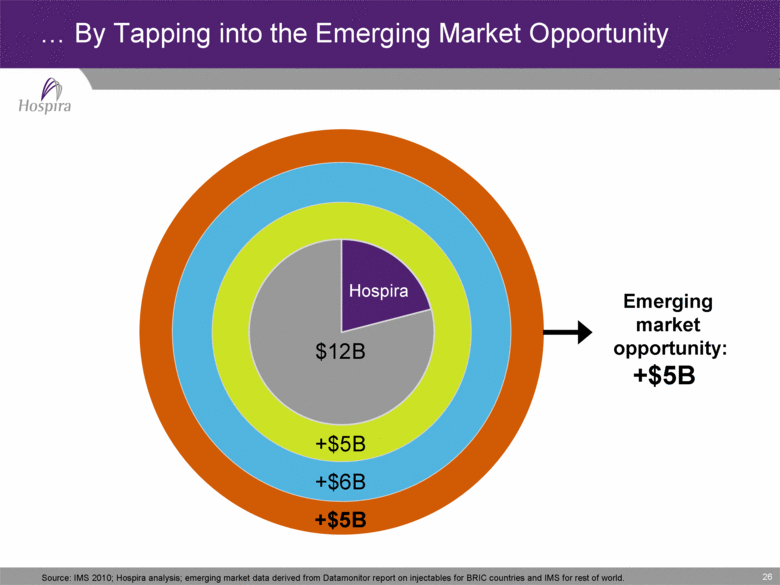
26 ...By Tapping into the Emerging Market Opportunity Hospira +$5B +$5B +$6B $12B Emerging market opportunity: +$5B Source: IMS 2010; Hospira analysis; emerging market data derived from Datamonitor report on injectables for BRIC countries and IMS for rest of world. |
|
|
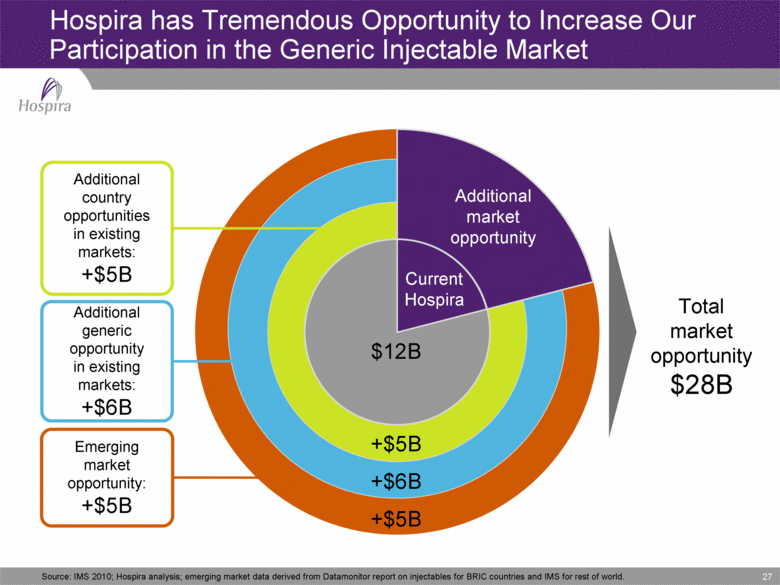
27 Hospira has Tremendous Opportunity to Increase Our Participation in the Generic Injectable Market Current Hospira Additional market opportunity +$5B +$5B +$6B $12B Total market opportunity $28B Additional country opportunities in existing markets: +$5B Additional generic opportunity in existing markets: +$6B Emerging market opportunity: +$5B Source: IMS 2010; Hospira analysis; emerging market data derived from Datamonitor report on injectables for BRIC countries and IMS for rest of world. |
|
|
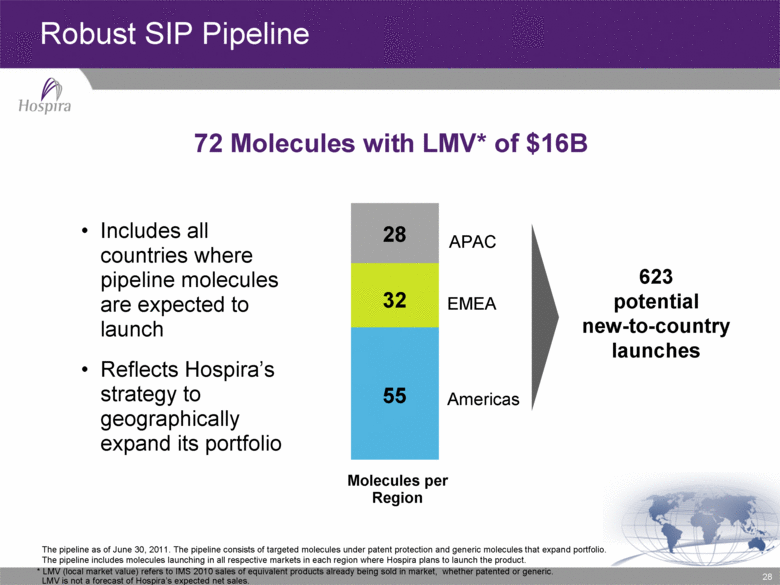
28 * LMV (local market value) refers to IMS 2010 sales of equivalent products already being sold in market, whether patented or generic. LMV is not a forecast of Hospira’s expected net sales. 55 32 28 APAC EMEA Americas Includes all countries where pipeline molecules are expected to launch Reflects Hospira’s strategy to geographically expand its portfolio 623 potential new-to-country launches Robust SIP Pipeline 72 Molecules with LMV* of $16B Molecules per Region The pipeline as of June 30, 2011. The pipeline consists of targeted molecules under patent protection and generic molecules that expand portfolio. The pipeline includes molecules launching in all respective markets in each region where Hospira plans to launch the product. |
|
|
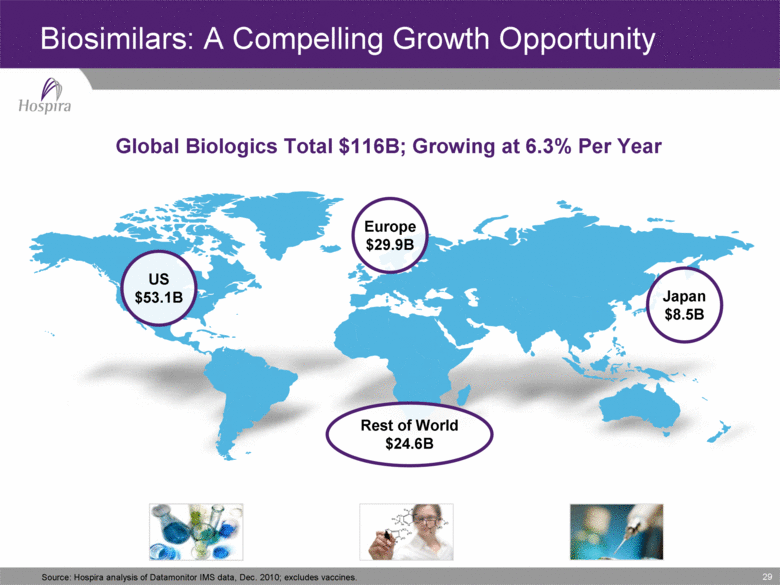
29 Source: Hospira analysis of Datamonitor IMS data, Dec. 2010; excludes vaccines. Global Biologics Total $116B; Growing at 6.3% Per Year Europe $29.9B Japan $8.5B US $53.1B Rest of World $24.6B Biosimilars: A Compelling Growth Opportunity |
|
|
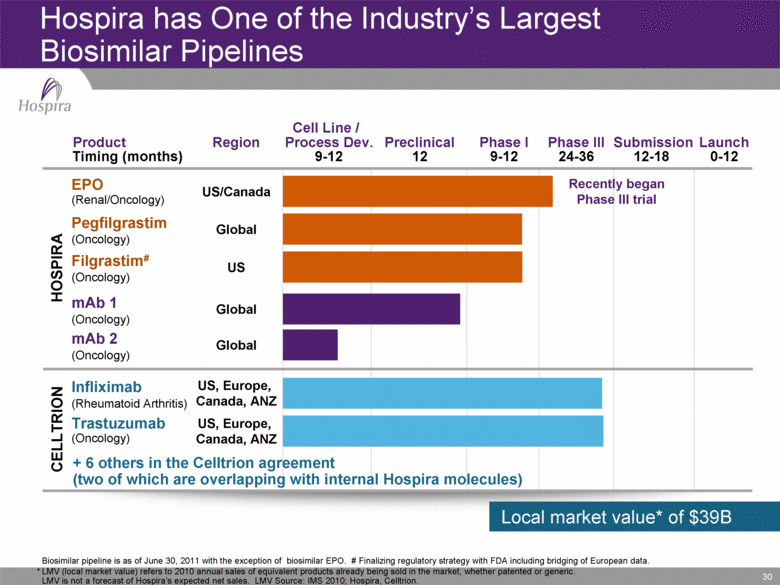
30 Hospira has One of the Industry’s Largest Biosimilar Pipelines * LMV (local market value) refers to 2010 annual sales of equivalent products already being sold in the market, whether patented or generic. LMV is not a forecast of Hospira’s expected net sales. LMV Source: IMS 2010; Hospira, Celltrion. Biosimilar pipeline is as of June 30, 2011 with the exception of biosimilar EPO. # Finalizing regulatory strategy with FDA including bridging of European data. Launch Submission Phase III Phase I Preclinical Cell Line / Process Dev. Region Product Timing (months) 0-12 12-18 24-36 9-12 12 9-12 US, Europe, Canada, ANZ Global US/Canada US Global Global US, Europe, Canada, ANZ CELLTRION HOSPIRA Trastuzumab (Oncology) EPO (Renal/Oncology) Filgrastim# (Oncology) mAb 1 (Oncology) mAb 2 (Oncology) + 6 others in the Celltrion agreement (two of which are overlapping with internal Hospira molecules) Pegfilgrastim (Oncology) Infliximab (Rheumatoid Arthritis) Local market value* of $39B Recently began Phase III trial |
|
|
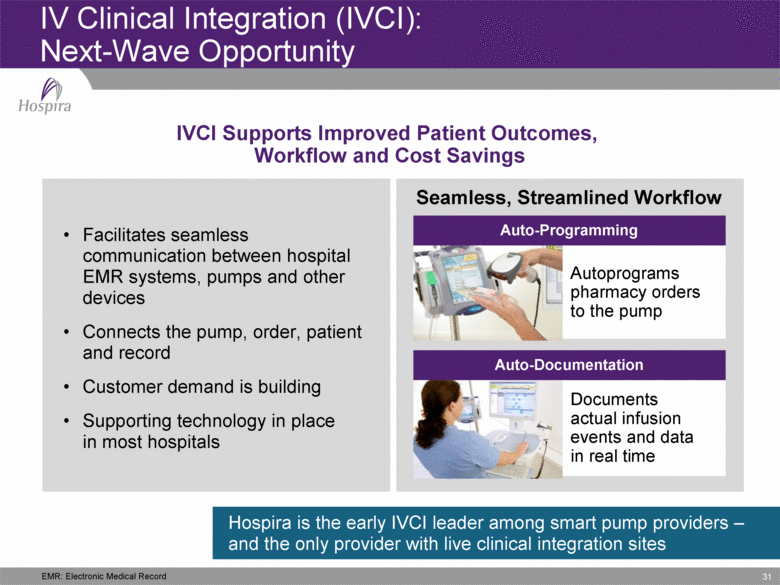
31 IV Clinical Integration (IVCI): Next-Wave Opportunity Facilitates seamless communication between hospital EMR systems, pumps and other devices Connects the pump, order, patient and record Customer demand is building Supporting technology in place in most hospitals IVCI Supports Improved Patient Outcomes, Workflow and Cost Savings Hospira is the early IVCI leader among smart pump providers – and the only provider with live clinical integration sites Seamless, Streamlined Workflow Autoprograms pharmacy orders to the pump Documents actual infusion events and data in real time Auto-Programming Auto-Documentation EMR: Electronic Medical Record |
|
|
32 Agenda Hospira overview Reinforcing the foundation Growth opportunities Summary |
|
|
33 The Hospira Investment Story Large, growing global markets Our markets are large and underpenetrated Significant international and emerging market opportunity Leveraging leadership position Significant channel strength and share requiring high levels of expertise and investment One-stop shop offering customers critical solutions Long-term growth potential Multiple growth drivers Positioned to benefit from healthcare megatrends Opportunity Position Growth |
|
|
34 Our Mandate is Clear Hospira is the World’s Leading Provider of Injectable Drugs and Infusion Technologies |
|
|
[LOGO] |