Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HeartWare International, Inc. | c26616e8vk.htm |
Exhibit 99.1

| 30th Annual J.P. Morgan Healthcare Conference Doug Godshall Chief Executive Officer and President January 9, 2012 I San Francisco |

| Safe Harbor Statement Forward-Looking Statements This presentation contains forward-looking statements that are based on our management's beliefs, assumptions and expectations and on information currently available to our management. Generally, you can identify forward-looking statements by terms such as "may," "will," "should," "could," "would," "expects," "plans," "anticipates," "believes," "estimates," "projects," "predicts," "potential" and similar expressions intended to identify forward-looking statements, which generally are not historical in nature. All statements that address operating performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements, including without limitation, our expectations regarding (a) regulatory submissions and approvals; (b) our clinical trials, including enrollment in and outcomes of our clinical trials; (c) product performance; (d) our ability to develop and commercialize our existing and new products; (e) our expectations for achieving product development and commercial milestones; and (f) continued favorable physician perception and acceptance of our products. The forward-looking statements reflect our current view about future events and are subject to risks, uncertainties and assumptions. Accordingly, you should not place undue reliance on our forward-looking statements. Except as required by law, we do not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. We may not actually achieve the plans, projections or expectations disclosed in our forward-looking statements, and actual results, developments or events could differ materially from those disclosed in the forward-looking statements. Forward-looking statements are subject to a number of risks and uncertainties, including without limitation those described in Part I, "Item 1A. Risk Factors" of our Form 10-K for the year ended December 31, 2010 and in our filings with the SEC and ASX. CAUTION: Investigational device. Limited by United States law to investigational use. |

| Leading a Small Revolution More than 1,700 HeartWare Ventricular Assist System Implants Globally HeartWare has grown to 330+ employees today Headquarters near Boston, Massachusetts (Framingham) Manufacturing in Miami, Florida Development and operations facility near Sydney Opened distribution facility for same-day service to German market CAUTION: Investigational device. Limited by United States law to investigational use. |
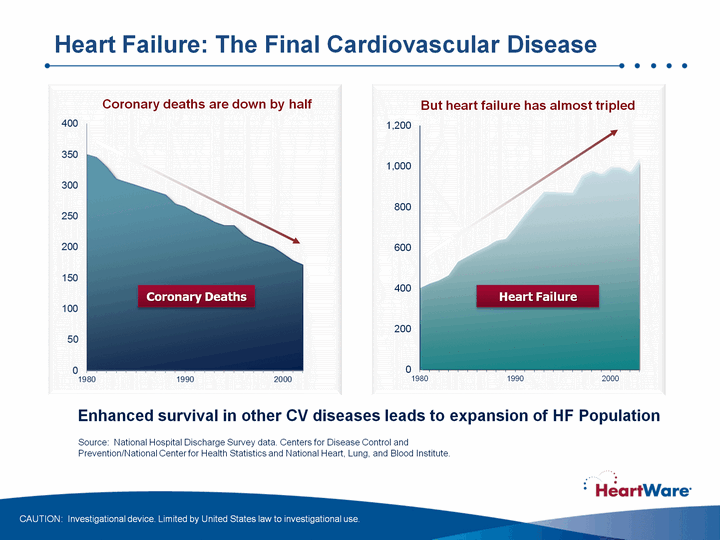
| Coronary deaths are down by half But heart failure has almost tripled (CHART) Heart Failure Source: National Hospital Discharge Survey data. Centers for Disease Control and Prevention/National Center for Health Statistics and National Heart, Lung, and Blood Institute. Enhanced survival in other CV diseases leads to expansion of HF Population Heart Failure: The Final Cardiovascular Disease CAUTION: Investigational device. Limited by United States law to investigational use. Coronary Deaths |
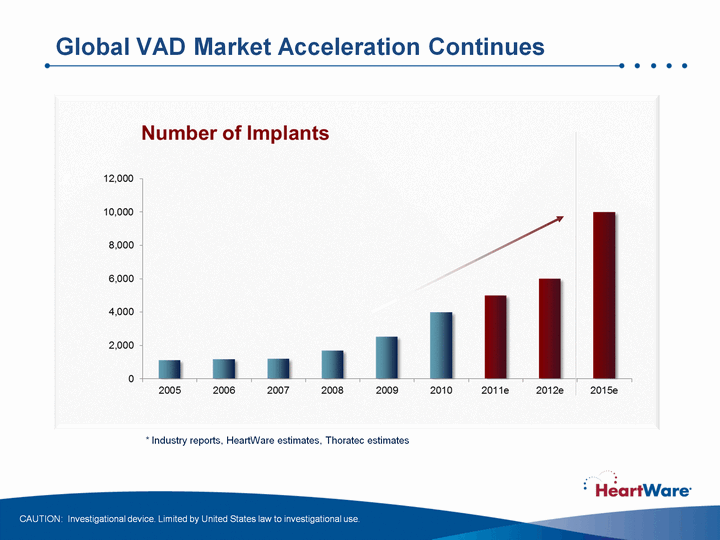
| Global VAD Market Acceleration Continues * Industry reports, HeartWare estimates, Thoratec estimates CAUTION: Investigational device. Limited by United States law to investigational use. |

| Significant Under-penetration of Transplant List ISHLT 2011 J Heart Lung Transplant. 2011 Oct; 30 (10): 1071-1132 * Includes LVAD, RVAD, TAH |
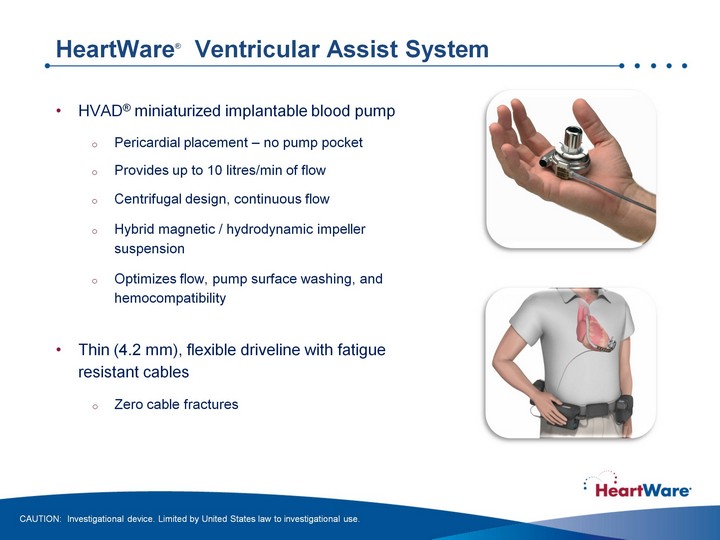
| HeartWare(r) Ventricular Assist System HVAD(r) miniaturized implantable blood pump Pericardial placement - no pump pocket Provides up to 10 litres/min of flow Centrifugal design, continuous flow Hybrid magnetic / hydrodynamic impeller suspension Optimizes flow, pump surface washing, and hemocompatibility Thin (4.2 mm), flexible driveline with fatigue resistant cables Zero cable fractures CAUTION: Investigational device. Limited by United States law to investigational use. |
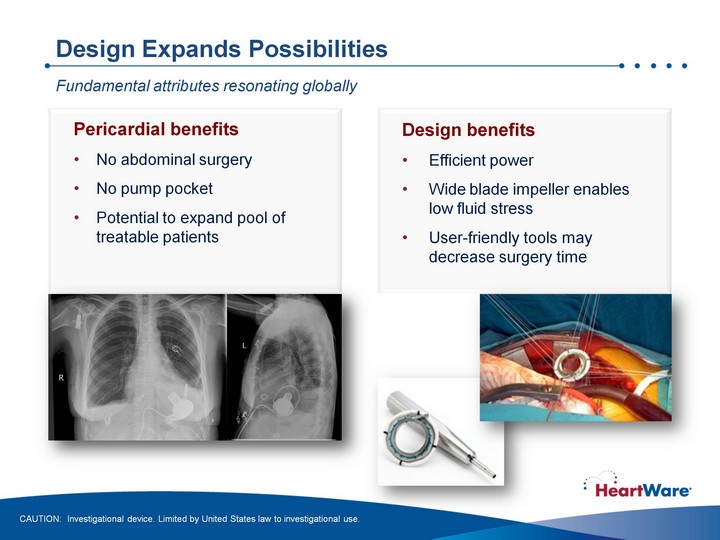
| Design Expands Possibilities Design benefits Efficient power Wide blade impeller enables low fluid stress User-friendly tools may decrease surgery time Pericardial benefits No abdominal surgery No pump pocket Potential to expand pool of treatable patients Fundamental attributes resonating globally CAUTION: Investigational device. Limited by United States law to investigational use. |
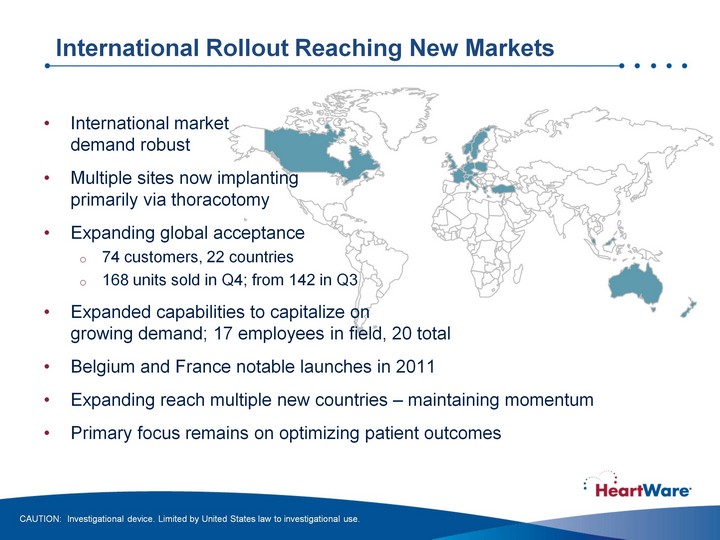
| International Rollout Reaching New Markets CAUTION: Investigational device. Limited by United States law to investigational use. International market demand robust Multiple sites now implanting primarily via thoracotomy Expanding global acceptance 74 customers, 22 countries 168 units sold in Q4; from 142 in Q3 Expanded capabilities to capitalize on growing demand; 17 employees in field, 20 total Belgium and France notable launches in 2011 Expanding reach multiple new countries - maintaining momentum Primary focus remains on optimizing patient outcomes |
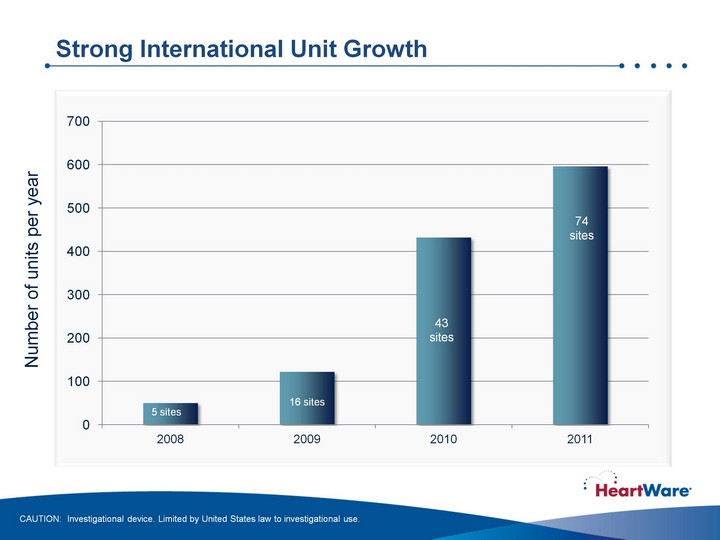
| Strong International Unit Growth Strong International Unit Growth CAUTION: Investigational device. Limited by United States law to investigational use. 5 sites Number of units per year |

| Geographic Revenue Breakdown Geographic Revenue Breakdown CAUTION: Investigational device. Limited by United States law to investigational use. |

| U.S. Clinical and Regulatory Tracking - HVAD(r) CAUTION: Investigational device. Limited by United States law to investigational use. Bridge-to- Transplant BTT Continued Access Destination Therapy DT Continued Access REVIVE-IT |
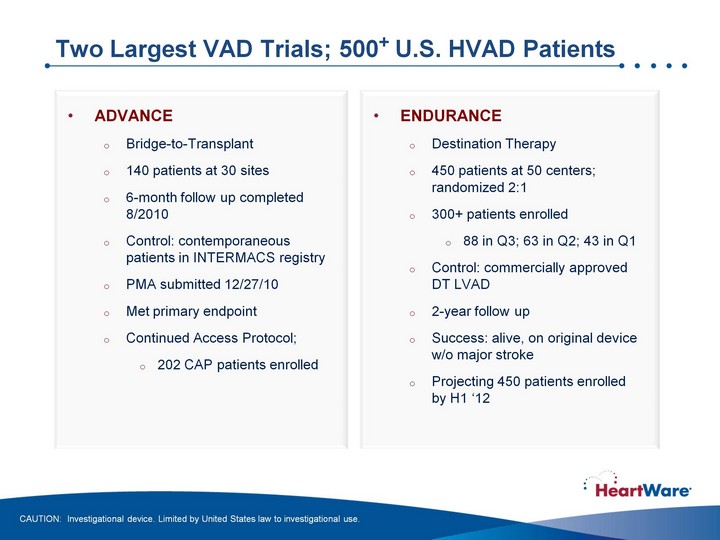
| Two Largest VAD Trials; 500+ U.S. HVAD Patients CAUTION: Investigational device. Limited by United States law to investigational use. ADVANCE Bridge-to-Transplant 140 patients at 30 sites 6-month follow up completed 8/2010 Control: contemporaneous patients in INTERMACS registry PMA submitted 12/27/10 Met primary endpoint Continued Access Protocol; 202 CAP patients enrolled ENDURANCE Destination Therapy 450 patients at 50 centers; randomized 2:1 300+ patients enrolled 88 in Q3; 63 in Q2; 43 in Q1 Control: commercially approved DT LVAD 2-year follow up Success: alive, on original device w/o major stroke Projecting 450 patients enrolled by H1 '12 |

| Overall Survival Comparison: SHFM vs. ADVANCE Figure 1: SHFM Estimated Survival Figure 1: SHFM Estimated Survival Seattle Heart Failure Model Comparison Wayne C. Levy, et al, ISHLT 2011 |

| Commercial Plan: Transition Existing Clinical Sites Phase I HTWR Clinical Sites 50 HVAD clinical sites approximate 70% US volume CAUTION: Investigational device. Limited by United States law to investigational use. |

| HeartWare Portfolio Progression 2011 2012 2013 CAUTION: Investigational device. Limited by United States law to investigational use. Shower Bag Redesigned Packaging New Apical Coring Tool Sintered Inflow Cannula Redesigned Controller Patient Sport Pack MVAD Clinical Studies Trans-apical Clinical Studies Fully Implantable GLP Studies New Patient Segment Technology (preclinical) Pre(preclinical) |

| Compelling Pipeline - Miniaturized VAD Design Expanded portfolio of MVAD designs Exploring new patient populations More than 100 animal studies conducted to date; strong early results GLP animal studies completed Q3 '11 First-in-man studies in H1 '12 Wide blade impeller with hybrid suspension allows significant miniaturization Ultra-thin driveline Simplified manufacturing Partial or full support attainable; Wear-less impeller suspension Will include novel new controller Pulsatility algorithms incorporated and software enhancements in process now All versions can eliminate sternotomy Designed to decrease invasiveness without decreasing efficacy CAUTION: Investigational device. Limited by United States law to investigational use. |
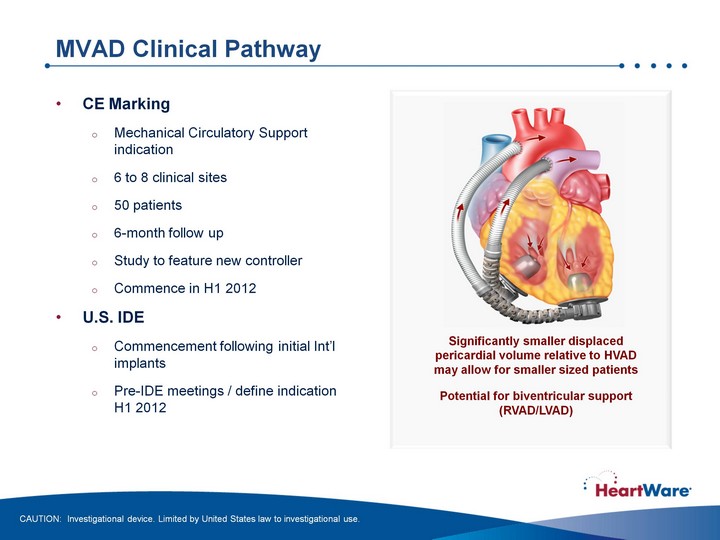
| MVAD Clinical Pathway CE Marking Mechanical Circulatory Support indication 6 to 8 clinical sites 50 patients 6-month follow up Study to feature new controller Commence in H1 2012 U.S. IDE Commencement following initial Int'l implants Pre-IDE meetings / define indication H1 2012 CAUTION: Investigational device. Limited by United States law to investigational use. Significantly smaller displaced pericardial volume relative to HVAD may allow for smaller sized patients Potential for biventricular support (RVAD/LVAD) |

| Fully Implantable VAD System - Dualis Agreement CAUTION: Investigational device. Limited by United States law to investigational use. Partnership with Dualis MedTech GmbH, a spin-off of the German Aerospace Centre (DLR) Agreement enables acceleration of wireless technology development and potential expansion for BiVAD technology application Competency in coil designs, biocompatible materials, and RF telemetry systems complement internal development effort Commenced work to configure technology for HVAD/MVAD mid-2011 Prior preclinical experience; more than a dozen animal models implanted Acute preclinical testing for HeartWare configuration mid-2012 Chronic preclinical testing by end of 2012 |

| Selected Financial Snapshot Revenue Preliminary Q4 2011 revenues $22M, highest quarter to date Preliminary 2011 revenues of $82M; up ~ 50% from 2010 (CHART) $ Millions Implant rate and growth likely to be variable in 2012 Capital structure Convertible debt $143M ($100 conversion price) Stock on issue: 14.1 million shares |

| Anticipated 2012 Milestones Initiate MVAD International clinical studies H1 2012 Prepare for PMA audit; potential advisory committee Submit BiVAD regulatory filing OUS H1 2012 Publication of ADVANCE data; manuscript submitted Enroll 450th patient in ENDURANCE DT study mid-year 2012 Request Continued Access Protocol for DT Validation of new Miami Lakes facility for US production Commence preclinical testing of fully implantable system US commercial launch Pediatric and BiVAD commencement in US CAUTION: Investigational device. Limited by United States law to investigational use. |

| Leading a Small Revolution CAUTION: Investigational device. Limited by United States law to investigational use. |
