Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HALOZYME THERAPEUTICS, INC. | c26615e8vk.htm |
Exhibit 99.1

| Halozyme Therapeutics, Inc. Thinking Outside the Cell(tm) J.P. Morgan Healthcare Conference January 11, 2012 Gregory I. Frost, Ph.D. President and Chief Executive Officer |

| Safe harbor All of the statements in this presentation that are not statements of historical facts constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements and all references to financial estimates are based upon management's current plans and expectations and are subject to a number of risks and uncertainties which could cause actual results to differ materially from such statements. While the Company believes that the assumptions concerning future events are reasonable, it cautions that there are inherent difficulties in anticipating or predicting certain important factors. A discussion of these factors, including risks and uncertainties, is set forth in the Company's annual and quarterly reports filed from time to time with the Securities and Exchange Commission. The Company disclaims any intention or obligation to revise or update any forward-looking statements, whether as a result of new information, future events, or otherwise. |
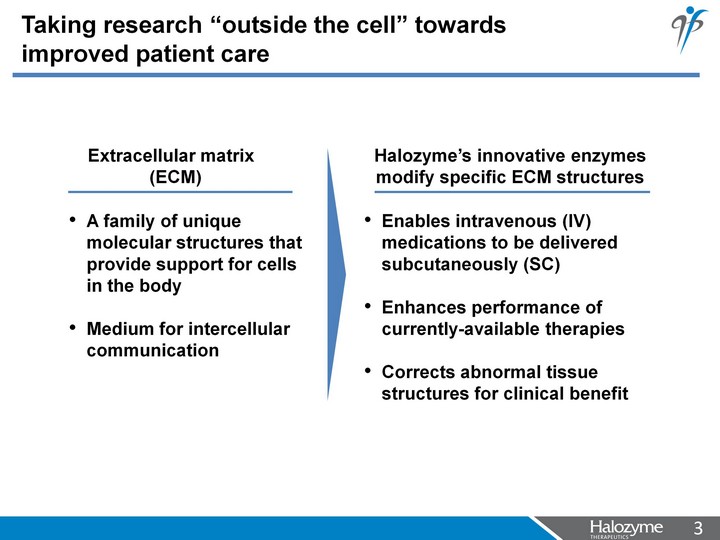
| Taking research "outside the cell" towards improved patient care A family of unique molecular structures that provide support for cells in the body Medium for intercellular communication Enables intravenous (IV) medications to be delivered subcutaneously (SC) Enhances performance of currently-available therapies Corrects abnormal tissue structures for clinical benefit Halozyme's innovative enzymes modify specific ECM structures Extracellular matrix (ECM) |

| rHuPH20 degrades hyaluronan (HA) in the ECM, clearing a pathway for therapeutic agents Inactivation of rHuPH20 and the return of the ECM rapidly restores tissue integrity. ...allowing exposure to capillaries and lymphatics. ...rHuPH20, our core technology, decreases local resistance to the injection of other molecules... The ECM creates resistance to bulk fluid flow... |
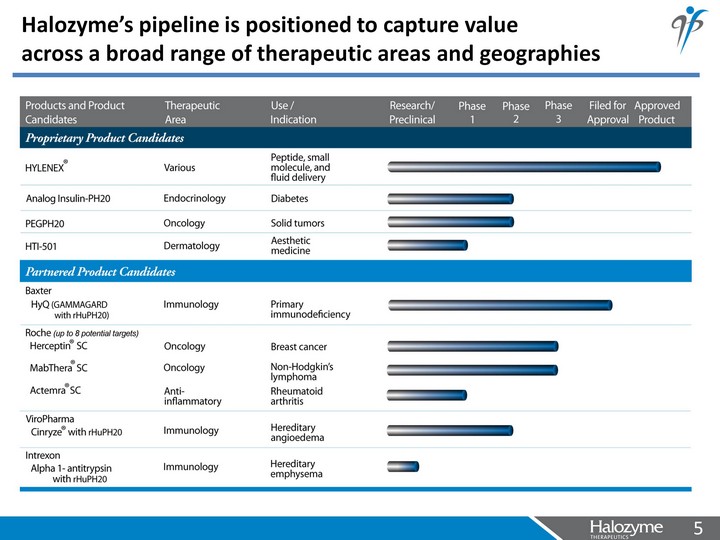
| Halozyme's pipeline is positioned to capture value across a broad range of therapeutic areas and geographies |
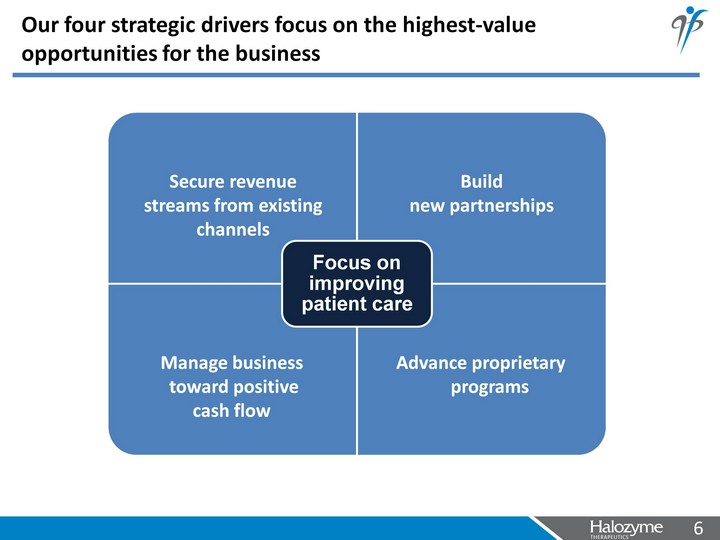
| Our four strategic drivers focus on the highest-value opportunities for the business Secure revenue streams from existing channels Build new partnerships Manage business toward positive cash flow Advance proprietary programs |

| Our partnered programs may increase the convenience, and safety of therapies while reducing the cost of care Alpha 1-antitrypsin (pre-clinical) |
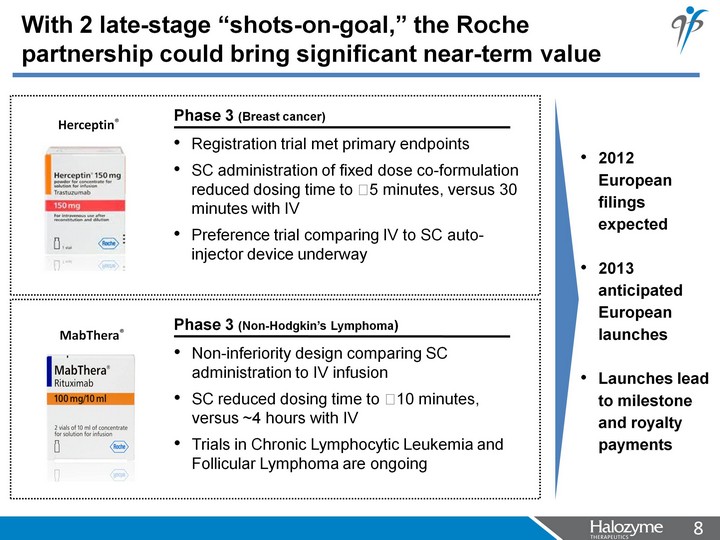
| With 2 late-stage "shots-on-goal," the Roche partnership could bring significant near-term value Registration trial met primary endpoints SC administration of fixed dose co-formulation reduced dosing time to ?5 minutes, versus 30 minutes with IV Preference trial comparing IV to SC auto- injector device underway Non-inferiority design comparing SC administration to IV infusion SC reduced dosing time to ?10 minutes, versus ~4 hours with IV Trials in Chronic Lymphocytic Leukemia and Follicular Lymphoma are ongoing 2012 European filings expected 2013 anticipated European launches Launches lead to milestone and royalty payments Phase 3 (Non-Hodgkin's Lymphoma) Phase 3 (Breast cancer) Herceptin(r) MabThera(r) |
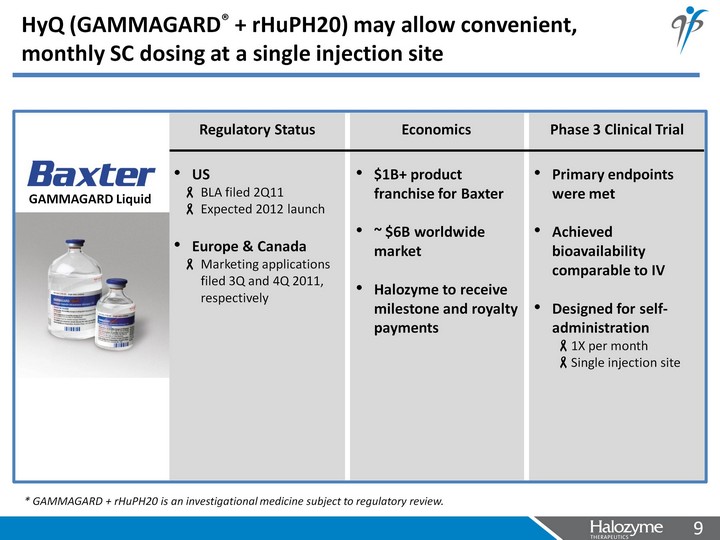
| HyQ (GAMMAGARD(r) + rHuPH20) may allow convenient, monthly SC dosing at a single injection site Economics $1B+ product franchise for Baxter ~ $6B worldwide market Halozyme to receive milestone and royalty payments Phase 3 Clinical Trial Primary endpoints were met Achieved bioavailability comparable to IV Designed for self- administration 1X per month Single injection site GAMMAGARD Liquid Regulatory Status US BLA filed 2Q11 Expected 2012 launch Europe & Canada Marketing applications filed 3Q and 4Q 2011, respectively * GAMMAGARD + rHuPH20 is an investigational medicine subject to regulatory review. |
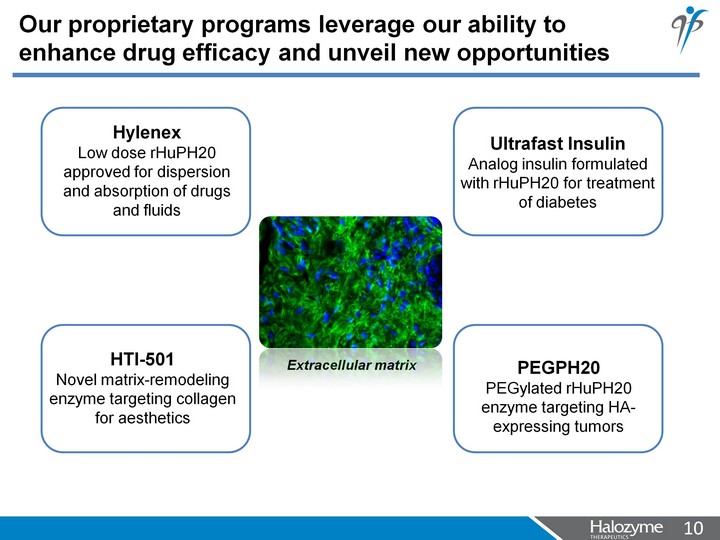
| Our proprietary programs leverage our ability to enhance drug efficacy and unveil new opportunities Hylenex Low dose rHuPH20 approved for dispersion and absorption of drugs and fluids Ultrafast Insulin Analog insulin formulated with rHuPH20 for treatment of diabetes PEGPH20 PEGylated rHuPH20 enzyme targeting HA- expressing tumors HTI-501 Novel matrix-remodeling enzyme targeting collagen for aesthetics Extracellular matrix |
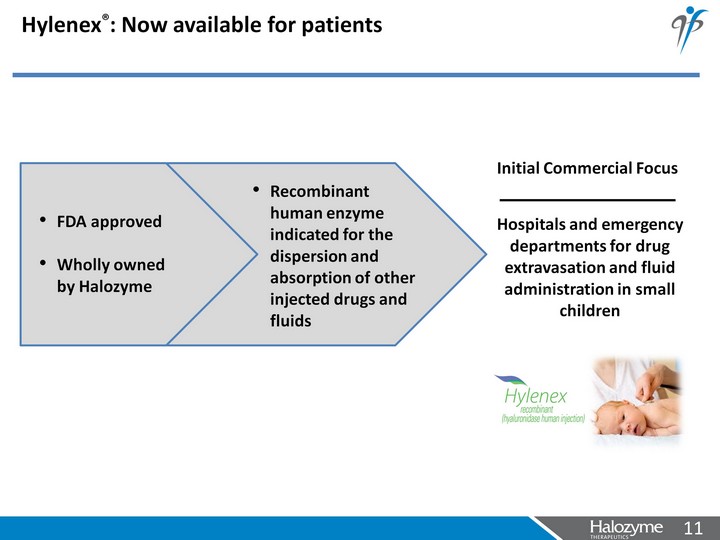
| Hylenex(r): Now available for patients Hospitals and emergency departments for drug extravasation and fluid administration in small children FDA approved Wholly owned by Halozyme Initial Commercial Focus Recombinant human enzyme indicated for the dispersion and absorption of other injected drugs and fluids |
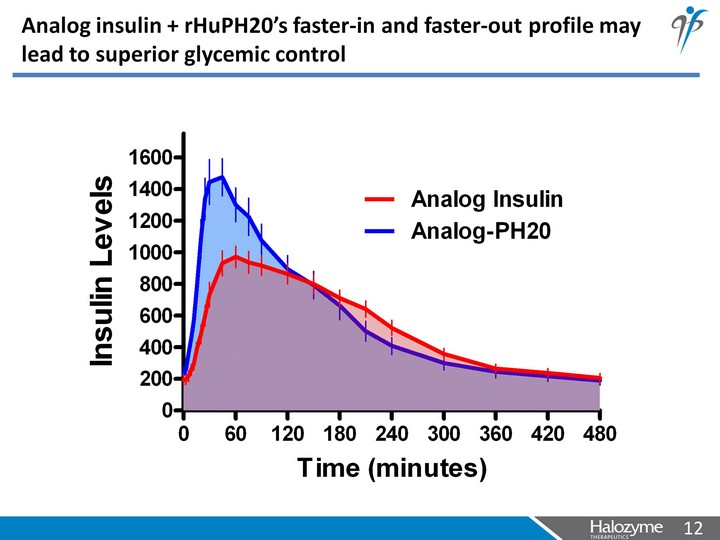
| Analog insulin + rHuPH20's faster-in and faster-out profile may lead to superior glycemic control |
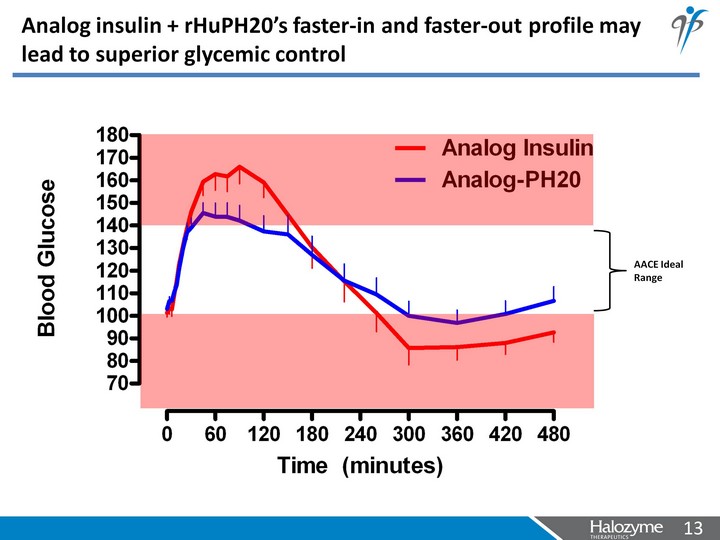
| Analog insulin + rHuPH20's faster-in and faster-out profile may lead to superior glycemic control AACE Ideal Range |
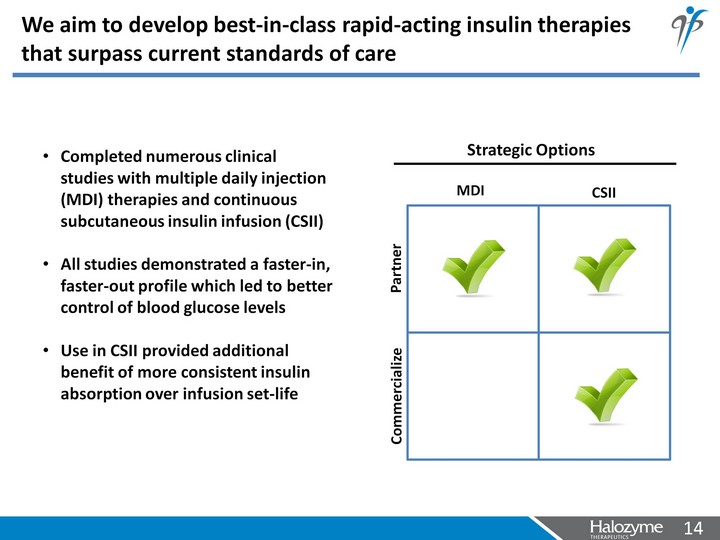
| We aim to develop best-in-class rapid-acting insulin therapies that surpass current standards of care Completed numerous clinical studies with multiple daily injection (MDI) therapies and continuous subcutaneous insulin infusion (CSII) All studies demonstrated a faster-in, faster-out profile which led to better control of blood glucose levels Use in CSII provided additional benefit of more consistent insulin absorption over infusion set-life Strategic Options MDI CSII Commercialize Partner |
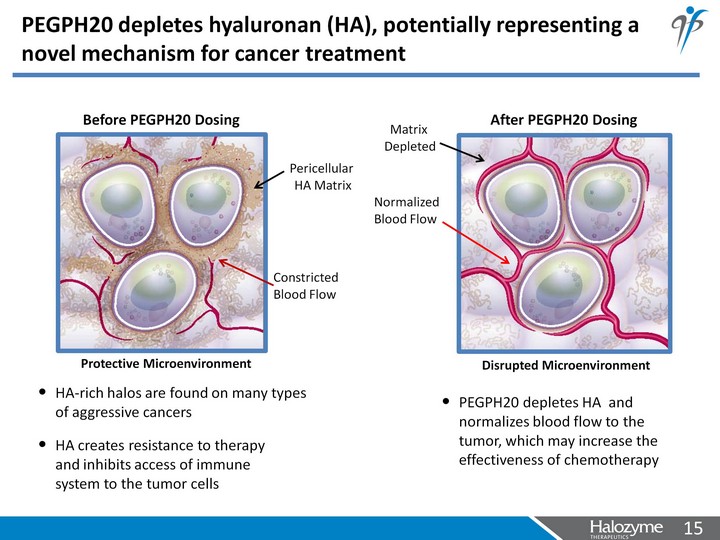
| PEGPH20 depletes hyaluronan (HA), potentially representing a novel mechanism for cancer treatment HA-rich halos are found on many types of aggressive cancers Pericellular HA Matrix Constricted Blood Flow Normalized Blood Flow Before PEGPH20 Dosing After PEGPH20 Dosing PEGPH20 depletes HA and normalizes blood flow to the tumor, which may increase the effectiveness of chemotherapy HA creates resistance to therapy and inhibits access of immune system to the tumor cells Protective Microenvironment Disrupted Microenvironment Matrix Depleted |

| With PEGPH20, our goal is to turn challenging cancers into a manageable disease Accumulation of HA is found in 20-30% of the most prevalent solid tumors, however, 87% of pancreatic ductal adenocarcinomas accumulate high HA Randomized phase 1b/2 study of gemcitabine in combination with PEGPH20 in patients with Stage IV pancreatic cancer is underway In Phase 1 studies, translation markers observed following IV administered PEGPH20 include: Reduced metabolic activity of the tumor Increased apparent diffusion and enhanced tumor perfusion Reduced tumor- and stroma-associated HA Dose-dependent increase in circulating HA catabolites |
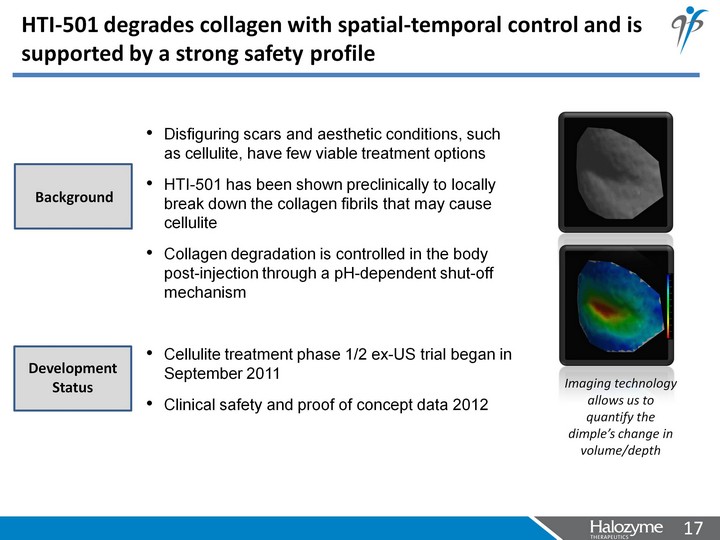
| Disfiguring scars and aesthetic conditions, such as cellulite, have few viable treatment options HTI-501 has been shown preclinically to locally break down the collagen fibrils that may cause cellulite Collagen degradation is controlled in the body post-injection through a pH-dependent shut-off mechanism Imaging technology allows us to quantify the dimple's change in volume/depth HTI-501 degrades collagen with spatial-temporal control and is supported by a strong safety profile Cellulite treatment phase 1/2 ex-US trial began in September 2011 Clinical safety and proof of concept data 2012 Background Development Status |
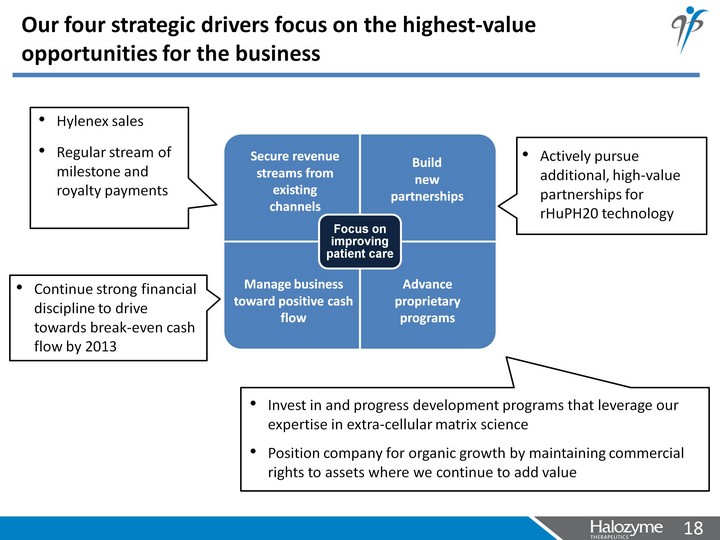
| Our four strategic drivers focus on the highest-value opportunities for the business Secure revenue streams from existing channels Build new partnerships Manage business toward positive cash flow Advance proprietary programs Hylenex sales Regular stream of milestone and royalty payments Actively pursue additional, high-value partnerships for rHuPH20 technology Invest in and progress development programs that leverage our expertise in extra-cellular matrix science Position company for organic growth by maintaining commercial rights to assets where we continue to add value Continue strong financial discipline to drive towards break- even cash flow by 2013 |
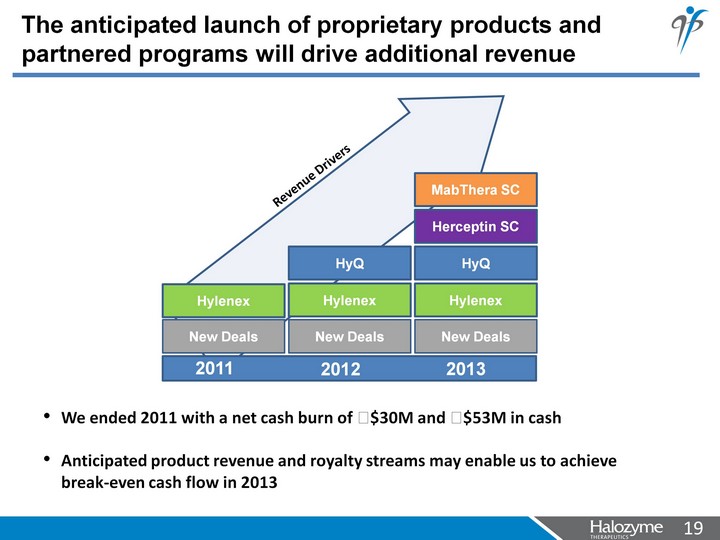
| The anticipated launch of proprietary products and partnered programs will drive additional revenue We ended 2011 with a net cash burn of ?$30M and ?$53M in cash Anticipated product revenue and royalty streams may enable us to achieve break-even cash flow in 2013 2011 2012 2013 New Deals Hylenex New Deals Hylenex HyQ New Deals Hylenex Herceptin SC HyQ MabThera SC Revenue Drivers |
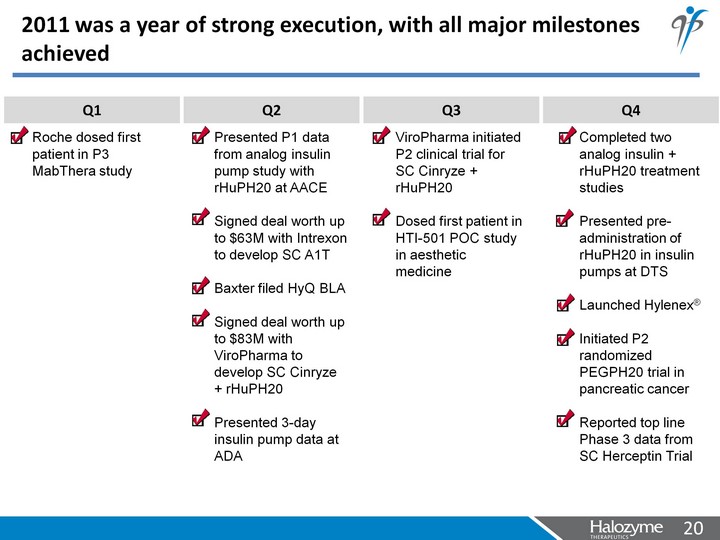
| 2011 was a year of strong execution, with all major milestones achieved Q1 Q2 Q3 Q4 Roche dosed first patient in P3 MabThera study Presented P1 data from analog insulin pump study with rHuPH20 at AACE Signed deal worth up to $63M with Intrexon to develop SC A1T Baxter filed HyQ BLA Signed deal worth up to $83M with ViroPharma to develop SC Cinryze + rHuPH20 Presented 3-day insulin pump data at ADA ViroPharma initiated P2 clinical trial for SC Cinryze + rHuPH20 Dosed first patient in HTI-501 POC study in aesthetic medicine Completed two analog insulin + rHuPH20 treatment studies Presented pre- administration of rHuPH20 in insulin pumps at DTS Launched Hylenex(r) Initiated P2 randomized PEGPH20 trial in pancreatic cancer Reported top line Phase 3 data from SC Herceptin Trial |

| Milestones (for proprietary and partnered programs) Present full data set from phase 3 SC Herceptin trial Present HTI-501 clinical proof-of-concept data File SC Herceptin marketing application in EU File SC MabThera marketing application in EU Launch HyQ (pending regulatory approval) Initiate phase 2 dose ranging study of Cinryze + rHuPH20 Disclose full data set from insulin + rHuPH20 treatment studies Present additional data from insulin pump trials 2012 milestones could be transformative |
