Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ENDO HEALTH SOLUTIONS INC. | d279229d8k.htm |
| EX-99.1 - PRESS RELEASE OF ENDO PHARMACEUTICALS HOLDINGS INC. DATED JANUARY 9, 2012 - ENDO HEALTH SOLUTIONS INC. | d279229dex991.htm |
 ENDO
PHARMACEUTICALS 30
th
Annual
J.P. Morgan Healthcare Conference
January 9, 2012
Exhibit 99.2
grow. collaborate. innovate.
thrive. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
2
FORWARD LOOKING STATEMENTS
This presentation contains forward-looking statements within the meaning of the Private
Securities Litigation
Reform
Act
of
1995.
Statements
including
words
such
as
“believes,”
“expects,”
“anticipates,”
“intends,”
“estimates,”
“plan,”
“will,”
“may,”
“look forward,”
“intend,”
“guidance,”
“future”
or similar
expressions are forward-looking statements. Because these statements reflect our
current views, expectations and beliefs concerning future events, these
forward-looking statements involve risks and uncertainties. Investors should note
that many factors, as more fully described under the caption “Risk
Factors”
in our Form 10-K, Form 10-Q and Form 8-K filings with the Securities and Exchange
Commission and as otherwise enumerated herein or therein, could affect our future
financial results and could cause our actual results to differ materially from those
expressed in forward-looking statements contained in our Annual Report on Form
10-K. The forward-looking statements in this presentation are qualified by
these risk factors. These are factors that, individually or in the aggregate, could cause our
actual results to differ materially from expected and historical results. We assume no
obligation to publicly update any forward-looking statements, whether as a result
of new information, future developments or otherwise. |
 grow.
collaborate. innovate. thrive. ENDO PHARMACEUTICALS
I.
Our Diversified Business
II.
Growth Drivers
III.
Commitment to Innovation
IV.
2011 Financial Guidance
V.
Value Creation in 2012 |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
FUTURE VALUE CREATION OPPORTUNITIES
4 |
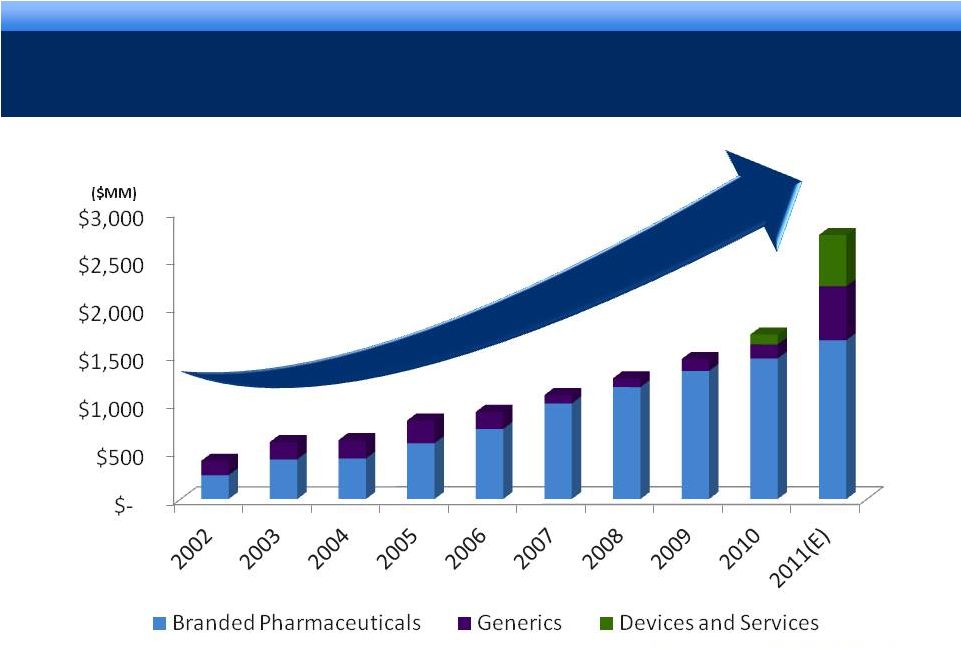 ©2011
Endo Pharmaceuticals SOLID TRACK RECORD OF SALES GROWTH
16% 3-YEAR CAGR FOR REVENUE*
5
Sustaining our Growth
* Revenue CAGR 2007-2010
($MM)
grow. collaborate. innovate. thrive. |
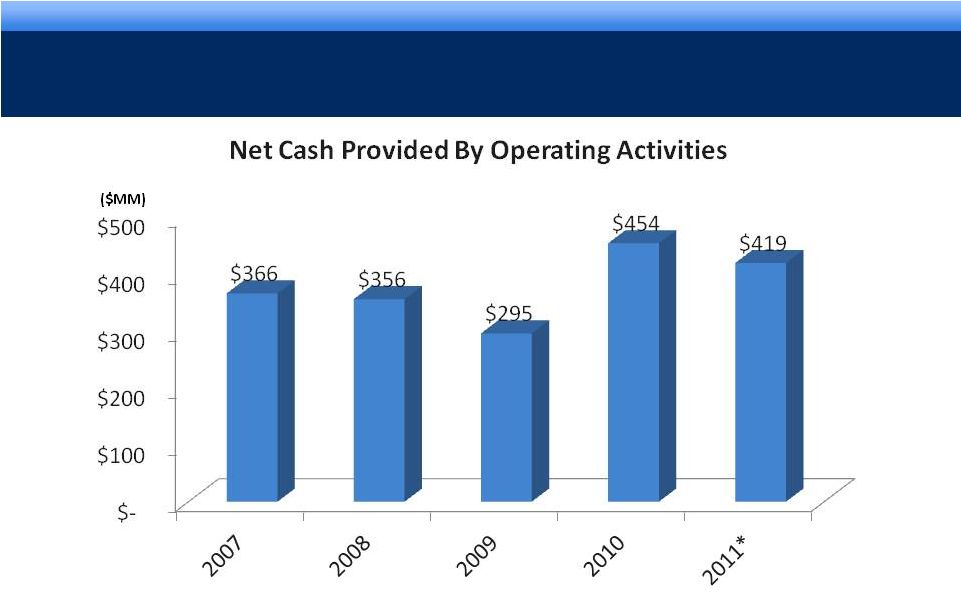 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
STRONG CASH FLOW GENERATION
6
* Year to date reported as of 9/30/2011 |
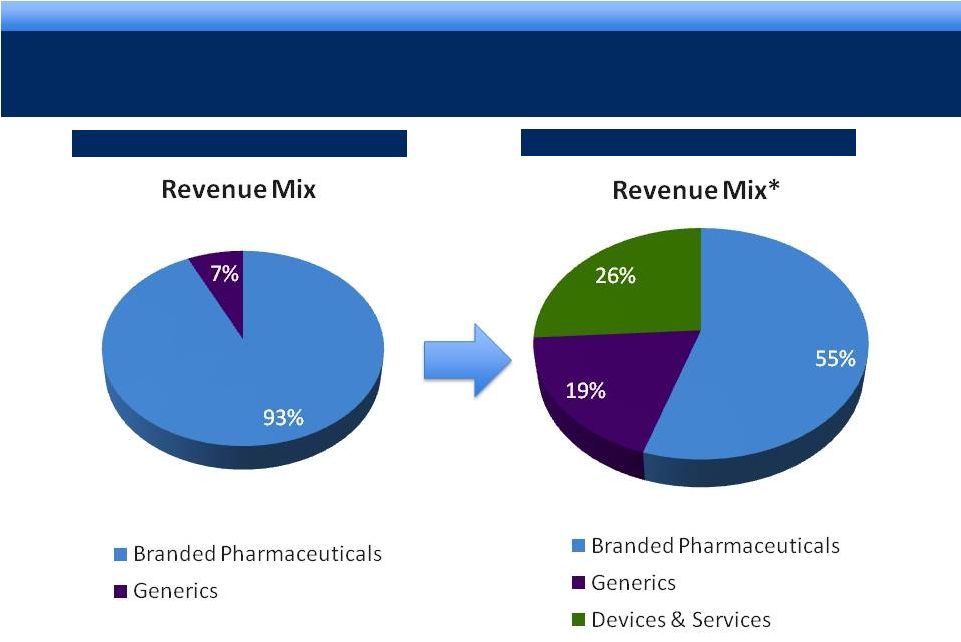 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
7
DIVERSIFIED HEALTHCARE SOLUTIONS COMPANY
Endo -
2011
Endo -
2008
* Pro forma -
TTM as of 9/30/2011. Includes full year of AMS and Qualitest.
|
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
8
SUSTAINING OUR GROWTH IN 2012
2012 Top Drivers of Revenue Growth:
1.
Branded Pharmaceuticals:
Opana ER
Voltaren Gel
2.
Qualitest
3.
AMS
Diversifying Endo has created a company with
near-term growth from multiple sources |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
BRANDED PHARMACEUTICALS DRIVERS
9
Voltaren®
Gel: A product with strong
long-term growth
November 2011 TRx growth:
Voltaren Gel ~37%
Expect 2012 revenue growth driven by:
Strong formulary access
Targeted promotion
Increasing share of prescription volumes
Strong
Prescription
Growth |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
BRANDED PHARMACEUTICALS DRIVERS
10
New Formulation of Opana ER Approved:
Patent protection through 2023
Opana ER Supply Chain Update:
Short-term supply constraints as a result of temporary shut down of Novartis
facility
Working with FDA and Novartis to resume production shortly
Currently expect full production levels of new formulation by end of Q1 or early
Q2
2012 Oxymorphone API quota allocation from DEA:
Significant quota
granted to support manufacture of
new
formulation
No quota
granted to support manufacture of
old
formulation
We believe this decision supports the introduction of new formulations of
extended
release
opioids
that
are
designed
to
potentially
reduce
certain
forms
of misuse and abuse. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
STRONG CORE BUSINESS SUPPORTING GROWTH
LIDODERM®
key component of our core business
Durable source of operating cash flow
Expect sales growth in 2012
Stable TRx trends and solid managed care positioning
12-year Commercial launch anniversary in September 2011
Differentiated product profile
Provides unique offering for HCPs and patients suffering
from PHN
11 |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
STRONG GENERICS GROWTH IN 2012
12
Expect continued revenue growth in Generics
Growth primarily driven by strong demand for current
commercial products ~600 SKUs
Specialty product lines create stability
~60% of revenues from Controlled Substance and Liquids
ANDA approvals and product launches supplement
growth
Capitalizing on demand opportunity –
investing to
increase manufacturing capacity by ~70% |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
13
AMS: PORTFOLIO OF GROWTH
Expect 2012 growth from all lines of business
Expansion in US and Non-US regions
Men’s
Health:
Driven
by
procedural
volume
growth
Erectile Restoration (ER) within US
Male Continence (MC) outside US
Women’s
Health:
Planning
for
a
return
to
growth
in
2012
and continued new product introductions
BPH:
Growth
driven
by
increasing
GreenLight
share
of
procedural volumes
International opportunities |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
Expect strong 2012 growth
Leading provider of urology services:
Lithotripsy
BPH laser
Cryosurgery
Service based relationships focused on:
Improving patient care
Enhancing practice economics
Recent addition of electronic medical record offerings:
Enhances HealthTronics’
service offerings
Adds significantly to our urology relationships
14
HEALTHTRONICS SERVICE BASED GROWTH |
 grow.
collaborate. innovate. thrive. •
Virtual Research
•
Channels within Pelvic Health and Pain
•
Diverse set of Therapeutic Opportunities:
o
Pain
o
Prostate Cancer
o
Bladder Cancer
o
Hormone Replacement
o
Sexual function
o
Incontinence
o
Malignant Disease
PARTNERSHIPS :
A KEY TO FUTURE VALUE CREATION
©2011 Endo Pharmaceuticals Inc. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
COMMITMENT TO INNOVATION
16
Branded Pharmaceuticals
Medical Devices
Generic Pharmaceuticals
ANDA
Filings
~50 Current
ANDA Reviews
ANDA
Approvals
Generic Development
Supplements strong
commercial base growth
•
Continually enhancing
existing products
•
Recent advances:
GreenLight™
XPS Laser
Console
MoXy™
Laser Fiber
AdVance™
XP (OUS)
•
Developing treatments in
new areas
Topas™
sling
Cryotherapy
•
Exploring new emerging
technologies
•
Key Therapeutic Areas
•
Pain
•
Oncology
•
Endocrinology
•
Semi-Virtual R&D Model
•
Global Partnerships
•
Discovery
•
Early Development
•
Development Pipeline
AVEED™
(NDA)
Long Acting Injectable Testosterone
BEMA Buprenorphine™
(Ph. III)
Pain
Urocidin™
(Ph. III)
Bladder Cancer
Androgen
Receptor
Antagonist
(Ph.
I)
Castration Resistant Prostate Cancer |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
17
NEW PHASE III BEMA Buprenorphine
Complements pain therapeutics portfolio
Draws upon existing expertise in the development and
commercialization of opioids
Key Financial Terms:
$30 million upfront payment to BDSI
$150 million in potential milestone payments
Contingent
on
IP,
Clinical
and
Regulatory
events
and
designated sales levels
Tiered royalties on net sales in the U.S. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
18
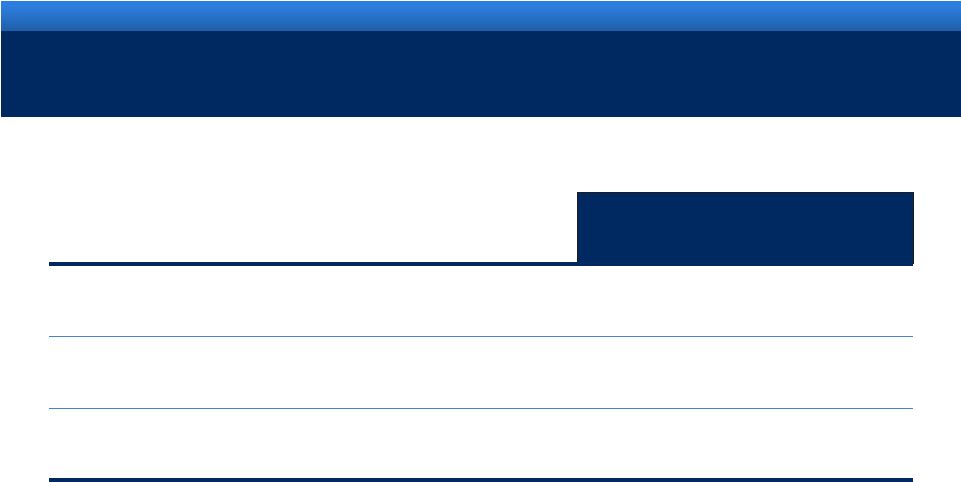
2011 ENDO GUIDANCE
Guidance
Revenue range
$2.72B -
$2.80B
Adjusted diluted EPS range
$4.55 -
$4.65
Reported (GAAP) diluted EPS range
$1.87 -
$1.97 |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
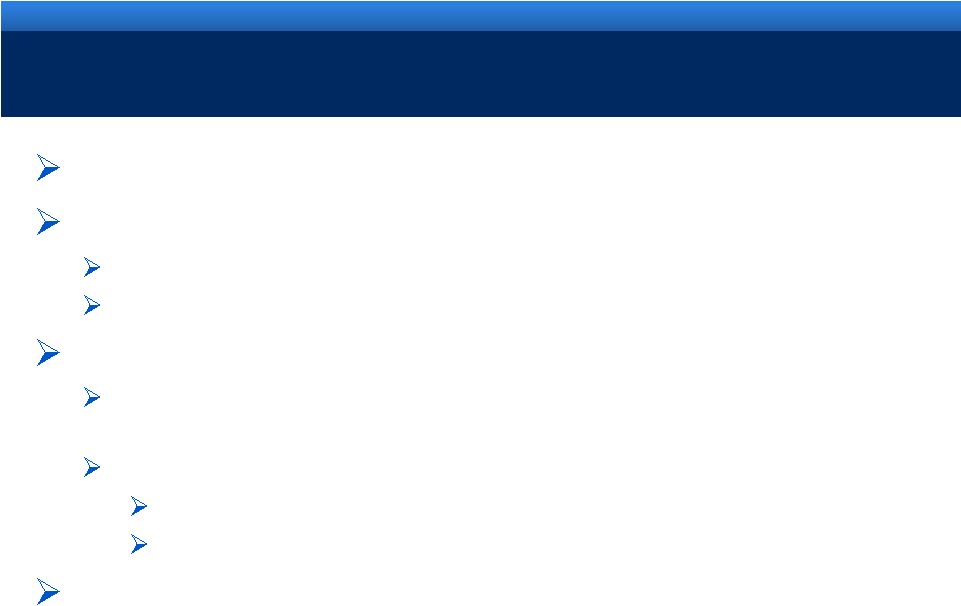
2011 KEY ACHIEVEMENTS
New formulation of Opana ER approved
Built a diversification-driven business model
Execution of Generics strategy through Qualitest
Expansion of therapeutic device offerings for Urologists with AMS
Create customer-centric opportunities
Endo’s Urology business: Diagnostics, Devices, Services, Pharmaceuticals
and Data
Launched Pilot programs in Urology
AMS Men’s Health and Fortesta Gel
Endocare cryoablation and AMS Green Light laser
Continued growth in Branded Pharmaceuticals
19 |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
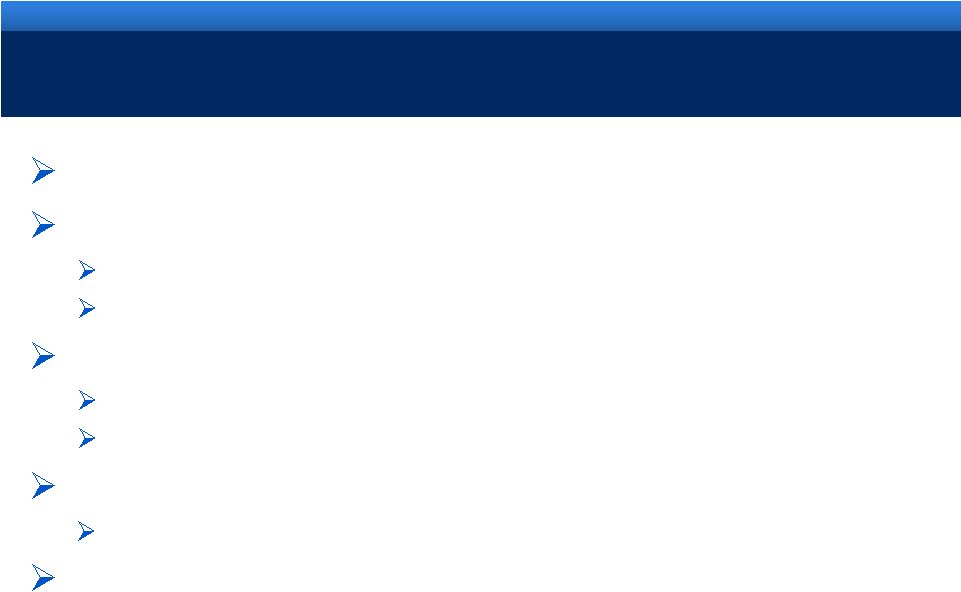
2012 VALUE CREATION OPPORTUNITIES
Successfully launch new formulation of Opana ER
Support the future growth of Qualitest
Invest capital to capture growing demand for products
Exceed cost synergies assumed at time of Qualitest acquisition
Invest in AMS to accelerate growth
AMS/Endo pilot program updates in first half 2012
Invest in R&D to accelerate advance of new products to market
Maximize operating cash flow to pay down debt
~$300M of debt repayments completed in 2011
Continue to evaluate complementary licensing and
acquisition opportunities aligned with current business
20 |
 ENDO
PHARMACEUTICALS grow. collaborate. innovate. thrive. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
22
22
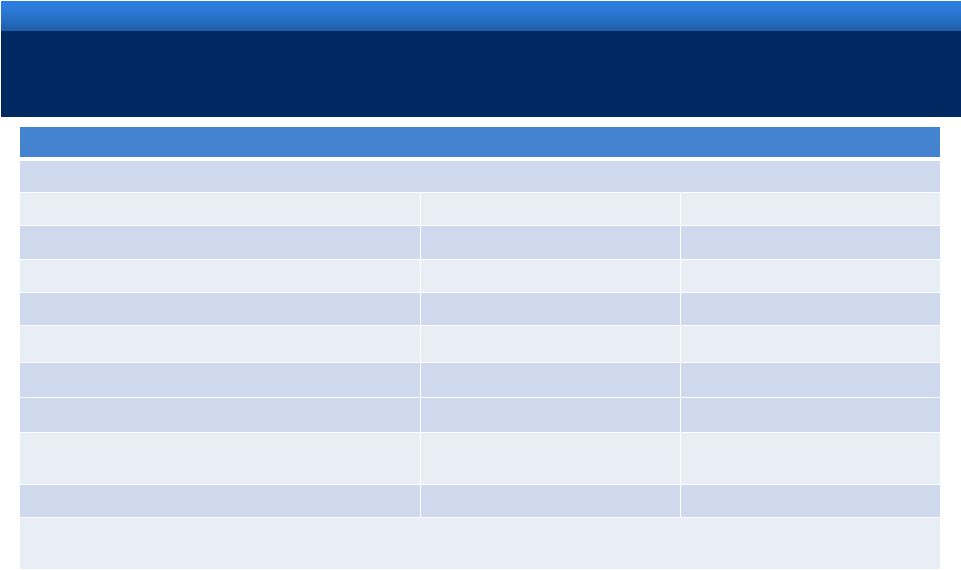
RECONCILIATION OF NON-GAAP MEASURES
22
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s
Current Report on Form 8-K filed today with the Securities and Exchange Commission
Reconciliation of Projected GAAP Diluted Earnings Per Share to Adjusted Diluted Earnings Per
Share Guidance for the Year Ending December 31, 2011 Lower End of Range
Upper End of Range
Projected GAAP diluted income per common share
$1.87
$1.97
Upfront and milestone-related payments to partners
$0.25
$0.25
Amortization of commercial intangible assets and inventory step-up
$2.10
$2.10
Acquisition and integration costs related to recent acquisitions.
$0.48
$0.48
Impairment of long-lived assets through September 30, 2011
$0.19
$0.19
Interest expense adjustment for ASC 470-20
$0.16
$0.16
Tax effect of pre-tax adjustments at the applicable tax rates and
certain other expected cash tax savings as a result of recent
acquisitions
($0.50)
($0.50)
Diluted adjusted income per common share guidance
$4.55
$4.65
The company's guidance is being issued based on certain assumptions including:
•Certain of the above amounts are based on estimates and there
can be no assurance that Endo will achieve these results
•Includes all completed business development transactions as of
January 9, 2012 |
 ENDO
PHARMACEUTICALS 30 Annual
J.P. Morgan Healthcare Conference
January 9, 2012
grow. collaborate. innovate.
thrive. th
|
