Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AMICUS THERAPEUTICS, INC. | c26618e8vk.htm |
| EX-99.2 - EXHIBIT 99.2 - AMICUS THERAPEUTICS, INC. | c26618exv99w2.htm |
Exhibit 99.1

| Corporate Overview At the Forefront of Therapies for Rare and Orphan DiseasesTM January 2012 |

| Safe Harbor This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations and financial conditions of Amicus including but not limited to preclinical and clinical development of Amicus' candidate drug products, the timing and reporting of results from preclinical studies and clinical trials evaluating Amicus' candidate drug products, the projected cash position for the Company, and business development and other transactional activities. Words such as, but not limited to, "look forward to," "believe," "expect," "anticipate," "estimate," "intend," "plan," "would," "should" and "could," and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus' forward-looking statements due to numerous known and unknown risks and uncertainties, including the "Risk Factors" described in our Annual Report on Form 10-K for the year ended December 31, 2010. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this news release to reflect events or circumstances after the date hereof. Slide 2 |

| Slide 3 Amicus is Business Led & Science Driven At the Forefront of Therapies for Rare and Orphan DiseasesTM Proprietary platform & IP Small molecules targeting misfolded and unstable proteins Stabilize & enhance patient's own enzyme; or Potential to stabilize & enhance ERT products for lysosomal storage disorders (LSDs) Global expertise in rare disease clinical research Clinical sites in over 20 countries Multiple Fabry Phase 3 & Phase 2 programs Pompe Phase 2 program underway 19.9% FOLD ownership stake $60M cash upfront + cost- sharing GSK to act as global commercial lead on Fabry Pharmacological Chaperone Platform Technology Global Clinical Capabilities & Pipeline Alliance with GSK Rare Diseases |

| Slide 4 Pharmacological Chaperones One Technology, Two Novel Applications Based on patient's own mutated enzyme Potential alternative to Enzyme Replacement Therapies (ERTs) Potential to improve ERT stability, uptake and activity & lower immunogenicity |

| Fabry - Migalastat HCl - Study 013 Pompe - AT2220 - Study 010 Other LSDS Fabry - Migalastat HCl - Study 011 Fabry - Migalastat HCl - Study 012 Parkinson's AT3375 Slide 5 Pharmacological Chaperones: Advanced Product Pipeline ERT Combination Therapy Preclinical Phase 1 Phase 2 Phase 3 Chaperone Monotherapy Marketing Application |

| Amicus & GSK Rare Diseases Alliance Strong Partnership Established Slide 6 Monotherapy & ERT Combination Therapy 75% global development costs paid by GSK (as of 2012) GSK has exclusive rights to commercialize Migalastat HCl globally Amicus eligible to receive up to $170M in milestones; tiered double-digit royalties on Migalastat HCl Close working relationship in rare diseases GSK equity ownership Migalastat HCl for Fabry Disease Product and Strategic Alliance |

| Slide 7 Migalastat HCl for Fabry Disease Three Key Clinical Studies Underway Phase 3 Monotherapy U.S. Registration Study Placebo controlled 67 patients Surrogate endpoint - kidney GL-3 Eligible for accelerated approval Data expected 3Q12 Pre-NDA meeting anticipated 2H12 STUDY 011 Phase 3 Monotherapy Global Registration Study Switch from ERT 50 patients 18-month clinical endpoint - kidney function Full enrollment targeted by YE12 STUDY 012 Phase 2 Chaperone- ERT combination Drug-drug interaction study Positive preliminary results in humans 2 doses of chaperone with Fabrazyme(r) and REPLAGAL(tm) Plasma PK & PD (skin biopsies) Expect to complete 1H12 STUDY 013 |

| Slide 8 Study 011 Phase 3 Confidence Phase 2 Experience Study 011 Design Contributes to Potential for Phase 3 Success 90+ years of patient experience 17 Phase 2 patients remain on migalastat HCl monotherapy Positive results on renal and urine GL-3 clearance (key biomarker) Long-term trends toward stabilization of kidney function Improved Histological Methodology BLISS-VM Methodology more advanced, sensitive & objective* vs. Thurberg-LM Phase 3 Observations Study 011 Low dropout rate High conversion to extension study Strict Entry Criteria Naive to ERT / no ERT in past 6 months Amenable mutations Urine GL-3 ^ 4x normal *Barisoni 2012 |

| Slide 9 Study 011 Patient Disposition to Date 67 Patients enrolled Low Drop Out Rate & High Conversion from Study 011 to Extension Study 44 to date completed 6 month TX period (19 ongoing) 44 continued in 6-month treatment extension (41 ongoing) 23 to date completed Study 011 (6-mo. TX + 6-mo. Extension) 23 enrolled in open-label extension studies (21 ongoing) |

| Slide 10 Fabry Market Continuing to Grow All Fabry Patients Potentially Eligible to Receive Migalastat HCl Upon Approval as Monotherapy or in Combination with ERT Projected Future Market Distribution Market Opportunity Chaperone-ERT Combination Chaperone Monotherapy* # of Fabry patients on treatment *Includes estimated size of undiagnosed population with amenable mutations; Spada 2006, Hwu 2009, Mechtler 2011 Sources: Leerink 2011, Cowen 2011, Genzyme/Shire 10Ks (est) $539M sales in 2010 growing to $1B+ (est) (CHART) |

| Chaperone-ERT Combination Technology Slide 11 |

| LSD ERT Market Potential Limitations of 1st Generation Technology LSD market currently dominated by ERTs... ...with multiple ERTs in development However, significant unmet needs remain due to potential limitations of 1st generation approach (ERT) "...on the basis of our studies, one of the major obstacles to enzyme replacement with alpha-Gal is the instability of the enzyme." Beutler and Mayes, 1977 Biochimica et Biophysica Acta "Unfortunately, none of the currently prescribed ERTs is able to treat all aspects of the (LSD) disorders equally. This is not surprising and was predicted following the embryonic attempts at ERT in the 1970s..." Wraith, 2006 J Inherit Metab Dis (Presented SSIEM 2005) "...(Physicians) note that Myozyme is clearly not a cure. They believe response can be variable from patient to patient, with some having excellent response, and others a minimal one." Cowen and Company, 2011 Orphan Disease Research Report Slide 12 Fabrazyme, Cerezyme, Myzoyme and Lumizyme are registered trademarks owned by Sanofi-Aventis VPRIV is a registered trademark owned by Shire |

| Photo by J Pastor/Univ of Florida Protein Instability Blood & Infusion Bag Slide 13 LSD Products Today Potential Issues Immunogenicity Dosing Limitations Duration of Infusion |

| Improving ERTs for Lysosomal Storage Disorders Pharmacological Chaperone Co-Administration Next Generation Therapy: potential for improving ERT Uncleared Substrate Circulation (Blood) Endosomal System Lysosome Pharmacological Chaperone (Oral) Unstable ERT in bag/blood Increased Tissue Exposure Slower ERT Clearance From Circulation Additional Substrate Reduction ~50% of Fabry patients |
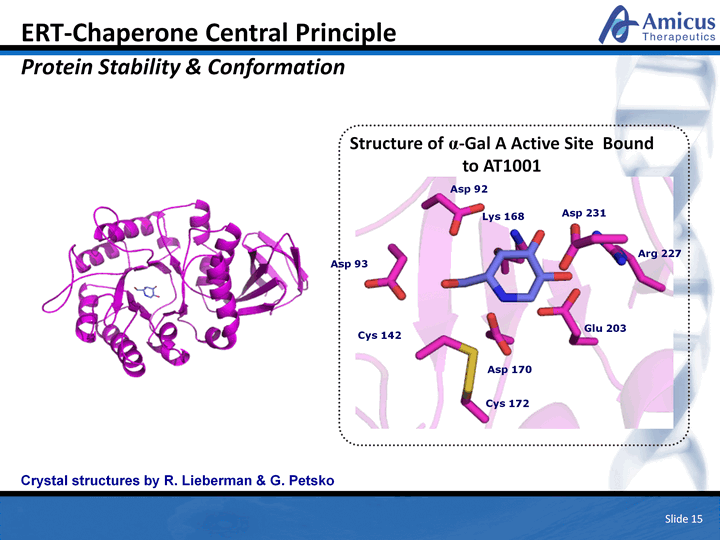
| Slide 15 ERT-Chaperone Central Principle Protein Stability & Conformation Crystal structures by R. Lieberman & G. Petsko Structure of ^-Gal A Active Site Bound to AT1001 |

| Slide 16 Fabry Study 013 Overview Study Population 18-24 male Fabry patients on ERT (all mutation types eligible) ERTs Evaluated 0.5 mg/kg Fabrazyme (every 2 weeks) 1.0 mg/kg Fabrazyme (every 4 weeks) 0.2 mg/kg Replagal (every 2 weeks) Migalastat HCl Doses Evaluated 150 mg 450 mg Endpoints Studied Safety ^-Gal A activity in plasma and in skin +/- Migalastat HCl Day 1 Day 2 (24 hours) Day 7 Baseline Skin Biopsy for ^-Gal A Activity (predose) Serial Blood Sampling for Plasma ^-Gal A Activity ERT Alone (period 1) ERT + Migalastat HCl (pd 2) Skin Biopsy for ^-Gal A Activity Skin Biopsy for ^-Gal A Activity Single dose, 2 hours prior to ERT infusion |

| Migalastat HCl 150 mg + Fabrazyme 1 mg/kg (n=2) Migalastat HCl 150 mg + Fabrazyme 0.5 mg/kg (n=4) Slide 17 Study 013 Plasma PK Preliminary Data (n = 6) Co-Administration Increases Levels of Active Enzyme in Plasma ~2.0- to 4.0-Fold vs. ERT Alone in First 6 Patients |

| 1.0 mg/kg Fabrazyme + 150mg migalastat (n=2) 0.5 mg/kg Fabrazyme + 150mg migalastat (n=3*) Study 013 Skin Biopsies - Preliminary Data (n = 6) Slide 18 ERT alone ERT + migalastat Fold Increase Day 2: 1.6, 2.1 Fold Increase Day 2: 2.8, 3.9, 1.1 (CHART) (CHART) Co-Administration Increases Levels of Active Enzyme in Skin at Day 2 vs. ERT Alone *1 Biopsy Sample Lost |
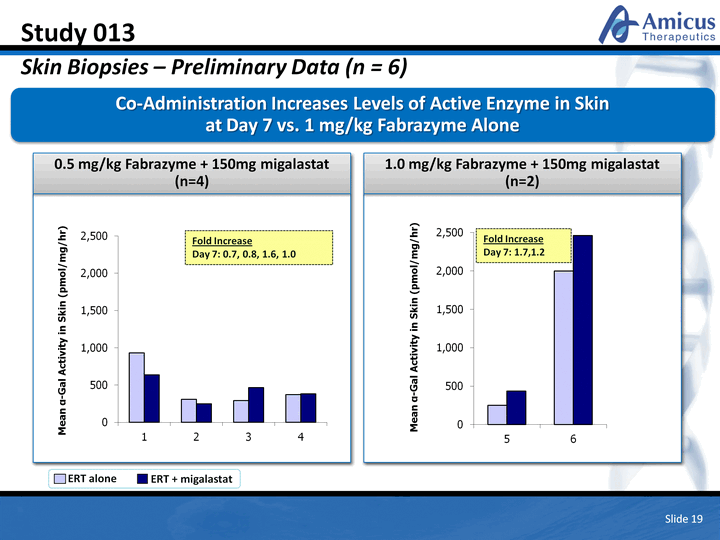
| 1.0 mg/kg Fabrazyme + 150mg migalastat (n=2) 0.5 mg/kg Fabrazyme + 150mg migalastat (n=4) Study 013 Skin Biopsies - Preliminary Data (n = 6) Co-Administration Increases Levels of Active Enzyme in Skin at Day 7 vs. 1 mg/kg Fabrazyme Alone Slide 19 ERT alone ERT + migalastat Fold Increase Day 7: 0.7, 0.8, 1.6, 1.0 Fold Increase Day 7: 1.7,1.2 (CHART) (CHART) |

| Slide 20 Study 013 Next Steps The following additional data are expected in 1H 2012: Fabrazyme (0.5 mg/kg) + 450 mg Migalastat Fabrazyme (1.0 mg/kg) + 450 mg Migalastat Replagal (0.2 mg/kg) + 150 mg and 450 mg Migalastat |

| Pompe Co-Administration Pompe Market Overview Worldwide population of ~5,000 Pompe patients1 Estimated true incidence is ~1 in 40,000 live births Literature suggests incidence of 1 in 20,000-100,000 live births (~2,000-10,000 patients) Significant unmet needs exist due to potential limitations of Myozyme/Lumizyme1,2 Outcomes vary greatly among treated patients Challenges of addressing muscle manifestations of disease Delivered relatively inefficiently to affected cells and organs Potential immunogenicity issues; antibody neutralization and infusion reactions 1Cowen and Company Orphan Disease Research Report, 2011 2BioMarin R&D Day, Dec 2011 Slide 21 |

| Preliminary Data from Study 010 Expected 1H 2012 Slide 22 Pompe Co-Administration Phase 2 PK/PD Study 010 Study Population 16 Pompe Patients on ERT ERT Evaluated Myozyme/Lumizyme AT2220 Doses Evaluated 4 increasing doses (single dose, given prior to ERT infusion) Endpoints Studied Safety GAA activity in plasma and in muscle +/- AT2220 |

| Parkinson's Slide 23 |

| Parkinson's - Gaucher Link Amicus at the Forefront 2011 MJFF approves second year to continue preclinical studies of AT3375 2010 MJFF awards $500,000 grant to focus on Parkinson's- implicated protein alpha-synuclein IP issued for use of chaperone to treat neurological disorders by enhancing Gcase IND-enabling work for chaperone AT3375 begins 2008 New series of chaperones identified, improve on AT2101 properties to target CNS 2007 Chaperone AT2101 increases activity of brain GCase, prevents accumulation of ^-synuclein in brain, and improves motor function in preclinical animal models 2006 MJFF awards $196,000 grant to support research into chaperones for Parkinson's disease Prevention of synuclein accumulation in the brain Untreated PC-Treated Vehicle 3 weeks 15 weeks 15 weeks Synuclein Synuclein/NeuN |

| Parkinson's - Gaucher Link Increasing Focus of Key Publications Acid ^^glucosidase mutants linked to Gaucher disease, Parkinson's and Lewy body dementia alter ^-synuclein processing Valerie Cullen, S. Pablo Sardi, Juliana Ng, Xu-Yu Hui, Ying Sun, Julianna J. Tomlinson, Piotr Kolodziej,Ilana Kahn, Paul Saftig, John Woulfe, Jean C. Rochet, Marcie A. Glicksman, Seng H. Cheng,Gregory L. Grabowski, Lamya S. Shihabuddin, Michael G. Schlossmacher Slide 25 |

| Strategic Vision, 2012 Milestones and Guidance Slide 26 |

| Continuum of Innovation Multiple Paths Forward for ERT-Chaperone Combinations We Envision Series of Incremental, Important Product Advances Unique to Each LSD Over This Decade Slide 27 Currently Marketed ERTs Currently Marketed ERTs Co-Administered with Chaperones Currently Marketed ERTs Co-Formulated with Chaperones Currently Marketed ERTs Co-Formulated with Chaperones and with Improved Delivery/Regimen Next Generation ERT Co-Formulated with Chaperones Next Generation ERT Co-Formulated with Chaperones and with Improved Delivery/Regimen |

| Slide 28 2012 Anticipated Milestones Building Shareholder Value Fabry Study 011 Phase 3 Data Q3 Study 012 Complete Enrollment Q4 Study 013 Complete 1H Pompe Study 010 Phase 2 Preliminary Data 1H Genetic Parkinsons '3375 IND Enabling Studies Complete Q4 |

| Slide 29 2012 Financial Guidance Estimated Beginning Cash: ~$60 M 2012 Cash Spend Guidance: $37 M to $43 M Expected Current Cash Runway: Mid Q3 2013 |

| Amicus Strategic Vision "We begin 2012 as a company with late stage clinical programs, a broad platform technology, a strong balance sheet and a key strategic partnership with GSK. We believe that these pillars of strength uniquely position Amicus to grow into a fully integrated biopharmaceutical company at the forefront of developing therapies for rare and orphan diseases," -John F. Crowley, Amicus Chairman & CEO (January 9, 2012) Slide 30 |
