Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Bausch Health Companies Inc. | d278394d8k.htm |
 2012 Financial
Guidance
Conference Call
January 6, 2012
Exhibit 99.1 |
 1
Forward-looking Statements
Forward-looking Statements
Certain statements made in this presentation may constitute forward-looking
statements, including, but not limited to, statements regarding
preliminary
results
and
guidance
with
respect
to
expected
revenues,
non-GAAP
cash
earnings
per
share,
adjusted
cash
flows
from operations,
product sales growth and
organic product
sales
growth,
integration-related activities and benefits,
synergies, launches
and
approvals
of
products,
the
impact
of
foreign
exchange
rates,
and
the
2012
strategic
initiatives
of
Valeant
Pharmaceuticals
International,
Inc.
(the
“Company”).
Forward-looking
statements
may
be
identified
by
the
use
of
the
words
“anticipates,”
“expects,”
“intends,”
“plans,”
“could,”
“should,”
“would,”
“may,”
“will,”
“believes,”
“estimates,”
“potential,”
or “continue”
and variations or similar
expressions.
These
statements
are
based
upon
the
current
expectations
and
beliefs
of
management
and
are
subject
to
certain
risks
and
uncertainties that could cause actual results to differ materially from those
described in the forward-looking statements. These risks and uncertainties
include, but are not limited to, risks and uncertainties discussed in the company's most recent annual or quarterly report
filed with the Securities and Exchange Commission ("SEC") and other risks and
uncertainties detailed from time to time in the Company's filings with the SEC
and the Canadian Securities Administrators ("CSA"), which factors are incorporated herein by reference.
Readers are cautioned not to place undue reliance on any of these forward-looking
statements. The Company undertakes no obligation to
update
any
of
these
forward-looking
statements
to
reflect
events
or
circumstances
after
the
date
of
this
presentation
or
to
reflect
actual outcomes.
Non-GAAP Information
To
supplement
the
financial
measures
prepared
in
accordance
with
generally
accepted
accounting
principles
(GAAP),
the
Company
uses
non-GAAP financial measures that exclude certain items. Management uses
non-GAAP financial measures internally for strategic decision making,
forecasting future results and evaluating current performance. By disclosing non-GAAP financial measures,
management intends to provide investors with a meaningful, consistent comparison of
the Company’s core operating results and trends for the periods
presented. Non-GAAP financial measures are not prepared in accordance with GAAP; therefore, the information is not
necessarily comparable to other companies and should be considered as a supplement
to, not a substitute for, or superior to, the corresponding
measures
calculated
in
accordance
with
GAAP.
The
Company
has
provided
preliminary
results
and
guidance
with
respect to cash earnings per share, adjusted cash flows from operations and organic
product growth rates, which are non-GAAP financial measures. The Company
has not provided a reconciliation of these preliminary and forward-looking non-GAAP financial
measures due to the difficulty in forecasting and quantifying the exact amount of the
items excluded from the non-GAAP financial measures that will be included
in the comparable GAAP financial measures. Note 1: The guidance in this presentation is
only effective as of the date given, January 6, 2012, and will not be updated or affirmed unless
and until the Company publicly announces updated or affirmed guidance. |
 2
Agenda
2011 Update
2012 Review
Other Updates |
 3
2011 Achievements
Acquisitions
Completed
11
acquisitions
–
PharmaSwiss,
Sanitas,
Ortho,
Dermik,
iNova,
Zovirax/Xerese,
Elidel
~$2.9b in total purchase price
Averaged 2.4x sales
Established new growth platforms in South East Asia, South Africa and Russia
Achieved
realized
synergies
of
$320
million
from
Biovail
merger
R&D Productivity and Product Launches
Potiga approved in U.S.
Trobalt approved and launched in Europe
IDP-108 –
2 positive Phase III trials
4 products approved
13 products launched in Canada, including Onsolis, Sublinox, Biacna, Aczone
100+ new OTC and Branded Generics products approved/launched in ex-US
markets Balance Sheet Management
Lowered average interest rate from ~7% to ~5.7%
Repurchased
16
million
common
shares
at
average
cost
of
~$40
per
share
under
previous
$1.8 billion securities repurchase program
Repurchased 1.5 million common shares at average cost of ~$42 per share under
current $1.5 billion program
Repurchased $100 million face value high yield debt at below par
Repurchased $205 million face value convertible preferred notes
Raised $4.65 billion in high yield debt and credit facilities
|
 4
2011 Update
Fourth Quarter 2011 Guidance
Revenue expected to be >$650 million
>8% organic growth
Cash
EPS
expected
to
be
between
$0.83
-
$0.87
Adjusted cash flows from operations expected to be >$230
million*
Currency headwinds higher than expected
~$40 million revenue impact
~$0.05 Cash EPS impact
Full Year 2011 pro forma** growth rates
~27% product sales growth over 2010
~8% organic product sales growth over 2010
~27% revenue growth over 2010
~39% adjusted cash flow from operations growth over 2010
* Excluding changes in working capital and cash restructuring charges as not yet
available ** Pro forma for 2010 Biovail/Valeant merger
See Note 1 regarding guidance |
 5
Agenda
2011 Update
2012 Review
Other Updates |
 6
2012 Guidance*
Total
revenues
expected
to
be
between
$3.1
-
$3.4
billion at constant currency rates
~30-40% increase over 2011
Currency fluctuations could have significant impact and not
included in range
Cash
EPS
expected
to
be
between
$3.95
–
$4.20
~40-45% increase over 2011
Adjusted Cash Flow from Operations >$1.2 billion
Up over $300 million or ~33% increase over 2011
Negative impact of ~$200 million due to divestitures and certain
generic entrants will be offset by organic growth and synergies
See Note 1 regarding guidance
* Excludes potential acquisitions
|
 7
Organic Growth Guidance
We will continue to measure and report each quarter
Strong organic growth remains a primary objective for each operating unit
However, our primary focus is on total cash returns to shareholders
All acquisitions subject to rigorous internal rate of return analysis
We complete different types of acquisitions
Most acquisitions focused on building long-term sustainable growth at very
attractive returns
e.g. Sanitas, iNova, PharmaSwiss
Some acquisitions offer even more attractive short-term returns, but are
dilutive to overall corporate organic growth in the longer term
e.g. Dermik
We will not provide specific annual organic growth
guidance for 2012 |
 8
New Segment Reporting
U.S. Dermatology
U.S. Neurology and Other
Canada/Australia
Emerging Markets
Latin America
Central/Eastern Europe
South East Asia/South Africa |
 9
U.S. Dermatology
Revenue
2012 expectations = $900 -
$950 million
60 -
75% increase over 2011
Key Growth Products
Zovirax/Xerese; Acanya; CeraVe; Atralin; Carac; Renova; Retin-A Micro
New product development
IDP –
108 –
onychomycosis
-
completed 2 successful Phase 3 trials
IDP –
107 –
oral acne
IDP –
118 –
psoriasis
Multiple life cycle management programs for current products ongoing
Integration of Ortho and Dermik
All management decisions made and communicated
Key 2012 agenda
Successful integration of Dermik
and Ortho to create the new “Valeant
Dermatology”
and “Valeant Aesthetics”
businesses |
 10
U.S Neurology
Revenue
2012
expectations
=
$675
-
$750
million
5-15% decrease over 2011
U.S. Neuro & Other <20% of total company revenue
Wellbutrin XL is < 4% of total company revenue
Key Products
Wellbutrin XL; Xenazine; Legacy Valeant tail products
New product launches
Trobalt/Potiga
Key 2012 agenda
Maximize Trobalt and Potiga
Manage segment to maximize cash flows |
 11
Canada
Revenue
2012
expectations
=
$350
-
$375
million
40-50% increase over 2011
Products under Meda JVs –
we do not book the revenue, only
our share of the profit (50%)
e.g. Sublinox; Onsolis
Key Products
Cesamet; Wellbutrin XL; Tiazac XC; Sublinox; COLD-FX
Key product launches in 2012
Opana, Lodalis
Key 2012 agenda
Continue to license in new products
Successfully build next generation of products through new
launches |
 12
Australia/New Zealand
Revenue
2012
expectations
=
$200
-
$250
million
> 150% increase over 2011
OTC Business
Cough/Cold –
Difflam, Andolex, DuroTuss (iNova)
Skincare –
Dermaveen Dr. Lewinns (Legacy Valeant)
Suncare –
Hamilton’s, Invisible Zinc, Reef (Legacy Valeant)
Key Rx Products
Metermine; Duromine
New product launches in 2012
Line extensions of skincare and cough/cold brands
1-2 new Rx brands
Key 2012 agenda
Successfully integrate iNova/Valeant |
 13
Emerging Markets
Revenue
2012 expectations = >$1.0 billion
40 -
50% over 2011
Key Markets
Central/Eastern Europe
Latin America
South East Asia/South Africa |
 14
Central/Eastern Europe
Revenue
2012 expectations = >$625 million
30-40% increase over 2011
Currency may continue to be a headwind
22 countries
Poland, Serbia, Hungary, Czech, Russia/Ukraine = ~80% of
revenue
Russia/Ukraine seen as new growth platforms
Export opportunities
Kazhakstan
and other former CIS countries
Key 2012 agenda
~250 product launches expected in 2012
Achieve cost synergies |
 15
Latin America
Revenue
2012 expectations = >$275 million
10-15% over 2011
2 major countries
Mexico
Brazil
Full Product Coverage
OTC
Branded Generics
Similares
Key 2012 agenda
Target area for future acquisitions |
 16
South East Asia/South Africa
Revenue
2012 expectations = ~$100 million
Own operations supplemented by distribution
agreements
Own
operations
–
Malaysia;
Philippines;
Thailand;
Hong
Kong;
South Africa
Distribution –
Vietnam; Indonesia; Singapore
Branded, Branded Generics and OTC business
Key 2012 Agenda
Establish own operations in other South East Asian markets
Cross launch products from other Valeant geographies
Aggressively pursue business development opportunities
|
 17
Agenda
2011 Update
2012 Review
Other Updates
Trobalt/Potiga
Update
Cost Synergy Program for 2012
Currency Analysis
Current Debt Structure
ISTA Update
New 2012 Strategic Objectives |
 18
Trobalt/Potiga Update
EU status
Launch roll out began in May 2011
Currently launched in ~12 countries
NICE recommendation secured
Still
early
-
less
than
6
months
of
data
in
most
countries
U.S. status
Approved June 2011
DEA scheduling completed December 2011
Launch meeting planned for late March 2012
$45 million milestone anticipated in Q2 2012
Extended Release Formulation
A
lead
candidate
identified
for
progressing
clinical
evaluation |
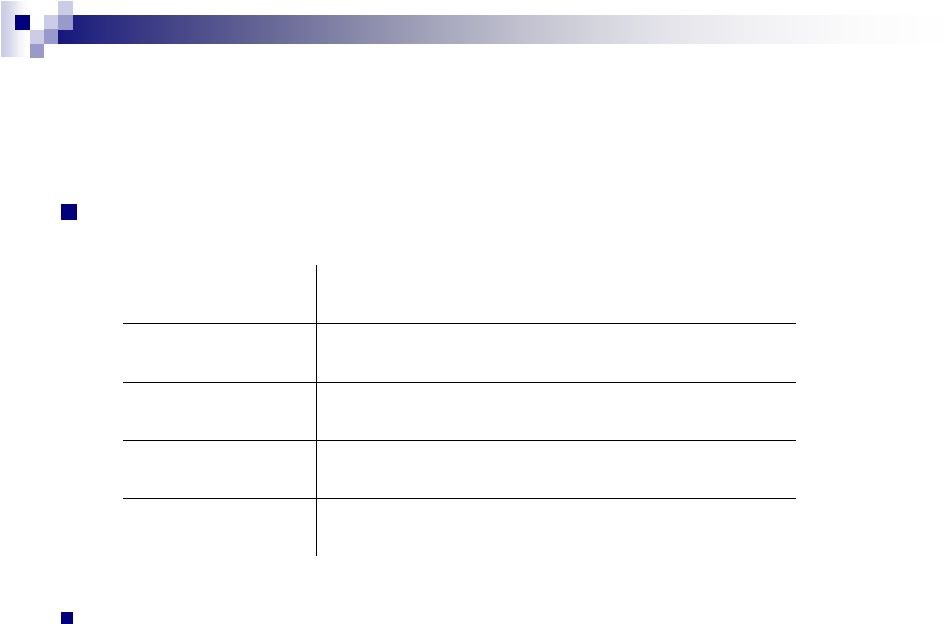 19
Cost Synergy Program in 2012
Expected cost synergies:
Total synergy run rates to be achieved by mid-year
Total Synergies
~$200 M
Canada (Afexa)
$15 M
Australia (iNova)
$25 M
U.S. Dermatology (Ortho & Dermik)
$85 M
Europe (PharmaSwiss
& Sanitas)
$75 M |
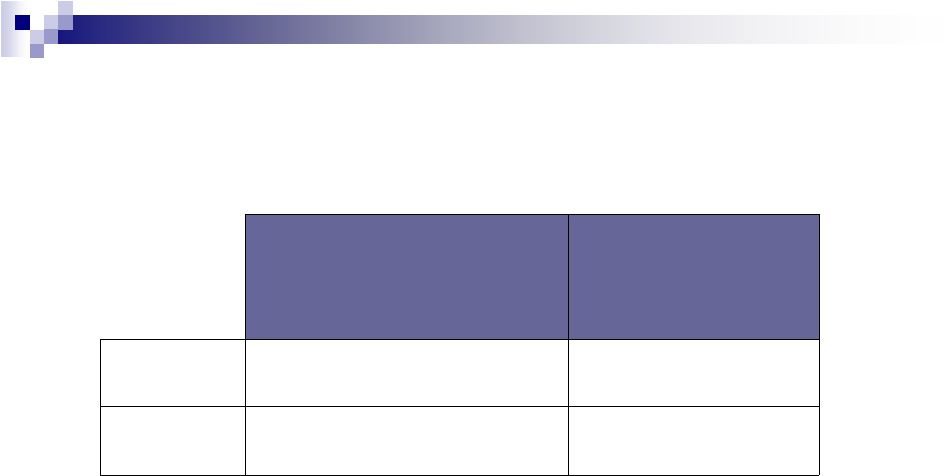 20
Currency Analysis
~($0.05)
~($40M)
Impact on Q411
actuals
when
compared to
forecasted rates
~($0.08)
Cash EPS:
~($75M)
Revenues:
Impact on 2012 budget
when compared to 2011
average rates |
 21
Current Debt Structure
Current debt = $6.7
billion
Average interest
rate = ~5.7%
Term Loan A =
$2.225 billion
Required annual
amortization in
2012 = 10%
Quarterly interest
expense = ~$100
million
Outstanding Principal
6.75% Senior Notes due 2017
$500,000,000
7.00% Senior Notes due 2020
$690,000,000
6.875% Senior Notes due 2018
$944,580,000
6.75% Senior Notes due 2021
$650,000,000
6.50% Senior Notes due 2016
$915,500,000
7.25% Senior Notes due 2022
$550,000,000
Term Loan (A and DD)
$2,225,000,000
Revolver
$220,000,000
Convertible Notes
$18,708,000 |
 22
ISTA Update
Opportunity to add a new growth platform
Merge Valeant’s small ophthalmology operations (<$50m) into
ISTA platform
Number of discussions with ISTA -
no agreement on
process or price
Proposed $6.50 per share
68% premium over 60-day volume weighted trading average of
$3.87
We are interested, but unwilling to spend too much
time
Deadline of January 31, 2012
ISTA announced they are pursuing “strategic
options”
We hope to have opportunity to negotiate a transaction
|
 23
New 2012 Strategic Initiatives
1)
Increase non-US revenues to approximately 50% of the
company
2)
Exceed $75M synergy run-rate in Europe by end of
2012
3)
Build / acquire at least once additional growth platform
4)
Exceed $1.5B in emerging market sales
5)
Continue to aggressively manage the Balance Sheet to
create shareholder value
6)
Become
a
top
15
global
pharma
company
(as
measured by Market Cap) by end of 2013 |
 2012 Financial
Guidance
Conference Call
January 6, 2012 |
