Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT OF GRANT THORNTON LLP - Parabel Inc. | d257673dex231.htm |
Table of Contents
As filed with the Securities and Exchange Commission on December 1, 2011
Registration No. 333-168742
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
to
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
PetroAlgae Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 2860 | 33-0301060 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
1901 S. Harbor City Blvd., Suite 600
Melbourne, FL 32901
(321) 409-7500
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Anthony John Phipps Tiarks
Chief Executive Officer
PetroAlgae Inc.
1901 S. Harbor City Blvd., Suite 600
Melbourne, FL 32901
(321) 409-7500
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
| Andrew J. Beck, Esq. Daniel P. Raglan, Esq. Torys LLP 1114 Avenue of the Americas New York, NY 10036 (212) 880-6000 |
James P. Dietz Chief Financial Officer PetroAlgae Inc. 1901 S. Harbor City Blvd., Suite 600 Melbourne, FL 32901 (321) 409-7500 |
Robert E. Buckholz, Jr., Esq. Sullivan & Cromwell LLP 125 Broad Street New York, NY 10004 (212) 558-4000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||||
| Non-accelerated filer | x | (Do not check if a smaller reporting company) | Smaller reporting company | ¨ |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We and the selling stockholders may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting offers to buy these securities in any jurisdiction where such offer or sale is not permitted.
Subject to Completion. Dated December 1, 2011.
Shares

Common Stock
This is an initial public offering of shares of the common stock of PetroAlgae Inc.
PetroAlgae is offering of the shares to be sold in this offering. The selling stockholders identified in this prospectus are offering an additional shares in this offering. PetroAlgae will not receive any of the proceeds from the sale of the shares being sold by the selling stockholders.
Our common stock is currently quoted on the OTCQB under the symbol “PALG.” On November 30, 2011, the last reported sale price of our common stock was $5.50. It is currently estimated that the initial public offering price per share will be between $ and $ .
Application has been made for listing on under the symbol “ ”.
Investing in our common stock involves a high degree of risk. See “Risk Factors” on page 15 to read about factors you should consider before buying shares of our common stock.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discount |
$ | $ | ||||||
| Proceeds, before expenses, to PetroAlgae |
$ | $ | ||||||
| Proceeds, before expenses, to the Selling Stockholders |
$ | $ | ||||||
The underwriters have the option, exercisable until the date which is days following the closing of this offering, to purchase up to an additional shares at the initial public offering price less the underwriting discount.
The underwriters expect to deliver the shares against payment in New York, New York on , 2012.
| Goldman, Sachs & Co. | UBS Investment Bank | Citigroup |
| Baird | Cowen and Company |
Prospectus dated , 2012.
Table of Contents
| Page | ||||
| 1 | ||||
| 11 | ||||
| 13 | ||||
| 15 | ||||
| 30 | ||||
| 31 | ||||
| 32 | ||||
| 33 | ||||
| 34 | ||||
| 35 | ||||
| 37 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
38 | |||
| 60 | ||||
| 79 | ||||
| 85 | ||||
| 93 | ||||
| 97 | ||||
| 99 | ||||
| 105 | ||||
| MATERIAL U.S. FEDERAL INCOME AND ESTATE TAX CONSIDERATIONS FOR NON-U.S. HOLDERS OF OUR COMMON STOCK |
107 | |||
| 110 | ||||
| 116 | ||||
| 117 | ||||
| 118 | ||||
| F-1 | ||||
Neither we nor the selling stockholders have authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses we have prepared. Neither we nor the selling stockholders take any responsibility for, and can provide any assurance as to the reliability of, any other information that others may give you. This prospectus is not an offer to sell these securities and it is not soliciting offers to buy these securities in any jurisdiction where such offer or sale is not permitted. The information contained in this prospectus is current only as of its date. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States, neither we, the selling stockholder nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
i
Table of Contents
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider before deciding to invest in our common stock. Before investing in our common stock, you should carefully read this entire prospectus, including our audited consolidated financial statements and the related notes thereto and the information set forth under the sections “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in each case included in this prospectus. Unless otherwise stated, (1) we use the terms “PetroAlgae,” “company,” “we,” “us” and “our” in this prospectus to refer to PetroAlgae Inc. and its sole operating subsidiary, PA LLC, (2) we use the term “selling stockholders” in this prospectus to refer to , and (3) information presented in this prospectus assumes that the underwriters do not exercise their option to purchase additional shares.
Our Company
We provide renewable technology and solutions to address the global demand for new economical sources of feed, food and fuel.
Our objective is to be the leading global provider of technology and processes for the commercial production of micro-crop biomass. We have developed proprietary technology, which we believe will allow our customer licensees to grow aquatic micro-crops at accelerated rates for conversion into products for both agriculture and energy markets. We seek to license this renewable technology to meet the significant and growing demand in these markets in both emerging and developed economies. Furthermore, we expect that our solution will deliver strong and consistent economic returns to our customer licensees as a consequence of continuous, predictable, year-round operations without the need for government subsidies.
Our strategy is to license and provide management support for micro-crop production facilities in equatorial regions around the world. Our solution is scalable and flexible, which we believe will allow our customer licensees to expand in order to meet market demand. We intend to generate revenue from licensing fees and royalties primarily from customer licensees. Our licensing approach is designed to minimize our capital expenditures, because our customer licensees will be expected to invest in the development and construction of the production facilities.
Our proprietary technology uses indigenous micro-crops that are not genetically modified and demonstrate an optimal growth profile for a particular geography and environment. These micro-crops will then be grown, harvested and processed in a manner that we believe will optimize the production of our micro-crop biomass, which our customer licensees can use to produce three products:
| Ÿ | Lemna Protein Concentrate, or LPC: LPC is a free-flowing powder containing a minimum 65% crude protein. We expect that our customer licensees will manufacture LPC for use in both animal and, potentially, human markets. Based on internal and third-party testing, we believe that LPC is similar in quality to fish meal and represents a potentially viable protein source for feed formulations in multiple industries, including aquaculture and swine. We believe that LPC can also be used as an alternative to kelp meal in fertilizer applications. |
| Ÿ | Lemna Meal, or LM: LM is a carbohydrate-rich free-flowing powder containing a minimum 15% crude protein. We expect that our customer licensees will manufacture LM for use in animal feed markets. Based on internal and third-party testing, we believe that LM is similar in quality to alfalfa meal and represents a potentially viable feed ingredient for formulations in multiple industries, including dairy and swine. We also believe that LM could be used in fertilizer and animal bedding applications. |
1
Table of Contents
| Ÿ | Biocrude: With a small change in process parameters (but not equipment), our processing system can produce Biocrude rather than LM. Biocrude is a renewable energy feedstock that, through the use of a variety of third-party conversion systems currently under development, could potentially be converted into renewable fuels. |
In 2011, we entered into a license agreement with a customer licensee in South America. See “Business—Our Customer Licensees—AIQ”. We are currently negotiating additional agreements with prospective customer licensees, including, among others, state-owned enterprises and multinational food companies. We expect that the successful execution of early projects will help validate our technology and ultimately enhance our ability to enter into commercial-scale agreements with other prospective customer licensees.
Our Markets
Our current product strategy is to enable customer licensees to sell our LPC product in the global animal feed market as a fish meal alternative and our LM product in the global animal feed market as an alternative to alfalfa meal. We believe that focusing in the near-term on the deployment of our technology for the production of animal feed helps to reduce the market risks associated with our solution, given the relatively high-value, high demand and low complexity of our animal feed products. We also believe that LPC is well positioned as an alternative to kelp meal, commonly used in fertilizers for turf grasses, such as those on golf courses, and that LM could potentially be used as an ingredient in organic fertilizers and in animal bedding applications.
Our research also indicates that LPC has the potential to be used in human food applications. We have commissioned and will continue to commission third-party testing regarding human food applications and are seeking regulatory approvals. If successful, we believe that the human use of our products would significantly increase the value of LPC.
We also expect to benefit from the development of third-party technology that will facilitate the commercial production of renewable fuel from Biocrude. We expect these efforts to provide our customer licensees with the option to pursue multiple fuel options with our Biocrude product, including use as a renewable energy feedstock that can be converted into fuel products or burned directly as a combustion fuel. The process of developing our fuel technology is ongoing through a number of joint development and collaboration efforts with petrochemical companies, which we believe will accelerate our fuel product platform commercialization.
2
Table of Contents
Our Industry
The food, feed and fuel markets are global and significant in size and the demand in these markets is expected to grow rapidly. We believe that our products will meet a portion of that growing demand. We also believe that our technology will deliver high production yields, particularly in equatorial regions, in which we expect the demand for our feed and food substitutes will be strong, and will enable our customer licensees to address the needs of the following markets.
Animal Feed
| Ÿ | Fish Meal: Fish meal, most of which is produced by the commercial fishing of wild schools of small fish, is a critical ingredient in the diets of nursery animals and aquaculture stocks. The fish meal supply is currently limited due to the effects of overfishing, while the demand for fish meal as a source of protein in animal diets continues to rise sharply. Global fish meal demand for aquafeed is expected to reach approximately 7 million metric tonnes in 2012 and to rise every year thereafter to approximately 16 million metric tonnes in 2020, a rate which is expected to significantly outpace supply. As a consequence, there is a large and growing need for an alternative protein source to address this supply and demand imbalance. |
Based on research conducted by the University of Idaho, we believe that LPC is strongly positioned as a fish meal alternative due to its nutritive qualities. We expect that our ability to locate production near sources of demand will provide our customer licensees with several commercial advantages, including the potential to reduce freight and duty import expenses, along with the ability to offer continuous production and just-in-time inventory management. Based on these factors, we expect the market price of LPC to be comparable to fish meal.
| Ÿ | Alfalfa Meal: Alfalfa meal is a premium forage ingredient, used to meet nutrition and growth requirements in numerous animal diets. The supply of alfalfa is limited due to the concentration of production in specific areas, such as the United States and Europe. Meanwhile, demand is continuing to rise, particularly in Asia. For dairy and swine alone, the global demand for alfalfa meal will be approximately 254 million metric tonnes in 2012, rising every year thereafter to approximately 262 million metric tonnes in 2020. |
Trials conducted by the University of Minnesota demonstrated that LM is a high quality alternative for alfalfa meal in diets for dairy cattle. Third-party testing is continuing with other animals that are customarily fed alfalfa, such as swine and horses. Based on LM’s nutritional similarity with alfalfa meal, we expect that its market price will be comparable.
Fertilizer
Kelp and other high nitrogen biomass can be used as organic fertilizers. However, the production of kelp is costly due to the limited areas in which it grows in the wild, as well as environmental regulations that limit the scale of its harvesting. We believe that LPC can be a lower-cost, potentially higher-value alternative to kelp. We also believe that LM could be used as a nitrogen source in organic fertilizers. We believe that LPC will be competitive in the specialty turf fertilizer market given its favorable chemical composition and the opportunity it presents to reduce potential environmental pollution as compared to other alternatives.
3
Table of Contents
Fortification of Basic Human Food Products
We are developing our technology to address the rising demand for human food, a market of particular relevance in the equatorial regions in which we believe our technology will be well-suited to grow. We believe that LPC can eventually serve the global market for fortification of basic food ingredients for malnourished populations, particularly in developing and emerging countries. Given the rapidly growing populations in regions such as South Asia, Africa and South America, we believe that there is a clear need for an alternative supply of high quality and economical protein to supplement and enhance basic foods, such as breads, tortillas, noodles and crackers.
Specialty Markets for Prepared Foods
In the long term, we believe we can develop a higher protein content product from lemna using alternative separation techniques. This would allow our customer licensees to access specialty markets for prepared foods for human consumption, such as baked goods, meats and frozen foods. Third-party research is continuing to validate our results and enhance our understanding of these properties. While this process change would increase the capital expenditure and operating costs associated with our technology, we anticipate that the increased value of the end product produced would in many cases justify the additional expenses.
Renewable Fuels
We expect that third-party technology will lead to the conversion of Biocrude into drop-in fuels. If the technology is successfully developed in this manner, we anticipate that our customer licensees will have the ability to create products that address the significant and continuing global demand for renewable fuel.
Our Solution and Customer Licensee Approach
We believe that our solution will result in the commercial scale production of renewable products specifically for the feed, food and fuel industries. Our technology platform primarily consists of four components: micro-crop selection and testing, growth and harvesting techniques, processing technology, and control systems. Our technology includes the selection of indigenous micro-crops that demonstrate an optimal profile for a particular geography and environment, which we believe will allow our customer licensees to grow, harvest and process these micro-crops efficiently and profitably. We have developed a scalable and flexible model based on micro-crop growth units of 150 hectare increments. This model is designed to be attractive to a wide range of entities, including agricultural customers with an interest in the sustainable production of feed products, larger operators of renewable fuel production facilities and financial investment groups.
The estimated capital expenditure for a 150 hectare facility as an entry point is $12 million (excluding the cost of land and improvements)—a model that we believe involves manageable costs, risks and build-out times, while also enabling our customer licensees to evaluate the commercial potential of the technology on a larger scale. Due to economies of scale, we believe that a 600 hectare unit would deliver an attractive internal rate of return on our customer licensees’ investment. Ultimately, we expect that our customer licensees will expand their facilities in 150 hectare increments at similar costs in order to complete facilities of up to 5,000 hectares. Depending on economies of scale, we expect that the capital expenditure for a 5,000 hectare facility will be approximately $375 million (excluding the cost of land and improvements). We believe that a license unit of this size will enable our customer licensees to deliver significant volumes of product to market, thereby maximizing internal rates of return on their investments.
4
Table of Contents
Our business model requires minimal capital expenditures from us and involves the licensing of our technology and processes to customer licensees for fees and royalties during the life of the license. Our customer licensees will be required to pay the upfront infrastructure costs as well as continued maintenance costs for their licensed facilities.
Since our inception in 2006, we have focused on continuously developing our technology with the objective of enabling our customer licensees to produce renewable feed, food and fuel products at commercial scale. We believe research and development is an ongoing effort and we continue to look for ways to improve our technology and processes so that our customer licensees will be able to scale up our technology efficiently and profitably. We have successfully built and operated a demonstration facility (approximately one hectare) and have extracted small field-scale product quantities for technical validation. We believe that this process of scaling up our technology from the laboratory scale to demonstration scale will allow us to reach commercial scale with our initial customer licensees.
Our Competitive Strengths
Our key competitive strengths are:
| Ÿ | Strong economic returns to our customer licensees without the need for government subsidies. We believe that our solution will present our customer licensees with the opportunity to invest profitably in renewable technology designed to meet the significant and growing demand for feed, food and fuel. The profitability of our technology and processes is not dependent on government subsidies; although any subsidies that we or our customer licensees receive will enhance the profitability of our technology and processes. |
| Ÿ | Highly scalable and flexible technology, with initial license units providing the basis for larger-scale operations in the future. To facilitate rapid deployment opportunities in the near future, we have developed a highly scalable and flexible model for potential customer licensees. Our solution involves the construction and operation of production facilities based on 150 hectare increments of micro-crop growth bioreactors. We expect each 150 hectare unit will be a profitable module that can be replicated as the components of a larger-scale project of up to 5,000 hectares. |
| Ÿ | Fully integrated, comprehensive solution that transforms simple, naturally occurring inputs, such as sunlight, water and indigenous micro-crops, into valuable outputs. We will provide our customer licensees with a comprehensive system that contains all components necessary for the creation of renewable products at commercial scale, from selection and testing of micro-crops to growth, harvesting and processing. In addition to a fully integrated technology platform, we expect to provide our customer licensees with extensive facility planning, design and construction/operation management services. |
| Ÿ | Product compatibility with the existing agriculture infrastructure. Our processes for producing LPC and LM are designed to be compatible with standard processing techniques that can be implemented using existing agricultural equipment and infrastructure. We expect both products to become direct substitutes for existing protein supplies in animal feed applications. Our technology can potentially provide a new source of supply where existing demand or expected growth in demand cannot be met solely by existing sources due to environmental and other constraints. |
| Ÿ | First mover advantage in providing renewable micro-crop technology to produce products that serve the global animal feed market. We believe that the deployment of our solution will place our customer licensees in a position to enter the global animal feed market as a first-mover in producing animal feed from renewable micro-crops. |
5
Table of Contents
| Ÿ | Experienced management team with a track record of innovative growth, value creation and commercial scaling of businesses. Our senior management team has broad expertise in guiding early-stage development companies to commercialization. Our management team has been involved in various technical and management roles within leading organizations across various industries. |
Our Business Strategy
Building on our competitive strengths, our objective is to become the leading global provider of technology and processes for the commercial production of micro-crop biomass for the agriculture and energy markets. Key elements of our strategy include:
| Ÿ | Rapid deployment and support of modular and highly scalable license units. Our customer licensees will be able to assemble our proprietary units from modular, cost-effective bioreactors and other ancillary equipment, which are currently operating at a smaller scale at our fully operational demonstration facility. These modular bioreactors are designed to enable rapid deployment at predictable cost and productivity levels. |
| Ÿ | Leveraging our capital-light licensing model. Our customer licensees will be required to pay the upfront infrastructure costs as well as continued maintenance costs for their licensed facilities. This business model allows us to make minimal capital expenditures while generating fees and royalties over the term of the license. |
| Ÿ | Expansion of our customer base on a global scale. We are continuing to expand our marketing efforts internationally. In 2011, we expanded our operations in China to further our focus in the region. We also recently expanded our sales and marketing efforts in both Africa and South America, which represent key locations where we expect to license our technology. In addition, we are establishing relationships and seeking opportunities with investment funds. |
| Ÿ | Further development of our technology and pursuit of additional applications for our products. We expect to further enhance our technology in order to provide our customer licensees with additional end market applications for our products along with the ability to capture higher economic returns. We expect these efforts to provide our customer licensees with access to markets for human food and renewable fuels. |
| Ÿ | Assisting our customer licensees in the identification of off-take partners. We will continue academic and commercial testing of our products to confirm their market viability. This third-party validation will assist us and our customer licensees in establishing off-take arrangements in which companies will purchase products produced by our customer licensees. |
| Ÿ | Creation of brand loyalty. We intend to become recognized as the leading global provider of technology for the commercial production of micro-crop biomass for agriculture and energy markets. We intend to become a recognized provider of green technology solutions. |
6
Table of Contents
Our Structure
Background
PetroAlgae Inc. is a holding company whose sole asset is its controlling equity interest in its operating subsidiary, PA LLC. We operate and control the business and affairs, and consolidate the financial results, of PA LLC. As of , we held % of the equity interests in PA LLC, with the remainder held by Valens-Calliope (as defined under “—The Exchange Transactions”) and certain of our employees.
PA LLC was originally founded in September 2006 by XL TechGroup, Inc., or XL Tech, as a company focused on developing technologies for the renewable energy market. In August 2008, XL Tech exchanged certain of its assets, including the equity it held in PA LLC, for the outstanding debt of XL Tech that was held by the Laurus-Valens Funds (as defined under “Our Principal Stockholders”). Subsequent to that exchange, the Laurus-Valens Funds transferred the equity they received and certain related debt to PetroTech Holdings Corp., or PetroTech, a holding company controlled by the Laurus-Valens Funds. In December 2008, PetroTech acquired a shell company that was quoted on the OTC Bulletin Board, or the OTCBB, and assigned its interest in PA LLC to this shell company, which it then renamed PetroAlgae Inc.
Our Principal Stockholders
Our principal stockholders consist of private investment entities that are managed by Laurus Capital Management, LLC, or LCM, and Valens Capital Management, LLC, or VCM. We refer to these private investment entities as the Laurus-Valens Funds. At the completion of this offering, the Laurus-Valens Funds will own directly or indirectly approximately % of our common stock (or % if the underwriters exercise their option in full) and approximately % of the equity interests in PA LLC. The Laurus-Valens Funds have invested in micro-cap and small private and publicly traded companies since 2001. For more information about our principal stockholders, see “Principal and Selling Stockholders.”
7
Table of Contents
Governance Matters
Prior to the completion of this offering, we will enter into a stockholders agreement with our principal stockholders, or the stockholders agreement. The stockholders agreement will become effective upon completion of this offering. The stockholders agreement will contain a voting agreement that provides, among other things, that:
| • | our principal stockholders will agree to vote any shares of our common stock they and their affiliates own pro rata with our unaffiliated stockholders (other than in connection with any vote relating to (i) a consolidation or merger of the company or any of its affiliates into or with any other person (other than affiliates of our principal stockholders); or (ii) a sale, lease or transfer of all or substantially all of our assets or capital stock to another person (other than affiliates of our principal stockholders)); and |
| • | our principal stockholders will not vote in favor of the removal of any of our independent directors unless our unaffiliated stockholders approve such removal. |
For more information regarding our governance, see “Relationships and Related Party Transactions — Stockholders Agreement.”
The Exchange Transactions
Prior to the acquisition of the shell company, our employees were compensated in part with equity incentives consisting of units in PA LLC. Since that acquisition, our employees have received equity incentives in PetroAlgae Inc. In order to provide liquidity for their equity interests in PA LLC and to facilitate the exchange of all equity interests in PA LLC, we have committed to the following:
| Ÿ | We will enter into an exchange agreement with certain of our employees, Valens U.S. SPV I, LLC, or Valens U.S., and Calliope Capital Corporation, or CCC, which, together with Valens U.S., we refer to as Valens-Calliope. Each of these entities and employees will also be a selling stockholder in this offering. The exchange agreement will give those employees and Valens-Calliope the right to exchange a portion of their units in PA LLC for shares of PetroAlgae Inc. common stock that they will then sell in this offering, subject to customary conversion rate adjustments for stock splits, stock dividends and reclassifications. |
| Ÿ | We will set aside $ from this offering to redeem up to units of PA LLC held by certain of our employees who are not party to the exchange agreement. |
| Ÿ | No sooner than months after the completion of this offering, we will file a registration statement that will cover the exchange from time to time of the aggregate number of shares of PetroAlgae Inc. common stock that will be issuable in exchange for the remaining units in PA LLC held by Valens-Calliope and our employees. |
We have reserved million shares of common stock for issuance to cover all exchanges of PA LLC units for shares of our common stock by our employees. We have reserved a further million shares of common stock for issuance to cover all such exchanges by Valens-Calliope.
8
Table of Contents
Our Organizational Structure
The following diagram depicts our organizational structure immediately following the consummation of this offering:
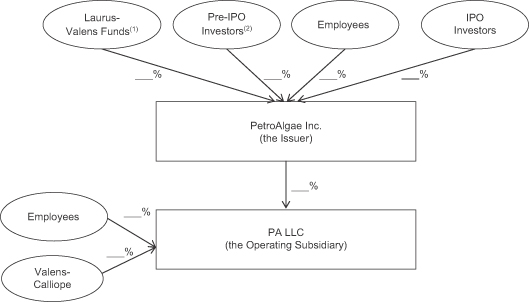
| (1) | The Laurus-Valens Funds hold their interests in PetroAlgae Inc. through PetroTech. |
| (2) | The Pre-IPO Investors consist of acquirors of our common stock pursuant to private placements, over-the-counter trading or the 2008 shell company transaction and which are not affiliated with the Laurus-Valens Funds. |
The following diagram depicts our organizational structure following the consummation of this offering and assuming that all of the equity interests of PA LLC are exchanged as described in “—The Exchange Transactions”:
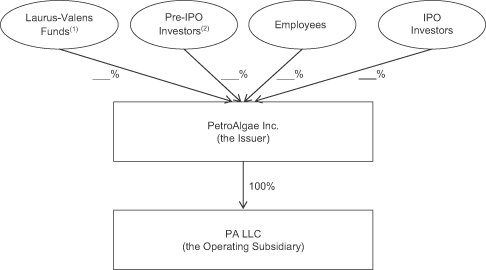
| (1) | The Laurus-Valens Funds hold their interests in PetroAlgae Inc. through PetroTech. |
| (2) | The Pre-IPO Investors consist of acquirors of our common stock pursuant to private placements, over-the-counter trading or the 2008 shell company transaction and which are not affiliated with the Laurus-Valens Funds. |
9
Table of Contents
Risk Factors
Our business is subject to numerous risks, as more fully described in the section entitled “Risk Factors” beginning on page 15 and elsewhere in this prospectus. These risks could materially and adversely impact our business, financial condition, operating results and cash flow, which could cause the trading price of our common stock to decline and could result in a partial or total loss of your investment. You should carefully consider such risks before deciding to invest in our common stock.
Our Corporate Information
PetroAlgae Inc. became a Delaware corporation on August 12, 2008 upon a change of domicile by its predecessor, a California corporation originally incorporated on April 15, 1988. Our principal executive office is located at 1901 S. Harbor City Blvd., Suite 600, Melbourne, FL 32901 and our telephone number is (321) 409-7500. Our website address is www.petroalgae.com. The information contained on, or that can be accessed through, our website is not part of, and is not incorporated into, this prospectus. We are currently a reporting company under Section 13 of the Securities Exchange Act of 1934, as amended, or the Exchange Act. “PetroAlgae”, the “PetroAlgae logo” and other trademarks or service marks of PetroAlgae Inc. appearing in this prospectus are the property of PetroAlgae Inc. This prospectus contains additional trade names, trademarks and service marks of others, which are the property of their respective owners and the inclusion of such trade names, trademarks or service marks in this prospectus does not signify a relationship with, or endorsement or sponsorship of us by, these other companies.
Industry and Market Data
Market data and industry statistics and forecasts used throughout this prospectus are based on independent industry publications, reports by market research firms and other published independent sources. Some data and other information are also based on our good faith estimates, which are derived from our review of internal surveys and independent sources. Certain information contained in this prospectus is based on results of studies conducted by the University of Idaho, the University of Minnesota, Purdue University, Kansas State University and Craft Technologies. We commissioned each of the aforementioned studies. Furthermore, to our knowledge, each of the studies cited in this prospectus represent the most recent information with respect to the subject matter of such study.
10
Table of Contents
| Common stock offered by us |
shares. |
| Common stock offered by the selling stockholders |
shares. |
| Common stock to be outstanding immediately after this offering(1) |
shares. |
| Common stock to be outstanding immediately after this offering, as adjusted(2) |
shares. |
| Voting rights |
Each share of our common stock entitles its holder to one vote on all matters to be voted on by stockholders generally. See “Description of Capital Stock—Common Stock.” |
| Use of proceeds |
We estimate that the net proceeds to us from this offering will be approximately $ (based on the midpoint of the range listed on the cover page of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us). |
| We will set aside $ of the net proceeds to redeem up to units of PA LLC held by certain of our employees who are not otherwise selling in this offering. We intend to use the remainder of the net proceeds from this offering for working capital and other general corporate purposes. |
| We will not receive any of the proceeds from the sale of shares by the selling stockholders. See “Use of Proceeds.” |
| Risk factors |
See “Risk Factors” and other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in shares of our common stock. |
| OTCQB symbol |
“PALG”. |
| Proposed symbol |
“ ”. |
| (1) | The number of shares of our common stock that will be outstanding immediately after this offering is based on shares of PetroAlgae common stock outstanding as of and excludes: |
| Ÿ | shares of our common stock issuable upon the exercise of options outstanding at a weighted average exercise price of $ per share; |
| Ÿ | shares of our common stock issuable upon the exercise of warrants outstanding at a weighted average exercise price of $ per share; |
11
Table of Contents
| Ÿ | shares of our common stock reserved for issuance under the 2009 Equity Compensation Plan; and |
| Ÿ | any exercise by the underwriters of their right to purchase up to an additional shares of our common stock from . |
| (2) | The number of shares of our common stock that will be outstanding immediately after this offering, as adjusted, is based on shares of PetroAlgae common stock outstanding as of and includes: |
| Ÿ | shares of our common stock offered by us in this offering; |
| Ÿ | shares of our common stock reserved for issuance to cover exchanges by our employees of their PA LLC units for common stock of PetroAlgae Inc.; and |
| Ÿ | shares of our common stock reserved for issuance to cover exchanges by Valens-Calliope of their PA LLC units for common stock of PetroAlgae Inc. |
and excludes:
| Ÿ | shares of our common stock issuable upon the exercise of options outstanding at at a weighted average exercise price of $ per share; |
| Ÿ | shares of our common stock issuable upon the exercise of warrants outstanding at at a weighted average exercise price of $ per share; |
| Ÿ | shares of our common stock reserved for issuance under the 2009 Equity Compensation Plan; and |
| Ÿ | any exercise by the underwriters of their right to purchase up to an additional shares of our common stock from . |
12
Table of Contents
SUMMARY CONSOLIDATED FINANCIAL DATA
The following summary consolidated financial data for the years ended December 31, 2007, 2008, 2009 and 2010 are derived from our audited consolidated financial statements. The summary audited consolidated financial data for the period beginning September 22, 2006 and ending December 31, 2010 is derived from our audited consolidated financial statements for such period that are included elsewhere in this prospectus. The summary unaudited consolidated interim financial data for the nine months ended September 30, 2010 and 2011 and as of September 30, 2011 are derived from our unaudited consolidated interim financial statements for such periods and dates that are included elsewhere in this prospectus. The unaudited consolidated interim financial statements were prepared on a basis consistent with our audited consolidated financial statements and include, in the opinion of management, all adjustments, consisting only of normal recurring adjustments, necessary for the fair presentation of the financial information contained in those statements. The historical results presented below are not necessarily indicative of financial results to be achieved in future periods. Prospective investors should read these summary consolidated financial data together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes included elsewhere in this prospectus.
| For the
Period From September 22, 2006 (Inception) Through December 31, |
(Unaudited) Nine Months Ended September 30, |
Pro Forma(1) | ||||||||||||||||||||||||||||||||||
| Years Ended December 31, | (Unaudited) Year Ended December 31, |
(Unaudited) Nine Months Ended September 30, |
||||||||||||||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2010 | 2010 | 2011 | 2010 | 2011 | ||||||||||||||||||||||||||||
| (in thousands, except for share and per share data) |
||||||||||||||||||||||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||||||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||
| Costs and expenses: |
||||||||||||||||||||||||||||||||||||
| Selling, general and administrative |
2,984 | 8,251 | 17,401 | 21,850 | 50,666 | 9,447 | 8,074 | 21,850 | 8,074 | |||||||||||||||||||||||||||
| Research and development |
4,863 | 9,866 | 15,085 | 17,424 | 48,469 | 11,184 | 8,463 | 17,424 | 8,463 | |||||||||||||||||||||||||||
| Interest expense—related party |
44 | 1,764 | 3,198 | 4,077 | 9,483 | 2,912 | 4,646 | 2,860 | 3,736 | |||||||||||||||||||||||||||
| Depreciation |
55 | 269 | 1,138 | 1,128 | 2,591 | 773 | 669 | 1,128 | 669 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total costs and expenses |
8,346 | 20,150 | 36,822 | 44,479 | 111,209 | 24,316 | 21,852 | 43,262 | 20,942 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Net loss before non-controlling interest |
(8,346 | ) | (20,150 | ) | (36,822 | ) | (44,479 | ) | (111,209 | ) | (24,316 | ) | (21,852 | ) | (43,262 | ) | (20,942 | ) | ||||||||||||||||||
| Non-controlling interest |
— | 162 | 6,554 | 6,469 | 13,186 | 3,842 | 2,797 | 6,285 | 2,681 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Net loss attributable to PetroAlgae Inc. |
$ | (8,346 | ) | $ | (19,988 | ) | $ | (30,268 | ) | $ | (38,010 | ) | $ | (98,023 | ) | $ | (20,474 | ) | $ | (19,055 | ) | $ | (36,977 | ) | $ | (18,261 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Basic and diluted common shares outstanding |
100,000,000 | 100,362,021 | 104,866,065 | 106,731,469 | 106,664,896 | 106,920,730 | 108,719,586 | 108,908,847 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Basic and diluted loss per share |
$ | (0.08 | ) | $ | (0.20 | ) | $ | (0.29 | ) | $ | (0.36 | ) | $ | (0.19 | ) | $ | (0.18 | ) | $ | (0.34 | ) | $ | (0.17 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
13
Table of Contents
| As of September 30, 2011 | ||||||||||||
| Actual | Pro Forma(1) |
Pro Forma As Adjusted(1)(2) |
||||||||||
| (Unaudited) | ||||||||||||
| (in thousands) | ||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||
| Cash |
$ | 6,715 | $ | 6,715 | $ | |||||||
| Property and equipment, net |
1,358 | 1,358 | ||||||||||
| Total assets |
8,324 | 8,324 | ||||||||||
| Current liabilities (excluding notes payable) |
12,232 | 9,310 | ||||||||||
| Notes payable—related party (current) |
72,053 | 62,053 | ||||||||||
| Total stockholders’ equity (deficit) |
(75,993 | ) | (63,071 | ) | ||||||||
| (1) | On a pro forma basis to give effect to the conversion (as of January 1, 2010 for statements of operations data and as of September 30, 2011 for balance sheet data) of all of our outstanding convertible debt of $10 million and accrued interest into common shares at a conversion price of $5.43 per share. |
| Ÿ | On the consolidated statements of operations: |
| Ÿ | $1.2 million and $0.9 million of interest expense was reversed for the year ended December 31, 2010 and the nine months ended September 30, 2011, respectively; and |
| Ÿ | net loss attributable to non-controlling interest of $0.2 million and $0.1 million was reversed for the year ended December 31, 2010 and the nine months ended September 30, 2011, respectively. |
| Ÿ | On the consolidated balance sheet: |
| Ÿ | the sum of accrued interest and notes payable – related party of $12.9 million was reclassified to reduce total stockholders’ deficit as of September 30, 2011. |
| (2) | On a pro forma as adjusted basis to give further effect to: |
| Ÿ | the sale by us of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the range listed on the cover page of this prospectus, and our receipt of the estimated net proceeds from that sale after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us; |
| Ÿ | the exchange of million shares of common stock reserved for issuance prior to the closing of this offering and that are issuable upon the exchange of units of PA LLC for common shares of PetroAlgae; and |
| Ÿ | our use of approximately $ million of the net proceeds of this offering to redeem certain of the equity units of PA LLC held by our employees. |
14
Table of Contents
Investing in our common stock involves a high degree of risk. You should carefully consider the following risk factors, as well as the other information in this prospectus, before deciding whether to invest in our common stock. Our business, prospects, financial condition or operating results could be materially adversely affected by any of these risks. The trading price of our common stock could decline as a result of any of these risks, and you could lose part or all of your investment in our common stock. When deciding whether to invest in our common stock, you should also refer to the other information in this prospectus, including our consolidated financial statements and related notes and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this prospectus. You should read the section entitled “Special Note Regarding Forward-Looking Statements” immediately following these risk factors for a discussion of what types of statements are forward-looking statements, as well as the significance of such statements in the context of this prospectus.
Risks Related to Our Business
We have a limited operating history.
We are a development stage company. As a result, we have no significant history of revenues, operating or net income, cash flows or the other financial performance metrics that will affect the future market price of our common stock. Any investment you make in our common stock will necessarily be speculative and based largely on your own views as to the future prospects for our business. If we are unable to generate sufficient revenues or profits, we may be unable to continue operating and you may lose your entire investment in our common stock.
In addition, because of this limited operating history, investors and research analysts could have more difficulty valuing our common stock, which could lead to significant variability in the value ascribed to our common stock by market participants. This, in turn, could lead to a high degree of volatility in the market price of our common stock, which could have adverse effects on our investors and on our ability to conduct financing activities, determine dividend policy and price any stock options we grant for compensation or other purposes.
We have a history of operating losses and our auditors have indicated that there is a substantial doubt about our ability to continue as a going concern.
To date, we have not been profitable and have incurred significant losses and cash flow deficits. For the fiscal years ended December 31, 2010, 2009, and 2008, we reported net losses before non-controlling interest of $44.5 million, $36.8 million, $20.2 million, respectively, and negative cash flow from operating activities of $22.8 million, $27.6 million, and $11.8 million, respectively. For the nine months ended September 30, 2011, we reported net losses of $19.1 million and negative cash flow from operating activities of $13.4 million. As of September 30, 2011, we had an aggregate accumulated deficit of $117.1 million. We anticipate that we will continue to report losses and negative cash flow for the next several years.
As a result of these net losses and cash flow deficits and other factors, our independent auditors issued an audit opinion with respect to our consolidated financial statements for the three years ended December 31, 2010 that indicated that there is a substantial doubt about our ability to continue as a going concern. Our financial statements do not include any adjustments that might result from the outcome of this uncertainty. These adjustments would likely include substantial impairment of the carrying amount of our assets and potential contingent liabilities that may arise if we are unable to fulfill various operational commitments. In addition, the value of our securities, including common stock issued in this offering, would be greatly impaired. Our ability to continue as a going concern is
15
Table of Contents
dependent upon generating sufficient cash flow from operations and obtaining additional capital and financing, including funds to be raised in this offering. If our ability to generate cash flow from operations is delayed or reduced and we are unable to raise additional funding from other sources, we may be unable to continue in business even if this offering is successful. For further discussion about our ability to continue as a going concern and our plan for future liquidity, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Ability to Continue as a Going Concern.”
We expect to rely on a small number of early-adopter customer licensees for substantially all of our revenue during our next several years of operation.
We expect to be reliant on a small number of customer licensees for substantially all of our revenue. During the next several years, our business will depend on the successful operation of the license units to which our initial contracts will relate.
Our initial contract is, and we expect that our additional early contracts will be, subject to significant conditionality. These contracts may be terminated by customer licensees if these conditions are not met. See “—Our initial contracts will be conditioned on pilot-scale facilities built by our customer licensees meeting certain levels of productivity, and the failure to meet those levels may lead to the termination of these contracts, which will materially adversely affect our ability to generate revenue.” In addition, these contracts are generally subject to termination in the event that we fail to satisfy our obligations under such contracts. Although we believe that we have satisfied, and intend to continue to satisfy, these obligations, there can be no assurance that we will be able to continue to do so in the future.
Furthermore, there is no assurance that our customer licensees will be able to successfully commercialize and distribute LPC, LM or Biocrude created using our technology. If our customer licensees are unable to sell the products that they produce with our technology, they may seek to terminate our contracts or renegotiate the terms. If any of these contracts are terminated or renegotiated on less favorable terms, if contracts are terminated for failure to meet conditions or if any of our customer licensees fail to meet their payment obligations to us, our ability to generate revenues will be materially adversely affected, and this could have a material adverse effect on our business, financial condition and results of operations.
Our dependence on certain turnkey contracts subjects us to certain legal and financial terms that could adversely affect us.
We expect that some of the contracts with our initial early-adopter customer licensees will provide for the build-out of a turnkey license unit of 150 hectares after the successful completion of the testing phase of our technology. This means that we will direct the general contractor of such units and we will be required to deliver the unit at a fixed cost. We will receive payment for the build-out of the license unit on a progress basis as the license unit is constructed. The difference in the amounts and timing of our payments to contractors and suppliers relative to payments we receive from our customer licensees exposes us to liquidity risk and could leave us vulnerable in the event we encounter challenges in building the facility or bringing it online, delays in achieving commercial viability with our production process, disputes with our customer licensees or other unanticipated events that may occur prior to the time we receive payment from our customer licensees. Because our customer licensees’ payments will be capped, we will bear the responsibility for construction costs in excess of those anticipated, which could cause us to suffer significant losses on these license units.
16
Table of Contents
Our success depends on future royalties we expect will be paid to us by our customer licensees, and we face the risks inherent in a royalty-based business model.
We are a licensing business without a substantial amount of tangible assets, and our long-term success depends on future royalties paid to us by customer licensees. The amount of royalty payments we expect to receive will be primarily based upon the revenues generated by our customer licensees’ operations, so we will be dependent on the successful operations of our customer licensees for a significant portion of our revenues. Furthermore, we expect that the terms of some of our early-adopter customer license contracts will provide for royalty payments in lieu of license fees, which will cause us to recognize revenue from these contracts only once the license units have been built and are operational and the end products are being sold by the customer licensee. We face risks inherent in a royalty-based business model, many of which are outside of our control, including those arising from our reliance on the management and operating capabilities of our customer licensees and the cyclicality of supply and demand for end-products produced using our technology. Should our customer licensees fail to achieve sufficient profitability in their operations, our royalty payments would be diminished and our business, financial condition and results of operations could be materially adversely affected.
Our initial contracts are or will be subject to significant conditionality, including that the pilot-scale facilities built by our customer licensees achieve certain minimum levels of projected investment return, and the failure to meet these conditions may lead to the termination of these contracts.
Our initial contract is, and we expect that our additional early contract will be, subject to significant conditionality. For example, our initial contract may be terminated if our customer licensee deems the performance of its pilot-scale facility to be unsatisfactory. See “Business—Our Customer Licensees—AIQ.” In the contracts that we are currently negotiating, our prospective customer licensees will be permitted to terminate their agreements if projected investment returns, based upon the performance of their pilot-scale facilities and other factors, do not meet minimum investment return requirements. We generally will not receive any revenues under these contracts unless such returns are met during the test periods prescribed in each such contract. We cannot provide any assurances that any or all of our customer licensees’ pilot-scale facilities will meet the minimum investment returns required under these contracts. If a pilot-scale facility does not meet the minimum investment return requirements, the relevant contract may be terminated by the customer licensee, which in turn could have a material adverse effect on our business, financial condition and results of operations and our ability to generate revenue.
Our proposed licensing terms may not be accepted by prospective customer licensees and, even if accepted, there can be no assurance that customer licensees will meet their payment obligations under these licensing terms.
Prospective customer licensees may not accept our planned license agreement model. For example, prospective customer licensees may be unwilling to pay the proposed licensing fees prior to or during the construction of our micro-crop production system or prior to full commercial deployment of the system. We expect that our initial contracts will generally be royalty-based and will not involve any significant payment of license fees, and there can be no assurance that future contracts will involve significant license fees. Therefore, even if we are successful in attracting new customer licensees for our technology, we may not realize revenue from such customer licensees in the form or time frame we currently anticipate. Furthermore, there is no guarantee that customer licensees will make payments in the time frame to which they agree. If prospective customer licensees are unwilling or unable to pay as anticipated, the timing of our revenues and incoming cash flows could be reduced or delayed, which could have a material adverse effect on our business, financial condition and results of operations.
17
Table of Contents
We may be unable to acquire and retain customer licensees.
Our main source of revenue will be in the form of licensing fees and royalties from our customer licensees. Therefore, obtaining a significant number of customer licensees is critical for our growth and operation. Because our technology has not previously been deployed in the marketplace, it is uncertain whether it will be accepted by prospective customer licensees and there is a risk that we will be unable to acquire and retain licensees due to a number of factors, including our proposed licensing fee and royalty structure, capital expenditure requirements or questions surrounding commercial feasibility of our technology. For example, the projected investment returns of our micro-crop production system and the processes and methodologies used to operate the system may not yield a result that is commercially attractive to prospective customer licensees (compared to competitive products or otherwise). Our micro-crop production system requires our customer licensees to incur large fixed capital costs, which could render the system cost prohibitive for prospective customer licensees. A failure to secure customer licensees in a timely manner could have a material adverse effect on our business, financial condition and results of operations.
We have yet to construct our technology on a commercial scale, and may be unable to solve technical and engineering challenges that would prevent our technology from being economically attractive to prospective customer licensees.
Although we have successfully built a fully operational demonstration facility (approximately one hectare) and have extracted small field-scale quantities of LPC, LM and Biocrude for technical validation, we have not demonstrated that our technology is viable on a commercial scale, which we define to mean an operation consisting of at least 150 hectares. All of the tests conducted to date by us with respect to our technologies have been performed on limited quantities of micro-crops, and we cannot provide any assurances that the same or similar results could be obtained at competitive cost on a large-scale commercial basis. We have never utilized this technology under the conditions or in the volumes that will be required to be profitable and cannot predict all of the difficulties that may arise. While our bioreactors are modular in design, a single commercial-scale facility would be substantially larger than our demonstration facility, and the implementation of our technology on that scale will require further research, development, design and testing. Such an undertaking involves significant uncertainties, and consequently the construction of a commercial-scale facility may face delays, failures or unexpected costs. Furthermore, we may not be able to successfully design a scalable, cost-effective system for the growth and harvesting of micro-crops.
In addition, once a customer licensee begins to scale up and replicate such a system, unforeseen factors and issues may arise which make any such system uneconomical, thus causing additional delays or outright failure. The production of LPC and LM from micro-crops involves complex outdoor aquatic systems with inherent risks, including weather, disease, and contamination. As a result, the operations of our customer licensees may be adversely affected from time to time by climatic conditions, such as severe storms, flooding, dry spells and changes in air and water temperature or salinity, and may also be adversely affected by pollution and disease. Any of these or other factors could cause production at commercial-scale operations to be significantly below those we project based on results we have achieved at our demonstration facility. Because we expect to rely on license fees and royalties for our revenues, any operational difficulties experienced by our customer licensees would have an adverse effect on our revenues and profitability, and such effects could be material.
If we encounter significant engineering or other obstacles in implementing our technology at commercial scale, or if our customer licensees experience significant operational difficulties, our business, financial condition, and results of operations could be materially adversely affected.
18
Table of Contents
The market may not accept the end-products produced by our technology.
The biomass produced by our micro-crop production system produces several end-products: principally LPC, LM and Biocrude. The commercial viability of our technology largely depends on the ability of our customer licensees to sell these end-products into existing markets.
It has yet to be proven that potential buyers for the specific end-products produced by our technology, such as feed companies, would ultimately purchase the end-products produced by our customer licensees. For example, market acceptance of our LPC and LM will be a function, among others, of digestibility and palatability (the assessment of which will require additional laboratory and field study). Should these studies not proceed favorably, the market for our LPC and LM may not materialize or could be materially diminished. Failure to establish such a market or any significant reduction in a customer licensee’s profitability would decrease the amount of any royalties we would otherwise receive and may result in their failing to pay us expected licensing fees. Any of these events may have a material adverse effect on our business, financial condition and results of operations.
The revenue and returns we believe our customer licensees will realize from our technology will be highly dependent on the market price of the end-products produced using our technology.
The economic viability of our technology depends on the ability of our customer licensees to generate sufficient revenues from the sale of LPC, LM and other potential end-products of our technology. These revenues, and the internal rates of return our customer licensees can expect from their investment in our technology, are sensitive to the market prices of these products. Should our customer licensees be unable to obtain an adequate price for these products, their profits would be lower than expected and they might incur losses.
If our customer licensees cannot operate at a reasonable level of profit or incur losses, they might cease operations, which would result in our losing license fees and royalty payments. In such a circumstance, it is also likely that we would face significant difficulties attracting new customer licensees. Either or both outcomes would likely have a materially adverse effect on our business, financial condition and results of operations.
The end-products produced by our technology are subject to industry and regulatory testing.
The end-products produced by our technology generally require industry or regulatory testing in the countries in which they are produced or sold. For example, LPC requires regulatory approvals before it can be used in animal feed or for human consumption. To date, we have obtained approval for the use of LPC in animal feed only in Indonesia. Regulatory approvals in other jurisdictions will be essential to the commercial viability of our technology. In addition, we have not yet submitted LPC’s application for human consumption for regulatory approval in any jurisdiction. Such approvals could take several years or longer to obtain, if they can be obtained at all. See “Business—Regulatory.”
Should the end-products produced by our technology fail to meet industry or regulatory requirements, there would be an adverse effect on the ability of our customer licensees to market the products of their micro-crop operations, which would adversely affect their profitability. Since we expect to rely on licensing fees and royalties for the substantial majority of our revenues, such events would also materially adversely affect our business, financial condition and results of operations. See “Business—Regulatory.”
19
Table of Contents
The assumptions underlying the projected revenues and profitability of our license units that we develop in conjunction with our customer licensees are inherently uncertain and are subject to significant business, economic, financial, regulatory and competitive risks and uncertainties that could cause our customer licensees’ actual results to differ materially from those projected.
The projected revenues and returns on investment of our license units that we develop in conjunction with our customer licensees are based on assumptions relating to construction costs and other capital costs associated with building a unit, operating expenses and productivity, end-product price ranges and numerous other assumptions, all of which are inherently uncertain and are subject to significant business, economic, meteorological, regulatory and competitive risks and uncertainties that could cause actual results to differ materially from those projected. If our license units do not achieve the projected results, our customer licensees may not generate revenues or profits sufficient for them to continue operating license units or the revenues that we receive from such license units may be significantly less than we anticipate. Any of the foregoing could have a material adverse effect on our business, financial condition and results of operations.
If a competitor were to achieve a technological breakthrough, our financial condition, results of operations and business could be negatively impacted.
There currently exist a number of businesses that are pursuing the use of algae, bacteria and other micro-crops and other methods for creating biomass, food and feed products, and alternative fuels. Should a competitor achieve a research and development, technological or biological breakthrough with significantly lower production costs, or if the costs of similar competing products were to fall substantially, we may have difficulty attracting customer licensees. In addition, competition from other technologies considered “green technologies” could decrease the demand for the end-products produced by our technology. Furthermore, competitors may have access to larger resources (capital or otherwise) that provide them with an advantage in the marketplace, which could result in a negative impact on our business, financial condition and results of operations.
Any competing technology that produces biomass of similar quality and on a more efficient cost basis than ours could render our technology obsolete. In addition, because we do not have any issued patents, we may not be able to preclude development of even directly competing technologies using the same methods, materials and procedures as we use to achieve our results. Any of these competitive forces may inhibit or materially adversely affect our ability to attract and retain customer licensees, or to obtain royalties or other fees from our customer licensees. This could have a material adverse effect on our financial condition, results of operations and business.
If we fail to establish, maintain and enforce intellectual property rights with respect to our technology, our financial condition, results of operations and business could be negatively impacted.
Our ability to establish, maintain and enforce intellectual property rights with respect to the technology that we license to our customer licensees will be a significant factor in determining our future financial and operating performance. We seek to protect our intellectual property rights by relying on a combination of patent, trade secret and copyright laws. We also use confidentiality and other provisions in our agreements that restrict access to and disclosure of our confidential know-how and trade secrets.
We have filed patent applications with respect to many aspects of our technologies, including those relating to our bioreactors, growth management systems and downstream processing technologies. However, we cannot provide any assurance that any of these applications will ultimately
20
Table of Contents
result in issued patents or, if patents are issued, that they will provide sufficient protections for our technology against competitors. See “—Although we have filed patent applications for some of our core technologies, we do not currently hold any issued patents and we may face delays and difficulties in obtaining these patents, or we may not be able to obtain such patents at all.”
Outside of these patent applications, we seek to protect our technology as trade secrets and technical know-how. However, trade secrets and technical know-how are difficult to maintain and do not provide the same legal protections provided by patents. In particular, only patents will allow us to prohibit others from using independently developed technology that is similar. If competitors develop knowledge substantially equivalent or superior to our trade secrets and technical know-how, or gain access to our knowledge through other means such as observation of our technology that embody trade secrets at customer sites which we do not control, the value of our trade secrets and technical know-how would be diminished.
While we strive to maintain systems and procedures to protect the confidentiality and security of our trade secrets and technical know-how, these systems and procedures may fail to provide an adequate degree of protection. For example, although we generally enter into agreements with our employees, consultants, advisors, customer licensees and strategic partners restricting the disclosure and use of trade secrets, technical know-how and confidential information, we cannot provide any assurance that these agreements will be sufficient to prevent unauthorized use or disclosure. In addition, some of the technology deployed at our customer sites which we do not control, may be readily observable by third parties who are not under contractual obligations of non-disclosure, which may limit or compromise our ability to continue to protect such technology as a trade secret.
Although we seek to secure intellectual property rights with respect to our technology where such rights are available, certain aspects of our technology cannot be protected as intellectual property. For example, unlike certain other companies operating in the alternative energy or biofuel industry, our technology does not involve the creation or use of manufactured or genetically engineered organisms. The micro-crops that our processes ultimately convert to LPC, LM and Biocrude are naturally-occurring species indigenous to the environments in which our customer licensees operate. Since we do not have, and cannot obtain, intellectual property rights with respect to these species, and consequently other companies are free to develop competing technologies to grow these organisms, it is important that we secure and protect our intellectual property rights in the technology that we license to customer licensees, including our processes and system for growing micro-crops at an optimal rate. If we fail to secure and protect these rights, other companies will be able to freely copy or otherwise reproduce our technology, which may in turn cause us to lose customer licensees and prospective customer licensees to those competitors.
While we are not currently aware of any infringement or other violation of our intellectual property rights, monitoring and policing unauthorized use and disclosure of intellectual property is difficult. If we learned that a third party was in fact infringing or otherwise violating our intellectual property, we may need to enforce our intellectual property rights through litigation. Litigation relating to our intellectual property may not prove successful and might result in substantial costs and diversion of resources and management attention.
From our customer licensee’s standpoint, the strength of the intellectual property under which we grant licenses can be a critical determinant of the value of these licenses. If we are unable to secure, protect and enforce our intellectual property, it may become more difficult for us to attract new customer licensees. Any such development could have a material adverse effect on our business, financial condition and results of operations.
21
Table of Contents
Although we have filed patent applications for some of our core technologies, we do not currently hold any issued patents and we may face delays and difficulties in obtaining these patents, or we may not be able to obtain such patents at all.
Patents are a key element of our intellectual property strategy. We have currently filed patent applications for six families of technologies, both in the United States and in foreign jurisdictions. These pending patent applications relate to, among other aspects of our technology, the design of our bioreactors, the physical and chemical treatment processes for biomass, our growth algorithms, multi-spectral cameras and imaging algorithms, the use of our processes to make Biocrude, LPC and LM, and our enterprise management system. Our patent applications are in the early stages and we have not received substantive feedback from relevant patent offices regarding the viability of our patent applications. It may take two or more years for any patents to issue from our applications, we cannot provide any assurance that any patents will ultimately be issued or that any patents that do ultimately issue will issue in a form that will adequately protect our commercial advantage.
Our ability to obtain patent protection for our technologies is uncertain due to a number of factors, including that we may not have been the first to make the inventions covered by our pending patent applications or to file patent applications for these inventions. We have not conducted any formal analysis of the prior art in the areas in which we have filed our patent applications, and the existence of any such prior art would bring the novelty of our technologies into question and could cause the pending patent applications to be rejected.
Further, changes in U.S. and foreign patent law may also impact our ability to successfully prosecute our patent applications. For example, the United States Congress and other foreign legislative bodies may amend their respective patent laws in a manner that makes obtaining patents more difficult or costly. Courts may also render decisions that alter the application of patent laws and detrimentally affect our ability to obtain patent protection.
Even if patents do ultimately issue from our patent applications, these patents may not provide meaningful protection or commercial advantage. Patents only provide protection for a 20-year period starting from the filing date and the longer a patent application takes to issue the less time there is to enforce it. Further, the claims under any patents that issue from our applications may not be broad enough to prevent others from developing technologies that are similar or that achieve similar results to ours. It is also possible that the intellectual property rights of others will bar us from licensing our technology and bar us or our customer licensees from exploiting any patents that issue from our pending applications. Numerous U.S. and foreign issued patents and pending patent applications owned by others exist in the fields in which we have developed and are developing our technology. These patents and patent applications might have priority over our patent applications and could subject our patent applications to invalidation. Finally, in addition to those who may claim priority, any patents that issue from our applications may also be challenged by our competitors on the basis that they are otherwise invalid or unenforceable.
We may be unable to fully enforce our intellectual property in certain countries.
Based on our current negotiations with prospective customer licensees, we expect that many of our customer licensees will be in certain countries, including China and Indonesia, where we may have difficulty enforcing our intellectual property rights due to the general nature of the intellectual property enforcement mechanisms that are in place in such countries. These jurisdictions may have weaker statutory protections for intellectual property, or judicial or administrative regimes that make enforcement of these protections more difficult than in the United States. As a result, we might not be able to pursue adequate remedies in the event that our intellectual property rights are violated. In particular, we might be unable to effectively prevent the continuing infringement of our intellectual property rights.
22
Table of Contents
To the extent that any material infringement of our intellectual property occurs for which we are unable to obtain a remedy, we are likely to suffer a loss of potential revenues, since we will not receive license fees or royalties for such infringing uses, and other existing and prospective customer licensees might become hesitant to pay license fees for a technology being used freely by their competitors in the marketplace. If the infringing uses became widespread, this could have a material adverse effect on our business, financial condition and results of operations.
We may face claims that we are violating the intellectual property rights of others.
We may face claims, including from direct competitors, other energy companies, scientists or research universities, asserting that our technology or the commercial use of such technology by our customer licensees or partners infringes or otherwise violates the intellectual property rights of others. We have not conducted infringement, freedom to operate or landscape analyses, and as a result we cannot be certain that our technologies and processes do not violate the intellectual property rights of others. We expect that we may increasingly be subject to such claims as we begin to earn revenues and our market profile grows.
We may also face infringement claims from the employees, consultants, agents and outside organizations we have engaged to develop our technology. While we have sought to protect ourselves against such claims through contractual means, we cannot provide any assurance that such contractual provisions are adequate, and any of these parties might claim full or partial ownership of the intellectual property in the technology that they were engaged to develop for us.
If we were found to be infringing or otherwise violating the intellectual property rights of others, we could face significant costs to implement work-around methods, and we cannot provide any assurance that any such work-around would be available or technically equivalent to our current technology. In such cases, we might need to license a third party’s intellectual property, although any required license might not be available to us on acceptable terms, or at all. If we are unable to work around such infringement or obtain a license on acceptable terms, we might face substantial monetary judgments against us or an injunction against continuing to license our technology, which might cause us to cease operations.
In addition, even if we are not infringing or otherwise violating the intellectual property rights of others, we could nonetheless incur substantial costs in defending ourselves in suits brought against us for alleged infringement. Also, if our license agreements provide that we will defend and indemnify our customer licensees for claims against them relating to any alleged infringement of the intellectual property rights of third parties in connection with such customer licensees’ use of our technologies, we may incur substantial costs defending and indemnifying our customer licensees to the extent they are subject to these types of claims. Such suits, even if without merit, would likely require our management team to dedicate substantial time to addressing the issues presented. Any party bringing claims might have greater resources than we do, which could potentially lead to our settling claims against which we might otherwise prevail on the merits.
Any claims brought against us or our customer licensees alleging that we have violated the intellectual property of others could have a material adverse effect on our business, financial condition and results of operations.
23
Table of Contents
We expect to rely on third parties, such as academic institutions and agriculture and energy companies.
We expect to rely on third parties, such as academic institutions and agriculture and energy companies, to aid in the development and marketing of our technology and processes. Should these third parties reduce their commitment to us or terminate their relationship with us, pursue competing relationships or attempt to develop or acquire products or services that compete with the end-products produced using our technology, such action could have a material adverse effect on our business, financial condition and results of operations.
We will have to devote significant management resources to our compliance with the requirements imposed by Section 404 of the Sarbanes-Oxley Act of 2002, which could harm our growth.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, and the rules and regulations promulgated by the Securities and Exchange Commission, or the SEC, to implement Section 404, we are required to include in our Annual Reports on Form 10-K a report by our management regarding the effectiveness of our internal control over financial reporting. The report must include, among other things, an assessment of the effectiveness of our internal control over financial reporting as of the end of our fiscal year, including a statement as to whether or not our internal control over financial reporting is effective. The Dodd-Frank Wall Street Reform and Consumer Protection Act exempts non-accelerated filers from complying with the requirement set forth in Section 404(b) of the Sarbanes-Oxley Act, which requires that reporting companies include in their annual reports an independent auditor’s attestation report on the effectiveness of the reporting company’s internal control over financial reporting. For the fiscal year ended December 31, 2011, we will not be an accelerated filer and will therefore be exempt from the requirements of Section 404(b) of the Sarbanes-Oxley Act. However, we expect that following the completion of this offering we will become an accelerated filer and will be required to comply with the requirements of Section 404(b) of the Sarbanes-Oxley Act, which will increase the diversion of our management’s limited resources and attention.
If we fail to manage future growth effectively, our business could be harmed.
We expect to experience rapid growth as we commercialize our technology. This growth will likely place significant demands on our management and on our operational and financial infrastructure. To manage our growth effectively, we must continue to improve and enhance our managerial, operational and financial controls, hire sufficient numbers of capable employees and upgrade our infrastructure. We must also manage an increasing number of new and existing relationships with customer licensees, suppliers, business partners and other third parties. These activities will require significant expenditures and allocation of valuable management resources. If we fail to maintain the efficiency of our organization as we grow, our revenues and profitability may be harmed, and we might be unable to achieve our business objectives.
We may be unable to recruit and retain the necessary personnel to support our future growth.
We currently rely on key individuals in the management and operation of our business. In order to continue the research and design of our micro-crop production system and to move to larger commercial scaling and deployment, it will be necessary for us to recruit a significant number of additional qualified individuals, including experienced management and specific technical experts. Any inability on our part to retain key employees or obtain the appropriate resources through hiring, outsourcing or through other contractors could delay or impair our ability to achieve successful results.
24
Table of Contents
Most of our planned production capacity will be in equatorial regions around the world, which will limit the number of prospective customer licensees willing to license our technology, and our business will be adversely affected if we do not operate effectively in those regions.
The economic viability of our technology depends on geographical proximity to the equator, and the operations of our customer licensees generally should be within approximately 15 degrees of latitude from the equator for optimal performance. As a result, prospective customer licensees that do not have access to these regions are unlikely to license our technology. Furthermore, we will be subject to risks associated with the concentration of our customer licensees’ operations in these regions. The occurrence of prolonged increases in temperature due to climate change or natural disasters, such as earthquakes or floods, localized extended outages of critical utilities or transportation systems or political, social or economic disruptions in these regions, could cause a significant interruption in the output and sales realized by our customer licensees. Such interruptions could have a material adverse effect on our business, financial condition and results of operations.
We face risks associated with our international operations, including unfavorable regulatory, political and tax conditions, which could harm our business.
We face risks associated with the international licensing activities we have engaged in and expect to engage in, including possible unfavorable regulatory, political and tax conditions, any of which could harm our business. We expect that our future customer licensees will be based in various jurisdictions around the world, each of which is subject to the legal, political, regulatory and social requirements and economic conditions in these jurisdictions. In particular, the legal systems in these jurisdictions, particularly with respect to commercial matters and property rights, may be less developed than in the United States or more uncertain in their application. Laws can be subject to varying or unpredictable interpretations, and prior court decisions may have limited or no precedential value. In addition, our ability to enforce our contractual rights in foreign jurisdictions might be limited, especially in relation to our customer licensees that are instrumentalities of foreign governments.
Our business relationships with international customer licensees are subject to foreign government taxes, regulations and permit requirements, and foreign tax and other laws. Our international licensee relationships are also subject to United States and foreign government trade restrictions, tariffs and price or exchange controls; the U.S. Foreign Corrupt Practices Act of 1977, as amended; preferences of foreign nations for domestic technologies; changes in diplomatic and trade relationships; political instability, natural disasters, war or events of terrorism; and the strength of international economies. Because we will rely on license fees and royalties from our customer licensees’ operations, any negative impact these factors have on our customer licensees could also have a material adverse effect on our business, financial condition and results of operations.
We expect to be exposed to fluctuations in foreign exchange rates.
We expect that substantially all of our revenues will be from customer licensees located outside the United States and that, over the long term, at least a substantial majority of our revenues will come from non-U.S. customer licensees. Under the license agreements that we expect to enter into in the future with non-U.S. customer licensees, all or a portion of our license and royalty fee revenue may be denominated in currencies other than the U.S. dollar. Many of our costs, however, including most of the costs of our employees who we expect to support the operations of our customer licensees, and our research and development costs, will be denominated in U.S. dollars. We expect that fluctuations in the value of these revenues and expenses as measured in U.S. dollars will affect our results of operations, and adverse currency exchange rate fluctuations may have a material impact on our future financial results. In particular, to the extent that the U.S. dollar appreciates in value relative to the currencies in which we receive revenue, our revenues will be lower in U.S. dollar terms, while many of our expenses will remain at the same level, resulting in a net negative impact on our profitability.
25
Table of Contents
Our quarterly operating results may fluctuate in the future, which may cause our stock price to decline.
Our quarterly operating results may fluctuate as a result of a variety of factors, many of which are outside of our control. Our fluctuations may be amplified by the uncertainty in timing of a relatively small number of large transactions we anticipate that we will consummate. Fluctuations in our quarterly operating results could cause our stock price to decline rapidly, may lead analysts to change their long-term model for valuing our common stock, could cause us to face short-term liquidity issues, may impact our ability to retain or attract key personnel, or cause other unanticipated issues. If our quarterly operating results fail to meet or exceed the expectations of research analysts or investors, the price of our common stock could decline substantially. We believe that our quarterly revenue and operating results may vary significantly in the future and that period-to-period comparisons of our operating results may not be meaningful. The results of any quarterly or annual periods should not be relied upon as indications of our future operating performance.
Risks Related to this Offering and Ownership of Our Common Stock
Following this offering, we will be controlled by our principal stockholders. As a result, our principal stockholders will be able to exert significant influence over matters requiring stockholder approval.
Following this offering, our principal stockholders will directly or through a single holding company own approximately % of our common stock (or % if the underwriters exercise their option in full) at the completion of this offering. Accordingly, our principal stockholders will have substantial influence over the outcome of certain corporate actions requiring stockholder approval, including the approval of significant corporate transactions, and it may in some instances exercise this influence in a manner that advances its best interests and not necessarily those of other stockholders. Although our principal stockholders will enter into the stockholders agreement requiring it to vote its stock in accordance with our unaffiliated stockholders’ vote in most cases, this requirement does not apply to merger or asset sales with entities that are not affiliated with our principal stockholders. Accordingly, our principal stockholders may vote its stock in a manner that has the effect of delaying, preventing or deterring a change in control of our company, which could deprive you of the opportunity to receive a premium for your common stock as part of a sale, could adversely affect the market price of our common stock and could impede our growth. See “Relationships and Related Party Transactions—Stockholders Agreement” and “Management—Structure of the Board of Directors.”
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us more difficult, limit attempts by our stockholders to replace or remove our current management and limit the market price of our common stock.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws, which we will adopt prior to the completion of this offering, may have the effect of delaying or preventing a change of control or changes in our management. Our amended and restated certificate of incorporation and amended and restated bylaws will include provisions that:
| Ÿ | authorize our board of directors to issue, without further action by the stockholders, up to shares of undesignated preferred stock; |
| Ÿ | require that any action to be taken by our stockholders be effected at a duly called annual or special meeting and not by written consent; |
| Ÿ | establish an advance notice procedure for stockholder proposals to be brought before an annual meeting, including proposed nominations of persons for election to our board of directors; |
26
Table of Contents
| Ÿ | establish that our board of directors is divided into three classes, with each class serving three-year staggered terms; |
| Ÿ | provide that our directors may be removed only for cause; and |
| Ÿ | provide that vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum. |
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our management by making it more difficult and time consuming for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the General Corporation Law of the State of Delaware, or DGCL, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with any “interested” stockholder (any stockholder with 15% or more of our capital stock) for a period of three years following the date on which the stockholder became an “interested” stockholder.
We became a public company through our acquisition of a public shell company pursuant to which we assumed all known and unknown potential liabilities of our predecessor entity.
We became a public company through the acquisition of a public shell company that did not have significant operations or assets at the time of the transaction, but previously operated as a company developing medical imaging technologies prior to declaring bankruptcy and subsequently undergoing bankruptcy liquidation in 1998. Although the bankruptcy decree discharged pre-existing liabilities, we may still be exposed to undisclosed liabilities related to the prior operations of the shell company and we could incur losses, damages or other costs as a result of such exposure. These losses, damages and costs from such undisclosed liabilities could be significant, and could have a material adverse effect on our business, financial condition and results of operations.
A total of , or %, of our total outstanding shares after the offering are restricted from immediate resale, but may be sold on a stock exchange in the near future. The large number of shares eligible for public sale or subject to rights requiring us to register them for public sale could depress the market price of our common stock.
The market price of our common stock could decline as a result of sales of a large number of shares of our common stock in the market after this offering, and the perception that these sales could occur may also depress the market price of our common stock. We will have shares of common stock outstanding after this offering, assuming no exercise of the underwriters’ option. Of these shares, the shares sold in this offering will be freely tradable in the United States, except for any shares purchased by our “affiliates” as defined in Rule 144, or Rule 144, under the Securities Act of 1933, as amended, or the Securities Act. The holders of shares of outstanding common stock have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of their common stock during the 180-day period beginning on the date of this prospectus, except with the prior written consent of Goldman, Sachs & Co., Citigroup Global Markets Inc. and UBS Securities LLC. After the expiration of the 180-day restricted period, these shares may be sold in the public market in the United States, subject to prior registration in the United States, if required, or reliance upon an exemption from U.S. registration, including as provided for by Rule 144 (subject, in the case of shares held by affiliates or control persons, to compliance with the volume and manner of sale restrictions prescribed thereby).
Shares that were outstanding prior to this offering and which are not subject to lock-up agreements, other than those shares held by our affiliates, will be freely tradable in the United States provided any holding period under Rule 144 has expired with respect to such shares, subject, in the case of shares held by our affiliates, to the volume and manner of sale restrictions imposed by Rule 144.
27
Table of Contents
No sooner than months following the completion of this offering, we will file a registration statement with the SEC covering the exchange from time to time of an aggregate of shares of our common stock issuable upon exchange of the outstanding limited liability company interests in PA LLC held by our employees or by Valens-Calliope. Any shares issued upon such exchange would be freely tradable by any exchanging holder subject, in the case of exchanging holders who are our affiliates, to the volume and manner of sale restrictions imposed by Rule 144. See “Shares Eligible for Future Sale—Rule 144.”
Sales of our common stock by our stockholders may make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate. These sales also could cause our stock price to fall and make it more difficult for you to sell shares of our common stock.
An active, liquid and orderly trading market for our common stock may not develop, our share price may be volatile, and you may be unable to sell your shares at or above the offering price.
Prior to this offering, there has been a limited public market for our common stock. We cannot predict the extent to which an expanded trading market will develop or how liquid that market might become. The initial public offering price for our shares will be determined by negotiations between us and representatives of the underwriters and may not be indicative of prices that will prevail in the trading market. In particular, because we do not have any significant operating history, valuation of our common stock might be more difficult than for companies with a substantial financial record. This might cause significant volatility in the market price of our common stock. The market price of shares of our common stock may also be subject to wide fluctuations in response to the many risk factors listed in this section and other factors beyond our control, including:
| Ÿ | actual or anticipated fluctuations in our key operating metrics, financial condition and operating results; |
| Ÿ | issuance of new or updated research or reports by securities analysts; |
| Ÿ | our announcement of actual results for a fiscal period that are higher or lower than projected or expected results, or our announcement of revenue or earnings guidance that is higher or lower than expected; |
| Ÿ | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| Ÿ | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| Ÿ | sales or expected sales of additional common stock; |
| Ÿ | announcements from, or operating results of, our competitors; or |
| Ÿ | general economic and market conditions. |
Furthermore, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political and market conditions, such as recessions, interest rate changes or international currency fluctuations, may cause our common stock price to decline. If our common stock price after this offering does not exceed the initial public offering price, you will not realize any return on your investment in our common stock and may lose some or all of your investment. In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns.
28
Table of Contents
We do not currently intend to pay dividends on our common stock and, consequently, your ability to achieve a return on your investment will depend solely on appreciation in the price of our common stock.
We have never declared or paid any cash dividends on our common stock and do not intend to do so for the foreseeable future. We currently intend to invest our future earnings, if any, to fund our growth. Therefore, you are not likely to receive any dividends on your common stock for the foreseeable future, and the success of an investment in shares of our common stock will depend upon any future appreciation in their value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
You will experience immediate and substantial dilution.
The initial public offering price will be substantially higher than the pro forma net tangible book value of each outstanding share of our common stock immediately after this offering. If you purchase our common stock in this offering, you will suffer immediate and substantial dilution. If previously granted warrants or options are exercised, you will experience additional dilution. As of , warrants to purchase shares of our common stock at a weighted-average exercise price of $ per share and options to purchase shares of our common stock at a weighted-average exercise price of $ were outstanding. For more information refer to the section of this prospectus entitled “Dilution.”
29
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including the sections entitled “Prospectus Summary,” “Risk Factors,” “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and “Business” contains forward-looking statements. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, business strategy and plans and our objectives for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, short term and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
You should read this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
30
Table of Contents
We estimate that the net proceeds we will receive from this offering will be approximately $ million, assuming an initial public offering price of $ per share, which is the midpoint of the range listed on the cover page of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We will not receive any of the proceeds from the sale of shares by the selling stockholders, although we will bear the costs, other than underwriting discounts and commissions, associated with those sales.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, which is the midpoint of the range listed on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering by $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated expenses payable by us.
We will set aside $ of the proceeds to redeem up to units of PA LLC held by certain of our employees who are not otherwise selling in this offering. We intend to use the remainder of the net proceeds from this offering for working capital and other general corporate purposes, including expanding our development, operations, demonstration facility, and marketing and sales departments.
Our management will have broad discretion in the application of the net proceeds of this offering. The amount we actually spend for the purposes described above will depend, in part, on the timing and amount of our future revenues and our future expenses. Investors will be relying on the judgment of our management regarding the application of the net proceeds of this offering. Pending these uses, we plan to invest these net proceeds in short-term, interest bearing obligations, investment grade instruments, certificates of deposit or direct or guaranteed obligations of the United States. The goal with respect to the investment of these net proceeds is capital preservation and liquidity so that such funds are readily available to fund our operations.
31
Table of Contents
We have never declared or paid dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support operations and to finance the growth and development of our business. We do not intend to declare or pay cash dividends on our common stock in the foreseeable future. Any future determination to pay dividends will be made at the discretion of our board of directors subject to applicable laws, and will depend upon our results of operations, financial condition, contractual restrictions, capital requirements and other factors that our board of directors may deem relevant.
32
Table of Contents
Our common stock is currently quoted on the OTCQB under the symbol “PALG”. There is a very limited public market for our common stock on the OTCQB. As a result, our common stock has experienced significant price fluctuations and volatility based on discrete trades of very modest volume.
The following table sets forth, for the periods indicated, the reported high and low sales prices of our common stock on the OTCQB. These quotations reflect inter-dealer prices, without retail mark-ups, mark-downs or commissions, involving our common stock during each calendar quarter, and may not represent actual transactions. The table also shows the average monthly trading volume for the periods indicated.
| Sales Price | Average Monthly Trading Volume |
|||||||||||
| High | Low | |||||||||||
| Fiscal 2011: |
||||||||||||
| Fourth Quarter (through November 30, 2011) |
$ | 13.50 | $ | 3.50 | 1,732 | |||||||
| Third Quarter |
$ | 25.00 | $ | 5.50 | 8,696 | |||||||
| Second Quarter |
$ | 25.00 | $ | 14.00 | 10,554 | |||||||
| First Quarter |
$ | 101.00 | $ | 8.80 | 16,482 | |||||||
| Fiscal 2010: |
||||||||||||
| Fourth Quarter |
$ | 25.00 | $ | 6.01 | 8,596 | |||||||
| Third Quarter |
$ | 26.00 | $ | 0.10 | 3,664 | |||||||
| Second Quarter |
$ | 20.00 | $ | 3.00 | 1,853 | |||||||
| First Quarter |
$ | 25.00 | $ | 11.60 | 2,326 | |||||||
| Fiscal 2009: |
||||||||||||
| Fourth Quarter |
$ | 24.95 | $ | 15.00 | 4,654 | |||||||
| Third Quarter |
$ | 40.00 | $ | 8.00 | 6,241 | |||||||
| Second Quarter |
$ | 11.00 | $ | 2.65 | 5,756 | |||||||
| First Quarter |
$ | 10.00 | $ | 2.00 | 94,704 | |||||||
| Fiscal 2008: |
||||||||||||
| Fourth Quarter |
$ | 7.00 | $ | 0.25 | 41,676 | |||||||
On November 30, the last reported sale price of our common stock on the OTCQB was $5.50 per common share. We have applied to have our common stock listed on under the symbol “ ”.
33
Table of Contents
The following table sets forth our capitalization as of September 30, 2011:
| Ÿ | on a pro forma basis to give further effect to the conversion of all of our outstanding convertible debt, including accrued interest, into 2,379,768 shares of common stock based on a conversion price of $5.43 per share. |
| Ÿ | on a pro forma as adjusted basis to give further effect to: |
| ¡ | the sale by us of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the range listed on the cover page of this prospectus, and our receipt of the estimated net proceeds from that sale after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us; |
| ¡ | the exchange of shares of common stock reserved for issuance prior to the closing of this offering and that are issuable upon the exchange of units of PA LLC for common shares of PetroAlgae; and |
| ¡ | our use of approximately $ of the net proceeds of this offering to redeem certain of the equity units of PA LLC held by our employees. |
The pro forma as adjusted information set forth in the table below is illustrative only and will adjust based on the actual initial public offering price and other terms of this offering determined at pricing.
You should read this table with the sections of this prospectus entitled “Selected Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes that are included elsewhere in this prospectus.
| As of September 30, 2011 | ||||||||||
| Actual | Pro Forma | Pro Forma as Adjusted | ||||||||
| (Unaudited) | ||||||||||
| Current Liabilities (excluding notes payable) |
$ | 12,232 | $ | 9,310 | ||||||
| Notes payable—related party (current) |
$ | 72,053 | $ | 62,053 | ||||||
| Stockholders’ equity (deficit): |
||||||||||
| Common stock, par value $0.001; 300,000,000 shares authorized; 106,920,730 shares issued and outstanding, actual; 109,300,498 shares issued and outstanding, pro forma; shares issued and outstanding, pro forma as adjusted |
$ | 107 | $ | 109 | ||||||
| Additional paid-in capital |
$ | 39,082 | $ | 52,002 | ||||||
| Accumulated deficit |
$ | (117,078 | ) | $ | (117,078 | ) | ||||
| Non-controlling interest |
$ | 1,896 | $ | 1,896 | ||||||
| Total stockholders’ equity (deficit) |
$ | (75,993 | ) | $ | (63,071 | ) | ||||
| Total capitalization |
$ | (3,940 | ) | $ | (1,018 | ) | ||||
The number of shares of our common stock set forth in the table above excludes:
| Ÿ | 1,700,750 shares of common stock issuable upon the exercise of options outstanding at September 30, 2011 at a weighted average exercise price of $5.50 per share; |
| Ÿ | 2,592,143 shares of common stock issuable upon the exercise of warrants outstanding at September 30, 2011, at a weighted average exercise price of $15.00 per share; and |
| Ÿ | shares of common stock reserved for future issuance under our stock-based compensation plans, consisting of 4,000,000 shares of common stock reserved for issuance under the 2009 Plan. |
34
Table of Contents
As of , we had a negative net tangible book value of approximately $ or $ per share of our common stock, based upon shares of our common stock outstanding on such date.
Our pro forma as adjusted net tangible book value as of was $ , or $ per share of our common stock. Pro forma as adjusted net tangible book value per share represents the amount of our total tangible assets reduced by the amount of our total liabilities and divided by the sum of:
| (i) | the shares of our common stock that will be outstanding following this offering; and |
| (ii) | the shares of our common stock that are issuable following this offering upon the exchange of units of PA LLC for shares of our common stock. |
Dilution in pro forma as adjusted net tangible book value per share to new investors in this offering represents the difference between the amount per share paid by purchasers of shares of our common stock in this offering and the pro forma as adjusted net tangible book value per share of our common stock immediately after the completion of this offering. After giving effect to the sale of the shares of our common stock offered by us in this offering at an assumed initial public offering price of $ per share, the mid-point of the range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of would have been $ , or $ per share of our common stock. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to existing stockholders and an immediate dilution of $ per share to new investors in our common stock. The following table illustrates this dilution on a per share basis.
| Assumed initial public offering price per share |
$ | |||
| Pro forma as adjusted net tangible book value per share as of , before giving effect to this offering |
$ | |||
| Increase in pro forma as adjusted net tangible book value per share attributed to new investors purchasing shares in this offering |
$ | |||
|
|
|
|||
| Pro forma as adjusted net tangible book value per share after giving effect to this offering |
$ | |||
|
|
|
|||
| Dilution per share to new investors in this offering |
$ | |||
|
|
|
A $1.00 increase or decrease in the assumed initial public offering price of $ per share would increase or decrease our pro forma as adjusted net tangible book value per share after this offering by $ per share and the dilution in pro forma as adjusted net tangible book value to new investors by $ per share, assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters exercise their option to purchase additional shares of our common stock in full, based on an assumed initial public offering price of $ per share, the pro forma as adjusted net tangible book value per share after this offering would be $ per share, and the dilution in pro forma as adjusted net tangible book value per share to new investors in this offering would be $ per share.
The following table summarizes, on a pro forma as adjusted basis as of and after giving effect to the offering, based on the assumed initial public offering price per share of $ , the midpoint of the range set forth on the cover of this prospectus.
35
Table of Contents
The differences between existing stockholders and new investors with respect to the number of shares of our common stock purchased from us, the total consideration paid to us and the average price per share paid:
| Shares Purchased |
Total Consideration | |||||||||||||||||
| Number | Percent | Amount (in thousands) |
Percent | Average Price Per Share |
||||||||||||||
| Existing stockholders |
% | $ | % | $ | ||||||||||||||
| New public investors |
||||||||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
| Total |
100 | % | $ | 100 | % | |||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
A $1.00 increase or decrease in the assumed initial public offering price of $ per share would increase or decrease, respectively, total consideration paid by new investors and total consideration paid by all stockholders by approximately $ , assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
If the underwriters exercise their option in full to acquire additional shares, our existing stockholders would own % and our new investors would own % of the total number of shares of our common stock outstanding upon the closing of this offering.
As of , warrants to purchase shares of our common stock at a weighted-average exercise price of $ per share and options to purchase shares of our common stock at a weighted-average exercise price of $ were outstanding.
36
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated financial data as of and for the years ended December 31, 2007, 2008, 2009 and 2010 are derived from our audited consolidated financial statements. The selected audited consolidated financial data for the period beginning September 22, 2006 and ending December 31, 2010 is derived from our audited consolidated financial statements for such period that are included elsewhere in this prospectus. The selected unaudited consolidated interim financial data for the nine months ended September 30, 2010 and 2011 and as of September 30, 2011 are derived from our unaudited consolidated interim financial statements for such periods and dates that are included elsewhere in this prospectus. The unaudited consolidated financial statements were prepared on a basis consistent with our audited consolidated interim financial statements and include, in the opinion of management, all adjustments, consisting only of normal recurring adjustments, necessary for the fair presentation of the financial information contained in those statements. The historical results presented below are not necessarily indicative of financial results to be achieved in future periods. Prospective investors should read these selected consolidated financial data together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes included elsewhere in this prospectus.
| Years Ended December 31, | For the Period From September 22, 2006 (Inception) Through December 31 |
Nine Months Ended September 30, |
||||||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2010 | 2010 | 2011 | ||||||||||||||||||||||
| (in thousands, except for share and per share data) |
||||||||||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||
| Costs and expenses: |
||||||||||||||||||||||||||||
| Selling, general and administrative |
2,984 | 8,251 | 17,401 | 21,850 | 50,666 | 9,447 | 8,074 | |||||||||||||||||||||
| Research and development |
4,863 | 9,866 | 15,085 | 17,424 | 48,469 | 11,184 | 8,463 | |||||||||||||||||||||
| Interest expense—related party |
444 | 1,764 | 3,198 | 4,077 | 9,483 | 2,912 | 4,646 | |||||||||||||||||||||
| Depreciation |
55 | 269 | 1,138 | 1,128 | 2,591 | 773 | 669 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total costs and expenses |
8,346 | 20,150 | 36,822 | 44,479 | 111,209 | 24,316 | 21,852 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss before non-controlling interest |
(8,346 | ) | (20,150 | ) | (36,822 | ) | (44,479 | ) | (111,209 | ) | (24,316 | ) | (21,852 | ) | ||||||||||||||
| Non-controlling interest |
— | 162 | 6,554 | 6,469 | 13,186 | 3,842 | 2,797 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss attributable to PetroAlgae Inc. |
$ | (8,346 | ) | $ | (19,988 | ) | $ | (30,268 | ) | $ | (38,010 | ) | $ | (98,023 | ) | $ | (20,474 | ) | $ | (19,055 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Basic and diluted common shares outstanding |
100,000,000 | 100,362,021 | 104,866,065 | 106,731,469 | 106,664,896 | 106,920,730 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Basic and diluted loss per share |
$ | (0.08 | ) | $ | (0.20 | ) | $ | (0.29 | ) | $ | (0.36 | ) | $ | (0.19 | ) | $ | (0.18 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| As of December 31, | As of September 30, |
|||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | ||||||||||||||||
| (in thousands, except for share data) |
||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Total assets |
$ | 720 | $ | 11,598 | $ | 8,592 | $ | 6,325 | $ | 8,323 | ||||||||||
| Total liabilities |
$ | 9,712 | $ | 26,803 | $ | 48,912 | $ | 62,829 | $ | 84,316 | ||||||||||
| Total stockholders’ deficit |
$ | (8,992 | ) | $ | (15,205 | ) | $ | (40,320 | ) | $ | (56,504 | ) | $ | (75,993 | ) | |||||
| Total common shares outstanding |
100,000,000 | 104,274,189 | 106,229,356 | 106,933,230 | 106,920,730 | |||||||||||||||
37
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with “Prospectus Summary—Summary Consolidated Financial Data,” “Selected Consolidated Financial Data” and our consolidated financial statements that are included elsewhere in this prospectus. In addition to historical data, this discussion contains forward-looking statements about our business, operations and financial performance based on current expectations that involve risks, uncertainties and assumptions. Our actual results may differ materially from those discussed in the forward-looking statements as a result of various factors, including, but not limited to, those discussed in the sections entitled “Risk Factors” and “Special Note Regarding Forward-Looking Statements” included elsewhere in this prospectus.
Overview
Our Business
We provide renewable technology and solutions to address the global demand for new economical sources of feed, food and fuel.
Our objective is to be the leading global provider of technology and processes for the commercial production of micro-crop biomass for agriculture and energy markets. We seek to license renewable technology to meet the significant and growing demand for agricultural products and energy in both emerging and developed economies. We believe that our solution will deliver to our customer licensees strong and consistent economic returns as a consequence of continuous, predictable, year-round operations without the need for government subsidies. We also expect our solution to provide social benefits, through job creation and community investment, as well as significant environmental advantages, through sustainable production that absorbs atmospheric carbon dioxide and does not compete with the existing food supply, require arable land, or pollute soil or water.
We have developed proprietary technology, consisting of integrated environmental management systems, which we believe will allow our customer licensees to grow aquatic micro-crops at accelerated rates. This solution utilizes locally available micro-crops and commercially available processing equipment to produce biomass-based products that can be sold directly into agriculture and energy markets.
Our strategy is to license and provide management support for production facilities in equatorial regions around the world. Our solution is scalable and flexible, which we believe will allow our customer licensees to expand facility capacity and increase production to meet market demand. We intend to generate revenue from licensing fees and royalties primarily from customer licensees that are engaged in the global agriculture and energy markets, as well as from investment groups. Our licensing approach is designed to minimize our capital expenditures, because our customer licensees will be expected to invest in the development and construction of the production facilities.
Our proprietary technology uses indigenous micro-crops (from the Lemnaceae family) that are not genetically modified and demonstrate an optimal growth profile for a particular geography and environment. These micro-crops will then be grown, harvested and processed in a manner that we believe will optimize the production of our micro-crop biomass. To date, the operations at our demonstration facility have deepened our understanding of the variables relating to the micro-crop productivity of our technology. Based on this work, as well as additional modeling, we believe that these micro-crops can be grown and harvested on a commercial scale. Our processes use industry-standard agricultural and food processing equipment in specialized configurations to produce products with consistent quality. During our process, the micro-crops are pressed to separate the pulp from the liquid. The liquid is further processed to create our Lemna Protein Concentrate, or LPC, while the pulp
38
Table of Contents
is converted to create our Lemna Meal, or LM, and Biocrude product. Our Biocrude product requires further third-party conversion to facilitate the production of renewable fuels. These products can then be used locally or shipped to end customer licensees.
We expect to generate our revenues from license fees and royalties that we will earn from license agreements, pursuant to which our customer licensees will license from us the right to build and operate the bioreactors and associated processing facilities that make up our technology platform.
Our Development
We have achieved a number of significant milestones since we were founded in September 2006. Our focus has been on the creation and development of growth and harvesting technologies for the commercial-scale production of micro-crop biomass that could be processed for sale into existing agriculture and energy markets. To do this, we committed significant resources to our research and development efforts, including completing a fully operational demonstration facility in June 2009 to test, refine and exhibit our technology and processes. Since the completion of our demonstration facility, we have conducted extensive internal and third-party testing (both customer and academic) of our products to validate their commercial viability. Although we continue to test and refine our technology and processes, we are now also focused on building our customer pipeline and entering into license agreements with prospective customer licensees.
Our key milestones to date are as follows:
| Ÿ | In 2006, we began the research and development of our growth and harvesting technologies. Our efforts focused on experimentation and testing, developing processes and equipment, and building the infrastructure and databases necessary to support our technology. |
| Ÿ | During 2007 and 2008, we developed our proprietary growth algorithms—the complex mathematical formulas we use to determine the correct harvesting density and sunlight exposure of the biomass. |
| Ÿ | In 2009, we completed our fully operational demonstration facility and established our business development team. This facility consists of a large bioreactor (approximately one hectare) and three smaller bioreactors that display our technology and processes. |
| Ÿ | In November 2009, the Indonesian Ministry of Agriculture approved our LPC product as an approved raw material for use in animal feed. |
| Ÿ | In May 2011, we entered into an agreement for the construction of a pilot-scale bioreactor in the Republic of Suriname. The construction of this bioreactor was completed in October 2011 and testing is ongoing. |
| Ÿ | In October 2011, we entered into a license agreement with Asesorias e Inversiones Quilicura S.A., or AIQ, a customer licensee in South America, with initial construction of a pilot-scale bioreactor having begun in September 2011. |
| Ÿ | In November 2011, China Energy Conservation and Environment Protection Group—CHONGQING INDUSTRY CO. LTD, or CECEP, a prospective Chinese customer licensee with which we are currently negotiating a license agreement, began construction of a pilot-scale bioreactor. |
Our Sales Cycle
Our sales and marketing process involves identifying prospective customer licensees and introducing them to our processes and products. We provide each prospective customer licensee with an economic analysis that provides an understanding of expected investment returns, and customer-specific deployment considerations. In addition, we permit prospective customer licensees to visit our demonstration facility. We also evaluate the financial capacity of prospective customer licensees in
39
Table of Contents
light of the capital expenditure required to construct our licensed facilities. Once these steps are complete, we and the prospective customer licensee seek to develop, sign and execute a license agreement, which is often phased, for the construction and operation of facilities in appropriate equatorial regions.
Financial Operations Overview
Revenue
We expect that the license agreements we enter into with our customer licensees will generally provide for the payment of license fees during the construction of our license units and ongoing royalty payments over the term of the license agreement as the licensed unit becomes operational. We may also earn other types of revenues. For a discussion of our revenue recognition policy, please see “—Critical Accounting Policies and Management Estimates—Revenue Recognition.”
We expect that our revenues will consist of the following:
License Fees. The timing and amount of the license fees we earn will be specific to each license agreement, but they may consist of equal periodic payments over a period of several years or more tailored provisions, including up-front payments at the time a license agreement is signed or payments due upon the achievement of certain specified technical or operational milestones. Some of our license agreements will provide for higher royalty payments in lieu of license fees.
Royalties. The timing and amount of royalty payments we receive will also be specific to a particular license agreement, but will generally be based on a fixed percentage of the gross revenues generated by our customer licensees. Our license agreements will include a transfer pricing mechanism, which will establish the amount of royalties to be paid by a customer licensee if it consumes the end-products in its own operations or sells the end-products at prices not set by the market.
Construction Revenue. To facilitate the capture of early customer licensees, we may agree to build the first portion of our customer licensees’ facilities for a fixed price. In general, revenues will be reported as construction progresses.
Other Revenues. We may also recognize revenue from various other sources, including customer support fees.
Factors Affecting our Revenue
We expect the following to be among the factors that will affect our revenue:
Entry into License Agreements. Our revenues will be almost entirely dependent on the number and terms of license agreements we enter into. Since we expect our sales cycles to be long, we believe that we will have a degree of visibility into our prospective pipeline for new license agreements.
Product Pricing. The market prices of the products produced by our technology will play an important role in determining future revenues. Since our royalty revenue will be based on the sales proceeds our customer licensees earn from such products, changes in the market prices of these products will directly impact those revenues. In addition, since long-term price trends in these markets will determine in part the return on investment our customer licensees earn from our technology, our technology will tend to be more attractive to our customer licensees if those prices are expected to trend upward or remain at high levels.
License Agreement Structure. We expect to tailor our license agreements to specific opportunities available. This may result in differences in the amount of fees paid and the timing of the payment of such fees. In addition to having effects on the timing of cash payments we receive, the structure of a license agreement will also have a significant effect on our ability to recognize revenue over the term of the contract. Since our license fee revenues will generally be recognized over the period of construction, a contract structure that provides for an uneven distribution of payments over that period might result in deferred revenues.
40
Table of Contents
Costs and Expenses
Our costs and expenses are expected to consist of the following:
Cost of Revenue. Cost of revenue will consist primarily of the costs associated with providing our customer licensees with the technical expertise necessary to assist in the deployment of the license units and ongoing support to optimize their operations. We expect the cost of personnel-related expenses to be the most significant component of these expenses, and we expect to continue to hire new employees, including employees in the jurisdictions in which our customer licensees will operate, in order to support our anticipated growth. These costs will be reported as costs of revenue over the period of the associated revenue recognition. We expect that costs related to customer deployment and support will include primarily employment-related costs for our personnel; travel-related expenses such as airfare, meals and lodging; and the cost of third-party experts that we will retain for technical, logistical and legal support, when necessary. With respect to turnkey agreements, we would recognize the cost of construction and equipment as a cost of revenue.
Selling, General and Administrative. Selling, general and administrative expenses consist primarily of personnel-related expenses, including consulting and overhead expenses, related to our executive, legal, finance, human resources and information technology functions. These expenses also include costs related to our sales function, including our marketing, public relations and regulatory costs. We expect selling, general and administrative expenses to increase as we incur additional listed company expenses, including increased accounting and legal costs, costs related to compliance with securities, corporate governance and other regulations, investor relations and higher insurance premiums. We also expect that the compensation costs associated with the marketing of our technology will increase. In addition, we expect to incur additional costs as we hire personnel and enhance our infrastructure to support the anticipated growth of our business.
Research and Development. Research and development expenses consist primarily of the costs of personnel engaged in the construction and operation of our demonstration facility, laboratory testing and experimentation, and product development. Research and development expenses also include the associated materials and equipment, supplies and related overheads associated with these functions. In addition, expenses include costs relating to the research, testing and build-out of various aspects of our technology and processes and costs relating to the testing of the products produced by our technology. We intend to continue to invest in research and development, including hiring additional technical personnel and using outside consultants for various research and development efforts.
Interest Expense—Related Party. We recognize interest expense on our debt obligations. To date, our debt financing has been provided exclusively by our principal stockholders.
Depreciation Expense. We capitalize assets used in our business that have an expected useful life that is longer than one year. Since many of our asset purchases relate to research and process testing and therefore have a useful life of less than one year, most of our expenditures on these items have not been capitalized. We depreciate capitalized property and equipment over estimated useful lives that generally range from two to seven years. As we grow our business, capitalized assets and depreciation expense will likely increase as we expand customer support and research and development facilities. Since, under the license agreements we expect to enter into, our customer licensees will purchase the materials associated with the construction of their license units, the significant associated capital expense and the related depreciation of those assets will not affect our financial results.
Income Taxes. Since our inception, we have incurred losses and have not recorded any U.S. federal, state or non-U.S. income tax. After the utilization of net operating loss and research and
41
Table of Contents
development tax credit carryforwards, we will pay U.S. federal and state or non-U.S. income tax on any profits we generate. We will also be subject to withholding of foreign taxes associated with income we generate from non-U.S. sources, which we expect to constitute a substantial majority of our revenues.
Non-controlling Interest. We do not wholly own PA LLC, our sole operating subsidiary. As of September 30, 2011, minority holders owned approximately 13% of PA LLC. Non-controlling interest represents the proportion of our net income (or loss) attributable to the minority holders and proportionally reduces the amount of reported earnings (or loss) in each period. We expect the current minority holdings to decrease over time as holders of PA LLC interests exchange those interests for shares of our common stock. As a result, the proportional reduction to our earnings will decrease, and the number of our shares outstanding will increase. See “Prospectus Summary—Our Structure.”
Factors Affecting our Costs and Expenses
We expect the following to be among the factors that will affect our costs and expenses:
The Growth of our Business. Our selling, general and administrative expenses are expected to increase in line with the growth of our business.
Continued Development of our Solution. In order to maintain our competitiveness, we will need to constantly refine our technology and processes, which will require significant ongoing research and development activity. The amount and nature of this activity will affect our research and development expenses from period to period.
Stock-based Compensation. We include stock-based compensation as part of selling, general and administrative expenses and research and development expenses according to the role of the grantee. We incur these expenses in connection with the grant of stock options and other equity awards to our directors, employees and consultants, and any subsequent modification of those awards. We apply the fair value method in accordance with authoritative guidance for determining the cost of stock-based compensation at the time of the grant. We made stock-based compensation grants of $8.3 million, $1.2 million, $3.0 million and $19.0 million during the nine months ended September 30, 2011 and the years ended December 31, 2010, 2009 and 2008, respectively. For a detailed discussion of stock-based compensation and our accounting for such grants, including the timing of expense recognition, see “—Critical Accounting Policies and Management Estimates—Stock-based Compensation.”
Ability to Continue as a Going Concern
We are currently in the development stage and have not yet recognized any revenue. Our financial statements are presented on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business.
We have never been profitable and we have incurred significant losses and cash flow deficits. For the fiscal years ended December 31, 2010, 2009 and 2008, we reported net losses before non-controlling interest of $44.5 million, $36.8 million and $20.2 million, respectively, and negative cash flow from operating activities of $22.8 million, $27.6 million and $11.8 million, respectively. For the nine months ended September 30, 2011 and 2010, and the period from September 22, 2006 (inception) to September 30, 2011, we reported net losses before non-controlling interest of $21.9 million, $24.3 million, and $133.1 million, respectively, and negative cash flow from operating activities of $13.4 million, $17.1 million, and $80.7 million, respectively. As of September 30, 2011, the Company had an aggregate accumulated deficit of $117.1 million. We anticipate that we will continue to report losses and negative cash flow during the remainder of 2011. As a result of these net losses and cash flow deficits and other factors, there is substantial doubt about our ability to continue as a going
42
Table of Contents
concern. However, we expect this uncertainty to be resolved if this offering is successful and we enter into additional license agreements pursuant to which we generate revenue. See “—Liquidity and Capital Resources.”
Our financial statements do not include any adjustments that might result from the outcome of this uncertainty. As of September 30, 2011, these adjustments would likely include the substantial impairment of the carrying amount of our assets of $1.6 million, which excludes cash and restricted cash of $6.7 million, and potential contingent liabilities that may arise if we are unable to fulfill various operational commitments. In addition, the value of our securities, including common stock and warrants, would be greatly impaired. Our ability to continue as a going concern is dependent upon generating sufficient cash flow from operations and obtaining additional capital and financing.
Results of Operations
Comparison of the Nine Months Ended September 30, 2010 and 2011
| Nine Months Ended September 30, | Variance | |||||||||||||||
| 2010 | 2011 | Amount | Percentage | |||||||||||||
| (Unaudited) | ||||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | N/M | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Costs and expenses: |
||||||||||||||||
| Selling, general and administrative |
9,447,354 | 8,074,208 | (1,373,146 | ) | (15)% | |||||||||||
| Research and development |
11,184,021 | 8,462,806 | (2,721,215 | ) | (24)% | |||||||||||
| Interest expense—related party |
2,912,155 | 4,646,195 | 1,734,040 | 60% | ||||||||||||
| Depreciation |
772,732 | 669,124 | (103,608 | ) | (13)% | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
24,316,262 | 21,852,333 | (2,463,929 | ) | (10)% | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss before non-controlling interest |
(24,316,262 | ) | (21,852,333 | ) | 2,463,929 | 10% | ||||||||||
| Net loss attributable to non-controlling interest |
3,842,232 | 2,797,625 | (1,044,607 | ) | (27)% | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss attributable to PetroAlgae Inc. |
$ | (20,474,030 | ) | $ | (19,054,708 | ) | 1,419,322 | 7% | ||||||||
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted common shares outstanding |
106,664,896 | 106,920,730 | 255,834 | N/M | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted loss per share |
$ | (0.19 | ) | $ | (0.18 | ) | $ | 0.01 | 5% | |||||||
|
|
|
|
|
|
|
|||||||||||
Revenue. No revenue has been recognized in the nine-month periods ended September 30, 2011 or 2010.
Total costs and expenses. For the nine months ended September 30, 2011 as compared to the nine months ended September 30, 2010, total costs and expenses decreased by $2.5 million, or 10.1%, to $21.9 million. The decrease in the comparable nine-month periods was primarily due to decreases in selling, general and administrative expenses and research and development expenses, partially offset by an increase in interest expense.
Selling, general and administrative expenses. Selling, general and administrative expenses include senior management and overhead costs, business development expenditures, system infrastructure support costs, finance and accounting costs, and intellectual property management costs. For the nine months ended September 30, 2011 as compared to the nine months ended September 30, 2010, selling, general and administrative expenses decreased by $1.4 million, or 14.5%, to $8.1 million. A selective headcount reduction resulted in $0.8 million savings during the nine months ended September 30, 2011 as compared to the same period in 2010. For the first nine months
43
Table of Contents
of 2011 as compared to 2010, equity compensation expense declined by $0.5 million as a result of termination-related forfeitures of employees whose roles were classified as selling, general and administrative.
Research and development expenses. Research and development costs include the research and experimentation of the various system components and the design and construction of the commercial-scale pilot system for customer licensee demonstration. During the nine months ended September 30, 2011 as compared to the same period in 2010, research and development expenses declined by $2.7 million, or 24.3%, to $8.5 million. Savings in the areas of payroll, external consultants, temporary labor and materials and supplies ($3.3 million for the nine-month period) were partially offset by an increase in equity compensation expense of $0.5 million, which was primarily related to the accelerated vesting of restricted ownership units held by an employee terminated during the period as well as the stock options issued during the first nine months of 2011. The savings mentioned above were also partially offset by an impairment charge of $0.4 million recorded during the first nine months of 2011 to reduce certain assets to their estimated realizable values.
Interest expense—related party. For the nine months ended September 30, 2011 as compared to the nine months ended September 30, 2010, interest expense—related party increased by $1.7 million, or 59.5%, to $4.6 million. These increases were due to additional funding of our operations through notes payable from related parties over the last year. The balance of these notes totaled $72.1 million at September 30, 2011 and $44.8 million at September 30, 2010.
Depreciation expense. For the nine months ended September 30, 2011 as compared to the same period in 2010, depreciation expense decreased by $104,000, or 13.4%, to $669,000. The decrease during the nine months ended September 30, 2011 as compared to the same period in 2010 was due to assets which were fully depreciated or impaired over the last year, offset partially by the addition of new capital assets.
Non-controlling interest. As of September 30, 2011 and 2010, non-controlling interests collectively owned approximately 12.7% and 13.2% of PA LLC, respectively, and have been attributed their respective portion of the loss of PA LLC. For the nine months ended September 30, 2011 and 2010, non-controlling interests were assigned $2.8 million and $3.8 million of the net loss, respectively.
Comparison of Years Ended December 31, 2009 and 2010
| December 31, 2009 |
December 31, 2010 |
Variance | ||||||||||||||
| Amount | Percentage | |||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | N/M | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Costs and expenses: |
||||||||||||||||
| Selling, general and administrative |
17,400,801 | 21,849,915 | 4,449,114 | 26% | ||||||||||||
| Research and development |
15,085,060 | 17,424,316 | 2,339,256 | 16% | ||||||||||||
| Interest expense—related party |
3,198,642 | 4,076,905 | 878,263 | 27% | ||||||||||||
| Depreciation |
1,137,733 | 1,128,249 | (9,484 | ) | N/M | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
36,822,236 | 44,479,385 | 7,657,149 | 21% | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss before non-controlling interest |
(36,822,236 | ) | (44,479,385 | ) | (7,657,149 | ) | (21)% | |||||||||
| Non-controlling interest |
6,554,358 | 6,469,356 | (85,002 | ) | N/M | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (30,267,878 | ) | $ | (38,010,029 | ) | $ | (7,742,151 | ) | (26)% | ||||||
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted common shares outstanding |
104,866,065 | 106,731,469 | (1,865,404 | ) | N/M | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted loss per share |
$ | (0.29 | ) | $ | (0.36 | ) | $ | (0.07 | ) | (24)% | ||||||
|
|
|
|
|
|
|
|||||||||||
44
Table of Contents
Revenue. We did not record any revenues in 2010 or 2009.
Total costs and expenses. Total costs and expenses increased by $7.7 million, or 20.8%, to $44.5 million in 2010 as compared to 2009, primarily due to an increase in selling, general and administrative expenses and research and development expenses for the reasons described below.
Selling, general and administrative expenses. Selling, general and administrative expenses increased by $4.4 million, or 25.6%, to $21.8 million, in 2010 as compared to 2009. The increase in 2010, as compared to 2009, was due to a $9.1 million increase in equity compensation expense which was primarily related to a modification of terms to restricted ownership units previously issued to certain of our officers. This increase was partially offset by decreases in compensation expenses and consulting expenses of $4.2 million. Compensation expenses within selling, general and administrative functions decreased in 2010 as compared to 2009 due to certain employee salary reductions as well as a 21.1% decrease in employee headcount. Expenses related to external consultants decreased 41% for the same period in an effort to improve the efficiency of our operations.
Research and development expenses. Research and development expenses increased by $2.3 million, or 15.5%, to $17.4 million, in 2010 as compared to 2009. Equity compensation expense increased by $2.5 million during 2010 as compared to 2009, primarily related to the modified terms of the restricted ownership units held by certain of our officers. Also, we recorded a $0.2 million impairment charge related to property and equipment. These increases were slightly offset by a $0.5 million decrease in spending as we needed less expensive materials and supplies for research and development efforts.
Interest expense—related party. Interest expense—related party increased by $0.9 million, or 27.5%, to $4.1 million in 2010 as compared to 2009. The increase was due to additional funding of our operations during 2010 through notes payable from related parties. The balance of these notes totaled $51.7 million at December 31, 2010 as compared to $35.1 million at December 31, 2009.
Depreciation expense. Depreciation expense remained relatively consistent in 2010 as compared to 2009, decreasing by $9,000, or 0.8%, to $1.1 million in 2010. We purchased fewer new assets in 2010 as compared to 2009, which was offset by a number of assets becoming fully depreciated during 2010.
Non-controlling interest. As of December 31, 2010 and 2009, non-controlling interest parties collectively owned approximately 13% and 18%, respectively, of PA LLC and have been attributed their respective portion of the loss of PA LLC. The decrease in the non-controlling interest percentage during 2010 was due to the June 2, 2010 transaction with Arizona Sciences and Technology Enterprises, LLC, or AzTE, which provided for recovery of the 1,000,000 Class A voting units of PA LLC and the cancellation of all existing and future obligations and agreements between us and AzTE in exchange for the return of certain AzTE-licensed technology and a payment of $0.6 million. The amount of loss assigned to non-controlling interests was $6.5 million in 2010 as compared to $6.6 million in 2009.
45
Table of Contents
Comparison of Years Ended December 31, 2008 and 2009
| December 31, 2008 |
December 31, 2009 |
Variance | ||||||||||||||
| Amount | Percentage | |||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | N/M | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Costs and expenses: |
||||||||||||||||
| Selling, general and administrative |
8,251,676 | 17,400,801 | 9,149,125 | 111% | ||||||||||||
| Research and development |
9,865,844 | 15,085,060 | 5,219,216 | 53% | ||||||||||||
| Interest expense—related party |
1,764,039 | 3,198,642 | 1,434,603 | 81% | ||||||||||||
| Depreciation |
268,691 | 1,137,733 | 869,042 | 323% | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
20,150,250 | 36,822,236 | 16,671,986 | 83% | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss before non-controlling interest |
(20,150,250 | ) | (36,822,236 | ) | (16,671,986 | ) | (83)% | |||||||||
| Non-controlling interest |
162,400 | 6,554,358 | 6,391,958 | 3,936% | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (19,987,850 | ) | $ | (30,267,878 | ) | $ | (10,280,028 | ) | (51)% | ||||||
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted common shares outstanding |
100,362,021 | 104,866,065 | 4,504,044 | N/M | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted loss per share |
$ | (0.20 | ) | $ | (0.29 | ) | $ | (0.09 | ) | (45)% | ||||||
|
|
|
|
|
|
|
|||||||||||
Revenue. We did not record any revenues in 2009 or 2008.
Total costs and expenses. Total costs and expenses increased by $16.7 million, or 83%, to $36.8 million in 2009 as compared to 2008, primarily due to an increase in selling, general and administrative expenses and research and development expenses as we expanded our operations.
Selling, general and administrative expenses. Selling, general and administrative expenses increased $9.1 million, or 111%, to $17.4 million in 2009 as compared to 2008. The increase in 2009, as compared to 2008, was due to a significant increase in the scope of our business development activities as well as increased compensation expense related to our enlarged management team. During the period from our inception to August 1, 2008, a portion of our selling, general and administrative functions, including the majority of business development activities, were provided by XL Tech and charged to us. The portion of selling, general and administrative expenses that arose from these related party charges was $2.2 million in 2008.
Research and development expenses. Research and development expenses increased $5.2 million, or 53%, to $15.1 million in 2009 as compared to 2008. The increase in 2009, as compared to 2008, was driven by a higher number of both external consultants and employees involved in research and development relating to various components of our system, as well as higher materials and equipment costs related to the design and construction of our demonstration facility.
Interest expense—related party. Interest expense on notes payable—related party increased by $1.4 million, or 81%, to $3.2 million in 2009 as compared to 2008. The increase in 2009, as compared to 2008, was due to additional funding of our operations through notes payable from related parties. The balance of these notes totaled $35.1 million at December 31, 2009 as compared to $23.6 million at December 31, 2008.
Depreciation expense. Depreciation expense increased by $0.9 million, or 323%, to $1.1 million in 2009 as compared to 2008. The increase in 2009, as compared to 2008, was due to an increase in our capital asset base as a result of the purchase of more laboratory equipment, computer equipment, furniture, and leasehold improvements in 2009 as compared to 2008.
46
Table of Contents
Non-controlling interest. As of December 31, 2009, non-controlling interest parties collectively owned approximately 18% of PA LLC and have been attributed their respective portion of the loss of PA LLC. The amount of loss assigned to non-controlling interests was $6.6 million in 2009 as compared to $0.2 million in 2008.
Critical Accounting Policies and Management Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with generally accepted accounting principles, or GAAP. The preparation of these consolidated financial statements require us to make estimates and assumptions based on experience and on various assumptions that we believe are reasonable under the circumstances that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the period. Actual results may differ from these estimates.
While our significant accounting policies are more fully described in Note 1 of our consolidated financial statements that are included elsewhere in the prospectus, we believe the following accounting policies are critical to the process of making significant judgments and estimates in the preparation of our consolidated financial statements.
Revenue Recognition
We will begin to record revenue when persuasive evidence of an arrangement exists, services have been rendered or product delivery has occurred, the sales price to the customer licensee is fixed or determinable, and collectability is reasonably assured. To the extent cash is received in advance of our performance or the availability of rights under a license agreement or such receipts are refundable at the customer licensee’s option, these amounts are reported as deferred revenue.
Cash and Cash Equivalents and Cash Concentrations
We consider all highly liquid investments with a remaining maturity of three months or less when purchased to be cash equivalents. At September 30, 2011, we had all of our cash on deposit at a single financial institution.
Fair Value of Financial Instruments
Fair value estimates discussed herein are based upon certain market assumptions and pertinent information available to management. The respective carrying values of certain on-balance-sheet financial instruments approximate their fair values. These financial instruments include cash and cash equivalents, accounts payable and accrued expenses. Fixed rate carrying values are assumed to approximate fair values for these financial instruments because they are short term in nature and their carrying amounts approximate the amounts expected to be received or paid.
The carrying value of our fixed-rate notes payable—related party approximate fair value based on the current market conditions for similar debt instruments.
Property and Equipment
Property and equipment is recorded at cost. Expenditures for major improvements and additions are added to property and equipment, while replacements, maintenance and repairs which do not extend the useful lives are expensed.
47
Table of Contents
Long-lived Assets
The carrying value of long-lived assets is reviewed on a regular basis for the existence of facts and circumstances that suggest permanent impairment. Specifically, our senior management considers in each reporting period the effectiveness of our significant assets to determine if impairment indicators such as physical deterioration, process change or technological change have resulted in underperformance, obsolescence or a need to replace such assets. We group our assets into three primary categories for this purpose. Due to the nature of our business, cash flows are not derived from specific asset groups, therefore this grouping is merely to aid our analysis.
| Ÿ | Office equipment, including leasehold improvements |
| Ÿ | Demonstration system and engineering equipment |
| Ÿ | Computer software and hardware—applications and networking |
We do not consider our net loss or cash outflow from operations to be an indicator of asset impairment as such financial results are typical for technology companies that are in the development stage. Rather, we evaluate the state of our technology, our process toward technical and business milestones, and relevant external market factors to ascertain whether a potential impairment has occurred. During 2010 and the nine months ended September 30, 2011, we identified certain changes in our process development plans that eliminated or changed the need or expected use of certain assets. As a result, impairment charges of $0.2 million and $0.4 million, respectively, were recorded as a reduction of property and equipment and an addition to research and development expense to reduce these assets to their estimated realizable value. No impairment indicators were noted in any of the years ended December 31, 2009, 2008 or 2007 or the nine-month period ended September 30, 2010 and our process of monitoring these assets was unchanged during these periods.
When we identify an impairment indicator, we measure the amount of the impairment based on the amount that the carrying value of the impaired asset exceeds our best internal estimate of the value of the asset while it remains in use, plus any proceeds expected from the eventual disposal of the impaired asset. Since we use most of our property and equipment to demonstrate our technology to prospective customer licensees and not to generate revenues or cash flows, a cash flow analysis of the categories above or the assets as a whole is not practicable. Instead, a depreciated replacement cost estimate is used for assets still in service and equipment secondary market quotes are obtained for assets planned for disposal. Depreciated replacement cost is determined by contacting vendors of the same or similar equipment or other assets to obtain an estimate of the market price of such assets, and then reducing this amount by a formula derived from the age of the equipment and the typical useful life of that equipment. For assets planned to be disposed, descriptions of the equipment are circulated to a number of purchasers of used machinery and equipment to solicit bids for such assets. Our best estimate of selling price based upon these purchasers’ responses is used to estimate the value of such assets, net of any costs to remove, recondition and/or ship the equipment to the buyer.
Income Taxes
We have not recorded any income tax expense or benefit for the four years ended December 31, 2010 and the nine months ended September 30, 2011 and 2010 primarily due to our history of operating losses. We follow Accounting Standards Codification, or ASC, 740, Income Taxes, for recording the provision for income taxes. Deferred tax assets and liabilities are computed based upon the difference between the financial statement and income tax basis of assets and liabilities using the enacted marginal tax rate applicable when the related asset or liability is expected to be realized or settled. Deferred income tax expenses or benefits are based on the changes in the asset or liability each period. If available evidence suggests that it is more likely than not that some portion or all of the deferred tax assets will not be realized, a valuation allowance is required to reduce the deferred tax
48
Table of Contents
assets to the amount that is more likely than not to be realized. Future changes in such valuation allowance are included in the provision for deferred income taxes in the period of change.
As required by ASC 740-10-05, Accounting for Uncertainty in Income Taxes, we recognize the financial statement effects of a tax position when it is more likely than not (defined as a likelihood of more than 50%), based on the technical merits, that the position will be sustained upon examination. The evaluation of whether a tax position meets the more-likely-than-not recognition threshold requires a substantial degree of judgment by management based on the individual facts and circumstances. Actual results could differ from these estimates.
PA LLC is organized as a limited liability company under the state law of Delaware. As a limited liability company that has elected to be taxed as a partnership, our earnings pass through to the members and are taxed at the member level. Accordingly, the income tax provision included in the accompanying consolidated financial statements represents the tax impact arising from our ownership interest in the operations related to PA LLC.
Because we have incurred an accumulated deficit during the development stage of $117.1 million as of September 30, 2011 and cannot predict with certainty our future profits, our deferred tax assets and the potential future benefit of our net operating losses, to the extent deductible for income tax purposes, are fully reserved.
Stock-based Compensation
We account for stock-based compensation in accordance with ASC 718, Stock Compensation. The Statement requires that the cost resulting from all stock-based transactions be recorded in the financial statements. The Statement establishes fair value as the measurement objective in accounting for stock-based payment arrangements with employees. The Statement also establishes fair value as the measurement objective for transactions in which an entity acquires goods or services from non-employees in stock-based payment transactions.
We have made the following equity-based compensation awards to our employees over the period from January 1, 2007 to September 30, 2011:
| Grant Type and Dates |
Number Granted |
Grant Date Fair Value(1) |
Fair Value Per Share |
|||||||||
| 2007 PA LLC profits interests |
674,500 | $ | 2,786,199 | $ | 4.13 | |||||||
| 2008 PA LLC profits interests |
2,816,471 | 19,046,340 | $ | 6.76 | ||||||||
| 2009 PetroAlgae Inc. stock options |
1,117,500 | 3,023,100 | $ | 2.71 | ||||||||
| 2010 PetroAlgae Inc. stock options |
322,000 | 1,223,600 | $ | 3.80 | ||||||||
| 2011 PetroAlgae Inc. stock options |
1,239,000 | 4,476,570 | $ | 3.61 | ||||||||
| 2011 PetroAlgae Inc. stock appreciation rights |
1,000,000 | 3,847,250 | $ | 3.85 | ||||||||
|
|
|
|||||||||||
| Total fair value at date of grant |
$ | 34,403,059 | ||||||||||
| Aggregate impact of forfeitures |
(6,154,640 | ) | ||||||||||
|
|
|
|||||||||||
| Equals: net historical and future expense |
$ | 28,249,019 | ||||||||||
|
|
|
|||||||||||
| (1) | Value reflects modifications made subsequent to original grant date, as discussed below. |
PA LLC profits interests. During 2007 and 2008, we granted the majority of our employees these interests as of the date that they began employment with PA LLC. These ownership interests give the recipient the right to participate in the income of PA LLC and any distribution that may arise from a liquidity event to the extent that such realized amounts exceed the ownership unit fair value at the date of grant. We determined the fair value of these grants using a probability-weighted present
49
Table of Contents
value calculation, as further described below. Upon the completion of this offering, these interests will become exchangeable into shares of our common stock. The total number of shares issuable upon such exchanges are included in the shares of our common stock reserved for issuance upon the exchange of units of PA LLC.
The fair value of the profit interests granted was calculated by modeling the cash impact of possible future liquidity event scenarios in relation to the economics of the agreements. The key steps in this process for each grant were as follows:
| Ÿ | The likelihood and expected value of an initial public offering, a sale to a strategic buyer, and a financial sale or leveraged buyout were estimated based on information that was available to management at the date of the grants; |
| Ÿ | The probability of achieving each scenario was estimated based upon our outlook at grant date based upon milestones accomplished at that point and future hurdles; |
| Ÿ | The probability-weighted future values were converted to future liquidity per share by dividing projected proceeds by the units outstanding before each grant; |
| Ÿ | The estimated fair value per unit at grant date (determined by employing discounted cash flow analyses to the business’ projections) was deducted from the estimated future value per unit to derive the gross potential profits interest for each grant; and |
| Ÿ | The gross profits interest was then discounted to present value based upon the weighted average time required to accomplish estimated liquidity events using a 60% discount rate, which is consistent with discount rates applied in the valuation of development stage technology companies. |
Upon issuance, the PA LLC profit interests grants contain restrictions that allow us to repurchase the shares for nominal consideration until a service period or other condition is met. This restriction is removed over a service period (generally four years) with regard to approximately 2.2 million of the interests with an initial grant date fair value of approximately $10.4 million. Consequently, the related compensation expense is recognized ratably over the remaining service period as a selling, general and administrative or research and development expense, depending upon the recipient’s role in us. Five senior officers received an aggregate 1.3 million profits interests that were subject to repurchase for nominal consideration until a significant liquidity event occurred. As of December 31, 2010, our principal stockholders authorized the modification of the terms of the grant to remove our right to repurchase the Interests held by all five officers, causing these grants to be immediately vested. This decision required a revaluation of the Interests held by the officers and immediate recognition of the calculated expense. Based upon current estimates and applying the same discounted cash flow methodology used as of the prior valuation dates, the compensation cost recognized during the fourth quarter of 2010 was $11.4 million, recorded as selling, general and administrative expense ($9.1 million) or research and development expense ($2.3 million) depending upon each officer’s role.
PetroAlgae Inc. stock options. These options to purchase our stock generally become exercisable on a ratable basis over four years beginning at each recipient’s date of hire. We determined the fair value of these options using the Black-Scholes model using the assumptions listed below. Compensation cost is recognized as a selling, general and administrative or research and development expense, depending upon the recipient’s role with us, over the required service period of each grant.
On June 30, 2011, our board of directors authorized the re-pricing of all outstanding stock options, changing the exercise price from $8.00 or $8.50 to $5.50 per option. In accordance with ASC 718-20-35-3, we computed the additional compensation cost as the fair value of the new awards in
50
Table of Contents
excess of the fair value of the original awards immediately before their terms were modified, using the Black-Scholes pricing model. This calculation used the following assumptions determined as of June 30, 2011:
| Ÿ | estimated fair value of the common stock of $5.10; |
| Ÿ | remaining terms ranging from 4.75 years to 5.89 years (depending on the date of the original grant); |
| Ÿ | risk free interest rate of 1.76%; |
| Ÿ | estimated volatility of 96.5%; and |
| Ÿ | dividend yield of 0.0%. |
The modification affected 1,240,000 options and resulted in aggregate additional compensation cost of $0.4 million. Of the total additional compensation cost, $0.2 million was expensed immediately as it related to options which were vested as of June 30, 2011, and the remaining $0.2 million will be expensed through 2014.
Additionally, on June 30, 2011, our board of directors authorized the grant of 616,000 options to employees, in the aggregate, to purchase common shares at an exercise price of $5.50 per share. Of the 616,000 options authorized, 351,000 were granted on June 30, 2011 and the remaining 265,000 were granted on July 21, 2011. The fair value of the options was calculated using the Black-Scholes pricing model with assumptions identical the ones listed above.
Due to the significant number of equity transactions that occurred during June of 2011, including the stock option modification and stock option issuance discussed above and the stock appreciation rights, or SARs, issuance discussed below, we re-examined our volatility estimate and consequently increased it from 75.2% to 96.5% on a prospective basis. We estimate volatility in accordance with SEC Staff Accounting Bulletin, or SAB, No. 107, “Share-based Payment” based on an analysis of expected volatility of share trading prices for a peer group of companies. Over a period of time similar to that of the expected option life, the volatility in share price of these alternative energy and clean-tech companies averaged 70.4%. To account for the fact that we are in the development stage and can be expected to have a higher volatility at our present stage than the average of the comparable companies, we adjusted upward our volatility estimate. Specifically, we estimated our share volatility by calculating the average volatility of those members of our peer group that exhibited volatility measures in the top quartile of the group. Using this approach, we determined that a volatility estimate of 96.5% is appropriate in our fair value calculations.
The Black-Scholes calculations resulted in the determination of the following total compensation cost for each grant after the modification:
| Grant Dates |
June 2009 | July 2009 | January 2010 | January 2011 | June and July 2011 |
|||||||||||||||
| Fair value per option |
$ | 2.70 | $ | 2.83 | $ | 3.80 | $ | 3.27 | $ | 3.96 | ||||||||||
| Number of options granted |
1,072,500 | 45,000 | 322,000 | 623,000 | 616,000 | |||||||||||||||
| Fair value |
$ | 2,895,750 | $ | 127,350 | $ | 1,223,600 | $ | 2,037,210 | $ | 2,439,360 | ||||||||||
| Expected term(1) |
4.75 years | 4.75 years | 5.15 years | 5.89 years | 6.25 years | |||||||||||||||
| (1) | In all cases, with the exception of June and July 2011 issuances, the expected term shown here represents the remaining term used in the Black-Scholes calculation at the time the stock options were re-valued at June 30, 2011. |
51
Table of Contents
PetroAlgae Inc. Stock Appreciation Rights. In November 2010, we issued one million SARs to Mr. Harris, our former President and Chief Operating Officer, under the 2009 Plan. The vesting of these SARs was tied to continued employment with us and the accomplishment of certain milestones. Mr. Harris resigned during the first quarter of 2011, forfeiting all such SARs. The impact on all periods presented was not material.
In June 2011, our board of directors authorized the grant of one million SARs at a base grant price of $5.50 per share to our newly appointed Chief Executive Officer under the 2009 Plan. The SARs have a ten-year term and will vest in equal quarterly installments over a two-year period. In the event of a change of control (as defined in the 2009 Plan) or a qualified public offering (as defined in the executive’s employment agreement), the SARs will become 100% vested. The grant date fair value of the SARs aggregated to $3.8 million and was calculated using the Black-Scholes pricing model with the following assumptions determined as of the date of grant: estimated fair value of the common stock of $5.10, expected term of 5.75 years, risk free interest rate of 1.55%, an estimated volatility of 96.5%, and a dividend yield of 0%. The fair value will be charged to operations over the remaining vesting periods commencing on the grant date.
Issuance of Shares and Warrants in Financing Transactions
Estimation of Common Stock Fair Value. In each reporting period, we have determined our best estimate of the fair value of our common stock by considering the following indicators of value:
| Ÿ | Level 3 valuations based upon a discounted cash flow analysis using inputs which were not externally observable, in this case our estimate of future cash flows from our business; |
| Ÿ | Prices paid in cash for shares of stock or securities units comprised of equity shares and five-year warrants to purchase equity shares; and |
| Ÿ | Over-The-Counter (“OTC”) quotation closing price and related statistics such as trading volume, volatility and recent price ranges. |
Level 3 estimated cash flow valuations. The Level 3 fair value estimate of our equity value was determined by applying discounted cash flow valuation techniques to expected future cash flows from our business as of each valuation date. Key assumptions utilized in this fair value estimate include the expected amount and timing of revenue from expected future sales of our technology licenses along with the associated cost of sales and other operating cash outflows. These cash flows are discounted to present value using a discount rate consistent with development stage technology companies and the resulting enterprise value was converted to an equity value per outstanding share.
The most significant uncertainty inherent in these calculations is the risk of obtaining and performing the contracts necessary to produce the estimated future revenues. In addition, the risk of collection of amounts that are expected to be due under projected contracts, the risk of unanticipated product development or delivery costs or timing delays and the risk of increased operating and overhead costs may each cause the estimates of future net cash flows from operations to vary from the assumptions that are made in these projections, and these variations may be material. Since we have not obtained or performed any commercial contracts to date, we do not have historical data on which to base our estimate of future revenues.
Because the Level 3 indications of value were able to take into account important information pertaining to the amount, timing and risk of our expected cash flows, they were given the most weight in our estimate of share value.
Third party transactions. From January 2009 through August 2010, we completed several private sales of equity shares and securities units comprised of both shares and warrants to purchase
52
Table of Contents
shares. The transactions were completed with both related and unrelated parties in exchange for cash. Given the size of these transactions and the sophisticated nature of the parties involved in their negotiation, these indications of value were given significant weight in our estimate of share value. However, because most of these transactions were with affiliates, sole or primary reliance was not placed on these values.
OTC Values. Our common stock is currently quoted on the OTCQB. A very small percentage of the issued and outstanding shares are available for active trading since approximately 94% of our outstanding shares are held by our principal stockholders. Due to the very limited size of our public float and the resulting low trading volume in our common stock, our public shares are susceptible to very large swings in price based on very few trades. As a result of these considerations, value indications provided by the OTC price quotation service were given the least weight of the three factors in our estimate of share value.
Estimation of Warrant Fair Value. We estimated the fair value of warrants issued during 2010 using the Black-Scholes valuation model, based on the estimated fair value of our common stock on the valuation date, an expected dividend yield of 0%, a risk-free interest rate based on constant maturity rates published by the U.S. Federal Reserve applicable to the remaining term of the instruments, an expected life equal to the remaining term of the instruments and an estimated volatility of 75.2%. No warrants have been issued during 2011.
Liquidity and Capital Resources
Overview
To date, we have financed our operations primarily through loans from, and debt securities issued to, our principal stockholders. To a lesser extent, we have also raised funds by issuing equity securities to unrelated third parties. Since our inception through September 30, 2011, we have raised in the aggregate $102.0 million in debt and equity investments, with $92.1 million of this total raised from our principal stockholders and $9.9 million raised from unrelated third parties.
We are a holding company whose sole asset is our controlling equity interest in our operating subsidiary, PA LLC. We operate and control all of the business and affairs, and consolidate the financial results, of PA LLC. PA LLC was originally founded in September 2006 by XL Tech, as a private company focused on developing technologies for the renewable energy market. From its inception through August 2008, PA LLC was financed by XL Tech. XL Tech provided $16.6 million of debt financing through August 2008. In August 2008, XL Tech exchanged certain of its assets, including the equity it held in PA LLC, for the outstanding debt of XL Tech that was held by our principal stockholders. In December 2008, our principal stockholders acquired a shell company that was quoted on the OTCBB and assigned its interest in PA LLC to this shell company, which it then renamed PetroAlgae Inc. Since August 2008, our principal stockholders have been primarily funding our operations.
Issuances of debt instruments. The majority of our capital has been debt financing provided by our principal stockholders, which is evidenced by promissory notes. Our fixed-rate notes, of which an aggregate principal amount of $52.5 million was outstanding at September 30, 2011, are secured by all of our assets, are each due on June 30, 2012 and each bear interest at an annual fixed rate of 12%. An earlier note with an outstanding balance of $19.6 million at September 30, 2011 bears interest at a floating rate of prime plus 2% and accrues interest on a payment-in-kind, or PIK, basis under which the accrued interest is added to the principal amount of the note. Subsequent to the end of the third quarter of 2011, our principal stockholders provided $4.4 million of additional debt financing to our operating subsidiary at an annual fixed rate of 12%.
53
Table of Contents
Issuance of common stock and warrants. In December 2008, our principal stockholder paid $10.0 million for 3.2 million shares of our common stock (approximately $3.15 per share), which was estimated to be the fair value of the shares at that time. In the second half of 2009 through August of 2010, we issued units comprised of a share of our common stock and an option to purchase a second share of common stock at any time over a five-year period at a price of $15.00. In each case, these units were issued for a cash price of $8.00. Over this period, our principal stockholders paid $10.0 million in cash for 1,250,000 units and unaffiliated third parties paid $9.9 million in cash for 1,235,000 units.
Operating and Capital Expenditure Requirements
We intend to apply the net proceeds from this offering, any cash we generate from operations and our cash balances to meet our anticipated operating and capital expenditure requirements. In the future, we expect our operating and capital expenditures to increase as we increase our headcount, expand our business activities, grow our customer licensee base and implement and enhance our technology and processes.
If our available cash balances and net proceeds from this offering are insufficient to satisfy our liquidity requirements, we may seek to sell equity or debt securities or enter into a credit facility. The sale of equity may result in dilution to our stockholders and any deficit we issue may have rights senior to those of our common stock. If we raise additional funds through the issuance of debt securities, these securities could contain covenants that would restrict our operations. Any credit facility would be likely to contain very significant restrictions on our business activities and might not be available to us on economically viable terms. We may require additional capital beyond our currently anticipated amounts. Additional capital may not be available on reasonable terms, or at all.
Our estimates of the period of time through which our financial resources will be adequate to support our operations and the costs to support research and development and our sales and marketing activities are forward-looking statements and involve risks and uncertainties and actual results could vary materially and negatively as a result of a number of factors, including the factors discussed in the section “Risk Factors” of this prospectus. We have based our estimates on assumptions that may prove to be wrong and we could utilize our available capital resources sooner than we currently expect.
Our short- and long-term capital requirements will depend on many factors, including the following:
| Ÿ | our ability to sign binding contracts and generate cash from operations; |
| Ÿ | our ability to control our costs; |
| Ÿ | the costs of competing with alternative technological developments; |
| Ÿ | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights or participating in litigation-related activities; and |
| Ÿ | our ability to consummate this offering. |
54
Table of Contents
Sources and Uses of Cash
At September 30, 2011, we had unrestricted cash and cash equivalents of $6.7 million compared to $1.1 million at December 31, 2010 and $2.7 million at December 31, 2009. The changes in the level of cash balances principally relates to timing of debt and equity transactions. The following table presents a summary of our cash and cash equivalents, and cash flows, for the periods indicated.
| Summary of Net Cash Flows | ||||||||||||||||||||
| For the year ended December 31, |
For the nine months ended September 30, |
|||||||||||||||||||
| 2008 | 2009 | 2010 | 2010 | 2011 | ||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Net cash used in operations |
$ | (11,849,362 | ) | $ | (27,602,288 | ) | $ | (22,783,504 | ) | $ | (17,114,869 | ) | $ | (13,423,545 | ) | |||||
| Net cash used in investing activities |
(812,660 | ) | (2,435,233 | ) | (1,034,916 | ) | (785,010 | ) | (526,958 | ) | ||||||||||
| Net cash provided by financing activities |
22,894,700 | 22,300,000 | 22,280,000 | 15,580,000 | 19,525,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (decrease) increase in cash |
$ | 10,232,678 | $ | (7,737,521 | ) | $ | (1,538,420 | ) | $ | (2,319,879 | ) | $ | 5,574,497 | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents-beginning of period |
$ | 184,145 | $ | 10,416,823 | $ | 2,679,302 | $ | 2,679,302 | $ | 1,140,882 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents-ending of period |
$ | 10,416,823 | $ | 2,679,302 | $ | 1,140,882 | $ | 359,423 | $ | 6,715,379 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Cash Flows from Operating Activities
Cash flows used in operating activities totaled $13.4 million in the nine months ended September 30, 2011, compared to $17.1 million in the same period in 2010. The decrease in cash usage was primarily attributed to decreased payroll costs due to a lower headcount ($2.4 million) and cost savings resulting from less utilization of external consultants ($0.9 million) for the nine months ended September 30, 2011 as compared to the same period in 2010.
During 2010, cash flows used in operating activities totaled $22.8 million, compared to $27.6 million in 2009. This decrease was primarily attributed to cost savings of $3.8 million in the area of external consultants.
During 2009, cash flows used in operating activities totaled $27.6 million as compared to $11.8 million in 2008. The increase in cash usage from 2008 to 2009 was primarily attributed to increased compensation expenses and external consultant expenses of $12.1 million to fund our research and product development activities and to build our operational and business development efforts.
Cash Flows from Investing Activities
Cash flows used in investing activities totaled $0.5 million in the nine months ended September 30, 2011, compared to $0.8 million in the same period during the prior year. The decline in expenditures between the two periods reflects the purchase of less equipment during 2011 as compared to 2010 since our technology development and laboratory testing are approaching completion.
During 2010, cash flows used in investing activities totaled $1.0 million, compared to $2.4 million in 2009. This decrease was due to the purchase of less equipment during 2010 than in 2009 because our demonstration facility had been completed in 2009.
55
Table of Contents
During 2009, cash flows used in investing activities totaled $2.4 million, compared to $0.8 million in 2008. These amounts represented the continued growth and expansion of our technology development assets, primarily from the construction of our demonstration facility, which was completed in 2009.
Cash Flows from Financing Activities
Cash flows provided by financing activities totaled $19.5 million in the nine months ended September 30, 2011, compared to $15.6 million in the same period during the prior year. For the nine months ended September 30, 2011 and 2010, cash raised from the issuance of common stock and warrants decreased by $7.6 million while borrowings under notes payable-related party increased by $11.0 million.
During 2010, cash flows provided by financing activities totaled $22.3 million, including $20.2 million funded by affiliates and $2.1 million funded by third parties, compared to $22.3 million in 2009, $14.5 million of which was funded by affiliates and $7.8 million of which was funded by third parties.
During 2008, cash flows provided by financing activities totaled $22.9 million. This consisted of debt and equity funding, all from affiliates.
Capital Expenditures
During the nine months ended September 30, 2011, we made total capital expenditures of $0.5 million, primarily related to our demonstration facility, including purchasing the land and upgrading certain equipment used in our processing facility. For the nine months ended September 30, 2010, our total capital expenditures were $0.8 million, which consisted of equipment used in furthering our research and development related to our technology. Our total capital expenditures during 2010 were $1.0 million, compared to $2.5 million during 2009. This decrease in capital spending is attributable to the completion of construction on our demonstration facility in 2009.
Senior Secured Credit Financings
Our debt is comprised of the following senior secured notes (payable to our principal stockholders or their affiliates) at September 30, 2011:
| Note Payable to Valens U.S. SPV I, LLC |
$ | 417,512 | ||
| ŸInterest accrues monthly at an annual rate of 12% |
||||
| ŸNote is due on June 30, 2012 |
||||
| Notes Payable to PetroTech |
42,047,089 | |||
| ŸInterest accrues monthly at an annual rate of 12% |
||||
| ŸNotes are due on June 30, 2012 |
||||
| Convertible Note Payable to PetroTech |
10,000,000 | |||
| ŸInterest accrues monthly at an annual rate of 12% Ÿ Note is due June 30, 2012 unless converted to common shares as described below |
||||
| Up to $25,000,000 Note Payable to PetroTech |
19,588,014 | |||
| ŸInterest payable monthly, and is drawn into note on a monthly basis at Prime + 2% (5.25% at September 30, 2011 and December 31, 2010) |
||||
| ŸNote is due on June 30, 2012 |
||||
| ŸPermits additional draws to fund interest. Maximum balance of this note is limited to $25,000,000. |
||||
|
|
|
|||
| Total Notes Payable—Related Party |
$ | 72,052,615 | ||
|
|
|
|||
56
Table of Contents
As of September 30, 2011, the principal balance of notes payable—related party has been reclassified to current liabilities as the maturity date is within one year. The notes payable—related party are secured by all of our assets. The convertible note payable allows the holder (at the holder’s option) to convert all or any portion of the issued and outstanding principal amount and/or accrued interest and fees then due into shares of our common stock at a fixed conversion price of $5.43 per share.
Each note payable and the related master security agreement and equity pledge and corporate guaranty agreement contain provisions that specify events of default which could lead to acceleration of the maturity of the debt. These provisions prohibit the encumbrance or sale of our assets and require maintenance and insurance of our assets. The loan agreements do not contain any required financial ratios or similar debt covenants. Generally, an event of default would arise if we became insolvent, filed for bankruptcy, allowed a change in control or had unresolved judgments against its assets. Through the date of this prospectus, none of these events had occurred.
Interest charged to operations on these notes was $4.6 million and $2.9 million during the nine months ended September 30, 2011 and 2010, respectively. Interest expense-related party, including amortization of original issue discount, was $4.1 million, $3.2 million and $1.8 million during the years ended December 31, 2010, 2009 and 2008, respectively.
The principal amount of debt obligations as of September 30, 2011 was $72.1 million, of which $19.6 million was outstanding at a floating rate of 2% over the prime interest rate and $52.5 million was outstanding at a fixed rate of 12%. Floating rate borrowings will lead to additional interest expense if interest rates increase.
Contractual Obligations
The following table discloses aggregate information about our contractual obligations and period in which payments are due as of September 30, 2011:
| Payments Due by Period | ||||||||||||||||||||||
| Total | Less than 1 year |
1-3 years | 3-5 years | More than 5 years |
||||||||||||||||||
| Debt: principal |
$ | 72,052,615 | $ | 72,052,615 | $ | — | $ | — | $ | — | ||||||||||||
| Debt: accrued interest to date(1) |
8,682,148 | 8,682,148 | — | — | — | |||||||||||||||||
| Debt: estimated future interest(2) |
5,506,728 | 5,506,728 | — | — | — | |||||||||||||||||
| Operating lease commitments |
989,140 | 527,762 | 461,378 | — | — | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
| Total contractual obligations |
$ | 87,230,631 | $ | 86,769,253 | $ | 461,378 | $ | — | $ | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
| (1) | Represents the amount of interest that has been accrued through the balance sheet date on $52.5 million of outstanding debt at a fixed annual rate of 12%. This amount is due at the June 30, 2012 maturity date of the related debt. |
| (2) | Estimated future interest represents the amount of interest expected to accrue until the maturity date of the related debt, which in all cases is June 30, 2012. The estimated amount is calculated from the current balance sheet date through maturity based upon the principal balance of each note and the applicable interest rate, which is compounded monthly at prime + 2% (assumed to be 5.25%) on $19.6 million of debt and calculated on a non-compounded basis at 12% on the remaining principal amount. |
Recent Transactions Affecting our Liquidity and Capital Resources
Equity issuances. We have not sold any shares during 2011.
Debt issuances. On each of October 5, 2011 and November 18, 2011, our principal stockholders funded $2.2 million to our operating subsidiary, PA LLC, pursuant to the terms of separate secured term notes. Each of these notes provides for interest at 12%, which is accrued as a PIK amount, and are due on June 30, 2012.
57
Table of Contents
Off-Balance Sheet Arrangements
As of September 30, 2011, we did not have any significant off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K.
Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
Our exposure to interest rate risk is related to our borrowings. The principal amount of our debt obligations as of September 30, 2011 was $72.1 million, of which $19.6 million was outstanding at a floating rate of 2% over the prime interest rate and $52.5 million was outstanding at a fixed rate of 12%. Floating rate borrowings will lead to additional interest expense if interest rates increase. We enter into loan arrangements when needed and borrowings are subject to interest rate risk because changes in the prime interest rate will have an effect on interest rate expense.
If interest rates would have increased by 1.0%, the additional interest expense for the nine months ended September 30, 2011 would have been $0.2 million. A similar increase in interest rates for the nine months ended September 30, 2010 would have resulted in approximately $0.1 million in additional interest expense.
Exchange Rate Risk
We are currently not subject to foreign exchange risk as a result of exposures to changes in currency exchange rates. Under the license agreements that we expect to enter into in the future with non-U.S. customer licensees, a portion of its license and royalty fee revenue may be denominated in currencies other than the U.S. dollar. Many of our costs, however, including most of the costs of our employees who we expect to support the operations of our customer licensees, and our research and development costs, will be denominated in U.S. dollars. We expect that fluctuations in the value of these revenues and expenses as measured in U.S. dollars will affect our results of operations, and adverse currency exchange rate fluctuations may have a material impact on our future financial results.
Commodity Price Risk
Our technology, once commercialized, will be used to produce protein for the agriculture market and biocrude for the energy market, each of which may directly or indirectly compete with existing commodities. In particular, we expect that the price our customer licensees will obtain from the sale of these products will most directly be impacted by market prices for protein, crude oil and petroleum-based fuels, respectively. These market prices, particularly for crude oil and petroleum-based fuels, can be volatile, and can be influenced by factors such as global demand, availability of supply, weather, political events and the market price of alternative forms of energy, all of which factors are beyond our control. Significant fluctuations in these commodity prices could impact the profitability of our technology to our customer licensees, and therefore the demand for our technology, and could also affect the amount of royalty revenues we receive from our customer licensees.
Recent Accounting Pronouncements
Adoption of New Accounting Standards
Revenue Recognition
In October 2009, the FASB issued Accounting Standards Update, or ASU, 2009-13, Multiple-Deliverable Revenue Arrangements, or ASU 2009-13. The new standard changes the requirements for establishing separate units of accounting in a multiple element arrangement and requires the allocation
58
Table of Contents
of arrangement consideration to each deliverable based on the relative selling price. The selling price for each deliverable is based on vendor-specific objective evidence, or VSOE, if available, third-party evidence if VSOE is not available, or estimated selling price if neither VSOE or third-party evidence is available. ASU 2009-13 is effective for revenue arrangements entered into in fiscal years beginning on or after June 15, 2010. The adoption of this new guidance did not have a material effect on our consolidated financial statements, nor does management expect it to have a material impact on our expected future revenues.
Fair Value
In January 2010, the FASB issued ASU 2010-6, Improving Disclosures About Fair Value Measurements, or ASU 2010-6, which requires reporting entities to make new disclosures about recurring or nonrecurring fair-value measurements, including significant transfers into and out of Level 1 and Level 2 fair-value measurements and information on purchases, sales, issuances, and settlements on a gross basis in the reconciliation of Level 3 fair-value measurements. ASU 2010-6 is effective for annual reporting periods beginning after December 15, 2009, except for Level 3 reconciliation disclosures which are effective for annual periods beginning after December 15, 2010. The adoption of ASU 2010-6 did not have a material impact on our consolidated financial statements.
Accounting Standards Not Yet Effective
In May 2011, the FASB issued ASU No. 2011-04, Fair Value Measurements and Disclosures (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and International Financial Reporting Standards, or ASU 2011-04, to provide a consistent definition of fair value and ensure that the fair value measurement and disclosure requirements are similar between U.S. GAAP and International Financial Reporting Standards. ASU 2011-04 changes certain fair value measurement principles and enhances the disclosure requirements, mainly for level 3 fair value measurements. ASU 2011-04 is effective for our fiscal year beginning January 1, 2012. Early adoption is not permitted. We are currently evaluating the impact of this new guidance, but does not expect it to have a material impact on our consolidated financial statements.
In June 2011, FASB issued ASU 2011-05, Comprehensive Income (Topic 220): Presentation of Comprehensive Income, or ASU 2011-05. ASU 2011-05 will require companies to present the components of net income and other comprehensive income either as one continuous statement or as two consecutive statements. It eliminates the option to present components of other comprehensive income as part of the changes in stockholders’ equity. The standard does not change the items which must be reported in other comprehensive income, how such items are measured or when they must be reclassified to net income. ASU 2011-05 is effective for interim and annual periods beginning after December 15, 2011, with early adoption permitted. The adoption of ASU 2011-05 will only impact presentation and will not have any effect on our consolidated financial statements or on our financial condition.
59
Table of Contents
Company Overview
Our Business
We provide renewable technology and solutions to address the global demand for new economical sources of feed, food and fuel.
Our objective is to be the leading global provider of technology and processes for the commercial production of micro-crop biomass for agriculture and energy markets. We seek to license renewable technology to meet the significant and growing demand for agricultural products and energy in both emerging and developed economies. We believe that our solution will deliver to our customer licensees strong and consistent economic returns as a consequence of continuous, predictable, year-round operations without the need for government subsidies. We also expect our solution to provide social benefits, through job creation and community investment, as well as significant environmental advantages, through sustainable production that absorbs atmospheric carbon dioxide and does not compete with the existing food supply, require arable land, or pollute soil or water.
We have developed proprietary technology, consisting of integrated environmental management systems, which we believe will allow our customer licensees to grow aquatic micro-crops at accelerated rates. This solution utilizes locally available micro-crops and commercially available processing equipment to produce biomass-based products that can be sold directly into agriculture and energy markets.
Our strategy is to license and provide management support for production facilities in equatorial regions around the world. Our solution is scalable and flexible, which we believe will allow our customer licensees to expand facility capacity and increase production to meet market demand. We intend to generate revenue from licensing fees and royalties primarily from customer licensees that are engaged in the global agriculture and energy markets, as well as from investment groups. Our licensing approach is designed to minimize our capital expenditures, because our customer licensees will be expected to invest in the development and construction of the production facilities.
Our proprietary technology uses indigenous micro-crops (from the Lemnaceae family) that are not genetically modified and demonstrate an optimal growth profile for a particular geography and environment. These micro-crops will then be grown, harvested and processed in a manner that we believe will optimize the production of our micro-crop biomass. To date, the operations at our demonstration facility have deepened our understanding of the variables relating to the micro-crop productivity of our technology. Based on this work, as well as additional modeling, we believe that these micro-crops can be grown and harvested on a commercial scale. Our processes use industry-standard agricultural and food processing equipment in specialized configurations to produce products with consistent quality. During our process, the micro-crops are pressed to separate the pulp from the liquid. The liquid is further processed to create our Lemna Protein Concentrate, or LPC, while the pulp is converted to create our Lemna Meal, or LM, and Biocrude product. Our Biocrude product requires further third-party conversion to facilitate the production of renewable fuels. These products can then be used locally or shipped to the end customer licensees and utilized in the markets depicted below.
60
Table of Contents
Our Products
The diagram below illustrates the products that we expect our technology to produce and their respective end markets.
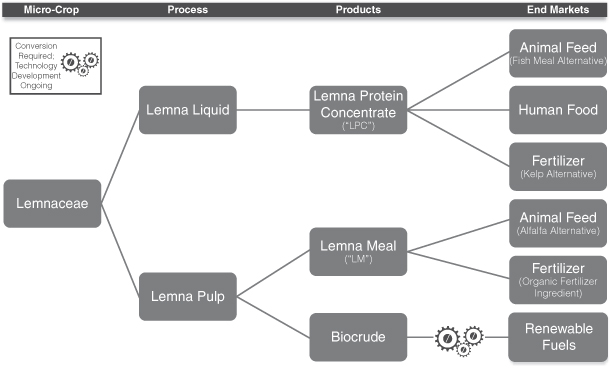
Our Markets
Our current product strategy is to enable customer licensees to sell our LPC product in the global animal feed market as a fish meal alternative and our LM product in the global animal feed market as an alternative to alfalfa meal. We believe that focusing in the near-term on the deployment of our technology for the production of animal feed helps to reduce the market risks associated with our solution, given the relatively high value, high demand, and low complexity of these products.
In addition, we believe that LPC is well-positioned as an alternative to kelp meal, commonly used in fertilizers for turf grasses, such as those on golf courses. In addition, we believe that LM could potentially be used as an ingredient in organic fertilizers and in animal bedding applications.
University studies and other third-party tests completed in 2011 indicate that LPC could potentially be used in human food applications. If the safety and viability of this use is confirmed, we believe that we will be successful in completing the process for regulatory approval required in various countries for the human use of our products, which would significantly increase the value of LPC. See “Business—Regulatory.”
We expect that the continued development of third-party fuel processes will facilitate the commercial production of renewable fuel from Biocrude. We expect these efforts to provide our customer licensees with the option to pursue multiple fuel options with our Biocrude product, including use as a renewable energy feedstock that can be converted into fuel products or burned directly as a
61
Table of Contents
combustion fuel. Biocrude has a high energy content as compared to other biomass feedstocks and does not contain fossil carbon. The process of developing our fuel technology is ongoing. We have completed a number of joint development and collaboration efforts with petrochemical companies that we believe will be of value in the progression of our fuel product platform commercialization.
Our Customer Licensees
We are currently negotiating agreements with prospective customer licensees, including, among others, state-owned enterprises and multinational food companies. We expect that the successful execution of early projects will help validate our technology and ultimately enhance our ability to enter into commercial-scale agreements with other customer licensees.
While we are a development stage company that has not generated any revenue and has experienced net losses since inception, we anticipate future revenues, profit and cash flows, given our recent achievement of important business and technological milestones that we believe will enable us to deliver to our current and future customer licensees solutions for the production of feed, food and fuel products at commercial scale, with attractive economic returns.
AIQ
On October 25, 2011, PA LLC entered into an amended and restated license agreement, or the AIQ Agreement, with AIQ. The AIQ Agreement provides for the construction of a pilot-scale bioreactor in the Republic of Chile and a framework for the subsequent build-out of a commercial-scale facility in South America, subject to the parties’ agreement that the pilot-scale bioreactor has been operated successfully.
The AIQ Agreement provides for three separate phases: the Preliminary Phase, Phase I and Phase II. In the Preliminary Phase, AIQ will construct and operate a pilot-scale bioreactor of 0.75 hectares to test and demonstrate the growth and harvesting aspects of our technology. We will grant AIQ a license to construct and operate the pilot-scale bioreactor in the Preliminary Phase in consideration for a limited license fee from AIQ.
If the parties agree that the Preliminary Phase has been operated successfully, we and AIQ will proceed to Phase I, during which the first commercial-scale unit increment of 150 hectares, including growth, harvesting and processing modules, will be constructed. We have agreed to construct the 150 hectare commercial growth unit on a turnkey basis, in return for payments from AIQ, billed on a progress basis, not to exceed a prescribed limit. After completion of Phase I, the parties will proceed to Phase II. In Phase II, AIQ will continue to expand the project in 150 hectare unit increments, including growth, harvesting and processing modules, until the facility forms a unit of up to 5,000 hectares. Under the AIQ Agreement, we will grant AIQ a license to use our technology to plan, construct and operate the Phase I and Phase II units and to sell the end products produced by these units in South America. We will receive a royalty on net sales of the products generated from every unit and unit increment during the 20-year term of the license.
Our Key Milestones
| Ÿ | In 2006, we began the research and development of our growth and harvesting technologies. Our efforts focused on experimentation and testing, developing processes and equipment, and building the infrastructure and databases necessary to support our technology. |
| Ÿ | During 2007 and 2008, we developed our proprietary growth algorithms—the complex mathematical formulas we use to determine the correct harvesting density and sunlight exposure of the biomass. |
62
Table of Contents
| Ÿ | In 2009, we completed our fully operational demonstration facility and established our business development team. This facility consists of a large bioreactor (approximately one hectare) and three smaller bioreactors that display our technology and processes. |
| Ÿ | In November 2009, the Indonesian Ministry of Agriculture approved our LPC product as an approved raw material for use in animal feed. |
| Ÿ | In May 2011, we entered into an agreement for the construction of a pilot-scale bioreactor in the Republic of Suriname. The construction of this bioreactor was completed in October 2011 and testing is ongoing. |
| Ÿ | In October 2011, we entered into a license agreement with AIQ, a customer licensee in South America, with initial construction of a pilot-scale bioreactor having begun in September 2011. |
| Ÿ | In November 2011, CECEP, a prospective Chinese customer licensee with which we are currently negotiating a license agreement, began construction of a pilot-scale bioreactor. |
Industry Overview
Our technology platform enables the production of LPC, LM and Biocrude. LPC and LM can currently be applied in the animal feed market, as well as fertilizer markets. We believe that, with further technology development, LPC could be applied in human food markets and Biocrude could be used as a feedstock for renewable fuels.
The feed, food and fuel markets are global and significant in size and the demand in these markets is expected to grow rapidly. We also believe our products will meet part of that growing demand.
According to data published by the World Health Organization in 2008, the current worldwide protein deficiency is at least 18 million metric tons per year, and is expected to grow. This shortage is particularly acute in developing countries. As population and per capita income grow in the developing world, the consumption of protein is expected to increase. As a consequence, the global feed and food industries require a new, economical source of adequate protein to eliminate this shortfall.
We believe that our technology will deliver high production yields, particularly in equatorial economies, in which we expect the demand for our feed and food substitutes will be strong, and will enable our customer licensees to address the needs of the following markets.
Animal Feed
Within the global animal feed market, we have developed our technology to address rising demand in the fish meal and alfalfa markets.
Fish Meal
Fish meal, most of which is produced by the commercial fishing of wild schools of small fish, is a critical ingredient in the diets of nursery animals and aquaculture stocks. The fish meal supply is currently limited due to the effects of overfishing, while the demand for fish meal as a source of protein in animal diets continues to rise sharply. Based on 2010 projections by Dr. Albert Tacon, global fish meal demand for aquafeed is expected to reach approximately 7 million metric tonnes in 2012 and to rise every year thereafter to approximately 16 million metric tonnes in 2020, a rate which will significantly outpace supply. As a consequence, there is a large and growing need for an alternative protein source to address this supply and demand imbalance.
Based on our internal assessment of its nutritive qualities, as well as the findings of a trial completed by the Aquaculture Research Institute at the University of Idaho in 2010, we believe that
63
Table of Contents
LPC is strongly positioned as a fish meal alternative. The study at the University of Idaho demonstrated that LPC can replace high-quality fish meal at levels up to 100% in feeds for tilapia. The study also found that LPC could be suitable as a fish meal replacement for other farmed fish species. In addition, LPC has been subject to third-party testing in poultry, nursery swine and salmon as an alternative to fish meal. Third-party trials are continuing in nursery swine, shrimp and other species. These trials form the basis of our belief that LPC is a viable fish meal alternative.
Furthermore, we believe that LPC would match the nutritive quality of fish meal while also providing our customer licensees with several commercial advantages. First, in addition to international sales opportunities, our solution would enable production in the end market, thereby avoiding the freight and import duty expenses that are typically associated with the cost of fish meal. Second, we expect the continuous production and just-in-time inventory management made possible by our year-round production and daily harvest to lead to significant savings by reducing storage costs and the need for forward purchasing. Third, as compared with fish meal, LPC would deliver a similar protein content and a more consistent quality, since the components of wild-caught fish exhibit significant variability. Our internal testing indicates that the amino acid profile of LPC is comparable to that of fish meal. Finally, based on LPC’s nutritional and growth similarity with fish meal, we expect that its market price will be comparable to fish meal.
Alfalfa Meal
Alfalfa meal is a premium forage ingredient, used to meet nutrition and growth requirements in numerous animal diets. The supply of alfalfa is limited due to the concentration of production in specific areas, such as the United States and Europe. According to a publication from the Food and Agriculture Organization, or the FAO, in December 2009, although these two regions represent 65 percent of global alfalfa production, production rates in both areas have dropped significantly in recent years. Meanwhile, demand is continuing to rise, particularly in Asia, which represents only 8 percent of global production but requires forage ingredients for its rapidly growing dairy and swine industries. For dairy and swine, our estimate—based on data we compiled by using the FAO’s Global Livestock Database and the United States Department of Agriculture’s Agriculture Marketing Service—is that the global demand for alfalfa meal will be approximately 254 million tones in 2012, rising every year thereafter to approximately 262 million metric tones in 2020.
Trials conducted by the University of Minnesota in 2011 demonstrated that LM is a high quality alternative for alfalfa meal in forage diets for dairy cattle. This testing demonstrated that LM performs as well as alfalfa meal in dairy cattle diets, with similar energy and dairy efficiency. Third-party testing is continuing with other animals that are customarily fed alfalfa, such as swine and horses. Based on LM’s nutritional similarity with alfalfa meal, we expect that its market price will be comparable.
Fertilizer
Kelp and other high nitrogen biomass can be used as organic fertilizers. However, the production of kelp is costly due to the limited areas in which it grows in the wild, as well as environmental regulations that limit the scale of its harvesting. We believe that LPC can be a lower-cost, potentially higher-value alternative to kelp. We also believe that LM could be used as a nitrogen source in organic fertilizers. We expect that LPC will be competitive in the specialty turf fertilizer market due to its high levels of nitrogen, phosphorus, and bio-stimulants, which promote strong growth. In addition, the chemical composition of the fertilizer containing the LPC will reduce run-off into nearby waterways—an environmentally damaging effect commonly associated with other fertilizer products. Due to LPC’s natural origin, any run-off would present a less harmful environmental impact than synthetic fertilizer run-off.
64
Table of Contents
Fortification of Basic Human Food Products
We are developing our technology to address the rising demand for human food, a market of particular relevance in the equatorial regions in which we believe our technology will be well-suited and in which we have focused our marketing strategy to a significant extent.
We believe that, pending further process enhancements and regulatory approvals, LPC can serve the global market for fortification of basic food ingredients for malnourished populations, particularly in developing and emerging countries. Given the rapidly growing populations in regions such as South Asia, Africa, and South America, we believe that there is a clear need for an alternative supply of high quality and economical protein to supplement and enhance basic foods, such as breads, tortillas, noodles and crackers.
Testing by Dr. Fadi Aramouni of the College of Animal Sciences and Industry at Kansas State University in 2011 indicated that, pending further process developments, there is potential for LPC to be used as an ingredient in common foods, such as tortillas, pasta, crackers and smoothies. We will continue to pursue internal and third-party research to explore the relationship between LPC’s nutritive properties and consumer acceptance in these basic human food applications.
Our planning indicates that this product application requires approximately one year of further technology development, to be followed by another year for regulatory approvals and product contract negotiations. By 2015, we anticipate that our technology will be licensed on a scale that would facilitate the production of LPC in quantities that would interest commercial buyers of protein for fortification of human food products.
Specialty Markets for Prepared Foods
In the long term, we believe we can develop a higher protein content product from lemna using alternative separation techniques. While this process change would increase the capital expenditure and operating costs associated with our technology, we anticipate that the increased value of the end product produced would in many cases justify the additional expenses. If we are able to achieve this objective, our customer licensees could access specialty markets for higher value prepared foods in which protein boosts nutritional or functional properties, such as baked goods, meats and frozen foods.
Furthermore, recent analyses completed by Purdue University and Craft Technologies (a leading testing laboratory that specializes in food and nutrient testing) in 2011, respectively, confirmed that LPC contains high levels of potentially valuable compounds and vitamins, which could provide significant human nutritional and health benefits. Third-party research is continuing to validate these results and enhance our understanding of these properties.
Renewable Fuels
We expect that third-party technology will lead to the conversion of Biocrude into drop-in fuels. If we are able to successfully develop our technology in this manner, we anticipate that our customer licensees will have the ability to create products that address the significant and continuing global demand for renewable fuel.
Our Solution and Customer Licensee Approach
We believe that our solution will result in the commercial scale production of renewable products specifically for the feed, food and fuel industries.
65
Table of Contents
To expand our initial base of prospective customer licensees and to enable our customer licensees to build-out more rapidly, we have developed a scalable and flexible model based on micro-crop growth units of 150 hectare increments. This model is designed to be attractive to a wide range of entities, including agricultural customer licensees with an interest in the sustainable production of feed products, larger operators of renewable fuel production facilities and financial investment groups.
The estimated capital expenditure for a 150 hectare facility as an entry point is $12 million (excluding the cost of land and improvements)—a model that we believe involves manageable costs, risks and build-out times, while also enabling our customer licensees to evaluate the commercial potential of the technology on a larger scale. Due to economies of scale, we believe that a 600 hectare unit, comprising four micro-crop growth units with a shared processing facility, would deliver an attractive internal rate of return on our customer licensees’ investment. Ultimately, we expect that our customer licensees will expand their facilities in 150 hectare increments at similar costs in order to complete facilities of up to 5,000 hectares. Depending on economies of scale, we expect that the capital expenditure for a 5,000 hectare facility will be approximately $375 million (excluding the cost of land and improvements). We believe that a license unit of this size will enable our customer licensees to deliver significant volumes of product to market, thereby maximizing the internal rates of return on their investments.
To facilitate the deployment of our solution, our sales and marketing strategy consists of targeting potential customer licensees that would be capable of deriving significant value from our technology and who may have also demonstrated interest in our technology. For the past three years, we have been communicating and building relationships with many of these potential customer licensees. We seek to ensure that potential customer licensees have access to suitable and economical sources of land, water, power and transportation for the efficient operation of our technology. We explain to our customer licensees that their productivity levels will vary based on their specific geographical location, climate and other factors. Please see “—Site Strategy.”
We expect that our early contracts will be conditional on pilot-scale projects meeting certain minimum performance levels on which we would base projections of commercial profitability. However, we would expect future customer licensees to proceed immediately to commercial-scale build-out.
Our Competitive Strengths
Our key competitive strengths are:
| Ÿ | Strong economic returns to our customer licensees without the need for government subsidies. |
We believe that our solution will present our customer licensees with the opportunity to invest profitably in a renewable technology designed to meet the significant and growing demand for feed, food and fuel. The profitability of our technology and processes is not dependent on government subsidies; although any subsidies that we or our customer licensees receive will enhance the profitability of our technology and processes. Our product platform has been developed to serve growing demand in large global markets.
| Ÿ | Highly scalable and flexible technology, with initial license units providing the basis for larger-scale operations in the future. |
To facilitate rapid deployment opportunities in the near future, we have developed a highly scalable and flexible model for potential customer licensees. Our solution involves the construction and operation of production facilities based on 150 hectare increments of micro-crop growth bioreactors. We expect each 150 hectare unit will be a profitable module that can be replicated as the components of a larger-scale project of up to 5,000 hectares. These units are expected to be immediately productive while the build-out of additional units proceeds. Initially, we believe that
66
Table of Contents
offering our customer licensees the opportunity to build-out in increments of 150 hectares will allow our customer licensees to quickly assess the technical and commercial viability of each unit.
| Ÿ | Fully integrated, comprehensive solution that transforms simple, naturally occurring inputs, such as sunlight, water and indigenous micro-crops, into valuable outputs. |
We will provide our customer licensees with a comprehensive system that contains all components necessary for the creation of renewable products at commercial scale, from selection and testing of micro-crops to growth, harvesting and processing. In addition to a fully integrated technology platform, we expect to add value through our extensive facility planning, design and construction/operation management services, including a procurement and supply chain system that will enable our customer licensees to acquire equipment in a cost-effective manner to promote an efficient and profitable build-out of our technology.
| Ÿ | Product compatibility with the existing agriculture infrastructure. |
Our processes for producing LPC and LM are designed to be compatible with standard processing techniques that can be implemented using existing agricultural equipment and infrastructure. Due to their nutritional characteristics, such as strong digestibility, palatability and amino acid profiles, we expect both products to become direct substitutes for existing protein supplies in animal feed applications. Our technology can potentially provide a new source of supply where existing demand or expected growth in demand cannot be met solely by existing sources due to environmental and other constraints.
| Ÿ | First mover advantage in providing renewable micro-crop technology to produce products that serve the global animal feed market. |
We believe that the deployment of our solution will place our customer licensees in a position to enter the global animal feed market as a first-mover in producing animal feed from renewable micro-crops.
| Ÿ | Experienced management team with a track record of innovative growth, value creation and commercial scaling of businesses. |
Our senior management team has broad expertise in guiding early-stage development companies to commercialization. Our management team has been involved in various technical and management roles within leading organizations across various industries.
Our Business Strategy
Building on our competitive strengths, our objective is to become the leading global provider of technology and processes for the commercial production of micro-crop biomass for the agriculture and energy markets. Key elements of our strategy include:
| Ÿ | Rapid deployment and support of modular and highly scalable license units. |
Our customer licensees will be able to assemble our proprietary units from modular, cost-effective bioreactors and other ancillary equipment, which are currently operating at a smaller scale at our fully operational demonstration facility. These modular bioreactors are designed to enable rapid deployment at predictable cost and productivity levels. We offer our customer licensees extensive support services, including pre-planning, construction management and supervision, equipment procurement on-site support and assistance with development of off-take agreements.
| Ÿ | Leveraging our capital-light licensing model. |
67
Table of Contents
Our customer licensees will be required to pay the upfront infrastructure costs as well as continued maintenance costs for their licensed facilities. This business model allows us to make minimal capital expenditures while generating fees and royalties over the term of the license.
| Ÿ | Expansion of our customer base on a global scale. |
We are continuing to expand our marketing efforts internationally. In 2011, we expanded our operations in China to further our focus in the region, as well as to assist with equipment procurement efforts to reduce capital costs for our customer licensees. We also recently expanded our sales and marketing efforts in both Africa and South America, which represent key locations where we expect to license our technology. In 2011, we entered into a license agreement with a customer licensee in South America. In addition, we are establishing relationships and seeking opportunities with investment funds that would be interested in sole source or co-investment opportunities.
| Ÿ | Further development of our technology and pursuit of additional applications for our products. |
We expect to further enhance our technology in order to provide our customer licensees with additional end market applications for our products along with the ability to capture higher economic returns. We expect these efforts to provide our customer licensees with access to markets for human food and renewable fuels. For example, in July 2011 we entered into an agreement with CRI Catalyst Company LP (CRI) to use Integrated Hydropyrolysis and Hydroconversion technology (IH²) for the conversion of our micro-crop residues into renewable fuels.
| Ÿ | Assisting our customer licensees in the identification of off-take partners. |
We will continue academic and commercial testing of our products to confirm their market viability. This third-party validation will assist us and our customer licensees in establishing off-take arrangements in which companies will purchase products produced by our customer licensees.
| Ÿ | Creation of brand loyalty. |
We intend to become recognized as the leading global provider of technology for the commercial production of micro-crop biomass for agriculture and energy markets. In the short term, we believe that our feed and fertilizer products will attract agricultural customer licensees and other investment partners. In the long term, we believe that we will have a substantial marketing opportunity with a variety of prospective customer licensees seeking sustainable, renewable fuel options, as well as customer licensees interested in creating and selling human food products. We intend to become a recognized provider of green technology solutions.
Product Development
We believe that our technology provides the basis for a broad product platform to bring to the agriculture and energy markets. Our current product development efforts are focused primarily on the production of animal feed and fertilizer, as well as protein for the fortification of basic food ingredients. We also plan to develop additional human food and renewable fuel products, and have already completed a number of tests with regard to these applications. However, these products will require enhancements to our technological process that are likely to increase the costs associated with our solution.
Our product strategy is based on the scalability and performance of our technology. With a licensing model based on highly scalable production facilities, we believe that our technology will facilitate the rapid and efficient production of end products in areas with large and growing demand.
The commercial viability of our technology largely depends on the ability of our customer licensees to sell into existing markets. Although we have achieved encouraging test results for several
68
Table of Contents
applications for our products, it has yet to be proven that there is a market for the specific end products produced by our technology. Please see “Risk Factors—The market may not accept the end-products produced by our technology.”
Lemna Protein Concentrate
LPC is a free-flowing powder containing a minimum 65% crude protein, which is extracted from non-genetically-modified plant species in the Lemnaceae family. Our customer licensees will manufacture LPC under internationally recognized process and quality control standards (including Association of American Feed Control Officials standards and the Codex Alimentarius) to enable approval for use in both animal and, potentially, human markets. Based on internal and third-party testing, we believe that LPC is similar in quality to fish meal and represents a potentially viable protein source for feed formulations in multiple industries, including aquaculture and swine.
Initially, LPC can be used as an alternative to fish meal. Fish meal is typically the most highly valued large source of protein for animal and aquaculture feeds. In addition, supplies have become restricted due to the effects of overfishing. We believe that LPC has multiple advantages over fish meal and is well-positioned to become a large scale alternative.
We believe that LPC could be well-suited to represent an alternative to kelp meal as a high-value fertilizer. Kelp is used in high-end fertilizers for applications such as golf course maintenance. LPC provides a natural source of nutrients as well as other bio-stimulants.
We also expect that LPC could serve as a nutritional enhancement to basic human food ingredients for breads, tortillas, noodles and crackers. The growing population in poorer parts of the world is often characterized by severe protein deficiencies. We believe that the addition of a relatively small proportion of LPC to basic foodstuffs could reduce or eliminate this protein deficiency in individuals without affecting the cooking and other properties of the food.
In the long term, we believe that significant potential exists for LPC in smaller, high-value niche markets, as a functional protein ingredient in packaged foods, and as a source of high-value nutritional additives.
Lemna Meal
LM is a free-flowing powder containing a minimum 15% crude protein, which is produced from the pulp of a non-genetically-modified plant species in the Lemnaceae family. The product contains an essential amino acid profile that is comparable to alfalfa—making it a competitive feed ingredient for a broad array of feed formulations in the dairy and swine industries.
Initially, our customer licensees will be able to use LM as an alternative to alfalfa meal. In addition, we believe that LM can be used as an ingredient in fertilizer or animal bedding.
Biocrude
With a small change in process conditions (but not equipment), our processing system can produce Biocrude rather than LM. Biocrude is a renewable energy feedstock that, through the use of a variety of third-party conversion systems currently under development, can be converted into renewable fuels or burned directly as a combustion fuel. Biocrude produces an energy content comparable to wood chips, and higher than most other agricultural biomass used as fuel, including palm waste, bagasse and corn stover. In addition, Biocrude does not contain fossil carbon and, as a cellulosic product, would qualify for cellulosic tax incentives, where available.
69
Table of Contents
Technology
Our technology consists of intellectual property that is a combination of multiple patent-pending systems, processes and configurations, trademarks, proprietary software and trade secrets. Based on the continued process refinements we make as a result of operations at our demonstration facility in Florida (see “—Scaling our Technology”), as well as our substantial overall investment in research and development, we continue to improve our technology and the ability of our customer licensees to bring valuable products to commercial markets.
Our technology platform primarily consists of four components: micro-crop selection and testing, growth and harvesting techniques, processing technology, and control systems. Our technology enables the selection of indigenous micro-crop species that demonstrate an optimal profile for a particular geography and environment, allowing our customer licensees to grow and harvest these micro-crops at economically attractive yields.
The diagram below illustrates the growth, harvesting and processing steps of our technological process.
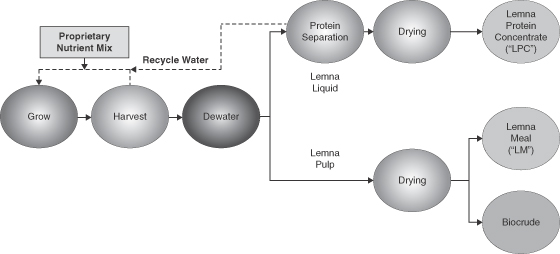
Selection and Testing
Our selection and testing approach is designed to identify naturally occurring micro-crops in regions of interest for our prospective customer licensees. We have substantial experience in this area. Over the past three years, through visits to regions of interest and analysis at our laboratories, our scientists have carefully analyzed a large number of possible micro-crop species suitable for the commercial-scale production of biomass in many regions throughout the world.
Our technology utilizes micro-crops that are indigenous to the region in which each customer licensee proposes to build facilities. We believe it is preferable to identify and choose dominant local species with a history of growth in the local environment. In our experience, dominant local species out-compete other organisms in open ponds.
We typically select several species from local areas to investigate. This process identifies potential micro-crops which can be grown in different seasons and climates. We then test the performance of the selected micro-crops and analyze the species composition.
Once a mix of viable species is chosen, we develop a site-specific plan to further qualify the desired location of the facility and subsequently grow and inoculate the mix at the site.
70
Table of Contents
Growth and Harvesting
Our micro-crops produce significantly more biomass per hectare than macro-crops, such as jatropha, palm, corn and soybeans. In addition, our micro-crops grow continuously and will be harvested throughout the year in equatorial climates, utilizing the available sunlight and consuming atmospheric carbon dioxide to create a predictable and consistent product yield.
The growth phase begins with open-air bioreactors, or production ponds, designed to provide controlled growing conditions. The bioreactors are specifically designed to be modular, which will enable our customer licensees to build-out in phases or to allow customization to various topologies. The bioreactor design will also provide our customer licensees with a reliable environment for large-scale production of biomass, including nutritional control, environmental control (such as water management) and monitoring control (for quality). The design of the bioreactors is intended to minimize capital and operational expenses, while maximizing the volume of material harvested for processing.
To help facilitate growth, we utilize a proprietary nutritional recipe that has been specifically tested and optimized for our micro-crops. The nutrients consist of commercially available fertilizers and minerals, although the composition and application remain proprietary to our system.
Our proprietary harvesting system helps to achieve optimal quality and yields for effective production. After harvesting, the biomass is typically transported to a nearby processing facility and approximately 98 percent of the water is recycled back into the ponds.
Production Process
Our process design is based on a configuration of equipment, infrastructure and knowledge that is specifically developed to optimize product yields and quality. The process consists of industry standard, commercially-available equipment that is commonly used in the agriculture and food processing industries in order to eliminate the risks and expense that would be associated with non-standard equipment. The essential processing steps remain similar across different projects, although the configuration of each facility varies depending on the local region of the customer licensee and the size of the facility.
After the biomass is transported from the bioreactors and dewatered, milling or grinding takes place. The milling operation essentially tears, grinds and shears the biomass into small particles. Next, the solids and the liquid of the lemna are separated. After the liquid is filtered and clarified, protein purification and concentration begins. Once extracted, the washed protein is dried to produce the final LPC, while the unused liquid is recycled to the bioreactors.
The second product produced by our process can be either LM or Biocrude. At the point of protein separation, the remaining solids are further processed into one of these two end products. This is accomplished through various pressing and drying techniques. A dryer is used to reduce the moisture content of the LM or the Biocrude to the desired application level, while not exceeding temperatures that may damage important characteristics of the final product.
These products can then be used locally or shipped to end users.
Control Systems
Our control systems comprise hardware and proprietary software throughout the production site. In the farming areas of the production site, the control system is designed to manage nutrient control and harvesting decisions. This will enable our customer licensees to focus on efficient management. In the processing facility of the production site, the control system is designed to optimize operations, ensure product quality and provide various safety measures.
71
Table of Contents
Data from each area of a customer licensee’s facility will be stored in a proprietary database for historical tracking of all key performance indicators. The report generation module will provide our customer licensees with the data and tracking needed to ensure efficiency, quality and output.
Each customer licensee’s software system will communicate key process data and productivity metrics to us to ensure ongoing efficient operations. Over time, we expect that this feature of the system will provide us with a significant competitive advantage, because we will be able to develop a clear understanding of operations around the world and use this information to enhance our processes and thereby offer increased efficiencies to future customer licensees.
In addition to daily operation and historical tracking, the system also features multiple mechanisms to safeguard all source code, object code and data. All information, including the encrypted source and object code, is remotely backed up on a regular basis.
Scaling our Technology
Since inception in 2006, we have focused on continuously developing our technology with the ultimate objective of enabling our customer licensees to produce renewable feed, food and fuel products at commercial scale. We believe research and development is an ongoing effort and we continue to look for ways to improve our technology and processes so that our customer licensees will be able to scale up our technology efficiently and profitably.
Although we have successfully built and operated a demonstration facility and have extracted small field-scale product quantities for technical validation, we have not demonstrated that our technology is viable on a commercial scale. While our bioreactors are modular in design, a single commercial-scale facility will be several orders of magnitude larger than our demonstration facility. Once a customer licensee begins to scale up and replicate such a system, unforeseen factors and issues may arise. Please see “Risk Factors—We have yet to construct our technology on a commercial scale, and may be unable to solve technical and engineering challenges that would prevent our technology from being economically attractive to prospective customer licensees.”
The process of scaling up our technology from the laboratory scale to demonstration scale to commercial scale has included the following key steps and milestones:
Laboratory-Scale
Beginning in 2007, we conducted initial important research and development at the NASA Kennedy Space Center (KSC) Space Life Sciences Laboratory. We leveraged the laboratory’s analysis capabilities for chemistry, molecular biology, microscopy and microbiology. The controlled environmental chambers allowed us to designate, control and manipulate environmental parameters, such as climate, temperature, day, night and seasonal cycles, to simulate growing conditions for research purposes as well as to understand climate conditions around the world. Our work at this facility increased our understanding of the likely performance of our technology in many parts of the world. Since mid-2011, we have continued our research efforts exclusively in our scientific laboratory in Fellsmere, Florida and at various customer sites around the world. Our Fellsmere laboratory has been developed for chemical analyses, culture storage, backup inoculum growth, bench top research experiments and fast turnaround analyses of field and product samples. Our work at this facility continues to further our technological understanding regarding product quality and environmental factors.
Demonstration-Scale
In June 2009, we completed our fully operational demonstration facility in Florida consisting of one large bioreactor (of approximately one hectare), three smaller bioreactors and an associated
72
Table of Contents
processing complex. This facility is not commercial scale and we cannot make any assurances that the results achieved at this facility can be replicated on a commercial scale. We use the facility to refine processing and growth reactor designs, generate test samples of our end products, and test solutions related to scalability.
In addition, through the use of our demonstration facility, we continue to evaluate new incremental technologies and processes to determine whether they would improve the economic proposition for our customer licensees. For example, in the past two years we have changed the configuration of our growth bioreactors from grid-based systems with static water to larger raceways with water movement systems that facilitate a consistent distribution of nutrition, as well as improved water quality, growth, harvesting and overall productivity.
Each customer licensee’s commercial-scale operations will include site and project-specific features, but the general conceptual and technical elements of the operations will be based on our demonstration facility. However, our technology has yet to be implemented beyond pilot scale and due to the inherent uncertainties in scaling up new technologies, including factors both within and beyond our control, we cannot guarantee the performance of our technology at commercial scale.
Pilot to Commercial Scale
In 2011, one customer licensee and two prospective customer licencees commenced construction of pilot-scale bioreactors in South America and Asia. If these pilot facilities are successful, these parties have expressed an intention to pursue commercial-scale projects, subject to further negotiation of license agreements.
In addition, the results of these projects will be important with respect to increasing our understanding of the performance of our technology in locations outside of the United States, including regions where we expect to achieve increased productivity and production yields.
If the pilot projects yield results that meet or exceed expectations, this would represent evidence of the viability of our technology and ultimately increase our ability to enter into agreements for commercial-scale projects.
Licensee Support Services
We believe that the provision of support services will be important to ensure the successful implementation of our technology system. As such, we offer project, construction management and operations support services to enable our customer licensees to scale up effectively. Examples of the licensee support services that we offer include:
| Ÿ | Pre-planning activities including site selection and qualification, preliminary project plan and cost analysis, contract development, equipment pricing and the finalization of the facility design. |
| Ÿ | Construction management activities including advising and overseeing construction activities by the chosen contractor to ensure that the site is developed effectively and the facility is constructed safely and efficiently. |
| Ÿ | Operations management activities including supplying a team of personnel to provide start-up operations and to train a customer licensee’s employees in the operation and maintenance of the facility. The principal goal of this team will be to optimize the facility output and efficiency. |
| Ÿ | Equipment procurement and supply chain management that will allow our customer licensees to obtain cost-effective equipment that meets our technical specifications. |
73
Table of Contents
In 2011, we supplemented our U.S.-based purchasing capabilities through the addition of a purchasing function focused on identification of sources of supply for the procurement of equipment and other materials in China and other Asian nations. Our expanded purchasing capabilities allow us to procure equipment and materials required by our customer licensees in a cost effective manner. We plan to continue to expand our staffing in our customer licensees’ regions to provide the support services listed above.
We expect to provide on-site customer support services to our customer licensees. We estimate the cost of these services will be approximately 20 to 25% of the ongoing fees we expect to receive in connection with the operation of a 5,000 hectare license unit.
In recent years, we have developed relationships that we believe will be valuable in terms of assisting our customer licensees to identify and enter into agreements with off-take partners. The characteristics of a suitable animal feed off-take partner include a strong understanding of the need for, and value of, alternative feed ingredients, and a strong understanding of the impact of diet on animal growth performance, as well as the knowledge, skills, and ability to properly assess and validate our products. Preferably, each off-take partner would have significant demand for our products.
Site Strategy
Our sales strategy targets customer licensees with the capacity to execute commercial-scale operations in regions on or close to the equator (generally within a range of 15 degrees North latitude to 15 degrees South latitude), due to the dependence of our technology on geographic proximity to the equator.
In determining appropriate sites, we also consider the following factors:
| Ÿ | the proximity of a potential licensee site to adequate infrastructure; |
| Ÿ | the availability of labor to construct and operate the facilities; |
| Ÿ | the availability of any state or local incentives; |
| Ÿ | the availability and/or potential for the creation of local markets in which end products may be sold; |
| Ÿ | the availability of economical and reliable sources of water and power; and |
| Ÿ | the stability and support of the local and national political environment. |
Competition
Our objective is to be the leading global provider of technology and processes for the commercial production of micro-crop biomass for agriculture and energy markets. In the near term, we expect the LPC and LM produced by our technology to compete primarily with fish meal and alfalfa, respectively. In these markets, our customer licensees will compete with the established providers of these products, including large international food and agriculture companies. In many cases these companies will have well-developed distribution systems and networks for their products, as well as sales and marketing programs in place to promote their products.
We believe that for LPC and LM to succeed in these markets, we must demonstrate to our customer licensees that they are comparable to both existing products and other alternative products that are being developed for the same markets based on some combination of cost, quality, availability of supply, compatibility with existing infrastructure, sustainability, and consumer preference characteristics. We believe LPC and LM will compete favorably with respect to all of these competitive factors.
74
Table of Contents
In the long term, we expect to develop human food applications for LPC, as well as applications of our Biocrude product for renewable fuel markets. Consequently, the products produced by our technology will compete in large, international agriculture and energy markets, including renewable fuels and transportation fuels markets. Many of our competitors in the fuels markets have resources and name recognition far in excess of ours. While we anticipate competition from these large, established companies, we may also seek to partner or collaborate with them.
For additional information, see “Risk Factors—If a competitor were to achieve a technological breakthrough, our operations and business could be negatively impacted.”
Intellectual Property
We have developed proprietary technology that we believe provides us with a competitive advantage. We seek to protect this technology through the use of a multilayered intellectual property protection strategy. Our intellectual property strategy is focused on a combination of patents, trade secrets and a proprietary control system.
As of December 2011, we have patent applications in the United States and foreign jurisdictions pending across the entire range of our technology and processes, including bioreactor construction, growth and harvesting, and processing of our micro-crops.
Our trade secrets complement and bolster the technology covered by patent applications and relate to each technological aspect of the system, such as growth and harvesting, nutrition, and processing.
Our proprietary control system and the accompanying algorithms manage the overall process to optimize yield. The control systems are protected through various multilayered security models that prevent unauthorized access to the software and source code. Under our licensing model, our customer licensees will not have access to our encrypted software, the underlying code or databases.
Raw Materials
Our processes primarily use four categories of raw materials: indigenous micro-crops (from the Lemnaceae family) that are not genetically modified and demonstrate an optimal growth profile for a particular geography and environment; fertilizer; water; and naturally-available elements, such as sunlight and carbon dioxide. The micro-crops our processes use will be indigenous to the regions in which our customer licensees operate and are available in the natural environment. The availability and quality of water in a specific location represents an important component of our site selection process. We intend to provide our customer licensees with our proprietary nutrient mix, which will consist of commercially-available ingredients.
Regulatory
To enable our customer licensees to sell the products produced by our technology in markets around the world, we will need to gain the necessary regulatory approvals. Our regulatory strategy consists of three main elements.
First, we organize and fund third-party trials at independent universities and other research institutions, with the objective of confirming or indicating the commercial viability and value of our products in various applications and markets. The results generated by these trials are critical in terms of informing our decisions to proceed with the relevant regulatory processes.
75
Table of Contents
Second, we conduct our own regulatory activities. For example, LPC and LM are subject to regulatory approvals required for animal feed. In November 2009, the Indonesian Ministry of Agriculture approved our LPC product as an approved raw material for use in animal feed. In 2012, we expect to receive similar regulatory approvals for other countries in which our customer licensees are most likely to be located, specifically Thailand, Chile, Philippines, China, Malaysia, and Vietnam. This will allow our customer licensees to sell our animal feed products at commercial scale in those jurisdictions.
We have also initiated the regulatory review process in the United States. We held an initial meeting with the FDA in April 2010. We presented an overview of our products and technology as well as the results of our research on lemna. As a result of this meeting, we agreed to prepare a white paper discussing the safety of lemna use in animal feed. We have also retained the services of a consultant to help expedite the regulatory process. We are currently pursuing the American Association of Feed Control Officials, or AAFCO, pathway for the use of LPC and LM as animal feed and have improved the design of our license units to be in compliance with AAFCO best management practices for animal feed production. We expect that the FDA will view our compliance with AAFCO best management practices in a favorable light and this will help the FDA to validate the products produced by our technology. We have not yet submitted a formal application for FDA approval.
Third, to help expedite the regulatory process, members of our product development and regulatory team will work directly with each customer licensee to understand the markets in which they intend to sell the products. Our team will assist each licensee in initiating and completing regulatory processes with the required regulatory bodies, as needed.
In relation to our products under development for human food markets, we intend to take advantage of widespread acceptance of the use of lemna in human nutrition to expedite regulatory approval for human consumption of LPC in India and China. Receiving regulatory approval for LPC in emerging markets, which represent the greatest potential source of demand, is an important priority, as we believe it would improve our economic proposition to customer licensees.
In addition, we believe that we will be able to obtain the required human approvals through the human GRAS (Generally Recognized as Safe by the AAFCO) pathway in the United States by leveraging the use of lemna in homeopathic medicine in other countries. We will also need to implement process improvements required to produce a product suitable for human consumption, including flavor enhancement, increase in purity, and a production line that meets food quality standards. We are in the initial stages of developing such enhancements.
Biocrude does not require any regulatory approvals for use as a fuel feedstock, but the products produced using Biocrude would require the same regulatory approvals as petroleum-based fuels, typically American Society for Testing and Materials standards in the United States.
Our products and the production facilities of our customer licensees will also be subject to inspection by federal, state, local and foreign environmental, health and safety authorities.
If we cannot meet the applicable requirements in countries in which we intend to use our technology to produce our products, then our business will be adversely affected . See “Risk Factors—Risks Related to Our Business—The end-products produced by our technology are subject to industry and regulatory testing.” We are unable to predict whether any governmental agency will adopt any laws or regulations that could have a material adverse effect on our operations. We will incur, capital and operating expenditures and other costs in the ordinary course of our business in complying with these laws and regulations.
76
Table of Contents
Environmental
Our licensed operations in the United States and abroad will be subject to a wide range of environmental laws and regulations at both the national and local levels. These laws and regulations govern, among other things, air emissions, wastewater discharges, solid and hazardous waste management, site remediation programs, and chemical use and management.
Pursuant to these laws and regulations, our customer licensees will be required to obtain and comply with a wide variety of environmental permits for different aspects of their operations. We are subject to similar laws with respect to the demonstration facility that we own. We believe that we are in material compliance with all current environmental laws and regulations to which we are subject. We currently estimate that any expenses incurred in maintaining compliance with these requirements will not materially affect our results of operations or cause us to exceed our level of anticipated capital expenditures. However, we cannot provide any assurance that regulatory requirements or permit conditions will not change, and we cannot predict the aggregate costs of additional measures that may be required to maintain compliance as a result of such changes or expenses.
We believe that the environmental risks we face are comparable to those faced by other companies in the agriculture or aquaculture markets.
Organization
Employees
We have a multidisciplinary team, including biologists, chemists, physicists, engineers and business executives. Currently, we have 69 direct, full-time, non-union employees, and we consider our employee relationships to be good.
We employ sales, marketing, and business development personnel, who have developed relationships throughout the agriculture and energy industries.
Research and Development
We believe research and development is an on-going effort and we continue to look for ways to improve our technology and processes. We have spent $9.9 million, $15.1 million, $17.4 million and $8.5 million during 2008, 2009, 2010 and the nine months ended September 30, 2011, respectively, on research and development activities. Through the use of our dedicated research and development bioreactors at our fully operational demonstration facility, we evaluate new incremental technologies and processes to determine if they improve our growth output. We believe that continued research and development efforts will allow us to further increase our micro-crop productivity, thereby increasing the economics for our customer licensees. We are continuing our research and development efforts to improve the value proposition of our technology and processes to our licensees and prospective customer licensees.
Facilities
Our corporate headquarters are located in Melbourne, Florida. This facility houses our executive and administrative offices, our finance, business development, operations and our information technology departments. It also includes meeting space and our computer network and communications infrastructure. We have leased 24,000 square feet at this facility through February 2014.
Our fully operational demonstration facility is located in Fellsmere, Florida. The facility provides demonstration-scale live operation of all aspects of our technological processes.
77
Table of Contents
We believe that our current facilities are suitable and adequate to meet our needs and that suitable space will be available to accommodate the foreseeable expansion if our operations.
Sales and Marketing
Our sales and marketing strategy consists of targeting customer licensees that are capable of deriving significant value from our technology. We employ sales and marketing personnel who have developed relationships throughout the agriculture and energy industries.
Legal Proceedings
We are not a party to any material litigation or proceeding and are not aware of any material litigation or proceeding, pending or threatened against us.
78
Table of Contents
Executive Officers and Directors
Our executive officers and directors, and their ages as of December 1, 2011, are set forth below:
| Name |
Age | Position(s) | ||||
| Anthony John Phipps Tiarks |
56 | Chief Executive Officer | ||||
| James P. Dietz |
47 | Chief Financial Officer | ||||
| Peter Sherlock |
55 | Chief Operating Officer | ||||
| John S. Scott |
60 | Non-Executive Chairman | ||||
| Sayan Navaratnam |
37 | Director | ||||
| Isaac D. Szpilzinger |
63 | Director | ||||
Anthony John Phipps Tiarks has been our Chief Executive Officer since June 2011. Prior to joining the Company, Mr. Tiarks was President and Chief Executive Officer of Liberty Aerospace, Inc., which he established in the United States in 2000 with the purpose of developing, certifying and delivering an entry level aircraft. Prior to starting Liberty Aerospace, Mr. Tiarks was a Managing Director in London of Donaldson, Lufkin & Jenrette, a U.S. investment bank, Chairman of Tide Brokers Limited, a wholesale money broker, and Managing Director of Tide (U.K.) Limited, an international foreign exchange fund management group. Mr. Tiarks has a B.S. in Systems and Management from City University, London, United Kingdom.
James P. Dietz has been our Chief Financial Officer since July 2011, and served as our Vice President of Finance and Accounting from May 2010 to July 2011. From May 2009 through April 2010, Mr. Dietz served as chief financial officer of U.S. Capital Holdings, LLC, an international private equity investor and developer, whose parent company is Tangshan Ganglu Iron & Steel Co., Ltd., a Chinese industrial company. Mr. Dietz also serves on the board of Global Income Trust (formerly known as MacQuarie CNL Global Income Trust, Inc.) a non-traded public real estate investment company, since his appointment as an independent director in November 2009. From May 2008 until May 2009, Mr. Dietz served as Vice President – Finance and Business Development for PACT, LLC, a real estate development company. From 1995 until December 2007, Mr. Dietz was with residential community developer WCI Communities, Inc., or WCI, and various predecessor firms, as its Chief Financial Officer. In August 2007, shareholders at WCI approved a new composition for WCI’s board of directors which had been submitted to them for vote at their annual meeting pursuant to an agreement between WCI and affiliates of Carl Icahn that resulted from a proxy contest. Mr. Dietz resigned as WCI’s Chief Financial Officer in December 2007. WCI filed a voluntary petition under Chapter 11 of the federal bankruptcy laws on August 4, 2008, from which it emerged on August 31, 2009. From 1993 to 1995, Mr Dietz managed asset-backed financing originations for GTE Leasing Corporation (a subsidiary of GTE, a predecessor to Verizon Communications). He began his professional career in December 1986 joining Arthur Andersen & Co where he advanced to manager, providing audit and financial consulting services to clients in the construction, real estate, healthcare and legal industries. Mr. Dietz earned a BA in accounting and economics from the University of South Florida in 1986. Mr. Dietz is a certified public accountant.
Peter Sherlock has been our Chief Operating Officer since July 2011. Between 1999 and 2011, Mr. Sherlock held the positions of Vice President, Product Development and Vice President/Chief Technology Officer at AuthenTec, Inc., a semiconductor, biometrics and security company. From 1996 until 1999, Mr. Sherlock established and directed the Raleigh (NC) Design Center for Integrated Device Technologies (IDT, California), a semiconductor electronics company. From 1990 until 1996, Mr. Sherlock held the positions of Vice President, Operations and Senior Vice President Business
79
Table of Contents
Development at IVEX Corporation, a real time visual simulation systems company, where he was recruited from the United Kingdom to work in the Atlanta, USA based operation. From 1977 until 1990, Mr. Sherlock spent his early career with Ferranti Computer Systems in the United Kingdom. Mr. Sherlock holds a First Class BSc in Electrical Engineering from the University of Salford, United Kingdom.
John S. Scott, Ph.D. was our Chief Executive Officer from August 2009 until June 16, 2011 and has been the Chairman of our board of directors since December 2008. In 2002, Dr. Scott founded XL Tech and has been its Chief Executive Officer since 2002 and a director since 2004. Since 2008, XL Tech has been an inactive holding company controlled in part by Dr. Scott. He has also been a director of XLTG LLC since 2010. He was a professor at the Universities of Maryland and Arizona, and a visiting professor at the University of North Carolina. Dr. Scott has published extensively on the development of successful technology enterprises and also lectures at business schools throughout the United States. Dr. Scott has served as a consultant to the United States and various European governments, and was a national research council fellow at NASA. He holds a B.Sc. in physics and astrophysics from Michigan State University and a Ph.D. in the astrophysics/physics dual program from the University of Arizona’s Steward Observatory. Dr. Scott’s experience with founding and developing technology companies, along with his academic credentials, enables him to provide important insight and guidance to our management team and board of directors.
Sayan Navaratnam has been a member of our board of directors and served on the board of PA LLC since December 2008. Since March 2009, Mr. Navaratnam has been a Senior Managing Director of Laurus Capital Management, LLC and a Senior Managing Director of Valens Capital Management, LLC, entities affiliated with our principal stockholders. Since 2004, he has been the Chairman of Creative Vistas, Inc., which installed and serviced broadband services to consumers and currently integrates security systems for commercial customers in Canada. From 2000 to 2003, Mr. Navaratnam was the Chief Operating Officer of ASPRO Technologies Ltd. ASPRO Technologies Ltd., which provided printer circuit boards to manufacturers of digital video recorders in the security industry and was sold in 2002 to private investors. He currently serves on the board of a number of other privately-held companies. Mr. Navaratnam holds an Honors Double Specialist degree in economics and political science from the University of Toronto. Mr. Navaratnam’s extensive background in technology, finance, operations, expertise in workout situations and experience as the chairman of a public company enables him to make a valuable contribution to our board of directors.
Isaac D. Szpilzinger has been a member of our board of directors since December 2008. He is an attorney in solo private practice licensed in the state of New York. From 1987 to 2006, Mr. Szpilzinger practiced with the law firm of Herzfeld & Rubin, P.C., where he was named a partner in 1994. He began his legal career in 1985 with a clerkship in the New York State Supreme Court, Appellate Division, Second Judicial Department. Mr. Szpilzinger holds a B.S. in Chemistry, cum laude, from Brooklyn College, and an M.A. in Chemistry and an Advanced Certificate in Educational Administration and Supervision from Brooklyn College. He also holds a J.D., cum laude, from New York Law School. Mr. Szpilzinger’s legal expertise and his academic background in the sciences enables him to provide valuable insight and guidance to our board of directors.
Structure of the Board of Directors
Our board of directors currently consists of three members: Dr. Scott, Mr. Navaratnam and Mr. Szpilzinger.
Upon the completion of this offering, our board of directors will consist of five directors: Mr. Tiarks, our current Chief Executive Officer, and four directors who will qualify as independent within the meaning of the applicable listing standards and Rule 10A-3 of the Exchange Act.
80
Table of Contents
Within six months of the completion of this offering, our board of directors will appoint two additional members to our board, each of whom will qualify as independent within the meaning of the applicable listing standards and Rule 10A-3 of the Exchange Act. Pursuant to the stockholders agreement, our principal stockholders will agree to maintain on our board of directors at least a majority of directors who are independent within the meaning of the applicable listing standards and Rule 10A-3 of the Exchange Act.
Upon completion of this offering, our bylaws will be amended and restated to provide that the authorized number of directors may be changed only by resolution of the board of directors, and our amended and restated certificate of incorporation will divide our board of directors into three classes of directors of the same or nearly the same number of directors (designated Class I, Class II and Class III) each with a staggered three-year term. At each annual meeting of stockholders, the directors whose terms then expire or their successors will be elected to serve from his or her time of election and qualification until the third annual meeting of stockholders following election or until their earlier death, resignation or removal.
Our amended and restated certificate of incorporation will provide that our directors may only be removed for cause. To remove a director for cause, 66 2/3% of the voting power of our outstanding voting stock must vote as a single class to remove the director at an annual or special meeting. Our amended and restated certificate of incorporation will also provide that, if a director is removed or if a vacancy occurs due to either an increase in the size of our board of directors or the death, resignation, removal or other cause, the vacancy will be filled solely by the affirmative vote of a majority of the remaining directors then in office, even if fewer than a quorum remain.
This classification of our board of directors, together with the ability of our stockholders to remove our directors only for cause, may have the effect of delaying or preventing a change in control or a change in management. See “Description of Capital Stock—Certain Provisions of our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws” for a discussion of anti-takeover provisions found in our amended and restated certificate of incorporation.
Our amended and restated certificate of incorporation will provide that, as soon as our principal stockholders and its affiliates beneficially own less than 25% of our outstanding common stock (as set forth on the cover of our then most recently filed annual report on Form 10-K or quarterly report on Form 10-Q), each of our directors’ terms will expire at the next annual meeting of stockholders and thereafter each director will be elected to serve from his or her time of election and qualification until our next annual meeting of stockholders or until his or her earlier death, resignation or removal.
In addition, prior to the completion of this offering, we will enter into the stockholders agreement. The stockholders agreement will become effective upon the completion of this offering. The stockholders agreement will contain a voting agreement that provides, among other things, that:
| Ÿ | our principal stockholders will agree to vote any shares of our common stock they and their affiliates own pro rata with our unaffiliated stockholders (other than in connection with any vote relating to (i) a consolidation or merger of the company or any of its affiliates into or with any other person (other than affiliates of our principal stockholders); or (ii) a sale, lease or transfer of all or substantially all of our assets or capital stock to another person (other than affiliates of our principal stockholders)); and |
| Ÿ | our principal stockholders will not vote in favor of the removal of any of our independent directors unless our unaffiliated stockholders approve such removal. |
For more information regarding our governance, see “Relationships and Related Party Transactions—Stockholders Agreement.”
Upon the completion of this offering, our common stock will be listed on . The rules of require that a majority of the members of our board of directors be independent within specified periods following the closing of this offering. We will not avail ourselves of the “controlled company” exemption
81
Table of Contents
provided for by the listing standards and therefore expect to fully comply with such requirements, including the maintenance of a majority independent board of directors and fully independent audit, compensation, and nominating and governance committees. Pursuant to the terms of the stockholders agreement, our principal stockholders will have the right to appoint one observer, or the observer, to our board of directors. The observer will be entitled to receive notice of all meetings of our board of directors and all information distributed in connection with such meetings and to attend such meetings. The observer will not have any voting rights. See “Relationships and Related Party Transactions—Stockholders Agreement.”
There will be no limit on the number of terms a director may serve on our board of directors. Our board of directors may consist of not less than one and not more than directors. The authorized number of directors may be changed only by vote of our stockholders. Our board of directors will have the ability to fill vacancies on our board of directors.
Our board of directors intends to be committed to strong corporate governance practices and to value independent board oversight as an essential component of managing corporate performance.
Board Committees
Our board of directors does not currently have an audit committee, compensation committee, or nominating and corporate governance committee. Prior to the completion of this offering, our board of directors will establish an audit committee, a compensation committee, and a nominating and corporate governance committee. Each of the members of these committees will meet the criteria for independence, and the functioning of these committees will comply with the requirements of, the Sarbanes-Oxley Act, the listing requirements of and SEC rules and regulations that will become applicable to us upon the consummation of this offering. Upon the completion of this offering, each of these committees will have adopted a written charter that will be available on our corporate website and will have the composition and responsibilities described below.
Audit Committee. Our audit committee will provide assistance to our board of directors in fulfilling its oversight responsibilities regarding:
| Ÿ | the integrity of financial statements; |
| Ÿ | our compliance with applicable legal and regulatory requirements; |
| Ÿ | the integrity of our financial reporting processes, including our systems of internal accounting and financial controls; |
| Ÿ | the performance of our internal audit function and independent registered public accounting firm; and |
| Ÿ | our financial policy matters. |
Our audit committee will determine and pre-approve the engagement of our independent registered public accounting firm to perform audit services and any permissible non-audit services. Our audit committee will also review and discuss with management and our registered independent public accounting firm the results of the annual audit and their reports regarding our accounting practices and systems of internal accounting controls, including significant issues that arise regarding accounting principles and financial statement presentation. Our audit committee will also evaluate the performance of our independent registered public accounting firm, determine whether to retain or terminate their services and take those actions as it deems necessary to satisfy itself that such accountants are independent of management. Our audit committee will also be responsible for establishing procedures for the receipt, retention and treatment of any complaints we receive regarding accounting, internal control or auditing matters.
82
Table of Contents
Upon the consummation of this offering, the members of our audit committee will be , and , with serving as the chairperson of our audit committee. Each member of our audit committee will be an independent director, as defined under the listing standards of and Rule 10A-3 of the Exchange Act. Each member of our audit committee will meet the applicable requirements for financial literacy. Our board of directors intends to appoint at least one director to our audit committee who will qualify as an “audit committee financial expert,” as defined under applicable SEC rules. Effective upon consummation of this offering, our audit committee will operate under a written charter that satisfies the applicable standards of the SEC and the .
Compensation Committee. Our compensation committee will:
| Ÿ | oversee our overall compensation structure, policies and programs; and |
| Ÿ | assess whether our compensation structure establishes appropriate incentives for officers and employees. |
Our compensation committee will review and approve corporate goals and objectives relevant to the compensation of our Chief Executive Officer and other executive officers, evaluate the performance of these officers in light of those goals and objectives and set the compensation of these officers based on such evaluations. In addition, our compensation committee will review and recommend to our board of directors any employment-related agreements, any proposed severance arrangements or change of control or similar agreements with these officers. Our compensation committee will also administer the issuance of stock options and other awards under any stock plans we may have. Our compensation committee will review and evaluate, at least annually, the performance of the compensation committee and its members and the adequacy of the charter of our compensation committee. Our compensation committee will also prepare a report on executive compensation, as required by SEC rules, to be included in our annual report and annual proxy statement.
The members of our compensation committee will be , and , with serving as the chairperson of the committee. Each member of our compensation committee will be an independent director under applicable listing standards, will be a non-employee director as defined in Rule 16b-3 under the Exchange Act and will be an outside director as that term is defined in Section 102(m) of the Code. Effective upon the consummation of this offering, our compensation committee will operate under a written charter that satisfies applicable listing standards.
Nominating and Corporate Governance Committee. Our nominating and corporate governance committee will be responsible for:
| Ÿ | developing and recommending to our board of directors criteria for identifying and evaluating candidates for directorships; and |
| Ÿ | making recommendations to our board of directors regarding candidates for election or reelection to our board of directors at each annual stockholders’ meeting. |
Our nominating and corporate governance committee, in recommending candidates for election to our board of directors, and our board of directors, in approving (and, in the case of vacancies, appointing) such candidates, will take into account many factors, including:
| Ÿ | diversity of personal background, perspective and experience; |
| Ÿ | personal and professional integrity; |
| Ÿ | experience in corporate management, operations or finance; |
| Ÿ | experience in our industry; and |
| Ÿ | experience as a board member of another publicly-held company. |
83
Table of Contents
Our board of directors will evaluate each individual in the context of our board of directors as a whole, with the objective of assembling a group that can best perpetuate the success of the business and represent stockholder interests through the exercise of sound judgment using its diversity of experience in these various areas. In addition, our nominating and corporate governance committee will be responsible for overseeing our corporate governance guidelines and reporting and making recommendations to our board of directors concerning corporate governance matters. Our nominating and corporate governance committee will be also responsible for making recommendations to our board of directors concerning the structure, composition and function of our board of directors and its committees.
The members of our nominating and corporate governance committee will be , and , with serving as chairperson of the compensation committee. Each member of the nominating and corporate governance committee will be independent under applicable listing standards. Effective upon the consummation of this offering, our nominating and corporate governance committee will operate under a written charter that satisfies the applicable standards of .
Overview of Risk Management
Our management is responsible for the day-to-day management of the risks we face, while our board of directors, as a whole and through its committees, has responsibility for the oversight of risk management. This oversight will be conducted primarily through committees of our board of directors, as disclosed in the descriptions of each of the committees below and in the charters of each of the committees. Our audit committee will be responsible for overseeing the management of our risks relating to accounting matters, financial reporting and legal and regulatory compliance. Our compensation committee will be responsible for overseeing the management of risks relating to our executive compensation programs and arrangements. Our nominating and corporate governance committee will be responsible for overseeing the management of our risks associated with the independence of our board of directors and potential conflicts of interest. While each committee will be responsible for evaluating certain risks and overseeing the management of such risks, our entire board of directors will be regularly informed through committee reports about such risks.
Executive Officers
Our executive officers are elected by, and serve at the discretion of, our board of directors.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics for all directors, officers and employees. The Code of Business Conduct and Ethics is available under the investor section of our website at www.petroalgae.com. A copy of the Code of Business Conduct and Ethics may also be obtained at no charge by written request to the attention of the Investor Relations Department at PetroAlgae Inc., 1901 S. Harbor City Blvd., Suite 600, Melbourne, FL 32901, telephone: (321) 409-7272, email: investorrelations@petroalgae.com.
Compensation Committee Interlocks and Insider Participation
David P. Szostak, our former Chief Financial Officer, is a director of XL Tech. John S. Scott, the Chairman of our board of directors, which has heretofore administered our executive compensation programs and initiatives, is the Chief Executive Officer of XL Tech.
84
Table of Contents
Summary Compensation Table
The following table provides information concerning compensation paid or accrued during the fiscal years ended December 31, 2008, 2009 and 2010 to our principal executive officers, our principal financial officers and our other most highly paid executive officers, collectively referred to as the named executive officers, determined during and at the end of the last fiscal year:
| Name and |
Fiscal year |
Salary | Bonus | Stock Awards(2) |
Option Awards |
Total Compensation |
||||||||||||||||||
| John S. Scott(6). |
2008 | $ | 451,923 | $ | 99,375 | $ | 3,576,000 | $ | — | $ | 4,127,298 | |||||||||||||
| Chairman of the Board and Chief Executive Officer |
2009 | 477,404 | (1) | — | — | — | 477,404 | |||||||||||||||||
| 2010 | 485,592 | (1) | — | 3,252,920 | — | 3,738,512 | ||||||||||||||||||
| David P. Szostak(5) |
2008 | $ | 271,114 | $ | 59,625 | $ | 1,206,900 | $ | — | $ | 1,537,679 | |||||||||||||
| Chief Financial Officer |
2009 | 225,000 | (1) | — | — | — | 225,000 | |||||||||||||||||
| 2010 | 225,298 | (1) | — | 1,097,861 | — | 1,323,159 | ||||||||||||||||||
| Gregory W. Haskell(5) |
2008 | $ | 115,385 | $ | 35,329 | $ | — | $ | — | $ | 150,714 | |||||||||||||
| Executive Vice President – Strategy |
2009 | 270,058 | (1) | — | — | 472,000 | 742,058 | |||||||||||||||||
| 2010 | 273,070 | (1) | 20,000 | — | — | 293,070 | ||||||||||||||||||
| Heinrich Gugger(5) |
2008 | $ | 230,000 | $ | — | $ | — | $ | — | $ | 230,000 | |||||||||||||
| Executive Vice President – Technology |
2009 | 88,684 | — | — | — | 88,684 | ||||||||||||||||||
| 2010 | 181,372 | — | — | 529,500 | 710,872 | |||||||||||||||||||
| Fred G. Tennant(5) |
2008 | $ | 150,385 | $ | 27,030 | $ | 276,500 | $ | — | $ | 453,915 | |||||||||||||
| Executive Vice President – Business Development |
2009 | 206,154 | — | — | — | 206,154 | ||||||||||||||||||
| 2010 | 212,076 | — | — | — | 212,076 | |||||||||||||||||||
| John Kinney |
2008 | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||
| Vice President – Corporate Development |
2009 | 46,731 | (3) | — | — | — | 46,731 | |||||||||||||||||
| 2010 | 135,000 | 5,600 | — | 176,500 | 317,100 | |||||||||||||||||||
| Ted Hollon(5) |
2008 | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||
| Deployment Program Manager |
2009 | — | — | — | — | — | ||||||||||||||||||
| 2010 | 220,673 | (4) | — | — | 88,250 | 308,923 | ||||||||||||||||||
| (1) | Includes certain amounts that, upon mutual agreement between PA LLC and the executive officers, have been withheld. The aggregate amount is approximately $80,000 and $310,000 for 2009 and 2010, respectively, for the officers referenced above. |
| (2) | Incentive equity awards in PA LLC are in the form of restricted units. When granted, the units are subject to repurchase by PA LLC. Based upon either a service period or the accomplishment of a liquidity event, the right of PA LLC to repurchase these units is removed. |
| (3) | Mr. Kinney joined the Company on August 24, 2009. |
| (4) | Mr. Hollon joined the Company on January 4, 2010. |
| (5) | During 2011, these individuals separated from the Company. |
| (6) | Dr. Scott resigned as Chief Executive Officer on June 16, 2011, but he remains non-executive Chairman of the Board. |
The following table summarizes the stock options granted to our named executive officers as of the end of the last fiscal year:
| Outstanding Equity Awards at Fiscal Year-End | ||||||||||||||||||||||||||||||||||||
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options Exercisable |
Number of Securities Underlying Unexercised Options Unexercisable |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options |
Option Exercise Price |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested |
Market Value of Shares or Stock That Have Not Vested |
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested |
|||||||||||||||||||||||||||
| John S. Scott |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| David P. Szostak |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Gregory W. Haskell |
100,000 | 100,000 | — | $ | 8.50 | 6/16/19 | — | — | — | — | ||||||||||||||||||||||||||
| Heinrich Gugger |
37,500 | 112,500 | — | $ | 8.00 | 1/26/20 | — | — | — | — | ||||||||||||||||||||||||||
| Fred G. Tennant |
— | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| John Kinney |
20,833 | 29,167 | — | $ | 8.00 | 1/26/20 | — | — | — | — | ||||||||||||||||||||||||||
| Ted Hollon |
— | 25,000 | — | $ | 8.00 | 1/26/20 | — | — | — | — | ||||||||||||||||||||||||||
85
Table of Contents
The following table summarizes the PA LLC equity incentives granted to our named executive officers as of the end of the last fiscal year:
| Grants of Plan Based Awards | ||||||||||||||||||||
| Name |
Grant Date |
Number of Restricted Units Underlying Grant (1) |
Number of Units Unrestricted as of 12/31/10(2) |
Fair Value per Unit |
Total Fair Value |
|||||||||||||||
| John S. Scott |
8/1/08 | 800,000 | 800,000 | $ | 8.54 | 6,828,920 | ||||||||||||||
| David P. Szostak |
8/1/08 | 270,000 | 270,000 | $ | 8.54 | 2,304,761 | ||||||||||||||
| Gregory W. Haskell |
— | — | — | — | — | |||||||||||||||
| Heinrich Gugger |
— | — | — | — | — | |||||||||||||||
| Fred G. Tennant |
1/15/07 | 50,000 | 37,500 | $ | 4.51 | 169,125 | ||||||||||||||
| 8/1/08 | 50,000 | 25,000 | $ | 5.53 | 138,250 | |||||||||||||||
| John Kinney |
— | — | — | — | ||||||||||||||||
| Ted Hollon |
— | — | — | — | ||||||||||||||||
| (1) | Incentive equity awards in PA LLC are in the form of restricted units. When granted, the units are subject to repurchase by PA LLC. Based upon either a service period or the accomplishment of a liquidity event, the right of PA LLC to repurchase these units is removed. |
| (2) | Employees holding Class B Membership Units that are not subject to repurchase by PA LLC will be able to exchange their equity interests for PetroAlgae Inc. common stock. |
Compensation Discussion and Analysis
Compensation philosophy and objectives. Our success depends, among other things, on attracting and retaining executive officers with the experience and skills in a number of different areas as we continue to further develop our business and technology. Historically, our principal stockholders played a significant role in establishing our executive compensation philosophy. In addition, those executive officers who were employees of XL Tech before the formation of PetroAlgae Inc. in December 2008 retained their base salaries when they became our employees.
We intend to establish a philosophy with respect to executive compensation policies and practices based on the alignment of each element of compensation with our short-term and long-term strategic objectives, while providing competitive compensation that enables us to attract and retain top-quality executive talent. We anticipate that the primary objectives of our compensation policies and practices for the named executive officers will be to:
| Ÿ | attract, retain, motivate and reward highly qualified and competent executives who have extensive industry experience through a mix of base salary, annual cash incentives and long-term equity incentives that encourage and recognize individual and company performance; and |
| Ÿ | provide incentives to increase and maximize stockholder value by: |
| ¡ | emphasizing equity-based compensation to more closely align the interests of executives with those of our stockholders; and |
| ¡ | structuring short-term compensation contingent upon the achievement of performance measures intended to reward performance year-over-year that we believe creates stockholder value in the short-term and over the long-term. |
We intend to adopt this philosophy because we believe that it will be critical to our continued success and the achievement of our short-term and long-term goals and objectives as a company for our stockholders.
86
Table of Contents
Administration. Since our inception, our board of directors has administered our executive compensation programs and initiatives. Our board of directors has also sought, and received, significant input from our principal stockholders with respect to the performance and compensation of our executive officers.
Prior to the completion of this offering, our board of directors will establish a compensation committee. We anticipate that our compensation committee will recommend, and our board of directors will adopt, a charter for our compensation committee which will delegate the responsibility for determining executive compensation and initiatives to our compensation committee. In accordance with our compensation committee’s charter, we anticipate that our compensation committee will have the authority to determine the annual compensation of our chief executive officer and other executive officers, and will regularly report its compensation decisions to the full board of directors. We also anticipate that our compensation committee will administer any equity compensation plans we may have, including our 2009 Equity Compensation Plan, or the 2009 Plan. See “—2009 Equity Compensation Plan.”
Our compensation committee may in the future engage compensation consultants familiar with our industry to advise the committee regarding certain compensation issues.
Compensation program. Based on and consistent with the philosophy and objectives stated above, our executive compensation program and practices will consist of the following elements:
| Ÿ | base salary; |
| Ÿ | annual cash incentive awards; |
| Ÿ | long-term equity incentive awards; and |
| Ÿ | benefits and perquisites. |
We have chosen these elements to remain competitive in attracting and retaining executive talent and to provide strong incentives for consistent high performance with current and potential financial rewards. We also plan to continue to provide employee benefits, such as health, dental and life insurance, to the named executive officers pursuant to plans that are generally available to our employees. We believe that our mix of compensation will instill in our executive officers the importance of achieving our short-term and long-term business goals and objectives and thereby increase stockholder value.
Additional details regarding elements of our executive compensation program are as follows:
Base salaries. Our board of directors established the base salary range for the named executive officers with significant input from our principal stockholders. In relation to long-term and short-term incentives, we view base salary as a less significant element of compensation than long-term equity, so the levels are less than those of peer companies. Our board of directors approves all increases in base salary for the named executive officers in advance and reviews salaries of executive officers at periodic intervals and awards increases, if appropriate. In assessing the amount and timing of salary adjustments, if any, our board of directors considers, and we anticipate that our compensation committee will consider, individual performance, changes in functions and responsibilities, if any, competitive salaries and peer comparisons, and relative positions within the company. At the end of 2008 and during 2009, certain former members of our management elected to defer a portion of their salary in order to assist us in preserving our financial position. Base salaries for all named executive officers for the fiscal years ended December 31, 2008, 2009 and 2010 are shown in the “Salary” column of the Summary Compensation Table above.
87
Table of Contents
Bonuses. Our executive officers participate in our annual cash bonus plan in which all of our full-time employees are eligible to participate. Our board of directors administers this bonus plan. Under this plan, each executive officer is eligible to receive a cash bonus amount based on a certain percentage of their salary. Our board of directors decides the amount of any bonus, as a percentage of such executive officer’s salary, for any given year. Our board of directors makes this determination based on the achievement by us of certain goals and objectives during the calendar year. Such goals and objectives are set forth in advance by the board of directors. To date, due to the development stage of our operations and specifically that we have not begun to generate net cash flows from operations, very little bonus compensation has been paid.
Long-term equity incentive compensation. We provide equity incentives pursuant to the 2009 Plan, which we adopted on June 17, 2009 and which seeks to give (i) our employees and the employees of PA LLC, (ii) certain consultants and advisors who perform services for us and our subsidiaries, and (iii) non-employee members of our board of directors and our parent and subsidiaries the opportunity to receive grants of incentive stock options, nonqualified stock options, stock awards and other stock-based awards. We believe that the 2009 Plan will encourage the participants to contribute materially to our growth, thereby benefiting our stockholders, and will align the economic interests of the participants with those of our stockholders.
Our board of directors currently administers the 2009 Plan. Our board of directors determines the individuals to whom awards shall be made under the 2009 Plan, as well as the type, size and terms of the awards, the time when the awards will be made and the duration of any applicable exercise or restriction period, including the criteria for exercisability and the acceleration of exercisability. Our board of directors may establish all other terms, conditions, and limitations applicable to awards, award programs and the shares of our common stock issued pursuant to such award programs, amend the terms of any previously issued award, and address any other matters arising under the 2009 Plan. Upon the consummation of this offering, our board of directors is expected to delegate each of the aforementioned responsibilities to our compensation committee.
Awards under the 2009 Plan may consist of grants of options, stock awards and other stock-based awards. Subject to certain adjustments, the aggregate number of shares of our common stock that may be issued pursuant to awards granted under the 2009 Plan may not exceed 4,000,000 shares of our common stock.
Employment Agreements
We do not have formal employment agreements with any of the named executive officers. See “Relationships and Related Party Transactions—Employment Agreements with Officers” for a description of our employment agreements with several of our current executive officers.
Other Executive Officer Perquisites
We generally do not provide any additional perquisites to the named executive officers except in certain limited circumstances to be approved by our board of directors or, after its establishment, our compensation committee.
Other Executive Officer Benefits
We provide the following benefits to our executive officers on the same basis as other eligible employees:
| Ÿ | health insurance; |
| Ÿ | vacation, personal holidays and sick days; |
88
Table of Contents
| Ÿ | life insurance; |
| Ÿ | short-term and long-term disability; and |
| Ÿ | a 401(k) plan. |
Impact of accounting and tax treatments. Section 162(m) of the Internal Revenue Code disallows a tax deduction for any publicly-held corporation for individual compensation exceeding $1.0 million in any taxable year for our chief executive officer and each of the other named executive officers (other than our chief financial officer), unless compensation is performance based. Our board of directors has not previously taken the deductibility limit imposed by Section 162(m) into consideration in setting compensation. We expect that our compensation committee will adopt a policy at some point in the future that, where reasonably practicable, will seek to qualify the variable compensation paid to our executive officers for an exemption from the deductibility limitations of Section 162(m). Until such policy is implemented, our board of directors and, after its establishment, our compensation committee may, in its judgment, authorize compensation payments that do not consider the deductibility limit imposed by Section 162(m) when it believes that such payments are appropriate to attract and retain executive talent.
Policy regarding the timing of equity awards. We have no program, plan or practice pertaining to the timing of stock option grants to executive officers coinciding with the release of material non-public information. We do not, as of yet, have any plans to implement such a program, plan or practice after the consummation of this offering. However, we intend to implement policies to ensure that equity awards are granted at fair market value on the date that the grant action occurs.
Policy regarding restatements. We do not have a formal policy regarding adjustment or recovery of awards or payments if the relevant performance measures upon which they are based are restated or otherwise adjusted in a manner that would reduce the size of the award or payment. Under those circumstances, we anticipate that our board of directors or, after its establishment, our compensation committee would evaluate whether adjustments or recoveries of awards were appropriate based upon the facts and circumstances surrounding the restatement.
Stock ownership policies. We have not established stock ownership or similar guidelines with respect to our executive officers. All of our executive officers currently have a direct or indirect, through their stock option holdings or otherwise, equity interest in us, and we believe that they regard the potential returns from these interests as a significant element of their potential compensation for services to us.
Pension benefits. None of the named executive officers participates in, or has an account balance in, a qualified or non-qualified defined benefit plan sponsored by us.
Non-qualified deferred compensation. None of the named executive officers participates in, or has an account balance in, a traditional non-qualified deferred compensation plan or other deferred compensation plans maintained by us.
2009 Equity Compensation Plan
Stock Options. We adopted the 2009 Plan on June 17, 2009. The 2009 Plan is intended to provide employees, consultants and others the opportunity to receive employee stock options and is limited to 4,000,000 shares of our common stock. The exercise price is equal to or greater than the fair value of the underlying common stock on the date the option is granted and its term does not exceed 10 years. Awards will not vest in full prior to the third anniversary of the award date. We reserved a pool of shares of our common stock for issuance to (i) our employees and the employees of PA LLC,
89
Table of Contents
(ii) certain consultants and advisors who perform services for us and our parent and subsidiaries, and (iii) non-employee members of our board of directors and our parent and subsidiaries pursuant to awards granted under the 2009 Plan. It provides for awards under three separate categories: (i) the option program pursuant to which eligible persons may be granted options, (ii) the stock award program pursuant to which eligible persons may be granted awards of our common stock, restricted stock, deferred stock and stock units, and (iii) the other stock-based awards program pursuant to which eligible persons may be granted awards that are based on our common stock as a bonus for achievement of performance goals or other conditions. The 2009 Plan is administered by our board of directors, which may delegate administrative responsibilities to a committee thereof. We expect that after the completion of this offering our board of directors will delegate the administration of the 2009 Plan to our compensation committee. Our board of directors has, and after its establishment our compensation committee will have, the authority to grant additional acceleration rights or waive any conditions relating to vesting, exercise, payment or distribution of any award upon a change of control to holders of options issued pursuant to the 2009 Plan.
We entered into stock option agreements, or SOAs, with 89 individuals, including 3 executive officers, to provide them with the option to purchase our common stock pursuant to the 2009 Plan. For the majority of the 86 individuals who were employees of PA LLC at the time we granted the option, the options vest ratably each year following each employee’s date of hire or the date of the grant so that the options are fully vested after four years. The three remaining SOAs provide for full vesting of the options over a four-year period, though not by equal numbers of shares each year. See “—2009 Equity Compensation Plan” for a more detailed analysis of the 2009 Plan.
A summary of stock option activity is as follows:
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Fair Value |
||||||||||
| Balance at December 31, 2008 |
— | $ | — | $ | — | |||||||
| Granted June 2009 |
1,072,500 | $ | 5.50 | $ | 2.70 | |||||||
| Granted July 2009 |
45,000 | $ | 5.50 | $ | 2.83 | |||||||
| Forfeited |
(200,000 | ) | $ | (5.50 | ) | $ | (2.70 | ) | ||||
|
|
|
|||||||||||
| Balance at December 31, 2009 |
917,500 | $ | 5.50 | $ | 2.37 | |||||||
| Granted January 2010 |
322,000 | $ | 5.50 | $ | 3.80 | |||||||
| Forfeited |
(247,750 | ) | $ | (5.50 | ) | $ | (2.95 | ) | ||||
|
|
|
|||||||||||
| Balance at December 31, 2010 |
991,750 | $ | 5.50 | $ | 2.68 | |||||||
| Granted January 2011 |
623,000 | $ | 5.50 | $ | 3.27 | |||||||
| Granted June 2011 |
351,000 | $ | 5.50 | $ | 3.96 | |||||||
| Granted July 2011 |
265,000 | $ | 5.50 | $ | 3.96 | |||||||
| Forfeited |
(530,000 | ) | $ | (5.50 | ) | $ | (2.92 | ) | ||||
|
|
|
|||||||||||
| Balance at September 30, 2011 |
1,700,750 | $ | 5.50 | $ | 3.47 | |||||||
On June 30, 2011, our board of directors authorized the re-pricing of all outstanding stock options, changing the exercise price from $8.00 or $8.50 to $5.50 per option. We computed the additional compensation cost as the fair value of the new awards in excess of the fair value of the original awards immediately before their terms were modified, using the Black-Scholes pricing model. This calculation used the following assumptions determined as of June 30, 2011: estimated fair value of the common stock of $5.10, remaining terms ranging from 4.75 years to 5.89 years (depending on the date of the original grant), risk free interest rate of 1.76%, an estimated volatility of 96.5%, and a dividend yield of 0%. The modification affected 1,240,000 options and resulted in aggregate additional compensation cost of $0.4 million. In the table above, the values shown for each grant prior to June 30, 2011 have been revised to reflect the new exercise price and recalculated fair.
90
Table of Contents
The weighted average grant date fair value for vested options as of September 30, 2011 was $3.11. The weighted average grant date fair value for unvested options as of September 30, 2011 was $3.66.
The weighted average contractual life of outstanding stock options at September 30, 2011 was 8.7 years. The weighted average contractual life of vested and unvested stock options at September 30, 2011 was 7.2 years and 9.4 years, respectively. As of September 30, 2011, a total of 574,917 of the options granted were exercisable and the fair value of such options was $1.8 million.
Stock awards program. The 2009 Plan provides for the grant of awards that are payable in shares of our common stock or denominated in units equivalent in value to shares of our common stock or are otherwise based on or related to shares of our common stock, restricted stock, deferred stock and stock units. These awards may not vest in full prior to the third anniversary of the award date; provided, however that our board of directors or a committee thereof may, in its sole discretion, grant awards that provide for accelerated vesting. At the discretion of our board of directors or the committee, these awards may be made subject to or may vest on an accelerated basis, upon the achievement of performance criteria related to a period of performance of not less than one year, which may be established on a companywide-basis or with respect to one more business units or divisions.
Other stock-based awards program. Our board of directors or a committee thereof may grant other stock-based awards, which are awards (other than those described above) that are based on or measured by our common stock, on such terms and conditions as our board of directors or a committee thereof determines. Other stock-based awards may be awarded subject to the achievement of performance goals or other conditions and may be payable in cash, common stock or any combination of the foregoing, as our board of directors or a committee thereof shall determine.
PA LLC Equity Incentive Plan
PA LLC has an equity compensation plan which allows it to grant employees and consultants awards of profits interests in restricted Class B ownership units, or the Interests, and is limited to 14% of the total outstanding units. PA LLC has granted 3,490,971 of its units to various employees through unit grant agreements. The unit grants generally vest over four years of continual service.
PA LLC granted 2,816,471 and 674,500 Interests during 2008 and 2007, respectively, to the majority of its employees as of the date that they began employment with PA LLC. As of September 30, 2011 and December 31, 2010, the granted Interests, net of Interests forfeited and repurchased by PA LLC, totaled 2,754,571 and 2,837,691, respectively, or 12.7% and 13.0%, respectively, of total outstanding units. The Interests give the recipient the right to participate in the income of PA LLC and any distribution that may arise from a liquidity event to the extent that such realized amounts exceed the ownership unit fair value at the date of grant.
The grants of Interests contain restrictions that allow PA LLC to repurchase the units for $0.01 per unit until a service period or other condition is met. This restriction is removed over a service period (generally four years) with regard to approximately 2.15 million Interests. On March 4, 2011, we modified the terms of the Interests granted to a former employee, causing the Interests to be immediately vested.
Five senior officers, including two executive officers, were granted an aggregate 1.34 million Interests that were subject to repurchase for $0.01 per unit until a significant liquidity event occurred. As of December 31, 2010, our principal stockholders authorized the modification of the terms of the grant to remove the right of PA LLC to repurchase the Interests held by all five officers, causing these
91
Table of Contents
grants to be immediately vested. This decision required a revaluation of the Interests held by the officers and immediate recognition of the calculated expense.
Stock Appreciation Rights
In November 2010, we issued one million Stock Appreciation Rights, or SARs to Mr. Harris, our president and chief operating officer, under the 2009 Plan. The vesting of these SARs was tied to continued employment with us and the accomplishment of certain milestones. Mr. Harris resigned during the first quarter of 2011, forfeiting all such SARs.
In June 2011, our board of directors authorized the grant of one million SARs at a base grant price of $5.50 per share to our newly-appointed chief executive officer under the 2009 Plan. The SARs have a ten-year term and will vest in equal quarterly installments over a two-year period. In the event of a change of control (as defined in the 2009 Plan) or a qualified public offering (as defined in the executive’s employment agreement), the SARs will become 100% vested.
401(k) Plan
We maintain a deferred savings retirement plan for our U.S. employees. The deferred savings retirement plan is intended to qualify as a tax-qualified plan under Section 401 of the Internal Revenue Code. Contributions to the deferred savings retirement plan are not taxable to employees until withdrawn from the plan. The deferred savings retirement plan provides that each participant may contribute his or her pre-tax compensation (up to a statutory limit, which is $16,500 in 2011). For employees 50 years of age or older, an additional catch-up contribution of $5,500 is allowable. Under the plan, each employee is fully vested in his or her deferred salary contributions. The deferred savings retirement plan also permits us to make additional discretionary contributions, subject to established limits and a vesting schedule.
Compensation of Directors
Our employee directors and any director appointed by our principal stockholders will not receive any additional compensation in connection with their service as directors. The compensation we pay our employee directors is discussed in “—Summary Compensation Table.” Currently, we pay our non-employee directors who are not affiliated with us a fee of $2,007 a month. Following this offering, we intend to pay our non-employee directors who are not affiliated with us a fee that commensurates with fees paid by peer companies for each meeting attended by them. Each non-employee director may also be entitled to participate in the 2009 Plan.
The following table summarizes the compensation we paid to our non-employee directors in 2010:
| Name |
Fees Earned or Paid in Cash ($) |
Stock Awards ($) |
Option Awards ($) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||
| Isaac Szpilzinger |
$ | 26,091 | — | — | — | $ | 26,091 | |||||||||||||
| Sayan Navaratnam |
— | — | — | — | — | |||||||||||||||
92
Table of Contents
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Stockholders Agreement
Prior to the completion of this offering, we will enter into the stockholders agreement. The stockholders agreement will become effective upon the completion of this offering. The stockholders agreement will contain a voting agreement that provides, among other things, that:
| Ÿ | our principal stockholders will agree to vote any shares of our common stock they and their affiliates own pro rata with our unaffiliated stockholders (other than in connection with any vote relating to (i) a consolidation or merger of the company or any of its affiliates into or with any other person (other than affiliates of our principal stockholders); or (ii) a sale, lease or transfer of all or substantially all of our assets or capital stock to another person (other than affiliates of our principal stockholders)); and |
| Ÿ | our principal stockholders will not vote in favor of the removal of any of our independent directors unless our unaffiliated stockholders approve such removal. |
At each stockholders’ meeting, our principal stockholders will be required to take all necessary or desirable actions within its control to effect the terms of the stockholders agreement, including with respect to the election of directors.
Pursuant to the terms of the stockholders agreement, our principal stockholders will have the right to appoint the observer to our board of directors. The observer will be entitled to receive notice of all meetings of our board of directors and all information distributed in connection with such meetings and to attend such meetings. The observer will not have any voting rights.
The stockholders agreement will also provide that we will not enter into any transaction with our principal stockholders or any of its affiliates without the prior written approval of a majority of the disinterested members of our board of directors.
The stockholders agreement will remain in effect so long as our principal stockholders and their affiliates beneficially own more than 25% of our outstanding common stock (as set forth on the cover of our then most recently filed annual report on Form 10-K or quarterly report on Form 10-Q).
Sublease from XL TechGroup, Inc.
As of December 31, 2010, we subleased space from XL Tech, on a month-to-month basis, at a current rate of $45,461 per month. The sublease was terminated in February 2011. Mr. Scott serves as a director and executive officer and is a principal shareholder of XL Tech. XL Tech founded and developed PA LLC from its inception.
Issuance of Shares to a Director
On December 19, 2008, in a private placement, we issued 1,000,000 shares of our common stock for a value of $3,150,000 to Nationwide Solutions Inc., or Nationwide Solutions, that is performing consulting services for us. Sayan Navaratnam, a director and an employee of our principal stockholders, had an interest in the transaction as the sole owner of Nationwide Solutions.
Issuance of Shares to Entities Affiliated with our Principal Stockholders
On May 5, 2010, April 7, 2010, March 1, 2010, October 9, 2009, September 29, 2009, September 14, 2009, September 4, 2009, and August 28, 2009, we issued and sold in the aggregate (i) 686,625 shares of our common stock to Valens U.S. SPV I, LLC for a purchase price of
93
Table of Contents
$8.00 per share and warrants to purchase 686,625 shares of our common stock at an exercise price of $15.00 per share, and (ii) 563,375 shares of our common stock to Valens Offshore SPV I, Ltd. for a purchase price of $8.00 per share and warrants to purchase 563,375 shares of our common stock at an exercise price of $15.00 per share. The aggregate proceeds from these private placements was $10,000,000.
On December 22, 2008, we issued and sold 666,667 shares of our common stock to Valens U.S. SPV I, LLC for a purchase price of $3.15 per share and 2,507,936 shares of our common stock to Valens Offshore SPV I, Ltd. for a purchase price of $3.15 for consideration of $2,100,000 and $7,900,000, respectively.
These were private placement transactions exempt from the registration requirements of the Securities Act pursuant to the exemption afforded by Section 4(2) thereof and/or Regulation D promulgated thereunder. We used the proceeds of these private placements to fund the working capital needs of PA LLC.
Employment Agreements with Officers
We entered into the following employment agreement with the following executives: (i) Anthony John Phipps Tiarks as our Chief Executive Officer, effective as of June 15, 2011 and (ii) Peter Sherlock as our Chief Operating Officer, effective as of July 21, 2011.
Each employment agreement has an initial term of one year and will continue on a year-to-year basis thereafter until the executive’s employment is not renewed or otherwise terminated as set forth therein.
Mr. Tiarks will receive the following compensation and benefits: (i) an annual base salary of $300,000 (subject to increase, but not decrease, in the sole discretion of our board of directors); (ii) a one-time signing bonus (before taxes) of $30,000; (iii) eligibility to receive an annual performance bonus based upon the achievement of targets established in our sole discretion; (iv) a grant of SARs on a base number of 1,000,000 of our shares at a base grant price of $5.50 per share; (v) eligibility to receive a special cash bonus upon the achievement of certain milestones; (vi) participation in a commission plan and (vii) a one-time bonus of $100,000 if we complete a total capital raise of at least $25,000,000. Mr. Sherlock will receive the following compensation and benefits: (i) an annual base salary of $220,000 (subject to increase, but not decrease, in the sole discretion of our board of directors); (ii) eligibility to receive an annual performance bonus based upon the achievement of targets established in our sole discretion; (iii) a grant of stock options on a base number of 200,000 of our shares at a base grant price of $5.50 per share; (iv) eligibility to receive a special cash bonus four times per year, each in the amount of $5,000, upon the achievement of certain milestones and (v) a one-time bonus of $100,000 if we complete a total capital raise of at least $25,000,000.
Each of the executives will also receive (i) eligibility to participate in all employee benefit plans and programs maintained from time to time for our employees and (ii) four weeks of annual paid vacation.
In the event the executive’s employment is terminated for any reason (including by expiration of the term of his employment agreement), the executive will be paid the following through the date of termination: (i) any accrued but unpaid base salary; (ii) reimbursement for any business expenses properly incurred; and (iii) vested benefits, if any, to which he may be entitled under our employee benefit plans or stock option plans, or, collectively, the Accrued Benefits. If the executive’s employment is terminated by us for “cause”, then he shall not be entitled to any further compensation or benefits other than the Accrued Benefits. If the executive’s employment is terminated by us “other than for
94
Table of Contents
cause”, then the executive shall be entitled to the Accrued Benefits and (subject to signing a mutual release) a lump sum amount equal to the number of days (at the base salary date) between the date of termination and the next anniversary of the execution of the his employment agreement. If the executive’s employment is terminated by him for “good reason”, then he shall be entitled to the Accrued Benefits and (subject to signing a mutual release) a lump sum amount equal to the number of days (at the base salary rate) between the date of termination and the next anniversary of the execution of his employment agreement. The executive can voluntarily resign his employment at any time, effective 31 days following the date on which a written notice to such effect is delivered to us. If the executive’s employment is terminated through a voluntary resignation and for no other reason, he shall be entitled to payment of the Accrued Benefits. In the event of termination of employment by reason of the executive’s death, his beneficiary or estate, as applicable, shall be entitled to the payment of the Accrued Benefits. Following a termination by the us for cause or voluntary resignation by the executive, each executive will be subject to a two-year non-competition and non-solicitation agreement. In addition, following termination of employment, each executive will be subject to a covenant not to disparage us.
In addition, in the event Mr. Tiarks’ employment is terminated by us through non-renewal of the term of his employment agreement, then Mr. Tiarks shall be entitled to the Accrued Benefits and (subject to signing a mutual release) a lump sum amount equal to the number of days (at the base salary rate) between the date of termination and the next anniversary of the execution of his employment agreement.
We also entered into an employment agreement with James P. Dietz as our Vice President of Accounting and Finance, effective as of May 10, 2010 and as amended on November 11, 2010. As of the date of this prospectus, Mr. Dietz serves as our Chief Financial Officer and his employment agreement remains in effect. Mr. Dietz will receive the following compensation and benefits: (i) an annual base salary of $200,000 (subject to adjustment, in the sole discretion of our board of directors), (ii) eligibility to receive a grant of stock options, (iii) 15 days of annual paid vacation, (iv) a one-time bonus of $100,000 if we compete a total capital raise of at least $25,000,000, (v) eligibility to receive an annual bonus of up to half his salary, (vi) eligibility to participate in all employee benefit plans and programs maintained from time to time for our employees, and (vii) reimbursement for relocation expenses not to exceed $5,000.
In the event Mr. Dietz’s employment is terminated (except by us for “cause”, by reason of his death or by reason of his disability), he will be eligible to receive an amount equal to two weeks of his then current-salary. If his employment is terminated by us for “cause”, then we will pay all compensation to which he is entitled to through the date of termination. In the event of termination of employment by reason of his death, his beneficiary or estate, as applicable, shall be entitled to the payment of any compensation then due and owing. In the event of termination of employment due to his disability, he shall be entitled to the payment of any compensation then due and owing. During Mr. Dietz’s term of employment and for two years after termination of his employment, Mr. Dietz will be subject to a non-disclosure covenant. If Mr. Dietz terminates his employment at any time for any reason or no reason at all, our obligations under his employment agreement cease.
Related Party Expenses
In 2008, we paid XL Tech $2.2 million as reimbursement for administrative, executive management, business development and other services. Since 2008, we have not made any additional payments to XL Tech, and we do not expect to make any additional payments to XL Tech with respect to these services.
95
Table of Contents
Assignment by our Principal Stockholders
On December 16, 2008, PetroTech acquired a shell company for $350,000 and, on December 19, 2008, assigned its entire interest in PA LLC to this shell company, which it then renamed PetroAlgae Inc.
Indemnification Agreements
Our amended and restated certificate of incorporation will contain provisions limiting the liability of directors, and our bylaws will provide that we will indemnify each of our directors to the fullest extent permitted under Delaware law. Our amended and restated certificate of incorporation and amended and restated bylaws will also provide our board of directors with the discretion to indemnify our officers and employees when determined appropriate by the board.
Policy on Future Related Party Transactions
Prior to the closing of this offering, our board of directors will adopt a written related party transaction policy setting forth the policies and procedures for the review and approval or ratification of related party transactions. This policy, which will become effective upon the closing of this offering, covers any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were or are to be a participant and a related person had or will have a direct or indirect material interest, as determined by the audit committee of our board of directors, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, and indebtedness, guarantees of indebtedness or employment by us of a related person. In reviewing any such proposal, our audit committee will be tasked with considering all relevant facts and circumstances, including, but not limited to, the commercial reasonableness of the terms, the benefit or perceived benefit, or lack thereof, to us, opportunity costs of alternate transactions, the materiality and character of the related person’s direct or indirect interest and the actual or apparent conflict of interest of the related person.
All related party transactions described in this section occurred prior to adoption of this policy and therefore were not subject to the approval and review procedures set forth in the policy. However, these transactions were reviewed and approved by our board of directors prior to the completion of the offering.
96
Table of Contents
PRINCIPAL AND SELLING STOCKHOLDERS
As of December 1, 2011, PetroTech held 2,417,215 shares of the common stock of PetroAlgae, representing 2.2% of the issued and outstanding common stock, and a $13.1 million promissory note convertible into a further 3,125,475 shares of common stock.
PetroTech is owned by the following private investment entities:
| Ÿ | Valens U.S.; |
| Ÿ | Valens Offshore SPV I, Ltd., or Valens SPV I; |
| Ÿ | Valens Offshore SPV II, Corp., or Valens SPV II; |
| Ÿ | Laurus Master Fund, Ltd. (In Liquidation), or the Fund, and its wholly-owned subsidiary, CCC; and |
| Ÿ | PSource Structured Debt Limited, or PSource. |
Valens U.S., Valens SPV I and Valens SPV II are each managed by VCM.
The Fund is in liquidation under the supervision of the Grand Court of the Cayman Islands. The joint official liquidators, or JOLs, have discretion over the management of the Fund and the disposition of its assets. LCM provides day-to-day investment management services to the Fund.
PSource is managed by LCM, subject to certain preapproval rights of the board of directors of PSource.
Eugene Grin and David Grin are the controlling principals of LCM and VCM.
We refer to the private investment entities described above, and that are managed by VCM and LCM, as our principal stockholders and also as the Laurus-Valens Funds. The Laurus-Valens Funds have invested in micro-cap and small private and publicly-traded companies since 2001.
The following table sets forth certain information with respect to the beneficial ownership of our common stock at December 1, 2011, as adjusted to reflect the sale of shares of our common stock offered by us in this offering, for:
| Ÿ | each person, or group of affiliated persons, who we know beneficially owns more than 5% of our outstanding shares of our common stock; |
| Ÿ | each of our directors; |
| Ÿ | each of our named executive officers; and |
| Ÿ | all of our current directors and executive officers as a group; and |
| Ÿ | the selling stockholders. |
We have determined beneficial ownership in accordance with the rules of the SEC. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the table below have sole voting and investment power with respect to all shares of our common stock that they beneficially own, subject to applicable community property laws.
97
Table of Contents
For purposes of the table below, we have assumed that shares of our common stock will be outstanding upon completion of this offering. In computing the number of shares of our common stock beneficially owned by a person and the percentage ownership of that person, we deemed to be outstanding all shares of our common stock subject to options, warrants or other convertible securities held by that person or entity that are currently exercisable or exercisable within 60 days of September 30, 2011. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.
Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o PetroAlgae Inc., 1901 S. Harbor City Blvd., Suite 600, Melbourne, FL 32901. Beneficial ownership representing less than 1% is denoted below with an asterisk (*).
| Shares of Our Common Stock Beneficially Owned Before This Offering |
Number of Shares Being Offered by Stockholder |
Shares of Our Common Stock Beneficially Owned After This Offering | ||||||||||||
| Name and Address of Beneficial Owners and |
Number of Shares |
Percentage of Class |
Number of Shares |
Percentage of Class | ||||||||||
| 5% Stockholders |
||||||||||||||
| PetroTech Holdings Corp.(1) |
104,424,603 | 97.7 | % | |||||||||||
| Directors and Executive Officers |
||||||||||||||
| Sayan Navaratnam |
1,000,000 | * | ||||||||||||
| John S. Scott |
— | * | ||||||||||||
| Isaac D. Szpilzinger |
— | * | ||||||||||||
| Anthony John Phipps Tiarks |
— | * | ||||||||||||
| James P. Dietz |
50,000 | * | ||||||||||||
| Peter Sherlock |
— | * | ||||||||||||
| All directors and officers as a group (six persons) |
1,050,000 | * | ||||||||||||
| Selling Stockholders |
||||||||||||||
|
(2) |
* | |||||||||||||
| (1) | PetroTech Holdings Corp., or PetroTech, is owned by Valens U.S. SPV I, LLC, a Delaware limited liability company, or Valens U.S., Valens Offshore SPV I, Ltd., a Cayman Islands limited company, or Valens SPV I, Valens Offshore SPV II, Corp., a Delaware corporation, or Valens SPV II, Laurus Master Fund, Ltd. (In Liquidation), a Cayman Islands limited company, or the Fund, Calliope Capital Corporation, a Delaware corporation, or CCC and PSource Structured Debt Limited, a Guernsey company, or PSource. The Fund, CCC and PSource are each managed by Laurus Capital Management, LLC, a Delaware limited liability company, or LCM. Valens U.S., Valens SPV I and Valens SPV II are each managed by Valens Capital Management, LLC, or VCM. Eugene Grin and David Grin, through other entities, are the controlling principals of LCM and VCM and share sole voting and investment power over all securities of the Company held by PetroTech. Eugene Grin and David Grin disclaim beneficial ownership of the securities of the Company held by PetroTech, except to the extent of such person’s pecuniary interest in PetroTech, if any. PetroTech pledged all of the common shares it holds to L.V. Administrative Services, an affiliate of the Laurus-Valens Funds, as administrator and collateral agent for Valens U.S., as security for a $417,511.92 promissory note made by PA LLC in favor of Valens U.S. |
| (2) | . |
98
Table of Contents
Prior to the completion of this offering, we will amend our restated certificate of incorporation and restated bylaws. We will file copies of the forms of our amended and restated certificate of incorporation and amended and restated bylaws as exhibits to the registration statement of which this prospectus is a part. The provisions of our amended and restated certificate of incorporation and amended and restated bylaws that will be in effect upon consummation of this offering and relevant sections of the DGCL are summarized below. The following description of our capital stock and provisions of our amended and restated certificate of incorporation and our amended and restated bylaws are summaries and are qualified by reference to our amended and restated certificate of incorporation and our amended and restated bylaws that will be in effect upon the closing of this offering.
Authorized Capital Stock
Our authorized capital stock will consist of shares, including: (i) shares of our common stock, par value $0.001 per share, and (ii) shares of preferred stock, $0.001 par value per share. As of September 30, 2011, we had outstanding 106,920,730 shares of our common stock, held of record by 393 stockholders, and no shares of preferred stock outstanding.
Common Stock
Holders of our common stock will be entitled to one vote per share on all matters submitted to a vote of stockholders, including the election of directors. Our common stockholders will not be entitled to cumulative voting in the election of directors. Subject to preferences that may be applicable to any outstanding shares of preferred stock, holders of our common stock will be entitled to receive ratably such dividends as may be declared by our board of directors out of funds legally available therefor if our board of directors, in its discretion, determines to issue dividends and only then at the times and in the amounts that our board of directors may determine. Upon the liquidation, dissolution or winding-up of our company, the holders of our common stock will be entitled to receive their ratable share of the net assets of our company available after payment of all debts and other liabilities, subject to the prior preferential rights and payment of liquidation preferences, if any, of any outstanding shares of preferred stock. Holders of our common stock will have no preemptive, subscription or redemption rights. There will be no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock will be subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate in the future.
Preferred Stock
Our board of directors will have the authority, subject to the limitations imposed by Delaware law, without any further vote or action by our stockholders, to issue preferred stock in one or more series and to fix the designations, powers, preferences, limitations and rights of the shares of each series, including:
| Ÿ | dividend rates; |
| Ÿ | conversion rights; |
| Ÿ | voting rights; |
| Ÿ | terms of redemption and liquidation preferences; and |
| Ÿ | the number of shares constituting each series. |
99
Table of Contents
Satisfaction of any dividend preferences of outstanding shares of preferred stock would reduce the amount of funds available for the payment of dividends on shares of our common stock. Holders of shares of preferred stock may be entitled to receive a preference payment in the event of our liquidation, dissolution or winding-up before any payment is made to the holders of shares of our common stock.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of our company and may adversely affect the market price of our common stock and the voting and other rights of the holders of our common stock. However, our board of directors may not, other than through an amendment to our amended and restated certificate of incorporation approved by our stockholders, adopt or approve any “rights plan,” “poison pill” or other similar plan, agreement or device designed to prevent or make more difficult a hostile takeover of our company by increasing the cost to a potential acquiror of such a takeover either through the issuance of new rights, shares of our common stock or preferred stock or any other security or device that may be issued to our stockholders other than all stockholders that carry severe redemption provisions, favorable purchase provisions or otherwise. This prohibition means that our board of directors will be prohibited from issuing preferred stock as a defensive mechanism to render more difficult or tend to discourage a merger, tender offer or proxy contest, the assumption of control by a holder of a large block of our securities or the removal of incumbent management.
There are no current agreements or understandings with respect to the issuance of preferred stock and our board of directors has no present intentions to issue any shares of preferred stock.
Stock Options
As of September 30, 2011, 1,700,750 shares of our common stock were issuable upon the exercise of stock options outstanding and exercisable at a weighted-average exercise price of $5.50 per share of which 574,917 shares with a weighted-average exercise price of $5.50 per share would be vested if purchased upon exercise of these options as of September 30, 2011. We have reserved 4 million shares of our common stock for issuance pursuant to outstanding stock options all of which remain available for issuance.
Warrants
As of September 30, 2011, 2,592,143 shares of our common stock were issuable upon the exercise of warrants outstanding and exercisable at an exercise price of $15.00. Below is a list of recent issuances:
On August 6, 2010, we issued a warrant to purchase 10,000 shares of our common stock at an exercise price of $15.00 per share.
On June 29, 2010, we issued a warrant to purchase 81,250 shares of our common stock at an exercise price of $15.00 per share.
On May 5, 2010, April 7, 2010, March 1, 2010, October 13, 2009, September 29, 2009, September 14, 2009, September 4, 2009 and August 28, 2009, we issued warrants to purchase 1,250,000 shares of our common stock in the aggregate at an exercise price of $15.00 per share.
On March 1, 2010, we issued a warrant to purchase 12,500 shares of our common stock at an exercise price of $15.00 per share.
100
Table of Contents
On February 6, 2010, we issued a warrant to purchase 6,250 shares of our common stock at an exercise price of $15.00 per share.
On December 24, 2009, we issued warrant to purchase 357,143 shares of our common stock at an exercise price of $15.00 per share.
On October 19, 2009, we issued a warrant to purchase 500,000 shares of our common stock at an exercise price of $15.00 per share.
On October 9, 2009, we issued a warrant to purchase 375,000 shares of our common stock at an exercise price of $15.00 per share.
Stockholder Registration Rights
Engineering, Automation & Design, Inc. is entitled to rights with respect to the registration of its shares under the Securities Act. These rights are provided under the terms of a registration rights agreement between us and the holder of those shares and include piggyback registration rights.
If we register any of our securities either for our own account or for the account of other security holders after the completion of this offering, the holder of these shares is entitled to include its shares in the registration at our expense. The underwriters of any underwritten offering have the right to limit the number of shares registered by these holders for marketing reasons, subject to certain limitations. All fees, costs and expenses of underwritten registrations will be borne by us and all selling expenses, including underwriting discounts and selling commissions, will be borne by the holder of the shares being registered.
The registration rights terminate with respect to the registration rights of the holder on the date when the holder can sell all of the holder’s shares in any three month period without registration under the Securities Act.
Certain Provisions of our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
Classified Board of Directors
Our amended and restated certificate of incorporation that will be in effect upon the closing of this offering will provide that our board of directors will be divided into three classes of directors, with the number of directors in each class to be as nearly equal as possible. Our classified board of directors staggers terms of the three classes and will be implemented through one, two and three-year terms for the initial three classes, followed in each case by full three-year terms. With a classified board of directors, only one-third of the members of our board of directors will be elected each year. This classification of directors will have the effect of making it more difficult and time consuming for stockholders to change the composition of our board of directors.
Size of Board of Directors and Removal of Directors
Our amended and restated certificate of incorporation and amended and restated bylaws that will be in effect upon the closing of this offering will provide that:
| Ÿ | the number of directors will be fixed from time to time exclusively pursuant to a resolution adopted by our board of directors, but must consist of not less than three directors, which will prevent stockholders from circumventing the provisions of our classified board of directors; |
| Ÿ | directors may be removed only for cause; and |
| Ÿ | vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum. |
101
Table of Contents
Stockholder Action By Written Consent
Pursuant to Section 228 of the DGCL, any action required to be taken at any annual or special meeting of the stockholders may be taken without a meeting, without prior notice and without a vote if a consent or consents in writing, setting forth the action so taken, is signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares of our stock entitled to vote thereon were present and voted, unless our amended and restated certificate of incorporation provides otherwise. Our amended and restated certificate of incorporation will provide that any action required or permitted to be taken by our stockholders may only be effected at a duly called annual or special meeting of our stockholders and may not be effected by consent in writing by such stockholders.
Requirements for Advance Notification of Stockholder Meeting, Nominations and Proposals
Our amended and restated bylaws will provide that special meetings of the stockholders must be called upon the request of holders of not less than % of the combined voting power of the voting stock, and may be called upon the request of our board of directors, or the chairman of the board, the vice chairman of the board (if any) or the chief executive officer.
Our amended and restated bylaws will establish an advance notice procedure for stockholder proposals to be brought before an annual or special meeting of our stockholders, including proposed nominations of persons for election to our board of directors. Stockholders at an annual or special meeting will only be able to consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of our board of directors or by a stockholder who was a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given our secretary timely written notice, in proper form, of the stockholder’s intention to bring that business before the meeting. Our amended and restated bylaws may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed or may discourage or defer a potential acquiror from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempting to obtain control of the company.
Our amended and restated bylaws will allow the presiding officer at a meeting of the stockholders to adopt rules and regulations for the conduct of meetings which may have the effect of precluding the conduct of certain business at a meeting if the rules and regulations are not followed. These provisions may also defer, delay or discourage a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to obtain control of our company.
Requirements for Amendment of Certificate and Bylaws
Our amended and restated certificate of incorporation will provide that the provisions in the amended and restated certificate of incorporation and our amended and restated bylaws relating to indemnification and exclusion of directors from personal liability may be amended only by a vote of % or more of all of the outstanding shares of our capital stock then entitled to vote. Our amended and restated bylaws will provide that our stockholders may amend our bylaws only by a vote of more than % of all of the outstanding shares of our capital stock then entitled to vote.
Delaware Law
We are subject to Section 203 of the DGCL, which, subject to certain exceptions, prohibits a Delaware corporation from engaging in any “business combination” (as defined below) with any interested stockholder for a period of three years following the date that such stockholder became an interested stockholder, unless: (1) prior to such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; (2) on consummation of the transaction that resulted in the stockholder
102
Table of Contents
becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding, for purposes of determining the voting stock outstanding, those shares owned (x) by persons who are directors and also officers and (y) by employee stock plans in which employee participants do not have the right to determine confidentiality whether shares held subject to this plan will be tendered in a tender or exchange offer; or (3) on or subsequent to such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder.
Section 203 of the DGCL defines generally “business combination” to include: (1) any merger or consolidation involving the corporation and the interested stockholder; (2) any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; (3) subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; (4) any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or (5) the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. In general, Section 203 defines an “interested stockholder” as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by such entity person.
Limitation of Liability and Indemnification
Our amended and restated certificate of incorporation and our amended and restated bylaws will provide indemnification for our directors and officers to the fullest extent permitted by the DGCL. In addition, as permitted by the DGCL, our amended and restated certificate of incorporation includes provisions that eliminate the personal liability of our directors for monetary damages resulting from breaches of certain fiduciary duties as a director. These provisions cannot be amended without the affirmative vote of % of the outstanding shares. The effect of this provision is to restrict our rights and the rights of our stockholders in derivative suits to recover monetary damages against a director for breach of fiduciary duties as a director, except that a director will be personally liable for:
| Ÿ | any breach of his or her duty of loyalty to us or our stockholders; |
| Ÿ | acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
| Ÿ | any transaction from which the director derived an improper personal benefit; and |
| Ÿ | improper distributions to stockholders. |
We also intend to maintain director and officer liability insurance, if available on reasonable terms.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and our amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
103
Table of Contents
As of the date of this prospectus, we are not aware of any pending litigation or proceeding involving any director, officer, employee or agent of our company where indemnification will be required or permitted, nor are we aware of any threatened litigation or proceeding that might result in a claim for indemnification.
Listing
We intend to list our common stock on the under the symbol “ ”.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A. The transfer agent’s address is 250 Royall Street, Canton, Massachusetts 02021, and its telephone number is (800) 662-7232.
104
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Shares of our common stock are currently listed on the OTCQB. Future sales of shares of our common stock in the public market after this offering, the perception that these sales could occur and the availability of shares for future sale could adversely affect the market price of our common stock prevailing from time to time and could impair our future ability to raise equity capital. We intend to apply to list our common stock on the .
Upon the closing of this offering, we will have outstanding an aggregate of shares of our common stock, assuming no exercise by the underwriters of their option to purchase additional shares from us and no exercise of then outstanding options or warrants. Of these shares, all shares sold in the offering will be freely tradeable without restriction or further registration under the Securities Act, except for any shares our “affiliates”, whose sales will be subject to the Rule 144 resale restrictions described below, other than the holding period requirement.
Shares that were outstanding prior to this offering and which are not subject to lock-up agreements, other than those shares held by our affiliates, will be freely tradable in the United States provided any holding period under Rule 144 has expired with respect to such shares, subject, in the case of shares held by our affiliates, to the volume and manner of sale restrictions imposed by Rule 144.
No sooner than months following the completion of the offering, we will file a registration statement with the SEC covering the exchange from time to time of an aggregate of shares of our common stock issuable upon exchange of the outstanding LLC interests in PA LLC held by our employees or by Valens-Calliope. Any shares issued upon such exchange would be freely tradable by any exchanging holder subject, in the case of exchanging holders who are our affiliates, to the volume and manner of sale restrictions imposed by Rule 144.
In addition, of the shares of our common stock that were subject to stock options outstanding as of , 2011, options to purchase shares of common stock were vested as of , 2011 and will be eligible for sale 180 days following the effective date of this offering, subject to extension as described in the section entitled “Underwriting.”
Rule 144
In general, a person who has beneficially owned restricted shares of our common stock for at least six months would be entitled to sell their securities provided that (i) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding, a sale and (ii) we are subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Persons who have beneficially owned restricted shares of our common stock for at least six months but who are our affiliates at the time of, or any time during the 90 days preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of either of the following:
| ¡ | 1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering assuming no exercise of the underwriters’ option to purchase additional shares, based on the number of shares of common stock outstanding as of , 2011; or |
| ¡ | the average weekly trading volume of our common stock on the during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Provided, in each case, that we are subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Such sales both by affiliates and by non-affiliates must also comply with
105
Table of Contents
the manner of sale, current public information and notice provisions of Rule 144. For purposes of Rule 144, an “affiliate” of an issuer is a person that directly, or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, such issuer.
Lock-Up Agreements
In connection with this offering, we, the selling stockholders, and all of our officers, directors and employees have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of their common stock or securities convertible into or exchangeable for shares of our common stock during the period from the date of this prospectus continuing through the date 180 days after the date of this prospectus, without the prior written consent of the representatives.
The 180-day restricted period described in the preceding paragraph will be automatically extended if: (1) during the last 17 days of the 180-day restricted period, the company issues an earnings release or announces material news or a material event; or (2) prior to the expiration of the 180-day restricted period, the company announces that it will release earnings results during the 15-day period following the last day of the 180-day period, in which case the restrictions described in the preceding paragraph will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release of the announcement of the material news or material event.
106
Table of Contents
MATERIAL U.S. FEDERAL INCOME AND ESTATE TAX
CONSIDERATIONS FOR NON-U.S. HOLDERS OF OUR COMMON STOCK
The following is a general discussion of the anticipated material U.S. federal income and estate tax consequences relating to the ownership and disposition of our common stock by Non-U.S. Holders, as defined below, who purchase our common stock in this offering and hold such common stock as capital assets within the meaning of Section 1221 of the U.S. Internal Revenue Code of 1986, as amended, or the Code. This discussion is based on currently existing provisions of the Code, existing and proposed U.S. Treasury Regulations promulgated thereunder, and administrative and judicial interpretation thereof, all as in effect or proposed on the date hereof and all of which are subject to change, possibly with retroactive effect or different interpretations. This discussion does not address all the tax consequences that may be relevant to specific holders in light of their particular circumstances or to holders subject to special treatment under U.S. federal income or estate tax laws, including but not limited to controlled foreign corporations, passive foreign investment companies, companies that accumulate earnings to avoid U.S. federal income tax, financial institutions, insurance companies, tax-exempt organizations, retirement plans, partnerships and their partners, other pass-through entities and their members, dealers in securities, brokers, U.S. expatriates and persons who have acquired our common stock as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment. This discussion does not address the U.S. state and local or non-U.S. tax consequences relating to the ownership and disposition of our common stock.
YOU ARE URGED TO CONSULT YOUR OWN TAX ADVISOR REGARDING THE U.S. FEDERAL TAX CONSEQUENCES OF OWNING AND DISPOSING OF OUR COMMON STOCK, AS WELL AS THE APPLICABILITY AND EFFECT OF ANY STATE, LOCAL OR NON-U.S. TAX LAWS.
As used in this discussion, the term “Non-U.S. Holder” refers to a beneficial owner of our common stock that for U.S. federal income tax purposes is not:
| (i) | an individual who is a citizen or resident of the U.S.; |
| (ii) | a corporation (or other entity taxable as a corporation) created or organized in or under the laws of the U.S. or any state thereof or the District of Columbia; |
| (iii) | an estate the income of which is subject to U.S. federal income tax regardless of its source; or |
| (iv) | a trust (a) if a U.S. court is able to exercise primary supervision over the administration of such trust and one or more U.S. persons have the authority to control all substantial decisions of such trust, or (b) that has in effect a valid election under applicable U.S. Treasury Regulations to be treated as a U.S. person. |
If a partnership (or other entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds our common stock, the tax treatment of a partner generally will depend upon the status of the partner and the activities of the partnership. If you are a partner of a partnership holding our common stock, we urge you to consult your own tax advisor.
Distributions
As described above under “Dividend Policy,” we do not intend to declare or pay cash dividends on our common stock in the foreseeable future. However, if we do make distributions on our common stock, those payments will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed our current and accumulated earnings and profits, they will constitute a return of capital and will first reduce a Non-U.S. Holder’s basis in our common stock, but not below zero, and then will be treated as a gain from the sale of stock as described below under “Sale, Exchange or Other Disposition.”
107
Table of Contents
We or a withholding agent will be required to withhold U.S. federal withholding tax from the gross amount of any dividends paid to a Non-U.S. Holder at a rate of 30%, unless (i) an applicable income tax treaty reduces or eliminates such tax, and a Non-U.S. Holder claiming the benefit of such treaty provides to us or such agent proper Internal Revenue Service, or IRS, documentation, or (ii) the dividends are effectively connected with a Non-U.S. Holder’s conduct of a trade or business in the U.S., or where an applicable income tax treaty provides, the dividends are attributable to a U.S. permanent establishment of such Non-U.S. Holder, and the Non-U.S. Holder provides to us or such agent proper IRS documentation. A Non-U.S. Holder described in (ii) in the preceding sentence generally will be subject to U.S. federal income tax with respect to such dividends at the same graduated individual or corporate rates applicable to U.S. persons, unless an applicable income tax treaty otherwise provides. Additionally, a Non-U.S. Holder that is a corporation could be subject to U.S. federal “branch profits tax” on effectively connected dividend income at a rate of 30% (or at a reduced rate under an applicable income tax treaty). If a Non-U.S. Holder is eligible for a reduced rate of U.S. federal withholding tax pursuant to an income tax treaty, such Non-U.S. Holder may obtain a refund of any excess amount withheld by timely filing an appropriate claim for refund with the IRS.
Sale, Exchange or Other Disposition
Generally, a Non-U.S. Holder will not be subject to U.S. federal income tax on gain realized upon the sale, exchange or other disposition of our common stock unless:
| (i) | the gain is effectively connected with such Non-U.S. Holder’s conduct of a trade or business in the U.S., or where an applicable income tax treaty provides, the gain is attributable to a U.S. permanent establishment of such Non-U.S. Holder; |
| (ii) | such Non-U.S. Holder is an individual present in the U.S. for 183 days or more in the taxable year of the sale, exchange or other disposition, and certain other conditions are met; or |
| (iii) | we are or have been a “U.S. real property holding corporation” for U.S. federal income tax purposes at any time during the shorter of the five-year period preceding such sale, exchange or other disposition or the period that such Non-U.S. Holder held our common stock. |
A Non-U.S. Holder described in (i) above will be required to pay tax on the net gain realized from such sale, exchange or other disposition under regular graduated U.S. federal income tax rates, and corporate Non-U.S. Holders described in (i) above may be subject to the branch profits tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. An individual Non-U.S. Holder described in (ii) above will be required to pay U.S. federal income tax at a rate of 30% on the gain realized from such sale, exchange or other disposition, which gain may be offset by other U.S. source capital losses realized during the same taxable year.
We do not believe that we have been, are currently, or are likely to become a U.S. real property holding corporation for U.S. federal income tax purposes. If we were to become a U.S. real property holding corporation, so long as our common stock is regularly traded on an established securities market and continues to be traded, a Non-U.S. Holder would be subject to U.S. federal income tax on any gain from the sale, exchange or other disposition of our common stock only if such Non-U.S. Holder actually or constructively owned more than 5% of our common stock during the shorter of the five-year period preceding such sale, exchange or other disposition or the period that such Non-U.S. Holder held our common stock.
Federal Estate Tax
Shares of our common stock owned or treated as owned by an individual who is not a citizen or resident of the U.S. (as defined for U.S. federal estate tax purposes) at the time of death are
108
Table of Contents
considered U.S. situs assets includible in such individual’s gross estate for U.S. federal estate tax purposes and therefore may be subject to U.S. federal estate tax unless an applicable estate tax treaty provides otherwise.
Information Reporting and Backup Withholding Tax
We must report annually to the IRS the amount of any dividends or other distributions we pay and the amount of tax we withhold, if any, on these distributions, regardless of whether withholding is required. Similarly, we must report to each Non-U.S. Holder the amount of any dividends or other distributions we pay to such Non-U.S. Holder and the amount of tax we withhold, if any, on these distributions, regardless of whether withholding is required. The IRS may make available copies of the information returns reporting those distributions and amounts withheld to the tax authorities in the country in which a Non-U.S. Holder resides, pursuant to the provisions of an applicable income tax treaty or exchange of information treaty.
Backup withholding tax (currently at a rate of 28% may also apply to payments made to a Non-U.S. Holder on or with respect to our common stock, unless the Non-U.S. Holder certifies as to its status as a Non-U.S. Holder under penalties of perjury, or otherwise establishes an exemption, and certain other conditions are satisfied. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder will be allowed as a refund or a credit against such Non-U.S. Holder’s U.S. federal income tax liability, provided that the required information is timely furnished to the IRS.
Legislation Relating to Foreign Accounts
Legislation enacted in 2010 will materially change the requirements for obtaining an exemption from U.S. withholding tax and impose withholding taxes on certain types of payments made to “foreign financial institutions” and certain other non-U.S. entities. In general, and depending on the specific facts and circumstances, the failure to comply with the additional certification, information reporting and other specified requirements will result in the imposition of a 30% withholding tax on “withholdable payments,” including payments of dividends and proceeds from the sale of our common stock, to certain Non-U.S. Holders. Pursuant to published guidance from the IRS and the U.S. Treasury Department, this legislation generally applies to payments of dividends made after December 31, 2013 and payments of gross proceeds made after December 31, 2014. Prospective investors should consult their tax advisors regarding this legislation and its potential effect on their ownership and disposition of our common stock.
109
Table of Contents
The company, the selling stockholders and the underwriters named below have entered into an underwriting agreement with respect to the shares being offered. Subject to certain conditions, each underwriter has severally agreed to purchase the number of shares indicated in the following table. Goldman, Sachs & Co., UBS Securities LLC and Citigroup Global Markets Inc. are the representatives of the underwriters.
| Underwriters |
Number of Shares | |
| Goldman, Sachs & Co. |
||
| UBS Securities LLC |
||
| Citigroup Global Markets Inc. |
||
| Robert W. Baird & Co. Incorporated |
||
| Cowen and Company, LLC |
||
|
| ||
| Total |
||
|
|
The underwriters are committed to take and pay for all of the shares being offered, if any are taken, other than the shares covered by the option described below unless and until this option is exercised.
The underwriters have an option to buy up to an additional shares from . They may exercise that option for days. If any shares are purchased pursuant to this option, the underwriters will severally purchase shares in approximately the same proportion as set forth in the table above.
The following tables show the per share and total underwriting discounts and commissions to be paid to the underwriters by the company and the selling stockholders. Such amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Paid by PetroAlgae Inc. |
No Exercise | Full Exercise | ||||||
| Per Share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
| Paid by the Selling Stockholders |
No Exercise | Full Exercise | ||||||
| Per Share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
Shares sold by the underwriters to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. If all the shares are not sold at the initial public offering price, the representatives may change the offering price and the other selling terms. The offering of the shares by the underwriters is subject to receipt and acceptance and subject to the underwriters’ right to reject any order in whole or in part.
In connection with this offering, we, the selling stockholders, and all of our officers, directors and employees have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of their common stock or securities convertible into or exchangeable for shares of our common stock during the period from the date of this prospectus continuing through the date 180 days after the date of this prospectus, without the prior written consent of the representatives.
The 180-day restricted period described in the preceding paragraph will be automatically extended if: (1) during the last 17 days of the 180-day restricted period, the company issues an earnings release or announces material news or a material event; or (2) prior to the expiration of the
110
Table of Contents
180-day restricted period, the company announces that it will release earnings results during the 15-day period following the last day of the 180-day period, in which case the restrictions described in the preceding paragraph will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release of the announcement of the material news or material event.
Prior to the offering, there has been only a limited public market for the shares. The initial public offering price has been negotiated among the company and the representatives. Among the factors considered in determining the initial public offering price of the shares, in addition to prevailing market conditions, will be the company’s historical performance, estimates of the business potential and earnings prospects of the company, an assessment of the company’s management and the consideration of the above factors in relation to market valuation of companies in related businesses.
An application has been made to list our common stock on under the symbol “ .” In order to meet one of the requirements for listing our common stock on , the underwriters have undertaken to sell lots of or more shares to a minimum of beneficial holders.
In connection with the offering, the underwriters may purchase and sell shares of our common stock in the open market. These transactions may include short sales, stabilizing transactions and purchases to cover positions created by short sales. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares from the in the offering. The underwriters may close out any covered short position by either exercising their option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase additional shares pursuant to the option granted to them. “Naked” short sales are any sales in excess of such option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of our common stock made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Purchases to cover a short position and stabilizing transactions, as well as other purchases by the underwriters for their own accounts, may have the effect of preventing or retarding a decline in the market price of the company’s stock, and together with the imposition of the penalty bid, may stabilize, maintain or otherwise affect the market price of our common stock. As a result, the price of our common stock may be higher than the price that otherwise might exist in the open market. If these activities are commenced, they may be discontinued at any time. These transactions may be effected on the , in the over-the-counter market or otherwise.
The underwriters do not expect sales to discretionary accounts to exceed five percent of the total number of shares offered.
We estimate that our share of the total expenses of the offering, excluding underwriting discounts and commissions, will be approximately $ .
111
Table of Contents
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a Relevant Member State), each underwriter has represented and agreed that with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State (the Relevant Implementation Date) it has not made and will not make an offer of shares to the public in that Relevant Member State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that it may, with effect from and including the Relevant Implementation Date, make an offer of shares to the public in that Relevant Member State at any time:
(a) to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
(b) to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than € 43 million and (3) an annual net turnover of more than € 50 million, as shown in its last annual or consolidated accounts;
(c) to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of the representatives for any such offer; or
(d) in any other circumstances which do not require the publication by the Issuer of a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression Prospectus Directive means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
United Kingdom
This prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive that are also (i) investment professionals falling within Article 19(5) of the Financial Services and markets Act 2000 (Financial Promotion) Order 2005, or the Order, or (ii) high net worth entities, and other persons to whom it may lawfully be communicated, falling with Article 49(2)(a) to (d) of the Order (each such person being referred to as a “relevant person”). This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom that is not a relevant person should not act or rely on this prospectus or any of its contents.
Hong Kong
The shares may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the
112
Table of Contents
Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Switzerland
The Prospectus does not constitute an issue prospectus pursuant to Article 652a or Article 1156 of the Swiss Code of Obligations, or CO, and the shares will not be listed on the SIX Swiss Exchange. Therefore, the Prospectus may not comply with the disclosure standards of the CO and/or the listing rules (including any prospectus schemes) of the SIX Swiss Exchange. Accordingly, the shares may not be offered to the public in or from Switzerland, but only to a selected and limited circle of investors, which do not subscribe to the shares with a view to distribution.
Australia
This prospectus is not a formal disclosure document and has not been, nor will be, lodged with the Australian Securities and Investments Commission. It does not purport to contain all information that an investor or their professional advisers would expect to find in a prospectus or other disclosure document (as defined in the Corporations Act 2001 (Australia)) for the purposes of Part 6D.2 of the Corporations Act 2001 (Australia) or in a product disclosure statement for the purposes of Part 7.9 of the Corporations Act 2001 (Australia), in either case, in relation to the securities.
The securities are not being offered in Australia to “retail clients” as defined in sections 761G and 761GA of the Corporations Act 2001 (Australia). This offering is being made in Australia solely to “wholesale clients” for the purposes of section 761G of the Corporations Act 2001 (Australia) and, as such, no prospectus, product disclosure statement or other disclosure document in relation to the securities has been, or will be, prepared.
This prospectus does not constitute an offer in Australia other than to wholesale clients. By submitting an application for our securities, you represent and warrant to us that you are a wholesale client for the purposes of section 761G of the Corporations Act 2001 (Australia). If any recipient of this prospectus is not a wholesale client, no offer of, or invitation to apply for, our securities shall be deemed to be made to such recipient and no applications for our securities will be accepted from such recipient. Any offer to a recipient in Australia, and any agreement arising from acceptance of such offer, is personal and may only be accepted by the recipient. In addition, by applying for our securities you undertake to us that, for a period of 12 months from the date of issue of the securities, you will not transfer any interest in the securities to any person in Australia other than to a wholesale client.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
113
Table of Contents
Where the shares are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired the shares under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (the Financial Instruments and Exchange Law) and each underwriter has agreed that it will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Law and any other applicable laws, regulations and ministerial guidelines of Japan.
France
Neither this prospectus nor any other offering material relating to the shares described in this prospectus has been submitted to the clearance procedures of the Autorité des marchés financiers or of the competent authority of another member state of the European Economic Area and notified to the Autorité des marchés financiers. The shares have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France. Neither this prospectus nor any other offering material relating to the shares has been or will be:
| Ÿ | released, issued, distributed or caused to be released, issued or distributed to the public in France; or |
| Ÿ | used in connection with any offer for subscription or sale of the shares to the public in France. |
Such offers, sales and distributions will be made in France only:
| Ÿ | to qualified investors (investisseurs qualifiés) and/or to a restricted circle of investors (cercle restreint d’investisseurs), in each case investing for their own account, all as defined in, and in accordance with articles L.411-2, D.411-1, D-411-2, D.734-1, D744-1, D.754-1 and D.764-1 of the French Code monétaire et financier; |
| Ÿ | to investment services providers authorized to engage in portfolio management on behalf of third parties; or |
| Ÿ | in a translation that, in accordance with article L.411-2-ll-1o -or-2o -or 3o of the French Code monétaire et financier and article 211-2 of the General Regulations (Règlement Général) of the Autorité des marchés financiers, does not constitute a public offer (appel public à l’épargne). |
The shares may be resold directly or indirectly, only in compliance with articles L.411-1, L411-2, L412-1 and L621-8 through L.621-8-3 of the French Code monétaire et financier.
The company and the selling stockholders have agreed to indemnify the several underwriters against certain liabilities, including liabilities under the Securities Act.
114
Table of Contents
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. Certain of the underwriters and their respective affiliates have, from time to time, performed, and may in the future perform, various financial advisory and investment banking services for the issuer, for which they received or will receive customary fees and expenses.
UBS AG, an affiliate of UBS Securities LLC, holds 500,000 shares of our common stock and a warrant to purchase 500,000 shares of our common stock at an exercise price of $15.00 per share. UBS AG purchased the securities from the company on October 19, 2009 in a private placement transaction for an aggregate purchase price of $4 million.
In the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of the issuer. The underwriters and their respective affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
115
Table of Contents
The validity of the shares of our common stock offered hereby will be passed upon for us by Torys LLP, New York, New York and for the underwriters by Sullivan & Cromwell LLP, New York, New York. As of the date of this prospectus, Torys LLP beneficially owns less than 1% of our common stock.
116
Table of Contents
The audited financial statements included in this prospectus and elsewhere in the registration statement have been included in reliance upon the report of Grant Thornton LLP, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing in giving said report.
117
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act for the shares of our common stock being offered by this prospectus. This prospectus, which is part of the registration statement, does not contain all of the information included in the registration statement and the exhibits. For further information about us and the unrestricted shares of our common stock offered by this prospectus, you should refer to the registration statement and its exhibits. You may read and copy any document that we file at the SEC’s public reference room located at 100 F Street, NE, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference rooms. SEC filings are also available to the public at the SEC’s website at www.sec.gov. Copies of documents we file with the SEC are also available on the our web site, www.petroalgae.com.
We are subject to the reporting and information requirements of the Exchange Act and, as a result, file periodic and current reports, proxy statements and other information with the SEC. We expect to make our periodic reports and other information filed with or furnished to the SEC, available, free of charge, through our website as soon as reasonably practicable after those reports and other information are filed with or furnished to the SEC. Additionally, these periodic reports, proxy statements and other information will be available for inspection and copying at the public reference room and website of the SEC referred to above.
118
Table of Contents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
| F-2 | ||||
| Consolidated Balance Sheets as of December 31, 2009 and 2010 |
F-3 | |||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| Consolidated Balance Sheets as of September 30, 2011 and December 31, 2010 |
F-24 | |||
| F-25 | ||||
| F-26 | ||||
| F-27 | ||||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
PetroAlgae, Inc.
We have audited the accompanying consolidated balance sheets of PetroAlgae, Inc. (a Development Stage Entity), a Delaware corporation, and subsidiary as of December 31, 2010 and 2009, and the related consolidated statements of operations and cash flows for each of the three years in the period ended December 31, 2010 and for the cumulative period from inception (September 22, 2006) to December 31, 2010 and the related consolidated statement of changes in stockholders’ deficit for the cumulative period from inception (September 22, 2006) to December 31, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of PetroAlgae, Inc. (A Development Stage Entity) and subsidiary as of December 31, 2010 and 2009, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2010 and for the period from inception (September 22, 2006) to December 31, 2010, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2, the Company incurred a net loss of $38,010,029 during the year ended December 31, 2010, and, as of that date, the Company’s current liabilities exceeded its current assets by $7,598,513 and its total liabilities exceeded its total assets by $56,504,381. These factors, among others, as discussed in Note 2 to the financial statements, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ GRANT THORNTON LLP
Orlando, Florida
March 31, 2011
F-2
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
| December 31, 2010 |
December 31, 2009 |
|||||||
| ASSETS |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 1,140,882 | $ | 2,679,302 | ||||
| Restricted cash |
2,325,000 | 2,000,000 | ||||||
| Prepaid expenses |
102,016 | 129,333 | ||||||
| Receivable |
— | 1,200,000 | ||||||
| Other current assets |
— | 341,726 | ||||||
|
|
|
|
|
|||||
| Total current assets |
3,567,898 | 6,350,361 | ||||||
| Property and equipment |
4,194,629 | 3,653,225 | ||||||
| Accumulated depreciation |
(2,292,952 | ) | (1,416,350 | ) | ||||
|
|
|
|
|
|||||
| Net property and equipment |
1,901,677 | 2,236,875 | ||||||
| Deposits |
24,814 | 5,200 | ||||||
| Other non-current assets |
830,375 | — | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 6,324,764 | $ | 8,592,436 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 1,569,715 | $ | 536,328 | ||||
| Accrued expenses |
2,189,561 | 1,617,191 | ||||||
| Accrued expenses - related party |
4,807,135 | 2,144,840 | ||||||
| Deferred revenue |
500,000 | — | ||||||
| Liabilities related to equity issuance |
100,000 | 7,500,000 | ||||||
| Other current liability |
2,000,000 | — | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
11,166,411 | 11,798,359 | ||||||
| Other liability |
— | 2,000,000 | ||||||
| Notes payable - related party |
51,662,734 | 35,114,096 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
62,829,145 | 48,912,455 | ||||||
|
|
|
|
|
|||||
| Commitments and Contingencies (Note 10) |
||||||||
| Stockholders’ deficit: |
||||||||
| Preferred stock - $.001 par value, 25,000,000 shares authorized; no shares issued or outstanding |
— | — | ||||||
| Common stock - $.001 par value, 300,000,000 shares authorized; |
||||||||
| 106,933,230 and 106,229,356 shares issued and outstanding at December 31, 2010 and 2009, respectively |
106,933 | 106,229 | ||||||
| Paid in capital |
37,823,248 | 24,115,788 | ||||||
| Deficit accumulated during the development stage |
(98,022,859 | ) | (60,012,830 | ) | ||||
|
|
|
|
|
|||||
| PetroAlgae Inc. stockholders’ deficit |
(60,092,678 | ) | (35,790,813 | ) | ||||
| Non-controlling interest |
3,588,297 | (4,529,206 | ) | |||||
|
|
|
|
|
|||||
| Total stockholders’ deficit |
(56,504,381 | ) | (40,320,019 | ) | ||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ deficit |
$ | 6,324,764 | $ | 8,592,436 | ||||
|
|
|
|
|
|||||
See accompanying notes to the consolidated financial statements.
F-3
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Consolidated Statements of Operations
| December 31, 2010 |
December 31, 2009 |
December 31, 2008 |
For the
Period From September 22, 2006 (Inception) Through December 31, 2010 |
|||||||||||||
| Revenue |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Costs and expenses: |
||||||||||||||||
| Selling, general and administrative |
21,849,915 | 17,400,801 | 8,251,676 | 50,666,429 | ||||||||||||
| Research and development |
17,424,316 | 15,085,060 | 9,865,844 | 48,468,997 | ||||||||||||
| Interest expense—related party |
4,076,905 | 3,198,642 | 1,764,039 | 9,483,058 | ||||||||||||
| Depreciation |
1,128,249 | 1,137,733 | 268,691 | 2,590,489 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
44,479,385 | 36,822,236 | 20,150,250 | 111,208,973 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss before non-controlling interest |
(44,479,385 | ) | (36,822,236 | ) | (20,150,250 | ) | (111,208,973 | ) | ||||||||
| Net loss attributable to non-controlling interest |
6,469,356 | 6,554,358 | 162,400 | 13,186,114 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to PetroAlgae Inc. |
$ | (38,010,029 | ) | $ | (30,267,878 | ) | $ | (19,987,850 | ) | $ | (98,022,859 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted common shares outstanding |
106,731,469 | 104,866,065 | 100,362,021 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted loss per share |
$ | (0.36 | ) | $ | (0.29 | ) | $ | (0.20 | ) | |||||||
|
|
|
|
|
|
|
|||||||||||
See accompanying notes to the consolidated financial statements.
F-4
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Consolidated Statement of Changes in Stockholders’ Deficit
Period From Inception (September 22, 2006) to December 31, 2010
| Common Stock Shares |
Amount | Paid in Capital | Non Controlling Interest |
Deficit Accumulated During the Development Stage |
Total | |||||||||||||||||||
| Shares issued at inception for cash |
100,000,000 | $ | 100,000 | $ | 388,532 | $ | — | $ | — | $ | 488,532 | |||||||||||||
| Net loss |
— | — | — | — | (1,410,724 | ) | (1,410,724 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance—December 31, 2006 |
100,000,000 | $ | 100,000 | $ | 388,532 | $ | — | $ | (1,410,724 | ) | $ | (922,192 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Amortization of PA LLC interests to employees |
— | — | 276,986 | — | — | 276,986 | ||||||||||||||||||
| Net loss |
— | — | — | — | (8,346,378 | ) | (8,346,378 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance—December 31, 2007 |
100,000,000 | $ | 100,000 | $ | 665,518 | $ | — | $ | (9,757,102 | ) | $ | (8,991,584 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Amortization of PA LLC interests to employees |
— | — | 2,240,041 | 200,000 | — | 2,440,041 | ||||||||||||||||||
| Shares issued for cash |
3,174,603 | 3,175 | 9,996,825 | — | — | 10,000,000 | ||||||||||||||||||
| Shares issued for unearned services |
1,000,000 | 1,000 | (1,000 | ) | — | — | — | |||||||||||||||||
| Amortization of unearned services |
— | — | 43,750 | — | — | 43,750 | ||||||||||||||||||
| Issuance of option as additional consideration for debt |
— | — | 1,453,000 | — | — | 1,453,000 | ||||||||||||||||||
| Reverse merger |
99,586 | 99 | — | — | — | 99 | ||||||||||||||||||
| Loss attributable to non controlling interest |
— | — | — | (162,400 | ) | — | (162,400 | ) | ||||||||||||||||
| Net loss |
— | — | — | — | (19,987,850 | ) | (19,987,850 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance—December 31, 2008 |
104,274,189 | $ | 104,274 | $ | 14,398,134 | $ | 37,600 | $ | (29,744,952 | ) | $ | (15,204,944 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Shares issued for services |
160,524 | 160 | 537,652 | — | — | 537,812 | ||||||||||||||||||
| Shares issued for cash |
500,000 | 500 | 3,999,500 | — | — | 4,000,000 | ||||||||||||||||||
| Shares issued with purchase price guaranty |
937,500 | 938 | (938 | ) | — | — | — | |||||||||||||||||
| Shares and warrants issued for other current assets |
357,143 | 357 | 3,017,787 | — | — | 3,018,144 | ||||||||||||||||||
| Amortization of PA LLC interests to employees |
— | — | — | 1,987,552 | — | 1,987,552 | ||||||||||||||||||
| Amortization of options issued to employees |
— | — | 588,653 | — | — | 588,653 | ||||||||||||||||||
| Amortization of unearned services |
— | — | 1,575,000 | — | — | 1,575,000 | ||||||||||||||||||
| Loss attributable to non controlling interest |
— | — | — | (6,554,358 | ) | — | (6,554,358 | ) | ||||||||||||||||
| Net loss |
— | — | — | — | (30,267,878 | ) | (30,267,878 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance—December 31, 2009 |
106,229,356 | $ | 106,229 | $ | 24,115,788 | $ | (4,529,206 | ) | $ | (60,012,830 | ) | $ | (40,320,019 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Return of common stock for other current asset |
(106,126 | ) | (106 | ) | (341,620 | ) | — | — | (341,726 | ) | ||||||||||||||
| Shares issued for cash |
810,000 | 810 | 6,479,190 | — | — | 6,480,000 | ||||||||||||||||||
| Shares issued with put option or purchase price guaranty, net of purchase price guaranty expirations |
— | — | 7,400,000 | — | — | 7,400,000 | ||||||||||||||||||
| Amortization of PA LLC interests to employees |
— | — | (794 | ) | 12,903,744 | — | 12,902,950 | |||||||||||||||||
| Amortization of options issued to employees |
— | — | 922,549 | — | — | 922,549 | ||||||||||||||||||
| Amortization of unearned services |
— | — | 1,531,250 | — | — | 1,531,250 | ||||||||||||||||||
| Loss attributable to non controlling interest |
— | — | — | (6,469,356 | ) | — | (6,469,356 | ) | ||||||||||||||||
| PA LLC units returned for surrendered technology license |
— | — | (2,283,115 | ) | 1,683,115 | — | (600,000 | ) | ||||||||||||||||
| Net loss |
— | — | — | — | (38,010,029 | ) | (38,010,029 | ) | ||||||||||||||||
| Balance—December 31, 2010 |
106,933,230 | $ | 106,933 | $ | 37,823,248 | $ | 3,588,297 | $ | (98,022,859 | ) | $ | (56,504,381 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
See accompanying notes to the consolidated financial statements.
F-5
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Consolidated Statements of Cash Flows
| December 31, 2010 |
December 31, 2009 |
December 31, 2008 |
For the Period From September 22, 2006 (Inception) Through December 31, 2010 |
|||||||||||||
| Cash flows from operating activities: |
||||||||||||||||
| Net loss |
$ | (44,479,385 | ) | $ | (36,822,236 | ) | $ | (20,150,250 | ) | $ | (111,208,973 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||||||
| Amortization of original issue discount |
375,000 | 562,000 | 516,000 | 1,453,000 | ||||||||||||
| Amortization of Company options |
922,549 | 588,653 | — | 1,511,202 | ||||||||||||
| Amoritization of unearned services |
1,531,250 | 1,575,000 | 43,750 | 3,150,000 | ||||||||||||
| Amortization of PA LLC interests to employees |
12,902,950 | 1,987,552 | 2,440,041 | 17,607,529 | ||||||||||||
| Expenses paid and interest added to notes payable—related party |
973,638 | 2,723,313 | 3,044,739 | 10,349,483 | ||||||||||||
| Expenses paid by issuance of common stock |
— | 1,214,230 | — | 1,214,230 | ||||||||||||
| Depreciation |
1,128,249 | 1,137,733 | 268,691 | 2,590,489 | ||||||||||||
| Impairment loss |
179,099 | — | — | 179,099 | ||||||||||||
| Loss on disposition of equipment |
62,766 | 126,830 | — | 189,596 | ||||||||||||
| Write-off of other non-current assets |
456,389 | — | — | 456,389 | ||||||||||||
| (Increase) in restricted cash |
(325,000 | ) | — | — | (325,000 | ) | ||||||||||
| (Increase) decrease in prepaid expenses |
7,703 | (19,056 | ) | (103,933 | ) | (121,630 | ) | |||||||||
| (Increase) decrease in deposits |
— | (200 | ) | 2,700 | (5,200 | ) | ||||||||||
| (Increase) in other non-current assets |
(109,720 | ) | — | — | (109,720 | ) | ||||||||||
| Increase (decrease) in accounts payable |
(88,563 | ) | (506,957 | ) | 712,384 | 501,418 | ||||||||||
| Increase (decrease) in accrued expenses |
3,179,571 | (169,150 | ) | 1,376,516 | 4,806,599 | |||||||||||
| Increase in deferred revenue |
500,000 | — | — | 500,000 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash used in operating activities |
$ | (22,783,504 | ) | $ | (27,602,288 | ) | $ | (11,849,362 | ) | $ | (67,261,489 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash flows from investing activities: |
||||||||||||||||
| Proceeds from sale of asset |
7,500 | 36,000 | — | 43,500 | ||||||||||||
| Acquisition of property and equipment |
(1,042,416 | ) | (2,471,233 | ) | (812,660 | ) | (4,904,361 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash used in investing activities |
$ | (1,034,916 | ) | $ | (2,435,233 | ) | $ | (812,660 | ) | $ | (4,860,861 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash flows from financing activities: |
||||||||||||||||
| Member contributions |
— | — | — | 488,532 | ||||||||||||
| Reverse merger |
— | — | 99 | 99 | ||||||||||||
| Common stock and warrants issued for cash |
6,480,000 | 11,500,000 | 10,000,000 | 27,980,000 | ||||||||||||
| Borrowings under notes payable—related party |
15,200,000 | 10,000,000 | 12,894,601 | 43,394,601 | ||||||||||||
| PA LLC units returned for cash and surrendered technology license |
(600,000 | ) | — | — | (600,000 | ) | ||||||||||
| Issuance of common stock and warrants for cash |
1,200,000 | 800,000 | — | 2,000,000 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash provided by financing activities |
$ | 22,280,000 | $ | 22,300,000 | $ | 22,894,700 | $ | 73,263,232 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net (decrease) increase in cash |
(1,538,420 | ) | (7,737,521 | ) | 10,232,678 | 1,140,882 | ||||||||||
| Cash and cash equivalents—beginning of period |
2,679,302 | 10,416,823 | 184,145 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash and cash equivalents—end of period |
$ | 1,140,882 | $ | 2,679,302 | $ | 10,416,823 | $ | 1,140,882 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Non-cash investing and financing activities: |
||||||||||||||||
| Liability incurred for other non-current asset |
$ | 721,000 | $ | — | $ | — | $ | 721,000 | ||||||||
| Stock and warrants issued for other liabilities, net |
$ | (7,400,000 | ) | $ | 7,500,000 | $ | — | $ | 100,000 | |||||||
| Common stock issued for unearned services |
$ | — | $ | — | $ | 3,150,000 | $ | 3,150,000 | ||||||||
| Issuance of shares for other current asset |
$ | — | $ | 1,200,000 | $ | — | $ | 1,200,000 | ||||||||
| Return of common stock for other current asset |
$ | (342,000 | ) | $ | — | $ | — | $ | (342,000 | ) | ||||||
| Liability incurred for restricted cash |
$ | — | $ | 2,000,000 | $ | — | $ | 2,000,000 | ||||||||
See accompanying notes to the consolidated financial statements.
F-6
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
December 31, 2010, 2009 and 2008
Note 1 Organization
PetroAlgae Inc. (the “Company”) began its operations through its controlled subsidiary, PA LLC (formerly known as PetroAlgae LLC), on December 19, 2008, as a result of the reverse acquisition described below. PA LLC was formed by XL TechGroup, LLC (“XL Tech”, a related party and its sponsor and parent until December 19, 2008) on September 22, 2006 as a Delaware limited liability company to develop technologies to commercially grow, harvest and process micro-crops. PA LLC is a renewable energy and food company whose primary business model is to license and deploy this technology to large customers who will commercially produce this biomass, which in turn will be processed into three end-products: a carbohydrate-rich solid material suitable as a renewable fuel feedstock, or as animal feed, and a protein supplement for animal feed and potentially human food products.
Reverse Acquisition
In August 2008, XL Tech exchanged certain of its assets, including the equity it held in PA LLC, for the outstanding debt of XL Tech that was held by our principal stockholder. Subsequent to that exchange, our principal stockholder transferred the equity it received and certain related debt to PetroTech Holdings Corp. (“PetroTech”), a holding company controlled by our principal stockholder. In December 2008, PetroTech acquired a shell company that traded on the OTC Bulletin Board and assigned its interest in PA LLC to this shell company, which it then renamed PetroAlgae Inc. As a result of the acquisition, PetroTech became the owner of 100,000,000 shares of common stock of PetroAlgae Inc. which, at the time, represented 99.9% of the total issued and outstanding common stock of PetroAlgae Inc. As the former members of PA LLC control PetroAlgae Inc. after the transaction, the merger was accounted for as a reverse acquisition under which, for accounting purposes, PA LLC is deemed to be the acquirer and PetroAlgae Inc., the legal acquirer, is deemed to be the acquired entity. No goodwill was recognized as PetroAlgae Inc. was a “shell” company. The former shareholders of PetroAlgae Inc. retained an aggregate of 99,586 shares of common stock, which were recorded at par value of $99.
Note 2 Going Concern
The Company is currently in the development stage at December 31, 2010 as it is continuing the development of its product and has not yet recognized any revenues. The Company intends to transition from the development stage to operational status during 2011 depending upon the timing and extent of success achieved in accomplishing its business development milestones. The Company’s consolidated financial statements are presented on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business.
The Company has never been profitable and has incurred significant losses and cash flow deficits. For the years ended December 31, 2010, 2009, 2008, and the period from September 22, 2006 (inception) to December 31, 2010, we reported net losses before non-controlling interest of $44.5 million, $36.8 million, $20.2 million, and $111.2 million, respectively, and negative cash flow from operating activities of $22.8 million, $27.6 million, $11.8 million, and $67.3 million, respectively. As of December 31, 2010, the Company has an aggregate accumulated deficit of $98.0 million. The Company anticipates that operations will continue to report losses and negative cash flow during 2011. As a result of these net losses, cash flow deficits, and other factors, there is a substantial doubt about our ability to continue as a going concern.
F-7
Table of Contents
The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. These adjustments would likely include substantial impairment of the carrying amount of our non-cash assets of $2.9 million and potential contingent liabilities that may arise if we are unable to fulfill various operational commitments. In addition, the value of our securities, including common stock and warrants, would be greatly impaired. Our ability to continue as a going concern is dependent upon generating sufficient cash flow from operations and obtaining additional capital and financing. The Company is currently seeking additional funding from its principal shareholder and unrelated third parties. If our ability to generate cash flow from operations is delayed or reduced and we are unable to raise additional funding, we may be unable to continue in business.
Note 3 Basis of Presentation
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its consolidated subsidiary, PA LLC. Non-controlling interests are accounted for based upon the value or cost attributed to their investment adjusted for the share of income or loss that relates to their percentage ownership of the entire Company. All intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America which require management to make estimates and assumptions. These estimates and assumptions affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expense. Actual results may differ from these estimates.
Revenue Recognition
The Company will begin to record revenue when persuasive evidence of an arrangement exists, services have been rendered or product delivery has occurred, the sales price to the customer is fixed or determinable, and collectability is reasonably assured. To the extent cash is received in advance of the Company’s performance or the availability of rights under a license agreement or such receipts are refundable at the customer’s option, these amounts are reported as deferred revenue.
Cash and Cash Equivalents and Cash Concentration
Cash and cash equivalents include unsecured money market funds and other liquid financial instruments with a maturity of three months or less at the date of purchase. At December 31, 2010 and 2009, the Company had approximately $1.1 million and $2.7 million, respectively, on deposit at a single financial institution. Accounts at this institution are insured by the Federal Deposit Insurance Corporation up to $0.25 million. The Company has not experienced any losses in such accounts.
Restricted Cash
Restricted cash consists of $2.0 million received by the Company related to a license for intellectual property which will be held in escrow until certain actions are taken by the licensee (see Notes 8 and 15). Also included in restricted cash as of December 31, 2010, is $0.3 million escrowed in accordance with the employment agreement for an executive.
F-8
Table of Contents
Fair Value of Financial Instruments
Fair value estimates discussed herein are based upon certain market assumptions and pertinent information available to management as of December 31, 2010. The respective carrying values of certain financial instruments approximate their fair values. These financial instruments include cash and cash equivalents, accounts payable, and accrued expenses. Fair values are assumed to approximate carrying values for these financial instruments because they are short term in nature and their carrying amounts approximate the amounts expected to be received or paid.
The carrying value of the Company’s fixed-rate notes payable-related party approximated their fair value based on the current market conditions for similar debt instruments.
Property and Equipment
Property and equipment is recorded at cost. Expenditures for major improvements and additions are added to property and equipment, while replacements, maintenance and repairs which do not extend the useful lives are expensed.
Long-Lived Assets
The carrying value of long-lived assets is reviewed on a regular basis for the existence of facts and circumstances that suggest permanent impairment. Specifically, senior management of the Company considers in each reporting period the effectiveness of the Company’s significant assets to determine if impairment indicators such as physical deterioration, process change or technological change have resulted in underperformance, obsolescence or a need to replace such assets. The Company groups its assets into three primary categories for this purpose. Due to the nature of the Company’s business, cash flows are not derived from specific asset groups, therefore this grouping is merely to aid the Company’s analysis.
| Ÿ | Office equipment, including leasehold improvements |
| Ÿ | Demonstration system and engineering equipment |
| Ÿ | Computer software and hardware—applications and networking |
The Company does not consider its net loss or cash outflow from operations to be an indicator of asset impairment as such financial results are typical for technology companies that are in the development stage. Rather, management evaluates the state of the technology, its process toward technical and business milestones, and relevant external market factors to ascertain whether a potential impairment has occurred. During the fourth quarter of 2010, the Company identified certain changes in its process development plans that eliminated or changed the need or expected use of certain assets. As a result, an impairment charge of $0.2 million to reduce these assets to their estimated realizable value was recorded as a reduction of property and equipment and an addition to research and development expense. No impairment indicators were noted in either of the years ended December 31, 2009 or 2008 and the Company’s process of monitoring these assets was unchanged during this period.
When the Company identifies an impairment indicator, it measures the amount of the impairment based on the amount that the carrying value of the impaired asset exceeds its best internal estimate of the value of the asset while it remains in use, plus any proceeds expected from the eventual disposal of the impaired asset. Since the Company uses most of its property and equipment to demonstrate its technology to prospective customers and not to generate revenues or cash flows, a cash flow analysis of the categories above or the assets as a whole is not practicable. Instead, a depreciated replacement cost estimate is used for assets still in service and equipment secondary market quotes are obtained
F-9
Table of Contents
for assets planned for disposal. Depreciated replacement cost is determined by contacting vendors of the same or similar equipment or other assets to obtain an estimate of the market price of such assets, and then reducing this amount by a formula derived from the age of the equipment and the typical useful life of that equipment. For assets planned to be disposed, descriptions of the equipment are circulated to a number of purchasers of used machinery and equipment to solicit bids for such assets. The Company’s best estimate of selling price based upon these purchasers’ responses is used to estimate the value of such assets, net of any costs to remove, recondition and/or ship the equipment to the buyer.
Income Taxes
The Company follows Accounting Standards Codification (“ASC”) 740, Income Taxes, for recording the provision for income taxes. Deferred tax assets and liabilities are computed based upon the difference between the financial statement and income tax basis of assets and liabilities using the enacted marginal tax rate applicable when the related asset or liability is expected to be realized or settled. Deferred income tax expenses or benefits are based on the changes in the asset or liability each period. If available evidence suggests that it is more likely than not that some portion or all of the deferred tax assets will not be realized, a valuation allowance is required to reduce the deferred tax assets to the amount that is more likely than not to be realized. Future changes in such valuation allowance are included in the provision for deferred income taxes in the period of change.
As required by ASC 740-10-05, Accounting for Uncertainty in Income Taxes, the Company recognizes the financial statement effects of a tax position when it is more likely than not (defined as a likelihood of more than 50%), based on the technical merits, that the position will be sustained upon examination. The evaluation of whether a tax position meets the more-likely-than-not recognition threshold requires a substantial degree of judgment by management based on the individual facts and circumstances. Actual results could differ from these estimates.
PA LLC is organized as a limited liability company under the state law of Delaware. As a limited liability company that has elected to be taxed as a partnership, the Company’s earnings pass through to the members and are taxed at the member level. Accordingly, the income tax provision included in the accompanying consolidated financial statements represents the tax impact to the Company arising from its ownership interest in the operations related to PA LLC.
Loss Per Share
Basic earnings (loss) per share is calculated by dividing net income (loss) by the weighted average number of common shares outstanding for the period. Diluted earnings (loss) per share is calculated by dividing net income (loss) by the weighted average number of common shares and dilutive common stock equivalents outstanding. During periods in which the Company incurs losses, common stock equivalents are not considered as their effect would be anti-dilutive.
The effect of 2,598,629 and 481,703 weighted average warrants and 1,083,475 and 626,151 weighted average options were not included for the years ended December 31, 2010 and 2009, respectively, as they would have had an anti-dilutive effect. There were no options or warrants outstanding during 2008.
Stock-Based Compensation
The Company accounts for stock-based compensation in accordance with ASC 718, Stock Compensation. This Statement requires that the cost resulting from all stock-based transactions be recorded in the consolidated financial statements. The Statement establishes fair value as the measurement objective in accounting for stock-based payment arrangements and requires all entities
F-10
Table of Contents
to apply a fair-value-based measurement in accounting for stock-based payment transactions with employees. The Statement also establishes fair value as the measurement objective for transactions in which an entity acquires goods or services from non-employees in stock-based payment transactions. (See Note 11).
Recently Issued Accounting Pronouncements
Adoption of New Accounting Standards
Accounting for the Transfers of Financial Assets
In June 2009, the Financial Accounting Standards Board (the “FASB”) issued new guidance relating to the accounting for transfers of financial assets. The new guidance, which was issued as SFAS No. 166, Accounting for Transfers of Financial Assets, an amendment to Statement of Financial Accounting Standards (“SFAS”) No. 140, was adopted into Codification in December 2009 through the issuance of Accounting Standards Updated (“ASU”) 2009-16. The new standard eliminates the concept of a “qualifying special-purpose entity,” changes the requirements for derecognizing financial assets, and requires additional disclosures in order to enhance information reported to users of financial statements by providing greater transparency about transfers of financial assets, including securitization transactions, and an entity’s continuing involvement in and exposure to the risks related to transferred financial assets. The new guidance is effective for fiscal years beginning after November 15, 2009. The Company’s adoption of the new guidance did not have a material effect on the Company’s consolidated financial statements.
Accounting for Variable Interest Entities
In June 2009, the FASB issued revised guidance on the accounting for variable interest entities. The revised guidance, which was issued as SFAS No. 167, Amending FASB Interpretation No. 46(R), was adopted into Codification in December 2009 through the issuance of ASU 2009-17 which is effective for calendar year reporting entities beginning on January 1, 2010. The revised guidance amends FASB Interpretation No. 46(R), consolidation of Variable Interest Entities, in determining whether an enterprise has a controlling financial interest in a variable interest entity. This determination identifies the primary beneficiary of a variable interest entity as the enterprise that has both the power to direct the activities of a variable interest entity that most significantly impacts the entity’s economic performance, and the obligation to absorb losses or the right to receive benefits of the entity that could potentially be significant to the variable interest entity. The revised guidance requires ongoing reassessments of whether an enterprise is the primary beneficiary and eliminates the quantitative approach previously required for determining the primary beneficiary. The Company’s adoption of the new guidance did not have a material effect on the Company’s consolidated financial statements.
Accounting Standards Not Yet Effective
Revenue Recognition
In April 2010, the FASB issued ASU 2010-17, Milestone Method of Revenue Recognition, a consensus of the FASB Emerging Issues Task Force (“ASU 2010-17”), which provides guidance on the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. ASU 2010-17 is effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010. The effect of ASU 2010-17 on the Company’s expected future revenues will depend upon the structure of the Company’s customer contracts and is still being analyzed.
In October 2009, the FASB issued ASU 2009-13, Multiple-Deliverable Revenue Arrangements (“ASU 2009-13”). The new standard changes the requirements for establishing separate units of accounting in a multiple element arrangement and requires the allocation of arrangement consideration
F-11
Table of Contents
to each deliverable based on the relative selling price. The selling price for each deliverable is based on vendor-specific objective evidence (“VSOE”) if available, third-party evidence if VSOE is not available, or estimated selling price if neither VSOE or third-party evidence is available. ASU 2009-13 is effective for revenue arrangements entered into in fiscal years beginning on or after June 15, 2010. The effect of this new guidance on the Company’s expected future revenues, which in turn depends upon the final structure of the Company’s contracts with customers, is still being analyzed.
Fair Value
In January 2010, the FASB issued ASU 2010-6, Improving Disclosures About Fair Value Measurements (“ASU 2010-6”), which requires reporting entities to make new disclosures about recurring or nonrecurring fair-value measurements, including significant transfers into and out of Level 1 and Level 2 fair-value measurements and information on purchases, sales, issuances, and settlements on a gross basis in the reconciliation of Level 3 fair-value measurements. ASU 2010-6 is effective for annual reporting periods beginning after December 15, 2009, except for Level 3 reconciliation disclosures which are effective for annual periods beginning after December 15, 2010. The adoption of ASU 2010-6 did not have a material impact on the Company’s consolidated financial statements nor is the portion effective in 2011 expected to have a material impact.
Note 4 Receivable
In December 2009, the Company entered into a master license and other agreements with Congoo, LLC which was later assigned to Green Science Energy, LLC, its controlled affiliate. The agreements provided for a $4.0 million payment to the Company, of which $2.8 million was received in 2009 and the remaining $1.2 million was recorded as a receivable and was subsequently received in January 2010. There were no receivables outstanding as of December 31, 2010.
Note 5 Property and Equipment
| As of December 31, | Estimated useful lives |
|||||||||||
| 2010 | 2009 | |||||||||||
| Leasehold improvements |
$ | 618,740 | $ | 613,863 | 2-5 years | |||||||
| Furniture and fixtures |
89,915 | 81,716 | 3-7 years | |||||||||
| Automobiles |
49,842 | 62,641 | 2-4 years | |||||||||
| Computer and office equipment |
268,426 | 230,068 | 1-5 years | |||||||||
| Engineering equipment |
2,757,086 | 2,276,908 | 2-7 years | |||||||||
| Software and networking |
410,620 | 388,029 | 1-5 years | |||||||||
|
|
|
|
|
|||||||||
| Total cost basis |
4,194,629 | 3,653,225 | ||||||||||
| Less accumulated depreciation |
(2,292,952 | ) | (1,416,350 | ) | ||||||||
|
|
|
|
|
|||||||||
| Total Property and Equipment |
$ | 1,901,677 | $ | 2,236,875 | ||||||||
|
|
|
|
|
|||||||||
Depreciation expense was $1.1 million, $1.1 million, $0.3 million, and $2.6 million for the years ended December 31, 2010, 2009, 2008, and the period from September 22, 2006 (inception) to December 31, 2010, respectively. During the fourth quarter of 2010, an impairment charge of $0.2 million was recorded as a reduction of property and equipment and an addition to research and development expense. No impairment charges were recorded for the years ended December 31, 2009 or 2008. Leasehold improvements are amortized over the shorter of the asset’s useful life or the remaining lease term.
Note 6 Other Non-Current Assets
Other non-current assets consists of legal and accounting costs associated with the Company’s filing of a registration statement with the SEC. If a resulting securities offering is successfully completed, these costs will be reclassified as a reduction of the resulting proceeds when added to paid
F-12
Table of Contents
in capital; otherwise, the costs will be expensed. During the fourth quarter of 2010, the Company performed an analysis of the likely future benefit of these costs and determined that $0.5 million should be expensed during the year ended December 31, 2010 and is included in selling, general and administrative expense on the accompanying consolidated statements of operations.
Note 7 Accrued Expenses
Accrued expenses are comprised of the following components:
| As of December 31, | ||||||||
| 2010 | 2009 | |||||||
| Accrued payroll and bonus |
$ | 806,860 | $ | 606,454 | ||||
| Accrued vacation compensation |
$ | 579,810 | $ | 651,839 | ||||
| Accrued legal, accounting and engineering fees |
$ | 497,265 | $ | 134,070 | ||||
| Other accruals |
$ | 305,626 | $ | 224,828 | ||||
|
|
|
|
|
|||||
| Total Accrued Expenses |
$ | 2,189,561 | $ | 1,617,191 | ||||
|
|
|
|
|
|||||
Note 8 Other Current Liability
In December 2009, the Company entered into an agreement with Green Science Energy, LLC (the “Licensee”) under which the Company received $2.0 million in exchange for the Licensee’s right to market and sell sub-licenses in Egypt and Morocco. The agreement provided that $2.0 million was to be placed in escrow and held subject to the election at the end of a 13-month period by the Licensee to either (a) continue its marketing and licensing efforts or (b) terminate the agreement, return the rights to PetroAlgae Inc. technology and, in return, receive a refund of the $2.0 million. During 2010, the $2.0 million was reclassified from other liability to other current liability as the 13-month period noted above expired in January of 2011 (see Note 15).
Note 9 Notes Payable—Related Party
During 2007 and 2008, the Company received funding from XL Tech in the form of a note with an interest rate of prime + 2%, with the interest drawing into the note after each month. In January 2008, an option to acquire 2,029,337 membership interests in PA LLC for 30 years at a price of less than one cent each was issued to XL Tech as additional consideration for funding under the note. The issuance of this option has been accounted for as an original issue discount on the related debt in the initial amount of approximately $1.5 million and has been amortized over the original term of the debt. The amortization of this discount of $0.4 million, $0.6 million, $0.5 million, and $1.5 million for the years ended December 31, 2010, 2009, 2008, and the period from September 22, 2006 (inception) to December 31, 2010, respectively, has been included in interest expense in the accompanying consolidated statements of operations.
In August 2008, the note and related option to acquire PA LLC membership interests that was held by XL Tech was acquired by PetroTech.
On July 24, 2009, the Company entered into agreements with its principal stockholder to restructure all of the Company’s loans. The Company, PA LLC, PetroTech, and LV Administrative Services, Inc., as administrative and collateral agent for PetroTech, fully executed an Omnibus Amendment, Joinder and Reaffirmation Agreement, which amended (i) the demand notes issued by PA LLC to PetroTech dated August 21, 2008, September 3, 2008, September 18, 2008 and September 25, 2008 in the aggregate principal amount of $7,222,089, (ii) the convertible demand notes issued by PA LLC to PetroTech dated April 24, 2009 and May 11, 2009 in the aggregate principal amount of $10,000,000, (iii) the demand note issued by PA LLC to Valens US SPV I, LLC in
F-13
Table of Contents
the principal amount of $417,512 dated August 8, 2008, ((iv) the promissory note dated June 12, 2008 issued by PA LLC in favor of XL Tech and assigned to PetroTech in the principal amount of $25,000,000 and (v) a master security agreement dated August 21, 2008 by PA LLC in favor of LV Administrative Services, Inc. on behalf of PetroTech. The Company also delivered an equity pledge agreement and a corporate guaranty in connection with the Omnibus Amendment, Joinder and Reaffirmation Agreement. Principally, the maturity of the loans was changed to the dates below.
During the year ended December 31, 2010, PetroTech funded a total of $15.2 million to PA LLC pursuant to the terms of twelve separate senior secured term notes, all of which are included in the $22.4 million notes payable in the following table. Notes payable consist of the following at December 31, 2010 and 2009:
| As of December 31, | ||||||||
| 2010 | 2009 | |||||||
| Note Payable to Valens U.S. SPV I, LLC |
$ | 417,512 | $ | 417,512 | ||||
| • Interest accrues monthly, at 12% per annum |
||||||||
| • Note is due on June 30, 2012 |
||||||||
| Notes Payable to PetroTech |
22,422,089 | 7,222,089 | ||||||
| • Interest accrues monthly, at 12% per annum |
||||||||
| • Notes are due on June 30, 2012 |
||||||||
| Convertible Note Payable to PetroTech |
10,000,000 | 10,000,000 | ||||||
| • Interest accrues monthly at 12% per annum |
||||||||
| • Note is due on June 30, 2012 if not converted to common shares as described below |
||||||||
| Up to $25,000,000 Note Payable to PetroTech |
18,823,133 | 17,849,495 | ||||||
| • Interest payable monthly and is drawn into note on a monthly basis at prime + 2% (5.25% at December 31, 2010) |
||||||||
| • Note is due on June 30, 2012 |
||||||||
| • Permits additional draws to fund interest. Maximum balance of this note is limited to $25,000,000 |
||||||||
| Subtotal |
51,662,734 | 35,489,096 | ||||||
| Original Issue Discount |
— | (375,000 | ) | |||||
|
|
|
|
|
|||||
| Total |
$ | 51,662,734 | $ | 35,114,096 | ||||
|
|
|
|
|
|||||
The notes payable—related party are collectively secured by all of the Company’s assets. The convertible note allows the holder to convert all or any portion of the issued and outstanding principal amount and/or accrued interest and fees then due into shares of common stock at a fixed conversion price of $5.43 per share. As of December 31, 2010, the intrinsic value of this conversion feature was $0.00. The intrinsic value is the difference between the estimated fair value of the Company’s stock and the conversion price.
Each note payable and the related master security agreement and equity pledge and corporate guaranty agreement contain provisions that specify events of default which could lead to acceleration of the maturity of the debt. These provisions prohibit the encumbrance or sale of the Company’s assets and require maintenance and insurance of the assets. The loan agreements do not contain any required financial ratios or similar debt covenants. Generally, an event of default would arise if the Company became insolvent, filed for bankruptcy, allowed a change in control or had unresolved judgments against its assets. Through the date of this annual report, none of these events have occurred.
F-14
Table of Contents
As of December 31, 2010 and 2009, interest in the amount of $4.8 million and $2.1 million, respectively, was accrued related to the notes payable—related party and is recorded in accrued expenses—related party on the accompanying consolidated balance sheets. During 2010 and 2009, additional accrued interest of $1.0 million and $0.9 million was rolled into the principal balance of the floating-rate note and is recorded in notes payable-related party on the accompanying consolidated balance sheets.
Interest charged to operations on these notes was $4.1 million, $3.2 million, $1.8 million, and $9.5 million during the years ended December 31, 2010, 2009, 2008, and the period from September 22, 2006 (inception) to December 31, 2010, respectively. No interest was capitalized during these periods.
Note 10 Leases
The Company has non-cancellable third-party operating leases for office space and certain land used in its business that expire through 2011. In addition, as of December 31, 2010, XL Tech sub leased office space to PetroAlgae Inc. on a month to month basis, at a current rate of $45,461 per month. This sub-lease was terminated in February 2011 (see Note 15).
The Company does not have any significant step rent, capital improvement, rent concession or other features in its office space or land leases.
Rental expense for all operating leases during 2010, 2009, 2008, and since inception was $0.8 million, $0.8 million, $0.4 million, and $2.1 million, respectively.
Note 11 Stockholders’ (Deficit) Equity
The Company’s equity consists of 300,000,000 shares of $.001 par value common stock, of which 106,933,230 and 106,229,356 shares had been issued and were outstanding as of December 31, 2010 and 2009, respectively. In addition, the Company may issue up to 25,000,000 shares of preferred stock. No preferred stock is outstanding.
Estimation of Common Stock Fair Value
In each reporting period, the Company has determined its best estimate of the fair value of its common stock by considering the following indicators of value:
| Ÿ | Level 3 valuations based upon a discounted cash flow analysis using inputs which were not externally observable, in this case the Company’s estimate of future cash flows from the Company’s business; and |
| Ÿ | Prices paid in cash for shares of stock or securities units comprised of equity shares and five-year warrants to purchase equity shares; and |
| Ÿ | Over-The-Counter (“OTC”) quotation closing price and related statistics such as trading volume, volatility and recent price ranges. |
Level 3 estimated cash flow valuations. The Level 3 fair value estimate of the Company’s equity value applied discounted cash flow valuation techniques to expected future cash flows from the Company’s business as of each valuation date. Key assumptions utilized in determining this fair value estimate include the expected amount and timing of revenue from expected future sales of the Company’s technology along with the associated cost of sales and other operating cash outflows. These cash flows are discounted back to present value using an interest rate consistent with development stage technology companies and the resulting enterprise value is converted to an equity value per outstanding share.
F-15
Table of Contents
The most significant uncertainty inherent in these calculations is the risk of obtaining and performing the contracts necessary to produce the estimated future revenues. In addition, the risk of collection of amounts that are expected to be due under projected contracts, the risk of unanticipated product development or delivery costs and the risk of increased operating and overhead costs may each cause the estimates of future revenues to vary from the assumptions that are made in these projections, and these variations may be material. Since the Company has not obtained or performed any commercial contracts to date, it does not have historical data on which to base its estimate of future revenues.
The fair value of the shares was estimated using the same Level 3 estimation method the Company used for its other share issuances, which applied valuation techniques to the Company’s expected future cash flow. The fair value of the warrants was estimated using the Black-Scholes valuation model, based on the estimated fair value of the common stock on the valuation date, an expected dividend yield of 0%, a risk-free interest rate based on constant maturity rates published by the U.S. Federal Reserve applicable to the remaining term of the instruments, an expected life equal to the remaining term of the instruments and an estimated volatility of 75.2%. Because the Level 3 indications of value were able to take into account important information pertaining to the amount, timing and risk of Company cash flows, they were given the most weight in our estimate of share value.
Third party transactions. The Company has completed several private sales of equity shares and securities units comprised of both equity shares and warrants to purchase additional equity shares over the reporting periods. The transactions were completed with both related and unrelated parties in exchange for cash. Given the size of these transactions and the sophisticated nature of the parties involved in their negotiation, these indications of value were given significant weight in the Company’s estimate of share value. However, because most of these transactions were with affiliates, sole or primary reliance was not placed on these values.
OTC Values. Through December 31, 2010, the Company’s common stock was traded on the OTC Bulletin Board. The shares became public through a reverse acquisition and a very small percentage of the issued and outstanding shares are available for active trading since approximately 94% of our outstanding shares are held by our controlling shareholder. Due to the very limited size of our public float and the resulting low trading volume in the Company’s common stock, its public shares are susceptible to unusual swings in price based on very few trades. As a result of these considerations, value indications provided by the OTC Bulletin Board, which are now provided by the OTC Pink Sheets, were given the least weight of the three factors in the Company’s estimate of share value.
Issuance of Common Stock and Warrants
On January 15, 2009, the Company entered into a Stock Purchase Agreement with Engineering Automation and Design Inc., a Nebraska corporation (“EAD”), pursuant to which we issued 151,057 shares of common stock as partial consideration, in advance, for certain services relating to the design, engineering, and construction of facilities for the growth, harvesting and processing of micro-crops for demonstration of our technology to potential customers. In February 2010, EAD returned 106,126 shares of common stock to the Company, which were canceled. The shares earned and retained by EAD under the related agreements were accounted for at their estimated fair value at issuance of approximately $144,678 (44,931 shares at $3.22 fair value per share) and charged to research and development expense. The shares returned in February of 2010, which had an estimated value of $341,726, were included in other current assets at December 31, 2009.
During December 2009, the Company issued approximately 9,500 shares of common stock valued at an estimated fair value of $5.50 per share for legal services.
F-16
Table of Contents
During December 2009, the Company issued 357,143 units consisting of one share of common stock and a warrant to purchase a share of common stock at $15.00 for a period of five years in exchange for a 30% interest in Green Sciences Energy, LLC, a subsidiary of Congoo, LLC. The units were valued at $8.00 per unit or $2.9 million. The $8.00 fair value per unit was determined based upon contemporaneous issuances of identical units for $8.00 in cash. In addition, the Company used its fair value estimate to allocate the value of the units issued between the fair values of the common stock and warrants of $2.0 million ($5.66 per share) and $0.9 million ($2.34 per warrant), respectively. This allocation indicated that a very small premium (less than 5%) should be attributed to the allocated share price compared to its fair value.
In addition, during December 2009, the Company issued an additional 250,000 warrants exercisable at $8.00 per share for a period of six months and 250,000 warrants exercisable at $15.00 per share for a period of six months. The Company estimated the fair value of the 500,000 additional warrants using the Black-Scholes option pricing model and the same assumptions stated above, resulting in an estimated fair value of the options of $0.2 million. The sum of the estimated fair value of the securities issued was approximately $3.0 million.
From February 12, 2010 to August 6, 2010, the Company sold 797,500 units, each unit consisting of one share of common stock and a warrant to purchase a share of common stock at $15.00 for a period of five years, for $8.00 per unit or $6.4 million as follows:
| Ÿ | 687,500 units to an affiliate; and |
| Ÿ | 110,000 units to third parties. |
The proceeds of the 797,500 units issued were allocated between the common stock and the warrants based upon their respective estimated fair values on the date of the issuance. This allocation attributed $4.5 million (or $5.52 to $5.60 per share) to the common stock issued and $1.9 million to the warrants issued (or $2.40 to $2.48 per warrant). For the warrants, the Company estimated the fair value using the Black-Scholes valuation model, based on the estimated fair value of the common stock on each valuation date ($5.89 to $6.67 per share), an expected dividend yield of 0%, a risk-free interest rate based on the yield of 5-year U.S. Treasury securities on each valuation date (1.78% to 2.62%), an expected life of five years and an estimated volatility of 75.2%.
The 687,500 units sold to the affiliate contained share price and warrant exercise price guarantees. The purchase price of the common stock and the exercise price of the warrants would have been adjusted in the event that the Company issued common stock or warrants at a price below $8.00 for common shares or a $15.00 exercise price for warrants for a period of six months from the purchase date. As of December 31, 2010, all of the aforementioned share price guaranty periods have expired and no adjustments were necessary.
On November 1, 2010, in anticipation of his impending employment as President and Chief Operating Officer, the Company issued and sold 12,500 shares of its common stock to this executive at a price of $8.00 per share. The shares contained a put option which gave the executive the option to sell any or all of the shares back to the Company for a purchase price of $8.00 at any time within one year of the original transaction. In accordance with ASC 480-10-25-8, the proceeds are recorded as liabilities related to equity issuance on the accompanying consolidated balance sheets.
Non-controlling Interest and PA LLC Equity Incentive Plan
During 2006, the Company issued 5% of the Class A voting units (1,000,000 units) of PA LLC as partial consideration for a license to certain technology from Arizona Technology Enterprises, LLC (“AzTE”). AzTE was granted anti-dilution rights and was entitled to 5% of the fully diluted capitalization of PA LLC.
F-17
Table of Contents
On June 2, 2010, the Company entered into an agreement with AzTE that provided for recovery of the 1,000,000 Class A voting units of PA LLC and the cancellation of all existing and future obligations and agreements between the Company and AzTE, in exchange for the return of certain AzTE-licensed technology and a payment of $0.6 million. The Company remitted the payment to AzTE on June 3, 2010 and the units of PA LLC were received and cancelled on June 3, 2010. The return of the Class A voting units of PA LLC effectively nullified the anti-dilution rights that were previously granted to AzTE.
The transaction with AzTE was accounted for as a return or repurchase of equity interests of PA LLC. The Company followed the guidance of ASC 810-10-45-23 and handled changes in the parent’s ownership by adjusting the carrying amount of the controlling and non-controlling interests based on the change in their respective ownership share in the subsidiary. This transaction increased the parent or controlling interest from approximately 82% to approximately 87%, which gave rise to a debit of $1.7 million to paid in capital attributable to PetroAlgae Inc., since PA LLC had a large retained deficit. In addition, the amount paid of $0.6 million was also recorded in paid in capital since it represents the cost to the controlling interest of acquiring a portion of the interest in the subsidiary that it did not previously own. The net effect of this transaction increased shareholders’ deficit attributable to PetroAlgae Inc. by $2.3 million.
PA LLC has an equity compensation plan which allows it to grant employees and consultants awards of profits interests in restricted Class B ownership units (the “Interests”) and is limited to 14% of the total outstanding units.
The Company granted 2,816,471 and 674,500 Interests during 2008 and 2007, respectively, to the majority of its employees as of the date that they began employment with the Company. As of December 31, 2010 and 2009, the granted Interests, net of Interests forfeited and repurchased by the Company, totaled approximately 2.8 million and 2.9 million, respectively, or 13.0% and 12.6%, respectively, of total outstanding units. These Interests give the recipient the right to participate in the income of PA LLC and any distribution that may arise from a liquidity event to the extent that such realized amounts exceed the ownership unit fair value at the date of grant.
The Company determined the fair value of these grants by estimating the proceeds that it may obtain from a range of possible future liquidity events such as a public equity offering or a Company sale. The timing of such liquidity events and the likelihood of their achievement was estimated based upon the information that existed as of the grant or valuation date. The weighted average future cash value was then discounted using a 60% per annum discount rate and the weighted average time from grant or valuation date to the liquidity event. This estimated cash present value was converted to a per share cash value and, based on the structure of the grant, the carrying amount per LLC unit was subtracted to determine the fair value of each of the Interests.
The grants of Interests contain restrictions that allow the Company to repurchase the units for $0.01 per unit until a service period or other condition is met. This restriction is removed over a service period (generally four years) with regard to approximately 2.15 million Interests. For the years ended December 31, 2010, 2009, and 2008, compensation expense related to these Interests was $1.5 million, $2.0 million, and $2.4 million, respectively. For the period from September 22, 2006 (inception) to December 31, 2010, compensation expense related to these Interests was $6.2 million. The related compensation expense is recognized as selling, general and administrative expense or research and development expense depending upon the recipient’s role in the Company. Amounts recognized as selling, general and administrative expense were $0.6 million, $1.1 million, $1.5 million, and $3.2 million for the years ended December 31, 2010, 2009, 2008, and the period from September 22, 2006 (inception) to December 31, 2010, respectively. Amounts recognized as research and development expense were $0.9 million, $0.9 million, $0.9 million, and $3.0 million for the years ended December 31, 2010, 2009, 2008, and the period from September 22, 2006 (inception) to December 31,
F-18
Table of Contents
2010, respectively. Unrecognized compensation cost related to 256,666 Interests which remain restricted totaled approximately $1.3 million as of December 31, 2010 and is expected to be expensed over the next two years. An aggregate of 111,000, 258,000, 156,000, and 653,000 of these Interests were forfeited during the years ended December 31, 2010, 2009, 2008, and the period from September 22, 2006 (inception) to December 31, 2010, respectively.
Five senior officers were granted an aggregate 1.34 million Interests that are subject to repurchase for $0.01 per unit until a significant liquidity event occurs. Because that condition had not yet been met, these grants were considered contingent and had not been amortized. The amount and nature of the liquidity event was changed in July 2009 from a requirement to obtain $150.0 million of distributions and/or debt repayments to the Company’s principal stockholder to a requirement to obtain $25.0 million of new liquidity for the Company. This change resulted in a $4.6 million increase in the unrecognized compensation expense to $6.0 million.
As of December 31, 2010, the Company’s principal stockholder modified the terms of the grant to remove the right of the Company to repurchase the Interests held by all five officers, causing these grants to be immediately vested. This decision required a revaluation of the Interests held by the officers and immediate recognition of the calculated expense. Based upon current estimates and applying the same discounted cash flow methodology used as of the prior valuation dates, the compensation cost was increased to $11.4 million during the fourth quarter of 2010 and recognized as selling, general and administrative expense ($9.1 million) or research and development expense ($2.3 million) depending upon each officer’s role in the Company.
As of December 31, 2010 and 2009, non-controlling interests collectively owned approximately 13.0% and 17.8%, respectively, of PA LLC, and have absorbed their respective portion of the loss of PA LLC. The amount of loss absorbed was $6.5 million, $6.6 million, and $0.2 million for the years ended December 31, 2010, 2009, and 2008, respectively. The amount of loss absorbed for the period from September 22, 2006 (inception) to December 31, 2010 was $13.2 million.
Stock Options
On June 17, 2009, the Company adopted the 2009 Equity Compensation Plan (the “2009 Plan”). The 2009 Plan is intended to provide employees, consultants and others the opportunity to receive incentive stock options and is limited to 4,000,000 shares of the Company’s common stock. The exercise price shall be equal to or greater than the fair value of the underlying common stock on the date the option is granted and its term shall not exceed 10 years. Awards shall not vest in full prior to the third anniversary of the award date.
On January 27, 2010, the Company granted its employees 322,000 options, in the aggregate, to purchase common shares at an exercise price of $8.00 per share. The options vest over a period of four years from the employee’s date of hire and expire 10 years from the grant date. The grant date fair value of the options aggregated to $1.1 million and was calculated using the following assumptions determined as of the date of grant: estimated fair value of the common stock of $5.68, expected term of 6.25 years, risk free interest rate of 2.8%, an estimated volatility of 75.2%, and a dividend yield of 0%. The expected term of options granted to employees was based upon the simplified method allowed for “plain vanilla” options as described by SEC SAB No. 107. The fair value will be charged to operations over the remaining vesting periods commencing on the grant date. The weighted average period over which options not vested through December 31, 2010 are expected to vest is 24 months. During the years ended December 31, 2010 and 2009, approximately $0.9 million and $0.6 million, respectively, was charged to operations related to all of these outstanding options. The fair value related to unexercisable stock options as of December 31, 2010 totaled approximately $1.2 million and is expected to be expensed over the next three years.
F-19
Table of Contents
A summary of stock option activity is as follows:
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Fair Value |
||||||||||
| Balance at December 31, 2008 |
— | $ | — | $ | — | |||||||
| Granted June 2009 |
1,072,500 | $ | 8.50 | $ | 2.36 | |||||||
| Granted July 2009 |
45,000 | $ | 8.00 | $ | 2.54 | |||||||
| Forfeited |
(200,000 | ) | $ | (8.50 | ) | $ | (2.36 | ) | ||||
| Balance at December 31, 2009 |
917,500 | $ | 8.48 | $ | 2.37 | |||||||
| Granted January 2010 |
322,000 | $ | 8.00 | $ | 3.53 | |||||||
| Forfeited |
(247,750 | ) | $ | (8.35 | ) | $ | (2.63 | ) | ||||
| Balance at December 31, 2010 |
991,750 | $ | 8.35 | $ | 2.68 | |||||||
|
|
|
|
|
|
|
|||||||
The weighted average grant date fair value for vested options as of December 31, 2010 and 2009 was $2.59 and $2.36, respectively. The weighted average grant date fair value for unvested options as of December 31, 2010 and 2009 was $2.73 and $2.37, respectively.
The weighted average contractual life of stock options at December 31, 2010 and 2009 was as follows:
| 2010 | 2009 | |||||||
| Vested |
3.1 yrs | 0.1 yrs | ||||||
| Unvested |
5.5 yrs | 9.4 yrs | ||||||
| Total outstanding |
8.7 yrs | 9.5 yrs | ||||||
As of December 31, 2010, a total of 360,708 of the options granted were exercisable and the fair value of such options was $0.9 million. Because the Company has very little history from which to estimate forfeiture of options or grants, it accounts for such forfeitures prospectively, that is, in the period in which they actually occur.
Inputs to both the Interest fair value calculation and the stock option Black-Scholes model are subjective and generally require significant judgment to determine. If, in the future, the Company determines that another method for calculating the fair value of its stock-based compensation is more reasonable, or if another method for calculating these input assumptions is prescribed by authoritative guidance, the fair value calculated for stock-based compensation could change significantly. Regarding stock options, higher volatility and longer expected terms generally result in an increase to stock-based compensation expense determined at the date of grant.
As of December 31, 2010, the aggregate intrinsic value of all stock options outstanding and expected to vest was $0.00 and the aggregate intrinsic value of currently exercisable stock options was $0.00. The intrinsic value of each option share is the difference between the estimated fair value of the Company’s stock and the exercise price of such option.
No stock options have been exercised as of December 31, 2010.
In November 2010, the Company issued one million Stock Appreciation Rights (or “SARs”) to its President and Chief Operating Officer under the 2009 Plan. The vesting of these SARs was tied to continued employment with the Company and the accomplishment of certain milestones.
F-20
Table of Contents
Note 12 Retirement Plan
Employees of PA LLC may participate in a 401k retirement plan sponsored by the Company. PA LLC will match the contribution made by participating employees up to 4% of their eligible salaries. Matching contributions were $0.2 million, $0.2 million, and $0.1 million during 2010, 2009, and 2008, respectively.
Note 13 Income Taxes
The Company has not recorded any income tax expense or benefit for the years ended December 31, 2010 and 2009 primarily due to our history of operating losses. Because the Company was a limited liability company and passed through its losses to its owners before the reverse acquisition (December 19, 2008), no federal or state tax benefit or provision was reported by the Company for periods before that date.
The reconciliation of income tax benefit at the statutory federal income tax rate of 35% to net income tax benefit for the years ended December 31, 2010 and 2009 is as follows:
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| U.S. federal benefit at statutory rate |
$ | (13,383,330 | ) | $ | (12,887,783 | ) | ||
| State income taxes, net of federal benefit |
(1,126,723 | ) | (1,243,056 | ) | ||||
| Nondeductible equity compensation |
4,516,310 | 695,627 | ||||||
| Other items |
20,215 | 22,372 | ||||||
| Prior year estimate to tax return adjustment |
(1,545,110 | ) | — | |||||
| Increase in valuation allowance |
11,518,638 | 13,412,840 | ||||||
|
|
|
|
|
|||||
| Income tax expense |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
The tax effects of temporary differences and carryforwards that give rise to significant portions of the deferred tax assets are as follows:
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Deferred tax assets |
||||||||
| Net operating loss and research and development credit carryforwards |
$ | 24,422,274 | $ | 14,300,976 | ||||
| Intangibles and fixed assets |
8,784,445 | 9,676,196 | ||||||
| Accruals and other items |
2,377,281 | 444,064 | ||||||
| Stock-based compensation |
582,947 | 227,073 | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
36,166,947 | 24,648,309 | ||||||
| Less: valuation allowance |
(36,166,947 | ) | (24,648,309 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
At December 31, 2010 and 2009 we had net operating loss carryforwards of approximately $60.4 million and $37.0 million, respectively, available to reduce future taxable income, if any, for federal and state income tax purposes. The net operating loss carryforwards expire between 2028 and 2030, and valuation allowances equal to the full amounts have been provided.
Utilization of the net operating loss carryforwards may be subject to an annual limitation if the Company experiences a tax code defined change in ownership in excess of percentage change limitations provided by the Internal Revenue Code of 1986 and similar state provisions. The annual limitations may result in the expiration of the net operating loss before utilization.
F-21
Table of Contents
The Company reviews its deferred tax assets on a regular basis to evaluate their recoverability based upon expected future reversals of deferred tax liabilities, projections of future taxable income, and tax planning strategies that it might employ to utilize such assets, including net operating loss carryforwards. Based on the positive and negative evidence of recoverability, the Company establishes a valuation allowance against the net deferred assets unless it is “more likely than not” that it will recover such assets through the above means. In the future, the Company’s evaluation of the need for the valuation allowance will be significantly influenced by its ability to achieve profitability and its ability to predict and achieve future projections of taxable income.
As required by ASC 740-10-05, Accounting for Uncertainty in Income Taxes, the Company recognizes the financial statement effects of a tax position when it is more likely than not (defined as a likelihood of more than 50%), based on the technical merits, that the position will be sustained upon examination. The evaluation of whether a tax position meets the more-likely-than-not recognition threshold requires a substantial degree of judgment by management based on the individual facts and circumstances. Actual results could differ from these estimates. The Company adopted ASC 740-10-05 on January 1, 2007.
The Company has been assessed late filing penalties by the Internal Revenue Service related to the returns for PA LLC for the tax years ended December 31, 2009 and 2008. Accordingly, the Company has recorded an accrual in the amount of $0.05 million in selling, general and administrative expenses for the year ended December 31, 2010. As of January 1, 2009 and December 31, 2009, the Company recorded no liability for uncertain tax positions.
The Company files U.S. and state income tax returns with varying statutes of limitations. Tax years 2007 and forward remain open to examination.
Note 14 Related Party Transactions
During 2008 and the period from September 22, 2006 (inception) to December 31, 2010, the Company paid XL Tech an aggregate of $2.2 million and $4.2 million, respectively, as reimbursement for administrative, executive management, business development, and other services which have been included in selling, general and administrative expense for those periods.
During 2010, 2009, and 2008, the Company incurred rent expense of $0.5 million, $0.5 million, and $0.2 million, respectively, in connection with office space sub-leased from XL Tech on a month-to-month basis.
During December 2008, the Company issued 1,000,000 shares of common stock for consulting services to be performed by a company owned by a board member through December 2010. These consulting services consist of general business and financial consulting and assistance in negotiating sales, financing, and other agreements. The fair value of the shares issued was $3.2 million which was initially recorded as unearned services, a reduction of paid in capital. During 2010, 2009, and 2008, $1.5 million, $1.6 million, and $0.1 million, respectively, was amortized as selling, general and administrative expense.
During 2010, the Company paid approximately $0.1 million for consulting services performed by related parties and affiliates of the Company. No amounts were payable to these parties at December 31, 2010.
F-22
Table of Contents
Note 15 Subsequent Events
Debt Financing
On January 3, 2011, January 21, 2011, February 11, 2011, February 24, 2011, and March 23, 2011, PetroTech funded $1.2 million, $1.0 million, $1.0 million, $1.2 million, and $1.0 million, respectively, to PA LLC pursuant to the terms of separate senior secured term notes. Each of these notes provide for interest at 12%, which is accrued as a payment-in-kind amount, and are due on June 30, 2012.
Disbursement of Escrowed Funds
In December 2009, the Company entered into an agreement with Green Science Energy, LLC (the “Licensee”) under which the Company received $2.0 million in exchange for the Licensee’s right to market and sell sub-licenses in Egypt and Morocco. In January 2011, the Company received timely notice from the Licensee of its election to surrender these licensing rights and comply with related provisions of the original contract in exchange for return of the $2.0 million it had paid, which the Company had placed in escrow. Accordingly, the $2.0 million was disbursed from escrow to the Licensee in January 2011.
Resignation of Executive Officer
On March 8, 2011, the Company announced the resignation of its President and Chief Operating Officer. In connection with the resignation of this executive officer, the Company entered into a separation agreement under which the Company made a severance payment of $0.3 million in exchange for a general release of all claims against the Company. The severance payment will be recorded as selling, general and administrative expense during the first quarter of 2011. Prior to the resignation of the President and Chief Operating Officer, this executive exercised his right to have the Company repurchase 12,500 shares of common stock for $100,000 that he purchased in November 2010.
Issuance of Options
On January 2, 2011, the Company granted certain employees 623,000 stock options at an exercise price of $8.00 per share. Generally, the options vest over a period of four years from the employee’s date of hire and expire 10 years from the grant date.
Lease Commitment
On March 23, 2011, the Company entered into a lease with an unrelated third-party of 24,000 square feet of headquarters office space in Melbourne, Florida. This three-year lease provides for monthly payments that increase from $20,000 to $28,000 over its term plus an allocated share of any increase in real estate tax, insurance and building maintenance costs. The cost of this lease will be evenly allocated over its 36-month term beginning in the first quarter of 2011.
Market for Common Shares
Effective February 23, 2011, the common stock of the Company became ineligible for quotation on the OTC Bulletin Board due to quoting inactivity pursuant to Rule 15c2-11 under the Securities Exchange Act of 1934, as amended. The Company’s common stock has been moved to OTC Link, which is operated by OTC Markets Group Inc. (formerly known as Pink OTC Markets Inc. or “Pink Sheets”). Quotations for the Company’s common stock can be obtained through the Pink Sheets website (www.pinksheets.com) under the symbol “PALG”.
F-23
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
| September 30, 2011 (Unaudited) |
December 31, 2010 |
|||||||
| ASSETS |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 6,715,379 | $ | 1,140,882 | ||||
| Restricted cash |
— | 2,325,000 | ||||||
| Prepaid expenses |
115,325 | 102,016 | ||||||
| Other current assets |
67,682 | — | ||||||
|
|
|
|
|
|||||
| Total current assets |
6.898,386 | 3,567,898 | ||||||
| Property and equipment |
4,282,494 | 4,194,629 | ||||||
| Accumulated depreciation |
(2,924,823 | ) | (2,292,952 | ) | ||||
|
|
|
|
|
|||||
| Net property and equipment |
1,357,671 | 1,901,677 | ||||||
| Deposits |
67,614 | 24,814 | ||||||
| Other non-current assets |
— | 830,375 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 8,323,671 | $ | 6,324,764 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 853,820 | $ | 1,569,715 | ||||
| Accrued expenses |
1,231,285 | 2,189,561 | ||||||
| Accrued expenses—related party |
9,555,043 | 4,807,135 | ||||||
| Deferred revenue |
591,749 | 500,000 | ||||||
| Liability related to equity issuance |
— | 100,000 | ||||||
| Current portion of notes payable—related party |
72,052,615 | — | ||||||
| Other current liabilities |
— | 2,000,000 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
84,284,512 | 11,166,411 | ||||||
| Deferred rent |
31,780 | — | ||||||
| Notes payable—related party |
— | 51,662,734 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
84,316,292 | 62,829,145 | ||||||
|
|
|
|
|
|||||
| Stockholders’ deficit: |
||||||||
| Preferred stock—$.001 par value, 25,000,000 shares authorized; no shares issued or outstanding at September 30, 2011 and December 31, 2010 |
— | — | ||||||
| Common stock—$.001 par value, 300,000,000 shares authorized; 106,920,730 and 106,933,230 shares issued and outstanding at September 30, 2011 and December 31, 2010, respectively |
106,921 | 106,933 | ||||||
| Paid in capital |
39,082,301 | 37,823,248 | ||||||
| Deficit accumulated during the development stage |
(117,077,567 | ) | (98,022,859 | ) | ||||
|
|
|
|
|
|||||
| PetroAlgae Inc. stockholders’ deficit |
(77,888,345 | ) | (60,092,678 | ) | ||||
| Non-controlling interest |
1,895,724 | 3,588,297 | ||||||
|
|
|
|
|
|||||
| Total stockholders’ deficit |
(75,992,621 | ) | (56,504,381 | ) | ||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ deficit |
$ | 8,323,671 | $ | 6,324,764 | ||||
|
|
|
|
|
|||||
See the accompanying notes to the consolidated financial statements.
F-24
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Consolidated Statements of Operations
(Unaudited)
| Three Months Ended September 30, |
Nine Months Ended September 30, |
For the Period From September 22, 2006 (Inception) Through September 30, 2011 |
||||||||||||||||||
| 2011 | 2010 | 2011 | 2010 | |||||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
| Costs and expenses: |
||||||||||||||||||||
| Selling, general and administrative |
2,206,280 | 2,718,105 | 8,074,208 | 9,447,354 | 58,740,637 | |||||||||||||||
| Research and development |
2,183,312 | 3,689,642 | 8,462,806 | 11,184,021 | 56,931,803 | |||||||||||||||
| Interest expense—related party |
1,767,864 | 1,060,121 | 4,646,195 | 2,912,155 | 14,129,253 | |||||||||||||||
| Depreciation |
188,912 | 258,712 | 669,124 | 772,732 | 3,259,613 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
6,346,368 | 7,726,580 | 21,852,333 | 24,316,262 | 133,061,306 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss before non-controlling interest |
(6,346,368 | ) | (7,726,580 | ) | (21,852,333 | ) | (24,316,262 | ) | (133,061,306 | ) | ||||||||||
| Net loss attributable to non-controlling interest |
805,989 | 1,019,909 | 2,797,625 | 3,842,232 | 15,983,739 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss attributable to PetroAlgae Inc. |
$ | (5,540,379 | ) | $ | (6,706,671 | ) | $ | (19,054,708 | ) | $ | (20,474,030 | ) | $ | (117,077,567 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted common shares outstanding |
106,920,730 | 106,917,360 | 106,920,730 | 106,664,896 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Basic and diluted loss per share |
$ | (0.05 | ) | $ | (0.06 | ) | $ | (0.18 | ) | $ | (0.19 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
See the accompanying notes to the consolidated financial statements.
F-25
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Consolidated Statements of Cash Flows
(Unaudited)
| Nine Months Ended September 30, |
For the Period From September 22, 2006 (Inception) Through September 30, 2011 |
|||||||||||
| 2011 | 2010 | |||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (21,852,333 | ) | $ | (24,316,262 | ) | $ | (133,061,306 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Amortization of original issue discount |
— | 375,000 | 1,453,000 | |||||||||
| Amortization of Company options |
698,801 | 687,468 | 2,210,003 | |||||||||
| Amortization of stock appreciation rights |
561,057 | — | 561,057 | |||||||||
| Amortization of unearned services |
— | 1,181,250 | 3,150,000 | |||||||||
| Amortization of PA LLC interests to employees |
1,104,235 | 1,234,813 | 18,711,764 | |||||||||
| Expenses paid and interest added to notes payable—related party |
764,881 | 726,034 | 11,114,364 | |||||||||
| Expenses paid by issuance of common stock |
— | — | 1,214,230 | |||||||||
| Depreciation |
669,124 | 772,732 | 3,259,613 | |||||||||
| Impairment loss |
409,674 | — | 588,773 | |||||||||
| (Gain) loss on disposition of equipment |
(7,834 | ) | 460 | 181,762 | ||||||||
| Write-off of other non-current assets |
830,375 | — | 1,286,764 | |||||||||
| Decrease in restricted cash |
325,000 | — | — | |||||||||
| (Increase) in prepaid expenses |
(13,309 | ) | (3,916 | ) | (134,939 | ) | ||||||
| (Increase) in other current assets |
(67,682 | ) | — | (67,682 | ) | |||||||
| (Increase) in deposits |
(42,800 | ) | — | (48,000 | ) | |||||||
| (Increase) in other non-current assets |
— | (27,483 | ) | (109,720 | ) | |||||||
| (Decrease) increase in accounts payable |
(592,210 | ) | 189,935 | (90,792 | ) | |||||||
| Increase in accrued expenses |
3,665,947 | 1,815,100 | 8,472,546 | |||||||||
| Increase in deferred revenue |
91,749 | 250,000 | 591,749 | |||||||||
| Increase in deferred rent |
31,780 | — | 31,780 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
$ | (13,423,545 | ) | $ | (17,114,869 | ) | $ | (80,685,034 | ) | |||
| Cash flows from investing activities: |
||||||||||||
| Proceeds from sale of asset |
16,520 | — | 60,020 | |||||||||
| Acquisition of property and equipment |
(543,478 | ) | (785,010 | ) | (5,447,839 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
$ | (526,958 | ) | $ | (785,010 | ) | $ | (5,387,819 | ) | |||
| Cash flows from financing activities: |
||||||||||||
| Member contributions |
— | — | 488,532 | |||||||||
| Reverse merger |
— | — | 99 | |||||||||
| Exercise of put option |
(100,000 | ) | — | (100,000 | ) | |||||||
| Common stock and warrants issued for cash |
— | 6,380,000 | 27,980,000 | |||||||||
| Borrowings under notes payable—related party |
19,625,000 | 8,600,000 | 63,019,601 | |||||||||
| PA LLC units returned for cash and surrendered technology license |
— | (600,000 | ) | (600,000 | ) | |||||||
| Issuance of common stock and warrants for cash |
— | 1,200,000 | 2,000,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
$ | 19,525,000 | $ | 15,580,000 | $ | 92,788,232 | ||||||
|
|
|
|
|
|
|
|||||||
| Net (decrease) increase in cash |
5,574,497 | (2,319,879 | ) | 6,715,379 | ||||||||
| Cash and cash equivalents—beginning of period |
1,140,882 | 2,679,302 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents—end of period |
$ | 6,715,379 | $ | 359,423 | $ | 6,715,379 | ||||||
|
|
|
|
|
|
|
|||||||
| Non-cash investing and financing activities: |
||||||||||||
| Liability incurred for other non-current asset |
$ | (555,000 | ) | $ | 1,152,000 | $ | 166,000 | |||||
| Stock and warrants issued for other liabilities, net |
$ | (100,000 | ) | $ | (4,500,000 | ) | $ | — | ||||
| Common stock issued for unearned services |
$ | — | $ | — | $ | 3,150,000 | ||||||
| Issuance of shares for other current asset |
$ | — | $ | — | $ | 1,200,000 | ||||||
| Return of common stock for other current asset |
$ | — | $ | (342,000 | ) | $ | (342,000 | ) | ||||
| Liability repaid with restricted cash |
$ | 2,000,000 | $ | — | $ | — | ||||||
See accompanying notes to the consolidated financial statements.
F-26
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
September 30, 2011
(Unaudited)
Note 1 Organization
PetroAlgae Inc. (the “Company”) began its operations through its controlled subsidiary, PA LLC (formerly known as PetroAlgae LLC), on December 19, 2008, as a result of the reverse acquisition described below. PA LLC was formed by XL TechGroup, LLC (“XL Tech”, a related party and its sponsor and parent until December 19, 2008) on September 22, 2006 as a Delaware limited liability company to develop technologies to commercially grow, harvest and process micro-crops. PA LLC is a technology development and licensing company that is currently working with prospective customer licensees to construct a biomass production platform that allows its licensees to grow aquatic micro-crops at a rate that substantially exceeds their natural growth rates. The Company expects the commercial-scale build-out of its technology to produce two products: a protein concentrate and a carbohydrate-rich biomass, referred to as the Company’s protein and biocrude products. The protein product is expected to be a highly effective substitute for high-value animal feedstocks such as fishmeal and may be further developed as a high-value human food ingredient. The biocrude product is suitable as an alfalfa feed substitute for grazing animals and may also be used to develop renewable fuels which are functionally compatible with the petroleum-based fuels they would replace.
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information and Rule 8-03 of Regulation S-X. They do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. The consolidated results of operations for the periods presented are not necessarily indicative of the results to be expected for the year ending December 31, 2011 or any future period. For further information, refer to the consolidated financial statements of the Company included in the Company’s Annual Report on Form 10-K, which was filed with the Securities and Exchange Commission (the “SEC”) on March 31, 2011.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its consolidated subsidiary, PA LLC. Non-controlling interests are accounted for based upon the value or cost attributed to their investment adjusted for the share of income or loss that relates to their percentage ownership of the entire Company. All intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates
The accompanying consolidated financial statements are prepared in accordance with U.S. GAAP which require management to make estimates and assumptions. These estimates and assumptions affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expense. Actual results may differ from these estimates.
Fair Value of Financial Instruments
Fair value estimates discussed herein are based upon certain market assumptions and pertinent information available to management as of September 30, 2011. The respective carrying values of
F-27
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
certain financial instruments approximate their fair values. These financial instruments include cash and cash equivalents, accounts payable, and accrued expenses. Carrying values are assumed to approximate fair values for these financial instruments because they are short term in nature and their carrying amounts approximate the amounts expected to be received or paid.
The carrying value of the Company’s fixed-rate notes payable-related party approximate their fair value based on the current market conditions for similar debt instruments.
Long-Lived Assets
The carrying value of long-lived assets is reviewed on a regular basis for the existence of facts and circumstances that suggest permanent impairment. Specifically, senior management of the Company considers in each reporting period the effectiveness of the Company’s significant assets to determine if impairment indicators, such as physical deterioration, process change or technological change, have resulted in underperformance, obsolescence or the need to replace such assets. When the Company identifies an impairment indicator, it measures the amount of the impairment based on the amount that the carrying value of the impaired asset exceeds its best internal estimate of the value of the asset while it remains in use, plus any proceeds expected from the eventual disposal of the impaired asset. Since the Company uses most of its property and equipment to demonstrate its technology to prospective customers and not to generate revenues or cash flows, a cash flow analysis of the assets is not practicable. Instead, a depreciated replacement cost estimate is used for assets still in service and equipment secondary market quotes are obtained for assets planned for disposal.
During the second and third quarters of 2011, the Company identified certain changes in its process development plans that eliminated or changed the need or expected use of certain assets. As a result, impairment charges of $0.1 million for the last three months and $0.4 million for the nine months ended September 30, 2011 were recorded to reduce these assets to their estimated realizable values. These charges reduced property and equipment and increased research and development expense during the nine months ended September 30, 2011. No other impairment indicators were noted in the nine months ended September 30, 2010 and the Company’s process of monitoring these assets was unchanged during this period.
Loss Per Share
Basic earnings (loss) per share is calculated by dividing net income (loss) by the weighted average number of common shares outstanding for the period. Diluted earnings (loss) per share is calculated by dividing net income (loss) by the weighted average number of common shares and dilutive common stock equivalents outstanding. During periods in which the Company incurs losses, common stock equivalents are not considered as their effect would be anti-dilutive.
The effect of 2,592,143 and 2,592,143 weighted average warrants and 1,743,880 and 1,597,878 weighted average options were not included for the three and nine months ended September 30, 2011, respectively, as they would have had an antidilutive effect. The effect of 2,588,773 and 2,600,815 weighted average warrants and 1,022,891 and 1,108,632 weighted average options were not included for the three and nine months ended September 30, 2010, respectively, as they would have had an antidilutive effect.
F-28
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
Reverse Acquisition
On December 19, 2008, a reverse acquisition was completed under which, for accounting purposes, PA LLC was deemed to be the acquirer and PetroAlgae Inc., the legal acquirer, was deemed to be the acquired entity. No goodwill was recognized as PetroAlgae Inc. was a “shell” company before the acquisition. The former stockholders of PetroAlgae Inc. retained an aggregate of 99,586 shares of common stock which were recorded at par value of $99.
Recently Issued Accounting Pronouncements
Adoption of New Accounting Standards
Revenue Recognition
In October 2009, the FASB issued ASU 2009-13, Multiple-Deliverable Revenue Arrangements (“ASU 2009-13”). The new standard changes the requirements for establishing separate units of accounting in a multiple element arrangement and requires the allocation of arrangement consideration to each deliverable based on the relative selling price. The selling price for each deliverable is based on vendor-specific objective evidence (“VSOE”) if available, third-party evidence if VSOE is not available, or estimated selling price if neither VSOE or third-party evidence is available. ASU 2009-13 is effective for revenue arrangements entered into in fiscal years beginning on or after June 15, 2010. The adoption of this new guidance did not have a material effect on the Company’s consolidated financial statements, nor does management expect it to have a material impact on the Company’s expected future revenues.
Fair Value
In January 2010, the FASB issued ASU 2010-6, Improving Disclosures About Fair Value Measurements (“ASU 2010-6”), which requires reporting entities to make new disclosures about recurring or nonrecurring fair-value measurements, including significant transfers into and out of Level 1 and Level 2 fair-value measurements and information on purchases, sales, issuances, and settlements on a gross basis in the reconciliation of Level 3 fair-value measurements. ASU 2010-6 is effective for annual reporting periods beginning after December 15, 2009, except for Level 3 reconciliation disclosures which are effective for annual periods beginning after December 15, 2010. The adoption of ASU 2010-6 did not have a material impact on the Company’s consolidated financial statements.
Accounting Standards Not Yet Effective
In May 2011, the FASB issued ASU No. 2011-04, Fair Value Measurements and Disclosures (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and International Financial Reporting Standards (“ASU 2011-04”) to provide a consistent definition of fair value and ensure that the fair value measurement and disclosure requirements are similar between U.S. GAAP and International Financial Reporting Standards. ASU 2011-04 changes certain fair value measurement principles and enhances the disclosure requirements, mainly for level 3 fair value measurements. ASU 2011-04 is effective for the Company’s fiscal year beginning January 1, 2012. Early adoption is not permitted. The Company is currently evaluating the impact of this new guidance, but does not expect it to have a material impact on the consolidated financial statements.
F-29
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
In June 2011, FASB issued ASU 2011-05, Comprehensive Income (Topic 220): Presentation of Comprehensive Income (“ASU 2011-05”). ASU 2011-05 will require companies to present the components of net income and other comprehensive income either as one continuous statement or as two consecutive statements. It eliminates the option to present components of other comprehensive income as part of the changes in stockholders’ equity. The standard does not change the items which must be reported in other comprehensive income, how such items are measured or when they must be reclassified to net income. ASU 2011-05 is effective for interim and annual periods beginning after December 15, 2011, with early adoption permitted. The adoption of ASU 2011-05 will only impact presentation and will not have any effect on the Company’s consolidated financial statements or on its financial condition.
Note 2 Going Concern
As of September 30, 2011, the Company was in the development stage as it continues to develop its products and has not yet recognized any revenues. The Company intends to transition from the development stage to operational status during 2012 depending upon the timing and its success in achieving its business development milestones. The Company’s consolidated financial statements are presented on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business.
The Company has never been profitable and has incurred significant losses and cash flow deficits. For the nine months ended September 30, 2011 and 2010, and the period from September 22, 2006 (inception) to September 30, 2011, it reported net losses before non-controlling interest of $21.9 million, $24.3 million, and $133.1 million, respectively, and negative cash flow from operating activities of $13.4 million, $17.1 million, and $80.7 million, respectively. As of September 30, 2011, the Company had an aggregate accumulated deficit of $117.1 million. The Company anticipates that it will continue to report losses and negative cash flow during the remainder of 2011. As a result of these net losses, cash flow deficits, and other factors, there is a substantial doubt about the Company’s ability to continue as a going concern.
The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. The Company’s ability to continue as a going concern is dependent upon generating sufficient cash flow from operations and obtaining additional capital and financing. The Company has been, and continues to be, wholly dependent on funding from its principal shareholder. The principal amount of debt obligations as of September 30, 2011 was $72.1 million. The principal balance of notes payable—related party and the interest payable thereon is due June 30, 2012. The Company has cash and cash equivalents of $6.7 million as of September 30, 2011 and anticipates having sufficient working capital to continue in business through June 30, 2012. However, if the Company’s ability to generate cash flow from operations is delayed beyond June of 2012 and it is unable to raise additional funding (including from its principal shareholder) and/or amend the term of its notes payable—related party, the Company will be unable to continue its business beyond that date.
F-30
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
Note 3 Other Non-Current Assets
Other non-current assets consists of legal and accounting costs associated with the Company’s filing of a registration statement with the SEC. These costs were capitalized with the intent of reclassifying them as a reduction of the resulting proceeds when the securities offering was successfully completed. During the fourth quarter of 2010, the Company reduced the originally incurred and capitalized costs by $0.5 million with a charge to selling, general and administrative costs. As of June 30, 2011, over 90 days had passed without substantive progress on the planned offering. As a result, based upon the application of SEC Staff Accounting Bulletin 99-1, topic 5A, the remaining offering costs of $0.8 million were charged to expense during the period and classified as selling, general and administrative costs during the second quarter of 2011.
Note 4 Accrued Expenses
Accrued expenses are comprised of the following components:
| September 30, 2011 |
December 31, 2010 |
|||||||
| Accrued payroll and bonus |
$ | 680,004 | $ | 806,860 | ||||
| Accrued vacation liability |
281,896 | 579,810 | ||||||
| Accrued legal, accounting and engineering fees |
50,147 | 497,265 | ||||||
| Other accruals |
219,238 | 305,626 | ||||||
|
|
|
|
|
|||||
| Total Accrued Expenses |
$ | 1,231,285 | $ | 2,189,561 | ||||
|
|
|
|
|
|||||
Note 5 Other Current Liabilities
In December 2009, the Company entered into an agreement with Green Science Energy, LLC (the “Licensee”) pursuant to which it received $2.0 million in exchange for the Licensee’s right to market and sell sub-licenses in Egypt and Morocco. In January 2011, the Company received timely notice from the Licensee of its election to surrender these licensing rights and to comply with related provisions of the original contract in exchange for the return of the $2.0 million it had paid, which the Company had placed in escrow. Accordingly, the $2.0 million was disbursed from escrow to the Licensee in January 2011.
F-31
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
Note 6 Notes Payable—Related Party
During the nine months ended September 30, 2011, PetroTech Holdings Corp. (“PetroTech”), a holding company controlled by the Company’s principal shareholder, funded a total of $19.6 million to PA LLC pursuant to the terms of separate senior secured term notes, all of which are included in the $42.0 million notes payable in the following table. Notes payable—related party consist of the following as of:
| September 30, 2011 |
December 31, 2010 |
|||||||
| Note Payable to Valens U.S. SPV I, LLC |
$ | 417,512 | $ | 417,512 | ||||
| • Interest accrues monthly, at an annual rate of 12% |
||||||||
| • Note is due on June 30, 2012 |
||||||||
| Notes Payable to PetroTech |
42,047,089 | 22,422,089 | ||||||
| • Interest accrues monthly at an annual rate of 12% |
||||||||
| • Notes are due on June 30, 2012 |
||||||||
| Convertible Note Payable to PetroTech |
10,000,000 | 10,000,000 | ||||||
| • Interest accrues monthly, at an annual rate of 12% |
||||||||
| • Note is due on June 30, 2012 unless converted to common shares as described below |
||||||||
| Up to $25,000,000 Note Payable to PetroTech |
19,588,014 | 18,823,133 | ||||||
| • Interest payable monthly, and is drawn into note on a monthly basis at Prime + 2% (5.25% at September 30, 2011 and December 31, 2010) |
||||||||
| • Note is due on June 30, 2012 |
||||||||
| • Permits additional draws to fund interest. Maximum balance of this note is limited to $25,000,000 |
||||||||
|
|
|
|
|
|||||
| Total |
$ | 72,052,615 | $ | 51,662,734 | ||||
|
|
|
|
|
|||||
As of September 30, 2011, the principal balance of notes payable—related party has been reclassified to current liabilities on the accompanying consolidated balance sheet as the maturity date is within one year. The notes payable—related party are secured by all of the Company’s assets. The convertible note payable to PetroTech allows the holder (at the holder’s option) to convert all or any portion of the issued and outstanding principal amount and/or accrued interest then due ($12,922,142 as of September 30, 2011) into shares of the Company’s common stock at a fixed conversion price of $5.43 per share.
Each note payable and the related master security agreement and equity pledge and corporate guaranty agreement contain provisions that specify events of default which could lead to acceleration of the maturity of the debt. These provisions prohibit the encumbrance or sale and require maintenance and insurance of the Company’s assets. The loan agreements do not contain any
F-32
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
required financial ratios or similar debt covenants. Generally, an event of default would arise if the Company became insolvent, filed for bankruptcy, allowed a change in control or had unresolved judgments against its assets. Through the date of this quarterly report on Form 10-Q, none of these events have occurred.
As of September 30, 2011 and December 31, 2010, interest in the amount of $8.7 million and $4.8 million, respectively, was accrued related to the notes payable—related party and is recorded in accrued expenses—related party on the accompanying consolidated balance sheets. During the nine months ended September 30, 2011, additional accrued interest of $0.8 million was added to the principal balance of the floating-rate note and is recorded in notes payable—related party on the accompanying consolidated balance sheets.
During the three months ended September 30, 2011 and 2010, interest charged to operations on these notes was $1.8 million and $1.1 million, respectively. Interest charged to operations on these notes during the nine months ended September 30, 2011 and 2010, and the period from September 22, 2006 (inception) to September 30, 2011 was $4.6 million, $2.9 million, and $14.1 million, respectively. No interest was capitalized during any of these periods.
Note 7 Stockholders’ Deficit
The Company’s equity consists of 300,000,000 shares of $.001 par value common stock, of which 106,920,730 and 106,933,230 shares had been issued and were outstanding as of September 30, 2011 and December 31, 2010, respectively. In addition, the Company may issue up to 25,000,000 shares of preferred stock. No preferred stock is outstanding.
Estimation of Common Stock Fair Value
In each reporting period, the Company has determined its best estimate of the fair value of its common stock by considering the following indicators of value:
| Ÿ | Level 3 valuations based upon a discounted cash flow analysis using inputs which were not externally observable, in this case the Company’s estimate of future cash flows from the Company’s business; and |
| Ÿ | Prices paid in cash for shares of stock or securities units comprised of equity shares and five-year warrants to purchase equity shares; and |
| Ÿ | Over-The-Counter (“OTC”) quotation closing price and related statistics such as trading volume, volatility and recent price ranges. |
Level 3 estimated cash flow valuations. The Level 3 fair value estimate of the Company’s equity value was determined by applying discounted cash flow valuation techniques to expected future cash flows from the Company’s business as of each valuation date. Key assumptions utilized in this fair value estimate include the expected amount and timing of revenue from expected future sales of the Company’s technology licenses along with the associated cost of sales and other operating cash outflows. These cash flows are discounted to present value using a discount rate consistent with development stage technology companies and the resulting enterprise value was converted to an equity value per outstanding share.
F-33
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
The most significant uncertainty inherent in these calculations is the risk of obtaining and performing the contracts necessary to produce the estimated future revenues. In addition, the risk of collection of amounts that are expected to be due under projected contracts, the risk of unanticipated product development or delivery costs or timing delays and the risk of increased operating and overhead costs may each cause the estimates of future net cash flows from operations to vary from the assumptions that are made in these projections, and these variations may be material. Since the Company has not obtained or performed any commercial contracts to date, it does not have historical data on which to base its estimate of future revenues.
Because the Level 3 indications of value were able to take into account important information pertaining to the amount, timing and risk of expected Company cash flows, they were given the most weight in the Company’s estimate of share value.
Third party transactions. From January 2009 through August 2010, the Company completed several private sales of equity shares and securities units comprised of both shares and warrants to purchase shares. The transactions were completed with both related and unrelated parties in exchange for cash. Given the size of these transactions and the sophisticated nature of the parties involved in their negotiation, these indications of value were given significant weight in the Company’s estimate of share value. However, because most of these transactions were with affiliates, sole or primary reliance was not placed on these values.
OTC Values. The Company’s common stock is currently traded on OTC Link, which is operated by OTC Markets Group Inc. (formerly known as Pink OTC Markets Inc. or Pink Sheets). A very small percentage of the issued and outstanding shares are available for active trading since approximately 94% of the Company’s outstanding shares are held by its controlling shareholder. Due to the very limited size of the Company’s public float and the resulting low trading volume in the Company’s common stock, its public shares are susceptible to very large swings in price based on very few trades. As a result of these considerations, value indications provided by the OTC price quotation service were given the least weight of the three factors in the Company’s estimate of share value.
Estimation of Warrant Fair Value
The Company estimated the fair value of warrants issued during 2010 using the Black-Scholes valuation model, based on the estimated fair value of the common stock on the valuation date, an expected dividend yield of 0%, a risk-free interest rate based on constant maturity rates published by the U.S. Federal Reserve applicable to the remaining term of the instruments, an expected life equal to the remaining term of the instruments and an estimated volatility of 75.2%. No warrants have been issued during 2011.
Issuance of Common Stock and Warrants
On November 1, 2010, in anticipation of Mr. Rob Harris’ impending employment as President and Chief Operating Officer, the Company issued and sold 12,500 shares of its common stock to Mr. Harris at a price of $8.00 per share. Mr. Harris also received a put option which gave him the option to sell any or all of the shares back to the Company for a purchase price of $8.00 at any time within one year of the original transaction. In accordance with Accounting Standards Codification (“ASC”) 480-10-25-8,
F-34
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
the proceeds were recorded as liabilities related to equity issuance on the accompanying consolidated balance sheet as of December 31, 2010. During January 2011, Mr. Harris exercised his option and the Company repurchased all 12,500 shares of common stock for $100,000.
PA LLC Equity Incentive Plan and Non-controlling Interest
PA LLC has an equity compensation plan which allows it to grant employees and consultants awards of profits interests in restricted Class B ownership units (the “Interests”) and is limited to 14% of the total outstanding units.
The Company granted 2,816,471 and 674,500 Interests during 2008 and 2007, respectively, to the majority of its employees as of the date that they began employment with the Company. As of September 30, 2011 and December 31, 2010, the granted Interests, net of Interests forfeited and repurchased by the Company, totaled approximately 2,754,571 and 2,837,691, respectively, or 12.7% and 13.0%, respectively, of total outstanding units. The Interests give the recipient the right to participate in the income of PA LLC and any distribution that may arise from a liquidity event to the extent that such realized amounts exceed the ownership unit fair value at the date of grant.
The Company determined the fair value of these grants by estimating the proceeds that it may obtain from a range of possible future liquidity events such as a public equity offering or a Company sale. The timing of such liquidity events and the likelihood of their achievement was estimated based upon the information that existed as of the grant or valuation date. The weighted average future cash value was then discounted using an annual discount rate of 60% and the weighted average time from grant or valuation date to the expected liquidity event. This estimated cash present value was converted to a per share cash value and, based on the structure of the grant, the carrying amount per Interest was subtracted to determine the fair value of each of the Interests.
The grants of Interests contain restrictions that allow the Company to repurchase the units for $0.01 per unit until a service period or other condition is met. This restriction is removed over a service period (generally four years) with regard to approximately 2.15 million Interests. On March 4, 2011, the Company modified the terms of the Interests granted to a former employee, causing the Interests to be immediately vested. This decision required a revaluation of the Interests and immediate recognition of additional compensation cost in the amount of $0.8 million, recorded as research and development expense, based upon the nature of the former employee’s role with the Company.
For the three months ended September 30, 2011 and 2010, compensation expense related to these Interests was $0.1 million and $0.3 million, respectively. For the nine months ended September 30, 2011 and 2010 and the period from September 22, 2006 (inception) to September 30, 2011, compensation expense related to these Interests was $1.1 million, $1.2 million, and $18.7 million, respectively. The related compensation expense is recognized as selling, general and administrative expense or research and development expense depending upon the recipient’s role in the Company. Amounts recognized as selling, general and administrative expense for the three months ended September 30, 2011 and 2010 were $0.0 million and $0.1 million, respectively. For the nine months ended September 30, 2011 and 2010 and the period from September 22, 2006 (inception) to September 30, 2011, compensation expense recognized as selling, general and administrative expense related to these Interests was $0.0 million, $0.6 million, and $12.4 million, respectively.
F-35
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
Amounts recognized as research and development expense for the three months ended September 30, 2011 and 2010 were $0.1 million and $0.2 million, respectively. For the nine months ended September 30, 2011 and 2010 and the period from September 22, 2006 (inception) to September 30, 2011, compensation expense recognized as research and development expense related to these Interests was $1.1 million, $0.6 million, and $6.3 million, respectively.
Unrecognized compensation cost related to 51,627 Interests which remain restricted totaled approximately $0.3 million as of September 30, 2011 and is expected to be expensed over the next 13 months. An aggregate of 83,000, 67,000, and 736,000 of these Interests were forfeited during the nine months ended September 30, 2011 and 2010, and the period from September 22, 2006 (inception) to September 30, 2011, respectively.
Five senior officers were granted an aggregate 1.34 million Interests that were subject to repurchase for $0.01 per unit until a significant liquidity event occurred. As of December 31, 2010, the Company’s principal shareholder authorized the modification of the terms of the grant to remove the right of the Company to repurchase the Interests held by all five officers, causing these grants to be immediately vested. This decision required a revaluation of the Interests held by the officers and immediate recognition of the calculated expense. Based upon current estimates and applying the same discounted cash flow methodology used as of the prior valuation dates, the compensation cost recognized during the fourth quarter of 2010 was $11.4 million, recorded as selling, general and administrative expense ($9.1 million) or research and development expense ($2.3 million) depending upon each officer’s role in the Company.
As of September 30, 2011 and December 31, 2010, non-controlling interests collectively owned approximately 12.7% and 13.0%, respectively, of PA LLC, and have absorbed their respective portion of the loss of PA LLC. The amount of loss absorbed was $0.8 million and $1.0 million for the three months ended September 30, 2011 and 2010, respectively. The amount of loss absorbed was $2.8 million, $3.8 million, and $16.0 million for the nine months ended September 30, 2011 and 2010, and for the period from September 22, 2006 (inception) to September 30, 2011.
Stock Options
On June 17, 2009, the Company adopted the 2009 Equity Compensation Plan (the “2009 Plan”). The 2009 Plan is intended to provide employees, consultants and others the opportunity to receive incentive stock options and is limited to 4,000,000 shares of the Company’s common stock. The exercise price shall be equal to or greater than the fair value of the underlying common stock on the date the option is granted and its term shall not exceed 10 years. Awards shall not vest in full prior to the third anniversary of the award date.
On January 1, 2011, the Company granted its employees 623,000 options, in the aggregate, to purchase common shares at an exercise price of $8.00 per share. The options generally vest over a period of four years from the grant date and expire 10 years from the grant date. The grant date fair value of the options aggregated to $1.9 million and was calculated using the following assumptions determined as of the date of grant: estimated fair value of the common stock of $5.10, expected term of 6.25 years, risk free interest rate of 2.0%, an estimated volatility of 75.2%, and a dividend yield of 0%. The expected term of options granted to employees was based upon the simplified method
F-36
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
allowed for “plain vanilla” options as described by SEC SAB No. 107. The fair value will be charged to operations over the remaining vesting periods commencing on the employee’s hire date or the grant date, depending upon terms of individual grants.
On June 30, 2011, the Company’s board of directors authorized the re-pricing of all outstanding stock options, changing the exercise price from $8.00 or $8.50 to $5.50 per option. In accordance with ASC 718-20-35-3, the Company computed the additional compensation cost as the fair value of the new awards in excess of the fair value of the original awards immediately before their terms were modified, using the Black-Scholes pricing model. This calculation used the following assumptions determined as of June 30, 2011: estimated fair value of the common stock of $5.10, remaining terms ranging from 4.75 years to 5.89 years (depending on the date of the original grant), risk free interest rate of 1.76%, an estimated volatility of 96.5%, and a dividend yield of 0%. The modification affected 1,240,000 options and resulted in aggregate additional compensation cost of $0.4 million. Of the total additional compensation cost, $0.2 million was expensed immediately as it related to options which were vested as of June 30, 2011, and $0.2 million will be expensed through 2014.
Due to the significant number of equity transactions that occurred during June of 2011, including the stock option modification discussed above and the stock option and the stock appreciation rights issuances discussed below, the Company re-examined its volatility estimate and consequently increased it from 75.2% to 96.5% on a prospective basis. The Company estimates volatility in accordance with SEC Staff Accounting Bulletin (“SAB”) No. 107, “Share-based Payment” based on an analysis of expected volatility of share trading prices for a peer group of companies. Over a period of time similar to that of the expected option life, the volatility in share price of these alternative energy and clean-tech companies averaged 70.4%. To account for the fact that the Company is in the development stage and can be expected to have a higher volatility at its present stage than the average of the comparable companies, the Company adjusted upward its volatility estimate. Specifically, the Company estimated its share volatility by calculating the average volatility of those members of its peer group that exhibited volatility measures in the top quartile of the group. Using this approach, the Company determined that a volatility estimate of 96.5% is appropriate in its fair value calculations.
On June 30, 2011, the Company’s board of directors authorized the grant of 616,000 options to employees, in the aggregate, to purchase common shares at an exercise price of $5.50 per share. Of the 616,000 options authorized, 351,000 were granted on June 30, 2011 and the remaining 265,000 were granted on July 21, 2011. The options generally vest over a period of four years from the grant date and expire 10 years from the grant date. The fair value of the options when granted aggregated to $2.4 million and was calculated using the Black-Scholes pricing model with the following assumptions determined as of the date of grant: estimated fair value of the common stock of $5.10, expected term of 6.25 years, risk free interest rate of 1.76%, an estimated volatility of 96.5%, and a dividend yield of 0%. The expected term of options granted to employees was based upon the simplified method allowed for “plain vanilla” options as described by SEC SAB No. 107. The fair value will be charged to operations over the remaining vesting periods commencing on the employee’s hire date or the grant date, depending upon terms of individual grants.
The weighted average period over which options not vested as of September 30, 2011 are expected to vest is 38 months. During the three months ended September 30, 2011 and 2010, approximately $0.2 million and $0.2 million, respectively, was charged to operations related to all of
F-37
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
these outstanding options. During the nine months ended September 30, 2011 and 2010, and for the period from September 22, 2006 (inception) to September 30, 2011, approximately $0.7 million, $0.7 million, and $2.2 million, respectively, was charged to operations related to all of these outstanding options. The fair value related to unexercisable stock options issued as of September 30, 2011 totaled approximately $4.1 million and is expected to be expensed over the next four years.
A summary of stock option activity is as follows:
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Fair Value |
||||||||||
| Balance at December 31, 2008 |
— | — | — | |||||||||
| Granted June 2009 |
1,072,500 | $ | 5.50 | $ | 2.70 | |||||||
| Granted July 2009 |
45,000 | $ | 5.50 | $ | 2.83 | |||||||
| Forfeited |
(200,000 | ) | $ | (5.50 | ) | $ | (2.70 | ) | ||||
|
|
|
|||||||||||
| Balance at December 31, 2009 |
917,500 | $ | 5.50 | $ | 2.37 | |||||||
| Granted January 2010 |
322,000 | $ | 5.50 | $ | 3.80 | |||||||
| Forfeited |
(247,750 | ) | $ | (5.50 | ) | $ | (2.95 | ) | ||||
|
|
|
|||||||||||
| Balance at December 31, 2010 |
991,750 | $ | 5.50 | $ | 2.68 | |||||||
| Granted January 2011 |
623,000 | $ | 5.50 | $ | 3.27 | |||||||
| Granted June 2011 |
351,000 | $ | 5.50 | $ | 3.96 | |||||||
| Granted July 2011 |
265,000 | $ | 5.50 | $ | 3.96 | |||||||
| Forfeited |
(530,000 | ) | $ | (5.50 | ) | $ | (2.92 | ) | ||||
|
|
|
|||||||||||
| Balance at September 30, 2011 |
1,700,750 | $ | 5.50 | $ | 3.47 | |||||||
|
|
|
|||||||||||
The weighted average grant date fair value for vested options as of September 30, 2011 was $3.11. The weighted average grant date fair value for unvested options as of September 30, 2011 was $3.66.
The weighted average contractual life of outstanding stock options at September 30, 2011 was 8.7 years. The weighted average contractual life of vested and unvested stock options at September 30, 2011 was 7.2 years and 9.4 years, respectively. As of September 30, 2011, a total of 574,917 of the options granted were exercisable and the fair value of such options was $1.8 million. Because the Company has very little history from which to estimate forfeiture of options or grants, it accounts for such forfeitures prospectively, that is, in the period in which they actually occur.
Inputs to both the Interests fair value calculation and the stock option Black-Scholes model are subjective and generally require significant judgment to determine. If, in the future, the Company determines that another method for calculating the fair value of its stock-based compensation is more reasonable, or if another method for calculating these input assumptions is prescribed by authoritative guidance, the fair value calculated for stock-based compensation could change significantly. Regarding stock options, higher volatility and longer expected terms generally result in an increase to stock-based compensation expense determined at the date of grant.
F-38
Table of Contents
PetroAlgae Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements—(Continued)
September 30, 2011
(Unaudited)
As of September 30, 2011, the aggregate intrinsic value of all stock options outstanding and expected to vest was $0.00 and the aggregate intrinsic value of currently exercisable stock options was $0.00. The intrinsic value of each option share is the difference between the estimated fair value of the Company’s stock and the exercise price of such option.
No stock options have been exercised as of September 30, 2011.
Stock Appreciation Rights
In November 2010, the Company issued one million Stock Appreciation Rights (“SARs”) to Mr. Harris, its President and Chief Operating Officer, under the 2009 Plan. The vesting of these SARs was tied to continued employment with the Company and the accomplishment of certain milestones. Mr. Harris resigned from the Company during the first quarter of 2011, forfeiting all such SARs. The impact on all periods presented was not material.
In June 2011, the board of directors authorized the grant of one million SARs at a base grant price of $5.50 per share to its newly appointed Chief Executive Officer under the 2009 Plan. The SARs have a ten-year term and will vest in equal quarterly installments over a two-year period. In the event of a change of control (as defined in the 2009 Plan) or a qualified public offering (as defined in the executive’s employment agreement), the SARs will become 100% vested. The grant date fair value of the SARs aggregated to $3.8 million and was calculated using the Black-Scholes pricing model with the following assumptions determined as of the date of grant: estimated fair value of the common stock of $5.10, expected term of 5.75 years, risk free interest rate of 1.55%, an estimated volatility of 96.5%, and a dividend yield of 0%. The fair value will be charged to operations over the remaining vesting periods commencing on the grant date. For the three and nine months ended September 30, 2011, compensation expense related to these SARs of $0.5 million and $0.6 million, respectively, was charged to selling, general and administrative expense.
Note 8 Related Party Transactions
As described in Note 6, the Company’s principal shareholder has funded notes payable in support of the Company’s operations. These notes payable provide for the accrual of interest through their June 30, 2012 maturity date and $8.7 million and $4.8 million, of such accrued interest is included in accrued expenses-related party as of September 30, 2011 and December 31, 2010, respectively. In addition, the Company’s principal shareholder has invoiced the Company for loan and corporate oversight expenses as well as out-of-pocket costs related to strategic and capital markets assistance. During the nine months ended September 30, 2011, the amount of such reimbursable costs invoiced to the Company was $0.7 million, which the Company has recorded as selling, general and administrative expense. The total amount of such accrued expenses as of September 30, 2011 was $0.9 million and is included in accrued expenses-related party on the accompanying consolidated balance sheet. The Company’s principal shareholder has indicated that it will not require repayment of these accrued amounts until significant new funding is obtained from an unaffiliated source.
Note 9 Subsequent Events
On October 5, 2011, PetroTech funded $2.2 million to PA LLC pursuant to the terms of a senior secured term note. The note provides for interest at an annual rate of 12%, which is accrued and added to accrued expenses—related party, and is due on June 30, 2012.
F-39
Table of Contents
Shares
PetroAlgae Inc.
Common Stock

Goldman, Sachs & Co.
UBS Investment Bank
Citigroup
Baird
Cowen and Company
Through and including , 2012 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Table of Contents
PART II INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses Of Issuance And Distribution |
The following table sets forth all expenses, other than the estimated underwriting discounts and commissions, payable by us in connection with this offering. All the amounts shown are estimates except the SEC registration fee, the Financial Industry Regulatory Authority, or FINRA, filing fee and the listing fee.
| SEC registration fee |
$14,260 | |
| FINRA filing fee |
$20,500 | |
| listing fee |
* | |
| Printing and engraving |
* | |
| Legal fees and expenses |
* | |
| Accounting fees and expenses |
* | |
| Transfer agent and registrar fees |
* | |
| Miscellaneous fees and expenses |
* | |
|
| ||
| Total |
* |
| * | To be included by amendment. |
| Item 14. | Indemnification Of Directors And Officers |
Pursuant to Section 145 of the Delaware General Corporation Law (the “DGCL”), a corporation shall have the power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than a derivative action by or in the right of such corporation) by reason of the fact that such person is or was a director, officer, employee or agent of such corporation, or serving at the request of such corporation in such capacity for another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, if such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of such corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
The DGCL also permits indemnification by a corporation under similar circumstances for expenses (including attorneys’ fees) actually and reasonably incurred by such persons in connection with the defense or settlement of a derivative action or suit, except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to such corporation unless the Delaware Court of Chancery or the court in which such action or suit was brought shall determine upon application that such person is fairly and reasonably entitled to indemnity for such expenses which such court shall deem proper.
To the extent a present or former director or officer is successful in the defense of such an action, suit or proceeding referenced above, or in defense of any claim, issue or matter therein, a corporation is required by the DGCL to indemnify such person for actual and reasonable expenses incurred in connection therewith. Expenses (including attorneys’ fees) incurred by such persons in defending any action, suit or proceeding may be paid in advance of the final disposition of such action, suit or proceeding upon, in the case of a current officer or director, receipt of an undertaking by or on behalf of such person to repay such amount if it is ultimately determined that such person is not entitled to be so indemnified.
The DGCL provides that the indemnification described above shall not be deemed exclusive of other indemnification that may be granted by a corporation pursuant to its bylaws, disinterested directors’ vote, stockholders’ vote and agreement or otherwise.
II-1
Table of Contents
Section 102(b)(7) of the DGCL enables a corporation, in its certificate of incorporation or an amendment thereto, to eliminate or limit the personal liability of a director to the corporation or its stockholders for monetary damages for violations of the directors’ fiduciary duty, except (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL (providing for liability of directors for unlawful payment of dividends or unlawful stock purchases or redemptions) or (iv) for any transaction from which a director derived an improper personal benefit. The Registrant’s amended and restated certificate of incorporation provides for such limitations on liability for its directors.
The DGCL also provides corporations with the power to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation in a similar capacity for another corporation, partnership, joint venture, trust or other enterprise, against any liability asserted against him or her in any such capacity or arising out of his or her status as such, whether or not the corporation would have the power to indemnify him or her against such liability as described above. The Registrant currently maintains liability insurance for its directors and officers. In connection with this offering, the Registrant will obtain additional liability insurance for its directors and officers. Such insurance would be available to its directors and officers in accordance with its terms.
The Registrant’s restated certificate of incorporation permits the Registrant to (A) indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative, or investigative (other than an action by or in the right of the Registrant), by reason of the fact that he is or was a director, officer, employee, fiduciary or agent of the Registrant or is or was serving at the request of the Registrant as a director, officer, employee, fiduciary or agent of another corporation, partnership, joint venture, trust, or other enterprise, against expenses (including attorney fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by him in connection with such action, suit, or proceeding, if he acted in good faith and in a manner he reasonably believed to be in the best interests of the Registrant and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. The termination of any action, suit, or proceeding by judgment, order, settlement, or conviction or equivalent shall not of itself create a presumption that the person did not act in good faith and in a manner which he reasonably believed to be in the best interests of the Registrant and, with respect to any criminal action or proceeding, had reasonable cause to believe his conduct was unlawful; (B) indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending, or completed action or suit by or in the right of the Registrant to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee, or agent of the Registrant or is or was serving at the request of the Registrant as a director, officer, employee, fiduciary or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses (including attorney fees) actually and reasonably incurred by him in connection with the defense or settlement of such action or suit if he acted in good faith and in a manner he reasonably believed to be in the best interests of the Registrant; but no indemnification shall be made in respect of any claim, issue, or matter as to which such person has been adjudged to be liable for negligence or misconduct in the performance of his duty to the Registrant unless and only to the extent that the court in which such action or suit was brought determines upon application that, despite the adjudication of liability, but in view of all circumstances of the case, such person is fairly and reasonably entitled to indemnification for such expenses which such court deems proper; (C) indemnify a director, officer, employee, fiduciary or agent of a corporation to the extent he has been successful on the merits in defense of any action, suit, or proceeding referred to in (A) or (B) above or in defense of any claim, issue, or matter therein, against expenses (including attorney fees) actually and reasonably incurred by him in connection therewith.
II-2
Table of Contents
Any indemnification under (A) or (B) above (unless ordered by a court) and as distinguished from (C) above will be made by the Registrant only as authorized in the specific case upon a determination that indemnification of the director, officer, employee, fiduciary or agent is proper in the circumstances because he has met the applicable standard of conduct set forth in (A) or (B) above. Such determination shall be made by the Registrant’s board of directors by a majority vote of a quorum consisting of directors who were not parties to such action, suit, or proceeding, or, if such a quorum is not obtainable or, even if obtainable, if a quorum of disinterested directors so directs, by independent legal counsel in a written opinion, or by the shareholders.
Expenses (including attorney fees) incurred in defending a civil or criminal action, suit, or proceeding may be paid by the Registrant in advance of the final disposition of such action, suit, or proceeding upon receipt of an undertaking by or on behalf of the director, officer, employee, fiduciary or agent to repay such amount unless it is ultimately determined that he is entitled to be indemnified by the Registrant as authorized in its restated certificate of incorporation.
The foregoing statements are subject to the full text of the Registrant’s restated certificate of incorporation, which is filed as Exhibit 3.1 hereto. Reference is made to the form of underwriting agreement to be filed as Exhibit 1 hereto for provisions providing that the underwriters are obligated under certain circumstances, to indemnify our directors, officers and controlling persons against certain liabilities under the Securities Act of 1933, as amended.
| Item 15. | Recent Sales Of Unregistered Securities |
| Ÿ | On November 1, 2010, we issued and sold 12,500 shares of our common stock to Rob Harris for a purchase price of $8.00 per share for an aggregate purchase price of $100,000. In February 2011, Rob Harris exercised his right to have us repurchase 12,500 shares of common stock for $100,000. |
| Ÿ | On August 6, 2010, we issued and sold 10,000 shares of our common stock to Jeff Peterson for a purchase price of $8.00 per share and a warrant to purchase 10,000 shares of our common stock at an exercise price of $15.00 per share for an aggregate purchase price of $80,000. |
| Ÿ | On June 29, 2010, we issued and sold 81,250 shares of our common stock to CNW Investment Company LLC for a purchase price of $8.00 per share and a warrant to purchase 81,250 shares of our common stock at an exercise price of $15.00 per share for an aggregate purchase price of $650,000. |
| Ÿ | On May 6, 2010, April 7, 2010, March 1, 2010, October 9, 2009, September 29, 2009, September 14, 2009, September 4, 2009 and August 28, 2009, we issued and sold in the aggregate (i) 686,625 shares of our common stock to Valens U.S. SPV I, LLC for a purchase price of $8.00 per share and warrants to purchase 686,625 shares of our common stock at an exercise price of $15.00 per share, and (ii) 563,375 shares of our common stock to Valens Offshore SPV I, Ltd. for a purchase price of $8.00 per share and warrants to purchase 563,375 shares of our common stock at an exercise price of $15.00 per share. The aggregate proceeds from these private placements was $10,000,000. |
| Ÿ | On December 22, 2008, we issued and sold 666,667 shares of our common stock to Valens U.S. SPV I, LLC for a purchase price of $3.15 per share and 2,507,936 shares of our common stock to Valens Offshore SPV I, Ltd. for a purchase price of $3.15 per share for consideration of $2,100,000 and $7,900,000, respectively. |
| Ÿ | On March 1, 2010, we issued and sold 12,500 shares of common stock to Crale Realty LLC for a purchase price of $8.00 per share and a warrant to purchase 12,500 shares of our common stock at an exercise price of $15.00 per share for an aggregate purchase price of $100,000. |
II-3
Table of Contents
| Ÿ | On February 6, 2010, we issued and sold 6,250 shares of our common stock to Lynn November for a purchase price of $8.00 per share and a warrant to purchase 6,250 shares of our common stock at an exercise price of $15.00 per share for an aggregate purchase price of $50,000. |
| Ÿ | On December 24, 2009, we issued and sold 357,143 shares of our common stock to Green Science Energy LLC and a warrant to purchase 357,143 shares of our common stock at an exercise price of $15.00 per share in exchange for 30% of the outstanding equity interests in Green Science Energy LLC. |
| Ÿ | On November 23, 2009, we issued 9,467 shares of our common stock to Torys LLP as consideration for legal services rendered. |
| Ÿ | On October 9, 2009, we issued and sold 375,000 shares of our common stock to Green Alternative Energy USA, LLC for a purchase price of $8.00 per share and a warrant to purchase 375,000 shares of our common stock at an exercise price of $15.00 per share for an aggregate purchase price of $3,000,000. |
| Ÿ | On October 19, 2009, we issued and sold 500,000 shares of our common stock to UBS AG for a purchase price of $8.00 per share and a warrant to purchase 500,000 shares of our common stock at an exercise price of $15.00 per share for an aggregate purchase price of $4,000,000. |
| Ÿ | On January 15, 2009, we issued 151,057 shares of our common stock to Engineering Automation and Design, Inc. as consideration for services rendered. |
| Ÿ | On December 19, 2008, we issued 1,000,000 shares of our common stock to Nationwide Solutions, Inc., the sole owner of which is Sayan Navaratnam (a director of the Company), as compensation for services rendered under a consulting agreement. |
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering, and the registrant believes the transactions were exempt from the registration requirements of the Securities Act of 1933 in reliance on Section 4(2) thereof, and the rules and regulations promulgated thereunder, as transactions by an issuer not involving a public offering. The recipients of securities in such transactions represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were affixed to the share certificates and instruments issued in such transactions. All recipients of securities above were accredited or sophisticated and either received adequate information about the registrant or had access, through their relationships with the registrant, to such information.
| Item 16. | Exhibits and Financial Statement Schedules |
| (a) | Exhibits. |
See the Exhibit Index beginning on page E-1, which follows the signature pages hereof and is incorporated herein by reference.
| (b) | Financial Statements Schedules |
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the consolidated financial statements or notes thereto.
| Item 17. | Undertakings |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is,
II-4
Table of Contents
therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post- effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Melbourne, State of Florida on the 1st day of December 2011.
| PETROALGAE INC. | ||
| By: |
/s/ Anthony John Phipps Tiarks | |
| Anthony John Phipps Tiarks Chief Executive Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Anthony John Phipps Tiarks and James P. Dietz and each of them, as his true and lawful attorney in fact and agent with full power of substitution, for him in any and all capacities, to sign any and all amendments to this registration statement (including post effective amendments or any abbreviated or subsequent registration statement and any amendments thereto filed pursuant to Rule 462(b) and any supplement to any prospectus included in this registration statement or any such amendment or any abbreviated or subsequent registration statement filed pursuant to Rule 462(b)), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney in fact, proxy and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney in fact, proxy and agent, or his substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated:
| Signatures |
Title |
Date | ||
| /s/ Anthony John Phipps Tiarks Anthony John Phipps Tiarks |
Chief Executive Officer (Principal Executive Officer) |
December 1, 2011 | ||
| /s/ James P. Dietz James P. Dietz |
Chief Financial Officer (Principal Financial and |
December 1, 2011 | ||
| /s/ John S. Scott John S. Scott |
Non-Executive Chairman |
December 1, 2011 | ||
| /s/ Sayan Navaratnam Sayan Navaratnam |
Director |
December 1, 2011 | ||
| /s/ Isaac D. Szpilzinger Isaac D. Szpilzinger |
Director |
December 1, 2011 | ||
II-6
Table of Contents
Exhibit Index
| Exhibit |
Description of Document | |||
| 1.1 | Form of Underwriting Agreement.* | |||
| 3.1 | Restated Certificate of Incorporation of PetroAlgae Inc.* | |||
| 3.2 | Bylaws of PetroAlgae Inc.* | |||
| 4.1 | Specimen Stock Certificate evidencing shares of common stock.* | |||
| 4.2 | Warrant, dated June 29, 2010, with CNW Investment Company LLC* | |||
| 4.3 | Warrant, dated August 6, 2010, with Jeff Peterson* | |||
| 4.4 | Warrant, dated March 1, 2010, with Crale Realty LLC* | |||
| 4.5 | Warrant, dated February 6, 2010, with Lynn November* | |||
| 4.6 | Warrant, dated December 24, 2009, with Green Science Energy LLC* | |||
| 4.7 | Warrant, dated October 9, 2009, with Valens U.S. SPV I, LLC* | |||
| 4.8 | Warrant, dated October 9, 2009, with Valens Offshore SPV Ltd.* | |||
| 4.9 | Warrant, dated October 9, 2009, with Green Alternative Energy USA, LLC* | |||
| 4.10 | Warrant, dated September 29, 2009, with Valens U.S. SPV I, LLC* | |||
| 4.11 | Warrant, dated September 29, 2009, with Valens Offshore SPV I, Ltd.* | |||
| 4.12 | Warrant, dated September 14, 2009, with Valens U.S. SPV I, LLC* | |||
| 4.13 | Warrant, dated September 14, 2009, with Valens Offshore SPV I, Ltd.* | |||
| 4.14 | Warrant, dated September 4, 2009, with Valens U.S. SPV I, LLC* | |||
| 4.15 | Warrant, dated September 4, 2009, with Valens Offshore SPV I, Ltd.* | |||
| 4.16 | Warrant, dated August 28, 2009, with Valens U.S. SPV I, LLC* | |||
| 4.17 | Warrant, dated August 28, 2009, with Valens Offshore SPV I, Ltd.* | |||
| 4.18 | Warrant, dated March 1, 2010, with Valens U.S. SPV I, LLC* | |||
| 4.19 | Warrant, dated March 1, 2010, with Valens Offshore SPV Ltd* | |||
| 4.20 | Warrant, dated April 7, 2010, with Valens U.S. SPV I, LLC* | |||
| 4.21 | Warrant, dated April 7, 2010, with Valens Offshore SPV I, Ltd* | |||
| 4.22 | Warrant, dated May 5, 2010, with Valens U.S. SPV I, LLC* | |||
| 4.23 | Warrant, dated May 5, 2010, with Valens Offshore SPV I, Ltd.* | |||
| 4.24 | Warrant, dated October 19, 2009, with UBS AG* | |||
| 4.25 | Registration Rights Agreement, dated March 9, 2009, between Engineering, Automation & Design, Inc. and the registrant* | |||
| 5.1 | Opinion of Torys LLP regarding legality.* | |||
| 10.1 | 2009 Equity Compensation Plan* | |||
| 10.4 | Securities Purchase Agreement, dated March 1, 2010, with Valens U.S. SPV I, LLC* | |||
| 10.5 | Securities Purchase Agreement, dated March 1, 2010, with Valens Offshore SPV I, Ltd.* | |||
| 10.6 | Securities Issuance Agreement, dated December 24, 2009, with Green Science Energy LLC* | |||
| 10.7 | Master License Agreement, dated December 24, 2009, between PA LLC and Congoo, LLC* | |||
E - 1
Table of Contents
| Exhibit |
Description of Document | |||
| 10.8 | Option Agreement, dated December 24, 2009, between PA LLC and Congoo, LLC* | |||
| 10.9 | Securities Purchase Agreement, dated October 19, 2009, with UBS AG* | |||
| 10.10 | Securities Purchase Agreement, dated October 9, 2009, with Green Alternative Energy USA, LLC* | |||
| 10.11 | Securities Purchase Agreement, dated October 9, 2009, with Valens U.S. SPV I, LLC* | |||
| 10.12 | Securities Purchase Agreement, dated October 9, 2009, with Valens Offshore SPV I, Ltd.* | |||
| 10.13 | Securities Purchase Agreement, dated September 29, 2009, with Valens U.S. SPV I, LLC * | |||
| 10.14 | Securities Purchase Agreement, dated September 29, 2009, with Valens Offshore SPV I, Ltd.* | |||
| 10.15 | Securities Purchase Agreement, dated September 14, 2009, with Valens U.S. SPV I, LLC* | |||
| 10.16 | Securities Purchase Agreement, dated September 14, 2009, with Valens Offshore SPV I, Ltd.* | |||
| 10.17 | Securities Purchase Agreement, dated September 4, 2009, with Valens U.S. SPV I, LLC* | |||
| 10.18 | Securities Purchase Agreement, dated September 4, 2009, with Valens Offshore SPV I, Ltd.* | |||
| 10.19 | Securities Purchase Agreement, dated August 28, 2009, with Valens U.S. SPV I, LLC* | |||
| 10.20 | Securities Purchase Agreement, dated August 28, 2009, with Valens Offshore SPV I, Ltd.* | |||
| 10.21 | Amended and Restated Master Security Agreement, dated July 28, 2009, among PA LLC and PetroAlgae Inc. in favor of LV Administrative Services, Inc. for the benefit of PetroTech Holdings Corp.* | |||
| 10.22 | Joinder Agreement, dated July 28, 2009, by PetroAlgae Inc. and PA LLC and delivered to LV Administrative Services, Inc., as administrative and collateral agent for PetroTech Holdings Corp.* | |||
| 10.23 | Equity Pledge Agreement, dated July 28, 2009, among PetroAlgae Inc. and LV Administrative Services, Inc., as administrative and collateral agent for PetroTech Holdings Corp.* | |||
| 10.24 | PetroAlgae Inc. Guaranty, dated July 28, 2009* | |||
| 10.25 | Omnibus Amendment, Joinder and Reaffirmation Agreement, dated July 28, 2009, among PetroAlgae Inc., PA LLC and LV Administrative Services, as administrative and collateral agent for Valens U.S. SPV I, LLC* | |||
| 10.26 | Second Amended and Restated Term Note, dated July 28, 2009, in the principal amount of $417,511.92, issued by PA LLC to Valens U.S. SPV I, LLC* | |||
| 10.27 | Amended and Restated Master Security Agreement, dated July 28, 2009, among PA LLC and PetroAlgae Inc. in favor of LV Administrative Services, Inc. for the benefit of Valens U.S. SPV I, LLC* | |||
| 10.28 | Joinder Agreement, dated July 28, 2009, by PetroAlgae Inc., PA LLC and PetroTech Holdings Corp. and delivered to LV Administrative Services, Inc., as administrative and collateral agent for Valens U.S. SPV I, LLC* | |||
| 10.29 | Second Amended and Restated Secured Term Note, dated July 28, 2009, in the principal amount of $7,222,089, issued by PA LLC to PetroTech Holdings Corp.* | |||
| 10.30 | Omnibus Amendment, Joinder and Reaffirmation Agreement, dated July 28, 2009, by and among PetroAlgae Inc., PA LLC and LV Administrative Services, Inc., as administrative and collateral agent for PetroTech Holdings Corp.* | |||
E - 2
Table of Contents
| Exhibit |
Description of Document | |||
| 10.31 | Amended and Restated Secured Convertible Note, dated July 28, 2009, in the principal amount of $10,000,000, issued by PA LLC to PetroTech Holdings Corp.* | |||
| 10.32 | Letter Agreement, dated May 12, 2009, with PetroTech Holdings Corp.* | |||
| 10.33 | Amendment One to Master License Agreement, dated April 30, 2009, by and between PA LLC and GTB Power Enterprise, Ltd.*† | |||
| 10.34 | Side Letter to Master License Agreement, dated March 5, 2009, between PA LLC and GTB Power Enterprise, Ltd.* | |||
| 10.35 | Master License Agreement, dated March 5, 2009, by and between PA LLC and GTB Power Enterprise, Ltd.*† | |||
| 10.36 | Stock Purchase Agreement, dated January 15, 2009, between PetroAlgae Inc. and Engineering Automation and Design, Inc.* | |||
| 10.37 | Demand Note, dated December 26, 2008, in the principal amount of $10,000,000, issued by PA LLC to PetroAlgae Inc.* | |||
| 10.38 | Consulting Agreement, dated December 19, 2008, between PetroAlgae Inc. and Nationwide Solutions Inc.* | |||
| 10.39 | Substitute Membership Agreement, dated December 19, 2008, between PetroTech Holdings Corp. and PetroAlgae Inc.* | |||
| 10.40 | Consent Agreement, dated September 24, 2008, made by Arizona Sciences and Technology Enterprises, LLC* | |||
| 10.41 | Consent Agreement, dated August 14, 2008, by and among Arizona Sciences and Technology Enterprises, LLC, PA LLC, LV Administrative Services, Inc. and PetroTech Holdings Corp.* | |||
| 10.42 | Promissory Note, dated June 12, 2008, issued by PA LLC to XL TechGroup, Inc. and assigned to PetroTech Holdings Corp., as amended by the Omnibus Amendment, Joinder and Reaffirmation Agreement, dated July 28, 2009, by and among PetroAlgae Inc., PA LLC and LV Administrative Services, Inc., as administrative and collateral agent for PetroTech Holdings Corp.* | |||
| 10.43 | Amended and Restated Limited Liability Company Agreement of PA LLC, dated February 16, 2007* | |||
| 10.44 | Securities Purchase Agreement, dated April 7, 2010, with Valens U.S. SPV I, LLC* | |||
| 10.45 | Securities Purchase Agreement, dated April 7, 2010, with Valens Offshore SPV I, Ltd.* | |||
| 10.46 | Securities Purchase Agreement, dated May 5, 2010, with Valens U.S. SPV I, LLC* | |||
| 10.47 | Securities Purchase Agreement, dated May 5, 2010, with Valens Offshore SPV I, Ltd.* | |||
| 10.48 | Secured Term Note, dated May 27, 2010, issued by PA LLC to PetroTech Holdings Corp.* | |||
| 10.49 | Secured Term Note, dated June 23, 2010, issued by PA LLC to PetroTech Holdings Corp.* | |||
| 10.50 | Form of Secured Term Note* | |||
| 10.51 | Stockholders Agreement, to be dated the date of this offering, among PetroAlgae Inc., , , and .* | |||
| 10.52 | Employment Agreement, dated as of June 15, 2011, by and among PetroAlgae Inc., PA LLC and Anthony John Phipps Tiarks.* | |||
| 10.53 | Employment Agreement, dated as of July 6, 2011, by and among PetroAlgae Inc., PA LLC and Peter Sherlock.* | |||
E - 3
Table of Contents
| Exhibit |
Description of Document | |||
| 10.54 | Employment Agreement, dated May 10, 2011, between PA LLC and James Dietz.* | |||
| 10.55 | Amended and Restated License Agreement, dated October 26, 2011, between PA LLC and Asesorias e Inversiones Quilicura S.A.* | |||
| 14.1 | Code of Business Conduct and Ethics.* | |||
| 21.1 | Subsidiary of the Registrant.* | |||
| 23.1 | Consent of Grant Thornton LLP, Independent Registered Public Accounting Firm. | |||
| 23.2 | Consent of Torys LLP (included in Exhibit 5.1).* | |||
| 24.1 | Power of Attorney (see page II-6 of this Form S-1). | |||
| 99.1 | Letter Agreement, dated August 15, 2008, between PetroTech Holdings Corp. and XL TechGroup, Inc.* | |||
| * | To be filed by amendment. |
| † | The Securities and Exchange Commission has granted confidential treatment pursuant to Rule 24b-2 under the Exchange Act for portions of this exhibit. In accordance with Rule 24b-2, these confidential portions have been omitted from this exhibit and filed separately with the Securities and Exchange Commission. |
E - 4
