Attached files
| file | filename |
|---|---|
| 10-Q - FORM 10-Q - IOVANCE BIOTHERAPEUTICS, INC. | v241247_10q.htm |
| EX-32.1 - EXHIBIT 32.1 - IOVANCE BIOTHERAPEUTICS, INC. | v241247_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - IOVANCE BIOTHERAPEUTICS, INC. | v241247_ex31-2.htm |
| EX-32.2 - EXHIBIT 32.2 - IOVANCE BIOTHERAPEUTICS, INC. | v241247_ex32-2.htm |
| EX-31.1 - EXHIBIT 31.1 - IOVANCE BIOTHERAPEUTICS, INC. | v241247_ex31-1.htm |
| EXCEL - IDEA: XBRL DOCUMENT - IOVANCE BIOTHERAPEUTICS, INC. | Financial_Report.xls |
Exhibit 10.1
[Lonza Letterhead]
LETTER OF INTENT
This Letter of Intent (“LOI”) is made and entered into as of November __, 2011 (the “Effective Date”) by and between Lonza Walkersville, Inc., a Delaware corporation with an address at 8830 Biggs Ford Road, Walkersville, MD 21793 (“Lonza”) and Genesis Biopharma, Inc., a Nevada corporation with an address at 10880 Wilshire Blvd., Suite 950, Los Angeles, CA 90024 (“Company”). The parties identified above are sometimes hereinafter individually referred to as a “Party” and collectively as the “Parties”.
|
|
1.
|
Lonza is engaged in the contract process development and manufacture of cell-based and biological products.
|
|
|
2.
|
Company is interested to have Lonza perform process development for the production of Cōntego™, an autologous cell therapy using tumor infiltrating lymphocytes for the treatment of Stage IV metastatic melanoma (“Product”) and to manufacture the Product for clinical trials to be performed by the Company.
|
|
|
3.
|
The Parties have decided to enter into this LOI in order to (i) permit technology transfer and process development to begin as of the Effective Date and (ii) expeditiously negotiate in good faith a final and detailed agreement regarding the technology transfer, process development, and manufacture of Product by Lonza (the “Agreement”). The services to be conducted by Lonza pursuant to this LOI are set forth in Exhibit A hereto (the “LOI Services”). Lonza will invoice Company for the LOI Services on a monthly basis and Company shall remit payment therefor on net 30 terms. In no event shall Company request or Lonza perform LOI Services incurring aggregate fees in excess of $500,000.
|
|
|
4.
|
The Parties agree that the LOI Services will be performed in accordance with the Standard Terms & Conditions attached hereto as Exhibit B (the “Terms”). The Terms are hereby incorporated into this Agreement by reference in their entirety.
|
|
|
5.
|
With respect to the negotiations and specific terms and conditions of the Agreement, the Parties wish to record the following intentions:
|
|
|
a.
|
The Parties intend to have (i) the Company transfer to Lonza all information and technology necessary for Lonza to perform process development for manufacture of Product, (ii) Lonza perform process development to optimize and/or develop a manufacturing process for Product, and (iii) Lonza manufacture, under current good manufacturing practices (“cGMP”), bulk purified Product.
|
|
|
b.
|
The LOI Services are expected to commence according to the timeline set forth in Exhibit A. A detailed schedule relating to the conduct of services under the Agreement shall be established between the Parties as part of the Agreement.
|
|
|
c.
|
The Parties intend that this LOI does not address all matters upon which agreement must be reached in order for the Agreement to be in a form and substance satisfactory to Lonza and the Company. The Parties intend that the transaction contemplated by this LOI will be subject to, and the Agreement will include, such terms and conditions as are customary in a transaction of this type, including but not limited to, representations, warranties, conditions, limitations on liability and indemnities.
|
|
|
d.
|
The Parties intend to enter into good faith negotiations, as part of the negotiation of the Agreement, regarding Lonza’s provision of long-term development and manufacturing services to the Company relating to Product, based, in part, upon the non-binding terms in Exhibit C. The Parties intend to commence negotiations on the Agreement as soon as possible upon execution of this LOI by both Parties and intend to execute the Agreement on or prior to January 13, 2012 (the “Target Date”). If the Agreement is not executed on or prior to the Target Date, this LOI shall terminate; provided, that this LOI may be extended once for a thirty (30) day period upon written notice by one Party to the other Party prior to termination.
|
|
|
6.
|
Under this LOI, Company makes a firm capacity reservation for Lonza’s process development and manufacturing capacity as follows:
|
|
|
a.
|
Company and Lonza agree that Company will pay a non-refundable capacity reservation fee of $500,000 (the “Reservation Fee”). Company will pay $250,000 within fourteen (14) days of Company’s signature of this LOI. The company will pay the balance of $250,000 by December 12, 2011. The Reservation Fee will serve as a firm reservation, by Lonza, of sufficient process development and manufacturing capacity/personnel resources to develop or produce Product on behalf of Company in accordance with the estimated time periods set forth herein. The Reservation Fee is non-refundable unless Lonza terminates this LOI under Section 7(iii), in which case Lonza shall refund the Reservation Fee to COMPANY, minus any compensation due Lonza under Section 8.
|
|
|
b.
|
The Reservation Fee is non-refundable, but upon signature of the Agreement, the Reservation Fee will immediately be fully creditable toward any costs or expenses charged to Company in accordance with the terms of the Agreement.
|
|
|
7.
|
This LOI shall be effective as of the Effective Date and will terminate upon the earlier of (i) signature of the Agreement, (ii) expiration of the Agreement negotiation period stated in Section 5.e. without execution of the Agreement, despite the good faith efforts of both Parties, or (iii) either Party giving 14 days prior written notice to the other Party, at any time or for any reason. Termination of this LOI will not affect the obligations set out in the Confidentiality Agreement referred to in Section 9 below.
|
|
|
8.
|
Lonza shall be entitled to compensation for the LOI Services as set forth in Exhibit A.
|
|
|
9.
|
The existence and contents of this LOI and all information disclosed to the other Party pertaining to the relationship between the Parties, including but not limited to negotiations with respect to the possible Agreement, shall be deemed confidential and are subject to the current Confidential Disclosure Agreement (the “Confidentiality Agreement”) between the Parties, dated 31 March 2011.
|
2
|
|
10.
|
This LOI merely records the intentions of the Parties and, with the exception of Sections 3, 4, 5.d., and 6-12, nothing in this LOI shall be binding upon either Party unless agreed to and confirmed in the Agreement.
|
|
|
11.
|
All services to be provided or work to be performed under this LOI will be performed in Lonza’s Walkersville facility and may be performed by Lonza or by any direct or indirect subsidiary of Lonza’s ultimate parent company at the Walkersville facility.
|
|
|
12.
|
The information contained in this LOI (including scope of work, estimated completion dates and deliverables) is based and conditioned on information supplied by and represented by Company.
|
[remainder of page intentionally left blank]
3
IN WITNESS WHEREOF, each Party hereto has caused this Agreement to be executed on its behalf by its duly authorized representative.
| GENESIS BIOPHARMA, INC. | LONZA WALKERSVILLE, INC. | |||
| By: | By: | |||
| Name: | Name: | |||
| Title: | Title: | |||
4
EXHIBIT A
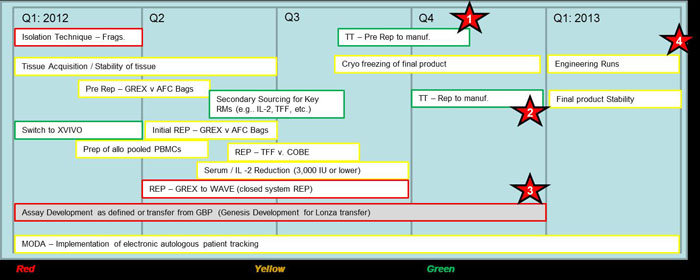
DESCRIPTION OF LOI SERVICES & PROJECT SCHEDULE
Performance of process development experiments as outlined below.
Estimate based upon time and materials for technology transfer, training, labor (project management, process development, etc.), reagents, testing and other activities to be defined in Statements of Work.
Requires approximately 6 FTEs over 5 quarters - plus materials.
Estimate: $3.2M + 25%
A-1
Exhibit B
STANDARD TERMS & CONDITIONS
1. PAYMENT: All payments to LONZA by COMPANY for work performed by LONZA under the LOI shall be in U.S. dollars and shall be by check, wire transfer, money order or other method of payment approved in writing by LONZA. Payment for materials and consumables (i.e. reimbursable costs) shall be due and payable upon receipt of the invoice sent by LONZA to COMPANY. Any fee, charge or other payment due to LONZA by COMPANY that is not paid within 30 days after it is due shall accrue interest, from the date when the same was due and payable, at the rate of eighteen percent (18%) per annum, payable on demand.
2. WARRANTY; REPRESENTATIONS: LONZA makes no representation or warranty regarding the PRODUCT's safety or effectiveness, or otherwise, except that LONZA shall perform activities as contemplated by the LOI. COMPANY acknowledges and agrees that LONZA and/or LONZA’s personnel (i) have not participated in the invention or testing of the PRODUCT, and (ii) have not evaluated the PRODUCT’s safety or suitability for use in humans or others. Other than quality control testing of the PRODUCT by LONZA as part of the work performed by LONZA under the LOI, LONZA shall not be in any way responsible for PRODUCT testing. COMPANY represents and warrants to LONZA that, to the best of its knowledge, (a) it has the requisite Intellectual Property rights related to the process and the PRODUCT and (b) performance of the LOI Services will not give rise to a potential cause of action by a third party against LONZA for infringement or another violation of Intellectual Property rights.
3. COMPANY SUPPLIED MATERIALS, CONSUMABLES AND EQUIPMENT: COMPANY shall be responsible to supply and/or pay for all materials and consumables (i.e. reimbursable costs). All materials and consumables shall be invoiced to COMPANY by LONZA at the relevant Acquisition Cost. “Acquisition Cost” shall mean the actual price paid by LONZA to any third party (net of any discounts, rebates, credits or the like) for any such materials, including, but not limited to, shipping and handling costs and customs duties incurred and paid by LONZA to that third party in connection with the acquisition of such materials, and also including five percent (5%) of such actual price to cover LONZA’s acquisition and storage costs for such materials. If additional equipment, other than what is supplied by LONZA, is needed to perform work under the LOI, COMPANY shall be responsible to supply and/or pay for the equipment.
4. PRODUCT/INTELLECTUAL PROPERTY: Neither COMPANY nor Lonza will acquire any right, title, or interest in any inventions (whether or not patentable), discoveries, improvements, data, information, reports and any and all related documentation made or conceived by the other party (collectively, "Intellectual Property") prior to the date hereof or independently of the LOI Services (“Background Intellectual Property”).
5. HAZARDOUS WASTE: LONZA shall provide COMPANY with prior written notice of any hazardous waste that may be generated by the work performed by LONZA under the LOI, only if such waste cannot be disposed of through LONZA’s standard waste disposal system. LONZA shall make any necessary special arrangements for disposal of such hazardous waste and COMPANY shall be solely responsible for all costs associated therewith.
6. TAXES: COMPANY represents and warrants that it is not subject to any sales and use taxes or other taxes (collectively, the “Taxes") resulting from the LOI Services.
7. LONZA PERSONNEL: COMPANY agrees not to solicit for employment (or for use as an independent contractor) LONZA employees during the term of the LOI and for a period of one (1) year thereafter.
8. DELIVERY OF PRODUCT SAMPLES, SHIPPING CHARGES: If the LOI is terminated and an Agreement has not been signed at the time of such termination, LONZA shall ship (i) the PRODUCT, (ii) COMPANY supplied equipment and samples, (iii) or any other COMPANY owned items in accordance with the COMPANY’s packing and shipping instructions, as provided by COMPANY to LONZA, and shall be by common carrier, unless otherwise specified by COMPANY. Delivery shall be F.O.B. Shipping Point (the LONZA facility). COMPANY shall provide its preferred carrier’s account number and shall pay for all shipping costs in connection with the delivery of each shipped item. LONZA’s responsibility ceases and COMPANY’s risk of loss arises, upon delivery of each shipped item to the common carrier or common carrier’s authorized agent.
B-1
9. INDEMNIFICATION: COMPANY shall indemnify and hold harmless LONZA from and against any and all third-party claims, damages, costs and expenses of any kind (including attorneys' and experts' fees) incurred by LONZA in connection with or relating to the PRODUCT and the LOI Services, including but not limited to, any third-party infringement claims.
10. LIMITATION OF LIABILITY:
COMPANY HEREBY AGREES THAT TO THE FULLEST EXTENT PERMITTED BY LAW, LONZA'S LIABILITY TO COMPANY FOR ANY AND ALL INJURIES, CLAIMS, LOSSES, EXPENSES, OR DAMAGES, WHATSOEVER, ARISING OUT OF OR IN ANY WAY RELATED TO THE WORK PERFORMED BY LONZA UNDER THE LOI, FROM ANY CAUSE OR CAUSES, INCLUDING, BUT NOT LIMITED TO, NEGLIGENCE, ERRORS, OMISSIONS OR STRICT LIABILITY, SHALL NOT EXCEED THE TOTAL CHARGES PAID BY COMPANY TO LONZA UNDER THE LOI. TO THE EXTENT THAT THIS CLAUSE CONFLICTS WITH ANY OTHER CLAUSE, THIS CLAUSE SHALL TAKE PRECEDENCE OVER SUCH CONFLICTING CLAUSE. IF APPLICABLE LAW PREVENTS ENFORCEMENT OF THIS CLAUSE, THEN THIS CLAUSE SHALL BE DEEMED MODIFIED TO PROVIDE THE MAXIMUM PROTECTION TO LONZA AS IS ALLOWABLE UNDER APPLICABLE LAW.
IN NO EVENT SHALL EITHER PARTY BE LIABLE TO THE OTHER OR ANY OF ITS AFFILIATES FOR ANY CONSEQUENTIAL, INCIDENTAL, INDIRECT, SPECIAL, PUNITIVE OR EXEMPLARY DAMAGES (INCLUDING, WITHOUT LIMITATION, LOST PROFITS, BUSINESS OR GOODWILL) SUFFERED OR INCURRED BY SUCH OTHER PARTY OR ITS AFFILIATES IN CONNECTION WITH THIS AGREEMENT, EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGES.
11. SECURITY PROCEDURES: COMPANY personnel authorized to have access to the LONZA facility in connection with the work performed by LONZA under the LOI shall abide by the security procedures established by LONZA. COMPANY shall be liable for any breaches of security by COMPANY personnel. All COMPANY personnel shall agree to abide by LONZA policies and standard operating procedures established by LONZA.
11. RECORDS: LONZA will maintain accurate records for all work performed by LONZA under the LOI. If process development work is performed under the LOI, LONZA will maintain accurate records of such process development work (the "Development Records"). COMPANY shall own the Development Records developed for COMPANY.
12. GOVERNING LAW: The LOI and any suits, disputes, actions or other legal proceedings related to or arising out of the work performed by LONZA under the LOI shall be governed by and construed in accordance with the internal laws of the State of Delaware, without giving effect to its conflicts of laws provisions.
13. SEVERABILITY: Each provision shall be treated as a separate and independent clause, and the unenforceability of any one clause shall in no way impair the enforceability of any of the other clauses herein. If one or more of the provisions shall for any reason be held to be excessively broad as to scope, activity, subject or otherwise so as to be unenforceable at law, such provision or provisions shall be construed by the appropriate judicial body by limiting or reducing it or them so as to be enforceable to the maximum extent compatible with the applicable law as it shall then appear.
14. DEFINITIONS: Capitalized terms not herein defined shall have the meanings ascribed to them in the LOI.
B-2
Exhibit C
Term Sheet
This Term Sheet is for discussion purposes only. This draft is not intended to - and does not - create any legally binding obligation on any party to agree to or consummate any transaction.
|
Item
|
Process Development and Manufacturing
|
|
|
Product(s) Covered
|
· |
Lonza technologies and research, development and manufacturing services (“Services”)
|
| · |
Genesis Biopharma, Inc.’s [GBI] autologous cell therapy - Cōntego™ (“Products”)
|
|
|
Business Relationship
|
Alliance wherein Lonza supports the Services needs for the Products as part of a long-term Development and Manufacturing Services Supply Agreement (“Agreement”), wherein GBI receives priority access to Lonza Services, including biologics production technologies and manufacturing capacity.
|
|
|
Term
|
Five (5) year initial term. Automatic renewal for two (2) one-year extension periods with mutual consent.
|
|
|
Lonza incentives to GBI
|
· |
Ongoing manufacturing strategy development and capacity planning, inclusive of evaluation of tax-advantaged zones and business continuity planning.
|
| · | Reserved manufacturing capacity in Walkersville, MD, for the production of Products for up to 600 patients in Phase III clinical trials to be conducted in the United States. | |
| · |
Process Development capacity and subject matter expert availability (on a fee-for-service basis) for continuous process improvement and manufacturing cost reduction activities.
|
|
|
GBI incentives to Lonza
|
Manufacturing Rights: Manufacture of not less than 90% of GBI’s demand for the Phase III clinical trials of the Products.
|
|
|
Effective Date
|
January 2012
|
|
|
Annual Pricing Adjustments
|
Services prices to be increased or decreased annually in-line with the U.S. Producer Price Index (“PPI”) rate of change.
|
|
|
Indemnity & Intellectual Property
|
Customary provisions to be negotiated by the parties.
|
|
|
Territories
|
Worldwide for both GBI and Lonza
|
|
|
Forecast
|
GBI will provide Lonza with 24 month rolling forecasts.
Lonza will make commercially reasonable efforts to meet GBI’s demand.
|
|
|
Payment
|
GBI shall make payments no later than 30 days after receiving an invoice from Lonza.
All Service fees to be paid in cash.
|
C-1
