Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - INSMED Inc | d258838d8k.htm |
 Developing Innovative Inhaled Treatments for Serious
Lung Infections
November 2011 |
 This
presentation contains forward-looking statements which are made pursuant to provisions of
Section 21E of the Securities Exchange Act of 1934. Investors are cautioned that such
statements in this presentation, including statements relating to our financial
position, results of operations, the status and the results of preclinical studies and
clinical trials and preclinical and clinical data described herein,
the
timing
of
responses
to
information
and
data
requests
from
FDA,
the
development
of our
products, and the business strategies, evaluations, plans and objectives of management,
constitute forward-looking statements which involve risks and uncertainties that
could cause actual results to differ materially from those anticipated by the
forward-looking statements. Our results may be affected by such factors as
the receipt and timing of FDA and other regulatory reviews and approvals, if at all,
competitive developments affecting our product development, delays in product development
or clinical trials, and patent disputes involving currently developing products. The
risks and uncertainties include, without limitation, we may experience unexpected
regulatory actions, delays or requests, our future clinical trials may not be
successful, we may be unsuccessful in developing our product candidates or receiving
necessary regulatory approvals, we may experience delays in our product development or
clinical trials, our product candidates may not prove to be commercially successful,
our expenses may be higher than anticipated and other risks and challenges detailed in our
filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form
10-K for
the
year
ended
December
31,
2010
and
our
Quarterly
Report
on
Form
10-Q
for
the
quarter
ended
September 30, 2011. Investors are cautioned not to place undue reliance on any
forward-looking statements
which
speak
only
as
of
the
date
of
this
presentation.
We
undertake
no
obligation
to
publicly release the results of any revisions to these forward-looking statements that may
be made to reflect events or circumstances that occur after the date of this release or
to reflect the occurrence of unanticipated events.
Safe Harbor Statement |
 2
Insmed: Value Proposition
Attractive
Late-Stage
Opportunity
Arikace®
(liposomal amikacin for inhalation), is Phase 3-ready for cystic
fibrosis
(CF)
Pseudomonas
and
Phase
2-ready
for
non-TB
mycobacteria
(NTM) lung infections
Amikacin is an FDA-approved antibiotic, long recognized as one of the most
effective treatments for gram-negative infections
Arikace has strong Phase 2 efficacy and safety data in CF
Compelling
Business Model
Arikace
has
global
annual
market
potential
of
over
$1
Billion
in
CF
and
NTM
Limited commercial infrastructure required
Orphan drug indications with high unmet need
Strong IP and potential for extended exclusivity
Strong Balance
Sheet &
Experienced
Management
As of end of 3Q 2011, company had ~$85 million in cash and no debt
Over
150
years
of
combined
experience
in
pharmaceutical
industry
and
drug development
Arikace®
is a potentially highly differentiated product opportunity that
offers a compelling business model in two orphan diseases
* Arikace®
is a registered trademark of Insmed Corporation |
 3
Arikace®
—
Update on Clinical Hold
Insmed announced in October 2011 that the FDA is continuing the clinical hold placed
in August 2011 on the Phase 3 clinical trials for Arikace in CF and NTM. The
Agency stated that based on the information provided to date it has insufficient
information to assess the risks of Arikace in CF and NTM patients following the
results of a long term rat inhalation study.
Agency has requested that Insmed conduct a 9-month inhalation dog toxicity study of
Arikace to determine if the findings in the rat inhalation carcinogenicity study
are also demonstrated in a non-rodent model, and to identify a more targeted
CF patient population/disease state where the risk profile of Arikace may be
more favourable Insmed was informed during further dialogue with the Agency that,
if the Company chooses to proceed, the required 9-month dog inhalation
toxicity study of ARIKACE can be conducted in parallel with the CF phase 3
clinical trials in human subjects FDA is requiring that Insmed conduct a phase 2
clinical trial in adult NTM patients intended to
provide proof-of-concept efficacy and safety data for ARIKACE in NTM before the
Company can proceed with a phase 3 clinical trial.
Based on the efficacy and safety data generated from the Phase 2
clinical trial program,
Insmed continues to believe Arikace®
has the potential to be an important treatment option
for CF and NTM patients
Insmed continuing with evaluation of potential next steps with ARIKACE program
|
 4
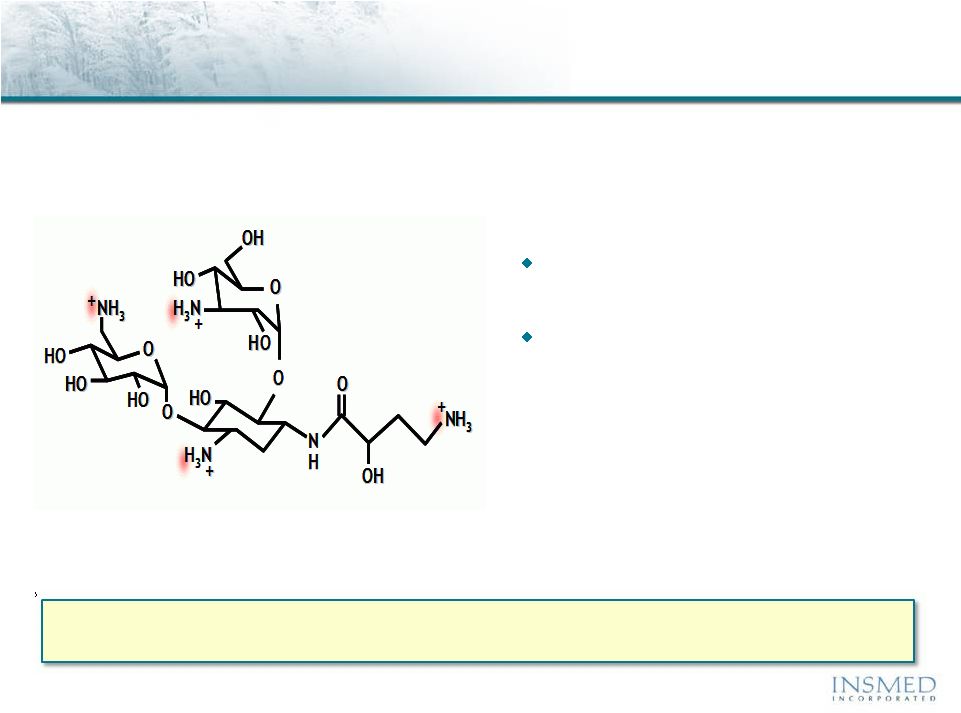
Arikace®: Amikacin Summary
Amikacin is an FDA-approved antibiotic with proven efficacy in the
treatment of gram-negative infections, including Pseudomonas and NTM
Member of the aminoglycoside
class of antibiotics
The value of the IV use has been
limited by issues of nephro-
toxicity and ototoxicity
Arikace (liposomal amikacin for inhalation) delivers high, sustained levels of drug to
the lung while reducing systemic exposure to well below established toxicity
levels |
 5
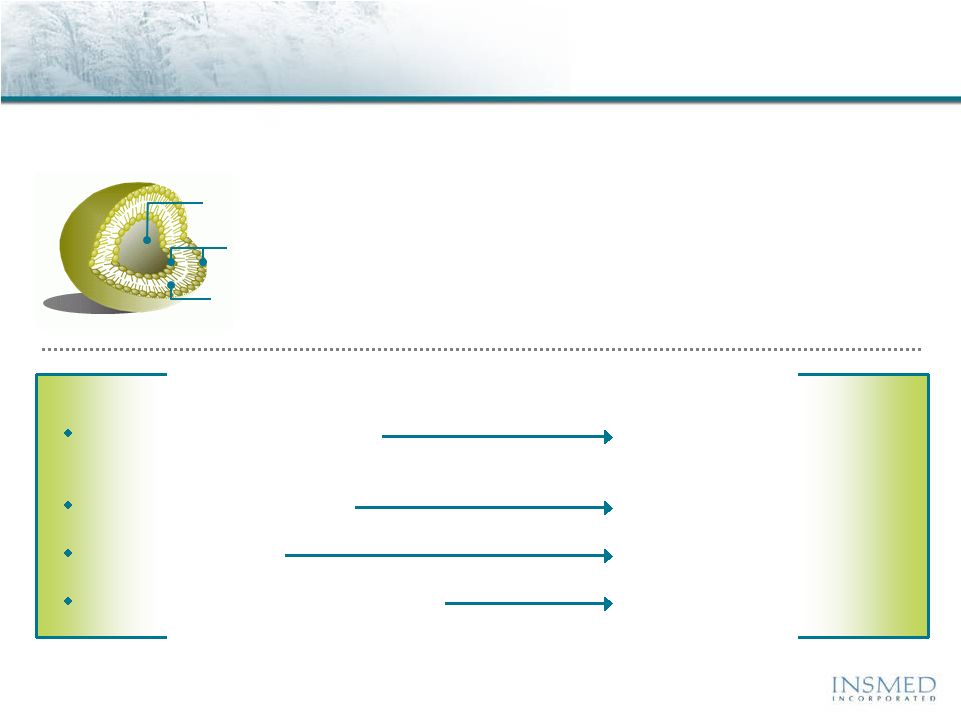
Arikace: Proprietary Liposomal Formulation
Engineered Specifically for Lung Delivery
Prolonged lung residence time
Minimal systemic exposure
Biofilm penetration
Preferential uptake into macrophages
Benefit
Arikace delivers the potency of Amikacin at the site of lung infection while
minimizing potential systemic toxicity
Potential
for
greater
efficacy
Addresses safety concerns
Once-a-day dosing and
potential for greater efficacy
Potential
for
greater
efficacy
Lipid Polar Head Groups
(at Both Surfaces)
Lipid Hydrophobic Chains
(Bi-Layer Interior)
Water Core (where Amikacin resides) |
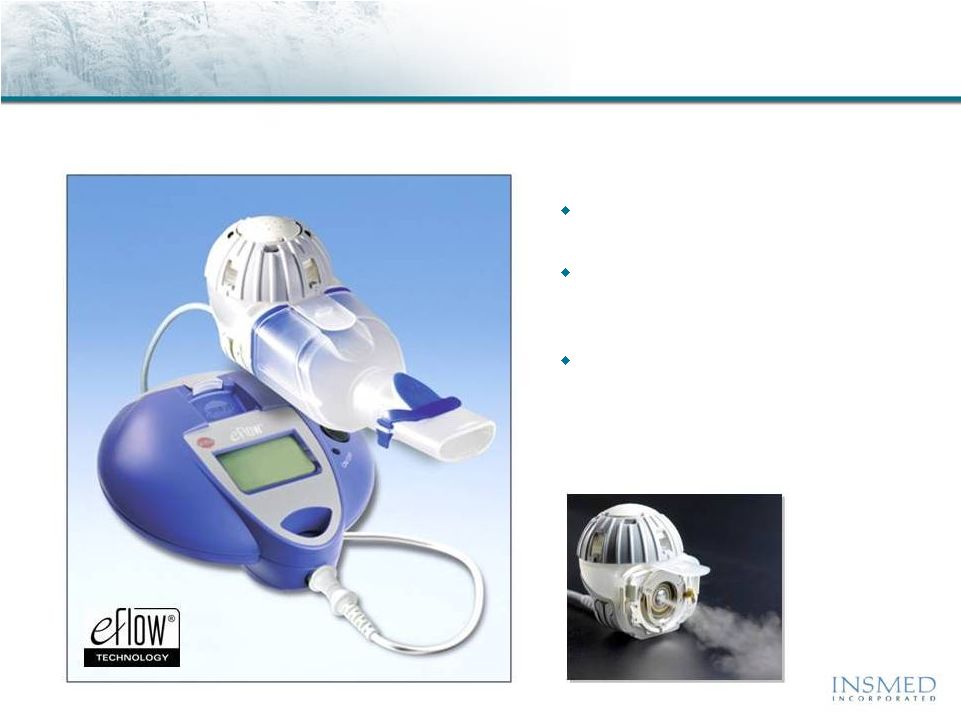 Arikace:
Delivery Using Proprietary eFlow ®
Technology
Arikace is delivered once daily via the state-of-the-art PARI Optimized,
Investigational eFlow Nebulizer System with Advanced Mesh Technology
Fast
drug delivery with efficient
lung deposition
Small, portable, silent and
cordless
device weighs less than
10 ounces.
eFlow Technology Device
exclusivity
from PARI Pharma for
15 years after first commercial
sale of Arikace
* eFlow
®
is a registered trademark of PARI Pharma GmbH
6 |
 7
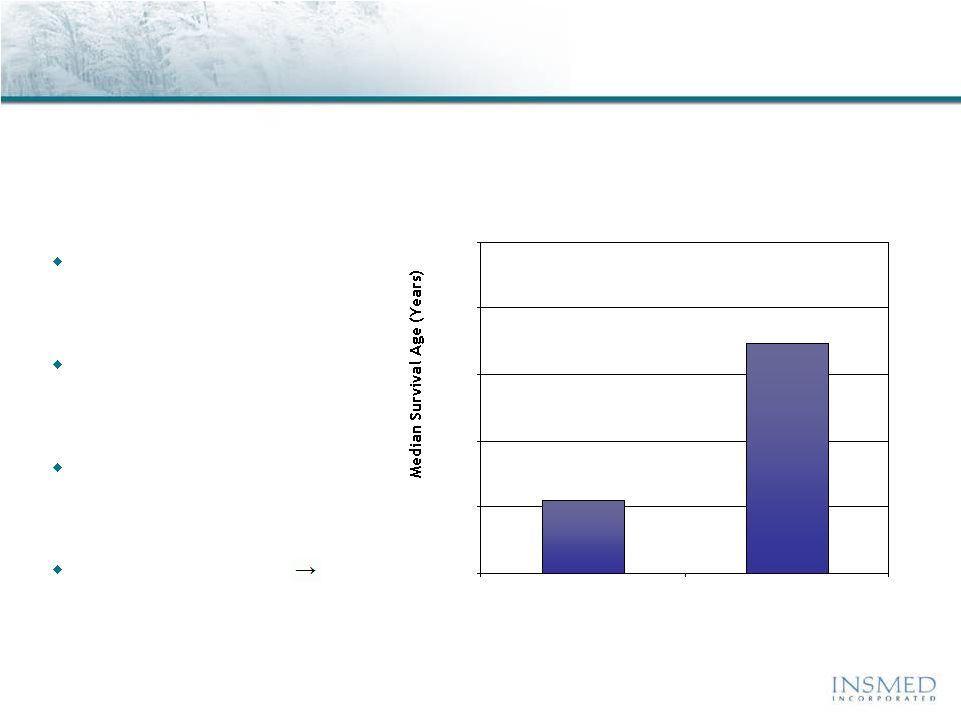
Arikace—Cystic Fibrosis
Epidemiology and Disease Description
Cystic fibrosis is a life-threatening disease with significant unmet needs
that is growing in prevalence
Affects about 30,000 children
and adults in the U.S. (70,000
worldwide)
Inherited disease that causes
thick, sticky mucus to build up
in the lungs
Despite expanded use of current
products, lung function often
continues to decline
High treatment burden
major compliance issue
20
25
30
35
40
45
1985
2008
U.S. CF Patients
Median Predicted Survival Age
Source: Adapted from Cystic Fibrosis Foundation, Patient Registry
2008 and 2007 Annual Reports, Bethesda, Maryland. |
 8
Arikace—Cystic Fibrosis
Need for New Inhaled Antibiotics
Current inhaled antibiotics produce modest efficacy in a limited
patient
population providing an opportunity for Arikace to become first-line
treatment
Current inhaled antibiotics are not indicated for a significant segment of
the CF population --
patients with FEV-1 % predicted of greater than 75%
Efficacy of current inhaled antibiotics declines from cycle to cycle and is
not sustained in the off-treatment period
Lung function continues to decline at an average of 1% to 3% per
year with
some patients experiencing much greater declines |
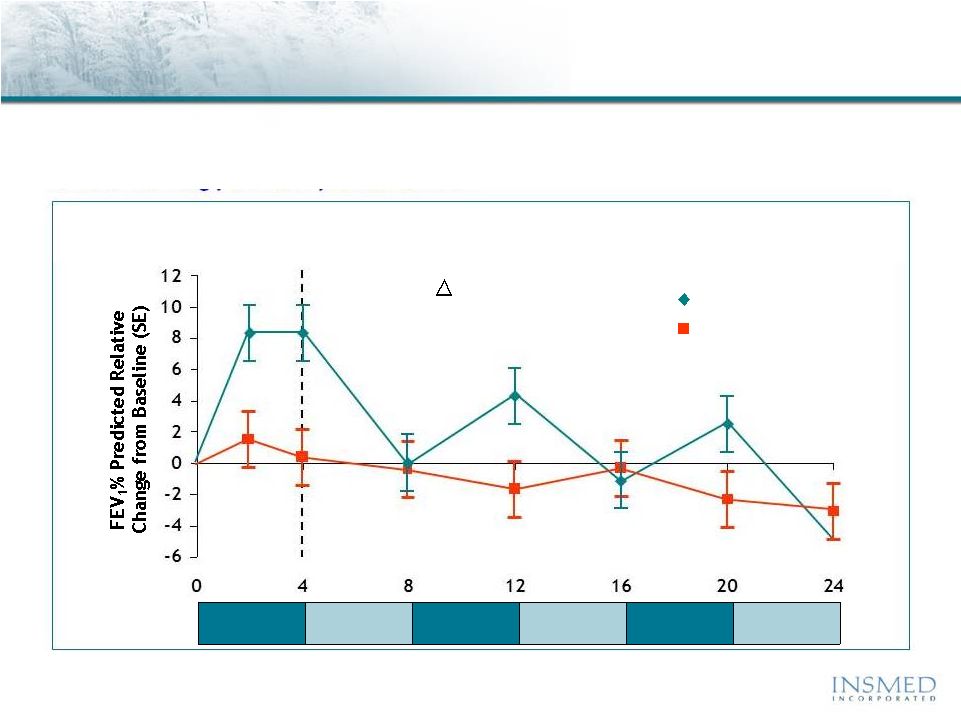 9
Aztreonam vs. Tobi
®
(inhaled tobramycin)
CF Phase 3 Trial Results: Pulmonary Function
Source: 2010 North American CF Conference Poster 305 and Slide Presentation,
10/10.
•Tobi
®
(Tobramycin Inhalation Solution) is a registered trademark of Novartis.
** AZLI = Aztreonam; TIS = Tobi
®
Lung function returned to baseline or lower during each off treatment
period and at the end of 24 weeks, both treatment groups showed a
decline in lung function from baseline
Lung Function
Adjusted
Mean
Relative
Change
in
FEV
1
%
Predicted
Week:
2
AZLI
TIS
+ 7.8
P
= 0.0001
95% CI (3.86, 11.73)
AZLI/
TIS
28 Days
AZLI/
TIS
28 Days
AZLI/
TIS
28 Days |
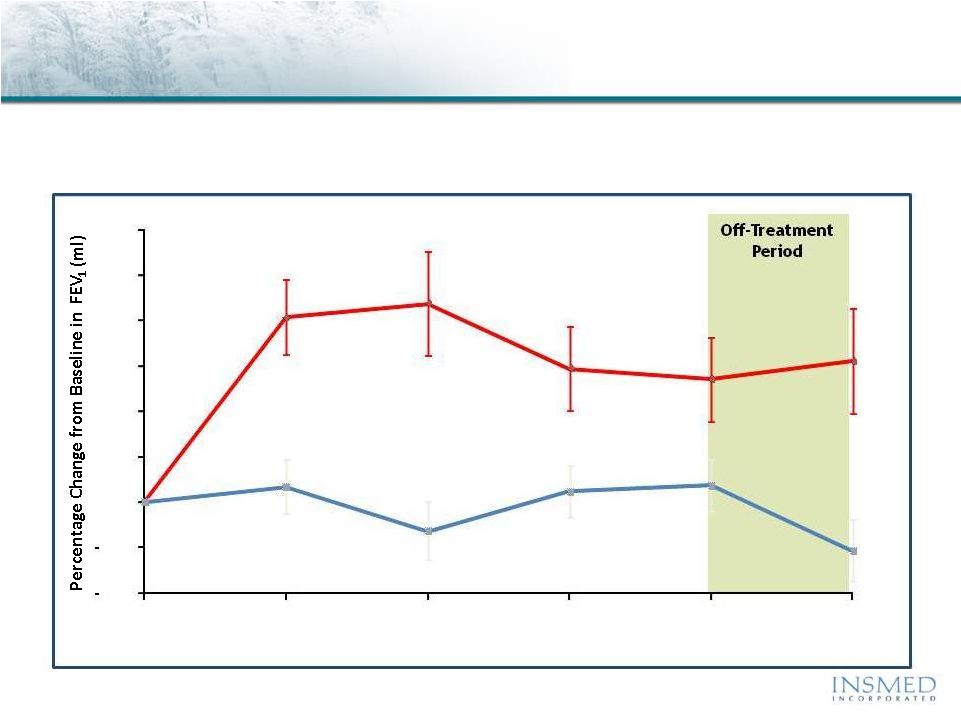 10
Arikace—Cystic Fibrosis
Phase 2 Pooled Results (560mg QD): Pulmonary Function
Mean (SE)
Arikace demonstrated statistically significant and clinically meaningful
improvement in pulmonary function throughout the 28-day treatment
period that was sustained through the off-treatment period
P = 0.033
P = 0.003
(36/36)
(36/35)
(33/36)
(32/35)
(34/35)
(34/34)
(N=Arikace/Placebo)
3%
0%
3%
6%
9%
12%
15%
18%
0
7
14
21
28
56
6%
Arikace
560mg
Placebo
Visit Day
% Change in FEV
(ml) vs. Baseline
(N)
1 |
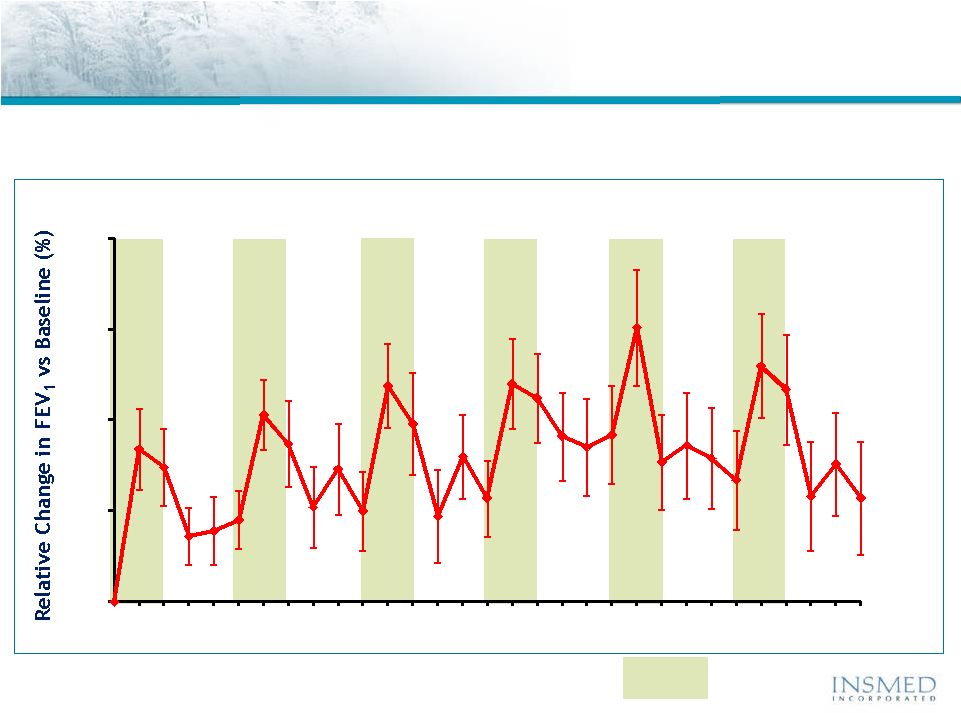 11
Visit Days
Patients
Receiving
560
mg
Arikace
®
Once
Daily
for
28
Days
and
Off-Treatment
for
56
Days
Arikace—Cystic Fibrosis
Open Label Extension (TR02-105): Duration of Response
Treatment
Period
* Significance at end of treatment over 6 cycles
** Significance 56 days off-treatment over 6 cycles
An open label extension study TR02-105 demonstrated the sustained efficacy
Of Arikace during and between multiple cycles of therapy
0
5
10
15
20
14
28
56
70
85
98
112
140
154
169
182
196
224
238
253
266
280
308
322
337
350
364
392
406
421
434
448
476
490
504
42
41
42
42
41
41
41
41
41
45
N=
45
47
46
44
45
47
46
46
45
44
44
44
43
43
43
43
44
43
45
p=0.0001**
p<0.0001*
41
47
Cycle
1
Cycle
2
Cycle
3
Cycle
4
Cycle
5
Cycle
6 |
 12
Arikace—Cystic Fibrosis
Phase III Program (pending FDA review and lifting of clinical hold)
Insmed had reached agreement with FDA and EMA on NDA/MAA path for CF
Patients with Pseudomonas lung infections
One E.U. Phase 3 (Arikace vs Tobi
®
Study, N = 300)
–
Primary End-Point: Relative Change in FEV-1 at week 24
•
Key Secondary End-Point: Time to Pulmonary
Exacerbation •
Key inclusion Criteria: FEV-1 % Predicted > 25%
–
Approximately 260 patients required to demonstrate non-inferiority at agreed upon
margin with 80% power
One U.S. Phase 3 (Arikace vs Placebo Study, N = 300)
–
Primary End-Point: Time to Pulmonary Exacerbation
•
Key Secondary End-Point: Relative Change in FEV-1
•
Key inclusion Criteria: FEV-1 % Predicted > 25%
–
Approximately 260 patients required to demonstrate superiority with 80% power
–
Estimate 50% reduction in frequency of exacerbations
–
Prospectively planned to evaluate pooled event rate to assess adequacy of sample
|
 13
Arikace—Non-TB Mycobacteria
Epidemiology and Disease Description
Diagnosis of NTM pulmonary
infections are growing
rapidly
Current treatment
approaches have limited
efficacy and are associated
with significant toxicity
Mean age of 66 years
66% treated with
antibiotics; mean of 7.6
courses per year
Average Length of Inpatient
Hospital Stay = 10.2 days
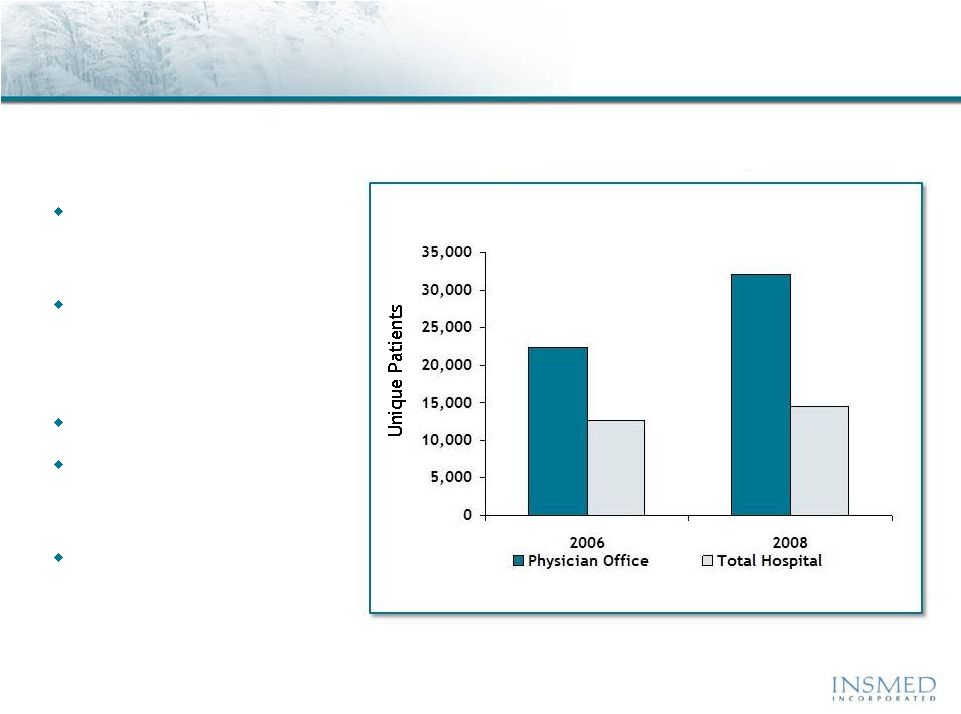
Non-TB mycobacteria (NTM) is an intracellular pathogen that can cause
severe pulmonary disease with limited effective treatment options
Source: SDI Healthcare Database, July 2009.
Total U.S. Patients Diagnosed with Non-TB Mycobacteria
Pulmonary Disease
32.0K +20%
2-Yr Growth
14.4K +7%
2-Yr Growth |
 14
Arikace—Non-TB Mycobacteria
Phase 2 Program (pending FDA review and lifting of clinical hold)
Trial Design and Patient Population:
–
Randomized, double-blind, placebo controlled phase 2 study in patients with
recalcitrant/persistent NTM lung disease who are on a stable ATS/IDSA
guidelines-based multi-drug treatment regimen
–
Patients receive Arikace or placebo daily for 84 days; then all patients can
receive Arikace 560 mg in an open-label manner for an additional 84 days
Key Inclusion Criteria: History of chronic infection with either Mycobacterium
avium complex or Mycobacterium abscessus or mixed infection with both species
Primary endpoint: Change in mycobacterial culture results from baseline to
end of treatment [Time
Frame:
84 days]
High unmet medical need enables a phase 2 program consisting of a single
study of approximately 100 patients |
 15
Projected Cash
at year end
2011
Approximately $72 to $75 million currently forecast
Current Overview: Capital Structure and Key Financials
Pro Forma
Balance Sheet
Pro forma cash of ~$85 million as of September 30, 2011
No Debt
Present Capital
Structure
25.8 M fully diluted shares following reverse stock split:
24.8 million Common Shares
1.0 million options, restricted stock and warrants
Insmed has a strong cash position and no debt |
 16
Insmed: Value Proposition
Attractive
Late-Stage
Opportunity
Arikace®
(liposomal amikacin for inhalation), is Phase 3-ready for cystic
fibrosis
(CF)
Pseudomonas
and
Phase
2-ready
for
non-TB
mycobacteria
(NTM) lung infections
Amikacin is an FDA-approved antibiotic, long recognized as one of the most
effective treatments for gram-negative infections
Arikace has strong Phase 2 efficacy and safety data in CF
Compelling
Business Model
Arikace
has
global
annual
market
potential
of
over
$1
Billion
in
CF
and
NTM
Limited commercial infrastructure required
Orphan drug indications with high unmet need
Strong IP and potential for extended exclusivity
Strong Balance
Sheet &
Experienced
Management
As of end of 3Q 2011, company had ~$85 million in cash and no debt
Over
150
years
of
combined
experience
in
pharmaceutical
industry
and
drug development
Arikace®
is a potentially highly differentiated product opportunity that
offers a compelling business model in two orphan diseases |
