Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Vericel Corp | a11-29782_18k.htm |
| EX-99.2 - EX-99.2 - Vericel Corp | a11-29782_1ex99d2.htm |
Exhibit 99.1
|
|
Patient-Specific Cellular Therapy (Ixmyelocel-T) is Safe and Improves Time to Treatment Failure in Patients with Critical Limb Ischemia and No Revascularization Options American Heart Assoc Scientific Sessions 2011 William Marston MD For the RESTORE-CLI Clinical Investigators Orlando, FL November 14, 2011 |
|
|
RESTORE-CLI Investigators Scott Berceli W Todd Bohannon Anthony Comerota Carlo Dall’Olmo Raul Guzman Brian Halloran Peter Henke Timothy Henry Colleen Johnson William Marston Jeff Martinez Farrell Mendelsohn Richard Powell Jorge Saucedo Patrick Stiff Edith Tzeng Omaida Velazquez |
|
|
Disclosures William Marston, MD TITLE: Chief, Division of Vascular Surgery Professor, Department of Surgery University of North Carolina FINANCIAL DISCLOSURE: Aastrom (Consultant/Advisory Board) |
|
|
Ixmyelocel-T: Description Autologous (patient-specific), expanded multicellular therapy Target population: CLI patients with no options for revascularization Cell source: Bone marrow Cell delivery: Twenty intramuscular injections in lower extremity |
|
|
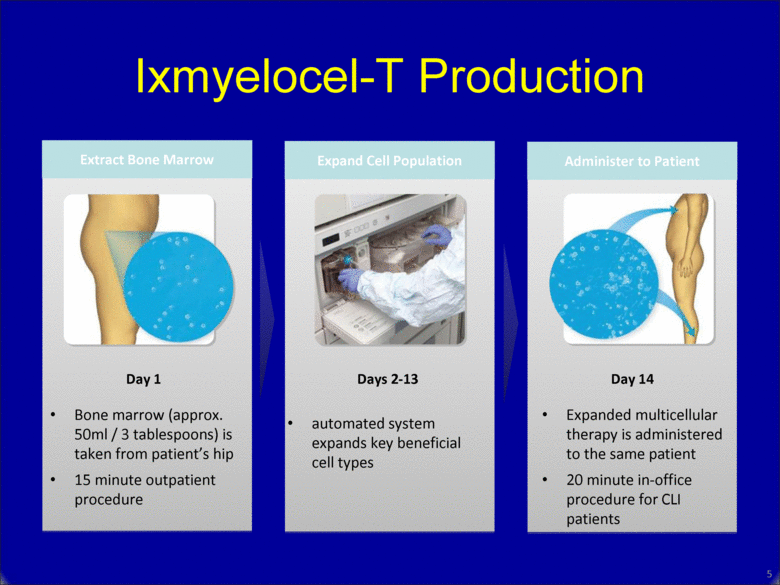
Ixmyelocel-T Production 5 Bone marrow (approx. 50ml / 3 tablespoons) is taken from patient’s hip 15 minute outpatient procedure automated system expands key beneficial cell types Expanded multicellular therapy is administered to the same patient 20 minute in-office procedure for CLI patients Day 1 Days 2-13 Day 14 Extract Bone Marrow Expand Cell Population Administer to Patient |
|
|
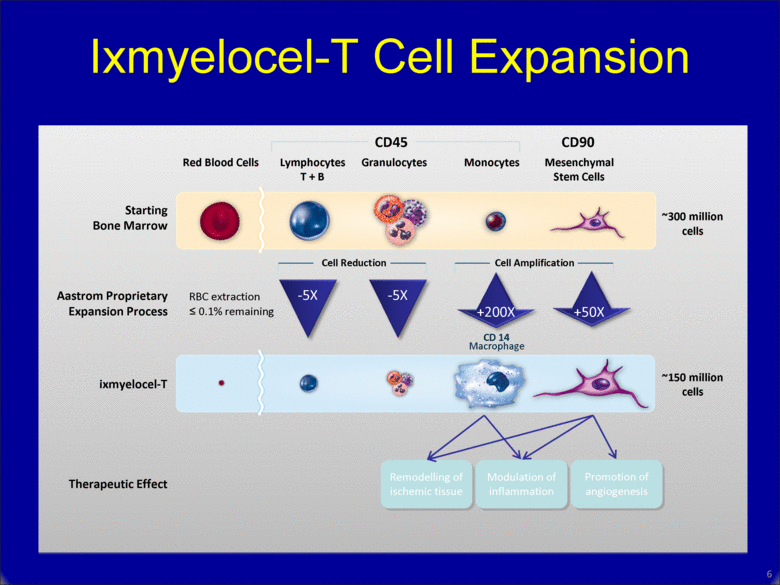
Ixmyelocel-T Cell Expansion 6 Starting Bone Marrow Red Blood Cells Lymphocytes T + B Granulocytes Monocytes Mesenchymal Stem Cells CD45 Aastrom Proprietary Expansion Process ixmyelocel-T Therapeutic Effect RBC extraction < 0.1% remaining Cell Reduction Cell Amplification -5X -5X +200X +50X ~300 million cells ~150 million cells Remodelling of ischemic tissue Modulation of inflammation Promotion of angiogenesis CD 14 Macrophage CD90 |
|
|
Ixmyelocel-T Has Multiple Biological Activities Remodeling of tissues Secretion of MMPs Phagocytosis and efferocytosis Contraction of ECM Promotion of angiogenesis Secretion of angiogenic cytokines Activation of eNOS Resolution of inflammation Secretion of anti-inflammatory cytokines Alternatively activated macrophages 7 |
|
|
Phase 2b RESTORE-CLI Study Design Randomized, placebo controlled Double-blind Powered as Phase 2 safety study 150 patients 18 active centers No-option CLI patients who had rest pain with or without baseline wounds 7 |
|
|
Key Inclusion Criteria Age 18-90 Diagnosed CLI Ischemic rest pain > 2 weeks duration Ulceration or gangrene of toe or foot Toe systolic pressure < 50 mmHg Ankle systolic pressure < 70 mmHg Infrainguinal occlusive arterial disease judged not amenable to revascularization 8 |
|
|
Key Exclusion Criteria Previous amputation at talus or above Failed ipsilateral revascularization within 2 weeks of randomization Active infection of target extremity HbA1C > 10% Untreated aorto-iliac occlusive disease Exposed tendon or bone in wound 9 |
|
|
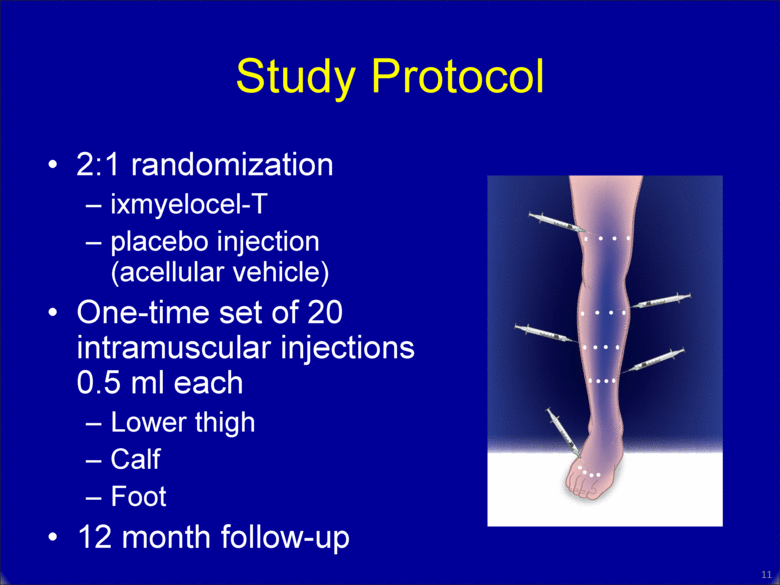
Study Protocol 2:1 randomization ixmyelocel-T placebo injection (acellular vehicle) One-time set of 20 intramuscular injections 0.5 ml each Lower thigh Calf Foot 12 month follow-up 10 |
|
|
Defined Study Outcomes Safety population: all pts randomized and aspirated Efficacy population: all pts randomized, aspirated and treated Primary (safety): All adverse events Primary efficacy: Time to first occurrence of treatment failure (TTF) Major amputation of treated leg All-cause mortality Doubling of wound total surface area from baseline De novo gangrene Secondary: Amputation-free survival (AFS) Major amputation of treated leg All-cause mortality 11 |
|
|
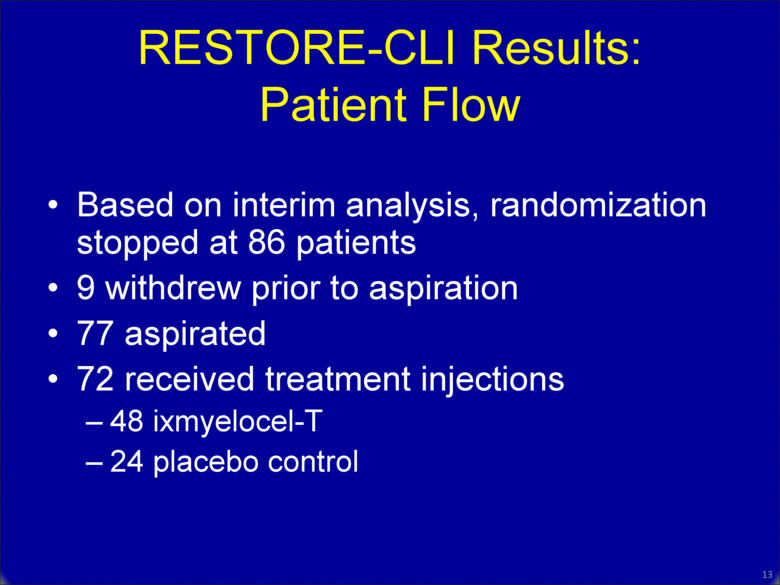
RESTORE-CLI Results: Patient Flow Based on interim analysis, randomization stopped at 86 patients 9 withdrew prior to aspiration 77 aspirated 72 received treatment injections 48 ixmyelocel-T 24 placebo control 12 |
|
|
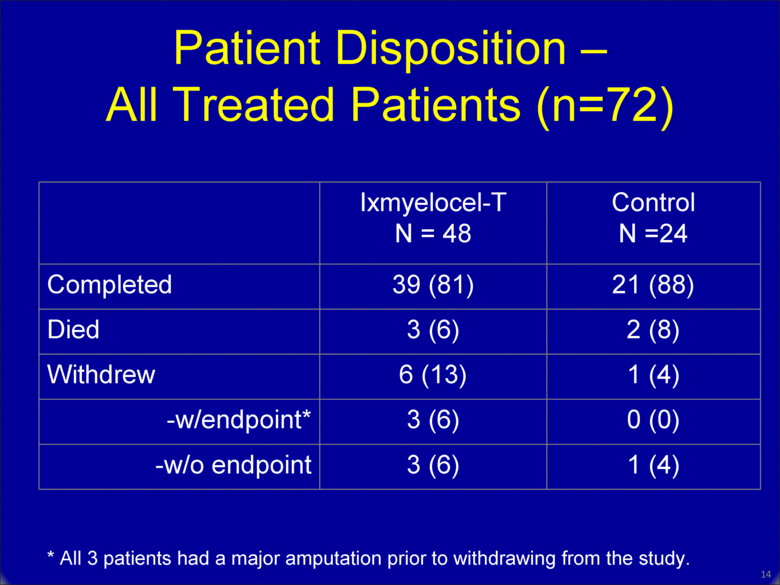
13 * All 3 patients had a major amputation prior to withdrawing from the study. Ixmyelocel-T N = 48 Control N =24 Completed 39 (81) 21 (88) Died 3 (6) 2 (8) Withdrew 6 (13) 1 (4) -w/endpoint* 3 (6) 0 (0) -w/o endpoint 3 (6) 1 (4) Patient Disposition – All Treated Patients (n=72) |
|
|
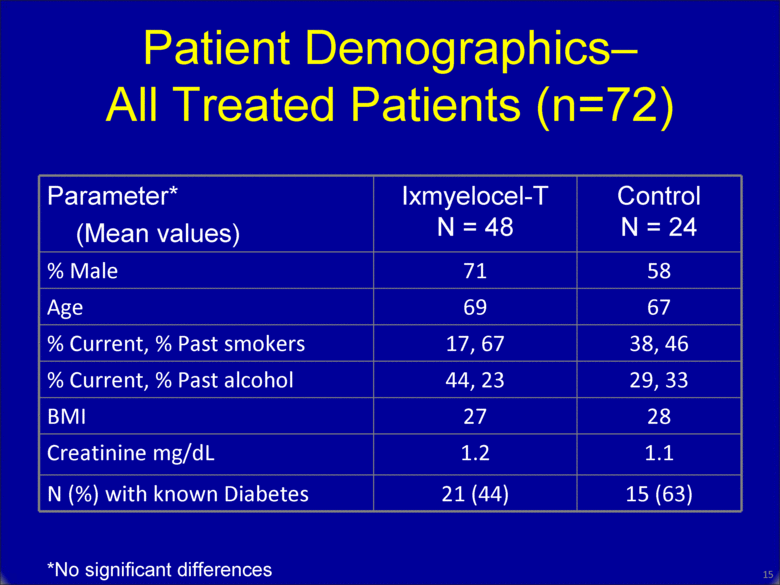
Parameter* (Mean values) Ixmyelocel-T N = 48 Control N = 24 % Male 71 58 Age 69 67 % Current, % Past smokers 17, 67 38, 46 % Current, % Past alcohol 44, 23 29, 33 BMI 27 28 Creatinine mg/dL 1.2 1.1 N (%) with known Diabetes 21 (44) 15 (63) Patient Demographics– All Treated Patients (n=72) *No significant differences 15 |
|
|
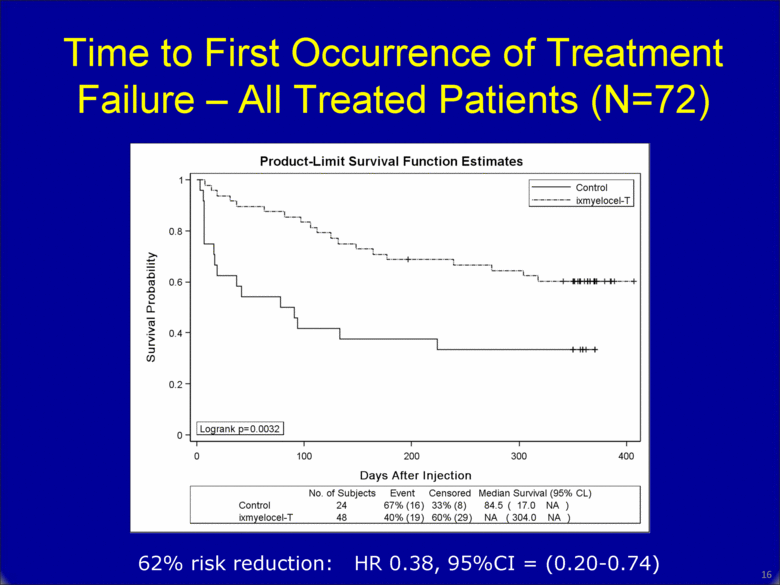
Time to First Occurrence of Treatment Failure – All Treated Patients (N=72) 62% risk reduction: HR 0.38, 95%CI = (0.20-0.74) 15 |
|
|
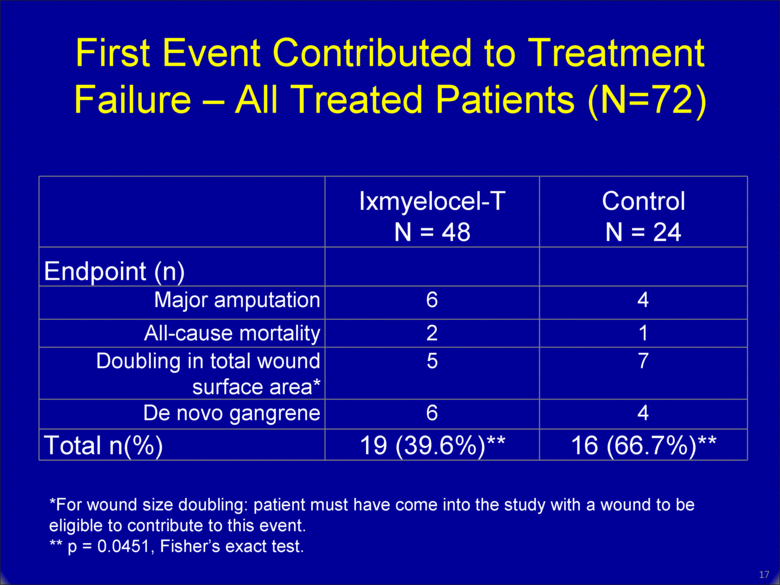
First Event Contributed to Treatment Failure – All Treated Patients (N=72) Ixmyelocel-T N = 48 Control N = 24 Endpoint (n) Major amputation 6 4 All-cause mortality 2 1 Doubling in total wound surface area* 5 7 De novo gangrene 6 4 Total n(%) 19 (39.6%)** 16 (66.7%)** *For wound size doubling: patient must have come into the study with a wound to be eligible to contribute to this event. ** p = 0.0451, Fisher’s exact test. 16 |
|
|
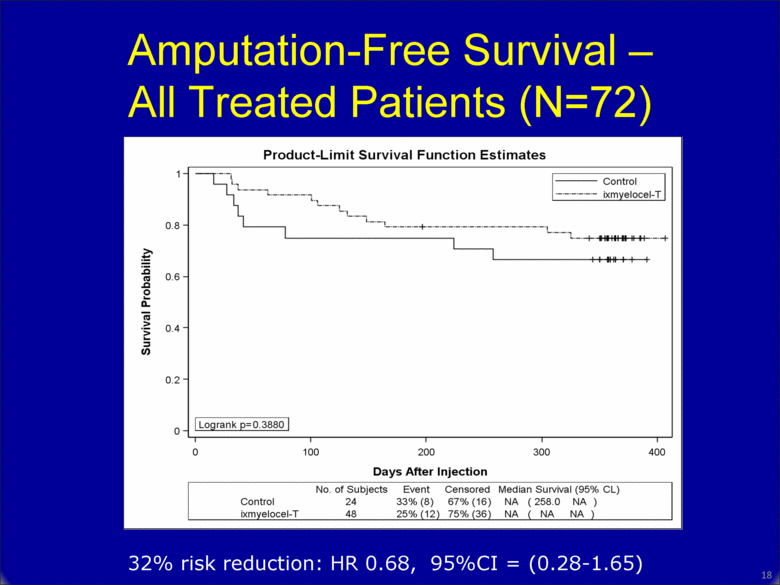
Amputation-Free Survival – All Treated Patients (N=72) 32% risk reduction: HR 0.68, 95%CI = (0.28-1.65) 17 |
|
|
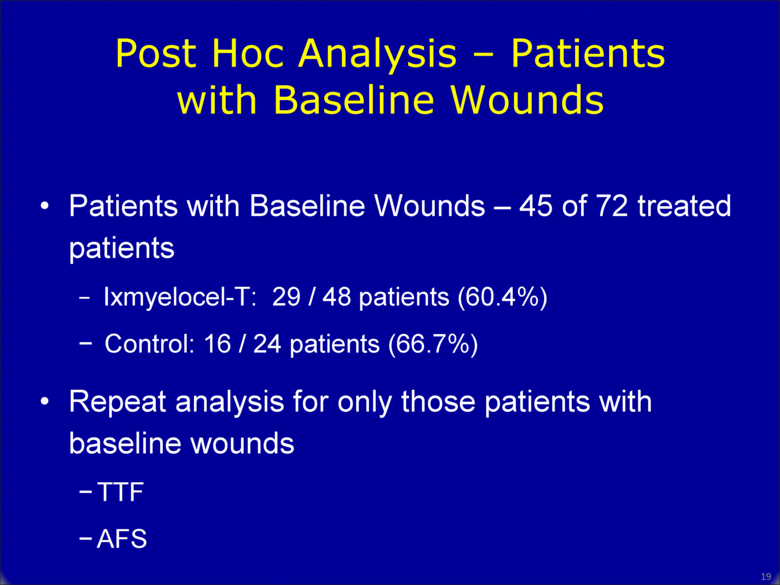
18 Patients with Baseline Wounds – 45 of 72 treated patients Ixmyelocel-T: 29 / 48 patients (60.4%) Control: 16 / 24 patients (66.7%) Repeat analysis for only those patients with baseline wounds TTF AFS Post Hoc Analysis – Patients with Baseline Wounds |
|
|
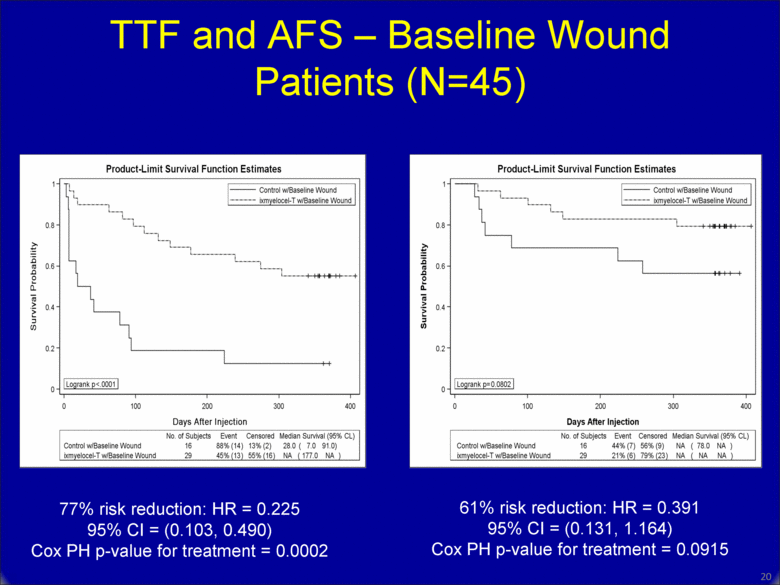
TTF and AFS – Baseline Wound Patients (N=45) 19 77% risk reduction: HR = 0.225 95% CI = (0.103, 0.490) Cox PH p-value for treatment = 0.0002 61% risk reduction: HR = 0.391 95% CI = (0.131, 1.164) Cox PH p-value for treatment = 0.0915 |
|
|
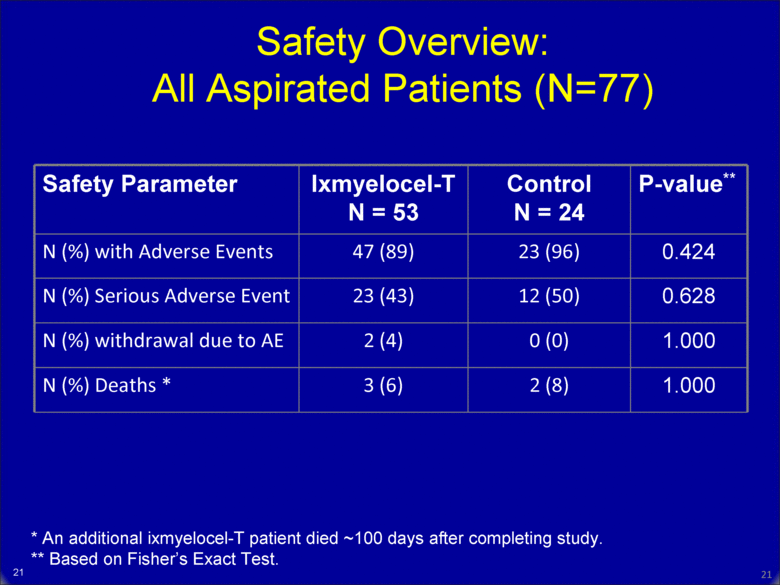
20 Safety Overview: All Aspirated Patients (N=77) Safety Parameter Ixmyelocel-T N = 53 Control N = 24 P-value** N (%) with Adverse Events 47 (89) 23 (96) 0.424 N (%) Serious Adverse Event 23 (43) 12 (50) 0.628 N (%) withdrawal due to AE 2 (4) 0 (0) 1.000 N (%) Deaths * 3 (6) 2 (8) 1.000 * An additional ixmyelocel-T patient died ~100 days after completing study. ** Based on Fisher’s Exact Test. 20 |
|
|
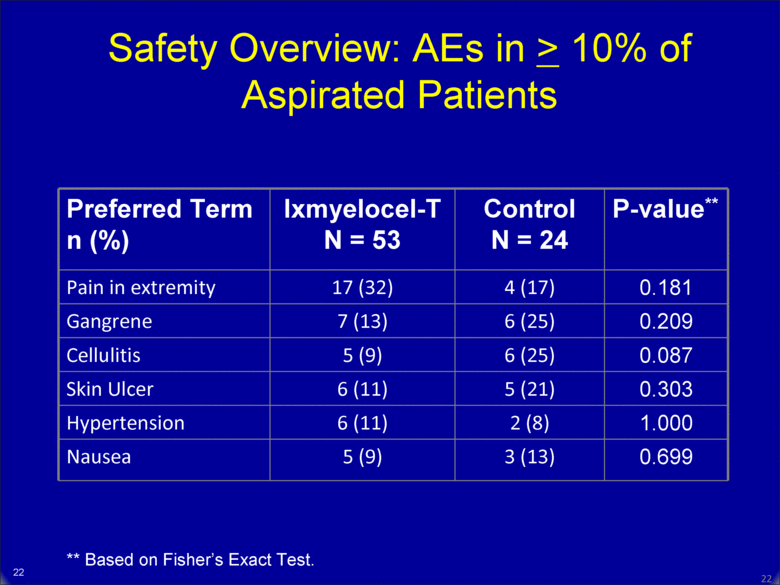
21 21 Safety Overview: AEs in > 10% of Aspirated Patients Preferred Term n (%) Ixmyelocel-T N = 53 Control N = 24 P-value** Pain in extremity 17 (32) 4 (17) 0.181 Gangrene 7 (13) 6 (25) 0.209 Cellulitis 5 (9) 6 (25) 0.087 Skin Ulcer 6 (11) 5 (21) 0.303 Hypertension 6 (11) 2 (8) 1.000 Nausea 5 (9) 3 (13) 0.699 ** Based on Fisher’s Exact Test. |
|
|
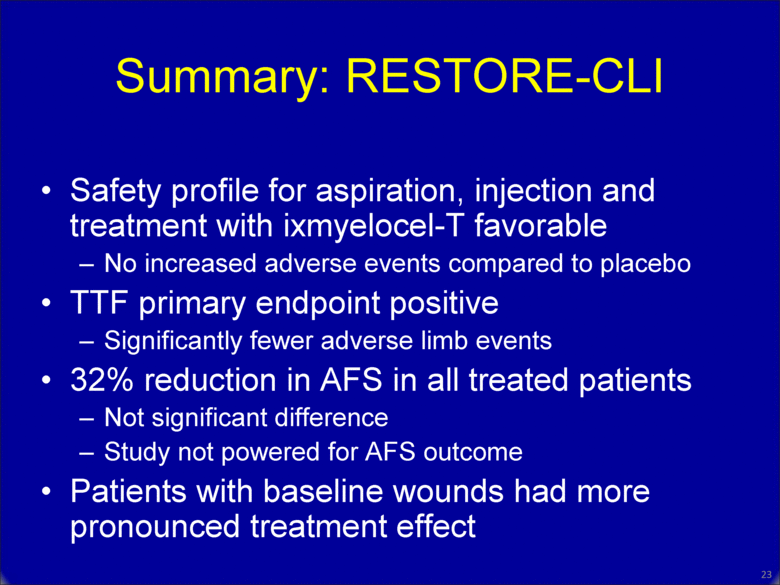
Summary: RESTORE-CLI Safety profile for aspiration, injection and treatment with ixmyelocel-T favorable No increased adverse events compared to placebo TTF primary endpoint positive Significantly fewer adverse limb events 32% reduction in AFS in all treated patients Not significant difference Study not powered for AFS outcome Patients with baseline wounds had more pronounced treatment effect 22 |
|
|
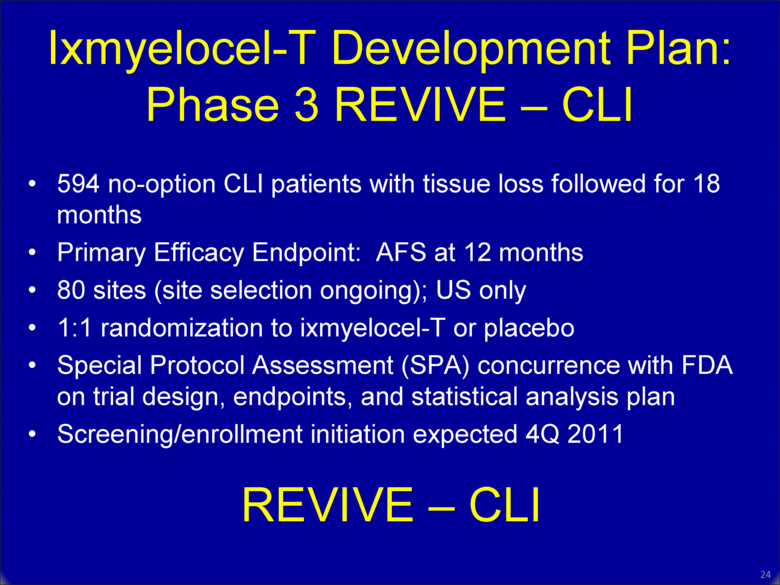
Ixmyelocel-T Development Plan: Phase 3 REVIVE – CLI 594 no-option CLI patients with tissue loss followed for 18 months Primary Efficacy Endpoint: AFS at 12 months 80 sites (site selection ongoing); US only 1:1 randomization to ixmyelocel-T or placebo Special Protocol Assessment (SPA) concurrence with FDA on trial design, endpoints, and statistical analysis plan Screening/enrollment initiation expected 4Q 2011 24 REVIVE – CLI |