Attached files
| file | filename |
|---|---|
| EX-31.1 - EXHIBIT 31.1 - Linkwell CORP | v238930_ex31-1.htm |
| EX-32.1 - EXHIBIT 32.1 - Linkwell CORP | v238930_ex32-1.htm |
| EX-31.4 - EXHIBIT 31.4 - Linkwell CORP | v238930_ex31-4.htm |
| EX-23.1 - EXHIBIT 23.1 - Linkwell CORP | v238930_ex23-1.htm |
| EX-31.3 - EXHIBIT 31.3 - Linkwell CORP | v238930_ex31-3.htm |
| EX-31.2 - EXHIBIT 31.2 - Linkwell CORP | v238930_ex31-2.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Amendment No. 2)
(Mark One)
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the Fiscal Year Ended December 31, 2010
OR
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
Commission file number: 000-24977
Linkwell Corporation
(Exact name of registrant as specified in its charter)
|
Florida
|
65-1053546
|
|
|
(State of Incorporation)
|
(I.R.S. Employer
Identification Number)
|
|
|
1104 Jiatang Road
Jiading District
Shanghai, China
|
201807
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
|
Securities registered under Section 12(b) of the Act:
|
Title of each class
|
Name of each exchange on which registered
|
|
|
None
|
Not Applicable
|
Securities registered pursuant to section 12(g) of the Act: common stock, par value $0.0005 per share
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “accelerated filer”, “large accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large Accelerated Filer o
|
Accelerated Filer o
|
Non-accelerated Filer o
(Do not check if a smaller
reporting company)
|
Smaller reporting company x
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates as of June 30, 2010, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $6,835,660.65.
The number of shares outstanding of capital stock as of March 29, 2011 was 86,605,475.
DOCUMENTS INCORPORATED BY REFERENCE
N/A.
EXPLANATORY NOTE
Linkwell Corporation (the “Company”) is filing this Amendment No. 2 to its Annual Report on Form 10-K/A (this “Amendment”) to its Annual Report on Form 10-K for the year ended December 31, 2010 filed with the Securities and Exchange Commission (the “SEC”) on March 31, 2011 (the “Original Report”), as previously amended by Form 10-K/A filed with the SEC on October 26, 2011 (“Amendment No. 1”), to amend the following:
1) Item 15 of Part IV, to include revised certifications in accordance with the language set forth in Item 601(b)(31) of Regulation S-K in response to SEC comments.
For the purposes of this Amendment, the Original Report and Amendment No. 1 have been amended and restated in their entirety. No other changes have been made to the Original Report. This Amendment speaks as of the original filing date of the Original Report, does not reflect facts or events that may have occurred subsequent to the filing date of the Original Report, and does not modify or update in any way any other disclosures made in the Original Report, or subsequent to any periods for which disclosure was otherwise provided in the Original Report. Accordingly, this Amendment should be read in conjunction with our filings with the SEC subsequent to the filing date of the Original Report, including any amendments thereto.
TABLE OF CONTENTS
|
Part I
|
||||
|
Item 1.
|
Business.
|
2 | ||
|
Item 1A.
|
Risk Factors.
|
20 | ||
|
Item 1B.
|
Unresolved Staff Comments.
|
28 | ||
|
Item 2.
|
Properties.
|
28 | ||
|
Item 3.
|
Legal Proceedings.
|
28 | ||
|
Item 4.
|
(Removed and Reserved).
|
28 | ||
|
Part II
|
||||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
29 | ||
|
Item 6.
|
Selected Financial Data.
|
29 | ||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations.
|
29 | ||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk.
|
36 | ||
|
Item 8.
|
Financial Statements and Supplementary Data.
|
36 | ||
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
|
36 | ||
|
Item9A.
|
Controls and Procedures.
|
36 | ||
|
Item 9B.
|
Other Information.
|
38 | ||
|
Part III
|
||||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance.
|
38 | ||
|
Item 11.
|
Executive Compensation.
|
40 | ||
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
41 | ||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence.
|
44 | ||
|
Item 14.
|
Principal Accounting Fees and Services.
|
45 | ||
|
Part IV
|
||||
|
Item 15.
|
Exhibits, Financial Statement Schedules.
|
46 | ||
|
Signatures
|
49 |
i
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION
Certain statements in this annual report contain or may contain forward-looking statements that are subject to known and unknown risks, uncertainties and other factors which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. These forward-looking statements were based on various factors and were derived utilizing numerous assumptions and other factors that could cause our actual results to differ materially from those in the forward-looking statements. These factors include, but are not limited to, our ability to increase our revenues, develop our brands, implement our strategic initiatives, economic, political and market conditions and fluctuations, government and industry regulation, U.S. and global competition, and other factors. Most of these factors are difficult to predict accurately and are generally beyond our control.
You should consider the areas of risk described in connection with any forward-looking statements that may be made in this annual report. Readers are cautioned not to place undue reliance on these forward-looking statements and readers should carefully review this annual report in its entirety, including the risks described in Part I, Item 1A, Risk Factors. Except for our ongoing obligations to disclose material information under the Federal securities laws, we undertake no obligation to release publicly any revisions to any forward-looking statements, to report events or to report the occurrence of unanticipated events. These forward-looking statements speak only as of the date of this annual report, and you should not rely on these statements without also considering the risks and uncertainties associated with these statements and our business.
OTHER PERTINENT INFORMATION
As used herein, unless the context indicates otherwise, the terms:
“Linkwell”, the “Company”, “we” and “us” refers to Linkwell Corporation,
a Florida corporation;
“Linkwell Tech” refers to our 90% owned subsidiary Linkwell Tech Group, Inc.,
a Florida corporation;
“LiKang Disinfectant” refers to Shanghai LiKang Disinfectant High-Tech Company, Limited,
a wholly-owned subsidiary of Linkwell Tech;
“LiKang Biological” refers to Shanghai LiKang Biological High-Tech Co., Ltd.,
a wholly owned subsidiary of LiKang Disinfectant;
“LiKang International” refers to Shanghai LiKang International Trade Co., Ltd.,
formerly a wholly owned subsidiary of LiKang Disinfectant which was sold to Linkwell International Trading Co., Limited on May 31, 2008.
We also use the following terms when referring to certain related parties:
“Shanhai” refers to Shanghai Shanhai Group, a Chinese company which used to be the minority owner of LiKang Disinfectant;
“Meirui” refers to Shanghai LiKang Meirui Pharmaceuticals High-Tech Co., Ltd., a company of which Shanhai is a majority shareholder;
“ZhongYou” refers to Shanghai ZhongYou Pharmaceutical High-Tech Co., Ltd., a company owned by Shanghai Jiuqing Pharmaceuticals Company, Ltd., whose 65% owner is Shanghai Ajiao Shiye Co. Ltd. Our Chairman and Chief Executive Officer Xuelian Bian is a 60% shareholder of Shanghai Ajiao Shiye Co. Ltd.
The People's Republic of China is herein referred to as China or the PRC.
The information which appears on our web site at www.linkwell.us is not part of this report.
1
PART I
ITEM 1. BUSINESS.
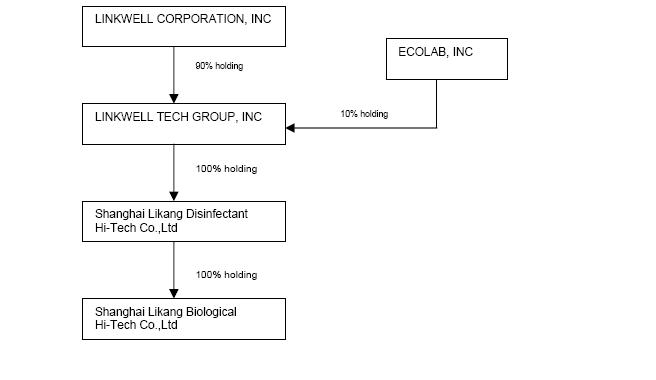
We operate under a holding company structure and currently have one direct operating subsidiary, Linkwell Tech Group Inc. (“Linkwell Tech”), a Florida corporation, of which we own 90%. On February 15, 2008, Linkwell Tech sold 10% of its issued and outstanding capital stock to Ecolab Inc., a Delaware corporation (“Ecolab”). Linkwell Tech owns 100% of Shanghai LiKang Disinfectant High-Tech Company, Limited (“LiKang Disinfectant”). LiKang Disinfectant's business is hospital disinfectant products which is our primary business. LiKang Disinfectant acquired 100% of LiKang Biological on March 5, 2009.
Linkwell Corporation, indirectly through LiKang Disinfectant, is engaged in the development, manufacture, sale and distribution of disinfectant health care products primarily to the medical industry in China. We have a national marketing and sales presence throughout all twenty-two provinces, as well as four autonomous regions, and four municipalities in China. We currently employ 33 full-time sales and marketing employees based in Shanghai. Shanghai ZhongYou Pharmaceutical High-Tech Co., Ltd, (“ZhongYou Pharmaceutical”) a company which is 65% owned by our Chairman and Chief Executive Officer Xuelian Bian, also sells our products using 62 independent sales representatives in China.
While we market our products to the medical industry in China, we are also making efforts to diversify and expand our products to reach the retail markets. We have made efforts to grow our customer base by expanding into the civil, industrial, livestock and agricultural disinfection markets of China. Currently we offer a variety of disinfectant products for the following applications:
· Skin and mucous membrane disinfectants;
· Hand disinfectants (external);
· Environment and surface disinfectants;
· Medical devices and equipment disinfectants;
· Machine disinfectants; and
· Animal disinfectants.
LiKang Disinfectant has 66 marketed products, 46 of which are certified by one or more government authority: the Chinese Ministry of Health, State Food and Drug Administration, or Ministry of Agriculture. China’s Ministry of Health approves products that require the highest level of licensing and have already granted 31 hygiene licenses to us. We also sell products which have been developed and manufactured by third parties. These parties manufacture disinfectant products that generated approximately 0.52% of our revenue for the fiscal year ended December 31, 2010. Products that we manufacture account for approximately 99.48% of our total net revenues for the fiscal year ended December 31, 2010.
Prior to May 31, 2008, we owned 100% of Shanghai LiKang International Trade Co. Ltd., through our subsidiary LiKang Disinfectant. The primary business of LiKang International was the import and export of a variety of products and services ranging from small medical equipment and chemical products to computers. On May 31, 2008, LiKang Disinfectant sold 100% of the capital stock of LiKang International to Linkwell International Trading Co., Ltd., a company registered in Hong Kong which is 100% owned by our Vice President and Director, Mr. Wei Guan.
2
On March 5, 2009, LiKang Disinfectant purchased 100% of LiKang Biological Company for approximately $291,792 (RMB 2,000,000) and 500,000 shares of our common stock.
As of 12/31/2010
Organizational Chart
Industry Background
In 2007, Frost & Sullivan stated, “The Chinese healthcare industry has been one of the fastest growing healthcare industries in the world. It is expected to become the fifth largest by 2010. Its growth is mainly driven by the government’s initiatives to simplify regulatory procedures, enhance trade relations, and attract foreign investment through friendly policies.”
According to the China Federation of Industrial Economics, China’s disinfectant industry is estimated to be well over $6.5 billion annually. The disinfectant industry in China may be characterized as an emerging industry, populated by approximately 1,000 small domestic manufacturers and distributors, and half a dozen large international companies with limited presence and products.
Major contributing factors responsible for the vigor of China’s disinfectant industry growth include the transition to a market economy, increasing health consciousness in the general population and increasing government health standards and education.
Increasing Domestic Demand
Since the shift to a market economy, the Chinese government has initiated several policies to improve public health and living standards and improve the Chinese healthcare industry. Consequently, these initiatives and traditional market forces have driven an increased demand for disinfectant products. According to Frost & Sullivan, China’s healthcare expenditures grew from 5.0% of GDP in 1999 to 6.7% in 2005, representing a growth rate of approximately 5%. During the same period, U.S. healthcare expenditures grew from 13.2% to 15.9%, representing a growth rate of approximately 3%.
3
After nearly 30 years of sustained economic growth in China, both the Chinese government and the public have become more concerned about the quality and cost of healthcare in China. A greater public awareness of the health benefits of our products, as well as growing public concern have led to a surge in interest for disinfectant products in China with consumers maintaining stockpiles of disinfectant products. Other factors that support the growth in demand for disinfectant products include:
· Rapidly increased infectious diseases spreading through the country, such as SARS, H1N1, AIDS, Super Bacteria and some other contagious diseases including cholera, turberculosis, etc.;
· Healthcare professionals and citizens wanting a healthcare system and hygienic standards as advanced as western countries;
· Ongoing government reforms in hospital sanitation, medical standards and disinfectant regulations;
· Government educational program increasing public awareness of public health and hygienic standards; and
· An increase in government investment in healthcare and medical services to achieve sustainable development of the disinfectant industry.
Recent Health Concerns in China
The most critical factors that triggered health concerns in China are the recent and recurring health crises that have led to several epidemics (see Table below) and potential pandemics. In response, the Chinese government has taken initiatives to improve public health and living standards, including the establishment of The Ministry of Public Health in China for the disinfectant industry in China.
|
Outbreak time
|
Location
|
Disease
|
Situation
|
|||
|
January 1988
|
Shanghai
|
Hepatitis A
|
310,000 reported cases of Hepatitis A, 47 deaths
|
|||
|
April - May 1998
|
Shenzhen
|
Sub-Tuberculosis bacillus disease
M. chelonae
|
Shenzhen Woman and Children Hospital reports an airborne infection. 168 patients infected, 46 severe cases
|
|||
|
November 2002
|
Throughout China
|
SARS
|
8,000 reported cases, 800 deaths
|
|||
|
June 24 - August 20, 2005
|
Sichuan Province
|
Streptococcus suis in swine and humans
|
204 reported cases of humans infected with the Swine streptococci in Sichuan, 38 deaths
|
|||
|
April 2005
|
Throughout China
|
Pulmonary tuberculosis, Hepatitis B
|
Pulmonary tuberculosis, Hepatitis B remain top two priorities on the infectious disease list in China
|
|||
|
June 2005
|
Tibet
|
Bubonic plague
|
Five infected cases reported, two deaths
|
|||
|
July-September 2005
|
Hunan, Fujian, Zhejiang provinces
|
Cholera
|
638 cases reported, two deaths
|
|||
|
August 2005
|
Guizhou, Ningxia, Liaoning, Jilin
|
Anthrax
|
140 cases reported, one death
|
|||
|
October 2005
|
Inner Mongolia, Hunan, Anhui, Liaoning, and Hubei provinces
|
Avian Flu
|
Three confirmed cases reported, two deaths
|
|||
|
October 2005
|
Zhejiang, Anhui provinces
|
Highly pathogenic bird flu
|
One confirmed case in each province reported
|
|||
|
March 24, 2006
|
Shanghai
|
Highly pathogenic bird flu
|
One confirmed case reported
|
|||
|
June 16,2006
|
Guangdong province
|
Highly pathogenic bird flu
|
One confirmed case reported
|
|||
|
August 14, 2006
|
XinJiang province
|
Highly pathogenic bird flu
|
One confirmed case reported
|
|||
|
January 9, 2007
|
Anhui province
|
Highly pathogenic bird flu
|
One confirmed case reported
|
|||
|
February 27, 2007
|
Fujian province
|
Highly pathogenic bird flu
|
One confirmed case reported
|
4
|
March 28, 2007
|
Anhui province
|
Highly pathogenic bird flu
|
One confirmed case reported
|
|||
|
May 24, 2007
|
People’s Liberation Army X department
|
Highly pathogenic bird flu
|
One confirmed case reported
|
|||
|
December 2, 2007
|
Jiangsu province
|
Highly pathogenic bird flu
|
One confirmed case reported
|
|||
|
December 6, 2007
|
Jiangsu province
|
Highly pathogenic bird flu
|
One confirmed case reported
|
|||
|
January-February , 2008
|
Xinjiang municipality
|
Measles Virus
|
11,000 cases reported, 21 deaths
|
|||
|
February 2008
|
Guangdong, Guangxi and Hunan provinces
|
Avian Flu
|
Three cases reported, three deaths
|
|||
|
April-May 2008
|
Nationwide
|
Hand, Foot and Mouth Disease
|
176,321 cases reported, 40 deaths
|
|||
|
January-September 2008
|
Nationwide
|
HIV
|
44,839 cases reported, 6,897 deaths
|
|||
|
October-November 2008
|
Hainan province
|
Cholera
|
42 cases reported
|
|||
|
October-November 2008
|
Hainan province
|
Diarrhea
|
351 cases reported
|
|||
|
January 2009
|
Nationwide
|
Avian Flu
|
Eight cases reported, five deaths
|
|||
|
April 2009
|
Worldwide
|
H1N1
|
12,220 deaths
|
|||
|
November 2009
|
Ukraine
|
Flu
|
1.4 million infected and 328 deaths
|
|||
|
January-December
2010
|
Nationwide
|
HIV
|
11,982 cases reported, 7,743 deaths
|
|||
|
October 2010
|
Ningxia Fujian
|
Super Bacteria
|
3 cases reported, 1 deaths
|
|||
|
January-December
2010
|
Nationwide
|
Cholera
|
157 cases reported
|
|||
|
January-December
2010
|
Nationwide
|
Hand, Foot and Mouth Disease
|
1,774,669, cases reported, 905 deaths
|
SARS - Severe Acute Respiratory Syndrome
In recent years, the Severe Acute Respiratory Syndrome (SARS) has threatened the community. SARS, which is a viral respiratory illness caused by a corona virus, called SARS-associated corona virus (SARS-CoV), was first reported in Asia in November 2002. Over the next few months, the illness spread to more than two dozen countries in North America, South America, Europe, and Asia before the SARS global outbreak of 2003 was contained. In April 2004, the Chinese Ministry of Health reported several new cases of possible SARS outbreaks in Beijing and the Anhui province, which is located in east-central China.
According to the Center for Disease Control of the central government of China, the common manner in which SARS appears to spread is by close person-to-person contact. The virus that causes SARS is thought to be transmitted most readily by respiratory droplets (“droplet spread”) when an infected person coughs or sneezes. Droplet spread occurs as germs from the cough or sneeze of an infected person are propelled a short distance (generally up to three feet) through the air and deposited on the mucous membranes of the mouth, nose, or eyes of nearby persons. The virus also can spread when a person touches a surface or object contaminated with infectious droplets and then touches his or her mouth, nose, or eye(s). Ultimately, there is much the global community does not know about SARS, and it is possible that the SARS virus might spread more broadly through the air (airborne spread) or by other ways that are not yet known.
5
Avian Influenza
In 2005, the threat of a global pandemic as a result of the avian flu began to capture the attention of the global community. The avian flu is a type of the A strain virus that infects birds. Typically, it is not common for humans to be infected with the virus via contact with birds, however, a few bird-to-human outbreaks have been reported and most have been in Asia. Humans were infected when they came into contact with sick birds or contaminated surfaces. In most cases, infected persons reported flu-like symptoms, but some had more serious complications, including pneumonia and acute respiratory distress. The avian flu has led to increased concerns for improved health conditions.
Leading up to February 5, 2009, there were 405 confirmed cases of highly pathogenic bird flu reported throughout the world that resulted in 254 deaths. Within China, there were 11 confirmed cases resulting in eight deaths during 2008.
H1N1
HINI is an influenza virus that had never been identified as a cause of infections in people before the current H1N1 pandemic. Genetic analyses of this virus have shown that it originated from animal influenza viruses and is unrelated to the human seasonal H1N1 viruses that have been in general circulation among people since 1977.
Antigenic analysis has shown that antibodies to the seasonal H1N1 virus do not protect against the pandemic H1N1 virus. However, other studies have shown that a significant percentage of people age 65 and older do have some immunity against the pandemic virus. This suggests that some people in older age groups may have some cross protection from exposure to viruses that have circulated in the more distant past.
Unlike typical seasonal flu patterns, the new virus caused high levels of summer infections in the northern hemisphere, and then even higher levels of activity during cooler months in this part of the world. After early outbreaks in North America in April 2009, the new influenza virus spread rapidly around the world. To date, many countries have confirmed infections from the new virus. In 2010, there were 7,123 H1N1 reported cases and 147 deaths in China.
The new virus has also led to patterns of death and illness not normally seen in influenza infections. Most of the deaths caused by the pandemic influenza have occurred among younger people, including those who were otherwise healthy. Pregnant women, younger children and people of any age with certain chronic lung or other medical conditions appear to be at higher risk of more complicated or severe illness. Many of the severe cases have been due to viral pneumonia, which is harder to treat than bacterial pneumonias usually associated with seasonal influenza. Many of these patients have required intensive care.
H1N1 flu viruses are spread mainly from person to person through coughing or sneezing. Sometimes people may become infected by touching an item with flu viruses on it and then touching their eyes, mouth, or nose. Providing sufficient facilities for hand washing and alcohol-based hand sanitizers in common workplace areas such as lobbies, corridors, and restrooms can reduce the chance of spread of the H1N1 virus. Studies have shown that the influenza virus can survive on environmental surfaces and can infect a person for up to 2-8 hours after being deposited on the surface. Disinfecting commonly touched hard surfaces in the workplace, such as work stations, counter tops, door knobs, and bathroom surfaces by wiping them down with disinfectant can reduce the chance of spread of the H1N1 virus.
China Health Standards
In July 2002, the Chinese Ministry of Public Health issued the 27th order of Ministry of Health of the People's Republic of China establishing national standards for the disinfection industry. The first criterion of the new order stipulated that disinfectant manufacturers in China must obtain a license to manufacture hygiene disinfectants. Secondly, prior to release, all disinfectant instruments must obtain the official hygiene permit document of both the local provincial hygiene administrative department and the Ministry of Public Health.
In June 2009, the Chinese Ministry of Public Health issued the Hygiene Standard of Manufacturers of Disinfection Products (2009) (“Hygiene Standard”) which updated the previously issued 2004 version. It specified plant layout, hygiene requirements for workplaces, requirements for production equipment, materials, warehouse and workers. The Hygiene Standard went into effect on January 1, 2010 and restricted market access of for certain small disinfectant companies due to the high Good Manufacturing Practice (“GMP”) standard, which should prove to be beneficial for the normalization of the disinfectant market.
6
The table below details the 30 licenses issued to LiKang Disinfectant by the Chinese Ministry of Public Health.
|
#
|
Products
|
Approved Date
|
||
|
1
|
An’erdian Skin Disinfectant
|
2010.6.11
|
||
|
2
|
An’erdian-type 2nd skin disinfectant
|
2010.6.11
|
||
|
3
|
An’erdian-type 3rd skin and mucous membrane disinfectant
|
2009.5.7
|
||
|
4
|
An’erdian pre-operation Skin Disinfectant
|
2010.6.11
|
||
|
5
|
An’erdian 0.1% PVP-I disinfectant
|
2010.6.11
|
||
|
6
|
Jifro disinfectant gel
|
2008.8.12
|
||
|
7
|
JifroTaixin disinfectant
|
2007.6.4
|
||
|
8
|
JifroSongning disinfectant
|
2008.8.12
|
||
|
9
|
Jifro surgical hand scrub
|
2009.12.22
|
||
|
10
|
Jifro washless surgical hand scrub
|
2010.6.11
|
||
|
11
|
PuTai Skin Disinfectant
|
2010.8.27
|
||
|
12
|
PuTai washless surgical hand scrub
|
2007.1.11
|
||
|
13
|
PuTai washless surgical hand foam disinfectant
|
2010.8.27
|
||
|
14
|
PuTai 4% Chlorhexidine gluconate surgical hand scrub
|
2010.8.27
|
||
|
15
|
Putai 2% Chlorhexidine gluconate disinfectant
|
2009.12.22
|
||
|
16
|
Dian’erkang compound iodine disinfectant
|
2007.11.14
|
||
|
17
|
Dian’erkang Iodophor disinfectant
|
2007.7.18
|
||
|
18
|
Dian’erkang PVP-I disinfectant
|
2009.5.7
|
||
|
19
|
Dian’erkang alcohol disinfectant
|
2007.12.12
|
||
|
20
|
Dian’erkang Aerosol Disinfectant
|
2007.11.14
|
||
|
21
|
Lineng glutaraldehyde disinfectant
|
2008.8.12
|
||
|
22
|
Aiershi disinfectant powder
|
2010.6.11
|
||
|
23
|
Aiershi disinfectant tablets
|
2010.6.11
|
||
|
24
|
Lvshaxing disinfectant tablets
|
2010.6.11
|
||
|
25
|
LiKang 84 disinfectant
|
2009.1.22
|
||
|
26
|
LiKang disinfectant detergent
|
2007.4.19
|
||
|
27
|
LiKang test paper of chlorine
|
2008.1.11
|
||
|
28
|
LiKang 1.8% Glutaraldehyde Monitors (Strip)
|
2009.11.3
|
||
|
29
|
LiKang steam pressure sterilization chemical indicator
|
2009.5.7
|
||
|
30
|
LiKang 121 steam pressure sterilization chemical indicator
|
2009.5.7
|
||
|
31
|
LiKang 132 steam pressure sterilization chemical indicator
|
2009.5.7
|
Product Lines
We market 66 products, which range from air disinfection machines to hot press bags, disinfection swabs, and disinfection indicators. Our products fall into five categories and six product types.
The Five Product Categories Include:
· Skin and Mucous Membrane Disinfectants – Eleven Products;
· Hand Disinfectants – Twelve Products;
· Environment and Surface Disinfectants – Eleven Products;
7
· Medical Devices and Equipment Disinfectants – Six Products;
· Air disinfection equipment – Seven Products; and
· Other products – Nineteen Products.
The Six Product Types Include:
· Liquids – gel;
· Tablets – powder;
· Aerosol;
· Chemical indicator;
· Disinfectant appliance; and
· Liquids – foam.
We believe our varied product line gives us a marketing advantage to build a national customer base for our products and services. Approximately 99.48% of our sales are derived from products we have internally developed and produced and 0.52% of our sales are produced by outside companies. The tables below offer a summary of our current product offerings:
Skin and Mucous Membrane Disinfectants
Skin and mucous membrane disinfectants target both external and internal applications. Prior to operations, incisions, or injections, the products are used to clean the skin surface. Mucous membrane disinfectants target internal germs located in the mouth, eye, perineum, and other internal sources. This product group accounted for approximately 48.51% of our sales in the 2010 fiscal year, and approximately 45.8% of our sales in the 2009 fiscal year. The table below lists our skin and/or mucous membrane disinfectants.
|
Product Names
|
Ingredients
|
Application
|
Industry Standard
|
|||
|
An’erdian Skin Disinfectant
|
iodine, alcohol
|
Skin disinfectant
|
Q/SUVE 20-2003
|
|||
|
An’erdian-type 3rd skin and mucous membrane disinfectant
|
iodine, chlorhexidine
|
Skin & mucous membrane disinfectant
|
Q/SUVE 22-2003
|
|||
|
Dian’erkang PVP-I disinfectant
|
Povidone-iodine
|
Skin & mucous membrane disinfectant
|
Q/SUVE 28-2004
|
|||
|
Dian’erkang alcohol disinfectant
|
alcohol
|
Skin disinfectant
|
Q/SUVE 08-2004
|
|||
|
PuTai Skin disinfectant
|
Chlorhexidine gluconate, alcohol
|
Skin disinfectant
|
Q/SUVE 37-2006
|
Hand Disinfectants
Hand disinfectants typically target the skin surface. Products are applied to the skin prior to medial procedures. This product group accounted for approximately 27.9% of our sales in the 2010 fiscal year and approximately 26.3% of our sales in the 2009 fiscal year. The table below lists our hand disinfectants.
|
Product Names
|
Ingredients
|
Application
|
Industry Standard
|
|||
|
Jifro antimicrobial hand washing
|
Chlorhexidine
|
Hand washing
|
Q/SUVE 04-2003
|
|||
|
Jifro disinfectant gel
|
DP300 (Triclosan)
|
Hand disinfectant
|
Q/SUVE 02-2003
|
|||
|
Jifro 4% Chlorhexidine gluconate surgical hand scrub
|
Chlorhexidine gluconate
|
Surgical hand disinfectant
|
Q/SUVE 09-2004
|
|||
|
PuTai washless surgical hand scrub
|
Chlorhexidine gluconate, alcohol
|
Hand disinfectant
|
Q/SUVE 39-2006
|
|||
|
PuTai washless surgical hand foam disinfectant
|
Chlorhexidine gluconate, alcohol
|
Hand disinfectant
|
Q/SUVE 38-2006
|
8
Environment and Surface Disinfectants
Environment and surface disinfectants target a variety of surfaces, such as floors, walls, tables, and medical devices. The products can also be applied to cloth materials including furniture and bedding. This product group accounted for approximately 9.76% of our sales in the 2010 fiscal year and approximately 12.5% of our sales in the 2009 fiscal year. The table below lists our environment and surface disinfectants.
|
Product Names
|
Ingredients
|
Application
|
Industry Standard
|
|||
|
Aiershi disinfectant tablets
|
Trichloroisocyanuric acid
|
Environment and surface disinfection
|
Q/SUVE 34-2004
|
|||
|
Lvshaxing disinfectant tablets
|
Dichloro dimethylhydantoin
|
Environment and surface disinfection
|
Q/SUVE 33-2003
|
|||
|
Dian’erkang aerosol disinfectant
|
Benzethonium Chloride
|
Environment and surface disinfection, preventing the spread of airborne viruses such as human influenza virus, SARS, and the Bird flu virus
|
Q/SUVE 07-2004
|
|||
|
Lvshaxing disinfectant granule
|
Dichloro dimethylhydantoin
|
Environment and surface disinfection
|
Q/SUVE 32-2003
|
|||
|
LiKang disinfectant detergent
|
Sodium hypochlorite
|
Surface disinfectant
|
Q/SUVE 37-2006
|
Medical Devices and Equipment Disinfectants
Medical devices and equipment disinfectants target medical equipment, including the sterilization of thermo sensitive instruments and endoscope equipment. This product group accounted for approximately 10.53% of our sales in the 2010 fiscal year and approximately 9.9% of our sales in the 2009 fiscal year. The table below lists our medical device and equipment disinfectants.
|
Product Names
|
Ingredients
|
Application
|
Industry Standard
|
|||
|
Dian’erkang 2% glutaraldehyde disinfectant
|
Glutaraldehyde
|
Disinfection and sterilization of device
|
Q/SUVE 10-2003
|
|||
|
Dian’erkang 2% glutaraldehyde disinfectant (sales to Olympus Corporation)
|
Glutaraldehyde
|
Disinfection and sterilization of endoscopes
|
Q/SUVE 10-2003
|
|||
|
Dian’erkang multi-enzyme rapid detergents
|
Multi-Enzyme
|
Rinsing and decontamination of device
|
Q/SUVE 14-2004
|
Disinfecting Apparatus Series
The disinfecting apparatus series is a line of disinfectants that target skin disinfection, air quality and disinfection results. This product group accounted for approximately 2.78% of our sales in the 2010 fiscal year and approximately 1.3% of our sales in the 2009 fiscal year. The table below lists our machine series disinfectants.
|
Product Names
|
Ingredients
|
Application
|
Industry Standard
|
|||
|
Lvshaxing LKQG-1000 air disinfection machine
|
Ozone, ultraviolet radiation, electrostatic
|
Air disinfection
|
Q/SUPE 09-2003
|
|||
|
An’erdian disinfection swab
|
An’erdian
|
Skin and disinfection
|
Q/NYMN07-2003
|
|||
|
LiKang test paper of chlorine
|
reagent
|
Indicates disinfectant concentration
|
Q/SUVE 40-2003
|
|||
|
LiKang 121 steam pressure sterilization chemical indicator (card and adhesive tape)
|
Indication oil
|
Indicates sterilization effect
|
Q/SUVE 16-2005
|
|||
|
LiKang 132 steam pressure sterilization chemical indicator (label)
|
Indication oil
|
Indicates sterilization effect
|
Q/SUVE 17-2005
|
|||
|
LiKang steam pressure sterilization chemical indicator
|
Indication oil
|
Indicates sterilization effect
|
Q/SUVE 18-2005
|
9
Retail products
In 2005, we began to expand our distribution to reach the retail markets. As a result, our products started being carried by hotels, schools, supermarkets, and drugstores. We have repackaged commercial disinfectant products for sale to the consumer market. Since October 1999, we redeveloped four separate products for distribution to the retail market. LiKang Disinfectant redeveloped the following products in the months and years listed:
|
· Jin Zhongda collutory (mouthwash)
|
October 1999
|
|
|
· Antibacterial lubricant
|
October 1999
|
|
|
· LiKang Disinfectant 84
|
August 2005
|
|
|
· Dian’erkang aerosol disinfectant
|
October 2005
|
Customers
We sell our products on a wholesale and retail basis to the medical community in China. We have approximately 6,000 or more active and recurring customers including hospitals, clinics, health centers, medical suppliers, and distribution companies throughout China. We maintain over 20 distribution contracts with wholesale dealers and agents. We generally offer our customers payment terms of four to six months to complete payment. For the fiscal year ended December 31, 2010, two affiliated entities that are our customers, ZhongYou Pharmaceutical and Shanghai Jiuqing Pharmaceuticals Co. Ltd., represented approximately 51.75% of our total net revenues.
Manufacturing
We operate two production facilities in Shanghai, one located in the Shanghai Jiading district and one located in the Shanghai Jinshan district. Products are manufactured primarily in liquid, tablet, and powder form. Approximately 99.48% of LiKang Disinfectant’s revenue for the 2010 fiscal year was derived from products manufactured in these two factories.
The Shanghai Jiading district factory is approximately 21,500 square feet, all of which is used for production. The main products produced at the Shanghai Jiading district factory are liquid and index disinfectant devices. The manufacturing facility has the capacity to produce approximately 9 million liters of liquid disinfectant annually. The manufacturing cycle for the liquids, from formulation to finished product, is one day.
The Shanghai Jinshan district factory is approximately 4,300 square feet and is used in the manufacture of the tablet and powder forms of disinfectants. The manufacturing capacity is 300 metric tons of tablet and 180 metric tons of powder disinfectant annually. The average manufacturing cycle for the tablets and powder, from formulation to finished product, is one day.
During the 2007 fiscal year, following GMP certification for both the factory and the equipment, we began utilizing the services of LiKang Biological, a related party, to manufacture some of our products including our An'erdian and Dian'erkang lines of disinfectants. On March 25, 2008, Linkwell Tech purchased 100% of the issued and outstanding stock of LiKang Biological.
10
We have found in our experience that products manufactured at GMP certified facilities utilizing GMP certified equipment can be sold at higher prices than similar products manufactured at non- GMP certified facilities. While GMP certified products cost more to produce, we are able to increase our selling prices proportionally. Our product packaging varies to meet different needs of the market. We package our liquid and gel disinfectants in popular sizes ranging from 40 ml to 5 liters. We package these tablets in 50 tablet, 100 tablet and 200 tablet bottles. Finally, we package our powder disinfectants in 250 gram and 500 gram containers.
We maintain an inventory of finished products equal to approximately 1 month of average sales. Currently, we are manufacturing at about 50% of full capacity based upon our current product demand, and we have the ability to increase to full capacity if demand continues to increase.
We have an in-house fulfillment and distribution operation, which is used to manage our supply chain, beginning with the placement of the order and continuing through order processing, packaging and shipping the products to each customer. We maintain inventory and fill customer orders from both the Jiading factory and the Jinshan factory.
Raw Materials
We purchase raw materials from six primary suppliers, and we have signed purchase contracts with these suppliers in an effort to ensure a steady supply of raw materials. We have maintained stable business relations with these suppliers for over 10 years, and we believe that our relationships with these primary suppliers will remain stable. In the event that these relationships falter, there are many other suppliers with the capability to supply our Company. We purchase raw materials on payment terms of 30 days to three months. Some of the suppliers import from foreign countries, as listed below, and we purchase directly from these suppliers.
The table below details the supply relationships for raw materials
|
Raw materials
|
Suppliers
|
Origin
|
||
|
Iodine
|
Shanghai Wenshui Chemical Co., Ltd
|
USA
|
||
|
Potassium iodide
|
Shanghai Wenshui Chemical Co., Ltd
|
Holland
|
||
|
Glutaraldehyde
|
Shanghai Jin an tang Hygienical Product Factory
|
Germany
|
||
|
Triclosan
|
Ciba Specialty Chemicals (China) LTD
|
Domestic
|
||
|
Alcohol
|
Shanghai Jangbo Chemical Co., L td
|
Domestic
|
||
|
Trichloroisocyanuric acid
|
Xuzhou Keweisi Disinfectant Co., Ltd
|
Domestic
|
Customer Service and Support
We believe that a high level of customer service and support is critical in retaining and expanding our customer base. Customer care representatives participate in ongoing training programs under the supervision of our training managers. These training sessions include a variety of topics such as product knowledge and customer service tips. Our customer care representatives respond to customers’ e-mails and calls that are related to order status, prices and shipping. If our customer care representatives are unable to respond to a customer’s inquiry at the time of the call, we strive to provide an answer within 24 hours. We believe our customer care representatives are a valuable source of feedback regarding customer satisfaction. Our customer returns and credits average approximately 1% of total sales.
New Product Development
We are committed to research and development. LiKang Disinfectant was created as a research and development organization by the Second Military Medical University (SMMU) of the Chinese Army in 1988. We develop our products internally and own all rights associated with these products.
11
In 2007, we commercialized four disinfectant products, including PuTai Skin Disinfectant, PuTai washless surgical hand scrub, PuTai washless surgical hand foam disinfectant and LiKang disinfectant detergent, which is an environmental and surface disinfectant. In 2008, eight products were in development, and licensing applications were filed for four of these eight products. In 2009, we commercialized two new disinfectant products, Jifro surgical hand scrub and Putai 2% Chlorhexidine gluconate disinfectant.
In 2010, we commercialized two new disinfectant products, An’erdian pre-operation Skin Disinfectant and An’erdian 0.1% PVP-I disinfectant, and we are also in the process of developing five additional products.
For the fiscal years ended December 31, 2010 and 2009, we spent approximately $311,268 and approximately $108,026, on research and development, respectively.
Marketing and Sales
We were formed in 1988 as a research and development organization by the Second Military Medical University (SMMU) of the Chinese Army. Our CEO, Mr. Xuelian Bian, was a member of the staff of SMMU. We believe that his relationships with alumni and business persons associated with SMMU provide us with certain marketing advantages. The university is a well recognized, prestigious institution in China and many of its graduates work at hospitals, medical suppliers and distribution companies throughout China in senior positions, which places them in the decision making process for purchasing the kind of products we sell. In addition, the students and faculty at the university provide a pool of talent from which we draw, both as potential employees or summer interns who go on to work at other companies, many of whom are customers or potential customers of our products. In marketing our products, we seek to leverage these relationships.
During the 2007 fiscal year, we expanded our distribution capability in the PRC. We have a national marketing and sales presence throughout all 22 provinces, as well as four autonomous regions, and four municipalities of China. We currently employ 33 full-time sales and marketing employees based in Shanghai. ZhongYou Pharmaceutical, an affiliate, also sells our products using 62 independent sales representatives in other provinces of China.
Approximately 48.25% of our sales are achieved by our proprietary sales force, while the remaining 51.75% are outsourced to independent dealers and agents. We compensate our proprietary salesman with a base salary plus commission. The sales representatives are located in each of China’s provinces. The external sales network currently covers hospitals in the following 22 provinces and municipalities including: Beijing, Guangdong, Tianjin, Fujian, Yunnan, Hainan, Jiangsu, Zhejiang, Anhui, Shandong, Henan, Hebei, Liaoning, Heilongjiang, Shaanxi, Gansu, Ningxia, Guizhou, Hunan, Sichuan, Xinjiang, Neimenggu. The independent sales representatives sell directly to the end-users.
Disinfectant Educational Center
On May 25, 2006, we entered into an agreement with China Pest Infestation Control and Sanitation Association, an association governed by the Chinese central government, to establish and operate a disinfectant educational center in Beijing, China. We are responsible for the establishment and development of the disinfectant educational center, as well as its management and funding. The China Pest Infestation Control and Sanitation Association is responsible for establishing a job training base in Beijing. We believe we were selected to participate in this program based upon our reputation and experience in the disinfectant industry.
We anticipate that the disinfectant educational center will offer a job training program to educate and train professionals to work in the disinfectant field. The disinfectant educational center will be a tuition based education program for which graduates will receive a license from the China Pest Infestation Control and Sanitation Association. After completion of the program, it is envisioned that a personnel exchange service center of the Chinese central government's Health Department will function much like a placement office and assist the center's graduates in securing positions with companies seeking to fill positions in the PRC. From time to time we may also recruit graduates from the disinfectant educational center to join our company.
12
In 2006, LiKang Disinfectant entered into an agreement with the China Pest Infestation Control Association, the Ministry of Health and the Beijing Olympic Game Committee to establish and operate a disinfectant educational center in Beijing, China. In accordance with the agreement, LiKang Disinfectant is responsible for the establishment of the disinfectant educational center, as well as its management and funding. As of December 31, 2007, we had provided the text books and technical standards for training. In 2008, prior to the Beijing Olympic Games starting, we held the first class for training the 2008 Beijing Olympic Staff. Out of the 51 participants in the training, 46 of them became qualified.
After the Wenchuan Earthquake occurred, LiKang Disinfectant entered into an agreement with the Ministry of Health, the National Patriot and Sanitation Committee, and the Dujiangyan National Disaster Headquarters to provide service to stricken areas. On June 6, 2008, Likang Disinfectant sent its own teams to Sichuan to provide sanitation technical training for over 270 staff as well as providing a qualification course and examinations to over 100 national disinfectors. On June 27, 2008, 76 national disinfectors had taken professional qualification exams and 67 national disinfectors were qualified. The passage rate was 88%.
In 2008, there were 325 participants that attended the Likang Disinfectant training course, of which 117 became qualified. In 2009, there were 2,320 participants that attended the Likang Disinfectant training course, of which 2,156 became qualified.
In 2010, there were 1,100 participants that attended the Likang Disinfectiant training course, of which 1,045 became qualified.
Intellectual Property
We have received eleven patents and have one pending patent application with National Property Right Administration of the PRC. The patent approval process can take up to 36 months. The following is a list of LiKang Disinfectant’s patents and pending patent applications:
|
Patent Category
|
Patent name
|
Patent No
|
Notes
|
|||
|
New invention
|
Low smell and stimulus contain chlorine disinfectant tablet, powder etc.
|
ZL 200410068135.8
|
Approved, expires August 2026
|
|||
|
New invention
|
A new skin & mucous membrane disinfectant including preparation methods
|
Application # 200410025305.4
|
Pending. Applied on 2004-11-12
|
|||
|
Appearance design
|
Bottle (with the wing stretch)
|
ZL 00 3 14391.0
|
Approved, expired on April 2010
|
|||
|
Appearance design
|
Packaging bottle
|
ZL 2003 3 0108274.5
|
Approved, expires November 2013
|
|||
|
Appearance design
|
Bottle
|
ZL 200530034239.2
|
Approved, expires December 2015
|
|||
|
Appearance design
|
Test paper box of chlorine
|
ZL 2004 3 0022740.2
|
Approved, expires May 2014
|
|||
|
Product Improvement
|
Improved heavy duty bottle
|
ZL 03 2 29616.9
|
Approved, expires March 2013
|
|||
|
Product Improvement
|
High strength water sterilizer with Model H ultraviolet lamp
|
ZL 03 2 10513.4
|
Approved, expires September 2013
|
|||
|
Product Improvement
|
Sewage application
|
ZL 2004 2 0037013.8
|
Approved, expires June 2014
|
|||
|
Product Improvement
|
Container with the vacuum pump
|
ZL 200420090682.1
|
Approved, expires June 2016
|
|||
|
Product Improvement
|
Multifunctional air disinfectant
|
ZL 200420037010.4
|
Approved, expires August 2015
|
|||
|
Product Improvement
|
Bracket for heavy duty bottle
|
ZL 200520039668.3
|
Approved, expires October 2016
|
13
We have nine product trademarks, of which four are registered trademarks with the China State Administration for industry and commerce trademark office. These trademarks cover our four major product lines, An’erdian, Jifro, Dian’erkang and Lvshaxing.
We are not a party to any confidentiality or similar agreement with any of our employees or any third parties regarding our intellectual property. It is possible that a third party could, without authorization, utilize our propriety technologies without our consent. We can give no assurance that our proprietary technologies will not otherwise become known or independently developed by competitors.
Competition
We operate in a fragmented, competitive national market for healthcare disinfectant products. According to a survey conducted in 2004 by the China Federation of Industrial Economics (CFIC), the disinfectant market in the PRC was approximately $6.25 billion (USD). While the disinfectant industry in China is an emerging industry, and the industry is populated with small regional players, we estimate that there are over 1,000 manufacturers and distributors of disinfectant products in China and a certain number of our major competitors distribute products similar to ours, including those which also prevent the spread of airborne viruses such as avian flu and SARS.
We also compete with foreign companies, including 3M, who are marketing a limited line of disinfectant products in China, as well as smaller, domestic manufacturers. Most domestic competitors offer a limited line of products and there are few domestic companies with a nationwide presence. We believe that our national marketing and sales presence throughout all twenty-two provinces, as well as four autonomous regions, and four municipalities of China gives us a competitive advantage over many other disinfectant companies in China.
In addition, prior to the adoption of industry standards in July 2002 by the central government of China, disinfectant products were generally marketed and sold based on pricing factors. We believe the recent standards implemented by the government and a growing middle income class will shift the customer demand from price to quality.
As a result of this heightened license and permit system, all disinfectant manufacturers must comply with "qualified disinfection product manufacturing enterprise requirements” established by the Ministry of Public Health. The requirements include standards for both hardware and software. Hardware includes facilities and machinery and software includes the technology to monitor the facilities. The requirements also encompass the knowledge and capability of both the production staff and quality control procedures.
Furthermore, we estimate that the government standards adopted in July 2002 have increased the barriers to enter into the disinfectant industry. We believe that the new standards may lead to fewer competitors as companies falter in their efforts to adhere to the new standards. The implementation of these improved production standards and licenses have effectively decreased the competitiveness of small and mid-size manufacturers. Compliance with the new standards is especially difficult for companies with limited product offerings and inferior technical content.
14
Competitive Advantages
We believe that the following are the principal competitive strengths that differentiate our Company from the majority of our competition:
· Strong sales and distribution network in China – This enables us to compete effectively with domestic competitors, as well as larger foreign-owned competitors.
· Product selection and availability – A number of our competitors are smaller, regional companies with a limited number of product offerings. We offer our customers a wide variety of disinfectant products and the ability to ship products to our customers on a timely basis throughout the PRC.
· Research and development – Our efforts to respond to market demand for new products have resulted in the issuance to us of 31 hygiene licenses by the Ministry of Public Health of the central government of China. Based upon our knowledge of our competitors, we do not believe any of our competitors have received as many licenses since the enactment of the licensing standards in July 2002.
· Strong product pipeline – We have a history of introducing three or four new products to the market each year. We have filed applications for four new products and have eight additional products in development.
· Manufacturing capacity – We are operating at 91% capacity to produce GMP certified products and we have the ability to increase capacity significantly at moderate costs.
· Customer services – Our sales personnel are thoroughly educated about our products, which enable them to better understand the needs of our customers. Our customer service representatives strive to answer questions immediately and, at a minimum, no later than 24 hours after a customer’s inquiry.
· Reliability and speed of delivery. We believe our products have developed a reputation of good quality and effectiveness and our manufacturing capabilities enable us to produce and ship products to our customers promptly.
· Customer service – Our customer service representatives participate in ongoing product training programs and we strive to respond to all customer inquiries within 24 hours.
· Price – We have developed relationships with a number of raw material suppliers which enables us to keep our costs low and thereby offer prices to our customers which are very competitive.
Our primary competitors in the sale of chemical disinfectants are 3M and Ace Disinfection Factory Co., Ltd. The primary competitors for instrument disinfectants are Chengdu Kangaking Instrument Co., Ltd. and Hangzhou Yangchi Medicine Article Co., Ltd. The primary competitors for chemical indicators are 3M and Shandong Xinhua Medical Instrument Co., Ltd. Domestic competition comes from regional companies which tend to offer products in small geographic areas and do not distribute their product lines throughout China.
Our primary competitors include:
|
Competitor
|
Products
|
|
|
3M Company
|
Hand disinfectant, skin and mucous disinfectant
|
|
|
Ace
|
Skin and mucous disinfectant
|
|
|
Chengdu Kangaking
|
Medical equipment and devices
|
|
|
Hangzhou Yangchi
|
Sterilized Q-tip
|
|
|
Shandong Xinhua
|
Chemical indicators
|
Our primary foreign competitor is 3M , which has had a presence in China for more than 20 years. 3M entered the hand disinfection market at the end of 2004 and primarily offers products in the areas of index and control devices and disinfectant machines. At present, 3M has five products for use in operating rooms and its products are found in provincial capital cities of China such as Shanghai, Beijing, Guangzhou, Hangzhou, Nanjing, Chengdu and Xi’an. 3M’s product line in China is relatively narrow, with few overlapping products between 3M and our Company.
15
Another foreign competitor is Johnson & Johnson, which established its operations in China in 1994. In China, Johnson & Johnson offers a variety of skin, hand, and medical equipment disinfectants. Prior to the recent initiatives taken by the government, disinfectant products were marketed based on pricing, and despite the brand awareness of Johnson & Johnson, its products did not have widespread reception among the community. Furthermore, Johnson & Johnson does not offer a wide variety of disinfectant products in China.
Government Regulations
Our business and operations are located in the PRC. We are subject to local food, drug, environmental laws related to certification of manufacturing and distributing of disinfectants. We are also licensed by the Shanghai City Government to manufacture and distribute disinfectants. We are in substantial compliance with all provisions of those licenses and have no reason to believe that they will not be renewed as required by the applicable rules of Shanghai. In addition, our operations must conform to general governmental regulations and rules for private companies conducting business in China.
Pursuant to the July 2002 Ministry of Public Health 27th Order of Ministry of Health of the People's Republic of China, all disinfectant manufacturers in China must obtain a license to manufacture hygiene disinfectants. Prior to such release, all disinfectant instruments must obtain the official hygiene permit document from the Ministry of Public Health and the approval of the provincial hygiene administrative department. The implementation of these improved production standards and licenses has effectively decreased the competitiveness of small to mid size manufacturers with single product and inferior technical content. Presently, we meet all standards initiated by this ordinance and we have been granted 28 hygiene licenses by the Ministry of Public Health.
We are also subject to various other rules and regulations, including the People’s Republic of China Infectious Disease Prevention and Cure Law, Disinfection Management Regulation, Disinfection Technique Regulation, Disinfection Product Manufacturer Sanitation Regulation, and Endoscope Rinse and Disinfection Technique Manipulation Regulation. We believe we are in material compliance with all of the applicable regulations.
Sanitary Standard for Producing Disinfectant products Enterprises 2009
To strengthen the supervision and standardize the behavior of disinfectant products, the People's Republic of China Ministry of Health have revised the old disinfectant manufacturing criteria and enacted the “ Sanitary Standard for Producing Disinfectant products Enterprises 2009 ” based on "Infectious Diseases Prevention Law" and "sterilization management approach" effective January 1, 2010.
According to the new guideline, the compounding, mixing, and subpackaging of Skin and mucous membrane disinfectants and antibacterial preparation (except for hand-washing products) should be processed in a workshop with a 300,000 level of air purification.
PRC Legal System
Since 1979, many laws and regulations addressing economic matters in general have been promulgated in the PRC. Despite the development of its legal system, the PRC does not have a comprehensive system of laws. In addition, the enforcement of existing laws may be uncertain and sporadic, and implementation and interpretation thereof inconsistent. The PRC judiciary is relatively inexperienced in enforcing the laws that exist, leading to a higher than usual degree of uncertainty as to the outcome of any litigation. Even where adequate laws exist in the PRC, it may be difficult to obtain swift and equitable enforcement of such laws, or to obtain enforcement of a judgment by a court of another jurisdiction. The PRC's legal system is based on written statutes and, therefore, legal precedents are often without any binding effect. The interpretation of PRC laws may be subject to policy changes reflecting domestic political changes. As the PRC legal system develops, the promulgation of new laws, changes to existing laws and the preemption of local regulations by national laws may adversely affect foreign investors. The trend of legislation over the past 20 years has, however, significantly enhanced the protection of foreign investors in the PRC. However, there can be no assurance that changes in such legislation or interpretation thereof will not have an adverse effect on our business operations or prospects.
16
Economic Reform Issues
Since 1979, the Chinese government has reformed its economic systems. Many reforms are unprecedented or experimental; therefore, they are expected to be refined and improved. Other political, economic and social factors, such as political changes, changes in the rates of economic growth, unemployment or inflation, or disparities in per capita of wealth between regions within China, could lead to further readjustment of the reform measures. We cannot predict if this refining and readjustment process may negatively affect our operations in future periods.
Over the last several years, China's economy has demonstrated a high growth rate. Recently, there have been indications that rates of inflation have increased. In response, the Chinese government has taken measures to curb the economy from expanding too rapidly. These measures have included devaluations of the Chinese currency as the result of inflation. This relative Renminbi (“RMB”) devaluation places some restrictions on the availability of domestic credit, the purchase capability of customers, and re-centralization of the approval process for some foreign product purchases. These measures alone may not succeed in slowing down the economy's excessive expansion or control inflation. The Chinese government may adopt additional measures to further combat inflation, including the establishment of freezes or restraints on certain projects or markets.
To date, China’s economic reforms have not adversely impacted our operations and are not expected to adversely impact operations in the foreseeable future; however, there is still no assurance that such reforms will not adversely affect us in the changes of China's political, economic, and social conditions and the changes in policies of the Chinese government. To be more specific, such as changes may include laws and regulations, measures which may be introduced to control inflation, changes in the rate or method of taxation, imposition of additional restrictions on currency conversion and remittance abroad, and reduction in tariff protection and other import restrictions.
As of December 31, 2010, we employed approximately 170 full time employees, including our executive officers. The numbers are listed as follows:
|
Department
|
Number of Employees
|
|
|
Administrative center
|
21
|
|
|
Accounting
|
11
|
|
|
Production
|
70
|
|
|
Logistics
|
18
|
|
|
Sales and Marketing Staff in Shanghai
|
33
|
|
|
Training
|
5
|
|
|
Research and Development
|
12
|
|
|
Total
|
170
|
17
U.S. Advisors
On May 1, 2008, we entered into a two year agreement with China Health Capital Group, Inc. (“CHC”) to provide us with financial and investment services. In connection with this agreement, on June 24, 2008, we issued 2,000,000 shares of common stock valued at $0.21 per share to CHC and recorded $420,000 as deferred compensation. We amortized $210,000 as stock-based compensation for the year ended December 31, 2009.
On June 27, 2008, Monarch Capital Fund, Ltd. exercised a warrant to purchase 100,000 shares of our common stock with a price of $0.20 per share. We received proceeds from this warrant exercise of $20,000 on June 24, 2008.
On July 1, 2009, we entered into a two year agreement with First Trust Group, Inc. In connection with this agreement, we issued 1,800,000 shares of our common stock valued at $0.09 per share to First Trust Group, Inc. and recorded $162,000 as deferred compensation. We amortized $121,500 as stock-based compensation for the year ended December 31, 2009.
History of Our Company
Linkwell Corporation (formerly Kirshner Entertainment & Technologies, Inc.) was incorporated in the State of Colorado on December 11, 1996. On May 31, 2000, we acquired 100% of HBOA.com, Inc. On December 28, 2000, we formed a new subsidiary, Aerisys Incorporated (“Aerisys”), a Florida corporation, to handle commercial private business. In June 2003, we formed our entertainment division and changed our name to reflect this new division. Effective as of March 31, 2004, we discontinued our entertainment division and our technology division, except for the Aerisys operations that continue on a limited basis.
On May 2, 2005, we entered into and consummated a share exchange with all of the shareholders of Linkwell Tech Group, Inc. (“Linkwell Tech”). Pursuant to the share exchange, we acquired 100% of the issued and outstanding shares of Linkwell Tech's common stock, in exchange for 36,273,470 shares of our common stock, which at closing represented approximately 87.5% of the issued and outstanding shares of our common stock. As a result of the transaction, Linkwell Tech became our wholly-owned subsidiary. Linkwell Tech was founded on June 22, 2004, as a Florida corporation. On June 30, 2004, Linkwell Tech acquired 90% of Shanghai LiKang Disinfectant High-Tech Company, Ltd. (“LiKang Disinfectant”), a PRC company, through a stock exchange. This transaction resulted in the formation of a U.S. holding company by the shareholders of LiKang Disinfectant as it did not result in a change in the underlying ownership interest of LiKang Disinfectant. LiKang Disinfectant is a science and technology enterprise founded in 1988. LiKang Disinfectant is involved in the development, production, marketing and sale, and distribution of disinfectant health care products.
LiKang Disinfectant has developed a line of disinfectant product offerings which are utilized by the hospital and medical industry in China. LiKang Disinfectant has developed a line of disinfectant product offerings. LiKang Disinfectant regards hospital disinfectant products as the primary segment of its business and has developed and manufactured several series of products in the field of skin mucous disinfection, hand disinfection, surrounding articles disinfection, medical instruments disinfection and air disinfection.
18
On June 30, 2005, our Board of Directors approved an amendment of our Articles of Incorporation to change the name of our Company to Linkwell Corporation. The effective date of the name change was after close of business on August 16, 2005.
On April 6, 2007, our subsidiary, Linkwell Tech, entered into two material stock purchase agreements as follows:
i) Linkwell Tech entered into an agreement (the “Biological Stock Purchase Agreement”) to acquire a 100% equity interest in Shanghai LiKang Biological High-Tech Company, Ltd. (“LiKang Biological”), a Chinese company, in a related party transaction with Mr. Xuelian Bian, the Company’s Chief Executive Officer, Mr. Wei Guan, the Company’s Vice-President and Director, and Shanghai Likang Pharmaceuticals Technology Co., Ltd. (“LiKang Pharmaceutical”), a PRC company. Before the Company entered into the Biological Stock Purchase Agreement, Mr. Bian and Mr. Guan owned 90% and 10% of LiKang Pharmaceutical, respectively. Mr. Bian and LiKang Pharmaceutical owned 60% and 40% of LiKang Biological, respectively. Pursuant to the terms of the Biological Stock Purchase Agreement, Mr. Bian and LiKang Pharmaceutical were to receive 1,000,000 shares of Linkwell Corporation’s restricted common stock.
Due to restrictions under PRC law that prohibited the consideration contemplated by the Biological Stock Purchase Agreement, the agreement did not close. As a result, on March 25, 2008, the parties agreed to enter into an amendment to the Biological Stock Purchase Agreement (“Biological Amendment”) in an effort to complete the stock purchase transaction. Pursuant to the terms of the Biological Amendment, the only material change to the Biological Stock Purchase Agreement related to the consideration paid by Linkwell Tech to Xuelian Bian and LiKang Pharmaceutical, which was changed from 1,000,000 shares of the Company’s common stock to 500,000 shares of common stock and $200,000. As of December 31, 2008, the Biological Stock Purchase Agreement was pending and required further approval from the PRC Ministry of Commerce. Due to the time consuming and complicated nature of the approval procedure, the parties agreed to enter into a second amendment to the Biological Stock Purchase Agreement (“Second Amendment”) in order to complete the purchase transactions timely and properly. Pursuant to the terms of the Second Amendment, the purchaser was changed from Linkwell Tech to LiKang Disinfectant, and, in addition, the consideration changed to RMB 2,000,000, approximately $292,500, and 500,000 shares of common stock. These changes were pertinent because an approval from the Ministry of Commerce in the People’s Republic of China would not be necessary if LiKang Disinfectant acquired 100% of the equity interest in LiKang Biological, as both companies are registered in the PRC. This transaction closed on March 5, 2009. During the quarter ended September 30, 2009, LiKang Disinfectant increased its investment into Likang Biological by injecting RMB 2.5 million cash and RMB 5.5 million in intangible assets (patents) into Likang Biological. Likang Biological is mainly engaged in producing the disinfectant concentrate.
ii) Linkwell Tech, which already owned a 90% equity interest in LiKang Disinfectant, entered into an agreement to purchase the remaining 10% equity interest of LiKang Disinfectant from Shanghai Shanhai Group (“Shanhai”), a non-affiliated Chinese entity (the “Disinfectant Stock Purchase Agreement”). Pursuant to the terms of the Disinfectant Stock Purchase Agreement, Shanhai was to receive 3,000,000 shares of Linkwell Corporation restricted common stock.
Due to restrictions under PRC law that prohibited the consideration then contemplated by the Disinfectant Stock Purchase Agreement, the agreement did not close. As a result of this, on March 25, 2008, the parties agreed to enter into an amendment to the Disinfectant Stock Purchase Agreement (“Disinfectant Amendment”) in an effort to complete the stock purchase transaction. Pursuant to the terms of the Disinfectant Amendment, the only material change to the Disinfectant Stock Purchase Agreement related to the consideration paid by Linkwell Tech to the Shanghai Shanhai Group for the remaining 10% equity interest, which was changed from 3,000,000 shares of our common stock to 1,500,000 shares of our common stock for $380,000. Due to the fluctuation of the applicable exchange rate, the cash consideration was increased to $399,057. The other terms of the Disinfectant Stock Purchase Agreement remained in full force and effect.
Linkwell Tech paid $395,800 to the Shanghai Shanhai Group on February 21, 2008 and paid $3,257 on April 18, 2008. A total of 1,500,000 shares were expected to be issued before the end of May 2008. The parties, however, agreed to extend the share issuance date until October 20, 2008. We valued the acquisition using the fair value of common shares at $0.19 per share and recorded an investment of $285,000. Including the cash payment of $399,057, the total investment for acquiring 10% equity interest in LiKang Disinfectant was $684,057. The cumulative minority interest of 10% equity interest in LiKang Disinfectant at March 25, 2008 was approximately $557,779. The difference between the total investment and the cumulative minority interest of $126,278 was deducted from retained earnings as dividends to the 10% minority shareholder, Shanghai Shanhai Group. As a result of the closing of the Disinfectant Stock Purchase Agreement, as amended, as of March 25, 2008, our 90% owned subsidiary Linkwell Tech owns 100% of the equity interest in LiKang Disinfectant.
19
On February 15, 2008, we entered into a stock purchase agreement with Ecolab Inc., a Delaware corporation (“Ecolab”), pursuant to which Ecolab agreed to purchase 888,889 of shares of Linkwell Tech, or 10% of the issued and outstanding capital stock of Linkwell Tech, for $2,000,000. On March 28, 2008 and June 4, 2008, Linkwell Tech received $200,000 and $1,388,559, respectively from Ecolab. Including a $400,000 loan that Linkwell Tech received from Ecolab and accrued interest thereon of $11,441, Linkwell Tech received a total investment of $2,000,000 from Ecolab. On May 31, 2008, our Company, Linkwell Tech and Ecolab entered into a Linkwell Tech Group Inc. Stockholders Agreement, whereby both the Company and Ecolab are subject to, and beneficiaries of, certain pre-emptive rights, transfer restrictions and take along rights relating to the shares of Linkwell Tech that the Company and Ecolab each hold. As of May 31, 2008, the principal and accrued interest of $11,441 on the short-term $400,000 loan became part of Ecolab’s investment and does not need to be repaid.
On May 31, 2008, LiKang Disinfectant entered into a stock purchase agreement under which it sold 100% of the capital stock of its wholly-owned subsidiary, LiKang International, to Linkwell International Trading Co., Ltd, a company registered in Hong Kong which is 100% owned by Mr. Wei Guan, our Company’s Vice President and Director. Pursuant to the terms of the agreement, LiKang Disinfectant received $291,754 (RMB 2,000,000) after the agreement was approved by the PRC Ministry of Commerce on March 27, 2008.
During the quarter ended September 30, 2009, LiKang Disinfectant invested certain of its intangible assets in Likang Biological. The Likang Disinfectant intangible assets were valued at approximately $2,000,000 and accounted for an approximately $800,000 increase in the registered capital of Likang Biological.
On December 21, 2009, we entered into a stock purchase agreement with Inner Mongolia Wuhai Chengtian Chemical Co., Ltd., a corporation organized under the laws of China (“Wuhai Chengtian”) and Honglin Li, a stockholder of Wuhai Chengtian, under which Likang Disinfectant purchased 35% of the outstanding capital stock of Wuhai Chengtian from Honglin Li in exchange for approximately $463,235 (3,150,000 RMB) and 4,000,000 shares of our common stock, $0.0005 par value per share (“Linkwell Shares”). Prior to this transaction, Likang Disinfectant owned 16% of the capital stock of Wuhai Chengtian. As a result of this transaction, LiKang Disinfectant now owns 51% of the capital stock of Wuhai Chengtian. Wuhai Chengtian manufactures materials we use to make certain of our disinfectant products.
We, along with Wuhai Chengtian, have been unable to obtain governmental tax approval of the transactions contemplated thereby. As such, on February 26, 2010, our Company, Linkwell Tech, Likang Disinfectant, Wuhai Chengtian and Honglin Li entered into Amendment No. 1 to the Stock Purchase Agreement (the “Amendment”) whereby the Stock Purchase Agreement has been amended such that the Exchange is now contingent upon the parties receiving governmental approval of the transaction. This transaction has not closed as of the date of this report.
ITEM 1A. RISK FACTORS.
Investing in our securities involves a great deal of risk. You should carefully consider the risks described below as well as other information provided to you in this document, including information in the section of this document entitled “Cautionary Statement Regarding Forward-Looking Statements.” The risks and uncertainties described below are not the only ones facing Linkwell. Additional risks and uncertainties not presently known to us or that we currently believe are immaterial may also impair our business operations. If any of the following risks actually occur, our Company’s business, financial condition or results of operations could be materially adversely affected, the value of our common stock could decline, and you may lose all or part of your investment. RISKS RELATED TO OUR COMPANY
20
We engage in a number of material transactions with related parties which could result in a conflict of interest involving our management.
We are heavily dependent on certain related party transactions in the ongoing conduct of our business. Sales to our related party represented approximately 51.75% of our total net revenues in the fiscal year of 2010 and approximately 36.60% of our total net revenues in fiscal 2009. This related party represented approximately 71.43% and approximately 58.6% of our accounts receivable at December 31, 2010 and 2009, respectively. These affiliated transactions may, from time to time, result in a conflict of interest for our management. Because these transactions are not subject to the approval of our shareholders, investors in our company are wholly dependent upon the judgment of our management in these related party transactions.
The management of our company is located in the PRC and we are heavily dependent upon advisory services of our U.S. advisors.
None of the current members of our management have any experience with the operation and regulatory framework of U.S. public companies. Additionally, these individuals are not fluent in English.
Until such time as we are able to expand our board of directors to include English-speaking individuals who also have experiences with the operation and regulatory framework applicable to U.S. public companies, we are heavily dependent upon our relationship with our U.S. advisors. If for any reason our advisors should fail to provide the contracted services at the anticipated levels or fail to extend their services, we may be unable to comply with the U.S. securities regulations on a timely basis. In this event, your ability to liquidate your investment would be negatively impacted and you could lose your entire investment in our Company.
As is customary in the PRC, we extend relatively long payment terms to our customers, which can negatively impact our cash flow. Our terms of sale generally require payment within four to six months, which is considerably longer than customary terms offered in the United States. For the 2010 fiscal year, the average time of payment on accounts receivable from related parties was six to eight months and from non-related third parties was four to five months. We would like to control the average time of payment on accounts receivable from related parties within 150 days.
We occasionally offer established customers, including related parties, longer payment terms of up to 240 days on new products as an incentive to purchase these products. We maintain a relatively low reserve for doubtful accounts when compared to a company operating in the U.S. Our payment terms may have the effect of adversely impacting our cash flow and our ability to fund our operations out of our operating cash flow. Our ability to continue to implement our growth strategy could suffer if our cash flow is adversely impacted, which may limit our ability to increase our revenues in the future.
Certain agreements to which we are a party and are material to our operations lack various legal protections, which are customarily contained in similar contracts prepared in the United States.
We are a Chinese company and all of our business and operations are conducted in China. We are a party to certain material contracts, including an agreement for the lease for our principal offices and manufacturing facility. While these contracts contain the basic business terms of the agreements between the parties, these contracts do not contain certain provisions which are customarily contained in similar contracts prepared in the U.S. For instance, our contracts may not include provisions such as representations and warranties of the parties, confidentiality and non-compete clauses, provisions outlining events of defaults, and/or termination and jurisdictional clauses. Because our material contracts omit these types of clauses, we may not have the same legal protections as we could have if the contracts contained these provisions. While we have not been subject to any adverse consequences as a result of the omission of these types of clauses, future events may occur which could lead to potential disputes. Contractual disputes may divert our management's time from our business operations and require us to expend additional funds to resolve possible disputes. This possible diversion of our management’s time may limit the time our management could otherwise devote to our business operation, and it could also limit the funds we have available to pay our ongoing operating expenses.
21
Each of our product groups operate in highly competitive businesses.
Each of our product groups is subject to competition from other manufacturers of similar products. There are approximately 1,000 manufacturers of similar disinfectant products in China, but only approximately 30 manufacturers, including our company, operate on a continuous basis. While we believe we are one of the leading manufacturers of disinfectant products in the PRC, from time to time, there may still be a sporadic oversupply of these products which can adversely impact our market share and competitive position in this product group. As a result, we may not be able to effectively compete in our product segments and may limit our ability to sustain our current level of operations or grow our revenue in future periods.
Because of the specialized, technical nature of the business, we are highly dependent on certain members of management, as well as our marketing, engineering and technical staff.
The loss of the services of our current management and skill employees could have a material and negative effect on our ability to effectively pursue our business strategy. In addition to manufacturing high volumes of our products and developing new products, we must attract, recruit and retain a sizeable workforce of technically competent employees, including additional skilled and experienced managerial, marketing, engineering and technical personnel. If we are unable to do so, our ability to expand our business and increase our revenue could be limited.
If we experience customer concentration, we may be exposed to all of the risks faced by our remaining material customers.
For the fiscal years ended December 31, 2010 and 2009 revenues from one customer, ZhongYou Pharmaceutical an affiliate, represented approximately 51.7% and approximately 33.4%, respectively, of our total net revenues. Unless we maintain multiple customer relationships, it is likely that we will experience periods during which we may be highly dependent on a limited number of customers. Dependence on a small number of customers could make it difficult for us to negotiate attractive prices for our products and could expose us to the risk of substantial losses if a single dominant customer stops conducting business with us. Moreover, to the extent that we are dependent on any single customer, we are subject to the risks faced by that customer to the extent that such risks impede the customer's ability to stay in business and make timely payments to us.
We depend on factories to manufacture our products, which may be insufficiently insured against damage or loss.
We have no direct business operation, other than our ownership of our subsidiaries located in China, and the results of operations and our financial condition are solely dependent on our subsidiaries' factories in China. We do not currently maintain insurance to protect against damage and loss to our facilities and other leasehold improvements. Therefore, any material damage to, or the loss of, any of our facilities due to fire, severe weather, flooding or other cause, would not be shared with an insurance company, and if large enough, would have a material and negative effect on our financial condition. If the damage was significant, we could be forced to stop operations until such time as the faculties could be repaired.
Our operations are subject to government regulation. If we fail to comply with the applicable regulations, our ability to operate in future periods could be in jeopardy.
We are subject to various state and local environmental laws related to our business. We are subject to local food, drug, environmental laws related to certification of manufacturing and distribution of our disinfectants. We are also licensed by the Shanghai City Government to manufacture and distribute disinfectants. While we are in substantial compliance with all provisions of those registrations, inspections, and licenses, and have no reason to believe that they will not be renewed as required by the applicable rules of the Central Government and the Shandong province, any non-renewal of these authorities could result in the cessation of our business activities.
22
Due to recent incidents of pollution to milk powder by Melamine and increased medical accidents and disputes, the government will impose more stringent restrictions over the quality of products and distributions in markets, given tighter control over food, drug and disinfectant industries, which would add costs to our operations from compliance of all provisions of restrictions. Any incompliance with provisions of these restrictions would jeopardize our ability to operate in future.
We may not have sufficient protection of certain of our intellectual property.
We utilize certain technologies in the purification of raw material used in our products which are proprietary in nature. We are not a party to any confidentiality or similar agreements with employees and third parties including consultants, vendors and customers and it not likely that we will enter into these types of agreements in the future. It is possible that our employees or a third party could, without authorization, utilize our propriety technologies without our consent. The unauthorized use of this proprietary information by third parties could adversely affect our business and operations as well as any competitive advantage we may have in our market segment. We may not have adequate remedies for the protection of our proprietary technologies and these proprietary technologies could become known or independently developed by competitors, in which event our ability to effectively compete could be in jeopardy.
We have not voluntarily implemented various corporate governance measures, in the absence of which, shareholders may have reduced protections against interested director transactions, conflicts of interest and other matters.
We are not subject to any law, rule or regulation requiring that we adopt any of the corporate governance measures that are required by the rules of national securities exchanges such as independent directors and audit committees. It is possible that if we were to adopt some or all of the corporate governance measures, shareholders would benefit from greater assurances that internal corporate decisions were being made by disinterested directors and that policies had been implemented to define responsible conduct. Prospective investors should bear consider our current lack of corporate governance measures in formulating their investment decisions.
RISKS RELATED TO DOING BUSINESS IN CHINA
All of our assets and operations are located in the PRC and are subject to changes resulting from the political and economic policies of the Chinese government.
Our business operations could be restricted by the political environment in the PRC. The PRC has operated as a socialist state since 1949 and is controlled by the Communist Party of China. In recent years, however, the government has introduced reforms aimed at creating a "socialist market economy" and policies have been implemented to allow business enterprises greater autonomy in their operations. Changes in the political leadership of the PRC may have a significant effect on laws and policies related to the current economic reform programs, other policies affecting business and the general political, economic and social environment in the PRC, including the introduction of measures to control inflation, changes in the rate or method of taxation, the imposition of additional restrictions on currency conversion and remittances abroad, and foreign investment. Moreover, economic reforms and growth in the PRC have been more successful in certain provinces than in others, and the continuation or increases of such disparities could affect the political or social stability of the PRC.
Although we believe that the economic reform and the macroeconomic measures adopted by the Chinese government have had a positive effect on the economic development of China, the future direction of these economic reforms is uncertain and the uncertainty may decrease the attractiveness of our company as an investment, which may in turn result in a decline in the trading price of our common stock.
23
The PRC government’s recent measures to curb inflation rates could adversely affect future results of operations.
In recent years, the Chinese economy has experienced periods of rapid expansion and high rates of inflation. Rapid economic growth can lead to growth in the money supply and rising inflation. In May 2010, the change in China’s Consumer Price Index increased by 3.1% according to the National Bureau of Statistics of China, or the NBS. If prices for our products rise at a rate that is insufficient to compensate for the rise in the costs of supplies, it may have an adverse effect on profitability. These factors have led to the adoption by the Chinese government, from time to time, of various corrective measures designed to restrict the availability of credit or regulate growth and contain inflation. High inflation may in the future cause the Chinese government to impose controls on credit and/or prices, or to take other action, which could inhibit economic activity in China, and thereby harm the market for our products.
The Chinese government exerts substantial influence over the manner in which we must conduct our business activities.
The PRC only recently has permitted provincial and local economic autonomy and private economic activities. The government of the PRC has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in the PRC or particular regions thereof. In such an event, we could be forced to cease operations.
Restrictions on currency exchange may limit our ability to receive and use our revenues effectively.
Because all of revenues are in the form of Renminbi, any future restrictions on currency exchanges may limit our ability to use revenue generated in Renminbi to fund any future business activities outside China or to make dividend or other payments in U.S. dollars. Although the Chinese government introduced regulations in 1996 to allow greater convertibility of the Renminbi for current account transactions, significant restrictions still remain, including primarily the restriction that foreign-invested enterprises may only buy, sell or remit foreign currencies, after providing valid commercial documents, at those banks authorized to conduct foreign exchange business. In addition, conversion of Renminbi for capital account items, including direct investment and loans, is subject to government approval in China, and companies are required to open and maintain separate foreign exchange accounts for capital account items. We cannot be certain that the Chinese regulatory authorities will not impose more stringent restrictions on the convertibility of the Renminbi, especially with respect to foreign exchange transactions.
We may be unable to enforce our rights due to policies regarding the regulation of foreign investments in China.
The PRC's legal system is a civil law system based on written statutes in which decided legal cases have little value as precedents, unlike the common law system prevalent in the United States. The PRC does not have a well-developed, consolidated body of laws governing foreign investment enterprises. As a result, the administration of laws and regulations by government agencies may be subject to considerable discretion and variation, and may be subject to influence by external forces unrelated to the legal merits of a particular matter. China's regulations and policies with respect to foreign investments are evolving. Definitive regulations and policies with respect to such matters as the permissible percentage of foreign investment and permissible rates of equity returns have not yet been published. Statements regarding these evolving policies have been conflicting and any such policies, as administered, are likely to be subject to broad interpretation and discretion and to be modified, perhaps on a case-by-case basis. The uncertainties regarding such regulations and policies present risks which may affect our ability to achieve our stated business objectives. If we are unable to enforce any legal rights we may have under our contracts or otherwise, our ability to compete with other companies in our industry could be limited which could result in a loss of revenue in future periods which could impact our ability to continue as a going concern.
24
Recent PRC regulations relating to acquisitions of PRC companies by foreign entities may create regulatory uncertainties that could restrict or limit our ability to operate, including our ability to pay dividends.
The PRC State Administration of Foreign Exchange, or SAFE, issued a public notice in January 2005 concerning foreign exchange regulations on mergers and acquisitions in China. The public notice states that if an offshore company controlled by PRC residents intends to acquire a PRC company, such acquisition will be subject to strict examination by the relevant foreign exchange authorities. The public notice also states that the approval of the relevant foreign exchange authorities is required for any sale or transfer by the PRC residents of a PRC company’s assets or equity interests to foreign entities for equity interests or assets of the foreign entities.
In April 2005, SAFE issued another public notice further explaining the January notice. In accordance with the April 2005 notice, if an acquisition of a PRC company by an offshore company controlled by PRC residents has been confirmed by a Foreign Investment Enterprise Certificate prior to the promulgation of the January 2005 notice, the PRC residents must each submit a registration form to the local SAFE branch with respect to their respective ownership interests in the offshore company, and must also file an amendment to such registration if the offshore company experiences material events, such as changes in the share capital, share transfer, mergers and acquisitions, spin-off transaction or use of assets in China to guarantee offshore obligations. The April 2005 notice also provides that failure to comply with the registration procedures set forth therein may result in restrictions on our PRC resident shareholders and our subsidiaries. Pending the promulgation of detailed implementation rules, the relevant government authorities are reluctant to commence processing any registration or application for approval required under the SAFE notices.
In addition, on August 8, 2006, the Ministry of Commerce (“MOFCOM”), joined by the State-Owned Assets Supervision and Administration Commission of the State Council, State Administration of Taxation, State Administration for Industry and Commerce, China Securities Regulatory Commission and SAFE, amended and released the Provisions for Foreign Investors to Merge and Acquire Domestic Enterprises, new foreign-investment rules which took effect September 8, 2006, superseding much, but not all, of the guidance in the prior SAFE circulars. These new rules significantly revise China’s regulatory framework governing onshore-offshore restructurings and how foreign investors can acquire domestic enterprises. These new rules signify greater PRC government attention to cross-border merger, acquisition and other investment activities, by confirming MOFCOM as a key regulator for issues related to mergers and acquisitions in China and requiring MOFCOM approval of a broad range of merger, acquisition and investment transactions. Further, the new rules establish reporting requirements for acquisition of control by foreigners of companies in key industries, and reinforce the ability of the Chinese government to monitor and prohibit foreign control transactions in key industries.
These new rules may significantly affect the means by which offshore-onshore restructurings are undertaken in China in connection with offshore private equity and venture capital financings, mergers and acquisitions. It is expected that such transactional activity in China in the near future will require significant case-by-case guidance from MOFCOM and other government authorities as appropriate. It is anticipated that application of the new rules will be subject to significant administrative interpretation, and we will need to closely monitor how MOFCOM and other ministries apply the rules to ensure its domestic and offshore activities continue to comply with PRC law. Given the uncertainties regarding interpretation and application of the new rules, we may need to expend significant time and resources to maintain compliance.
It is uncertain how our business operations or future strategy will be affected by the interpretations and implementation of the SAFE notices and new rules. Our business operations or future strategy could be adversely affected by the SAFE notices and the new rules. For example, we may be subject to more stringent review and approval process with respect to our foreign exchange activities.
Failure to comply with the United States Foreign Corrupt Practices Act could subject us to penalties and other adverse consequences.
We are subject to the United States Foreign Corrupt Practices Act, which generally prohibits United States companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in the PRC. We can make no assurance, however, that our employees or other agents will not engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
25
If relations between the United States and the PRC worsen, investors may be unwilling to hold or buy our common stock and our common stock price may decrease.
At various times during recent years, the United States and the PRC have had significant disagreements over political and economic issues. Controversies may arise in the future between these two countries. Any political or trade controversies between the United States and PRC, whether or not directly related to our business, could reduce the price of our common stock.
We may have difficulty establishing adequate management, legal and financial controls in the PRC.
PRC companies have historically not adopted a Western style of management and financial reporting concepts and practices, which includes strong corporate governance, internal controls and, computer, financial and other control systems. In addition, we may have difficulty in hiring and retaining a sufficient number of qualified employees to work in the PRC. As a result of these factors, we may experience difficulty in establishing management, legal and financial controls, collecting financial data and preparing financial statements, books of account and corporate records and instituting business practices that meet Western standards. Therefore, we may, in turn, experience difficulties in implementing and maintaining adequate internal controls. Any such deficiencies, weaknesses or lack of compliance could have a material adverse effect on our business.
RISKS RELATED TO OUR COMMON STOCK
We have not voluntarily implemented various corporate governance measures, in the absence of which, stockholders may have more limited protections against interested director transactions, conflicts of interest and similar matters.
Recent Federal legislation, including the Sarbanes-Oxley Act of 2002, has resulted in the adoption of various corporate governance measures designed to promote the integrity of the corporate management and the securities markets. Some of these measures have been adopted in response to legal requirements. Others have been adopted by companies in response to the requirements of national securities exchanges, such as the NYSE or The NASDAQ Stock Market, on which their securities are listed. Among the corporate governance measures that are required under the rules of national securities exchanges are those that address board of directors' independence, audit committee oversight, and the adoption of a code of ethics. Although we have adopted a Code of Ethics, we have not yet adopted any of these other corporate governance measures and, since our securities are not yet listed on a national securities exchange, we are not required to do so. We have not adopted corporate governance measures such as an audit or other independent committees of our board of directors as we presently do not have any independent directors. If we expand our board membership in the future to include additional independent directors, we may seek to establish an audit and other committees of our Board of Directors. It is possible that if we were to adopt some or all of these corporate governance measures, stockholders would benefit from somewhat greater assurances that internal corporate decisions were being made by disinterested directors and that policies had been implemented to define responsible conduct. Prospective investors should bear in mind our current lack of corporate governance measures in formulating their investment decisions.
Provisions in our Articles of Incorporation and bylaws may delay or prevent a takeover which may not be in the best interests of our stockholders.
Provisions in our Articles of Incorporation and bylaws may be deemed to have anti-takeover effects, which include when and by whom special meetings of our shareholders may be called, and may delay, defer or prevent a takeover attempt. In addition, certain provisions of the Florida Statutes may also be deemed to have certain anti-takeover effects, including that a control of shares acquired in excess of certain specified thresholds will not possess any voting rights unless these voting rights are approved by a majority of a corporation's disinterested shareholders.
26
In addition, our Articles of Incorporation authorize the issuance of up to 10,000,000 shares of preferred stock, with such rights and preferences as may be determined from time to time by our Board of Directors, of which no shares are currently outstanding. Our Board of Directors may, without shareholder approval, issue preferred stock with dividends, liquidation, conversion, voting or other rights that could adversely affect the voting power or other rights of the holders of our common stock.
Our stock price may fluctuate and could subject our Company to litigation.
The market price of our common stock may fluctuate significantly in response to a number of factors, some of which are beyond our control. These factors include:
· Quarterly variations in operating results;
· Changes in accounting treatments or principles;
· Additions or departures of key personnel;
· Stock market price and volume fluctuations of publicly-traded companies in general and Chinese-based companies in particular; and
· General political, economic and market conditions.
Because our common stock currently trades below $5.00 per share, our common stock is considered a "penny stock" which can adversely affect its liquidity.
As a result of the trading price of our common stock being less than $5.00 per share, our common stock is considered a "penny stock" and is subject to the requirements of Rule 15g-9 under the Securities Exchange Act of 1934. Under this rule, broker/dealers who recommend low-priced securities to persons other than established customers and accredited investors must satisfy special sales practice requirements. The broker/dealer must make an individualized written suitability determination for the purchaser and receive the purchaser's written consent prior to the transaction.
SEC regulations also require additional disclosure in connection with any trades involving a "penny stock," including the delivery of a disclosure schedule explaining the penny stock market and its associated risks prior to any penny stock transaction. These requirements severely limit the liquidity of securities in the secondary market because few brokers or dealers are likely to undertake these compliance requirements. In addition, other risks associated with trading in penny stocks include price fluctuations and the lack of a liquid market.
Our common stock trades in the Over the Counter Market and there can be no assurance that an established trading market will develop.
Our common stock trades in the Pink Sheets and has in the past traded on Over The Counter Bulletin Board (“OTCBB”) until our common stock was deleted due to failure to meet the broker-dealer requirements of Rule 15c2-11 of the Exchange Act of 1934, as amended. Such Rule includes a requirement that at least one broker-dealer quotes our common stock each trading day and the removal from the system if this requirement is failed for a specific number of consecutive trading days. Our common stock does not have an established trading market.
In order to be listed on the OTCBB, at least one broker-dealer member of FINRA must file a Form 15c2-11 application and become a market maker on the system and remain on the system quoting a market for our common stock. Also, in order to maintain the quotation of our shares of common stock on the OTCBB, we must remain a reporting company under the Securities Exchange Act of 1934 (the “Exchange Act”). This requires us to comply with the periodic reporting and proxy statement requirements of the Exchange Act. It is possible that our common stock could be listed on the OTCBB and again removed from the OTCBB and then be traded on the potentially less desirous Pink Sheets. In either venue, an investor may find it difficult to obtain accurate quotations as to the market value of the common stock. In addition, if we failed to meet the criteria set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling the common stock, which may further affect its liquidity. This would also make it more difficult for us to raise additional capital.
27
ITEM 1B. UNRESOLVED STAFF COMMENTS.
N/A.
ITEM 2. PROPERTIES.
Our facilities include our principal executive offices, located at 1104 Jiatang Road Jiading District, Shanghai China 201807. We currently lease another office building and warehouse space, which consists of approximately 1,060 square meter, from Shanghai Shanhai Group, an unaffiliated third party, for approximately $32,000 annually. The lease will expire in November 2011. Our other executive office is located at Room 701-704, No 11 Guotai Road, Yangpu District, Shanghai, under leases entered into on July 16, 2010 for approximately $131,000 annually. The lease agreement will expire on December 31, 2011.
Until August 2005, we leased approximately 21,500 square feet of manufacturing space from Shanghai LiKang Pharmaceutical Technology Company, Limited, an affiliate, under a lease originally expiring December 2006 for approximately $11,500 annually. In August 2005 we purchased this building, which includes an assignment of the land use permit for $333,675.
ITEM 3. LEGAL PROCEEDINGS.
We are a defendant in a lawsuit filed in November 2009 in the Supreme Court of the State of New York, County of New York, by two warrant holders of our Company, seeking damages of at least $800,000. As of March 31, 2011, we are vigorously defending our position in this litigation matter and have not made for a provision with regards to this lawsuit in the event of an unfavorable outcome. We have filed a motion to dismiss the complaint and proceedings relating to the motion to dismiss are ongoing. We are also currently in the process of conducting settlement negotiations; however, there is no certainty as to the terms and conditions of the settlement.
ITEM 4. (REMOVED AND RESERVED).
N/A.
28
PART II
ITEM 5. MARKET FOR COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Our common stock is currently being quoted on the Pink Sheets under the trading symbol PINK:LWLL. Until February 23, 2011, our common stock was listed for quotation on the Over The Counter Bulletin Board (“OTCBB”) under the symbol OTC:LWLL. As of February 23, 2011, our common stock, along with the securities of over 600 other issuers, was removed from the OTCBB automated quotation system due to failure to comply with Rule 15c2-11 and was delisted to the Pink Sheets. The removal of our common stock from the OTCBB was not due to the failure to meet quality standards or any delinquencies in reporting. On February 23, 2011, our trading symbol changed to “PINK:LWLL.” The reported high and low closing price for the common stock as reported on the OTC Bulletin Board are shown below. Trading in the common stock on the OTCBB and Pink Sheets has been limited and sporadic and the quotations set forth below are not necessarily indicative of actual market conditions. The quotations reflect inter-dealer prices, without retail mark-up, markdown or commission, and may not represent actual transactions.
|
High
|
Low
|
|||||||
|
Fiscal 2009
|
||||||||
| $ | 0.07 | $ | 0.03 | |||||
|
Second quarter ended June 30, 2009
|
$ | 0.22 | $ | 0.04 | ||||
|
Third quarter ended September 30, 2009
|
$ | 0.24 | $ | 0.07 | ||||
|
Fourth quarter ended December 31, 2009
|
$ | 0.19 | $ | 0.13 | ||||
|
Fiscal 2010
|
||||||||
|
First quarter ended March 31, 2010
|
$ | 0.16 | $ | 0.11 | ||||
|
Second quarter ended June 30, 2010
|
$ | 0.18 | $ | 0.11 | ||||
|
Third quarter ended September 30, 2010
|
$ | 0.14 | $ | 0.08 | ||||
|
Fourth quarter ended December 31, 2010
|
$ | 0.12 | $ | 0.07 | ||||
On March 29, 2011, the last sale price of our common stock was $0.08. As of March 29, 2011, there were approximately 109 record owners of our common stock.
Dividend Policy
We have never paid cash dividends on our common stock. Payment of dividends will be within the sole discretion of our Board of Directors and will depend, among other factors, upon our earnings, capital requirements and our operating and financial condition. At the present time, our anticipated financial capital requirements are such that we intend to follow a policy of retaining earnings in order to finance the development of our business.
While we have no current intention of paying dividends on our common stock, should we decide in the future to do so, as a holding company, our ability to pay dividends and meet other obligations will depend upon the receipt of dividends or other payments from our operating subsidiaries. In addition, our operating subsidiaries, from time to time, may be subject to restrictions on their ability to make distributions to us, including situations such as restrictive covenants in loan agreements, restrictions on the conversion of local currency into U.S. dollars or other hard currency and regulatory restrictions.
ITEM 6. SELECTED FINANCIAL DATA.
N/A.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OR FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
OVERVIEW
We operate under a holding company structure and currently have one direct 90% owned operating subsidiary, Linkwell Tech Group Inc. (“Linkwell Tech”), a Florida corporation. Linkwell Tech owns 100% of Shanghai LiKang Disinfectant High-Tech Company, Limited (“LiKang Disinfectant”). On February 15, 2008, Linkwell Tech sold 10% of its issued and outstanding capital stock to Ecolab Inc., a Delaware corporation (“Ecolab”). On March 5, 2009, LiKang Disinfectant purchased 100% of LiKang Biological Company for approximately $292,500 (RMB 2,000,000) and 500,000 shares of our common stock.
Linkwell Corporation, through Linkwell Tech’s wholly-owned subsidiaries, LiKang Disinfectant and Likang Biological, is engaged in the development, manufacture, sale and distribution of disinfectant health care products primarily to the medical industry in China.
29
Since 1988, we have developed, manufactured and distributed disinfectant health care products primarily to the medical industry in China. In the last few years, China has experienced a variety of public health crises, such as the outbreak of SARS, which demonstrated the need for increased health standards in China. In response, since the beginning in 2002, the Chinese government has undertaken various initiatives to improve public health and living standards, including continuing efforts to educate the public about the need for proper sanitation procedures and the establishment of production standards for the disinfectant industry in China. As a result of this heightened license and permit system, all disinfectant manufacturers must comply with “qualified disinfection product manufacturing enterprise requirements” established by the Ministry of Public Health. The requirements include standards for hardware, such as facilities and machinery, and software, including the technology to monitor the facilities, as well as the heightened knowledge and capability of the production staff regarding quality control procedures. Following the adoption of the industry standards in 2002, we were granted thirty-one hygiene licenses by the Ministry of Public Health.
We believe that government standards adopted in July 2002 have increased the barriers for competitors to enter into the disinfectant industry in China. The implementation of these improved production standards and license requirements has effectively decreased the competitive landscape as it pertains to small to medium size manufacturers, since the new standards are especially difficult for companies with limited product offerings and inferior technical content. In addition, prior to the adoption of industry standards, disinfectant products were generally marketed and sold based on price as opposed to quality. We believe that as a result of the adoption of industry standards, the marketplace is evolving with a more stringent focus on product quality, which we believe will enable us to increase our base of commercial customers thereby increasing our revenues.
Historically, our focus has been on the commercial distribution of our products. Our customers include hospitals, medical suppliers and distribution companies throughout China. We have made efforts to expand our distribution to reach the retail markets. We have repackaged certain of our commercial disinfectant products for sale to the consumer market and have commenced upon expanding our customer base to include hotels, schools, supermarkets and pharmacies. By virtue of the Chinese government's continuing focus on educating the Chinese population about the benefits of proper sanitation procedures, we believe that another key to increasing our revenue is the continued expansion of the retail distribution of our products.
The disinfectant industry in China is an emerging industry that is populated with small, regional companies. We estimate that there are in excess of 1,000 manufacturers and distributors of disinfectant products in China; however, most domestic competitors offer a limited line of products and there are only a few domestic companies with a nationwide presence. We believe that our national marketing and sales presence throughout all 22 provinces, as well as four autonomous regions and four municipalities in China, give us a competitive advantage over many other disinfectant companies in China and will enable us to leverage the brand awareness for our products with commercial customers to the retail marketplace.
Our present manufacturing facilities and production capacities are sufficient for the foreseeable future, and we believe that we have the assets and capital available to us necessary to enable the increase of our revenue in future periods as the market for disinfectant products in China continues to increase. We will continue to focus our efforts on the retail market for our products, as well as expanding our traditional base of commercial customers. In addition, we may also consider the possible acquisition of independent sales networks, which could be used to increase our product distribution capacity and align our company with small, regional companies in the industry.
30
RESULTS OF OPERATIONS
Comparison of the Years Ended December 31, 2010 and 2009
The table below sets forth the results of operations for the year ended December 31, 2010 as compared to the year ended December 31, 2009 accompanied by the percentage of changes.
|
|
2010
|
2009
|
||||||||||||||
|
|
$
|
% of Sales
|
$
|
% of Sales
|
||||||||||||
|
Net Revenue
|
||||||||||||||||
|
Non-related companies
|
$
|
6,517,018
|
48.3
|
%
|
$
|
9,299,120
|
63.4
|
%
|
||||||||
|
Related companies
|
6,989,387
|
51.7
|
%
|
5,367,662
|
36.6
|
%
|
||||||||||
|
Total Net Revenue
|
13,506,405
|
100
|
%
|
14,666,782
|
100
|
%
|
||||||||||
|
Cost of Sales
|
6,942,366
|
51.4
|
%
|
6,501,335
|
44.3
|
%
|
||||||||||
|
Gross Profit
|
6,564,039
|
48.6
|
%
|
8,165,447
|
55.7
|
%
|
||||||||||
|
Selling Expense
|
1,262,502
|
9.3
|
%
|
1,598,134
|
10.9
|
%
|
||||||||||
|
General and Administrative
|
3,881,847
|
28.8
|
%
|
2,409,104
|
16.4
|
%
|
||||||||||
|
Total Operating Expenses
|
5,144,349
|
38.1
|
%
|
4,007,238
|
27.3
|
%
|
||||||||||
|
Income from Operations
|
1,419,690
|
10.5
|
%
|
4,158,209
|
28.4
|
%
|
||||||||||
|
Other Income (Expenses), net
|
9,852
|
0.1
|
%
|
(121,897)
|
(0.8)
|
%
|
||||||||||
|
Income tax expense
|
(366,879
|
)
|
(2.7
|
)%
|
(594,078
|
)
|
(4.1
|
)%
|
||||||||
|
Net Income including non-controlling interest
|
1,062,663
|
7.9
|
%
|
3,442,234
|
23.5
|
%
|
||||||||||
|
Less: net income attributable non-controlling interest
|
(124,259
|
)
|
(1.0
|
)%
|
(381,441
|
)
|
(2.6
|
)%
|
||||||||
|
Net Income attributable to Linkwell Corp
|
938,404
|
6.9
|
%
|
3,060,793
|
20.9
|
%
|
||||||||||
NET REVENUE
Net revenue for the year ended December 31, 2010 was $13,506,405 as compared to net revenue of $14,666,782 for 2009, a decrease of $1,160,377, or approximately 7.9%. This decrease in revenue was due to adecrease average selling price of 10%. Due to the intensive competition in the market in China, we lowered the selling price for most of our products in order to maintain our market share. Of our total net revenue for 2010, $6,989,387, or approximately 51.7%, was attributable to related parties as compared to net revenue of $5,367,662, or approximately 36.6%, in 2009.
COST OF SALES
Cost of sales includes raw materials and manufacturing costs, which include labor, rent and an allocated portion of overhead expenses, such as utilities, directly related to product production. For the year ended December 31, 2010, cost of revenue amounted to $6,942,366, or approximately 51.4% of net revenue, as compared to the cost of revenue of $6,501,335, or approximately 44.3% of net revenue in 2009. The increase in cost of revenue as a percentage of revenue was mainly due to the increased price of raw material resulting from overall price inflation in China compared to 2009.
GROSS PROFIT
Gross profit for the year ended December 31, 2010 was $6,564,039, or approximately 48.6% of net revenue, as compared to $8,165,447, or approximately 55.7% of net revenue for 2009. The decrease in gross profit margin was mainly due to the decrease of the average selling price, and an increase of cost of revenue as a percentage of revenue.
31
OPERATING EXPENSES
Total operating expenses consisted of selling, general and administrative expenses. For the year ended December 31, 2010, total operating expenses were $5,144,349, an increase of $1,137,111, or approximately 28.4%, from total operating expenses of $4,007,738 for 2009. The increase in operating expenses was mainly due to increased payroll, consulting fees, and research and development expenses for new products and technology.
NONCONTROLLING INTEREST
On February 15, 2008, we entered into a stock purchase agreement with Ecolab, pursuant to which Ecolab purchased and Linkwell Tech sold 888,889 of its shares, or 10% of the issued and outstanding capital stock of Linkwell Tech, for a total of $2,000,000. On March 28, 2008 and June 4, 2008, Linkwell Tech received $200,000 and $1,388,559, respectively, from Ecolab. Linkwell Tech received a total of $2,000,000 from Ecolab, inclusive of a $400,000 loan that Ecolab released to Linkwell Tech and accrued interest of $11,441. On May 31, 2008, Linkwell Tech and Ecolab entered into a Linkwell Tech Group Inc. Stockholders Agreement, whereby both our Company and Ecolab are subject to, and are the beneficiaries of, certain pre-emptive rights, transfer restrictions and take along rights relating to the shares of Linkwell Tech that we and Ecolab each hold. As of May 31, 2008, the principal and accrued interest of $11,441 on the short-term $400,000 loan became part of Ecolab’s investment and no longer needed to be repaid. After this transaction, Ecolab became a 10% minority interest holder of Linkwell Tech. For the year ended December 31, 2010, we had a minority interest expense of $124,259 as compared to $381,441 in 2009.
NET INCOME
Our net income for the year ended December 31, 2010 was $938,404 compared to $3,060,793 in 2009, a decrease of $2,122,389 or 69.3%. Net income as a percentage of revenue was 6.9% for the year ended December 31, 2010, while it was 20.9% in 2009. This decrease in net income was attributable to decreased sales and increased cost of sales, and increased general and administrative expenses in 2010.
LIQUIDITY AND CAPITAL RESOURCES
As shown in the accompanying financial statements, our working capital increased $1,652,007, or approximately 14.1%, from $11,562,651 on December 31, 2009 to $13,214,658 on December 31, 2010. With the expansion of our businesses, we anticipate the need to utilize our capital resources in the near future. In addition to our working capital, we intend to obtain required capital through a combination of bank loans and the sale of our equity securities. Although we are not party to any commitments or agreements at this time to provide us with additional bank financing or to sell our securities, we are optimistic that we will be able to obtain additional capital resources to fund our business expansions.
We currently have no material commitments for capital expenditures. As of December 31, 2010, we had a total of $1,132,469 in outstanding short term loans, which will mature in 2011. Other than our working capital and loans, we presently have no other alternative capital resources available to us. We plan to build additional product lines and upgrade our manufacturing facilities in order to expand our production capacity and improve the quality of our products. Based on our preliminary estimates, upgrades and expansion will require additional capital of approximately $1 million.
We need to raise additional capital to meet the demands described above. We may raise additional capital through the sale of equity securities. There can be no assurances that any additional debt or equity financing will be available to us on acceptable terms, if at all. The inability to obtain debt or equity financing could have a material adverse effect on our operating results, and as a result, we could be required to cease or significantly reduce our operations, seek a merger partner or sell additional securities on terms that may be disadvantageous to shareholders.
32
The following is a summary of cash provided by or used in each of the indicated types of activities during the years ended December 31, 2010 and 2009:
|
2010
|
2009
|
|||||||
|
Cash provided by (used in):
|
||||||||
|
Operating Activities
|
$
|
2,082,114
|
|
$
|
292,353
|
|||
|
Investing Activities
|
(876,653)
|
|
(1,317,186)
|
|
||||
|
Financing Activities
|
(311,608)
|
1,090,368
|
||||||
NET CASH FROM OPERATING ACTIVITES
Net cash provided by operating activities for 2010 was $2,082,114, as compared to net cash provided by operating activities $292,353 in 2009, an increase of cash inflow of $1,789,761. The increase in cash inflow in 2010 was mainly a result of decreased account receivables outstanding due to our timely collection and increased account payable and accrued expenses despite decreased net income as compared to 2009.
NET CASH FROM INVESTING ACTIVITIES
Net cash used in investing activities for 2010 was $876,653 as compared to net cash used in investing activities of $1,317,186 in 2009, a decrease of $440,533. Cash used in investing activities in 2010 mainly consisted of $564,296 for construction in progress and $312,357 for purchase of equipment, while in 2009, we spent $1.17 million for purchase of fixed assets.
NET CASH FROM FINANCING ACTIVITIES
Net cash used by financing activities was $311,608 for the year ended December 31, 2010 compared to net cash provided by financing activities of $1,090,368 in 2009. This was primarily a result of a $1,855,590 increase in due from related parties, an increase of $820,147 due to related parties, and $723,835 in proceeds from short-term loans. In comparison to 2009, we had a $380,618 cash inflow from short term loans, a $1,378,357 decrease in due from related parties and a $746,596 repayment of short term loans.
CRITICAL ACCOUNTING POLICIES
The preparation of our consolidated financial statements in conformity with accounting principles generally accepted in the United States requires us to make estimates and judgments that affect our reported assets, liabilities, revenues, and expenses, and the disclosure of contingent assets and liabilities.
We base our estimates and judgments on historical experience and on various other assumptions we believe to be reasonable under the circumstances. Future events, however, may differ markedly from our current expectations and assumptions. While there are a number of significant accounting policies affecting our consolidated financial statements, we believe the following critical accounting policies involve the most complex, difficult and subjective estimates and judgments: allowance for doubtful accounts; income taxes; stock-based compensation; asset impairment and option value.
While our significant accounting policies are more fully described in Note 1 to our consolidated financial statements, we believe the following accounting policies are the most critical to help develop a full understanding and evaluation this management discussion and analysis.
33
ALLOWANCE FOR DOUBTFUL ACCOUNTS
We maintain an allowance for doubtful accounts to reduce amounts to their estimated realizable value. A considerable amount of judgment is required when we assess the realization of accounts receivables, including assessing the probability of collection and the current credit-worthiness of each customer. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, an additional provision for doubtful accounts could be required. We initially record a provision for doubtful accounts based on our historical experiences, and we then adjust this provision at the end of each reporting period based on a detailed assessment of our accounts receivables and allowance for doubtful accounts. In estimating the provision for doubtful accounts, we consider, among other factor: (i) the aging of the accounts receivable; (ii) trends within and ratios involving the age of the accounts receivable; (iii) the customer mix in each of the aging categories and the nature of the receivable; (iv) our historical provision for doubtful accounts; (v) the credit worthiness of the customer; and (vi) the economic conditions of the customer's industry as well as general economic conditions.
REVENUE RECOGNITION
Our revenue recognition policies are in compliance with SEC Staff Accounting Bulletin (“SAB”) 104 (codified in FASB ASC Topic 480). We record revenue when persuasive evidence of an arrangement exists, services have been rendered or product delivery has occurred, the sales price to the customer is fixed or determinable, and collectibility is reasonably assured. The following policies reflect specific criteria for our various revenue streams.
Our revenue from the sale of products to related parties are recorded when the goods are shipped to the customers from our related parties. Upon shipment, title passes, and collectibility is reasonably assured. We receive purchase orders from our related parties on an as need basis from the related party customers. Generally, the related party does not hold our inventory. If the related party has inventory on hand at the end of a reporting period, the sale is reversed and the inventory is included in our balance sheet.
INCOME TAXES
We account for income taxes in accordance with Accounting for Income Taxes, which prescribes the use of the liability method. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts and the tax basis of assets and liabilities. Deferred tax assets and liabilities are measured using the enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. We then assess the likelihood that our deferred tax assets will be recovered from future taxable income and to the extent we believe that recovery is not likely, we establish a valuation allowance. To the extent we establish a valuation allowance, or increase or decrease this allowance in a period, we increase or decrease our income tax provision in our statement of operations. If any of our estimates of our prior period taxable income or loss prove to be incorrect, material differences could impact the amount and timing of income tax benefits or payments for any period. In addition, as a result of the significant change in our ownership, our future use of its existing net operating losses may be limited.
We currently operate in the PRC, however, our operations could change in the near future and we could be subject to tax liability involving a consideration of uncertainties in the application and interpretation of complex tax regulations in a multitude of jurisdictions across operations in other countries.
We recognize potential liabilities and record tax liabilities for anticipated tax audit issues in the U.S. and other tax jurisdictions based on our estimate of whether, and the extent to which, additional taxes will be due. The tax liabilities are a reflected net of realized tax loss carry forwards. We adjust these reserves upon specific events; however, due to the complexity of some of these uncertainties, the ultimate resolution may result in a payment that is different from our current estimate of the tax liabilities. If our estimate of tax liabilities proves to be less than the ultimate assessment, an additional charge to expense would result. If payment of these amounts ultimately proves to be less than the recorded amounts, the reversal of the liabilities would result in tax benefits being recognized in the period when the contingency has been resolved and the liabilities are no longer necessary.
Changes in tax laws, regulations, agreements and treaties, foreign currency exchange restrictions or our level of operations or profitability in each taxing jurisdiction could have an impact upon the amount of income taxes that we provide during any given year.
34
FOREIGN CURRENTY TRANSLATION AND COMPREHENSIVE INCOME (LOSS)
Our functional currency is the Chinese Yuan - Renminbi (“RMB”). For financial reporting purposes, RMB was translated into United States dollars ("USD") as the reporting currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenue and expenses are translated at the average rate of exchange prevailing during the reporting period. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of shareholders' equity as "Accumulated other comprehensive income." Gains and losses resulting from foreign currency transactions are included in income. There has been no significant fluctuation in exchange rate for the conversion of RMB to USD after the balance sheet date.
SEGMENT REPORTING
SFAS No. 131, "Disclosures about Segments of an Enterprise and Related Information" (codified in FASB ASC Topic 280) requires use of the “management approach” model for segment reporting. The management approach model is based on the way a company's management organizes segments within the company for making operating decisions and assessing performance. Reportable segments are based on products and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company.
SFAS 131 has no effect on our Company's financial statements as substantially all of our Company's operations are conducted in one industry segment. All of our Company's assets are located in the PRC.
RECENT ACCOUNTING PRONOUNCEMENTS
On July 1, 2009, we adopted Accounting Standards Update (“ASU”) No. 2009-01, “Topic 105 - Generally Accepted Accounting Principles - amendments based on Statement of Financial Accounting Standards No. 168, The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles” (“ASU No. 2009-01”). ASU No. 2009-01 re-defines authoritative GAAP for nongovernmental entities to be only comprised of the FASB Accounting Standards Codification (“Codification”) and, for SEC registrants, guidance issued by the SEC. The Codification is a reorganization and compilation of all then-existing authoritative GAAP for nongovernmental entities, except for guidance issued by the SEC. The Codification is amended to effect non-SEC changes to authoritative GAAP. Adoption of ASU No. 2009-01 only changed the referencing convention of GAAP in Notes to the Consolidated Financial Statements.
On February 25, 2010, the FASB issued ASU No. 2010-09 Subsequent Events Topic 855 “Amendments to Certain Recognition and Disclosure Requirements,” effective immediately. The amendments in the ASU remove the requirement for an SEC filer to disclose a date through which subsequent events have been evaluated in both issued and revised financial statements. Revised financial statements include financial statements revised as a result of either correction of an error or retrospective application of US GAAP. The FASB believes these amendments remove potential conflicts with the SEC’s literature. The adoption of this ASU did not have a material impact on our consolidated financial statements.
On March 5, 2010, the FASB issued ASU No. 2010-11 Derivatives and Hedging Topic 815 “Scope Exception Related to Embedded Credit Derivatives.” This ASU clarifies the guidance within the derivative literature that exempts certain credit related features from analysis as potential embedded derivatives requiring separate accounting. The ASU specifies that an embedded credit derivative feature related to the transfer of credit risk that is only in the form of subordination of one financial instrument to another is not subject to bifurcation from a host contract under ASC 815-15-25, Derivatives and Hedging — Embedded Derivatives — Recognition. All other embedded credit derivative features should be analyzed to determine whether their economic characteristics and risks are “clearly and closely related” to the economic characteristics and risks of the host contract and whether bifurcation is required. The ASU is effective for the Company on July 1, 2010. Early adoption is also permitted. The adoption of this ASU did not have a material impact on our consolidated financial statements.
35
In April 2010, the FASB codified the consensus reached in Emerging Issues Task Force Issue No. 08-09, “Milestone Method of Revenue Recognition.” FASB ASU No. 2010-17 provides guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research and development transactions. FASB ASU No. 2010-17 is effective for fiscal years beginning on or after June 15, 2010, and is effective on a prospective basis for milestones achieved after the adoption date. We do not expect this ASU will have a material impact on its financial position or results of operations when it adopts this update on January 1, 2011.
In April 2010, the FASB issued an authoritative pronouncement on the effect of denominating the exercise price of a share-based payment award in the currency of the market in which the underlying equity securities trades and that currency is different from (1) entity's functional currency, (2) functional currency of the foreign operation for which the employee provides services, and (3) payroll currency of the employee. The pronouncement clarifies that an employee share-based payment award with an exercise price denominated in the currency of a market in which a substantial portion of the entity's equity securities trades should not be considered to contain a condition that is not a market, performance, or service condition, and therefore should be considered an equity award assuming all other criteria for equity classification are met. The pronouncement is for interim and annual periods beginning on or after December 15, 2010, and will be applied prospectively. Affected companies will be required to record a cumulative catch-up adjustment for all awards outstanding as of the beginning of the annual period in which the guidance is adopted. This amendment is not expected to have a material impact on our financial statements.
OFF-BALANCE SHEET ARRANGEMENTS
We have not entered into any other financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our stocks and classified as shareholder’s equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
N/A.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
Our financial statements are contained in pages F-1 through F-21 which appear at the end of this annual report.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2010. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to our management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
36
Based on the evaluation of our disclosure controls and procedures as of December 31, 2010, our principal executive officer concluded that, as of such date, our disclosure controls and procedures were not effective to ensure that (i) information required to be disclosed by our Company in reports that we file or submit under the Securities Exchange Act of 1934 is accumulated and communicated to our Company’s management, including the Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure by our Company; and (ii) information required to be disclosed by our Company in reports that we file or submit under the Securities Exchange Act of 1934 is record, processed, summarized and reported within the time period specific in the Securities and Exchange Act rules and forms.
Internal Control Over Financial Reporting
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting for the company. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principals and includes those policies and procedures that:
· Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
· Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of our Company are being made only in accordance with authorizations of our management and directors; and
· Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2010. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in the report entitled, "Internal Control-Integrated Framework."
Based on our assessment, management concluded that, as of December 31, 2010, our internal control over financial reporting is not effective due to a material weakness. This material weakness is that all of our employees and accounting staff are located in the PRC and we do not presently have a chief financial officer, comptroller or similarly titled senior financial officer who is bilingual and experienced in the application of U.S. GAAP. We have taken the steps to eliminate this material weakness including the hiring of additional accounting consulting staff to review and oversee our application of generally accepted accounting principles in the United States to bring additional financial expertise to our organization and to facilitate the flow of information to our independent accountants. This accounting consulting staff has assisted us in implementing additional practices to ensure that we (i) properly accrue undeclared and unpaid dividends, (ii) properly record related party transactions, and (iii) properly account for changes in loans payable. However, until we expand our full time staff to include a bilingual senior financial officer who has the requisite experience necessary, as well as supplement the accounting knowledge of our staff, notwithstanding the guidance provided to us by the accounting consulting staff we could continue to have material weaknesses in our disclosure controls that may lead to restatements of our financial statements.
37
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit us to provide only management’s report in this annual report.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f)) that occurred during the fourth quarter ended December 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION.
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVRNANCE.
Directors and Executive Officers
|
Name
|
Age
|
Positions
|
||
|
Xuelian Bian
|
45
|
Chief Executive Officer, President and Chairman of the Board
|
||
|
Wei Guan
|
47
|
Vice President and Director
|
Mr. Xuelian Bian has served as our Chairman of the Board, Chief Executive Officer and President since May 2, 2005. Simultaneously, he has served as Chief Executive Officer, President and a Director of Linkwell Tech since its inception in June 2004 and as General Manager of LiKang Disinfectant since 1993. From 1990 to 1993, he was a project assistant in charge of science and technology achievement application in the Second Military Medical University, Shanghai, China. From 1986 to 1990, Mr. Bian was a member of the technical staff in the Epidemiological Institute in the Second Military Medical University. Mr. Bian contributed to the compilation of "Disinfection - Antiseptic - Anticorrosion - Preservation" and "Modern Disinfection Study" of which the first book laid the foundation for Chinese disinfectant study. Mr. Bian started related research with his colleagues on the microbiology sterilization effect examination, high strength ultraviolet lamp tube and decontaminating apparatus prior to the inception of LiKang Disinfectant. Mr. Bian graduated from the China Army Second Military Medical University in 1990 with a bachelor’s degree in public health.
Mr. Wei Guan has served as our Vice President and a member of our Board of Directors since May 2, 2005. He has served as Vice President of Linkwell Tech since its inception in June 2004 and vice General Manager of Shanghai LiKang Disinfectant Company, Limited since 2002. From 1987 to 1990, Mr. Guan worked at Hunan Machinery Importing & Exporting Corporation as a member of management. From 1990 to 2002, he worked for the Division of Importing and Export at Worldbest Group as a general manager. Mr. Guan graduated from Hunan University in Changsha, Hunan Province with a bachelor’s degree in Industry Foreign Trading in 1987.
38
There are no familial relationships between any of the executive officers and directors. Each director is elected at our annual meeting of stockholders and holds office until the next annual meeting of stockholders, or until his successor is elected and qualified.
Key Employees
Mr. Chun Ming Huang, age 42, has served as Chief Operating Officer of our Company since 2005. From 2001 until joining us in 2005, Mr. Huang served as the Associate Director of the Analytical Department of WuXi Pharma Tech. Mr Huang received his master’s degree in pharmaceuticals from the Second Military Medical University in 1992.
Ms. Gendi Li, age 58, has served as LiKang Disinfectant's Controller since 2003. From 1996 to 2003, Ms. Li was employed as an Executive Accountant and Financial Manager for QiaoFu Construction Holding Company (Shanghai). From 1993 to 1996, Ms. Li was employed as an Executive Accountant and Head of the Finance Department at Shanghai Yuxin Machinery Co., Ltd. From 1968 to 1993, Ms. Li was employed in various financial positions, including Executive Accountant, and Head of the Finance Department at First Plastic Machinery Factory. Ms. Li graduated from the Shanghai Finance and Economics Institute.
Mr. Wensheng Sun, age 42, has been LiKang Disinfectant's Vice-General Manager for Production since 1995 and has held the same position at LiKang Disinfectant since 1995 following completion of his Master’s degree in Medicine at the Second Military Medical University School of Pharmacy.
Mr. Rick Wang, age 35, currently serves as our Secretary. Mr. Wang joined LiKang Disinfectant in 1999 and received his bachelor’s degree from the Public Health Department of Xinjiang Medical College in 1997.
Code of Business Conduct and Ethics
In December 2005, we adopted a Code of Business Conduct and Ethics applicable to our Chief Executive Officer, principal financial and accounting officers and persons performing similar functions. A Code of Business Conduct and Ethics is a written standard designed to deter wrongdoing and to promote:
· Honest and ethical conduct,
· Full, fair, accurate, timely and understandable disclosure in regulatory filings and public statements, compliance with applicable laws, rules and regulations,
· The prompt reporting violation of the code, and
· Accountability for adherence to the Code.
We will provide a copy, without charge, to any person desiring a copy of the Code of Business Conduct and Ethics, by written request to, 1104 Jiatang Road Jiading District, Shanghai China 201807, Attention: Corporate Secretary.
Committees of the Board of Directors
Our Board of Directors has not established any committees, including an Audit Committee, a Compensation Committee or a Nominating Committee or any committees performing a similar function. The functions of those committees are being undertaken by the board of directors as a whole. Because we do not have any independent directors, our Board of Directors believes that the establishment of committees of the Board would not provide any benefits to our Company and could be considered more form than substance.
We do not have a policy regarding the consideration of any director candidates which may be recommended by our stockholders, including the minimum qualifications for director candidates, nor has our Board of Directors established a process for identifying and evaluating director nominees. We have not adopted a policy regarding the handling of any potential recommendation of director candidates by our stockholders, including the procedures to be followed. Our Board of Directors has not considered or adopted any of these policies as we have never received a recommendation from any stockholder for any candidate to serve on our Board of Directors. Given that all of our operations are located in the PRC and our lack of directors and officers insurance coverage, we do not anticipate that any of our stockholders will make such a recommendation in the near future. While there have been no nominations of additional directors proposed, in the event such a proposal is made, all members of our Board of Directors will participate in the consideration of director nominees.
39
None of our directors is an “audit committee financial expert” within the meaning of Item 407(d)(5) of Regulation S-K. In general, an “audit committee financial expert” is an individual member of the audit committee or Board of Directors who:
· Understands generally accepted accounting principles and financial statements;
· Is able to assess the general application of such principles in connection with accounting for estimates, accruals and reserves;
· Has experience preparing, auditing, analyzing or evaluating financial statements comparable to the breadth and complexity to our financial statements;
· Understands internal controls over financial reporting; and
· Understands audit committee functions.
Our Board of Directors is comprised of individuals who were integral to our formation and who are involved in our day to day operations. While we would prefer that one or more of our directors be an audit committee financial expert, none of these individuals who have been key to our development have professional backgrounds in finance or accounting. When we are able to expand our Board of Directors to include one or more independent directors, we intend to establish an Audit Committee. It is our intention that one or more of these independent directors will also qualify as an audit committee financial expert. Our securities are not quoted on an exchange that has requirements that a majority of our Board members be independent and we are not currently otherwise subject to any law, rule or regulation requiring that all or any portion of our Board of Directors include “independent” directors, nor is our Board of Directors required to establish or maintain an Audit Committee or any other committee.
ITEM 11. EXECUTIVE COMPENSATION.
Summary Compensation Table
The following table summarizes all compensation recorded by us in each of the last two completed fiscal years for our principal executive officer. Our principal executive officer also serves as our principal financial officer. No other executive officer received total annual compensation exceeding $100,000.
SUMMARY COMPENSATION TABLE
|
Name and principal position (a)
|
Year
(b)
|
Salary
($)
(c)
|
Bonus
($)
(d)
|
Stock
Awards
($)
(e)
|
Option
Awards
($)
(f)
|
Non-Equity Incentive Plan Compensation
($)
(g)
|
Non-qualified Deferred Compensation Earnings
($)
(h)
|
All
Other
Compensations
($)
(i)
|
Total
($)
(j)
|
||||||||||||
|
Xuelian Bian
|
2010
|
12,800 | 12,800 | ||||||||||||||||||
|
Chief Executive Officer, principal executive officer, and principal financial officer
|
2009
|
12,800 | 12,800 | ||||||||||||||||||
40
Employment Agreements
We are not a party to any employment agreements.
Compensation of Directors
Our Board of Directors is presently comprised of our executive officers who do not receive compensation for their services as directors. At such time as we expand our Board of Directors to include independent members, we will establish a policy for the compensation of those members.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Securities Authorized For Issuance under Equity Compensation Plans The following table sets forth securities authorized for issuance under equity compensation plans, including individual compensation arrangements, by us under our Stock Option Plan and any compensation plans not previously approved by our stockholders as of December 31, 2010.
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights
|
Weighted average exercise price of outstanding options, warrants and rights
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in first column)
|
||||||||||
|
Plan category
|
||||||||||||
|
Plans approved by our stockholders:
|
||||||||||||
|
2005 Equity Compensation Plan
|
2,000,000 | 0.21 | 8,000,000 | |||||||||
|
Plans not approved by stockholders:
|
N/A | N/A | N/A | |||||||||
|
None
|
||||||||||||
Stock Option Plans
2005 Equity Compensation Plan
On June 28, 2005, our Board of Directors adopted our 2005 Equity Compensation Plan under which 5,000,000 shares of our common stock were for issuance upon the exercise of options or stock grants under the plan. Our officers, directors, key employees and consultants are eligible to receive stock grants and non-qualified options under the Plan. Only our employees are eligible to receive incentive options. The purpose of the 2005 Equity Compensation Plan is to encourage stock ownership by our officers, directors, key employees and consultants, and to give such persons a greater personal interest in the success of our business and an added incentive to continue to advance and contribute to us. Shares used for stock grants and plan options may be authorized and unissued shares or shares reacquired by us, including shares purchased in the open market. Shares covered by plan options which terminate unexercised will again become available for grant as additional options, without decreasing the maximum number of shares issuable under the plan, although such shares may also be used by us for other purposes.
41
Our Board of Directors, or a committee of the Board, administers the 2005 Equity Compensation Plan including, without limitation, the selection of the persons who will be awarded stock grants and granted options, the type of options to be granted, the number of shares subject to each option and the exercise price. Plan options may either be options qualifying as incentive stock options under Section 422 of the Internal Revenue Code of 1986, as amended, or non-qualified options. In addition, the plan allows for the inclusion of a reload option provision, which permits an eligible person to pay the exercise price of the option with shares of common stock owned by the eligible person and receive a new option to purchase shares of common stock equal in number to the tendered shares. Any incentive option granted under the Plan must provide for an exercise price of not less than 100% of the fair market value of the underlying shares on the date of grant, but the exercise price of any incentive option granted to an eligible employee owning more than 10% of our outstanding common stock must not be less than 110% of fair market value on the date of the grant. The exercise price of non-qualified options shall be determined by the Board of Directors, but shall not be less than the par value of our common stock on the date the option is granted.
The term of each plan option and the manner in which it may be exercised is determined by the Board of Directors or the committee, provided that no option may be exercisable more than 10 years after the date of its grant and, in the case of an incentive option granted to an eligible employee owning more than 10% of the common stock, no more than five years after the date of the grant. The plan provides that, with respect to incentive stock options, the aggregate fair market value (determined as of the time the option is granted) of the shares of common stock, with respect to which incentive stock options are first exercisable by any option holder during any calendar year shall not exceed $1,000,000. Unless the plan is approved by our shareholders within one year of the effective date, no incentive stock options may be granted and all incentive stock options that may have been previously granted shall automatically be converted into non-qualified stock options.
The plan provides that, if our outstanding shares are increased, decreased, exchanged or otherwise adjusted due to a share dividend, forward or reverse share split, recapitalization, reorganization, merger, consolidation, combination or exchange of shares, an appropriate and proportionate adjustment shall be made in the number or kind of shares subject to the plan or subject to unexercised options and in the purchase price per share under such options. Any adjustment, however, does not change the total purchase price payable for the shares subject to outstanding options. In the event of our proposed dissolution or liquidation, a proposed sale of all or substantially all of our assets, a merger or tender offer for our shares of common stock, the Board of Directors may declare that each option granted under the plan shall terminate as of a date to be fixed by the Board of Directors; provided that not less than 30 days written notice of the date so fixed shall be given to each participant holding an option, and each such participant shall have the right, during the period of 30 days preceding such termination, to exercise the participant's option, in whole or in part, including as to options not otherwise exercisable.
Plan options are exercisable by delivery of written notice to us stating the number of shares with respect to which the option is being exercised, together with full payment of the purchase price therefore. Payment is to be in the form of cash, checks, certified or bank cashier's checks, promissory notes secured by the shares issued through exercise of the related options, shares of common stock or in such other form or combination of forms which may be acceptable to the Board of Directors, provided that any loan or guarantee by us of the purchase price may only be made upon resolution of the Board that such loan or guarantee is reasonably expected to benefit us.
All plan options are nonassignable and nontransferable, except by will or by the laws of descent and distribution, during the lifetime of the optionee, and may be exercised only by such optionee. If an optionee shall die while our being an employee or within three months after termination of employment by us due to his/her disability, retirement, or otherwise, such options may be exercised, to the extent that the optionee shall have been entitled to do so on the date of death or termination of employment, by the person or persons to whom the optionee's right under the option pass by will or applicable law, or if no such person has such right, by his executors or administrators.
In the event of termination of employment due to the optionee’s death or disability, the optionee's options may be exercised not later than the expiration date specified in the option or one year after the optionee's death, whichever date is earlier, or in the event of termination of employment due to retirement or otherwise, not later than the expiration date specified in the option or one year after the optionee's death, whichever date is earlier. If an optionee is terminated by us because of disability and such optionee has not died within the following three months, the options may be exercised, to the extent that the optionee shall have been entitled to do so at the date of the termination of employment, at any time, or from time to time, but not later than the expiration date specified in the option or one year after termination of employment, whichever date is earlier.
42
If an optionee's employment terminates for any reason other than death or disability, then the optionee may exercise his/her options to the same extent that the options were exercisable on the date of termination, for up to three months following such termination, or on or before the expiration date of the options, whichever occurs first. In the event that the optionee was not entitled to exercise the options at the date of termination or if the optionee does not exercise such options (which were then exercisable) within the time specified herein, the options will terminate. If an optionee's employment terminates for any reason other than death, disability or retirement, all rights to exercise the option will terminate not later than 90 days following the date of such termination of employment.
The Board of Directors may amend, suspend or terminate the plan at any time. Unless the plan shall have been earlier suspended or terminated by the Board of Directors, the 2005 Equity Compensation Plan terminates on June 28, 2015.
As of January 22, 2008, 5,000,000 shares of our restricted common stock had been granted under the Equity Compensation Plan. No shares of common stock (plus any shares that might in the future be returned to the Equity Compensation Plan as a result of the termination, expiration, or forfeiture of options) remained available for future grant under the Equity Compensation Plan as of such date. Management determined that the number of shares of common stock then available for issuance under the Equity Compensation Plan was insufficient to meet its needs to provide for awards to the plan participants.
On January 22, 2008, our Board of Directors approved and adopted an amendment to our 2005 Equity Compensation Plan, subject to stockholder approval. On January 22, 2008, stockholders owning approximately 53.9% of the outstanding shares of our common stock approved the amendment to the Equity Compensation Plan by action taken by written consent without a meeting in accordance with the Florida Business Corporation Act. No further vote of our stockholders is required to approve the amendment to the 2005 Equity Compensation Plan. Such approval by our stockholders became effective 20 calendar days after the date such Information Statement was first mailed to our stockholders.
The amendment to our 2005 Equity Compensation Plan increased the number of shares of our common stock with respect to which awards may be granted under the 2005 Equity Compensation Plan from 5,000,000 to 15,000,000. On May 1, 2008, we entered into a two year agreement with China Health Capital Group, Inc. (“CHC”) to provide us with financial and investment services. In connection with this agreement, on June 24, 2008, we issued 2,000,000 shares of Common Stock under the 2005 Equity Compensation Plan valued at $0.21 per share to CHC and recorded $420,000 as deferred compensation.
Security Ownership of Certain Beneficial Owners and Management
As of December 31, 2010 we had 86,605,475 shares of our common stock issued and outstanding. The following table sets forth information regarding the beneficial ownership of our common stock as of December 31, 2010 by:
· Each person known by us to be the beneficial owner of more than 5% of our common stock;
· Each of our directors;
· Each of our executive officers; and
· Our executive officers, directors and director nominees as a group.
43
Unless otherwise indicated, the business address of each person listed is in care of 1104 Jiatang Road Jiading District, Shanghai China 201807. The percentages in the table have been calculated on the basis of treating as outstanding for a particular person, all shares of our common stock outstanding on that date and all shares of our common stock issuable to that holder in the event of exercise of outstanding options, warrants, rights or conversion privileges owned by that person at that date which are exercisable within 60 days of that date. Except as otherwise indicated, the persons listed below have sole voting and investment power with respect to all shares of our common stock owned by them, except to the extent that power may be shared with a spouse.
|
Name of Beneficial Owner
|
Amount and Nature of Beneficial Ownership
|
Percentage of Class 1
|
||||||
|
Xuelian Bian
|
20,420,919 | 23.6 | % | |||||
|
Wei Guan
|
13,602,551 | 15.7 | % | |||||
|
All officers and directors as a group (two persons)
|
34,023,470 | 39.3 | % | |||||
|
Holders of 5% or more of the total number of shares outstanding
|
||||||||
1 Calculated based on 86,605,475 shares outstanding as March 29, 2011.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Linkwell Tech's wholly-owned subsidiary, LiKang Disinfectant, is engaged in business activities with four related parties: Shanghai ZhongYou Pharmaceutical High-Tech Co., Ltd., (“ZhongYou”), Shanghai LiKang Biological High-Tech Co., Ltd. (“Likang Biological”), Shanghai Jiuqing Pharmaceuticals Company, Ltd. (“Shanghai Jiuqing”) and Linkwell International Trading Co., Ltd (“Linkwell Trading ”). Shanghai LiKang Meirui Pharmaceuticals High-Tech Co., Ltd (“Meirui”), a company of which Shanhai is a majority shareholder, and had owned 68% of its Meirui equity shares used to be a related party. However, due to the fact that Linkwell Tech acquired Shanghai Shanhai’s 10% equity interest in LiKang Disinfectant for total consideration of $684,057, which included the cash payment of $399,057 and 1,500,000 common shares at value of $0.19 per share. As a result of this transaction, Meirui was no longer a related party. Likang Disinfectant completed its acquisition of Likang Biological on March 3, 2009; therefore, Likang Biological was no longer a related party of our Company and since then, all the sales and purchases with Likang Biological were eliminated for the consolidation.
Shanghai ZhongYou Pharmaceutical High-Tech Co., Limited.
Our Chairman and Chief Executive Officer, Xuelian Bian, and Vice President and Director, Wei Guan, own 90% and 10% respectively, of the capital stock of ZhongYou. In March 2007, Wei Guan sold his 10% interest to Bing Chen, President of LiKang Disinfectant. In August 2007, Xuelian Bian sold his 90% shares to his mother, Xiuyue Xing. In October 2007, the two new shareholders, Bing Chen (10%) and Xiuyue Xing (90%) sold all of their shares in ZhongYou to Shanghai Jiuqing Pharmaceuticals Company, Ltd., whose 100% owner is Shanghai Ajiao Shiye Co. Ltd. Mr. Bian is a 60% shareholder of Shanghai Ajiao Shiye Co. Ltd. For the years ended December 31, 2010 and 2009, we recorded net revenues of $6,987,576 and $4,983,962 to ZhongYou respectively. At December 31, 2010 and 2009, accounts receivable from sales to ZhongYou were $6,701,229 and $3,322,044, respectively. In general, accounts receivable due from ZhongYou are payable in cash and are due within 4 to 6 months, which approximate normal business terms with independent third parties.
Shanghai Jiuqing Pharmaceuticals Company, Limited.
Shanghai Ajiao Shiye Co. Ltd. owns 100% of Shanghai Jiuqing Pharmaceuticals Company, Ltd. (“Shanghai Jiuqing”). Xuelian Bian is a 60% shareholder of Shanghai Ajiao Shiye Co. Ltd. For the years ended December 31, 2010 and 2009, we recorded net revenues of $1,811 and $3,222 to Shanghai Jiuqing, respectively. At December 31, 2010 and 2009, accounts receivable from sales to Shanghai Jiuqing were $88,278 and $85,451, respectively.
As of December 31, 2010, accounts receivable from sales to all other related parties was $2,100,204.
44
Other related party transactions
There were no other related party transactions.
Director Independence
None of the members of our Board of Directors are “independent” as defined by Rule 4200(a)(14) of the Financial Industry Regulatory Authority (FINRA) Rules.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
Sherb & Co., LLP served as our independent registered public accounting firm for fiscal 2010 and 2009. The following table shows the fees that were billed for the audit and other services provided by the firm for fiscal 2010 and 2009.
|
Fiscal 2010
|
Fiscal 2009
|
|||||||
|
Audit Fees
|
$ | 80,000 | $ | 80,000 | ||||
|
Audit-Related Fees
|
0 | 0 | ||||||
|
Tax Fees
|
0 | 0 | ||||||
|
All Other Fees
|
0 | 0 | ||||||
|
Total
|
$ | 80,000 | $ | 80,000 | ||||
Audit Fees — This category includes the audit of our annual financial statements, review of financial statements included in our Form 10-Q Quarterly Reports and services that are normally provided by the independent auditors in connection with engagements for those fiscal years. This category also includes advice on audit and accounting matters that arose during, or as a result of, the audit or the review of interim financial statements.
Audit-Related Fees — This category consists of assurance and related services by the independent auditors that are reasonably related to the performance of the audit or review of our financial statements and are not reported above under “Audit Fees.” The services for the fees disclosed under this category include consultation regarding our correspondence with the SEC and other accounting consultations.
Tax Fees — This category consists of professional services rendered by our independent auditors for tax compliance and tax advice. The services for the fees disclosed under this category include tax return preparation and technical tax advice.
All Other Fees — This category consists of fees for other miscellaneous items.
Our Board of Directors has adopted a procedure for pre-approval of all fees charged by our independent auditors. Under the procedure, the Board approves the engagement letter with respect to audit, tax and review services. Other fees are subject to pre-approval by the Board, or, in the period between meetings, by a designated member of Board. Any such approval by the designated member is disclosed to the entire Board at the next meeting. The audit and tax fees paid to the auditors with respect to fiscal year 2010 were pre-approved by the entire Board of Directors.
45
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
(a) Documents to be filed as part of this annual report:
|
(1)
|
Financial Statements – All consolidated financial statements of the Company as set forth under pages F1- F21 of this annual report.
|
|
(2)
|
Financial Statement Schedules – N/A
|
|
(3)
|
Exhibits.
|
|
Exhibit No.
|
Description
|
|
|
3.1
|
Articles of Incorporation (1)
|
|
|
3.2
|
Articles of Amendment to Articles of Incorporation (2)
|
|
|
3.3
|
Articles of Amendment to Articles of Incorporation (3)
|
|
|
3.4
|
Articles of Amendment to Articles of Incorporation (4)
|
|
|
3.5
|
Articles of Amendment to the Articles of Incorporation (9)
|
|
|
3.6
|
Bylaws (1)
|
|
|
3.7
|
Articles of Amendment to the Articles of Incorporation (10)
|
|
|
4.1
|
Form of common stock purchase warrant (5)
|
|
|
4.2
|
Form of Class A and Class B Common Stock Purchase Warrants (9)
|
|
|
10.1
|
Linkwell Corporation 2005 Equity Compensation Plan (8)
|
|
|
10.2
|
Form of agreement between Shanghai LiKang Disinfectant High-Tech Company, Limited and its customers (10)
|
|
|
10.3
|
Form of agreement between Shanghai LiKang Disinfectant High-Tech Company, Limited and its suppliers (10)
|
|
|
10.4
|
Sales Agreement dated as of January 1, 2005 between Shanghai LiKang Disinfectant High-Tech Co., Ltd. and Shanghai LiKang Meirui Pharmaceutical High-Tech Co., Ltd. (18)
|
|
|
10.5
|
Lease Agreement effective January 1, 2005 between Shanghai LiKang Disinfectant High-Tech Co., Ltd. and Shanghai Shanhai Group for principal executive offices (18)
|
|
|
10.6
|
Lease Agreement effective January 1, 2002 between Shanghai LiKang Pharmaceuticals Co., Ltd. and Shanghai LiKang Disinfectant High-Tech Co. Ltd. (18)
|
|
|
10.7
|
Lease Agreement effective January 1, 2005 between Shanghai Jinshan Zhuhang Plastic Lamp Factory, Ltd. and Shanghai LiKang Disinfectant High-Tech Co. Ltd. (18)
|
|
|
10.8
|
Manufacturing Agreement dated as of January 1, 2005 between Shanghai LiKang Disinfectant High-Tech Co., Ltd. and Shanghai LiKang Meirui Pharmaceutical High-Tech Co., Ltd. (18)
|
|
|
10.9
|
Lease Agreement dated December 15, 2004 between Shanghai Shanhai Group and Shanghai LiKang Disinfectant High-Tech Co., Ltd. (18)
|
|
|
10.10
|
Lease Agreement dated August 11, 2005 between Shanghai Henglain Industrial Co., Ltd. and Shanghai LiKang Disinfectant High-Tech Co., Ltd. (18)
|
|
|
10.11
|
Lease Agreement dated September 16, 2005 between Shanghai Henglain Industrial Co., Ltd. and Shanghai LiKang Disinfectant High-Tech Co., Ltd. (18)
|
|
|
10.12
|
Disinfection Education Center Agreement dated May 25, 2006 between Shanghai LiKang Disinfectant High-Tech Co., Ltd. and China Pest Infestation Control and Sanitation Association (18)
|
|
|
10.13
|
Stock Purchase Agreement, dated April 6, 2007, by and among the Company, Linkwell Tech, Xuelian Bian and LiKang Pharmaceutical (20)
|
|
|
10.13(a)
|
Amendment to Stock Purchase Agreement, dated March 25, 2008 by and among the Company, Linkwell Tech, Xuelian Bian and LiKang Pharmaceutical (21)
|
|
|
10.14
|
Stock Purchase Agreement, dated April 6, 2007, by and among the Company, Linkwell Tech, Xuelian Bian and LiKang Pharmaceutical (20)
|
|
|
10.14(a)
|
Amendment to Stock Purchase Agreement, dated March 25, 2008, by and among the Company, Linkwell Tech and Shanghai Shanhai (21)
|
|
|
10.15
|
Loan agreement between LiKang Disinfectant and LiKang Biological, as translated, dated January 2, 2007 (22)
|
|
|
10.16
|
Stock Purchase Agreement dated February 15, 2008, among Linkwell Corporation, Linkwell Tech Group, Inc., and Ecolab Inc. (23)
|
46
|
10.17
|
Linkwell Tech Group, Inc. Stockholders Agreement, dated May 30, 2008, by and among Linkwell Tech Group, Inc., Linkwell Corp. and Ecolab Inc. (24)
|
|
|
10.18
|
Registration Rights Agreement, dated May 30, 2008, by and among Ecolab Inc. and Linkwell Corp.
(24)
|
|
|
10.19
|
Stock Purchase Agreement dated May 29, 2008, by and between Shanghai Likang Disinfectant Hi-Tech Co., Ltd, and Hong Kong Linkwell International Trading Company (25)
|
|
|
10.20
|
Amended and Restated Stock Purchase Agreement, dated March 5, 2009, by and among the Linkwell Corp., Linkwell Tech Group, Inc., Shanghai Likang Biological High-Tech Co., Ltd., Shanghai Likang Disinfectant Hi-Tech Co., Ltd., Xuelian Bian and Shanghai Likang Pharmaceutical Technology Co., Ltd. (26)
|
|
10.21
|
Stock Purchase Agreement, dated December 21, 2009, by and among Linkwell Corp., Linkwell Tech Group Inc., Shanghai Likang Disinfectant Hi-Tech Co., Ltd., Inner Mongolia Wuhai Chengtian Chemical Co., Ltd. and Honglin Li. (27)
|
|
|
10.22
|
Amendment No. 1 to the Stock Purchase Agreement, dated February 26, 2010, by and among Linkwell Corp., Linkwell Tech Group Inc., Shanghai Likang Disinfectant Hi-Tech Co., Ltd., Inner Mongolia Wuhai Chengtian Chemical Co., Ltd. and Honglin Li. (28)
|
|
|
14.1
|
Code of Business Conduct and Ethics (18)
|
|
|
21.1
|
Subsidiaries of the Registrant (29)
|
|
|
23.1
|
Consent of Sherb & Co., LLP, filed herewith
|
|
| 31.1 | Amended Certification of President and Chief Executive Officer (Principal Executive Officer) Pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Form 10-K), filed herewith. | |
| 31.2 | Amended Certification of Principal Financial Officer Pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes Oxley Act of 2002 (Form 10-K), filed herewith. | |
| 31.3 | Certification of President and Chief Executive Officer (Principal Executive Officer) Pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Form 10-K/A), filed herewith | |
| 31.4 | Certification of Principal Financial Officer Pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes Oxley Act of 2002 (Form 10-K/A), filed herewith | |
| 32.1 | Certification of President and Chief Executive Officer (Principal Executive Officer) and Principal Financial Officer Pursuant to Rule 13a-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, filed herewith |
|
(1)
|
Incorporated by reference to the Report on Form 8-K as filed on December 8, 1999.
|
|
(2)
|
Incorporated by reference to the Report on Form 8-K as filed on December 27, 2001.
|
|
(3)
|
Incorporated by reference to the annual report on Form 10-KSB for the fiscal year ended December 31, 2002.
|
|
(4)
|
Incorporated by reference to the Report on Form 8-K as filed on March 17, 2005.
|
|
(5)
|
Incorporated by reference to the Report on Form 8-K as filed on April 15, 2005.
|
|
(6)
|
Incorporated by reference to the Report on Form 8-K as filed on January 31, 2002.
|
|
(7)
|
Incorporated by reference to the Report on Form 8-K as filed on February 1, 2005.
|
|
(8)
|
Incorporated by reference to the Report on Form 8-K as filed on August 17, 2006.
|
|
(9)
|
Incorporated by reference to the Report on Form 8-K as filed on August 22, 2006.
|
|
(10)
|
Incorporated by reference to the Report on Form 8-K as filed on September 15, 2006.
|
|
(11)
|
Incorporated by reference to the Report on Form 8-K as filed on October 14, 2006.
|
|
(12)
|
Incorporated by reference to the Report on Form 8-K as filed on October 27, 2006.
|
|
(13)
|
Incorporated by reference to the Report on Form 8-K as filed on October 27, 2006.
|
|
(14)
|
Incorporated by reference to the registration statement on Form S-8, SEC File No. 333-138297 as filed on October 30, 2006.
|
|
(15)
|
Incorporated by reference to the registration statement on Form SB-2, SEC File No. 333-139752, as amended, as initially filed on December 29, 2006.
|
|
(16)
|
Incorporated by reference to the registration statement on Form S-8, SEC File No. 333-125871, as filed on June 16, 2005.
|
47
|
(17)
|
Incorporated by reference to the registration statement on Form S-8, SEC File No. 333-121963, as filed on January 11, 2005.
|
|
(18)
|
Incorporated by reference to the registration statement on Form SB-2, SEC File No. 333-131666, as amended, as initially filed on February 8, 2006.
|
|
(19)
|
Incorporated by reference to the by reference to the annual report on Form 10-KSB for the fiscal year ended December 31, 2006.
|
|
(20)
|
Incorporated by reference to the Report on Form 8-K as filed on April 14, 2007.
|
|
(21)
|
Incorporated by reference to the Report on Form 8-K as filed on March 28, 2008
|
|
(22)
|
Incorporated by reference to the by reference to the quarterly report on Form 10-QSB for the quarter ended March 31, 2007.
|
|
(23)
|
Incorporated by reference to the annual report on Form 10-KSB as filed on April 15, 2008
|
|
(24)
|
Incorporated by reference to the Report on Form 8-K as filed on June 5, 2008.
|
|
(25)
|
Incorporated by reference to the by reference to the quarterly report on Form 10-QSB for the quarter ended August 26, 2008.
|
|
(26)
|
Incorporated by reference to the Report on Form 8-K as filed on March 10, 2009.
|
|
(27)
|
Incorporated by reference to the Report on Form 8-K as filed on December 28, 2009.
|
|
(28)
|
Incorporated by reference to the Report on Form 8-K/A as filed on March 4, 2010.
|
| (29) |
Incorporated by reference to the Annual Report on Form 10-K as filed on March 31, 2011.
|
48
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Linkwell Corporation
|
|||
|
By:
|
/s/ Xuelian Bian
|
||
|
Xuelian Bian
CEO, President,
|
|||
|
Principal Executive Officer,
|
|||
|
Principal Financial and Accounting Officer
|
|||
| Dated: November 9, 2011 | |||
49
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders
Linkwell Corporation and Subsidiaries
Shanghai, China
We have audited the accompanying consolidated balance sheets of Linkwell Corporation and Subsidiaries as of December 31, 2010 and 2009 and the related consolidated statements of operations, changes in shareholders’ equity, and cash flows for the year ended December 31, 2010 and 2009. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purposes of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining on a test basis, evidence supporting the amount and disclosures in the combined financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Linkwell Corporation and Subsidiaries as of December 31, 2010 and 2009, and the results of their operation and their cash flows for the year ended December 31, 2010 and 2009, in conformity with accounting principles generally accepted in the United States of America.
/s/ Sherb & Co., LLP
Certified Public Accountants
New York, NY
March 31, 2011
F-1
LINKWELL CORPORATION AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
CONTENTS
| Report of Independent Registered Public Accounting Firm. |
F-1
|
|
|
Consolidated Financial Statements:
|
||
|
Consolidated Balance Sheet
|
F-3
|
|
|
Consolidated Statements of Operations
|
F-4
|
|
|
Consolidated Statements of Stockholders' Equity
|
F-5
|
|
|
Consolidated Statements of Cash Flows
|
F-6
|
|
|
Notes to Consolidated Financial Statements
|
F-7 to F-21
|
F-2
LINKWELL CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
|
AS OF
DECEMBER 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
CURRENT ASSETS
|
||||||||
|
Cash and cash equivalent
|
$ | 3,170,529 | $ | 2,144,360 | ||||
|
Accounts receivable, net
|
2,949,855 | 4,033,718 | ||||||
|
Accounts receivable - related parties, net
|
7,376,366 | 5,708,355 | ||||||
|
Due from related party
|
2,100,204 | 90,013 | ||||||
|
Other receivables
|
152,337 | 254,166 | ||||||
|
Inventories, net
|
2,072,976 | 2,055,986 | ||||||
|
Prepaid expenses and other current assets
|
957,633 | 823,088 | ||||||
|
Total current assets
|
18,779,900 | 15,109,686 | ||||||
|
NON-CURRENT ASSETS
|
||||||||
|
Property, plant and equipment, net
|
2,192,295 | 2,057,758 | ||||||
|
Deposit
|
554,000 | 544,000 | ||||||
|
Intangible assets
|
410,918 | 513,648 | ||||||
|
Total non-current assets
|
3,157,213 | 3,115,406 | ||||||
|
TOTAL ASSETS
|
$ | 21,937,113 | $ | 18,225,092 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES
|
||||||||
|
Loans payable
|
$ | 1,132,469 | $ | 380,774 | ||||
|
Accounts payable and accrued expenses
|
2,338,609 | 2,092,995 | ||||||
|
Advances from customers
|
286,914 | 142,138 | ||||||
|
Taxes payable
|
365,375 | 257,824 | ||||||
|
Other payables
|
61,247 | 154,673 | ||||||
|
Due to related parties
|
1,380,628 | 518,631 | ||||||
|
Total current liabilities
|
5,565,242 | 3,547,035 | ||||||
|
Put option liability
|
2,400,000 | 2,400,000 | ||||||
|
Total liability
|
7,965,242 | 5,947,035 | ||||||
|
COMMITMENT AND CONTIGENCY
|
||||||||
|
STOCKHOLDERS' EQUITY
|
||||||||
|
Preferred Stock, No par value; 10,000,000 authorized, no shares issued and outstanding at December 31, 2010 and 2009, respectively
|
- | - | ||||||
|
Common Stock, $.0005 par value, 150,000,000 authorized, 86,605,475 shares issued and outstanding at December 31, 2010 and 2009, respectively
|
43,303 | 43,303 | ||||||
|
Additional paid-in capital
|
7,474,021 | 7,474,021 | ||||||
|
Statutory surplus reserve
|
802,749 | 802,749 | ||||||
|
Deferred compensation
|
(109,042 | ) | (307,542 | ) | ||||
|
Retained earnings
|
4,188,519 | 3,250,115 | ||||||
|
Accumulated other comprehensive income
|
1,066,622 | 633,970 | ||||||
|
Total Linkwell Corporation stockholder's equity
|
13,466,172 | 11,896,616 | ||||||
|
NONCONTROLLING INTEREST
|
505,699 | 381,441 | ||||||
|
TOTAL STOCKHOLDERS' EQUITY
|
13,971,871 | 12,278,057 | ||||||
|
TOTAL LIABILITIES AND STOCKHOLDER'S EQUITY
|
$ | 21,937,113 | $ | 18,225,092 | ||||
See accompanying notes to these consolidated financial statements.
F-3
LINKWELL CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
|
YEARS ENDED
DECEMBER 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
NET SALES
|
||||||||
|
Non-related companies
|
$ | 6,517,018 | $ | 9,299,120 | ||||
|
Related companies
|
6,989,387 | 5,367,662 | ||||||
|
Total Net Sales
|
13,506,405 | 14,666,782 | ||||||
|
COST OF SALES
|
6,942,366 | 6,501,335 | ||||||
|
GROSS PROFIT
|
6,564,039 | 8,165,447 | ||||||
|
OPERATING EXPENSES
|
||||||||
|
Selling expenses
|
1,262,502 | 1,598,134 | ||||||
|
General and administrative
|
3,881,847 | 2,409,104 | ||||||
|
Total Operating Expenses
|
5,144,349 | 4,007,238 | ||||||
|
INCOME FROM OPERATIONS
|
1,419,690 | 4,158,209 | ||||||
|
OTHER INCOME (EXPENSES)
|
||||||||
|
Other income
|
98,901 | (71,451 | ) | |||||
|
Interest income
|
152 | 4,752 | ||||||
|
Interest expense
|
(89,201 | ) | (55,198 | ) | ||||
|
Total Other Income (Expenses), net
|
9,852 | (121,897 | ) | |||||
|
INCOME BEFORE INCOME TAX
|
1,429,542 | 4,036,312 | ||||||
|
INCOME TAX EXPENSE
|
(366,879 | ) | (594,078 | ) | ||||
|
|
||||||||
|
NET INCOME INCLUDING NONCONTROLLING INTEREST
|
1,062,663 | 3,442,234 | ||||||
|
LESS: NET INCOME ATTRIBUTABLE TO NONCONTROLLING INTEREST
|
(124,259 | ) | (381,441 | ) | ||||
|
NET INCOME ATTRIBUTABLE TO LINKWELL CORP
|
938,404 | 3,060,793 | ||||||
|
OTHER COMPREHENSIVE INCOME
|
||||||||
|
Foreign currency translation
|
432,652 | (397,051 | ) | |||||
|
COMPREHENSIVE INCOME
|
$ | 1,371,056 | $ | 2,663,742 | ||||
|
BASIC AND DILUTED INCOME PER COMMON SHARE
|
||||||||
|
Basic earnings per shares from continuing operation
|
$ | 0.01 | $ | 0.04 | ||||
|
Diluted earnings per shares from continuing operation
|
$ | 0.01 | $ | 0.04 | ||||
|
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING:
|
||||||||
|
Basic
|
86,605,475 | 79,275,338 | ||||||
|
Diluted
|
86,605,475 | 79,324,441 | ||||||
See accompanying notes to these consolidated financial statements.
F-4
LINKWELL CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
YEARS ENDED DECEMBER 31, 2010 AND 2009
|
Additional
|
Accumulated
Other
|
Total
|
||||||||||||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Statutory
|
Accumulated
|
Deferred
|
Comprehensive
|
Stockholders'
|
||||||||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Reserve
|
Deficit
|
Compensation
|
Income
|
Equity
|
|||||||||||||||||||||||||
|
Balance at January 1, 2009
|
77,955,475 | $ | 38,978 | $ | 6,512,346 | $ | 561,222 | $ | 430,849 | $ | (318,556 | ) | $ | 1,031,021 | $ | 8,255,860 | ||||||||||||||||
|
Common stock issued for services
|
4,150,000 | 2,075 | 394,925 | - | - | (397,000 | ) | - | - | |||||||||||||||||||||||
|
Common stock issued for acquisitions
|
4,500,000 | 2,250 | 566,750 | - | - | - | - | 569,000 | ||||||||||||||||||||||||
|
Amortization of deferred compensation
|
- | - | - | - | - | 408,014 | - | 408,014 | ||||||||||||||||||||||||
|
Adjustment to statutory reserve
|
- | - | - | 241,527 | (241,527 | ) | - | - | - | |||||||||||||||||||||||
|
Net income for the year
|
- | - | - | - | 3,060,793 | - | - | 3,060,793 | ||||||||||||||||||||||||
|
Foreign currency translation adjustment
|
- | - | - | - | - | - | (397,051 | ) | (397,051 | ) | ||||||||||||||||||||||
|
Balance at December 31, 2009
|
86,605,475 | 43,303 | 7,474,021 | 802,749 | 3,250,115 | (307,542 | ) | 633,970 | 11,896,616 | |||||||||||||||||||||||
|
Amortization of deferred compensation
|
- | - | - | - | - | 198,500 | - | 198,500 | ||||||||||||||||||||||||
|
Net income for the year
|
- | - | - | - | 938,404 | - | - | 938,404 | ||||||||||||||||||||||||
|
Foreign currency translation adjustment
|
- | - | - | - | - | - | 432,652 | 432,652 | ||||||||||||||||||||||||
|
Balance at December 31, 2010
|
86,605,475 | $ | 43,303 | $ | 7,474,021 | $ | 802,749 | $ | 4,188,519 | $ | (109,042 | ) | $ | 1,066,622 | $ | 13,466,172 | ||||||||||||||||
See accompanying notes to these consolidated financial statements.
F-5
LINKWELL CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
YEARS ENDED
DECEMBER 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||
|
Income including noncontrolling interest
|
$ | 1,062,663 | $ | 3,442,234 | ||||
|
Adjustments to reconcile income including noncontrolling
|
||||||||
|
Interest to net cash provided by operating activities:
|
||||||||
|
Deferred compensation
|
198,500 | 408,014 | ||||||
|
Depreciation and amortization
|
344,702 | 160,447 | ||||||
|
Allowance for doubtful accounts
|
450,960 | (328,323 | ) | |||||
|
Allowance for doubtful accounts-related party
|
263,096 | (58,646 | ) | |||||
|
Loss from disposal of property
|
1,233 | - | ||||||
|
Increase(decrease) in current assets
|
||||||||
|
Accounts receivable
|
731,848 | (435,660 | ) | |||||
|
Accounts receivable - related party
|
(1,927,647 | ) | (2,721,551 | ) | ||||
|
Other receivables
|
106,634 | 72,720 | ||||||
|
Inventories
|
45,790 | 143,350 | ||||||
|
Deposits, prepaid expenses and other current assets
|
486,726 | 263,027 | ||||||
|
Increase(decrease) in current liabilities
|
||||||||
|
Accounts payable and accrued expenses
|
82,895 | (826,978 | ) | |||||
|
Advances from customers
|
137,322 | 50,443 | ||||||
|
Taxes payable
|
97,392 | 123,276 | ||||||
|
NET CASH PROVIDED BY OPERATING ACTIVITIES
|
2,082,114 | 292,353 | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||
|
Cash acquired from acquisition of Likang Biological
|
- | 145,747 | ||||||
|
Acquisition of Likang Biological-related party
|
- | (292,783 | ) | |||||
|
Construction in progress
|
(564,296 | ) | - | |||||
|
Purchase of property, plant and equipment
|
(312,357 | ) | (1,170,150 | ) | ||||
|
NET CASH USED IN INVESTING ACTIVITIES
|
(876,653 | ) | (1,317,186 | ) | ||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||
|
Change in due to related parties
|
820,147 | 77,989 | ||||||
|
Change in due from related parties
|
(1,855,590 | ) | 1,378,357 | |||||
|
Repayment for loans payable
|
- | (746,596 | ) | |||||
|
Proceeds from loans payable
|
723,835 | 380,618 | ||||||
|
NET CASH (USED IN) PROVIDED BY FINANCING ACTIVITIES
|
(311,608 | ) | 1,090,368 | |||||
|
EFFECT OF EXCHANGE RATE ON CASH
|
132,317 | 6,138 | ||||||
|
NET INCREASE IN CASH AND CASH EQUIVALENTS
|
1,026,169 | 71,673 | ||||||
|
CASH AND CASH EQUIVALENTS, BEGINNING OF YEAR
|
2,144,360 | 2,072,687 | ||||||
|
CASH AND CASH EQUIVALENTS, END OF YEAR
|
$ | 3,170,529 | $ | 2,144,360 | ||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION:
|
||||||||
|
Cash paid for:
|
||||||||
|
Interest
|
$ | 76,923 | $ | 54,520 | ||||
|
Income taxes
|
$ | 376,660 | $ | 595,612 | ||||
See accompanying notes to these consolidated financial statements.
F-6
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2010 AND 2009
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
ORGANIZATION
Linkwell Corporation (formerly Kirshner Entertainment & Technologies, Inc.) (the “Company”) was incorporated in the State of Colorado on December 11, 1996. On May 31, 2000, the Company acquired 100% of HBOA.com, Inc. On December 28, 2000, the Company formed a new subsidiary, Aerisys Incorporated (“Aerisys”), a Florida corporation, to handle commercial private business. In June 2003, the Company formed its entertainment division and changed its name to reflect this new division. Effective as of March 31, 2003, the Company discontinued its entertainment division and its technology division, except for the Aerisys operations that continue on a limited basis.
On May 2, 2005, the Company entered into and consummated a share exchange with all of the shareholders of Linkwell Tech Group, Inc. (“Linkwell Tech”). Pursuant to the share exchange, the Company acquired 100% of the issued and outstanding shares of Linkwell Tech's common stock, in exchange for 36,273,470 shares of our common stock, which at closing represented approximately 87.5% of the issued and outstanding shares of the Company’s common stock. As a result of the transaction, Linkwell Tech became our wholly-owned subsidiary. For financial accounting purposes, the exchange of stock was treated as a recapitalization of Kirshner with the former shareholders of the Company retaining 7,030,669 or approximately 12.5% of the outstanding stock. The consolidated financial statements reflect the change in the capital structure of the Company due to the recapitalization and in the operations of the Company and its subsidiaries for the periods presented.
Linkwell Tech was founded on June 22, 2004, as a Florida corporation. On June 30, 2004, Linkwell Tech acquired 90% of Shanghai LiKang Disinfectant High-Tech Company, Ltd. (“LiKang Disinfectant”), a PRC company, through a stock exchange. This transaction resulted in the formation of a U.S. holding company by the shareholders of LiKang Disinfectant as it did not result in a change in the underlying ownership interest of LiKang Disinfectant. LiKang Disinfectant is a science and technology enterprise founded in 1988. LiKang Disinfectant is involved in the development, production, marketing and sale, and distribution of disinfectant health care products.
LiKang Disinfectant has developed a line of disinfectant product offerings which are utilized by the hospital and medical industry in China. LiKang Disinfectant has developed a line of disinfectant product offerings. LiKang Disinfectant regards hospital disinfectant products as the primary segment of its business and has developed and manufactured several series of products in the field of skin mucous disinfection, hand disinfection, surrounding articles disinfection, medical instruments disinfection and air disinfection.
On June 30, 2005, the Company's Board of Directors approved an amendment of its Articles of Incorporation to change the name of the Company to Linkwell Corporation. The effective date of the name change was after close of business on August 16, 2005.
On April 6, 2007, the Company’s subsidiary, Linkwell Tech, entered into two material stock purchase agreements as follows:
i) Linkwell Tech entered into an agreement (the “Biological Stock Purchase Agreement”) to acquire a 100% equity interest in Shanghai LiKang Biological High-Tech Company, Ltd. (“LiKang Biological”), a Chinese company, in a related party transaction with Mr. Xuelian Bian, the Company’s Chief Executive Officer, Mr. Wei Guan, the Company’s Vice-President and Director, and Shanghai Likang Pharmaceuticals Technology Co., Ltd. (“LiKang Pharmaceutical”), a PRC company. Before the Company entered into the Biological Stock Purchase Agreement, Mr. Bian and Mr. Guan owned 90% and 10% of LiKang Pharmaceutical, respectively. Mr. Bian and LiKang Pharmaceutical owned 60% and 40% of LiKang Biological, respectively. Pursuant to the terms of the Biological Stock Purchase Agreement, Mr. Bian and LiKang Pharmaceutical were to receive 1,000,000 shares of Linkwell Corporation’s restricted common stock.
F-7
Due to restrictions under PRC law that prohibited the consideration contemplated by the Biological Stock Purchase Agreement, the agreement did not close. As a result, on March 25, 2008, the parties agreed to enter into an amendment to the Biological Stock Purchase Agreement (“Biological Amendment”) in an effort to complete the stock purchase transaction. Pursuant to the terms of the Biological Amendment, the only material change to the Biological Stock Purchase Agreement related to the consideration paid by Linkwell Tech to Xuelian Bian and LiKang Pharmaceutical, which was changed from 1,000,000 shares of the Company’s common stock to 500,000 shares of common stock and $200,000. As of December 31, 2008, the Biological Stock Purchase Agreement was pending and required further approval from the PRC Ministry of Commerce. Due to the time consuming and complicated nature of the approval procedure, the parties agreed to enter into a second amendment to the Biological Stock Purchase Agreement (“Second Amendment”) in order to complete the purchase transactions timely and properly. Pursuant to the terms of the Second Amendment, the purchaser was changed from Linkwell Tech to LiKang Disinfectant, and, in addition, the consideration changed to RMB 2,000,000, approximately $292,500, and 500,000 shares of common stock. These changes were pertinent because an approval from the Ministry of Commerce in the People’s Republic of China would not be necessary if LiKang Disinfectant acquired 100% of the equity interest in LiKang Biological, as both companies are registered in the PRC. This transaction closed on March 5, 2009. During the quarter ended September 30, 2009, LiKang Disinfectant increased its investment into Likang Biological by injecting RMB 2.5 million cash and RMB 5.5 million in intangible assets (patents). Likang Biological is mainly engaged in producing the disinfectant concentrate.
ii) Linkwell Tech, which already owned a 90% equity interest in LiKang Disinfectant, entered into an agreement to purchase the remaining 10% equity interest of LiKang Disinfectant from Shanghai Shanhai Group (“Shanhai”), a non-affiliated Chinese entity (the “Disinfectant Stock Purchase Agreement”). Pursuant to the terms of the Disinfectant Stock Purchase Agreement, Shanhai was to receive 3,000,000 shares of Linkwell Corporation restricted common stock.
Due to restrictions under PRC law that prohibited the consideration then contemplated by the Disinfectant Stock Purchase Agreement, the agreement did not close. As a result of this, on March 25, 2008, the parties agreed to enter into an amendment to the Disinfectant Stock Purchase Agreement (“Disinfectant Amendment”) in an effort to complete the stock purchase transaction. Pursuant to the terms of the Disinfectant Amendment, the only material change to the Disinfectant Stock Purchase Agreement related to the consideration paid by Linkwell Tech to the Shanghai Shanhai Group for the remaining 10% equity interest, which was changed from 3,000,000 shares of Common Stock to 1,500,000 shares of Common Stock for $380,000. Due to the fluctuation of the applicable exchange rate, the cash consideration was increased to $399,057. The other terms of the Disinfectant Stock Purchase Agreement remained in full force and effect.
Linkwell Tech paid $395,800 to the Shanghai Shanhai Group on February 21, 2008 and paid $3,257 on April 18, 2008. A total of 1,500,000 shares were expected to be issued before the end of May 2008. The parties, however, agreed to extend the share issuance date until October 20, 2008. The Company valued the acquisition using the fair value of common shares at $0.19 per share and recorded an investment of $285,000. Including the cash payment of $399,057, the total investment for acquiring 10% equity interest in LiKang Disinfectant was $684,057. The cumulative minority interest of 10% equity interest in LiKang Disinfectant at March 25, 2008 was approximately $557,779. The difference between the total investment and the cumulative minority interest of $126,278 was deducted from retained earnings as dividends to the 10% minority shareholder, Shanghai Shanhai Group. As a result of the closing of the Disinfectant Stock Purchase Agreement, as amended, as of March 25, 2008, our 90% owned subsidiary Linkwell Tech owns 100% of the equity interest in LiKang Disinfectant.
On February 15, 2008, the Company entered into a stock purchase agreement with Ecolab Inc., a Delaware corporation (“Ecolab”), pursuant to which Ecolab agreed to purchase 888,889 of shares of Linkwell Tech, or 10% of the issued and outstanding capital stock of Linkwell Tech, for $2,000,000. On March 28, 2008 and June 4, 2008, Linkwell Tech received $200,000 and $1,388,559, respectively from Ecolab. Including a $400,000 loan that Linkwell Tech received from Ecolab and accrued interest thereon of $11,441, Linkwell Tech received a total investment of $2,000,000 from Ecolab. On May 31, 2008, the Company, Linkwell Tech and Ecolab entered into a Linkwell Tech Group Inc. Stockholders Agreement, whereby both the Company and Ecolab are subject to, and beneficiaries of, certain pre-emptive rights, transfer restrictions and take along rights relating to the shares of Linkwell Tech that the Company and Ecolab each hold. As of May 31, 2008, the principal and accrued interest of $11,441 on the short-term $400,000 loan became part of Ecolab’s investment and does not need to be repaid.
On May 31, 2008, LiKang Disinfectant entered into a stock purchase agreement under which it sold 100% of the capital stock of its wholly-owned subsidiary, LiKang International, to Linkwell International Trading Co., Ltd, a company registered in Hong Kong which is 100% owned by Mr. Wei Guan, the Company’s Vice President and Director. Pursuant to the terms of the agreement, LiKang Disinfectant received $291,754 (RMB 2,000,000) after the agreement was approved by the PRC Ministry of Commerce on March 27, 2008.
BASIS OF PRESENTATION
The financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America and are expressed in US dollars. All material intercompany transactions and balances have been eliminated in the consolidation.
F-8
Certain reclassifications have been made to the prior year to conform to current year’s presentation. The consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”). The consolidated financial statements of the Company include the accounts of its 90% owned subsidiary, Linkwell Tech, and 100% owned subsidiaries LiKang Disinfectant and Likang Biological. All significant inter-company balances and transactions have been eliminated.
USE OF ESTIMATES
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates. Significant estimates in the years ended December 31, 2010 and 2009 include the allowance for doubtful accounts, stock-based compensation, useful life of property and equipment, inventory reserve and option value.
NON-CONTROLLING INTEREST
Effective January 1, 2009, the Company adopted Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 810, “Consolidation,” which established new standards governing the accounting for and reporting of noncontrolling interests (NCIs) in partially owned consolidated subsidiaries and the loss of control of subsidiaries. Certain provisions of this standard indicate, among other things, that NCIs (previously referred to as minority interests) be treated as a separate component of equity, not as a liability (as was previously the case), that increases and decreases in the parent’s ownership interest that leave control intact be treated as equity transactions rather than as step acquisitions or dilution gains or losses, and that losses of a partially owned consolidated subsidiary be allocated to the NCI even when such allocation might result in a deficit balance. This standard also requires changes to certain presentation and disclosure requirements. Losses attributable to the NCI in a subsidiary may exceed the NCI’s interests in the subsidiary’s equity. The excess attributable to the NCI is attributed to those interests. The NCI shall continue to be attributed its share of losses even if that attribution results in a deficit NCI balance.
FAIR VALUE OF FINANCIAL INSTRUMENTS
For certain of the Company’s financial instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued expenses, advances from customers, short-term loans payable and amounts due from or to related parties, the carrying amounts approximate their fair values due to their short maturities. ASC Topic 820, “Fair Value Measurements and Disclosures,” requires disclosure of the fair value of financial instruments held by the Company. ASC Topic 825, “Financial Instruments,” defines fair value, and establishes a three-level valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The carrying amounts reported in the consolidated balance sheets for receivables and current liabilities each qualify as financial instruments and are a reasonable estimate of their fair values because of the short period of time between the origination of such instruments and their expected realization and their current market rate of interest. The three levels of valuation hierarchy are defined as follows:
Level 1 inputs to the valuation methodology are quoted prices for identical assets or liabilities in active markets.
Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instruments.
Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The Company analyzes all financial instruments with features of both liabilities and equity under ASC 480, “Distinguishing Liabilities from Equity,” and ASC 815.
As of December 31, 2010, the Company did not identify any assets and liabilities that are required to be presented on the balance sheet at fair value.
CASH AND CASH EQUIVALENTS
For purposes of the consolidated statements of cash flows, the Company considers all highly liquid instruments purchased with a maturity of three months or less and money market accounts to be cash equivalents.
F-9
ACCOUNTS RECEIVABLE
The Company has a policy of reserving for uncollectible accounts based on its best estimate of the amount of probable credit losses in its existing accounts receivable. The Company periodically reviews its accounts receivable to determine whether an allowance is necessary based on an analysis of past due accounts and other factors that may indicate that the realization of an account may be in doubt. Account balances deemed to be uncollectible are charged to the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. As of December 31, 2010 and 2009, the Company had established that, based on a review of its third party accounts receivable outstanding balances, allowances for doubtful accounts existed in the amounts of $952,232 and $476,491, respectively. As of December 31, 2010 and 2009, the Company had established that, based on a review of its related party accounts receivable outstanding balances, allowances for doubtful accounts existed in the amounts of $598,033 and $319,200, respectively.
INVENTORIES
Inventories, consisting of raw materials, work in process and finished goods related to the Company’s products are stated at the lower of cost or market utilizing the weighted average method. The valuation of inventory requires the Company to estimate obsolete or excess inventory based on analysis of future demand for our products. Due to the nature of the Company’s business and our target market, levels of inventory in the distribution channel, changes in demand due to price changes from competitors and introduction of new products are not significant factors when estimating the Company’s excess or obsolete inventory. If inventory costs exceed expected market value due to obsolescence or lack of demand, inventory write-downs may be recorded as deemed necessary by management for the difference between the cost and the market value in the period that impairment is first recognized. As of December 31, 2010 and 2009, the reserve for obsolete inventory amounted to $0.
PROPERTY AND EQUIPMENT
Property and equipment are carried at cost. Depreciation is provided using the straight-line method over the estimated economic lives of the assets, which range from five to twenty years. The cost of repairs and maintenance are expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in the income statement in the year of disposition.
IMPAIRMENT OF LONG-LIVED ASSETS
Long-lived assets, which include property, plant and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable. Based on its review, the Company believes that, during the years ended December 31, 2010 and 2009, there were no significant impairment of its long lived assets.
ADVANCES FROM CUSTOMERS
As of December 31, 2010 and 2009, advances from customers were $286,914 and $142,138, respectively. These advances consisted of prepayments from third party customers to the Company for merchandise that had not yet been shipped by the Company. The Company will recognize the prepayments as revenue as customers take delivery of the goods, in compliance with its revenue recognition policy.
INCOME TAXES
The Company utilizes Financial Accounting Standard Board (“FASB”) Accounting Standard Codification (“ASC” Topic 740) , “Accounting for Income Taxes,” which requires recognition of deferred tax assets and liabilities for expected future tax consequences of events that were included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
F-10
The Company adopted the provisions of the Financial Accounting Standards Board's ("FASB") Interpretation No. 48, Accounting for Uncertainty in Income Taxes, (codified in FASB ASC Topic 740). When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority.
BASIC AND DILUTED EARNINGS PER SHARE
The Company presents net income (loss) per share (“EPS”) in accordance with FASB ASC Topic 740, “Earnings per Share”. Accordingly, basic income (loss) per share is computed by dividing income (loss) available to common shareholders by the weighted average number of shares outstanding, without consideration for common stock equivalents. Diluted net income per share is computed by dividing the net income by the weighted-average number of common shares outstanding as well as common share equivalents outstanding for the period determined using the treasury-stock method for stock options and warrants and the if-converted method for convertible notes. The Company has made an accounting policy election to use the if-converted method for convertible securities that are eligible to common stock dividends, if declared. If the if-converted method was anti-dilutive (that is, the if-converted method resulted in a higher net income per common share amount than basic net income per share calculated under the two-class method), then the two-class method was used to compute diluted net income per common share, including the effect of common share equivalents. Diluted earnings per share reflects the potential dilution that could occur based on the exercise of stock options or warrants, unless such exercise would be anti-dilutive, with an exercise price of less than the average market price of the Company’s common stock.
The following table presents a reconciliation of basic and diluted earnings per share:
|
|
2010
|
2009
|
||||||
|
Net income
|
$
|
938,404
|
$
|
3,060,793
|
||||
|
Weighted average shares outstanding - basic
|
86,605,475
|
79,275,338
|
||||||
|
Effect of dilutive securities:
|
||||||||
|
Unexercised warrants
|
-
|
49,103
|
||||||
|
Weighted average shares outstanding - diluted
|
86,605,475
|
79,324,441
|
||||||
|
Earnings per share from continuing operation - basic
|
$
|
0.01
|
$
|
0.04
|
||||
|
Earnings per share from continuing operation - diluted
|
$
|
0.01
|
$
|
0.04
|
||||
REVENUE RECOGNITION
The Company's revenue recognition policies are in compliance with FASB ASC Topic 605. The Company records revenue when an arrangement exists, services have been rendered or product delivery has occurred, the sales price to the customer is fixed or determinable, and collectibility is reasonably assured. The following policies reflect specific criteria for the various revenue streams of the Company. The Company's revenues from the sale of products are recorded when the goods are shipped, title passes, and collectibility is reasonably assured.
F-11
The Company's revenues from the sale of products to related parties are recorded when the goods are shipped to the customers from our related parties. Upon shipment, title passes, and collectibility is reasonably assured. The Company receives purchase orders from our related parties on an as need basis from the related party customers. Generally, the related party does not hold the Company’s inventory. If the related party has inventory on hand at the end of a reporting period, the sale is reversed and the inventory is included on the Company’s balance sheet.
STATEMENT OF CASH FLOWS
In accordance with FASB ASC Topic 230, “Statement of Cash Flows,” cash flows from the Company's operations are calculated based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statement of cash flows may not necessarily agree with changes in the corresponding balances on the balance sheets.
CONCENTRATION OF BUSINESS AND CREDIT RISK
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and trade accounts receivable. The Company places its cash with high credit quality financial institutions in the U.S. and in China. The majority of the Company’s bank accounts in banks located in the PRC are not covered by any type of protection similar to that provided by the FDIC on funds held in U.S banks. As of December 31, 2010 and 2009, the Company maintained cash in the U.S., in a financial institution insured by the FDIC in the amounts of approximately $2,315 and $142,230, respectively.
Almost all of the Company's sales are credit sales which are primarily to customers whose ability to pay is dependent upon the industry economics prevailing in these areas; however, concentrations of credit risk with respect to trade accounts receivables are limited due to generally wide distribution of our products and shorter payment terms than customary in the PRC. The Company also performs ongoing credit evaluations of its customers to help further reduce credit risk. For the years ended at December 31, 2010 and 2009, sales to related parties accounted for 52% and 37% of net revenue, respectively.
The Company is operating in People’s Republic of China, which may give rise to significant foreign currency risks from fluctuations and the degree of volatility of foreign exchange rates between the U.S. dollar and the RMB.
Payments of dividends may be subject to some restrictions.
FOREIGN CURRENCY TRANSLATION AND COMPREHENSIVE INCOME
The Company primarily operates in the PRC. The financial position and results of operations of the subsidiaries are determined using the local currency (“Renminbi” or “RMB”) as the functional currency.
Translation from RMB into United States dollars (“USD” or “$”) for reporting purposes is performed by translating the results of operations denominated in foreign currency at the weighted average rates of exchange during the reporting periods. Assets and liabilities denominated in foreign currencies at the balance sheet dates are translated at the market rate of exchange in effect at that date. The registered equity capital denominated in the functional currency is translated at the historical rate of exchange at the time of capital contribution. All translation adjustments resulting from the translation of the financial statements into USD are reported as a component of accumulated other comprehensive income in shareholders’ equity. The exchange rates used in translation from RMB to USD amount were published by People’s Bank of the People’s Republic of China.
|
For the year ended
December 31, 2010
|
For the year ended
December 31, 2009
|
|||
|
Balance sheet items, except for the registered and paid-in capital and retained earnings, as of the year ended December 31, 2010 and 2009
|
US$1=RMB6.6227
|
US$1=RMB6.8282
|
||
|
Statements of operations and statements of cash flows for the year ended December 31, 2010 and 2009
|
US$1=RMB6.7695
|
US$1=RMB6.831
|
F-12
SHIPPING COSTS
Shipping costs are included in selling expenses and totaled $481,110 and $441,950 for the years ended December 31, 2010 and 2009, respectively.
ADVERTISING
Incurred advertising expenses include selling expenses. For the years ended December 31, 2010 and 2009, advertising expenses amounted to $90,806 and $108,026, respectively.
STOCK-BASED COMPENSATION
The Company accounts for its stock-based compensation in accordance with FASB ASC Topics 718 & 505 . The Company recognizes in the income statement the grant-date fair value of stock options and other equity-based compensation issued to employees and non-employees (consultants).
The cost of stock-based compensation awards issued to non-employees for services are recorded at either the fair value of the services rendered or the instruments issued in exchange for such services, whichever is more readily determinable, using the measurement date guidelines enumerated in Emerging Issues Task Force Issue, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services”.
REGISTRATION RIGHTS AGREEMENTS
The Company accounts for payment arrangements under registration rights agreement in accordance with FASB ASC Topic 815 , which requires the contingent obligation to make future payments or otherwise transfer consideration under a registration payment arrangement, whether issued as a separate agreement or included as a provision of a financial instrument or other agreement, be separately recognized and measured in accordance with FASB ASC Topic 450 .
The Company has adopted “Effect of a Liquidated Damages Clause on a Freestanding Financial Instrument. Accordingly, the Company classifies as liability instruments, the fair value of registration rights agreements when such agreements (i) require it to file, and cause to be declared effective under the Securities Act, a registration statement with the SEC within contractually fixed time periods, and (ii) provide for the payment of liquidating damages in the event of its failure to comply with such agreements. Accordingly, (i) registration rights with these characteristics are accounted for as derivative financial instruments at fair value and (ii) contracts that are (a) indexed to and potentially settled in an issuer's own stock and (b) permit gross physical or net share settlement with no net cash settlement alternative are classified as equity instruments.
RESEARCH AND DEVELOPMENT COST
Research and development costs are expensed as incurred and included in general and administrative expenses. These costs primarily consist of cost of materials used and salaries paid for the development department of the Company and fees paid to third parties. Research and development costs for the years ended December 31, 2010 and 2009 were $311,268 and $108,026, respectively.
SEGMENT REPORTING
FASB ASC Topic 280 "Disclosures about Segments of an Enterprise and Related Information" requires use of the “management approach” model for segment reporting. The management approach model is based on the way a company's management organizes segments within the company for making operating decisions and assessing performance. Reportable segments are based on products and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company.
FASB ASC Topic 280 has no effect on the Company's financial statements as substantially all of the Company's operations are conducted in one industry segment. All of the Company's assets are located in the PRC.
F-13
RECENT ACCOUNTING PRONOUNCEMENTS
On July 1, 2009, the Company adopted Accounting Standards Update (“ASU”) No. 2009-01, “Topic 105 - Generally Accepted Accounting Principles - amendments based on Statement of Financial Accounting Standards No. 168, The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles” (“ASU No. 2009-01”). ASU No. 2009-01 re-defines authoritative GAAP for nongovernmental entities to be only comprised of the FASB Accounting Standards Codification (“Codification”) and, for SEC registrants, guidance issued by the SEC. The Codification is a reorganization and compilation of all then-existing authoritative GAAP for nongovernmental entities, except for guidance issued by the SEC. The Codification is amended to effect non-SEC changes to authoritative GAAP. Adoption of ASU No. 2009-01 only changed the referencing convention of GAAP in Notes to the Consolidated Financial Statements.
On February 25, 2010, the FASB issued ASU No. 2010-09 Subsequent Events Topic 855 “Amendments to Certain Recognition and Disclosure Requirements,” effective immediately. The amendments in the ASU remove the requirement for an SEC filer to disclose a date through which subsequent events have been evaluated in both issued and revised financial statements. Revised financial statements include financial statements revised as a result of either correction of an error or retrospective application of US GAAP. The FASB believes these amendments remove potential conflicts with the SEC’s literature. The adoption of this ASU did not have a material impact on the Company’s consolidated financial statements.
On March 5, 2010, the FASB issued ASU No. 2010-11 Derivatives and Hedging Topic 815 “Scope Exception Related to Embedded Credit Derivatives.” This ASU clarifies the guidance within the derivative literature that exempts certain credit related features from analysis as potential embedded derivatives requiring separate accounting. The ASU specifies that an embedded credit derivative feature related to the transfer of credit risk that is only in the form of subordination of one financial instrument to another is not subject to bifurcation from a host contract under ASC 815-15-25, Derivatives and Hedging — Embedded Derivatives — Recognition. All other embedded credit derivative features should be analyzed to determine whether their economic characteristics and risks are “clearly and closely related” to the economic characteristics and risks of the host contract and whether bifurcation is required. The ASU is effective for the Company on July 1, 2010. Early adoption is permitted. The adoption of this ASU did not have a material impact on the Company’s consolidated financial statements.
In April 2010, the FASB codified the consensus reached in Emerging Issues Task Force Issue No. 08-09, “Milestone Method of Revenue Recognition.” FASB ASU No. 2010-17 provides guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research and development transactions. FASB ASU No. 2010-17 is effective for fiscal years beginning on or after June 15, 2010, and is effective on a prospective basis for milestones achieved after the adoption date. The Company does not expect this ASU will have a material impact on its financial position or results of operations when it adopts this update on January 1, 2011.
In April 2010, the FASB issued an authoritative pronouncement on the effect of denominating the exercise price of a share-based payment award in the currency of the market, in which the underlying equity securities trades and that currency is different from the (1) entity's functional currency, (2) functional currency of the foreign operation for which the employee provides services, and (3) payroll currency of the employee. The pronouncement clarifies that an employee share-based payment award with an exercise price denominated in the currency of a market in which a substantial portion of the entity's equity securities trades should not be considered to contain a condition that is not a market, performance, or service condition, and therefore should be considered an equity award assuming all other criteria for equity classification are met. The pronouncement is for interim and annual periods beginning on or after December 15, 2010, and will be applied prospectively. Affected companies will be required to record a cumulative catch-up adjustment for all awards outstanding as of the beginning of the annual period in which the guidance is adopted. This amendment is not expected to have a material impact on the Company’s financial statements.
A variety of proposed or otherwise potential accounting standards are currently under study by standard setting organizations and various regulatory agencies. Due to the tentative and preliminary nature of those proposed standards, management has not determined whether implementation of such proposed standards would be material to the consolidated statements.
F-14
NOTE 2 – INVENTORIES
A summary of inventories by major category as of December 31, 2010 and 2009 were as follows:
|
2010
|
2009
|
|||||||
|
Raw materials
|
$
|
820,293
|
$
|
870,559
|
||||
|
Work-in-process
|
31,398
|
2,604
|
||||||
|
Finished goods
|
1,221,285
|
1,182,823
|
||||||
|
Net inventories
|
$
|
2,072,976
|
$
|
2,055,986
|
||||
NOTE 3 – PROPERTY AND EQUIPMENT
For the years ended December 31, 2010 and 2009, property and equipment consisted of the following:
|
|
Estimated
Useful Life
(In years)
|
2010
|
2009
|
|||||||
|
Office equipment and furniture
|
3-7
|
$
|
404,130
|
$
|
356,950
|
|||||
|
Autos and trucks
|
5
|
327,479
|
294,554
|
|||||||
|
Manufacturing equipment
|
2-10
|
826,136
|
637,910
|
|||||||
|
Building
|
5-20
|
1,643,684
|
1,528,404
|
|||||||
|
Subtotal
|
3,201,429
|
2,817,818
|
||||||||
|
Less: Accumulated depreciation
|
(1,009,134
|
)
|
(760,060
|
)
|
||||||
|
Property and equipment, net
|
$
|
2,192,295
|
$
|
2,057,758
|
||||||
For the years ended December 31, 2010 and 2009, depreciation expenses amounted to $241,972 and $160,447, respectively.
NOTE 4 – PREPAID EXPENSE AND OTHER CURRENT ASSETS
The Company had prepaid expenses and other current assets of $957,633 and $823,088 at December 31, 2010 and 2009, respectively, including $576,804 and $559,445 (RMB 3.82 million) prepaid land use right application fees. The Company constructed the building on this land in 2010, and is waiting for the approval and issuance of title from the government; the Company expects to receive the titles of the land and building by the end of 2011.
NOTE 5 - INTANGIBLE ASSETS
For the years ended December 31, 2010, intangible assets consisted of customers lists arising from the acquisition of Likang Biological, amortizing over 5 years. Net intangible assets as of December 31, 2010 and 2009 totaled $410,918 and $513,648, respectively. Amortization expenses for the years ended December 31, 2010 and 2009 were $102,730 and $0, respectively. Annual amortization expenses for the next five years from December 31, 2010 are expected to be: $102,730, $102,730, $102,730, $102,728 and $0.
NOTE 6 - DEPOSITS
For the years ended December 31, 2010 and 2009, deposits mainly represented prepaid application fee of $544,000 for the deposit of purchasing an acquisition target in China.
F-15
NOTE 7 – LOANS PAYABLE
Loans payable consisted of the following for the years ended December 31, 2010 and December 31, 2009:
|
|
2010
|
2009
|
||||||
|
Loans from Shanghai Rural Commercial Bank, Dachang Branch due on June 16, 2010 with interest rate at 6.90% per annum, Guaranteed by Shanhai Group (RMB 2,600,000)
|
$
|
-
|
$
|
380,774
|
||||
|
Loans from Shanghai Rural Commercial Bank, Dachang Branch due on March 15, 2011 with interest rate 5.31% per annum. Guaranteed by Shanhai Group and Mr. Bian, Chairman of the Company (RMB 4,900,000)
|
739,880
|
-
|
||||||
|
Loans from Shanghai Rural Commercial Bank, Dachang Branch due on July 13, 2011 with interest rate 5.31% per annum. Guaranteed by Shanhai Group and Mr. Bian, Chairman of the Company (RMB 2,600,000)
|
392,589
|
-
|
||||||
|
$
|
1,132,469
|
$
|
380,774
|
|||||
NOTE 8 – RELATED PARTY TRANSACTIONS
Linkwell Tech's wholly-owned subsidiary, LiKang Disinfectant, is engaged in business activities with four related parties: Shanghai ZhongYou Pharmaceutical High-Tech Co., Ltd., (“ZhongYou”), Shanghai LiKang Biological High-Tech Co., Ltd. (“Likang Biological”), Shanghai Jiuqing Pharmaceuticals Company, Ltd. (“Shanghai Jiuqing”) and Linkwell International Trading Co., Ltd. (“Linkwell Trading ”). Shanghai LiKang Meirui Pharmaceuticals High-Tech Co., Ltd. (“Meirui”), a company of which Shanhai is a majority shareholder, and had owned 68% of its Meirui equity shares, used to be a related party, in that it formally owned 10% of Likang Disinfectant. However, Linkwell Tech acquired Shanhai’s 10% equity interest in LiKang Disinfectant for total consideration of $684,057, which included the cash payment of $399,057 and 1,500,000 common shares at $0.19 per share. As a result of this transaction, Meirui was no longer a related party. Likang Disinfectant completed its acquisition of Likang Biological on March 31, 2009, thus, making Likang Biological no longer a related party to the Company. Since then, all the sales and purchase with Likang Biological were eliminated during the consolidation.
The Company’s Chairman and Chief Executive Officer, Xuelian Bian, and Vice President and Director, Wei Guan, own 90% and 10% respectively, of the capital stock of ZhongYou. In March 2007, Wei Guan sold his 10% shares to Bing Chen, the President of LiKang Disinfectant. In August 2007, Xuelian Bian sold his 90% shares to his mother, Xiuyue Xing. In October 2007, the two new shareholders, Bing Chen (10%) and Xiuyue Xing (90%) sold all of their shares in ZhongYou to Shanghai Jiuqing, whose 100% owner is Shanghai Ajiao Shiye Co. Ltd. Mr. Bian is currently a 60% shareholder of Shanghai Ajiao Shiye Co. Ltd.
For the years ended December 31, 2010 and 2009, the Company recorded sales of $6,987,576 and $4,983,962 to ZhongYou, respectively. For the years ended December 31, 2010 and 2009, accounts receivables from sales to ZhongYou were $6,701,229 and $3,322,044, respectively.
Shanghai Ajiao Shiye Co. Ltd. owns 100% of Shanghai Jiuqing Pharmaceuticals Company, Ltd (“Shanghai Jiuqing”). Xuelian Bian is a 60% shareholder of Shanghai Ajiao Shiye Co. Ltd. For the years ended December 31, 2010 and 2009, the Company recorded sales of $1,811 and $3,222 to Shanghai Jiuqing, respectively. At December 31, 2010 and 2009, accounts receivable from sales to Shanghai Jiuqing were $88,278 and $85,451, respectively.
For the years ended December 31, 2010, due from other related parties represented short-term advances and other receivables other than the receivables from sales, including due from Wuhai Likang $1,567,337, Likang Trading $301,992 and due from other related parties $230,875. As of December 31, 2009, due from other related parties were$90,013.
On December 31, 2010, the Company owed its management $54,000, Zhongyou $1,025,621, Wuhai Chengtian $217,434 and other related parties $83,573. As of December 31, 2009, the Company owed its management $54,000, Zhongyou $175,520, and other related parties of $289,111.
NOTE 9 – TAXES PAYABLE
Taxes payable consisted of the following for the years ended December 31, 2010 and 2009, respectively:
|
2010
|
2009
|
|||||||
|
Income tax payable
|
$
|
132,140
|
$
|
136,823
|
||||
|
Value added tax payable
|
225,810
|
114,720
|
||||||
|
Other taxes payable
|
7,425
|
6,281
|
||||||
|
$
|
365,375
|
$
|
257,824
|
|||||
F-16
NOTE 10 –PUT OPTION LIABILITY
On February 15, 2008, the Company entered into a stock purchase agreement with Ecolab pursuant to which Ecolab agreed to purchase and Linkwell Tech agreed to sell 888,889 of its shares, or 10% of the issued and outstanding capital stock of Linkwell Tech for $2,000,000. On May 31, 2008, the Company, Linkwell Tech and Ecolab entered into a Stockholders Agreement (“Stockholders Agreement”), whereby both the Company and Ecolab are subject to, and the benefit of, certain pre-emptive rights, transfer restrictions and take along rights relating to the shares of Linkwell Tech, the Company and Ecolab each hold.
Pursuant to the terms of the Stockholders Agreement, Ecolab has an option (“Put Option”) to sell the 888,889 shares (“Shares”) of common stock, par value $0.001, of Linkwell Tech that Ecolab purchased under the Stock Purchase Agreement back to Linkwell Tech in exchange for, as determined by Linkwell, cash in the amount of $2,400,000 or the lesser of (a) 10,000,000 shares of Linkwell common stock, or (b) such number of shares of Linkwell common stock as is determined by dividing 3,500,000 by the average daily closing price of Linkwell common stock for the twenty days on which Linkwell shares of common stock were traded on the OTC Bulletin Board prior to the date the Put Option is exercised (“Put Shares”). The Put Option is exercisable during the period between the second and fourth anniversaries of May 30, 2008, or upon the occurrence of certain events including material breach by Linkwell Tech or its subsidiaries, of the Consulting Agreement, Distributor Agreements or Sales Representative Agreement entered into in connection with the Stock Purchase Agreement.
Under the Stockholders Agreement, Ecolab also has a call option (“Call Option”), exercisable if Linkwell is subject to a change of control transaction, to require the Company to sell to Ecolab all of the equity interests in Linkwell Tech, or any of Linkwell Tech’s subsidiaries, then owned by the Company. The Company recognized the maximum expenses of the Put Option and the Call Option described above as $2,400,000 put option liability.
NOTE 11 - INCOME TAXES
The Company is subject to income taxes by entity on income arising in or derived from the tax jurisdiction in which each entity is domiciled.
Linkwell Corp and Linkwell Tech were incorporated in the U.S. and have net operating losses (NOL) for income tax purposes. Linkwell Corp and Linkwell Tech had net operating loss carry forwards for income taxes of approximately $2,259,000 on December 31, 2010 which may be available to reduce future years’ taxable income as NOL’s can be carried forward up to 20 years from the year the loss is incurred. Under IRC section 382, certain of these loss carry-forward amounts may be limited due to the more than 50% change in ownership which took place during 2004. The Company’s management believes that the realization of benefits from these losses are uncertain due to the Company’s limited operating history and continuing losses. Accordingly, a 100% valuation allowance has been provided on the deferred tax asset of approximately $862,000.
Likang Disinfectant and Likang Biological are governed by the Income Tax Law of the PRC concerning privately-run enterprises, which are generally subject to tax at a statutory rate of 25% on income reported in the statutory financial statements after appropriated tax adjustments. Likang Disinfectant is subject to preferential income tax rate of 12.5% for 2010 and 2009; Likang Biological was subject preferential income tax rate 20% for 2009.
The following table reconciles the U.S. statutory rates to the Company’s effective tax rate for the years ended December 31, 2010 and 2009:
|
2010
|
2009
|
|||||||
|
US statutory rates
|
34.0
|
%
|
34.0
|
%
|
||||
|
State income tax, net of federal benefit
|
4.0
|
%
|
4.0
|
%
|
||||
|
Tax rate difference between China and U.S.
|
(17.1
|
)%
|
(14.8
|
)%
|
||||
|
Effect of tax holiday on PRC taxable income
|
(18.3
|
)%
|
(12.4)
|
%
|
||||
|
Other
|
6.9
|
%
|
(1.3)
|
%
|
||||
|
Valuation allowance on US NOL
|
16.2
|
%
|
5.2
|
%
|
||||
|
Tax per financial statements
|
25.7
|
%
|
14.7
|
%
|
||||
F-17
NOTE 12 - STATUTORY RESERVES
Pursuant to the PRC’s corporate law effective as of January 1, 2006, the Company is now only required to maintain one statutory reserve by appropriating from its after-tax profit before declaration or payment of dividends. The statutory reserve represents restricted retained earnings.
Surplus Reserve Fund
The Company is now only required to transfer 10% of its net income, as determined under PRC accounting rules and regulations, to a statutory surplus reserve fund until such reserve balance reaches 50% of the Company’s registered capital.
The surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance after such issue is not less than 25% of the registered capital.
Common Welfare Fund
The common welfare fund is a voluntary fund that provides that the Company can elect to transfer 5% to 10% of its net income to this fund. This fund can only be utilized on capital items for the collective benefit of the Company’s employees, such as construction of dormitories, cafeteria facilities, and other staff welfare facilities. This fund is non-distributable other than upon liquidation. The Company did not make any reserve to this fund during the years ended December 31, 2010 and 2009.
NOTE 13 – STOCKHOLDERS’ EQUITY
Common Stock
In September 2006, the Company entered into a three-year agreement with a consultant to provide business development and management services. In connection with this agreement, the Company issued 500,000 shares of the Company’s common stock. The Company valued these services using the fair value of common shares on the grant date at $0.185 per share and recorded deferred consulting expense of $92,500 to be amortized over the service period. The deferred consulting expense has been fully expensed in 2009.
On May 1, 2008, the Company entered into a two year agreement with China Health Capital Group, Inc. (“CHC”) to provide the Company with financial and investment services. In connection with this agreement, on June 24, 2008, the Company issued 2,000,000 shares of Common Stock valued at $0.21 per share to CHC and recorded $420,000 as deferred compensation. This compensation has been fully expensed in 2009.
On July 1, 2009, the Company entered into a two year agreement with FirsTrust Group, Inc. In connection with this agreement, the Company issued 1,800,000 shares of Common Stock valued at $0.09 per share to FirsTrust Group, Inc. and recorded $162,000 as deferred compensation. The Company had accumulated amortization of $121,500 for stock-based compensation as of December 31, 2010. This agreement has a penalty to the stock recipient for non-compliance to its terms.
On August 1, 2009, the Company entered into a two year agreement with Shanghai Hai Mai Law Firm. In connection with this agreement, the Company issued 2,350,000 shares of Common Stock valued at $0.10 per share to Shanghai Hai Mai Law Firm and recorded $235,000 as deferred compensation. The Company had accumulated amortization of $166,458 for stock-based compensation as of December 31, 2010. This agreement has a penalty to the stock recipient for non-compliance to its terms.
On December 30, 2009, the Company issued 4,000,000 shares of common stock for acquiring an acquisition target in China for $544,000 accounted for as a deposit as of December 31, 2009. The Company is in the process of closing the acquisition, which is contingent upon receiving governmental approval of the acquisition.
F-18
On December 30, 2009, the Company issued 500,000 shares of common stock for paying the stock consideration portion of the acquisition price for Likang Biological.
Common Stock Warrants
During the years ended December 31, 2010 and 2009, there were no warrants granted or exercised. At December 31, 2010, all the warrants were expired.
NOTE 14 – ACQUISITION OF LIKANG BIOLOGICAL
On March 5, 2009, the Company closed the acquisition of all the outstanding capital stock of Likang Biological. The purchase price for Likang Biological was RMB2,000,000 (approximately $292,500) and 500,000 shares of common stock equivalent to approximately US$25,000, which was determined by multiplying the 500,000 shares by the average stock price of Linkwell Corp. two days before and two days after the closing date . This acquisition was a related party transaction. Mr. Xuelian Bian, the Company’s Chief Executive Officer, owned 90% of LiKang Pharmaceutical. Mr. Bian and LiKang Pharmaceutical owned 60% and 40% of LiKang Biological, respectively.
For convenience of reporting the acquisition for accounting purposes, March 1, 2009 was designated as the acquisition date.
The following table summarizes the fair values of the assets acquired and liabilities assumed at the date of acquisition.
|
Cash
|
$
|
145,749
|
||
|
Other receivables
|
121,573
|
|||
|
Inventory
|
986,043
|
|||
|
Property and equipment
|
340,542
|
|||
|
Construction in progress
|
834,401
|
|||
|
Intangible assets
|
469,084
|
|||
|
Accounts payable
|
(970,235
|
)
|
||
|
Other current liabilities
|
(1,609,657
|
)
|
||
|
Purchase price
|
$
|
317,500
|
The following unaudited pro forma consolidated results of operations of the Company for the year ended December 31, 2009 presents the operations of the Company and Likang Biological as if the acquisition of Likang Biological occurred on January 1, 2009. The pro forma results are not necessarily indicative of the actual results that would have occurred had the acquisitions been completed as of the beginning of the periods presented, nor are they necessarily indicative of future consolidated results.
|
For the year
ended
December 31,
2009
|
||||
|
Net revenue
|
$ |
14,806,452
|
||
|
Cost of revenue
|
6,625,574
|
|||
|
Gross profit
|
8,180,878
|
|||
|
Selling expense
|
1,598,134
|
|||
|
General & administrative expense
|
2,436,968
|
|||
|
Total operating expenses
|
4,035,102
|
|||
|
Income from operations
|
4,145,776
|
|||
|
Non-operating (expenses), net
|
(121,964)
|
|||
|
Income from discontinued operations
|
-
|
|||
|
Income before income tax
|
4,023,812
|
|||
|
Income tax
|
594,078
|
|||
|
Net income including noncontrolling interest
|
3,429,734
|
|||
|
Less: noncontrolling interest
|
381,440
|
|||
|
Net income
|
$ |
3,048,294
|
||
F-19
NOTE 15- COMMITMENTS
On July 16, 2008, the Company entered a two-year lease agreement for its administrative office which expired on July 15, 2010, for monthly rent of $4,400 (RMB 30,237) until April of 2009, and $9,470 (RMB 64,669) after April of 2009. The Company renewed this lease after the expiration and monthly rent increased to $11,000 (RMB 73,173). The lease will expire at December 31, 2011.
On November 1, 2008, the Company entered another two-year lease agreement for its branch office which expired on October 31, 2010, for monthly rent of $3,500 (RMB 24,000). The Company renewed this lease after the expiration. The new lease will expire on October 31, 2011.
Future minimum rental payments required under these operating leases are as follows:
|
Year Ended December 31,
|
||||
|
2011
|
$
|
171,224
|
||
NOTE 16 – CONTINGENCY
The Company is a defendant in a lawsuit filed in November 2009 in the Supreme Court of the State of New York, County of New York, by two warrant holders of the Company, seeking damages of at least $800,000. As of March 31, 2011, the Company is vigorously defending its position in this litigation matter and has not made a provision with regards to this lawsuit in the event of an unfavorable outcome. The Company filed a motion to dismiss the complaint, but that motion has been withdrawn without prejudice to reinstatement.
NOTE 17 – OPERATING RISK
|
(a)
|
Country risk
|
Currently, the Company’s revenues are primarily derived from the sale of a line of disinfectant product to customers in the PRC. The Company hopes to expand its operations to countries outside the PRC, however, such expansion has not been commenced and there are no assurances that the Company will be able to achieve such an expansion successfully. Therefore, a downturn or stagnation in the economic environment of the PRC could have a material adverse effect on the Company’s financial condition.
|
(b)
|
Products risk
|
In addition to competing with other domestic manufacturers of disinfectant product offerings, the Company competes with larger U.S. companies who have greater funds available for expansion, marketing, research and development and the ability to attract more qualified personnel. These U.S. companies may be able to offer products at a lower price. There can be no assurance that the Company will remain competitive should this occur.
|
(c)
|
Exchange risk
|
The Company cannot guarantee that the current exchange rate will remain steady; therefore there is a possibility that the Company could post the same amount of profit for two comparable periods. This is because a fluctuating exchange rate may post a higher or lower profit depending on exchange rate of Renminbi converted to US dollars on that day. The exchange rate could fluctuate depending on changes in the political and economic environments without notice.
|
(d)
|
Political risk
|
Currently, the PRC is in a period of growth and is openly promoting business development in order to bring more business into the country. Additionally, the PRC currently allows a Chinese corporation to be owned by a United States corporation. If the PRC government changes laws or regulations relating to the ownership of a Chinese corporation, then the Company's ability to operate the PRC subsidiaries could be affected.
F-20
NOTE 18 – SUBSEQUENT EVENTS
In accordance with ASC 855, “Subsequent Events,” the Company has evaluated subsequent events after the balance sheet date of December 31, 2010. The Company has reported all required subsequent events.
F-21
