Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REVA Medical, Inc. | c24249e8vk.htm |
Exhibit 99.1

| Novel Methods to Evaluate Bioresorbable Scaffolds REVA Medical Program Update Dr. Alexandre Abizaid |
| Disclosure Statement of Financial Interest Consulting Fees REVA Medical, Inc. Within the past 12 months, I have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company |

| To develop a stent that provides the benefits of metal stents without their permanency Strength Maintain lumen (passage) in vessel Maintain support throughout healing process Performance Expansion range to accommodate "real world" clinical use Visibility Visualize entire stent during and after deployment Resorption Dissolves once its job is done leaving the patient free of a permanent implant Goals of the REVA Program Intent is to drive meaningful clinical benefits |
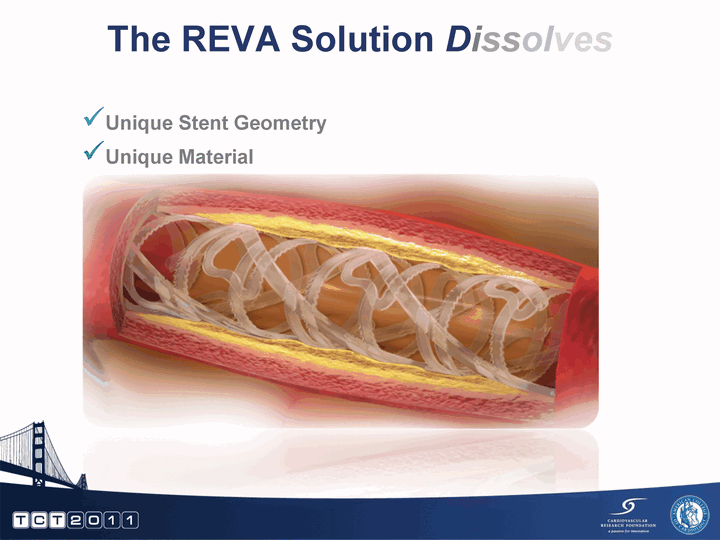
| The REVA Solution Dissolves Unique Stent Geometry Unique Material |

| ReZolve(tm) Sirolimus-Eluting Bioresorbable Coronary Stent Drug-eluting (Sirolimus) Stronger and More Resilient Polymer Optimized Uniform Design |
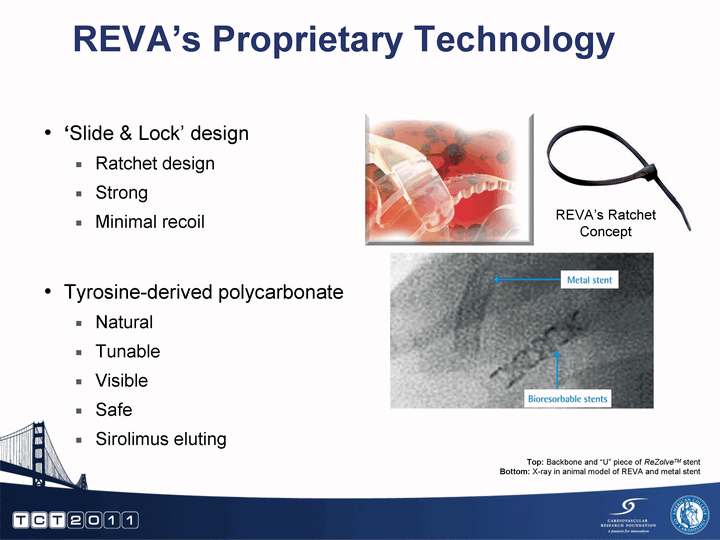
| 'Slide & Lock' design Ratchet design Strong Minimal recoil Tyrosine-derived polycarbonate Natural Tunable Visible Safe Sirolimus eluting REVA's Proprietary Technology Top: Backbone and "U" piece of ReZolveTM stent Bottom: X-ray in animal model of REVA and metal stent REVA's Ratchet Concept |

| ReZolve(tm) Elutes Sirolimus 80µg dose Sirolimus with abluminal delivery Delivery kinetics match commercial -limus eluting stents Drug embedded in stent polymer on the surface Thin coating layer (^ 5µm) Reproducible coating process |
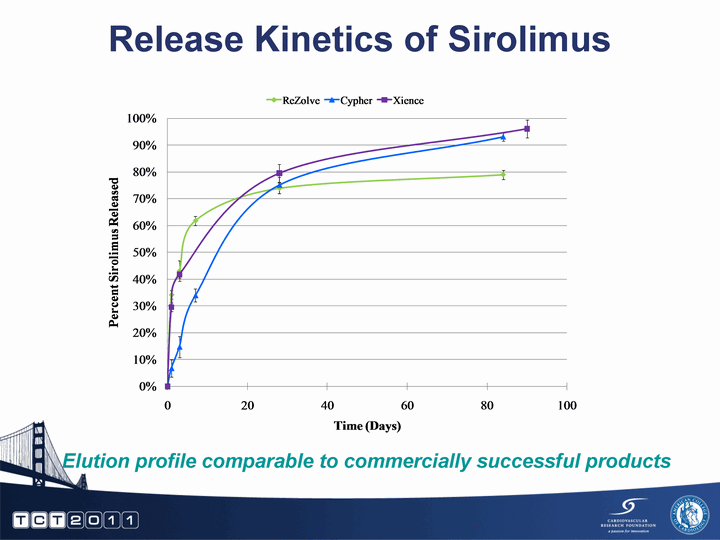
| Release Kinetics of Sirolimus Elution profile comparable to commercially successful products |

| Bench and Preclinical Testing |

| Enhanced Test Methods Demonstrate Robustness Stent Type Cycles Cycles Time Time Stent Type Range Average Range Average ReZolve 127,202 - 507,854 246,230 29 - 120 Hrs 62 Hrs Metal Control 61,986 - 108,248 80,956 14.5 - 25 Hrs 18.9 Hrs |
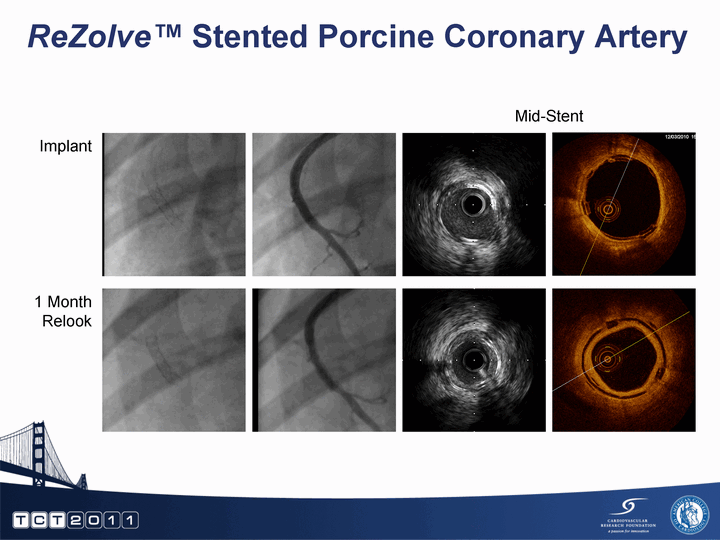
| ReZolve(tm) Stented Porcine Coronary Artery Implant 1 Month Relook Mid-Stent |

| 3 Months 6 Months 12 Months ReZolve(tm) Preclinical Time Sequence (bare) |

| Endothelialization 3-month SEM Metal Control ReZolve(tm) Stent |

| Novel Simulated Lesion Preclinical Models |

| Porcine Chronic Partial Occlusion ("CPO") X-ray visible, highly mineralized plugs Watanabe Rabbit Hypercholesterolemic "soft" plaque Simulated Lesion Models Pig and Rabbit Challenge Tests |

| Angiographic Images of Moderate CPO Lesion Pre-Stenting ~55% Stenosis Post-Stenting |

| OCT Images of Moderate CPO Lesion Pre-Stenting Note: After Pre-dilation with balloon Post-Stenting |
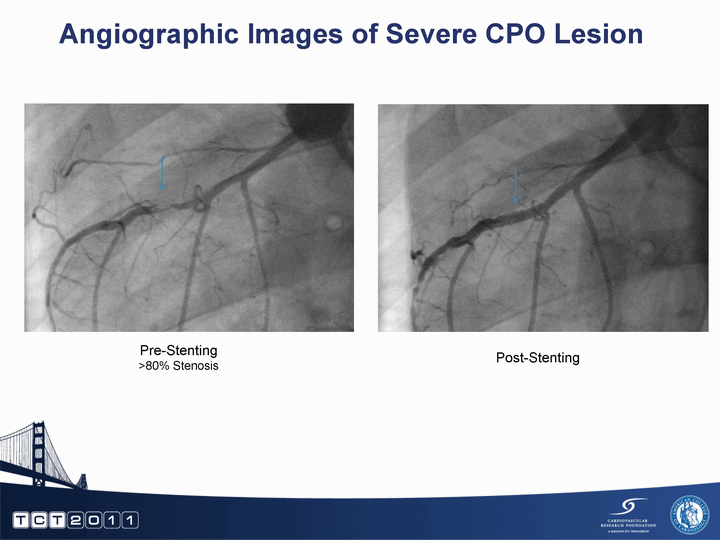
| Angiographic Images of Severe CPO Lesion Pre-Stenting >80% Stenosis Post-Stenting |

| OCT Images of Severe CPO Lesion Pre-Stenting Note: After Pre-dilation with balloon Post-Stenting |

| ReZolve(tm) vs. Metal Control ReZolve Metal Control |

| Watanabe Rabbit vs. Human Coronary Lesion Watanabe Aortic Lesion Human LAD Lesion |

| ReZolve(tm) Stent Performance at One Month Distal Reference (non-stented) Mid Stent (stented) Proximal Reference (non-stented) |

| RESTORE Clinical Trial ReZolve Sirolimus-Eluting Bioresorbable Coronary Stent Pilot Safety Study 50 Patient enrollment Sites in Brazil & Europe Principal Investigator: Dr. Alexandre Abizaid Primary Endpoint(s): Freedom from ischemic-driven target lesion revascularization at 6 months Quantitative measurements at 12 months (QCA/IVUS) |

| Status of Pilot Clinical Trial Pilot clinical trial to begin this quarter All required regulatory approvals have been received to initiate the trial in both Brazil and Germany Pursuing additional clinical sites in Europe Pilot clinical trial will likely involve up to eleven hospitals |

| Thank you |

| Supplemental Slides |

| Fatigue Resistance Under Load |

| Lockout Robustness Rail Fails Before Lockout Tooth |

| ISO-10993 Testing Shows Polymer Safety |

| Known Polymer Molecular Breakdown |
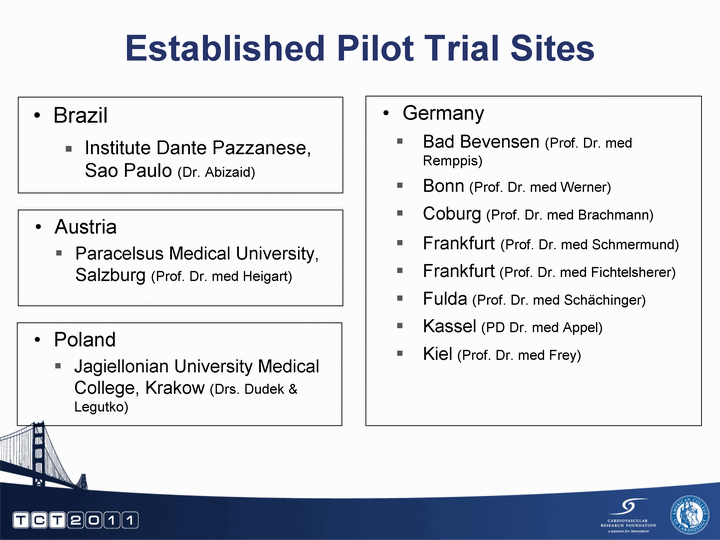
| Brazil Institute Dante Pazzanese, Sao Paulo (Dr. Abizaid) Established Pilot Trial Sites Germany Bad Bevensen (Prof. Dr. med Remppis) Bonn (Prof. Dr. med Werner) Coburg (Prof. Dr. med Brachmann) Frankfurt (Prof. Dr. med Schmermund) Frankfurt (Prof. Dr. med Fichtelsherer) Fulda (Prof. Dr. med Schachinger) Kassel (PD Dr. med Appel) Kiel (Prof. Dr. med Frey) Austria Paracelsus Medical University, Salzburg (Prof. Dr. med Heigart) Poland Jagiellonian University Medical College, Krakow (Drs. Dudek & Legutko) |
