Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ardea Biosciences, Inc./DE | d254070d8k.htm |
| EX-99.1 - PRESS RELEASE - Ardea Biosciences, Inc./DE | d254070dex991.htm |
 Ardea Biosciences
Company Update
November 7, 2011
Exhibit 99.2 |
 2
Safe Harbor Statement
Statements contained in this presentation regarding matters that are not historical facts are
“forward-looking statements” within the meaning of the Private Securities
Litigation Reform Act of 1995. Because such statements are subject to risks and
uncertainties, actual results may differ materially from those expressed or implied by
such forward-looking statements. Such statements include, but are not limited
to, statements regarding: Ardea’s goals, its preclinical and clinical trial plans,
timelines and milestones, its expectations about the size of its markets and commercial
potential of its compounds, expected results of future clinical trials, expected properties of
compounds under development, financial position, cash usage, licensing and partnering
opportunities, liquidity and anticipated milestones. Risks that contribute to the
uncertain nature of the forward-looking statements include: risks related to the
outcomes of preclinical and clinical trials, risks related to regulatory approvals,
delays in commencement of preclinical and clinical tests, costs associated with
internal development, and the outcome of our business development activities, including
collaboration or licensing agreements. These and other risks and uncertainties
are described more fully in Ardea’s most recently filed SEC documents, including its
Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, under the
headings "Risk Factors." All forward-looking statements contained in
this presentation speak only as of the date of this presentation, and Ardea undertakes
no obligation to update such statements to reflect events that occur or circumstances
that exist after the date hereof or otherwise. |
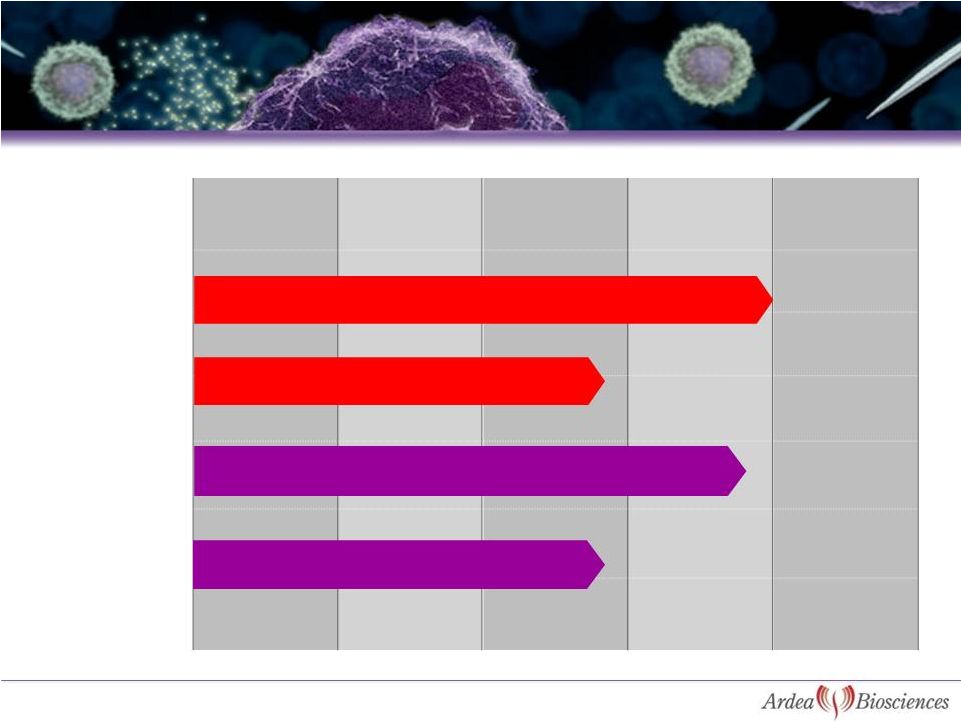 Discovery
Preclinical
Phase 1
Phase 2
Phase 3
MEKI + gemcitabine for advanced
pancreatic cancer
3
Gout
Lesinurad
(RDEA594)
RDEA3170
Next Generation
URAT1
BAY 86-9766
(RDEA119)
BAY 86-9766
(RDEA119)
Gout
Status of Development Programs
MEKI + sorafenib for primary liver cancer |
 HYPERURICEMIA/GOUT
4 |
 5
1. Perez-Ruiz F, Arthritis Rheum 2002;47:356-360
Allopurinol does a very poor job of treating severe disease
(Images are courtesy of Nicola Dalbeth)
Velocity of Tophi Reduction
1
Gout Unmet Medical Need
Severe Disease Is Not Infrequent
0
0.2
0.4
0.6
0.8
1
1.2
1.4
1.6
Combination
Benzbromarone
Allopurinol |
 Gout is caused
by abnormally elevated levels of serum uric acid (>6.8 mg/dL) Increasing incidence
and severity in US: approximately 8 million patients –
~ 90% of patients are “under-excretors”
of uric acid
•
Lesinurad increases urinary excretion of uric acid; returns excretion to normal levels
•
Allopurinol and febuxostat reduce production, which reduces excretion further
•
Less than half of the
2.5 million patients currently taking allopurinol are flare free
•
The
combination
of
lesinurad
and
xanthine
oxidase
inhibitors
may
produce
greater
reductions
in
uric
acid
and
speed
up
clearance
of
tophi
and
body
burden
of
uric
acid
Hyperuricemia
linked
to
elevated
hypertension
in
adults
1
and
children
2
,
increased
mortality
in
Chronic
Kidney
Disease
3
and
possibly
other
cardiovascular risk factors
4,5
,
including elevated C-Reactive Protein
–
Asymptomatic hyperuricemia is treated in Japan
6
Hyperuricemia/Gout
1. Int Urol Nephrol 2007;39:1227-33 ; 2. D Feig, B Soletsky, R Johnson. JAMA.
2008;300(8):924-932; 3. Am J Kidney Dis 2009;53:796-803; 4. JAMA. 2008;300(8):924-932;
5. Chen Abstract 2088 ACR2010 |
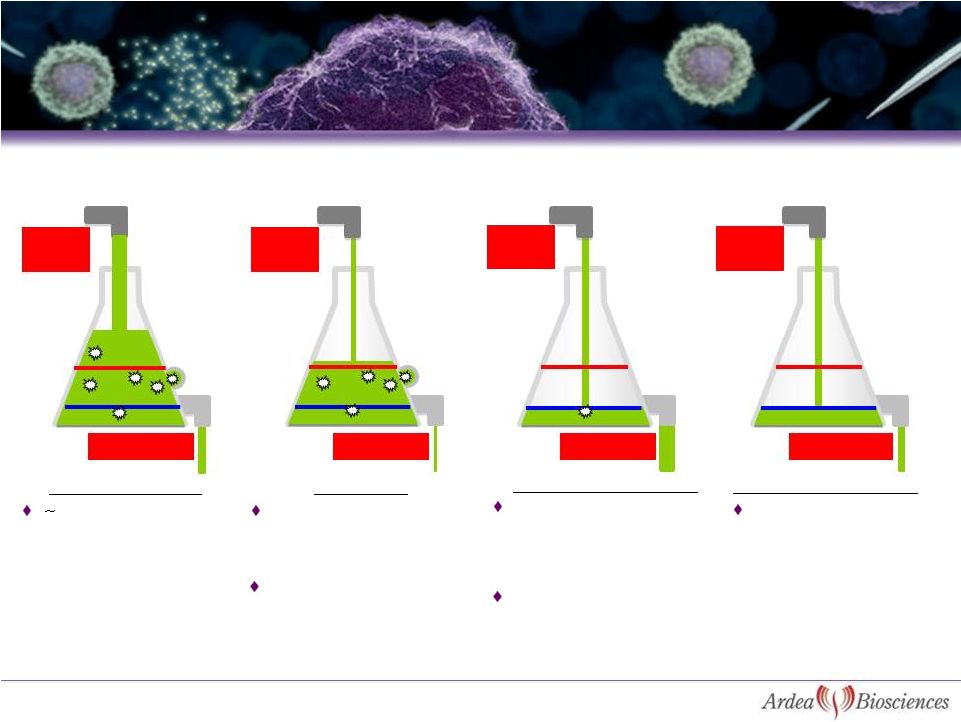
 >99%
Reabsorption
Excretion
~100% of uric acid is initially filtered through
glomerular filtration
URAT1
Enomoto; Urat1 identification in Nature May2002
D Levinson & L Sorensen; Renal Handling of Uric Acid
Proximal Tubule
RDEA594
7
URAT1 Inhibitors Increase the Urinary
Excretion of Uric Acid |
 Lesinurad + XO-Inhibitor
Treated Patient
XO-Inhibitor
Treated Patient
Typical Gout
Patient
Long-term
Treated Patient
Typical Gout Patient
XO-Inhibitor
Lesinurad + XO-Inhibitor
Lesinurad + XO-Inhibitor
1. Perez-Ruiz F, Arthritis Rheum 2002;47:356-360;
8
Why Allopurinol Only Works in 30-40% of Patients
and the Addition of Lesinurad Works in 80-90%
600-800
mg/day
<400
mg/day
<400
mg/day
400 mg/day
200 mg/day
600 mg/day
<400 mg/day
90% of patients are
under-excretors of uric
acid
Decrease production of
uric acid, but also
decrease excretion
Only 30-50% of patients
(ITT analysis) reach target
of sUA < 6mg/dL in
clinical trials
Combination with XO-
inhibitor can reach
Krystexxa-like (70-80%)
reductions of sUA
When all solid urate
is mobilized, input
and output should be
in balance
Allopurinol + URAT1
inhibitor has been shown
to resolve tophi faster
than allopurinol
alone
1
<400
mg/day |
 9
Allopurinol
Results
From
Uloric®
US
package
Insert*
APEX
28-week Study
FACT
52-week Study
CONFIRMS
28-week Study
Allopurinol Dose
(no. of patients)
300/100mg QD
(n=263)
300mg QD
(n=242)
300/100mg QD
(n=755)
% patients with sUA <6 mg/dL
39%
36%
42%
* LOCF analysis used for determination of response rates
~60% of patients on allopurinol did not reach
<6 mg/dL and ~90% didn’t
reach <5 mg/dL, which is needed for patients with tophaceous disease
Response rates are only 29-38% with rigorous Intent-To-Treat (ITT) analysis
With ITT analysis, response rates for febuxostat are:
–
Febuxostat 40 mg –
38% response rate
–
Febuxostat 80 mg –
48-60% response rates
Competitive Landscape -
Allopurinol
Results From Large Phase 3 Studies |
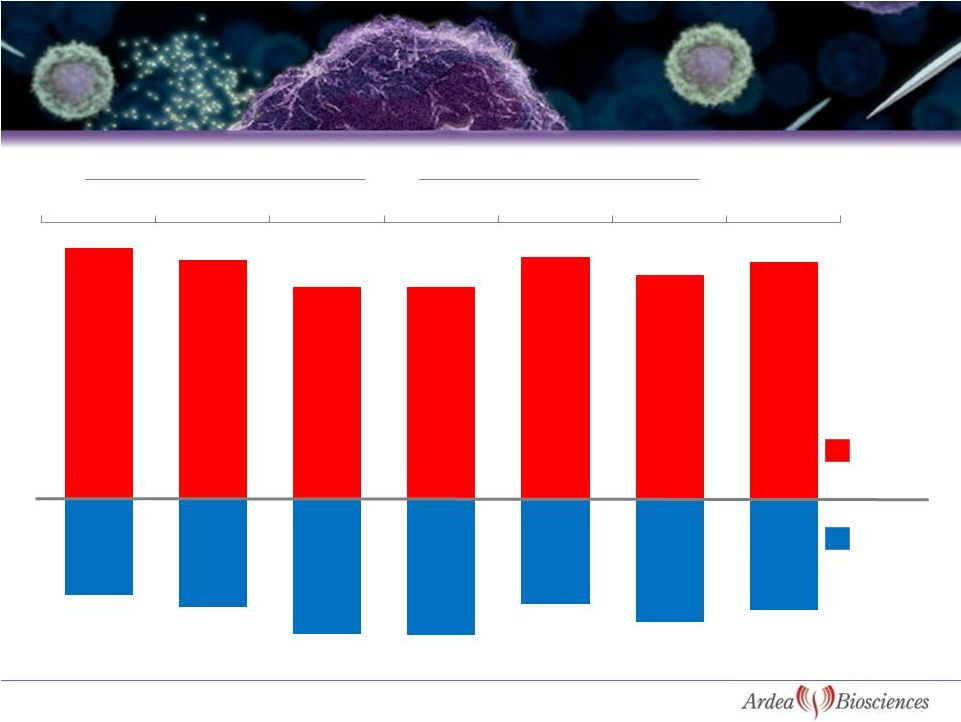 Source: BioTrends Chart Review 2010, n=786 (patients on allopurinol and
febuxostat) 10
Majority of Patients Do Not Reach sUA Target of < 6 mg/dL
on Urate Lowering Therapy (ULT) in Clinical Practice
Mild
Moderate
Severe
Mild
Moderate
Severe
Total
Allopurinol
Febuxostat
27%
35%
30%
61%
31%
32%
73%
65%
70%
39%
69%
68%
61%
39%
sUA>
6mg/dL
sUA<
6mg/dL |
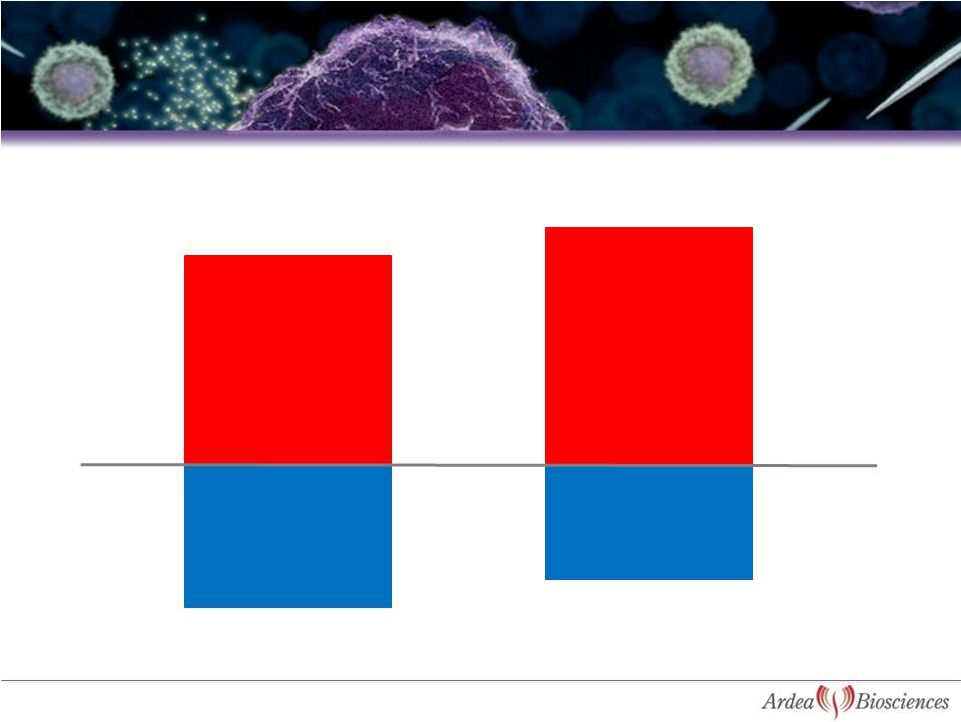 11
ULT Treated
Not Treated
Source: BioTrends Chart Review 2010, n=1039 (all patients)
Percent of patients that experienced gout flares in last 12 months
Majority of Patients (ULT Treated or Not) Are Still
Having Flares in Clinical Practice
41%
35%
59%
65%
Had Gout Flares
Had Gout Flares
No Gout Flares
No Gout Flares |
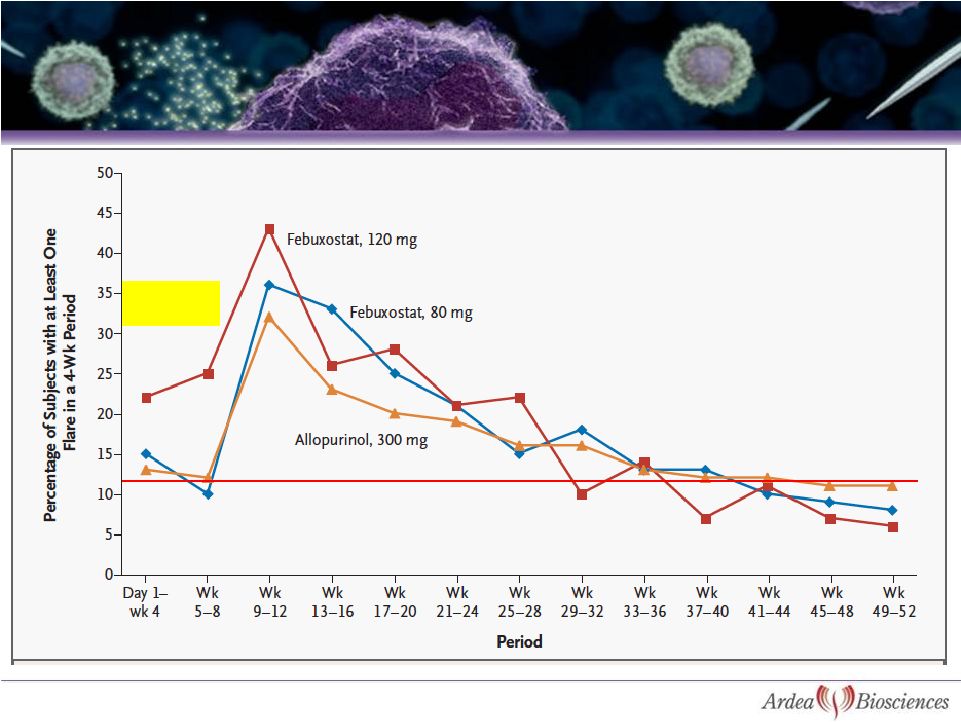 12
Colchicine
Prophylaxis
•
Sharp rise in flares after two
months of colchicine was stopped
•
Improvement in flares with more
potent therapy not seen until after
week 44
•
Same flare rate with allopurinol at
1 month and 1 year
Febuxostat FACT Study |
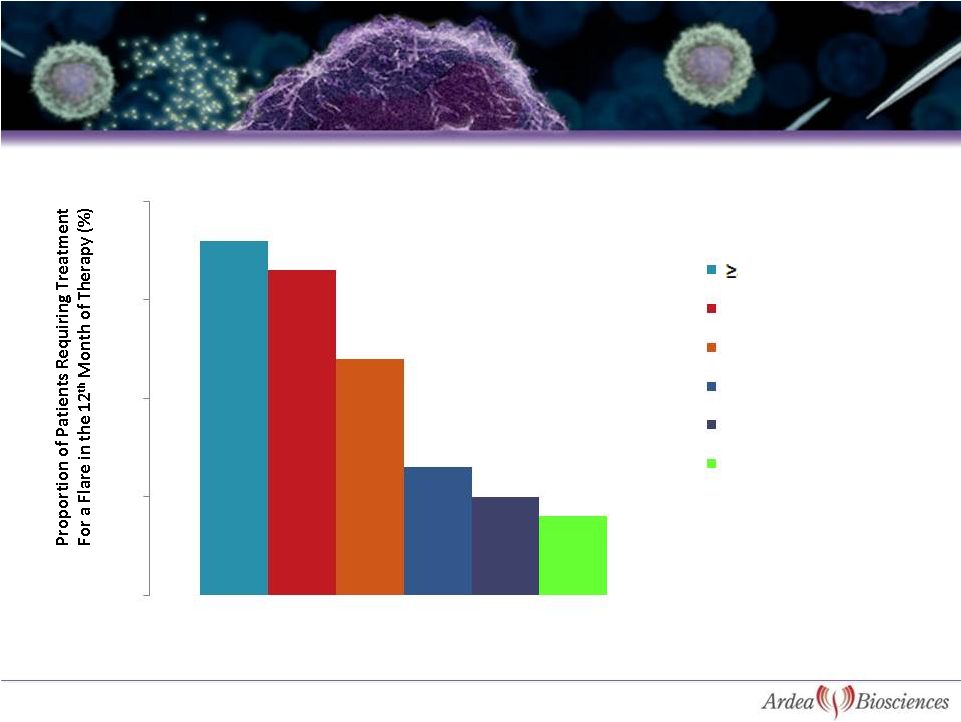 13
Abstract 758: MA Becker, et al.
ACR Boston November 2007
Serum Urate in the 12
th
Month of
Treatment by 1 mg/dL
Flare Rate in Month 12 From FACT Trial Shows
Clear Relationship to sUA
0
5
10
15
20
7.0
-
<8.0 mg/dl
6.0 -
<7.0 mg/dl
5.0 -
<6.0 mg/dl
4.0 -
<5.0 mg/dl
<4.0 mg/dl
8.0 mg/dl |
 Diagnosed
Gout
8.3M
(1) BioTrends Chart Review
2010, for severe gout patients not responding to current therapy (n=1039); (2) NHANES; (3) Estimate NHANES & extrapolation from various sources including IMS RX
data; (4) BioTrends
Chart Review 2010, 68% of patients are not adequately responding based on sUA > 6 mg/dL, and 59% of patients had a flare in the prior year (n=1039).
14
90%
59-68%
Allopurinol
Responders
Gout Pts
on ULT
2.6M
Allo Inadequate
Responders
1.5-1.7M
4
Increasing prevalence/aging
population
Education, advertising (DTC)
Allopurinol
Intolerant
125 -
250K
Allopurinol
Treated
2.5M
Improved Compliance
Growing evidence of CV risk
Gout Market Breakdown
2
3
Patients
Restarting
Therapy
Trx Refractory
Gout
~100K
1 |
 Placebo
2-4 weeks
4 weeks
2 weeks
Population:
–
208
gout
patients
with
serum
urate
(sUA)
6
mg/dL
while
receiving
a
stable
dose
of allopurinol for at least 6 weeks
Duration:
–
4-week double-blind treatment period with doses escalated weekly
Endpoints:
–
Primary: Mean reduction in sUA at Week 4
–
Key Secondary: proportion of subjects with sUA < 6.0 mg/dL at Week 4
–
Safety and tolerability of the combination versus allopurinol alone
Colchicine
Treatment
(0.5
mg
–
0.6
mg/day)
Randomize
if no gout
flare during 2
weeks of
colchicine
15
Allopurinol
Treatment
(200
mg
–
600
mg/day)
Screening
Period:
Patients
must have
sUA >
6
mg/dL on
stable
dose of
allopurinol
Study 203: Lesinurad Phase 2b Combination Study
with Allopurinol in Allopurinol-Refractory Patients
Off drug
Off drug
Off drug
Placebo
Placebo
200 mg Lesinurad
400 mg Lesinurad
600 mg Lesinurad
200 mg
200 mg
400 mg |
 p<0.0001
16
ITT Population
p<0.0001
p<0.0001
Baseline sUA =
6.7 6.4
6.9
7.3
(mg/dL)
P-values are for comparison to placebo group using an ANCOVA model
Study 203: Primary Endpoint -
Mean Percent
Change in Serum Urate at Week 4
3
-16
-22
-30
-35
-30
-25
-20
-15
-10
-5
0
5
Placebo
Lesinurad 200mg +
Allopurinol
Lesinurad 400mg +
Allopurinol
Lesinurad 600mg +
Allopurinol |
 17
p<0.0001
p<0.0001
p<0.0001
* Patients with missing Week 4 results are analyzed as treatment failures, regardless of the
reason for the missing data p-values are for comparison to allopurinol alone (placebo
group) using a Fisher’s exact test (N = 72)
(N = 46)
(N = 42)
(N = 48)
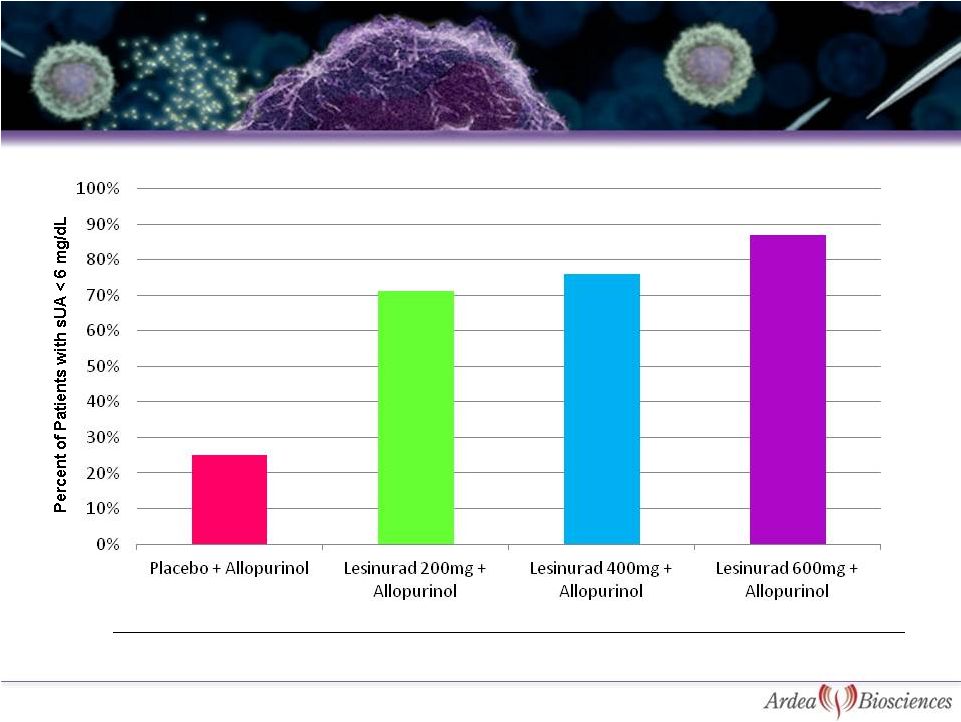
Study 203: Percent of Patients with sUA < 6
mg/dL at Week 4 –
Intent to Treat Analysis*
25%
63%
74%
79%
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
Placebo + Allopurinol
Lesinurad 200mg +
Allopurinol
Lesinurad 400mg +
Allopurinol
Lesinurad 600mg +
Allopurinol |
 18
p<0.0001
p<0.0001
p<0.0001
LOCF analysis used in febuxostat US package insert
Study 203: Percent of Patients w/ Serum Urate
< 6 mg/dL at Last Visit –
LOCF Analysis*
28%
71%
76%
87%
* Last observation carried forward analysis (LOCF) uses a prior week’s sUA results when
Week 4 results are missing
p-values are for comparison to allopurinol alone (placebo group) using a
Fisher’s exact test
(N = 46)
(N = 72)
(N = 48)
(N = 42) |
 19
Study 203 Lesinurad Allopurinol Add-on
Lesinurad Consistently Lowers sUA Across Renal
Function Categories |
 20
Adverse Events
Allopurinol + Lesinurad Dose Group
Allopurinol +
200mg
(N=46)
n (%)
400mg
(N=42)
n (%)
600mg
(N=48)
n (%)
Combined
Lesinurad
(N=136)
n (%)
Pooled
Placebo
(N=72)
n (%)
Any Adverse Event
1 (2.2)
4 (9.5)
5 (10.4)
10 (7.4)
10 (13.9)
Diarrhea
0
1 (2.4)
0
1 (0.7)
2 (2.8)
Dyspepsia
0
0
1 (2.1)
1 (0.7)
1 (1.4)
Lipase Increased
0
2 (4.8)
0
2 (1.5)
1 (1.4)
Hematuria
0
1 (2.4)
0
1 (0.7)
2 (2.8)
Nausea
0
0
0
0
2 (2.8)
*AEs reported by a total of 2 or more subjects that were considered possibly
related to drug; excludes gout flares EULAR 2011 update
Incidence of the Most Frequent Adverse
Events* |
 21
EULAR 2011 update
Study 203: Safety Continued
No Serious Adverse Events (SAEs) in main study
5 discontinuations due to adverse events in main study:
2 combination patients (urticaria, elevated lipase)
1 allopurinol patient (hematuria) and 2 baseline patients on
allopurinol + colchicine (QT prolongation, elevated CK)
One patient died on allopurinol and colchicine prior to receiving
lesinurad. |
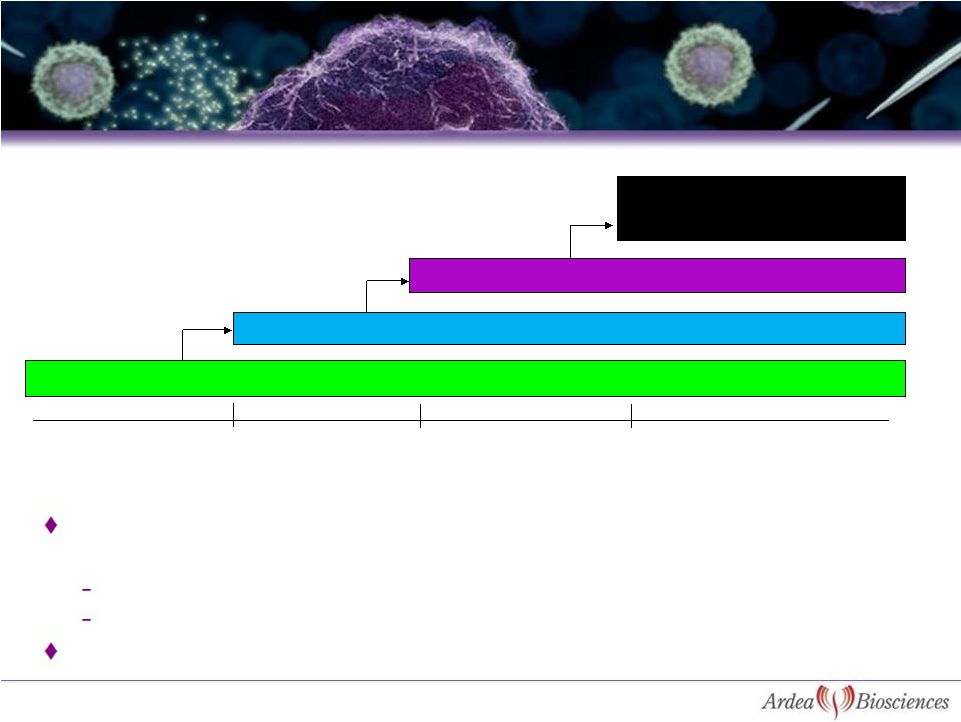 After a
minimum of two week washout, patients were eligible for restarting lesinurad / matched
placebo in blinded extension period
126 patients restarted at a dose of lesinurad 200 mg / matched placebo at Week
0 The dose may be escalated for response
Allopurinol continued throughout washout and extension phase
Lesinurad 200mg / matched placebo + Allopurinol
Lesinurad 400mg / matched placebo + Allopurinol
Lesinurad 600mg / matched placebo + Allopurinol
Optional -
Lesinurad 600mg/ matched
placebo + increased Allopurinol (in
100 mg increments)
Weeks 0 -
4
Weeks 9 -
12
Weeks 5 -
8
Weeks 15 -
44
22
Study 203 -
Optional Blinded Extension Period |
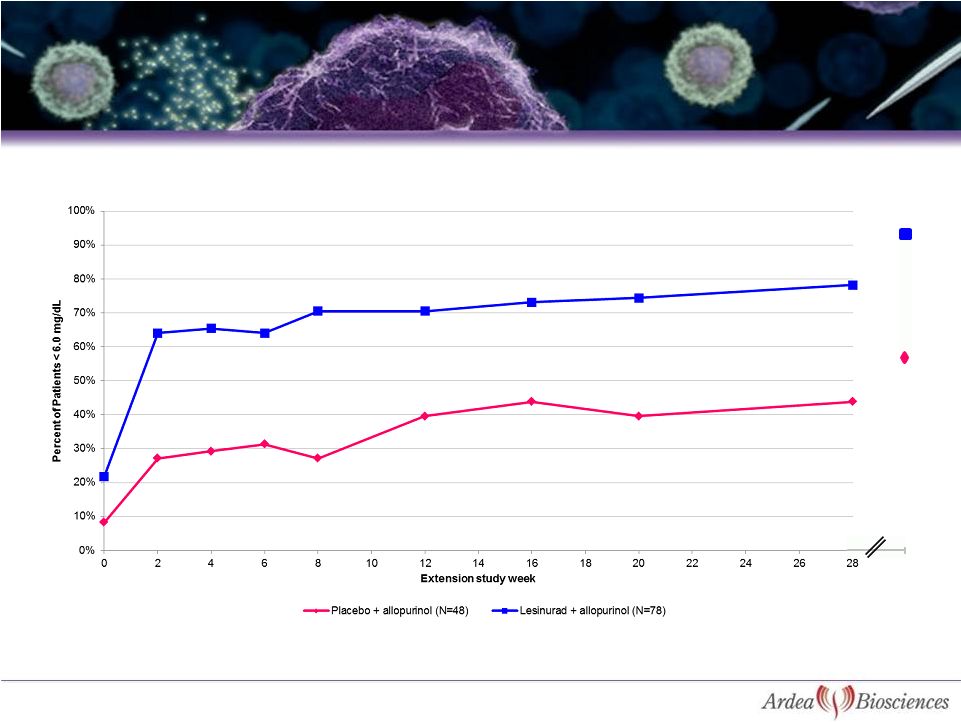 90%
Lesinurad sample sizes before LOCF:
78
76 71 75
73
71
68
66 31
Data presented with LOCF analysis through Week 28; Week 44 is observed cases
23
Study 203: Patients Continue to Respond in Long-Term
Extension With 90% Response at 44 Weeks
Placebo sample sizes before LOCF:
48
46 47 48
46
43
40
36 11
44
55% |
 24
Study 203: Patients Receiving Lesinurad 200 mg or
400 mg Have >90% Response at 44 Weeks
Lesinurad group does not include patients who were escalated to 600 mg at any time. LOCF imputation used
through Week 28. Week 44 is observed cases (11 placebo, 21 lesinurad) |
 n
= 22 21 18
19 19
20
19
19
13
12
After 4 weeks, non-responding patients can be escalated to 400 mg
and then later to 600 mg; patients that moved to higher doses are
excluded
from
this
analysis
to
see
the
effect
of
just
the
200
mg
dose
Mean Change in sUA for Patients Staying on Lesinurad 200 mg
Confidential
Study 203: Patients on Lesinurad 200mg
Continue to Decline for 16 Weeks |
 26
~40% higher drop-out rate on placebo than lesinurad, with ~3-times as many
patients in the lesinurad arms completing 44 weeks of blinded extension;
after 44 weeks most placebo patients are switched to lesinurad
Consistently lower flare rates on lesinurad 200 mg than placebo months
6-12 4 SAEs; none considered treatment-related (angina pectoris,
cerebral artery embolism resulting in death, muscle rupture, bursitis
infective) 30 discontinuations or study completions:
-
14 allopurinol alone (placebo) patients and 16 lesinurad + allopurinol
combination patients
6 patients (2 on allopurinol alone and 4 on lesinurad plus allopurinol)
discontinued due to adverse events (none were considered an SAE):
-
1 patient on allopurinol alone and 3 patients on lesinurad plus allopurinol
were discontinued due to elevated serum creatinine; there were no
differences in mean serum creatinine between lesinurad and placebo out
to Week 44
03 October 2011 data cut-off
Study 203 Extension: Safety |
 Population:
–
21
gout
patients
with
hyperuricemia
(sUA
=
8
mg/dL)
•
febuxostat 40 mg panel: 12 patients with median sUA of 9.2 mg/dL
•
febuxostat 80 mg panel: 9 patients with median sUA of 10.4 mg/dL
Objectives:
–
Plasma
PK
and
urinary
excretion
of
lesinurad
in
combination
with
febuxostat
–
PK of colchicine alone and in combination with febuxostat or both febuxostat and
lesinurad
–
Effect of febuxostat alone and in combination with lesinurad on serum urate
concentrations and urinary urate excretion
febuxostat 80mg QD
7 days
7 days
7 days
Screening
Period
-21 day to day 0
Colchicine Treatment
febuxostat 80mg QD +
400mg lesinurad
febuxostat 80mg QD +
600mg lesinurad
febuxostat 40mg QD
febuxostat 40mg QD +
400mg lesinurad
febuxostat 40mg QD +
600mg lesinurad
27
Lesinurad Study 111 –
Phase 1b Febuxostat and
Lesinurad Dose Titration Study in Gout Patients |
 febuxostat 40 mg
febuxostat 80 mg
All 600 mg combination values are at least
p<0.05 versus 400 mg combination
All combination values are
p<0.001 versus to
febuxostat alone
28
All combination values are
p<0.001 compared to
febuxostat alone
All 600 mg combination values are at least
p<0.05 versus 400 mg combination
RDEA594 Study 111 –
Mean sUA and
Percent Change in sUA
0
2
4
6
8
10
12
Week 1
Week
2 Week 3
febuxostat
Febuxostat+
lesinurad
400 mg
Febuxostat+
lesinurad
600 mg
-100
-80
-60
-40
-20
0
Week 1
Week
2 Week 3
febuxostat
febuxostat+l
lesinurad
400 mg
febuxostat+l
lesinurad
600 mg |
 *
P<0.05, **P<0.01,***P<0.001 versus FBX 40 mg alone # P<0.05,
## P<0.01,### P<0.001 versus FBX 80 mg alone ***
**
**
*
*
###
##
#
#
#
##
###
29
Study 111: Combining Lesinurad and Febuxostat
Produces Much Greater Responses than Can Be
Achieved with Highest Dose of Febuxostat
0%
20%
40%
60%
80%
100%
120%
FBX 40
mg
(n=12)
FBX 40 mg +
Lesinurad 400 mg
FBX 40 mg
+
Lesinurad 600 mg
FBX 80
mg
(n=9)
FBX 80 mg +
Lesinurad 400 mg
FBX 80 mg
+
Lesinurad 600 mg
< 6 mg/dL
< 5 mg/dL
< 4 mg/dL
< 3 mg/dL |
 21
days 2 weeks
4 weeks
2 weeks
30
Labs
X X
X
X
X
X
XX X
Study 202 -
Phase 2b Lesinurad
Monotherapy -
Design
Screening
Period
Washout
of urate
lowering
therapy
200 mg Lesinurad
Off drug
Off drug
Off drug
200 mg
Placebo
400 mg Lesinurad
200 mg
400 mg
600 mg Lesinurad
Colchicine Treatment
Randomize
if no gout
flare during
1-2 weeks of
colchicine
Off drug
Population:
–
gout patients with hyperuricemia (serum uric acid = 8 mg/dL)
–
total of 123 patients in 4 treatment arms
Duration:
–
8 wks: 2-wk run-in, 4-wk treatment, 2-wk follow-up: dose was titrated up
weekly Endpoints:
–
Proportion of subjects with sUA level < 6.0 mg/dL at Week 4
–
Safety and tolerability of the combination versus placebo |
 31
ITT population
n=
27
31
33
32
P=0.0001
P<0.0001
Plasma urate assay using direct LC-MS method of analysis.
Study 202 –
Phase 2b Monotherapy Study
Response Rates for Urate Reduction
0%
13%
42%
60%
0%
10%
20%
30%
40%
50%
60%
70%
Placebo
Lesinurad 200 mg
Lesinurad 400 mg
Lesinurad 600 mg |
 Baseline sUA
32
ITT population
n=
27
31
33
32
Plasma urate assay for response using direct LC-MS method of analysis.
Study 202 –
Response Rates for Plasma
Urate Reduction by Baseline Urate Levels
0%
10%
20%
30%
40%
50%
60%
70%
80%
Placebo
Lesinurad 200 mg
Lesinurad 400 mg
Lesinurad 600 mg
sUA >10 mg/dL
sUA < 10 mg/dL
P=0.0004
P=0.0095
P<0.0001 |
 33
Lesinurad Consistently Lowers sUA Across
Renal Function Categories |
 34
*Adverse events reported by at least 2 subjects that were considered at least possibly
related to treatment No Serious Adverse Events
Two discontinuations due to adverse events, both on 400 mg dose:
one patient with vertigo, and
one patient with elevated SCr that returned to normal range while receiving
lesinurad No clinically relevant lipase or ALT elevations on lesinurad; one
on placebo Study 202 -
Incidence of the Most Frequent
Adverse Events*
Adverse Events
200mg
(N=31)
400mg
(N=33)
600mg
(N=32)
Placebo
(N=27
)
Any Adverse Event
7%
15%
16%
15%
Diarrhea
3%
0
3%
4%
Dyspepsia
0
6%
0
0
Headache
0
3%
3%
7%
Lesinurad Dose Group |
 Long-term
Allopurinol
Safety
Trial
(LASSO)
–
open-label
interventional
study
of
allopurinol
in
gout patients otherwise eligible for our Phase 3 studies
Combination Study of Lesinurad in Allopurinol Standard of Care Inadequate Responders
(CLEAR #1) –
randomized, placebo-controlled trial of lesinurad added to allopurinol in
patients not reaching sUA target with allopurinol alone; North America
Combination Study of Lesinurad in Allopurinol Standard of Care Inadequate Responders
(CLEAR #2) –
randomized, placebo-controlled trial of lesinurad added to allopurinol in
patients not reaching sUA target with allopurinol alone; Global
Lesinurad Monotherapy in Gout Subjects Intolerant to Xanthine Oxidase Inhibitors (LIGHT) – randomized, placebo-controlled trial of
lesinurad monotherapy in patients where febuxostat and/or
allopurinol
are
contraindicated
(due
to
intolerance,
drug
interactions,
co-morbidities,
etc); Global
Combination Treatment Study in Subjects with Tophaceous Gout Using Lesinurad and
Febuxostat
(CRYSTAL)
–
randomized,
placebo-controlled
trial
of
lesinurad
in
combination
with febuxostat for the treatment of hyperuricemia in gout patients with tophi (resolution of
tophi is a key secondary endpoint); Global
Total
patients
planned
for
Phase
3
studies:
2000
–
2500
35
Lesinurad Phase 3 Program |
 Population:
–
Up to 2500 pts with hyperuricemia (sUA
8 mg/dL) at ~300 centers
Design:
–
Provide therapy with allopurinol at the medically appropriate doses; monitor
patients for a minimum of 2 months prior to their being eligible for
enrollment into Phase 3 program (~3 months or greater on allopurinol prior
to Phase 3 baseline) Objectives:
–
Generate patients for the Phase 3 program with well-documented inadequate
efficacy or intolerance to allopurinol (should accelerate enrollment of
Phase 3 program)
36
Allopurinol prescribed at
labeled dose
Long-term Allopurinol Safety Study Evaluating Outcomes
in Gout Patients (LASSO -
N. America & Global)
Phase 3 - Allopurinol SoC Inadequate Responders North America Phase 3 - Allopurinol SoC Inadequate Responders NA
and ROW Phase 3 – Allopurinol
Intolerant Study |
 Population:
–
Gout patients with sUA > 6.5 mg/dL on stable dose of allopurinol between 300 mg
to 800 mg (200 mg acceptable for moderate renal impairment) for
12 weeks
–
2 gout flares in preceding 12 months
Endpoints:
–
Primary: proportion of subjects with sUA level < 6.0 mg/dL after 6 months
–
Key Secondary: Studies would be pooled for assessment of gout flare rate, tophi
resolution and quality of life measurements. Prospective efficacy analysis in
subjects treated with >300 mg/day.
Screening
Period
Colchicine for gout flare prophylaxis
37
RDEA594
200 mg + ALLO 200-800 mg
RDEA594
400 mg + ALLO 200-800 mg
3 weeks
1 week
Month 6 Primary Endpoint (sUA)
Month 12 Secondary Endpoints
RDEA594
Placebo+ ALLO 200-800 mg
Combination Study of LEsinurad in Allopurinol Standard of Care Inadequate
Responders (CLEAR #1 & #2)
|
 2
wks 1 wk
Month 6 primary endpoint (sUA)
Month 12 Secondary Endpoints
Population:
–
Gout patients with hyperuricemia (sUA dependent on previous therapy)
and
1 measureable tophus
–
Prospective assessment of patients not responding to FBX 80 mg during
run-in period
Endpoints:
–
Primary: proportion of subjects with sUA level < 5.0 mg/dL after 24 wks
–
Key Secondary: tophus resolution, tophus response, and HAQ-DI at
month 12
105
pts:
Lesinurad
200mg
+FBX
80
mg
Colchicine for gout flare prophylaxis
38
105
pts:
Lesinurad
Placebo
+FBX
80
mg
105
pts:
Lesinurad
400mg
+FBX
80
mg
Combination TReatment
StudY in Subjects with TophAeous
Gout Using LesInurad and Febuxostat (CRYSTAL
-
Global) |
 Population:
–
Gout patients with sUA
6.5 mg/dL with medical history in which XO
therapy is contraindicated (e.g., hypersensitivity, intolerance,
or toxicity)
Endpoints:
–
Primary: Proportion of patients whose sUA levels are < 6.0 mg/dL by 6
months
39
RDEA594 400 mg
RDEA594 Placebo
Screening
Period
Colchicine for gout flare prophylaxis
RDEA594 400mg
RDEA594 400 mg extension
4 Weeks
Month 6 primary endpoint (sUA)
LesInurad Monotherapy in
Gout Subjects Intolerant to Xanthine Oxidase Inhibitors (
LIGHT
-
Global) |
 RDEA3170
40 |
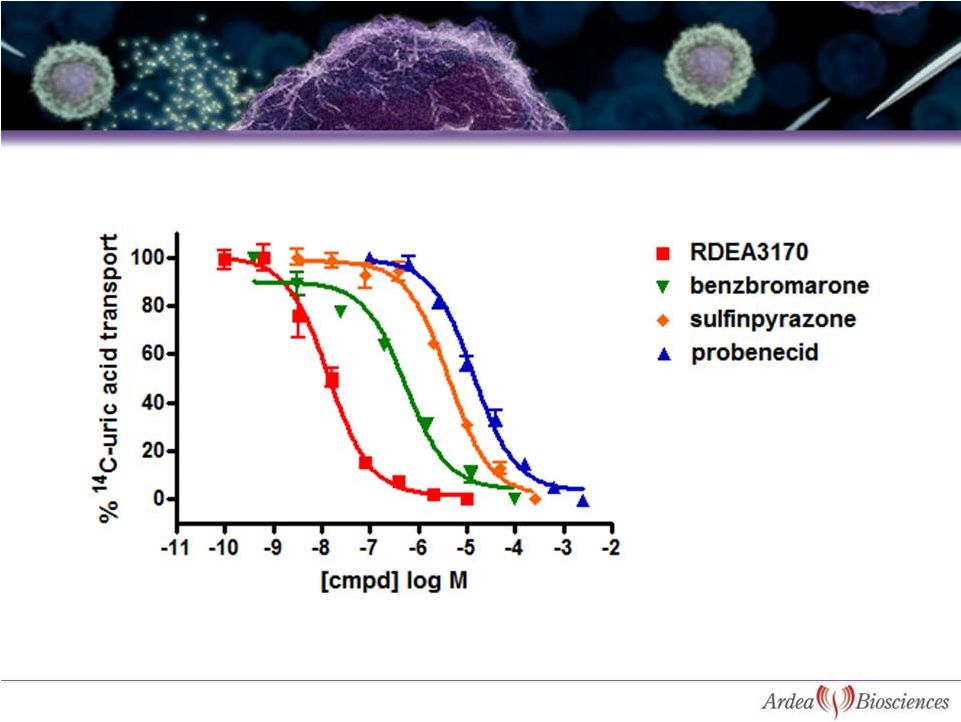 41
Comparison Between Uricosuric Agents for
Inhibition of Uric Acid Transport by URAT1 |
 N=6, fasted state
60%
Sustained
Reduction
in
sUA
After
a
Single
Dose
of
RDEA3170
40
mg
0
12
24
36
48
60
72
Time (hr)
-80
-70
-60
-50
-40
-30
-20
-10
0 |
 CANCER
43 |
 Nexavar
MEK
Ras
Raf
c-Myc
Herceptin, Erbitux
Proliferation
Angiogenesis
Differentiation
Apoptosis
cPLA2
PDE4
MNK1/2
Elk-1
MAPKAPK1/3
BAY86-9766
(RDEA119)
RTK
Bcl-2
Mcl-1
c-Jun
Tarceva
GF
ERK2
ERK2
44
BAY80-6946
The Age of Targeted Cancer Treatments
mTOR
AKT
PI3K |
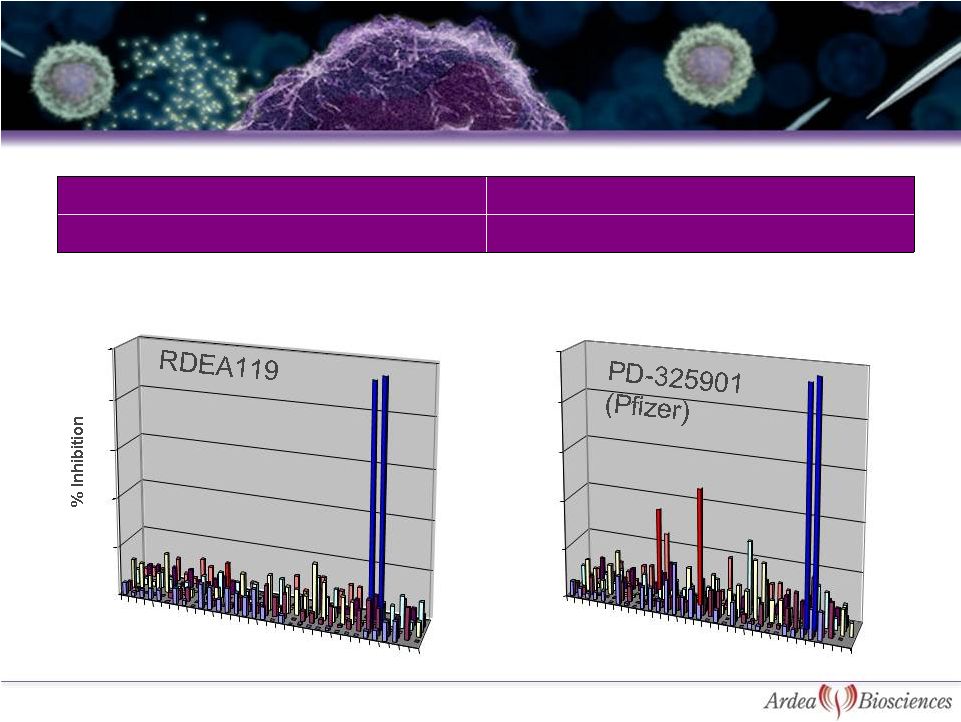 MEK1/2 Enzyme IC
50
17-50 nM
Cellular pERK EC
50
2.5-8.7 nM*
>100-fold selectivity in kinase panel of 205 enzymes at 10 µM**
*Cell lines: Colo205, A375, A431, HT-29
** In-house data
MEK1 & MEK2
40
60
80
100
45
RDEA119 is Potent, Highly Specific MEK
Inhibitor
1
6
11
16
21
26
31
0
20
1
6
11
16
21
26
31
0
20
40
60
80
100
MEK1 & MEK2
Ron
SRC |
 46
0
20
40
60
80
100
120
control
Sorafenib-
3.5uM
RDEA119-
0.1uM
RDEA119
+Sorafenib
Huh7, day 6
control
Sorafenib-3.5uM
RDEA119 -
0.25uM
RDEA119
+Sorafenib
HepG2, day 6
Synergy between RDEA119 and Sorafenib in
Hepatoma Cancer Lines |
 A Phase 2
Trial of BAY86-9766 Plus Sorafenib as First Line Systemic Treatment for
Hepatocellular Carcinoma (HCC) A Multi-center, Phase 1/2 Study of BAY86-9766 in
Combination With Gemcitabine in Patients With Locally Advanced Inoperable or Metastatic
Pancreatic Cancer
Phase 1b Trial of the Combination of PI3K Inhibitor BAY80-6946 and
Allosteric-MEK Inhibitor BAY86-9766 in Subjects With Advanced Cancer
Phase 1 Study of Single Agent BAY86-9766 in Japanese Patients With
Advanced or Refractory Solid Tumors
First data expected mid-2012
47
*Further details are available on www.clinicalTrials.gov and
www.bayerpharma.com/en/research_and_development/clinical_trials Current Bayer Clinical
Trials with BAY86-9766* |
 MEK program
licensed to Bayer HealthCare on April 27, 2009 –
$35 million upfront license fee
–
$15 million milestone received January 2011 for initiation of Phase 2
study in primary liver cancer by Bayer (NCT01304177)
–
Bayer has also initiated a Phase 1/2 combination study with
gemcitabine in advanced pancreatic cancer (NCT01251640)
–
Additional $7.5 million milestone due upon initiation of a second
Phase 2 study in a different indication
–
Payments could total $407 million, excluding royalties
–
Low double-digit royalties on worldwide sales
48
MEK Inhibitor Program |
 26.8 million
common shares outstanding Summary Statement of Operations
(In thousands, except per share data)
Nine Months Ended
September 30, 2011
Revenue
Operating expenses
Other income, net
$ 5,636
61,460
17
NET LOSS
$(55,807)
NET LOSS PER SHARE
$(2.11)
Condensed Balance Sheet Data
(In thousands)
September 30,
2011
Dec. 31, 2010
Cash, cash equivalents & ST invest
Receivables
Total assets
Total stockholders’
equity
$122,732
$1,918
$129,795
$109,550
$80,612
$16,959
$100,454
$77,123
49
Financial Position |
