Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - QUESTCOR PHARMACEUTICALS INC | d248366d8k.htm |
 NASDAQ:
QCOR
NASDAQ:
QCOR
October 2011
Exhibit 99.1 |
 2
Safe Harbor Statement
Note: Except for the historical information contained herein, this press release contains
forward-looking statements that have been made pursuant to the Private Securities
Litigation Reform Act of 1995. These statements relate to future events or our future
financial performance. In some cases, you can identify forward-looking statements by terminology such as
“believes,” “continue,” “could,” “estimates,”
“expects,” “growth,” “may,” “plans,” “potential,” “should,” “substantial” or “will”
or the negative of such terms and other comparable terminology. These statements are only
predictions. Actual events or results may differ materially. Factors that could cause
or contribute to such differences include, but are not limited to, the following: Our
reliance on Acthar for substantially all of our net sales and profits; Reductions in vials used per prescription
resulting from changes in treatment regimens by physicians or patient compliance with
physician recommendations; The complex nature of our manufacturing process and
the potential for supply disruptions or other business disruptions; The lack of patent
protection for Acthar; and the possible FDA approval and market introduction of competitive products; Our
ability to generate revenue from sales of Acthar to treat on-label indications associated
with NS, and our ability to develop other therapeutic uses for Acthar including SLE;
Research and development risks, including risks associated with Questcor's work in the
area of nephrotic syndrome and potential work in the area of SLE, and our reliance on third-parties to conduct
research and development and the ability of research and development to generate successful
results; Regulatory changes or other policy actions by governmental authorities and
other third parties in connection with U.S. health care reform or efforts to reduce
federal and state government deficits; Our ability to receive high reimbursement levels from third party
payers; An increase in the proportion of our Acthar unit sales comprised of
Medicaid-eligible patients and government entities; Our ability to estimate
reserves required for Acthar used by government entities and Medicaid-eligible patients and
the impact that unforeseen invoicing of historical Medicaid prescriptions may have upon our
results; Our ability to operate within an industry that is highly regulated at both the
Federal and state level; Our ability to effectively manage our growth, including the
expansion of our NS selling effort, and our reliance on key personnel; The impact to our business caused by
economic conditions; Our ability to protect our proprietary rights; Our ability to maintain
effective controls over financial reporting; The risk of product liability lawsuits;
Unforeseen business interruptions; Volatility in Questcor's monthly and quarterly
Acthar shipments and end-user demand, as well as volatility in our stock price; and Other risks discussed in
Questcor's annual report on Form 10-K for the year ended December 31, 2010, and other
documents filed with the Securities and Exchange Commission.
The risk factors and other information contained in these documents should be considered in
evaluating Questcor's prospects and future financial performance. |
 Questcor
A biopharmaceutical company
whose product helps patients with serious,
difficult-to-treat medical conditions
A biopharmaceutical company
whose product helps patients with serious,
difficult-to-treat medical conditions
3 |
 Questcor
Overview Flagship Product:
Flagship Product:
•
Profitable, cash flow positive, $180M* in cash, debt-free
•
Profitable, cash flow positive, $180M* in cash, debt-free
•
19 approved indications
•
19 approved indications
Key Markets:
Key Markets:
•
Multiple Sclerosis, Nephrotic Syndrome, Infantile Spasms
•
Combined market opportunity exceeds $1.5 billion
•
Multiple Sclerosis, Nephrotic Syndrome, Infantile Spasms
•
Combined market opportunity exceeds $1.5 billion
Strategy:
Strategy:
•
Grow Acthar sales in each key market
•
Develop on-label Lupus market for Acthar
•
Grow Acthar sales in each key market
•
Develop on-label Lupus market for Acthar
Financials:
Financials:
* As of 10/21/11
4 |
 History of
Acthar 1952
First
Approved
1950
1950
1978
MS Indication Added
2000
2000
2001
Questcor Acquires
Acthar
2010
Label Modernized
19 Indications
2007
Questcor Changes
Strategy
2010
2010
5 |
 Significant
Barriers to Entry 6 |
 QCOR Strategy
– Sell More Acthar
7
Systemic Lupus
Erythematosus
Multiple Sclerosis (MS)
Multiple Sclerosis (MS)
Nephrotic Syndrome (NS)
Nephrotic Syndrome (NS)
Infantile Spasms (IS)
Infantile Spasms (IS) |
 8
Acthar and MS
•
Neurodegenerative
disorder
•
Acute treatment for
relapses
•
Treatment for 1-2
weeks*
•
$40K-$50K/Rx
Inadequate
Inadequate
Response to
Response to
Steroids
Steroids
Poor
Poor
Venous
Venous
Access
Access
Problematic
Problematic
Steroid Side
Steroid Side
Effects
Effects
Acthar
Acthar
when
when
“Steroids are
“Steroids are
not suitable”
not suitable”
*Based on prescriptions written
8 |
 9
MS Script -
Record of Consistent Growth
New Paid Rxs
New Paid Rxs
Notes: Historical trend information is not necessarily indicative of future results.
Chart includes "Related Conditions" - diagnoses that are
either alternative descriptions of the condition or are closely related to the medical
condition which is the focus of the chart. Yellow numbers in the bars
show the number of MS sales people making calls at the end of the
quarter. |
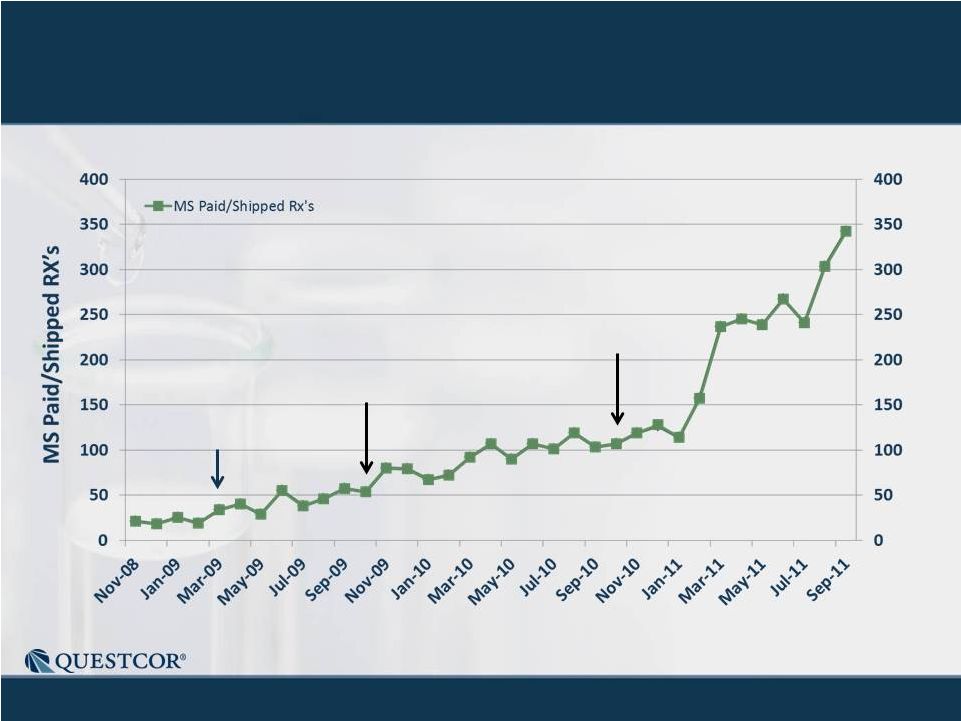 Monthly MS
Scripts Have 160% CAGR 15-30 reps
30-38 reps
38-77 reps
10 |
 11
•
Doubled sales force: 38 to 77 sales reps Nov 2010
•
Q3-2011 results
–
Q3-11 new, paid Rxs up 174% vs. Q3-10
–
MS about 65% of QCOR net sales*
•
Estimated $150M annualized run-rate*
–
About 450 prescribers in Q3
–
September was a record month
•
Doubled sales force: 38 to 77 sales reps Nov 2010
•
Q3-2011 results
–
Q3-11 new, paid Rxs up 174% vs. Q3-10
–
MS about 65% of QCOR net sales*
•
Estimated $150M annualized run-rate*
–
About 450 prescribers in Q3
–
September was a record month
MS Trends
*Based on Company estimates
**As of 9/30/11 |
 12
•
Characterized by excessive spilling of protein from the
kidneys into the urine (proteinuria)
•
Can result in end-stage renal disease (ESRD), dialysis,
transplant
•
Significant unmet need
–
Few treatment options
•
Treatment for 4-6 months*
•
$150K-250K/Rx
•
Characterized by excessive spilling of protein from the
kidneys into the urine (proteinuria)
•
Can result in end-stage renal disease (ESRD), dialysis,
transplant
•
Significant unmet need
–
Few treatment options
•
Treatment for 4-6 months*
•
$150K-250K/Rx
Acthar and Nephrotic Syndrome (NS)
*Based on prescriptions written |
 13
NS Scripts –
Off to a Good Start
New Paid Rxs
New Paid Rxs
Notes: Historical trend information is not necessarily indicative of future results.
Chart includes "Related Conditions" - diagnoses that are
either alternative descriptions of the condition or are closely related to the medical
condition which is the focus of the chart. Yellow numbers in the bars
show the number of NS sales people making
calls
at
the
end
of
the
quarter.
Q3’
11
included
expansion
and training of new sales people.
11
4
8
7
18
45
60
Q1 ‘10
Q2 ‘10
Q4 ‘10
Q3 ‘10
Q1 ‘11
Q2 ‘11
Q3 ‘11
5 |
 14
NS Market Size |
 15
•
Hired 5 reps to sell Acthar to nephrologists
–
Initiated
sales
efforts
in
early
March
2011
–
Q1
2011
new,
paid
NS
Rxs:
18
–
Q2
2011
new,
paid
NS
Rxs:
45
–
Q3
2011
new,
paid
NS
Rxs:
60
•
Expanded NS selling effort: 5 to 28 NS during Q3
•
Planned sales calls to increase in Q4 by 7X over Q2
•
6 month course of therapy creates future vial demand
•
Hired 5 reps to sell Acthar to nephrologists
–
Initiated
sales
efforts
in
early
March
2011
–
Q1
2011
new,
paid
NS
Rxs:
18
–
Q2
2011
new,
paid
NS
Rxs:
45
–
Q3
2011
new,
paid
NS
Rxs:
60
•
Expanded NS selling effort: 5 to 28 NS during Q3
•
Planned sales calls to increase in Q4 by 7X over Q2
•
6 month course of therapy creates future vial demand
NS Sales Trends |
 16
•
Treatment Resistant Idiopathic Membranous
Nephropathy
•
Dose response trial
–
Randomized, double blinded 3 arm study with 2 different dosage
regimens of Acthar and placebo
–
n=84 (approximate), 35 centers (approximate)
–
Endpoint is reduction of proteinuria
•
Trial milestones
–
“First look”
data available early 2013
–
Final reporting late 2013
•
Treatment Resistant Idiopathic Membranous
Nephropathy
•
Dose response trial
–
Randomized, double blinded 3 arm study with 2 different dosage
regimens of Acthar and placebo
–
n=84 (approximate), 35 centers (approximate)
–
Endpoint is reduction of proteinuria
•
Trial milestones
–
“First look”
data available early 2013
–
Final reporting late 2013
NS
Phase
IV
Company
Sponsored
Study |
 17
•
Devastating, refractory form of childhood epilepsy
–
Very poor developmental outcome if inadequately treated
•
Not responsive to standard anti-epileptic drugs
•
Ultra-rare orphan disorder
–
1,500 to 2,000 patients annually
•
Typically occurs in children less than 2 years old
•
Characterized by
–
“spasms”
–
a specific type of seizure
–
“hypsarrhythmia”
–
abnormal EEG pattern
•
Devastating, refractory form of childhood epilepsy
–
Very poor developmental outcome if inadequately treated
•
Not responsive to standard anti-epileptic drugs
•
Ultra-rare orphan disorder
–
1,500 to 2,000 patients annually
•
Typically occurs in children less than 2 years old
•
Characterized by
–
“spasms”
–
a specific type of seizure
–
“hypsarrhythmia”
–
abnormal EEG pattern
Infantile Spasms |
 18
•
Standard of care for years, FDA approval 10/15/10
•
Crisis therapy
•
Treatment for 2-4 weeks*
•
In a randomized, single-blinded, controlled study, 87%
of patients achieved overall response (no spasms and
no hypsarrhythmia) at two weeks versus 29% on
prednisone
•
$100K-$125K/Rx
–
About half of patients receive drug for free
•
Standard of care for years, FDA approval 10/15/10
•
Crisis therapy
•
Treatment for 2-4 weeks*
•
In a randomized, single-blinded, controlled study, 87%
of patients achieved overall response (no spasms and
no hypsarrhythmia) at two weeks versus 29% on
prednisone
•
$100K-$125K/Rx
–
About half of patients receive drug for free
Acthar and IS
*Based on prescriptions written |
 19
•
Targeting select institutions
–
Promotion effort being narrowed as market is maturing
–
Creates selling time for Acthar MS reps to target NS
•
Significant variability in quarterly Rxs
•
Q3-2011 new, paid Rxs within historic range
–
Acthar currently used to treat 40-50% of IS patients
•
Targeting select institutions
–
Promotion effort being narrowed as market is maturing
–
Creates selling time for Acthar MS reps to target NS
•
Significant variability in quarterly Rxs
•
Q3-2011 new, paid Rxs within historic range
–
Acthar currently used to treat 40-50% of IS patients
IS Sales Trends |
 20
•
Specialty Sales Force
–
Main focus on MS (time split is ~ 80%/15%/5% on MS/NS/IS)
–
77 representatives, 13 regional managers, one national director
•
Nephrology Sales Force
–
Focus 100% on Nephrotic Syndrome
–
28 representatives, 4 regional managers, one national director
–
Full force commenced selling activity by 10/3/11
•
Combined Forces will be calling on
–
>4,000 neurologists
–
>3,000 nephrologists
–
about 100 key children’s hospitals
•
Specialty Sales Force
–
Main focus on MS (time split is ~ 80%/15%/5% on MS/NS/IS)
–
77 representatives, 13 regional managers, one national director
•
Nephrology Sales Force
–
Focus 100% on Nephrotic Syndrome
–
28 representatives, 4 regional managers, one national director
–
Full force commenced selling activity by 10/3/11
•
Combined Forces will be calling on
–
>4,000 neurologists
–
>3,000 nephrologists
–
about 100 key children’s hospitals
Total Acthar Sales Force |
 21
Immediate
Acthar
Growth
Opportunities
*Represents estimated net sales market opportunity based on internal company estimates
MS
Build on sales
momentum,
good market
headroom
Market size
$500M+*
NS
Significantly
expanded
selling effort
to 28 reps
during Q3
Market size
$1B+*
IS
Targeted sales
strategy
Market size
$100M*
•
•
•
•
•
•
-
-
- |
 22
•
High unmet need
•
Serious health risk if unsuccessfully treated
•
Difficult to treat
•
Multiple on-label indications for Acthar
–
Exacerbations
–
Maintenance therapy
–
Lupus nephritis
•
Large patient population
•
High unmet need
•
Serious health risk if unsuccessfully treated
•
Difficult to treat
•
Multiple on-label indications for Acthar
–
Exacerbations
–
Maintenance therapy
–
Lupus nephritis
•
Large patient population
Systemic
Lupus
Erythematosus
(Lupus) |
 23
Financials
Profitable
Profitable
Debt Free
Debt Free
Cash Flow Positive
Cash Flow Positive |
 24
Net Sales ($M)
Net Sales ($M)
Gross Margin
Gross Margin
Operating Income ($M)
Operating Income ($M)
Fully Diluted, GAAP EPS
Fully Diluted, GAAP EPS
$59.8
$59.8
94%
94%
$33.6
$33.6
$0.35
$0.35
$31.3
$31.3
93%
93%
$16.8
$16.8
$0.18
$0.18
Q3-2011
Q3-2011
Q3-2010
Q3-2010
Record Net Sales (up 91%) and Solid Earnings (EPS up 94%)
Record Net Sales (up 91%) and Solid Earnings (EPS up 94%)
•
Third quarter vials shipped: 2,910
•
Third quarter cash flow from operations: $32.6M
•
Medicaid reserves continue to appear adequate
•
No shares repurchased
Q3-2011 Financial Results |
 *After return
of $78 million of cash to shareholders through share
repurchases.
Cash / ST Investments
Cash / ST Investments
Accounts Receivable
Accounts Receivable
$180M*
$180M*
$24M
$24M
10/21/11
10/21/11
25
Debt-free balance sheet
Debt-free balance sheet
Questcor
is
Cash
Flow
Positive |
 26
26
Share Repurchases: 15 Million Shares
•
2.2 Million Preferred shares repurchased
•
13.2 Common shares repurchased
•
$78 million returned to shareholders in stock
buybacks
•
62.7 million shares currently outstanding
•
4.3 million shares remain on buyback authorization
•
2.2 Million Preferred shares repurchased
•
13.2 Common shares repurchased
•
$78 million returned to shareholders in stock
buybacks
•
62.7 million shares currently outstanding
•
4.3 million shares remain on buyback authorization
Repurchases significantly improve EPS
Repurchases significantly improve EPS |
 27
•
Sustain effort and momentum with MS
•
Execute with expanded NS selling effort
•
Maintain and gradually grow IS sales
•
Explore Systemic Lupus Erythematosus (Lupus) as another
vertical market
•
Develop other markets for Acthar
–
Acthar is its own pipeline with many other on-label
and many possible other therapeutic uses
–
Further define and develop the unique
characteristics of Acthar
•
No business development efforts planned
•
Sustain effort and momentum with MS
•
Execute with expanded NS selling effort
•
Maintain and gradually grow IS sales
•
Explore Systemic Lupus Erythematosus (Lupus) as another
vertical market
•
Develop other markets for Acthar
–
Acthar is its own pipeline with many other on-label
and many possible other therapeutic uses
–
Further define and develop the unique
characteristics of Acthar
•
No business development efforts planned
Go Forward Plan -
Sell More Acthar |
 28
Investment Highlights
High margins provide good operating
leverage
Announced new vertical market -- Lupus
Sales in MS and NS are growing rapidly, yet
market penetration is low
Acthar is approved for 19 indications-many
in large markets with sizable unmet need
Acthar has sustainable competitive advantages-
without FDA approval risk
Profitable, cash flow positive, no debt
|
 29
29
NASDAQ:
QCOR
NASDAQ:
QCOR
October 2011
October 2011 |
