Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - BIOLIFE SOLUTIONS INC | blfs_8k.htm |

Investor Presentation
October 2011
October 2011
OTCBB: BLFS

OCTOBER 2011 INVESTOR PRESENTATION
2
Safe Harbor Statement
This presentation contains forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995 including, but not limited to,
statements about BioLife Solutions, Inc. (the “Company”) and its future operating
results, strategies, and product development plans. These forward-looking statements
are based on current expectations and assumptions that are subject to risks and
uncertainties. Actual results could differ materially from the results expressed or
implied in these forward-looking statements. Factors that may cause or contribute to
such differences are more fully discussed, as are other factors, in Part I, Item1A. “Risk
Factors” of the Company’s Form 10-K for the fiscal year ended December 31, 2010,
which was filed with the SEC on March 28, 2011. In addition, any forward-looking
statements represent our estimates only as of today and should not be relied upon as
representing our estimates as of any subsequent date. While the Company may elect
to update forward-looking statements at some point in the future, the Company
specifically disclaims any obligation to do so except as may be legally necessary, even if
the Company’s estimates should change.
Private Securities Litigation Reform Act of 1995 including, but not limited to,
statements about BioLife Solutions, Inc. (the “Company”) and its future operating
results, strategies, and product development plans. These forward-looking statements
are based on current expectations and assumptions that are subject to risks and
uncertainties. Actual results could differ materially from the results expressed or
implied in these forward-looking statements. Factors that may cause or contribute to
such differences are more fully discussed, as are other factors, in Part I, Item1A. “Risk
Factors” of the Company’s Form 10-K for the fiscal year ended December 31, 2010,
which was filed with the SEC on March 28, 2011. In addition, any forward-looking
statements represent our estimates only as of today and should not be relied upon as
representing our estimates as of any subsequent date. While the Company may elect
to update forward-looking statements at some point in the future, the Company
specifically disclaims any obligation to do so except as may be legally necessary, even if
the Company’s estimates should change.
OCTOBER 2011 INVESTOR PRESENTATION
3
Leading Innovator Serving $500M Market
NEXT-
GENERATION
PRODUCTS
GENERATION
PRODUCTS
SCALABLE
PRODUCTION
FACILITY
PRODUCTION
FACILITY
LARGE,
GROWING
CUSTOMER BASE
GROWING
CUSTOMER BASE
Proprietary, best-in-
class biopreserveration
media products for
cells, tissues and
organs
class biopreserveration
media products for
cells, tissues and
organs
$25MM annual
revenue capacity in a
scalable GMP facility
revenue capacity in a
scalable GMP facility
300+ customers and key
distributors in strategic
market segments:
distributors in strategic
market segments:
Regenerative
Medicine
Medicine
Biobanking
Drug
Discovery
Discovery

OCTOBER 2011 INVESTOR PRESENTATION
4
Products and Services
cGMP, Serum-free, protein-free biopreservation
media products for cells, tissues, and organs
media products for cells, tissues, and organs
HypoThermosol®
CryoStor®
BloodStor®
Contract
aseptic media
formulation,
fill and
finish services
aseptic media
formulation,
fill and
finish services
Hypothermic
storage &
shipping media
storage &
shipping media
Cryopreservation
freeze media
freeze media
Cord blood stem
cell freeze media
cell freeze media

Target Markets
OCTOBER 2011 INVESTOR PRESENTATION
5
Biobanking
Umbilical cord blood banks, adult
stem cell banks, tissue banks,
biorepositories
stem cell banks, tissue banks,
biorepositories
Drug
Discovery
Discovery
Pharmaceutical companies, cell
suppliers, toxicity testing labs
suppliers, toxicity testing labs
Regenerative
Medicine
Medicine
Commercial cell therapy and tissue
engineering companies, hospital
based stem cell transplant centers,
university-based research labs
engineering companies, hospital
based stem cell transplant centers,
university-based research labs

Regenerative Medicine Opportunity
Use of traditional, archaic “home brew” cocktail results
in yield loss and limited shelf life of precious cells
intended for clinical applications.
in yield loss and limited shelf life of precious cells
intended for clinical applications.
Developers face increasing quality and regulatory
pressure to switch to pre-formulated, engineered media
and reagents.
pressure to switch to pre-formulated, engineered media
and reagents.
Fragile, live cells from source materials such as blood, tissue,
and organs are enabling the development of biologic-based
therapies and treatments for the leading causes of death and
disability.
and organs are enabling the development of biologic-based
therapies and treatments for the leading causes of death and
disability.
These must be transported from the processing lab to the
bedside in a refrigerated or frozen state to preserve viability,
quality, and potency. This is Biopreservation!
bedside in a refrigerated or frozen state to preserve viability,
quality, and potency. This is Biopreservation!
OCTOBER 2011 INVESTOR PRESENTATION
6

OCTOBER 2011 INVESTOR PRESENTATION
Pre-formulated Ÿ cGMP Ÿ Serum-free Ÿ Protein-free
BioLife Products Meet The Opportunity
§ Enable 2x to 3x
longer shelf-life
of cell and tissue
-based clinical
therapies -
several days!
longer shelf-life
of cell and tissue
-based clinical
therapies -
several days!
§ Increase the
yield and
viability of cells
in the clinical
dose
yield and
viability of cells
in the clinical
dose
7

OCTOBER 2011 INVESTOR PRESENTATION
8
CryoStor® & HypoThermosol® In Customer Clinical Trials
Any successful commercial application could result in $1-2M revenue per year
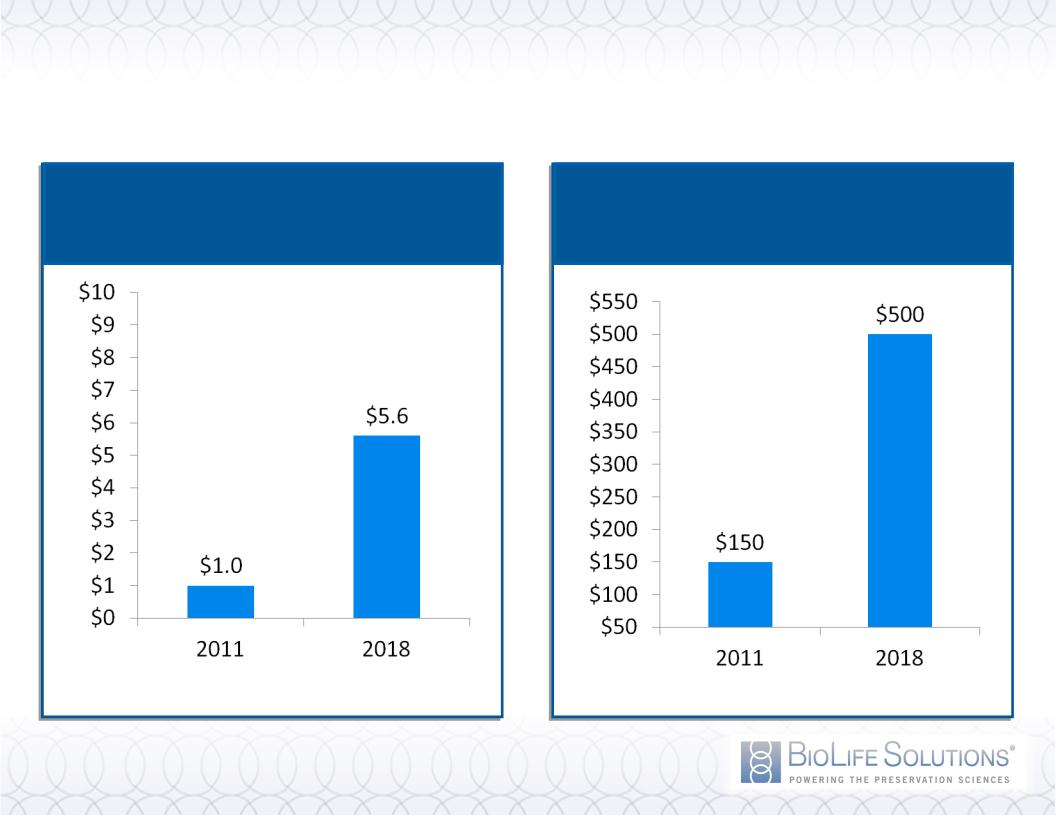
Rapidly Expanding Market
OCTOBER 2011 INVESTOR PRESENTATION
9
Source: VisionGain
Source: Management estimates, Axis Research Mind
Regenerative Medicine Market
Growth (Billions)
Growth (Billions)
Demand for Biopreservation
Media (Millions)
Media (Millions)
Why Our Products Are In High Demand
Outperform all other
biopreservation media
biopreservation media
Highest quality - GMP
Customer service
BioLife Preserved Cells
Competitive Products
OCTOBER 2011 INVESTOR PRESENTATION
10

OCTOBER 2011 INVESTOR PRESENTATION
11
Competition
Home-Brew Biopreservation Media

OCTOBER 2011 INVESTOR PRESENTATION
12
How Our Products Are Different
BioLife Biopreservation Media Advantages
Proprietary formula, optimized to protect cells from low temperature stress
Proprietary formula, optimized to protect cells from low temperature stress
Mitigates apoptosis & necrosis
Mitigates apoptosis & necrosis
Free radical scavengers
Free radical scavengers
Energy substrates
Energy substrates
pH buffers
pH buffers
Pre-Formulated - no mixing required
Pre-Formulated - no mixing required
cGMP manufactured
cGMP manufactured
USP or highest quality components
USP or highest quality components
USP sterility tested
USP sterility tested
USP endotoxin tested
USP endotoxin tested
Bioassay tested
Bioassay tested
FDA Master File
FDA Master File
OCTOBER 2011 INVESTOR PRESENTATION
13
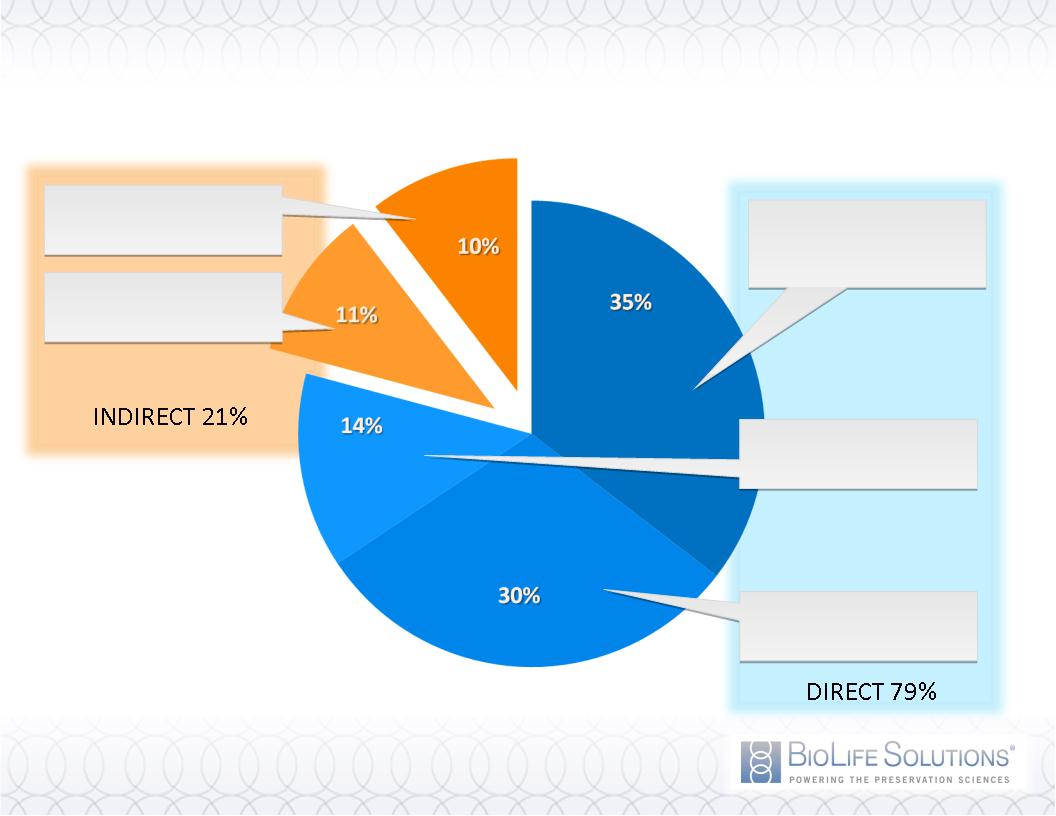
Estimated 2010 Segment Revenues
Regenerative
Medicine
Medicine
Drug Discovery
BioBanking
Distributor
Contract Mfg
Diverse Customer Base
OCTOBER 2011 INVESTOR PRESENTATION
14
Industry Leading Customers

§ 7 issued patents
§ 5 submitted applications
§ Over 200 third-party issued
and pending patents citing
BioLife products
and pending patents citing
BioLife products
INTELLECTUAL PROPERTY
EMBEDDED TECHNOLOGY
OCTOBER 2011 INVESTOR PRESENTATION
Proprietary, High-Value Technology
Critical supplier status with leading
companies:
companies:
§Regenerative medicine
§Cord blood banks
§Suppliers of cells used in drug
screening
screening
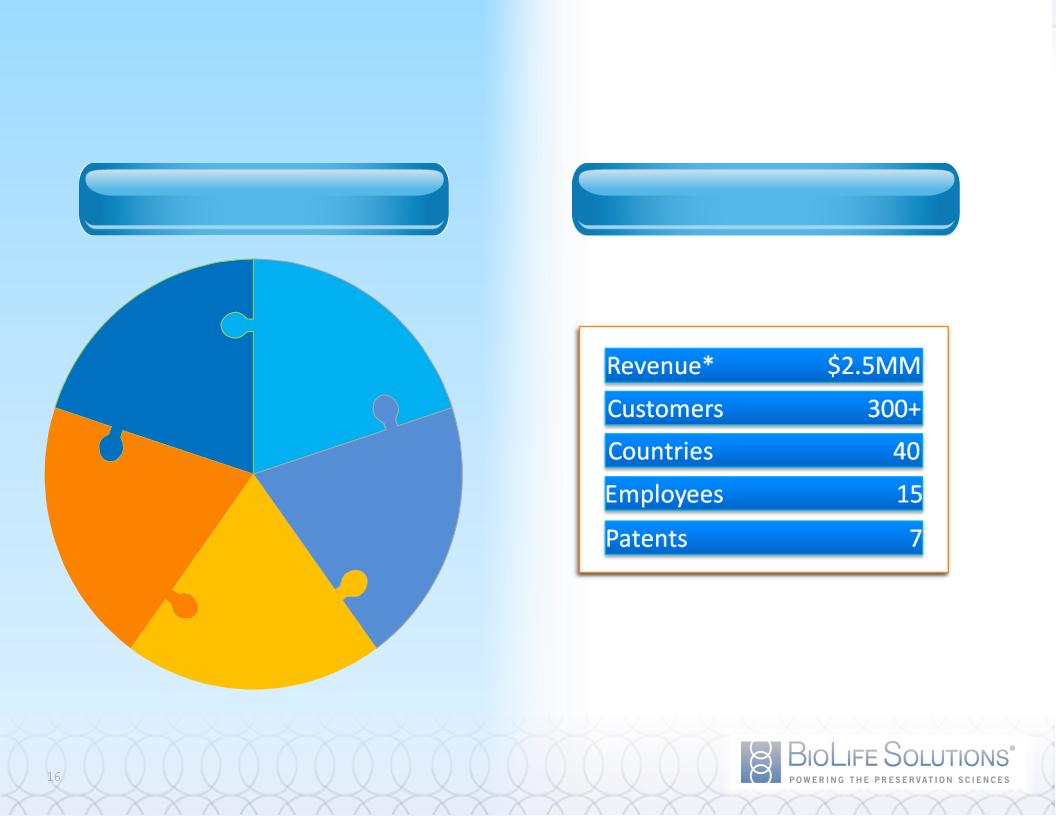
Revenue Model Key to Record Growth
REVENUE STREAMS
HIGHLIGHTS
Drug
Discovery
Discovery
Regnerative
Medicine
Medicine
Contract
Mfg
Mfg
Distributor
Biobanking
OCTOBER 2011 INVESTOR PRESENTATION
* Trailing 12 Months

OCTOBER 2011 INVESTOR PRESENTATION
17
Growth Trend
Record Revenue in Last 5 Quarters
Investment Summary
OCTOBER 2011 INVESTOR PRESENTATION
18
• Best-in-class,
proprietary, first-
choice market
position
proprietary, first-
choice market
position
• Leading supplier
of clinical grade
biopreservation
media products
of clinical grade
biopreservation
media products
• BioLife technology is
embedded in
numerous current &
development stage
clinical products
embedded in
numerous current &
development stage
clinical products
• Significant revenue
upside upon
regulatory approval
of customer
products
upside upon
regulatory approval
of customer
products
• High-quality,
scalable GMP
manufacturing
facility
scalable GMP
manufacturing
facility
• ISO 13485 certified
• FDA Master Files
• 30% - 50% annual
revenue growth
revenue growth
• Moving to the
NASDAQ market
NASDAQ market

OTCBB:
BLFS
BLFS
http://biolifesolutions.com
