Attached files
| file | filename |
|---|---|
| EX-10 - LEASE AGREEMENT - TherapeuticsMD, Inc. | ex-10.htm |
| EX-3.1 - CERTIFICATE - TherapeuticsMD, Inc. | ex-3_1.htm |
| EX-99.4 - CONSENT OF PUBLIC ACCOUNTING FIRM - TherapeuticsMD, Inc. | ex-99_4.htm |
| EX-99.5 - PRESS RELEASE - TherapeuticsMD, Inc. | ex-99_5.htm |
| EX-10.2 - STOCK OPTION AGREEMENT - TherapeuticsMD, Inc. | ex-10_2.htm |
| EX-10.3 - STOCK PURCHASE WARRANT - TherapeuticsMD, Inc. | ex-10_3.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 4, 2011
THERAPEUTICSMD, INC.
(Exact name of registrant as specified in its charter)
|
Nevada
|
000-16731
|
87-0233535
|
|
(State or other jurisdiction of incorporation)
|
(Commission File Number)
|
(IRS Employer Identification No.)
|
951 Broken Sound Parkway NW, Suite 320, Boca Raton, FL 33487
(Address of principal executive offices and Zip Code)
(516) 961-1911
(Registrant’s telephone number, including area code)
AMHN, INC.
10611 N. Hayden Rd., Suite D106, Scottsdale, AZ 85260
(Former Name and Address of Registrant)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
o
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
|
o
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a -12)
|
|
o
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d -2(b))
|
|
o
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e -4(c))
|
TABLE OF CONTENTS
|
Cautionary Statement Concerning Forward-Looking Information
|
3
|
|
Explanatory Note
|
3
|
|
4
|
|
|
4
|
|
|
Agreement and Plan of Merger with VitaMedMD, LLC
|
4
|
|
Form 10 Information
|
6
|
|
The Business
|
6
|
|
Reports to Security Holders
|
23
|
|
Risk Factors
|
24
|
|
Risks Related to VitaMed's Business and Industry
|
24
|
|
Risks Related to VitaMed's Dependence on Third Parties
|
35
|
|
Risks Related to the Company's Common Stock
|
36
|
|
Trends, Risks and Uncertainties
|
41
|
|
Selected Financial Information
|
42
|
|
Management's Discussion and Analysis or Plan of Operations
|
43
|
|
Summary of Key Results
|
43
|
|
Properties
|
47
|
|
Security Ownership of Certain Beneficial Owners and Management
|
48
|
|
Directors and Executive Officers
|
49
|
|
Executive Compensation
|
53
|
|
Certain Relationships and Related Transactions and Director Independence
|
57
|
|
Legal Proceedings
|
59
|
|
Market Price of and Dividends on Common Equity and Related Stockholder Matters
|
59
|
|
Indemnification of Directors and Officers
|
60
|
|
Financial Statements
|
62
|
|
62
|
|
|
63
|
|
|
63
|
|
|
64
|
|
2
CAUTIONARY STATEMENT CONCERNING FORWARD-LOOKING INFORMATION
This report contains certain forward-looking statements (as such term is defined in Section 27A of the Securities Act of 1933, as amended (the "Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act") and information relating to the Company that are based on the current beliefs of the Company’s management as well as assumptions made by and information currently available to management, including statements related to the markets for the Company’s products, general trends and trends in the Company’s operations or financial results, plans, expectations, estimates and beliefs. When used in this report, the words "anticipate," "believe," "estimate,"
"expect," "intend," "plan," "predict," "opinion," "will" and similar expressions and their variants, as they relate to the Company or the Company’s management, may identify forward-looking statements. Such statements reflect the Company’s judgment as of the date of this report with respect to future events, the outcome of which is subject to certain risks, including the risk factors described herein, which may have a significant impact on the Company’s business, operating results or financial condition. You are cautioned that these forward-looking statements are inherently uncertain. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results or outcomes may vary materially from those described herein. See Item 2.01, Form 10
Information, Risk Factors for examples of factors, risks and uncertainties that could cause actual outcomes and results to be materially different from those projected or assumed in our forward-looking statements. The Company undertakes no obligation to update forward-looking statements.
EXPLANATORY NOTE
Upon the closing of the Agreement and Plan of Merger as more fully described below (the "Merger"), TherapeuticsMD, Inc., a Nevada corporation formerly known as AMHN, Inc. ("Therapeutics," "AMHN," or the "Company"), became the parent company of VitaMedMD, LLC, a Delaware limited liability company ("VitaMed"). Unless otherwise provided in this Current Report on Form 8-K (the "Report"), all references in this Report to "we," "us," "our Company," "our," "Therapeutics," the "Company," or the "Registrant" refers to Therapeutics. Unless otherwise indicated in this Report, all references in this Report to the Company’s Board of Directors shall refer to the Board of Directors of Therapeutics which was reconstituted
upon the closing of the Merger. The business of Therapeutics following the Merger primarily consists of those of its subsidiary, VitaMed. This Report contains summaries of the material terms of various agreements executed in connection with the Merger and related transactions.
3
|
Item 1.01
|
Entry into a Material Definitive Agreement.
|
On July 18, 2011, AMHN, Inc., a Nevada corporation ("AMHN" or the "Company") entered into an Agreement and Plan of Merger ("Merger Agreement") by and among VitaMedMD, LLC, a Delaware limited liability company ("VitaMed") and VitaMed Acquisition, LLC, a Delaware limited liability company and wholly owned subsidiary of the Company ("Merger Sub"), pursuant to which the Company would acquire 100% of VitaMed. The proposed acquisition was to be accomplished by the merger of Merger Sub with and into VitaMed with VitaMed being the surviving limited liability company (the "Merger") in accordance with the Limited Liability Company Act of the State of Delaware. The
Merger became effective upon the filing of the Certificate of Merger with the Secretary of State of the State of Delaware on October 4, 2011 (the "Effective Time").
In preparation of and prior to the closing of the Merger Agreement, the Company completed the following required corporate actions with an effective date of October 3, 2011:
|
·
|
a reverse split of its outstanding shares of Common Stock on a ratio of 1 for 100 (the "Reverse Split"),
|
|
·
|
an increase of its authorized shares of Common Stock to 250,000,000,
|
|
·
|
a change in the name of the Company to TherapeuticsMD, Inc., and
|
|
·
|
an amendment to the Company's Long Term Incentive Compensation Plan ("LTIP") to increase the authorized shares for issuance thereunder to 25,000,000.
|
The Merger Agreement was filed as an exhibit to the Current Report on Form 8-K filed with the Securities and Exchange Commission (the "Commission") on July 21, 2011. The closing of the Merger Agreement is further discussed in Item 201, Completion of Acquisition or Disposition of Assets, Agreement and Plan of Merger with VitaMedMD, LLC below.
|
Item 2.01
|
Completion of Acquisition or Disposition of Assets.
|
AGREEMENT AND PLAN OF MERGER WITH VITAMEDMD, LLC
On October 4, 2011, the Closing Date of the Merger Agreement, the Company acquired 100% of VitaMed in exchange for the issuance of shares of the Company's Common Stock, as more fully described below (the "Merger"). In accordance with the provisions of this triangulated merger, Merger Sub was merged with and into VitaMed as of the Effective Date. Upon consummation of the Merger Agreement and all transactions contemplated therein, the separate existence of Merger Sub ceased and VitaMed became a wholly owned subsidiary of the Company. .
Corporate Actions Prior to Closing of Agreement
On July 18, 2011, prior to and in anticipation of the Merger, the sole member of the Company's Board of Directors (the "Sole Director") and the Company's majority shareholder owning 53.7% of the Company's then-outstanding shares of Common Stock ("Consenting Shareholder") consented to and approved the following corporate actions:
|
·
|
A Reverse Split of the Company's 16,575,209 issued and outstanding shares of Common Stock. As a result of the Reverse Split, each share of Common Stock outstanding on the July 28, 2011 (the "Record Date"), without any action on the part of the holder thereof, became one one-hundredth of a share of Common Stock. The Reverse Split decreased the number of outstanding shares of the Company's Common Stock by approximately 99% resulting in 165,856 shares outstanding after the Reverse Split. The effectuation of the Reverse Split did not result in a change in the relative equity position or voting power of the shareholders of the Company.
|
4
|
·
|
An increase in the Company's number of shares of Common Stock authorized for issuance to 250,000,000.
|
|
·
|
A change in the name of the Company to TherapeuticsMD, Inc.
|
|
·
|
An amendment to the Company's LTIP to increase the shares authorized for issuance thereunder to 25,000,000.
|
The effective date for the above-mentioned corporate actions was October 3, 2011.
Exchange of Securities
At the Effective Time, all outstanding membership units of VitaMed (the "Units") were exchanged for shares of the Company's Common Stock. In addition, all outstanding VitaMed options ("Options") and VitaMed warrants ("Warrants") were exchanged and converted into options and warrants for the purchase of the Company's Common Stock ("Company Options" and "Company Warrants"). All Units, Options and Warrants were exchanged on a pro-rata basis for shares of the Company's Common Stock which in the aggregate totaled 70,000,000 shares, resulting in a conversion ratio calculated by the sum of all outstanding Units, Options and Warrants divided by 70,000,000 (the
"Conversion Ratio"). Pursuant to the Conversion Ratio, the Company will issue 58,407,331 shares of the Company's Common Stock in exchange for the outstanding Units and will reserve for issuance an aggregate of 10,365,281 shares issuable upon the exercise of the Company's Options. The Company assumed VitaMed warrants that were originally issued in conjunction with the sale of VitaMed Promissory Notes, and pursuant to the Conversion Ratio, will issue Company Warrants for the purchase of an aggregate of 613,718 shares of the Company's Common Stock. The Company also assumed VitaMed's obligation to subsequently issue warrants to affiliates in consideration for their guarantee of a bank loan for the benefit of VitaMed (the "Reserved Warrants"). Pursuant to the Conversion Ratio, the Reserved Warrants for the purchase of 613,710 shares of the Company's Common
Stock will be issued to certain officers and directors of the Company once they are earned.
Aggregate Beneficial Ownership of Therapeutics' Common Stock After the Transaction
After giving effect to the Reverse Split, and taking into consideration the issuance of the 58,407,331 aforementioned shares in exchange for the Units, the number of shares of the Company's Common Stock issued and outstanding is 58,573,187 of which the members of VitaMed own approximately 99%.
All shares of the Company's Common Stock issued in exchange for the Units, and to be issued upon exercise of the Company Options and Warrants, are subject to a lock-up agreement for a period of eighteen (18) months from the Closing.
The aggregated beneficial ownership of the Company's shares of outstanding Common Stock on a fully diluted basis is as follows:
|
●
|
The members who exchanged their Units in connection with the Merger acquired an aggregate beneficial ownership of approximately ninety-nine percent (99%) of the issued and outstanding shares of Common Stock of the Company; and
|
|
●
|
Shareholders beneficially owning 100% of the shares of the Company's Common Stock immediately prior to the consummation of the Transaction were diluted to an aggregate beneficial ownership of approximately one percent (1%) of the issued and outstanding shares of Common Stock of the Company.
|
5
A discussion of beneficial ownership of the Company's directors, officers and principal shareholders is set forth herein at Security Ownership of Certain Beneficial Owners and Management.
FORM 10 INFORMATION
THE BUSINESS
Corporate Overview and History of Therapeutics, Inc.
The Company was incorporated in Utah in 1907 under the name Croff Mining Company. The Company changed its name to Croff Oil Company in 1952 and in 1996 changed its name to Croff Enterprises, Inc. In the twenty (20) years prior to 2008, Croff's operations consisted entirely of oil and natural gas leases. Due to a spin-off of its operations in December 2007, Croff had no business operations or revenue source and had reduced its operations to a minimal level although it continued to file reports required under the Exchange Act. As a result of the spin-off, Croff was a "shell company" under the rules of the Commission. In July 2009, the Company (i) closed a transaction to
acquire America's Minority Health Network, Inc. as a wholly owned subsidiary, (ii) ceased being a shell company, and (iii) experienced a change in control in which the former shareholders of America's Minority Health Network, Inc. acquired control of the Company.
On September 14, 2009, the Company changed its name to AMHN, Inc.
On June 11, 2010, the Company closed a transaction to acquire Spectrum Health Network, Inc. as a wholly owned subsidiary.
On July 20, 2010, the Company filed Articles of Conversion and Articles of Incorporation to redomicile in the State of Nevada and changed the par value of its shares of capital stock to $0.001 per share. On July 31, 2010, the Company transferred the assets of America's Minority Health Network, Inc. to a secured noteholder in exchange for the satisfaction of debt associated therewith.
On February 15, 2011, the Company transferred the assets of Spectrum Health Network, Inc. to a secured noteholder in exchange for the satisfaction of debt associated therewith and in exchange for an Exclusive Licensing, Distribution and Advertising Sales Agreement under which the Company sells subscription services and advertising on the Spectrum Health Network for commissions.
On August 3, 2011, in anticipation of closing the Merger, the Company filed Amended and Restated Articles of Incorporation to change its name to TherapeuticsMD, Inc. and to increase the shares of Common Stock authorized for issuance to 250,000,000.
On October 4, 2011, the Company closed the Merger. Unless otherwise stated or unless the context otherwise requires, the description of our business set forth below is provided on a combined basis, taking into account our newly-acquired wholly owned subsidiary, VitaMed.
The Company maintains a website at www.therapeuticsmd.com.
Corporate Overview and History of VitaMedMD, LLC
VitaMed is a specialty pharmaceutical company organized as a limited liability company in the State of Delaware on May 13, 2008. VitaMed has developed a patent-pending technology and business methodology to market both over-the-counter ("OTC") and prescription versions of nutritional supplements, drugs, medical foods and other medical products directly to consumers with the recommendation of their physician. VitaMed’s business model creates unique value propositions for patients, physician/providers and insurance payors by eliminating much of the inefficiencies associated with the traditional sales, marketing and distribution models. VitaMed offers superior-quality products for a lower overall cost to
patients and payors while increasing efficiencies for physicians.
6
VitaMed's technology allows it to collect and analyze data from various sources to improve patient compliance and education, facilitate product development and provide immediate feedback on effectiveness of therapies. The result is increased efficiency and communication between the patient, physician/provider and insurance payor, ultimately creating improved outcomes for all. This combination of efficient distribution and technology provides measurable customer benefits differentiates VitaMed from existing competitors in the market.
VitaMed was founded by Robert Finizio and Brian Bernick, M.D. to provide pregnant women with alternatives to traditionally overpriced prescription vitamins. In January of 2009, VitaMed's completed formulation of its first products, a prenatal multivitamin and a vegan docosahexaenoic acid ("DHA") supplement. VitaMed contracted with Lang Naturals, Inc. to produce its first products in March of 2009. In the first half of 2009, VitaMed began to hire management and sales
personnel, design its consumer sales website and deploy its business model. VitaMed's first product sales occurred in June 2009 with sales focused primarily in south Florida. In September 2010, VitaMed achieved a milestone of $1 million in total sales.
VitaMed's new product development continues to focus on the women’s health market place. Based on the analysis of the proprietary data collected, VitaMed has developed and released eight new products since the introduction of its first prenatal vitamin:
· January 2010 – Single pill prenatal vitamin
· February 2020 – Stretch mark cream
· September 2010 - Menopause supplement, Iron supplement and Scar Guard
· February 2011 – Vitamin D3 50,000 IU and 1,000 IU
· May 2011 – Calcium with Vitamin D
As VitaMed continues its product development efforts for both new products and refinements to existing products, it is also looking to find proprietary ingredients that can be licensed on an exclusive basis for use in women’s healthcare that can help further differentiate its products from the competition.
VitaMed has not been involved in any bankruptcy, receivership or any similar proceeding, and except for the subject Merger set forth herein, has not had or been a party to any material reclassifications, mergers or consolidations since inception.
VitaMed's primary SIC code is 2833 – Medicinal Chemicals and Botanical Products. VitaMed maintains a website at www.vitamedmd.com.
Overview of Industry and Market
Healthcare and Pharmaceutical Market
According to statistics compiled by Kaiser Family Foundation, a non-profit foundation focusing on the major healthcare issues facing the United States, healthcare expenditures were approximately $2.5 trillion in 2009 (or 17.6% of our nation's economy or Gross Domestic Product (GDP)), up from 7.2% of GDP in 1970 and 12.5% of GDP in 1990. In 2009, healthcare spending in the U.S. averaged $8,086 per person.
Recently, healthcare reimbursements by Medicare and Medicaid have been reduced to accommodate federal and state budget deficits. This change in physician reimbursement has had an adverse financial impact on physicians in that the costs associated with administration of a medical practice have exceeded the revenues received from providing services to patients. Moreover, as healthcare becomes increasingly consumer driven, patients are seeking more information, control and convenience which place additional time and financial pressures on physicians. These changes have prompted many physicians in the United States to search for tools and solutions to improve practice
efficiency and increase revenue.
7
Pharmaceuticals are a major cost driver in U.S. healthcare. In a report issued by Centers for Medicare and Medicaid Services ("CMS"), the total national spending on prescription drugs, both private and public, from retail outlets reached $250 billion last year, or real per capita spending of $806. In 2009, prescription drugs accounted for approximately ten percent of all national healthcare spending. Total national spending on prescription drugs, both private and public, from retail outlets "increased on average by about 10 percent a year from 1998 through 2009 — faster than the average 6.7 percent a year increase in total U.S. health expenditures
for the same period."
Women’s Health Market
The U. S. Census Bureau projects that there were approximately 150 million women and 146 million men living in the U.S. in 2010. Women are major consumers of health care services, negotiating not only their own complex health care but often managing care for their family members as well. Their reproductive health needs, greater rates of health problems and longer life spans as compared with men make women's' relationships with the health care system complex. According to a 2004 study by the Department of U.S. Health and Human Services, women's health care spending was 57% of the total health care expenditures. According to US Department of Health Services the number of infant births in the U.S. in 2009
was 4,131,019. Women are also more likely to be low-income and often face the added challenge of balancing work with family health and care giving responsibilities. For the one in five women who are uninsured, access to high quality, comprehensive care is even more difficult.
U.S. Dietary Supplement Market
According to a survey conducted by Ipsos-Public Affairs for the Council for Responsible Nutrition, 65% of U.S. adults used dietary supplements in 2010. According to the 2009 U.S. Nutrition Industry Overview by the Nutrition Business Journal (NBJ), a division of Penton Media, Inc. that provides strategic market and competitive analysis of the global nutrition industry, U.S. sales of dietary supplements (including vitamins, herbs, meal supplements, sports nutrition and specialty supplements) grew 6.0% to $26.9 billion in 2009. NBJ is forecasting U.S. sales of dietary supplements to grow at a rate of 6.0% per
year for the next four years reaching $34 billion by 2013. Steady growth reflects customers’ purchases of these natural products to protect their health and ward off more expensive medical visits and prescription drugs. The dietary supplement industry is highly fragmented with products sold through multiple channels including retailers such as mass merchants, grocery stores, drug stores and specialty retailers, direct mail, catalogs, multi-level marketers and the Internet. U.S. sales of dietary supplements through the Internet grew significantly faster than the overall category increasing approximately 18% in 2009 to $1.2 billion and accounted for an estimated 4.3% of the total U.S. dietary supplement category. According to the NBJ 2010 Direct-to-Consumer Selling Report, Internet sales of dietary supplements are expected to grow at an 18% compound annual growth rate (CAGR) over
the next four years, reaching $2.3 billion by 2013.
8
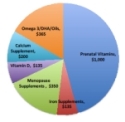 |
The market for supplements in the women's health market is estimated at $2 billion annually (see illustration to left). A common misperception by healthcare providers is that prescription Nutrition and Medical Foods (i.e., prenatal vitamins) are drugs that require approval of and fall under the drug manufacturing standards of the U.S. Food and Drug Administration ("FDA"). The fact is that prescription nutritional products are dietary supplements, NOT drugs, even though they may be dispensed through a pharmacy to fulfill a doctor's prescription. Our business model is designed to transform this large market currently burdened by unnecessary costs and inefficiencies. |
Our Business Model
VitaMed is a specialty pharmaceutical company that has developed a patent-pending technology and business methodology to market both OTC and prescription versions of nutritional supplements, medical foods, drugs and other medical products directly to consumers or via retail pharmacies under the direct supervision of a physician. VitaMed’s business model creates a unique value proposition for patients, physicians/providers and payors by eliminating much of the inefficiencies associated with the traditional sales, marketing and distribution models. VitaMed offers superior-quality products for a lower overall cost to patients and payors while increasing efficiencies for physicians.
At the core of our business model is our patent-pending information technology platform, OPERA™. This technology allows us to collect critical data from various sources that is continuously evaluated and analyzed by VitaMed. This transformation of data is what allows VitaMed to provide significant value to patients, providers and payors by focusing on the areas of customer satisfaction and service, product strategy and development, market intelligence and Phase IV drug studies.1
|
As healthcare becomes increasingly consumer driven, patients are seeking more information, control and convenience which place additional time and financial pressures on physicians. Physicians are looking for improved ways to provide better service to their patients. A recent study by IMS Health Incorporated, the leading provider of information servcies for the healthcare industry, concludes that physicians desire fewer but more encompassing relationships with companies that can provide more valuable information, deliver more relevant services, and better respond to specific needs of their practice and patients. VitaMed meets this
challenge by focusing on the opportunities in women’s health, specifically the OB/GYN market, to provide a better customer experience for physician and patient.
|
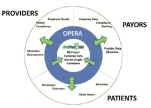 |
1Phase IV trial is also known as postmarketing surveillance trial. Phase IV trials involve the safety surveillance and ongoing technical support of a drug after it receives permission to be sold. Phase IV studies may be required by regulatory authorities or may be undertaken by the sponsoring company for competitive or other reasons.
9
Our business model is designed to achieve better outcomes for patient, physician and payor.
|
·
|
VitaMed offers the highest quality products incorporating patented ingredients like chelated iron and life’s DHA™ into its formulations while maintaining value pricing. This results in greater patient acceptance and satisfaction of our products versus the competition.
|
|
·
|
VitaMed is able to show physician practices that by recommending VitaMed products, the practice is able to realize office efficiencies and cost savings over prescribing competing prescription products.
|
|
·
|
Through the use of our data collection, VitaMed is able to provide to physician practices with statistics and data that show they have helped reduced the cost of patient care and improved patient compliance.
|
|
·
|
Physician practices that choose to dispense products directly to their patients from their offices earn revenue from the sale of the products. Additionally, selected physicians that participate in our studies can receive compensation for their time and services.
|
|
·
|
Our statistical data indicates that a high level of patient compliance is achieved as a result of VitaMed’s direct interaction with patients which supplements patient education.
|
|
·
|
Improved patient education, a high level of patient compliance and reduced cost of products all result in lower cost of care for payors.
|
Sales Strategy
Although our national sales force is similar to that of a traditional pharmaceutical company in that the sales representatives are calling on OB/GYN practices to provide education and sampling, our sales representatives are more customer centric in their sales approach. Our sales representatives offer more than just differences in our products from the competition; they are able to offer an array of partnering opportunities to promote efficiency and cost savings. Our OPERA™ technology allows us to collect and analyze critical data from various inputs allowing VitaMed to provide significant value to patients, providers and payors.
Our national rollout strategy is to focus first on the largest metropolitan areas. In order accelerate the sales ramp in a new territory, VitaMed employs a national sales/large practice sales effort to identify key practices in a new or expanding market. Concurrently with our provider sales effort, VitaMed is working with both commercial insurance and Medicaid insurance payors for partnerships in which the payor can support the recommendation of VitaMed’s products for the benefit of patient, physician and payor with the end result of providing better outcomes for all three constituents.
In general, a better outcome is to provide patients with the best products and care at the best value. Having an assortment of high quality product options that can be recommended by both the physician and payor is the foundation of providing options to the patient.
At the forefront of our sales approach is the philosophy that the physician should recommend products based only on what is best for the patient. The physician and patient also have the option to ask for an alternative recommendation of a similar product.
10
Our Products
Our vitaMedMD® brand includes a full range of products targeted for women’s health and associated with pregnancy, child birth, nursing, post birth and menopause. The specific products include: prenatal vitamins, DHA, iron supplements, calcium supplements, Vitamin D supplements, women's multivitamins, natural (non-hormonal) menopause relief, and scar reduction creams. Our products are detailed below.
Prenatal Plus (Combo Pack)
Prenatal Plus is a two pill combo pack that contains a complete multivitamin with 18 essential vitamins and minerals and 300 mg. of life’s DHA™ (a trademarked product of Martek Bioscience Corporation). Uniquely, it is a 100% Vegetarian and Vegan and Kosher Certified. Based on the latest medical and scientific research, we have optimized many of the forms and nutrients found in our latest version. All minerals, including Iron, Zinc, Selenium, Copper, Manganese and Molybdenum are chelated to improve absorption and tolerability. The citrus-flavored tablet is small and easy to swallow. The fact that the
DHA is plant based (most DHA comes from fish-based sources) is important to many pregnant women due to concerns over contamination and taste of fish-based DHA.
Prenatal 1
Prenatal 1 is a single dose daily multivitamin that provides 14 vitamins, minerals and 200 mg. of vegetarian DHA. Prenatal 1 uses only vegetarian life’s DHA™ and is 100% fish- and animal-free. Each convenient, easy-to-swallow softgel also features .975 mcg. of Folic Acid with Vitamin C, Iron and Zinc.
Iron Complete
Iron Complete is a doctor-recommended iron replacement supplement with a unique 3-weeks-on/1-week-off dosing schedule that helps maximize absorption and enhances tolerability. It is formulated with 150 mg. of chelated Iron to help improve tolerability and limit typical side effects associated with iron replacements. Each easy-to-swallow single tablet serving also includes 800 mcg. of Folic Acid, plus Vitamins C and E, and Succinic Acid to aid in absorption.
Transitions Menopause Relief
Transitions Menopause Relief offers a natural solution for hot flashes, night sweats and mood disturbances. Each single tablet dosage delivers 120 mg. of Lifenol®, a patented, well-studied female hops extract recognized for its potency and support in alleviating hot flashes, plus Black Cohosh and plant phytoestrogens. It also includes Calcium (as Calcium Citrate) and Vitamin D3 for added bone support. Transitions Menopause Relief offers women relief from their symptoms without the risk of Hormone Replacement Therapy (HRT).
Calcium + Vitamin D
VitaMed's Calcium + Vitamin D is a doctor-formulated, dietary supplement that helps preserve beneficial levels of Calcium and Vitamin D in the body. Each convenient two tablet serving delivers the recommended dietary allowance of Calcium for most adults. This product provides 1,200 mg. of Calcium as Calcium Carbonate and Calcium Citrate blend, readily absorbable and digestible, and can be taken on an empty stomach. It also includes 1,000 IU of Vitamin D3 to enhance absorption and support bone health.
11
Vitamin D3 50,000 IU and Vitamin D3 1,000 IU
Vitamin D3 50,000 IU and 1,000 IU are doctor-formulated dietary supplements that help replenish and maintain beneficial levels of Vitamin D in the body. Sustaining adequate levels of Vitamin D in the body is essential to bone health, enhancing the absorption of Calcium and Phosphorus. Vitamin D3, also known as Cholecalciferol, is considered the most preferred form of Vitamin D as it is the most active form of the nutrient. Vitamin D3 50,000 IU and 1,000 IU are used in the dietary management of Vitamin D deficiency and should be used under medical supervision. Vitamin D3 50,000 IU and 1,000 IU are ideal for pregnant, breastfeeding and menopausal women needing
to sustain adequate levels of Vitamin D.
Stretch Mark Body Cream
VitaMed's Stretch Mark Body Cream contains naturally-derived ingredients, including Peptides, Shea Butter, Sweet Almond Oil and Fruit Extracts, that hydrate, soothe and pamper skin to make it softer, smoother and younger-looking. It helps reduce the appearance of stretch marks, scars, and other skin irregularities; intensely hydrates and replenishes skin’s moisture; diminishes the look of fine lines and wrinkles and encourages the fading of age spots and sun spots. Backed by clinical and scientific testing, Stretch Mark Body Cream is hypoallergenic, paraben-free and non-comedogenic.
Scar Reduction Body Cream
VitaMed's Scar Reduction Body Cream is rich in vitamins and naturally-derived extracts. Backed by independent clinical and scientific testing, it helps minimize the size and appearance of old and new scars; helps reduce scar tissue; diminish the appearance of fine line and wrinkles; and encourages the fading of age spots. It is paraben-free, non-comedogenic and hypoallergenic.
Products in Development
VitaMed's market objective is to develop an entire suite of products that are condition specific and geared to the women’s health sector. Our sales force has developed strong relationships and partnerships in the OB/GYN market segment to sell our current products. We have also established relationships with some of the largest OB/GYN practices in the country. By delivering additional products through the same sales channel we can leverage our already deployed assets to increase our sales and improve profitability.
In the next 12 months, VitaMed intends to introduce its first medical food prescription products. Our focus is to introduce products in which we use propriety or patented molecules or ingredients that will differentiate our products from the competition. We currently have five new products in development and plan to introduce our first new product by the third quarter of 2012. VitaMed is also planning to introduce prenatal vitamins with a folic acid that is better tolerated by some women. Folic acid is a key ingredient in prenatal vitamins and certain types of folic acid significantly enhance the bio-availability of folic
acid. We intend to enhance our current line of OTC prenatal vitamins by adding this more-efficiently absorbed folic acid.
Raw Materials for Our Products
All raw materials and ingredients for our proprietary products are purchased from a group of third-party suppliers specializing in raw material manufacturing, processing and specialty distribution. Our manufacturers maintain multiple supply and purchasing relationships throughout the raw materials marketplace to provide an uninterrupted supply of product to meet our manufacturing requirements.
12
Manufacturing of Our Products
Our products are manufactured and regulated by the same FDA quality standards (Controls Used for Manufacturing, Processing, Packing, or Holding Dietary Supplements for FDA 21 CFR Part 110/111 CGMP Regulations ("CFR 111")) and current good manufacturing practices ("cGMP") as prescription nutritional therapies. In addition, we use some of the same manufacturing facilities as our prescription competition, we conduct two additional un-required certificates of analysis on every lot to ensure quality, and we employ an outside third party to enforce rigorous quality audits.
All of our manufacturing is performed by third party manufacturers. Over 90% of our manufacturing is done by Lang Naturals, Inc. ("Lang"), a full-service, private label and corporate brand manufacturer specializing in premium health benefit driven™ products, including medical foods, nutritional supplements, beverages, bars, and functional foods in the dietary supplement category. Lang provides VitaMed a variety of additional services including development processes, prototype development, raw materials sourcing, regulatory review and packaging production. At present, our relationship with Lang
is excellent and we intend to continue to use them as our third party manufacturer for most of our products. In the event our relationship with Lang terminates for any reason, there are a number of other manufacturers available to VitaMed; accordingly, management is of the opinion that such termination would not have a material adverse effect on VitaMed's business.
Quality Control for Our Products
A quality assurance team establishes process controls and documents and tests every stage of the manufacturing process to ensure we meet product specifications and that our finished dietary supplements contain the correct ingredients, purity, strength, and composition in compliance with FDA regulations. We test incoming raw materials and finished goods to ensure they meet or exceed FDA and U.S. Pharmacopeia standards including quantitative and qualitative assay and microbial and heavy metal contamination.
Our manufacturers' quality and production standards are designed to meet or exceed the latest FDA regulations. To ensure the highest quality, our manufacturing operations are audited by AIB International, Inc. ("AIB") for independent cGMP certification. AIB is an independent, not-for-profit organization that offers programs and services to augment and support the work of regulatory officials around the country, including standards development, product testing and certification, and onsite audits and inspections. The manufacturing facilities we use are also ISO 9001 certified which is a
family of standards related to quality management systems and are designed to help organizations ensure they meet the needs of customers. In addition, our manufacturers are hazard analysis critical control point ("HACCP") certified which is a systematic preventive approach for food and pharmaceutical safety that addresses physical, chemical and biological hazards as a means of prevention rather than finished product inspection.
Customer Service
Our goal is 100% customer satisfaction by consistently delivering superior customer experiences; before, during and after the sale. To achieve this goal, we maintain a fully staffed customer care center for both inbound and outbound customer service using the most current technologies to respond to customer's via incoming and outgoing calls, e-mails and live-chat. We believe our customer service initiatives allow us to establish and maintain long-term customer relationships and facilitate repeat visits and purchases.
Our fully staffed customer care center has representatives available to answer customer questions and to accept customer orders. Our staff has access to real time inventory data to determine if a product is in stock so as to properly manage customer expectations. Our customer care center systems provide a seamless customer experience through our toll-free telephone number, e-mail and live-chat features. Our representatives receive regular training so that they can effectively and efficiently field questions from current and prospective customers and are also trained not to answer questions that
should be directed to a customer’s physician. Having a quality customer care center allows our representatives to provide an array of valuable data in the areas of sales, market research, quality assurance, lead generation and customer retention.
13
Our Return Policy
Customers may return or exchange products for any reason by returning the product within thirty (30) days of receipt. We will refund the entire purchase price, less shipping. The customer is responsible for the cost of returning the products to us except cases where the product is being returned because of a defect or an error made in our order fulfillment. If the purchased product exceeded a thirty-day supply, the unused product must be returned to receive the full refund. All unopened products may be exchanged for different products; the customer will be responsible for the difference in price if the replacement product is more
expensive or we will refund the difference if the replacement product is less expensive.
Our Quality Guarantee
We proudly stand behind the quality of our products. Our guarantee makes it easy, convenient and safe for customers to purchase our products. Under our quality guarantee we:
|
·
|
ensure the potency and quality of our vitamin products,
|
|
·
|
help physicians/providers and payors deliver the best possible outcomes to patients by deliveringbetter information on patient compliance and satisfaction,
|
|
·
|
provide a 30-day money back guarantee for all of our products, and
|
|
·
|
ensure a safe, secure online shopping experience through our encrypted website.
|
We value frequent communication with and feedback from our customers in order to continue to improve our offerings and services.
Intellectual Property
We rely on a combination of patent, copyright, trademark and trade secret laws, confidentiality procedures and contractual provisions to protect our proprietary rights with respect to our technology and proprietary information. We have registered the name, vitaMedMD® as a trademark with the USPTO for use in connection with dietary and nutritional supplements and have trademark applications pending for TherapeuticsMD™, and OPERA™. We believe our trademarks to be valuable and identified strongly with our brand.
Issuance of a federally registered trademark creates a rebuttable presumption of ownership of the mark; however, it is subject to challenge by others claiming first use in the mark in some or all of the areas in which it is used.
Federally registered trademarks have a perpetual life, as long as they are maintained and renewed on a timely basis and used properly as trademarks, subject to the rights of third parties to seek cancellation of the trademarks if they claim priority or confusion of usage. We believe our patents and trademarks are valuable and provide us certain benefits in marketing our products. We intend to actively protect our patents, trademarks, trade secrets and other intellectual property.
14
The Company has the following U.S. trademark registrations and pending trademark applications:
| vitaMedMD® [word] |
U.S. Reg. No. 3842265
|
|
| vitaMedMD® [design] |
U.S. Reg. No. 3835805
|
|
| OPERA™ |
U.S. Application No. 85118845
|
|
| TherapeuticsMD™ |
U.S. Application No. 85371566
|
|
| VITAMEDMD™ |
U.S. Application No. 85371567
|
On September 17, 2009, we filed for patent protection on a System and Method of Ongoing Evaluation Reporting and Analysis (U.S. patent application Ser. No.: 12561515). The patent application is currently pending in the USPTO.
OPERA™ is our patent-pending information technology platform used in our business. The deployment of OPERA™ and the further development and deployment of related technology creates a sustainable competitive advantage that has led to our market share growth. We are currently developing additional intellectual property in the following areas:
|
·
|
OPERA™ business process patents
|
|
·
|
Physician/provider portal; a key way to gather and share physician data
|
|
·
|
Mobile applications linked to the OPERA™ system
|
|
·
|
New product technologies and formulations
|
As we continue to develop proprietary intellectual property, we will expand our protection by applying for additional patents around the business process for OPERA™ and patents on future technologies, including developing mobile applications to more effectively communicate with patients. As we examine our current product offerings and new product pipeline, we are in the process of modifying and developing new formulations that will enable us to gain patent protection for these products.
Generally our nutritional product formulations are proprietary in that in designing them, we attempt to blend an optimal combination of nutrients that appear to have beneficial impact based upon scientific literature and input from physicians; however, as formal clinical studies have in most instances not been conducted by us to validate the intended health benefits of our products, we are generally prohibited by the FDA from making disease treatment and prevention claims in the promotion of our products that use these formulations.
While we seek broad coverage under our patent applications, there is always a risk that an alteration to the process may provide sufficient basis for a competitor to avoid infringement claims. In addition, patents expire and we cannot provide any assurance that any patents will be issued from our pending application or that any potentially issued patents will adequately protect our intellectual property.
Online Commerce
A vast majority of VitaMed's sales are completed online. The Internet has continued to increase its influence over communication, content and commerce. According to Forrester Research, an independent research company providing advice to global leaders in business and technology, U.S. online retail sales increased 12.6% from 2009. Forrester projects online retail sales to grow at a 10% CAGR to $278.9 billion by 2015. We believe several factors will contribute to this increase including convenience, expanded range of available products and services, improved security and electronic payment technology, increased access to broadband Internet connections and widespread consumer confidence and acceptance
of the Internet as a means of commerce.
15
Growth Strategy
VitaMed has exceptional opportunities to expand its business. There are five key pillars to our growth strategy:
1. Geographic Expansion - We have experienced rapid growth in our initial sales territories (principally Florida, Texas, Southern California and Georgia). We are now expanding to additional markets and increased our sales team to 27 people as of September 30, 2011. In the next 12 months, we intend to expand to 60 territories with a focus on major markets.
2. Introduction of New Products through Existing Sales Channel - Through our unique offerings like e-commerce, wholesale opportunities and OPERA, we are able to develop a much stronger partnership with OB/GYN practices than in traditional pharma. This gives us the ability to bring significant new products and services to these practices. We have an aggressive pipeline of new products and are also able to offer our sales channel capability to other companies that are looking to penetrate the OB/GYN market.
3. Large Group Practices – Due to our unique partnership offerings, we have developed strong relationships with many of the largest OB/GYN practices. Because of the savings and the data that come with our model, we are particularly attractive to large practices that can use this data in negotiating their contracts. Once the leaders of a large practice accept our model there is rapid adoption by the other practitioners in that group.
4. Direct to Consumer - In addition to our physician channel, we have a unique direct-to-consumer channel to drive customer retention, acquisition and revenue growth. Consumers that go to our website are usually sent there by a healthcare provider, so they arrive with a bias that the site is credible and believable. After their initial order, over 60% of our customers sign up for "auto-refill" so they can continue to receive the product without placing an order each month. In addition, to the initial product sales, a satisfied customer provides us with continued sales
opportunities throughout the customer's life cycle which increases the overall value of each customer. The loyalty of our customer base helps build traffic revenue through social media.
5. VitaMed is working with both commercial insurance and Medicaid insurance payors to create relationships in which the payor can support the recommendation of our products for the benefit of patient, provider and payor with the end result of providing better outcomes for all.
6. Software Services and Licensing - OPERA is a powerful tool for reducing costs and improving efficiencies. As such, other companies are interested in licensing this technology for use in enhancing their own marketing strategies. Additionally, OPERA is very well suited to facilitate data gathering for Phase 4 drug studies, which will provide another source of future revenue.
The key elements of our growth strategy include (i) the hiring of additional in-field sales representatives, including national sales and local sales personnel, (ii) additions to our product line and (iii) opening new distribution channels. Over time, we believe our growth strategy will increase net sales while maintaining or increasing our gross margins,
Competition and Our Competitive Advantage
The specialty pharmaceutical industry, including the Women’s Health market in which we primarily participate, is defined by rapidly advancing technologies, extreme competition and a focus on proprietary products. We face competition from numerous sources, including commercial pharmaceutical companies, pharmacy retailers, specialty retailers, on-line retailers, biotechnology organizations, academic institutions, government agencies and private and public research institutions. Our current products compete with existing and new therapies that may become available in the future.
16
Our competition may have larger pools of financial resources and more sophisticated expertise in research and development, manufacturing, clinical trials, regulatory pathways and marketing approved products than we do. These competitors are also recruiting and retaining exceptional sales and management personnel. Usually, competition to our currently marketed products have distinguished brand names, are distributed by large pharmaceutical companies with sizable amounts of resources and have achieved widespread acknowledgement in the healthcare market. Small or early stage companies may also prove to be serious competition, predominantly through collaborative
agreements with large and established companies. While we have experience in OTC products, we have never developed a medical food or FDA-approved drug. With respect to FDA-approval process, we are at a competitive disadvantage to many companies with significantly more experience than we have in developing these drugs.
We believe our business model creates a unique value proposition for patients, providers and payors by eliminating much of the inefficiencies associated with the traditional sales, marketing and distribution models. We believe we compete favorably; however, the nature and extent to which our competitors implement various pricing and promotional activities in response to increasing competition and our response to these competitive actions, could adversely affect our profitability.
User friendly shopping experience
Our website is designed to attract natural search traffic while providing a convenient, educational, secure and efficient shopping experience. Our website and sales collateral includes specific and detailed information about our products which helps our customers make informed purchases. Our website uses secure encryption technology designed to protect our customers’ personal and credit card information and to prevent its unauthorized use. Our customer service representatives take orders and answer product and technical questions through our toll-free telephone number. Customers are also able to reach our customer service representatives via email or the
live-chat feature on our website. We seek to respond within 24 hours to all email requests received between Monday and Friday. We also facilitate repeat customer orders through our Autoship feature.
Technology Infrastructure and Operations
Our website is supported by a technology infrastructure designed to provide a superior customer experience, including simplicity, speed and security. We are able to monitor our website and services in real time. We also track and manage our inventory, order fulfillment, customer service and marketing through state-of-the-art technologies that allow us to integrate this data as part of our OPERA™ system. In summary, our technology allows us to collect critical data from various sources that we continuously evaluate and analyze. This transformation of data is what allows us to provide significant value to patients, providers and payors by
focusing on the areas of customer satisfaction and service, product strategy and development, market intelligence and post marketing surveillance.
We follow rigorous industry standards to protect our internal operations and the personal information we collect from our customers. We do not sell or disclose the personal information of our customers. We continue to maintain and upgrade our technology framework to assure compliance with the high levels of security defined by the Payment Card Industry Data Security Standard, the standard created to increase controls around cardholder data to reduce credit card fraud.
17
Government Regulation
Although our current products do not specifically require approval, we are subject to federal and state consumer protection laws, including laws protecting the privacy of consumer non-public information and regulations prohibiting unfair and deceptive acts and trade practices. In particular, under federal and state financial privacy laws and regulations, we must provide:
· notice to consumers of our policies on sharing non-public information with third parties;
· advance notice of any changes to our policies, and
· with limited exceptions, provide consumers the right to prevent sharing of their non-public personal information with unaffiliated third parties.
The growth and demand for eCommerce could result in more stringent consumer protection laws that impose additional compliance burdens on online retailers. These consumer protection laws could result in substantial compliance costs and could interfere with the conduct of our business.
There is currently great uncertainty in many states whether or how existing laws governing issues such as property ownership, sales and other taxes, and libel and personal privacy apply to the Internet and commercial online retailers. These issues may take years to resolve. For example, tax authorities in a number of states, as well as a Congressional advisory commission, are currently reviewing the appropriate tax treatment of companies engaged in online commerce and new state tax regulations may subject us to additional state sales and income taxes. New legislation or regulation, the application of laws and regulations from jurisdictions whose laws do not
currently apply to our business, or a change in application of existing laws and regulations to the Internet and commercial online services could result in significant additional taxes on our business. These taxes could have an adverse effect on our results of operations.
Our products are subject to extensive regulation in the U.S. The FDA enforces the Federal Food, Drug and Cosmetic Act (FDCA) and related regulations which govern the identity, purity, quality, strength, and composition of dietary supplements and regulate the formulation, manufacture, packaging, labeling, holding, sale, and distribution of dietary supplements, foods and over-the-counter (OTC) drugs, and prohibit the sale of misbranded and adulterated dietary supplements and dietary supplements that by the intention of the manufacturer or distributor or label or labeling claims are unapproved new drugs.
The Federal Trade Commission (FTC) enforces the Federal Trade Commission Act (FTCA) and related regulations which govern the advertising and advertising acts and practices associated with the promotion and sale of these products. The U.S. Postal Inspection Service enforces federal laws governing fraudulent use of the mail. Regulation of certain aspects of the dietary supplement business at the federal level is also governed by the Consumer Product Safety Commission (CPSC) (e.g., concerning the presence of adulterated substances, such as toxic levels of lead or iron, that render products unsafe for consumption and require an ordered recall), the Department of
Agriculture (e.g., for products that are intended for ingestion as dietary supplements for animals) and the Environmental Protection Agency (e.g., in the methods of disposal used for certain dietary ingredients, such as colloidal silver). Federal and state anti-kick back statutes, the Ethics in Patient Referrals Act, false claims statutes and HIPPA also apply to our business.
The FDCA has been amended several times affecting provisions that concern dietary ingredients and dietary supplements, including by the Dietary Supplement Health and Education Act of 1994 (DSHEA). DSHEA formally defined what may be sold as a dietary supplement, defined statements of nutritional support and the conditions under which they may lawfully be used, and included provisions that permit the FDA to regulate manufacturing practices and labeling claims peculiar to dietary supplements. "Dietary supplements" are defined as vitamins, minerals, herbs, other botanicals, amino acids and other dietary substances that are used to supplement the diet,
as well as concentrates, constituents, extracts, metabolites, or combinations of such dietary ingredients. Generally, under DSHEA, dietary ingredients that were on the market before October 15, 1994 may be used in dietary supplements without notifying the FDA. However, a "new" dietary ingredient (i.e., a dietary ingredient that was not marketed in the U.S. before October 15, 1994) must be the subject of a new dietary ingredient notification submitted to the FDA unless the ingredient has been "present in the food supply as an article used for food" without having been "chemically altered." A new dietary ingredient notification must provide the FDA with evidence of a "history of use or other evidence of safety" which establishes that use of the dietary ingredient "will reasonably be expected to be safe." A new dietary ingredient notification must be submitted to the FDA at
least 75 days before the new dietary ingredient can be marketed. There can be no assurance that the FDA will accept evidence purporting to establish the safety of any new dietary ingredients that we may want to market and the FDA’s refusal to accept such evidence could prevent the marketing of such dietary ingredients.
18
Increased FDA enforcement could lead the FDA to challenge dietary ingredients already on the market as "illegal" under the FDCA because of the failure to file a new dietary ingredient notification or because the substance may be one found to be the subject of an investigational new drug application for which clinical trials have commenced and been publicized.
The FDA generally prohibits labeling a dietary supplement with any "health claim" (i.e., any statement associating a nutrient with prevention, but not treatment, of a disease or health-related condition), unless the claim is pre-approved by the FDA. The FDA prohibits disease treatment claims entirely when made for a dietary supplement; however, "statements of nutritional support," including so-called "structure/function claims" are permitted to be included in labeling for dietary supplements without FDA pre-approval. Such statements may describe how a particular dietary ingredient affects the structure, function or general well-being of the body, or the mechanism of
action by which a dietary ingredient may affect the structure, function or well-being of the body, but such statements may not state that a dietary supplement will reduce the risk or incidence of a disease unless such claim has been reviewed and approved by the FDA. A company that uses a statement of nutritional support in labeling must possess evidence substantiating that the statement is truthful and not misleading. Such statements must be submitted to the FDA no later than thirty days after first marketing the product with the statement and must be accompanied by the following FDA mandated label disclaimer: "This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease." There can be no assurance that the FDA will not determine that a particular statement of nutritional support that
we want to use is an unacceptable disease claim or an unauthorized nutrient-disease relationship claim otherwise permitted with FDA approval as a "health claim." Such a determination might prevent the use of such a claim.
Medical foods are specially formulated and intended for the dietary management of a disease that has distinctive nutritional needs that cannot be met by normal diet alone. They were defined in the Food and Drug Administration’s 1988 Orphan Drug Act Amendments and are subject to the general food and safety labeling requirements of the Federal Food, Drug, and Cosmetic Act. Medical foods are distinct from the broader category of foods for special dietary use and from traditional foods that bear a health claim. In order to be considered a medical food the product must, at a minimum:
|
·
|
be a food for oral ingestion or tube feeding (nasogastric tube);
|
|
·
|
be labeled for the dietary management of a specific medical disorder, disease or condition for which there are distinctive nutritional requirements; and
|
|
·
|
be intended to be used under medical supervision. Medical foods require a prescription from a physician.
|
In addition, DSHEA provides that certain "third-party literature," such as a reprint of a peer-reviewed scientific publication linking a particular dietary ingredient with health benefits, may "in connection with the sale of a dietary supplement to consumers" be exempt from labeling regulation. However, the FDA has adopted an "intent to use" doctrine whereby such literature, even if exempt from labeling, may nonetheless form the basis for an agency determination that the literature in context reveals a company's intent to sell a dietary ingredient or dietary supplement as a drug, thereby rendering the supplement an unlawful, unapproved new
drug. Because the "intent to use" doctrine is predicated on a subjective assessment of all facts and circumstances associated with the promotion and sale of a dietary supplement, we cannot know whether any particular piece of literature otherwise exempt from labeling will be deemed by the FDA unlawful for use in association with the sale of the dietary ingredient or dietary supplement.
19
As authorized by the FDCA, the FDA has adopted and is implementing Good Manufacturing Practices (GMPs) specifically for dietary supplements. These GMPs impose extensive process controls on the manufacture, holding, labeling, packaging, and distribution of dietary supplements. They require that every dietary supplement be made in accordance with a master manufacturing record, that each step in the manufacture, holding, labeling, packaging, and distribution be defined with written standard operating procedures, monitored, and documented, and that any deviation in manufacture, holding, labeling, packaging, or distribution be contemporaneously documented, assessed by a
quality control expert, and corrected through documented corrective action steps (whether through an intervention that restores the product to the specifications in the master manufacturing record or to document destruction of the non-conforming product). The GMPs are designed to ensure documentation, including testing results that confirm the identity, purity, quality, strength, and composition of dietary supplements. In addition, GMPs require a company to make and keep written records of every product complaint that is related to GMPs. The written record of the product complaint must include the following: the name and description of the dietary supplement; the batch, lot, or control number of the dietary supplement, if available; the date the complaint was received and the name, address, or telephone number of the person making the complaint, if available; the nature of the
complaint, including, if known, how the product was used; the reply to the complainant, if any; and findings of the investigation and follow-up action taken when an investigation is performed. The regulations directly affect all who manufacture the dietary supplements we sell. The FDA may deem any dietary supplement adulterated, whether presenting a risk of illness or injury or not, based on a failure to comply with any one or more process controls in the GMP regulations. If deemed adulterated, a dietary supplement may not be lawfully sold and may have to be recalled from the market. It is possible that the FDA will find one or more of the process controls implemented by us, by our contract manufacturers, or by those whose dietary supplements we sell to be inadequate and, thus, requiring corrective action, requiring any one or more of the dietary supplements we sell to be unlawful for
sale, or resulting in a judicial order that may impair our ability to manufacture, market, and sell dietary supplements.
The FDA also requires adverse event notices on labels and serious adverse event reporting for all supplements and OTC drugs. An "adverse event" is defined by statute to include "any health-related event associated with the use of a dietary supplement that is adverse." Only serious adverse events must be reported to the FDA. A "serious adverse event" is an adverse event that: results in death, a life-threatening experience, inpatient hospitalization, a persistent or significant disability or incapacity, or a congenital anomaly or birth defect; or requires, based on reasonable medical judgment, a medical or surgical intervention to prevent an
outcome described above.
The regulation of medical foods and dietary supplements may increase or become more restrictive in the future. There can be no assurance that, if more stringent statutes are enacted for dietary supplements, or if more stringent regulations are promulgated, we will be able to comply with such statutes or regulations without incurring substantial expense.
The FDA regulates the formulation, manufacturing, packaging, labeling and distribution of OTC drug products pursuant to a "monograph" system that specifies active drug ingredients that are generally recognized as safe and effective for particular uses. If an OTC drug is not in compliance with the applicable FDA monograph, the product generally cannot be sold without first obtaining FDA approval of a new drug application, which can be a long and expensive procedure. The homeopathic drugs that we sell are regulated as non-prescription, OTC drugs. These products must generally meet the standards set forth in the Homeopathic Pharmacopeia of the United States and claims
made for them must not deviate from those contained in specific homeopathic treatises recognized by the FDA as appropriate for use. If these requirements are not met, the FDA can consider the products unapproved new drugs and prohibit their sale.
20
The FDA has broad authority to enforce the provisions of the FDCA concerning medical foods, dietary supplements and OTC drugs, including powers to issue a public "warning letter" to a company to quarantine and prohibit the sale of products deemed adulterated or misbranded, to publicize information about illegal products, to request a voluntary recall of illegal products from the market, to request that the Department of Justice initiate a seizure action, an injunction action or a criminal prosecution in U.S. courts, and to seek disgorgement from a federal court of all proceeds received from the sale of products deemed misbranded or adulterated. For instance, the FDA
recently announced that any unapproved new drug introduced after September 19, 2011 will be subject to immediate enforcement action, without prior notice and without regard to the enforcement priorities set out in CPG 440.100. The FDA will continue to apply the enforcement priorities established in 2006. These give a higher priority to enforcement actions involving drugs in certain high-risk categories, such as drugs that pose a potential safety risk or lack evidence of effectiveness.
The FTC exercises jurisdiction over the advertising of medical foods, dietary supplements and OTC drugs. In recent years, the FTC has instituted numerous enforcement actions against dietary supplement companies for making false or misleading advertising claims and for failing to adequately substantiate claims made in advertising. These enforcement actions have often resulted in consent decrees and the payment of civil penalties and/or restitution by the companies involved. The FTC also regulates other aspects of consumer purchases including, but not limited to, promotional offers of savings compared policies, telemarketing, continuity plans, and "free"
offers.
We are also subject to regulation under various state, local and international laws that include provisions governing, among other things, the formulation, manufacturing, packaging, labeling, advertising and distribution of dietary supplements and OTC drugs. For example, Proposition 65 in the State of California is a list of substances deemed to pose a risk of carcinogenicity or birth defects at or above certain levels. If any such ingredient exceeds the permissible levels in a dietary supplement, cosmetic, or drug, the product may be lawfully sold in California only if accompanied by a prominent warning label alerting consumers that the product contains an
ingredient linked to cancer or birth defect risk. Private attorney general actions as well as California attorney general actions may be brought against non-compliant parties and can result in substantial costs and fines.
Applicable federal and state healthcare laws and regulations, include, but are not limited to, the following:
|
·
|
The federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid.
|
|
·
|
The Ethics in Patient Referrals Act, commonly referred to as the Stark Law, and its corresponding regulations, prohibit physicians from referring patients for designated health services reimbursed under the Medicare and Medicaid programs to entities with which the physicians or their immediate family members have a financial relationship or an ownership interest, subject to narrow regulatory exceptions.
|
|
·
|
The federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government.
|
21
|
·
|
HIPAA imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information.
|
|
·
|
The federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services.
|
|
·
|
Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government.
|
Efforts to ensure that our business arrangements with third parties comply with applicable healthcare laws and regulations could be costly. Although VitaMed's regulatory counsel has assisted VitaMed in establishing business practices compliant with applicable laws, it is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our past or present operations, including activities conducted by our sales team or agents, are found to be in violation of any of these laws or any other
governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from third party payor programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians, providers or entities with whom we do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Many aspects of these laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of subjective interpretations which increases the risk of potential violations. In addition, these laws and their interpretations are subject to change. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and damage our reputation.
In addition, from time to time in the future, we may become subject to additional laws or regulations administered by the FDA, the FTC, or by other federal, state, local or foreign regulatory authorities, to the repeal of laws or regulations that we generally consider favorable, such as DSHEA, or to more stringent interpretations of current laws or regulations. We are not able to predict the nature of such future laws, regulations, repeals or interpretations, and we cannot predict what effect additional governmental regulation, if and when it occurs, would have on our business in the future. Such developments could, however, require reformulation of certain products
to meet new standards, recalls or discontinuance of certain products not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of certain products, additional or different labeling, additional scientific substantiation, additional personnel or other new requirements. Any such developments could have a material adverse effect on our business.
Research and Development Activities
For the years ended December 31, 2010 and 2009, we estimate we spent $65,402 and $23,343, respectively, on research and development activities. For the period ended June 30, 2011, we estimate we spent $42,741 on research and development activities. None of these research and development costs will be borne directly by our customers.
22
Environmental Laws
We depend on third parties to support us in manufacturing and developing all of our products and do not directly handle, store or transport hazardous materials or waste products. We depend on these third parties to abide by all applicable federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous materials and waste products. We do not anticipate the cost of complying with these laws and regulations to be material.
Employees
Immediately after the Closing of the Merger, we had 44 full-time and two part-time employees. Additionally, from time to time, we hire temporary contract employees. None of our employees are covered by a collective bargaining agreement and we are unaware of any union organizing efforts. We have never experienced a major work stoppage, strike or dispute. We consider our relationship with our employees to be good.
Corporate Information
Our corporate headquarters are located at 951 Broken Sound Parkway NW, Suite 320, Boca Raton, FL 33487. Our telephone number is 800-728-0009 and our fax number is 561-431-3389.
REPORTS TO SECURITYHOLDERS
We are required to file annual, quarterly and current reports, proxy statements and other information with the Commission and our filings are available to the public over the Internet at the Commission's website at http://www.sec.gov. The public may read and copy any materials filed by us with the Commission at the Public Reference Room at 100 F Street NE, Washington, D.C. 20549, on official business days during the hours of 10:00 am to 3:00 pm. The public may obtain information on the operation of the Public Reference Room by calling the Commission at 800-732-0330. You
may obtain further information about our Company at our websites: http://www.therapeuticsmd.com and http://www.vitamedmd.com.
23
RISK FACTORS
INVESTING IN OUR COMMON STOCK INVOLVES A HIGH DEGREE OF RISK. PROSPECTIVE INVESTORS SHOULD CAREFULLY CONSIDER THE RISKS DESCRIBED BELOW, TOGETHER WITH ALL OF THE OTHER INFORMATION INCLUDED IN OR REFERRED TO IN THIS REPORT, BEFORE PURCHASING SHARES OF OUR COMMON STOCK. THERE ARE NUMEROUS AND VARIED RISKS, KNOWN AND UNKNOWN, THAT MAY PREVENT US FROM ACHIEVING OUR GOALS. THE RISKS DESCRIBED BELOW ARE NOT THE ONLY ONES WE WILL FACE. IF ANY OF THESE RISKS ACTUALLY OCCUR, OUR BUSINESS, FINANCIAL CONDITION OR RESULTS OF OPERATION MAY BE MATERIALLY ADVERSELY AFFECTED. IN SUCH CASE, THE TRADING
PRICE OF OUR COMMON STOCK COULD DECLINE AND INVESTORS IN OUR COMMON STOCK COULD LOSE ALL OR PART OF THEIR INVESTMENT.
Risks Related to VitaMed's Business and Industry
We have a limited operating history and have losses which we expect to continue into the future. There is no assurance our future operations will result in profitable revenues. If we cannot generate sufficient revenues to operate profitably, we may suspend or cease operations.
VitaMed was organized in Delaware in May 13, 2008. We have a limited operating history as we have been selling our products for less than two years. To date, our efforts have been primarily focused on the development and testing of our products and business model. We have concentrated in building our customer base and our brand name in selected markets, primarily in Florida. We face many of the risks and difficulties inherent in introducing new products and services. Through June 30, 2011, our net loss from inception is approximately $6,359,000. Our ability to achieve and maintain profitability and positive cash flow is dependent, among other things, upon:
|
·
|
Further development and launching of our proprietary technology,
|
|
·
|
acceptance of our products by physicians and consumers,
|
|
·
|
our ability to negotiate satisfactory payment agreements with insurance companies, and
|
|
·
|
our ability to continue to manage satisfactory relationships with manufacturers of our products.
|
Based upon current plans, we expect to incur operating losses in future periods because we expect to incur expenses which will exceed revenues for an unknown period of time. We can provide no assurance that we will be successful in generating sufficient revenues to support operations in the future. Failure to generate sufficient revenues may cause us to go out of business and you could lose your investment.
Based on our historical financials, there is uncertainty as to our ability to continue as a going concern.
In the event that we are unable to achieve or sustain profitability or are otherwise unable to secure external financing, we may not be able to meet our obligations as they come due, raising substantial doubts as to our ability to continue as a going concern. Any such inability to continue as a going concern may result in our security holders losing their entire investment. Our financial statements, which have been prepared in accordance with generally accepted accounting principles, contemplate that we will continue as a going concern and do not contain any adjustments that might result if we were unable to continue as a going concern. Notwithstanding the foregoing, our cash flow
deficiencies raise substantial doubt as to our ability to continue as a going concern. Also, our existing and anticipated working capital needs, the acceleration or modification of our expansion plans, lower than anticipated revenues, or increased expenses or other events will affect our ability to continue as a going concern.
24
We anticipate incurring operating losses and negative cash flows in the foreseeable future resulting in uncertainty of future profitability and limitation on our operations.
We anticipate that we will continue to incur operating losses and negative cash flows in the foreseeable future and will accumulate increasing deficits as we increase our expenditures for (i) infrastructure, (ii) sales and marketing, (iii) inventory, (iv) personnel, and (v) general operating expenses. Any increases in our operating expenses will require us to achieve significant revenue before we can attain profitability. In the event that we are unable to achieve profitability or raise sufficient funding to cover our losses, we may not be able to meet our obligations as they come due, raising substantial doubts as to our ability to continue as a going concern.
We will need substantial additional funding and may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs, commercialization efforts or acquisition strategy.
We have made significant investments for sales, marketing, securing commercial quantities of our current products from our manufacturers, and distribution. We expect to use revenue from sales of our current products to fund a portion of the costs for establishing and expanding our sales and marketing infrastructure and for future product development. However, we will need additional funding for these purposes and may be unable to raise capital when needed or on attractive terms which would force us to delay, reduce or eliminate our development programs or commercialization efforts.
As of June 30, 2011, we had approximately $78,000 of cash and cash equivalents. We believe that our existing cash and cash equivalents, along with revenues from product sales, will not be sufficient to enable us to fund our operating expenses and capital expenditure requirements for the next twelve (12) months. To the extent this capital is insufficient to meet our future capital requirements, we will need to finance our cash needs through public or private equity offerings, debt financings, corporate collaboration and licensing arrangements or other financing alternatives. Additional equity or debt financing, or corporate
collaboration and licensing arrangements, may not be available on acceptable terms, if at all.
We intend to raise additional funds by issuing equity securities and our stockholders will experience dilution. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any debt financing or additional equity that we raise may contain terms, such as liquidation and other preferences, which are not favorable to us or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish valuable rights to our technologies, future revenue streams or product candidates or
to grant licenses on terms that may not be favorable to us. If additional financing is not available when required or is not available on acceptable terms, we may be unable to fund expansion, successfully promote our brand name, develop or enhance our services, take advantage of business opportunities, or respond to competitive pressures or unanticipated requirements, any of which could seriously harm our business and reduce the value of your investment.
The commercial success of our products will depend upon the degree of market acceptance by physicians, patients, healthcare payers and others in the medical community.
Any products that we bring to the market may not gain market acceptance by physicians, patients, healthcare payers and others in the medical community. If our products do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not be profitable. The degree of market acceptance of our products is primarily dependent on the willingness of physicians to prescribe these therapies and the willingness of the target patient population to use our products over the products of our competition.
25
We may not be able to build our brand awareness and successfully market our products.
Development and awareness of our brand will depend largely upon our success in increasing our customer base. In order to attract and retain customers and to promote and maintain our brand in response to competitive pressures, management plans to significantly increase our marketing and advertising budgets particularly in our field sales force. If we are unable to economically promote or maintain our brand, our business, results of operations and financial condition could be severely harmed.
Our business may be affected by unfavorable publicity or lack of consumer acceptance.
We are highly dependent upon consumer acceptance of the safety, efficacy and quality of our products, as well as similar products distributed by other companies. Consumer acceptance of a product can be significantly influenced by scientific research or findings, national media attention and other publicity about product use. A product may be received favorably resulting in high sales associated with that product that may not be sustainable as consumer preferences change. Future scientific research or publicity could be unfavorable to our industry or any of our particular products and may not be consistent with earlier favorable research or publicity. A
future research report or publicity that is perceived by our consumers as less than favorable or that may question earlier favorable research or publicity could have a material adverse effect on our ability to generate revenue. Adverse publicity in the form of published scientific research, statements by regulatory authorities or otherwise, whether or not accurate, that associates consumption of our product or any other similar product with illness or other adverse effects, or that questions the benefits of our product or a similar product, or that claims that such products are ineffective could have a material adverse effect on our business, reputation, financial condition or results of operations.
Our success is dependent on market acceptance.
Our product may not achieve sufficient market acceptance to allow us to become profitable. To be successful, we must develop and market products that are widely accepted at profitable prices. A failure to develop acceptable product offerings will adversely affect our revenues and ability to achieve profitability.
Our success depends on how efficiently we respond to changing consumer preferences and demand.
Our continued success depends, in part, on our ability to anticipate and respond to changing consumer trends and preferences. We may not be able to respond in a timely or commercially appropriate manner to these changes. Our failure to accurately predict these trends could negatively impact our inventory levels, sales and consumer opinion of us as a source for the latest product. The success of our new product offerings depends upon a number of factors, including our ability to:
· accurately anticipate customer needs;
· innovate and develop new products;
· successfully commercialize new products in a timely manner;
· competitively price our product in the market;
· procure and maintain product in sufficient volumes and in a timely manner; and
· differentiate our product offerings from those of our competitors.
If we do not introduce new products, make enhancements to existing products or maintain the appropriate inventory levels to meet customers’ demand in a timely manner, our business, results of operations and financial condition could be materially and adversely affected.
26
Our success is tied to the adequacy of the Internet infrastructure.
Our future revenues and profits, if any, substantially depend upon the continued widespread use of the Internet as an effective medium of business and communication. Certain factors over which we have no control may negatively affect our business. Factors which could reduce the widespread use of the Internet include:
|
·
|
actual or perceived lack of security of information or privacy protection;
|
|
·
|
possible disruptions, computer viruses or other damage to the Internet servers or to users' computers; and
|
|
·
|
excessive governmental regulation.
|
The content of our website could expose us to significant liability.
Because we post product information and other content on our website, we face potential liability for, among other things, copyright infringement, patent infringement, trademark infringement, defamation, unauthorized practice of medicine, false or misleading advertising and other claims based on the nature and content of the materials we post. Although we maintain general liability insurance, our insurance may not cover potential claims of this type or may not be adequate to indemnify us for all liability that may be imposed. Any imposition of liability that is not covered by insurance, or is in excess of insurance coverage, could materially adversely affect our
business, financial condition or results of operations.
The requirement to protect our customers’ personal information may subject us to potential liability.
If our customers’ personal or credit card information is misappropriated by us or third parties that breach our network security, we could be subject to liability. This liability could include claims for unauthorized purchases with credit card information, impersonation, or similar fraud claims for alleged violations of state or federal laws governing security protocols for the safekeeping of customers’ personal or credit card information. This liability could also include claims for other misuses of personal information, including unauthorized marketing purposes. These claims could result in litigation against us. Liability for misappropriation of this
information could adversely affect our business, financial condition or operating results. In addition, the FTC and state agencies have been investigating various Internet companies regarding their use of customers’ personal information. We could incur additional expenses if new regulations regarding the use of personal information are introduced or if government agencies investigate our privacy practices.
We rely on encryption and authentication technology licensed from third parties to provide the security and authentication necessary to effect secure transmission of confidential information such as customer credit card numbers. We cannot provide assurance that advances in computer capabilities, new discoveries in the field of cryptography or other events or developments will not result in a compromise or breach of the algorithms that we use to protect our customers’ transaction data. If any such compromise of our security were to occur, it could harm our reputation, business, prospects, financial condition and results of operations. A party who is able to
circumvent our security measures could misappropriate proprietary information or cause interruptions in our operations. We may be required to expend significant capital and other resources to protect against such security breaches or to resolve problems caused by such breaches. We cannot assure you that our security measures will prevent security breaches or that failure to prevent such security breaches will not harm our business, financial condition or results of operations.
27
We are subject to regulations relating to privacy and data.
We are subject to increasing regulation at the federal, state and international levels relating to privacy and the use of personal user information. For example, we are subject to various telemarketing laws that regulate the manner in which we may solicit future suppliers and customers. Such regulations, along with increased governmental or private enforcement, may increase the cost of growing our business. In addition, many jurisdictions have laws that limit the use of personal information gathered or require companies to establish privacy policies. The FTC has adopted regulations regarding the collection and use of personal identifying information obtained from
children under thirteen years of age. Proposed legislation in this country and existing laws in foreign countries require companies to establish procedures to notify users of privacy and security policies, obtain consent from users for the collection and use of personal information, and/or provide users with the ability to access, correct and delete personal information stored by us. From time to time, Congress has proposed legislation regarding data security and privacy protection. Any enacted data protection regulations may restrict our ability to collect demographic and personal information which could be costly or harm our marketing efforts and could require us to implement new and potentially costly processes, procedures and/or protective measures.
Our network and communications systems are vulnerable to system interruption and damage.
Our ability to receive and fulfill orders promptly and accurately is critical to our success and largely depends on the efficient and uninterrupted operation of our computer and communications hardware and software systems. We may experience periodic system interruptions that impair the performance of our transaction systems or make our website inaccessible to our customers. These system interruptions may prevent us from efficiently accepting and fulfilling orders, sending out promotional emails and other customer communications in a timely manner, introducing new products and features on our website, promptly responding to customers, or providing services to third
parties. Frequent or persistent interruptions in our services could cause current or potential customers to believe that our systems are unreliable which could cause them to avoid our website, drive them to our competitors and harm our reputation. To minimize future system interruptions, we must continue to add software and hardware, improve our systems and network infrastructure to accommodate increases in website traffic and sales volume, and replace aging hardware and software. We may be unable to promptly and effectively upgrade and expand our systems and integrate additional functionality into our existing systems. In addition, upgrades to our system may cause existing systems to fail or operate incorrectly. Any unscheduled interruption in our services could result in fewer orders, additional operating expenses, or reduced customer satisfaction, any of which would harm our
business, financial condition and operating results. In addition, the timing and cost of upgrades to our systems and infrastructure may substantially affect our ability to maintain profitability.
Our systems and operations, and those of our suppliers and Internet service providers, are vulnerable to damage or interruption from fire, flood, earthquakes, power loss, server failure, telecommunications and Internet service failure, acts of war or terrorism, computer viruses and denial-of-service attacks, physical or electronic break-ins, sabotage, human error and similar events. Any of these events could lead to system interruptions, order fulfillment delays, and loss of critical data for us, our suppliers, or our Internet service providers, and could prevent us from accepting and fulfilling customer orders. Any significant interruption in the availability or
functionality of our website or our customer processing, distribution, or communications systems, for any reason, could seriously harm our business, financial condition, and operating results. The occurrence of any of these factors could have a material adverse effect on our business, financial condition or results of operations.
28
We are subject to a number of risks related to credit cards.
We expect a majority of our Internet orders will be paid for using a credit card or debit card. For credit and debit card payments, we pay interchange and other fees which may increase over time and raise our operating costs and lower our profit margins. We are also subject to payment card association operating rules, certification requirements and rules governing electronic funds transfers which could change or be reinterpreted to make it difficult or impossible for us to comply. If we fail to comply with these rules or requirements, we may be subject to fines and higher transaction fees and lose our ability to accept credit and debit card payments from our
customers. In addition, we have and may continue to suffer losses as a result of orders placed with fraudulent credit and debit card data. We do not carry insurance against the risk of credit card fraud, so the failure to adequately control fraudulent credit card transactions could reduce our net revenue and our gross profit percentage. We have implemented technology to help us detect the fraudulent use of credit card information. Under current practices, a merchant is liable for fraudulent credit card transactions when the merchant does not obtain a cardholder’s signature. A failure to adequately control fraudulent credit card transactions would result in significantly higher credit card-related costs and could have a material adverse effect on our business, financial condition or results of operations.
We may be affected by the inability of our retail customers to access consumer credit.
Many of our customers use credit cards to pay for our product and services. Because of the current global economic downturn, credit card issuers have tightened their consumer lending standards which has resulted in decreased credit card limits and increased interest rates and fees. If our customers lose access to consumer credit or determine that the use of credit is prohibitively expensive, our business, financial condition or results of operations could be materially and adversely affected.
Failure to manage growth effectively could prevent us from achieving our goals.
Our strategy envisions a period of rapid growth that may impose a significant burden on our administrative and operational resources. Our ability to effectively manage growth will require us to substantially expand the capabilities of our administrative and operational resources and to attract, train, manage and retain qualified management and other personnel. Our failure to successfully manage growth could result in our sales not increasing commensurately with capital investments. Our inability to successfully manage growth could materially adversely affect our business.
Any failure to adequately expand a direct sales force will impede our growth.
We expect to be substantially dependent on a direct sales force to attract new business and to manage customer relationships. We plan to expand our direct sales force and believe that there is significant competition for qualified, productive direct sales personnel with advanced sales skills and technical knowledge. Our ability to achieve significant growth in revenue in the future will depend, in large part, on our success in recruiting, training and retaining sufficient direct sales personnel. Recent hires and planned hires may not become as productive as expected and we may be unable to hire sufficient numbers of qualified individuals in the future in the markets where we do business. While there
presently exists a high rate of unemployment, if we are unable to hire and develop sufficient numbers of productive sales personnel our business prospects could suffer.
If we are unable to attract, hire and retain qualified sales and management personnel, the commercial opportunity for our products may be diminished.
Currently, our sales force consists of 27 full-time sales representatives. We may not be able to attract, hire, train and retain qualified sales and sales management personnel. If we are not successful in our efforts to maintain and grow a qualified sales force, our ability to independently market and promote our products may be impaired. In such an event, we would likely need to establish collaboration, co-promotion, distribution or other similar arrangement to market and sell our products. However, we might not be able to enter into such an arrangement on favorable terms, if at all. Even if we are able to effectively maintain a qualified sales force, our sales
force may not be successful in commercializing our products.
29
If we fail to attract and retain key personnel, or to retain our executive management team, we may be unable to successfully develop or commercialize our products.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified managerial personnel. We are highly dependent upon our executive management team. The loss of the services of any one or more of the members of our executive management team could delay or prevent the successful completion of some of our development and commercialization objectives.
Recruiting and retaining qualified sales and marketing personnel is critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our development and commercialization strategy. Our consultants and advisors may also be employed by companies and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
We may encounter difficulties in managing our growth which could disrupt our operations.
To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the inexperience of our management team in managing a company during a period of such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business
plans or disrupt our operations.
We are attempting to grow our business rapidly in terms of the geographical size of our distribution and the number of customers we serve. The execution of our business plan will require substantial growth in terms of management and operations. There can be no assurance that we will be able to effectively manage any expansion of our business. Management’s inability to manage our growth effectively could have a material adverse effect on our business, financial condition and results of operations. Our
failure to manage growth effectively could result in increased costs and harm our ability to introduce additional markets resulting in a reduction of expected revenue.
There is no assurance that we will successfully manage our efforts to:
• expand, train, manage and retain our employee base
• expand and improve our customer service and support systems and distribution network
• increase our manufacturing capabilities while controlling cost of goods sold
• capitalize on new opportunity in the competitive marketplace
• control our expenses
The strains posed by these demands are magnified by the emerging nature of our operations. Any significant growth will place a strain on our operational, human and financial resources and will also increase our operating complexity as well as the level of responsibility for both existing and new management personnel. Our ability to effectively manage our growth will depend on the continued development of plans, systems and controls for our operational, financial and management needs and on our ability to expand, train and manage our employee base.
30
A failure to maintain optimal inventory levels to meet commercial demand for our products could harm our reputation and subject us to financial losses.
Our ability to maintain optimal inventory levels to meet commercial demand depends on the performance of third-party contract manufacturers. In some instances our products have unique ingredients used under license arrangements. If our manufacturers are unsuccessful in obtaining raw materials, if we are unable to manufacture and release inventory on a timely and consistent basis, if we fail to maintain an adequate level of product inventory, if inventory is destroyed or damaged or if our inventory reaches its expiration date, patients might not have access to our products, our reputation and brands could be harmed and physicians may be less
likely to recommend our products in the future, each of which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Product liability lawsuits against us could cause us to incur substantial liabilities and limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the sale of our currently marketed products and any other products that we develop. If we cannot successfully defend ourselves against claims that our products caused injuries, we will incur substantial liabilities. Any product liability claim against us could result in increased costs and adversely affect our reputation with our customers, which in turn could adversely affect our business, financial condition or results of operations.
The amount of insurance that we currently hold may not be adequate to cover all liabilities that we may incur. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost and we may not be able to obtain insurance coverage that will be adequate to satisfy any liability that may arise. We cannot assure you that our insurance will be sufficient to cover our losses. We do not view insurance, by itself, as a material mitigant to these business risks. Any losses that are not completely covered by our insurance could have a material adverse effect on our business, financial condition or results of
operations.
We may be subject to product recalls that could negatively affect our business.
We may be subject to product recalls, withdrawals or seizures if any of the products we formulate, manufacture or sell are believed to cause injury or illness or if we are alleged to have violated governmental regulations in the manufacture, labeling, promotion, sale or distribution of any of our products. A recall, withdrawal or seizure of any of our products could materially and adversely affect consumer confidence in our brands and lead to decreased demand for our products. In addition, a recall, withdrawal or seizure of any of our products would require significant management attention, would likely result in substantial and unexpected expenditures and could
materially and adversely affect our business, financial condition or results of operations.
If we are unable to obtain and maintain protection for intellectual property relating to our technology and products, the value of our technology and products will be adversely affected.
Our success will depend in part on our ability to obtain and maintain protection for the intellectual property covering or incorporated into our technology and products. The patent situation in the field of pharmaceuticals is highly uncertain and involves complex legal and scientific questions. We rely upon patents, trade secret laws and confidentiality agreements to protect our technology and products. We may not be able to obtain patent rights relating to our technology or products and pending patent applications to which we have rights may not issue as patents or if issued, may not issue in a form that will be advantageous to us. Even if issued, any patents
issued to us may be challenged, narrowed, invalidated, held to be unenforceable or circumvented. Changes in either patent laws or in interpretations of patent laws in the United States may diminish the value of our intellectual property or narrow the scope of our patent protection. Currently we do not have any approved patents on our products or technology although we have one patent pending for our OPERA™ information reporting system.
31
Trademark protection of our products may not provide us with a meaningful competitive advantage.
We use trademarks on most of our currently marketed products and believe that having distinctive marks is an important factor in marketing those products. Distinctive marks may also be important for any additional products that we successfully develop and commercially market; however, we generally do not expect our marks to provide a meaningful competitive advantage over other branded or generic products. We believe that efficacy, safety, convenience, and price are and are likely to continue to be more important factors in the commercial success of our products. If we initiate legal proceedings to seek to protect our trademarks, the costs of these proceedings could
be substantial and it is possible that our efforts could be unsuccessful.
Proposed federal regulation may subject us to future taxation risks.
We do not collect sales or other taxes on shipments of most of our goods into most states in the U.S. Proposed federal legislation would subject each facility used in the manufacture and distribution of dietary supplements to an annual tax and reporting requirement. The proceeds of the tax would be dedicated to increased inspections of companies that manufacture, distribute and hold dietary supplements. Taxes of this kind could adversely affect our ability to remain in business, could restrict the type or kind of product we sell or could require significant expenditures to ensure compliance. Currently, U.S. Supreme Court decisions restrict the imposition of
obligations to collect state and local sales and use taxes with respect to sales made over the Internet. However, a number of states, as well as the U.S. Congress, have been considering initiatives that could limit or supersede the Supreme Court’s position regarding sales and use taxes on Internet sales. If any of these initiatives were successful, we could be required to collect sales and use taxes in additional states. The imposition by state and local governments of various taxes upon Internet commerce could create administrative burdens for us, reduce our competitive advantage over traditional retailers and decrease our future sales. Our warehousing and expected manufacturing centers, and any future expansion of them, along with other aspects of our evolving business, may result in additional sales and other tax obligations. One or more states or foreign countries may seek to
impose sales or other tax collection obligations on out-of-jurisdiction eCommerce companies. Effective June 2008, New York imposed such a sales tax obligation requirement on online retailers that use New York residents to directly or indirectly refer potential customers, via a link on an Internet website or otherwise, to the online retailer. A successful assertion by one or more states or foreign countries that we should collect sales or other taxes on the sale of merchandise or services could result in substantial tax liabilities for past sales, decrease our ability to compete with traditional retailers and otherwise harm our business, financial condition or results of operations.
Our relationships with customers and payors are subject to applicable laws related to fraud, abuse and other healthcare regulations which could expose us to criminal sanctions, civil penalties, contractual damages, reputation harm, and diminished profits and future earnings.
Healthcare providers, physicians and others play a primary role in the recommendation and prescription of our products. Our arrangements with third party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products.
32
Applicable federal and state healthcare laws and regulations, include, but are not limited to, the following:
|
·
|
The federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid.
|
|
·
|
The Ethics in Patient Referrals Act, commonly referred to as the Stark Law, and its corresponding regulations, prohibit physicians from referring patients for designated health services reimbursed under the Medicare and Medicaid programs to entities with which the physicians or their immediate family members have a financial relationship or an ownership interest, subject to narrow regulatory exceptions.
|
|
·
|
The federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government.
|
|
·
|
HIPAA imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information.
|
|
·
|
The federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services.
|
|
·
|
Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government.
|
Efforts to ensure that our business arrangements with third parties comply with applicable healthcare laws and regulations could be costly. Although VitaMed's regulatory counsel has assisted VitaMed in establishing business practices compliant with applicable laws, it is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our past or present operations, including activities conducted by our sales team or agents, are found to be in violation of any of these laws or any other
governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from third party payor programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians, providers or entities with whom we do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Many aspects of these laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of subjective interpretations which increases the risk of potential violations. In addition, these laws and their interpretations are subject to change. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and damage our reputation.
33
We are required to comply with new and existing government regulation.
The processing, formulation, manufacturing, packaging, labeling, advertising, distribution and sale of our product are subject to regulation by several U.S. federal agencies, including the FDA, the Federal Trade Commission, or FTC, the Postal Service, the Consumer Product Safety Commission, the Department of Agriculture and the Environmental Protection Agency, as well as various state, local and international laws and agencies of the localities in which our product are sold. Government regulations may prevent or delay the introduction or require the reformulation of our product.
The FDA regulates, among other things, the manufacture, composition, safety, labeling, marketing and distribution of prescription medicine, medical foods, dietary supplements and food products. The FDA may also determine that certain advertising and promotional claims, statements or activities are not in compliance with applicable laws and regulations and may determine that a particular statement is an unacceptable drug claim or an unauthorized version of a food or dietary supplement "health claim." Failure to comply with FDA or other regulatory requirements could prevent us from marketing particular dietary supplement product or subject us to
administrative, civil or criminal penalties.
The FTC exercises jurisdiction over the advertising of dietary supplements and has instituted numerous enforcement actions against dietary supplement companies for failing to have adequate substantiation for claims made in advertising or for using false or misleading advertising claims. The FTC routinely polices the market for deceptive dietary supplement advertising and accepts and reviews complaints from the public concerning such advertising. The FTC also regulates deceptive advertising claims and promotional offers of savings compared to "regular" prices. The National Advertising Division, or NAD, of the Council of Better Business Bureaus oversees an
industry-sponsored self-regulatory system that permits competitors to resolve disputes over advertising claims, including promotions for savings off of regular prices. The NAD has no enforcement authority of its own but may refer promotions to the FTC that the NAD views as violating FTC guides or rules. Violations of these orders could result in substantial monetary penalties.
If the estimates that we make, or the assumptions upon which we rely, in preparing our financial statements prove inaccurate, our future financial results may vary from expectations.
Our financial statements have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of our financial statements requires us to make estimates and judgments that affect the reported amounts of our assets, liabilities, stockholders’ equity, revenues and expenses, the amounts of charges accrued by us and related disclosure of contingent assets and liabilities. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. We cannot assure you, therefore, that there may not be material fluctuations between our estimates and the
actual results.
The current global economic downturn or recession may negatively affect our business.
The current global economic downturn or recession could negatively affect our sales because many consumers consider the purchase of our products discretionary. We cannot predict the timing or duration of the economic slowdown or recession or the timing or strength of a subsequent recovery, worldwide, or in the specific end markets we serve. If the markets for our product significantly deteriorate due to the economic situation, our business, financial condition or results of operations could be materially and adversely affected.
34
Risks Related to VitaMed's Dependence on Third Parties
We use third parties to manufacture all of our products and product candidates. This may increase the risk that we will not have sufficient quantities of our products or such quantities at an acceptable cost, which could result in development and commercialization of our products being delayed, prevented or impaired.
We do not own or operate, and do not currently have plans to establish, any manufacturing facilities for our products. We have limited personnel with experience in product manufacturing and we do not have the facilities and capabilities to manufacture any of our products on a commercial scale. We currently rely, and expect to continue to rely, on third parties for the supply of the ingredients in our products and for the manufacture of the finished forms of these products and packaging. The current manufacturers of our products are, and any future third party manufacturers that we enter into arrangements with will likely be, our sole suppliers of our
products for a significant period of time. These manufacturers are commonly referred to as single source suppliers for each individual product.
If any of these manufacturers should become unavailable to us for any reason, we may be unable to conclude arrangements with replacements on favorable terms, if at all, and may be delayed in identifying and qualifying such replacements. In any event, identifying and qualifying a new third party manufacturer could involve significant costs associated with the transfer of the ingredients or finished product manufacturing process. Reliance on third party manufacturers entails risks that we would not be subject to if we manufactured our own products, including:
| ● | reliance on third party for regulatory compliance and quality assurance; | |
| ● | the possible breach of the manufacturing arrangement by the third party because of factors beyond our control; and | |
| ● | the possible termination or nonrenewal of the manufacturing relationship by the third party, based on its own business priorities, at a time that is costly or inconvenient for us. |
Our products may compete with other products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that are both capable of manufacturing for us and willing to do so. If the third parties that we engage to manufacture a product for commercial sale should cease to continue to do so for any reason, we likely would experience delays in obtaining sufficient quantities of our products to meet commercial demand while we identify and qualify replacement suppliers. Our current and anticipated future dependence upon others for the manufacture of our products may adversely affect our profit
margins and our ability to develop and commercialize products on a timely and competitive basis.
We rely on our third party manufacturers for compliance with applicable regulatory requirements. This may increase the risk of sanctions being imposed on us or on a manufacturer of our products which could result in our inability to obtain sufficient quantities of these products.
Our manufacturers may not be able to comply with cGMP regulations or other regulatory requirements or similar regulatory requirements outside the United States. Our manufacturers are subject to registration requirements and unannounced inspections by the FDA. Our failure, or the failure of our third party manufacturers to comply with applicable requirements could result in sanctions being imposed on us.
Any sanctions could significantly and adversely affect supplies of our products, our business and our financial condition. Furthermore, if we fail to obtain the required commercial quantities on a timely basis and at commercially reasonable prices, we may be unable to meet demand for our products, if approved, and would lose potential revenues.
35
Our dependence on third party manufacturers may cause an unexpected interruption or shortage in, or a significant increase, in the cost of raw materials used to manufacture our products.
An unexpected interruption of supply or a significant increase in the cost of raw materials, whether to us or to our contract manufacturers for any reason, such as regulatory requirements, import restrictions, loss of certifications, disruption of distribution channels as a result of weather, terrorism or acts of war, or other events, could result in significant cost increases and/or shortages of our product. Our inability to obtain a sufficient amount of product or to pass through higher cost of products we offer could have a material adverse effect on our business, financial condition or results of operations.
Risks Related to the Company's Common Stock
FINRA sales practice requirements may limit a stockholder's ability to buy and sell our stock.
The Financial Industry Regulatory Authority ("FINRA") has adopted rules that relate to the application of the Commission's penny stock rules in trading our securities and require that a broker/dealer have reasonable grounds for believing that the investment is suitable for that customer, prior to recommending the investment. Prior to recommending speculative, low priced securities to their non-institutional customers, broker/dealers must make reasonable efforts to obtain information about the customer's financial status, tax status, investment objectives and other information. Under interpretations of these rules, the FINRA believes that there is a high probability that speculative, low priced
securities will not be suitable for at least some customers. The FINRA requirements make it more difficult for broker/dealers to recommend that their customers buy our Common Stock which may have the effect of reducing the level of trading activity and liquidity of our Common Stock. Further, many brokers charge higher transactional fees for penny stock transactions. As a result, fewer broker/dealers may be willing to make a market in our Common Stock thereby reducing a shareholder's ability to resell shares of our Common Stock.
If we fail to comply with the rules under the Sarbanes-Oxley Act related to accounting controls and procedures or if material weaknesses or other deficiencies are discovered in our internal accounting procedures, our stock price could decline significantly.
Section 404 of the Sarbanes-Oxley Act requires annual management assessments of the effectiveness of our internal controls over financial reporting and a report by our independent auditors addressing these assessments. We are in the process of documenting and testing our internal control procedures, and we may identify material weaknesses in our internal control over financial reporting and other deficiencies. If material weaknesses and deficiencies are detected, it could cause investors to lose confidence in our Company and result in a decline in our stock price and consequently affect our financial condition. In addition, if we fail to
achieve and maintain the adequacy of our internal controls, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act. Moreover, effective internal controls, particularly those related to revenue recognition, are necessary for us to produce reliable financial reports and are important to helping prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our Common Stock could drop significantly. In addition, we cannot be certain that additional material weaknesses or significant deficiencies in our internal controls will not be discovered
in the future.
Because there is a limited public trading market for our Common Stock, you may not be able to resell your stock.
There is currently a limited public trading market for our Common Stock and there is no assurance that a more active trading market will ever develop. As such, you may have to hold your shares for an extended period of time before you are able to sell them, if at all.
36
Our Board of Directors may issue and fix the terms of shares of our Preferred Stock without stockholder approval, which could adversely affect the voting power of holders of our Common Stock or any change in control of our Company.
Our Articles of Incorporation authorize the issuance of up to 10,000,000 shares of "blank check" preferred stock, $0.001 par value per share (the "Preferred Stock"), with such designation rights and preferences as may be determined from time to time by the Board of Directors. Our Board of Directors is empowered, without shareholder approval, to issue shares of Preferred Stock with dividend, liquidation, conversion, voting or other rights which could adversely affect the voting power or other rights of the holders of our Common Stock. In the event of such issuances, the Preferred Stock could be used, under certain circumstances, as a method
of discouraging, delaying or preventing a change in control of our Company.
Our shares are considered "penny stocks" which imposes additional sales practice requirements on broker/dealers; as such many broker/dealers may not want to make a market in our shares which could affect your ability to sell your shares in the future.
Our shares are considered "penny stocks" covered by section 15(g) of the Exchange Act, and Rules 15g-1 through 15g-6 promulgated thereunder, which imposes additional sales practice requirements on broker/dealers who sell our securities to persons other than established customers and accredited investors (generally institutions with assets in excess of $5,000,000 or individuals with net worth in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly with their spouses). Since our shares are covered by section 15(g) of the Securities Exchange Act of 1934, many broker/dealers may not want to make a market in our shares or conduct any transactions in our shares. As such, your ability
to dispose of your shares may be adversely affected.
Future sales by our stockholders may negatively affect our stock price and our ability to raise funds in new stock offerings.
Sales of our Common Stock in the public market could lower the market price of our Common Stock. Sales may also make it more difficult for us to sell equity securities or equity-related securities in the future at a time and price that our management deems acceptable or at all. Of the 58,573,187 shares of Common Stock outstanding after the Closing of the Merger, 31,985 shares are freely tradable without restriction by stockholders who are not our affiliates. The remaining 58,541,202 shares of Common Stock are "restricted securities" with an aggregate of 37,649,371 shares held by our affiliates, all of which shares may be resold in the public market only
when released from the provisions of a lock up agreement, when and if registered pursuant to an exemption from registration, or pursuant to the applicable requirements of Rule 144.
We do not expect to pay dividends and investors should not buy our Common Stock expecting to receive dividends.
We do not anticipate that we will declare or pay any dividends in the foreseeable future. Consequently, you will only realize an economic gain on your investment in our Common Stock if the price appreciates. You should not purchase our Common Stock expecting to receive cash dividends. Since we do not pay dividends, and if we are not successful in establishing an orderly trading market for our shares, then you may not have any manner to liquidate or receive any payment on your investment. Therefore our failure to pay dividends may cause you to not see any return on your investment even if we are successful in our business operations. In addition, because we do not
pay dividends we may have trouble raising additional funds which could affect our ability to expand our business operations.
37
Securities analysts may not cover our Common Stock and this may have a negative impact on our Common Stock’s market price.
The trading market for our Common Stock may depend on the research and reports that securities analysts publish about us or our business. We do not have any control over these analysts. There is no guarantee that securities analysts will cover our Common Stock. If securities analysts do not cover our Common Stock, the lack of research coverage may adversely affect our Common Stock’s market price, if any. If we are covered by securities analysts who downgrade our stock, our stock price would likely decline. If one or more of these analysts ceases to cover us or fails to publish regularly reports on us, we could lose visibility in the financial markets, which could cause our stock price or trading volume to
decline.
We are likely to raise additional funds, finance acquisitions or develop strategic relationships by issuing capital stock.
We have financed our operations, and we expect to continue to finance our operations, acquisitions and develop strategic relationships, by issuing equity or convertible debt securities, which could significantly reduce the percentage ownership of our existing stockholders. Furthermore, any newly issued securities could have rights, preferences and privileges senior to those of our existing Common Stock. Moreover, any issuances by us of equity securities may be at or below the prevailing market price of our Common Stock and in any event may have a dilutive impact on your ownership interest, which could cause the market price of our Common Stock to decline.
We may also raise additional funds through the incurrence of debt, and the holders of any debt we may issue would have rights superior to your rights in the event we are not successful and are forced to seek the protection of the bankruptcy laws.
A significant business or product announcement by us or our competitors may cause fluctuations in our stock price.
The market price of our Common Stock may be subject to substantial volatility as a result of announcements by us or other companies in our industry, including our collaborators. Announcements that may subject the price of our Common Stock to substantial volatility include announcements regarding:
· our operating results, including the amount and timing of sales of our products;
· the availability and timely delivery of a sufficient supply of our products;
· the acquisition of technologies or products by us or our competitors;
· the development of new technologies or products by us or our competitors;
· regulatory actions with respect to our products or those of our competitors; and
· significant acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors.
Insiders have substantial control over the outstanding shares of the Company's Common Stock and could delay or prevent a change in corporate control, including a transaction in which the combined company’s stockholders could sell or exchange their shares for a premium.
Our directors and executive officers beneficially own an aggregate of approximately 65% of our outstanding shares of Common Stock. As a result, our directors and executive officers, if acting together, have the ability to affect the outcome of matters submitted to stockholders for approval, including the election and removal of directors and any merger, consolidation or sale of all or substantially all of our assets. In addition, these persons acting together will have the ability to control our management and affairs. Accordingly, this concentration of ownership may harm the value of our Common Stock by:
38
· delaying, deferring or preventing a change in control;
· impeding a merger, consolidation, takeover or other business combination; or
· discouraging a potential acquirer from making an acquisition proposal or otherwise attempting to obtain control.
Resale of shares of our Common Stock could materially adversely affect the market price of our Common Stock.
We issued an aggregate of 58,407,331 shares of Common Stock to the members of VitaMed pursuant to the Merger. These shares were issued pursuant to an exemption from the registration requirements of the 1933 Act and are therefore "restricted securities" as defined in Rule 144. In addition to being subject to restrictions on transfer imposed under federal securities laws, each holder of the newly issued shares entered into a lock up agreement, which among other things, restricts the sale or transfer of these shares for specified periods. Although we have no current plans to do so, we may waive the restrictions on transfer under these lock
up agreements in the future. When the shares covered under the lock up agreements become available for resale, sales of a substantial number of shares of our Common Stock in the public market, or the perception that these sales could occur, could materially adversely affect the market price of our Common Stock.
Our operating results are likely to fluctuate from period to period.
We anticipate that there may be fluctuations in our future operating results. Potential causes of future fluctuations in our operating results may include:
· period-to-period fluctuations in financial results;
· issues in manufacturing products;
· unanticipated potential product liability claims;
· the introduction of technological innovations or new commercial products by competitors;
· the entry into, or termination of, key agreements, including key strategic alliance agreements;
· the initiation of litigation to enforce or defend any of our intellectual property rights;
· the loss of key employees;
· regulatory changes;
· failure of any of our products to achieve commercial success;
· general and industry-specific economic conditions that may affect research and development expenditures;
· future sales of our Common Stock; and
· changes in the structure of healthcare payment systems resulting from proposed healthcare legislation or otherwise.
Moreover, stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of our Common Stock.
Our stock price is subject to fluctuation which may cause an investment in our Common Stock to suffer a decline in value.
The market price of our Common Stock may fluctuate significantly in response to factors that are beyond our control. The stock market in general has recently experienced extreme price and volume fluctuations. The market prices of securities of pharmaceutical and biotechnology companies have been extremely volatile and have experienced fluctuations that often have been unrelated or disproportionate to the operating performance of these companies. These broad market fluctuations could result in extreme fluctuations in the price of our Common Stock which could cause a decline in the value of our Common Stock.
39
In the past, following periods of volatility in the market price of a company’s securities, stockholders have often instituted class action securities litigation against those companies. Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could significantly harm our financial condition, results of operations and reputation.
We will incur increased costs as a result of being a public company.
As a public company, we will incur significant legal, accounting and other expenses. We expect the laws, rules and regulations governing public companies to increase our legal and financial compliance costs and to make some activities more time-consuming and costly.
Our management will be devoting substantial time to comply with public company regulations.
As a public company, we incur significant legal, accounting and other expenses. In addition, the Sarbanes-Oxley Act, as well as rules subsequently implemented by the Commission, impose various requirements on public companies with respect to corporate governance practices. Moreover, these rules and regulations increase legal and financial compliance costs and make some activities more time-consuming and costly.
In addition, the Sarbanes-Oxley Act requires, among other things, that our management maintain adequate disclosure controls and procedures and internal control over financial reporting. In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management and, as applicable, our independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our compliance with Section 404 will require us to incur substantial accounting and related expenses and expend significant
management efforts. If we are not able to comply with the requirements of Section 404, or if we or our independent registered public accounting firm identifies deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, our financial reporting could be unreliable and misinformation could be disseminated to the public.
Any failure to develop or maintain effective internal control over financial reporting or difficulties encountered in implementing or improving our internal control over financial reporting could harm our operating results and prevent us from meeting our reporting obligations. Ineffective internal controls also could cause our stockholders and potential investors to lose confidence in our reported financial information, which would likely have a negative effect on the trading price of our Common Stock. In addition, investors relying upon this misinformation could make an uninformed investment decision, and we could be subject to sanctions or investigations by the
Commission or other regulatory authorities or to stockholder class action securities litigation.
We may incur significant costs to be a public company to ensure compliance with corporate governance and accounting requirements and we may not be able to absorb such costs.
We may incur significant costs associated with our public company reporting requirements, costs associated with newly applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the Commission. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits
and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these newly applicable rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. In addition, we may not be able to absorb these costs of being a public company which will negatively affect our business operations.
40
The lack of substantial public company experience of our management team could adversely impact our ability to comply with the reporting requirements of U.S. securities laws.
Our management team has limited experience in working with public companies which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley Act of 2002. Such responsibilities include complying with federal securities laws and making required disclosures on a timely basis. Our senior management may not be able to implement programs and policies in an effective and timely manner that adequately respond to such increased legal, regulatory compliance and reporting requirements, including the establishing and maintaining internal controls over
financial reporting. Any such deficiencies, weaknesses or lack of compliance could have a materially adverse effect on our ability to comply with the reporting requirements of the Securities Exchange Act of 1934 which is necessary to maintain our public company status. If we were to fail to fulfill those obligations, our ability to continue as a public company would be in jeopardy in which event you could lose your entire investment in our company.
TRENDS, RISKS AND UNCERTAINTIES
Competition has increased as the vitamin and supplements industry shifts towards a greater Internet presence. This competitive environment continues to drive margin pressure as deep discounting results from aggressive customer acquisition and retention actions. The medical foods and dietary supplement industry and our performance are affected by demographic trends as well as trends affecting health and lifestyle preferences and consumer spending. Changes in these trends and other factors that we may not foresee may also impact our business, including potential regulatory actions by the FDA and the FTC that may affect the viability of a given product that
we offer. Our business allows us to respond to changing trends by introducing new products and adjusting our product mix and pricing. We plan to continue to expand our product offering. We have sought to identify what we believe to be the most significant risks to our business, but we cannot predict whether, or to what extent, any of such risks may be realized nor can we guarantee that we have identified all possible risks that might arise. Investors should carefully consider all such risk factors before making an investment decision with respect to our Common Stock.
41
SELECTED FINANCIAL INFORMATION
The following table sets forth summary historical consolidated financial and other operating data for VitaMed. The information set forth below should be read in conjunction with the information under Item 9.01 – Financial Statements and Exhibits," "Management's Discussion and Analysis and Plan of Operations and the consolidated financial statements and related notes and the financial statements included elsewhere in this Report.
Balance Sheet items reflect information as of June 30, 2011 and December 31, 2010 and 2009. Statement of Operations items reflect information for the six months ended June 30, 2011 and the twelve months ended December 31, 2010 and 2009.
|
December 31,
|
||||||||||||
|
June 30, 2011
|
2010
|
2009 | ||||||||||
|
TOTAL ASSETS
|
$ | 1,152,879 | $ | 1,197,253 | $ | 585,404 | ||||||
|
TOTAL LIABILITIES
|
1,011,805 | 232,842 | 101,774 | |||||||||
|
TOTAL MEMBERS’ EQUITY
|
141,074 | 964,411 | 483,630 | |||||||||
|
TOTAL LIABILITIES AND MEMBERS’ EQUITY
|
$ | 1,152,879 | $ | 1,197,253 | $ | 585,404 | ||||||
|
Years Ended
December 31,
|
||||||||||||
|
Period Ended
June 30, 2011
|
2010
|
2009
|
||||||||||
|
REVENUE, NET
|
$ | 994,159 | $ | 1,241,921 | $ | 221,192 | ||||||
|
COSTS OF REVENUE
|
444,849 | 556,390 | 205,097 | |||||||||
|
OPERATING EXPENSES
|
2,551,909 | 3,739,144 | 1,304,603 | |||||||||
|
LOSS BEFORE INCOME TAXES
|
(2,002,599 | ) | (3,053,613 | ) | (1,228,508 | ) | ||||||
|
PROVISION FOR INCOME TAXES
|
0 | 0 | 0 | |||||||||
|
NET LOSS
|
$ | (2,002,599 | ) | $ | (3,053,613 | ) | $ | (1,228,508 | ) | |||
42
MANAGEMENT’S DISCUSSION AND ANALYSIS AND PLAN OF OPERATIONS
This section refers to VitaMedMD. The following discussion and analysis provides information which VitaMed believes to be relevant to an assessment and understanding of its results of operations and financial condition. This discussion should be read together with VitaMed's financial statements and the notes to the financial statements for the years ended December 31, 2010 and 2009 and six months ended June 30, 2011, which were included as exhibits to the Definitive Information Statement on Schedule 14C ("Schedule 14C") filed with the Commission on September 12, 2011, which Schedule 14C and exhibits are incorporated herein by reference. The information
set forth and discussed in this Management’s Discussion and Analysis and Plan of Operations is derived from the referenced financial statements and the related notes thereto of VitaMed and should be read in conjunction therewith. Additionally, this Management’s Discussion and Analysis and Plan of Operation contain certain statements that are not strictly historical and are "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995 and involve a high degree of risk and uncertainty. Actual results may differ materially from those projected in the forward-looking statements due to other risks and uncertainties that exist in VitaMed's operations, development efforts and business environment, the other risks and uncertainties described in the section entitled
Cautionary Note Regarding Forward-Looking Statements at the front of this Report and our Risk Factors section herein. All forward-looking statements included herein are based on information available to the Company as of the date hereof and we assume no obligation to update any such forward-looking statement.
The separate financial statements of AMHN and the Management’s Discussion and Analysis and Plan of Operation with respect to the AMHN financial statements are contained in AMHN's Form 10-Q filed with the Commission on August 15, 2011, which filing, financial statements and exhibits are incorporated herein by reference.
Unaudited Pro-forma Consolidated Financial Statements were included as exhibits to the Schedule 14C filed with the Commission on September 12, 2011, which Schedule 14C and exhibits are incorporated herein by reference.
Basis of Presentation of Financial Information
On October 4, 2011, the Closing Date of the Merger, the Company acquired 100% of VitaMed in exchange for the issuance of shares of the Company's Common Stock. In accordance with the provisions of this triangulated merger, Merger Sub was merged with and into VitaMed as of the Effective Date. Upon consummation of the Merger Agreement and all transactions contemplated therein, the separate existence of Merger Sub ceased, the Company is the surviving parent corporation and VitaMed is its wholly owned subsidiary. As a result of the Merger, the primary business of the Company became that of VitaMed. Our management's discussion and analysis is based on the financial results of
VitaMed.
SUMMARY OF KEY RESULTS
Overview
Our Company's primary business is that of our subsidiary, VitaMed. We also continue to sell subscriptions for and advertising on the Spectrum Health Network. VitaMed is a limited liability company organized in the State of Delaware on May 13, 2008. VitaMed is a specialty pharmaceutical company that has developed a patent-pending technology and business methodology to market both OTC and prescription versions of nutritional supplements, medical foods, drugs, medical foods and other medical products directly to consumers or via retail pharmacies under the direct supervision of a physician. VitaMed's business model creates a unique value proposition for patients, physicians/providers and payors by eliminating much
of the inefficiencies associated with the traditional sales, marketing and distribution models.
43
VitaMed offers superior-quality products for a lower overall cost to patient and payors while increasing efficiencies for the physician.
VitaMed's information technology system collects and analyzes data designed to improve patient compliance and education, facilitate product development and provide immediate feedback on effectiveness of therapies. The result is increased efficiency and communication between the patient, physician/provider and insurance payor, ultimately creating improved outcomes for all. This combination of simplified distribution and information technology provides measurable customer benefits and clear differentiation from existing competitors in the market.
VitaMed is currently concentrating on the non-prescription nutrient market place in the area of women's health. VitaMed's current products include prenatal vitamins, Iron and Calcium supplements, menopause supplement, stretch mark cream and scar guard cream.
This section refers to VitaMed. The following discussion and analysis provides information which VitaMed believes to be relevant to an assessment and understanding of its results of operations and financial condition. This discussion should be read together with VitaMed's financial statements and the notes to the financial statements for the years ended December 31, 2010 and 2009 and six months ended June 30, 2011, which were included as exhibits to the Schedule 14C filed with the Commission on September 12, 2011, which Schedule 14C and exhibits are incorporated herein by reference. The reported results will not necessarily reflect future results of operations or
financial condition.
Material Changes in Financial Condition and Results of Operations
As of June 30, 2011, VitaMed had cash of $78,255, a decrease of $344,684 from December 31, 2010. Current liabilities increased $778,963 to $1,011,805 at June 30, 2011 from $232,842 at December 31, 2010, while working capital decreased $836,879 to $(10,609) at June 30, 2011 from $826,270 at December 31, 2010.
Results of Operations
The following discussion of VitaMed's financial condition and results of operations should be read in conjunction with its financial statements incorporated herein by reference above. This discussion should not be construed to imply that the results discussed herein will necessarily continue into the future, or that any conclusion reached herein will necessarily be indicative of actual operating results in the future. Such discussion represents only the best present assessment of VitaMed's management. Historical financial information presented for the six months ended June 30, 2011 and the year ended December 31, 2010 is that of
VitaMed.
Results of Operations – Comparison of Six Months Ended June 30, 2011 and 2010
For the six months ended June 30, 2011 and 2010, VitaMed had net revenue totaling $994,159 and $468,128, respectively. This improvement of $526,031 in sales and the corresponding $260,721 increase in cost of goods sold is a result of the expansion of VitaMed's product line and an increase in sales.
During the six months ended June 30, 2011 and 2010, VitaMed's operating expense totaled $2,512,174 and $1,569,923, respectively. This increase of approximately $942,000 is primarily a result of:
| ● | an increase of approximately $482,000 in salaries, benefits, commissions, and incentives related to an increase in the management and sales teams; |
44
| ● | an increase of approximately $33,000 in non-cash compensation related to the issuance of options to purchase Units of VitaMed; | ||
| ● | an increase in professional fees of approximately $122,000 required by the Merger; | ||
| ● | an increase in other sales, general and administrative expenses of approximately $278,000 related to the marketing, advertising, promotions, rent, travel, communications, and vehicle expenses; | ||
| ● | an increase in research and amortization expenses of $22,000 related to the testing of current products' stability and packaging; and | ||
| ● | an increase in depreciation expense of approximately $5,000 related to the purchase of property and equipment. |
Other income (expenses) increased approximately $40,000 related to the amortization of debt discount associated with certain promissory notes issued by VitaMed.
For the six months ended June 30, 2011 and 2010, VitaMed recorded a net loss of $2,002,599 compared to $1,285,923 for the same period in 2010, an increase of $716,676.
Results of Operations - Comparison of Years Ended December 31, 2010 and 2009
For the years ended December 31, 2010 and 2009, VitaMed had net revenue totaling $1,241,921 and $221,192, respectively. This improvement of $1,020,729 in sales and the corresponding $351,293 increase in cost of goods sold is a result of the expansion of VitaMed's product line and an increase in sales.
During the years ended December 31, 2010 and 2009, VitaMed's operating expense totaled $3,739,144 and $1,309,356, respectively. This increase of approximately $2,430,000 is primarily a result of:
| ● | an increase of approximately $1,279,000 in salaries, benefits, commissions, and incentives related to an increase in the management and sales teams; | ||
| ● | an increase of approximately $168,000 in non-cash compensation related to the issuance of options to purchase Units of VitaMed; | ||
| ● | an increase in other sales, general and administrative expenses of approximately $922,000 related to the marketing, advertising, promotions, rent, travel, communications, and vehicle expenses; | ||
| ● | an increase in research and amortization expenses of $42,000 related to the testing of current products' stability and packaging; and | ||
| ● | an increase in depreciation expense of approximately $19,000 related to the purchase of property and equipment. |
For the years ended December 31, 2010 and 2009, VitaMed recorded a net loss of $3,053,613 compared to $1,288,508 for the same period in 2009, an increase of $1,765,105.
Effects of Inflation
During the periods for which financial information is presented for VitaMed, VitaMed does not believe that its business and operations were materially affected by inflation.
45
Liquidity and Capital Resources
VitaMed began its business in 2008 and has not yet attained a level of revenue to allow it to meet its current overhead. The Company's management does not contemplate attaining profitable operations until late 2013, nor is there any assurance that such an operating level can ever be achieved. The Company will be dependent upon obtaining additional financing in order to adequately fund working capital, infrastructure, manufacturing expenses and significant marketing/investor related expenditures to gain market recognition, so that it can achieve a level of revenue adequate to support its cost structure, none of which can be assured. Management believes
that the Company will need approximately $5 million over the next twelve months. While initial operations have been funded with private placements of equity and bridge loans, there can be no assurance that adequate financing will continue to be available, and, if available, be on terms that are favorable.
Recent Events
On June 1, 2011, VitaMed sold Promissory Notes ("Notes") in the aggregate of $500,000 with accompanying warrants ("VitaMed Warrants") to purchase an aggregate of 500,000 Units of VitaMed. The Notes bear interest at the rate of four percent (4%) per annum and are due at the earlier of (i) the six (6) month anniversary of the date hereof and (ii) such time as VitaMed receives the proceeds of a promissory note issued in an amount of not less than $1,000,000 (the "Funding"). Upon the closing of the Funding on July 18, 2011, as more fully described in the following paragraph, two of the Notes in the aggregate of $200,000 were paid in
full. By mutual agreement, the remaining Notes in the aggregate of $300,000 were extended until the closing of the Merger. On October 6, 2011, Notes in the aggregate of $50,000 were paid in full, $50,000 were extended to October 17, 2011, and $100,000 were extended to December 4, 2011. By mutual agreement, the remaining Notes in the aggregate of $100,000 will be subsequently converted into shares of the Company's Common Stock at $0.38 per share.
On July 18, 2011, VitaMed sold two Senior Secured Promissory Notes ("Secured Notes") in the amount of $500,000 each and also entered into a Security Agreement under which VitaMed pledged all of its assets to secure the obligation. The Secured Notes bear interest at the rate of six percent (6%) per annum and are due on the one (1) year anniversary of the date of the Secured Notes.
In September and October, 2011, VitaMed sold Convertible Promissory Notes ("Notes") in the aggregate of $530,160. The Notes bear interest at the rate of four percent (4%) per annum and are due December 31, 2011. At maturity, the Notes are convertible at the option of the noteholder into shares of the Company's Common Stock at $0.38 per share.
Outlook
VitaMed sells its products to the women’s health segment of the health care market. We intend to sell a variety of products including medical foods, nutritional supplements and ancillary products that address women's health needs. We plan to sell our products online and through physician's offices and pharmacies.
Our sales and business models are linked together by our patent-pending information technology platform, OPERA. The information collected monthly from physicians on clinical effectiveness, patient satisfaction and development needs and the demographic and usage data of our customers enhances our sales and marketing efforts. The combination of these data sources affords significant value to patients, physicians/providers and payors by providing the ability to quantify and track savings, monitor compliance, provide educational content, improve patient satisfaction and provide input on clinical needs for future product development. The benefits achieved
through the use of OPERA allow our sales force to deliver a portfolio of competitively-superior products.
46
We currently employ a sales force of approximately 27 individuals and plan to expand our sales force to over 60 individuals in the next 12 months. Our sales force currently calls on OB/GYN physicians and healthcare payors. When VitaMed begins selling prescription drugs, our sales force will make calls to pharmacies and distributors to the prescription drug market. Our sales force focuses on the top 40 Metropolitan Statistical Areas ("MSA’s") in the United States.
We believe that the combination of our expanded product line, our OPERA system and increased sales force will help us increase sales and become profitable in the next couple of years.
This Outlook section and other portions of this document include certain "forward-looking statements." These forward-looking statements are based largely on the current expectations of management and are subject to a number of assumptions, risks and uncertainties. Our actual results could differ materially from these forward-looking statements.
Off-Balance Sheet Arrangements
None.
PROPERTIES
The Company's principal offices, and that of its subsidiary, are located at 951 Broken Sound Parkway NW, Suite 320, Boca Raton, FL 33487. On July 9, 2009, VitaMed entered into a 45-month lease for approximately 7,130 square feet of office and warehouse space (the "Lease"). Over the term of the Lease, the Company will pay an average monthly cost of $9,352 which includes base rent, common area fees, taxes and insurance. Terms of the Lease provide for an extension for an additional two-year period. The Company's management believes that the leased premises are suitable and adequate to meet its needs. (See
Lease Agreement, Exhibit 10.00, filed herewith and incorporated herein by reference.)
47
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
As of the date of this filing, the following table sets forth certain information with respect to the beneficial ownership of our Common Stock by (i) each shareholder known by us to be the beneficial owner of more than five percent (5%) of our Common Stock, (ii) by each of our current directors and executive officers as identified herein, and (iii) all of the Company’s directors and executive officers as a group. Each person has sole voting and investment power with respect to the shares of Common Stock, except as otherwise indicated. Beneficial ownership is determined in accordance with the rules of the Commission and generally includes
voting or investment power with respect to securities. Shares of Common Stock, Company Options and Company Warrants and convertible securities that are currently exercisable or convertible into shares of Common Stock within sixty (60) days of the date of this document, are deemed to be outstanding and to be beneficially owned by the person holding the Options, Warrants, or convertible securities for the purpose of computing the percentage ownership of the person, but are not treated as outstanding for the purpose of computing the percentage ownership of any other person. This table does not include any shares which may be issuable upon the conversion of outstanding convertible securities held by certain non-affiliated investors who may limit their respective ownership to less than five percent (5%). Unless specified otherwise, the address for each beneficial owner
listed herein is 951 Broken Sound Parkway NW, Suite 320, Boca Raton, FL 33487.
|
Name and Address of Beneficial Owner
|
Title of Class
|
Number of Shares Beneficially Owned(1)
|
Percent of Class
|
|||
|
Robert G. Finizio
Chairman and Chief Executive Officer
|
Common Stock
|
23,552,668(2)
|
37.63%
|
|||
|
John C.K. Milligan, IV
President, Secretary and Director
|
Common Stock
|
8,367,631(3)
|
13.24%
|
|||
|
Daniel A. Cartwright
Chief Financial Officer, Vice President Finance, Treasurer
|
Common Stock
|
-0-
|
-0-
|
|||
|
Mitchell L. Krassan
Executive Vice President, Chief Strategy Officer
|
Common Stock
|
431,646(4)
|
0.70%
|
|||
|
Brian Bernick, M.D.
Director
|
Common Stock
|
10,572,221(5)
|
16.87%
|
|||
|
All directors and executive officers as a group ( 5 persons):
|
Common Stock
|
42,924,166(6)
|
64.57%
|
________________
|
(1)
|
Unless otherwise noted, we believe that all shares are beneficially owned and that all persons named in the table have sole voting and investment power with respect to all shares of Common Stock owned by them. Applicable percentage of ownership is based on 61,204,766 shares of Common Stock currently outstanding as adjusted for each shareholder.
|
|
(2)
|
This amount includes 22,161,586 shares directly owned by Finizio and 1,391,082 shares due to Finizio upon exercise of vested options. The percentage of class for Finizio is based on 62,595,848 shares which would be outstanding if all of Finizio's vested options were exercised.
|
|
(3)
|
This amount includes (i) 6,368,018 shares directly owned by Milligan, (ii) 1,938,241 shares due to Milligan upon exercise of vested options, and (iii) 61,372 shares due to Milligan upon exercise of warrants. The percentage of class for Milligan is based on 63,204,379 shares which would be outstanding if all of Milligan's vested options and warrants were exercised.
|
|
(4)
|
This amount includes 431,646 shares due to Krassan upon exercise of vested options. The percentage of class for Krassan is based on 61,636,412 shares which would be outstanding if all of Krassan's vested options were exercised.
|
|
(5)
|
This amount includes (i) 9,119,766 shares beneficially owned by BF Investment Enterprises, Ltd., a company controlled by Mr. Bernick ("BF Investment"), (ii) 1,391,082 shares due to BF Investment upon exercise of vested options, and (iii) 61,372 shares due to BF Investment upon exercise of warrants. The percentage of class for Bernick is based on 62,657,220 shares which would be outstanding if all of BF Investment's vested options and warrants were exercised.
|
|
(6)
|
This amount includes all shares directly and indirectly owned by all officers and directors and all shares to be issued directly and indirectly upon exercise of options and warrants, The percentage of class for all officers and directors is based on 66,479,561 which would be outstanding if all of the officers' and directors' vested options and warrants were exercised.
|
48
DIRECTORS AND EXECUTIVE OFFICERS
The following individuals serve as the directors and executive officers of our Company and subsidiary. Our directors hold office until the next annual meeting of shareholders or until their successors have been elected and qualified. The executive officers of our Company are appointed by and serve at the pleasure of our board of directors. All officers and directors of our subsidiary are appointed by the Company's Board of Directors. All of the Company's officers and directors were elected or appointed on October 4, 2011.
|
NAME
|
AGE
|
POSITION
|
|
Robert G. Finizio
|
40
|
Chairman, Chief Executive Officer of the Company
Chief Executive Officer of VitaMed
|
|
John C. K. Milligan, IV
|
49
|
President, Secretary, Director of the Company
President, Secretary of VitaMed
|
|
Daniel A. Cartwright
|
53
|
Chief Financial Officer, Vice President Finance, Treasurer of the Company
Chief Financial Officer, Vice President Finance, Treasurer of VitaMed
|
|
Mitchell L. Krassan
|
46
|
Executive Vice President, Chief Strategy Officer of the Company
Executive Vice President, Chief Strategy Officer of VitaMed
|
|
Brian Bernick, M.D.
|
42
|
Director of the Company
|
There are no arrangements or understandings between any of the above-listed officers and directors pursuant to which they were selected to serve as an officer and/or director.
Identification of Certain Significant Employees
None.
Business Experience
The following is a brief account of the education and business experience during at least the past five years of each director and executive officer our Company and newly acquired operating subsidiary, indicating the person's principal occupation during that period, and the name of the organization in which such occupation and employment were carried out.
49
|
Robert G. Finizio – Chairman, Chief Executive Officer of the Company
Chairman, Chief Executive Officer of VitaMed
|
Robert G. Finizio was elected Chairman and appointed Chief Executive Officer of TherapeuticsMD, Inc. on October 4, 2011. On the same day, the Company's Board of Directors appointed him to serve as Chairman and Chief Executive Officer of VitaMed which is now a wholly owned subsidiary of the Company. As co-founder of VitaMed, from April 2008 to October 4, 2011, Mr. Finizio served as Chief Executive Officer and Director. From August 2001 to February 2008, Mr. Finizio co-founded and served as President of Care Fusion, LLC and then as Chief Executive Officer of CareFusion, Inc. CareFusion, Inc. is a global leader in healthcare technology and
equipment and provider of integrated technology, software, services and equipment to healthcare institutions worldwide. Mr. Finizio managed CareFusion's growth from inception to over 70 employees and 200 hospital customers prior to its acquisition by Cardinal Health. Mr. Finizio's early business experience was with Omnicell Technologies (OMCL) and Endoscopy Specialists (TFX) in the healthcare IT and surgical space, respectively. Mr. Finizio earned a BA from the University of Miami.
|
John C.K. Milligan, IV – President, Secretary, Director of the Company
President Secretary of VitaMed
|
John C.K. Milligan, IV was appointed President, Secretary and Director of TherapeuticsMD, Inc. on October 4, 2011. On the same day, the Company's Board of Directors appointed him to serve as President and Secretary of VitaMed which is now a wholly owned subsidiary of the Company. From December 2008 to October 4, 2011, Mr. Milligan served as President and Director of VitaMed. From August 2001 to February 2008, Mr. Milligan co-founded and served as Vice President and General Manager of Care Fusion, LLC and then as President and Chief Operating Officer of CareFusion, Inc. CareFusion, Inc. is a global leader in healthcare technology and
equipment and provider of integrated technology, software, services and equipment to healthcare institutions worldwide. Mr. Milligan led the post-acquisition integration into the $3.5 billion business unit and the transition of CareFusion's finance, staff, and product portfolio into publicly-traded $80 billion pharmaceutical distributor and healthcare technology provider. From 1997 to 2001, Mr. Milligan was Vice President, Sales and Operations for Omnicell, Inc., a provider of healthcare, supply chain management systems and services, where he increased revenues from under $3 million to over $25 million.
|
Daniel A. Cartwright – Chief Financial Officer, Vice President of Finance, and Treasurer of the Company
Chief Financial Officer, Vice President of Finance, Treasurer of VitaMed
|
Daniel A. Cartwright was appointed Chief Financial Officer, Vice President of Finance, and Treasurer of TherapeuticsMD, Inc. on October 4, 2011. On the same day, the Company's Board of Directors appointed him to serve as Chief Financial Officer, Executive Vice President of Finance and Treasurer of VitaMed which is now a wholly owned subsidiary of the Company. From July 2011 to October 4, 2011, Mr. Cartwright served as Chief Financial Officer of VitaMed. From May 1996 to July 2011, Mr. Cartwright served as Chief Financial Officer and Executive Vice President of Circle F Ventures, LLC, an Arizona venture capital firm which made
investments in more than fifty companies. During the same period, Mr. Cartwright served as Chief Financial Officer and Treasurer of Fleming Securities, a registered broker dealer involved with raising capital for public and private companies, where he was instrumental in raising over $250 million in funding. From 1993 to 1996, Mr. Cartwright served as Chief Financial Officer of American Wireless Systems, a provider of entertainment video services. Mr. Cartwright holds several federal securities licenses including Series 7, 24, 27 and 63. Mr. Cartwright currently serves as a member of the Board of Directors of Antenna Technologies Company, Inc., a private engineering firm, and of Primetrica, Inc., a private information research company for the telecommunications industry. Mr. Cartwright earned his B.S. in Accounting from Arizona State
University.
50
|
Mitchell L. Krassan – Executive Vice President, Chief Strategy Officer of the Company
Executive Vice President, Chief Strategy Officer of VitaMed
|
Mitchell L. Krassan was appointed Executive Vice President and Chief Strategy Officer of TherapeuticsMD, Inc. on October 4, 2011. On the same day, the Company's Board of Directors appointed him to serve as Executive Vice President and Chief Strategy Officer of VitaMed. From April 2010 to October 4, 2011, Mr. Krassan served as Chief Strategy and Performance Officer of VitaMedMD, LLC, a Delaware limited liability company, which is now a wholly owned subsidiary of TherapeuticsMD, Inc. His duties included assisting the Chief Executive Officer with creating, communicating, executing and sustaining strategic initiatives. In addition, he
was responsible for capturing and leveraging business performance data to effect improvements and innovations in business necessary for successful strategy execution. From October 1997 to present, Mr. Krassan has been a partner with EquiMark Limited, a private investment partnership. From November 1994 to July 1997, Mr. Krassan served as Chief Financial Officer and Chief Operating Officer of The Reich Group/Telespectrum Worldwide, a fully-integrated direct marketing firm that provided clients expertise in market research and analysis, strategic planning, marketing, creative and production services, telemarketing and database development. The Reich Group became the lead company in a roll-up and $180 million IPO of Telespectrum Worldwide. Mr. Krassan earned a B.S. in Accounting from University of Maryland, received his certification as a CPA in
the State of Maryland, and earned his MBA in Management from New York University.
Brian Bernick, M.D. – Director of the Company
Dr. Brian Bernick was elected as a Director of TherapeuticsMD, Inc. on October 4, 2011. As co-founder of VitaMed, Dr. Bernick served on VitaMed's Board of Directors since inception. Dr. Bernick is a practicing and board certified Obstetrician/Gynecologist with twenty years of clinical medical experience. Dr. Bernick is the past Chairman of the Department of Obstetrics and Gynecology at Boca Raton Regional Hospital and has severed as a member of its Medical Executive Board. He has served on the Board of Directors of the Palm Beach Medical Society and VitalMD Group Holding, LLC, the largest physician-owned and managed group of
obstetricians/gynecologists in Florida covering more than 250 physicians/practices. Dr. Bernick is the recipient of several national and regional awards including the American Medical Association Foundation's Leadership Award and was recognized by both Super Doctors and National Consumers Survey for being in the top 5% of doctors. He provides medical education in conjunction with Emory University and Florida Atlantic University School of Nursing and Medicine. Dr. Bernick earned a BA in Economics from Northwestern University and a doctorate in medicine from the University of Chicago Medical School. He completed his residency at the University of Pennsylvania.
Family Relationships
There are no family relationships between any of our directors or executive officers.
Other Directorships
Other than as indicated within this section at Business Experience, none of the Company's directors hold or have been nominated to hold a directorship in any company with a class of securities registered pursuant to Section 12 of the Exchange Act (the "Act") or subject to the requirements of Section 15(d) of the Securities Act of 1933 or company registered as an investment company under the Investment Company Act of 1940.
51
Involvement in Certain Legal Proceedings
Currently, and for the past five years, none of our directors or executive officers have been involved in any legal proceeding concerning (i) any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; (ii) any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); (iii) being subject to any order, judgment or decree, not subsequently reversed, suspended, or vacated, of any court of competent jurisdiction
permanently or temporarily enjoining, barring, suspending or otherwise limiting involvement in any type of business, securities or banking activity; or (iv) being found by a court, the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law (and the judgment has not been reversed, suspended or vacated).
Committees of the Board
The Company has not yet appointed an Audit Committee or Nominating Committee. Until such time as appointments are made, the Company's Board of Directors will serve in those capacities.
Codes of Conduct
On December 31, 2009, the Company's board of directors approved (i) a Code of Business Conduct and Ethics for each director and executive officer, (ii) a Code of Ethics for Financial Executives for all officers with financial oversight responsibilities, and (iii) an Insider Trading Policy for each director and executive officer. A form of the Code of Business Conduct and Ethics, Code of Ethics for Financial Executives, and Insider Trading Policy was attached as an exhibit to the Company's Form 10-K for year ended December 31, 2009 and is included herein by reference. The Company will provide a copy of these policies free of charge upon written request.
The Code of Business Conduct and Ethics is applicable to all directors and executive officers of the Company. This code is intended to focus the members of the Board of Directors and each executive officer on areas of ethical risk, provide guidance to directors and executive officers to help them recognize and deal with ethical issues, provide mechanisms to report unethical conduct, and help foster a culture of honesty and accountability. All members of the Board of Directors and all executive officers are required to sign this code on an annual basis.
The Code of Ethics is applicable to all financial executives and any other senior officer with financial oversight responsibilities. This code governs the professional and ethical conduct of the Company's financial executives, and directs that they: (i) act with honesty and integrity; (ii) provide information that is accurate, complete, objective, relevant, and timely; (iii) comply with federal, state, and local rules and regulations; (iv) act in good faith with due care, competence and diligence; and (v) respect the confidentiality of information acquired in the course of their work and not use the information acquired for personal gain. All
of the Company's financial executives are required to sign this code on an annual basis.
The Insider Trading Policy is applicable to all directors and officers. Insider trading generally refers to the buying or selling of a security in breach of a fiduciary duty or other relationship of trust and confidence while in possession of material, non-public information about the security. Insider trading violations may also include "tipping" such information, securities trading by the person "tipped," and securities trading by those who misappropriate such information. The scope of insider trading violations can be wide reaching. As such, our Board of Directors has adopted an Insider Trading Policy that outlines the definitions
of insider trading, the penalties and sanctions determined, and what constitutes material, non-public information. Illegal insider trading is against the policy of the Company as such trading can cause significant harm to the reputation for integrity and ethical conduct of the Company. Individuals who fail to comply with the requirements of the policy are subject to disciplinary action, at the sole discretion of the Company, including dismissal for cause. All members of the Company's Board of Directors and all executive officers are required to ratify the terms of this policy on an annual basis.
52
By written consent executed immediately after the Closing of the Transaction, the Company's Board of Directors ratified the Code of Business Conduct and Ethics, Code of Ethics for Financial Executives and Insider Trading Policy and all officers and directors executed same.
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Exchange Act requires directors, officers, and the persons who beneficially own more than 10% of Common Stock of certain companies to file reports of ownership and changes in ownership with the Commission. To date, we believe that all such reports have been timely filed.
EXECUTIVE COMPENSATION
The following table lists the compensation of the principal executive officers of the Company prior to and after the Closing of the Merger outlined herein. The compensation of the Company's former principal executive officers includes compensation for each of the years ending ended December 31, 2010 and 2009 as well as the compensation paid from January 1, 2011 through the Closing of the Merger. The compensation of the Company's current principal executive officers includes compensation paid by VitaMed from January 1, 2011 through September 30, 2011. No compensation has been recorded for any executive officers of the Company from September 30, 2011 through the date of this
Report.
(Remainder of page intentionally left blank.)
53
The following information includes the dollar value of base salaries, bonus awards, the number of non-qualified stock options ("Options") granted and certain other compensation, if any, whether paid or deferred. The aggregated Options granted to the Company's current executive officers to date were originally issued by VitaMed and were converted into Company Options as of the Closing of the Merger pursuant to the Conversion Ratio.
|
Annual Compensation
|
Long Term Compensation
|
|||||
|
Name and
Principal Position
|
Fiscal Year
End
|
Salary ($)
|
Bonus ($)
|
All other and annual Compensation and LTIP Payouts ($)
|
Securities under Options/
SARS Granted (#)
|
Restricted Shares or Restricted Share Units
(#)
|
|
Robert G. Finizio*
Chief Executive Officer
|
2011
|
114,000
|
-0-
|
45,554
|
-0-
|
-0-
|
|
2010
|
140,282
|
-0-
|
45,554
|
-0-
|
-0-
|
|
|
2009
|
1,846
|
-0-
|
45,554
|
1,472,910
|
-0-
|
|
|
John C.K. Milligan*
President, Secretary
|
2011
|
114,000
|
-0-
|
63,471
|
-0-
|
-0-
|
|
2010
|
144,787
|
-0-
|
63,471
|
-0-
|
-0-
|
|
|
2009
|
1,846
|
-0-
|
63,471
|
2,052,255
|
-0-
|
|
|
Daniel A. Cartwright*
Chief Financial Officer, Vice President Finance, Treasurer
|
2011
|
22,406
|
-0-
|
-0-
|
-0-
|
-0-
|
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
|
2009
|
-0-
|
-0-
|
-0-
|
-0-
|
||
|
Mitchell L. Krassan*
Chief Strategy Officer
|
2011
|
80,385
|
-0-
|
53,426
|
-0-
|
-0-
|
|
2010
|
15,096
|
-0-
|
31,945
|
902,158
|
-0-
|
|
|
2009
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
|
Jeffrey D. Howes(1)
Former Principal Executive Officer
|
2011
|
15,000
|
-0-
|
-0-
|
-0-
|
-0-
|
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
|
2009
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
|
Robert Cambridge(2)
Former Principal Executive Officer
|
2011
|
5,000
|
-0-
|
-0-
|
-0-
|
-0-
|
|
2010
|
60,000
|
-0-
|
-0-
|
-0-
|
-0-
|
|
|
2009
|
26,000
|
-0-
|
-0-
|
-0-
|
-0-
|
|
|
Sky Kelley(3)
Former Chief Executive Officer
|
2011
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
|
2009
|
50,679
|
-0-
|
-0-
|
-0-
|
34,235
|
|
|
Gregory R. Woodhill(4)
Former Chief Executive Officer
|
2011
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
|
2009
|
-0-
|
-0-
|
3,500
|
-0-
|
-0-
|
|
|
Gerald L. Jensen
Former Principal Executive Officer
|
2011
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
2010
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
|
2009
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|
____________________
*Officers appointed at the Closing of the Merger.
54
|
(1)
|
Mr. Howes served as the Company's sole officer and director from February 15, 2011 through the Closing of the Merger for which services he received an aggregate of $15,000 pursuant to a consulting arrangement with the Company.
|
|
(2)
|
Mr. Cambridge's compensation for 2011 including consulting fees from January 1 through February 15, 2011. Compensation for 2009 included consulting fees from July 2009 to December 2009. Mr. Cambridge's compensation was invoiced and paid through his consulting company.
|
|
(3)
|
Includes compensation paid by America's Minority Health Network from June 2009 through December 2009. Also includes 3,423,422 shares of the Company's restricted Common Stock valued at $291,667.
|
|
(4)
|
Mr. Woodhill was not an employee of the Company; he received $500 per month for his services pursuant to a consulting arrangement with the Company.
|
As of the Closing of the Merger, the compensation arrangement with the Company's former sole officer was terminated.
Compensation Arrangements with Executive Officers
There are no employment agreements or consulting agreements with any of the Company's executive officers. All executive officers are employed through a verbal compensation arrangement.
Director Compensation
The Company does not pay cash fees to directors who attend regularly scheduled and special board meetings; however, we may reimburse out-of-state directors for costs associated with travel and lodging to attend such meetings. Our directors may also be granted non-qualified stock options ("Options") from time to time under the Company's LTIP. For the year ended December 31, 2010, the Company had paid zero compensation to its directors. The following table shows compensation paid to VitaMed's directors for services rendered during year ended December 31, 2010. The valuation methodology used to determine the fair value of the Options
issued during the year was the Black-Scholes-Merton option-pricing model, an acceptable model in accordance with ASC 718.
|
Name (a)
|
Fees earned or paid in cash
($)
(b)
|
Stock awards
($)
(c)
|
Option awards
($)
(d)
|
Non-equity incentive plan com-
pensation
($)
(e)
|
Nonqualified
deferred
compensation
earnings
($)
(f)
|
All
other
compen-sation
($)
(g)
|
Total
($)
(h)
|
|
Robert G. Finizio
|
0
|
0
|
0
|
0
|
0
|
0
|
|
|
John C.K. Milligan, IV
|
0
|
0
|
0
|
0
|
0
|
0
|
|
|
Nick Segal*
|
0
|
0
|
13,986
|
0
|
0
|
0
|
13,986
|
|
Alan Wurtzel*
|
0
|
0
|
13,986
|
0
|
0
|
0
|
13,986
|
|
Brian Bernick
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
Larry Lewin*
|
0
|
0
|
13,986
|
0
|
0
|
0
|
13,986
|
________________________
|
|
*These individuals are not serving as directors of the Company post Merger, but were directors of VitaMed prior to the Merger through the Closing of the Merger.
|
55
Outstanding Equity Awards at Fiscal Year End and To Date
At fiscal year ended December 31, 2010, the Company had no outstanding equity awards. The table below shows equity awards currently outstanding for the Company's executive officers as of the filing date of this Report, which equity awards consists solely of non-qualified stock options issued under the LTIP (the "Options"). The Options were originally issued by VitaMed and were converted into Company Options as of the Closing of the Merger pursuant to the Conversion Ratio.
| Option Awards | Stock Awards | ||||||||
|
Name
|
Number of Securities Underlying Unexercised Options
(#) Exercisable
|
Number of Securities Underlying Unexercised Options
(#) Unexercis-able
|
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options
(#)
|
Option Exercise Price
($)
|
Option Expiry Date
|
Number of Shares
or Units of Stock That Have Not Vested (#)
|
Market Value of Shares or Units of Stock That Have Not Vested
($)
|
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested
(#)
|
Equity Incentive Plan Awards Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)
|
| Robert G. Finizio, Chairman, CEO | 1,391,082(1) | 81,828(1) | -0- | $0.10 | 01/01/19 | -0- | -0- | -0- | -0- |
|
John C.K. Milligan, IV, President, Secretary, Director
|
1,938,241(1) | 114,014(1) | -0- | $0.10 | 01/01/19 |
-0-
|
-0- | -0- | -0- |
| Daniel A. Cartwright, VP-Finance, CFO, Treasurer | -0- | -0- | -0- | -0- | N/A | -0- | -0- | -0- | -0- |
|
Mitchell Krassan,
|
73,646(2) | -0- | -0- | $0.19 |
05/01/20
|
-0- |
-0-
|
-0-
|
-0- |
| Exec. Vice President | 23,015(3) | 69,042(3) | -0- | $0.19 | 05/01/20 | -0- | -0- | -0- | -0- |
| 265,943(4) | 470,512(4) | -0- | $0.20 | 05/01/20 | -0- | -0- | -0- | -0- | |
| Brian Bernick, Director | 1,391,082(1) | 81,828(1) | -0- | $0.10 | 01/01/19 | -0- | -0- | -0- | -0- |
___________________
|
|
(1) The options granted on January 1, 2009 vest monthly on the first of each month over three years.
|
|
|
(2) All 73,646 underlying shares vested on May 1, 2011.
|
|
|
(3) The options granted on May 1, 2010 vest annually on the anniversary date over four years.
|
|
|
(4) The options granted on September 1, 2010 vest monthly on the first of each month over three years.
|
56
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
Promissory Notes
On June 1, 2011, VitaMed sold Promissory Notes ("Notes") in the aggregate of $500,000 with accompanying warrants ("VitaMed Warrants") to purchase an aggregate of 500,000 Units of VitaMed. The Notes bear interest at the rate of four percent (4%) per annum and are due at the earlier of (i) the six (6) month anniversary of the date of issuance and (ii) such time as VitaMed receives the proceeds of a promissory note issued in an amount of not less than $1,000,000 (the "Funding"). Upon the closing of the Funding on July 18, 2011, as more fully described in the following paragraph, two of the Notes in the aggregate of $200,000 were paid in
full. By mutual agreement, the remaining Notes in the aggregate of $300,000 were extended until the Closing of the Merger. On October 6,, 2011, Notes in the aggregate of $50,000 were paid in full, $50,000 were extended to October 17, 2011, and $100,000 were extended to December 4, 2011. By mutual agreement, the remaining Notes in the aggregate of $100,000 will be subsequently converted into shares of the Company's Common Stock at $0.38 per share.
On July 18, 2011, VitaMed sold two Senior Secured Promissory Notes ("Secured Notes") in the amount of $500,000 each and also entered into a Security Agreement under which VitaMed pledged all of its assets to secure the obligation. The Secured Notes bear interest at the rate of six percent (6%) per annum and are due on the one (1) year anniversary of the date of the Secured Notes.
In September and October, 2011, VitaMed sold Convertible Promissory Notes ("Notes") in the aggregate of $534,160. The Notes bear interest at the rate of four percent (4%) per annum and are due December 1, 2011. The Notes are convertible at the option of the noteholder into shares of the Company's Common Stock at $0.38 per share.
Loans from Affiliates
Between May 30 and June 30, 2011, VitaMed issued short-term Promissory Notes ("Notes") in the aggregate of $200,000 to certain officers and directors of VitaMed. The Notes bear interest at the rate of four percent (4%) per annum. On October 6, 2011, Notes in the aggregate of $50,000 were paid in full, $100,000 were extended to December 4, 2011 and $50,000 will subsequently be converted into shares of the Company's Common Stock at $0.38 per share.
Lock Up Agreements
As required by of the Merger Agreement, a Lock Up Agreement ("Agreement") was entered into between the Company and security holders covering the aggregate of 70,000,000 shares of the Company's Common Stock issued pursuant to the Merger or reserved for issuance pursuant to Company Options and Warrants. Each security holder agreed that from the date of the Agreement until eighteen (18) months thereafter (the "Lock-Up Period"), they would not make or cause any sale of the Company's securities. After the completion of the Lock-Up Period, the security holder agreed not to sell or dispose of more than 2.5 percent (2.5%) of the aggregate Common Stock
or shares reserved for issuance for Company Options and Company Warrants per quarter over the following twelve (12) month period (the “Dribble Out Period”). Upon the completion of the Dribble Out Period, the Agreements shall terminate.
57
Lang Agreement
As previously mentioned, VitaMed purchases approximately 90% of its products from Lang. After the Closing of the Merger, one of the majority owners of Lang is a minority shareholder of the Company. As part of the manufacturing relationship with Lang, Lang provides VitaMed credit terms under a separate financing agreement. VitaMed has entered into a manufacturing contract with Lang on each of its products and believes that the contracted rates are at or below current market rates.
Pernix Stock Purchase
On September 8, 2011, the Company entered into a Stock Purchase Agreement with Pernix Therapeutics, LLC, a Louisiana limited liability company ("Pernix"), and solely for the purposes of Section 5 of the Stock Purchase Agreement, VitaMedMD. Pursuant to the terms of the Stock Purchase Agreement, Pernix agreed to purchase 2,631,579 shares of the Company's Common Stock (the "Shares") at a purchase price of $0.38 per share for a total purchase price of $1,000,000 ("Purchase Price"). The closing of the Stock Purchase Agreement took place on October 5, 2011. In connection with the Stock Purchase Agreement, the Company and Pernix entered into a Lock-Up Agreement which, among other things, restricts the sale, assignment, transfer, encumbrance and other
disposition of the Shares issued to Pernix. Pursuant to the terms of the Lock-Up Agreement, Pernix agreed that for a period of twelve (12) months from the date of the Lock-Up Agreement, it would not make or cause any sale of the Shares (the "Lock-Up Period"). After the completion of the Lock-Up Period, Pernix agreed not to sell or dispose of more than five percent (5%) of the Shares per quarter for the following twelve (12) month period. The foregoing descriptions of the Stock Purchase Agreement and Lock-Up Agreement are qualified, in their entirety, by reference to each agreement, copies of which were filed as exhibits to the Current Report on Form 8-K filed with the Commission on September 8, 2011, which Form 8-K and exhibits are incorporated herein by reference.
Non-qualified Stock Options
The Company issued Non-Qualified Stock Options to its executive officers, directors, significant employees, and non-executive employees as further outlined herein at Recent Sales of Unregistered Securities.
Warrants
In conjunction with the Closing of the Merger, the Company assumed VitaMed warrants that were originally issued to certain officers and directors in conjunction with the sale of VitaMed Promissory Notes, and pursuant to the Conversion Ratio, issued Company Warrants for the purchase of an aggregate of approximately 122,744 shares of the Company's Common Stock. The Company also assumed VitaMed's obligation to subsequently issue warrants to certain officers and directors in consideration for guaranteeing a VitaMed bank loan (the "Reserved Warrants"). Pursuant to the Conversion Ratio, the Reserved Warrants for the purchase of 409,140 shares of the
Company's Common Stock will be issued to certain officers and directors of the Company once they are earned.
Director Independence
There are no members of our Board of Directors that qualify as independent directors although our securities are not currently traded on an exchange or on NASDAQ which would require that the Board of Directors include a majority of directors that are independent.
58
LEGAL PROCEEDINGS
Currently, we are not a party to any pending legal proceedings and are not aware of any proceeding threatened or contemplated against us by any governmental authority or other party.
MARKET PRICE OF AND DIVIDENDS ON COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Our Common Stock is traded on the OTC Bulletin Board under the symbol "AMHND." The following table shows the price range of our Common Stock for each quarter ended during the last two fiscal years and for the first three quarters of the current year. The below quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not represent actual transactions. Prices listed are historic prices and were not adjusted to reflect the 3:1 Forward Split that was effective on July 27, 2009 or the 1:100 Reverse Split that was effective on October 3, 2011.
|
Quarter Ended
|
High
|
Low
|
|
Fiscal Year 2011
|
||
|
Third Quarter
|
$0.04
|
$0.01
|
|
Second Quarter
|
$0.07
|
$0.01
|
|
First Quarter
|
$0.10
|
$0.02
|
|
Fiscal Year 2010
|
||
|
Fourth Quarter
|
$0.15
|
$0.03
|
|
Third Quarter
|
$0.90
|
$0.06
|
|
Second Quarter
|
$1.34
|
$0.25
|
|
First Quarter
|
$1.60
|
$0.10
|
|
Fiscal Year 2009
|
||
|
Fourth Quarter
|
$1.55
|
$0.51
|
|
Third Quarter
|
$1.50
|
$0.20
|
|
Second Quarter
|
$1.01
|
$0.51
|
|
First Quarter
|
$1.50
|
$1.01
|
Holders
As of October 11, 2011, after giving effect to the Closing of the Merger and the issuance of shares required thereunder, there are approximately 340 holders of record of our Common Stock.
Dividends
We do not anticipate that we will declare or pay any dividends in the foreseeable future. Our current policy is to retain earnings, if any, to fund operations, and the development and growth of our business. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, operation results, capital requirements, applicable contractual restrictions, restrictions in our organizational documents, and any other factors that our Board of Directors deems relevant.
59
Securities Authorized for Issuance under Equity Compensation Plans
On September 25, 2009, the Company's Board of Directors adopted the AMHN, Inc. 2009 Long Term Incentive Compensation Plan (the "LTIP") to provide financial incentives to employees, members of the Board, and advisers and consultants of the Company who are able to contribute towards the creation of or who have created stockholder value by providing them stock options and other stock and cash incentives (the "Awards"). The Awards available under the LTIP consist of stock options, stock appreciation rights, restricted stock, restricted stock units, performance stock, performance units, EVA awards, and other stock or cash awards as described in the LTIP. The
LTIP was amended to reserve an aggregate of 25,000,000 shares of the Company's Common Stock for issuance thereunder. Prior to the Merger, no awards had been issued under the LTIP.
In conjunction with the Closing of the Merger, the Company issued Company Options for the purchase of an aggregate of 10,365,281 shares of the Company's Common Stock. The following table shows the number of securities to be issued upon exercise of outstanding Company Options.
|
Plan Category
|
Number of Securities to be issued upon exercise of outstanding options
(a)
|
Weighted-average exercise price of
outstanding options
(b)
|
Number of securities remaining
available for future issuance under
equity compensation plans
(excluding securities reflected in column (a)
(c)
|
|
Equity compensation plans not approved by security holders
|
-0-
|
-0-
|
-0-
|
|
Equity compensation plan (LTIP) approved by security holders
|
10,365,281
|
$0.147
|
14,364,719
|
|
Total
|
10,365,281
|
$0.147
|
14,364,719
|
Common Stock Purchase Warrants
In conjunction with the Closing of the Merger, the Company assumed VitaMed warrants that were originally issued in conjunction with the sale of VitaMed Promissory Notes, and pursuant to the Conversion Ratio, issued Company Warrants for the purchase of an aggregate of 613,718 shares of the Company's Common Stock. The Company also assumed VitaMed's obligation to subsequently issue warrants to affiliates in consideration for their guarantee of a bank loan for the benefit of VitaMed (the "Reserved Warrants"). Pursuant to the Conversion Ratio, the Reserved Warrants for the purchase of 613,710 shares of the Company's Common Stock will be issued to
certain officers and directors of the Company once they are earned.
INDEMNIFICATION OF OFFICERS AND DIRECTORS
Section 145 of the Nevada Corporation Law provides in relevant parts as follows:
60
(1) A corporation shall have power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative, or investigative (other than an action by or in the right of the corporation) by reason of the fact that he is or was a director, officer, employee, or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise, against expenses (including attorneys' fees), judgments, fines, and amounts paid in
settlement actually and reasonably incurred by him in connection with such action, suit, or proceeding if he acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. The termination of any action, suit, or proceeding by judgment, order, settlement, conviction, or on a plea of nolo contendere or its equivalent, shall not, of itself, create a presumption that the person did not act in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and with respect to any criminal action or proceeding, had reasonable cause to believe that his conduct was unlawful.
(2) A corporation shall have power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending, or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee, or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other enterprise against expenses (including attorneys' fees) actually and reasonably incurred by him in connection with the defense or settlement of such action
or suit if he acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made in respect of any claim, issue, or matter as to which such person shall have been adjudged to be liable for negligence or misconduct in the performance of his duty to the corporation unless and only to the extent that the court in which such action or suit was brought shall determine on application that, despite the adjudication of liability but in view of all circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which such court shall deem proper.
(3) To the extent that a director, officer, employee, or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit, or proceeding referred to in (1) or (2) of this subsection, or in defense of any claim, issue or matter therein, he shall be indemnified against expenses (including attorneys' fees) actually and reasonably incurred by him in connection therewith.
(4) The indemnification provided by this section shall not be deemed exclusive of any other rights to which those seeking indemnification may be entitled under any bylaws, agreement, vote of stockholders or disinterested directors or otherwise, both as to action in his official capacity and as to action in another capacity while holding such office, and shall continue as to a person who has ceased to be a director, officer, employee, or agent and shall inure to the benefit of the heirs, executors, and administrators of such a person.
The foregoing discussion of indemnification merely summarizes certain aspects of indemnification provisions and is limited by reference to the above discussed sections of the Nevada Corporation Law.
The Company's Articles of Incorporation and Bylaws provide that the Company may indemnify to the full extent of its power to do so, all directors, officers, employees, and/or agents. Insofar as indemnification by the Company for liabilities arising under the Securities Act may be permitted to officers and directors of the Company pursuant to the foregoing provisions or otherwise, the Company is aware that in the opinion of the Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
61
FINANCIAL STATEMENTS
See information contained in Item 9.01 below.
Item 3.02 Unregistered Sales of Equity Securities.
Securities Issued Pursuant to Merger
As previously mentioned herein, pursuant to and in conjunction with the Closing of the Merger, the Company:
|
·
|
issued 58,407,331 shares of its Common Stock to the members of VitaMed;
|
|
·
|
issued Company Options for the purchase of an aggregate of 10,365,281 underlying shares of its Common Stock;
|
|
·
|
issued Company Warrants for the purchase of an aggregate of 613,718 underlying shares of its Common Stock, and
|
|
·
|
reserved 613,710 underlying shares of its Common Stock for issuance upon exercise of Company Warrants to guarantee a bank loan.
|
The 58,407,331 shares issued to the members of VitaMed were issued with a restrictive legend that the shares had not been registered under the Securities Act of 1933. The exchange of the securities pursuant to the Merger was conducted pursuant to the exemption from registration provided by Regulation D of the Securities Act and Section 4(2) of the Securities Act.
Securities Issued Pursuant to Stock Purchase Agreement with Pernix Therapeutics, LLC
On September 8, 2011, the Company entered into a Stock Purchase Agreement with Pernix Therapeutics, LLC, a Louisiana limited liability company ("Pernix"), and solely for the purposes of Section 5 of the Stock Purchase Agreement, VitaMedMD. Pursuant to the terms of the Stock Purchase Agreement, Pernix agreed to purchase 2,631,579 shares of the Company's Common Stock (the "Shares") at a purchase price of $0.38 per share for a total purchase price of $1,000,000 ("Purchase Price"). The closing of the Stock Purchase Agreement took place on October 5, 2011 wherein Pernix wired the Purchase Price for the benefit of the Company to VitaMed and the
Company will issue the Shares.
In connection with the Stock Purchase Agreement, the Company and Pernix entered into a Lock-Up Agreement of even date therewith, which, among other things, will restrict the sale, assignment, transfer, encumbrance and other disposition of the Shares issued to Pernix. Pursuant to the terms of the Lock-Up Agreement, Pernix agreed that for a period of twelve (12) months from the date of the Lock-Up Agreement, it would not make or cause any sale of the Shares (the "Lock-Up Period"). After the completion of the Lock-Up Period, Pernix agreed not to sell or dispose of more than five percent (5%) of the Shares per quarter for the following twelve (12)
month period.
The foregoing descriptions of the Stock Purchase Agreement and Lock-Up Agreement are qualified, in their entirety, by reference to each agreement, copies of which were attached as exhibits to the Current Report on Form 8-K filed with the Commission on September 14, 2011, which filing and exhibits are incorporated by reference.
62
Item 5.01 Changes in Control of Registrant.
As previously mentioned herein, pursuant to the Closing of the Merger, the Company issued 58,407,331 shares of its Common Stock to the members of VitaMed in exchange for 100% of their ownership thereof. Prior to the subject Merger, the members of VitaMed owned no shares of the Company.
On the Closing Date of the Merger, and after giving effect to (i) the Reverse Split and (ii) the issuance of the Company's Common Stock in exchange for all of the outstanding units of VitaMed, there are 58,573,187 shares of the Company's Common Stock issued and outstanding. The aggregated beneficial ownership on a fully diluted basis is as follows:
|
●
|
The members who exchanged their units of VitaMed in connection with the Merger acquired an aggregate beneficial ownership of 58,407,331 shares or approximately ninety-nine percent (99%) of the issued and outstanding shares of the Company's Common Stock, and
|
|
●
|
Shareholders beneficially owning 100% of the shares of the Company's Common Stock immediately prior to the consummation of the Merger were diluted to an aggregate beneficial ownership of 165,856 shares or approximately one percent (1%) of the Company's issued and outstanding shares of Common Stock.
|
In conjunction with the Closing of the Merger, the Company's sole officer and director prior to the Merger resigned and those individuals designated by VitaMed became the Company's officers and directors. There are no other arrangements or understandings regarding the Merger that are not outlined within the aforementioned Merger Agreement.
Item 5.02 Departure of Directors or Principal Officers; Election of Directors; Appointment ofCertain Officers; Compensatory Arrangements of Certain Officers.
Change in the Directors Serving on our Board
In connection with the Merger, the sole director serving on the Company's Board of Directors immediately prior to the Merger resigned and the following individuals were elected as directors:
Robert G. Finizio, Chairman
John C.K. Milligan
Brian Bernick, M.D.
Information on each of the new directors is set forth herein at Directors and Executive Officers.
Change in Officers
In connection with the Merger, the sole officer of the Company immediately prior to the Merger resigned and the following individuals were named as officers:
| Robert G. Finizio | Chief Executive Officer | |
| John C.K. Milligan | President, Secretary | |
| Daniel A. Cartwright | Chief Financial Officer, Vice President Finance, Treasurer | |
| Mitchell Krassan | Executive Vice President, Chief Strategy Officer |
The above officers hold the same positions in VitaMed. Officers serve at the pleasure of the Company's Board of Directors. Information on each of the new officers is set forth herein at Directors and Executive Officers.
63
| Item 9.01 | Financial Statements and Exhibits. | |
|
(a)
|
Financial Statements of Business Acquired: | |
|
See Financial Statements of VitaMed for six months ended June 30, 2011 and for yearsended December 31, 2010 and 2009 filed as exhibits to the 14C filed with the Commissionon September 12, 2011.
|
||
| (b) | Pro Forma Financial Information: | |
|
See Unaudited Proforma Consolidated Financial Statements filed as exhibits to the 14C filedwith the Commission on September 12, 2011.
|
||
| (c) |
Shell Company Transactions:
|
|
|
None.
|
||
| (d) |
Exhibits:
|
|
Exh. No.
|
Date
|
Document
|
|
2.1
|
July 18, 2011
|
Agreement and Plan of Merger by and among AMHN, Inc., VitaMedMD, LLC and VitaMed Acquisition, LLC(1)
|
|
10.1
|
n/a
|
Long Term Incentive Plan, as amended(2)
|
|
10.4
|
n/a
|
Lock Up Agreement, form of(1)
|
|
10.5
|
September 8, 2011
|
Stock Purchase Agreement between the Company and Pernix Therapeutics, LLC(3)
|
|
10.6
|
September 8, 2011
|
Lock-Up Agreement between the Company and Pernix Therapeutics, LLC(3)
|
|
99.1
|
n/a
|
Audited Financial Statements for VitaMedMD, LLC for years ended December 31, 2010 and 2009(2)
|
|
99.2
|
n/a
|
Unaudited Financial Statements for VitaMedMD, LLC for six months ended June 30, 2011 and 2010(2)
|
|
99.3
|
n/a
|
Unaudited Consolidated Proforma Financial Statements for AMHN, Inc. reflecting VitaMed acquisition as of December 31, 2010 and June 30, 2011(2)
|
____________________________
|
(1)(2)
(3)
|
Filed as an exhibit to Form 8-K filed with the Commission on July 21, 2011.
Filed as an exhibit to Definitive Information Statement on Schedule 14C filed with the Commission on September 12, 2011.
Filed as an exhibit to Form 8-K filed with the Commission on September 14, 2011.
|
64
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: October 11, 2011 | THERAPEUTICSMD, INC. | ||
| By: | /s/Robert G. Finizio | ||
|
Robert G. Finizio, Chief Executive Officer
|
|||
65
