Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Precipio, Inc. | v236826_8k.htm |

Transgenomic, Inc. Craig - Hallum 2nd Annual Alpha Select Conference October 6, 2011 Offering innovative products and services for personalized medicine (OTCBB: TBIO) 1

2 Forward - Looking Statements Certain statements in this presentation constitute “forward - looking statements” of Transgenomic within the meaning of the Private Securities Litigation Reform Act of 1995, which involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from any future results, performance or achievements expressed or implied by such statements. Forward - looking statements include, but are not limited to, those with respect to management's current views and estimates of future economic circumstances, industry conditions, company performance and financial results, including the ability of the Company to grow its 4 involvement in the diagnostic products and services markets. The known risks, uncertainties and other factors affecting these forward - looking statements are described from time to time in Transgenomic's filings with the Securities and Exchange Commission. Any change in such factors, risks and uncertainties may cause the actual results, events and performance to differ materially from those referred to in such statements. Accordingly, the Company claims the protection of the safe harbor for forward - looking statements contained in the Private Securities Litigation Reform Act of 1995 with respect to all statements contained in this press release. All information in this press release is as of the date of the release and Transgenomic does not undertake any duty to update this information, including any forward - looking statements, unless required by law.
3 • Founded 1997 • Global Footprint • Employees: 167 • O/S 49.2 Million • 2011 Proj. Revenue $32M • Projected Income Breakeven in 2012 • Cash into 2012 Transgenomic Today

Transgenomic Core Strengths • Transgenomic is a 13 year market leader in DNA mutation DISCOVERY and DETECTION • Our technologies are both extremely sensitive for detecting low level mutations and enable scanning for ALL mutations in a specimen rather than technologies which are targeted for known mutations only.
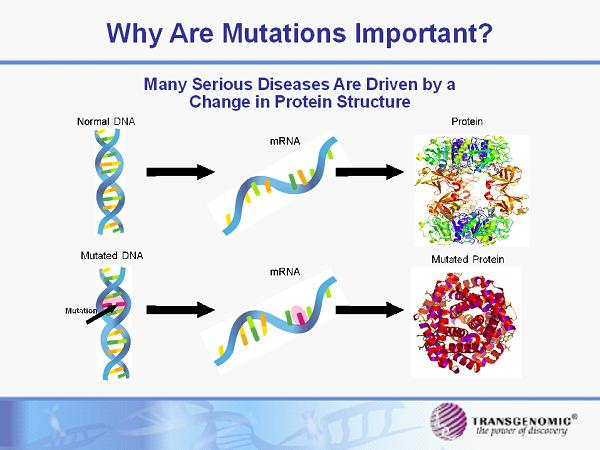
Why Are Mutations Important? Many Serious Diseases Are Driven by a Change in Protein Structure

…In Cancer 6 Cancer is a Progressive Genetic Disorder Mutation Disease Progression Treatment Challenges Ultra - sensitive DNA Detection Heterogeneity of cancer demands highly sensitive mutation scanning methods for early detection of important genetic changes
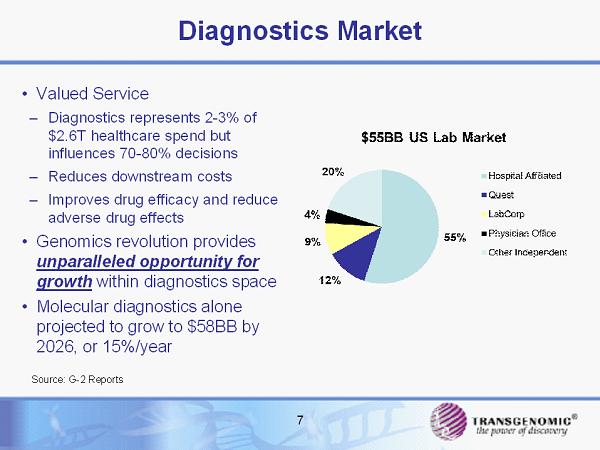
Diagnostics Market • Valued Service – Diagnostics represents 2 - 3% of $2.6T healthcare spend but influences 70 - 80% decisions – Reduces downstream costs – Improves drug efficacy and reduce adverse drug effects • Genomics revolution provides unparalleled opportunity for growth within diagnostics space • Molecular diagnostics alone projected to grow to $58BB by 2026, or 15%/year 7 Source: G - 2 Reports
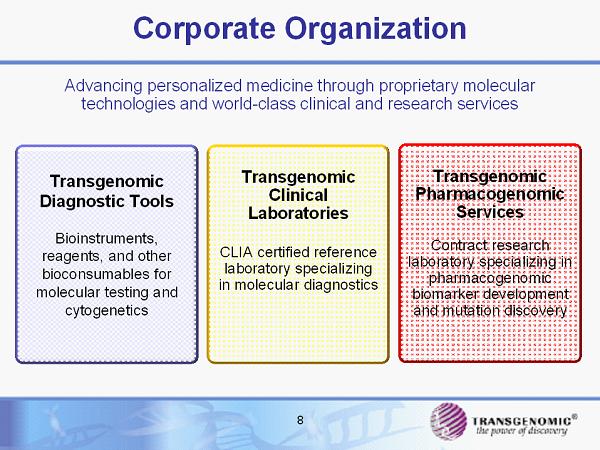
8 Advancing personalized medicine through proprietary molecular technologies and world - class clinical and research services Corporate Organization Transgenomic Diagnostic Tools Bioinstruments, reagents, and other bioconsumables for molecular testing and cytogenetics Transgenomic Clinical Laboratories CLIA certified reference laboratory specializing in molecular diagnostics Transgenomic Pharmacogenomic Services Contract research laboratory specializing in pharmacogenomic biomarker development and mutation discovery

Strong Revenue Platform 9 (Pro forma) ($ In ‘000)

Strong Integration Management 10

Our Pathway to Market Leadership 11 Complementary Businesses Drive Revenue, Supporting Innovation and High - Value Product Development, Building to Long - term Profitability Areas of Focus Oncology Cardiology Neurology Clinical Labs Companion Diagnostics Diagnostic Tools Pharmacogenomic Services Proprietary Technology

12 Transgenomic Pharmacogenomic Services

13 Pharmacogenomic Services • Initial commercial vehicle for our proprietary technologies • Studies completed or on - going with top pharma companies • Market leading DNA mutation detection for key cancer gene mutation studies employing Sanger sequencing, SURVEYOR and ICE COLD - PCR • ICE COLD - PCR Assays for key cancer gene mutations in blood are a key future process enhancement driving growth • Also offer CGH, methylation and gene expression assays • Pharmacogenomic projects support new assay and technology development

14 Transgenomic Clinical Laboratories
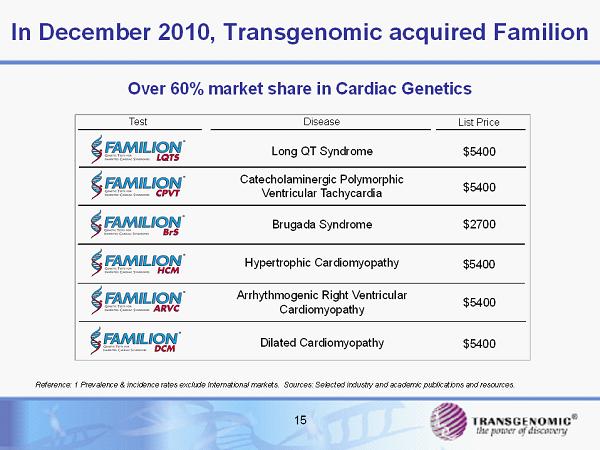
15 In December 2010, Transgenomic acquired Familion Test Long QT Syndrome List Price Disease Hypertrophic Cardiomyopathy Brugada Syndrome Catecholaminergic Polymorphic Ventricular Tachycardia Arrhythmogenic Right Ventricular Cardiomyopathy $5400 $2700 $5400 $5400 $5400 Dilated Cardiomyopathy $5400 Reference: 1 Prevalence & incidence rates exclude International markets. Sources: Selected industry and academic publication s a nd resources. Over 60% market share in Cardiac Genetics
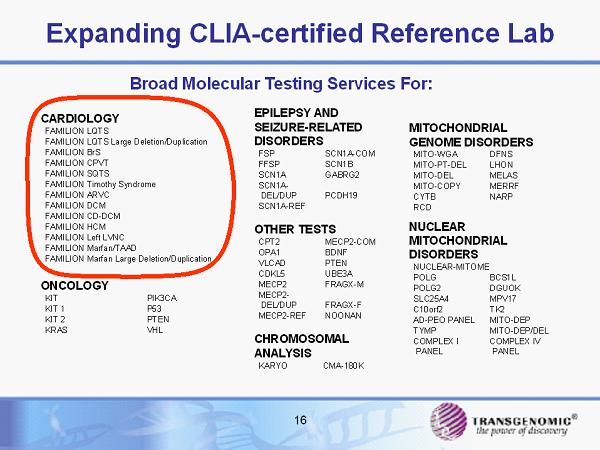
16 Expanding CLIA - certified Reference Lab MITOCHONDRIAL GENOME DISORDERS MITO - WGA DFNS MITO - PT - DEL LHON MITO - DEL MELAS MITO - COPY MERRF CYTB NARP RCD NUCLEAR MITOCHONDRIAL DISORDERS NUCLEAR - MITOME POLG BCS1L POLG2 DGUOK SLC25A4 MPV17 C10orf2 TK2 AD - PEO PANEL MITO - DEP TYMP MITO - DEP/DEL COMPLEX I PANEL COMPLEX IV PANEL EPILEPSY AND SEIZURE - RELATED DISORDERS FSP SCN1A - COM FFSP SCN1B SCN1A GABRG2 SCN1A - DEL/DUP PCDH19 SCN1A - REF OTHER TESTS CPT2 MECP2 - COM OPA1 BDNF VLCAD PTEN CDKL5 UBE3A MECP2 FRAGX - M MECP2 - DEL/DUP FRAGX - F MECP2 - REF NOONAN CHROMOSOMAL ANALYSIS KARYO CMA - 180K CARDIOLOGY FAMILION LQTS FAMILION LQTS Large Deletion/Duplication FAMILION BrS FAMILION CPVT FAMILION SQTS FAMILION Timothy Syndrome FAMILION ARVC FAMILION DCM FAMILION CD - DCM FAMILION HCM FAMILION Left LVNC FAMILION Marfan/TAAD FAMILION Marfan Large Deletion/Duplication ONCOLOGY KIT PIK3CA KIT 1 P53 KIT 2 PTEN KRAS VHL Broad Molecular Testing Services For:

17 Successful Integration of CLDA Assets • Accelerates Transgenomic to ~ $32 million run rate with addition of $10.5 million in molecular lab testing sales • Brings state - of - the - art laboratory, key scientific, billing, customer service and marketing employees & expertise • Enabled consolidation of company’s lab services • Took 2 businesses that were losing over $8MM per year to break even (modified EBITDA) in 2011 • Adds over 280 million covered lives under reference lab testing contracts • Adds additional proprietary biomarkers with significant potential • Purchase price $15.4 million (cash/notes), supported by Third Security (RJ Kirk, CEO)

18 Transgenomic DNA Tools
» WAVE ® , SURVEYOR ® Scan, BLOCker™ - Sequencing and ICE COLD - PCR are breakthrough methodologies that enhance the ability of Sanger sequencing to detect and identify mutations at or significantly beyond the levels achieved with allele - specific probes or next generation sequencing » SURVEYOR Scan kits can be run on multiple platforms → Ability to meet any research or testing need » BLOCker - Sequencing and ICE COLD - PCR are run on standard Sanger sequencing equipment found in most molecular labs Technologies with Broad Appeal 19
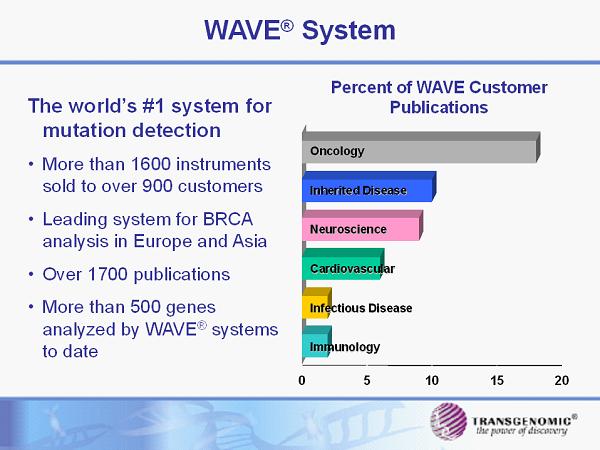
0 5 10 15 20 Percent of WAVE Customer Publications Oncology Inherited Disease Neuroscience Cardiovascular Infectious Disease Immunology WAVE ® System The world’s #1 system for mutation detection • More than 1600 instruments sold to over 900 customers • Leading system for BRCA analysis in Europe and Asia • Over 1700 publications • More than 500 genes analyzed by WAVE ® systems to date

21 WAVE ® MCE and SURVEYOR ® Scan Transgenomic’s new platform offers cutting - edge technology defined by their best in class sensitivity and comprehensive scanning ability for detecting mutations
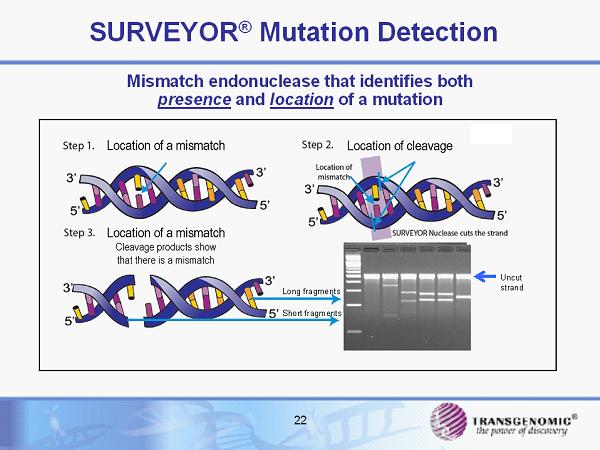
22 SURVEYOR ® Mutation Detection Mismatch endonuclease that identifies both presence and location of a mutation Long fragments Short fragments Uncut strand
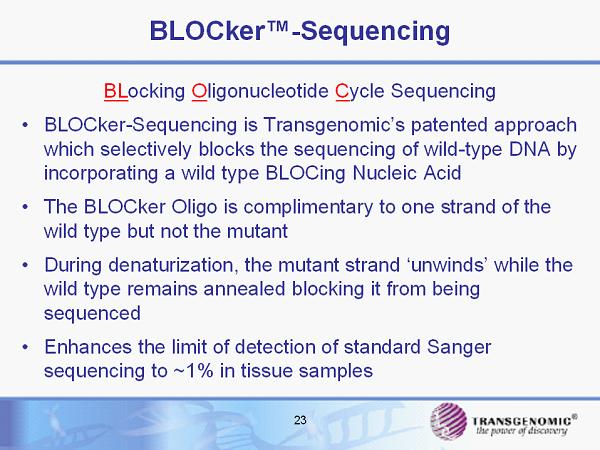
BLOCker™ - Sequencing BL ocking O ligonucleotide C ycle Sequencing • BLOCker - Sequencing is Transgenomic’s patented approach which selectively blocks the sequencing of wild - type DNA by incorporating a wild type BLOCing Nucleic Acid • The BLOCker Oligo is complimentary to one strand of the wild type but not the mutant • During denaturization, the mutant strand ‘unwinds’ while the wild type remains annealed blocking it from being sequenced • Enhances the limit of detection of standard Sanger sequencing to ~1% in tissue samples 23
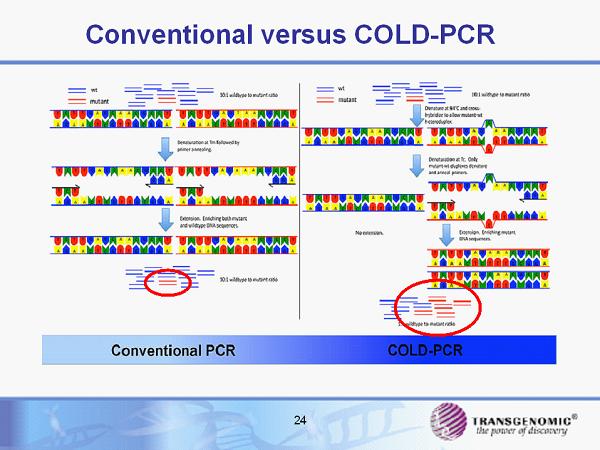
Conventional versus COLD - PCR 24

ICE COLD - PCR I mproved and C omplete E nrichment CO amplification at L ower D enaturation temperatures – PCR • Patented enhancement to COLD - PCR demonstrating reproducible detection at very high analytic sensitivity (100 to 1000 fold over “normal” Sanger sequencing) for detection of DNA in blood and body fluids • Specifically enhances amplification of mutated DNA • Enable detection of cancer gene mutations for early diagnosis of disease, monitoring of disease, recurrence and therapeutic response • Exclusively licensed from Dana Farber for Sanger sequencing and pyrosequencing, non - exclusive for RT - PCR, next gen sequencing Several collaborations beginning to further validate ICE COLD - PCR 25
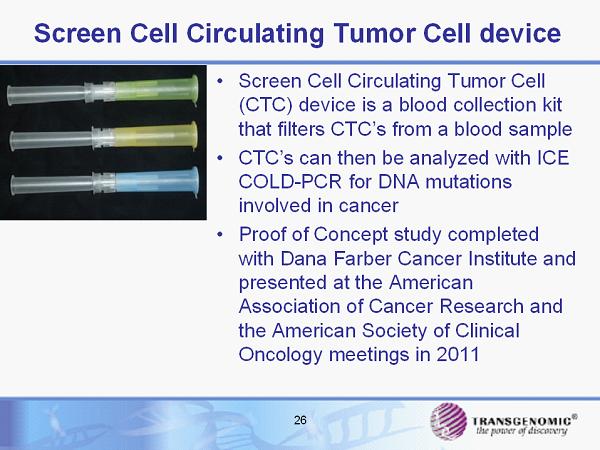
26 Screen Cell Circulating Tumor Cell device • Screen Cell Circulating Tumor Cell (CTC) device is a blood collection kit that filters CTC’s from a blood sample • CTC’s can then be analyzed with ICE COLD - PCR for DNA mutations involved in cancer • Proof of Concept study completed with Dana Farber Cancer Institute and presented at the American Association of Cancer Research and the American Society of Clinical Oncology meetings in 2011
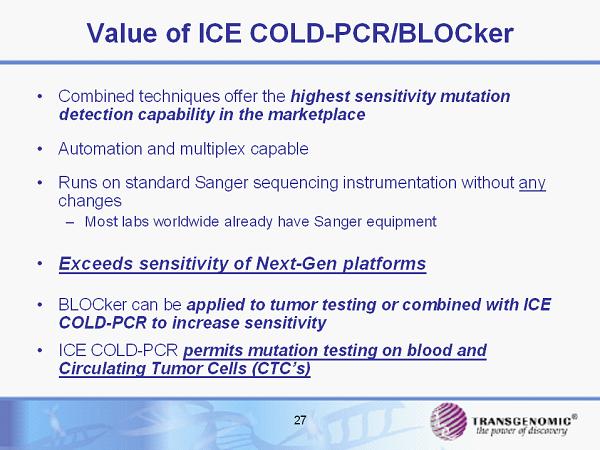
27 Value of ICE COLD - PCR/BLOCker • Combined techniques offer the highest sensitivity mutation detection capability in the marketplace • Automation and multiplex capable • Runs on standard Sanger sequencing instrumentation without any changes – Most labs worldwide already have Sanger equipment • Exceeds sensitivity of Next - Gen platforms • BLOCker can be applied to tumor testing or combined with ICE COLD - PCR to increase sensitivity • ICE COLD - PCR permits mutation testing on blood and Circulating Tumor Cells (CTC’s)
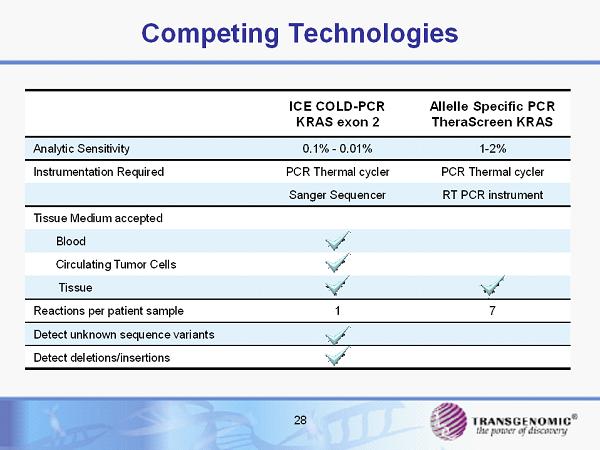
Competing Technologies 28 ICE COLD - PCR KRAS exon 2 Allelle Specific PCR TheraScreen KRAS Analytic Sensitivity 0.1% - 0.01 % 1 - 2% Instrumentation Required PCR Thermal cycler PCR Thermal cycler Sanger Sequencer RT PCR instrument Tissue Medium accepted Blood Circulating Tumor Cells Tissue Reactions per patient sample 1 7 Detect unknown sequence variants Detect deletions/insertions
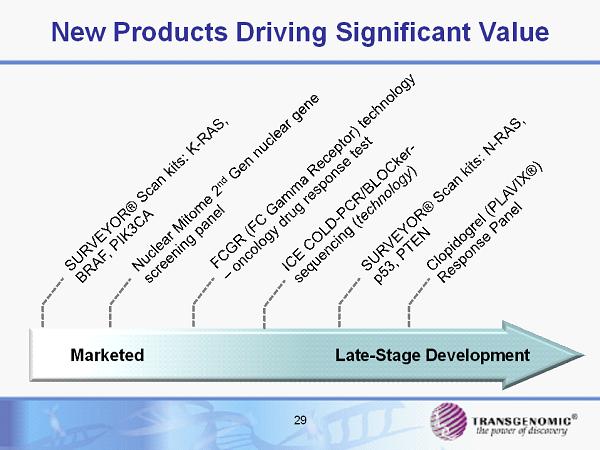
29 New Products Driving Significant Value Marketed Late - Stage Development

30 Integrated Strategy for Growth • Expand consolidated reference laboratory business following successful integration of CLDA assets – Expand testing menu – License new markers – Acquire and integrate complementary businesses • Leverage mutation detection sensitivity leadership to build and market best - in - class assay panels in our Services and Tools businesses targeting key oncology genes • Continue licensing activities for panel and technology build - out • Achieve high - margin profitability through innovations that bridge drug development and areas of high unmet medical need
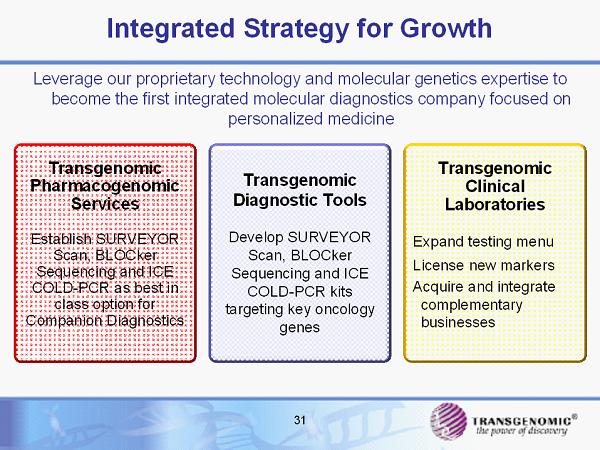
31 Leverage our proprietary technology and molecular genetics expertise to become the first integrated molecular diagnostics company focused on personalized medicine Integrated Strategy for Growth Transgenomic Diagnostic Tools Develop SURVEYOR Scan, BLOCker Sequencing and ICE COLD - PCR kits targeting key oncology genes Transgenomic Clinical Laboratories Expand testing menu License new markers Acquire and integrate complementary businesses Transgenomic Pharmacogenomic Services Establish SURVEYOR Scan, BLOCker Sequencing and ICE COLD - PCR as best in class option for Companion Diagnostics

Transgenomic, Inc. Craig - Hallum 2 nd Annual Alpha Select Conference October 6, 2011 (OTCBB: TBIO) 32 Thank You
