Attached files
| file | filename |
|---|---|
| EX-31.1 - SECTION 302 PRESIDENT & CEO CERTIFICATION - BG Medicine, Inc. | d231413dex311.htm |
| 10-Q/A - FORM 10-Q AMENDMENT NO. 1 - BG Medicine, Inc. | d231413d10qa.htm |
| EX-31.2 - SECTION 302 VICE PRESIDENT & CFO CERTIFICATION - BG Medicine, Inc. | d231413dex312.htm |
Exhibit 10.1
SUPPLY AGREEMENT
This Supply Agreement (“Agreement”) is effective as of the 15th day of March, 2011 (the “Effective Date”), between BG Medicine, Inc., a Delaware corporation with offices at 610N Lincoln Street, Waltham, MA 02451 (“BGM”), and Health Diagnostic Laboratory, Inc., a Virginia corporation with offices at 737 N. 5th Street, Suite 103, Richmond, VA 23219 (“HDL”).
WHEREAS, BGM has developed an assay for measuring Galectin-3 levels in a human specimen, which has been shown to provide clinically useful information that could be used by physicians as an aid in the stratification of patients diagnosed with heart failure, among other potential clinical indications; and
WHEREAS, HDL is engaged in the business of providing laboratory testing services; and
WHEREAS, HDL desires to purchase kits from BGM for the performance of BGM’s Galectin-3 assay, and BGM desires to sell such kits to HDL in accordance with the terms and conditions of this Agreement.
NOW, THEREFORE, HDL and BGM hereby agree as follows:
1. Definitions. For purposes of this Agreement, the terms below shall have the meanings set forth below. Additional terms are defined above and throughout the Agreement.
“Affiliate” means, with respect to a party to this Agreement, any current or future Entity which controls, is controlled by, or is under common control with such party. For purposes of this definition only, “control” means direct or indirect ownership of at least fifty percent (50%) of the shares of the subject Entity entitled to vote in the election of directors (or, in the case of an Entity that is not a corporation, for the election of the corresponding managing authority).
“AMA” means the American Medical Association.
“Change of Control” means a transaction or event (or series of related transactions or events) as a result of which the holders of the outstanding voting stock of a party as of the Effective Date cease to own a majority of the outstanding voting stock of such party.
“Clinical Lab” means any Entity engaged in the business of providing clinical laboratory products and/or services.
“CMO” means any Entity with which BGM contracts for the manufacture of Manual Test Kits.
“Desired [***] Change” means an [***] for galectin-3 testing (that would include the Manual Test) with a Medicare national limitation amount (“NLA”) equal to or greater than [***].
“Entity” means a person, corporation, partnership, association, limited liability company, unincorporated organization, firm, or other entity.
1
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
“FDA” means the United States Food and Drug Administration or any successor agency thereto.
“FDA Approval” means final approval or clearance by the FDA through a premarket approval application (PMA), 510(k) premarket notification (PMN), or other applicable regulatory pathways which are now or may become available to market and sell the Testing Services for commercial purposes in the United States for use with regard to humans.
“Galectin-3” means the protein known as Galectin-3.
“GMP” shall mean the current Good Manufacturing Practice regulations and the Quality System Regulations promulgated by the FDA, including 21 C.F.R. Part 820 et seq., as such regulations may be amended from time to time, and such equivalent regulations or standards of countries outside the United States as may be applicable to activities conducted hereunder.
“Kit” means an in vitro diagnostic medical device, as defined in regulation at 21 C.F.R. § 809.3(a), that is in a kit form and that requires FDA Approval, which can be used to perform the Test. The kit contains the test reagents, microtiter plates, calibrator and quality control materials.
“Kit Price” means (i) [***] per Manual Test Kit until the Desired [***] Change has been approved by the [***], and (ii) [***] per Manual Test Kit after the Desired [***] has been [***] and a [***].
“Manual Test” means a version of the Test which is performed using handheld pipettors, with technologist intervention to move the samples through the steps of the testing protocol.
“Manual Test Kit” means a Kit for performing the Manual Test, as described on Exhibit A attached hereto.
“Regulatory Authority” means any governmental authority with jurisdiction over the manufacture, distribution, use, and marketing of in vitro diagnostics and/or new drug products intended for human use, including the FDA.
“Specifications” means the specifications and descriptions for the Manual Test Kit set forth in the FDA cleared package insert and the manufacturer specifications.
“Term” means the period beginning on the Effective Date and ending upon expiration or termination of this Agreement.
“Territory” means the United States of America
“Test” means an assay that measures Galectin-3 in a human specimen by enzyme-linked immunosorbent assay (ELISA) on a microtiter plate platform, as such assay may be improved or otherwise modified from time to time during the Term with 90 day notice to HDL.
“Testing Service” means the performance of the Manual Test for third parties.
2
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
2. Supply of Manual Test Kits.
2.1. Purchase Orders. HDL will submit to BGM purchase orders for Manual Test Kits from time to time. All purchase orders shall specify the type and quantity of Manual Test Kits being ordered, the requested delivery date, and the requested delivery location, which shall be in the Territory. HDL shall not submit purchase orders containing any other terms. If HDL does submit a purchase order containing other terms then, in addition to any other remedies available to BGM, such terms shall automatically be deemed rejected by BGM, and in no event shall BGM’s failure to object to such terms or conditions, or BGM’s provision of products specified in such purchase order, be deemed acceptance of such terms.
2.2. Acceptance of Orders. Upon receipt of each purchase order, [***] the purchase order. BGM agrees to supply the Manual Test Kits to HDL in accordance with the terms of each accepted purchase order to the extent consistent with Section 2.1 and the other provisions of this Agreement.
2.3. Labeling; Packaging. All Manual Test Kits shall be labeled, packaged and shipped in accordance with applicable law.
2.4. Shipping Charges; Risk of Loss. All shipments shall be made FOB delivery address with the shipping charges paid by HDL. BGM shall bear all risk of loss until such time as delivery is made to HDL.
2.5. Quality Control Testing. All production batches of Manual Test Kits shall be manufactured in accordance with the current Good Manufacturing Practice regulations and the Quality System Regulations promulgated by the FDA, including 21 C.F.R. Part 820 and tested in accordance with appropriate quality control testing procedures prior to shipment to HDL, and Manual Test Kits failing such testing shall not be shipped to HDL.
2.6. Supply to Affiliates. If any Affiliate of HDL located in the Territory desires to purchase Manual Test Kits from BGM under the terms of this Agreement, then BGM shall provide such Affiliate with all of the benefits hereof and treat such Affiliate as HDL for the purposes of this Agreement and such Affiliates shall have all the obligations of HDL hereunder. HDL unconditionally guarantees all of the obligations contained in this Agreement of its Affiliates purchasing Manual Test Kits from BGM pursuant to this Agreement.
3. Inclusion of Test in Panel; Right of First Negotiation.
3.1. Inclusion of Test in Panel. HDL shall include the Test in its standard menu of lab tests offered by HDL.
3.2. Right of First Negotiation. If BGM intends to enter into an agreement with a third party under which such party would have the right to offer or sell the multivariate biomarker blood-based test for atherothrombotic cardiovascular disease currently being developed by BGM under the name “AMIPredict,” In the event HDL performs a minimum of [***] galectin-3 assays per [***] for a minimum of [***] prior to [***], BGM shall, before entering into any such agreement, provide HDL with written notice of such intention, and negotiate with HDL in good faith for a period of [***] regarding the terms upon which BGM might enter into such an agreement with HDL. If the parties are unable to agree on such terms within such [***] period, BGM shall have no further obligation to HDL with respect to such test.
3
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
4. Payments to BGM.
4.1. Prices. HDL agrees to pay the Kit Price for each Manual Test Kit purchased by HDL and its Affiliates.
4.2. Invoicing; Payment Terms and Taxes. BGM will provide an invoice to HDL for all Manual Test Kits ordered and shipped to HDL or its Affiliates, which shall be payable by HDL within thirty (30) days after the date of HDL’s receipt of the invoice. HDL is taxable based on the shipment destination and all applicable local/state taxes are the responsibility of HDL. In those states where BGM collects local/state sales taxes, BGM will add these taxes to the invoices and remit to the appropriate taxing authority. Invoices shall be mailed to HDL at the following address:
ATTN: Accounts Payable
Health Diagnostic Laboratory, Inc.
737 N. 5th Street, Suite 103
Richmond, VA 23219
5. Support and Assistance.
5.1. Support to be Provided by BGM. BGM agrees to provide the following clinical market development resources, programs and assistance as reasonably requested by HDL for the Testing Services (at no additional cost to HDL):
5.1.1. BGM will manage clinical studies it performs concerning the Test, clinical publications relating to the Test, and maintain the supporting data and other sources of proof concerning the safety, accuracy and efficacy of the Test;
5.1.2. BGM will use commercially reasonable efforts to maintain relationships with key opinion leaders in the field associated with the Test;
5.1.3. BGM will use commercially reasonable efforts to manage and fund society and clinical speakers bureaus which may be beneficial for the Test;
5.1.4. BGM will use commercially reasonable efforts to offer CME and other medical education programs relating to the Test;
5.1.5. BGM will use commercially reasonable efforts to provide field sales support for the Test in coordination with HDL;
5.1.6. BGM will supply HDL and its Affiliates with marketing and promotional information for the Test for inclusion by HDL in the materials created by HDL pursuant to Section
5.2. Support to be Provided by HDL. HDL agrees to provide the following marketing support and market education assistance during the Term of this Agreement (at no additional cost to BGM):
4
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
5.2.1. HDL will train its sales and service representatives (including without limitation communicating the core messages and value proposition for the Test) to enable them to sell the Testing Service by calling on relevant physician segments;
5.2.2. HDL will [***] to [***] who have [***];
5.2.3. HDL will cooperate in good faith with BGM in the participation and promotion (with BGM representatives) of a reasonable number of BGM-funded events (including without limitation “Lunch and Learns”, grand rounds, and professional/medical seminars), provided HDL receives reasonable prior notice of such events;
5.2.4. HDL will [***] with a [***] of the [***] for [***].
6. Kit Quality, Warranties, and Legal Standards.
6.1. BGM Warranties. BGM represents, warrants and covenants to HDL that:
6.1.1. All Manual Test Kits shall be free from material defects when shipped to HDL and its Affiliates and shall conform to their Specifications for a minimum shelf life of the longer of (i) [***] from the date of delivery.
6.1.2. BGM will convey to HDL and its Affiliates good and full title to all Manual Test Kits delivered, free and clear of any security interests, liens, claims or encumbrances;
6.1.3. To BGM’s knowledge, neither BGM nor any of its employees or agents rendering services pursuant to this Agreement is under investigation by any Regulatory Authority, including the FDA, for activities that could form the basis of a debarment action or is presently debarred pursuant to the Generic Drug Enforcement Act of 1992, 21 U.S.C. § 335a, or any other similar law of any Regulatory Authority; and BGM shall notify HDL promptly upon any learning of any inquiry concerning or the commencement of any such investigation or proceeding involving BGM or any person or entity related to or involved in BGM’s performance of its obligations under this Agreement;
6.1.4. BGM’s personnel and consultants have, and shall have, all training, licenses, approvals, certifications, immunizations, equipment and information to the extent required by law, including specifically 21 C.F.R. Part 820 (to the extent applicable), and consistent with the standard of care in the industry, including those actions reasonably necessary for safely and properly performing the obligations under this Agreement, and BGM will use commercially reasonable efforts to ensure that all such training, licenses, approvals, certifications, immunizations, equipment and information are properly maintained throughout the conduct of BGM’s activities under this Agreement;
6.1.5. BGM is and will remain in compliance with all applicable regulatory and legal requirements, as interpreted and enforced by Regulatory Authorities, including but not limited to registration and listing requirements under 21 C.F.R. Part 807 (to the extent applicable), and the Quality System Regulations under 21 C.F.R. Part 820;
6.1.6. Neither BGM nor, to its knowledge, any of its personnel, have been involved in an investigation or in research that was terminated, as the term “termination” is used in 21 C.F.R. § 812.3(q), nor, to BGM’s knowledge, have they been subjected to any restrictions or sanctions related to allegations of research or professional misconduct;
5
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
6.1.7. None of the Manual Test Kits supplied by BGM shall be adulterated or misbranded (within the meaning of the U.S. Food Drug and Cosmetic Act, as amended, 21 U.S.C. 301 et. seq., and the regulations promulgated thereunder) when shipped by BGM to HDL;
6.2. Records. BGM shall, at all times, keep accurate records with respect to Manual Test Kits produced and sold to HDL pursuant to this Agreement, as required by applicable law, including without limitation GMP.
6.3. HDL Warranties. HDL represents, warrants and covenants to BGM that:
6.3.1. HDL’s obligations and responsibilities under this Agreement (including without limitation the performance of Manual Tests) will be performed in compliance with applicable federal, state and local laws, rules and regulations, as interpreted and enforced by Regulatory Authorities;
6.3.2. HDL shall use the Manual Test Kits only for the purpose of providing the Testing Service. HDL shall not resell or otherwise transfer Manual Test Kits to any Entity, except that HDL may transfer Manual Test Kits to one or more Affiliates that provide, or assist HDL in providing, the Testing Service;
6.3.3. To HDL’s knowledge, neither HDL nor any of its employees or agents rendering services pursuant to this Agreement is under investigation by any Regulatory Authority, including the FDA, for activities that could form the basis of a debarment action or is presently debarred pursuant to the Generic Drug Enforcement Act of 1992, 21 U.S.C. § 335a, or any other similar law of any Regulatory Authority; and HDL shall notify BGM promptly upon any learning of any inquiry concerning or the commencement of any such investigation or proceeding involving BGM or any person or entity related to or involved in BGM’s performance of its obligations under this Agreement;
6.3.4. HDL’s personnel and consultants have, and shall have, all training, licenses, approvals, certifications, immunizations, equipment and information to the extent required by law, and consistent with the standard of care in the industry, including those actions reasonably necessary for safely and properly performing the obligations under this Agreement, and HDL will use commercially reasonable efforts to ensure that all such training, licenses, approvals, certifications, immunizations, equipment and information are properly maintained throughout the conduct of HDL’s activities under this Agreement;
6.3.5. HDL is and will remain in compliance with all applicable regulatory and legal requirements, as interpreted and enforced by Regulatory Authorities, including but not limited to registration and listing requirements under 21 C.F.R. Part 807 (to the extent applicable), and the Quality System Regulations under 21 C.F.R. Part 820; and
6.3.6. Neither HDL nor, to its knowledge, any of its personnel, have been involved in an investigation or in research that was terminated, as the term “termination” is used in 21 C.F.R. § 812.3(q), nor, to HDL’s knowledge, have they been subjected to any restrictions or sanctions related to allegations of research or professional misconduct.
6
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
7. Term; Termination.
7.1. Term. This Agreement shall commence as of the Effective Date and, unless earlier terminated pursuant to the terms of this Agreement, shall continue until the five (5) year anniversary of the Effective Date. Upon expiration of the foregoing period, this Agreement shall automatically terminate unless extended by mutual written agreement of the parties.
7.2. Termination for Breach. This Agreement may be terminated upon sixty (60) days prior written notice by either party for failure of the other party to comply with the terms of this Agreement (with the notice including a reasonable description of such failure), unless the party in default remedies its failure within such sixty (60) day notice period.
7.3. Effect of Termination. Upon the expiration or termination of this Agreement for any reason, (i) HDL shall be responsible for the payment of all issued and outstanding invoices for Manual Test Kits delivered as of the effective date of expiration or termination hereof and any Manual Test Kits delivered and accepted under subsection (ii) hereof; (ii) BGM shall complete delivery of Manual Test Kits under all purchase orders then existing according to the terms of this Agreement; (iii) each party shall promptly return any confidential information of the other Party (including without limitation all copies) except for one copy which may be retained solely for archival/legal purposes; and (iv) the provisions of Sections 1 and 6 through 10, as well as any other obligations which by their terms continue after expiration or termination of this Agreement, shall survive the expiration or termination of this Agreement for any reason and remain binding upon the parties. Notwithstanding the expiration or termination of this Agreement for any reason, HDL and its Affiliates shall be permitted, following such termination, to use any Manual Test Kits it has already purchased to perform Tests and sell the Testing Services.
8. Confidentiality. The parties acknowledge that they may exchange confidential or proprietary information as a result of this Agreement, including without limitation the Specifications and other information related to the Manual Test Kits. Each party agrees that, during the term of this Agreement and thereafter, it shall not, directly or indirectly, (i) use the other party’s confidential or proprietary information for any reason other than to perform its obligations or exercise its rights under this Agreement, or (ii) disclose or otherwise make available the other party’s confidential or proprietary information to any third Parties, except in either case as authorized by such other party in writing.
9. Limitations of Liability.
9.1. Indemnification by BGM. BGM agrees to indemnify, defend, and hold harmless HDL, its Affiliates, and their respective employees, officers, directors, agents, successors, and assigns from and against any and all liabilities, obligations, losses, fines, costs, penalties, assessments, deficiencies, demands, actions, suits, proceedings, judgments, expenses or damages of any nature (including without limitation attorneys’ fees) resulting from claims of third parties arising from or relating to (i) any breach of this Agreement by BGM (including without limitation breach of any representations, warranties, covenants or obligations of BGM herein) or (ii) any misrepresentations made by BGM’s field representatives or other employees or agents to third parties concerning the Test or Testing Services. However, the foregoing rights to indemnity shall not apply to the extent that such claim results from HDL’s negligence or intentionally harmful misconduct, HDL’s breach of this Agreement, or from the modification or improper handling,
7
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
storage or use of any Manual Test Kit by HDL or a third party outside of BGM’s control or without BGM’s permission or any incorrect reporting of results from the performance of Manual Tests. In the event of a lawsuit or other action in connection with which HDL is seeking indemnification from BGM hereunder, HDL agrees to give timely notice of the lawsuit or action to BGM and to cooperate with BGM in the defense of the lawsuit or action, at BGM’s expense.
9.2. Indemnification by HDL. HDL agrees to indemnify, defend, and hold harmless BGM, its Affiliates, and their respective employees, officers, directors, agents, successors, and assigns from and against any and all liabilities, obligations, losses, fines, costs, penalties, assessments, deficiencies, demands, actions, suits, proceedings, judgments, expenses or damages of any nature (including without limitation attorneys’ fees) resulting from claims of third parties arising from or relating to (i) any breach of this Agreement by HDL (including without limitation breach of any representations, warranties, covenants or obligations of HDL herein), (ii) any misrepresentations made by HDL’s field representatives or other employees or agents to third parties concerning the Test or Testing Services, or (iii) HDL and/or its Affiliates’ improper handling, storage or use of any Manual Test Kits or any incorrect reporting of results from the performance of Manual Tests. However, the foregoing rights to indemnity shall not apply to the extent that such claim results from BGM’s negligence or intentionally harmful misconduct or BGM’s breach of this Agreement. In the event of a lawsuit or other action in connection with which BGM is seeking indemnification from HDL hereunder, BGM agrees to give timely notice of the lawsuit or action to HDL and to cooperate with HDL in the defense of the lawsuit or action, at HDL’s expense.
9.3. Warranty Disclaimer. EXCEPT AS OTHERWISE EXPRESSLY PROVIDED IN THIS AGREEMENT, NEITHER PARTY MAKES ANY WARRANTY WITH RESPECT TO ANY TECHNOLOGY, GOODS, SERVICES, RIGHTS OR OTHER SUBJECT MATTER OF THIS AGREEMENT AND EACH PARTY HEREBY DISCLAIMS ALL WARRANTIES, EXPRESS OR IMPLIED, INCLUDING, WITHOUT LIMITATION, WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
9.4. Limited Liability. NOTWITHSTANDING ANYTHING TO THE CONTRARY IN THIS AGREEMENT, NEITHER PARTY SHALL BE LIABLE TO THE OTHER PARTY OR ANY OF ITS AFFILIATES FOR ANY SPECIAL, PUNITIVE, INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES, INCLUDING, WITHOUT LIMITATION, LOST PROFITS OR LOST REVENUES, EXCEPT FOR (A) DAMAGES INCLUDED IN THIRD PARTY CLAIMS FOR WHICH A PARTY IS ENTITLED TO INDEMNIFICATION FROM THE OTHER PARTY, OR (B) DAMAGES ARISING FROM INTENTIONAL MISCONDUCT OR GROSS NEGLIGENCE OF THE OTHER PARTY. THE TOTAL LIABILITY OF EACH PARTY, REGARDLESS OF WHETHER SUCH LIABILITY IS BASED ON BREACH OF CONTRACT, TORT, STRICT LIABILITY, BREACH OF WARRANTIES, FAILURE OF ESSENTIAL PURPOSE OR OTHERWISE, UNDER THIS AGREEMENT OR WITH RESPECT TO THE MANUAL TEST KITS SHALL BE LIMITED TO THE AMOUNTS RECEIVED BY BGM FROM HDL UNDER THIS AGREEMENT, PROVIDED, THAT THE FOREGOING SHALL NOT LIMIT THE PARTIES’ OBLIGATIONS TO PROVIDE INDEMNIFICATION WITH RESPECT TO THIRD PARTY CLAIMS PURSUANT TO SECTION 9.1 OR 9.2, AS APPLICABLE.
8
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
10. Miscellaneous.
10.1. Governing Law. This Agreement and the rights and obligations of the parties hereunder shall be governed by and construed in accordance with the laws of the State of New York, without regard to its conflicts of laws principles.
10.2. Benefit; Assignment. The rights, duties, and obligations of the parties under this Agreement shall inure to the benefit and shall be binding upon their respective successors and permitted assigns. Neither this Agreement nor the respective rights, duties, obligations, and responsibilities of either party under this Agreement may be assigned, subcontracted or otherwise transferred, in whole or in part, to any other Entity without the prior written consent of the other party, which consent shall not be unreasonably withheld; provided, however, that without such consent (i) BGM shall have the right to have Manual Test Kits manufactured by a CMO, and (ii) either party may assign this Agreement in whole, but not in part, to any purchaser of all or substantially all of its assets to which this Agreement relates, or to an Affiliate or to any successor corporation resulting from a Change of Control.
10.3. Relationship. The relationship between BGM and HDL is solely that of seller, on the one hand, and buyer, on the other hand, and nothing in this Agreement shall constitute either party as the agent, partner or legal representative of the other party for any purpose whatsoever; nor shall either party hold itself out as such. Neither party shall have any authority to bind or commit the other party in any manner or for any purpose.
10.4. Notices. Any notice, request or communication given, made or sent pursuant to the terms of this Agreement shall be made in writing and shall be deemed duly given: (i) on the day delivered personally, (ii) three (3) days after being sent registered or certified mail, return receipt requested, or (iii) one day after the date communicated via facsimile (with the original being sent the same day by registered or certified mail, return receipt requested) to the other party at the following addresses and numbers (or to such other addresses and numbers as either party hereto may hereafter designate in writing):
| BGM | HDL | |
| BG Medicine, Inc. | Health Diagnostic Laboratory, Inc. | |
| Attn: President | Attn: President | |
| 610N Lincoln Street | 737 N. 5th Street, Suite 103 | |
| Waltham, MA 02451 | Richmond, VA 23219 | |
| Fax: (781) 895-1119 | Fax: (804) 343-2704 | |
10.5. Change in Law. The terms of this Agreement are intended to be in compliance with all federal, state and local statutes, regulations and ordinances applicable on the Effective Date. The parties agree to execute amendments as may be necessary for the continuing compliance with the aforementioned applicable laws, as additional regulations are promulgated or become final and effective.
10.6. Severability; Headings; Counterparts; Amendment; Waiver; Entire Agreement. If any term or provision of this Agreement shall be held invalid or unenforceable, the remaining items hereof shall not be affected, but shall be valid and enforced to the fullest extent permitted by law.
9
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
The headings used in this Agreement are intended for guidance only and shall not be considered part of the written understanding between the parties hereto. This Agreement may be executed in counterparts in order to provide each party with a fully-executed original hereof. Except as otherwise provided herein, this Agreement may not be changed, modified or amended except by an agreement in writing signed by both parties. The waiver by any party to this Agreement of any breach or violation of any provisions of this Agreement by any other party hereto shall not operate as a waiver of any other or subsequent breach. All Exhibits attached hereto are hereby incorporated by reference. This Agreement reflects the complete understanding of the parties and constitutes their entire agreement, superseding all prior negotiations, representations, agreements, understandings, and statements regarding its subject matter.
10.7. Force Majeure. Neither party shall be liable for loss, damage, detention or delay resulting from any cause whatsoever beyond its reasonable control or resulting from a force majeure, including, without limitation, fire, flood, strike, lockout, civil or military authority, insurrection, war, or embargo, and delivery dates shall be extended to the extent of any delays resulting from the foregoing or similar causes. The party so affected shall give prompt notice to the other party of such cause, and shall take whatever reasonable steps are necessary to relieve the effect of such cause as rapidly as reasonably possible. The party giving such notice shall be excused from such of its obligations hereunder for so long as it is so disabled; provided, however, that such affected party commences and continues to take reasonable and diligent actions to cure such cause. Notwithstanding the foregoing, nothing in this Section 10.7 shall excuse or suspend the obligation to make any payment due hereunder in the manner and at the time provided.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
10
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
IN WITNESS WHEREOF, HDL and BGM have caused this Supply Agreement to be executed by their duly authorized officer as of the Effective Date.
| BG Medicine, Inc. | Health Diagnostic Laboratory, Inc. | |||||||
| By: | /s/ Pieter Muntendam | By: | /s/ Tonya Mallory 3/15/11 | |||||
| Printed Name: | Pieter Muntendam | Printed Name: | Tonya Mallory | |||||
| Title: | President and CEO | Title: | Pres & CEO | |||||
11
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit A
Manual Test Kit Description (FDA Approved Product Insert)
12
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.

Table of Contents
| Intended Use |
3 | |||
| Summary and Explanation of the Test |
3 | |||
| Principle of the Test Procedure |
3 | |||
| Kit Contents |
4 | |||
| Storage Instructions |
4 | |||
| Specimen Collection and Storage |
5 | |||
| Warnings and Precautions |
5 | |||
| Materials Required But Not Provided |
6 | |||
| Quality Control |
9 | |||
| BGM Galectin-3 Controls (supplied with kit) |
9 | |||
| Additional quality control (optional for users) |
9 | |||
| BGM Galectin-3 Test Procedure |
10 | |||
| Preparation of Reagents |
10 | |||
| Detailed Assay Procedure |
10 | |||
| Procedural Notes |
18 | |||
| Calculation of Results |
20 | |||
| Measuring Range of the Assay |
22 | |||
| Limitations |
23 | |||
| Clinical Studies and Interpretation of Results |
24 | |||
| Interpretation |
34 | |||
| Interpretation Relative to Natriuretic Peptides |
35 | |||
| Performance Characteristics |
36 | |||
| Precision |
36 | |||
| Clinical Laboratory Precision |
36 | |||
| Detection Limit |
37 | |||
| Cross Reactivity |
38 | |||
| Linearity |
39 | |||
| Interfering Substances |
39 | |||
| High Dose Hook Effect |
42 | |||
| Dilution Parallelism |
43 | |||
| Sample Matrices |
43 | |||
| Reference Range Study |
44 | |||
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 1 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
| References |
45 | |||
| Technical Support Contact Information: |
46 | |||
| Glossary of Symbols: |
46 | |||
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 2 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
BGM Galectin-3™
(Galectin-3 Assay)
Intended Use
BGM Galectin-3™ is an in vitro diagnostic device that quantitatively measures galectin-3 in serum or EDTA-plasma by enzyme linked immunosorbent assay (ELISA) on a microtiter plate platform, to be used in conjunction with clinical evaluation as an aid in assessing the prognosis of patients diagnosed with chronic heart failure (HF).
Summary and Explanation of the Test
Galectin-3 is a structurally unique member of a family of beta-galactoside-binding lectins. Expression of galectin-3 has been associated with the epithelium and inflammatory cells including macrophages, neutrophils and mast cells. Galectin-3 has been implicated in a variety of biological processes important in heart failure including myofibroblast proliferation, fibrogenesis, tissue repair, cardiac remodeling and inflammation.
Principle of the Test Procedure
BGM Galectin-3 is a microtiter plate-based ELISA for the quantitative determination of galectin-3 levels in human serum and plasma.
BGM Galectin-3 utilizes two monoclonal antibodies against galectin-3. One rat monoclonal anti-mouse galectin-3 antibody is coated onto the surface of the wells in a microtiter plate and serves as the capture antibody to bind galectin-3 molecules in samples, while the other mouse monoclonal anti-human galectin-3 antibody is provided in solution and functions as the tracer antibody for detecting galectin-3 molecules bound to the capture antibody. The microtiter plate is ready to use.
Standards, quality control materials, and patient specimens are introduced in duplicate into the wells and incubated for 60 minutes. During this incubation, the galectin-3 present in the standards and specimens binds to the capture antibody coated onto the well surface. A subsequent wash step removes all unbound material introduced with the samples, including unbound galectin-3.
The tracer antibody, a horseradish peroxidase (HRP)-labeled anti-galectin-3 antibody, is then introduced into the well and incubated for 60 minutes. During this time, an antibody-antigen-antibody complex is formed.
After a wash step to remove any unbound tracer antibody, the tetramethylbenzidine (TMB) substrate is added, yielding a blue color in the presence of HRP. The color development is stopped after 20 minutes by the addition of sulfuric acid, changing the color to yellow, which can be read at an absorbance of 450 nm.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 3 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
The absorbance is proportional to the galectin-3 levels in the specimens. The test results of the specimens are read from the standard curve.
Kit Contents
Each BGM Galectin-3 kit (box) contains the following:
| Qty |
Name |
Description |
Abbreviation | |||
| 1 plate |
Plate | Ready-to-use microtiter plate coated with rat monoclonal anti-mouse galectin-3 monoclonal antibody | (P) | |||
| 1 bottle |
Assay Buffer* | Phosphate buffered saline with 1% bovine serum albumin (45 mL) | (AB) | |||
| 1 bottle |
TMB substrate | Tetramethylbenzidine (15 mL) | (TS) | |||
| 1 bottle |
Stop solution | 0.5M sulfuric acid (10 mL) | (ST) | |||
| 2 bottles |
Wash buffer concentrate* | 0.5M Tris buffered saline (2 x 50 mL; 10X concentrate) | (WC) | |||
| 1 bottle |
Tracer concentrate* | Horseradish peroxidase (HRP) labeled mouse monoclonal anti-human galectin-3 monoclonal antibody (0.45 mL) | (TC) | |||
| 2 vials |
Standard | Recombinant human galectin-3, 12 ng per vial (lyophilized) | (S1) | |||
| 2 vials |
Low Quality Control (QC) † | Low QC material, Recombinant human galectin-3 in protein matrix (lyophilized) | (C1) | |||
| 2 vials |
High Quality Control (QC) † | High QC material, Recombinant human galectin-3 in protein matrix (lyophilized) | (C2) | |||
| 2 |
Plate seals | Adhesive plastic plate seals | ||||
| * | Contains ProClin® as a preservative. |
| † | Contains processed human plasma tested negative or nonreactive for anti-HIV-1/2, anti-HCV and HBsAg when tested by an FDA approved method. |
Storage Instructions
Store the assay kit at 2-8°C upon receipt. Expiration dates of the reagents are printed on the labels. Return all kit components to 2-8°C immediately after use. Reconstituted or diluted kit components may be stored for a maximum of 10 days at 2-8°C.
Note: The expiration dates of the reagents can only be assured if the reagents are stored properly, and, in case of repeated use of one reagent, the reagent is not contaminated by the first handling.
Do not expose assay reagents to strong light (e.g. direct sunlight) during storage or use.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 4 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Specimen Collection and Storage
| • | BGM Galectin-3 is validated for use with human serum and EDTA-plasma only. |
| • | Blood should be collected using standard venous blood collection techniques and equipment. |
Centrifugation and separation of the serum or plasma from the cellular components should occur as soon as possible following collection.
| • | Serum (no additive, red top) tubes and EDTA (lavender top) tubes have been tested with BGM Galectin-3. Other tube types and anticoagulants have not been evaluated. It is the user’s responsibility to test other tube types. |
| • | The recommended specimen volume for BGM Galectin-3 is 30 µL which is sufficient volume for duplicate measurements. |
| • | Serum or EDTA-plasma with visible hemolysis (i.e. specimens with visible pink or red color) should not be used as falsely elevated galectin-3 levels will occur. |
| • | If necessary, serum or plasma may be stored for future analysis. Endogenous human galectin-3 has been tested and shown to be stable under the following conditions: |
| Storage Condition (temperature) |
Specimen Stability | |
| 22-28°C | 22 days | |
| 2-8°C | 22 days | |
| -20°C or -70°C* | at least 12 months |
| * | Stability studies for specimens stored at -20°C or -70°C are on-going. |
Galectin-3 in human serum and EDTA-plasma has been shown to be stable for 9 freeze-thaw cycles after storage at -20°C or -70°C.
Warnings and Precautions
| • | For in vitro diagnostic use. |
| • | For use by healthcare professionals. |
| • | Results should be interpreted along with clinical findings and other laboratory test results. |
| • | Levels of galectin-3 in blood may be increased in patients with certain forms of advanced cancer and other conditions associated with organ fibrosis. BGM Galectin-3 results should be interpreted with caution in such patients. |
| • | The BGM Galectin-3 assay is not indicated for detection, diagnosis, prognosis, or any uses associated with any type of cancer, conditions associated with organ fibrosis, or any other condition not noted under Intended Use. |
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 5 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
| • | Handle all serum and EDTA-plasma specimens as potentially biohazardous material. Follow universal precautions and handle specimens as potentially contaminated and as if capable of transmitting an infectious agent. |
| • | Dispose of waste in accordance with local requirements. A Material Safety Data Sheet is available upon request. |
| • | Avoid contact of assay reagents or specimens with skin, eyes, or mucous membranes. In the event of contact with skin or eyes, wash immediately with water. |
| • | Avoid contact of substrate solution with oxidizing agents and metal. |
| • | Reagents contain the active ingredient 5-chloro-2-methyl-thiazol-3-one and 2-methylthiazol-3-one, as a biocide preservative. |
Materials Required But Not Provided
| • | Deionized water |
| • | Adjustable pipettes (for pipetting 30, 50, 100, 125, 270, 300, 900 µL volumes), 8 Channel Adjustable Multi-Pipette (for pipetting 50, 100 µL volumes) and appropriate tips |
| • | Multi-channel pipette reservoirs (polypropylene) |
| • | One of the following transfer vessel(s) to prepare sample dilutions and to facilitate transfer (pipetting) of samples into the BGM Galectin-3 antibody-coated plate: |
| • | Disposable borosilicate glass or polypropylene or other low protein-binding plastic test tubes or vials for dilution of standard, tracer, TMB-substrate, controls and patient specimens |
| • | Non-binding 96-well U-bottom microtiter plate for use as a transfer plate |
| • | Mechanical microplate washer as defined in Table 1 or suitable wash buffer bottle for manual plate washing |
Table 1: Minimum Recommended Plate Washer Requirements for BGM Galectin-3*
| Plate Washer Feature |
Minimum Recommended Requirements for Use in BGM Galectin-3 | |
| Plate Size |
Support 96 well flat-bottom plate in 1x8 strips | |
| Number of cycles |
Support at least 4 cycles/wash | |
| Dispense Volume |
Support dispense volume of 400 µL/ well | |
| Accuracy |
< 5% | |
| Precision |
< 5% CV | |
| Residual Volume/Well |
< 3 µL | |
| Soak Time |
Support soak time interval of 15 seconds | |
| Fluid reservoir capacity |
Support at least 500 mL |
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 6 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
| * | Each laboratory should ensure proper validation of their equipment and software for use with BGM Galectin-3. |
| • | Absorbent paper towels for plate blotting after washes |
| • | Microtiter plate reader capable of reading at 450 nm and other characteristics as defined in Table 2: |
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 7 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Table 2: Minimum Recommended Plate Reader Requirements for BGM Galectin-3*
| Plate Reader Feature |
Minimum Recommended Requirements for Use with BGM Galectin-3 | |
| Detection method |
Absorbance | |
| Wavelength range |
Able to measure at 450 nm | |
| Band width |
< 10 nm | |
| Dynamic range |
0.000 to 3.000 OD | |
| Resolution |
0.001 OD | |
| Accuracy |
+1.0% or 0.010 from 0 to 2.0 OD; < 2.0% to 3.0 OD | |
| Reproducibility |
+1.0% or 0.010 from 0 to 2.0 OD; < 1.5% to 3.0 OD | |
| Linearity |
+1.0% from 0 to 2.0 OD; +2.0% to 3.0 OD | |
| Plate type |
Compatible with 96 well standard flat-bottom microplates | |
| Read method |
Endpoint | |
| Read temperature |
Able to achieve the above performance at room temperature (20–25°C) |
| * | Each laboratory should ensure proper validation of their equipment and software for use with BGM Galectin-3. |
| • | Appropriate software to perform curve fitting of the standard (calibration) curve. Note that many microtiter plate readers have curve-fitting software built-in, otherwise separate curve-fitting software should be obtained. It is important that the curve-fitting software includes one of the curve-fitting methods that is suitable for use with the BGM Galectin-3 assay, which include: |
| • | Third-order polynomial (cubic) with least squares optimization (avoid use of “cubic-spline” curve fit methods) |
| • | Four-parameter logistic (4PL) |
| • | Five-parameter logistic (5PL) |
In addition, use of a 1/Y2 weighting scheme, if available, is recommended for all three curve fitting methods as curve fitting results tend to be slightly improved through the use of this particular weighting scheme. However, reliable results can be obtained with or without the use of a 1/Y2 weighting scheme.
For additional information, please contact Technical Support
at 1-877-665-0077, option 2 or via email at
tsupport@bg-medicine.com.
| • | Additional plate seals, if desired, can be procured (Corning 3095) |
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 8 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Quality Control
BGM Galectin-3 Controls (supplied with kit)
Description and Intended use: The BGM Galectin-3 assay kit includes two lyophilized quality control materials (C1 and C2) intended to monitor the performance of the BGM Galectin-3 assay. The lower control (C1) is formulated with a target galectin-3 concentration of 18.4 ng/mL and is designed to monitor the performance of patient specimens near the clinical cutoff level of 17.8 ng/mL and specimens in the lower or normal range corresponding to the lower end of the standard curve. The higher control (C2) is formulated with approximately 69 ng/mL galectin-3 and is designed to monitor the performance of the assay for patient specimens with higher galectin-3 values and the upper end of the standard curve.
Assigned values: The BGM Galectin-3 Controls are assayed materials which are supplied with assigned target values and QC ranges that are printed on the vials and the accompanying Value Assignment Sheet. Lot-specific ranges have been assigned with multiple operators over multiple days and are based on the mean galectin-3 values ± 3 standard deviations (mean ± 3 SD). These values and associated ranges are provided as guidelines to the end user. It is recommended that each clinical laboratory confirm the suitability of the assigned ranges or establish their own ranges based on their own test system and criteria.
Composition and operating instructions: The controls are comprised of galectin-3 in a protein matrix to closely simulate the composition of patient specimens. Controls should be prepared and analyzed with the same procedure as patient samples (i.e. 1:10 dilution in Assay Buffer and duplicate measurement) to ensure adequate assay performance including preparatory and operational steps.
Frequency for QC: It is recommended that both QC levels (C1 and C2) be prepared and analyzed for each analytical run of the BGM Galectin-3 assay. In addition, QC materials should be used in accordance with local, state and/or federal regulations or accreditation requirements.
Additional quality control (optional for users)
It is recommended that clinical laboratories consider additional quality control (QC) materials to enhance their overall quality control strategy. Labs may use patient pools with galectin-3 concentrations at specific clinical decision levels or cut-points to supplement the BGM Galectin-3 controls that are supplied with the kit. Users are referred to CLSI C24-A3 for additional information.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 9 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
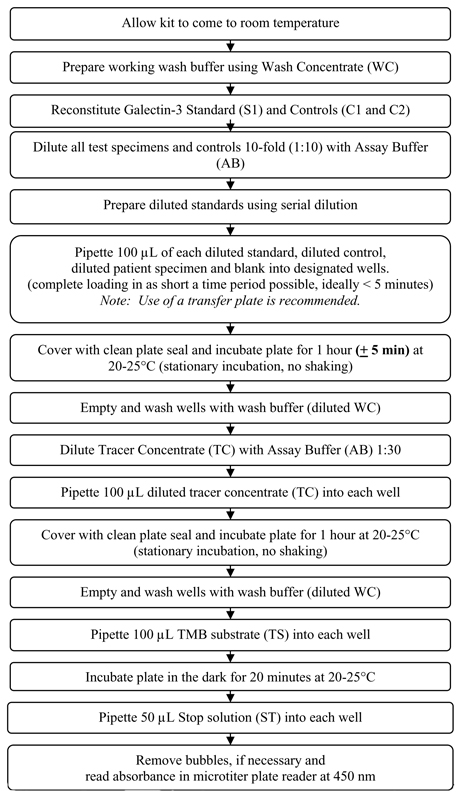
BGM Galectin-3 Test Procedure
(Refer to flow chart in Figure 2)
Preparation of Reagents
| 1. | Prior to use, allow all kit components to equilibrate to room temperature for a minimum of 30 minutes. Note: Return all kit components to storage at 2-8°C after use. |
| 2. | Plate (P): After opening the pouch, number the top surface of each strip with permanent ink in case they are unintentionally released from the plate frame. Note: Plates are designed for partial use. If partial use is desired, unneeded strips may be removed from the frame and returned to the plate pouch for storage. Do not remove the desiccant from the pouch. Securely reseal the plate pouch and return to storage for a maximum of 14 days at 2-8°C. When the first partial plate assay is complete, save the plate frame for the second partial run. |
| 3. | Wash Concentrate (WC): Each WC bottle contains 10x concentrated wash buffer. Using deionized water, prepare a 1:10 dilution of the WC. The entire fill volume may be diluted and stored or smaller aliquots may be prepared for a given day’s experiment. If a plate washer is to be used to perform washes, ensure that adequate wash volume is prepared to account for priming the plate washer (check manufacturer’s recommendation). Diluted buffer may be stored for a maximum of 10 days at 2-8°C. |
Detailed Assay Procedure
Note: All samples (patient specimens, controls and calibrators) must be diluted prior to the start of the testing procedure. Sample dilution should never be performed in the wells of the antibody-coated plate. In order to allow samples to react with the coated antibody for approximately the same duration, sample transfer should be completed across the plate in five minutes or less. In order to facilitate rapid sample transfer, use of transfer vessel(s) and a multi-channel pipette is recommended.
| 1. | Reconstitute Galectin-3 Standard (S1) |
BGM Galectin-3 is calibrated with a set of seven standards that are prepared by serial dilution of the standard (S1) that is supplied with each kit. The calibration range is 0.156 ng/mL to 10.0 ng/mL.
Open vacuum sealed vial slowly to avoid loss of material due to aerosol formation. Note: To avoid potential cross-contamination, write “S1” on the stopper with permanent ink. Before use, reconstitute one vial of the galectin-3 standard (S1) with 300 µL of deionized water, immediately followed by addition of 900 µL of Assay Buffer. Allow the vial to
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 10 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
stand for a minimum of 15 minutes at room temperature with periodic vortexing and gentle inversion ensuring that the reconstitution water wets the entire surface area inside the vial. Complete dissolution of the standard is critical. Verify dissolution is complete prior to use. After use, remaining reconstituted standard may be stored for a maximum of 10 days at 2-8°C, if reuse is desired.
| 2. | Reconstitute Galectin-3 Controls (C1 and C2) |
The controls are comprised of a protein matrix spiked with recombinant human galectin-3. The BGM Galectin-3 Controls are supplied with assigned QC ranges that are printed on the vials and in the accompanying Value Assignment Sheet. Ranges are lot-specific and the user must confirm the appropriate range with each new lot of a BGM Galectin-3 kit. If an assay control result is determined to be out of range, the assay should be repeated.
Open vacuum sealed vials slowly to avoid loss of material due to aerosol formation. Note: To avoid potential cross-contamination, write “C1 or “C2”, as applicable on the appropriate stopper of each vial with permanent ink. Reconstitute one vial of C1 and C2 with 250 µL deionized water. Allow the vials to stand for a minimum of 15 minutes at room temperature with periodic vortexing and gentle inversion ensuring that the reconstitution water wets the entire surface area inside the vial. Verify complete dissolution prior to use. After use, remaining reconstituted controls may be stored for a maximum of 10 days at 2-8°C, if reuse is desired.
| 3. | Define a Plate Map |
Designate microtiter plate wells for each of the controls, test specimens, diluted standards and blank. All samples should be tested in duplicate (i.e. blank, diluted standards, controls and test specimens).
| 4. | Prepare Diluted Specimens and Blank |
While the standard and controls are reconstituting, dilute each test specimen 10-fold (1:10) using the Assay Buffer (AB). Final dilution volume should be sufficient for duplicate measurement, thus it is recommended that a minimum of 30 µL of serum or EDTA-plasma be used to prepare a (i.e. 30 µL specimen + 270 µL AB). An assay blank should be prepared using just Assay Buffer (AB).
Dilutions must be performed externally in a separate transfer vessel (i.e. off-line and not in the BGM Galectin-3 antibody-coated plate). Recommended transfer vessels are a non-binding 96-well U-bottom microtiter plate (“transfer plate”) or disposable test tubes composed of borosilicate glass, polypropylene or other low protein-binding plastic. If a transfer plate is used, ensure plate is clean by inspecting for dust particles prior to use and make sample dilutions in the corresponding wells per the plate map defined in step 3 above. Mix each dilution by performing multiple aspiration & dispense cycles with the pipette (if transfer plate is used), or by vortexing or inversion (if test tubes are used).
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 11 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Note: BGM Galectin-3 is designed to analyze samples (patient specimens and controls) that are diluted 10-fold (1:10) in Assay Buffer prior to analysis. This provides the proper sample to reagent ratio that yields optimal results within the measurement range up to 94.8 ng/mL. Patient specimens that yield galectin-3 results greater than 94.8 ng/mL should NOT be further diluted.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 12 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
| 5. | Prepare Diluted Controls |
Dilute each reconstituted Control (C1 and C2) 10-fold (1:10) using the Assay Buffer (AB) in transfer vessels (i.e., in designated wells of transfer plate, or disposable test tubes composed of borosilicate glass, polypropylene or other low protein-binding plastic). Mix each dilution by pipette aspiration, vortexing or inversion. It is recommended that a minimum of 30 µL of the reconstituted control be used for the dilution (i.e. 30 µL C1 or C2 + 270 µL AB). Dilutions must be performed externally (i.e. off-line and not in the BGM Galectin-3 antibody-coated plate). After use, remaining reconstituted or diluted (1:10) control material may be stored for a maximum of 10 days at 2-8°C, if reuse is desired.
| 6. | Prepare Set of Diluted Standards |
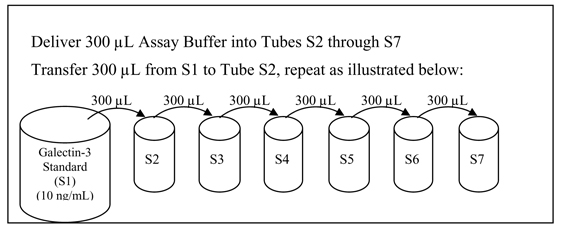
Dilute the standards immediately before use. Label 6 disposable test tubes with numbers S2 to S7 according to the dilution scheme illustrated in Figure 1. Pipette 300 µL Assay Buffer (AB) into each labeled tube. Next, pipette 300 µL Galectin-3 Standard (S1) to tube S2 and mix gently by pipette aspiration, vortexing or inversion. Then, transfer 300 µL from tube S2 to tube S3 and mix gently, transfer 300 µL from tube S3 to S4, etc. If using a transfer plate, pipette 300uL of S1 through S7 into the corresponding wells of the transfer plate per the plate map defined in step 3 above. After use, remaining reconstituted or diluted standard material may be stored for a maximum of 10 days at 2-8°C, if reuse is desired.
Figure 1: Serial Dilution Scheme for Preparation of Standards
| 7. | Prepare Samples for Transfer |
Samples are transferred from the transfer vessel (i.e. transfer plate or test tubes) using a multi-channel pipette. If a transfer plate was used as the transfer vessel, the samples are already prepared for transfer to the BGM Galectin-3 antibody-coated plate using
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 13 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
adjustable or multi-channel pipette. If test tubes or vials were used as the transfer vessel, arrange all test tubes in a suitable rack corresponding to the sample order per the plate map defined in step 3 above so samples can be readily transferred to the BGM Galectin-3 antibody-coated plate using a multi-channel pipette.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 14 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
| 8. | Transfer Samples |
Transfer should be completed within 5 minutes, regardless of method.
Transfer 100 µL of each sample (blank, diluted standards, diluted controls, and diluted test specimens) to duplicate wells of the BGM Galectin-3 antibody-coated plate using a multi-channel pipette and according to the plate map defined in step 3 above.
| 9. | Seal and Incubate |
Cover the wells with a clean plate seal and incubate for 1 hour ± 5 minutes at 20-25°C without shaking. The incubation time at this step is critical. The plate should incubate for 1 hour ± 5 minutes. Use of a timer is strongly recommended.
| 10. | Remove Seal and Wash |
Carefully remove the plate seal and wash wells with diluted wash buffer.
Mechanical washer: 400 µL per well, 4 cycles. Dispensed wash should remain in wells a minimum of 15 seconds before aspiration step. After the fourth wash, empty wells by tapping on an absorbent paper towel. Inspect wells for any remaining wash and repeat tapping on absorbent paper towel if necessary.
Note: Prior to mechanical washing, ensure wash/aspirator tips have been adjusted to be close to the bottom of the wells but not touching or scratching the surface. If the mechanical washer model does not have the ability to adjust the washer wash/aspirator tip height, an additional wash cycle may be added if blank wells are inconsistent or the absorbance reading is too high.
Manual wash: Empty wells, add 300 µL wash buffer per well and soak for 15 seconds; empty wells by tapping on an absorbent paper towel. Repeat 3 more times for a total of 4 wash cycles.
| 11. | Prepare Diluted Tracer |
Dilute the Tracer Concentrate 1:30 with the Assay Buffer according to the dilution scheme shown in Table 3. After use, remaining diluted Tracer material may be stored for a maximum of 10 days at 2-8°C, if reuse is desired.
Note: This step may be performed while the plate is being washed.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 15 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Table 3: Recommended Dilution Scheme for Tracer Concentrate (30x)*
| Number of Strips |
Assay (µL) |
Tracer (µL) |
Number of Strips |
Assay (µL) |
Tracer (µL) | |||||
| 1 | 928 | 32 | 7 | 6496 | 224 | |||||
| 2 | 1856 | 64 | 8 | 7424 | 256 | |||||
| 3 | 2784 | 96 | 9 | 8352 | 288 | |||||
| 4 | 3712 | 128 | 10 | 9280 | 320 | |||||
| 5 | 4640 | 160 | 11 | 10208 | 352 | |||||
| 6 | 5568 | 192 | 12 | 11020 | 380 |
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 16 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
| 12. | Add Diluted Tracer |
Pipette 100 µL diluted tracer solution to each well.
| 13. | Seal and Incubate |
Cover the wells with a clean plate seal and incubate 1 hour at 20-25°C without shaking.
| 14. | Remove Seal and Wash |
Carefully remove plate seal and wash wells with diluted wash buffer.
Mechanical washer: 400 µL per well, 4 cycles. Dispensed wash should remain in wells a minimum of 15 seconds before aspiration step. After the fourth wash, empty wells by tapping on an absorbent paper towel. Inspect wells for any remaining wash and repeat tapping on absorbent paper towel if necessary.
Manual wash: Empty wells, add 300 µL wash buffer per well and soak for 15 seconds; empty wells by tapping on an absorbent paper towel. Repeat 3 more times for a total of 4 wash cycles.
| 15. | Add TMB Substrate and Incubate in the Dark |
Pipette 100 µL TMB-substrate (TS) to each well and incubate the plate for 20 minutes at 20-25°C in the dark. Note: Avoid pipetting directly from the TS bottle. Pour volume needed into intermediate 15 mL conical tube to measure volume needed, then transfer to the reservoir.
| 16. | Add Stop Solution |
Pipette 50 µL stop solution (ST) to each well. Mix well by drawing up and down using a clean pipette tip, or by gently tapping the side of the plate. The contents of the well will turn from blue to yellow. Note: Other steps have required 100 µL; this step requires only 50 µL.
| 17. | Remove Bubbles |
Check for and remove any bubbles from the liquid surface of each well. Remove any dirt or liquid from the well exterior. Note: Bubbles may be removed by using a clean pipette tip to gently touch and burst the air bubble; be certain to use a clean tip for each well.
| 18. | Measure Absorbance |
Measure the absorbance of each well in a microtiter plate reader at 450 nm within 30 minutes of adding the stop solution.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 17 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Procedural Notes
| • | All components in the kit must be at room temperature prior to use. |
| • | Do not pool reagents from different lots. |
| • | All samples should be assayed in duplicate, including blank, diluted standards, diluted controls and all diluted patient specimens. |
| • | Controls (C1 and C2) should be treated exactly like patient specimens (i.e. diluted 1:10, assayed in duplicate, checked for agreement). |
| • | Do not use any reconstituted material without visually verifying complete dissolution before sampling. |
| • | To avoid potential cross-contamination, do not mix up stoppers for the controls and standard. Labeling stoppers is recommended. |
| • | It is critical to keep the loading time of diluted standards, controls and test specimens into the BGM Galectin-3 plate within 5 minutes to reduce within plate variability. Use of a transfer plate and a multi-channel pipette are strongly advised. |
| • | Use clean, dedicated reagent trays and pipette tips for dispensing the conjugate and substrate reagents. |
| • | After each loading step, check for and remove any bubbles from the liquid surface of each well. Note: Bubbles may be removed by using a clean pipette tip to gently touch and burst the air bubble; be certain to use a clean tip for each well. |
| • | Do not use a shaker during plate incubation periods. |
| • | Do not reuse plate seals. If partial use is desired, plate seals may be cut. |
| • | If partial plate use is desired, retain plate frame for reuse. |
| • | Discard all consumed reagent upon completion of procedure in compliance with local biohazardous waste regulations. |
| • | Exposure to acids will inactivate the conjugate. |
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 18 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Figure 2: Overview of the BGM Galectin-3 Test Procedure
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 19 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Calculation of Results
BGM Galectin-3 is based on traditional spectrophotometry and a multi-point standard (calibration) curve. After completing the assay steps, the absorbance of each specimen is read at 450 nm using the microplate reader. The absorbance is proportional to the concentration of galectin-3 in the specimens. Galectin-3 concentrations in the specimens and controls are based on the relationship of the absorbance of the specimens compared to that of the standards, which have a known concentration of galectin-3 and should be assigned using the following procedure:
| • | Verify that the average absorbance of the blank is less than the average absorbance of the lowest standard. If the absorbance of the blank exceeds the absorbance of the lowest standard (e.g. the low standard absorbance is negative when the blank absorbance is subtracted), the entire plate should be repeated. |
| • | Subtract the average absorbance of the blank from each individual replicate absorbance of the standards, controls and test specimens. |
| • | Calculate the average absorbance for each of the blank-corrected standards. Use the average of the blank-corrected absorbance for each standard to generate the standard curve using either 4-parameter logistic (4PL), 5-parameter logistic (5PL) or third order polynomial (cubic) curve fitting with least squares optimization (note: avoid use of “Cubic-Spline” curve fit methods). See ‘Materials Required but Not Provided’ section for more details. |
| • | Calculate concentrations for each of the duplicate measurements of unknown test specimens and controls based upon the selected curve fit equation using the corresponding blank-corrected absorbances. Multiply the measured concentration of specimens and controls by 10 (dilution factor of specimens and controls). |
| • | Calculate the average, standard deviation, and coefficient of variation (CV) of the assigned concentration for each set of duplicate controls and test specimens. |
| • | The coefficient of variation (CV) of the duplicate measurements of controls and test specimens should be within 20%. Specimens with duplicate CVs greater than 20% should be re-analyzed. If either of the controls has a duplicate CV greater than 20%, the entire plate is rejected and all specimens should be re-analyzed. |
| • | Verify that the average concentration of the duplicate measurement for each control is within the corresponding acceptable range. If the average concentration of either control is out of the acceptable range, the assay should be repeated. |
| • | Report the average concentration of the duplicate measurement of each test specimen as the galectin-3 concentration. |
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 20 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
For reference, a representative standard curve is shown in Figure 3 and representative values for the absorbance of each diluted standard are shown in Table 4. Note: The figure and table are shown for informational purposes only and should not be used to derive test results.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 21 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Figure 3: A Representative Standard Curve
Note: The standard curve in Figure 3 is for illustration only.
Do not use to derive test results. A new standard curve should be established for each assay.
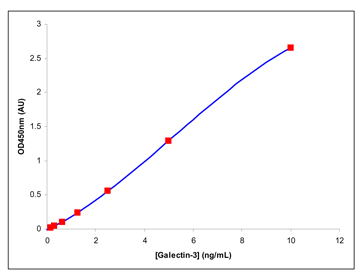
Table 4: A Representative Standard Curve and Typical Absorbance Values at 450 nm
Note: The standard curve data in Table 4 is for illustration only. Do not use data in Table 4 to derive test results. A new standard curve should be established for each assay.
| Dilution |
Galectin-3 Concentration (ng/mL) |
Abs450 nm | ||
| 1 | 10.0 | 2.690 | ||
| 2 | 5.0 | 1.326 | ||
| 3 | 2.5 | 0.595 | ||
| 4 | 1.25 | 0.269 | ||
| 5 | 0.625 | 0.131 | ||
| 6 | 0.313 | 0.082 | ||
| 7 | 0.156 | 0.054 | ||
| Blank | 0 | 0.033 |
Measuring Range of the Assay
The BGM Galectin-3 measuring range is 1.4 to 94.8 ng/mL with clinical specimens.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 22 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
The assay is calibrated with seven standards spanning the range of approximately 0.1 to 10.0 ng/mL. Each test sample (i.e. control or patient specimen) is pre-diluted 1:10 prior to assay allowing the measurement to occur within the range bracketed by the calibrators.
Limitations
| • | BGM Galectin-3 is intended to be used only in patients with chronic heart failure and should not be used for diagnosis of heart failure. |
| • | There is a possibility that factors such as technical or procedural errors, as well as additional substances in blood specimens that are not listed below, may interfere with the test and cause erroneous results. |
| • | Hemolysis is known to interfere with measurement of galectin-3 using the BGM Galectin-3 assay. Serum or EDTA-plasma with visible hemolysis should not be used because falsely elevated galectin-3 results may occur. |
| • | Presence of human anti-mouse antibodies (HAMA) or rheumatoid factor (RF) may interfere with the BGM Galectin-3 assay, which could cause falsely elevated results. The BGM Galectin-3 assay should not be used in patients with known HAMA or RF. BGM Galectin-3 results should be interpreted with caution in patients with a history of therapeutic use of murine monoclonal antibodies (IgG) or their fragments, or who have known autoimmune disorders. |
| • | Specimens with high levels of gamma globulins (> 2.5 g/dL) may cause false elevation in results. Galectin-3 results from patients with diseases associated with hyperglobulinemia, such as multiple myeloma, should be interpreted with caution. |
| • | DO NOT further dilute patient samples with galectin-3 levels greater than the upper limit of the measurement range (94.8 ng/mL). Recovery of samples that are not diluted at the recommended 1:10 dilution may yield variable results. Report as “galectin-3 value exceeds the upper limit of the measurement range” or utilize language consistent with laboratory or institutional policies. |
| • | Spike/recovery experiments should be avoided to assess the accuracy of BGM Galectin-3 as recovery of exogenously spiked recombinant human galectin-3 does not adequately represent the behavior of endogenous native galectin-3 in clinical specimens. |
| • | Blood samples should be collected in serum (no additive, red top) tubes or EDTA (lavender top) tubes only. Other tube types and anticoagulants have not been evaluated for use with BGM Galectin-3. It is the user’s responsibility to test other tube types. |
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 23 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Clinical Studies and Interpretation of Results
To validate the clinical effectiveness of the BGM Galectin-3 assay, galectin-3 levels were measured in a set of 895 banked EDTA-plasma samples from participants in the United States and Canada in a controlled multi-center clinical study, the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study. The HF-ACTION study involved 2,331 chronic HF patients with left ventricular dysfunction and with NYHA class II, III or IV symptoms. The average age of the 895 participants whose galectin-3 levels were assessed in the clinical validation study was 58 years, 29% were female, and 36% were non-white. Sensitivity analysis was performed comparing the set of 895 HF-ACTION subjects having evaluable galectin-3 values with all other HF-ACTION participants, and it was found that the clinical validation results based on the evaluable set of subjects were robust and representative of the larger study population. The median follow-up time was approximately 30 months. Participants were categorized based on galectin-3 risk categories defined below:
The derived galectin-3-dependent risk categories are as follows:
| • | galectin-3 greater than 25.9 ng/mL |
| • | galectin-3 between 17.8 and 25.9 ng/mL |
| • | galectin-3 less than or equal to 17.8 ng/mL |
For the clinical validation study, Cox regression models were used to evaluate the association of baseline galectin-3 levels in HF patients with the endpoints of: (i) composite of all-cause mortality and all-cause hospitalization, (ii) cardiovascular mortality, (iii) composite of cardiovascular mortality and heart failure-related hospitalization, and (iv) all-cause mortality. Galectin-3 levels were found to be significantly associated with increased risk of each of these endpoints in Cox regression models (Table 5, Table 7, Table 9 and Table 12). Galectin-3 remained significantly associated with increased risk upon adjustment for baseline risk factors of age, gender, NYHA functional classification, left ventricular ejection fraction, diabetes status, and smoking status. Figure 4 displays Kaplan Meier curves for the composite endpoint of all-cause mortality or all-cause hospitalization, by baseline galectin-3 category. Figure 5, Figure 6, and Figure 7 display cumulative probabilities for events for the endpoints of the composite of all-cause mortality and all-cause hospitalization, cardiovascular mortality,
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 24 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
and the composite of cardiovascular mortality and heart failure-related hospitalization in the clinical validation study, by baseline galectin-3 category at timepoints of 6, 12, 24 and 36 months after baseline.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 25 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
All-Cause Mortality and All-Cause Hospitalization
Table 5: Hazard Ratios for All-Cause Mortality and All-Cause Hospitalization Events for HF Subjects in the Clinical Validation Study
| Hazard Ratio (95% CI, p value) | ||||||
| Galectin-3 Category |
£ 17.8 ng/mL | 17.8-25.9 ng/mL | > 25.9 ng/mL | |||
| Number of Subjects |
647 | 170 | 78 | |||
| Galectin-3* |
1.0 | 1.35 (1.10-1.65, p= 0.004) |
1.46 (1.11-1.92, p= 0.006) | |||
| * | adjusted for baseline risk factors: age, gender, NYHA functional classification, left ventricular ejection fraction, diabetes status, and smoking status. |
Figure 4: Kaplan-Meier Curves for the Composite Endpoint of All-Cause Mortality or All-Cause Hospitalization, for HF Subjects in the Clinical Validation Study, by Baseline Galectin-3 Level
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 26 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Figure 5: Cumulative Probability of Event for the Composite Endpoint of All-Cause Mortality and All-Cause Hospitalization, at Various Time Points and By Baseline Galectin-3 Level, for HF Subjects in the Clinical Validation Study
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 27 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
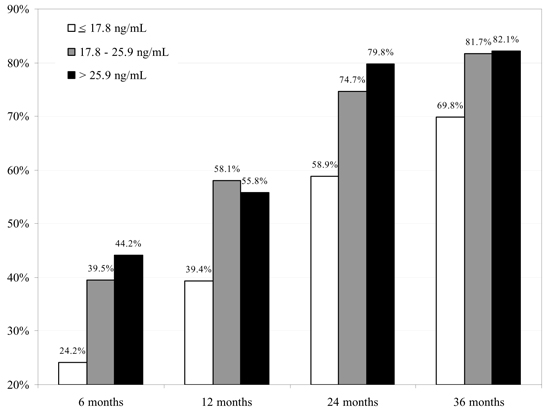
Table 6: Cumulative Probability (with 95% Confidence Intervals) of Event for the Composite Endpoint of All-Cause Mortality and All-Cause Hospitalization, at Various Time Points and By Baseline Galectin-3 Level, for HF Subjects in the Clinical Validation Study
| Cumulative Probability of All-Cause Mortality and All-Cause Hospitalization (95% CI) by Galectin-3 Category and Time Point (in percent) | ||||||||
| Galectin-3 Category |
6 months |
12 months | 24 months | 36 months | ||||
| £ 17.8 ng/mL |
24.2% (21.1%-27.7%) | 39.4 (35.7-43.3) | 58.9 (55.0-62.9) | 69.8 (65.8-73.7) | ||||
| 17.8-25.9 ng/mL |
39.5 (32.5-47.3) | 58.1 (50.8-65.7) | 74.7 (67.7-81.1) | 81.7 (74.9-87.6) | ||||
| > 25.9 ng/mL |
44.2 (33.9-55.9) | 55.8 (45.2-67.1) | 79.8 (69.9-88.2) | 82.1 (72.0-90.0) | ||||
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 28 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Cardiovascular Mortality
Table 7: Hazard Ratios for Cardiovascular Mortality Events for HF Subjects in the Clinical Validation Study
| Hazard Ratio (95% CI, p value) | ||||||
| Galectin-3 Category |
£17.8 ng/mL | 17.8-25.9 ng/mL | > 25.9 ng/mL | |||
| Number of Subjects |
647 | 170 | 78 | |||
| Galectin-3* |
1.0 | 1.91 (1.28-2.86, p= 0.002) |
2.33 (1.43-3.80, p < 0.001) | |||
| * | adjusted for baseline risk factors: age, gender, NYHA functional classification, left ventricular ejection fraction, diabetes status, and smoking status. |
Figure 6: Cumulative Probability of Event for the Endpoint of Cardiovascular Mortality, at Various Time Points and By Baseline Galectin-3 Level, for HF Subjects in the Clinical Validation Study
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 29 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
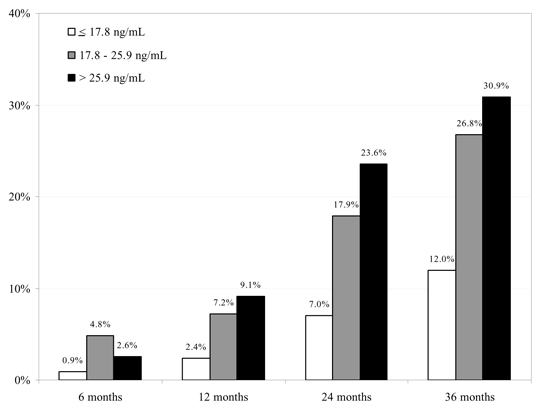
Table 8: Cumulative Probability (with 95% Confidence Intervals) of Event for the Cardiovascular Mortality, at Various Time Points and By Baseline Galectin-3 Level, for HF Subjects in the Clinical Validation Study
| Cumulative Probability of Cardiovascular Mortality (95% CI) by Galectin-3 Category and Time Point (in percent) | ||||||||
| Galectin-3 Category |
6 months |
12 months | 24 months | 36 months | ||||
| £ 17.8 ng/mL |
0.9% (0.4%-2.1%) | 2.4(1.4-3.9) | 7.0(5.2-9.3) | 12.0(9.4-15.2) | ||||
| 17.8-25.9 ng/mL |
4.8(2.4-9.3) | 7.2(4.1-12.3) | 17.9(12.7-24.9) | 26.8(20.0-35.5) | ||||
| > 25.9 ng/mL |
2.6(0.6-9.9) | 9.1(4.4-18.1) | 23.6(15.0-36.0) | 30.9(20.4-45.0) | ||||
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 30 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Cardiovascular Mortality and Heart Failure-Related Hospitalization
Table 9: Hazard Ratios for Cardiovascular Mortality and Heart Failure-Related Hospitalization Events for HF Subjects in the Clinical Validation Study
| Hazard Ratio (95% CI, p value) | ||||||
| Galectin-3 Category |
£ 17.8 ng/mL | 17.8-25.9 ng/mL | > 25.9 ng/mL | |||
| Number of Subjects |
647 | 170 | 78 | |||
| Galectin-3* |
1.0 | 1.51 (1.14-2.00, p= 0.004) |
1.70 (1.19-2.42, p= 0.004) | |||
| * | adjusted for baseline risk factors: age, gender, NYHA functional classification, left ventricular ejection fraction, diabetes status, and smoking status. |
Figure 7: Cumulative Probability of Event for the Composite Endpoint of Cardiovascular Mortality and Heart Failure-Related Hospitalization, at Various Time Points and By Baseline Galectin-3 Level, for HF Subjects in the Clinical Validation Study
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 31 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
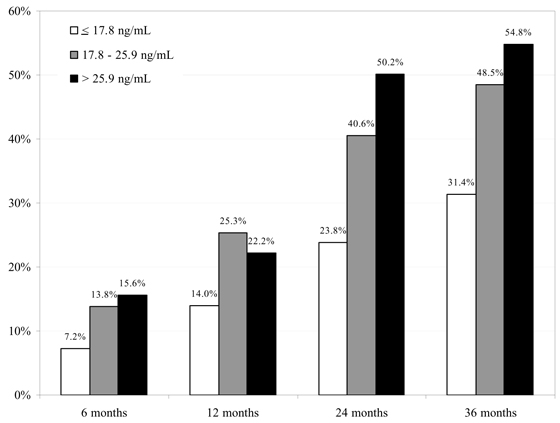
Table 10: Cumulative Probability (with 95% Confidence Intervals) of Event for Cardiovascular Mortality and Heart Failure-Related Hospitalization, at Various Time Points and By Baseline Galectin-3 Level, for HF Subjects in the Clinical Validation Study
| Cumulative Probability of Cardiovascular Mortality and Heart Failure- Related Hospitalization (95% CI) by Galectin-3 Category and Time Point (in percent) | ||||||||
| Galectin-3 Category |
6 months |
12 months | 24 months | 36 months | ||||
| £ 17.8 ng/mL |
7.2% (5.4%-9.5%) | 14.0 (11.5-17.0) | 23.8 (20.6-27.4) | 31.4 (27.6-35.6) | ||||
| 17.8-25.9 ng/mL |
13.8 (9.4-20.1) | 25.3 (19.4-32.7) | 40.6 (33.4-48.7) | 48.5 (40.5-57.2) | ||||
| > 25.9 ng/mL |
15.6 (9.2-25.8) | 22.2 (14.4-33.2) | 50.2 (38.9-62.8) | 54.8 (42.9-67.6) | ||||
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 32 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
All-Cause Mortality
Figure 8: Kaplan-Meier Curves for the Endpoint of All-Cause Mortality, for HF Subjects in the Clinical Validation Study, by Baseline Galectin-3 Level
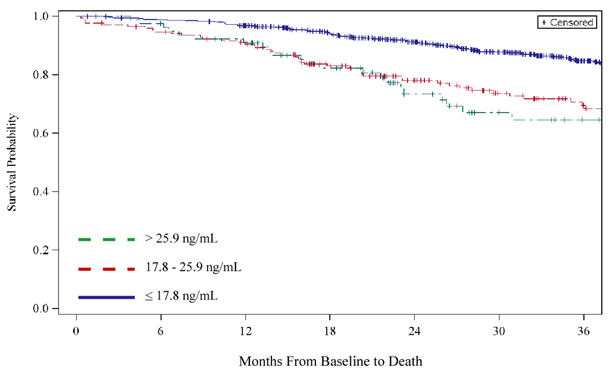
Table 11: Cumulative Probability (with 95% Confidence Intervals) of Event for the Endpoint of All-Cause Mortality, at Various Time Points and By Baseline Galectin-3 Level, for HF Subjects in the Clinical Validation Study
| Cumulative Probability of All-Cause Mortality Event (95% CI) by Galectin-3 Category and Time Point (in percent) | ||||||||
| Galectin-3 Category |
6 months |
12 months | 24 months | 36 months | ||||
| £ 17.8 ng/mL |
1.2% (0.6%-2.5%) | 3.3 (2.1-5.0) | 8.7 (6.7-11.3) | 15.3 (12.4-18.8) | ||||
| 17.8-25.9 ng/mL |
5.3 (2.8-10.0) | 8.9 (5.5-14.4) | 22.0 (16.3-29.4) | 30.5 (23.4-39.1) | ||||
| > 25.9 ng/mL |
2.6 (0.6-9.9) | 9.1 (4.4-18.1) | 26.6 (17.5-39.1) | 35.5 (24.5-49.5) | ||||
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 33 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Table 12: Hazard Ratios for All-Cause Mortality Events for HF Subjects in the Clinical Validation Study
| Hazard Ratio (95% CI, p value) | ||||||
| Galectin-3 Category |
£ 17.8 ng/mL | 17.8-25.9 ng/mL | > 25.9 ng/mL | |||
| Number of Subjects |
647 | 170 | 78 | |||
| Galectin-3* |
1.0 | 1.84 (1.28-2.64, p= 0.001) |
2.06 (1.31-3.23, p= 0.002) | |||
| * | adjusted for baseline risk factors: age, gender, NYHA functional classification, left ventricular ejection fraction, diabetes status, and smoking status. |
Interpretation
The BGM Galectin-3 assay results should be interpreted in conjunction with clinical evaluation as an aid in assessing the prognosis of patients diagnosed with chronic heart failure.
Patients with chronic heart failure with galectin-3 levels over 17.8 ng/mL were found to have a higher risk of adverse outcomes including mortality or hospitalization compared to patients with levels below 17.8 ng/mL. Galectin-3 levels between 17.8 ng/mL and 25.9 ng/mL should be interpreted with caution because these values lie within the reference range.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 34 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Interpretation Relative to Natriuretic Peptides
Galectin-3 and natriuretic peptides are measures of separate and distinct biological processes. Each marker provides independent and complementary information on the prognosis of patients with chronic heart failure.
Table 13 illustrates this for N-terminal pro B-type natriuretic peptide (NT-proBNP) in the clinical validation study by evaluating primary endpoint event rates by categories of galectin-3 and NT-proBNP.
Table 13: Event Rates at 6, 12, 24 and 36 Months for the Composite Endpoint of All-Cause Mortality and All-Cause Hospitalization, by Galectin-3 Category and NT-proBNP level, for HF Subjects in the Clinical Validation Study. The median value for NT-proBNP in the Clinical Validation Study was 848 pg/mL.
| Galectin-3 £ 17.8
ng/ mL and NT-proBNP £ median |
Galectin-3 £ 17.8
ng/mL and NT-proBNP > median or Galectin-3 > 17.8 ng/mL and NT-proBNP £ median |
Galectin-3 > 17.8 ng/mL and NT-proBNP > median |
||||||||||
| Event rate at 6 months |
19.4 | % | 31.8 | % | 42.7 | % | ||||||
| Event rate at 12 months |
32.0 | % | 50.0 | % | 58.0 | % | ||||||
| Event rate at 24 months |
55.3 | % | 71.1 | % | 85.7 | % | ||||||
| Event rate at 36 months |
76.1 | % | 85.8 | % | 93.0 | % | ||||||
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 35 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Performance Characteristics
Precision
Precision of BGM Galectin-3 was assessed in an evaluation according to the CLSI EP5-A2 guideline. Six (6) EDTA-plasma pools spanning a range of galectin-3 concentrations were analyzed in duplicate with two (2) runs per day over twenty (20) days using one (1) reagent lot, two (2) operators and one (1) microtiter plate reader. Estimates of within-run, run-to-run, day-to-day and total precision were calculated and met acceptance criteria. Results are summarized in Table 14.
Table 14: Precision of BGM Galectin-3
| Test Specimen # |
Galectin-3 mean (ng/mL) |
Within run | Run to run | Day to day | Total | |||||||||||||
| SD | CV% | SD | CV% | SD | CV% | SD | CV% | |||||||||||
| 1 |
6.1 | 0.3 | 5.7 | 0.6 | 10.5 | 0.0 | 0.0 | 0.7 | 12.0 | |||||||||
| 2 |
17.6 | 0.4 | 2.1 | 0.7 | 3.8 | 0.5 | 2.8 | 0.9 | 5.1 | |||||||||
| 3 |
20.7 | 0.7 | 3.4 | 1.4 | 6.7 | 0.3 | 1.7 | 1.6 | 7.7 | |||||||||
| 4 |
26.3 | 0.6 | 2.2 | 0.8 | 3.0 | 0.5 | 2.1 | 1.1 | 4.2 | |||||||||
| 5 |
46.2 | 1.1 | 2.4 | 1.6 | 3.6 | 0.5 | 1.1 | 2.0 | 4.4 | |||||||||
| 6 |
72.2 | 2.4 | 3.3 | 4.3 | 6.0 | 2.9 | 4.0 | 5.7 | 8.0 | |||||||||
Table 14 shows the results of the precision evaluation with EDTA-plasma pools. An additional experiment was performed using serum pools which yielded similar results. Additional test pools at multiple galectin-3 concentrations were also tested and yielded similar results.
Clinical Laboratory Precision
Precision was also evaluated at three (3) CLIA-certified clinical laboratories according the CLSI EP5-A2 guideline. The study included testing of EDTA-plasma pools spanning three (3) galectin-3 concentrations, across two (2) reagent lots, using three (3) different models of microtiter plate readers, and a total of four (4) different operators. Total testing days were 17, 18 and 20 days across the three sites, yielding 110 unique analytical runs. Results from each of the CLIA laboratories were within acceptable limits. Within run and total imprecision estimates are summarized in Table 15.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 36 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Table 15: Clinical Laboratory Precision - Within Run and Total Imprecision
| CLIA Lab |
# days, # runs |
Galectin-3 mean (ng/mL) |
Within Run | Total | ||||||||
| SD | CV% | SD | CV% | |||||||||
| A |
20 days, 40 runs |
6.0 | 0.30 | 5.0 | 0.46 | 7.7 | ||||||
| 20.1 | 0.59 | 2.9 | 1.20 | 6.0 | ||||||||
| 68.3 | 2.71 | 4.0 | 9.97 | 14.6 | ||||||||
| B |
17 days, 34 runs |
6.7 | 0.36 | 5.4 | 0.63 | 9.4 | ||||||
| 21.6 | 0.56 | 2.6 | 1.55 | 7.2 | ||||||||
| 75.5 | 1.71 | 2.3 | 12.75 | 16.9 | ||||||||
| C |
18 days, 36 runs |
6.3 | 0.46 | 7.3 | 0.59 | 9.4 | ||||||
| 21.2 | 0.71 | 3.3 | 1.19 | 5.6 | ||||||||
| 71.5 | 2.16 | 3.0 | 6.25 | 8.7 | ||||||||
Detection Limit
The limit of detection and limit of quantitation of BGM Galectin-3 were established according to the recommendation of the CLSI EP17-A guideline. The limit of blank (LoB) was determined as the 95th percentile value of forty-eight (48) replicate measurements of the BGM Galectin-3 Assay Buffer. The limit of detection (LoD) was LoD = LoB + cßSDs, where SDs is the pooled standard deviations from four (4) serum samples with different levels of galectin-3, each of which was measured in sixteen (16) replicates (for a total of sixty-four (64) measurements) and cßis the 95th percentile of the standard Gaussian distribution corrected for the degree of freedom. The Limit of quantitation (LoQ) was specified as the lowest galectin-3 concentration of the serum samples closest to while above the LoD, which is 1.32 ng/mL. For this sample, the coefficient of variation (CV) for the galectin-3 measurement was 10.4%.
| Limit of Blank (LoB): |
LoB = 0.86 ng/mL | |
| Limit of Detection (LoD): |
LoD = 1.13 ng/mL | |
| Limit of Quantitation (LoQ): |
LoQ = 1.32 ng/mL |
Note: The LoQ does not represent the lower end of the measuring range and should not be used for that purpose. The measuring range is 1.4 to 94.8 ng/mL as reported in the Measuring Range and Linearity sections of this package insert.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 37 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Cross Reactivity
BGM Galectin-3 displayed no significant cross-reactivity when tested in the presence of the following compounds: galectin-1, galectin-2, galectin-4, galectin-7, galectin-8, galectin-9, galectin-12, collagen I and collagen III, all at a concentration of 500 ng/mL. The mean % cross-reactivity of the above potential cross-reactants is at or below 0.3%.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 38 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Linearity
The linearity of BGM Galectin-3 was established according to the recommendations of the Clinical and Laboratory Standards Institute Evaluation Protocol 6 (CLSI EP6-A guideline). Samples were prepared to span a clinically-meaningful measurement range of galectin-3 concentrations. Linearity of BGM Galectin-3 was demonstrated between 1.4 and 94.8 ng/mL. These linearity data are shown Figure 9.
Figure 9: Linearity of BGM Galectin-3
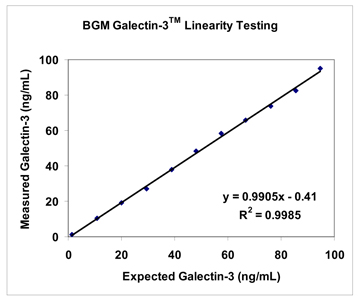
Interfering Substances
BGM Galectin-3 was evaluated for the effects of potential interfering substances, both endogenous and exogenous, according to the recommendations of the CLSI EP7-A guideline, using an interference acceptance limit of +/-10%. Conjugated bilirubin (up to 16.8 mg/dL), unconjugated bilirubin (up to 40.3 mg/dL), albumin (BSA, up to 12 g/dL), triglycerides (up to 3000 mg/dL), cholesterol (up to 747 mg/dL), and creatinine (up to 5 mg/dL) do not show any significant interference in the assay based on the interference acceptance limit (+/- 10%). Purified hemoglobin (up to 500 mg/dL) does not show significant interference in BGM Galectin-3; however, packed blood cell lysate does show interference.
Human anti-mouse antibodies (HAMA) and rheumatoid factor (RF) cause significant positive interference and rheumatoid factor (RF) greater than 50 IU/mL causes significant
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 39 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
positive interference with BGM Galectin-3. High levels of gamma globulins (> 2.5 g/dL) may cause false elevation in galectin-3 levels. This information is summarized in Table 16.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 40 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Table 16: Endogenous Interference Summary
| Potential interfering substance |
Result of interference study based on an interference acceptance limit of +/- 10% | |
| Conjugated bilirubin |
No significant interference up to 16.8 mg/dL | |
| Unconjugated bilirubin |
No significant interference up to 40.3 mg/dL | |
| Albumin |
No significant interference up to 12 g/dL | |
| Triglycerides |
No significant interference up to 3000 mg/dL | |
| Cholesterol |
No significant interference up to 747 mg/dL | |
| Creatinine |
No significant interference up to 5 mg/dL | |
| Purified hemoglobin |
No significant interference up to 500 mg/dL | |
| Whole blood lysate |
Hemolyzed specimens should not be used with BGM Galectin-3™ | |
| Human anti-mouse antibodies (HAMA) |
Specimens from patients with HAMA should not be used with BGM Galectin-3™ | |
| Rheumatoid Factor (RF) |
Interference seen at levels > 50 IU/mL | |
| Gamma globulins |
Interference seen at levels > 2.5 g/dL |
BGM Galectin-3 measurements were not significantly affected when tested in the presence of thirty-four (34) common pharmaceutical substances; including HF drugs (refer to Table 17). All analytes fall within the interference acceptance limit of +/- 10%.
Table 17: Common Drugs That Did Not Show Interference with BGM Galectin-3
| Acetaminophen |
Carvedilol | Dopamine | Lisinopril | Quinidine | ||||
| Acetylsalicylic acid |
Captopril | Enalaprilat | Losartan | Ramipril | ||||
| Amlodipine |
Chloramphenicol | Furosemide | Lovastatin | Spironolactone | ||||
| Ampicillin |
Diclofenac | Hydrochlorothiazide | Methyldopa | Theophylline | ||||
| Ascorbic Acid |
Digoxin | Ibuprofen | Metoprolol | Verapamil | ||||
| Atenolol |
Diltiazem | Indomethacin | Naproxen | Warfarin | ||||
| Caffeine |
Disopyramide | Lidocaine | Nifedipine |
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 41 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
High Dose Hook Effect
There is no high dose hook effect at galectin-3 levels up to 500 ng/mL.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 42 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Dilution Parallelism
Dilution parallelism was evaluated by analyzing ten (10) clinical specimens with endogenous native galectin-3 concentrations from 21.6 ng/mL to 88.5 ng/mL at 1:20, 1:40, 1:80 and 1:160 dilutions. The grand mean recovery was 97.6%. For patient samples, dilute ten-fold (1:10) prior to measurement according to the instructions provided in the Procedure Section. Dilutions other than ten-fold are not recommended.
Sample Matrices
The BGM Galectin-3 assay has been validated for use with EDTA-plasma or serum samples. The equivalence of these sample matrices were demonstrated in a study of forty-nine (49) matched serum and EDTA-plasma samples with values spanning the measurement range. The regression equation is shown in the x/y scatter plot in Figure 10.
Figure 10: BGM Galectin-3 Serum and EDTA-Plasma Regression
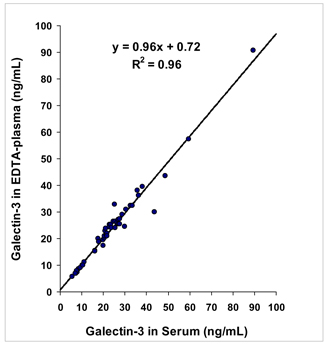
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 43 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Reference Range Study
A reference distribution for galectin-3 was determined through an observational study. Galectin-3 levels were measured in 1,099 banked plasma samples from a population of apparently healthy subjects without known heart disease but that otherwise resemble, by age and gender distribution, the HF patient population. Specimens were from women between the ages of 60 and 80 years (n=575) and men between the ages of 55 and 80 (n=524). This reference population comprised individuals of different ethnic background, as follows: Black or African-American (n=307, 27.9%), Caucasian (n=691, 62.9%), Hispanic (n=42, 3.8%), Asian or Pacific Islander (n=30, 2.7%), and not specified (n=29, 2.6%). All subjects had detectable galectin-3 levels (min-max, 3.2 - 94.6 ng/mL) within the measuring range of BGM Galectin-3. Blood plasma samples were collected from study participant into tubes containing EDTA. The blood was processed and blood plasma was subsequently frozen at -80°C or colder. Table 18 summarizes the galectin-3 distribution results. The 97.5th percentile of the galectin-3 distribution from this reference population is 26.2 ng/mL.
Each laboratory should establish a reference range that is representative of the patient population to be evaluated. Additionally, each laboratory should consider their current practice in the evaluation of heart failure patients at each institution.
Table 18: Distribution of Galectin-3 Levels in Subjects without Known Heart Disease
| Percentile |
Galectin-3 (ng/mL) | |
| 2.5th | 5.4 | |
| 5th | 6.3 | |
| 25th | 9.7 | |
| 50th | 12.4 | |
| 75th | 15.6 | |
| 90th | 19.0 | |
| 95th | 22.1 | |
| 97.5th | 26.2 |
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 44 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
References
Statistical Quality Control for Quantitative Measurement Procedures: Principles and Definitions; Approved Guideline – Third Edition. CLSI document C24-A3 [ISBN 1-56238-613-1]. Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS), 2006.
Christenson RH, Duh SH, Wu AH, et al. Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin Biochem 2010 May;43(7-8):683-90.
Gabius HJ. Cell surface glycans: the why and how of their functionality as biochemical signals in lectin-mediated information transfer. Crit Rev Immunol 2006;26:43-79.
Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA 2006;103:5060-5.
Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol 1996;156:3939-44.
Liu YH, D’Ambrosio M, Liao TD, et al. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol 2009;296:H404-12.
Sano H, Hsu DK, Yu L, et al. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol 2000;165:2156-64.
Sharma U, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004;110:3121-8.
de Boer RA, Voors AA, Muntendam P, et al. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail 2009;11:811-7.
Whellan DJ, O’Connor CM, Lee KL, et al. HF-ACTION Trial Investigators. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007 Feb;153(2):201-11.
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 45 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Manufactured for:

Technical Support Contact Information:
Tel: 1-877-665-0077
Fax: 1-877-313-8877
Email: tsupport@bg-medicine.com
To Place an Order:
Tel: 1-877-665-0077
Fax: 1-877-313-8877
Email: customer@bg-medicine.com
Revision: FDA LABELING
Date of Issuance: March, 2011
U.S. Patents Pending: Patent Application # 61/109,366 and Patent Application # 10/575,745
Trademarks: ProClin® is a registered trademark of Rohm and Haas Company.
Glossary of Symbols:
| BG Medicine, Inc., 610N Lincoln Street, Waltham, MA 02451 | Document: LAB-IVD-001 R07 | |
| Tel: (+1) 781.890.1199, Fax: (+1) 781.895.1119 | Effective: 09Mar11 |
Page 46 of 46
Portions of this Exhibit, indicated by the mark “[***],” were omitted and have been filed separately with the Securities and Exchange Commission pursuant to the Registrant’s application requesting confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
