Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - TENGION INC | form8k.htm |

1
Rodman & Renshaw 13th Annual Healthcare Conference
Rodman & Renshaw 13th Annual Healthcare Conference
September 2011
September 2011

2
Certain statements in this presentation may constitute forward looking statements within the meaning
of the Private Securities Litigation Reform Act of 1995. Although Tengion believes that these
statements are based upon reasonable assumptions within the bounds of its knowledge of its
business and operations, there are a number of factors that may cause actual results to differ from
these statements.
of the Private Securities Litigation Reform Act of 1995. Although Tengion believes that these
statements are based upon reasonable assumptions within the bounds of its knowledge of its
business and operations, there are a number of factors that may cause actual results to differ from
these statements.
For instance there can be no assurance that: (i) the Company will be able to find a suitable candidate
for the position of chief executive officer or that the Company will be able to retain such person on
mutually agreeable terms; (ii) the Company's Neo-Urinary Conduit clinical trial will not be placed on
clinical hold by the Food and Drug Administration, or FDA; (iii) patients enrolled in the Company's Neo
-Urinary Conduit clinical trial will not experience additional adverse events, which could delay clinical
trials or cause the Company to terminate the development of the Neo-Urinary Conduit; (iv) the
Company will be able to successfully enroll patients in its clinical trials, including its initial clinical trial
for the Neo-Urinary Conduit; (v) the results of the clinical trial for the Neo-Urinary Conduit will support
further development of that product candidate; (vi) data from the Company's ongoing preclinical
studies will continue to be supportive of advancing its preclinical product candidates; (vii) the
Company will be able to progress its product candidates that are undergoing preclinical testing,
including the Neo-Kidney Augment, into clinical trial; and (viii) the Company will be able to obtain the
capital it needs to develop its product candidates and continue its operations.
for the position of chief executive officer or that the Company will be able to retain such person on
mutually agreeable terms; (ii) the Company's Neo-Urinary Conduit clinical trial will not be placed on
clinical hold by the Food and Drug Administration, or FDA; (iii) patients enrolled in the Company's Neo
-Urinary Conduit clinical trial will not experience additional adverse events, which could delay clinical
trials or cause the Company to terminate the development of the Neo-Urinary Conduit; (iv) the
Company will be able to successfully enroll patients in its clinical trials, including its initial clinical trial
for the Neo-Urinary Conduit; (v) the results of the clinical trial for the Neo-Urinary Conduit will support
further development of that product candidate; (vi) data from the Company's ongoing preclinical
studies will continue to be supportive of advancing its preclinical product candidates; (vii) the
Company will be able to progress its product candidates that are undergoing preclinical testing,
including the Neo-Kidney Augment, into clinical trial; and (viii) the Company will be able to obtain the
capital it needs to develop its product candidates and continue its operations.
For additional factors which could cause actual results to differ from expectations, reference is made
to the reports filed by the Company with the Securities and Exchange Commission under the
Securities Exchange Act of 1934, as amended. The forward looking statements in this presentation
are made only as of the date hereof and the Company disclaims any intention or responsibility for
updating predictions or expectations in this presentation.
to the reports filed by the Company with the Securities and Exchange Commission under the
Securities Exchange Act of 1934, as amended. The forward looking statements in this presentation
are made only as of the date hereof and the Company disclaims any intention or responsibility for
updating predictions or expectations in this presentation.
Forward Looking Statements

3
Tengion products harness the body’s natural ability to regenerate
§ Unique and productive research platform
Neo-Urinary Conduit provides alternative to standard of care for bladder
cancer patients
cancer patients
§ Three patients implanted so far at Johns Hopkins and Univ of Chicago with
staggered enrollment
staggered enrollment
– Investigators gaining important insights and making modifications to surgical
approach
approach
§ Patient screening for enrollment currently active; anticipated enrollment of up to 10
patients
patients
§ Significant unmet need
Neo-Kidney Augment delays progression of kidney failure
§ Four animal models of chronic kidney failure published and/or presented
§ FDA interaction anticipated by end of year to clarify path to clinical trials
Company Highlights
Company Highlights
4
INTEGRATED
PLATFORM
Industrialization
Cells
Biomaterials
Surgical Implantation
Humans have limited capacity to regenerate
Our platform uniquely catalyzes human tissue regeneration
Catalyzing Regeneration in the Body
Catalyzing Regeneration in the Body

6
Urologic Franchise
7
Benefit of
Tengion
Products
Tengion
Products
Current
Standard of
Care
Standard of
Care
Primary Patient
Populations
(US and EU)
Populations
(US and EU)
Bowel used to create a tube to
transport urine from ureters to
abdominal wall into ostomy bag
transport urine from ureters to
abdominal wall into ostomy bag
Neo-Urinary Conduit
Phase I
Bladder cancer (20K/yr)
Bowel used to create a bladder
to carry and store urine inside the
patient for excretion via urethra
to carry and store urine inside the
patient for excretion via urethra
Neo-Bladder Replacement
Pre-IND
Bladder cancer (1.6K/yr)
Eliminate the use of bowel and
reduce the associated complications and side effects
Neo-Bladder Augment
Bowel used to increase
bladder capacity and
decrease pressure
bladder capacity and
decrease pressure
Phase II
Spina Bifida / SCI* (1K/yr)
*SCI = Spinal Cord Injury
Urologic Products to Enhance or Replace Bladder
Urologic Products to Enhance or Replace Bladder
8
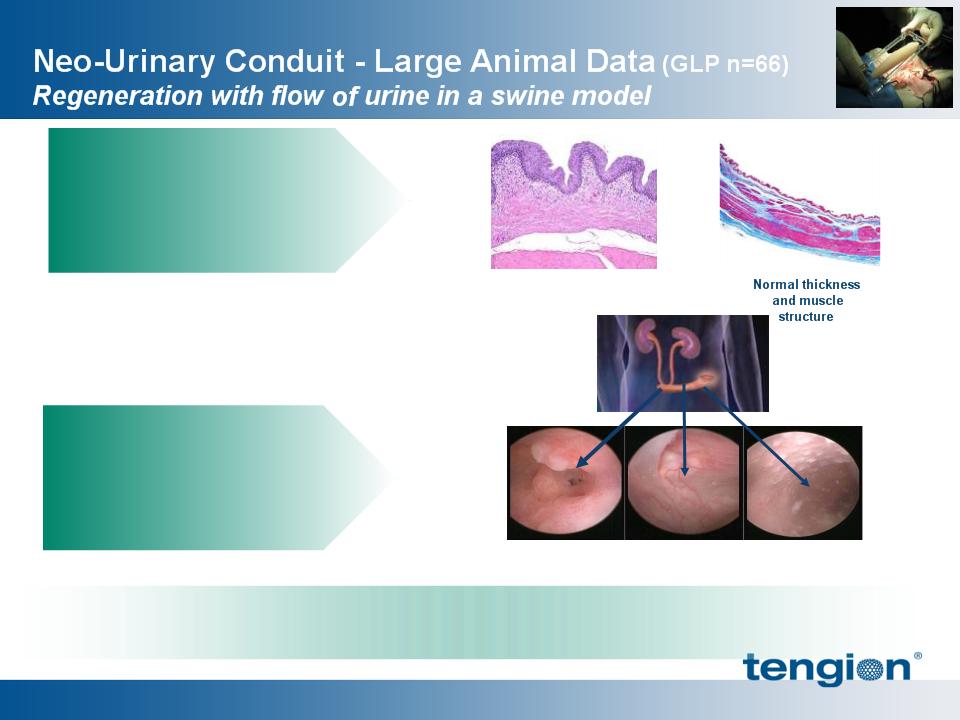
Native-like regeneration at
three months with no
immune rejection
three months with no
immune rejection
Normal lining
Source: Tengion IND #14218; Presented at International Society for Stem Cell Research, 2009
Regeneration of normal
lining from ureters to skin
with no urine absorption
and no mucus secretion
lining from ureters to skin
with no urine absorption
and no mucus secretion
Junction
with ureter
with ureter
Conduit
Junction
with skin
with skin
The product candidate catalyzed regeneration of a conduit made of
bladder tissue, allowing for unobstructed urine flow

9
Up to 10 patients with primary bladder cancer requiring cystectomy
§ Three patients implanted to date at University of Chicago and Johns Hopkins
Hospital
Hospital
Sequential enrollment of initial patients
§ Allows for modifications to surgical procedure as needed
§ Same 2 surgeons at initial implants to define procedure
Primary efficacy assessment
§ Conduit integrity and patency at 1 year
Neo-Urinary Conduit: Initial Clinical Trial
Open label study to define surgical procedure and safety
Open label study to define surgical procedure and safety
Neo-Urinary Conduit: Initial Clinical Trial
Open label study to define surgical procedure and safety
Open label study to define surgical procedure and safety

10
May 2011
§ Assessment conducted with investigators and DSMB following complications
and discussed with FDA
and discussed with FDA
June - August 2011
§ FDA grants orphan-drug designation to Neo-Urinary Conduit
§ Additional clinical data collected
§ Outcomes reviewed and surgical approach revised
– Modifications focused on vascular supply and stoma
September 2011
§ Enrollment resumed
– Stagger 15 weeks between patients 4 and 5 for observation
– Future timelines dependent upon outcomes
Neo-Urinary Conduit: Recent Progress
Neo-Urinary Conduit: Recent Progress

11
Neo-Kidney Augment
Neo-Kidney Augment

12
100,000 new dialysis patients each year in the US
§ 350,000 currently on dialysis
§ 20% annual mortality
§ $77,000 annual cost per patient
§ $39 billion in direct US costs annually for end stage kidney disease
Neo-Kidney Augment Overview
Intended to delay the need for dialysis or transplantation
Intended to delay the need for dialysis or transplantation
Neo-Kidney Augment Overview
Intended to delay the need for dialysis or transplantation
Intended to delay the need for dialysis or transplantation
Biopsy Cell Isolation & Culture Cell Selection Dose Preparation Delivery
(2-3 weeks) (<1 day) (2 weeks)
cells + carrier
injectable format
injectable format

13
Initial data published in September, 2010
§ Aggressive preclinical rodent model of chronic kidney disease
§ Preserved functional kidney mass, slowed progression, improved survival at 6 months
Diabetic kidney failure
§ Aggressive diabetic, obese, hypertensive rodent model of kidney failure
§ Slowed kidney failure progression and improved survival at 1 year
Human kidney cells in nude rat kidney failure
§ Reversed kidney failure at 3 months
Large animal kidney failure
§ Observations consistent with small animal results
§ Effects seen as early as 7 weeks, with persistent effects reported at 9 months
Neo-Kidney Augment
Preclinical Data
Preclinical Data
Neo-Kidney Augment
Preclinical Data
Preclinical Data
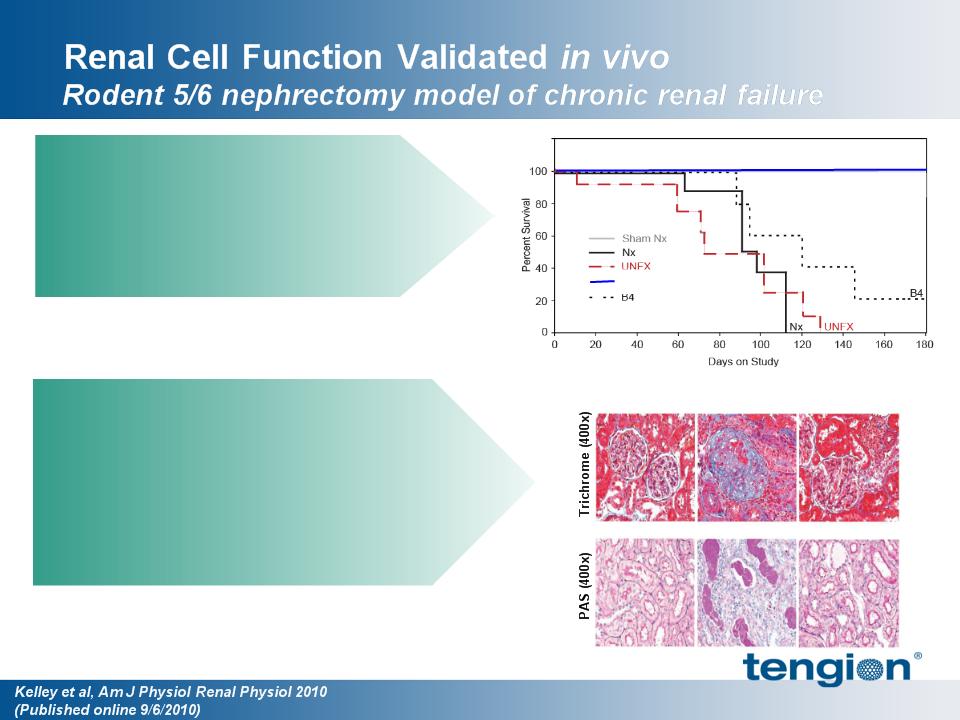
14
NKA
NKA
SHAM NX
HEALTHY NX NX + NKA
Renal cells delivered after chronic
disease state established enhanced
survival and renal function
disease state established enhanced
survival and renal function
§ sCREAT sustained at >200%
Renal cells improved multiple
structural and physiologic parameters
§ Enhanced survival
– 100% vs. 0% (Nx)
§ Improved glomerular function
§ Improved tubular function

15
Initial data published in September, 2010
§ Aggressive preclinical rodent model of chronic kidney disease
§ Preserved functional kidney mass, slowed progression, improved survival at 6 months
Diabetic kidney failure
§ Aggressive diabetic, obese, hypertensive rodent model of kidney failure
§ Slowed kidney failure progression and improved survival at 1 year
Human kidney cells in nude rat kidney failure
§ Reversed kidney failure at 3 months
Large animal kidney failure
§ Observations consistent with small animal results
§ Effects seen as early as 7 weeks, with persistent effects reported at 9 months
Neo-Kidney Augment
Preclinical Data
Preclinical Data
Neo-Kidney Augment
Preclinical Data
Preclinical Data
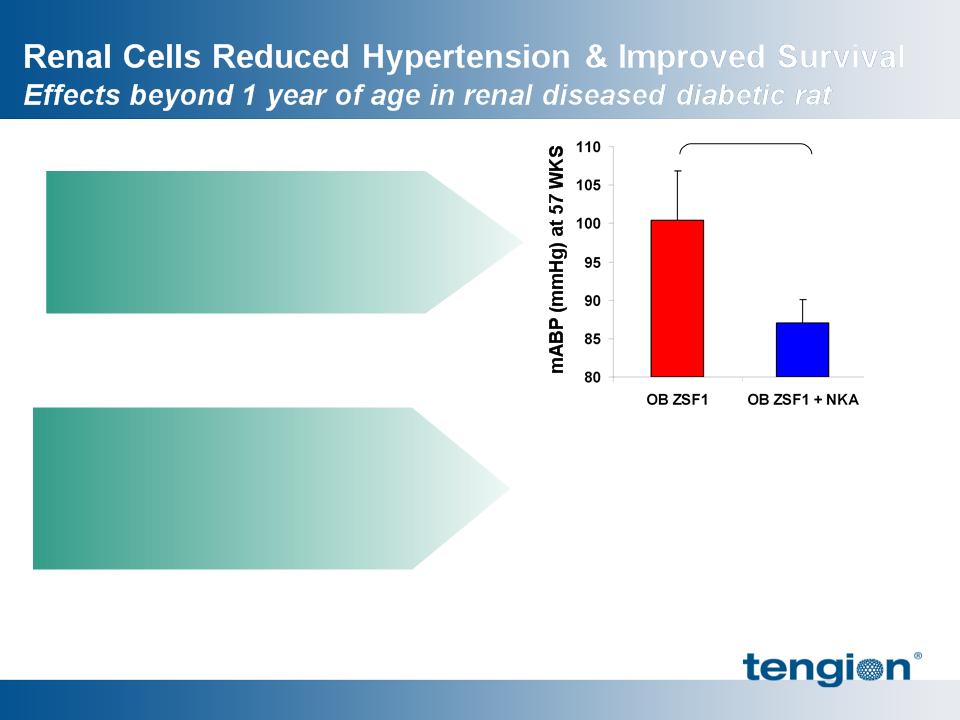
16
|
Treatment
Group |
63-week
Survival |
|
Untreated
OB ZSF1
|
20% (1/5)
|
|
OB ZSF1
+ NKA |
100% (5/5)
|
p = 0.042
Renal Cells reduced mean arterial
blood pressure (MABP)
significantly
blood pressure (MABP)
significantly
Renal Cells support survival
beyond 50% mortality time point
for OB ZSF1
beyond 50% mortality time point
for OB ZSF1

17
Initial data published in September, 2010
§ Aggressive preclinical rodent model of chronic kidney disease
§ Preserved functional kidney mass, slowed progression, improved survival at 6 months
Diabetic kidney failure
§ Aggressive diabetic, obese, hypertensive rodent model of kidney failure
§ Slowed kidney failure progression and improved survival at 1 year
Human kidney cells in nude rat kidney failure
§ Reversed kidney failure at 3 months
Large animal kidney failure
§ Observations consistent with small animal results
§ Effects seen as early as 7 weeks, with persistent effects reported at 9 months
Neo-Kidney Augment
Preclinical Data
Preclinical Data
Neo-Kidney Augment
Preclinical Data
Preclinical Data
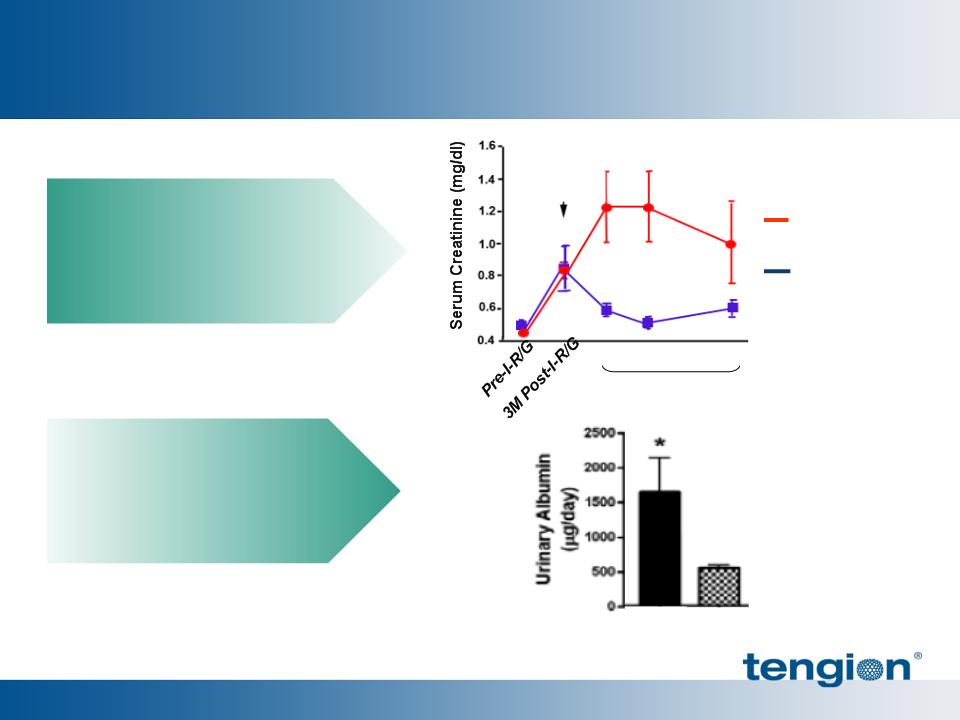
18
Human-derived cells
prevent renal failure in
CKD Nude Rats for 3 mo.
prevent renal failure in
CKD Nude Rats for 3 mo.
Human-derived cells
improve CKD Nude Rat
nephron function
improve CKD Nude Rat
nephron function
I-R/G Injury +
Human Renal Cells
Human Renal Cells
I-R/G Injury
Treatment
1 4 12
weeks post-treatment
*
*
* p<0.05
Human
Renal Cells
NoTx
Human Kidney Tissue Regeneration in Nude Rats
Human Kidney Tissue Regeneration in Nude Rats
Delayed progression of CKD and stabilized renal function
Delayed progression of CKD and stabilized renal function

19
Initial data published in September, 2010
§ Aggressive preclinical rodent model of chronic kidney disease
§ Preserved functional kidney mass, slowed progression, improved survival at 6 months
Diabetic kidney failure
§ Aggressive diabetic, obese, hypertensive rodent model of kidney failure
§ Slowed kidney failure progression and improved survival at 1 year
Human kidney cells in nude rat kidney failure
§ Reversed kidney failure at 3 months
Large animal kidney failure
§ Observations consistent with small animal results
§ Effects seen as early as 7 weeks, with persistent effects reported at 9 months
Neo-Kidney Augment
Preclinical Data
Preclinical Data
Neo-Kidney Augment
Preclinical Data
Preclinical Data
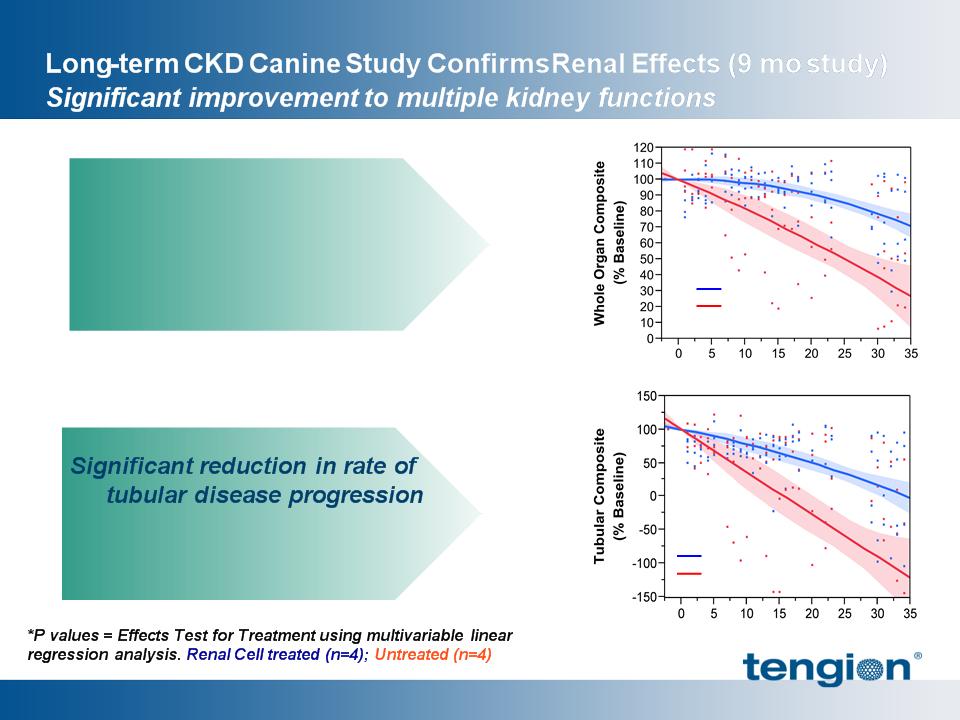
20
Treated
Untreated
Weeks Post Renal Cell Treatment
Treated
Untreated
Weeks Post Renal Cell Treatment
§ Electrolyte,UPC
p < 0.0001*
Improvement and stabilization
of whole kidney functions
of whole kidney functions
§ Glomerular, Tubular and
Collecting duct functions
Collecting duct functions
p < 0.0001*

21
Mechanism of Action Studies
NKA modifying widely accepted mechanisms
NKA modifying widely accepted mechanisms
§ Engraftment
§ Renal cells persist for at least 6 months at low levels
§ Expansion post-implantation
§ NKA SRC and Resident nephron cellular components expand post-
implantation
implantation
§ Attenuation of inflammatory and fibrosis pathways
§ Substantially reduced gene and protein expression of key biochemical
and molecular pathways: TGFb >50%; PAI-1 >50%; Fibronectin 50%
and molecular pathways: TGFb >50%; PAI-1 >50%; Fibronectin 50%
§ NKA effects on chronic kidney disease can be linked to direct
and indirect mechanisms of selected regenerative cells.
and indirect mechanisms of selected regenerative cells.
Ilagan et al, Amer Soc of Nephrology, 2010; Bruce et al, Exp Biol, 2011;
Kelley et al ISCT, 2010; Kelly et al TERMIS, 2010

22
Corporate
Corporate
23
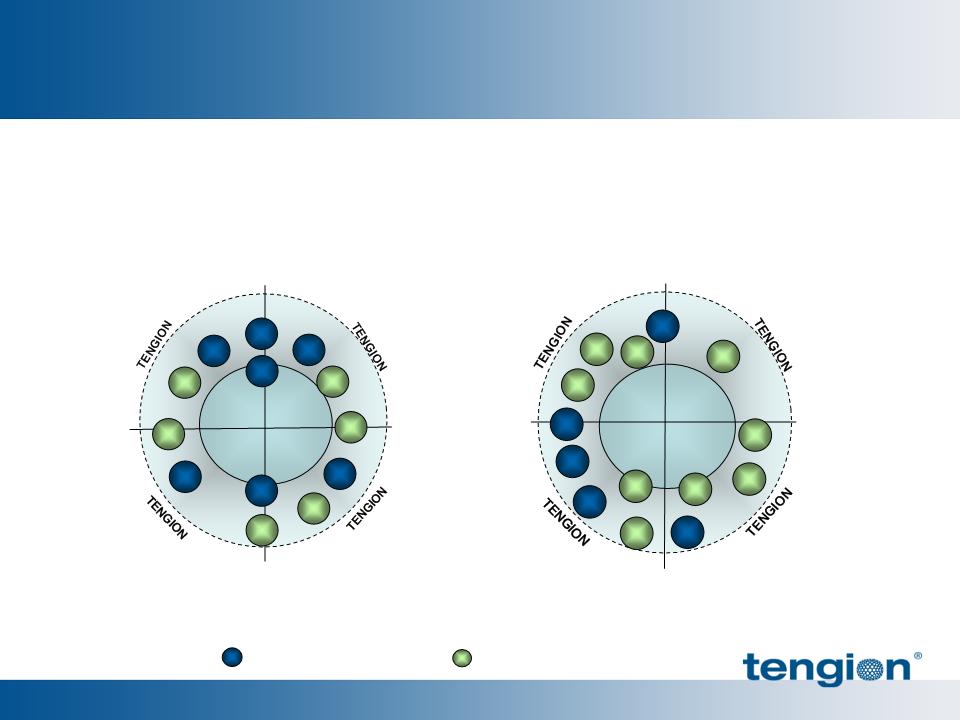
IP and Barriers to Entry
Issued patent protection to 2021, plus recent applications pending
§ 28 US and 97 international patents and patent applications
§ Core patents cover composition, design and methods of manufacture*
Plus know-how, trade secrets and integrated capabilities associated with
the discovery, development and manufacturing of our product candidates
the discovery, development and manufacturing of our product candidates
CELLS
DEVICE /
MATERIAL
PROCESS
THX USE
Urologic Franchise
= Patent Applications
= Issued Patents
Neo-Kidney Augment
CELLS
DEVICE /
MATERIAL
PROCESS
THX USE
* Individual patents may cover multiple elements
24
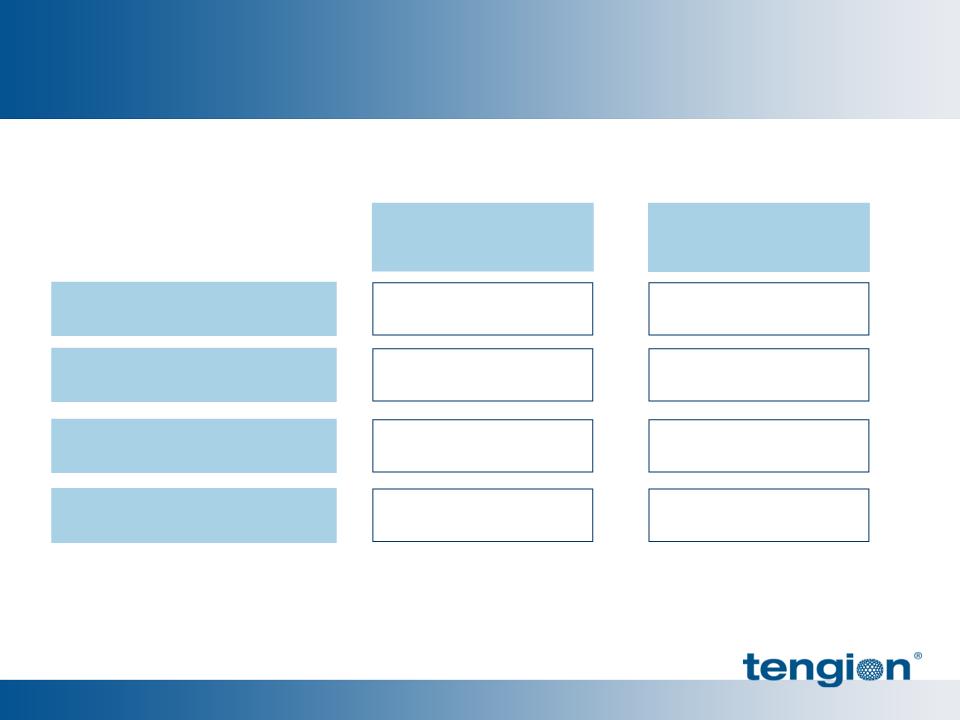
Financial Overview
Financial Overview
June 30, 2011
Cash and Investments
Long-Term Debt
Adjusted Net Loss - YTD
Outstanding Shares
$25.2 M
$5.6 M
$13.1 M
23.5 M
$31.5 M
$5.9 M
$6.5 M
23.5 M
March 31, 2011

25
Tengion products harness the body’s natural ability to regenerate
§ Unique and productive research platform
Neo-Urinary Conduit provides alternative to standard of care for bladder
cancer patients
cancer patients
§ Three patients implanted so far at Johns Hopkins and Univ of Chicago with
staggered enrollment
staggered enrollment
– Investigators gaining important insights and making modifications to surgical
approach
approach
§ Patient screening for enrollment currently active; anticipated enrollment of up to 10
patients
patients
§ Significant unmet need
Neo-Kidney Augment delays progression of kidney failure
§ Four animal models of chronic kidney failure published and/or presented
§ FDA interaction anticipated by end of year to clarify path to clinical trials
Company Highlights
Company Highlights

26
