Attached files
| file | filename |
|---|---|
| 8-K - OPEXA THERAPEUTICS, INC. 8-K - Acer Therapeutics Inc. | a6858248.htm |
Exhibit 99.1

Opexa Therapeutics, Inc. Corporate Presentation September 2011 (NASDAQ: OPXA) Neil K. Warma President & CEO

Forward-Looking Statements This presentation contains forward-looking statements which are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The forward-looking statements in this presentation do not constitute guarantees of future performance. Investors are cautioned that statements in this presentation which are not strictly historical statements, including, without limitation, statements regarding the Company’s clinical development plans for Tovaxin, constitute forward-looking statements. Such forward-looking statements are subject to a number of risks and
uncertainties that could cause actual results to differ materially from those anticipated, including, without limitation, risks associated with the Company’s capital position, the ability of the Company to enter into and benefit from a partnering arrangement for the Company’s product candidate, Tovaxin, on reasonably satisfactory terms (if at all), and our dependence (if partnered) on the resources and abilities of any partner for the further development of Tovaxin, our ability to compete with larger, better financed pharmaceutical and biotechnology companies, new approaches to the treatment of our targeted diseases, our expectation of incurring continued losses, our uncertainty of developing a marketable product, our ability to raise additional capital to continue our treatment development program and to undertake and complete any further clinical studies for Tovaxin, the
success of our clinical trials, our ability to develop and commercialize products, our ability to obtain required regulatory approvals, our compliance with all Food and Drug Administration regulations, our ability to obtain, maintain and protect intellectual property rights (including for Tovaxin), the risk of litigation regarding our intellectual property rights, the success of third party development and commercialization efforts with respect to products covered by intellectual property rights transferred by the Company, our limited manufacturing capabilities, our dependence on third-party manufacturers, our ability to hire and retain skilled personnel, our volatile stock price, and other risks detailed in our filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this presentation. We assume no obligation or undertaking to
update or revise any forward-looking statements contained herein to reflect any changes in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. You should, however, review additional disclosures we make in our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K filed with the SEC. Opexa Corporate Presentation 2 Opexa Therapeutics, Inc.

Opexa: a Leading Cell Therapy Company •Pioneering the development of cell therapies for autoimmune diseases through proprietary T-cell platform •Lead drug candidate Tovaxin® shows promising clinical efficacy in Multiple Sclerosis patients •Promising efficacy and favorable safety profile supports multiple late stage clinical trials •cGMP manufacturing facility supports clinical development needs and allows close oversight of quality control and costs •Strong management team to execute on opportunity Corporate Presentation 3 Opexa Therapeutics, Inc.
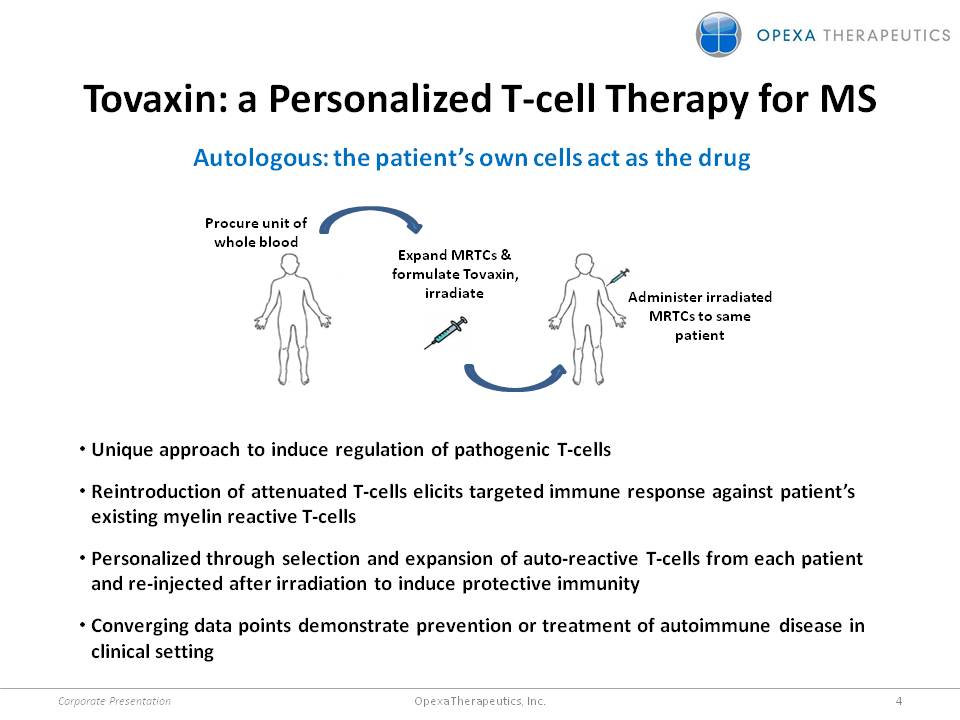
Tovaxin: a Personalized T-cell Therapy for MS Autologous: the patient’s own cells act as the drug •Unique approach to induce regulation of pathogenic T-cells •Reintroduction of attenuated T-cells elicits targeted immune response against patient’s existing myelin reactive T-cells •Personalized through selection and expansion of auto-reactive T-cells from each patient and re-injected after irradiation to induce protective immunity •Converging data points demonstrate prevention or treatment of autoimmune disease in clinical setting Administer irradiated MRTCs to same patient Expand MRTCs & formulate Tovaxin, irradiate Procure unit of whole blood Corporate
Presentation 4 Opexa Therapeutics, Inc.

Tovaxin highlights •5 clinical studies have been conducted (in Relapsing Remitting and Secondary Progressive MS patients), enrolling 356 patients with 182 treated with Tovaxin •Over 850 Tovaxin preparations have been manufactured: reproducibility and consistency established, commercially viable process implemented •Safety demonstrated and clinical efficacy promising across broad spectrum of patients for relapsing and progressive MS •Multiple commercial opportunities: –Secondary Progressive MS •Promising efficacy shown in slowing disease progression and reducing relapse rates •Significantly differentiates Tovaxin –Relapsing Remitting MS •FDA
concurrence for Phase 3 studies •Clinical benefit shown with reduction of relapse rates and improvement in disability •Mechanism supports combination therapy •Dose and regimen have been confirmed as effective and practical •In-house cGMP manufacturing enables close control of process and COGS •Opexa owns 100% worldwide rights for all indications Corporate Presentation 5 Opexa Therapeutics, Inc.
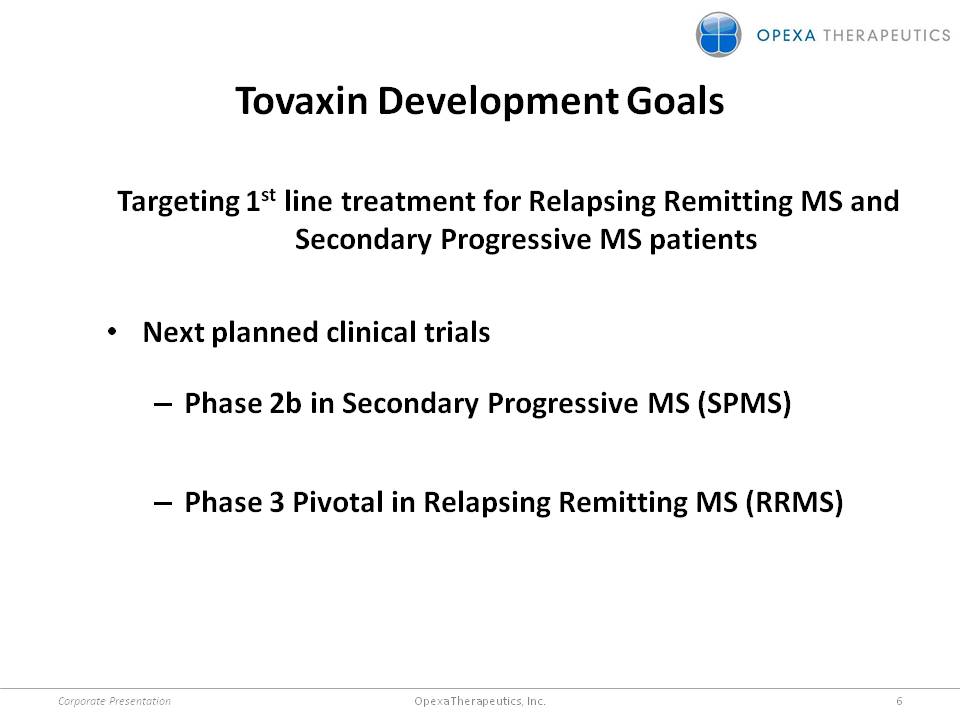
Tovaxin Development Goals Targeting 1stline treatment for Relapsing Remitting MS and Secondary Progressive MS patients •Next planned clinical trials –Phase 2bin Secondary Progressive MS (SPMS) –Phase 3 Pivotal in Relapsing Remitting MS (RRMS) Corporate Presentation 6 Opexa Therapeutics, Inc.

Business Strategy for Secondary Progressive MS (SPMS) •Supportive data –36 patients treated in three clinical studies –80% showed no disease progression, significant reduction in relapse rate –Well tolerated, no Serious Adverse Events (SAEs) –Supports Tovaxin mechanism of action •Possibility for streamlined clinical development path to approval (i.e. single pivotal study) •Strong interest from Key Opinion Leaders, clinical investigators and patients •Area of increased focus by Pharma companies •Positive result supports RRMS indication •Substantial unmet medical need in SPMS –Only one product currently approved for SPMS indication with
limited use due to cardio toxicity •Positive data could differentiate Tovaxin even further from competitors •Rapid study accrual possible due to limited treatment options Corporate Presentation 7 Opexa Therapeutics, Inc.
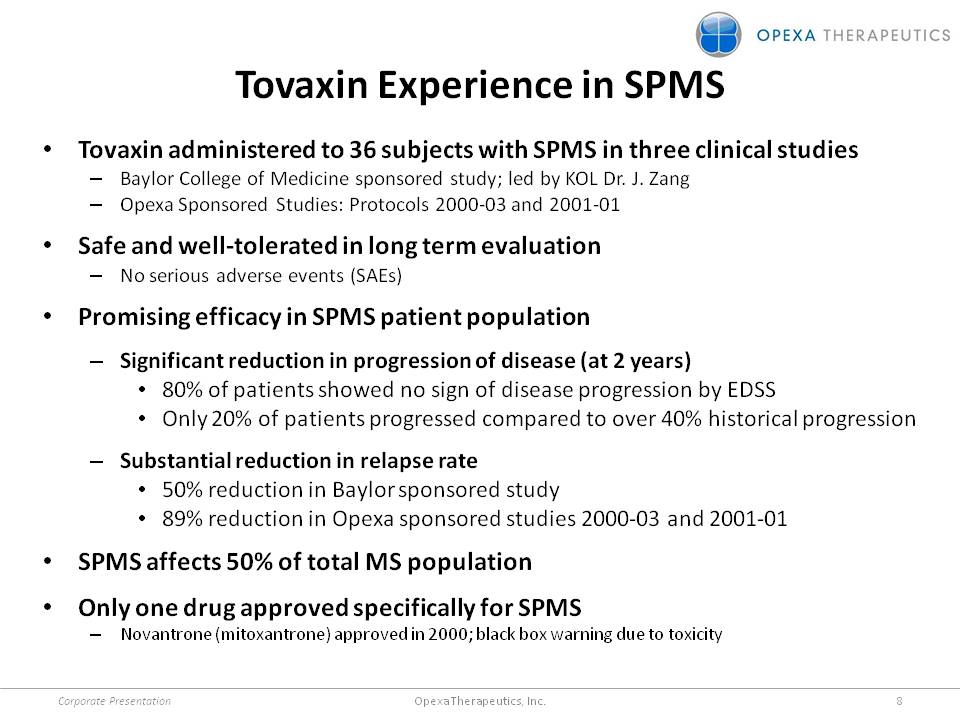
Tovaxin Experience in SPMS •Tovaxin administered to 36 subjects with SPMS in three clinical studies –Baylor College of Medicine sponsored study; led by KOL Dr. J. Zang –Opexa Sponsored Studies: Protocols 2000-03 and 2001-01 •Safe and well-tolerated in long term evaluation –No serious adverse events (SAEs) •Promising efficacy in SPMS patient population –Significant reduction in progression of disease (at 2 years) •80% of patients showed no sign of disease progression by EDSS •Only 20% of patients progressed compared to over 40% historical progression –Substantial reduction in relapse rate •50% reduction in Baylor sponsored study
•89% reduction in Opexa sponsored studies 2000-03 and 2001-01 •SPMS affects 50% of total MS population •Only one drug approved specifically for SPMS –Novantrone (mitoxantrone) approved in 2000; black box warning due to toxicity Corporate Presentation 8 Opexa Therapeutics, Inc.
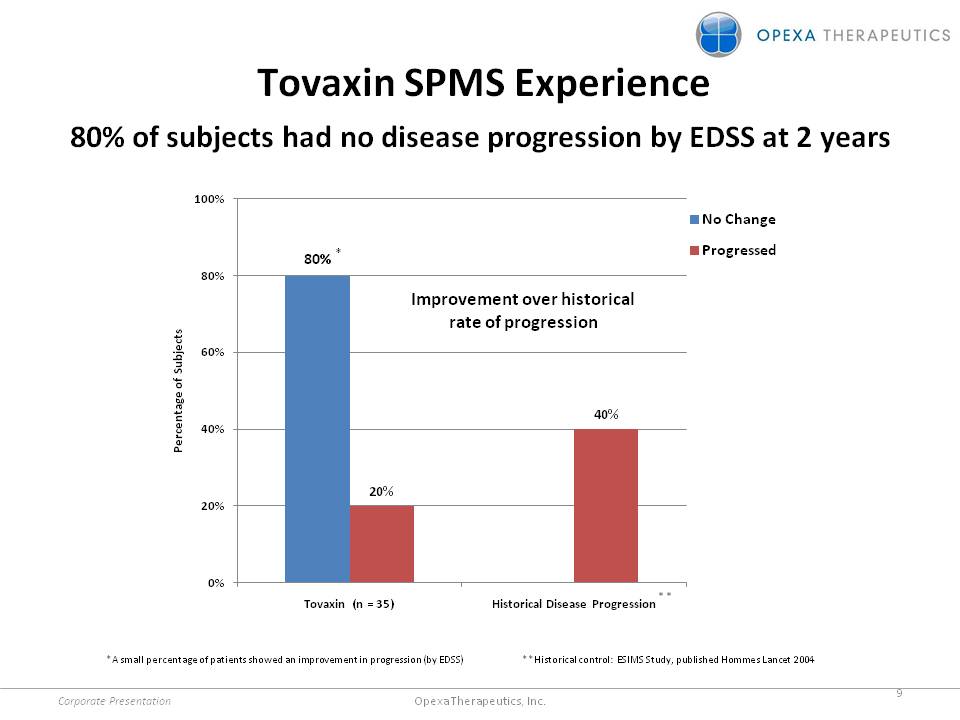
Tovaxin SPMS Experience 80% of subjects had no disease progression by EDSS at 2 years No Change Progressed 80%* 20% 40% Percentage of Subjects 0% 20% 40% 60% 80% 100% Improvement over historical rate of progression Tovaxin (n = 35) Historical Disease Progression ** *A small percentage of patients showed an improvement in progression (by EDSS) **Historical control: ESIMS Study, published Hommes Lancet 2004 Corporate Presentation 9 Opexa Therapeutics, Inc.
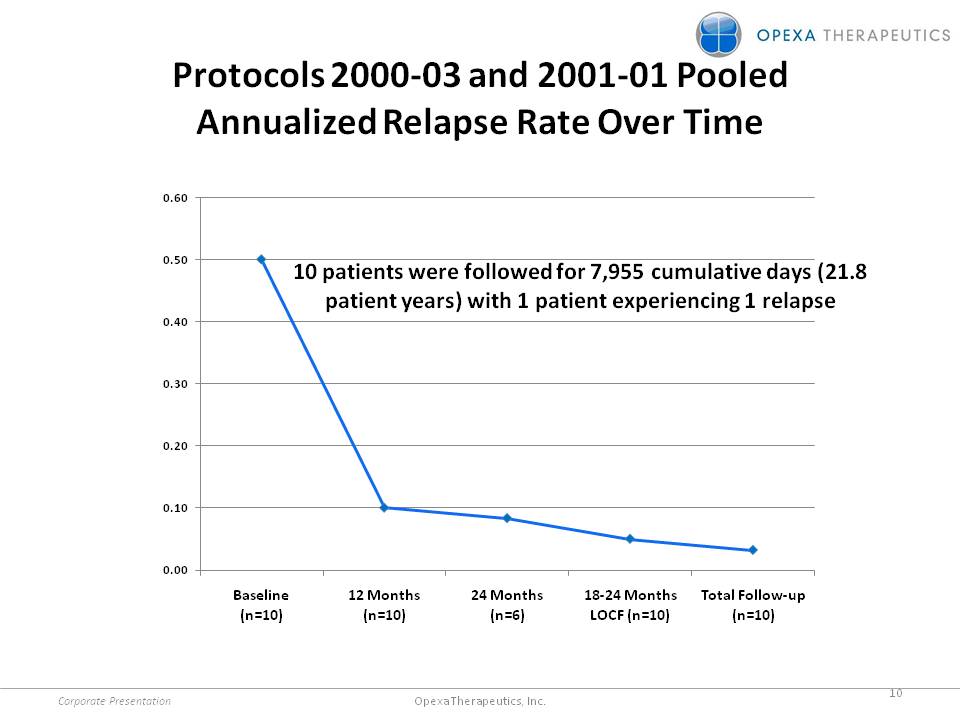
Protocols 2000-03 and 2001-01 Pooled Annualized Relapse Rate Over Time patients were followed for 7,955 cumulative days (21.8 patient years) with 1 patient experiencing 1 relapse 0.00 0.10 0.20 0.30 0.40 0.50 0.60 Baseline (n=10) 12 Months (n=10) 24 Months (n=6) 18-24 Months LOCF (n=10) Total Follow-up (n=10) Corporate Presentation 10 Opexa Therapeutics, Inc.
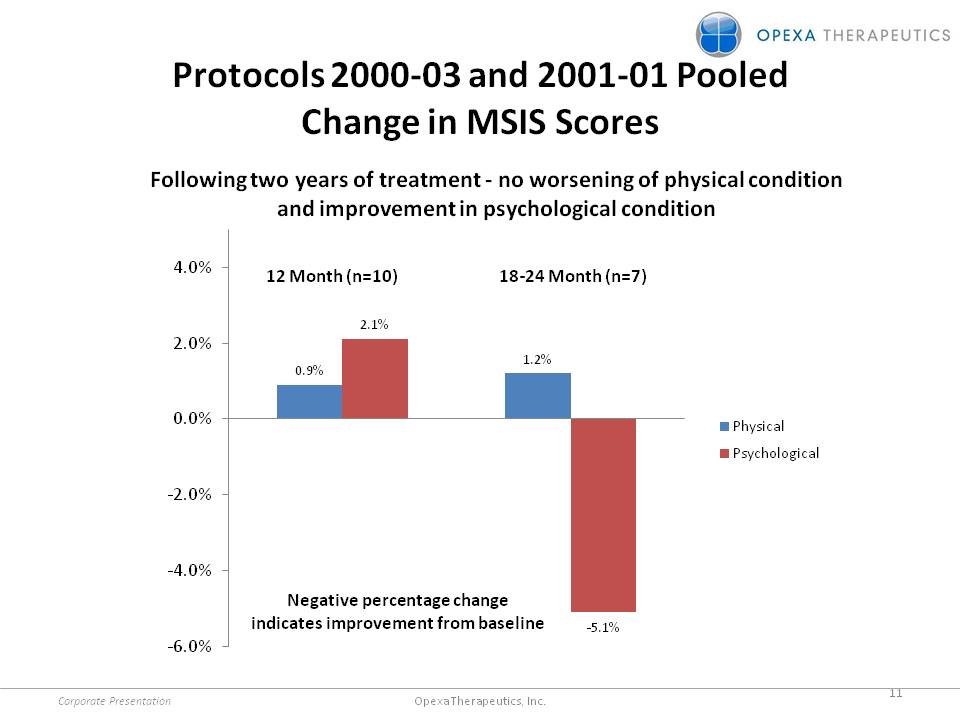
Protocols 2000-03 and 2001-01 Pooled Change in MSIS Scores Following two years of treatment -no worsening of physical condition and improvement in psychological condition 12 Month (n=10) 18-24 Month (n=7) 0.9% 2.1% 1.2% -5.1% Physical Psychological 4.0% 2.0% 0.0% -2.0% -4.0% -6.0% Negative percentage change indicates improvement from baseline Corporate Presentation 11 Opexa Therapeutics, Inc.
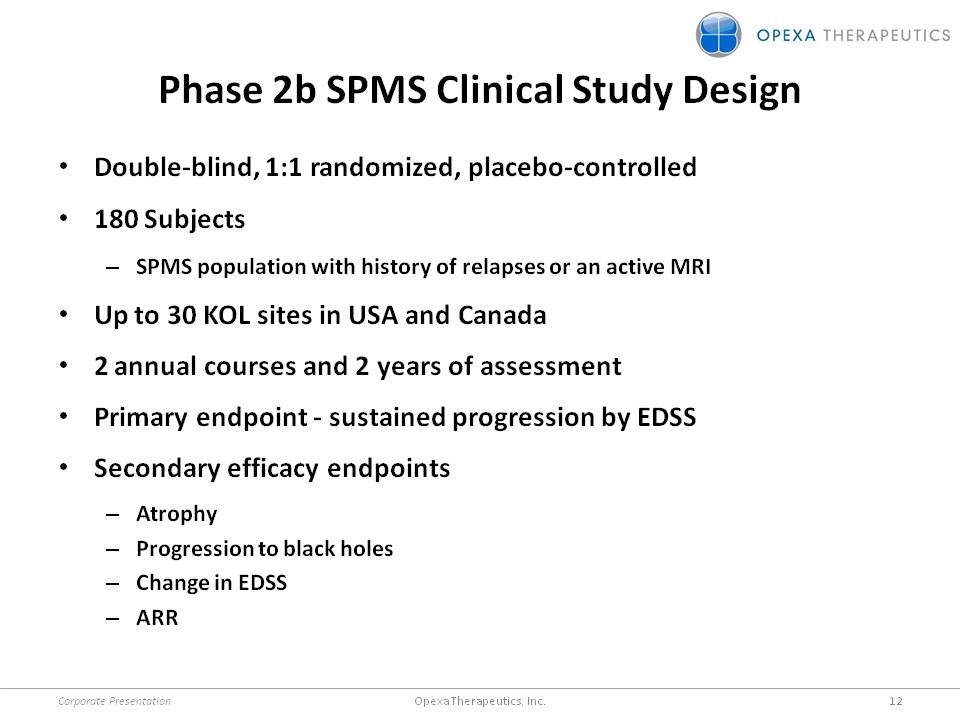
Phase 2b SPMS Clinical Study Design •Double-blind, 1:1 randomized, placebo-controlled •180 Subjects –SPMS population with history of relapses or an active MRI •Up to 30 KOL sites in USA and Canada •2 annual courses and 2 years of assessment •Primary endpoint -sustained progression by EDSS •Secondary efficacy endpoints –Atrophy –Progression to black holes –Change in EDSS –ARR Corporate Presentation 12 Opexa Therapeutics, Inc.

Relapsing Remitting MS Status •Completed Phase 2bclinical study in 150 RRMS patients •Completed two End of Phase 2meetings with FDA •Phase 3 pivotal studies are planned •Successful meeting with Health Canada to pave way for conducting clinical studies in Canada •Tovaxin positioned well in RRMS –superior safety, promising efficacy –Newly diagnosed –Non-compliant patients key targets Corporate Presentation 13 Opexa Therapeutics, Inc.
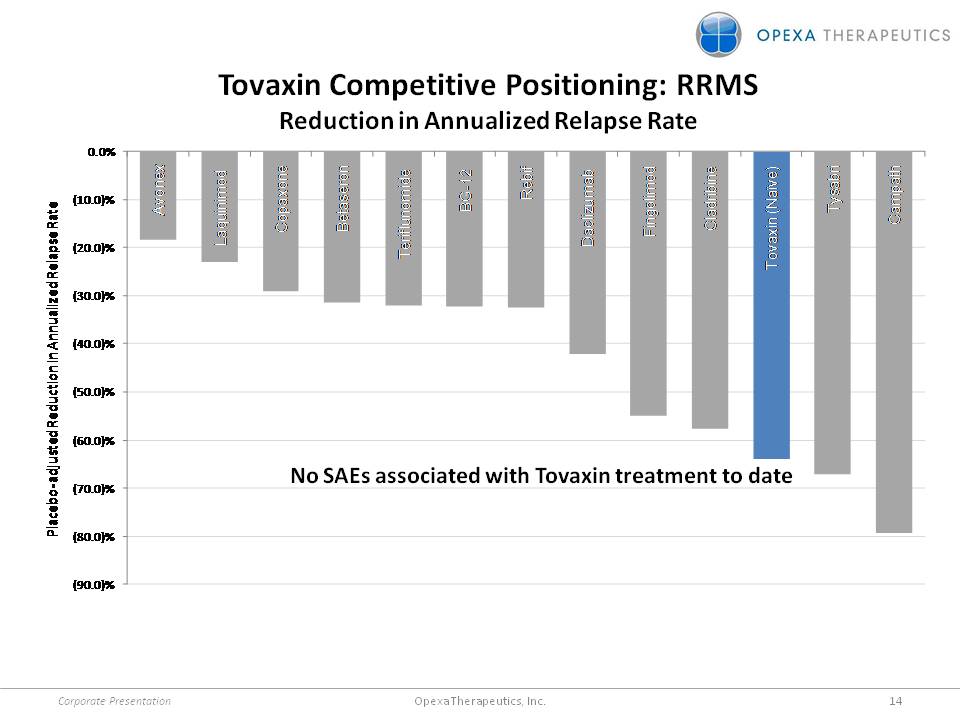
Tovaxin Competitive Positioning: RRMS Reduction in Annualized Relapse Rate Placebo-adjusted Reduction in Annualized Relapse Rate No SAEs associated with Tovaxin treatment to date Corporate Presentation 14 Opexa Therapeutics, Inc.
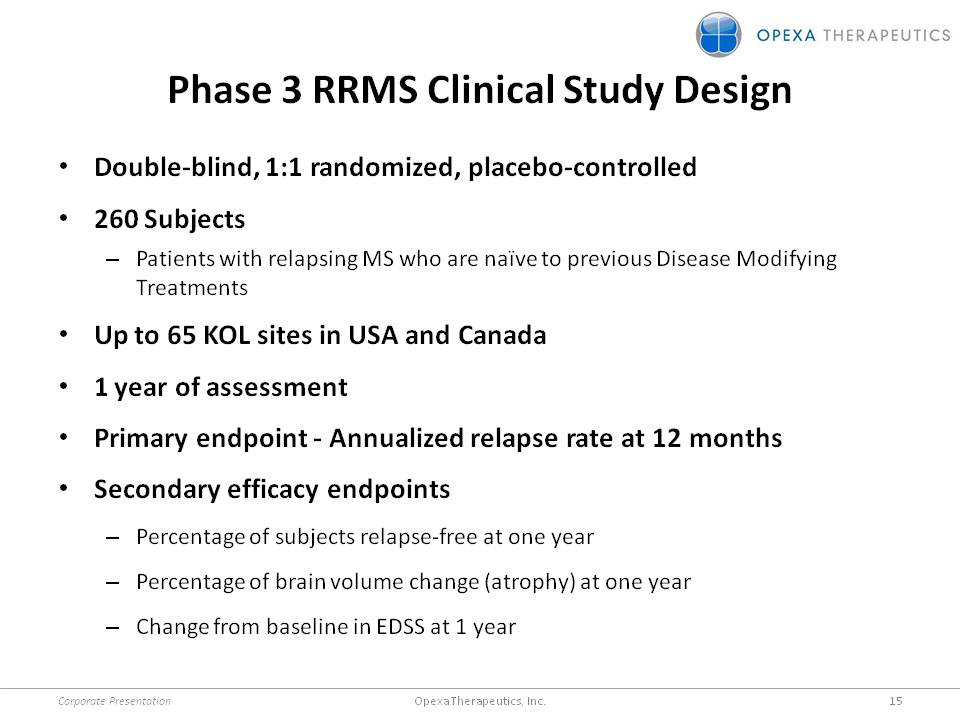
Phase 3 RRMS Clinical Study Design •Double-blind, 1:1 randomized, placebo-controlled •260 Subjects –Patients with relapsing MS who are naïve to previous Disease Modifying Treatments •Up to 65 KOL sites in USA and Canada •1 year of assessment •Primary endpoint -Annualized relapse rate at 12 months •Secondary efficacy endpoints –Percentage of subjects relapse-free at one year –Percentage of brain volume change (atrophy) at one year –Change from baseline in EDSS at 1 year Corporate Presentation 15 Opexa Therapeutics, Inc.
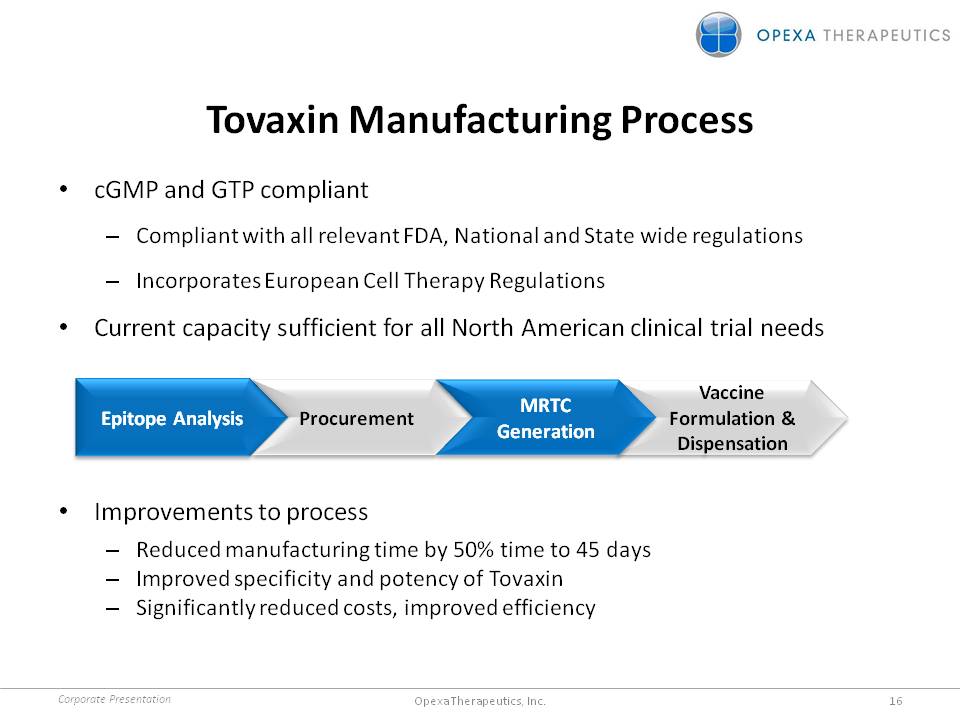
Tovaxin Manufacturing Process •cGMP and GTP compliant –Compliant with all relevant FDA, National and State wide regulations –Incorporates European Cell Therapy Regulations •Current capacity sufficient for all North American clinical trial needs Epitope Analysis Procurement MRTC Generation Vaccine Formulation & Dispensation •Improvements to process –Reduced manufacturing time by 50% time to 45 days –Improved specificity and potency of Tovaxin –Significantly reduced costs, improved efficiency Corporate Presentation 16 Opexa Therapeutics, Inc.

Previous 12 Month Company Milestones Q4’10 Secured FDA concurrence for Phase 3 RRMS studies -Completed two formal End of Phase 2meetings with FDA -Manufacturing, full CMC Review -Clinical Review, Future Development Plan Q1’11 Secured financing to advance toward Phase 3 trial initiation -Gross proceeds $8.5 million Q2’11 Presented Tovaxin data at the American Academy of Neurology (AAN) Meeting Q2’11 Executed strategic agreements with the American Red Cross and the Blood Group Alliance, Inc. to provide blood collection services to support future clinical development of Tovaxin Q2’11 Initiated the design and development of a proprietary Web-based system to manage patient and
product flow throughout future clinical studies Q3’11 Met with Health Canada’s Biologics and Genetics Therapies Directorate as part of the process to secure approval for Opexa to conduct a portion of future clinical development in Canada Corporate Presentation 17 Opexa Therapeutics, Inc.

Targeted Near-Term Company Milestones •Secure financing to advance clinical development •Initiate discussions with European Medicines Agency (EMA) •Evaluate further expansion of platform to other indications and geographical territories Corporate Presentation 18 Opexa Therapeutics, Inc.
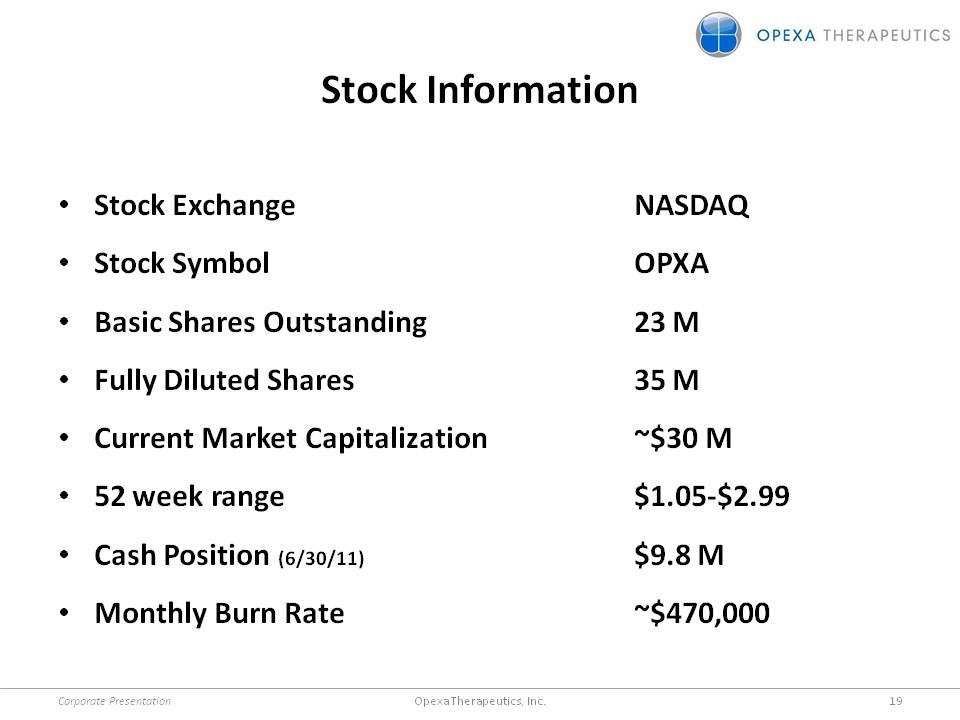
Stock Information •Stock Exchange NASDAQ •Stock Symbol OPXA •Basic Shares Outstanding 23 M •Fully Diluted Shares 35 M •Current Market Capitalization ~$30 M •52 week range $1.05-$2.99 •Cash Position (6/30/11) $9.8 M •Monthly Burn Rate ~$470,000 Corporate Presentation 19 Opexa Therapeutics, Inc.
