Attached files
Table of Contents
As filed with the Securities and Exchange Commission on August 16, 2011
Registration No. 333-173154
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 6
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Insys Therapeutics, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware |
2834 |
51-0327886 | ||
| (State or Other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
10220 South 51st Street, Suite 2
Phoenix, AZ 85044-5231
(602) 910-2617
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Michael L. Babich
President and Chief Executive Officer
Insys Therapeutics, Inc.
10220 South 51st Street, Suite 2
Phoenix, AZ 85044-5231
(602) 910-2617
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent for Service)
Copies to:
| Matthew T. Browne, Esq. Charles S. Kim, Esq. Sean M. Clayton, Esq. Cooley LLP 4401 Eastgate Mall San Diego, CA 92121 (858) 550-6000 |
Cheston J. Larson, Esq. Divakar Gupta, Esq. Matthew T. Bush, Esq. Latham & Watkins LLP 12636 High Bluff Drive, Suite 400 San Diego, CA 92130 (858) 523-5400 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended (the “Securities Act”), check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer |
x (Do not check if a smaller reporting company) | Smaller reporting company | ¨ |
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of each class of securities to be registered | Proposed maximum aggregate |
Amount of registration fee | ||
| Common Stock, $0.0002145 par value per share |
$55,000,000 | $6,386(2) | ||
|
| ||||
|
| ||||
| (1) | Estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(o) under the Securities Act. Includes the offering price of shares that the underwriters have the option to purchase to cover over-allotments, if any. |
| (2) | Previously paid. |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
| PROSPECTUS | SUBJECT TO COMPLETION, DATED AUGUST 16, 2011 |

Shares
Common Stock
This is an initial public offering of Insys Therapeutics, Inc. We are offering shares of common stock. We currently estimate that the initial public offering price of our common stock will be between $ and $ per share.
We have filed an application for our common stock to be listed on the Nasdaq Global Market under the symbol “INRX.”
Investing in our common stock involves risk. See “Risk Factors” beginning on page 11.
| Per Share | Total | |||||||
| Initial price to public |
$ | $ | ||||||
| Underwriting discounts and commissions |
$ | $ | ||||||
| Proceeds, before expenses, to Insys Therapeutics, Inc. |
$ | $ | ||||||
We have granted to the underwriters an option to purchase up to additional shares of common stock to cover over-allotments, if any, exercisable at any time until 30 days after the date of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares on or about , 2011.
| Wells Fargo Securities | JMP Securities |
Oppenheimer & Co.
Prospectus dated , 2011.
Table of Contents
| Page | ||||
| 1 | ||||
| 11 | ||||
| 50 | ||||
| 52 | ||||
| 52 | ||||
| 53 | ||||
| 55 | ||||
| Unaudited Pro Forma Condensed Consolidated Financial Information |
57 | |||
| 64 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
66 | |||
| 86 | ||||
| 115 | ||||
| 122 | ||||
| 145 | ||||
| 151 | ||||
| 153 | ||||
| 157 | ||||
| Material U.S. Federal Income Tax Consequences to Non-U.S. Holders of Our Common Stock |
159 | |||
| 163 | ||||
| 169 | ||||
| 169 | ||||
| 169 | ||||
| F-1 | ||||
You should rely only on the information contained in this prospectus and in any free writing prospectus that we may provide to you in connection with this offering. Neither we nor any of the underwriters has authorized anyone to provide you with information different from, or in addition to, that contained in this prospectus or any such free writing prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. Neither we nor any of the underwriters is making an offer to sell or seeking offers to buy these securities in any jurisdiction where or to any person to whom the offer or sale is not permitted. The information in this prospectus is accurate only as of the date on the front cover of this prospectus and the information in any free writing prospectus that we may provide you in connection with this offering is accurate only as of the date of that free writing prospectus. Our business, financial condition, results of operations and future growth prospects may have changed since those dates.
For investors outside the United States: neither we nor any of the underwriters has done anything that would permit this offering or possession or distribution of this prospectus or any free writing prospectus we may provide to you in connection with this offering in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus and any such free writing prospectus outside of the United States.
i
Table of Contents
This summary highlights information contained in other parts of this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before investing in shares of our common stock and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus. You should read the entire prospectus carefully, especially “Risk Factors” and our financial statements and the notes to these financial statements, before deciding to buy shares of our common stock.
Overview
We are a specialty pharmaceutical company that develops and seeks to commercialize innovative pharmaceutical products that target the unmet needs of cancer patients, with an initial focus on cancer-supportive care. We have assembled a product pipeline targeting cancer-supportive care and cancer therapy that we believe can be developed cost efficiently and, if approved, commercialized through a targeted commercial organization. Our supportive care product candidates include Subsys, a proprietary, fast-acting sublingual fentanyl spray for the treatment of breakthrough cancer pain, or BTCP, and our family of dronabinol product candidates for the treatment of chemotherapy-induced nausea and vomiting, or CINV, and appetite stimulation in AIDS patients. Subsys and our generic Dronabinol SG Capsule product candidates are both under review for marketing approval by the U.S. Food and Drug Administration, or FDA. We are also developing proprietary cancer therapeutics, the most advanced of which is LEP-ETU, an improved formulation of paclitaxel, the active ingredient in the cancer drugs Taxol and Abraxane.
We focus our research and development efforts on product candidates that utilize innovative formulations to address the clinical shortcomings of existing commercial pharmaceutical products. We intend to build a capital-efficient commercial organization to market Subsys and our other proprietary products, if approved. We expect to utilize an incentive-based sales model similar to that employed by Sciele Pharma, Inc. and other companies previously led by members of our board, including our founder and Executive Chairman.
The National Cancer Institute estimates that as of January 1, 2008, there were approximately 12.0 million people in the United States who had been previously diagnosed or were living with cancer. Debilitating side effects and symptoms such as pain, nausea and vomiting are prevalent in cancer patients and generally are caused by their disease as well as the radiation or chemotherapy treatment regimens intended to eradicate or inhibit the progression of the cancer. These side effects, among others, can impact a patient’s quality of life and ability to tolerate cancer treatment regimens. We believe effective supportive care is an important component in the treatment of cancer that is not adequately addressed by existing marketed therapies. By focusing on supportive care products, we believe we can contribute to the improvement of cancer patient outcomes and survival rates.
We are led by a management team and board of directors with substantial experience founding and managing pharmaceutical and related companies. Our founder and Executive Chairman, Dr. John N. Kapoor, has held executive management and board positions at Sciele Pharma and OptionCare, Inc., among others. Dr. Kapoor has also had significant experience with cancer-supportive care products, including Marinol, while he was Chairman of Unimed Pharmaceuticals, Inc. Our President and Chief Executive Officer, Michael L. Babich, has board and management experience at Alliant Pharmaceuticals, Inc. and EJ Financial Enterprises, Inc. Our Director of Scientific Development, Dr. Daniel D. Von Hoff, is a renowned oncologist and a founder of ILEX Oncology, Inc. Dr. Von Hoff previously led the development of several approved cancer and cancer-supportive care therapies including drugs such as Campath, Camptosar and Clofarabine. Our Chief Medical Officer, Dr. Larry Dillaha, previously served as the Chief Medical Officer of Sciele Pharma. We intend to leverage the
1
Table of Contents
experience of our management team to build Insys into a leading specialty pharmaceutical company focused on commercializing innovative therapies that address unmet medical needs of cancer patients.
Our Product Candidates
The following table summarizes certain information regarding our most advanced product candidates:
| Franchise |
Product Candidate |
Regulatory Pathway |
Indication |
Status | ||||||
| Spray | Subsys |
505(b)(2) | BTCP in Opioid-Tolerant Patients |
NDA Accepted; PDUFA goal date January 4, 2012 | ||||||
| Dronabinol |
Dronabinol SG Capsule | ANDA | CINV and Appetite Stimulation in Patients with AIDS |
ANDA Submitted3 | ||||||
| Dronabinol RT Capsule
|
sANDA1 | } | Pending4 | |||||||
| Dronabinol Oral Solution | 505(b)(2)1 | CINV and Appetite Stimulation in Patients with AIDS2 |
Pre-Phase 35 | |||||||
| Dronabinol Inhalation Device
|
505(b)(2)1 | Preclinical | ||||||||
| Dronabinol IV Solution | 505(b)(2)1 | Preclinical | ||||||||
| Oncology |
LEP-ETU | TBD | Metastatic Breast Cancer2 | Phase 2 | ||||||
| 1 | Anticipated regulatory pathway |
| 2 | Initial targeted indication |
| 3 | Abbreviated New Drug Application, or ANDA, under expedited review |
| 4 | Supplemental ANDA, or sANDA, expected to be filed in the first quarter of 2012, assuming approval of Dronabinol SG Capsule ANDA in the third quarter of 2011 |
| 5 | End-of-Phase 2 meeting completed; planning to initiate pivotal bioequivalence study |
Subsys
Subsys is a proprietary, single-use product that delivers fentanyl, an opioid analgesic, in seconds for transmucosal absorption underneath the tongue. In March 2011, we submitted a New Drug Application, or NDA, to the FDA for Subsys for the treatment of BTCP in opioid-tolerant patients. The FDA notified us in May 2011 that it had accepted the NDA for review and initially assigned a Prescription Drug User Fee Act, or PDUFA, goal date of January 4, 2012 for its review of the NDA. BTCP is characterized by sudden, often unpredictable, episodes of intense pain which can peak in severity at three to five minutes despite background pain medication. Subsys is the only transmucosal product to show statistically significant pain relief when measuring the sum of pain intensity difference at five minutes in a Phase 3 BTCP clinical trial using fentanyl. We believe this product is further differentiated by ease and speed of administration relative to the most widely-prescribed treatment alternatives. BTCP occurs in 50 to 90% of patients with cancer pain based on industry publications. According to IMS Health, transmucosal immediate-release fentanyl, or TIRF, products generated $440 million in U.S. sales in 2010. We believe this market has the potential to expand if faster-acting and more convenient products such as Subsys are approved by the FDA and this product class is more effectively promoted to oncologists and pain specialists. We currently plan to market Subsys in the United States, if approved, through a targeted sales force of approximately 50 to 75 representatives.
Dronabinol Product Family
We are developing a portfolio of dronabinol product candidates for the treatment of CINV and appetite stimulation in patients with AIDS, as well as other indications where dronabinol could have
2
Table of Contents
potential therapeutic benefits. Dronabinol, the active ingredient in Marinol, is a synthetic cannabinoid whose chemical name is delta-9-tetrahydrocannabinol, or THC. In 2010, dronabinol products generated approximately $142 million in U.S. sales. Our portfolio consists of two product candidates intended to be generic equivalents to Marinol in addition to three proprietary formulations, including Dronabinol Oral Solution. We believe our family of dronabinol products, if approved, has the potential to capture a broader share of the CINV market, which, according to IMS Health, generated $1.9 billion in U.S. sales in 2010.
We produce dronabinol active pharmaceutical ingredient, or API, for our product candidates at our U.S.-based, state-of-the-art manufacturing facility, which we believe provides us with a significant competitive advantage. We believe that this facility has the capacity to supply sufficient commercial quantities of the API for our dronabinol product candidates for the foreseeable future. In May 2011, we entered into a supply and distribution agreement with Mylan Pharmaceuticals Inc., or Mylan, for the distribution of Dronabinol SG and RT Capsules within a defined geographic area.
Dronabinol SG Capsule. Dronabinol SG Capsule, the most advanced product candidate in our dronabinol family, is a dronabinol soft gelatin capsule intended to be a generic equivalent to Marinol. In June 2010, we submitted an amendment to our ANDA to the FDA for this product candidate. If approved, we intend to commercialize Dronabinol SG Capsule with the aim of generating near-term cash flows to help fund the commercialization of Subsys and the development of our proprietary dronabinol and other product candidates, as well as validating our dronabinol supply chain and internal manufacturing capabilities.
Dronabinol RT Capsule. Dronabinol RT Capsule is a proprietary dronabinol soft gel capsule that is stable at room temperature. We intend to submit an sANDA to the FDA for Dronabinol RT Capsule following the approval of Dronabinol SG Capsule. We believe Dronabinol RT Capsule, if approved, would offer convenience advantages to distributors, pharmacies and patients, as product labeling for Marinol requires storage at refrigerated temperatures.
Dronabinol Oral Solution. Dronabinol Oral Solution is a proprietary synthetic THC in an oral liquid formulation which may offer advantages, including more consistent bioavailability, faster onset of action and more flexible dose titration. We have completed an end-of-Phase 2 meeting with the FDA and plan to initiate a pivotal bioequivalence study for this product candidate in the second half of 2011. Marinol is characterized by a highly variable bioavailability and an onset of action that ranges from 30 minutes to one hour. In our Phase 1 clinical trial, Dronabinol Oral Solution demonstrated a more reliable absorption profile and rapid onset of action as compared to Marinol. We believe these product attributes, coupled with increased acceptance of THC as a therapeutic alternative, could result in Dronabinol Oral Solution capturing market share and potentially expanding the market for dronabinol-based products.
Cancer Therapeutics
In addition to our cancer-supportive care products, we intend to develop proprietary cancer therapeutics targeting limitations of existing commercial products.
LEP-ETU. LEP-ETU, our most advanced proprietary cancer therapeutic, is a proprietary NeoLipid liposomal, or microscopic membrane-like structure created from lipids, formulation that incorporates paclitaxel. LEP-ETU recently completed a successful Phase 2 clinical trial of 70 patients with metastatic breast cancer. We are developing this product candidate to improve efficacy and reduce paclitaxel-related side effects. According to IMS Health, paclitaxel products generated $393 million in U.S. sales in 2010.
3
Table of Contents
Our Strategy
Our goal is to become a leading specialty pharmaceutical company focused on commercializing and developing innovative therapies that address unmet medical needs of cancer patients. Key elements of our strategy are to:
| • | Obtain FDA approval of Subsys. |
| • | Build a capital-efficient commercial organization to market Subsys and complementary products. |
| • | Obtain FDA approval of Dronabinol SG Capsule and Dronabinol RT Capsule, and commercialize these products through our May 2011 distribution agreement with Mylan. |
| • | Develop innovative dronabinol formulations to expand usage of synthetic THC for CINV and appetite stimulation in AIDS patients, as well as other indications. |
| • | Advance clinical development of LEP-ETU for the treatment of cancer. |
| • | Add commercial products or product candidates to our portfolio that complement our core competencies. |
Risks Associated with Our Business
Our business and our ability to execute our business strategy are subject to a number of risks that you should be aware of before you decide to buy our common stock. In particular, you should consider the following risks, which are discussed more fully in “Risk Factors:”
| • | We have not had commercial sales of any of our product candidates and may never become profitable. |
| • | We are highly dependent on the success of Subsys, Dronabinol SG Capsule and our other product candidates, and we cannot give any assurance that any of these product candidates will receive regulatory approval or acceptable Drug Enforcement Administration, or DEA, classification, or be successfully commercialized. |
| • | We face significant competition from both branded and generic products, and our operating results will suffer if we fail to compete effectively. |
| • | We are subject to numerous complex regulations and failure to comply with these regulations, or the cost of compliance with these regulations, may harm our business. |
| • | The anticipated development of a Risk Evaluation and Mitigation Strategies, or REMS, program for Subsys could cause significant delays in the approval process and would add additional layers of regulatory requirements that could significantly impact our ability to commercialize Subsys and dramatically reduce its market potential. |
| • | We have recently taken a number of significant actions aimed at growing our business and will need to further increase the size and complexity of our organization in the future, and we may experience difficulties in managing our growth and executing our growth strategy. |
| • | If we are unable to establish sales and marketing capabilities or execute on our sales and marketing strategy, including through our distribution agreement with Mylan, we may not be able to effectively market and sell any of our products, if approved, and generate product revenue. |
| • | We produce our dronabinol API internally and may encounter manufacturing failures that could delay the preclinical and clinical development or regulatory approval of our dronabinol product candidates, or their commercial production if approved. |
4
Table of Contents
| • | We rely on third parties to manufacture our product candidates, supply API and conduct our clinical trials. |
| • | If we fail to attract and keep management and other key personnel, as well as our board members, we may be unable to successfully develop or commercialize our product candidates and implement our business plan. |
| • | We may not be able to obtain and enforce patent rights or other intellectual property rights that cover our product candidates and are of sufficient breadth to prevent third parties from competing against us. |
| • | Our founder, Executive Chairman and principal stockholder can individually control our direction and policies, and his interests may be adverse to the interests of our stockholders. |
Corporate Information
We were incorporated as Oncomed Inc. in Delaware in June 1990, and subsequently changed our name to NeoPharm, Inc. On October 29, 2010, we entered into an Agreement and Plan of Merger with Insys Therapeutics, Inc., a Delaware corporation, and ITNI Merger Sub Inc., our wholly-owned subsidiary and a Delaware corporation. On November 8, 2010, pursuant to the Agreement and Plan of Merger, ITNI Merger Sub Inc. merged with and into Insys Therapeutics, Inc., and Insys Therapeutics, Inc. survived as our wholly-owned subsidiary. We refer to this transaction herein as the Merger. Following the Merger, our wholly-owned subsidiary, Insys Therapeutics, Inc., changed its name to Insys Pharma, Inc. and we changed our name to Insys Therapeutics, Inc. In connection with the Merger, all of the outstanding shares of common stock of Insys Pharma prior to the Merger were exchanged for 319,667 shares of our common stock and 14,864,607 shares of our newly-created convertible preferred stock. Each share of our convertible preferred stock is convertible into 0.57 shares of our common stock. As a result of the Merger, 95% of our common stock on an as-converted basis was held by the then-existing stockholders of Insys Pharma.
Our principal executive offices are located at 10220 South 51st Street, Suite 2, Phoenix, Arizona and our telephone number is (602) 910-2617. Our corporate website address is www.insysrx.com. We do not incorporate the information contained on, or accessible through, our website into this prospectus, and you should not consider it part of this prospectus.
For convenience in this prospectus, “Insys,” “we,” “us,” and “our” refer to Insys Therapeutics, Inc. and its subsidiaries taken as a whole, unless otherwise noted. We have applied for registration of the trademark “Insys Therapeutics, Inc.” in logo format, along with the trademarks “Insys” and “Subsys” with the United States Patent and Trademark Office. The trademark “Insys Therapeutics, Inc” in logo format is officially registered on the Principal Register of the United States Patent and Trademark Office. The trademark “Subsys” is allowed and will be eligible for registration on the Principal Register after it is used in commerce. This prospectus also contains trademarks and tradenames of other companies, and those trademarks and tradenames are the property of their respective owners.
5
Table of Contents
The Offering
| Common stock offered |
shares (or shares if the underwriters’ over-allotment option is exercised in full) |
| Common stock to be outstanding after this offering |
shares (or shares if the underwriters’ over-allotment option is exercised in full) |
| Use of proceeds from this offering |
We intend to use the net proceeds from this offering to fund the commercialization of Subsys and Dronabinol SG Capsule, if approved; to fund the development of Dronabinol RT Capsule, Dronabinol Oral Solution, Dronabinol Inhalation Device, LEP-ETU and our other early-stage product candidates; for potential product licensing and acquisitions; and for working capital and for other general corporate purposes. Please see the section entitled “Use of Proceeds.” |
| Risk factors |
You should read the “Risk Factors” section of this prospectus for a discussion of certain of the factors to consider carefully before deciding to purchase any shares of our common stock. |
| Proposed Nasdaq Global Market symbol |
INRX |
The number of shares of our common stock that will be outstanding after this offering is based on shares outstanding as of June 30, 2011 (after giving effect to the conversion of our convertible preferred stock outstanding as of such date into an aggregate of 8,528,860 shares of our common stock and the conversion of $ million in aggregate principal amount of notes and accrued interest thereon owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming a conversion date of , 2011 and an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, both of which will occur automatically immediately prior to the closing of this offering), and excludes:
| • | 540,102 shares of our common stock issuable upon the exercise of outstanding options as of June 30, 2011 under our equity incentive plans, with a weighted average exercise price of $9.76 per share; |
| • | 1,079,133 shares of our common stock issuable upon the exercise of outstanding options as of June 30, 2011 under the Insys Pharma, Inc. equity incentive plan, with a weighted average exercise price of $1.83 per share; and |
| • | 3,000,000 shares of our common stock reserved for future issuance under our 2011 equity incentive plan, 2011 non-employee directors’ stock award plan and 2011 employee stock purchase plan, each of which will become effective upon the signing of the underwriting agreement for this offering. |
Unless otherwise stated, all information contained in this prospectus assumes:
| • | the conversion of all of our outstanding convertible preferred stock into an aggregate of 8,528,860 shares of common stock automatically immediately prior to the closing of this offering; |
| • | the conversion of $ million in aggregate principal amount of notes and accrued interest thereon owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming a conversion date of , 2011 and an initial |
6
Table of Contents
| public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, immediately prior to the closing of this offering; |
| • | the filing of our amended and restated certificate of incorporation and adoption of our amended and restated bylaws, which will occur upon the closing of this offering; and |
| • | no exercise of the underwriters’ over-allotment option to purchase additional shares. |
The information in this prospectus also reflects a 1-for-61 reverse stock split of our common stock that was effected on July 14, 2011.
7
Table of Contents
Summary Financial Data
The following tables set forth our summary financial data. The summary financial data for the years ended December 31, 2010, 2009 and 2008 are derived from our audited financial statements appearing elsewhere in this prospectus. The statement of operations data for the six months ended June 30, 2011 and 2010 and the selected balance sheet data as of June 30, 2011 have been derived from our unaudited consolidated financial statements appearing elsewhere in this prospectus. The unaudited financial statements have been prepared on a basis consistent with our audited financial statements included in this prospectus and include, in our opinion, all adjustments, consisting only of normal recurring adjustments, necessary to state fairly our financial position as of June 30, 2011 and results of operations for the six months ended June 30, 2011 and 2010. You should read this summary financial data in conjunction with the financial statements and related notes and the information under the headings “Selected Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Unaudited Pro Forma Condensed Consolidated Financial Information” appearing elsewhere in this prospectus. Our historical results are not necessarily indicative of our future results.
On October 29, 2010, we entered into an Agreement and Plan of Merger with Insys Therapeutics, Inc., a Delaware corporation, and ITNI Merger Sub Inc., our wholly-owned subsidiary and a Delaware corporation. On November 8, 2010, pursuant to the Agreement and Plan of Merger, ITNI Merger Sub Inc. merged with and into Insys Therapeutics, Inc., and Insys Therapeutics, Inc. survived as our wholly-owned subsidiary. We refer to this transaction as the Merger. Following the Merger, our wholly-owned subsidiary, Insys Therapeutics, Inc., changed its name to Insys Pharma, Inc. and we changed our name to Insys Therapeutics, Inc. In connection with the Merger, all of the outstanding shares of common stock of Insys Pharma prior to the Merger were exchanged for 319,667 shares of our common stock and 14,864,607 shares of our newly-created convertible preferred stock. Each share of our convertible preferred stock is convertible into 0.57 shares of our common stock. As a result of the Merger, 95% of our common stock on an as-converted basis was held by the then-existing stockholders of Insys Pharma. Since Insys Pharma is the acquiring entity for accounting purposes, the financial statements for all periods up to and including the November 8, 2010 Merger date are the financial statements of the entity that is now our subsidiary, Insys Pharma. The financial statements for all periods subsequent to the November 8, 2010 Merger date are the consolidated financial statements of Insys Therapeutics, Inc. and Insys Pharma. However, for all periods, the financial statements are labeled “Insys Therapeutics, Inc.” financial statements. In addition, the audited financial statements of NeoPharm for the years ended December 31, 2009 and 2008 and the unaudited financial statements for the nine months ended September 30, 2010 and 2009 are also included in this prospectus.
The summary unaudited pro forma condensed consolidated statement of operations data for the six months ended June 30, 2011 and for the year ended December 31, 2010 below is based on the historical consolidated statements of operations of Insys Therapeutics, Inc. and NeoPharm, giving effect to the Merger, the conversion of our convertible preferred stock outstanding as of June 30, 2011 and December 31, 2010 into 8,528,860 shares of our common stock, the issuance after each period presented of additional notes payable to trusts controlled by our Executive Chairman and principal stockholder, and the conversion of the resulting $ million in aggregate principal amount of notes and actual accrued interest thereon through , 2011 owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, as if such transactions had occurred on January 1, 2010. The unaudited pro forma condensed consolidated statement of operations data is based on the estimates and assumptions set forth in the notes to the unaudited pro forma condensed consolidated financial statements. Please see the section entitled “Unaudited Pro Forma Condensed Consolidated Financial Information.” These estimates and assumptions are preliminary and subject to change, and have been made solely for the
8
Table of Contents
purposes of developing such pro forma information. The summary unaudited pro forma condensed consolidated statement of operations data is not necessarily indicative of the combined results of operations to be expected in any future period or the results that actually would have been realized had the entities been a single entity during the period presented.
| Pro Forma | Actual | Pro Forma | Actual | |||||||||||||||||||||||||
| Six Months Ended June 30, | Year Ended December 31, | |||||||||||||||||||||||||||
| 2011 |
2011 | 2010 | 2010 |
2010 | 2009 | 2008 | ||||||||||||||||||||||
| (In thousands, except share and per share data) |
||||||||||||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||||||||||
| Revenues |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||
| Research and development |
3,807 | 3,807 | 5,240 | 13,244 | 10,428 | 8,982 | 14,729 | |||||||||||||||||||||
| General and administrative |
4,595 | 4,595 | 2,015 | 5,265 | 3,539 | 4,504 | 10,221 | |||||||||||||||||||||
| Loss on settlement of vendor dispute |
— | — | — | — | — | — | 1,104 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total operating expenses |
8,402 | 8,402 | 7,255 | 18,509 | 13,967 | 13,486 | 26,054 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Loss from operations: |
(8,402 | ) | (8,402 | ) | (7,255 | ) | (18,509 | ) | (13,967 | ) | (13,486 | ) | (26,054 | ) | ||||||||||||||
| Other income |
102 | 102 | 26 | 1,532 | 797 | 31 | 780 | |||||||||||||||||||||
| Interest income (expense), net. |
— | (888 | ) | (473 | ) | 57 | (1,148 | ) | (999 | ) | (1,913 | ) | ||||||||||||||||
| Income tax benefit |
— | — | — | — | 575 | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss |
(8,300 | ) | (9,188 | ) | (7,702 | ) | (16,920 | ) | (13,743 | ) | (14,454 | ) | (27,187 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss allocable to preferred stockholders |
— | 8,414 | 7,425 | — | 13,144 | 13,932 | 26,205 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss allocable to common stockholders |
$ | (8,300 | ) | $ | (774 | ) | $ | (277 | ) | $ | (16,920 | ) | $ | (599 | ) | $ | (522 | ) | $ | (982 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Basic and diluted net loss per common share |
$ | $ | (0.99 | ) | $ | (0.87 | ) | $ | $ | (1.54 | ) | $ | (7.05 | ) | $ | (19.09 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Weighted average common shares outstanding, basic and diluted(1) |
784,020 | 319,423 | 388,449 | 74,063 | 51,438 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| (1) | Please see Note 2 to our audited financial statements appearing elsewhere in this prospectus for an explanation of the method used to calculate the net loss per common share and the number of common shares used in the computation of historical per share amounts. |
9
Table of Contents
| As of June 30, 2011 | ||||||||||||
| Actual | Pro Forma | Pro Forma As Adjusted(1) |
||||||||||
| (Unaudited) | ||||||||||||
| (In thousands) | ||||||||||||
| Balance Sheet Data: |
||||||||||||
| Cash and cash equivalents |
$ | 17 | $ | $ | ||||||||
| Total current assets |
1,524 | |||||||||||
| Total assets |
14,644 | |||||||||||
| Total current liabilities |
46,381 | |||||||||||
| Total liabilities |
48,779 | |||||||||||
| Total stockholders’ equity (deficit) |
(34,135 | ) | ||||||||||
| (1) | A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, total current assets, total assets and total stockholders’ equity by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
The pro forma balance sheet data as of June 30, 2011 above gives effect to (1) the filing of our amended and restated certificate of incorporation which will occur upon the closing of this offering, (2) the conversion of our convertible preferred stock outstanding as of such date into 8,528,860 shares of our common stock, which will occur automatically immediately prior to the closing of this offering, (3) the issuance of an additional $ million in aggregate principal amount of notes payable to trusts controlled by our Executive Chairman and principal stockholder subsequent to June 30, 2011 and (4) the conversion of $ million in aggregate principal amount of notes and accrued interest thereon owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming a conversion date of , 2011 and an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, immediately prior to the closing of this offering, which amount includes the $ million in aggregate principal amount of notes issued subsequent to June 30, 2011 and accrued interest on all notes payable through , 2011. The pro forma as adjusted balance sheet data as of June 30, 2011 above gives further effect to our receipt of the estimated net proceeds from the sale of shares of common stock by us in this offering at an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
10
Table of Contents
An investment in shares of our common stock involves a high degree of risk. You should carefully consider the following information about these risks, together with the other information appearing elsewhere in this prospectus, before deciding to invest in our common stock. The occurrence of any of the following risks could have a material adverse effect on our business, financial condition, results of operations and future growth prospects. In these circumstances, the market price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Financial Position
We have not had commercial sales of any of our product candidates and may never become profitable.
We have accumulated a large deficit since inception that has primarily resulted from the significant research and development expenditures we have made. We expect that our losses will continue to be substantial for at least the short term and that our operating and general and administrative expenses will be significant and increase as we transition to a public company and in connection with our planned research and development and commercialization efforts, including our anticipated creation of a commercial organization. For the six months ended June 30, 2011 and the year ended December 31, 2010, we had a consolidated net loss of $9.2 million and $13.7 million, respectively. As of June 30, 2011, we had a consolidated accumulated deficit of $94.9 million.
Our ability to become profitable depends upon our ability to generate significant continuing revenues. To generate revenues, we must succeed, either alone or with others, in developing, obtaining regulatory approval and acceptable DEA classification for, and manufacturing, selling and marketing, our product candidates, and in particular, Subsys and Dronabinol SG Capsule.
To date, our product candidates have not generated any revenues from commercial sales, and we do not know if or when we will generate any such revenue. Our ability to generate revenue depends on a number of factors, including, but not limited to:
| • | achievement of regulatory approval and acceptable DEA classification for our product candidates, and in particular for Subsys, Dronabinol SG Capsule and Dronabinol RT Capsule; |
| • | successfully manufacturing commercial quantities of our product candidates at acceptable cost levels if regulatory approvals are obtained; |
| • | successful sales, distribution and marketing of our products, if approved, including execution on our plans to build a capital-efficient commercial organization and successful partnering with third parties; |
| • | successful completion of formulation development, preclinical studies and clinical trials for our product candidates, including Dronabinol Oral Solution, Dronabinol Inhalation Device, Dronabinol IV Solution and LEP-ETU. |
Because of the numerous risks and uncertainties associated with our development efforts and other factors, we are unable to predict when we will generate revenues or become profitable, if ever. Even if we do achieve profitability, we may not be able to sustain or increase profitability on an ongoing basis.
We have significant and increasing cash burn and may require additional funding.
Our operations have consumed substantial amounts of cash since inception. Our cash flow used for operating activities for the six months ended June 30, 2011 and the year ended December 31, 2010 was $7.7 million and $15.0 million, respectively. We expect our operating and general and administrative expenses and cash used for operations to continue to be significant and increase substantially as we transition to a public company and in connection with our planned research, development and commercialization efforts, including our anticipated creation of a commercial organization. We believe
11
Table of Contents
that the net proceeds from this offering and our existing cash and cash equivalents, together with interest thereon, will be sufficient to fund our operations through at least the next 12 months. We have based these estimates, however, on assumptions that may prove to be wrong, and we could spend our available financial resources much faster than we currently expect. Further, we may need to raise additional capital following this offering to fund our operations and continue to conduct clinical trials to support potential regulatory approval of marketing applications.
The amount and timing of our future funding requirements will depend on many factors, including, but not limited to:
| • | the timing of FDA approval and DEA classification of Subsys, Dronabinol SG Capsule and our other product candidates, if at all; |
| • | the timing and amount of revenue from sales of any of our product candidates, if approved, or revenue from grants or other sources; |
| • | the rate of progress and cost of our clinical trials and other product development programs for our dronabinol product candidates, LEP-ETU product candidate and any other product candidates that we may develop, in-license or acquire; |
| • | costs of establishing or outsourcing sales, marketing and distribution capabilities; |
| • | costs and timing of completion of outsourced commercial manufacturing supply arrangements for each product candidate; |
| • | costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights associated with our product candidates; |
| • | costs of operating as a public company; |
| • | the effect of competing technological and market developments; |
| • | our ability to acquire or in-license products and product candidates, technologies or businesses; |
| • | personnel, facilities and equipment requirements; and |
| • | the terms and timing of any collaborative, licensing, co-promotion or other arrangements that we may establish. |
Raising additional funds by issuing securities or through licensing or lending arrangements may cause dilution to you, restrict our operations or require us to relinquish proprietary rights.
We may need to raise additional funds to finance future cash needs through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. We cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that we raise additional capital by issuing equity securities or convertible debt, your ownership will be diluted. Any future debt financing into which we enter may impose upon us covenants that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, redeem our stock, make certain investments and engage in certain merger, consolidation or asset sale transactions. Any borrowings under any future debt financing will need to be repaid, which creates additional financial risk for us, particularly if our business or prevailing financial market conditions are not conducive to paying-off or refinancing our outstanding debt obligations. In addition, if we raise additional funds through corporate collaboration and licensing arrangements, it may be necessary to relinquish potentially valuable rights to our product candidates, or grant licenses on terms that are not favorable to us.
If we are unable to raise additional capital when required or on acceptable terms, we may be required to significantly delay, scale back or discontinue one or more of our product development
12
Table of Contents
programs or commercialization efforts, or other aspects of our business plan. We also may be required to relinquish, license or otherwise dispose of rights to product candidates or products that we would otherwise seek to develop or commercialize ourselves on terms that are less favorable than might otherwise be available. In addition, our ability to achieve profitability or to respond to competitive pressures would be significantly limited.
Risks Related to Our Business and Industry
We are highly dependent on the success of Subsys, Dronabinol SG Capsule and our other product candidates, and we cannot give any assurance that any of these product candidates will receive regulatory approval or acceptable DEA classification, or be successfully commercialized.
We currently have no drug products for sale and, to date, we have not successfully commercialized any products. We have expended significant time, resources and effort on the development of our product candidates, and our future results of operations depend heavily on our ability to obtain regulatory approval and acceptable DEA classification, if applicable, for and successfully commercialize our product candidates. Moreover, we do not have internal new drug discovery capabilities, and our primary focus is on developing improved formulations and delivery methods for existing FDA-approved products. In the near-term, we are highly dependent on our ability to obtain regulatory approval for Dronabinol SG Capsule and Subsys.
There can be no guarantee that the FDA will accept any of our submissions and approve any of our product candidates on our anticipated timelines, or at all, including our Dronabinol SG Capsule ANDA and NDA for Subsys. As part of PDUFA, the FDA has a goal to review and act on a percentage of all submissions in a given time frame. The general review goal for a drug application is 10 months for a standard application and six months for a priority review application. The FDA’s review goals are subject to change, and it is unknown whether the review of our NDA filing for Subsys, or a filing for any of our other product candidates, will be completed within the FDA’s review goals or will be delayed. Moreover, the duration of the FDA’s review may depend on the number and types of other NDAs that are submitted to the FDA around the same time period. In addition, the FDA may identify new or additional deficiencies related to our submissions. These deficiencies could require us to take a number of actions that could have a material adverse impact on our operations and business plan, including requiring us to undertake additional time-consuming and expensive clinical trials and manufacturing and testing activities, which could cause significant delay in the review and potential approval of our product candidates, or prevent us from receiving approval at all. Moreover, with respect to Dronabinol SG Capsule, any requirement by the FDA to produce additional test batches could result in significant increased costs and also prevent or significantly delay the potential approval of Dronabinol SG Capsule. In addition, the success of Dronabinol SG Capsule is also important in terms of generating near-term cash flows to help fund the commercialization of Subsys and the development of our proprietary dronabinol and other product candidates, validate our dronabinol supply chain and internal manufacturing capabilities, and allow us to file a supplement to our ANDA for our Dronabinol RT Capsule product candidate.
If we do not obtain regulatory approval and acceptable DEA classification, if applicable, for and successfully commercialize our product candidates, and in particular for Dronabinol SG Capsule or Subsys, on our anticipated timelines or at all, we may be unable to generate sufficient revenues to sustain and grow our business, our reputation would be harmed, our competitive position would be compromised, and our business, financial condition and results of operations will be materially adversely affected. In addition, delays in obtaining regulatory approval for any of our product candidates increases the chances that our competition will produce and obtain regulatory approval for competing products before us, which would likely have a material negative impact upon our competitive position and ability to generate revenue from sales of any approved products. This in turn could have a material adverse effect on our ability to execute on our business plan, develop our other product candidates or achieve or maintain profitability.
13
Table of Contents
With respect to Dronabinol SG Capsule, prior to 2008, the FDA issued two “major deficiency” letters citing various deficiencies relating to our ANDA for our hard gelatin capsule formulation of this product candidate. In response to the FDA’s request, we made new registration batches of soft gelatin capsules and performed a new bioequivalence study. This study was completed and data from this study along with responses to other deficiencies was submitted to the FDA in the form of a major amendment in June 2010. On October 15, 2010, we received a letter from the FDA expressing the need for clarifications related to bioequivalence and we responded to this letter on November 15, 2010. In December 2010, we received a quality deficiency “minor” letter from the FDA requesting information and clarification regarding the raw material components, composition of the three proposed dosage strengths and container closure components, to which we responded in January 2011. Separately in January 2011, we received another deficiency letter from the FDA related to labeling comments to which we also responded in January 2011. On February 10, 2011, we submitted an electronic Final Product Label in response to a request from the FDA. On March 8, 2011, we submitted a labeling amendment in response to an additional request from the FDA. The labeling comments and requests from the FDA related to revisions to our proposed labeling components for consistency with Marinol labeling and certain formatting changes. On July 7, 2011, the FDA notified us of three “telephone” deficiencies to our ANDA for Dronabinol SG Capsule. The “telephone” deficiencies we received related to the request for revised shell formulation information, a blank batch record and a filing by the holder of the Subsys drug master file. The shell formulation information had previously been submitted to the FDA by our third party manufacturer and the holder of the Subsys drug master file had previously submitted the requested filing. On July 11, 2011, we notified the FDA of the prior submissions, and we responded to the FDA’s request for the blank batch record. We believe our response has adequately addressed all the deficiencies. There can be no assurance, however, that the FDA will approve the Dronabinol SG Capsule ANDA based on our response, and the FDA may identify additional deficiencies related to our ANDA. As a general matter, amendments to ANDAs submitted in response to major deficiency letters or amendment requests are given the same review priority as original, non-reviewed ANDAs by the Office of Generic Drugs, or OGD. They are generally placed into the 180-day queue and reviewed in accordance with OGD’s first in-first reviewed procedures. In contrast, ANDA amendments submitted in response to minor FDA deficiency letters or amendment requests are generally given a higher priority review than major amendments because they often mean an ANDA is close to approval and should, therefore, be given priority. The FDA generally reviews minor amendments within 30 to 60 days. As a general matter, “telephone” deficiencies primarily relate to administrative or minor technical issues, and the FDA endeavors to review responses to “telephone” deficiencies upon receipt. Any failure to adequately respond to the FDA’s requests and deficiency notifications could delay approval of Dronabinol SG Capsule and have a material adverse effect on our business plan.
We face significant competition from both branded and generic products, and our operating results will suffer if we fail to compete effectively.
Our industry is characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. We face competition from many different sources, including pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, drug delivery companies, academic institutions, government agencies and private and public research institutions, many of which have significantly greater financial, technical and other resources than us.
If Subsys receives regulatory approval, it will compete against numerous branded and generic products already being marketed and potentially those which are or will be in development. In the BTCP market, physicians often treat BTCP with a variety of short-acting opioid medications, including morphine, morphine and codeine derivatives and fentanyl. Some currently marketed products against which we will likely directly compete include Cephalon, Inc.’s Fentora and Actiq, BioDelivery Sciences International’s Onsolis, Nycomed International Management GmbH’s Instanyl and ProStrakan Group plc’s Abstral. Some generic fentanyl products against which we will compete are marketed by TEVA Pharmaceuticals USA and Watson Pharmaceuticals, Inc. In addition, we are aware of numerous companies developing other treatments and technologies for rapid delivery of opioids to treat BTCP,
14
Table of Contents
including transmucosal, transdermal, nasal spray, inhaled delivery systems and sublingual delivery systems, among others. For example, Archimedes Pharma Ltd.’s Lazanda, a fentanyl nasal spray solution which has been approved in Europe and in the United States. Additionally, we are aware of companies with product candidates in late stage development for BTCP, including AcelRx Pharmaceuticals, Inc.’s ARX-02 and Akela Pharma Inc.’s Fentanyl TAIFUN, both of which are Phase 3 ready. If these treatments and technologies are successfully developed and approved, they could represent significant additional competition to Subsys. We also expect that sales and marketing efforts for other fentanyl products will increase if and when a classwide REMS program is approved by the FDA, which could make it more difficult for us to compete with a smaller and lower cost sales force.
With respect to our dronabinol product candidates, and in particular our generic Dronabinol SG Capsule, the market in which we will compete is challenging in part because generic products generally do not benefit from patent protection. If any of our dronabinol product candidates, and in particular Dronabinol SG Capsule, receive the requisite regulatory approval and acceptable DEA classification and are marketed, the competition from generic products which we will encounter may have an effect on our product prices, market share, revenues and profitability. We or our distributor may not be able to differentiate any products that we may market from those of our competitors, successfully develop or introduce new products that are less costly or offer better performance than those of our competitors, or offer purchasers of our products payment and other commercial terms as favorable as those offered by our competitors. In addition, there are a number of established therapies and products already commercially available and under development by other companies that treat the indications for which we are developing dronabinol products and with which our product candidates will compete if approved. If we receive regulatory approval and acceptable DEA classification for our dronabinol product candidates, these product candidates will compete against therapies and products such as Abbott Laboratories’ Marinol, Marinol generics and Valeant Pharmaceutical International Inc.’s Cesamet. Moreover, Par Pharmaceutical Companies Inc. markets an approved generic version of Marinol and we believe that other companies are pursuing regulatory approval for generic dronabinol products. We cannot give any assurance that any such other companies will not obtain regulatory approval or acceptable DEA classification for, or commercialize their generic dronabinol products on a more rapid timeline or more successfully than us.
Moreover, our products will compete with non-synthetic cannabinoid drugs, including therapies such as GW Pharmaceuticals plc’s Sativex, especially in many countries outside of the United States where non-synthetic cannabinoids are legal and in the United States if non-synthetic cannabinoids are legalized. The DEA’s proposed rule issued on November 1, 2010, if finalized, would classify naturally-derived dronabinol derived from plant material as a Schedule III controlled substance if part of an approved ANDA. In addition, literature has been published arguing the benefits of natural cannabis, or marijuana, over dronabinol, and there are a number of states that have already enacted laws legalizing medicinal marijuana. Irrespective of its potential medical applications, there is some support in the United States for legalization of marijuana. We also cannot assess the extent to which patients utilize marijuana illegally to alleviate CINV, instead of using prescribed therapies such as approved dronabinol products. Furthermore, in the treatment of CINV, physicians typically offer conventional anti-nausea drugs prior to initiating chemotherapy, such as sanofi-aventis’ Anzemet, Eisai Inc./Helsinn Group’s Aloxi, Roche Holding AG’s Kytril, MonoSol Rx’s Zuplenz and GlaxoSmithKline plc’s Zofran and its generic equivalents, as well as Neurokinin 1 receptor antagonists on the market including ProStrakan’s SANCUSO and Merck & Co., Inc.’s Emend. To the extent that our proprietary dronabinol products compete in a broader segment of the CINV market, we will also face competition from these products.
Additionally, we are aware of companies with product candidates in late stage development for CINV, including A.P. Pharma’s APF530, which has received a Complete Response Letter from the FDA, Aphios Corp.’s Zindol, which is in Phase 2/3 development, Roche Holding/Helsinn Group’s netupitant, which is in Phase 3 development, and Tesaro’s Rolapitant, which is in Phase 2 development. If these products are successfully developed and approved over the next few years, they could represent significant competition for our dronabinol family of product candidates, if any are approved.
15
Table of Contents
Our LEP-ETU product candidate, if approved for the treatment of metastatic breast cancer or other cancer indications, will compete with the leading taxanes currently on the market, including those with formulations that specifically incorporate paclitaxel as the active ingredient such as Bristol-Myers Squibb’s Taxol and its generic equivalents and Celgene Corporation’s Abraxane, as well as other taxanes, such as sanofi-aventis’ Taxotere. Furthermore, LEP-ETU could face future competition, if approved, from Cornerstone Pharmaceuticals’ new formulation of paclitaxel known as EmPac, which is undergoing preclinical studies. In addition, LEP-ETU would compete with other cytotoxic agents beyond the taxane class, including capecitabine, gemcitabine, ixabepilone and navelbine. Additionally, there are numerous biotechnology and pharmaceutical companies that currently have extensive development efforts and resources within oncology. Abbott Laboratories, Amgen Inc., AstraZeneca PLC., Bayer AG, Biogen Idec Inc., Eisai Co., Ltd., F. Hoffmann- LaRoche Ltd., Johnson and Johnson, Merck and Co., Inc., Novartis AG, Onyx Pharmaceuticals Inc., Pfizer Inc., sanofi-aventis and Takeda Pharmaceutical Co. Ltd., are among some of the leading companies researching and developing new compounds in oncology.
We will also face competition from third parties in obtaining allotments of fentanyl and dronabinol under applicable DEA annual quotas, recruiting and retaining qualified personnel, establishing clinical trial sites and enrolling patients in clinical trials, and in identifying and acquiring or in-licensing new products and product candidates.
New developments, including the development of other drug technologies and delivery methods, occur in the pharmaceutical and life sciences industries at a rapid pace. Compared to us, many of our potential competitors have substantially greater:
| • | research and development resources, including personnel and technology; |
| • | regulatory experience; |
| • | drug development, clinical trial and drug marketing and commercialization experience; |
| • | experience and expertise in intellectual property rights; |
| • | name recognition; and |
| • | capital resources. |
As a result of these and other factors, our competitors may obtain FDA approval of their products more rapidly than us or may obtain patent protection or other intellectual property rights that limit our ability to develop or commercialize our product candidates. Our competitors may also develop products that are more effective, better tolerated, subject to fewer or less severe side effects, more useful, more widely-prescribed or accepted, or less costly than ours. If we receive regulatory approvals for our products, sales and marketing efficiency are likely to be significant competitive factors. Our plan is to build a commercial organization without using third-party sales or marketing channels in the United States for most of our proprietary product candidates if approved, and there can be no assurance that we can develop these capabilities in a manner that will be capital efficient and competitive with the sales and marketing efforts of our competitors, especially since some or all of those competitors could expend greater economic resources than we do and/or employ third-party sales and marketing channels.
We are subject to numerous complex regulations and failure to comply with these regulations, or the cost of compliance with these regulations, may harm our business.
The research, testing, development, manufacturing, quality control, approval, labeling, packaging, storage, recordkeeping, promotion, advertising, marketing, distribution, possession and use of our product candidates, among other things, are subject to regulation by numerous governmental authorities in the United States and elsewhere. The FDA regulates drugs under the Federal Food, Drug and Cosmetic Act, or FDC Act, and implementing regulations. Noncompliance with any applicable regulatory requirements can result in refusal to approve products for marketing, warning letters, product recalls or seizure of products, total or partial suspension of production, prohibitions or
16
Table of Contents
limitations on the commercial sale of products or refusal to allow the entering into of federal and state supply contracts, fines, civil penalties and/or criminal prosecution. Additionally, the FDA and comparable governmental authorities have the authority to withdraw product approvals that have been previously granted. Moreover, the regulatory requirements relating to our products may change from time to time and it is impossible to predict what the impact of any such changes may be.
We are developing product candidates that are controlled substances as defined in the Controlled Substances Act of 1970, or CSA, which establishes, among other things, certain registration, production quotas, security, recordkeeping, reporting, import, export and other requirements administered by the DEA. The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have high potential for abuse, no currently accepted medical use in the United States and lack accepted safety for use under medical supervision, and may not be marketed or sold in the United States. Except for research and industrial purposes, a pharmaceutical product may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest risk of abuse and Schedule V substances the lowest relative risk of abuse among such substances. Fentanyl is listed by the DEA as a Schedule II substance under the CSA. Dronabinol in sesame oil and encapsulated in a soft gelatin capsule in the form previously approved by the FDA for the commercial sale of Marinol is currently listed by the DEA as a Schedule III substance under the CSA. Dronabinol in bulk or other product forms is currently classified by the DEA as a Schedule I substance under the CSA. If the FDA approves formulations of dronabinol which differ from Marinol, the DEA will have to make a scheduling determination and place the products in a schedule other than Schedule I in order for such products to be marketed to patients in the United States.
The manufacture, shipment, storage, sale and use, among other things, of controlled substances that are pharmaceutical products are subject to a high degree of regulation. For example, generally all Schedule II substance prescriptions, such as prescriptions for fentanyl, must be written and signed by a physician, physically presented to a pharmacist and may not be refilled without a new prescription.
The DEA also conducts periodic inspections of certain registered establishments that handle controlled substances. Facilities that conduct research, manufacture, distribute, import or export controlled substances must be registered to perform these activities and have the security, control and inventory mechanisms required by the DEA to prevent drug loss and diversion. Failure to maintain compliance, particularly non-compliance resulting in loss or diversion, can result in regulatory action that could have a material adverse effect on our business, results of operations, financial condition and prospects. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to restrict, suspend or revoke those registrations. In certain circumstances, violations could lead to criminal proceedings.
Individual states also have controlled substances laws. Though state controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule our product candidates as well. While some states automatically schedule a drug when the DEA does so, other states schedule drugs through rulemaking or a legislative action. State scheduling may delay commercial sale of any product for which we obtain federal regulatory approval and adverse scheduling could have a material adverse effect on the commercial attractiveness of such product. We or our partners must also obtain separate state registrations, permits or licenses in order to be able to obtain, handle, and distribute controlled substances for clinical trials or commercial sale, and failure to meet applicable regulatory requirements could lead to enforcement and sanctions by the states in addition to those from the DEA or otherwise arising under federal law.
The anticipated development of a REMS program for Subsys could cause significant delays in the approval process and would add additional layers of regulatory requirements that could significantly impact our ability to commercialize Subsys and dramatically reduce its market potential.
Section 505-1 of the FDC Act permits the FDA to require sponsors to submit a proposed REMS program to ensure the safe use of the drugs in question following commercial approval. A REMS program
17
Table of Contents
is a strategic safety program that the FDA requires to ensure that the benefits of a drug continue to outweigh its risks. In determining whether a REMS program is necessary, the FDA must consider the size of the population likely to use the drug, the seriousness of the disease or condition to be treated, the expected benefit of the drug, the duration of treatment, the seriousness of known or potential adverse events, and whether the drug is a new molecular entity. A REMS program may be required to include various elements, such as a medication guide, patient package insert, a communication plan, elements to assure safe use, and an implementation system, and must include a timetable for assessment of the REMS program. Elements to assure safe use can restrict the prescribing, sale and distribution of drug products.
In September 2007 and in December 2007, the FDA issued a safety alert to healthcare professionals and consumers concerning recent reports of deaths and other adverse events in patients using approved TIRF products. The FDA has determined that TIRF products will be required to have a REMS program to ensure that the benefits of the drugs continue to outweigh the serious risks of overdose, abuse, misuse, addiction and serious complications due to medication errors. A classwide REMS program is being developed jointly by all manufacturers and IND application holders of TIRF products, and we participate actively in this program. This REMS program is expected to be a single shared program across the TIRF class of products. We expect that the FDA will approve a classwide REMS program in the second half of 2011. In addition, we continue to pursue a REMS program specific to our company as a potential alternative to the classwide REMS program.
There can be no assurance that the FDA will approve the classwide REMS program or our alternative individual REMS program that we submitted as part of our NDA submission on our anticipated timeline, or at all. Delays in the REMS program approval process could result in significant delays in the approval process for Subsys. In addition, as part of the REMS program relating to Subsys, the FDA could require significant restrictions, such as restrictions on the prescribing, distribution and patient use of the product, which could significantly impact our ability to effectively commercialize Subsys and dramatically reduce its market potential.
We have recently taken a number of significant actions aimed at growing our business and will need to further increase the size and complexity of our organization in the future, and we may experience difficulties in managing our growth and executing our growth strategy.
Our management and personnel, systems and facilities currently in place may not be adequate to support our business plan and future growth. In November 2010, we completed the Merger, which resulted in Insys Pharma becoming our wholly-owned subsidiary. Prior to the Merger, Insys Pharma in September 2009 obtained assets which have given us the ability to manufacture our supply of dronabinol API internally through our manufacturing facility in Texas. Both of these transactions have significantly increased the complexity of our business operations. We recently expanded our management team and board of directors. In addition, we grew the number of our full-time employees from 12 as of December 31, 2009 to 28 as of July 15, 2011, due in large part to the Merger and subsequent organic growth. We will need to further expand our managerial, operational, financial and other resources, and build a sales force and marketing infrastructure, in order to manage and fund our future operations and clinical trials, continue our research and development activities, and commercialize our product candidates, if approved.
Our need to effectively manage our operations, growth and various projects requires that we:
| • | continue to improve our operational, financial and management controls and reporting systems and procedures; |
| • | attract and retain sufficient numbers of talented employees; |
| • | manage our clinical trials effectively; |
| • | manage our internal dronabinol production operations effectively and in a cost effective manner; |
18
Table of Contents
| • | manage our development efforts effectively while carrying out our contractual obligations to licensors, contractors and other third parties; and |
| • | continue to improve our facilities. |
In addition, historically, we have utilized and continue to utilize the services of part-time outside consultants to perform a number of tasks for us, including tasks related to accounting and finance, clinical trial management, regulatory affairs, formulation development and other drug development functions. For example, in addition to seeking advice from our scientific advisory board, we utilize consultants for tasks such as state licensing procurement and accounting and book-keeping services. Our growth strategy may also entail expanding our use of consultants to implement these and other tasks going forward. Because we rely on consultants for certain functions of our business, we will need to be able to effectively manage these consultants to ensure that they successfully carry out their contractual obligations and meet expected deadlines. There can be no assurance that we will be able to manage our existing consultants or find other competent outside consultants, as needed, on economically reasonable terms, or at all. If we are not able to effectively expand our organization by hiring new employees and expanding our use of consultants, we may be unable to successfully implement the tasks necessary to further develop and commercialize our product candidates and, accordingly, may not achieve our research, development and commercialization goals.
If we are unable to establish sales and marketing capabilities or execute on our sales and marketing strategy, we may not be able to effectively market and sell any approved products and generate product revenue.
We do not currently have an organization for the sales, marketing and distribution of pharmaceutical products, and we must build this organization, make arrangements with third parties or rely on current distribution partners to perform these functions in order to commercialize any products we successfully develop and for which we obtain regulatory approvals and acceptable DEA classifications, if applicable.
If Dronabinol SG Capsule or Dronabinol RT Capsule is approved, we intend to distribute these products through Mylan pursuant to our May 2011 supply and distribution agreement. In the event that Mylan fails to adequately commercialize these product candidates, if approved or because it lacks adequate financial or other resources, decides to focus on other initiatives or otherwise, our business, financial condition, results of operations and prospects would be harmed. In addition, our agreement with Mylan may be terminated early by either party under certain circumstances. If Mylan terminated its agreement with us, we may not be able to secure an alternative distributor on a timely basis or at all, in which case we may be required to commercialize Dronabinol SG Capsule or Dronabinol RT Capsule on our own which would be costly and require significant time and resources. In such an event, our ability to generate revenues from the sale of Dronabinol SG Capsule or Dronabinol RT Capsule, if approved, would be materially harmed.
If Subsys or our other proprietary dronabinol product candidates are approved, we expect to utilize an incentive-based sales model to market those products similar to that employed at Sciele Pharma and other companies previously led by members of our board, including our founder and Executive Chairman. Under this model, we expect to maintain a smaller and lower cost commercial organization than many of our competitors, which could hinder our efforts to broadly market any products that we are able to commercialize as compared to our competitors. The establishment of a commercial organization may be costly and time consuming and could delay any product launch. We cannot be certain that we will be able to successfully develop these capabilities on the economic terms we currently anticipate, if at all. If we are unable to establish our sales and marketing capabilities or any other capabilities as currently anticipated, we will need to consider alternatives, such as entering into arrangements with third parties to market and sell such products. Such an arrangement would likely result in significantly greater sales and marketing expenses or lower revenues than currently estimated in our business plan.
19
Table of Contents
Moreover, although we expect to rely on a sales model similar to that employed at companies previously led by members of our board of directors, we cannot assure you that we will be able to effectively implement such a model to market our products.
To the extent we receive the requisite approvals to market our product candidates internationally, we plan to enter into arrangements with third parties to market and sell our products as opposed to building an international commercial organization. We currently possess limited sales and marketing resources and may not be successful in establishing our own commercial organization for the United States or in establishing arrangements with third parties for international sales on acceptable terms, if at all.
We produce our dronabinol API internally and may encounter manufacturing failures that could delay the preclinical and clinical development or regulatory approval of our dronabinol product candidates, or their commercial production if approved.
Any performance failure on the part of our internal dronabinol API manufacturing operations could delay the preclinical and clinical development or regulatory approval of our dronabinol product candidates, and harm our reputation. Our internal manufacturing operations may encounter difficulties involving, among other things, production yields, regulatory compliance, quality control and quality assurance, obtaining DEA quotas which allow us to produce dronabinol in the quantities needed to execute on our business plan, as well as shortages of qualified personnel. Approval of our dronabinol product candidates could be delayed, limited or denied if the FDA does not approve and maintain the approval of our manufacturing processes and facilities. In addition, we may encounter difficulties with the manufacturing processes required to manufacture commercial quantities of dronabinol or the quantities needed for our preclinical studies or clinical trials. We are especially prone to such difficulties because we have no experience producing dronabinol in commercial quantities. Such difficulties could result in delays in our preclinical studies, clinical trials and regulatory submissions, in the commercialization of our product candidates if approved, or, in the recall or withdrawal of approved products from the market. If we fail to produce the required commercial quantities or quantities needed for our preclinical studies and clinical trials on a timely basis and upon terms that we find acceptable, we may be unable to meet demand for any of our product candidates that may receive approval, and could lose potential revenue.
We are only aware of two other manufacturers that are able to produce dronabinol in the United States. We are aware of only five manufacturers that hold Drug Master Files for the production of dronabinol in the United States. Because dronabinol is a controlled substance, inability to manufacture dronabinol in the United States would have a material adverse effect on our business given the regulatory restrictions associated with obtaining authorization to import and transport controlled substances into the United States. Moreover, we believe dronabinol is difficult to produce and if there was any problem in manufacturing it internally, we may not be able to identify a third party to manufacture it for us in a cost effective manner, if at all.
We must comply with current good manufacturing practices, or cGMP, enforced by the FDA through its facilities inspection program and review of submitted technical information. In addition, we must obtain and maintain necessary DEA and state registrations, and must establish and maintain processes to assure compliance with DEA and state requirements governing, among other things, the storage, handling, security, recordkeeping and reporting for controlled substances. We must also apply for and receive a quota for these products. Any failure to comply with these requirements may result in penalties, including fines and civil penalties, suspension of production, suspension or delay in product approvals, product seizure or recall, operating restrictions, criminal prosecutions or withdrawal of product approvals, any of which could significantly and adversely affect our business. If the safety of any drug product or component is compromised due to a failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize the affected product candidate, and we may be held liable for any injuries sustained as a result. Any of these factors could cause a delay or termination of preclinical studies and clinical trials, regulatory
20
Table of Contents
submissions, approvals or commercialization of our product candidates if approved, entail higher costs or result in our being unable to effectively commercialize our approved products. Certain changes in our dronabinol API manufacturing processes or procedures, including a change in the location where the material is manufactured, generally require prior FDA, or foreign regulatory authority, review and/or approval. We may need to conduct additional preclinical studies and clinical trials to support approval of such changes. This review and approval process may be costly and time-consuming, and could delay or prevent the launch of a product candidate.
We have no internal manufacturing capabilities other than for our dronabinol API, and if we fail to develop and maintain supply and manufacturing relationships with various third parties, we may be unable to develop or commercialize many of our product candidates.
We rely on a number of third parties for the development of our product candidates and their commercialization, if approved. Our ability to develop and commercialize many of our product candidates depends, in part, on our ability to successfully obtain the API (or starting materials for the API, as the case may be) and outsource most if not all of the aspects of their manufacturing at competitive costs, in accordance with regulatory requirements and in sufficient quantities for clinical testing and eventual commercialization. If we fail to develop and maintain supply relationships with these third parties, we may be unable to develop or commercialize our product candidates.
Manufacturers and suppliers are subject to regulatory requirements covering, among other things, manufacturing, testing, quality control and recordkeeping relating to our product candidates, and are subject to ongoing inspections by FDA, DEA and other regulatory agencies. We purchase the fentanyl API utilized in connection with Subsys and the starting materials for our dronabinol API from several third parties. We do not have long-term agreements with any of these parties, but rather purchase material on a purchase order basis. Moreover, some of the starting material for our dronabinol API is difficult to procure and produce. Our ability to obtain fentanyl API and the starting materials for our dronabinol API in sufficient quantities and quality, and on a timely basis, is critical to the successful completion of our related preclinical studies and clinical trials and the timeliness of their commercial sale. There is no assurance that these suppliers will continue to produce the materials in the quantities and quality and at the times they are needed, if at all, especially in light of the fact that we intend to significantly increase our orders for these materials in the near future, if approved. Moreover, the replacement of any of these suppliers, particularly the supplier of the starting material for our dronabinol API that is difficult to produce, could lead to significant delays and increase in our costs.
Our Dronabinol SG Capsule is manufactured and packaged by Catalent Pharma Solutions, and we have contracted with Mylan for the distribution of our Dronabinol SG Capsule. Our Subsys sub-component manufacturing is performed by AptarGroup, Inc., with the final fill, assembly and packaging of Subsys performed by DPT Lakewood, LLC, or DPT. We have contracts in place with Catalent Pharma Solutions, Mylan, AptarGroup and DPT. If there are problems relating to the equipment utilized to manufacture Subsys, we will be responsible for fixing or replacing that equipment. Any requirement to do so could result in unexpected costs and expenses and delay the production of this product candidate which could in turn negatively impact our business and development efforts.
The manufacture of pharmaceutical products generally requires significant expertise and capital investment, often including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products often encounter difficulties in production, particularly in scaling up and validating initial production. These problems can include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. We cannot assure you that any such issues relating to the manufacture of any of our products will not occur in the future. Additionally, our manufacturers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. If our manufacturers were to encounter any of these difficulties, or otherwise fail to comply with their
21
Table of Contents
contractual obligations, our ability to commercially launch any approved product candidates or provide any product candidates for preclinical studies or clinical trials could be jeopardized. Any delay or interruption in our ability to commercialize approved product candidates will result in the loss of potential revenues and could adversely affect our ability to gain market acceptance for these products. In addition, any delay or interruption in the supply of preclinical study or clinical trial supplies could delay the completion of those studies or trials, increase the costs associated with maintaining our programs and, depending upon the period of delay, require us to commence new studies or trials at additional expense or terminate studies or trials completely.
Moreover, the facilities used by our third-party manufacturers must be approved by the applicable regulatory authorities. We do not control the manufacturing processes of third-party manufacturers and are currently completely dependent on them. If any of our third-party manufacturers cannot successfully manufacture product that conforms to our specifications and the applicable regulatory authorities’ strict regulatory requirements, they will not be able to secure or maintain regulatory approval for the manufacturing facilities. In addition, we have no control over the ability of third-party manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or any other applicable regulatory authorities do not approve these facilities for the manufacture of our product candidates or if they withdraw any such approval in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market any approved product candidates.
Failures by the third-party suppliers or manufacturers could materially adversely affect our business and delay or impede the development and commercialization of our product candidates, and could have a material adverse effect on our business, results of operations, financial condition and prospects. And in the event we need to replace a current supplier or manufacturer, we cannot assure you that we would be able to do so on a timely basis or in a cost effective manner, or at all.
If we fail to attract and keep management and other key personnel, as well as our board members, we may be unable to successfully develop or commercialize our product candidates and implement our business plan.
Our ability to compete in the highly competitive biotechnology and pharmaceuticals industries depends upon our ability to attract and retain highly qualified managerial, scientific, medical and other personnel. We are highly dependent on our management, scientific and medical personnel, as well as our board members, including our founder and Executive Chairman, Dr. John N. Kapoor, our President and Chief Executive Officer, Michael L. Babich, our Director of Scientific Development, Dr. Daniel D. Von Hoff, and our Chief Medical Officer, Dr. Larry Dillaha. The loss of the services of any of these individuals could delay or prevent the development and commercialization of our product candidates and negatively impact our ability to successfully implement our business plan. If we lose the services of any of these individuals, we may not be able to find suitable replacements on a timely basis or at all, and our business would likely be harmed as a result. We do not maintain “key man” insurance policies on the lives of these individuals or the lives of any of our other employees. We employ all of our executive officers and key personnel on an at-will basis and their employment can be terminated by us or them at any time, for any reason and without notice. In order to retain valuable employees at our company, in addition to salary and cash incentives, we provide incentive stock options that vest over time. The value to employees of stock options that vest over time will be significantly affected by movements in our stock price that are beyond our control, and may at any time be insufficient to counteract offers from other companies.
We may not be able to attract or retain qualified management and other key personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses, particularly in the Phoenix, Arizona area where we are headquartered and nearby geographic locales such as Southern California. Our industry has experienced a high rate of turnover of management personnel in recent years. As such, we could have difficulty attracting experienced
22
Table of Contents
personnel to our company and may be required to expend significant financial resources in our employee recruitment and retention efforts. Many of the other biotechnology and pharmaceutical companies with whom we compete for qualified personnel have greater financial and other resources, different risk profiles and longer histories in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high quality candidates than that which we have to offer. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that will impede significantly the achievement of our business objectives, our ability to raise additional capital and our ability to implement our business strategy.
In addition, we have scientific and clinical advisors who assist us in formulating our development and clinical strategies. These advisors are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. In addition, our advisors may have arrangements with other companies to assist those companies in developing products or technologies that may compete with ours.
Our short operating history as a combined company and the recent additions to our management team and board of directors make it difficult to evaluate our business and prospects and may increase the risk of your investment.
In November 2010, we completed the Merger, which resulted in Insys Pharma becoming our wholly-owned subsidiary. We therefore have a short operating history as a combined company. Moreover, our business has had limited operations in the several years immediately prior to the Merger. Our Chief Executive Officer joined Insys Pharma in 2007 and was appointed as our Chief Executive Officer in March 2011. Our Chief Financial Officer was promoted to that position from Corporate Controller in March 2011. Our Chief Medical Officer joined Insys Pharma in April 2010. Our Director of Scientific Development started working with us in December 2010. Moreover, several members of our board of directors joined us in March 2011. In addition, we have not yet demonstrated an ability to obtain regulatory approval for or commercialize any product candidate. Consequently, any predictions about our future performance may not be as accurate as they could be if we had a history of successful development and commercialization of pharmaceutical products or if our management team and board or directors had been with us and working together for a longer period of time.
Failure to obtain or maintain Schedule III classification for any of our dronabinol product candidates would substantially limit our ability to produce and commercialize any such product candidates.
The DEA generally regulates dronabinol as a Schedule I controlled substance, except in the case of the FDA-approved Marinol product and its generics, which are Schedule III controlled substances. Schedule I controlled substances have high potential for abuse, no currently accepted medical use in the United States and lack accepted safety for use under medical supervision and may not lawfully be commercially sold or marketed to patients. After the initial FDA approval of Marinol in 1985, the DEA scheduled dronabinol in sesame oil and encapsulated in a soft gelatin capsule as a Schedule II substance. In 1999, the DEA promulgated a regulation that reclassified this formulation as a Schedule III controlled substance. This regulation directly corresponds to the product characteristics of Marinol, whose sponsor had petitioned the DEA for the scheduling change. DEA regulations currently limit the formulation of FDA-approved dronabinol products that are classified in Schedule III. Specifically, classification in Schedule III is limited to “dronabinol (synthetic) in sesame oil and encapsulated in a soft gelatin capsule in” an FDA-approved product. There is a possibility that some generic versions of Marinol would not meet these specific conditions, and therefore, would not be classified as a Schedule III substance, but rather would be considered as Schedule I products until otherwise scheduled for marketing. Currently, several products are the subject of ANDAs under review by the FDA, including our application for Dronabinol SG Capsule. On November 1, 2010, the DEA issued a Notice of Proposed Rulemaking concerning the listing of approved drug products containing dronabinol in Schedule III. The DEA proposed rulemaking would
23
Table of Contents
amend the scheduling regulations to expand the Schedule III listing of dronabinol to include formulations having naturally-derived dronabinol and in hard gelatin capsules. If this ruling is allowed, it may increase the number of generics approved as we believe there are active ANDAs which utilize naturally-derived dronabinol and hard gelatin capsule technology. The DEA may ultimately schedule Dronabinol SG Capsule and Dronabinol RT Capsule, if approved, under a schedule other than Schedule III. These product candidates are also subject to regulation by state-controlled substance authorities. We cannot assure you that Dronabinol SG Capsule and Dronabinol RT Capsule, if approved, will be classified as Schedule III substances, in the timeline that we currently anticipate, or at all.
In addition, because the DEA currently regulates the scheduling of dronabinol on a product-specific basis as opposed to regulating all dronabinol-containing products under one schedule, we believe that the DEA will also need to make individual scheduling decisions with respect to our proprietary dronabinol product candidates if approved, based on, among other factors, assessments of the drug abuse potential for each of our formulations. Therefore, even if the DEA agrees to classify Dronabinol SG Capsule under Schedule III, because our other proprietary dronabinol product candidates will, if approved, represent novel dosage forms, and in the case of the Dronabinol Inhalation Device, a novel route of administration for dronabinol, the DEA may determine that stricter scheduling controls than those applicable to Schedule III controlled substances are appropriate for the additional product candidates. In fact, these product candidates will likely default to Schedule I until the DEA completes a scheduling action for them. Moreover, there may be significant delay in the issuance of DEA’s scheduling decisions with respect to our products following FDA approval, if such approval is granted. Even with FDA approval, we will not be able to market any of our controlled substance products until the DEA has issued a scheduling decision with respect to each drug product.
Because the restrictions on the manufacture, sale, distribution, prescribing, and dispensing of Schedule II substances are greater than for Schedule III substances, failure to obtain Schedule III classification for our dronabinol product candidates could significantly impact our anticipated ability to produce and commercialize any such dronabinol products and would have a material adverse effect on our business and ability to generate revenue. For example, Schedule II drugs or substances generally may not be dispensed without the written prescription of a practitioner, and prescriptions for these drugs or substances may not be refilled. Although the DEA regulates the frequency of Schedule III prescription refills, physicians may call in the prescriptions and they may be refilled. A failure by the DEA to respond favorably to our classification petition before, or in a timely manner after, FDA approval of our dronabinol product candidates, and in particular our Dronabinol SG Capsule product candidate, or a refusal by the DEA to grant our request to schedule our dronabinol product candidates under Schedule III, if approved by the FDA, would have an adverse impact on our ability to promptly or effectively commercialize such products.
Even if we obtain regulatory approvals and acceptable DEA classifications for our fentanyl and dronabinol product candidates or any other product candidate, if those products do not achieve broad market acceptance among physicians, patients, hospitals, healthcare payors and the medical community, we will likely generate limited revenues from sales of those products.
Even if our product candidates receive regulatory approval and acceptable DEA classification, if applicable, they may not gain market acceptance among physicians, patients, healthcare payors, the medical community and other third parties. Coverage and reimbursement of our product candidates by third-party payors, including government payors, generally is also necessary for commercial success. The degree of market acceptance of any of our approved products will depend on a number of factors, including:
| • | the clinical indications for which the product is approved; |
| • | our ability to provide acceptable evidence of safety and efficacy, and acceptance by physicians and patients of the product as a safe and effective treatment; |
24
Table of Contents
| • | the relative convenience and ease of administration, and with respect to Dronabinol RT Capsule, our expectation that this product will be preferred over cool or refrigerated storage versions of dronabinol; |
| • | pricing and cost effectiveness; |
| • | a final REMS program applicable to Subsys; |
| • | the prevalence and severity of any adverse side effects; |
| • | warnings or limitations contained in a product’s FDA-approved labeling; |
| • | the DEA and state scheduling classification; |
| • | our ability to maintain compliance with regulatory requirements; |
| • | the availability of alternative treatments, and the perceived advantages of one product over alternative treatments; |
| • | the effectiveness of our sales, marketing and distribution strategies, particularly the targeted commercial organization we anticipate building and the efforts of our distribution partners, including Mylan; and |
| • | our ability to obtain sufficient third-party coverage or reimbursement. |
Our ability to successfully sell generic products in particular, such as Dronabinol SG Capsule if approved, depends in large part on the acceptance of those products by third parties such as wholesalers, pharmacies, physicians and patients. Although the brand-name products generally have been marketed safely for many years prior to the introduction of a generic alternative, there is a possibility that one of our generic products could produce an unanticipated clinical side effect, or be considered less effective or less convenient, or otherwise inferior, to the branded product, which could result in an adverse effect on our ability to achieve acceptance by third parties.
In addition, fentanyl and dronabinol treatments can be costly to third-party payors and patients. Accordingly, hospitals and physicians may resist prescribing our products and third-party payors and patients may not purchase our products due to cost. If any of our product candidates are approved and receive acceptable DEA classifications but do not achieve an adequate level of acceptance by physicians, patients, hospitals, healthcare payors and the medical community, we may not generate sufficient revenue from these products and we may not become or remain profitable.
Furthermore, the potential market for dronabinol products may not expand as anticipated or may even decline based on numerous factors, including the introduction of superior alternative products and regulatory action negatively impacting the dronabinol market. Moreover, even if we obtain regulatory approval for our dronabinol product candidates and they are successfully commercialized, there is no guarantee that introduction of improved formulations of dronabinol will result in expansion of the dronabinol market or permit us to gain share in that market or maintain or increase any market share we may capture. New dronabinol products that we introduce could potentially replace our then currently marketed dronabinol products, thus not impacting the overall size of the market or increasing our overall share of that market. If we are unable to expand the market for the medical use of dronabinol or gain, maintain or increase market share in that market, this failure would have a material adverse effect on our ability to execute on our business plan and ability to generate revenue.
Even if our Dronabinol SG Capsule is approved by the FDA and classified as a Schedule III substance by the DEA, the FDA may not conclude that our planned approach for regulatory approval for Dronabinol RT Capsule is appropriate and may require us to provide additional data prior to approval.
If our Dronabinol SG Capsule is approved by the FDA and classified as a Schedule III substance by the DEA, we intend to submit an sANDA for a dronabinol soft gel formulation that is stable at room
25
Table of Contents
temperature, or Dronabinol RT Capsule. If the FDA does not allow us to pursue the sANDA pathway, we may be required to conduct additional clinical trials, provide additional data and information, and meet additional standards for regulatory approval. If this were to occur, the time and financial resources required to obtain FDA approval for Dronabinol RT Capsule, and the complications and risks associated with this product candidate, may increase. Moreover, the inability to obtain regulatory approval of Dronabinol RT Capsule through an sANDA would likely delay our ability to market this product on the timeline anticipated, and potentially result in competitors’ products reaching the market more quickly than our product candidate, which could materially adversely impact our competitive position and prospects. Even if we are allowed to pursue regulatory approval via an sANDA, we cannot assure you that Dronabinol RT Capsule will receive the requisite approvals and acceptable DEA classification for commercialization.
If the FDA does not conclude that certain of our product candidates satisfy the requirements for the Section 505(b)(2) regulatory approval pathway, or if the requirements for such product candidates under Section 505(b)(2) are not as we expect, the approval pathway for those product candidates will likely take significantly longer, cost significantly more and entail significantly greater complications and risks than anticipated, and in either case may not be successful.
We are developing several proprietary dronabinol product candidates, including Dronabinol Oral Solution, Dronabinol Inhalation Device and Dronabinol IV Solution, for which we intend to seek FDA approval through the Section 505(b)(2) regulatory pathway. In addition, we have submitted an NDA for approval of Subsys under Section 505(b)(2) of the FDC Act. Section 505(b)(2), if applicable to us under the FDC Act would allow an NDA we submit to FDA to rely in part on data in the public domain or the FDA’s prior conclusions regarding the safety and effectiveness of approved compounds, which could expedite the development program for our product candidates by potentially decreasing the amount of clinical data that we would need to generate in order to garner FDA approval. If the FDA does not allow us to pursue the Section 505(b)(2) regulatory pathway as anticipated, we may need to conduct additional clinical trials, provide additional data and information, and meet additional standards for regulatory approval. If this were to occur, the time and financial resources required to obtain FDA approval for these product candidates, and complications and risks associated with these product candidates, would likely substantially increase. We would likely need to obtain substantially more additional funding than currently anticipated, which could result in significant dilution to the ownership interests of our then existing stockholders to the extent we issue equity securities or convertible debt. We cannot assure you that we would be able to obtain such additional financing on terms acceptable to us, if at all. Moreover, inability to pursue the Section 505(b)(2) regulatory pathway would likely result in new competitive products reaching the market more quickly than our product candidates, which would likely materially adversely impact our competitive position and prospects. Even if we are allowed to pursue the Section 505(b)(2) regulatory pathway, we cannot assure you that our product candidates will receive the requisite approvals for commercialization.
In addition, notwithstanding the approval of a number of products by the FDA under Section 505(b)(2) over the last few years, certain brand-name pharmaceutical companies and others have objected to the FDA’s interpretation of Section 505(b)(2). If the FDA’s interpretation of Section 505(b)(2) is successfully challenged, the FDA may be required to change its 505(b)(2) policies and practices, which could delay or even prevent the FDA from approving any NDA that we submit under Section 505(b)(2). In addition, the pharmaceutical industry is highly competitive, and Section 505(b)(2) NDAs are subject to special requirements designed to protect the patent rights of sponsors of previously approved drugs that are referenced in a Section 505(b)(2) NDA. These requirements may give rise to patent litigation and mandatory delays in approval of our NDAs for up to 30 months or longer depending on the outcome of any litigation. It is not uncommon for a manufacturer of an approved product to file a citizen petition with the FDA seeking to delay approval of, or impose additional approval requirements for, pending competing products. If successful, such petitions can significantly delay, or even prevent, the approval of the new product. However, even if the FDA
26
Table of Contents
ultimately denies such a petition, the FDA may substantially delay approval while it considers and responds to the petition. In addition, even if we are able to utilize the Section 505(b)(2) regulatory pathway, there is no guarantee this would ultimately lead to accelerated drug development or earlier approval.
Moreover, even if our product candidates are approved under Section 505(b)(2), the approval may be subject to limitations on the indicated uses for which the products may be marketed or to other conditions of approval, or may contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the products.
Annual DEA quotas on the amount of dronabinol allowed to be produced in the United States and our specific allocation of dronabinol by the DEA could significantly limit the clinical development of our dronabinol product candidates as well as the production or sale of any dronabinol product candidates for which we obtain regulatory approval.
Dronabinol, a Schedule I substance, is subject to the DEA’s production and procurement quota scheme. The DEA establishes annually an aggregate quota for the amount of dronabinol that may be produced in the United States based on the DEA’s estimate of the quantity needed to meet legitimate scientific and medicinal needs. This limited aggregate amount of dronabinol that the DEA allows to be produced in the United States each year is allocated among individual companies, who must submit applications annually to the DEA for individual production and procurement quotas. We are required to obtain an annual quota from the DEA in order to manufacture and produce dronabinol. The DEA may adjust aggregate production quotas and individual production and procurement quotas from time to time during the year and has substantial discretion in deciding whether or not to make such adjustments. The DEA’s aggregate production quota for dronabinol was 312.5 kilograms for each of 2005, 2007, 2008 and 2009. The aggregate production quota for 2006 was 338 kilograms and for 2010 was 264 kilograms. For 2011, this aggregate production quota was increased to 393.0 kilograms. For 2011, we were allocated what we believe is a sufficient quantity of dronabinol to meet our currently anticipated testing and production needs through 2011. However, we may need additional amounts of dronabinol in future years to implement our business plan.
In terms of the allocation of our dronabinol quota for 2011, a significant portion will be used for our planned production of Dronabinol SG and Dronabinol RT Capsules, if approved. Because we are allocated a finite amount of dronabinol for 2011, changes in the amount of dronabinol used for a certain purpose will impact other parts of our business plan utilizing dronabinol.
We do not know what amounts of dronabinol other companies developing dronabinol product candidates may have requested for 2011 or will request in future years. The DEA, in assessing factors such as medical need, abuse potential and other policy considerations, may have chosen to set the aggregate dronabinol quota for 2011 lower than the total amount requested by the companies, and may do so in the future. Though companies are permitted to petition the DEA to increase the aggregate quota for dronabinol in a given year after it is initially established, there is no guarantee the DEA would act promptly or favorably upon such a petition. The success of our business plan will depend in part on our being able to expand the overall market for the medical use of dronabinol by introducing new dronabinol formulations, and to sell significant amounts of our approved dronabinol products. In order to do so, we will need to receive from the DEA significantly increased allotments of dronabinol quotas over time and likely an increase in the aggregate annual quota. Any delay or refusal by the DEA in establishing quotas necessary for us to execute on our business plan could negatively impact our preclinical studies and clinical trials, as well as our ability to sell any approved products, which would in turn have a material adverse effect on our business, our ability to execute on our business plan, our financial position and results of operations, our prospects, and our ability to generate revenue to fund the development of our other product candidates.
27
Table of Contents
Clinical trials for our product candidates are expensive, time consuming, uncertain and susceptible to change, delay or termination.
Clinical trials are very expensive, time consuming and difficult to design and implement. Even if the results of our clinical trials are favorable, we estimate that the clinical trials for a number of our product candidates will continue for several years and may take significantly longer than expected to complete. In addition, we, the FDA, an Institutional Review Board, or other regulatory authorities, including state and local, may suspend, delay or terminate our clinical trials at any time, or the DEA could suspend or terminate the registrations and quota allotments we require in order to procure and handle controlled substances, for various reasons, including:
| • | lack of effectiveness of any product candidate during clinical trials; |
| • | discovery of serious or unexpected toxicities or side effects experienced by study participants or other safety issues; |
| • | slower than expected rates of subject recruitment and enrollment rates in clinical trials; |
| • | difficulty in retaining subjects who have initiated a clinical trial but may withdraw at any time due to adverse side effects from the therapy, insufficient efficacy, fatigue with the clinical trial process or for any other reason; |
| • | delays or inability in manufacturing or obtaining sufficient quantities of materials for use in clinical trials, in particular obtaining sufficient quantities of dronabinol and fentanyl due to regulatory and manufacturing constraints; |
| • | inadequacy of or changes in our manufacturing process or product formulation; |
| • | delays in obtaining regulatory authorization to commence a study, or “clinical holds” or delays requiring suspension or termination of a study by a regulatory agency, such as the FDA, before or after a study is commenced; |
| • | DEA-related recordkeeping, reporting, or security violations at a clinical site, leading the DEA or state authorities to suspend or revoke the site’s controlled substance license and causing a delay or termination of planned or ongoing studies; |
| • | changes in applicable regulatory policies and regulations; |
| • | delays or failure in reaching agreement on acceptable terms in clinical trial contracts or protocols with prospective clinical trial sites; |
| • | uncertainty regarding proper dosing; |
| • | unfavorable results from ongoing clinical trials and preclinical studies; |
| • | failure of our contract research organizations, or CROs, or other third-party contractors to comply with all contractual and regulatory requirements or to perform their services in a timely or acceptable manner; |
| • | failure by us, our employees, our CROs or their employees to comply with all applicable FDA, DEA or other regulatory requirements relating to the conduct of clinical trials or the handling, storage, security and recordkeeping for controlled substances; |
| • | scheduling conflicts with participating clinicians and clinical institutions; |
| • | failure to design appropriate clinical trial protocols; |
| • | insufficient data to support regulatory approval; |
| • | inability or unwillingness of medical investigators to follow our clinical protocols; |
| • | difficulty in maintaining contact with subjects during or after treatment, which may result in incomplete data; or |
28
Table of Contents
| • | regulatory concerns with cannabinoid or opioid products generally and the potential for abuse of the drugs. |
Generally, there is a high rate of failure for drug candidates proceeding through clinical trials. We may suffer significant setbacks in our clinical trials similar to the experience of a number of other companies in the pharmaceutical and biotechnology industries, even after receiving promising results in earlier trials. Further, even if we view the results of a clinical trial to be positive, the FDA or other regulatory authorities may disagree with our interpretation of the data. In the event that that the FDA does not approve our NDA for Subsys or our ANDA for Dronabinol SG Capsule, or we abandon or are delayed in our clinical development efforts related to our other dronabinol and fentanyl product candidates, LEP-ETU or any of our other product candidates, we may not be able to generate sufficient revenues or obtain financing to continue our operations or become profitable, we may not be able to execute on our business plan effectively, our reputation in the industry and in the investment community would likely be significantly damaged and our stock price would likely decrease significantly.
The results of preclinical studies and clinical trials are not necessarily predictive of future results, and our current product candidates may not have favorable results in later studies or trials.
Companies frequently suffer significant setbacks in advanced clinical trials, even after earlier clinical trials have shown promising results. Favorable results in our early studies or trials may not be repeated in later studies or trials, including continuing preclinical studies and large-scale clinical trials, and our product candidates in later stage trials may fail to show desired safety and efficacy despite having progressed through earlier trials. Preclinical data and limited clinical results for our product candidates may differ from results from studies in larger numbers of subjects drawn from more diverse populations treated for longer periods of time. Preclinical data also may not predict the ability of the product candidates to achieve or sustain the desired effects in the intended population or to do so safely. Unfavorable results from ongoing preclinical studies or clinical trials could result in delays, modifications or abandonment of ongoing or future clinical trials. In addition, we may report top-line data from time to time, which is based on a preliminary analysis of key efficacy and safety data, and is subject to change following a more comprehensive review of the data related to the applicable clinical trial. Any of our planned later stage clinical trials may not be successful for a variety of reasons, including the clinical trial designs, the failure to recruit or enroll a sufficient number of suitable subjects, undesirable side effects and other safety concerns, and the inability to demonstrate efficacy or safety.
We rely on third parties to conduct and oversee our clinical trials. If these third parties do not meet our deadlines or otherwise conduct the trials as required we may not be able to obtain regulatory approval for or commercialize our product candidates when expected or at all.
We rely on third-party CROs to conduct and oversee our clinical trials. For example, for our proprietary LEP-ETU product candidate, we contracted with Excel Life Sciences based out of India to serve as our master CRO for our Phase 2 clinical trial in metastatic breast cancer. We also intend to engage a CRO to conduct our Dronabinol Oral Solution pivotal bioequivalence study.
We also rely upon various medical institutions, clinical investigators and contract laboratories to conduct our trials in accordance with our clinical protocols and all applicable regulatory requirements, including the FDA’s good clinical practice regulations and DEA and state regulations governing the handling, storage, security and recordkeeping for controlled substances. These CROs and third parties play a significant role in the conduct of these trials and the subsequent collection and analysis of data from the clinical trials. We rely heavily on these parties for the execution of our clinical and preclinical studies, and control only certain aspects of their activities.
If any of our clinical trial sites terminate their involvement in one of our clinical trials for any reason, we may experience the loss of follow-up information on patients enrolled in our ongoing clinical trials unless we are able to transfer the care of those patients to another qualified clinical trial site. In addition,
29
Table of Contents
principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive cash or equity compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, the integrity of the data generated at the applicable clinical trial site may be questioned by the FDA.
We conducted clinical trials outside the United States and the FDA may not accept data from any such trial. Furthermore we may choose to conduct additional trials in any of our product lines outside the United States and the FDA may not accept data from such trials.
We have conducted and may in the future choose to conduct one or more of our clinical trials outside the United States. For example, our Phase 3 Subsys safety trial was conducted at 46 sites in the United States and 10 sites in India. In addition, we have conducted a Phase 2 clinical trial in India on patients with metastatic breast cancer for LEP-ETU. In addition, though we are not conducting this trial, we are currently providing the dronabinol API for and paying certain monitoring fees in connection with an ongoing Phase 3 clinical trial of 492 patients for the use of dronabinol in the treatment of multiple sclerosis, or MS, in the United Kingdom. Although the FDA may accept data from clinical trials conducted outside the United States, acceptance of such study data by the FDA is subject to certain conditions. For example, the study must be well designed and conducted and performed by qualified investigators in accordance with ethical principles. The study population must also adequately represent the U.S. population, and the data must be applicable to the U.S. population and U.S. medical practice in ways that the FDA deems clinically meaningful. The FDA has advised us that the patient population in which our clinical studies are conducted should be representative of the population for whom we intend to label the product in the United States. In addition, such studies would be subject to the applicable local laws and FDA acceptance of the data would be dependent upon its determination that the studies also complied with all applicable U.S. laws and regulations. There can be no assurance the FDA will accept data from trials conducted outside of the United States. If the FDA does not accept any such data, that would likely result in the need for additional trials, which would be costly and time-consuming and delay aspects of our business plan.
We are subject to uncertainty relating to healthcare reform measures and reimbursement policies which, if not favorable to our product candidates, could hinder or prevent our product candidates’ commercial success.
Our ability to commercialize any approved product candidates successfully will depend in part on the extent to which governmental authorities, private health insurers and other third-party payors establish appropriate coverage and reimbursement levels for our product candidates and related treatments. As a threshold for coverage and reimbursement, third-party payors generally require that drug products have been approved for marketing by the FDA. Third-party payors are increasingly imposing additional requirements and restrictions on coverage and limiting reimbursement levels for medical products. For example, federal and state governments reimburse covered prescription drugs at varying rates below average wholesale price. These restrictions and limitations influence the purchase of healthcare services and products. Legislative proposals to reform healthcare or reduce costs under government insurance programs may result in lower reimbursement for our product candidates or exclusion of our product candidates from coverage and reimbursement programs. The cost containment measures that healthcare payors and providers are instituting and the effect of any healthcare reform could significantly reduce our revenues from the sale of any approved product. We cannot provide any assurances that we will be able to obtain third-party coverage or reimbursement for our product candidates in whole or in part.
In the United States, there have been a number of legislative and regulatory changes to the healthcare system in ways that could affect our future revenues and profitability and the future revenues and profitability of our potential customers. For example, the Medicare Part D prescription drug benefit allows beneficiaries to obtain prescription drug coverage from private sector plans, which can limit the number of prescription drugs that are covered in each therapeutic category and class on their formularies. If our products are not widely included on the formularies of these plans, our ability to
30
Table of Contents
market our products to the Medicare population could be harmed. The State Medicaid programs also generally provide reimbursement for our commercial products, at reimbursement rates that are below the published average wholesale price and that vary from state to state. In return for including pharmaceutical products in the Medicaid programs, manufacturers have agreed to pay a rebate to state Medicaid agencies that provide reimbursement for those products. Pursuant to the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, or PPACA, an increase in the Medicaid rebate rate from 15.1 to 23.1% became effective on January 1, 2010, and the volume of rebated drugs has been expanded to include beneficiaries in Medicaid managed care organizations, effective as of March 23, 2010. The PPACA also includes a 50% discount on brand name drugs for Medicare Part D participants in the coverage gap. The law also revises the definition of “average manufacturer price” for reporting purposes (effective October 1, 2010), which could increase the amount of the Medicaid drug rebates paid to states once the provision is effective.
The PPACA is expected to substantially impact the U.S. pharmaceutical industry and how health care is financed by both governmental and private insurers. Some of the specific PPACA provisions, among other things:
| • | establish annual, non-deductible fees on any entity that manufactures or imports certain branded prescription drugs and biologics, beginning 2011; |
| • | increase minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program, retroactive to January 1, 2010, to 23.1% and 13.0% of the average manufacturer price, or AMP, for branded and generic drugs, respectively; |
| • | redefine a number of terms used to determine Medicaid drug rebate liability, including average manufacturer price and retail community pharmacy, effective October 2010; |
| • | extend manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations, effective March 23, 2010; |
| • | expand eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals beginning April 2010 and by adding new mandatory eligibility categories for certain individuals with income at or below 133.0% of the Federal Poverty Level beginning 2014, thereby potentially increasing manufacturers’ Medicaid rebate liability; |
| • | establish a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research; |
| • | require manufacturers to participate in a coverage gap discount program, under which they must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs being covered under Medicare Part D, beginning 2011; and |
| • | increase the number of entities eligible for discounts under the Public Health Service pharmaceutical pricing program, effective January 2010. |
There also have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare costs to contain or reduce costs of healthcare may adversely affect one or more of the following:
| • | our ability to set a price that we desire for our products; |
| • | our ability to generate revenues and achieve profitability; |
| • | the future revenues and profitability of our potential customers, suppliers and collaborators; and |
31
Table of Contents
| • | the availability of capital. |
In certain foreign markets, the pricing of prescription drugs is subject to government control and reimbursement may, in some cases, be unavailable. In the United States, given recent federal and state government initiatives directed at lowering the total cost of healthcare, Congress and state legislatures will likely continue to focus on healthcare reform, the cost of prescription drugs and the changes to the Medicare and Medicaid programs. While we cannot predict the full outcome of any such legislation, it may result in decreased reimbursement for prescription drugs, which may further exacerbate industry-wide pressure to reduce prescription drug prices. This could harm our ability to market our products and generate revenues. It is also possible that other proposals having a similar effect will be adopted.
If we fail to comply with healthcare regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
In addition to FDA and DEA restrictions on the marketing of pharmaceutical products and federal and state restrictions on distributing and prescribing these products, several other types of state and federal laws have been applied to protect individually identifiable health information and restrict certain marketing practices in the pharmaceutical and medical device industries. These healthcare laws include The Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HIPAA, and fraud and abuse statutes such as the anti-kickback and false claims statutes.
HIPAA mandates, among other things, standards relating to the privacy and security of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. In addition, many states have enacted comparable laws addressing the privacy and security of health information, some of which are more stringent then HIPAA. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and criminal penalties. The costs of compliance with these laws and potential liability associated with failure to do so could adversely affect our business.
The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or, in return for, purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not, in all cases, meet all of the criteria for safe harbor protection from anti-kickback liability.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. In addition, HIPAA created federal criminal laws that prohibit executing a scheme to defraud any health care benefit program or making false statements relating to health care matters. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would be reimbursed by federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the company’s marketing of the product for unapproved, and thus non-reimbursable, uses. There are also federal “sunshine” laws that require transparency regarding financial arrangements with health care providers, such as the reporting and disclosure requirements imposed by PPACA on drug manufacturers regarding any “transfer of value” made or distributed to prescribers and other health care providers.
32
Table of Contents
Sanctions under these laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines and imprisonment.
The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Some states, such as California, Massachusetts and Vermont, also mandate implementation of corporate compliance programs to ensure compliance with these laws.
Because of the breadth of these laws and the fact that their provisions are open to a variety of interpretations, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Moreover, recent health care reform legislation has strengthened these laws. For example, PPACA amends the intent requirement of the federal anti-kickback and criminal health care fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the federal false claims laws. We also expect there will continue to be federal and state laws and/or regulations, proposed and implemented, that could impact our operations and business. The extent to which future legislation or regulations, if any, relating to health care fraud abuse laws and/or enforcement, may be enacted or what effect such legislation or regulation would have on our business remains uncertain. Any government challenge of our business practices could have a material adverse effect on our business, financial condition and results of operations.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA regulations, provide accurate information to the FDA, comply with applicable manufacturing standards, comply with federal and state healthcare fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, or illegal misappropriation of drug product, which could result in regulatory sanctions and serious harm to our reputation. We have adopted a Code of Business Conduct and Ethics, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
We use hazardous materials, chemicals and controlled substances in our research and development and manufacturing activities and we may incur significant costs complying with the environmental, health and safety laws and regulations that govern such use. In addition, if we fail to comply with the applicable environmental, health and safety regulations, we could be exposed to significant liabilities.
Our research and development as well as manufacturing activities involve the use of potentially harmful hazardous materials, chemicals and controlled substances that could be hazardous to human health and safety or the environment and which are subject to a variety of federal, state and local laws
33
Table of Contents
and regulations governing their use, generation, manufacture, storage, handling and disposal. These materials and various wastes resulting from their use are stored at our facility pending ultimate use and disposal. We cannot completely eliminate the risk of contamination, which could cause:
| • | an interruption of our research and development and manufacturing efforts; |
| • | injury to our employees and others; |
| • | environmental damage resulting in costly clean up; and |
| • | liabilities under federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. |
In such an event, we may be held liable for any resulting damages, and any such liability could exceed our resources. Although we carry insurance in amounts and type that we consider commercially reasonable, we do not carry specific biological or hazardous waste insurance coverage.
Since the starting materials we utilize to manufacture dronabinol are sourced out of India, we are exposed to a number of risks and uncertainties associated with that geographic region.
The suppliers of the starting materials we utilize to manufacture dronabinol are located in India. This exposes us to a number of risks and uncertainties outside our control. India has suffered political instability in the past due to various factors including the failure of any party to win an absolute majority in the Indian Parliament for several years. There have also been armed conflicts between India and neighboring Pakistan. Moreover, extremist groups within India and neighboring Pakistan have from time to time targeted Western interests. In addition, India is susceptible to natural disasters such as earthquakes and floods. Political instability, future hostilities with countries such as Pakistan, targeting of our interests by extremist attacks, and earthquakes or other natural disasters in India could harm our operations and impede our ability to produce dronabinol on our anticipated timeline, or at all.
If we fail to successfully develop, identify and acquire or in-license additional products or product candidates, we may have limited growth opportunities.
As resources allow and opportunities present themselves, we intend to enhance our pipeline of new products and product candidates through acquisition or in-licensing. The success of this strategy will depend upon our ability to effectively identify, select and acquire or in-license pharmaceutical products or product candidates.
The process of proposing, negotiating and implementing the acquisition or in-license of a product or product candidate is lengthy and complex. Other companies, including some with substantially greater financial, marketing and sales resources, may compete with us for these products or product candidates. We have limited resources to identify and execute acquisition or in-licensing transactions. Even if we acquire or in-license additional products or product candidates, we have limited resources to integrate the acquired or licensed assets into our current infrastructure. In addition, we may devote resources to potential acquisition or in-licensing opportunities that are never completed, or we may fail to realize the anticipated benefits of such efforts after expenditure of considerable time and resources. We may not be able to acquire the rights to additional products or product candidates on terms that we find acceptable, or at all.
Future acquisition and in-licensing transactions may entail numerous operational and financial risks including:
| • | exposure to unknown liabilities; |
| • | disruption of our business and diversion of our management’s time and attention to the development of these products or product candidates; |
| • | incurrence of substantial debt or dilutive issuances of securities to pay for acquisitions or in-licensing transactions; and |
34
Table of Contents
| • | high acquisition and integration costs. |
Product candidates that we acquire or in-license will likely require additional development efforts prior to commercial sale, including product development, extensive clinical testing and approval by the FDA and other applicable regulatory authorities. All product candidates are prone to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate will not be shown to be sufficiently safe or effective for approval by regulatory authorities. In addition, we cannot provide assurance that any products or product candidates that we acquire or in-license will be manufactured profitably or achieve market acceptance.
We may engage in strategic transactions that could impact our liquidity, increase our expenses and present significant distractions to our management.
From time to time we may consider strategic transactions, such as acquisitions of companies, asset purchases and out-licensing or in-licensing of products, product candidates or technologies. For example, in November 2010, we completed the Merger which resulted in Insys Pharma becoming our wholly-owned subsidiary. Previously, in September 2009, Insys Pharma obtained assets which have given us the ability to manufacture our supply of dronabinol API internally through our manufacturing facility in Texas. Additional potential transactions include a variety of different business arrangements, spin-offs, strategic partnerships, joint ventures, restructurings, divestitures, business combinations and investments. Any such transaction may require us to incur non-recurring or other charges, may increase our near and long-term expenditures and may pose significant integration challenges or disrupt our management or business, which could harm our operations and financial results.
We may not realize any benefits and may experience detriments as a result of the combination of Insys Therapeutics and Insys Pharma.
We may not realize any benefits from the integration of the businesses of Insys Therapeutics and Insys Pharma. The timely, efficient and successful integration of the businesses will entail execution of a number of tasks, including the following:
| • | integrating the business, operations, different locations, research and development functions and technologies of the companies; |
| • | retaining and assimilating the key personnel of each company; |
| • | managing the varying regulatory approval processes, intellectual property protection strategies and other activities of the companies; |
| • | retaining strategic partners of each company and attracting new strategic partners; |
| • | creating uniform standards, controls, procedures, policies and information systems, including with respect to disclosure controls and procedures and internal control over financial reporting; and |
| • | meeting the challenges inherent in efficiently managing an increased number of employees, including the need to implement appropriate systems, policies, benefits and compliance programs. |
We may encounter difficulties successfully managing a larger and more diverse organization and may encounter significant delays in achieving successful management of our organization. Integration of our business operations will involve risks and may not be successful. These risks include the following:
| • | the potential disruption of ongoing business and distraction of our management; |
| • | the potential strain on our financial and managerial controls and reporting systems and procedures; |
| • | our inability to manage the research and development, regulatory and reimbursement approval, and other activities of our businesses; |
| • | unanticipated or greater than anticipated expenses and potential delays related to integration of the operations, technology and other resources of our businesses; |
35
Table of Contents
| • | the impairment of relationships with employees and suppliers as a result of any integration of new management personnel or other activities; and |
| • | potential unknown liabilities associated with the strategic combination and the combined operations. |
We may not succeed in addressing these risks or any other problems encountered in connection with the integration of our businesses. The inability to integrate successfully the operations, technology and personnel of our businesses, or any significant delay in achieving integration, could have a material adverse effect on our business, results of operations and prospects, and on the market price of our common stock.
Our results of operations and liquidity needs could be materially negatively affected by market fluctuations and economic downturn.
Our results of operations and liquidity could be materially negatively affected by economic conditions generally, both in the United States and elsewhere around the world. Domestic and international equity and debt markets have experienced and may continue to experience heightened volatility and turmoil based on domestic and international economic conditions and concerns. In the event these economic conditions and concerns continue or worsen and the markets continue to remain volatile, our results of operations and liquidity could be adversely affected by those factors in many ways, including making it more difficult for us to raise funds if necessary.
We may incur substantial liabilities from any product liability claims if our insurance coverage for those claims is inadequate.
We face an inherent risk of product liability exposure related to the testing, manufacturing and marketing of our product candidates in human clinical trials, and will face an even greater risk if we sell our product candidates commercially. Common adverse events for dronabinol products include: abdominal pain, nausea, vomiting, dizziness, euphoria, paranoid reaction, somnolence, abnormal thought patterns, and difficulty with concentration/attention. Common adverse events for fentanyl products include: nausea, dizziness, somnolence, vomiting, asthenia or lack of energy and strength and anxiety. An individual may bring a liability claim against us if one of our product candidates causes, or merely appears to have caused, an injury. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for our product candidates; |
| • | product recall or withdrawal from the market; |
| • | impairment to our business reputation or acceptance in the medical community; |
| • | withdrawal of clinical trial participants; |
| • | costs of related litigation; |
| • | distraction of management’s attention from our primary business; |
| • | substantial monetary awards to patients or other claimants; |
| • | loss of revenues; and |
| • | the inability to commercialize our product candidates. |
We have obtained product liability insurance coverage for our clinical trials with a $1 million per occurrence and $2 million aggregate limit, together with an umbrella policy of $3 million for each occurrence and $3 million aggregate limit. Our insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly
36
Table of Contents
expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost, in sufficient amounts or upon adequate coverage terms to protect us against losses due to liability. We intend to expand our insurance coverage to include the sale of commercial products if regulatory approval is obtained for any of our product candidates. However, we may be unable to obtain this product liability insurance on commercially reasonable terms or with insurance coverage that will be adequate to satisfy any liability that may arise. Large judgments have been awarded in class action or individual lawsuits based on drugs that had unanticipated side effects, including side effects which are less severe than those of our product candidates. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
Our ability to utilize our net operating loss carryforwards, or NOLs, and research and development income tax credit carryforwards may be limited.
Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, substantial changes in our ownership may limit the amount of NOLs and research and development income tax credit carryforwards that could be utilized annually in the future to offset taxable income, if any. Specifically, this limitation may arise in the event of a cumulative change in ownership of our company of more than 50% within a three-year period. Any such annual limitation may significantly reduce the utilization of the NOLs before they expire. The closing of this offering, together with the other transactions that have occurred since our inception, may trigger an ownership change pursuant to Sections 382 and 383, which could further limit the amount of NOLs that could be utilized annually in the future to offset taxable income, if any. Any such limitation, whether as the result of prior transactions, sales of common stock by our existing stockholders or additional sales of common stock by us after this offering, could have an adverse effect on our results of operations.
On November 8, 2010, Insys Therapeutics, Inc. effected the Merger in a transaction that was accounted for as a reverse acquisition and resulted in a change of 50% or more of the ownership of NeoPharm. As of the Merger date, NeoPharm had $274.0 million of federal NOLs which were scheduled to expire in tax years 2011 to 2029. Under Section 382 of the Code, our utilization of the pre-Merger federal NOLs of NeoPharm to offset our post-Merger federal taxable income is significantly limited due to the Merger. Prior to the Merger, NeoPharm had completed a partial analysis of ownership changes under Section 382 of the Code to determine if a change in control of NeoPharm had occurred. Based on NeoPharm’s partial analysis, no change in control was identified, based on the review of eight test dates covering a four-year period ended December 31, 2007. A complete formal analysis of ownership change would have to be performed in order to obtain certainty that a change in control of NeoPharm had not occurred prior to the Merger, which could further limit the utilization of the NeoPharm pre-Merger NOLs by us. Based on the above, we have estimated the amount of pre-Merger federal NOLs of NeoPharm that are available to offset our post-Merger income is limited to approximately $158,000 a year for 20 years, or cumulatively $3.2 million. For state income tax purposes, we have $274.0 million of state NOLs related to NeoPharm operations. We have placed a valuation allowance on our deferred tax assets, which include the federal and state NOLs, for it is not more likely than not that such amounts will be realized.
Our product candidates contain controlled substances, the use of which may generate public controversy.
Fentanyl is a Schedule II controlled substance narcotic derivative and despite the strict regulations on the marketing, prescribing and dispensing of Schedule II substances, illicit use and abuse of fentanyl products is well-documented. Moreover dronabinol, though synthetic, is a cannabinoid. Since our product candidates contain controlled substances, regulatory approval of these product candidates may generate public controversy. Political and social pressures and adverse publicity could lead to delays in, and increased expenses for, and limit or restrict the introduction and marketing of our product candidates.
37
Table of Contents
Import/export regulations and tariffs may change and increase our operating costs.
We are subject to risks associated with the regulations relating to the import and export of products and materials. In particular, for dronabinol, we obtain the two chemicals that are used as starting materials for its production from suppliers in India. We cannot predict whether the import and/or export of our products will be adversely affected by changes in, or enactment of new quotas, duties, taxes or other charges or restrictions imposed by India or any other country in the future. Any of these factors could have a material adverse effect on our operating costs.
Currency exchange rate fluctuations may increase our costs.
The exchange rate between the U.S. dollar and non-U.S. currencies in which we trade to conduct our business have and will likely fluctuate in the future. Any appreciation in the value of these non-U.S. currencies would result in higher expenses for our company. We do not have any hedging arrangements to protect against such exchange rate exposures.
38
Table of Contents
Risks Relating to Our Intellectual Property
We may not be able to obtain and enforce patent rights or other intellectual property rights that cover our product candidates, such as Subsys, Dronabinol RT Capsule, Dronabinol Oral Solution, Dronabinol Inhalation Device, Dronabinol IV Solution and LEP-ETU and are of sufficient breadth to prevent third parties from competing against us.
Our success with respect to our product candidates, such as Subsys, Dronabinol RT Capsule, Dronabinol Oral Solution, Dronabinol Inhalation Device, Dronabinol IV Solution and LEP-ETU will depend in part on our ability to obtain and maintain patent protection in both the United States and other countries, to preserve our trade secrets, and to prevent third parties from infringing upon our proprietary rights on our product candidates. Our ability to protect any of our approved drug products from unauthorized or infringing use by third parties depends in substantial part on our ability to obtain and maintain valid and enforceable patents. Fentanyl, dronabinol and paclitaxel have been approved for many years and therefore our ability to obtain any patent protection is limited. Composition of matter patents on active pharmaceutical ingredients are a particularly effective form of intellectual property protection for pharmaceutical products as they apply without regard to any method of use or other type of limitation. However, we will not be able to obtain composition of matter patents or methods of use patents that cover the active pharmaceutical ingredients in any of our product candidates. As a result, competitors who obtain the requisite regulatory approval can offer products with the same active ingredients as our product candidates so long as the competitors do not infringe any formulation patents that we may obtain or license, if any.
The only patent protection that we can expect will cover, our fentanyl, dronabinol and LEP product candidates consists of patents relating to formulations and methods of treatment using certain formulations. Formulation patents protect the product only when competitors use a similar formulation. However, this type of patent does not limit a competitor from making and marketing a product that is intended to be used in the same indication as long they use a different dosage form and/or formulation. Any formulation patents that we may obtain may be too narrow in scope and thus easily circumvented by competitors.
We have multiple pending patent applications in the United States and in some foreign jurisdictions that attempt to cover our formulations for our fentanyl, dronabinol, and LEP product candidates. We have no issued patents in the United States, or in many foreign countries, that pertain to either fentanyl or dronabinol formulations. We can give no assurances that any patents will issue, that if they do issue, they will provide sufficient protection against competitors, or that they would be valid and enforceable. In addition, two of the issued patents for LEP-ETU expire in July 2018 and the third one expires in June 2019.
Due to evolving legal standards relating to patentability, validity, enforceability and claim scope of patents covering pharmaceutical inventions, our ability to maintain, obtain and enforce patents is uncertain and involves complex legal and factual questions. Accordingly, rights under any patents we may obtain or license may not provide us with sufficient protection for our product candidates and products to afford a commercial advantage against competitive products or processes, including those from branded and generic pharmaceutical companies. In addition, we cannot guarantee that any patents will issue from any pending or future patent applications owned by or licensed to us. Even if patents have issued or will issue, we cannot guarantee that the claims of these patents are or will be held valid or enforceable by the courts or will provide us with any significant protection against competitive products or otherwise be commercially valuable to us. Patent applications in the United States are maintained in confidence for up to 18 months after their filing. In some cases, however, patent applications remain confidential in the United States Patent and Trademark Office, or USPTO, for the entire time prior to issuance as a U.S. patent. Similarly, publication of discoveries in scientific or patent literature often lag behind actual discoveries. Consequently, we cannot be certain that we or our licensors were the first to invent, or the first to file patent applications on our product candidates or products. In the event that a third party has also filed a U.S. patent application relating to our drug
39
Table of Contents
product or a similar invention, we may have to participate in interference proceedings declared by the USPTO to determine priority of invention in the United States. The costs of these proceedings could be substantial and it is possible that our efforts would be unsuccessful, resulting in a loss of our U.S. patent position.
In addition, third parties may challenge our in-licensed patents and any of our own patents that we may obtain, which could result in the invalidation or unenforceability of some or all of the relevant patent claims. Litigation or other proceedings to enforce or defend intellectual property rights is very complex, expensive, and may divert our management’s attention from our core business and may result in unfavorable results that could adversely affect our ability to prevent third parties from competing with us.
The laws of some foreign jurisdictions do not provide intellectual property rights to the same extent as in the United States and many companies have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. If we encounter such difficulties in protecting or are otherwise precluded from effectively protecting our intellectual property in foreign jurisdictions, our business prospects could be substantially harmed. The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents.
The degree of future protection of our proprietary rights is uncertain. Patent protection may be unavailable or severely limited in some cases and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| • | we might not have been the first to file patent applications for these or similar inventions; |
| • | we might not have been the first to make the inventions covered by each of our pending patent applications and issued patents; |
| • | others may independently develop similar or alternative technologies or duplicate any of our technologies; |
| • | the patents of others may have an adverse effect on our business; |
| • | it is possible that none of our or our licensors’ pending patent applications will result in issued patents; |
| • | any patents we obtain or our licensors’ issued patents may not encompass commercially viable products, may not provide us with any competitive advantages, or may be challenged by third parties; |
| • | any patents we obtain or our in-licensed issued patents may not be valid or enforceable; or |
| • | we may not develop additional proprietary technologies that are patentable. |
If we or our licensors fail to appropriately prosecute and maintain patent protection for these product candidates or other product candidates that we may develop, our ability to develop and commercialize these or any other product candidates we develop may be adversely affected and we may not be able to prevent competitors from making, using and selling competing products. This failure to properly protect the intellectual property rights relating to these product candidates could have a material adverse effect on our business, financial condition and results of operation.
Proprietary trade secrets and unpatented know-how are also very important to our business. Although we have taken steps to protect our trade secrets and unpatented know-how, by entering into confidentiality agreements with third parties, and confidential information and inventions agreements with certain employees, consultants and advisors, third parties may still obtain this information or we
40
Table of Contents
may be unable to protect our rights. There can be no assurance that binding agreements will not be breached, that we would have adequate remedies for any breach, or that our trade secrets and unpatented know-how will not otherwise become known or be independently discovered by our competitors. If trade secrets are independently discovered, we would not be able to prevent their use. Enforcing a claim that a third party illegally obtained and is using our trade secrets or unpatented know-how is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secret information.
We are a defendant in a lawsuit to seek rescission of certain invention assignments, and if we do not prevail, any resulting rescission of invention assignments could have a material adverse impact on our business by preventing us from obtaining exclusive patent rights covering certain of our product candidates.
Although we expect all of our employees to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidential information and inventions agreements, we cannot provide any assurances that all such agreements have been duly executed or will be held enforceable.
For example, in September 2009, Insys Pharma and certain of its officers and directors, as well as their spouses, were named as defendants in a lawsuit in Arizona Superior Court brought by Santosh Kottayil, certain of his family members and a trust of which Dr. Kottayil is the trustee. Dr. Kottayil formerly served as President, Chief Scientific Officer and a director of Insys Pharma, among other positions. The complaint brought a cause of action, among others, seeking to rescind Dr. Kottayil’s assignment to Insys Pharma of his interest in all of the fentanyl and dronabinol patent applications we own and to recover the benefits of those interests. Insys Pharma and the other defendants answered and filed counter-claims to Dr. Kottayil’s complaint. If the patent assignments are successfully rescinded, we will not have exclusive patent rights covering our fentanyl and dronabinol product candidates, and such exclusive patent rights may not be available to us on acceptable terms, if at all, which would have a material adverse effect on our business. If the assignments are rescinded, Kottayil could assign his interest in the fentanyl and dronabinol patent applications to a competitor and we would not be able to prevent generic copies of our products. Please see the section entitled “Legal Proceedings.”
We license patent rights from third-party owners. If such owners do not properly maintain or enforce the patents underlying such licenses, our competitive position and business prospects may be harmed.
We are party to a number of license agreements that give us rights to third-party intellectual property that may be necessary or useful for our business. We may enter into additional license agreements to use third- party intellectual property in the future. Our success will depend in part on the ability of our licensors to obtain, maintain and enforce patent protection for their intellectual property, in particular, those patents to which we have secured exclusive rights. Our licensors may not successfully prosecute the patent applications to which we are licensed. Even if patents issue from these patent applications, our licensors may fail to maintain these patents, may determine not to pursue litigation against other companies that are infringing these patents, or may pursue such litigation less aggressively than we would, and in certain licenses, we only maintain the right to enforce the patents if the applicable licensor fails or decides to not take any action. In addition, our licensors may terminate their agreements with us in the event we breach the applicable license agreement and fail to cure the breach within a specified period of time. Without protection for the intellectual property we license, other companies might be able to offer substantially identical products for sale, which could adversely affect our competitive business position and harm our business prospects.
41
Table of Contents
If we are sued for infringing intellectual property rights of third parties, it will be costly and time consuming to defend such litigation, and an unfavorable outcome in that litigation may have a material adverse effect on our business.
Our commercial success also depends upon our ability and the ability of our future collaborators to develop, manufacture, market and sell our product candidates and to use our proprietary technologies without infringing the proprietary rights of third parties. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing products. Furthermore, we may not have identified all U.S. and foreign patents or published applications that affect our business either by potentially blocking our ability to commercialize our product candidates or by covering similar technologies that affect our target markets. Because patent applications can take many years to issue, there may be currently pending applications, which may later result in issued patents that our product candidates or proprietary technologies may infringe.
We may be exposed to, or threatened with, future litigation by third parties having patent or other intellectual property rights alleging that our product candidates and/or proprietary technologies infringe their intellectual property rights. If one of these patents was found to cover our product candidates, proprietary technologies or their uses or manufacturer, we or our future collaborators could be required to pay damages and could be unable to commercialize our product candidates or to use our proprietary technologies unless we or they obtained a license to the patent. A license may not be available to us or our future collaborators on acceptable terms, if at all. Thus, we could be prevented from commercializing the product for many years until the patent expires which would have a material adverse effect on our business. In addition, during litigation, the patent holder could obtain a preliminary injunction or other equitable right which could prohibit us from making, using or selling our products, technologies or methods.
There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries generally. And in particular, the generic drug industry is characterized by frequent litigation between generic drug companies and branded drug companies. If a third party claims that we infringe its intellectual property rights, we may face a number of issues, including, but not limited to:
| • | infringement and other intellectual property claims which, with or without merit, may be expensive and time-consuming to litigate and may divert our management’s attention from our core business; |
| • | substantial damages for infringement, including treble damages and attorneys’ fees, which we may have to pay if a court decides that the product or proprietary technology at issue infringes on or violates the third party’s rights; |
| • | a court prohibiting us from selling or licensing the product or using the proprietary technology unless the third party licenses its technology to us, which it is not required to do; |
| • | if a license is available from the third party, we may have to pay substantial royalties, fees and/or grant cross licenses to our technology; and |
| • | redesigning our product candidates or processes so they do not infringe, which may not be possible or may require substantial funds and time. |
We have not conducted an extensive search of patents issued to third parties, and no assurance can be given that third-party patents containing claims covering our product candidates, technology or methods do not exist, have not been filed, or could not be filed or issued. Because of the number of patents issued and patent applications filed in our technical areas or fields, we believe there is a significant risk that third parties may allege they have patent rights encompassing our products, technology or methods. Other product candidates that we may in-license or acquire could be subject to similar risks and uncertainties.
42
Table of Contents
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed alleged trade secrets of their other clients or former employers to us.
As is common in the biotechnology and pharmaceutical industry, certain of our employees were formerly employed by other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Moreover, we engage the services of consultants to assist us in the development of our product candidates, many of whom were previously employed at or may have previously been or are currently providing consulting services to, other biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may be subject to claims that these employees and consultants or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers or their former or current customers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. For example, on November 2, 2007, we received a letter from counsel for Solvay Pharmaceuticals, Inc. and Unimed Pharmaceuticals, Inc., or Abbott (Solvay/Unimed), in which Abbott (Solvay/Unimed) asserted that we may have used its confidential and proprietary information in the development of our dronabinol technology and products, and requested various information and written assurances from us. Abbott (Solvay/Unimed)’s letter contains broad allegations concerning its Marinol manufacturing and business strategy, but does not specifically identify any particular confidential or proprietary information allegedly used by us. The allegations appear to be based primarily on the fact that our founder worked for Abbott (Solvay/Unimed) several years ago. We responded to Abbott (Solvay/Unimed) denying these allegations on November 14, 2007 and informed them at that time that we do not intend to comply with any of the requests made in their letter. On January 25, 2008, we received another letter from counsel for Solvay that mainly reasserted the allegations and repeated the request for information and assurances made in the November 2, 2007 letter. In response, on January 30, 2008, we responded to Solvay, once again denying their assertions and informing them we do not intend to comply with their requests. After this latest response, we have had no further communications. There can be no assurance, however, that Abbott (Solvay/Unimed) will not take further legal action with respect to such assertions or allegations. Any such litigation would likely be protracted, expensive, a distraction to our management team, not viewed favorably by investors and other third parties, and may potentially result in an unfavorable outcome.
Risks Related to This Offering and Ownership of Our Common Stock
Our Executive Chairman and principal stockholder can individually control our direction and policies, and his interests may be adverse to the interests of our stockholders.
As of June 30, 2011, our founder, Executive Chairman and principal stockholder, Dr. John N. Kapoor, beneficially owned approximately % of our capital stock outstanding as of June 30, 2011, after giving effect to the issuance of shares of our common stock upon conversion of notes beneficially owned by him and accrued interest thereon immediately prior to the closing of this offering, assuming a conversion date of , 2011 and an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus. Upon the closing of this offering, assuming no exercise of the underwriters’ option to purchase additional shares, Dr. Kapoor will beneficially own approximately % of our outstanding shares of common stock. By virtue of his holdings, Dr. Kapoor can and will continue to be able to effectively control the election of the members of our board of directors, our management and our affairs and prevent corporate transactions such as mergers, consolidations or the sale of all or substantially all of our assets that may be favorable from our standpoint or that of our other stockholders or cause a transaction that we or our other stockholders may view as unfavorable. Accordingly, this concentration of ownership may harm the market price of our common stock by:
| • | delaying, deferring or preventing a change in control; |
| • | impeding a merger, consolidation, takeover or other business combination involving us; or |
43
Table of Contents
| • | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
In addition, sales of shares beneficially owned by Dr. Kapoor could be viewed negatively by third parties and have a negative impact on our stock price. Moreover, upon his passing, we cannot assure you as to how these shares will be distributed and subsequently voted.
Moreover, trusts controlled by Dr. Kapoor have been the sole source of financing for Insys Pharma to date. As of June 30, 2011, we owed $43.7 million in secured debt and accrued interest to Dr. Kapoor’s trusts. While this outstanding debt will convert into shares of our common stock upon completion of this offering, we may in the future issue additional debt to entities controlled by Dr. Kapoor and Dr. Kapoor’s interest as a holder of our debt may conflict with your interest as a holder of our common stock.
If we are unable to successfully remediate the material weakness in our internal control over financial reporting, the accuracy and timing of our financial reporting may be adversely affected.
In connection with the audit of our consolidated financial statements for the year ended December 31, 2010, our management and independent registered public accounting firm identified a material weakness in our internal control over financial reporting. A material weakness is a control deficiency, or a combination of control deficiencies, that results in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected. Our management and independent registered public accounting firm did not perform an evaluation of our internal control over financial reporting as of December 31, 2010 in accordance with the provisions of the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley Act. Had we and our independent registered public accounting firm performed an evaluation of our internal control over financial reporting in accordance with the provisions of the Sarbanes-Oxley Act, additional control deficiencies may have been identified by management or our independent registered public accounting firm, and those control deficiencies could have also represented one or more material weaknesses.
Our management and independent registered public accounting firm identified a material weakness related to a lack of sufficient staff with appropriate training in GAAP and SEC rules and regulations with respect to financial reporting. This deficiency resulted in a more than remote likelihood that a material misstatement of our annual and interim financial statements would not be prevented or detected. As a result, audit adjustments to our financial statements were identified during the course of the audit. Currently, we have only one designated finance and accounting employee, our new Chief Financial Officer, and rely on consultants to provide many accounting, book-keeping and administrative services. In an effort to remediate this material weakness, we intend to hire additional finance and accounting personnel, build our financial management and reporting infrastructure, and further develop and document our accounting policies and financial reporting procedures in 2011 and 2012. For example, we have begun a search for a controller and expect to fill that position in 2011. We cannot assure you that we will be successful in these hiring efforts or that these measures will significantly improve or remediate the material weakness described above. We also cannot assure you that we have identified all or that we will not in the future have additional material weaknesses. Accordingly, material weaknesses may still exist when we report on the effectiveness of our internal control over financial reporting for purposes of our attestation required by reporting requirements under the Securities Exchange Act of 1934, as amended, or the Exchange Act, or Section 404 of the Sarbanes-Oxley Act after this offering. The standards required for a Section 404 assessment under the Sarbanes-Oxley Act will require us to implement additional corporate governance practices and adhere to a variety of reporting requirements and complex accounting rules. These stringent standards require that our audit committee be advised and regularly updated on management’s review of internal controls. Our management may not be able to effectively and timely implement controls and procedures that adequately respond to the increased regulatory compliance and reporting requirements that will be applicable to us as a public company. If we fail to staff our accounting and finance function adequately or maintain internal controls
44
Table of Contents
adequate to meet the demands that will be placed upon us as a public company, including the requirements of the Sarbanes-Oxley Act, we may be unable to report our financial results accurately or in a timely manner and our business and stock price may suffer.
Maintaining and improving our financial controls and the requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract and retain qualified board members.
As a public company, we will be subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act and the Nasdaq Stock Market Rules, or Nasdaq rules. The requirements of these rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and may also place undue strain on our personnel, systems and resources. The Exchange Act will require, among other things, that we file annual, quarterly and current reports with respect to our business and financial condition.
The Sarbanes-Oxley Act will require, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. Ensuring that we have adequate internal financial and accounting controls and procedures in place is a costly and time-consuming effort that needs to be re-evaluated frequently. We are in the process of documenting, reviewing and, where appropriate, improving our internal controls and procedures in preparation for compliance with the SEC regulations adopted pursuant to Section 404 of the Sarbanes-Oxley Act, which requires annual management assessments of the effectiveness of our internal control over financial reporting and, if we are an accelerated filer, a report by our independent auditors addressing these assessments. Both we and potentially our independent auditors will be testing our internal controls in connection with the Section 404 requirements and could, as part of that documentation and testing, identify material weaknesses, significant deficiencies or other areas for further attention or improvement. Implementing any appropriate changes to our internal controls may require specific compliance training for our directors, officers and employees, entail substantial costs to modify our existing accounting systems, and take a significant period of time to complete. Such changes may not, however, be effective in maintaining the adequacy of our internal controls, and any failure to maintain that adequacy, or consequent inability to produce accurate financial statements on a timely basis, could increase our operating costs and could materially impair our ability to operate our business. Moreover, effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent fraud. As a result, our failure to satisfy the requirements of Section 404 on a timely basis could result in the loss of investor confidence in the reliability of our financial statements, which in turn could cause the market value of our common stock to decline.
In accordance with Nasdaq rules, we will be required to maintain a majority independent board of directors. We also expect that the various rules and regulations applicable to public companies will make it more difficult and more expensive for us to maintain directors’ and officers’ liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to maintain coverage. If we are unable to maintain adequate directors’ and officers’ insurance, our ability to recruit and retain qualified directors, especially those directors who may be deemed independent for purposes of Nasdaq rules, and officers will be significantly curtailed.
Compliance with these reporting rules, Sarbanes-Oxley Act and Nasdaq requirements will require us to build out our accounting and finance staff. Our Chief Financial Officer is the only dedicated accounting and finance employee currently employed by us. We will need to expand our accounting and financing staff, and our failure to adequately do so would harm our ability to comply with the requirements listed above.
45
Table of Contents
We expect that the price of our common stock will fluctuate substantially.
Following this offering, the market price for our common stock is likely to be volatile, in part because there has not been a true public market for the common stock of our combined entity prior to this offering. In addition, the market price of our common stock may fluctuate significantly in response to a number of factors, most of which we cannot control, including:
| • | the development status of our product candidates and whether and when any of our product candidates receive regulatory approval or acceptable scheduling by the DEA, including the regulatory status of our NDA for Subsys and sANDA for Dronabinol SG Capsule; |
| • | the results of our preclinical studies and clinical trials; |
| • | variations in the level of expenses related to our product candidates or preclinical and clinical development programs, including relating to the timing of invoices, from and other billing practices of, our CROs and clinical trial sites; |
| • | our execution of our manufacturing, sales and marketing, and other aspects of our business plan; |
| • | price and volume fluctuations in the overall stock market; |
| • | changes in operating performance and stock market valuations of other pharmaceutical companies; |
| • | market conditions or trends in our industry or the economy as a whole; |
| • | our execution of collaborative, co-promotion, licensing or other arrangements, and the timing of payments we may make or receive under these arrangements; |
| • | the public’s response to press releases or other public announcements by us or third parties, including our filings with the SEC and announcements relating to litigation, intellectual property or cannabinoids, dronabinol or fentanyl impacting us or our business; |
| • | the financial projections we may provide to the public, any changes in these projections or our failure to meet these projections; |
| • | changes in financial estimates by any securities analysts who follow our common stock, our failure to meet these estimates or failure of those analysts to initiate or maintain coverage of our common stock; |
| • | ratings downgrades by any securities analysts who follow our common stock; |
| • | the development and sustainability of an active trading market for our common stock; |
| • | future sales of our common stock by our officers, directors and significant stockholders; |
| • | other events or factors, including those resulting from war, incidents of terrorism, natural disasters or responses to these events; and |
| • | changes in accounting principles. |
In addition, the stock markets, and in particular the Nasdaq Global Market, have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many pharmaceutical companies. Stock prices of many pharmaceutical companies have fluctuated in a manner unrelated or disproportionate to the operating performance of those companies. In the past, stockholders have instituted securities class action litigation following periods of market volatility. If we were involved in securities litigation, we could incur substantial costs and our resources and the attention of management could be diverted from our business.
46
Table of Contents
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of our company, the trading price for our stock would be negatively impacted. If we obtain securities or industry analyst coverage and if one or more of the analysts who covers us downgrades our stock or publishes inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our stock could decrease, which could cause our stock price and trading volume to decline.
There may not be a viable public market for our common stock.
Although our common stock was once traded on the Nasdaq Capital Market and some of our common stock is currently quoted on the Pink Sheets, a centralized electronic quotation service for over-the-counter securities, immediately prior to this offering there is no liquid public market on which our common stock is actively and readily traded. The initial public offering price of our common stock for this offering will be determined through negotiations between us and the representatives of the underwriters, and may not be indicative of the market price of our common stock following this offering. If you purchase shares of our common stock, you may not be able to resell those shares at or above the initial public offering price. We cannot predict the extent to which investor interest in our company will lead to the development of an active trading market on the Nasdaq Global Market or otherwise or how liquid that market might become. An active public market for our common stock may not develop or be sustained after the offering. If an active public market does not develop or is not sustained, it may be difficult for you to sell your shares of common stock at a price that is attractive to you, or at all.
Future sales of our common stock or securities convertible into our common stock may depress our stock price.
Sales of a substantial number of shares of our common stock or securities convertible into our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. After this offering, we will have outstanding shares of common stock based on the number of shares outstanding as of , 2011. This includes the shares that we are selling in this offering, which may be resold in the public market immediately unless held by an affiliate of ours. 464,353 of the remaining shares were outstanding prior to the Merger and, unless held by an affiliate of ours, substantially all of these shares will also be eligible for resale on the public market immediately, and of the remaining shares may be sold after the expiration of lock-up agreements at least 180 days after the date of this prospectus pursuant to Rule 144 or Rule 701 under the Securities Act of 1933, as amended, or the Securities Act, unless held by an affiliate of ours, as more fully described in the section entitled “Shares Eligible for Future Sale.”
Moreover, we also intend to register all shares of common stock that we may issue after this offering under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to the lock-up agreements described above and in the section entitled “Underwriting—Lock-Up Agreements.”
If a large number of shares of our common stock or securities convertible into our common stock are sold in the public market after they become eligible for sale, the sales could reduce the trading price of our common stock and impede our ability to raise future capital.
47
Table of Contents
Anti-takeover provisions in our charter documents and Delaware law might deter acquisition bids for us that you might consider favorable.
Our amended and restated certificate of incorporation and bylaws contain provisions that may make the acquisition of our company more difficult without the approval of our board of directors. These provisions:
| • | establish a classified board of directors so that not all members of our board are elected at one time; |
| • | authorize the issuance of undesignated preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval, and which may include rights superior to the rights of the holders of common stock; |
| • | prohibit stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders; |
| • | provide that the board of directors is expressly authorized to make, alter, or repeal our bylaws; and |
| • | establish advance notice requirements for nominations for elections to our board or for proposing matters that can be acted upon by stockholders at stockholder meetings. |
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law which, subject to certain exceptions, prohibits stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us. These anti-takeover provisions and other provisions under Delaware law could discourage, delay or prevent a transaction involving a change in control of our company, even if doing so would benefit our stockholders. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing so as to cause us to take certain corporate actions you desire.
If you purchase shares of common stock sold in this offering, you will incur immediate and substantial dilution.
If you purchase shares of common stock in this offering, you will incur immediate and substantial dilution in the pro forma as adjusted amount of $ per share, because the initial public offering price of $ is substantially higher than the pro forma as adjusted net book value per share of our outstanding common stock as of June 30, 2011. This dilution is due in large part to the fact that our earlier investors paid substantially less than the initial public offering price when they purchased their shares. Moreover, investors who purchase shares of common stock in this offering will contribute approximately % of our total funding to date but will own only % of our outstanding shares. In addition, you may also experience additional dilution upon future equity issuances, including upon conversion of any outstanding debt, or the exercise of stock options to purchase common stock granted to our employees, consultants and directors under our stock option and equity incentive plans. Please see the section entitled “Dilution.”
Because management has broad discretion as to the use of the net proceeds from this offering, you may not agree with how we use them, and such proceeds may not be applied successfully.
Our management will have considerable discretion over the use of proceeds from this offering. We intend to use the net proceeds from this offering:
| • | to fund the commercialization of Subsys and Dronabinol SG Capsule, if approved; |
| • | to fund the development of Dronabinol RT Capsule, Dronabinol Oral Solution, Dronabinol Inhalation Device, LEP-ETU and our other early-stage product candidates; and |
48
Table of Contents
| • | for working capital and general corporate purposes. |
In addition, a portion of the net proceeds may also be used to acquire or license products, technologies or businesses. However, we do not currently have any specific plans for use of the net proceeds from this offering, nor have we performed studies or made preliminary decisions with respect to the best use of the capital resources resulting from this offering. As such, our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not necessarily improve our operating results or enhance the value of our common stock. You will be relying on the judgment of our management concerning these uses and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. The failure of our management to apply these funds effectively could result in unfavorable returns and uncertainty about our prospects, each of which could cause the price of our common stock to decline.
We have never paid dividends on our capital stock, and we do not anticipate paying any cash dividends in the foreseeable future.
The continued operation and expansion of our business will require substantial funding. Accordingly, we do not anticipate that we will pay any cash dividends on shares of our common stock for the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our board of directors and will depend upon results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant. Accordingly, if you purchase shares in this offering, realization of a gain on your investment will depend on the appreciation of the price of our common stock, which may never occur. Investors seeking cash dividends in the foreseeable future should not purchase our common stock.
49
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. The forward-looking statements are contained principally in the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| • | our ability to successfully complete preclinical and clinical development of, obtain regulatory approval and acceptable DEA classifications for, and commercialize our product candidates on our expected timeframes or at all; |
| • | the content and timing of submissions to and decisions made by the FDA, the DEA and other regulatory agencies; |
| • | the safety and efficacy of our product candidates; |
| • | the benefits of our product candidates, especially in comparison to competitors’ products and product candidates; |
| • | the actions of our competitors and success of competing drugs that are or may become available; |
| • | the effects of government regulation and regulatory developments, and our ability and the ability of the third parties with whom we engage to comply with applicable regulatory requirements; |
| • | our expectations regarding the development of a REMS program for Subsys; |
| • | our ability to effectively manage our anticipated future growth; |
| • | our ability to successfully integrate the operations of Insys Therapeutics, Inc. and Insys Pharma, Inc., and realize any expected benefits from the Merger; |
| • | our ability to effectively remediate the material weakness in our internal control over financial reporting; |
| • | our ability to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act; |
| • | our ability to manufacture, or otherwise secure the manufacture of, sufficient amounts of our API for preclinical studies and clinical trials and, if approved, products for commercialization activities; |
| • | the performance of our manufacturers, CROs and other third parties, over whom we have limited control; |
| • | our ability to successfully execute on our commercialization strategy for any approved product candidate, including the performance of Mylan under our distribution agreement for Dronabinol SG Capsule and Dronabinol RT Capsule, and building a sufficient commercial organization to sell and market Subsys and certain of our other proprietary products; |
| • | our ability to realize any cost-savings associated with our anticipated plan to build a capital-efficient commercial organization to market Subsys and certain of our other proprietary product candidates; |
| • | the rate and degree of market acceptance of any approved product candidates; |
50
Table of Contents
| • | the size and growth of the potential markets for any approved product candidates and our ability to serve or impact the size of those markets; |
| • | our expectations regarding DEA quotas; |
| • | the anticipated regulatory pathways for our product candidates; |
| • | our ability to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of any of our product candidates that may be approved for sale; |
| • | our ability to operate our business without infringing the intellectual property rights of others; |
| • | our expectations regarding ongoing litigation related matters; |
| • | our anticipated use of the net proceeds from this offering; |
| • | our ability to attract and keep management and other key personnel; and |
| • | our ability to effectively transact business in foreign countries. |
In some cases, you can identify these statements by terms such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or the negative of those terms, and similar expressions. These forward-looking statements reflect our management’s beliefs and views with respect to future events and are based on estimates and assumptions as of the date of this prospectus and are subject to risks and uncertainties. We discuss many of these risks in greater detail under the heading “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Given these uncertainties, you should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this prospectus are excluded from the safe harbor protection provided by the Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act.
You should read this prospectus and the documents that we reference in this prospectus, and have filed as exhibits to the registration statement of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in this prospectus by these cautionary statements.
Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
51
Table of Contents
We estimate that we will receive net proceeds of approximately $ million (or approximately $ million if the underwriters’ over-allotment option is exercised in full) from the sale of the shares of common stock offered by us in this offering, based on an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The principal purposes of this offering are to obtain additional capital to support our operations, to create an active public market for our common stock and to facilitate our future access to the public equity markets. We intend to use the net proceeds from this offering as follows:
| • | approximately $15 million to fund the commercialization of Subsys and Dronabinol SG Capsule, if approved; |
| • | approximately $10 million to fund the development of Dronabinol RT Capsule, Dronabinol Oral Solution, Dronabinol Inhalation Device, LEP-ETU and our other early-stage product candidates; and |
| • | the remainder to fund working capital and other general corporate purposes. |
We may also use a portion of the net proceeds to in-license, acquire or invest in complementary businesses or products; however, we have no current commitments or obligations to do so. Pending their use, we plan to invest the net proceeds from this offering in short- and intermediate-term interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
We believe that the net proceeds from this offering and our existing cash and cash equivalents, together with interest thereon, will be sufficient to fund our operations for at least the next 12 months.
The amounts and timing of our actual expenditures will depend on numerous factors, including the regulatory action relating to our pending Dronabinol SG Capsule ANDA and Subsys NDA, the progress of our preclinical and clinical trials, and other development and commercialization efforts, as well as the amount of cash used in our operations. Therefore, the amount actually spent for the purposes described above may vary significantly. We also may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in the application of the net proceeds.
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our board of directors may deem relevant.
52
Table of Contents
The following table sets forth our cash and cash equivalents and capitalization as of June 30, 2011 on:
| • | an actual basis; |
| • | a pro forma basis to give effect to (1) the filing of our amended and restated certificate of incorporation which will occur upon the closing of this offering, (2) the conversion of our convertible preferred stock outstanding as of such date into 8,528,860 shares of our common stock which will occur automatically immediately prior to the closing of this offering, (3) the issuance of an additional $ million in aggregate principal amount of notes payable to trusts controlled by our Executive Chairman and principal stockholder subsequent to June 30, 2011 and (4) the conversion of $ million in aggregate principal amount of notes and accrued interest thereon owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming a conversion date of , 2011 and an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, immediately prior to the closing of this offering, which amount includes the $ million in aggregate principal amount of notes issued subsequent to June 30, 2011 and accrued interest on all notes payable through , 2011; and |
| • | a pro forma as adjusted basis to give further effect to the sale of shares of common stock by us in this offering at an assumed initial offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
The information in this table is illustrative only and our capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read the information in this table together with our financial statements and accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this prospectus.
| As of June 30, 2011 | ||||||||||||
| Actual | Pro Forma | Pro Forma As Adjusted (1) |
||||||||||
| (Unaudited) | ||||||||||||
| (In thousands, except per share data) |
||||||||||||
| Cash and cash equivalents |
$ | 17 | $ | $ | ||||||||
|
|
|
|
|
|
|
|||||||
| Debt, current and long-term |
$ | 43,705 | $ | $ | ||||||||
| Stockholders’ equity: |
||||||||||||
| Common stock, $0.0002145 par value: 750,000,000 shares authorized and 784,020 shares issued and outstanding, actual; 200,000,000 shares authorized and shares issued and outstanding, pro forma; 200,000,000 shares authorized and shares issued and outstanding, pro forma as adjusted |
— | |||||||||||
| Convertible preferred stock, $0.01 par value: 15,000,000 shares authorized and 14,864,607 shares issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted |
149 | |||||||||||
| Preferred stock, $0.001 par value: no shares authorized, issued or outstanding, actual; 10,000,000 shares authorized and no shares issued and outstanding, pro forma and pro forma as adjusted… |
— | |||||||||||
| Additional paid-in capital |
60,596 | |||||||||||
| Notes receivable from stockholders |
(21 | ) | ||||||||||
| Deficit accumulated during the development stage |
(94,859 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity (deficit) |
(34,135 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | 9,570 | $ | $ | ||||||||
|
|
|
|
|
|
|
|||||||
53
Table of Contents
| (1) | A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) each of the pro forma as adjusted cash and cash equivalents, additional paid-in capital, total stockholder’s equity and total capitalization by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
The number of shares of our common stock outstanding as of June 30, 2011 on an actual, pro forma and pro forma as adjusted basis excludes:
| • | 540,102 shares of our common stock issuable upon the exercise of outstanding options as of June 30, 2011 under our equity incentive plans, with a weighted average exercise price of $9.76 per share; |
| • | 1,079,133 shares of our common stock issuable upon the exercise of outstanding options as of June 30, 2011 under the Insys Pharma, Inc. equity incentive plan, with a weighted average exercise price of $1.83 per share; and |
| • | 3,000,000 shares of common stock reserved for future issuance under our 2011 equity incentive plan, 2011 non-employee directors’ stock award plan and 2011 employee stock purchase plan, each of which will become effective upon the signing of the underwriting agreement for this offering. |
54
Table of Contents
If you invest in our common stock in this offering, your ownership interest will be diluted to the extent of the difference between the initial public offering price per share of our common stock and the pro forma as adjusted net tangible book value (deficit) per share of our common stock after this offering. The historical net tangible book deficit of our common stock as of June 30, 2011 was $39.5 million, or $4.25 per share of common stock, based on the number of shares of common stock outstanding as of June 30, 2011, without giving effect to the conversion of our outstanding convertible preferred stock or outstanding notes and accrued interest thereon into shares of our common stock immediately prior to the closing of this offering. Historical net tangible book value (deficit) per share is determined by dividing the number of shares of our common stock outstanding as of June 30, 2011 into the amount of our total tangible assets (total assets less intangible assets) less total liabilities allocable to holders of our common stock.
The pro forma net tangible book value as of June 30, 2011 of $ million, or $ per share of our common stock, represents our historical net tangible book deficit as of June 30, 2011 after giving effect to (1) the filing of our amended and restated certificate of incorporation which will occur upon the closing of this offering, (2) the conversion of all of our outstanding convertible preferred stock into an aggregate of 8,528,860 shares of common stock which will occur automatically immediately prior to the closing of this offering (3) the issuance of an additional $ million in aggregate principal amount of notes payable to trusts controlled by our Executive Chairman and principal stockholder subsequent to June 30, 2011 and (4) the conversion of $ million in aggregate principal amount of notes and accrued interest thereon owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming a conversion date of , 2011 and an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, immediately prior to the closing of this offering, which amount includes the $ million in aggregate principal amount of notes issued subsequent to June 30, 2011 and accrued interest on all notes payable through , 2011.
Investors participating in this offering will incur immediate, substantial dilution. After giving further effect to the sale of shares of common stock by us in this offering at an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of June 30, 2011 would have been $ million, or $ per share. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to existing stockholders, and an immediate dilution of $ per share to investors participating in this offering. The following table illustrates this per share dilution:
| Assumed initial public offering price per share |
$ | |||||||
| Historical net tangible book value (deficit) per share as of June 30, 2011 |
$ | (4.25 | ) | |||||
| Pro forma increase in net tangible book value per share as of June 30, 2011 attributable to the conversion of convertible preferred stock |
$ | — | ||||||
| Pro forma in net tangible book value (deficit) per share as of June 30, 2011 attributable to the issuance of additional notes payable and the conversion of outstanding notes and accrued interest |
$ | |||||||
|
|
|
|||||||
| Pro forma net tangible book value (deficit) per share as of June 30, 2011 |
$ | |||||||
| Increase in pro forma net tangible book value per share attributable to investors participating in this offering |
$ | |||||||
|
|
|
|||||||
| Pro forma as adjusted net tangible book value per share after this offering |
$ | |||||||
|
|
|
|||||||
| Pro forma as adjusted dilution per share to investors participating in this offering |
$ | |||||||
|
|
|
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted net tangible book value per share after this offering by approximately $ per share and the dilution in pro forma as adjusted net tangible book value per share to
55
Table of Contents
investors participating in this offering by approximately $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters exercise in full their over-allotment option to purchase additional shares at the assumed initial public offering price of $ per share, the pro forma as adjusted net tangible book value per share after the offering would be $ per share, the increase in pro forma net tangible book value per share to existing stockholders would be $ per share and the dilution to new investors would be $ per share.
The following table summarizes, on the pro forma as adjusted basis described above, as of June 30, 2011, the differences between the number of shares of common stock purchased from us, the total effective cash consideration paid to us, and the average price per share paid to us by our existing stockholders and by investors participating in this offering at an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, before deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| Shares Purchased | Total Consideration | Average Price Per Share |
||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||
| Existing stockholders before this offering |
% | $ | % | $ | ||||||||||||||
| Investors participating in this offering |
% | % | ||||||||||||||||
|
|
|
|
|
|
|
|
||||||||||||
| Total |
100 | % | $ | 100 | % | $ | ||||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) the total consideration paid by investors participating in this offering by approximately $ million, and increase (decrease) the percent of total consideration paid by investors participating in this offering by %, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters’ over-allotment option to purchase additional shares is exercised in full, the number of shares of common stock held by existing stockholders will be further reduced to % of the total number of shares of common stock to be outstanding after this offering, and the number of shares of common stock held by investors participating in this offering will be further increased to shares or % of the total number of shares of common stock to be outstanding after this offering.
The number of shares of our common stock outstanding as of June 30, 2011 on an actual, pro forma and pro forma as adjusted basis excludes:
| • | 540,102 shares of our common stock issuable upon the exercise of outstanding options as of June 30, 2011 under our equity incentive plans, with a weighted average exercise price of $9.76 per share; and |
| • | 1,079,133 shares of our common stock issuable upon the exercise of outstanding options as of June 30, 2011 under the Insys Pharma, Inc. equity incentive plan, with a weighted average exercise price of $1.83 per share. |
In addition, effective upon the signing of the underwriting agreement for this offering, an aggregate of 3,000,000 shares of our common stock will be reserved for issuance under our 2011 equity incentive plan, our 2011 non-employee directors’ stock award plan and our 2011 employee stock purchase plan, respectively, and these share reserves will also be subject to automatic annual increases in accordance with the terms of the plans. Furthermore, we may choose to raise additional capital through the sale of equity or convertible debt securities due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that any of these options are exercised, new stock awards are issued under our equity incentive plans or we issue additional shares of common stock or other equity or convertible debt securities in the future, investors participating in this offering will experience further dilution.
56
Table of Contents
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED
FINANCIAL INFORMATION
Introductory Note
On October 29, 2010, we entered into an Agreement and Plan of Merger with Insys Therapeutics, Inc., a Delaware corporation (the entity now known as Insys Pharma), and ITNI Merger Sub Inc., our wholly-owned subsidiary and a Delaware corporation. On November 8, 2010, pursuant to the Agreement and Plan of Merger, ITNI Merger Sub Inc. merged with and into Insys Therapeutics, Inc., and Insys Therapeutics, Inc. survived as our wholly-owned subsidiary. We refer to this transaction herein as the Merger. All of the outstanding share capital of Insys Therapeutics, Inc. was exchanged for newly-issued shares of common stock and convertible preferred stock of NeoPharm. As a result of the Merger, Insys Therapeutics, Inc. became a wholly-owned subsidiary of NeoPharm and changed its name to Insys Pharma, Inc. NeoPharm then changed its name to Insys Therapeutics, Inc.
The Merger was accounted for as a reverse acquisition under the provisions of Accounting Standards Codification, or ASC, 805, Business Combinations. Pursuant to the Merger agreement, all of the common stock of the entity now known as Insys Pharma prior to the Merger was exchanged for 319,667 shares of NeoPharm common stock and 14,864,607 shares of newly-created NeoPharm convertible preferred stock. The convertible preferred stock is convertible into common stock on a one-for-0.57 basis and, until converted, will be entitled to the voting, dividend and liquidation rights of the same number of shares of common stock into which it is convertible. Immediately subsequent to the Merger, the former NeoPharm stockholders owned 5% of the combined entity on an as-converted basis.
As additional consideration, the NeoPharm board of directors approved the distribution, immediately after the Merger, of non-transferable contingent payment rights, or CPRs, to its stockholders of record as of November 5, 2010. These rights entitle the pre-Merger stockholders of NeoPharm to receive cash payments aggregating $20.0 million (equivalent to $0.70402 per share) if, prior to the five year anniversary of the Merger, the FDA approves a new drug application for any one or more of the NeoPharm drugs that were under development at the time of the Merger. The distribution would be payable within nine months of FDA approval. The fair value of this contingent payment was determined to be $1.8 million based on the assumed probability of this event. See Note 10 to the consolidated financial statements appearing elsewhere in this prospectus.
Basis of Presentation
The following unaudited pro forma condensed consolidated financial information for the year ended December 31, 2010 and the six months ended June 30, 2011 was prepared in accordance with SEC Regulation S-X, Article 11, giving effect to the accounting acquisition of NeoPharm, as described above, as well as certain related pro forma adjustments, all of which are described in the notes accompanying this unaudited pro forma condensed consolidated financial information.
The determination of the accounting acquirer was based on a review of the pertinent facts and circumstances. The identification of the acquiring entity in this instance is subjective and was based on a number of factors outlined in ASC 805-10-55-12, which are as follows:
| • | the relative voting rights in the combined entity after the business combination; |
| • | the existence of a large minority voting interest in the combined entity if no other owner or organized group of owners has a significant voting interest; |
| • | the composition of the governing board of directors of the combined entity; |
| • | the composition of the senior management of the combined entity; |
| • | the terms of the exchange of equity interests; |
| • | the relative size of each entity; |
57
Table of Contents
| • | which party initiated the transaction; and |
| • | other qualitative factors. |
After consideration of the factors outlined above, it was determined that Insys Therapeutics, Inc. (entity now known as Insys Pharma) was the accounting acquirer in this transaction based on the following:
| • | Immediately following the Merger, Insys Therapeutics, Inc. stockholders held a 95% stake on an as-converted basis in the combined company and held a greater than 50% ownership in the combined entity after giving consideration to the exercise of vested, in-the-money stock options, leading to the conclusion that this criterion favored Insys Therapeutics, Inc. as the accounting acquirer. |
| • | Immediately following the consummation of the Merger, Insys Therapeutics, Inc.’s board members comprised all of the combined company’s board of directors, leading to the conclusion that this criterion favored Insys Therapeutics, Inc. as the accounting acquirer. |
| • | Immediately following the consummation of the Merger, Insys Therapeutics, Inc. management team members comprised all of the senior management positions of the combined company, leading to the conclusion that this criterion favored Insys Therapeutics, Inc. as the accounting acquirer. |
| • | Ownership interests received by the parties in the combined company were the result of a value-for-value exchange, and given neither companies’ stock was actively and readily traded, any valuation would have been inherently subjective and may not have provided a clear indication as to a premium (if any) being paid by either party, leading to the conclusion that this was a neutral criterion not favoring either entity as the accounting acquirer. |
| • | Income statement and book value of assets indicated that Insys Therapeutics, Inc. was more significant, leading to the conclusion that this criterion favored Insys Therapeutics, Inc. as the accounting acquirer. |
| • | Insys Therapeutics, Inc. initiated the transaction discussions, led the transaction negotiations, and the combined company’s operations were anticipated to be headquartered at Insys Therapeutics, Inc.’s location going forward, leading to the conclusion that this criterion favored Insys Therapeutics, Inc. as the accounting acquirer. |
The Merger was accounted for using the “acquisition method” of accounting. Under the acquisition method of accounting, the purchase price is required to be allocated to the underlying tangible and intangible assets acquired and liabilities assumed based on their respective fair market values. Any purchase price in excess of the fair market value of the acquired tangible and intangible assets is required to be allocated to goodwill in our condensed consolidated balance sheet and amounted to $0.1 million.
We performed appraisals necessary to derive preliminary fair values of the tangible and intangible assets acquired and liabilities assumed, the amounts of assets and liabilities arising from contingencies, and the amount of goodwill to be recognized as of the Merger date, and the related preliminary allocation of the purchase price. The purchase price for accounting purposes equals the fair value of the outstanding shares of NeoPharm just prior to the Merger. While NeoPharm’s outstanding common stock options remain outstanding after the Merger, their value as of the Merger date was de minimis. We believe diversifying NeoPharm’s drug product candidates with our drug product candidates to enable the combined company to better access the capital markets, and gaining access to our management and board with significant commercial experience in cancer supportive care areas, provided the primary motivation for NeoPharm to enter the Merger.
The unaudited pro forma condensed consolidated statements of operations information for the six months ended June 30, 2011 and for the year ended December 31, 2010 are based on the consolidated
58
Table of Contents
statements of operations of Insys Therapeutics, Inc. for the respective periods and the unaudited consolidated financial statements of NeoPharm for the period from January 1, 2010 to November 8, 2010 (the date of the Merger), and gives effect to:
| • | the Merger; |
| • | the issuance after each period presented of additional notes payable to trusts controlled by our Executive Chairman and principal stockholder; |
| • | the conversion of the resulting $ million in aggregate principal amount of notes and actual accrued interest thereon through , 2011 owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus; and |
| • | the conversion of all outstanding shares of our preferred stock into 8,528,860 shares of common stock, |
as if all such transactions had occurred on January 1, 2010.
The unaudited pro forma condensed consolidated financial information presented for the year ended December 31, 2010 is preliminary and subject to change, is provided for illustrative purposes only and is not necessarily indicative of the results that would have been achieved had the Merger been completed as of the date indicated or that may be achieved in future periods. The unaudited pro forma condensed consolidated statement of operations for the year ended December 31, 2010 does not include:
| • | the effects of any non-recurring costs or income/gains resulting from: |
| • | professional fees and other direct or indirect costs incurred in connection with the Merger, |
| • | accelerated stock-based compensation expense resulting from the Merger, or |
| • | income tax benefits resulting directly from the Merger; |
| • | the costs related to restructuring or integration activities that we may implement subsequent to the closing of the Merger; and |
| • | the realization of any cost savings from operating efficiencies, synergies or other restructurings that may result from the Merger. |
This unaudited pro forma condensed consolidated financial information should be read in conjunction with the historical consolidated financial statements of Insys Therapeutics, Inc. and NeoPharm and the related notes thereto and other information included elsewhere in this prospectus.
59
Table of Contents
An unaudited pro forma condensed consolidated balance sheet as of June 30, 2011 that gives effect to the above transactions is not presented primarily because the Merger is already fully reflected in our actual June 30, 2011 balance sheet. The impact of the assumed conversion of all outstanding shares of our preferred stock into 8,528,860 shares of our common stock as of June 30, 2011 would result in a reclassification within our stockholders’ equity (deficit) of $0.1 million between capital accounts. The impact of the assumed issuance of additional notes and conversion of the resulting outstanding notes and accrued interest into shares of our common stock would result in additional cash of $ million and a reclassification of $ million of debt and accrued interest into stockholders’ equity (deficit).
INSYS THERAPEUTICS, INC.
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS
SIX MONTHS ENDED JUNE 30, 2011
(in thousands, except share and per share data)
| Insys Therapeutics, Inc. |
Pro Forma Adjustments See Note 2 |
Pro Forma | ||||||||||
| Revenues |
$ | — | $ | — | $ | — | ||||||
| Operating expenses: |
||||||||||||
| Research and development |
3,807 | — | 3,807 | |||||||||
| General and administrative |
4,595 | — | 4,595 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
8,402 | — | 8,402 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations: |
(8,402 | ) | — | (8,402 | ) | |||||||
| Other income |
102 | — | 102 | |||||||||
| Interest expense, net. |
(888 | ) | 888 | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(9,188 | ) | $ | 888 | (8,300 | ) | ||||||
|
|
|
|||||||||||
| Net loss allocable to preferred stockholders |
8,414 | — | ||||||||||
|
|
|
|
|
|||||||||
| Net loss allocable to common stockholders |
$ | (774 | ) | $ | (8,300 | ) | ||||||
|
|
|
|
|
|||||||||
| Loss per common share |
$ | (0.99 | ) | $ | ||||||||
|
|
|
|
|
|||||||||
| Weighted average common shares outstanding |
784,020 | |||||||||||
|
|
|
|
|
|
|
|||||||
60
Table of Contents
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED FINANCIAL INFORMATION
INSYS THERAPEUTICS, INC.
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS
FISCAL YEAR ENDED DECEMBER 31, 2010
(in thousands, except share and per share data)
| Insys Therapeutics, Inc. |
NeoPharm, Inc. | Pro Forma Adjustments (See Note 2) |
Pro Forma Combined |
|||||||||||||
| Revenues |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
10,428 | 2,921 | (105 | ) | 13,244 | |||||||||||
| General and administrative |
3,539 | 2,140 | (414 | ) | 5,265 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
13,967 | 5,061 | (519 | ) | 18,509 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(13,967 | ) | (5,061 | ) | 519 | (18,509 | ) | |||||||||
| Other income |
797 | 735 | — | 1,532 | ||||||||||||
| Interest income (expense), net |
(1,148 | ) | 55 | 1,150 | 57 | |||||||||||
| Income tax benefit |
575 | — | (575 | ) | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(13,743 | ) | $ | (4,271 | ) | $ | 1,094 | (16,920 | ) | |||||||
|
|
|
|
|
|||||||||||||
| Net loss allocable to preferred stockholders |
13,144 | — | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Net loss allocable to common stockholders |
$ | (599 | ) | $ | (16,920 | ) | ||||||||||
|
|
|
|
|
|||||||||||||
| Loss per common share |
$ | (1.54 | ) | $ | ||||||||||||
|
|
|
|
|
|||||||||||||
| Weighted average common shares outstanding |
388,449 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||
NOTES TO UNAUDITED PRO FORMA CONDENSED CONSOLIDATED FINANCIAL INFORMATION
NOTE 1 – PRELIMINARY PURCHASE PRICE ALLOCATION — NEOPHARM
The assets acquired and liabilities assumed in the Merger are as follows (in thousands):
| Amount | ||||
| Cash |
$ | 143 | ||
| Prepaid and other current assets |
429 | |||
| Fixed assets |
144 | |||
| Other assets |
371 | |||
| Identifiable intangible assets |
5,300 | |||
| Goodwill |
103 | |||
|
|
|
|||
| Total assets acquired |
$ | 6,490 | ||
|
|
|
|||
| Accounts payable and accrued expenses |
$ | (1,693 | ) | |
| Unfavorable lease liability |
(120 | ) | ||
| Deferred tax liability |
(575 | ) | ||
| Contingent liability to NeoPharm stockholders |
(1,829 | ) | ||
|
|
|
|||
| Total liabilities acquired |
$ | (4,217 | ) | |
|
|
|
|||
| Net assets acquired |
$ | 2,273 | ||
|
|
|
|||
61
Table of Contents
We have assessed the fair values of the acquired assets and assumed liabilities and allocated the purchase price accordingly. This valuation resulted in the recording of in-process research and development, or IPR&D, as an intangible long-lived asset in the amount of $5.3 million. The fair value of the IPR&D was determined primarily through the use of the cost approach. The cost approach relies on historical costs incurred adjusted for estimated wasted efforts and taxes. A deferred tax liability of approximately $0.6 million was generated as a result of purchase accounting at the Merger date. Accordingly, we released $0.6 million of the valuation allowance on our deferred tax assets which created an income tax benefit as of the date of the Merger and offsets this deferred tax liability. The fair value of the contingent consideration of $1.8 million was determined based on the estimated probability of any payment being made to the prior NeoPharm stockholders in 2015, discounted to present value at a rate of 15%. Any subsequent changes in the estimated fair value of this contingent consideration will be recorded in our Statement of Operations.
The $2.3 million fair value of NeoPharm at the time of the Merger was derived from a valuation that was conducted for the post-Merger combination of NeoPharm and Insys Therapeutics, Inc. This valuation exercise utilized the Income Approach using Probability Weighted Expected Return Method, or PWERM. This approach involves the estimation of future potential outcomes for the company, as well as values and probabilities associated with each respective potential outcome. The fair value of the post-Merger combined entity was determined to be $4.88 per common share, and this fair value per common share was then applied to the 464,353 shares of NeoPharm common stock outstanding at the time of the Merger.
NOTE 2 – PRO FORMA ADJUSTMENTS
The research and development and general and administrative pro forma adjustments for the year ended December 31, 2010 relate to the:
| • | additional amortization for the period from January 1, 2010 through November 8, 2010 that would have been recorded on the unfavorable lease liability resulting from the Merger in the amount of ($18,000); |
| • | the elimination of stock-based compensation expense recorded in the NeoPharm historical results relating to acceleration of the vesting of the NeoPharm stock options upon the Merger in the amount of ($0.2 million); and |
| • | the elimination of direct Merger-related expenses recorded in both the Insys and NeoPharm historical results in the amount of ($0.3 million). |
No pro forma amortization is reflected for the IPR&D in either period as the related product candidates are not expected to be in commercial production within one year of the Merger date.
The interest expense, net pro forma adjustments, in both periods represents the elimination of interest expense that would have been avoided due to the assumed conversion of the related notes into shares of common stock.
The income tax benefit pro forma adjustment for the year ended December 31, 2010 represents the elimination of a deferred income tax benefit recorded in the Insys historical results related to the reduction of its income tax valuation allowance as a direct result of the Merger.
The weighted average shares outstanding pro forma adjustment for the year ended December 31, 2010 reflects:
| • | the deemed issuance of 464,353 common shares to the NeoPharm stockholders on the Merger date; |
| • | the conversion of preferred shares into 8,528,860 shares of common stock; and |
62
Table of Contents
| • | the conversion of $ million in aggregate principal amount of notes and actual accrued interest thereon through , 2011 owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus. |
The weighted average shares outstanding pro forma adjustment for the six months ended June 30, 2011 reflects:
| • | the conversion of all outstanding shares of our preferred stock into 8,528,860 shares of common stock; and |
| • | the conversion of $ million in aggregate principal amount of notes and actual accrued interest thereon through , 2011 owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus. |
While the conversion of the preferred shares into common shares for both periods did increase the pro forma weighted average shares outstanding, it had no impact on earnings per share because the historical computation of per share losses is done under the two class method which essentially treats the preferred shares as converted common shares.
63
Table of Contents
The following tables set forth our selected financial data. The selected statements of operations data for the years ended December 31, 2010, 2009 and 2008 and the selected balance sheet data as of December 31, 2010 and 2009 are derived from our audited financial statements appearing elsewhere in this prospectus. The selected statements of operations data for the years ended December 31, 2007 and 2006 and the selected balance sheet data as of December 31, 2008, 2007 and 2006 are derived from our audited financial statements not included in this prospectus. The selected statement of operations data for the six months ended June 30, 2011 and 2010, and for the period from October 2002 (date of inception) to June 30, 2011 and the selected balance sheet data as of June 30, 2011 have been derived from our unaudited consolidated financial statements appearing elsewhere in this prospectus. The unaudited financial statements have been prepared on a basis consistent with our audited financial statements included in this prospectus and include, in our opinion, all adjustments, consisting only of normal recurring adjustments, necessary to state fairly our financial position as of June 30, 2011 and results of operations for the six months ended June 30, 2011 and 2010. You should read this selected financial data in conjunction with the financial statements and related notes and the information under the headings “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Unaudited Pro Forma Condensed Consolidated Financial Information” appearing elsewhere in this prospectus. Our historical results are not necessarily indicative of our future results.
On October 29, 2010, we entered into an Agreement and Plan of Merger with Insys Therapeutics, Inc., a Delaware corporation, and ITNI Merger Sub Inc., our wholly-owned subsidiary and a Delaware corporation. On November 8, 2010, pursuant to the Agreement and Plan of Merger, ITNI Merger Sub Inc. merged with and into Insys Therapeutics, Inc., and Insys Therapeutics, Inc. survived as our wholly-owned subsidiary. We refer to this transaction as the Merger. Following the Merger, our wholly-owned subsidiary, Insys Therapeutics, Inc., changed its name to Insys Pharma, Inc. and we changed our name to Insys Therapeutics, Inc. In connection with the Merger, all of the outstanding shares of common stock of Insys Pharma prior to the Merger were exchanged for 319,667 shares of our common stock and 14,864,607 shares of our newly-created convertible preferred stock. Each share of our convertible preferred stock is convertible into 0.57 shares of our common stock. As a result of the Merger, 95% of our common stock on an as-converted basis was held by the then-existing stockholders of Insys Pharma. Since Insys Pharma is the acquiring entity for accounting purposes, the financial statements for all periods up to and including the November 8, 2010 Merger date are the financial statements of the entity that is now our subsidiary, Insys Pharma. The financial statements for all periods subsequent to the November 8, 2010 Merger date are the consolidated financial statements of Insys Therapeutics, Inc. and Insys Pharma. However, for all periods, the financial statements are labeled “Insys Therapeutics, Inc.” financial statements. In addition, the audited financial statements of NeoPharm for the years ended December 31, 2009 and 2008 and the unaudited financial statements for the nine months ended September 30, 2010 and 2009 are also included in this prospectus.
The selected unaudited pro forma condensed consolidated statement of operations data for the six months ended June 30, 2011 and for the year ended December 31, 2010 below is based on the historical consolidated statements of operations of Insys Therapeutics, Inc. and NeoPharm, giving effect to the Merger, the conversion of our convertible preferred stock outstanding as of June 30, 2011 and December 31, 2010 into 8,528,860 shares of our common stock and the issuance of an additional $ million in aggregate principal amount of notes payable to trusts controlled by our Executive Chairman and principal stockholder subsequent to June 30, 2011 and the conversion of $ million in aggregate principal amount of notes and actual accrued interest thereon through , 2011 owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, as if such transactions had occurred on January 1, 2010. The unaudited pro forma condensed consolidated statement of operations data is based on the estimates and assumptions set forth in the notes to the unaudited pro forma condensed consolidated financial statement. These estimates and assumptions are preliminary and subject to change, and have
64
Table of Contents
been made solely for the purposes of developing such pro forma information. The selected unaudited pro forma condensed consolidated statement of operations data is not necessarily indicative of the combined results of operations to be expected in any future period or the results that actually would have been realized had the entities been a single entity during the period presented.
| Pro Forma | Actual | Pro Forma | Actual | |||||||||||||||||||||||||||||||||||||
| Six Months Ended June 30, 2011 |
Period from October, 2002 (inception) to June 30, 2011 |
|||||||||||||||||||||||||||||||||||||||
| Six Months Ended June 30, |
Year Ended December 31, | |||||||||||||||||||||||||||||||||||||||
| 2011 | 2010 | 2010 | 2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||||||||||||||||||||||||
| (Unaudited) |
||||||||||||||||||||||||||||||||||||||||
| (In thousands, except share and per share data) | ||||||||||||||||||||||||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||||||||||||||||||||||
| Revenues |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||||||||||||||
| Research and development |
3,807 | 61,300 | 3,807 | 5,240 | 13,244 | 10,428 | 8,982 | 14,729 | 13,723 | 5,707 | ||||||||||||||||||||||||||||||
| General and administrative |
4,595 | 27,048 | 4,595 | 2,015 | 5,265 | 3,539 | 4,504 | 10,221 | 2,999 | 571 | ||||||||||||||||||||||||||||||
| Loss on settlement of vendor dispute |
— | 1,104 | — | — | — | — | — | 1,104 | — | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Total operating expenses |
8,402 | 89,452 | 8,402 | 7,255 | 18,509 | 13,967 | 13,486 | 26,054 | 16,722 | 6,278 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Loss from operations: |
(8,402 | ) | (89,452 | ) | (8,402 | ) | (7,255 | ) | (18,509 | ) | (13,967 | ) | (13,486 | ) | (26,054 | ) | (16,722 | ) | (6,278 | ) | ||||||||||||||||||||
| Other income |
102 | 1,710 | 102 | 26 | 1,532 | 797 | 31 | 780 | — | — | ||||||||||||||||||||||||||||||
| Interest expense, net. |
— | (7,692 | ) | (888 | ) | (473 | ) | 57 | (1,148 | ) | (999 | ) | (1,913 | ) | (1,739 | ) | (727 | ) | ||||||||||||||||||||||
| Income tax benefit |
— | 575 | — | — | — | 575 | — | — | — | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Net loss |
(8,300 | ) | $ | (94,859 | ) | (9,188 | ) | (7,702 | ) | (16,920 | ) | (13,743 | ) | (14,454 | ) | (27,187 | ) | (18,461 | ) | (7,005 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Net loss allocable to preferred stockholders |
— | 8,414 | 7,425 | — | 13,144 | 13,932 | 26,205 | 17,794 | 6,752 | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Net loss allocable to common stockholders |
$ | (8,300 | ) | $ | (774 | ) | $ | (277 | ) | $ | (16,920 | ) | $ | (599 | ) | $ | (522 | ) | $ | (982 | ) | $ | (667 | ) | $ | (253 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Basic and diluted net loss per common share |
$ | $ | (0.99 | ) | $ | (0.87 | ) | $ | $ | (1.54 | ) | $ | (7.05 | ) | $ | (19.09 | ) | $ | (25.58 | ) | $ | (11.19 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Weighted average common shares outstanding, basic and diluted (1) |
784,020 | 319,423 | 388,449 | 74,063 | 51,438 | 26,080 | 22,617 | |||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| (1) | Please see Note 2 to our audited financial statements appearing elsewhere in this prospectus for an explanation of the method used to calculate the net loss per common share and the number of common shares used in the computation of historical per share amounts. |
| As of June 30, 2011 |
As of December 31, | |||||||||||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||
| (In thousands) | ||||||||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||||||
| Cash and cash equivalents |
$ | 17 | $ | 64 | $ | 143 | $ | 528 | $ | 9 | $ | 17 | ||||||||||||
| Total current assets |
1,524 | 1,147 | 953 | 3,997 | 43 | 21 | ||||||||||||||||||
| Total assets |
14,644 | 14,755 | 8,241 | 5,553 | 9,952 | 1,707 | ||||||||||||||||||
| Total current liabilities, including debt |
46,381 | 37,970 | 23,387 | 5,046 | 9,748 | 4,631 | ||||||||||||||||||
| Total long term debt |
— | — | — | 14,888 | 29,465 | 8,623 | ||||||||||||||||||
| Total liabilities |
48,779 | 40,277 | 23,772 | 20,070 | 39,213 | 13,456 | ||||||||||||||||||
| Total stockholders’ equity (deficit) |
(34,135 | ) | (25,522 | ) | (15,531 | ) | (14,517 | ) | (29,261 | ) | (11,749 | ) | ||||||||||||
65
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with “Selected Financial Data” and our financial statements and related notes included elsewhere in this prospectus. This discussion and analysis and other parts of this prospectus contain forward-looking statements based upon current beliefs, plans and expectations that involve risks, uncertainties and assumptions. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under “Risk Factors” and elsewhere in this prospectus. You should carefully read the “Risk Factors” section of this prospectus to gain an understanding of the important factors that could cause actual results to differ materially from our forward-looking statements. Please see the section entitled “Special Note Regarding Forward-Looking Statements.”
Overview
We are a specialty pharmaceutical company that develops and seeks to commercialize innovative pharmaceutical products that target the unmet needs of cancer patients, with an initial focus on cancer-supportive care. We focus our research and development efforts on product candidates that utilize innovative formulations to address the clinical shortcomings of existing commercial pharmaceutical products. We have two product candidates, our proprietary fentanyl spray Subsys and our generic Dronabinol SG Capsule, under review for marketing approval by the FDA. We intend to build a capital-efficient commercial organization to market Subsys and our other proprietary products, if approved.
In March 2011, we submitted an NDA to the FDA for Subsys, a sublingual spray for the treatment of BTCP in opioid-tolerant patients. The FDA notified us in May 2011 that it had accepted the NDA for review and initially assigned a PDUFA goal date of January 4, 2012 for its review of the NDA. Subsys is a proprietary, single-use product that delivers fentanyl, an opioid analgesic, in seconds for transmucosal absorption underneath the tongue.
In June 2010, we submitted an amendment to our ANDA for Dronabinol SG Capsule. Dronabinol SG Capsule is a dronabinol soft gelatin capsule intended to be a generic equivalent to Marinol, a currently approved treatment for CINV and appetite stimulation in patients with AIDS. Dronabinol SG Capsule is the first in our family of dronabinol products. If Dronabinol SG Capsule is approved by the FDA, we intend to submit an ANDA to the FDA for a proprietary dronabinol soft gel formulation that is stable at room temperature, which we refer to as Dronabinol RT Capsule. Our most advanced proprietary formulation of dronabinol, Dronabinol Oral Solution, is an orally administered liquid formulation. We have completed an end-of-Phase 2 meeting with the FDA and plan to initiate a pivotal bioequivalence study for this product candidate in the second half of 2011. We produce our clinical and commercial supply of dronabinol API in our U.S.-based, state-of-the-art dronabinol manufacturing facility, which we believe provides us with a significant competitive advantage.
In addition to our cancer-supportive care products, we are developing proprietary cancer therapeutics, the most advanced of which is LEP-ETU, which recently completed a Phase 2 clinical trial of 70 patients with metastatic breast cancer. LEP-ETU is a proprietary NeoLipid liposomal, or microscopic membrane-like structure created from lipids, formulation that incorporates paclitaxel, the active ingredient in the cancer chemotherapy drugs Taxol and Abraxane.
Insys Therapeutics, Inc. was incorporated in the state of Delaware in October 2002, and we maintain our headquarters in Phoenix, Arizona. On November 8, 2010, Insys Therapeutics, Inc. effected the Merger in a transaction that was accounted for as a reverse acquisition. All of the outstanding share capital of Insys Therapeutics, Inc. was exchanged for newly-issued shares of common stock and convertible preferred stock of NeoPharm. As a result of the Merger, Insys Therapeutics, Inc. became a wholly-owned subsidiary of NeoPharm and changed its name to Insys Pharma. As of immediately prior to the Merger, our Executive Chairman, Dr. John N. Kapoor, was the chairman of NeoPharm’s board of directors and beneficial holder of more than 5% of NeoPharm’s common stock. As a result of the Merger, 95% of our common stock, on an as-converted basis, was held by the then-existing
66
Table of Contents
stockholders of Insys Pharma. NeoPharm then changed its name to Insys Therapeutics, Inc. Since Insys Pharma is the acquiring entity for accounting purposes, the financial statements for all periods up to and including the November 8, 2010 Merger date are the financial statements of the entity that is now Insys Pharma. The financial statements for all periods subsequent to the November 8, 2010 Merger date are the consolidated financial statements of Insys Therapeutics, Inc. and Insys Pharma. All of the discussion in this Management’s Discussion and Analysis of Financial Condition and Results of Operations will reflect this financial presentation of these entities. However, for all periods, the financial statements are labeled “Insys Therapeutics, Inc.” financial statements.
We are a development-stage company. To date, we have generated no revenues and have incurred significant losses. We have financed our operations and internal growth through the issuance of promissory notes to The John N. Kapoor Trust and the Kapoor Children 1992 Trust, some of which have been converted into shares of our common stock, as well as through the sale of shares of our common stock. These trusts are controlled by our founder, Executive Chairman and principal stockholder, Dr. John N. Kapoor. We have devoted substantially all of our efforts to research and development activities, including preclinical studies and clinical trials. Our net loss was $9.2 million for the six months ended June 30, 2011 and $13.7 million for the year ended December 31, 2010. As of June 30, 2011, we had an accumulated deficit of $94.9 million. This accumulated deficit is attributable primarily to our research and development activities.
We are focusing our efforts and capital resources on obtaining approval for Subsys and Dronabinol SG Capsule, developing our other proprietary product candidates, and commercializing Subsys and our other proprietary products through a capital-efficient commercial organization and Dronabinol SG Capsule and Dronabinol RT Capsule through a distribution agreement with a leading generic pharmaceutical company.
We are subject to risks and uncertainties common to biopharmaceutical companies in the development stage, including, but not limited to, obtaining regulatory approval and acceptable DEA classification for our product candidates, dependence upon market acceptance of any approved products, risks associated with intellectual property, pricing and reimbursement, intense competition, development of markets and distribution channels and dependence on key personnel.
Our ultimate success is dependent upon our ability to successfully develop, obtain approval for and market our product candidates. We anticipate we will continue to incur net losses for at least the next several years as we:
| • | incur expenses for the regulatory approval of Subsys and our Dronabinol SG Capsule and the development of our other product candidates, including Dronabinol Oral Solution; |
| • | establish sales and marketing capabilities for the anticipated U.S. commercial launch of Subsys; |
| • | expand our corporate infrastructure to support growth and commercialization activities and transition to operating as a public company; |
| • | increase general and administrative expenses associated with the commercialization of our Dronabinol SG Capsule and Dronabinol RT Capsule product candidates, if approved, through a potential distribution agreement with a leading generic pharmaceutical company; and |
| • | advance the clinical development of LEP-ETU and other product candidates either currently in our pipeline or that we may in-license or acquire in the future. |
As of June 30, 2011, we had cash and cash equivalents of $17,000. We believe that the net proceeds from this offering and our existing cash and cash equivalents, together with interest thereon, will be sufficient to fund our operations for at least 12 months. However, we may need additional financing in the event that we do not obtain regulatory approval for our product candidates when expected, or if approved, the future sales of our product candidates do not generate sufficient revenues to fund operations. Failure to raise capital if and when needed would have a negative impact on our financial condition and our ability to pursue our business strategies.
67
Table of Contents
Basis of Presentation
Revenues
To date, we have generated no revenues. We do not expect to begin generating any revenues unless any of our product candidates receive marketing approval from the FDA.
Research and Development Expenses
Research and development expenses, including those for NeoPharm, consist of costs associated with our preclinical studies and clinical trials, and other expenses related to our drug development efforts. Our research and development expenses consist primarily of:
| • | external research and development expenses incurred under agreements with third-party CROs and investigative sites, third-party manufacturers and consultants; |
| • | employee-related expenses, which include salaries, benefits and stock-based compensation for the personnel involved in our preclinical and clinical drug development activities; and |
| • | facilities, depreciation and other allocated expenses, and equipment and laboratory supplies. |
To date, our research and development efforts have been focused primarily on product candidates from our fentanyl, dronabinol and LEP-ETU programs. Research and development expenses for product candidates historically developed by NeoPharm, including expenses relating to development of LEP-ETU, have been consolidated since the effective date of the Merger on November 8, 2010. From inception to June 30, 2011, we have incurred $61.3 million in total research and development expenses.
The following table provides a breakdown of our research and development expenses over the past three years, and the six months ended June 30, 2011 and 2010 (in millions):
| Cumulative Period from January 1, 2007 to June 30, 2011(1) |
Six Months Ended June 30, |
Year Ended December 31, | ||||||||||||||||||||||
| 2011 | 2010 | 2010 | 2009 | 2008 | ||||||||||||||||||||
| Fentanyl(2) |
$ | 26.0 | $ | 0.5 | $ | 3.1 | $ | 5.7 | $ | 6.4 | $ | 4.6 | ||||||||||||
| Dronabinol(2) |
11.7 | 0.6 | 1.0 | 1.7 | 2.2 | 5.5 | ||||||||||||||||||
| LEP-ETU(2) |
0.8 | 0.8 | — | — | — | — | ||||||||||||||||||
| Internal research and development costs(3) |
12.9 | 1.9 | 1.1 | 3.0 | 0.4 | 4.6 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 51.4 | $ | 3.8 | $ | 5.2 | $ | 10.4 | $ | 9.0 | $ | 14.7 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| (1) | Prior to January 1, 2007, a number of research and development expenses were incurred in connection with multiple product candidates and families, and therefore were not attributable to one specific product candidate family. We concluded that it was not practicable to attempt to track expenses by product candidate prior to such date. Therefore, the table above does not reflect a breakdown of our research and development expenses by product candidates or family for the inception to date period, but rather from January 1, 2007 to June 30, 2011. |
| (2) | Consists primarily of direct research and development costs related to product development. |
| (3) | Comprised primarily of salary and benefits, depreciation, facilities expenses and stock-based compensation allocated to our research and development activities. |
We expect research and development expenses to increase as we continue our planned preclinical studies and clinical trials for our product candidates, particularly our proprietary dronabinol and LEP-ETU product candidates. Clinical development timelines, likelihood of regulatory approval and commercialization and associated costs are uncertain and therefore can vary significantly. We anticipate determining which research and development projects to pursue as well as the level of funding available for each project based on the scientific and preclinical and clinical results of each product candidate and related regulatory action.
68
Table of Contents
Several actions and additional financial investment are necessary to complete development of our Dronabinol SG Capsule product candidate in preparation for our anticipated commercialization, if approved. For example, we plan to complete multiple process validation batches aimed to meet FDA regulatory requirements for a commercial launch of Dronabinol SG Capsule. It is anticipated that the financial requirement for these process related steps will be $1.1 million incurred over a 12-month period. We believe this investment and the processes undertaken as described above will allow us to complete development of Dronabinol SG Capsule in anticipation of potential commercial launch.
Due to the risks inherent in conducting preclinical studies and clinical trials, the regulatory approval process and the costs of preparing, filing and prosecuting patent applications, our development completion dates and costs will vary significantly for each product candidate and are very difficult to estimate. The lengthy process of seeking regulatory approvals and the subsequent compliance with applicable regulations require the expenditure of substantial additional resources. Any failure by us to obtain, or any delay in obtaining, regulatory approvals or acceptable DEA classifications for our product candidates could cause our research and development expenditures to increase significantly and, in turn, have a material adverse effect on our results of operations.
General and Administrative Expenses
General and administrative expenses consist primarily of:
| • | personnel-related expenses, including stock-based compensation costs; |
| • | depreciation and amortization charges allocated to general and administrative expense; |
| • | costs related to raising capital and becoming a public reporting company; |
| • | facilities-related expenses; and |
| • | business development-related expenses. |
Our general and administrative expenses have historically been significant, and we expect these expenses to increase as we expand our infrastructure to support increased commercialization efforts relating to Subsys, Dronabinol SG Capsule and our other product candidates, if those product candidates are approved by the FDA. We also anticipate incurring additional expenses as a public company following the closing of this offering as a result of additional legal, accounting and corporate governance expenses, including costs associated with tax return preparations, accounting support services, expenses related to compliance with the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, expenses related to filing annual, quarterly and other reports and documents with the Securities and Exchange Commission, directors’ fees, directors’ and officers’ insurance premiums, expenses related to listing and transfer agent fees, and investor relations expenses.
If our NDA for Subsys is approved, we anticipate hiring a head of sales and marketing and thereafter building a sales force, supplemented by additional representatives as deemed necessary in the future. We anticipate incurring these costs sometime in the first half of 2012, depending on related regulatory action.
Interest Expense and Interest Income
Interest expense consists primarily of the interest accrued on outstanding promissory notes payable to The John N. Kapoor Trust and the Kapoor Children 1992 Trust. These trusts are controlled by our founder, Executive Chairman and principal stockholder, Dr. John N. Kapoor. The interest rate on these promissory notes is the applicable prime rate plus 2%, which was 5.25% at June 30, 2011. As of June 30, 2011, we had $43.7 million in debt owed to these trusts, including accrued interest of $6.1 million, all of which is payable on demand.
Interest income consists of amounts received from our interest-bearing checking account.
69
Table of Contents
Other Income
Other income consists primarily of one-time credits for cash received related to awards for government grants and other various items.
Unaudited Pro Forma Condensed Consolidated Financial Information
The unaudited pro forma condensed consolidated statement of operations information for the year ended December 31, 2010 is based on the historical consolidated statements of operations of Insys Therapeutics, Inc. and NeoPharm, giving effect to the Merger as if it had occurred on January 1, 2010. This pro forma information was prepared utilizing:
| • | the audited consolidated financial statements of Insys Therapeutics, Inc. for the year ended December 31, 2010 and the unaudited consolidated financial statements of NeoPharm for the period from January 1, 2010 to November 8, 2010, the Merger date; |
| • | the preliminary purchase price allocation of the Merger, a summary of which is included in Note 1 to the pro forma information included in the section entitled “Unaudited Pro Forma Condensed Consolidated Financial Information;” and |
| • | the assumptions and adjustments described in the notes to such pro forma financial information. |
The unaudited pro forma condensed consolidated financial information for the year ended December 31, 2010 is preliminary and subject to change, is provided for illustrative purposes only and is not necessarily indicative of the results that would have been achieved had the Merger been completed as of the date indicated or that may be achieved in future periods. In addition, the unaudited pro forma condensed consolidated statement of operations does not include:
| • | the effects of any non-recurring costs or income/gains resulting from: |
| • | professional fees and other direct or indirect costs incurred in connection with the Merger, |
| • | accelerated stock-based compensation expense resulting from the Merger, or |
| • | income tax benefits resulting directly from the Merger; |
| • | the costs related to restructuring or integration activities that we may implement subsequent to the closing of the Merger; and |
| • | the realization of any cost savings from operating efficiencies, synergies or other restructurings that may result from the Merger. |
The unaudited pro forma condensed consolidated financial information for the year ended December 31, 2010 should be read in conjunction with the historical consolidated audited financial statements of Insys Therapeutics, Inc. and NeoPharm and the related notes thereto and other information included elsewhere in this prospectus.
The unaudited pro forma condensed consolidated financial information for the six months ended June 30, 2011 is based on our historical consolidated statements of operations, giving effect to the transactions described in the notes to such pro forma financial information as if such transactions had occurred on January 1, 2010. The unaudited pro forma condensed consolidated financial information for the six months ended June 30, 2011 should be read in conjunction with our historical consolidated unaudited financial statements and the related notes thereto and other information included elsewhere in this prospectus.
Internal Control Over Financial Reporting
In connection with the audit of our consolidated financial statements for the year ended December 31, 2010, our management and independent registered public accounting firm identified a material weakness in our internal control over financial reporting, as defined in rules established by the
70
Table of Contents
Public Company Accounting Oversight Board, or PCAOB. This material weakness related to a lack of sufficient staff with appropriate training in GAAP and SEC rules and regulations with respect to financial reporting. As a result, audit adjustments to our financial statements were identified during the course of the audit. Currently, we have only one designated finance and accounting employee, our new Chief Financial Officer, and rely on consultants to provide many accounting, book-keeping and administrative services. In an effort to remediate this material weakness, we intend to hire additional finance and accounting personnel, build our financial management and reporting infrastructure, and further develop and document our accounting policies and financial reporting procedures in 2011 and 2012. For example, we have begun a search for a controller and expect to fill that position in 2011. We cannot assure you that we will be successful in these hiring or remediation efforts, or that any of these measures will significantly improve or remediate the material weakness described above.
Assessing our staffing and training procedures to improve our internal control over financial reporting is an ongoing process. We are currently not required to comply with Section 404 of the Sarbanes-Oxley Act, and are therefore not required to make an assessment of the effectiveness of our internal control over financial reporting. As a result, our management did not perform an evaluation of our internal control over financial reporting as of December 31, 2010. Further, our independent registered public accounting firm has not been engaged to express, nor have they expressed, an opinion on the effectiveness of our internal control over financial reporting. We also currently do not have an internal audit function.
For the year ending December 31, 2012, pursuant to Section 404 of the Sarbanes-Oxley Act, management will be required to deliver a report that assesses the effectiveness of our internal control over financial reporting. Under current SEC rules, if we are an accelerated filer, our independent registered public accounting firm will also be required to deliver an attestation report on the effectiveness of our internal control over financial reporting beginning with the year ending December 31, 2012.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosed amounts of contingent assets and liabilities, and our reported expenses. We evaluate our estimates and judgments related to these estimates on an ongoing basis. We base our estimates of the carrying values of assets and liabilities that are not readily apparent from other sources on historical experience and on various other factors that we believe are reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
We believe that the following accounting policies are critical to a full understanding of our reported financial results. Our significant accounting policies are more fully described in Note 1 of our audited consolidated financial statements appearing elsewhere in this prospectus.
Research and Development Expenses
As a development-stage company, research and development is our most significant expenditure. Our research and development expenses consist of expenses incurred in developing and testing our product candidates. These expenses include, among other things, salaries, benefits, stock-based compensation costs, consulting fees and costs reimbursed to third parties under license and research agreements, expenses related to regulatory filings for our drug candidates, facilities, depreciation and other allocated expenses, and equipment and laboratory supplies. We expense our research and development costs as incurred and expect little variability between the estimates recorded and actual research and development expenses. As we continue to develop product candidates and proceed through the various preclinical studies and clinical trials required for possible FDA approvals, we
71
Table of Contents
expect that research and development expenses will increase in absolute dollars, but, if and when we begin generating revenues as anticipated, decrease as a percentage of revenues going forward.
Acquisitions, Goodwill and Other Intangible Assets
We account for acquired businesses using the acquisition method of accounting in accordance with GAAP accounting rules for business combinations which requires that the assets acquired and liabilities assumed be recorded at the date of acquisition at their respective fair values. Any excess of the purchase price over the estimated fair values of net assets acquired is recorded as goodwill. Any excess of the fair value of assets acquired and liabilities assumed over the purchase price is recorded as a bargain purchase gain. The fair value of intangible assets, including developed product and in-process research and development, is based on significant judgments made by management. The valuations and useful life assumptions are based on information available near the acquisition date and are based on expectations and assumptions that are considered reasonable by management. In our assessment of the fair value of identifiable intangible assets acquired in the Merger, management used valuation techniques and made various assumptions in determining the valuation. Our analysis and financial projections are based on management’s prospective operating plans and the historical performance of the acquired business. In connection with the Merger on November 8, 2010, we engaged consultants to assist management in the following:
| • | developing an understanding of the economic and competitive environment for the industry in which we and the acquired company participate; |
| • | identifying the intangible assets acquired; |
| • | reviewing the Merger agreements and other relevant documents made available; |
| • | interviewing our employees, including the employees of the acquired company, regarding the history and nature of the Merger, historical and expected financial performance, product lifecycles and roadmap, and other factors deemed relevant to our valuation analysis; |
| • | performing additional market research and analysis deemed relevant to our valuation analysis; |
| • | estimating the fair values and recommending useful lives of the acquired intangible assets; and |
| • | preparing a narrative report detailing methods and assumptions used in the valuation of the intangible assets. |
All work performed by consultants was discussed and reviewed in detail by management to determine the estimated fair values of the intangible assets. The judgments made in determining estimated fair values assigned to assets acquired and liabilities assumed, as well as asset lives, can materially impact our results of operations.
Federal and State Income Taxes
Prior to November 8, 2010, the subsidiary entity that is now known as Insys Pharma was subject to taxation under the provisions of Subchapter S of the Code in the United States, and, as a result, the federal and state income tax liabilities of that entity were the responsibility of its stockholders. Accordingly, no provision was made for federal or state income taxes of that entity, since it was the personal responsibility of the individual stockholders of that entity to separately report their proportionate share of its taxable income or loss. As of November 8, 2010, as a result of the Merger, the subsidiary entity that is now known as Insys Pharma became a Subchapter C Corporation and became subject to U.S. federal and state income tax at the corporate level. The effect of the change in tax status was to recognize a one-time non-cash tax benefit of $3.0 million to establish a $3.0 million net deferred tax asset for the future tax consequences attributable to differences between the financial statement and income tax bases of its assets and liabilities as of November 8, 2010. A full valuation allowance was recorded against this net deferred tax asset as it is not more likely than not that it will be realized.
72
Table of Contents
On November 8, 2010, Insys Therapeutics, Inc. effected the Merger in a transaction that was accounted for as a reverse acquisition and resulted in a change of 50% or more of the ownership of NeoPharm. As of the Merger date, NeoPharm had $274.0 million of federal NOLs which were scheduled to expire in tax years 2011 to 2029. Under Section 382 of the Code, our utilization of the pre-Merger federal NOLs of NeoPharm to offset our post-Merger federal taxable income is significantly limited due to the Merger. Prior to the Merger, NeoPharm had completed a partial analysis of ownership changes under Section 382 of the Code to determine if a change in control of NeoPharm had occurred. Based on NeoPharm’s partial analysis, no change in control was identified, based on the review of eight test dates covering a four-year period ended December 31, 2007. A complete formal analysis of ownership change would have to be performed in order to obtain certainty that a change in control of NeoPharm had not occurred prior to the Merger, which could further limit the utilization of the NeoPharm pre-Merger NOLs by us.
Based on the above, we have estimated the amount of pre-Merger federal NOLs of NeoPharm that are available to offset our post-Merger income is limited to approximately $158,000 each year for 20 years, or cumulatively $3.2 million. For state income tax purposes, we have $274.0 million of state NOLs relating to NeoPharm. We have placed a valuation allowance on its deferred tax assets, which include the federal and state NOLs, for it is not more likely than not that such amounts will be realized.
We account for our deferred income tax assets and liabilities based on differences between the financial reporting and tax bases of assets and liabilities, and NOLs and other tax credit carryforwards. These items are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in the period that includes the enactment date.
We record a valuation allowance to reduce the deferred income tax assets to the amount that is more likely than not to be realized. In making such determination, management considers available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax planning strategies and recent financial operations.
We recognize a tax benefit from uncertain tax positions when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits of the position. We recognize interest accrued on unrecognized tax benefits and penalties in income tax expense.
Stock-Based Compensation
We account for stock-based compensation under the guidance of the Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, 718: Compensation-Stock Compensation. Under this standard, the fair value of each share-based payment award is estimated on the date of grant using an option pricing model that meets certain requirements and the expense is amortized ratably over the vesting period. We currently use the Black-Scholes option pricing model to estimate the fair value of our share-based payment awards. The determination of the fair value of share-based payment awards utilizing the Black-Scholes model is highly subjective and requires judgment, and a number of assumptions, including expected volatility, risk-free interest rate, expected term and expected dividend yield.
For the six months ended June 30, 2011 and the years ended December 31, 2010 and 2008 (there were no grants in 2009) the fair value of stock options was estimated at the grant date using the following assumptions:
| Six Months Ended June 30, 2011 |
Year Ended December 31, | |||||
| 2010 | 2008 | |||||
| Expected volatility |
109.2% | 100.0% – 110.8% | 123.1% – 123.2% | |||
| Risk-free interest rate |
3.5% | 2.5% – 2.9% | 3.3% – 3.5% | |||
| Expected term (in years) |
6.5 – 9.7 | 5.0 – 6.0 | 6.0 | |||
| Expected dividend yield |
0.0% | 0.0% | 0.0% | |||
73
Table of Contents
Expected Volatility. Prior to the Merger, we did not have a history of market prices for our common stock and since the Merger, we do not have what we consider a sufficiently actively and readily traded market for our common stock to use historical market prices for our common stock to estimate volatility. Accordingly, we estimate the expected stock price volatility for our common stock by taking the median historical stock price volatility for industry peers based on daily price observations over a period equivalent to the expected term of the stock option grants. Industry peers consist of other public companies in the pharmaceutical industry similar in size, stage of life cycle and financial leverage. We did not rely on the implied volatilities of traded options in our industry peers’ common stock, because either the term of those traded options was much shorter than the expected term of our stock option grants, or the volume of activity was relatively low.
Risk-Free Interest Rate. Generally, the risk-free interest rate assumption is based on observed interest rates appropriate for the terms of our awards. For grants made in 2011, 2010 and 2008, the risk-free interest rate assumption was based on zero coupon U.S. Treasury instruments whose term was consistent with the expected term of our stock option grants.
Expected Term. Generally, the expected term of the awards is based on a simplified method which defines the life as the average of the contractual term of the options and the weighted average vesting period for all open tranches. We have very little historical information to develop reasonable expectations about future exercise patterns and post-vesting employment termination behavior for our stock option grants. As a result, for stock option grants made to employees during the six months ended June 30, 2011, and the years ended December 31, 2010 and 2008, the expected term was estimated using the simplified method allowed under Securities and Exchange Commission Staff Accounting Bulletin No. 107, Share-Based Payment.
Expected Dividend Yield. The dividend yield assumption is based on our history and expectation of paying no dividends.
We periodically evaluate the assumptions used to value our awards. If factors change, such as changes in the expected term of the options granted or the fair value of our common stock, and we employ different assumptions, stock-based compensation expense may differ significantly from what we may have recorded in the past. If there are any modifications or cancellations of the underlying unvested securities, we may be required to accelerate, increase or cancel any remaining unearned stock-based compensation expense. Future stock-based compensation expense and unearned stock-based compensation will increase to the extent that we grant additional equity awards to employees or we assume unvested equity awards in connection with acquisitions.
We have granted to our employees options to purchase common stock at exercise prices equal to the fair market value of the underlying stock at the time of each grant, as determined by our board of directors. The board of directors considered objective and subjective factors in determining the estimated fair value of our common stock on each option grant date, including, among others:
| • | the development status of our product candidates and regulatory issues encountered during the relevant period; |
| • | the composition of the management team and employees; |
| • | developments in the industry and our targeted markets; |
| • | the actual financial condition and results of operations relative to our formal operating plan during the relevant period; |
| • | lack of earnings and dividend paying capacity; |
| • | limited sources of funding and dependence on one investor for financing; |
| • | lack of revenues and estimated revenue forecasts; |
| • | risks and volatility associated with us, our industry and our peers; |
74
Table of Contents
| • | the illiquidity of our capital stock; |
| • | the likelihood of a liquidity event; |
| • | certain equity award and stock restrictions; and |
| • | concentration in control of ownership. |
In addition to considering such factors in determining the fair value of our common stock and common stock options, our board of directors, with the assistance of management, conducted valuations of our common stock as of April 15, 2008, May 31, 2009 and February 28, 2010. We used a valuation methodology that is consistent with the practices recommended by the American Institute of Certified Public Accountants, or AICPA, Audit and Practice Aid Series Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the Practice Aid. In determining the appropriate method to use in valuing our common stock, we used the Income Approach, specifically the Discounted Cash Flow, or DCF, method which is the suggested method for a development stage company that is in Stage 3 as defined in the Practice Aid.
The DCF method is predicated on the concept that the fair market value of a business and its common stock is equal to the present value of cash flows earned during the forecast period, plus the value at the end of that period referred to as its terminal value. The primary assumptions applied in the DCF valuation analysis are revenue, costs of goods sold, research and development expenses, selling, general and administrative expenses, depreciation, capital expenditures, debt free working capital, notes receivable, federal and state tax rates and notes outstanding.
Once the total equity capital is calculated, the analysis then determines the discount rate (cost of equity) applied to the forecasted economics based on venture capital rates of return for biotechnology companies. Insys was considered a high risk investment given its early stage of development. The residual value was calculated using the “Gordon Growth Model.”
In July 2008, we granted options to purchase a total of 299,418 shares of our common stock (voting and non-voting) with an exercise price of $22.57 per share (reflecting the retroactive effect of a one-for-1,500,000 reverse stock split effective on June 5, 2009, a 1,862,623-for-one stock split on February 22, 2010, and the Merger). Utilizing the DCF method described above, it was determined that the indicated total invested capital value was $70 million and equity value after debt totaled $30 million. The overall weighted average cost of capital used in this calculation was 35%. The discounts related to lack of control (-10%) and lack of marketability (-30%) were then taken into account, due to the fact that we were still in a development stage and had a simple capital structure with only one major investor. The adjusted equity value was then determined to be $18.0 million, or $22.57 per share, of our common stock (voting and non-voting) using the DCF method.
In 2009, we granted no options and all of the previously granted options were cancelled at the time that we effected a one-for-1,500,000 reverse stock split and cancelled all of the resulting fractional shares.
In February and April 2010, we granted options to purchase a total of 1,138,804 shares of our common stock (voting and non-voting) with an exercise price of $1.83 per share (reflecting the retroactive effect of a one for-1,500,000 reverse stock split effective on June 5, 2009, a 1,862,623-for-one stock split on February 22, 2010, and the Merger). Utilizing the DCF method described above, it was determined that the indicated total invested capital was $48.4 million and equity value after debt totaled $26.2 million. The overall weighted average cost of capital used in this calculation was 40%. The discounts related to minority interests (-15%) and lack of marketability (-30%) were then taken into account. The adjusted equity value was determined to be $14.4 million, or $1.83 per share. There were several key internal and external factors which contributed to the decline of the value of our common stock between July 2008 and February 2010. These key factors included:
| • | In 2008, a competitor of ours received approval for a generic form of Marinol, resulting in a downward revision in our estimated market opportunity for our lead product lines. |
75
Table of Contents
| • | During 2009, macro-economic conditions continued to deteriorate, making it difficult for private biotechnology companies to raise additional capital to fund their operations. Financings that closed during this period were at significant discounts to prior rounds of financing, and there was a high level of uncertainty regarding whether we would be able to obtain sufficient funding to continue long-term operations. |
| • | In May 2009, we received notice from the FDA that our Dronabinol HG Capsule application was denied and that it would be in our best interest to abandon the project. At the time, Dronabinol HG Capsule was our only potential near-term source of revenue and prior to the FDA notice we attributed a significant portion of the overall value of our company to this product candidate. At this time we also froze spending on our other Dronabinol line extensions. |
| • | As of February 2010, we were pursuing the development of our Dronabinol SG Capsule and were conducting the bioequivalence study but did not yet know the results of the study or whether we would be able to resubmit our application to the FDA. Additionally, as of this time our debt had increased to $22.3 million and we were reliant on one major investor. |
| • | Between July 2008 and February 2010, sales of Dronabinol decreased substantially, which caused us to lower our projected future earnings if we were to ever commercialize a Dronabinol product. Also during this period, the FDA began scrutinizing prescriptions of Fentanyl products to patients who do not fit indicated patient profiles or for off label uses. We believe this resulted in lower sales of Fentanyl products, which in turn caused us to revise our projected revenues from Subsys, if approved. |
On February 15, 2011, we conducted another valuation exercise utilizing the Income Approach using Probability Weighted Expected Return Method, or PWERM. This approach involves the estimation of future potential outcomes for us, as well as values and probabilities associated with each respective potential outcome. The common stock per share value determined using this approach is ultimately based upon probability-weighted per share values resulting from the various future scenarios, which can include an initial public offering, merger or sale, dissolution, or continued operation as a private company. Under a probability-weighted expected return method, in accordance with the AICPA guidelines, the value of the common stock is estimated based upon an analysis of future values for the enterprise assuming various future outcomes. Share value is based upon the probability-weighted present value of future expected investment returns, considering each of the possible future outcomes available to the enterprise, as well as the rights of each share class.
We first determined the value of our equity using the Income Approach, the Market Approach (which calculates the equity value based on the Public Company Market Multiple Method, or PCMMM), and the Cost Approach. The values from these various approaches were then used to conclude a future value of Insys through a PWERM analysis as described below. PWERM was determined to be the most appropriate methodology due to Insys’ shorter expected time horizon to a potential liquidity event, as well as management’s ability to more accurately assess potential exit events and associated probabilities. The following likelihoods of various scenarios were used for this analysis:
| • | Initial public offering in 6 months – 35% probability (Market Approach used); |
| • | Initial public offering in 1 year – 20% probability (Market Approach used); |
| • | Acquisition in 1 year – 15% probability (Income Approach used); |
| • | Acquisition in 1.5 years – 10% probability (Income Approach used); |
| • | Liquidation in 1.5 years – 10% probability (Cost Approach used); and |
| • | Remain private – 10% probability (Income Approach used). |
The value under the income approach was determined using the DCF method. The discount rate used was 35% after assessing rates of returns on investments in development-stage biotechnology companies. We used similar primary assumptions as used in prior year valuations in which the Income
76
Table of Contents
Approach was used. Under the Income Approach using the DCF method, it was determined that our equity value was $69.9 million after a 10% lack of control adjustment. Our value under the acquisition scenario and in the scenario where we remained private was based on our equity value concluded from the Income Approach.
For PCMMM, we selected guideline companies which represented the most comparable publicly traded companies with operations similar to ours at the valuation date and analyzed the enterprise value, or EV, to future revenue multiples for each of these companies. Initial public offering multiples from transactions that occurred between October 2008 and June 2009 were excluded due to the economic turmoil and instability in the capital markets during that time frame. We also cross-referenced forward initial public offering multiples and considered adjustments to reflect the differences between us and the guideline companies in terms of growth, profitability and risk. Based on this analysis, we arrived at an estimated equity value of $93 million. Our value under the initial public offering scenario was based on the selected multiple from the PCMMM of the market approach.
Our value under the liquidation scenario was determined based on a cost approach, in which the book value of our equity as of December 2010 (including NeoPharm) was assumed to approximate our fair market value in connection with a liquidation in 1.5 years. The book value of our equity as of December 2010 was negative. As a result, liquidation proceeds to equity holders under this scenario were determined to be zero.
The following is a summary of the PWERM values as of February 15, 2011:
| Scenario |
Scenario Probability |
Time to Event (years) |
Common Shares | |||||||||||||
| Value Per Share | Probability Weighted Value |
|||||||||||||||
| IPO (6 months) |
35 | % | 0.5 | $ | 7.93 | $ | 2.44 | |||||||||
| IPO (1 year) |
20 | % | 1.0 | 6.71 | 1.22 | |||||||||||
| Acquisition (1 year) |
15 | % | 1.0 | 6.71 | 1.22 | |||||||||||
| Acquisition (1.5 years) |
10 | % | 1.5 | 6.71 | 0.61 | |||||||||||
| Liquidation (1.5 years) |
10 | % | 1.5 | — | — | |||||||||||
| Remain private |
10 | % | NA | 6.71 | 0.61 | |||||||||||
| Total |
100 | % | $ | 6.71 | ||||||||||||
| 25% downward adjustment for lack of marketability |
|
($ | 1.83 | ) | ||||||||||||
| Concluded value per share (minority, non-marketable basis) (rounded) |
|
$ | 4.88 | |||||||||||||
In March 2011, we granted options to purchase a total of 508,491 shares of our common stock with an exercise price of $4.88 per share which was based on the valuation of our common stock in February 2011 as described above, but prior to consideration of the initial public offering and the underlying corporate activities that supported the offering. We subsequently reassessed the fair market value of our common stock after giving consideration to our proposed initial public offering and the underlying corporate activities that supported the offering and determined that for accounting purposes the fair market value of our common stock in March 2011 was $15.00 per share.
77
Table of Contents
The following table sets forth the number of options granted, the fair market value per share underlying the options based on the analyses set forth above, the total fair market value of the option shares, the exercise price and the intrinsic value, if any, for each option grant made by us since January 1, 2008:
| Option Date |
Total Options Granted (1) |
Fair Market Value Per Share |
Total Fair Market Value |
Exercise Price |
Total Exercise Price |
Intrinsic Value (2) |
||||||||||||||||||
| July 9, 2008 |
2,574 | $ | 22.57 | $ | 58,095 | $ | 22.57 | $ | 58,095 | $ | — | |||||||||||||
| July 25, 2008 |
296,844 | $ | 22.57 | $ | 6,699,769 | $ | 22.57 | $ | 6,699,769 | — | ||||||||||||||
| February 22, 2010 |
1,026,678 | $ | 1.83 | $ | 1,878,821 | $ | 1.83 | $ | 1,878,821 | — | ||||||||||||||
| April 5, 2010 |
112,126 | $ | 1.83 | $ | 205,191 | $ | 1.83 | $ | 205,191 | — | ||||||||||||||
| March 28, 2011 |
508,491 | $ | 15.00 | $ | 7,627,365 | $ | 4.88 | $ | 2,481,436 | 5,145,929 | ||||||||||||||
| Total Options |
||||||||||||||||||||||||
| Options outstanding vested at June 30, 2011 |
1,118,851 | $ | 4.64 | (3) | $ | 5,191,469 | $ | 4.27 | (3) | $ | 4,777,494 | |||||||||||||
| Options outstanding unvested at June 30, 2011 |
500,381 | $ | 14.26 | (3) | $ | 7,135,433 | $ | 4.88 | (3) | $ | 2,441,859 | |||||||||||||
| (1) | Represents shares of both voting and non-voting common stock. |
| (2) | The intrinsic value of the options granted on July 9, 2008, July 25, 2008, February 22, 2010 and March 28, 2011 is defined as the positive difference between the fair market value of our common stock (voting and non-voting) at the date of grant (as determined by the valuation methods described above) and the exercise price of the options. The intrinsic value of the total options outstanding (vested and unvested) at June 30, 2011 is defined as the positive difference between the estimated offering price of our common stock in this offering and the weighted average exercise price of the outstanding options. |
| (3) | Weighted average fair market value and exercise price. |
Stock-based compensation expense was $570,000 and $1.3 million for the six months ended June 30, 2011 and 2010, respectively, and $1.4 million, $2.5 million and $7.8 million for the years ended December 31, 2010, 2009 and 2008, respectively. Included in stock-based compensation expense for the years ended December 31, 2009 and 2008 was $3.2 million and $3.9 million, respectively, resulting from the difference between the fair market value per share of a controlling equity interest in us and the fair market value per share of the minority, non-marketable interest relating to certain issuances of shares of our common stock to The John N. Kapoor Trust and Dr. John N. Kapoor. On December 29, 2009, debt and accrued interest totaling $11.5 million was converted into 253,414 shares and on July 25, 2008, debt and accrued interest totaling $24.2 million was converted into 38,112 shares. See Note 10, “NeoPharm Merger” to the Insys Therapeutics consolidated financial statements located elsewhere in this prospectus.
In connection with the one-for-1,500,000 reverse stock split on June 2, 2009, we cancelled all options outstanding at that time. This resulted in a reversal of stock-based compensation expense that had been previously recorded for all of the outstanding options that had not vested as of the date cancelled. The total reversal of stock-based compensation expense related to this cancellation and employee terminations resulted in an offset to our stock-based compensation expense of $0.7 million for the year ended December 31, 2009.
Results of Operations
Comparison of Six Months Ended June 30, 2011 to Six Months Ended June 30, 2010
Revenues. We did not recognize any revenues during the six months ended June 30, 2011 or 2010.
Research and Development Expenses. For the six months ended June 30, 2011, research and development expenses were $3.8 million. Of this amount, $2.7 million related to Insys Pharma
78
Table of Contents
operations and $1.1 million was associated with the NeoPharm operations. Of these research and development expenses, $0.5 million were for direct costs attributable to our fentanyl program and $0.6 million were direct costs attributable to our dronabinol programs. The $1.1 million expenses associated with the NeoPharm operations were for staffing and for direct costs related to the clinical trials for LEP-ETU. For the six months ended June 30, 2010, research and development expenses were $5.2 million and all related to the Insys Pharma operations. We estimate $3.1 million of these research and development expenses was attributable to our fentanyl program and $1.0 million was attributable to our dronabinol programs. The $1.4 million, or 27%, decrease in research and development expenses between the six months ended June 30, 2011 and the six months ended June 30, 2010 is primarily the result of decreased expenses in connection with the completion of our clinical trials for Subsys and Dronabinol SG Capsule products and decreased stock-based compensation expenses, partly offset by the inclusion of expenses associated with NeoPharm’s operations in the six months ended June 30, 2011. Total research and development stock-based compensation expense for the six months ended June 30, 2011 was $0.1 million, a decrease of $0.5 million from the 2010 period. The decrease is primarily attributable to a longer vesting period for the shares granted in March 2011, as compared to the vesting period for the shares granted in February 2010.
General and Administrative Expenses. General and administrative expenses were $4.6 million for the six months ended June 30, 2011, an increase of $2.6 million, or 130%, from $2.0 million for the six months ended June 30, 2010. This increase was primarily due to the payment of approximately $1.5 million of regulatory filing fees for the submission of the NDA for the fentanyl drug product and general and administrative expenses of approximately $1.3 million related to NeoPharm’s operations.
Interest Expense. Net interest expense was $0.9 million for the six months ended June 30, 2011, an increase of $0.4 million, or 80%, from $0.5 million for the six months ended June 30, 2010. This increase was primarily a result of greater amounts outstanding under promissory notes payable to The John N. Kapoor Trust and the Kapoor Children 1992 Trust during the six months ended June 30, 2011 as compared to the six months ended June 30, 2011. As of June 30, 2011 and December 31, 2010, the aggregate principal balance of these notes payable was $37.6 million and $29.7 million, respectively, excluding accrued interest expense payable of $6.1 million and $5.2 million, respectively.
Other Income, Net. Other income, net of $0.1 million for the six months ended June 30, 2011 was primarily the result of a $0.3 million grant awarded to us under a program administered by the U.S. federal government for certain types of qualifying therapeutic discovery projects, partially offset by the $0.2 million accretion of our contingent payment obligation to the pre-Merger NeoPharm stockholders.
Comparison of Year Ended December 31, 2010 to Year Ended December 31, 2009
Revenues. We did not recognize any revenues during the years ended December 31, 2010 or 2009.
Research and Development Expenses. For the year ended December 31, 2010, research and development expenses were $10.4 million. Of this amount, $10.2 million related to Insys Pharma operations and $0.2 million was associated with the NeoPharm operations. Of these research and development expenses, $5.7 million were for direct costs attributable to our fentanyl program and $1.7 million were direct costs attributable to our dronabinol programs. The $0.2 million expenses associated with the NeoPharm operations were for staffing and for direct costs related to the clinical trials for LEP-ETU. For the year ended December 31, 2009, research and development expenses were $9.0 million. We estimate $6.4 million of these research and development expenses was attributable to our fentanyl program and $2.2 million was attributable to our dronabinol programs. The $1.4 million, or 16%, increase in research and development expenses between the year ended December 31, 2010 and the year ended December 31, 2009 is primarily the result of increased stock-based compensation expenses, as well as increased personnel costs in connection with an increase in staffing at the Dronabinol API manufacturing facility that was obtained in the third quarter of 2009. Total research and development stock-based compensation expense for the year ended December 31, 2010 was $0.7 million, an increase of $1.1 million over 2009. The total cancellation of all stock options outstanding and
79
Table of Contents
employee terminations in 2009 resulted in a $0.4 million net reversal of research and development stock-based compensation expense for the year ended December 31, 2009.
General and Administrative Expenses. General and administrative expenses were $3.5 million for the year ended December 31, 2010, a decrease of $1.0 million, or 21%, from $4.5 million for the year ended December 31, 2009. This decrease was primarily due to lower stock-based compensation expenses, reduced legal expenses and decreased salaries due to a restructuring of the executive team.
Interest Expense. Net interest expense was $1.1 million for the year ended December 31, 2010, an increase of $0.1 million, or 15%, from $1.0 million for the year ended December 31, 2009. This increase was primarily a result of greater amounts outstanding under promissory notes payable to The John N. Kapoor Trust and the Kapoor Children 1992 Trust during the year ended December 31, 2010 as compared to the year ended December 31, 2009. As of December 31, 2010 and December 31, 2009, the aggregate principal balance of these notes payable was $29.7 million and $14.5 million, respectively, excluding accrued interest expense payable of $5.2 million and $4.1 million, respectively.
Other Income. Other income of $0.8 million for the year ended December 31, 2010 was primarily the result of grants awarded to us under a program administered by the U.S. federal government for certain types of qualifying therapeutic discovery projects.
Income Tax Benefit. An income tax benefit of $0.6 million that was recorded for the year ended December 31, 2010 was primarily due to the release of a portion of our valuation allowance on deferred tax assets to offset the deferred tax liability created as a result of the Merger on November 8, 2010. Prior to November 8, 2010, the federal and state income tax liabilities of Insys Pharma were the responsibility of its stockholders and, accordingly, no provision was made for federal or state income taxes in fiscal years 2009 and 2008 and prior thereto.
Comparison of Year Ended December 31, 2009 to Year Ended December 31, 2008
Revenues. We did not recognize any revenues during the years ended December 31, 2009 or 2008.
Research and Development Expenses. For the year ended December 31, 2009, research and development expenses were $9.0 million. Of these research and development expenses, approximately $6.4 million were direct costs attributable to the fentanyl program and $2.2 million were direct costs attributable to dronabinol programs. For the year ended December 31, 2008, research and development expenses were $14.7 million. Of these research and development expenses, approximately $4.6 million were direct costs attributable to the fentanyl program and $5.5 million was attributable to dronabinol programs. The $5.7 million, or 39%, decrease in research and development expenses between the year ended December 31, 2009 and the year ended December 31, 2008 resulted primarily from the discontinuation of the Dronabinol HG Capsule product candidate program in 2009, partially offset by expenses for additional outside consultants hired as we expanded our development of the Dronabinol RT Capsule, Dronabinol Oral Solution and Dronabinol Inhalation Device product candidates.
General and Administrative Expenses. General and administrative expenses were $4.5 million for the year ended December 31, 2009, a decrease of $5.7 million, or 56%, from $10.2 million for the year ended December 31, 2008. This decrease was primarily due to reduced expenses related to stock-based compensation and lower executive salaries due to restructuring. We had also incurred expenses in 2008 in connection with a contemplated initial public offering which led to higher expenses in 2008 as compared to 2009.
Interest Expense and Interest Income. Net interest expense was $1.0 million for the year ended December 31, 2009, a decrease from $1.9 million for the year ended December 31, 2008. This decrease was primarily due to a decline in the principal balance of the demand and promissory notes payable to entities affiliated with Dr. John N. Kapoor during 2009 as compared to 2008 primarily as a result of the
80
Table of Contents
conversion of a portion of such debt to equity that took place in July 2008. With no further borrowing for the rest of the year, the principal balance of these notes payable, excluding $4.1 million of accrued interest payable, remained $14.3 million as of December 31, 2008.
Other Income. Other income decreased from $0.8 million for the year ended December 31, 2008 to approximately $31,000 for the year ended December 31, 2009. Included in other income for the year ended December 31, 2008 was a one-time credit that was recorded in connection with the settlement of a dispute with a supplier.
Liquidity and Capital Resources
Sources of Liquidity
We have incurred losses since our inception. As of June 30, 2011, we had an accumulated deficit of $94.9 million. We have financed our operations through the issuance of promissory notes to The John N. Kapoor Trust and the Kapoor Children 1992 Trust, which are controlled by our founder, Executive Chairman and principal stockholder. During the six months ended June 30, 2011, we received net proceeds of $7.9 million, and during the years ended December 31, 2010, 2009 and 2008, we received net proceeds of $15.1 million, $11.5 million and $9.5 million, respectively, from the issuance of such promissory notes.
As of June 30, 2011, we and Insys Pharma had $43.7 million in debt, including accrued interest of $6.1 million, under the promissory notes payable to The John N. Kapoor Trust and the Kapoor Children 1992 Trust, and $17,000 in cash and cash equivalents. Upon the closing of this offering, these notes, and other notes issued by us or Insys Pharma to trusts controlled by Dr. Kapoor, including accrued interest, will convert into shares of our common stock at the price to the public of the shares sold in this offering.
Cash Flows
The following table shows a summary of our cash flows for the periods indicated (in millions):
| Six Months Ended June 30, |
Year Ended December 31, |
|||||||||||||||||||
| 2011 | 2010 | 2010 | 2009 | 2008 | ||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Cash and cash equivalents at beginning of period |
$ | 0.1 | $ | 0.1 | $ | 0.1 | $ | 0.5 | $ | 0.0 | ||||||||||
| Cash provided by (used in): |
||||||||||||||||||||
| Operating activities |
(7.8 | ) | (6.5 | ) | (15.0 | ) | (10.2 | ) | (15.4 | ) | ||||||||||
| Investing activities |
(0.2 | ) | (0.1 | ) | (0.2 | ) | (1.6 | ) | (0.3 | ) | ||||||||||
| Financing activities |
7.9 | 6.5 | 15.1 | 11.4 | 16.2 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (decrease) increase in cash and cash equivalents |
$ | (0.1 | ) | $ | (0.1 | ) | $ | 0.1 | $ | 0.4 | $ | 0.5 | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of period |
$ | 0.0 | $ | 0.0 | $ | 0.1 | $ | 0.1 | $ | 0.5 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Net Cash Used in Operating Activities. Net cash used in operating activities was $7.8 million and $6.5 million for the six months ended June 30, 2011 and 2010, respectively, and $15.0 million, $10.2 million and $15.4 million for the years ended December 31, 2010, 2009 and 2008, respectively. The net cash used in each of these periods primarily reflects the net loss for those periods, offset in part by depreciation, stock-based compensation expense and non-cash interest expense and is also impacted by changes in other current liabilities.
Net Cash Used in Investing Activities. Net cash used in investing activities was $0.2 million and $0.1 for the six months ended June 30, 2011 and 2010, respectively, and $0.2 million, $1.6 million and $0.3 million for the years ended December 31, 2010, 2009 and 2008, respectively. The significant increase in net cash used in investing activities during the year ended December 31, 2009, primarily reflects the purchase of equipment, leasehold improvements and API related to the manufacturing facility for dronabinol which we obtained in 2009.
81
Table of Contents
Net Cash Provided by Financing Activities. Net cash provided by financing activities was $7.9 million and $6.5 million for the six months ended June 30, 2011 and 2010, respectively, and $15.1 million, $11.4 million and $16.2 million for the years ended December 31, 2010, 2009 and 2008, respectively. Net cash provided by financing activities was primarily attributable to the promissory notes payable to The John N. Kapoor Trust and The Kapoor Children 1992 Trust.
Our cash flows for the remainder of 2011 and beyond will depend on a variety of factors, including anticipated regulatory approvals, revenues from commercialization of approved products and funding requirements, as well as timing of the closing of this offering and our use of net offering proceeds as described in this prospectus under the heading “Use of Proceeds.” Until we obtain regulatory approval and commence sales of our products, we expect our net cash outflows to continue increasing as we expand research and development, manufacturing, regulatory and sales and marketing activities and operate as a public company.
Funding Requirements
We believe that the net proceeds from this offering and our existing cash and cash equivalents, together with interest thereon, will be sufficient to fund our anticipated operating expenses and capital expenditures for at least the next 12 months.
As of June 30, 2011, we had $0.4 million of undrawn funds available under a note payable to The John N. Kapoor Trust. During the third quarter of 2011, we borrowed the remaining amount available under this note and issued one additional note of $1.0 million to The John N. Kapoor Trust to fund our regulatory filings for Subsys, working capital and general purposes. We have borrowed an aggregate of $ from The John N. Kapoor Trust as of , 2011. We expect that our funding requirements will be for development and commercialization of our product candidates, payments under contract manufacturing agreements and working capital and general purposes. In February 2011, The John N. Kapoor Trust agreed to fund the operations of Insys on an as-needed basis through March 31, 2012.
Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to predict the amounts of increased capital outlays and operating expenditures associated with our current anticipated product introduction, clinical trials and preclinical studies. Our funding requirements will depend on numerous factors, including:
| • | timing of FDA approval and DEA classification of Subsys, Dronabinol SG Capsule and other product candidates, if at all; |
| • | the timing and amount of revenue from sales of any of our product candidates, if approved, or revenue from grants or other sources; |
| • | rate of progress and cost of our clinical trials and other product development programs for our dronabinol, fentanyl product and LEP-ETU product candidates and any other product candidates that we may develop, in-license or acquire; |
| • | costs of establishing or outsourcing sales, marketing and distribution capabilities; |
| • | costs and timing of completion of outsourced commercial manufacturing supply arrangements for each product candidate; |
| • | costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights associated with our product candidates; |
| • | costs of operating as a public company; |
| • | the effects of competing technological and market developments; |
| • | our ability to acquire or in-license products and product candidates, technologies or businesses; |
82
Table of Contents
| • | personnel, facilities and equipment requirements; and |
| • | terms and timing of any collaborative, licensing, co-promotion or other arrangements that we may establish. |
We believe that if approved, our generic Dronabinol SG Capsule will begin providing us with revenues that we intend to use toward the commercialization of our other product candidates, if approved, and toward general and administrative expenses. However, until we can consistently generate significant cash from sales of our product candidates and other operations, we expect to continue to fund our operations primarily from the net proceeds from offerings of our equity securities, including this offering, and from the issuance of notes payable to trusts controlled by Dr. John N. Kapoor. We cannot be sure that our existing cash and cash equivalents will be adequate, or that additional financing will be available when needed, or that, if available, financing will be obtained on terms favorable to us or our stockholders. Having insufficient funds may require us to delay, scale back or eliminate some or all of our research or development programs or to relinquish greater or all rights to product candidates at an earlier stage of development or on less favorable terms than we would otherwise choose. If we raise additional funds by issuing equity securities, substantial dilution to existing stockholders will likely result. If we raise additional funds by incurring debt obligations, the terms of the debt will likely require significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business.
Contractual Obligations
The following table summarizes our outstanding contractual obligations as of December 31, 2010 (in millions):
| Payments due by period | ||||||||||||||||
| Less than One Year (2011) |
2-3 Years | 4-5 Years | Total | |||||||||||||
| Operating leases |
$ | 0.7 | $ | 1.7 | $ | 0.5 | $ | 2.9 | ||||||||
| Promissory notes payable (including accrued interest)1 |
34.9 | — | — | 34.9 | ||||||||||||
| Future interest on promissory notes2 |
1.6 | — | — | 1.6 | ||||||||||||
| Clinical trial expenses3 |
0.3 | — | — | 0.3 | ||||||||||||
| Manufacturing agreement expenses4 |
1.5 | — | — | 1.5 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| TOTAL |
$ | 39.0 | $ | 1.7 | $ | 0.5 | $ | 41.2 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | These promissory notes and related accrued interest are payable upon demand. For purposes of this table, the notes and interest are assumed to be required to be paid by December 31, 2011. As of June 30, 2011, total promissory notes including accrued interest have increased by $8.8 million to $43.7 million. Upon the closing of this offering, these notes, and other notes issued by us or Insys Pharma to trusts controlled by Dr. Kapoor, including accrued interest, will convert into shares of our common stock at the price to the public of the shares sold in this offering. |
| (2) | Estimated interest at an assumed interest rate of 5.25%, based on the prevailing prime interest rate as of December 31, 2010. |
| (3) | Remaining commitments for clinical trial agreements for our fentanyl and LEP-ETU product candidates. |
| (4) | Estimated minimum purchase obligations contingent on commercialization of product and amounts reasonably likely to be paid in future periods to contract manufacturers for the Dronabinol SG Capsule and Subsys product candidates. As of August 5, 2011, these estimated minimum purchase obligations have increased by $4.2 million, $3.7 million, $2.1 million and $10.0 million for the periods from 2-3 years, 4-5 years, greater than 5 years and in total, respectively. Approximately $9.5 million of this total obligation is subject to approval of the related product candidates. |
83
Table of Contents
In addition, we may be required to make future payments to our licensors based on the achievement of milestones set forth in various in-licensing agreements. In most cases, these milestone payments are based on the achievement of development or regulatory milestones, including the exercise of options to obtain licenses related to specific disease targets, commencement of various phases of clinical trials, filing of product license applications, approval of product licenses from the FDA or foreign regulatory agencies, and the first commercial sale of a related product. Payment for the achievement of milestones under our in-license agreements is highly speculative and subject to a number of contingencies. The aggregate amount of additional milestone payments that we could be required to pay under all of our in-license agreements in effect at December 31, 2010 is approximately $2.9 million, all of which is related to university and government collaborations which are currently on hold or in clinical development. These amounts assume that all remaining milestones associated with the related milestone payments are met. In the event that product license approval for any of the related products is obtained, we may be required to make royalty payments in addition to these milestone payments. Although we believe that in the distant future some of the milestones contained in our in-license agreements may be achieved, it is highly unlikely that a significant number of them will be achieved. Because the milestones are highly contingent and we have limited control over whether the development and regulatory milestones will be achieved, we are not in a position to reasonably estimate how much, if any, of the potential milestone payments will ultimately be paid, or when. Additionally, under the in-license agreements, many of the milestone events are related to progress in clinical trials which will take at least several years to achieve.
Moreover, in connection with the Merger, each of the pre-Merger stockholders of NeoPharm was distributed a contingent payment right, or CPR, for each share of NeoPharm common stock then-held by such stockholder. Each CPR entitles the holder to receive a pro rata share of up to an aggregate of $20.0 million, payable in cash, if, within five years of the Merger, one of the NeoPharm product candidates that was in development prior to the Merger receives FDA approval. Of these product candidates, we are actively developing LEP-ETU, but we cannot predict when, if ever, this product candidate will receive FDA approval, and we believe the probability of making this payment is low.
Quantitative and Qualitative Disclosure About Market Risk
Our exposure to market risk includes our cash and cash equivalents, which we may invest in high-quality financial instruments. Our cash may be subject to interest rate risk and could fall in value if interest rates were to increase. Additionally, the interest expense we incur on our outstanding debt is subject to interest rate risk because it is based on the prime rate, and as a result, our obligations may increase in the future if interest rates were to increase. We do not hedge interest rate exposure. Because most of our transactions are denominated in U.S. dollars, we do not have any material exposure to fluctuations in currency exchange rates.
Recently Issued Accounting Pronouncements
In January 2010, the FASB issued amended guidance on fair value measurements and disclosures. The new guidance requires additional disclosures regarding fair value measurements, amends disclosures about postretirement benefit plan assets, and provides clarification regarding the level of disaggregation of fair value disclosures by investment class. This guidance is effective for interim and annual reporting periods beginning after December 15, 2009, except for certain Level 3 activity disclosure requirements that will be effective for reporting periods beginning after December 15, 2010. Accordingly, we adopted this amendment on January 1, 2010, except for the additional Level 3 requirements which were adopted on January 1, 2011. The adoption had no impact on our consolidated financial statements.
In April 2010, the FASB issued an accounting standard update which provides guidance on the criteria to be followed in recognizing revenue under the milestone method. The milestone method of recognition allows a vendor who is involved with the provision of deliverables to recognize the full
84
Table of Contents
amount of a milestone payment upon achievement, if, at the inception of the revenue arrangement, the milestone is determined to be substantive as defined in the standard. The guidance is effective on a prospective basis for milestones achieved in fiscal years and interim periods within those fiscal years, beginning on or after June 15, 2010. Early adoption is permitted. We adopted this guidance on January 1, 2011 and it did not have a material impact on our consolidated financial statements.
In December 2010, the FASB issued guidance relating to the disclosure of supplementary pro forma information for business combinations. This guidance specifies that if a public entity presents comparative financial statements, the entity should disclose revenue and earnings of the combined entity as though the business combination(s) that occurred during the current year had occurred as of the beginning of the comparable prior annual reporting period only. This guidance also expands the supplemental pro forma disclosures to include a description of the nature and amount of material, nonrecurring pro forma adjustments directly attributable to the business combination included in the reported pro forma revenue and earnings. This guidance is effective prospectively for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2010. Early adoption is permitted. We adopted this guidance in 2010 and the disclosures relating to the Merger in Note 10 to our audited consolidated financial statements included elsewhere in this prospectus are made based on this guidance.
85
Table of Contents
Overview
We are a specialty pharmaceutical company that develops and seeks to commercialize innovative pharmaceutical products that target the unmet needs of cancer patients, with an initial focus on cancer-supportive care. We focus our research and development efforts on product candidates that utilize innovative formulations to address the clinical shortcomings of existing commercial pharmaceutical products. We have two product candidates, our proprietary fentanyl spray Subsys and our generic Dronabinol SG Capsule, under review for marketing approval by the U.S. Food and Drug Administration, or FDA. We intend to build a capital-efficient commercial organization to market Subsys and our other proprietary products, if approved. We expect to utilize an incentive-based sales model similar to that employed by Sciele Pharma, Inc. and other companies previously led by members of our board, including our founder and Executive Chairman.
In March 2011, we submitted a New Drug Application, or NDA, to the FDA for Subsys, a sublingual spray for the treatment of breakthrough cancer pain, or BTCP, in opioid-tolerant patients. The FDA notified us in May 2011 that it had accepted the NDA for review and initially assigned a Prescription Drug User Fee Act, or PDUFA, goal date of January 4, 2012 for its review of the NDA. BTCP is characterized by sudden, often unpredictable, episodes of intense pain which can peak in severity at three to five minutes despite background pain medication. Subsys is a proprietary, single-use product that delivers fentanyl, an opioid analgesic, in seconds for transmucosal absorption underneath the tongue. In our pivotal Phase 3 clinical trial, Subsys demonstrated statistically significant pain relief at five minutes, which represents a result that has not been reported by any other competitor in this class of products for the treatment of BTCP. We believe this product is further differentiated by ease and speed of administration relative to the most widely-prescribed current treatments, including Actiq, a lozenge which requires patient manipulation for up to 15 minutes to fully dissolve, and Fentora, a buccal tablet which takes 14 to 25 minutes to fully disintegrate. According to IMS Health, transmucosal immediate-release fentanyl, or TIRF, products generated $440 million in U.S. sales in 2010. We believe the TIRF market has the potential to expand if faster-acting and more convenient products such as Subsys are approved by the FDA and the product class is more effectively promoted to oncologists and pain specialists. If approved, we currently plan on marketing Subsys in the United States through a targeted sales force of approximately 50 to 75 representatives.
In June 2010, we submitted an amendment to our Abbreviated New Drug Application, or ANDA, to the FDA for Dronabinol SG Capsule. Dronabinol SG Capsule is a dronabinol soft gelatin capsule intended to be a generic equivalent to Marinol, a currently approved treatment for chemotherapy-induced nausea and vomiting, or CINV, and appetite stimulation in patients with AIDS. Dronabinol, the active ingredient in Marinol, is a synthetic cannabinoid whose chemical name is delta-9-tetrahydrocannabinol, or THC. Dronabinol SG Capsule is the first in our family of dronabinol products that we are developing for the treatment of CINV and appetite stimulation in patients with AIDS, as well as other indications where synthetic THC could have potential therapeutic benefits, including central nervous system, or CNS, disorders such as multiple sclerosis, or MS. If approved, we intend to commercialize Dronabinol SG Capsule with the aim of generating near-term cash flows to help fund the commercialization of Subsys and the development of our proprietary dronabinol and other product candidates, as well as to validating our dronabinol supply chain and internal manufacturing capabilities. If Dronabinol SG Capsule is approved by the FDA, we intend to submit a supplemental ANDA, or sANDA, to the FDA for a proprietary dronabinol soft gel formulation that is stable at room temperature, which we refer to as Dronabinol RT Capsule. We believe this formulation, if approved, would offer convenience advantages to distributors, pharmacies and patients, as product labeling for Marinol requires storage at refrigerated temperatures. Finally, we believe we have an additional competitive advantage by producing our clinical and commercial supply of dronabinol active pharmaceutical ingredient, or API, in our U.S.-based, state-of-the-art dronabinol manufacturing facility.
86
Table of Contents
We are also developing additional proprietary formulations of dronabinol, the most advanced of which is Dronabinol Oral Solution, an orally administered liquid formulation. We have completed an end-of-Phase 2 meeting with the FDA and plan to initiate a pivotal bioequivalence study for this product candidate in the second half of 2011. As Marinol is characterized by a highly variable bioavailability and an onset of action that ranges from 30 minutes to one hour, we believe a significant need exists for a dronabinol product with a more consistent bioavailability and faster onset of action. In our Phase 1 clinical trial, Dronabinol Oral Solution demonstrated a more reliable absorption profile and rapid onset of action as compared to Marinol, which we believe will offer dosing and efficacy advantages. We believe these product attributes, coupled with increased acceptance of THC as a therapeutic alternative, could result in Dronabinol Oral Solution capturing market share and potentially expanding the market for dronabinol-based products, which, in 2010, generated approximately $142 million in U.S. sales. Importantly, we believe our family of dronabinol products, if approved, has the potential to capture a broader share of the CINV market, which, according to IMS Health, generated $1.9 billion in U.S. sales in 2010.
The National Cancer Institute estimates that as of January 1, 2008, there were approximately 12.0 million people in the United States who had been previously diagnosed or were living with cancer. According to the American Cancer Society, the number of patients with cancer continues to increase as the population ages and diagnosis, treatment and survival rates improve due to higher standards of care and greater patient access to health care. Cancer patients often suffer from symptoms such as pain, nausea, vomiting, fatigue, weight loss and anemia as a result of their cancer or radiation and chemotherapy treatments intended to eradicate or inhibit the growth of cancerous cells and tumors. Pain is a widely prevalent symptom of cancer patients, of whom it is estimated that between 50 to 90% also suffer from BTCP. We believe that the acute pain episodes of BTCP patients are not adequately managed by oncologists and pain specialists, creating an opportunity for us to educate these medical professionals and promote effective BTCP management using Subsys. According to a 2004 study by the American Society of Clinical Oncology, it is estimated that 60 to 80% of all cancer patients who receive chemotherapy experience nausea and vomiting associated with their therapy. We believe current therapies do not adequately address the needs of many of these patients. Supportive care is an important component in the treatment of cancer patients, as suggested by an August 2010 article in the New England Journal of Medicine indicating that improved supportive care in cancer patients prolonged median survival by over two months. By focusing on supportive care products, we believe we can contribute to the improvement of cancer patient outcomes and survival rates.
In addition to our cancer-supportive care products, we are developing a portfolio of proprietary cancer therapeutics, the most advanced of which is LEP-ETU, which recently completed a Phase 2 clinical trial in metastatic breast cancer. LEP-ETU is a proprietary NeoLipid liposomal, or microscopic membrane-like structure created from lipids, formulation that incorporates paclitaxel, the active ingredient in the cancer chemotherapy drugs Taxol and Abraxane. We are developing this product candidate to improve efficacy and reduce paclitaxel-related side effects. The Phase 2 clinical trial enrolled 70 patients and LEP-ETU demonstrated a tumor response rate of 24.6% in the 69 eligible patients. According to IMS Health, paclitaxel products generated $393 million in U.S. sales in 2010.
We are led by a management team and board of directors with substantial experience founding and managing pharmaceutical and related companies. Our founder and Executive Chairman, Dr. John N. Kapoor, has held executive management and board positions at Sciele Pharma and OptionCare, Inc., among others. Dr. Kapoor has also had significant experience with cancer-supportive care products, including Marinol while he was Chairman of Unimed Pharmaceuticals, Inc. Our President and Chief Executive Officer, Michael L. Babich, has board and management experience at Alliant Pharmaceuticals, Inc. and EJ Financial Enterprises, Inc. Our Director of Scientific Development, Dr. Daniel D. Von Hoff, is a renowned oncologist and a founder of ILEX Oncology, Inc. Dr. Von Hoff previously led the development of several approved cancer and cancer-supportive care therapies including drugs such as Campath, Camptosar and Clofarabine. Our Chief Medical Officer, Dr. Larry Dillaha, served as the Chief Medical Officer of Sciele Pharma. We intend to leverage the experience of
87
Table of Contents
our management team to build Insys into a leading specialty pharmaceutical company focused on commercializing innovative therapies that address unmet medical needs of cancer patients.
Strategy
The key elements of our strategy are to:
| • | Obtain FDA approval of Subsys. In March 2011, we submitted an NDA to the FDA for Subsys with a proposed Risk Evaluation and Mitigation Strategies, or REMS, program. The FDA notified us in May 2011 that it had accepted the NDA for review and initially assigned a PDUFA goal date of January 4, 2012 for its review of the NDA. In addition, we are currently working jointly with companies in the TIRF space in developing a classwide REMS program. |
| • | Build a capital-efficient commercial organization to market Subsys and complementary products. We intend to commercialize Subsys and our proprietary dronabinol products through a capital-efficient commercial organization utilizing an incentive-based sales model similar to that employed by Sciele Pharma and other companies previously led by members of our board of directors, including our founder and Executive Chairman. We intend to target our product detailing efforts primarily towards oncologists, pain specialists and centers that focus on supportive care. We also intend to launch a related marketing campaign directed at patient advocacy groups, clinicians, researchers and the academic community. |
| • | Obtain FDA approval of Dronabinol SG Capsule and Dronabinol RT Capsule, and commercialize these products through our distribution agreement with Mylan Pharmaceuticals Inc., or Mylan. In June 2010, we submitted an amendment to our ANDA for Dronabinol SG Capsule. If our Dronabinol SG Capsule ANDA is approved, we intend to submit an sANDA for Dronabinol RT Capsule. We have partnered with Mylan to distribute Dronabinol SG and RT Capsules, if approved. If approved, we intend to use cash flows from Dronabinol SG and RT Capsules to help fund the commercialization of Subsys and the development of our proprietary dronabinol and other product candidates. |
| • | Develop innovative dronabinol formulations to expand usage of synthetic THC for CINV and appetite stimulation in AIDS patients, as well as other indications. We believe there is an unmet patient need for a more reliable and effective synthetic THC for treating CINV and appetite stimulation in patients with AIDS, as well as other indications. We plan to initiate a pivotal bioequivalence study for our proprietary Dronabinol Oral Solution in the second half of 2011. Our Dronabinol Oral Solution has demonstrated what we believe is a promising product profile in Phase 1 clinical development. We are also evaluating our proprietary Dronabinol Inhalation Device and Dronabinol IV Solution in preclinical testing. Since dronabinol is difficult to import, procure and produce, we have acquired a U.S.-based, state-of-the-art dronabinol manufacturing facility, which we anticipate will be able to supply the API for all of our dronabinol product candidates. |
| • | Advance clinical development of LEP-ETU. The results from the Phase 2 clinical trial of LEP-ETU in 70 patients with metastatic breast cancer demonstrated a tumor reduction response rate of 24.6% for our product candidate in the 69 eligible patients. Based on the results of the Phase 2 clinical trial, we intend to further develop this product candidate in indications such as breast, gastric and ovarian cancers. |
| • | Add commercial products or product candidates to our portfolio that complement our core competencies. We believe our core competencies include the development of superior formulations of existing compounds as well as building and operating a capital-efficient, incentive-based commercial organization. We intend to leverage our proprietary spray technology and know-how to develop additional existing compounds that we believe would benefit from a more rapid onset of action and convenient administration. We may also pursue opportunities to acquire commercial products or product candidates that could further leverage our planned cancer-supportive care commercial organization. |
88
Table of Contents
Our Product Candidates
The following table summarizes certain information regarding our most advanced product candidates:
| Franchise |
Product Candidate |
Regulatory Pathway |
Indication |
Status | ||||||
| Spray | Subsys |
505(b)(2) | BTCP in Opioid-Tolerant Patients |
NDA Accepted; PDUFA goal date January 4, 2012 | ||||||
| Dronabinol |
Dronabinol SG Capsule | ANDA | CINV and Appetite Stimulation in Patients with AIDS |
ANDA Submitted3 | ||||||
| Dronabinol RT Capsule
|
sANDA1 | } | Pending4 | |||||||
| Dronabinol Oral Solution | 505(b)(2)1 | CINV and Appetite Stimulation in Patients with AIDS2 |
Pre-Phase 35 | |||||||
| Dronabinol Inhalation Device
|
505(b)(2)1 | Preclinical | ||||||||
| Dronabinol IV Solution | 505(b)(2)1 | Preclinical | ||||||||
| Oncology |
LEP-ETU | TBD | Metastatic Breast Cancer2 | Phase 2 | ||||||
| 1 | Anticipated regulatory pathway |
| 2 | Initial targeted indication |
| 3 | ANDA under expedited review |
| 4 | sANDA expected to be filed in the first-quarter of 2012, assuming approval of Dronabinol SG Capsule ANDA in the third quarter of 2011 |
| 5 | End-of-Phase 2 meeting completed; planning to initiate pivotal bioequivalence study |
Subsys Product Candidate
In March 2011, we submitted an NDA to the FDA for Subsys for the treatment of BTCP in opioid-tolerant patients. The FDA notified us in May 2011 that it had accepted the NDA for review and initially assigned a PDUFA goal date of January 4, 2012 for its review of the NDA. We believe that Subsys has the potential to address certain key limitations of currently-marketed fentanyl formulations by providing more rapid onset of pain control, increased patient convenience, a higher level of bioavailability and a lower level of gastrointestinal, or GI, side effects. We submitted our NDA via the 505(b)(2) regulatory pathway. If we receive FDA approval for Subsys, we intend to build a capital-efficient, incentive-based commercial organization to market this product primarily to oncologists, pain specialists and centers that focus on supportive care. In addition, we may conduct post-marketing clinical trials to seek to establish other advantages that Subsys may have over existing fentanyl products.
Fentanyl is an opioid analgesic approved in the United States for acute and chronic pain management. Depending upon the type of pain, physicians currently prescribe fentanyl in three forms of administration: injectable, transmucosal, or delivery by diffusion through the mucous membranes of the mouth, and transdermal, or delivery through the skin. Fentanyl imitates natural biochemicals found in the body that moderate pain and block the transmission of pain signals that travel along nerves to the brain. We believe these properties make fentanyl a potent and effective therapy for use in patients with cancer who suffer from acute or breakthrough episodes of pain.
Subsys is a proprietary, single-use product developed to treat BTCP through the delivery of a liquid fentanyl formulation in 100, 200, 400, 600 and 800 microgram, or mcg, dosages. We have also tested Subsys in 1200 and 1600 mcg doses, which are delivered by consecutively spraying two 600 and 800 mcg unit dose spray products, respectively. The mechanism by which the liquid is delivered is a highly consistent, one-step process in which a plume of fentanyl is generated by the actuation of the device.
89
Table of Contents
The plume disperses a small volume of liquid across the surface area of the sublingual mucosa and facilitates rapid absorption by the body.
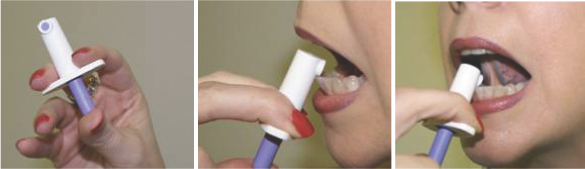
Cancer Pain Market Overview
Cancer pain can occur as a result of tumors pressing on nerves, damage caused by cancer cells in bone and treatments for cancer such as chemotherapy, radiation therapy or surgery. Many cancer patients experiencing pain suffer from two types of pain: (1) persistent or continuous pain, which is typically managed by long-acting or sustained-release drugs taken by patients on a regular schedule, and (2) breakthrough pain, which can be severe and sudden, and may require a stronger, fast-acting medication. Opioids are the most widely-prescribed treatment for cancer pain followed by medications commonly used to treat inflammatory pain, such as corticosteroids, anesthetics, non-steroidal anti-inflammatory drugs, anticonvulsants and antidepressants. A report published by Worldwide Marketing Research estimated that the value of the U.S. cancer pain market was $3.1 billion in 2008 and will increase to $5.3 billion by 2018.
BTCP is characterized by sudden, often unpredictable, episodes of intense pain which peak in severity at three to five minutes despite background pain medication. These episodes can last several minutes to an hour, and usually occur several times per day. Pain is a widely prevalent symptom of cancer patients, of whom it is estimated that between 50 to 90% suffer from BTCP, which is particularly difficult to treat due to its severity, rapid onset and the often unpredictable nature of its occurrence. Physicians typically treat BTCP with a variety of short-acting opioid medications, including morphine, morphine and codeine derivatives and fentanyl.
Morphine and morphine and codeine derivatives have been available for decades in immediate-release forms of tablets, capsules or liquids that are ingested by the patient. More recently approved short-acting opioid-based fentanyl formulations utilize transmucosal delivery in an attempt to improve upon existing fentanyl therapies. Cephalon, Inc.’s Actiq, approved by the FDA in 1998 and now available in several generic options, is an oral transmucosal lozenge, and Fentora, approved by the FDA in 2006, is a fentanyl buccal tablet. BioDelivery Sciences International, Inc.’s Onsolis, a soluble film placed on the buccal area after wetting the inside of the cheek with saliva or water, was approved in 2009 by the FDA. Most recently, in January 2011, ProStrakan Group plc’s received FDA approval for Abstral, an immediate-release transmucosal sublingual tablet. According to IMS Health, oral fentanyl products indicated for BTCP generated $440 million in U.S. sales in 2010. Although these existing therapies provide improvements over oral opioids, we believe that the current treatment options have limitations and that there remains a significant unmet need for therapies that provide faster pain relief, more convenient dose administration and a better pharmacokinetic, or PK, profile.
Limitations of Existing Therapies
We believe that the BTCP market is underserved due to the limitations of existing therapies, which include:
| • | Time until statistically significant pain relief: Patients suffering from BTCP require rapid pain relief as peak intensity of episodic breakthrough pain can occur between three and five minutes |
90
Table of Contents
| from the onset of pain symptoms. The peak effect of transmucosal delivery systems may be delayed as it may take up to 14 to 30 minutes for the lozenge or tablet to fully dissolve and be absorbed. In addition, oral immediate-release opioids are metabolized in the liver and consequently may take up to 30 to 45 minutes to become effective. |
| • | Inconvenient delivery: We believe current commercially available therapies do not adequately address patient ease of use and convenience needs. Existing BTCP therapies can require an administration period of several minutes, disrupt daily activities and cause patient discomfort. For example, some products require patients to place lozenges between their cheeks and lower gums and rub the lozenge from side to side over a 15-minute period. In addition, patients with dry mouth and oral mucositis may experience difficulty in using some current commercially available therapies. |
| • | Pharmacokinetic profile: Actiq, the current market leader, and its generic equivalents achieve bioavailability of approximately 50% and require 15 to 30 minutes for absorption. Up to half of the delivered dose of competing treatments is swallowed and is absorbed slowly through the GI tract, which we believe may delay the onset of pain relief and contribute to side effects. |
| • | Limited dosage forms: Actiq and its generic equivalents are available in six dosage strengths ranging from 200 to 1600 mcg. No other commercially available BTCP therapies are offered in the 1200 and 1600 mcg dosage range. According to IMS Health, approximately 45% of the U.S. sales of Actiq in 2010 were in the 1200 and 1600 mcg doses. |
Our Solution
We believe Subsys’s formulation and sublingual delivery mechanism will offer several advantages over current FDA-approved transmucosal treatment alternatives, and these advantages may lead to improved patient compliance and expanded medical use of fentanyl for BTCP. Such advantages include:
| • | Statistically significant pain relief in five minutes: Subsys is the only product to show statistically significant pain relief when measuring the sum of pain intensity difference at five minutes in a Phase 3 BTCP clinical trial using fentanyl. We believe that Subsys is able to achieve this rapid delivery of fentanyl through sublingual delivery because there is a high density of blood vessels beneath the tongue and the thin layer in the mucosa enables higher absorption. The product sprays in a manner that is designed to maximize the area covered by the product. |
| • | Administered in seconds: Subsys is administered in one step using a small handheld delivery system that sprays fentanyl beneath the patient’s tongue. This delivery mechanism allows for administration in a matter of seconds, rather than the 14 to 30 minutes or more required for Actiq and Fentora. Further, Subsys can be administered without moistening the tongue or cheek, allowing for administration in cancer patients suffering from dry mouth and oral mucositis. |
| • | Superior pharmacokinetic profile: As compared to Actiq’s PK profile, Subsys’s PK profile is characterized by higher peak blood concentrations, which are achieved at a more rapid rate. This profile is, in part, due to greater than 85% absorption occuring transmucosally, resulting in higher bioavailability. Because a small volume of liquid is sprayed on to the sublingual mucosa, we believe this method of administration reduces the amount of liquid swallowed and subsequently absorbed via the digestive system. As a result, we believe that less fentanyl is exposed to first-pass metabolism in the liver. |
| • | Full range of dosage strengths allows for proper titration and better pain relief: If approved, Subsys will be available in the most complete spectrum of dosage levels per pain episode in the TIRF market, at 100, 200, 400, 600, 800, 1200 and 1600 mcg. We believe it is important to offer a product in all dose ranges for the treatment of BTCP, as all branded products, and, to our knowledge, all product candidates currently in development, are not, or will not be, available in the 1200 and 1600 mcg dosage strengths. |
91
Table of Contents
Completed Clinical Trials
We have completed two Phase 3 clinical trials and two Phase 1 clinical trials involving an aggregate of over 500 patients to support our NDA for Subsys.
Phase 3 Clinical Trials
Our Phase 3 clinical program for Subsys was comprised of a 130-patient safety and efficacy trial and a 300-patient safety trial. We conducted these clinical trials based on guidance received from the FDA in our December 2007 end-of-Phase-2 meeting and subsequent dialogue.
Our Phase 3 safety and efficacy trial was a randomized, double-blind, placebo-controlled study conducted at 27 U.S. clinical sites. Patients enrolled in the study experienced one to four BTCP episodes during a four-day screening period and were opioid-tolerant, defined as actively using long-acting oral opioids or transdermal fentanyl as a background analgesic and short-acting solid oral opioids to manage breakthrough episodes. Prior to entering the treatment period, patients were titrated to establish the optimal dose of Subsys to relieve their BTCP. Patients could receive 100, 200, 400, 600, 800, 1200 (2 x 600 mcg) and 1600 mcg (2 x 800 mcg) doses of Subsys. Of the 130 patients enrolled in the safety and efficacy trial, 92 were evaluated in the efficacy analysis. The primary endpoint of this study was pain relief at 30 minutes using the sum of pain intensity differences, or SPID, at 30 minutes for Subsys versus placebo. We also evaluated the secondary endpoints of SPID across time periods ranging from five minutes to 60 minutes post-administration as well as safety, tolerability and acceptability. Subsys met all primary and secondary endpoints with statistical significance and, notably, demonstrated statistically significant pain relief at five minutes. Statistical significance is a measure of the strength of a conclusion that can be drawn from the data. Clinical trial results are considered statistically significant when the probability of the results occurring by chance, rather than from the efficacy of the drug candidate, is sufficiently low. Statistical significance is measured by the probability value, or p-value. A clinical trial result with a p-value of less than 0.05 means that the probability of the same trial results occurring randomly or by chance is less than 5%, and is generally considered to be statistically significant. For our efficacy study, our primary endpoint had a p-value of <0.0001 and all secondary endpoints had p-values of <0.05. The results of this study are presented below.
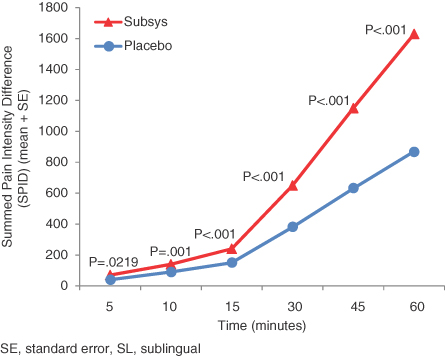
92
Table of Contents
Our Phase 3 safety trial was a three-month open-label study conducted at 46 sites in the United States and 10 sites in India. Patients enrolled in the study included those rolled-over from the Phase 3 safety and efficacy study as well as new patients that met the same major inclusion criteria. The new patients were also titrated to the optimal dose of Subsys for the study period. The primary endpoint of this study was safety over a three-month treatment period. We were required to enroll 300 patients in this study, of which 150 needed to complete 90 days on the treatment. No serious adverse events were reported in this study. The most common adverse events observed in this trial were principally those identified as typical of fentanyl products, including sleepiness, dizziness, nausea, vomiting and shortness of breath.
Phase 1 Clinical Trials
We have conducted two Phase 1 clinical trials evaluating the absorption rate, bioavailability and PK of Subsys. The results of our Phase 1 open-label trial in 21 healthy, normal volunteers, completed in April 2007, compared the rate of absorption and availability of the active drug to the patients on Subsys relative to patients receiving Actiq and a fentanyl intravenous injection, or fentanyl IV. These results are presented below.
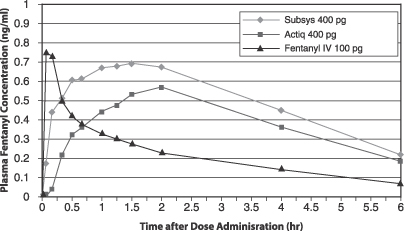
In the trial, we observed Subsys reaching higher peak blood concentration, or Cmax, than Actiq, as well as a more rapid rate of absorption, or Tmax. Subsys had a mean Cmax of 0.813 nanograms per milliliter, or ng/mL, versus 0.607 ng/mL for Actiq. In addition, Subsys reached maximum concentration in the body in approximately 1.3 hours versus 1.7 hours for Actiq. We also observed that Subsys remained in the body at higher levels when compared to the same dose of Actiq. As expected, we observed that fentanyl IV achieved a higher Cmax more rapidly than Subsys, but that its plasma concentration in the body declined much more rapidly than Actiq and Subsys. Cmax for fentanyl IV was 0.929 ng/mL and time to maximum plasma concentration was 0.16 hours.
Data from our Phase 1 clinical trial relative to PK results supports our belief in the relatively rapid absorption of fentanyl using Subsys. The data further illustrates that the duration of action of Subsys is comparable to Actiq, providing support for our belief that Subsys may be a faster and more convenient alternative to existing treatment options. A second Phase 1 single-site trial was completed in 49 patients evaluating PK data across five different doses of Subsys. The results suggest that the administration of fentanyl using a sublingual spray is dose-proportional over a 100 to 800 mcg range.
The final Phase 1 study was conducted in 18 patients with Grade 1 and Grade 2 mucositis. The results of this study showed no statistically significant variation in plasma blood levels in patients with mucositis compared to those without mucositis.
93
Table of Contents
Risk Evaluation and Mitigation Strategies (REMS)
In September 2007 and in December 2007, the FDA issued a safety alert to healthcare professionals and consumers concerning recent reports of deaths and other adverse events in patients using approved TIRF products. The FDA has determined that TIRF products will be required to have a REMS program to ensure that the benefits of the drugs continue to outweigh the serious risks of overdose, abuse, misuse, addiction and serious complications due to medication errors. Each REMS program includes, or will include, a Medication Guide, elements to assure safe use including prescriber certification or training, dispenser certification, and documentation of safe-use conditions, an implementation system, and a timetable for submission of assessments. This shared REMS program is being developed jointly by all manufacturers and Investigational New Drug, or IND, application holders of TIRF products. We participate actively in this collaborative development effort. This REMS program is expected to be a single shared system across the TIRF class of products. We expect that the FDA will approve a classwide REMS program in the second half of 2011. In addition, we continue to pursue an Insys-specific REMS program strategy as a potential alternative to the classwide REMS program. As a result, we believe the timeline for FDA approval of the classwide REMS program will not be an impediment to the approval of Subsys.
Dronabinol Product Candidates
We are developing a portfolio of dronabinol product candidates for the treatment of CINV and appetite stimulation in patients with AIDS, as well as other indications. This portfolio includes Dronabinol SG Capsule, for which we submitted an amendment to the ANDA to the FDA in June 2010, and our proprietary Dronabinol Oral Solution, for which we intend to begin a pivotal clinical trial in the third quarter of 2011. In addition, we intend to seek regulatory approval for Dronabinol RT Capsule through an sANDA filing upon approval of Dronabinol SG Capsule and we are evaluating proprietary inhaled and intravenous formulations of dronabinol in preclinical studies.
Dronabinol, the active ingredient in Marinol, is a synthetic form of THC. THC is an orally active cannabinoid which, like other cannabinoids, has complex effects on the CNS. Approved by the FDA in 1985, Marinol is indicated for the treatment of CINV in patients who have failed to respond adequately to conventional treatments, as well as for the treatment of appetite loss associated with weight loss in patients with AIDS. Marinol is formulated in sesame oil and encapsulated in soft gelatin capsules and must be stored in cool storage conditions or in a refrigerator.
Market Overview
CINV is a commonly known side effect of chemotherapy that can have a significant negative impact on quality of patient life. CINV is classified into five categories:
| • | Acute: Occurs within 24 hours of chemotherapy administration. |
| • | Delayed: Occurs more than 24 hours after chemotherapy administration, with peak intensity two to three days post-administration and duration of up to one week. |
| • | Anticipatory: Occurs prior to treatment. |
| • | Breakthrough: Occurs after use of antiemetic agents. |
| • | Refractory: Occurs after failed use of breakthrough therapy. |
The majority of chemotherapy patients experience at least one type of CINV. The National Comprehensive Cancer Network, or NCCN, estimates that 70 to 80% of patients undergoing chemotherapy experience vomiting, with 10 to 44% experiencing anticipatory vomiting. Predictive factors for developing CINV can include: age of less than 50 years, female gender, vomiting during previous chemotherapy, pregnancy-induced nausea/vomiting, history of motion sickness and anxiety. In addition to generally affecting patient quality of life, CINV can result in weakness, weight loss,
94
Table of Contents
electrolyte imbalance, dehydration or anorexia. According to a study published by Ballatori, et al in 2007, 90% of patients who experienced CINV reported an impact on daily activities.
Although the pathophysiology of CINV is not clearly understood, it is thought that chemotherapeutic agents cause vomiting by activating neurotransmitter receptors located in the chemoreceptor trigger zone, GI tract, and vomiting center, or VC. Activation of the VC directly or through the chemoreceptor trigger zone results in stimulation of the salivation and respiratory centers as well as control of the pharyngeal, GI and abdominal muscles. This stimulation can trigger the body to retch and vomit.
Treatment of CINV is highly patient-specific and is based on the emetogenic potential of the chemotherapy regimen. According to IMS Health, U.S. sales for drugs treating CINV were $1.9 billion in 2010, though published reports suggest that current therapies are not entirely effective. A 2004 report published in Cancer estimated that approximately 35% of patients treated with CINV therapies continue to experience acute nausea, with 13% of CINV patients experiencing acute vomiting after first-line treatment.
Limitations of Existing Therapies
We believe that the synthetic cannabinoid market is underserved due to the limitations of existing therapies, which include:
| • | Cool storage requirement: Marinol and its generic equivalents require cool storage conditions at 8 to 15°C or in a refrigerator. We believe this cool storage requirement limits the market potential for Marinol by making its treatment of CINV and appetite stimulation in patients with AIDS inconvenient for patients and physicians and also increasing costs and storage burdens for pharmacies and distributors. |
| • | Delayed onset of action: Marinol is only available in a capsule formulation, which must be dissolved and digested before it is metabolized in the patient’s liver, where the drug is broken down by enzymes. We believe that this capsule formulation and digestion process delays onset of action and relief of nausea and vomiting. After oral administration, Marinol has an onset of action of approximately 30 minutes to one hour and peak effect at two to four hours. |
| • | Side effects: Marinol side effects include euphoria, dizziness and confusion. Such side effects are greater or more severe in patients taking higher doses. The variability levels among patients vary widely, making it difficult to predict the level or severity of side effects experienced by different patients. |
| • | Lower level of efficacy: Due to the capsule formulation and digestion process of Marinol, only 10 to 20% of an administered dose of Marinol reaches the systemic circulation in the body. This poor absorption profile significantly reduces the bioavailability of the API in patients using its capsule formulation which may result in lower efficacy. |
| • | Lack of flexibility in dosing: Marinol and its generic equivalents are only available in 2.5, 5.0 and 10.0 milligram, or mg, soft-gelatin capsules. The fixed dosage amounts may cause patients to ingest improper dosage amounts, which can result in increased side effects and/or a lower level of efficacy. |
Our Solutions
We believe our family of dronabinol products has the potential to address many of the limitations that exist in synthetic cannabinoid products by providing a number of key advantages, including:
| • | Room temperature storage: Dronabinol RT Capsule, if approved, will allow for storage in room temperature conditions, thereby allowing more storage flexibility and convenience to distributors, physicians, pharmacies and patients. |
95
Table of Contents
| • | Faster onset of action: Dronabinol Oral Solution is a liquid solution and therefore is absorbed faster than a capsule formulation which has to dissolve in the GI tract. We believe that quicker absorption will lead to faster onset of action for an oral solution product. Separately, we believe that our Dronabinol Inhalation Device and Dronabinol IV Solution product candidates, currently in preclinical studies, will further enhance dronabinol’s onset of action due to their delivery into the lungs and systemic circulation, respectively, thereby bypassing first-pass metabolism in the liver. |
| • | Reduced side effects: Based on our 18-patient PK study, we believe Dronabinol Oral Solution has lower patient-to-patient variability which should lead to more consistent patient responses. Due to higher absorption rates anticipated for our Dronabinol Inhalation Device, we anticipate that lower dosages of this formulation will be required as compared to Marinol. |
| • | Level of efficacy: By bypassing first-pass metabolism in the liver, we believe both Dronabinol IV Solution and Dronabinol Inhalation Device will demonstrate lower patient-to-patient variability compared to Marinol and, as a result, greater efficacy. |
| • | Flexibility in dosing: Dronabinol Oral Solution allows for greater flexibility across the dosing range versus the fixed dosing necessitated by Marinol. We believe that giving physicians and patients an improved product with the opportunity to more properly titrate may increase market acceptance of dronabinol. |
Dronabinol SG Capsule
In June 2010, we submitted an amendment to our ANDA for Dronabinol SG Capsule to the FDA. If approved, Dronabinol SG Capsule will be a generic version of Marinol. Dronabinol SG Capsule is a simple solution of dronabinol in sesame oil that is encapsulated in soft gelatin. Dronabinol SG Capsule will be supplied in three dosage strengths containing 2.5, 5 or 10 mg. For the three dosage strengths, the percent composition of the capsule fill contents is identical to that of the respective three dosage strengths of Marinol capsules, the reference listed drug. If Dronabinol SG Capsule is approved by the FDA, we intend to commercialize this product candidate through Mylan pursuant to our May 2011 supply and distribution agreement.
We believe that Dronabinol SG Capsule, if approved, will provide us with near-term cash flow to help fund the commercialization of Subsys and the development of our proprietary dronabinol and other product candidates.
Clinical Trials and Regulatory Status
From 2006 to 2008, we pursued the development of a hard gelatin dronabinol capsule as a generic alternative to Marinol. In August 2009, we terminated this program based on interactions with the FDA and committed to pursue approval of a soft gelatin capsule. Prior to 2008, the FDA issued two “major deficiency” letters citing various deficiencies relating to our ANDA for the hard gelatin capsule formulation. In response to the FDA’s request, we made new registration batches of soft gelatin capsules and performed a new bioequivalence study. This study was completed and data from this study along with responses to other deficiencies was submitted to the FDA in the form of a major amendment in June 2010. On October 15, 2010, we received a letter from the FDA expressing the need for clarifications related to bioequivalence and we responded to this letter on November 15, 2010. In December 2010, we received a quality deficiency “minor” letter from the FDA requesting information and/or clarification regarding the raw material components, composition of the three proposed dosage strengths and container closure components, to which we responded in January 2011. Separately in January 2011, we received another deficiency letter from the FDA related to labeling comments to which we also responded in January 2011. The FDA subsequently had additional labeling related requests, to which we responded in February and March of 2011. The labeling comments and requests from the FDA related to revisions to our proposed labeling components for consistency with Marinol labeling and certain
96
Table of Contents
formatting changes. On July 7, 2011, the FDA notified us of three “telephone” deficiencies to our ANDA for Dronabinol SG Capsule. The “telephone” deficiencies we received related to the request for revised shell formulation information, a blank batch record and a filing by the holder of the Subsys drug master file. The shell formulation information had previously been submitted to the FDA by our third party manufacturer and the holder of the Subsys drug master file had previously submitted the requested filing. On July 11, 2011, we notified the FDA of the prior submissions, and we responded to the FDA’s request for the blank batch record. We believe our response has adequately addressed all the deficiencies. There can be no assurance, however, that the FDA will approve the Dronabinol SG Capsule ANDA based on our response, and the FDA may identify additional deficiencies related to our ANDA. As a general matter, amendments to ANDAs submitted in response to major deficiency letters or amendment requests are given the same review priority as original, non-reviewed ANDAs by the Office of Generic Drugs, or OGD. They are generally placed into the 180-day queue and reviewed in accordance with OGD’s first in-first reviewed procedures. In contrast, ANDA amendments submitted in response to minor FDA deficiency letters or amendment requests are generally given a higher priority review than major amendments because they often mean an ANDA is close to approval and should, therefore, be given priority. The FDA generally reviews minor amendments within 30 to 60 days. As a general matter, “telephone” deficiencies primarily relate to administrative or minor technical issues, and the FDA endeavors to review responses to “telephone” deficiencies upon receipt. If approved by the FDA, Dronabinol SG Capsule could be the second FDA-approved generic version of Marinol.
Dronabinol RT Capsule
Dronabinol RT Capsule is a line extension of the Dronabinol SG Capsule, and is part of our strategy of developing additional products by applying improved formulations to existing pharmaceutical compounds. Dronabinol RT Capsule, for which we have filed a patent application, includes an antioxidant additive to stabilize the synthetic THC at room temperature. We believe that this formulation, if approved, will eliminate the need for cool or refrigerated storage, a requirement for Marinol and Dronabinol SG Capsule, and will make treatment of CINV and appetite stimulation in patients with AIDS, as well as other indications, more convenient for patients and physicians, while concurrently reducing costs and storage burdens for pharmacies. As a result, while some patients may switch from Dronabinol SG Capsule to Dronabinol RT Capsule, we believe that Dronabinol RT Capsule, if approved, could help us further penetrate and potentially expand the market for medicinal dronabinol drugs as patients, physicians, pharmacists and payors recognize the convenience of a room temperature treatment option.
Regulatory Status. If our ANDA for Dronabinol SG Capsule is approved by the FDA in the third quarter of 2011, we intend to file an sANDA seeking FDA approval for Dronabinol RT Capsule in the first quarter of 2012.
Dronabinol Oral Solution
Dronabinol Oral Solution is a proprietary synthetic THC in an oral liquid formulation, which contains ingredients to enhance and sustain absorption. Because it is a liquid formulation as opposed to a capsule, we believe that this product candidate may provide increased flexibility in dosing, more convenient delivery and improved absorption profile in patients. We believe these attributes may ultimately increase patient compliance because of better efficacy and fewer side effects, which we believe may permit us to further penetrate and expand the market for the medical use of dronabinol.
Clinical Trials and Regulatory Status. In early 2009, we conducted an 18-patient crossover bioavailability and PK study comparing Dronabinol Oral Solution with Marinol. The key outcomes from the study were rapid onset of action observed for the Dronabinol Oral Solution, as evidenced by 83% of subjects achieving the threshold plasma level of THC in 15 minutes with the Dronabinol Oral Solution, as compared to no patients at 15 minutes in the Marinol group, and less patient-to-patient variability was observed with the Dronabinol Oral Solution as compared to Marinol, where inter-patient variability was
97
Table of Contents
reduced at some time points by almost 50%. We conducted an end-of-Phase 2 meeting with the FDA in May 2010. After the meeting and upon receiving feedback from the FDA, we submitted a bioequivalence study design to the FDA. Our current plan is to initiate this study in the second half of 2011.
LEP-ETU Product Candidate
Paclitaxel is an anti-microtubule agent that prevents cell division by promoting the assembly and stabilization of microtubules. It is active in a broad spectrum of malignancies. Our LEP-ETU product candidate is a proprietary formulation of a liposomal delivery system for paclitaxel consisting of small uniformly sized liposomes.
We believe there are several limitations with current paclitaxel therapies, including resistance to the drug, severe hypersensitivity reaction and the lack of efficacy in many types of cancers. Based on early preclinical data, we believe LEP-ETU has the potential to address many of the limitations of existing paclitaxel therapies by providing a number of key advantages, which may include better antitumor activity, no allergic reactions and potentially more activity in the tumor types for which current paclitaxel formulations are not approved for use. We believe small-sized trials may help us demonstrate improved efficacy in additional cancer indications.
We have conducted a Phase 2 clinical trial in India in patients with metastatic breast cancer, the results of which have not yet been presented to the FDA. We have currently evaluated tumor response rates using National Cancer Institute sanctioned criteria from 70 patients. Results from the evaluation demonstrated that LEP-ETU achieved a tumor response rate of 24.6% in the 69 eligible patients. We intend to discuss the overall dataset with the FDA in the second half of 2011.
Other Product Candidates
Our other product candidates include other dronabinol line extensions and dronabinol combination products, fentanyl label expansion and analogues of morphine-6-O-sulfate.
Other Dronabinol Line Extensions and Dronabinol Combination Products. We plan to develop additional dronabinol delivery systems, including Dronabinol Inhalation Device and Dronabinol IV Solution. We are considering the use of dronabinol for additional treatment indications, including the treatment of MS. In addition, we are evaluating and developing product candidates that combine dronabinol with other compounds for the treatment of pain disorders and cancer cachexia, a progressive loss of body weight in patients with cancer.
Proprietary Spray Technology. We are committed to leveraging our existing spray technology to acquire or in-license products or product candidates that complement our core competencies. Our scientific advisory team has identified other potential candidates for this technology platform that will focus on utilizing capital-efficient 505(b)(2) regulatory pathway strategies to help mitigate clinical and financial risk.
Fentanyl Label Expansion. We plan to investigate using fentanyl as a treatment option in other indications such as the areas of neuropathic and post-operative breakthrough pain relief. We also plan to investigate additional fentanyl delivery methods.
Morphine-6-O-Sulfate. We have licensed a morphine derivative from the University of Kentucky. Preliminary studies suggest that this morphine derivative may prolong analgesic response several hours longer than morphine and it is reported to be several times more potent. These advantages could offer improved efficacy and a reduced side effect profile. We plan to continue the development of this compound in the future.
98
Table of Contents
Sales and Marketing
Key members of our management and board have extensive experience in building and implementing capital-efficient, incentive-based commercial organizations as well as commercializing cancer-supportive care products, including dronabinol. If approved, we intend to first commercialize Subsys and then Dronabinol Oral Solution through a U.S.-based sales and marketing organization focused on cancer-supportive care. Specifically, we currently plan to market Subsys in the United States, if approved, through a targeted sales force comprised of approximately 50 to 75 representatives. We intend to build this commercial organization utilizing an incentive-based model similar to that employed by Sciele Pharma and other companies previously led by members of our board, including our founder and Executive Chairman. This model employs a pay structure where a majority of the compensation paid to sales representatives comes in the form of potential bonuses based on sales performance. Our product detailing efforts will focus primarily on oncologists, pain specialists and centers that cater to supportive care. We also intend to launch a marketing campaign directed at patient advocacy groups, clinicians, researchers and the academic community. Subject to marketing approval in the relevant countries, we intend to engage sales, marketing and distribution partners in Europe, Asia and Latin America to sell our product candidates in those territories.
We believe the key factors in successful adoption of Subsys will include taking share from leading fentanyl products and expanding the BTCP market further by building awareness of its rapid onset of action, convenient dosing, reduced side effect profile and broader range of dosage strengths. To successfully commercialize our family of proprietary dronabinol products, we intend to focus our commercial efforts on taking share from Marinol and its generic alternatives as well as further expanding into a broader segment of the CINV market by developing awareness of our product attributes relative to currently available dronabinol products.
We entered into a supply and distribution agreement effective as of May 20, 2011 with Mylan, pursuant to which we engaged Mylan to exclusively distribute our Dronabinol SG Capsule within the United States. The agreement has a seven-year term commencing upon the first commercial sale of the Dronabinol SG Capsule product candidate with automatic one-year renawals following the initial term. Pursuant to the terms of the agreement, we are required to order our Dronabinol SG Capsule from Catalent Pharma Solutions, LLC, or Catalent, for shipment to Mylan in accordance with certain specifications, and ensure that Catalent uses commercially reasonable efforts to maintain enough Dronabinol SG Capsule inventory to satisfy Mylan’s purchase orders. Under the terms of the agreement, we are obligated to pay Mylan a royalty between 10% and 20% on Mylan’s net product sales, and a single digit percentage fee on such sales for distribution and storage services. The parties are required to agree to technical protocols and specific responsibilities for handling quality complaints related to our Dronabinol SG Capsule. The parties will mutually determine the timing for launching our Dronabinol SG Capsule; however, we may terminate the agreement if Mylan fails to launch our Dronabinol SG Capsule within 120 days after its is approval by the FDA. Mylan may terminate the agreement in the event of a negative outcome of a quality audit of our and/or Catalent’s manufacturing facilities. Additionally, we or Mylan may terminate the agreement effective upon 45 days’ prior written notice to the other party if the other party commits a material breach of the agreement and fails to cure such breach within the 45-day period or effective upon notice if the other party becomes insolvent. If approved, we intend to utilize near-term cash flows from our generic dronabinol products to help fund the commercialization of Subsys and the development of our proprietary dronabinol and other product candidates.
Manufacturers and Suppliers
We produce dronabinol, the API in our family of generic and proprietary dronabinol products, internally at our U.S.-based, state-of-the-art manufacturing facility. We believe that we have the capacity to manufacture sufficient dronabinol to meet all projected needs for our family of dronabinol products in our current facility. We believe this investment in a wholly-owned facility gives us a significant
99
Table of Contents
competitive advantage since dronabinol, a Schedule I material, is not easy to procure, is difficult to import into the United States and has a limited number of suppliers domestically.
The chemical materials for this API are sourced from independent suppliers and are manufactured utilizing well-established chemical techniques. Our manufacturing facility utilizes these chemical materials to produce dronabinol through a series of synthetic reactions and purification cycles. We believe that our suppliers are equipped to meet our current and future chemical material needs for the development and commercialization of our dronabinol-based product candidates. In March 2011, we entered into a commercial manufacturing and packaging agreement with Catalent pursuant to which we engaged Catalent on an exclusive basis to provide processing and packaging services with respect to our Dronabinol SG Capsule. Pursuant to the terms of the agreement, we are required to supply Catalent with the API for our Dronabinol SG Capsule, and following approval by the FDA, are required to purchase a minimum number of units of our Dronabinol SG Capsule pursuant to quarterly purchase orders. For units ordered, we are required to pay Catalent a per-unit fee, plus annual product maintenance fees. The initial term of the agreement is five years, unless earlier terminated, and automatically renews for additional periods of two years, unless we or Catalent give the other party at least 12 months’ prior written notice of its desire to terminate the agreement. Additionally, we or Catalent may terminate the agreement effective upon 60 days’ prior written notice to the other party if the other party commits a material breach of the agreement and fails to cure such breach within the 60-day period, if the other party becomes insolvent or upon 24 months’ prior written notice to the other party. Catalent has been selected for its specific competencies in manufacturing processes and materials.
Subsys is manufactured by contract manufacturers and sub-component fabricators. AptarGroup, Inc., a dispensing system company based in Illinois, developed the sublingual spray device that we used in our clinical development and intend to use for our commercial product, if approved. We entered into a supply agreement effective as of March 7, 2011 with AptarGroup pursuant to which AptarGroup supplies us with the delivery system to administer Subsys. We also granted AptarGroup the exclusive option to supply us with all of our requirements for Subsys drug delivery systems, and all other drug delivery systems for drugs we may develop in the future. In addition, under the agreement, AptarGroup is required to supply us exclusively with devices to administer Subsys, which obligation is dependent on several factors, including exclusivity payments to AptarGroup of less than $1.0 million, purchase order levels and our efforts to seek certain market approval for Subsys in Europe. We are required to provide AptarGroup with rolling quarterly forecasts of our requirement for Subsys drug delivery systems. Under certain circumstances, such forecasts are non-binding; however, some portions of such forecasts may constitute a firm commitment to purchase delivery systems to administer Subsys. The agreement has a term of five years from the effective date; however, either party may terminate the agreement (i) if the other party makes an assignment for the benefit of its creditors or a receiver or custodian is appointed for it or its business is placed under attachment, garnishment or other process involving a significant portion of its business, (ii) if the other party commits a material breach of the agreement and fails to commence and diligently pursue a remedy for any such material breach, (iii) if the other party becomes insolvent, or (iv) upon written notice from the terminating party if we do not receive approval of the NDA for Subsys by January 1, 2013.
We entered into a manufacturing agreement effective as of May 24, 2011 with DPT Lakewood, LLC, or DPT, pursuant to which we engaged DPT on an exclusive basis to provide processing and packaging services with respect to Subsys. Pursuant to the terms of the agreement, we are required to supply DPT with the API for Subsys, and to pay manufacturing and materials fees related to the production, packaging, administration and carrying cost of Subsys. DPT is required to manufacture Subsys in accordance with certain specifications and to conduct product testing prior to delivery. Unless terminated earlier, the initial term of the agreement will continue until December 31st of the fifth calendar year following the year in which DPT first manufactures Subsys. Thereafter, the agreement will automatically renew for 24-month periods unless either party provides notice at least 24 months prior to the expiration of the initial term. We or DPT may terminate the agreement effective upon 60 days’ prior
100
Table of Contents
written notice to the other party if the other party commits a material breach of the agreement and fails to cure such breach within the 60-day period or effective upon notice to the other party if the other party becomes insolvent.
AptarGroup and DPT have been selected for their specific competencies in manufacturing, product design and materials. FDA regulations require that materials be produced under cGMPs or QSR, as required for the respective unit operation within the manufacturing process. We believe both key suppliers have sufficient capacity to meet our projected product requirements.
Competition
Our industry is characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. We face competition from many different sources, including pharmaceutical and biotechnology companies, pharmaceutical and generic drug companies, drug delivery companies and academic and research institutions. We believe the key competitive factors that will affect the development and commercial success of our product candidates include, but are not limited to, onset of action, bioavailability, efficacy, convenience of dosing and distribution, safety, cost and tolerability profile. Many of our potential competitors have substantially greater financial, technical and human resources and greater experience in the development of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Consequently, our competitors may develop products for the treatment of BTCP, CINV and appetite stimulation in patients with AIDS, cancer or other indications we pursue that are more effective, better tolerated, more useful and less costly, and they may also be more successful in manufacturing and marketing their products. We will also face competition in recruiting and retaining qualified personnel, establishing clinical trial sites and patient registration for clinical trials and in identifying and in-licensing new product candidates.
Subsys Product Candidate
Subsys, if approved, would compete against branded and generic TIRF products indicated to treat BTCP as well as branded and generic immediate-release opioid products such as oxycodone, hydrocodone, morphine and hydromorphone, which are widely used for acute pain episodes including BTCP. Existing products include Cephalon Inc.’s Fentora and Actiq, BioDelivery Sciences International, Inc.’s Onsolis, ProStrakan Group plc’s Abstral, Nycomed International Management GmbH’s Instanyl and Archimedes Pharma Ltd.’s PecFent. Generic fentanyl products are marketed by TEVA Pharmaceuticals USA and Watson Pharmaceuticals, Inc. We would also face competition from generic drugs such as morphine, non-steroidal anti-inflammatory products, opioids and non opioid analgesics such as acetaminophen.
Additionally, we are aware of companies with product candidates in late stage development for BTCP, including AcelRx Pharmaceuticals, Inc.’s ARX-02 (Phase 3 ready) and Akela Pharma Inc.’s Fentanyl TAIFUN (Phase 3). If these technologies are successfully developed and approved over the next few years, they could represent significant competition for Subsys.
Dronabinol Family of Product Candidates
If approved for the treatment of CINV and appetite stimulation in patients with AIDS, our dronabinol family of product candidates will directly compete against Abbott Laboratories’ Marinol and Valeant Pharmaceutical International, Inc.’s Cesamet. Par Pharmaceutical Companies Inc. markets an approved generic version of Marinol, and Watson Pharmaceuticals, Inc. markets an authorized version of Marinol. We believe that other companies are pursuing regulatory approval for generic dronabinol products. We cannot give any assurance that other companies will not obtain regulatory approval or commercialize their dronabinol products before we do.
In the treatment of CINV, physicians typically offer conventional anti-nausea agents prior to initiating chemotherapy, such as sanofi-aventis’ Anzemet, Eisai Inc./Helsinn Group’s Aloxi, Roche
101
Table of Contents
Holding AG’s Kytril, Par Pharmaceutical Companies Inc.’s Zuplenz and GlaxoSmithKline plc’s Zofran, as well as Neurokinin 1 receptor antagonists on the market including ProStrakan Group plc’s SANCUSO and Merck & Co’s Emend. To the extent that our proprietary dronabinol products compete in a broader segment of the CINV market, we will also face competition from these products.
We will compete with non-synthetic cannabinoid drugs and therapies such as GW Pharmaceuticals’ Sativex, especially in the many countries outside of the United States where natural-based cannabinoids are legal. We also cannot assess the extent to which patients utilize natural cannabis, marijuana, to alleviate CINV or loss of appetite in the case of patients with AIDS, or other indications for which synthetic THC has a therapeutic benefit, instead of using prescribed therapies such as our dronabinol products.
Additionally, we are aware of companies in late stage development for CINV product candidates, including A.P. Pharma, Inc.’s APF530, which has received a Complete Response Letter from the FDA, Aphios Corp.’s Zindol, which is in Phase 2/3 development, Roche Holding/Helsinn Group’s netupitant, which is in Phase 3 development, and Tesaro, Inc.’s rolapitant, which is in Phase 2 development. If these technologies are successfully developed and approved over the next few years, they could represent significant competition for our dronabinol family of product candidates, if any are approved.
LEP-ETU Product Candidate
LEP-ETU, if approved for the treatment of metastatic breast cancer or other cancer indications, will compete with the leading taxanes currently on the market, including those with formulations that specifically incorporate paclitaxel as the active ingredient such as Bristol-Myers Squibb’s Taxol and its generic equivalents and Celgene Corporation’s Abraxane, as well as other taxane derivatives, such as sanofi-aventis’ Taxotere. We are also aware of Cornerstone Pharmaceutical, Inc.’s new formulation of paclitaxel known as EmPac, which is in preclinical testing. In addition, LEP-ETU would compete with other cytotoxic agents beyond the taxane class, including capecitabine, gemcitabine, ixabepilone and navelbine.
Additionally, there are numerous biotechnology and pharmaceutical companies with extensive development efforts and resources within oncology. Abbott Laboratories, Amgen Inc., AstraZeneca PLC., Bayer AG, Biogen Idec Inc., Eisai Co., Ltd., F. Hoffmann- LaRoche Ltd., Johnson and Johnson, Merck and Co., Inc., Novartis AG, Onyx Pharmaceuticals Inc., Pfizer Inc., sanofi- aventis and Takeda Pharmaceutical Co. Ltd., are among some of the leading companies researching and developing new compounds in oncology.
Intellectual Property
The success of most of our product candidates will depend in large part on our ability to:
| • | obtain and maintain patent and other legal protections for the proprietary technology, inventions and improvements we consider important to our business; |
| • | prosecute our patent applications and defend our issued patents; |
| • | preserve the confidentiality of our trade secrets; and |
| • | operate without infringing the patents and proprietary rights of third parties. |
We intend to continue to seek appropriate patent protection for certain of our lead compounds and product candidates, drug delivery systems, molecular modifications, as well as other proprietary technologies and their uses by filing patent applications in the United States and selected other countries. We intend for these patent applications to cover, where possible, claims for compositions of matter, medical uses, processes for preparation, processes for delivery and formulations.
As of June 30, 2011, we owned or had licensed from third parties a total of 13 issued U.S. utility patents and nine pending U.S. utility patent applications. The U.S. patents licensed to us will expire in
102
Table of Contents
2012 through 2022 and the U.S. patents and patent applications that we own, if they issue, will expire in 2018 through 2028. Some of the issued patents and pending applications, if issued, may also be eligible for patent term adjustment and patent term restoration, thereby extending their patent terms.
Fentanyl
The fentanyl patent portfolio currently consists of two U.S. pending patent applications and some foreign counterparts. We do not currently have any issued U.S. patents in our fentanyl patent portfolio. The claims of these applications currently cover at least formulations and methods of use relating to Subsys. Any patents that issue from our pending patent applications will expire in 2027 and 2028.
Dronabinol
Our dronabinol patent portfolio currently consists of three U.S. pending patent applications and some foreign counterparts. We do not currently have issued patents in our dronabinol patent portfolio. The claims of these applications currently cover at least formulations and methods of use relating to Dronabinol RT Capsule and Dronabinol Oral Solution. In addition, the patent applications include claims that would also cover other dronabinol extension products, such as an inhalation device, an IV Solution, a sublingual spray, a transdermal gel and an ophthalmic drop or ointment. Any patents that issue from our pending patent applications will expire between 2025 and 2028.
Other
Our LEP-ETU patent portfolio currently consists of three issued U.S. patents, one pending U.S. patent application, and some foreign counterparts. These patents and applications relate to liposomal paclitaxel formulations methods of treating cancer. We currently license two issued U.S. patents as well as some corresponding foreign patents and patent applications from Georgetown University, although we expect to terminate this license effective October 2011. The current formulation of LEP-ETU is protected in a pending U.S. patent application that, if it issues, will expire in 2024. Some of the corresponding foreign counterparts have already issued into patents.
The rest of our patent portfolio relates to patents and applications owned or licensed by Insys and directed to other potential product candidates.
Although we believe our rights under these patents and patent applications provide a competitive advantage, the patent positions of pharmaceutical and biotechnology companies are highly uncertain and involve complex legal and factual questions. We may not be able to develop products or processes, and may not be able to obtain issued patents from pending applications. Even if patents are granted, the allowed claims may not be sufficient to adequately protect the technology owned by or licensed to us. Any patents or patent rights that we obtain carry some risk of being circumvented, challenged or invalidated by our competitors. As described under the section “Business — Legal Proceedings,” a former officer of Insys Pharma has sought to rescind his assignment of his inventions concerning fentanyl and dronabinol patent applications described above. Ownership and inventorship disputes may arise for other patents and applications that we own or license.
We also rely on trade secrets, proprietary know-how and continuing innovation to develop and maintain our competitive position, especially when we do not believe that patent protection is appropriate or can be obtained. We intend to require each of our employees, consultants and advisors to execute proprietary information and inventions assignment agreement before they begin providing services to us. Among other things, this agreement will obligate our employee, consultant or advisor to refrain from disclosing any of our confidential information received during the course of providing services and, with some exceptions, to assign to us any inventions conceived or developed during the course of these services. We also require confidentiality agreements from third parties that receive our confidential information.
103
Table of Contents
The biotechnology and biopharmaceutical industries are characterized by the existence of a large number of patents and frequent litigation based on allegations of patent infringement. As our current and potential product candidates and others based upon our proprietary technologies progress toward commercialization, the possibility of an infringement claim against us increases. While we attempt to be certain that our products and proprietary technologies do not infringe other parties’ patents and other proprietary rights, competitors or other parties may assert that we infringe on their proprietary rights.
We have conducted certain clearance searches of issued U.S. patents for our fentanyl and LEP formulations and have not conducted extensive clearance searches for our other products, and cannot guarantee that the searches we have done were comprehensive and, therefore, whether these or any of our product candidates, delivery devices, or methods of using, making or delivering our product candidates infringe the patents searched, or that other patents do not exist that cover our product candidates, delivery devices or these methods. Interpreting patent claims involves complex legal and scientific questions and it is difficult to assess whether or not our product candidates would infringe any patent. Likewise, it is difficult to predict whether or not third-party patent applications will issue and what claim scope they may obtain. If we conclude that any patents, or patent applications once they issue as patents, that we may identify from such searches cover our product candidates, we cannot guarantee that we will be able to formulate around such patents at all or without material delay or whether we can obtain reasonable license terms from their owners, if at all. There may also be other pending patent applications that are unknown to us and, if granted, may prevent us from marketing our product candidates. Other product candidates that we may develop, either internally or in collaboration with others, could be subject to similar uncertainties. If a product is found to infringe a third-party patent, we could be prevented from developing and selling that product. Please see the section entitled “Risk Factors — Risks Relating to Our Intellectual Property.”
Environmental and Safety Matters
We use hazardous materials, including chemicals, biological agents and compounds that could be dangerous to human health and safety or the environment. Our operations also produce hazardous waste products. Federal, state and local laws and regulations govern, among other things, the use, generation, manufacture, storage, handling and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair our drug development efforts.
In addition, we cannot entirely eliminate the risk of accidental injury or contamination from these materials or wastes. If one of our employees was accidentally injured as a result of the use, storage, handling or disposal of these materials or wastes, the medical costs related to his or her treatment is within the coverage terms of our workers’ compensation insurance policy. However, we do not carry specific biological or hazardous waste insurance coverage and our property and casualty and general liability insurance policies specifically exclude coverage for damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury, we could be held liable for damages or penalized with fines in an amount exceeding our resources, and our clinical trials or regulatory approvals could be suspended.
Governmental Regulation
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act, or the FDC Act, and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions,
104
Table of Contents
such as FDA refusal to approve pending IND applications and NDAs, or issue warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
FDA approval is required before any new unapproved drug or dosage form, including a new use of a previously approved drug, can be marketed in the United States. Pharmaceutical product development in the United States typically involves, among other things, preclinical laboratory and animal tests, the submission to the FDA of a notice of claimed investigational exemption via an IND application, which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease indicated for treatment.
Preclinical tests include laboratory evaluation of product chemistry, stability, formulation and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND application along with other information including information about product chemistry, manufacturing and controls and a proposed clinical trial protocol. Long-term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND application is submitted. A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has not placed a clinical hold on the IND application within this 30-day period, the clinical trial proposed in the IND application may begin.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted in compliance with federal regulations, good clinical practices, or GCP, which include the requirement that all research subjects provide their informed consent in writing for their participation in any clinical trial, as well as under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing in U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND application.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human patients, the drug is tested to assess safety, metabolism, PK, pharmacological actions, side effects associated with increasing doses and, if possible, early evidence of effectiveness. Phase 2 usually involves trials in a limited patient population to evaluate the effectiveness of the drug for a particular indication or indications, dosage tolerance and optimum dosage, and identify possible adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to establish the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In some cases, the FDA may condition approval on the sponsor’s agreement to conduct additional clinical trials to further assess the drug’s safety and effectiveness after approval. Such post-approval studies are typically referred to as Phase 4 studies.
105
Table of Contents
The current FDA standards of approving new pharmaceutical products are more stringent than those that were applied in the past. These standards were not applied to many established products currently on the market, including certain opioid products. As a result, the FDA does not have as extensive safety databases on these products as on some products developed more recently. We believe the FDA has recently expressed an intention to develop safety data for certain products, including many opioids. In particular, the FDA has expressed interest in specific impurities that may be present in a number of opioid narcotic active pharmaceutical ingredients, such as oxycodone. Based on certain structural characteristics, these impurities may have the potential to cause mutagenic effects. If, after testing, such effects are ultimately demonstrated to exist, more stringent controls on the levels of these impurities may be required for FDA approval of products containing these impurities, such as oxymorphone. Any additional testing or remedial measures that may be necessary could result in increased costs for, or delays in, obtaining approval for certain of our products in development.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the United States. The NDA must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls, and proposed labeling, among other things. The cost of preparing and submitting an NDA is substantial. Under federal law, the submission of most NDAs is additionally subject to a substantial application user fee, currently $1,542,000, and the manufacturer and/or sponsor under an approved NDA are also subject to annual product and establishment user fees, currently $86,520 per product and $497,200 per establishment. These fees are typically increased annually.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. Under the Prescription Drug User Fee Act the FDA has agreed to certain performance goals in the review of NDAs. The FDA has a goal of reviewing applications for non-priority drug products within ten months. The review process may be extended by the FDA for three additional months to consider certain information or clarification regarding information already provided in the submission. The FDA may also refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless the facility demonstrates compliance with current cGMPs and the NDA contains data that provides substantial evidence that the drug is safe and effective for the indication sought in the proposed labeling. Additionally, the FDA will typically inspect one or more clinical trial sites to assure compliance with GCPs before approving an NDA.
After the FDA evaluates the NDA and the manufacturing facilities, it may issue an approval letter, or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two to six months depending on the type of information included.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy and may impose other conditions, including labeling or distribution restrictions or other risk-management mechanisms which can materially affect the potential market and profitability of the drug. Further, if there are any modifications to the drug, including changes in indications, labeling, or manufacturing processes or facilities, we may be required to submit and obtain FDA approval for a new or supplemental NDA, which
106
Table of Contents
may require us to develop additional data or conduct additional preclinical studies and clinical trials. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
The FDA may require sponsors of investigational drugs to submit proposed REMS in order to ensure that the benefits of the drugs continue to outweigh the risks. Sponsors of certain drug applications approved without a REMS program may also be required to submit a proposed REMS program if the FDA becomes aware of new safety information and makes a determination that a REMS program is necessary. A REMS program can include a Medication Guide, Patient Package Insert, a communication plan, elements to assure safe use, and an implementation system, and must include a timetable for assessment of the REMS program. Elements to assure safe use can restrict the prescribing, sale and distribution of drug products. In February 2009, the FDA notified manufacturers of branded and generic extended release opioid products that they may be required to submit a REMS program to reduce the risks associated with misuse and abuse. The FDA later notified manufacturers of transmucosal fentanyl products that they must submit a REMS program. As of February 2011, a REMS program had been approved for two fentanyl products, Onsolis and Abstral. Both of these REMS include elements to assure safe use.
The Hatch-Waxman Act
Abbreviated New Drug Applications (ANDAs)
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent with claims that cover the applicant’s product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential competitors in support of approval of an ANDA. An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown to be bioequivalent to the listed drug. ANDA applicants are not required to conduct or submit results of preclinical or clinical tests to prove the safety or effectiveness of their drug product, but are required to conduct bioequivalence testing, which compares the bioavailability of their drug product to that of the listed drug to confirm chemical and therapeutic equivalence. Drugs approved in this way are commonly referred to as generic versions of the listed drug, and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book. Specifically, the applicant must certify that: (1) the required patent information has not been filed; (2) the listed patent has expired; (3) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (4) the listed patent is invalid or will not be infringed by the new product. A certification that the new product will not infringe the already approved product’s listed patents or that such patents are invalid is called a Paragraph IV certification. If the applicant does not challenge the listed patents via a Paragraph IV certification, the FDA will not approve the ANDA application until all the listed patents claiming the referenced product have expired.
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification prevents the FDA from approving the ANDA until the earlier of 30 months from the date of the lawsuit, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant. As an incentive for the rapid development of generic drug products, the first ANDA(s) filed that challenges a
107
Table of Contents
listed patent by filing a Paragraph IV certification may be granted a 180-day marketing exclusivity period during which the FDA may not approve another ANDA for the same product. There may be multiple such “first filers.” The 180-day marketing exclusivity period is triggered either by commercial launch of any first-filed ANDA approved product or from the date of a court decision finding the challenged patent to be invalid, unenforceable or not infringed, whichever is first. The 180-day exclusivity can be forfeited, among other reasons, if the first filed and approved ANDA is not marketed, does not obtain tentative approval or the challenged patent expires.
The ANDA application also will not be approved until any non-patent market exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired. Federal law provides an exclusive period of five years following approval of a drug containing no previously approved active ingredients, during which ANDAs for generic versions of those drugs cannot be submitted unless the submission contains a Paragraph IV challenge to a listed patent, in which case the submission may be made four years following the original product approval. Federal law additionally provides for a period of three years of exclusivity following approval of a drug that contains previously approved active ingredients but is approved in a new dosage form, route of administration or combination, or for a new use, the approval of which was required to be supported by new clinical trials conducted by or for the sponsor. The FDA cannot grant effective approval of an ANDA based on that listed drug during this three-year period.
Section 505(b)(2) Regulatory Pathway
Most drug products obtain FDA marketing approval pursuant to an NDA or an ANDA. A third alternative is a special type of NDA, commonly referred to as a Section 505(b)(2) NDA. Section 505(b)(2) of the FDC Act enables the applicant to rely, in part, on the FDA’s findings of safety and efficacy for an existing product, or published literature, in support of its application.
505(b)(2) NDAs often provide an alternate regulatory pathway to FDA approval for new or improved formulations or new uses of previously approved products. Specifically, Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The applicant may rely upon the FDA’s findings from preclinical or clinical studies conducted for an approved product. The FDA may also require 505(b)(2) applicants to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new product candidate for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant. To the extent that the Section 505(b)(2) applicant is relying on findings of safety or efficacy for an already approved product, the applicant is subject to existing exclusivity for the reference product and is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would. Thus approval of a Section 505(b)(2) NDA can be stalled until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earlier of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the Section 505(b)(2) applicant.
Post-Approval FDA Requirements
Once an NDA is approved, a product will be subject to extensive and ongoing post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. FDA post-market regulations also include, among other things, requirements relating to drug listing, recordkeeping, periodic reporting, product sampling and distribution, manufacturing and reporting of
108
Table of Contents
adverse events arising from use of the product. Failure to comply with these regulatory requirements may result in restrictions on the marketing or manufacturing of the product, recall or market withdrawal, fines, warning letters, refusal to approve pending applications, suspension or revocation of approvals, product seizure or detention, injunctions and/or the imposition of civil or criminal penalties.
Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase 4 testing, a REMS program and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product.
In addition, quality control as well as drug manufacture, packaging, and labeling procedures must continue to conform to cGMPs after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA during which the agency inspects manufacturing facilities to access compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs. The FDA and comparable state regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
The distribution of prescription pharmaceutical products is also subject to the Prescription Drug Marketing Act, or PDMA, which governs the distribution of drugs and drug samples at the federal level, and sets minimum standards for the licensing and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Controlled Substances; Drug Enforcement Administration (DEA)
We intend to sell products that are “controlled substances” as defined in the federal Controlled Substances Act of 1970, or CSA, which establishes registration, security, recordkeeping, reporting, storage and other requirements administered by the Drug Enforcement Administration, or DEA. States impose similar requirements. The DEA regulates entities that handle controlled substances and the equipment and raw materials used in their manufacture and packaging, in order to prevent loss and diversion into illicit channels of commerce.
The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have high potential for abuse, no currently accepted medical use in the United States and lack accepted safety for use under medical supervision, and may not be marketed or sold in the United States. Except for research and industrial purposes, a pharmaceutical product may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest risk of abuse and Schedule V substances the lowest relative risk of abuse among such substances. Fentanyl, the active ingredient in one of our products, is listed by the DEA as a Schedule II substance under the CSA. Consequently, its manufacture, shipment, storage, sale and use are subject to a high degree of regulation. For example, generally all Schedule II drug prescriptions must be signed by a physician and physically presented to a pharmacist before filling and may not be refilled without a new prescription.
109
Table of Contents
Dronabinol is listed by the DEA as a Schedule I substance, but when formulated in sesame oil and encapsulated in a soft gelatin capsule, it is listed as a Schedule III substance. DEA regulations currently limit the formulation of FDA-approved dronabinol products that are classified in Schedule III. Specifically, classification in Schedule III is limited to “dronabinol (synthetic) in sesame oil and encapsulated in a soft gelatin capsule in” an FDA-approved product. There is a concern that some generic versions of Marinol would not meet these specific conditions, and therefore, would not be classified as a Schedule III substance, but rather would be considered as Schedule I products until otherwise scheduled for marketing. Currently, several products are the subject of pending ANDAs, including our application for Dronabinol SG Capsule, under review by the FDA. On November 1, 2010, the DEA issued a Notice of Proposed Rulemaking concerning the listing of approved drug products containing dronabinol in Schedule III. The DEA proposed rulemaking would amend the scheduling regulations to expand the Schedule III listing of dronabinol to include formulations containing naturally-derived dronabinol and formulations encapsulated in hard gelatin capsules. If this ruling is allowed, it may increase the number of generics approved as we believe there are active ANDAs which utilize naturally-derived dronabinol and hard gelatin capsule technology.
DEA registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, activity and controlled substance schedule. For example, separate registrations are needed for import and manufacturing, and each registration will specify which schedules of controlled substances are authorized to be handled under that registration.
The DEA typically inspects certain facilities to review their security controls, recordkeeping and reporting prior to issuing a registration. Security requirements vary by controlled substance schedule, with the most stringent requirements applying to Schedule I and Schedule II substances. Security measures required by the DEA include background checks on employees and physical control of inventory through measures such as vaults, cages, surveillance cameras and inventory reconciliations. Records must be maintained for the handling of all controlled substances, and periodic reports made to the DEA, for example distribution reports for Schedule I and II controlled substances, Schedule III substances that are narcotics, and other designated substances. Reports must also be made for thefts or losses of any controlled substance, suspicious orders, and to obtain authorization to destroy any controlled substance. In addition, special authorization and notification requirements apply to imports and exports.
A DEA quota system controls and limits the availability and production of controlled substances in Schedule I or II. This includes manufacturing of the active pharmaceutical ingredient and production of dosage forms. Distributions of any Schedule I or II controlled substance must also be accompanied by special order forms, with copies provided to the DEA. Absent Marinol-like formulation and encapsulation exception, dronabinol is a Schedule I controlled substance and, therefore, subject to the DEA’s production and procurement quota scheme. The DEA establishes annually an aggregate quota for how much total dronabinol may be produced in the United States based on the DEA’s estimate of the quantity needed to meet legitimate scientific and medicinal needs. This limited aggregate amount of dronabinol that the DEA allows to be produced in the United States each year is allocated among individual companies, who must submit applications annually to the DEA for individual manufacturing and procurement quotas. We or our partners, including our contract manufacturers, must obtain an annual quota from the DEA in order to produce or procure any Schedule I or Schedule II substance, including dronabinol and fentanyl. The DEA may adjust aggregate production quotas and individual manufacturing quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments. Our, or our contract manufacturers’, quota of the active ingredient may not be sufficient to meet commercial demand or complete clinical trials. Any delay or refusal by the DEA in establishing our, or our contract manufacturers’, quota for controlled substances could delay or stop our clinical trials or product launches which could have a material adverse effect on our business, financial position and results of operations.
110
Table of Contents
The DEA conducts periodic inspections of certain registered establishments that handle controlled substances. Failure to maintain compliance with applicable requirements, particularly as manifested in loss or diversion, can result in enforcement action that could have a material adverse effect on our business, results of operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to restrict, suspend or revoke those registrations. In certain circumstances, violations could result in criminal proceedings.
Individual states also regulate controlled substances, and we and our contract manufacturers will be subject to state regulation on distribution of these products, including licensing, recordkeeping and security.
Anti-Kickback, False Claims Laws & The Prescription Drug Marketing Act
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Violations of the anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
The Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, amends the intent requirement of the federal anti-kickback and criminal health care fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the federal false claims laws.
Coverage and Reimbursement
Our ability to commercialize any approved product candidates successfully will depend in part on the extent to which governmental authorities, private health insurers and other third-party payors establish appropriate coverage and reimbursement levels for our product candidates and related treatments. As a threshold for coverage and reimbursement, third-party payors generally require that drug products have been approved for marketing by the FDA. Third-party payors are increasingly
111
Table of Contents
imposing additional requirements and restrictions on coverage and limiting reimbursement levels for medical products. For example, federal and state governments reimburse covered prescription drugs at varying rates below average wholesale price. These restrictions and limitations influence the purchase of healthcare services and products. Legislative proposals to reform healthcare or reduce costs under government insurance programs may result in lower reimbursement for our product candidates or exclusion of our product candidates from coverage and reimbursement programs. The cost containment measures that healthcare payors and providers are instituting and the effect of any healthcare reform could significantly reduce our revenues from the sale of any approved product. We cannot provide any assurances that we will be able to obtain third-party coverage or reimbursement for our product candidates in whole or in part.
Healthcare Privacy and Security Laws
We may be subject to various privacy and security regulations, including but not limited to the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or collectively, HIPAA. HIPAA mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common healthcare transactions, as well as standards relating to the privacy and security of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. In addition, many states have enacted comparable laws addressing the privacy and security of health information, some of which are more stringent then HIPAA. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and criminal penalties.
Approval Outside the United States
In order to market any product outside of the United States, we must comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy and governing, among other things, clinical trials and commercial sales and distribution of our products. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods, and may be otherwise complicated by our product candidates being controlled substances such as synthetic cannabinoids and fentanyl. The time required to obtain approval in other countries might differ from and be longer than that required to obtain FDA approval and DEA classification. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others.
To date, we have not initiated any discussions with the European Medicines Agency or any other foreign regulatory authorities with respect to seeking regulatory approval for any indication in Europe or in any other country outside the United States. As in the United States, the regulatory approval process in Europe and in other countries is a lengthy, challenging and inherently uncertain process.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Employees
As of July 15, 2011, we employed 28 full-time employees, consisting of 21 employees in research, development and regulatory affairs, and 17 in management, administration, finance and facilities. As of the same date, 10 of our employees had a Ph.D. or M.D. degree. None of our employees is subject to a collective bargaining agreement and we consider our relationship with our employees to be good.
112
Table of Contents
Scientific Advisory Board
We have established a scientific advisory board consisting of industry experts with knowledge of our target markets. Our scientific advisors generally meet twice a year as a group to assist us in formulating our research, development, clinical and sales and marketing strategies. Some individual scientific advisors consult with and meet informally with us on a more frequent basis. Our scientific advisors are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. In addition, our scientific advisors may have arrangements with other companies to assist those companies in developing products or technologies that may compete with ours.
Properties
We lease approximately 16,000 square feet of office and lab space in Phoenix, Arizona under a lease agreement that expires in October 2012. We have an option to extend this lease for an additional five years and a right of first offer to purchase the facility. In addition, we are responsible for expenses associated with the use and maintenance of our Arizona facility, such as utility and common area maintenance expenses. We believe that the Phoenix, Arizona facility is adequate to meet our current needs, and that suitable additional or alternative space will be available in the future on commercially reasonable terms. We also have a 9,000 square facility in Lake Bluff, Illinois which houses a laboratory and office space, the lease for which expires in 2015. Lastly, we own our U.S.-based, state-of-the-art dronabinol manufacturing facility, which is located in Texas and housed five employees as of June 30, 2011.
Legal Proceedings
In September 2009, Insys Pharma, Inc. and certain of its officers and directors, as well as their spouses, were named as defendants in a lawsuit in Arizona Superior Court brought by Santosh Kottayil, Ph.D., certain of his family members and a trust of which Dr. Kottayil is the trustee. Dr. Kottayil formerly served as President, Chief Scientific Officer and a director of Insys Pharma, among other positions. The complaint brought a cause of action for statutory and common law appraisal of Dr. Kottayil’s Insys Pharma common stock. The cause of action for appraisal relates to a one-for-1,500,000 reverse stock split that Insys Pharma effected in June 2009, which resulted in Dr. Kottayil’s ownership position becoming a fractional share of Insys Pharma common stock. Following the reverse stock split, Insys Pharma cancelled all resulting fractional shares, including the fractional share held by Dr. Kottayil, and offered a cash payment in lieu of the fractional shares. The complaint also brought causes of action for breach of fiduciary duty and negligent misrepresentation in the defendants’ dealings with Dr. Kottayil on the subject of his compensation and stock ownership in Insys Pharma. In January 2010, the plaintiffs added claims seeking to rescind Dr. Kottayil’s assignment to Insys Pharma of his interest in all of the fentanyl and dronabinol patent applications we own and to recover the benefits of those interests. Dr. Kottayil is seeking, among other relief, the fair value of his Insys Pharma common stock as of June 2, 2009, compensatory and punitive damages, and rescission of all assignments to Insys Pharma of his interest in the patent applications, as well as attorneys’ fees, costs and interest.
In February 2010, Insys Pharma and the other defendants answered and filed counter-claims to Dr. Kottayil’s amended complaint. The counter-claims include actions for breach of fiduciary duty, fraud and negligence with respect to the time during which Dr. Kottayil was employed at Insys Pharma. The counter-claims, among other relief, seek compensatory and punitive damages. We do not expect a trial of this action to take place before 2012, although an earlier date is possible. We are not able at this time to estimate the range of potential loss or any potential recovery from the counter-claims, nor are we able to predict the outcome of this litigation. If the patent assignments are successfully rescinded, we may not have exclusive patent rights covering our fentanyl and dronabinol product candidates, and such patent rights may not be available to us on acceptable terms, if at all, which would have a material adverse effect on our business. We intend to vigorously defend against the plaintiffs’ claims and pursue our counter-claims.
113
Table of Contents
Insys Pharma has received letters from the counsel of Solvay Pharmaceuticals and Unimed Pharmaceuticals, Inc., who market and sell Marinol, asserting that Insys Pharma’s founder may have utilized their confidential and proprietary information for the ANDA of dronabinol. Solvay Pharmaceuticals and Unimed Pharmaceuticals are requesting various information and written assurances from Insys Pharma related to the ANDA of dronabinol. The matter has been handled out of court thus far and we intend to vigorously defend each and every claim and request made in the letters if the complaint escalates. Management is unable to estimate the potential outcome or range of possible loss, if any. Any such litigation could be protracted, expensive, and potentially subject to an unfavorable outcome.
In 2001, we and certain of our former officers were named in a complaint, which alleged various violations of the federal securities laws in connection with our public statements regarding our LEP-ETU drug product candidate during the period from October 31, 2001 through April 19, 2002. In November 2002, we moved to have the complaint dismissed. This motion to dismiss was granted in part and denied in part in February 2003. In November 2004, the plaintiffs filed a motion to amend and a motion for summary adjudication. The motion to amend sought to include certain pre-class period statements in the complaint. The motion for summary adjudication asked the Court to rule that certain statements made in an arbitration award regarding the LEP drug product candidate be deemed facts established in this proceeding. In February 2007, the Court entered an order denying both the plaintiffs’ motion to amend and the plaintiffs’ motion for summary adjudication. Fact and expert discovery is closed. In March 2008, the dispositive motion filing deadline, we filed a motion for summary judgment. On March 31, 2010, the Court granted in part and denied in part our motion for summary judgment. The Court dismissed the plaintiff’s claims based on statements made before January 14, 2002 but held that there was a genuine issue of material fact as to whether we could be liable for statements made between January 14, 2002 and April 19, 2002. On April 27, 2010, the Court set a trial date of February 22, 2011 and also set a settlement conference date of July 27, 2010. On October 25, 2010, the parties entered into a Stipulation of Settlement which set forth the terms and conditions for a proposed settlement of the litigation and for dismissal of the litigation with prejudice. The parties have agreed to settle the litigation for $3,350,000 in cash to be distributed to eligible class members of the plaintiff. At the settlement hearing on March 17, 2011, the Court gave final approval of the settlement, which was paid by our insurers. None of our company’s current directors or officers were named in this complaint.
114
Table of Contents
Executive Officers, Key Persons and Directors
The following table sets forth certain information regarding our executive officers, key persons and directors as of June 30, 2011:
| Name |
Age | Position(s) | ||||
| Michael L. Babich |
34 | President, Chief Executive Officer and Director | ||||
| Martin McCarthy |
53 | Chief Financial Officer | ||||
| Larry Dillaha, M.D. |
47 | Chief Medical Officer | ||||
| Daniel D. Von Hoff, M.D. |
63 | Director of Scientific Development | ||||
| John N. Kapoor, Ph.D. |
67 | Director and Executive Chairman of the Board | ||||
| Patrick P. Fourteau(1)(2)(3) |
63 | Director | ||||
| Pierre Lapalme(2)(3) |
70 | Director | ||||
| Steven Meyer(1)(2) |
55 | Director | ||||
| Brian Tambi(1)(3) |
68 | Director | ||||
| (1) | Member of the audit committee. |
| (2) | Member of the compensation committee. |
| (3) | Member of the nominating and corporate governance committee. |
Michael L. Babich has served on our board of directors since November 2010. In connection with the Merger with Insys Pharma, of which company Mr. Babich was a director and the Chief Operating Officer, the Merger agreement provided that Mr. Babich would be elected to our board of directors following the closing of the Merger. Mr. Babich has also served as our President since November 2010 and was appointed as our Chief Executive Officer in March 2011. From March 2007 until the Merger in November 2010, Mr. Babich served as the Chief Operating Officer and a director of Insys Pharma. Prior to that, from 2001 to 2007 Mr. Babich worked at EJ Financial Enterprises, Inc., a venture capital firm specializing in early stage and startup investments primarily in the healthcare sector. During his time at EJ Financial Enterprises, Mr. Babich held various roles and worked on various projects, including private equity transactions, asset management and strategic consulting for both public and private companies. Prior to his work at EJ Financial Enterprises, Mr. Babich worked at the Northern Trust Corporation managing mid- to large-cap portfolios for high net worth individuals. Mr. Babich also has served as a director and in management roles at Alliant Pharmaceuticals, Inc. Mr. Babich received a B.A. from the University of Illinois at Urbana-Champaign and an MBA from the Kellogg School of Management at Northwestern University. The board of directors believes that Mr. Babich’s business expertise, including his experience working with the investment community, provides him the operational expertise, breadth of knowledge and valuable understanding of our industry to qualify him to serve on our board of directors and as our President and Chief Executive Officer.
Martin McCarthy has served as our Chief Financial Officer since March 2011. From November 2007 until the Merger in November 2010, Mr. McCarthy served as our Corporate Controller and Acting Chief Financial Officer. From November 2006 until June 2007, Mr. McCarthy served as Chief Financial Officer for 411 Solutions, Inc., an information technology services firm. Prior to that, from August 2001 to November 2006, Mr. McCarthy served as Director of Finance for the AmerisourceBergen Technology Group of AmerisourceBergen Corporation, a Fortune 50 pharmaceutical services company. From September 1998 to August 2001, Mr. McCarthy served as Director of Finance for Yesmail.com, an internet marketing and services company and division of infoGroup (formerly InfoUSA). From September 1990 to August 1998, Mr. McCarthy served as Director of Financial Accounting & Treasury Services at Tang Industries, Inc., a privately held diversified holding company. Mr. McCarthy has also been employed by the accounting firms of Deloitte & Touche LLP and PricewaterhouseCoopers LLP from September 1984 to August 1990. He is a member of the Illinois and American Institutes of Certified Public Accountants. Mr. McCarthy received a B.S. in Accounting from Indiana University.
115
Table of Contents
Larry Dillaha, M.D. has served as our Chief Medical Officer since March 2011. Prior to joining our company, he served as Executive Vice-President and Chief Medical Officer for Shionogi and Co., Ltd. (formerly Sciele Pharma, Inc. and First Horizon Pharmaceutical Corp.). While at Shionogi/Sciele, Dr. Dillaha oversaw the development and successful FDA filings of numerous compounds integral to the success of the company. He has extensive experience interacting with the FDA and designing successful drug development plans. Prior to serving as an officer of Shionogi/Sciele, Dr. Dillaha served as Medical Director for sanofi-aventis, a multinational pharmaceutical company, where he was involved in several major clinical studies for the company’s lead compounds. Dr. Dillaha also serves as a member of the board of directors of InVasc Therapeutics, Inc., a pharmaceutical company. Dr. Dillaha earned his M.D. degree as well as a B.A. in Biology from the University of Tennessee.
Daniel D. Von Hoff, M.D. has served as our Director of Scientific Development and consultant since March 2011. He is currently Physician in Chief, Distinguished Professor and Director of Clinical Translational Research Division at the Translational Genomics Research Institute in Phoenix, Arizona. He is also Chief Scientific Officer for US Oncology and for Scottsdale Healthcare’s Clinical Research Institute and holds an appointment as Professor of Medicine, Mayo Clinic, Scottsdale, AZ. Dr. Von Hoff was involved in the initial development of many of the anticancer agents now used routinely, including: mitoxantrone, fludarabine, paclitaxel, docetaxel, gemcitabine, irinotecan, nelarabine, capecitabine, lapatinib and others. Dr. Von Hoff has published more than 569 papers, 135 book chapters and over 1,000 abstracts. Dr. Von Hoff received the 2010 David A. Karnofsky Memorial Award from the American Society of Clinical Oncology for his outstanding contributions to cancer research leading to significant improvements in patient care. Dr. Von Hoff served on the National Cancer Advisory Board from 2004 to 2010. He is the former President of the American Association for Cancer Research, the world’s largest cancer research organization, a Fellow of the American College of Physicians and a member and former board member of the American Society of Clinical Oncology. He is a founder of ILEX Oncology, Inc., acquired by Genzyme after ILEX had two agents, alemtuzumab and clofarabine, approved by the FDA for patients with leukemia. Dr. Von Hoff is a co-founder and the Editor Emeritus of Investigational New Drugs — The Journal of New Anticancer Agents; and Editor-in-Chief of Molecular Cancer Therapeutics. He is a co-founder of the AACR/ASCO Methods in Clinical Cancer Research Workshop.
John N. Kapoor, Ph.D. has served on our board of directors since our formation in 1990 and has served as Chairman from 1990 to 2004 and Executive Chairman since June 2006. Dr. Kapoor also served as a director of Insys Pharma from its inception in 2002. Dr. Kapoor has served as the President and Chairman of the board of directors of EJ Financial Enterprises since forming the company in 1990. Dr. Kapoor is also the Managing Partner of Kapoor-Pharma Investments, an investment company that he founded in 2000. Dr. Kapoor serves as the chairman of the board of directors of Akorn, Inc., a publicly traded specialty pharmaceutical company, where he served as the Chief Executive Officer from March 2001 to December 2002 and from May 1996 to November 1998. Dr. Kapoor also served as the chairman of the board of directors of Sciele Pharma and OptionCare, Inc., a specialty pharmaceutical services company, where he served as Chief Executive Officer from August 1993 to April 1996. Dr. Kapoor received his Ph.D. in Medicinal Chemistry from the State University of New York at Buffalo and a B.S. in Pharmacy from Bombay University in India. The board believes that Dr. Kapoor’s leadership experience in the biopharmaceutical industry and his success as a venture capitalist add valuable expertise and insight to our board of directors and uniquely qualify him to serve as our Executive Chairman.
Patrick P. Fourteau has served on our board of directors since March 2011. From August 2007 until January 2009, Mr. Fourteau served as a director of Insys Pharma. Mr. Fourteau served as President and Chief Executive Officer of Shionogi from 2008 until 2010. Prior to the acquisition of Sciele by Shionogi and Co. Ltd., Mr. Fourteau served as President and CEO of Sciele Pharma from 2003 until 2008 and served on the board of directors of Sciele from 2004 until 2008. He is a seasoned pharmaceutical industry executive having over 30 years of healthcare industry experience, with particular expertise in executing sales strategies for pharmaceutical products. Mr. Fourteau served as President of Worldwide
116
Table of Contents
Sales of inVentiv Health, Inc. from 2000 to 2002. Mr. Fourteau served as President of various divisions of St. Jude Medical, Inc. from 1995 to 2000 and as an Executive of Eli Lilly and Company prior to 1995. Mr. Fourteau earned his B.A. and M.A. in Mathematics from the University of California, Berkeley and an MBA from Harvard University. The board believes that Mr. Fourteau’s leadership experience in the pharmaceutical industry will add valuable expertise and insight to our board of directors.
Pierre Lapalme has served on our board of directors since March 2011. Mr. Lapalme has also served as a member of the Board of Directors of BioMarin Pharmaceutical Inc., a biopharmaceutical company, since January 2004. From 1995 until his retirement in 2003, he served as the President and Chief Executive Officer of North America Ethypharm, Inc., a drug delivery company. Throughout his career, Mr. Lapalme held numerous senior management positions in the pharmaceutical industry, including Chief Executive Officer and Chairman of the Board of Rhône-Poulenc Pharmaceuticals, Inc., from 1979 to 1994, and Senior Vice President and General Manager of North America Ethicals, divisions of Rhône-Poulenc Rorer, Inc. (which in 1999 merged with Hoechst AG to form Aventis, which then went on to merge with Sanofi-Synthélabo forming sanofi-aventis). Mr. Lapalme served on the board of the National Pharmaceutical Council and was a board member of the Pharmaceutical Manufacturers Association of Canada where he played a role in reinstituting certain patent protection for pharmaceuticals. Mr. Lapalme studied at the University of Western Ontario and INSEAD France. The board believes that Mr. Lapalme’s experience in the pharmaceutical industry gives him a valuable understanding of our industry which qualify him to serve as a director on our board.
Steven Meyer has served on our board of directors since November 2010. The terms of the Merger provided that Mr. Meyer would be elected to our board of directors following the closing of the Merger. From August 2007 until the Merger, Mr. Meyer served as a director of Insys Pharma. Since November 2005, Mr. Meyer has served as the Chief Financial Officer of JVM Realty Corporation, a private investment firm specializing in the acquisition, re-positioning and management of real estate for investors. Prior to that, Mr. Meyer was employed by Baxter International Incorporated, a global healthcare company, where he served as Corporate Treasurer from January 1997 to July 2004. Mr. Meyer earned his MBA in finance and accounting from the Kellogg Graduate School of Management at Northwestern University and his B.A. in Economics from the University of Illinois in Campaign-Urbana. He is an Illinois Certified Public Accountant. The board believes that Mr. Meyer’s management experience and his knowledge of the finance and healthcare industries give him a valuable understanding of our industry which qualifies him to serve as a director on our board.
Brian Tambi has served on our board of directors since November 2010. In connection with our merger with Insys Pharma, of which company Mr. Tambi was a director, the merger agreement provided that Mr. Tambi would be elected to our board of directors following the closing of the Merger. Mr. Tambi currently serves as a member of the board of directors of Akorn, Inc., a publicly traded pharmaceuticals company. From August 2007 until the Merger, Mr. Tambi served as a director of Insys Pharma. Since forming the company in January 2007, Mr. Tambi has served as the Chairman of the Board, President and Chief Executive Officer of BrianT Laboratories LLC, a pharmaceutical company currently focused on developing, manufacturing and marketing combinations of leading single agent drugs and delivery systems. Dr. Kapoor is an investor in BrianT Laboratories. Prior to that, since 1995 Mr. Tambi served as the Chairman, President and Chief Executive Officer of Morton Grove Pharmaceuticals, Inc. and he currently serves as Morton Grove’s non-executive Chairman of the Board. Prior to Morton Grove, Mr. Tambi served as President of Ivax North American Pharmaceuticals and as a member of the board of directors of Ivax Corporation (acquired by Teva Pharmaceutical Industries Ltd.), a publicly traded pharmaceutical company. Mr. Tambi also served as Chief Operating Officer of Fujisawa USA, Inc., a subsidiary of Fujisawa Pharmaceutical Company, Ltd. Mr. Tambi also held executive positions at Lyphomed, Inc. and Bristol-Myers Squibb. He earned his MBA in International Finance & Economics in 1974 and his B.S. in Corporate Finance in 1972, both from Syracuse University. The board believes that Mr. Tambi’s drug development and commercialization expertise as well as his experience in the finance sector will bring important strategic insight to our board of directors.
117
Table of Contents
BOARD COMPOSITION
Our business and affairs are organized under the direction of our board of directors, which currently consists of six members. The primary responsibilities of our board of directors are to provide oversight, strategic guidance, counseling and direction to our management. Our board of directors meets on a regular basis and additionally as required.
Our board of directors has determined that four of our six directors, Patrick P. Fourteau, Pierre Lapalme, Steven Meyer and Brian Tambi, are independent directors, as defined by Rule 5605(a)(2) of the Nasdaq Listing Rules.
Effective upon the closing of this offering, we will divide our board of directors into three classes, as follows:
| • | Class I, which will consist of Steven Meyer and Brian Tambi, whose terms will expire at our annual meeting of stockholders to be held in 2012; |
| • | Class II, which will consist of Pierre Lapalme and Michael L. Babich, whose terms will expire at our annual meeting of stockholders to be held in 2013; and |
| • | Class III, which will consist of Patrick P. Fourteau and John N. Kapoor, Ph.D., whose terms will expire at our annual meeting of stockholders to be held in 2014. |
At each annual meeting of stockholders to be held after the initial classification, the successors to directors whose terms then expire will serve until the third annual meeting following their election and until their successors are duly elected and qualified. The authorized size of our board of directors is currently seven members. The authorized number of directors may be changed only by resolution of the board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed between the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of the board of directors may have the effect of delaying or preventing changes in our control or management. Our directors may be removed for cause by the affirmative vote of the holders of at least 66- 2/3% of our voting stock.
Board Leadership Structure
Our board of directors is currently chaired by our Executive Chairman, Dr. Kapoor. As a general policy, our board of directors believes that separation of the positions of Chairman and Chief Executive Officer reinforces the independence of the board of directors from management, creates an environment that encourages objective oversight of management’s performance and enhances the effectiveness of the board of directors as a whole. As such, Mr. Babich serves as our President and Chief Executive Officer while Dr. Kapoor serves as our Executive Chairman of the board of directors but is not an officer. We expect and intend the positions of Chairman of the board of directors and Chief Executive Officer to be held by two individuals in the future as well.
Role of the Board in Risk Oversight
One of the key functions of our board of directors is informed oversight of our risk management process. The board of directors does not have a standing risk management committee, but rather administers this oversight function directly through the board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure and our audit committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The audit committee also monitors compliance with legal and regulatory requirements. Our nominating and corporate governance committee monitors the effectiveness of our
118
Table of Contents
corporate governance practices, including whether they are successful in preventing illegal or improper liability-creating conduct. Our compensation committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking.
Board Committees
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee.
Audit Committee
Our audit committee consists of Patrick P. Fourteau, Steven Meyer and Pierre Lapalme. Our board of directors has determined that each of the members of our audit committee satisfies the Nasdaq Stock Market and SEC independence requirements. Mr. Meyer serves as the chair of our audit committee. The functions of this committee include, among other things:
| • | evaluating the performance, independence and qualifications of our independent auditors and determining whether to retain our existing independent auditors or engage new independent auditors; |
| • | reviewing and approving the engagement of our independent auditors to perform audit services and any permissible non-audit services; |
| • | monitoring the rotation of partners of our independent auditors on our engagement team as required by law; |
| • | prior to engagement of any independent auditor, and at least annually thereafter, reviewing relationships that may reasonably be thought to bear on their independence, and assessing and otherwise taking the appropriate action to oversee the independence of our independent auditor; |
| • | reviewing our annual and quarterly financial statements and reports, including the disclosures contained under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and discussing the statements and reports with our independent auditors and management; |
| • | reviewing with our independent auditors and management significant issues that arise regarding accounting principles and financial statement presentation, and matters concerning the scope, adequacy and effectiveness of our financial controls; |
| • | reviewing with management and our auditors any earnings announcements and other public announcements regarding material developments; |
| • | establishing procedures for the receipt, retention and treatment of complaints received by us regarding financial controls, accounting or auditing matters and other matters; |
| • | preparing the audit committee report that the SEC requires in our annual proxy statement; |
| • | reviewing and providing oversight of any related-person transactions in accordance with our related-person transaction policy and reviewing and monitoring compliance with legal and regulatory responsibilities, including our code of business conduct and ethics; |
| • | reviewing our major financial risk exposures, including the guidelines and policies to govern the process by which risk assessment and risk management is implemented; |
| • | reviewing on a periodic basis our investment policy; and |
| • | reviewing and evaluating on an annual basis the performance of the audit committee, including compliance of the audit committee with its charter. |
119
Table of Contents
Our board of directors has determined that Steven Meyer qualifies as an audit committee financial expert within the meaning of SEC regulations and meets the financial sophistication requirements of the NASDAQ Listing Rules. In making this determination, our board has considered Mr. Meyer’s formal education and the nature and scope of experience that he has previously had with public companies. Both our independent registered public accounting firm and management periodically meet privately with our audit committee.
Compensation Committee
Our compensation committee consists of Patrick P. Fourteau, Pierre Lapalme and Brian Tambi. Patrick P. Fourteau serves as the chair of our compensation committee. Each member of our compensation committee is a non-employee director, as defined in Rule 16b-3 promulgated under the Exchange Act, is an outside director, as defined pursuant to Section 162(m) of the Code and satisfies the Nasdaq Stock Market independence requirements. The functions of this committee include, among other things:
| • | reviewing, modifying and approving (or if it deems appropriate, making recommendations to the full board of directors regarding) our overall compensation strategy and policies; |
| • | reviewing and approving the compensation and other terms of employment of our executive officers; |
| • | reviewing and approving performance goals and objectives relevant to the compensation of our executive officers and assessing their performance against these goals and objectives; |
| • | reviewing and approving (or if it deems it appropriate, making recommendations to the full board of directors regarding) the equity incentive plans, compensation plans and similar programs advisable for us, as well as modifying, amending or terminating existing plans and programs; |
| • | evaluating risks associated with our compensation policies and practices and assessing whether risks arising from our compensation policies and practices for our employees are reasonably likely to have a material adverse effect on us; |
| • | reviewing and approving (or if it deems it appropriate, making recommendations to the full board of directors regarding) the type and amount of compensation to be paid or awarded to our non-employee board members; |
| • | establishing policies with respect to votes by our stockholders to approve executive compensation as required by Section 14A of the Exchange Act and determining our recommendations regarding the frequency of advisory votes on executive compensation; |
| • | reviewing and assessing the independence of compensation consultants, legal counsel and other advisors as required by Section 10C of the Exchange Act; |
| • | administering our equity incentive plans; |
| • | establishing policies with respect to equity compensation arrangements; |
| • | reviewing the competitiveness of our executive compensation programs and evaluating the effectiveness of our compensation policy and strategy in achieving expected benefits to us; |
| • | reviewing and approving the terms of any employment agreements, severance arrangements, change in control protections and any other compensatory arrangements for our executive officers; |
| • | reviewing the adequacy of its charter on a periodic basis; |
| • | reviewing with management and approving our disclosures under the caption “Compensation Discussion and Analysis” in our periodic reports or proxy statements to be filed with the SEC; |
120
Table of Contents
| • | preparing the compensation committee report that the SEC requires in our annual proxy statement; and |
| • | reviewing and assessing on an annual basis the performance of the compensation committee. |
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee consists of Brian Tambi, Patrick P. Fourteau and Steven Meyer. Our board of directors has determined that each of the members of this committee satisfies the Nasdaq Stock Market independence requirements. Mr. Tambi serves as the chair of our nominating and corporate governance committee. The functions of this committee include, among other things:
| • | identifying, reviewing and evaluating candidates to serve on our board of directors consistent with criteria approved by our board of directors; |
| • | determining the minimum qualifications for service on our board of directors; |
| • | evaluating director performance on the board and applicable committees of the board and determining whether continued service on our board is appropriate; |
| • | evaluating, nominating and recommending individuals for membership on our board of directors; |
| • | evaluating nominations by stockholders of candidates for election to our board of directors; |
| • | considering and assessing the independence of members of our board of directors; |
| • | developing a set of corporate governance policies and principles, including a code of business conduct and ethics, periodically reviewing and assessing these policies and principles and their application, and recommending to our board of directors any changes to such policies and principles; |
| • | considering questions of possible conflicts of interest of directors as such questions arise; |
| • | reviewing the adequacy of its charter on an annual basis; and |
| • | annually evaluating the performance of the nominating and corporate governance committee. |
121
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
Overview
Our board of directors, together with our compensation committee, have administered our executive compensation program. Following this offering, our compensation committee will have the primary role of overseeing our compensation and benefits plans and policies, administering our stock plans and reviewing and approving all compensation decisions relating to our executive officers.
Our Philosophy
Our executive compensation programs are guided by several key beliefs of our board of directors and compensation committee:
| • | Competition. Compensation should reflect the competitive marketplace, so we can retain, attract, and motivate talented executives. Compensation provided to executives should remain competitive relative to compensation paid by companies of similar size and stage of development operating in the pharmaceutical industry, taking into account our relative performance, financial position and our own strategic objectives. |
| • | Accountability for Product and Patent Development. Compensation should be tied, in part, to the growth of our business through development of patents and licenses and other product pipeline related activities, as well as the commercial performance of any approved products. |
| • | Accountability for Financial Performance. Compensation should be tied, in part, to financial performance, so that executive officers are held accountable through their compensation for contributions to our performance as a whole and through the performance of the businesses for which they are responsible. |
| • | Accountability for Individual Performance. Compensation should be tied, in part, to the executive officer’s individual contributions to our overall performance. Our board of directors and compensation committee considers individual performance as well as performance of the businesses and responsibility areas that an executive officer oversees, and weighs these factors as appropriate in assessing a particular executive officer’s performance. |
| • | Alignment with Stockholder Interests. Compensation should be tied, in part, to our stock performance through equity awards to align executives’ interests with those of our stockholders. |
| • | Mix of Compensation. Compensation should include short-term and long-term components, including cash and equity-based compensation, and should reward performance that consistently meets or exceeds expectations. |
Application of our Philosophy
Our executive compensation program aims to encourage our executive officers to continually pursue our strategic opportunities while effectively managing the risks and challenges inherent to our business. Specifically, we have created an executive compensation package that we believe is most appropriate to provide incentives to our executive officers and reward them for achieving the following goals:
| • | develop a culture that embodies a passion for our business, creative contribution and a drive to achieve success for our business; |
| • | provide leadership to the organization in such a way as to maximize the results of our business operations; |
| • | lead us by demonstrating forward thinking in the operation, development and expansion of our business; |
122
Table of Contents
| • | effectively manage organizational resources to derive the greatest value possible from each dollar invested; and |
| • | take strategic advantage of market opportunities to expand and grow our business. |
The components of our executive compensation program not only aim to be competitive in our industry, but also to be fair relative to (i) compensation paid to other professionals within our organization; (ii) our short- and long-term performance and (iii) the value we deliver to our stockholders. We seek to maintain a performance-oriented culture and a compensation approach that rewards our executive officers when we achieve our goals and objectives, while putting at risk an appropriate portion of their compensation against the possibility that our goals and objectives may not be achieved. Overall, our approach is designed to tie the compensation of our executive officers to (x) the achievement of short and longer term goals and objectives; (y) their willingness to challenge and improve existing policies and structures and (z) their capability to take advantage of unique opportunities and overcome difficult challenges within our business.
Role of Chief Executive Officer in Compensation Decisions
Our Chief Executive Officer typically evaluates the performance of other executive officers and employees, along with the performance of the company as a whole, and makes recommendations to the board of directors and compensation committee with respect to salary adjustments, bonuses and stock option grants. Each of the board of directors and compensation committee exercises its own independent discretion in setting salary adjustments and discretionary cash and equity-based awards for all executive officers. The Chief Executive Officer is not present during deliberations or voting with respect to his own compensation.
Components of Executive Compensation
Our current executive compensation program consists of three components: short-term compensation (including base salary and annual bonuses), long-term equity-based incentives and benefits.
Short-Term Compensation
We utilize short-term compensation, including base salary, annual adjustments to base salary and annual bonuses, to motivate and reward our executive officers in accordance with our performance-based program. Each executive officer’s short-term compensation components are tied to an annual assessment of his or her performance in the prior year.
Our compensation amounts historically have been highly individualized, resulted from arm’s length negotiations and were based on a variety of informal factors including, in addition to the factors listed above, our financial status, our need for that particular position to be filled and the compensation levels of our other executive officers, each as of the time of the applicable compensation decision. In addition, we consider the competitive market for corresponding positions within comparable geographic areas and industries. Although members of our board of directors and compensation committee possess general knowledge regarding the compensation given to some of the executive officers of other companies in our industry, the board of directors and compensation committee have each historically applied its subjective discretion to make compensation decisions and have not formally benchmarked executive compensation against a particular set of comparable companies or used a formula to set the compensation for our executives in relation to survey data. We anticipate that our recently reconstituted compensation committee will more formally benchmark executive compensation against a peer group of comparable companies in the future. We also anticipate that our compensation committee may make adjustments in executive compensation levels in the future as a result of this more formal benchmarking process.
123
Table of Contents
Base Salary. Base salaries for our executives are established based on the executive officer’s seniority, position and functional role and level of responsibility. The base salary of each executive officer is reviewed on an annual basis and adjustments are made to reflect performance-based factors, our current financial position, as well as competitive conditions. Increases are considered within the context of our overall merit increase structure as well as individual and market competitive factors. We do not apply specific formulas to determine increases. Generally, executive officer base salaries are adjusted based on a review of:
| • | the executive’s general performance during the applicable review period; |
| • | an assessment of the executive’s professional effectiveness, consisting of a portfolio of competencies that include leadership, commitment, creativity and organizational accomplishment; and |
| • | the executive’s knowledge, skills and attitude, focusing on ability to drive results. |
In January 2010, our board of directors and compensation committee approved base salaries for our named executive officers for 2010 in an amount equal to $340,000 for Dr. Aquilur Rahman, our then-current President, Chief Executive Officer and Chief Scientific Advisor, $178,750 for Dr. Shahid Ali, our Executive Vice President, Research & Development, and $151,250 for Martin McCarthy, our Chief Financial Officer. In September 2010, due to our diminishing cash resources and then-current cash burn rate, our board of directors, together with input from our executive officers, reduced base salaries for our then-current named executive officers by 25%. This decrease in base salary was not a reflection of the performance of our executive officers, but merely intended to preserve our cash resources and reduce our cash burn rate. In November 2010, following the Merger, Dr. Rahman left the company, and Mr. Babich become our acting President. As our acting President, Mr. Babich’s base salary was $181,771, which was the same base salary he received as Chief Operating Officer of Insys Pharma prior to the Merger. Mr. Babich’s base salary while Chief Operating Officer of Insys Pharma was established by its board of directors based on (i) the scope of his responsibilities and individual experience, (ii) the company’s overall performance, including its progress towards research and development goals, and (iii) the pay provided to executives by companies of similar size and stage of development operating in the pharmaceutical industry. In March 2011, the board of directors, in connection with its annual review of executive compensation, approved base salaries for our executive officers in an amount equal to $365,000 for Mr. Babich, $175,000 for Mr. McCarthy and $225,000 for Dr. Dillaha. In approving these base salary adjustments, the board of directors considered our current financial status, the growth of our company and attainment of development milestones, and the fact that we are in the process of actively pursuing an initial public offering.
Annual Bonuses. Our executive officers’ annual bonuses are discretionary and tied to the achievement of corporate objectives, functional area objectives and/or individual performance objectives and a thorough review of the applicable performance results of the company, business, function and/or individual during the applicable period. Final determinations as to discretionary bonus levels are based in part on the achievement of these company-wide and personal objectives, as well as the assessment as to the overall development of our business and corporate accomplishments. Based on a review of such factors, final bonus amounts, if any, are determined at the sole discretion of the compensation committee and our board of directors. For 2010, due to our diminishing cash resources and then-current cash burn rate, no discretionary cash bonuses were paid. Additionally, no bonuses were paid to executives of Insys Pharma during 2010. Discretionary bonuses for 2011, if any, will be determined by our compensation committee and our board of directors and paid at the end of 2011 or in early 2012. Pursuant to the terms of their employment agreements, Mr. Babich and Dr. Dillaha are each eligible to receive a discretionary bonus for 2011 of up to 80% and 60%, respectively, of their respective base salaries in 2011.
Long-Term Equity-Based Compensation
Our long-term compensation program consists solely of stock option grants. Stock option grants made to executive officers are designed to provide them with incentives to execute their
124
Table of Contents
responsibilities in such a way as to generate long-term benefit to us and our stockholders. Through possession of stock options, our executive officers participate in the long-term results of their efforts, whether by appreciation of our company’s value or the impact of business setbacks, either company-specific or industry-based. Additionally, stock options provide a means of retaining our executive officers, in that they are in almost all cases subject to vesting over an extended period of time.
Upon joining us, each executive officer is granted an initial option award that is primarily based on competitive conditions applicable to such officer’s specific position. These competitive conditions are primarily based on the supply and demand for executive officers of that type at the time of hiring, which may be evidenced by competing offers for a particular individual. In addition, our board of directors and compensation committee considers the number of options held by other executive officers within our company. We believe this strategy is consistent with the approach of other companies at the same stage of development in our industry and, in our board of directors’ and compensation committee’s view, is appropriate for aligning the interests of our executive officers with those of our stockholders over the long term.
Periodic awards to executive officers are made based on an assessment of their sustained performance over time, their ability to impact results that drive value to our stockholders and their organization level. Option awards are not granted at regular intervals or automatically to our executive officers. Our Chief Executive Officer periodically reviews the performance of our executive officers on the bases noted above and recommends to our board of directors and compensation committee any option awards deemed appropriate.
During 2010, our named executive officers were awarded stock options in the amounts indicated in the section below entitled “Grants of Plan-Based Awards,” which our board of directors or compensation committee, based upon input from our Chief Executive Officer, believed provided the named executive officers with sufficient incentive to execute their responsibilities in such a way as to generate long-term benefit to us and our stockholders.
Benefits
We provide the following benefits to our executive officers on the same basis as the benefits provided to all employees:
| • | health and dental insurance; |
| • | life insurance; |
| • | short- and long-term disability; and |
| • | 401(k) plan. |
We believe that these benefits are consistent with those offered by other companies and specifically with those companies with which we compete for employees.
Our Competitive Market
The market for experienced management with the knowledge, skills and experience our organization requires is highly competitive. Our objective is to attract and retain the most highly qualified executives to manage each of our business functions. In doing so, we draw upon a pool of talent that is highly sought after by other companies in our industry and other industries that also produce the requisite skills we seek.
We believe that our ability to offer significant upside potential through equity-based compensation gives us a competitive advantage compared to many larger or more established companies with which we compete for executives. Nonetheless, we must also offer cash compensation to our existing and prospective executive officers through base salaries and cash bonuses that are competitive in the marketplace and allow them to satisfy their day to day financial requirements.
125
Table of Contents
We also compete on the basis of our vision of future success, our culture and company values, and the excellence of our other management personnel.
Total Compensation
We intend to continue our strategy of compensating our executive officers at competitive levels, with the opportunity to impact their total annual compensation through performance-based compensation that includes both cash and equity elements. Our approach to total executive compensation is designed to drive results that maximize our financial performance and deliver value to our stockholders. In light of our compensation philosophy, we believe that the total compensation package for our executives should continue to consist of base salary, annual bonus and long-term equity-based awards.
Evolution of our Compensation Approach
Our compensation approach is necessarily tied to our stage of development as a company. Accordingly, the specific direction, emphasis and components of our executive compensation program will continue to evolve as our company and its underlying business strategy continue to grow and develop. In the future, we may engage a compensation consultant to assist our board of directors and compensation committee in continuing to evolve our executive compensation program, and we may look to programs implemented by comparable public companies in refining our compensation approach, including the benchmarking of our compensation using a formal peer group. Additionally, we expect that our executive compensation policies may further evolve as we transition from a research and development organization to a commercial organization.
Summary Compensation Table
The following table provides information regarding the compensation earned during the year ended December 31, 2010 by each person serving in 2010 as our Chief Executive Officer or our Chief Financial Officer and our other most highly compensated executive officer serving in 2010. We refer to these executives as our named executive officers.
| Name and Principal Position |
Year | Salary ($) |
Option Awards ($)(1) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||
| Michael L. Babich(2) |
2010 | 181,771 | (3) | 446,109 | (4) | — | 627,880 | |||||||||||||
| President and Chief Executive Officer |
||||||||||||||||||||
| Martin McCarthy(5) |
2010 | 151,250 | 7,830 | 9,988 | (6) | 169,068 | ||||||||||||||
| Chief Financial Officer |
||||||||||||||||||||
| Shahid Ali, Ph.D.(7) |
2010 | 178,750 | 13,050 | 11,523 | (8) | 203,323 | ||||||||||||||
| Executive Vice President, Research and Development |
||||||||||||||||||||
| Aquilur Rahman, Ph.D.(9) |
2010 | 290,417 | 39,150 | — | 329,567 | |||||||||||||||
| Former President, Chief Executive Officer and Chief Scientific Advisor |
||||||||||||||||||||
| (1) | Represents the full grant date fair value of stock option awards calculated in accordance with FASB ASC Topic 718. For a discussion of valuation assumptions for stock option awards to Mr. Babich, see Note 6, “Stock-Based Compensation” to the Insys Therapeutics consolidated financial statements located elsewhere in this prospectus. For a discussion of valuation assumptions for stock option awards to Mr. McCarthy, Dr. Ali and Dr. Rahman, see Note 3, “Stock-Based Compensation” to the NeoPharm consolidated financial statements located elsewhere in this prospectus. |
126
Table of Contents
| (2) | Prior to the Merger, Mr. Babich was the President and Chief Operating Officer of Insys Pharma. Upon closing the Merger, Mr. Babich became our President and Chief Operating Officer. Mr. Babich became our Chief Executive Officer in March 2011. |
| (3) | Represents salary paid to Mr. Babich during 2010 by both Insys Pharma and us. Of this amount, $154,407 was paid by Insys Pharma prior to the Merger. |
| (4) | Upon closing of the Merger in November 2010, we assumed outstanding options to purchase shares of common stock of Insys Pharma and converted these options into options to purchase shares of our common stock. The value of Mr. Babich’s stock options shown represent the conversion of an option to purchase up to an aggregate of 32,786 shares of common stock of Insys Pharma into an option to purchase up to an aggregate of 121,876 shares of our common stock and the conversion of an option to purchase up to an aggregate of 59,803 shares of common stock of Insys Pharma into an option to purchase up to an aggregate of 222,303 shares of our common stock. |
| (5) | Mr. McCarthy served as our Corporate Controller and Acting Financial Officer until March 2011, at which time he became our Chief Financial Officer. |
| (6) | Includes payment of a car allowance of $4,800 and payment of qualified non-elective contributions to Mr. McCarthy’s 401(k) account of $5,188. |
| (7) | Following the Merger, Dr. Ali ceased to be an executive officer. |
| (8) | Includes payment of a car allowance of $4,800 and payment of qualified non-elective contributions to Dr. Ali’s 401(k) account of $6,723. |
| (9) | In connection with the Merger, Dr. Rahman resigned effective November 8, 2010. |
Employment Agreements with Executive Officers
Employment agreements or written offer letters are used from time to time on a case by case basis, to attract and/or to retain executives. We currently maintain written employment agreements with Mr. Babich and Dr. Dillaha.
Employment Agreement with Mr. Babich. We entered into an employment agreement with Mr. Babich in April 2011 setting forth the terms of Mr. Babich’s employment as our President and Chief Executive Officer. Pursuant to the agreement, Mr. Babich is paid an annual salary of $365,000 and is eligible to receive a performance bonus of up to 80% of his base salary for 2011. Mr. Babich’s employment is at-will, and either we or Mr. Babich may terminate the agreement at any time without cause and without notice. However, if we terminate Mr. Babich without cause, or if Mr. Babich resigns for good reason, and Mr. Babich signs a release in our favor, Mr. Babich will be entitled to receive salary continuation for a period of 12 months following his termination date, as well as an additional severance payment equal to his prorated target bonus for the year in which he is terminated, and all of Mr. Babich’s unvested stock options and equity awards will immediately vest in full.
Employment Agreement with Dr. Dillaha. We entered into an employment agreement with Dr. Dillaha in April 2011 setting forth the terms of Dr. Dillaha’s employment as our Chief Medical Officer. Pursuant to the agreement, Dr. Dillaha is paid an annual salary of $225,000 and is eligible to receive a performance bonus of up to 60% of his base salary for 2011. Dr. Dillaha’s employment is at-will, and either we or Dr. Dillaha may terminate the agreement at any time without cause and without notice. However, if we terminate Dr. Dillaha without cause, or if Dr. Dillaha resigns for good reason, and Dr. Dillaha signs a release in our favor, Dr. Dillaha will be entitled to receive salary continuation for a period of 12 months following his termination date, as well as an additional severance payment equal to his prorated target bonus for the year in which he is terminated, and all of Dr. Dillaha’s unvested stock options and equity awards will immediately vest in full.
127
Table of Contents
Potential Payments Upon Termination or Change in Control
Payments Made Upon Termination. Regardless of the manner in which a named executive officer’s employment terminates, the named executive officer is entitled to receive amounts earned during his term of employment, including salary and unused vacation pay.
Potential Termination-Based Payments. During 2010, none of our executive officers had the right to receive payments upon termination of their services, except for potential accelerated vesting of stock options under our equity incentive plans in the event of certain corporate transactions. In April 2011, we entered into employment agreements with Mr. Babich and Dr. Dillaha providing for certain termination-based payments described above under “—Employment Agreements with Executive Officers.” For more information regarding accelerated vesting of stock options under our equity incentive plans in the event of certain corporate transactions, please see “— Employee Benefit Plans” below.
The following table sets forth potential payment value of other benefits receivable by our current executive officers upon a termination of employment without cause or resignation for good reason or upon a change in control. Our compensation committee may in its discretion revise, amend or add to the benefits if it deems advisable. The table below reflects amounts payable to our executive officers assuming the termination occurred on, and their employment was terminated on, December 31, 2010 and, if applicable, a change of control also occurred on such date:
| Upon Termination Without Cause or Resignation for Good Reason |
Upon Change in Control (1) | |||||||||||||
| Name |
Salary ($) | Bonus ($) | Value of Accelerated Vesting ($)(2) |
Total ($) | Value of Accelerated Vesting ($)(2) | |||||||||
| Michael L. Babich (3) |
365,000 | 292,000 | ||||||||||||
| Larry Dillaha (3) |
225,000 | 135,000 | ||||||||||||
| Martin McCarthy |
— | — | ||||||||||||
| (1) | See “—Equity Benefit Plans” for a more detailed description of the effect of accelerated vesting in the event of change in control transactions under our equity incentive plans. |
| (2) | The value of accelerated vesting is equal to an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover of this prospectus, multiplied by the number of shares subject to accelerated vesting, less the stock option exercise price. |
| (3) | In April 2011, we entered into employment agreements with Mr. Babich and Dr. Dillaha which provide for severance payments and acceleration of unvested equity awards upon a termination for cause or resignation for good reason. Prior to April 2011, neither Mr. Babich or Dr. Dillaha was entitled to such benefits for a qualifying termination or resignation. |
Grants of Plan-Based Awards
Stock options granted to our named executive officers were granted under our 2006 Equity Incentive Plan, except for options granted to former employees of Insys Pharma, which were granted under the Insys Pharma, Inc. Amended and Restated Equity Incentive Plan. Following February 2009 and prior to the Merger, the exercise price per share of each stock option granted by us was equal to the mean between the lowest and highest reported sales prices of our common stock on the OTC market on the grant date. For options granted by Insys Pharma, the exercise price per share of each stock option was based on the per share fair market value of Insys Pharma common stock, as determined in good faith by the Insys Pharma board of directors on the grant date.
128
Table of Contents
The following table sets forth certain information regarding grants of plan-based awards to our named executive officers during the year ended December 31, 2010.
| Name |
Grant Date | All Other Option Awards: Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($/Sh)(1) |
Grant Date Fair Value of Stock and Option Awards ($)(2) |
||||||||||||
| Michael L. Babich |
2/22/10 | 121,876 | (3) | 1.83 | (4) | 155,181 | ||||||||||
| 2/22/10 | 222,303 | (3) | 1.83 | (4) | 290,927 | |||||||||||
| Martin McCarthy |
2/10/10 | 491 | (5) | 18.91 | 7,830 | |||||||||||
| Shahid Ali, Ph.D. |
2/10/10 | 819 | (5) | 18.91 | 13,050 | |||||||||||
| Aquilur Rahman, Ph.D.(7) |
2/10/10 | 2,459 | (5)(6) | 18.91 | 39,150 | |||||||||||
| (1) | Represents the mean between the lowest and highest reported sales prices of our common stock on the OTC market as of the grant date. For options granted by Insys Pharma, represents the per share fair market value of Insys Pharma common stock, as determined in good faith by the Insys Pharma board of directors on the grant date. |
| (2) | Represents the full grant date fair value of stock option awards calculated in accordance with FASB ASC Topic 718. For a discussion of valuation assumptions for stock option awards to Mr. Babich, see Note 6, “Stock-Based Compensation” to the Insys Therapeutics consolidated financial statements located elsewhere in this prospectus. For a discussion of valuation assumptions for stock option awards to Mr. McCarthy, Dr. Ali and Dr. Rahman, see Note 3, “Stock-Based Compensation” to the NeoPharm consolidated financial statements located elsewhere in this prospectus. |
| (3) | Upon closing of the Merger in November 2010, we assumed outstanding options to purchase shares of common stock of Insys Pharma and converted such options into options to purchase shares of our common stock. The shares underlying Mr. Babich’s stock options shown represent the conversion of an option to purchase up to an aggregate of 32,786 shares of common stock of Insys Pharma into an option to purchase up to an aggregate of 121,876 shares of our common stock and the conversion of an option to purchase up to an aggregate of 59,803 shares of common stock of Insys Pharma into an option to purchase up to an aggregate of 222,303 shares of our common stock. |
| (4) | The exercise prices of Mr. Babich’s stock option awards shown are based on options to purchase shares of common stock of Insys Pharma at an exercise price of $6.10, converted to give effect to the exchange rate applicable to our assumption and conversion of such options as a result of the Merger in November 2010. |
| (5) | Shares subject to vesting pursuant to these stock option grants became immediately vested upon the closing of the Merger in November 2010. |
| (6) | In November 2010 our board of directors extended the period during which Dr. Rahman’s unexercised stock options are exercisable to allow for exercise at any time during the full ten-year term of the stock option. |
| (7) | In connection with the Merger, Dr. Rahman ceased to be our President and Chief Executive Officer and Chief Scientific Advisor. |
129
Table of Contents
Outstanding Equity Awards as of December 31, 2010
The following table sets forth certain information regarding equity awards granted to our named executive officers that were outstanding as of December 31, 2010.
| Option Awards | ||||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options – Exercisable (#) |
Number
of Securities Underlying Unexercised Options – Unexercisable (#) |
Option Exercise Price ($)(1) |
Option Expiration Date ($) |
||||||||||||
| Michael L. Babich |
60,938 | (1) | 60,938 | (2)(3) | 1.83 | (4) | 2/22/2020 | |||||||||
| 222,303 | (1) | — | 1.83 | (4) | 2/22/2020 | |||||||||||
| Martin McCarthy |
327 | — | 66.49 | 11/5/2017 | ||||||||||||
| 491 | — | 4.88 | 2/9/2019 | |||||||||||||
| 491 | — | 18.91 | 2/10/2020 | |||||||||||||
| Shahid Ali, Ph.D. |
70 | — | 840.58 | 2/1/2012 | ||||||||||||
| 213 | — | 538.02 | 12/30/2012 | |||||||||||||
| 147 | — | 1,214.51 | 2/17/2014 | |||||||||||||
| 162 | — | 484.95 | 4/28/2015 | |||||||||||||
| 132 | — | 760.06 | 1/25/2016 | |||||||||||||
| 245 | — | 433.71 | 12/5/2016 | |||||||||||||
| 819 | — | 60.39 | 8/16/2017 | |||||||||||||
| 1,229 | — | 4.88 | 2/9/2019 | |||||||||||||
| 819 | — | 18.91 | 2/10/2020 | |||||||||||||
| Aquilur Rahman, Ph.D. |
3,278 | — | 60.39 | 8/16/2017 | ||||||||||||
| 2,459 | — | 4.88 | 2/9/2019 | |||||||||||||
| 2,459 | — | 18.91 | 2/10/2020 | |||||||||||||
| (1) | Represents mean between the lowest and highest reported sales prices of our common stock on the OTC market as of the grant date. For options granted by Insys Pharma, represents the per share fair market value of Insys Pharma common stock, as determined in good faith by the Insys Pharma board of directors on the grant date. |
| (2) | Upon closing of the Merger in November 2010, we assumed outstanding options to purchase shares of common stock of Insys Pharma and converted such options into options to purchase shares of our common stock. The shares underlying Mr. Babich’s stock options shown represent the conversion of an option to purchase up to an aggregate of 32,786 shares of common stock of Insys Pharma into an option to purchase up to an aggregate of 121,876 shares of our common stock and the conversion of an option to purchase up to an aggregate of 59,803 shares of common stock of Insys Pharma into an option to purchase up to an aggregate of 222,303 shares of our common stock. |
| (3) | These options became fully vested on February 22, 2011. |
| (4) | The exercise prices of Mr. Babich’s stock option awards shown are based on options to purchase shares of common stock of Insys Pharma at an exercise price of $6.10, converted to give effect to the exchange rate applicable to our assumption and conversion of such options as a result of the Merger in November 2010. |
Pension Benefits
Our named executive officers did not participate in or have account balances in qualified or nonqualified defined benefit plans sponsored by us. Our board of directors or compensation committee may elect to adopt qualified or nonqualified benefit plans in the future if it determines that doing so is in our best interests.
130
Table of Contents
Nonqualified Deferred Compensation
Our named executive officers did not participate in or have account balances in nonqualified defined contribution plans or other nonqualified deferred compensation plans maintained by us. Our board of directors or compensation committee may elect to provide our executive officers and other employees with nonqualified defined contribution or other nonqualified deferred compensation benefits in the future if it determines that doing so is in our best interests.
Director Compensation
Prior to the Merger, our board of directors had adopted a compensation policy applicable to all non-employee directors. This compensation policy provided that each such non-employee director received the following compensation for service on our board of directors:
| • | an annual cash retainer of $24,000 for service as chairman of the board of directors; |
| • | an annual cash retainer of $22,500 for service as chairman of the nominating and corporate governance committee; |
| • | an annual cash retainer of $20,000 for service as chairman of the audit committee; |
| • | an annual cash retainer of $10,000 for service as chairman of the compensation committee; |
| • | an annual cash retainer of $10,000, $10,000 and $4,000 for service as a member of the nominating and corporate governance committee, the audit committee and the compensation committee, respectively; and |
| • | an annual option grant to purchase shares of our common stock. |
The following table provides information regarding the compensation earned during the year ended December 31, 2010 by our non-employee directors during that year.
| Name |
Fees Earned or Paid in Cash ($) |
Option Awards ($)(2)(3) |
Total ($) |
|||||||||
| Frank Becker(1) |
29,750 | 14,500 | 44,250 | |||||||||
| Bernard Fox, M.D.(1) |
31,937 | 14,500 | 46,437 | |||||||||
| Paul Freiman(1) |
26,250 | 14,500 | 40,750 | |||||||||
| John N. Kapoor, Ph.D.(4) |
21,000 | 21,750 | 42,750 | |||||||||
| Rao Akella, M.D. |
— | — | — | |||||||||
| Brian Tambi |
— | — | — | |||||||||
| Steven Meyer |
— | — | — | |||||||||
| (1) | Resigned as a member of our board of directors following the Merger. |
| (2) | Represents the full grant date fair value of stock option awards calculated in accordance with FASB ASC Topic 718. For a discussion of valuation assumptions, see Note 3, “Stock-Based Compensation” to the NeoPharm consolidated financial statements located elsewhere in this prospectus. |
| (3) | The aggregate number of shares subject to each director’s outstanding option awards as of December 31, 2010 was as follows: Mr. Becker 1,024 shares; Dr. Fox 1,024 shares; Mr. Freiman 1,024 shares; Dr. Kapoor 1,536 shares; Dr. Akella 23,518 shares; Mr. Tambi 17,885 shares; and Mr. Meyer 17,885 shares. |
| (4) | From June 2008 until September 2010, Dr. Kapoor served as Chief Executive Officer of Insys Pharma but received no compensation in this capacity. |
131
Table of Contents
Following the year ended December 31, 2010, our board of directors adopted a new compensation policy applicable to all of our non-employee directors. This compensation policy provides that each such non-employee director will receive the following compensation for service on our board of directors:
| • | an annual cash retainer of $40,000 for service as chairman of the board of directors; |
| • | an annual cash retainer of $30,000 for service as a member of the board of directors; |
| • | an additional annual cash retainer of $3,000 for service as chairman of the audit committee, compensation committee or the nominating and corporate governance committee; |
| • | an annual cash retainer of $1,500, $2,500 and $2,500 for service as a member of the nominating and corporate governance committee, the audit committee and the compensation committee, respectively; |
| • | upon first joining our board of directors, an automatic initial grant of an option to purchase 4,000 shares of our common stock vesting monthly over one year following the grant date; and |
| • | for each non-employee director whose term continues on January 1st of each year, an automatic annual grant of an option to purchase 2,000 shares of our common stock vesting monthly over one year following the grant date. |
Equity Benefits Plans
2006 Equity Incentive Plan
We adopted our 2006 equity incentive plan, or the 2006 plan, in April 2006. As of June 30, 2011, no shares of common stock have been issued upon the exercise of options granted under our 2006 plan, options to purchase 537,178 shares of common stock were outstanding and 110,362 shares remained available for future grant. Upon the effective date of this offering, no further option grants will be made under our 2006 plan. We intend to grant all future equity awards under our 2011 equity incentive plan, or the 2011 plan and our 2011 non-employee directors’ stock award plan, or the directors’ plan. However, all stock options granted under our 2006 plan will continue to be governed by the terms of our 2006 plan.
Eligibility. Our 2006 plan permits us to grant stock awards, including stock options, restricted stock, stock appreciation rights, performance units, performance shares and other stock awards to our employees, directors and consultants. Our board of directors has granted only stock options under our 2006 plan. A stock option may be an incentive stock option within the meaning of Section 422 of the Code, or a nonstatutory stock option.
Stock option provisions generally. In general, the duration of a stock option granted under our 2006 plan cannot exceed 10 years. No later than the grant date of any option, the exercise price of such stock option is required to be determined; provided, however, that our board of directors may elect to determine the exercise price as of the date the grantee is hired or promoted (or similar event), if the grant date occurs not more than 90 days after the date of hiring, promotion or other event. The exercise price of a stock option (other than an incentive stock option) shall not be less than 85% of the fair market value of our common stock on the grant date. If, and to the extent deemed necessary by our board of directors with respect to a nonstatutory stock option granted to a named executive officer, the price to be paid for each share of our common stock upon exercise of such stock option shall be less than 100% of the fair market value of a share of our common stock on the date such stock option is granted, the exercisability of such stock option must be subject to one or more of the performance goals set forth in the 2006 plan that will enable such stock option to qualify as “performance-based compensation” under regulations promulgated under Section 162(m) of the Code.
Incentive stock options may be granted only to our employees or employees of any designated subsidiary of ours as permitted under the applicable provisions of the Code. The aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to which incentive
132
Table of Contents
stock options are exercisable for the first time by an optionholder during any calendar year under all of our stock plans may not exceed $100,000. No incentive stock option may be granted to any person who, at the time of the grant, owns or is deemed to own stock possessing more than 10% of our total combined voting power unless (a) the option exercise price is at least 110% of fair market value of the stock subject to the option on the date of grant and (b) the term of the incentive stock option does not exceed five years from the date of grant.
Effect on stock options of certain change in control events. Unless otherwise provided in an award agreement, if we experience a change in control, stock options held by individuals whose service has not terminated prior to the change in control will be accelerated in full and shall be immediately exercisable in full on the date of such change in control.
Other provisions. Our board of directors will appropriately adjust the class and the maximum number of shares subject to the 2006 plan in the event of a consolidation of shares, stock dividend or stock split. Our board of directors may also provide for cash settlement and/or make such other adjustments to the terms of the outstanding awards as it deems appropriate in the context of a transaction in which our stockholders receive capital stock of another corporation in exchange for their shares in a merger, consolidation, acquisition of property or stock, separation or reorganization.
1998 Equity Incentive Plan
We adopted our 1998 equity incentive plan, as amended and restated, or the 1998 plan, in July 1998. The 1998 plan provided for the grant of stock awards, primarily stock options, to employees, directors, and consultants to acquire up to 75,409 shares of our common stock. The 1998 plan also permitted the grant of performance shares, performance units and bonus stock. The 1998 plan terminated in accordance with its terms on July 23, 2008 and accordingly no further shares can be granted pursuant to this plan. As of June 30, 2011, options to purchase 2,924 shares of common stock were outstanding and no shares remained available for future grant. Following the approval of the 2006 plan by our stockholders in June 2006, no further awards were made under the 1998 plan. We intend to grant all future equity awards under our 2011 plan and our directors’ plan. However, all stock options granted under our 1998 plan will continue to be governed by the terms of our 1998 plan.
Option awards under the 1998 plan were generally granted with an exercise price equal to the closing price of our common stock on the date of grant, but may have been granted with an exercise price of not less than 85% of the fair market value of our common stock. Option awards under the 1998 plan typically had a 10-year life and vested ratably on the first four anniversaries of the grant, subject to continuous employment. Stock awards granted to our non-employee directors under the 1998 plan typically vest one year from the date of grant. Outstanding awards issued under the 1998 plan vested immediately upon a change in control.
Effect on stock options of certain change of control events. If we experience a change in control, all stock options will be accelerated in full and shall be immediately exercisable in full on the date of such change in control.
Insys Pharma, Inc. Amended and Restated Equity Incentive Plan
In connection with the Merger, on November 8, 2010, we assumed all of the outstanding stock options granted under Insys Pharma, Inc’s amended and restated equity incentive plan, or the Insys Pharma plan. Subsequent to the Merger, these stock options were adjusted to cover shares of our common stock at the exchange ratios set forth in the applicable Merger Agreement. As of June 30, 2011, options to purchase an aggregate of 1,079,133 shares of our common stock under the Insys Pharma plan were outstanding. The Insys Pharma plan was terminated and we will not grant additional equity awards under the Insys Pharma plan.
Share Reserve. Except with respect to the outstanding options referenced above, no shares of our common stock remain reserved or available for issuance under the Insys Pharma plan.
133
Table of Contents
Administration. Our board of directors administers the Insys Pharma plan, but the board may delegate authority to administer the Insys Pharma plan to a committee that complies with applicable law. Our board of directors has broad authority to administer the Insys Pharma plan.
Eligibility. The Insys Pharma plan permitted for the grant of nonstatutory stock options to key employees, non-employee directors and consultants, and permitted for the grant of incentive stock options within the meaning of Section 422 of the Code to employees.
Stock option provisions generally. In general, the duration of a stock option granted under the Insys Pharma plan cannot exceed ten years. An incentive stock option may be transferred only on death, but a nonstatutory stock option may be transferred as permitted by our board of directors or other permitted plan administrator. In addition, our board of directors may amend, modify, extend, cancel or renew any outstanding option or may waive any restrictions or conditions applicable to any outstanding option.
The aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to which incentive stock options are exercisable for the first time by an optionholder during any calendar year under all of our stock plans may not exceed $100,000. An incentive stock option granted to a person who at the time of grant owns or is deemed to own more than 10% of the total combined voting power of all classes of our outstanding stock or any of our affiliates must have a term of no more than five years and an exercise price that is at least 110% of fair market value at the time of grant.
Effect on stock options of certain change in control events. If we experience a change in control, stock options held by individuals whose service has not terminated prior to the change in control will be immediately exercisable in full on the date of such change in control.
Other provisions. If there is a transaction or event which changes our stock that does not involve our receipt of consideration, such as a merger, consolidation, reorganization, stock dividend or stock split, our board of directors will appropriately adjust the class and the maximum number of shares subject to the Insys Pharma plan.
2011 Equity Incentive Plan
Our board of directors adopted and our stockholders approved the 2011 plan in August 2011. The 2011 plan will become effective immediately upon the signing of the underwriting agreement related to this offering. Our board of directors may terminate the 2011 plan at any time, however, incentive stock options may no longer be granted after the day before the tenth anniversary of the date the board of directors adopted the 2011 plan. The purpose of the 2011 plan is to attract, retain and motivate selected employees, consultants and directors through the granting of stock-based compensation awards and cash-based performance bonus awards. The 2011 plan is also designed to permit us to make cash-based awards and equity-based awards intended to qualify as “performance-based compensation” under Section 162(m) of the Code.
Stock Awards. The 2011 plan provides for the grant of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance-based stock awards and other forms of equity compensation, or collectively, stock awards. In addition, the 2011 plan provides for the grant of performance cash awards. Incentive stock options may be granted only to employees, subject to certain limitations described below. All other awards may be granted to employees, including officers, as well as directors and consultants.
The principal features of the 2011 plan are summarized below. This summary is qualified in its entirety by reference to the text of the 2011 plan, which is filed as an exhibit to the registration statement of which this prospectus is a part.
Share reserve. Following this offering, initially, the aggregate number of shares of our common stock that may be issued pursuant to stock awards under the 2011 plan after the 2011 plan becomes
134
Table of Contents
effective is 2,540,102 shares, which number is the sum of (1) the number of shares reserved for future issuance under the 2006 plan and the 1998 plan at the time the 2011 plan becomes effective, (2) an additional number of 1,817,153 new shares plus an additional number of shares in an amount not to exceed 540,102 shares, that are subject to outstanding stock awards granted under the 2006 plan or the 1998 plan that expire or terminate for any reason prior to their exercise or settlement and would otherwise return to the 2006 plan or the 1998 plan reserve, respectively. The number of shares of our common stock reserved for issuance under the 2011 plan will automatically increase on January 1 of each year, starting on January 1, 2012 and continuing through January 1, 2021, by the lesser of (a) 5% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year or (b) 700,000 shares. The maximum number of shares that may be issued pursuant to the exercise of incentive stock options under the 2011 plan is 5,500,000 shares.
No person may be granted stock awards covering more than 1,000,000 shares of our common stock under the 2011 plan during any calendar year pursuant to stock options, stock appreciation rights or other stock awards whose value is determined by reference to an increase over an exercise or strike price of at least 100% of the fair market value on the date of grant. In addition, no person may be granted a performance stock award covering more than 1,000,000 shares or a performance cash award covering more than 1,000,000 shares in any calendar year. Such limitations are designed to help assure that any deductions to which we would otherwise be entitled with respect to such stock awards will not be subject to the $1,000,000 limitation on the income tax deductibility of compensation paid per covered executive officer imposed by Section 162(m) of the Code.
If a stock award granted under the 2011 plan expires or otherwise terminates without being exercised in full, or is settled in cash, the shares of our common stock not acquired pursuant to the stock award again become available for subsequent issuance under the 2011 plan. In addition, the following types of shares under the 2011 plan may become available for the grant of new stock awards under the 2011 plan: (a) shares that are forfeited to or repurchased by us prior to becoming fully vested; (b) shares withheld to satisfy income or employment withholding taxes; (c) shares used to pay the exercise price of an option in a net exercise arrangement; and (d) shares tendered to us to pay the exercise price of an option. As of the date hereof, no shares of our common stock have been issued under the 2011 plan.
Administration. Our board of directors has delegated its authority to administer the 2011 plan to our compensation committee. The compensation committee is required to consist of two or more “outside directors” within the meaning of Section 162(m) of the Code and/or two or more “non-employee directors” for the purposes of Rule 16b-3 under the Exchange Act. Subject to the terms of the 2011 plan, our board of directors or an authorized committee, referred to as the plan administrator, determines recipients, dates of grant, the numbers and types of stock awards to be granted and the terms and conditions of the stock awards, including the period of their exercisability and vesting. Subject to the limitations set forth below, the plan administrator will also determine the exercise price of options granted, the consideration (if any) to be paid for restricted stock awards and the strike price of stock appreciation rights.
The plan administrator has the authority to reprice any outstanding stock award (by reducing the exercise price of any outstanding option, canceling an option in exchange for cash or another equity award or any other action that may be deemed a repricing under generally accepted accounting provisions) under the 2011 plan without the approval of our stockholders.
Stock options. Incentive and nonstatutory stock options are granted pursuant to incentive and nonstatutory stock option agreements adopted by the plan administrator. The plan administrator determines the exercise price for a stock option, within the terms and conditions of the 2011 plan, provided that, except in the case of certain incentive stock options, as described below, the exercise price of a stock option cannot be less than 100% of the fair market value of our common stock on the date of grant. Options granted under the 2011 plan vest at the rate specified by the plan administrator.
135
Table of Contents
The plan administrator determines the term of stock options granted under the 2011 plan, up to a maximum of 10 years, except in the case of certain incentive stock options, as described below. Unless the terms of an optionholder’s stock option agreement provide otherwise, if an optionholder’s relationship with us, or any of our affiliates, ceases for any reason other than for cause, disability or death, the optionholder may exercise any vested options for a period of three months following the cessation of service. If an optionholder’s service relationship with us is terminated for cause, then the option terminates immediately. If an optionholder’s service relationship with us or any of our affiliates ceases due to disability or death, or an optionholder dies within the period (if any) specified in the award agreement following cessation of service, the optionholder or a beneficiary may exercise any vested options for a period of 12 months in the event of disability and 18 months in the event of death. The option term may be extended in the event that exercise of the option following termination of service is prohibited by applicable securities laws. In no event, however, may an option be exercised beyond the expiration of its maximum term.
Acceptable consideration for the purchase of common stock issued upon the exercise of a stock option will be determined by the plan administrator and may include (a) cash, check, bank draft or money order, (b) a program developed under Regulation T as promulgated by The Federal Reserve Board, (c) the tender of common stock previously owned by the optionholder, (d) a net exercise of the option and (e) other legal consideration approved by the plan administrator.
Unless the plan administrator provides otherwise, options generally are not transferable except by will, the laws of descent and distribution, or pursuant to a domestic relations order. An optionholder may, however, designate a beneficiary who may exercise the option following the optionholder’s death.
Limitations on incentive stock options. Incentive stock options may be granted only to our employees. The aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to incentive stock options that are exercisable for the first time by an optionholder during any calendar year under all of our stock plans may not exceed $100,000. No incentive stock option may be granted to any person who, at the time of the grant, owns or is deemed to own stock comprising more than 10% of our total combined voting power or that of any of our affiliates unless (a) the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant and (b) the term of the incentive stock option does not exceed five years from the date of grant.
Restricted stock awards. Restricted stock awards are granted pursuant to restricted stock award agreements adopted by the plan administrator. Restricted stock awards may be granted in consideration for (a) cash, check, bank draft or money order, (b) past or future services rendered to us or our affiliates, or (c) any other form of legal consideration. Shares of common stock acquired under a restricted stock award may, but need not, be subject to a share repurchase option or forfeiture restriction in our favor in accordance with a vesting schedule to be determined by the plan administrator. Rights to acquire shares under a restricted stock award may be transferred only upon such terms and conditions as set by the plan administrator. Except as otherwise provided in the applicable award agreement, restricted stock awards that have not vested will be forfeited or subject to repurchase upon the participant’s cessation of continuous service for any reason.
Restricted stock unit awards. Restricted stock unit awards are granted pursuant to restricted stock unit award agreements adopted by the plan administrator. Restricted stock unit awards may be granted in consideration for any form of legal consideration. A restricted stock unit award may be settled by cash, delivery of stock, a combination of cash and stock as deemed appropriate by the plan administrator, or in any other form of consideration set forth in the restricted stock unit award agreement. Additionally, dividend equivalents may be credited in respect of shares covered by a restricted stock unit award. Except as otherwise provided in the applicable award agreement, restricted stock units that have not vested will be forfeited upon the participant’s cessation of continuous service for any reason.
136
Table of Contents
Stock appreciation rights. Stock appreciation rights are granted pursuant to stock appreciation rights agreements adopted by the plan administrator. The plan administrator determines the strike price for a stock appreciation right which cannot be less than 100% of the fair market value of our common stock on the date of grant. Upon the exercise of a stock appreciation right, we will pay the participant an amount equal to the product of (a) the excess of the per share fair market value of our common stock on the date of exercise over the strike price, multiplied by (b) the number of shares of common stock with respect to which the stock appreciation right is exercised. A stock appreciation right granted under the 2011 plan vests at the rate specified in the stock appreciation right agreement as determined by the plan administrator.
The plan administrator determines the term of stock appreciation rights granted under the 2011 plan, up to a maximum of 10 years. If a participant’s service relationship with us, or any of our affiliates, ceases for any reason other than for cause, disability or death, then the participant, or the participant’s beneficiary, may exercise any vested stock appreciation right for three months (or such longer or shorter period specified in the stock appreciation right agreement) after the date such service relationship ends. If a participant’s service relationship with us or any of our affiliates ceases due to death or disability, or a participant dies within the period (if any) specified in the stock appreciation rights agreement following cessation of service, the participant or beneficiary may exercise any vested stock appreciation rights for a period of 12 months in the event of disability and 18 months in the event of death. In no event, however, may a stock appreciation right be exercised beyond the expiration of its term.
Performance awards. The 2011 plan permits the grant of performance stock awards and performance cash awards that may qualify as performance-based compensation that is not subject to the $1,000,000 limitation on the income tax deductibility of compensation paid per covered executive officer imposed by Section 162(m) of the Code. To assure that the compensation attributable to performance-based awards will so qualify, our committee can structure such awards so that stock will be issued or paid pursuant to such award only upon the achievement of certain pre-established performance goals during a designated performance period. The maximum benefit number of shares that may be granted to a participant in any calendar year attributable to performance stock awards may not exceed 1,000,000 shares of common stock and the maximum value that may be granted to a participant in any calendar year attributable to performance cash awards may not exceed $1,000,000.
Other stock awards. The plan administrator may grant other awards based in whole or in part by reference to our common stock. The plan administrator will set the number of shares under the award and all other terms and conditions of such awards.
Changes to capital structure. In the event that there is a specified type of change in our capital structure, such as a stock split, appropriate adjustments will be made to (a) the class and maximum number of shares reserved under the 2011 plan, (b) the maximum number of shares by which the share reserve may increase automatically each year, (c) the class and maximum number of shares subject to options, stock appreciation rights and performance stock awards and performance cash awards that can be granted in a calendar year, (d) the class and maximum number of shares that may be issued upon exercise of incentive stock options and (e) the number of shares and exercise price or strike price, if applicable, of all outstanding stock awards.
Corporate transactions. In the event of certain significant corporate transactions, our board of directors has the discretion to:
| • | arrange for the assumption, continuation, or substitution of a stock award by a surviving or acquiring entity or parent company; |
| • | arrange for the assignment of any reacquisition or repurchase rights held by us in respect of shares issued pursuant to a stock award to the surviving or acquiring entity; |
| • | accelerate the vesting of a stock award and provide for its termination prior to the effective time |
137
Table of Contents
| of the corporate transaction; |
| • | arrange for the lapse of any reacquisition or repurchase rights held by us with respect to the stock award; |
| • | make a payment equal to the excess of (a) the value of the property that the participant would have received upon the exercise of the stock award over (b) any exercise price otherwise payable in connection with the stock award; or |
| • | cancel or arrange for the cancellation of the stock award, to the exact non-vested or exercised prior to the effective time of the corporate transaction in exchange for such cash consideration, if any, determined by our board of directors. |
Changes in control. Our board of directors has the discretion to provide for additional acceleration of vesting and exercisability of a stock award upon or after a change in control in a participant’s award agreement. For example, the board may provide that a stock award will immediately vest as to all or any portion of the shares subject to the stock award in the event a participant’s service with us or a successor entity is terminated actually or constructively within a designated period following the occurrence of certain specified change in control transactions. Stock awards held by participants under the 2011 plan will not vest automatically on such an accelerated basis unless specifically provided in the participant’s applicable award agreement. For purposes of the 2011 plan, a change in control is the consummation of one or more of the following events:
| • | a transaction in which one person or a group acquires stock that, combined with stock previously owned, represents more than 50% of our voting power; |
| • | a merger, consolidation or similar transaction involving us (directly or indirectly) in which our stockholders immediately before the transaction do not own at least 50% of the outstanding securities or voting power of the surviving entity following such transaction in each case in substantially the same proportions as their outstanding voting securities immediately prior to the transaction; |
| • | the approval by our stockholders or our board of directors of our complete liquidation or dissolution; |
| • | a sale, lease, exclusive license or other disposition of all or substantially all of our consolidated assets, other than to an entity in which more than 50% of the voting power is owned by our stockholders in substantially the same proportions as their ownership of our voting securities immediately prior to such transaction; or |
| • | a majority of our board of directors is replaced by persons whose appointment or election is not endorsed by a majority of our board of directors. |
Dissolution or Liquidation. In the event of our dissolution or liquidation, except as otherwise provided in the award agreement, all outstanding stock awards under the 2011 plan other than vested and outstanding shares subject to stock awards will terminate immediately prior to the completion of such dissolution or liquidation and shares of common stock subject to our repurchase rights or to a forfeiture condition may be repurchased or reacquired by us. Our board of directors may, however, in its sole discretion, cause some or all such stock awards to become fully vested, exercisable and/or no longer subject to repurchase or forfeiture before the dissolution or liquidation is completed, but contingent upon its completion.
Plan Suspension, Termination. Our board of directors has the authority to suspend or terminate the 2011 plan at any time provided that such action does not impair the existing rights of any participant.
Securities laws and federal income taxes. The 2011 plan is designed to comply with various securities and federal tax laws as follows:
Securities laws. The 2011 plan is intended to conform to all applicable provisions of the Securities Act and Exchange Act and any and all regulations and rules promulgated by the SEC
138
Table of Contents
thereunder, including, without limitation, Rule 16b-3. The 2011 plan will be administered, and options will be granted and may be exercised, only in such a manner as to conform to such laws, rules and regulations.
Section 409A of the Code. Certain awards under the 2011 plan may be considered “nonqualified deferred compensation” for purposes of Section 409A of the Code, which imposes certain additional requirements regarding the payment of deferred compensation. Generally, if at any time during a taxable year a nonqualified deferred compensation plan fails to meet the requirements of Section 409A of the Code, or is not operated in accordance with those requirements, all amounts deferred under the 2011 plan and all other equity incentive plans for the taxable year and all preceding taxable years, by any participant with respect to whom the failure relates, are includable in gross income for the taxable year to the extent not subject to a substantial risk of forfeiture and not previously included in gross income. If a deferred amount is required to be included in income under Section 409A of the Code, the amount also is subject to interest and an additional income tax. The interest imposed is equal to the interest at the underpayment rate plus one percentage point, imposed on the underpayments that would have occurred had the compensation been includable in income for the taxable year when first deferred, or if later, when not subject to a substantial risk of forfeiture. The additional federal income tax is equal to 20% of the compensation required to be included in gross income. In addition, certain states, including California, have laws similar to Section 409A of the Code, which impose additional state penalty taxes on such compensation.
Section 162(m) of the Code. In general, under Section 162(m) of the Code, income tax deductions of publicly held corporations may be limited to the extent total compensation (including, but not limited to, base salary, annual bonus, and income attributable to stock option exercises and other non-qualified benefits) for certain executive officers exceeds $1,000,000 (less the amount of any “excess parachute payments” as defined in Section 280G of the Code) in any taxable year of the corporation. However, under Section 162(m) of the Code, the deduction limit does not apply to certain “performance-based compensation” established by an independent compensation committee that is adequately disclosed to, and approved by, stockholders. In particular, stock options and stock appreciation rights will satisfy the “performance-based compensation” exception if the awards are made by a qualifying compensation committee, the 2011 plan sets the maximum number of shares that can be granted to any person within a specified period and the compensation is based solely on an increase in the stock price after the grant date. Specifically, the option exercise price must be equal to or greater than the fair market value of the stock subject to the award on the grant date.
We have attempted to structure the 2011 plan in such a manner that the compensation attributable to stock options, stock appreciation rights and other performance-based awards which meet the other requirements of Section 162(m) will not be subject to the $1,000,000 limitation. We have not, however, requested a ruling from the IRS or an opinion of counsel regarding this issue.
2011 Non-Employee Directors’ Stock Award Plan
Our board of directors adopted and our stockholders approved the directors’ plan in August 2011. The directors’ plan will become effective immediately upon the execution and delivery of the underwriting agreement for this offering. The directors’ plan will terminate at the discretion of our board of directors. The purpose of the directors’ plan is to retain the services of non-employee directors, secure and retain the services of new non-employee directors and provide incentives for such persons to exert maximum efforts towards our success by giving them an opportunity to benefit from increases in value of our common stock. The directors’ plan provides for the automatic grant of nonstatutory stock options to purchase shares of our common stock to our non-employee directors. The directors’ plan also provides for the discretionary grant of nonstatutory stock options, stock appreciation rights, restricted stock awards, restricted stock units, and other stock awards.
139
Table of Contents
Share Reserve. An aggregate of 350,000 shares of our common stock are reserved for issuance under the directors’ plan. This amount will be increased annually on January 1, from 2012 until 2021, by the lesser of 150,000 shares or the aggregate number of shares of our common stock subject to awards granted under the directors’ plan during the immediately preceding fiscal year. However, our board of directors will have the authority to designate a lesser number of shares by which the share reserve will be increased.
Shares of our common stock subject to stock awards that have expired or otherwise terminated under the directors’ plan without having been exercised in full shall again become available for grant under the directors’ plan. Shares of our common stock issued under the directors’ plan may be previously unissued shares or reacquired shares bought on the market or otherwise. Any shares that are (a) forfeited to or repurchased by us prior to becoming fully vested, (b) withheld, required or not issued to satisfy withholding obligations, or (c) used to pay the exercise of a stock award shall again become available for the grant of awards under the directors’ plan.
Administration. Our board of directors has delegated its authority to administer the directors’ plan to our compensation committee. The compensation committee must consist of two or more “non-employee directors” pursuant to the Rule 16b-3 of the Exchange Act.
Stock Options. Stock options will be granted pursuant to stock option agreements. The exercise price of the options granted under the directors’ plan will be equal to 100% of the fair market value of our common stock on the date of grant. Initial grants vest in equal monthly installments over 12 months after the date of grant and annual grants vest in equal monthly installments over 12 months after the date of grant.
In general, the term of stock options granted under the directors’ plan may not exceed ten years. Unless the terms of an optionholder’s stock option agreement provide otherwise, if an optionholder’s service relationship with us, or any affiliate of ours, ceases due to death or disability, the optionholder or his or her beneficiary may then exercise any vested options for a period of 12 months in the event of disability, or 18 months in the event of death. If an optionholder’s service with us or any affiliate ceases for any other reason, the optionholder may exercise the vested options for up to three months following cessation of service. Additionally, stock option agreements generally provide that the optionholder may exercise the vested options for up to 12 months after the termination of the optionholder’s service relationship at any time within the 12 months following a change in control.
Acceptable consideration for the purchase of our common stock issued under options pursuant to the directors’ plan may include cash, check, bank draft or money order, by “net” exercise, by delivery of common stock previously owned by the optionholder or pursuant to a program developed under Regulation T as promulgated by the Federal Reserve Board.
Generally, an optionholder may not transfer a stock option other than by will or the laws of descent and distribution. However, an optionholder may transfer an option under certain circumstances with our written consent if a Form S-8 registration statement is available for the exercise of the option and the subsequent resale of the shares. In addition, an optionholder may designate a beneficiary who may exercise the option following the optionholder’s death.
Non-discretionary Grants
| • | Initial Grant. Any person who becomes a non-employee director for the first time after the closing of this offering will automatically receive an initial grant of an option to purchase shares of our common stock upon his or her election or appointment, subject to adjustment by our board of directors from time to time. These options will vest in equal monthly installments over 12 months. These initial grants may also be issued in the form of other types of stock awards if so determined by our board of directors. |
| • | Annual Grant. In addition, any person who is a non-employee director on the date of each annual meeting of our stockholders automatically will be granted, on the annual meeting date, |
140
Table of Contents
| beginning with our first annual meeting following the closing this offering annual meeting, an option to purchase 2,000 shares of our common stock, or the annual grant, subject to adjustment by our board of directors from time to time. These options will vest in equal monthly installments over 12 months. These annual grants may also be issued in the form of other types of stock awards if so determined by our board of directors. |
Discretionary Grants In addition to the non-discretionary grants noted above, our board of directors may grant stock awards to one or more non-employee directors in such numbers and subject to such other provisions as it shall determine. These awards may be in the form of stock options, stock appreciation rights, restricted stock units, restricted stock or other stock awards and shall vest pursuant to vesting schedules to be determined by our board of directors in its sole discretion.
Changes to Capital Structure. In the event there is a specified type of change in our capital structure not involving the receipt of consideration by us, such as a stock split or stock dividend, the class and number of shares reserved under the directors’ plan, the maximum number of shares by which the share reserve may increase automatically each year, the class and number of shares subject to the initial and annual grants and the class and number of shares and exercise price of all outstanding stock awards will be appropriately adjusted.
Corporate transactions. In the event of certain significant corporate transactions, our board of directors has the discretion to:
| • | arrange for the assumption, continuation, or substitution of a stock award by a surviving or acquiring entity or parent company; |
| • | arrange for the assignment of any reacquisition or repurchase rights held by us in respect of shares issued pursuant to a stock award to the surviving or acquiring entity; |
| • | accelerate the vesting of a stock award and provide for its termination prior to the effective time of the corporate transaction; |
| • | arrange for the lapse of any reacquisition or repurchase rights held by us with respect to the stock award; |
| • | make a payment equal to the excess of (a) the value of the property that the participant would have received upon the exercise of the stock award over (b) any exercise price otherwise payable in connection with the stock award; or |
| • | cancel or arrange for the cancellation of the stock award, to the exact non-vested or exercised prior to the effective time of the corporate transaction in exchange for such cash consideration, if any, determined by our board of directors. |
Change in Control Transactions. In the event of a change in control transaction, the vesting of options held by non-employee directors whose service has not terminated may be accelerated in full according to the provisions of the award agreement. Additionally, if a non-employee director is terminated within the 12 months following a change in control, the non-employee director may exercise vested options for up to 12 months following termination according to the provisions of the award agreement. A change in control is the occurrence of one or more of the following events:
| • | a transaction in which one person or a group acquires stock that, combined with stock previously owned, controls more than 50% of our value or voting power; |
| • | a merger, consolidation or similar transaction involving us (directly or indirectly) in which our stockholders immediately before the transaction do not own at least 50% of the outstanding securities following such transaction; |
| • | our complete liquidation or dissolution; |
141
Table of Contents
| • | a sale, lease, exclusive license or other disposition of all or substantially all of our assets, other than to an entity in which more than 50% of the voting power is owned by our stockholders in substantially the same proportions as their ownership of our voting securities immediately prior to such transaction; or |
| • | a majority of our board of directors is replaced by persons whose appointment or election is not endorsed by a majority of our board of directors. |
Plan Amendments. Our board of directors will have the authority to amend or terminate the directors’ plan. However, no amendment or termination of the directors’ plan will adversely affect any rights under awards already granted to a participant unless agreed to by the affected participant. We will obtain stockholder approval of any amendment to the directors’ plan that is required by applicable law.
2011 Employee Stock Purchase Plan
Our board of directors adopted and our stockholders approved our 2011 employee stock purchase plan, or the 2011 purchase plan, in August 2011. The purpose of the 2011 purchase plan is to assist us in retaining the services of new employees and securing the services of new and existing employees while providing incentives for such individuals to exert maximum efforts toward our success.
Share reserve. Upon the closing of this offering, the 2011 purchase plan authorizes the issuance of 650,000 shares of our common stock pursuant to purchase rights granted to our employees or to employees of our designated affiliates. The number of shares of our common stock reserved for issuance will automatically increase on January 1 of each calendar year, from January 1, 2012 through January 1, 2021, by the least of (a) 1.5% of the total number of shares of our common stock outstanding on December 31st of the preceding calendar year, (b) 200,000 shares, or (c) a number determined by our board of directors that is less than (a) or (b). The 2011 purchase plan is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Code. As of the date hereof, no shares of our common stock have been purchased under the 2011 purchase plan.
Administration. Our board of directors has delegated its authority to administer the 2011 purchase plan to our compensation committee. The 2011 purchase plan is implemented through a series of offerings of purchase rights to eligible employees. Under the 2011 purchase plan, we may specify offerings with durations of not more than 27 months, and may specify shorter purchase periods within each offering. Each offering will have one or more purchase dates on which shares of our common stock will be purchased for employees participating in the offering. An offering may be terminated under certain circumstances.
Payroll deductions. Generally, all regular employees, including executive officers, employed by us or by any of our designated affiliates, may participate in the 2011 purchase plan and may contribute, normally through payroll deductions, up to 20% of their earnings for the purchase of our common stock under the 2011 purchase plan. Unless otherwise determined by our board of directors, common stock will be purchased for accounts of employees participating in the 2011 purchase plan at a price per share equal to the lower of (a) 85% of the fair market value of a share of our common stock on the first date of an offering or (b) 85% of the fair market value of a share of our common stock on the date of purchase.
Limitations. Employees may have to satisfy one or more of the following service requirements before participating in the 2011 purchase plan, as determined by our board of directors: (a) customarily employed for more than 20 hours per week, (b) customarily employed for more than five months per calendar year or (c) continuous employment with us or one of our affiliates for a period of time not to exceed two years. No employee may purchase shares under the 2011 purchase plan at a rate in excess of $25,000 worth of our common stock based on the fair market value per share of our common stock at the date such rights are granted in an offering for each year such a purchase right is outstanding.
142
Table of Contents
Finally, no employee will be eligible for the grant of any purchase rights under the 2011 purchase plan if immediately after such rights are granted, such employee has voting power over 5% or more of our outstanding capital stock measured by vote or value pursuant to Section 424(d) of the Code.
Changes to capital structure. In the event that a change in our capital structure occurs as a result of actions such as a stock split, merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or similar transaction, the board of directors will make appropriate adjustments to (a) the class and number of shares reserved for issuance under the 2011 purchase plan, (b) the class and maximum number of shares by which the share reserve may increase automatically each year, (c) the class and number of shares and purchase price of all outstanding purchase rights and (d) the class and number of shares subject to any purchase limits under each ongoing offering.
Corporate transactions. In the event of the consummation of certain significant corporate transactions, including a sale of all our assets, the sale or disposition of 90% of our outstanding securities, or a merger consolidation or similar transaction where we are not the surviving corporation in the transaction or we are the surviving corporation but our common stock is converted or exchanged into other property by virtue of the transaction, any then-outstanding rights to purchase our stock under the 2011 purchase plan may be assumed, continued or substituted for by any surviving or acquiring entity (or its parent company). If the surviving or acquiring entity (or its parent company) elects not to assume, continue or substitute for such purchase rights, then the participants’ accumulated payroll contributions will be used to purchase shares of our common stock within 10 business days prior to such corporate transaction, and such purchase rights will terminate immediately thereafter.
Plan Amendment, Termination. Our board of directors has the authority to amend, suspend or terminate the 2011 purchase plan at any time. However, any such amendment, suspension or termination generally may not impair any benefits, privileges, entitlements or obligations under outstanding purchase rights without the consent of employees granted such purchase rights. We will obtain stockholder approval of any amendment to the 2011 purchase plan as required by applicable law.
401(k) Plan
We maintain a defined contribution employee retirement plan, or 401(k) plan, for our employees. Our executive officers are also eligible to participate in the 401(k) plan on the same basis as our other employees. The 401(k) plan is intended to qualify as a tax-qualified plan under Section 401(k) of the Code. The plan provides that each participant may contribute up to the lesser of 100% of his or her pre-tax compensation or the statutory limit, which is $16,500 for calendar year 2011. Participants that are 50 years or older can also make “catch-up” contributions, which in calendar year 2011 may be up to an additional $5,500 above the statutory limit. The 401(k) plan provides for us to make qualified non-elective contributions on behalf of all eligible participants. Employees are eligible to receive the non-elective contribution if they were eligible to participate in the 401(k) plan at any time during the year. The contribution is 3% of an employee’s eligible compensation. Under the 401(k) plan, each participant is fully vested in his or her deferred salary contributions when contributed. Participant contributions are held and invested, pursuant to the participant’s instructions, by the plan’s trustee.
Compensation Committee Interlocks and Insider Participation
We have established a compensation committee which has and will make decisions relating to compensation of our executive officers. Our board of directors appointed Patrick P. Fourteau, Pierre Lapalme and Brian Tambi to serve on the compensation committee. None of these individuals has ever been an executive officer or employee of ours. None of our executive officers currently serves, or has served during the last completed fiscal year, on the compensation committee or board of directors of any other entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
143
Table of Contents
Limitation of Liability and Indemnification
Our amended and restated certificate of incorporation, which will become effective upon the closing of this offering, limits the liability of directors to the maximum extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
| • | breach of their duty of loyalty to the corporation or its stockholders; |
| • | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| • | transaction from which the directors derived an improper personal benefit. |
Our amended and restated certificate of incorporation, which will become effective upon the closing of this offering, does not eliminate a director’s duty of care and, in appropriate circumstances, equitable remedies, such as injunctive or other forms of non-monetary relief, which remain available under Delaware law. These limitations also do not affect a director’s responsibilities under any other laws, such as the federal securities laws or other state or federal laws. Our amended and restated bylaws, which will become effective upon the closing of this offering, provide that we will indemnify our directors and executive officers, and may indemnify other officers, employees and other agents, to the fullest extent permitted by law. Our amended and restated bylaws, which will become effective upon the closing of this offering, also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding and also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in connection with their services to us, regardless of whether our amended and restated bylaws permit such indemnification. We have obtained a policy of directors’ and officers’ liability insurance.
We have entered, and intend to continue to enter, into separate indemnification agreements with our directors and executive officers, in addition to the indemnification provided for in our amended and restated bylaws. These agreements, among other things, require us to indemnify our directors and executive officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or executive officers, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Except as otherwise disclosed under the heading “Business-Legal Proceedings” in the Business section of this prospectus, at present, there is no pending litigation or proceeding involving any of our directors or executive officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
144
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following includes a summary of transactions since January 1, 2008 to which we have been a party, in which the amount involved in the transaction exceeded $120,000, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under “Compensation Discussion and Analysis.”
The Merger
We acquired our wholly-owned subsidiary, Insys Pharma, Inc., in the Merger on November 8, 2010. As of immediately prior to the Merger, our Executive Chairman, Dr. John N. Kapoor, was the Chairman of our board of directors and beneficial holder of more than 5% of our common stock. Dr. Kapoor was also a director and majority stockholder of Insys Pharma at the time of the Merger. In connection with the Merger, each share of Insys Pharma common stock was exchanged for (i) 0.134 shares of our common stock and (ii) 0.102 shares of our convertible preferred stock. Each share of convertible preferred stock issued pursuant to the Merger is convertible into 0.57 shares of our common stock. The following table shows the number of shares of our common stock acquired as a result of the Merger by our directors, executive officers or beneficial owners of more than 5% of our capital stock:
| Insys Pharma Stockholder |
Relationship to our Company at Time of the Merger |
Number of Insys Pharma Shares Held |
Shares of our Common Stock Issued upon the Merger |
Shares of our Convertible Preferred Stock Issued upon the Merger |
Shares of our Common Stock Issuable Upon Conversion of our Convertible Preferred Stock |
Total Common Stock on an As- Converted Basis |
||||||||||||||||||
| The John N. Kapoor Trust dated 9/20/89 |
(a | ) | 1,903,316 | 254,774 | 11,846,877 | 6,797,388 | 7,052,162 | |||||||||||||||||
| John N. Kapoor 1999 Descendants Trust |
(a | ) | 91,604 | 12,261 | 570,176 | 327,150 | 339,411 | |||||||||||||||||
| Kapoor Children’s 1992 Trust |
(a | ) | 305,348 | 40,873 | 1,900,588 | 1,090,501 | 1,131,374 | |||||||||||||||||
| Robert Kapoor |
(b | ) | 2,241 | 300 | 13,953 | 8,005 | 8,305 | |||||||||||||||||
| Vijay Kapoor |
(b | ) | 2,241 | 300 | 13,953 | 8,005 | 8,305 | |||||||||||||||||
| Michael L. Babich |
(c | ) | 3,015 | 408 | 18,996 | 10,899 | 11,307 | |||||||||||||||||
| (a) | Controlled by Dr. Kapoor, a director and greater than 5% stockholder. |
| (b) | Family member of Dr. Kapoor, a director and greater than 5% stockholder. |
| (c) | Mr. Babich is our current President and Chief Executive Officer and a director, and was the Chief Operating Officer and a director of Insys Pharma immediately prior to the Merger. |
Prior to the Merger, our compensation committee approved an amendment to our 2006 Equity Incentive Plan. The amendment provided that each stock option to purchase shares of our common stock held by the members of our board of directors at the time, comprised of Frank Becker, Dr. Bernard Fox, Paul Freiman, Dr. John N. Kapoor and Dr. Aquilur Rahman, would remain outstanding through the term of the option notwithstanding termination of the director’s relationship with our company as a director, employee or consultant.
In connection with the Merger, all outstanding unvested stock options to purchase shares of our common stock as of immediately prior to the Merger, including those held by Dr. Kapoor, a current director, and Martin McCarthy, our current Chief Financial Officer, became vested and immediately exercisable.
We issued to each of our stockholders of record as of immediately prior to the effective time of the Merger a contingent payment right, or CPR, for each share of our common stock then-held. Each CPR entitles the holder to receive a pro rata share of an aggregate of $20.0 million, payable in cash, if, within five years of the effective date of the Merger, one of the NeoPharm product candidates in development
145
Table of Contents
prior to the Merger receives FDA approval. Of these product candidates, we are actively developing LEP-ETU, but we cannot predict when, if ever, this product candidate will receive FDA approval. Based on his ownership of our common stock at the time of the Merger, Dr. Kapoor, who was a director and 5% stockholder of NeoPharm and Insys Pharma, received, along with certain family members and entities controlled by him, the majority of the CPRs issued in connection with the Merger.
We also assumed the outstanding Insys Pharma stock options in connection with the Merger. Please see “Stock Options Granted to Executive Officers and Directors” below.
Sale of Securities
In connection with the promissory note dated February 15, 2008, discussed below, Insys Pharma, pursuant to a conversion agreement, issued a warrant to The JNK Trust to purchase up to an aggregate of 18,917 shares of Insys Pharma common stock (reflecting the retroactive effect of a one-for-1,500,000 reverse stock split effective on June 5, 2009, a 1,862,623-for-one stock split effective on February 22, 2010 and the Merger). This warrant expired in February 2011.
In July 2008, Insys Pharma issued 38,112 shares of its non-voting common stock (reflecting the retroactive effect of a one-for-1,500,000 reverse stock split effective on June 5, 2009, a 1,862,623-for-one stock split effective on February 22, 2010 and the Merger) to The JNK Trust in exchange for cancellation of a series of promissory notes with an aggregate principal amount of $23.0 million and accrued interest of $1.2 million. As additional compensation to The JNK Trust for cancellation of these notes, Insys Pharma sold shares of its voting common stock to various affiliates of Dr. Kapoor, as set forth in the following table (which such share amounts reflect a one-for-1,500,000 reverse stock split effective on June 5, 2009, and a 1,862,623-for-one stock split effective on February 22, 2010 and the Merger):
| Name |
Shares of Insys Pharma Common Stock(1) |
Purchase Price | ||||||
| Rani Aneja 1992 Trust |
156 | $ | 99,383 | |||||
| Jagdish Lal Mehra 1992 Trust |
156 | $ | 99,383 | |||||
| Gopal Mehra 1992 Trust |
156 | $ | 99,383 | |||||
| Banarsi Das Mehra 1992 Trust |
156 | $ | 99,383 | |||||
| Kamalavati Mehra 1992 Trust |
156 | $ | 99,383 | |||||
| Hillock Family 1992 Trust |
1,095 | $ | 695,681 | |||||
| John K. Kapoor 1999 Descendants Trust |
13,559 | $ | 8,608,636 | |||||
| Vijay Kapoor |
40 | $ | 25,767 | |||||
| Bob Kapoor 1992 Trust |
272 | $ | 173,000 | |||||
| (1) | See Note 10, “NeoPharm Merger” to the Insys Therapeutics consolidated financial statements located elsewhere in this prospectus. |
146
Table of Contents
Loan Transactions
Since January 1, 2008, we and Insys Pharma have entered into various loan arrangements with entities controlled by Dr. Kapoor, our founder, Executive Chairman and principal stockholder, pursuant to which we or Insys Pharma have issued secured promissory notes and secured demand notes. Below is a summary of certain information relating to such notes for each of the six months ended June 30, 2011 and the years ended December 31, 2010, 2009 and 2008:
Loan transactions with entities affiliated with Dr. John N. Kapoor (in thousands)
| Six
Months Ended June 30, 2011 |
Years
Ended December 31, |
|||||||||||||||
| 2010 | 2009 | 2008 | ||||||||||||||
| Principal amount of promissory and demand notes issued |
$ | 7,919 | $ | 15,145 | $ | 11,498 | $ | 9,514 | ||||||||
| Largest aggregate principal amount outstanding |
37,607 | 29,687 | 25,814 | 40,421 | ||||||||||||
| Interest expense accrued on notes payable |
888 | 1,150 | 1,037 | 1,931 | ||||||||||||
| Principal and interest repaid |
— | — | — | 3,141 | ||||||||||||
| Principal and interest converted to equity |
— | — | 11,549 | 24,197 | ||||||||||||
In addition, during the third quarter of 2011, we issued an additional note of $1.0 million to The John N. Kapoor Trust. The foregoing note carries interest at the prime rate plus 2% per annum and is currently payable on demand. All of the notes are secured against the assets of the issuing entity. As of June 30, 2011, we and Insys Pharma had $43.7 million in outstanding indebtedness, including accrued interest, pursuant to these notes and other notes issued by us or Insys Pharma in prior years to trusts controlled by Dr. Kapoor. Upon the closing of this offering, these notes, including accrued interest, will convert into shares of our common stock at the price to the public of the shares sold in this offering.
Employment Arrangements
We currently maintain written employment agreements with our President and Chief Executive Officer, Michael Babich, and our Chief Medical Officer, Dr. Larry Dillaha. For more information, refer to “Employment Agreements with Executive Officers” under “Compensation Discussion and Analysis.”
147
Table of Contents
Stock Options Granted to Executive Officers and Directors
We have granted stock options under our 2006 Equity Incentive Plan to our executive officers and directors. The table below summarizes the stock option grants made to such persons since January 1, 2008.
| Name |
Grant Date |
Shares of Our
Common Stock Subject to Option Grants |
Exercise Price Per Share |
|||||||
| Frank Becker Former Director |
August 22, 2008 | 819 | $ | 25.62 | ||||||
| July 24, 2009 | 819 | $ | 14.03 | |||||||
| August 2010 | 1,024 | $ | 17.69 | |||||||
| Bernard Fox, M.D. Former Director |
August, 2008 | 819 | $ | 25.62 | ||||||
| July 2009 | 819 | $ | 14.03 | |||||||
| August 2010 | 1,024 | $ | 17.69 | |||||||
| Paul Freiman Former Director |
August 2008 | 819 | $ | 25.62 | ||||||
| July 2009 | 819 | $ | 14.03 | |||||||
| August 2010 | 1,024 | $ | 17.69 | |||||||
| John N. Kapoor, Ph.D. Executive Chairman |
August 2008 | 1,229 | $ | 25.62 | ||||||
| July 2009 | 1,229 | $ | 14.03 | |||||||
| August 2010 | 1,536 | $ | 17.69 | |||||||
| Aquilur Rahman, Ph.D. Former Director and Former President and Chief Executive Officer |
February 2009 | 2,459 | $ | 4.88 | ||||||
| February 2010 | 2,459 | $ | 18.91 | |||||||
| Martin McCarthy Chief Financial Officer |
February 2009 | 491 | $ | 4.88 | ||||||
| February 2010 | 491 | $ | 18.91 | |||||||
| March 2011 | 40,491 | $ | 4.88 | |||||||
| Michael L. Babich Director / President and Chief Executive Officer |
March 2011 | 98,480 | $ | 4.88 | ||||||
| Richard Mallery Former Director |
March 2011 | 24,590 | $ | 4.88 | ||||||
| Pierre Lapalme Director |
March 2011 | 24,590 | $ | 4.88 | ||||||
| Patrick P. Fourteau Director |
March 2011 | 24,590 | $ | 4.88 | ||||||
| Steven Meyer Director |
March 2011 | 22,950 | $ | 4.88 | ||||||
| Brian Tambi Director |
March 2011 | 22,950 | $ | 4.88 | ||||||
| Larry Dillaha, M.D. Chief Medical Officer |
March 2011 | 51,807 | $ | 4.88 | ||||||
148
Table of Contents
In connection with the Merger, stock options to purchase shares of Insys Pharma common stock outstanding as of immediately prior to the Merger were converted into options, or Converted Options, to purchase shares of our common stock. The Merger did not affect the vesting schedule of the Converted Options. Each Converted Option was converted to an option to purchase a number of shares of our common stock approximately equal to the product of the number of shares of Insys Pharma common stock subject to the option multiplied by 3.717, at an exercise price per share of our common stock approximately equal to the quotient of the exercise price per share of Insys Pharma common stock subject to the option divided by 3.717. The table below shows the number of shares of our common stock purchasable by each of our executive officers and directors who received Converted Options in the Merger, with the corresponding exercise prices.
| Name |
Shares of Insys Pharma Common Stock Subject to Options |
Exercise Price Per Share |
Shares of our Common Stock Subject to Converted Options |
Adjusted Exercise Price Per Share |
||||||||||||
| Michael L. Babich Director / President and Chief Executive Officer |
89,538 | $ | 6.10 | 341,041 | $ | 1.83 | ||||||||||
| Dr. Larry Dillaha Chief Medical Officer |
30,163 | $ | 6.10 | 112,126 | $ |
1.83 |
| |||||||||
| Brian Tambi Director |
3,918 | $ | 6.10 | 17,885 | $ |
1.83 |
| |||||||||
| Steven Meyer Director |
3,926 | $ | 6.10 | 17,885 | $ | 1.83 | ||||||||||
For further information regarding stock option grants to our executive officers and directors, please see the section entitled “Compensation Discussion and Analysis.”
Indemnification Agreements
We have entered, and intend to continue to enter, into separate indemnification agreements with our directors and executive officers, in addition to the indemnification provided for in our amended and restated bylaws. These agreements, among other things, require us to indemnify our directors and executive officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or executive officers, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Related Party Director
One of our former directors, Richard Mallery, who was appointed by our board of directors in March 2011, is a partner at Snell & Wilmer LLP, one of the outside law firms we engage for legal services. We plan to engage Snell & Wilmer in 2011; however, we anticipate that this engagement will result in substantially less than 5% of Snell & Wilmer’s gross revenues for its 2011 fiscal year.
149
Table of Contents
Policies and Procedures for Transactions with Related Persons
We have adopted a written related-person transactions policy that sets forth our policies and procedures regarding the identification, review, consideration and oversight of “related-person transactions.” For purposes of our policy only, a “related-person transaction” is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any “related person” are participants involving an amount that exceeds $120,000.
Transactions involving compensation for services provided to us as an employee, consultant or director are not considered related-person transactions under this policy. A related person is any executive officer, director or a holder of more than 5% of our common stock, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, where a transaction has been identified as a related-person transaction, management must present information regarding the proposed related-person transaction to our audit committee (or, where review by our audit committee would be inappropriate, to another independent body of our board of directors) for review. The presentation must include a description of, among other things, the material facts, the direct and indirect interests of the related persons, the benefits of the transaction to us and whether any alternative transactions are available. To identify related-person transactions in advance, we rely on information supplied by our executive officers, directors and certain significant stockholders. In considering related-person transactions, our audit committee or other independent body of our board of directors takes into account the relevant available facts and circumstances including, but not limited to:
| • | the risks, costs and benefits to us; |
| • | the impact on a director’s independence in the event the related person is a director, immediate family member of a director or an entity with which a director is affiliated; |
| • | the terms of the transaction; |
| • | the availability of other sources for comparable services or products; and |
| • | the terms available to or from, as the case may be, unrelated third parties or to or from our employees generally. |
In the event a director has an interest in the proposed transaction, the director must recuse himself or herself from the deliberations and approval.
150
Table of Contents
The following table sets forth information regarding beneficial ownership of our common stock outstanding as of June 15, 2011 by:
| • | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock; |
| • | each of our directors; |
| • | our named executive officer; and |
| • | all of our directors and executive officers as a group. |
The percentage ownership information shown in the table is based upon 9,312,880 shares of common stock outstanding as of June 15, 2011, which assumes the conversion of all of our outstanding convertible preferred stock into 8,528,860 shares of common stock, which will occur immediately prior to the closing of this offering. The percentage ownership information assumes no exercise of the underwriters’ over-allotment option to purchase additional shares.
Information with respect to beneficial ownership has been furnished by each director, officer or beneficial owner of more than 5% of our common stock. We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, these rules require inclusion of shares of common stock issuable pursuant to the exercise of stock options or warrants that are either immediately exercisable or exercisable on or before August 14, 2011, which is 60 days after June 15, 2011. These shares are deemed to be outstanding and beneficially owned by the person holding those options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
151
Table of Contents
Except as otherwise noted below, the address for each person or entity listed in the table is c/o Insys Therapeutics, Inc., 10220 South 51st Street, Suite 2, Phoenix, Arizona 85044.
| Number of Shares Beneficially Owned |
Percentage of Shares Beneficially Owned |
|||||||||||
| Name and Address of Beneficial Owner |
Before Offering |
After Offering |
||||||||||
| 5% or Greater Stockholders |
||||||||||||
| The John N. Kapoor Trust dated September 20, 1989 |
7,087,162 | 76.1 | % | % | ||||||||
| 1925 W. Field Ct., Ste. 300 |
||||||||||||
| Lake Forest, IL 60045 |
||||||||||||
| The Kapoor Children’s 1992 Trust |
1,131,374 | 12.1 | % | % | ||||||||
| 1925 W. Field Ct., Ste. 300 |
||||||||||||
| Lake Forest, IL 60045 |
||||||||||||
| Named Executive Officer and Directors |
||||||||||||
| John N. Kapoor, Ph.D.(1) |
8,625,353 | 92.6 | % | % | ||||||||
| Michael L. Babich(2) |
352,348 | 3.6 | % | % | ||||||||
| Larry Dillaha, M.D.(3) |
88,411 | * | ||||||||||
| Steven Meyer(4) |
20,436 | * | ||||||||||
| Brian Tambi(5) |
20,436 | * | ||||||||||
| Aquilar Rahman, Ph.D.(6) |
8,196 | * | ||||||||||
| Martin McCarthy(8) |
4,879 | * | ||||||||||
| Shahid Ali, Ph.D.(7) |
3,836 | * | ||||||||||
| Patrick P. Fourteau |
2,049 | * | ||||||||||
| Pierre Lapalme |
2,049 | * | ||||||||||
| All current executive officers and directors as a group (eight persons)(9) |
9,119,797 | 93.1 | % | % | ||||||||
| * | Represents beneficial ownership of less than 1%. |
| (1) | Includes 1,262 shares held by Dr. Kapoor in his individual capacity; 3,994 shares that Dr. Kapoor has the right to acquire from us within 60 days of June 15, 2011 pursuant to the exercise of stock options; 7,087,166 shares held by The John N. Kapoor Trust, dated September 20, 1989, of which Dr. Kapoor is the sole trustee and sole beneficiary; and 18,763 shares held by EJ Financial/NEO Management, L.P., of which Dr. Kapoor is Managing General Partner. Also includes 1,131,374 shares held by The Kapoor Children’s 1992 Trust, or the Children’s 1992 Trust, for which Dr. Kapoor is the grantor; 339,411 shares held by The John N. Kapoor 1999 Descendants Trust, or the Descendants Trust, for which Dr. Kapoor is the grantor; 6,221 shares held by The John and Editha Kapoor Charitable Foundation, or the Charitable Foundation, of which Dr. Kapoor is a joint trustee; 30,722 shares held by The John N. Kapoor 1994-A Annuity Trust, or the Annuity Trust, of which the sole trustee is Dr. Rao Akella, who is an employee of EJ Financial Enterprises, Inc., of which Dr. Kapoor is the sole stockholder and President; 6,444 shares held by four trusts which have been established for Dr. Kapoor’s children, or the Children’s Trusts, of which the sole trustee is Dr. Akella. Dr. Kapoor does not have or share voting, investment or dispositive power with respect to the shares owned by the Annuity Trust or the Children’s Trusts and Dr. Kapoor disclaims beneficial ownership of these shares as well as the shares held by the Children’s 1992 Trust, the Descendants Trust and the Charitable Foundation. |
| (2) | Includes 11,293 shares held by Mr. Babich and 341,041 shares that Mr. Babich has the right to acquire from us within 60 days of June 15, 2011 pursuant to the exercise of stock options. |
| (3) | Represents 88,411 shares that Dr. Dillaha has the right to acquire from us within 60 days of June 15, 2011 pursuant to the exercise of stock options. |
| (4) | Represents 20,436 shares that Mr. Meyer has the right to acquire from us within 60 days of June 15, 2011 pursuant to the exercise of stock options. |
| (5) | Represents 20,436 shares that Mr. Tambi has the right to acquire from us within 60 days of June 15, 2011 pursuant to the exercise of stock options. |
| (6) | Represents 8,196 shares that Dr. Rahman has the right to acquire from us within 60 days of June 15, 2011 pursuant to the exercise of stock options. |
| (7) | Represents 3,836 shares that Dr. Ali has the right to acquire from us within 60 days of June 15, 2011 pursuant to the exercise of stock options. |
| (8) | Includes 196 shares held by Mr. McCarthy and 4,683 shares that Mr. McCarthy has the right to acquire from us within 60 days of June 15, 2011 pursuant to the exercise of stock options. |
| (9) | Includes 485,148 shares that our current executive officers and directors as a group have the right to acquire from us within 60 days of June 15, 2011 pursuant to the exercise of stock options. |
152
Table of Contents
Upon the closing of this offering and the filing of our amended and restated certificate of incorporation, our authorized capital stock will consist of 200,000,000 shares of common stock, par value $0.0002145 per share, and 10,000,000 shares of preferred stock, par value $0.001 per share. The following is a summary of the rights of our common and preferred stock and some of the provisions of our amended and restated certificate of incorporation and amended and restated bylaws, which will become effective upon the closing of this offering, and of the Delaware General Corporation Law. This summary is not complete. For more detailed information, please see our amended and restated certificate of incorporation, as amended, amended and restated certificate of designations, as amended, and amended and restated bylaws, which are filed as exhibits to the registration statement of which this prospectus is a part, as well as the relevant provisions of the Delaware General Corporation Law.
Common Stock
On June 30, 2011, there were 784,020 shares of our common stock outstanding, held of record by 139 stockholders. As of June 30, 2011, there were 1,619,232 shares of our common stock subject to outstanding options. Based on (i) 784,020 shares of our common stock outstanding as of June 30, 2011, (ii) the conversion of 14,864,607 shares of convertible preferred stock into 8,528,860 shares of common stock immediately prior to the closing of this offering, (iii) the conversion of $ million in aggregate principal amount of notes and accrued interest thereon owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming a conversion date of , 2011 and an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, immediately prior to the closing of this offering, and (iv) the issuance of shares of common stock in this offering, there will be shares of our common stock outstanding upon the closing of this offering.
Voting. Our common stock is entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, including the election of directors, and does not have cumulative voting rights. Accordingly, the holders of a majority of the shares of our common stock entitled to vote in any election of directors can elect all of the directors standing for election.
Dividends. Subject to preferences that may be applicable to any then outstanding preferred stock, the holders of common stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of legally available funds.
Liquidation. In the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities, subject to the satisfaction of any liquidation preference granted to the holders of any outstanding shares of preferred stock.
Rights and Preferences. Holders of our common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock that we may designate and issue in the future.
Fully Paid and Nonassessable. All of our outstanding shares of common stock are, and the shares of common stock to be issued in this offering will be, fully paid and nonassessable.
Contingent Payment Rights. In connection with the Merger, each of our stockholders immediately prior to the closing of the Merger, on November 8, 2010 was distributed a contingent payment right, or CPR, for each share of our common stock held on that date. Each CPR entitles the holder to receive a pro rata share of up to an aggregate of $20.0 million, payable in cash, if, within five years of the Merger, one of the NeoPharm product candidates that was in development prior to the Merger receives FDA approval.
153
Table of Contents
Preferred Stock
As of June 30, 2011, there were 14,864,607 shares of convertible preferred stock outstanding, held of record by 21 stockholders. Pursuant to our amended and restated certificate of designations, as amended, each share of convertible preferred stock will automatically convert into shares of our common stock immediately prior to the closing of this offering, at the then-applicable conversion ratio. Each share of our convertible preferred stock is convertible into 0.57 shares of our common stock. Accordingly, immediately prior to the closing of this offering, the outstanding shares of convertible preferred stock will automatically convert into 8,528,860 shares of our common stock.
Following this offering, under our amended and restated certificate of incorporation, our board of directors will have the authority, without further action by the stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power, impair the liquidation rights of our common stock or otherwise adversely affect the rights of holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in our control and may adversely affect the market price of our common stock and the voting and other rights of the holders of common stock. We have no current plans to issue any shares of preferred stock.
Anti-Takeover Effects of Provisions of Our Amended and Restated Certificate of Incorporation, Our Bylaws and Delaware Law
Delaware Anti-Takeover Law. We are subject to Section 203 of the Delaware General Corporation Law, or Section 203. Section 203 generally prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless:
| • | prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| • | the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (a) shares owned by persons who are directors and also officers and (b) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66-2/3% of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
154
Table of Contents
| • | subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; |
| • | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; and |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws. Provisions of our amended and restated certificate of incorporation and amended and restated bylaws, which will become effective upon the closing of this offering, may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock. Among other things, our amended and restated certificate of incorporation and amended and restated bylaws:
| • | permit our board of directors to issue up to 10,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate (including the right to approve an acquisition or other change in our control); |
| • | provide that the authorized number of directors may be changed only by resolution of the board of directors; |
| • | provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
| • | divide our board of directors into three classes; |
| • | require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent; |
| • | provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner, and also specify requirements as to the form and content of a stockholder’s notice; |
| • | do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); and |
| • | provide that special meetings of our stockholders may be called only by the chairman of the board, our Chief Executive Officer or by the board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors. |
The amendment of any of these provisions, with the exception of the ability of our board of directors to issue shares of preferred stock and designate any rights, preferences and privileges thereto, would require approval by the holders of at least 66-2/3% of our then outstanding common stock.
155
Table of Contents
Nasdaq Global Market Listing
We have applied for listing of our common stock on the Nasdaq Global Market under the symbol “INRX.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A. The transfer agent and registrar’s address is 350 Indiana Street, Suite 750, Golden, Colorado 80401.
156
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Although our common stock was once traded on the Nasdaq Capital Market and our common stock is currently quoted on the Pink Sheets, immediately prior to this offering, we do not believe that there is currently a liquid public market on which our common stock is actively and readily traded. Future sales of substantial amounts of common stock in the public market could adversely affect prevailing market prices. Furthermore, since a relatively limited number of our outstanding shares will be available for sale shortly after this offering due to contractual and legal restrictions on resale described below, sales of substantial amounts of common stock in the public market after these restrictions lapse could adversely affect the prevailing market price for our common stock as well as our ability to raise equity capital in the future.
Based on 784,020 shares of common stock outstanding as of June 30, 2011, the conversion of 14,864,607 shares of convertible preferred stock into 8,528,860 shares of common stock immediately prior to the closing of this offering the conversion of $ million in aggregate principal amount of notes and accrued interest thereon owed to trusts controlled by our Executive Chairman and principal stockholder into shares of common stock, assuming a conversion date of , 2011 and an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, immediately prior to the closing of this offering, and the issuance of shares of common stock in this offering, upon the closing of this offering, shares of common stock will be outstanding, assuming no exercise of the underwriters’ over-allotment option and no exercise of outstanding stock options. All of the shares sold in this offering will be freely tradable unless held by an affiliate of ours. Immediately prior to the Merger, there were 464,353 shares of our common stock outstanding, and we expect that substantially all of these shares will also be freely tradable after this offering unless held by an affiliate of ours. Except as set forth below, the remaining 8,848,527 shares of common stock outstanding upon the closing of this offering will be restricted as a result of securities laws or lock-up agreements. These remaining shares will generally become available for sale in the public market under Rule 144 or Rule 701 upon expiration of lock-up agreements at least 180 days after the date of this offering or longer if the lock-up period is extended, in certain circumstances, subject to volume limitations pursuant to Rule 144.
Rule 144
In general, under Rule 144 as currently in effect, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, any person who is not an affiliate of ours and has held their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell shares without restriction, provided current public information about us is available. In addition, under Rule 144, any person who is not an affiliate of ours and has held their shares for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares immediately upon the closing of this offering without regard to whether current public information about us is available. Beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is an affiliate of ours and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of restricted shares within any three-month period that does not exceed the greater of:
| • | 1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering; or |
| • | the average weekly trading volume of our common stock on the Nasdaq Global Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Sales of restricted shares under Rule 144 held by our affiliates are also subject to requirements regarding the manner of sale, notice and the availability of current public information about us. Rule 144 also provides that affiliates relying on Rule 144 to sell shares of our common stock that are not restricted shares must nonetheless comply with the same restrictions applicable to restricted shares, other than the holding period requirement.
157
Table of Contents
Notwithstanding the availability of Rule 144, the holders of substantially all of our restricted shares have entered into lock-up agreements as described below and their restricted shares will become eligible for sale at the expiration of the restrictions set forth in those agreements.
Rule 701
Under Rule 701, shares of our common stock acquired upon the exercise of currently outstanding options or pursuant to other rights granted under our stock plans may be resold by:
| • | persons other than affiliates, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, subject only to the manner-of-sale provisions of Rule 144; and |
| • | our affiliates, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, subject to the manner-of-sale and volume limitations, current public information and filing requirements of Rule 144, in each case, without compliance with the six-month holding period requirement of Rule 144. |
As of June 30, 2011, options to purchase a total of 1,619,232 shares of common stock were outstanding, of which 1,118,851 were vested. Of the total number of shares of our common stock issuable under these options, substantially all are subject to contractual lock-up agreements with us or the underwriters described below under the section entitled “Underwriting — Lock-Up Agreements” and will become eligible for sale at the expiration of those agreements unless held by an affiliate of ours.
Lock-Up Agreements
As described under the section entitled “Underwriting — Lock-Up Agreements” below, we, each of our directors and officers, the holders of substantially all of the other shares of our common stock outstanding prior to this offering, other than shares outstanding prior to the Merger, and the holders of substantially all of our options outstanding prior to this offering, have agreed, subject to specified exceptions, not to, directly or indirectly, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of any shares of our common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock, without the prior written consent of Wells Fargo Securities, LLC and JMP Securities LLC, for a period of 180 days from the date of the final prospectus for the offering, or longer if the lock-up period is extended.
Wells Fargo Securities, LLC and JMP Securities LLC, may, in their sole discretion, at any time or from time to time and without notice, release for sale in the public market all or any portion of the shares restricted by the terms of the lock-up agreements.
Equity Incentive Plans
We intend to file with the SEC a registration statement on Form S-8 under the Securities Act covering the shares of common stock reserved for issuance under our 2011 plan, our directors’ plan and our 2011 purchase plan. The registration statement is expected to be filed and become effective as soon as practicable after the closing of this offering. Accordingly, shares registered under the registration statement will be available for sale in the open market following its effective date, subject to Rule 144 volume limitations and the lock-up agreements described above, if applicable.
158
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO
NON-U.S. HOLDERS OF OUR COMMON STOCK
The following summary describes the material U.S. federal income and estate tax consequences of the acquisition, ownership and disposition of our common stock acquired in this offering by Non-U.S. Holders (as defined below). This discussion does not address all aspects of U.S. federal income and estate taxes and does not deal with foreign, state and local consequences that may be relevant to Non-U.S. Holders in light of their particular circumstances, nor does it address U.S. federal tax consequences other than income and estate taxes. Special rules different from those described below may apply to certain Non-U.S. Holders that are subject to special treatment under the Code, such as financial institutions, insurance companies, tax-exempt organizations, broker-dealers and traders in securities, U.S. expatriates, “controlled foreign corporations,” “passive foreign investment companies,” corporations that accumulate earnings to avoid U.S. federal income tax, persons that hold our common stock as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or integrated investment or other risk reduction strategy, partnerships and other pass-through entities, and investors in such pass-through entities or an entity that is treated as a disregarded entity for U.S. federal income tax purposes (regardless of its place of organization or formation). Such Non-U.S. Holders are urged to consult their own tax advisors to determine the U.S. federal, state, local and other tax consequences that may be relevant to them. Furthermore, the discussion below is based upon the provisions of the Code, and Treasury regulations, rulings and judicial decisions thereunder as of the date hereof, and such authorities may be repealed, revoked or modified, perhaps retroactively, so as to result in U.S. federal income and estate tax consequences different from those discussed below. We have not requested a ruling from the U.S. Internal Revenue Service, or IRS, with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS will agree with such statements and conclusions. This discussion assumes that the Non-U.S. Holder holds our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment).
The following discussion is for general information only and is not tax advice. Persons considering the purchase of our common stock pursuant to this offering should consult their own tax advisors concerning the U.S. federal income and estate tax consequences of acquiring, owning and disposing of our common stock in light of their particular situations as well as any consequences arising under the laws of any other taxing jurisdiction, including any state, local or foreign tax consequences.
For the purposes of this discussion, a “Non-U.S. Holder” is, for U.S. federal income tax purposes, a beneficial owner of common stock that has not been excluded from this discussion and is not a U.S. Holder. A “U.S. Holder” means a beneficial owner of our common stock that is for U.S. federal income tax purposes (a) an individual who is a citizen or resident of the United States, (b) a corporation or other entity treated as a corporation created or organized in or under the laws of the United States, any state thereof or the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source or (d) a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
Distributions
Subject to the discussion below, distributions, if any, made on our common stock to a Non-U.S. Holder of our common stock to the extent made out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles) generally will constitute dividends for U.S. tax purposes and will be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required to provide us with a properly executed IRS Form W-8BEN, or other appropriate form, certifying the Non-U.S. Holder’s entitlement to benefits under that treaty. In the case
159
Table of Contents
of a Non-U.S. Holder that is an entity, Treasury Regulations and the relevant tax treaty provide rules to determine whether, for purposes of determining the applicability of a tax treaty, dividends will be treated as paid to the entity or to those holding an interest in that entity. If a Non-U.S. Holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries. If you are eligible for a reduced rate of U.S. federal withholding tax under an income tax treaty, you should consult with your own tax advisor to determine if you are able to obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS.
We generally are not required to withhold tax on dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment that such holder maintains in the United States) if a properly executed IRS Form W-8ECI, stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial institution or other agent, to such agent). In general, such effectively connected dividends will be subject to U.S. federal income tax, on a net income basis at the regular graduated rates, unless a specific treaty exemption applies. A corporate Non-U.S. Holder receiving effectively connected dividends may also be subject to an additional “branch profits tax,” which is imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) on the corporate Non-U.S. Holder’s effectively connected earnings and profits, subject to certain adjustments.
To the extent distributions on our common stock, if any, exceed our current and accumulated earnings and profits, they will constitute a non-taxable return of capital and will first reduce your adjusted basis in our common stock, but not below zero, and then will be treated as gain and taxed in the same manner as gain realized from a sale or other disposition of common stock as described in the next section.
Gain on Disposition of Our Common Stock
A Non-U.S. Holder generally will not be subject to U.S. federal income tax with respect to gain realized on a sale or other disposition of our common stock unless (a) the gain is effectively connected with a trade or business of such holder in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment that such holder maintains in the United States), (b) the Non-U.S. Holder is a nonresident alien individual and is present in the United States for 183 or more days in the taxable year of the disposition and certain other conditions are met, or (c) we are or have been a “United States real property holding corporation” within the meaning of Code Section 897(c)(2) at any time within the shorter of the five-year period preceding such disposition or such holder’s holding period. In general, we would be a United States real property holding corporation if interests in U.S. real estate comprised (by fair market value) at least half of our business assets. We believe that we are not, and do not anticipate becoming, a United States real property holding corporation. Even if we are treated as a United States real property holding corporation, gain realized by a Non-U.S. Holder on a disposition of our common stock will not be subject to U.S. federal income tax so long as (1) the Non-U.S. Holder owned, directly, indirectly and constructively, no more than 5% of our common stock at all times within the shorter of (i) the five-year period preceding the disposition or (ii) the holder’s holding period and (2) our common stock is regularly traded on an established securities market. There can be no assurance that our common stock will continue to qualify as regularly traded on an established securities market.
If you are a Non-U.S. Holder described in (a) above, you will be required to pay tax on the net gain derived from the sale at regular graduated U.S. federal income tax rates, unless a specific treaty exemption applies, and corporate Non-U.S. Holders described in (a) above may be subject to the additional branch profits tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. If you are an individual Non-U.S. Holder described in (b) above, you will be required
160
Table of Contents
to pay a flat 30% tax on the gain derived from the sale, which gain may be offset by U.S. source capital losses (even though you are not considered a resident of the United States).
Information Reporting Requirements and Backup Withholding
Generally, we or certain financial middlemen must report information to the IRS with respect to any dividends we pay on our common stock including the amount of any such dividends, the name and address of the recipient, and the amount, if any, of tax withheld. A similar report is sent to the holder to whom any such dividends are paid. Pursuant to tax treaties or certain other agreements, the IRS may make its reports available to tax authorities in the recipient’s country of residence.
Dividends paid by us (or our paying agents) to a Non-U.S. Holder may also be subject to U.S. backup withholding. U.S. backup withholding generally will not apply to a Non-U.S. Holder who provides a properly executed IRS Form W-8BEN or otherwise establishes an exemption. The current backup withholding rate is 28%.
Under current U.S. federal income tax law, U.S. information reporting and backup withholding requirements generally will apply to the proceeds from a disposition of our common stock effected by or through a U.S. office of any broker, U.S. or foreign, except that information reporting and such requirements may be avoided if the holder provides a properly executed IRS Form W-8BEN or otherwise meets documentary evidence requirements for establishing Non-U.S. Holder status or otherwise establishes an exemption. Generally, U.S. information reporting and backup withholding requirements will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through a non-U.S. office of a non-U.S. broker. Information reporting and backup withholding requirements may, however, apply to a payment of disposition proceeds if the broker has actual knowledge, or reason to know, that the holder is, in fact, a U.S. person. For information reporting purposes, certain brokers with substantial U.S. ownership or operations will generally be treated in a manner similar to U.S. brokers.
If backup withholding is applied to you, you should consult with your own tax advisor to determine if you are able to obtain a tax benefit or credit with respect to such backup withholding.
Recently Enacted Legislation Affecting Taxation of Our Common Stock Held by or Through Foreign Entities
Recently enacted legislation generally will impose a U.S. federal withholding tax of 30% on dividends and the gross proceeds from a disposition of our common stock paid after December 31, 2012 to a foreign financial institution (as specifically defined for this purpose) unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners). The legislation also will generally impose a U.S. federal withholding tax of 30% on dividends and the gross proceeds from a disposition of our common stock paid after December 31, 2012 to a non-financial foreign entity unless such entity provides the withholding agent with either a certification that it does not have any substantial direct or indirect U.S. owners or provides information regarding direct and indirect U.S. owners of the entity. Under certain circumstances, a Non-U.S. Holder might be eligible for refunds or credits of such taxes. Holders are encouraged to consult with their own tax advisors regarding the possible implications of the legislation on their investment in our common stock.
Federal Estate Tax
An individual Non-U.S. Holder who is treated as the owner of, or has made certain lifetime transfers of, an interest in our common stock will be required to include the value thereof in his or her gross estate for U.S. federal estate tax purposes, and may be subject to U.S. federal estate tax unless an
161
Table of Contents
applicable estate tax treaty provides otherwise, even though such individual was not a citizen or resident of the United States at the time of his or her death.
THE PRECEDING DISCUSSION OF U.S. FEDERAL INCOME AND ESTATE TAX CONSIDERATIONS IS FOR GENERAL INFORMATION ONLY. IT IS NOT TAX ADVICE. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAW.
162
Table of Contents
Subject to the terms and conditions set forth in an underwriting agreement, we have agreed to sell to the underwriters named below, and the underwriters, for whom Wells Fargo Securities, LLC and JMP Securities LLC are acting as joint-book running managers and representatives, have severally agreed to purchase, the respective numbers of shares of common stock appearing opposite their names below:
| Underwriter |
Number of Shares | |
| Wells Fargo Securities, LLC |
||
| JMP Securities LLC |
||
| Oppenheimer & Co. Inc |
||
|
| ||
| Total |
All of the shares to be purchased by the underwriters will be purchased from us.
The underwriting agreement provides that the obligations of the several underwriters are subject to various conditions, including approval of legal matters by counsel. The shares of common stock are offered by the underwriters, subject to prior sale, when, as and if issued to and accepted by them. The underwriters reserve the right to withdraw, cancel or modify the offer and to reject orders in whole or in part.
The underwriting agreement provides that the underwriters are obligated to purchase all the shares of common stock offered by this prospectus if any are purchased, other than those shares covered by the over-allotment option described below. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated.
Over-Allotment Option
We have granted a 30-day option to the underwriters to purchase up to a total of additional shares of our common stock from us at the initial public offering price per share less the estimated underwriting discounts and commissions per share, as set forth on the cover page of this prospectus, and less any dividends or distributions declared, paid or payable on the shares that the underwriters have agreed to purchase from us but that are not payable on such additional shares, to cover over-allotment, if any. If the underwriters exercise this option in whole or in part, then the underwriters will be severally committed, subject to the conditions described in the underwriting agreement, to purchase the additional shares of our common stock in proportion to their respective commitments set forth in the prior table.
Discounts and Commissions
Shares sold by the underwriters to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus and to certain dealers at that price less a concession of not more than $ per share, of which up to $ per share may be reallowed to other dealers. After the initial offering, the public offering price, concession and reallowance to dealers may be changed.
163
Table of Contents
The following table summarizes the underwriting discounts and commissions and the proceeds, before expenses, payable to us, both on a per share basis and in total, assuming either no exercise or full exercise by the underwriters of their overallotment option:
| Total | ||||||||||||
| Per Share | Without Option |
With Option |
||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discounts and commissions |
$ | $ | $ | |||||||||
| Proceeds, before expenses, to us |
$ | $ | $ | |||||||||
We estimate that the expenses of this offering payable by us, not including underwriting discounts and commissions, will be approximately $ .
Indemnification of Underwriters
The underwriting agreement provides that we will indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, or contribute to payments that the underwriters may be required to make in respect of those liabilities.
Lock-Up Agreements
We, each of our directors and officers, the holders of substantially all of the other shares of our common stock outstanding prior to this offering, other than shares outstanding prior to the Merger, and the holders of substantially all of our options outstanding prior to this offering, have agreed, subject to specified exceptions, that, without the prior written consent of Wells Fargo Securities, LLC and JMP Securities LLC, we and they will not, during the period beginning on and including the date of this prospectus through and including the date that is the 180th day after the date of this prospectus, directly or indirectly:
| • | issue (in the case of us), offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of any shares of our common stock or other capital stock or any securities convertible into or exercisable or exchangeable for our common stock or other capital stock; |
| • | in the case of us, file or cause the filing of any registration statement under the Securities Act with respect to any shares of our common stock or other capital stock or any securities convertible into or exercisable or exchangeable for our common stock or other capital stock, other than registration statements on Form S-8 filed with the SEC after the closing date of this offering; or |
| • | enter into any swap or other agreement, arrangement, hedge or transaction that transfers to another, in whole or in part, directly or indirectly, any of the economic consequences of ownership of our common stock or other capital stock or any securities convertible into or exercisable or exchangeable for our common stock or other capital stock, |
whether any transaction described in any of the foregoing bullet points is to be settled by delivery of our common stock or other capital stock, other securities, in cash or otherwise, or publicly announce an intention to do any of the foregoing. Moreover, if:
| • | during the last 17 days of the lock-up period, we issue an earnings release or material news or a material event relating to us occurs; or |
| • | prior to the expiration of the lock-up period, we announce that we will release earnings results or become aware that material news or a material event relating to us will occur during the 16-day period beginning on the last day of the lock-up period, |
164
Table of Contents
the restrictions described in the immediately preceding sentence will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release or the occurrence of the material news or material event, as the case may be, unless Wells Fargo Securities, LLC and JMP Securities LLC waive, in writing, that extension.
Wells Fargo Securities, LLC and JMP Securities LLC may, in their sole discretion and at any time or from time to time, without notice, release all or any portion of the shares or other securities subject to the lock-up agreements. Any determination to release any shares or other securities subject to the lock-up agreements would be based on a number of factors at the time of determination, which may include the market price of the common stock, the liquidity of the trading market for the common stock, general market conditions, the number of shares or other securities proposed to be sold or otherwise transferred and the timing, purpose and terms of the proposed sale or other transfer.
Nasdaq Global Market Listing
We have applied to have our common stock listed on the Nasdaq Global Market under the symbol “INRX.”
Stabilization
In order to facilitate this offering of our common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the market price of our common stock. Specifically, the underwriters may sell more shares of common stock than they are obligated to purchase under the underwriting agreement, creating a short position. A short sale is covered if the short position is no greater than the number of shares of common stock available for purchase by the underwriters under the over-allotment option. The underwriters may close out a covered short sale by exercising the over-allotment option or purchasing common stock in the open market. In determining the source of common stock to close out a covered short sale, the underwriters may consider, among other things, the market price of common stock compared to the price payable under the over-allotment option. The underwriters may also sell shares of common stock in excess of the over-allotment option, creating a naked short position. The underwriters must close out any naked short position by purchasing shares of common stock in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after the date of pricing of this offering that could adversely affect investors who purchase in this offering.
As an additional means of facilitating this offering, the underwriters may bid for, and purchase, common stock in the open market to stabilize the price of our common stock, so long as stabilizing bids do not exceed a specified maximum. The underwriting syndicate may also reclaim selling concessions allowed to an underwriter or a dealer for distributing common stock in this offering if the underwriting syndicate repurchases previously distributed common stock to cover syndicate short positions or to stabilize the price of the common stock.
The foregoing transactions, if commenced, may raise or maintain the market price of our common stock above independent market levels or prevent or retard a decline in the market price of the common stock.
The foregoing transactions, if commenced, may be effected on the Nasdaq Global Market or otherwise. Neither we nor any of the underwriters makes any representation that the underwriters will engage in any of these transactions and these transactions, if commenced, may be discontinued at any time without notice. Neither we nor any of the underwriters makes any representation or prediction as to the direction or magnitude of the effect that the transactions described above, if commenced, may have on the market price of our common stock.
165
Table of Contents
Discretionary Accounts
The underwriters have informed us that they do not intend to confirm sales to accounts over which they exercise discretionary authority in excess of 5% of the total number of shares of common stock offered by them.
Pricing of this Offering
Prior to this offering, there has been no public market for our common stock. Consequently, the initial public offering price for our common stock was determined between us and the representative of the underwriters. The factors considered in determining the initial public offering price included:
| • | prevailing market conditions; |
| • | our results of operations and financial condition; |
| • | financial and operating information and market valuations with respect to other companies that we and the representative of the underwriters believe to be comparable or similar to us; |
| • | the present state of our development; and |
| • | our future prospects. |
An active trading market for our common stock may not develop. It is possible that the market price of our common stock after this offering will be less than the initial public offering price. In addition, the estimated initial public offering price range appearing on the cover of this preliminary prospectus is subject to change as a result of market conditions or other factors.
Relationships
The underwriters and/or their respective affiliates may in the future provide various financial advisory, investment banking, commercial banking and other financial services to us, for which they may receive compensation.
Sales Outside the United States
No action has been or will be taken in any jurisdiction (except in the United States) that would permit an initial public offering of the common stock, or the possession, circulation or distribution of this prospectus or any other material relating to us or the common stock in any jurisdiction where action for that purpose is required. Accordingly, the common stock may not be offered or sold, directly or indirectly, and neither of this prospectus nor any other offering material or advertisements in connection with the common stock may be distributed or published, in or from any country or jurisdiction except in compliance with any applicable rules and regulations of any such country or jurisdiction.
Each of the underwriters may arrange to sell common stock offered by this prospectus in certain jurisdictions outside the United States, either directly or through affiliates, where they are permitted to do so. In that regard, Wells Fargo Securities, LLC may arrange to sell shares in certain jurisdictions through an affiliate, Wells Fargo Securities International Limited, or WFSIL. WFSIL is a wholly-owned indirect subsidiary of Wells Fargo & Company and an affiliate of Wells Fargo Securities, LLC. WFSIL is a U.K. incorporated investment firm regulated by the Financial Services Authority. Wells Fargo Securities is the trade name for certain corporate and investment banking services of Wells Fargo & Company and its affiliates, including Wells Fargo Securities, LLC and WFSIL.
166
Table of Contents
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of any shares of common stock which are the subject of the offering contemplated by this prospectus (the “Shares”) may not be made in that Relevant Member State except that an offer to the public in that Relevant Member State of any Shares may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
(a) to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
(b) to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000; and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts;
(c) to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of the representatives of the underwriters; or
(d) in any other circumstances falling within Article 3(2) of the Prospectus Directive,
provided that no such offer of Shares shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer to the public” in relation to any Shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any Shares to be offered so as to enable an investor to decide to purchase any Shares, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in the Relevant Member State and the expression “Prospectus Directive” means Directive 2003/71 EC (including the 2010 PD Amending Directive, in the case of Early Implementing Member States) and includes any relevant implementing measure in each Relevant Member State and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
United Kingdom
This prospectus and any other material in relation to the shares described herein is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the meaning of Article 2(1)(e) of the Prospective Directive (“qualified investors”) that also (i) have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, or the Order, (ii) who fall within Article 49(2)(a) to (d) of the Order or (iii) to whom it may otherwise lawfully be communicated (all such persons together being referred to as “relevant persons”). The shares are only available to, and any invitation, offer or agreement to purchase or otherwise acquire such shares will be engaged in only with, relevant persons. This offering memorandum and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other person in the United Kingdom. Any person in the United Kingdom that is not a relevant person should not act or rely on this prospectus or any of its contents.
The distribution of this prospectus in the United Kingdom to anyone not falling within the above categories is not permitted and may contravene the Financial Services and Markets Act of 2000. No person falling outside those categories should treat this prospectus as constituting a promotion to him, or act on it for any purposes whatever. Recipients of this prospectus are advised that we, the underwriters and any other person that communicates this prospectus are not, as a result solely of communicating this prospectus, acting for or advising them and are not responsible for providing recipients of this prospectus with the protections which would be given to those who are clients of any aforementioned entities that is subject to the Financial Services Authority Rules.
167
Table of Contents
France
The prospectus supplement and the accompanying prospectus (including any amendment, supplement or replacement thereto) have not been approved either by the Autorité des marchés financiers or by the competent authority of another State that is a contracting party to the Agreement on the European Economic Area and notified to the Autorité des marchés financiers; no security has been offered or sold and will be offered or sold, directly or indirectly, to the public in France within the meaning of Article L. 411-1 of the French Code Monétaire et Financier except to permitted investors, or Permitted Investors, consisting of persons licensed to provide the investment service of portfolio management for the account of third parties, qualified investors (investisseurs qualifiés) acting for their own account and/or a limited circle of investors (cercle restreint d’investisseurs) acting for their own account, with “qualified investors” and “limited circle of investors” having the meaning ascribed to them in Articles L. 411-2, D. 411-1, D. 411-2, D. 411-4, D. 744-1, D. 754-1 and D. 764-1 of the French Code Monétaire et Financier; none of this prospectus supplement and the accompanying Prospectus or any other materials related to the offer or information contained therein relating to our securities has been released, issued or distributed to the public in France except to Permitted Investors; and the direct or indirect resale to the public in France of any securities acquired by any Permitted Investors may be made only as provided by Articles L. 411-1, L. 411-2, L. 412-1 and L. 621-8 to L. 621-8-3 of the French Code Monétaire et Financier and applicable regulations thereunder.
Notice to the Residents of Germany
This document has not been prepared in accordance with the requirements for a securities or sales prospectus under the German Securities Prospectus Act (Wertpapierprospektgesetz), the German Sales Prospectus Act (Verkaufsprospektgesetz), or the German Investment Act (Investmentgesetz). Neither the German Federal Financial Services Supervisory Authority (Bundesanstalt fur Finanzdienstleistungsaufsicht — BaFin) nor any other German authority has been notified of the intention to distribute the securities in Germany. Consequently, the securities may not be distributed in Germany by way of public offering, public advertisement or in any similar manner AND THIS DOCUMENT AND ANY OTHER DOCUMENT RELATING TO THE OFFERING, AS WELL AS INFORMATION OR STATEMENTS CONTAINED THEREIN, MAY NOT BE SUPPLIED TO THE PUBLIC IN GERMANY OR USED IN CONNECTION WITH ANY OFFER FOR SUBSCRIPTION OF THE SECURITIES TO THE PUBLIC IN GERMANY OR ANY OTHER MEANS OF PUBLIC MARKETING. The securities are being offered and sold in Germany only to qualified investors which are referred to in Section 3, paragraph 2 no. 1, in connection with Section 2, no. 6, of the German Securities Prospectus Act. This document is strictly for use of the person who has received it. It may not be forwarded to other persons or published in Germany.
Switzerland
This document does not constitute a prospectus within the meaning of Art. 652a of the Swiss Code of Obligations. The shares of common stock may not be sold directly or indirectly in or into Switzerland except in a manner which will not result in a public offering within the meaning of the Swiss Code of Obligations. Neither this document nor any other offering materials relating to the shares of common stock may be distributed, published or otherwise made available in Switzerland except in a manner which will not constitute a public offer of the shares of common stock in Switzerland.
168
Table of Contents
The validity of the shares of common stock being offered by this prospectus will be passed upon for us by Cooley LLP, San Diego, California. The underwriters are being represented by Latham & Watkins LLP, San Diego, California.
The financial statements of Insys Therapeutics, Inc. as of December 31, 2010 and 2009, and for each of the three years in the period ended December 31, 2010, and the financial statements of NeoPharm, Inc. as of December 31, 2009 and 2008, and for the years then ended included in this Prospectus have been so included in reliance on the reports of BDO USA, LLP, an independent registered public accounting firm (the report on the NeoPharm financial statements contains an explanatory paragraph regarding NeoPharm’s ability to continue as a going concern) appearing elsewhere herein, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the shares of common stock being offered by this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the common stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street NE, Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street NE, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities. You may also request a copy of these filings, at no cost, by writing us at 10220 South 51st Street, Suite 2, Phoenix, Arizona 85044 or telephoning us at (602) 910-2617.
Upon the closing of this offering, we will be subject to the information reporting requirements of the Exchange Act, and we will file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available for inspection and copying at the public reference room and web site of the SEC referred to above. We also maintain a website at www.insysrx.com, at which, following the closing of this offering, you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website incorporated by reference in, and is not part of, this prospectus.
169
Table of Contents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
| Insys Therapeutics, Inc. |
||||
| F-2 | ||||
| Consolidated Balance Sheets as of June 30, 2011 (unaudited), and December 31, 2010 and 2009 |
F-3 | |||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| NeoPharm, Inc. |
||||
| F-29 | ||||
| Consolidated Balance Sheets as of December 31, 2009 and 2008 |
F-30 | |||
| Consolidated Statements of Operations for the Years Ended December 31, 2009 and 2008 |
F-31 | |||
| Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2009 and 2008 |
F-32 | |||
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2009 and 2008 |
F-33 | |||
| F-34 | ||||
| NeoPharm, Inc. |
||||
| Condensed Consolidated Balance Sheets as of September 30, 2010 and December 31, 2009 |
F-50 | |||
| F-51 | ||||
| F-52 | ||||
| Notes to Unaudited Condensed Consolidated Financial Statements |
F-53 | |||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Insys Therapeutics, Inc.
Phoenix, Arizona
We have audited the accompanying consolidated balance sheets of Insys Therapeutics, Inc., a development-stage company, as of December 31, 2010 and 2009 and the related consolidated statements of operations and cash flows for each of the three years in the period ended December 31, 2010, and the statements of stockholders’ deficit for each of the eight years in the period ended December 31, 2010 and for the period from October 2002 (inception) through December 31, 2002. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Insys Therapeutics, Inc. at December 31, 2010 and 2009, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2010 in conformity with accounting principles generally accepted in the United States of America.
/s/ BDO USA, LLP
Chicago, Illinois
March 29, 2011, except for the July 2011 reverse stock split described in Note 13 which is as of July 14, 2011
F-2
Table of Contents
(A Development-Stage Company)
BALANCE SHEETS
(in U.S. $ 000’s, except par value and share data)
| Pro Forma Stockholders' Deficit as of June 30, 2011 (see Note 1) |
As of June 30, 2011 |
As of December 31, |
||||||||||||||
| 2010 | 2009 | |||||||||||||||
| (Unaudited) |
||||||||||||||||
| ASSETS |
||||||||||||||||
| Current Assets |
||||||||||||||||
| Cash and cash equivalents |
$ | 17 | $ | 64 | $ | 143 | ||||||||||
| Inventory |
780 | 780 | 780 | |||||||||||||
| Prepaid expenses and other assets |
727 | 303 | 30 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
1,524 | 1,147 | 953 | |||||||||||||
| Fixed assets, net |
5,858 | 6,290 | 6,516 | |||||||||||||
| Intangible asset |
5,300 | 5,300 | — | |||||||||||||
| Goodwill |
103 | 103 | — | |||||||||||||
| Other assets |
1,859 | 1,915 | 772 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 14,644 | $ | 14,755 | $ | 8,241 | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT |
||||||||||||||||
| Current Liabilities |
||||||||||||||||
| Accounts payable and accrued expenses |
$ | 2,168 | $ | 2,564 | $ | 3,543 | ||||||||||
| Other current liabilities |
508 | 508 | 1,241 | |||||||||||||
| Notes payable to related party, including interest |
43,705 | 34,898 | 18,603 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
46,381 | 37,970 | 23,387 | |||||||||||||
| Contingent payment obligation |
1,988 | 1,829 | — | |||||||||||||
| Other long-term liabilities |
410 | 478 | 385 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
48,779 | 40,277 | 23,772 | |||||||||||||
| Commitments and contingencies (see Notes 9 and 12) |
||||||||||||||||
| Stockholders’ Deficit |
||||||||||||||||
| Common stock (par value $0.0002145 per share, 750,000,000 shares authorized as of June 30, 2011 and 50,000,000 shares authorized as of December 31, 2010 and 2009; 784,020, 785,362 and 317,465 shares issued and outstanding as of June 30, 2011, December 31, 2010 and 2009, respectively) |
$ | 2 | — | — | — | |||||||||||
| Convertible preferred stock (par value $0.001 per share, 15,000,000 shares authorized, 14,864,607, 14,864,607 and 14,824,584 shares issued and outstanding as of June 30, 2011, December 31, 2010 and 2009, respectively) |
— | 149 | 149 | 149 | ||||||||||||
| Additional paid in capital |
60,743 | 60,596 | 60,026 | 56,269 | ||||||||||||
| Deficit accumulated during the development stage |
(94,859 | ) | (94,859 | ) | (85,671 | ) | (71,928 | ) | ||||||||
| Notes receivable from stockholders |
(21 | ) | (21 | ) | (26 | ) | (21 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total stockholders’ deficit |
$ | (34,135 | ) | (34,135 | ) | (25,522 | ) | (15,531 | ) | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities and stockholders’ deficit |
$ | 14,644 | $ | 14,755 | $ | 8,241 | ||||||||||
|
|
|
|
|
|
|
|||||||||||
See accompanying notes to financial statements.
F-3
Table of Contents
(A Development-Stage Company)
STATEMENTS OF OPERATIONS
(in U.S. $000’s, except share and per share data)
| Period from October 2002 (inception) through June 30, 2011 |
Six Months Ended June 30, |
Years Ended | ||||||||||||||||||||||
| 2011 | 2010 | 2010 | 2009 | 2008 | ||||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||
| Revenues |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Research and development |
61,300 | 3,807 | 5,240 | 10,428 | 8,982 | 14,729 | ||||||||||||||||||
| General and administrative |
27,048 | 4,595 | 2,015 | 3,539 | 4,504 | 10,221 | ||||||||||||||||||
| Loss on settlement of vendor dispute |
1,104 | — | — | — | — | 1,104 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
89,452 | 8,402 | 7,255 | 13,967 | 13,486 | 26,054 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss from operations |
(89,452 | ) | (8,402 | ) | (7,255 | ) | (13,967 | ) | (13,486 | ) | (26,054 | ) | ||||||||||||
| Other Income, net |
1,710 | 102 | 26 | 797 | 31 | 780 | ||||||||||||||||||
| Interest expense |
(7,932 | ) | (888 | ) | (474 | ) | (1,150 | ) | (1,038 | ) | (1,940 | ) | ||||||||||||
| Interest income |
240 | — | 1 | 2 | 39 | 27 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss before income taxes |
(95,434 | ) | (9,188 | ) | (7,702 | ) | (14,318 | ) | (14,454 | ) | (27,187 | ) | ||||||||||||
| Income tax benefit |
575 | — | — | 575 | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
$ | (94,859 | ) | (9,188 | ) | (7,702 | ) | (13,743 | ) | (14,454 | ) | (27,187 | ) | |||||||||||
|
|
|
|||||||||||||||||||||||
| Net loss allocable to preferred stockholders |
8,414 | 7,425 | 13,144 | 13,932 | 26,205 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss allocable to common stockholders |
$ | (774 | ) | $ | (277 | ) | $ | (599 | ) | $ | (522 | ) | $ | (982 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Basic and diluted net loss per common share |
$ | (0.99 | ) | $ | (0.87 | ) | $ | (1.54 | ) | $ | (7.05 | ) | $ | (19.09 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Basic and diluted weighted average common shares outstanding |
784,020 | 319,423 | 388,449 | 74,063 | 51,438 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
See accompanying notes to financial statements.
F-4
Table of Contents
(A Development-Stage Company)
STATEMENTS OF STOCKHOLDERS’ DEFICIT
(in U.S. $ 000’s)
| Common Stock | Convertible Preferred Stock |
Additional Paid-In Capital |
Deficit Accumulated During Development- Stage |
Notes Receivable from Stockholders |
Total | |||||||||||||||||||||||||||
| No. of Shares |
Amount | No. of Shares |
Amount | |||||||||||||||||||||||||||||
| Initial capitalization, October, 2002 |
21,558 | $ | 1,002,524 | $ | 10 | $ | 30 | $ | — | $ | — | $ | 40 | |||||||||||||||||||
| Net loss |
— | — | — | — | — | (133 | ) | — | (133 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2002 |
21,558 | — | 1,002,524 | 10 | 30 | (133 | ) | — | (93 | ) | ||||||||||||||||||||||
| Net loss |
— | — | — | — | — | (751 | ) | — | (751 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2003 |
21,558 | — | |
1,002,524 |
|
10 | 30 | (884 | ) | — | (844 | ) | ||||||||||||||||||||
| Net loss |
— | — | — | — | — | (1,111 | ) | — | (1,111 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2004 |
21,558 | — | |
1,002,524 |
|
10 | 30 | (1,995 | ) | — | (1,955 | ) | ||||||||||||||||||||
| Shares awarded to executive |
839 | — | 39,059 | — | — | — | — | — | ||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | (2,826 | ) | — | (2,826 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2005 |
22,397 | — | 1,041,583 | 10 | 30 | (4,821 | ) | — | (4,781 | ) | ||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | 23 | — | — | 23 | ||||||||||||||||||||||||
| Exercise of common stock options |
890 | — | 41,398 | — | 56 | — | (42 | ) | 14 | |||||||||||||||||||||||
| Net loss |
— | — | — | — | — | (7,005 | ) | — | (7,005 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2006 |
23,287 | — | 1,082,981 | 10 | 109 | (11,826 | ) | (42 | ) | (11,749 | ) | |||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | 617 | — | — | 617 | ||||||||||||||||||||||||
| Shares issued to The John N. Kapoor Trust and family members in lieu of payment of accrued interest |
3,478 | — | 161,752 | 2 | 238 | — | — | 240 | ||||||||||||||||||||||||
| Exercise of common stock options |
1,328 | — | 61,776 | 1 | 91 | — | — | 92 | ||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | (18,461 | ) | — | (18,461 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2007 |
28,093 | — | 1,306,509 | 13 | 1,055 | (30,287 | ) | (42 | ) | (29,261 | ) | |||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | 7,786 | — | — | 7,786 | ||||||||||||||||||||||||
| Shares issued, net of repurchases |
1,437 | — | 66,857 | 1 | 1,338 | — | — | 1,339 | ||||||||||||||||||||||||
| Shares issued to The John N. Kapoor Trust |
13,559 | — | 630,500 | 6 | 8,603 | — | — | 8,609 | ||||||||||||||||||||||||
| Shares issued to The John N. Kapoor Trust and family members in lieu of payment of notes payable and accrued interest |
38,112 | — | 1,772,217 | 18 | 24,179 | — | — | 24,197 | ||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | (27,187 | ) | — | (27,187 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2008 |
81,201 | — | 3,776,083 | 38 | 42,961 | (57,474 | ) | (42 | ) | (14,517 | ) | |||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | 2,450 | 2,450 | ||||||||||||||||||||||||||
| Shares returned in repayment of employee loans |
(897 | ) | — | (41,748 | ) | — | (33 | ) | — | 21 | (12 | ) | ||||||||||||||||||||
| Shares repurchased |
(14,911 | ) | — | (693,395 | ) | (7 | ) | (540 | ) | — | — | (547 | ) | |||||||||||||||||||
| Shares issued to The John N. Kapoor Trust and family members in lieu of payment of notes payable and accrued interest |
253,414 | — | 11,783,644 | 118 | 11,431 | — | — | 11,549 | ||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | (14,454 | ) | — | (14,454 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2009 |
318,807 | — | 14,824,584 | 149 | 56,269 | (71,928 | ) | (21 | ) | (15,531 | ) | |||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | 1,445 | — | — | 1,445 | ||||||||||||||||||||||||
| NeoPharm, Inc. merger |
464,353 | — | — | — | 2,273 | — | — | 2,273 | ||||||||||||||||||||||||
| Exercise of common stock options |
860 | — | 40,023 | — | 39 | — | (5 | ) | 34 | |||||||||||||||||||||||
| Net loss |
— | — | — | — | — | (13,743 | ) | (13,743 | ) | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2010 |
784,020 | — | 14,864,607 | 149 | 60,026 | (85,671 | ) | (26 | ) | (25,522 | ) | |||||||||||||||||||||
| Stock-based compensation expense (unaudited) |
— | — | — | — | 570 | — | — | 570 | ||||||||||||||||||||||||
| Repayment of employee loans (unaudited) |
— | — | — | — | — | — | 5 | 5 | ||||||||||||||||||||||||
| Net loss (unaudited) |
— | — | — | — | — | (9,188 | ) | — | (9,188 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at June 30, 2011 (unaudited) |
784,020 | $ | — | 14,864,607 | $ | 149 | $ | 60,596 | $ | (94,859 | ) | $ | (21 | ) | $ | (34,135 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
See accompanying notes to financial statements.
F-5
Table of Contents
(A Development-Stage Company)
STATEMENTS OF CASH FLOWS
(in U.S. $ 000’s)
| Period from October 2002 (inception) through June 30, 2011 |
Six Months Ended June 30, |
Years Ended December 31, | ||||||||||||||||||||||
| 2011 | 2010 | 2010 | 2009 | 2008 | ||||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||
| Cash flows from operating activities: |
||||||||||||||||||||||||
| Net loss |
$ | (94,859 | ) | $ | (9,188 | ) | $ | (7,702 | ) | $ | (13,743 | ) | $ | (14,454 | ) | $ | (27,187 | ) | ||||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||||||||||||||
| Depreciation and amortization of fixed assets |
2,396 | 654 | 173 | 754 | 348 | 324 | ||||||||||||||||||
| Loss on settlement of vendor dispute |
1,104 | — | — | — | — | 1,104 | ||||||||||||||||||
| Stock-based compensation |
12,891 | 570 | 1,336 | 1,445 | 2,450 | 7,786 | ||||||||||||||||||
| Interest expense, accrued on notes payable |
7,862 | 888 | 474 | 1,150 | 1,037 | 1,931 | ||||||||||||||||||
| Interest income, accrued on notes receivable |
(190 | ) | — | — | — | (37 | ) | — | ||||||||||||||||
| Deferred income tax benefit |
(575 | ) | — | — | (575 | ) | — | — | ||||||||||||||||
| Loss on disposal of assets |
9 | — | — | — | — | — | ||||||||||||||||||
| Accretion of contingent payment obligation |
159 | 159 | — | — | — | — | ||||||||||||||||||
| Changes in assets and liabilities, net of NeoPharm merger: |
||||||||||||||||||||||||
| Prepaid expenses and other assets |
(1,787 | ) | (368 | ) | — | (616 | ) | (770 | ) | 1,866 | ||||||||||||||
| Accounts payable, accrued expenses, and other current liabilities |
1,630 | (459 | ) | (784 | ) | (3,389 | ) | 1,231 | (1,206 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net cash used in operating activities |
(71,360 | ) | (7,744 | ) | (6,503 | ) | (14,974 | ) | (10,195 | ) | (15,382 | ) | ||||||||||||
| Cash flows from investing activities: |
||||||||||||||||||||||||
| Purchase of fixed assets |
(4,314 | ) | (222 | ) | (72 | ) | (384 | ) | (1,601 | ) | (307 | ) | ||||||||||||
| Cash received in merger |
143 | — | — | 143 | — | — | ||||||||||||||||||
| Advances made under notes receivable |
(5,735 | ) | — | — | — | — | — | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net cash used in investing activities |
(9,906 | ) | (222 | ) | (72 | ) | (241 | ) | (1,601 | ) | (307 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Cash flows from financing activities: |
||||||||||||||||||||||||
| Proceeds from note payable to related party |
75,018 | 7,919 | 6,449 | 15,145 | 11,498 | 9,514 | ||||||||||||||||||
| Principal payments on capital lease obligations |
(687 | ) | — | — | (9 | ) | (87 | ) | (113 | ) | ||||||||||||||
| Proceeds from exercise of stock options |
105 | — | — | — | — | — | ||||||||||||||||||
| Initial capitalization |
40 | — | — | — | — | — | ||||||||||||||||||
| Repurchase of common stock |
(52 | ) | — | — | — | — | (52 | ) | ||||||||||||||||
| Stock issued to related parties |
8,609 | — | — | — | — | 8,609 | ||||||||||||||||||
| Stock issued to others |
1,391 | — | — | — | — | 1,391 | ||||||||||||||||||
| Payments of notes payable |
(3,141 | ) | — | — | — | — | (3,141 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net cash provided by financing activities |
81,283 | 7,919 | 6,449 | 15,136 | 11,411 | 16,208 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net (decrease) increase in cash and cash equivalents |
17 | (47 | ) | (126 | ) | (79 | ) | (385 | ) | 519 | ||||||||||||||
| Cash and cash equivalents, beginning of period |
— | 64 | 143 | 143 | 528 | 9 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Cash and cash equivalents, end of period |
$ | 17 | $ | 17 | $ | 17 | $ | 64 | $ | 143 | $ | 528 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Supplemental disclosure of cash flow information: |
||||||||||||||||||||||||
| Shares issued in lieu of payment of notes payable and accrued interest |
$ | — | $ | — | $ | — | $ | 189 | $ | 396 | ||||||||||||||
| Cash paid for interest |
$ | — | $ | — | $ | — | $ | 1 | $ | 8 | ||||||||||||||
The Company completed a merger with NeoPharm, Inc. on November 8, 2010 that was accounted for as a reverse acquisition. All of the Company's common stock prior to the merger was exchanged for 19,499,989 shares of NeoPharm common stock and 14,864,607 shares of newly-created NeoPharm convertible preferred stock (see Note 10).
See accompanying notes to financial statements.
F-6
Table of Contents
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS
| 1. | INTRODUCTION AND BASIS OF PRESENTATION |
Organization
Insys Therapeutics, Inc. was incorporated in the state of Delaware in October 2002 and maintains its headquarters in Phoenix, Arizona.
On November 8, 2010, Insys Therapeutics, Inc. effected a merger with NeoPharm, Inc. (“NeoPharm”), a Delaware corporation incorporated in 1990, in a transaction that was accounted for as a reverse acquisition (see Note 10). Dr. John N. Kapoor, the Chairman of the board of directors and 96% stockholder of Insys Therapeutics, Inc. was also the Chairman of the board of directors and a 21% stockholder of NeoPharm at the time of the merger. Accordingly, NeoPharm’s board of directors established a Special Committee consisting solely of independent directors not affiliated with Dr. Kapoor and Insys Therapeutics, Inc. to evaluate the proposed transaction and strategic alternatives. The Special Committee retained its own advisers and counsel, received a fairness opinion from an investment bank and unanimously recommended the merger to the NeoPharm board of directors, which also unanimously approved the transaction. All of the outstanding share capital of Insys Therapeutics, Inc. was exchanged for newly-issued shares of common stock and convertible preferred stock of NeoPharm. As a result of the merger, Insys Therapeutics, Inc. became a wholly-owned subsidiary of NeoPharm and changed its name to Insys Pharma, Inc. (“Insys Pharma”). NeoPharm then changed its name to Insys Therapeutics, Inc.
Insys Therapeutics, Inc., together with its wholly-owned subsidiary, Insys Pharma, Inc. is hereafter referred to as “the Company.” Since Insys Pharma is the acquiring entity for accounting purposes, the financial statements for all periods up to and including the November 8, 2010 merger date are the financial statements of the entity that is now the subsidiary Insys Pharma. The financial statements for all periods subsequent to the November 8, 2010 merger date are the consolidated financial statements of Insys Therapeutics, Inc. and Insys Pharma. All of the discussion in this section will reflect this financial presentation of these entities. However, for all periods, the financial statements are labeled “Insys Therapeutics, Inc.” financial statements.
The Company is a specialty pharmaceutical company that develops and seeks to commercialize innovative pharmaceutical products that target the unmet needs of cancer patients, with an initial focus on cancer-supportive care. The Company focuses its research and development efforts on product candidates that utilize innovative formulations to address the clinical shortcomings of existing commercial pharmaceutical products.
Basis of Presentation and Accounting Policies
The accompanying financial statements are prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). The Company is considered a development-stage entity and has disclosed inception-to-date information within these financial statements. The Company is in the process of seeking regulatory approval for certain of its product candidates.
Amounts presented have been rounded to the nearest thousand except percentages and share and per share data, unless otherwise noted.
The equity accounts and all share and per share data of the Company have been retroactively adjusted to reflect a 10-for-one stock split on October 11, 2005, a 2.5-for-one stock split on June 15, 2006, a one-for-2.35 reverse stock split on January 17, 2008, a one-for-1,500,000 reverse stock split on June 2, 2009, a 1,862,623-for-one stock split on February 22, 2010, a one-for-61 reverse stock split on July 14, 2011 and the November 8, 2010 merger with NeoPharm (see Note 10).
F-7
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expense during the reporting period. Actual results could differ from those estimates.
Unaudited Interim Financial Statements
The accompanying consolidated balance sheet as of June 30, 2011, the consolidated statements of operations and of cash flows for the six months ended June 30, 2011 and 2010, and for the cumulative period from October 2002 (inception) to June 30, 2011, and the consolidated statement of stockholders’ deficit for the six months ended June 30, 2011 are unaudited. The unaudited interim consolidated financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to state fairly the Company’s financial position as of June 30, 2011 and results of operations and cash flows for the six months ended June 30, 2011 and 2010 and for the cumulative period from October 2002 (inception) to June 30, 2011. The financial data and other information disclosed in these notes to the consolidated financial statements related to the six month periods and the cumulative period from October 2002 (inception) to June 30, 2011 are unaudited. The results for the six months ended June 30, 2011 are not necessarily indicative of the results to be expected for the year ending December 31, 2011 or for any other interim period or for any future year.
Unaudited Pro Forma Stockholders’ Deficit
The unaudited pro forma stockholders’ deficit as of June 30, 2011 has been prepared assuming upon closing of an initial public offering (“IPO”), all outstanding convertible preferred stock will automatically convert into an aggregate of 8,528,860 shares of common stock. The shares of common stock issuable in the initial public offering described in Note 13 and the related estimated net proceeds and the shares of common stock issuable upon the conversion of outstanding promissory notes and accrued interest into common stock immediately prior to the closing of an IPO are excluded from such pro forma information.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. The carrying value of those investments approximates their fair market value due to their short maturity and liquidity.
Inventories
Inventories consist principally of raw materials related to the Company’s dronabinol product candidate and are valued at the lower of cost or market. Inventory costs are capitalized prior to regulatory approval and product launch based on management’s judgment of probable future commercial use and net realizable value of the inventory. Such judgment incorporates the Company’s knowledge and best estimate of where the relevant product is in the regulatory process, the Company’s required investment in the product, market conditions, competing products and the Company’s economic expectations for the product post-approval relative to the risk of manufacturing the product prior to approval. In evaluating the recoverability of inventories produced in preparation for product launches, the Company considers the probability that revenue will be obtained from the future sale of the related inventory together with the status of the product within the regulatory approval process, as well as the market for the product in its current state. The Company could be required to permanently
F-8
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
write down previously capitalized costs related to pre-approval or pre-launch inventory upon a change in such judgment, due to a denial or delay of approval by regulatory bodies, a delay in commercialization, or other potential factors including product expiration.
Fixed Assets
Fixed assets are recorded at cost and depreciated using the straight-line method over their estimated useful lives. Maintenance and repairs that do not extend the life of assets are charged to expense when incurred. When properties are disposed of, the related costs and accumulated depreciation are removed from the accounts and any gain or loss is reported in the period the transaction takes place.
Fixed assets are reviewed for impairment whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted cash flows expected to be generated by the asset. If the carrying amount exceeds its estimated future undiscounted cash flows, an impairment charge is recognized by the amount by which the carrying amount exceeds the fair value of the asset.
Acquisitions, Goodwill and Other Intangible Assets
The Company accounts for acquired businesses using the acquisition method of accounting in accordance with GAAP, which requires that the assets acquired and liabilities assumed be recorded at the date of acquisition at their respective fair values. Any excess of the purchase price over the estimated fair values of net assets acquired is recorded as goodwill. Any excess of the fair value of assets acquired and liabilities assumed over the purchase price is recorded as a bargain purchase gain. The fair value of intangible assets, which consist of in-process research and development (“IPR&D”), is based on significant judgments made by management. The valuations and useful life assumptions are based on information available near the acquisition date and are based on expectations and assumptions that are considered reasonable by management. In the Company’s assessment of the fair value of identifiable intangible assets acquired in the NeoPharm merger, management used valuation techniques and made various assumptions which are further described in Note 10.
The Company expects to review the IPR&D, which currently has an indefinite useful life, for impairment at least annually in the fourth fiscal quarter, or more frequently if an event occurs creating the potential for impairment, until such time as the research and development efforts are completed or abandoned. If the research and development efforts are abandoned, the related costs will be written off in the period of such determination. If the research and development efforts are completed successfully, the related assets will be amortized over the estimated useful life of the underlying products. The Company will amortize the cost of identified intangible assets using amortization methods that reflect the pattern in which the economic benefits of the intangible assets are consumed or otherwise realized. The Company reviews intangible assets that have finite useful lives when an event occurs creating the potential for impairment. The Company reviews for impairment by examining facts or circumstances, either external or internal, indicating that the Company may not recover the carrying value of the asset. The Company measures impairment losses related to long-lived assets based on the amount by which the carrying amounts of these assets exceed their fair values. The Company measures fair value generally based on the estimated future cash flows. The Company’s analysis is based on available information and on assumptions and projections that it considers to be reasonable and supportable. If necessary, the Company will perform subsequent calculations to measure the amount of the impairment loss based on the excess of the carrying value over the fair value of the impaired assets.
F-9
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
Income Taxes
Prior to November 8, 2010, the entity now known as Insys Pharma was subject to taxation under the provisions of Subchapter S of the U.S. Internal Revenue Code of 1986, as amended (the “Code”), and, as a result, the federal and state income tax liabilities of this entity were the responsibility of its stockholders. Accordingly, no provision was made for federal or state income taxes, since it was the personal responsibility of the individual stockholders of this entity to separately report their proportionate share of its taxable income or loss. As of November 8, 2010, as a result of the merger with NeoPharm, the entity now known as Insys Pharma became a Subchapter C Corporation and became subject to U.S. federal and state income tax at the corporate level. The effect of this change in the tax status was to recognize a one-time non-cash tax benefit of $3.0 million to establish a $3.0 million net deferred tax asset for the future tax consequences attributable to differences between the financial statement and income tax bases of its assets and liabilities as of November 8, 2010. The Company recorded a full valuation allowance against this net deferred tax asset.
The Company accounts for its deferred income tax assets and liabilities based on differences between the financial reporting and tax bases of assets and liabilities, and net operating loss carryforwards (the “NOLs”) and other tax credit carryforwards. These items are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in the period that includes the enactment date.
The Company records a valuation allowance to reduce the deferred income tax assets to the amount that is more likely than not to be realized. In making such determination, management considers all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax planning strategies and recent financial operations.
The Company recognizes a tax benefit from uncertain tax positions when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigations processes, based on the technical merits of the position. The Company recognizes interest accrued on unrecognized tax benefits and penalties in income tax expense.
Revenue Recognition
The Company will recognize revenue when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. From inception through June 30, 2011, the Company has not recognized any revenue.
Research and Development Expenses
Research and development (“R&D”) costs are expensed when incurred. These costs consist of external research and development expenses incurred under agreements with third-party contract research organizations and investigative sites, third-party manufacturing organizations and consultants; employee-related expenses, which include salaries, benefits and stock-based compensation for the personnel involved in our preclinical and clinical drug development activities; and facilities expense, depreciation and other allocated expenses, and equipment and laboratory supplies.
Stock-Based Compensation Expenses
Stock-based compensation cost is estimated at the grant date based on the fair value of the award, and the cost is recognized as expense ratably over the vesting period. The Company uses the Black-Scholes option pricing model for estimating the grant date fair value of stock options. Determining the
F-10
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
assumptions that are inputs of the model is highly subjective and requires judgment. Prior to the merger with NeoPharm, the Company did not have a history of market prices for its common stock and since the merger, it does not have what it considers a sufficiently actively and readily traded market for its common stock to use historical market prices for its common stock to estimate volatility. Accordingly, the Company estimates the expected stock price volatility for its common stock by taking the median historical stock price volatility for industry peers based on daily price observations over a period equivalent to the expected term of the stock option grants. The expected term is based on a simplified method which defines the life as the average of the contractual term of the options and the weighted average vesting period for all open employee awards. The risk-free interest rate for the expected term of the option is based on the average market rate on U.S. treasury securities in effect during the quarter in which the options were granted. The dividend yield assumption is based on the Company’s history and expectation of paying no dividends.
Leases
The Company’s operating leases with scheduled rent increases are accounted for on a straight-line basis over the lease term. Capital leases are accounted for in accordance with Accounting Standards Codification (“ASC”) 840, Leases.
Segment Information
ASC 280, Segment Reporting, establishes standards for reporting information about operating segments. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision-maker, or decision-making group, in deciding how to allocate resources and in assessing performance. Based on the Company’s integration and management strategies, the Company operates in a single operating segment.
Recent Accounting Pronouncements
In January 2010, the Financial Accounting Standards Board (the “FASB”) issued amended guidance on fair value measurements and disclosures. The new guidance requires additional disclosures regarding fair value measurements, amends disclosures about post-retirement benefit plan assets and provides clarification regarding the level of disaggregation of fair value disclosures by investment class. This guidance is effective for interim and annual reporting periods beginning after December 15, 2009, except for certain Level 3 activity disclosure requirements that became effective for reporting periods beginning after December 15, 2010. Accordingly, the Company adopted this amendment on January 1, 2010, except for the additional Level 3 requirements which were adopted on January 1, 2011. The adoption had no impact on the Company’s consolidated financial statements.
In April 2010, the FASB issued an accounting standard update which provides guidance on the criteria to be followed in recognizing revenue under the milestone method. The milestone method of recognition allows a vendor who is involved with the provision of deliverables to recognize the full amount of a milestone payment upon achievement, if, at the inception of the revenue arrangement, the milestone is determined to be substantive as defined in the standard. The guidance is effective on a prospective basis for milestones achieved in fiscal years and interim periods within those fiscal years, beginning on or after June 15, 2010. The Company adopted this guidance on January 1, 2011, but the adoption did not have an impact on the Company’s consolidated financial statements.
In December 2010, the FASB issued guidance relating to the disclosure of supplementary pro forma information for business combinations. This guidance specifies that if a public entity presents
F-11
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
comparative financial statements, the entity should disclose revenue and earnings of the combined entity as though the business combination(s) that occurred during the current year had occurred as of the beginning of the comparable prior annual reporting period only. This guidance also expands the supplemental pro forma disclosures to include a description of the nature and amount of material, nonrecurring pro forma adjustments directly attributable to the business combination included in the reported pro forma revenue and earnings. This guidance is effective prospectively for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2010. Early adoption is permitted. The Company adopted this guidance in 2010 and the disclosures relating to the NeoPharm merger in Note 10 are made based on this guidance.
Reclassifications
Certain reclassifications have been made to conform prior period consolidated financial statements and notes to current period presentation.
| 2. | NET LOSS PER SHARE |
The Company computes the net loss per common share using the two-class method as its convertible preferred shares meet the definition of a participating security and thereby share in the net income or loss of the Company on a ratable basis with the common stockholders. The convertible preferred shares portion of the net loss for the six months ended June 30, 2011 and June 30, 2010 was 91.6% and 96.4%, respectively, and 95.6%, 96.4% and 96.4% for the years ended December 31, 2010, 2009 and 2008, respectively. Basic net loss per common share is computed by dividing the net loss allocable to the common stockholders by the weighted average number of common shares outstanding during the period. The diluted loss per share further includes any common shares available to be issued upon exercise of outstanding stock options if such inclusion would be dilutive.
The following table sets forth the computation of basic and diluted net loss per common share (in U.S.$ 000’s):
| Six Months Ended June 30, | Year Ended December 31, | |||||||||||||||||||
| 2011 | 2010 | 2010 | 2009 | 2008 | ||||||||||||||||
| Numerator: |
||||||||||||||||||||
| Total net loss |
$ | (9,188 | ) | $ | (7,702 | ) | $ | (13,743 | ) | $ | (14,454 | ) | $ | (27,187 | ) | |||||
| Net loss allocable to participating securities |
$ | 8,414 | $ | 7,425 | $ | 13,144 | $ | 13,932 | $ | 26,205 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss allocable to common stockholders |
$ | (774 | ) | $ | (277 | ) | $ | (599 | ) | $ | (522 | ) | $ | (982 | ) | |||||
| Denominator: |
||||||||||||||||||||
| Weighted average common shares outstanding |
784,020 | 319,423 | 388,449 | 74,063 | 51,438 | |||||||||||||||
| Basic and diluted net loss per common share |
$ | (0.99 | ) | $ | (0.87 | ) | $ | (1.54 | ) | $ | (7.05 | ) | $ | (19.09 | ) | |||||
As the Company has incurred a net loss for all periods presented, basic and diluted per share amounts are the same, since the effect of potential common share equivalents is anti-dilutive. Anti-dilutive share equivalents included 1,619,232 and 1,026,688 as of June 30, 2011 and 2010, respectively, and 1,112,356, zero and 281,093 outstanding stock options as of December 31, 2010, 2009 and 2008, respectively.
F-12
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
| 3. | FIXED ASSETS |
Fixed assets are comprised of the following (in U.S. $ 000’s):
| Estimated Useful Life (in years) |
As of June 30, 2011 |
As of December 31, | ||||||||||||||
| 2010 | 2009 | |||||||||||||||
| Computer equipment |
3-5 | $ | 148 | $ | 132 | $ | 106 | |||||||||
| Scientific equipment |
5-7 | 4,317 | 4,118 | 3,783 | ||||||||||||
| Furniture |
5-7 | 108 | 108 | 87 | ||||||||||||
| Equipment under capital lease |
7 | 96 | 96 | 96 | ||||||||||||
| Leasehold improvements |
* | 3,521 | 3,514 | 3,368 | ||||||||||||
| Less accumulated depreciation and amortization |
* | * | (2,332 | ) | (1,678 | ) | (924 | ) | ||||||||
|
|
|
|
|
|
|
|||||||||||
| Fixed assets, net |
$5,858 | $ | 6,290 | $ | 6,516 | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| * | The estimated useful life of the leasehold improvements is the lesser of the lease term or five years. |
| ** | Amortization expense related to assets under capital lease is included in depreciation expense and accumulated depreciation. |
Total depreciation expense for the six months ended June 30, 2011 and 2010 was $654,000 and $173,000, respectively, and $754,000, $348,000 and $324,000 for the years ended December 31, 2010, 2009 and 2008, respectively.
| 4. | DRONABINOL API MANUFACTURING FACILITY |
The Company produces its clinical and commercial supply of dronabinol active pharmaceutical ingredient (“API”) in its U.S.-based wholly-owned state-of-the-art dronabinol manufacturing facility.
The Company was previously party to an Exclusive Purchase and Supply Agreement, as amended (“Exclusive Purchase and Supply Agreement”), with a third-party supplier, Austin Pharma, LLC, which provided that the Company would either advance funds to Austin Pharma to purchase equipment or, acquire by purchase or lease for this supplier’s use, the equipment necessary to produce the API in the Company’s dronabinol product candidate. As of December 31, 2008, the Company had advanced funds to Austin Pharma under the Exclusive Purchase and Supply Agreement totaling approximately $6,587,000, including accrued interest, in the form of notes receivable to purchase equipment and build out the Company’s leased dronabinol manufacturing facility. A portion of the advances was made interest free. As the Company would not ordinarily loan funds at less than prevailing market rates, the Company concluded that there were unstated rights or privileges relating to the purchase price of the API to be supplied to the Company under the Exclusive Purchase and Supply Agreement. The Company therefore imputed interest on these advances using the Company’s effective borrowing rate of 9.75%, discounted the notes receivable, and established an intangible asset of approximately $865,000 (representing the favorable purchase price which would then be amortized as the Company acquired the API product).
Upon review of the Exclusive Purchase and Supply Agreement, as well as the various promissory notes and loans made by the Company to Austin Pharma under the Exclusive Purchase and Supply Agreement, the Company determined that the provisions under ASC 810, Consolidation, regarding the consolidation of variable interest entities applied to Austin Pharma. Austin Pharma qualified as a variable interest entity and the Company had variable interests in Austin Pharma. The Company’s variable interests in Austin Pharma consisted of the promissory notes, loans to Austin Pharma for the
F-13
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
purchase of equipment, and the Exclusive Purchase and Supply Agreement. As a result of these variable interests, the Company conducted an analysis to determine if the Company was the primary beneficiary of Austin Pharma. Based on the results of the analysis conducted, Austin Pharma’s parent company was deemed to be the primary beneficiary of Austin Pharma and therefore Austin Pharma was not consolidated into the Company’s financial statements.
Austin Pharma ceased operations in late 2008 as none of the Company’s dronabinol product candidates had been approved by the U.S. Food and Drug Administration (“FDA”) at that time. Subsequently, a settlement dispute ensued between the parties over the facility operations and funding. To resolve the dispute with Austin Pharma, in September 2009, the Company entered into an agreement to purchase certain assets, including equipment, leasehold improvements and API inventory, for $1,944,000, assume the lease of the manufacturing facility and cancel repayment of all advances on the notes receivable. Approximately $1,250,000 of the purchase price was paid upon execution of the agreement; the remaining payment of $694,000 was deferred and paid in full in September 2010. Austin Pharma’s parent also agreed to forgive $1,931,000 of accounts payable owed by the Company as part of the transaction. These payables had accumulated for the production of the API. The Company had an appraisal done by a third-party valuation firm which estimated the fair value of the assets purchased, and the facility lease and other liabilities. The following table summarizes the Company’s loss as a result of the transaction:
Net assets recorded prior to settlement (in U.S. $ 000’s):
| Notes and interest receivable |
$ | 5,722 | ||
| Intangible asset |
865 | |||
| Accounts payable |
(1,931 | ) | ||
| 4,656 | ||||
| Cash paid for settlement |
1,944 | |||
| $ | 6,600 | |||
| Fair value of net assets exchanged: |
||||
| Fixed Assets |
$ | 5,296 | ||
| Inventory |
780 | |||
| Liabilities assumed |
(580 | ) | ||
| Fair value of net assets exchanged |
$ | 5,496 | ||
| Net loss recorded on settlement |
$ | (1,104 | ) | |
Based on the above, the Company recorded a net loss on settlement of $1,104,000 in the Company’s statement of operations for the year ended December 31, 2008 to reduce the carrying value of the notes, write-off the corresponding intangible asset and reduce the accounts payable owed to Austin Pharma.
F-14
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
| 5. | NOTES PAYABLE—RELATED PARTY |
The Company has issued several promissory and demand notes (“Kapoor Notes”) payable in favor of two trusts controlled by the Company’s founder, Executive Chairman and principal stockholder, The John N. Kapoor Trust (“The JNK Trust”) and the Kapoor Children 1992 Trust. The Company draws on the Kapoor Notes as needed to pay its expenses. In general, unless otherwise noted, the principal and interest are due upon maturity. The notes carry interest at the prime rate plus 2.0% (5.25% as of June 30, 2011 and December 31, 2010). The following is a summary of the outstanding Kapoor Notes:
From 2002 to June 30, 2011, the Company issued a series of promissory and demand notes payable totaling $62,655,000 in favor of The JNK Trust and the Kapoor Children 1992 Trust. The cumulative promissory notes issued through December 31, 2010 totaled $55,098,000. In 2008, the Company repaid approximately $3,141,000 of these notes. Additionally, a portion of the notes were converted into equity in 2008 and 2009—refer to Note 7. The JNK Trust also agreed to fund the Company on an as-needed basis through March 31, 2012. Approximately $379,000 remains available for borrowing as of June 30, 2011 and $299,000 remained available for borrowing as of December 31, 2010 before additional notes were issued during the first and second quarter of 2011. These notes bear interest at 5.25% as of June 30, 2011 and December 31, 2010. The principal and interest are due on demand. The outstanding principal approximated $24,944,000, $17,387,000 and $2,242,000 as of June 30, 2011 and December 31, 2010 and 2009, respectively. The Company had not repaid the principal or interest accrued on these notes as of June 30, 2011 or December 31, 2010 and they are currently payable on demand.
In connection with one of these notes issued in February of 2008, the Company issued a warrant to The JNK Trust to purchase up to an aggregate of 18,917 shares of the Company, which expired in February 2011. The fair value of this warrant was deemed to be de minimus.
The Company issued a promissory note payable for $12,300,000 in favor of The JNK Trust on October 11, 2005. This note bears interest at 5.25% as of June 30, 2011 and December 31, 2010. The principal and interest were due upon maturity, which was October 11, 2010. The Company had not repaid the principal or interest accrued on this note as of June 30, 2011 or December 31, 2010 and it is currently payable on demand.
Total interest accrued on these notes approximated $6,099,000, $5,211,000 and $4,061,000 as of June 30, 2011, December 31, 2010 and 2009, respectively. Interest expense was approximately $888,000 and $474,000 for the six months ended June 30, 2011 and 2010, respectively, and $1,150,000, $1,038,000 and $1,940,000 for the years ended December 31, 2010, 2009 and 2008, respectively.
The balance payable, including interest, was approximately $43,705,000, $34,898,000 and $18,603,000 as of June 30, 2011, December 31, 2010 and 2009, respectively.
| 6. | STOCK-BASED COMPENSATION |
The Company currently has the following stock-based incentive plans:
2006 Equity Incentive Plan
The Company’s 2006 Equity Incentive Plan (the “2006 Plan”) provides for the grant of stock awards, including stock options, restricted stock, stock appreciation rights, performance units, performance shares and other stock awards, to the Company’s employees, directors and consultants. The 2006 Plan was adopted in April 2006. In March 2011, the 2006 Plan was amended to increase the total shares
F-15
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
available for future grant to 647,540 shares under the 2006 Plan. In March 2011, the Company granted options to purchase a total of 508,491 shares of our common stock to its employees and a nonemployee consultant. These options vest monthly over a three or four year period. As of June 30, 2011, options to purchase 537,175 shares of common stock were outstanding and 110,365 shares remained available for future grant. As of December 31, 2010, options to purchase 29,686 shares of common stock were outstanding and 26,051 remained available for future grant.
Awards under the 2006 Plan generally consist of stock options that have an exercise price equal to the fair market value of the Company’s common stock on the date of grant, a 10-year term, and vest ratably over four years, subject to continuous employment. Stock awards granted to the Company’s non-employee directors under the 2006 Plan typically vest one year from the date of grant. Awards under the 2006 Plan vest immediately upon a change in control. Although the 2006 Plan provides for the issuance of performance units and performance shares, the Company has not made grants of these types of awards.
1998 Equity Incentive Plan
The Company’s 1998 Equity Incentive Plan (the “1998 Plan”) provides for the grant of stock awards, primarily stock options, to employees, directors and consultants. The 1998 Plan also permitted the grant of performance shares, performance units and bonus stock. The 1998 Plan was adopted in July 1998. Following the approval of the 2006 Plan by the Company’s stockholders in June 2006, no further awards were made under the 1998 Plan. As of June 30, 2011 and December 31, 2010, options to purchase 2,924 and 3,537 shares of common stock were outstanding, respectively, and no shares remained available for future grant because the 1998 Plan terminated in 2008.
Stock option awards under the 1998 Plan generally have an exercise price equal to the fair market value of the Company’s common stock on the date of grant, but may have been granted with an exercise price of not less than 85% of the fair market value of the Company’s common stock. Stock option awards under the 1998 Plan typically have a 10-year term and vest ratably on the first four anniversaries of the grant, subject to continuous employment. Stock awards granted to the Company’s non-employee directors under the 1998 Plan typically vest one year from the date of grant. Awards under the 1998 Plan vest immediately upon a change in control.
Insys Pharma, Inc. Amended and Restated Equity Incentive Plan
Insys Pharma, Inc.’s Amended and Restated Equity Incentive Plan (the “Plan”) provides for the grant of stock options to employees, directors and consultants to acquire Insys Pharma, Inc.’s voting and non-voting common stock. The Plan was originally adopted by Insys Pharma, Inc. in December 2002 and was amended and restated in June 2006. In connection with the merger in November 2010, all of the outstanding options granted under the Plan were assumed by the Company and were converted into options to purchase shares of the Company’s common stock at the exchange ratio set forth in the merger agreement. As of June 30, 2011 and December 31, 2010, options to purchase an aggregate of 1,079,133 shares of the Company’s common stock under the Plan were outstanding. The number of shares underlying unvested options outstanding under the Plan as of June 30, 2011 and December 31, 2010 was 28,032 and 198,048 shares, respectively. The Plan has been terminated and the Company will not grant additional equity awards under the Plan.
Option awards under the Plan are generally granted with an exercise price equal to the fair market value of Insys Pharma, Inc.’s common stock on the date of grant. Option awards under the Plan typically have a 10-year life and vest within the first two years of the grant, subject to continuous employment. Option awards granted to Insys Pharma, Inc.’s non-employee consultants under the Plan typically vest
F-16
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
within two years from the date of grant. These options are marked to market at each reporting period. The expense associated with these adjustments has historically been immaterial.
Amounts recognized in the statements of operations with respect to the Company’s stock-based compensation plans were as follows (in U.S. $ 000’s):
| Six Months Ended June 30, | Year Ended December 31, | |||||||||||||||||||
| 2011 | 2010 | 2010 | 2009 | 2008 | ||||||||||||||||
| Research and development |
$ | 113 | $ | 641 | $ | 693 | $ | (389 | ) | $ | 1,912 | |||||||||
| General and administrative |
457 | 695 | 752 | 2,839 | 5,874 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total cost of stock-based compensation plans during period |
$ | 570 | $ | 1,336 | $ | 1,445 | $ | 2,450 | $ | 7,786 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The table above also includes the compensation expense recorded in 2010 and 2008 associated with the debt to equity conversions — refer to Note 7 for further description.
The Company has never capitalized, or recognized an income tax benefit from, stock-based compensation.
The following table summarizes employee stock option awards during the six months ended June 30, 2011 and the years ended December 31, 2010, 2009 and 2008:
| No. of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (in years) |
Aggregate Intrinsic Value (in U.S. $ millions) |
|||||||||||||
| Outstanding on December 31, 2007 |
31,930 | $ | 12.81 | $ | — | |||||||||||
| Granted |
299,418 | $ | 22.57 | |||||||||||||
| Cancelled |
(50,255 | ) | $ | 19.52 | ||||||||||||
| Exercised |
— | — | ||||||||||||||
|
|
|
|||||||||||||||
| Outstanding on December 31, 2008 |
281,093 | $ | 21.96 | 9.49 | $ | — | ||||||||||
| Granted |
— | — | ||||||||||||||
| Cancelled |
(281,093 | ) | $ | 21.96 | ||||||||||||
| Exercised |
— | — | ||||||||||||||
|
|
|
|||||||||||||||
| Outstanding on December 31, 2009 |
— | — | — | $ | — | |||||||||||
| Exercisable on December 31, 2009 |
— | — | ||||||||||||||
| Granted |
1,138,804 | $ | 1.83 | |||||||||||||
| NeoPharm Merger |
33,554 | $ | 114.07 | |||||||||||||
| Cancelled |
(36,103 | ) | $ | 1.83 | ||||||||||||
| Exercised |
(23,899 | ) | $ | 1.83 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding on December 31, 2010 |
1,112,356 | $ | 4.88 | 8.54 | $ | 3.3 | ||||||||||
| Exercisable on December 31, 2010 |
914,307 | $ | 5.49 | 8.41 | $ | 2.7 | ||||||||||
| Granted |
508,491 | $ | 4.88 | |||||||||||||
| Cancelled |
(1,615 | ) | $ | 426.39 | ||||||||||||
| Exercised |
— | |||||||||||||||
|
|
|
|||||||||||||||
| Outstanding on June 30, 2011 |
1,619,232 | $ | 4.27 | 8.97 | $ | 19.4 | ||||||||||
| Exercisable on June 30, 2011 |
1,118,851 | $ | 4.27 | 8.79 | $ | 14.3 | ||||||||||
Insys Pharma’s board of directors approved approximately 35,723 options for issuance in 2010 which, pursuant to the terms of the Plan, could not be exercised and accordingly, are not reflected in the above table.
F-17
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
In connection with the one-for-1,500,000 reverse stock split on June 2, 2009, the Company cancelled all options outstanding at that time. This resulted in a reversal of stock-based compensation expense that had been previously recorded for all of the outstanding options that had not vested as of the date cancelled. The total reversal of stock-based compensation expense related to this cancellation and employee terminations resulted in negative stock-based compensation expense associated with stock options of $709,000 for the year ended December 31, 2009.
The aggregate intrinsic value for stock options outstanding and exercisable is defined as the difference between the fair market value of the Company’s common stock and the exercise price of the stock options. The estimated aggregate intrinsic value of options exercised during the year ended December 31, 2010 was approximately $39,000. As of June 30, 2011 and December 31, 2010, the unrecognized stock-based compensation costs related to the Company’s outstanding stock options expected to vest was $6.6 million and $82,000, respectively, and will be recognized over an estimated weighted average amortization period of 3.1 and 0.3 years, respectively.
Stock Option Valuation Information
The Company currently uses the Black-Scholes option pricing model to estimate the fair value of its stock-based payment awards. The determination of the fair value of stock-based payment awards utilizing the Black-Scholes model is affected by our stock price and a number of assumptions, including expected volatility, risk-free interest rate, expected term, expected dividends yield and expected forfeiture rate. Prior to the merger, the Company did not have a history of market prices of its common stock and since the merger, it does not have what it considers a sufficiently active and readily traded market for its common stock to use historical market prices for its common stock to estimate volatility. Accordingly, the Company estimates volatility in accordance with Staff Accounting Bulletin No. 107 using historical volatilities of similar public entities. The risk-free interest rate assumption is based on observed interest rates appropriate for the terms of our awards. The life of the employee awards is based on a simplified method which defines the life as the average of the contractual term of the options and the weighted average vesting period for all open tranches. The dividend yield assumption is based on the Company’s history and expectation of paying no dividends. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The weighted-average estimated fair value of employee stock options granted as calculated using the Black-Scholes model and the related weighted-average assumptions follow:
| Six Months Ended June 30, 2011 |
Year Ended December 31, | |||||
| 2010 | 2008 | |||||
| Expected volatility |
109.2% | 100.0% – 110.8% | 123.1% – 123.2% | |||
| Risk-free interest rate |
3.5% | 2.5% – 2.9% | 3.3% – 3.5% | |||
| Expected term (in years) |
6.5 – 9.7 | 5.0 – 6.0 | 6.0 | |||
| Expected dividend yield |
0.00% | 0.0% | 0.0% | |||
For the six months ended June 30, 2011 the weighted average estimated fair value per option granted was $13.90. The 508,491 options granted in March 2011 were issued with an exercise price of $4.88 per share which reflected the estimated fair value of the Company’s common stock in February 2011 prior to consideration of the initial public offering and the underlying corporate activities that supported that offering. The fair value of these options was computed based on an estimated fair value of the underlying common stock equal to $15.00 after the additional consideration related to this offering were taken into consideration. For the years ended December 31, 2010 and 2008, the weighted average estimated fair value per option granted was $1.22 and $20.13, respectively.
F-18
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
| 7. | EQUITY |
The share data presented in the balance sheet and statement of stockholders’ deficit and the share and per share data presented in the statement of operations have been retroactively adjusted to account for the a one-for-2.35 reverse stock split on January 17, 2008, a one-for-1,500,000 reverse stock split on June 2, 2009, a 1,862,623-for-one stock split on February 22, 2010, a one-for-61 reverse stock split on July 14, 2011 and the November 8, 2010 merger with NeoPharm, Inc. (see Note 10).
On December 29, 2009, debt and accrued interest payable to The JNK Trust and Dr. John N. Kapoor totaling $11,549,000 was converted into 253,414 shares of the Company’s common stock, which was based on the then existing fair market value per share of a minority, non-marketable interest in the Company. On July 25, 2008, The JNK Trust converted approximately $24,197,000 of debt and accrued interest into 38,112 shares of the Company’s common stock. On July 25, 2008, the Company sold 13,559 shares of the Company’s common stock to The JNK Trust for total proceeds of $8,609,000. Certain of these transactions were based on the then fair market value per share of a minority, non-marketable interest in the Company. Compensation expense of $3,160,000 and $3,942,000 was recognized in 2009 and 2008, respectively, for the conversions based on the difference between the fair market value per share of a 100% equity interest in the Company and the fair market value per share of the minority, non-marketable interest.
In connection with the one-for-1,500,000 reverse stock split on June 2, 2009, the Company agreed to repurchase common shares from those stockholders which were left with only fractional shares after the reverse stock split. The Company recorded a liability of $547,000 to these stockholders as of December 31, 2009 relating to this repurchase of 14,911 aggregate shares. As of June 30, 2011 and December 31, 2010, the remaining liability is approximately $508,000 and is included in “Other current liabilities” on the Company’s consolidated balance sheet.
In March 2011, total authorized shares of the Company’s common stock was increased from 50,000,000 shares to 750,000,000 shares.
| 8. | INCOME TAXES |
From inception through November 8, 2010, Insys Therapeutics, Inc. operated as a Subchapter S Corporation for income tax purposes. Losses incurred through November 7, 2010 were reported on the stockholders’ tax returns and are not available to the Company as NOLs. Since that time, losses incurred result in NOLs which can be used to offset possible future taxable income of the Company.
On November 8, 2010, Insys Therapeutics, Inc. effected a merger with NeoPharm in a transaction that was accounted for as a reverse acquisition and resulted in a change of 50% or more of the ownership of NeoPharm. As of the merger date, NeoPharm had approximately $274.0 million of federal NOLs which were scheduled to expire in tax years 2011 to 2029. Under Section 382 of the Code, the Company’s utilization of the pre-merger federal NOLs of NeoPharm to offset the Company’s post-merger federal taxable income is significantly limited due to the merger. Prior to the merger, NeoPharm had completed a partial analysis of ownership changes under Section 382 of the Code to determine if a change in control of NeoPharm had occurred. Based on NeoPharm’s partial analysis, no change in control was identified, based on the review of eight test dates covering a four-year period ended December 31, 2007. A complete formal analysis of ownership change would have to be performed in order to obtain certainty that a change in control of NeoPharm had not occurred prior to the merger, which could further limit the utilization of the NeoPharm pre-merger NOLs by the Company.
F-19
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
Based on the above, the Company has estimated the amount of pre-acquisition federal NOLs of NeoPharm that are available to offset post- merger income of the Company is limited to approximately $158,000 a year for 20 years or cumulatively $3.2 million. For state income tax purposes, the Company has approximately $274.0 million of state NOLs related to NeoPharm’s operations. The Company has placed a valuation allowance on its deferred tax assets, which include the federal and state NOLs, for it is not more likely than not that such amounts will be realized.
The Company’s federal statutory tax rate is 35.0% while its effective tax rate was 4.07% in 2010.
Effective Tax Rate Reconciliation:
| 2010 | 2009 | 2008 | ||||||||||
| U.S. statutory tax rate |
35.00 | % | 35.00 | % | 35.00 | % | ||||||
| Increase (reduction) in income taxes resulting from: |
||||||||||||
| Tax (expense)/benefit of Subchapter S status |
(31.95 | )% | (35.00 | )% | (35.00 | )% | ||||||
| Tax benefit of change in tax status |
17.34 | % | — | — | ||||||||
| Tax benefit related to merger with NeoPharm |
4.07 | % | — | — | ||||||||
| Change in valuation allowance |
(20.39 | )% | — | — | ||||||||
| Total benefit |
4.07 | % | 0 | % | 0 | % | ||||||
The tax effects of temporary differences and carryforwards that give rise to the deferred tax assets and liabilities are comprised of the following as of December 31, 2010 (in U.S. $ 000’s):
| Deferred Tax Assets: |
||||
| NOLs |
$ | 21,147 | ||
| Start-up expenditures |
3,506 | |||
| Stock based compensation |
618 | |||
| Expenses not currently deductible for tax purposes |
259 | |||
| Gross deferred tax assets |
25,530 | |||
| Deferred tax asset valuation allowance |
(23,102 | ) | ||
| Net deferred tax asset |
2,428 | |||
| Deferred Tax Liabilities: |
||||
| In-process research and development |
(2,242 | ) | ||
| Property and equipment |
(186 | ) | ||
| Net deferred tax assets |
$ | — | ||
The valuation allowance increased from $0 to $23.1 million during the year ended December 31, 2010. In assessing the realization of deferred tax assets, the Company considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. The Company also considers the scheduled reversal of deferred tax liabilities, projected future taxable income or losses, and tax planning strategies in making this assessment. Based upon the Company’s history of tax losses and projections for future taxable income over the periods in which the deferred tax assets are deductible, the Company does not believe realization of these tax assets is more likely than not. As such, a full valuation allowance for the deferred tax assets has been established.
F-20
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
As of June 30, 2011 and December 31, 2010, 2009 and 2008, the Company has not recorded any reserves for uncertain tax positions and has not recorded any interest and penalties. It is the Company’s policy to recognize interest and penalties related to unrecognized tax benefits as income tax expense. The Company’s tax years subsequent to 2006 remain open to examination by federal and state taxing authorities. In addition, NeoPharm’s pre-merger NOLs remain open to examination.
| 9. | COMMITMENTS |
Lease Commitments
The Company leases facilities under non-cancelable operating lease agreements. Future minimum commitments for these operating leases in place as of June 30, 2011, with a remaining non-cancelable lease term in excess of one year, are as follows (in U.S. $ 000’s):
| Year Ended December 31, |
Amount | |||
| 2011 (six months) |
$ | 346 | ||
| 2012 |
642 | |||
| 2013 |
467 | |||
| 2014 |
475 | |||
| 2015 |
255 | |||
| Thereafter |
200 | |||
|
|
|
|||
| Total |
$ | 2,385 | ||
|
|
|
|||
Future minimum commitments for these operating leases as of December 31, 2010 were as follows (in U.S. $000’s):
| Year Ended December 31, |
Amount | |||
| 2011 |
$ | 731 | ||
| 2012 |
725 | |||
| 2013 |
467 | |||
| 2014 |
475 | |||
| 2015 |
255 | |||
| Thereafter |
200 | |||
|
|
|
|||
| Total |
$ | 2,853 | ||
|
|
|
|||
Dr. John N. Kapoor, the Company’s Executive Chairman and principal stockholder, guarantees the lease commitments under one of these operating leases totaling $968,000 and $1,035,000 as of June 30, 2011 and December 31, 2010, respectively.
The terms of certain lease agreements provide for rental payments on a graduated basis. The Company recognizes rent expense on the straight-line basis over the lease period and has accrued for rent expense incurred but not paid. Rent expense under operating leases for the six months ended June 30, 2011 and 2010 was approximately $324,000 and $163,000, respectively, and for the years ended December 31, 2010, 2009, 2008 was approximately $377,000, $237,000 and $208,000, respectively.
Defined Contribution Retirement Plan (401(k) Plan)
The Company sponsors a 401(k) plan covering all full-time employees. Participants may contribute up to the legal limit. The 401(k) plan provides for employee contributions, but the Company does not make any matching contributions.
F-21
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
Contractual Commitments
Purchase and Supply Agreements
(1) DPT Lakewood, Inc. (“DPT”) – The Company has an agreement with DPT for the completion of work related to the Subsys clinical trial. The remaining estimated contractual obligation is approximately $210,000 as of June 30, 2011 and was approximately $300,000 as of December 31, 2010. DPT is the Company’s contractor which manufactures and packages Subsys. In May 2011, the Company entered into a purchase and supply agreement with DPT for the commercial manufacturing of Subsys. The agreement has an initial term continuing until December 31st of the fifth year in which DPT first manufactures Subsys, followed by automatic 24-month renewal periods. Under the terms of the agreement, the Company is obligated to purchase a minimum annual quantity from DPT. Estimated minimum purchase obligations to DPT are $8,900,000 over the course of the initial term of the agreement.
(2) Aptar Pharma/Pfeiffer of America (“Aptar”) – The Company has an agreement with Aptar for the completion of work related to the Subsys trial. The remaining estimated contractual obligation is approximately $300,000 as of June 30, 2011 and was approximately $368,000 as of December 31, 2010. Aptar is the Company’s contractor which manufactures Subsys. In May 2011, the Company entered into a purchase and supply agreement with Aptar for the commercial manufacturing of the Subsys device. Under the terms of the agreement, the Company is obligated to pay an upfront fee to Aptar during the year following the commercial launch of Subsys.
(3) Catalent Pharma Solutions (“Catalent”) – The Company has an agreement with Catalent for the manufacturing of validation batches for the Dronabinol SG Capsule product candidate. The remaining estimated contractual obligation to be paid is approximately $560,000 as of June 30, 2011 and was approximately $1,108,000 as of December 31, 2010.
(4) Mylan Pharmaceuticals Inc. (“Mylan”) – In May 2011, the Company entered into a supply and distribution agreement with Mylan for the commercial sale within a defined geographic area of the Dronabinol SG Capsule product candidate. The agreement has a term commencing upon the first commercial sale of the Dronabinol SG Capsule product candidate with automatic renewals following the initial term. Under the terms of the agreement, the Company is obligated to pay Mylan a royalty between 10% and 20% on Mylan’s net product sales, and a single digit percentage fee on such sales for distribution and storage services. The Company’s minimum obligation to Mylan for royalties and fees related to the distribution and storage of the Dronabinol SG Capsule is estimated to be $608,000 over the course of the initial term of the agreement.
Clinical Trial and Research Agreements
(1) Omnicare Clinical Research (“Omnicare”) – The Company entered into an agreement with Omnicare for the safety and efficacy studies and trials of Subsys. As of June 30, 2011, the estimated contractual obligation to be paid to Omnicare is zero and was approximately $100,000 as of December 31, 2010.
(2) Excel Life Sciences – The Company has an agreement with Excel Life Sciences for the safety and efficacy studies and trials of the Company’s LEP-ETU product candidate. As of June 30, 2011, the estimated contractual obligation to be paid to Excel Life Sciences is approximately $74,000 and was approximately $221,000 as of December 31, 2010.
F-22
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
(3) University of Plymouth – The Company entered into an agreement with the University of Plymouth in relation to the supply of the Company’s Dronabinol SG Capsule product candidate for clinical research studies in the treatment of human subjects with multiple sclerosis. Although the agreement can be terminated at any time by either party, the Company expects that the study will be completed in 2012. There is no payment due to the University as of June 30, 2011 or December 31, 2010.
| 10. | NEOPHARM MERGER |
As described in Note 1, on November 8, 2010, the Company completed a merger with NeoPharm that was accounted for as a reverse acquisition under the provisions of ASC 805, Business Combinations. Pursuant to the merger agreement, all of the outstanding common stock of Insys Therapeutics prior to the merger was exchanged for 319,667 shares of NeoPharm common stock and 14,864,607 shares of newly-created NeoPharm convertible preferred stock. The convertible preferred stock is convertible into common stock on a one-to-0.57 basis and, until converted, will be entitled to the voting and dividend rights of the same number of shares of common stock into which it is convertible. For presentation purposes in these financial statements, it was assumed that the convertible preferred stock was issued in this ratio in conjunction with each issuance of common stock for the period from inception through the date of the merger. Immediately following the transaction, the former NeoPharm stockholders owned five percent of the combined entity on an as-converted basis. The deemed purchase price of NeoPharm was $2.3 million, equal to the estimated fair value of the NeoPharm common shares that the prior NeoPharm stockholders retained in the merger. This estimated fair value of NeoPharm common shares was derived from a valuation that was conducted for the post-merger combined entity that resulted in a combined entity fair value price per common share of $4.88. This combined entity fair value price per common share was then applied to the 464,353 shares of NeoPharm common stock outstanding at the time of the merger.
As additional consideration, the NeoPharm board approved the distribution, immediately after the merger, of non-transferable contingent payment rights to its stockholders of record as of November 5, 2010. These rights entitle the pre-merger stockholders of NeoPharm to receive cash payments aggregating $20.0 million (equivalent to $0.70402 per share) if, prior to the five year anniversary of the merger, the FDA approves a new drug application for any one or more of the NeoPharm product candidates that were under development at the time of the merger. The distribution is payable within nine months of FDA approval. The fair value of this contingent payment was determined to be approximately $1.8 million based on the assumed probability of any payment being made to the prior NeoPharm stockholders in 2015, discounted to present value at a rate of 15% and such accretion was $159,000 in the six months ended June 30, 2011. Any subsequent changes in the estimated fair value of this contingent consideration will be recorded in the Statement of Operations.
F-23
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
The assets acquired and liabilities assumed in the merger are as follows (in U.S. $ 000’s):
| Amount | ||||
| Cash |
$ | 143 | ||
| Prepaid and other current assets |
429 | |||
| Fixed assets |
144 | |||
| Other assets |
371 | |||
| Identifiable intangible assets |
5,300 | |||
| Goodwill |
103 | |||
|
|
|
|||
| Total assets acquired |
$ | 6,490 | ||
|
|
|
|||
| Accounts payable and accrued expenses |
$ | (1,693 | ) | |
| Unfavorable lease liability |
(120 | ) | ||
| Deferred tax liability |
(575 | ) | ||
| Contingent liability to NeoPharm stockholders |
(1,829 | ) | ||
|
|
|
|||
| Total liabilities acquired |
$ | (4,217 | ) | |
|
|
|
|||
| Net assets acquired |
$ | 2,273 | ||
|
|
|
|||
The Company has assessed the fair values of the acquired assets and assumed liabilities and allocated the purchase price accordingly. This valuation resulted in the recording of in-process research and development (“IPR&D”) as an intangible long-lived asset in the amount of $5.3 million, of which $4.2 million related to LEP-ETU and $1.1 million related to IL-13.
LEP-ETU is a liposomal formulation of the widely used cancer drug, paclitaxel. At the time of the merger, a Phase 2 clinical trial for LEP-ETU was ongoing in India and approximately 75% of the patients had been enrolled for that trial. The overseas location of this trial created certain inherent logistical and management challenges for the Company. In order to complete this study, the Company needed to fully enroll the trial, have the data captured and analyzed, and have a final study report written. As of June 30, 2011, the trial was completely enrolled and related data were being captured and analyzed. The Company anticipates having a final study report by around the third quarter of 2011. The estimated additional costs the Company will incur to complete this study are approximately $150,000. Given that the final study report is yet to be evaluated, the Company is not certain if the study will yield a positive result on the efficacy analysis or if any safety issues will be identified in the final analysis.
IL-13 is a potential therapeutic agent for the treatment of idiopathic pulmonary fibrosis (“IPF”) and asthma. Prior to the merger, an IND was submitted by NeoPharm for a Phase 1 study in IPF, and that submission was on hold by the FDA at the time of the merger and remains on hold as of June 30, 2011. IL-13 was also granted an orphan drug designation for this indication. All work done at the time of the merger was preclinical and no human clinical trials had been performed. The complexity and uniqueness of this project is quite extensive since the agent is a combination of a protein and an endotoxin and must be maintained at the right temperature in a solution. The planned delivery system for the agent is a nebulizer which is still in a state of refinement. Additionally, there are Chemistry, Manufacturing and Control (“CMC”) challenges that must be overcome in order to have a commercially viable product. A significant amount of additional work remains on this indication and the chances of success at this point for a commercially viable product using the agent are low at this point in time. The next step the Company will need to undertake with respect to IL-13 is to get the clinical hold released by working with a different type of nebulizer that will allow more drug product to be delivered via an
F-24
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
inhaled pathway. The anticipated timeline for getting this hold potentially released is in 2012. In parallel, the Company will be working with animal models to determine the feasibility of IL-13 working effectively in humans.
The fair value of the IPR&D was determined primarily through the use of the cost approach. The cost approach relies on historical costs incurred adjusted for estimated wasted efforts and taxes. A deferred tax liability of approximately $575,000 was generated as a result of purchase accounting at the merger date. Accordingly, the Company released $575,000 of the valuation allowance on its deferred tax assets which created an income tax benefit as of the date of the merger and offsets this deferred tax liability. The fair value of the contingent consideration of approximately $1.8 million was determined based on the estimated probability of any payment being made. Goodwill resulting from the merger is not tax deductible.
The following table contains unaudited condensed consolidated pro forma results of operations for the six months ended June 30, 2010 and the years ended December 31, 2010 and 2009, giving retroactive effect to the merger as if it closed on January 1, 2009 (in U.S. $ 000’s, except share and per share amounts):
| Six
Months Ended June 30, 2010 |
Year Ended December 31, | |||||||||||
| 2010 | 2009 | |||||||||||
| Net revenues |
$ | — | $ | — | $ | — | ||||||
| Net loss |
$ | (10,145 | ) | $ | (18,070 | ) | $ | (21,915 | ) | |||
| Basic and diluted net loss per common share |
$ | (1.14 | ) | $ | (1.83 | ) | $ | (1.22 | ) | |||
| Weighted average common shares outstanding, basic and diluted |
785,385 | 787,413 | 541,257 | |||||||||
The pro forma disclosures in the table above include adjustments to reflect results that are more representative of the combined results of the transaction if they had occurred on January 1, 2009. The pro forma results include the operating results of NeoPharm for applicable periods prior to the merger, adjusted for amortization of unfavorable lease liability established in purchase accounting and the elimination of direct merger costs and accelerated stock-based compensation. Additionally, the tax benefit recorded by the Company as a result of the merger is also eliminated from the pro forma results.
This pro forma information is presented for illustrative purposes only and may not be indicative of the results of operation that would have actually occurred. In addition, future results may vary significantly from the results reflected in the pro forma information.
| 11. | FAIR VALUE MEASUREMENT |
In September 2006, the FASB issued new guidance now codified as ASC 820, Fair Value Measurements and Disclosures. The new guidance defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles in the U.S. (“U.S. GAAP”), and expands disclosures about fair value measurements and was adopted by the Company in 2008. In February 2008, the FASB issued new guidance now codified in ASC 820 which delays the effective date for non-financial assets and liabilities that are not measured or disclosed on a recurring basis to fiscal years beginning after November 15, 2008 and was adopted by the Company in 2009.
F-25
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
The standard describes three levels of inputs that may be used to measure fair value:
Level 1 - Quoted prices in active markets for identical assets or liabilities.
Level 2 - Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 - Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The Company utilized a cost approach to value NeoPharm’s IPR&D since the IPR&D, which is currently in development, has not yet been proven in the marketplace. The to-date expenses on the acquired IPR&D were inflation adjusted from the year in which they were incurred to bring such expenses to a price level as of the valuation date. In the aggregate, this resulted in a total inflation adjustment to the acquired IPR&D of $692,000. The inflation adjusted to-date expenses were then adjusted for estimated wasted efforts and overhead expenses incurred for each of the respective projects. The estimated wasted efforts (40% of such expenses for LEP-ETU and 20% for IL-13) were based on management’s evaluation of the design and execution of NeoPharm’s clinical and pre-clinical studies for each of the respective projects. On this balance price level adjusted expense, an additional 15% was added for overhead expenses. The adjustment made for estimated overhead expenses of 15% was based on NeoPharm’s actual overhead expenses relative to direct project costs for the year prior to the Merger. No additional adjustment for opportunity cost was applicable given the nature of the asset and high speculation on commercialization time frames. Consequently, the IPR&D is considered a Level 3 measurement. To determine the expected value of the contingent rights and payment obligation, the Company probability-weighted (17.5%) the likelihood of the contingent consideration payment (which would occur in mid 2015 if it were to take place) and discounted the weighted payment to present value. The contingent rights and payment obligation is considered a Level 3 measurement and relate to the product candidates LEP-ETU, LE-DT and IL-13.
| 12. | LEGAL MATTERS |
In September 2009, Insys Pharma and certain of its officers and directors, as well as their spouses, were named as defendants in a lawsuit in Arizona Superior Court brought by Santosh Kottayil, Ph.D., certain of his family members and a trust of which Dr. Kottayil is the trustee. Dr. Kottayil formerly served as President, Chief Scientific Officer and a director of Insys Pharma, among other positions. The complaint brought a cause of action for statutory and common law appraisal of Dr. Kottayil’s Insys Pharma common stock. The cause of action for appraisal relates to a one-for-1,500,000 reverse stock split that Insys Pharma effected in June 2009, which resulted in Dr. Kottayil’s ownership position becoming a fractional share of Insys Pharma common stock. Following the reverse stock split, Insys Pharma cancelled all resulting fractional shares, including the fractional share held by Dr. Kottayil, and offered a cash payment in lieu of the fractional shares. The complaint also states causes of action for breach of fiduciary duty and negligent misrepresentation in the defendants’ dealings with Dr. Kottayil on the subject of his compensation and stock ownership in Insys Pharma. In January 2010, the plaintiffs
F-26
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
added claims seeking to rescind Dr. Kottayil’s assignment to Insys Pharma of his interest in all of the fentanyl and dronabinol patent applications the Company owns and to recover the benefits of those interests. Dr. Kottayil is seeking, among other relief, the fair value of his Insys Pharma common stock as of June 2, 2009, compensatory and punitive damages, and rescission of all assignments to Insys Pharma of his interest in the patent applications, as well as attorneys’ fees, costs and interest.
In February 2010, Insys Pharma and the other defendants answered and filed counter-claims to Dr. Kottayil’s amended complaint. The counter-claims include actions for breach of fiduciary duty, fraud and negligence with respect to the time during which Dr. Kottayil was employed at Insys Pharma. The counter-claims, among other relief, seek compensatory and punitive damages. The Company does not expect a trial of this action to take place until at least 2012, although an earlier date is possible. The Company is not able at this time to estimate the range of potential loss or any potential recovery from the counter-claims, nor is it able to predict the outcome of this litigation. If the patent assignments are successfully rescinded, the Company will not have exclusive patent rights covering its fentanyl and dronabinol product candidates, and such exclusive patent rights may not be available to the Company on acceptable terms, if at all, which would have a material adverse effect on the Company’s business. If the assignments are rescinded, Dr. Kottayil could assign his interest in the fentanyl and dronabinol patent applications to a competitor and the Company would not be able to prevent generic copies of its products. The Company intends to vigorously defend against the plaintiffs’ claims and pursue its counter-claims.
Insys Pharma has received letters from the counsel of Solvay Pharmaceuticals and Unimed Pharmaceuticals (who market and sell the branded version of dronabinol, Marinol) asserting that one of Insys Pharma’s founders may have utilized their confidential and proprietary information for the abbreviated new drug application of dronabinol. Solvay and Unimed are requesting various information and written assurances from Insys Pharma related to the ANDA of dronabinol. The matter has been handled out of court thus far and the Company intends to vigorously defend each and every claim and request made in the letters if the complaint escalates. Management is unable to estimate the potential outcome or range of possible loss, if any. Any such litigation could be protracted, expensive, and potentially subject the Company to an unfavorable outcome.
In 2001, NeoPharm and certain of its former officers were named in a complaint, which alleged various violations of the federal securities laws in connection with NeoPharm’s public statements regarding its LEP-ETU product candidate during the period from October 31, 2001 through April 19, 2002. In November 2002, the Company moved to have the complaint dismissed. This motion to dismiss was granted in part and denied in part in February 2003. In November 2004, the plaintiffs filed a motion to amend and a motion for summary adjudication. The motion to amend sought to include certain pre-class period statements in the complaint. The motion for summary adjudication asked the Court to rule that certain statements made in an arbitration award regarding the LEP-ETU product candidate be deemed facts established in this proceeding. In February 2007, the Court entered an order denying both the plaintiffs’ motion to amend and the plaintiffs’ motion for summary adjudication. Fact and expert discovery is closed. In March 2008, the dispositive motion filing deadline, NeoPharm filed a motion for summary judgment. On March 31, 2010, the Court granted in part and denied in part the Company’s motion for summary judgment. The Court dismissed the plaintiffs’ claims based on statements made before January 14, 2002 but held that there was a genuine issue of material fact as to whether the Company could be liable for statements made between January 14, 2002 and April 19, 2002. On April 27, 2010, the Court set a trial date of February 22, 2011 and also set a settlement conference date of July 27, 2010. On October 25, 2010, the parties entered into a Stipulation of Settlement which set forth the terms and conditions for a proposed settlement of the litigation and for dismissal of the litigation with
F-27
Table of Contents
INSYS THERAPEUTICS, INC.
(A Development-Stage Company)
NOTES TO FINANCIAL STATEMENTS — (Continued)
prejudice. The parties have agreed to settle the litigation for $3,350,000 in cash to be distributed to eligible class members of the plaintiff. At the settlement hearing on March 17, 2011, the Court gave final approval of the settlement, which was paid by the Company’s insurers. None of NeoPharm’s current directors or officers were named in this complaint.
| 13. | SUBSEQUENT EVENTS |
The Company evaluated subsequent events through March 29, 2011, the date the annual financial statements were originally filed with the SEC and re-evaluated events through August 16, 2011, the date the interim financial statements were originally filed with the SEC.
Initial Public Offering
On July 15, 2011, the Company filed an amended registration statement with the SEC in response to comments received from the SEC pertaining to the original registration statement that was filed with the SEC in March 2011 relating to an initial public offering of its common stock. There can be no assurance the Company will be successful in this offering.
Subsequent Funding
During the third quarter of 2011, the Company borrowed the remaining amounts available under the 2010 JNK Trust notes of $379,000. The Company also issued an additional $1.0 million demand note payable in favor of The JNK Trust from which the Company has borrowed an aggregate of $734,000 as of August 15, 2011.
2011 Equity Plans
In August 2011, the Company’s board of directors adopted and its stockholders approved the Company’s 2011 Equity Incentive Plan, 2011 Non-Employee Directors’ Stock Award Plan and the 2011 Employee Stock Purchase Plan, all of which will become effective immediately upon the signing of the underwriting agreement pursuant to the Company’s initial public offering of its common stock.
The 2011 Equity Incentive Plan provides for the discretionary grant of incentive stock options and other stock awards and forms of equity compensation to the Company’s employees and consultants. There will initially be an aggregate of 2,540,102 shares of the Company’s common stock available for issuance under this plan.
The 2011 Employee Stock Purchase Plan provides for the discounted purchase of the Company’s common stock by employees of the Company and its designated affiliates. There will initially be an aggregate of 650,000 shares of the Company’s common stock available for issuance under this plan.
The 2011 Non-Employee Directors’ Stock Award Plan provides for the automatic grant of nonstatutory stock options and other discretionary stock awards to the Company’s non-employee directors. There will initially be an aggregate of 350,000 shares of the Company’s common stock available for issuance under this plan.
Reverse Stock Split
On July 14, 2011, the Company effected a one-for-61 reverse stock split of its common stock. The accompanying financial statements and notes to the financial statements give retroactive effect to the reverse stock split for all periods presented.
F-28
Table of Contents
The Board of Directors and Stockholders
NeoPharm, Inc.
Lake Bluff, Illinois
We have audited the accompanying consolidated balance sheets of NeoPharm, Inc. as of December 31, 2009 and 2008 and the related consolidated statements of operations, stockholders’ equity, and cash flows for the years then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of NeoPharm, Inc. at December 31, 2009 and 2008 and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations and has not secured additional financing to further the development of its drug product candidates which raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ BDO USA, LLP
Chicago, Illinois
July 9, 2010
F-29
Table of Contents
Consolidated Balance Sheets
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Assets |
||||||||
| Current Assets |
||||||||
| Cash and cash equivalents |
$ | 5,042,000 | $ | 7,298,000 | ||||
| Auction rate securities |
12,393,000 | — | ||||||
| Put option on auction rate securities |
1,557,000 | — | ||||||
| Prepaid expenses and other current assets |
310,000 | 338,000 | ||||||
| Total Current Assets |
19,302,000 | 7,636,000 | ||||||
| Fixed assets, net of accumulated depreciation |
250,000 | 366,000 | ||||||
| Auction rate securities |
— | 12,321,000 | ||||||
| Put option on auction rate securities |
— | 2,379,000 | ||||||
| Other assets |
650,000 | 651,000 | ||||||
| Total Assets |
$ | 20,202,000 | $ | 23,353,000 | ||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current Liabilities |
||||||||
| Current maturities of long-term debt |
$ | 13,950,000 | $ | — | ||||
| Interest expense payable |
61,000 | |||||||
| Accounts payable |
67,000 | 222,000 | ||||||
| Accrued clinical trial expenses |
854,000 | 1,438,000 | ||||||
| Accrued compensation |
583,000 | 796,000 | ||||||
| Accrued research and development |
368,000 | 163,000 | ||||||
| Accrued manufacturing expenses |
61,000 | 739,000 | ||||||
| Current maturities of capital lease obligations |
36,000 | 34,000 | ||||||
| Other accrued expenses |
99,000 | 150,000 | ||||||
| Total Current Liabilities |
16,079,000 | 3,542,000 | ||||||
| Long-Term Debt, less current maturities (Note 6) |
— | 9,201,000 | ||||||
| Deferred Rent |
36,000 | 18,000 | ||||||
| Capital Lease Obligations |
3,000 | 40,000 | ||||||
| Total Liabilities |
16,118,000 | 12,801,000 | ||||||
| Commitments and Other Matters |
||||||||
| Stockholders’ Equity |
||||||||
| Preferred stock, $.01 par value; 15,000,000 shares authorized, none issued and outstanding |
— | — | ||||||
| Common stock, $.0002145 par value; 50,000,000 shares authorized, 28,498,814 and 28,497,049 shares issued and outstanding, respectively |
6,000 | 6,000 | ||||||
| Additional paid-in capital |
291,256,000 | 290,998,000 | ||||||
| Accumulated other comprehensive income |
735,000 | — | ||||||
| Accumulated deficit |
(287,913,000 | ) | (280,452,000 | ) | ||||
| Total Stockholders’ Equity |
4,084,000 | 10,552,000 | ||||||
| Total Liabilities and Stockholders’ Equity |
$ | 20,202,000 | $ | 23,353,000 | ||||
See accompanying notes to consolidated financial statements.
F-30
Table of Contents
Consolidated Statements of Operations
| Year ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| Revenue |
$ | — | $ | — | ||||
| Operating Expenses |
||||||||
| Research and development |
3,598,000 | 4,827,000 | ||||||
| Selling, general, and administrative |
2,911,000 | 4,315,000 | ||||||
| Employee termination costs |
303,000 | — | ||||||
| Facility consolidation costs |
— | (63,000 | ) | |||||
| Gain on sale of equipment and furniture |
(21,000 | ) | (350,000 | ) | ||||
| Total operating expenses |
6,791,000 | 8,729,000 | ||||||
| Operating Loss |
(6,791,000 | ) | (8,729,000 | ) | ||||
| Unrealized loss on auction rate securities put option |
(735,000 | ) | — | |||||
| Interest income |
217,000 | 596,000 | ||||||
| Interest expense |
(152,000 | ) | (85,000 | ) | ||||
| Net Loss |
$ | (7,461,000 | ) | $ | (8,218,000 | ) | ||
| Net Loss Per Share, basic and diluted |
$ | (0.26 | ) | $ | (0.29 | ) | ||
| Weighted Average Shares Outstanding, basic and diluted |
28,498,814 | 28,492,543 | ||||||
See accompanying notes to consolidated financial statements.
F-31
Table of Contents
Consolidated Statements of Stockholders’ Equity
| Shares of Common Stock |
Par Value |
Additional Paid-in Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Income |
Total Stockholders’ Equity |
|||||||||||||||||||
| Balance, at January 1, 2008 |
28,488,550 | $ | 6,000 | $ | 290,481,000 | $ | (272,234,000 | ) | $ | — | $ | 18,253,000 | ||||||||||||
| Stock-based compensation |
— | — | 317,000 | — | — | 317,000 | ||||||||||||||||||
| Issuance and compensation associated with restricted common stock |
— | — | 197,000 | — | — | 197,000 | ||||||||||||||||||
| Issuance and common stock to employees stock purchase plan |
8,499 | — | 3,000 | — | — | 3,000 | ||||||||||||||||||
| Net loss |
— | — | — | (8,218,000 | ) | — | (8,218,000 | ) | ||||||||||||||||
| Balance, at December 31, 2008 |
28,497,049 | 6,000 | 290,998,000 | (280,452,000 | ) | — | 10,552,000 | |||||||||||||||||
| Stock-based compensation |
— | — | 234,000 | — | — | 234,000 | ||||||||||||||||||
| Issuance and compensation associated with restricted common stock |
— | — | 24,000 | — | — | 24,000 | ||||||||||||||||||
| Issuance and common stock to employees stock purchase plan |
1,765 | — | — | — | — | — | ||||||||||||||||||
| Unrealized gain on investments in auction rate securities |
— | — | — | — | 735,000 | 735,000 | ||||||||||||||||||
| Net loss |
— | — | — | (7,461,000 | ) | — | (7,461,000 | ) | ||||||||||||||||
| Balance, at December 31, 2009 |
28,498,814 | $ | 6,000 | $ | 291,256,000 | $ | (287,913,000 | ) | $ | 735,000 | $ | 4,084,000 | ||||||||||||
See accompanying notes to consolidated financial statements.
F-32
Table of Contents
Consolidated Statements of Cash Flows
| Year ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| Cash flows from operating activities |
||||||||
| Net loss |
$ | (7,461,000 | ) | $ | (8,218,000 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities |
||||||||
| Depreciation and amortization |
135,000 | 174,000 | ||||||
| Stock-based compensation expense |
258,000 | 514,000 | ||||||
| Gain on sale of equipment and furniture |
— | (18,000 | ) | |||||
| Unrealized loss on auction rate securities put option |
735,000 | — | ||||||
| Changes in assets and liabilities |
||||||||
| Interest receivable on auction rate securities |
— | 57,000 | ||||||
| Prepaid expenses and other assets |
29,000 | 87,000 | ||||||
| Accounts payable |
(155,000 | ) | (362,000 | ) | ||||
| Other current liabilities |
(1,259,000 | ) | (2,000 | ) | ||||
| Deferred rent |
18,000 | — | ||||||
| Net cash used in operating activities |
(7,700,000 | ) | (7,768,000 | ) | ||||
| Cash flows from investing activities |
||||||||
| Proceeds from sales of marketable securities |
750,000 | 5,003,000 | ||||||
| Purchase of equipment and furniture |
(19,000 | ) | (145,000 | ) | ||||
| Proceeds from sales of equipment and furniture |
— | 19,000 | ||||||
| Net cash provided by investing activities |
731,000 | 4,877,000 | ||||||
| Cash flows from financing activities |
||||||||
| Proceeds from employee stock purchase plan |
— | 3,000 | ||||||
| Proceeds from borrowings, net |
4,750,000 | 9,200,000 | ||||||
| Repayment of capital lease obligation |
(37,000 | ) | (36,000 | ) | ||||
| Net cash provided by financing activities |
4,713,000 | 9,167,000 | ||||||
| Net (Decrease) Increase in Cash and Cash Equivalents |
(2,256,000 | ) | 6,276,000 | |||||
| Cash and Cash Equivalents, at beginning of year |
7,298,000 | 1,022,000 | ||||||
| Cash and Cash Equivalents, at end of year |
$ | 5,042,000 | $ | 7,298,000 | ||||
See accompanying notes to consolidated financial statements.
F-33
Table of Contents
Notes to Consolidated Financial Statements
| 1. | Nature of Operations and Principal Accounting Policies |
Nature of Operations
NeoPharm, Inc. (“we”, “us,” “our”, or the “Company”), a Delaware corporation, was incorporated on June 15, 1990, under the name of OncoMed, Inc. In March 1995, we changed our name to NeoPharm, Inc. During 2004, we established a wholly-owned subsidiary, NeoPharm EU Limited, to comply with regulatory requirements enacted for clinical trials conducted in the European Union. All of our assets are located in the United States.
We are a biopharmaceutical company engaged in the research, development and commercialization of drugs for the treatment of various cancers and other diseases. Our corporate office and research and development facility is located in Lake Bluff, Illinois and we had 17 active employees as of December 31, 2009.
Basis of Presentation
The consolidated financial statements include the accounts of NeoPharm, Inc. and its wholly owned subsidiary, NeoPharm EU Limited, and have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). As of and through December 31, 2009, the subsidiary had nominal assets and had not conducted any business. All significant intercompany accounts and transactions are eliminated in consolidation.
Amounts presented have been rounded to the nearest thousand.
The accompanying financial statements have been prepared in conformity with U.S. GAAP, which contemplates continuation of the Company as a going concern. Although the Company has not yet been able to secure any additional financing to further the development of its drug product candidates, management is currently involved in discussions to secure additional financing. The Company has the ability to decelerate spending in order to maximize its cash balance until financing is secured. However, there is no assurance that the Company will be successful in these efforts and therefore, there is substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that might be necessary in the event the Company cannot continue in existence.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expense during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
We consider all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. Included with cash are cash equivalents of $182,000 and $7.1 million as of December 31, 2009 and 2008, respectively. The carrying value of these investments approximates their fair market value due to their short maturity and liquidity.
The Company has cash in excess of federally insured limits, however, it does not believe it is exposed to any significant credit risk.
F-34
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
Auction Rate Securities and Put Option on Auction Rate Securities
Investments in auction rate securities have scheduled maturities greater than 90 days at the time of purchase, are classified as available-for-sale securities and are recorded at fair value (see Note 5 below). Temporary changes in the estimated fair market value of the securities are recorded through other comprehensive income. Other-than-temporary changes are recorded in operations.
The Company adopted guidance within Accounting Standards Codification (“ASC”) No. 825, “Financial Instruments,” in 2008 for the put option on auction rate securities. Accordingly, the put option is recorded at fair value and marked to market at each reporting period. All changes in the estimated fair market value of the put option are recorded in operations.
Refer to Note 4 for further discussion regarding the Company’s auction rate securities and put option on auction rate securities.
Fixed Assets
Fixed assets are recorded at cost and depreciated using the straight-line method over their estimated useful lives. Maintenance and repairs that do not extend the life of assets are charged to expense when incurred. When properties are disposed of, the related costs and accumulated depreciation are removed from the accounts and any gain or loss is reported in the period the transaction takes place. Total depreciation expense for the years ended December 31, 2009 and 2008, was $135,000 and $174,000, respectively.
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted cash flows expected to be generated by the asset. If the carrying amount exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount exceeds the fair value of the asset.
Other Assets
Other assets as of December 31, 2009 and 2008 consist of cash held by banks as collateral for two standby letters of credit issued by the bank in favor of the Company. The cash held by the banks is restricted as to use for the term of the standby letter of credit. One letter of credit for $175,000 is for the capitalized lease on office equipment and the other letter of credit for $470,000 is for the lease on the facility into which the Company has moved in the first quarter of 2009.
Accrued Compensation
At December 31, 2009, accrued compensation consists of accrued severance of $253,000, accrued bonuses and related payroll taxes of $218,000, accrued 401(k) expenses of $68,000 and other accrued compensation of $44,000. At December 31, 2008, accrued compensation consisted of accrued severance of $374,000, accrued bonuses of $305,000, accrued 401(k) expenses of $68,000 and other accrued compensation of $49,000. The accrued severance liability as of December 31, 2008 was satisfied with the payment of $350,000 in March 2009.
Income Taxes
We account for income taxes under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the
F-35
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
financial statement carrying amounts of existing assets and liabilities and their respective tax basis and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. We provide valuation allowances against the deferred tax asset for amounts which are not considered “more likely than not” to be realized.
Revenue Recognition
We recognize revenue when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. The Company recognized no revenue in 2009 or 2008.
Research and Development
Research and development, or R&D, costs are expensed when incurred. These costs include, among other things, consulting fees and costs reimbursed to third parties under license and research agreements described in Note 11, Commitments. Payments related to the acquisition of technology rights, for which development work is in process, are expensed as incurred and are considered a component of R&D costs. We charge the indirect cost of administering R&D activities to R&D expense.
Stock-Based Compensation
The Company has a stock-based employee compensation plan that covers the Company’s employees and is more fully described in Note 3. The Company measures compensation expense under the plan based on the estimated fair value of the awards on the grant dates and amortizes the expense over the options’ vesting periods.
Leases
The Company accounts for scheduled rent increase provisions in lease agreements by recognizing rent expense on a straight-line basis over the lease term. Our capital lease is accounted for under the provision of ASC 840, Leases.
Contingencies
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, and penalties and other sources are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated. Recoveries from other parties are recorded when realized.
Fair Value of Financial Instruments
Financial instruments consist of cash, cash equivalents, short-term investments, accounts receivable, auction rate securities and accounts payable (see Note 5 below). The carrying value of these financial instruments is a reasonable estimate of fair value.
F-36
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
Recently Issued Accounting Pronouncements
Accounting Standards Codifications
In June 2009, the FASB issued SFAS No. 168, “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles — a replacement of FASB Statement No. 162” (ASC 105). ASC 105 establishes the FASB Accounting Standards Codification (Codification) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. All guidance contained in the Codification carries an equal level of authority. The Codification does not change current U.S. GAAP, but is intended to simplify user access to all authoritative U.S. GAAP by providing all the authoritative literature related to a particular topic in one place. On the effective date of ASC 105, the Codification superseded all then-existing non-SEC accounting and reporting standards. All other non-grandfathered non-SEC accounting literature not included in the Codification will become non-authoritative. ASC 105 is effective for financial statements issued for interim and annual periods ended after September 15, 2009. The Company has revised all references to the authoritative accounting principles to reflect the Codification.
Fair Value Measurements and Disclosures
In September 2006, the FASB issued ASC 820, “Fair Value Measurements and Disclosures,” (ASC 820). ASC 820 defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements. ASC 820-10 was effective for financial statements issued for fiscal years beginning after November 15, 2007. In February 2008, the FASB delayed the effective date of ASC 820 for non-financial assets and liabilities which are not measured at fair value on a recurring basis (at least annually) until fiscal years beginning after November 15, 2008. In October 2008, FASB provided guidance in determining the fair value of a financial asset when the market for that financial asset is not active. The adoption of ASC 820 did not have a material effect on the financial statements.
Subsequent Events
In May 2009, the FASB issued ASC 855, “Subsequent Events” (ASC 855), which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before the financial statements are issued or are available to be issued. It requires the disclosure of the date through which an entity has evaluated subsequent events and the basis for that date. The Company adopted ASC 855 in 2009.
Reclassifications
Certain reclassifications have been made to conform prior period consolidated financial statements and notes to current period presentation.
| 2. | Net Loss Per Share |
| Year ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| Numerator |
||||||||
| Net loss |
$ | (7,461,000 | ) | $ | (8,218,000 | ) | ||
| Denominator |
||||||||
| Weighted average shares outstanding |
28,498,814 | 28,492,453 | ||||||
| Loss per share – basic and diluted |
$ | (0.26 | ) | $ | (0.29 | ) | ||
F-37
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
As we have incurred net losses in each of the years presented, basic and diluted loss per share amounts are the same. Common share equivalents of 1,742,901 and 1,535,950 at December 31, 2009 and 2008, respectively, have been excluded from the computation since the effect of their assumed conversion would be anti-dilutive.
| 3. | Stock-Based Compensation |
We currently sponsor the following stock-based payment plans:
2006 Equity Incentive Plan
In June 2006, our stockholders approved the NeoPharm, Inc. 2006 Equity Incentive Plan, or the 2006 Plan. The 2006 Plan originally provided for the issuance of stock options, non-vested stock, restricted stock, performance units or performance share awards to employees, directors and consultants convertible to up to 1,000,000 shares of our common stock. In 2007, the Board of Directors approved resolutions increasing the total shares available for issuance under the 2006 Plan to 3,400,000 shares and increasing the number of shares of common stock that may be granted to any grantee during any calendar year, or earned by any grantee under any performance based award during any calendar year, from 500,000 to 750,000 shares. In 2008, the board approved an increase in the number of shares of the Company’s common stock that may be awarded under the 2006 Plan as restricted stock or bonus stock from 500,000 to 1,500,000 and this board resolution was approved by the stockholders at the Annual Meeting of Stockholders held in August 2008. Awards under the 2006 Plan generally consist of stock options having an exercise price equal to the average of the lowest and highest reported sale prices of our common stock on the date of grant; vest ratably over four years; have a 10-year term; and are subject to continuous employment. Stock awards granted to our non-employee directors under the 2006 Plan typically vest one year from the date of grant. Awards under the 2006 Plan vest immediately upon a change in control, as defined in the 2006 Plan. Although the 2006 Plan provides for the issuance of performance units and performance shares, we have not made grants of these types of awards. As of December 31, 2009 and December 31, 2008, 2,250,889 and 1,979,639 shares, respectively, were available for issuance under the 2006 Plan.
2006 Employee Stock Purchase Plan
In June 2006, our stockholders approved the 2006 Employee Stock Purchase Plan, or the Purchase Plan, under which eligible employees may purchase shares of common stock quarterly through payroll deductions. An aggregate of 100,000 shares of common stock may be issued under the Purchase Plan. The price per share under the Purchase Plan is 85% of the lower of the closing price of the common stock on (i) the first business day of the plan period or (ii) the purchase date. The Purchase Plan imposes a limitation upon a participant’s right to acquire common stock if immediately after or prior to purchase, the employee owns five percent or more of the total combined voting power or value of our common stock. During the year ended December 31, 2009, 1,765 shares were issued under the Purchase Plan and the corresponding compensation expense was not material. During the year ended December 31, 2008, 8,499 shares were issued resulting in compensation expense of $3,000. As of December 31, 2009, 36,131 shares remain available for issuance.
The 1998 Plan
Our 1998 Equity Incentive Plan, or the 1998 Plan, provided for the grant of awards, primarily stock options, to employees, directors, and consultants to acquire up to 4,600,000 shares of our common stock. Following the June 2006 stockholder approval of the 2006 Plan, no further awards have been or will be
F-38
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
made under the 1998 Plan. Option awards under the 1998 Plan were generally granted with an exercise price equal to the closing price of our common stock on the date of grant, but may have been granted with an exercise price of not less than 85% of the fair market value of our common stock. Option awards under the 1998 Plan typically had a 10-year life and vested ratably on the first four anniversaries of the grant, subject to continuous employment. Stock awards granted to our non-employee directors under the 1998 Plan typically vest one year from the date of grant. Outstanding awards issued under the 1998 Plan vested immediately upon a change in control, as defined in the 1998 Plan.
Amounts recognized in the consolidated statements of operations with respect to our stock-based compensation plans were as follows:
| Year ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| Research and development |
$ | 142,000 | $ | 160,000 | ||||
| Selling, general and administrative |
116,000 | 354,000 | ||||||
| Total cost of stock-based payment plans during period |
$ | 258,000 | $ | 514,000 | ||||
We have never capitalized, or recognized an income tax benefit from, stock-based compensation.
The following is a summary of activity relating to option awards to employees and non-employee directors:
| Shares | Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life in Years |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding at December 31, 2007 |
1,252,326 | $ | 5.35 | — | $ | — | ||||||||||
| Granted |
280,500 | $ | 0.42 | — | — | |||||||||||
| Exercised |
— | — | — | — | ||||||||||||
| Forfeited, expired and or cancelled |
(132,375 | ) | $ | 2.33 | — | — | ||||||||||
| Outstanding at December 31, 2008 |
1,400,451 | $ | 4.65 | 7.12 | — | |||||||||||
| Granted |
800,250 | $ | 0.12 | — | — | |||||||||||
| Exercised |
— | — | — | — | ||||||||||||
| Forfeited, expired and or cancelled |
(457,800 | ) | $ | 2.69 | — | — | ||||||||||
| Outstanding at December 31, 2009 |
1,742,901 | $ | 3.08 | 6.79 | — | |||||||||||
| Exercisable at December 31, 2009 |
910,401 | $ | 5.50 | 4.88 | $ | — | ||||||||||
As of December 31, 2009 we expect to recognize $171,000 of unrecognized share-based compensation for our outstanding options over a weighted average period of 2.1 years.
Restricted Stock Awards
In June 2007, 180,665 restricted shares of common stock were granted to the Chief Executive Officer (CEO) of the Company, with a weighted average fair value of $1.36 per share and a four year vesting period. As of the October 2009, such CEO was terminated. 50% of the shares which had not vested were forfeited.
F-39
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
Stock Option Valuation Information
In August 2008, the Company granted 225,000 stock options to non-employee directors with a weighted average fair value of $0.29 and a one-year vesting period. In August 2008, the Company granted 50,000 stock options to our Chief Executive Officer and in August, October and December 2008, also granted 5,500 stock options to other employees. All of these employee stock option grants have a weighted average fair value of $0.31 and a four-year vesting period.
In February 2009, the Company granted 575,250 stock options to its employees and a non-employee consultant with a weighted average fair value of $0.06 and a four-year vesting period. In July 2009, the Company granted 225,000 stock options to non-employee directors with a weighted average fair value of $0.19 and a one-year vesting period.
The Company has estimated the fair value of NeoPharm’s stock-based compensation using the Black-Scholes option-pricing model. This model requires the use of subjective assumptions that have a significant impact on the fair value estimate. We have based our assumptions regarding expected volatility on the historic volatility of our common stock, the risk-free interest rate on the U.S. Treasury yield curve in effect at the time of grant and the expected term of options using the “Simplified Method” in accordance with SAB No. 107, “Share-Based Payment.” The weighted-average estimated fair value of employee stock options granted during 2009 and 2008 were calculated using the Black-Scholes option-pricing model and the related weighted-average assumptions:
| Year ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| Expected volatility |
109.42 | % | 80.74 | % | ||||
| Risk-free interest rate |
2.42 | % | 3.45 | % | ||||
| Expected term (in years) |
5.5 | 5.8 | ||||||
| Expected dividend yield |
— | — | ||||||
| 4. | Investments In and Put Option on Auction Rate Securities (“ARS”) |
The fair value of the Company’s investments in ARS as of December 31, 2009 and 2008 as estimated by the investment bank which holds these auction rate securities is as follows (in thousands):
| Year ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| State government agencies, at par value |
$ | 13,950 | $ | 14,700 | ||||
| Less: decline in estimated fair value |
(1,550 | ) | (2,400 | ) | ||||
| Net estimated fair value of investments in auction rate securities |
$ | 12,400 | $ | 12,300 | ||||
Investments in ARS have scheduled maturities greater than 90 days at the time of purchase, and are therefore classified as available-for-sale securities and recorded at fair value on our consolidated balance sheet. In 2008, the Company entered into a settlement agreement with this investment bank, which gives the Company rights to sell all of the Company’s ARS at par value back to the investment bank at any time during the period between June 30, 2010 and July 2, 2012 under the terms of a non-transferable rights offering. These Rights represent a legally enforceable firm commitment from the investment bank. Accordingly, the Company has recorded a put option of $1.55 million and $2.4 million as of December 31, 2009 and 2008, respectively, for the difference between the par value and fair value
F-40
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
of the auction rate securities. Any unrealized gains and losses and the auction rate securities put option that have been recorded in connection with the fair value accounting for the auction rate securities will be reversed and eliminated as of the June 30, 2010 par value redemption date and the Company will not incur any financial loss as a result thereof.
The estimated fair value of the ARS increased by $735,000 from December 31, 2008 to December 31, 2009. In accordance with the applicable accounting literature, this increase was recorded through other comprehensive income. The corresponding decrease of $735,000 in the estimated fair value of the put option is recorded as an unrealized loss in the Company’s consolidated statement of operations for the year ended December 31, 2009. As of December 31, 2008, the cumulative loss recognizing the other-than-temporary decline in the estimated fair value of the ARS was offset in our consolidated statement of operations by the gain recorded in recognition of the fair value of the put option.
Issuers of the Company’s ARS redeemed a total of $750,000 of these securities during 2009 at par value. There were no redemptions in 2008. The proceeds from this redemption were used to immediately repay the ARS loan which was made by this investment bank (see Note 6 below).
| 5. | Fair Value Measurement |
The Company’s financial assets and liabilities, which include only the ARS and Put Option, are recorded in our financial statements at fair value. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The Company follows a hierarchal disclosure framework which prioritizes and ranks the level of market observable inputs used in measuring fair value. The three levels of the hierarchy are as follows:
| Level 1 – |
Uses unadjusted quoted prices that are available in active markets for identical assets or liabilities as of the reporting date. | |
| Level 2 – |
Uses inputs other than Level 1 that are either directly or indirectly observable as of the reporting date through correlation with market data, including quoted prices for similar assets and liabilities in active markets and quoted prices in markets that are not active. Level 2 also includes assets and liabilities that are valued using models or other pricing methodologies that do not require significant judgment since the input assumptions used in the models, such as interest rates and volatility factors, are corroborated by readily observable data. | |
| Level 3 – |
Uses inputs that are unobservable and are supported by little or no market activity and reflect the use of significant management judgment. These values are generally determined using pricing models for which the assumptions utilize management’s estimates of market participant assumptions. | |
At December 31, 2009 and 2008, investments in the Company’s student loan-backed ARS are considered Level 3 assets and fair value measurements have been estimated by the investment bank which holds NeoPharm’s ARS using an income-approach model (discounted cash-flow analysis). The model considers factors that reflect assumptions that market participants would use in pricing similar securities, including, the collateral underlying the investments, the creditworthiness of the counterparty, expected future cash-flows, including the next time the security is expected to have a successful auction, and risks associated with the uncertainties of the current market, the formula applicable to each security which defines the interest rate paid to investors in the event of a failed auction, forward projections of
F-41
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
the interest rate benchmarks specified in such formulas, the likely timing of principal repayments, the probability of full repayment considering the guarantees by the U.S. Department of Education of the underlying student loans, guarantees by other third parties, and additional credit enhancements provided through other means, and publicly available pricing data for recently issued student loan-backed securities which are not subject to auctions.
The fair value of the Company’s auction rate securities as estimated by the investment bank approximates $12.4 million and 12.3 million, respectively, as of December 31, 2009 and 2008, which is $1.55 million and $2.4 million, respectively, less than their par value. These differences represent the estimated fair value of the Put Option as of December 31, 2009 and 2008, based on the Rights the Company obtained in the Settlement Agreement described in Note 4 above. This Put Option is also considered a Level 3 asset.
| 6. | Auction Rate Securities Loans |
NeoPharm has borrowed from the investment bank that holds our ARS amounts up to the full par value of such auction rate securities under a series of successive credit agreements executed with the investment bank during 2009 and 2008. In connection with the Settlement Agreement with this investment bank discussed in Note 4 above, the Company has the right, in the form of a put option, as well as the intent to redeem all of our ARS at par value as of June 30, 2010 and repay the ARS loan in full plus any accrued and unpaid interest expense. Approximately $13.9 million and $9.2 million were outstanding under these credit agreements as of December 31, 2009 and 2008, respectively. Borrowings are collateralized by the Company’s ARS and interest is based on an annual rate equal to the sum of the prevailing LIBOR plus 125 basis points. This rate was 1.24% and 1.69% as of December 31, 2009 and 2008, respectively. Interest expense on the ARS loans cannot exceed interest income on the ARS investments on a cumulative basis under the no net cost terms of the underlying credit agreements with the investment bank.
Included in interest expense in our consolidated statements of operations for the years ended December 31, 2009 and 2008 was interest on all short-term and long-term ARS loans of $149,000 and $80,000, respectively.
| 7. | Fixed Assets |
Fixed assets are comprised of the following as of December 31:
| Estimated Useful Life (Years) |
2009 | 2008 | ||||||||||
| Computer equipment |
3 | $ | 1,339,000 | $ | 1,326,000 | |||||||
| Scientific equipment |
5 | 3,247,000 | 3,242,000 | |||||||||
| Furniture |
7 | 307,000 | 307,000 | |||||||||
| Equipment under capital lease |
5 | 43,000 | 43,000 | |||||||||
| Leasehold improvements |
* | 107,000 | 107,000 | |||||||||
| Less accumulated depreciation |
* | * | (4,793,000 | ) | (4,659,000 | ) | ||||||
| Fixed assets, net |
$ | 250,000 | $ | 366,000 | ||||||||
| * | The estimated useful life of leasehold improvements is the lesser of the lease term or five years. |
| ** | Amortization expense related to assets under capital lease is included in depreciation expense and accumulated depreciation. |
F-42
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
| 8. | Stockholders’ Equity |
In February 2009, the Company voluntarily delisted its common stock from the NASDAQ Capital Market and began trading on the Pink Sheets, a centralized electronic quotation service for over-the-counter securities. The Company also voluntarily deregistered its common stock with the SEC in February 2009, and immediately suspended the Company’s obligation to file periodic reports under the Securities and Exchange Act of 1934, as amended, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and Proxy Statements. The deregistration with the SEC reduced the internal and external costs of public company compliance and enabled the Company to conserve cash and reallocate resources more appropriately to development of its novel drug technologies.
In April 2009, the NeoPharm board of directors approved the termination of the Company’s Stockholder Rights Plan, which accelerated the current expiration date from July 28, 2013 to May 1, 2009. The Company had maintained a Stockholder Rights Plan whereby rights to purchase shares of Series A Participating Preferred Stock became exercisable by our stockholders in the event a non-excluded party acquired, or attempted to acquire, 15% or more of the Company’s outstanding common stock.
| 9. | Income Taxes |
From inception through October, 1995, we operated as an S Corporation for income tax purposes. Losses incurred during this period were reported on the stockholders’ tax returns, and are not available to the Company as NOLs. Since that time, losses incurred result in NOLs which could be used to offset possible future taxable income.
The NOLs will expire as follows:
| Year ending December 31, |
||||
| 2011 |
$ | 1,882,000 | ||
| 2012 |
1,969,000 | |||
| 2018 |
3,122,000 | |||
| 2020 |
3,296,000 | |||
| 2021 |
12,500,000 | |||
| 2022 |
35,488,000 | |||
| 2023 |
52,200,000 | |||
| 2024 |
57,597,000 | |||
| 2025 |
40,990,000 | |||
| 2026 |
33,073,000 | |||
| 2027 |
11,916,000 | |||
| 2028 |
8,667,000 | |||
| 2029 |
7,214,000 | |||
| Total NOLs |
$ | 269,914,000 | ||
We have general business credit carryforwards of approximately $6,984,000 which expire in the period 2011-2029, and an alternative minimum tax credit of approximately $40,000 which can be carried forward for an indefinite period.
Under Section 382 of the Code, if an ownership change of more than 50% occurs there are annual limitations on the amount of NOLs and other deductions which would be available to us. Accordingly, our ability to offset any future federal taxable income with NOLs arising before any such ownership
F-43
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
changes may be limited. The Company has completed a partial analysis of ownership change and no change in control was identified, based on the review of eight test dates covering a four-year period ended December 31, 2007. A complete formal analysis of ownership change would have to be performed in order to obtain certainty that a change in control has not occurred. However, such limitations would not have a material impact on the financial statements as the NOLs are fully reserved. Our federal statutory tax rate is 35% while our effective tax rate is 0%. Differences between the federal statutory and effective tax rates result from the establishment of a valuation allowance to reduce the carrying value of deferred tax assets to zero.
Significant components of the Company’s deferred tax assets and (liabilities) as of December 31 are as follows:
| Year ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| NOLs |
$ | 105,193,000 | $ | 102,412,000 | ||||
| General business credit carryforwards |
6,984,000 | 6,766,000 | ||||||
| Charitable contribution carryforwards |
72,000 | 183,000 | ||||||
| Alternative minimum tax credit carryforwards |
40,000 | 40,000 | ||||||
| Depreciation |
46,000 | 49,000 | ||||||
| Non-deductible stock-based compensation expense |
944,000 | 851,000 | ||||||
| Expense not currently deductible for tax purposes |
57,000 | 52,000 | ||||||
| Total deferred tax assets |
113,336,000 | 110,353,000 | ||||||
| Valuation allowance |
(113,336,000 | ) | (110,353,000 | ) | ||||
| Net deferred tax assets |
$ | — | $ | — | ||||
The valuation allowance increased by $2,983,000 and $3,385,000 in the years ended December 31, 2009 and 2008, respectively. In assessing the realizability of deferred tax assets, we consider whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. We also consider the scheduled reversal of deferred tax liabilities, projected future taxable income or losses, and tax planning strategies in making this assessment. Based upon our history of tax losses and projections for future taxable income over the periods in which the deferred tax assets are deductible, we do not believe realization of these tax assets is more likely than not. As such, we have established full valuation allowances for the deferred tax assets.
| 10. | Employee Severance Costs |
The Company recorded a charge of $303,000 in the consolidated statement of operations in the fourth quarter of 2009 for salary continuation payments to be made to the Company’s former CEO over a 12-month period beginning at the time our CEO left the Company in October 2009 in accordance with his employment agreement.
| 11. | Commitments |
License and Research Agreements
From time to time we enter into license and research agreements with third parties. As of December 31, 2009, we had significant agreements with four parties, as described below.
F-44
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
Georgetown University
We have entered into two license agreements with Georgetown University whereby we obtained an exclusive worldwide license to use certain technologies. In exchange for the grant of one of these exclusive licenses that is related to taxane derivatives, we agreed to pay Georgetown University a royalty, ranging from 1.25% to 2.50% of any net sales from our products incorporating such technologies as covered by the licensed patents. The royalty will be payable for the life of the related patents. Additionally, we may be obligated to pay $400,000 upon entering into any sublicense agreement and $250,000 upon approval of an NDA.
In July 2007, we entered into an exclusive license to use certain antisense technologies covered by certain US patents. In exchange for the grant of this license, we paid Georgetown University a non-refundable license issue fee of $10,000 and are liable for yearly maintenance fees of $20,000. In addition, we agreed to pay Georgetown University a royalty of 2.75% of net sales from our products incorporating these technologies and 50% of any royalties received from sublicensees. We may also be obligated to make milestone payments totaling $900,000 upon achievement of certain objectives.
National Institutes of Health
We entered into an exclusive worldwide licensing agreement with the National Institute of Health, or, NIH, in 1997 to develop and commercialize IL13-PE38QQR (Cintredekin Besudotox). The agreement required us to pay NIH a $75,000 non-refundable license issue payment and minimum annual royalty payments of $10,000, which increase to $25,000 after the first commercial sale. The agreement further provides for us to make milestone payments to NIH of up to $585,000 and royalties of up to 3.50% based on any future product sales. We made the first milestone payment of $25,000 to NIH in November 1999 after the filing of the U.S. Investigational New Drug (“IND”) application for IL13-PE38QQR. We are required to pay the costs of filing and maintaining product patents on the licensed patents. The agreement extends to the expiration of the last to expire of the patents on the licensed patents, if not terminated earlier. The agreement may be terminated by mutual consent of NIH and us. Either party may terminate if the other party breaches a material term or condition and such breach is not cured within a certain period of time. Also, either party may unilaterally terminate by giving advanced notice.
On May 30, 2006, we entered into a non-exclusive Patent License Agreement with the NIH providing us with a non-exclusive license to utilize a patented process owned by the U.S. government relating to convection enhanced delivery, or CED, for us to use with drugs, including Cintredekin Besudotox in the treatment of gliomas, in the United States, its territories and possessions. Under the terms of this Patent License Agreement, we have paid NIH a non-creditable, nonrefundable license issue royalty of $5,000 and have agreed to pay a nonrefundable, minimum annual royalty of $2,000, which will be credited against earned royalties, which are fixed at one-half of one percent (0.5%) on aggregate future product sales over $100 million. An additional benchmark royalty of $20,000 is payable within 80 days of receiving approval from the U.S. FDA of approval to use the licensed CED process in administrating a drug for the treatment of gliomas. Pursuant to an amendment to this Patent License Agreement entered into in August 2006, we expanded the field of use to cover the treatment of cancer, were given the right to sublicense our rights and extended the time for us to reach certain benchmarks. In return for these additional rights, we agreed to pay additional sublicensing royalties one and one-half percent (1.5%), to a maximum of $200,000, on the fair market value of any upfront consideration received for granting a sublicense.
In June 2007, we entered into an exclusive worldwide license agreement with the NIH to develop and commercialize IL13-PE38QQR (Cintredekin Besudotox) for use in the treatment of asthma and pulmonary fibrosis. Upon entering the contract, we paid NIH a non-refundable license issue royalty of $125,000 and have agreed to pay an annual royalty of $20,000, which will be credited against earned
F-45
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
royalties, which are fixed at four percent of net sales, including those of sublicensees. In addition, we may be obligated to make milestone payments totaling $1,410,000 upon achievement of certain objectives. We are required to pay the costs of filing and maintaining product patents on the licensed patents. The agreement extends to the expiration of the last to expire of the patents on the licensed patents, if not terminated earlier. The agreement may be terminated by mutual consent of NIH and us. Either party may terminate if the other party breaches a material term or condition and such breach is not cured within a certain period of time. Also, either party may unilaterally terminate by giving advanced notice.
U.S. Food and Drug Administration
In 1997 the Company entered into a Cooperative Research and Development Agreement (the “CRADA”) with the FDA. Pursuant to the CRADA, we committed to work to commercialize the IL13-PE38QQR chimeric protein which we licensed from NIH. The FDA agreed to collaborate on the clinical development and commercialization of IL13-PE38QQR. In September 2005, the Company and the FDA agreed to extend the term and funding of the CRADA through July 2009 for $165,000 per year. In 2009 the term was extended for another year, through July of 2010, for $25,000.
Lovelace Respiratory Research Institute
In the third quarter of 2008, the Company entered into an agreement to pay $1.1 million for the performance of a preclinical inhalation toxicology study in non-human primates for our Cintredekin Besudotox IL13-PE38QQR therapeutic agent for the treatment of pulmonary fibrosis. Under the terms of this agreement, the Company paid $200,000 upon execution of the agreement, $500,000 in the fourth quarter of 2008 and $135,000 in the second quarter of 2009. All of these amounts are included in research and development expense in the consolidated statement of operations for their respective years. The $280,000 remaining balance under this agreement was accrued in research and development expense in the consolidated statement of operations upon completion of the final study report in the fourth quarter of 2009, and subsequently paid in the first quarter of 2010.
Clinical Trial Commitments
As of December 31, 2009, we had clinical trial agreements with various parties, as described below.
Providence Portland Medical Center
In December 2009, the Company entered into an agreement with Providence Portland Medical Center for the completion of a Phase 2 clinical trial with the use of LE-DT for the treatment of advanced prostate cancer. The agreement contains milestone payments which are based upon stages of completion of the Phase 2 clinical trials. The $10,000 start up fee payable to Providence Portland Medical Center upon entering the contract was accrued in research and development expense in the consolidated statement of operations in the fourth quarter of 2009. The total obligation for the Phase 2 clinical trial at this site is estimated to be $400,000, but is dependent on the number of patients that are enrolled and their ultimate progression in the study.
Georgetown University
In January 2010, we entered into an agreement with Georgetown University Medical Center for the completion of a Phase 2 clinical trial with the use of LE-DT for the treatment of locally advanced or metastatic pancreatic cancer. The total obligation for the Phase 2 clinical trial at this site is estimated to be $400,000, but is dependent on the number of patients that are enrolled and their ultimate progression in the study (See Note 13 below).
F-46
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
Excel Life Science
In April 2010, the Company entered into a new agreement with Excel Life Science (“Excel”) for the enrollment of an additional 35 patients in a Phase 2 clinical trial with the use of LEP-ETU for the treatment of metastatic breast cancer. Previously the Company had completed an initial Phase 2 trial under an agreement with Excel in which 35 patients were enrolled in a similar study using LEP-ETU to treat breast cancer. The total obligation for the new agreement is $368,000, and it contains milestone payments which are based upon various stages of completion of the Phase 2 clinical trial. The Company paid Excel $74,000 for the initial milestone at the signing of the new agreement (See Note 13 below).
Consulting Agreements
On January 1, 2010, the Company entered into a consulting agreement with Dr. Aquilur Rahman (the “Agreement”) to serve as its President and Chief Executive Officer. Dr. Rahman was subsequently elected to the Company’s board of directors in February 2010. Under terms of the Agreement, Dr. Rahman will be compensated at the annual rate of $340,000. The Agreement supersedes Dr. Rahman’s previous consulting agreement under which he served as the Company’s Chief Scientific Advisor. Dr. Rahman will continue to serve in that role as well under the new Agreement.
Lease Commitments
The Company has a noncancelable operating lease for office and research and development space which expires in March 2015 and requires NeoPharm to pay all executory costs. Rental payments include minimum rentals, plus contingent rentals based on common area maintenance expenses and property taxes.
Rental expense for the years ended December 31, 2009 and 2008 consisted of the following:
| 2009 | 2008 | |||||||
| Minimum rentals |
$ | 276,000 | $ | 272,000 | ||||
| Contingent rentals |
64,000 | 85,000 | ||||||
| Rental expense |
$ | 340,000 | $ | 357,000 | ||||
The Company has an agreement which runs through June 2012 for information technology network equipment and services for the Company’s information services applications. The total of these payments for the 30-month period is approximately $133,000.
Future minimum lease payments under noncancelable operating leases as of December 31, 2009 are:
| Year ended or ending December 31, |
||||
| 2010 |
$ | 358,000 | ||
| 2011 |
330,000 | |||
| 2012 |
308,000 | |||
| 2013 |
290,000 | |||
| 2014 |
290,000 | |||
| Later years |
84,000 | |||
| Total minimum lease payments |
$ | 1,660,000 | ||
F-47
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
The Company has a five-year capital lease on five copy machines which expires in January 2011. Their gross asset value in Fixed Assets-Furniture was $161,000 at the beginning of the lease in February 2006. Included in the capitalized amount is imputed interest of $28,000. These copiers were included in an impairment writedown in the third quarter of 2007. The impairment for these copiers was $64,000 leaving a gross asset value at December 31, 2007 of $43,000. Depreciation expense for the capitalized copiers was $13,000 and $16,000 for the years ended 2009 and 2008, respectively. Minimum future lease payments under a capital lease as of December 31, 2009 are as follows:
| For the year ended December 31, 2010 |
$ | 38,000 | ||
| For the year ending December 31, 2011 |
3,000 | |||
| Total minimum lease payments |
41,000 | |||
| Less amount representing interest |
(2,000 | ) | ||
| Present value of minimum lease payments |
$ | 39,000 | ||
| 12. | Contingencies |
NeoPharm and certain of the Company’s former officers have been named in a consolidated amended complaint, which alleges various violations of the federal securities laws in connection with our public statements regarding our LEP-ETU product candidate during the period from October 31, 2001 through April 19, 2002. In November 2002, the Company moved to have the complaint dismissed. This motion to dismiss was granted in part and denied in part in February 2003. In November 2004, the plaintiffs filed a motion to amend and a motion for summary adjudication. The motion to amend sought to include certain pre-class period statements in the complaint. The motion for summary adjudication asked the Court to rule that certain statements made in an arbitration award regarding the LEP-ETU product candidate be deemed facts established in this proceeding. In February 2007, the Court entered an order denying both the plaintiffs’ motion to amend and the plaintiffs’ motion for summary adjudication. Fact and expert discovery is closed. In March 2008, the dispositive motion filing deadline, NeoPharm filed a motion for summary judgment. On March 31, 2010, the Court granted in part and denied in part the Company’s motion for summary judgment. The Court dismissed the plaintiffs’ claims based on statements made before January 14, 2002 but held that there was genuine issue of material fact as to whether the Company could be liable for statements made between January 14, 2002 and April 19, 2002. On April 27, 2010, the Court set a trial date of February 22, 2011 and also set a settlement conference date of July 27, 2010. None of NeoPharm’s current directors or officers are named in this complaint. The Company intends to vigorously defend each and every claim in the complaint. Management is unable to estimate the potential outcome or range of possibilities, if any. The Company maintains insurance coverage to mitigate the financial impact of any potential loss.
The Company entered into various contractual arrangements, primarily during the fourth quarter of 2006 and the first quarter of 2007, under take or pay agreements, as amendments to the original contract with Diosynth RTP, Inc. (“Diosynth”). These contractual arrangements were made to secure access to manufacturing capacity for the potential manufacture and regulatory advancement of Cintredekin Besudotox through early 2008. As a result of Diosynth’s failure to complete work that it was contractually required to perform, as well as the FDA’s decision to require additional Phase 3 clinical testing of Cintredekin Besudotox, the Company advised Diosynth that the timing of further work to support a potential BLA submission must be delayed. Diosynth indicated that such a delay constituted a default under the Company’s contract. In response, the Company invoked the dispute resolution provisions of the contract in an attempt to resolve these and other differences between the two companies. In the fourth quarter of 2008, Diosynth filed a request for mediation. In connection with the mediation, which commenced in June 2009, the Company asserted its own claim against Diosynth for the recovery of payments made to Diosynth for work that was never started or satisfactorily completed. In December
F-48
Table of Contents
NeoPharm, Inc.
Notes to Consolidated Financial Statements — (Continued)
2009, the Company entered into a Settlement Agreement with Diosynth to avoid further mediation and arbitration and paid Diosynth $150,000. The Company recorded a credit to research and development expenses of $550,000 in the fourth quarter of 2009 to adjust the accrual for manufacturing expenses related to Diosynth to the amount of the settlement payment.
The employment of Mr. Guillermo Herrera, the former CEO of the Company, was terminated in March 2007 and the Company recorded a charge of $425,000 to selling, general and administrative expense in the consolidated statement of operations in the second quarter of 2007 for salary continuation payments to be made to Mr. Herrera over a 12-month period in accordance with his severance agreement. In May 2007, Mr. Herrera’s attorney filed a suit seeking, in addition to the salary continuation payments, payment of $121,500 for his 2006 bonus and $25,000 for a salary increase for 2007. Subsequent to the filing of this suit, the Company determined that under the terms of his employment agreement the Company should not be responsible for the payment of severance and terminated further payments. Mr. Herrera filed an Amended Complaint in February 2008, alleging breach of his employment agreement with the Company, defamation, and tortious presentation of the plaintiff in a false light, and seeking an additional $363,612 representing the remaining severance payments, plus attorneys’ fees and costs. In March 2009, the Company entered into an agreement to settle the Herrera litigation and paid $350,000, which the Company had reserved for in accrued compensation on the consolidated balance sheet as of December 31, 2008.
The Company is from time to time subject to claims and litigation arising in the ordinary course of business. The Company intends to defend vigorously any such litigation that may arise under all defenses that would be available to it. In the opinion of management, the ultimate outcome of those proceedings of which management is aware, even if adverse to the Company, will not have a material adverse effect on the Company’s consolidated financial position or results of operations. While the Company maintains insurance to cover the use of our drug product candidates in clinical trials, the Company does not presently maintain insurance covering the potential commercial use of our drug product candidates and there is no assurance that the Company will be able to obtain or maintain such insurance on acceptable terms.
| 13. | Subsequent Events |
The Company has evaluated subsequent events through July 9, 2010, the date the financial statements were available for issuance. The following events were identified:
The Company entered into an agreement with Georgetown University Medical Center in January 2010 for the completion of a Phase 2 clinical trial with the use of LE-DT for the treatment of locally advanced or metastatic pancreatic cancer. The total obligation for the Phase 2 clinical trial at this site is estimated to be $400,000, but is dependent on the number of patients that are enrolled and their ultimate progression in the study (See Note 12 above).
The Company entered into an agreement with Excel Life Sciences in March 2010 for the enrollment of an additional 35 patients in a Phase 2 clinical trial with the use of LEP for the treatment of metastatic breast cancer. The total obligation for the new agreement is $368,000, and it contains milestone payments which are based upon various stages of completion of the Phase 2 clinical trial. The Company paid Excel $74,000 for the initial milestone at the signing of the new agreement (See Note 11 above).
Subsequent to December 31, 2009, the Company entered into an agreement with H. Lee Moffitt Cancer Center and Research Institute Hospital for the completion of a Phase 2 clinical trial with the use of LE-DT for the treatment of metastatic castrate resistant prostate cancer. The total obligation for the Phase 2 clinical trial at this site is estimated to be $400,000, but is dependent on the number of patients that are enrolled and their ultimate progression in the study (See Note 11 above).
F-49
Table of Contents
Condensed Consolidated Balance Sheets
| September 30, 2010 |
December 31, 2009 |
|||||||
| (unaudited) | ||||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 518,000 | $ | 5,042,000 | ||||
| Investments in auction rate securities |
— | 12,393,000 | ||||||
| Put option on auction rate securities |
— | 1,557,000 | ||||||
| Prepaid expenses and other current assets |
487,000 | 310,000 | ||||||
| Total current assets |
1,005,000 | 19,302,000 | ||||||
| Fixed assets, net of accumulated depreciation |
154,000 | 250,000 | ||||||
| Other assets |
371,000 | 650,000 | ||||||
| Total assets |
$ | 1,530,000 | $ | 20,202,000 | ||||
| Liabilities and stockholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Short-term debt: auction rate securities loan |
$ | — | $ | 13,950,000 | ||||
| Interest expense payable: auction rate securities loan |
— | 61,000 | ||||||
| Accounts payable |
208,000 | 67,000 | ||||||
| Accrued clinical trial expenses |
839,000 | 854,000 | ||||||
| Accrued compensation |
112,000 | 583,000 | ||||||
| Accrued research and development expenses |
22,000 | 368,000 | ||||||
| Accrued manufacturing expenses |
— | 61,000 | ||||||
| Obligations under capital lease |
12,000 | 36,000 | ||||||
| Other accrued expenses |
105,000 | 99,000 | ||||||
| Total current liabilities |
1,298,000 | 16,079,000 | ||||||
| Deferred rent |
44,000 | 36,000 | ||||||
| Obligations under capital lease |
— | 3,000 | ||||||
| Total long-term liabilities |
44,000 | 39,000 | ||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.01 par value; 15,000,000 shares authorized: none issued and outstanding |
||||||||
| Common stock, $0.0002145 par value; 50,000,000 shares authorized: 28,408,482 and 28,498,814 shares issued and outstanding, respectively |
6,000 | 6,000 | ||||||
| Additional paid-in capital |
291,382,000 | 291,256,000 | ||||||
| Accumulated other comprehensive income |
— | 735,000 | ||||||
| Accumulated deficit |
(291,200,000 | ) | (287,913,000 | ) | ||||
| Total stockholders’ equity |
188,000 | 4,084,000 | ||||||
| Total liabilities and stockholders’ equity |
$ | 1,530,000 | $ | 20,202,000 | ||||
The accompanying notes to the condensed consolidated financial statements are an integral part of these financial statements.
F-50
Table of Contents
Condensed Consolidated Statements of Operations
Nine Months Ended September 30, 2010 and September 30, 2009
(unaudited)
| Nine Months Ended | ||||||||
| September 30, 2010 |
September 30, 2009 |
|||||||
| Total revenue |
$ | — | $ | — | ||||
| Expenses: |
||||||||
| Research and development |
2,503,000 | 2,708,000 | ||||||
| Selling, general, and administrative |
1,575,000 | 2,452,000 | ||||||
| Gain on sale of equipment and furniture |
— | (21,000 | ) | |||||
| Total expenses |
4,078,000 | 5,139,000 | ||||||
| Loss from operations |
(4,078,000 | ) | (5,139,000 | ) | ||||
| Gain (loss) on auction rate securities put option |
736,000 | (736,000 | ) | |||||
| Net interest income (expense) |
55,000 | 64,000 | ||||||
| Net loss |
$ | (3,287,000 | ) | $ | (5,811,000 | ) | ||
| Net loss per share—Basic and diluted |
$ | (0.12 | ) | $ | (0.20 | ) | ||
| Weighted average shares outstanding—Basic and diluted |
28,408,482 | 28,498,814 | ||||||
The accompanying notes to the condensed consolidated financial statements are an integral part of these financial statements.
F-51
Table of Contents
Condensed Consolidated Statements of Cash Flows
Nine Months Ended September 30, 2010 and September 30, 2009
(unaudited)
| Nine Months Ended | ||||||||
| September 30, 2010 |
September 30, 2009 |
|||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (3,287,000 | ) | $ | (5,811,000 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
85,000 | 104,000 | ||||||
| Stock-based compensation expense |
126,000 | 308,000 | ||||||
| Gain (loss) on auction rate securities put option |
(736,000 | ) | 736,000 | |||||
| Changes in assets and liabilities: |
||||||||
| Prepaid expenses and other assets |
102,000 | 17,000 | ||||||
| Accounts payable |
141,000 | (51,000 | ) | |||||
| Other current liabilities |
(947,000 | ) | (1,344,000 | ) | ||||
| Deferred rent |
8,000 | 14,000 | ||||||
| Net cash and cash equivalents used in operating activities |
(4,508,000 | ) | (6,027,000 | ) | ||||
| Cash flows from investing activities: |
||||||||
| Proceeds from sales of marketable securities |
13,950,000 | 350,000 | ||||||
| Purchase of equipment and furniture |
— | (5,000 | ) | |||||
| Proceeds from sale of equipment and furniture |
11,000 | — | ||||||
| Net cash and cash equivalents provided by investing activities |
13,961,000 | 345,000 | ||||||
| Cash flows from financing activities: |
||||||||
| Proceeds from short-term debt |
— | 14,350,000 | ||||||
| Proceeds from long-term debt |
— | (9,200,000 | ) | |||||
| Repayment of short-term debt |
(13,950,000 | ) | — | |||||
| Repayment of capital lease obligation |
(27,000 | ) | (26,000 | ) | ||||
| Net cash and cash equivalents (used in) provided by financing activities |
(13,977,000 | ) | 5,124,000 | |||||
| Net decrease in cash and cash equivalents |
(4,524,000 | ) | (558,000 | ) | ||||
| Cash and cash equivalents, beginning of period |
5,042,000 | 7,298,000 | ||||||
| Cash and cash equivalents, end of period |
$ | 518,000 | $ | 6,740,000 | ||||
The accompanying notes to the condensed consolidated financial statements are an integral part of these financial statements.
F-52
Table of Contents
Notes to Condensed Consolidated Financial Statements
(unaudited)
| 1. | Basis of Presentation |
Neopharm, Inc. is a biopharmaceutical company engaged in the research, development and commercialization of drugs for treatment of various cancers and other diseases.
The unaudited condensed consolidated financial statements of NeoPharm and its wholly owned subsidiary (the “Company”) as of September 30, 2010 and for the nine months ended September 30, 2010 and 2009 have been prepared on the same basis as the audited consolidated financial statements as of and for the year ended December 31, 2009, and in the opinion of management, reflect all adjustments – consisting of only normal and recurring adjustments – necessary to present fairly the Company’s financial position as of September 30, 2010 and the results of operations and cash flows for the nine months ended September 30, 2010 and 2009. The condensed consolidated results of operations for the nine months ended September 30, 2010 are not indicative of the results that may be expected for a full year. These financial statements do not include all of the necessary disclosures and should be read in conjunction with the Company’s year end consolidated financial statements.
The condensed consolidated financial statements include the accounts of NeoPharm and its wholly owned subsidiary, NeoPharm EU Limited, and have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). As of and through September 30, 2010, the subsidiary had nominal assets and had not conducted any business. All significant intercompany accounts and transactions are eliminated in consolidation.
Amounts presented have been rounded to the nearest thousand.
Recent Accounting Pronouncements
In January 2010, the Financial Accounting Standards Board (the “FASB”) issued amended guidance on fair value measurements and disclosures. The new guidance requires additional disclosures regarding fair value measurements, amends disclosures about postretirement benefit plan assets, and provides clarification regarding the level of disaggregation of fair value disclosures by investment class. This guidance is effective for interim and annual reporting periods beginning after December 15, 2009, except for certain Level 3 activity disclosure requirements that will be effective for reporting periods beginning after December 15, 2010. Accordingly, we adopted this amendment on January 1, 2010, except for the additional Level 3 requirements which will be adopted in 2011. The adoption is not expected to have a material impact on the Company’s consolidated financial statements.
In April 2010, the FASB issued an accounting standard update which provides guidance on the criteria to be followed in recognizing revenue under the milestone method. The milestone method of recognition allows a vendor who is involved with the provision of deliverables to recognize the full amount of a milestone payment upon achievement, if, at the inception of the revenue arrangement, the milestone is determined to be substantive as defined in the standard. The guidance is effective on a prospective basis for milestones achieved in fiscal years and interim periods within those fiscal years, beginning on or after June 15, 2010. Early adoption is permitted. The adoption is not expected to have an impact on our consolidated financial statements.
Reclassifications
Certain reclassifications have been made to conform prior period consolidated financial statements and notes to current period presentation.
F-53
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expense during the reporting period. Actual results could differ from those estimates.
Research and Development
Research and development (“R&D”) costs are expensed when incurred. These costs include, among other things, salary, stock-based compensation, consulting fees and costs reimbursed to third parties under license and research agreements described in Note 9, Commitments. Payments related to the acquisition of technology rights, for which development work is in process, are expensed as incurred and are considered a component of R&D costs. The Company charges the indirect cost of administering R&D activities to R&D expense.
Stock-Based Compensation
Stock-based compensation cost is estimated at the grant date based on the fair value of the award, and the cost is recognized as expense ratably over the vesting period. The Company uses the Black-Scholes model for estimating the grant date fair value of stock options. Determining the assumptions that enter into the model is highly subjective and requires judgment. The Company has based its assumptions regarding expected volatility on the historical volatility of its common stock, the risk-free interest rate on the U.S. Treasury yield curve in effect at the time of grant, and the expected term of options using the “Simplified Method” in accordance with SAB No. 107, “Share Based Payment.” The life of the awards is based on a simplified method which defines the life as the average of the contractual term of the options and the weighted average vesting period for all open tranches. The risk-free interest rate for the expected term of the option is based on the average market rate on U.S. treasury securities in effect during the quarter in which the options were granted. The dividend yield reflects historical experience as well as future expectations over the expected term of the option.
| 2. | Net Loss Per Share |
The following table sets forth the computation of basic and diluted net loss per share:
| Nine Months Ended September 30, |
||||||||
| 2010 | 2009 | |||||||
| Numerator: |
||||||||
| Net loss |
$ | (3,287,000 | ) | $ | (5,811,000 | ) | ||
| Denominator: |
||||||||
| Weighted average shares outstanding |
28,408,482 | 28,498,814 | ||||||
| Loss per share – basic and diluted |
$ | (0.12 | ) | $ | (0.20 | ) | ||
As the Company has incurred net losses in each of the periods presented, basic and diluted loss per share amounts are the same. Common share equivalents of 2,047,551 and 1,742,901 at September 30, 2010 and December 31, 2009, respectively, have been excluded from the computation since the effect of their assumed conversion would be anti-dilutive.
F-54
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
| 3. | Stock-Based Compensation |
The Company has stock-based employee incentive plans that cover the Company’s employees. The Company measures compensation expense under the plan based on the estimated fair value of the awards on the grant dates and amortizes the expense over the options’ vesting periods.
The Company currently has in place the following stock-based incentive plans:
2006 Equity Incentive Plan
In June 2006, the Company’s stockholders approved the NeoPharm 2006 Equity Incentive Plan (the “2006 Plan”). The 2006 Plan originally provided for the issuance of stock options, non-vested stock, restricted stock, performance units or performance share awards to employees, directors and consultants convertible to up to 1,000,000 shares of the Company’s common stock. In 2007, the board of directors approved resolutions increasing the total shares available for issuance under the 2006 Plan to 3,400,000 shares and increasing the number of shares of common stock that may be granted to any grantee during any calendar year, or earned by any grantee under any performance based award during any calendar year, from 500,000 to 750,000 shares. In 2008, the board approved an increase in the number of shares of the Company’s common stock that may be awarded under the 2006 Plan as restricted stock or bonus stock from 500,000 to 1,500,000 and this board resolution was approved by the stockholders at the Annual Meeting of Stockholders held in August 2008. Awards under the 2006 Plan generally consist of stock options having an exercise price equal to the average of the lowest and highest reported sale prices of our common stock on the date of grant; vest ratably over four years; have a 10-year term; and are subject to continuous employment. Stock awards granted to our non-employee directors under the 2006 Plan typically vest one year from the date of grant. Awards under the 2006 Plan vest immediately upon a change in control, as defined in the 2006 Plan. Although the 2006 Plan provides for the issuance of performance units and performance shares, the Company has not made grants of these types of awards. As of September 30, 2010 and December 31, 2009, 2,555,539 and 2,250,889 shares, respectively, were available for issuance under the 2006 Plan.
2006 Employee Stock Purchase Plan
In June 2006, the Company’s stockholders approved the 2006 Employee Stock Purchase Plan (the “Purchase Plan”), under which eligible employees may purchase shares of common stock quarterly through payroll deductions. An aggregate of 100,000 shares of the Company’s common stock may be issued under the Purchase Plan. The price per share under the Purchase Plan is 85% of the lower of the closing price of the common stock on (i) the first business day of the plan period or (ii) the purchase date. The Purchase Plan imposes a limitation upon a participant’s right to acquire common stock if immediately after or prior to purchase, the employee owns five percent or more of the total combined voting power or value of the Company’s common stock. During the nine months ended September 30, 2009, 1,765 shares were issued under the Purchase Plan and the corresponding compensation expense was not material. There were no shares issued during the nine months ended September 30, 2010 and a total of 36,131 shares remain available for issuance as of that date.
The 1998 Plan
The Company’s 1998 Equity Incentive Plan (the “1998 Plan”) provided for the grant of awards, primarily stock options, to employees, directors, and consultants to acquire up to 4,600,000 shares of the Company’s common stock. Following the June 2006 stockholder approval of the 2006 Plan, no further awards have been or will be made under the 1998 Plan. Option awards under the 1998 Plan were generally granted with an exercise price equal to the closing price of the Company’s common stock on
F-55
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
the date of grant, but may have been granted with an exercise price of not less than 85% of the fair market value of the Company’s common stock. Option awards under the 1998 Plan typically had a 10-year life and vested ratably on the first four anniversaries of the grant, subject to continuous employment. Stock awards granted to our non-employee directors under the 1998 Plan typically vest one year from the date of grant. Outstanding awards issued under the 1998 Plan vested immediately upon a change in control, as defined in the 1998 Plan.
Amounts recognized in the consolidated statements of operations with respect to the Company’s stock-based incentive plans were as follows:
| Nine Months Ended September 30, |
||||||||
| 2010 | 2009 | |||||||
| Research and development |
$ | 81,000 | $ | 124,000 | ||||
| Selling, general and administrative |
45,000 | 184,000 | ||||||
| Total cost of stock-based payment plans during period |
$ | 126,000 | $ | 308,000 | ||||
The Company has never capitalized, or recognized an income tax benefit from, stock-based compensation.
The following is a summary of activity relating to option awards to employees and nonemployee directors:
| Shares | Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life in Years |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding at December 31, 2009 |
1,742,901 | $ | 3.08 | 6.79 | $ | — | ||||||||||
| Granted |
615,500 | 0.30 | — | — | ||||||||||||
| Forfeited, expired and or cancelled |
(310,850 | ) | 5.53 | — | — | |||||||||||
| Outstanding at September 30, 2010 |
2,047,551 | 1.86 | 6.27 | |||||||||||||
| Exercisable at September 30, 2010 |
1,063,989 | $ | 3.29 | 4.48 | $ | — | ||||||||||
As of September 30, 2010, there was $189,000 of unrecognized stock-based compensation for the Company’s outstanding options. As described above in the 1998 and 2006 Plans, the options vest immediately upon change in control. As described the Subsequent Events (see Note 11 below), in November 2010, all the options were vested and all of the unrecognized compensation was expensed.
Restricted Stock Awards
In June 2007, 180,665 restricted shares of common stock were granted to the Chief Executive Officer (CEO) of the Company, with a weighted average fair value of $1.36 per share and a four year vesting period. As of the October 2009, the CEO’s employment was terminated and 50% of the shares which had not vested were forfeited.
F-56
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
Stock Option Valuation Information
In February 2010, the Company granted 334,250 stock options to its employees and a nonemployee consultant with a weighted average fair value of $0.25 and a four-year vesting period. In August 2010, the Company granted 281,250 stock options to non-employee directors with a weighted average fair value of $0.23 and a one-year vesting period. The Company has estimated the fair value of the Company’s stock-based compensation using the Black-Scholes option-pricing model. This model requires the use of subjective assumptions that have a significant impact on the fair value estimate. The Company has based its assumptions regarding expected volatility on the historical volatility of its common stock, the risk-free interest rate on the U.S. Treasury yield curve in effect at the time of grant, and the expected term of options using the “Simplified Method” in accordance with SAB No. 107, “Share-Based Payment.” The estimated weighted-average fair value of employee stock options granted during 2010 was calculated using the Black-Scholes option-pricing model and the related weighted average assumptions are as follows:
| Nine Months Ended September 30, 2010 |
||||
| Expected volatility |
107.13 | % | ||
| Risk-free interest rate |
3.14 | % | ||
| Expected term (in years) |
5.5 | |||
| Expected dividend yield |
— | % | ||
| 4. | Investments In and Put Option on Auction Rate Securities (“ARS”) |
The Company’s investments in auction rate securities (“ARS”) had scheduled maturities greater than 90 days at the time of purchase, and were therefore classified as available-for-sale securities and recorded at fair value on its consolidated balance sheet.
The Company adopted guidance within Accounting Standards Codification (“ASC”) No. 825, Financial Instruments, in 2008 for the put option on ARS. Accordingly, the put option was recorded at fair value and marked to market at each reporting period. All changes in the estimated fair market value of the put option were recorded in operations.
The fair value of the Company’s investments in ARS as of December 31, 2009 as estimated by the investment bank which held these auction rate securities was as follows:
| December 31, 2009 |
||||
| State government agencies, at par value |
$ | 13,950,000 | ||
| Less: decline in estimated fair value |
(1,550,000 | ) | ||
| Net estimated fair value of investments in auction rate securities |
$ | 12,400,000 | ||
In 2008, the Company entered into a settlement agreement with this investment bank which had invested in the ARS on the Company’s behalf, which settlement agreement gave the Company rights to sell all of the Company’s ARS at par value back to the investment bank at any time during the period between June 30, 2010 and July 2, 2012 under the terms of a non-transferable rights offering. The Company exercised its rights on June 30, 2010. These rights represented a legally enforceable firm commitment from the investment bank. Accordingly, the Company recorded a put option (the “Put Option”) of $1.55 million as of December 31, 2009, for the difference between the par value and fair
F-57
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
value of the ARS. The unrealized gains and losses and the ARS put option that had been recorded in connection with the fair value accounting for the ARS and were reversed and eliminated as of their redemption on June 30, 2010.
The estimated fair value of the auction rate securities increased by $735,000 from December 31, 2008 to September 30, 2009. In accordance with the applicable accounting literature, this increase was recorded through Other Comprehensive Income. The corresponding decrease of $735,000 in the estimated fair value of the put option was recorded as an unrealized loss in the Company’s Condensed Consolidated Statement of Operations for the nine months ended September 30, 2009.
Issuers of the Company’s ARS redeemed a total of $750,000 of these securities during 2009 at par value. The proceeds from this redemption were used to immediately repay the ARS loan which was made by this investment bank (see Note 6 below).
On June 30, 2010, the Company exercised its put option and redeemed all of its ARS investments at full par value and repaid its ARS loan in full with the proceeds of this redemption. The redemption resulted in the reversal of the $735,000 unrealized loss previously recorded in connection with the ARS.
| 5. | Fair Value Measurement |
The Company’s financial assets and liabilities, which included only the ARS and Put Option, were recorded in the Company’s financial statements at fair value as of December 31, 2009 and prior to the June 30, 2010 redemption at full par value. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The Company follows a hierarchal disclosure framework which prioritizes and ranks the level of market observable inputs used in measuring fair value. The three levels of the hierarchy are as follows:
| Level 1 – |
Uses unadjusted quoted prices that are available in active markets for identical assets or liabilities as of the reporting date. | |
| Level 2 – |
Uses inputs other than Level 1 that are either directly or indirectly observable as of the reporting date through correlation with market data, including quoted prices for similar assets and liabilities in active markets and quoted prices in markets that are not active. Level 2 also includes assets and liabilities that are valued using models or other pricing methodologies that do not require significant judgment since the input assumptions used in the models, such as interest rates and volatility factors, are corroborated by readily observable data. | |
| Level 3 – |
Uses inputs that are unobservable and are supported by little or no market activity and reflect the use of significant management judgment. These values are generally determined using pricing models for which the assumptions utilize management’s estimates of market participant assumptions. | |
At December 31, 2009, investments in the Company’s student loan backed ARS were considered Level 3 assets and fair value measurements had been estimated by the investment bank which held the Company’s ARS using an income-approach model (discounted cash-flow analysis). The model considered factors that reflect assumptions that market participants would use in pricing similar securities, including the collateral underlying the investments, the creditworthiness of the counterparty, expected future cash-flows, including the next time the security is expected to have a successful auction,
F-58
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
risks associated with the uncertainties of the current market, the formula applicable to each security which defines the interest rate paid to investors in the event of a failed auction, forward projections of the interest rate benchmarks specified in such formulas, the likely timing of principal repayments the probability of full repayment considering the guarantees by the U.S. Department of Education of the underlying student loans, guarantees by other third parties, and additional credit enhancements provided through other means, and publicly available pricing data for recently issued student loan asset-backed securities which are not subject to auctions.
The fair value of the Company’s ARS as estimated by the investment bank approximated $12.3 million as of December 31, 2009, which was $1.55 million less than their par value. This difference represented the estimated fair value of the Put Option as of December 31, 2009, based on the rights the Company obtained pursuant to the settlement agreement described above. This Put Option was also considered a Level 3 asset.
| 6. | ARS Loans |
As described in Note 4 above, on June 30, 2010, the Company exercised its Put Option and redeemed all of its ARS investments at full par value, reversing all previously recorded unrealized losses and repaying its ARS loan in full with the proceeds of this redemption.
The Company had previously borrowed from the investment bank that held its ARS amounts up to the full par value of such ARS under a series of successive credit agreements executed with the investment bank during 2009 and 2008. In connection with the settlement agreement with this investment bank discussed in Note 4 above, the Company had the right, in the form of the Put Option, as well as the intent to redeem all of its ARS at par value as of June 30, 2010 and repay the ARS loan in full plus any accrued and unpaid interest expense. Approximately $13.9 million was outstanding under these credit agreements as of December 31, 2009. Borrowings were collateralized by the Company’s ARS and interest was based on an annual rate equal to the sum of the prevailing LIBOR plus 125 basis points. Interest expense on the ARS loans could not exceed interest income on the ARS investments on a cumulative basis under the no net cost terms of the underlying credit agreements with the investment bank. Included in interest expense in the Company’s consolidated statements of operations for the nine months ended September 30, 2010 and 2009 was interest on all short-term and long-term ARS loans of $85,000 and $103,000, respectively.
| 7. | Fixed Assets |
Fixed assets are recorded at cost and depreciated using the straight-line method over their estimated useful lives. Maintenance and repairs that do not extend the life of assets are charged to expense when incurred. When properties are disposed of, the related costs and accumulated depreciation are removed from the accounts and any gain or loss is reported in the period the transaction takes place. Total depreciation expense for the nine months ended September 30, 2010 and 2009, was $85,000 and $104,000, respectively.
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted cash flows expected to be generated by the asset. If the carrying amount exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount exceeds the fair value of the asset. No impairment charges were recorded during the nine months ended September 30, 2010 and 2009.
F-59
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
| 8. | Stockholders’ Equity |
In February 2009, the Company voluntarily delisted its common stock from the NASDAQ Capital Market and began trading on the Pink Sheets, a centralized electronic quotation service for over-the-counter securities. The Company also voluntarily deregistered its common stock with the SEC in February 2009, and immediately suspended the Company’s obligation to file periodic reports under the Securities and Exchange Act of 1934, as amended, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and Proxy Statements on Schedule 14A.
In April 2009, the NeoPharm board of directors approved the termination of the Company’s Stockholder Rights Plan, which accelerated the current expiration date from July 28, 2013 to May 1, 2009. The Company had maintained a Stockholder Rights Plan whereby rights to purchase shares of Series A Participating Preferred Stock became exercisable by the Company’s stockholders in the event a non-excluded party acquired, or attempted to acquire, 15% or more of the Company’s outstanding common stock.
| 9. | Commitments |
License and Research Agreements
From time to time the Company enters into license and research agreements with third parties. As of September 30, 2010, the Company had significant agreements with four parties, as described below.
Georgetown University
The Company entered into two license agreements with Georgetown University whereby it obtained an exclusive worldwide license to use certain technologies. In exchange for the grant of one of these exclusive licenses that is related to taxane derivatives, the Company agreed to pay Georgetown University a royalty, ranging from 1.25 to 2.50% of any net sales from its products incorporating such technologies as covered by the licensed patents. The royalty will be payable for the life of the related patents. Additionally, the Company may be obligated to pay $400,000 upon entering into any sublicense agreement and $250,000 upon approval of an NDA.
In July 2007, the Company entered into an exclusive license to use certain antisense technologies covered by certain U.S. patents. In exchange for the grant of this license, the Company paid Georgetown University a non-refundable license issue fee of $10,000 and is liable for yearly maintenance fees of $20,000. In addition, the Company agreed to pay Georgetown University a royalty of 2.75% of net sales from our products incorporating these technologies and 50% of any royalties received from sub licensees. The Company may also be obligated to make milestone payments totaling $900,000 upon achievement of certain objectives.
National Institutes of Health
The Company entered into an exclusive worldwide licensing agreement with the NIH in 1997 to develop and commercialize IL13-PE38QQR (Cintredekin Besudotox), the Company’s product candidate for the treatment of pulmonary fibrosis. The agreement required the Company to pay NIH a $75,000 non-refundable license issue payment and minimum annual royalty payments of $10,000, which increase to $25,000 after the first commercial sale. The agreement further provides for the Company to make milestone payments to NIH of up to $585,000 and royalties of up to 3.50% based on any future product sales. We made the first milestone payment of $25,000 to NIH in November 1999 after the filing of the U.S. Investigational New Drug IND application for IL13-PE38QQR (Cintredekin Besudotox). The
F-60
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
Company is required to pay the costs of filing and maintaining product patents on the licensed patents. The agreement extends to the expiration of the last to expire of the patents on the licensed patents, if not terminated earlier. The agreement may be terminated by mutual consent of NIH and the Company. Either party may terminate if the other party breaches a material term or condition and such breach is not cured within a certain period of time. Also, either party may unilaterally terminate by giving advanced notice.
On May 30, 2006 the Company entered into a non-exclusive Patent License Agreement with the NIH providing us with a non-exclusive license to utilize a patented process owned by the U.S. government relating to convection enhanced delivery (“CED”), for the Company to use with drugs, including IL13-PE38QQR (Cintredekin Besudotox) in the treatment of gliomas, in the U.S., its territories and possessions. Under the terms of this Patent License Agreement, the Company has paid NIH a noncreditable, nonrefundable license issue royalty of $5,000 and has agreed to pay a nonrefundable, minimum annual royalty of $2,000, which will be credited against earned royalties, which are fixed at one-half of one percent on aggregate future product sales over $100 million. An additional benchmark royalty of $20,000 is payable within 30 days of receiving approval from the FDA of approval to use the licensed CED process in administrating a drug for the treatment of gliomas. Pursuant to an amendment to this Patent License Agreement entered into in August 2006, the Company expanded the field of use to cover the treatment of cancer, were given the right to sublicense the Company’s rights and extended the time for the Company to reach certain benchmarks. In return for these additional rights, the Company agreed to pay additional sublicensing royalties one and one-half percent, to a maximum of $200,000, on the fair market value of any upfront consideration received for granting a sublicense.
In June 2007, the Company entered into an exclusive worldwide license agreement with the NIH to develop and commercialize IL13-PE38QQR (Cintredekin Besudotox) for use in the treatment of asthma and pulmonary fibrosis. Upon entering the contract, the Company paid NIH a non-refundable license issue royalty of $125,000 and has agreed to pay an annual royalty of $20,000, which will be credited against earned royalties, which are fixed at four percent of net sales, including those of sub licensees. In addition, the Company may be obligated to make milestone payments totaling $1,410,000 upon achievement of certain objectives. The Company is required to pay the costs of filing and maintaining product patents on the licensed patents. The agreement extends to the expiration of the last to expire of the patents on the licensed patents, if not terminated earlier. The agreement may be terminated by mutual consent of NIH and the Company. Either party may terminate if the other party breaches a material term or condition and such breach is not cured within a certain period of time. Also, either party may unilaterally terminate by giving advanced notice.
U.S. Food and Drug Administration
In 1997 the Company entered into a Cooperative Research and Development Agreement (the “CRADA”), with the FDA. Pursuant to the CRADA, the Company committed to commercialize the IL13-PE38QQR chimeric protein which the Company licensed from NIH. The FDA agreed to collaborate on the clinical development and commercialization of IL13-PE38QQR. In September 2005, the Company and the FDA agreed to extend the term and funding of the CRADA through July 2009 for $165,000 per year. In 2009 the term was extended for another year, through July of 2010, for $25,000.
Lovelace Respiratory Research Institute
In the third quarter of 2008, the Company entered into an agreement to pay $1.1 million for the performance of a preclinical inhalation toxicology study in non-human primates for its IL13-PE38QQR (Cintredekin Besudotox) product candidate. Under the terms of this agreement, the Company paid
F-61
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
$200,000 upon execution of the agreement, $500,000 in the fourth quarter of 2008 and $135,000 in the second quarter of 2009. All of these amounts are included in research and development expense in the Consolidated Statement of Operations for their respective years. The $280,000 remaining balance under this agreement was accrued in research and development expense in the Consolidated Statement of Operations upon completion of the final study report in the fourth quarter of 2009, and subsequently paid in the first quarter of 2010.
Clinical Trial Commitments
As of September 30, 2010, the Company had clinical trial agreements with various parties, as described below.
Georgetown University
In January 2010, the Company entered into an agreement with Georgetown University Medical Center for the completion of a Phase 2 clinical trial with the use of LE-DT for the treatment of locally advanced or metastatic pancreatic cancer. The total obligation for the Phase 2 clinical trial at this site is estimated to be $400,000, but is dependent on the number of patients that are enrolled and their ultimate progression in the study. As of September 30, 2010, the Company had recorded a liability of $66,000 for patient enrollment costs. To date, there have been no further patients enrolled.
Excel Life Science
In April 2010, the Company entered into a new agreement with Excel Life Science (“Excel”) for the enrollment of an additional 35 patients in a Phase 2 clinical trial with the use of LEP-ETU for the treatment of metastatic breast cancer. Previously the Company had completed an initial Phase 2 trial under an agreement with Excel in which 35 patients were enrolled in a similar study using LEP-ETU to treat breast cancer. The total obligation for the new agreement is $368,000, and it contains milestone payments which are based upon various stages of completion of the Phase 2 clinical trial. As of September 30, 2010, the Company has paid Excel two milestone payments totaling $147,000 for the signing of the letter of intent and the enrollment of the first patient. Subsequent to September 30, 2010, a third milestone payment of $74,000 was made for the enrollment of the 35th patient.
Consulting Agreements
On January 1, 2010, the Company entered into a consulting agreement with Dr. Aquilur Rahman (the “Agreement”) to serve as its President and Chief Executive Officer. Dr. Rahman was subsequently elected to the Company’s Board of Directors in February 2010. Under terms of the Agreement, Dr. Rahman was compensated at the annual rate of $340,000. The Agreement superseded Dr. Rahman’s previous consulting agreement under which he served as the Company’s Chief Scientific Advisor. Dr. Rahman served in that role as well under the new Agreement until his resignation effective at the time of the merger with the company now known as Insys Pharma, Inc. (see Note 11 below.)
| 10. | Contingencies |
NeoPharm and certain of the Company’s former officers have been named in a consolidated amended complaint, which alleges various violations of the federal securities laws in connection with public statements regarding the Company’s LEP-ETU product candidate during the period from October 31, 2001 through April 19, 2002. In November 2002, the Company moved to have the complaint dismissed. This motion to dismiss was granted in part and denied in part in February 2003. In November 2004, the plaintiffs filed a motion to amend and a motion for summary adjudication. The motion to amend sought to include certain pre-class period statements in the complaint. The motion for summary
F-62
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
adjudication asked the Court to rule that certain statements made in an arbitration award regarding the LEP-ETU drug product candidate be deemed facts established in that proceeding. In February 2007, the Court entered an order denying both the plaintiffs’ motion to amend and the plaintiffs’ motion for summary adjudication. Fact and expert discovery is closed. In March 2008, the dispositive motion filing deadline, NeoPharm filed a motion for summary judgment. On March 31, 2010, the Court granted in part and denied in part the Company’s motion for summary judgment. The Court dismissed the plaintiffs’ claims based on statements made before January 14, 2002 but held that there was a genuine issue of material fact as to whether the Company could be liable for statements made between January 14, 2002 and April 19, 2002. On April 27, 2010, the Court set a trial date of February 22, 2011 and also set a settlement conference date of July 27, 2010. On October 25, 2010, the parties entered into a Stipulation of Settlement which set forth the terms and conditions for a proposed settlement of the litigation and for dismissal of the litigation with prejudice. The parties have agreed to settle the litigation for $3.35 million in cash for distribution to eligible class members which was paid by the Company’s insurers. At the settlement hearing on March 17, 2011, the Court gave final approval of the settlement, which was paid by the Company’s insurers. None of NeoPharm’s current directors or officers are named in this complaint.
The Company entered into various contractual arrangements, primarily during the fourth quarter of 2006 and the first quarter of 2007, under take or pay agreements, as amendments to the original contract with Diosynth RTP, Inc. (“Diosynth”). These contractual arrangements were made to secure access to manufacturing capacity for the potential manufacture and regulatory advancement of Cintredekin Besudotox through early 2008. As a result of Diosynth’s failure to complete work that it was contractually required to perform, as well as the FDA’s decision to require additional Phase 3 clinical testing of Cintredekin Besudotox, the Company advised Diosynth that the timing of further work to support a potential BLA submission must be delayed. Diosynth indicated that such a delay constituted a default under the contract with the Company. In response, the Company invoked the dispute resolution provisions of the contract in an attempt to resolve these and other differences between the two companies. In the fourth quarter of 2008, Diosynth filed a request for mediation. In connection with the mediation, which commenced in June 2009, the Company asserted its own claim against Diosynth for the recovery of payments made to Diosynth for work that was never started or satisfactorily completed. In June 2009, the Company entered into a Settlement Agreement and agreed to pay Diosynth $150,000 to avoid further mediation and arbitration. The Company recorded a credit to research and development expenses of $550,000 in its Condensed Consolidated Statement of Operations for the nine months ended September 30, 2009 to adjust the accrual for manufacturing expenses related to Diosynth to the amount of the settlement payment. In the fourth quarter of 2009, the Company paid Diosynth $150,000 pursuant to the Settlement Agreement.
The Company is from time to time subject to claims and litigation arising in the ordinary course of business. The Company intends to defend vigorously any such litigation that may arise under all defenses that would be available to the Company. In the opinion of management, the ultimate outcome of those proceedings of which management is aware, even if adverse to the Company, will not have a material adverse effect on the Company’s consolidated financial position or results of operations. While the Company maintains insurance to cover the use of its drug product candidates in clinical trials, the Company does not presently maintain insurance covering the potential commercial use of its product candidates and there is no assurance that the Company will be able to obtain or maintain such insurance on acceptable terms.
| 11. | Subsequent Events |
On November 8, 2010, the Company completed a merger with Insys Therapeutics, now known as Insys Pharma, which was accounted for as a reverse acquisition of the Company by Insys Therapeutics.
F-63
Table of Contents
NeoPharm, Inc.
Notes to Condensed Consolidated Financial Statements — (Continued)
All of the common stock of Insys Therapeutics, Inc. prior to the merger was exchanged for 19,499,989 shares of NeoPharm common stock and 14,864,607 shares of newly-created convertible preferred stock. The convertible preferred stock is convertible into common stock on a one-for-35 basis and, until converted, will be entitled to the voting, dividend and liquidation rights of the same number of shares of common stock into which it is convertible. As a result of the merger, Insys Therapeutics became a wholly-owned subsidiary of NeoPharm and changed its name to Insys Pharma, Inc. and NeoPharm then changed its name to Insys Therapeutics, Inc. Subsequent to the merger, the former NeoPharm stockholders own 5% of the combined entity. Upon effectiveness of the Merger, the officers and directors of NeoPharm resigned and were replaced by the officers and directors of Insys Therapeutics.
As additional consideration, the NeoPharm board approved the distribution, immediately after the merger, of non-transferable contingent payment rights to its stockholders of record as of November 5, 2010. These rights entitle the holders of NeoPharm stock prior to the merger to receive cash payments aggregating $20.0 million (equivalent to $0.70402 per share) if, prior to the five year anniversary of the merger, the FDA approves an NDA for any one or more of the NeoPharm product candidates that were under development at the time of the merger. The distribution would be payable within nine months of FDA approval.
The Company evaluated events through March 29, 2011, the date these financial statements were originally filed with the SEC and re-evaluated events through May 6, 2011, the date Amendment No. 1 to Form S-1 was filed with the SEC.
F-64
Table of Contents

Shares
Common Stock
PROSPECTUS
, 2011
Wells Fargo Securities
JMP Securities
Oppenheimer & Co.
Through and including , 2011 (25 days after the commencement of this offering), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This delivery is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to their unsold allotments or subscriptions.
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution. |
The following table sets forth all costs and expenses, other than underwriting discounts and commissions, payable by us in connection with the sale of the common stock being registered. All amounts shown are estimates except for the SEC registration fee, the FINRA filing fee and the Nasdaq Global Market filing fee.
| Amount paid or to be paid |
||||
| SEC registration fee |
$ | 6,386 | ||
| FINRA filing fee |
6,000 | |||
| Nasdaq Global Market filing fee |
125,000 | |||
| Blue sky qualification fees and expenses |
25,000 | |||
| Printing and engraving expenses |
300,000 | |||
| Legal fees and expenses |
1,200,000 | |||
| Accounting fees and expenses |
200,000 | |||
| Transfer agent and registrar fees and expenses |
15,000 | |||
| Miscellaneous expenses |
122,614 | |||
| Total |
$ | 2,000,000 | ||
| Item 14. | Indemnification of Directors and Officers. |
We are incorporated under the laws of the State of Delaware. Section 145 of the Delaware General Corporation Law provides that a Delaware corporation may indemnify any persons who were, are, or are threatened to be made, parties to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director, employee or agent of such corporation, or is or was serving at the request of such corporation as an officer, director, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided that such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was illegal. A Delaware corporation may indemnify any persons who were, are, or are threatened to be made, a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person is or was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests except that no indemnification is permitted without judicial approval if the officer or director is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses (including attorneys’ fees) actually and reasonably incurred.
Our amended and restated certificate of incorporation and amended and restated bylaws, each of which will become effective upon the closing of this offering, provide for the indemnification of our directors and officers to the fullest extent permitted under the Delaware General Corporation Law.
II-1
Table of Contents
Section 102(b)(7) of the Delaware General Corporation Law permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
| • | transaction from which the director derives an improper personal benefit; |
| • | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payment of dividends or redemption of shares; or |
| • | breach of a director’s duty of loyalty to the corporation or its stockholders. |
Our amended and restated certificate of incorporation includes such a provision. Expenses incurred by any officer or director in defending any such action, suit or proceeding in advance of its final disposition shall be paid by us upon delivery to us of an undertaking, by or on behalf of such director or officer, to repay all amounts so advanced if it shall ultimately be determined that such director or officer is not entitled to be indemnified by us.
Section 174 of the Delaware General Corporation Law provides, among other things, that a director who willfully or negligently approves of an unlawful payment of dividends or an unlawful stock purchase or redemption, may be held liable for such actions. A director who was either absent when the unlawful actions were approved or dissented at the time may avoid liability by causing his or her dissent to such actions to be entered in the books containing minutes of the meetings of the board of directors at the time such action occurred or immediately after such absent director receives notice of the unlawful acts.
As permitted by the Delaware General Corporation Law, we have entered into indemnity agreements with each of our directors and executive officers, that require us to indemnify such persons against any and all costs and expenses (including attorneys’, witness or other professional fees) actually and reasonably incurred by such persons in connection with any action, suit or proceeding (including derivative actions), whether actual or threatened, to which any such person may be made a party by reason of the fact that such person is or was a director or officer or is or was acting or serving as an officer, director, employee or agent of Insys or any of its affiliated enterprises. Under these agreements, we are not required to provided indemnification for certain matters, including:
| • | indemnification beyond that permitted by the Delaware General Corporation Law; |
| • | indemnification for any proceeding with respect to the unlawful payment of remuneration to the director or officer; |
| • | indemnification for certain proceedings involving a final judgment that the director or officer is required to disgorge profits from the purchase or sale of our stock |
| • | indemnification for proceedings involving a final judgment that the director’s or officer’s conduct was in bad faith, knowingly fraudulent or deliberately dishonest or constituted willful misconduct or a breach of his or her duty of loyalty, but only to the extent of such specific determination; |
| • | indemnification for proceedings or claims brought by an officer or director against us or any of our directors, officers, employees or agents, except for claims to establish a right of indemnification or proceedings or claims approved by our board of directors or required by law; |
| • | indemnification for settlements the director or officer enters into without our consent; or |
| • | indemnification in violation of any undertaking required by the Securities Act or in any registration statement that we file. |
The indemnification agreements also set forth certain procedures that will apply in the event of a claim for indemnification thereunder.
II-2
Table of Contents
Except as otherwise disclosed under the heading “Legal Proceedings” in the Business section of this registration statement, there is at present no pending litigation or proceeding involving any of our directors or executive officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
We have an insurance policy in place that covers our officers and directors with respect to certain liabilities, including liabilities arising under the Securities Act or otherwise.
We plan to enter into an underwriting agreement which provides that the underwriters are obligated, under some circumstances, to indemnify our directors, officers and controlling persons against specified liabilities, including liabilities under the Securities Act.
Reference is made to the following documents filed as exhibits to this registration statement regarding relevant indemnification provisions described above and elsewhere herein:
| Exhibit Document |
Number | |||
| Form of Underwriting Agreement |
1.1 | |||
| Form of Registrant’s Amended and Restated Certificate of Incorporation to become effective upon the closing of this offering |
3.2 | |||
| Form of Registrant’s Amended and Restated Bylaws to become effective upon the closing of this offering |
3.4 | |||
| Form of Indemnity Agreement |
10.1 | |||
| Item 15. | Recent sales of unregistered securities. |
The following sets forth information regarding all unregistered securities sold by us since January 1, 2008:
| (1) | Between February 12, 2009 and November 8, 2010, we granted stock options to purchase up to an aggregate of 13,373 shares of our common stock to employees, consultants and directors under our 2006 Equity Incentive Plan at exercise prices ranging from $17.69 and $25.32 per share. Except for options to purchase 442 shares of our common stock, all of these options have since vested. Of these options, as of March 31, 2011, no options to purchase shares of common stock have been exercised and options to purchase 13,287 shares of common stock remain exercisable. |
| (2) | In November 2010, we acquired Insys Pharma, Inc. in the Merger. In connection with the Merger, we issued 319,667 shares of our common stock and 14,864,607 shares of our convertible preferred stock to the stockholders of Insys Pharma, and also assumed stock options of Insys Pharma, which were converted into stock options to purchase up to an aggregate of 1,129,872 shares of our common stock. |
| (3) | On January 24, 2011, we and Insys Pharma issued demand notes to The John N. Kapoor Trust dated September 20, 1989 in an aggregate principal amount of $1.5 million. |
| (4) | On February 11, 2011, we issued a demand note to The John N. Kapoor Trust dated September 20, 1989 in an aggregate principal amount of $2.0 million. |
| (5) | On March 21, 2011, we issued a demand note to The John N. Kapoor Trust dated September 20, 1989 in an aggregate principal amount of $1.5 million. |
| (6) | On March 28, 2011, we granted stock options to purchase up to an aggregate of 508,491 shares of our common stock to employees, consultants and directors under our 2006 Equity Incentive Plan at an exercise price of $4.88 per share. |
| (7) | On April 27, 2011, we issued a demand note to The John N. Kapoor Trust dated September 20, 1989 in an aggregate principal amount of $1.0 million. |
| (8) | On May 27, 2011, we issued a demand note to The John N. Kapoor Trust dated September 20, 1989 in an aggregate principal amount of $1.0 million. |
II-3
Table of Contents
| (9) | On June 28, 2011, we issued a demand note to The John N. Kapoor Trust dated September 20, 1989 in an aggregate principal amount of $1.0 million. |
| (10) | On July 26, 2011, we issued a demand note to The John N. Kapoor Trust dated September 20, 1989 in an aggregate principal amount of $1.0 million. |
All of the offers, sales and issuances of the securities described in paragraph (1), and the offers and issuances of options to purchase an aggregate of 198,043 shares of our common stock described in paragraph (6), were deemed to be exempt from registration under the Securities Act in reliance on Rule 701 in that the transactions were under compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of such securities were our employees, directors or bona fide consultants and received the securities under our 2006 Equity Incentive Plan. Appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions had adequate access, through employment, business or other relationships, to information about us.
The offers, sales, and issuances of the securities described in paragraph (2) were deemed to be exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act as transactions by an issuer not involving a public offering. The recipients of securities in each of these transactions acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof.
The offers, sales, and issuances of the securities described in paragraphs (3), (4), (5), (7), (8), (9) and (10) and the offers and issuances of options to purchase an aggregate of 310,448 shares of our common stock described in paragraph (6), were deemed to be exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act and Rule 506 promulgated under Regulation D promulgated thereunder as transactions by an issuer not involving a public offering. The recipients of securities in each of these transactions acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions was an accredited investor within the meaning of Rule 501 of Regulation D under the Securities Act and had adequate access, through employment, business or other relationships, to information about us.
| Item 16. | Exhibits and financial statement schedules. |
(a) Exhibits.
| Exhibit |
Description of document | |
| 1.1(1) | Form of Underwriting Agreement. | |
| 2.1(1) | Agreement and Plan of Merger Among the Registrant, Insys Therapeutics, Inc. and ITNI Merger Sub Inc. dated October 29, 2010. | |
| 3.1(1) | Registrant’s Amended and Restated Certificate of Incorporation, as amended and as currently in effect. | |
| 3.2(1) | Form of the Registrant’s Amended and Restated Certificate of Incorporation to become effective upon the closing of this offering. | |
| 3.3(1) | Registrant’s Bylaws, as currently in effect. | |
| 3.4(1) | Form of the Registrant’s Amended and Restated Bylaws to become effective upon the closing of this offering. | |
| 3.5(1) | Amended and Restated Certificate of Designations, Preferences and Rights of Convertible Preferred Stock of Insys Therapeutics, Inc. | |
| 3.6(1) | Certificate of Amendment of Amended and Restated Certificate of Designations, Preferences and Rights of Convertible Preferred Stock of Insys Therapeutics, Inc. | |
II-4
Table of Contents
| Exhibit |
Description of document | |
| 4.1(1) | Form of Common Stock Certificate of the Registrant. | |
| 5.1† | Opinion of Cooley LLP. | |
| 10.1+(1) | Form of Indemnity Agreement by and between the Registrant and its directors and officers. | |
| 10.2+(1) | Insys Therapeutics, Inc. 1998 Equity Incentive Plan, as amended. | |
| 10.3+(1) | Insys Therapeutics, Inc. 2006 Equity Incentive Plan, as amended. | |
| 10.4+(1) | Insys Pharma, Inc. Amended and Restated Equity Incentive Plan. | |
| 10.5+ | 2011 Equity Incentive Plan and Form of Stock Option Agreement and Form of Stock Option Grant Notice thereunder. | |
| 10.6+ | 2011 Non-Employee Directors’ Stock Award Plan and Form of Stock Option Agreement and Forms of Stock Option Grant Notice thereunder. | |
| 10.7+ | 2011 Employee Stock Purchase Plan and Form of Offering Document thereunder. | |
| 10.8+(1) | Employment Agreement by and between the Registrant and Michael Babich dated April 29, 2011. | |
| 10.9+(1) | Employment Agreement by and between the Registrant and Larry Dillaha dated April 29, 2011. | |
| 10.10(1) | Lease dated as of March 12, 2007 between the Insys Pharma, Inc. and First Industrial, L.P. as predecessor in interest to Kachina Investments, LLC. | |
| 10.11(1) | Lease Agreement dated as of December 20, 2007, as amended, between the Registrant and Chicago Title Land Trust Company, as successor trustee to LaSalle Bank National Association, as successor trustee to American National Bank and Trust Company of Chicago, as Trustee under Trust Agreement dated March 16, 1987 and known as Trust No. 10207306. | |
| 10.12*(1) | Softgel Commercial Manufacturing and Packaging Agreement dated as of March 21, 2011 between the Registrant and Catalent Pharma Solutions, LLC. | |
| 10.13*(1) | Supply and Distribution Agreement dated as of May 20, 2011 by and between the Registrant and Mylan Pharmaceuticals Inc. | |
| 10.14*(1) | Manufacturing Agreement dated as of May 24, 2011 by and between the Registrant and DPT Lakewood, LLC. | |
| 10.15* | Supply Agreement dated as of March 7, 2011 by and between the Registrant and AptarGroup, Inc. | |
| 21.1(1) | Subsidiaries of the Registrant. | |
| 23.1 | Consent of BDO USA, LLP Independent Registered Public Accounting Firm | |
| 23.2 | Consent of Cooley LLP. Reference is made to Exhibit 5.1. | |
| 24.1(1) | Power of Attorney. | |
| † | To be filed by amendment. |
| + | Indicates management contract or compensatory plan. |
| * | Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC. |
| (1) | Previously filed. |
II-5
Table of Contents
(b) Financial statement schedules.
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or the notes thereto.
| Item 17. | Undertakings. |
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the Underwriting Agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The Registrant hereby undertakes that:
| (a) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (b) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-6
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Phoenix, State of Arizona, on the 16th day of August, 2011.
| INSYS THERAPEUTICS, INC. | ||
| By: |
/s/ MICHAEL L. BABICH | |
| Michael L. Babich | ||
| President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /S/ MICHAEL L. BABICH Michael L. Babich |
President, Chief Executive Officer and Member of the Board of Directors (Principal Executive Officer) |
August 16, 2011 | ||
| /S/ MARTIN MCCARTHY Martin McCarthy |
Chief Financial Officer (Principal Financial and Accounting Officer) | August 16, 2011 | ||
| /S/ JOHN N. KAPOOR* John N. Kapoor, Ph.D. |
Executive Chairman of the Board of Directors | August 16, 2011 | ||
| /S/ PATRICK P. FOURTEAU* Patrick P. Fourteau |
Member of the Board of Directors | August 16, 2011 | ||
| /S/ STEVEN MEYER* Steven Meyer |
Member of the Board of Directors | August 16, 2011 | ||
| /S/ BRIAN TAMBI* Brian Tambi |
Member of the Board of Directors | August 16, 2011 | ||
| /S/ PIERRE LAPALME* Pierre Lapalme |
Member of the Board of Directors | August 16, 2011 | ||
* Pursuant to Power of Attorney
| By: |
/S/ MICHAEL L. BABICH Michael L. Babich Attorney-in-Fact |
|||||
II-7
Table of Contents
EXHIBIT INDEX
| Exhibit |
Description of document | |
| 1.1(1) | Form of Underwriting Agreement. | |
| 2.1(1) | Agreement and Plan of Merger Among the Registrant, Insys Therapeutics, Inc. and ITNI Merger Sub Inc. dated October 29, 2010. | |
| 3.1(1) | Registrant’s Amended and Restated Certificate of Incorporation, as amended and as currently in effect. | |
| 3.2(1) | Form of the Registrant’s Amended and Restated Certificate of Incorporation to become effective upon the closing of this offering. | |
| 3.3(1) | Registrant’s Bylaws, as currently in effect. | |
| 3.4(1) | Form of the Registrant’s Amended and Restated Bylaws to become effective upon the closing of this offering. | |
| 3.5(1) | Amended and Restated Certificate of Designations, Preferences and Rights of Convertible Preferred Stock of Insys Therapeutics, Inc. | |
| 3.6(1) | Certificate of Amendment of Amended and Restated Certificate of Designations, Preferences and Rights of Convertible Preferred Stock of Insys Therapeutics, Inc. | |
| 4.1(1) | Form of Common Stock Certificate of the Registrant. | |
| 5.1† | Opinion of Cooley LLP. | |
| 10.1+(1) | Form of Indemnity Agreement by and between the Registrant and its directors and officers. | |
| 10.2+(1) | Insys Therapeutics, Inc. 1998 Equity Incentive Plan, as amended. | |
| 10.3+(1) | Insys Therapeutics, Inc. 2006 Equity Incentive Plan, as amended. | |
| 10.4+(1) | Insys Pharma, Inc. Amended and Restated Equity Incentive Plan. | |
| 10.5+ | 2011 Equity Incentive Plan and Form of Stock Option Agreement and Form of Stock Option Grant Notice thereunder. | |
| 10.6+ | 2011 Non-Employee Directors’ Stock Award Plan and Form of Stock Option Agreement and Forms of Stock Option Grant Notice thereunder. | |
| 10.7+ | 2011 Employee Stock Purchase Plan and Form of Offering Document thereunder. | |
| 10.8+(1) | Employment Agreement by and between the Registrant and Michael Babich dated April 29, 2011. | |
| 10.9+(1) | Employment Agreement by and between the Registrant and Larry Dillaha dated April 29, 2011. | |
| 10.10(1) | Lease dated as of March 12, 2007 between the Insys Pharma, Inc. and First Industrial, L.P. as predecessor in interest to Kachina Investments, LLC. | |
| 10.11(1) | Lease Agreement dated as of December 20, 2007, as amended, between the Registrant and Chicago Title Land Trust Company, as successor trustee to LaSalle Bank National Association, as successor trustee to American National Bank and Trust Company of Chicago, as Trustee under Trust Agreement dated March 16, 1987 and known as Trust No. 10207306. | |
| 10.12*(1) | Softgel Commercial Manufacturing and Packaging Agreement dated as of March 21, 2011 between the Registrant and Catalent Pharma Solutions, LLC. | |
Table of Contents
| Exhibit |
Description of document | |
| 10.13*(1) | Supply and Distribution Agreement dated as of May 20, 2011 by and between the Registrant and Mylan Pharmaceuticals Inc. | |
| 10.14*(1) | Manufacturing Agreement dated as of May 24, 2011 by and between the Registrant and DPT Lakewood, LLC. | |
| 10.15* | Supply Agreement dated as of March 7, 2011 by and between the Registrant and AptarGroup, Inc. | |
| 21.1(1) | Subsidiaries of the Registrant. | |
| 23.1 | Consent of BDO USA, LLP, Independent Registered Public Accounting Firm. | |
| 23.2 | Consent of Cooley LLP. Reference is made to Exhibit 5.1. | |
| 24.1(1) | Power of Attorney. | |
| † | To be filed by amendment. |
| + | Indicates management contract or compensatory plan. |
| * | Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC. |
| (1) | Previously filed. |
