Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - INSMED Inc | d8k.htm |
 Developing Innovative Inhaled Treatments for Serious
Lung Infections
August 2011
EXHIBIT 99.1 |
 This
presentation contains forward-looking statements which are made pursuant to provisions of
Section 21E of the Securities Exchange Act of 1934. Investors are cautioned that such
statements in this presentation, including statements relating to our financial
position, results of operations, the status and the results of preclinical studies and
clinical trials and preclinical and clinical data described herein,
the
timing
of
responses
to
information
and
data
requests
from FDA, the development of our
products, and the business strategies, plans and objectives of management, constitute
forward- looking statements which involve risks and uncertainties that could cause
actual results to differ materially from those anticipated by the forward-looking
statements. Our results may be affected by such factors as the receipt and timing
of FDA and other regulatory reviews and approvals, if at all, competitive developments
affecting our product development, delays in product development or clinical trials,
and patent disputes involving currently developing products. The risks and uncertainties
include, without limitation, we may experience unexpected regulatory actions, delays or
requests, our future clinical trials may not be successful, we may be unsuccessful in
developing our product candidates or receiving necessary regulatory approvals, we may
experience delays in our product development or clinical trials, our product candidates
may not prove to be commercially successful, our expenses may be higher than
anticipated and other risks and challenges detailed in our filings with the U.S.
Securities and Exchange Commission, including our Annual Report on Form 10-K for the year
ended December 31, 2010 and our Quarterly Report on Form 10-Q for the quarter ended June
30, 2011. Investors are cautioned not to place undue reliance on any
forward-looking statements which speak
only
as
of
the
date
of
this
presentation.
We
undertake
no
obligation to publicly release the
results of any revisions to these forward-looking statements that may be made to reflect
events or circumstances that occur after the date of this release or to reflect the
occurrence of unanticipated events.
Safe Harbor Statement |
 2
Experienced, Successful Combined Management Team
Don Hayden
Chairman
30 Years Experience
15 Years as a Senior Executive, BMS
5 Years Leading Entrepreneurial Biotech Companies
Tim Whitten
President & CEO
25 Years Experience
Commercial
Leadership
–
Oncology,
Immunology,
&
CV
BMS, Pharmacyclics
Kevin Tully CGA
Chief Financial
Officer
30 Years Experience in Finance, Commercial & Manufacturing
International Experience in US, Canada and Europe
Insmed, Tenneco, UCB
Renu Gupta, MD
EVP, Development
& Chief Medical
Officer
25 Years Experience
Infectious Disease Specialty (CHOP); ID & ONC Drug Dev
BMS, Novartis, Covance
Nick LaBella
Chief Scientific
Officer
30 Years Experience
Clinical & Regulatory Leadership; Drug Development
Watson, Sandoz, Cardiokine
Andi Drucker
SVP, General
Counsel & Corp Sec
25 years of legal experience in biotech and pharmaceuticals
Extensive in-house experience including as public company GC
PuriCore, FMC, Novavax, Auxilium
Nick Gurreri
SVP, Commercial
Operations and BD
>20 Years Experience
ID, CV and Ophthalmology Commercial/Marketing
Pfizer, Pharmacia, BMS
The management team has more than 150 years experience in drug
development and commercialization |
 3
Insmed: Value Proposition
Attractive
Late-Stage
Opportunity
Arikace®
(liposomal amikacin for inhalation), is Phase 3-ready for cystic
fibrosis
(CF)
Pseudomonas
and
non-TB
mycobacteria
(NTM)
lung
infections
Amikacin is an FDA-approved antibiotic, long recognized as one of the most
effective treatments for gram-negative infections
Arikace has strong Phase 2 efficacy and safety data
Compelling
Business Model
Arikace
has
global
annual
market
potential
of
over
$1
Billion
in
CF
and
NTM
Limited commercial infrastructure required
Orphan drug indications with high unmet need
Strong IP and potential for extended exclusivity
Strong Balance
Sheet &
Experienced
Management
As of end of 2Q 2011, company had ~$94 million in cash and no debt
Over
150
years
of
combined
experience
in
pharmaceutical
industry
and
drug development
Arikace®
is a potentially highly differentiated phase 3 product opportunity
that offers a compelling business model in two orphan diseases
|
 4
Arikace®
—
Update on Clinical Hold
Insmed announced on 8/1/11 that the FDA placed the Phase 3 clinical trials
for Arikace in CF and NTM on clinical hold based on the Agency's
initial
review of findings from a rat inhalation carcinogenicity study
Insmed believes it has sound scientific rationale for the findings and expects to
supply the requested information and data to FDA before end of August
FDA has advised Insmed that the Agency will respond to the Company within 30
days following receipt of Insmed’s complete response to FDA’s requests
Based on the efficacy and safety data generated from the Phase 2
clinical trial
program, Insmed continues to believe Arikace®
has the potential to be an
important treatment option for CF and NTM patients
Depending upon timing of FDA’s review and response, the Company is hopeful
that the phase 3 clinical trial program for Arikace®
can be resumed during
4Q ‘11 |
 5
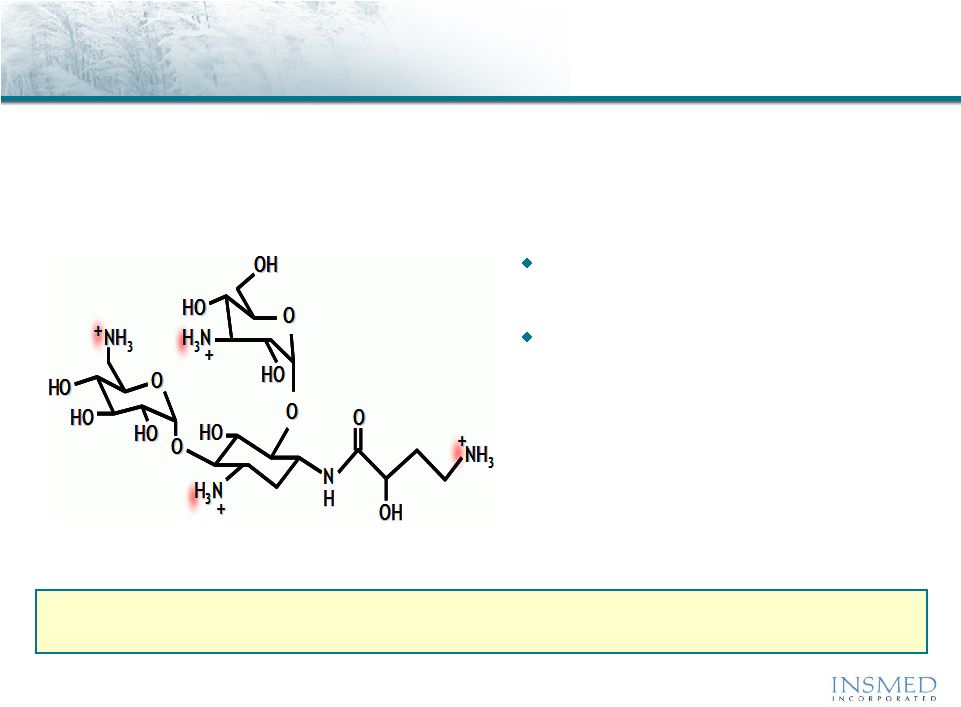
Arikace™: Amikacin Summary
Amikacin is an FDA-approved antibiotic with proven efficacy in the
treatment of gram-negative infections, including Pseudomonas and NTM
Member of the aminoglycoside
class of antibiotics
The value of the IV use has been
limited by issues of nephro-
toxicity and ototoxicity
Arikace (liposomal amikacin for inhalation) delivers high, sustained levels of drug to
the lung while reducing systemic exposure to well below established toxicity
levels |
 6
Arikace: Proprietary Liposomal Formulation
Engineered Specifically for Lung Delivery
Prolonged lung residence time
Minimal systemic exposure
Biofilm penetration
Preferential uptake into macrophages
Benefit
Lipid Polar Head Groups
(at Both Surfaces)
Lipid Hydrophobic Chains
(Bi-Layer Interior)
Water Core (where Amikacin resides)
Arikace delivers the potency of Amikacin at the site of lung infection while
minimizing potential systemic toxicity
Potential for greater efficacy
Addresses safety concerns
Once-a-day dosing and
potential for greater efficacy
Potential for greater efficacy |
 7
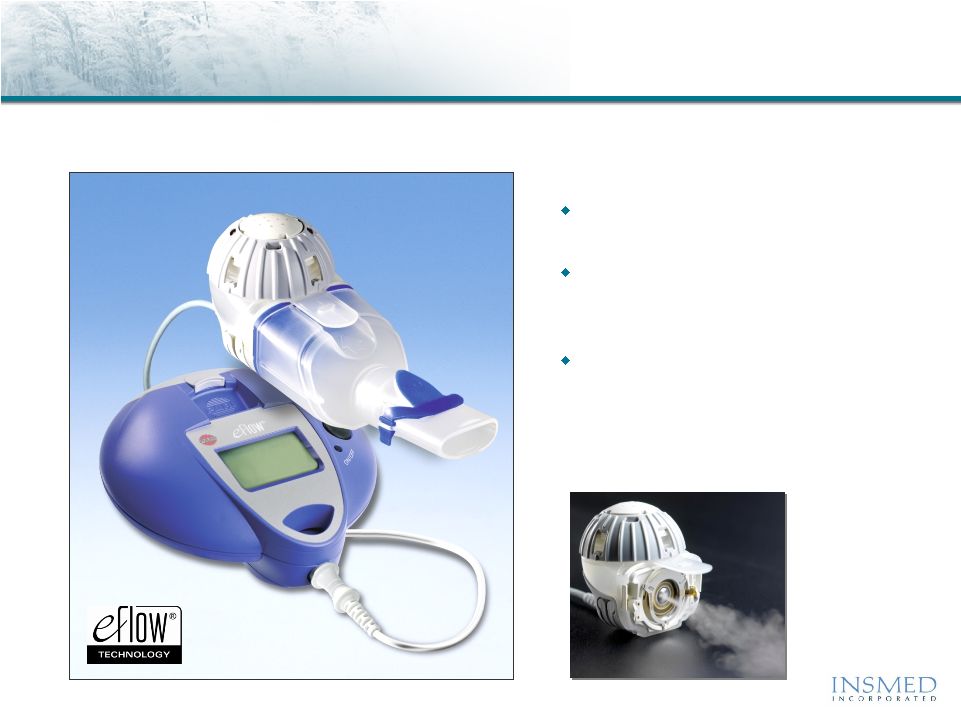
Arikace: Delivery Using Proprietary eFlow Technology
®
Arikace is delivered once daily via the state-of-the-art PARI Optimized,
Investigational eFlow
®
Nebulizer System with Advanced Mesh Technology
Fast
drug delivery with efficient
lung deposition
Small, portable, silent and
cordless
device weighs less than
10 ounces.
eFlow Technology Device
exclusivity
from PARI Pharma for
15 years after first commercial
sale of Arikace |
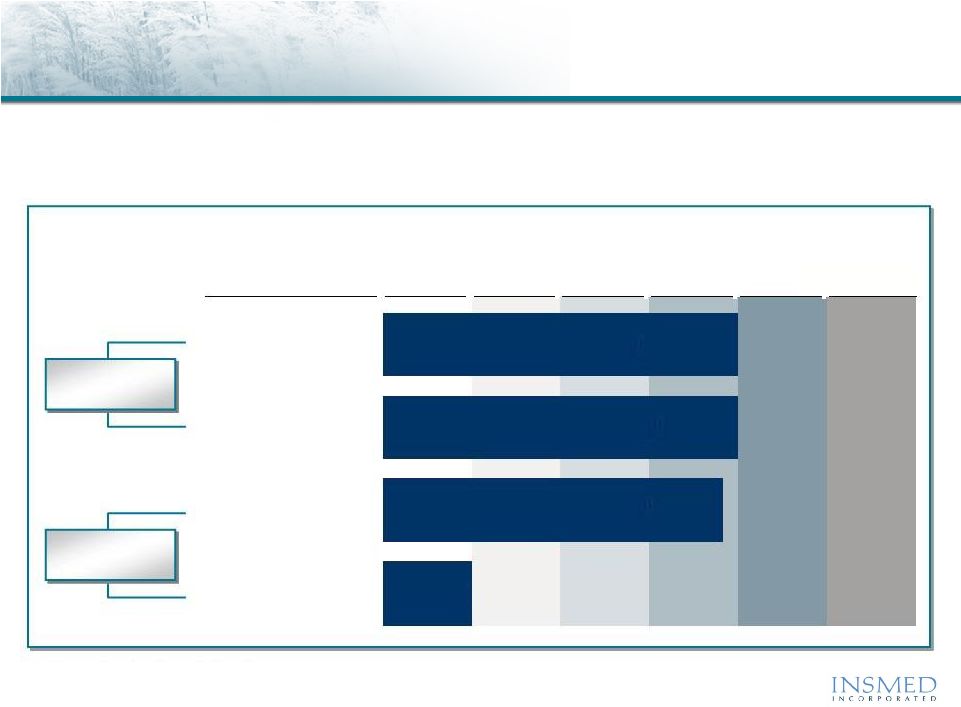 8
Global Market Potential
($ in Millions)
Arikace: “Pipeline in a Product”
Arikace offers efficient, rapid sequential entry into growing markets that we
believe require limited commercial infrastructure
Source: Based on Insmed estimates.
Product
Discovery
Preclinical
Phase 1
Phase 2
Cystic Fibrosis (CF)
$500 -
$1,000
Non-TB
Mycobacterium
$500 -
$750
Non-CF Bronchiectasis
$750+
Other Opportunities
Multiple
Lead
Indications
Follow-on
Opportunities
Phase 3
Approval |
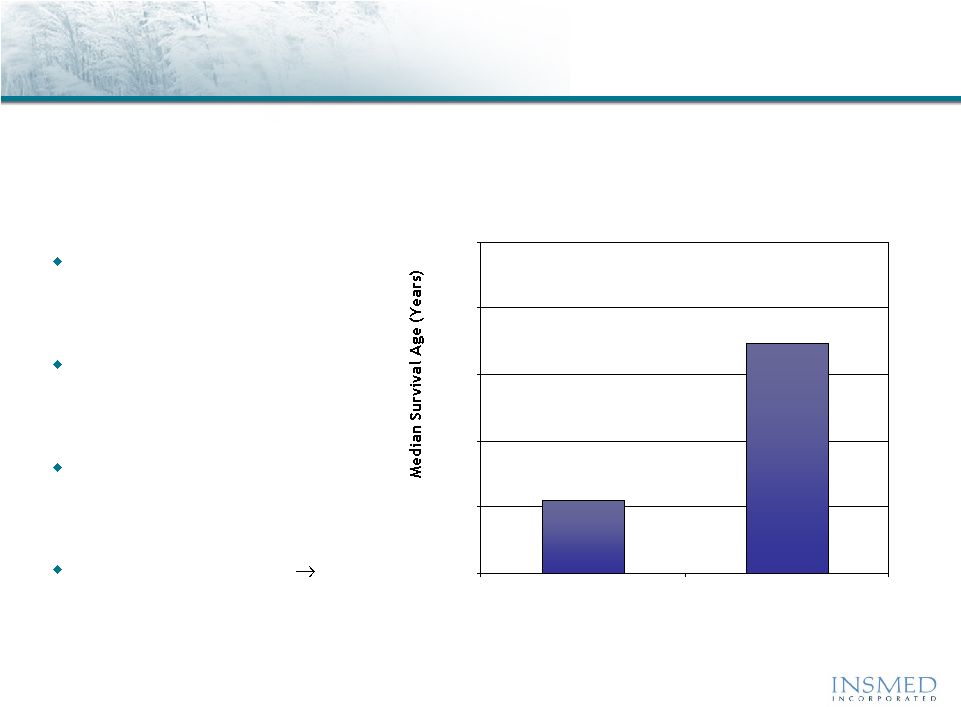 9
Arikace—Cystic Fibrosis
Epidemiology and Disease Description
Cystic fibrosis is a life-threatening disease with significant unmet needs
that is growing in prevalence
Affects about 30,000 children
and adults in the U.S. (70,000
worldwide)
Inherited disease that causes
thick, sticky mucus to build up
in the lungs
Despite expanded use of current
products, lung function often
continues to decline
High treatment burden
major compliance issue
20
25
30
35
40
45
1985
2008
U.S. CF Patients
Median Predicted Survival Age
Source: Adapted from Cystic Fibrosis Foundation, Patient Registry
2008 and 2007 Annual Reports, Bethesda, Maryland. |
 10
Arikace—Cystic Fibrosis
Need for New Inhaled Antibiotics
Current inhaled antibiotics produce modest efficacy in a limited
patient
population providing an opportunity for Arikace to become first-line
treatment
Current inhaled antibiotics are not indicated for a significant segment of
the CF population --
patients with FEV-1 % predicted of greater than 75%
Efficacy of current inhaled antibiotics declines from cycle to cycle and is
not sustained at all in the off-treatment period
Lung function continues to decline at an average of 1% to 3% per
year with
some patients experiencing much greater declines |
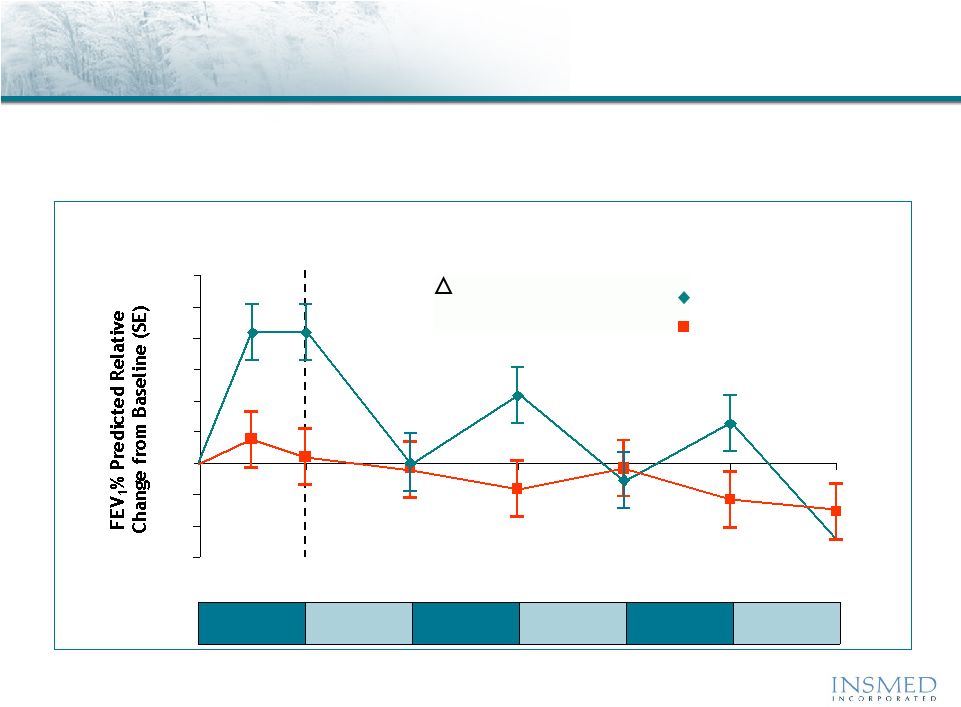 11
-6
-4
-2
0
2
4
6
8
10
12
0
4
8
12
16
20
24
Aztreonam vs. Tobi
®
(inhaled tobramycin)
CF Phase 3 Trial Results: Pulmonary Function
Lung Function
Adjusted
Mean
Relative
Change
in
FEV
1
%
Predicted
Source: 2010 North American CF Conference Poster 305 and Slide Presentation,
10/10. •Tobi
®
(Tobramycin
Inhalation
Solution)
is
a
registered
trademark
of
Novartis.
** AZLI = Aztreonam; TIS = Tobi
®
Lung function returned to baseline or lower during each off treatment
period and at the end of 24 weeks, both treatment groups showed a
decline in lung function from baseline
Week:
2
AZLI
TIS
+ 7.8
P
= 0.0001
95% CI (3.86, 11.73)
AZLI/
TIS
28 Days
AZLI/
TIS
28 Days
AZLI/
TIS
28 Days |
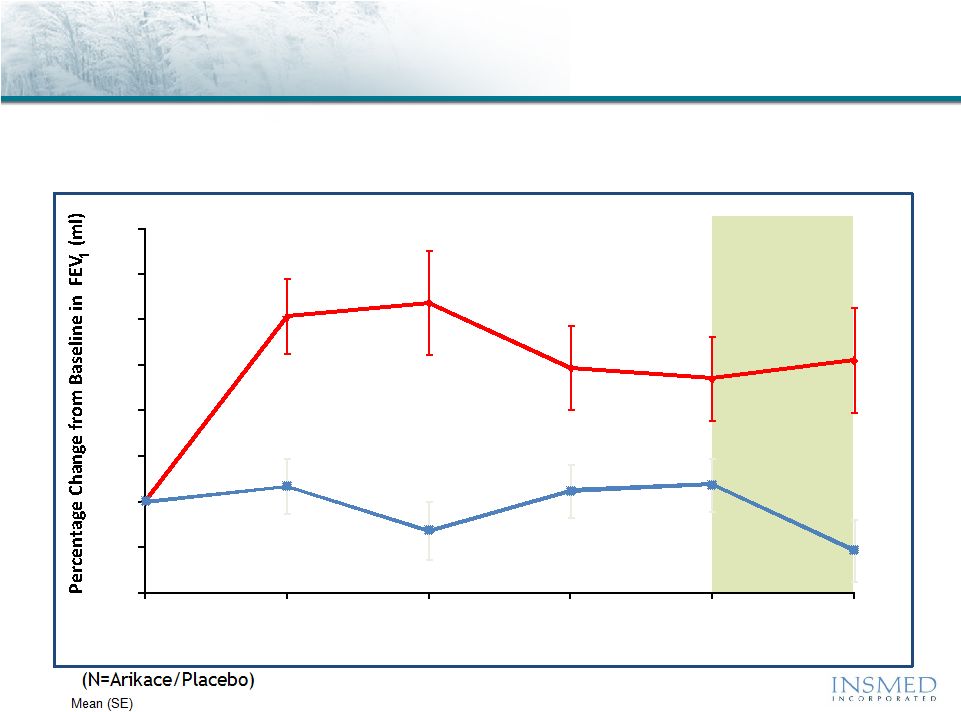 12
Off-Treatment
Period
P = 0.033
P = 0.003
(36/36)
(36/35)
(33/36)
(32/35)
(34/35)
(34/34)
Arikace—Cystic Fibrosis
Phase 2 Pooled Results (560mg QD): Pulmonary Function
(N)
Arikace demonstrated statistically significant and clinically meaningful
improvement in pulmonary function throughout the 28-day treatment
period that was sustained through the off-treatment period
-6%
-3%
0%
3%
6%
9%
12%
15%
18%
0
7
14
21
28
56
Visit Day
% Change in FEV
1
(ml) vs. Baseline
Arikace
560mg
Placebo |
 13
Visit Days
Patients
Receiving
560
mg
Arikace
®
Once
Daily
for
28
Days
and
Off-Treatment
for
56
Days
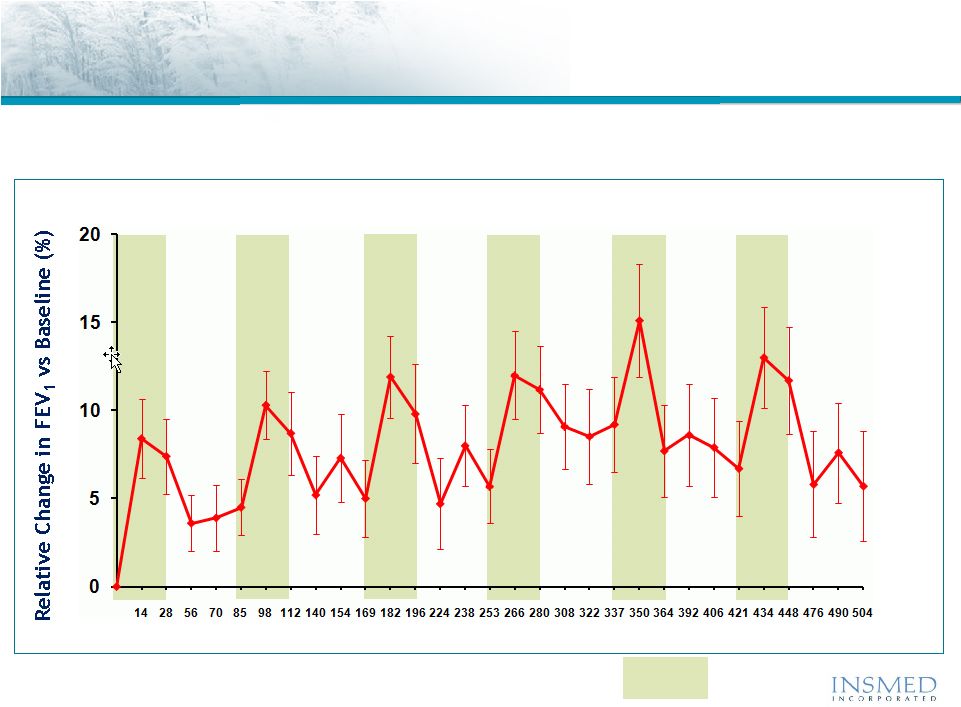
Arikace—Cystic Fibrosis
Open Label Extension (TR02-105): Duration of Response
42
41
42
42
41
41
41
41
41
45
Treatment
Period
* Significance at end of treatment over 6 cycles
** Significance 56 days off-treatment over 6 cycles
N=
45
47
46
44
45
47
46
46
45
44
44
44
43
43
43
43
44
43
45
p=0.0001**
p<0.0001*
41
47
Cycle
1
Cycle
2
Cycle
3
Cycle
4
Cycle
5
Cycle
6
An open label extension study TR02-105 demonstrated the sustained efficacy
Of Arikace during and between multiple cycles of therapy |
 14
Arikace—Cystic Fibrosis
Phase III Program (pending FDA review and lifting of clinical hold)
Insmed had reached agreement with FDA and EMA on NDA/MAA path for CF
Patients with Pseudomonas lung infections
One E.U. Phase 3 (Arikace vs Tobi
®
Study, N = 300)
–
Primary End-Point: Relative Change in FEV-1 at week 24
•
Key Secondary End-Point: Time to Pulmonary
Exacerbation •
Key inclusion Criteria: FEV-1 % Predicted > 25%
–
Approximately 260 patients required to demonstrate non-inferiority at agreed upon
margin with 80% power
One U.S. Phase 3 (Arikace vs Placebo Study, N = 300)
–
Primary End-Point: Time to Pulmonary Exacerbation
•
Key Secondary End-Point: Relative Change in FEV-1
•
Key inclusion Criteria: FEV-1 % Predicted > 25%
–
Approximately 260 patients required to demonstrate superiority with 80% power
–
Estimate 50% reduction in frequency of exacerbations
–
Prospectively planned to evaluate pooled event rate to assess adequacy of sample
|
 15
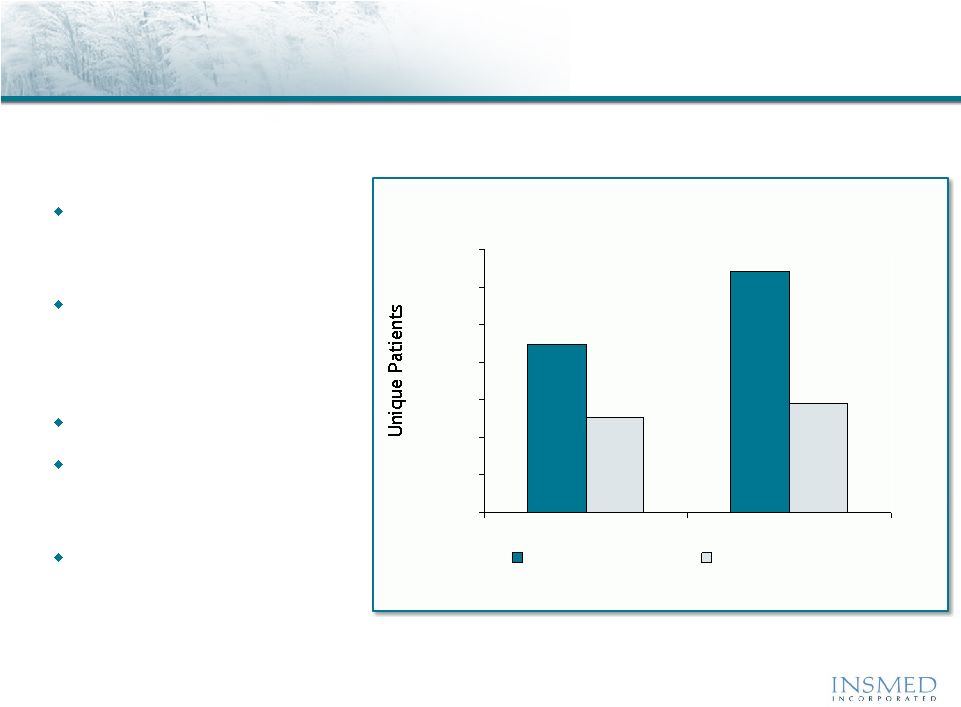
Arikace—Non-TB Mycobacteria
Epidemiology and Disease Description
Diagnosis of NTM pulmonary
infections are growing
rapidly
Current treatment
approaches have limited
efficacy and are associated
with significant toxicity
Mean age of 66 years
66% treated with
antibiotics; mean of 7.6
courses per year
Average Length of Inpatient
Hospital Stay = 10.2 days
Non-TB mycobacteria (NTM) is an intracellular pathogen that can cause
severe pulmonary disease with limited effective treatment options
Source: SDI Healthcare Database, July 2009.
0
5,000
10,000
15,000
20,000
25,000
30,000
35,000
2006
2008
Physician Office
Total Hospital
Total U.S. Patients Diagnosed with Non-TB Mycobacteria
Pulmonary Disease
32.0K +20%
2-Yr Growth
14.4K +7%
2-Yr Growth |
 16
Arikace—Non-TB Mycobacteria
Summary of Opportunity
Opportunity for accelerated path to market for chronic treatment
of NTM
High unmet need
Current treatment often includes chronic use of poorly formulated, compounded
solutions of amikacin
Arikace opportunity: provide superior efficacy in NTM treatment by better penetrating
macrophages where NTM bacteria reside while limiting systemic drug
exposure If approved, Arikace would be the first drug approved for NTM lung
disease in more than a decade
IND filed for primary efficacy study in February 2011 and clearance from FDA to
proceed received in March
Clinical trial costs and execution partially supported by a CRADA with NIH
|
 17
Arikace—Non-TB Mycobacteria
Phase 3 Program (pending FDA review and lifting of clinical hold)
Trial Design and Patient Population:
–
Randomized, double-blind, placebo controlled phase 3 study in patients with
recalcitrant/persistent NTM lung disease who are on a stable ATS/IDSA
guidelines-based multi-drug treatment regimen
–
Patients receive Arikace or placebo daily for 84 days; then all patients can
receive Arikace 560 mg in an open-label manner for an additional 84 days
Key Inclusion Criteria: History of chronic infection with either Mycobacterium
avium complex or Mycobacterium abscessus or mixed infection with both species
Primary endpoint: Change in mycobacterial culture results from baseline to
end of treatment [Time
Frame:
84 days]
High unmet medical need enables a phase 3 program consisting of a single
study of approximately 100 patients |
 18
Projected Cash
at year end
2011
Approximately $70 to $75 million currently forecast
Current Overview: Capital Structure and Key Financials
Pro Forma
Balance Sheet
Pro forma cash of ~$94 million as of June 30, 2011
No Debt
Present Capital
Structure
25.8 M fully diluted shares following reverse stock split:
24.8 million Common Shares
1.0 million options, restricted stock and warrants
Insmed has a strong cash position and no debt |
 19
Arikace Intellectual Property and Exclusivity
Composition-of-matter patent issued in U.S. with protection to October 2026
Additional applications under review in the U.S. and E.U. including an E.U.
composition- of-matter application
Orphan drug status provides additional protection and financial benefits
eFlow Technology Device exclusivity from PARI Pharma for 15 years after first
commercial sale of Arikace
Liposomal delivery technology provides additional regulatory hurdle
Insmed has extensive patent coverage and other exclusive agreements
protecting composition, use, and delivery of Arikace |
 20
Insmed: Value Proposition
Attractive
Late-Stage
Opportunity
Arikace®
(liposomal amikacin for inhalation), is Phase 3-ready for cystic
fibrosis
(CF)
Pseudomonas
and
non-TB
mycobacteria
(NTM)
lung
infections
Amikacin is an FDA-approved antibiotic, long recognized as one of the most
effective treatments for gram-negative infections
Arikace has strong Phase 2 efficacy and safety data
Compelling
Business Model
Arikace
has
global
annual
market
potential
of
over
$1
Billion
in
CF
and
NTM
Limited commercial infrastructure required
Orphan drug indications with high unmet need
Strong IP and potential for extended exclusivity
Strong Balance
Sheet &
Experienced
Management
As of end of 2Q 2011, company had ~$94 million in cash and no debt
Over
150
years
of
combined
experience
in
pharmaceutical
industry
and
drug development
Arikace®
is a potentially highly differentiated phase 3 product opportunity
that offers a compelling business model in two orphan diseases
|
