As filed with the U.S. Securities and
Exchange Commission on July 22, 2011
Registration
No. 333-174583
UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C.
20549
Amendment No. 2
to
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
MYRIANT CORPORATION
(Exact name of Registrant as
specified in its charter)
| |
|
|
|
|
|
Delaware
|
|
2869
|
|
26-4724442
|
(State or other jurisdiction
of
incorporation or organization)
|
|
(Primary Standard Industrial
Classification Code Number)
|
|
(I.R.S. Employer
Identification Number)
|
1 Pine Hill Drive, Batterymarch Park II, Suite 301,
Quincy, MA 02169
(617) 657-5200
(Address, including zip code,
and telephone number, including area code, of Registrant’s
principal executive offices)
Stephen J. Gatto
Chief Executive Officer
Myriant Corporation
1 Pine Hill Drive
Batterymarch Park II, Suite 301
Quincy, MA 02169
(617) 657-5200
(Name, address, including zip
code, and telephone number, including area code, of agent for
service)
Copies to:
| |
|
|
|
Byron S. Kalogerou
|
|
Donald J. Murray
|
|
David A. Cifrino
|
|
Dewey & LeBoeuf LLP
|
|
McDermott Will & Emery LLP
|
|
1301 Avenue of the Americas
|
|
28 State Street
|
|
New York, NY 10019-6092
|
|
Boston, MA 02109
|
|
Telephone: (212) 259-8000
|
|
Telephone:
(617) 535-4000
|
|
Facsimile: (212) 259-6333
|
|
Facsimile:
(617) 535-3800
|
|
|
Approximate date of commencement of proposed sale to the
public: As soon as practicable after the
effective date of this Registration Statement.
If any of the securities being registered on this Form are to be
offered on a delayed or continuous basis pursuant to
Rule 415 under the Securities Act of 1933, check the
following
box. o
If this Form is filed to register additional securities for an
offering pursuant to Rule 462(b) under the Securities Act,
check the following box and list the Securities Act registration
statement number of the earlier effective registration statement
for the same
offering. o
If this Form is a post-effective amendment filed pursuant to
Rule 462(c) under the Securities Act, check the following
box and list the Securities Act registration statement number of
the earlier effective registration statement for the same
offering. o
If this Form is a post-effective amendment filed pursuant to
Rule 462(d) under the Securities Act, check the following
box and list the Securities Act registration statement number of
the earlier effective registration statement for the same
offering. o
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in
Rule 12b-2
of the Exchange Act. (Check one):
| |
|
|
|
|
|
|
|
Large accelerated
filer o
|
|
Accelerated
filer o
|
|
Non-accelerated
filer þ
|
|
Smaller reporting
company o
|
|
|
|
|
|
(Do not check if a smaller reporting company)
|
|
|
CALCULATION
OF REGISTRATION FEE
| |
|
|
|
|
|
|
|
|
|
|
Proposed Maximum
|
|
|
Amount of
|
Title of Each Class of
|
|
|
Aggregate Offering
|
|
|
Registration
|
|
Securities to be Registered
|
|
|
Price(1)
|
|
|
Fee
|
|
Common Stock, $0.0001 par value
|
|
|
$125,000,000
|
|
|
$14,512.50(2)
|
|
|
|
|
|
|
|
|
|
|
| (1)
|
Estimated solely for the purpose of computing the amount of the
registration fee pursuant to Rule 457(o) under the
Securities Act of 1933. Includes the offering price of
additional shares that the underwriters have the option to
purchase.
|
| |
| (2)
|
Previously paid.
|
The Registrant hereby amends this Registration Statement on
such date or dates as may be necessary to delay its effective
date until the Registrant shall file a further amendment which
specifically states that this Registration Statement shall
thereafter become effective in accordance with Section 8(a)
of the Securities Act of 1933 or until the Registration
Statement shall become effective on such date as the Commission,
acting pursuant to said Section 8(a), may determine.
The
information contained in this preliminary prospectus is not
complete and may be changed. We may not sell these securities
until the registration statement filed with the Securities and
Exchange Commission is effective. This preliminary prospectus is
not an offer to sell these securities and we are not soliciting
offers to buy these securities in any jurisdiction where the
offer or sale is not permitted.
|
SUBJECT TO
COMPLETION ,
2011
PRELIMINARY PROSPECTUS
Shares
Common Stock
This is the initial public offering of our common stock. No
public market currently exists for our common stock. We are
offering all of the shares of common stock offered by this
prospectus. We expect the public offering price to be between
$ and
$ per share.
We have applied to list our common stock on The Nasdaq Global
Market under the symbol “MYRT.”
Investing in our common stock involves a high degree of risk.
Before buying any shares, you should carefully read the
discussion of material risks of investing in our common stock in
“Risk factors” beginning on page 11 of this
prospectus.
Neither the Securities and Exchange Commission nor any state
securities commission has approved or disapproved of these
securities or determined if this prospectus is truthful or
complete. Any representation to the contrary is a criminal
offense.
| |
|
|
|
|
|
|
|
|
|
|
|
Per Share
|
|
Total
|
|
|
|
Public offering price
|
|
$
|
|
|
|
$
|
|
|
|
Underwriting discounts and commissions
|
|
$
|
|
|
|
$
|
|
|
|
Proceeds, before expenses, to us
|
|
$
|
|
|
|
$
|
|
|
The underwriters have the option to purchase from us up to an
additional shares of our
common stock at the public offering price, less the underwriting
discounts and commissions payable by us, to cover
over-allotments, if any, within 30 days from the date of
this prospectus. If the underwriters exercise this option in
full, the total underwriting discounts and commissions will be
$ , and our total proceeds, after
underwriting discounts and commissions but before expenses, will
be $ .
The underwriters are offering the common stock as set forth
under “Underwriting.” Delivery of the shares will be
made on or
about ,
2011.
Morgan Joseph
TriArtisan
Prospectus
dated ,
2011
You should rely only on the information contained in this
prospectus. We and the underwriters have not authorized anyone
to provide you with information different from that contained in
this prospectus. We are offering to sell, and seeking offers to
buy, shares of common stock only in jurisdictions where offers
and sales are permitted. The information contained in this
prospectus is accurate only as of the date on the front cover of
this prospectus, or such other dates as are stated in this
prospectus, regardless of the time of delivery of this
prospectus or of any sale of our common stock.
TABLE OF
CONTENTS
| |
|
|
|
|
|
|
|
Page
|
|
|
|
|
|
|
ii
|
|
|
|
|
|
1
|
|
|
|
|
|
10
|
|
|
|
|
|
12
|
|
|
|
|
|
30
|
|
|
|
|
|
32
|
|
|
|
|
|
33
|
|
|
|
|
|
34
|
|
|
|
|
|
36
|
|
|
|
|
|
38
|
|
|
|
|
|
40
|
|
|
|
|
|
58
|
|
|
|
|
|
61
|
|
|
|
|
|
87
|
|
|
|
|
|
95
|
|
|
|
|
|
132
|
|
|
|
|
|
134
|
|
|
|
|
|
140
|
|
|
|
|
|
142
|
|
|
|
|
|
146
|
|
|
|
|
|
153
|
|
|
|
|
|
153
|
|
|
|
|
|
153
|
|
|
|
|
|
F-1
|
|
| EX-10.20 |
| EX-23.1 |
Conventions
that apply to this prospectus
Unless the context otherwise requires, in this prospectus:
|
|
|
| |
•
|
“the company,” “we,”
“us” and “our” refer to
Myriant Corporation and its subsidiaries, or its predecessor
prior to July 16, 2009, as the context requires.
|
| |
| |
•
|
“CBOT” refers to the Chicago Board of Trade.
|
| |
| |
•
|
“EIA” refers to the U.S. Energy
Information Association.
|
| |
| |
•
|
“USDA” refers to U.S. Department of
Agriculture.
|
| |
| |
•
|
“DOE” refers to U.S. Department of Energy.
|
Certain market data presented in this prospectus have been
derived from data published by government agencies and
commodities exchanges, as well as private sector organizations.
These agencies, exchanges and organizations include those listed
above as well as leading chemical industry consulting firms.
Certain target market size information presented in this
prospectus has been calculated by us (as further described
below) based on such data. Unless otherwise indicated, market
data included in this prospectus were derived as discussed
below. None of these entities has endorsed or otherwise passed
upon the computations, accuracy or presentations of data
calculated on the basis of data published by these entities. In
addition, you should read our cautionary statement in the
section entitled “Special note regarding forward-looking
statements.”
With respect to our calculation of product market volumes and
market sizes contained in this prospectus:
|
|
|
| |
•
|
product market volumes are provided solely to show the magnitude
of the potential markets for biobased chemical intermediates and
the products derived from them. They are not intended to be
projections of our biochemical production volumes or sales;
|
| |
| |
•
|
target market sizes are calculated based on estimated product
market volumes consumed within the petrochemicals industry
multiplied by current market prices; and
|
| |
| |
•
|
volume data with respect to target market sizes are derived from
data included in various industry publications, surveys and
forecasts. We have converted these sizes into volumes of
biochemical equivalent as follows:
|
|
|
|
| |
•
|
we calculate the size of the biochemicals markets by
substituting volumes of biochemical equivalent to the volume of
products currently used to serve these markets; and
|
| |
| |
•
|
for consistency in measurement, where necessary, we convert
market sizes into pounds.
|
ii
Prospectus
summary
This summary highlights information contained elsewhere in
this prospectus and does not contain all of the information you
should consider in making your investment decision. You should
read this summary together with the more detailed information,
including our financial statements and the related notes,
appearing elsewhere in this prospectus. In particular, you
should carefully consider the matters discussed in “Risk
factors” before making an investment decision.
We are a development stage company with a history of losses.
We have generated minimal revenues and have had limited
sales.
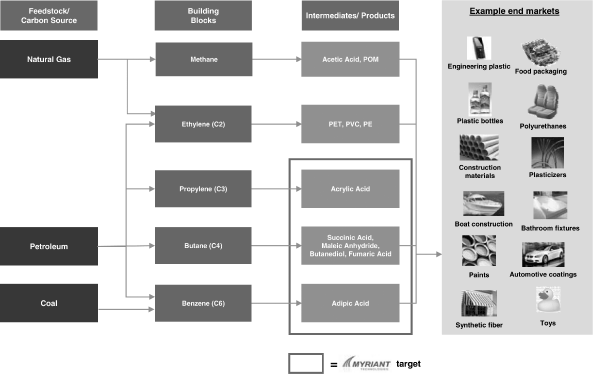
OVERVIEW
We are an industrial biotechnology company focused on becoming a
low-cost producer of biobased chemicals. We have designed a
process that uses proprietary microorganisms, which we call
“biocatalysts,” to convert a variety of sugars into
chemical precursors, called “intermediates.” We plan
to sell those “biobased” intermediates into the large
and growing petrochemicals industry.
Petrochemical companies manufacture and sell a broad range of
chemicals used to make thousands of industrial and consumer
products. Historically, these chemicals have been sourced from
oil, natural gas and coal. These sources, or
“feedstocks,” are extremely rich in carbon-based
molecules, called organic molecules. Organic molecules will
readily react with other organic or inorganic chemicals, and by
controlling these reactions, industrial chemists can convert
organic intermediates into thousands of different chemicals with
specific properties tailored to commercial applications. In
recent years, however, the petrochemicals industry has become
increasingly challenged by a variety of factors, including the
rising and volatile prices of fossil-fuels, increasing
regulation of the sourcing and processing of fossil fuel based
feedstock and concerns over depleting supplies of fossil fuels.
One solution to these challenges is to use industrial sugars to
produce biobased chemicals.
We have developed a proprietary technology platform that we
believe will enable us to manufacture biobased chemical
intermediates to replace petroleum-based chemicals in the same
applications using a broad range of renewable feedstocks, rather
than petroleum-based feedstocks. Through a process known as
directed evolution, we adapt microorganisms to convert sugars
into organic chemical intermediates with greater productivity
and yield compared to other known bioproduction processes. We
believe that we can produce our target chemical intermediates at
an average of half the cost of traditional petrochemical
intermediates at a wide range of oil and industrial sugar prices
without relying on government subsidies.
We are using our technology to produce, at competitive costs,
biobased chemical intermediates using commercial-scale unit
operations and multiple feedstocks. Our technology combines
proprietary microorganisms, or biocatalysts, and a fermentation
process capable of using a variety of renewable feedstocks to
create biobased chemical intermediates. We have successfully
produced several of these intermediates at laboratory and pilot
scale and have commercialized our first product, lactic acid,
through a licensee. We are preparing to commercialize our second
product, succinic acid. We refer to succinic acid produced using
our bioproduction processes as “biosuccinic acid.” Our
biosuccinic acid is chemically identical to succinic acid
produced from petrochemicals.
We plan to market our biobased chemical intermediates for use in
chemical reaction processes requiring the same or similar
intermediates as an input. We refer to this as a “drop
in” product. We also plan to market our products to replace
different chemicals in various manufacturing processes.
Replacing a different chemical input is possible by adjusting
the chemical reaction process and either ending up with a
functionally similar or the same end product. We believe that
our market opportunities include biosuccinic acid (a
$7.5 billion market at current market prices), fumaric acid
(a $1.7 billion market at current market prices), acrylic
acid (a $14.5 billion market at current market prices) and
lactic acid (forecast to eventually become a multi-billion pound
market).
We believe that our technology can efficiently produce biobased
chemicals at high yields while consuming less feedstock by using
an anaerobic process (that is, a reaction process that does not
require oxygen) that consumes, rather than produces, carbon
dioxide, resulting in a reduced carbon footprint and higher
yield of the target product. Based on current commercial-scale
costs and using 95 Dextrose, a widely
1
available industrial sugar, as a feedstock, we estimate that the
production process for our biosuccinic acid, will be
cost-competitive with petroleum-based processes down to $45 per
barrel of oil.
Furthermore, our biocatalysts can consume sugars from a variety
of sources, including glucose from corn and grain sorghum,
sucrose from sugarcane, cellulosic feedstocks from waste
biomass, and glycerol. Cellulosic feedstocks are non-food based
feedstocks derived from agricultural, forestry and other types
of organic wastes. In terms of their chemical composition,
cellulosic feedstocks are a mixture of polymers, or chains, of
sugars with five or six carbon atoms per sugar molecule. Xylose
is an example of a five-carbon sugar. Glucose is an example of a
six-carbon sugar. Our biocatalysts can consume both five-carbon
sugars and six-carbon sugars derived from cellulosic feedstocks,
which we believe will lower the cost of feedstocks when they
become more widely available. This is because cellulosic
feedstocks tend to be much cheaper than food-based feedstocks,
resulting in lower overall product manufacturing cost. We plan
to use the lowest cost regional feedstocks or a combination of
low-cost feedstocks in our manufacturing plants.
We have entered into or intend to enter into strategic
relationships with international companies to accelerate the
global commercialization of our products and development of our
biochemical production capabilities. For example, we have signed
a non-binding memorandum of understanding to enter into a joint
development agreement with Johnson Matthey PLC’s
subsidiary, Davy Process Technology Limited, or Davy, a
developer and licensor of advanced process technologies used to
produce petrochemicals. Under that agreement, upon successful
completion of testing and engineering using our biosuccinic
acid, Davy would guarantee the use of our biosuccinic acid in
plants using the Davy process to manufacture butanediol, a
widely used petrochemical. Davy believes that its butanediol
process accounts for approximately 1.2 billion pounds or
27% of total global capacity, and 50% of plant capacity
installed since 1992. We have also signed exclusive alliance
agreements with ThyssenKrupp’s subsidiary Uhde GmbH, or
Uhde, a chemical plant engineering company, and its
U.S. subsidiary, under which Uhde will integrate our
fermentation and its separation technology in biochemical
manufacturing plants and guarantee the performance of those
plants to facilitate access to project finance. In addition,
through PTT Chemical International Private Limited, or CH Inter,
a subsidiary of PTT Chemical Public Company Limited, or PTTCH, a
Thailand-based petrochemical producer, we can access a breadth
of commercial and technical expertise and extensive knowledge
and infrastructure in Asian markets.
In January 2006, we granted Purac Biochem BV, or Purac, a
wholly-owned subsidiary of CSM N.V., an exclusive, worldwide and
royalty-bearing license to use our technology to produce, market
and sell D(−) lactic acid, as well as its derivatives and
by-products. Purac has been using our technology to produce
D(−) lactic acid at commercial scale since 2008.
We are currently building a 30 million pound biosuccinic
acid plant in Lake Providence, Louisiana, or the Louisiana
Plant, which we expect will begin commercial operations during
the first quarter of 2013. We are funding the construction of
the Louisiana Plant in part with a $50 million cost-sharing
award from the U.S. Department of Energy, or the DOE. We
intend to expand the annual production capacity of this plant to
approximately 170 million pounds by the end of the first
quarter of 2014. We are also negotiating a letter of intent with
Uhde for an industrial biochemical facility for the production
of biosuccinic acid at the Infraleuna Chemical Site in Leuna,
Germany. The plant, which we expect would commence operations in
the first half of 2012, would utilize our technologies to
produce biosuccinic acid and ammonium sulfate in accordance with
our product specifications. We have also signed a memorandum of
understanding with China National BlueStar (Group) Co. Ltd., or
BlueStar, to develop a proposal for a jointly-owned
220 million pound biosuccinic acid plant in Nanjing, China,
the biosuccinic acid requirements of which would be exclusively
supplied by the Company. BlueStar is currently producing BDO
utilizing a process licensed from Davy.
We have already produced 24 metric tons of biosuccinic acid in
support of internal and customer/vendor sampling and testing
programs. We scaled up these quantities from an initial
fermentation vessel size of five liters to 50,000 liters from
January 2008 to February 2011 at various locations. The
commercial
scale-up of
the Louisiana Plant represents a two-fold
scale-up of
the fermentation already commercialized by Purac in the
production of our biobased D(−) lactic acid. We have
contracts committing customers to buy 100% of their annual
succinic acid requirements from us, consuming a substantial
portion, and prospectively all, of the
2
Louisiana Plant’s initial capacity based on these
customers’ current stated forecasts for biosuccinic acid
demand. We are in discussions with prospective customers who
have indicated interest in purchasing the biosuccinic acid that
we would produce from the additional capacity created upon the
expansion of the Louisiana Plant.
Our
Commercial Products
Biosuccinic
Acid
We are currently focused on commercializing and producing
biosuccinic acid. Succinic acid is a chemical building block
that is used as an intermediate in the production of numerous
large-volume products, including plastics, fibers,
coatings, solvents and cosmetics. The U.S. Department of
Energy and others have identified biosuccinic acid as one of the
five most promising “building block” chemicals that
can be produced commercially from biomass rather than fossil
fuels. This product represents a market opportunity of
$7.5 billion. We believe we will be a low-cost producer of
biosuccinic acid relative to producers using either traditional
petroleum-based processes, or other known bioproduction
techniques.
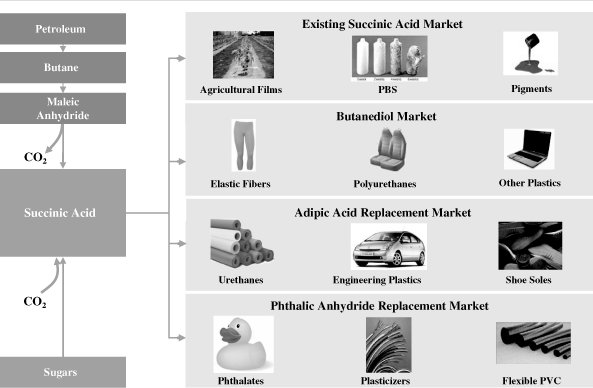
There is currently a small existing merchant market for succinic
acid for use in pigments, solvents, detergents, metal plating
and PBS polymers. In addition to targeting the existing succinic
acid market, we plan to sell our biosuccinic acid as a drop-in
or replacement chemical in the following target markets:
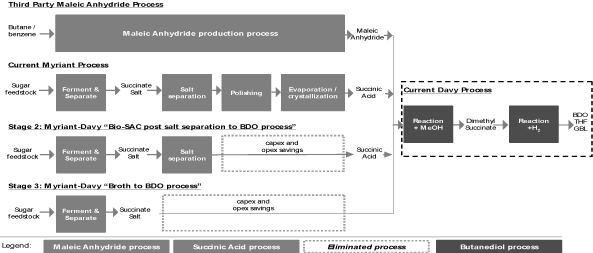
Butanediol
Butanediol, or BDO, is a chemical intermediate with end markets
in a wide variety of everyday products, including engineered
plastics, biodegradable food packaging, adhesive tapes, foams,
fibers such as elastane (better known as
Spandex®
and
Lycra®)
and coatings. Approximately 27% of global BDO production
capacity utilizes a proprietary production process developed by
Davy, in which maleic anhydride, or MAN, an organic
intermediate, is converted to dimethyl succinate, a derivative
of succinic acid, and then to BDO. Today, petroleum-derived MAN
is produced from benzene, a carcinogen, or butane. Through our
memorandum of understanding with Davy, we are negotiating a
joint development agreement with the goal of targeting BDO
plants using the Davy process to replace petroleum-derived MAN
with our biosuccinic acid. This raw material replacement would
enable our customers to produce BDO from renewable resources
with a reduced carbon footprint, higher renewable content and
reduced price volatility compared to the existing Davy BDO
production process. We are also exclusively working with Davy to
integrate and optimize each other’s processes to further
reduce the costs of producing biosuccinic acid for BDO
applications.
Adipic
Acid
Adipic acid is a chemical intermediate derived from benzene and
used in a range of polymers and plastics applications, which are
in turn used in the production of a wide variety of end
products, including flexible foams, garment linings, packaging,
filters, fuel tank linings and adhesive tapes with automotive,
electrical, surgical and industrial applications. Our
biosuccinic acid can be substituted for adipic acid. Several
prospective customers have already tested our biosuccinic acid
as an adipic acid replacement, and one customer has signed a
supply contract with us for that purpose.
Phthalic
Anhydride
Phthalic anhydride is a chemical intermediate used as a raw
material to produce plasticizers, coatings, and a wide variety
of everyday plastics used in food wrap films, flexible PVC
piping, flexible wire jackets, and toys. As a result of
increasing government regulation of this class of chemicals and
growing public awareness of their potential health risks,
producers are looking for phthalic anhydride replacements.
Succinate esters, chemicals derived from succinic acid, have
performance characteristics similar to phthalic anhydride and
can be used as a replacement. Several prospective customers have
already tested our biosuccinic acid as a phthalic anhydride
replacement, and one customer, The Chemical Company, has signed
a supply contract with us for that purpose.
3
D(−)
lactic acid
Lactic acid is derived from the fermentation of sugars and can
be converted, through a process called polymerization, into
polylactic acid, or PLA. PLA is used in a range of everyday
products, including packaging, apparel, bottles, durable goods,
films, bedding, non-wovens, and plastic dining utensils. The
market for PLA is currently limited because conventional PLA
cannot be used in applications requiring a resistance to heat.
Our D(−) lactic acid can be used to produce D-PLA, which,
when combined with conventional PLA, forms a polymer called
stereo-complex PLA, or SC-PLA. This polymer addresses the heat
stability problem that has limited the adoption of PLA. SC-PLA
also offers greater strength and crystallinity than PLA,
enabling its use in higher-value applications such as engineered
and high-performance plastics. We believe that growth in demand
for SC-PLA, which requires D(−) lactic acid as a key
input, will significantly expand the PLA market beyond its
current size. In June 2008, our technology was commercialized
for the production of D(−) lactic acid through our
licensee, Purac, who pays us royalties based on sales. Market
forecasts indicate that the market for D(−) lactic acid
will eventually exceed one billion pounds.
OUR
PRODUCT PIPELINE
Our technology platform provides for the manufacture of several
additional biochemicals utilizing the same or similar
microorganisms.
Fumaric
Acid
Fumaric acid is currently used as a preservative in food and
beverages, as a sizing agent to protect and help the spread of
ink in paper production, and as an input in the production of
alkyd resins, chemicals used in paints and coatings. Given that
fumaric acid is chemically equivalent to a combination of MAN
and water, it can potentially be used as a replacement for MAN
in all MAN-based applications, including the production of
unsaturated polyester resins (UPR), chemicals used in
construction, marine, and automotive products. We are currently
developing a biobased fumaric acid utilizing the same E. coli
bacteria used to produce our biosuccinic acid. We plan to
address both the existing fumaric acid market and the potential
market for fumaric acid as a replacement for MAN in UPRs and
other applications, which represent a combined market for
fumaric acid of $1.7 billion at current prices. We are one
year into our development cycle for fumaric acid and anticipate
scaling the technology to pilot scale in 2012.
Acrylic
Acid
Acrylic acid is one of the most versatile and widely used
industrial chemicals with applications in superabsorbents,
coatings, adhesives, sealants, textiles, paper chemicals, and
plastic additives. The acrylic acid market is estimated at
$14.5 billion at current market prices. We have achieved
proof of concept at laboratory scale and are in the first year
of our development phase for acrylic acid. We expect to enter
pilot phase in 2012.
OUR
COMPETITIVE STRENGTHS
We believe the following competitive strengths differentiate us
from both traditional chemical producers and other biobased
chemical producers:
Validated
proprietary technology
Our proprietary technology platform, validated in our
laboratories and third-party facilities and commercialized with
our licensee, Purac, is based on a single-step anaerobic process
that allows our biocatalysts to grow and simultaneously produce
the targeted product in the fermentation vessel. This anaerobic
process, in the case of our biosuccinic acid and biobased
fumaric acid, consumes carbon dioxide when producing the target
product, rather than releases it, resulting in greater
productivity and yield relative to other known bioproduction
techniques.
4
Low-cost
producer of sustainable biobased products
Our biobased products seek to address large and growing
industrial chemical markets. We believe we can produce these
products at an average of half the cost of traditional
petrochemical intermediates at a wide range of oil and
industrial sugar prices without relying on government subsidies.
We believe our manufacturing cost profile will allow us to offer
customers sustainable, biobased inputs for existing processes at
prices competitive with petroleum-based inputs while providing
us with an attractive return on capital. We will also market our
biosuccinic acid as a substitute for other petroleum-based
products, such as adipic acid and phthalic anhydride, reducing
our customers’ exposure to the price volatility of oil.
Feedstock
flexible
We have produced biosuccinic acid at laboratory and pilot scale
from a wide variety of sugars, including 95 Dextrose, grain
sorghum, sucrose from sugarcane, cellulosic sugars from waste
biomass, and glycerol. Our biocatalysts consume both five-carbon
sugars and six-carbon sugars, which we believe will provide a
competitive advantage when cellulosic feedstocks become more
widely available due to their lower cost. We plan to use the
lowest cost regional feedstocks or a combination of low cost
feedstocks in our future plants.
Commercialized
product
Our technology platform was commercialized for the production of
D(−) lactic acid through our licensee, Purac, one of the
world’s leading lactic acid producers. Purac uses our
technology to address the thermal stability problems associated
with polylactic acid, or PLA, by producing SC-PLA, a biopolymer
created by combining D-PLA with L-PLA. SC-PLA offers higher
strength and crystallinity and superior heat resistance relative
to L-PLA, enabling its use in higher-value applications such as
engineered and high-performance plastics.
Strategic
relationships with leaders in the fields of chemicals, process
technology, and engineering
We are working with international companies to create key
strategic partnerships to accelerate the global
commercialization of our products and development of our
biochemical production capabilities. Our memorandum of
understanding with Davy contemplates that upon successful
completion of testing and engineering using our biosuccinic
acid, Davy would guarantee its butanediol process licensees that
they can use our biosuccinic acid in their butanediol process in
place of petroleum-derived MAN without significant additional
capital expenditures. We have entered into an exclusive alliance
with Uhde in which it will integrate our fermentation and its
separation technology in biochemical manufacturing plants and
guarantee the performance of those plants, to facilitate access
to project finance. In addition, through CH Inter and PTTCH, we
can access a breadth of commercial and technical expertise and
extensive knowledge and infrastructure in Asian markets.
Profitable
unit-level economics without subsidies or mandates
We do not rely on green premiums, regulatory policies,
subsidies, mandates, or tariffs to make our products
commercially viable. Based on our testing, projections, and
experience to date, we believe that our technology platform will
allow us to offer our products at a price that is competitive
with petroleum-based chemicals. We believe capital expenditures
required for production of our products are consistent on a per
pound basis with traditional chemical plants and have the
potential to achieve a more attractive return on capital.
Scale-up
and signed customer contracts
We scaled up the production of our biosuccinic acid from an
initial fermentation vessel size of five liters to 50,000 liters
between January 2008 and February 2011 at various locations,
completing our scale-up at Fermic’s “toll
manufacturing facility”. A toll manufacturing facility is a
plant which companies use to contract manufacture products or to
scale-up
technologies to production levels. The owner of the facility
provides the equipment, labor, utilities and raw materials for a
monthly fee to manufacture a given product. Using biosuccinic
acid samples produced at Fermic and supplied to prospective
customers, we have signed contracts with three customers who
have agreed to buy up to 100% of their succinic acid
requirements from our
5
30 million pound Louisiana Plant. We are in discussions
with prospective customers who have indicated interest in
purchasing the biosuccinic acid that we would produce from the
additional capacity created upon the expansion of the Louisiana
Plant to 170 million pounds.
Experienced
team with a demonstrated track record
The members of our management team have more than 100 combined
years of experience, and with other companies have developed,
scaled, built and operated biotechnology and chemical
businesses. Our team has achieved success in (i) developing
microorganisms and new products, (ii) developing the
fermentation and downstream processes of industrial
biotechnology, (iii) employing process development and
project management of large-scale plants from start to finish,
(iv) financing and constructing large-scale capital
projects, (v) commercializing and navigating product
approvals at global and multinational companies, and
(vi) managing global business functions, including pricing,
sourcing, customer interfacing, budgeting, and capital planning.
OUR
STRATEGY
Our strategy is to become a low-cost producer of biobased
chemical intermediates that can drop in or replace traditional
petroleum-based industrial chemicals. Key elements of our
strategy include:
|
|
|
| |
•
|
developing drop-in and replacement products for large, existing
markets
|
| |
| |
•
|
leveraging and establishing partnerships to achieve commercial
success
|
| |
| |
•
|
targeting drop-in applications to drive market penetration
|
| |
| |
•
|
securing customer contracts to support production capacity
expansion
|
| |
| |
•
|
expanding internationally to markets with the greatest business
opportunities
|
| |
| |
•
|
capitalizing on feedstock flexibility to further reduce our costs
|
SUMMARY
RISK FACTORS
Our business is subject to numerous risks that could prevent us
from successfully implementing our business strategy. These
risks are discussed more fully in “Risk factors”
beginning on page 11 of this prospectus, and include the
following:
|
|
|
| |
•
|
We are an early stage company with a history of losses. We
expect to incur losses for at least the next several years, and
we may never achieve profitability.
|
| |
| |
•
|
We have never operated a commercial-scale plant or produced our
lead product, biosuccinic acid, in commercial volumes, and as a
result, we may encounter unforeseen difficulties in constructing
and operating large-scale commercial facilities.
|
| |
| |
•
|
Our biochemical products, including biosuccinic acid, may not be
accepted by customers in the petrochemicals industry,
particularly if we cannot meet our customers’ product
specifications.
|
| |
| |
•
|
We will be dependent initially on three customers for sales of
our lead product, biosuccinic acid. The product requirements of
these customers may be less than the initial capacity of our
Louisiana Plant.
|
| |
| |
•
|
Our initial production of biosuccinic acid will be conducted at
a single location. Any delays or disruptions in production that
occur at this plant may prevent us from generating revenue from
the sales of our product.
|
| |
| |
•
|
We expect to face competition for our biochemical products,
including biosuccinic acid, often from companies with greater
resources and experience.
|
| |
| |
•
|
A significant decline in the price of petroleum and
petroleum-based products may reduce demand for our biosuccinic
acid and our other biochemical intermediates.
|
| |
| |
•
|
We are dependent on third parties with whom we have entered into
strategic commercial relationships.
|
6
|
|
|
| |
•
|
Some of our key operating strategies, including those involving
Davy and PTTCH, depend upon negotiating and executing binding
agreements.
|
| |
| |
•
|
Our rights to key intellectual property are licensed to us.
Termination of the underlying agreements would be highly
detrimental to us.
|
| |
| |
•
|
We may not be able to enforce our intellectual property rights,
including our trade secrets, especially in the international
markets in which we expect to operate.
|
CORPORATE
INFORMATION
We were formed in Delaware on April 3, 2009 as Myriant
Technologies LLC, a limited liability company. On July 16,
2009, through a series of separation transactions, the holders
of BioEnergy International, LLC, a Delaware limited liability
company, or the Predecessor Company, acquired membership units
of Myriant Technologies LLC, which simultaneously acquired the
assets and liabilities of the chemical intermediates business of
the Predecessor Company. For further information with respect to
our reorganization, see Management’s discussion and
analysis and Note 1 to Consolidated Financial Statements.
We filed a certificate of conversion and a certificate of
incorporation in Delaware on January 10, 2011, converting
into a corporation originally named Myriant Corp. Our second
amended and restated certificate of incorporation will be filed
and take effect following the completion of this offering.
Our principal executive offices are located at 1 Pine Hill
Drive, Batterymarch Park II, Suite 301, Quincy, MA 02169,
and our telephone number is
(617) 657-5200.
Our website address is www.myriant.com. Information contained on
our website is not incorporated by reference into this
prospectus, and you should not consider information contained on
our website to be part of this prospectus.
Our logos,
“Myriant®”,
“Myriant
Technologies®”,
“Chemistry Refined
Naturally®”
and other trademarks or service marks of Myriant Corporation
appearing in this prospectus are the property of Myriant
Corporation. This prospectus contains additional trade names,
trademarks and service marks of other companies. We do not
intend our use or display of other companies’ trade names,
trademarks or service marks to imply relationships with, or
endorsement or sponsorship of us by, these other companies.
7
THE
OFFERING
|
|
|
|
Common stock offered by us |
|
shares
(or
shares if the underwriters exercise in full their over-allotment
option to purchase additional shares). |
| |
|
Common stock to be outstanding after this offering |
|
shares
(or shares
if the underwriters exercise in full their over-allotment option
to purchase additional shares). |
| |
|
Proposed Nasdaq Global Market symbol |
|
MYRT |
| |
|
Use of proceeds |
|
We expect that the net proceeds to us from the sale of the
shares of common stock we are offering will be
$ million, assuming a public
offering price of $ per share (the
mid-point of the price range set forth on the cover page of this
prospectus) and after deducting underwriting discounts and
commissions and estimated offering expenses. If the underwriters
exercise their over-allotment option in full, the net proceeds
to us would be approximately
$ million. |
| |
|
|
|
We intend to use the net proceeds of this offering to finance,
in part, the construction of the Louisiana Plant (approximately
$103 million, including working capital, fees and
expenses), to fund our expected equity contribution to the
planned expansion of the Louisiana Plant (approximately
$60 million, which includes working capital, fees and
expenses), to pay the dividend accruing on the Class A
common stock (which totaled $1,025,753 as of March 31,
2011), to fund research and development expenses (which we
estimate will be approximately $25.5 million in the
aggregate for the two fiscal years following the completion of
this offering) and for working capital and other general
corporate purposes, which will include expenses and the costs
associated with being a public company. |
| |
|
|
|
We may also use a portion of the net proceeds to acquire other
complementary businesses, products or technologies or to make
other strategic investments. We currently have no agreements or
commitments for any specific acquisitions or investments at this
time. |
| |
|
|
|
The potential uses of net proceeds from this offering represent
our current intentions based upon our present business plans and
conditions. We cannot guarantee the specific amount of the net
proceeds that will be used to develop, construct and operate our
biochemical production capabilities globally, fund working
capital or be used for other general corporate purposes. |
| |
|
|
|
Please see “Use of proceeds.” |
| |
|
Risk factors |
|
See “Risk factors” beginning on page 11 of this
prospectus for a discussion of factors you should carefully
consider before deciding to invest in our common stock. |
The number of shares of common stock to be outstanding after
this offering is based on the number of shares outstanding as of
June 27, 2011, assumes the obligatory exercise of warrants
exercisable for 17,134 shares of common stock at an exercise
price of $6.00 per share and warrants exercisable for
89,656 shares of
8
common stock at an exercise price of $10.00 per share
assuming an initial public offering in excess of 1.5 times the
exercise price of the warrants, and excludes:
|
|
|
| |
•
|
1,162,043 shares of common stock issuable upon the exercise
of options issued under our 2011 Omnibus Incentive Plan and
outstanding as of July 21, 2011 at a weighted average
exercise price of $12.38 per share;
|
| |
| |
•
|
506,250 shares issuable under restricted stock units
granted subject to vesting conditions under the 2011 Omnibus
Incentive Plan;
|
| |
| |
•
|
59,231 shares of common stock issuable upon the exercise of
warrants outstanding as of March 31, 2011 at an exercise
price of $13.00 per share;
|
| |
| |
•
|
2,139,483 shares of our common stock reserved for future
issuance under our 2011 Omnibus Incentive Plan, plus any annual
increases in the number of shares of common stock reserved for
future issuance as provided for in such plan, as described in
“Management — Employee Benefit and Stock
Plans; and
|
| |
| |
•
|
500,000 shares of common stock reserved for issuance under
our 2011 employee stock purchase plan, which will become
effective upon completion of this offering.
|
In addition, except as otherwise indicated, all information in
this prospectus:
|
|
|
| |
•
|
gives effect to the conversion of all of our outstanding shares
of convertible common stock into 14,259,858 shares of
common stock upon completion of this offering;
|
| |
| |
•
|
assumes no exercise of the underwriters’ option to purchase
additional shares; and
|
| |
| |
•
|
reflects the filing of our second amended and restated
certificate of incorporation upon completion of this offering.
|
9
Summary
selected financial data
The following summary selected financial data is derived from
financial information of us and BioEnergy International, LLC, or
the Predecessor Company. We were separated from the Predecessor
Company through a series of transactions effective as of
July 16, 2009. The financial data set forth below as of and
for the periods ended December 31, 2006, 2007 and 2008 are
derived from the historical financial statements of the
Predecessor Company. The results of operations set forth below
for the year ended December 31, 2009 represent the pro
forma combined financial results for the Predecessor Company
(through July 15) and us (from July 16). A more detailed
description of the foregoing is set forth under “Selected
historical financial data” and “Management’s
discussion and analysis of financial condition and results of
operations.” Potential investors are urged to carefully
consider that information as well as the information contained
in our and the Predecessor Company’s consolidated financial
statements and related notes included elsewhere in this
prospectus.
Consolidated
Statements of Operations Data:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Predecessor Company
|
|
|
Myriant Corporation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
July 16,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year
|
|
|
Fiscal Year
|
|
|
Fiscal Year
|
|
|
January 1,
|
|
|
2009
|
|
|
Fiscal Year
|
|
|
|
|
|
|
|
|
|
|
Ended
|
|
|
Ended
|
|
|
Ended
|
|
|
2009
|
|
|
(Inception)
|
|
|
Ended
|
|
|
Three Months Ended
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
to July 15,
|
|
|
to December 31,
|
|
|
December 31,
|
|
|
March 31,
|
|
|
|
|
2006
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
2009
|
|
|
2010
|
|
|
2010
|
|
|
2011
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License fee revenue
|
|
$
|
267,065
|
|
|
$
|
396,515
|
|
|
$
|
344,860
|
|
|
$
|
71,833
|
|
|
$
|
221,711
|
|
|
$
|
258,241
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Management fee revenue-related party
|
|
|
—
|
|
|
|
—
|
|
|
|
262,212
|
|
|
|
267,318
|
|
|
|
—
|
|
|
|
3,557,574
|
|
|
|
600,166
|
|
|
|
2,519
|
|
|
Development fee revenue-related party
|
|
|
—
|
|
|
|
—
|
|
|
|
3,125,714
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Government awards
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,419,043
|
|
|
|
1,516,323
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
267,065
|
|
|
|
396,515
|
|
|
|
3,732,786
|
|
|
|
339,151
|
|
|
|
221,711
|
|
|
|
14,234,858
|
|
|
|
2,116,489
|
|
|
|
2,519
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of license fee revenue
|
|
|
108,432
|
|
|
|
135,813
|
|
|
|
110,020
|
|
|
|
22,373
|
|
|
|
73,859
|
|
|
|
84,600
|
|
|
|
—
|
|
|
|
—
|
|
|
Research and development
|
|
|
653,469
|
|
|
|
1,499,577
|
|
|
|
4,679,935
|
|
|
|
3,770,721
|
|
|
|
2,793,085
|
|
|
|
15,904,717
|
|
|
|
2,552,100
|
|
|
|
1,883,603
|
|
|
Project development
|
|
|
706,666
|
|
|
|
1,764,244
|
|
|
|
2,179,965
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
General and administrative expense
|
|
|
1,547,540
|
|
|
|
3,166,646
|
|
|
|
5,699,186
|
|
|
|
5,438,073
|
|
|
|
4,979,186
|
|
|
|
12,673,247
|
|
|
|
2,806,285
|
|
|
|
3,034,498
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
3,016,107
|
|
|
|
6,566,280
|
|
|
|
12,669,106
|
|
|
|
9,231,167
|
|
|
|
7,846,130
|
|
|
|
28,662,564
|
|
|
|
5,358,385
|
|
|
|
4,918,101
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(2,749,042
|
)
|
|
|
(6,169,765
|
)
|
|
|
(8,936,320
|
)
|
|
|
(8,892,016
|
)
|
|
|
(7,624,419
|
)
|
|
|
(14,427,706
|
)
|
|
|
(3,241,896
|
)
|
|
|
(4,915,582
|
)
|
|
Other income (expense), net:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
1,477
|
|
|
|
138,395
|
|
|
|
60,222
|
|
|
|
5,385
|
|
|
|
8,051
|
|
|
|
87,611
|
|
|
|
84,844
|
|
|
|
6,328
|
|
|
Interest expense
|
|
|
(260,268
|
)
|
|
|
(1,959,909
|
)
|
|
|
(1,636,062
|
)
|
|
|
(1,352,767
|
)
|
|
|
(915,804
|
)
|
|
|
(4,470,478
|
)
|
|
|
(628,386
|
)
|
|
|
(12,606,762
|
)
|
|
Miscellaneous income
|
|
|
600
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8,000
|
|
|
Gain(loss) on foreign currency exchange
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(21,721
|
)
|
|
|
—
|
|
|
|
(5,235
|
)
|
|
|
(3,853
|
)
|
|
|
11,638
|
|
|
Changes in fair value of warrant liability
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,092,643
|
|
|
|
(515,108
|
)
|
|
|
2,592,979
|
|
|
|
(823,582
|
)
|
|
|
2,461
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net
|
|
|
(258,191
|
)
|
|
|
(1,821,514
|
)
|
|
|
(1,575,840
|
)
|
|
|
723,540
|
|
|
|
(1,422,861
|
)
|
|
|
(1,795,123
|
)
|
|
|
(1,370,977
|
)
|
|
|
(12,578,335
|
)
|
|
Net loss
|
|
|
(3,007,233
|
)
|
|
|
(7,991,279
|
)
|
|
|
(10,512,160
|
)
|
|
|
(8,168,476
|
)
|
|
|
(9,047,280
|
)
|
|
|
(16,222,829
|
)
|
|
|
(4,612,873
|
)
|
|
|
(17,493,917
|
)
|
|
Dividend on Class A common stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,025,753
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders
|
|
$
|
(3,007,233
|
)
|
|
$
|
(7,991,279
|
)
|
|
$
|
(10,512,160
|
)
|
|
$
|
(8,168,476
|
)
|
|
$
|
(9,047,280
|
)
|
|
$
|
(16,222,829
|
)
|
|
$
|
(4,612,873
|
)
|
|
$
|
(18,519,670
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Predecessor Company
|
|
|
Myriant Corporation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
July 16,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year
|
|
|
Fiscal Year
|
|
|
Fiscal Year
|
|
|
January 1,
|
|
|
2009
|
|
|
Fiscal Year
|
|
|
|
|
|
|
|
|
|
|
Ended
|
|
|
Ended
|
|
|
Ended
|
|
|
2009
|
|
|
(Inception)
|
|
|
Ended
|
|
|
Three Months Ended
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
to July 15,
|
|
|
to December 31,
|
|
|
December 31,
|
|
|
March 31,
|
|
|
|
|
2006
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
2009
|
|
|
2010
|
|
|
2010
|
|
|
2011
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
Net loss per unit/share attributable to common
stockholders-basic and diluted
|
|
$
|
(0.69
|
)
|
|
$
|
(1.72
|
)
|
|
$
|
(2.14
|
)
|
|
$
|
(1.55
|
)
|
|
$
|
(1.30
|
)
|
|
$
|
(2.31
|
)
|
|
$
|
(0.66
|
)
|
|
$
|
(2.29
|
)
|
|
Weighted average number of units outstanding- basic and diluted
|
|
|
4,358,126
|
|
|
|
4,655,011
|
|
|
|
4,918,668
|
|
|
|
5,285,807
|
|
|
|
6,953,079
|
|
|
|
7,009,251
|
|
|
|
6,982,215
|
|
|
|
8,092,943
|
|
|
Net loss used in computing pro forma net loss per share of
common stock-basic and diluted (unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(18,519,670
|
)
|
|
Pro forma net loss per common share (unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
(0.83
|
)
|
|
Weighted average shares used in computing pro forma basic and
diluted net loss per common share (unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22,352,801
|
|
Balance
Sheet Data:
| |
|
|
|
|
|
|
|
|
|
|
|
March 31, 2011
|
|
|
|
Actual
|
|
As Adjusted(1)
|
|
|
|
(Unaudited)
|
|
(Unaudited)
|
|
|
|
Cash, cash equivalents and restricted cash
|
|
$
|
50,692,707
|
|
|
$
|
—
|
|
|
Working capital
|
|
|
31,985,396
|
|
|
|
|
|
|
Total assets
|
|
|
61,134,435
|
|
|
|
|
|
|
Warrant liability
|
|
|
2,199,585
|
|
|
|
840
|
|
|
Stockholders’ equity
|
|
|
54,967,658
|
|
|
|
57,112,796
|
|
|
|
|
|
(1) |
|
Adjusted for the issuance of 639,170 shares of common stock
upon the exercise of warrants on April 12, 2011, and the
issuance and sale of the shares of our common stock in this
offering (assuming a public offering price of
$ per share, which is the
mid-point of the price range set forth on the cover page of this
prospectus, and after underwriting discounts and commissions and
our expected offering expenses) and the receipt of the net
proceeds from the offering. |
11
Risk
factors
Investing in our common stock involves a high degree of risk.
You should carefully consider the following risk factors, as
well as the other information in this prospectus, before
deciding whether to invest in shares of our common stock. The
occurrence of any of the events described below could harm our
business, financial condition, results of operations and growth
prospects. In such an event, the trading price of our common
stock may decline and you may lose all or part of your
investment.
WE ARE AN
EARLY STAGE COMPANY WITH AN UNPROVEN PRODUCT
We are
a development stage company with a history of losses. We expect
to incur losses for at least the next several years, and we may
not achieve or maintain profitability.
We are a development stage company. To date, we have generated
no product sales of any significance, and the minimal revenues
we have generated have been generated primarily through
licensing royalties and government awards. We, and before us,
the Predecessor Company, have a history of losses, including
losses of $10.5 million in 2008, $17.2 million in 2009
and $16.2 million in 2010, as well as a loss of
$17.5 million for the three months ended March 31,
2011. As of March 31, 2011, we had an accumulated deficit
of $17.5 million, resulting from losses incurred by us
since our conversion to a subchapter C corporation in January
2011. Our cumulative losses (including those of our Predecessor
Company) total $72.4 million. We expect to incur losses and
negative cash flow from operating activities for at least the
next several years, and we may never achieve profitability. In
addition, as detailed below, we expect to incur substantial
costs associated with the construction of the Louisiana Plant.
As a result, even if our revenues increase substantially, we
expect that our expenses will exceed revenues for at least the
next several years. If we never become profitable, or if the
time required to become profitable is longer than we expect, we
may not be able to continue our business. Even if we become
profitable, we may not be able to sustain or increase
profitability on a quarterly or annual basis.
Our
processes and resulting products are unproven at commercial
scale.
While the manufacturing of organic compounds from fossil-fuel
feedstocks has a long history of process development and
optimization, we have limited experience operating our biobased
technology and have only done so at pilot scale. To successfully
commercialize our biosuccinic acid and other biochemical
intermediates, we must manufacture our products on a
cost-competitive basis at commercial scale and in accordance
with customer quality specifications. We have only manufactured
a variety of chemical intermediates at laboratory and pilot
scale, with the exception of D(−) lactic acid, which has
been manufactured at commercial scale by our licensee, Purac
Biochem BV, or Purac, a wholly-owned subsidiary of CSM N.V., a
leading global chemical producer. However, our biosuccinic acid
technology, and that of other products that we attempt to
commercialize, may not perform at commercial scale in the same
manner as we have seen at laboratory or pilot scale. Moreover,
our technology may not achieve customer acceptance or generate
significant product sales for a variety of reasons, many of
which are described in detail below.
OUR
BUSINESS MODEL AND OPERATING STRATEGIES HAVE INHERENT
RISKS
We
have never built a commercial-scale plant or produced our
products, including biosuccinic acid, in commercial volumes, and
as a result we may encounter unforeseen difficulties in
constructing a large scale facility.
Our first large-scale commercialization initiative is producing
biosuccinic acid and selling it into the petrochemicals market
as a chemical intermediate. To date, we have produced limited
quantities of biosuccinic acid for customer/vendor sampling and
validation. While we have reached performance targets for
certain aspects of the commercial production of biosuccinic
acid, we have not yet done so in a commercial-scale plant or in
commercial volumes, and we may not be able to do so in a timely
or cost-effective manner.
To commercialize our biosuccinic acid, we are constructing a
30 million pound, 392,000 square-foot plant at the
Port of Lake Providence, Louisiana. Constructing a production
facility of this type and size is a complex and
12
lengthy undertaking that requires sophisticated,
multi-disciplinary planning and precise execution. Despite the
detailed planning and the time and money that we have invested
and will continue to invest in the construction of this plant,
it may not perform as expected. For example, our biocatalyst may
perform less efficiently than it did in smaller-scale
production. Design parameters for mixing, heat transfer and flow
rates may need to be adjusted to meet required product or volume
specifications. Production rate, concentration and purity may
vary dramatically from our expectations. We may need to install
additional equipment to achieve desired specifications, which
could delay commercialization and increase costs. In addition,
contaminants in our feedstock could reduce the purity of the
biosuccinic acid that we produce and require us to invest in
more costly processes or equipment. We may encounter these or
other operational challenges, and we may be unable to devise
workable solutions. Furthermore, our processes require us to
maintain certain levels of product purity to assure consistent
product quality. In the event an impurity is introduced in a
particular production batch of our biosuccinic acid, the product
quality may be reduced or the product rendered unsalable, which
would affect our profitability.
We have entered into an agreement pursuant to which, among other
things, we are collaborating with ThyssenKrupp’s subsidiary
Uhde GmbH, and its U.S. subsidiary, or Uhde, in the design,
engineering and procurement for the Louisiana Plant. If Uhde
does not perform as expected, we could experience significant
delays and cost overruns.
Developing this plant also subjects us to many financial
uncertainties. We have created financial forecasts for
construction costs, construction time, output and operating
costs of the plant. Our decision to proceed with the
construction of the Louisiana Plant is based on our analysis of
these forecasts. However, these forecasts are based on a variety
of observations and assumptions, some involving considerable
judgment on our part, which could prove to be inaccurate.
Inherent risks associated with the novel nature of this project,
inaccurate assumptions about our operating metrics and the fact
that neither we nor anyone else has built or operated a
biosuccinic acid facility at a commercial scale could result in:
|
|
|
| |
•
|
significant delays in constructing the Louisiana Plant and
commercializing our product;
|
| |
| |
•
|
greater operating costs than anticipated;
|
| |
| |
•
|
cost overruns and the need for significant additional
expenditure of time and capital; and
|
| |
| |
•
|
lower than expected revenues and profit margins generated from
the Louisiana Plant.
|
We may
experience unexpected challenges and setbacks in operating our
Louisiana Plant.
Our success depends on our ability to operate the Louisiana
Plant so as to produce biosuccinic acid efficiently and
cost-effectively in a timely manner. We have never operated a
commercial-scale biosuccinic acid facility and we may be
required to expend significant time and money to develop our
operational capabilities. Although our management team has
significant experience in chemical intermediates and
fermentation technology, the skills and knowledge gained in
these fields and in operating smaller-scale biosuccinic acid
production facilities may prove insufficient for successful
operation of a commercial-scale biosuccinic acid facility.
Accordingly, we may encounter significant difficulties in
operating the Louisiana Plant.
The production of biosuccinic acid requires multiple, integrated
steps, including:
|
|
|
| |
•
|
obtaining the feedstocks;
|
| |
| |
•
|
generating fermentable sugars;
|
| |
| |
•
|
fermentation by organisms to produce succinic salts and ammonium
succinate from the fermentable sugars;
|
| |
| |
•
|
separation of the biosuccinic acid and ammonium-salt fractions;
|
| |
| |
•
|
purification of the biosuccinic acid; and
|
| |
| |
•
|
storage and distribution of the resulting biosuccinic acid.
|
13
The technological and logistical challenges associated with each
of these and other processes involved in production, marketing,
sale and distribution of biosuccinic acid are substantial. We
may not be able to resolve any difficulties that arise in a
timely or cost-effective manner.
In addition, while certain members of our management team have
experience in procuring large quantities of feedstocks for other
production facilities we have never sourced large quantities of
feedstocks, and we have no experience storing
and/or
distributing significant volumes of biosuccinic acid. We may not
be able to do so cost-effectively. We may also have difficulty
in recruiting, training and retaining qualified personnel to
operate the Louisiana Plant. Failure to meet the operational
challenges of developing and managing our production processes
would have a material adverse effect on our business, financial
condition and results of operations.
We may
not achieve overall profitability due to higher costs of
producing biosuccinic acid and other biochemicals.
The cost to produce biosuccinic acid and other chemical
intermediates is highly dependent on the cost and usage of
various process chemicals, such as sulfuric acid and ammonia.
Although the chemical and nutrient usage quantities are based on
predictable chemical reactions, the actual consumption required
to produce biosuccinic acid on a commercial scale may be
greater, affecting production cost and impacting production
volumes. Although there are indices that show the pricing of the
process chemicals used for production that closely track to our
end products, there are no assurances that the indices are valid
or, if valid, that current prices will not later change. We
cannot control the cost of these process chemicals, and we could
underestimate the volume required to operate at commercial
scale. These uncertainties could affect our costs and margins.
Moreover, our operating and other financial projections for our
Louisiana Plant assume that we will sell 100% of the volume of
ammonium sulfate, or AMS, that we will produce as a co-product
of our biosuccinic acid manufacturing process. We have entered
into a contract with Wilson Industrial Sales Company, Inc. to
sell all of the AMS produced at the Louisiana Plant during the
term of the contract. AMS is a co-product of our biosuccinic
acid production process and is commonly used as a soil
fertilizer. If the quality of the AMS co-product does not meet
market specifications, we may not be able to sell AMS at the
anticipated price or in the anticipated quantity. Also, if the
nature and quantity of the wastewater generated in the
manufacturing process is substantially higher than in pilot
testing, our wastewater treatment design may be undersized,
affecting our ability to operate the Louisiana Plant at full
capacity, if at all.
Fluctuations
in the price of feedstocks may affect our cost
structure.
An increase in the price of feedstocks would change our cost
structure and therefore our profit margins. At certain levels,
prices may make our products uneconomical to produce, as we may
be unable to pass the full amount of feedstock cost increases on
to our customers. The prices of many of these feedstocks are
cyclical and volatile. While our biocatalysts are able to
process a variety of feedstocks to make chemical intermediates,
our ability to deliver products to our customers in a timely and
cost-effective fashion may be impacted by the need to substitute
feedstocks because of price fluctuations.
We may
experience delayed production, reduced output and reduced
revenues if the cost or availability of feedstocks or other
factors require us to change feedstocks.
The production of our products will require large volumes of
feedstocks. Although our process can utilize a wide variety of
feedstocks, we cannot predict the future availability of such
feedstocks or be sure that our suppliers, who we expect to
supply the feedstocks necessary to produce our products, will be
able to supply it in sufficient quantities or in a timely
manner. The supply of feedstocks might be impacted by
growing-season disruptions, crop yields, crop disease, droughts,
floods, infestations, natural disasters, farming decisions or
government policies and subsidies. In particular, weather
conditions have historically caused volatility in the sugar
industries by causing crop failures or reduced harvests.
Excessive rainfall can adversely affect the supply of certain
feedstocks by reducing the sugar content and limiting
growers’ ability to harvest the crop. Crop disease and
pestilence can adversely affect growth, potentially rendering
unusable all or a substantial portion
14
of affected harvests. The limited amount of time during which
certain feedstocks retain their sugar content after harvest also
limits our ability to substitute supply, if necessary.
We
cannot assure you that our biochemicals, including biosuccinic
acid, will be accepted by our target customers.
If we fail to successfully market our biosuccinic acid and other
chemical intermediates to our target customers, our business,
financial condition and results of operations will be adversely
affected. The markets we intend to enter first are those for
chemical intermediates used by large consumer products or
chemical companies. In entering these markets, we intend to sell
our products as alternatives to current petroleum-based
intermediates. Although a significant market currently exists
for succinic acid produced from petroleum, to our knowledge,
biosuccinic acid has never been produced or sold at a commercial
scale. Many potential chemical industry customers have invested
substantial amounts of time and money in developing
petroleum-based production channels. These potential customers
for our products, including biosuccinic acid, generally have
well-developed manufacturing processes and, in many instances,
arrangements with suppliers of the components of their products.
Pre-existing contractual commitments, unwillingness to invest in
new infrastructure, distrust of new production methods and
long-term relationships with current suppliers may all slow
market acceptance of our biosuccinic acid and our other
biochemicals. Furthermore, most producers of plastics and
specialty chemicals that rely on succinic acid as a chemical
intermediate have been producing their succinic acid
requirements internally. As such, there is only a small merchant
market for succinic acid.
We
will be dependent initially on three customers.
We have supply agreements with three customers requiring that
they purchase 100% of their succinic acid requirements from us.
These supply agreements do not require these customers to
purchase a minimum quantity of succinic acid from us. Although
we expect that these purchasers will consume a substantial
portion, and prospectively all, of the Louisiana Plant’s
production capacity, we cannot be certain that these
customers’ requirements will meet our expectations.
Two of these customer contracts require us to negotiate initial
pricing of our product and to renegotiate pricing on a quarterly
basis. These and other provisions of these contracts could
result in disputes or stand-offs with these customers,
disrupting our product sales. We are in discussions with these
customers to agree on a pricing formula, but we may not succeed
in doing so.
In addition, substantially all of our revenues will be derived,
initially and for the foreseeable future, from these three
customers. This customer concentration increases the risk of
volatility in our revenues and operating results. Moreover,
under specified circumstances, these customers are permitted to
cancel their agreements with us. The loss or reduction of
business from any one or more of these customers could
materially adversely affect our revenues, financial condition
and results of operations. We have indications of interest from
others to purchase quantities of our biosuccinic acid. However,
even if one of these substitute purchasers were willing to
purchase excess supply, we would likely experience a temporary
disruption in product sales, which would adversely affect our
revenues.
We
have limited experience in structuring arrangements with
customers for the purchase of our biochemicals, including
biosuccinic acid, and we may not be successful in this essential
aspect of our business.
To date, we have generated limited revenues from sales of our
commercial products and have limited experience operating in our
customers’ industries and interacting with the customers
that we are targeting. Developing that expertise may take longer
than we expect and will require that we expand and improve our
sales and marketing infrastructure. These activities could delay
our ability to capitalize on the opportunities that our
technology and products present, and may prevent us from
achieving commercialization of our initial products. Our target
customers are generally much larger than we are and have
substantially longer operating histories in the specialty
chemicals industry than we have. As a result, we may not be
effective in negotiating
15
or managing the terms of our relationships with these companies,
which could adversely affect our future results of operations.
We may
be unable to produce biosuccinic acid and other biobased
intermediates in accordance with customer
specifications.
It is critically important that we are able to meet
customers’ product and volume specifications. If we fail to
do so, prospective customers will not purchase from us and
existing customers could terminate their agreements with us.
While our biosuccinic acid will be sold in various grades,
failure to successfully meet the specifications of our customers
and potential customers for biosuccinic acid and other chemical
intermediates would decrease demand for our production and
significantly hinder market adoption of our product.
Even if we produce our products at our contractual or targeted
specifications, as the case may be, we may face delays or
reduced demand for our product related to current or future
customer qualification trials that could take several months.
For our biosuccinic acid to be accepted, our customers may need
to test and certify it for use in their processes and, in some
cases, determine whether products that contain our biosuccinic
acid satisfy additional third-party specifications. We may need
to demonstrate to our customers that our biosuccinic acid does
not contain impurities that cause our product to behave
differently than the petroleum-based equivalent in a way that
impacts their end product quality. Our customers, in turn, may
need to validate the use of our biosuccinic acid in products
produced for third parties. Meeting these suitability standards
could be a time-consuming and expensive process, and we may
invest substantial time and resources into such qualification
efforts without ultimately securing approval by our customers.
This could materially and adversely impact revenues until
customer qualification is achieved and maintained.
A
significant decline in the price of petroleum and
petroleum-based products may reduce demand for our biosuccinic
acid.
Based on our current financial modeling, if the price of oil
falls below $45 per barrel for a sustained period, we will be
unable to manufacture biosuccinic acid as a cost-effective
alternative to competing petroleum-based products, which will
result in lost sales and would adversely impact our operating
results. We anticipate that biosuccinic acid will be marketed as
an alternative to corresponding petroleum-based products. World
prices for oil have fluctuated widely in recent years. For
example, during 2008 the average market price per barrel of West
Texas Intermediate crude oil ranged from a low of $30.81 to a
high of $145.66 and was $98.14 as of July 20, 2011. We
expect that prices will continue to fluctuate in the future.
Declining oil prices, or the perception of a future decline in
oil prices, may adversely affect the prices we can obtain from
our potential customers or prevent potential customers from
entering into agreements with us to buy our products.
We
expect to face competition for our biochemical intermediates,
often from companies with greater resources and experience than
us.
In the industrial biochemical market, we expect to face vigorous
competition from both the traditional, largely petroleum-based
chemicals that are currently used in our target markets and from
alternatives to these existing products, such as other biobased
chemicals. We may not compete effectively against incumbent
petroleum-based products. Petroleum-based products have
dominated the market for many years, and there is substantial
existing infrastructure designed for petroleum-based
intermediates, which may impede our ability to establish a
position in these markets. Producers of these incumbent products
include global oil companies, large international chemical
companies and other companies specializing in specific
chemicals. We may also compete in one or more of these markets
with products that are offered as alternatives to the
traditional petroleum-based or other traditional products being
offered in these markets. Our potential customers generally have
well-developed manufacturing processes and arrangements with
suppliers of the chemical components of their products and may
resist changing these processes and components. Many of our
prospective competitors are better capitalized, with larger
research and development departments and budgets, and have
well-developed distribution systems and networks for their
products.
16
In addition, our potential customers frequently impose lengthy
and complex product qualification procedures on their suppliers,
influenced by consumer preference, manufacturing considerations
such as process changes and capital and other costs associated
with transitioning to alternative components, supplier operating
history, regulatory issues, product liability and other factors.
Satisfying these processes may take many months or years. If we
are unable to convince these potential customers that our
biosuccinic acid and our other chemical intermediates are
comparable or superior to the alternatives that they currently
use, we will not be successful in entering these markets.
We believe the primary competitive factors in the industrial
biochemical market are:
|
|
|
| |
•
|
product price;
|
| |
| |
•
|
product performance and other measures of quality;
|
| |
| |
•
|
infrastructure compatibility of products;
|
| |
| |
•
|
sustainability; and
|
| |
| |
•
|
dependability of supply.
|
We believe that for our biochemicals to succeed in the market,
we must demonstrate that our products are comparable, attractive
alternatives to existing products and to any alternative
products that are being developed for the same markets based on
some combination of product cost, availability, performance and
consumer preference characteristics. We may not be able to
compete effectively against these other products.
Technological
innovation could render our products and processes
obsolete.
The biochemical industry is characterized by rapid technological
change. Our success will depend on our ability to maintain a
competitive position with respect to technological advances. Our
technologies and products may be rendered obsolete or
uneconomical by technological advances, more efficient and
cost-effective biocatalysts or entirely different approaches
developed by one or more of our competitors.
WE ARE
HIGHLY DEPENDENT ON THIRD PARTIES WITH WHICH WE HAVE ENTERED
INTO, OR ARE SEEKING TO ENTER INTO, STRATEGIC COMMERCIAL
RELATIONSHIPS
Our
commercialization strategy relies heavily on negotiating a
definitive agreement with Davy Process Technology.
We plan to market our biosuccinic acid as a drop-in chemical
intermediate for use in established processes for the production
of industrial and specialty chemicals. In February 2011, we
signed a non-binding memorandum of understanding with Davy
Process Technology Limited, or Davy, to enter into a definitive
joint development agreement. Under the agreement, Davy would
test and approve our biosuccinic acid as a drop-in feedstock
replacement for petroleum-derived maleic anhydride within
Davy’s process for the production of butanediol, or BDO.
The proposed joint development agreement would require Davy to
perform, at its expense, the engineering, development and pilot
plant work necessary for it to guarantee to its customers that
our biosuccinic acid will work for existing Davy process
production facilities. We cannot be certain that Davy would be
satisfied with its findings, that Davy would offer the guarantee
sought or, if the use of our biosuccinic acid is guaranteed for
Davy BDO process licensees, that Davy would expend the resources
necessary to continue to guarantee our biosuccinic acid for use
in its process. If we do not enter into the proposed joint
development agreement, our business and prospects could be
materially and adversely affected.
BDO producers that use Davy’s process represent a
substantial portion of our target customer base. If Davy will
not guarantee that our biosuccinic acid will work in the Davy
BDO process, our market for biosuccinic acid will be
dramatically reduced.
17
Our
plant construction and project finance strategy relies heavily
on our relationship with Uhde.
In September 2009 and October 2009, we signed two Alliance
Agreements with Uhde. These contracts require that Uhde and we
work collaboratively and exclusively to incorporate each
party’s proprietary technology in the development of our
plant. Pursuant to the Alliance Agreements, Uhde will integrate
our fermentation technology with its separation technology in
the plant design and, on a
project-by-project
basis, provide process and performance guarantees on mutually
agreeable terms for our biochemical plants around the world. If
we and Uhde are unable to agree upon the terms and conditions by
which Uhde would provide process and performance guarantees for
the operational outputs of our plant, we could have difficulty
performing our obligations to our customers, and it could become
more difficult for us to secure and maintain project financing
for our proposed industrial biochemical plants on terms
favorable to us, if at all. Since adapting our technology to
commercial-scale production and building plants to use our
technology is a major part of our commercialization strategy,
losing our exclusive alliance with Uhde would likely slow our
technological and commercial development. It could also force us
to find a new contractor with less experience than Uhde in
designing and building industrial biochemical plants or to
invest the time and resources necessary to build plants on our
own. This could substantially hinder our ability to expand our
production capacity and could severely impact our performance.
If Uhde fails to fulfill its obligations to us under our
agreements or our competitors obtain access to Uhde’s
expertise, our ability to realize continued development and
commercial benefits from our alliance could be impaired.
Accordingly, if we lose our exclusive alliance with Uhde or Uhde
terminates or breaches its agreements with us, our business and
prospects could be adversely affected.
Our
potential relationship with PTTCH may have a substantial impact
on us.
We are negotiating a joint venture agreement and licensing
arrangement with PTT Chemical Public Company Limited, or PTTCH,
a large Thailand-based petrochemical producer and the parent
company of our largest stockholder, which we envision we will
finalize and execute shortly. As part of the joint venture, we
would grant a license to the joint venture under our
intellectual property to commercialize our technology
exclusively within the ASEAN countries. The license agreement
under discussion would provide for payments to us with respect
to royalties based on product sales, maintenance fees and our
pro rata share of joint venture earnings. The joint venture also
calls for a research and development collaboration between us
and PTTCH to, among other things, validate and optimize our
biocatalysts and regional feedstocks for use in the ASEAN
countries.
The consummation of our joint venture and licensing agreement
with PTTCH is subject to the negotiation and execution of
transaction documentation, including intellectual property
licenses and sublicenses. We may not enter into the joint
venture and licensing agreement with PTTCH on the timing we
expect or at all. If the joint venture company is formed, we do
not know whether we will receive any benefits from it. If the
proposed joint venture and licensing agreement with PTTCH is not
consummated, our business and prospects could be adversely
affected.
We
rely on our other strategic partners and collaborators, and our
failure to successfully manage these relationships could delay
us from developing and commercializing many of our products and
achieving or sustaining profitability.
We expect that our ability to maintain and manage collaborations
in our markets, like those we intend to establish with Davy,
Uhde and PTTCH, will be significant factors to the success of
our business. We have limited or no control over the amount or
timing of resources that any third party commits to negotiating
a collaboration with us or, if negotiated and entered into, the
timing or amount of resources that a collaboration partner will
commit. Davy, PTTCH or any other third party with which we are
in negotiations may experience a change of policy or priorities
and may discontinue negotiations with us. Any of our strategic
partners or collaborators may fail to perform their obligations
as expected. These strategic partners and collaborators may
breach or terminate their agreements with us or otherwise fail
to conduct their collaborative activities successfully and in a
timely manner. Further, our strategic partners and collaborators
may not develop products arising out of our collaborative
arrangements or devote sufficient resources to the development,
manufacture, marketing, or sale of our existing and future
products. Moreover, disagreements with a strategic partner or
18
collaborator regarding strategic direction, economics of our
relationship, intellectual property or other matters could
develop, and any such conflict could reduce our ability to enter
into future collaboration agreements and negatively impact our
relationships with one or more existing strategic partners or
collaborators. If any of these events occur, or if we fail to
maintain our agreements with our strategic partners and
collaborators, we may not be able to commercialize our existing
and future products, further develop our business, or generate
sufficient revenues to support our operations.
Additionally, our business could be negatively impacted if any
of our strategic partners or collaborators undergoes a change of
control or assigns the rights or obligations under any of our
agreements. If any of our strategic partners or collaborators
were to assign these agreements to our competitors or to a third
party who is not willing to work with us on the same terms or
commit the same resources as the current strategic partner or
collaborator, our business and prospects could be adversely
affected.
OTHER
RISKS ASSOCIATED WITH OUR BUSINESS OPERATIONS
We may
not be successful in identifying market needs for new
technologies and in developing new products to meet those
needs.
The success of our business model depends in part on our ability
to identify additional market opportunities for our
biochemicals. The materials and manufacturing technologies we
research and develop are new and continuously changing and
advancing. The biochemicals that are derived from these
technologies may not be applicable or compatible with demands in
existing or future markets. Furthermore, we may not be able to
identify new opportunities as they arise for our products since
future applications of any given product may not be readily
determinable, and we cannot reasonably estimate the size of any
markets that may develop. If we are not able to successfully
develop new products, we may be unable to expand our business
beyond biosuccinic acid.
Ethical,
legal and social concerns about genetically engineered products
and processes, and similar concerns about feedstocks that could
be used for food production, could limit or prevent the use of
our products, processes and technologies and limit our
revenues.
The use of genetically-engineered products and processes is
subject to laws and regulations in many countries, some of which
are new or still evolving. Public attitudes about the safety and
environmental hazards of genetically-engineered products and
processes, and ethical concerns over genetic research, could
influence public acceptance of our technology, process and
products.
Our ability to develop and commercialize one or more of our
technologies, products, or processes could be limited by the
following additional factors:
|
|
|
| |
•
|
public attitudes regarding, and potential changes to laws
governing, ownership of genetic material, which could harm our
intellectual property rights with respect to our genetic
material and discourage others from supporting, developing or
commercializing our products, processes and technologies;
|
| |
| |
•
|
public attitudes and ethical concerns surrounding production of
feedstocks on land which could be used to grow food, which could
influence public acceptance of our technologies, products and
processes; and
|
| |
| |
•
|
governmental reaction to negative publicity concerning
genetically engineered organisms, which could result in greater
government regulation of genetic research and derivative
products or feedstocks produced on land that could be used to
grow food, which could result in greater government regulation
of feedstock sources.
|
The subjects of genetically engineered organisms and food versus
fuel have received negative publicity, which has aroused public
debate. This adverse publicity could lead to greater regulation
and trade restrictions on imports of genetically engineered
products or feedstocks grown on land suitable for food
production. These trends could result in increased expenses,
delays or other impediments to our programs or the public
acceptance and commercialization of our products.
19
Our
initial production of biosuccinic acid will be conducted at a
single location, which makes us susceptible to
disasters.
Our biosuccinic acid production will initially be conducted at a
single location in Lake Providence, Louisiana. We plan to take
precautions to safeguard this facility from natural and other
disasters, through insurance, hazard protection, health and
safety protocols and off-site storage of critical research
results and computer data. However, a natural disaster, such as
a fire, flood, hurricane, tornado, earthquake or an
international act of sabotage or terrorism, could cause
substantial delays in our operations, damage or destroy our
manufacturing equipment, feedstocks, or product inventory, and
cause us to incur additional expenses. The insurance we maintain
against natural disasters may not be adequate to cover our
losses in any particular case. For example, the Louisiana Plant
will be located on a site adjacent to the Mississippi River.
Recent flooding on the river has brought disastrous results to
businesses, farms and residences located in the vicinity. While
to date we have experienced no flooding, we could be affected by
similar events in the future.
If we
lose key personnel or are unable to attract and retain
additional key personnel, it could harm our research and
development efforts, delay the commercialization of our
products, delay launch of products in our development pipeline
and impair our ability to meet our business
objectives.
Our business involves complex operations spanning a variety of
disciplines and demanding a management team and employee
workforce that is knowledgeable in the many areas necessary for
our operations. The loss of any key member of our management
team or key research and development or operational employees,
or the failure to attract and retain such employees, could
prevent us from developing and commercializing our products for
our target markets and executing our business plans.
We may not be able to attract or retain qualified employees due
to the intense competition for qualified personnel among
biotechnology and other technology-based businesses,
particularly in the biorenewables area, or due to the scarcity
of personnel with the qualifications or experience necessary for
our business. Hiring, training and successfully integrating
qualified personnel into our operation is lengthy and expensive.
The market for qualified personnel is very competitive because
of the limited number of people available with the necessary
technical skills and understanding of our technology and
anticipated products. If we are not able to attract and retain
the necessary personnel to accomplish our business objectives,
we may experience staffing constraints that will adversely
affect our ability to support our internal research and
development programs or satisfy customer demands for our
products. In particular, our product development and research
and development programs are dependent on our ability to attract
and retain highly skilled scientific, technical and operational
personnel. Competition for such personnel from numerous
companies and academic and other research institutions may limit
our ability to do so on acceptable terms, or at all. All of our
employees are at-will employees, which means that either the
employee or we may terminate their employment at any time.
We use
hazardous materials in our business and must comply with
applicable environmental laws and regulations. Any claims
relating to improper handling, storage or disposal of these
materials or noncompliance with applicable laws and regulations
could be time consuming and costly and could adversely affect
our business and results of operations.
We use hazardous chemicals and biological materials in our
business and are subject to a variety of federal, state and
local laws and regulations governing the use, generation,
manufacture, storage, handling and disposal of these materials
both in the U.S. and overseas. Although we have implemented
safety procedures for handling and disposing of these materials
and waste products, we cannot be sure that our safety measures
are compliant with legal requirements or adequate to eliminate
the risk of accidental injury or contamination. In the event of
contamination or injury, we could be held liable for any
resulting damages, and any liability could exceed our insurance
coverage. There can be no assurance that we will not violate
environmental, health and safety laws as a result of human
error, accident, equipment failure or other causes. Compliance
with applicable environmental laws and regulations is expensive
and time consuming, and the failure to comply with past,
present, or future laws could result in the imposition of fines,
third-party property damage, product liability and personal
injury claims, investigation and remediation costs, the
suspension of production, or a cessation of operations. Our
liability in such an event may exceed our total assets.
Liability under
20
environmental laws can be joint and several and without regard
to comparative fault. Environmental laws could become more
stringent over time, imposing greater compliance costs and
increasing risks and penalties associated with violations, which
could impair our research, development or production efforts and
harm our business. Accordingly, violations of present and future
environmental laws could restrict our ability to expand
facilities, or pursue certain technologies, and could require us
to acquire equipment or incur potentially significant costs to
comply with environmental regulations.
WE ARE
SUBJECT TO A VARIETY OF UNCERTAINTIES AND RISKS RELATING TO OUR
AND THIRD PARTIES’ INTELLECTUAL PROPERTY
Our
patent rights may not provide commercially meaningful protection
against competition.
Our success will depend in part on our ability to obtain patents
and other intellectual property rights to protect our products
from competition. We have adopted a strategy of seeking patents
and patent licenses in the U.S. and in certain foreign
countries with respect to certain technologies used in, or
relating to, our products and processes.
The scope and validity of patents and success in prosecuting
patent applications involve complex legal and factual questions,
and the issuance, scope, validity, and enforceability of a
patent cannot be predicted with any certainty. Patents issued or
licensed to us may be challenged, invalidated or circumvented.
Moreover, third parties could practice our inventions in secret
and in territories where we do not have patent protection. Such
third parties may then try to sell or import products made using
our inventions in and into the U.S. or other territories.
We may be unable to prove that such products were made using our
inventions. Additional uncertainty may result from patent reform
legislation proposed by the U.S. Congress and other
national governments and from legal precedent as handed down by
the U.S. Court of Appeals for the Federal Circuit, the
U.S. Supreme Court and the courts of other countries, as
they determine legal issues concerning the scope, validity and
construction of patent claims. Because patent applications in
the U.S. and many foreign jurisdictions are typically not
published until 18 months after filing, or in some cases
not at all, and because publication of discoveries in the
scientific literature often lags behind the actual discoveries,
there is additional uncertainty as to the inventorship, and
therefore the validity, of any issued patents. Accordingly, we
cannot be certain that any of our patent applications will
result in issued patents, or even if issued, be sure of their
validity or enforceability. Moreover, we cannot predict whether
any of our patent rights will be broad enough in scope to
provide commercial advantage and prevent circumvention. In any
event, patents are enforceable only for a limited term.
We may
not be able to enforce our intellectual property rights
throughout the world.
We plan in the future to build, or partner with others in
building, manufacturing facilities using our technologies in
countries other than the United States. However, the laws of
some foreign countries do not protect intellectual property
rights to the same extent as federal and state laws in the
U.S. Many companies have encountered significant problems
in protecting and enforcing intellectual property rights in
certain foreign jurisdictions. The legal systems of certain
countries, particularly certain developing countries, do not
favor the enforcement of patents and other intellectual property
protection, particularly those relating to bioindustrial
technologies. This could make it difficult for us to stop the
infringement of our patents or misappropriation of our other
intellectual property rights. Proceedings to enforce our patents
and other proprietary rights in foreign jurisdictions could
result in substantial costs and divert our efforts and attention
from other aspects of our business. Accordingly, our efforts to
enforce our intellectual property rights in such countries may
be inadequate to obtain a significant commercial advantage from
the intellectual property that we develop.
We may
not be able to operate our business without infringing
third-party patents.
Our ability to commercialize our proposed products depends on
our ability to develop, manufacture, market and sell our
proposed products without infringing the proprietary rights of
third parties. Numerous U.S. and foreign patents and
pending patent applications owned by third parties exist in
fields that relate to our proposed products and our underlying
methodologies and discoveries. Third parties may allege that our
21
proposed products or our methods infringe their intellectual
property rights. If we are found to infringe their intellectual
property rights, we could be prohibited from commercializing the
infringing product unless we obtain a license to use the
technology covered by the patent or are able to design around
the patent. We may be unable to obtain a license on terms
acceptable to us, if at all, and we may not be able to redesign
our products, biocatalysts or processes to avoid infringement.
Even if we are able to redesign our products, biocatalysts or
processes to avoid an infringement claim, our efforts to design
around the patent may lead to an inferior or more costly
product. A court could also order us to pay compensatory damages
for any infringement, plus prejudgment interest and could, in
addition, treble the compensatory damages and award attorney
fees. These damages could be substantial and could harm our
reputation, business, financial condition and operating results.
A court also could enter orders that temporarily, preliminarily
or permanently prohibit us and our customers from making, using,
selling or offering to sell one or more of our products, or
could enter an order mandating that we undertake certain
remedial activities.
In
addition to our patent rights, we rely in part on trade secrets
to protect our technology. Trade secrets can be difficult to
protect and enforce.
We rely on trade secrets to protect some of our technology,
particularly where we do not believe patent protection is
appropriate or obtainable. However, trade secrets are difficult
to maintain and protect. Our strategy for
scale-up of
production requires us to share confidential information with
our business partners and other parties. Our business
partners’ employees, consultants, contractors or scientific
and other advisors may unintentionally or willfully disclose our
proprietary information to competitors. Enforcement of claims
that a third party has illegally obtained and is using trade
secrets is expensive, time consuming and uncertain. In addition,
foreign courts are sometimes less willing than U.S. courts
to protect trade secrets. If our competitors independently
develop equivalent knowledge, methods and know-how, we would not
be able to assert our trade secrets against them. Our failure to
obtain or maintain trade secret protection could adversely
affect our competitive business position.
If our
biocatalysts, or the genes that code for our biocatalysts, are
stolen, misappropriated or reverse engineered, others could use
these biocatalysts or genes to produce competing chemical
intermediates.
A number of third parties, including various collaborators,
tolling plant operators, university scientists and researchers,
customers and those involved in the shipping and handling of our
biocatalysts and our primary fermentation products, have access
to our proprietary biocatalysts. If our biocatalysts, or the
genes that code for our biocatalysts, were stolen,
misappropriated or reverse engineered based on our disclosures
in our patent applications, they could be used by other parties
for their own commercial gain. If this were to occur, it could
be difficult for us to discover or challenge this type of use,
especially in countries with limited intellectual property
protection.
Our
rights to key intellectual property are licensed to us.
Termination of the related agreements would be highly
detrimental to us.
We are a party to certain license agreements, including our
license agreements with the University of Florida Research
Foundation, Inc., pursuant to which we license key intellectual
property underlying technology used in our business. These
license agreements impose various diligence, milestone, payment,
royalty, insurance and other obligations on us. If we fail to
comply with any of these obligations, the licensors may have the
right to reduce an exclusive license of intellectual property to
a nonexclusive license or to terminate the license completely,
in which case our competitors may gain access to these important
licensed technologies or we may be unable to develop or market
products covered by the licensed intellectual property. If we
lose rights that are important to our biosuccinic acid or other
biochemical production, our business would be materially
adversely affected. We may enter into additional licenses in the
future, and if we fail to comply with obligations under those
agreements, we could suffer similar consequences.
22
Certain
colleges and universities that license intellectual property to
us receive funding from U.S. government agencies, which could
negatively affect our intellectual property
rights.
Some of the research undertaken by the university technology
offices with which we have relationships have been funded by
grants from U.S. government agencies. When new technologies
are developed with U.S. government funding, the government
obtains certain rights in any resulting patents and technical
data, generally including, at a minimum, a nonexclusive license
authorizing the government to use the invention or technical
data for noncommercial purposes. U.S. government funding
must be disclosed in any resulting patent applications, and our
rights in such inventions will normally be subject to government
license rights, periodic progress reporting, foreign
manufacturing restrictions and march-in rights. March-in rights
refer to the right of the U.S. government, under certain
limited circumstances, to require us to grant a license to
technology developed under a government grant to a responsible
applicant, or, if we refuse, to grant such a license itself.
March-in rights can be triggered if the government determines
that we have failed to work sufficiently towards achieving
practical application of a technology or if action is necessary
to alleviate health or safety needs, to meet requirements of
federal regulations or to give preference to U.S. industry.
If we breach the terms of our grants, the government may gain
rights to the intellectual property developed in our related
research.
Furthermore, the terms of our research grants from
U.S. government agencies may prohibit us from using the new
technologies we have developed using those grants in
non-U.S. manufacturing
plants, which could adversely affect our business. Under the
Bayh-Dole Act of 1980, a party that acquires an exclusive
license for an invention that was funded in whole or in part by
a federal research grant is subject to the following government
rights:
|
|
|
| |
•
|
products using the invention that are sold in the U.S. are to be
manufactured substantially in the U.S., unless a waiver is
obtained;
|
| |
| |
•
|
the government may force the granting of a license to a third
party who will make and sell the needed product if the licensee
does not pursue reasonable commercialization of a needed product
using the invention; and
|
| |
| |
•
|
the U.S. government may use the invention for its own needs.
|
If we fail to meet these guidelines, we would lose our exclusive
rights to these inventions and we would lose potential revenue
derived from these inventions.
We may
not retain exclusive rights to intellectual property created as
a result of our collaborations.
We are negotiating a joint development agreement with Davy and a
joint venture and licensing arrangement with PTTCH and have
established a partnership with Uhde, each of which involves, or
would involve if a binding agreement were signed, joint venture
research and development efforts. We share, and would share, to
various degrees, intellectual property we jointly develop. Such
provisions may limit our ability to gain commercial benefit from
some of the intellectual property we develop and may lead to
costly or time-consuming disputes with parties with whom we have
commercial relationships over rights to certain innovations.
Patent
disputes and litigations can be expensive.
Because of the uncertainties involved in the issuance and
enforcement of patents, and the value of a broadly interpreted
patent, patent disputes and litigations are common. We may
become involved in patent disputes relating to infringement of
our technology, with third-parties asserting “blocking
patents,” with our licensors or licensees, with
collaborators and with employees, among others. Patent disputes
can take years to resolve, can be very costly and can result in
loss of rights, injunctions and substantial penalties. Moreover,
patent disputes and related proceedings can distract
management’s attention and interfere with running the
business.
23
FINANCING
UNCERTAINTIES CREATE RISKS FOR US
We may
require substantial additional financing to achieve our goals.
If we cannot raise capital when needed or on acceptable terms,
we could be forced to delay, limit, reduce or terminate our
development and commercialization efforts.
Since our inception, most of our resources have been dedicated
towards research and development and otherwise investigating the
technological and commercial feasibility of our technology in
our laboratories and at third-party tolling facilities. We have
consumed substantial amounts of capital since our inception in
2009 for our research and development activities. For the year
ended December 31, 2010, we used $9.4 million to fund
our operating and investing activities. Although we believe our
existing cash resources of $50.7 million as of
March 31, 2011, including the $15.2 million of
restricted cash for the Louisiana Plant, plus the proceeds of
this offering, will be sufficient to fund our anticipated cash
requirements for at least the next 24 months, we may
require significant additional financing in the future to fund
our operations. We believe that we will continue to expend
substantial resources for the foreseeable future on further
developing our technologies and developing commercial-scale
industrial biochemical plants. These expenditures will include
costs associated with research and development, developing,
building and operating industrial biochemical plants, acquiring
or constructing storage facilities and negotiating agreements
for the purchase of feedstocks and the sale of biosuccinic acid
and other biochemicals that we plan to produce. In addition,
unanticipated costs may arise. Because the costs of developing
our technology at a commercial scale are highly uncertain, we
cannot estimate with certainty the amounts necessary to
successfully commercialize our production.
To date, we have funded our operations primarily through equity
issuances, debt and government awards. We believe that the net
proceeds from this offering, together with our existing cash and
cash equivalents and government awards, will allow us to take a
substantial step toward implementing our strategy. However,
based on our current plans and expectations, we will require
additional funding to achieve our production and sales
objectives for the next several years. In addition, the cost of
preparing, filing, prosecuting, maintaining and enforcing
patent, trademark and other intellectual property rights and
defending ourselves against potential claims by others that we
are violating their intellectual property rights may be
significant. Moreover, our plans and expectations may change as
a result of factors currently unknown to us, and we may need
additional funds sooner than planned. We may also choose to seek
additional capital sooner than required due to favorable market
conditions or strategic considerations.
Our future capital requirements will depend on many factors,
including:
|
|
|
| |
•
|
the timing of, and costs involved in, developing and scaling up
our technologies for commercial-scale production of biosuccinic
acid and other chemical intermediates;
|
| |
| |
•
|
the timing of, and costs involved in, developing and building
the Louisiana Plant and other industrial biochemical
manufacturing plants;
|
| |
| |
•
|
the cost of operating and maintaining our manufacturing plants;
|
| |
| |
•
|
our ability to gain market acceptance for biosuccinic acid;
|
| |
| |
•
|
the requirements for biosuccinic acid of our initial three
customers and whether they comply with their supply agreements
with us by purchasing those requirements from us;
|
| |
| |
•
|
our ability to negotiate additional supply arrangements for our
biosuccinic acid and other biochemicals and the timing and terms
of those sales;
|
| |
| |
•
|
our ability to negotiate supply agreements for the purchase of
the feedstocks required in our production process and for the
sale of biosuccinic acid and other biochemicals we produce and
the timing and terms of those agreements;
|
| |
| |
•
|
our ability to sell ammonium sulfate, a co-product of producing
biosuccinic acid, and the timing and terms of those sales;
|
24
|
|
|
| |
•
|
our ability to enter into binding collaboration agreements with
Davy, PTTCH and others, to maintain those relationships and the
timing and terms of those arrangements; and
|
| |
| |
•
|
the cost of preparing, filing, prosecuting, maintaining,
defending and enforcing patent, trademark and other intellectual
property claims, including litigation costs and the outcome of
such litigation.
|
Additional funds may not be available when we need them, on
terms that are acceptable to us, or at all. If needed funds are
not available to us on a timely basis, we may be required to
delay, limit, reduce or terminate:
|
|
|
| |
•
|
our research and development activities;
|
| |
| |
•
|
our plans to build biosuccinic acid and other biochemical
manufacturing plants;
|
| |
| |
•
|
our production of biosuccinic acid at our plants; and
|
| |
| |
•
|
our ability to discover, develop,
scale-up and
produce additional biochemicals and products from our pipeline.
|
Additional financing may not be available to us when and if we
need it, on commercially reasonable terms, or at all. Until we
can generate significant continuing revenues, we expect to
satisfy our future cash needs through strategic collaborations,
private or public sales of our securities, debt financings,
governmental awards, or by licensing our technology. Further,
additional funding may significantly dilute existing
stockholders.
Our
government award from the DOE is subject to certain conditions
and obligations.
We are the recipient of an award from the U.S. Department
of Energy providing for a 50/50 cost share for our Louisiana
Plant, with up to $50 million reimbursable by the DOE. We
have already received and spent $10.4 million under this
award as of March 31, 2011. The terms of this award require
us, and we intend, to use the funds to design, build and operate
our Louisiana Plant to develop
U.S.-based
production capabilities for renewable chemicals derived from
grain sorghum and other lignocellulosic feedstocks for a period
of approximately 1,100 hours, which we anticipate will not
have an impact on our overall business plan. Under this award,
we are required to fund an additional $39.6 million in
cost-sharing expenses to build the Louisiana Plant and to
reserve $15.2 million in cash contingency. Generally,
government award agreements have fixed terms and may be
terminated, modified or recovered by the granting agency under
certain conditions. If the Department of Energy later terminates
its funding agreement with us, our ability to complete the
construction of the Louisiana Plant could be impaired, which
could harm our business.
The
planned expansion of our Louisiana Plant will depend on
infrastructure improvements that will be paid for and undertaken
by the Lake Providence Port Commission and the State of
Louisiana.
The Lake Providence Port Commission and the State of Louisiana
have committed a combined total of $16 million to support
infrastructure improvements at the site of our Louisiana Plant,
$6 million of which has already been spent on engineering
and related fees. These infrastructure improvements will be
necessary to support the proposed expansion of the Louisiana
Plant. If the Lake Providence Port Commission or the State of
Louisiana later terminate their commitments to provide funding
for and make the infrastructure improvements referenced above,
production prospects could be impaired, which could harm our
business.
We may seek to obtain additional government awards and subsidies
in the future to offset all or a portion of the costs of
building or retrofitting new biochemical manufacturing plants
and research and development activities. We cannot be certain
that we will be able to secure any such additional government
awards or subsidies. Our existing funding or any new government
awards that we may obtain may be terminated, modified or
recovered by the granting governmental body under certain
conditions. Various governments have grant, loan and subsidy
programs focused on the development of clean technologies,
including alternatives to petroleum-based products and the
reduction of carbon emissions. Such government programs could
lead to increased funding for our competitors or a rapid
increase in the number of competitors within those markets.
25
We may
experience shortfalls in our funding of the Louisiana
Plant.
We expect to pay for the completion of the Louisiana Plant,
including construction and
start-up
costs, with the net proceeds of the $50 million award from
the DOE and $10 million in infrastructure improvements from
the Lake Providence Port Commission and the State of Louisiana
and the balance from our own funds. We have estimated the
$103 million cost to complete the Louisiana Plant, which
includes working capital, fees and expenses, based upon a
competitive bidding process for various equipment and
construction packages designed by our engineering partners. Most
of the equipment and construction packages were provided on a
lump sum basis, so the costs for these are firm, but are subject
to additional costs for design changes. Cost overruns or other
unexpected difficulties could result in additional costs in
excess of the amount that we have reserved for construction
contingencies, which could require additional funding. Such
funds may not be available when we need them, on terms that are
acceptable to us or at all, which could delay or prevent our
initial commercial production of biosuccinic acid. If there are
difficulties, delays or other unforeseen issues relating to the
construction or operation of the Louisiana Plant, our total
investment in the plant may increase significantly and the
revenue from sales, if any, of biosuccinic acid and our other
biochemical intermediates produced at the plant and the
distribution of profits, if any, may be delayed.
OTHER
RISKS YOU WOULD FACE AS AN INVESTOR
A
significant portion of our total outstanding shares of common
stock is restricted from immediate resale but may be sold into
the market in the near future. This could cause the market price
of our common stock to drop significantly, even if our business
is doing well.
Sales of a substantial number of shares of our common stock in
the public market could occur at any time. These sales, or the
perception in the market that the holders of a large number of
shares of common stock intend to sell shares, could reduce the
market price of our common stock. When this offering is
completed, assuming the sale
of shares
of common stock in this offering, our three largest stockholders
as
of ,
2011 will beneficially own, collectively,
approximately % of our outstanding
common stock. If one or more of them were to sell a substantial
portion of the shares they hold, it could cause our stock price
to decline.
Upon completion of this offering we will
have
outstanding shares of common stock, which excludes shares
subject to outstanding options and warrants. All of the shares
we sell in this offering (except any acquired by our affiliates)
will be freely
tradable. shares
of common stock will be subject to a
180-day
contractual
lock-up with
the underwriters (which
lock-up
period may be released at any time and as to any number of
shares by the underwriters). Upon expiration of the lockup
agreements, these shares will be eligible for immediate resale,
subject in some cases to the volume and other restrictions of
Rule 144 under the Securities Act of 1933, as amended, or
the Securities Act. These shares represent a substantial
majority of our total shares outstanding, and sales of these
shares upon expiration of the
lock-up
could significantly depress our share price.
In addition, as of March 31, 2011, there were
1,534,975 shares issuable under currently outstanding
options and warrants (of which 639,170 warrants were exercised
on April 12, 2011 at an exercise price of $0.01 per share)
that if issued will become eligible for sale in the public
market to the extent permitted by any applicable vesting
requirements, the
lock-up
agreements and Rules 144 and 701 under the Securities Act.
Moreover, after this offering, the holders of an aggregate of
approximately shares
of our outstanding common stock (including shares of our common
stock issued and issuable upon the exercise of options or
warrants), will have rights, subject to some conditions, to
require us to file registration statements covering their shares
and to include their shares in registration statements that we
may file for ourselves or other stockholders.
We also intend to
register shares
of common stock that have been reserved for issuance under our
2011 Omnibus Incentive Plan and our 2011 employee stock purchase
plan. To the extent any of these registered shares are issued,
subject to any applicable vesting requirements they can be
freely sold in the public market subject to the
180-day
lock-up
periods under the
lock-up
agreements described in the “Underwriting” section of
this prospectus.
26
Concentration
of ownership among our existing officers, directors and
principal stockholders may prevent other stockholders from
influencing significant corporate decisions and depress our
stock price.
Assuming
that shares
are sold in this offering, our officers, directors and existing
stockholders who beneficially own at least 5% of our stock will
together beneficially own
approximately % of our
outstanding common stock following the completion of this
offering. As of March 31, 2011, PTT Chemical International
Private Limited, a wholly-owned subsidiary of PTTCH, Plainfield
Finance II LLC/Plainfield Direct LLC and Norwood LDK, LLC,
the entity through which our chief executive officer, Stephen J.
Gatto, holds his shares in the company, beneficially owned
approximately 47.9%, 26.4% and 13.9% of our common stock,
respectively. If these officers, directors, and principal
stockholders or a group of our principal stockholders act
together, they will be able to exert a significant degree of
influence over our management and affairs and control matters
requiring stockholder approval, including the election of
directors and approval of mergers or other business combination
transactions. The interests of this concentration of ownership
may not always coincide with our interests or the interests of
other stockholders. For instance, officers, directors, and
principal stockholders, acting together, could cause us to enter
into transactions or agreements that we would not otherwise
consider. Similarly, this concentration of ownership may have
the effect of delaying or preventing a change in control of our
company otherwise favored by our other stockholders. This
concentration of ownership could depress our stock price.
Purchasers
in this offering will experience immediate and substantial
dilution in the book value of their investment.
The initial public offering price in this offering will be
substantially higher than the tangible book value per share of
shares of our common stock immediately after the offering.
Tangible book value means the total value of our tangible assets
less our total liabilities divided by the number of shares of
our common stock outstanding. We call the difference between the
price per share paid by investors in this offering and the
tangible book value per share immediately after this offering
“book value dilution,” or simply “dilution.”
To the extent outstanding options and warrants to purchase
shares of common stock are exercised, there will be further
dilution. For further information on this calculation, see
“Dilution” elsewhere in this prospectus.
We
have broad discretion in the use of net proceeds from this
offering and may not use them effectively.
Although we currently intend to use the net proceeds from this
offering in the manner described in “Use of proceeds”
elsewhere in this prospectus, we will have broad discretion in
the application of the net proceeds. Our failure to apply these
net proceeds effectively could affect our ability to continue to
develop and sell our products and grow our business, which could
cause the value of your investment to decline.
We
will incur significant increased costs as a result of operating
as a public company, and our management will be required to
devote substantial time to new compliance
initiatives.
We have never operated as a public company. As a public company,
we will incur significant legal, accounting and other expenses
that we did not incur as a private company. In addition, the
Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, as well
as related rules implemented by the Securities and Exchange
Commission, or the SEC, and The Nasdaq Stock Market, impose
various requirements on public companies. Our management and
other personnel will need to devote a substantial amount of time
to these compliance initiatives. Moreover, these rules and
regulations will increase our legal and financial compliance
costs and will make some activities more time-consuming and
costly. For example, we expect these rules and regulations to
make it more expensive for us to maintain director and officer
liability insurance.
If we
fail to maintain an effective system of internal controls, we
might not be able to report our financial results accurately or
prevent fraud; in that case, our stockholders could lose
confidence in our financial reporting, which would harm our
business and could negatively impact the price of our
stock.
Effective internal controls are necessary for us to provide
reliable financial reports and prevent fraud. In addition, SEC
rules implemented in accordance with Section 404 of the
Sarbanes-Oxley Act of 2002 will
27
require us to evaluate and report on and, in the event we become
an “accelerated filer” as defined in SEC rules, our
independent registered public accounting firm to attest to, our
internal control over financial reporting beginning with our
Annual Report on
Form 10-K
for the year ending December 31, 2012. The process of
implementing our internal controls and complying with
Section 404 will be expensive and time consuming and will
require significant attention of management. We cannot be
certain that these measures will ensure that we implement and
maintain adequate controls over our financial processes and
reporting in the future. Even if we conclude, and our
independent registered public accounting firm concurs, that our
internal control over financial reporting provides reasonable
assurance regarding the reliability of financial reporting and
the preparation of financial statements for external purposes in
accordance with generally accepted accounting principles,
because of its inherent limitations, internal control over
financial reporting may not prevent or detect fraud or
misstatements. Failure to implement required new or improved
controls, or difficulties encountered in their implementation,
could harm our results of operations or cause us to fail to meet
our reporting obligations. If we or our independent registered
public accounting firm determine a “material
weakness,” as defined in SEC rules, exists in our internal
control over financial reporting, the disclosure of that
determination, even if quickly remedied, could reduce the
market’s confidence in our financial statements and harm
our stock price. In addition, non-compliance with
Section 404 could subject us to a variety of administrative
sanctions, including SEC action, ineligibility for short form
resale registration, the suspension or delisting of our common
stock from the stock exchange on which it is listed and the
inability of registered broker-dealers to make a market in our
common stock, which would further reduce our stock price and
could harm our business.
We are
subject to anti-takeover provisions in our certificate of
incorporation and bylaws and under Delaware law that could delay
or prevent an acquisition of our company, even if the
acquisition would be beneficial to our
stockholders.
Provisions in our second amended and restated certificate of
incorporation, our amended and restated bylaws and Delaware law
may delay or prevent an acquisition of us. These provisions may
also frustrate or prevent any attempts by our stockholders to
replace or remove our current management by making it more
difficult for stockholders to replace members of our board of
directors, who are responsible for appointing the members of our
management team. Although we believe these provisions together
provide for an opportunity to receive higher bids by requiring
potential acquirers to negotiate with our board of directors,
they would apply even if an offer to acquire our company may be
considered beneficial by some stockholders. These provisions are
described in detail under the caption “Description of
capital stock.”
Our
share price may be volatile and you may be unable to sell your
shares at or above the offering price.
The initial public offering price for our shares will be
determined by negotiations between us and representatives of the
underwriters and may not be indicative of prices that will
prevail in the trading market. The market price of shares of our
common stock could be subject to wide fluctuations in response
to many risk factors listed in this section, and others beyond
our control, including:
|
|
|
| |
•
|
actual or anticipated fluctuations in our financial condition
and operating results;
|
| |
| |
•
|
the position of our cash and cash equivalents;
|
| |
| |
•
|
actual or anticipated changes in our growth rate relative to our
competitors;
|
| |
| |
•
|
actual or anticipated fluctuations in our competitors’
operating results or changes in their growth rate;
|
| |
| |
•
|
announcements of technological innovations by us, our partners
or our competitors;
|
| |
| |
•
|
announcements by us, our partners or our competitors of
significant acquisitions, strategic partnerships, joint ventures
or capital commitments;
|
| |
| |
•
|
the entry into, modification or termination of licensing
arrangements;
|
| |
| |
•
|
the entry into, modification or termination of research,
development, commercialization, supply or distribution
arrangements;
|
28
|
|
|
| |
•
|
additions or losses of customers;
|
| |
| |
•
|
additions or departures of key management or scientific
personnel;
|
| |
| |
•
|
competition from existing products or new products that may
emerge;
|
| |
| |
•
|
issuance of new or updated research reports by securities or
industry analysts;
|
| |
| |
•
|
fluctuations in the valuation of companies perceived by
investors to be comparable to us;
|
| |
| |
•
|
disputes or other developments related to proprietary rights,
including patents, litigation matters and our ability to obtain
patent protection for our technologies;
|
| |
| |
•
|
announcement or expectation of additional financing efforts;
|
| |
| |
•
|
sales of our common stock by us or our stockholders;
|
| |
| |
•
|
share price and volume fluctuations attributable to inconsistent
trading volume levels of our shares;
|
| |
| |
•
|
general market conditions in our industry; and
|
| |
| |
•
|
general economic and market conditions, including the recent
financial crisis.
|
Furthermore, the stock markets have experienced extreme price
and volume fluctuations that have affected and continue to
affect the market prices of equity securities of many companies.
These fluctuations often have been unrelated or disproportionate
to the operating performance of those companies. These broad
market and industry fluctuations, as well as general economic,
political and market conditions such as recessions, interest
rate changes or international currency fluctuations, may
negatively impact the market price of shares of our common
stock. If the market price of shares of our common stock after
this offering does not exceed the initial public offering price,
you may not realize any return on your investment in us and may
lose some or all of your investment. In the past, companies that
have experienced volatility in the market price of their stock
have been subject to securities class action litigation. We may
be the target of this type of litigation in the future.
Securities litigation against us could result in substantial
costs and divert our management’s attention from other
business concerns, which could seriously harm our business.
A significant portion of our total outstanding shares of common
stock is restricted from immediate resale but may be sold into
the market in the near future. This could cause the market price
of our common stock to drop significantly, even if our business
is doing well.
29
Special
note regarding forward-looking statements
This prospectus contains forward-looking statements that involve
risks and uncertainties. The forward-looking statements are
contained principally in the sections entitled “Prospectus
summary,” “Risk factors,” “Management’s
discussion and analysis of financial condition and results of
operations” and “Business.” These statements
relate to future events or our future financial or operational
performance and involve known and unknown risks, uncertainties
and other factors that could cause our actual results, levels of
activity, performance or achievement to differ materially from
those expressed or implied by these forward-looking statements.
These risks and uncertainties are contained principally in the
section entitled “Risk factors.”
Forward-looking statements include all statements that are not
historical facts. In some cases, you can identify
forward-looking statements by terms such as “may,”
“will,” “should,” “could,”
“would,” “expects,” “plans,”
“anticipates,” “believes,”
“estimates,” “projects,”
“predicts,” “potential,” or the negative of
those terms, and similar expressions and comparable terminology
intended to identify forward-looking statements. These
statements reflect our current views with respect to future
events and are based on assumptions and subject to risks and
uncertainties. Because forward-looking statements are inherently
subject to risks and uncertainties, some of which cannot be
predicted or quantified, you should not rely on these
forward-looking statements as guarantees of future events. These
forward-looking statements represent our estimates and
assumptions only as of the date of this prospectus and, except
as required by law, we undertake no obligation to update or
revise publicly any forward-looking statements, whether as a
result of new information, future events or otherwise after the
date of this prospectus.
In particular, forward-looking statements in this prospectus
include statements about:
|
|
|
| |
•
|
the timing and cost of constructing, operating and expanding the
Louisiana Plant and other biochemical production facilities;
|
| |
| |
•
|
our expectations of how our biobased production technology will
perform at ongoing, commercial scale, especially as to its
efficiency and cost;
|
| |
| |
•
|
our access to capital, including to the remaining portion of our
award from the DOE;
|
| |
| |
•
|
the commercial
scale-up of
our industrial biochemical production, including the timing and
volume of our future production;
|
| |
| |
•
|
the purchase needs of our initial three customers and our
ability to negotiate pricing terms with them and their
performance under our supply agreements with them;
|
| |
| |
•
|
the availability of suitable and cost-competitive feedstocks;
|
| |
| |
•
|
our ability to gain market acceptance for biosuccinic acid and
other biochemicals;
|
| |
| |
•
|
our ability to produce and sell ammonium sulfate;
|
| |
| |
•
|
the expected applications of our products and potential products
and markets;
|
| |
| |
•
|
the expected cost-competitiveness and relative performance
attributes of our biosuccinic acid and the products derived from
it;
|
| |
| |
•
|
the achievement of advances in our technology platform;
|
| |
| |
•
|
the timing of commercial sales of our products, including the
timing and terms of supply agreements for the biosuccinic acid
that we will produce;
|
30
|
|
|
| |
•
|
our ability to enter into collaborations with Davy, PTTCH and
other commercial enterprises, the timing of any such
collaboration and our success in obtaining the hoped-for
benefits from our current collaborations and any such future
arrangements that we may negotiate;
|
| |
| |
•
|
the cost of protecting intellectual property rights and/or
defending against patent infringement claims, and our ability to
compete in the event of an adverse outcome in any legal or
administrative proceeding regarding intellectual property rights
or patent infringement;
|
| |
| |
•
|
customer certification, approval and acceptance of our products;
|
| |
| |
•
|
the anticipated sizes of the markets for our products;
|
| |
| |
•
|
the future price and volatility of renewable feedstocks; and
|
| |
| |
•
|
the future price and volatility of petroleum and products
derived from petroleum.
|
31
Use of
proceeds
We estimate that we will receive net proceeds of approximately
$ million from the sale
of shares
of common stock in this offering based on an assumed initial
public offering price of $ per
share (the mid-point of the price range set forth on the cover
page of this prospectus) and after deducting the estimated
underwriting discounts and commissions and estimated offering
expenses payable by us. A $1.00 increase (decrease) in the
assumed initial public offering price of
$ per share (the mid-point of the
price range set forth on the cover page of this prospectus)
would increase (decrease) the net proceeds to us from this
offering by $ million,
assuming that the number of shares offered by us, as set forth
on the cover page of this prospectus, remains the same and after
deducting the estimated underwriting discounts and commissions
and estimated offering expenses payable by us. If the
underwriters exercise their option to
purchase
additional shares, we estimate that our net proceeds will be
approximately $ million based
on an assumed initial public offering price of
$ per share (the mid-point of the
price range set forth on the cover page of this prospectus).
We currently intend to use all or a portion of the net proceeds
of this offering, together with
$ million of remaining
proceeds from a government award, $10 million in
infrastructure improvements to be contributed by the Lake
Providence Port Commission and the State of Louisiana and
$ million of existing cash,
cash equivalents and restricted cash to complete the
30 million pound per year plant at the Port of Lake
Providence, Louisiana, or the Louisiana Plant (approximately
$103 million, which includes working capital, fees and
expenses), to fund our expected equity contribution to the
expansion of the Louisiana Plant to 170 million pounds per
year (approximately $60 million, which includes working
capital, fees and expenses), to pay the dividend accruing on the
Class A common stock upon the closing of this offering
(which totaled $1,025,753 as of March 31, 2011) and to fund
research and development expenses (which we estimate will be
approximately $25.5 million in the aggregate for the two
fiscal years following the completion of this offering) and for
working capital and other general corporate purposes, which will
include expenses and the costs associated with being a public
company. We may also use a portion of the net proceeds to
acquire other complementary businesses, products or technologies
or to make other strategic investments. Although we do not have
any current plans or agreements for any specific acquisitions or
investments at this time, we believe opportunities may exist
from time to time to expand our current business through
strategic investments or acquisitions with other companies,
products or technologies.
The potential uses of net proceeds from this offering represent
our current intentions based upon our present business plans and
business conditions. We cannot guarantee the specific amount of
the net proceeds that will be used to develop, construct and
operate our biochemical production capabilities globally, fund
working capital or be used for other general corporate purposes.
Until we apply the net proceeds of this offering to its intended
uses, we intend to invest the net proceeds in interest-bearing
demand deposit accounts or short-term investment-grade
securities. We cannot predict whether these temporary
investments of the net proceeds will yield a favorable return,
or any return at all.
32
Dividend
policy
We have never declared or paid cash dividends on shares of our
common or convertible common stock and currently do not plan to
declare or pay cash dividends in the foreseeable future, other
than the dividend accruing on the Class A common stock at
the rate per annum of 8% per share compounded annually on a
cumulative basis, which is to be paid upon the conversion of
Class A common stock into common stock upon the closing of
this offering. As of March 31, 2011, accrued Class A
common stock dividends totaled $1,025,753. We expect to retain
our future earnings, if any, for use in the operation and
expansion of our business. The payment of cash dividends in the
future, if any, will be at the discretion of our board of
directors and will depend upon such factors as earnings levels,
capital requirements, requirements under the Delaware General
Corporation Law, restrictions and covenants pursuant to any
other credit facilities or debt indentures we may enter into,
our overall financial condition and any other factors deemed
relevant by our board of directors.
33
Capitalization
The following table sets forth our cash, cash equivalents and
restricted cash and our capitalization as of March 31, 2011:
|
|
|
| |
•
|
on an actual basis; and
|
| |
| |
•
|
on a pro forma basis after giving effect to the conversion of
all of our outstanding Class A common stock and
Class B common stock into 14,259,858 shares of common
stock and payment of the Class A common stock cumulative 8%
dividend in the amount accrued through March 31, 2011 of
$1,025,753 upon completion of this offering, issuance of
639,170 shares of common stock upon the exercise of
warrants on April 12, 2011 and the related warrant
liability transfer to additional paid in capital in the amount
of $2,198,745, and the inclusion of 186,491 shares of
restricted stock issued under the 2011 Omnibus Incentive
Plan; and
|
| |
| |
•
|
on a pro forma, as adjusted basis to reflect the pro forma
adjustments described above, the completion of this offering and
our receipt of the estimated net proceeds from this offering,
based on an assumed initial public offering
of shares
at a price of $ per share (the
mid-point of the price range set forth on the cover page of this
prospectus) and after deducting the estimated underwriting
discounts and commissions and estimated offering expenses
payable by us; including the obligatory exercise of warrants
exercisable for 17,134 shares of common stock at an
exercise price of $6.00 per share and warrants exercisable
for 89,656 shares of common stock at an exercise price of
$10.00 per share; and the increase to 100,000,000 shares of
our common stock authorized for issuance, among other things,
effected by the filing of a second amended and restated
certificate of incorporation.
|
The pro forma and pro forma, as adjusted, information below is
illustrative only and our capitalization following the
completion of this offering will be adjusted based on the actual
initial public offering price and other terms of this offering
determined at pricing. You should read this table together with
“Management’s discussion and analysis of financial
condition and results of operations” and our consolidated
financial statements and the accompanying notes appearing
elsewhere in this prospectus.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of March 31, 2011
|
|
|
|
|
|
|
|
|
|
|
Pro Forma as
|
|
|
|
|
Actual
|
|
|
Pro Forma
|
|
|
Adjusted
|
|
|
|
|
Cash, cash equivalents and restricted cash
|
|
$
|
50,692,707
|
|
|
$
|
49,673,346
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Class A common stock, $0.0001 par value per share:
11,214,953 shares authorized and outstanding, actual;
11,214,953 shares authorized, no shares outstanding, pro
forma; no shares authorized or outstanding, pro forma, as
adjusted
|
|
$
|
1,121
|
|
|
$
|
0
|
|
|
|
—
|
|
|
Class B common stock, $0.0001 par value per share:
3,044,905 shares authorized and outstanding, actual;
3,044,905 authorized, no shares outstanding, pro forma; no
shares authorized or outstanding, pro forma, as adjusted
|
|
|
304
|
|
|
|
0
|
|
|
|
—
|
|
|
Preferred stock, $0.0001 par value per share: No shares
authorized or outstanding, actual and pro forma;
5,000,000 shares authorized, no shares outstanding, pro
forma, as adjusted
|
|
|
—
|
|
|
|
—
|
|
|
$
|
0
|
|
|
Common stock, $0.0001 par value per share:
30,000,000 shares authorized; 8,494,650 outstanding,
actual; 30,000,000 shares authorized,
23,580,169 shares outstanding, pro forma;
100,000,000 shares
authorized, shares
issued and outstanding, pro forma, as adjusted
|
|
|
849
|
|
|
|
2,358
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
72,459,301
|
|
|
|
74,664,355
|
|
|
|
|
|
|
Accumulated deficit
|
|
|
(17,493,917
|
)
|
|
|
(18,519,670
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
54,967,658
|
|
|
|
56,147,043
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total capitalization
|
|
$
|
54,967,658
|
|
|
$
|
56,147,043
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
34
Each $1.00 increase or decrease in the assumed initial public
offering price of $ per share (the
mid-point of the price range set forth on the cover page of this
prospectus) would increase (decrease), our pro forma, as
adjusted cash and cash equivalents, additional paid-in capital,
total stockholders’ equity and total capitalization by
approximately $ million,
assuming that the number of shares offered by us, as set forth
on the cover page of this prospectus, remains the same and after
deducting the estimated underwriting discounts and commissions
and estimated offering expenses payable by us.
The number of shares of common stock shown as issued and
outstanding in the table set forth above is based on the number
of shares outstanding as of March 31, 2011, and excludes:
|
|
|
| |
•
|
186,491 shares of restricted stock issued under the 2011
Omnibus Incentive Plan and outstanding (except all of these
shares are included in the pro forma and pro forma as adjusted
columns);
|
| |
| |
•
|
506,250 shares issuable under restricted stock units
granted subject to vesting conditions under our 2011 Omnibus
Incentive Plan;
|
| |
| |
•
|
1,162,043 shares of common stock issuable upon the exercise
of options issued under our 2011 Omnibus Incentive Plan and
outstanding as of July 21, 2011 at a weighted average
exercise price of $12.38 per share;
|
| |
| |
•
|
805,191 shares of common stock issuable upon the exercise
of warrants outstanding as of March 31, 2011 at a weighted
average exercise price of $2.21 per share (except, as noted,
639,170 of such shares issued upon the exercise of common stock
warrants at an exercise price of $0.01 per share on
April 12, 2011 are reflected in the pro forma and pro forma
as adjusted columns, and the obligatory exercise of warrants
exercisable for 17,134 shares of common stock at an
exercise price of $6.00 per share and warrants exercisable for
89,656 shares of common stock at an exercise price of
$10.00 per share, with net proceeds to the company of
$999,364, are reflected in the pro forma as adjusted column);
|
| |
| |
•
|
2,139,483 shares of our common stock reserved for future
issuance under our 2011 Omnibus Incentive Plan plus any annual
increases in the number of shares of common stock reserved for
future issuance as provided for in such plan, as described in
“Management — Employee Benefits and Stock
Plans”; and
|
| |
| |
•
|
500,000 shares of common stock reserved for issuance under
our 2011 employee stock purchase plan, which will become
effective upon completion of this offering.
|
35
Dilution
If you invest in our common stock, your interest will be diluted
to the extent of the difference between the public offering
price per share of our common stock and the pro forma, as
adjusted net tangible book value per share of our common stock
after this offering.
Our pro forma net tangible book value at March 31, 2011 was
$56.1 million, or $2.38 per share of common stock. Pro
forma net tangible book value per share represents total
tangible assets less total liabilities, divided by the number of
outstanding shares of common stock on March 31, 2011, after
giving effect to the conversion of all of our outstanding
Class A common stock and Class B common stock into
shares of our common stock upon completion of this offering, the
exercise on April 12, 2011 of 639,170 warrants at an
exercise price of $0.01 per share, or the Warrant Exercise, and
the related warrant liability transfer to Additional Paid in
Capital in the amount of $2,198,745. Our pro forma, as adjusted
net tangible book value at March 31, 2011, after giving
effect to the Warrant Exercise and the sale by us
of shares
of common stock in this offering at an assumed initial public
offering price of $ per share (the
mid-point of the price range set forth on the cover page of this
prospectus) and after deducting the estimated underwriting
discounts and commissions and estimated offering expenses
payable by us, would have been approximately
$ million, or
$ per share. This represents an
immediate increase in pro forma, as adjusted net tangible book
value of $ per share to existing
stockholders and an immediate dilution of
$ per share to new investors
purchasing shares of our common stock in this offering at the
assumed initial public offering price of
$ per share (the mid-point of the
price range set forth on the cover page of this prospectus),
subject to adjustment to reflect the actual offering price. The
following table illustrates this per share dilution:
| |
|
|
|
|
|
|
|
|
|
Assumed initial public offering price per share
|
|
|
|
|
|
$
|
|
|
|
Pro forma net tangible book value per share at March 31,
2011
|
|
$
|
2.38
|
|
|
|
|
|
|
Increase in pro forma net tangible book value per share
attributable to this offering
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pro forma, as adjusted net tangible book value per share after
this offering
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dilution per share to new investors
|
|
|
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
If the underwriters exercise their over-allotment option in
full, the pro forma as adjusted net tangible book value will
increase to $ per share,
representing an immediate increase to existing stockholders of
$ per share and an immediate
dilution of $ per share to new
investors.
A $1.00 increase (decrease) in the assumed initial public
offering price of $ per share (the
mid-point of the price range set forth on the cover page of this
prospectus) would increase (decrease) our pro forma, as adjusted
net tangible book value by
$ million, the pro forma, as
adjusted net tangible book value per share by
$ per share and the dilution in
the pro forma net tangible book value to new investors in this
offering by $ per share, assuming
the number of shares offered by us, as set forth on the cover
page of this prospectus, remains the same and after deducting
the estimated underwriting discounts and commissions and
estimated offering expenses payable by us.
The following table shows, as of March 31, 2011, the number
of shares of common stock purchased from us, the total
consideration paid to us and the average price paid per share by
existing stockholders and by new investors purchasing common
stock in this offering at an assumed initial public offering
price of $ per share (the
mid-point of the price range set forth on the cover page of this
prospectus), before deducting the estimated underwriting
discounts and commissions and estimated offering expenses
payable by us. The discussion and tables in this section
regarding dilution are based on 23,580,169 shares of common
stock issued and outstanding as of March 31, 2011 and the
$6.00 and $10.00 per share warrant exercise and assumes the
conversion of all of our
36
Class A common stock and Class B common stock into an
aggregate of 14,259,858 shares of our common stock upon the
completion of this offering and inclusion of the 186,491 shares
of restricted stock.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares Purchased
|
|
|
Total Consideration
|
|
|
Average Price per
|
|
|
|
|
Number
|
|
|
Percent
|
|
|
Amount
|
|
|
Percent
|
|
|
Share
|
|
|
|
|
Existing stockholders
|
|
|
23,580,169
|
|
|
|
|
|
|
$
|
74,666,713
|
|
|
|
|
|
|
$
|
3.17
|
|
|
New investors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
|
|
|
|
|
|
|
$
|
|
|
|
|
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The table above, and the information below, assumes that our
existing stockholders do not purchase any shares in this
offering.
A $1.00 increase (decrease) in the assumed initial public
offering price of $ per share (the
mid-point of the price range set forth on the cover page of this
prospectus) would increase (decrease) total consideration paid
by new investors, total consideration paid by all stockholders
and the average price per share paid by all stockholders by
$ million,
$ million and
$ , respectively, assuming the
number of shares offered by us, as set forth on the cover page
of this prospectus, remains the same and before deducting the
underwriting discount and estimated offering expenses payable by
us.
The discussion and tables in this section regarding dilution
assume the obligatory exercise of warrants exercisable for
17,134 shares of common stock at an exercise price of $6.00
per share and warrants exercisable for 89,656 shares of common
stock at an exercise price of $10.00 per share assuming an
initial public offering in excess of 1.5 times the exercise
price of the warrants, and exclude:
|
|
|
| |
•
|
506,250 shares issuable under restricted stock units
granted subject to vesting conditions under the 2011 Omnibus
Incentive Plan;
|
| |
| |
•
|
1,162,043 shares of common stock issuable upon the exercise
of options issued under the 2011 Omnibus Incentive Plan and
outstanding as of July 21, 2011 at a weighted average
exercise price of $12.38 per share;
|
| |
| |
•
|
59,231 shares of common stock issuable upon the exercise of
warrants outstanding as of March 31, 2011 at an exercise
price of $13.00 per share;
|
| |
| |
•
|
2,139,483 shares of our common stock reserved for future
issuance under our 2011 Omnibus Incentive Plan, plus any annual
increases in the number of shares of common stock reserved for
future issuance as provided for in such plan, as described in
“Management — Employee Benefit and Stock
Plans”; and
|
| |
| |
•
|
500,000 shares of common stock reserved for issuance under
our 2011 employee stock purchase plan, which will become
effective upon completion of this offering.
|
If the underwriters exercise their option to purchase additional
shares in full, the following will occur:
|
|
|
| |
•
|
the number of shares of our common stock held by existing
stockholders would decrease to % of
the total number of shares of our common stock outstanding after
this offering; and
|
| |
| |
•
|
the number of shares of our common stock held by new investors
would increase to approximately %
of the total number of shares of our common stock outstanding
after this offering.
|
To the extent that outstanding options or warrants are
exercised, you will experience further dilution. If all options
and warrants outstanding as of March 31, 2011 had been
exercised on such date, our pro forma net tangible book value as
of March 31, 2011 would have been $60.3 million, or
$2.48 per share, and the pro forma, as adjusted net tangible
book value after this offering would have been
$ million, or
$ per share, causing dilution to
new investors of $ per share.
In addition, we may choose to raise additional capital due to
market conditions or strategic considerations even if we believe
we have sufficient funds for our current or future operating
plans. To the extent that we raise additional capital through
the sale of equity or convertible debt securities, the issuance
of such securities would result in further dilution to our
stockholders.
37
Selected
historical financial data
The following selected historical financial data should be read
together with our historical consolidated financial statements
and the accompanying notes, included elsewhere in this
prospectus, and “Management’s discussion and analysis
of financial condition and results of operations.” The
selected historical financial data in this section is not
intended to replace our historical financial statements and the
accompanying notes. For purposes of the disclosure contained in
this section, “Myriant”, “Company”,
“we”, “us” and “our” refer only to
Myriant Corporation and its subsidiaries.
We derived the historical financial statement data as of and for
the fiscal year ended December 31, 2008 and for the period
from January 1, 2009 to July 15, 2009 from the audited
consolidated financial statements of BioEnergy International,
LLC, or the Predecessor Company, included elsewhere in this
prospectus, and as of December 31, 2009 and 2010, for the
period from July 16, 2009 (inception) to December 31,
2009, and for the fiscal year ended December 31, 2010 from
our audited consolidated financial statements, included
elsewhere in this prospectus. We derived the financial statement
data for the years ended December 31, 2007 and 2006 from
the Predecessor’s unaudited financial statements, which are
not included in this prospectus.
The consolidated statements of operations data for the three
months ended March 31, 2010 and 2011 and the consolidated
balance sheet data as of March 31, 2011 are derived from
our unaudited interim consolidated financial statements included
elsewhere in this prospectus. The unaudited interim consolidated
financial statements have been prepared on the same basis as our
audited annual consolidated financial statements and, in the
opinion of management, reflect all adjustments, which include
only normal recurring adjustments, necessary to state fairly our
financial position as of March 31, 2011 and results of
operations for the three months ended March 31, 2010 and
2011. Operating results for the three months ended
March 31, 2011 are not necessarily indicative of the
results that may be expected for the year ended
December 31, 2011. The data should be read in conjunction
with the consolidated financial statements, related notes, and
other financial information included elsewhere in this
prospectus.
The table below sets forth our and the Predecessor
Company’s respective consolidated results of operations for
the periods shown. The inception of the Company was
July 16, 2009. The 2008 results reflect the results of the
Predecessor Company for the year ended December 31, 2008.
The 2009 results reflect the results of the Predecessor Company
for the period from January 1, 2009 through July 15,
2009, which we refer to as the 2009 Predecessor Period, and for
us for the period from July 16, 2009 to December 31,
2009, which we refer to as the 2009 Successor Period.
The operations of the Predecessor Company and our company were
substantially the same as all assets and liabilities transferred
from the Predecessor Company to us, with the exception of
certain of the Predecessor Company’s debt and the
Predecessor Company’s investment in BioEnergy Holding LLC,
as described above. The most notable changes that resulted from
the transfer of the chemical intermediates business from the
Predecessor Company to us are as follows:
|
|
|
| |
•
|
The Predecessor Company’s investment in BioEnergy Holding
LLC was not transferred to us. This did not have a significant
financial impact as there were no earnings or invested capital
in the investment.
|
| |
| |
•
|
The Predecessor Company’s note payable to BioEnergy Holding
LLC, in the amount of approximately $10.0 million at such
time with interest at 6%, was not transferred to us.
|
| |
| |
•
|
The Predecessor Company’s warrant liability in the amount
of $1.5 million was not transferred to us.
|
Based on our analysis, we concluded that (i) the results of
the Predecessor Company’s operations for the year ended
December 31, 2008 were comparable to the results of the
operations for the 2009 Predecessor Period combined with the
2009 Successor Period and (ii) the results of operations
for the 2009 Predecessor Period combined with the 2009 Successor
Period were comparable to our results of operations for the year
ended December 31, 2010.
38
The financial statements included in this prospectus do not
reflect results of operations, financial position and cash flows
as if the Predecessor Company’s biochemical business had
been operated as a stand-alone company during all periods
presented prior to July 16, 2009 (inception).
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Predecessor Company
|
|
|
Myriant Corporation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year
|
|
|
Fiscal Year
|
|
|
Fiscal Year
|
|
|
January 1, 2009
|
|
|
Period from
|
|
|
Fiscal Year
|
|
|
Three Months Ended
|
|
|
|
|
Ended
|
|
|
Ended
|
|
|
Ended
|
|
|
to July 15,
|
|
|
July 16, 2009 (Inception)
|
|
|
Ended
|
|
|
March 31,
|
|
|
|
|
December 31, 2006
|
|
|
December 31, 2007
|
|
|
December 31, 2008
|
|
|
2009
|
|
|
to December 31, 2009
|
|
|
December 31, 2010
|
|
|
2010
|
|
|
2011
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License fee revenue
|
|
$
|
267,065
|
|
|
$
|
396,515
|
|
|
$
|
344,860
|
|
|
$
|
71,833
|
|
|
$
|
221,711
|
|
|
$
|
258,241
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Management fee revenue-related party
|
|
|
—
|
|
|
|
—
|
|
|
|
262,212
|
|
|
|
267,318
|
|
|
|
—
|
|
|
|
3,557,574
|
|
|
|
600,166
|
|
|
|
2,519
|
|
|
Development fee revenue-related party
|
|
|
—
|
|
|
|
—
|
|
|
|
3,125,714
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Government awards
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,419,043
|
|
|
|
1,516,323
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
267,065
|
|
|
|
396,515
|
|
|
|
3,732,786
|
|
|
|
339,151
|
|
|
|
221,711
|
|
|
|
14,234,858
|
|
|
|
2,116,489
|
|
|
|
2,519
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of license fee revenue
|
|
|
108,432
|
|
|
|
135,813
|
|
|
|
110,020
|
|
|
|
22,373
|
|
|
|
73,859
|
|
|
|
84,600
|
|
|
|
—
|
|
|
|
—
|
|
|
Research and development
|
|
|
653,469
|
|
|
|
1,499,577
|
|
|
|
4,679,935
|
|
|
|
3,770,721
|
|
|
|
2,793,085
|
|
|
|
15,904,717
|
|
|
|
2,552,100
|
|
|
|
1,883,603
|
|
|
Project development
|
|
|
706,666
|
|
|
|
1,764,244
|
|
|
|
2,179,965
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
General and administrative expense
|
|
|
1,547,540
|
|
|
|
3,166,646
|
|
|
|
5,699,186
|
|
|
|
5,438,073
|
|
|
|
4,979,186
|
|
|
|
12,673,247
|
|
|
|
2,806,285
|
|
|
|
3,034,498
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
3,016,107
|
|
|
|
6,566,280
|
|
|
|
12,669,106
|
|
|
|
9,231,167
|
|
|
|
7,846,130
|
|
|
|
28,662,564
|
|
|
|
5,358,385
|
|
|
|
4,918,101
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(2,749,042
|
)
|
|
|
(6,169,765
|
)
|
|
|
(8,936,320
|
)
|
|
|
(8,892,016
|
)
|
|
|
(7,624,419
|
)
|
|
|
(14,427,706
|
)
|
|
|
(3,241,896
|
)
|
|
|
(4,915,582
|
)
|
|
Other income (expense), net
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
1,477
|
|
|
|
138,395
|
|
|
|
60,222
|
|
|
|
5,385
|
|
|
|
8,051
|
|
|
|
87,611
|
|
|
|
84,844
|
|
|
|
6,328
|
|
|
Interest expense
|
|
|
(260,268
|
)
|
|
|
(1,959,909
|
)
|
|
|
(1,636,062
|
)
|
|
|
(1,352,767
|
)
|
|
|
(915,804
|
)
|
|
|
(4,470,478
|
)
|
|
|
(628,386
|
)
|
|
|
(12,606,762
|
)
|
|
Miscellaneous income
|
|
|
600
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8,000
|
|
|
Gain(loss) on foreign currency exchange
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(21,721
|
)
|
|
|
—
|
|
|
|
(5,235
|
)
|
|
|
(3,853
|
)
|
|
|
11,638
|
|
|
Changes in fair value of warrant liability
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,092,643
|
|
|
|
(515,108
|
)
|
|
|
2,592,979
|
|
|
|
(823,582
|
)
|
|
|
2,461
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net
|
|
|
(258,191
|
)
|
|
|
(1,821,514
|
)
|
|
|
(1,575,840
|
)
|
|
|
723,540
|
|
|
|
(1,422,861
|
)
|
|
|
(1,795,123
|
)
|
|
|
(1,370,977
|
)
|
|
|
(12,578,335
|
)
|
|
Net loss
|
|
|
(3,007,233
|
)
|
|
|
(7,991,279
|
)
|
|
|
(10,512,160
|
)
|
|
|
(8,168,476
|
)
|
|
|
(9,047,280
|
)
|
|
|
(16,222,829
|
)
|
|
|
(4,612,873
|
)
|
|
|
(17,493,917
|
)
|
|
Dividend on Class A common stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,025,753
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders
|
|
$
|
(3,007,233
|
)
|
|
$
|
(7,991,279
|
)
|
|
$
|
(10,512,160
|
)
|
|
$
|
(8,168,476
|
)
|
|
$
|
(9,047,280
|
)
|
|
$
|
(16,222,829
|
)
|
|
$
|
(4,612,873
|
)
|
|
$
|
(18,519,670
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per unit/share attributable to common
stockholders — basic and diluted
|
|
$
|
(0.69
|
)
|
|
$
|
(1.72
|
)
|
|
$
|
(2.14
|
)
|
|
$
|
(1.55
|
)
|
|
$
|
(1.30
|
)
|
|
$
|
(2.31
|
)
|
|
$
|
(0.66
|
)
|
|
$
|
(2.29
|
)
|
|
Weighted average number of units outstanding- basic and diluted
|
|
|
4,358,126
|
|
|
|
4,655,011
|
|
|
|
4,918,668
|
|
|
|
5,285,807
|
|
|
|
6,953,079
|
|
|
|
7,009,251
|
|
|
|
6,982,215
|
|
|
|
8,092,943
|
|
|
Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and restricted cash
|
|
$
|
1,108,795
|
|
|
|
7,335,110
|
|
|
|
624,838
|
|
|
|
|
|
|
|
1,467,781
|
|
|
|
938,197
|
|
|
|
952,958
|
|
|
|
50,692,707
|
|
|
Working capital
|
|
|
852,780
|
|
|
|
7,263,300
|
|
|
|
(725,839
|
)
|
|
|
|
|
|
|
(2,158,612
|
)
|
|
|
(6,484,047
|
)
|
|
|
259,761
|
|
|
|
31,985,396
|
|
|
Total assets
|
|
|
4,797,908
|
|
|
|
9,172,118
|
|
|
|
4,761,835
|
|
|
|
|
|
|
|
13,169,940
|
|
|
|
12,450,937
|
|
|
|
14,119,911
|
|
|
|
61,134,435
|
|
|
Current and long term notes payable
|
|
|
7,651,771
|
|
|
|
6,065,567
|
|
|
|
11,040,091
|
|
|
|
|
|
|
|
11,802,522
|
|
|
|
20,846,719
|
|
|
|
15,558,424
|
|
|
|
—
|
|
|
Warrant liability
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
2,640,435
|
|
|
|
3,289,625
|
|
|
|
3,565,123
|
|
|
|
2,199,585
|
|
|
Stockholders equity (deficit)
|
|
|
(3,270,782
|
)
|
|
|
1,769,985
|
|
|
|
(8,338,239
|
)
|
|
|
|
|
|
|
(3,843,892
|
)
|
|
|
(19,540,777
|
)
|
|
|
(8,143,177
|
)
|
|
|
54,967,658
|
|
39
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
The following discussion and analysis of our financial
condition and results of operations should be read in
conjunction with our consolidated financial statements and
related notes and the historical consolidated financial
statements and related notes of our Predecessor Company included
elsewhere in this prospectus. In addition to historical
financial information, the following discussion contains
forward-looking statements that involve risks and uncertainties.
Our actual results may differ materially from those discussed
below. Factors that could cause or contribute to these
differences include those discussed below and elsewhere in this
prospectus, particularly in “Risk factors.”
OVERVIEW
We are an industrial biotechnology company focused on becoming a
low-cost producer of high-value biochemicals that
“drop-in” and substitute for traditional
petroleum-based industrial chemicals. We have developed a
proprietary technology platform that we believe will enable us
to manufacture a variety of drop-in chemicals and replacement
chemicals for large and growing markets using a broad range of
low-cost, renewable and readily available feedstocks. Our
technology platform, which we have validated in our laboratories
and third-party tolling facilities and commercialized with our
licensee, Purac Biochem BV, or Purac, a wholly-owned subsidiary
of CSM N.V., is based on a single-step anaerobic fermentation
process that allows our microorganisms to grow and
simultaneously produce the target product, resulting in greater
productivity and yield relative to other known bioproduction
processes. We believe that we can produce our target high-value
chemical intermediates at an average of half the cost of
traditional petrochemical intermediates at a wide range of oil
and industrial sugar prices without relying on government
subsidies.
We have entered into or intend to enter into strategic
relationships with international companies to accelerate the
global commercialization of our products and development of our
biochemical production capabilities. For example, we signed a
non-binding memorandum of understanding, or MOU, to enter into a
definitive joint development agreement with Johnson Matthey
PLC’s subsidiary Davy Process Technology Limited, or Davy,
a developer and licensor of advanced process technologies. Under
that agreement, Davy would, upon successful completion of
testing and engineering using our biobased succinic acid, or
biosuccinic acid, guarantee to its butanediol process licensees
that they could use our biosuccinic acid in their butanediol
process in place of petroleum-derived maleic anhydride without
significant additional capital expenditures. We have also signed
exclusive alliance agreements with ThyssenKrupp’s
subsidiary Uhde GmbH, or Uhde, a chemical plant engineering
company, and its U.S. subsidiary, under which Uhde will
integrate our fermentation technology with its separation
technology in the plant design and, on a
project-by-project
basis, provide process and performance guarantees for our future
plants on mutually agreeable terms. In addition, we plan to
leverage our partnership with PTT Chemical International Private
Limited, or CH Inter, our largest stockholder and a subsidiary
of PTT Chemical Public Company Limited, or PTTCH, a large
Thailand-based petrochemical producer, to access CH Inter’s
and PTTCH’s breadth of commercial and technical expertise
and extensive knowledge and infrastructure in Asian markets.
Our technology platform enables us to manufacture a variety of
drop-in chemicals and replacement chemicals using a broad range
of renewable feedstocks. Our proprietary microorganisms can
metabolize diverse sugars to create various chemical
intermediates such as biosuccinic acid, lactic acid, fumaric
acid and acrylic acid. We have in-licensed patent rights
relating to certain core technologies from the University of
Florida. Prior to our spin-off, the Predecessor Company,
BioEnergy International, LLC, a Delaware limited liability
company, developed a process to produce D(−) lactic acid
using our technology and licensed this process, with associated
patent rights, to Purac. These licenses were assigned to us as
part of the restructuring described above. The D(−) lactic
acid made and sold by Purac represents the first commercial
product utilizing our biobased, fermentation technology.
We have signed contracts with three customers who have agreed to
buy 100% of each of their annual requirements of succinic acid
from us. We expect these three customers will consume a
substantial portion, and prospectively all, of this plant’s
annual production capacity, based on the customers’ current
stated forecasts of their demand. We intend to enter into
similar supply arrangements in connection with the planned
40
expansion of our Louisiana Plant to an annual production
capacity of 170 million pounds. In addition to biosuccinic
acid, we plan to sell ammonium sulfate, or AMS, commonly used as
a soil fertilizer, which is generated as a co-product of our
process. We signed a contract with Wilson Industrial Sales
Company, Inc. to sell all of the AMS produced at the Louisiana
Plant during the term of the contract.
We estimate that the total cost to complete our Louisiana Plant
will be approximately $103 million, which includes working
capital, fees and expenses. We, along with the Lake Providence
Port Commission and the State of Louisiana, have already
invested over $13 million to prepare the plant’s site
for construction. In addition, we were awarded $50 million
from the U.S. Department of Energy, or the DOE, in federal
cost-share funding under the American Recovery and Reinvestment
Act of 2009, which will enable us to complete the Louisiana
Plant. This award was made in two phases. The first phase of
engineering and piloting, referred to as Budget Period 1, or
BP1, was completed on December 31, 2010 and we have
received the $10.4 million maximum amount allowed for BP1.
The second construction phase, referred to as Budget Period 2,
or BP2, with an allowable amount under the award of
$39.6 million, is ongoing. Costs eligible for reimbursement
in BP2 include those related to the Louisiana Plant’s
construction, equipment purchases, testing and operation. As of
March 31, 2011, we have not received any of the
$39.6 million allowable for BP2. Once the Louisiana Plant
is completed, we plan to make significant investments in
infrastructure to accommodate the planned expansion. We intend
to finance our equity contribution to the expansion of our
Louisiana Plant with a combination of cash on hand and a portion
of the net proceeds received from this initial public offering
of our common stock. We may implement other financing plans if
financially attractive options become available.
We may also use a portion of the net proceeds to acquire other
complementary businesses, products or technologies or to make
other strategic investments. Although we do not have any current
plans or agreements for any specific acquisitions or investments
at this time, we believe opportunities may exist from time to
time to expand our current business through strategic
investments or acquisitions with other companies, products or
technologies.
The potential uses of net proceeds from this offering represent
our current intentions based upon our present business plans and
business conditions. As of the date of this prospectus, we
cannot guarantee the specific amount of the net proceeds that
will be used to develop, construct and operate our biochemical
production capabilities globally, fund working capital or be
used for other general corporate purposes.
We have also signed a memorandum of understanding with China
National BlueStar (Group) Co. Ltd., or BlueStar, to develop a
proposal for a jointly-owned 220 million pound biosuccinic
acid plant in Nanjing, China, the biosuccinic acid requirements
of which would be exclusively supplied by the Company. BlueStar
is currently producing BDO utilizing a process licensed from
Davy.
We scaled up the production of our biosuccinic acid from an
initial fermentation vessel of five liters to 50,000 liters from
January 2008 to February 2011 at various locations. This process
was completed at the tolling plant owned by Fermic, S.A. de
C.V., or Fermic, in Mexico City. This
scale-up of
biosuccinic acid was executed in ten fold increments over prior
capacity and validated assumed commercial-scale cost metrics to
be cost-competitive down to $45 per barrel of oil. The Fermic
tolling facility has operated under our supervision for over a
year and a half and produced 24 metric tons of biosuccinic acid
for us in support of our internal customer/vendor sampling and
testing programs.
We were formed in Delaware in April 2009 as a limited liability
company for the purpose of succeeding to the chemical
intermediates business of our Predecessor Company. The
Predecessor Company was focused on the development of
proprietary biocatalysts to produce renewable fuels and biobased
chemical intermediates for use by energy companies, specialty
chemical companies and manufacturers of a wide range of consumer
products. To differentiate the biochemical business from its
renewable fuels business, the Predecessor Company underwent a
restructuring in July 2009 whereby the Predecessor Company
assigned to us the assets and liabilities of the chemical
intermediates business while leaving the renewable fuels
business, along with its related assets and liabilities,
consisting principally of an investment in BioEnergy Holding LLC
and debt, with the Predecessor Company. Following this
assignment, our ownership interests were distributed pro rata to
the Predecessor Company’s investors.
41
Following our spin-off, we have focused on developing our
technology to produce biosuccinic acid, lactic acid, fumaric
acid and acrylic acid. We are currently building a
30 million pound biosuccinic acid plant in Lake Providence,
Louisiana, or the Louisiana Plant, which we expect will begin
commercial operations during the first quarter of 2013. We
intend to expand the annual production capacity of this plant to
approximately 170 million pounds by the end of the first
quarter of 2014.
We are a development stage company. To date, we have generated
minimal revenues and no product sales. We and the Predecessor
Company have incurred losses since our inception, including
losses of $10.5 million in 2008, $17.2 million in 2009
and $16.2 million in 2010, as well as a loss of
$17.5 million for the three months ended March 31,
2011. As of that date, we had an accumulated deficit of
$17.5 million which reflects our conversion to a subchapter
C corporation in January 2011. Our cumulative losses (including
those of our Predecessor Company) total $72.4 million. We
expect most of our expenditures over the next 24 months
will go towards constructing and expanding the Louisiana Plant
and designing and planning additional manufacturing facilities.
We do not expect to record meaningful product revenue until the
Louisiana Plant becomes operational, which we project will occur
in the first quarter of 2013. We expect to incur losses and
negative cash flow from operating activities for the foreseeable
future.
The financial statements and data included in this prospectus
represent the financial position and results of operations:
|
|
|
| |
•
|
of the Predecessor Company for 2008 and 2009 (through
July 15, the effective date of the restructuring); and
|
| |
| |
•
|
of Myriant for the remainder of 2009 and thereafter.
|
Since our spin-off from the Predecessor Company, we have
committed substantial resources to the development of our
biobased fermentation technology, including the construction of
our Louisiana Plant, and expect our operations to diverge
substantially from those of the Predecessor Company in the
future. As such, the historical results of operations set forth
in this prospectus do not necessarily provide a reliable basis
against which our future performance can be measured.
OPERATING
RESULTS DISCUSSION
As described above, our historical results of operations are not
representative of our expected future financial results. Set
forth below is a description of our principal items of
historical revenues and expenses as well as a description of
factors we expect to be important to our future financial
position and results of operations. Please refer to
“Special note regarding forward-looking statements”
for cautionary information relating to the forward-looking
statements included in the discussion below.
Revenues
and Operating Expenses
Revenues
The historical revenues we report in this prospectus were
derived from the following sources:
|
|
|
| |
•
|
License fee revenue represents royalty payments received from
Purac under our license agreement. We licensed to Purac the
right to make and sell D(−) lactic acid using our
technology in return for royalties based on product sales. We
have recognized only limited revenues from this source and do
not expect it to be a source of significant future revenues.
|
| |
| |
•
|
Management and development fee revenue — related party
represents payments made to us for consulting and management
services we provided to the owners of a biofuel production
facility owned by affiliated entities located in Clearfield
County, Pennsylvania. We terminated this agreement in January
2011 and will not receive additional revenues from this
arrangement.
|
| |
| |
•
|
Government awards represent payments under our award from the
DOE. Through December 31, 2010, payments received under the
award were recognized as revenues in the period during which the
related costs were incurred. Following the completion of Budget
Period 1 in December 2010, payments made
|
42
|
|
|
| |
|
under this award will no longer be recognized as revenue but
rather will be capitalized as construction in progress.
|
We expect that, once we begin commercial production, our
revenues will be largely derived from product sales of
biosuccinic acid and, to a lesser extent, ammonium sulfate.
Product sales will be largely dependent on:
|
|
|
| |
•
|
Demand — Demand will vary not only with market
acceptance of our product but also the fluctuating need for the
consumer, industrial and other end-market products that
biosuccinic acid is used to manufacture.
|
| |
| |
•
|
Pricing — We intend to be price competitive in
the sale of biosuccinic acid. The extent to which we can
maintain pricing will have a direct impact on our revenue. Also,
pricing for biosuccinic acid is subject to fluctuations in the
manufacturing cost, or purchase price for, biosuccinic acid in
the market generally.
|
| |
| |
•
|
Capacity — Assuming sufficient demand for our
product, our revenues will depend on the throughput capacity of
our manufacturing facility. Capacity will depend on a variety of
factors, including the efficiency of our biobased production
process and the operational
start-up of
our production facilities. We do not expect feedstock
availability to constrain capacity.
|
| |
| |
•
|
Ammonium sulfate sales — AMS, commonly used as
soil fertilizer, is a co-product of our biosuccinic acid
production process. We expect to sell all of the AMS we generate
and have entered into a contract with Wilson Industrial Sales
Company, Inc. to supply it with all of the liquid ammonium
sulfate produced at the Louisiana Plant.
|
Cost of
goods sold
To date, the cost of license fee revenue has been our sole cost
of goods sold and represents royalty and other amounts paid to
the University of Florida, or the University, under our license
agreement with it. We are obligated to pay the University
royalties on product sales at a rate that declines with
increasing volume. We are also obligated to share with the
University any license fees we receive from sublicensing the
relevant patent rights. We do not expect these payments to
represent a substantial cost to us.
Upon completion of our Louisiana Plant, our cost of goods sold
will depend principally on:
|
|
|
| |
•
|
Fermentation efficiency — Yields and
productivity will not be known with certainty until our
Louisiana Plant is fully operational. Based on our experience to
date, we believe that our fermentation technology will result in
attractive product yields. We likely will engage in continuous
refinement of the fermentation process once operating at
commercial scale to achieve the highest possible yield and
productivity. As a product of biological processes and chemical
separation and purification, yields and productivity will likely
fluctuate, resulting in
period-to-period
fluctuations of costs of goods sold.
|
| |
| |
•
|
Plant operations — Beyond the product yield
from our fermentation technology, there are numerous processes,
including product separation and purification and deriving
sugars from available feedstocks, that can significantly affect
overall operational efficiency.
|
| |
| |
•
|
Cost of feedstock — After completing
start-up and
operational trials on sorghum-based sugars at the Louisiana
Plant, we will transition to widely available, low-cost
industrial sugars such as 95 Dextrose. This carbon source is
derived from corn not sold for human consumption. Its cost and
availability are tied to factors we cannot control, such as the
weather in crop-producing areas, trends in the commodities
markets and government policies. We believe that, as a whole, 95
Dextrose represents a more dependable and less cost-volatile
feedstock than that used in other biobased production
facilities. Nevertheless, fluctuations in our acquisition costs
of feedstock could have a significant impact on our cost of
goods sold. We anticipate our purchase volumes will not have a
significant impact on the market price of 95 Dextrose. In
addition, we believe there is an ample market supply of 95
Dextrose to supply our commercial operations.
|
43
Gross
Margin
Our gross margins will depend principally on the factors
described above. While our operations will have a significant
fixed-cost component, we do not expect that under-utilization of
our manufacturing facilities will be a principal driver of our
gross margins, since we are designing our manufacturing
facilities to run at full capacity based on customer demand. For
example, we currently have signed agreements with customers
that, based on their current stated forecasts, would consume a
substantial portion, and prospectively all, of our expected
capacity of the Louisiana Plant’s initial 30 million
pound annual production capacity.
Research
and Development Expenses
Research and development expenses represent costs incurred for
internal projects. These costs include salaries and other
personnel-related expenses, travel costs, facility costs,
piloting costs, supplies and depreciation of laboratory
equipment, as well as research consultants and the cost of
funding research at universities and other research
institutions. These costs are expensed as incurred. Costs to
acquire technologies that are utilized in research and
development and that have no alternative future use are expensed
when incurred.
Sales and
Marketing
We do not currently record a material amount of sales and
marketing expense. We expect that these costs will grow
significantly in conjunction with future revenue growth.
General
and Administrative Expenses
General and administrative expenses represent personnel-related
costs, travel costs, consulting, marketing and professional
fees, depreciation and occupancy-related costs not associated
with our research and development facility. After completion of
this offering, we anticipate incurring a significant increase in
general and administrative expenses as we incur additional
compliance costs as a public company. These increases will
likely include increased costs for insurance, costs related to
the hiring of additional personnel and payments to consultants,
lawyers and accountants. We also expect to incur significant
costs to comply with corporate governance, internal controls and
similar requirements applicable to public companies.
Results
of Operations
The following table sets forth consolidated results of
operations for us and the Predecessor Company for the periods
shown. Our inception date is July 16, 2009. The 2008
results of operations set forth in this prospectus reflect the
results of the Predecessor Company. The 2009 results reflect the
Predecessor Company’s results for the period from
January 1, 2009 through July 15, 2009, which we refer
to as the 2009 Predecessor Period, and our results from July 16
(inception) through year end, which we refer to as the 2009
Successor Period.
Our operations and those of the Predecessor Company were
substantially the same, as all assets and liabilities were
contributed from the Predecessor Company to us with the
exception of certain of the Predecessor Company’s debt and
the Predecessor Company’s investment in BioEnergy Holding
LLC, as described above. The most notable changes that resulted
from the transfer of the chemical intermediates business from
the Predecessor Company to us are set forth below:
|
|
|
| |
•
|
The Predecessor Company’s investment in BioEnergy Holding
LLC was not transferred to us. This did not have significant
financial impact as there were no earnings or invested capital
in the investment.
|
| |
| |
•
|
The Predecessor Company’s note payable to BioEnergy Holding
LLC, in the amount of $10.0 million with interest at 6%,
was not transferred to us.
|
| |
| |
•
|
The Predecessor Company’s warrant liability of
approximately $1.5 million was not transferred to us.
|
Based on our analysis, we concluded that (i) the results of
the Predecessor Company’s operations for the year ended
December 31, 2008 were comparable to the combined results
of the operations for the 2009 Predecessor Period and the 2009
Successor Period, and (ii) the combined results of
operations for the 2009
44
Predecessor Period and the 2009 Successor Period were comparable
with our operations for the year ended December 31, 2010.
For the reasons stated under “Overview —
Operating Results Discussion,” our historical results of
operations are not representative of our expected future
operating results.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Predecessor Company
|
|
|
Myriant Corporation
|
|
|
|
|
Fiscal Year
|
|
|
Period from
|
|
|
Period from
|
|
|
Fiscal Year
|
|
|
Three Months Ended
|
|
|
|
|
Ended
|
|
|
January 1, 2009
|
|
|
July 16, 2009 (Inception)
|
|
|
Ended
|
|
|
March 31,
|
|
|
|
|
December 31, 2008
|
|
|
to July 15, 2009
|
|
|
to December 31, 2009
|
|
|
December 31, 2010
|
|
|
2010
|
|
|
2011
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License fee revenue
|
|
$
|
344,860
|
|
|
$
|
71,833
|
|
|
$
|
221,711
|
|
|
$
|
258,241
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Management fee revenue-related party
|
|
|
262,212
|
|
|
|
267,318
|
|
|
|
—
|
|
|
|
3,557,574
|
|
|
|
600,166
|
|
|
|
2,519
|
|
|
Development fee revenue-related party
|
|
|
3,125,714
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Government awards
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,419,043
|
|
|
|
1,516,323
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
3,732,786
|
|
|
|
339,151
|
|
|
|
221,711
|
|
|
|
14,234,858
|
|
|
|
2,116,489
|
|
|
|
2,519
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of license fee revenue
|
|
|
110,020
|
|
|
|
22,373
|
|
|
|
73,859
|
|
|
|
84,600
|
|
|
|
—
|
|
|
|
—
|
|
|
Research and development
|
|
|
4,679,935
|
|
|
|
3,770,721
|
|
|
|
2,793,085
|
|
|
|
15,904,717
|
|
|
|
2,552,100
|
|
|
|
1,883,603
|
|
|
Project development
|
|
|
2,179,965
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
General and administrative expense
|
|
|
5,699,186
|
|
|
|
5,438,073
|
|
|
|
4,979,186
|
|
|
|
12,673,247
|
|
|
|
2,806,285
|
|
|
|
3,034,498
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
12,669,106
|
|
|
|
9,231,167
|
|
|
|
7,846,130
|
|
|
|
28,662,564
|
|
|
|
5,358,385
|
|
|
|
4,918,101
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(8,936,320
|
)
|
|
|
(8,892,016
|
)
|
|
|
(7,624,419
|
)
|
|
|
(14,427,706
|
)
|
|
|
(3,241,896
|
)
|
|
|
(4,915,582
|
)
|
|
Other income (expense), net:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
60,222
|
|
|
|
5,385
|
|
|
|
8,051
|
|
|
|
87,611
|
|
|
|
84,844
|
|
|
|
6,328
|
|
|
Interest expense
|
|
|
(1,636,062
|
)
|
|
|
(1,352,767
|
)
|
|
|
(915,804
|
)
|
|
|
(4,470,478
|
)
|
|
|
(628,386
|
)
|
|
|
(12,606,762
|
)
|
|
Miscellaneous income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8,000
|
|
|
Gain (loss) on foreign currency exchange
|
|
|
—
|
|
|
|
(21,721
|
)
|
|
|
—
|
|
|
|
(5,235
|
)
|
|
|
(3,853
|
)
|
|
|
11,638
|
|
|
Change in fair value of warrant liability
|
|
|
—
|
|
|
|
2,092,643
|
|
|
|
(515,108
|
)
|
|
|
2,592,979
|
|
|
|
(823,582
|
)
|
|
|
2,461
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net
|
|
|
(1,575,840
|
)
|
|
|
723,540
|
|
|
|
(1,422,861
|
)
|
|
|
(1,795,123
|
)
|
|
|
(1,370,977
|
)
|
|
|
(12,578,335
|
)
|
|
Net loss
|
|
|
(10,512,160
|
)
|
|
|
(8,168,476
|
)
|
|
|
(9,047,280
|
)
|
|
|
(16,222,829
|
)
|
|
|
(4,612,873
|
)
|
|
|
(17,493,917
|
)
|
|
Dividend on Class A common stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,025,753
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders
|
|
$
|
(10,512,160
|
)
|
|
$
|
(8,168,476
|
)
|
|
$
|
(9,047,280
|
)
|
|
$
|
(16,222,829
|
)
|
|
$
|
(4,612,873
|
)
|
|
$
|
(18,519,670
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per unit/share attributable to common
stockholders-basic and diluted
|
|
$
|
(2.14
|
)
|
|
$
|
(1.55
|
)
|
|
$
|
(1.30
|
)
|
|
$
|
(2.31
|
)
|
|
$
|
(0.66
|
)
|
|
$
|
(2.29
|
)
|
|
Weighted average number of units outstanding — basic
and diluted
|
|
|
4,918,668
|
|
|
|
5,285,807
|
|
|
|
6,953,079
|
|
|
|
7,009,251
|
|
|
|
6,982,215
|
|
|
|
8,092,943
|
|
45
Comparison
of three months ended March 31, 2010 and 2011
The following table shows the amounts of the listed items from
our consolidated statements of operations for the periods
presented, showing
period-over-period
changes:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
|
|
|
|
March 31,
|
|
|
Increase
|
|
|
|
|
2010
|
|
|
2011
|
|
|
(Decrease)
|
|
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Management fee revenue-related party
|
|
$
|
600,166
|
|
|
$
|
2,519
|
|
|
$
|
(597,647
|
)
|
|
Government awards
|
|
|
1,516,323
|
|
|
|
—
|
|
|
|
(1,516,323
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
2,116,489
|
|
|
|
2,519
|
|
|
|
(2,113,970
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
2,552,100
|
|
|
|
1,883,603
|
|
|
|
(668,497
|
)
|
|
General and administrative expense
|
|
|
2,806,285
|
|
|
|
3,034,498
|
|
|
|
228,213
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
5,358,385
|
|
|
|
4,918,101
|
|
|
|
(440,284
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(3,241,896
|
)
|
|
|
(4,915,582
|
)
|
|
|
(1,673,686
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
84,844
|
|
|
|
6,328
|
|
|
|
(78,516
|
)
|
|
Interest expense
|
|
|
(628,386
|
)
|
|
|
(12,606,762
|
)
|
|
|
(11,978,376
|
)
|
|
Miscellaneous income
|
|
|
—
|
|
|
|
8,000
|
|
|
|
8,000
|
|
|
Gain (loss) on foreign currency exchange
|
|
|
(3,853
|
)
|
|
|
11,638
|
|
|
|
15,491
|
|
|
Change in fair value of warrant liability
|
|
|
(823,582
|
)
|
|
|
2,461
|
|
|
|
826,043
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net
|
|
|
(1,370,977
|
)
|
|
|
(12,578,335
|
)
|
|
|
(11,207,358
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(4,612,873
|
)
|
|
$
|
(17,493,917
|
)
|
|
$
|
(12,881,044
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Revenues: Total revenues declined to
$3,000 for the three months ended March 31, 2011 from
$2.1 million for the prior-year period. Management fee
revenue-related party declined to $3,000 for the three months
ended March 31, 2011 from $600,000 for the prior-year
period as we terminated our management agreement with a related
party in January 2011. Government award revenue declined to $0
for the three months ended March 31, 2011 from
$1.5 million for the prior-year period. Prior to entering
into the construction phase of the Louisiana Plant, all receipts
under the DOE award were recorded as revenue and the
corresponding expenditures were recorded as expense in
accordance with our policy. In February 2011, we entered into
the construction phase of the project, and as a result, all
future costs and related reimbursements under the award will be
capitalized as construction in progress.
Research and development: Research and
development expense declined to $1.9 million for the three
months ended March 31, 2011 from $2.6 million during
the prior-year period. This decline was driven by lower
engineering and piloting costs associated with the Fermic
tolling facility.
General and administrative: General and
administrative expenses increased to $3.0 million for the
three months ended March 31, 2011 from $2.8 million
during the prior-year period. This increase related principally
to stock-based compensation expense incurred as a result of the
institution of our 2011 Omnibus Incentive Plan.
Other income (expense), net: Interest expense
increased to $12.6 million for the three months ended
March 31, 2011 from $628,000 during the prior-year period.
The conversion of senior convertible notes into equity in
January 2011 resulted in a charge to interest expense to fully
amortize the remaining value ascribed to the warrants issued as
well as an interest charge associated with the beneficial
conversion feature of the notes. During the three months ended
March 31, 2010, we incurred warrant liability expense of
$824,000, which resulted from a change in the fair value and a
re-pricing of certain warrants. No similar charge was recorded
in 2011.
46
Comparison
of the 2009 Predecessor Period combined with the 2009 Successor
Period, with the year ended December 31, 2010
The financial results for the year ended December 31, 2009
described below reflect the combined results of the 2009
Predecessor Period and the 2009 Successor Period, which, as
discussed under the heading “Selected historical financial
data” above, we believe is a comparable presentation to our
financial results for the year ended December 31, 2010.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Predecessor
|
|
|
Myriant
|
|
|
Combined
|
|
|
Myriant
|
|
|
|
|
|
|
|
January 1, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
through
|
|
|
July 16, 2009
|
|
|
Year Ended
|
|
|
Year Ended
|
|
|
|
|
|
|
|
July 15,
|
|
|
through
|
|
|
December 31,
|
|
|
December 31,
|
|
|
Increase
|
|
|
|
|
2009
|
|
|
December 31, 2009
|
|
|
2009
|
|
|
2010
|
|
|
(Decrease)
|
|
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License fee revenue
|
|
$
|
71,833
|
|
|
$
|
221,711
|
|
|
$
|
293,544
|
|
|
$
|
258,241
|
|
|
$
|
(35,303
|
)
|
|
Management fee revenue-related party
|
|
|
267,318
|
|
|
|
—
|
|
|
|
267,318
|
|
|
|
3,557,574
|
|
|
|
3,290,256
|
|
|
Government awards
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,419,043
|
|
|
|
10,419,043
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
339,151
|
|
|
|
221,711
|
|
|
|
560,862
|
|
|
|
14,234,858
|
|
|
|
13,673,996
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of license fee revenue
|
|
|
22,373
|
|
|
|
73,859
|
|
|
|
96,232
|
|
|
|
84,600
|
|
|
|
(11,632
|
)
|
|
Research and development
|
|
|
3,770,721
|
|
|
|
2,793,085
|
|
|
|
6,563,806
|
|
|
|
15,904,717
|
|
|
|
9,340,911
|
|
|
General and administrative expense
|
|
|
5,438,073
|
|
|
|
4,979,186
|
|
|
|
10,417,259
|
|
|
|
12,673,247
|
|
|
|
2,255,988
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
9,231,167
|
|
|
|
7,846,130
|
|
|
|
17,077,297
|
|
|
|
28,662,564
|
|
|
|
11,585,267
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(8,892,016
|
)
|
|
|
(7,624,419
|
)
|
|
|
(16,516,435
|
)
|
|
|
(14,427,706
|
)
|
|
|
2,088,729
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
5,385
|
|
|
|
8,051
|
|
|
|
13,436
|
|
|
|
87,611
|
|
|
|
74,175
|
|
|
Interest expense
|
|
|
(1,352,767
|
)
|
|
|
(915,804
|
)
|
|
|
(2,268,571
|
)
|
|
|
(4,470,478
|
)
|
|
|
(2,201,907
|
)
|
|
Gain (loss) on foreign currency exchange
|
|
|
(21,721
|
)
|
|
|
—
|
|
|
|
(21,721
|
)
|
|
|
(5,235
|
)
|
|
|
16,486
|
|
|
Changes in fair value of warrant liability
|
|
|
2,092,643
|
|
|
|
(515,108
|
)
|
|
|
1,577,535
|
|
|
|
2,592,979
|
|
|
|
1,015,444
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net
|
|
|
723,540
|
|
|
|
(1,422,861
|
)
|
|
|
(699,321
|
)
|
|
|
(1,795,123
|
)
|
|
|
(1,095,802
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(8,168,476
|
)
|
|
$
|
(9,047,280
|
)
|
|
$
|
(17,215,756
|
)
|
|
$
|
(16,222,829
|
)
|
|
$
|
992,927
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues: Revenues increased to
$14.2 million for the year ended December 31, 2010
from $561,000 for the prior year. This increase was driven by an
increase in management fees of $3.3 million reflecting the
start up of operations at BioEnergy Holding’s biofuels
facility and receipt of funds under the DOE award. Under the
terms of the award, the initial stage consisted of engineering
and product development efforts designed to prove the
feasibility of our technology. Because these efforts consisted
principally of determining technological feasibility and
piloting the technology, all costs and related award receipts
were recorded in the statement of operations as expenses and
award revenue, respectively. Award receipts amounting to
$10.4 million were recorded as revenue during 2010. We also
recognized a slight decrease in license fee revenue attributable
to exchange rate fluctuations as the contract is denominated in
Euros.
Cost of license fee revenue: Cost of license
fee revenue decreased to $85,000 for the year ended
December 31, 2010 from $96,000 in the prior year. This
decrease related to lower license fee revenue recorded in 2010,
as the cost represents a royalty payment to the University of
Florida based on a percentage of revenues achieved.
47
Research and development: Research and
development expense increased to $15.9 million for the year
ended December 31, 2010 from $6.6 million in the prior
year. This increase was primarily driven by increases in
engineering costs of $7.0 million and laboratory costs of
$1.6 million related to piloting activities associated with
our biosuccinic acid.
General and administrative expense: General
and administrative expenses increased to $12.7 million for
the year ended December 31, 2010 from $10.4 million in
the prior year. This increase was primarily driven by increases
in personnel-related costs of $1.0 million, bad debt
expense of $546,000 and travel costs of $463,000. The personnel
and travel costs reflected higher headcount in 2010. The
increase in bad debt expense resulted from the establishment of
a bad debt reserve on an amount advanced to a related party of
the Predecessor Company in conjunction with the termination of
our management agreement with that related party.
Other income (expense), net: Our interest
expense increased to $4.5 million for the year ended
December 31, 2010 from $2.3 million in the prior year,
which reflected an increase in the balance of our senior
convertible notes during 2010. We recognized a gain associated
with our warrant liability of $2.6 million for 2010, which
was an increase of $1.0 million over the prior year. This
reflected a decrease in the fair value of our warrants from 2009.
Comparison
of year ended December 31, 2008 to the 2009 Predecessor
Period combined with the 2009 Successor Period
The 2008 results reflect the results of the Predecessor Company
for the year ended December 31, 2008. The financial results
for the year ended December 31, 2009 reflect the 2009
Predecessor Period combined with the 2009 Successor Period.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Myriant
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
Predecessor
|
|
|
July 16, 2009
|
|
|
Combined
|
|
|
|
|
|
|
|
|
|
|
January 1, 2009
|
|
|
(Inception)
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
through
|
|
|
through
|
|
|
Year Ended
|
|
|
|
|
|
|
|
December 31,
|
|
|
July 15,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
Increase
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2009
|
|
|
2009
|
|
|
(Decrease)
|
|
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License fee revenue
|
|
$
|
344,860
|
|
|
$
|
71,833
|
|
|
$
|
221,711
|
|
|
$
|
293,544
|
|
|
$
|
(51,316
|
)
|
|
Management fee revenue-related party
|
|
|
262,212
|
|
|
|
267,318
|
|
|
|
—
|
|
|
|
267,318
|
|
|
|
5,106
|
|
|
Development fee revenue
|
|
|
3,125,714
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,125,714
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
3,732,786
|
|
|
|
339,151
|
|
|
|
221,711
|
|
|
|
560,862
|
|
|
|
(3,171,924
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of license fee revenue
|
|
|
110,020
|
|
|
|
22,373
|
|
|
|
73,859
|
|
|
|
96,232
|
|
|
|
(13,788
|
)
|
|
Research and development
|
|
|
4,679,935
|
|
|
|
3,770,721
|
|
|
|
2,793,085
|
|
|
|
6,563,806
|
|
|
|
1,883,871
|
|
|
Project development
|
|
|
2,179,965
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,179,965
|
)
|
|
General and administrative expense
|
|
|
5,699,186
|
|
|
|
5,438,073
|
|
|
|
4,979,186
|
|
|
|
10,417,259
|
|
|
|
4,718,073
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
12,669,106
|
|
|
|
9,231,167
|
|
|
|
7,846,130
|
|
|
|
17,077,297
|
|
|
|
4,408,191
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(8,936,320
|
)
|
|
|
(8,892,016
|
)
|
|
|
(7,624,419
|
)
|
|
|
(16,516,435
|
)
|
|
|
(7,580,115
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
48
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Myriant
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
Predecessor
|
|
|
July 16, 2009
|
|
|
Combined
|
|
|
|
|
|
|
|
|
|
|
January 1, 2009
|
|
|
(Inception)
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
through
|
|
|
through
|
|
|
Year Ended
|
|
|
|
|
|
|
|
December 31,
|
|
|
July 15,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
Increase
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2009
|
|
|
2009
|
|
|
(Decrease)
|
|
|
|
|
Other income (expense), net:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
60,222
|
|
|
|
5,385
|
|
|
|
8,051
|
|
|
|
13,436
|
|
|
|
(46,786
|
)
|
|
Interest expense
|
|
|
(1,636,062
|
)
|
|
|
(1,352,767
|
)
|
|
|
(915,804
|
)
|
|
|
(2,268,571
|
)
|
|
|
(632,509
|
)
|
|
Gain (loss) on foreign currency exchange
|
|
|
—
|
|
|
|
(21,721
|
)
|
|
|
—
|
|
|
|
(21,721
|
)
|
|
|
(21,721
|
)
|
|
Changes in fair value of warrant liability
|
|
|
—
|
|
|
|
2,092,643
|
|
|
|
(515,108
|
)
|
|
|
1,577,535
|
|
|
|
1,577,535
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net
|
|
|
(1,575,840
|
)
|
|
|
723,540
|
|
|
|
(1,422,861
|
)
|
|
|
(699,321
|
)
|
|
|
876,519
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(10,512,160
|
)
|
|
$
|
(8,168,476
|
)
|
|
$
|
(9,047,280
|
)
|
|
$
|
(17,215,756
|
)
|
|
$
|
(6,703,596
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues: Revenues decreased to $561,000 for
the year ended December 31, 2009 from $3.7 million for
the prior year. The decline was principally due to the decline
in development fee revenue. In 2008, the Predecessor Company
received fees in conjunction with services provided to a related
party in closing a financing transaction to construct a biofuels
facility. We performed no such services in 2009 nor do we
anticipate any such revenue in the future.
Cost of license fee revenue: Cost of license
fee revenue decreased to $96,000 for the year ended
December 31, 2009 from $110,000 in the prior year. This
decrease related to decreased license fee revenue recorded in
2009, as the cost represents a royalty payment to the University
of Florida based on a percentage of revenues achieved.
Research and development: Research and
development expense increased to $6.6 million for the year
ended December 31, 2009 from $4.7 million during the
prior year. This increase was primarily driven by increases in
personnel-related costs of $1.3 million, laboratory and
engineering costs of $347,000 and depreciation expense of
$252,000. The increase in personnel and laboratory and
engineering costs reflected higher headcount as we expanded our
research and development department. The increase in
depreciation expense reflected a full year of depreciation in
2009 on equipment purchased throughout the year in 2008.
Project development: Project development
expenses declined to $0 for the year ended December 31,
2009 from $2.2 million in the prior year. These expenses
included engineering and personnel costs associated with the
development of a biofuels facility for a related party in 2008.
No such activity occurred in 2009.
General and administrative: General and
administrative expenses increased to $10.4 million for the
year ended December 31, 2009 from $5.7 million in the
prior year. This was primarily driven by increases in
personnel-related costs of $2.3 million, legal and
professional fees of $1.0 million and stock-based
compensation of $487,000. The increase in personnel-related
costs reflected higher headcount as we expanded our operations.
Legal and professional fees increased as we were involved with
various contract negotiations throughout 2009.
Other income and expense, net: Interest
expense increased to $2.3 million for the year ended
December 31, 2009 from $1.6 million in the prior year,
which reflected an increase in the balance of our senior
convertible notes during 2009. We recognized a gain on warrant
liability of $1.6 million for 2009 in conjunction with
changes in the fair value of warrants classified as a liability
on our balance sheet.
Liquidity
and Capital Resources
From July 16, 2009 (inception) through December 31,
2010, we funded our operations primarily through
$13.7 million from the sale of senior convertible notes,
$10.4 million from the DOE award, $480,000 from license
fees and $3.6 million from management fees. In January
2011, we completed a $60 million equity
49
financing with CH Inter, a wholly-owned subsidiary of PTTCH,
which resulted in net proceeds of $57.1 million. To date,
we have not generated any revenues from the sale of biosuccinic
acid. As of March 31, 2011, our cash and cash equivalents
totaled $50.7 million, of which $15.6 million was, in
accordance with the terms of our DOE award, restricted as a
reserve to cover any contingencies that arise in connection with
construction of our Louisiana Plant.
We currently intend to use all or a portion of the net proceeds
of this offering, together with $39.6 million of remaining
proceeds from a government award, $10 million in
infrastructure improvements to be undertaken by the Lake
Providence Port Commission and the State of Louisiana and
$50.7 million of existing cash and cash equivalents, to
complete the Louisiana Plant (approximately $103 million,
which includes working capital, fees and expenses), to fund our
expected equity contribution to the expansion of the Louisiana
Plant to 170 million pounds per year (estimated at
$60 million, which includes working capital, fees and
expenses), to pay the dividend accruing on the Class A
common stock (which totaled $1,025,753 as of March 31,
2011) and to fund working capital, research and development
expenses and other general corporate purposes, which will
include expenses and the costs associated with being a public
company.
We may also use a portion of the net proceeds to acquire other
complementary businesses, products or technologies or to make
other strategic investments. Although we do not have any current
plans or agreements for any specific acquisitions or investments
at this time, we believe opportunities may exist from time to
time to expand our current business through strategic
investments or acquisitions with other companies, products or
technologies.
Based on our current operating plan, we believe that the
anticipated net proceeds from this offering, together with the
remaining DOE award funds and our existing cash and cash
equivalents, will provide adequate funds for ongoing operations,
planned capital expenditures and working capital requirements
for at least the next 24 months. Successful completion of
our research and development program and, ultimately, the
attainment of profitable operations are dependent upon future
events, including completion of our development activities
resulting in commercial products and technology, obtaining
financing to complete our development activities, obtaining
financing to construct production facilities, market acceptance
and demand for our products and services and attracting and
retaining qualified personnel.
The following table sets forth the major sources and uses of
cash for each of the periods set forth below. The 2008 results
reflect the results of the Predecessor Company for the year
ended December 31, 2008. The 2009 results reflect the
combined results of the Successor Company and the Predecessor
Company for the year ended December 31, 2009.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Predecessor Company
|
|
Myriant
|
|
Combined
|
|
Myriant
|
|
|
|
|
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
July 16, 2009
|
|
Year
|
|
|
|
Three Months
|
|
|
|
Year Ended
|
|
January 1, 2009
|
|
(Inception) to
|
|
Ended
|
|
Year Ended
|
|
Ended
|
|
|
|
December 31,
|
|
to July 15,
|
|
December 31,
|
|
December 31,
|
|
December 31,
|
|
March 31,
|
|
|
|
2008
|
|
2009
|
|
2009
|
|
2009
|
|
2010
|
|
2011
|
|
|
|
Net cash used in operating activities
|
|
$
|
(7,006,013
|
)
|
|
$
|
(8,236,413
|
)
|
|
$
|
(6,330,465
|
)
|
|
$
|
(14,566,878
|
)
|
|
$
|
(9,034,096
|
)
|
|
$
|
(6,814,219
|
)
|
|
Net cash provided by (used in) investing activities
|
|
$
|
(3,004,285
|
)
|
|
$
|
(2,910,589
|
)
|
|
$
|
2,199,920
|
|
|
$
|
(710,669
|
)
|
|
$
|
(373,170
|
)
|
|
$
|
(15,789,293
|
)
|
|
Net cash provided by financing activities
|
|
$
|
3,283,054
|
|
|
$
|
10,845,519
|
|
|
$
|
5,175,367
|
|
|
$
|
16,020,886
|
|
|
$
|
8,926,591
|
|
|
$
|
57,151,813
|
|
Operating
activities
Our primary uses for cash from operating activities are
personnel-related expenses, research and development-related
expenses, including costs associated with piloting our
technology, and engineering and permitting costs associated with
our Louisiana Plant.
Cash used in operating activities of $6.8 million during
the three months ended March 31, 2011 reflected our net
loss of $17.5 million offset by non-cash charges totaling
$13.3 million and changes in operating assets and
liabilities of $(2.6 million). Non-cash charges included
non-cash interest expense and amortization of debt discounts of
$12.6 million, stock based compensation of $470,000,
depreciation and amortization of $237,000
50
and a gain from the change in the fair value of warrant
liabilities of $2,500. The net use of cash from our operating
assets and liabilities of $2.6 million primarily reflected
the payment of engineering invoices that had been accrued at
year end.
Cash used in operating activities of $9.0 million in 2010
reflected our net loss of $16.2 million offset in part by
non-cash charges totaling $3.3 million and changes in
operating assets and liabilities of $3.8 million. Non-cash
charges included non-cash interest expense and amortization of
debt discounts of $4.4 million, gain from change in fair
value of warrant liabilities of $2.6 million, depreciation
and amortization of $1.0 million and stock-based
compensation of $526,000. The net source of cash from our
operating assets and liabilities of $3.8 million primarily
reflected accrued engineering costs that were payable in 2011.
Cash used in operating activities of $14.6 million in 2009
reflected our net loss of $17.2 million offset in part by
non-cash charges totaling $2.0 million and changes in
operating assets and liabilities of $651,000. Non-cash charges
included non-cash interest expense and amortization of debt
discounts of $2.2 million, change in fair value of warrant
liabilities of $(1.6 million), depreciation of $841,000 and
stock-based compensation of $488,000. The net source of cash
from our operating assets and liabilities of $651,000 primarily
reflected increases in accrued liabilities.
Cash used in operating activities of $7.0 million in 2008
reflected our net loss of $10.5 million offset in part by
non-cash charges totaling $2.1 million and changes in our
operating assets and liabilities of $1.4 million. Non-cash
charges included non-cash interest expense and amortization of
the value ascribed to the warrants of $1.6 million and
depreciation of $478,000. The net source of cash from our
operating assets and liabilities of $1.4 million primarily
reflected growth in accounts payable and accrued liabilities due
to the growth of the business.
Investing
activities
Our investing activities consist primarily of capital
expenditures.
During the three months ended March 31, 2011, cash used in
investing activities included $15.2 million in restricted
cash associated with the construction of our Louisiana Plant and
$583,000 for capital expenditures net of government award
receipts of $479,000.
In 2010, cash used in investing activities was primarily related
to $422,000 of capital expenditures.
In 2009, cash used in investing activities was primarily related
to $633,000 of capital expenditures, along with $107,000 in
deposits associated with facility leases.
In 2008, cash used in investing activities was primarily related
to $3.0 million of capital expenditures which was
principally associated with the expansion of our laboratory.
Financing
activities
During the three months ended March 31, 2011, cash provided
by financing activities was $57.2 million, primarily due to
the net proceeds of $57.1 million from our sale of equity
to CH Inter, a strategic investor.
In 2010, cash provided by financing activities was
$8.9 million, primarily due to net proceeds of
$9.7 million from the issuance of senior convertible notes
offset by repayment of a $1.7 million note payable.
In 2009, cash provided by financing activities was
$16.0 million, primarily due to $9.5 million in
proceeds from the exercise of warrants, $5.8 million in
proceeds from the issuance of senior convertible notes and the
receipt of a note payable in the amount of $1.7 million.
In 2008, cash provided by financing activities was
$3.3 million, primarily due to proceeds of
$3.8 million from the issuance of senior convertible notes.
51
Contractual
Obligations and Commitments
The following summarizes the future commitments arising from our
contractual obligations at December 31, 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Less Than
|
|
|
|
|
|
|
|
|
More Than
|
|
|
|
|
Total
|
|
|
1-Year
|
|
|
1-3 Years
|
|
|
3-5 Years
|
|
|
5 Years
|
|
|
|
|
Debt
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
$
|
0
|
|
|
Capital Lease Obligations
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Operating Lease Obligations
|
|
|
2,265,770
|
|
|
|
1,048,827
|
|
|
|
1,207,897
|
|
|
|
9,046
|
|
|
|
0
|
|
|
Purchase Obligations
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Other Long-Term Liabilities
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
2,265,770
|
|
|
$
|
1,048,827
|
|
|
$
|
1,207,897
|
|
|
$
|
9,046
|
|
|
$
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Off-Balance
Sheet Arrangements
We did not have during the periods presented, and we do not
currently have any relationships with unconsolidated entities,
such as entities often referred to as structured finance or
special purpose entities, established for the purpose of
facilitating off-balance sheet arrangements or other
contractually narrow or limited purposes.
The Predecessor Company holds an investment in BioEnergy
Holding, a variable interest entity, or VIE. At
December 31, 2009 and 2008, management determined that the
Predecessor Company was not the primary beneficiary of the VIE
and, as such, concluded that they were not required to
consolidate BioEnergy Holding. For investments that are not
required to be consolidated, the Predecessor Company followed
the equity method of accounting. As there were no earnings or
invested capital in the investment, there were no amounts
recorded with respect to this investment in 2008 and 2009.
QUANTITATIVE
AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest
rate risk
We had unrestricted cash and cash equivalents totaling
$1.0 million, $564,000 and $35.1 million at
December 31, 2009, December 31, 2010 and
March 31, 2011, respectively. These amounts were invested
primarily in demand deposit savings accounts and are held for
working capital purposes. The primary objective of our
investment activities is to preserve our capital for the purpose
of funding our operations. We do not enter into investments for
trading or speculative purposes. We believe that we do not have
material exposure to changes in fair value as a result of
changes in interest rates. Declines in interest rates, however,
will reduce future investment income.
CRITICAL
ACCOUNTING POLICIES AND ESTIMATES
Our consolidated financial statements have been prepared in
conformity with generally accepted accounting principles in the
United States of America, or the U.S., and include our accounts
and the accounts of our wholly-owned subsidiaries, Myriant LP
LLC and Myriant Lake Providence, Inc. The preparation of our
consolidated financial statements requires us to make estimates,
assumptions and judgments that affect the reported amounts of
assets and liabilities and disclosure of contingent assets and
liabilities at the date of the financial statements, and the
reported amounts of revenues and expenses during the applicable
periods. Management bases its estimates, assumptions and
judgments on historical experience and on various other factors
that are believed to be reasonable under the circumstances.
Different assumptions and judgments would change the estimates
used in the preparation of our consolidated financial
statements, which, in turn, could change the results from those
reported. Our management evaluates its estimates, assumptions
and judgments on an ongoing basis.
While our significant accounting policies are more fully
described in Note 2 to our consolidated financial
statements included in this prospectus, we believe that the
following accounting policies are the most critical
52
to aid you in fully understanding and evaluating our reported
financial results and reflect the more significant judgments and
estimates that we use in the preparation of our consolidated
financial statements.
Stock-based
compensation
We have adopted the provisions of Financial Accounting Standards
Board (FASB) Accounting Standards Codification (ASC) 718,
Compensation — Stock Compensation. Compensation
costs related to all equity instruments are recognized at the
grant date fair value of the awards.
Prior to 2011, Myriant was organized as a limited liability
company. In certain instances, employees and non-employee
advisors were granted membership units with vesting provisions.
In those instances, management estimated the fair value of the
award and recognized compensation expense over the vesting
period on a straight-line basis.
Membership
unit and common stock valuations
In the absence of a public trading market, we determined a
reasonable estimate of the then current fair value of our common
stock for purposes of granting stock-based compensation based on
multiple criteria. We determined the fair value of our common
stock utilizing methodologies, approaches and assumptions
consistent with the American Institute of Certified Public
Accountants Practice Aid, “Valuation of
Privately-Held-Company Equity Securities Issued as
Compensation” (AICPA Practice Aid). In addition, we
exercised judgment in evaluating and assessing the foregoing
based on several factors including:
|
|
|
| |
•
|
the nature and history of our business;
|
| |
| |
•
|
our historical operating and financial results;
|
| |
| |
•
|
the market value of companies that are engaged in a similar
business to ours;
|
| |
| |
•
|
the lack of marketability of our common stock;
|
| |
| |
•
|
the price at which shares of our membership units have been sold;
|
| |
| |
•
|
our progress in developing our technology;
|
| |
| |
•
|
our progress towards producing biosuccinic acid at commercial
scale at the Louisiana Plant;
|
| |
| |
•
|
the risks associated with transferring our biosuccinic acid
production technology to full commercial scale;
|
| |
| |
•
|
the overall inherent risks associated with our business at the
time stock option grants and restricted shares were
approved; and
|
| |
| |
•
|
the overall equity market conditions and general economic trends.
|
We considered the factors outlined above, as well as the results
of an independent outside valuation performed as of
January 14, 2011. We used an option-pricing method, as well
as other factors outlined above, to estimate the fair value of
our membership units and common stock as follows:
| |
|
|
|
|
|
Fair Value
|
|
Valuation Date
|
|
per Unit
|
|
|
|
December 31, 2008
|
|
$10.00
|
|
July 15, 2009
|
|
$8.48
|
|
December 31, 2009
|
|
$6.00
|
|
January 14, 2011
|
|
$3.45-$5.35
|
In January 2009, we completed a valuation to estimate the fair
market value of the membership units of our Predecessor Company
as of December 31, 2008. To determine our estimated
enterprise value, we applied an income-based approach and a
market based approach based upon negotiations with potential
investors at that point in time. This valuation included the
estimated value of the Predecessor Company’s investment in
BioEnergy Holding LLC. Using a 30% discount for the lack of
marketability of our membership units, we
53
estimated a fair market value at December 31, 2008 of
$10.00 per unit. This value was supported by existing investors
exercising warrants at $10.00 per unit during that period of
time. We used this fair market value per unit for units granted
between December 1, 2008 and July 15, 2009.
In July 2009, we separated from the Predecessor Company. The
principal asset that was not transferred to us was the
Predecessor Company’s investment in BioEnergy Holding LLC.
At July 15, 2009, we estimated the value of the Predecessor
Company’s investment in BioEnergy Holding LLC by
discounting the estimated future cash flows of BioEnergy Holding
LLC using a discount rate of 15%. This discount rate was lower
than the rate applied to Myriant as the cash flows associated
with the biofuels facility were supported by an offtake
agreement and were more predictable in nature. This investment
was estimated to have a $1.52 per unit value. Accordingly, the
value of the units associated with our business was estimated at
$8.48 per unit. We used this fair market value per unit for
units granted between July 16, 2009 and December 31,
2009.
In January 2010, we completed a valuation to estimate the fair
market value of our membership units as of December 31,
2009 using an income based approach. To determine our estimated
enterprise value, we discounted expected future cash flows by
40% which resulted in an estimated fair market value at
December 31, 2009 of $6.00 per unit. We used this fair
market value per unit for units granted between January 1,
2010 and October 31, 2010.
In January 2011, we consummated a $60 million equity
financing with CH Inter, a wholly-owned subsidiary of PTTCH,
which resulted in net proceeds of $57.1 million. In
conjunction with this transaction, we converted from a limited
liability company to a Subchapter C corporation with three
classes of common stock. CH Inter purchased Class A common
stock at a price of $5.35 per share. At this time, a valuation
was done to estimate the fair value of our common stock as of
January 14, 2011. To determine our estimated enterprise
value, we applied a market-based approach based upon the
investment in our Class A common stock by CH Inter. We used
the option-pricing method to allocate the estimated enterprise
value between the three classes of stock. Within the allocation
model, we applied two liquidity event scenarios. The event
scenarios were a merger or acquisition, or M&A, within
2 years and an initial public offering, or an IPO, within
1 year. The risk free rate employed was 0.29% and 0.59% for
the IPO and M&A scenarios, respectively, which represented
the published yield on the one- and two-year U.S. Treasury
notes. Stock price volatility was estimated at 65% for both
scenarios based upon an analysis of historical volatilities of
public companies deemed comparable to us. We then weighted the
probability of the M&A scenario at 40% and the IPO scenario
at 60%. Using this method, we estimated a fair market value per
common share at January 14, 2011 of $3.45. We used this
fair market value per common share for the restricted shares and
options granted between January 14, 2011 and March 31,
2011.
No single event caused the valuation of our common stock to
decline from December 31, 2008 to March 2011; rather, it
was a combination of factors that led to the changes in the fair
value of the underlying common stock:
|
|
|
| |
•
|
The separation of our business from our Predecessor Company
resulted in a reduction in value associated with the investment
in BioEnergy Holding LLC which remained with the Predecessor
Company.
|
|
|
|
| |
•
|
The estimated cash flows from our license agreement for
D(−) lactic acid have not expanded as rapidly as
originally anticipated.
|
| |
| |
•
|
We completed our equity financing with a strategic investor in
January 2011. The value of the company negotiated during this
financing resulted in the creation of three classes of stock.
The preferences afforded the Class A and Class B
common shares resulted in a lower value ascribed to the common
stock.
|
There is inherent uncertainty in these estimates and if we had
made different assumptions than those described above, the
amount of stock-based compensation expense, net loss and net
loss per share could have been significantly different.
54
In conjunction with our conversion to a Subchapter C
corporation, we adopted the 2011 Omnibus Incentive Plan. The
following table summarizes the stock options granted from the
inception of the plan through March 31, 2011 with their
exercise prices, the fair value of the underlying common stock
and the intrinsic value per share, if any:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
Exercise Price
|
|
Fair
|
|
Intrinsic
|
|
Date of Issuance
|
|
Options
|
|
per Share
|
|
Value
|
|
Value
|
|
|
|
January 31, 2011
|
|
|
279,543
|
|
|
$
|
5.35
|
|
|
$
|
3.45
|
|
|
$—
|
|
March 31, 2011
|
|
|
263,750
|
|
|
$
|
3.45
|
|
|
$
|
3.45
|
|
|
$—
|
Significant
factors, assumptions and methodologies used in determining fair
value
We have estimated the fair value of our stock option grants
using the Black-Scholes option-pricing method. We calculate the
estimated volatility rate based on selected comparable public
companies, due to a lack of historical information regarding the
volatility of our stock price. We will continue to analyze the
historical stock price volatility assumption as more historical
data for our common stock becomes available. Due to our limited
history of grant activity, we calculate the expected life of
options granted using the “simplified method”
permitted by the SEC as the arithmetic average of the total
contractual term of the option and its vesting period. The
risk-free interest rate assumption was based on the
U.S. Treasury yield curve in effect during the year of
grant for instruments with a term similar to the expected life
of the related option. No dividends are expected to be paid.
Forfeitures have been estimated by us based upon our historical
and expected forfeiture experience.
The fair value of stock options granted for the three months
ended March 31, 2011 were estimated using the following
assumptions:
| |
|
|
|
|
|
Three Months Ended
|
|
|
|
March 31, 2011
|
|
|
|
Risk free interest rate
|
|
2.02-2.41%
|
|
Expected dividend yield
|
|
0%
|
|
Expected volatility factor
|
|
65%
|
|
Expected option life (years)
|
|
5-6
|
|
Expected forfeitures
|
|
5%
|
Our Predecessor Company recognized stock-based compensation
expense of $0 and $280,000 for the year ended December 31,
2008 and the period from January 1, 2009 to July 15,
2009, respectively, all of which was attributable to membership
unit grants. This amount was recorded as general and
administrative expense. We recognized a total of $208,000 in
stock-based compensation expense for the period from
July 16, 2009 to December 31, 2009, all of which was
attributable to membership unit grants and included in general
and administrative expense. We recognized $526,000 in
stock-based compensation expense for 2010, all of which was
attributable to membership unit grants. Of this amount, $235,000
was recorded as research and development expense and $291,000
was recorded as general and administrative expense. In the three
months ended March 31, 2010 and 2011, we recognized a total
of $150,000 and $470,000 in stock-based compensation expense,
respectively, of which $0 and $241,000, respectively, was
attributable to employee stock options and $150,000 and
$229,000, respectively, was attributable to membership units and
restricted stock.
Estimation
of fair value of warrants to purchase stock
In accordance with FASB ASC 815, Derivatives and
Hedging, all warrants issued by us that are exercisable for
common stock are accounted for as derivatives and recognized in
the consolidated balance sheets as fair value of warrant
liabilities at their estimated fair value. We determined that
this treatment was appropriate because the common stock
underlying the warrants has down-round protection. At inception,
July 16, 2009, we recorded these liabilities at their fair
value of $2.1 million.
As of December 31, 2009 and 2010, the fair value of stock
warrants was estimated to be $2.6 million and
$3.3 million, respectively, using the Black-Scholes option
pricing model . We recorded $515,000 and
55
$2.6 million of non-cash charges related to the change in
fair value of stock warrants for the years ended
December 31, 2009 and 2010, respectively, and $824,000 and
$2,500 for the three months ended March 31, 2010 and 2011,
respectively. These warrant liabilities are marked to fair value
from July 16, 2009 resulting in the recognition of gain or
loss in our consolidated statements of operations as gain or
loss from change in fair value of warrant liabilities from that
date.
Stock warrants were initially issued by us in connection with
the issuance of senior convertible debt and promissory notes.
The warrants were not issued with the intent of effectively
hedging any exposures to cash flow, market or foreign currency
risks. The warrants do not qualify for hedge accounting, and as
such, all future changes in the fair value of these warrants
will be recognized currently in earnings until such time as the
warrants are exercised, expire or are converted to common stock.
The warrants do not trade in an active market, and as such, we
estimated the fair value of these warrants using an
option-pricing model with the following assumptions:
| |
|
|
|
|
|
|
|
|
|
|
|
July 16, 2009
|
|
December 31, 2009
|
|
December 31, 2010
|
|
March 31, 2011
|
|
|
|
Risk free interest rate
|
|
2.46%
|
|
2.69%-3.04%
|
|
0.66%
|
|
0.80%
|
|
Expected volatility
|
|
49%-51%
|
|
49%-51%
|
|
38%
|
|
29%
|
|
Expected time to liquidity event
|
|
5-6 years
|
|
5-6 years
|
|
1-2 years
|
|
1-2 years
|
To value our common stock warrants as of March 31, 2011, we
first estimated our enterprise value and then allocated this
value to the underlying classes of equity using the
option-pricing method as outlined in the AICPA Practice Aid. We
used the option-pricing method to calculate our overall
estimated enterprise value, using a scenario analysis
incorporating probabilities of future events for existing
stockholders of an IPO and an M&A transaction as described
previously.
There is inherent uncertainty in these estimates and if we had
made different assumptions than those described above, the
amount of our loss on change in fair value of common stock
warrants, net loss and net loss per share amounts could have
been significantly different.
The table below summarizes the common stock warrants that were
issued by us and recorded as a liability as of July 16,
2009, December 31, 2009, December 31, 2010 and
March 31, 2011. The warrants listed in the table below and
issued in 2009 were transferred from our Predecessor Company at
the time of the transaction.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant
|
|
|
Number of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares
|
|
|
Warrant
|
|
|
|
|
|
Fair Value of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding
|
|
|
Shares
|
|
|
|
|
|
Warrants at
|
|
|
Fair Value of
|
|
|
Fair Value of
|
|
|
Fair Value of
|
|
|
|
|
at July 16,
|
|
|
Outstanding
|
|
|
|
|
|
July 16,
|
|
|
Warrants at
|
|
|
Warrants at
|
|
|
Warrants at
|
|
|
|
|
2009
|
|
|
at March 31,
|
|
|
Exercise
|
|
|
2009
|
|
|
December 31,
|
|
|
December 31,
|
|
|
March 31,
|
|
|
Year of Issuance
|
|
(Inception)
|
|
|
2011
|
|
|
Price
|
|
|
(Inception)
|
|
|
2009
|
|
|
2010
|
|
|
2011
|
|
|
|
|
2010
|
|
|
—
|
|
|
|
17,134
|
|
|
$
|
6.00
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
147,487
|
|
|
$
|
754
|
|
|
2009
|
|
|
766,347
|
|
|
|
89,656
|
|
|
|
10.00
|
|
|
|
1,233,819
|
|
|
|
1,754,935
|
|
|
|
896
|
|
|
|
80
|
|
|
2009
|
|
|
482,308
|
|
|
|
59,231
|
|
|
|
13.00
|
|
|
|
803,846
|
|
|
|
885,500
|
|
|
|
178
|
|
|
|
6
|
|
|
2010
|
|
|
—
|
|
|
|
639,170
|
|
|
|
0.01
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,141,064
|
|
|
|
2,198,745
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,248,655
|
|
|
|
805,191
|
|
|
|
|
|
|
$
|
2,037,665
|
|
|
$
|
2,640,435
|
|
|
$
|
3,289,625
|
|
|
$
|
2,199,585
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
recognition
Substantially all of our revenue is related to a government
award and related party management fees. Revenue under this
award is recognized in the period during which the related costs
are incurred, provided that the conditions under the awards have
been met and only perfunctory obligations are outstanding. We do
not expect further revenue from awards as all future award
receipts will be recorded as a reduction in the carrying value
of our production facility. Additionally, our management fee
agreement with a related party was terminated effective January
2011. No future management fee revenue is expected. At
March 31, 2011, the Company’s only existing source of
revenue is from its license agreement for D(−) lactic
acid, which is
56
recognized when Purac informs us on a quarterly basis of volumes
produced with a reconciliation at the end of the year for the
minimum royalty when relevant.
Impairment
of long-lived assets
In accordance with FASB ASC 360, Property, Plant, and
Equipment, we assess impairment of long-lived assets, which
include property, plant and equipment, for recoverability when
events or changes in circumstances indicate that their carrying
amount may not be recoverable. Circumstances which could trigger
a review include, but are not limited to, significant decreases
in the market price of the asset; significant adverse changes in
the business climate, legal or regulatory factors; accumulation
of costs significantly in excess of the amount originally
expected for the acquisition or construction of the asset;
current period cash flow or operating losses combined with a
history of losses or a forecast of continuing losses associated
with the use of the asset; or expectations that the asset will
more likely than not be sold or disposed of significantly before
the end of its estimated useful life.
The Company assesses the impairment of long-lived assets when
events or changes in circumstances suggest that the fair value
of the assets could be less than their net book value. In those
circumstances, the Company assesses long-lived assets for
impairment by determining their fair value based upon the
forecasted, undiscounted cash flows the assets are expected to
generate plus the net proceeds expected from the sale of the
asset. An impairment loss would be recognized when the fair
value is less than the carrying value of the asset group.
There were no indications of impairment of long-lived assets for
the three months ended March 31, 2011 and the years ended
December 31, 2010 and 2009.
We have not yet generated positive cash flows from operations on
a sustained basis, and such cash flows may not materialize for a
significant period in the future, if ever. Additionally, we may
make changes to our business plan that will result in changes to
the expected cash flows from long-lived assets. As a result, it
is possible that future evaluations of long-lived assets may
result in impairment.
We make estimates and judgments about future undiscounted cash
flows. Although our cash flow forecasts are based on assumptions
that are consistent with our plans, there is significant
exercise of judgment involved in determining the cash flow
attributable to a long-lived asset over its estimated remaining
useful life. As a result, the carrying amounts of our long-lived
assets could be reduced through impairment charges in the future.
Recent
Accounting Pronouncements
In January 2010, the FASB issued Accounting Standards Update
(ASU)
No. 2010-06,
Fair Value Measurements and Disclosures — Improving
Disclosures about Fair Value Measurements, that requires
entities to make new disclosures about recurring or
non-recurring fair value measurements and provides clarification
of existing disclosure requirements. This amendment requires
disclosures about transfers into and out of Levels 1 and 2
and separate disclosures about purchases, sales, issuances, and
settlements relating to Level 3 measurements. It also
clarifies existing fair value disclosures about the level of
disaggregation and about inputs and valuation techniques used to
measure fair value. This amendment is effective for periods
beginning after December 15, 2009, except for the
requirement to provide the Level 3 activity of purchases,
sales, issuances, and settlements, which will be effective for
fiscal years beginning after December 15, 2010. The
adoption of this standard did not have a material impact on the
consolidated financial statements.
57
Industry
We are an industrial biotechnology company focused on becoming a
low-cost producer of high-value biochemicals that drop-in and
substitute for traditional petroleum-based industrial chemicals.
We have developed a proprietary technology platform that we
believe will enable us to manufacture a variety of
drop-in
chemicals and replacement chemicals for large and growing
markets using a broad range of low-cost, renewable and readily
available feedstocks. Our technology platform is based on a
single-step anaerobic fermentation process that allows our
microorganisms to grow and simultaneously produce the target
product, resulting in greater productivity and yield relative to
other known bioproduction processes. We believe that we can
produce our target high-value chemical intermediates at an
average of half the cost of traditional petrochemical
intermediates at a wide range of oil and industrial sugar prices
without relying on government subsidies. We have entered into or
intend to enter into strategic relationships with international
companies to accelerate the global commercialization of our
products and development of our biochemical production
capabilities. We are currently building a 30 million pound
biosuccinic acid plant in Lake Providence, Louisiana, which we
expect will begin commercial operations during the first quarter
of 2013.
Our vision is to be the pre-eminent global low-cost producer of
green, high-value biochemical intermediates.
INDUSTRY
OVERVIEW
The global chemical market is estimated at approximately $1.2
trillion in annual sales. Chemicals are generally produced
through the conversion of a feedstock into a higher-value
product through a series of chemical reactions.
Organic chemicals are a class of chemicals based on or
containing carbon atoms. Petrochemicals, which are organic
chemicals, have traditionally been derived from fossil fuels
such as petroleum, natural gas or coal. Organic chemicals can
also be produced from renewable carbon sources such as
plant-derived matter, including sugars, oils and cellulosic
materials. These renewable plant sources are the basis of the
biochemicals industry.
Organic chemical building blocks are used by the petrochemical
industry to produce, through different chemical reactions,
downstream chemicals used in thousands of industrial and
consumer applications. These building blocks are classified
according to the number of carbon atoms per molecule. For
example, ethylene, the building block for such materials as
polyethylene and ethylene glycol, has two carbon atoms per
molecule (C2), is primarily derived from natural gas found in
the U.S. and the Middle East, and is considered a light
feedstock (i.e., its molecules contain few carbon atoms).
Building blocks with three (C3), four (C4) and six (C6) carbon
atoms are most commonly derived from a heavier petroleum
feedstock. Supplies of these building blocks are influenced in
part by the broader supply and demand dynamics of fossil-based
feedstocks. For example, in the U.S., due to the widening
pricing spread between natural gas and oil, many cracker
operators are using lighter natural gas feedstocks to earn
higher margins, thus limiting the production of heavier C3, C4
and C6 building block chemicals, which are derived from
higher-priced petroleum feedstocks.
We are targeting high-value chemicals traditionally derived from
these heavier feedstocks. Given their supply and demand
dynamics, proprietary nature and barriers to entry, these target
chemicals generally command higher prices than traditional
commodity chemicals. We are targeting the C3, C4, and C6
chemicals shown in the diagram below.
58
PETROCHEMICAL
PRODUCT CHAIN
Feedstock cost and availability are important factors for
chemical producers seeking to manufacture products with
predictable and competitive costs. The overwhelming majority of
chemicals are currently derived from fossil fuel sources.
According to industry estimates, a substantial majority of all
chemicals could be produced from renewable feedstocks. Given the
limited availability and the increasingly volatile price of most
fossil-based feedstocks, chemical and energy companies such as
BASF, Dow, DuPont, DSM and Total are exploring alternatives,
including using biobased inputs such as plant-derived sugars,
cellulosic sugars and plant-based oils, to increase availability
of feedstocks and reduce price volatility.
The market for biobased chemicals was estimated to be between
$130 and $180 billion in 2010. By 2025, biobased chemical
products are expected to comprise over 20% of the chemicals
market at $490 to $610 billion (8-9% CAGR). Currently,
chemical producers use biobased materials in a variety of
products, such as in the production of fatty acids from
plant-based oils for use as surfactants in detergents,
cosmetics, synthetic lubricants and plastics. For example,
DuPont and Tate & Lyle have developed and are
operating a commercial-scale facility in Tennessee using
renewable feedstocks to produce propanediol used in
DuPont’s
Sorona®
product line of textile fibers and fabrics.
We believe that biotechnology has the potential to revolutionize
the competitive dynamics of the petrochemicals market. This
technology can substitute biobased chemicals for existing fossil
fuel-based chemicals to produce drop-in chemicals and
replacement chemicals that are less price volatile as compared
to petroleum-based alternatives and are of identical quality.
Biobased chemicals can be molecularly identical or perform
identically to fossil fuel chemicals for which they substitute.
This drop-in attribute allows downstream customers to use the
same manufacturing processes designed and optimized for the use
of traditional chemicals, allowing them to immediately
substitute in biobased chemicals without long qualification
steps or significant additional capital expenditures.
KEY
FACTORS DRIVING DEMAND FOR BIOBASED CHEMICALS
Finite
supply of fossil fuels
Petrochemical manufacturers and energy producers compete for
petroleum, natural gas and coal. These carbon sources are finite
resources with continuously growing demand. The global
population is growing at a rate of over 80 million people
per year, resulting in higher industrial output, powered
principally by fossil fuels. Emerging markets (defined by the
International Monetary Fund’s World Economic Outlook
Database as
59
150 emerging and developing countries) are expected to continue
to grow rapidly, experiencing approximately 6% annual real GDP
growth over the next 10 years, leading to increased demand
for energy. For example, China and India’s combined share
of energy consumption has nearly doubled in the past
20 years, rising from 10% of the world’s total energy
consumption in 1990 to more than 20% in 2010, leading to
pressure on feedstock availability and pricing pressure on
chemicals production.
High
and volatile price of petroleum
According to the EIA, global petroleum prices have risen over
300% in the past 10 years from $25.89 on May 18, 2001
to $108.48 on May 20, 2011. Chemical companies are
continually evaluating feedstock alternatives to mitigate high
input prices, maintain or improve margins and limit the need for
price increases for their downstream products, which reduce
demand and drive product substitution. Additionally, volatile
prices for fossil fuels have generated financial and economic
uncertainty for chemical producers. For instance, the average
market price per barrel of West Texas Intermediate crude oil
ranged from $145.66 in July 2008 to $30.81 in December 2008
and has increased to $98.14 as of July 20, 2011. Chemical
manufacturers are seeking feedstocks with stable pricing to
generate more predictable profit and cash flows.
Shortages
of petroleum-based feedstocks
Steam crackers convert both petroleum-based feedstock (e.g.,
naphtha) and natural gas-based feedstock (e.g., ethane) into
building blocks used in the production of chemicals. Recently,
the low price of natural gas relative to petroleum in the
U.S. has driven many cracker operators to switch from
petroleum-based feedstocks to natural gas-based feedstocks to
earn higher margins. Cracking natural gas-based feedstocks
yields lower quantities of C3 and heavier chemical building
blocks relative to yields resulting from cracking
petroleum-based feedstock. The recent shift away from cracking
petroleum-based feedstocks has reduced the supply of C3 and
heavier chemical building blocks. Examples of affected chemicals
include propylene (an input to acrylic acid) and benzene (an
input to adipic acid and phthalic acid). Scarcity of such
chemicals is expected to continue in the future as crackers
continue to switch to natural gas-based feedstock, particularly
in North America and the Middle East.
Low
price volatility of biobased chemicals
alternatives
Advances in the fields of industrial biotechnology and metabolic
engineering are enabling the manufacture of biobased
alternatives for a broad set of chemicals at lower production
costs than petroleum-based chemicals. These biobased chemicals
can substitute for existing fossil fuel-based chemicals, are
less price volatile, and can be of identical quality. We believe
that these qualities will drive industry adoption of biobased
chemicals.
Improving
economics drive increasing demand for “green”
products
Market demand for “green” products is growing as a
result of consumer preferences shifting towards products that
are renewable and environmentally friendly. Leading consumer
products companies, such as
Coca-Cola,
Wal-Mart, and Proctor & Gamble, are responding by
introducing green products with a reduced carbon footprint and
higher renewable content. Many of these initiatives have been
hampered by the historically higher costs, or “green
premium,” of manufacturing products using
environmentally-friendly methods versus traditional
cost-optimized processes. Cost-competitive, biobased chemicals
would enable end-product vendors to address the growing demand
for green products without sacrificing margins.
Increasing
regulatory pressure and public awareness
Concerns over energy independence, product toxicity and
greenhouse gas emissions generated in the production and
consumption of fossil fuels have increased government regulation
of the petrochemical industry, such as the U.S.’s Clean Air
Act and Toxic Substances Control Act (TSCA) and the European
Union’s REACH regulation. Growing public awareness of and
concern over the production of atmospheric greenhouse gases and
regulation of
CO2
as a pollutant is increasing pressure on petrochemical companies
to find replacements for current petroleum-based processes that
generate large quantities of
CO2
(such as BDO from butane or benzene and adipic acid from
benzene) with newer biobased processes (such as our bioprocesses
that produce biosuccinic acid) with reduced carbon footprints.
60
Business
COMPANY
OVERVIEW
We are an industrial biotechnology company focused on becoming a
low-cost producer of high-value biochemicals that drop-in and
substitute for traditional petroleum-based industrial chemicals.
We have developed a proprietary technology platform that we
believe will enable us to manufacture a variety of
drop-in
chemicals and replacement chemicals for large and growing
markets using a broad range of low-cost, renewable and readily
available feedstocks. Our technology platform, which we have
validated in our laboratories and third-party tolling facilities
and commercialized with our licensee, Purac Biochem BV, or
Purac, a wholly-owned subsidiary of CSM N.V., is based on a
single-step anaerobic fermentation process that allows our
microorganisms to grow and simultaneously produce the target
product, resulting in greater productivity and yield relative to
other known bioproduction processes. We believe that we can
produce our target high-value chemical intermediates at an
average of half the cost of traditional petrochemical
intermediates at a wide range of oil and industrial sugar prices
without relying on government subsidies.
We have entered into or intend to enter into strategic
relationships with international companies to accelerate the
global commercialization of our products and development of our
biochemical production capabilities. For example, we signed a
non-binding memorandum of understanding, or MOU, to enter into a
definitive joint development agreement with Johnson Matthey
PLC’s subsidiary Davy Process Technology Limited, or Davy,
a developer and licensor of advanced process technologies. Under
that agreement, Davy would, upon completion of testing and
engineering of our biosuccinic acid, guarantee to its butanediol
process licensees that they could use our biosuccinic acid in
their butanediol process in place of petroleum-derived maleic
anhydride without significant additional capital expenditures.
We have also signed exclusive alliance agreements with
ThyssenKrupp’s subsidiary Uhde GmbH, or Uhde, a chemical
plant engineering company, and its U.S. subsidiary, to
integrate our fermentation technology with Uhde’s
separation technology in the plant design and, on a
project-by-project
basis, provide process and performance guarantees for our future
plants on mutually agreeable terms to facilitate access to
project finance. In addition, we plan to leverage our
partnership with PTT Chemical International Private Limited, or
CH Inter, our largest stockholder and a subsidiary of PTT
Chemical Public Company Limited, or PTTCH, a large
Thailand-based petrochemical producer, to access CH Inter’s
and PTTCH’s breadth of commercial and technical expertise
and extensive knowledge and infrastructure in Asian markets.
We have validated our proprietary technology as cost-competitive
using commercial-scale unit operations and multiple feedstocks.
Our technology platform combines proprietary microorganisms, or
biocatalysts, and a fermentation process capable of using
diverse industrial sugars to create various chemical
intermediates, such as succinic acid (a $7.5 billion market
at current prices), fumaric acid (a $1.7 billion market),
acrylic acid (a $14.5 billion market) and lactic acid
(forecast to eventually become a multi-billion pound market). We
believe that our technology is capable of efficiently producing
organic chemicals at high yields while consuming less feedstock
because we use an anaerobic process that consumes, rather than
releases, atmospheric carbon dioxide, or
CO2.
For example, our models show that we can convert the same amount
of sugar needed to make 100 million gallons of ethanol into
approximately 1.4 billion pounds of biosuccinic acid,
thereby yielding potential sales of $1.2 billion from
biosuccinic acid versus $250 million from ethanol based on
today’s market prices. Overall, our improved feedstock
efficiency allows us to achieve significant cost advantages
relative to competitors who use either traditional
petroleum-based processes or other known bioproduction
techniques, allowing us to realize attractive margins and return
on capital.
Based on current commercial-scale cost metrics, we estimate that
our production process for biosuccinic acid will be
cost-competitive with petroleum-based processes down to $45 per
barrel of oil. We believe, as a result, that we can radically
transform the 1,4 butanediol, or BDO, and adipic acid markets,
using renewable feedstocks while reducing our customers’
carbon footprint. Using this same technology platform, we also
plan to address even larger markets, such as acrylic acid.
Our biosuccinic acid is a drop-in product that can be used in a
number of petrochemical manufacturing processes. We are able to
accomplish this because our technology produces a biosuccinic
acid that is the same
61
molecule as petroleum-based succinic acid currently used in
existing markets. Our customers benefit from our chemical
intermediates because we produce these high-value chemicals at
costs that have lower volatility than petroleum inputs. We
believe we can replicate this model for many widely used
petrochemicals.
We are currently building a 30 million pound biosuccinic
acid plant in Lake Providence, Louisiana, or the Louisiana
Plant, and have contracts committing customers to buy 100% of
each customer’s annual succinic acid requirements from us,
consuming a substantial portion, and prospectively all, of the
Louisiana Plant’s initial capacity based on the
customers’ current stated forecasts. We anticipate that the
Louisiana Plant will begin commercial operations during the
first quarter of 2013. We intend to expand the annual production
capacity of this plant to approximately 170 million pounds
by the end of the first quarter of 2014. We are also negotiating
a letter of intent with Uhde for an industrial biochemical
facility for the production of biosuccinic acid at the
Infraleuna Chemical Site in Leuna, Germany. The plant, which we
expect would commence operations in the first half of 2012,
would utilize our technologies to produce biosuccinic acid and
ammonium sulfate in accordance with our product specifications.
We have also signed a memorandum of understanding with China
National BlueStar (Group) Co. Ltd., or BlueStar, to develop a
proposal for a jointly-owned 220 million pound biosuccinic
acid plant in Nanjing, China, the biosuccinic acid requirements
of which would be exclusively supplied by the Company. BlueStar
is currently producing BDO utilizing a process licensed from
Davy.
TECHNOLOGY
We are applying our expertise in metabolic engineering, directed
evolution, fermentation technology and chemical engineering to
design methods for producing high-value biochemicals at
competitive prices. Our team of 36 scientists and researchers,
including 17 with Ph.D. degrees, have developed a proprietary
technology platform that we use to produce high-value
biochemicals at high yield and productivity. We accomplished
this by developing biocatalysts, microorganisms with altered
metabolic pathways, that grow and simultaneously produce target
biochemicals from a variety of feedstocks without the use of
expensive complex nutrients. Our ability to couple the growth of
our biocatalysts to target biochemical production improves
productivity and yield and limits by-product formation, thereby
reducing the need for expensive post-conversion unit operations.
This reduces the risks of commercial
scale-up and
enables low-cost fermentations. Our process is anaerobic and
therefore does not release
CO2;
in addition, in the production of biosuccinic acid, we introduce
CO2
as an additional carbon source, thereby using less feedstock and
reducing the carbon footprint of our products. Using our
experience in industrial chemical engineering, we develop and
scale-up
fermentation processes that efficiently recover industrial
biochemicals from fermentation broth in high purities. To
separate and purify our target biochemicals, we use
off-the-shelf
equipment that has been deployed and proven at commercial scale
for other organic products.
FEEDSTOCKS
Our biocatalysts are feedstock flexible and can consume sugars
from a variety of sources, including glucose from corn and grain
sorghum, sucrose from sugarcane, cellulosic sugars from waste
biomass (e.g., bagasse and wood waste) and glycerol, a
by-product of biodiesel facilities. After completing
start-up and
operational trials on sorghum-based sugars at the Louisiana
Plant, we plan to transition to low cost sugars such as 95
Dextrose. The price of 95 Dextrose, an intermediate product of
the corn wet-milling process, is significantly less volatile
than that of corn. Between May 2001 and May 2011, corn prices on
the CBOT ranged from $1.94 to $7.33 per bushel while 95 Dextrose
ranged from $0.13 to $0.20 per pound.
Our management team has extensive experience in the commercial
scale-up and
operation of plants utilizing cellulosic-derived sugars spanning
more than 20 years, having built and operated three
separate pilot plants with various production capabilities. This
experience includes all aspects of feedstock preparation,
pre-treatment, hydrolysis and fermentation. We have made
hydrolysate, containing both five carbon sugars (C5s such as
xylose) and six carbon sugars (C6s such as glucose), which we
have converted to both biosuccinic and lactic acid at the same
efficiencies as traditional sugars. Our biocatalysts can consume
both five carbon and six carbon sugars, which we believe
positions us to be a first mover when cellulosic feedstocks
become more
62
widely available. We plan to use the most cost-effective
regional feedstocks or a combination of feedstocks in our future
plants.
COMMERCIAL
SCALE-UP
Our technology platform has been deployed on a commercial scale
since June 2008 at Purac’s manufacturing facility in
Barcelona, Spain for the production of D(−) lactic acid.
Purac is a worldwide leader in the production of lactic acid. In
January 2006, we granted Purac an exclusive, worldwide and
royalty-bearing license under our licensed patents and
technologies to produce, market and sell D(−) lactic acid,
its derivatives and by-products. Purac produces D(−)
lactic acid using our technology in 200,000 liter fermenters.
The scale-up
of the Louisiana Plant represents a two fold
scale-up as
compared to the production of D(−) lactic acid as
commercialized by Purac and is comparable in nature.
We have scaled up our biosuccinic acid production from an
initial fermentation vessel size of five liters to
50,000 liters from January 2008 to February 2011 beginning
in our laboratories, and continuing at Uhde’s Leipzig,
Germany pilot plant, at Wisconsin BioProducts, a contract
fermenter, and at Fermic. We entered into a contract
manufacturing arrangement with Fermic to validate our technology
at a larger scale and produce samples for customers and
equipment vendors. Using Fermic’s 20,000 liter fermenters
represented a more than 1,000 fold increase in fermenter size
compared to the fermenters in our R&D laboratory. We began
to make product in our labs with five liter fermenters,
then transitioned to 500 liter fermenters at Fermic and then
rapidly progressed to Fermic’s 20,000 liter
fermenters. Using Fermic’s site, personnel, and equipment,
we produced a total of 24 metric tons of biosuccinic acid.
Myriant reimbursed Fermic for raw materials, the use of
Fermic’s personnel, and the rental of Fermic’s
equipment. All product made under this arrangement belonged
exclusively to Myriant and was sent to customers, vendors, and
our laboratories for testing. For a period of 20 months, we
conducted our tests, further developed our process, and made
product samples.
PARTNERSHIPS
We have entered into or intend to enter into strategic
relationships with international companies to accelerate the
global commercialization of our products and the development of
our biochemical production capabilities. Our partners include
international process design, engineering and licensing firms
that serve the chemicals industry, such as Davy and Uhde. These
arrangements will help validate our products and processes for
the commercial production of biosuccinic acid. For example, we
expect that Davy will perform, at its expense, all of the
engineering, development and pilot plant work at its facilities
needed to validate our biosuccinic acid for its process
technology and, upon successful testing of our biosuccinic acid,
would guarantee to its licensees that our biosuccinic acid will
work in replacement of MAN in its BDO production process. This
approach takes advantage of the existing plants that use the
Davy process for BDO production (approximately 1.2 billion
pounds or 27% of total global capacity and 50% of capacity
installed since 1992) and would enable Davy process licensees to
use our biosuccinic acid in their production process without
significant additional capital expenditures. To accelerate
development, construction and financing of our production
facilities, we have signed exclusive alliance agreements with
Uhde, an international engineering company focused on the design
and construction of chemical, refining and other industrial
plants. Uhde will integrate our fermentation technology with its
separation technology in the plant design and provide process
and performance guarantees on mutually agreed terms facilitating
access to project finance.
We plan to leverage our partnership with CH Inter and PTTCH to
access CH Inter’s and PTTCH’s breadth of commercial
and technical expertise and extensive knowledge in Asian
markets. In addition, we are negotiating a joint venture and
licensing arrangement with PTTCH that will enable us to
commercialize our technology and develop biochemical plants in
the ASEAN countries.
TARGET
MARKETS
The following markets are established chemical markets
traditionally dependent on petroleum-based feedstocks for which
we are developing biobased alternatives.
63
Succinic
Acid ($7.5 billion market)
Succinic acid is a chemical building block that is used as an
intermediate in the production of numerous high-volume products
including plastics, fibers, coatings, solvents and cosmetics.
The U.S. Department of Energy has identified succinic acid
as one of the five most promising “building block”
chemicals that can be produced commercially from biomass rather
than fossil fuels, as can be seen in the following figure.
Biosuccinic
Acid Applications
Succinic acid is conventionally produced from benzene or butane.
To produce succinic acid, benzene or butane is first oxidized to
produce maleic anhydride, or MAN, releasing roughly half the raw
material mass as
CO2,
after which MAN is reduced to succinic acid through hydrolysis.
Succinic acid is produced in existing chemical processes that
use MAN as an input, and is most commonly produced as a captive
intermediate rather than as a merchant product. Examples of
high-volume production processes that go through succinic acid
include BDO, gamma-butyrolactone (GBL), and tetrahydrofuran
(THF). We plan to produce biosuccinic acid that will feed into
existing processes that use succinic acid as an intermediate,
replacing MAN with biosuccinic acid and thereby eliminating
plant unit operations and saving capital costs.
There is currently a small existing merchant market for succinic
acid which we plan to sell our biosuccinic acid into. Succinic
acid is currently produced by Royal DSM N.V. and several small
producers in China and India, and is used as an input in the
production of pigments, solvents, detergents, metal plating, and
polybutylene succinate (PBS) polymers. PBS polymers are
biodegradable, and can be used to replace conventional plastics
in applications such as flexible packaging, agricultural films
and compostable bags. The PBS polymers market is currently
small, but we expect it to grow over time as demand for
biodegradable plastics increases.
64
In addition to targeting the existing succinic acid market, we
plan to sell our biosuccinic acid in the following target
markets:
Butanediol
BDO is a high-value chemical intermediate with end markets in
polymer resins, fibers, coatings and other downstream chemical
products. Total global BDO capacity is estimated at
approximately 4.5 billion pounds per year. Half of global
BDO is used as an intermediate in the production of THF, which
is an intermediate in the production of elastic fibers such as
elastane, better known as
Spandex®
and
Lycra®.
The rest of global BDO production is used as a raw material for
polybutylene terephtalate (PBT) engineering polymers,
polyurethanes and other applications. Growth drivers for BDO
demand include increasing demand for elastic fibers in China and
recovering demand for PBT engineering resins in the U.S.
Roughly two-thirds of global BDO is captively used by BDO
producers. BDO is produced through four main processes: the
Reppe process (41% of global capacity), the Davy process (27%),
the Propylene Oxide process (21%) and the Mitsubishi process
(7%). The largest producers of BDO globally include BASF, Dairen
Chemical, LyondellBasell, ISP, Invista and China National
BlueStar Corporation.
The Davy process was commercialized in the 1990s as a
lower-cost, more efficient alternative to the Reppe process and
is based on the conversion of MAN into dimethyl succinate and
then into BDO. Succinic acid can be used as a drop-in
replacement for MAN in this process. This process represents the
fastest growing BDO technology. The Davy process accounts for
50% of BDO plant capacity built since 1992, and its licensed
installed base is currently estimated at 1.2 billion pounds
per year. The largest Davy process producers include China
National BlueStar Corporation, Sinopec, BASF/Petronas, Gulf
Advanced Chemical Industries and Sanwei.
We plan to sell our biosuccinic acid as a drop-in in the
butanediol market.
Adipic
Acid
Adipic acid is a major chemical intermediate derived from
benzene and used in a range of polymers and plastics
applications, which are in turn used in the production of
specialty flexible foams for textile laminations and garment
linings, packaging, filters and fuel tank linings and for
PVC-based adhesive tapes, such as automotive decals, labels and
electrical, surgical and industrial tapes. Over half of the
adipic acid produced globally is used to produce nylon 6,6
fibers and engineering resins. The remainder of adipic acid is
used to produce polyester polyols, plasticizers, and other
polymer applications. The total market size of adipic acid is
estimated at $6.9 billion at current prices.
The adipic acid market has been negatively impacted by the
limited supply of benzene, which is the traditional building
block used to produce adipic acid. Periodic supply shortages and
high prices in benzene have led to volatility in the
availability and cost of adipic acid. In addition, capacity
rationalization during the recent recession combined with a
rebound in demand for adipic acid has further contributed to
shortages and price increases. Adipic acid production also
generates large quantities of pollutants such as carbon dioxide
and nitrous oxide. Emissions of these pollutants attract
regulatory pressure and can lead to higher taxes and regulatory
requirements to add equipment to reduce emissions. Biosuccinic
acid, which can be sold as a replacement for adipic acid in
non-nylon applications, offers an alternative with potentially
less volatile prices and green attributes.
The major producers of adipic acid include Invista, Rhodia,
Ascend Performance Materials and BASF.
We plan to sell our biosuccinic acid as a replacement chemical
in the adipic acid market.
Phthalic
Anhydride
Phthalic anhydride, or the anhydride of phthalic acid, is a
major chemical intermediate used as a raw material to produce
plasticizers, coatings and polymer resins. Plasticizers account
for approximately half of global phthalic anhydride consumption,
with the remainder used in alkyd resins for surface coatings,
unsaturated polyester resins and polyester polyols. Plasticizers
are used in, among other products, wire/cable
65
jacketing, garden hoses, medical tubing and PVC film. Polyester
resins are used in pipes, ducts, bathroom components, automotive
parts and swimming pools. The total market size of phthalic
anhydride is estimated at $6.2 billion at current prices.
Phthalic anhydride is generally produced from o-xylene, a
benzene derivative, but can also be produced from naphthalene or
an o-xylene/naphthalene blend. Because phthalic anhydride
markets are linked to benzene, recent global shortages in
benzene have driven up prices and reduced available supply.
Health concerns have recently been raised regarding the use of
phthalic anhydride derivatives, or phthalates in consumer
plastics. Studies have found that animals exposed to phthalates
experience adverse health effects including reduced testosterone
production, reduced estrogen production, birth defects and liver
cancer. Citing health risks, the U.S. government in 2008
enacted a permanent ban on three phthalates and a temporary ban
on three others from composing more than 0.1% of any
children’s product for ages 12 and under. In 2005, the
E.U. enacted a similar ban on the use of six phthalates in
children’s products, and in 2011 Canada restricted the use
of six phthalates above 1,000 milligrams per kilogram in
children’s toys and child care products. Phthalates were
also the subject of a recent U.S. law suit in which two
consumer advocacy groups sued the U.S. Consumer Product
Safety Commission (CPSC) alleging the commission acted illegally
by failing to adequately regulate toxic phthalates in consumer
products.
As a result of increasing government regulation of phthalates
and growing public awareness of their potential health risks,
producers are looking for non-phthalate chemicals to use as
replacements. Succinate esters, derived from succinic acid, have
similar performance characteristics to phthalates. Some of our
prospective customers have already tested replacing phthalates
with succinates and one customer has signed a contract with us
for that purpose.
The major global producers of phthalic anhydride include BASF,
Nan Ya, ExxonMobil, UPC Group and Aekyung Petrochemical.
We plan to sell our biosuccinic acid as a replacement chemical
in the phthalic anhydride market. We have entered into a supply
agreement with The Chemical Company to supply it with
biosuccinic acid in accordance with the terms set forth in the
section titled “Customers” on page 72 of this
prospectus.
Lactic
Acid (forecast to eventually become a multi-billion pound
market)
Lactic acid exists in nature as a mixture of two forms:
D(−) lactic acid and L(+) lactic acid. These molecules
contain the same elements but are mirror images of each other,
like a right and left hand. The production of the polylactic
acid, or PLA, uses this mixture of lactic acid. PLA has an
inherent weakness in heat stability, compared to other polymers
such as polyethylene and polypropylene. PLA polymers melt at
approximately 70 degrees Celsius, which makes it unsuitable for
many applications. Combining D(−) lactic acid in higher
concentration raises the melting point of PLA to
200 degrees Celsius, which opens up many new applications
to PLA. Thus, there is significant value in producing pure
D(−) lactic acid.
Since D(−) and L(+) lactic acid are so similar, they are
difficult to separate into pure product. Prior to the
development of our technology, D(−) and L(+) lactic acid
were separated from each other through an expensive chemical
separation process. As a result of this costly separation
process, use of pure D(−) lactic acid in PLA has not been
commercially viable. Our technology allows for the production of
D(−) lactic acid alone or L(+) lactic acid alone in one
step through biosynthesis, which simplifies and lessens the cost
of the purification process. We believe our low cost position in
the production of D(−) lactic acid would thus create an
economically viable solution for manufacturers looking to
enhance the performance of PLA.
Until recently, SC-PLA could not be produced because there was
no commercial supply of D(−) lactic acid. In 2008, Purac,
one of the world’s largest producers of lactic acid, began
commercial production of D(−) lactic acid using
bioproduction technology licensed from us.
66
Fumaric
Acid ($1.7 billion market)
Fumaric acid is currently used as a preservative in food and
beverages, as a sizing agent in paper production and as an input
for alkyd resins used in paints and coatings. The existing
fumaric acid market size is estimated at $319 million at
current prices. Fumaric acid can also be used as a replacement
for MAN in the production of unsaturated polyester resins (UPR),
which are used in construction, marine and automotive products.
This replacement is possible because fumaric acid is chemically
equivalent to a combination of MAN and water. Fumaric acid is
not used as a MAN replacement today because it is not currently
produced at a cost that is competitive with the MAN production
cost. The market for which fumaric acid can potentially replace
MAN, excluding MAN used in the Davy BDO process, is estimated at
$1.4 billion at current prices. The existing fumaric market
and MAN market are together estimated at $1.7 billion at
current prices.
The major global fumaric producers include Bartek Ingredients,
Polynt, Thirumlai, DSM Fine Chemicals, UPC Technology, Weinan
Shuangqiao, Proaroma Industria e Comercio, Tate & Lyle
and Yongsan Chemicals.
We plan to sell our fumaric acid as both a drop-in to the
fumaric acid market and as a replacement for MAN.
Acrylic
Acid ($14.5 billion market)
Acrylic acid, one of the most versatile monomers, is used to
impart hardness, tackiness and durability to thousands of
polymer formulations. Its two main derivatives are acrylates and
polyacrylic acid. Acrylates, which are esters of acrylic acid,
are used in superabsorbents, coatings, adhesives, sealants,
textiles, paper chemicals and plastic additives. The acrylic
acid market size is estimated at $14.5 billion. Major
growth applications for acrylates include radiation-curable
coatings, adhesives, sealants and acrylic fibers.
Acrylic acid is produced primarily from propylene.
Propylene-based production involves adding oxygen to propylene
to produce acrolein, an unsaturated aldehyde, which is further
oxidized to acrylic acid. Acrylate esters can then be produced
through esterification of acrylic acid with an alcohol (e.g.,
n-butanol, ethanol, methanol). Major global producers of acrylic
acid include BASF, Dow, Nippon Shokubai, Arkema, Formosa
Plastics, Evonik Stockhausen and Jiangsu Jurong.
We plan to sell our acrylic acid as a drop-in chemical in the
acrylic acid market.
OUR
COMMERCIAL PRODUCTS
Biosuccinic
Acid
We are currently focused on commercializing and producing
biosuccinic acid. This product represents a market opportunity
of $7.5 billion. Biosuccinic acid can be used to make a
range of high-volume, high-value chemical products, including
BDO, polybutylene succinate, or PBS, tetrahydrofuran, or THF,
and as a substitute for adipic acid and phthalic anhydride.
These products are primarily used in the production of
polyurethane fibers, solvents and engineered plastics. We
believe we can be a low-cost producer of biosuccinic acid
relative to producers using either traditional petroleum-based
processes, including petroleum-derived maleic anhydride, or
other known bioproduction techniques.
We believe we can achieve commercial-scale cost targets for our
biosuccinic acid and have already had more than 24 metric tons
of biosuccinic acid made for us at the Fermic tolling plant for
our internal testing and customer/vendor sampling and
validation. Customer feedback has been positive and has resulted
in three supply contracts and one signed letter of intent. We
estimate that these contracts will consume a substantial
portion, and prospectively all, of the initial annual production
capacity available at our Louisiana Plant. We are planning to
expand the plant to 170 million pounds by the end of the
first quarter of 2014 and have a pipeline of customers in place
that have indicated interest in purchasing our biosuccinic acid
that would be sourced from this additional annual production
capacity.
Through our relationship with Davy, we intend to target BDO
plants that use the Davy process to replace their
petroleum-derived maleic anhydride with our biosuccinic acid.
This will allow our customers to produce BDO from renewable
resources with a reduced carbon footprint and higher renewable
content relative to the
67
traditional Davy production processes. We are also working with
Davy to integrate and optimize our respective processes to
reduce the number of unit processing operations, thereby further
reducing our overall cost of biosuccinic acid production for use
in BDO applications.
D(−)
Lactic Acid
In June 2008, our technology platform was commercialized for the
production of D(−) lactic acid through our licensee,
Purac. This is the first product based on our technology. Purac
pays us royalties based on sales. Our D(−) lactic acid is,
to our knowledge, the only pure D(−) form of lactic acid
on the market, which allows it to be cost-effectively alloyed
with PLA as a stereocomplex (SC-PLA), addressing the thermal
stability problem that has limited the adoption of traditional
PLA (L-PLA). We believe that biobased SC-PLA has the potential
to enter higher-value markets such as engineered plastic, which
will expand SC-PLA’s market. As a result, market forecasts
predict the market for D(−) lactic acid will eventually
exceed one billion pounds. The conversion of feedstock through
our D(−) lactic acid to PLA can be visualized in four
general steps:

We are currently in the development phase of our
stage-gated
process to use non-food cellulosic biomass to make D(−)
and L(+) lactic acid. Our stage-gate process is described in
more detail in the section titled “Our Technology
Platform” on page 72 of this prospectus. When the second
generation process is completed, we will have the ability to
produce and sell D(−) and L(+) lactic acid from our own
plants. We have no obligation to license the second generation
process to Purac. D(−) lactic acid and L(+) lactic acid
are together forecast to become a multi-billion pound market.
We intend to finance the completion of this development with a
combination of cash on hand and a portion of the net proceeds
received from this initial public offering of our common stock.
OUR
PRODUCT PIPELINE
Our technology platform provides for the manufacture of several
additional products through the use of metabolic pathways
related to those that we used to develop both our lactic acid
and biosuccinic acid technologies.
Fumaric
Acid
We are developing a biobased fumaric acid utilizing the same E.
coli-based technology used to produce our biosuccinic acid.
Fumaric acid is an important ingredient used in food
preservation, as a sizing agent in paper production and as an
input to alkyd resins used in paints and coatings. Fumaric acid
can also be used as a replacement for MAN in all MAN-based
applications, because fumaric acid is chemically equivalent to a
combination of MAN and water. The most significant of these
applications is the production of UPRs, where fumaric acid can
be used on a drop-in basis to replace MAN. We are in initial
discussions with major UPR producers regarding the use of
biobased fumaric acid in UPR production. Including both the
existing fumaric acid market and the market for which fumaric
acid can replace MAN, but excluding MAN used in Davy BDO plants,
we believe that fumaric acid represents a $1.7 billion
market. We are one year into our development cycle for fumaric
acid, and we expect to enter the
scale-up
phase in 2012.
Acrylic
Acid
We are currently in the discovery stages of developing a
biobased process for making acrylic acid, one of the most
versatile monomers. We have achieved proof of concept at
laboratory scale and are in the first year of our development
phase for acrylic acid. We expect to enter the pilot phase in
our cycle in 2012.
68
OUR
COMPETITIVE STRENGTHS
We believe that the following strengths differentiate us from
both traditional chemical producers and other biobased chemical
producers:
Validated proprietary technology. Our
technology has the following features:
|
|
|
| |
•
|
single-step anaerobic process allows our biocatalysts to grow
and simultaneously produce the target product, resulting in
greater productivity and yield relative to other known
bioproduction techniques;
|
| |
| |
•
|
allows fermentation of diverse sugars to create multiple
high-value biochemicals;
|
| |
| |
•
|
enables us to produce a broad set of C3, C4 and C6 based
chemicals;
|
| |
| |
•
|
reduces carbon footprint for our products by consuming rather
than producing
CO2;
|
| |
| |
•
|
limits by-product creation, lowering purification costs; and
|
| |
| |
•
|
organism that thrives in a minimal salt medium with no need for
corn steep liquor, yeast extract or other expensive raw
materials.
|
Low-cost producer of sustainable biobased
products. Our drop-in and replacement products
address large existing industrial chemical markets at an average
of half of the cost of traditional petrochemical intermediates
at both today’s and a wide range of oil and industrial
sugar prices. We believe our low-cost manufacturing process will
allow us to offer customers sustainable, biobased inputs to
existing processes at stable prices competitive with
petroleum-based inputs. For example, Davy-licensed BDO producers
could offer a green product and reduce their exposure to price
volatility of petroleum-derived MAN by substituting our
biosuccinic acid. In addition to stable raw material pricing,
Davy approval of our biosuccinic acid for use in its BDO process
would enable its process licensees to use our biosuccinic acid
in their production process without significant additional
capital expenditure. We also plan to offer our biosuccinic acid
as a replacement for other petroleum-based products such as
adipic acid and phthalic anhydride, thus reducing price
volatility faced by purchasers of those products.
Feedstock flexible. Using bioproduction
processes, we have successfully made biosuccinic acid from
95 Dextrose, grain sorghum, sucrose from sugarcane,
cellulosic sugars from waste biomass (e.g. bagasse and wood
waste) and glycerol, which is a by-product of biodiesel
facilities. Our biocatalysts consume both five carbon sugars
(C5s such as xylose) and six carbon sugars (C6s such as
glucose), which we believe positions us to be one of the first
movers when cellulosic feedstocks become more widely available.
We plan to use the most cost-effective regional feedstocks or
combination of feedstocks in our future plants, such as cassava
in Thailand, sugarcane in Brazil, corn-derived 95 Dextrose in
the United States and waste biomass and cellulosics throughout
the world.
Commercialized product. Our technology
platform has been commercialized for the production of
D(−) lactic acid through our licensee, Purac. Purac uses
our process to produce D(−) lactic acid, which is an input
to SC-PLA, a biopolymer that addresses the thermal stability
problems associated with conventional PLA (L-PLA). SC-PLA offers
higher strength and crystallinity and superior heat resistance
relative to L-PLA, enabling its use in higher value applications
such as engineered and high-performance plastics. We know of no
other biocatalyst capable of producing pure D(−) lactic
acid in one step via bio-synthesis.
Strategic relationships with international companies in the
fields of chemicals, process technology and
engineering. We have entered into or intend to
enter into strategic relationships with international companies
to accelerate the global commercialization of our products and
the development of our biochemical production capabilities. We
are collaborating or negotiating with two international process
design, engineering and licensing firms to the chemical
industry, Davy and Uhde, to help validate our products and
processes through developing process and performance guarantees
for the commercial production of biosuccinic acid. Our
memorandum of understanding with Davy contemplates that if Davy
successfully completes engineering and testing of our
biosuccinic acid, Davy would guarantee to its licensees that our
biosuccinic acid will work in replacement of MAN in its BDO
production process. This approach takes advantage of the
existing installed asset base that uses the Davy process for BDO
production and would enable Davy process licensees to use our
biosuccinic acid in their production process without significant
additional capital expenditures. To accelerate development,
construction and project financing of our production facilities,
we have signed exclusive alliance
69
agreements with Uhde, an international engineering company
focused on the design and construction of chemical, refining and
other industrial plants, providing for Uhde to integrate our
fermentation technology with its separation technology in the
plant design and, on a
project-by-project
basis, to provide process and performance guarantees on mutually
agreed terms for our future plants, facilitating access to
project finance.
We also plan to leverage our partnership with CH Inter and PTTCH
to access CH Inter’s and PTTCH’s breadth of commercial
and technical expertise and extensive knowledge in Asian
markets. In addition, we are negotiating a joint venture and
licensing arrangement with PTTCH to commercialize our
intellectual property and develop biochemical plants in the
ASEAN countries.
Profitable unit-level economics without subsidies or
mandates. We do not rely on regulatory policies,
subsidies, mandates or tariffs to make our products commercially
viable. Based on our testing, projections and experience to
date, we believe that our technological platform will allow us
to offer our products at prices that are competitive with
petroleum-based chemicals, without reliance on green premiums or
government subsidies. We believe capital expenditures required
for production of our products are consistent on a per-pound
basis with traditional chemicals plants and provide a more
attractive return on capital.
Scale-up
and signed customer contracts. We have scaled up
the production of our biosuccinic acid from an initial five
liters to 50,000 liters from January 2008 to February 2011. This
scale-up was
executed in ten fold increments over prior capacity and was
executed successfully, providing the basis of the
commercial-scale cost metrics that will make our production
process for our biosuccinic acid cost-competitive down to $45
per barrel of oil. Under our supervision, the Fermic tolling
facility produced 24 metric tons of biosuccinic acid for us in
support of our internal and customer/vendor sampling and testing
programs of over 100 current and potential customers. Our
biosuccinic acid was validated through sampling and testing in
analytical labs, application labs and pilot plants by 26 major
chemical companies. We anticipate entering into additional
contracts with a variety of customers prior to construction of
new production facilities. We are negotiating additional supply
agreements for the supply of our products from our Louisiana
Plant expansion with a variety of large, well-established
chemical companies.
Experienced team with a demonstrated track
record. Members of our management team have years
of experience in developing, scaling, building and operating
biotechnology and chemical businesses, and have led several
companies and projects with a demonstrated track record of
achievement and technological process. Accomplishments include:
|
|
|
| |
•
|
developing microorganisms and new products (D(−) lactic
acid, cellulosic ethanol, DuPont’s
Suva®,
riboflavin);
|
| |
| |
•
|
developing the fermentation and downstream processes of
industrial biotechnology (D(−) lactic acid, cellulosic
ethanol, riboflavin);
|
| |
| |
•
|
employing process development and project management of large
scale plants from start to finish (BASF’s $1.3 billion
steam cracker and the world’s largest butadiene extraction
unit);
|
| |
| |
•
|
financing and constructing large scale capital projects;
|
| |
| |
•
|
commercializing and navigating product approvals at global and
multinational companies (D(−) lactic acid and
DuPont’s
Suva®);
|
| |
| |
•
|
constructing and operating a $270 million, 100 million
gallon biofuel facility; and
|
| |
| |
•
|
managing global business functions, including pricing, sourcing,
customer interfacing, budgeting, and capital planning.
|
70
OUR
STRATEGY
Our strategy is to become a low-cost producer of high-value
biochemicals that drop-in and substitute for traditional
petroleum-based industrial chemicals. Key elements of our
strategy include:
Developing drop-in and replacement products for large
existing markets. We intend to commercialize a
broad set of chemicals with large markets. Through our licensee
Purac, we commercialized our first high-value chemical,
D(−) lactic acid, and we are currently constructing a
commercial-scale production facility for our biosuccinic acid (a
$7.5 billion market) product. In addition, we are
developing new biocatalysts and processes to produce fumaric
acid (a $1.7 billion market) and acrylic acid (a
$14.5 billion market). We intend to finance the development
of such new biocatalysts and processes with a combination of
cash on hand and a portion of the net proceeds received from
this initial public offering of our common stock.
Leveraging and establish partnerships to achieve commercial
success. We believe that our technology and
engineering partnership with Uhde and potential partnership with
Davy will enable us to accelerate the global commercialization
of our products and the development of our biochemical
production capabilities and provide us with a channel to market
for high-value biochemicals. We intend to leverage our strategic
partnership with CH Inter and PTTCH to access their breadth of
commercial and technical expertise and extensive knowledge and
infrastructure in Asian markets. We are currently in discussions
with potential partners to accelerate all elements of our
business plan.
Targeting drop-in applications to drive market
penetration. We intend to initially target
drop-in applications for our products to drive market adoption.
We also plan to pursue replacement applications for our products
to expand our market position. For example, our biosuccinic acid
commercialization strategy involves targeting drop-in products
for BDO manufacturing and existing biosuccinic acid
applications, where we intend to drive adoption to increase
productivity without the need for significant additional capital
expenditure by our targeted customers. We also plan to market
our biosuccinic acid as a replacement for adipic acid in
applications where manufacturers can use it to make products of
identical quality with lower price volatility and greater
environmental sustainability.
Rapidly penetrating existing BDO market. We
signed a non-binding memorandum of understanding, or MOU, with
Davy to enter into a definitive joint development agreement,
pursuant to which Davy would approve our biosuccinic acid for
use in BDO plants that use the Davy process, which would provide
us with an immediately available market for biosuccinic acid.
This represents roughly 27% of the BDO market, or
1.2 billion pounds per year. We believe our biosuccinic
acid, if successfully guaranteed by Davy for use in its BDO
process, will enable Davy process licensees to use our
biosuccinic acid in their production process without significant
additional capital expenditure.
Securing customer contracts to support production capacity
expansion. We plan to secure customer contracts
in advance of expanding our production capacity to mitigate
capacity utilization risks. We have signed contracts with three
customers who have agreed to buy 100% of their succinic acid
from our Louisiana Plant to replace petroleum-based succinic
acids, adipic acid and phthalates. As of May 2011, we were in
discussions with over 100 prospective customers that have
indicated interest in purchasing our biosuccinic acid that would
be sourced from the additional capacity created upon the
expansion of the Louisiana Plant to 170 million pounds.
Expanding internationally to markets with the greatest
business opportunities. We plan to compete in the
broad petrochemical market by deploying our technology and
establishing manufacturing operations globally. We expect that
Asia will be responsible for a majority of the growth of demand
in global markets. Our strategic relationship with CH Inter and
PTTCH will enhance our ability to develop, finance, build and
operate plants in the region.
Capitalizing on our feedstock flexibility to further reduce
cost. Our biocatalysts are feedstock flexible and
can consume sugars from a variety of biomass sources. We plan to
use the most cost-effective regional feedstocks or combination
of feedstocks in our future plants, such as cassava in Thailand,
sugarcane in Brazil and corn-derived 95 Dextrose in the United
States, as well as waste biomass
71
and cellulosics throughout the world. This strategy is designed
to expand the geographic scope of our business while further
improving the economics of our products.
OUR
TECHNOLOGY PLATFORM
Our proprietary technology platform, which we have validated in
our laboratories and third-party tolling facilities and
commercialized with our licensee, Purac, is based on a
single-step anaerobic fermentation process (that is, a chemical
reaction process that does not require oxygen). This process
allows our biocatalysts to grow and simultaneously produce the
target product, resulting in greater productivity and yield
relative to other known bioproduction techniques, rather than a
two-step process of first growing biocatalysts aerobically and
then switching to an aerobic fermentation process. Aerobic
growth uses more feedstock than anaerobic growth, reducing yield
and increasing costs. For this reason, we can produce
high-value biochemical intermediates at an average of half the
cost of traditional petrochemical processes at a wide range of
oil and feedstock prices without reliance on government
subsidies or a “green premium”. We intend to
capitalize on the feedstock flexibility of our processes to
tailor manufacturing plants to utilize low cost, readily
available feedstocks.
Our detailed understanding of many of the biochemical pathways
within microbial cells has allowed us to create engineered
microbes that produce desirable biochemicals. Such engineered
microbes can be grown in suitable media and used as biocatalysts
to create desired biochemicals.
Our team of 36 scientists and researchers, including 17 with
Ph.D. degrees, couple the understanding of the biochemical
pathways of microorganisms with our expertise in metabolic
engineering and metabolic evolution to create unique and novel
proprietary biocatalysts that produce target biochemicals at
high yields. We believe that our biocatalysts are unique in
their ability to grow and simultaneously produce target
biochemicals in minimal growth medium and without the use of
expensive complex nutrients. Our ability to couple the growth of
our biocatalysts to target biochemical production improves
productivity and yield and limits by-product formation thereby
reducing the need for expensive post-conversion unit operations.
In addition, our biocatalysts have the capacity to produce
target biochemicals through one-step anaerobic growth as opposed
to aerobic growth. Aerobic growth results in greater variability
and less predictability in the growth of the biocatalysts and in
commercial scale production of target biochemicals due to
technical difficulties in providing uniform oxygen partial
pressure throughout a large volume fermentation vessel. On the
other hand, the growth under anaerobic growth conditions is
uniform. Our biocatalysts are produced through metabolic
engineering and metabolic evolution and do not contain any
foreign genes.
72
Once we have established laboratory scale production of target
biochemicals using our biocatalysts, our expertise in
fermentation technology allows us to scale up to commercial
scale production with consistent productivity and yield. The
chart below indicates where our products stand in our
stage-gated commercialization process. A “stage-gate
process” is a roadmap for advancing a product from the
exploration stage to commercialization. The stage gate divides
the development activities into distinct stages separated by
decision points that do not allow further progress until the
decision makers elect to proceed based on the results of the
particular stage. At each “gate” meeting, the decision
makers review technical progress up to that point, estimated
costs of proceeding to the next stage and benefits of the new
product or technology. The decision to proceed to the next stage
or terminate the project is based on this review.
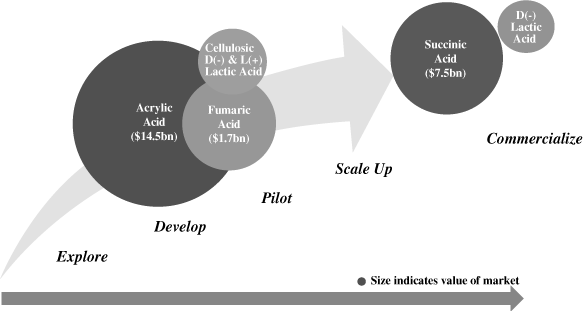
The D(-) lactic acid production process is at commercial scale.
We are in the process of commercializing our biosuccinic acid
process. Our current development efforts are focused on
developing biocatalysts for the production of fumaric and
acrylic acid from a variety of sources, including glucose from
corn and grain sorghum, sucrose from sugarcane, cellulosic
sugars from waste biomass and glycerol. Our platform provides
flexibility and we are able to produce biocatalysts that are
based on a variety of micro-organisms, including gram negative
bacteria, such as E. coli, gram positive bacteria, such as
bacillus, and yeasts. Our platform is directed toward selecting
the most efficient microorganism to create biocatalysts for the
production of desired biochemicals in a cost-effective manner.
We have developed biocatalysts with the ability at laboratory
scale to utilize a variety of different biobased feedstocks,
including glucose, xylose, sucrose, glycerol and hydrolysates
derived from woody lignocellulosic feedstock.
With our extensive experience in industrial chemical engineering
processes, we have recovered high-value biochemicals from
fermentation broth with high purities. To separate and purify
our products, we use off-the shelf equipment that has proved
useful in the large-scale recovery of other organic compounds.
Through research and development, metabolic engineering and
directed-evolution processes, we seek to remove chemical
pathways in organisms that may create unwanted by-products. This
enables the elimination of purification steps in the chemical
manufacturing process thereby reducing capital costs.
CUSTOMERS
As of April 2011, we have entered into the following contracts
for the supply of biosuccinic acid as described below. Except as
noted, in each case, pricing is to be determined by future
negotiations with the
73
customers. However, we intend to develop and incorporate pricing
formulas into our contracts that will be based, in part, on the
market prices of certain reference commodities.
Johann Haltermann Ltd. We entered into a
supply agreement in June 2010 with Johann Haltermann Ltd., or
Haltermann, previously the Dow Haltermann Custom Processing
division of the Dow Chemical Company, to supply it with 100% of
its requirements for succinic acid, not to exceed
20 million pounds annually. Haltermann is a leading
supplier of a wide range of chemicals. Haltermann has been a
partner with virtually all of the top chemical, automotive, and
engine companies since 1981. Our contract with Haltermann has a
five-year term.
Piedmont Chemical Industries I, LLC. We
entered into a supply agreement in January 2010 with Piedmont
Chemical Industries I, LLC, or Piedmont, to supply it with
100% of its requirements for succinic acid, with a target volume
of five million pounds annually at a fluctuating price based in
part on certain reference commodities. Piedmont is a subsidiary
of Piedmont Chemical Industries, Inc., which has over seventy
years of experience in the chemical industry serving customers
in a wide range of specialty chemicals. Our contract with
Piedmont has a five-year term.
The Chemical Company. We entered into a supply
agreement in May 2010 with The Chemical Company, or TCC, to
supply it with 100% of its requirements for succinic acid, with
a target volume of five million pounds of biosuccinic acid
annually. TCC is a leading manufacturer and distributor of
specialty and commodity chemicals with over 40 years of
experience in the industry. Its chemicals are used in the
cosmetics, pharmaceuticals, petroleum, plastics, and food and
paper industries. Our contract with TCC has an initial five-year
term.
As of April 2011, we have entered into the following non-binding
arrangement for the supply of biosuccinic acid:
Showa Denko Europe GmbH. We entered into a
non-binding Heads of Agreement in February 2010 with Showa Denko
Europe GmbH, or Showa Denko, outlining a relationship in which
Showa Denko would purchase our biosuccinic acid in replacement
of petroleum-based succinic acid for use in the production of
polybutylene succinate (PBS), a type of biodegradable polyester,
outside of North America. The Heads of Agreement also sets the
terms by which we would work with Showa Denko to develop the
North American market for polybutylene succinate.
As of April 2011, we have entered into the following arrangement
for the supply of liquid ammonium sulfate:
Wilson Industrial Sales Company, Inc. We
entered into a supply agreement with Wilson Industrial Sales
Company, Inc., or Wilson, to supply it with 100% of the liquid
ammonium sulfate produced at the Louisiana Plant. Wilson is a
supplier of virgin chemicals to manufacturers in a variety of
industries. Liquid ammonium sulfate is a co-product of the
manufacturing of our biosuccinic acid. Wilson has agreed to
purchase all of the liquid ammonium sulfate produced at the
Louisiana Plant for a period of five years and three months,
beginning in October 2011, which is automatically renewable for
two year terms.
PARTNERSHIPS
Davy
Process Technology
Davy develops licenses and advanced process technologies for the
manufacture of oil and gas, petrochemicals, commodity chemicals,
fine chemicals and pharmaceuticals. Davy is a developer and
licensor of advanced process technologies for the commercial
production of BDO and its derivatives tetrahydrofuran (THF), and
gamma-buttyrolactone (GBL), which are all chemical
intermediaries used in the manufacture of elastic fibers
(Lycra®/Spandex®),
polyurethanes and other plastics. Davy has licensed 11 BDO
plants (18 trains) with an aggregate capacity in excess of
610,000 metric tons per year. The Davy licensed process has been
used in approximately 50% of all new BDO capacity built since
1992.
On February 9, 2011, we signed a non-binding memorandum of
understanding, or MOU, with Davy to enter into a definitive
joint development agreement. The agreement would provide for the
testing and approval of our biosuccinic acid as a drop-in
feedstock replacement for petroleum-derived maleic anhydride
within the Davy process. Under the proposed joint development
agreement, Davy would perform, at its expense, all of the
engineering,
74
development and pilot plant work at its facilities in Teeside,
England and, upon successful testing of our biosuccinic acid,
guarantee that our biosuccinic acid will work for the production
of BDO at commercial scale. This approach takes advantage of the
existing BDO plants that use the Davy process, enabling them to
use our biosuccinic acid in their production process without
significant additional capital expenditure. This would provide
us with an immediately available market for biosuccinic acid in
existing and future BDO plants that use the Davy process to
replace biosuccinic acid produced from MAN in the production of
BDO, representing a market opportunity for our biosuccinic acid
of 1.8 billion pounds per year. Our biosuccinic acid would
enable Davy process licensees to reduce their exposure to
petroleum price volatility and carbon footprint and sell a green
product.
In addition, we are collaborating exclusively with Davy to
integrate and optimize our biosuccinic acid technology with the
Davy BDO technology for the production of biobased BDO. This
development work will aim to further integrate and optimize our
biosuccinic acid production process with Davy’s BDO
production process by eliminating intermediate unit process
operations. If successful, we believe this process integration
would drive down our overall operating and capital costs for BDO
applications, further enhancing our competitive position in this
market.
The following graphic demonstrates the current integration of
our process and the Davy process for the production of biobased
BDO from sugar as well as the expected impact of our further
integration and optimization:
The technology and know-how necessary to implement Stage 2 in
the above graphic currently exist, but require further testing
to validate and implement. The technology and know-how necessary
to implement Stage 3 in the above graphic does not currently
exist and would need to be developed.
Uhde
GmbH, a subsidiary of the ThyssenKrupp Group
In September 2009 and October 2009, we signed two Alliance
Agreements with Uhde and its U.S. subsidiary. Uhde an
international engineering company focused on the design and
construction of chemical, refining, and other industrial plants.
Pursuant to the Alliance Agreements, we exclusively cooperate
with each other in our research and development efforts to
jointly develop and optimize our fermentation process and
Uhde’s separation process for the production of biosuccinic
acid in our planned production facilities, including at the
Louisiana Plant. We also exclusively cooperate in our business
development efforts to develop commercial scale biosuccinic acid
plants globally, subject to certain conditions and limitations.
Uhde has agreed to act exclusively as our engineering,
procurement and construction contractor for commercial scale
biosuccinic acid plants, except in certain limited
circumstances. Under the terms of the Alliance Agreements, if an
identified project requires the provision of process and
performance guarantees, Uhde will be prepared to negotiate such
guarantees on mutually agreeable terms, thus lowering our cost
of capital by facilitating access to project finance.
75
PTT
Chemical International Private Limited
In January 2011, we closed a $60 million strategic equity
investment by PTT Chemical International Private Limited, or CH
Inter, our largest stockholder and a subsidiary of PTT Chemical
Public Company Limited, or PTTCH, a large Thailand-based
petrochemical producer, which investment resulted in net
proceeds of $57.1 million. PTTCH, through its subsidiaries,
produces and distributes various petrochemical and chemical
products in Thailand and internationally. This investment
creates a strategic relationship with a major petrochemical
company in Asia that provides significant future partnering and
commercial opportunities for us throughout Asia.
In addition, we intend to establish a joint venture company with
PTTCH for the ASEAN countries (Brunei, Cambodia, Indonesia,
Laos, Malaysia, Myanmar, Philippines, Singapore, Thailand and
Vietnam), of which PTTCH would own 54% and we would own the
remaining 46%. Under the proposed joint venture and licensing
arrangement, we would grant a license to the joint venture
company to commercialize our intellectual property exclusively
within the ASEAN countries for which we would receive a
technology transfer fee, royalty income, maintenance fees and
our pro rata share of earnings of the joint venture company.
Specifically, the joint venture company would sublicense our
intellectual property for the production of biochemicals in the
ASEAN countries. PTTCH would have the primary responsibility to
develop, structure and scope the parameters of such projects for
consideration by the joint venture company. We would have the
right to participate in such projects as an equity investor or
sponsor provided our investment in any such project was limited
to our proportionate equity interest that we hold in the joint
venture company. In every case, we would receive priority
royalties and intellectual property maintenance fees from the
projects for the use of our intellectual property.
The joint venture also calls for a research and development
collaboration between PTTCH and us to, among other things,
validate and optimize our biocatalysts and regional feedstocks
in connection with our intellectual property for use in the
ASEAN countries. By combining PTTCH’s presence, research
and development capabilities and our technology and intellectual
property, we believe that the joint venture will further drive
technologies for the manufacturing of green chemicals using the
abundant high quality biobased feedstocks available in Thailand
and elsewhere in the ASEAN countries.
China
National BlueStar
On May 10, 2011, we signed a memorandum of understanding
with China National BlueStar (Group) Co. Ltd., or BlueStar, a
Davy process licensee for the production of BDO. BlueStar is
recognized as one of the leading BDO producers in Asia and will
have 120,000 metric tons of butanediol production capacity in
2011 at its BDO plant in Nanjing, China. Pursuant to the
memorandum of understanding, we intend to develop a proposal for
the construction of a biosuccinic acid plant to be located
adjacent to its plant and enter into an agreement for the
exclusive supply of biosuccinic acid to BlueStar. This proposed
jointly-owned biosuccinic acid plant would have the capacity to
produce 220 million pounds of biosuccinic acid on an annual
basis, of which 185 million pounds would be dedicated for
use in BlueStar’s BDO plant. We would plan to sell the
balance into the merchant market in that region.
Milestones
Achieved and Commercialization Roadmap
Our principal commercial milestones achieved to date and our
commercialization roadmap of future activities include:
| |
|
|
|
Commercial License to Purac
|
|
May 2008
|
|
We granted Purac, one of the world’s largest producers of
lactic acid, a full royalty-bearing exclusive license to use our
D(−) lactic acid strain technology to commercially produce
D(−) lactic acid.
|
|
D(−) Lactic Acid Commercial
Scale-Up
|
|
Summer 2008
|
|
Our first D(−) lactic acid strain was scaled up to
commercial scale (200,000 liter fermenters) at Purac’s
facility in Spain.
|
76
| |
|
|
|
OmniGene Bioproducts Acquisition
|
|
July 2008
|
|
We acquired equipment, organisms, intellectual property and
know-how, including the proprietary strain development systems
of OmniGene Bioproducts. In addition, we acquired the entire
OmniGene team of scientists and engineers with a long track
record of commercial success in molecular biology, cell biology
and fermentation technology (e.g., invention of the process to
manufacture riboflavin).
|
|
Successful Pilot Plant Fermentation
|
|
July 2009
|
|
We completed our first successful pilot fermentation of
biosuccinic acid to 5,000 liter fermenters at Wisconsin
Bioproducts.
|
|
Uhde Exclusive Alliance
|
|
September / October 2009
|
|
We signed a global exclusive partnership with Uhde for the
design, engineering, procurement and construction of biosuccinic
acid manufacturing plants and to provide process and performance
guarantees on mutually agreed terms for such plants.
|
|
$50 Million Department of Energy Award
|
|
December 2009
|
|
The U.S. Department of Energy awarded us $50 million in federal
cost share funding for our Louisiana Plant, which was the only
award focused exclusively on biobased chemicals rather than
biofuels.
|
|
Biosuccinic Acid
Scale-Up
|
|
|
|
December 2009
|
|
We scaled our biosuccinic acid process using 20,000 liter
fermenters while improving yield at the tolling plant owned by
Fermic in Mexico City
|
|
Showa Denko Heads of Agreement
|
|
May 2010
|
|
We entered into a non-binding Heads of Agreement with Showa
Denko. Pursuant to that Heads of Agreement, we intend to
establish a relationship in which Showa Denko would purchase our
biosuccinic acid for use in polybutylene succinate, or PBS,
production and we will work with Showa Denko to develop the
North American market for PBS.
|
|
First Customer Commitment to Purchase Biosuccinic
Acid
|
|
May 2010
|
|
We signed a contract with TCC to supply all of its biosuccinic
acid requirements with a target of 5 million pounds on an annual
basis from our Louisiana Plant.
|
|
Second Customer Commitment to Purchase Biosuccinic
Acid
|
|
June 2010
|
|
We signed a contract with Haltermann to supply all of its
succinic acid requirements of not more than 20 million
pounds on an annual basis from our Louisiana Plant.
|
|
PTT Chemical International Private Limited (CH Inter)
Strategic Investment
|
|
January 2011
|
|
CH Inter, a subsidiary of PTTCH, a large Thailand-based
petrochemical producer, made a $60 million strategic equity
investment in us, which resulted in net proceeds of
$57.1 million. We also agreed to establish a joint venture
company to license our intellectual property for construction of
biochemical plants in the ASEAN countries.
|
|
Third Customer Commitment to Purchase Biosuccinic
Acid
|
|
January 2011
|
|
We signed a contract with Piedmont to supply all of its succinic
acid requirements with a target of 5 million pounds on an annual
basis from our Louisiana Plant.
|
77
| |
|
|
|
Construction Phase of Louisiana Plant
|
|
January 2011
|
|
We entered Budget Period 2, the construction phase (pursuant to
the DOE award), of our Louisiana Plant.
|
|
Davy Exclusive Partnership
|
|
February 2011
|
|
We signed a non-binding memorandum of understanding with Davy
for the testing and approval of our biosuccinic acid technology
as a drop-in feedstock replacement for petroleum-derived MAN
within the Davy process for the production of biobased BDO. We
are negotiating a joint development agreement with Davy to
reflect the terms of this memorandum of understanding.
|
|
Commitment to Purchase Liquid Ammonium Sulfate
|
|
April 2011
|
|
We signed a contract with Wilson to supply it with all of the
liquid ammonium sulfate produced at the Louisiana Plant.
|
|
China National BlueStar Memorandum of Understanding
|
|
May 2011
|
|
We signed a memorandum of understanding with BlueStar to develop
a proposal for the construction of a biosuccinic acid plant in
Nanjing, China, and to enter into an agreement to be the
exclusive supplier of biosuccinic acid to BlueStar.
|
|
Louisiana Plant Commercial Operations
|
|
First Quarter 2013
|
|
We anticipate commencing commercial operations of the Louisiana
Plant.
|
|
Louisiana Plant Expansion
|
|
First Quarter 2014
|
|
We anticipate that the expanded Louisiana Plant, with an annual
production capacity of over 170 million pounds, will become
operational.
|
Our
Production Capabilities
Scale-up
and pilot plant operation
Over the past year and a half, we have produced 24 metric tons
of biosuccinic acid in support of the sampling and testing
programs of our current and prospective customers at the Fermic
tolling plant primarily using their 20,000 liter fermenters
while improving yield. To make these samples, we deployed a
process from feedstock to product, validating process economics
and generating the engineering data required for the
construction of a commercial scale plant. The commercial
scale-up of
the Louisiana Plant represents a two fold
scale-up
from the fermentation already commercialized by Purac in the
production of D(−) lactic acid based on our technology
platform. All the separation and purification equipment used is
off-the-shelf
and is customarily used at even larger scale for other organic
acids and sugar applications.
Louisiana
Plant
We plan to complete construction of the Louisiana Plant and
begin commercial operations during the first quarter of 2013. We
have completed preliminary engineering and design planning with
two multinational engineering, design and construction
companies: Uhde, which built Purac’s largest lactic acid
plant, and CH2M HILL, which built DuPont and Tate &
Lyle’s biopropanediol plant. We, along with the Lake
Providence Port Commission and the State of Louisiana, have
previously made over $13 million in improvements to the
site. The site has access to significant biomass feedstock
providers as well as rail and barge transportation. The
Department of Energy has conducted an Environmental Assessment
of the project and has issued a “Finding of No Significant
Impact” (FONSI) in December 2010. A state permit covering
the Louisiana Plant’s industrial wastewater discharges has
been issued, as has a minor source permit covering its air
pollutant emissions. It is not believed that any federal air
pollution control permits will be needed for the plant, given
the limited amount of expected emissions.
78
We plan to expand the Louisiana Plant to 170 million pounds
from its initial 30 million pound annual production
capacity and commence commercial operations by the end of the
first quarter of 2014. We are identifying the demand for this
additional capacity and are negotiating commercial supply
agreements. We believe the current group of potential customers
to which we are speaking will have demand in excess of the
planned expansion capacity of the Louisiana Plant. The
infrastructure for the 30 million pound Louisiana Plant
includes certain design elements intended to support the
expanded capacity of the plant.
We were awarded $50 million in 2009 by the
U.S. Department of Energy, or DOE, in federal cost share
funding under the American Recovery and Reinvestment Act of 2009
for the financing of the construction of the Louisiana Plant. We
were chosen as the only biobased chemical-focused, as opposed to
biofuels-focused, award recipient from hundreds of applicants.
Current cash on hand, including the $15.2 million in
construction cash contingency reserve that the DOE requires us
to maintain for the project, and government funds, including
$10 million in infrastructure improvements to be undertaken
by the Lake Providence Port Commission and the State of
Louisiana, will enable us to construct the 30 million pound
Louisiana Plant. Under the terms of the award, we are required,
and we intend, to use grain sorghum as the initial feedstock for
a period of approximately 1,100 hours, which we do not
anticipate will have an impact on our overall business plan. We
procured the services of Vogelbush USA under the terms of a
services agreement entered into in March 2010 for the process
engineering necessary to convert the grain sorghum into
fermentable sugars. The DOE award is structured as a cost-share
funding arrangement. During each design, construction and
operational phase, we submit certain of our plant-related
expenses for reimbursement by the DOE. The DOE matches our
expenditures related to the plant, including research and
development and certain other indirect corporate costs, up to a
predetermined limit during each phase, not to exceed
$50 million in the aggregate. This award was made in two
phases. The first phase of engineering and piloting, referred to
as Budget Period 1, or BP1, was completed on December 31,
2010 and we have received $10.4 million, the maximum amount
allowed for BP1. The second construction phase, referred to as
Budget Period 2, or BP2, with an allowable amount of
$39.6 million, is ongoing. Costs eligible for reimbursement
in BP2 include those related to the Louisiana Plant’s
construction, equipment purchases, testing and operation. As of
March 31, 2011, we have not received any of the
$39.6 million allowable under the award for BP2.
Future
Manufacturing Facilities
We recognize the importance of establishing biochemical
facilities located in close proximity to our customers and are
developing additional manufacturing plants.
We signed a memorandum of understanding with China National
BlueStar (Group) Co. Ltd., or BlueStar, a Davy process licensee
for the production of BDO, on May 10, 2011. BlueStar is
recognized as one of the leading BDO producers in Asia and will
have 120,000 metric tons of butanediol production capacity in
2011 at its plant in Nanjing, China. Pursuant to the memorandum
of understanding, we intend to develop a proposal for the
construction of a jointly-owned biosuccinic acid plant to be
located adjacent to BlueStar’s BDO plant in Nanjing, China,
and to enter into an agreement to be the exclusive supplier of
biosuccinic acid to BlueStar. This proposed biosuccinic acid
plant would have the capacity to produce 220 million pounds
of biosuccinic acid on an annual basis, of which
185 million pounds would be dedicated for use in
BlueStar’s BDO plant. We would plan to sell the balance
into the merchant market in that region.
We are also negotiating a letter of intent with Uhde for an
industrial biochemical facility for the production of
biosuccinic acid at the Infraleuna Chemical Site in Leuna,
Germany. The plant, which we expect would commence operations in
the first half of 2012, would utilize our technologies to
produce biosuccinic acid and ammonium sulfate in accordance with
our product specifications. In its first year of operation, we
expect the plant would produce 500 tons of biosuccinic acid
and 600 tons of ammonium sulfate. To date, we have begun
the technical analysis in conjunction with Uhde in order to
ready the plant for its intended purpose.
As part of our strategic relationship with CH Inter and PTTCH,
we are evaluating feedstocks and markets for the construction of
a plant in an ASEAN country to support PTTCH’s interest in
BDO, PBS and other potential chemical markets. We are also
continuing to evaluate the potential for the development of
additional biochemical manufacturing plants in a variety of
locations, including other parts of greater China, including
79
Taiwan, and Korea based on a number of factors, including
customer demand, feedstock accessibility, potential partners,
and access to capital.
INTELLECTUAL
PROPERTY
Our success depends in part on our proprietary technology for
which we seek intellectual property protection under patent,
copyright, trademark and trade secret laws and by contract
through confidentiality agreements, and our ability to operate
without infringing the proprietary rights of third parties.
Intellectual property protection of our technology is important
so that we may offer our customers and partners proprietary
products and services that are not available from our
competitors and so that we can exclude our competitors from
using technology that we have developed or exclusively licensed
from third parties. If competitors in our industry have access
to the same technology as we do, our competitive position may be
adversely affected.
As of July 21, 2011, we have filed three United States
provisional patent applications and five patent applications
under the Patent Cooperation Treaty. These patent applications
cover our technology, including biocatalysts and methods for
making our products, which support our chemical intermediates
business. In addition to our patent portfolio, we maintain
significant trade secrets and know-how related to processes and
systems for producing our products.
As of July 21, 2011, we have exclusively licensed rights to
two granted United States Patents and 33 pending patent
applications (including 12 national stage applications being
filed) in the United States and various foreign jurisdictions,
which licenses expire between November 5, 2023 and
November 21, 2031. These licensed patents and patent
applications are owned by the University of Florida and cover
enabling technology, including biocatalysts and methods for the
production of lactic acid, biosuccinic acid, malic acid, acetic
acid and pyruvic acid. Our licenses allow us to freely practice
the licensed invention.
We and our leadership have a long-standing working relationship
with the University of Florida for the development of technology
related to our business. We financially support research at the
University of Florida that is related to our business under a
Research Agreement and have a first option to license,
non-exclusively or exclusively at our election, any and all
technology developed under the Research Agreement. We cannot
assure you that additional technology will be developed under
the Research Agreement, or that we will exercise our option to
license any such technology.
We are aware of U.S. patents issued to third parties that
relate to biocatalysts and methods of using biocatalysts to
produce succinic acid. We believe that the properly construed
claims of the U.S. patents do not cover the biocatalyst or
processes we use to produce biosuccinic acid. If it is
determined that they do encompass our biocatalyst or processes,
we will likely be required to seek a license to these patents.
We are also aware of published U.S. and international
patent applications that are owned by third parties, which
relate to various biocatalysts, and methods for making and using
biocatalysts to produce succinic acid.
We cannot assure you that we will be successful in obtaining
licenses to the technology of third parties that may be required
or desirable to conduct our business in the future, or that such
technology can be licensed on terms agreeable to us and at a
reasonable cost. Our business may be adversely affected if we
are not successful in obtaining such licenses. It is possible
that the parties that currently license or sublicense patents to
us, or patents which we may later acquire, license or
sublicense, may be successfully challenged or invalidated in
whole or in part. It is also possible that we may not obtain
issued patents from our filed applications, and may not be able
to obtain patents that cover other inventions we seek to
protect. Under appropriate circumstances, we may sometimes
permit certain intellectual property to lapse or go abandoned.
Due to uncertainties inherent in prosecuting patent
applications, sometimes patent applications are rejected and we
may subsequently abandon them. It is also possible that we may
develop products or technologies that will not be patentable or
that the patents of others will limit or preclude our ability to
do business. In addition, any patent issued to us may provide us
with little or no competitive advantage, in which case we may
abandon such patent or license it to another entity. Our efforts
to protect our proprietary rights may not be adequate and our
competitors may independently develop technology or products
that are similar to or compete with ours. Patent, trademark and
trade secret laws afford only limited protection for our
80
technology platform and products. The laws of many countries do
not protect our proprietary rights to as great an extent as do
the laws of the U.S. Despite our efforts to protect our
proprietary rights, unauthorized parties may attempt to operate
using aspects of our intellectual property or products or to
obtain and use information that we regard as proprietary. Third
parties may also design around our proprietary rights, which may
render our protected technology and products less valuable. In
addition, if any of our products or technologies is covered by
third-party patents or other intellectual property rights, we
could be subject to various enforcement, infringement or other
legal actions. We cannot assure you that our technology
platform, biocatalysts, processes and products do not infringe
patents held by others or that they will not in the future.
Some of our technology is dependent upon proprietary information
that is developed through the knowledge, skills and expertise of
our scientific, research and technical personnel, which are not
patentable. We require our employees, advisors, and consultants
to execute confidentiality and invention assignment agreements
upon commencing a relationship with us, to protect our rights to
and maintain the confidentiality of our trade secrets and other
proprietary information. These agreements prohibit unauthorized
disclosure of confidential information, require assignment of
inventions and require disclosure of certain information,
including discoveries, ideas and inventions, made or developed
by our employees, advisors, and consultants. We cannot assure
you that these agreements will not be breached, or that our
trade secrets and proprietary information will not become known
to others or independently developed by others.
Litigation may be necessary to enforce our intellectual property
rights, to protect our trade secrets, to determine the validity
and scope of the proprietary rights of others or to defend
against claims of infringement, invalidity, misappropriation or
other allegations. Any such litigation could result in
substantial costs and diversion of our resources.
As of July 21, 2011, our trademark applications for
“Myriant Technology”, “Myriant”,
“Chemistry Refined Naturally” and a logo design have
been allowed at the United States Patent and Trademark Office.
The trademark “Myriant” has been registered in the
European Union.
COMPETITION
We expect that our products will compete in the industrial
biochemicals market. The major U.S. and European companies
in this market include BioAmber, Royal DSM N.V. (through a joint
venture with Roquette Fréres S.A.), BASF (through a
partnership with Purac) and Genomatica. We believe that our
products will also compete in the broader petrochemical market.
We believe that the primary competitive factors in the biobased
chemicals market are:
• cost of production;
• capital requirements;
• feedstock flexibility;
• technology, including yields and productivity;
• ability to develop drop-in and replacement products
for existing large markets; and
• ability to reach commercial production levels.
We believe that the primary competitive factors in the global
petrochemicals market are:
• cost of production;
• capital requirements;
• the price and reliability of the supply of
feedstock; and
• technology, including production yields.
Our competitors include large chemical companies. Many of these
are better capitalized, with larger research and development
departments and budgets, and have well-developed distribution
systems and
81
networks for their products. These companies have relationships
with our potential customers and have sales and marketing
programs in place to promote their products.
FACILITIES
Louisiana
Plant
On June 16, 2011, Myriant Lake Providence, Inc., our
wholly-owned subsidiary, entered into a lease (superceding and
replacing all prior agreements between the parties, their
predecessors and affiliates) with the Lake Providence Port
Commission, a political subdivision of the State of Louisiana,
for the premises located at 420 Port Road, Lake Providence,
Louisiana. The primary term of the lease is for a period
commencing on June 16, 2011, and continuing 20 years,
subject to extension by the Company for two additional terms of
10 years each.
Laboratories
Our research and development activities are concentrated in a
17,760 square foot laboratory located in Woburn,
Massachusetts. The lease for the laboratory expires in March
2013. The Woburn laboratory is equipped to conduct numerous
types of genetic engineering work to develop new biocatalysts
for the production of a number of biochemicals and testing the
yield and productivity of those new biocatalysts. In addition,
the Woburn laboratory has equipment required for the processing
of various biomass feedstocks and downstream processing of
fermentation broth to recover various biochemicals. Moreover, we
are also engaging contract research laboratories and academic
institutions to address specific technical issues in its
developmental programs.
Headquarters
Our corporate headquarters are located in Quincy, Massachusetts,
where we occupy approximately 11,000 square feet of office
space. The lease for the Quincy headquarters expires in January
2012.
REGULATORY
OVERVIEW
We are subject to various federal, state and local environmental
laws and regulations, including those relating to pollutant
discharges into the environment, the management of hazardous
materials, the protection of endangered species and the health
and safety of our employees. These laws and regulations require
the procurement of environmental permits in addition to those
already in place and compliance with health, safety and
environmental requirements for construction and operation of our
Louisiana Plant. In addition, future expansion of the plant may
require installation of pollution control equipment and
implementation of operational changes to limit environmental
impacts that are significant in cost. Furthermore, such laws,
regulations and permit conditions can result in substantial
liabilities and the potential for permit revocations and
facility shutdowns.
In addition to actual plant operations, liabilities could arise
from investigation and cleanup of environmental contamination at
the plant or at an off-site location where we arranged for the
disposal of hazardous substances generated at the Louisiana
Plant. We may also be subject to third party claims alleging
property damage or personal injury due to the release of or
exposure to such hazardous substances. However, we are not aware
of any material environmental liabilities relating to
contamination at the Louisiana Plant site which is situated on
previously undeveloped property.
In addition, new laws, new regulations, new interpretations of
existing laws or regulations, future governmental enforcement of
environmental laws or other developments could result in
significant expenditures. For example, promulgation of a more
stringent standard for an existing source of air pollution, or
of a standard for a newly identified pollutant, could trigger a
significant expenditure for procurement and operation of
pollution control equipment where emissions of the pollutant
from the Louisiana Plant exceed the relevant applicability
threshold. Future construction of a new facility, or expansion
of the Louisiana Plant, also might
82
trigger application of new standards not relevant in the past,
like those governing hazardous air pollutants. Such standards
may also limit our operational flexibility.
As a condition to granting the permits necessary for operating
our facilities, regulators could likewise make demands that
increase our construction and operating costs, and result in the
procurement of additional financing. In addition, failure to
obtain and comply with all applicable permits and licenses could
halt construction and subject us to future claims. We therefore
cannot guarantee procurement or compliance with the terms of all
permits needed to complete our Louisiana Plant.
EMPLOYEES
As of July 21, 2011, we had 66 employees, of which 36
were engaged in research and development activities. Of the 36
individuals engaged in research and development activities, 17
of them have Ph.D. degrees. Based on our current business plan,
we expect to increase the number of employees significantly,
particularly in the areas of research and development and
engineering and operations. None of our employees are
represented by a labor union and we consider our employee
relations to be good.
STRATEGIC
AND SCIENTIFIC ADVISORY PANELS
We have formed both a Strategic Advisory Panel and a Scientific
Advisory Panel. While members of these panels do not participate
in managing our operations, they provide us with advice,
insights, contacts and other assistance based on their extensive
experience and expertise.
Strategic
Advisory Panel
The company’s Strategic Advisory Panel advises the
company’s board of directors and management on the
company’s strategic decision making process and other
matters, including business and organizational development,
technology, policy and outreach. Members of our Strategic
Advisory Panel enter into a Strategic Advisory Panel Agreement
with us upon their appointment. This agreement is for a term of
one year and is automatically renewable for additional one year
periods unless either the panel or the member provides notice at
least 10 days prior to the anniversary date. Additionally,
the Panel or the member may terminate the agreement at any time
and for any reason. Members of the Strategic Advisory Panel are
compensated for their service to the company. Upon entering into
the agreement, the member is granted restricted stock of the
company in a value equal to $75,000, which is issued pursuant to
our 2011 Omnibus Equity Incentive Plan. Each year thereafter,
members are granted restricted stock of the company in a value
equal to $35,000. Members are also entitled to reimbursement
from the company for all reasonable
out-of-pocket
expenses incurred in connection with his or her services to the
Strategic Advisory Panel.
The current members of the Strategic Advisory Panel are as
follows:
Norman Augustine: Mr. Augustine has
decades of private and public sector experience, including
serving as the former President and Chief Executive Officer of
Lockheed Martin and as Acting Secretary of the Army. He was
recently named as chairman of the Review of United States Human
Space Flight Plans Committee, which is tasked to review
NASA’s plans for the Moon, Mars and beyond. He is a current
or former member of the Board of Directors of ConocoPhillips,
Black & Decker, Procter & Gamble and
Lockheed Martin, and of the Board of Trustees of Colonial
Williamsburg. He is a Regent of the University System of
Maryland, Trustee Emeritus of Johns Hopkins and a former member
of the Board of Trustees of the Massachusetts Institute of
Technology, or MIT, and Princeton University, his alma mater. He
is an Advisory Board member to the Department of Homeland
Security, was a member of the Hart/Rudman Commission on National
Security, and has served for 16 years on the
President’s Council of Advisors on Science and Technology.
He has been presented the National Medal of Technology and
received the Joint Chiefs of Staff Distinguished Public Service
Award. He is a five-time recipient of the Department of
Defense’s Distinguished Service Medal. He co-authored
The Defense Revolution and Shakespeare In Charge
and is the author of Augustine’s Laws and
Augustine’s Travels. He was selected by Who’s
Who in America and the Library of Congress as one of “Fifty
Great Americans.” He was Chairman and Principal Officer of
the American Red Cross for nine years, Chairman of the National
Academy of Engineering, President and
83
Chairman of the Association of the United States Army, Chairman
of the Aerospace Industries Association and Chairman of the
Defense Science Board. He is a former President of the American
Institute of Aeronautics and Astronautics and the Boy Scouts of
America.
Brian Ferguson: Mr. Ferguson is the
former CEO and Chairman of Eastman Chemical Company, a global
chemical company engaged in the manufacture and sale of a broad
portfolio of chemicals, plastics and fibers. He served as CEO
and Chairman of Eastman from January 2002 through June 2008, and
as Executive Chairman of the Board from June 2008 through
December 2010. Prior to his roles as CEO/Chairman, Ferguson was
President of Eastman’s Chemicals Group where he had direct
responsibility for all chemicals-based business organizations,
as well as the manufacturing, sales, pricing and product
management, technology and geographical aspects of the business
group. Ferguson graduated from Arizona State University with a
Bachelors Degree in Chemical Engineering in 1977.
Mr. Ferguson has served as a director of Nextera Energy,
Inc. (formerly FPL Group) since 2005. He was Chairman of the
American Chemistry Council in 2010, and was a member of the
Business Roundtable and the board of the National Association of
Manufacturers prior to his retirement from Eastman.
Robert McFarlane: After a distinguished career
of public service, culminating in his appointment to President
Ronald Reagan’s cabinet as his National Security Advisor,
Robert McFarlane founded his own energy development company,
Global Energy Investors LLC, sponsoring major international
power projects in Brazil, Pakistan, the Philippines and China.
He has also served as a consultant to foreign governments on
energy, infrastructure and privatization policies.
Mr. McFarlane has been affiliated with the company since
its inception. Apart from his business activities,
Mr. McFarlane remains a respected figure in international
affairs. He is frequently called upon to advise the
U.S. and other governments, the U.S. Congress and the
news media. Prior to his career in the public sector,
Mr. McFarlane saw extensive combat as a Marine Corps
officer after graduation from the U.S. Naval Academy. Later
he was chosen as an Olmsted Scholar and was awarded a
Master’s Degree in Strategic Studies from the Institut de
Hautes Études Internationales in Switzerland.
R. James Woolsey: R. James Woolsey is Chairman
of Woolsey Partners LLC and a Venture Partner with Lux Capital
Management. He also Chairs the Board of the Foundation for
Defense of Democracies, is a Senior Fellow at Yale
University’s Jackson Institute for Global Affairs, Of
Counsel to the law firm of Goodwin Procter, and chairman of the
Strategic Advisory Group of Paladin Capital Corporation. He
previously was a Venture Partner and Senior Advisor to
VantagePoint Venture Partners. In 2009 he was the Annenberg
Distinguished Visiting Fellow at the Hoover Institution at
Stanford University. Before he joined VantagePoint in March
2008, Mr. Woolsey was a Partner with Booz Allen Hamilton in
McLean, Virginia, specializing in energy and security issues,
and prior to that a partner with Shea & Gardner in
Washington, D.C., specializing in commercial litigation and
alternative dispute resolution (arbitration and mediation). He
practiced at the firm for 22 years on four different
occasions and served in the U.S. Government five times for
12 years, holding Presidential appointments in two
Republican and two Democratic administrations. He has served on
numerous corporate and non-profit boards. From time to time, he
speaks publicly and contributes articles to newspapers and other
periodicals on such issues as national security, energy, foreign
affairs and intelligence.
Scientific
Advisory Panel
The company’s Scientific Advisory Panel advises the
company’s board of directors and management as to the
technology, business, organizational development, policy and
outreach related to proprietary cellulosic technology and
patented microbial fermentation, including the strategy of
commercializing biorefineries with biocatalysts that convert low
cost sugars into targeted, high-value renewable biochemicals and
biofuels, and makes non-binding recommendations to the board of
directors and management of the company. Members of our
Scientific Advisory Panel enter into a Scientific Advisory Panel
Agreement with us upon their appointment. This agreement is for
a term of three years and is renewable for additional three year
periods at our invitation and the acceptance by the member.
Members of the Scientific Advisory Panel are compensated for
their service to the company. Members receive an annual stipend
in a value equal to $25,000, 60% of which is comprised of
restricted stock issued pursuant to our 2011 Omnibus Equity
Incentive Plan, and 40% of which is comprised of cash.
Additionally, members are entitled to a payment of $2,000 per
meeting day for attendance at any Scientific
84
Advisory Panel meetings. Members are also entitled to
reimbursement from the Company for all reasonable
out-of-pocket
expenses incurred in connection with his or her services to the
Scientific Advisory Panel.
The current members of the Scientific Advisory Panel are as
follows:
Dr. Stephen J.
Benkovic: Dr. Benkovic is an Evan Pugh
Professor of Chemistry and Eberly Chair in Chemistry at The
Pennsylvania State University, and has focused on the assembly
and kinetic characteristics of the proteins responsible for DNA
replication; the importance of dynamic coupling of proximal and
distal residues in the catalytic cycle of dihydrofolate
reductase; the intracellular observation of de novo
purine biosynthesis, and the development of novel cyclic
peptides for modulating protein/protein interactions. Benkovic
has 555 publications and is the recipient of the 2009 National
Medal of Science and the National Academy of Science Award in
Chemical Sciences in 2011.
Dr. Lew Christopher: Dr. Christopher
is Director of the Center for Bioprocessing Research and
Development, and Associate Professor of Chemical and Biological
Engineering, South Dakota School of Mines and Technology, Rapid
City, South Dakota. After earning his Ph.D. degree, he moved to
South Africa where he held positions with the University of the
Free State, South Africa, beginning as a researcher with the
Department of Microbial, Biochemical and Food Microbiology.
Subsequently, Dr. Christopher held positions of increasing
responsibility. In 2004 he was appointed Professor and Head of
Sappi Biotechnology Laboratory and in 2007, Managing Director of
Biorefineries, International, South Africa. Dr. Christopher
is a prolific scientist, having published 53 peer reviewed
articles, one book, and nine book chapters. He holds seven
patents, has given 150 presentations at national and
international meetings and 20 invited lectures in Europe, India,
Japan, South Africa and the United States.
Dr. Lonnie Ingram: Dr. Lonnie Ingram
is currently a Distinguished Professor of Microbiology at the
University of Florida and Director of The University of Florida
Center for Renewable Chemicals. Dr. Ingram’s team
developed the four patents that serve as our critical technology
base. Dr. Ingram’s research has centered on the
production of ethanol for automotive fuels and value-added
chemicals from renewable cellulosic waste materials (yard trash,
sugarcane bagasse, citrus pulp, etc.). His research has been
reported in over 200 publications concerning various aspects of
biotechnology with more than 20 issued national and
international patents, including the U.S. Landmark Patent
No. 5,000,000 for bioethanol. Dr. Ingram is a member
of the U.S. National Academy of Sciences, a fellow of the
Society of Industrial Microbiology, and a member the American
Academy of Microbiology. His research achievements have been
recognized by the U.S. Department of Agriculture, or USDA, in
the form of the Distinguished Service Award, the highest award
presented for research by USDA.
Dr. Gregory
Stephanopoulos: Dr. Stephanopoulos is a
Professor of Chemical Engineering at MIT. He has also been the
Taplin Professor at the Harvard-MIT Division of Health Sciences
and Technology, or HST, since 2001, Instructor of Bioengineering
at Harvard Medical School since 1997, and the W.H. Dow Professor
of Chemical Engineering and Biotechnology at MIT. Upon finishing
his doctorate in 1978, Dr. Stephanopoulos joined the
chemical engineering faculty of the California Institute of
Technology, or Caltech, and in 1985 he was appointed Professor
of Chemical Engineering at MIT where he has since remained. He
has served as an Associate Director of the Biotechnology Process
Engineering Center from 1990 to 1997 and a Member of the
International Faculty of the Technical University of Denmark in
2001. Dr. Stephanopoulos’ current research focuses on
two areas including metabolic engineering and its applications
to the production of biochemicals and specialty chemicals, as
well as mammalian cell physiology as it pertains to diabetes and
metabolism.
Dr. Janet
Westpheling: Dr. Westpheling is currently a
Professor in the Department of Genetics at the University of
Georgia. Dr. Westpheling’s research relates to
rate-limiting step in the conversion of lignocellulosic biomass
from crop plants such as poplar or switchgrass to biofuels, such
as ethanol and biomaterials, the recalcitrance of these complex
substrates. Dr. Westpheling is on the editorial board of
the Journal of Bacteriology. In 2005, Dr. Westpheling was a
member of the National Research Council
85
Committee on the Development and Acquisition of Medical
Countermeasures Against Biological Warfare Agents. In 2000,
Dr. Westpheling received the Creative Research Medal from
the University of Georgia.
Dr. Huimin Zhao: Dr. Zhao has been
the Centennial Endowed Chair Professor of chemical and
biomolecular engineering at the University of Illinois at
Urbana-Champaign (UIUC) since August 2008. Prior to joining UIUC
in 2000, he was a project leader at the Industrial Biotechnology
Laboratory of the Dow Chemical Company. Dr. Zhao has
authored and co-authored over 100 research articles and 15
pending or issued patents. Dr. Zhao served as a consultant
for over 10 companies. His primary research interests are
in the development and applications of synthetic biology tools
to address society’s most daunting challenges in human
health and energy.
LEGAL
PROCEEDINGS
We are not currently party to any material legal proceedings.
86
Management
EXECUTIVE
OFFICERS, KEY EMPLOYEES AND DIRECTORS
The following table sets forth certain information about our
current executive officers and directors and their ages as of
March 31, 2011.
| |
|
|
|
|
|
|
|
Name
|
|
Age
|
|
Position(s)
|
|
|
|
Stephen J. Gatto
|
|
|
50
|
|
|
Chairman, Chief Executive Officer and Director
|
|
Ralph A. Tapia
|
|
|
60
|
|
|
Executive Vice President and Chief Financial Officer
|
|
A. Cenan Ozmeral, Ph.D.
|
|
|
61
|
|
|
Executive Vice President and Chief Operating Officer
|
|
Joseph P. Glas, Ph.D.
|
|
|
73
|
|
|
Chief Technology Officer
|
|
Samuel McConnell
|
|
|
51
|
|
|
Senior Vice President, Corporate Development
|
|
Jeffrey Gatto
|
|
|
51
|
|
|
Senior Vice President, Engineering and Operations
|
|
Rudy E. Fogleman, Jr.
|
|
|
61
|
|
|
Vice President of Manufacturing, North America
|
|
Puntip Oungpasuk
|
|
|
50
|
|
|
Director
|
|
Narongsak Jivakanun(1)
|
|
|
42
|
|
|
Director
|
|
Thitipong Jurapornsiridee(2)
|
|
|
41
|
|
|
Director
|
|
Steven M. Sisselman(1)(2)
|
|
|
52
|
|
|
Director
|
|
Keith Carter(1)(2)
|
|
|
38
|
|
|
Director
|
|
|
|
|
(1) |
|
Member of the compensation committee |
| |
|
(2) |
|
Member of the audit committee |
Stephen J. Gatto has served as a director of the company
since July 16, 2009 and has served as Chairman and Chief
Executive Officer of the company since July 16, 2009.
Mr. Gatto formed the company on April 3, 2009, which
was spun off from the Predecessor Company, which Mr. Gatto
formed in 2004 and became its Chief Executive Offier and
Chairman of the Board of Directors. Pursuant to his employment
agreement with the company, Mr. Gatto is required to devote
substantially all of his business time to the performance of his
duties to the company. Accordingly, Mr. Gatto has no
continuing operational role with the Predecessor Company and
resigned as its Chief Executive Officer in June 2011. He remains
an investor in the Predecessor Company and its Chairman. The
company does not envision any potential conflicts of interest
with the Predecessor Company and our board and management would
address any such conflict in accordance with their fiduciary
duties and the company’s code of business conduct and
ethics and related person transaction policy that the company
intends to adopt upon completion of this offering,
Mr. Gatto has been involved in the biotechnology industry
for over 19 years, including founding a biofuels company
and serving on numerous Congressional and United States
Department of Energy and Department of Agriculture committees.
Mr. Gatto earned an Associate of Science degree in
Information Services and a Bachelor of Science degree in
Marketing, both from Southern New Hampshire University. The
board concluded that Mr. Gatto should serve as director because
of his significant management and leadership experience in the
biotechnology industry and his extensive marketing and
operational expertise.
Ralph A. Tapia has served as Executive Vice President and
Chief Financial Officer of the company since May 2011.
Mr. Tapia previously served as our Vice President,
Corporate Controller of the company since September 2006. Prior
to his joining the company, Mr. Tapia explored a career in
financial planning, serving as an uncompensated employee of
Waddell Reed from May 2006 to August 2006. In all,
Mr. Tapia has over 25 years of experience in corporate
accounting and financial management. In the past he has
financially managed energy, water/wastewater and biofuel
construction projects valued in excess of $1.5 billion,
covering site work to full operations, and has been a Vice
President, Finance at three NYSE listed companies.
Mr. Tapia earned a Bachelor of Science degree in Accounting
from San Jose State University and a Master of Business
Administration degree from Southern New Hampshire University.
87
Rudy E. Fogleman, Jr. has served as our Vice President of
Manufacturing, North America since July 2006. Prior to joining
the company, Mr. Fogleman was General Manager at BCI
Louisiana from 1994 to July 2006, and General Manager of Biocom
USA Limited from 1988 to 1994. In total, Mr. Fogleman has
over 21 years of experience in operating ethanol plants,
including biomass-based pilot plant operations and
100 million gallon grain alcohol sites. Mr. Fogleman
received a Bachelor of Science degree in Business Administration
and a Master of Business Administration from the University of
Southwestern Louisiana (Lafayette).
A. Cenan Ozmeral, Ph.D. has served as Executive
Vice President and Chief Operating Officer of the company since
March 2011, prior to which Dr. Ozmeral served as Executive
Vice President and General Manager of Specialty Chemicals since
November 2008. Prior to Dr. Ozmeral’s involvement with
the company, Dr. Ozmeral spent 29 years at BASF, a
leading chemical manufacturer, where he developed BASF’s
specialty chemicals and polymers business. While at BASF,
Dr. Ozmeral oversaw several major capital investments and
mergers and acquisitions by BASF, and, as Vice President,
managed business units in Petrochemicals, Industrial Organics
and Plasticizer Chemicals between 1997 and 2005. From 2005 to
November 2008, Dr. Ozmeral served as Vice President Acrylic
Dispersions and XSB Latex Business, NAFTA Region, and was
responsible for both manufacturing and research and development
operations, had full profit and loss responsibility and oversaw
the activities of 25 scientists and 75 technicians. He was also
responsible for 1,4 butanediol and derivatives marketing and
sales in North America and gained extensive experience in
marketing of specialty diols, intermediates and agrochemicals.
Dr. Ozmeral holds a Master of Business Administration
degree from Louisiana State University, a Ph.D. from the
University of New Orleans, a Master of Science degree from
Pennsylvania State University and a Bachelor of Science degree
from Robert College, all in Chemistry.
Joseph P. Glas, Ph.D. has served as Chief Technology
Officer of the company since February 7, 2011. From 2005 to
February 2011, Dr. Glas served as our Executive Vice
President of Research and Development. Prior to joining the
company, from 1999 to 2002, Dr. Glas was Senior Vice
President of Research & Development for BC
International. Prior to BC International, Dr. Glas has
34 years of experience with E.I. du Pont de Nemours and
Company, or DuPont, as Vice President and General Manager of
DuPont Biotechnology, including a senior role in the formation
of DuPont Life Sciences, during which time DuPont’s
bio-propanediol technology was being developed. Dr. Glas
was also the Vice President and General Manager of DuPont’s
fluorocarbon business where he spearheaded the development of
substitute products that did not cause deterioration of the
ozone layer. Dr. Glas received his Master of Science degree
and Ph. D. in Chemical Engineering from University of Illinois
and his Bachelor of Arts degree in Chemistry from Rockhurst
College.
Samuel McConnell has served as Senior Vice President,
Corporate Development of the company since August 2006. From
2002 to July 2006, Mr. McConnell served as a Managing
Director at Decker Energy International overseeing power plant
acquisitions, greenfield development, and financing. Prior to
that, he worked for various companies in the independent power
industry that focused on renewable energy, such as Wheelabrator
Technologies and Citizens Power. Mr. McConnell has
20 years of experience in project finance and development,
and has experience in greenfield development, acquisitions,
power marketing/contract restructuring, fuel supply, project
finance and asset optimization. Mr. McConnell received a
Bachelor of Arts degree in Economics from the University of
Virginia and a Master of Business Administration degree from
Cornell University’s Johnson Graduate School of Management.
Jeffrey Gatto has served as Senior Vice President,
Engineering and Operations of the company since April 1,
2011, and previously served in an acting capacity for the same
role as a consultant from July 2010 through March 2011. Prior to
his involvement with the company, from September 2008 to May
2010, Mr. Gatto was Vice President of Supply Chain and
Operational Excellence at Tyco International; from 1999 to
September 2008, he held several Vice President positions at
3Com, and from 1997 to 1999, he served as Vice President of
Operations for BC International, and successfully piloted and
scaled up BCI’s C5 hydrolysis and fermentation technology.
Mr. Gatto has over 25 years of international
operations experience in high technology industries, with
expertise in engineering and
scale-up of
new technologies, supply chain transformation, divisional
management, and strategic planning. Mr. Gatto received a
Masters in Electrical Engineering from Syracuse University, and
a Bachelors of Mechanical Engineering from Tufts University.
88
Puntip Oungpasuk has served as a director of the company
since January 13, 2011. Since 2005, Mrs. Oungpasuk has
been Executive Vice President, Strategy &
International Affairs of PTT Chemical Public Company Limited, or
PTTCH. Mrs. Oungpasuk has been instrumental in driving the
growth and development of PTTCH since 2005. Mrs. Oungpasuk
currently oversees PTTCH’s business strategy, business
development, corporate planning, corporate strategy and
portfolio management, science and innovation, and international
businesses. From 2003 to 2005, Mrs. Oungpasuk was Senior
Vice President and Chief of Corporate Finance and Strategy of
Thai Olefins Plc. At present, Mrs. Oungpasuk also serves as
director for various companies such as PTT Chemical
International Private Limited, PTT Chemical International (Asia
Pacific ROH) Limited, Bio Creation Co., Ltd., Emery
Oleochemicals (M) Sdn. Bhd. and Vinythai Public Company
Limited. Mrs. Oungpasuk received a B.S. in Chemical
Engineering from Prince Songkhla University in Thailand and
received her Master of Business Administration from Thammasart
University in Thailand. Mrs. Oungpasuk brings to the board
valuable corporate development and strategic planning expertise
acquired during the course of her career in the Thai chemicals
industry.
Narongsak Jivakanun has served as a director of the
company since January 13, 2011. Since January 2010,
Mr. Jivakanun has been the Chief Executive Officer of PTT
Chemical International Private Limited — the
international investment arm of PTTCH and, since October 2009,
has also been the Chief Executive Officer of PTT Chemical
International (Asia Pacific ROH) Limited. From February 2008 to
September 2009, Mr. Jivakanun served as Vice President,
International Business for PTTCH, the parent company of PTT
Chemical International Private Limited, with extensive
experience in the petrochemical, chemicals, oil refinery and
industrial gases industry, in business and project development,
strategy and planning roles. From May 2007 to January 2008,
Mr. Jivakanun served as special project head for PTTCH.
From 1997 to April 2007, Mr. Jivakanun served as project
development director (last position) for Thai Industrial Gases
Public Company Limited. Mr. Jivakanun also serves as a
director of Emery Oleochemicals (M) Sdn Bhd (for which he
serves as an alternate director), Emery Oleochemicals HK
Limited, Bio Creation Company Limited, Bio Spectrum Company
Limited (for which he serves as chairman of the board of
directors) and Sermkit Textile Company Limited. Previously,
Mr. Jivakanun served as a director of TIG HyCO Limited, a
position which he resigned in 2007. Mr. Jivakanun received
his Master of Science in Chemical Engineering from Oregon State
University and his Bachelor of Science in Chemical Engineering
from Chulalongkorn University in Thailand. Mr. Jivakanun
brings to the board more than ten years of development and
strategic planning expertise acquired in various executive
leadership roles in the petrochemicals industry.
Thitipong Jurapornsiridee has served as a director of the
company since January 13, 2011. Since July 2009,
Mr. Jurapornsiridee has been the Vice President —
Corporate Finance Policies and Investor Relations for PTTCH and
he previously served in an acting capacity in that role from
July 2008 to June 2009. Mr. Jurapornsiridee also serves as
a director of Bio Spectrum Co., Ltd. From December 2005 to July
2008, Mr. Jurapornsiridee was Investor Relations
Division Manager for PTTCH. His experience covers financial
strategies and investor relations, particularly in the olefins
and petrochemical industries. Mr. Jurpornsiridee’s
experience in investor relations and in the petrochemicals
industry along with his accounting and financial management
expertise make him a unique and valuable resource for the
Company.
Steven M. Sisselman has served as a director of the
company since July 16, 2009. From January 2006 to the
present, Mr. Sisselman has served as the Executive Vice
President and Chief Operating Officer of both Itera
International Energy Corp, as well as its parent company, Itera
USA, Inc. From January 1996 to December 2005, he served as Vice
President of Business Development with Itera International
Energy Corp., a subsidiary of The Itera International Group of
companies, operating in the United States natural gas, real
estate and commodity business, where he was responsible for
strategic acquisitions in the Western Hemisphere. Since May
2004, Mr. Sisselman has served as a director of Dune
Energy, Inc. and is currently Chairman of the Corporate
Governance Committee. Dune Energy, Inc. is engaged in oil and
gas exploration and production in the U.S. Gulf Coast.
Mr. Sisselman received a Bachelor of Science degree in
Business Administration with a major in Finance from the
University of Florida. Mr. Sisselman was chosen as director
because of his
15-year
tenure in the energy industry and his corresponding knowledge of
various operational and development aspects of the energy
industry.
89
Keith Carter has served as a director of the company
since July 21, 2010. Since July 2006, Mr. Carter has
been a Managing Director at Plainfield Asset Management, a hedge
fund based in Greenwich, Connecticut where he is responsible for
private and public market investments in the industrial and
financial sectors. Prior to joining Plainfield in July 2006,
Mr. Carter was a Vice President from 2005 to 2006 of
Briscoe Capital Management LLC, a hedge fund focused on
non-investment grade leveraged loans to companies owned by
financial sponsors. From 2001 to 2005, Mr. Carter was an
Associate at Heartland Industrial Partners, a private equity
fund in Greenwich, Connecticut, which specialized in industrial
investments. While at Heartland, Mr. Carter served for two
years as Director of Corporate Development for Metaldyne
Corporation, an auto parts manufacturer in Detroit, Michigan.
From 1998 to 2001, Mr. Carter was an Associate in the
Leveraged Finance Group at Deutsche Banc Alex Brown, formerly
Bankers Trust Alex Brown. From 1996 to 1998,
Mr. Carter worked in the Fixed Income Mortgage Group at
Morgan Stanley & Co. Mr. Carter started his
career in 1994 as an Analyst at S.N. Phelps & Co., a
distressed fund in Greenwich, Connecticut. Currently,
Mr. Carter is also a member of the board of directors of
Wolverine Tube, Inc. Mr. Carter received a Master of
Business Administration degree from Columbia University’s
Graduate School of Business and a Bachelor of Arts degree from
St. Lawrence University. Mr. Carter has a strong background
in finance and management and thus brings to the board valuable
insight into various aspects of the Company’s business.
Stephen J. Gatto and Jeffrey Gatto are brothers.
BOARD
COMPOSITION
We currently have six directors, of which three are designated
by the current holders of our Class A common stock and
three are designated by the current holders of our Class B
common stock. Under the terms of our amended and restated
certificate of incorporation and the voting agreement among us,
the holders of our Class A common stock, Class B
common stock and certain of the holders of our common stock
designate the members of our board of directors. Upon the
completion of this offering, all of these provisions will
terminate and there will be no further contractual obligations
regarding the election of our directors.
Under the terms of our second amended and restated certificate
of incorporation and amended and restated bylaws to become
effective upon completion of this offering, our board may
establish the authorized number of directors from time to time
by resolution. Upon the completion of this offering, our board
plans to increase the authorized number of directors on our
board of directors to nine, which will be divided into three
classes with staggered three-year terms. Independent directors
will be appointed to fill the seats created by the increase in
the size of the board of directors. At each annual meeting of
stockholders, the successors to directors whose terms then
expire will be elected to serve from the time of election and
qualification until the third annual meeting following election.
After the completion of this offering, our directors will be
divided among the three classes as follows:
|
|
|
| |
•
|
the Class I directors will be Puntip Oungpasuk, Keith
Carter, and an additional director to be appointed, and their
terms will expire at the annual meeting of stockholders to be
held in 2012;
|
| |
| |
•
|
the Class II directors will be Thitipong Jurapornsiridee,
Steven M. Sisselman, and an additional director to be appointed,
and their terms will expire at the annual meeting of
stockholders to be held in 2013; and
|
| |
| |
•
|
the Class III directors will be Stephen J. Gatto, Narongsak
Jivakanun, and an additional director to be appointed, and their
terms will expire at the annual meeting of stockholders to be
held in 2014.
|
Any additional directorships resulting from an increase in the
number of directors will be distributed among the three classes
so that, as nearly as possible, each class will consist of
one-third of the directors. The division of our board of
directors into three classes with staggered three-year terms may
delay or prevent a change of our management or a change of
control at our company.
Our second amended and restated certificate of incorporation
will provide that the authorized number of directors may be
changed only by resolution of the board of directors. In
addition, our second amended and restated certificate of
incorporation and amended and restated bylaws will provide that
our directors may be removed only for cause by the affirmative
vote of the holders of at least a majority of the votes that all
our
90
stockholders would be entitled to cast in an annual election of
directors. Any vacancy on our board of directors, including a
vacancy resulting from an enlargement of our board of directors,
may be filled only by vote of a majority of our directors then
in office.
DIRECTOR
INDEPENDENCE
Under Rule 5605 and Rule 5615(b) of The Nasdaq Stock
Market, independent directors must comprise a majority of a
listed company’s board of directors within one year of
listing. In addition, The Nasdaq Stock Market rules require
that, subject to specified exceptions, each member of a listed
company’s audit committee (and, if any, its compensation
and nominating and governance committees) be independent. Audit
committee members must also satisfy the independence criteria
set forth in
Rule 10A-3
under the Securities Exchange Act of 1934, as amended, or the
Exchange Act. Under Rule 5605(a)(2) of The Nasdaq Stock
Market, a director will only qualify as an “independent
director” if, in the opinion of that company’s board
of directors, that person does not have a relationship that
would interfere with the exercise of independent judgment in
carrying out the responsibilities of a director. To be
considered to be independent for purposes of
Rule 10A-3,
a member of an audit committee of a listed company may not,
other than in his or her capacity as a member of the audit
committee, the board of directors, or any other board committee:
(i) accept, directly or indirectly, any consulting,
advisory, or other compensatory fee from the listed company or
any of its subsidiaries; or (ii) be an affiliated person of
the listed company or any of its subsidiaries.
Our board of directors undertook a review of its composition,
the composition of its committees and the independence of each
director. Based upon information requested from and provided by
each director concerning his background, employment and
affiliations, including family relationships, our board of
directors has determined that Messrs. Sisselman and Carter, who
are current non-employee directors, have no relationship that
would interfere with the exercise of independent judgment in
carrying out the responsibilities of a director and that each of
these directors is “independent” as that term is
defined under Nasdaq Rule 5605(a)(2). In making this
determination, our board of directors considered the
relationships that each non-employee director and nominee has
with our company and all other facts and circumstances our board
of directors deemed relevant in determining their independence,
including the beneficial ownership of our capital stock by each
non-employee director and nominee.
BOARD
COMMITTEES
Our board of directors has established standing audit and
compensation committees, each of which will have the composition
and responsibilities described below upon the closing of this
offering.
Audit
committee
Our audit committee oversees our corporate accounting and
financial reporting process. Upon the completion of this
offering, the audit committee will operate under a written
charter that satisfies the applicable standards of the SEC and
The Nasdaq Stock Market, and which provides that, among other
matters, the audit committee will appoint the independent
registered public accounting firm; evaluate the independent
registered public accounting firm’s qualifications,
independence and performance; determine the engagement of the
independent registered public accounting firm; review and
approve the scope of the annual audit and audit fee; discuss
with management and the independent registered public accounting
firm the results of the annual audit and the review of our
quarterly consolidated financial statements; approve the
retention of the independent registered public accounting firm
to perform any proposed permissible non-audit services; monitor
the rotation of partners of the independent registered public
accounting firm on our engagement team as required by law;
review our consolidated financial statements and our
management’s discussion and analysis of financial condition
and results of operations to be included in our annual and
quarterly reports to be filed with the SEC; review our critical
accounting policies and estimates; and annually review the audit
committee charter and the committee’s performance. The
members of our audit committee include Mr. Jurapornsiridee,
Mr. Sisselman and Mr. Carter, each currently a
non-employee member of our board of directors. Prior to the
completion of this offering, our board of directors will have
made a determination as to whether each of the committee members
meets the requirements for independence and financial literacy
applicable to audit
91
committee members under rules and regulations of The Nasdaq
Stock Market and the SEC, including the independence criteria
set forth in
Rule 10A-3
under the Exchange Act. We envision that one of the new
independent directors will also sit on this committee and will
meet these requirements.
Compensation
committee
Our compensation committee reviews and recommends policies
relating to compensation and benefits of our officers and
employees. The compensation committee operates under a written
charter under which the committee reviews and approves corporate
goals and objectives relevant to compensation of our chief
executive officer and other executive officers, evaluates the
performance of these officers in light of those goals and
objectives, and sets the compensation of these officers based on
such evaluations. The compensation committee has the authority
after the offering to grant stock options and other awards under
our stock plans. The compensation committee will review and
evaluate, at least annually, the performance of the compensation
committee and its members, including compliance of the
compensation committee with its charter. The members of our
compensation committee will include Mr. Jivakanur,
Mr. Sisselman and Mr. Carter, each currently a
non-employee member of our board of directors. Prior to the
completion of this offering, our board of directors will have
made a determination as to whether each of the committee members
meets the requirements for independence under the applicable
rules and regulations of The Nasdaq Stock Market. We envision
that one of the new independent directors will also sit on this
committee and will meet these requirements.
CODE OF
BUSINESS CONDUCT AND ETHICS
Our board of directors will adopt a code of business conduct and
ethics in connection with this offering. The code will apply to
all of our employees, officers (including our principal
executive officer, principal financial officer, principal
accounting officer or controller, or persons performing similar
functions), including directors and consultants. Upon the
effectiveness of the registration statement of which this
prospectus forms a part, the full text of our code of business
conduct and ethics and the charters of our standing committees
will be posted on our website at www.myriant.com. We expect that
any amendments to the code, or any waivers of its requirements,
will be disclosed on our website. The inclusion of our website
address in this prospectus does not include or incorporate by
reference the information on our website into this prospectus.
CORPORATE
GOVERNANCE GUIDELINES
Our board of directors has adopted corporate governance
guidelines to be effective upon the closing of this offering to
assist the board in the exercise of its duties and
responsibilities and to serve the best interests of our company
and our stockholders. Upon the closing of this offering, these
guidelines, which provide a framework for the conduct of our
board’s business, will provide:
|
|
|
| |
•
|
that the board of directors’ principal responsibility is to
oversee the management of the company;
|
| |
| |
•
|
criteria for board membership;
|
| |
| |
•
|
that a majority of the members of the board shall be independent
directors;
|
| |
| |
•
|
limits on a board member’s service on boards of directors
of other public companies;
|
| |
| |
•
|
for the appointment of a lead independent director;
|
| |
| |
•
|
that the independent directors meet regularly in executive
session;
|
| |
| |
•
|
that at least annually, the board and its committees will
conduct a self-evaluation; and
|
| |
| |
•
|
that directors have complete access to all officers and
employees.
|
COMPENSATION
COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
None of the members of our compensation committee is or has been
an officer or employee of our company. None of our executive
officers currently serves, or in the past year has served, as a
member of the
92
board of directors or compensation committee (or other committee
serving an equivalent function) of any entity that has one or
more executive officers serving on our board of directors or
compensation committee. See “Certain relationships and
related person transactions” for a description of related
party transactions and relationships since our formation.
DIRECTOR
COMPENSATION
Members of our board, whether or not employees, received no
additional compensation for their services as directors in 2010.
Effective upon completion of this offering, our board has
established a director compensation program for our non-employee
directors with the following components:
Initial
equity grants
Each of the non-employee directors serving on the board as of
the offering shall receive an immediate, one-time grant of
14,000 stock options and 6,000 restricted stock units. Directors
who join the board after the offering shall receive an
immediate, one-time grant of equity equal to $160,000, with
seventy percent being provided in the form of stock options
(based on a Black-Scholes value) and thirty percent being
provided in the form of restricted stock units. These awards
will vest one-third on each anniversary of the grant date while
the director remains in service with us except that one-third of
the initial equity grants awarded to the non-employee directors
serving on the board as of the offering shall vest on the first
annual stockholders’ meeting following the offering (and
not the first anniversary of our offering). The stock options
will have a 10 year term subject to earlier termination
upon leaving the board. Non-employee directors who join the
board after this offering will also receive these equity awards
upon first becoming elected to the board.
Annual
cash retainer
Non-employee directors will earn an annual cash retainer of
$30,000. The annual retainer will be pro-rated for non-employee
directors who serve on the board for only a portion of the year.
The annual retainer normally will be paid quarterly. In
addition, the respective chairs of our audit committee and
compensation committee will receive an additional cash retainer
of $15,000 and $12,000, respectively.
Meeting
fees
Directors will receive $1,500 for each board meeting attended.
Committee members will receive $1,000 for each committee meeting
attended. Meeting fees are payable in cash.
Annual
equity awards
An annual award of 6,000 stock options and 4,000 deferred stock
units will be granted to each of our non-employee directors who
are serving on the board for 2011 following the completion of
this offering. For years after 2011, the annual equity award
will be equal to $100,000, with sixty percent being provided in
the form of stock options (based on a Black Scholes value) and
forty percent being provided in the form of restricted stock
units. These equity awards will be fully vested on the grant
date. Annual equity awards will be granted on a pro-rata basis
for service on the board for less than a year.
Exercise
price for stock options
The exercise price of each stock option awarded shortly after
this offering as part of an initial equity grants to our
non-employee directors will be equal to the average closing
price of our common stock during the
thirty-day
period following the closing of this offering. The exercise
price for all other stock options will be the closing price of
our common stock as quoted on The Nasdaq Global Market on the
date of grant.
Equity
awards subject to the 2011 Omnibus Incentive Plan
Awards of stock options, restricted stock units and deferred
stock units under this policy are subject to the terms and
conditions of our 2011 Omnibus Incentive Plan.
93
Purchase
of Our Stock
The directors will be allowed to purchase the same amount of
shares of our stock on a discounted basis under our 2011 Omnibus
Incentive Plan as employees may purchase shares under our
Employee Stock Purchase Plan. In addition, directors may direct
us to purchase shares of our common stock at fair market value
with their annual cash retainer.
No
other compensation
No other compensation, in the form of cash or otherwise, is paid
to non-employee directors other than reimbursement of their
reasonable expenses incurred in connection with attending board
and committee meetings. Employee directors do not receive any
compensation for their service as directors.
94
Executive
Compensation
COMPENSATION
DISCUSSION AND ANALYSIS
This Compensation Discussion and Analysis, or CD&A,
explains our executive compensation program as it relates to the
following “named executive officers” for 2010 whose
compensation information is presented in the tables following
this discussion in accordance with SEC rules:
| |
|
|
|
Name
|
|
Position
|
|
|
|
Stephen J. Gatto
|
|
Chairman and Chief Executive Officer
|
|
Ralph A. Tapia
|
|
Executive Vice President and Chief Financial Officer
|
|
A. Cenan Ozmeral, Ph.D.
|
|
Executive Vice President and Chief Operating Officer
|
|
Samuel G. McConnell
|
|
Senior Vice President, Corporate Development
|
|
Rudy E. Fogleman, Jr.
|
|
Vice President of Manufacturing, North America
|
The total compensation for these named executive officers is set
forth in the Summary Compensation Table that follows this
CD&A.
This discussion contains forward-looking statements that are
based on our current plans, considerations, expectations and
determinations regarding future compensation programs. Actual
compensation programs that we adopt may differ materially from
currently planned programs as summarized in this discussion.
The
objectives of our compensation program
The primary objectives of our compensation and benefits programs
for our named executive officers for 2010 were to:
|
|
|
| |
•
|
enable us to attract, retain and motivate talented executives to
lead the company,
|
| |
| |
•
|
focus our named executive officers on achieving key business
objectives by providing the opportunity to earn annual incentive
awards that place a substantial portion of total annual
compensation at risk depending upon corporate and individual
performance, and
|
| |
| |
•
|
align the interests of our named executive officers with those
of our stockholders through the use of equity compensation.
|
Establishment
of our compensation committee
The current members of our compensation committee and the
responsibilities and duties of our compensation committee are
discussed in the section titled “Compensation
Committee” located elsewhere in this prospectus.
Additionally, the compensation committee is responsible for,
among other duties, recommending compensation levels for our
Chief Executive Officer and reviewing his performance, and
reviewing compensation levels recommended by our Chief Executive
Officer for our other executive officers and reviewing the
assessment of their performance conducted by our Chief Executive
Officer. Our compensation committee will decide how much and
what type of compensation and benefits will be provided to our
named executive officers after the offering. During 2010, our
board of directors made all compensation decisions with respect
to the named executive officers. Our anticipated initial
compensation and benefits programs described below are not
necessarily indicative in all respects of how we will compensate
our named executive officers in the future. Among other things,
future compensation may take into account increased duties and
responsibilities of the named executive officers following the
offering.
Role
of our chief executive officer in compensation
decisions
While our board of directors had the ultimate authority and
responsibility for approving all compensation for our named
executive officers in 2010, our Chief Executive Officer played
an active role in such decisions except with respect to his own
compensation. At the beginning of the year, our Chief Executive
Officer recommended annual corporate and divisional objectives,
which were reviewed and approved by the board of
95
directors and formed the basis for our annual operating budget.
The status of our corporate and divisional objectives, as well
as our performance relative to these objectives, was reviewed
and discussed with the board of directors. At the end of the
year, actual performance was measured against corporate and
divisional goals and results against target performance were
determined by the compensation committee. With respect to
individual performance, the Chief Executive Officer reviewed and
evaluated the performance of the other named executive officers
to determine their individual contribution to achievement of
these objectives, as well as their achievement of individual
objectives. Our board of directors separately reviewed the Chief
Executive Officer’s performance based on its evaluation of
the company’s overall performance, the Chief Executive
Officer’s individual contributions to achievement of key
objectives and his leadership of the company. Our board of
directors used this information in its subjective discretion to
determine adjustments to compensation and the amount of annual
performance incentives.
Data
used to make compensation decisions in 2010
In making decisions regarding the compensation of our named
executive officers, our board of directors generally considered
compensation and survey data for similarly situated executives
at a comparison group of companies it considered to be our peer
group at the beginning of 2010. Data from this group of
companies was primarily used to gauge the reasonableness and
competitiveness of executive compensation decisions in 2010.
With the assistance of our prior compensation consultant, Iker
Consulting, we used the following sources of information during
2010 to evaluate our executive compensation practices and make
compensation decisions for our named executive officers:
|
|
|
| |
•
|
Select Peer Group: Pacific Ethanol, Verenium, 3Com, Metabolix,
Aventine
|
| |
| |
•
|
Survey: Strategic Industries Rewards Solutions (SIRS), benchmark
survey published by ORC Worldwide (now William M. Mercer),
updated as of April 10, 2010. This survey covered a total
of 539 companies in 7 industries and
20 sub-industries.
|
We did not apply a specific weighting to either data source when
making compensation comparisons. These data sources were used by
the Board in its discretion in making compensation decisions.
2011
Executive compensation review
The compensation committee engaged Radford — an Aon
consulting company, in March 2011 to conduct a comprehensive
review of executive compensation and equity compensation
practices. Specifically, this review included base salary,
annual incentives and equity compensation, including the number
of shares to reserve for future equity awards and the level of
equity incentives that are appropriate to award in connection
with this offering. Radford evaluated compensation data from the
following sources for this review:
|
|
|
| |
•
|
Public Surveys: Hay Group Total Remuneration Survey (limited to
the chemical industry, May 2010), Radford Global Life Sciences
Survey (focusing on public technology and alternative energy
companies with between 25 and 200 employees, April 2010)
|
| |
| |
•
|
Named Peers: The following 21 public companies were approved by
the compensation committee in March 2011 because it was
determined that they exhibited similar financial profiles (fewer
than 500 employees, less than $300 million in revenue
and a market value between $200 million and
$1.5 billion) across multiple relevant industries
(Chemical, Biotech, Clean Tech) relevant to the alternative
energy and biochemical industry: Amyris, Inc., Codexis, Inc.,
Gevo, Inc., Metabolix, Inc., Aceto Corporation, Balchem
Corporation, Cambrex Corporation, Innophos Holdings, Inc.,
Innospec Inc., OMNOVA Solutions Inc., A123 Systems, Inc.,
American Superconductor Corporation, EnerNOC, Inc., FuelCell
Energy, Inc., Alnylam Pharmaceuticals, Inc., ARIAD
Pharmaceuticals, Inc., Geron Corporation, Halozyme Therapeutics,
Inc., ImmunoGen, Inc., NPS Pharmaceuticals, Inc. and Optimer
Pharmaceuticals, Inc.
|
| |
| |
•
|
Recent IPO Peers: The following 12 public companies were
approved by the compensation committee in March 2011 because it
was determined that they exhibit a similar financial profile as
the Named Peers, and additionally completed an initial public
offering within the past 18 months: Amyris, Inc., Codexis, Inc.,
Gevo, Inc., STR Holdings, Inc., A123 Systems, Inc., Generac
Holdings Inc., NuPathe
|
96
|
|
|
| |
|
Inc., Alimera Sciences, Inc., Anthera Pharmaceuticals, Inc.,
Aveo Pharmaceuticals, Inc., Tengion, Inc. and Zogenix, Inc.
|
Information from the Public Surveys and the Named Peers was used
to evaluate salary and annual incentive compensation levels and
grants of long-term incentive awards. The Recent IPO Peers were
used to evaluate equity ownership levels for the named executive
officers, total dilution (both before and after IPO), total
potential wealth transfer and share reserve levels. As discussed
below under the heading “Long-Term Equity Incentive
Awards,” significant equity grants were recommended by
Radford in connection with this offering.
Executive
compensation program
We provide three basic forms of direct compensation to our
executive officers: base salary, annual performance incentives
and long-term equity incentive awards. Total compensation for
our named executive officers may vary significantly from year to
year based on company, functional area, and individual
performance. Further, the value of equity awards made to our
named executive officers will vary in value based on our stock
price.
Base
salary
Base salary is intended to provide all our named executive
officers with a fair and competitive base level of compensation
that reflects their job function, organizational level,
experience and tenure, and sustained performance over time. Our
named executive officers have been eligible for salary increases
on an annual basis at the board’s discretion. Increases are
considered within the context of our overall budgetary
parameters before more specific individual and market
competitive factors and overall economic factors are considered.
We do not apply specific formulas for individual salary
adjustments. We budgeted 3% of payroll for salary increases for
all employees in the aggregate for the first quarter of 2011.
Salary increases made in April 2011 for the named executive
officers ranged from 2.5% to 3.5%, with the amount of increase
based on a subjective review of overall individual performance
by the compensation committee after consultation with the Chief
Executive Officer as reflected in performance ratings. Effective
April 2011, we increased the base salaries of our named
executive officers to the following levels:
| |
|
|
|
|
|
Name
|
|
Base Salary
|
|
|
|
Stephen J. Gatto
|
|
$
|
566,500
|
|
|
Ralph A. Tapia
|
|
$
|
192,610
|
|
|
A. Cenan Ozmeral, Ph.D.
|
|
$
|
310,500
|
|
|
Samuel G. McConnell
|
|
$
|
211,150
|
|
|
Rudy E. Fogleman, Jr.
|
|
$
|
211,020
|
|
Mr. Tapia’s base salary was increased to $270,000 in
connection with his promotion to Executive Vice President and
Chief Financial Officer in May 2011.
Annual
performance incentives
Annual performance incentives are intended to reward our named
executive officers for achieving corporate, division and
individual objectives on an annual basis. Each named executive
officer’s target annual performance incentive for 2010
(expressed as a percentage of his 2010 base salary and, in the
case of Mr. Fogleman, expressed as a percentage of his
salary of $192,000 effective in March 2011) was as follows:
| |
|
|
|
|
|
|
|
Target
|
|
Name
|
|
Percentage
|
|
|
|
Stephen J. Gatto
|
|
|
200
|
%
|
|
Ralph A. Tapia
|
|
|
25
|
%
|
|
A. Cenan Ozmeral, Ph.D.
|
|
|
50
|
%
|
|
Samuel G. McConnell
|
|
|
50
|
%
|
|
Rudy E. Fogleman, Jr.
|
|
|
35
|
%
|
Mr. Gatto’s target percentage for 2011 will be
decreased for the 2011 performance year to 100%. This adjustment
was made in light of the equity awards granted to Mr. Gatto
in connection with this offering as
97
described under the heading “Long-Term Equity Incentive
Awards.” Mr. Tapia’s target percentage for the
2011 performance year will be increased to 40%. Mr. Tapia’s
target annual performance incentive was increased from 25% to
40% as part of his promotion to Executive Vice President and
Chief Financial Officer in May 2011.
2010
Annual performance incentives
Set forth below is a summary of the plan formula, performance
goals applicable to our named executive officers for 2010 and
the level of achievement realized as compared to target
performance that resulted in payments. The annual incentive
target award that could have been earned for 2010 is set forth
in the Grants of Plan-Based Awards in 2010 Fiscal Year Table.
The same annual incentive structure is being used for 2011.
Radford has recommended that we re-evaluate the structure of the
annual incentive for the 2012 performance year, and our annual
incentive program in future years may vary significantly from
the summary set forth below.
The total dollar amount of the annual performance incentive that
could be earned by a named executive officer for 2010 was
determined under the following formula with respect to each
performance goal:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Performance Weight for Performance Goal
|
|
X
|
|
Results vs. Target
|
|
X
|
|
Individual Modifier
|
|
X
|
|
Target
Award
|
|
=
|
|
Earned Payment
|
For purposes of this formula:
|
|
|
| |
•
|
Performance Goal is a corporate performance goal or divisional
performance goal.
|
| |
| |
•
|
Performance Weight is the percentage of the target award that is
based on attaining a performance goal
|
| |
| |
•
|
Results vs. Target, expressed as a percentage, represents the
extent to which the corporate performance goals or divisional
performance goals were met at target for 2010, as determined by
the compensation committee.
|
| |
| |
•
|
Individual Modifier means the adjustment that is made to the
annual performance incentive based on individual performance as
determined by our board upon recommendation by the Chief
Executive Officer (or, in the case of our Chief Executive
Officer, by our board of directors).
|
| |
| |
•
|
Target Award is a dollar amount equal to the percentage of the
named executive officer’s 2010 base salary (or, in the case
of Mr. Fogleman, his 2011 base salary) that could be earned
based on performance at target with respect to all performance
goals.
|
Earned Payment is the dollar value of the annual performance
that was earned by the named executive officer for 2010. The
Performance Weight for some of the Performance Goals depended
upon attaining multiple operational objectives. The results
reported in the Results vs. Target column of the following
tables reflect in some instances that not all of the operational
objectives for a Performance Goal were met. In addition, an
operational objective listed under a Performance Goal may have
been partially achieved during 2010. In that case, the
compensation committee evaluated the extent to which the
operational objective had been achieved after consultation with
the Chief Executive Officer and, in some cases, determined in
its discretion the extent to which Results vs. Target should
reflect partial completion of such operational objective.
Any corporate or division performance that exceeds target
performance only results in a payment to the extent awarded by
the compensation committee in its discretion.
Corporate
performance goals — Gatto and Tapia
The annual performance incentives for Mr. Gatto and
Mr. Tapia were based entirely on corporate performance
goals. The compensation committee determined that the Results
vs. Target with respect to corporate performance goals for 2010
was 72.5%. Set forth below is a table setting forth each
corporate performance goal and its weighting based on actual
results.
98
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Results vs.
|
|
Performance Goal
|
|
Weighting
|
|
Target
|
|
|
|
Begin construction of Louisiana Plant; develop and begin
construction of an in-house integrated pilot plan
|
|
|
35
|
%
|
|
|
24.5
|
%
|
|
Execute a third-party tolling agreement and begin production;
complete sampling; complete scale up at Fermic
|
|
|
10
|
%
|
|
|
5
|
%
|
|
Execute memorandum of understanding and finalize contracts with
customers; complete two strategic partnerships
|
|
|
40
|
%
|
|
|
38
|
%
|
|
New product development; broaden internal capabilities; execute
memorandum of understanding with targeted companies; expand
intellectual property portfolio
|
|
|
10
|
%
|
|
|
0
|
%
|
|
Maintain bank model parameters
|
|
|
5
|
%
|
|
|
5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
Total of Corporate Goals Achieved
|
|
|
|
|
|
|
72.5
|
%
|
|
|
|
|
|
|
|
|
|
|
Corporate
and division performance goals — Ozmeral, McConnell
and Fogleman
The annual performance incentives for Mr. Ozmeral,
Mr. McConnell and Mr. Fogleman were based 60% on
corporate performance goals (as discussed above with respect to
Mr. Gatto and Mr. Tapia) and 40% on divisional
performance goals. The 40% allocable to divisional goals for
Mr. Ozmeral, Mr. McConnell and Mr. Fogleman was
based on the following:
| |
|
|
|
Name
|
|
Division
|
|
|
|
Ozmeral
|
|
50% Specialty Chemicals
|
|
|
|
50% Research & Development
|
|
McConnell
|
|
100% Corporate Development
|
|
Fogleman
|
|
100% Operations
|
Our board determined that the Results vs. Target with respect to
performance goals for 2010 for the Specialty Chemicals Division
was 81%. Set forth below is a table setting forth each
divisional performance goal for the Specialty Chemicals Division
and its weighting based on actual results.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Results vs.
|
|
Performance Goal
|
|
Weighting
|
|
Target
|
|
|
|
Execute tolling agreement (i.e., operation
start-up,
generate $3M revenue)
|
|
|
10
|
%
|
|
|
0
|
%
|
|
Begin construction on Louisiana Plant: safely execute plan (less
than 1.0 Total Recordable Incident Rate), Outside Battery Limits
for Louisiana Plant expansion, infrastructure for expansion,
Inside Battery Limits plans
|
|
|
40
|
%
|
|
|
36
|
%
|
|
Complete scale up at Fermic (i.e., safely execute
plan — less than 1.0 Total Recordable Incident Rate)
|
|
|
10
|
%
|
|
|
10
|
%
|
|
Finalize contracts with certain customers; execute two
partnerships (including a specified party); execute two
additional MOUs with customers
|
|
|
40
|
%
|
|
|
35
|
%
|
|
|
|
|
|
|
|
|
|
|
|
Total of Specialty Chemicals Division Goals Achieved:
|
|
|
|
|
|
|
81
|
%
|
|
|
|
|
|
|
|
|
|
|
99
The compensation committee determined that the Results vs.
Target with respect to performance goals for 2010 for the
Research & Development Division was 81%. Set forth
below is a table setting forth each divisional performance goal
for the Research & Development Division and its
weighting based on actual results.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Results vs.
|
|
Performance Goal
|
|
Weighting
|
|
Target
|
|
|
|
Achieve biosuccinic acid costs at target
|
|
|
50
|
%
|
|
|
40
|
%
|
|
Optimize production process
|
|
|
30
|
%
|
|
|
26
|
%
|
|
Provide basic data packaged for Front End Loading/2, PFDs, heat
and material balance, equipment list
|
|
|
20
|
%
|
|
|
15
|
%
|
|
|
|
|
|
|
|
|
|
|
|
Total of Research & Development Division Goals
Achieved
|
|
|
|
|
|
|
81
|
%
|
|
|
|
|
|
|
|
|
|
|
The compensation committee determined that the Results vs.
Target with respect to performance goals for 2010 for the
Corporate Development Division, after a 25% discretionary
adjustment, was 81%. Set forth below is a table setting forth
each divisional performance goal for the Corporate Development
Division, its weighting based on actual results and the
discretionary adjustment.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results vs.
|
|
|
Performance Goal
|
|
Weighting
|
|
|
Target
|
|
|
|
|
Establish partnership with chemical channel partner; downstream
end user company (demand-pull); upstream process or feedstock
provider
|
|
|
40
|
%
|
|
|
16
|
%
|
|
Develop government affairs plan; production tax credit; loan
guarantee (written confirmation of qualification form from the
DOE); confidential goal with DOE
|
|
|
20
|
%
|
|
|
20
|
%
|
|
Development of confidential pilot plan project
|
|
|
40
|
%
|
|
|
20
|
%
|
|
|
|
|
|
|
|
|
|
|
|
Total of Corporate Development Division
|
|
|
|
|
|
|
56
|
%
|
|
Discretionary Adjustment for Achieving Clearfield Support
Goals
|
|
|
|
|
|
|
25
|
%
|
|
|
|
|
|
|
|
|
|
|
|
Total (including discretionary adjustment)
|
|
|
|
|
|
|
81
|
%
|
|
|
|
|
|
|
|
|
|
|
Our board provided the 25% discretionary adjustment to reflect
additional support services provided by Mr. McConnell for
Clearfield that were not performance goals identified early in
2010. These support services consisted primarily of the
company’s provision of management support to BioEnergy
Management Services, LLC, a subsidiary of the Predecessor
Company, and included negotiating biofuel sales agreements,
plant operating analysis, negotiations with lenders and support
of an arbitration matter to which neither the company, the
Predecessor Company nor BioEnergy Management Services, LLC is a
party.
The compensation committee determined that the Results vs.
Target with respect to performance goals for 2010 for the
Operations Division was 100%. The performance goals for the
Operations Division primarily related to activities that were
attributable to the Predecessor Company prior to our separation
from the Predecessor Company.
Individual
modifier
In making decisions regarding the annual performance incentive,
our board considered the performance of each named executive
officer relative to key corporate objectives and each
officer’s individual goals. The maximum individual modifier
due to individual achievement is 150%, and the minimum
individual modifier due to individual achievement is 50%. No
specific weight is given to any of these factors in determining
the individual modifier.
The 2010 individual modifier for Mr. Gatto, our Chairman
and Chief Executive Officer, was 88.5%. Key individual
objectives taken into account by the board in its discretion in
assigning this individual modifier were completion of the PTT
financing, completion of the DOE award and finalization of the
Louisiana Plant.
100
The 2010 individual modifier for Mr. Tapia, our Executive
Vice President and Chief Financial Officer, was 100%. Key
objectives taken into account by the board in its discretion in
assigning this individual modifier were successfully managing
internal budgeting, completing the audited financial statements
on time, internal system upgrades and conversion of Clearfield
biofuel facility construction loans to term loans.
The 2010 individual modifier for Mr. Ozmeral, our Executive
Vice President and Chief Operating Officer, was 150%. Key
objectives taken into account by the board in its discretion in
assigning this individual modifier were executing the
third-party tolling agreement, customer approval of samples,
finalizing contracts with certain customers and supporting
external financing.
The 2010 individual modifier for Mr. McConnell, our Senior
Vice President of Corporate Development, was 95%. Key objectives
taken into account by the board in its discretion in assigning
the individual modifier were based substantially on the overall
operation of the Corporate Development division.
The 2010 individual modifier for Mr. Fogleman, our Vice
President of Manufacturing, North America, was 150%. Key
objectives taken into account by the board in its discretion in
assigning the individual modifier were increasing wet cake
sales, completing the Perdue transaction, operating within
approved budget and plant operational capabilities.
2010
annual performance incentive calculation
Set forth below is the calculation of the 2010 annual
performance incentive.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Performance
|
|
Performance
|
|
|
|
Results vs.
|
|
|
|
Individual
|
|
|
|
Target
|
|
|
|
Earned
|
|
Name
|
|
Goal
|
|
Weight
|
|
X
|
|
Target
|
|
X
|
|
Modifier
|
|
X
|
|
Award
|
|
=
|
|
Payment
|
|
|
|
Stephen J. Gatto
|
|
|
Corporate
|
|
|
|
100
|
%
|
|
|
|
|
|
|
72.5
|
%
|
|
|
|
|
|
|
88.5
|
%
|
|
|
|
|
|
$1,100,000
|
|
|
|
$
|
705,788
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Earned Payment:
|
|
|
|
$
|
705,788
|
|
|
Ralph A. Tapia
|
|
|
Corporate
|
|
|
|
100
|
%
|
|
|
|
|
|
|
72.5
|
%
|
|
|
|
|
|
|
100
|
%
|
|
|
|
|
|
$46,750
|
|
|
|
$
|
33,894
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Earned Payment:
|
|
|
|
$
|
33,894
|
|
|
A. Cenan Ozmeral, Ph.D.
|
|
|
Corporate
|
|
|
|
60
|
%
|
|
|
|
|
|
|
72.5
|
%
|
|
|
|
|
|
|
150
|
%
|
|
|
|
|
|
$150,000
|
|
|
|
$
|
97,875
|
|
|
|
|
|
Specialty Chemical
|
|
|
|
20
|
%
|
|
|
|
|
|
|
81
|
%
|
|
|
|
|
|
|
150
|
%
|
|
|
|
|
|
$150,000
|
|
|
|
$
|
36,450
|
|
|
|
|
|
Research &
|
|
|
|
20
|
%
|
|
|
|
|
|
|
81
|
%
|
|
|
|
|
|
|
150
|
%
|
|
|
|
|
|
$150,000
|
|
|
|
$
|
36,450
|
|
|
|
|
|
Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Earned Payment:
|
|
|
|
$
|
170,775
|
|
|
Samuel G. McConnell
|
|
|
Corporate
|
|
|
|
60
|
%
|
|
|
|
|
|
|
72.5
|
%
|
|
|
|
|
|
|
95
|
%
|
|
|
|
|
|
$103,000
|
|
|
|
$
|
42,565
|
|
|
|
|
|
Corporate
|
|
|
|
40
|
%
|
|
|
|
|
|
|
81
|
%
|
|
|
|
|
|
|
95
|
%
|
|
|
|
|
|
$103,000
|
|
|
|
$
|
31,703
|
|
|
|
|
|
Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Earned Payment:
|
|
|
|
$
|
74,268
|
|
|
Rudy E. Fogleman, Jr.
|
|
|
Corporate
|
|
|
|
60
|
%
|
|
|
|
|
|
|
72.5
|
%
|
|
|
|
|
|
|
150
|
%
|
|
|
|
|
|
$67,200
|
|
|
|
$
|
43,848
|
|
|
|
|
|
Operations
|
|
|
|
40
|
%
|
|
|
|
|
|
|
100
|
%
|
|
|
|
|
|
|
150
|
%
|
|
|
|
|
|
$67,200
|
|
|
|
$
|
40,320
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Earned Payment:
|
|
|
|
$
|
84,168
|
|
Form
of payment of annual performance incentives
Annual performance incentives that were earned by our named
executive officers in 2010 were made half in cash and half in
the form of stock options (except that Mr. Tapia received
his annual incentive for 2010 70% in cash and 30% in stock
options). Providing a significant percentage of the annual
performance incentive in the form of stock options allowed us to
preserve our cash to fund operating activities and to provide a
meaningful incentive to increase the value of the company. Going
forward as a public company, we anticipate having a greater
percentage of total compensation provided in the form of cash
incentives when there are operating profits.
A stock option was granted for each $2.08 of earned annual
incentive not paid in cash. Stock options were granted at an
exercise price of $3.45, which reflected the per share value of
our common stock on the grant date. The $2.08 conversion factor
reflects a modified Black-Scholes stock option value. The common
stock and stock option values were determined by our board. The
stock options earned as annual incentives for 2010 were granted
in March 2011 and are reflected in the Grants of Plan-Based
Awards in Fiscal Year 2010 Table and the Outstanding Equity
Awards at 2010 Fiscal Year-End Table.
101
LONG-TERM
EQUITY INCENTIVE AWARDS
We typically have made an initial equity award to new employees
and periodic grants at other times as approved by our board in
connection with our annual performance incentives and
promotions. Grants of stock options have been made with the
intention that the exercise price be at least equal to the then
current fair market value of a share of our common stock on the
date of grant, as determined by our board.
Radford advised our compensation committee that the equity
ownership of our named executive officers significantly lagged
in comparison to named executive officers at the Recent IPO
Peers. Radford reported that the comparable executive had an
equity interest in the company of approximately 0.25% (excluding
shares acquired by Mr. Gatto through the spinoff of the
company from the Predecessor Company as an investment through
Norwood LDK, LLC) versus an average of approximately 0.68%
for similarly situated executives at the Recent IPO Peers. In
addition, the equity held by our named executive officers had
limited retentive value as more than half of these awards (other
than equity held by Messrs. Ozmeral and Fogleman) are
vested. In order to bring the equity ownership levels of our
named executive officers in line with competitive market
practices, to align the interests of our named executive
officers with those of our stockholders, and to be able to
provide a significant performance and retention incentive for
our named executive officers and other key performers in light
of our anticipated future growth:
|
|
|
| |
•
|
we increased the number of shares reserved for issuance under
the our 2011 Omnibus Incentive Plan by 3,250,000 shares to
4,189,130 shares,
|
| |
| |
•
|
we included a provision as part of the 2011 Omnibus Incentive
Plan allowing the board discretion beginning in 2012 and ending
in 2016 to annually increase the number of shares available for
issuance by up to the lesser of 5% of the outstanding shares of
our common stock as of the 30th day after the completion of
this offering or 2 million shares,
|
| |
| |
•
|
we granted time-based restricted stock units to our named
executive officers in May 2011 in order to provide a significant
retention incentive, and
|
| |
| |
•
|
we granted stock options subject to a performance-based vesting
schedule to our named executive officers in May 2011 at an
exercise price of $19.36 to motivate them to increase the value
of our stock after the offering.
|
Approximately
two-thirds
of the equity holdings of the named executive officers that are
attributable to employment with the company are unvested as of
May 27, 2011.
Restricted
Stock Units
The named executive officers received the following number of
time-based restricted stock units (RSUs) on May 27, 2011:
| |
|
|
|
|
|
Name
|
|
No. of RSUs
|
|
|
|
|
Stephen J. Gatto
|
|
|
247,500
|
|
|
Ralph A. Tapia
|
|
|
56,250
|
|
|
A. Cenan Ozmeral, Ph.D.
|
|
|
112,500
|
|
|
Samuel G. McConnell
|
|
|
22,500
|
|
|
Rudy E. Fogleman, Jr.
|
|
|
22,500
|
|
The time-based restricted stock units shall vest in equal
one-third (1/3) installments provided that the named executive
officer is then employed by us. If the named executive
officer’s employment is terminated prior to a scheduled
vesting date, the RSUs shall be forfeited unless the named
executive officer suffers a long-term disability or his
employment is terminated in a manner that would entitle him to
severance benefits under his employment agreement. The
restricted stock units will become fully vested upon a change in
control (as defined in the 2011 Omnibus Incentive Plan) provided
that the named executive officer is then employed us. Vested
restricted stock units will be settled by delivering shares of
our common stock to the named executive officers upon vesting.
In no event shall the restricted stock units become vested and
shares shall not be issued if this offering is not completed by
May 27, 2012.
102
Stock
Options
The named executive officers received the following
performance-based stock options on May 27, 2011:
| |
|
|
|
|
|
|
|
Shares Underlying
|
|
Name
|
|
Stock Options
|
|
|
|
Stephen J. Gatto
|
|
|
302,500
|
|
|
Ralph A. Tapia
|
|
|
68,750
|
|
|
A. Cenan Ozmeral, Ph.D.
|
|
|
137,500
|
|
|
Samuel G. McConnell
|
|
|
27,500
|
|
|
Rudy E. Fogleman, Jr.
|
|
|
27,500
|
|
The stock options shall be subject to a ten-year term and shall
only be exercisable upon satisfying both performance and
continued employment requirements. The performance requirement
with respect to 50% of the stock options is based upon achieving
a targeted level of EBITDA with respect to operations of a
specified division not later than December 31, 2013. The
performance requirement with respect to the other 50% of the
stock options is based upon achieving a targeted milestone for
efficient production of a new product. If the performance
criteria for a stock option has been met, the named executive
officer may exercise that option provided that he is then
employed by us. The requirement to remain employed by us will be
waived if the executive suffers a long-term disability or dies
prior to attaining a performance requirement. The stock options
will become fully vested upon a change in control (as defined in
the 2011 Omnibus Incentive Plan) provided that the named
executive officer is then employed by us. The options shall be
cancelled if this offering is not completed by May 27, 2012.
On March 1, 2010, Messrs. Tapia and Ozmeral each
received an award of restricted membership units in our
predecessor Myriant Technologies, LLC as follows:
| |
|
|
|
|
|
|
|
|
|
No. of Restricted
|
|
|
|
|
Name
|
|
Membership Units
|
|
|
Vesting
|
|
|
|
Ralph A. Tapia
|
|
|
20,000
|
|
|
12,000 units to vest equally on the first 3 anniversaries
of the grant date and 8,000 units to vest upon achievement
of a targeted level of EBITDA with respect to operations of a
specified division not later than December 31, 2012,
provided, in each case, that Mr. Tapia was then employed by
us
|
|
A. Cenan Ozmeral, Ph.D.
|
|
|
48,000
|
|
|
24,000 units to vest equally on the first 3 anniversaries
of the grant date and 24,000 units to vest upon achievement
of a targeted level of EBITDA with respect to certain operations
not later than December 31, 2012, provided, in each case,
that Mr. Ozmeral was then employed by us
|
Upon the conversion of the Predecessor Company into a
corporation on January 10, 2011, each of these awards was
converted into a restricted share award under the 2011 Omnibus
Incentive Plan in which each unit was substituted for a share of
our common stock.
Mr. Tapia’s equity grant was awarded as a retention
incentive in connection with the negotiation of a new employment
agreement in March 2010. In January 2011, Mr. Tapia
negotiated a replacement of the restricted full-value equity
award with a nonqualified stock option to purchase
20,000 shares of our common stock, subject to a
10-year
term. This stock option will become exercisable with respect to
4,000 shares of our common stock on each of the first 3
anniversaries of the grant date and will become exercisable with
respect to the remaining 8,000 shares upon the
company’s achievement of a targeted level of EBITDA with
respect to
103
operations of a specified division not later than
December 31, 2013, in each case, subject to
Mr. Tapia’s continued employment.
In March 2011, we amended the vesting conditions applicable to
Mr. Ozmeral’s 48,000 restricted share award and a
prior award of 66,666 restricted units granted by the
Predecessor Company as follows:
| |
|
|
|
|
|
No. of Shares
|
|
|
Amended Vesting Conditions
|
|
|
|
|
48,000
|
|
|
30,000 shares to vest equally on the first three
anniversaries of the grant date (provided that Mr. Ozmeral
is then employed by us), and 18,000 shares to vest upon
achievement of a targeted level of EBITDA with respect to
operations of a specified division not later than
December 31, 2013 (provided Mr. Ozmeral is employed by
us on December 31, 2013)
|
|
|
66,666
|
|
|
33,333 shares to vest equally on the first three
anniversaries of November 20, 2008 (provided that
Mr. Ozmeral is then employed by us), and 33,333 shares
to vest upon the successful completion of the construction of
the Louisiana Plant at or below cost by December 31, 2012
(provided Mr. Ozmeral is employed by us on
December 31, 2012)
|
Stock
Ownership Guidelines
We anticipate our compensation committee will establish stock
ownership requirements for our named executive officers to more
closely align their interests with the long-term interests of
our stockholders. We believe this commitment to stock ownership
will play a significant role in driving our success and creating
long-term stockholder value.
Benefits
and perquisites
We provide our executive officers with generally the same
benefits as those provided to all other salaried employees, such
as health insurance, group life insurance, short- and long-term
disability, and a 401(k) plan with company match. We provided
our Chief Executive Officer an auto allowance and a subsidy for
tax and financial planning services in accordance with his
employment agreement.
Employment
agreements
We have entered into employment agreements with our named
executive officers. These employment agreements provide for base
salary, annual discretionary bonuses and employee benefits over
specified terms of employment. Each of these agreements provides
for certain payments and other benefits if the executive’s
employment terminates under certain circumstances other than for
“cause,” including in connection with a “change
in control.” Payments are subject to signing a release and
our executives are subject to non-competition, non-solicitation
and confidentiality restrictions. See the subsection
“Discussion Concerning Summary Compensation Table and
Grants of Plan-Based Awards Table” for a description of the
agreement terms impacting current compensation and
“Potential Payments upon Termination or Change in
Control” for a description of applicable severance and
change in control benefits.
On May 27, 2011, we authorized a new employment agreement
for Mr. Tapia to retain his services as Executive Vice
President and Chief Financial Officer, and with
Mr. Fogleman to retain his services as Vice President of
Manufacturing, North America. The compensation committee
subsequently reviewed the employment agreements with our named
executive officers and, after receiving advice from its
compensation consultant, made the following changes:
|
|
|
| |
•
|
the term of all employment agreements will be three years (until
July 22, 2014) subject to one-year extensions
|
| |
| |
•
|
incentive payments will be subject to any compensation recovery
policy that we adopt from time to time
|
104
|
|
|
| |
•
|
payments from the company to the named executive officers are
subject to being limited to an amount that will not trigger
golden parachute excise taxes if doing so maximizes the net
after tax benefit payable in connection with a change in control
|
| |
| |
•
|
an increase to the severance protection provided to our chief
executive officer in the event of either a termination of his
employment by us without cause or resignation due to good reason
at any time from one times salary to two times the sum of his
then-current annual salary and the annual bonus, if any, that
was earned by him in the year immediately preceding the year of
such employment termination (with the annual bonus instead being
the target bonus for the year of termination or resignation if
the termination or resignation triggering the severance is
within the two year period beginning on a change in control (as
defined in the 2011 Omnibus Incentive Plan as in effect or the
date of this prospectus), subject to the chief executive officer
signing and complying with an extended non-compete and
non-solicitation agreement for an additional 12 months (for a
total restricted period of 24 months)
|
| |
| |
•
|
an increase to the severance protection provided to our chief
operating officer in the event of either a termination of his
employment by us without cause or resignation due to good reason
at any time from one times salary to one and one-half times the
sum of his then-current annual salary and the annual bonus, if
any, that was earned by him in the year immediately preceding
the year of such employment termination (with the annual bonus
instead being the target bonus for the year of termination or
resignation if the termination or resignation triggering the
severance is within the two year period beginning on a change in
control (as defined in the 2011 Omnibus Incentive Plan as in
effect or the date of this prospectus), subject to the chief
operating officer signing and complying with an extended
non-compete and non-solicitation agreement for an additional 6
months (for a total restricted period of 18 months)
|
| |
| |
•
|
in addition to the current one year cash severance benefit under
the prior employment agreements, the named executive officers
other than the chief executive officer and chief operating
officer shall be entitled to receive a lump sum cash payment in
the event of a termination of employment by us without cause or
by the executive for good reason, in each case, within
12 months after a change in control equal to an additional
six months of salary so long as the named executive officer
signs and complies with an extended non-compete and
non-solicitation agreement for an additional six months (for a
total restricted period of 18 months in the case of our
other named executive officers)
|
|
|
|
| |
•
|
severance benefits will include continued medical and dental
benefits for a period of time after employment termination equal
to the number of months of salary included in the
executive’s cash severance
|
|
|
|
| |
•
|
beginning in 2011, the target annual equity award that may be
earned by our chief executive officer and chief operating
officer shall be 100% of base salary
|
No tax
gross-up
payments will be made by the company for golden parachute excise
taxes. The compensation committee decided to make these changes
for the chief executive officer and chief operating officer in
connection with renegotiating their employment agreements. The
compensation committee made these changes with respect to the
other named executive officers in order to ensure that a cash
severance payment is made within the first six months following
an involuntary employment termination (other than for cause)
that occurs one year within a change in control so long as the
named executive officer agrees to extend the post-employment
restrictions described above. After we become a public company,
the one year cash severance benefit under the prior employment
agreement that will be continued going forward, if triggered,
cannot be paid six months and a day after employment termination
in order to comply with applicable federal tax laws. The
compensation committee decided to provide medical and dental
continuation as described above consistent with market practices
as evaluated by the compensation consultant.
Our compensation committee believes that change in control and
severance protection are important parts of the overall
compensation program for our named executive officers. Change in
control provisions help to secure the continued employment and
dedication of our named executive officers, notwithstanding any
concern that they might have regarding their own continued
employment prior to or following a change in control, and
105
to promote a continuity of management during a corporate
transaction. Severance provisions are used primarily to attract,
retain and motivate individuals with the requisite experience
and ability to drive our success. Severance arrangements also
serve, in part, as consideration to secure commitments from our
named executive officers not to compete with us after
termination of their employment.
On June 24, 2011, Mr. Gatto terminated his consulting
agreement with our Predecessor Company. His employment agreement
requires that he spend substantially all of his business time to
the performance of his duties to the company.
Tax
Considerations
Section 162(m) of the Code generally disallows a deduction
for compensation in excess of $1.0 million paid to certain
named executive officers. Qualifying performance-based
compensation is not subject to the deduction limitation if
specified requirements are met. We generally intend to structure
the performance-based portion of our executive compensation,
when feasible, to comply with exemptions from
Section 162(m) so that the compensation remains tax
deductible to us. However, our board of directors or
compensation committee may, in its judgment, authorize
compensation payments that do not comply with the exemptions
from Section 162(m) when it believes that such payments are
appropriate to attract and retain executive talent.
SUMMARY
COMPENSATION TABLE FOR FISCAL YEAR 2010
The following table summarizes compensation earned by our Chief
Executive Officer, Chief Financial Officer, and each of our
three other most highly compensated officers during the year
ended December 31, 2010. In this prospectus, we refer to
these officers as our named executive officers.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incentive
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock
|
|
|
Option
|
|
|
Plan
|
|
|
All Other
|
|
|
|
|
|
|
|
|
|
|
Salary
|
|
|
Awards
|
|
|
Awards
|
|
|
Compensation
|
|
|
Compensation
|
|
|
Total
|
|
|
Name and Principal Position
|
|
Year
|
|
|
($)
|
|
|
($)(1)
|
|
|
($)(2)
|
|
|
($)(3)
|
|
|
($)(4)
|
|
|
($)
|
|
|
|
|
Stephen J. Gatto,
|
|
|
2010
|
|
|
$
|
478,750
|
(5)
|
|
|
—
|
|
|
$
|
352,894
|
|
|
$
|
352,894
|
|
|
$
|
57,680
|
|
|
$
|
1,242,218
|
|
|
Chairman and Chief Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ralph A. Tapia,
|
|
|
2010
|
|
|
$
|
180,831
|
|
|
$
|
120,000
|
(6)
|
|
$
|
10,169
|
|
|
$
|
23,726
|
|
|
$
|
7,491
|
|
|
$
|
342,217
|
|
|
Executive Vice President and Chief Financial Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A. Cenan Ozmeral Ph.D.,
|
|
|
2010
|
|
|
$
|
287,500
|
|
|
$
|
288,000
|
|
|
$
|
85,388
|
|
|
$
|
85,388
|
|
|
$
|
10,396
|
|
|
$
|
756,672
|
|
|
Executive Vice President and Chief Operating Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Samuel G. McConnell,
|
|
|
2010
|
|
|
$
|
202,769
|
|
|
|
—
|
|
|
$
|
37,134
|
|
|
$
|
37,134
|
|
|
$
|
8,249
|
|
|
$
|
285,286
|
|
|
Senior Vice President, Corporate Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rudy E. Fogleman, Jr.,
|
|
|
2010
|
|
|
$
|
196,242
|
|
|
|
—
|
|
|
$
|
42,084
|
|
|
$
|
42,084
|
|
|
$
|
7,734
|
|
|
$
|
288,144
|
|
|
Vice President of Manufacturing, North America
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Reflects the aggregate grant date fair value of the restricted
membership unit awards that were granted by the Predecessor
Company on March 1, 2010. These awards are further
addressed in the All Other Stock Awards Number of Shares of
Stock or Units Column in the Grants of Plan-Based Awards in 2010
Fiscal Year Table. |
| |
|
(2) |
|
Reflects the total dollar amount of stock options earned under
the 2010 annual incentive. The value of the stock options was
calculated using the modified Black-Scholes stock option value
of $2.08 as determined by our board. In conducting its analysis
regarding the Black-Scholes value of the stock options, the
following assumptions were used: (i) stock price was valued
at $3.45, (ii) the risk-free rate was estimated to be 2.3%,
(iii) expected life was estimated to be six years, and
(iv) volatility was estimated at 65%. There can be no
assurance that these options will ever be exercised (in which
case no value will be realized by the |
106
|
|
|
|
|
|
named executive officers) or that the value on exercise will
equal the fair value. These awards are further addressed in the
All Other Stock Awards Number of Shares of Stock or Units Column
in the Grants of Plan-Based Awards in 2010 Fiscal Year Table. |
| |
|
(3) |
|
Reflects the total dollar amount earned under the 2010 annual
incentive that was paid in cash. These awards are further
addressed in the Estimated Future Payouts under Non-Equity
Incentive Plan column in the Grants of Plan-Based Awards in 2010
Fiscal Year Table. The portion of our annual incentive that was
granted in the form of stock options is reflected in the Option
Awards column of this Summary Compensation Table. |
| |
|
(4) |
|
The compensation represented by the amounts for 2010 set forth
in the All Other Compensation column of the Summary Compensation
Table for the named executive officers are detailed in the
following table. |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Qualified
|
|
|
|
|
|
|
Name and
|
|
Savings
|
|
Group Term Life
|
|
|
|
|
|
Principal Position
|
|
Plan(a)
|
|
Insurance(b)
|
|
Perquisites(c)
|
|
Total(d)
|
|
|
|
Stephen J. Gatto,
|
|
|
—
|
|
|
$
|
138
|
|
|
$
|
57,542
|
|
|
$
|
57,680
|
|
|
Chairman and Chief Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ralph A. Tapia,
|
|
$
|
7,223
|
|
|
$
|
258
|
|
|
|
—
|
|
|
$
|
7,491
|
|
|
Executive Vice President and Chief Financial Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A. Cenan Ozmeral Ph.D.,
|
|
$
|
10,000
|
|
|
$
|
396
|
|
|
|
—
|
|
|
$
|
10,396
|
|
|
Executive Vice President and Chief Operating Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Samuel G. McConnell,
|
|
$
|
8,111
|
|
|
$
|
138
|
|
|
|
—
|
|
|
$
|
8,249
|
|
|
Senior Vice President, Corporate Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rudy E. Fogleman, Jr.,
|
|
$
|
7,338
|
|
|
$
|
396
|
|
|
|
—
|
|
|
$
|
7,734
|
|
|
Vice President of Manufacturing, North America
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
(a)
|
Consists of matching contribution made under the company’s
401(k) plan.
|
|
|
|
| |
(b)
|
Reflects the cost of term life insurance coverage that the
company provided named executive officers on a discriminatory
basis.
|
|
|
|
| |
(c)
|
The following perquisites were provided to Mr. Gatto during
2010: $36,818, reflecting Mr. Gatto’s company-provided
auto, and $20,724, reflecting a reimbursement the company made
to Mr. Gatto in connection with his 2010 self-employment
tax per the terms of his employment agreement with the company.
No other named executive officers received perquisites during
2010.
|
|
|
|
| |
(d)
|
Reflects the total amount set forth in the All Other
Compensation column to the Summary Compensation Table.
|
|
|
|
|
(5) |
|
Represents $360,000 of cash compensation earned by
Mr. Gatto as a consultant for the period from
January 1, 2010 through May 15, 2010 under a prior
consulting agreement with us and $118,750 of salary earned by
Mr. Gatto on and after May 16, 2010 under an
employment agreement. |
| |
|
(6) |
|
Mr. Tapia’s restricted membership unit award was
cancelled and was replaced with a nonqualified stock option
award that was granted on January 31, 2011. This award is
further described in the All Other Options Awards-Number of
Securities Underlying Options column in the Grants of Plan-Based
Awards in Fiscal Year 2010 Table. |
107
GRANTS OF
PLAN-BASED AWARDS IN FISCAL YEAR 2010
The following table shows information regarding grants of equity
and non-equity awards to our named executive officers during the
year ended December 31, 2010.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
|
|
All Other
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock
|
|
Option
|
|
|
|
Grant
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Awards:
|
|
Awards:
|
|
Exercise
|
|
Date Fair
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
Number of
|
|
or Base
|
|
Value of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares of
|
|
Securities
|
|
Price of
|
|
Stock and
|
|
|
|
|
|
Estimated Future Payouts Under Non-Equity Incentive Plan
Awards
|
|
Estimated Future Payouts Under Equity Incentive Plan
Awards
|
|
Stock or
|
|
Underlying
|
|
Option
|
|
Options
|
Name and
|
|
|
|
Threshold
|
|
Target
|
|
Threshold
|
|
Target
|
|
Maximum
|
|
Units
|
|
Options
|
|
Awards
|
|
Awards
|
|
Principal Position
|
|
Grant Date
|
|
(#)
|
|
($)(1)
|
|
(#)
|
|
(#)
|
|
(#)
|
|
(#)
|
|
(#)
|
|
($/Sh)
|
|
($)
|
|
|
|
Stephen J. Gatto,
|
|
|
3/23/2011
|
|
|
|
0
|
|
|
$
|
1,100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
169,660
|
(2)
|
|
$
|
3.45
|
|
|
$
|
352,894
|
(3)
|
|
Chairman and Chief Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ralph A. Tapia,
|
|
|
3/1/2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20,000
|
(4)
|
|
|
|
|
|
|
—
|
|
|
$
|
120,000
|
(5)
|
|
Executive Vice President and Chief
|
|
|
3/23/2011
|
|
|
|
0
|
|
|
$
|
46,750
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,889
|
(2)
|
|
$
|
3.45
|
|
|
$
|
10,169
|
(3)
|
|
Financial Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A. Cenan Ozmeral Ph.D.,
|
|
|
3/1/2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
48,000
|
(6)
|
|
|
|
|
|
|
—
|
|
|
|
288,000
|
(5)
|
|
Executive Vice President and Chief
|
|
|
3/23/2011
|
|
|
|
0
|
|
|
$
|
150,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
41,052
|
(2)
|
|
$
|
3.45
|
|
|
|
85,388
|
(3)
|
|
Operating Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Samuel G. McConnell,
|
|
|
3/23/2011
|
|
|
|
0
|
|
|
$
|
103,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
17,853
|
(2)
|
|
$
|
3.45
|
|
|
$
|
37,134
|
(3)
|
|
Senior Vice President, Corporate Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rudy E. Fogleman, Jr.,
|
|
|
3/23/2011
|
|
|
|
0
|
|
|
$
|
67,200
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20,233
|
(2)
|
|
$
|
3.45
|
|
|
$
|
42,084
|
(3)
|
|
Vice President of Manufacturing, North America
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Represents total amount of annual incentive that could be paid
for the 2010 performance year. The committee retained the
discretion to pay this as either cash or equity. The amounts
reported in the All Other Option Awards: Number of Securities
Underlying Options column above satisfied 50% of the annual
incentive earned for 2010 (or 30% in the case of Mr. Tapia). |
| |
|
(2) |
|
Represents the number of stock options granted to each named
executive officer under the annual incentive for the 2010
performance year. Each option was granted with an exercise price
of $3.45, was fully vested upon the grant date, and is subject
to a 10-year
term. A stock option at an exercise price of $3.45 per share was
earned for each $2.08 of annual performance incentive,
reflecting the modified Black-Scholes stock option value as
determined by our board. |
| |
|
(3) |
|
The value of the stock options was calculated using the modified
Black-Scholes stock option value of $2.08 as determined by our
board. There can be no assurance that the options will ever be
exercised (in which case no value will be realized by the
executive) or that the value on exercise will equal the fair
value. |
| |
|
(4) |
|
On March 1, 2010, Mr. Tapia was granted 20,000
restricted membership units in the Predecessor Company prior to
its conversion to a corporation. This award was later cancelled.
In replacement of the cancelled restricted membership units,
Mr. Tapia was granted a stock option on January 31,
2011 to purchase 20,000 shares of our common stock, with an
exercise price of $3.45 and subject to a
10-year
term. The option shall become exercisable as follows:
(i) with respect to 4,000 shares on each of the first
three anniversaries of March 1, 2010, and (ii) with
respect to 8,000 shares upon the company’s achievement
of a targeted level of EBITDA with respect to operations of a
specified division not later than December 31, 2013
(subject, in each case, to Mr. Tapia’s continued
employment with the company on such vesting dates). |
| |
|
(5) |
|
Reflects the 2010 aggregate grant date fair value of the
restricted membership unit awards as of March 1, 2010 of
$6.00 per unit. |
| |
|
(6) |
|
On March 1, 2010, Mr. Ozmeral was granted 48,000
restricted membership units in the Company prior to its
conversion to a corporation. This award was converted into a
restricted stock award on a one-share for
one-unit
basis in January 2011 and vests as follows:
(i) 10,000 shares shall vest on each of the first
three anniversaries of March 1, 2010, and (ii) 18,000
restricted shares shall vest upon the company’s achievement
of a targeted level of EBITDA with respect to operations of a
specified division not later than |
108
|
|
|
|
|
|
December 31, 2013 (in each case, subject to
Mr. Ozmeral’s continued employment with the company on
such vesting dates). |
DISCUSSION
CONCERNING SUMMARY COMPENSATION TABLE AND GRANTS OF PLAN-BASED
AWARDS TABLE
Employment
Agreements
Executive employment agreements with our named executive
officers provide for base salary, annual bonus opportunities,
participation in our benefit plans and post-termination benefits
and obligations. With respect to the 2011 calendar year, the
employment agreements for our chief executive officer and chief
operating officer provide them with the opportunity to earn
target equity awards equal to 100% of base salary, payable in
the form of stock options, restricted stock units or other forms
of equity compensation as determined by the compensation
committee, provided that any such award shall be subject to the
satisfaction of company and individual performance criteria and
other criteria as determined by the compensation committee.
Each of the executive employment agreements for our named
executive officers has a term that expires on July 22,
2014, subject thereafter to automatic renewal for successive
one-year periods absent one year advance written notice (or
60 days advance written notice in the case of any other
named executive officer) to the contrary by either party prior
to the end of the then current term. Non-renewal of an
employment agreement does not trigger the payment of severance.
Each executive employment agreement specifies a minimum level of
base salary for the named executive officer, but gives our
compensation committee authority to increase the base salary
from time to time. The minimum base salary for each of our named
executive officers, as set forth in the executive employment
agreements is described below:
| |
|
|
|
|
|
Named Executive Officer
|
|
Base Salary
|
|
|
|
|
Stephen J. Gatto
|
|
$
|
550,000
|
|
|
Ralph A. Tapia
|
|
$
|
270,000
|
|
|
A. Cenan Ozmeral Ph.D.
|
|
$
|
300,000
|
|
|
Samuel G. McConnell
|
|
$
|
206,000
|
|
|
Rudy E. Fogleman, Jr.
|
|
$
|
211,020
|
|
Mr. Gatto’s executive employment agreement, as in
effect in 2010, provided that he shall be eligible to receive an
annual bonus at a target of 200% of his base salary, with the
actual amount determined by our board of directors. Under his
executive employment agreement, Mr. Gatto specifically
waived all entitlements to any bonuses previously offered to him
while he acted as a consultant to the company, including,
without limitation (i) 200% of his annual consulting fee
upon the closing of a capital raise equal to or greater than
$40 million and (ii) a bonus of $1.46 million
upon the completion of the Louisiana Plant. For 2011,
Mr. Gatto’s target bonus will be reduced to 100% of
base salary.
The executive employment agreements for the other named
executive officers provide for an annual bonus target as
described in the annual performance incentives section of the
Compensation Discussion and Analysis.
The executive employment agreements also provide that each
executive is entitled to, among other things, participation in
any equity incentive plan and employee benefit programs on the
same basis as similarly-situated executives (except that the
chief executive officer and chief operating officer shall be
eligible for the target equity awards described above): paid
vacation and reimbursement of business expenses. In addition,
Mr. Gatto’s employment agreement provides for a
vehicle allowance of $2,083.33 per month and standard tax
preparation and planning services of up to $18,000 per year.
The executive employment agreements with our named executive
officers also provide for severance payments upon termination of
employment by us without cause, termination by the executive for
good reason, and termination of employment for any reason within
twelve months following a change in control. See
109
“Potential payments upon termination or change in
control” below for a description of these provisions in the
employment agreements.
OUTSTANDING
EQUITY AWARDS AT 2010 FISCAL YEAR END
The following table shows the grants of stock awards to our
named executive officers that were outstanding on
December 31, 2010, the last day of our fiscal year.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option Awards
|
|
|
|
Stock Awards
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
incentive
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
plan awards:
|
|
|
|
|
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
Equity
|
|
market or
|
|
|
|
|
|
|
|
|
|
Incentive
|
|
|
|
|
|
|
|
|
|
incentive
|
|
payout
|
|
|
|
|
|
|
|
|
|
Plan
|
|
|
|
|
|
|
|
|
|
plan
|
|
value of
|
|
|
|
|
|
|
|
|
|
Awards:
|
|
|
|
|
|
|
|
Market
|
|
awards:
|
|
unearned
|
|
|
|
|
|
Number of
|
|
|
|
Number
|
|
|
|
|
|
Number of
|
|
value of
|
|
number
|
|
shares,
|
|
|
|
|
|
securities
|
|
Number of
|
|
of securities
|
|
|
|
|
|
shares or
|
|
shares or
|
|
of unearned
|
|
units, or
|
|
|
|
|
|
underlying
|
|
securities
|
|
underlying
|
|
|
|
|
|
units of
|
|
units of
|
|
shares,
|
|
other
|
|
|
|
|
|
unexercised
|
|
underlying
|
|
unexercised
|
|
Option
|
|
Option
|
|
stock that
|
|
stock that
|
|
units or other
|
|
rights that
|
|
|
|
|
|
options (#)
|
|
unexercised
|
|
unearned
|
|
Exercise
|
|
Expiration
|
|
have not
|
|
have not
|
|
rights
|
|
have
|
Name And
|
|
|
|
exercisable
|
|
options
|
|
options
|
|
Price
|
|
Date
|
|
vested
|
|
vested ($)
|
|
that have
|
|
not vested
|
|
Principal Position
|
|
Grant Date
|
|
(1)
|
|
unexercisable (#)
|
|
(#)
|
|
($)
|
|
(2)
|
|
(#)
|
|
(3)
|
|
not vested (#)
|
|
($)(3)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stephen J. Gatto,
|
|
|
3/23/2011
|
|
|
|
169,660
|
(4)
|
|
|
—
|
|
|
|
—
|
|
|
$
|
3.45
|
|
|
|
3/23/2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chairman and Chief Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ralph A. Tapia,
|
|
|
3/1/2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12,000
|
(5)
|
|
$
|
41,400
|
|
|
|
8,000
|
(6)
|
|
$
|
27,600
|
|
|
Executive Vice
|
|
|
1/31/2011
|
|
|
|
|
|
|
|
1,897
|
(7)
|
|
|
|
|
|
$
|
5.35
|
|
|
|
1/31/2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
President and Chief
|
|
|
1/31/2011
|
|
|
|
16,500
|
(8)
|
|
|
—
|
|
|
|
|
|
|
$
|
5.35
|
|
|
|
3/23/2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial Officer
|
|
|
3/23/2011
|
|
|
|
4,889
|
(4)
|
|
|
—
|
|
|
|
—
|
|
|
$
|
3.45
|
|
|
|
3/23/2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A. Cenan Ozmeral Ph.D.,
|
|
|
11/20/2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11,111
|
(9)
|
|
$
|
38,333
|
|
|
|
33,333
|
(10)
|
|
$
|
114,999
|
|
|
Executive Vice
|
|
|
3/1/2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20,000
|
(11)
|
|
$
|
69,000
|
|
|
|
18,000
|
(12)
|
|
$
|
62,100
|
|
|
President and Chief
|
|
|
1/31/2011
|
|
|
|
—
|
|
|
|
5,755
|
(7)
|
|
|
—
|
|
|
$
|
5.35
|
|
|
|
1/31/2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Officer
|
|
|
3/23/2011
|
|
|
|
41,052
|
(4)
|
|
|
—
|
|
|
|
—
|
|
|
$
|
3.45
|
|
|
|
3/21/2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Samuel G. McConnell,
|
|
|
1/31/2011
|
|
|
|
|
|
|
|
4,918
|
(7)
|
|
|
|
|
|
$
|
5.35
|
|
|
|
1/31/2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Senior Vice
|
|
|
3/23/2011
|
|
|
|
17,853
|
(4)
|
|
|
—
|
|
|
|
—
|
|
|
$
|
3.45
|
|
|
|
3/23/2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
President, Corporate
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rudy E. Fogleman, Jr.,
|
|
|
1/31/2011
|
|
|
|
|
|
|
|
3,610
|
(7)
|
|
|
|
|
|
$
|
5.35
|
|
|
|
1/31/2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vice President of
|
|
|
3/23/2011
|
|
|
|
20,233
|
(4)
|
|
|
—
|
|
|
|
—
|
|
|
$
|
3.45
|
|
|
|
3/23/2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Manufacturing, North America
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Represents fully exercisable stock options. |
| |
|
(2) |
|
All stock options have a 10 year term subject to earlier
expiration on account of employment termination as follows: the
post-termination exercise period being the lesser of
(i) the remaining term, (ii) thirty days, or
(iii) in the event of (a) a termination due to death,
disability or retirement, 1 year, (b) termination
without cause or for good reason, 90 days, or
(c) termination for cause, the date of termination. |
| |
|
(3) |
|
The market value of the stock awards is determined by
multiplying the number of shares by $3.45. |
| |
|
(4) |
|
These stock options were granted in connection with the 2010
annual incentive and were fully exercisable upon the grant date.
For more information on these awards, see the discussion in the
Compensation Discussion and Analysis under 2010 annual
performance incentives. |
| |
|
(5) |
|
These restricted membership units were granted to Mr. Tapia
on March 1, 2010 and were scheduled to vest on the first
three anniversaries of such grant date. However, this award was
later cancelled and replaced with a non-qualified stock option.
See the footnotes to the Grants of Plan-Based Awards in Fiscal
Year 2010 Table for a description of that option. |
| |
|
(6) |
|
These restricted membership units were granted to Mr. Tapia
on March 1, 2010 and were scheduled to vest upon the
company’s achievement of a targeted level of EBITDA with
respect to operations of a specified division. However, this
award was later cancelled and replaced with a non-qualified
stock option. See the footnotes to the Grants of Plan-Based
Awards in Fiscal Year 2010 Table for a description of that
option. |
| |
|
(7) |
|
These stock options were granted in connection with the 2009
annual incentive and will become exercisable in one-third
increments on the first, second, and third anniversary dates of
the grant, subject to continued employment with us. Vesting of
these options will be accelerated if the executive becomes
entitled |
110
|
|
|
|
|
|
to receive any severance benefits due to employment termination
within the twelve month period after a change in control. |
| |
|
(8) |
|
Options to acquire units in the predecessor company were
initially pledged to Mr. Tapia on September 18, 2006
in connection with the commencement of his employment. Upon the
adoption of the 2011 Omnibus Incentive Plan on January 12,
2011, the company granted Mr. Tapia a fully vested
non-qualified stock option award in recognition of past services. |
| |
|
(9) |
|
These restricted shares will vest on November 20, 2011,
provided Mr. Ozmeral remains employed by the company
through such date. |
| |
|
(10) |
|
These restricted shares will vest upon the successful completion
of the construction of the Louisiana Plant at or below cost by
December 31, 2012, provided Mr. Ozmeral remains
employed by the company through such date. |
| |
|
(11) |
|
These restricted shares shall vest in one-half increments on the
second and third anniversary dates of March 1, 2010 so long
as the executive remains employed by us. |
| |
|
(12) |
|
These restricted shares will vest upon the company’s
achievement of a targeted level of EBITDA with respect to
operations of a specified division not later than
December 31, 2013, provided Mr. Ozmeral remains
employed by the company through such date. |
STOCK
VESTED IN FISCAL YEAR 2010
The following table shows the stock awards to our named
executive officers that vested by December 31, 2010, the
last day of our fiscal year. No stock options were exercised
during the year ended December 31, 2010.
| |
|
|
|
|
|
|
|
|
|
|
|
Stock Awards
|
|
|
|
Number of Shares
|
|
Value Realized on
|
|
Name and Principal Position
|
|
Acquired on Vesting(#)
|
|
Vesting($)(1)
|
|
|
|
Stephen J. Gatto,
|
|
|
—
|
|
|
|
—
|
|
|
Chairman and Chief Executive Officer
|
|
|
|
|
|
|
|
|
|
Ralph A. Tapia,
|
|
|
—
|
|
|
|
—
|
|
|
Executive Vice President and Chief Financial Officer
|
|
|
|
|
|
|
|
|
|
A. Cenan Ozmeral Ph.D.,
|
|
|
11,111
|
(2)
|
|
$
|
38,833
|
|
|
Executive Vice President and Chief Operating Officer
|
|
|
|
|
|
|
|
|
|
Samuel G. McConnell,
|
|
|
—
|
|
|
|
—
|
|
|
Senior Vice President, Corporate Development
|
|
|
|
|
|
|
|
|
|
Rudy E. Fogleman, Jr.,
|
|
|
—
|
|
|
|
—
|
|
|
Vice President of Manufacturing, North America
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
The value realized is calculated by multiplying the number of
shares vested by $3.45, the per share value of our common stock
determined by our board. |
| |
|
(2) |
|
This represents shares of stock that vested on November 20,
2010. |
PENSION
BENEFITS
We do not maintain any defined benefit pension plans.
NONQUALIFIED
DEFERRED COMPENSATION
We do not maintain any nonqualified deferred compensation plans.
POTENTIAL
PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
The following is a summary of the severance and change in
control benefits provided to each of our named executive
officers under employment agreements as in effect July 22,
2011.
111
Employment Termination Due to Death, Disability, for Cause,
or Without Good Reason. Upon the termination of
an executive’s employment due to the executive’s death
or disability or without good reason prior to a change in
control, we shall provide the executive with the following
payments
and/or
benefits (the “Accrued Amounts”):
|
|
|
| |
•
|
unpaid base salary through the date of termination;
|
| |
| |
•
|
any accrued vacation in accordance with company policy;
|
| |
| |
•
|
reimbursement for any unreimbursed expenses incurred through the
date of termination;
|
| |
| |
•
|
accrued and vested benefits under a Section 401(k)
plan; and
|
| |
| |
•
|
all other benefits to which the executive may be entitled under
the terms of any applicable compensation program or arrangement,
equity or perquisite plan or program, including but not limited
to any applicable insurance benefits.
|
Employment Termination Without Cause or for Good Reason for
the Chief Executive Officer and Chief Operating
Officer. If we terminate at any time the
executive’s employment without “cause” or the
executive terminates his employment for “good reason,”
each as defined below, we are obligated to provide him with the
following payments
and/or
benefits, subject to the executive’s execution of a release:
|
|
|
| |
•
|
the Accrued Amounts (as defined above);
|
| |
| |
•
|
in the case of our chief executive officer, two cash payments
over a period of six months and a day equal in total to two
times the sum of his then current annual salary and the annual
bonus, if any, that was earned by him in the year immediately
preceding the year of such employment termination (with the
annual bonus instead being the target bonus for the year of
termination or resignation if the termination or resignation
triggering the severance is within the two year period beginning
on a change in control (as defined in the 2011 Omnibus Incentive
Plan as in effect as of the date of this prospectus), subject to
the chief executive officer signing and complying with an
extended non compete and non solicitation agreement for an
additional 12 months (for a total restricted period of
24 months);
|
| |
| |
•
|
in the case of our chief operating officer, two cash payments
over the period of six months and a day equal in total to one
and one half times the sum of his then current annual salary and
the annual bonus, if any, that was earned by him in the year
immediately preceding the year of such employment termination
(with the annual bonus instead being the target bonus for the
year of termination or resignation if the termination or
resignation triggering the severance is within the two year
period beginning on a change in control (as defined in the 2011
Omnibus Incentive Plan as in effect as of the date of this
prospectus), subject to the chief operating officer signing and
complying with an extended non compete and non solicitation
agreement for an additional 6 months (for a total
restricted period of 18 months);
|
| |
| |
•
|
a pro rata portion of his annual bonus for the performance year
in which the executive’s termination occurs, payable at the
time that annual bonuses are paid to other senior executives or,
if required to comply with applicable tax laws, six months and a
day following the date of his termination, (determined in each
case by multiplying the executive’s target bonus by a
fraction, the numerator of which is the number of days during
the performance year of termination that the executive was
employed by the company and the denominator of which is
365); and
|
| |
| |
•
|
24 months of continued medical and dental insurance
coverage in the case of our chief executive officer and
18 months of continued medical and dental coverage in the
case of our chief operating officer.
|
As described above in the Compensation Discussion and Analysis,
the increased severance amounts are subject to signing and
complying with an extended non compete and non solicitation
agreement for 24 months in the case of the chief executive
officer and 18 months in the case of the chief operating
officer.
112
Employment Termination Without Cause or for Good Reason for
Named Executive Officers other than the Chief Executive Officer
and the Chief Operating Officer:
|
|
|
| |
•
|
the Accrued Amounts (as defined above);
|
| |
| |
•
|
cash equal to the executive’s then-current annual base
salary, payable in a single lump sum on the 60th day
following the date of his termination or, if required to comply
with applicable tax laws, six months and a day following the
date of his termination;
|
| |
| |
•
|
a pro rata portion of his annual bonus for the performance year
in which the executive’s termination occurs, payable at the
time that annual bonuses are paid to other senior executives or,
if required to comply with applicable tax laws, six months and a
day following the date of his termination, (determined in each
case by multiplying the executive’s target bonus by a
fraction, the numerator of which is the number of days during
the performance year of termination that the executive was
employed by the company and the denominator of which is
365); and
|
| |
| |
•
|
12 months of continued medical and dental insurance
coverage.
|
As further described above in the Compensation Discussion and
Analysis, an employment agreement amendment authorized by our
Board provides that each named executive officer other than the
Chief Executive Officer or Chief Financial Officer may become
entitled to an additional lump sum payment equal to six months
of salary and an additional six months of continued medical and
dental coverage in the case of termination of employment by us
without cause or the named executive officer’s termination
for good reason during the twelve month period following a
change in control.
Qualifying Termination during the Protected
Period. If, during the “protected
period” (as defined below), a named executive officer
resigns without good reason, we are obligated to provide him
with the following payments
and/or
benefits, subject to the executive’s execution of a release:
|
|
|
| |
•
|
the Accrued Amounts;
|
| |
| |
•
|
a payment equal to one-times the executive’s then-current
annual base salary plus, in the case of the chief executive
officer and chief operating officer, one times his full then
current target bonus, payable in a single lump sum on the 60th
day following the date of his termination or, if required to
comply with applicable tax laws, six months and a day following
the date of his termination;
|
| |
| |
•
|
all of the executive’s outstanding and unvested stock
options and other equity awards shall become fully
vested (except that in the case of chief executive officer
and chief operating officer, all of their equity awards
automatically vest solely upon a change in control);
|
| |
| |
•
|
a pro rata portion of his annual bonus for the performance year
in which the executive’s termination occurs, payable at the
time that annual bonuses are paid to other senior executives
(determined by multiplying the executive’s target bonus by
a fraction, the numerator of which is the number of days during
the performance year of termination that the executive was
employed by the company and the denominator of which is
365); and
|
| |
| |
•
|
12 months of continued medical and dental insurance
coverage.
|
For this purpose, the “protected period” shall mean
the period beginning on the effective date of a change in
control (as defined in the 2011 Omnibus Incentive Plan as in
effect on the date of this prospectus) and ending on the first
anniversary of such change in control.
“Cause” for purposes of the executive employment
agreements consists of:
|
|
|
| |
i.
|
The failure of the executive to perform any of his or her
material duties to the company, which in the case of the chief
executive officer and chief operating officer has a material
adverse impact on us;
|
|
|
|
| |
ii.
|
The failure or refusal to comply in a material way with the
company’s code of conduct, conflict of interest or other
material employment policies which, in the case of the chief
executive officer and chief operating officer, relates in a
material way to their service with us;
|
113
|
|
|
| |
iii.
|
Any act or omission to act by the executive (other than the
executive’s resignation or retirement) that would
reasonably be likely to have the effect of injuring the
reputation, business or business relationships of the company;
|
|
|
|
| |
iv.
|
Acts of theft, embezzlement, fraud, dishonesty,
misrepresentation or falsification of documents or records
involving the company;
|
|
|
|
| |
v.
|
A breach of the terms of the executive’s confidentiality
agreement, or any other material agreement between the executive
and the company, after giving effect to the notification
provisions, if any, and the mechanisms to remedy or cure a
breach, if appropriate, as described in any such
agreement; or
|
|
|
|
| |
vi.
|
The executive is convicted of or takes a plea of nolo contendere
for a felony or a crime involving moral turpitude.
|
In the event the company determines that it may be necessary to
terminate the executive’s employment for cause as defined
in paragraphs (i) through (v) above, and such event of
cause is capable of being cured, the executive shall have
30 days from the date of receipt of written notice from the
company to cure such event. Should the executive fail to cure
the event of cause in accordance with the written notice, his
employment shall be terminated immediately upon expiration of
this thirty (30) day period. If the cause as defined in
paragraphs (i) through (v) above is not capable of
being cured, in each case, the executive’s employment shall
be terminated immediately upon receiving written notice from the
company.
Termination for “good reason” for purposes of the
executive employment agreements means the occurrence of one of
the following conditions without the executive’s consent:
|
|
|
| |
i.
|
A material adverse change in the nature or scope of the
executive’s responsibilities, authorities, powers,
functions or duties;
|
|
|
|
| |
ii.
|
A material reduction in the executive’s base salary, except
for
across-the-board
salary reductions similarly affecting all, or substantially all,
similarly-situated employees; or
|
|
|
|
| |
iii.
|
The company requires the executive to relocate to a location
outside of a 50 mile radius from Quincy, Massachusetts (or,
in the case of Mr. Fogleman, to a location outside of a
50-mile
radius from the Louisiana Plant).
|
Notwithstanding the foregoing, the conditions described
immediately above in (i.) through (iii.) shall not
give rise to a termination by an executive for good reason,
unless the executive has notified the company in writing within
30 days of the initial occurrence of such condition, the
company has failed to correct the condition within 60 days
after the company’s receipt of such written notice, and the
executive actually terminates employment with the company within
90 days of the initial occurrence of the condition.
Notwithstanding the foregoing, a suspension of an
executive’s title and authority while on administrative
leave due to a reasonable belief that the executive has engaged
in misconduct, whether or not the suspected misconduct
constitutes cause for employment termination, shall not be
considered good reason.
Change
in Control
The employment agreements with our chief executive officer and
chief operating officer provide for all equity awards granted to
them to fully vest upon a change in control, provided that the
executive is then employed by us. All of the restricted stock
units and performance based stock options granted to the named
executive officers in May 2011 that are described in the
Compensation Discussion and Analysis above fully vest upon a
change in control. The definition of change in control for this
purpose is the same as used for a Qualified Termination During
the Protected Period as described above.
In addition, unless otherwise determined in a specific case by
majority vote of the Board, a change in control shall not be
deemed to have occurred solely because (i) the Company,
(ii) a subsidiary, (iii) any one or more members of
the executive management of the Company or their affiliates,
(iv) any employee stock ownership plan or any other employee
benefit plan of the Company or any subsidiary or (v) any
combination of the persons referred to in the preceding clauses
(i) through (iv) becomes the actual or beneficial
owner
114
(within the meaning of Rule 13d-3 promulgated under the
Securities and Exchange Act of 1934, as amended) of at least 50%
of the combined voting power of the voting securities of the
Company then outstanding after giving effect to such acquisition.
Post
Termination Payment Table
The following table sets forth the estimated potential payments
upon termination or a change in control for each of the named
executive officers, based on the assumptions following the table
and assuming such event occurred on December 31, 2010. The
amounts set forth in the Post Termination Payment Table for
Mr. Tapia were calculated based on the severance and change
in control benefits in effect for him in 2010. Mr. Fogleman
was not subject to any severance or change in control benefits
in 2010.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination Without Cause or for Good Reason
|
|
Termination Without Cause or for Any Reason Within One(1)
Year Following A Change in Control
|
|
|
|
Name
|
|
Salary
|
|
Bonus(1)
|
|
Total
|
|
Salary
|
|
Bonus
|
|
Equity Acceleration
|
|
Total
|
|
|
|
Stephen J. Gatto
|
|
$
|
550,000
|
|
|
$
|
1,100,000
|
|
|
$
|
1,650,000
|
|
|
$
|
550,000
|
|
|
$
|
1,100,000
|
|
|
$
|
0
|
|
|
$
|
1,650,000
|
|
|
Ralph A. Tapia
|
|
$
|
93,500
|
|
|
$
|
46,750
|
|
|
$
|
140,250
|
|
|
$
|
93,500
|
|
|
$
|
46,750
|
|
|
$
|
0
|
|
|
$
|
140,250
|
|
|
A. Cenan Ozmeral Ph.D.
|
|
$
|
300,000
|
|
|
$
|
150,000
|
|
|
$
|
450,000
|
|
|
$
|
300,000
|
|
|
$
|
150,000
|
|
|
$
|
284,432
|
|
|
$
|
734,432
|
|
|
Samuel G. McConnell
|
|
$
|
206,000
|
|
|
$
|
103,000
|
|
|
$
|
309,000
|
|
|
$
|
206,000
|
|
|
$
|
103,000
|
|
|
$
|
0
|
|
|
$
|
309,000
|
|
|
Rudy E. Fogleman(2)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
(1) |
|
Based on the target bonus percentages as in effect on
December 31, 2010. |
| |
|
(2) |
|
Mr. Fogleman did not have an employment agreement with us
in 2010. |
Assumptions Regarding Post Termination Payment
Table. The table presented above was prepared as
though each named executive officer’s employment was
terminated on December 31, 2010 using a stock price of
$5.35. The amounts under the column labeled “Termination
without Cause or for Any Reason Within One (1) Year
Following A Change In Control” assume that a change in
control occurred on December 31, 2010. We are required by
the SEC to use these assumptions. With those assumptions taken
as a given, we believe that the remaining assumptions listed
below, which are necessary to produce these estimates and
reflect solely our interpretation of the company’s
contractual obligations, are reasonable in the aggregate.
However, the executives’ employment was not terminated on
December 31, 2010, and a change in control did not occur on
that date. There can be no assurance that a termination of
employment, a change in control or both would produce the same
or similar results as those described if either or both of them
occur on any other date or at any other price of our common
stock, or if any assumption is not in fact correct.
The following assumptions were used for these tables:
|
|
|
| |
•
|
in determining estimated severance benefits, the salary for each
executive’s year of employment termination equals his
salary in effect on December 31, 2010,
|
| |
| |
•
|
stock options and restricted stock vested on December 31,
2010, with respect to a executive’s termination of
employment by us without cause or a termination by the executive
for any reason within one (1) year of a change in control
for each named executive officer, and
|
| |
| |
•
|
stock options and restricted shares that become vested due to an
executive’s termination of employment by us without cause
or a termination by the executive for any reason within one year
of a change in control are valued in the tables above based on
their intrinsic value on December 31, 2010 (i.e., the
difference between the stock’s fair market value and the
exercise or purchase price, if any).
|
Amount Not Covered by Post Termination Payment
Table. The table presented above does not include
the value of stock options that were vested as of
December 31, 2010 that are described in the Outstanding
Equity Awards at 2010 Fiscal Year End Table, the value of
increased severance benefits available under new employment
agreements extended to Mr. Tapia and Mr. Fogleman in
May 2011, the enhancement to severance benefits under employment
agreement changes authorized by the compensation committee in
2011
115
and described in the Compensation Discussion &
Analysis, and the potential acceleration value with respect to
equity awards that were granted after December 31, 2010.
EMPLOYEE
BENEFIT AND STOCK PLANS
Myriant
Technologies, Inc. 2011 Omnibus Incentive Plan
On January 12, 2011, we established the Myriant
Technologies, Inc. 2011 Omnibus Incentive Plan. On May 27,
2011, we increased the shares subject to this plan as described
below, and our stockholders subsequently approved the amendment
and restatement of this plan, which we refer to in this
prospectus as the “2011 Omnibus Incentive Plan”. The
purposes of the 2011 Omnibus Incentive Plan are to attract,
reward and retain highly competent persons, as employees,
directors and consultants, to provide additional incentives to
such individuals by aligning their interests with those of our
company’s stockholders, and to promote the success of the
business of our company. To accomplish these purposes, the 2011
Omnibus Incentive Plan will provide for the issuance of stock
options, stock appreciation rights, restricted shares,
restricted share units, or other equity-based awards.
The following description summarizes the features of the 2011
Omnibus Incentive Plan.
Summary
of plan terms
Shares Subject to the 2011 Omnibus Incentive
Plan. An aggregate of 4,189,130 shares of
our common stock was reserved under the 2011 Omnibus Incentive
Plan. The number of shares reserved for issuance under the 2011
Omnibus Incentive Plan shall be subject to an annual increase on
January 1st of each year following the completion of
this offering until 2016 equal to the lesser of
(i) two million shares of our common stock,
(ii) five percent (5%) of the outstanding shares of our
common stock as of the 30th day following the closing of
this offering, and (iii) such lesser amount determined by
our board of directors. The number of shares of our common stock
authorized for grant under the 2011 Omnibus Incentive Plan,
including shares that may be added as described above, is
subject to adjustment for certain significant corporate events
as described below. Shares related to awards that terminate in
whole or in part by expiration, termination, surrendering,
cancellation, or otherwise without issuance of shares shall
again be available for future grant or sale under the 2011
Omnibus Incentive Plan, subject to any limitations imposed by
the Code applicable to incentive stock options. Shares not
issued upon exercise of a stock option or stock appreciation
right, shares delivered to us to pay the option exercise price
upon exercise and shares delivered to us to pay for withholding
taxes shall be available for grant under the 2011 Omnibus
Incentive Plan.
As soon as practicable after the completion of this offering, we
intend to file with the SEC a registration statement on
Form S-8
under the Securities Act covering the shares issuable under the
2011 Omnibus Incentive Plan. This registration statement will
become effective immediately upon filing, and shares covered by
this registration statement will thereupon be eligible for sale
in the public markets, subject to Rule 144 limitations
applicable to affiliates and any
lock-up
agreements.
Plan Administration. The 2011 Omnibus
Incentive Plan will be administered by our compensation
committee. The 2011 Omnibus Incentive Plan is
structured so that awards under it may qualify as
performance-based compensation exempt from Section 162(m)
of the Code. Nevertheless, we intend to make equity grants under
the IPO transition rule in order to maximize our tax deductions.
Our compensation committee, or in certain cases our Chief
Executive Officer, will determine who shall receive awards under
the 2011 Omnibus Incentive Plan, the number of shares of stock
and/or
dollars covered by such award, and the terms and conditions of
each award. Within the terms of the 2011 Omnibus Incentive Plan,
our compensation committee may accelerate the vesting of any
award. Our compensation committee may also modify any equity
award after the grant date, including adjusting the exercise
price, as well as cancel or substitute any awards. In addition,
our compensation committee will interpret the 2011 Omnibus
Incentive Plan and may adopt any administrative rules,
regulations, procedures and guidelines governing the 2011
Omnibus Incentive Plan or any awards granted under the 2011
Omnibus Incentive Plan as it deems to be appropriate.
Eligibility. Our compensation committee may
grant awards to employees, consultants, and directors; provided,
however, only employees shall be eligible to receive incentive
stock options.
116
Types of Awards. The following types of awards
may be made under the 2011 Omnibus Incentive Plan. All of the
awards described below are subject to the conditions,
limitations, restrictions, vesting and forfeiture provisions
determined by our compensation committee (or in certain cases,
our Chief Executive Officer), in its sole discretion, subject to
such limitations as are provided in the 2011 Omnibus Incentive
Plan.
Nonqualified Stock Options. An award of a
nonqualified stock option grants a participant the right to
purchase a certain number of shares of our common stock during a
specified term in the future, after a vesting period, at an
exercise price equal to at least 100% of the fair market value
of a share of our common stock on the grant date. The term of a
nonqualified stock option may not exceed 10 years from the
date of grant. The exercise price may be paid with cash or a
cash equivalent approved by the compensation committee. A
nonqualified stock option is an option that does not meet the
qualifications of an incentive stock option as described below.
Incentive Stock Options. An incentive stock
option is a stock option that meets the requirements of
Section 422 of the Code, which include an exercise price of
no less than 100% of fair market value on the grant date, a term
of no more than 10 years, and that the option be granted
from a plan that has been approved by stockholders.
Stock Appreciation Rights. A stock
appreciation right (“SAR”) entitles the participant to
receive an amount equal to the difference between the fair
market value of our common stock on the exercise date and the
exercise price of the SAR (which may not be less than 100% of
the fair market value of a share of our common stock on the
grant date), multiplied by the number of shares subject to the
SAR. Payment to a participant upon the exercise of a SAR may be
in cash or shares of our common stock. Our compensation
committee may grant SARs in tandem with options or independent
of them.
Restricted Shares. A restricted share award is
an award of outstanding shares of our common stock that does not
vest until after a specified period of time, or satisfaction of
other vesting conditions as determined by our compensation
committee, and which may be forfeited if conditions to vesting
are not met. Participants are generally entitled to receive all
dividends and distributions paid with respect to shares subject
to their restricted share award. If such dividends or
distributions are paid in shares, such shares shall be issued to
the participant as an additional restricted share award, subject
to the same terms, conditions, and vesting restrictions, if any,
as the restricted share award with respect to which they were
paid. If any such dividends or distributions are paid in cash,
such cash payments shall be subject to the same restrictions as
the restricted share award and shall be accumulated during any
period of restriction applicable to the award and paid (or
forfeited) when the award vests (or is forfeited). Participants
are also generally entitled to vote the shares subject to their
restricted share award, subject to the terms of any voting
agreement they may have exercised with us.
Restricted Share Units. A restricted share
unit is an award denominated in shares of our common stock that
may be settled either in shares or cash, subject to terms and
conditions determined by our compensation committee (or in
certain cases, our Chief Executive Officer). Participants do not
have voting rights, but our compensation committee may authorize
the payment of dividend equivalent payments during the vesting
period.
Performance Awards. The 2011 Omnibus Incentive
Plan authorizes our compensation committee to grant
performance-based awards, which may be payable in shares, share
units, or cash. Performance awards would vest and become payable
upon the achievement of performance objectives within a period
of time specified by our compensation committee. Our
compensation committee may, in its discretion, authorize the
payment of dividend equivalent payments with respect to any
performance award paid in stock.
The performance awards may be subject to the achievement of
specified performance objectives. Performance objectives with
respect to any award may include any one or more financial or
operational objectives or a combination thereof, as established
by the compensation committee in its sole discretion, which may
be applicable on a company-wide basis
and/or with
respect to operating units, divisions, subsidiaries, acquired
businesses, minority investments, partnerships, or joint
ventures, as set forth below:
|
|
|
| |
•
|
increasing the company’s net sales;
|
| |
| |
•
|
achieving a target level of earnings (including gross earnings;
earnings before certain deductions, such as interest, taxes,
depreciation, or amortization; or earnings per share);
|
117
|
|
|
| |
•
|
achieving a target level of income (including net income or
income before consideration of certain factors, such as
overhead) or a target level of gross profits for the company, an
affiliate, or a business unit;
|
| |
| |
•
|
achieving a target return on the company’s (or an
affiliate’s) sales, revenues, capital, assets, or
stockholders’ equity;
|
| |
| |
•
|
maintaining or achieving a target level of appreciation in the
price of the shares;
|
| |
| |
•
|
increasing the company’s (or an affiliate’s) market
share to a specified target level;
|
| |
| |
•
|
achieving or maintaining a share price that meets or exceeds the
performance of specified stock market indices or other
benchmarks over a specified period;
|
| |
| |
•
|
achieving a level of share price, earnings, or income
performance that meets or exceeds performance in comparable
areas of peer companies over a specified period;
|
| |
| |
•
|
achieving specified reductions in costs or targeted levels in
costs;
|
| |
| |
•
|
achieving specified improvements in collection of outstanding
accounts or specified reductions in non-performing debts;
|
| |
| |
•
|
achieving a level of cash flow;
|
| |
| |
•
|
introducing one or more products into one or more new markets;
|
| |
| |
•
|
acquiring a prescribed number of new customers in a line of
business;
|
| |
| |
•
|
achieving a prescribed level of productivity within a business
unit;
|
| |
| |
•
|
completing specified projects within or below the applicable
budget;
|
| |
| |
•
|
completing acquisitions of other businesses or integrating
acquired businesses;
|
| |
| |
•
|
expanding into other markets;
|
| |
| |
•
|
scientific or regulatory achievements;
|
| |
| |
•
|
implementation, completion or attainment of measurable
objectives with respect to research, development, patents,
inventions, products, projects or facilities and other key
performance indicators; and
|
| |
| |
•
|
similar objectives as may be determined by the compensation
committee from time to time.
|
Our compensation committee may, in its sole discretion, reduce
(but not increase) the number of shares deliverable or amounts
payable under any performance-based award after the applicable
performance objectives have been satisfied.
Other Forms of Equity Award. The 2011 Omnibus
Incentive Plan provides our compensation committee the
discretion to grant other awards payable in shares, such as
deferred stock units, unrestricted shares, securities
convertible into shares or a right to purchase shares at a
discount to the then current fair market value. In the event of
such an award, the committee would determine the terms and
conditions of such award, including any vesting criteria
applicable thereto.
Forfeiture Provisions. Our compensation
committee may provide by rule or regulation or in any award
agreement, or may determine in any individual case, the
circumstances in which awards shall be paid or forfeited in the
event a participant ceases to provide services to us prior to
the end of a performance period, period of restriction or the
exercise, vesting or settlement of such award.
Adjustments. Our compensation committee shall
make appropriate equitable adjustments to the maximum number of
shares available for issuance under the 2011 Omnibus Incentive
Plan and other limits stated in the 2011 Omnibus Incentive Plan,
the number of shares covered by outstanding awards, the grant,
purchase, or exercise prices, if any, of all outstanding awards,
performance measures applicable to outstanding awards, and the
kind of shares available for grant and covered by outstanding
awards, as our compensation committee, in its sole discretion,
may determine to be equitably required to prevent dilution or
enlargement of the rights of
118
participants. These changes may be made to reflect changes in
our capital structure (including a change in the number of
shares of common stock outstanding) on account of any
extraordinary dividend or other extraordinary distribution that
occurs in respect of the shares, or any reclassification,
recapitalization, stock split (including a stock split in the
form of a stock dividend), reverse stock split, reorganization,
merger, consolidation,
split-up,
spin-off, combination, repurchase, or exchange of shares or
other securities of the company or any similar, unusual or
extraordinary corporate transaction (or event in respect of the
shares), including a change in control.
In addition, in connection with a merger, consolidation, or
similar transaction, our compensation committee may grant awards
under the 2011 Omnibus Incentive Plan in substitution for stock
and stock-based awards held by employees, directors, consultants
or advisors of another corporation (an “Acquired
Corporation”) in connection with a merger, consolidation or
similar transaction involving such Acquired Corporation and the
company or an affiliate or the acquisition by the company or an
affiliate of property or stock of the Acquired Corporation. Any
substitute awards granted under the 2011 Omnibus Incentive Plan
shall not count against the share limitations described above.
Accelerated Vesting in Connection with a Change in
Control. Except as otherwise determined by the
Committee or as required under an employment agreement, equity
awards that vest based on continued employment with us will
become 100% vested immediately upon a termination of employment
by the company or the employee resigning for good reason within
the twelve month period that begins on a change in control. Good
reason for purposes of this provision under the 2011 Omnibus
Incentive Plan means either a material adverse change in the
employee’s responsibilities, authority, powers, functions
or duties, a material reduction in base salary or a relocation
to a office more than 50 miles from the then current office
location. Except as otherwise provided in an employment
agreement, awards that vest based on performance conditions
shall only be subject to accelerated vesting as determined in
the discretion of the compensation committee.
Change in Control A change in control under
the 2011 Omnibus Incentive Plan occurs if there is either:
(1) A merger or consolidation in which the company is a
party or a subsidiary of the company is a party and the company
issues shares of its capital stock pursuant to such merger or
consolidation, except any such merger or consolidation involving
the company or a subsidiary in which the shares of capital stock
of the company outstanding immediately prior to such merger or
consolidation continue to represent, or are converted into or
exchanged for shares of capital stock that represent,
immediately following such merger or consolidation at least a
majority, by voting power, of the capital stock of (a) the
surviving or resulting corporation or (b) if the surviving
or resulting corporation is a wholly owned subsidiary of another
corporation immediately following such merger or consolidation,
the parent corporation of such surviving or resulting
corporation (provided that, for the purpose of this definition,
all shares issuable upon the exercise of stock options
outstanding immediately prior to such merger or consolidation or
upon conversion of any other convertible securities outstanding
immediately prior to such merger or consolidation shall be
deemed to be outstanding immediately prior to such merger or
consolidation and, if applicable, converted or exchanged in such
merger or consolidation on the same terms as the actual
outstanding Shares are converted or exchanged); or
(2) the acquisition by any person or group, other than
holders of capital stock of the Company on January 13,
2011, of a majority of the voting power of the outstanding
shares of capital stock of the Company (other than a financing
transaction, the proceeds of which are paid to the Company and
not directly or indirectly to any stockholders of the Company
including but not limited to an initial public offering); or
(3) the sale, lease, transfer, exclusive license, or other
disposition, in a single transaction or series of related
transactions, by the Company and its subsidiaries of all or
substantially all the assets of the Company and its subsidiaries
taken as a whole or the sale or disposition (whether by merger
or otherwise) of one or more subsidiaries of the Company if
substantially all of the assets of the Company and its
subsidiaries taken as a whole are held by such subsidiary or
subsidiaries, except where such sale, lease, transfer, exclusive
license or other disposition is to a wholly owned subsidiary of
the Company; or
(4) the sale or transfer of all of the company’s and
its subsidiaries’ right, title and interest in and to, or
encumbrance of title to (other than through a license), all
material intellectual property rights of the Company and its
subsidiaries, other than licensing transactions in the ordinary
course of business of the
119
Company or its subsidiaries such as corporate partnering and
commercial licensing transactions; provided, however that this
paragraph (4) shall not apply to any equity awards granted
after May 27, 2011.
In addition, unless otherwise determined in a specific case by
majority vote of the Board, a change in control shall not be
deemed to have occurred solely because (i) the company,
(ii) a subsidiary, (iii) any one or more members of
the executive management of the Company or their affiliates,
(iv) any employee stock ownership plan or any other
employee benefit plan of the Company or any subsidiary or
(v) any combination of the persons referred to in the
preceding clauses (i) through (iv) becomes the actual
or beneficial owner (within the meaning of
Rule 13d-3
promulgated under the Securities and Exchange Act of 1934, as
amended) of at least 50% of the combined voting power of the
voting securities of the company then outstanding after giving
effect to such acquisition.
Amendment and Termination. The 2011 Omnibus
Incentive Plan may be amended or terminated by our board of
directors or compensation committee at any time; provided, no
amendment shall impair the rights of any participant or
beneficiary under an outstanding award unless consented to in
writing by the participant. No participant consent will be
required for any amendment required to comply with an applicable
law. The company shall obtain stockholder approval for any such
amendment to the extent required by applicable laws.
Grant of
Awards under the 2011 Omnibus Incentive Plan
Set forth below is a summary of the stock options and restricted
stock awards that we have awarded under the 2011 Omnibus
Incentive Plan. Future equity grants under the 2011 Omnibus
Incentive Plan (as well as any performance-based cash incentives
granted thereunder) thereafter will be made at the discretion of
our compensation committee.
| |
|
|
|
|
|
Share Reserve
|
|
Number of Shares
|
|
|
|
|
Shares originally authorized under the 2011 Omnibus Incentive
Plan
|
|
|
939,130
|
|
|
|
|
|
|
|
|
Total number of shares approved on May 27, 2011 for the
2011 Plan
|
|
|
3,250,000
|
|
|
|
|
|
|
|
|
Total number of equity awards granted January 12, 2011
through June 24, 2011 under the 2011 Plan
|
|
|
2,712,762
|
|
|
|
|
|
|
|
|
Total shares available for grant under the 2011 Plan on
June 24, 2011
|
|
|
1,476,368
|
*
|
|
|
|
|
|
|
|
|
|
|
* |
|
The Board may annually increase the number of shares available
under the 2011 Plan beginning in 2012 and ending in 2016 by up
to the lesser of 5% of the outstanding shares of our stock as of
the 30th day after completion of this offering or 2 million
shares. |
Federal
Income Tax Consequences of 2011 Omnibus Incentive Plan
Awards
The following is a brief summary of the principal United States
federal income tax consequences of transactions under the 2011
Omnibus Incentive Plan, based on current United States federal
income tax laws. This summary is not intended to be exhaustive,
does not constitute tax advice and, among other things, does not
describe state, local or foreign tax consequences, which may be
substantially different.
Nonqualified Stock Options. Generally, a
participant will not recognize taxable income on the grant or
vesting of a nonqualified stock option. Upon the exercise of a
nonqualified stock option, a participant will recognize ordinary
income in an amount equal to the difference between the fair
market value of our common stock received on the date of
exercise and the option cost (number of shares purchased
multiplied by the exercise price per share). We will ordinarily
be entitled to a deduction on the exercise date equal to the
ordinary income recognized by the participant upon exercise.
Incentive Stock Options. No taxable income is
recognized by a participant on the grant or vesting of an ISO.
If a participant holds the shares acquired for at least one year
from the exercise date and does not sell or otherwise dispose of
the shares for at least two years from the grant date, the
participant’s gain or loss upon a subsequent sale will be
long-term capital gain or loss equal to the difference between
the amount realized on the sale and the participant’s basis
in the shares acquired. In this case, we will not be entitled to
a deduction
120
by reason of the grant or exercise of the ISO; however the
excess of the fair market value over the exercise price of the
shares acquired is an item of adjustment in computing
alternative minimum tax of the participant. If a participant
sells or otherwise disposes of the shares acquired without
satisfying the required minimum holding period, such
“disqualifying disposition” will give rise to ordinary
income equal to the excess of the fair market value of the
shares acquired on the exercise date or, if less, the amount
realized upon disqualifying disposition over the
participant’s tax basis in the shares acquired. We will
ordinarily be entitled to a deduction equal to the amount of the
ordinary income resulting from a disqualifying disposition.
Stock Appreciation Rights. Generally, a
participant will not recognize taxable income upon the grant or
vesting of a Stock Appreciation Right, or SAR, but will
recognize ordinary income upon the exercise of a SAR in an
amount equal to the cash amount received upon exercise (if the
SAR is cash-settled) or the difference between the fair market
value of our common stock received from the exercise of the SAR
and the amount, if any, paid by the participant in connection
with the exercise of the SAR. The participant will recognize
ordinary income upon the exercise of a SAR regardless of whether
the shares of our common stock acquired upon the exercise of the
SAR are subject to further restrictions on sale or
transferability. The participant’s basis in the shares will
be equal to the ordinary income attributable to the exercise and
the amount, if any, paid in connection with the exercise of the
SAR. Upon the exercise of a SAR, we will ordinarily be entitled
to a deduction in the amount of the ordinary income recognized
by the participant.
Restricted Shares. A participant generally
will not be taxed at the time of a restricted share award but
will recognize taxable income when the award vests or otherwise
is no longer subject to a substantial risk of forfeiture. The
amount of taxable income will be the fair market value of the
shares at the time of vesting.
Participants may elect to be taxed at the time of grant by
making an election under Section 83(b) of the Code within
30 days of the award date. If a restricted share award
subject to the Section 83(b) election is subsequently
canceled, no deduction will be allowed for the amount previously
recognized as income, and no tax previously paid will be
refunded. Unless a participant makes a Section 83(b)
election, dividends paid to a participant on shares of an
unvested restricted share award will be taxable to the
participant as ordinary income. If the participant made a
Section 83(b) election, the dividends will be taxable to
the participant as dividend income.
We will ordinarily be entitled to a deduction at the same time
and in the same amounts as the ordinary income recognized by the
participant. Unless a participant has made a Section 83(b)
election, we will also be entitled to a deduction, for federal
income tax purposes, for dividends paid on unvested restricted
share awards.
Restricted Share Units. A participant
generally will not be subject to income tax at the time of grant
of a restricted share unit award or upon vesting but will
recognize taxable income upon receiving shares under the award
and any cash that is attributable to dividend equivalents.
Restricted share units are subject to the Federal Insurance
Contribution Act tax upon vesting. The amount of taxable income
will be the fair market value of the shares at the time of
issuance. No Section 83(b) election is available for
restricted share units.
We will ordinarily be entitled to a deduction at the same time
and in the same amounts as the ordinary income recognized by the
participant. We will also be entitled to a deduction, for
federal income tax purposes, for cash dividend equivalent
payments, if any, on restricted share units.
Other Equity-Based Awards. A participant will
generally not recognize taxable income on a deferred stock award
until shares subject to the award are distributed. The amount of
this ordinary income will be the fair market value of the shares
of our common stock on the date of distribution. Any dividend
equivalents paid on unvested deferred stock awards are taxable
as ordinary income when paid to the participant. We will
ordinarily be entitled to a deduction at the same time and in
the same amounts as the ordinary income recognized by the
participant. We will also be entitled to a deduction, for
federal income tax purposes, on any dividend equivalent payments
made to the participant.
A participant will generally recognize taxable income on the
grant of unrestricted shares, in an amount equal to the fair
market value of the shares on the grant date. We will ordinarily
be entitled to a deduction at the same time and in the same
amounts as the ordinary income recognized by the participant.
121
Cash Awards. A participant will generally
recognize taxable income upon the payment of a cash award, in an
amount equal to the amount of the cash received. We will
ordinarily be entitled to a deduction at the same time and in
the same amounts as the ordinary income recognized by the
participant.
Withholding. To the extent required by law, we
will withhold from any amount paid in settlement of an award
amounts of withholding and other taxes due or take other action
as we deem advisable to enable ourselves to satisfy withholding
and tax obligations related to any awards.
2011
Employee stock purchase plan
Background. We plan to adopt and implement a
2011 employee stock purchase plan designed to enable
eligible employees to periodically purchase shares of our common
stock at a discount. Purchases will initially be accomplished
through participation in discrete monthly offering periods, at
purchase prices that are 15% below the closing price for our
shares on the last date of the applicable purchase period. Our
2011 employee stock purchase plan is intended to qualify as
an employee stock purchase plan under Section 423 of the
Code, received stockholder approval and will become effective
upon consummation of our initial public offering.
Share reserve. We expect that we will
initially reserve 500,000 shares of our common stock for
issuance under our 2011 employee stock purchase plan.
Administration. Our compensation committee
will administer our 2011 employee stock purchase plan.
Employees who are five percent stockholders, or would become
five percent stockholders as a result of their participation in
our 2011 employee stock purchase plan, as well as seasonal
employees (i.e., less than 5 months per year) and temporary
employees (i.e. less than 20 hours per week) are ineligible
to participate in our 2011 employee stock purchase plan. We
may impose additional restrictions on eligibility as well. Under
our 2011 employee stock purchase plan, eligible employees
will be able to acquire shares of our common stock by
accumulating funds through payroll deductions. Our eligible
employees will be able to select a rate of payroll deduction
between one percent and 25% of their cash compensation. We will
also have the right to amend or terminate our 2011 employee
stock purchase plan, except that, subject to certain exceptions,
no such action may adversely affect any outstanding rights to
purchase stock under the plan. Our 2011 employee stock
purchase plan will terminate on the 10th anniversary of our
initial public offering, unless it is terminated earlier by our
board of directors.
Purchase rights. When an offering period
commences, our employees who meet the eligibility requirements
for participation in that offering period will be automatically
granted a non-transferable option to purchase shares in that
offering period. Each offering period may run for no more than
27 months and consist of no more than five purchase periods
consistent with applicable tax requirements under
section 423 of the code. An employee’s participation
will automatically end upon termination of employment for any
reason.
No participant will have the right to purchase our shares at a
rate which, when aggregated with purchase rights under all our
employee stock purchase plans that are also outstanding in the
same calendar year(s), have a fair market value of more than
$25,000, determined as of the first day of the applicable
offering period, for each calendar year in which such right is
outstanding. The purchase price for shares of our common stock
purchased under our 2011 employee stock purchase plan will
initially be 85% of the fair market value of our common stock on
the last trading day of each purchase period in the applicable
offering period, although our 2011 employee stock purchase
plan authorizes the purchase price to be 85% of the lesser of
the fair market value of our common stock on (i) the first
trading day of the applicable offering period and (ii) the
last trading day of each purchase period in the applicable
offering period. In no event may an employee purchase more than
1,250 shares during a calendar year.
Change in control. In the event of a corporate
transaction (as defined in our 2011 employee stock purchase
plan), the offering period for such purchase rights will be
shortened and end on a new purchase date immediately prior to
the consummation of the corporate transaction, and no new
offering period will commence.
122
Confidentiality,
non-competition, non-solicitation and inventions
agreement
Each of our named executive officers has entered into a standard
form agreement with respect to confidential information,
non-competition, non-solicitation and inventions. Among other
things, this agreement obligates each employee to refrain from
disclosing any of our proprietary information received during
the course of employment and, with some exceptions, to assign to
us any inventions conceived or developed during the course of
employment. Additionally, each employee is subject to certain
non-competition and non-solicitation covenants during the course
of employment and for a period of one year thereafter.
Certain
relationships and related party transactions
We describe below transactions, since our formation
(April 3, 2009), in which we are involved as a party or
otherwise and:
|
|
|
| |
•
|
the amounts involved exceeded or will exceed $120,000; and
|
| |
| |
•
|
a director, executive officer, holder of more than 5% of our
common stock or any member of their immediate family had or will
have a direct or indirect material interest.
|
STOCK
ISSUANCES
Issuance
of Class A common stock
On January 13, 2011, we sold an aggregate of
11,214,953 shares of Class A common stock at a price
of $5.35 per share for gross proceeds of approximately
$60 million, which resulted in net proceeds of
$57.1 million. The table below sets forth the number of
shares of Class A common stock sold to our directors,
executive officers and 5% stockholders and their affiliates.
| |
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
|
|
|
Shares of
|
|
Aggregate
|
|
|
|
Class A
|
|
Purchase
|
|
Investor
|
|
Common Stock
|
|
Price
|
|
|
|
PTT Chemical International Private Limited(1)(2)(3)
|
|
|
11,214,953
|
|
|
$
|
60,000,000
|
|
|
|
|
|
(1) |
|
Puntip Oungpasuk is one of our directors and is the Executive
Vice President of Strategy and International Affairs of PTT
Chemical Public Company Limited. PTT Chemical Public Company
Limited is the parent corporation of PTT Chemical International
Private Limited. |
| |
|
(2) |
|
Narongsak Jivakanun is one of our directors and is the chief
executive officer of PTT Chemical International Private
Limited. |
| |
|
(3) |
|
Thitipong Jurapornsiridee is one of our directors and is the
Vice President of Corporate Finance of PTT Chemical Public
Company Limited. PTT Chemical Public Company Limited is the
parent corporation of PTT Chemical International Private
Limited. |
Issuance
of Class B common stock
On January 13, 2011, we entered into a Debt Conversion
Agreement with certain of our investors holding our 15% secured
convertible promissory notes and warrants to purchase shares of
our common stock. Pursuant to the agreement, such investors
converted the principal and accrued interest of such promissory
notes and used a portion of the proceeds from the conversion
thereof to purchase shares of common stock pursuant to their
warrants at a purchase price of $6.00 per share. The outstanding
balance of such notes was used to purchase shares of
Class B common stock at a price of $5.35 per share for
gross cancellation of debt proceeds of approximately
$23 million. The table below sets forth the number of
shares of common stock and Class B common stock issued to
our directors, executive officers and 5% stockholders and their
affiliates upon the conversion of the notes.
123
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
Shares of
|
|
|
|
|
|
Number of
|
|
Class B
|
|
Amount of
|
|
|
|
Shares of
|
|
Common
|
|
Debt
|
|
Investor
|
|
Common Stock
|
|
Stock
|
|
Converted
|
|
|
|
Entities affiliated with Plainfield Direct LLC(1)(2)
|
|
|
1,007,565
|
|
|
|
2,846,870
|
|
|
$
|
21,276,144
|
|
|
Norwood LDK, LLC(3)
|
|
|
34,744
|
|
|
|
50,476
|
|
|
$
|
478,511
|
|
|
Entities affiliated with Itera Ethanol LLC(4)
|
|
|
75,069
|
|
|
|
147,559
|
|
|
$
|
1,239,857
|
|
|
|
|
|
(1) |
|
Includes 1,007,565 shares of common stock held by
Plainfield Finance II LLC and 2,846,870 shares of
Class B common stock held by Plainfield Direct LLC.
Subsequently, Plainfield Direct LLC transferred its shares of
Class B common stock to Plainfield Finance II LLC. |
| |
|
(2) |
|
Keith Carter is one of our directors and is a managing
director of Plainfield Asset Management, which is the parent
company of each of Plainfield Direct LLC and Plainfield
Finance II LLC. |
| |
|
(3) |
|
The managing member of Norwood LDK, LLC is Stephen J. Gatto,
the chief executive officer of the company, and the other
members of Norwood LDK, LLC are members of Mr. Gatto’s
immediate family. At the time of the agreement, Stephen J. Gatto
entered into such agreement personally, as Mr. Gatto
provided a loan to Norwood LDK, LLC for the purchase of the
securities described herein, which will be repaid by Norwood
LDK, LLC, and the securities were immediately registered in the
name of Norwood LDK, LLC. |
| |
|
(4) |
|
Includes 28,673 shares of common stock held by Itera
Ethanol, LLC, 46,396 shares of common stock and
82,942 shares of Class B common stock held by Green
Chem Holdings LLC and 64,617 shares of Class B common
stock held by Green Chem Second Edition, LLC. Steven Sisselman
is one of our directors and is an executive officer of each of
Itera Ethanol, LLC Green Chem Holdings LLC and Green Chem Second
Edition, LLC. |
2010
Bridge financing
In November 2010, we sold secured convertible promissory notes,
or the Bridge Notes, to certain of our investors in the
aggregate principal amount of $1.7 million. The Bridge
Notes accrued interest at a rate of 15% per annum and had a
maturity date of November 2015. Payments on the Bridge Notes
were deferred until November 2012. In January 2011, in
connection with the issuance of Class B common stock and
the Debt Conversion Agreement described above, the full
principal amount of and accrued but unpaid interest on the
Bridge Notes, along with the full principal amount of and
accrued but unpaid interest on the other notes of the company
outstanding, was first used to purchase an aggregate of
1,117,378 shares of common stock at an exercise price of
$6.00 per share, with the remaining balance used to purchase an
aggregate of 3,044,905 shares of Class B common stock.
In connection with the Bridge Notes, we issued warrants to
purchase an aggregate of 913,100 units of interest at an
exercise price of $0.01 per share to the purchasers of the
Bridge Notes. Upon our conversion to a corporation in January
2011, the warrants were converted to warrants to purchase shares
of our common stock. The warrants may be exercised at any time
prior to their respective expiration dates, which are each
December 15, 2015.
The table below sets forth the principal amount of the Bridge
Notes and the shares of common stock issuable upon the exercise
of the related warrants sold to our directors, executive
officers and 5% stockholders and their affiliates.
| |
|
|
|
|
|
|
|
|
|
|
|
Shares of
|
|
|
|
|
|
Common Stock
|
|
Aggregate
|
|
|
|
Issuable upon
|
|
Principal
|
|
|
|
the Exercise of
|
|
Amount of the
|
|
Investor
|
|
Warrants
|
|
Bridge Notes
|
|
|
|
Plainfield Direct LLC(1)(2)
|
|
|
639,170
|
|
|
$
|
1,190,000
|
|
|
Green Chem Second Edition LLC(3)(4)
|
|
|
182,620
|
|
|
$
|
340,000
|
|
|
Norwood LDK, LLC(5)(6)
|
|
|
91,310
|
|
|
$
|
170,000
|
|
124
|
|
|
|
(1) |
|
Keith Carter is one of our directors and is a managing
director of Plainfield Asset Management, which is the parent
company of Plainfield Direct LLC. |
| |
|
(2) |
|
Subsequently, Plainfield Direct LLC transferred its warrants
to Plainfield Finance II LLC, and the warrants were
exercised in April 2011 for an aggregate purchase price of
$6,391.70. |
| |
|
(3) |
|
Steven Sisselman is one of our directors and is an executive
officer of Green Chem Second Edition LLC. |
| |
|
(4) |
|
Green Chem Second Edition LLC exercised its warrants in March
2011 for an aggregate purchase price of $1,826.20. |
| |
|
(5) |
|
The managing member of Norwood LDK, LLC is Stephen J. Gatto,
the chief executive officer of the company, and the other
members of Norwood LDK, LLC are members of Mr. Gatto’s
immediate family. At the time of the agreement, Stephen J. Gatto
entered into such agreement personally, as Mr. Gatto
provided a loan to Norwood LDK, LLC for the purchase of the
securities described herein, which will be repaid by Norwood
LDK, LLC, and the securities were immediately registered in the
name of Norwood LDK, LLC. |
| |
|
(6) |
|
Norwood LDK, LLC exercised its warrants in March 2011 for an
aggregate purchase price of $913.10. |
July
2010 financing
In July 2010, we sold secured convertible promissory notes, or
the July 2010 Notes, to certain of our investors in the
aggregate principal amount of $3.0 million. The July 2010
Notes accrued interest at a rate of 15% per annum and had a
maturity date of October 10, 2013. In January 2011, in
connection with the issuance of Class B common stock and
the Debt Conversion Agreement described above, the full
principal amount of and accrued but unpaid interest on the July
2010 Notes, along with the full principal amount of and accrued
but unpaid interest on the other notes of the company
outstanding, was first used to purchase an aggregate of
1,117,378 shares of common stock at an exercise price of
$6.00 per share, with the remaining balance used to purchase an
aggregate of 3,044,905 shares of Class B common stock.
The table below sets forth the principal amount of the July 2010
Notes sold to our directors, executive officers and 5%
stockholders and their affiliates.
| |
|
|
|
|
|
|
|
Aggregate
|
|
|
|
Principal
|
|
|
|
Amount of the
|
|
|
|
July 2010
|
|
Investor
|
|
Notes
|
|
|
|
Plainfield Direct LLC(1)
|
|
$
|
2,575,000
|
|
|
Green Chem Holdings LLC(2)
|
|
$
|
300,000
|
|
|
Norwood LDK, LLC(3)
|
|
$
|
125,000
|
|
|
|
|
|
(1) |
|
Keith Carter is one of our directors and is a managing
director of Plainfield Asset Management, which is the parent
company of Plainfield Direct LLC. |
| |
|
(2) |
|
Steven Sisselman is one of our directors and is an executive
officer of Green Chem Second Edition, LLC. |
| |
|
(3) |
|
The managing member of Norwood LDK, LLC is Stephen J. Gatto,
the chief executive officer of the company, and the other
members of Norwood LDK, LLC are members of Mr. Gatto’s
immediate family. At the time of the agreement, Stephen J. Gatto
entered into such agreement personally, as Mr. Gatto
provided a loan to Norwood LDK, LLC for the purchase of the
securities described herein, which will be repaid by Norwood
LDK, LLC, and the securities were immediately registered in the
name of Norwood LDK, LLC. |
February
2010 financing
In February 2010, we sold secured convertible promissory notes,
or the February 2010 Notes, to certain of our investors in the
aggregate principal amount of $5.0 million. The February
2010 Notes accrued interest at a rate of 15% per annum and had a
maturity date of October 10, 2013. In January 2011, in
connection with the issuance of Class B common stock and
the Debt Conversion Agreement described above, the full
principal amount of and accrued but unpaid interest on the
February 2010 Notes, along with the full principal amount
125
of and accrued but unpaid interest on the other notes of the
company outstanding, was first used to purchase an aggregate of
1,117,378 shares of common stock at an exercise price of
$6.00 per share, with the remaining balance used to purchase an
aggregate of 3,044,905 shares of Class B common stock.
In connection with the February 2010 Notes, we exchanged
warrants to purchase units of interest which we had previously
issued to our investors at exercise prices of $10.00 per share
and $13.00 per share, for warrants to purchase units of interest
at an exercise price of $6.00 per share. In addition, we issued
new warrants to purchase an aggregate of 37,744 shares of
units of interest at an exercise price of $6.00 per share. Upon
our conversion to a corporation in January 2011, the warrants
were converted to warrants to purchase shares of our common
stock. The warrants may be exercised at any time prior to their
respective expiration dates, which are August 15, 2015.
In accordance with the note purchase agreement entered into in
connection with the issuance of the February 2010 Notes, Norwood
LDK, LLC was required to forfeit a portion of its units of
interest until such time as a qualified investor acknowledged
that its customary due diligence condition had been met with
respect to a potential equity financing. Norwood LDK, LLC
forfeited 267,864 units of interest to the investors in the
notes in proportion to their ownership interest in the February
2010 Notes pursuant to this provision of the note purchase
agreement.
The table below sets forth the principal amount of the February
2010 Notes and the shares of common stock issuable upon the
exercise of the related warrants sold to our directors,
executive officers and 5% stockholders and their affiliates.
| |
|
|
|
|
|
|
|
|
|
|
|
Shares of
|
|
Aggregate
|
|
|
|
Common Stock
|
|
Principal
|
|
|
|
Issuable upon
|
|
Amount of the
|
|
|
|
the Exercise of
|
|
February
|
|
Investor
|
|
Warrants
|
|
2010 Notes
|
|
|
|
Plainfield Direct LLC(1)(2)
|
|
|
1,007,565
|
|
|
$
|
4,350,000
|
|
|
Entities affiliated with Itera Ethanol, LLC(3)(4)
|
|
|
46,568
|
|
|
$
|
500,000
|
|
|
Norwood LDK, LLC(5)(6)
|
|
|
34,744
|
|
|
$
|
150,000
|
|
|
|
|
|
(1) |
|
Keith Carter is one of our directors and is a Managing
Director of Plainfield Asset Management, which is the parent
company of Plainfield Direct LLC. |
| |
|
(2) |
|
Subsequently, Plainfield Direct LLC transferred its warrants
to Plainfield Finance II LLC, and the warrants were
exercised in January 2011 pursuant to the Debt Conversion
Agreement |
| |
|
(3) |
|
Includes 22,818 warrants and a note in the principal amount
of $350,000 held by Green Chem Holdings LLC and 23,750 warrants
and a note in the principal amount of $150,000 held by Itera
Ethanol, LLC. Steven Sisselman is one of our directors and is an
executive officer of Green Chem Holdings LLC and Itera Ethanol,
LLC. |
| |
|
(4) |
|
Green Chem Holdings LLC exercised its warrants in January
2011 pursuant to the Debt Conversion Agreement. Itera Ethanol,
LLC exercised all but 1,922 of its warrants in January 2011
pursuant to the Debt Conversion Agreement. |
| |
|
(5) |
|
The managing member of Norwood LDK, LLC is Stephen J. Gatto,
the chief executive officer of the company, and the other
members of Norwood LDK, LLC are members of Mr. Gatto’s
immediate family. At the time of the agreement, Stephen J. Gatto
entered into such agreement personally, as Mr. Gatto
provided a loan to Norwood LDK, LLC for the purchase of the
securities described herein, which will be repaid by Norwood
LDK, LLC, and the securities were immediately registered in the
name of Norwood LDK, LLC. |
| |
|
(6) |
|
The warrants issued hereunder were exercised in January 2011
pursuant to the Debt Conversion Agreement. |
November
2009 financing
In November 2009, we sold secured convertible promissory notes,
or the November 2009 Notes, to Plainfield Direct LLC in the
principal amount of $2.0 million. The November 2009 Notes
accrued interest at a
126
rate of 15% per annum and had a maturity date of
October 10, 2013. In January 2011, in connection with the
issuance of Class B common stock and the Debt Conversion
Agreement described above, the full principal amount of and
accrued but unpaid interest on the November 2009 Notes, along
with the full principal amount of and accrued but unpaid
interest on the other notes of the company outstanding, was
first used to purchase an aggregate of 1,117,378 shares of
common stock at an exercise price of $6.00 per share, with the
remaining balance used to purchase an aggregate of
3,044,905 shares of Class B common stock.
The table below sets forth the principal amount of the November
2009 Notes sold to our directors, executive officers and 5%
stockholders and their affiliates.
| |
|
|
|
|
|
|
|
Aggregate
|
|
|
|
Principal
|
|
|
|
Amount of the
|
|
|
|
November 2009
|
|
Investor
|
|
Notes
|
|
|
|
Plainfield Direct LLC(1)
|
|
$
|
2,000,000
|
|
|
|
|
|
(1) |
|
Keith Carter is one of our directors and is a Managing
Director of Plainfield Asset Management, which is the parent
company of Plainfield Direct LLC. |
October
2009 financing
In October 2009, we sold secured convertible promissory notes,
or the October 2009 Notes, to Plainfield Direct LLC in the
principal amount of $2.0 million. The October 2009 Notes
accrued interest at a rate of 15% per annum and had a maturity
date of October 10, 2013. In January 2011, in connection
with the issuance of Class B common stock and the Debt
Conversion Agreement described above, the full principal amount
of and accrued but unpaid interest on the October 2009 Notes,
along with the full principal amount of and accrued but unpaid
interest on the other notes of the company outstanding, was
first used to purchase an aggregate of 1,117,378 shares of
common stock at an exercise price of $6.00 per share, with the
remaining balance used to purchase an aggregate of
3,044,905 shares of Class B common stock.
The table below sets forth the principal amount of the October
2009 Notes sold to our directors, executive officers and 5%
stockholders and their affiliates.
| |
|
|
|
|
|
|
|
Aggregate
|
|
|
|
Principal
|
|
|
|
Amount of the
|
|
|
|
October 2009
|
|
Investor
|
|
Notes
|
|
|
|
Plainfield Direct LLC(1)
|
|
$
|
2,000,000
|
|
|
|
|
|
(1) |
|
Keith Carter is one of our directors and is a Managing
Director of Plainfield Asset Management, which is the parent
company of Plainfield Direct LLC. |
Predecessor
Notes
In July 2009, our Predecessor Company transferred certain of its
assets to us. At such time, we entered into a Transfer,
Assignment and Assumption Agreement pursuant to which we assumed
certain promissory notes in the aggregate principal amount of
$5.5 million of the Predecessor Company issued to
Plainfield Direct LLC, or the Predecessor Notes. The Predecessor
Notes accrued interest at a rate of 15% per annum and had a
maturity date of October 10, 2013. In January 2011, in
connection with the issuance of Class B common stock and
the Debt Conversion Agreement described above, the full
principal amount of and accrued but unpaid interest on the
October 2009 Notes, along with the full principal amount of and
accrued but unpaid interest on the other notes of the company
outstanding, was first used to purchase an aggregate of
1,117,378 shares of common stock at an exercise price of
$6.00 per share, with the remaining balance used to purchase an
aggregate of 3,044,905 shares of Class B common stock.
The table below sets forth the principal amount of the
Predecessor Notes sold to our directors, executive officers and
5% stockholders and their affiliates.
127
| |
|
|
|
|
|
|
|
Aggregate
|
|
|
|
Principal
|
|
|
|
Amount of the
|
|
|
|
Predecessor
|
|
Investor
|
|
Notes
|
|
|
|
Plainfield Direct LLC(1)
|
|
$
|
5,500,000
|
|
|
|
|
|
(1) |
|
Keith Carter is one of our directors and is a Managing
Director of Plainfield Asset Management, which is the parent
company of Plainfield Direct LLC. |
Acquisition
of Myriant LP LLC
In July 2009, we purchased all of the issued and outstanding
units of Myriant LP LLC (then known as Bionol Lake Providence,
LLC) from certain of our investors in exchange for
1,000,001 of our membership interests, which were converted into
an equivalent number of shares of our common stock upon our
conversion to a corporation.
The table below sets forth the number of shares of our common
stock issued to our directors, executive officers and 5%
stockholders and their affiliates.
| |
|
|
|
|
|
|
|
Number of
|
|
|
|
Shares of
|
|
Investor
|
|
Common Stock
|
|
|
|
Plainfield Finance II LLC(1)
|
|
|
596,702
|
|
|
Itera Ethanol LLC(2)
|
|
|
77,491
|
|
|
Rudy E. Fogleman, Jr.
|
|
|
14,145
|
|
|
|
|
|
(1) |
|
Keith Carter is one of our directors and is a Managing
Director of Plainfield Asset Management, which is the parent
company of Plainfield Finance II LLC. |
| |
|
(2) |
|
Steven Sisselman is one of our directors and is the Executive
Vice President and Chief Operating Officer of Itera
International Energy Corp. and Itera USA, Inc., which are each
affiliated with Itera Ethanol LLC. |
Warrant
Issuance Agreement
In connection with the separation of our business from the
Predecessor Company, we issued warrants to purchase an aggregate
of 766,437 units of interest at an exercise price of $10.00
per unit to certain of our investors. Upon our conversion to a
corporation in January 2011, the warrants were converted to
warrants to purchase shares of our common stock. The warrants
may be exercised at any time prior to their respective
expiration dates, which are August 15, 2015.
The table below sets forth the number of shares of our common
stock issuable upon the exercise of warrants issued to our
directors, executive officers and 5% stockholders and their
affiliates.
| |
|
|
|
|
|
|
|
Shares of
|
|
|
|
Common Stock
|
|
|
|
Issuable upon
|
|
|
|
the Exercise of
|
|
Investor
|
|
Warrants
|
|
|
|
Plainfield Finance II LLC(1)(2)
|
|
|
584,488
|
|
|
Itera Ethanol LLC(3)(4)
|
|
|
92,203
|
|
|
|
|
|
(1) |
|
Keith Carter is one of our directors and is a Managing
Director of Plainfield Asset Management, which is the parent
company of Plainfield Finance II LLC. |
| |
|
(2) |
|
In connection with the February 2010 Notes, the exercise
price of the warrants issued hereunder was amended to $6.00 per
share. In connection with the Debt Conversion Agreement
described above, these warrants were exercised at an exercise
price of $6.00 per share. |
| |
|
(3) |
|
Steven Sisselman is one of our directors and is the Executive
Vice President and Chief Operating Officer of Itera
International Energy Corp. and Itera USA, Inc., which are each
affiliated with Itera Ethanol LLC. |
128
|
|
|
|
(4) |
|
In connection with the February 2010 Notes, the exercise
price of the warrants issued hereunder was amended to $6.00 per
share. In connection with the Debt Conversion Agreement
described above, all but 1,922 of the shares were issued to
Itera Ethanol LLC at an exercise price of $6.00 per share. |
Additionally, we issued warrants to purchase an aggregate of
423,077 units of interest at an exercise price of $13.00
per share to Plainfield Finance II LLC. Upon our conversion
to a corporation in January 2011, the warrants were converted to
warrants to purchase shares of our common stock. The warrants
may be exercised at any time prior to their respective
expiration dates, which are August 15, 2015.
The table below sets forth the number of shares of our common
stock issuable upon the exercise of warrants issued to our
directors, executive officers and 5% stockholders and their
affiliates.
| |
|
|
|
|
|
|
|
Shares of
|
|
|
|
Common Stock
|
|
|
|
Issuable upon
|
|
|
|
the Exercise of
|
|
Investor
|
|
Warrants
|
|
|
|
Plainfield Finance II LLC(1)(2)
|
|
|
423,077
|
|
|
|
|
|
(1) |
|
Keith Carter is one of our directors and is a Managing
Director of Plainfield Asset Management, which is the parent
company of Plainfield Finance II LLC. |
| |
|
(2) |
|
In connection with the February 2010 notes, the exercise
price of the warrants issued hereunder was amended to $6.00 per
share. In connection with the Debt Conversion Agreement
described above, these warrants were exercised at an exercise
price of $6.00 per share. |
Contribution
and Exchange Agreement
In July 2009, in connection with the separation of our business
from the Predecessor Company, the members of the Predecessor
Company were issued our units of interest in exchange for an
equivalent number of units of interest of the Predecessor
Company. Immediately thereafter, we made a distribution of the
units of interest of the Predecessor Company to our members.
Upon our conversion to a corporation in January 2011, the units
were converted to an equivalent number of shares of our common
stock.
The table below sets forth the number of shares of our common
stock issued to our directors, executive officers and 5%
stockholders and their affiliates.
| |
|
|
|
|
|
|
|
Number of
|
|
|
|
Shares of
|
|
Investor
|
|
Common Stock
|
|
|
|
Norwood LDK, LLC(1)
|
|
|
3,348,306
|
|
|
Plainfield Finance II LLC(2)
|
|
|
1,027,891
|
|
|
Itera Ethanol LLC(3)
|
|
|
404,397
|
|
|
Rudy E. Fogleman, Jr.
|
|
|
29,371
|
|
|
Joseph P. Glas
|
|
|
29,371
|
|
|
Samuel G. McConnell
|
|
|
29,371
|
|
|
|
|
|
(1) |
|
Stephen J. Gatto is the managing member of Norwood LDK, LLC
and he and his immediate family are the only members thereof.
Mr. Gatto is also the chief executive officer of the
company. |
| |
|
(2) |
|
Keith Carter is one of our directors and is a Managing
Director of Plainfield Asset Management, which is the parent
company of Plainfield Finance II LLC. |
| |
|
(3) |
|
Steven Sisselman is one of our directors and is the Executive
Vice President and Chief Operating Officer of Itera
International Energy Corp. and Itera USA, Inc., which are each
affiliated with Itera Ethanol LLC. |
MANAGEMENT
SERVICE AGREEMENT
In February 2008, the Predecessor Company entered into an
Administrative Services and Construction Management Agreement
and an Operations and Maintenance Agreement, or Services
Agreements, with Bionol Clearfield LLC, a wholly owned
subsidiary of BioEnergy Holding LLC, or Holding, of which
approximately
129
24% of the equity of which is held by the Predecessor Company.
Our chief executive officer, Stephen J. Gatto, beneficially owns
approximately 56% of the Predecessor Company, has been the
chairman of its board of directors since 2004 and was its chief
executive officer from 2004 to June 2011. He currently has no
operational role with the Predecessor Company. In addition,
certain of our other stockholders, Plainfield Finance II
LLC, Itera Ethanol LLC, Green Chem Holdings LLC and Specialty
Chemicals LLC in the aggregate hold approximately 24% of the
Predecessor Company. Pursuant to the agreements, the Predecessor
Company was required to provide certain services including
general services
(day-to-day
management), provide personnel for general services, bookkeeping
and accounting services, preparation and distribution of
financial reports, ensure compliance with financing documents
and project documents, maintain insurance, obtain any permits
required, marketing services and construction related services.
In July 2009, concurrent with the transfer of the biochemicals
business to us, the Services Agreements were subcontracted to us
through the Administrative Services, Construction Management,
Operations and Maintenance Subcontract, or Management Services
Agreement, pursuant to which the Predecessor Company would pay
us up to $3,900,000 annually to perform all of the services
described above. This fee represented fair market value for the
services. In January 2011, the Management Service Agreement was
terminated.
The Predecessor Company recognized related party revenues
totaling $267,318 and $3,387,926 for the period from
January 1, 2009 to July 15, 2009 and the year ended
December 31, 2008, respectively. We recognized revenues of
$2,519, $600,166, $3,557,575 and $3,560,094 under this
arrangement for the three months ended March 31, 2011 and
2010 (unaudited), the year ended December 31, 2010 and for
the period from inception to March 31, 2011 (unaudited). No
revenues were recognized in 2009.
Amounts due from related parties at March 31, 2011
(unaudited), December 31, 2010 and 2009 under this
agreement were $30,412, $87,439 and $1,116,188, respectively.
ARRO
BUILDING SERVICES AND STEVEN G. MACK
In August 2010, we entered into an independent contractor
agreement with Arro Building Services, pursuant to which Arro
Building Services will assist the company in its expected
transition to a new office facility. Arro Building Services is
responsible for interfacing with the company’s current real
estate companies, visiting potential real estate sites, lease
negotiations, logistics and other duties on an as needed or as
requested basis. Additionally, Arro Building Services is
responsible for all facilities management at the company’s
corporate headquarters and laboratory facility. Arro Building
Services is entitled to receive $10,000 per month in its
capacity as a consultant. The Predecessor Company paid Arro
Building Services $179,034 and $115,432 in 2008 and 2009,
respectively for such services. The company paid Arro Building
Services $84,326 and $148,610 in 2009 and 2010, respectively,
and has paid Arro Building Services $57,583 to date in 2011. The
president of Arro Building Services is Steven G. Mack, who is a
former director of the company. Biomac, LLC, a stockholder of
the company, is owned by Mr. Mack.
CLEAR
CREEK CAPITAL AND NEAL ROY
In October 2010, we entered into a consulting agreement with
Clear Creek Capital, LLC, or Clear Creek, pursuant to which we
retained Clear Creek to render consulting services for us in
connection with the construction, ownership and operation of the
Louisiana Plant. Pursuant to this agreement, Clear Creek’s
services include assisting us in identifying, pursuing,
structuring and executing government programs, loan guarantees,
grants or other incentives, meeting with our development
partners and third-party consultants, recommending and working
with professional services firms as required and evaluating
documentation with respect to contracts, permits and other
matters as we request. In consideration for these services, we
have agreed to pay Clear Creek a fee not to exceed an aggregate
of $250,000 upon the achievement of certain milestones. In
addition, Clear Creek will be entitled to additional placement
fees upon obtaining certain government loans and tax credits for
the company. The company paid Clear Creek $21,250 in April 2011
and $25,000 in May 2011 with respect to its assistance with
services rendered in connection with our application for a loan
guarantee under the U.S. Department of Agriculture’s
Rural Development Business & Industry Loan Guarantee
program. Neal Roy is a managing director of Clear Creek.
Mr. Roy formerly served as a director of the company. In
addition, Mr. Roy is a stockholder of the company. The
company paid Neal Roy $11,163 and $14,885 in December 2010 and
March 2011, respectively, for his travel to Thailand in
connection with
130
negotiations with PTT Chemical International Private Limited
over its January 2011 investment in the company, and $5,246 for
board meetings and miscellaneous travel expenses in 2010.
JEFFREY
GATTO CONSULTING AGREEMENT
On July 13, 2010, we entered into a consulting agreement
with Jeffrey Gatto, pursuant to which he would provide
consulting services with respect to the construction of our
Louisiana Plant. Mr. Jeffrey Gatto was entitled to receive
$5,000 per month and 2,799 shares of common stock as
compensation in his capacity as a consultant, with the
opportunity to receive additional shares of common stock upon
reaching certain milestones. Mr. Jeffrey Gatto is the
brother of Stephen J. Gatto, the chief executive officer of the
company. The board of directors approved this transaction on
September 14, 2010. On December 22, 2010, the board of
directors approved the hiring of Mr. Jeffrey Gatto as
Senior Vice President, Engineering and Operations, conditioned
upon Mr. Jeffrey Gatto entering into an employment
agreement with the company. Mr. Jeffery Gatto entered into
such employment agreement on March 29, 2011, which
supersedes the consulting agreement.
OTHER
TRANSACTIONS
We have entered into employment and offer letter agreements with
certain of our executive officers that, among other things,
provide for certain severance and change of control benefits.
For a description of these agreements, see “Executive
Compensation — Employment Agreements.”
We have granted stock options and restricted stock to our
executive officers and certain of our directors. For a
description of these equity grants, see “Executive
Compensation — Grants of Plan-Based Awards in 2010
Table.”
We have entered into indemnification agreements with our
directors and will enter into indemnification agreements with
our new directors before the completion of this offering. See
“Description of capital stock — Limitation on
Liability and Indemnification Matters.”
POLICIES
AND PROCEDURES FOR RELATED PARTY TRANSACTIONS
Our board of directors intends to adopt a written related person
transaction policy to set forth the policies and procedures for
the review and approval or ratification of related person
transactions. This policy will cover, with certain exceptions
set forth in Item 404 of
Regulation S-K
under the Securities Act, any transaction, arrangement or
relationship, or any series of similar transactions,
arrangements or relationships in which we were or are to be a
participant, the amount involved exceeds $120,000, and a related
person had or will have a direct or indirect material interest,
including, without limitation, purchases of goods or services by
or from the related person or entities in which the related
person has a material interest, indebtedness, guarantees of
indebtedness and employment by us of a related person.
131
Principal
stockholders
The following table sets forth information about the beneficial
ownership of our common stock as of March 31, 2011, by:
|
|
|
| |
•
|
each person, or group of affiliated persons, known to us to be
the beneficial owner of more than 5% of our common stock;
|
| |
| |
•
|
each named executive officer and each director; and
|
| |
| |
•
|
all of our executive officers and directors as a group.
|
Unless otherwise noted below, the address of each beneficial
owner listed in the table is
c/o Myriant
Corporation, 1 Pine Hill Drive, Batterymarch Park II,
Suite 301, Quincy, MA 02169. We have determined beneficial
ownership in accordance with the rules of the SEC. Except as
indicated by the footnotes below, we believe, based on the
information furnished to us, that the persons and entities named
in the tables below have sole voting and investment power with
respect to all shares of common stock that they beneficially
own, subject to applicable community property laws.
In computing the number of shares of common stock beneficially
owned by a person and the percentage ownership of that person,
we deemed outstanding shares of common stock subject to options
or warrants held by that person that are currently exercisable
or exercisable within 60 days of March 31, 2011. We
did not deem these shares outstanding, however, for the purpose
of computing the percentage ownership of any other person.
We have calculated the percentage of beneficial ownership prior
to and after the offering based on 23,393,678 shares of
common stock outstanding in the March 31, 2011 Pro Forma
and assuming the conversion of all of our Class A common
stock and Class B common stock into an aggregate of
14,259,858 shares of our common stock upon the completion
of this offering, the Warrant Exercise and no exercise of the
underwriters’ overallotment option.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Shares
|
|
Percentage of Shares
|
|
|
|
Beneficially Owned
|
|
Beneficially Owned
|
|
|
|
Prior to the
|
|
After the
|
|
Prior to the
|
|
After the
|
|
Name and Address of Beneficial Owner
|
|
Offering
|
|
Offering
|
|
Offering
|
|
Offering
|
|
|
|
5% Stockholders:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PTT Chemical International Private Limited(1)
|
|
|
11,214,953
|
|
|
|
|
|
|
|
47.9
|
%
|
|
|
|
|
|
Entities affiliated with Plainfield(2)
|
|
|
6,351,240
|
|
|
|
|
|
|
|
26.4
|
%
|
|
|
|
|
|
Norwood LDK, LLC(3)
|
|
|
3,241,569
|
|
|
|
|
|
|
|
13.9
|
%
|
|
|
|
|
|
Named executive officers and directors:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stephen J. Gatto(4)
|
|
|
3,411,229
|
|
|
|
|
|
|
|
14.5
|
%
|
|
|
|
|
|
A. Cenan Ozmeral(5)
|
|
|
155,718
|
|
|
|
|
|
|
|
*
|
|
|
|
|
|
|
Ralph A. Tapia(6)
|
|
|
25,389
|
|
|
|
|
|
|
|
*
|
|
|
|
|
|
|
Samuel McConnell(7)
|
|
|
61,910
|
|
|
|
|
|
|
|
*
|
|
|
|
|
|
|
Rudy E. Fogleman, Jr.(8)
|
|
|
64,290
|
|
|
|
|
|
|
|
*
|
|
|
|
|
|
|
Puntip Oungpasuk(9)
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
Narongsak Jivakanun(10)
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
Thitipong Jurapornsiridee(9)
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
Steven Sisselman(11)
|
|
|
851,784
|
|
|
|
|
|
|
|
3.6
|
%
|
|
|
|
|
|
Keith Carter(12)
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
All executive officers and directors as a group
(12 persons)(13)
|
|
|
4,688,393
|
|
|
|
|
|
|
|
20.0
|
%
|
|
|
|
|
|
|
|
|
* |
|
Represents beneficial ownership of less than 1% of the
outstanding shares of our common stock. |
| |
|
(1) |
|
The address for PTT Chemical International Private Limited is
391B Orchard Road, #15-05/08, Ngee Ann City Tower B, Singapore
238874. |
132
|
|
|
|
(2) |
|
Includes 3,504,370 shares held by Plainfield
Finance II LLC and 2,846,870 shares held by Plainfield
Direct LLC. The address for each entity is
c/o Plainfield
Asset Management LLC, 333 Ludlow Street, Stamford, CT 06902.
Includes 639,170 shares held by Plainfield Finance II
LLC issued upon the exercise of its common stock warrants on
April 12, 2011. |
| |
|
(3) |
|
Stephen J. Gatto is the managing member of Norwood LDK, LLC
and the chief executive officer of the company. |
| |
|
(4) |
|
Includes 3,241,569 shares held by Norwood LDK, LLC, of
which Stephen J. Gatto is the managing member and he and his
immediate family are the sole members and he has the ability to
direct the disposition and voting of such shares and includes
169,660 shares issuable pursuant to the exercise of an
option to purchase common stock. |
| |
|
(5) |
|
Includes 114,666 shares, of which 82,444 are currently
subject to a right of forfeiture, and includes
41,052 shares issuable pursuant to the exercise of an
option to purchase common stock. |
| |
|
(6) |
|
Includes 25,389 shares issuable pursuant to the exercise
of an option to purchase common stock. |
| |
|
(7) |
|
Includes 17,853 shares issuable pursuant to the exercise
of an option to purchase common stock. |
| |
|
(8) |
|
Includes 20,233 shares issuable pursuant to the exercise
of an option to purchase common stock. |
| |
|
(9) |
|
Excludes 11,214,953 shares beneficially owned by PTT
Chemical International Private Limited. Ms. Oungpasuk and
Mr. Jurapornsiridee are employed by PTT Chemical Public
Company Limited or its affiliates and do not have any voting or
dispositive power with respect to such shares.
Ms. Oungpasuk serves as a director of PTT Chemical
International Private Limited. |
| |
|
(10) |
|
Excludes 11,214,953 shares beneficially owned by PTT
Chemical International Private Limited. Mr. Jivakanun is
the chief executive officer of PTT Chemical International
Private Limited, however the voting and dispositive power of the
shares held by PTT Chemical International Private Limited is
held by its board of directors. Mr. Jivakanun is not a
member of the board of directors of PTT Chemical International
Private Limited. |
| |
|
(11) |
|
Includes 373,784 shares beneficially owned by Itera
Ethanol LLC, which includes 1,922 shares issuable pursuant
to the exercise of a warrant at an exercise price of $6.00 per
share, 230,762 shares beneficially owned by Green Chem
Holdings, LLC and 247,238 shares beneficially owned by
Green Chem Second Edition LLC. Mr. Sisselman is the
president of each of Itera Ethanol, LLC, Green Chem Holdings,
LLC and Green Chem Second Edition LLC and has the ability to
direct the voting or disposition of such shares. |
| |
|
(12) |
|
Excludes 3,504,370 shares beneficially owned by
Plainfield Finance II LLC and 2,846,870 shares
beneficially owned by Plainfield Direct LLC. Mr. Carter is
a Managing Director of Plainfield Asset Management, which is the
parent company of each of Plainfield Direct LLC and Plainfield
Finance II LLC, however Mr. Carter does not have any
voting or disposition power with respect to such shares. |
| |
|
(13) |
|
See footnotes 3 through 8 and 11. Also includes
44,057 shares and 8,210 shares issuable pursuant to
the exercise of an option to purchase common stock held by
Joseph P. Glas and 109,047 shares (of which 75,000 are
currently subject to a right of forfeiture) held by Jeffrey
Gatto. |
133
Description
of capital stock
GENERAL
Upon the completion of this offering, we will have authorized
under our second amended and restated certificate of
incorporation 100,000,000 shares of common stock,
$0.0001 par value per share, and 5,000,000 shares of
preferred stock, $0.0001 par value per share. The following
information gives effect to the filing of our second amended and
restated certificate of incorporation upon completion of the
offering.
As of July 21, 2011, there were outstanding:
|
|
|
| |
•
|
23,580,169 shares of our common stock held by approximately
40 stockholders (assuming the conversion of all of our
Class A common stock and Class B common stock into an
aggregate of 14,259,858 shares of our common stock upon the
completion of this offering);
|
| |
| |
•
|
506,250 shares issuable under the restricted stock units
granted subject to vesting conditions under the 2011 Omnibus
Incentive Plan;
|
| |
| |
•
|
1,162,043 shares of our common stock issuable upon exercise
of outstanding stock options; and
|
| |
| |
•
|
166,021 shares of our common stock issuable upon exercise
of outstanding warrants.
|
The following description of our capital stock and provisions of
our second amended and restated certificate of incorporation and
amended and restated bylaws to be in effect upon the completion
of this offering are summaries. Copies of these documents have
been filed with the SEC as exhibits to our registration
statement, of which this prospectus forms a part. The
descriptions of the common stock and preferred stock reflect
changes to our capital structure that will occur upon the
closing of this offering. Currently, there is no established
public trading market for our common stock.
COMMON
STOCK
Dividends
Subject to preferences that may be applicable to any
then-outstanding convertible common stock, holders of our common
stock are entitled to receive dividends, if any, as may be
declared from time to time by our board of directors out of
legally available funds.
Voting
rights
Each holder of our common stock is entitled to one vote for each
share on all matters submitted to a vote of the stockholders,
including the election of directors. Our stockholders do not
have cumulative voting rights in the election of directors.
Accordingly, holders of a majority of the voting shares are able
to elect all of the directors.
Liquidation
In the event of our liquidation, dissolution or winding up,
holders of our common stock will be entitled to share ratably in
the net assets legally available for distribution to
stockholders after the payment of all of our debts and other
liabilities and the satisfaction of any liquidation preference
granted to the holders of any then outstanding shares of
preferred stock.
Rights
and preferences
Holders of our common stock have no preemptive, conversion,
subscription or other rights, and there are no redemption or
sinking fund provisions applicable to our common stock. The
rights, preferences and privileges of the holders of our common
stock are subject to and may be adversely affected by the rights
of the holders of shares of any series of our preferred stock
that we may designate in the future.
134
PREFERRED
STOCK
Upon the completion of this offering, our board of directors
will have the authority, without further action by our
stockholders, to issue up to 5,000,000 shares of preferred
stock in one or more series and to fix the rights, preferences,
privileges and restrictions thereof. These rights, preferences
and privileges could include dividend rights, conversion rights,
voting rights, terms of redemption, liquidation preferences,
sinking fund terms and the number of shares constituting any
series or the designation of such series, any or all of which
may be greater than the rights of common stock. The issuance of
our preferred stock could adversely affect the voting power of
holders of common stock and the likelihood that such holders
will receive dividend payments and payments upon liquidation. In
addition, the issuance of preferred stock could have the effect
of delaying, deferring or preventing a change of control of our
company or other corporate action. Upon completion of this
offering, no shares of preferred stock will be outstanding, and
we have no present plan to issue any shares of preferred stock.
WARRANTS
The company has issued warrants to purchase shares of its common
stock at an exercise price of $6.00 per share, or the $6
Warrants, to Itera Ethanol LLC and Specialty Chemicals LLC. The
company has issued warrants to purchase shares of its common
stock at an exercise price of $10.00 per share, or the $10
Warrants, to NGP Capital Resources Company and Camulos BioEnergy
Partners LLC. The $6 Warrants and the $10 Warrants have
substantially similar terms, with the exception of the exercise
price. The $6 Warrants and the $10 Warrants expire on
August 15, 2015. Investors holding the $6 Warrants or the
$10 Warrants have the option to exercise such warrants at any
time upon payment of the exercise price in cash. In the event of
an initial public offering meeting certain pricing requirements,
the investors holding the $6 Warrants and the $10 Warrants will
be obligated to exercise these warrants. Under the terms of each
such warrant, if the price per share of our common stock in a
fully underwritten, firm commitment public offering exceeds 1.5
times the exercise price of the warrants, the investor will be
obligated to exercise such warrants. The $6 Warrants and the $10
Warrants were issued to the investors named above in connection
with the separation of our business from the Predecessor
Company. Pursuant to the February 2010 financing, described
herein, the warrants issued to Itera Ethanol LLC and Specialty
Chemicals LLC were amended to provide for an exercise price of
$6.00 per share, rather than $10.00 per share, in consideration
for such investors’ participation in the February 2010
financing.
The company has also issued warrants to purchase shares of its
common stock at an exercise price of $13.00 per share, or the
$13 Warrants, to Access Shipping Limited Partnership, or Access
Shipping. The $13 Warrants have substantially similar terms to
the $6 Warrants and the $10 Warrants, with the exceptions noted
herein. The $13 Warrants expire on September 14, 2014.
There is no automatic exercise provision of the $13 Warrants.
Access Shipping has the option to exercise the $13 Warrants on a
cashless basis, whereby Access Shipping may notify the company
that it wishes to exercise all or any portion of the $13
Warrants. The company will issue that number of shares of common
stock equal to the number of warrant shares exercised multiplied
by the difference of the then-current market price and the
exercise price, divided by the then-current market price.
The following table sets forth information about outstanding
warrants to purchase shares of our common stock as of
March 31, 2011 (excluding warrants exercisable for
639,170 shares of common stock that were exercised on
April 12, 2011).
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Maximum
|
|
Exercise
|
|
|
|
|
|
Number of
|
|
Price per
|
|
Expiration
|
|
Class of Stock
|
|
Shares
|
|
Share ($)
|
|
Date
|
|
|
|
Common
|
|
|
17,134
|
|
|
$
|
6.00
|
|
|
August 15, 2015
|
|
Common
|
|
|
89,656
|
|
|
$
|
10.00
|
|
|
August 15, 2015
|
|
Common
|
|
|
59,231
|
|
|
$
|
13.00
|
|
|
September 14, 2014
|
135
REGISTRATION
RIGHTS
We are party to an investors’ rights agreement which
provides that holders of 21,285,762 shares of our common
stock, including shares of common stock issuable upon the
conversion of our convertible Class A common stock and
Class B common stock in connection with this offering, and
shares of common stock issuable upon the exercise of outstanding
warrants and options, have the right in specified circumstances
to require us to register their shares under the Securities Act
for resale to the public. These shares are referred to as
registrable securities.
Set forth below is a summary of the registration rights held by
holders of registrable securities pursuant to this agreement.
Demand
registration
Beginning on the earlier of 180 days after the completion
of this offering and January 13, 2014, the holders of at
least 30% of the outstanding registrable securities can request
that we file a registration statement under the Securities Act
in order to register all or any part of the registrable
securities held by such holders, subject to certain conditions
and limitations. We are not required to make any such
registration if we have previously effected two such
registrations on behalf of the holders of registrable
securities. The aggregate registrable securities requested to be
registered pursuant to such request must have an anticipated
aggregate public offering price, net of underwriting discounts
and commissions, of at least $10 million.
If our board of directors believes in good faith that it would
be materially detrimental to us and our stockholders to proceed
with a registration at the time the demand is made, we may delay
the registration once in any
12-month
period for a period not to exceed 90 days. Also, if the
holders of registrable securities requesting registration
request that the shares be offered for distribution through an
underwriting, the number of registrable securities to be
registered may be reduced upon the advice of the underwriters
for the offering and the number of registrable securities to be
registered shall be allocated among such holders of registrable
securities; provided that shares of such holders of registrable
securities shall not be reduced unless all other shares are
first entirely excluded from the underwriting.
Piggyback
registration
Subject to certain limitations, holders of registration rights
pursuant to the investors’ rights agreement have unlimited
rights to request that their registrable securities be included
in any registration of our common stock that we initiate.
The holders of registration rights have waived their rights to
include any of their registrable securities in this offering.
Form S-3
registration
After we have qualified for registration on
Form S-3,
which will not occur until at least 12 months after we have
become a publicly-reporting company, the holders of at least 20%
of the outstanding registrable securities may request in writing
that we effect registration of its or their shares on
Form S-3,
provided that the offering proceeds of the shares proposed to be
registered on behalf of our stockholders, net of underwriting
discounts and commissions, in each registration is at least
$1 million.
If our board of directors believes in good faith that it would
be materially detrimental to us and our stockholders to proceed
with a registration at the time the demand is made, we may delay
the registration once in any
12-month
period for a period not to exceed 90 days. In addition, we
are not required to make any registration on
Form S-3
under the investors’ rights agreement if we have effected
two registrations pursuant to the
Form S-3
registration rights on behalf of the holders of registrable
securities within 12 months prior to the request.
136
Transferability
The registration rights are generally transferable to any
transferee who acquires at least 500,000 shares of
registrable securities from the transferor.
Expenses
Generally, we are required to bear all registration and selling
expenses incurred in connection with the demand, piggyback and
Form S-3
registrations described above, other than underwriting discounts
and commissions. We are also required to bear the reasonable
fees and expenses, not to exceed $35,000, of one counsel for the
selling stockholders in each registration.
ANTI-TAKEOVER
PROVISIONS
Second
amended and restated certificate of incorporation and bylaws to
be in effect upon the completion of this offering
Our second amended and restated certificate of incorporation to
be in effect upon the completion of this offering will provide
for our board of directors to be divided into three classes,
with staggered three-year terms. Only one class of directors
will be elected at each annual meeting of our stockholders, with
the other classes continuing for the remainder of their
respective three-year terms. Because our stockholders do not
have cumulative voting rights, our stockholders holding a
majority of the shares of common stock outstanding will be able
to elect all of our directors. Our second amended and restated
certificate of incorporation and amended and restated bylaws to
be effective upon the completion of this offering will provide
that all stockholder actions must be effected at a duly called
meeting of the stockholders and not by a consent in writing, and
that only our board of directors may call a special meeting of
the stockholders.
Our second amended and restated certificate of incorporation
will require a
662/3%
stockholder vote for the adoption, amendment or repeal of any
provision of our amended and restated bylaws and for the
amendment or repeal of certain provisions of our second amended
and restated certificate of incorporation relating to the
classification of our board of directors, the requirement that
stockholder actions be effected at a duly called meeting, and
the designated parties entitled to call a special meeting of the
stockholders. The combination of the classification of our board
of directors, the lack of cumulative voting and the
662/3%
stockholder voting requirements will make it more difficult for
our existing stockholders to replace our board of directors as
well as for another party to obtain control of us by replacing
our board of directors. Because our board of directors has the
power to retain and discharge our officers, these provisions
could also make it more difficult for existing stockholders or
another party to effect a change in management. In addition, the
authorization of undesignated preferred stock makes it possible
for our board of directors to issue preferred stock with voting
or other rights or preferences that could impede the success of
any attempt to change our control.
These provisions may have the effect of deterring hostile
takeovers or delaying changes in our control or management.
These provisions are intended to enhance the likelihood of
continued stability in the composition of our board of directors
and its policies and to discourage certain types of transactions
that may involve an actual or threatened acquisition of us.
These provisions are designed to reduce our vulnerability to an
unsolicited acquisition proposal. The provisions also are
intended to discourage certain tactics that may be used in proxy
fights. However, such provisions could have the effect of
discouraging others from making tender offers for our shares
and, as a consequence, they also may inhibit fluctuations in the
market price of our shares that could result from actual or
rumored takeover attempts. Such provisions may also have the
effect of preventing changes in our management.
137
Section 203
of the Delaware General Corporation Law
We are subject to Section 203 of the Delaware General
Corporation Law, which prohibits a Delaware corporation from
engaging in any business combination with any interested
stockholder for a period of three years after the date that such
stockholder became an interested stockholder, with the following
exceptions:
|
|
|
| |
•
|
before such date, the board of directors of the corporation
approved either the business combination or the transaction that
resulted in the stockholder becoming an interested holder;
|
| |
| |
•
|
upon completion of the transaction that resulted in the
stockholder becoming an interested stockholder, the interested
stockholder owned at least 85% of the voting stock of the
corporation outstanding at the time the transaction began,
excluding for purposes of determining the voting stock
outstanding (but not the outstanding voting stock owned by the
interested stockholder) those shares owned (i) by persons
who are directors and also officers and (ii) employee stock
plans in which employee participants do not have the right to
determine confidentially whether shares held subject to the plan
will be tendered in a tender or exchange offer; or
|
| |
| |
•
|
on or after such date, the business combination is approved by
the board of directors and authorized at an annual or special
meeting of the stockholders, and not by written consent, by the
affirmative vote of at least
662/3%
of the outstanding voting stock that is not owned by the
interested stockholder.
|
In general, Section 203 defines business combination to
include the following:
|
|
|
| |
•
|
any merger or consolidation involving the corporation and the
interested stockholder;
|
| |
| |
•
|
any sale, transfer, pledge or other disposition of 10% or more
of the assets of the corporation involving the interested
stockholder;
|
| |
| |
•
|
subject to certain exceptions, any transaction that results in
the issuance or transfer by the corporation of any stock of the
corporation to the interested stockholder;
|
| |
| |
•
|
any transaction involving the corporation that has the effect of
increasing the proportionate share of the stock or any class or
series of the corporation beneficially owned by the interested
stockholder; or
|
| |
| |
•
|
the receipt by the interested stockholder of the benefit of any
loss, advances, guarantees, pledges or other financial benefits
by or through the corporation.
|
In general, Section 203 defines an “interested
stockholder” as an entity or person who, together with the
person’s affiliates and associates, beneficially owns, or
is an affiliate or associate of the corporation and within three
years prior to the time of determination of interested
stockholder status did own, 15% or more of the outstanding
voting stock of the corporation.
LIMITATIONS
OF LIABILITY AND INDEMNIFICATION MATTERS
Our second amended and restated certificate of incorporation and
amended and restated bylaws, each to be effective upon the
completion of this offering, will provide that we will indemnify
our directors, officers, employees and agents to the fullest
extent permitted by the Delaware General Corporation Law, which
prohibits our second amended and restated certificate of
incorporation from limiting the liability of our directors for
the following:
|
|
|
| |
•
|
any breach of the director’s duty of loyalty to us or to
our stockholders;
|
| |
| |
•
|
acts or omissions not in good faith or that involve intentional
misconduct or a knowing violation of law;
|
| |
| |
•
|
unlawful payment of dividends or unlawful stock repurchases or
redemptions; and
|
| |
| |
•
|
any transaction from which the director derived an improper
personal benefit.
|
If Delaware law is amended to authorize corporate action further
eliminating or limiting the personal liability of a director,
then the liability of our directors will be eliminated or
limited to the fullest extent
138
permitted by Delaware law, as so amended. Our second amended and
restated certificate of incorporation does not eliminate a
director’s duty of care and, in appropriate circumstances,
equitable remedies, such as injunctive or other forms of
nonmonetary relief, remain available under Delaware law. This
provision also does not affect a director’s
responsibilities under any other laws, such as the federal
securities laws or other state or federal laws. Under our
amended and restated bylaws, we will also be empowered to enter
into indemnification agreements with our directors, officers,
employees and other agents and to purchase insurance on behalf
of any person whom we are required or permitted to indemnify.
In addition to the indemnification required in our second
amended and restated certificate of incorporation and amended
and restated bylaws, we have entered into indemnification
agreements with certain of our directors and officers, and will
enter into new indemnification agreements with each of our
current directors, officers and certain employees before the
completion of this offering. These agreements provide for the
indemnification of our directors, officers and certain employees
for all reasonable expenses and liabilities incurred in
connection with any action or proceeding brought against them by
reason of the fact that they are or were our agents. We believe
that these provisions in our second amended and restated
certificate of incorporation and amended and restated bylaws and
indemnification agreements are necessary to attract and retain
qualified persons as directors and officers. Furthermore, we
have obtained director and officer liability insurance to cover
liabilities our directors and officers may incur in connection
with their services to us. This description of the
indemnification provisions of our second amended and restated
certificate of incorporation, our amended and restated bylaws
and our indemnification agreements is qualified in its entirety
by reference to these documents, each of which is attached as an
exhibit to the registration statement of which this prospectus
is a part.
The limitation of liability and indemnification provisions in
our second amended and restated certificate of incorporation and
amended and restated bylaws may discourage stockholders from
bringing a lawsuit against directors for breach of their
fiduciary duties. They may also reduce the likelihood of
derivative litigation against directors and officers, even
though an action, if successful, might benefit us and our
stockholders. A stockholder’s investment may be harmed to
the extent we pay the costs of settlement and damage awards
against directors and officers pursuant to these indemnification
provisions. Insofar as indemnification for liabilities arising
under the Securities Act may be permitted to our directors,
officers and controlling persons pursuant to the foregoing
provisions, or otherwise, we have been advised that, in the
opinion of the SEC, such indemnification is against public
policy as expressed in the Securities Act, and is, therefore,
unenforceable. There is no pending litigation or proceeding
naming any of our directors or officers as to which
indemnification is being sought, nor are we aware of any pending
or threatened litigation that may result in claims for
indemnification by any director or officer.
THE
NASDAQ GLOBAL MARKET LISTING
We have applied to have our common stock approved for listing on
The Nasdaq Global Market under the trading symbol
“MYRT”.
TRANSFER
AGENT AND REGISTRAR
The transfer agent and registrar for our common stock is
Computershare Limited.
139
Shares
eligible for future sale
Prior to this offering, there has been no public market for our
common stock. Future sales of our common stock in the public
market, or the availability of such shares for sale in the
public market, could adversely affect market prices prevailing
from time to time. As described below, only a limited number of
shares will be available for sale shortly after this offering
due to contractual and legal restrictions on resale.
Nevertheless, sales of our common stock in the public market
after such restrictions lapse, or the perception that those
sales may occur, could adversely affect the prevailing market
price at such time and our ability to raise equity capital in
the future.
Based on the number of shares of common stock outstanding as of
March 31, 2011 and the issuance of 639,170 shares of
common stock upon the exercise of common stock warrants on
April 12, 2011, upon completion of this
offering, shares
of common stock will be outstanding, assuming no exercise of the
underwriters’ option to purchase additional shares, the
conversion of all of our Class A common stock and
Class B common stock into an aggregate of
14,259,858 shares of our common stock upon the completion
of this offering, and no exercise of options or warrants after
March 31, 2011 (other than the warrant exercise described
above). All of the shares sold by us in this offering will be
freely tradable unless purchased by our affiliates. Of the
remaining 23,393,678 shares of common stock outstanding
after this
offering,
will be restricted as a result of securities laws or
lock-up
agreements as described below. Following the expiration of the
lock-up
period, all shares will be eligible for resale in compliance
with Rule 144 to the extent such shares have been released
from any repurchase option that we may hold. “Restricted
securities” as defined under Rule 144 were issued and
sold by us in reliance on exemptions from the registration
requirements of the Securities Act. These shares may be sold in
the public market only if registered or pursuant to an exemption
from registration, such as Rule 144 under the Securities
Act.
RULE 144
In general, under Rule 144 of the Securities Act, as in
effect on the date of this prospectus, a person (or persons
whose shares are aggregated) who has beneficially owned
restricted stock for at least six months, will be entitled to
sell in any three-month period a number of shares that does not
exceed the greater of:
|
|
|
| |
•
|
1% of the number of shares of common stock then
outstanding, shares
immediately after this offering
(or shares
if the underwriters’ option to purchase additional shares
is exercised in full); or
|
| |
| |
•
|
the average weekly trading volume of our common stock on The
Nasdaq Global Market during the four calendar weeks immediately
preceding the date on which the notice of sale is filed with the
SEC.
|
Sales pursuant to Rule 144 are subject to requirements
relating to manner of sale, notice and availability of current
public information about us. A person (or persons whose shares
are aggregated) who is not deemed to be an affiliate of ours for
90 days preceding a sale, and who has beneficially owned
restricted stock for at least one year is entitled to sell such
shares without complying with the manner of sale, public
information, volume limitation or notice provisions of
Rule 144. Rule 144 will not be available to any
stockholders until we have been subject to the reporting
requirements of the Exchange Act for 90 days.
RULE 701
Rule 701 under the Securities Act, as in effect on the date
of this prospectus, permits resales of shares in reliance upon
Rule 144 but without compliance with certain restrictions
of Rule 144, including the holding period requirement. Most
of our employees, executive officers or directors who purchased
shares under a written compensatory plan or contract may be
entitled to rely on the resale provisions of Rule 701, but
all holders of Rule 701 shares are required to wait
until 90 days after the date of this prospectus before
selling their shares. However, substantially all
Rule 701 shares are subject to
lock-up
agreements as described below and under “Underwriting”
included elsewhere in this prospectus and will become eligible
for sale upon the expiration of the restrictions set forth in
those agreements.
140
LOCK-UP
AGREEMENTS
We, along with our directors, executive officers and all of our
other holders of 1% or more of our common stock, on an
as-converted and as-exercised basis, have agreed that for a
period of 180 days following the date of this prospectus,
we or they will not offer, sell, contract to sell, pledge, or
otherwise dispose of, directly or indirectly, any shares of
common stock or any securities convertible into or exercisable
or exchangeable for shares of common stock, or enter into any
swap, hedge or other arrangement that transfers to another, in
whole or in part, any of the economic consequences of ownership
of the common stock whether any of these transactions are to be
settled by delivery of our common stock or other securities, in
cash or otherwise, or publicly disclose the intention to make
any offer, sale, pledge or disposition, or to enter into any
transaction, swap, hedge or other arrangement, subject to
specified exceptions. UBS Securities LLC may, in its sole
discretion, at any time without prior notice, release all or any
portion of the shares from the restrictions in any such
agreement.
The 180-day
restricted period described in the preceding paragraph will be
extended if:
|
|
|
| |
•
|
during the last 15 calendar days plus three business days of the
180-day
restricted period we issue an earnings release or material news
or a material event relating to us occurs; or
|
| |
| |
•
|
prior to the expiration of the
180-day
restricted period, we announce that we will release earnings
results during the
16-day
period beginning on the last day of the
180-day
period,
|
in which case the restrictions described in the preceding
paragraph will continue to apply until the date that is 15
calendar days plus three business days after the date on which
the issuance of the release or the material news or material
event occurred, unless such extension is waived, in writing, by
UBS Securities LLC on behalf of the underwriters.
The restrictions set forth above do not apply to certain
issuances by us and certain transfers by our stockholders, which
are described in “Underwriting — No Sales of
Similar Securities.”
STOCK
PLANS
As soon as practicable after the completion of this offering, we
intend to file a
Form S-8
registration statement under the Securities Act to register
shares of our common stock subject to options outstanding or
reserved for issuance under our 2011 Omnibus Incentive Plan and
our 2011 employee stock purchase plan. This registration
statement will become effective immediately upon filing, and
shares covered by the registration statement of which this
prospectus is a part will upon their issuance be eligible for
sale in the public markets, subject to Rule 144 limitations
applicable to affiliates and any
lock-up
agreements. For a more complete discussion of our stock plans,
see “Management — Employee Benefit and Stock
Plans.”
141
Material
United States federal income and estate tax consequences to
non-U.S.
holders
The following is a summary of material U.S. federal income
and estate tax consequences to
non-U.S. holders
(as defined below) of the acquisition, ownership and disposition
of our common stock issued pursuant to this offering. This
discussion is not a complete analysis of all of the potential
U.S. federal income and estate tax consequences relating
thereto, nor does it address any gift tax consequences or any
tax consequences arising under any state, local or foreign tax
laws, or any other U.S. federal tax laws. This discussion
is based on the Internal Revenue Code of 1986, or the Code,
Treasury Regulations promulgated thereunder, judicial decisions,
and published rulings and administrative pronouncements of the
Internal Revenue Service, or IRS, all as in effect as of the
date of this offering. These authorities may change, possibly
retroactively, or may be subject to different interpretation,
resulting in U.S. federal income and estate tax
consequences different from those discussed below. No ruling has
been or will be sought from the IRS with respect to the matters
discussed below, and there can be no assurance that the IRS will
not take a contrary position regarding the tax consequences of
the acquisition, ownership or disposition of our common stock,
or that any such contrary position would not be sustained by a
court.
This discussion is limited to
non-U.S. holders
who purchase our common stock issued pursuant to this offering
and who hold our common stock as a “capital asset”
within the meaning of Section 1221 of the Code (generally,
property held for investment). This discussion does not address
all of the U.S. tax consequences that may be relevant to a
particular holder in light of such holder’s particular
circumstances. This discussion also does not consider any
specific facts or circumstances that may be relevant to holders
subject to special rules under the U.S. federal income tax
laws, including, without limitation:
|
|
|
| |
•
|
U.S. expatriates or former long-term residents of the U.S.;
|
| |
| |
•
|
partnerships and their partners;
|
| |
| |
•
|
real estate investment trusts;
|
| |
| |
•
|
regulated investment companies;
|
| |
| |
•
|
“controlled foreign corporations;”
|
| |
| |
•
|
“passive foreign investment companies;”
|
| |
| |
•
|
banks, insurance companies, or other financial institutions;
|
| |
| |
•
|
brokers, dealers, or traders in securities, commodities or
currencies;
|
| |
| |
•
|
tax-exempt organizations;
|
| |
| |
•
|
retirement plans;
|
| |
| |
•
|
persons subject to the alternative minimum tax;
|
| |
| |
•
|
persons holding our common stock as part of a straddle, hedge,
conversion transaction, constructive sale, or other integrated
transaction; or
|
| |
| |
•
|
persons who have acquired our common stock as compensation or
otherwise in connection with the performance of services.
|
PROSPECTIVE INVESTORS ARE URGED TO CONSULT THEIR TAX ADVISORS
REGARDING THE PARTICULAR UNITED STATES FEDERAL INCOME AND ESTATE
TAX CONSEQUENCES TO THEM OF ACQUIRING, OWNING AND DISPOSING OF
OUR COMMON STOCK, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER
ANY STATE, LOCAL OR FOREIGN TAX LAWS AND ANY OTHER UNITED STATES
FEDERAL TAX LAWS.
142
DEFINITION
OF NON-U.S.
HOLDER
For purposes of this discussion, a
non-U.S. holder
is any beneficial owner of our common stock that is not a
“U.S. person” or a partnership (or other entity
treated as a partnership) for U.S. federal income tax
purposes. A U.S. person is any of the following:
|
|
|
| |
•
|
an individual citizen or resident of the U.S.;
|
| |
| |
•
|
a corporation (or other entity treated as a corporation for
U.S. federal tax purposes) created or organized under the
laws of the U.S., any state thereof or the District of Columbia;
|
| |
| |
•
|
an estate the income of which is subject to U.S. federal
income tax regardless of its source; or
|
| |
| |
•
|
a trust (i) the administration of which is subject to the
primary supervision of a U.S. court and all substantial
decisions of which are controlled by one or more
U.S. persons or (ii) that has a valid election in
effect under applicable Treasury Regulations to be treated as a
U.S. person.
|
If a partnership or other entity or arrangement treated as a
partnership for U.S. federal income tax purposes holds our
common stock, the tax treatment of a partner will generally
depend upon the status of the partner and the activities of the
partnership. If you are a partner of a partnership holding our
common stock, we urge you to consult your tax advisor.
DISTRIBUTIONS
ON OUR COMMON STOCK
If we make cash or other property distributions on our common
stock, such distributions will generally constitute dividends
for U.S. federal income tax purposes to the extent paid
from our current or accumulated earnings and profits, as
determined under U.S. federal income tax principles.
Amounts not treated as dividends for U.S. federal income
tax purposes will constitute a return of capital and will first
be applied against and reduce a holder’s adjusted tax basis
in the common stock, but not below zero. Distributions in excess
of our current and accumulated earnings and profits and in
excess of a
non-U.S. holder’s
tax basis in its shares will be treated as gain realized on the
sale or other disposition of the common stock and will be
treated as described under “Gain on Disposition of Our
Common Stock” below.
Dividends paid to a
non-U.S. holder
of our common stock generally will be subject to
U.S. federal withholding tax at a rate of 30% of the gross
amount of the dividends, or such lower rate specified by an
applicable income tax treaty. To receive the benefit of a
reduced treaty rate, a
non-U.S. holder
must furnish to us or our paying agent a valid IRS
Form W-8BEN
(or applicable successor form) certifying such holder’s
qualification for the reduced rate. This certification must be
provided to us or our paying agent prior to the payment of
dividends and must be updated periodically.
If a
non-U.S. holder
holds our common stock in connection with the conduct of a trade
or business in the U.S., and dividends paid on the common stock
are effectively connected with such holder’s
U.S. trade or business, the
non-U.S. holder
will be exempt from U.S. federal withholding tax. To claim
the exemption, the
non-U.S. holder
must generally furnish to us or our paying agent a properly
executed IRS
Form W-8ECI
(or applicable successor form) and must update such form
periodically.
Any dividends paid on our common stock that are effectively
connected with a
non-U.S. holder’s
U.S. trade or business generally will be subject to
U.S. federal income tax on a net income basis at the
regular graduated U.S. federal income tax rates in much the
same manner as if such holder were a resident of the U.S.,
unless an applicable income tax treaty provides otherwise. A
non-U.S. holder
that is a foreign corporation also may be subject to a branch
profits tax equal to 30% (or such lower rate specified by an
applicable income tax treaty) of a portion of its effectively
connected earnings and profits for the taxable year, as adjusted
for certain items.
Non-U.S. holders
are urged to consult any applicable income tax treaties that may
provide for different rules.
A
non-U.S. holder
who claims the benefit of an applicable income tax treaty
generally will be required to satisfy applicable certification
and other requirements prior to the distribution date.
Non-U.S. holders
that do not timely provide us or our paying agent with the
required certification, but which qualify for a reduced
143
treaty rate, may obtain a refund of any excess amounts withheld
by timely filing an appropriate claim for refund with the IRS.
Non-U.S. holders
should consult their tax advisors regarding their entitlement to
benefits under a relevant income tax treaty.
GAIN ON
DISPOSITION OF OUR COMMON STOCK
A
non-U.S. holder
generally will not be subject to U.S. federal income tax on
any gain realized upon the sale or other disposition of our
common stock, unless:
|
|
|
| |
•
|
the gain is effectively connected with the
non-U.S. holder’s
conduct of a trade or business in the U.S. and, in the case
of a
non-U.S. holder
otherwise eligible for the benefits of an applicable income tax
treaty, attributable to a permanent establishment maintained by
the
non-U.S. holder
in the U.S.;
|
| |
| |
•
|
the
non-U.S. holder
is an individual present in the U.S. for 183 days or
more during the taxable year of the disposition, and certain
other requirements are met; or
|
| |
| |
•
|
our common stock constitutes a “U.S. real property
interest” by reason of our status as a U.S. real
property holding corporation, or USRPHC, for U.S. federal
income tax purposes at any time within the shorter of
(i) the five-year period ending on the date of the
disposition or (ii) the
non-U.S. holder’s
holding period for our common stock. We will be a USRPHC if the
fair market value of our U.S. real property interests
equals or exceeds 50% of the fair market value of our
(A) U.S. real property interests, (B) foreign
real property interests, and (C) other assets which are
used or held for use in a trade or business.
|
Unless an applicable income tax treaty provides otherwise, gain
described in the first bullet point above will be subject to
U.S. federal income tax (but not the Medicare contribution
tax for taxable years beginning after December 31,
2012) on a net income basis at the regular graduated
U.S. federal income tax rates in much the same manner as if
such holder were a resident of the U.S. Further,
non-U.S. holders
that are foreign corporations also may be subject to a branch
profits tax equal to 30% (or such lower rate specified by an
applicable income tax treaty) of a portion of its effectively
connected earnings and profits for the taxable year, as adjusted
for certain items.
Gain described solely in the second bullet point above will be
subject to U.S. federal income tax at a flat 30% rate (or
such lower rate specified by an applicable income tax treaty),
but may be offset by U.S. source capital losses (even
though the individual is not considered a resident of the U.S.).
With respect to gain described in the third bullet point above,
we believe we are not currently and do not anticipate becoming a
USRPHC for U.S. federal income tax purposes. Even if we
become a USRPHC, however, so long as our common stock is
regularly traded on an established securities market, such
common stock will be treated as U.S. real property
interests in the hands of a
non-U.S. holder
only if the
non-U.S. holder
actually or constructively holds more than 5% of our common
stock during the applicable period that is specified in the Code.
Non-U.S. holders
are urged to consult any applicable income tax treaties that may
provide for different rules.
FEDERAL
ESTATE TAX
Shares of our common stock that are owned or treated as owned by
an individual who is not a citizen or resident of the
U.S. (as specially defined for U.S. federal estate tax
purposes) at the time of death will be included in the
individual’s gross estate for U.S. federal estate tax
purposes, unless an applicable estate tax or other treaty
provides otherwise, and therefore may be subject to
U.S. federal estate tax.
INFORMATION
REPORTING AND BACKUP WITHHOLDING
We must report annually to the IRS and to each
non-U.S. holder
the amount of distributions on our common stock paid to such
holder and the amount of tax withheld, if any, with respect to
those distributions. These information reporting requirements
apply even if no withholding was required because the
distributions
144
were effectively connected with the holder’s conduct of a
U.S. trade or business, or withholding was reduced or
eliminated by an applicable income tax treaty. This information
also may be made available by the IRS under a specific treaty or
agreement to the tax authorities in the country in which the
non-U.S. holder
resides or is established.
Backup withholding at the current rate of 28% may apply to
distribution payments to a
non-U.S. holder
of our common stock and information reporting and backup
withholding at the same rate may apply to the payments of the
proceeds of a sale of our common stock within the U.S. or
through certain
U.S.-related
financial intermediaries, unless the
non-U.S. holder
furnishes to us or our paying agent the required certification
as to its
non-U.S. status,
such as by providing a valid IRS
Form W-8BEN
or IRS
Form W-8ECI,
or certain other requirements are met. Notwithstanding the
foregoing, backup withholding may apply if either we have or our
paying agent has actual knowledge, or reason to know, that the
holder is a U.S. person that is not an exempt recipient.
Backup withholding is not an additional tax. Any amounts
withheld under the backup withholding rules may be allowed as a
refund or a credit against a
non-U.S. holder’s
U.S. federal income tax liability, provided the required
information is timely furnished to the IRS.
Non-U.S. holders
should consult their tax advisors regarding the application of
the information reporting and backup withholding rules to them.
RECENT
LEGISLATIVE DEVELOPMENTS
Recently enacted legislation will generally require, for
payments made after December 31, 2012, withholding at a
rate of 30% on dividends in respect of, and gross proceeds from
the sale or other disposition of, our common stock held by or
through certain foreign financial institutions (including
investment funds), unless such institution enters into an
agreement with the Secretary of the Treasury to report, on an
annual basis, information with respect to accounts or interests
in the institution held by certain U.S. persons and by
certain
non-U.S. entities
that are wholly or partially owned by U.S. persons.
Accordingly, the entity through which our common stock is held
will affect the determination of whether such withholding is
required. Similarly, dividends in respect of, and gross proceeds
from the sale or other disposition of, our common stock held by
an investor that is a non-financial
non-U.S. entity
will be subject to withholding at a rate of 30%, unless such
entity either (i) certifies to us that such entity does not
have any “substantial United States owners” or
(ii) provides certain information regarding the
entity’s “substantial United States owners,”
which we will in turn provide to the Secretary of the Treasury.
The recently enacted legislation requires the Secretary of the
Treasury to coordinate the withholding rules of the new
legislation and the withholding rules of other provisions of the
Code (such as the withholding rules discussed above under
“— Distributions on Our Common Stock” and
“— Information Reporting and Backup
Withholding”). Furthermore, although there can be no
assurances in this regard, it is possible that if a beneficial
owner of a payment is entitled to treaty benefits and the
recently enacted legislation results in withholding that
overly-taxes the beneficial owner, the beneficial owner may be
eligible for a credit or refund, provided the beneficial owner
complies with procedures to be established by the Secretary of
the Treasury.
Non-U.S. holders
are encouraged to consult with their tax advisors regarding the
possible implications of the legislation on their investment in
our common stock.
145
Underwriting
We are offering the shares of our common stock described in this
prospectus through the underwriters named below. UBS Securities
LLC, J.P. Morgan Securities LLC, Citigroup Global Markets
Inc. and Piper Jaffray & Co. are acting as joint
book-running managers of this offering and as the
representatives of the underwriters. Morgan Joseph TriArtisan
LLC is acting as co-manager of this offering. We have entered
into an underwriting agreement with the representatives. Subject
to the terms and conditions of the underwriting agreement, each
of the underwriters has severally agreed to purchase the number
of shares of common stock listed next to its name in the
following table.
| |
|
|
|
|
|
Underwriters
|
|
Number of Shares
|
|
|
|
|
UBS Securities LLC
|
|
|
|
|
|
J.P. Morgan Securities LLC
|
|
|
|
|
|
Citigroup Global Markets Inc.
|
|
|
|
|
|
Piper Jaffray & Co.
|
|
|
|
|
|
Morgan Joseph TriArtisan LLC
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
The underwriting agreement provides that the underwriters must
buy all of the shares if they buy any of them. However, the
underwriters are not required to pay for the shares covered by
the underwriters’ over-allotment option described below.
Our common stock is offered subject to a number of conditions,
including:
|
|
|
| |
•
|
receipt and acceptance of our common stock by the
underwriters; and
|
| |
| |
•
|
the underwriters’ right to reject orders in whole or in
part.
|
We have been advised by the representatives that the
underwriters intend to make a market in our common stock but
that they are not obligated to do so and may discontinue making
a market at any time without notice.
In connection with this offering, certain of the underwriters
and other securities dealers may distribute prospectuses
electronically.
OVER-ALLOTMENT
OPTION
We have granted the underwriters an option to buy up to an
aggregate
of
additional shares of our common stock. The underwriters may
exercise this option solely for the purpose of covering
over-allotments, if any, made in connection with this offering.
The underwriters have 30 days from the date of this
prospectus to exercise this option. If the underwriters exercise
this option, they will each purchase additional shares
approximately in proportion to the amounts specified in the
table above.
COMMISSIONS
AND DISCOUNTS
Shares sold by the underwriters to the public will initially be
offered at the initial offering price set forth on the cover of
this prospectus. Any shares sold by the underwriters to
securities dealers may be sold at a discount of up to
$ per share from the initial
public offering price. Sales of shares made outside of the
U.S. may be made by affiliates of the underwriters. If all
the shares are not sold at the initial public offering price,
the representatives may change the offering price and the other
selling terms. Upon execution of the underwriting agreement, the
underwriters will be obligated to purchase the shares at the
prices and upon the terms stated therein. The representatives of
the underwriters have informed us that they do not expect to
sell more than an aggregate
of
percent of the total number of shares of common stock offered by
them to accounts over which such representatives exercise
discretionary authority.
146
The following table shows the per share and total underwriting
discounts and commissions we will pay to the underwriters
assuming both no exercise and full exercise of the
underwriters’ option to purchase up
to
additional shares.
| |
|
|
|
|
|
|
|
|
|
|
|
No Exercise
|
|
|
Full Exercise
|
|
|
|
|
Per share
|
|
$
|
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
We estimate that the total expenses of this offering payable by
us, not including the underwriting discounts and commissions,
will be approximately
$ million.
NO SALES
OF SIMILAR SECURITIES
We, our executive officers and directors, the holders of all of
our outstanding shares of common and preferred stock and the
holders of all of our currently outstanding common stock
warrants have entered into
lock-up
agreements with the underwriters. Under these agreements, we and
each of these persons may not, without the prior written
approval of UBS Securities LLC offer, sell, contract to sell,
pledge, or otherwise dispose of, directly or indirectly, or
hedge our common stock or securities convertible into or
exchangeable or exercisable for our common stock, except in the
circumstances described below. These restrictions will be in
effect for a period of 180 days after the date of this
prospectus, which period is subject to extension in the
circumstances described in the paragraph below. At any time and
without public notice, UBS Securities LLC may, in its sole
discretion, release some or all the securities from these
lock-up
agreements.
The restrictions set forth above shall not apply to our issuance
of shares of our common stock described below:
|
|
|
| |
•
|
the issuance by us of shares of common stock upon the exercise
of options or warrants disclosed as outstanding in this
prospectus;
|
| |
| |
•
|
the issuance to employees or directors of restricted stock
grants not vesting during, or stock options not exercisable
during, the
lock-up
period pursuant to stock plans described in this prospectus;
|
| |
| |
•
|
the filing of registration statements on
Form S-8
relating to shares of our common stock that may be issued
pursuant to stock plans described in this prospectus; and
|
| |
| |
•
|
the issuance by us of up to 10% of the number of shares of
common stock outstanding immediately after the completion of
this offering in connection with any acquisition or strategic
investment, provided that the recipient of these shares executes
a lock-up agreement for the remainder of the lock-up period.
|
The restrictions set forth above shall not apply to the
following types of transfers of shares of our common stock, or
securities convertible into or exchangeable or exercisable for
our common stock, by any of our directors, executive officers or
the holders of our shares or warrants in the following
circumstances:
|
|
|
| |
•
|
a bona fide gift or gifts, or transfers made to a former spouse
pursuant to a qualified domestic relations order, provided that
the recipient thereof executes a
lock-up
agreement for the remainder of the
lock-up
period;
|
| |
| |
•
|
a disposition to any trust for the direct or indirect benefit of
the holder
and/or the
holder’s immediate family, provided, that (i) the
disposition does not involve a disposition for value and
(ii) the trust executes a
lock-up
agreement for the remainder of the
lock-up
period;
|
| |
| |
•
|
in the case of a corporation, limited liability company or
partnership, a transfer to a wholly-owned subsidiary thereof, or
to the direct or indirect stockholders, members or partners or
other affiliates thereof; provided, that (i) the transfer
does not involve a disposition for value, (ii) the
transferee executes a
lock-up
agreement for the remainder of the
lock-up
period, and (iii) no filing pursuant to Section 16 of
the Exchange Act is required nor will such a filing be made as a
result of the transfer;
|
147
|
|
|
| |
•
|
transfers that occur by will or other testamentary document or
by the rules of intestate succession upon the death of a
locked-up
party, provided that no filing pursuant to Section 16 of
the Exchange Act is required or will be made as a result of the
transfer; and
|
| |
| |
•
|
the disposition of shares of common stock acquired in open
market transactions after the offering; provided that no filing
pursuant to Section 16 of the Exchange Act is required or
will be made as a result of the disposition.
|
In addition, two of our stockholders, Plainfield Direct LLC and
Plainfield Finance II LLC, holding in the aggregate
6,351,240 shares of our common stock, may, following the
earlier of (i) completion, abandonment or termination of
this offering or (ii) November 11, 2011, sell or
otherwise dispose of all, but not less than all, of their
combined shares of capital stock to a single purchaser or group
of purchasers in a single, private transaction, so long as each
such purchaser (A) agrees in writing with the Underwriters
to be bound by the terms of a substantially similar lock-up
agreement, (B) agrees to be bound by the Investor Rights
Agreement dated as of January 13, 2011 by and among us and
the investors named therein and (C) is not a competitor of
ours as determined in the reasonable opinion of our board of
directors.
INDEMNIFICATION
We have agreed to indemnify the several underwriters against
certain liabilities, including certain liabilities under the
Securities Act. If we are unable to provide this
indemnification, we have agreed to contribute to payments the
underwriters may be required to make in respect of those
liabilities.
NASDAQ
GLOBAL MARKET QUOTATION
We have applied to list our common stock on The Nasdaq Global
Market under the symbol “MYRT”.
PRICE
STABILIZATION, SHORT POSITIONS
In connection with this offering, the underwriters may engage in
activities that stabilize, maintain or otherwise affect the
price of our common stock, including:
|
|
|
| |
•
|
stabilizing transactions;
|
| |
| |
•
|
short sales;
|
| |
| |
•
|
purchases to cover positions created by short sales;
|
| |
| |
•
|
imposition of penalty bids; and
|
| |
| |
•
|
syndicate covering transactions.
|
Stabilizing transactions consist of bids or purchases made for
the purpose of preventing or retarding a decline in the market
price of our common stock while this offering is in progress.
These transactions may also include making short sales of our
common stock, which involve the sale by the underwriters of a
greater number of shares of common stock than they are required
to purchase in this offering and purchasing shares of common
stock on the open market to cover short positions created by
short sales. Short sales may be “covered short sales,”
which are short positions in an amount not greater than the
underwriters’ over-allotment option referred to above, or
may be “naked short sales,” which are short positions
in excess of that amount.
The underwriters may close out any covered short position by
either exercising their over-allotment option, in whole or in
part, or by purchasing shares in the open market. In making this
determination, the underwriters will consider, among other
things, the price of shares available for purchase in the open
market as compared to the price at which they may purchase
shares through the over-allotment option.
Naked short sales are in excess of the over-allotment option.
The underwriters must close out any naked short position by
purchasing shares in the open market. A naked short position is
more likely to be created if the underwriters are concerned that
there may be downward pressure on the price of the common stock
in the open market that could adversely affect investors who
purchased in this offering.
148
The underwriters also may impose a penalty bid. This occurs when
a particular underwriter repays to the underwriters a portion of
the underwriting discount received by it because the
representatives have repurchased shares sold by or for the
account of that underwriter in stabilizing or short covering
transactions.
As a result of these activities, the price of our common stock
may be higher than the price that otherwise might exist in the
open market. If these activities are commenced, they may be
discontinued by the underwriters at any time. The underwriters
may carry out these transactions on The Nasdaq Global Market, in
the
over-the-counter
market or otherwise.
DETERMINATION
OF OFFERING PRICE
Prior to this offering, there was no public market for our
common stock. The initial public offering price will be
determined by negotiation among us and the representatives of
the underwriters. The principal factors to be considered in
determining the initial public offering price include:
|
|
|
| |
•
|
the information set forth in this prospectus and otherwise
available to the representatives;
|
| |
| |
•
|
our history and prospects and the history and prospects for the
industry in which we compete;
|
| |
| |
•
|
our past and present financial performance and an assessment of
our management;
|
| |
| |
•
|
our prospects for future earnings and the present state of our
development;
|
| |
| |
•
|
the general condition of the securities markets at the time of
this offering;
|
| |
| |
•
|
the recent market prices of, and demand for, publicly traded
common stock of generally comparable companies; and
|
| |
| |
•
|
other factors deemed relevant by the underwriters and us.
|
AFFILIATIONS
The underwriters and their respective affiliates are full
service financial institutions engaged in various activities,
which may include securities trading, commercial and investment
banking, financial advisory, investment management, investment
research, principal investment, hedging, financing and brokerage
activities. The underwriters and their affiliates may from time
to time in the future engage with us and perform services for us
in the ordinary course of their business for which they will
receive customary fees and expenses. In the ordinary course of
their various business activities, the underwriters and their
respective affiliates may make or hold a broad array of
investments and actively trade debt and equity securities (or
related derivative securities) and financial instruments
(including bank loans) for their own account and for the
accounts of their customers, and such investment and securities
activities may involve securities
and/or
instruments of us or our subsidiaries. The underwriters and
their respective affiliates may also make investment
recommendations
and/or
publish or express independent research views in respect of
these securities or instruments and may at any time hold, or
recommend to clients that they acquire, long
and/or short
positions in these securities and instruments.
DIRECTED
SHARE PROGRAM
At our request, the underwriters have reserved up
to % of the shares of common stock
being sold in this offering for sale to our employees and
consultants and other designated persons having a relationship
with us at the initial public offering price through a directed
share program. Any sale to these persons will be made by Piper
Jaffray & Co. through a directed share program. The
number of shares available for sale to the general public in
this offering will be reduced to the extent that these reserved
shares are purchased by these persons. Any reserved shares not
purchased by these persons will be offered by the underwriters
to the general public on the same basis as the other shares in
this offering.
149
NOTICE TO
INVESTORS
Notice
to prospective investors in European Economic Area
In relation to each member state of the European Economic Area
that has implemented the Prospectus Directive (each, a relevant
member state), other than Germany, with effect from and
including the date on which the Prospectus Directive is
implemented in that relevant member state (the relevant
implementation date), an offer of securities described in this
prospectus may not be made to the public in that relevant member
state other than:
|
|
|
| |
•
|
to any legal entity which is a qualified investor as defined in
the Prospectus Directive;
|
| |
| |
•
|
by the Managers to fewer than 100, or, if the Relevant Member
State has implemented the relevant provisions of the 2010 PD
Amending Directive, 150, natural or legal persons (other than
qualified investors as defined in the Prospectus Directive), as
permitted under the Prospectus Directive, subject to obtaining
the prior consent of the Bookrunners for any such offer; or
|
| |
| |
•
|
in any other circumstances falling within Article 3(2) of
the Prospectus Directive.
|
provided that no such offer of securities shall require us or
any underwriter to publish a prospectus pursuant to
Article 3 of the Prospectus Directive.
For purposes of this provision, the expression an “offer of
securities to the public” in any relevant member state
means the communication in any form and by any means of
sufficient information on the terms of the offer and the
securities to be offered so as to enable an investor to decide
to purchase or subscribe for the securities, as the expression
may be varied in that member state by any measure implementing
the Prospectus Directive in that member state, and the
expression “Prospectus Directive” means Directive
2003/71/EC (and amendments thereto, including the 2010 PD
Amending Directive, to the extent implemented in the Relevant
Member State), and includes any relevant implementing measure in
the Relevant Member State, and includes any relevant
implementing measure in each relevant member state. The
expression 2010 PD Amending Directive means Directive 2010/73/EU.
We have not authorized and do not authorize the making of any
offer of securities through any financial intermediary on their
behalf, other than offers made by the underwriters with a view
to the final placement of the securities as contemplated in this
prospectus. Accordingly, no purchaser of the securities, other
than the underwriters, is authorized to make any further offer
of the securities on behalf of us or the underwriters.
The EEA selling restriction is in addition to any other selling
restrictions set out in this prospectus.
Notice
to prospective investors in Australia
This offering memorandum is not a formal disclosure document and
has not been, nor will be, lodged with the Australian Securities
and Investments Commission. It does not purport to contain all
information that an investor or their professional advisers
would expect to find in a prospectus or other disclosure
document (as defined in the Corporations Act 2001 (Australia))
for the purposes of Part 6D.2 of the Corporations Act 2001
(Australia) or in a product disclosure statement for the
purposes of Part 7.9 of the Corporations Act 2001
(Australia), in either case, in relation to the securities.
The securities are not being offered in Australia to
“retail clients” as defined in sections 761G and
761GA of the Corporations Act 2001 (Australia). This offering is
being made in Australia solely to “wholesale clients”
for the purposes of section 761G of the Corporations Act
2001 (Australia) and, as such, no prospectus, product disclosure
statement or other disclosure document in relation to the
securities has been, or will be, prepared.
This offering memorandum does not constitute an offer in
Australia other than to wholesale clients. By submitting an
application for our securities, you represent and warrant to us
that you are a wholesale client for the purposes of
section 761G of the Corporations Act 2001 (Australia). If
any recipient of this offering memorandum is not a wholesale
client, no offer of, or invitation to apply for, our securities
shall be deemed to be made to such recipient and no applications
for our securities will be accepted from such recipient. Any
150
offer to a recipient in Australia, and any agreement arising
from acceptance of such offer, is personal and may only be
accepted by the recipient. In addition, by applying for our
securities you undertake to us that, for a period of
12 months from the date of issue of the securities, you
will not transfer any interest in the securities to any person
in Australia other than to a wholesale client.
Notice
to prospective investors in Hong Kong
Our securities may not be offered or sold in Hong Kong, by means
of this prospectus or any document other than (i) to
“professional investors” within the meaning of the
Securities and Futures Ordinance (Cap.571, Laws of Hong Kong)
and any rules made thereunder, or (ii) in circumstances
which do not constitute an offer to the public within the
meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong),
or (iii) in other circumstances which do not result in the
document being a “prospectus” within the meaning of
the Companies Ordinance (Cap.32, Laws of Hong Kong). No
advertisement, invitation or document relating to our securities
may be issued or may be in the possession of any person for the
purpose of issue (in each case whether in Hong Kong or
elsewhere) which is directed at, or the contents of which are
likely to be accessed or read by, the public in Hong Kong
(except if permitted to do so under the securities laws of Hong
Kong) other than with respect to the securities which are or are
intended to be disposed of only to persons outside Hong Kong or
only to “professional investors” within the meaning of
the Securities and Futures Ordinance (Cap. 571, Laws of Hong
Kong) and any rules made thereunder.
Notice
to prospective investors in Japan
Our securities have not been and will not be registered under
the Financial Instruments and Exchange Law of Japan (the
Financial Instruments and Exchange Law) and our securities will
not be offered or sold, directly or indirectly, in Japan, or to,
or for the benefit of, any resident of Japan (which term as used
herein means any person resident in Japan, including any
corporation or other entity organized under the laws of Japan),
or to others for re-offering or resale, directly or indirectly,
in Japan, or to a resident of Japan, except pursuant to an
exemption from the registration requirements of, and otherwise
in compliance with, the Financial Instruments and Exchange Law
and any other applicable laws, regulations and ministerial
guidelines of Japan.
Notice
to prospective investors in Singapore
This document has not been registered as a prospectus with the
Monetary Authority of Singapore and in Singapore, the offer and
sale of our securities is made pursuant to exemptions provided
in sections 274 and 275 of the Securities and Futures Act,
Chapter 289 of Singapore (“SFA”). Accordingly,
this prospectus and any other document or material in connection
with the offer or sale, or invitation for subscription or
purchase, of our securities may not be circulated or
distributed, nor may our securities be offered or sold, or be
made the subject of an invitation for subscription or purchase,
whether directly or indirectly, to persons in Singapore other
than (i) to an institutional investor as defined in
Section 4A of the SFA pursuant to Section 274 of the
SFA, (ii) to a relevant person as defined in
section 275(2) of the SFA pursuant to Section 275(1)
of the SFA, or any person pursuant to Section 275(1A) of
the SFA, and in accordance with the conditions specified in
Section 275 of the SFA or (iii) otherwise pursuant to,
and in accordance with the conditions of, any other applicable
provision of the SFA, in each case subject to compliance with
the conditions (if any) set forth in the SFA. Moreover, this
document is not a prospectus as defined in the SFA. Accordingly,
statutory liability under the SFA in relation to the content of
prospectuses would not apply. Prospective investors in Singapore
should consider carefully whether an investment in our
securities is suitable for them.
Where our securities are subscribed or purchased under
Section 275 of the SFA by a relevant person which is:
(a) by a corporation (which is not an accredited investor
as defined in Section 4A of the SFA) the sole business of
which is to hold investments and the entire share capital of
which is owned by one or more individuals, each of whom is an
accredited investor; or
151
(b) for a trust (where the trustee is not an accredited
investor) whose sole purpose is to hold investments and each
beneficiary of the trust is an individual who is an accredited
investor,
shares of that corporation or the beneficiaries’ rights and
interest (howsoever described) in that trust shall not be
transferable for six months after that corporation or that trust
has acquired the shares under Section 275 of the SFA,
except:
(1) to an institutional investor (for corporations under
Section 274 of the SFA) or to a relevant person defined in
Section 275(2) of the SFA, or any person pursuant to an
offer that is made on terms that such shares of that corporation
or such rights and interest in that trust are acquired at a
consideration of not less than S$200,000 (or its equivalent in a
foreign currency) for each transaction, whether such amount is
to be paid for in cash or by exchange of securities or other
assets, and further for corporations, in accordance with the
conditions, specified in Section 275 of the SFA;
(2) where no consideration is given for the
transfer; or
(3) where the transfer is by operation of law.
In addition, investors in Singapore should note that the
securities acquired by them are subject to resale and transfer
restrictions specified under Section 276 of the SFA, and
they, therefore, should seek their own legal advice before
effecting any resale or transfer of their securities.
Notice
to prospective investors in Switzerland
The Prospectus does not constitute an issue prospectus pursuant
to Article 652a or Article 1156 of the Swiss Code of
Obligations (“CO”) and the shares will not be listed
on the SIX Swiss Exchange. Therefore, the Prospectus may not
comply with the disclosure standards of the CO
and/or the
listing rules (including any prospectus schemes) of the SIX
Swiss Exchange. Accordingly, the shares may not be offered to
the public in or from Switzerland, but only to a selected and
limited circle of investors, which do not subscribe to the
shares with a view to distribution.
Notice
to prospective investors in United Kingdom
This prospectus is only being distributed to and is only
directed at: (1) persons who are outside the United
Kingdom; (2) investment professionals falling within
Article 19(5) of the Financial Services and Markets Act
2000 (Financial Promotion) Order 2005 (the “Order”);
or (3) high net worth companies, and other persons to whom
it may lawfully be communicated, falling within
Article 49(2)(a) to (d) of the Order (all such persons
falling within (1)-(3) together being referred to as
“relevant persons”). The shares are only available to,
and any invitation, offer or agreement to subscribe, purchase or
otherwise acquire such shares will be engaged in only with,
relevant persons. Any person who is not a relevant person should
not act or rely on this prospectus or any of its contents.
152
Legal
matters
The validity of our common stock offered by this prospectus will
be passed upon for us by McDermott Will & Emery LLP,
Boston, Massachusetts. Dewey & LeBoeuf LLP, New York,
New York, is counsel to the underwriters in connection with this
offering.
Experts
The consolidated financial statements appearing in this
Prospectus and Registration Statement have been audited by
McGladrey & Pullen, LLP, an independent registered
public accounting firm, as stated in their report appearing
elsewhere herein, which report expresses an unqualified opinion
and includes an explanatory paragraph referring to the
Company’s status as a development stage enterprise and the
Predecessor Company’s change in the method of accounting
for warrants and are included in reliance upon such report and
upon the authority of such firm as experts in accounting and
auditing.
Where you
can find additional information
We have filed with the SEC, a registration statement on
Form S-1
under the Securities Act, with respect to the shares of our
common stock offered hereby. This prospectus does not contain
all of the information set forth in the registration statement
and the exhibits and schedules thereto. Some items are omitted
in accordance with the rules and regulations of the SEC. For
further information with respect to us and the common stock
offered hereby, we refer you to the registration statement and
the exhibits and schedules filed therewith. Statements contained
in this prospectus as to the contents of any contract, agreement
or any other document are summaries of the material terms of
such contract, agreement or other document. A copy of the
registration statement, and the exhibits and schedules thereto,
may be inspected without charge at the public reference
facilities maintained by the SEC at 100 F Street,
N.E., Washington, D.C. 20549. Copies of these materials may
be obtained by writing to the Public Reference Section of the
SEC at 100 F Street, N.E., Washington, D.C.
20549. Please call the SEC at
1-800-SEC-0330
for further information on the operation of the public reference
facility. The SEC maintains a website that contains reports,
proxy and information statements and other information regarding
registrants that file electronically with the SEC. The address
of the SEC’s website is
http://www.sec.gov.
Upon completion of this offering, we will become subject to the
information and periodic reporting requirements of the Exchange
Act and, in accordance therewith, will file periodic reports,
proxy statements and other information with the SEC. Such
periodic reports, proxy statements and other information will be
available for inspection and copying at the public reference
room and website of the SEC referred to above. We maintain a
website at www.myriant.com. You may access our annual report on
Form 10-K,
quarterly reports on
Form 10-Q,
current reports on
Form 8-K
and amendments to those reports filed or furnished pursuant to
Section 13(a) or 15(d) of the Exchange Act with the SEC
free of charge at our website as soon as reasonably practicable
after such material is electronically filed with, or furnished
to, the SEC. The reference to our website address does not
constitute incorporation by reference of the information
contained on our website.
153
Report of
Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders
Myriant Corporation and Subsidiaries (a development stage
enterprise)
Quincy, Massachusetts
We have audited the accompanying consolidated balance sheets of
Myriant Corporation and Subsidiaries (formerly Myriant
Technologies LLC and Subsidiaries) (a development stage
enterprise) (the “Company”) as of December 31,
2010 and 2009, and the related consolidated statements of
operations, changes in members’ equity (deficit), and cash
flows of Myriant Corporation and Subsidiaries (a development
stage enterprise) for the year ended December 31, 2010 and
the period from July 16, 2009 (inception) to
December 31, 2009 and of BioEnergy International, LLC (the
“Predecessor Company”) for the period from
January 1, 2009 through July 15, 2009 and for the year
ended December 31, 2008. These financial statements are the
responsibility of the Company’s and Predecessor
Company’s management. Our responsibility is to express an
opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. The Company and its Predecessor
Company are not required to have, nor were we engaged to perform
an audit of their internal control over financial reporting. Our
audit included consideration of internal control over financial
reporting as a basis for designing audit procedures that are
appropriate in the circumstances, but not for the purpose of
expressing an opinion on the effectiveness of the Company’s
and its Predecessor Company’s internal control over
financial reporting. Accordingly, we express no such opinion. An
audit also includes examining, on a test basis, evidence
supporting the amounts and disclosures in the financial
statements, assessing the accounting principles used and
significant estimates made by management, as well as evaluating
the overall financial statement presentation. We believe that
our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred
to above present fairly, in all material respects, the financial
position of Myriant Corporation and Subsidiaries (a development
stage enterprise) as of December 31, 2010 and 2009, and the
results of operations and cash flows for Myriant Corporation and
Subsidiaries (a development stage enterprise) for the year ended
December 31, 2010 and for the period from July 16,
2009 (inception) through December 31, 2009 and of the
Predecessor Company for the period from January 1, 2009
through July 15, 2009 and for the year ended
December 31, 2008 in conformity with U.S. generally
accepted accounting principles.
As discussed in Note 1 to the consolidated financial
statements, the Company is a development stage enterprise.
As discussed in Note 4 to the consolidated financial
statements, the Predecessor Company changed its method of
accounting for warrants upon the adoption of
ASC 814-40,
“Contracts in an Entity’s Own Stock” on
January 1, 2009.
/s/ McGladrey & Pullen, LLP
Boston, Massachusetts
May 27, 2011
F-2
Myriant
Corporation and Subsidiaries (A Development Stage
Enterprise)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
ASSETS
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
35,112,448
|
|
|
$
|
564,147
|
|
|
$
|
1,044,822
|
|
|
Accounts receivable
|
|
|
—
|
|
|
|
244,504
|
|
|
|
221,576
|
|
|
Government award receivable
|
|
|
478,960
|
|
|
|
1,458,518
|
|
|
|
—
|
|
|
Due from related parties
|
|
|
30,412
|
|
|
|
87,439
|
|
|
|
540,398
|
|
|
Prepaid expenses
|
|
|
197,631
|
|
|
|
319,916
|
|
|
|
379,806
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
35,819,451
|
|
|
|
2,674,524
|
|
|
|
2,186,602
|
|
|
Debt issuance costs
|
|
|
—
|
|
|
|
13,750
|
|
|
|
44,197
|
|
|
Due from related parties
|
|
|
—
|
|
|
|
—
|
|
|
|
575,790
|
|
|
Restricted cash
|
|
|
15,580,259
|
|
|
|
374,050
|
|
|
|
422,959
|
|
|
Property and equipment, net
|
|
|
9,734,725
|
|
|
|
9,388,613
|
|
|
|
9,940,392
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
61,134,435
|
|
|
$
|
12,450,937
|
|
|
$
|
13,169,940
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ AND MEMBERS’ EQUITY
(DEFICIT)
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
337,924
|
|
|
$
|
3,917,532
|
|
|
$
|
435,863
|
|
|
Accrued liabilities
|
|
|
3,496,131
|
|
|
|
3,782,706
|
|
|
|
1,981,357
|
|
|
Current portion of secured convertible notes-related party
|
|
|
—
|
|
|
|
1,458,333
|
|
|
|
250,000
|
|
|
Note payable
|
|
|
—
|
|
|
|
—
|
|
|
|
1,677,994
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
3,834,055
|
|
|
|
9,158,571
|
|
|
|
4,345,214
|
|
|
Deferred rent
|
|
|
133,137
|
|
|
|
155,132
|
|
|
|
153,655
|
|
|
Secured convertible notes-related party, net of current portion
|
|
|
—
|
|
|
|
19,388,386
|
|
|
|
9,874,528
|
|
|
Warrant liability
|
|
|
2,199,585
|
|
|
|
3,289,625
|
|
|
|
2,640,435
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
6,166,777
|
|
|
|
31,991,714
|
|
|
|
17,013,832
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ and members’ equity (deficit)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Class A common stock, $.0001 par value per share;
11,214,953, 0 and 0 shares authorized, issued and
outstanding at March 31, 2011, December 31, 2010 and
December 31, 2009, respectively. Aggregate liquidation
preference of $61,025,753 at March 31, 2011 (unaudited)
|
|
|
1,121
|
|
|
|
—
|
|
|
|
—
|
|
|
Class B common stock, $.0001 par value per share;
3,044,905, 0 and 0 shares authorized, issued and
outstanding at March 31, 2011, December 31, 2010 and
December 31, 2009, respectively. Aggregate liquidation
preference of $16,290,242 at March 31, 2011 (unaudited)
|
|
|
304
|
|
|
|
—
|
|
|
|
—
|
|
|
Common stock, $.0001 par value per share; 30,000,000, 0 and
0 shares authorized at March 31, 2011,
December 31, 2010 and December 31, 2009, respectively;
8,494,650 (unaudited), 0 and 0 shares issued and
outstanding at March 31, 2011, December 31, 2010 and
December 31, 2009, respectively
|
|
|
849
|
|
|
|
—
|
|
|
|
—
|
|
|
Members’ equity
|
|
|
—
|
|
|
|
5,729,332
|
|
|
|
5,203,388
|
|
|
Additional paid in capital
|
|
|
72,459,301
|
|
|
|
—
|
|
|
|
—
|
|
|
Accumulated deficit
|
|
|
(17,493,917
|
)
|
|
|
(25,270,109
|
)
|
|
|
(9,047,280
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ and members’ equity (deficit)
|
|
|
54,967,658
|
|
|
|
(19,540,777
|
)
|
|
|
(3,843,892
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ and members’
equity (deficit)
|
|
$
|
61,134,435
|
|
|
$
|
12,450,937
|
|
|
$
|
13,169,940
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-3
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
For the
Three Months Ended March 31, 2011 and 2010 (Unaudited),
Year Ended December 31, 2010 and Periods from July 16,
2009 (Inception) to December 31, 2009 and March 31,
2011 (Unaudited) Predecessor Company Statement of Operations for
the Period January 1, 2009 to July 15, 2009 and
For the Year Ended December 31, 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Myriant Corporation
|
|
|
BioEnergy International, LLC (Predecessor Company)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
For the
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
July 16, 2009
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
|
|
(Inception) to
|
|
|
July 16, 2009
|
|
|
Period from
|
|
|
|
|
|
|
|
March 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
(Inception) to
|
|
|
January 1 to
|
|
|
December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2010
|
|
|
2009
|
|
|
March 31, 2011
|
|
|
July 15, 2009
|
|
|
2008
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
License fee revenue
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
258,240
|
|
|
$
|
221,711
|
|
|
$
|
479,951
|
|
|
$
|
71,833
|
|
|
$
|
344,860
|
|
|
Management fee revenue-related party
|
|
|
2,519
|
|
|
|
600,166
|
|
|
|
3,557,575
|
|
|
|
—
|
|
|
|
3,560,094
|
|
|
|
267,318
|
|
|
|
262,212
|
|
|
Development fee revenue-related party
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,125,714
|
|
|
Government awards
|
|
|
—
|
|
|
|
1,516,323
|
|
|
|
10,419,043
|
|
|
|
—
|
|
|
|
10,419,043
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
2,519
|
|
|
|
2,116,489
|
|
|
|
14,234,858
|
|
|
|
221,711
|
|
|
|
14,459,088
|
|
|
|
339,151
|
|
|
|
3,732,786
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of license fee revenue
|
|
|
—
|
|
|
|
—
|
|
|
|
84,600
|
|
|
|
73,859
|
|
|
|
158,459
|
|
|
|
22,373
|
|
|
|
110,020
|
|
|
Research and development
|
|
|
1,883,603
|
|
|
|
2,552,100
|
|
|
|
15,904,717
|
|
|
|
2,793,085
|
|
|
|
20,581,405
|
|
|
|
3,770,721
|
|
|
|
4,679,935
|
|
|
Project development
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,179,965
|
|
|
General and administrative expense
|
|
|
3,034,498
|
|
|
|
2,806,285
|
|
|
|
12,673,247
|
|
|
|
4,979,186
|
|
|
|
20,686,931
|
|
|
|
5,438,073
|
|
|
|
5,699,186
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
4,918,101
|
|
|
|
5,358,385
|
|
|
|
28,662,564
|
|
|
|
7,846,130
|
|
|
|
41,426,795
|
|
|
|
9,231,167
|
|
|
|
12,669,106
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(4,915,582
|
)
|
|
|
(3,241,896
|
)
|
|
|
(14,427,706
|
)
|
|
|
(7,624,419
|
)
|
|
|
(26,967,707
|
)
|
|
|
(8,892,016
|
)
|
|
|
(8,936,320
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
6,328
|
|
|
|
84,844
|
|
|
|
87,611
|
|
|
|
8,051
|
|
|
|
101,990
|
|
|
|
5,385
|
|
|
|
60,222
|
|
|
Interest expense
|
|
|
(12,606,762
|
)
|
|
|
(628,386
|
)
|
|
|
(4,470,478
|
)
|
|
|
(915,804
|
)
|
|
|
(17,993,044
|
)
|
|
|
(1,352,767
|
)
|
|
|
(1,636,062
|
)
|
|
Gain (loss) on foreign currency exchange
|
|
|
11,638
|
|
|
|
(3,853
|
)
|
|
|
(5,235
|
)
|
|
|
—
|
|
|
|
6,403
|
|
|
|
(21,721
|
)
|
|
|
—
|
|
|
Miscellaneous income
|
|
|
8,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8,000
|
|
|
|
—
|
|
|
|
—
|
|
|
Changes in fair value of warrant liability
|
|
|
2,461
|
|
|
|
(823,582
|
)
|
|
|
2,592,979
|
|
|
|
(515,108
|
)
|
|
|
2,080,332
|
|
|
|
2,092,643
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net
|
|
|
(12,578,335
|
)
|
|
|
(1,370,977
|
)
|
|
|
(1,795,123
|
)
|
|
|
(1,422,861
|
)
|
|
|
(15,796,319
|
)
|
|
|
723,540
|
|
|
|
(1,575,840
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(17,493,917
|
)
|
|
|
(4,612,873
|
)
|
|
|
(16,222,829
|
)
|
|
|
(9,047,280
|
)
|
|
|
(42,764,026
|
)
|
|
|
(8,168,476
|
)
|
|
|
(10,512,160
|
)
|
|
Dividend on Class A common stock
|
|
|
(1,025,753
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,025,753
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders
|
|
$
|
(18,519,670
|
)
|
|
$
|
(4,612,873
|
)
|
|
$
|
(16,222,829
|
)
|
|
$
|
(9,047,280
|
)
|
|
$
|
(43,789,779
|
)
|
|
$
|
(8,168,476
|
)
|
|
$
|
(10,512,160
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per unit/share attributable to common
stockholders-basic and diluted:
|
|
$
|
(2.29
|
)
|
|
$
|
(0.66
|
)
|
|
$
|
(2.31
|
)
|
|
$
|
(1.30
|
)
|
|
$
|
(6.12
|
)
|
|
$
|
(1.55
|
)
|
|
$
|
(2.14
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of units outstanding- basic and diluted
|
|
|
8,092,943
|
|
|
|
6,982,215
|
|
|
|
7,009,251
|
|
|
|
6,953,079
|
|
|
|
7,150,339
|
|
|
|
5,285,807
|
|
|
|
4,918,668
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-4
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
MEMBERS’
EQUITY (DEFICIT)
For the
Three Months Ended March 31, 2011 (Unaudited), Year Ended
December 31, 2010 and the Periods from July 16, 2009
(Inception) to December 31, 2009 and March 31, 2011
(Unaudited) and Predecessor Company Statement of Changes in
Stockholders’ and Members’ Deficit for the Period from
January 1, 2009 to July 15, 2009 and for the Year
Ended December 31, 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
Class A
|
|
|
Class B
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
Stockholders’
|
|
|
|
|
Common Stock
|
|
|
Common Stock
|
|
|
Common Stock
|
|
|
|
|
|
Members’
|
|
|
Paid In
|
|
|
Accumulated
|
|
|
and Members’
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Units
|
|
|
Equity
|
|
|
Capital
|
|
|
Deficit
|
|
|
Equity (Deficit)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Predecessor Company
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2007
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
4,918,668
|
|
|
$
|
13,557,045
|
|
|
$
|
—
|
|
|
$
|
(11,787,060
|
)
|
|
$
|
1,769,985
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued in connection with issuance of secured
convertible notes-related party
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
403,846
|
|
|
|
—
|
|
|
|
—
|
|
|
|
403,846
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(10,512,160
|
)
|
|
|
(10,512,160
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,December 31, 2008
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
4,918,668
|
|
|
|
13,960,891
|
|
|
|
—
|
|
|
|
(22,299,220
|
)
|
|
|
(8,338,329
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adoption of warrant liability accounting standard
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,941,845
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,941,845
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants exercised, net of issuance costs
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
950,000
|
|
|
|
9,455,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
9,455,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of member units
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
48,198
|
|
|
|
279,920
|
|
|
|
—
|
|
|
|
—
|
|
|
|
279,920
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital distribution
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(77,343
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(77,343
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biochemicals transfer to Myriant
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,386,660
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,386,660
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(8,168,476
|
)
|
|
|
(8,168,476
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ending balance, July 15, 2009
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
5,916,866
|
|
|
$
|
22,063,283
|
|
|
$
|
—
|
|
|
$
|
(30,467,696
|
)
|
|
$
|
(8,404,413
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Successor Company
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning balance, July 16, 2009
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contribution of Biochemicals business
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
5,916,866
|
|
|
|
(2,386,660
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,386,660
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contribution of Myriant Lake Providence
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,000,001
|
|
|
|
7,382,455
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,382,455
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of membership units
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
65,147
|
|
|
|
207,593
|
|
|
|
—
|
|
|
|
—
|
|
|
|
207,593
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(9,047,280
|
)
|
|
|
(9,047,280
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2009
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,982,014
|
|
|
|
5,203,388
|
|
|
|
—
|
|
|
|
(9,047,280
|
)
|
|
|
(3,843,892
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of membership units
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
57,399
|
|
|
|
525,944
|
|
|
|
—
|
|
|
|
—
|
|
|
|
525,944
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(16,222,829
|
)
|
|
|
(16,222,829
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2010
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,039,413
|
|
|
|
5,729,332
|
|
|
|
—
|
|
|
|
(25,270,109
|
)
|
|
|
(19,540,777
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of Myriant Technologies, LLC into C corporation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,039,413
|
|
|
|
704
|
|
|
|
(7,039,413
|
)
|
|
|
(5,729,332
|
)
|
|
|
(19,541,481
|
)
|
|
|
25,270,109
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature-senior secured notes
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,445,199
|
|
|
|
—
|
|
|
|
10,445,199
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of senior secured notes, accrued interest and
exercise of warrants to equity
|
|
|
—
|
|
|
|
—
|
|
|
|
3,044,905
|
|
|
|
304
|
|
|
|
1,117,378
|
|
|
|
112
|
|
|
|
—
|
|
|
|
—
|
|
|
|
23,139,354
|
|
|
|
—
|
|
|
|
23,139,770
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of Class A common stock
|
|
|
11,214,953
|
|
|
|
1,121
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
59,998,879
|
|
|
|
—
|
|
|
|
60,000,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock issuance costs
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,907,953
|
)
|
|
|
—
|
|
|
|
(2,907,953
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of $.01 common stock warrants
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
273,930
|
|
|
|
27
|
|
|
|
—
|
|
|
|
—
|
|
|
|
945,033
|
|
|
|
—
|
|
|
|
945,060
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
63,929
|
|
|
|
6
|
|
|
|
—
|
|
|
|
—
|
|
|
|
380,270
|
|
|
|
—
|
|
|
|
380,276
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(17,493,917
|
)
|
|
|
(17,493,917
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2011 (unaudited)
|
|
|
11,214,953
|
|
|
$
|
1,121
|
|
|
|
3,044,905
|
|
|
$
|
304
|
|
|
|
8,494,650
|
|
|
$
|
849
|
|
|
|
—
|
|
|
$
|
—
|
|
|
$
|
72,459,301
|
|
|
$
|
(17,493,917
|
)
|
|
$
|
54,967,658
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-5
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
For the
Three Months Ended March 31, 2011 and 2010 (Unaudited),
Year Ended December 31, 2010 and Periods from July 16,
2009 (Inception) to December 31, 2009 and March 31,
2011 (Unaudited) Predecessor Company Statement of Cash Flows for
the Period January 1, 2009 to July 15, 2009 and
For the Year Ended December 31, 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BioEnergy International, LLC
|
|
|
|
|
Myriant Corporation
|
|
|
(Predecessor Company)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the
|
|
|
For the
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
July 16, 2009
|
|
|
July 16, 2009
|
|
|
Period from
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
|
|
(Inception) to
|
|
|
(Inception) to
|
|
|
January 1 to
|
|
|
|
|
|
|
|
March 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
March 31,
|
|
|
July 15,
|
|
|
December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2010
|
|
|
2009
|
|
|
2011
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(unaudited)
|
|
|
(unaudited)
|
|
|
|
|
|
|
|
|
(unaudited)
|
|
|
|
|
|
|
|
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(17,493,917
|
)
|
|
$
|
(4,612,873
|
)
|
|
$
|
(16,222,829
|
)
|
|
$
|
(9,047,280
|
)
|
|
$
|
(42,764,026
|
)
|
|
$
|
(8,168,476
|
)
|
|
$
|
(10,512,160
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating
activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
236,972
|
|
|
|
221,281
|
|
|
|
973,858
|
|
|
|
422,014
|
|
|
|
1,632,844
|
|
|
|
418,970
|
|
|
|
478,429
|
|
|
Non-cash interest expense
|
|
|
12,606,762
|
|
|
|
610,515
|
|
|
|
4,433,409
|
|
|
|
894,574
|
|
|
|
17,934,745
|
|
|
|
1,352,767
|
|
|
|
1,636,062
|
|
|
Stock-based compensation
|
|
|
470,275
|
|
|
|
150,114
|
|
|
|
525,944
|
|
|
|
207,593
|
|
|
|
1,203,812
|
|
|
|
279,920
|
|
|
|
—
|
|
|
Changes in fair value of warrant liability
|
|
|
(2,461
|
)
|
|
|
823,582
|
|
|
|
(2,592,979
|
)
|
|
|
515,108
|
|
|
|
(2,080,332
|
)
|
|
|
(2,092,643
|
)
|
|
|
—
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
244,504
|
|
|
|
221,576
|
|
|
|
(22,928
|
)
|
|
|
(213,681
|
)
|
|
|
7,895
|
|
|
|
223,334
|
|
|
|
75,662
|
|
|
Government award receivable
|
|
|
979,558
|
|
|
|
(1,516,323
|
)
|
|
|
(1,458,518
|
)
|
|
|
—
|
|
|
|
(478,960
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Prepaid expenses
|
|
|
122,285
|
|
|
|
133,900
|
|
|
|
59,890
|
|
|
|
(147,198
|
)
|
|
|
34,977
|
|
|
|
221,543
|
|
|
|
592,488
|
|
|
Accounts payable
|
|
|
(3,579,608
|
)
|
|
|
165,365
|
|
|
|
3,481,669
|
|
|
|
434,863
|
|
|
|
336,924
|
|
|
|
(569,955
|
)
|
|
|
393,734
|
|
|
Accrued liabilities
|
|
|
(376,594
|
)
|
|
|
543,615
|
|
|
|
1,786,911
|
|
|
|
741,103
|
|
|
|
2,151,420
|
|
|
|
112,124
|
|
|
|
200,011
|
|
|
Deferred rent
|
|
|
(21,995
|
)
|
|
|
(74,685
|
)
|
|
|
1,477
|
|
|
|
(137,561
|
)
|
|
|
(158,079
|
)
|
|
|
(13,997
|
)
|
|
|
129,761
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(6,814,219
|
)
|
|
|
(3,333,933
|
)
|
|
|
(9,034,096
|
)
|
|
|
(6,330,465
|
)
|
|
|
(22,178,780
|
)
|
|
|
(8,236,413
|
)
|
|
|
(7,006,013
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment
|
|
|
(1,062,044
|
)
|
|
|
(93,722
|
)
|
|
|
(422,079
|
)
|
|
|
(324,395
|
)
|
|
|
(1,808,518
|
)
|
|
|
(308,237
|
)
|
|
|
(2,787,313
|
)
|
|
Government Award
|
|
|
478,960
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
478,960
|
|
|
|
—
|
|
|
|
—
|
|
|
Restricted cash
|
|
|
(15,206,209
|
)
|
|
|
50,406
|
|
|
|
48,909
|
|
|
|
(53,610
|
)
|
|
|
(15,210,910
|
)
|
|
|
(53,352
|
)
|
|
|
(16,972
|
)
|
|
Cash transferred in biochemicals business to Myriant
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,577,925
|
|
|
|
2,577,925
|
|
|
|
(2,549,000
|
)
|
|
|
—
|
|
|
Acquisition of property and equipment
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(200,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities
|
|
|
(15,789,293
|
)
|
|
|
(43,316
|
)
|
|
|
(373,170
|
)
|
|
|
2,199,920
|
|
|
|
(13,962,543
|
)
|
|
|
(2,910,589
|
)
|
|
|
(3,004,285
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments of debt issuance costs
|
|
|
—
|
|
|
|
(25,000
|
)
|
|
|
(102,158
|
)
|
|
|
(56,750
|
)
|
|
|
(158,908
|
)
|
|
|
(12,600
|
)
|
|
|
(236,960
|
)
|
|
Warrant exercise proceeds
|
|
|
2,739
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,739
|
|
|
|
9,500,000
|
|
|
|
—
|
|
|
Warrant exercise issuance cost
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(45,000
|
)
|
|
|
—
|
|
|
Capital distribution
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(77,343
|
)
|
|
|
—
|
|
|
Issuance of Class A common stock
|
|
|
60,000,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
60,000,000
|
|
|
|
—
|
|
|
|
—
|
|
|
Payment of stock issuance costs
|
|
|
(2,907,953
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,907,953
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Repayments of (advances to) due from related parties
|
|
|
57,027
|
|
|
|
(364,097
|
)
|
|
|
1,028,749
|
|
|
|
(467,883
|
)
|
|
|
617,893
|
|
|
|
(269,538
|
)
|
|
|
(229,986
|
)
|
|
Issuance of senior convertible notes
|
|
|
—
|
|
|
|
5,000,000
|
|
|
|
9,700,000
|
|
|
|
4,000,000
|
|
|
|
13,700,000
|
|
|
|
1,750,000
|
|
|
|
3,750,000
|
|
|
Issuance (repayment) of note payable
|
|
|
—
|
|
|
|
(1,700,000
|
)
|
|
|
(1,700,000
|
)
|
|
|
1,700,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
57,151,813
|
|
|
|
2,910,903
|
|
|
|
8,926,591
|
|
|
|
5,175,367
|
|
|
|
71,253,771
|
|
|
|
10,845,519
|
|
|
|
3,283,054
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
|
34,548,301
|
|
|
|
(466,346
|
)
|
|
|
(480,675
|
)
|
|
|
1,044,822
|
|
|
|
35,112,448
|
|
|
|
(301,483
|
)
|
|
|
(6,727,244
|
)
|
|
Cash and cash equivalents, beginning of period
|
|
|
564,147
|
|
|
|
1,044,822
|
|
|
|
1,044,822
|
|
|
|
—
|
|
|
|
—
|
|
|
|
308,841
|
|
|
|
7,036,085
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
35,112,448
|
|
|
$
|
578,476
|
|
|
$
|
564,147
|
|
|
$
|
1,044,822
|
|
|
$
|
35,112,448
|
|
|
$
|
7,358
|
|
|
$
|
308,841
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flow information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid
|
|
$
|
—
|
|
|
$
|
17,871
|
|
|
$
|
32,309
|
|
|
$
|
34,000
|
|
|
$
|
66,309
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash transactions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued in connection with issuance of secured
convertible notes—related party
|
|
$
|
—
|
|
|
$
|
101,105
|
|
|
$
|
3,242,169
|
|
|
$
|
87,662
|
|
|
$
|
3,329,831
|
|
|
$
|
188,463
|
|
|
$
|
403,846
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contribution of Myriant Lake Providence
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
7,382,455
|
|
|
$
|
7,382,455
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contribution of biochemicals business
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
(2,386,660
|
)
|
|
$
|
(2,386,660
|
)
|
|
$
|
2,386,660
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of secured convertible notes-related party plus
accrued interest into Class B common stock and exercise of
common stock warrants
|
|
$
|
23,139,770
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
23,139,770
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of $.01 warrants
|
|
$
|
942,321
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
942,321
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted stock reserved for issuance
|
|
$
|
90,000
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
90,000
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-6
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
|
|
|
1.
|
Organization
and Business
|
The consolidated financial statements include the accounts of
Myriant Technologies LLC (Myriant) and its wholly-owned
subsidiaries, Myriant LP LLC (formerly Myriant Lake Providence,
LLC) and Myriant Lake Providence, Inc. (MLPI),
(collectively, the Company). Effective January 10, 2011,
Myriant converted to a Subchapter C corporation and changed its
name to Myriant Technologies, Inc. and subsequently changed its
name to Myriant Corporation.
Myriant, a development stage enterprise, was formed
July 16, 2009 by the holders of BioEnergy International,
LLC, or BEI or the Predecessor Company. BEI had been engaged in
developing and delivering technologies to produce
environmentally beneficial fuels and specialty chemicals from
traditional feedstocks and diverse biomass. BEI planned to
operate multi-product biorefineries, initially focusing on
developing or acquiring critical mass of conventional technology
ethanol plants. Upon completion of these facilities, BEI
intended to utilize its proprietary technologies to produce
high-value specialty chemicals and renewable fuels. BEI holds a
22.05% ownership interest in a variable interest entity,
BioEnergy Holding LLC, or Holding. Holding has a wholly-owned
subsidiary, Bionol Clearfield LLC, or Clearfield. Clearfield is
engaged in the operation of a 100 million gallon ethanol
facility in Clearfield, Pennsylvania. BEI had provided
development and construction services to Clearfield under a
management fee arrangement since 2007. Subsequent to the
restructuring described below, BEI entered into a subcontractor
agreement with Myriant in which Myriant would provide these
management services. This agreement was terminated in January
2011.
In order to differentiate BEI’s biochemical business from
its other activities, BEI underwent a restructuring in 2009.
BEI’s investors formed Myriant in order to separate
BEI’s biochemical business into a separate entity.
Effective July 16, 2009, in order to effect this
transaction, the holders of BEI membership units contributed
their ownership units in BEI to Myriant in exchange for
5,916,866 membership units in Myriant. In conjunction with this
exchange, the holders of BEI distributed the assets and
liabilities of the biochemicals business to Myriant but retained
its investment in Holding, its $10.0 million note payable
to Holding and its $1.5 million warrant liability. These
assets and liabilities were not transferred to Myriant.
Additionally, as part of this restructuring, the holders of
Bionol Lake Providence, or LP, contributed their ownership
interest in LP to Myriant in exchange for 1,000,001 Myriant
membership units. LP consisted principally of land improvements
on a site in Lake Providence, Louisiana, which will be used to
construct a biochemicals facility. The Predecessor Company
financial statements do not include LP. LP had no ongoing
operations or liabilities in the prior period. In connection
with this transaction, the entity’s name was changed to
Myriant Lake Providence LLC. Because these transactions occurred
between entities under common control, all assets and
liabilities were transferred at their historical cost. The
following table summarizes the assets and liabilities
transferred to Myriant in the transaction:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biochemicals
|
|
|
LP
|
|
|
Eliminations
|
|
|
Total
|
|
|
Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
2,549,000
|
|
|
$
|
28,925
|
|
|
$
|
—
|
|
|
$
|
2,577,925
|
|
|
Accounts receivable
|
|
|
7,895
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,895
|
|
|
Prepaid expenses
|
|
|
232,408
|
|
|
|
200
|
|
|
|
—
|
|
|
|
232,608
|
|
|
Restricted cash
|
|
|
369,349
|
|
|
|
—
|
|
|
|
—
|
|
|
|
369,349
|
|
|
Property and equipment
|
|
|
2,732,052
|
|
|
|
7,305,959
|
|
|
|
—
|
|
|
|
10,038,011
|
|
|
Debt issuance costs
|
|
|
222,122
|
|
|
|
—
|
|
|
|
—
|
|
|
|
222,122
|
|
|
Other long term receivables
|
|
|
648,305
|
|
|
|
50,291
|
|
|
|
(50,291
|
)
|
|
|
648,305
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
6,761,131
|
|
|
$
|
7,385,375
|
|
|
$
|
(50,291
|
)
|
|
$
|
14,096,215
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-7
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biochemicals
|
|
|
LP
|
|
|
Eliminations
|
|
|
Total
|
|
|
Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities
|
|
$
|
1,303,063
|
|
|
$
|
2,920
|
|
|
$
|
(50,291
|
)
|
|
$
|
1,255,692
|
|
|
Deferred rent
|
|
|
291,216
|
|
|
|
—
|
|
|
|
—
|
|
|
|
291,216
|
|
|
Secured convertible notes-related party
|
|
|
5,515,847
|
|
|
|
—
|
|
|
|
—
|
|
|
|
5,515,847
|
|
|
Warrant liability
|
|
|
2,037,665
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,037,665
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
9,147,791
|
|
|
|
2,920
|
|
|
|
(50,291
|
)
|
|
|
9,100,420
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Members’ equity (deficit)
|
|
|
(2,386,660
|
)
|
|
|
7,382,455
|
|
|
|
—
|
|
|
|
4,995,795
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and members’ equity (deficit)
|
|
$
|
6,761,131
|
|
|
$
|
7,385,375
|
|
|
$
|
(50,291
|
)
|
|
$
|
14,096,215
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In January 2010, the Company received a $50 million award
in federal cost share funding from the United States Department
of Energy (DOE) for the purpose of constructing our Louisiana
Plant for the Company’s biosuccinic acid. Under the terms
of the award, the Company is required to provide matching funds.
The award consists of two phases. The initial phase, referred to
as Budget Period 1, or BP1, consists of engineering and product
development efforts designed to prove the feasibility of the
Company’s technology. This portion of the award amounted to
$10.4 million. At the completion of BP1 on
December 31, 2010, the DOE determined the technology to be
viable and authorized the Company to enter into Budget Period 2,
or BP2 which consists of the construction and operation of the
facility. The award funds allowable during BP2 amount to
$39.6 million. Because the efforts associated with BP1
consist principally of determining technological feasibility and
piloting the Company technology, all costs associated with BP1
have been recorded in the statement of operations as biochemical
and research and development expense. The related DOE award
receipts associated with BP1 have been recorded as government
award revenue. Since the entry into BP2, costs associated with
the construction of the facility are capitalized and the related
award receipts will be recorded as a reduction to the basis of
the facility.
In connection with the receipt of this award, the Company formed
Myriant Lake Providence, Inc., or MLPI, a wholly-owned
subsidiary, to execute the tasks outlined in the award.
Since inception, the Company has devoted its efforts principally
to research and development, business planning, recruiting
management and technical staff, and fund raising. The Company
has not commenced its planned principal operations. As a result,
the Company is considered a development stage enterprise.
The Company is subject to a number of risks similar to other
companies in the development stage, including, but not limited
to, raising additional capital, lack of marketing and sales
history and dependence on key personnel and protection of its
proprietary technology. If it does not successfully develop and
commercialize its technologies, the Company will be unable to
achieve profitability.
The Company has a history of operating losses and it expects to
continue to incur operating losses over the next few years. The
future viability of the Company is largely dependent on its
ability to raise additional capital to finance its operations.
The Company raised $60 million from a strategic investor,
PTT Chemical International Private Limited, or CH Inter, in
January 2011, which resulted in net proceeds of
$57.1 million. Management believes that this infusion of
capital coupled with the remaining funds available under the DOE
award will allow the Company to operate for at least the next
twelve months.
F-8
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
|
|
|
2.
|
Summary
of Significant Accounting Policies
|
Basis
of Presentation
The consolidated financial statements include the accounts of
Myriant Corporation and its wholly-owned subsidiaries, Myriant
LP LLC and Myriant Lake Providence, Inc. All significant
intercompany balances and transactions have been eliminated. The
Company has included the accounts of its Predecessor Company,
BioEnergy International, LLC and Subsidiaries, for the period
from January 1, 2009 to July 15, 2009 and for the year
ended December 31, 2008. The financial statements have been
prepared in accordance with accounting standards set by the
Financial Accounting Standards Board (FASB). The FASB sets
generally accepted accounting principles (GAAP) to ensure
financial condition, results of operations, and cash flows are
consistently reported. References to GAAP issued by the FASB in
these footnotes are to the FASB Accounting Standards
Codification (FASB ASC).
Unaudited
Interim Financial Information
The interim consolidated financial statements and related
disclosures as of March 31, 2011, for the three months
ended March 31, 2011 and 2010, and for the cumulative
period from July 16, 2009 (date of inception) to
March 31, 2011 are unaudited and have been prepared in
accordance with the rules and regulations of the Securities and
Exchange Commission (SEC). The unaudited interim consolidated
financial statements have been prepared on the same basis as the
audited consolidated financial statements and, in the opinion of
management, reflect all adjustments of a normal recurring nature
considered necessary to present fairly the Company’s
financial position as of March 31, 2011, the results of its
operations and its cash flows for the three months ended
March 31, 2011 and 2010, and for the cumulative period from
July 16, 2009 (date of inception) to March 31, 2011.
The financial data and other information disclosed in these
notes to the consolidated financial statements as of
March 31, 2011, the three months ended March 31, 2011
and 2010, and for the period from July 16, 2009 (date of
inception) to March 31, 2011 are unaudited. The results of
operations for the three months ended March 31, 2011 are
not necessarily indicative of the results that may be expected
for the year ending December 31, 2011.
Unaudited
Pro Forma Information
The unaudited pro forma net loss per share as of March 31,
2011 reflects the automatic conversion of all outstanding shares
of convertible Class A and Class B common stock as of
that date into 14,259,858 shares of common stock, which
will occur upon the closing of the Company’s proposed
initial public offering. The convertible Class A and
Class B common stock converts to common stock on an assumed
one for one basis for all series of Class A and
Class B common stock. See Note 10 for conversion ratio
adjustments that may be applicable upon future events. Pro forma
net loss per share reflects the assumed conversion of all
outstanding shares of convertible Class A and Class B
common stock as noted above.
Variable
Interest Entity — Predecessor Company
A variable interest entity (VIE) is a corporation, partnership,
trust, or any legal structure used for business purposes where
equity investors do not provide sufficient financial resources
for the entity to support its activities. Generally accepted
accounting principles require a VIE to be consolidated by a
company if that company is the primary beneficiary of the VIE.
The primary beneficiary of the VIE is an entity that is subject
to a majority of the risk of loss from the VIE’s
activities, or entitled to receive the majority of the
entity’s residual returns, or both. As noted above, our
Predecessor Company holds an investment in Holding, a variable
interest entity. For the periods included in these financial
statements, management determined that they were not the primary
beneficiary of the VIE primarily because the Predecessor Company
had not provided any equity capital, loans or loan guarantees
and had limited economic exposure to losses. Since the
Predecessor Company was not the primary beneficiary of the VIE,
it was not required to consolidate Holding.
F-9
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Since the investment in Holding was not required to be
consolidated, the Predecessor Company followed the equity method
of accounting. Under the equity method of accounting, an
investment is stated at initial cost and adjusted for subsequent
additional investment and the Predecessor Company’s
proportionate share of earnings or losses and distributions
until the investment balance is reduced to zero. Since there
were no earnings or invested capital in the investment, there
was no investment amount recorded in the accompanying
consolidated financial statements of the Predecessor Company. As
of July 15, 2009, Holdings had accumulated losses totaling
approximately $18,133,000.
The Successor Company, Myriant, does not hold any equity
interest nor does it have economic exposure to absorb losses or
receive benefits, nor does it have the ability or power to
direct activities that impact the significant performance of the
VIE (Holding). Myriant had a subcontract agreement with the VIE
(see Note 4).
Revenue
Recognition
The Company’s revenues are derived from the following:
|
|
|
| |
•
|
A license agreement with a single customer, Purac, that requires
the customer to make royalty payments to utilize the
Company’s proprietary technology. The license agreement
relates to the Company’s technology associated with D(-)
lactic acid. Revenue is recognized quarterly based upon the
customer’s production of D(-) lactic acid. The contract
contains a minimum royalty provision of 200,000 Euros annually
($282,000 U.S. dollars at March 31, 2011 (unaudited)). To
the extent that the customer has not met the minimum requirement
by year end, the balance required to meet the minimum
requirement will be recorded in the fourth quarter when earned.
|
| |
| |
•
|
A management fee arrangement with a related party in which the
Company provides management support to the customer’s
ethanol facility. The management fee is invoiced quarterly in
advance. The quarterly fee is recognized ratably over the period
the services are performed.
|
| |
| |
•
|
A government award from the United States Department of Energy.
The award provides us with cost reimbursement for certain types
of expenditures associated with our research and development
activities associated with our biosuccinic acid product.
Revenues from the award are recognized in the period during
which the related costs are incurred, provided that the
conditions under which the award was provided have been met.
|
In all instances, revenue is recognized provided that persuasive
evidence of an arrangement exists, the fee is determinable,
collectability is reasonably assured and customer acceptance
terms have been satisfied.
Foreign
Currency Transactions
The Company’s functional currency is the U.S. dollar.
The Company has a licensing agreement with a customer, in which
payments received are denominated in Euro. All foreign currency
transaction gains and losses are recorded as a component of
other income (expense) in the statement of operations. Foreign
currency gains and losses were a gain of $11,638, loss of
$3,853, loss of $5,235 and gain of $6,403 for the three months
ended March 31, 2011 (unaudited) and March 31, 2010
(unaudited), the year ended December 31, 2010 and the
period from July 16, 2009 (inception) to March 31,
2011 (unaudited), respectively. Foreign currency gains and
losses were a loss of $21,721 and $0 for the Predecessor Company
for the period from January 1, 2009 through July 15,
2009 and the year ended December 31, 2008, respectively.
Research
and Development
Costs incurred in the research and development of the
Company’s products are expensed as incurred.
Project
Development Costs
Costs incurred in the development of construction projects prior
to debt financing and issuance of notice to proceed to begin
construction are expensed as incurred. These costs generally
include permitting, engineering, consulting, legal and other
professional fees.
F-10
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Use of
Estimates
The preparation of financial statements in conformity with
generally accepted accounting principles require management to
make estimates and assumptions. These estimates and assumptions
affect the amounts reported in the accompanying consolidated
financial statements. Significant estimates include valuation of
warrants and stock-based compensation. Actual results could
differ from those estimates.
Environmental
Liabilities
The Company’s operations are subject to environmental laws
and regulations adopted by various governmental authorities in
the jurisdictions in which it operates. These laws require the
Company to investigate and remediate the effects of the release
or disposal of materials at its locations. Accordingly, the
Company has adopted policies, practices and procedures in the
areas of pollution control, occupational health and the
production, handling, storage and use of hazardous materials to
prevent material environmental or other damage, and to limit the
financial liability which could result from such events.
Environmental liabilities are recorded when the Company’s
liability is probable and the costs can be reasonably estimated.
No environmental liabilities have been recorded as of
March 31, 2011 (unaudited), December 31, 2010 and 2009.
Net
Loss Per Unit/Share
Basic net loss per unit/share is computed by dividing the net
loss attributable to membership units/common shares for the
period by the weighted average number of membership units/shares
outstanding during the period. Diluted net loss per unit/share
is computed by dividing net loss attributable to membership
units/common shares for the period by the weighted-average
number of dilutive membership units/shares outstanding during
the period.
Dilutive membership units/shares outstanding are calculated by
adding to the weighted average membership units/shares
outstanding any potential (unissued) membership units/shares
based on the treasury stock method. At December 31, 2010
and 2009 the Company had only one class of membership units. At
March 31, 2011, the Company had more than one class of
common stock.
Diluted net loss per unit/share is the same as basic net loss
per unit/share for all periods presented because any potential
dilutive units/shares were anti-dilutive. Such potentially
dilutive membership units/shares are excluded from the
computation of diluted net loss per unit/share when the effect
would be to reduce net loss per unit/share. Therefore, in a
period when a loss is reported, the calculation of basic and
dilutive loss per unit/share results in the same value. In a
period that net income is achieved, the Company will report
earnings per share using the two class method.
F-11
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
The following table summarizes the Company’s calculation of
historical net loss per unit/share attributable to Myriant
Corporation members/stockholders:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BioEnergy International LLC
|
|
|
|
|
Myriant Corporation
|
|
|
(Predecessor Company)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Period
|
|
|
For the Period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
from July 16, 2009
|
|
|
from July 16, 2009
|
|
|
Period from
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
|
|
(Inception) to
|
|
|
(Inception) to
|
|
|
January 1 to
|
|
|
|
|
|
|
|
March 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
March 31,
|
|
|
July 15,
|
|
|
December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2010
|
|
|
2009
|
|
|
2011
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Historical net loss per unit/share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(17,493,917
|
)
|
|
$
|
(4,612,873
|
)
|
|
$
|
(16,222,829
|
)
|
|
$
|
(9,047,280
|
)
|
|
$
|
(42,764,026
|
)
|
|
$
|
(8,168,476
|
)
|
|
$
|
(10,512,160
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dividend on Class A common stock
|
|
|
(1,025,753
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,025,753
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders
|
|
$
|
(18,519,670
|
)
|
|
$
|
(4,612,873
|
)
|
|
$
|
(16,222,829
|
)
|
|
$
|
(9,047,280
|
)
|
|
$
|
(43,789,779
|
)
|
|
$
|
(8,168,476
|
)
|
|
$
|
(10,512,160
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per unit/share attributable to common
stockholders-basic and diluted:
|
|
$
|
(2.29
|
)
|
|
$
|
(0.66
|
)
|
|
$
|
(2.31
|
)
|
|
$
|
(1.30
|
)
|
|
$
|
(6.12
|
)
|
|
$
|
(1.55
|
)
|
|
$
|
(2.14
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average units/common shares used in computing net loss
per unit/share-basic and diluted
|
|
|
8,092,943
|
|
|
|
6,982,215
|
|
|
|
7,009,251
|
|
|
|
6,953,079
|
|
|
|
7,150,339
|
|
|
|
5,285,807
|
|
|
|
4,918,668
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pro forma net loss per share (unaudited):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Numerator:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss used in computing pro forma net loss per share of
common stock-basic and diluted
|
|
$
|
(18,519,670
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominator:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted weighted average common shares
|
|
|
8,092,943
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pro forma adjustment to reflect weighted average of assumed
conversion of convertible Class A and Class B common
stock
|
|
|
14,259,858
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares used in computing pro forma basic and
diluted net loss per common share:
|
|
|
22,352,801
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pro forma net loss per common share:
|
|
$
|
(0.83
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following potentially dilutive securities were excluded from
the calculation of diluted net loss per unit/share during each
period as the effect was anti-dilutive:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BioEnergy International LLC
|
|
|
|
|
Myriant Corporation
|
|
|
(Predecessor Company)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Period
|
|
|
For the Period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
from July 16, 2009
|
|
|
from July 16, 2009
|
|
|
Period from
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
|
|
(Inception) to
|
|
|
(Inception) to
|
|
|
January 1 to
|
|
|
|
|
|
|
|
March 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
March 31,
|
|
|
July 15,
|
|
|
December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2010
|
|
|
2009
|
|
|
2011
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible Class A common stock upon conversion to common
stock (as converted basis)
|
|
|
11,214,953
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
11,214,953
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible Class B common stock upon conversion to common
stock (as converted basis)
|
|
|
3,044,905
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,044,905
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants to purchase common shares
|
|
|
805,191
|
|
|
|
1,283,399
|
|
|
|
2,196,499
|
|
|
|
1,248,655
|
|
|
|
805,191
|
|
|
|
5,273,077
|
|
|
|
6,088,462
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested restricted shares
|
|
|
186,491
|
|
|
|
222,597
|
|
|
|
156,072
|
|
|
|
151,642
|
|
|
|
186,491
|
|
|
|
208,040
|
|
|
|
261,572
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding options to purchase common stock
|
|
|
543,293
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
543,293
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
15,794,833
|
|
|
|
1,505,996
|
|
|
|
2,352,571
|
|
|
|
1,400,297
|
|
|
|
15,794,833
|
|
|
|
5,481,117
|
|
|
|
6,350,034
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income
Taxes
As of December 31, 2010, the Company consisted of limited
liability companies (Myriant and MLP) and a C corporation
(MLPI). Accordingly, Myriant and MLP’s items of income and
expense flowed through to the members’ individual tax
returns. The income taxes on the consolidated statements of
operations will consist of state and federal income tax
provisions related to MLPI. Effective January 10, 2011,
Myriant converted to a
F-12
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
C corporation and is included in the income tax provision
during the three month period ended March 31, 2011. Income
taxes are provided for the tax effects of transactions reported
in the financial statements and consist of taxes currently due
plus deferred taxes related to temporary differences arising
from assets and liabilities whose bases are different for
financial reporting and income tax purposes.
Deferred taxes are provided on the asset and liability method
whereby deferred tax assets are recognized for deductible
temporary differences and net operating loss and tax credit
carryforwards, and deferred tax liabilities are recognized for
taxable temporary differences. Temporary differences are the
differences between reported amounts of assets and liabilities
and their tax bases. Deferred tax assets and liabilities are
adjusted for the effects of changes in tax laws and rates on the
date of enactment.
The Company follows guidance for income taxes, which prescribes
a recognition threshold and measurement standard for the
financial statement recognition and measurement of an income tax
position taken or expected to be taken in a tax return. For all
periods presented there were no tax positions that met the
more-likely-than-not recognition threshold at the effective date.
The Company accounts for interest and penalties related to
uncertain tax positions, if any, as part of tax expense. MLPI
was incorporated in 2010 and as such is not subject to state and
federal income tax examinations by tax authorities for years
before 2010.
Cash
and Cash Equivalents
The Company considers all highly liquid investments with an
original maturity of three months or less at the date of
purchase to be cash equivalents. Cash and cash equivalents
consist of accounts held in bank checking and money market
savings accounts.
Concentrations
of Credit Risk and Significant Customers
The Company’s principal credit risk relates primarily to
its cash and cash equivalents and accounts receivable. Cash and
cash equivalents consist of overnight investments and money
market accounts maintained at various accredited financial
institutions which, at times, may exceed federally insured
limits. The Company has not experienced any losses in such
accounts and does not believe that it is subject to any unusual
risk beyond the normal risk associated with commercial banking
relationships. At March 31, 2011 (unaudited), one customer
accounted for 100% of revenue and accounts and grants
receivable. At December 31, 2010 and 2009, one and two
customers, respectively, accounted for 100% of revenue and
accounts and government award receivable. To minimize credit
risk, the Company performs ongoing credit evaluations of
customer financial conditions.
Restricted
Cash
The Company maintains a restricted cash account which contains
deposits associated with leases associated with its corporate
headquarters and laboratory facility. The restricted cash
account consists of certificates of deposit and totaled
$374,616, $374,050 and $422,959 as of March 31, 2011
(unaudited), December 31, 2010 and 2009, respectively.
At March 31, 2011 (unaudited), restricted cash also
includes $15,205,643 of money market savings accounts that are
required for a contingency fund under the government award
agreement with the Department of Energy. The contingency funds
will be restricted throughout the construction of the facility
which is expected to be completed in 2012.
F-13
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Accounts
and Government Award Receivables
The Company records receivables under a license agreement with a
single customer and the award agreement with the Department of
Energy. Unbilled accounts receivable, included in government
award receivable, totaled $342,828 as of March 31, 2011
(unaudited), relating to work that has been performed or
services that have been received, though invoicing has not yet
occurred under the DOE reimbursement contract. All of the
unbilled receivables are expected to be billed and collected
within the next 12 months. There were no unbilled
receivables as of December 31, 2010 and 2009. Accounts
receivable are stated at invoice amounts less an allowance for
doubtful accounts. As of March 31, 2011 (unaudited),
December 31, 2010 and 2009, no allowance for doubtful
accounts has been recorded, based on the expected full
collection of the accounts and government award receivable.
Fair
Value Measurements
The Company follows current accounting standards for fair value
measurements which define fair value as “the price that
would be received from selling an asset or paid to transfer a
liability in an orderly transaction between market participants
at the measurement date.” When determining the fair value
measurements for assets and liabilities required or permitted to
be recorded at fair value, the Company considers the principal
or most advantageous market in which it would transact, and it
considers assumptions that market participants would use when
pricing the asset or liability.
An entity is required to maximize the use of observable inputs
and minimize the use of unobservable inputs when measuring fair
value. A fair value hierarchy has been established based on the
level of independent, objective evidence surrounding the inputs
used to measure fair value. A financial instrument’s
categorization within the fair value hierarchy is based upon the
lowest level of input that is significant to the fair value
measurement. The inputs are prioritized into three levels that
may be used to measure fair value:
Level 1 — applies to assets or liabilities
for which there are quoted prices in active markets for
identical assets or liabilities.
Level 2 — applies to assets or liabilities
for which there are inputs other than quoted prices that are
observable for the asset or liability such as quoted prices for
similar assets or liabilities in active markets; quoted prices
for identical assets or liabilities in markets with insufficient
volume or infrequent transactions; or model derived valuations
in which significant inputs are observable or can be derived
principally from, or corroborated by, observable market data.
Level 3 — applies to assets or liabilities
for which there are unobservable inputs to the valuation
methodology that are significant to the measurement of the fair
value of the assets and liabilities.
Debt
Issuance Costs
Debt issuance costs are costs incurred to obtain debt financing
and are included in the accompanying consolidated balance sheet
and are amortized as interest expense over the term of the
related financing using the effective interest rate method.
Property
and Equipment
Property and equipment are recorded at cost and depreciated over
their estimated useful lives using the straight line method.
Leasehold improvements are amortized over the shorter of the
lease term or the estimated useful life of the related asset.
Upon retirement or sale, the cost of the assets disposed of and
the related accumulated depreciation are removed from the
accounts and any resulting gain or loss is credited or charged
to income. Repairs and maintenance costs are expensed as
incurred.
F-14
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Construction
in Progress
Construction in progress includes costs incurred in the future
development of the Company’s manufacturing facility. The
Company capitalizes hard costs such as land and land
improvements when they are incurred. Soft costs such as salaries
and benefits, professional fees, and permitting fees are
capitalized when management concludes that the construction of a
project is probable. The Company deems a project to be probable
upon acquisition of a project site, receipt of all site specific
permits required to commence construction, the receipt of all
contracts required to obtain funding, the receipt of a
construction contract, and the receipt of debt or equity
financing that supports the project scope. Upon completion of
the facility, the Company will allocate value to land, building
and equipment based on the accumulation of these costs. Based on
this allocation, the Company will begin to depreciate the assets
over their useful lives which will be determined at that time.
Capitalized soft costs, which consisted principally of internal
labor costs, amounted to $504,279 for the three months ended
March 31, 2011 (unaudited), and the period from inception
to March 31, 2011 (unaudited). The Company did not
capitalize soft costs during the year ended December 31,
2010 and the period from July 16, 2009 (inception) to
December 31, 2009 and the Predecessor Company period from
January 1, 2009 to July 15, 2009 and December 31,
2008.
Government
Awards
In January 2010, the Company secured a government award from the
United States Department of Energy (DOE) to construct our
Louisiana Plant for its biosuccinic acid. This award was
separated into two phases. The first, Budget Period 1, or BP1,
involved engineering and laboratory work to establish the
technological feasibility of the Company’s product and
process. Costs incurred in BP1 are charged to expense as
incurred and reimbursements from the DOE are recorded as
revenue. The second phase, Budget Period 2, or BP2, involves the
construction of the facility upon attainment of technological
feasibility. With the approval of BP2 in January 2011, all costs
associated with the project are being capitalized and DOE
reimbursements are being recorded as a reduction to the cost of
the facility.
Interest
Capitalization
Interest is capitalized on construction in progress based upon
rates applicable to borrowings outstanding during the period. If
the Company’s facility is being financed with tax exempt
bonds, interest income associated with these borrowings will be
offset against the expense capitalized. Interest capitalization
ceases when the construction is substantially complete and the
asset is available for use or if construction is halted. As of
March 31, 2011 (unaudited), December 31, 2010 and
2009, the Company has capitalized interest expense totaling
$1,193,675. No interest was capitalized in the three months
ended March 31, 2011 (unaudited) or the year ended
December 31, 2010 as there was no interest expense or the
project was not active during that period.
Warrant
Liability
The Company classifies its warrants as liabilities because the
strike prices of the warrants are subject to adjustment based
upon a new issuance of member units or shares. The liability is
carried at fair value, and any adjustment to fair value at each
balance sheet date is recorded as a component of other income
(expense).
Impairment
of Long-Lived Assets
The Company assesses the impairment of long-lived assets when
events or changes in circumstances suggest that the fair value
of the assets could be less than their net book value. In those
circumstances, the Company assesses long-lived assets for
impairment by determining their fair value based upon the
forecasted, undiscounted cash flows the assets are expected to
generate plus the net proceeds expected from the sale of the
asset. An impairment loss would be recognized when the fair
value is less than the carrying value of the asset group.
F-15
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
There were no indications of impairment of long-lived assets for
the three months ended March 31, 2011 (unaudited) and the
years ended December 31, 2010 and 2009.
Asset
Retirement Obligations
The Company considers the effects of potential asset retirement
obligations upon the purchase of long lived assets. If the
Company determines that there is a potential asset retirement
obligation and the fair value of the liability can be reasonably
estimated, the Company will recognize a liability for the asset
retirement obligation. If there is uncertainty with respect to
the timing
and/or
method of settlement of the retirement obligation, the
recognition of the liability will not be deferred. Management
has determined that no asset retirement obligations exist as of
March 31, 2011 (unaudited), December 31, 2010 and 2009.
Deferred
Rent
The Company is a party to certain lease agreements that provide
for increasing rent payments. The Company recognizes the total
rent payments during the lease term on the straight line basis.
The difference between the straight line rent expense and
amounts paid are recorded as deferred rent.
Stock-Based
Compensation
Stock-based compensation for awards to employees and
non-employees is measured at the grant date based on the fair
value of the awards and is recognized as expense over the
required service period of the award. During January 2011, the
Company established the 2011 Omnibus Incentive Plan, or the
Plan, concurrent with Myriant converting to a Subchapter C
corporation. The Company estimates the fair value of stock
options issued to employees and non-employees using the
Black-Scholes option pricing model. Restricted common stock
grants issued are accounted for at the estimated fair value of
the underlying common stock.
Advertising
The Company expenses advertising costs in the period in which
they are incurred. Advertising expense was $28,467, $14,627,
$98,762, $18,372 and $145,601 for the three months ended
March 31, 2011 and 2010 (unaudited), the year ended
December 31, 2010 and the period from July 16, 2009
(inception) to December 31, 2009 and March 31, 2011
(unaudited), respectively. There was no advertising expense for
the Predecessor Company for the period from January 1, 2009
to July 15, 2009 and the year ended December 31, 2008.
Recent
Accounting Pronouncements
In January 2010, the FASB issued Accounting Standards Update
(ASU)
No. 2010-06,
Fair Value Measurements and Disclosures—Improving
Disclosures about Fair Value Measurements, that requires
entities to make new disclosures about recurring or
non-recurring fair value measurements and provides clarification
of existing disclosure requirements. This amendment requires
disclosures about transfers into and out of Levels 1 and 2
and separate disclosures about purchases, sales, issuances, and
settlements relating to Level 3 measurements. It also
clarifies existing fair value disclosures about the level of
disaggregation and about inputs and valuation techniques used to
measure fair value. This amendment is effective for periods
beginning after December 15, 2009, except for the
requirement to provide the Level 3 activity of purchase,
sales, issuances, and settlements, which will be effective for
fiscal years beginning after December 15, 2010. The
adoption of this standard did not have a material impact on the
consolidated financial statements.
F-16
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
The carrying amounts of the Company’s financial assets and
liabilities approximate fair value due to the short term nature
of the financial statement accounts. Management believes that
the Company’s debt obligations bear interest rates which
approximate prevailing market rates for instruments with similar
characteristics and, accordingly, the carrying value for these
instruments approximates fair value.
As of March 31, 2011 (unaudited), December 31, 2010
and 2009, there were no transactions measured at fair value on a
non-recurring basis. Assets and liabilities measured at fair
value on a recurring basis as of March 31, 2011,
December 31, 2010 and 2009 were as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurement Using
|
|
|
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
|
Total
|
|
|
2011 (Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant liability
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
(2,199,585
|
)
|
|
$
|
(2,199,585
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurement Using
|
|
|
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
|
Total
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant liability
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
(3,289,625
|
)
|
|
$
|
(3,289,625
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurement Using
|
|
|
|
|
Level 1
|
|
|
Level 2
|
|
|
Level 3
|
|
|
Total
|
|
|
2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant liability
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
(2,640,435
|
)
|
|
$
|
(2,640,435
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company’s warrants do not trade in an active market,
and as such, the Company estimates the fair value of these
warrants using the Black-Scholes option pricing method. The
following table summarizes assumptions used in the analysis
| |
|
|
|
|
|
|
|
|
|
March 31, 2011
|
|
2010
|
|
2009
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
Expected volatility
|
|
29%
|
|
38%
|
|
49–51%
|
|
Expected life
|
|
1-2 years
|
|
1-2 years
|
|
5-6 years
|
|
Dividend yield
|
|
0%
|
|
0%
|
|
0%
|
|
Risk free interest rate
|
|
0.8%
|
|
0.66%
|
|
2.69-3.04%
|
|
Fair value per unit
|
|
$0.01-$3.44
|
|
$0.01-$3.44
|
|
$1.45-$2.29
|
The risk free interest rate assumption is based upon observed
interest rates from the U.S. treasury yield curve
appropriate for the expected term of the warrants. The expected
volatility is based upon the historical volatility of peer
companies. The Company has not paid any dividends on common
stock and does not anticipate paying dividends for the
foreseeable future. The computation of the expected warrant term
is based upon their contractual term and estimates of when an
automatic exercise event will occur.
F-17
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
The following table provides a summary of the warrant liability
at March, 31, 2011, December 31, 2010 and 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2011
|
|
|
2010
|
|
|
2009
|
|
|
|
|
Units
|
|
|
Liabilities
|
|
|
Units
|
|
|
Liabilities
|
|
|
Units
|
|
|
Liabilities
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$10.00 warrants
|
|
|
89,656
|
|
|
$
|
80
|
|
|
|
89,656
|
|
|
$
|
896
|
|
|
|
766,347
|
|
|
$
|
1,754,935
|
|
|
$13.00 warrants
|
|
|
59,231
|
|
|
|
6
|
|
|
|
59,231
|
|
|
|
178
|
|
|
|
482,308
|
|
|
|
885,500
|
|
|
$6.00 warrants
|
|
|
17,134
|
|
|
|
754
|
|
|
|
1,134,512
|
|
|
|
147,487
|
|
|
|
—
|
|
|
|
—
|
|
|
$0.01 warrants
|
|
|
639,170
|
|
|
|
2,198,745
|
|
|
|
913,100
|
|
|
|
3,141,064
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
805,191
|
|
|
$
|
2,199,585
|
|
|
|
2,196,499
|
|
|
$
|
3,289,625
|
|
|
|
1,248,655
|
|
|
$
|
2,640,435
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table provides a roll forward of the fair value of
the warrant liability:
| |
|
|
|
|
|
|
|
|
|
|
|
Units
|
|
|
Value
|
|
|
|
|
Warrant liability at date of adoption (July 16, 2009)
|
|
|
—
|
|
|
$
|
—
|
|
|
Issuance in connection with secured convertible notes- related
party
|
|
|
59,231
|
|
|
|
87,662
|
|
|
Exercises
|
|
|
—
|
|
|
|
—
|
|
|
Transfer of warrant liability from BEI
|
|
|
1,189,424
|
|
|
|
2,037,665
|
|
|
Changes in fair value of warrant liability
|
|
|
—
|
|
|
|
515,108
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant liability at December 31, 2009
|
|
|
1,248,655
|
|
|
|
2,640,435
|
|
|
Issuance in connection with secured convertible notes-related
party
|
|
|
947,844
|
|
|
|
3,242,169
|
|
|
Exercises
|
|
|
—
|
|
|
|
—
|
|
|
Changes in fair value of warrant liability
|
|
|
—
|
|
|
|
(2,592,979
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Warrant liability at December 31, 2010
|
|
|
2,196,499
|
|
|
|
3,289,625
|
|
|
Exercises and conversions
|
|
|
(1,391,308
|
)
|
|
|
(1,087,579
|
)
|
|
Changes in fair value of warrant liability
|
|
|
—
|
|
|
|
(2,461
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Warrant liability at March 31, 2011 (unaudited)
|
|
|
805,191
|
|
|
$
|
2,199,585
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4
|
Related
Party Transactions
|
Secured
Convertible Notes Payable
Prior to the transfer to Myriant, BEI had entered into an
agreement with an investor to issue up to $5,500,000 in Senior
Convertible Notes. These notes bore interest at 15% and interest
was to be deferred until October 10, 2009. At such time,
the outstanding balance of principal and interest was to be
repaid on a monthly basis over 48 months with the final
payment due on October 10, 2013. In connection with this
agreement, BEI had issued 423,077 warrants with an exercise
price of $13 per unit. These warrants were ascribed a relative
fair value of $593,222 using the Black-Scholes option pricing
model. In computing the value of the warrants, BEI assumed a
risk free interest rate of 2.5%, a life of 6.7 years,
expected volatility of 46% and no future dividends. The relative
fair value of the warrants was recorded as interest expense over
the term of the note under the interest method using an
effective rate of 26.5%. Upon adoption of
ASC 815-40
“Contracts in an Entity’s Own Stock,” the
warrants have been recorded as a liability effective
January 1, 2009. These notes were transferred to Myriant in
conjunction with the restructuring described in Note 1.
On October 30, 2009, the Company entered into an amended
note agreement with this investor. Under the terms of the new
agreement, the maximum borrowing capacity was increased from
$5,500,000 to $9,500,000 and interest and principal payments on
outstanding advances of $5,500,000 were deferred until
October 10,
F-18
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
2010. At such time, the outstanding balance of principal and
interest on any new or existing advances was to be repaid on a
monthly basis over 36 months with the first payment
commencing two years from the original closing date with final
payment due November 30, 2014. This amendment met the
definition of a debt extinguishment; therefore the Company
recorded a $160,000 write off of the debt issuance costs related
to the original loan.
On February 8, 2010, the Company entered into a third
amended note agreement with this investor along with additional
investors. Under the terms of the new agreement, the maximum
borrowing capacity was increased from $9,500,000 to $14,500,000.
In conjunction with this financing, the Company adjusted the
strike price of 676,691 $10 warrants and 423,077 $13 warrants to
$6. Additionally, 34,744 new $6 warrants were issued to an
investor in exchange for its participation in the current
financing. The Company used the Black-Scholes option pricing
model to determine the value of the warrants that were
re-priced. In computing the value of the warrants, the Company
assumed a risk free interest rate of 2.3%, a life of
5.5 years, expected volatility of 51% and no future
dividends. The change in value amounted to $859,548, which was
recorded as a change in warrant liability in the statement of
operations with a corresponding increase to the warrant
liability.
The 34,744 warrants issued in conjunction with this financing
were also valued using the Black-Scholes option pricing model.
In computing the value of the warrants, the Company assumed a
risk free interest rate of 2.3%, a life of 5.5 years,
expected volatility of 51% and no future dividends. The residual
fair value of the warrants was $101,105, and was recorded as
interest expense over the term of the note under the interest
method using an effective interest rate of 31.7%.
On July 29, 2010, the fourth amended note agreement
increased the maximum borrowing capacity from $14,500,000 to
$17,500,000.
On November 16, 2010, the Company and the note holders
entered into a fifth amended note agreement to defer interest
and principal payments on the existing advances of $17,500,000
until October 10, 2011. At such time, the outstanding
balance of principal and interest on any existing advances was
to be repaid on a monthly basis over 36 months with final
payment due October 10, 2014. This amendment met the
definition of a debt extinguishment; therefore the Company
recorded an immaterial write off of debt issuance costs. There
were no additional available borrowings on this note agreement
at December 31, 2010.
On November 5, 2010, the Company entered into a bridge note
agreement with certain investors to issue up to $1,700,000 in
Secured Convertible Notes. These notes bore interest at 15% and
payments were to be deferred until November 5, 2012. At
such time, the outstanding balance of principal and interest was
to be repaid on a monthly basis over 36 months with the
final payment due in November 2015. In connection with this
agreement, the Company issued 913,100 warrants with an exercise
price of $.01 per unit. These warrants were ascribed a value of
$3,141,064 using the Black-Scholes option pricing model. In
computing the value of the warrants, the Company assumed a risk
free interest rate of 0.38%, a life of 1.6 years, expected
volatility of 38% and no future dividends. The residual fair
value ascribed was greater than the proceeds received from the
notes. Accordingly, $1,700,000 of the residual fair value was
recorded as interest expense over the term of the note under the
interest method using an effective interest rate of 43.2%. The
remaining value of the warrants of $1,441,064 was recorded as
interest expense with a corresponding increase to the warrant
liability. There were no additional available borrowings on this
note agreement at December 31, 2010.
The $17,500,000 note payable agreements and the $1,700,000
bridge note agreement were convertible to member units at an
applicable conversion rate equal to the lowest price per
Conversion Unit for any Conversion Units issued by the Company
to a third party between the closing date and the date of
conversion and was also adjustable for anti-dilution effects. If
a security is convertible from inception but contains conversion
terms that change upon the occurrence of a future event, any
contingent beneficial conversion feature should be measured
using the commitment date unit price and recognized in earnings
when the
F-19
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
contingency is resolved. The applicable conversion rate had a
contingent conversion feature that would reduce the conversion
price if the fair value of the underlying unit declined after
the commitment date below a specified price. When the reduction
in price occurs, a beneficial conversion is recognized. At
December 31, 2010 and 2009, the note agreements were not
convertible since there were no equity issuances that occurred
subsequent to the debt financing that would reset the conversion
price. Therefore, no beneficial conversion was recorded at
December 31, 2010 and 2009. These notes contained a
mandatory conversion feature in the event that the Company
closes an equity transaction in which the price per unit equals
or exceeds $5.70 per unit and the net proceeds to the Company
equals or exceeds $15,000,000.
Effective January 13, 2011, the Company completed a
$60 million equity financing with a strategic investor, CH
Inter, in exchange for 11,214,953 shares of Class A
common stock, which financing resulted in net proceeds of
$57.1 million. This transaction triggered the existing note
payable and the bridge note agreements to be convertible and set
the conversion rate to $5.35 per share. As a result, the Company
recorded a beneficial conversion as of the date of this event
totaling $10,445,199 which was recorded as interest expense and
additional paid in capital. As a condition of the CH Inter
closing, existing investors converted their note payable and
bridge note agreements into Class B common stock and
exercised 1,117,378 of their $6 warrants to the extent
outstanding in exchange for common stock. Any debt remaining
after the warrants were exercised was converted to Class B
common stock at $5.35 per share. As a result of this
transaction, all debt that existed at December 31, 2010 was
converted to equity. At the date of conversion, $22,994,510 of
debt was converted into equity, of which $3,794,510 related to
accrued interest.
F-20
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Interest
expense
The Company and its Predecessor Company have had significant
borrowings from related parties. The following table summarizes
interest expense for all periods presented:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Myriant Corporation
|
|
|
Predecessor Company
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
July 16,
|
|
|
July 16,
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year
|
|
|
2009
|
|
|
2009
|
|
|
January 1,
|
|
|
Fiscal Year
|
|
|
|
|
Three Months Ended
|
|
|
Ended
|
|
|
(Inception)
|
|
|
(Inception)
|
|
|
2009
|
|
|
Ended
|
|
|
|
|
March 31,
|
|
|
December 31,
|
|
|
to December 31,
|
|
|
to March 31,
|
|
|
to July 15,
|
|
|
December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2010
|
|
|
2009
|
|
|
2011
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
Secured convertible notes payable:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest
|
|
$
|
10,540,525
|
|
|
$
|
508,301
|
|
|
$
|
4,066,192
|
|
|
$
|
542,806
|
|
|
$
|
15,149,523
|
|
|
$
|
473,969
|
|
|
$
|
103,045
|
|
|
Non-cash interest expense
|
|
|
2,052,487
|
|
|
|
26,698
|
|
|
|
198,168
|
|
|
|
66,595
|
|
|
|
2,317,250
|
|
|
|
53,723
|
|
|
|
22,460
|
|
|
Debt issuance cost amortization
|
|
|
13,750
|
|
|
|
5,914
|
|
|
|
99,447
|
|
|
|
183,552
|
|
|
|
296,749
|
|
|
|
19,746
|
|
|
|
7,692
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total secured convertible note interest
|
|
|
12,606,762
|
|
|
|
540,913
|
|
|
|
4,363,807
|
|
|
|
792,953
|
|
|
|
17,763,522
|
|
|
|
547,438
|
|
|
|
133,197
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note payable(1):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
322,132
|
|
|
|
601,145
|
|
|
Non-cash interest expense
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
483,197
|
|
|
|
901,720
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total note payable interest
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
805,329
|
|
|
|
1,502,865
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total related party interest
|
|
|
12,606,762
|
|
|
|
540,913
|
|
|
|
4,363,807
|
|
|
|
792,953
|
|
|
|
17,763,522
|
|
|
|
1,352,767
|
|
|
|
1,636,062
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Third party interest:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest
|
|
|
—
|
|
|
|
17,871
|
|
|
|
37,069
|
|
|
|
20,510
|
|
|
|
57,579
|
|
|
|
—
|
|
|
|
—
|
|
|
Non-cash interest expense
|
|
|
—
|
|
|
|
36,444
|
|
|
|
36,444
|
|
|
|
51,218
|
|
|
|
87,662
|
|
|
|
—
|
|
|
|
—
|
|
|
Debt issuance cost amortization
|
|
|
—
|
|
|
|
33,158
|
|
|
|
33,158
|
|
|
|
51,123
|
|
|
|
84,281
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Third party interest on note payable
|
|
|
—
|
|
|
|
87,473
|
|
|
|
106,671
|
|
|
|
122,851
|
|
|
|
229,522
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total interest expense
|
|
$
|
12,606,762
|
|
|
$
|
628,386
|
|
|
$
|
4,470,478
|
|
|
$
|
915,804
|
|
|
$
|
17,993,044
|
|
|
$
|
1,352,767
|
|
|
$
|
1,636,062
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
The note payable above was a $10 million note between the
Predecessor Company and related party investors. This note was
not transferred to the Successor Company at the time of the
restructuring. |
Management
Services Agreement
During February 2008, the Predecessor Company entered into a
$4,000,000 Administrative Services and Construction Management
Agreement and an Operations and Maintenance Agreement, or
Services Agreements, with Clearfield, a wholly-owned subsidiary
of Holding. Under these agreements, the Predecessor Company was
required to provide certain services including general services
(day-to-day
management), provide personnel for general services, bookkeeping
and accounting services, preparation and distribution of
financial reports, ensure compliance with financing documents
and project documents, maintain insurance, obtain any permits
required, marketing services and construction related services.
The Predecessor Company recognized related party revenues
totaling $267,318 and $3,387,926 for the period from
January 1, 2009 to July 15, 2009 and the year ended
December 31, 2008, respectively.
The Services Agreements referred to above were subcontracted to
the Successor Company in July 2009, under which the Predecessor
Company would pay the Successor Company up to $3,900,000
annually to perform all of the services described above. This
fee represented fair market value for these services. This
F-21
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
subcontract agreement was concurrent with the transfer of the
biochemicals business to Myriant and the Predecessor Company
continued to retain its ownership in Holding. The Successor
Company recognized revenues of $2,519, $600,166, $3,557,575 and
$3,560,094 under this arrangement for the three months ended
March 31, 2011 and 2010 (unaudited), the year ended
December 31, 2010 and for the period from inception to
March 31, 2011 (unaudited). No revenues were recognized in
2009. There were no transactions between the Successor Company
and Predecessor Company during the periods presented requiring
elimination.
In January 2011, this agreement was terminated. Amounts due from
related parties at March 31, 2011 (unaudited),
December 31, 2010 and 2009 under this agreement were
$30,412, $87,439 and $1,116,188, respectively.
Consulting
Agreements
The Company maintains a consulting arrangement with an entity
owned by a stockholder and former director of the Company under
which this entity provides facility and real estate transaction
support to the Company. Consulting expense related to this
agreement amounted to $46,327, $33,328, $148,609, $84,326 and
$279,262 for the three months ended March 31, 2011 and 2010
(unaudited), the year ended December 31, 2010 and the
periods from July 16, 2009 to December 31, 2009 and
March 31, 2011 (unaudited), respectively. The Predecessor
Company recognized expense of $115,432 and $179,033 for the
period from January 1, 2009 to July 15, 2009 and the
year ended December 31, 2008, respectively.
In July 2010, the Company entered into a consulting arrangement
with a relative of the Company’s chairman and chief
executive officer. This individual provided engineering support
in connection with the Louisiana Plant. The Company recognized
consulting expense of approximately $60,000, $145,000 and
$205,000 for the three months ended March 31, 2011
(unaudited), the year ended December 31, 2010 and the
period from inception to March 31, 2011 (unaudited),
respectively.
In October 2010, the Company entered into a consulting
arrangement with another entity controlled by a stockholder and
former director of the Company. This agreement relates to
administrative support related to the construction of the
Company’s facility in Louisiana. This entity is entitled to
receive a fee of up to $250,000 upon the achievement of
acceptance of an application for certain government awards or
loans. The Company recognized consulting expense of $21,250
relating to the achievement of one of the targets for the three
months ended March 31, 2011 (unaudited) and the period from
inception to March 31, 2011 (unaudited), respectively.
F-22
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
|
|
|
5.
|
Property
and Equipment
|
Property and equipment consisted of the following at
March 31, 2011, December 31, 2010 and 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Estimated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Useful
|
|
|
March 31,
|
|
|
|
|
|
|
|
|
|
|
Life
|
|
|
2011
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(Years)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
Land
|
|
|
|
|
|
$
|
48,750
|
|
|
$
|
48,750
|
|
|
$
|
48,750
|
|
|
Furniture and fixtures
|
|
|
7
|
|
|
|
321,114
|
|
|
|
321,114
|
|
|
|
312,265
|
|
|
Equipment
|
|
|
5
|
|
|
|
2,523,309
|
|
|
|
2,516,224
|
|
|
|
2,208,063
|
|
|
Computers and software
|
|
|
3
|
|
|
|
891,135
|
|
|
|
799,497
|
|
|
|
750,244
|
|
|
Leasehold improvements
|
|
|
5
|
|
|
|
702,757
|
|
|
|
702,757
|
|
|
|
590,757
|
|
|
Construction in progress
|
|
|
—
|
|
|
|
7,697,328
|
|
|
|
7,212,967
|
|
|
|
7,330,241
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12,184,393
|
|
|
|
11,601,309
|
|
|
|
11,240,320
|
|
|
Less — Accumulated depreciation
|
|
|
|
|
|
|
(2,449,668
|
)
|
|
|
(2,212,696
|
)
|
|
|
(1,299,928
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
9,734,725
|
|
|
$
|
9,388,613
|
|
|
$
|
9,940,392
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation expense was $236,972, $221,281, $973,858, $422,014
and $1,632,844 for the three months ended March 31, 2011
and 2010 (unaudited), the year ended December 31, 2010 and
the periods from inception to December 31, 2009 and
March 31, 2011 (unaudited), respectively. Depreciation
expense was $418,970 and $478,429 for the Predecessor Company
period January 1, 2009 to July 15, 2009 and for the
year ended December 31, 2008, respectively. Construction in
progress consists of land and land improvements in Lake
Providence, Louisiana. It is expected that the Company will
construct the Louisiana Plant on this property. As of
March 31, 2011, construction has not yet commenced. The
Company has assessed the completion date for the facility to be
in late 2012 and anticipates the total cost to complete is
approximately $103 million. As of March 31, 2011
(unaudited), the construction in progress balance included
$478,960 of government award receipts.
Accrued liabilities consisted of the following at March 31,
2011, December 31, 2010 and 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31,
|
|
|
|
|
|
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
Payroll related
|
|
$
|
2,424,018
|
|
|
$
|
3,006,803
|
|
|
$
|
1,342,368
|
|
|
Professional fees
|
|
|
224,062
|
|
|
|
497,986
|
|
|
|
307,395
|
|
|
License fees
|
|
|
80,686
|
|
|
|
81,501
|
|
|
|
73,858
|
|
|
Other
|
|
|
767,365
|
|
|
|
196,416
|
|
|
|
257,736
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
3,496,131
|
|
|
$
|
3,782,706
|
|
|
$
|
1,981,357
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On September 18, 2009, the Company entered into a short
term promissory note agreement with an equipment leasing company
for $1,700,000. The note bore interest at 10% and was secured by
the Company’s equipment. The loan was payable on the
earlier of a) the Company achieving additional financing or
b) March 15, 2010. Interest was payable monthly in
arrears on the first business day of each month. At
December 31, 2009, the amount owed under this arrangement
amounted to $1,714,438, which included accrued interest of
$14,438. The promissory note was paid in full in February 2010.
F-23
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
In conjunction with the issuance of this note, the Company
issued 59,231 warrants to purchase Company membership units at
$13. These warrants expire September 18, 2014. At the time
of the transaction, a value of $87,662 was ascribed to these
warrants using the Black-Scholes option pricing model. In
computing the value of the warrants, Myriant assumed a risk free
interest rate of 3.5%, a life of 5 years, expected
volatility of 48% and no future dividends. The residual fair
value ascribed was recorded as interest expense over the term of
the loan under the interest method using an effective rate of
12.2%. The Company recognized interest expense of $36,444,
$51,218 and $87,662 related to this note for the year ended
December 31, 2010 and the periods from inception to
December 31, 2009 and 2010, respectively. The following
table summarizes the balance at December 31, 2010 and 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
Principal
|
|
$
|
—
|
|
|
$
|
1,700,000
|
|
|
Accrued interest
|
|
|
—
|
|
|
|
14,438
|
|
|
Warrant
|
|
|
—
|
|
|
|
(36,444
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
—
|
|
|
$
|
1,677,994
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8.
|
Employee
Benefit and Bonus Plans
|
The Company has established a defined contribution savings plan
under Section 401(k) of the Internal Revenue Code. This
plan covers substantially all employees who meet minimum age and
service requirements and allows participants to defer a portion
of their annual compensation on a pre-tax basis. The Company
matches employee contributions to a maximum of 4% of
compensation. Company contributions for the three months ended
March 31, 2011 and 2010 (unaudited), the year ended
December 31, 2010 and the periods from inception to
December 31, 2009 and March 31, 2011 (unaudited)
totaled $67,479, $38,643, $191,199, $78,432 and $337,110,
respectively. The Predecessor Company contributions for the
period January 1, 2009 to July 15, 2009 and for the
year ended December 31, 2008 totaled $115,361 and $136,139,
respectively. At March 31, 2011 (unaudited),
December 31, 2010 and 2009, the Company’s employer
contribution payable of $11,330, $7,267 and $6,135,
respectively, was included in accrued liabilities.
The Company maintains a discretionary bonus plan for all
employees. The decision of whether to award an annual bonus and
the amount of such bonus is made at the sole discretion of the
Board of Directors. The Company recognized bonus expense of
$645,537, $419,065, $1,470,425, $324,279 and $2,440,241 for the
three months ended March 31, 2011 and 2010 (unaudited), the
year ended December 31, 2010 and the periods from inception
to December 31, 2009 and March 31, 2011 (unaudited),
respectively. The Predecessor Company recognized bonus expense
of $837,107 and $220,400 for the period from January 1,
2009 to July 15, 2009 and for the year ended
December 31, 2008, respectively.
|
|
|
9.
|
Stock-Based
Compensation
|
Effective January 10, 2011, the Company adopted the Plan.
The Plan permits the grant of 939,130 incentive stock options,
non qualified stock options, stock appreciation rights,
restricted stock, unrestricted stock or other equity based
awards. The vesting period and maturity of each equity
instrument is determined at the date of grant. The term of an
option cannot exceed 10 years.
The Company, formerly organized as a limited liability company,
granted membership units to employees and non-employees in the
normal course of business. The existing membership units
reserved were converted to common shares upon the Company’s
conversion to a C corporation in January 2011. In certain
instances, these grants have vesting provisions. The Company
ascribes a value to these grants at the date of grant. In the
case of grants with vesting provisions, the value ascribed to
the units is recognized ratably over the requisite service or
vesting period. At inception, the Company had 208,040 unvested
membership units which were transferred to Myriant as part of
the restructuring with BEI. Grants contain both performance and
time vesting.
F-24
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Restricted
stock and membership units
The following table provides the grant activity since inception:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted Average
|
|
|
Time
|
|
|
Performance
|
|
|
|
|
|
|
|
Grant Date Fair Value
|
|
|
Vesting
|
|
|
Vesting
|
|
|
Total
|
|
|
|
|
Unvested grants at inception
|
|
$
|
8.48
|
|
|
|
124,708
|
|
|
|
83,332
|
|
|
|
208,040
|
|
|
Vested
|
|
|
(8.48
|
)
|
|
|
(56,398
|
)
|
|
|
—
|
|
|
|
(56,398
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested grants at December 31, 2009
|
|
|
8.48
|
|
|
|
68,310
|
|
|
|
83,332
|
|
|
|
151,642
|
|
|
Grants
|
|
|
5.33
|
|
|
|
97,188
|
|
|
|
32,000
|
|
|
|
129,188
|
|
|
Vested
|
|
|
(7.34
|
)
|
|
|
(39,461
|
)
|
|
|
—
|
|
|
|
(39,461
|
)
|
|
Forfeited
|
|
|
(8.48
|
)
|
|
|
(35,298
|
)
|
|
|
(49,999
|
)
|
|
|
(85,297
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested grants at December 31, 2010
|
|
|
6.16
|
|
|
|
90,739
|
|
|
|
65,333
|
|
|
|
156,072
|
|
|
Grants
|
|
|
3.45
|
|
|
|
96,000
|
|
|
|
15,000
|
|
|
|
111,000
|
|
|
Vested
|
|
|
(4.40
|
)
|
|
|
(54,581
|
)
|
|
|
—
|
|
|
|
(54,581
|
)
|
|
Forfeited
|
|
|
(6.00
|
)
|
|
|
(12,000
|
)
|
|
|
(14,000
|
)
|
|
|
(26,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested grants at March 31, 2011 (unaudited)
|
|
$
|
5.40
|
|
|
|
120,158
|
|
|
|
66,333
|
|
|
|
186,491
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The performance vesting units will immediately vest upon the
Company achieving specific target earnings and milestones in
2012 and 2013. If the performance condition is not met, the
shares would be forfeited. The targets included the successful
completion of the Lake Providence Plant at or below budget by
December 31, 2012 and the achievement of aggregate EBITDA
of $10 million by December 31, 2013. The Company does
not believe it is probable that the targets will be met, and as
such, no compensation has been recorded to date in connection
with these grants. The Company will continue to reassess the
probability of achieving these targets at each reporting period
to determine whether compensation should be recorded.
In addition to the grants noted above, the Company issued 9,346,
3,014, 8,749 and 17,938 shares to Company advisors for the
three months ended March 31, 2011 and 2010 (unaudited) and
the period from July 16, 2009 (inception) to
December 31, 2009 and the year ended December 31,
2010. At March 31, 2011 (unaudited), there is unrecognized
compensation expense of approximately $924,000 related to
restricted share awards, which is expected to be recognized over
a period of three years.
F-25
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Common
stock options
Upon establishing the Plan, the Company issued stock options to
employees. Options granted contain both time and performance
vesting criteria. A summary of stock option activity is
presented below:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Average
|
|
|
|
|
|
|
|
Number of
|
|
|
Exercise
|
|
|
Remaining
|
|
|
Aggregate
|
|
|
|
|
Options
|
|
|
Price
|
|
|
Term (Years)
|
|
|
Intrinsic Value
|
|
|
|
|
Options outstanding, December 31, 2010
|
|
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
543,293
|
|
|
|
4.43
|
|
|
|
|
|
|
|
|
|
|
Cancelled or forfeited
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options outstanding, March 31, 2011 (Unaudited)
|
|
|
543,293
|
|
|
$
|
4.43
|
|
|
|
9.91
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options fully vested and exercisable, March 31, 2011
(Unaudited)
|
|
|
274,293
|
|
|
$
|
4.28
|
|
|
|
9.93
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options expected to vest, March 31, 2011 (Unaudited)
|
|
|
253,500
|
|
|
$
|
4.64
|
|
|
|
9.91
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In 2011, we granted 15,500 stock options with performance based
vesting, which had an exercise price of $3.45 per share. These
options would immediately vest upon the Company achieving
specific target earnings and milestones in 2012 and 2013, but
would be forfeited if these performance conditions are not
achieved. The targets included the successful completion of the
Lake Providence Plant at or below budget by December 31,
2012 and the achievement of aggregate EBITDA of $10 million
by December 31, 2013. The Company does not believe it is
probable that the targets will be met, and as such, no
compensation has been recorded to date in connection with these
grants. The Company will continue to reassess the probability of
achieving these targets at each reporting period to determine
whether compensation should be recorded.
Additional information related to stock options is summarized
below:
| |
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
Ended
|
|
|
|
|
March 31, 2011
|
|
|
|
|
(Unaudited)
|
|
|
|
|
Weighted average grant date fair value of awards
|
|
$
|
1.73
|
|
|
Intrinsic value of options exercised
|
|
|
—
|
|
|
Proceeds received from the exercise of options
|
|
|
—
|
|
|
Grant date fair value of options vested
|
|
$
|
138,692
|
|
As of March 31, 2011 (unaudited), the Company had
approximately $417,000 of total unrecognized compensation
expense related to stock options which is expected to be
recognized over a period of three years.
The Company settles employee stock option exercises with newly
issued common shares. No tax benefits were realized by the
Company as no options have been exercised since the inception of
the plan.
F-26
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Information about stock options outstanding and exercisable at
March 31, 2011 (unaudited), is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options Outstanding
|
|
|
Options Exercisable
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
|
Average
|
|
|
Average
|
|
|
|
|
|
Number of
|
|
|
Remaining
|
|
|
Number of
|
|
|
Exercise
|
|
|
Remaining
|
|
|
Exercise Price
|
|
|
Options
|
|
|
Term (Years)
|
|
|
Options
|
|
|
Price
|
|
|
Term (Years)
|
|
|
|
|
$
|
3.45
|
|
|
|
263,750
|
|
|
|
10
|
|
|
|
153,750
|
|
|
$
|
3.45
|
|
|
|
10
|
|
|
$
|
5.35
|
|
|
|
279,543
|
|
|
|
10
|
|
|
|
120,543
|
|
|
$
|
5.35
|
|
|
|
10
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
543,293
|
|
|
|
|
|
|
|
274,293
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The fair value of the stock options granted was estimated using
the following assumptions:
| |
|
|
|
|
|
Three Months
|
|
|
|
Ended
|
|
|
|
March 31, 2011
|
|
|
|
(Unaudited)
|
|
|
|
Risk free interest rate
|
|
2.02%-2.41%
|
|
Expected dividend yield
|
|
0%
|
|
Expected volatility factor
|
|
65%
|
|
Expected option life (years)
|
|
5-6
|
|
Expected forfeitures
|
|
5%
|
The risk free interest rate was based upon the
U.S. Treasury curve in effect at the time of the grant for
instruments with a term similar to the expected life of the
related option. The volatility factor was determined based upon
management’s estimate using inputs from comparable public
companies. Due to the Company’s limited history of grant
activity, the expected life of options granted was estimated
using the “simplified method” where the expected life
equals the arithmetic average of the vesting term and the
original contractual term of the options. No dividends are
expected to be paid.
F-27
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Stock-based compensation expense included in the Company’s
consolidated statements of operations is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Myriant Corporation
|
|
|
Predecessor Company
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
July 16,
|
|
|
July 16,
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year
|
|
|
2009
|
|
|
2009
|
|
|
January 1,
|
|
|
Fiscal Year
|
|
|
|
|
Three Months Ended
|
|
|
Ended
|
|
|
(Inception)
|
|
|
(Inception)
|
|
|
2009
|
|
|
Ended
|
|
|
|
|
March 31,
|
|
|
December 31,
|
|
|
to December 31,
|
|
|
to March 31,
|
|
|
to July 15,
|
|
|
December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2010
|
|
|
2009
|
|
|
2011
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
Stock options issued to employees:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
$
|
43,524
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
—
|
|
|
|
43,524
|
|
|
|
—
|
|
|
|
—
|
|
|
General and administrative
|
|
|
197,943
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
197,943
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
241,467
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
241,467
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted stock issued to employees:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
14,607
|
|
|
|
100,934
|
|
|
|
145,084
|
|
|
|
—
|
|
|
|
141,607
|
|
|
|
—
|
|
|
|
—
|
|
|
General and administrative
|
|
|
91,957
|
|
|
|
31,095
|
|
|
|
173,854
|
|
|
|
155,099
|
|
|
|
420,910
|
|
|
|
154,920
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
106,564
|
|
|
|
132,029
|
|
|
|
318,938
|
|
|
|
155,099
|
|
|
|
562,517
|
|
|
|
154,920
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted stock issued to non employees:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
—
|
|
|
|
—
|
|
|
|
89,544
|
|
|
|
—
|
|
|
|
107,628
|
|
|
|
—
|
|
|
|
—
|
|
|
General and administrative
|
|
|
122,244
|
|
|
|
18,085
|
|
|
|
117,462
|
|
|
|
52,494
|
|
|
|
292,200
|
|
|
|
125,000
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
122,244
|
|
|
|
18,085
|
|
|
|
207,006
|
|
|
|
52,494
|
|
|
|
399,828
|
|
|
|
125,000
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total share based compensation
|
|
$
|
470,275
|
|
|
$
|
150,114
|
|
|
$
|
525,944
|
|
|
$
|
207,593
|
|
|
$
|
1,203,812
|
|
|
$
|
279,920
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of March 31, 2011 (unaudited), options for the purchase
of an aggregate of 543,293 shares of common stock and
186,491 unvested shares of restricted stock are outstanding
under the Plan. The number of options and restricted stock
remaining to be granted totaled 14,438 as of March 31, 2011
(unaudited).
|
|
|
10.
|
Stockholders’
and Members’ Equity
|
Effective January 10, 2011, the Company converted from a
limited liability company to a Subchapter C corporation. Upon
conversion, the Company’s authorized classes of capital
stock consist of 11,214,953 shares of $.0001 par value
Class A common stock, 3,044,905 shares of
$.0001 par value Class B common stock and
30,000,000 shares of $.0001 par value common stock.
All warrants described below were converted to warrants to
purchase common stock.
At inception, certain warrants issued by BEI were carried over
to Myriant. Under the terms of the transaction, 766,347 warrants
to purchase membership units at $10 and 423,077 warrants to
purchase membership units at $13 were transferred to Myriant. In
conjunction with this transaction, the lives of the warrants
were extended, and the increase in incremental value was
reflected in the warrant liability at July 16, 2009 with
the offset to stockholders’ and members’ equity. These
warrants were currently exercisable and expire on
August 15, 2015 and October 15, 2015, respectively. In
November 2009, the Company issued an additional 59,231 warrants
with an exercise price of $13 in connection with the receipt of
an equipment loan. These warrants are currently exercisable and
expire on September 18, 2014.
In February 2010, the Company adjusted the price of certain $10
and $13 warrants to $6 and concurrently issued 34,744 additional
$6 warrants. Additionally, the Company issued 913,100 warrants
with an exercise price of $.01 in connection with a bridge loan.
These warrants expire November 5, 2015.
F-28
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Effective January 13, 2011, the Company completed a
$60 million equity financing with a strategic investor, CH
Inter, in exchange for 11,214,953 shares of Class A
common stock, which resulted in net proceeds of
$57.1 million. This transaction triggered the existing note
payable and the bridge note agreements to be convertible and set
the conversion rate to $5.35 per share. As a result, the Company
recorded a beneficial conversion as of the date of this event
totaling $10,445,199 which was recorded as interest expense and
additional paid in capital. As a condition of the CH Inter
closing, existing investors converted their note payable and
bridge note agreements into Class B common stock and
exercised 1,117,378 of their $6 warrants to the extent
outstanding in exchange for common stock. Any debt remaining
after the warrants were exercised was converted to Class B
common stock at $5.35 per share. As a result of this
transaction, all debt that existed at December 31, 2010 was
converted to equity. At the date of conversion, $22,994,510 of
debt was converted into equity, of which $3,794,510 related to
accrued interest.
The following table summarizes the number of membership units or
common stock reserved for issuance at March 31, 2011,
December 31, 2010 and 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
Exercise of $10 warrants
|
|
|
89,656
|
|
|
|
89,656
|
|
|
|
766,347
|
|
|
Exercise of $13 warrants
|
|
|
59,231
|
|
|
|
59,231
|
|
|
|
482,308
|
|
|
Exercise of $6 warrants
|
|
|
17,134
|
|
|
|
1,134,512
|
|
|
|
—
|
|
|
Exercise of $.01 warrants
|
|
|
639,170
|
|
|
|
913,100
|
|
|
|
—
|
|
|
Convertible Class A common stock
|
|
|
11,214,953
|
|
|
|
—
|
|
|
|
—
|
|
|
Convertible Class B common stock
|
|
|
3,044,905
|
|
|
|
—
|
|
|
|
—
|
|
|
Unvested employee and non employee membership units
|
|
|
186,491
|
|
|
|
156,072
|
|
|
|
151,642
|
|
|
Units reserved for future option grants
|
|
|
543,293
|
|
|
|
216,591
|
|
|
|
118,593
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
15,794,833
|
|
|
|
2,569,162
|
|
|
|
1,518,890
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In addition to the membership units or common stock reserved
above for issuance, the Company has also reserved membership
units or common stock for the conversion of the secured
convertible notes-related party plus accrued interest as of
December 31, 2010 and 2009 totaling $22,899,184 and
$10,574,057, respectively. As of December 31, 2010, the
notes were not convertible since the conversion rate is set upon
the occurrence of an event relating to a new equity issuance.
During January 2011, these notes were converted into
3,044,905 shares of Class B common stock.
Class A
common stock
Class A common stock accrues dividends at the rate per
annum of 8% per share compounded annually on a cumulative basis
and is payable if declared by the Board of Directors, upon the
conversion of Class A common stock into common stock,
including in connection with the closing of the Company’s
proposed initial public offering, or in the event of any
liquidation, dissolution or winding up of the Company, whether
or not the dividend was declared. At March 31, 2011
(unaudited), the Company has not accrued Class A dividends
since they do not become a corporate liability until declared.
Had the Company declared dividends as of March 31, 2011
(unaudited), the total accrued dividends would have been
$1,025,753. The dividends accumulated for the period on
cumulative Class A common stock are included in the net
loss when computing net loss per share. In the event of a
liquidation event, the holders of Class A common stock are
entitled to be paid out of the assets of the corporation
available for distribution to stockholders before any payment
can be made to holders of Class B common stock and common
stock in an amount equal to the Class A common original
issue price of $5.35 plus any accrued and unpaid dividends.
Class A common stock holders are entitled to one vote for
each share. Class A common shares do not have redemption
rights.
F-29
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Each share of Class A common stock shall be convertible, at
the option of the holder, at any time and without the payment of
additional consideration by the holder, into such number of
fully paid and non-assessable shares of common stock as is
determined by dividing the Class A common original issue
price by the Conversion Price in effect at the time of
conversion. The Conversion Price shall initially be equal to
$5.35. Such initial conversion price, and the rate at which
shares of Class A common stock may be converted into shares
of common stock could be subject to downward adjustment.
Class B
common stock
Dividends on Class B common stock may only be paid if the
dividends are declared by the unanimous vote of all members of
the board of directors and all amounts payable to holders of
Class A common stock have been paid. In the event of a
liquidation event, the holders of Class B common stock are
entitled to be paid out of the assets of the Company available
for distribution to stockholders after payments made to holders
of Class A common stock but before any payment can be made
to holders of common stock in an amount equal to the
Class B common original issue price of $5.35 plus any
dividends declared and unpaid. Class B common stock holders
are entitled to one vote for each share. Class B common
shares do not have redemption rights.
Each share of Class B common stock shall be convertible, at
the option of the holder, at any time and without the payment of
additional consideration by the holder, into such number of
fully paid and nonassessable shares of common stock as is
determined by dividing the Class B common original issue
price by the Conversion Price in effect at the time of
conversion. The Conversion Price shall initially be equal to
$5.35. Such initial conversion price, and the rate at which
shares of Class B common stock may be converted into shares
of common stock could be subject to downward adjustment.
Common
stock
Each share of common stock is entitled to one vote. The holders
of common stock are entitled to receive dividends whenever funds
are legally available and when declared by the board of
directors, subject to the prior rights of all holders of all
classes of stock outstanding.
Conversion
Feature
The Class A and B common stock have a mandatory conversion
feature which is triggered upon either of:
(a) the closing of the sale of shares of common stock to
the public at a price per share equal to or greater than the
quotient obtained by dividing $350,000,000 by the total number
of shares of common stock outstanding on an as-converted, fully
diluted basis immediately before giving effect to such sale of
shares, in a firm-commitment underwritten public offering
pursuant to an effective registration statement under the
Securities Act of 1933, as amended, resulting in at least
$50,000,000 of proceeds, net of the underwriting discount and
commissions, to the Company or
(b) the date and time, or the occurrence of an event,
specified by vote or written consent of the holders of a
majority of the then outstanding shares of Class A common
stock.
The Company has determined that the conversion option included
in the Class A and B common stock does not meet the
definitions of a derivative due to the fact there is no net
settlement provisions as of March 31, 2011. If a security
is convertible from inception but contains conversion terms that
change upon the occurrence of a future event, any contingent
beneficial conversion feature should be measured using the
commitment date stock price and recognized in earnings when the
contingency is resolved. The applicable conversion rate has a
contingent conversion feature that will reduce the conversion
price if the Company issues stock at a price below the original
conversion rate of $5.35. When the reduction in price occurs, a
beneficial conversion will be recognized. As of March 31,
2011, there was no beneficial conversion for the Class A
and B common stock.
F-30
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
|
|
|
11.
|
Commitments
and Contingencies
|
Lease
Agreements
The Company has entered into non-cancelable operating leases for
its corporate headquarters, research facilities and certain
equipment. The lease term includes a rent escalation each year,
which the Company is recognizing on a straight line basis over
the term of the lease. The cumulative difference in straight
line rent expense and actual rent paid is a liability included
in deferred rent, which totaled $125,270, $131,065 and $153,655
as of March 31, 2011 (unaudited), December 31, 2010
and 2009, respectively. The Company also received a tenant
improvement allowance from the landlord in the amount of
$280,000, which is being amortized over the term of the lease as
a reduction in rent expense and has also been included in
property and equipment. The unamortized tenant improvement
allowance was $87,214 and $104,025 as of March 31, 2011
(unaudited) and December 31, 2010, and is also included in
deferred rent. This amount was not material as of
December 31, 2009.
Future minimum lease payments under these non-cancelable
operating leases at March 31, 2011 (unaudited) are as
follows:
| |
|
|
|
|
|
2011
|
|
$
|
761,042
|
|
|
2012
|
|
|
968,725
|
|
|
2013
|
|
|
163,840
|
|
|
|
|
|
|
|
|
|
|
$
|
1,893,607
|
|
|
|
|
|
|
|
Rent expense for the three months ended March 31, 2011 and
2010 (unaudited), the year ended December 31, 2010 and the
period from July 16, 2009 (inception) through
December 31, 2009 and March 31, 2011 (unaudited) was
$224,153, $247,630, $895,202, $261,189 and $1,380,544,
respectively, related to these leases. The Predecessor Company
incurred rent expense of $460,940 and $717,136 for the period
January 1, 2009 to July 15, 2009 and for the year
ended December 31, 2008, respectively.
Additionally, the Company had entered into a lease agreement
with the Lake Providence Port Authority, or the Port, to lease
land which will be used to build the Louisiana Plant.
Non-cancellable payments are due through 2015. Currently, the
Company makes lease payments to the Port. The lease term
includes a rent escalation each year which the Company is
recognizing on a straight line basis over the term of the lease.
The cumulative difference in straight line rent expense and
actual rent paid is included in deferred rent as a prepaid of
$79,347 and $79,958 as of March 31, 2011 (unaudited) and
December 31, 2010. Rent expense for the three months ended
March 31, 2011 and 2010 (unaudited), the year ended
December 31, 2010 and the period from inception to
December 31, 2009 and March 31, 2011 (unaudited) was
$20,389, $23,018, $46,035, $52,110 and $118,534, respectively.
Future minimum lease payments under this agreement are as
follows at March 31, 2011 (unaudited):
| |
|
|
|
|
|
2011
|
|
$
|
19,167
|
|
|
2012
|
|
|
32,670
|
|
|
2013
|
|
|
25,246
|
|
|
2014
|
|
|
17,416
|
|
|
2015
|
|
|
9,046
|
|
|
|
|
|
|
|
|
|
|
$
|
103,545
|
|
|
|
|
|
|
|
The Company has an aircraft lease agreement expiring in June
2011, which can be extended for subsequent three month periods.
Rent expense for the three months ended March 31, 2011 and
2010 (unaudited), the year ended December 31, 2010 and the
periods from inception to December 31, 2009 and
March 31, 2011 (unaudited) were approximately $20,000,
$62,000, $196,000, $81,000 and $297,000, respectively.
F-31
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Litigation
The Company is subject to legal proceedings, claims and
litigations arising in the ordinary course of business. At the
balance sheet dates presented, there were no outstanding claims
or litigation.
Significant
Contractual Agreements
The Company entered in to a non-binding memorandum of
understanding, or MOU, with Johnson Matthey PLC’s
subsidiary Davy Process Technology Limited, or Davy, a developer
and licensor of advanced process technologies, to enter into a
definitive joint development agreement that would (a) allow
Davy to qualify Myriant’s biosuccinic acid for use in
Davy’s BDO process and (b) collaborate on the use of
biosuccinic acid salts prior to purification steps in the Davy
BDO process, or the developed process. The parties shall
collaborate on the developed process to achieve overall
optimized operating and capital costs. The parties will jointly
offer the developed process to existing Davy customers who wish
to use biosuccinic acid as a replacement for maleic anhydride
feedstock and potential new Davy customers seeking new build
opportunities that may utilize biosuccinic acid. As of
March 31, 2011 (unaudited), there were no amounts incurred
under this agreement.
The Company has entered into a number of exclusive license
agreements with the University of Florida. Under the various
agreements, the Company is obligated to pay the University
royalties on product sales at a rate that declines with
increasing volume. There were no product sales for the periods
presented. The Company is also obligated to pay the University a
percentage of any license fees earned by sublicensing the
relevant patent rights to third parties. The Company has
sublicensed certain of the patent rights from the University of
Florida to Purac. License fees paid to the University of Florida
amounted to $0, $0, $84,600, $73,859 and $158,459 for the three
months ended March 31, 2011 and 2010 (unaudited), the year
ended December 31, 2010 and the periods from July 16,
2009 to December 31, 2009 and March 31, 2011
(unaudited), respectively. The Predecessor Company recognized
expense of $22,373 and $110,020 for the period from
January 1, 2009 to July 15, 2009 and the year ended
December 31, 2008, respectively.
In September 2009 and October 2009, the Company signed two
alliance agreements with ThyssenKrupp’s subsidiary Uhde
GmbH, or Uhde, and its U.S. subsidiary. Uhde is an
international engineering company focused on the design and
construction of chemical, refining, and other industrial plants.
Pursuant to these agreements, the parties will exclusively
cooperate with each other in research and development efforts to
jointly develop and optimize the Company’s fermentation
process and Uhde’s separation process for the production of
biosuccinic acid. The parties also exclusively cooperate in
their business development efforts to develop commercial scale
biosuccinic acid plants globally, subject to certain conditions
and limitations. Uhde has agreed to act exclusively as the
Company’s engineering, procurement and construction
contractor for commercial scale biosuccinic acid plants, except
in certain limited circumstances. Under the terms of these
agreements, if an identified project requires the provision of
process and performance guarantees, Uhde will be prepared to
negotiate such guarantees on mutually agreeable terms. No costs
have been incurred under this agreement through March 31,
2011 (unaudited).
In December 2009, the Company entered into a toll manufacturing
agreement with Fermic SA de CV (Fermic). Under the terms of the
agreement, Fermic produces biosuccinic acid for the Company and
the Company makes payments based upon production milestones
achieved. The Company recognized expense of $349,500, $629,888,
$1,405,450 and $1,754,950 for the three months ended
March 31, 2011 and 2010 (unaudited), the year ended
December 31, 2010 and the period from July 16, 2009 to
March 31, 2011 (unaudited), respectively. The Predecessor
Company did not recognize any expense related to this agreement.
The Company discontinued use of this facility in April 2011.
F-32
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
The current tax provision is computed on the basis used by
Myriant in filing its income tax returns. Due to the
Company’s losses from its inception, there is no tax
provision on the accompanying consolidated statements of
operations. The Company’s net deferred income tax expense
(benefit) represents the change in the deferred income taxes
from the beginning to the end of the respective period.
Myriant was incorporated on January 10, 2011 and therefore,
had no activity prior to that date. MLPI was incorporated on
January 13, 2010 but did not commence operations until July
2010. Accordingly, no deferred tax activity existed at
March 31, 2010 (unaudited).
Net deferred tax assets consisted of the following components as
of March 31, 2011 and December 31, 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
March 31,
|
|
|
December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
Capitalized
start-up
costs
|
|
$
|
3,050,000
|
|
|
$
|
3,050,000
|
|
|
Net operating losses
|
|
|
2,733,000
|
|
|
|
1,100,000
|
|
|
Intangible assets
|
|
|
3,785,000
|
|
|
|
—
|
|
|
Construction in progress
|
|
|
612,000
|
|
|
|
—
|
|
|
Other
|
|
|
674,000
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10,854,000
|
|
|
|
4,150,000
|
|
|
Less: valuation allowance
|
|
|
(10,854,000
|
)
|
|
|
(4,150,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net balance
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
As of March 31, 2011 (unaudited) and December 31,
2010, Myriant had federal and state net operating loss
carryforwards of approximately $6,840,000 and $2,730,000
respectively, which may be used to offset future taxable income.
Management has determined that it is more likely than not that
Myriant will not recognize the full benefit of the federal and
Massachusetts deferred tax asset and, as a result, the deferred
tax asset was reduced by a full valuation adjustment of
approximately $10,854,000 and $4,150,000 as of March 31,
2011 (unaudited) and December 31, 2010, respectively. All
deferred tax assets have been calculated at Myriant’s
incremental income tax rates for the period ended March 31,
2011 (unaudited).
Reconciling items from income tax computed at the statutory
federal rate for the year ended December 31, 2010 for MLPI
were as follows. During the year ended December 31, 2009,
all entities were limited liability companies and therefore
there was no income tax provision presented.
| |
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
|
|
Federal income tax at statutory rate
|
|
|
35.00
|
%
|
|
State income taxes, net of federal benefits
|
|
|
5.00
|
%
|
|
Valuation allowance
|
|
|
(40.00
|
)%
|
|
|
|
|
|
|
|
Effective tax rate
|
|
|
0.00
|
%
|
|
|
|
|
|
|
On July 11, 2008, the Predecessor Company acquired certain
assets of Omnigene BioProducts, Inc., or Omnigene, pursuant to
the terms of an asset purchase agreement. The consideration paid
included $200,000 in cash and a contingent payment of $50,000.
The contingent payment was payable upon the Omnigene founders
remaining employed with the Company until July 2009. The assets
acquired included certain lab equipment and a research and
development workforce as well as certain strains, organisms and
patents owned by
F-33
Myriant
Corporation and Subsidiaries (A Development Stage Enterprise)
Notes to
Consolidated Financial
Statements — (Continued)
Omnigene. Management estimated that the fair value of the lab
equipment acquired was $200,000. The strains, organisms and
patents acquired were ascribed no value as the projects were
determined to be in the conceptualization phase. The
Company’s operating results include the results of Omnigene
since the date of acquisition. The contingent payment was
recorded to compensation expense and included in research and
development expenses when earned in July 2009. Had this
transaction been consummated at January 1, 2008, the
Predecessor Company would have incurred approximately $300,000
in additional research and development expense.
In making a determination of business segments, the Company gave
consideration to the Chief Executive Officer’s (or the
Chief Operating Decision Maker’s) review of financial
information and the organizational structure of our management
team. Based upon this review, the Company concluded that, at the
present time, resources are allocated and other decisions made
based upon consolidated financial information. Therefore, the
Company has determined that it operates in one business segment.
All of the Company’s long lived assets are located in North
America. The following chart presents revenue based upon
customer location:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Myriant Corporation
|
|
|
Predecessor Company
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
July 16,
|
|
|
July 16,
|
|
|
Period from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year
|
|
|
2009
|
|
|
2009
|
|
|
January 1,
|
|
|
Fiscal Year
|
|
|
|
|
Three Months Ended
|
|
|
Ended
|
|
|
(Inception)
|
|
|
(Inception)
|
|
|
2009
|
|
|
Ended
|
|
|
|
|
March 31,
|
|
|
December 31,
|
|
|
to December 31,
|
|
|
to March 31,
|
|
|
to July 15
|
|
|
December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2010
|
|
|
2009
|
|
|
2011
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(Unaudited)
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
North America:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Management fee revenue-related party
|
|
$
|
2,519
|
|
|
$
|
600,166
|
|
|
$
|
3,557,575
|
|
|
$
|
—
|
|
|
$
|
3,560,094
|
|
|
|
267,318
|
|
|
|
262,212
|
|
|
Development fee revenue-related party
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,125,714
|
|
|
Government grants
|
|
|
—
|
|
|
|
1,516,323
|
|
|
|
10,419,043
|
|
|
|
—
|
|
|
|
10,419,043
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
2,519
|
|
|
|
2,116,489
|
|
|
|
13,976,618
|
|
|
|
—
|
|
|
|
13,979,137
|
|
|
|
267,318
|
|
|
|
3,387,926
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Europe:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
License fee revenue
|
|
|
—
|
|
|
|
—
|
|
|
|
258,240
|
|
|
|
221,711
|
|
|
|
479,951
|
|
|
|
71,833
|
|
|
|
344,860
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
$
|
2,519
|
|
|
$
|
2,116,489
|
|
|
$
|
14,234,858
|
|
|
$
|
221,711
|
|
|
$
|
14,459,088
|
|
|
$
|
339,151
|
|
|
$
|
3,732,786
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On April 12, 2011, an investor exercised the remaining
639,170 $0.01 warrants. The Company received $6,391 in proceeds
from this transaction.
On June 16, 2011, the Company entered into a lease with the
Lake Providence Park Commission, a political subdivision of the
State of Louisiana, for the premises located at 420 Port Road,
Lake Providence, Louisiana (unaudited). The primary term of the
lease is for a period commencing on June 16, 2011 and
continuing 20 years from such date. The Company has two
options to extend the lease for two additional terms of
10 years each. Annual lease payments are as follows:
years 1 and 2 – $375,000; years 3
through 14 – $500,000;
years 15-20 – $219,540, subject to CPI
adjustments in year 18.
In May 2011, the Company entered into a non-binding memorandum
of understanding, or MOU, with China National Bluestar (Group)
Co. Ltd, to develop a proposal for a jointly-owned
220 million pound biosuccinic acid plant in Nanjing, China.
No costs have been incurred under this agreement.
The Company evaluated subsequent events through the date of this
filing.
F-34
Shares
MYRIANT CORPORATION
Common Stock
PROSPECTUS
Morgan Joseph
TriArtisan
Through and
including ,
2011 (the
25th day
after commencement of this offering), all dealers that buy,
sell, or trade shares of our common stock, whether or not
participating in this offering, may be required to deliver a
prospectus. This delivery requirement is an addition to the
obligation of dealers to deliver a prospectus when acting as
underwriters and with respect to their unsold allotments or
subscriptions.
PART II
INFORMATION
NOT REQUIRED IN PROSPECTUS
|
|
|
ITEM 13.
|
OTHER
EXPENSES OF ISSUANCE AND DISTRIBUTION
|
The following table sets forth the fees and expenses, other than
underwriting discounts and commissions, payable in connection
with the registration of the common stock hereunder. All amounts
are estimates except the SEC registration fee, the FINRA filing
fee and The Nasdaq Global Market listing fee.
| |
|
|
|
|
|
Securities and Exchange Commission registration fee
|
|
$
|
14,512.50
|
|
|
FINRA filing fee
|
|
|
13,000.00
|
|
|
Nasdaq Global Market listing fee
|
|
|
150,000.00
|
|
|
Printing and engraving expenses
|
|
|
*
|
|
|
Legal fees and expenses
|
|
|
*
|
|
|
Accounting fees and expenses
|
|
|
*
|
|
|
Transfer agent and registrar fees
|
|
|
*
|
|
|
Miscellaneous expenses
|
|
|
*
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
*
|
|
|
|
|
|
|
|
|
|
|
ITEM 14.
|
INDEMNIFICATION
OF DIRECTORS AND OFFICERS
|
Section 145 of the Delaware General Corporation Law
provides that a corporation may indemnify its directors and
officers from certain expenses in connection with legal
proceedings and permits a corporation to include in its charter
documents, and in agreements between the corporation and its
directors and officers, provisions expanding the scope of
indemnification beyond that specifically provided by this
section.
The Registrant’s second amended and restated certificate of
incorporation provides for the indemnification of directors to
the fullest extent permissible under Delaware law.
The Registrant’s amended and restated bylaws provide for
the indemnification of officers, directors and third parties
acting on the Registrant’s behalf if such persons act in
good faith and in a manner reasonably believed to be in and not
opposed to the Registrant’s best interest, and, with
respect to any criminal action or proceeding, such indemnified
party had no reason to believe his or her conduct was unlawful.
The Registrant has entered into indemnification agreements with
each of its directors, and will enter into new indemnification
agreements with each of its directors and executive officers
before the completion of this offering, in addition to the
indemnification provisions provided for in its charter
documents. The Registrant intends to enter into indemnification
agreements with any new directors and executive officers in the
future.
The underwriting agreement (to be filed as Exhibit 1.1
hereto) will provide for indemnification by the underwriters of
the Registrant, the Registrant’s executive officers and
directors, and indemnification of the underwriters by the
Registrant for certain liabilities, including liabilities
arising under the Securities Act of 1933, as amended, in
connection with matters specifically provided in writing by the
underwriters for inclusion in the registration statement.
The Registrant intends to purchase and maintain insurance on
behalf of any person who is or was a director or officer against
any loss arising from any claim asserted against him or her and
incurred by him or her in that capacity, subject to certain
exclusions and limits of the amount of coverage.
|
|
|
ITEM 15.
|
RECENT
SALES OF UNREGISTERED SECURITIES
|
Since our formation (April 3, 2009), we have issued and
sold the following unregistered securities:
1. In July 2009, we issued an aggregate of
5,894,762 units of interest to members of BioEnergy
International, LLC in exchange for an equivalent number of units
of interest of BioEnergy International, LLC.
II-1
2. In July 2009, we issued warrants to purchase
423,077 units of interest at an exercise price of $13.00
per unit to Plainfield Finance Corporation. The warrants may be
exercised any time prior to October 10, 2015.
3. In July 2009, we issued warrants to purchase an
aggregate of 766,347 units of interest at an exercise price
of $10.00 per unit to Plainfield Finance Corporation, Itera
Ethanol LLC, NGP Capital Resources Company and Camulos BioEnergy
Partners LLC. The warrants may be exercised any time prior to
October 10, 2015.
4. In July 2009, we issued an aggregate of
1,000,001 units of interest to Plainfield Finance
Corporation, Itera Ethanol LLC, NGPC Asset Holdings V, LP
and Camulos BioEnergy Partners LLC in exchange for membership
interests such parties owned in Bionol Lake Providence, LLC.
5. In July 2009, we assumed from BioEnergy International,
LLC those certain 15% secured convertible promissory notes in
the aggregate principal amount of $5,500,000 payable to
Plainfield Direct Inc.
6. In September 2009, we issued and sold a secured
promissory note in the principal amount of $1,700,000 to Access
Shipping Limited Partnership. The Registrant issued warrants to
purchase 39,231 units of interest at an exercise price of
$13.00 per unit to Access Shipping Limited Partnership.
7. In October 2009, we issued warrants to purchase
20,000 units of interest at an exercise price of $13.00 per
unit to Access Shipping Limited Partnership.
8. In October 2009, we issued and sold a 15% secured
convertible promissory note in the principal amount of
$2,000,000 payable to Plainfield Direct Inc.
9. In November 2009, we issued and sold a 15% secured
convertible promissory note in the principal amount of
$2,000,000 payable to Plainfield Direct Inc.
10. In February 2010, we issued and sold 15% secured
convertible promissory notes in the aggregate principal amount
of $5,000,000 to Plainfield Direct Inc., Itera Ethanol LLC,
Green Chem Holdings LLC and Stephen J. Gatto and issued warrants
to purchase 34,744 units of interest at an exercise price
of $6.00 per unit to Stephen J. Gatto.
11. In July 2010, we issued and sold 15% secured
convertible promissory notes in the aggregate principal amount
of $3,000,000 to Plainfield Direct Inc., Green Chem Holdings LLC
and Stephen J. Gatto.
12. In November 2010, we issued and sold 15% secured
convertible promissory notes in the aggregate principal amount
of $1,700,000 to Plainfield Direct LLC, Green Chem Second
Edition LLC and Stephen J. Gatto and issued warrants to purchase
an aggregate of 913,100 units of interest at an exercise
price of $0.01 per unit to Plainfield Direct LLC, Green Chem
Second Edition LLC and Stephen J. Gatto.
13. In January 2011, we converted from a limited liability
company to a corporation and all of our warrants converted to
warrants to purchase an equivalent number of shares of the
Registrant’s common stock.
14. In January 2011, we converted an aggregate of
$22,994,511.46 of our issued and outstanding promissory notes to
Plainfield Direct LLC, Plainfield Finance II LLC, Itera
Ethanol LLC, Green Chem Holdings LLC, Green Chem Second Edition
LLC and Stephen J. Gatto. Proceeds from this conversion were
used to exercise an aggregate of 1,117,378 warrants to purchase
shares of the Registrant’s common stock. The remainder of
the proceeds were converted into the issuance of an aggregate of
3,044,905 shares of the Registrant’s Class B
common stock.
15. In January 2011, we issued and sold an aggregate of
11,214,953 shares of its Class A common stock to PTT
Chemical International Private Limited.
II-2
16. In January 2011, we granted stock options to purchase
an aggregate of 279,543 shares of its common stock at an
exercise price of $5.35 per share to our employees and
consultants. We also awarded an aggregate of 440,110 shares
of its restricted common stock to employees and consultants of
the Registrant.
17. In March 2011, Green Chem Second Edition LLC exercised
warrants to purchase 182,620 shares of our common stock at
an exercise price of $0.01 per share.
18. In March 2011, Norwood LDK, LLC exercised warrants to
purchase 91,310 shares of our common stock at an exercise
price of $0.01 per share.
19. In March 2011, the Registrant granted stock options to
purchase an aggregate of 263,750 shares of its common stock
at an exercise price of $3.45 per share to our employees and
consultants.
20. In April 2011, Plainfield Finance II LLC exercised
warrants to purchase 639,170 shares of our common stock at
an exercise price of $0.01 per share.
The issuance of securities described above in
paragraphs 1-15,
17-18 and 20 were exempt from registration under the Securities
Act of 1933, as amended, in reliance on Section 4(2) of the
Securities Act of 1933, as amended, or Regulation D or
Regulation S promulgated thereunder, as transactions by an
issuer not involving any public offering. The purchasers of the
securities in these transactions represented that they were
accredited investors and that they were acquiring the securities
for investment only and not with a view toward the public sale
or distribution thereof. Such purchasers received written
disclosures that the securities had not been registered under
the Securities Act of 1933, as amended, and that any resale must
be made pursuant to a registration statement or an available
exemption from registration. All purchasers either received
adequate financial statement or non-financial statement
information about us or had adequate access, through their
relationship with us, to financial statement or non-financial
statement information about us. The sale of these securities was
made without general solicitation or advertising.
The issuance of securities described above in paragraphs 16
and 19 were exempt from registration under the Securities Act of
1933, as amended, in reliance on Rule 701 of the Securities
Act of 1933, as amended, pursuant to compensatory benefit plans
or agreements approved by the Registrant’s board of
directors.
All certificates representing the securities issued in these
transactions described in this Item 15 included appropriate
legends setting forth that the securities had not been offered
or sold pursuant to a registration statement and describing the
applicable restrictions on transfer of the securities. There
were no underwriters employed in connection with any of the
transactions set forth in this Item 15.
II-3
|
|
|
ITEM 16.
|
EXHIBITS AND
FINANCIAL STATEMENT SCHEDULES
|
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description
|
|
|
|
|
1
|
.1*
|
|
Form of Underwriting Agreement
|
|
|
3
|
.1#
|
|
Amended and Restated Certificate of Incorporation of the
Registrant, as currently in effect
|
|
|
3
|
.2*
|
|
Form of Second Amended and Restated Certificate of Incorporation
of the Registrant, to be in effect upon completion of the
offering
|
|
|
3
|
.3#
|
|
Bylaws of the Registrant, as currently in effect
|
|
|
3
|
.4*
|
|
Form of Amended and Restated Bylaws of the Registrant, to be in
effect upon completion of the offering
|
|
|
4
|
.1*
|
|
Form of the Registrant’s Common Stock Certificate
|
|
|
4
|
.2#
|
|
Investors’ Rights Agreement, dated January 13, 2011
|
|
|
4
|
.3#
|
|
Voting Agreement, dated January 13, 2011
|
|
|
4
|
.4#
|
|
Right of First Refusal and Co-Sale Agreement, dated
January 13, 2011
|
|
|
4
|
.5#
|
|
Warrant to purchase shares of Common Stock issued to Access
Shipping Limited Partnership, dated January 13, 2011
|
|
|
4
|
.6#
|
|
Warrant to purchase shares of Common Stock issued to Camulos
BioEnergy Partners, LLC, dated January 13, 2011
|
|
|
4
|
.7#
|
|
Warrant to purchase shares of Common Stock issued to NGP Capital
Resources Company, dated January 13, 2011
|
|
|
4
|
.8#
|
|
Warrant to purchase shares of Common Stock issued to Itera
Ethanol, LLC, dated January 13, 2011
|
|
|
4
|
.9#
|
|
Warrant to purchase shares of Common Stock issued to Specialty
Chemicals, LLC, dated January 13, 2011
|
|
|
5
|
.1*
|
|
Opinion of McDermott Will & Emery LLP
|
|
|
10
|
.1#
|
|
Assistance Agreement by and between BioEnergy International, LLC
and the United States of America Department of Energy, effective
January 28, 2010
|
|
|
10
|
.2†#
|
|
Alliance Agreement by and between the Registrant and Uhde
Corporation of America, dated September 18, 2009
|
|
|
10
|
.3†#
|
|
Global Alliance Agreement by and between the Registrant and Uhde
GmbH, dated October 6, 2009
|
|
|
10
|
.4†#
|
|
Supply Contract by and between the Registrant and Johann
Haltermann Ltd., dated June 15, 2010
|
|
|
10
|
.5†#
|
|
Supply Contract by and between the Registrant and The Chemical
Company, dated May 18, 2010
|
|
|
10
|
.6†#
|
|
Supply Contract by and between the Registrant and Piedmont
Chemical Industries I, LLC, dated January 18, 2010
|
|
|
10
|
.7†#
|
|
Supply Contract by and between the Registrant and Wilson
Industrial Sales Company, Inc., dated April 12, 2011
|
|
|
10
|
.8†#
|
|
Amended and Restated License Agreement by and between BioEnergy
International, LLC and PURAC Biochem BV, dated May 19, 2008
|
|
|
10
|
.9#
|
|
South East Asia Joint Venture Term Sheet by and between the
Registrant and PTT Chemical Public Company Limited, dated
January 13, 2011
|
|
|
10
|
.10#
|
|
Consulting Agreement by and between the Registrant and Arro
Building Services, dated August 31, 2010
|
|
|
10
|
.11#
|
|
Consulting Agreement by and between the Registrant and Clear
Creek Capital, LLC, dated October 14, 2010
|
|
|
10
|
.12*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and Stephen J. Gatto, dated July 22, 2011
|
|
|
10
|
.13*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and A. Cenan Ozmeral, dated July 22, 2011
|
II-4
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description
|
|
|
|
|
10
|
.14*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and Samuel McConnell, dated July 22, 2011
|
|
|
10
|
.15*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and Ralph A. Tapia, dated July 22, 2011
|
|
|
10
|
.16*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and Jeffrey Gatto, dated July 22, 2011
|
|
|
10
|
.17#
|
|
Lease Agreement by and between BioEnergy International, LLC and
Two Batterymarch Park LLC/NFPA, dated September 26, 2007
|
|
|
10
|
.18#
|
|
Lease Agreement by and between BioEnergy International, LLC and
Cummings Properties LLC, dated September 14, 2007, as
amended December 11, 2007, February 11, 2008,
May 20, 2008, September 19, 2008, September 25,
2008 and April 16, 2009
|
|
|
10
|
.19#
|
|
Lease by and between Myriant Lake Providence Inc. and Lake
Providence Port Commission, dated June 16, 2011
|
|
|
10
|
.20
|
|
2011 Incentive Plan of the Registrant
|
|
|
10
|
.21#
|
|
Form of Restricted Stock Unit Award Notification and Agreement
under the 2011 Omnibus Incentive Plan
|
|
|
10
|
.22#
|
|
Form of Stock Option Grant Notification and Agreement under the
2011 Omnibus Incentive Plan
|
|
|
10
|
.23#
|
|
Form of Indemnification Agreement between the Registrant and its
directors and officers (as entered into by Stephen J. Gatto,
Steven M. Sisselman, Keith Carter, Puntip Oungpasuk, Narongsak
Jivakanun and Thitipong Jurapornsiridee)
|
|
|
10
|
.24†#
|
|
Standard Exclusive License Agreement with Sublicensing Terms,
Agreement No. A5029 by and between BioEnergy International,
LLC and the University of Florida Research Foundation, Inc.
dated February 2, 2006, as amended October 6, 2006,
September 22, 2008, June 8, 2009 and April 26,
2010
|
|
|
10
|
.24.1†#
|
|
Fifth Amendment to License Agreement No. A5029 by and
between Myriant Technologies Inc. and the University of Florida
Research Foundation, Inc. dated May 31, 2011
|
|
|
10
|
.25†#
|
|
Standard Exclusive License Agreement with Sublicensing Terms,
Agreement No. A6126 by and between BioEnergy International,
LLC and the University of Florida Research Foundation, Inc.
dated November 30, 2007, as amended April 26, 2010,
September 15, 2010 and May 20, 2011
|
|
|
10
|
.25.1†#
|
|
Fourth Amendment to License Agreement No. A6126 by and
between Myriant Technologies Inc. and the University of Florida
Research Foundation, Inc. dated May 31, 2011
|
|
|
10
|
.26†#
|
|
Standard Exclusive License Agreement with Sublicensing Terms,
Agreement No. A4779 by and between BioEnergy International,
LLC and the University of Florida Research Foundation, Inc.
dated October 3, 2005
|
|
|
10
|
.26.1†#
|
|
First Amendment to License Agreement No. A4779 by and
between Myriant Technologies Inc. and the University of Florida
Research Foundation, Inc. dated May 31, 2011
|
|
|
10
|
.27†#
|
|
Standard Exclusive License Agreement with Sublicensing Terms,
Agreement No. A4780 by and between BioEnergy International,
LLC and the University of Florida Research Foundation, Inc.
dated October 3, 2005
|
|
|
10
|
.27.1†#
|
|
First Amendment to License Agreement No. A4780 by and
between Myriant Technologies Inc. and the University of Florida
Research Foundation, Inc. dated May 31, 2011
|
|
|
10
|
.28†#
|
|
Standard Exclusive License Agreement, Agreement No. A10176
by and between Myriant Technologies Inc. and the University of
Florida Research Foundation, Inc. dated June 15, 2011
|
|
|
10
|
.29†#
|
|
Standard Exclusive License Agreement, Agreement No. A10550
by and between Myriant Technologies Inc. and the University of
Florida Research Foundation, Inc. dated June 15, 2011
|
|
|
10
|
.30#
|
|
Transfer, Assignment and Assumption Agreement by and between
BioEnergy International, LLC and Myriant Technologies LLC, dated
July 16, 2009
|
|
|
10
|
.31#
|
|
Form of Scientific Advisory Panel Agreement
|
|
|
10
|
.32#
|
|
Form of Strategic Advisory Panel Agreement
|
|
|
10
|
.33#
|
|
Debt Conversion Agreement by and among Myriant Technologies,
Inc. and the parties named therein, dated January 13, 2011
|
II-5
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description
|
|
|
|
|
10
|
.34†#
|
|
Services Agreement, Agreement Number MYSAB-001, by and between
Myriant Technologies, LLC and Vogelbusch USA dated
March 17, 2010
|
|
|
10
|
.35#
|
|
Toll Manufacturing Agreement by and between BioEnergy
International, LLC and Fermic Sa de CV, dated May 18, 2009,
as amended December 18, 2009
|
|
|
10
|
.36#
|
|
Summary of Terms of Stephen J. Gatto Consulting Arrangement
|
|
|
10
|
.37#
|
|
Independent Contractor Agreement (Individual) by and between
Myriant Technologies LLC and Jeffrey Gatto, dated July 13,
2010
|
|
|
10
|
.38#
|
|
Administrative Services, Construction Management, Operations and
Maintenance Subcontract by and between BioEnergy Management
Services, LLC and Myriant Technologies LLC, dated July 16,
2009
|
|
|
10
|
.39*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and Rudy E. Fogleman, Jr., dated July 22, 2011
|
|
|
21
|
.1#
|
|
List of Subsidiaries
|
|
|
23
|
.1
|
|
Consent of McGladrey & Pullen, LLP
|
|
|
23
|
.2*
|
|
Consent of McDermott Will & Emery LLP (included in
Exhibit 5.1)
|
|
|
24
|
.1#
|
|
Power of Attorney (contained on signature page)
|
|
|
|
|
* |
|
To be filed by amendment. |
| |
|
† |
|
Certain portions have been omitted pursuant to a confidential
treatment request. Omitted information has been filed separately
with the SEC. |
| |
|
# |
|
Previously filed. |
Schedules not listed above have been omitted because the
information required to be set forth therein is not applicable
or is shown in the financial statements or notes thereto.
Insofar as indemnification for liabilities arising under the
Securities Act of 1933, as amended, may be permitted to
directors, officers and controlling persons of the Registrant
pursuant to the foregoing provisions, or otherwise, the
Registrant has been advised that in the opinion of the
Securities and Exchange Commission such indemnification is
against public policy as expressed in the Securities Act of
1933, as amended, and is, therefore, unenforceable. In the event
that a claim for indemnification against such liabilities (other
than the payment by the Registrant of expenses incurred or paid
by a director, officer or controlling person of the Registrant
in the successful defense of any action, suit or proceeding) is
asserted by such director, officer or controlling person in
connection with the securities being registered, the Registrant
will, unless in the opinion of its counsel the matter has been
settled by controlling precedent, submit to a court of
appropriate jurisdiction the question whether such
indemnification by it is against public policy as expressed in
the Securities Act of 1933, as amended, and will be governed by
the final adjudication of such issue.
The Registrant hereby undertakes that:
(a) The Registrant will provide to the underwriters at the
closing as specified in the underwriting agreement, certificates
in such denominations and registered in such names as required
by the underwriters to permit prompt delivery to each purchaser.
II-6
(b) For purposes of determining any liability under the
Securities Act of 1933, as amended, the information omitted from
a form of prospectus filed as part of this registration
statement in reliance upon Rule 430A and contained in the
form of prospectus filed by the Registrant pursuant to
Rule 424(b)(1) or (4) or 497(h) under the Securities
Act of 1933, as amended, shall be deemed to be part of this
registration statement as of the time it was declared effective.
(c) For the purpose of determining any liability under the
Securities Act of 1933, as amended, each post-effective
amendment that contains a form of prospectus shall be deemed to
be a new registration statement relating to the securities
offered therein, and the offering of such securities at that
time shall be deemed to be the initial bona fide offering
thereof.
II-7
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, the Registrant has duly caused this Amendment
No. 2 to the Registration Statement on
Form S-1
to be signed on its behalf by the undersigned, thereunto duly
authorized, in the City of Boston, The Commonwealth of
Massachusetts, on the 22nd day of July, 2011.
MYRIANT CORPORATION
Stephen J. Gatto
Chief Executive Officer
Pursuant to the requirements of the Securities Act of 1933, as
amended, this Amendment No. 2 to the Registration Statement
has been signed by the following persons in the capacities and
on the dates indicated.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
/s/ Stephen
J. Gatto
Stephen
J. Gatto
|
|
Chief Executive Officer (Principal Executive Officer) and
Chairman of the Board of Directors
|
|
July 22, 2011
|
|
|
|
|
|
|
|
/s/ Ralph
A. Tapia
Ralph
A. Tapia
|
|
Executive Vice President and Chief Financial Officer (Principal
Financial and Accounting Officer)
|
|
July 22, 2011
|
|
|
|
|
|
|
|
*
Puntip
Oungpasuk
|
|
Director
|
|
July 22, 2011
|
|
|
|
|
|
|
|
*
Narongsak
Jivakanun
|
|
Director
|
|
July 22, 2011
|
|
|
|
|
|
|
|
*
Thitipong
Jurapornsiridee
|
|
Director
|
|
July 22, 2011
|
|
|
|
|
|
|
|
*
Steven
M. Sisselman
|
|
Director
|
|
July 22, 2011
|
|
|
|
|
|
|
|
*
Keith
Carter
|
|
Director
|
|
July 22, 2011
|
|
|
|
|
|
|
|
|
|
*
|
|
/s/ Stephen
J. Gatto
Stephen
J. Gatto,
Attorney-in-Fact
|
|
|
|
|
II-8
EXHIBIT INDEX
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description
|
|
|
|
|
1
|
.1*
|
|
Form of Underwriting Agreement
|
|
|
3
|
.1#
|
|
Amended and Restated Certificate of Incorporation of the
Registrant, as currently in effect
|
|
|
3
|
.2*
|
|
Form of Second Amended and Restated Certificate of Incorporation
of the Registrant, to be in effect upon completion of the
offering
|
|
|
3
|
.3#
|
|
Bylaws of the Registrant, as currently in effect
|
|
|
3
|
.4*
|
|
Form of Amended and Restated Bylaws of the Registrant, to be in
effect upon completion of the offering
|
|
|
4
|
.1*
|
|
Form of the Registrant’s Common Stock Certificate
|
|
|
4
|
.2#
|
|
Investors’ Rights Agreement, dated January 13, 2011
|
|
|
4
|
.3#
|
|
Voting Agreement, dated January 13, 2011
|
|
|
4
|
.4#
|
|
Right of First Refusal and Co-Sale Agreement, dated
January 13, 2011
|
|
|
4
|
.5#
|
|
Warrant to purchase shares of Common Stock issued to Access
Shipping Limited Partnership, dated January 13, 2011
|
|
|
4
|
.6#
|
|
Warrant to purchase shares of Common Stock issued to Camulos
BioEnergy Partners, LLC, dated January 13, 2011
|
|
|
4
|
.7#
|
|
Warrant to purchase shares of Common Stock issued to NGP Capital
Resources Company, dated January 13, 2011
|
|
|
4
|
.8#
|
|
Warrant to purchase shares of Common Stock issued to Itera
Ethanol, LLC, dated January 13, 2011
|
|
|
4
|
.9#
|
|
Warrant to purchase shares of Common Stock issued to Specialty
Chemicals, LLC, dated January 13, 2011
|
|
|
5
|
.1*
|
|
Opinion of McDermott Will & Emery LLP
|
|
|
10
|
.1#
|
|
Assistance Agreement by and between BioEnergy International, LLC
and the United States of America Department of Energy, effective
January 28, 2010
|
|
|
10
|
.2†#
|
|
Alliance Agreement by and between the Registrant and Uhde
Corporation of America, dated September 18, 2009
|
|
|
10
|
.3†#
|
|
Global Alliance Agreement by and between the Registrant and Uhde
GmbH, dated October 6, 2009
|
|
|
10
|
.4†#
|
|
Supply Contract by and between the Registrant and Johann
Haltermann Ltd., dated June 15, 2010
|
|
|
10
|
.5†#
|
|
Supply Contract by and between the Registrant and The Chemical
Company, dated May 18, 2010
|
|
|
10
|
.6†#
|
|
Supply Contract by and between the Registrant and Piedmont
Chemical Industries I, LLC, dated January 18, 2010
|
|
|
10
|
.7†#
|
|
Supply Contract by and between the Registrant and Wilson
Industrial Sales Company, Inc., dated April 12, 2011
|
|
|
10
|
.8†#
|
|
Amended and Restated License Agreement by and between BioEnergy
International, LLC and PURAC Biochem BV, dated May 19, 2008
|
|
|
10
|
.9#
|
|
South East Asia Joint Venture Term Sheet by and between the
Registrant and PTT Chemical Public Company Limited, dated
January 13, 2011
|
|
|
10
|
.10#
|
|
Consulting Agreement by and between the Registrant and Arro
Building Services, dated August 31, 2010
|
|
|
10
|
.11#
|
|
Consulting Agreement by and between the Registrant and Clear
Creek Capital, LLC, dated October 14, 2010
|
|
|
10
|
.12*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and Stephen J. Gatto, dated July 22, 2011
|
|
|
10
|
.13*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and A. Cenan Ozmeral, dated July 22, 2011
|
|
|
10
|
.14*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and Samuel McConnell, dated July 22, 2011
|
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description
|
|
|
|
|
10
|
.15*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and Ralph A. Tapia, dated July 22, 2011
|
|
|
10
|
.16*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and Jeffrey Gatto, dated July 22, 2011
|
|
|
10
|
.17#
|
|
Lease Agreement by and between BioEnergy International, LLC and
Two Batterymarch Park LLC/NFPA, dated September 26, 2007
|
|
|
10
|
.18#
|
|
Lease Agreement by and between BioEnergy International, LLC and
Cummings Properties LLC, dated September 14, 2007, as
amended December 11, 2007, February 11, 2008,
May 20, 2008, September 19, 2008, September 25,
2008 and April 16, 2009
|
|
|
10
|
.19#
|
|
Lease by and between Myriant Lake Providence Inc. and Lake
Providence Port Commission, dated June 16, 2011
|
|
|
10
|
.20
|
|
2011 Omnibus Incentive Plan of the Registrant
|
|
|
10
|
.21#
|
|
Form of Restricted Stock Unit Award Notification and Agreement
under the 2011 Omnibus Incentive Plan
|
|
|
10
|
.22#
|
|
Form of Stock Option Grant Notification and Agreement under the
2011 Omnibus Incentive Plan
|
|
|
10
|
.23#
|
|
Form of Indemnification Agreement between the Registrant and its
directors and officers (as entered into by Stephen J. Gatto,
Steven M. Sisselman, Keith Carter, Puntip Oungpasuk, Narongsak
Jivakanun and Thitipong Jurapornsiridee)
|
|
|
10
|
.24†#
|
|
Standard Exclusive License Agreement with Sublicensing Terms,
Agreement No. A5029 by and between BioEnergy International,
LLC and the University of Florida Research Foundation, Inc.
dated February 2, 2006, as amended October 6, 2006,
September 22, 2008, June 8, 2009 and April 26,
2010
|
|
|
10
|
.24.1†#
|
|
Fifth Amendment to License Agreement No. A5029 by and
between Myriant Technologies Inc. and the University of Florida
Research Foundation, Inc. dated May 31, 2011
|
|
|
10
|
.25†#
|
|
Standard Exclusive License Agreement with Sublicensing Terms,
Agreement No. A6126 by and between BioEnergy International,
LLC and the University of Florida Research Foundation, Inc.
dated November 30, 2007, as amended April 26, 2010,
September 15, 2010 and May 20, 2011
|
|
|
10
|
.25.1†#
|
|
Fourth Amendment to License Agreement No. A6126 by and
between Myriant Technologies Inc. and the University of Florida
Research Foundation, Inc. dated May 31, 2011
|
|
|
10
|
.26†#
|
|
Standard Exclusive License Agreement with Sublicensing Terms,
Agreement No. A4779 by and between BioEnergy International,
LLC and the University of Florida Research Foundation, Inc.
dated October 3, 2005
|
|
|
10
|
.26.1†#
|
|
First Amendment to License Agreement No. A4779 by and
between Myriant Technologies Inc. and the University of Florida
Research Foundation, Inc. dated May 31, 2011
|
|
|
10
|
.27†#
|
|
Standard Exclusive License Agreement with Sublicensing Terms,
Agreement No. A4780 by and between BioEnergy International,
LLC and the University of Florida Research Foundation, Inc.
dated October 3, 2005
|
|
|
10
|
.27.1†#
|
|
First Amendment to License Agreement No. A4780 by and
between Myriant Technologies Inc. and the University of Florida
Research Foundation, Inc. dated May 31, 2011
|
|
|
10
|
.28†#
|
|
Standard Exclusive License Agreement, Agreement No. A10176
by and between Myriant Technologies Inc. and the University of
Florida Research Foundation, Inc. dated June 15, 2011
|
|
|
10
|
.29†#
|
|
Standard Exclusive License Agreement, Agreement No. A10550
by and between Myriant Technologies Inc. and the University of
Florida Research Foundation, Inc. dated June 15, 2011
|
|
|
10
|
.30#
|
|
Transfer, Assignment and Assumption Agreement by and between
BioEnergy International, LLC and Myriant Technologies LLC, dated
July 16, 2009
|
|
|
10
|
.31#
|
|
Form of Scientific Advisory Panel Agreement
|
|
|
10
|
.32#
|
|
Form of Strategic Advisory Panel Agreement
|
|
|
10
|
.33#
|
|
Debt Conversion Agreement by and among Myriant Technologies,
Inc. and the parties named therein, dated January 13, 2011
|
|
|
10
|
.34†#
|
|
Services Agreement, Agreement Number MYSAB-001, by and between
Myriant Technologies, LLC and Vogelbusch USA dated
March 17, 2010
|
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description
|
|
|
|
|
10
|
.35#
|
|
Toll Manufacturing Agreement by and between BioEnergy
International, LLC and Fermic SA de CV, dated May 18, 2009,
as amended December 18, 2009
|
|
|
10
|
.36#
|
|
Summary of Terms of Stephen J. Gatto Consulting Arrangement
|
|
|
10
|
.37#
|
|
Independent Contractor Agreement (Individual) by and between
Myriant Technologies LLC and Jeffrey Gatto, dated July 13,
2010
|
|
|
10
|
.38#
|
|
Administrative Services, Construction Management, Operations and
Maintenance Subcontract by and between BioEnergy Management
Services, LLC and Myriant Technologies LLC, dated July 16,
2009
|
|
|
10
|
.39*
|
|
Amended and Restated Employment Agreement by and between the
Registrant and Rudy E. Fogleman, Jr., dated July 22, 2011
|
|
|
21
|
.1#
|
|
List of Subsidiaries
|
|
|
23
|
.1
|
|
Consent of McGladrey & Pullen, LLP
|
|
|
23
|
.2*
|
|
Consent of McDermott Will & Emery LLP (included in
Exhibit 5.1)
|
|
|
24
|
.1#
|
|
Power of Attorney (contained on signature page)
|
|
|
|
|
* |
|
To be filed by amendment. |
| |
|
† |
|
Certain portions have been omitted pursuant to a confidential
treatment request. Omitted information has been filed separately
with the SEC. |
| |
|
# |
|
Previously filed. |