Attached files
 Annual
Shareholders Meeting Company Update
14 July 2011
Exhibit 99.2 |
 Forward Looking
Statement Statements made in this presentation may be forward-looking statements
within the meaning of Federal Securities laws that are subject to certain risks and
uncertainties and involve factors that may cause actual results to differ materially
from those projected or suggested. Factors that could cause actual results to differ
materially from those in forward-looking statements include, but are not limited
to: (i) the ability of Marina Biotech to obtain
additional
funding;
(ii)
the
ability
of
Marina
Biotech
to
attract
and/or
maintain
manufacturing, research, development and commercialization partners; (iii) the ability of
Marina
Biotech
and/or
a
partner
to
successfully
complete
product
research
and
development, including preclinical and clinical studies and commercialization; (iv) the
ability of Marina Biotech and/or a partner to obtain required governmental approvals;
and (v) the ability of Marina Biotech and/or a partner to develop and commercialize
products that can compete favorably with those of competitors. Additional factors
that could cause actual results to differ materially from those projected or
suggested in any forward-looking statements are contained in Marina
Biotech’s most recent periodic reports on Form 10-K and Form 10-Q which
are filed with the Securities and Exchange Commission. Marina Biotech assumes no
obligation to update and supplement forward-looking statements because of
subsequent events. 2 |
 Broad Nucleic
Acid-Based Platform Provides a Superior Drug Discovery Engine
Appropriate
therapeutic
approach
to
targets
not
typically
suited
to
small
molecule or monoclonal antibody therapeutics
Capitalizes on distinct endogenous intracellular processes for protein
down-regulation
Allows for a scientific and risk adjusted pursuit of a specific nucleic acid-
based therapeutic modality
Established regulatory path through FDA and EMA
Multiple nucleic acid-based therapeutics are in clinical development
Two such therapeutics have been approved
3 |
 4
Pfizer and Nucleic Acid-based Therapeutics
Tacere
shRNA
(TT-033): Three separate, expressed RNAi elements targeting the
conserved regions of the Hepatitis C virus entrapped in an AAV protein coat
$145 MM deal (upfront and milestone payments have been made through Feb
2010)
Quark
siRNA
(PF-04523655): Chemically-modified, unformulated siRNA compound
targeting proprietary target, RTP-801, for the treatment of wet age-related
macular degeneration (wet-AMD) and diabetic macular edema (DME)
Terms
not
disclosed
but
Pfizer
upped
milestone
payments
in
March
2011
to
support extended Phase II trials
Santaris
:
LNA
chemistry
for
single-stranded
RNA-targeted
therapies
$17 MM upfront (included $10 MM in equity) in 2010; $14 MM in 2011 and up
to $600 MM in downstream milestones
ssRNA |
 Nucleic Acid
Therapeutic “Toolbox” Constructs
Dicer length siRNA
RISC length siRNA
microRNA mimetics
microRNA antagonists
miniRNAs
Single-stranded RNA/DNA
5
Chemistries
UNA –
Non-nucleotide acyclic
monomers –
greater flexibility
CRN –
nucleotide analogs with a
linker connecting the C2’
and C4’
Delivery
DiLA
2
–
Combinations of amino acid head-groups, spacers, and
alkyl chains (tails)
SMARTICLES –
Combinations of anionic and cationic lipids
E. Coli –
Non-pathogenic bacteria engineered to produce, deliver
and release interfering RNA mediators (shRNA) to targeted tissue
Conjugation –
Peptides, etc. (phage display library) |
 Critical Needs
for a Broad Nucleic Acid- Based Therapeutics Discovery Engine
Multiple
chemistries to construct single and double-stranded
oligonucleotides with suitable affinity, stability and minimization of off-
target activity
Unlocked Nucleotide Analogs (UNA)
Conformationally Restricted Nucleotides (CRN)
With
multiple
delivery
approaches
Multiple carriers ranging from saline to bacteria to liposomal
(DiLA
2
/SMARTICLES
®
)
Multiple routes of administration (oral, local and systemic)
Leading to
multiple nucleic acid-based therapeutic approaches
Double-stranded RNAi-based therapeutics
Single-stranded oligonucleotide therapeutics
MicroRNA-based therapeutics
6 |
 RNAi
RDNA+
/chi
Marina Biotech’s Nucleic Acid-based
Therapeutic Platform
7
“undruggable target”
for a specific
indication
ssRNA/DNA
dsRNA
antagomir
mimetic
CRN/RNA
+ delivery
CRN/RNA
UNA/CRN
+ delivery
CRN/RNA
+ delivery
CRN/RNA
Plasmid +
bacteria
UNA +
delivery
Molecular Target
Local/Systemic
Oral
mRNA
microRNA
tk
tau
muRNA/muRNA+
RNAi
RDNA
RNA
RNA+
chi |
 /mu
mu
Selection of a Nucleic Acid-based
Therapeutic Approach
Target, indication and patient phenotype determine the most appropriate
combination of technologies and thus the specific nucleic acid-based
modality(ies) to pursue in preclinical proof-of-concept
Determine target and intended biology
Specific mRNA(s) to down-regulate a specific protein(s)
microRNA to up-/down-regulate multiple mRNAs/proteins
For down-regulation, use a mimetic
For up-regulation, use an antagomir
Select delivery approach based on construct and site (tissue/organ)
tkRNAi
tauRNAi
DNA+
–
delivery
of
ssDNA/RNA
A
When appropriate, multiple technologies can be evaluated to determine
the best approach
8
–
delivery
of
shRNA
to
mucosal
epithelium
RNAi
–
delivery
of
dsRNA
in
a
liposomal
formulation
RNAi
RDNA
RNA+
–
delivery
of
ssDNA/RNA
or
dsRNA
RNA
/mu
RDNA+
–
delivery
of
ssDNA/RNA
chi
/chi
mu |
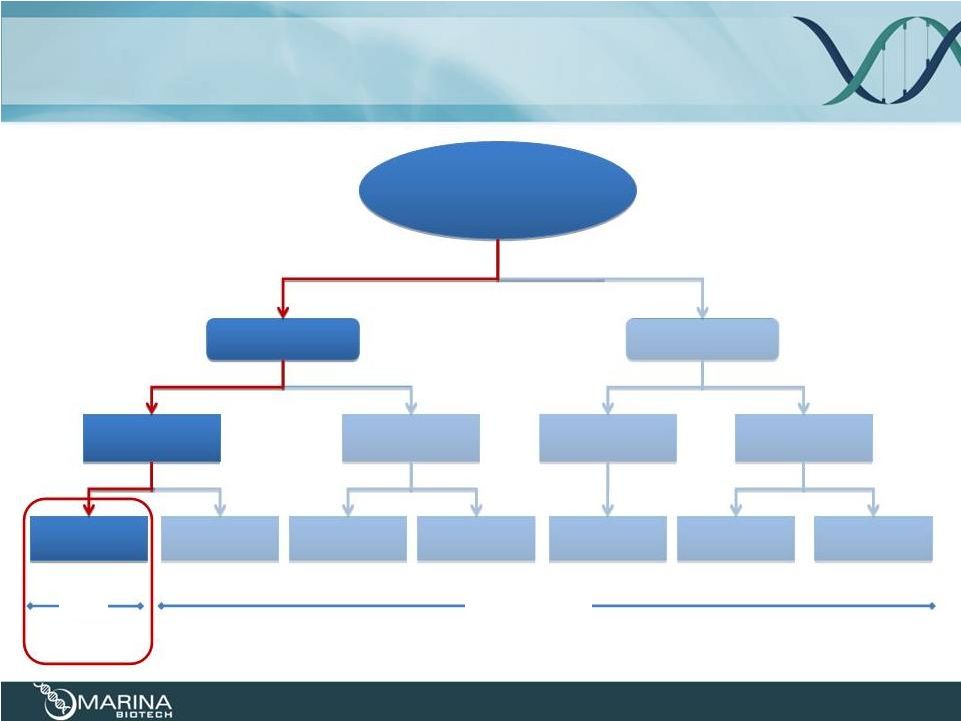 transKingdom
RNAi Platform
9
“undruggable target”
for a specific
indication
Molecular Target
mRNA
(protein)
ssDNA/RNA
dsRNA
antagomir
mimetic
microRNA
CRN-RNA
+ delivery
CRN-RNA
UNA/CRN
+ delivery
CRN-RNA
+ delivery
CRN/RNA
Plasmid +
bacteria
UNA +
delivery
Local/Systemic
Oral
tk
RNAi |
 About
transKingdom RNA interference (tkRNAi™)
10
Non-pathogenic bacteria engineered to produce, deliver and release
interfering RNA mediators (shRNA) to targeted tissue
Efficient delivery to the epithelium of the gastrointestinal tract via oral
administration
Safe
and
well
tolerated
daily
dosing
of
up
to
10
11
cfu
(colony
forming
units) in non-human primates for 9 months
Phase 1b/2a clinical trial to treat Familial Adenomatous Polyposis
(FAP) –
a genetic pre-cancerous polyposis disease with orphan drug
status
Exclusive worldwide license from Beth Israel Deaconess Medical
Center/Harvard |
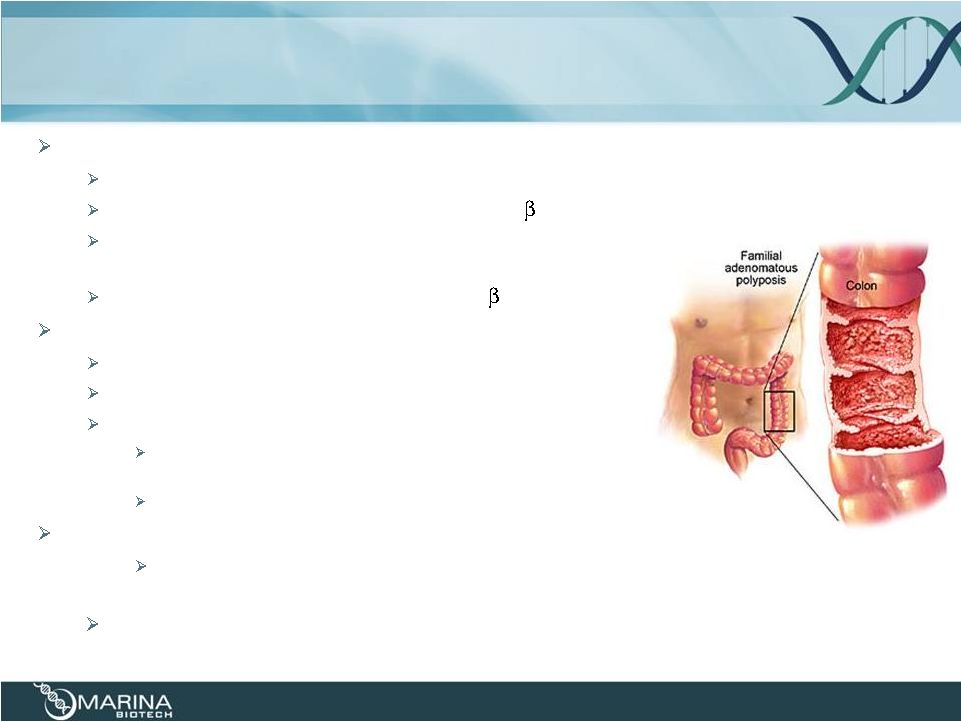 Familial
Adenomatous Polyposis (FAP) Rare hereditary disease
Mutation in Adenomatous Polyposis Coli (APC) gene
Causes
dysregulation
and
accumulation
of
-catenin
Results in numerous colon polyps appearing in early
adolescence with potential for rapid disease progression
Clinical
drug
product,
CEQ508,
targets
-catenin
oncogene
Unmet medical need
~80,000 worldwide (orphan status)
Near 100% risk of colon cancer if untreated
Treatment options:
Surgical intervention (colectomy) is the only available treatment
to prevent colon cancer progression
No generally accepted pharmaceutical approach is available
U.S. FAP Market Potential: >$100 MM
Opportunities to expand into sporadic CRC, other polyposis syndromes and other
GI cancers
Potential for 4Q2014 launch and first RNAi drug to market
11 |
 Actual and
Potential Clinical, Regulatory and Commercial Timelines
12
December 2009 –
IND granted in U.S.
September 2010 –
Patent grant in the EP protecting tkRNAi
December 2010 –
Received FDA Orphan Drug Designation
January 2011 –
Completed LTT study in Non-Human Primates
June 2011 –
Completed dosing in Cohort 1
1Q2012 –
Complete dose escalating phase of 1b/2a
Delayed dosing of Cohort 2 until 4Q2011
Remedying GMP-related issues not a safety issue
Informed the FDA of our decision to delay dosing
2Q2012 –
Complete stable dose phase of 1b/2a
4Q2013 –
Complete Pivotal Phase 2
3Q2014 –
Expedited/accelerated FDA review and approval
4Q2014 –
Launch –
first RNAi drug to market |
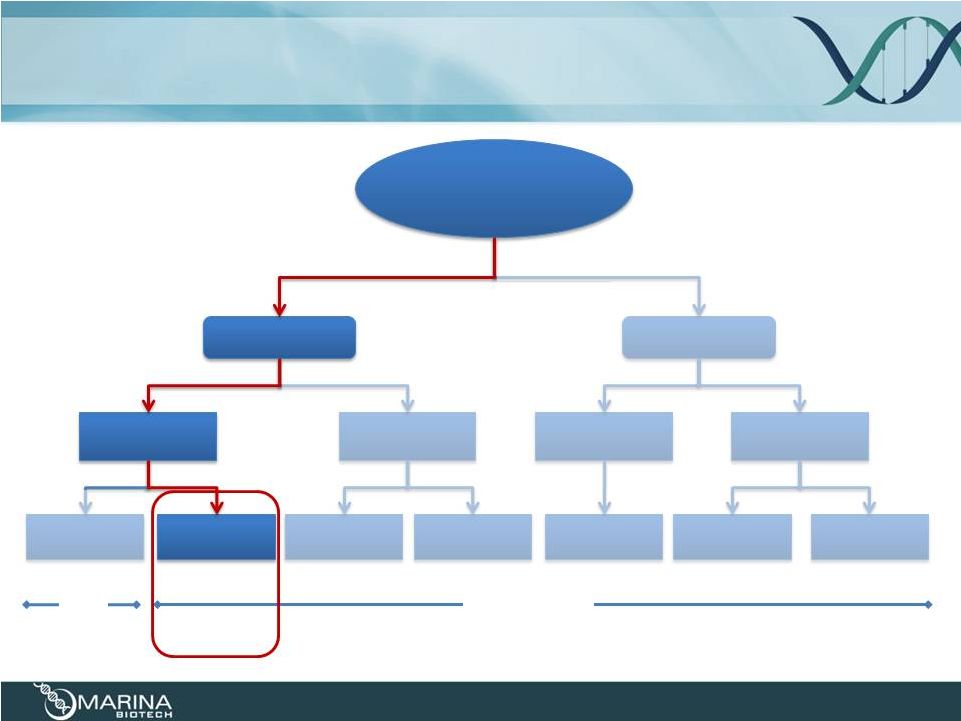 RNAi
Platform 13
“undruggable target”
for a specific
indication
ssDNA/RNA
dsRNA
mRNA
antagomir
mimetic
microRNA
CRN/RNA
+ delivery
CRN/RNA
UNA/CRN
+ delivery
CRN/RNA
+ delivery
CRN/RNA
Plasmid +
bacteria
UNA +
delivery
Molecular Target
RNAi
tau
Local/Systemic
Oral
tau |
 About
tauRNA interference (tauRNAi) 14
Combination of highly active UsiRNA constructs and liposomal
delivery systems
UsiRNA –
Substitution of unlocked nucleobase analogs (UNA) within
siRNA; therapeutic use proprietary to Marina Biotech
DiLA
-
Di-Alkylated
Amino
Acids
for
liposomal-based
delivery;
novel
compounds proprietary to Marina Biotech
Smarticles -
Lipid-based amphoteric liposomes for oligonucleotide delivery
(in Phase I Clinical Trial by ProNAi via systemic delivery)
Pairing of the UsiRNA with the most appropriate liposomal delivery
system provides greater efficacy and specificity for RNAi
2 |
 Publications
on UsiRNAs and Systemic and
Local
Delivery
with
DiLA
15
Mol Ther. 2011 Apr 19. (PMID:21505423)
An Amino Acid-based Amphoteric Liposomal Delivery System for Systemic
Administration of siRNA.
Adami RC, Seth S, Harvie P, Johns R, Fam R, Fosnaugh K, Zhu T, Farber K, McCutcheon
M, Goodman TT, Liu Y, Chen Y, Kwang E, Templin MV, Severson G, Brown T,
Vaish N, Chen F, Charmley P, Polisky B, Houston ME Jr. Marina Biotech, Inc.,
Bothell, Washington, USA. Nucleic Acids Res. 2011 Mar
1;39(5):1823-32. Improved specificity of gene silencing by siRNAs
containing unlocked nucleobase analogs. Vaish N, Chen F, Seth S, Fosnaugh K,
Liu Y, Adami R, Brown T, Chen Y, Harvie P, Johns R, Severson G, Granger B, Charmley P,
Houston M, Templin MV, Polisky B.
Marina Biotech Inc., 3830 Monte Villa Parkway, Bothell, WA 98021, USA
Mol Ther. 2011 Mar 1. (PMID: 21364537)
RNAi-based Therapeutics Targeting Survivin and PLK1 for Treatment of Bladder
Cancer. Seth
S,
Matsui
Y,
Fosnaugh
K,
Liu
Y,
Vaish
N,
Adami
R,
Harvie
P,
Johns
R,
Severson
G,
Brown
T,
Takagi
A,
Bell
S,
Chen
Y,
Chen
F,
Zhu T, Fam R, Maciagiewicz I, Kwang E, McCutcheon M, Farber K, Charmley P, Houston
Jr ME, So A, Templin MV, Polisky B. Discovery Research and Pharmaceutical
Development, Marina Biotech Inc., Bothell, Washington, USA 2
|
 Marina
Biotech/Debiopharm: Bladder Cancer R&D Collaboration
Exclusive agreement for the research, development and
commercialization of Marina Biotech’s pre-clinical program
in bladder cancer
First agreement to license an RNAi-based therapeutic,
delivered with a lipid-
or liposomal-based formulation, to a
pharma company
Debiopharm is responsible for development and
commercialization of any products
Debiopharm will pay:
All research and development costs for the program
Up to $24 million based on predefined research and development
milestones
Royalties on sales of products
16 |
 ssDNA/RNA
Technology 17
“undruggable target”
for a specific
indication
ssDNA/RNA
dsRNA
mRNA
antagomir
mimetic
microRNA
CRN/RNA
+ delivery
CRN/RNA
UNA/CRN
+ delivery
CRN/RNA
+ delivery
CRN/RNA
Plasmid +
bacteria
UNA +
delivery
RDNA/ RDNA+
Molecular Target
Local/Systemic
Oral
chi
chi |
 Conformationally Restricted
Nucleotides (CRN)
CRNs
are
nucleotide
analogs
with
a
linker
connecting
the
C2’
and
C4’
carbons of ribose
CRN and LNA lock the ribose ring into a stable conformation and increase
the hybridization affinity to the mRNA or microRNA target
Distinguished
from
LNA
by
a
longer
linker
and
different
location
of
the
oxygen atom
Places the oxygen in an optimal position for stability and affinity
RNA
CRN
LNA
18 |
 CRN-Substitution Results in Less
Ribose Ring Puckering
19
CRNs are distinguished by a linker whose length results in less ribose
ring puckering
Conformation of CRN more closely resembles that found in RNA
CRN
LNA
Puckering of the ribose
ring at the 2’
carbon |
 Less Ribose
Ring Puckering May Result in Lower Structural Distortion
20
CRN substitution within an oligo may result in lower structural distortion
when hybridized to a complementary mRNA
Figure Adapted from Eichert et al.,
Nucleic Acid res. 2010 Oct 1 38(19):6729-36
RNA/CRN
Distortion of
Duplex Structure
The rigid structure imparted by multiple
LNA
substitutions
“stretches”
the
duplex
CRNs have greater flexibility by virtue of
the longer linker and would be expected
to maintain an overall structure similar to
an all-RNA structure
Therefore CRN substituted single-
stranded RNA/DNA may have promising
drug-like properties |
 Therapeutic
Approaches Using CRN Technology
21
mRNA Translational Blockers
Substitute CRN at ends of DNA-RNA hybrid compounds thus preserving
DNA in the interior and allowing interaction with RNAse H
microRNA Antagonists
Substitute CRN throughout strand to maximize hybridization affinity
Additional modifications (e.g., 2’O-methyl) can be included within the
construct for increased affinity and greater nuclease stability
= CRN |
 Advantages of
CRN Technology 22
Increased affinity with CRN substitution maintains structural orientation
thus allowing for variable lengths
Standard 19-
to 22-mers
Greater number of substitutions in 14-
to 16-mers
Fully substituted 7-
to 9-mers targeting microRNA seed region
Delivery can be achieved with multiple formulations
Simple formulations (e.g., saline) can be used in many situations (liver,
lung, kidney, some tumors)
If
appropriate,
encapsulation
within
a
liposomal
formulation
for
systemic
or
local administration to certain tissues/organs |
 CRN-Related Therapeutic Modalities
Aptamers
Therapeutics
Diagnostics
Delivery/cell surface ligands
Exon skipping oligonucleotide
Minor/Major Groove Binders/Triplex forming oligonucleotides
Ribozymes
Splice junction inhibitors
Steric blockers
tRNA/tRNA suppressors
bridge (adapter molecule) between the mRNA and the protein
“Suppress”
the phenotypic effect of a coding mutation
23 |
 Other Uses for
CRN technology Primer/Probes for PCR
qRT-PCR
5’
and 3’
RACE
Single, multiplex and allele specific PCR
Capture Probes
In
situ
hybridization/Fluorescence
in
situ
hybridization
SNP genotyping
Expression analysis
Monitor/identify exon skipping
Monitor/identify splice variants
Research/Diagnostics agents
Aptamers
Bacterial strain/virus identification
Single cell miRNA analysis
24 |
 microRNA
(miRNA) Technology Local/Systemic
Oral
“undruggable target”
for a specific
indication
microRNA
mimetic
antagomir
UNA/CRN
+ delivery
CRN-RNA
+ delivery
CRN/RNA
mRNA
dsRNA
ssDNA/RNA
Plasmid +
bacteria
UNA +
delivery
CRN-RNA
+ Delivery
CRN-RNA
25 |
 microRNA
Mimetic Approach Molecular Target
Local/Systemic
Oral
“undruggable target”
for a specific
indication
microRNA
mimetic
antagomir
UNA/CRN
+ delivery
CRN-RNA
+ delivery
CRN-RNA
mRNA
dsRNA
ssDNA/RNA
Plasmid +
bacteria
UNA +
delivery
CRN-RNA
+ delivery
CRN-RNA
26
mu
RNA+ |
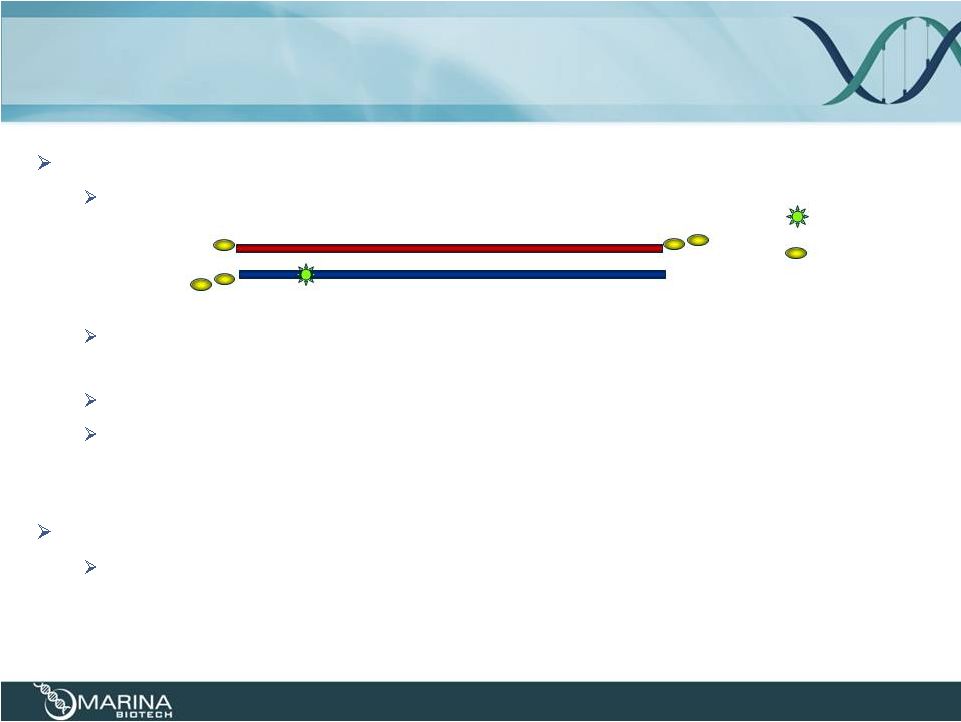 microRNA
Mimetic using UNA & CRN 27
MicroRNA mimetic -
dsRNA
Substitute with UNA and CRN
UNA –
increase nuclease resistance and decrease cytokine induction;
inhibition of passenger strand activity
CRN –
increase hybridization affinity; nuclease resistance
Additional modifications (e.g., 2’O-methyl) can be included within the
construct for nuclease stability
Duplex
construct
–
likely
to
require
delivery
for
most
indications
DiLA
2
-based
liposomes
or
Smarticles
(amphoteric
liposomes)
= CRN
= UNA |
 Delivery of a
miR-34 Mimetic in a Smarticles Formulation Inhibits Tumor Growth
Inhibition of Liver Cancer Biomarker
Alpha Feto Protein Levels
Inhibition of Tumor Growth
Tumor Weight (g)
miR-NC
miR-34
Untreat.
Cont. miR-NC miR-34
Smarticles
formulation
provided
effective
delivery
to
orthotopic
liver
tumors
Significant reduction in tumor growth and liver tumor specific biomarker
28 |
 Local/Systemic
Oral
Molecular Target
“undruggable target”
for a specific
indication
microRNA
mimetic
antagomir
UNA/CRN
+ delivery
CRN-RNA
+ delivery
CRN/RNA
mRNA
dsRNA
ssDNA/RNA
Plasmid +
bacteria
UNA +
delivery
CRN-RNA
+ delivery
CRN-RNA
29
mu
RNA/
mu
RNA+ |
 microRNA
Antagonist using CRN 30
MicroRNA antagonists (Antimir) therapeutics
Substitute CRN throughout the strand to maximize the hybridization affinity
between antimir and the target microRNA
Allows for use of shorter length sequences
Additional modifications (e.g., phosphorothioate and 2’O-methyl) can be
included within the construct for nuclease stability and increase affinity
Delivery formulation
Simple formulations (e.g., saline) can be used as the vehicle for and can
be used as the vehicle in many situations
Encapsulation within a liposomal formulation for systemic or local
administration may be required for some tissues/organs
= CRN |
 CRNs Increase
Antimir Activity CRN substitution increases the activity of an Antimir with >90%
inhibition of luciferase activity
No activity against a scrambled target sequence (not shown)
31
Dual luciferase assay; Average of triplicate; HeLa cells co-transfected with
psiCHECK2-miR21plasmid % Luciferase (Renilla) Activity Remaining
Construct
0%
20%
40%
60%
80%
100%
miR-21
Antimir-21
Unmodified
Antimir-21
(CRN)
Antimir-21
(LNA)
5 nM
25 nM
5 nM
25 nM
5 nM
25 nM
5 nM
25 nM |
 Antimir
Conc. (nM) CRN-substituted Antimir
LNA-substituted Antimir
Unmodified Antimir
CRN-substituted
Antimir
is
highly
potent,
with
an
IC
50
of
0.2
nM
Similar potency for CRN-
and LNA-substituted versions
50% Inhibition
Dual luciferase assay; HeLa cells co-transfected with psiCHECK2-miR21plasmid;
Unmodified Antimir all RNA; CRN-substituted Antimir has DNA and P=S
backbone CRNs Increase Antimir Potency
32 |
 Molecular
Target Local/Systemic
Oral
“undruggable target”
for a specific
indication
microRNA
mimetic
antagomir
UNA/CRN
+ delivery
CRN/RNA
+ delivery
CRN/RNA
mRNA
dsRNA
ssRNA/DNA
Plasmid +
bacteria
UNA +
delivery
CRN/RNA
+ delivery
CRN/RNA
Marina Biotech’s Nucleic Acid-based
Therapeutic Platform
33
tk
RNAi
tau
RNAi
chi
RDNA/
chi
RDNA+
mu
RNA+
mu
RNA/ |
 Resourcing a
Broad Discovery Engine –
Funded
by
Marina
Biotech
Includes funding CEQ508 Phase 1b/2a Trial
–
Funded by outside sources
siRNA in liposomal delivery for local administration funded by Debiopharm
(Marina Biotech UsiRNA and liposomal delivery)
–
Funded
by
Marina
Biotech
and
outside
sources
ssDNA in liposomal delivery for systemic administration funded by ProNAi (ProNAi DNA
decoy in Marina Biotech delivery)
ssRNA/DNA in saline/liposomal delivery for systemic administration funded by Marina
Biotech
–
Funded
by
Marina
Biotech
and
outside
sources
microRNA mimetic in liposomal delivery for systemic administration funded by
Mirna Therapeutics (Mirna mimetic in Marina Biotech delivery)
microRNA antagonists in saline for systemic administration funded by Marina
Biotech
34
tk
RNAi
tau
RNAi
chi
RDNA/
chi
RDNA+
mu
RNA/
mu
RNA+ |
 2010-2011
Achievements Initiated patient dosing in an FAP Phase 1b/2a Clinical Trial and
completed dosing the first cohort
Established first ever partnership around a liposomal delivered
RNAi compound with Debiopharm for the development and
commercialization a bladder cancer therapeutic
Established use of CRNs within single-stranded oligonucleotides
including microRNA antagonists
Established broadest single-company nucleic acid-based drug
discovery engine in the industry
Published three scientific manuscripts in peer-reviewed journals
describing UsiRNA platform, DiLA2 delivery technology and pre-
clinical data from the bladder cancer program
35 |
 Thank
You 36 |
