Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Heart Tronics, | ht8k-june232011.htm |
| EX-99.2 - PRESS RELEASE - Heart Tronics, | ex99-2.htm |
Exhibit 99.1

CORPORATE UPDATE
Heart Tronics, Inc. (“Heart Tronics”, “the company,” “we,” “us,” “our” and similar terms) is providing this update to provide some information to our shareholders and the markets relating to our company, pending such time as we can update our financial and otherwise bring our SEC filings current. This filing is not intended to address all matters that would ordinarily be included in any SEC filing, and we do not represent that it address, in full or in part, all matters that any shareholder may deem to be material.
|
Overview
|
Heart Tronics is a medical device company focused on researching, developing and marketing medical devices which monitor and measure physiological signals in order to detect diseases that impact an individual’s health. Physiological signals are small bioelectrical signals generated by the body.
Our initial product, the Heart Tronics Heart Monitoring System, is an FDA 510(k)-cleared heart monitoring system that uses our proprietary signal acquisition technology to acquire, amplify and process physiological signals associated with a patient’s cardiovascular system. Heart monitor systems are used in a variety of medical settings. For example, they are used to collect physiological data for electrocardiogram or “ECG” tests for the purpose of detecting and identifying cardiovascular disease, and also used to monitor the condition of the heart during surgical procedures.
Our Fidelity 100 Heart Monitoring System is a system of equipment (i.e., our Fidelity 100 Patient Module, electrodes and lead sets, and a computer used to collect, process, interpret and store signal data) used to monitor and record changes in physiological signals associated with a patient’s cardiovascular system. The Fidelity 100 Module, which is the core of our heart monitoring system, was originally designed, engineered, fabricated and tested by Battelle Memorial Institute, Health and Life Sciences (“Battelle Memorial Institute”). Battelle Memorial Institute is a global science and technology enterprise that designs, develops and commercializes technology and manages laboratories for customers. The Model 100 Module, as designed and engineered by Battelle Memorial Institute, has the capability to collect and transmit raw signal data for all ECG applications. We are currently in discussions with Battelle Memorial Institute to update our Fidelity 100 Monitoring System and our proprietary software system to account for recent developments, as well as their use in certain foreign countries, pending receipt of adequate working capital.
Our corporate offices are located at 14942 Gault Street, Van Nuys, California 91405. Our telephone number is (818) 782-2189. Our common stock (“common shares”) currently trade on the Pink Sheets under the symbol HRTT.
Since the second half of 2008, we have operated at minimal level due to a lack of financial resources attributable, in principal part, to our inability to raise working capital in light of a pending class action lawsuit. As part of such reduction in business activity, we have not marketed our principal product, the Fidelity 100 Heart Monitoring System, since 2008. The class action lawsuit has now been settled (see “Litigation Settlements” below), and Heart Tronics is now positioned (pending the receipt of adequate working capital) to ramp up operations, recommence sales and marketing activities, and bring our SEC filings current.
-1-
|
Products and Technology
|
Overview: Description Of Heart Monitor Systems And ECGs
A heart monitor system is a system of equipment (i.e., heart monitor, electrodes and lead sets, and signal processing and interpretive equipment) used to monitor and record changes in physiological signals associated with a patient’s cardiovascular system. The principal use of heart monitor systems is to collect physiological data for electrocardiogram or “ECG” tests for the purpose of detecting and identifying cardiovascular disease. Other uses include the monitoring of the heart during different medical procedures. An ECG gives the cardiologist important information about the heart. For example, by examining changes in waveforms from 0.67 Hz to 40 Hz frequency range, a cardiologist can identify irregularities in the heart’s rate and rhythm, known as arrhythmia, which constitutes approximately 5% of heart disease. By examining changes in waveforms in the broader 0.05 Hz to 150 Hz frequency range, a cardiologist can identify other types of heart disease, including damage to the heart muscles or tissue resulting from (1) decreased blood flow attributable to the narrowing of the arteries, known as cardiac ischemia, (2) enlargement of the heart resulting from additional effort attributable to the hardening of the heart muscle, known as hypertrophy, and (3) the existence of past or presently occurring heart attacks.
When an ECG test is ordinarily conducted in a resting setting, the physiological signals from the patient’s heart are displayed through a heart monitor system called a 12-lead ECG, based on acquiring a signal from ten electrodes, one of which is attached to each of the patient’s arms, six to the chest and one to each leg. The placement of the ten electrodes enables the heart to be examined for different diseases. Physiological signals generated by the heart are amplified and recorded in the form of a series of waveforms that can be displayed on a screen or printed on paper for interpretation by a cardiologist. Any irregularity in heart rhythm, damage or stress to the heart muscle will result in a deviation from a normal waveform.
There are three settings under which ECGs are normally taken: (1) the resting (or clinical) setting where the patient is immobile; (2) the stress setting where the patient’s heart is subjected to additional physical stress due to physical exertion tested in a controlled environment; and (3) the ambulatory setting where the patient’s heart is tested over an extended period of time while he or she is mobile and conducting his or her everyday activities.
ECGs administered in the resting setting are generally taken (1) on an annual or periodic basis for typically older patients as part of their annual or regular physical examination; (2) under emergency or exigent circumstances when an individual complains of symptoms typically associated with heart disease such as chest pains, shortness of breath or heart palpitations; or (3) as part of surgeries and medical procedures, such as heart surgery.
ECGs administered in the stress setting are given while the patient exercises, e.g., on a treadmill, step machine or exercise cycle to enable the cardiologist to monitor, among other things, the patient’s heart behavior under conditions of physical stress. Exercise can exacerbate cardiovascular abnormalities that are not present at rest and it can be used to determine the adequacy of cardiac function. Similar to an ambulatory ECG, this allows the cardiologist to identify different heart disease such as cardiac ischemia and cardiac hypertrophy as well as the existence of past or presently occurring heart attacks that may not be evident under a resting or simple ambulatory ECG test conditions. Indeed, many physicians administer a stress ECG after administering a resting ECG.
-2-
ECGs administered in the ambulatory setting are given in an attempt to identify so-called “paroxysmal” heart disease—that is, problems that come and go, and that are not apparent in the low-activity states where a standard resting ECG is typically taken. Examples of paroxysmal heart disease are cardiac ischemia and cardiac hypertrophy. Additionally, the existence of past or presently occurring heart attacks can escape detection without longer-term monitoring in a physically active or stressful setting.
Description Of Problems In Taking ECGs Using Current Technologies (Other Than Heart Tronics)
The accurate reproduction of the shape of the waveform is of vital importance insofar as the signal represented by the waveform represents the specific type of heart disease that the physician is attempting to diagnose. The principal technical issues in administering ECGs using current technology (other than that of Heart Tronics) are their inability to differentiate or discriminate (particularly in higher and lower frequency ranges) signals directly related to heart disease and signals attributable to ambient noise from the surrounding environment, including (1) signals generated by the other organs, muscles and systems of the body, whether from movement or the performance by those organs of their bodily functions (”physiological noise”), and (2) signals generated by sources external to the body, such as electronic equipment, lights or engines (“environmental noise”). This ambient noise, whether physiological or environmental in nature, is commonly referred to as an “artifact”.
As previously discussed, signals (and consequentially displayed waveforms) associated with the full spectrum of heart diseases range from 0.05 to 150 Hz, which is the frequency range recommended for diagnostic review by the American Heart Association, the American College of Cardiology, the Heart Rhythm Society, and the International Society For Computerized Electrocardiology. Although there is physiological and environmental noise present in the mid-frequency ranges (0.5 Hz to 40 Hz), the heart signal is nevertheless strong enough in these frequency levels to be generally discerned, albeit it remains distorted by ambient noise. In the lower (0.05 to 0.67 Hz) and upper (40 to 150 Hz) frequency ranges, however, the heart signal does not stand-out from the ambient noise, and it is therefore exceptionally difficult for current ECG technology to differentiate or discriminate the heart signals from artifact. As a consequence, since most heart diseases (with the principal exception of arrhythmia) demonstrate frequencies in these broader ranges, this portion of the signal in the upper and lower ranges is, as a practical matter, of no or little value, while the mid-frequency portion while discernable is nevertheless distorted by the artifact. This unsatisfactory situation not only makes diagnosis difficult, due to the poor quality of the available data, but can lead to misdiagnosis since the artifact may indicate the presence of another cardiac disease or a non-cardiac condition.
The clearest signals currently attainable by conventional ECG systems is in the resting setting in the 0.5 Hz to 40 Hz range. Here, the patient lie in the supine position while the ECG is being taken, remaining as still as possible to reduce ambient noise caused by physical movement. These ECGs are typically taken in areas that are shielded from environmental sources of artifact to further minimum distortion. Another method to reduce ambient noise is to reduce the sensitivity of the monitoring equipment, although this alternative results in a significant loss of signal quality, the ability to read certain signal intricacies and a decrease in the physicians’ ability to correctly diagnose heart disease due to established error in the ECG.
However, as discussed above, it is equally important to take ECGs in the stress setting in order to identify heart disease that is not evident in the resting setting, as well as ECGs taken with Holter monitors in the ambulatory setting during daily activities in order to identify paroxysmal heart disease. Unfortunately, the physical movements associated with both of these activities, as well as the inability to control artifact associated with environmental sources, generate significant artifact that further distort signal quality.
-3-
Manufacturers of conventional ECG devices have attempted to address the ambient noise issues, whether in a resting, stress or ambulatory setting, through sophisticated computer software processing. By way of example, one conventional ECG software methodology is to (1) filter out all signals, both heart and from physiological or environmental sources, in the lower 0.05 to 0.67 Hz and upper (40 to 150 Hz) frequency ranges, and (2) filter out “selected” signals deemed to represent artifact from the mid frequency ranges. The problem with filtering is that it either eliminates or omits vital data in cases where it filters out all original signals such as the case in the upper and lower frequency ranges, or alters original signal in the case of mid-frequency ranges. In such cases the physician ends up with incomplete or distorted data.
Another conventional ECG software methodology is to reconstruct the waveform by using various techniques, such as producing an “average” waveform. The problem with this approach is that the final waveform is one based upon mathematical assumptions and algorithms, which have limited utility as a general matter and ultimately result in the physician again ending up with incomplete or distorted data.
Given the software limitations in handling noise and the technical limitations of the algorithms used in processing signals, the American Heart Association and American College of Cardiology each state that computer processing is not completely reliable and advise cardiologists to look at the raw data and not to rely solely upon software-processed data.
Conventional ECG technology, including digital filtering and processing, merely compound the problem, insofar as they take distorted data to begin with, and then process the distorted data in a manner that does not accurate reproduce the original signal and, equally bad, omit information in the higher and lower frequency ranges, all of which further compound the issue. Simply put, a physician’s diagnosis is only as good as his data, and bad or misleading data can often result in misdiagnosis and the failure to provide proper treatment. Heart Tronics’s technology significantly mitigates, and in some cases totally eliminates, these problems.
Description Of ECG Quality Using Heart Tronics Technology
The solution afforded by Heart Tronics’s ECG technology is simple. Rather than acquiring distorted and incomplete signal data in the first place (i.e., the “front end”), and then attempting to make it accurate on the “back end”, our technology acquires complete and accurate signal data that is not affected by artifact on the front end, thereby avoiding the need to process and clean the data on the back end. Signal data acquired through our proprietary signal acquisition technology is highly specific (i.e., the heart signal is only minimally affected by ambient noise from physical movement or the surrounding environment), is highly sensitive (i.e., accurately reflects extremely small changes in the signal data), and faithfully reproduces the heart signals for diagnostic purposes. In addition, given the specificity of our signal acquisition technology, the entire 0.05 to 150 Hz frequency spectrum necessary to review in the opinion of the American Heart Association, the American College of Cardiology, the Heart Rhythm Society, and the International Society For Computerized Electrocardiology for the purpose of diagnosing the full spectrum of heart disease may be examined for diagnostic purposes. To our knowledge, no other competitor offers the ability to collect clean, undistorted and highly accurate signals, or the ability to acquire the entire 0.05 to 150 Hz frequency spectrum, as afforded by our proprietary signal acquisition technology. Indeed, no competitor to our knowledge claims any ability to procure specific, artifact-free signals. The abilities of our technology facilitates better diagnosis and treatment insofar as the physician has more specific, accurate and complete signal data. Ultimately, our proprietary signal acquisition technology will facilitate “greater diagnostic yield”, a medical term which means that the physician can more accurately and expeditiously diagnose the cardiac disease or condition, leading to better patient outcomes.
-4-
The ability of the Fidelity 100 to provide more specific, accurate and complete signal data, can be easily demonstrated and explained by the following graphic, which compares two ECG print-outs taken during a cardiac surgical procedure (seen in the background) performed at a major hospital.
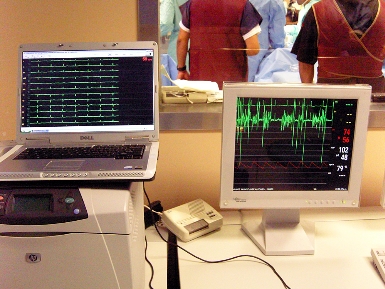
The readout on the left is from the Fidelity 100 Heart Monitor, while the read-out on the right is from a “state of the art” heart monitor manufactured by a large competitor. The Fidelity 100 readout shows the waveform of the normal or proper heart function from all eight leads. The read-out from the “state of the art” monitor, on the other hand, shows only one lead (on the top) which has any similarity whatsoever to a normal waveform. The data from the second lead is confusing and essentially meaningless, representing noise and artifacts present during the procedure, even though there is none indicated on the Heart Tronics read-out. The other leads show no data whatsoever. The significance of the foregoing is that not only does the Fidelity 100 monitor consistently give accurate signals from all leads in all cases, it also avoid “false positives” relating to inaccurate information. Specifically, since, as a practical matter, the meaning of the signal from the second lead on the “state of the art” monitor is meaningless, the physician can only “speculate” as to what is going on with the heart, and can potentially misdiagnose the condition of the heart.
-5-
As previously discussed, the taking of stress ECGs, which are necessary to identify cardiovascular abnormalities that only become evident under conditions of physical stress, are also highly problematic due to the high level of physiologically-based ambient noise created by the body while under stress. Moreover, as also previously discussed, the digital filtering and processing used to address this issue does not always provide reliable results, leading the American Heart Association and American College of Cardiology to advise cardiologists and other physicians to look at the raw data and not to rely solely upon software-processed data.
|
The ability of the Fidelity 100 to provide an accurate and consistent signal in an ambulatory setting notwithstanding the ambient noise can be easily demonstrated and explained by the graphic to the right. In this graphic, you can see a readout showing the waveform of the normal or proper heart function from all twelve leads while Willie Gault, the famous retired NFL wide receiver and past Olympic gold medal winner (110 meter hurdler and member of the USA world record-setting 4X100 meter relay team, and present track and field world record holder in innumerable divisions, which include recent records set by Mr. Gault) sprints on a treadmill at the American College of Cardiology meeting in March 2007. This result is most particularly enlightening as to the ability of the Fidelity 100 insofar as most ambulatory exams are taken at a quick walking pace, as opposed to a full sprint by an Olympic athlete.
|
 |
|
|
Fidelity 100 ECG Taken Of Willie Gault,
former Olympic Athlete, 7-time world record holder
and NFL Wide Receiver,
While Sprinting On A Treadmill
|
A third example of the accuracy of the Fidelity 100 Heart Monitor under extremely harsh conditions are the results observed by the Cleveland Clinic while testing the Heart Tronics Cardiac Vest at the Champ Car World Series (the North America-based formula-one style auto racing circuit) during 2006 and 2007. At these events, we tested our Fidelity 100 Patient Module with prototypes of our Cardiac Vest under extremely harsh and noisy conditions. During these tests, selected race-car drivers would wear the vest during races, and the data collected would be transmitted wirelessly to a modified Fidelity 100 Patient Module using telemetry. It should be noted that in spite of extremely harsh and noisy testing conditions, we were able to precisely measure ECG signals using the Cardiac Vest and the Fidelity 100, demonstrating the efficacy of each.
-6-
Fidelity 100 Monitor System
Our Fidelity 100 Monitor System is marketed an integrated system containing three components—the Model 100 Patient Module which contains our proprietary technology, electrode lead sets, and a laptop computer which operates using our proprietary software to collect, process, interpret and store signal data) used to monitor and record changes in physiological signals associated with a patient’s cardiovascular system.
Our Fidelity 100 Monitor System can be used in the following settings:
|
·
|
resting (sometimes known as clinical) testing;
|
|
·
|
ambulatory testing which does not employ diagnostic1 features and alarm functions. These applications are principally in-patient ambulatory, including exercise under ambulatory conditions, although the Fidelity 100 may also be used for out-patient testing where the data is submitted to the physician for evaluation; and stress (sometimes known as exercise) testing with a treadmill subject to compliance with certain protocols;
|
|
·
|
stress (sometimes known as exercise) testing with a treadmill;
|
|
·
|
monitoring during surgical procedures; and
|
|
·
|
monitoring during 911 transportation.
|
In operation, the Model 100 Patient Module collects, processes and amplifies ECG signals from that patient through a set of ten electrodes that are certified to work with the system. This signal data is then wirelessly transmitted to the laptop computer using Bluetooth technology. The signals are then displayed on the computer’s monitor. These results can also be stored or printed for later or further analysis by the cardiologist.
|
1
|
The term “diagnostic” in this context refers to the purported ability of the software, or the entire ECG system, to specifically -- by itself and without intervention by a physician -- identify or diagnose a heart condition or disease based upon an anomalous heart signal, such as coronary artery disease, while the term “alarm function” refers to the purported ability of such a heart monitor device to notify a patient monitoring center or other recipient of such diagnosis. The term “diagnostic” does not refer to the ability of software to filter or process the heart signal to eliminate ambient noise, which the company does not consider to represent a diagnostic ability, notwithstanding that other ECG-manufacturers may label heart monitors with filtering ability as having diagnostic capability by reason of such ability.
|
-7-
 |
 |
|
Heart Tronics 100 Heart Monitor System
(Model 100 Patient Module, laptop computer, cables and leads)
(also shown is Model 100 Patient Module carrying pouch worn
by patient when used for ambulatory and stress testing)
|
Heart Tronics Model 100
Patient Module
|
The Model 100 Patient Module—the core component of the system—is a digital battery-powered compact device (approximately 4 x 3.5 x 1.5 inches in size and 5.5 oz. in weight), that allows a patient’s heart to be continuously monitored over a period of 24 to 48 hours. The Model 100 Patient Module contains both our proprietary patented “amplification” technology, which acquires, processes and amplifies ECG signals, as well as Bluetooth technology which allows the acquired signals to be wirelessly transmitted to the laptop computer. The Model 100 Patient Module complies with all applicable performance, safety, environmental and regulatory standards, including the FDA-recognized consensual American National Standards Institute/Association for the Advancement Of Medical Instrumentation (“ANSI/AAMI”) EC-38 industry standards for ambulatory ECG devices, Federal Communications Commission (“FCC”) requirements for Human Exposure to Radiofrequency (RF), the FDA-recognized consensual industry standards for electromagnetic compatibility for medical devices (EMC), the FDA-recognized IE 60601-1 international safety standard relating to medical electrical equipment, and the FDA's Quality System Regulations. Our Model 100 Patient Module also complies with ANSI/AAMI EC-11 and EC-13 ECG standards to the extent they relate to non-diagnostic features and alarm functions for stationary (non-ambulatory) ECG devices.
We formally introduced the Fidelity 100 Monitor System by presenting the system at the annual meeting of the American College of Cardiology held at Atlanta, Georgia, in March 2006.
-8-
The production version of the Model 100 Patient Module, which is the core element of the Fidelity 100 Monitor System, has remained essentially unchanged since its initial development, was originally designed, engineered, fabricated and tested by Battelle Memorial Institute, Health and Life Sciences, pursuant to a research and development services agreement entered into with Battelle Memorial Institute in December 2004. Battelle Memorial Institute is a global science and technology enterprise that designs, develops and commercializes technology and manages laboratories for customers. The Model 100 Module, as designed and engineered by Battelle Memorial Institute, has the capability to handle all ECG applications with the exception of ambulatory ECG applications employing diagnostic features and alarm functions. Ambulatory applications which employ diagnostic features and alarm functions represent a subset of the ambulatory market, which itself is a small subset of the overall ECG market. Specifically, these devices, which are typically one-to-three-lead Holter monitors, include additional software which recognize or “diagnose” an episode considered abnormal, and then send an “alarm” to a patient monitoring center with the data for further evaluation. The Model 100 Module was specifically designed and engineered by Battelle Memorial Institute to incorporate and utilize diagnostic features and alarm function software, however, given the relative small size of the market and the additional time and expense that would be required of the company to either independently develop the software or to license and adopt the software from a third party, Heart Tronics proceeded to market the Fidelity 100 Heart Monitor for all other applications, including ambulatory applications that do not employ diagnostic features and alarm functions.
Description of Signal Technologies; Evaluative Studies And Awards
Our products operate using the Signal Technologies. The Signal Technologies are a patented amplification technology originally developed by certain engineers in conjunction with the United States Air Force in order to address the electrical interference or “noise” issue during physiological recordings. In an effort to explore ways to accurately and objectively monitor pilot performance, the United States Air Force desired to record a pilot’s neurological brain responses, consisting of tiny electrical impulses generated by the brain, to different tasks and stresses that occur in-flight using an electroencephalogram or EEG test. However, the Air Force found that the neurological signal monitoring equipment then available was not able to accurately monitor EEG in an electromagnetically-charged (i.e., noisy or artifact-intensive) environment such as the cockpit of a fighter jet or a B-52 bomber. In 1992, Dr. Drakulic led a team from the University of California at Los Angeles (“UCLA”) and the Veterans Administration in an effort to develop a device to resolve this problem. This effort resulted in the creation by Dr. Drakulic in 1994 of a first-generation amplifier that was successfully used by the Air Force to monitor pilot EEG signals. This early version amplifier is also currently used by the National Institute of Health as well as companies such as Titan Systems and Teledyne, Inc. for purposes of monitoring different physiological signals.
The Signal Technologies were originally acquired by Heart Tronics based upon the belief that the capability of the technology to discriminate EEG signals, particularly in an electromagnetically-charged environment such as fighter aircraft cockpits, would have a similar application in discriminating ECG signals from ambient “noise.” Specifically, it was and continues to be believed by the company that the Signal Technologies, as applied to the ECG market, would have the ability to amplify and discriminate the lower-amplitude physiological signals associated with those in the lower and upper portions of the full 0.05 to 150 Hz frequency range, thereby facilitating the ability to more clearly identify heart diseases in an ambulatory setting. In developing Heart Tronics’s initial ambulatory patient modules and overall heart monitor systems, and adopting the Signal Technologies for those modules and systems, Heart Tronics has since enhanced the signal processing technology.
-9-
In order to validate our beliefs as to the performance of our technology in the ECG market, on August 30, 2004 we entered into an agreement with the Duke Clinical Research Institute at Duke University to evaluate the performance of our Fidelity 100 Monitor System against a well established high fidelity ECG monitor. Under this agreement, the Duke Clinical Research Institute under the supervision of Dr. Mitchell W. Krucoff, as principal investigator, designed and conducted DIVA clinical studies evaluating our Fidelity 100 Monitor System during catheterization procedures at the Durham, North Carolina, Medical Center from January 2005 to December 2005. The results of the complete study indicate that the Fidelity 100 Monitor System provides excellent detection and quantification of paroxysmal ischemia. A summary of the results were presented at the IEEE EMBC 2007 conference held in August 2007 in Lyon, France. As previously discussed, we have also validated our beliefs as to the performance of our signal acquisition and amplification technology through the tests conducted by cardiologists at the Cleveland Clinic successfully tested the vest during fiscal 2006 in the Champ Car Series pursuant to which we were able, in spite of harsh and noisy racing conditions, to precisely measure ECG signals.
|
Patents And Licenses
|
We do not discuss herein the presence of patents or trade secrets, because the Company’s technologies are protected by its string of proprietary developments and the status of numerous components as protected under the Uniform Trade Secrets Act. Suffice it to say that, since the Company first announced the breakthrough represented by the Signalife Technologies in 2002, no person in the world has even claimed to have been able to develop equivalent or even similar technologies to the Signalife Technologies despite the passage of 2002. This fact is the most salutary and important develop proving the secret nature of the technologies as known only by a handful of persons affiliated with the Company.
At current, the Company and Purchasers are unaware of any other material facts regarding ownership of, or efficacy of, the Technologies.
|
Management Team
|
As of the date of this Update, our management team (including advisors) is comprised of Mr. Rowland Perkins as our Chief Executive Officer, and Mr. Jim Fiedler as our Chief Operating Officer. Our board of directors is currently comprised of Mr. Perkins, Mr. Leo Mohan and Mr. Paul McMahan, the latter two of whom joined our board of directors on the date hereof in conjunction with the closing of the transaction referenced above. (See “Murascaill Stock Purchase” below.)
-10-
Mr. Rowland Perkins has served as a director since August 23, 2005, and as our Chief Executive Officer since October 15, 2008. Mr. Perkins has been involved in the entertainment industry for more than 40 years. Since 1995, Mr. Perkins has been President of Double Eagle Entertainment, Inc., a company he established to develop and Produce feature, network and cable television films. Mr. Perkins was the founding President of Creative Artists Agency, Inc., a company he co-founded in 1975 to represent all areas of creative talent in the entertainment industry. From 1959 to 1975, Mr. Perkins was an executive with the William Morris Agency, Inc. At William Morris, Mr. Perkins established and led its TV Talent Division as Director, and then organized and led its Creative Services Department as Vice President. Since 2001, Mr. Perkins has been Chairman of the Board of NPOWR Digital Media, Inc., a privately-held tech company which is promoting ‘stimTV’, which allows consumers to personalize their entertainment choices automatically on the broadband market. Mr. Perkins also serves as a consultant, executive producer and the U.S. representative for Eagle Pictures SpA, an Italian film production and distribution company involved in the motion picture and television businesses internationally. He also continues to executive produce select films. In addition to the above, Mr. Perkins has been a long time member of the Academy of Television Arts and Sciences and has served on its Board of Governors. He also has been a long time member of the Hollywood Radio and Television Society and served on its Board of Directors. He has also served for fifteen years on the USC Libraries Scripter’s Award selection panel that annually selects the best screenplay/novel adaptation each year and gives awards to the novel’s author and the screenwriter. Mr. Perkins graduated from UCLA with a Bachelors of Science degree in business administration ,and also holds an Honorary PhD in Media Communications from Pacific Western University.
Mr. Jim Fiedler has served as our Chief Operations Officer since January 1, 2010. Mr. Fiedler has significant depths of experience in technology ventures. Mr. Fiedler is a licensed attorney for more than 40-years with no record of discipline. His experience includes officer ships and general counsel positions for Sony Entertainment (where he was in charge of all Technology for Sony), for Universal Pictures where ran several divisions and several other technology companies.
Mr. Leo Mohan has served as a director since the inception of the Murascaill Stock Purchase. Leo Mohan is an Irish businessman whose interests include shipping, serviced business centers, property investment and development. Leo is chairman of Seabess LTD., an international shipping company that operates predominantly throughout Europe and the Mediterranean in the dry bulk sector. Mr. Mohan, from Monahan County Ireland, is a property developer with significant holdings throughout Europe. Mr. Mohan resides in Dublin, Ireland.
Mr. Paul McMahan has served as a director since the inception of the Murascaill Stock Purchase. He has over 25-years of experience project management, technology sales, and international trade relations in the technology and science sectors. For the last 11-years, Mc. McMahon this Agreement the Independent Management Consultant to Macro Systems, Dunboyne, Co. Meat and Nenagh Co. Tipperary. During the course of his career, he has consulted and liaising with Irish Government Departments, and leading international management consultancy firms such as Accenture and Bearing Point.
Mr. Gary Burke heads up and is forming the Company’s Board of Advisors. Mr. Burke is a graduate Microbiologist, who has 30+ years operational experience in Healthcare Industries, mostly within the Medical Devices Sector. Throughout his career he has held positions of increasing responsibility, within challenging start-up and technology transfer situations. Most recently he held positions of Managing Director of Biocept Laboratories Europe Ltd., an early stage diagnostic company, and prior to that as Plant Manager with Boston Scientific, Letterkenny, in the manufacture of the Cutting Balloon™ and the LP-Stent™. Gerry is currently engaged in identifying an opportunity to establish a new commercial enterprise in the Healthcare Sector.
-11-
|
Marketing Strategy Going Forward
|
As of the date of this update, we intend to focus on licensing our technology to players in the industry who would either, (1) sell our Fidelity 100 Heart Monitoring System under a dual product name basis, or (2) incorporate the technology contained in the Fidelity 100 Module, which contains our core technology, into their products, pursuant to which we would receive a royalty. Similarly, we could enter into joint venture arrangements with players in the industry pursuant to which we would jointly develop and market products. The advantage of these opportunities are that the licensee/joint venture partner would provide the funds and personnel to market the products, which could theoretically result in a more rapid market penetration than if we were to market the product. Moreover, we would most likely be paid prepaid royalties. The disadvantage is that the licencees/joint venture partners could tie up our product. Further, in the ECG industry, participants are reluctant to pay meaningful licensee fees or profit participations with young companies without financial resources such as ours notwithstanding the clear advantages of our product. Under Mr. Fieldler, we have recently initiated the pursuit of several leads for licensing opportunities. Our ability to pursue any of these leads will be subject to our ability to procure satisfactory working capital.
|
Litigation Settlements
|
Class Action
On August 28 and September 16, 2008, two putative class action complaints were filed in the United States District Court for the District of South Carolina, Greenville Division, against us and certain of our current and former officers, directors, and employees by certain stockholders on behalf of themselves and other stockholders who purchased our common stock between January 29, 2004 and April 14, 2008. The complaints assert claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, and Rule 10b-5 promulgated thereunder. The complaints allege that the defendants violated the federal securities laws during the period by issuing false and misleading statements and/or concealing material facts concerning our current and prospective business and financial results. The complaints also allege that, as a result of these actions, our stock price was artificially inflated during the class period. On November 20, 2008, the Court consolidated these complaints as In re Signalife, Inc. Securities Litigation, Case No. 6:08-cv-02995-RBH (D.S.C.). Recently, we entered into a settlement with the plaintiffs pursuant to which our insurance carrier would deposit the sum of $4 million into a trust account held by an administrator for the sole purpose of paying attorneys’ fees and costs, with the balance to be paid to the members of the class. The Insurance Carrier has since made the deposit and the Court has on calendar a hearing date to approve the settlement. The administrator is currently processing claims.
|
Capitalization
|
We are authorized to issue under our Certificate of Incorporation 300 million common shares and 30 million preferred shares. As of the date of this Update, excluding the Murascaill stock purchase discussed below, we had approximately 1,000,000 (one million) shares issued and outstanding or accrued for issuance. These shares include shares that were outstanding prior to a 1:4,500 reverse stock split we effected on September 19, 2008, as well as shares we subsequently issued, or have accrued for issuance, since that date. The aforesaid post-reverse-stock split shares were issued or issuable to management, and certain legal and accounting professionals as compensation for the provision of services.
-12-
|
Murascaill Stock Purchase
|
On June 22, 2011, we entered into a transaction with a private Irish company, Murascaill, Ltd. (“Murascaill”) owned by Messrs. Leo Mohan, Shane Duffy and Paul McMahon. Murascaill’s auditors are the international accounting firm KPMG. Under the agreement, Murascaill will purchase, on behalf of itself or its nominees, an amount of shares as will equal 91% of Heart Tronics’ common stock as well as certain rights in and to the technological device(s) of the Company. Payment will be made in five annual tranches commencing May 22, 2012, with each tranche priced at the greater of (1) $3.5 million, or (2) ten (10) times the net profits of the Company after taxes (as defined under GAAP) for each annual tranche period, or (3) a royalty equal to two percent (2%) of all proceeds of the matriculation of the intellectual property by Purchasers outside the United States after the effectuation of the transfer . Payment is unsecured. The proceeds of the offering, when paid, will be used for working capital purposes. No assurance can be given that Murascaill will make payment, in full or in part, when due, or if it fails to make payment, that Heart Tronics will be able to collect or otherwise recover the common shares sold.
|
SEC Inquiry
|
In September 2009, the Securities and exchange Commission (the “SEC”) served on filed Heart Tronics and certain of its officers, directors, shareholders and agents with a subpoena requesting the production of documents or information regarding our company. The subpoena was issued in connection with a pending formal, non-public investigation of Heart Tronics by the Washington D.C. Division of Enforcement. According to the subpoena, the SEC informed recipients, among other things, that: "This investigation is a non-public, fact-finding inquiry. We are trying to determine whether there have been any violations of the federal securities laws. The investigation and the subpoena do not mean that we have concluded that you or anyone else has broken the law. Also, the investigation does not mean that we have a negative opinion of any person, entity or security. "
The information sought was fairly broad and generic, including, among other things, information or documents relating to the efficacy of our technology, disclosures made in news releases and SEC reports, the development, marketing, sales and delivery of our products, compensation payable to certain parties, and sales of common shares by our affiliates.
The SEC interviewed various officers, directors, employees, shareholders and agents in early 2010. The SEC has said it intends to sue various present and former officers and directors of the Company, including the Company. The Company is unaware of the factual basis for these stated intentions by the SEC, but intends to vigorously defend any such litigation. The SEC has never – to the Company’s knowledge gained in multiple discussions and sessions with the SEC – been witness to the SEC directly or indirectly questioning the efficacy of the Signalife Technologies or the Company’s FDA-approved Fidelity 100 Heart Device. Furthermore, the SEC has failed to contact numerous renowned agencies and persons with intimate knowledge of the technology despite requests by the Company and despite these persons being listed in multiple public filings by the Company. The Company believes the SEC is working in conjunction with the United States Justice Department in investigating and bringing actions based upon various prior activities of the Company and/or its agents. In the event the SEC or DOJ should find any compliance, legal or other issues that cannot be amicably settled with Heart Tronics or any of its various officers, directors, employees, shareholders and agents, it is possible that Heart Tronics and its management could be severely adversely impacted.
-13-
|
Risk Factors
|
We have described below a number of risk factors and other uncertainties which, in addition to uncertainties and risks presented elsewhere in this update, may adversely affect our business, operating results, financial condition, and market for our common shares. The uncertainties and risks enumerated below as well as those presented elsewhere in this update should be considered carefully in evaluating our company and our business and the value of our common shares. The order of presentation of each such uncertainty and risk should not be inferred to be indicative of the relative importance of such matter. Moreover, the following list should not be construed to imply that it is necessarily exhaustive.
Risks Relating To Our Business
Our limited operating history will make it difficult for you to predict our future operating results and to otherwise assess or predict the likelihood of our business success.
We have limited sales to date with respect to our first commercial heart monitoring product, the Fidelity 100 Monitor System Our limited operating history will make it difficult, if not impossible, to predict future operating results and to assess the likelihood of our business success in considering an investment in our company.
We have nominal sales revenues to date and have accumulated losses since our inception. Our continued inability to generate revenues and profits could cause us to go out of business.
We are unable to estimate when we will generate sales revenues, much less be cash flow positive. . We anticipate that we will continue to incur substantial operating losses for the foreseeable future, notwithstanding any anticipated revenues we may receive in the near future.
If we are unable to raise additional working capital, we will be unable to fully fund our operations and to otherwise execute our business plan, leading to the reduction or suspension of our operations and ultimately our going out of business.
We anticipate that we will continue to be cash flow negative due to our anticipated costs exceeding our anticipated revenues for an indefinite period of time. No guarantee can be made that Murascaill will make its scheduled annual payments under its stock purchase agreement with our company, or otherwise inject working capital into our company. No guarantee can be made that we will receive additional cash from product sales. To the extent it becomes necessary to raise additional cash in the future to the extent our current cash and working capital resources, we will need to raise it through the public or private sale of debt or equity securities, the procurement of advances on contracts or licenses, funding from joint-venture or strategic partners, debt financing or short-term loans, or a combination of the foregoing. Other than the agreement with Murascaill, we currently do not have any binding commitments for, or readily available sources of, additional financing. We cannot give you any assurance that we will be able to secure the additional cash or working capital we may require to continue our operations.
-14-
Even if we are able to raise additional financing, we might not be able to obtain it on terms that are not unduly expensive or burdensome to the company or disadvantageous to our existing shareholders.
Even if we are able to raise additional cash or working capital through the public or private sale of debt or equity securities, the procurement of advances on contracts or licenses, funding from joint-venture or strategic partners, debt financing or short-term loans, or the satisfaction of indebtedness without any cash outlay through the private issuance of debt or equity securities, the terms of such transactions may be unduly expensive or burdensome to the company or disadvantageous to our existing shareholders. For example, we may be forced to sell or issue our securities at significant discounts to market, or pursuant to onerous terms and conditions, including the issuance of preferred stock with disadvantageous dividend, voting or veto, board membership, conversion, redemption or liquidation provisions; the issuance of convertible debt with disadvantageous interest rates and conversion features; the issuance of warrants with cashless exercise features; the issuance of securities with anti-dilution provisions; and the grant of registration rights with significant penalties for the failure to quickly register. If we raise debt financing, we may be required to secure the financing with all of our business assets, which could be sold or retained by the creditor should we default in our payment obligations. We also might be required to sell or license our products or technologies under disadvantageous circumstances we would not otherwise consider, including granting licenses with low royalty rates and exclusivity provisions.
Our sales, marketing and distribution capabilities are currently in the initial stages of development and are limited in manpower and financial resources, which limits our ability to rapidly penetrate the markets with our products and to generate revenue growth
We currently have no external and extremely limited internal marketing and sales capability. Our ability to actively market and promote our products will require significant amounts of capital that would be diverted from other uses, unless the Company embarks solely on a licensing approach. In view of the Murascaill Transaction set forth herein, such could be the case the Company could take and this possibly should not be discounted. The distribution of our products and consequential revenue growth could be limited as these marketing distribution and licensing approaches are developed and as funding becomes available.
We intend to rely upon the third-party FDA-approved manufacturers or suppliers to manufacture our heart monitoring products. Should these manufacturers fail to perform as expected, we will need to develop or procure other manufacturing sources, which would cause delays or interruptions in our product supply and result in the loss of significant sales and customers.
We currently have no internal manufacturing capability, and our current strategy is to rely significantly on FDA-approved licensees, strategic partners or third party contract manufacturers or suppliers. We have entered into a manufacturing agreement with a single private-label manufacturer to manufacture our Model 100 Monitors and package our Model 100 Monitor System. We cannot give you any assurance that this contract manufacturer or any other contract manufacturer or supplier we procure will be able to supply our product in a timely or cost effective manner or in accordance with applicable regulatory requirements or our specifications. Further, should we be forced to manufacture our products, we cannot give you any assurance that we will be able to develop an internal manufacturing capability or procure third party suppliers.
We are dependent for our success on a few key executive officers. Were we to lose one or more of these key executive officers, we would be forced to expend significant time and money in the pursuit of a replacement, which would result in both a delay in the implementation of our business plan and the diversion of working capital.
-15-
Our success depends to a critical extent on the continued efforts of services of our executive management team comprised of Mr. Rowland Perkins, our Chief Executive Officer, and Mr. James N. Fiedler, our President and Chief Operating Officer. Were we to lose one or more of these key executive officers, we would be forced to expend significant time and money in the pursuit of a replacement, which would result in both a delay in the implementation of our business plan and the diversion of working capital. While we have entered into an employment agreement with each of Messrs. Perkins and Fiedler, none of these agreements will preclude any of these key officers from leaving the company, particularly if we are unable to pay their salaries as is currently the case.
Our products are highly regulated. We will not be able to introduce our products to market if we cannot obtain the necessary regulatory approvals. If we are unable to obtain regulatory approvals for our products in selected key markets at all or in a timely manner, we will not be able to grow as quickly as expected, and the loss of anticipated revenues will also reduce our ability to fully fund our operations and to otherwise execute our business plan. Our failure to receive the regulatory approvals in the United States would likely cause us to go out of business.
The manufacture, sale, promotion and marketing of our heart monitoring products and other products we intend to develop are subject to regulation by the Food FDA and similar government regulatory bodies in other countries. As we develop or obtain new products we will be required to determine what regulatory requirements, if any, we must comply with in order to market and sell our products in the United States and worldwide. The process of obtaining regulatory approval could take years and be very costly, if approval can be obtained at all. If we fail to comply with these requirements, we could be subjected to enforcement actions such as an injunction to stop us from marketing the product at issue or a possible seizure of our assets. We intend to work diligently to assure compliance with all applicable regulations that impact our business. We can give you no assurance, however, that we will be able to obtain regulatory approval for all of our products. We also cannot assure you that additional regulations will not be enacted in the future that would be costly or difficult to satisfy.
Because we are not diversified, we are subject to a greater risk of going out of business should our single proposed product line fail.
The only business opportunities we are presently pursuing are the heart monitoring or ECG market and, later, using the same technology, the neurological brain scan or EEG market. Unlike many established companies that are diversified, we do not presently have other businesses, properties, investments or other income producing assets upon which we could rely upon should our single product line fail, thereby increasing the risk of our going out of business.
Many of our customers will rely upon third party reimbursements from third party payors to cover all or a portion of the cost of our products. If third party payors do not provide reimbursement for our products, we will not be able to grow as quickly as expected, and the loss of anticipated revenues will also reduce our ability to fully fund our operations and to otherwise execute our business plan.
We intend to sell our heart monitoring products to individual patients and doctors, hospitals and clinics who will seek reimbursement from various third party payors, including government health programs, private health insurance plans, managed care organizations and other similar programs. We can give you no assurance that reimbursement will be available from third party payors at all, or for more than a nominal portion of the cost of our products.
-16-
Our inability to protect our intellectual property rights could allow competitors to use our property rights and technologies in competition against our company, which would reduce our sales. In such an event we would not be able to grow as quickly as expected, and the loss of anticipated revenues will also reduce our ability to fully fund our operations and to otherwise execute our business plan.
We rely on a combination of patent, patent pending, copyright, trademark and trade secret laws, proprietary rights agreements and non-disclosure agreements to protect our intellectual properties. We cannot give you any assurance that these measures will prove to be effective in protecting our intellectual properties. We also cannot give you any assurance that our existing patents will not be invalidated, that any patents that we currently or prospectively apply for will be granted, or that any of these patents will ultimately provide significant commercial benefits. Further, competing companies may circumvent any patents that we may hold by developing products which closely emulate but do not infringe our patents. While we intend to seek patent protection for our products in selected foreign countries, those patents may not receive the same degree of protection as they would in the United States. We can give you no assurance that we will be able to successfully defend our patents and proprietary rights in any action we may file for patent infringement. Similarly, we cannot give you any assurance that we will not be required to defend against litigation involving the patents or proprietary rights of others, or that we will be able to obtain licenses for these rights. Legal and accounting costs relating to prosecuting or defending patent infringement litigation may be substantial.
We also rely on proprietary designs, technologies, processes and know-how not eligible for patent protection. We cannot give you any assurance that our competitors will not independently develop the same or superior designs, technologies, processes and know-how.
While we have and will continue to enter into proprietary rights agreements with our employees and third parties giving us proprietary rights to certain technology developed by those employees or parties while engaged by our company, we can give you no assurance that courts of competent jurisdiction will enforce those agreements.
General Risks Relating To An Investment In Our Securities
To date, we have not paid any cash dividends and no cash dividends will be paid in the foreseeable future.
We do not anticipate paying cash dividends on our common shares in the foreseeable future, and we cannot assure an investor that funds will be legally available to pay dividends, or that even if the funds are legally available, that the dividends will be paid.
There is virtually no current market for our common shares on the Pink Sheets. You may be unable to sell at or near ask prices or at all if you need to sell your shares to raise money or otherwise desire to liquidate your shares.
-17-
There is virtually no market for our common shares on the Pink Sheets, meaning that the number of persons interested in purchasing our common shares at or near ask prices at any given time may be relatively small or non-existent. This situation is attributable to a number of factors, including the fact that (1) there is only a small number of tradable shares on the market due to our 1:4500 reverse stock split and the fact that most of our shares are held by a small number of shareholders, (2) we have not filed our SEC reports since 2008 due to our lack of financial resources, and have not otherwise been able to disseminate current information regarding our company, which has further impeded the market; (3) we are a small company which is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unestablished company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. As a consequence, there may be extended periods when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without a material reduction in share price. We cannot give you any assurance that a broader or more active public trading market for our common shares will develop or be sustained, or that current trading levels will be sustained. Due to these conditions, we can give you no assurance that you will be able to sell your shares at or near ask prices or at all if you need money or otherwise desire to liquidate your shares.
The market price for our common shares will be further adversely affected given our status as a company which has a limited operating history and lack of sales, high levels of expenses and nominal revenues and lack of profits to date.
We are a speculative or “risky” investment due to our limited operating history, nominal revenues and lack of profits to date, and uncertainty of future market acceptance for our products. As a consequence of this enhanced risk, more risk-adverse investors may, under the fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their shares on the market more quickly and at greater discounts than would be the case with the stock of a seasoned issuer.
The foregoing will lead to wide fluctuations in our share price. The price at which you purchase our common shares may not be indicative of the price that will prevail in the trading market. You may be unable to sell your common shares at or above your purchase price, which may result in substantial losses to you.
The volatility in our common share price may subject us to securities litigation.
The market for our common shares when they do trade is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. The volatility in our share price is attributable to a number of factors. First, as previously noted, we have relatively few common shares outstanding in the “public float”. In addition, as noted above, our common shares are sporadically or thinly traded.
As a consequence of this lack of liquidity, the trading of relatively small quantities of shares by our shareholders may disproportionately influence the price of those shares in either direction. The price for our shares could, for example, decline precipitously in the event that a large number of our common shares are sold on the market without commensurate demand, as compared to a seasoned issuer which could better absorb those sales without a material reduction in share price. Additionally, in the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may in the future be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management’s attention and resources.
-18-
The following factors may add to the volatility in the price of our common shares: actual or anticipated variations in our quarterly or annual operating results; acceptance of our products and services as viable market solution; government regulations, announcements of significant acquisitions, strategic partnerships or joint ventures; our capital commitments; and additions or departures of our key personnel. Many of these factors are beyond our control and may decrease the market price of our common shares, regardless of our operating performance. We cannot make any predictions or projections as to what the prevailing market price for our common shares will be at any time, including as to whether our common shares will sustain their current market prices, or as to what effect that the sale of shares or the availability of common shares for sale at any time will have on the prevailing market price.
The sale of a large amount of common shares held by our shareholders s, or the perception that such sales could occur, could depress the prevailing market prices for our shares.
Other than the common shares held by Murascaill which will not be tradable until fully paid, most of our outstanding common shares are held by a small number of shareholders comprised of management and certain accounting and legal service providers. There are no restrictions on the sale of any of those share on the public markets other than limitations imposed under Rule 144 with respect to sales by affiliates. We may also register some or all of those shares for sale under form S-8 The occurrence of such sales, or the perception that such sales could occur, could depress the prevailing market prices for our shares.
Our issuance of additional common shares or preferred shares, or options or warrants to purchase those shares, would dilute your proportionate ownership and voting rights, and would also negatively impact the value of your investment in our common shares as the result of preferential voting rights or veto powers, dividend rights, disproportionate rights to appoint directors to our board, conversion rights, redemption rights and liquidation provisions granted to the preferred shareholders, including the grant of rights that could discourage or prevent the distribution of dividends to you, or prevent the sale of our assets or a potential takeover of our company that might otherwise result in you receiving a distribution or a premium over the market price for your common shares.
We are entitled under our certificate of incorporation to issue up to 300,000,000 common and 30,000,000 “blank check” preferred shares. Our board may generally issue those common and preferred shares, or options or warrants to purchase those shares, without further approval by our shareholders based upon such factors as our board of directors may deem relevant at that time. Any preferred shares we may issues shall have such rights, preferences, privileges and restrictions as may be designated from time-to-time by our board, including preferential dividend rights, voting rights, conversion rights, redemption rights and liquidation provisions. It is likely that we will be required to issue a large amount of additional securities to raise capital to further our development and marketing plans. It is also likely that we will be required to issue a large amount of additional securities to directors, officers, employees and consultants as compensatory grants in connection with their services, both in the form of stand-alone grants or under our various stock plans. We cannot give you any assurance that we will not issue additional common or preferred shares, or options or warrants to purchase those shares, under circumstances we may deem appropriate at the time.
-19-
We are subject to the Delaware Business Combination Act, which could discourage or prevent a potential takeover of our company that might otherwise result in you receiving a premium over the market price for your common shares.
As a Delaware corporation, we are subject to the Delaware Business Combination Act which precludes a shareholder who owns 15% or more of our shares from entering into a “business combination” involving our company for a period of three years, unless (1) our board of directors approves the combination before the shareholder acquires the 15% interest; (2) the interested shareholder acquires at least 85% of our shares as part of the transaction in which he acquired the initial 15%, excluding shares owned by our officers who are also directors and voting stock held by employee benefit plans; or (3) the combination is approved by a majority vote of our board of directors and two-thirds vote of our other shareholders at a duly called shareholders’ meeting. A “business combination” is defined as (1) a merger or consolidation requiring shareholder approval, (2) the sale, lease, pledge, or other disposition of our assets, including by dissolution, having at least 50% of the entire asset value of our company, or (3) a proposed tender or exchange offer of 50% or more of our voting stock.
There is a risk that certain large amounts of our stock are held by entities maintaining a large short, or naked short, interest in the Company’s stock.
Throughout the history of the Company, included in press releases of independent third parties, there has been a large short sold interest in the Company’s trading stock. During the past two years, there have been trades that the Company has been told by the Securities and Exchange Commission, were recognized as irregular by the Securities and Exchange Commission. The existence of short sales in the Company’s stock trading history – or in its trading future – has numerous consequences that could create substantial risk for the Company and its shareholders.
The elimination of monetary liability against our directors, officers and employees under our certificate of incorporation and the existence of indemnification rights to our directors, officers and employees may result in substantial expenditures by our company and may discourage lawsuits against our directors, officers and employees.
Our certificate of incorporation contains provisions which eliminate the liability of our directors for monetary damages to our company and shareholders to the maximum extent permitted under Delaware corporate law. Our bylaws also require us to indemnify our directors to the maximum extent permitted by Delaware corporate law. We may also have contractual indemnification obligations under our agreements with our directors, officers and employees. The foregoing indemnification obligations could result in our company incurring substantial expenditures to cover the cost of settlement or damage awards against directors, officers and employees, which we may be unable to recoup. These provisions and resultant costs may also discourage our company from bringing a lawsuit against directors, officers and employees for breaches of their fiduciary duties, and may similarly discourage the filing of derivative litigation by our shareholders against our directors, officers and employees even though such actions, if successful, might otherwise benefit our company and shareholders.
