Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AIkido Pharma Inc. | a11-14918_18k.htm |
Exhibit 99.1
Exhibit 99.1: Spherix Incorporated Overview Presentation, June 15, 2011
|
|
Spherix Incorporated (NASDAQ: SPEX) June 2011 |
|
|
Forward-Looking Statements This presentation contains forward-looking statements made pursuant to provisions of Section 21E of the Securities Exchange Act of 1934. Investors are cautioned that such statements, including statements relating to planned clinical study design, regulatory and business strategies, plans and objectives of management and growth opportunities for existing or proposed products, constitute forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those anticipated by the forward-looking statements. The risks and uncertainties include, without limitation, risks that product candidates may fail in the clinic or may not be successfully marketed or manufactured, we may lack financial resources to complete development of D-tagatose, the FDA may interpret the results of studies differently than us, competing products may be more successful, demand for new pharmaceutical products may decrease, the biopharmaceutical industry may experience negative market trends, our continuing efforts to develop D-tagatose may be unsuccessful, our common stock could be delisted from the Nasdaq Capital Market, and other risks and challenges detailed in our filings with the U.S. Securities and Exchange Commission. You are cautioned not to place undue reliance on any forward-looking statements that speak only as of the date of this presentation. We undertake no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this presentation or to reflect the occurrence of unanticipated events. |
|
|
Company Description Core expertise in scientific and technical aspects of drug and food development Operates two subsidiaries Biospherics: Pharmaceutical development of D-tagatose and pipeline products Dyslipidemias Atherosclerosis Metabolic Syndrome and Diabetes Spherix Consulting: Scientific consulting on food and drug approvals for clients worldwide |
|
|
Investment Highlights D-tagatose and SPX-106 show promise in multiple clinical applications Hypertriglyceridemia ($26 billion treatment market) Diabetes ($175 billion treatment market) Preclinical and Phase 2 data show significant lowering of triglycerides Much faster and less expensive development pathway vs. diabetes Clinical development program initiated Cash position sufficient to fund initial triglyceride studies Phase 3 study demonstrated statistically significant reductions in HbA1c in patients with mild Type 2 diabetes Pursuing partnership(s) for further clinical development |
|
|
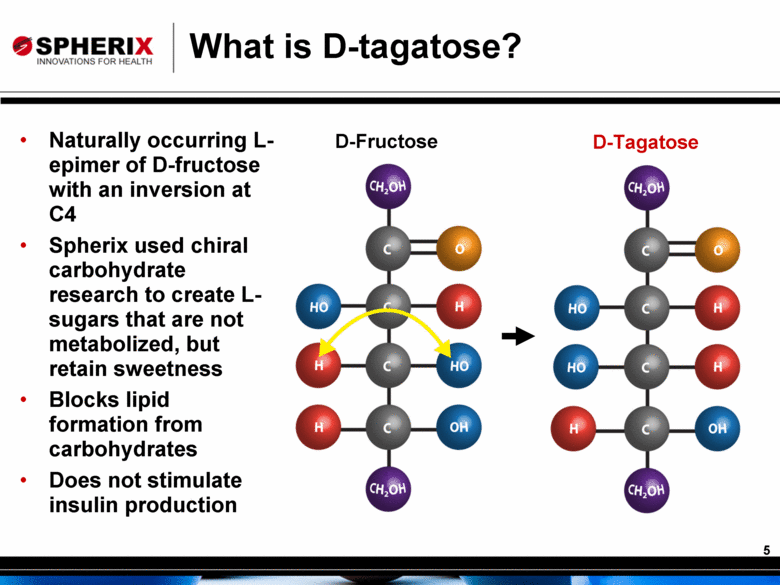
What is D-tagatose? D-Tagatose D-Fructose Naturally occurring L-epimer of D-fructose with an inversion at C4 Spherix used chiral carbohydrate research to create L-sugars that are not metabolized, but retain sweetness Blocks lipid formation from carbohydrates Does not stimulate insulin production |
|
|
The D-Tagatose Difference May provide a safety advantage over current agents Approved as GRAS by FDA; approved as Novel Food in EU; ADI “not specified” by JECFA Provides lipoprotein and glycemic control through a mechanism of action unlike any agent currently marketed in the U.S. “Sugar blocker” with potential fat, liver and gut mechanisms that may modify blood lipid and post-prandial glucose levels |
|
|
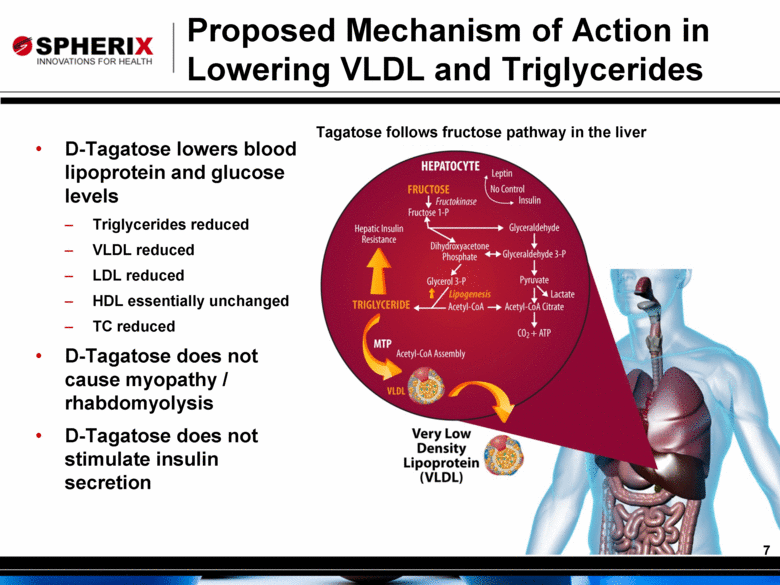
Proposed Mechanism of Action in Lowering VLDL and Triglycerides D-Tagatose lowers blood lipoprotein and glucose levels Triglycerides reduced VLDL reduced LDL reduced HDL essentially unchanged TC reduced D-Tagatose does not cause myopathy / rhabdomyolysis D-Tagatose does not stimulate insulin secretion Tagatose follows fructose pathway in the liver 7 |
|
|
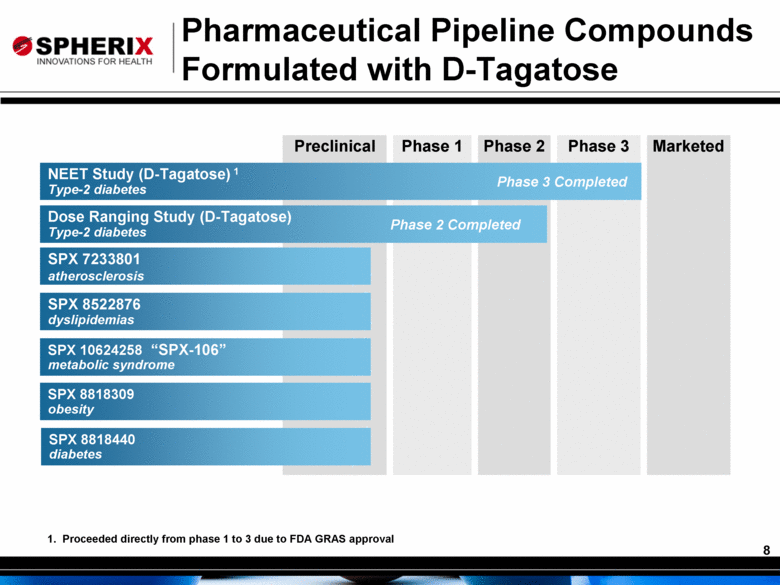
Preclinical Phase 1 Phase 2 Phase 3 Marketed Pharmaceutical Pipeline Compounds Formulated with D-Tagatose SPX 8522876 dyslipidemias Dose Ranging Study (D-Tagatose) Type-2 diabetes Phase 2 Completed SPX 7233801 atherosclerosis SPX 10624258 metabolic syndrome SPX 8818309 obesity SPX 8818440 diabetes NEET Study (D-Tagatose) 1 Type-2 diabetes Phase 3 Completed 1. Proceeded directly from phase 1 to 3 due to FDA GRAS approval “SPX-106” |
|
|
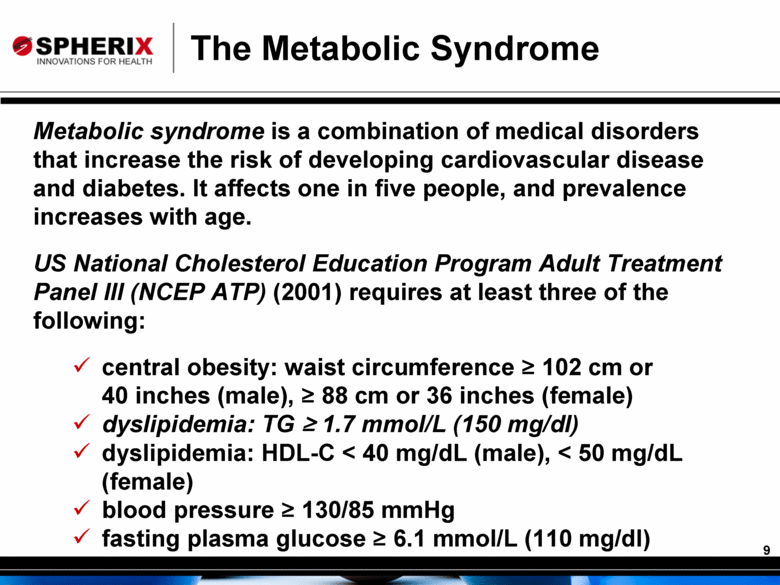
The Metabolic Syndrome Metabolic syndrome is a combination of medical disorders that increase the risk of developing cardiovascular disease and diabetes. It affects one in five people, and prevalence increases with age. US National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP) (2001) requires at least three of the following: central obesity: waist circumference ≥ 102 cm or 40 inches (male), ≥ 88 cm or 36 inches (female) dyslipidemia: TG ≥ 1.7 mmol/L (150 mg/dl) dyslipidemia: HDL-C < 40 mg/dL (male), < 50 mg/dL (female) blood pressure ≥ 130/85 mmHg fasting plasma glucose ≥ 6.1 mmol/L (110 mg/dl) |
|
|
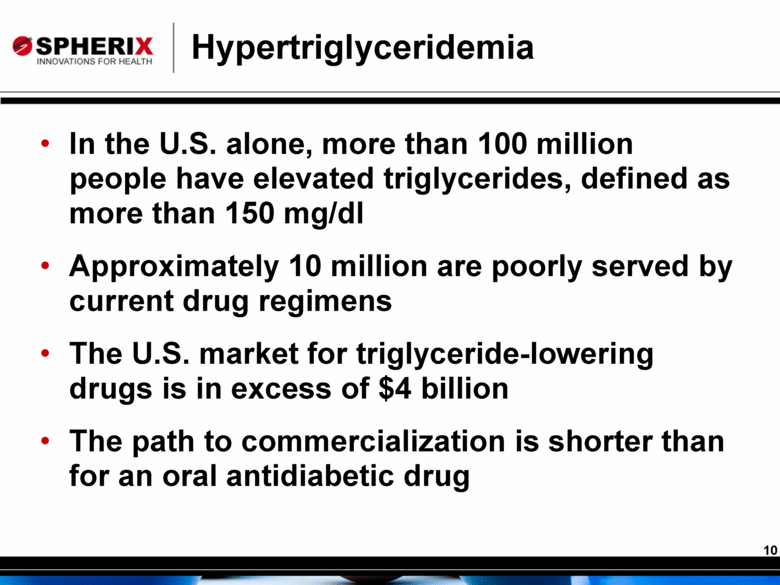
Hypertriglyceridemia In the U.S. alone, more than 100 million people have elevated triglycerides, defined as more than 150 mg/dl Approximately 10 million are poorly served by current drug regimens The U.S. market for triglyceride-lowering drugs is in excess of $4 billion The path to commercialization is shorter than for an oral antidiabetic drug |
|
|
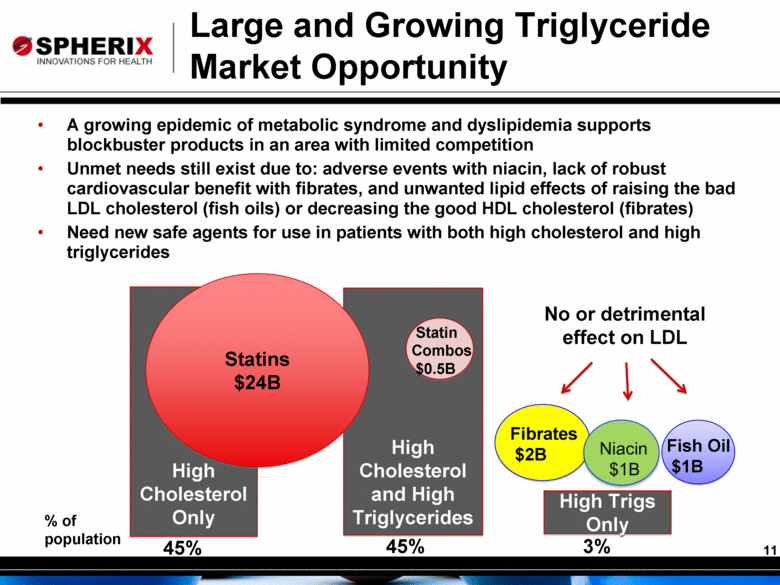
Large and Growing Triglyceride Market Opportunity A growing epidemic of metabolic syndrome and dyslipidemia supports blockbuster products in an area with limited competition Unmet needs still exist due to: adverse events with niacin, lack of robust cardiovascular benefit with fibrates, and unwanted lipid effects of raising the bad LDL cholesterol (fish oils) or decreasing the good HDL cholesterol (fibrates) Need new safe agents for use in patients with both high cholesterol and high triglycerides High Cholesterol Only High Cholesterol and High Triglycerides High Trigs Only Statins $24B Fibrates $2B Fish Oil $1B 45% 45% 3% Statin Combos $0.5B No or detrimental effect on LDL % of population Niacin $1B |
|
|
The Metabolic Diseases Crisis-Hypertriglyceridemia In the US alone, >100 million people have elevated triglycerides (> 150 mg/dl) Associated with other lipid abnormalities and the metabolic syndrome abdominal obesity, insulin resistance, low high-density lipoprotein (HDL), and hypertension, which are linked to coronary artery disease Preclinical work and interim Phase 2 D-tagatose data support pursuit of this indication for development Good commercial opportunity! Large, but poorly-served market due to side effects of current products, with few competitors and low promotional intensity |
|
|
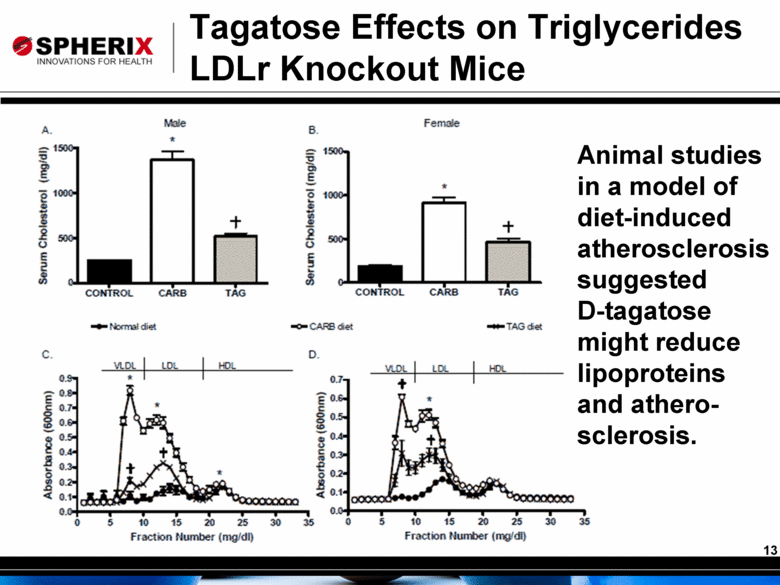
Tagatose Effects on Triglycerides LDLr Knockout Mice Animal studies in a model of diet-induced atherosclerosis suggested D-tagatose might reduce lipoproteins and athero-sclerosis. |
|
|
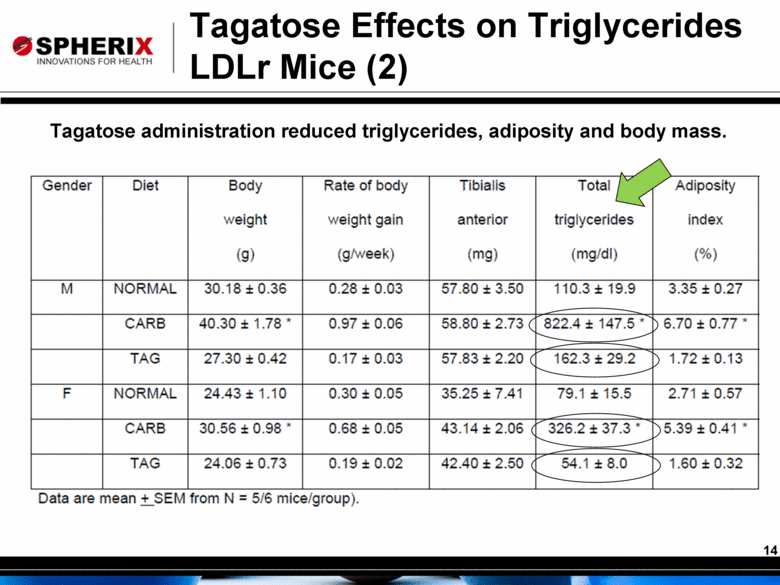
Tagatose Effects on Triglycerides LDLr Mice (2) Tagatose administration reduced triglycerides, adiposity and body mass. |
|
|
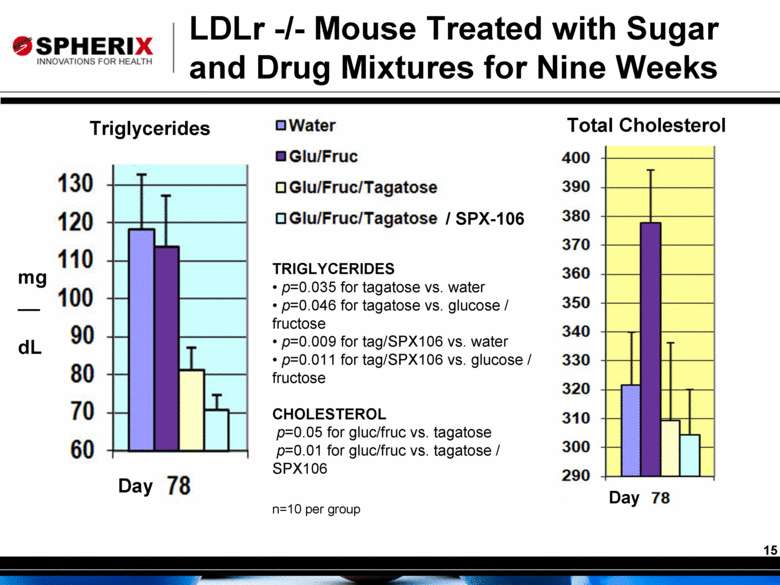
/ SPX-106 Triglycerides Total Cholesterol LDLr -/- Mouse Treated with Sugar and Drug Mixtures for Nine Weeks mg __ dL Day Day TRIGLYCERIDES p=0.035 for tagatose vs. water p=0.046 for tagatose vs. glucose / fructose p=0.009 for tag/SPX106 vs. water p=0.011 for tag/SPX106 vs. glucose / fructose CHOLESTEROL p=0.05 for gluc/fruc vs. tagatose p=0.01 for gluc/fruc vs. tagatose / SPX106 n=10 per group |
|
|
The SPX-106/tagatose combination is the most effective of the treatments for lowering triglycerides. The SPX-106 dose is responsible for lowering most of the triglycerides in the blood, followed by tagatose. D-tagatose lowers triglycerides by -3.1 mg/dl per g/kg/dose of the sugar, while the SPX-106 lowers triglycerides by -372 mg/dl per g/kg/dose of the drug. Both glucose and fructose raise triglycerides. The SPX-106/tagatose combination is the most effective of the treatments for lowering cholesterol. The SPX-106 concentration is responsible for lowering most of the cholesterol in the mice blood, followed by tagatose. D-tagatose lowers cholesterol by -3.9 mg/dl per g/kg/dose of the sugar, while the SPX-106 lowers cholesterol by -629 mg/dl per g/kg/dose of the drug. Both glucose and fructose raise cholesterol. Peak Factor Analysis Results for the LDLr -/- Mice |
|
|
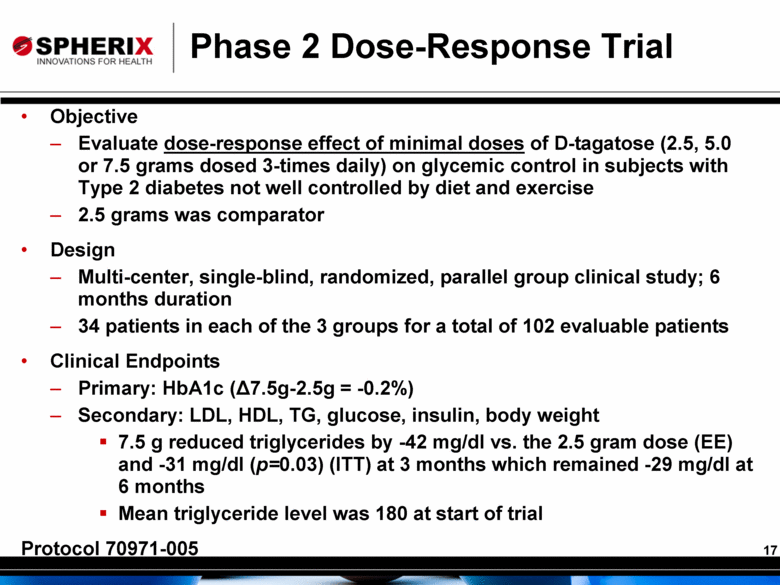
Phase 2 Dose-Response Trial Objective Evaluate dose-response effect of minimal doses of D-tagatose (2.5, 5.0 or 7.5 grams dosed 3-times daily) on glycemic control in subjects with Type 2 diabetes not well controlled by diet and exercise 2.5 grams was comparator Design Multi-center, single-blind, randomized, parallel group clinical study; 6 months duration 34 patients in each of the 3 groups for a total of 102 evaluable patients Clinical Endpoints Primary: HbA1c (Δ7.5g-2.5g = -0.2%) Secondary: LDL, HDL, TG, glucose, insulin, body weight 7.5 g reduced triglycerides by -42 mg/dl vs. the 2.5 gram dose (EE) and -31 mg/dl (p=0.03) (ITT) at 3 months which remained -29 mg/dl at 6 months Mean triglyceride level was 180 at start of trial Protocol 70971-005 |
|
|
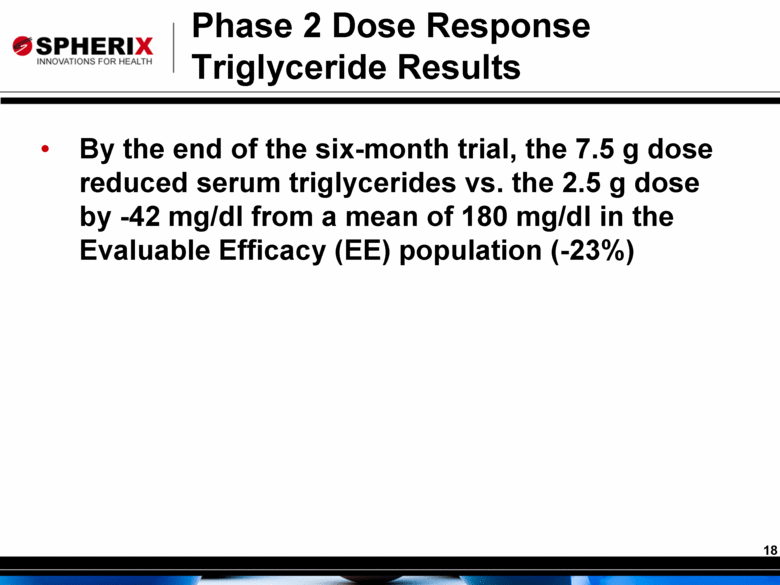
By the end of the six-month trial, the 7.5 g dose reduced serum triglycerides vs. the 2.5 g dose by -42 mg/dl from a mean of 180 mg/dl in the Evaluable Efficacy (EE) population (-23%) Phase 2 Dose Response Triglyceride Results |
|
|
Unlike some other drugs, D-tagatose lowered triglycerides without elevating VLDL D-tagatose in the 7.5 g dose reduced LDL vs. the 2.5 g dose by -11 mg/dl by the third month of treatment The reduction essentially held steady at the six-month end-of-study visit (-10 mg/dl). HDL was unchanged, increasing only between 0.3 and 1.4 mg/dl over the entire course of the study vs. comparator Phase 2 Dose Response Triglyceride Results |
|
|
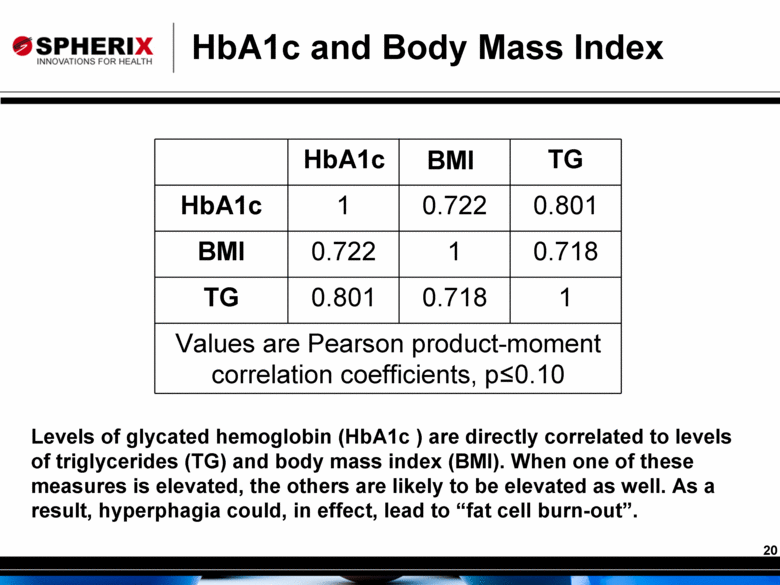
HbA1c and Body Mass Index HbA1c BMI TG HbA1c 1 0.722 0.801 BMI 0.722 1 0.718 TG 0.801 0.718 1 Values are Pearson product-moment correlation coefficients, p≤0.10 Levels of glycated hemoglobin (HbA1c ) are directly correlated to levels of triglycerides (TG) and body mass index (BMI). When one of these measures is elevated, the others are likely to be elevated as well. As a result, hyperphagia could, in effect, lead to “fat cell burn-out”. |
|
|
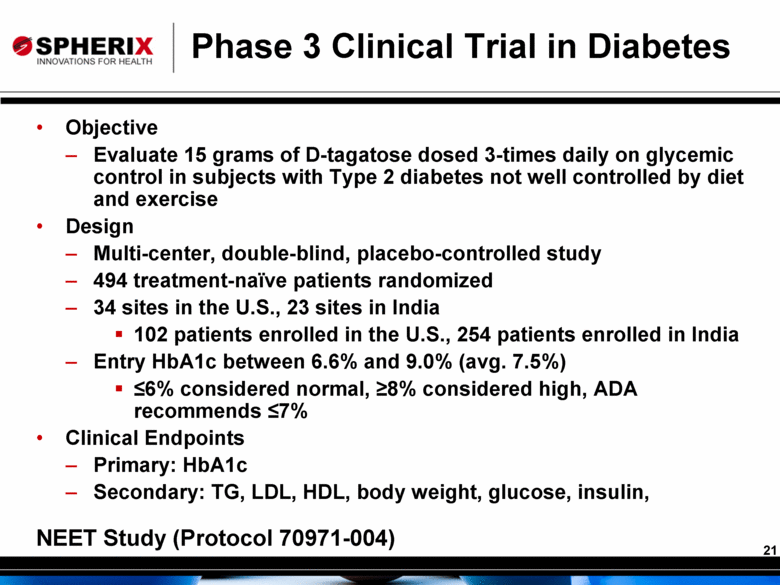
Phase 3 Clinical Trial in Diabetes Objective Evaluate 15 grams of D-tagatose dosed 3-times daily on glycemic control in subjects with Type 2 diabetes not well controlled by diet and exercise Design Multi-center, double-blind, placebo-controlled study 494 treatment-naïve patients randomized 34 sites in the U.S., 23 sites in India 102 patients enrolled in the U.S., 254 patients enrolled in India Entry HbA1c between 6.6% and 9.0% (avg. 7.5%) ≤6% considered normal, ≥8% considered high, ADA recommends ≤7% Clinical Endpoints Primary: HbA1c Secondary: TG, LDL, HDL, body weight, glucose, insulin, NEET Study (Protocol 70971-004) |
|
|
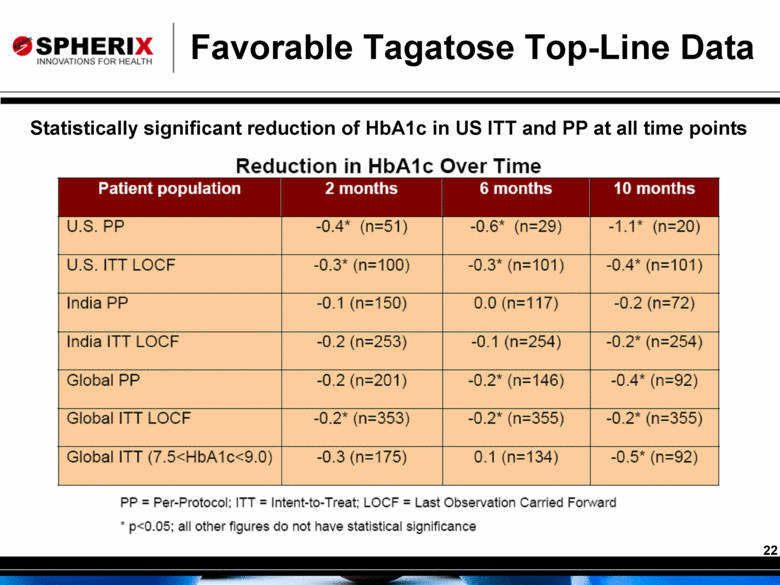
Favorable Tagatose Top-Line Data Statistically significant reduction of HbA1c in US ITT and PP at all time points |
|
|
Other Diabetes Clinical Findings Patients with ≥1 treatment-emergent adverse events in the active group (163) was comparable to the placebo group (166) No serious adverse event deemed treatment related No episodes of hypoglycemia or pancreatitis were reported among any trial subjects The study was not powered for significance in secondary endpoints (e.g., triglycerides) |
|
|
Intellectual Property Protection Patents include: Ten national filings based on PCT publication # 2010/054001 as anti-metabolic, atherosclerosis, obesity & diabetes methods and composition with a 2nd pharmaceutical agent US patent # 5,447,917 D-tagatose as anti-hyperglycemic agent US patent # 5,356,879 D-tagatose as anti-hyperglycemic agent New Chemical Entity Exclusivity (Hatch-Waxman) – U.S. 5-year exclusivity (no aNDA’s accepted) usually granted to new drug products containing chemical entities never previously approved by FDA either alone or in combination Essentially ~6 years exclusivity (base case) Pediatric Exclusivity – U.S. 6 months exclusivity for conducting studies in pediatric population Added to end of all existing marketing exclusivity and patent periods New Chemical Entity Exclusivity – E.U. 10 years exclusivity granted to NCE in European Union |
|
|
Experienced Leadership Claire L. Kruger, Ph.D., Chief Executive Officer Toxicologist with 20 years of consulting experience; primary area of expertise is in pharmaceuticals, consumer products and foods, where she provides scientific, regulatory, and strategic support to clients in both the US and international regulatory arenas Robert A. Lodder, Ph.D., President Founder of InfraReDx, Inc., Prescient Medical, Inc., and Escent Technologies, Inc. Professor of Pharmaceutical Sciences at the College of Pharmacy, University of Kentucky Medical Center. Joint appointments in Electrical & Computer Engineering and in Chemistry. Inventor with over 100 publications and 17 patents. Robert L. Clayton, Chief Financial Officer 16 years of experience in finance and accounting, including 5 years in public accounting; previously served as Director of Finance and Controller for Spherix Katherine M. Brailer, Corporate Secretary and Director of Administrative Services 25 years of experience in all aspects of administrative services, including 10 years as an Officer of Spherix |
|
|
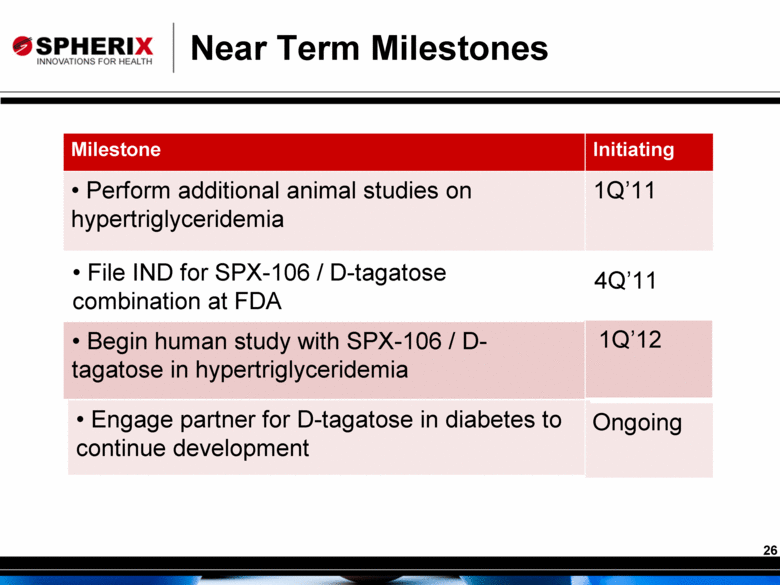
Near Term Milestones Milestone Initiating Perform additional animal studies on hypertriglyceridemia 1Q’11 Begin human study with SPX-106 / D-tagatose in hypertriglyceridemia 1Q’12 Engage partner for D-tagatose in diabetes to continue development Ongoing File IND for SPX-106 / D-tagatose combination at FDA 4Q’11 |
|
|
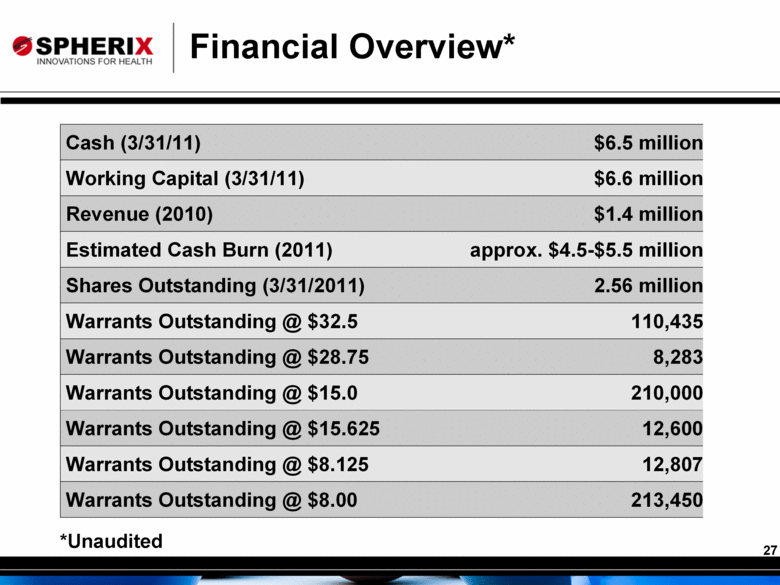
Financial Overview* *Unaudited Cash (3/31/11) $6.5 million Working Capital (3/31/11) $6.6 million Revenue (2010) $1.4 million Estimated Cash Burn (2011) approx. $4.5-$5.5 million Shares Outstanding (3/31/2011) 2.56 million Warrants Outstanding @ $32.5 110,435 Warrants Outstanding @ $28.75 8,283 Warrants Outstanding @ $15.0 210,000 Warrants Outstanding @ $15.625 12,600 Warrants Outstanding @ $8.125 12,807 Warrants Outstanding @ $8.00 213,450 |
|
|
Investment Highlights D-tagatose and SPX-106 show promise in multiple clinical applications Hypertriglyceridemia ($26 billion treatment market) Diabetes ($175 billion treatment market) Preclinical and Phase 2 data show significant lowering of triglycerides Much faster and less expensive development pathway vs. diabetes Clinical development program initiated Cash position sufficient to fund initial triglyceride studies Phase 3 study demonstrated statistically significant reductions in HbA1c in patients with mild Type 2 diabetes Pursuing partnership(s) for further clinical development |