Attached files
| file | filename |
|---|---|
| EX-32.1 - Bohai Pharmaceuticals Group, Inc. | v222146_ex32-1.htm |
| EX-32.2 - Bohai Pharmaceuticals Group, Inc. | v222146_ex32-2.htm |
| EX-31.2 - Bohai Pharmaceuticals Group, Inc. | v222146_ex31-2.htm |
| EX-31.1 - Bohai Pharmaceuticals Group, Inc. | v222146_ex31-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
|
x
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the quarterly period ended March 31, 2011
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from to
Commission File Number: 000-53401
Bohai Pharmaceuticals Group, Inc.
(Exact name of registrant as specified in its charter)
|
Nevada
|
98-0588402
|
|
(State or other jurisdiction of
|
(I.R.S. Employer Identification No.)
|
|
incorporation or organization)
|
|
c/o Yantai Bohai Pharmaceuticals Group Co. Ltd.
|
|
|
No. 9 Daxin Road, Zhifu District
|
|
|
Yantai, Shandong Province, China
|
264000
|
|
(Address of principal executive offices)
|
(Zip Code)
|
Registrant’s telephone number (including area code): +86(535)-685-7928
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer or a smaller reporting company. See definition of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer ¨
|
Accelerated filer ¨
|
|
|
Non-accelerated filer ¨
|
Smaller reporting company x
|
|
|
(Do not check if a smaller reporting company)
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ Nox
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Date File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ¨
As of May 12, 2011, there were 17,821,085 shares of company common stock issued and outstanding.
Bohai Pharmaceuticals Group, Inc.
Quarterly Report on Form 10-Q
TABLE OF CONTENTS
|
PART I – FINANCIAL INFORMATION
|
||
|
Cautionary Note Regarding Forward-Looking Statements
|
||
|
Item 1.
|
Financial Statements (unaudited)
|
|
|
Condensed Consolidated Balance Sheets as of March 31, 2011 and June 30, 2010 (audited)
|
1 | |
|
Condensed Consolidated Statements of Income and Comprehensive Income for the Three and Nine Months ended March 31, 2011 and 2010
|
2 | |
|
Condensed Consolidated Statements of Changes in Stockholders’ Equity for the Nine Months Ended March 31, 2011
|
3 | |
|
Condensed Consolidated Statements of Cash Flows for the Nine Months ended March 31, 2011 and 2010
|
4 | |
|
Notes to Condensed Consolidated Financial Statements
|
5 | |
|
Item 2.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
35 |
|
Item 3.
|
Quantitative and Qualitative Disclosures About Market Risk
|
49 |
|
Item 4(T).
|
Controls and Procedures
|
49 |
|
PART II – OTHER INFORMATION
|
||
|
Item 1.
|
Legal Proceedings
|
51 |
|
Item 2.
|
Unregistered Sales of Equity Securities and Use of Proceeds
|
51 |
|
Item 3.
|
Defaults Upon Senior Securities
|
51 |
|
Item 4.
|
Removed and Reserved
|
51 |
|
Item 5.
|
Other Information
|
51 |
|
Item 6.
|
Exhibits
|
51 |
|
SIGNATURES
|
52 | |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
In addition to historical information, this Quarterly Report on Form 10-Q contains forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from those reflected in such forward-looking statements. We cannot give any guarantee that the plans, intentions or expectations described in the forward looking statements will be achieved. All forward-looking statements involve significant risks and uncertainties, and actual results may differ materially from those discussed in the forward-looking statements as a result of various factors, including those factors described in the “Risk Factors” section of our Annual Report for the fiscal year ended June 30, 2010 (the “2010 10-K”). Readers should carefully review such risk factors as well as factors described in other documents that we file from time to time with the Securities and Exchange Commission.
In some cases, you can identify forward-looking statements by terminology such as “guidance,” “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “projects,” “potential,” “proposed,” “intended,” or “continue” or the negative of these terms or other comparable terminology. You should read statements that contain these words carefully, because they discuss our expectations about our future operating results or our future financial condition or state other “forward-looking” information. There may be events in the future that we are not able to accurately predict or control. You should be aware that the occurrence of any of the events described in our risk factors and other disclosures could substantially harm our business, results of operations and financial condition, and that upon the occurrence of any of these events, the trading price of our securities could decline. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, growth rates, and levels of activity, performance or achievements. Factors that may cause actual results, our performance or achievements, or industry results, to differ materially from those contemplated by such forward-looking statements include, without limitation:
|
|
·
|
our ability to obtain sufficient working capital to support our business plans;
|
|
|
·
|
our ability to expand our product offerings and maintain the quality of our products;
|
|
|
·
|
the availability of Chinese government granted rights to exclusively manufacture or co-manufacture our products;
|
|
|
·
|
the availability of Chinese national healthcare reimbursement of our products;
|
|
|
·
|
our ability to manage our expanding operations and continue to fill customers’ orders on time;
|
|
|
·
|
our ability to maintain adequate control of our expenses allowing us to realize anticipated revenue growth;
|
|
|
·
|
our ability to maintain or protect our intellectual property;
|
|
|
·
|
our ability to maintain our proprietary technology;
|
|
|
·
|
the impact of government regulation in China and elsewhere, including the support provided by the Chinese government to the Traditional Chinese Medicine and healthcare sectors in China;
|
|
|
·
|
our ability to implement product development, marketing, sales and acquisition strategies and adapt and modify them as needed;
|
|
|
·
|
our ability to integrate any future acquisitions;
|
|
|
·
|
our implementation of required financial, accounting and disclosure controls and procedures and related corporate governance policies; and
|
|
|
·
|
our ability to anticipate and adapt to changing conditions in the Traditional Chinese Medicine and healthcare industries resulting from changes in government regulations, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics.
|
Readers are cautioned not to place undue reliance on our forward-looking statements, which reflect management’s opinions only as of the date thereof. We undertake no obligation to revise or publicly release the results of any revision of our forward-looking statements, except as required by law.
BOHAI PHARMACEUTICALS GROUP, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
AS OF MARCH 31, 2011 AND JUNE 30, 2010
|
As of
|
As of
|
|||||||
|
March 31,
|
June 30,
|
|||||||
|
2011
|
2010
|
|||||||
|
(unaudited)
|
||||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 10,665,517 | $ | 17,149,082 | ||||
|
Restricted cash
|
220,043 | 576,019 | ||||||
|
Accounts receivable
|
14,798,323 | 10,409,527 | ||||||
|
Other receivables and prepayments
|
2,006,268 | 1,449,590 | ||||||
|
Amount due from equity holder
|
- | 40,160 | ||||||
|
Inventories
|
1,997,545 | 748,422 | ||||||
|
Total current assets
|
29,687,697 | 30,372,801 | ||||||
|
Non-current assets
|
||||||||
|
Property, plant and equipment, net
|
7,925,075 | 7,895,042 | ||||||
|
Prepayment for land use right
|
14,839,957 | 7,343,654 | ||||||
|
Intangible assets
|
25,278,154 | 17,342,772 | ||||||
|
Deferred fees on convertible notes
|
719,819 | 1,562,617 | ||||||
|
Total non-current assets
|
48,763,005 | 34,144,085 | ||||||
|
TOTAL ASSETS
|
$ | 78,450,702 | $ | 64,516,886 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$ | 1,466,525 | $ | 741,621 | ||||
|
Other accrued liabilities
|
4,909,783 | 2,984,988 | ||||||
|
Amount due to equity holder
|
11,980 | - | ||||||
|
Income taxes payable
|
945,678 | 700,326 | ||||||
|
Short-term borrowings
|
905,618 | 4,398,849 | ||||||
|
Total current liabilities
|
8,239,584 | 8,825,784 | ||||||
|
Non-current liabilities
|
||||||||
|
Derivative liabilities - investor and agent warrants
|
2,300,325 | 5,481,928 | ||||||
|
Convertible notes, net of discount
|
438,743 | 124,820 | ||||||
|
Total non-current liabilities
|
2,739,068 | 5,606,748 | ||||||
|
TOTAL LIABILITIES
|
10,978,652 | 14,432,532 | ||||||
|
STOCKHOLDERS' EQUITY
|
||||||||
|
Common stock , $0.001 par value, 150,000,000 shares authorized, 17,821,085 and 16,500,000 shares issued and outstanding as of March 31, 2011 and June 30, 2010, respectively
|
17,821 | 16,500 | ||||||
|
Additional paid-in capital
|
18,320,431 | 15,317,621 | ||||||
|
Accumulated other comprehensive income
|
2,771,433 | 626,584 | ||||||
|
Statutory reserves
|
2,201,817 | 2,201,817 | ||||||
|
Retained earnings
|
44,160,548 | 31,921,832 | ||||||
|
Total stockholders’ equity
|
67,472,050 | 50,084,354 | ||||||
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY
|
$ | 78,450,702 | $ | 64,516,886 | ||||
See accompanying notes to the unaudited condensed consolidated financial statements
1
BOHAI PHARMACEUTICALS GROUP, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF
INCOME AND COMPREHENSIVE INCOME
FOR THE THREE AND NINE MONTHS ENDED MARCH 31, 2011 AND 2010
(UNAUDITED)
|
For The Three Months Ended
|
For The Nine Months Ended
|
|||||||||||||||
|
March 31,
|
March 31,
|
|||||||||||||||
|
2011
|
2010
|
2011
|
2010
|
|||||||||||||
|
Net revenues
|
$ | 22,153,412 | $ | 15,323,878 | $ | 61,289,991 | $ | 46,072,455 | ||||||||
|
Cost of revenues
|
5,213,548 | 2,841,385 | 13,341,860 | 8,205,715 | ||||||||||||
|
Gross profit
|
16,939,864 | 12,482,493 | 47,948,132 | 37,866,740 | ||||||||||||
|
Selling, general and administrative expenses
|
12,845,962 | 9,202,873 | 32,565,981 | 28,208,753 | ||||||||||||
|
Income from operations
|
4,093,901 | 3,279,620 | 15,382,151 | 9,657,987 | ||||||||||||
|
Other income (expenses)
|
||||||||||||||||
|
Other income
|
1,783 | - | 99,901 | 18,864 | ||||||||||||
|
Interest income
|
11,176 | - | 40,673 | - | ||||||||||||
|
Amortization of deferred financing fees
|
(232,200 | ) | (253,577 | ) | (736,224 | ) | (253,577 | ) | ||||||||
|
Interest expenses
|
(554,428 | ) | (381,700 | ) | (2,203,775 | ) | (538,008 | ) | ||||||||
|
Other expenses
|
(546 | ) | - | (2,468 | ) | (22,092 | ) | |||||||||
|
Change in fair value of derivative liabilities
|
263,118 | 1,083,350 | 3,181,603 | 1,083,350 | ||||||||||||
|
Total other income (expenses)
|
(511,097 | ) | 448,073 | 379,710 | 288,537 | |||||||||||
|
Income before provision for income taxes
|
3,582,804 | 3,727,693 | 15,761,860 | 9,946,524 | ||||||||||||
|
Provision for income taxes
|
(897,458 | ) | (585,135 | ) | (3,523,145 | ) | (2,193,931 | ) | ||||||||
|
Net income
|
$ | 2,685,346 | $ | 3,142,558 | $ | 12,238,716 | $ | 7,752,593 | ||||||||
|
Comprehensive income:
|
||||||||||||||||
|
Net income
|
2,685,346 | 3,142,558 | 12,238,716 | 7,752,593 | ||||||||||||
|
Other comprehensive income
|
||||||||||||||||
|
Unrealized foreign currency translation gain
|
415,307 | (157,384 | ) | 2,144,849 | (108,823 | ) | ||||||||||
|
Comprehensive income
|
$ | 3,100,653 | $ | 2,985,174 | $ | 14,383,564 | $ | 7,643,770 | ||||||||
|
Earnings per common share
|
||||||||||||||||
|
Basic
|
$ | 0.15 | $ | 0.20 | $ | 0.72 | $ | 0.48 | ||||||||
|
Diluted
|
$ | 0.14 | $ | 0.16 | $ | 0.59 | $ | 0.37 | ||||||||
|
Weighted average common shares outstanding
|
||||||||||||||||
|
Basic
|
17,544,163 | 16,078,472 | 16,988,489 | 16,193,659 | ||||||||||||
|
Diluted
|
22,808,885 | 21,745,139 | 22,439,202 | 22,084,170 | ||||||||||||
See accompanying notes to the unaudited condensed consolidated financial statements
2
BOHAI PHARMACEUTICALS GROUP, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF
CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE NINE MONTHS ENDED MARCH 31, 2011
(UNAUDITED)
|
Common stock
|
Additional
|
Accumulated
other
|
Total
|
|||||||||||||||||||||||||
|
Shares
outstanding
|
Amount
|
paid-in
capital
|
comprehensive
income
|
Statutory
reserves
|
Retained
Earnings
|
Stockholders’
Equity
|
||||||||||||||||||||||
|
Balance at June 30, 2010
|
16,500,000 | $ | 16,500 | $ | 15,317,622 | $ | 626,584 | $ | 2,201,817 | $ | 31,921,832 | $ | 50,084,354 | |||||||||||||||
|
Net income for the period
|
- | - | - | - | - | 12,238,716 | 12,238,716 | |||||||||||||||||||||
|
Stock based compensation
|
45,000 | 45 | 160,455 | - | - | - | 160,500 | |||||||||||||||||||||
|
Option based compensation
|
- | - | 23,844 | - | - | - | 23,844 | |||||||||||||||||||||
|
Conversion of convertible notes
|
527,703 | 528 | 948,304 | - | - | - | 948,832 | |||||||||||||||||||||
|
Sale of common stock
|
748,382 | 748 | 1,870,207 | 1,870,955 | ||||||||||||||||||||||||
|
Foreign currency translation difference
|
- | - | - | 2,144,849 | - | - | 2,144,849 | |||||||||||||||||||||
|
Balance at March 31, 2011
|
17,821,085 | $ | 17,821 | $ | 18,320,431 | $ | 2,771,433 | $ | 2,201,817 | $ | 44,160,548 | $ | 67,472,050 | |||||||||||||||
See accompanying notes to the unaudited condensed consolidated financial statements
3
BOHAI PHARMACEUTICALS GROUP, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE NINE MONTHS ENDED MARCH 31, 2011 AND 2010
(UNAUDITED)
|
For the Nine Months Ended
|
||||||||
|
March 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Cash flows from operating activities
|
||||||||
|
Net income
|
$ | 12,238,716 | $ | 7,752,593 | ||||
|
Adjustments to reconcile net income to net cash used in operating activities:
|
||||||||
|
Depreciation
|
260,577 | 224,656 | ||||||
|
Loss on disposal of property, plant and equipment
|
1,908 | 10,942 | ||||||
|
Accretion of beneficial conversion feature
|
1,029,487 | - | ||||||
|
Amortization of deferred fees on convertible notes
|
736,224 | 253,577 | ||||||
|
Interest expense on convertible notes
|
339,842 | 121,126 | ||||||
|
Change in fair value of warrants
|
(3,181,603 | ) | (1,083,350 | ) | ||||
|
Stock and option based compensation
|
184,344 | - | ||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
(Increase) in accounts receivable
|
(3,946,124 | ) | (433,728 | ) | ||||
|
(Increase)/decrease in other receivables and prepayments
|
(496,515 | ) | 4,127,297 | |||||
|
Decrease in amount due from equity holder
|
- | 1,465,000 | ||||||
|
(Increase) in inventories
|
(1,201,923 | ) | (662,830 | ) | ||||
|
Increase (decrease) in accrued liabilities
|
158,949 | (10,407,917 | ) | |||||
|
Increase/ (decrease) in accounts payable
|
686,541 | (128,858 | ) | |||||
|
Increase in other payable
|
1,633,817 | - | ||||||
|
Increase in income taxes payable
|
216,325 | 2,905,998 | ||||||
|
Increase in restricted cash
|
355,976 | - | ||||||
|
Net cash provided by operating activities
|
9,016,541 | 4,144,506 | ||||||
|
Cash flows used in investing activities
|
||||||||
|
Purchases of property, plant and equipment
|
(14,619 | ) | (280,804 | ) | ||||
|
Proceeds from disposal of property, plant and equipment
|
4,491 | - | ||||||
|
Purchase of leased land use rights
|
(7,111,204 | ) | - | |||||
|
Purchase of intangible assets
|
(7,186,059 | ) | - | |||||
|
Net cash used in investing activities
|
(14,307,391 | ) | (280,804 | ) | ||||
|
Cash flows from financing activities
|
||||||||
|
Proceeds from borrowings
|
890,772 | 4,381,153 | ||||||
|
Repayment of borrowings
|
(4,483,801 | ) | (5,860,000 | ) | ||||
|
Repayment from related party
|
53,147 | - | ||||||
|
Cash fees on placement agent and other financing costs
|
- | (1,570,000 | ) | |||||
|
Proceeds from issuance of convertible promissory notes
|
- | 12,000,000 | ||||||
|
Proceeds from sale of common stock
|
1,870,955 | - | ||||||
|
Net cash flows (used in) provided by financing activities
|
(1,668,927 | ) | 8,951,153 | |||||
|
Effect of foreign currency translation on cash and cash equivalents
|
476,212 | 266,544 | ||||||
|
Net (decrease) increase in cash and cash equivalents
|
(6,483,565 | ) | 13,081,399 | |||||
|
Cash and cash equivalents at beginning of period
|
17,149,082 | 2,493,510 | ||||||
|
Cash and cash equivalents at end of period
|
$ | 10,665,517 | $ | 15,574,909 | ||||
|
Cash paid during the period for:
|
||||||||
|
Interest paid
|
$ | 811,582 | $ | 466,135 | ||||
|
Income taxes paid
|
$ | 3,306,820 | $ | 2,305,073 | ||||
|
Supplemental cash flow information
|
||||||||
|
Non-cash investing and financing activities:
|
||||||||
|
Common stock issued upon conversion of convertible notes and accrued interest
|
$ | 948,832 | $ | - | ||||
|
Placement agent warrants issued
|
$ | - | $ | 582,454 | ||||
See accompanying notes to the unaudited condensed consolidated financial statements
4
BOHAI PHARMACEUTICALS GROUP, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND NINE MONTHS ENDED MARCH 31, 2011 AND 2010
|
1.
|
ORGANIZATION AND PRINCIPAL ACTIVITIES
|
Bohai Pharmaceuticals Group, Inc., or the Company (formerly known as Link Resources, Inc.), was incorporated under the laws of the State of Nevada on January 9, 2008. Until January 5, 2010, our principal office was located in Calgary, Alberta, Canada. Prior to January 5, 2010, we were a public “shell” company in the exploration stage since our formation had not yet realized any revenues from our planned operations.
Pursuant to a Share Exchange Agreement, dated January 5, 2010 (the “Share Exchange Agreement” and the transactions contemplated thereby, the “Share Exchange”), the Company acquired Chance High International Limited, a British Virgin Islands company, or Chance High, from Chance High’s shareholders, or the Chance High shareholders, and, as a result, acquired Chance High’s indirect, controlled affiliate, Yantai Bohai Pharmaceuticals Group Co., Ltd., or Bohai, a Chinese company engaged the production, manufacturing and distribution in the People’s Republic of China (“China” or the “PRC”) of herbal medicines, including capsules and other products, based on traditional Chinese medicine.
The closing of the Share Exchange, or the Closing, took place on January 5, 2010, or the Closing Date. On the Closing Date, pursuant to the terms of the Share Exchange Agreement, the Company acquired all of the outstanding equity securities, or the Chance High shares, of Chance High from the Chance High Shareholders, and the Chance High Shareholders transferred and contributed all of their Chance High Shares to the Company. In exchange, we issued to Chance High Shareholders an aggregate of 13,162,500 newly issued shares of common stock, par value $0.001 per share, or the Common Stock. In addition, pursuant to the terms of the Share Exchange Agreement, Anthony Zaradic, the former President and Chief Executive Officer of the Company, cancelled a total of 1,500,000 shares of Common Stock.
Chance High owns 100% of the issued and outstanding capital stock of a Chinese wholly-foreign owned enterprise, Yantai Shencaojishi Pharmaceuticals Co., Ltd., or the WFOE. On December 7, 2009, the WFOE entered into a series of variable interest entity contractual agreements, or the VIE Agreements, with Bohai and its three shareholders, including Mr. Hongwei Qu, currently the Company’s Chairman, Chief Executive Officer and President, pursuant to which WFOE effectively assumed management of the business activities of Bohai and has the right to appoint all executives and senior management and the members of the board of directors of Bohai.
Chance High, WFOE and Bohai are referred to herein collectively and on a consolidated basis as the “Company” or “we”, “us” or “our” or similar terminology.
The VIE Agreements are comprised of a series of agreements, including a Consulting Services Agreement, Operating Agreement and Proxy Agreement, through which WFOE has the right to advise, consult, manage and operate Bohai for an annual fee in the amount of Bohai’s yearly net profits after tax. Additionally, Bohai’s shareholders pledged their rights, titles and equity interest in Bohai as security for WFOE to collect consulting and services fees provided to Bohai through an equity pledge agreement. In order to further reinforce WFOE’s rights to control and operate Bohai, Bohai’s shareholders granted WFOE an exclusive right and option to acquire all of their equity interests in Bohai through an option agreement.
5
|
1.
|
ORGANIZATION AND PRINCIPAL ACTIVITIES - Continued
|
On January 29, 2010, we entered into an agreement and plan of merger, the sole purpose of which was to effect a change of our corporate name from Link Resources Inc. to Bohai Pharmaceuticals Group, Inc.
We are engaged in the production, manufacturing and distribution of herbal pharmaceuticals based on traditional Chinese medicine, or TCM, in the People’s Republic of China. We are based in the city of Yantai, Shandong Province, China, and our operations are exclusively in China.
|
2.
|
BASIS OF PREPARATION
|
The accompanying unaudited condensed consolidated financial statements of our company and our subsidiaries at March 31, 2011 and for the three and nine months ended March 31, 2011 and 2010 reflect all adjustments (consisting only of normal recurring adjustments) that, in the opinion of management, are necessary to present fairly our consolidated financial position and results of operations for the periods presented. Operating results for the three and nine months ended March 31, 2011 are not necessarily indicative of the results that may be expected for the year ending June 30, 2011. The accompanying condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements and the notes thereto included in our Annual Report on Form 10-K filed with the Securities and Exchange Commission on September 28, 2010.
The accompanying unaudited condensed consolidated financial statements for our company, our subsidiaries and our variable interest entity (Bohai) have been prepared in accordance with accounting principles generally accepted in the United States of America, or the US, for interim financial information and with the instructions to Form 10-Q and Article 8-03 of Regulation S-X. Operating results for interim periods are not necessarily indicative of results that may be expected for the fiscal year as a whole.
The Share Exchange was accounted for as a reverse recapitalization effected as of January 5, 2010. Although we legally acquired Chance High and its controlled subsidiary Bohai, for accounting purposes, Chance High and Bohai are considered to be the accounting acquirers and Link Resources, Inc. as the accounting acquiree. As a result, the historical consolidated financial statements for periods prior to January 5, 2010 are those of Chance High and Bohai and the operating results, financial position and cash flows of our company (formerly known as Link Resources, Inc.) are consolidated only from its acquisition on January 5, 2010. As the transaction between our company and Chance High and its subsidiaries is treated as reverse acquisition, no goodwill was recorded. Intercompany transactions and balances are eliminated in consolidation.
|
3.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
Basis of Presentation and Consolidation
We adopted FAS ASC 810-10-15-14 and also ASC 810-10-05-8, which requires that a Variable Interest Entity, or VIE, to be consolidated by a company if that company is entitled to receive a majority of the VIE’s residual returns and has the direct ability to make decisions on all operating activities of the voting right of the VIE. We controls Bohai through the VIE Agreements described in Note 1 and accordingly it is consolidated for all periods presented.
The Operating Agreement provides that the WFOE has the direct ability to make decisions on all the operating activities and exercise all voting rights of Bohai, the Company’s VIE.
6
|
3.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES – Continued
|
Under Consultant Service Agreement entered between WFOE and Bohai on December 7, 2009, Bohai agreed to pay all of its net income to WOFE quarterly as a consulting fee. Accordingly, WOFE has the right to receive the expected residual returns of Bohai.
Under the above mentioned contractual arrangement, our company qualifies as the primary beneficiary of such controlling financial interest in Bohai as operating under ASC 810-10-15-14, an Interpretation of Accounting Research Bulletin No. 51. The results of subsidiaries or VIEs acquired prior to the date of Share Exchange Agreement on January 5, 2010 are included in the consolidated financial statements.
As of March 31, 2011, the particulars of our company’s subsidiaries and VIE are as follows:
|
Name of Company
|
Place of
incorporation
|
Date of
incorporation
|
Attributable
equity interest
|
Issued Capital
(US Dollars)
|
||||||||
|
Chance High International Limited
|
British Virgin Islands
|
July 2, 2009
|
100% | $ | 50,000 | |||||||
|
Yantai Shencaojishi
Pharmaceuticals Co., Ltd.
|
People’s Republic of China
|
November 25, 2009
|
100% | $ | 10,000,000 | |||||||
|
Yantai Bohai Pharmaceuticals Group Co., Ltd.
|
People’s Republic of China
|
July 8, 2004
|
* | $ |
2,918,000
|
|||||||
|
*
|
We have an indirect controlling interest in Bohai under the VIE Agreements entered on December 7, 2009, which are described in Note 1 above.
|
Initial measurement of VIE: we initially measured the assets, liabilities, and non-controlling interests of the VIEs at their carrying amount as of the date of the acquisition.
Accounting after initial measurement of VIE: subsequent accounting for the assets, liabilities, and non- controlling interest of a consolidated VIE are accounted for as if the entity were consolidated based on voting interests and the usual accounting rules for which the VIE operates are applied as they would to a consolidated subsidiary as follows:
|
·
|
Carrying amounts of the VIE are consolidated into the financial statements of the Company as the primary beneficiary, or Primary Beneficiary, or PB; and
|
|
·
|
Inter-company transactions and balances, such as revenues and costs, receivables and payables between or among the Primary Beneficiary and the VIE(s) are eliminated in their entirety.
|
7
|
3.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES – Continued
|
The carrying amount and classification of Yantai Bohai’s assets and liabilities included in the Consolidated Balance Sheets are as follows:
|
March 31, 2011
|
June 30, 2010
|
|||||||
|
(unaudited)
|
||||||||
|
Total current assets*
|
$
|
45,056,495
|
$
|
28,177,777
|
||||
|
Total assets*
|
78,259,724
|
53,415,591
|
||||||
|
Total current liabilities**
|
19,654,445
|
9,005,735
|
||||||
|
Total liabilities**
|
$
|
19,654,445
|
$
|
9,005,735
|
||||
* Including intercompany accounts of $1,627,814 and $394,821 as at March 31, 2011 and June 30, 2010 be eliminated in consolidation.
** Including intercompany accounts of $11,471,035 and $457,004 as at March 31, 2011 and June 30, 2010 be eliminated in consolidation
Economic and Political Risks
Our operations are conducted solely in the PRC. There are significant risks associated with doing business in the PRC, among others, political, economic, legal and foreign currency exchange risks. Our results may be adversely affected by changes in the political and social conditions in the PRC, and by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion, remittances abroad, and rates and methods of taxation, among other things.
Use of Estimates
In preparing the condensed consolidated financial statements in conformity with US GAAP, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. These accounts and estimates include, but are not limited to, the valuation of accounts receivable, inventories, deferred income taxes, the estimation on useful lives of plant and machinery, and the fair value of derivative liabilities. Actual results could differ from those estimates.
Fair Value Measurements and Fair Value of Financial Instruments
We adopted the guidance of Accounting Standards Codification, or ASC, 820 for fair value measurements, which clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2 - Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other then quoted prices that are observable, and inputs derived from or corroborated by observable market data.
8
|
3.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES – Continued
|
Level 3 - Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
The carrying amounts reported in the balance sheets for cash, accounts receivable, other receivables, short-term borrowings, accounts payable and accrued expenses, customer advances, and amounts due from related parties approximate their fair market value based on the short-term maturity of these instruments.
ASC 825-10 “Financial Instruments,” allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair value option may be elected on an instrument-by-instrument basis and is irrevocable, unless a new election date occurs. If the fair value option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings at each subsequent reporting date. We use Level 3 inputs to value our derivative liabilities.
The following table reflects gains and losses for the three and nine months ended March 31, 2011 for all financial assets and liabilities categorized as Level 3 as of March 31, 2011.
|
Liabilities:
|
||||
|
Balance of derivative liabilities as of December 31, 2010
|
$
|
2,563,443
|
||
|
Change in the fair value of derivative liabilities
|
(263,118)
|
|||
|
Balance of derivative liabilities as of March 31, 2011
|
$
|
2,300,325
|
||
|
Liabilities:
|
||||
|
Balance of derivative liabilities as of June 30, 2010
|
$
|
5,481,928
|
||
|
Change in the fair value of derivative liabilities
|
(3,181,603)
|
|||
|
Balance of derivative liabilities as of March 31, 2011
|
$
|
2,300,325
|
||
Estimating fair values of derivative financial instruments require the development of significant and subjective estimates that may, and are likely to, change over the duration of the instrument with related changes in internal and external market factors. In addition, valuation techniques are sensitive to changes in the trading market price of our Common Stock and its estimated volatility. Because derivative financial instruments are initially and subsequently carried at fair values, our net income may include significant charges or credits as these estimates and assumptions change.
The potential credit risk to our company is mainly attributable to its accounts receivable and bank balances. We have policies in place to ensure that we will only accept customers from countries which are politically stable and customers with an appropriate credit history. In addition, all bank balances are on deposit with financial institutions with high-credit quality. Accordingly, we do not consider that we are subject to significant credit risk.
Our interest rate risk is primarily attributable to our borrowings, all of which have fixed interest rates. We do not use interest rate swaps to hedge our exposure to interest rate risk.
Cash and Cash Equivalents
We consider all highly liquid investments purchased with original maturities of three months or less to be cash equivalents. We maintain bank accounts in the PRC and restricted cash accounts and a checking account in the United States of America. The restricted cash accounts were created for interest payments due to convertible note holders and payments for investor relations activities in the US.
9
|
3.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES – Continued
|
Concentrations of Credit Risk
Financial instruments which potentially subject us to concentrations of credit risk consist principally of cash and trade accounts receivable. Substantially all of our cash is maintained with state-owned banks within the PRC, and no deposits are covered by insurance. We have not experienced any losses in such accounts and believe we are not exposed to any risks on our cash in bank accounts. A significant portion of our sales are credit sales which are primarily to customers whose ability to pay is dependent upon the industry economics prevailing in these areas; however, concentrations of credit risk with respect to trade accounts receivables is limited due to generally short payment terms. We also perform ongoing credit evaluations of our customers to help further reduce credit risk.
At March 31, 2011 and June 30, 2010, our cash balances by geographic area were as follows:
|
|
|
March 31, 2011
|
June 30, 2010
|
|
||||||||||||
|
|
|
(unaudited)
|
|
|||||||||||||
|
Country:
|
||||||||||||||||
|
United States
|
$
|
31,829
|
0.3
|
%
|
$
|
-
|
-
|
%
|
||||||||
|
China
|
10,633,688
|
99.7
|
%
|
17,149,082
|
100.0
|
%
|
||||||||||
|
Total cash and cash equivalents
|
$
|
10,665,517
|
100.0
|
%
|
$
|
17,149,082
|
100.0
|
%
|
||||||||
Accounts Receivable
Accounts receivable consists of amounts due from customers. We extend unsecured credit to our customers in the ordinary course of business but mitigate the associated risks by performing credit checks and actively pursuing past due accounts. An allowance for doubtful accounts is established and determined based on management’s assessment of known requirements, aging of receivables, payment history, the customer’s current credit worthiness and the economic environment. As of March 31, 2011 and June 30, 2010, no allowance for doubtful accounts was deemed necessary based on management’s assessment.
Inventories
Inventories are valued at the lower of cost or market with cost is determined using the weighted average method. Finished goods inventories consist of raw materials, direct labor and overhead associated with the manufacturing process. In assessing the ultimate realization of inventories, management makes judgments as to future demand requirements compared to current or committed inventory levels. Our reserve requirements generally increase/decrease due to management’s projected demand requirements, market conditions and product life cycle changes. As of March 31, 2011 and June 30, 2010, we did not make any allowance for slow-moving or defective inventories.
Intangible Assets
Intangible assets consist of “Pharmaceutical Formulas”, which were acquired with indefinite useful lives. These intangible assets are measured initially at cost and not subject to amortization and will be tested for impairment annually or more frequently if there is indication of impairment. If the carrying amount exceeds fair value, an impairment loss would be recognized. Subsequently reversal of a recognized impairment loss is prohibited. There was no impairment of the intangible assets as of March 31, 2011 and June 30, 2010.
10
|
3.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES – Continued
|
Property, Plant and Equipment
Property, plant and equipment are carried at cost and are depreciated on a straight-line basis over the estimated useful lives of the assets. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the year of disposition. We examine the possibility of decreases in the value of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable.
Included in property and equipment was construction-in-progress which consisted of factories and office buildings under construction and machinery pending installation and includes the costs of construction, machinery and equipment, and any interest charges arising from borrowings used to finance these assets during the period of construction or installation. No provision for depreciation is made on construction-in-progress until such time as the relevant assets are completed and ready for their intended use. The principal annual rates are as follows:
|
Leasehold land and buildings
|
30 to 40 years
|
|
|
Motor vehicles
|
10 years
|
|
|
Plant and machinery
|
10 years
|
|
|
Office equipment
|
5 years
|
Accounting for the Impairment of Long-Lived Assets
We use ASC Topic 360, which addresses financial accounting and reporting for the impairment or disposal of long-lived assets. We periodically evaluate the carrying value of long-lived assets to be held and used in accordance with ASC Topic 360. ASC Topic 360 requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amounts. In that event, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the long-lived assets. Loss on long-lived assets to be disposed of is determined in a similar manner, except that fair market values are reduced for the cost of disposal. Based on our review, we believe that, as of March 31, 2011 and June 30, 2010, there were no impairments of our long-lived asset.
Foreign Currency Translation
Our reporting currency is the U.S. dollar. We maintain our consolidated financial statements in the functional currency. Our functional currency is the Chinese Renminbi, or RMB. For our subsidiaries and affiliates whose functional currencies are the RMB, results of operations and cash flows are translated at average exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period, and equity is translated at historical exchange rates. As a result, amounts relating to assets and liabilities reported on the statements of cash flows may not necessarily agree with the changes in the corresponding balances on the balance sheets. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive income. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchanges rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods. All of our revenue transactions are transacted in the functional currency. We do not enter any material transaction in foreign currencies and, accordingly, transaction gains or losses have not had, and are not expected to have a material effect on our results of operations.
11
|
3.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES – Continued
|
Assets and liabilities are translated at the exchange rates at the balance sheet dates and revenue and expenses are translated at the average exchange rates using the following exchange rates:
|
|
Nine months ended
March 31, 2011
|
Year ended
June 30, 2010
|
Nine months ended
March 31, 2010
|
||||||
|
Period end US$: RMB exchange rate
|
6.57010
|
6.80860
|
6.84560
|
||||||
|
Average periodic US$: RMB exchange rate
|
6.67960
|
6.83667
|
6.85094
|
||||||
RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US dollar at the rates used in translation.
Revenue Recognition
Revenue represents the invoiced value of goods sold recognized upon the delivery of goods to distributors. Pursuant to the guidance of ASC Topic 605 and ASC Topic 36, revenue is recognized when all of the following criteria are met:
|
·
|
Persuasive evidence of an arrangement exists;
|
|
·
|
Delivery has occurred or services have been rendered;
|
|
·
|
The seller’s price to the buyer is fixed or determinable; and
|
|
·
|
Collectability is reasonably assured.
|
We account for sales returns by establishing an accrual in an amount equal to our estimate of sales recorded for which the related products are expected to be returned. We determine the estimate of the sales return accrual primarily based on our historical experience regarding sales returns, but also by considering other factors that could impact sales returns. These factors include levels of inventory in the distribution channel, estimated shelf life, product discontinuances, and price changes of competitive products, introductions of generic products and introductions of competitive new products. For the three and nine months ended March 31, 2011 and 2010, our sales return rate is low and deemed immaterial and accordingly, no provision for sales returns was recorded.
Cost of Revenue
Cost of revenue consists primarily of raw material costs, labor cost, overhead costs associated with the manufacturing process and related expenses which are directly attributable to our revenues.
12
|
3.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES – Continued
|
Stock-based Compensation
Stock based compensation is accounted for based on the requirements of the Share-Based Payment topic of ASC 718 which requires recognition in the financial statements of the cost of employee and director services received in exchange for an award of equity instruments over the employee or director’s requisite service period (presumptively, the vesting period). The FASB Accounting Standards Codification also requires measurement of the cost of employee and director services received in exchange for an award based on the grant-date fair value of the award.
Pursuant to ASC Topic 505-50, for share-based payments to consultants and other third-parties, compensation expense is determined at the “measurement date.” The expense is recognized over the vesting period of the award. Until the measurement date is reached, the total amount of compensation expense remains uncertain. We record compensation expense based on the fair value of the award at the reporting date. The awards to consultants and other third-parties are then revalued, or the total compensation is recalculated based on the then current fair value, at each subsequent reporting date.
Research and Development Costs
Research and development costs are charged as an expense when incurred and included in operating expenses. Research and development costs totaled $190,440 and $146,640 for the three months ended March 31, 2011 and 2010, respectively. Research and development costs totaled $562,261 and $442,046 for the nine months ended March 31, 2011 and 2010, respectively.
Shipping Costs
Shipping costs are included in selling, general and administrative expens and totaled $230,938 and $326,097 for the three months ended March 31, 2011 and 2010, respectively, and totaled $598,525 and $608,500 for the nine months ended March 31, 2011 and 2010, respectively,
Advertising and Promotion
Advertising and promotion is expensed as incurred. Advertising and promotion expenses were included in selling, general and administrative expenses and amounted to $3,962,454 and $2,767,549 for the three months ended March 31, 2011 and 2010, respectively, and amounted to $10,461,435 and $8,732,252 for the nine months ended March 31, 2011 and 2010, respectively.
Income Taxes
We are governed by the Income Tax Law of the People’s Republic of China and the Internal Revenue Code of the United States. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry-forwards. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the statements of income and comprehensive income in the periods that includes the enactment date.
13
|
3.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES – Continued
|
Comprehensive Income
Comprehensive income is defined to include all changes in equity except those resulting from investments by owners and distributions to owners. Among other disclosures, all items that are required to be recognized under current accounting standards as components of comprehensive income are required to be reported in a financial statement that is presented with the same prominence as other financial statements. Our current components of other comprehensive income are the foreign currency translation adjustment.
Commitments and Contingencies
Liabilities for loss contingencies arising from claims, assessments, litigation, fines and penalties and other sources are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
Earnings Per Share
We report basic earnings per share in accordance with ASC Topic 260, “Earnings Per Share”. Basic earnings/ (loss) per share is computed by dividing net income/ (loss) by weighted average number of shares of Common Stock outstanding during the period. Diluted earnings per share is computed by dividing net income by the weighted average number of shares of Common Stock, Common Stock equivalents and potentially dilutive securities outstanding during the period. Common equivalent shares are excluded from the computation in periods for which they have an anti-dilutive effect. Stock options for which the exercise price exceeds the average market price over the period are anti-dilutive and, accordingly, are excluded from the calculation. At March 31, 2011, we had 5,225,000 Common Stock equivalents from convertible notes and stock options to purchase 26,000 shares of Common Stock that could potentially dilute future earnings per share. Warrants to purchase 6,600,000 shares of Common Stock were outstanding during the three and nine months ended March 31, 2011, but were excluded from the computation of diluted earnings per share as their effect would have been anti-dilutive.
Reclassification
Sales tax of $231,870 and $729,975 for the three and nine months ended March 31, 2010, respectively, have been reclassified from net revenue to cost of revenue to confirm with the current presentation. The reclassification has no impact on the net income for the three and nine months ended March 31, 2010.
Recent Accounting Pronouncements Not Yet Adopted
In April 2010, the FASB issued ASU 2010-13, Compensation-Stock Compensation (Topic 718): Effect of Denominating the Exercise Price of a Share-Based Payment Award in the Currency of the Market in Which the Underlying Equity Security Trades - a consensus of the FASB Emerging Issues Task Force. The amendments in this Update are effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2010. Earlier application is permitted. We do not expect the provisions of ASU 2010-13 to have a material effect on our position, results of operations or cash flows.
14
|
3.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES – Continued
|
In December 2010, FASB issued ASU No. 2010-28, Intangibles - Goodwill and Other (ASC Topic 350). Under Topic 350 on goodwill and other intangible assets, testing for goodwill impairment is a two-step test. When a goodwill impairment test is performed (either on an annual or interim basis), an entity must assess whether the carrying amount of a reporting unit exceeds its fair value (Step 1). If it does, an entity must perform an additional test to determine whether goodwill has been impaired and to calculate the amount of that impairment (Step 2). The amendments in this update modify Step 1 of the goodwill impairment test for reporting units with zero or negative carrying amounts. For those reporting units, an entity is required to perform Step 2 of the goodwill impairment test if it is more likely than not that a goodwill impairment exists. In determining whether it is more likely than not that a goodwill impairment exists, an entity should consider whether there are any adverse qualitative factors indicating that an impairment may exist. The qualitative factors require that goodwill of a reporting unit be tested for impairment between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. The amendments in this update are effective for fiscal years, and interim periods within those years, beginning after December 15, 2010. Early adoption is not permitted. As we do not have any significant intangible assets, we believe that the impact of adopting this update will not be material on our consolidated results of operations and financial position.
In December 2010, FASB issued Accounting Standards Update (ASU) No. 2010-29, Business Combinations (ASC Topic 805). The amendments in this update specify that if a public entity presents comparative financial statements, the entity should disclose revenue and earnings of the combined entity as though the business combination(s) that occurred during the current year had occurred as of the beginning of the comparable prior annual reporting period only. The amendments also improve the usefulness of the pro forma revenue and earnings disclosures by requiring a description of the nature and amount of material, nonrecurring pro forma adjustments that are directly attributable to the business combination(s). The amendments in this update are effective for fiscal years, and interim periods within those years, beginning after December 15, 2010. Early adoption is permitted. As we did not enter into any business combinations in fiscal year 2010, we believe that the adoption this update will not have any material impact on our financial statement disclosures. However, if we enter into material business combinations in the future, the adoption of this update may have significant impact on our financial statement disclosures.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on our consolidated financial statements upon adoption.
|
4.
|
OTHER RECEIVABLES AND PREPAYMENTS
|
Other receivables and prepayments consist of the following:
15
4. OTHER RECEIVABLES AND PREPAYMENTS - Continued
|
|
As of
|
As of
|
||||
|
March 31, 2011
|
June 30, 2010
|
|||||
|
(unaudited)
|
||||||
|
Prepayment for advertising and promotion
|
$ | 1,749,349 | $ | 1,198,484 | ||
|
Prepayment for director and office insurance
|
5,417 | 29,792 | ||||
|
Advance to suppliers
|
8,048 | - | ||||
|
Other receivables
|
243,454 | 221,314 | ||||
|
Total other receivables and prepayments
|
$ | 2,006,268 | $ | 1,449,590 | ||
|
5.
|
AMOUNT DUE FROM EQUITY HOLDER
|
Amount due from equity holder consists of the following:
|
|
As of
|
As of
|
||||||
|
March 31, 2011
|
June 30, 2010
|
|||||||
|
(unaudited)
|
||||||||
|
Mr. Hongwei Qu
|
$ | - | $ | 40,160 | ||||
The amount due from an equity holder (the Company’s Chairman, President and Chief Executive Officer) as of June 30, 2010 is unsecured, non-interest bearing. The balance of $40,160 was repaid in July 2010.
|
6.
|
INVENTORIES
|
Inventories consist of the following:
|
As of
|
As of
|
|||||||
|
March 31, 2011
|
June 30, 2010
|
|||||||
|
(unaudited)
|
||||||||
|
Raw materials
|
$ | 973,043 | $ | 445,693 | ||||
|
Finished goods
|
1,024,503 | 302,729 | ||||||
|
Total inventories
|
$ | 1,997,545 | $ | 748,422 | ||||
16
|
7.
|
INTANGIBLE ASSETS
|
Intangible assets consist of the following:
|
|
As of
|
As of
|
||||||
|
March 31, 2011
|
June 30, 2010
|
|||||||
|
(unaudited)
|
||||||||
|
Pharmaceuticals formulas, at cost
|
$ | 25,278,154 | $ | 17,342,772 | ||||
On December 9, 2010, we entered into an Intangible Assets Transfer Agreement with Shandong Daxin Microbiology Pharmaceutical Industry Co., Ltd. (“Daxin”), an unrelated party, pursuant to which Daxin transferred to us all rights and title for 14 State Food and Drug Administration previously approved traditional Chinese medicine formulas. The aggregate purchase price of approximately $7,186,100 (RMB 48,000,000) has been paid by March 31, 2011. The 14 new formulas consist of two new product categories, powder and pellet formulations, which are the most popular product formulations under Chinese government’s Essential Drug List (EDL). Additionally, 4 of the 14 formulas are included in the EDL and an additional 5 medicines are included in the National Drug Reimbursement List (NDRL). Inclusion on EDL or NDRL allows for up to 100% insurance coverage by the Chinese government.
|
8.
|
PROPERTY, PLANT AND EQUIPMENT, NET
|
Property, plant and equipment consisted of the following:
|
|
As of
|
As of
|
||||||
|
March 31, 2011
|
June 30, 2010
|
|||||||
|
(unaudited)
|
||||||||
|
Leasehold land and buildings
|
$ | 7,906,455 | $ | 7,629,498 | ||||
|
Plant equipment
|
1,279,825 | 1,238,343 | ||||||
|
Office equipment
|
103,129 | 81,799 | ||||||
|
Motor vehicles
|
421,947 | 414,648 | ||||||
|
Total
|
9,711,355 | 9,364,288 | ||||||
|
Less: accumulated depreciation
|
1,786,280 | 1,469,246 | ||||||
|
Property, plant and equipment, net
|
$ | 7,925,075 | $ | 7,895,042 | ||||
Depreciation expense for the three months ended March 31, 2011 and 2010 amounted to $87,847 and $84,518, respectively. Depreciation expense for the nine months ended March 31, 2011 and 2010 amounted to $260,577 and $224,656, respectively.
As of March 31, 2011 and June 30, 2010, we have pledged plant machinery having a carrying amount of $457,565 and $534,102, respectively to secure a bank loan of Bohai.
17
|
9.
|
SHORT-TERM BORROWINGS
|
Bohai obtained several short-term loan facilities from financial institution in the PRC. Short-term borrowings as of March 31, 2011 consisted of the following:
|
Loan from
financial
institution
|
Loan period
|
Annual
interest rate
|
Secured by
|
Amount
|
|||||||
|
Yantai Laishan Rural Credit Union
|
September 21, 2010 to September 20, 2011
|
9.03 | % |
Bohai’s machinery and vehicles
|
$ | 608,819 | |||||
|
Yantai Laishan Rural Credit Union
|
September 21, 2010 to September 20, 2011
|
6.90 | % |
Yantai Jiahua Medical Equipment Co. Ltd
|
296,799 | ||||||
| $ | 905,618 | ||||||||||
Short-term borrowings as of June 30, 2010 consisted of the following:
|
Loan from
financial
institution
|
Loan period
|
Annual
interest rate
|
Secured by
|
Amount
|
|||||||
|
China Construction Bank
|
February 24, 2010 to February 23, 2011
|
5.84 | % |
Shandong Dai Xin Heavy Industries Co. Ltd.
|
$ | 3,524,954 | |||||
|
Yantai Laishan Rural Credit Union
|
September 28, 2009 to September 26, 2010
|
9.03 | % |
Bohai’s machinery and vehicles
|
587,492 | ||||||
|
Yantai Laishan Rural Credit Union
|
September 28, 2009 to September 26, 2010
|
6.90 | % |
Yantai Jiahua Medical Equipment Co. Ltd
|
286,403 | ||||||
| $ | 4,398,849 | ||||||||||
|
10.
|
COMMON STOCK
|
We are authorized to issue 150 million shares of Common Stock, par value $0.001 per share. Holders of Common Stock are entitled to one vote for each share held of record on each matter submitted to a vote of shareholders. Holders of Common Stock do not have a cumulative voting right, which means that the holders of more than one half of our outstanding shares of Common Stock, subject to the rights of the holders of preferred stock, if any, can elect all of our directors, if they choose to do so. In this event, the holders of the remaining shares of Common Stock would not be able to elect any directors. Subject to the prior rights of any class or series of preferred stock which may from time to time be outstanding, if any, holders of Common Stock are entitled to receive ratably, dividends when, as, and if declared by our Board of Directors out of funds legally available for that purpose and, upon our liquidation, dissolution, or winding up, are entitled to share ratably in all assets remaining after payment of liabilities and payment of accrued dividends and liquidation preferences on the preferred stock, if any. Holders of Common Stock have no preemptive rights and have no rights to convert their Common Stock into any other securities. The outstanding Common Stock is duly authorized and validly issued, fully-paid, and non-assessable. Except as required or permitted by law or our charter documents, all stockholder action is taken by the vote of a majority of the outstanding shares of Common Stock present at a meeting of stockholders at which a quorum consisting of a majority of the outstanding shares of Common Stock is present in person or by proxy.
18
|
10.
|
COMMON STOCK - Continued
|
On January 21, 2011, we closed a financing transaction under which we sold an aggregate of 748,382 shares of Common Stock to a total of 42 individual investors at $2.50 per share, for total gross proceeds of $1,870,955. The shares were sold pursuant to separate subscription agreements between us and each investor. All investors are domiciled in and citizens of the People's Republic of China.
Notes with an aggregate face amount of $1,050,000 and interest of $5,406 on the $1,050,000 Notes were converted into 527,703 shares of Common Stock during the nine months ended March 31, 2011.
Restricted Stock Awards
On June 4, 2010, we issued 120,000 shares of restricted Common Stock to our Chief Financial Officer for three years of service. The restricted stock vests in three equal annual installments over the term of employment. For the three and nine months ended March 31, 2011, the Company recognized $22,000 and $73,000 of the restricted stock as compensation expenses.
On November 10, 2010, we issued 25,000 shares of restricted Common Stock to a third party to create investor awareness programs, which shares vested immediately. For the three and nine months ended March 31, 2011, the Company recognized $24,000 and $52,500 of the restricted stock as general and administrative expenses.
On January 5, 2011, we issued 20,000 shares of restricted Common Stock to a third party to create investor awareness programs, which shares vested immediately. For the three and nine months ended March 31, 2011, the Company recognized $35,000 of the restricted stock as general and administrative expenses.
|
11.
|
EARNINGS PER SHARE
|
Basic earnings per share are computed on the basis of the weighted average number of shares of Common Stock outstanding during the period. Diluted earnings per share is computed on the basis of the weighted average number of shares of Common Stock plus the effect of dilutive potential common shares outstanding during the period using the if-converted method for the convertible notes and the treasury stock method for warrants. The following table sets forth the computation of basic and diluted net income per common share:
19
|
11.
|
EARNINGS PER SHARE - Continued
|
|
Three
months
ended
|
Three
months
ended
|
Nine months
ended
|
Nine months
ended
|
|||||||||||||
|
March 31,
2011
|
March 31,
2010
|
March 31,
2011
|
March 31,
2010
|
|||||||||||||
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
|||||||||||||
|
Net income available for common shareholders
|
$ | 2,685,346 | $ | 3,142,558 | $ | 12,238,716 | $ | 7,752,593 | ||||||||
|
Effective interest charge on convertible note
|
438,376 | 347,793 | 986,842 | 347,793 | ||||||||||||
|
Net income for diluted earnings per common share
|
$ | 3,123,722 | $ | 3,490,351 | $ | 13,225,558 | $ | 8,100,386 | ||||||||
|
Three
months
ended
|
Three
months
ended
|
Nine months
ended
|
Nine months
ended
|
|||||||||||||
|
March 31,
2011
|
March 31,
2010
|
March 31,
2011
|
March 31,
2010
|
|||||||||||||
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
|||||||||||||
|
Basic weighted average common stocks outstanding
|
17,544,163 | 16,078,472 | 16,988,489 | 16,193,659 | ||||||||||||
|
Effect of dilutive securities:
|
||||||||||||||||
|
Warrants - incremental shares based on assumed proceeds & repurchases
|
- | - | - | - | ||||||||||||
|
Options - incremental shares based on assumed proceeds & repurchases
|
- | - | 75 | - | ||||||||||||
|
Restricted stock
|
14,722 | - | 9,398 | - | ||||||||||||
|
Common shares if converted from Convertible Notes
|
5,250,000 | 5,666,667 | 5,441,240 | 5,890,511 | ||||||||||||
|
Diluted weighted average for common stocks outstanding
|
22,808,885 | 21,745,139 | 22,439,202 | 22,084,170 | ||||||||||||
|
Earnings per share:
|
||||||||||||||||
|
Basic
|
$ | 0.15 | $ | 0.20 | $ | 0.72 | $ | 0.48 | ||||||||
|
Diluted
|
$ | 0.14 | $ | 0.16 | $ | 0.59 | $ | 0.37 | ||||||||
20
|
11.
|
EARNINGS PER SHARE - Continued
|
Warrants to purchase 6,600,000 shares of Common Stock and stock options to purchase 26,000 shares of Common Stock were outstanding during the three and nine months ended March 31, 2011 but were excluded from the computation of diluted earnings per share as their effect would have been anti-dilutive.
Warrants to purchase 6,600,000 shares of Common Stock were outstanding during the three and nine months ended March 31, 2010 but were excluded from the computation of diluted earnings per share as their effect would have been anti-dilutive
|
12.
|
OTHER ACCRUED LIABILITIES
|
Other accrued liabilities as of March 31, 2011 and June 30, 2010 are consisted of the following:
|
|
As of
|
As of
|
||||||
|
March 31, 2011
|
June 30, 2010
|
|||||||
|
(unaudited)
|
||||||||
|
Accrued selling expenses
|
$ | 3,233,082 | $ | 1,541,383 | ||||
|
Accrued staff costs
|
259,220 | 221,810 | ||||||
|
Value added tax payable
|
998,700 | 686,478 | ||||||
|
Other taxes payable
|
150,509 | 78,370 | ||||||
|
Other accrued expenses
|
268,272 | 456,947 | ||||||
|
Total other accrued liabilities
|
$ | 4,909,783 | $ | 2,984,988 | ||||
|
13.
|
CONVERTIBLE PROMISSORY NOTES AND WARRANTS
|
On January 5, 2010, pursuant to a Securities Purchase Agreement, or securities purchase agreement, with 128 accredited investors, or the Investors, we sold 6,000,000 units for aggregate gross proceeds of $12,000,000, each unit consisting of an 8% senior convertible promissory note, or Notes, in the principal amount of $2 and one Common Stock purchase warrant, or Warrant. By agreement with the Investors, each investor received: (i) a single Note representing the aggregate number of Notes purchased by them as part of the units (each, a “Note” and collectively, the “Notes”) and (ii) a single Investor Warrant representing the aggregate number of Investor Warrants purchased by them as part of the units.
The Notes bear interest at 8% per annum, payable quarterly in arrears on the last day of each fiscal quarter of our company. No principal payments are required until maturity of the Notes on January 5, 2012. Each Note, plus all accrued but unpaid interest thereon, is convertible, in whole but not in part, at any time at the option of the holder, into shares of Common Stock at a conversion price of $2.00 per share, subject to adjustment as set forth in the Note. The Notes issued have face amounts that range from $43,200 to $500,000.
21
|
13.
|
CONVERTIBLE PROMISSORY NOTES AND WARRANTS - Continued
|
The conversion price of the Notes is subject to standard anti-dilution adjustments for stock splits and similar events. In addition, if we issue or sell any additional shares of Common Stock or instruments convertible or exchangeable for Common Stock at a price per share less than the conversion price then in effect or without consideration, then the conversion price upon each such issuance will be adjusted to that price determined by multiplying the conversion price then in effect by a fraction: (1) the numerator of which is the sum of (x) the number of shares of Common Stock outstanding immediately prior to the issuance of such additional shares of Common Stock plus (y) the number of shares of Common Stock which the aggregate consideration for the total number of such additional shares of Common Stock so issued would purchase at a price per share equal to the conversion price then in effect, and (2) the denominator of which is the number of shares of Common Stock outstanding immediately after the issuance of such additional shares of Common Stock. Notwithstanding any provision of the Note to the contrary, no adjustment will cause the conversion price to be less than $1.00, as adjusted for any stock dividend, stock split, stock combination, reclassification or similar transaction.
Effective as of June 30, 2010, we entered into an Amendment and Agreement (the "A&A") with the Investors, pursuant to which the Company and the Investors agreed to make certain amendments to the Notes and the Warrants. Pursuant to the Amendment, the anti-dilution protection provisions in the Notes and the Warrants were eliminated and a provision specifically precluding net cash settlement by us of the Notes and the Warrants was added. In return, and subject to certain non-financing exceptions, we agreed not to issue any new equity securities at a price per share below $2.20 until the earlier of (i) January 5, 2013 or (ii) the date on which, collectively with any prior conversions or exercises of Notes and Warrants, 75% of the principal face value of the Notes in the aggregate has been converted into shares of Common Stock and Warrants representing, in the aggregate, 75% of the aggregate shares of Common Stock underlying the Warrants have been exercised. This Amendment did not change the Company’s accounting for the Notes and the Warrants described below.
On and effective as of March 30, 2011, the Company entered into a Termination Agreement (the "Termination Agreement") with Euro Pacific Capital, Inc., as investor representative (the "Investor Representative"), pursuant to which the Company and the Investor Representative agreed to terminate the aforementioned A&A, by and between the Company and the Investor Representative. As a result of the Termination Agreement, the A&A and each of its provisions were terminated. After further study, the Company concluded that the original purpose of the A&A (to mitigate the impact of certain non-cash embedded derivative liabilities associated with the Notes, Warrants and Agent Warrants) would not be achieved. Therefore, the Company determined and agreed with the Investor Representative to terminate the A&A and to thereby restore the Notes, Warrants and Agent Warrants to their original terms.
The Notes contain certain events of default, including non-payment of interest or principal when due, bankruptcy, failure to maintain a listing of the Common Stock or to make required filings on a timely basis. No premium is payable by us if an event of default occurs. However, upon an Event of Default, and provided no more than 50% of the aggregate face amount of the Notes have been converted, the Investors holding Notes have the right to receive a portion, based on their pro-rata participation in the transaction, of 1,000,000 shares of our Common Stock that have been placed in escrow by our principal stockholder. The shares in escrow will be returned to the principal stockholder when 50% of the aggregate face amount of the Notes has been converted or, if later, when the Notes are repaid.
The Investor Warrants expire on January 5, 2013 and may be exercised by the holder at any time to purchase one share of Common Stock at an exercise price of $2.40 per share (subject to adjustment as set forth in the Investor Warrants). The exercise price of the Investor Warrants is subject to adjustment in the same manner as the conversion price of the Notes described above, except that the exercise price will not be adjusted to less than $1.20, as adjusted for any stock dividend, stock split, stock combination, reclassification or similar transaction. The Investor Warrants may only be exercised for cash and do not permit the holder to perform a cashless exercise.
22
|
13.
|
CONVERTIBLE PROMISSORY NOTES AND WARRANTS - Continued
|
In connection with the sale of the units, we paid our placement agents a cash fee of $1,200,000. In addition, the placement agents received warrants, the Placement Agent Warrants and, together with the Investor Warrants, the Warrants to purchase 600,000 shares of Common Stock, which agent warrants are substantially identical to the Investor Warrants, except that, pursuant to separate lock-up agreements executed by the holders of the Placement Agent Warrants, the Placement Agent Warrants are not exercisable until the six month anniversary of the later of: (i) the date of effectiveness of the registration statement registering the resale of the Common Stock underlying the Notes and Warrants or (ii) the date of commencement of sales in connection with such registration statement.
In addition to the placement agent fee, we paid $370,000 of legal and other expenses. As required by the Securities Purchase Agreement, $500,000 of the proceeds from the sale of the units were placed in escrow to pay investor relations expenses to be incurred by us and $240,000, equivalent to one quarter’s interest expense on the Notes, was also placed in escrow. The interest escrow will be released to us at such time as 75% of all shares underlying the Notes have been issued upon conversion of Notes. After payment of the placement agent fees and other expenses and the amounts required to be placed in escrow, we received net proceeds of $9,690,000. At March 31, 2011 and June 30, 2010, $220,043 and $576,019, respectively, remained in escrow and is included in restricted cash.
We also entered into a Registration Rights Agreement with the Investors. We agreed to file, no later than March 6, 2010, a registration statement to register the shares underlying the Notes and the Warrants and to have such registration statement effective no later than August 13, 2010. The required registration statement was filed on March 2, 2010 and became effective on August 12, 2010. Accordingly, we did not incur any registration delay payments.
Valuation
At the time the Notes and Warrants were issued, there had not been any market activity for the Common Stock. Accordingly, determining the fair value of the Common Stock required us to make complex and subjective judgments. We estimated the value of our enterprise as of January 5, 2010 based on a review of the enterprise value derived from the use of market and income valuation approaches. We also reviewed an asset-based approach to assess whether the result of such an approach was consistent with the value derived from the market and income valuation approaches. The market approach was based on the market price to earnings multiple for companies considered by management to be comparable to us. The income approach was based on applying discount rates to estimated future net income. The estimated enterprise value was then allocated to our existing outstanding Common Stock, the Notes and the Warrants using the option pricing method. The option pricing method was based on the two year period to maturity of the Notes and the three year period to expiration of the Warrants, risk-free interest rates commensurate with those periods and the expected volatility used was based on a review of the historical volatility of companies considered by management to be comparable to us.
Based on the allocation of the estimated enterprise value, we estimated the fair value of the Common Stock at $2.28 per share, as of January 5, 2010. The Investor Warrants and the Placement Agent Warrants were valued at $5,824,538 and $582,454, respectively, based on the estimated fair value of the Common Stock of $2.28, a term equal to the remaining life of the Warrants, an expected dividend yield of 0%, a risk-free interest rate of 1.57% based on constant maturity rates published by the U.S. Federal Reserve applicable to the remaining life of the Warrants and estimated volatility of 65%, based on a review of the historical volatility of companies considered by management to be comparable to us. As noted above, prior to the June 30, 2010 Amendment described above, the Warrants contained a down-round anti-dilution protection feature. As of January 5, 2010, the value of this feature was not considered to be material and no adjustment was made for it in the estimated fair value of the Warrants.
23
|
13.
|
CONVERTIBLE PROMISSORY NOTES AND WARRANTS - Continued
|
Accounting for Convertible Notes
At January 5, 2010 and March 31, 2011, the conversion options embedded in the Notes are not derivative instruments as defined in FASB ASC 815-10-15-83 because the Notes do not permit or require net settlement, there is no market mechanism outside the contracts that permits net settlement and the shares to be received on conversion of the Notes are not readily convertible to cash. At the time the Notes were issued, there had not been any market activity for the Common Stock. Although market trading activity for our Common Stock has developed, the Notes can be exercised only in whole but not in part and through March 31, 2011 and continuing, there has been insufficient trading volume to permit the shares to be received on conversion of each Note to be readily sold in the market, thus precluding the shares to be received by the holder of each Note from being readily convertible to cash.
In future periods, whether or not the embedded conversion option in each Note is considered to be a derivative instrument will depend on whether or not the aggregate number of shares to be received on exercise of each of the Notes, which Notes can be exercised only in whole but not in part, could be readily sold in the market without significantly affecting the market price of the Common Stock, thus permitting the shares received by the holder of each Note to be readily convertible to cash. At each reporting date, the Company will re- evaluate each Note, based on the level of activity in the market for the Common Stock at that time, to determine whether or not the embedded conversion option in each Note is a derivative instrument. Depending on the trading volume for the Common Stock that develops in the future and the face amount of each Note, the embedded conversion option may be considered a derivative instrument for some Notes but not for others and its status as a derivative instrument may vary from period to period.
FASB ASC 815-10-15-74 provides that a contract which would otherwise meet the definition of a derivative instrument but that is both (a) indexed to a company’s own stock and (b) classified in stockholders’ equity in the statement of financial position would not be considered a derivative financial instrument. FASB ASC 815-40-15 and 815-40-25 provide guidance for determining whether those two criteria are met. Because the Company’s functional currency is the Renminbi but the Notes are denominated in U.S. Dollars, FASB ASC 815-40-15-7I provides that the embedded conversion options are not considered to be indexed only to our Common Stock. Furthermore, prior to the June 30, 2010 Amendment described above, the criteria that the instruments be indexed only to the Common Stock was also not met because the conversion price of the Notes would be reduced if we issued securities at a lower exercise or conversion price. Because the requirement that the instruments be indexed only to the Common Stock is not met, the exemption in FASB ASC 815-10-15-74 will not be available and we will account for the embedded conversion options in the Notes as derivative instrument liabilities, if and when the shares to be issued on conversion are considered to be readily convertible to cash.
Derivative financial instruments are initially measured at their fair value. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported as charges or credits to income. If and when the embedded conversion option in any of the Notes first qualifies as a derivative instrument, the fair value at that time of the embedded derivative instrument will be re-classified and separately recognized and subsequently marked-to-market each reporting period, as long as the embedded conversion option continues to qualify as a derivative instrument. If the embedded conversion option ceases to be a derivative instrument, it will be marked-to-market as of the date of re-classification but thereafter will no longer be marked-to-market.
24
|
13.
|
CONVERTIBLE PROMISSORY NOTES AND WARRANTS - Continued
|
Warrants
Because our functional currency is the Renminbi but the Warrants are denominated in U.S. Dollars, the Warrants are not considered to be indexed only to our Common Stock. Furthermore, prior to the June 30, 2010 Amendment described above, the criteria that the instruments be indexed only to the Common Stock was also not met because the exercise price of the Warrants would be reduced if we issued securities at a lower exercise or conversion price. In accordance with ASC 815-10-S99-4, the Warrants (including the Placement Agent Warrants) are accounted for at fair value, with changes in their fair value charged or credited to income each period.
At January 5, 2010, the Investor Warrants were valued at $5,824,538, as described above. At March 31, 2011, the Investor Warrants were re-valued at $2,091,205 using a binomial model, based on the closing market price on that date of $1.70, a term equal to the remaining life of the Warrants, an expected dividend yield of 0%, a risk-free interest rate of 0.68% based on constant maturity rates published by the U.S. Federal Reserve applicable to the remaining life of the Warrants and estimated volatility of 55%, based on a review of the historical volatility of companies considered by management to be comparable to our company. The effect of the down-round anti-dilution protection was not considered to be material and no adjustment was made for it in the estimated fair value of the Investor Warrants.
The Placement Agent Warrants were initially valued at $582,454, as described above. The cost of these instruments, together with the cash fees paid to the placement agents and the other fees and expenses paid by us, as described above, in the aggregate amount of $2,152,454, have been deferred and are being amortized on a straight-line basis over the two year period to maturity of the Notes. At March 31, 2011, the Placement Agent Warrants were re-valued at $209,120, based on the closing market price on that date of $1.70, a term equal to the remaining life of the Warrants, an expected dividend yield of 0%, a risk-free interest rate of 0.68% and estimated volatility of 55%. The effect of the down-round anti-dilution protection was not considered to be material and no adjustment was made for it in the estimated fair value of the Placement Agent Warrants.
The aggregate change in the value of the Investor and Placement Agent Warrants for the three and nine months ended March 31, 2011 of $263,118 and $3,181,603, respectively, has been recorded as a gain on the consolidated statement of income. The aggregate change in the value of the Investor and Placement Agent Warrants for the three and nine months ended March 31, 2010 of $1,083,350 has been recorded as a gain on the consolidated statement of income.
The following table summarizes all of our warrants outstanding as of March 31, 2011:
25
|
13.
|
CONVERTIBLE PROMISSORY NOTES AND WARRANTS - Continued
|
|
Exercise Price
|
||||||||||||
|
Warrant
|
Exercisable
|
per Common
|
||||||||||
|
Shares
|
Shares
|
Stock Range
|
||||||||||
|
Balance, June 30, 2010
|
6,600,000 | 6,000,000 | $ | 2.4 | ||||||||
|
Granted or vested during the nine months ended March 31, 2011
|
- | 600,000 | 2.4 | |||||||||
|
Exercised during the nine months ended March 31, 2011
|
- | - | - | |||||||||
|
Expired during the nine months ended March 31, 2011
|
- | - | - | |||||||||
|
Balance, March 31, 2011 (unaudited)
|
6,600,000 | 6,600,000 | $ | 2.4 | ||||||||
The following table summarizes the weighted average remaining contractual life and exercise price of our outstanding warrants.
|
Warrants Outstanding
|
||||||||||||||||||
|
Number
|
||||||||||||||||||
|
Outstanding
|
Weighted
|
Weighted Average
|
||||||||||||||||
|
Number
|
Currently
|
Average
|
Exercise Price of
|
|||||||||||||||
|
Outstanding
|
Exercisable
|
Remaining
|
Warrants
|
|||||||||||||||
|
Exercise
|
at March 31, |
at
|
Contractual Life
|
currently
|
||||||||||||||
|
Price
|
2011
|
March 31, 2011
|
(Years)
|
exercisable
|
||||||||||||||
| $ | 2.40 | 6,600,000 | 6,600,000 | 1.77 | $ | 2.40 | ||||||||||||
Convertible Notes
The Investor Warrants were initially recorded at their fair value of $5,824,538 and the remainder of the $12,000,000 gross proceeds received from the Investors of $6,175,462 was allocated to the Notes. Based on the proceeds allocated to the Notes, the Notes are convertible into Common Stock at an effective conversion price of approximately $1.03 per share. Because the effective conversion price is less than the fair value of the Common Stock at the time the Notes were issued, the Company recognized a beneficial conversion feature, which was limited to the amount of proceeds allocated to the Notes of $6,175,462. The Notes were initially recorded at a carrying value of zero and are being amortized, together with interest accruing on the Notes, to their maturity value over the period to maturity, at an effective interest rate of approximately 540% per annum. Interest expense for the three and nine months ended March 31, 2011 was $438,376 and $986,842, respectively. Interest expense for the three and nine months ended March 31, 2010 was $347,793. As of March 31, 2011, Notes with an aggregate face amount of $1,550,000 and interest of $5,406 on the Notes were converted into 777,703 shares of Common Stock.
26
|
13.
|
CONVERTIBLE PROMISSORY NOTES AND WARRANTS - Continued
|
The convertible note liability is as follows at March 31, 2011 and June 30, 2010:
|
|
As of
|
As of
|
||||
|
March 31, 2011
|
June 30, 2010
|
|||||
|
(unaudited)
|
||||||
|
Convertible notes payable, at face value
|
$ | 10,450,000 | $ | 11,500,000 | ||
|
Less: unamortized beneficial conversion feature and warrants discount on convertible notes
|
(10,011,257 | ) | (11,375,180 | ) | ||
|
Convertible notes, net
|
$ | 438,743 | $ | 124,820 | ||
Escrowed Shares
As of January 5, 2010 and at March 31, 2011, our principal stockholder is obligated to deliver 1,000,000 shares of Common Stock to the Investors if certain Events of Default occur (as defined in the Notes). The fair value of this obligation is not considered to be material as the probability of such events occurring is currently considered to be minimal. Accordingly, at March 31, 2011 no liability for this obligation has been recognized.
|
14.
|
DEFERRED FEES ON CONVERTIBLE NOTES
|
We incurred total placement fees of $2,152,454 in connection with our private placement of Convertible Notes (see Note 13) that occurred on January 5, 2010. The placement fees are being amortized on a straight line basis over the two year expected life of the Convertible Notes, starting on the date of closing, January 5, 2010.
|
As of
|
As of
|
|||||||
|
|
March 31, 2011
|
June 30, 2010
|
||||||
|
(Unaudited)
|
||||||||
|
Deferred fees, beginning balance
|
$ | 1,562,617 | $ | 2,152,454 | ||||
|
Transferred to equity on conversion
|
(106,574 | ) | (79,120 | ) | ||||
|
Amortization of deferred fees
|
(736,224 | ) | (510,717 | ) | ||||
|
Deferred Fee, ending balance
|
$ | 719,819 | $ | 1,562,617 | ||||
|
15.
|
STOCK OPTIONS
|
On October 13, 2010, we granted stock options to two directors for the purchase of 26,000 shares of our Common Stock at an exercise price of $2.00 per share. The options vest immediately on the grant date and expire five years from the date of issuance.
27
|
15.
|
STOCK OPTIONS- Continued
|
These options have been valued at $23,844. We use a binomial option pricing model to calculate the grant date fair value of the options, with the following assumptions: no dividend yield, expected volatility of 70%, risk free interest rate of 0.3%, expected term of 2.5 years.
The following table summarizes the weighted average remaining contractual life and exercise price of our outstanding options as of March 31, 2011:
|
Options Outstanding
|
||||||||||||||||||
|
Number
|
||||||||||||||||||
|
Outstanding
|
Weighted
|
Weighted Average
|
||||||||||||||||
|
Number
|
Currently
|
Average
|
Exercise Price of
|
|||||||||||||||
|
Outstanding
|
Exercisable
|
Remaining
|
Warrants
|
|||||||||||||||
|
Exercise
|
at March 31,
|
at
|
Contractual Life
|
currently
|
||||||||||||||
|
Price
|
2011
|
March 31, 2011
|
(Years)
|
exercisable
|
||||||||||||||
| $ | 2.00 | 26,000 | 26,000 | 4.54 | $ | 2.00 | ||||||||||||
We account for share-based payments in accordance with ASC 718. Accordingly, we expense the fair value of awards granted to the directors. Total compensation expense related to the stock options for the three months and nine months ended March 31, 2011 was $23,844 and was recorded as general and administrative expense.
A summary of our stock option activity as of March 31, 2011, and changes during nine months ended March 31, 2011 is presented in the following table:
|
Exercise Price
|
||||||||||||
|
Option
|
Vested
|
per Common
|
||||||||||
|
Shares
|
Shares
|
Stock Range
|
||||||||||
|
Balance, June 30, 2010
|
- | - | $ | - | ||||||||
|
Granted or vested during the nine months ended March 31, 2011
|
26,000 | 26,000 | $ | 2.0 | ||||||||
|
Exercised during the nine months ended March 31, 2011
|
- | - | $ | - | ||||||||
|
Expired during the nine months ended March 31, 2011
|
- | - | $ | - | ||||||||
|
Balance, March 31, 2011 (unaudited)
|
26,000 | 26,000 | $ | 2.0 | ||||||||
28
|
16.
|
SELLING, GENERAL AND ADMINISTRATIVE EXPENSE
|
For the three and nine months ended March 31, 2011 and 2010, selling, general and administrative expenses consisted of the following:
|
|
Three months
ended
|
Three months
ended
|
Nine months
ended
|
Nine months
ended
|
||||||||||||
|
|
March 31, 2011
|
March 31, 2010
|
March 31, 2011
|
March 31, 2010
|
||||||||||||
|
|
(Unaudited)
|
(Unaudited)
|
(Unaudited)
|
(Unaudited)
|
||||||||||||
|
Travel and accommodation
|
$ | 3,197,894 | $ | 1,824,750 | $ | 7,215,635 | $ | 4,880,926 | ||||||||
|
Advertising and promotion
|
3,962,454 | 2,767,549 | 10,461,435 | 8,732,252 | ||||||||||||
|
Audit fees
|
12,483 | - | 101,534 | - | ||||||||||||
|
Commission
|
1,155,155 | 1,060,462 | 2,628,125 | 3,464,415 | ||||||||||||
|
Conference
|
1,269,264 | 738,898 | 4,161,191 | 3,070,497 | ||||||||||||
|
Depreciation
|
10,421 | 9,397 | 30,427 | 27,872 | ||||||||||||
|
Staff costs
|
966,557 | 608,263 | 2,106,130 | 1,571,400 | ||||||||||||
|
Research and development cost
|
190,440 | 146,640 | 562,261 | 442,046 | ||||||||||||
|
Other operating expenses
|
2,081,292 | 2,046,914 | 5,299,241 | 6,019,345 | ||||||||||||
|
Total selling, general and administrative expenses
|
$ | 12,845,962 | $ | 9,202,873 | $ | 32,565,981 | $ | 28,208,753 | ||||||||
|
17.
|
INTEREST EXPENSE
|
For the three and nine months ended March 31, 2011 and 2010, interest expense consisted of the following:
29
|
17.
|
INTEREST EXPENSE – Continued
|
|
|
Three months
ended
|
Three months
ended
|
Nine months
ended
|
Nine months
ended
|
||||||||
|
|
March 31, 2011
|
March 31, 2010
|
March 31, 2011
|
March 31, 2010
|
||||||||
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
|||||||||
|
Interest on short-term bank borrowings and notes payable
|
$ | 19,659 | $ | 33,907 | $ | 187,446 | $ | 190,215 | ||||
|
Amortization of beneficial conversion feature and warrants discount on convertible notes converted
|
96,393 | - | 1,029,487 | - | ||||||||
|
Effective interest charge on Convertible Notes
|
438,376 | 347,793 | 986,842 | 347,793 | ||||||||
|
Total interest expenses
|
$ | 554,428 | $ | 381,700 | $ | 2,203,775 | $ | 538,008 | ||||
|
18.
|
INCOME TAXES
|
For the three and nine months ended March 31, 2011 and 2010, income tax expense consisted of the following:
|
Three months
ended March
31, 2011
|
Three months
ended March
31, 2010
|
Nine months
ended March
31, 2011
|
Nine months
ended March
31, 2010
|
|||||||||||||
| Current taxes |
(unaudited)
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
||||||||||||
|
United States
|
$ | - | $ | - | $ | - | $ | - | ||||||||
|
PRC
|
897,458 | 585,135 | 3,523,145 | 2,193,931 | ||||||||||||
|
Deferred taxes
|
||||||||||||||||
|
United States
|
(274,959 | ) | 226,560 | (778,776 | ) | 226,560 | ||||||||||
|
PRC
|
- | (836 | ) | - | (1,336 | ) | ||||||||||
| - | ||||||||||||||||
|
Change in valuation allowance
|
274,959 | (225,724 | ) | 778,776 | (225,224 | ) | ||||||||||
|
Total income tax expenses
|
$ | 897,458 | $ | 585,135 | $ | 3,523,145 | $ | 2,193,931 | ||||||||
30
|
18.
|
INCOME TAXES
|
As of March 31, 2011, we incurred $1,908,432 of net operating losses carry forwards available for federal tax purposes that may be used to offset future taxable income and will begin to expire in 2029, if unutilized. we provided for a full valuation allowance against the deferred tax assets of $648,867 on the expected future tax benefits from the net operating loss carry forwards as the management believes it is more likely than not that these assets will not be realized in the future.
PRC Tax
PRC’s legislative body, the National People’s Congress, adopted the unified Enterprise Income Tax (“EIT”) Law on March 16, 2007. This tax law replaces the existing separate income tax laws for domestic enterprises and foreign-invested enterprises and became effective on January 1, 2008. Under the new tax law, a unified income tax rate is set at 25% for both domestic enterprises and foreign-invested enterprises. However, there will be a transition period for enterprises, whether foreign-invested or domestic, that are currently receiving preferential tax treatments granted by relevant tax authorities. Enterprises that are subject to an enterprise income tax rate lower than 25% may continue to enjoy the lower rate and will transit into the new rate over a five year period beginning on the effective date of the EIT Law. Enterprises that are currently entitled to exemptions for a fixed term may continue to enjoy such treatment until the exemption term expires. Preferential tax treatments may continue to be granted to industries and projects that qualify for such preferential treatments under the new law.
United States Tax
We are subject to income tax in the United States. No provision for income tax in the United States has been made as we had no taxable income for the three and nine months ended March 31, 2011 and 2010. The statutory tax rate is 34%.
The table below summarizes the differences between the U.S. statutory federal rate and our effective tax rate and as follows for the three and nine months ended March 31, 2011 and 2010:
|
Three months
ended March 31,
2011
|
Three months
ended March
31, 2010
|
Nine months
ended March
31, 2011
|
Nine months
ended March
31, 2010
|
|||||||||||||
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
|||||||||||||
|
United States Tax at statutory rate
|
$ | 1,218,153 | $ | 1,267,416 | $ | 5,359,032 | $ | 3,381,818 | ||||||||
|
Foreign tax rate difference
|
(379,746 | ) | (197,564 | ) | (1,428,436 | ) | (677,299 | ) | ||||||||
|
Change in valuation allowance
|
274,959 | (225,724 | ) | 778,776 | (225,224 | ) | ||||||||||
|
Permanent difference
|
(159,222 | ) | (258,993 | ) | (454,508 | ) | (285,364 | ) | ||||||||
|
Other reconciling items
|
(56,687 | ) | - | (731,719 | ) | - | ||||||||||
|
Income tax expense
|
$ | 897,458 | $ | 585,135 | $ | 3,523,145 | $ | 2,193,931 | ||||||||
31
|
18.
|
INCOME TAXES - Continued
|
Our deferred tax assets as of March 31, 2011 and June 30, 2010 are as follows:
|
As of March 31, 2011
|
As of June 30, 2010
|
|||||||
|
(unaudited)
|
||||||||
|
Deferred tax asset:
|
||||||||
|
Net operating loss carry forward
|
$ | 648,867 | $ | 308,406 | ||||
|
Stock options and warrants
|
438,315 | - | ||||||
|
Total gross deferred tax asset
|
1,087,182 | 308,406 | ||||||
|
Less: valuation allowance
|
(1,087,182 | ) | (308,406 | ) | ||||
|
Net deferred tax asset
|
$ | - | $ | - | ||||
During the three months ended March 31, 2011 and 2010, the valuation allowance increased by $274,959 and decreased by $225,224, respectively. During the nine months ended March 31, 2011 and 2010, the valuation allowance increased by $778,776 and decreased by $225,224, respectively.
VAT
Certain of our revenues are subject to output VAT generally calculated at 6%, 13% and 17% of the selling price. Input credit relating to input VAT paid on purchases can be used to offset the output VAT.
|
Three months
ended March 31,
2011
|
Three months
ended March 31,
2010
|
Nine months
ended March 31,
2011
|
Nine months
ended March 31,
2010
|
|||||||||||||
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
|||||||||||||
|
Net value added tax paid
|
$ | 3,001,042 | $ | 2,086,644 | $ | 8,262,278 | $ | 6,627,557 | ||||||||
|
19.
|
COMMITMENTS AND CONTINGENCIES
|
As of May 2009, we entered into a contract with Yantai Tianzheng Medicine Research and Development Co. to perform research and development on two new pharmaceutical products, namely Fern Injection and Forsythia Capsule, on behalf of us. The total contract price is approximately $2,283,100 (RMB 15,000,000). Yantai Tianzheng Medicine Research and Development Co. is committed to complete all research work require for the clinical trial within 3 years. As of March 31, 2011, we have paid $1,263,299 (RMB 8,300,000) and the remainder contract amount will be paid progressively in installments. The final payment will be paid upon obtaining new drug certification from the related government department. There are no other foreseeable material commitments or contingencies as of March 31, 2011.
32
|
20.
|
SIGNIFICANT CONCENTRATIONS
|
|
(a)
|
Customer Concentrations
|
We do not have concentrations of business with any customer constituting greater than 10% of our gross sales for the three and nine months ended March 31, 2011 and 2010.
We have not experienced any significant difficulty in collecting its accounts receivable in the past and is not aware of any financial difficulties being experienced by its major customers.
|
(b)
|
Supplier Concentrations
|
We have the following concentrations of business with each supplier constituting greater than 10% of our purchases of raw materials or other supplies:
|
Three months
ended
|
Three months
ended
|
Nine months
ended
|
Nine months
ended
|
|||||||||||||
|
March 31,
2011
|
March 31,
2010
|
March 31,
2011
|
March 31,
2010
|
|||||||||||||
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
|||||||||||||
|
Shandong Yantai Medicine Procurement and Supply Station
|
18.8 | % | 15.7 | % | 18.6 | % | 15.0 | % | ||||||||
|
Anguo Jinkangdi Chinese Herbal Medicine Co. Ltd
|
10.6 | % | * | 11.3 | % | * | ||||||||||
|
Anhui DeChang Pharmaceutical Co. Ltd.
|
* | 15.0 | % | * | 12.9 | % | ||||||||||
|
* Constitutes less than 10% of the Company’s purchase.
|
||||||||||||||||
|
21.
|
STATUTORY RESERVES
|
According to the laws and regulations in the PRC, we are required to provide for certain statutory funds, namely, reserve fund by an appropriation from net profit after taxes but before dividend distribution based on the local statutory financial statements of the PRC company prepared in accordance with the accounting principles and relevant financial regulations.
In the PRC, we are required to allocate at least 10% of our net profit to the reserve fund until the balance of such fund has reached 50% of its registered capital. Appropriation of enterprise expansion fund are determined at the discretion of it directors. We had satisfied statutory reserve requirement by the first quarter of the fiscal year 2010, no further allocation to the statutory reserve is required.
The reserve fund can only be used, upon approval by the relevant authority, to offset accumulated losses or increase capital. The enterprise expansion fund can only be used to increase capital upon approval by the relevant authority.
33
|
22.
|
SUBSEQUENT EVENTS
|
We have performed an evaluation of subsequent events through the date of these financial statements are issued.
34
Item 2. Management’s Discussion and Analysis of Financial Conditions of Operations.
The following discussion and analysis of financial condition and results of operations relates to the operations and financial condition reported in our unaudited condensed consolidated financial statements for the three months and nine months ended March 31, 2011 and 2010, and should be read in conjunction with such financial statements and related notes included in this report. Those statements in the following discussion that are not historical in nature should be considered to be forward looking statements that are inherently uncertain. Actual results and the timing of the events may differ materially from those contained in these forward looking statements due to a number of factors, including those discussed in the “Cautionary Note on Forward Looking Statements” set forth elsewhere in this Report.
Overview
We were incorporated under the laws of the State of Nevada on January 9, 2008. Since January 5, 2010, our business consists of the production, manufacturing and distribution of herbal pharmaceuticals in the People’s Republic of China (which we refer to as China or the PRC) which is based on Traditional Chinese Medicine, or TCM. We are based in the city of Yantai, Shandong Province, China.
Our medicines are intended to address rheumatoid arthritis, viral infections, gynecological diseases, cardio vascular issues and respiratory diseases. We obtained Drug Approval Numbers in China for 29 varieties of TCM products in 2004 and 14 varieties of TCM products in 2010. We currently produce 15 varieties of such products in seven delivery systems: tablets, granules, capsules, syrup, concentrated powder, tincture and medicinal wine. Of these 15 products, 8 are prescription drugs and 7 are over-the-counter products. Of our current products: (i) 5 have “exclusive” status, meaning that we are the only company that manufactures these products in China; (ii) 2 have Certificates of Protected Variety of Traditional Chinese Medicine (Grade Two) issued by the State Food and Drug Administration of China (“SFDA”), which gives us exclusive or near exclusive rights to manufacture and distribute these products, and (iii) 1 has received Chinese patent protection with a duration of 20 years.
Prior to January 5, 2010, we were a public “shell” company operating under the name “Link Resources, Inc.” On January 5, 2010, we consummated a share exchange transaction (the “Share Exchange”) pursuant to which we acquired Chance High, the parent company of Yantai Bohai Pharmaceuticals Group Co. Ltd., our principal operating subsidiary, which is a Chinese variable interest entity that we (through a Chinese wholly-owned foreign enterprise subsidiary) control through certain contractual arrangements. Our current organizational structure is summarized below:
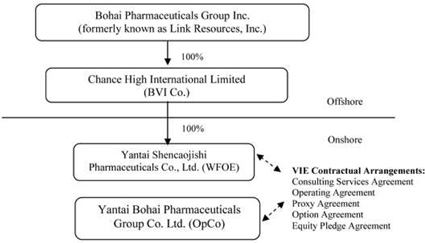
35
Use of Non-GAAP Financial Measures
We make reference to financial measures (so called “Non-GAAP” measures) not complied in full accordance with generally accepted accounting principals (“GAAP”) in portions of this “Management’s Discussion of Financial Condition and Results of Operations”. Generally, a Non-GAAP financial measure is a numerical measure of a company’s performance, financial position or cash flow that either excludes or includes amounts that are not normally excluded or included in the most directly comparable measure calculated and presented in accordance with GAAP. Management believes that investors may find it useful to review our financial results that exclude certain non-cash income and expense, namely changes in fair value of our warrants, the accretion of beneficial conversion features on convertible notes, and option and stock-based compensation shown in the chart below, of $85,725 and $1,083,351 income for the three months ended March 31, 2011 and 2010, respectively, and $1,967,772 and $1,083,351 income for the nine months ended March 31, 2011 and 2010, respectively, due to the adoption of the Financial Accounting Standards Board’s (“FASB”) ASC 815 accounting standard as discussed in footnote 13 to the accompanying financial statements.
Management believes that these Non-GAAP financial measures are useful to investors in that they provide supplemental information to possibly better understand the underlying business trends and operating performance of our company. We use these Non-GAAP financial measures to evaluate operating performance. However, Non-GAAP financial measures should not be considered as an alternative to net income or any other performance measures derived in accordance with GAAP.
|
Three Months Ended
|
Nine Months Ended
|
|||||||||||||||||||||||
|
March 31,
|
March 31,
|
|||||||||||||||||||||||
|
Increase
|
Increase
|
|||||||||||||||||||||||
|
2011
|
2010
|
(Decrease)
|
2011
|
2010
|
(Decrease)
|
|||||||||||||||||||
|
Net income available to common shareholders -GAAP
|
$ | 2,685,346 | $ | 3,142,558 | $ | (457,212 | ) | $ | 12,238,716 | $ | 7,752,593 | $ | 4,486,123 | |||||||||||
|
Add back (subtract):
|
||||||||||||||||||||||||
|
Change in fair value of warrants
|
(263,118 | )(a) | (1,083,351 | )(a) | 820,233 | (3,181,603 | )(a) | (1,083,351 | )(a) | (2,098,252 | ) | |||||||||||||
|
Accretion of beneficial conversion features on convertible notes converted
|
96,393 | (a) | - | (a) | 96,393 | 1,029,487 | (a) | - | (a) | 1,029,487 | ||||||||||||||
|
Change in option and equity based compensation
|
81,000 | (b) | - | (b) | 81,000 | 184,344 | (b) | - | (b) | 184,344 | ||||||||||||||
|
Adjusted net income available to common shareholders -non-GAAP
|
$ | 2,599,621 | $ | 2,059,207 | $ | 540,414 | $ | 10,270,944 | $ | 6,669,242 | $ | 3,601,702 | ||||||||||||
|
Net income margins non-GAAP
|
11.7 | % | 13.4 | % | (1.7 | )% | 16.8 | % | 14.5 | % | 2.3 | % | ||||||||||||
|
Basic earning per share – GAAP
|
$ | 0.15 | $ | 0.20 | $ | (0.05 | ) | $ | 0.72 | $ | 0.48 | $ | 0.24 | |||||||||||
|
Add back (Subtract):
|
||||||||||||||||||||||||
|
Change in fair value of warrants
|
(0.01 | )(a) | (0.07 | )(a) | 0.05 | (0.19 | )(a) | (0.07 | )(a) | (0.12 | ) | |||||||||||||
|
Accretion of beneficial conversion features on convertible notes converted
|
0.01 | (a) | 0.00 | (a) | 0.01 | 0.06 | (a) | 0.00 | (a) | 0.06 | ||||||||||||||
|
Change in option and equity based compensation
|
0.00 | (b) | 0.00 | (b) | 0.00 | 0.01 | (b) | 0.00 | (b) | 0.01 | ||||||||||||||
|
Adjusted basic earning per share non-GAAP
|
$ | 0.15 | $ | 0.13 | $ | 0.01 | $ | 0.60 | $ | 0.41 | $ | 0.19 | ||||||||||||
|
Diluted earning per share-GAAP
|
$ | 0.14 | $ | 0.16 | $ | (0.02 | ) | $ | 0.59 | $ | 0.37 | $ | 0.22 | |||||||||||
|
Add back (Subtract):
|
||||||||||||||||||||||||
|
Change in fair value of warrants
|
(0.01 | )(a) | (0.05 | )(a) | 0.04 | (0.14 | )(a) | (0.05 | )(a) | (0.09 | ) | |||||||||||||
|
Accretion of beneficial conversion features on convertible notes converted
|
0.00 | (a) | 0.00 | (a) | 0.00 | 0.05 | (a) | 0.00 | (a) | 0.05 | ||||||||||||||
|
Change in option and equity based compensation
|
0.00 | (b) | 0.00 | (b) | 0.00 | 0.01 | (b) | 0.00 | (b) | 0.01 | ||||||||||||||
|
Adjusted diluted earning per share non-GAAP
|
$ | 0.14 | * | $ | 0.11 | $ | 0.03 | * | $ | 0.50 | * | $ | 0.32 | $ | 0.18 | |||||||||
|
Weighted average number of shares
|
||||||||||||||||||||||||
|
Basic
|
17,544,163 | 16,078,472 | 16,988,489 | 16,193,659 | ||||||||||||||||||||
|
Diluted
|
22,808,885 | 21,745,139 | 22,439,202 | 22,084,170 | ||||||||||||||||||||
* Numbers may not add up due to rounding differences
36
|
(a)
|
We adopted the provisions of FASB accounting standard, ASC 815, which provides standards with respect to determining whether an instrument (or embedded feature) is indexed to an entity’s own stock (See note 13). As a result of these measurements, we recognized non-cash income of $263,118 and $3,181,603 for the three and nine months ended March 31, 2011, respectively, from changes in fair value for investor and agent warrants and non-cash expense of $96,393 and $1,029,487 for three and nine months ended March 31, 2011, respectively, from the accretion of beneficial conversion features on convertible notes.
|
|
(b)
|
We record stock-based compensation expense pursuant to FASB’s accounting standard regarding stock compensation which requires companies to measure compensation cost for consultants and stock-based employee compensation plans at fair value at the grant date and recognize the expense over the employee's requisite service period. Under ASC Topic 718, our expected volatility assumption is based on the historical volatility of our stock or the expected volatility of similar entities. The expected life assumption is primarily based on historical exercise patterns and employee post-vesting termination behavior. The risk-free interest rate for the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant. For the three months and nine months ended March 31, 2011, we recognized $81,000 and $160,500 of restricted stock as compensation expense. For the three months and nine months ended March 31, 2011, we recognized $0 and $23,844, respectably as compensation expenses in connection with the issuance of stock options.
|
Principal Factors Affecting Our Financial Performance
We believe that the following factors will continue to affect our financial performance:
Sales of Key Products
Our top selling products as a percentage of total net revenue consist of the following:
|
For the three months ended
|
For the nine months ended
|
|||||||||||||||
|
March 31
|
March 31
|
|||||||||||||||
|
2011
|
2010
|
2011
|
2010
|
|||||||||||||
|
Tongbi Capsules
|
27.4 | % | 28.5 | % | 27.4 | % | 22.6 | % | ||||||||
|
Tongbi Tablets
|
14.2 | % | 14.7 | % | 14.8 | % | 14.2 | % | ||||||||
|
Lung Nourishing Syrup
|
26.1 | % | 27.6 | % | 26.6 | % | 25.2 | % | ||||||||
|
Others
|
32.3 | % | 29.2 | % | 31.2 | % | 38.0 | % | ||||||||
|
Total
|
100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | ||||||||
We expect that a significant portion of our future revenue will continue to be derived from sales of our top three products.
We held the Certificates of Protected Variety of Traditional Chinese Medicine (Grade Two) issued by the SFDA for Tongbi Capsules and Anti-flu Granules which gave exclusive or near-exclusive rights to manufacture and distribute these two medicines. Tongbi Capsules’ certificates expired in September 2009 and we filed an application for extending the protection period on March 12, 2009. The certificate will remain active while application is under review. Lung Nourishing Syrup received a patent with duration of 20 years from the State Intellectual Property Office of the PRC and the patent will expire on September 12, 2027.
37
Experienced Management
Management’s marketing strategies and business relationships gives us the ability to expand our product market areas, which provides us with leverage to acquire less sophisticated operators, increase production volumes, and implement quality standards. Our future prospects depend substantially on the continued services of our senior management team, especially our President, Chief Executive Officer and Chairman of the Board, Mr. Qu.
Price Control of Drugs by PRC Government
The State Development and Reform Commission of the PRC (“SDRC”) and the price administration bureaus of the relevant provinces of the PRC in which the pharmaceutical products are manufactured are responsible for the retail price control over our pharmaceutical products. The SDRC sets the price ceilings for certain pharmaceutical products in the PRC. All of our products except those under the protection periods are subject to such price controls and could affect our future revenue growth. However, due to the direct support of TCM by the Chinese government, China’s immense market, and our protected drugs, we are optimistic regarding our continuous growth potential for TCM in China.
Financial Highlights
|
|
▪
|
Net revenues for the three months ended March 31, 2011 increased 45% to $22.2 million compared to the same period in 2010. Net revenues for the nine months ended March 31, 2011 increased 33% to $61.3 million compared to the same period in 2010.
|
|
|
o
|
Net revenues for the five new products introduced in April 2010 increased 9.4% for the three months ended March 31, 2011 compared to the previous quarter.
|
|
|
o
|
Sales were mostly derived from our lead products, Lung Nourishing Syrup, Tongbi Capsules and Tongbi Tablets, which together represented 67.7% of our total net revenues for the three months ended March 31, 2011. During the quarter, we have added 200 level 2 hospitals and10 new drug store chains to our national network of retail locations in China currently selling the company's Lung Nourishing Syrup.
|
|
|
o
|
61% of net revenue was derived from sales of prescription products and 39% was from Over-the-Counter products for the three months ended March 31, 2011.
|
|
|
▪
|
Non-GAAP net income for the three months ended March 31, 2011 increased 26.2% to $2.6 million compared to the same period in 2010. GAAP Net income for the three months ended March 31, 2011 decreased 14.5% to $2.7 million compared to the same period in 2010 (See above Use of Non-GAAP Financial Measures).
|
|
|
o
|
Net income from operations increased 24.8% to $4.1 million this quarter compared to the same quarter last year
|
|
|
o
|
Net income margin decreased from 20.5% for the quarter ended March 31, 2010 to 12.1% this quarter. The decrease was mainly due to net decrease in certain non-cash gains such as fair value of our warrants this quarter and increase in selling related expenses, which could potentially increase our revenues in the near future.
|
|
|
o
|
Including in the net income was non-cash income of $263,118 and $1,083,351 for changes in fair value of warrants and non-cash charges for approximately $177,393 and $0 for restricted stocks and option base compensations as well as unamortized beneficial conversion for convertible notes this quarter compared to the same quarter last year
|
38
|
|
▪
|
Basic earnings per share increased to $0.72 and diluted earnings per share increased to $0.59 for the nine months ended March 31, 2011.
|
|
|
o
|
Non-GAAP diluted earnings per share increased 56% to $0.50 for the nine months ended March 31, 2011 compared to the same period in 2010.
|
|
|
o
|
Non-GAAP basic earnings per share increased 46% to $0.60 for the nine months ended March 31, 2011 compared to the same period in 2010.
|
|
|
▪
|
Including restricted cash, our total cash balance was $10.9 million and cash flow from operating activities was $9.0 million for the nine months ended March 31, 2011.
|
|
|
o
|
Total cash and cash equivalents decreased by $6.5 million at March 31, 2011 compared to June 30, 2010.
|
|
|
o
|
Major cash payments activities for the nine months ended March 31, 2011 included $7.1 million for the purchase of prepaid land use rights from the Shandong provincial government for future factory expansion, $7.2 million for the purchase of intangible assets, and repayment of short term bank loan of $4.5 million to China Construction Bank.
|
Operating Results
Comparison of the three months ended March 31, 2011 and 2010
The following table sets forth key components of our results of operations for the three months ended March 31, 2011 and 2010, in US dollars:
|
For The Three Months Ended
|
||||||||||||||||
|
March 31,
|
||||||||||||||||
|
Percentage
|
||||||||||||||||
|
Increase
|
||||||||||||||||
|
2011
|
2010
|
Difference
|
(Decrease)
|
|||||||||||||
|
Net revenues
|
$ | 22,153,412 | $ | 15,323,878 | $ | 6,829,534 | 44.6 | % | ||||||||
|
Cost of revenue
|
(5,213,548 | ) | (2,841,385 | ) | (2,372,163 | ) | 83.5 | % | ||||||||
|
Gross profit
|
16,939,864 | 12,482,493 | 4,457,371 | 35.7 | % | |||||||||||
|
Selling, general, and administrative expenses
|
(12,845,962 | ) | (9,202,873 | ) | (3,643,089 | ) | 39.6 | % | ||||||||
|
Income from operations
|
4,093,901 | 3,279,620 | 814,281 | 24.8 | % | |||||||||||
|
Total other income (expenses)
|
(511,097 | ) | 448,073 | (959,170 | ) | (214.1 | )% | |||||||||
|
Income before provision for income taxes
|
3,582,804 | 3,727,693 | (144,889 | ) | (3.9 | )% | ||||||||||
|
Provision for income taxes
|
(897,458 | ) | (585,135 | ) | (312,323 | ) | 53.4 | % | ||||||||
|
Net income
|
$ | 2,685,346 | $ | 3,142,558 | $ | (457,212 | ) | (14.5 | )% | |||||||
39
The following table sets forth key components as a percentage of net revenue for the three months ended March 31, 2011 and 2010:
|
For The Three Months Ended
|
||||||||
|
March 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Net revenues
|
100.0 | % | 100.0 | % | ||||
|
Cost of sales
|
(23.5 | ) % | (18.5 | )% | ||||
|
Gross profit
|
76.5 | % | 81.5 | % | ||||
|
Selling, general, and administrative expenses
|
(58.0 | )% | (60.1 | )% | ||||
|
Income from operations
|
18.5 | % | 21.4 | % | ||||
|
Total other income(expenses)
|
(2.3 | )% | 2.9 | % | ||||
|
Income before provision for income taxes
|
16.2 | % | 24.3 | % | ||||
|
Provision for income taxes
|
(4.1 | )% | (3.8 | )% | ||||
|
Net income
|
12.1 | % | 20.5 | % | ||||
Net Revenues
Net revenues are comprised of sales of 15 traditional Chinese medicines in China. Net revenues for the three months ended March 31, 2011 increased by $6,829,534 or 44.6% to $22,153,412 as compared to $15,323,878 for the three months ended March 31, 2010. This increase was primarily due to a net increase of 37.6% of revenues from our three lead products, Lung Nourishing Syrup, Tongbi Capsules and Tongbi Tablets, which together accounted for 68% of our total net revenues. All of our lead products are listed for coverage and reimbursement under the national medical insurance program starting in December 2009. The increase was also due to sales from five new products for approximately $1.4 million, or 5.6% of total net revenue for the three months ended March 31, 2011. There were no such net revenues for the three months ended March 31, 2010. The sale of our prescription drug products for the three months ended March 31, 2011 represented 61% for total net revenues compared to 60% from the same period in last year. The increase in prescription sales was due to increases in sales from our two prescription drugs, Tongbi Capsules and Tongbi Tablets.
During the quarter, we have added 200 level 2 hospitals and10 new drug store chains to our national network of retail locations in China currently selling the company's Lung Nourishing Syrup. As a result, we now sell Lung Nourishing Syrup in approximately 1,600 level 2 hospitals and 36 drug store chains across China. We anticipate our overall net revenues will continue to increase due to a national medical and health plan initiated by the Chinese government in 2009, which will eventually cover individual health insurance for over 90% of China’s population by 2011 and includes traditional Chinese medicines for coverage and reimbursement from hospitals and medical centers all over China.
40
Cost of Revenues
Cost of revenues is comprised of raw material costs, labor costs, overhead costs associated with the manufacturing processes and related expenses which are directly attributable to our revenues. Our cost of revenues for the three months ended March 31, 2011 was $5,213,548 as compared to $2,841,385 for the three months ended March 31, 2010, representing an increase of $2,372,163, or 83.5%. The increase in cost of revenues was mainly attributable to the increase in total costs of raw material, labor, and overhead associated with an increase in overall sales and five new products we introduced this year. We also experienced an increase in unit costs of raw material for two other products, Danqi Tablet and Anti-Flu Granules, in the quarter ended March 31, 2011 compared to the same quarter last year.
Gross Profit
Gross profit represents the difference between net revenues and cost of revenues. We achieved gross profit of $16,939,864 for the three months ended March 31, 2011, compared to $12,482,493 for the same fiscal quarter in 2010, representing an increase of $4,457,371, or 35.7. Our overall gross profit margins as a percentage of net revenues decreased by approximately 5.0% this fiscal quarter compared to the same quarter last year. The decrease in gross profit margin was principally due to lower gross profit margins from our five new products resulting from higher manufacturing costs and lower unit sales prices when introducing the 5 new products into the market. The decrease of gross margin was also due to increase in unit costs of raw material for two of our products, Danqi Tablet s and Anti-Flu Granules. If we excluded gross profit for the five new products from our total gross profit this fiscal quarter, the total gross profit margin would be 81.2%, which was in line with gross profit margin of 81.5% for the three months ended March 31, 2010.
Selling, General and Administrative Expenses
Our selling, general and administrative expenses increased by $3,643,089 to $12,845,962, for the three months ended March 31, 2011 compared to $9,202,873 for the same fiscal quarter end in 2010. This increase was mainly attributable to an increase in advertising and promotional expenses for approximately $1,194,905, travel and accommodation expenses of $1,373,144, conference expenses of $530,366 and staff expenses of $358,294 during the quarter ended March 31, 2011 compared to the same fiscal quarter last year. The overall increase in selling, general, and administrative expenses was related to services supporting an overall increase in sale activities and new product promotions. We believe that with additional expenses incurred this quarter in supporting our overall growth in sales will eventually expand our sale volumes for the year to come. As a result of our additional selling activities this quarter, we added 200 level 2 hospitals and10 new drug store chains to our national network of retail locations in China currently selling the company's Lung Nourishing Syrup. The percentage of selling, general, and administrative expenses to net revenues was 58.0% and 60.1% for the three months ended March 31, 2011 and 2010, respectively, representing a decrease of 3.5% as a percentage of net revenues.
Total Other Income (Expenses)
Total other income (expenses) are comprised of interest income (expense), changes in fair value of derivative instruments, non-operating income (expense), and amortization of deferred financing fees. Total other expenses were $511,097 for the quarter ended March 31, 2011 compared to total other income of $448,073 in the quarter ended March 31, 2010, an increase of other expenses for $959,170. The increase was principally due to an increase in non-cash effective interest charges for $90,583, an increase of non-cash accretion of beneficial conversion features on convertible notes for $96,393, and a decrease in non-cash gain in fair value of warrants of $820,232 for convertible notes in connection with our private placement on January 5, 2010.
41
Provision for Income Tax
Our provisions for income taxes for the three months ended March 31, 2011 and 2010 were $897,458 and $585,135, an increase of $312,323 or 53.4% from this quarter over the same quarter in last year. The increase in income tax was principally due to an increase in taxable income under the PRC law (see Note 18 to the accompanying financial statements).
Net Income
We had net income of $2,685,346 for the three months ended March 31, 2011, as compared to net income of $3,142,558 for the three months ended March 31, 2010, a decrease of $457,212, or 14.5 %. This translates into basic net income per common share of $0.15 and $0.20, and diluted net income per common share of $0.14 and $0.16, for the three months ended March 31, 2011 and 2010, respectively. The decrease in net income was primarily attributable to an increase in total gross profit of $4,457,371 offset by a decrease on gains for non-cash related activities such as changes in fair value of our warrants, accretion of beneficial conversion features on convertible notes, and option and stock-based compensation for a total of $997,626, an increase in selling, general and administrative expenses of $3,643,090, and an increase in tax provision of $312,323 this fiscal quarter compared to the same quarter in prior year.
Net income margin was 12.1% this fiscal quarter compared to 20.5% for the same fiscal quarter in last year, representing a decrease of 8.4%. The decrease in net income margin for the three months ended March 31, 2011 over the period in the last fiscal year was principally due to a decrease in net gains for non-cash related activities such as changes in fair value of our warrants, unamortized beneficial conversion features on convertible notes converted, and option and stock-based compensation for a total of $997,626. If we excluded such net gains, the net income margins would be 11.7% this fiscal quarter compared to 13.4% for the same quarter in last year.
We had a Non-GAAP net income of $2,599,621 for the three months ended March 31, 2011, as compared to Non-GAAP net income of $2,059,207 for the three months ended March 31, 2010, an increase in Non-GAAP net income of $540,414, or 26.2 %. This translates to basic Non-GAAP net income per common share of $0.15 and $0.13, and Non-GAAP diluted net income per common share of $0.14 and $0.11, for the three months ended March 31, 2011 and 2010, respectively (See Use of Non-GAAP Financial Measures in Item 2)
Total other income included a non-cash gain on change in fair value of investor and agent warrants of $263,117 for the three months ended March 31, 2011 compared to a non-cash gain of $1,083,351 for the same period in 2010. Total other income this quarter was also comprised of non-cash charges for unamortized BCF on warrant discount of $96,393 for convertible notes in connection with our private placement on January 5, 2010.
Comparison of the nine months ended March 31, 2011 and 2010
The following table sets forth key components of our results of operations for the nine months ended March 31, 2011 and 2010, in US dollars:
42
|
For The Nine Months Ended
|
||||||||||||||||
|
March 31,
|
||||||||||||||||
|
Percentages
|
||||||||||||||||
|
2011
|
2010
|
Difference
|
Increase
|
|||||||||||||
|
Net revenues
|
$ | 61,289,991 | $ | 46,072,455 | $ | 15,217,536 | 33.0 | % | ||||||||
|
Cost of revenues
|
(13,341,860 | ) | (8,205,715 | ) | (5,136,145 | ) | 62.6 | % | ||||||||
|
Gross profit
|
47,948,132 | 37,866,740 | 10,081,392 | 26.6 | % | |||||||||||
|
Selling, general, and administrative expenses
|
(32,565,981 | ) | (28,208,753 | ) | (4,357,228 | ) | 15.4 | % | ||||||||
|
Income from operations
|
15,382,151 | 9,657,987 | 5,724,164 | 59.3 | % | |||||||||||
|
Total other income (expenses)
|
379,710 | 288,537 | 91,173 | 31.6 | % | |||||||||||
|
Income before provision for income taxes
|
15,761,860 | 9,946,524 | 5,815,336 | 58.5 | % | |||||||||||
|
Provision for income taxes
|
(3,523,145 | ) | (2,193,931 | ) | (1,329,214 | ) | 60.6 | % | ||||||||
|
Net income
|
$ | 12,238,716 | $ | 7,752,593 | $ | 4,486,123 | 57.9 | % | ||||||||
The following table sets forth key components as a percentage of net revenue for the nine months ended March 31, 2011 and 2010:
|
For The nine Months Ended
|
||||||||
|
March 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Net revenues
|
100.0 | % | 100.0 | % | ||||
|
Cost of sales
|
(21.8 | )% | (17.8 | )% | ||||
|
Gross profit
|
78.2 | % | 82.2 | % | ||||
|
Selling, general, and administrative expenses
|
(53.1 | )% | (61.2 | )% | ||||
|
Income from operations
|
25.1 | % | 21.0 | % | ||||
|
Total other income(expenses)
|
0.6 | % | 0.6 | % | ||||
|
Income before provision for income taxes
|
25.7 | % | 21.6 | % | ||||
|
Provision for income taxes
|
(5.7 | )% | (4.8 | )% | ||||
|
Net income
|
20.0 | % | 16.8 | % | ||||
43
Net Revenues
Net revenues are comprised of sales of 15 traditional Chinese medicines in China. Net revenues for the nine months ended March 31, 2011 increased by $15,217,536 or 33.0% to $61,289,991 as compared to $46,072,455 for the nine months ended March 31, 2010. This increase was primarily due to a net increase of 37.1% in revenues from our three lead products, Lung Nourishing Syrup, Tongbi Capsules and Tongbi Tablets, which together accounted for over 68.5% of our total net revenues. All of our lead products are listed for coverage and reimbursement under national medical insurance program starting in December 2009. The increase was also due to sales from five new products for approximately $3.4 million, or 5.6% of total net revenue for the nine months ended March 31, 2011. There was no such revenue for the nine months ended March 2010. The sale of our prescription drug products for the nine months ended March 31, 2011 represented 60.9% of total net revenue compared to 59.4% for the same period in last year. The increase in prescription sales was due to increases in sales from our two prescription drugs, Tongbi Capsules and Tongbi Tablets.
During the fiscal quarter ended March 31, 2011, we added 200 level 2 hospitals and10 new drug store chains to our national network of retail locations in China currently selling the company's Lung Nourishing Syrup. As a result, we now sell Lung Nourishing Syrup in approximately 1,600 level 2 hospitals and 36 drug store chains across China. We anticipate our overall net revenues will continue to increase due to a national medical and health plan initiated by the Chinese government in 2009, which will eventually cover individual health insurance for over 90% of China’s population by 2011 and includes traditional Chinese medicines for coverage and reimbursement from hospitals and medical centers all over China.
Cost of Revenues
Cost of revenues is comprised of raw material costs, labor cost, overhead costs associated with the manufacturing processes and related expenses which are directly attributable to the Company’s revenues. Our cost of revenues for the nine months ended March 31, 2011 was $13,341,860 as compared to $8,205,715 for the nine months ended March 31, 2010, representing an increase of $5,136,145, or 62.6%. The increase in cost of revenues was mainly attributable to an increase in total cost of raw material, labor, and overhead as a result of an increase in overall sales and five new products we introduced this year as well as an increase in unit costs of raw material for two other products, Danqi Tablet and Anti-Flu Granules, for the nine months ended March 31, 2011 compared to the same periods in last year.
Gross Profit
Gross profit represents the difference between net revenues and cost of revenues. We achieved gross profit of $47,948,132 for the nine months ended March 31, 2011, compared to $37,866,740 for the same periods in 2010, representing an increase of $10,081,392, or 26.6%, over the same periods of fiscal year 2010. Our overall gross profit margins as a percentage of net revenues decreased by approximately 4.0% this fiscal year to date compared to the same periods last year. The decrease of gross profit margin was principally due to lower gross profit margins from our five new products resulting from higher manufacturing costs and lower unit sales prices when introducing the 5 new products into the market. The decrease of gross margin was also due to increase in unit costs of raw material for two of our products, Danqi Tablet s and Anti-Flu Granules. If we excluded gross profit for the five new products from the total gross profit for the nine months ended March 31, 2010, the gross profit margin would be 82.4%, which was in line with gross profit margin of 82.2% for the nine months ended March 31, 2010.
Selling, General and Administrative Expenses
Our selling, general and administrative expenses increased by $4,357,228 to $32,565,981, for the nine months ended March 31, 2011 compared to $28,208,753 for the same fiscal periods end in 2010. This increase was mainly attributable to an increase in advertising and promotion expenses of approximately $1,729,183 and travel and accommodation expenses for $2,334,709 in the quarter ended March 31, 2011 compared to the same fiscal quarter last year. The overall increase in selling, general, and administrative expenses was related to services supporting an overall increase in sales activities and new product promotions. The percentage of selling, general, and administrative expenses to net revenues was 53.1% and 61.2% for the nine months ended March 31, 2011 and 2010, respectively, representing a decrease of 8.1% as a percentage of net revenues as we continue to improve operating margins by leveraging fixed cost infrastructure and increasing capacity utilization. The overall increase in sales activities year to date, including the addition of 200 level 2 hospitals and10 new drug store chains to our national network of retail locations in China in this quarter, will eventually expand our sales volumes for the year to come.
44
Total Other Income (Expenses)
Total other income (expenses) are comprised of interest income (expense), changes in fair value of derivative instruments, non-operating income (expense), and amortization of deferred financing fees. Total other income was $379,710 for the nine months ended March 31, 2011 compared to total other income of $288,537 for the period ended March 31, 2010, an increase of $91,173. The increase in other income was principally due to an increase in amortization of deferred financing fees of approximately $483,000, an increase of non-cash accretion of beneficial conversion features on convertible notes converted and effective interest charges of $1,666,000 offset by an increase in non-cash gain in fair value of warrants for $2,098,000 for convertible notes in connection with our private placement on January 5, 2010.
Provision for Income Tax
Our provisions for income taxes for the nine months ended March 31, 2011 and 2010 were $3,523,145 and $2,193,931, an increase of $1,329,214 or 60.6% from this fiscal year to date over the same period in last year. The increase in income tax was principally due to an increase in taxable income under the PRC law (see footnote 18).
Net Income
We had a net income of $12,238,716 for the nine months ended March 31, 2011, as compared to net income of $7,752,593 for the nine months ended March 31, 2010, an increase in net income of $4,486,123, or 57.9 %. This translates into basic net income per common share of $0.72 and $0.48, and diluted net income per common share of $0.59 and $0.37, for the nine months ended March 31, 2011 and 2010, respectively. The increase in net income was primarily attributable to an increase in total gross profit of $10,081,391 offset by an increase in selling, general and administrative expenses of $4,357,227 and an increase in the tax provision of $1,329,214 this fiscal year to date compared to the same period in prior year.
Net income margin was 20.0% for the nine months ended March 31, 2011 compared to 16.8% for the same period last year, an increase of 3.2%. The increase in net income margin for the nine months ended March 31, 2011 over the period in the previous fiscal year was principally due to an increase in net gains on non-cash related activities such as changes in fair value of our warrants, accretion of beneficial conversion features on convertible notes converted, and option and stock-based compensation for a total of $884,421. If we excluded such net gains, the net income margin would be 16.8% this fiscal year to date compared to 14.5% for the same period in last year.
We had Non-GAAP net income of $10,270,944 for the nine months ended March 31, 2011, as compared to Non-GAAP net income of $6,669,242 for the nine months ended March 31, 2010, an increase in Non-GAAP net income of $3,601,702, or 54.0 %. This translates into basic Non-GAAP net income per common share of $0.60 and $0.41, and Non-GAAP diluted net income per common share of $0.50 and $0.32, for the nine months ended March 31, 2011 and 2010, respectively (See Use of Non-GAAP Financial Measures in Item 2)
45
Total other income included a non-cash gain on change in fair value of investor and agent warrants of $3,181,603 for the nine months ended March 31, 2011 compared to $1,083,351 for the same period in 2010.
Total other income for the nine months ended March 31, 2011 also comprised of non-cash charges for unamortized BCF on warrant discount of $1,029,487 for convertible notes in connection with our private placement on January 5, 2010.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate adequate amounts of cash to meet its needs for cash. As of March 31, 2011, we had cash and cash equivalents of $10,665,517 and restricted cash of $220,043, substantially almost all of which is located in financial institutions in China. The following table provides detailed information about our net cash flow for financial statement periods presented in this report:
Summary of Cash Flow Statements
|
For the nine months ended
|
||||||||
|
March 31
|
||||||||
|
2011
|
2010
|
|||||||
|
Net cash provided by operating activities
|
$ | 9,016,541 | $ | 4,144,506 | ||||
|
Net cash (used in) investing activities
|
(14,307,391 | ) | (280,804 | ) | ||||
|
Net cash provided by (used in) financing activities
|
(1,668,927 | ) | 8,951,153 | |||||
|
Effect of foreign currency translation on cash and cash equivalents
|
476,212 | 266,544 | ||||||
|
Net (decrease) increase in cash and cash equivalent
|
$ | (6,483,565 | ) | $ | 13,081,399 | |||
On January 5, 2010, pursuant to a Securities Purchase Agreement with 128 accredited investors, we sold 6,000,000 units for aggregate gross proceeds of $12,000,000, each unit consisting of an 8% senior convertible promissory note in the principal amount of $2.0, or the Notes, and one common stock purchase warrant, or the Warrants. The Notes bear interest at 8% per annum, payable quarterly in arrears on the last day of each of our fiscal quarter s. No principal payments are required until maturity of the Notes on January 5, 2012. Each Note, plus all accrued but unpaid interest thereon, is convertible, in whole but not in part, at any time at the option of the holder, into shares of the Company ’ s common stock, par value $0.001 per share, at a conversion price of $2.00 per share, subject to adjustment as set forth in the Note.
Effective as of June 30, 2010, we entered into an Amendment and Agreement (which we refer to herein as the Amendment) with the representative of the investors, pursuant to which we and the Investors agreed to make certain amendments to the Notes and the Warrants. Pursuant to the Amendment, the anti-dilution protection provisions i n the Notes and the Warrants were eliminated and a provision specifically precluding net cash settlement by the Company of the Notes and the Warrants was added. In return, and subject to certain non-financing exceptions, we agreed not to issue any new equity securities at a price per share below $2.20 until the earlier of (i) January 5, 2013 or (ii) the date on which, collectively with any prior conversions or exercises of Notes and Warrants, 75% of the principal face value of the Notes in the aggregate ha s been converted into shares of Common Stock and Warrants representing, in the aggregate, 75% of the aggregate shares of Common Stock underlying the Warrants have been exercised. Effective March 30, 2011, we and the representative of the investors agreed to terminate the Amendment. This termination took place because, after further study, we concluded that the original purpose of the Amendment (to mitigate the impact of certain non-cash embedded derivative liabilities associated with the Notes and Warrants) would not be achieved. Therefore, we determined and agreed with the representative of the investors to terminate the Amendment and to thereby restore the Notes, Warrants and warrants issued to the placement agents in offering to their original terms.
46
Comparison of Nine Months Ended March 31, 2011 and 2010
Net Cash Provided by Operating Activities
Net cash provided by operating activities totaled $9,016,541 for the nine months ended March 31, 2011 as compared to net cash provided by operating activities of $4,144,506 for the nine months ended March 31, 2010. The increase in net cash provided by operating activities was primarily due to increases in net income and other payable balances offset by increases in accounts receivable and inventory balances. We expect our cash flow from operating activities to maintain at positive flow due to strong support of TCM products from the Chinese government in that a national medical and health plan initiated by the Chinese government in 2009, which will eventually cover individual health insurance over 90% of China’s population by 2011 and includes traditional Chinese medicines for coverage and reimbursement from hospitals and medical centers all over China.
Net Cash Used In Investing Activities
Net cash used in investing activities was $14,307,391 for the nine months ended March 31, 2011 and $280,804 for the nine months ended March 31, 2010. The increase in cash used in investing activities was due to our cash payment of approximately $7.1 million for the purchase of leased land use rights from the Shandong provincial government in January 2010. The aggregate purchase price is approximately $14,839,957 (RMB 97,500,000). The increase in cash used in investing activities was also due to cash payment of $7,186,059 for purchase of 14 State approved TCM formulas.
Net Cash Used in (Provided by) Financing Activities
Net cash used in financing activities totaled $1,668,927 for the nine months ended March 31, 2011 as compared to net cash provided by financing activities of $8,951,153 for the same period in 2010. The reason for the decrease in cash provided by financing activities was due to net cash received of $10.4 million from convertible notes issued in connection with our private placement on January 5, 2010.
Cash Position
As of March 31, 2011, we had cash of $10,665,517 as compared to $17,149,082 as of June 30, 2010, a decrease of $6,483,565. This decrease was due primarily to increase in cash from operating activities of approximately $9.0 million and cash receipt of approximately $1.9 million from restricted shares issued to Chinese investors offset by cash payment of approximately $7.1 million for the purchase of leased land use rights from the Shandong provincial government, cash payment of $7.1 million for 14 State approved TCM formulas and cash payment of $4.5 million for short term bank loan.
We believe that we can meet our liquidity and capital requirements for our ongoing operations from our currently available working capital and maintain our operations at our current levels.
47
Critical Accounting Policies and Estimates
As discussed in Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations, of our Annual Report on Form 10-K for the fiscal year ended June 30, 2010, we consider our use of estimates, account receivables, revenue recognition, inventories, property plant and equipment, and income taxes to be the most critical in understanding the judgments that are involved in preparing our consolidated financial statements. There have been no significant changes to these estimates in the three and nine months ended March 31, 2011.
Recent Accounting Pronouncements Adopted
See Note 3 to the accompanying condensed consolidated financial statements included in Item 1, Financial Information, of this Quarterly Report on Form 10-Q.
Recent Accounting Pronouncements Not Yet Adopted
See Note 3 to the accompanying condensed consolidated financial statements included in Item 1, Financial Information, of this Quarterly Report on Form 10-Q.
Obligations under Material Contracts
The following table summarizes our contractual obligations as of March 31, 2011, and the effect these obligations are expected to have on our liquidity and cash flows in future periods.
|
Payments Due by Period
|
||||||||||||||||||||
|
Total
|
Less than 1 year
|
1-3 Years
|
4-5 years
|
5 Years+
|
||||||||||||||||
|
Contractual Obligations:
|
||||||||||||||||||||
|
Bank loans
|
$ | 905,618 | $ | 905,618 | $ | - | $ | - | $ | - | ||||||||||
|
Research and development
|
1,019,771 | - | 1,019,771 | - | - | |||||||||||||||
|
Convertible notes
|
10,500,000 | 10,500,000 | - | - | - | |||||||||||||||
|
Raw material purchase obligations *
|
1,433,944 | 1,433,944 | - | - | - | |||||||||||||||
|
Total Contractual Obligations:
|
$ | 13,859,333 | $ | 12,839,562 | $ | 1,019,771 | $ | - | $ | - | ||||||||||
|
*
|
Various raw material purchase contracts, contractual obligation fulfilled in April 2011.
|
Other than discussed above, there are no other foreseeable material commitments or contingencies as of March 31, 2011.
Off-Balance Sheet Arrangements
We did not engage in any “off-balance sheet arrangements” (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) during the nine months ended March 31, 2011. We have not entered into any financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as stockholder ’ s equity or that are not reflected in our condensed consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk, or credit support to us or engages in leasing, hedging, or research and development services with us.
48
Item 3. Quantitative and Qualitative Disclosures about Market Risk
Not applicable
Item 4T. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
As of March 31, 2011, the quarterly period covered by this report, our President and Chief Executive Officer and our Chief Financial Officer (the “Certifying Officers”), conducted evaluations of the Company’s disclosure controls and procedures. As defined under Sections 13a–15(e) and 15d–15(e) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), the term “disclosure controls and procedures” means controls and other procedures of an issuer that are designed to ensure that information required to be disclosed by the issuer in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, including the Certifying Officers, to allow timely decisions regarding required disclosures.
Based on this evaluation, the Certifying Officers have concluded that our disclosure controls and procedures were, due to certain significant deficiencies, not effective to ensure that material information is recorded, processed, summarized and reported by our management on a timely basis in order to comply with our disclosure obligations under the Exchange Act and the rules and regulations promulgated thereunder.
A significant deficiency is a deficiency, or a combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness(within the meaning of PCAOB Auditing Standard No. 5) yet important enough to merit attention by those responsible for oversight of a company’s financial reporting.
The significant deficiencies identified by the Certifying Officers continue to be as described in our Annual Report on Form 10-K for the fiscal year ending June 30, 2010, which was filed with the SEC on September 29, 2010. As a result, the Certifying Officers and our board of directors are continuing to asses on our internal control over financial reporting and our disclosure controls and procedures in an attempt to address such significant deficiencies.
Changes in Internal Control over Financial Reporting
Subject to the foregoing disclosure, there were no changes in our internal control over financial reporting during the three months ended March 31, 2011 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
49
Limitations on the Effectiveness of Internal Controls
Readers are cautioned that our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will necessarily prevent all fraud and material error. An internal control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any control design will succeed in achieving its stated goals under all potential future conditions. Over time, control may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate.
50
Item 1. Legal Proceedings
From time to time, we may become involved in various lawsuits and legal proceedings, which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business. We are currently not aware of any such legal proceedings or claims that will have, individually or in the aggregate, a material adverse affect on our business, financial condition or operating results.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 4. Removed and Reserved
Item 5. Other Information
None.
Item 6. Exhibits
(a) Exhibits
|
Exhibit
Number
|
Description of Exhibit
|
|
|
31.1*
|
Certification of Principal Executive Officer pursuant to Rule 13a-14 and Rule 15d-14(a), promulgated under the Securities and Exchange Act of 1934, as amended.
|
|
|
31.2*
|
Certification of Principal Financial Officer pursuant to Rule 13a-14 and Rule 15d 14(a), promulgated under the Securities and Exchange Act of 1934, as amended.
|
|
|
32.1*
|
Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Chief Executive Officer).
|
|
|
32.2*
|
|
Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Chief Financial Officer).
|
* Filed herewith
51
SIGNATURES
In accordance with the requirements of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Bohai Pharmaceuticals Group, Inc.
|
||
|
May 16, 2011
|
By:
|
/s/ Hongwei Qu
|
|
Hongwei Qu
|
||
|
Chief Executive Officer
|
||
|
(Principal Executive Officer)
|
||
|
May 16, 2011
|
By:
|
/s/ Gene Hsiao
|
|
Gene Hsiao
|
||
|
Chief Financial Officer
|
||
|
(Principal Accounting Officer)
|
||
52
