Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - WRIGHT MEDICAL GROUP INC | Financial_Report.xls |
| EX-32 - EX-32 - WRIGHT MEDICAL GROUP INC | g27126exv32.htm |
| EX-31.2 - EX-31.2 - WRIGHT MEDICAL GROUP INC | g27126exv31w2.htm |
| EX-31.1 - EX-31.1 - WRIGHT MEDICAL GROUP INC | g27126exv31w1.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
(Mark One)
| þ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2011
or
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 000-32883
WRIGHT MEDICAL GROUP, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware (State or Other Jurisdiction of Incorporation or Organization) |
13-4088127 (IRS Employer Identification Number) |
|
| 5677 Airline Road Arlington, Tennessee (Address of Principal Executive Offices) |
38002 (Zip Code) |
(901) 867-9971
(Registrant’s Telephone Number, Including Area Code)
(Registrant’s Telephone Number, Including Area Code)
Indicate by check mark whether the registrant: (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12
months (or for such shorter period that the registrant was required to file such reports),
and (2) has been subject to such filing requirements for the past 90 days.
þ Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate Web site, if any, every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12
months (or for such shorter period that the registrant was required to submit and post such
files.) þ Yes o No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated
filer, a non-accelerated filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2
of the Exchange Act.
| Large accelerated filer þ | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller Reporting Company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Exchange Act). o Yes þ No
As of May 9, 2011, there were 39,020,799 shares of common stock outstanding.
WRIGHT MEDICAL GROUP, INC.
TABLE OF CONTENTS
| Page Number |
||||||||
| 1 | ||||||||
| 1 | ||||||||
| 2 | ||||||||
| 3 | ||||||||
| 4 | ||||||||
| 15 | ||||||||
| 24 | ||||||||
| 25 | ||||||||
| 26 | ||||||||
| 26 | ||||||||
| 26 | ||||||||
| 26 | ||||||||
| 26 | ||||||||
| 26 | ||||||||
| 26 | ||||||||
| 30 | ||||||||
| EX-31.1 | ||||||||
| EX-31.2 | ||||||||
| EX-32 | ||||||||
| EX-101 INSTANCE DOCUMENT | ||||||||
| EX-101 SCHEMA DOCUMENT | ||||||||
| EX-101 CALCULATION LINKBASE DOCUMENT | ||||||||
| EX-101 LABELS LINKBASE DOCUMENT | ||||||||
| EX-101 PRESENTATION LINKBASE DOCUMENT | ||||||||
| EX-101 DEFINITION LINKBASE DOCUMENT | ||||||||
SAFE-HARBOR STATEMENT
This quarterly report contains “forward-looking statements” as defined under U.S. federal
securities laws. These statements, including statements regarding potential actions by the USAO, independent monitor, OIG, and other agencies or their potential impact, reflect management’s current knowledge, assumptions, beliefs,
estimates, and expectations and express management’s current views of future performance, results,
and trends and may be identified by their use of terms such as “anticipate,” “believe,” “could,”
“estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “will,” and other similar
terms. Forward-looking statements are subject to a number of risks and uncertainties that could
cause our actual results to materially differ from those described in the forward-looking
statements. Readers should not place undue reliance on forward-looking statements. Such statements
are made as of the date of this quarterly report, and we undertake no obligation to update such
statements after this date.
Risks and uncertainties that could cause our actual results to materially differ from those
described in forward-looking statements include those discussed in our filings with the Securities
and Exchange Commission (including those described in Item 1A of our Annual Report on Form 10-K for
the year ended December 31, 2010, under the heading, “Risk Factors” and in Item 1A of Part II and
elsewhere in this report), and the following:
| • | the impact of our settlement of the federal investigation into our consulting arrangements with orthopaedic surgeons relating to our hip and knee products in the United States, including our compliance with the Deferred Prosecution Agreement (“DPA”) through September 2011 (which could, by its terms, be extended for a further six months) and the Corporate Integrity Agreement (“CIA”) through September 2015. Our failure to comply with the Deferred Prosecution Agreement or the Corporate Integrity Agreement could expose us to significant liability including, but not limited to, extension of the term of the DPA by up to 6 months, exclusion from federal healthcare program participation, including Medicaid and Medicare, which would have a material adverse effect on our financial condition, results of operations and cash flows, potential prosecution, including under the previously-filed criminal complaint, civil and criminal fines and penalties, and additional litigation cost and expense. A breach of the DPA or the CIA could result in an event of default under the Senior Credit Facility, which in turn could result in an event of default under the Indenture; |
Table of Contents
| • | demand for and market acceptance of our new and existing products; | |
| • | recently enacted healthcare reform legislation and its future implementation, possible additional legislation, regulation and other governmental pressures in the United States or globally, which may affect pricing, reimbursement, taxation and rebate policies of government agencies and private payers or other elements of our business; | |
| • | tax reform measures, tax authority examinations and associated tax risks and potential obligations; | |
| • | our ability to identify business development and growth opportunities for existing or future products; | |
| • | product quality or patient safety issues, leading to product recalls, withdrawals, launch delays, sanctions, seizures, litigation, or declining sales; | |
| • | individual, group or class action alleging products liability claims, including an increase in the number of claims during any period; | |
| • | future actions of the FDA or any other regulatory body or government authority that could delay, limit or suspend product development, manufacturing or sale or result in seizures, injunctions, monetary sanctions or criminal or civil liabilities; | |
| • | our ability to enforce our patent rights or patents of third parties preventing or restricting the manufacture, sale or use of affected products or technology; | |
| • | the impact of geographic and product mix on our sales; | |
| • | retention of our sales representatives and independent distributors; | |
| • | inventory reductions or fluctuations in buying patterns by wholesalers or distributors; | |
| • | our ability to realize the anticipated benefits of restructuring initiatives; and | |
| • | any impact of the commercial and credit environment on us and our customers and suppliers. |
Table of Contents
PART I — FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS (unaudited).
WRIGHT MEDICAL GROUP, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(In thousands, except share data)
(unaudited)
(In thousands, except share data)
(unaudited)
| March 31, | December 31, | |||||||
| 2011 | 2010 | |||||||
Assets: |
||||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 154,839 | $ | 153,261 | ||||
Marketable securities |
14,840 | 19,152 | ||||||
Accounts receivable, net |
107,622 | 105,336 | ||||||
Inventories |
171,738 | 166,339 | ||||||
Prepaid expenses |
5,793 | 5,333 | ||||||
Deferred income taxes |
32,133 | 32,026 | ||||||
Other current assets |
19,184 | 16,143 | ||||||
Total current assets |
506,149 | 497,590 | ||||||
Property, plant and equipment, net |
161,430 | 158,247 | ||||||
Goodwill |
54,647 | 54,172 | ||||||
Intangible assets, net |
15,813 | 16,501 | ||||||
Marketable securities |
10,071 | 17,193 | ||||||
Deferred income taxes |
4,616 | 4,125 | ||||||
Other assets |
7,091 | 7,411 | ||||||
Total assets |
$ | 759,817 | $ | 755,239 | ||||
Liabilities and Stockholders’ Equity: |
||||||||
Current liabilities: |
||||||||
Accounts payable |
$ | 22,081 | $ | 15,862 | ||||
Accrued expenses and other current liabilities |
58,760 | 54,409 | ||||||
Current portion of long-term obligations |
8,617 | 1,033 | ||||||
Total current liabilities |
89,458 | 71,304 | ||||||
Long-term debt and capital lease obligations |
173,269 | 201,766 | ||||||
Deferred income taxes |
5,814 | 5,705 | ||||||
Other liabilities |
11,343 | 5,492 | ||||||
Total liabilities |
$ | 279,884 | $ | 284,267 | ||||
Commitments and contingencies (Note 11) |
||||||||
Stockholders’ equity: |
||||||||
Common stock, $.01 par value,
authorized: 100,000,000 shares; issued and
outstanding: 39,180,164 shares at March 31, 2011 and
39,171,501 shares at December 31, 2010 |
380 | 379 | ||||||
Additional paid-in capital |
392,795 | 390,098 | ||||||
Accumulated other comprehensive income |
24,844 | 22,173 | ||||||
Retained earnings |
61,914 | 58,322 | ||||||
Total stockholders’ equity |
479,933 | 470,972 | ||||||
Total liabilities and stockholders’ equity |
$ | 759,817 | $ | 755,239 | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
1
Table of Contents
WRIGHT MEDICAL GROUP, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
(unaudited)
(In thousands, except per share data)
(unaudited)
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2011 | 2010 | |||||||
Net sales |
$ | 135,386 | $ | 131,244 | ||||
Cost of sales 1 |
38,768 | 40,141 | ||||||
Gross profit |
96,618 | 91,103 | ||||||
Operating expenses: |
||||||||
Selling, general and administrative 1 |
74,825 | 76,438 | ||||||
Research and development 1 |
9,207 | 9,835 | ||||||
Amortization of intangible assets |
690 | 649 | ||||||
Restructuring charges |
— | 544 | ||||||
Total operating expenses |
84,722 | 87,466 | ||||||
Operating income |
11,896 | 3,637 | ||||||
Interest expense, net |
1,835 | 1,508 | ||||||
Other expense, net |
4,459 | 132 | ||||||
Income before income taxes |
5,602 | 1,997 | ||||||
Provision for income taxes |
2,010 | 2,522 | ||||||
Net income (loss) |
$ | 3,592 | $ | (525 | ) | |||
Net income (loss) per share (Note 9): |
||||||||
Basic |
$ | 0.09 | $ | (0.01 | ) | |||
Diluted |
$ | 0.09 | $ | (0.01 | ) | |||
Weighted-average number of shares outstanding-basic |
38,033 | 37,540 | ||||||
Weighted-average number of shares outstanding-diluted |
38,327 | 37,540 | ||||||
| 1 | These line items include the following amounts of non-cash, stock-based compensation expense for the periods indicated: |
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2011 | 2010 | |||||||
Cost of sales |
$ | 347 | $ | 340 | ||||
Selling, general and administrative |
2,068 | 2,267 | ||||||
Research and development |
445 | 398 | ||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
2
Table of Contents
WRIGHT MEDICAL GROUP, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
(unaudited)
(In thousands)
(unaudited)
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2011 | 2010 | |||||||
Operating activities: |
||||||||
Net income (loss) |
$ | 3,592 | $ | (525 | ) | |||
Adjustments to reconcile net income to net cash provided by
operating activities: |
||||||||
Depreciation |
9,442 | 8,436 | ||||||
Stock-based compensation expense |
2,860 | 3,005 | ||||||
Amortization of intangible assets |
690 | 649 | ||||||
Amortization of deferred financing costs |
336 | 246 | ||||||
Deferred income taxes |
(767 | ) | (924 | ) | ||||
Write off of deferred financing costs |
2,926 | — | ||||||
Excess tax benefit from stock-based compensation arrangements |
(1 | ) | (93 | ) | ||||
Non-cash restructuring charges |
— | 121 | ||||||
Other |
(1,312 | ) | 1,061 | |||||
Changes in assets and liabilities (net of acquisitions): |
||||||||
Accounts receivable |
51 | (2,638 | ) | |||||
Inventories |
(5,204 | ) | 1,455 | |||||
Prepaid expenses and other current assets |
(3,118 | ) | 3,684 | |||||
Accounts payable |
6,096 | 6,400 | ||||||
Accrued expenses and other liabilities |
2,557 | 7,810 | ||||||
Net cash provided by operating activities |
18,148 | 28,687 | ||||||
Investing activities: |
||||||||
Capital expenditures |
(10,085 | ) | (11,603 | ) | ||||
Acquisitions of businesses |
— | (237 | ) | |||||
Purchase of intangible assets |
(61 | ) | (751 | ) | ||||
Investment in held-to-maturity marketable securities |
— | 20,090 | ||||||
Sales and maturities of available-for-sale marketable securities |
11,538 | — | ||||||
Investment in available-for-sale marketable securities |
— | (11,435 | ) | |||||
Proceeds from sale of assets |
5,500 | — | ||||||
Net cash provided by (used in) investing activities |
6,892 | (3,936 | ) | |||||
Financing activities: |
||||||||
Issuance of common stock |
78 | 206 | ||||||
Financing under factoring agreement, net |
— | 5 | ||||||
Payments of deferred financing costs |
(2,887 | ) | — | |||||
Payments of capital leases |
(261 | ) | (55 | ) | ||||
Redemption of convertible senior notes |
(170,889 | ) | — | |||||
Proceeds from term loan borrowings |
150,000 | — | ||||||
Excess tax benefit from stock-based compensation arrangements |
1 | 93 | ||||||
Net cash (used in) provided by financing activities |
(23,958 | ) | 249 | |||||
Effect of exchange rates on cash and cash equivalents |
496 | (370 | ) | |||||
Net increase in cash and cash equivalents |
1,578 | 24,630 | ||||||
Cash and cash equivalents, beginning of period |
153,261 | 84,409 | ||||||
Cash and cash equivalents, end of period |
$ | 154,839 | $ | 109,039 | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
3
Table of Contents
WRIGHT
MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(UNAUDITED)
1. Summary of Significant Accounting Policies
Basis of Presentation. The unaudited condensed consolidated interim financial statements of Wright
Medical Group, Inc. have been prepared in accordance with accounting principles generally accepted
in the United States (U.S.) for interim financial information and the instructions to Quarterly
Report on Form 10-Q and Rule 10-01 of Regulation S-X. Certain information and footnote disclosures
normally included in financial statements prepared in accordance with accounting principles
generally accepted in the U.S. have been condensed or omitted pursuant to these rules and
regulations. Accordingly, these unaudited condensed consolidated interim financial statements
should be read in conjunction with our consolidated financial statements and related notes included
in our Annual Report on Form 10-K for the year ended December 31, 2010, as filed with the U.S.
Securities and Exchange Commission (SEC).
In the opinion of management, these unaudited condensed consolidated interim financial statements
reflect all adjustments necessary for a fair presentation of our interim financial results. All
such adjustments are of a normal and recurring nature. The results of operations for any interim
period are not indicative of results for the full fiscal year.
The accompanying unaudited condensed consolidated interim financial statements include our accounts
and those of our wholly-owned domestic and international subsidiaries. Intercompany accounts and
transactions have been eliminated in consolidation.
The preparation of these financial statements requires management to make estimates and assumptions
that affect the reported amounts of assets and liabilities and the disclosure of contingent
liabilities at the dates of the financial statements and the amounts of revenues and expenses
during the reporting periods. Actual amounts realized or paid could differ from those estimates.
Revenue Recognition. Our revenues are primarily generated through two types of customers, hospitals
and surgery centers, and stocking distributors, with the majority of our revenue derived from sales
to hospitals. Our products are primarily sold through a network of employee sales representatives
and independent sales representatives in the U.S. and by a combination of employee sales
representatives, independent sales representatives, and stocking distributors outside the U.S.
Revenues from sales to hospitals are recorded when the hospital takes title to the product, which
is generally when the product is surgically implanted in a patient. We record revenues from sales
to our stocking distributors outside the U.S. at the time the product is shipped to the stocking
distributor.
In 2011, we entered into a trademark license agreement (License Agreement) with KCI Medical
Resources, a subsidiary of Kinetic Concepts, Inc. The License Agreement provides KCI Medical
Resources with a non-transferable license to use Wright’s trademarks associated with its
GRAFTJACKET® line of products in connection with the marketing and
distribution of KCI Medical Resources’ soft tissue graft containment products used in the wound
care field, subject to certain exceptions. License revenue is being recognized over the life of the
agreement on a straight line basis.
Derivative Instruments. We account for derivative instruments and hedging activities under
Financial Accounting Standards Board (FASB) Accounting Standards Codification (FASB ASC) Topic 815,
Derivatives and Hedging (FASB ASC 815). Accordingly, all of our derivative instruments are recorded
in the accompanying condensed consolidated balance sheets as either an asset or liability and measured at
fair value. The changes in the derivative’s fair value are recognized currently in earnings unless
specific hedge accounting criteria are met.
We employ a derivative program using 30-day foreign currency forward contracts to mitigate the risk
of currency fluctuations on our intercompany receivable and payable balances that are denominated
in foreign currencies. These forward contracts are expected to offset the transactional gains and
losses on the related intercompany balances. These forward contracts are not designated as hedging
instruments under FASB ASC 815. Accordingly, the changes in the fair value and the settlement of
the contracts are recognized in the period incurred in the accompanying condensed consolidated statements of
operations.
Additionally, we entered into an interest rate swap to hedge our variable interest rate
obligations. The interest rate swap has been accounted for as a cash flow hedge in accordance with
FASB ASC Topic 815. See Note 6 for further disclosure on our interest rate swap.
4
Table of Contents
WRIGHT MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
Fair
Value of Financial Instruments and Immaterial Error Correction. The carrying values of cash and cash equivalents, accounts
receivable, and accounts payable approximate the fair values of these financial instruments as of
March 31, 2011 and December 31, 2010 due to their short maturities.
The carrying amount of debt outstanding pursuant to the credit agreement (the term loan)
approximates fair value as interest rates on these instruments approximate current market rates.
The $29.1 million of our convertible senior notes are carried at cost. The estimated fair value of
the senior notes was approximately $27.8 million at March 31, 2011 based on a limited number of
trades and does not necessarily represent the value at which the entire convertible note portfolio
can be retired.
Pursuant to the requirements of the FASB ASC Topic 820, Fair Value Measurements and Disclosures,
our financial assets and liabilities measured at fair value on a recurring basis are classified and
disclosed in one of the following three categories:
| Level 1: | Financial instruments with unadjusted, quoted prices listed on active market exchanges. | ||
| Level 2: | Financial instruments determined using prices for recently traded financial instruments with similar underlying terms as well as directly or indirectly observable inputs, such as interest rates and yield curves that are observable at commonly quoted intervals. | ||
| Level 3: | Financial instruments that are not actively traded on a market exchange. This category includes situations where there is little, if any, market activity for the financial instrument. The prices are determined using significant unobservable inputs or valuation techniques. |
We use a third-party provider to determine fair values of our available for sale marketable
securities. The third-party provider receives market prices for each marketable security from a
variety of industry standard data providers, security master files from large financial
institutions and other third-party sources with reasonable levels of price transparency. The
third-party provider uses these multiple prices as inputs into a pricing model to determine a
weighted average price for each security. We classify our U.S. Treasury bills and bonds as Level 1
based upon quoted prices in active markets. All other marketable securities are classified as Level
2 based upon the other than quoted prices with observable market data. These include municipal
debt securities, U.S agency debt securities, corporate debt securities, certificates of deposits
and time deposits. During the three months ended March 31, 2011, we began investing in commercial
paper with original maturity dates of three months or less. Our commercial paper is classified as
a Level 2 and is included in our Cash and cash equivalents balance as of March 31, 2011.
During the quarter ended March 31, 2011, we corrected an immaterial error in the footnotes to our
2010 Form 10-K related to the fair value hierarchy classification of certain available for sale
marketable securities. As of December 31, 2010, municipal debt securities, U.S. agency debt
securities, and corporate debt securities with fair values of $897,000, $14.5 million, and $3.2
million, respectively, all of which are Level 2 fair value measurements, were incorrectly
classified as Level 1 fair value measurements. The table below has been corrected to reflect the
appropriate fair value hierarchy classification as of December 31, 2010. This error is not
considered material to the 2010 consolidated financial statements.
As part of the acquisition of EZ Concepts Surgical Device Corporation, d/b/a EZ Frame, completed in
2010, we may be obligated to pay contingent consideration of up to $400,000 upon the achievement of
certain revenue milestones. The $356,000 fair value of the contingent consideration was determined
using a discounted cash flow model and probability adjusted estimates of the future earnings and is
classified in Level 3. This obligation is included in current liabilities in our condensed
consolidated balance sheet. Changes in the fair value of contingent consideration will be recorded
in our consolidated statements of operations.
The following table summarizes the valuation of our financial instruments measured at fair value on
a recurring basis (in thousands):
5
Table of Contents
WRIGHT MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
| Prices with | ||||||||||||||||
| Quoted Prices | Other | Prices with | ||||||||||||||
| in Active | Observable | Unobservable | ||||||||||||||
| Markets | Inputs | Inputs | ||||||||||||||
| Total | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
At March 31, 2011 |
||||||||||||||||
Assets |
||||||||||||||||
Cash and cash equivalents |
$ | 154,839 | $ | 79,862 | $ | 74,977 | $ | — | ||||||||
Available-for-sale marketable securities |
||||||||||||||||
Municipal debt securities |
$ | 892 | $ | — | $ | 892 | $ | — | ||||||||
U.S. agency debt securities |
8,509 | — | 8,509 | — | ||||||||||||
Corporate debt securities |
3,163 | — | 3,163 | — | ||||||||||||
U.S. government debt securities |
7,534 | 7,534 | — | — | ||||||||||||
Total available-for-sale marketable
securities |
20,098 | 7,534 | 12,564 | — | ||||||||||||
Held-to-maturity time deposits |
4,813 | — | 4,813 | — | ||||||||||||
Interest rate swap |
243 | — | 243 | — | ||||||||||||
| $ | 179,993 | $ | 87,396 | $ | 92,597 | $ | — | |||||||||
Liabilities |
||||||||||||||||
Contingent consideration |
356 | — | — | 356 | ||||||||||||
| $ | 356 | $ | — | $ | — | $ | 356 | |||||||||
| Prices with | ||||||||||||||||
| Quoted Prices | Other | Prices with | ||||||||||||||
| in Active | Observable | Unobservable | ||||||||||||||
| Markets | Inputs | Inputs | ||||||||||||||
| Total | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
At December 31, 2010 |
||||||||||||||||
Assets |
||||||||||||||||
Cash and cash equivalents |
$ | 153,261 | $ | 153,261 | $ | — | $ | — | ||||||||
Available-for-sale marketable securities |
||||||||||||||||
Municipal debt securities |
$ | 897 | $ | — | $ | 897 | $ | — | ||||||||
U.S. agency debt securities |
14,511 | — | 14,511 | — | ||||||||||||
Certificates of deposits |
38 | — | 38 | — | ||||||||||||
Corporate debt securities |
3,183 | — | 3,183 | |||||||||||||
U.S. government debt securities |
13,045 | 13,045 | — | — | ||||||||||||
Total available-for-sale marketable
securities |
31,674 | 13,045 | 18,629 | — | ||||||||||||
Held-to-maturity time deposits |
4,671 | — | 4,671 | — | ||||||||||||
| $ | 189,606 | $ | 166,306 | $ | 23,300 | $ | — | |||||||||
Liabilities |
||||||||||||||||
Contingent consideration |
$ | 356 | $ | — | $ | — | $ | 356 | ||||||||
| $ | 356 | $ | — | $ | — | $ | 356 | |||||||||
6
Table of Contents
WRIGHT MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
2. Inventories
Inventories consist of the following (in thousands):
| March 31, | December | |||||||
| 2011 | 31, 2010 | |||||||
Raw materials |
$ | 8,697 | $ | 8,962 | ||||
Work-in-process |
26,179 | 24,723 | ||||||
Finished goods |
136,862 | 132,654 | ||||||
| $ | 171,738 | $ | 166,339 | |||||
3. Marketable Securities
We have historically invested in treasury bills, government and agency bonds, and certificates of
deposit with maturity dates of less than 12 months. Our investments in these marketable securities
are classified as available-for-sale securities in accordance with FASB ASC Topic 320, Investments
— Debt and Equity Securities. These securities are carried at their fair value, and all unrealized
gains and losses are recorded within other comprehensive income. In the third quarter of 2010, we
invested in a bank deposit with a maturity date of 12 months. This investment, which is classified
as held-to-maturity, is carried at its amortized cost. Marketable securities are classified as
short-term for those expected to mature or be sold within 12 months and the remaining portion is
classified as long-term. The cost of investment securities sold is determined by the specific
identification method.
As of March 31, 2011 and December 31, 2010, we had current marketable securities totaling $14.8
million and $19.2 million, respectively, consisting of investments in corporate, municipal and
government bonds, certificates of deposits, and treasury bills, all of which are valued at fair
value using a market approach. In addition, we had noncurrent marketable securities totaling $10.1
million and $17.2 million as of March 31, 2011 and December 31, 2010, respectively, consisting of
investments in corporate, municipal, government, and agency bonds, all of which are valued at fair
value using a market approach.
The following tables present a summary of our marketable securities (in thousands):
| Gross | Gross | |||||||||||||||
| Amortized | Unrealized | Unrealized | Estimated | |||||||||||||
| Cost | Gains | (Losses) | Fair Value | |||||||||||||
At March 31, 2011 |
||||||||||||||||
Available-for-sale marketable securities |
||||||||||||||||
Municipal debt securities |
$ | 890 | $ | 2 | $ | — | $ | 892 | ||||||||
U.S. agency debt securities |
8,500 | 9 | — | 8,509 | ||||||||||||
Corporate debt securities |
3,154 | 9 | — | 3,163 | ||||||||||||
U.S. government debt securities |
7,518 | 16 | — | 7,534 | ||||||||||||
Total available-for-sale marketable
securities |
20,062 | 36 | — | 20,098 | ||||||||||||
Held-to-maturity time deposits |
4,813 | — | — | 4,813 | ||||||||||||
Total marketable securities |
$ | 24,875 | $ | 36 | $ | — | $ | 24,911 | ||||||||
7
Table of Contents
WRIGHT MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
| Gross | Gross | |||||||||||||||
| Amortized | Unrealized | Unrealized | Estimated | |||||||||||||
| Cost | Gains | (Losses) | Fair Value | |||||||||||||
At December 31, 2010 |
||||||||||||||||
Available-for-sale marketable securities |
||||||||||||||||
Municipal debt securities |
$ | 897 | $ | — | $ | — | $ | 897 | ||||||||
U.S. agency debt securities |
14,501 | 11 | (1 | ) | 14,511 | |||||||||||
Certificates of deposits |
38 | — | — | 38 | ||||||||||||
Corporate debt securities |
3,176 | 7 | — | 3,183 | ||||||||||||
U.S. government debt securities |
13,027 | 18 | — | 13,045 | ||||||||||||
Total available-for-sale marketable
securities |
31,639 | 36 | (1 | ) | 31,674 | |||||||||||
Held-to-maturity time deposits |
4,671 | — | — | 4,671 | ||||||||||||
Total marketable securities |
$ | 36,310 | $ | 36 | $ | (1 | ) | $ | 36,345 | |||||||
The maturities of available-for-sale and held-to-maturity debt securities at March 31, 2011 are as
follows:
| Available-for-Sale | Held-to-maturity | |||||||||||||||
| Cost Basis | Fair Value | Cost Basis | Fair Value | |||||||||||||
Due in one year or less |
$ | 10,004 | $ | 10,027 | $ | 4,813 | $ | 4,813 | ||||||||
Due after one year
through two years |
7,058 | 7,069 | — | — | ||||||||||||
Due after two years |
3,000 | 3,002 | — | — | ||||||||||||
| $ | 20,062 | $ | 20,098 | $ | 4,813 | $ | 4,813 | |||||||||
4. Property, Plant and Equipment, Net
Property, plant and equipment consist of the following (in thousands):
| March 31, | December 31, | |||||||
| 2011 | 2010 | |||||||
Property, plant and equipment, at cost |
$ | 335,510 | $ | 323,146 | ||||
Less: Accumulated depreciation |
(174,080 | ) | (164,899 | ) | ||||
| $ | 161,430 | $ | 158,247 | |||||
5. Long-Term Debt and Capital Lease Obligations
Long-term debt and capital lease obligations consist of the following (in thousands):
| March 31, | December 31, | |||||||
| 2011 | 2010 | |||||||
Capital lease obligations |
$ | 2,775 | $ | 2,799 | ||||
Term loan |
150,000 | — | ||||||
Convertible senior notes |
29,111 | 200,000 | ||||||
| 181,886 | 202,799 | |||||||
Less: current portion |
(8,617 | ) | (1,033 | ) | ||||
| $ | 173,269 | $ | 201,766 | |||||
In November 2007, we issued $200 million of 2.625% Convertible Senior Notes due 2014 (“Notes”)
maturing on December 1, 2014. The Notes pay interest semiannually at an annual rate of 2.625% and
are convertible into shares of our common stock at an initial conversion rate of 30.6279 shares per
$1,000 principal amount of the Notes subject to adjustment upon the occurrence of specified events,
which represents an initial conversion price of $32.65 per share. The holder of the Notes may
convert at any time on or prior to the close of business on the business day immediately preceding
the maturity date of Notes. Beginning on December 6, 2011, we may redeem the notes, in whole or in
part, at a redemption price equal to 100% of the principal amount of the notes, plus accrued and
unpaid interest, if the closing price of our common stock has exceeded 140% of the conversion price
for at least 20 days during any consecutive 30-day trading period. Additionally, if we experience a
fundamental change event, as defined in the indenture governing the Notes (“Indenture”), the
holders may require us to purchase for cash all or a portion of the notes, for 100% of the
principal amount of the notes, plus accrued and unpaid interest. If upon a fundamental
8
Table of Contents
WRIGHT MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
change event, a holder elects to convert its Notes, we may, under certain circumstances, increase
the conversion rate for the Notes surrendered. The Notes are unsecured obligations and are
effectively subordinated to (i) all of our existing and future secured debt, including our
obligations under our credit agreement, to the extent of the value of the assets securing such
debt, and (ii) because the Notes are not guaranteed by any of our subsidiaries, to all liabilities
of our subsidiaries.
On February 10, 2011, we announced the commencement of a tender offer to purchase for cash any and
all of our outstanding Notes. Upon expiration on March 11, 2011, we purchased $170.9 million
aggregate principal amount of the Notes. As a result of this transaction, we recognized
approximately $4.1 million for the write off of pro-rata unamortized deferred financing fees and
for bank and legal fees associated with the purchase. As of March 31, 2011, $29.1 million aggregate
principal amount of the Notes remain outstanding.
On February 10, 2011, we entered into an amended and restated revolving credit agreement (“Senior
Credit Facility”). This Senior Credit Facility has revolver availability of $200 million and
availability in a delayed draw term loan of up to $150 million. The total availability can be
increased by up to an additional $100 million at our request and subject to the agreement of the
lenders. Borrowings under the Senior Credit Facility will bear interest at the sum of a base rate
or a Eurodollar rate plus an applicable margin that ranges from 0.0% to 2.75%, depending on the
type of loan and our consolidated leverage ratio. The term of the Senior Credit Facility extends
through February 10, 2016. As a result of this transaction, we incurred deferred financing charges
of approximately $2.9 million, which will be amortized over the term of the Senior Credit Facility.
In March 2011, to fund the purchase of the Convertible Notes, we borrowed $150 million under the
delayed draw term loan (“Term Loan”) facility available under our Senior Credit Facility. The Term
Loan will bear interest at a one month LIBOR rate, plus a margin based on Wright Medical’s
consolidated leverage ratio as defined in the Senior Credit Facility. As of March 31, 2011, the one
month LIBOR was 0.25% and the applicable margin was 1.75%. Quarterly repayments of the original
principal amount of the Term Loan are required under the Senior Credit Facility, with the remaining
principal amount due on February 10, 2016.
In March 2011, we entered into an interest rate swap agreement which we designated as cash flow
hedge of the underlying variable rate obligation on our Term Loan. We did not have any interest
rate swap agreements outstanding as of December 31, 2010. See Note 6 for additional information
regarding the interest rate swap agreement.
6. Derivative Instruments and Hedging Activities
We account for derivatives in accordance with FASB ASC Topic 815, Derivatives and Hedging, which
establishes accounting and reporting standards requiring that derivative instruments be recorded on
the balance sheet as either an asset or liability measured at fair value. Additionally, changes in
the derivative’s fair value shall be recognized currently in earnings unless specific hedge
accounting criteria are met. If hedge accounting criteria are met for cash flow hedges, the
changes in a derivative’s fair value are recorded in shareholders’ equity as a component of Other
comprehensive income, net of tax. These deferred gains and losses are recognized in income in the
period in which the hedge item and hedging instrument affect earnings.
Interest Rate Hedging
On March 14, 2011, we entered into an interest rate swap intended to hedge our variable interest
rate obligations with respect to a portion of the our Senior Credit Facility discussed in Note 5.
This interest rate swap is a contract to exchange fixed rate payments for floating rate payments
over the life of the agreement without the exchange of the underlying notional amount. The
notional amount of the interest rate swap is used to measure interest to be paid or received and
does not represent the amount of exposure to credit loss.
As of March 31, 2011, we had a $150 million loan outstanding under our Senior Credit Facility and
one interest rate swap with a notional amount of $50 million. Under the terms of the interest rate
swap agreement, we receive interest on the $50 million notional amount based on one-month LIBOR and
we pay a fixed rate of 1.74%. This swap effectively converted $50 million of our variable-rate
borrowings to fixed-rate borrowings beginning on March 31, 2011 and through February 27, 2015. The
fair value of the interest rate swap as of March 31, 2011 was $243,000 and is classified as Other
assets in our condensed consolidated balance sheet.
9
Table of Contents
WRIGHT MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
In accordance with ASC Topic 815, we designated the above interest rate swap as a cash flow hedge
and formally documented the relationship between the interest rate swap and the fixed rate
borrowing, as well as its risk management objective and strategy for undertaking the hedge
transaction. This process included linking the derivative to the specific liability or asset on the balance sheet. We assessed whether the
derivative used in the hedging transaction was highly effective in offsetting changes in the cash
flows of the hedged item at inception and will test both
retrospectively and prospectively on an
ongoing basis. The effective portion of unrealized gains (losses) on the derivative instrument used
in the hedging transaction will be deferred as a component of Accumulated other comprehensive
income (AOCI) and will be recognized in earnings at the time the hedged item affects earnings. Any
ineffective portion of the change in fair value will be immediately recognized in earnings. At
March 31, 2011, because there was no ineffective portion of the interest rate swap, the total fair
value of the asset was recorded to AOCI.
Counterparty Credit Risk
We manage our concentration of counterparty credit risk on its derivative instruments by limiting
acceptable counterparties to major financial institutions with investment grade credit ratings, and
by actively monitoring their credit ratings on an on-going basis. Therefore, we consider the credit
risk of the counterparties to be low.
The following table summarizes the fair value and the presentation in the condensed consolidated balance
sheet as of March 31, 2011:
| Location on | ||||||||||||
| condensed | Amount of gain | |||||||||||
| consolidated | Fair value as of | recognized in AOCI | ||||||||||
| balance sheet | March 31, 2011 | (Effective Portion) | ||||||||||
Interest rate swap |
Other assets | $ | 243,000 | $ | 243,000 | |||||||
Derivatives not Designated as Hedging Instruments
We employ a derivative program using 30-day foreign currency forward contracts to mitigate the risk
of currency fluctuations on our intercompany receivable and payable balances that are denominated
in foreign currencies. These forward contracts are expected to offset the transactional gains and
losses on the related intercompany balances. These forward contracts are not designated as hedging
instruments under FASB ASC Topic 815. Accordingly, the changes in the fair value and the settlement
of the contracts are recognized in the period incurred in the accompanying condensed consolidated statements
of operations. At March 31, 2011, we had no foreign currency contracts outstanding.
7. Goodwill and Intangible Assets
Changes in the carrying amount of goodwill occurring during the three months ended March 31, 2011,
are as follows (in thousands):
Goodwill at December 31, 2010 |
$ | 54,172 | ||
Foreign currency translation |
475 | |||
Goodwill at March 31, 2011 |
$ | 54,647 | ||
10
Table of Contents
WRIGHT MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
The components of our identifiable intangible assets are as follows (in thousands):
| March 31, 2011 | December 31, 2010 | |||||||||||||||
| Accumulated | Accumulated | |||||||||||||||
| Cost | Amortization | Cost | Amortization | |||||||||||||
Distribution channels |
$ | 21,970 | $ | 21,820 | $ | 20,719 | $ | 20,563 | ||||||||
Completed technology |
10,508 | 4,023 | 12,627 | 6,162 | ||||||||||||
Licenses |
5,610 | 2,143 | 5,613 | 2,040 | ||||||||||||
Customer relationships |
3,888 | 1,184 | 3,888 | 1,087 | ||||||||||||
Trademarks |
2,706 | 679 | 2,706 | 633 | ||||||||||||
Other |
2,596 | 1,616 | 2,859 | 1,426 | ||||||||||||
| 47,278 | $ | 31,465 | 48,412 | $ | 31,911 | |||||||||||
Less: Accumulated amortization |
(31,465 | ) | (31,911 | ) | ||||||||||||
Intangible assets, net |
$ | 15,813 | $ | 16,501 | ||||||||||||
Based on the intangible assets held at March 31, 2011, we expect to amortize approximately
$2.5 million for the full year of 2011, $2.3 million in 2012, $2.0 million in 2013, $1.8 million in
2014, and $1.7 million in 2015.
8. Stock-Based Compensation
Amounts recognized within the condensed consolidated financial statements are as follows (in
thousands):
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2011 | 2010 | |||||||
Total cost of share-based payment plans |
$ | 2,826 | $ | 2,924 | ||||
Amounts capitalized as inventory and intangible
assets |
(316 | ) | (262 | ) | ||||
Amortization of capitalized amounts |
350 | 343 | ||||||
Charged against income before income taxes |
2,860 | 3,005 | ||||||
Amount of related income tax benefit |
(847 | ) | (836 | ) | ||||
Impact to net income (loss) |
$ | 2,013 | $ | 2,169 | ||||
Impact to basic earnings per share |
$ | 0.05 | $ | 0.06 | ||||
Impact to diluted earnings per share |
$ | 0.05 | $ | 0.06 | ||||
In the three-month period ended March 31, 2011, we granted approximately 16,000 stock options,
11,000 non-vested shares of common stock, and 4,500 restricted stock units at weighted-average fair
values of $6.44, $15.42 and $16.51, respectively, which will be recognized on a straight line basis
over the requisite service period of four years. As of March 31, 2011, we had approximately 3.7
million stock options (of which approximately 2.9 million were exercisable), 1.1 million non-vested
shares of common stock, 13,000 stock-settled phantom stock units, and 117,000 restricted stock
units outstanding.
As of March 31, 2011, we had $18 million of total unrecognized compensation cost related to
unvested stock-based compensation arrangements granted to employees, which is expected to be
recognized over a weighted-average period of 2.3 years.
9. Earnings Per Share
FASB ASC Topic 260, Earnings Per Share, requires the presentation of basic and diluted earnings per
share. Basic earnings per share is calculated based on the weighted-average number of shares of
common stock outstanding during the period. Diluted earnings per share is calculated to include any
dilutive effect of our common stock equivalents. Our common stock equivalents consist of stock
options, non-vested shares of common stock, stock-settled phantom stock units, restricted stock
units, and convertible debt. The dilutive effect of the stock options, non-vested shares of common
stock, stock-settled phantom stock units, and restricted stock units is calculated using the
treasury-stock method. The
11
Table of Contents
WRIGHT MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
dilutive effect of convertible debt is calculated by applying the “if-converted” method. This assumes an add-back of interest, net of income taxes, to net income as
if the securities were converted at the beginning of the period.
The weighted-average number of shares outstanding for basic and diluted earnings per share is as
follows (in thousands):
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2011 | 2010 | |||||||
Weighted-average number of shares outstanding,
basic |
38,033 | 37,540 | ||||||
Common stock equivalents |
294 | — | ||||||
Weighted-average number of shares outstanding,
diluted |
38,327 | 37,540 | ||||||
For the three-month period ending March 31, 2010, 283,000 common stock equivalents have been
excluded from the computation of diluted net loss per share, because their effect is anti-dilutive
as a result of our net loss. Additionally, the following potential common shares were excluded from
common stock equivalents as their effect would have been anti-dilutive (in thousands):
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2011 | 2010 | |||||||
Stock options |
3,727 | 3,564 | ||||||
Non-vested shares, restricted stock units, and
stock-settled phantom stock units |
103 | 290 | ||||||
Convertible debt |
4,962 | 6,126 | ||||||
10. Other Comprehensive Income
The difference between our net income (loss) and our comprehensive income (loss) is attributable to
foreign currency translation, unrealized gains and losses (net of taxes) on our derivative
instrument, unrealized gains and losses on our available-for-sale marketable securities, and
adjustments related to our minimum pension liability in Japan. The following table provides a
reconciliation of net income (loss) to comprehensive income (loss) (in thousands):
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2011 | 2010 | |||||||
Net income (loss) |
$ | 3,592 | $ | (525 | ) | |||
Changes in foreign currency translation |
2,523 | (2,918 | ) | |||||
Unrealized gain on derivative instrument, net of
taxes of $95 |
148 | — | ||||||
Unrealized (loss) gain on marketable securities |
(5 | ) | 46 | |||||
Minimum pension liability adjustment |
5 | 4 | ||||||
Comprehensive income (loss) |
$ | 6,263 | $ | (3,393 | ) | |||
11. Commitments and Contingencies
In December 2007, we received a subpoena from the United States Department of Justice (DOJ) through
the United States Attorney’s Office for the District of New Jersey (USAO) requesting documents for
the period January 1998 through the present related to any consulting and professional service
agreements with orthopaedic surgeons in connection with hip or knee joint replacement procedures or
products. This subpoena was served shortly after
12
Table of Contents
WRIGHT MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
several of our knee and hip competitors agreed to resolutions with the DOJ after being subjects of investigations involving the same subject matter.
On September 29, 2010, our wholly-owned subsidiary, Wright Medical Technology, Inc. (WMT) entered
into a 12-month Deferred Prosecution Agreement (DPA) with the USAO and a Civil Settlement Agreement
(CSA) with the United States. Under the DPA, the USAO filed a criminal complaint in the United
States District Court for the District of New Jersey charging us with conspiracy to commit
violations of the Anti-Kickback Statute (42 U.S.C. § 1320a-7b) during the years 2002 through 2007.
The USAO has the discretion to extend the term of the DPA by up to six months. The court deferred prosecution of the criminal complaint during the term of the DPA. If
we comply with the provisions of the DPA, the USAO will seek dismissal of the criminal complaint.
Pursuant to the CSA, WMT settled civil and administrative claims relating to the matter for a
payment of $7.9 million without any admission by WMT. In conjunction with the CSA, WMT also entered
into a five year Corporate Integrity Agreement (CIA) with the Office of the Inspector General of
the United States Department of Health and Human Services. Pursuant to the DPA, an independent
monitor will review and evaluate WMT’s compliance with its
obligations under the DPA. The DPA and CIA were filed as
Exhibits 10.3 and 10.2, respectively, to the Company's current report
on Form 8-K filed on September 30, 2010. The DPA has also been posted
to the Company's website.
As a result of the work of the independent monitor and WMT’s compliance program, the Board of
Directors became aware of facts indicative of possible compliance issues. At the direction of the
Nominating, Compliance and Governance Committee of the Board of Directors of WMT’s parent, Wright
Medical Group, Inc. (“WMGI”), WMGI and WMT have conducted an internal investigation with the
assistance of outside counsel. The Board of Directors of WMGI received a report from outside
counsel. On May 4, 2011, pursuant to Paragraph 20 of the DPA, WMT provided written notice to the
independent monitor and the USAO of “credible evidence of serious wrongdoing.” The same notice was
also provided to OIG. The Board of WMGI also took a number of measures to enhance WMT’s compliance
environment. WMT and the independent monitor continue their investigative activities pursuant to
the DPA, and communications between WMT, the independent monitor, the USAO and OIG are ongoing.
On
May 5, 2011, we received a letter from the United States
Attorney’s Office for the District of New Jersey (USAO) pursuant
to Paragraph 50 of the Deferred Prosecution Agreement (DPA) stating
that the USAO believes that WMT has knowingly and willfully breached
material provisions of the DPA. As required by the terms of the DPA, the letter provides WMT an
opportunity to make a presentation within three weeks of receipt of the letter. WMT intends to
make a presentation in response to the letter. WMT’s presentation could address whether any breach
has occurred, whether any breach was knowing or willful, materiality, and whether any breach has
been cured. Pursuant to the DPA, we expect that the USAO will not take any further action until
consideration of WMT’s presentation.
The DPA and CIA impose certain obligations on WMT to maintain compliance with U.S. healthcare
regulatory laws. Our failure to do so could expose us to significant liability including, but not
limited to, extension of the term of the DPA by up to six months, exclusion from federal healthcare
program participation, including Medicaid and Medicare, which would
have a material adverse effect on our financial condition, results of
operations and cash flows, potential prosecution, including under the
previously-filed criminal complaint, civil and criminal fines or penalties, and additional
litigation cost and expense. A breach of the DPA or the CIA could result in an event of default
under the Senior Credit Facility, which in turn could result in an event of default under the
Indenture. If not extended, our obligations under the DPA expire as of September 29, 2011, while
our obligations under the CIA expire as of September 29, 2015.
An estimate of the amount of any possible contingency cannot be made
at this time.
In addition to the USAO and OIG, other governmental agencies, including state authorities, could
conduct investigations or institute proceedings that are not precluded by the terms of the
settlements reflected by the DPA and the CIA. In addition, the settlement with the USAO and OIG
could increase our exposure to lawsuits by potential whistleblowers under the federal false claims
acts, based on new theories or allegations arising from the allegations made by the USAO. The costs
of defending or resolving any such investigations or proceedings could have a material adverse
effect on our financial condition, results of operations and cash flows.
As of March 31, 2011, the trade receivable balance due from our stocking distributor in Turkey was
$8.6 million, of which a significant portion is past due. We have a reserve of $5.6 million against
this balance as of March 31, 2011. It is possible that the future realization of this accounts
receivable balance could be more or less than the remaining unreserved balance of $3.0 million.
13
Table of Contents
WRIGHT MEDICAL GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(UNAUDITED)
In addition to the stocking distributor in Turkey, our next ten largest international stocking
distributors have net trade receivable balances totaling approximately $17 million as of March 31,
2011. However, it is at least reasonably possible that changes in global economic conditions and/or
local operating and economic conditions in the regions these distributors operate, or other
factors, could affect the future realization of these accounts receivable balances.
In addition to those noted above, we are subject to various other legal proceedings, product
liability claims, and other matters which arise in the ordinary course of business. In the opinion
of management, the amount of liability, if any, with respect to these matters, will not materially
affect our consolidated results of operations or financial position.
14
Table of Contents
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
General
The following management’s discussion and analysis of financial condition and results of operations
describes the principal factors affecting the results of our operations, financial condition, and
changes in financial condition for the three month period ended March 31, 2011. This discussion
should be read in conjunction with the accompanying unaudited financial statements, our Annual
Report on Form 10-K for the year ended December 31, 2010, which includes additional information
about our critical accounting policies and practices and risk
factors, and Note 11 of Part I of
this report.
Executive Overview
Company Description. We are a global orthopaedic medical device company specializing in the design,
manufacture, and marketing of devices and biologic products for extremity, hip, and knee repair and
reconstruction. Extremity hardware includes implants and other devices to replace or reconstruct
injured or diseased joints and bones of the foot, ankle, hand, wrist, elbow, and shoulder, which we
generally refer to as either foot and ankle or upper extremity products. We are a leading provider
of surgical solutions for the foot and ankle market. Reconstructive devices are used to replace or
repair knee, hip, and other joints and bones that have deteriorated or been damaged through disease
or injury. Biologics are used to repair or replace damaged or diseased bone, to stimulate bone
growth and to provide other biological solutions for surgeons and their patients. Within these
markets, we focus on the higher-growth sectors of the orthopaedic industry, such as the foot and
ankle market, as well as on the integration of our biologic products into reconstructive procedures
and other orthopaedic applications. Our extensive foot and ankle product portfolio, our over 180
specialized foot and ankle sales representatives, and our increasing level of training of foot and
ankle surgeons has resulted in our being a recognized leader in the foot and ankle market. We have
been in business for over 60 years and have built a well-known and respected brand name.
Principal Products. We primarily sell devices and biologic products for extremity, hip, and knee
repair and reconstruction. We specialize in extremity and biologic products used by extremity
focused surgeon specialists for the reconstruction, trauma, and arthroscopy markets. Our biologics
sales encompass a broad portfolio of products designed to stimulate and augment the natural
regenerative capabilities of the human body. We also sell orthopaedic products not considered to be
part of our knee, hip, extremity, or biologic product lines.
Significant Quarterly Business Developments. Net sales increased 3% in the first quarter of 2011 to
$135.4 million, compared to net sales of $131.2 million in the first quarter of 2010. In the first
quarter of 2011, we recorded net income of $3.6 million, compared to a net loss of $525,000 for the
first quarter of 2010, primarily as a result of decreased expenses relating to ongoing governmental
inquiries, and improved gross margins.
Our first quarter domestic sales remained relatively flat in 2011; however, we achieved 12% growth
within our extremity line. Our domestic extremities growth is primarily attributable to higher
sales volume of our foot and ankle products, in particular our INBONE™ products, our
ORTHOLOC™ Polyaxial Locked Plating System, and our VALOR® Hindfoot Fusion
Nail System which was launched in June 2010. Domestic sales of our biologic products decreased by
2% in the first quarter of 2011 as compared to the same period in 2010. Our domestic knee sales
were flat and our domestic hip sales declined 15%, in the first quarter of 2011 as compared to the
same period in 2010 due to lower levels of unit sales volume as well as lower pricing, both of
which are impacted by market conditions throughout the industry.
Our international sales increased 7% to $57.4 million in the first quarter of 2011, compared to
$53.5 million in the first quarter of 2010. This increase in sales in the first quarter of 2011
compared to 2010 is primarily the result of increased sales in Japan, Latin America and Australia
and favorable currency exchange rates.
In January 2011, we announced the extension of our supply agreement with LifeCell Corporation, a
business unit of Kinetic Concepts, Inc. (KCI) for the supply of GRAFTJACKET® Regenerative Tissue
Matrix through December 2018 for orthopaedic markets. In addition, we entered into an agreement
with KCI to license our GRAFTJACKET® brand to KCI for exclusive use in wound markets for an $8.5
million payment, of which $5.5 million was received during the three months ended March 31, 2011,
as well as payments based on future sales of licensed products. The license will be amortized over
the life of the license agreement and was not material for the quarter ended March 31, 2011. The
license agreement is expected to have a negative impact on our global sales
15
Table of Contents
growth rate of
approximately
1% to 2% in 2011 and our U.S. biologics sales growth rate of 8% to 15%. However, we expect minimal
impact on our earnings results for 2011.
In February 2011, we announced that we had commenced a tender offer for any and all of our
outstanding Notes. Upon expiration of the tender offer, we used the proceeds from a $150 million
borrowing under the Term Loan facility available under our Senior Credit Facility and cash on hand
to fund the purchase of all $170.9 million of the Notes validly tendered in the tender offer and
not withdrawn prior to the expiration date. Following the closing of the tender offer, $29.1
million aggregate principal amount of the Notes remain outstanding.
Subsequent
Events. On April 5, 2011, we announced that our Board of Directors elected David D.
Stevens, the Chairman of our Board of Directors, as interim President and Chief Executive Officer,
effective April 4, 2011. Mr. Stevens replaced Gary D. Henley, who resigned as President and Chief
Executive Officer, and as a director, effective April 4, 2011. Our Board has initiated a CEO search
and succession process. Mr. Stevens is not a candidate in that process, and our Board expects that
Mr. Stevens will serve on an interim basis only, until that process is complete and a new President
and Chief Executive Officer is selected. Mr. Stevens remains as Chairman of the Board.
Mr. Henley resigned without “Good Reason” under his Employment Agreement dated April 2, 2009, as
amended. In general, we will be obligated to pay Mr. Henley accrued salary earned through April 4,
2011 and the value of accrued but untaken vacation, and to reimburse him for unreimbursed business
expenses, but Mr. Henley is not entitled to severance pay under the Employment Agreement.
In addition, on April 5, 2011, we announced that Frank S. Bono, Senior Vice President and Chief
Technology Officer, was terminated for cause effective April 4, 2011.
On May 4, 2011, we announced that Thomas L. McAllister was appointed interim General Counsel and
Secretary, replacing Raymond C. Kolls, Senior Vice President, General
Counsel and Secretary. The Board has not yet established any
compensation arrangements for Mr. McAllister’s
service as interim General Counsel and Secretary. Mr.
Kolls resigned without “Good Reason.” Because Mr. Kolls’ resignation was without “Good Reason,”
the Company has no obligations to him other than payment of accrued obligations.
In addition, Alicia M. Napoli, Vice President, Clinical & Regulatory Affairs, and Cary P. Hagan,
Sr. Vice President, Commercial Operations — Europe, Middle East and Africa, also resigned from the
Company without “Good Reason” effective May 3 and 4, 2011, respectively.
Opportunities and Challenges. Our results of operations can be substantially affected not only by
global economic conditions, but also by local operating and economic conditions, which can vary
substantially by market. Unfavorable conditions can depress sales in a given market and may result
in actions that adversely affect our margins, constrain our operating flexibility, or result in
charges which are unusual or non-recurring. The current state of the global economy has negatively
impacted industry growth rates in both U.S. and international markets, and we are unable to predict
when these markets will return to historical rates of growth.
In our domestic markets, we expect that an expansion of our focused foot and ankle sales force and
new product offerings will continue to favorably impact our extremities business in the remainder
of 2011. We believe that our hip and knee business will be unfavorably impacted by market
conditions during the remainder of the year.
Significant Industry Factors. Our industry is affected by numerous competitive, regulatory, and
other significant factors. The growth of our business relies on our ability to continue to develop
new products and innovative technologies, obtain regulatory clearance and compliance for our
products, protect the proprietary technology of our products and our manufacturing processes,
manufacture our products cost-effectively, respond to competitive pressures specific to each of our
geographic markets, including our ability to enforce non-compete agreements, and successfully
market and distribute our products in a profitable manner. We, and the entire industry, are subject
to extensive governmental regulation, primarily by the United States Food and Drug Administration
(FDA). Failure to comply with regulatory requirements could have a material adverse effect on our
business. Additionally, our industry is highly competitive and has recently experienced increased
pricing pressures, specifically in the areas of reconstructive joint devices.
In December 2007, we received a subpoena from the United States Department of Justice (DOJ) through
the United States Attorney’s Office for the District of New Jersey (USAO) requesting documents for
the period January 1998 through the present related to any consulting and professional service
agreements with orthopaedic surgeons in connection with hip or knee joint replacement procedures or
products. This subpoena was served shortly after
16
Table of Contents
several of our knee and hip competitors agreed to resolutions with the DOJ after being subjects of
investigations involving the same subject matter.
On September 29, 2010, our wholly-owned subsidiary, Wright Medical Technology, Inc. (WMT) entered
into a 12-month Deferred Prosecution Agreement (DPA) with the USAO and a Civil Settlement Agreement
(CSA) with the United States. Under the DPA, the USAO filed a criminal complaint in the United States District Court for the District
of New Jersey charging us with conspiracy to commit violations of the Anti-Kickback Statute (42 U.S.C § 1320a-7b) during the years 2002 through 2007.
The USAO has the discretion to extend the term of the DPA by up to six months. The court deferred prosecution of the criminal complaint during the term of the DPA.
If we comply with the provisions of the DPA, the USAO will seek dismissal of the criminal complaint.
Pursuant to
the CSA, WMT settled civil and administrative claims relating to the matter for a payment of $7.9
million without any admission by WMT. In conjunction with the CSA, WMT also entered into a five
year Corporate Integrity Agreement (CIA) with the Office of the Inspector General of the United
States Department of Health and Human Services. Pursuant to the DPA, an independent monitor will
review and evaluate WMT’s compliance with its obligations under
the DPA. The DPA and CIA were filed as Exhibits 10.3 and 10.2,
respectively, to the Company’s current report on Form 8-K filed on
September 30, 2010. The DPA has also been posted to the Company’s
website.
As a result of the work of the independent monitor and WMT’s compliance program, the Board of
Directors became aware of facts indicative of possible compliance issues. At the direction of the
Nominating, Compliance and Governance Committee of the Board of Directors of WMT’s parent, Wright
Medical Group, Inc. (“WMGI”), WMGI and WMT have conducted an internal investigation with the
assistance of outside counsel. The Board of Directors of WMGI received a report from outside
counsel. On May 4, 2011, pursuant to Paragraph 20 of the DPA, WMT provided written notice to the
independent monitor and the USAO of “credible evidence of serious wrongdoing.” The same notice was
also provided to OIG. The Board of WMGI also took a number of measures to enhance WMT’s compliance
environment. WMT and the independent monitor continue their investigative activities pursuant to
the DPA, and communications between WMT, the independent monitor, the USAO and OIG are ongoing.
On May 5, 2011, we received a letter from the United States Attorney’s Office for the District of New Jersey (USAO)
pursuant to Paragraph 50 of the Deferred Prosecution Agreement (DPA) stating that the USAO believes that WMT has
knowingly and willfully breached material provisions of the DPA. As required by the terms of the DPA, the letter
provides WMT an opportunity to make a presentation within three weeks of receipt of the letter. WMT intends to make
a presentation in response to the letter. WMT’s presentation could address whether any breach has occurred, whether
any breach was knowing or willful, materiality, and whether any breach has been cured. Pursuant to the DPA, we expect
that the USAO will not take any further action until consideration of WMT’s presentation.
The DPA and CIA impose certain obligations on WMT to maintain compliance with U.S. healthcare
regulatory laws. Our failure to do so could expose us to significant liability including, but not
limited to, extension of the term of the DPA by up to six months, exclusion from federal healthcare
program participation, including Medicaid and Medicare, which would
have a material adverse effect on our financial condition, results of
operations and cash flows, potential prosecution, including under the
previously-filed criminal complaint, civil and criminal fines or penalties, and additional
litigation cost and expense. A breach of the DPA or the CIA could result in an event of default
under the Senior Credit Facility, which in turn could result in an event of default under the
Indenture. If not extended, our obligations under the DPA expire as of September 29, 2011, while
our obligations under the CIA expire as of September 29, 2015. An estimate of the amount of any possible contingency cannot be made at this time.
In addition to the USAO and OIG, other governmental agencies, including state authorities, could
conduct investigations or institute proceedings that are not precluded by the terms of the
settlements reflected by the DPA and the CIA. In addition, the settlement with the USAO and OIG
could increase our exposure to lawsuits by potential whistleblowers under the federal false claims
acts, based on new theories or allegations arising from the allegations made by the USAO. The costs
of defending or resolving any such investigations or proceedings could have a material adverse
effect on our financial condition, results of operations and cash flows.
A detailed discussion of these risks and other factors is provided in Item 1A of our Annual Report
on Form 10-K for the year ended December 31, 2010 and elsewhere in this report.
17
Table of Contents
Results of Operations
Comparison of three months ended March 31, 2011 to three months ended March 31, 2010
The following table sets forth, for the periods indicated, our results of operations expressed as
dollar amounts (in thousands) and as percentages of net sales:
| Three Months Ended March 31, | ||||||||||||||||
| 2011 | 2010 | |||||||||||||||
| Amount | % of Sales | Amount | % of Sales | |||||||||||||
Net sales |
$ | 135,386 | 100.0 | % | $ | 131,244 | 100.0 | % | ||||||||
Cost of sales1 |
38,768 | 28.6 | % | 40,141 | 30.6 | % | ||||||||||
Gross profit |
96,618 | 71.4 | % | 91,103 | 69.4 | % | ||||||||||
Operating expenses: |
||||||||||||||||
Selling, general and administrative1 |
74,825 | 55.3 | % | 76,438 | 58.2 | % | ||||||||||
Research and development1 |
9,207 | 6.8 | % | 9,835 | 7.5 | % | ||||||||||
Amortization of intangible assets |
690 | 0.5 | % | 649 | 0.5 | % | ||||||||||
Restructuring charges |
— | 0.0 | % | 544 | 0.4 | % | ||||||||||
Total operating expenses |
84,722 | 62.6 | % | 87,466 | 66.6 | % | ||||||||||
Operating income |
11,896 | 8.8 | % | 3,637 | 2.8 | % | ||||||||||
Interest expense, net |
1,835 | 1.4 | % | 1,508 | 1.1 | % | ||||||||||
Other expense, net |
4,459 | 3.3 | % | 132 | 0.1 | % | ||||||||||
Income before income taxes |
5,602 | 4.1 | % | 1,997 | 1.5 | % | ||||||||||
Provision for income taxes |
2,010 | 1.5 | % | 2,522 | 1.9 | % | ||||||||||
Net income |
$ | 3,592 | 2.7 | % | $ | (525 | ) | (0.4 | %) | |||||||
| 1 | These line items include the following amounts of non-cash, stock-based compensation expense for the periods indicated: |
| Three Months Ended | ||||||||||||||||
| March 31, | ||||||||||||||||
| % of | % of | |||||||||||||||
| 2011 | Sales | 2010 | Sales | |||||||||||||
Cost of sales |
$ | 347 | 0.2 | % | $ | 340 | 0.3 | % | ||||||||
Selling, general and administrative |
2,068 | 1.5 | % | 2,267 | 1.7 | % | ||||||||||
Research and development |
445 | 0.3 | % | 398 | 0.3 | % | ||||||||||
18
Table of Contents
The following table sets forth our net sales by product line for the periods indicated (in
thousands) and the percentage of year-over-year change:
| Three Months Ended March 31, | ||||||||||||
| 2011 | 2010 | % Change | ||||||||||
Hip products |
$ | 45,897 | $ | 46,285 | (0.8 | %) | ||||||
Knee products |
32,833 | 32,418 | 1.3 | % | ||||||||
Extremity products |
34,273 | 30,104 | 13.8 | % | ||||||||
Biologics products |
19,307 | 19,792 | (2.5 | %) | ||||||||
Other |
3,076 | 2,645 | 16.3 | % | ||||||||
Total net sales |
$ | 135,386 | $ | 131,244 | 3.2 | % | ||||||
The following graphs illustrate our product line net sales as a percentage of total net sales
for the three months ended March 31, 2011 and 2010:
Product Line Sales as a Percentage of Total Net Sales
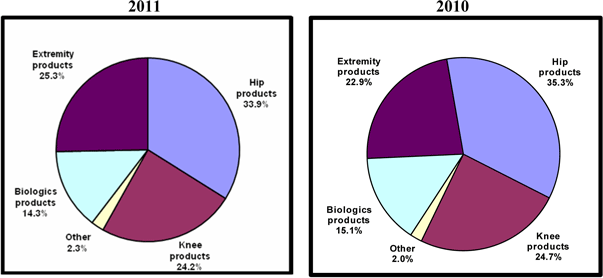
Net Sales. Overall, our net sales increased 3% in the first quarter of 2011 compared to the first
quarter of 2010. We experienced continued growth in our extremity product line, which increased 14%
over prior year, as well as growth of 1% in our knee line, while we experienced declines of 1% and
2% in our hip and biologics product lines, respectively. Geographically, our domestic net sales
totaled $77.9 million in the first quarter of 2011 and $77.7 million in the first quarter of 2010,
representing 58% and 59% of total net sales, respectively, with relatively flat growth in 2011
compared to 2010. Our international net sales totaled $57.4 million in the first quarter of 2011,
compared to $53.5 million in the first quarter of 2010, representing growth of 7%. This increase is
primarily a result of increased sales in Japan, Latin America, and Australia and favorable currency
exchange rates.
Our extremity product line net sales increased to $34.3 million in the first quarter of 2011,
representing growth of 14% over the first quarter of 2010. Domestically, extremity product sales
increased 12% over the first quarter of 2010, due to higher levels of sales of our foot and ankle
products and our upper extremity products. Our international extremity sales increased 21% compared
to the same period in 2010 primarily due to increased sales in Canada, Japan and Germany.
19
Table of Contents
Our knee product net sales increased 1% to $32.8 million in the first quarter of 2011 from $32.4
million during the same period in 2010. Domestically, knee sales were flat from the prior year as
increased unit sales were offset by a decrease in pricing. International knee sales increased due
to higher levels of sales in Australia and Latin America.
Our hip product net sales totaled $45.9 million during the first quarter of 2011, representing a 1%
decrease from the prior year. Our domestic hip sales decreased 15% over prior year due to both
decreased unit volumes and decreased
pricing. Internationally, hip sales increased 9% over prior year primarily due to increased sales
in Japan and Latin America.
Net sales of our biologics products totaled $19.3 million in the first quarter of 2011,
representing a 2% decrease over the first quarter of 2010. In the U.S., our biologics sales
decreased 2% in 2011. Sales increases in our PRO-STIM™ Osteoinductive Bone Graft
Substitute, were offset by continued declines of our GRAFTJACKET® tissue
repair and containment membranes. Our international biologics sales decreased in the first quarter
of 2011, as compared to the same period in 2010, primarily due to decreased sales to certain of our
international stocking distributors.
Cost of Sales. Our cost of sales as a percentage of net sales decreased from 30.6% in the first
quarter of 2010 to 28.6% in the first quarter of 2011, due to favorable manufacturing expenses,
which was partially offset by unfavorable geographic mix, as our more profitable domestic sales
decreased as a percentage of total sales. Our cost of sales included 0.2 and 0.3 percentage points
of non-cash, stock-based compensation expense in 2011 and 2010, respectively. Our cost of sales and
corresponding gross profit percentages can be expected to fluctuate in future periods depending
upon changes in our product sales mix and prices, distribution channels and geographies,
manufacturing yields, period expenses, levels of production volume, cost of raw materials, and
currency exchange rates.
Selling, General and Administrative. Our selling, general and administrative expenses as a
percentage of net sales totaled 55.3% in the first quarter of 2011, a 2.9 percentage point decrease
from 58.2% in the first quarter of 2010. Selling, general and administrative expense for the first
quarter of 2011 included $2.1 million of non-cash, stock based compensation expense (1.5% of net
sales) and $2.2 million of costs associated with our DPA (1.6% of net sales). During the first
quarter of 2010, selling, general and administrative expense included $2.3 million of non-cash,
stock based compensation expense (1.7% of net sales) and $8.1 million of expenses related to U.S.
government inquiries (6.1% of net sales). The decrease in selling, general and administrative
expenses as a percentage of sales during the first quarter of 2011 is primarily the result of lower
levels of expenses associated with U.S. government inquiries.
We anticipate that our selling, general and administrative expenses will increase in absolute
dollars to the extent that additional growth in net sales results in increases in sales commissions
and royalty expense associated with those sales and requires us to expand our infrastructure.
Further, in the near term, we anticipate that these expenses may increase as a percentage of net
sales as we make strategic investments in order to grow our business, as we incur expenses
associated with our compliance with the DPA, and as our spending related to the global compliance
requirements of our industry increases.
Research and Development. Our investment in research and development activities represented
approximately 6.8% of net sales in the first quarter of 2011, as compared to 7.5% of net sales in
the first quarter of 2010. Our research and development expenses include approximately $0.4 million
(0.3% of net sales) of non-cash, stock-based compensation expense in both the first quarter of 2011
and 2010.
We anticipate that our research and development expenditures will remain relatively flat as a
percentage of net sales, but will increase in absolute dollars as we continue to increase our
investment in product development initiatives and clinical studies to support regulatory approvals
and provide expanded proof of the efficacy of our products.
Amortization of Intangible Assets. Charges associated with the amortization of intangible
assets in the first quarter of 2011 were flat compared to the same period in 2010. Based on the
intangible assets held as of March 31, 2011, we expect to recognize amortization expense of
approximately $2.5 million for the full year of 2011, $2.3 million in 2012, $2.0 million in 2013,
$1.8 million in 2014, and $1.7 million in 2015.
Interest Expense, Net. Interest expense, net, consists of interest expense of $2.0 million during
the first quarter of 2011 and $1.6 million during the first quarter of 2010, primarily from
borrowings under our Convertible Senior Notes due 2014, offset by interest income $0.1 million
during the first quarters of 2011 and 2010, generated by our invested cash balances and investments
in marketable securities. The amounts of interest income we realize in 2011 and beyond are subject
to variability, dependent upon both the rate of invested returns we realize and the amount of
20
Table of Contents
excess cash balances on hand. Additionally, the amount of interest expense we incur is subject to
variability dependent upon the change in LIBOR rates and our consolidated leverage ratio.
Other Income, Net. Our Other income, net increased from $0.1 million in the first three months of
2010 to $4.5 million in the first three months of 2011. The increase is attributable to the
recognition of approximately $4.1
million of expenses for the write off of pro-rata unamortized deferred financing fees and for bank
and legal fees associated with the purchase of all $170.9 million of the Notes validly tendered in
the tender offer.
Provision for Income Taxes. We recorded tax provisions of $2.0 million and $2.5 million in the
first quarter of 2011 and 2010, respectively. During the first quarter of 2011, our effective tax
rate was approximately 35.9% as compared to 126.3% in the first quarter of 2010. This decrease is
primarily attributable to an unfavorable 85.5 percentage point impact in the first quarter of 2010
due to the discrete tax effect of the $8.0 million charge to record management’s estimate of the
monetary payment for the potential settlement of the ongoing DOJ investigation.
Seasonal Nature of Business
We traditionally experience lower sales volumes in the third quarter than throughout the rest of
the year as many of our products are used in elective procedures, which generally decline during
the summer months, typically resulting in selling, general and administrative expenses and research
and development expenses as a percentage of sales that are higher during this period than
throughout the rest of the year. In addition, our first quarter selling, general and administrative
expenses include additional expenses that we incur in connection with the annual meeting held by
the American Academy of Orthopaedic Surgeons. This meeting, which is the largest orthopaedic
meeting in the world, features the presentation of scientific papers and instructional courses for
orthopaedic surgeons. During this three-day event, we display our most recent and innovative
products to these surgeons.
Liquidity and Capital Resources
The following table sets forth, for the periods indicated, certain liquidity measures (in
thousands):
| As of | ||||||||
| March 31, | As of | |||||||
| 2011 | December 31, 2010 | |||||||
Cash and cash equivalents |
$ | 154,839 | $ | 153,261 | ||||
Short-term marketable securities |
14,840 | 19,152 | ||||||
Long-term marketable securities |
10,071 | 17,193 | ||||||
Working capital |
416,691 | 426,286 | ||||||
Line of credit availability |
200,000 | 100,000 | ||||||
During 2010, we began investing in long-term marketable securities with maturity dates ranging from
17 to 36 months, consisting of investments in government, agency, and corporate bonds. As of March
31, 2011, the weighted average maturity for these investments is 20 months.
Operating Activities. Cash provided by operating activities was $18.1 million for the first three
months of 2011 as compared to $28.7 million for the first three months of 2010. The decrease in
operating cash flow is primarily attributable to increased spending in working capital, primarily
for inventory due to new product launches.
Investing Activities. Our capital expenditures totaled approximately $10.1 million and $11.6
million in the first three months of 2011 and 2010, respectively. Our industry is capital
intensive, particularly as it relates to surgical instrumentation. Historically, our capital
expenditures have consisted of purchased surgical instruments, manufacturing equipment, research
and testing equipment, computer systems, and office furniture and equipment. We expect to incur
capital expenditures of approximately $50 million in 2011.
Additionally, in 2011, we generated proceeds of approximately $5.5 million related to the sale of a
license to KCI for the exclusive use of our GRAFTJACKET® brand in wound markets.
Financing Activities. During the first three months of 2011, cash used in financing activities
totaled $24.0 million compared to the first three months of 2010 when cash provided by financing
activities totaled $249,000. The change is primarily attributable to the payments to fund the
purchase of all $170.9 million of the Notes validly tendered in the tender offer being offset by
the cash proceeds from a $150 million borrowing under the Term Loan.
21
Table of Contents
During 2007, we issued $200 million of Notes, which generated net proceeds of $193.5 million. The
notes pay interest semiannually at an annual rate of 2.625%. The notes are convertible into shares
of our common stock at an initial conversion rate of 30.6279 shares per $1,000 principal amount of
the notes, which represents a conversion price of $32.65 per share.
On February 10, 2011, we announced the commencement of a tender offer to purchase for cash any and
all of our outstanding Notes. Upon expiration on March 11, 2011, we purchased $170.9 million
aggregate principal amount of the Notes. We will make scheduled interest payments in 2011 related
to the remaining convertible notes totaling $765,000.
On February 10, 2011, we entered into the Senior Credit Facility. This Senior Credit Facility has
revolver availability of $200 million and availability in a delayed draw term loan of up to $150
million. The total availability can be increased by up to an additional $100 million at our request
and subject to the agreement of the lenders. Borrowings under the Senior Credit Facility will bear
interest at the sum of a base rate or a Eurodollar rate plus an applicable margin that ranges from
0.0% to 2.75%, depending on the type of loan and our consolidated leverage ratio. The term of the
Senior Credit Facility extends through February 10, 2016.
In March 2011, to fund the purchase of the Notes, we borrowed $150 million under the Term Loan
available under our Senior Credit Facility. The Term Loan will bear interest at a one month LIBOR
rate plus a margin based on Wright Medical’s consolidated leverage ratio as defined in the Senior
Credit Facility. As of March 31, 2011, the one month LIBOR was 0.25% and the applicable margin was
1.75%. Quarterly repayments of the original principal amount of the Term Loan are required under
the Senior Credit Facility, with the remaining principal amount due on February 10, 2016.
The payment of our indebtedness under the Senior Credit Facility is secured by pledges of 100% of
the capital stock of our U.S. subsidiaries directly owned by a Loan Party and 65% of the capital
stock of our material foreign subsidiaries, and is guaranteed by our material domestic
subsidiaries. The Senior Credit Facility contains customary financial and non-financial covenants.
Upon the occurrence of an event of default, the lenders may declare that all principal, interest
and other amounts owed are immediately due and payable and may exercise any other available right
or remedy. The events of default include, but are not limited to, non-payment of amounts owed,
failure to perform covenants, breach of representations and warranties, institution of insolvency
proceedings, entry of certain judgments, and occurrence of a change in control.
Other Liquidity Information
We have funded our cash needs since 2000 through various equity and debt issuances and through cash
flow from operations. In 2007, we issued $200 million of Notes, which generated net proceeds
totaling $193.5 million. In 2011, we purchased $170.9 million aggregate principal amount of the
Notes outstanding, which we funded through a delayed draw term loan of $150 million under our
Senior Credit Facility and cash on hand.
Although it is difficult for us to predict our future liquidity requirements, we believe that
our current cash and cash equivalents balance of $154.8 million, our marketable securities balances
totaling $24.9 million, our existing available credit line of $200 million, and our expected cash
flow from our 2011 operations will be sufficient for the foreseeable future to fund our working
capital requirements and operations, permit anticipated capital expenditures in 2011 of
approximately $50 million, and meet our contractual cash obligations in 2011.
Critical Accounting Policies and Estimates
Information on judgments related to our most critical accounting policies and estimates is
discussed in Item 7 of our Annual Report on Form 10-K for the year ended December 31, 2010. Certain
of our more critical accounting estimates require the application of significant judgment by
management in selecting the appropriate assumptions in determining the estimate. By their nature,
these judgments are subject to an inherent degree of uncertainty. We develop these judgments based
on our historical experience, terms of existing contracts, our observance of trends in the
industry, information provided by our customers, and information available from other outside
sources, as appropriate. Actual results may differ from these judgments under different assumptions
or conditions. Different, reasonable estimates could have been used for the current period.
Additionally, changes in accounting estimates are reasonably likely to occur from period to period.
Both of these factors could have a material impact on the presentation of our financial condition,
changes in financial condition or results of operations. All of our significant accounting policies
are more fully described in Note 2 to our consolidated financial statements set forth in our
22
Table of Contents
Annual
Report on Form 10-K for the year ended December 31, 2010. There have been no significant
modifications to the policies related to our critical accounting estimates since December 31, 2010.
23
Table of Contents
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Interest Rate Risk
Our interest rate risk relates primarily to our U.S. dollar LIBOR-indexed borrowings of $150
million. We have used an interest rate derivative instrument to manage our earnings and cash flow
exposure to changes in interest rates by utilizing an interest rate swap. This interest rate
derivative instrument will fix the interest rate on a portion ($50 million) of our LIBOR-indexed
floating-rate borrowings effective March 31, 2011.
Based on our outstanding borrowings at March 31, 2011, a 0.25% increase in interest rates would
have increased interest expense on the unhedged portion of our debt by $250,000 on an annualized
basis, and a decrease in rates would have an insignificant impact on interest expense due to the
current low LIBOR rates.
Foreign Currency Exchange Rate Risk
Fluctuations in the rate of exchange between the U.S. dollar and foreign currencies could adversely
affect our financial results. Approximately 31% and 29% of our total net sales were denominated in
foreign currencies during the three months ended March 31, 2011 and for the year ended December 31,
2010, respectively, and we expect that foreign currencies will continue to represent a similarly
significant percentage of our net sales in the future. Cost of sales related to these sales are
primarily denominated in U.S. dollars; however, operating costs related to these sales are largely
denominated in the same respective currencies, thereby partially limiting our transaction risk
exposure. For sales not denominated in U.S. dollars, an increase in the rate at which a foreign
currency is exchanged for U.S. dollars will require more of the foreign currency to equal a
specified amount of U.S. dollars than before the rate increase. In such cases, if we price our
products in the foreign currency, we will receive less in U.S. dollars than we did before the rate
increase went into effect. If we price our products in U.S. dollars and our competitors price their
products in local currency, an increase in the relative strength of the U.S. dollar could result in
our prices not being competitive in a market where business is transacted in the local currency.
A substantial majority of our sales denominated in foreign currencies are derived from European
Union countries, which are denominated in the euro; from Japan, which are denominated in the
Japanese yen; from the United Kingdom, which are denominated in the British pound; and from Canada,
which are denominated in the Canadian dollar. Additionally, we have significant intercompany
receivables from our foreign subsidiaries which are denominated in foreign currencies, principally
the euro, the yen, the British pound, and the Canadian dollar. Our principal exchange rate risk,
therefore, exists between the U.S. dollar and the euro, the U.S. dollar and the yen, the U.S.
dollar and the British pound, and the U.S. dollar and the Canadian dollar. Fluctuations from the
beginning to the end of any given reporting period result in the revaluation of our foreign
currency-denominated intercompany receivables and payables, generating currency translation gains
or losses that impact our non-operating income and expense levels in the respective period.
As discussed in Note 2 to our consolidated financial statements set forth in our Annual Report on
Form 10-K for the year ended December 31, 2010, we enter into certain short-term derivative
financial instruments in the form of foreign currency forward contracts. These forward contracts
are designed to mitigate our exposure to currency fluctuations in our intercompany balances
principally denominated in euros, Japanese yen, British pounds, and Canadian dollars. Any change in
the fair value of these forward contracts as a result of a fluctuation in a currency exchange rate
is expected to be offset by a change in the value of the intercompany balance. These contracts are
effectively closed at the end of each reporting period.
24
Table of Contents
ITEM 4. CONTROLS AND PROCEDURES.
Disclosure Controls and Procedures
We have established disclosure controls and procedures, as such term is defined in Rule 13a-15(e)
under the Securities Exchange Act of 1934. Our disclosure controls and procedures are designed to
ensure that material information relating to us, including our consolidated subsidiaries, is made
known to our principal executive officer and principal financial officer by others within our
organization. Under the supervision and with the participation of our management, including our
principal executive officer and principal financial officer, we conducted an evaluation of the
effectiveness of our disclosure controls and procedures as of March 31, 2011 to ensure that the
information required to be disclosed by us in the reports that we file or submit under the
Securities Exchange Act of 1934 is recorded, processed, summarized, and reported within the time
periods specified in the SEC’s rules and forms. Disclosure controls and procedures include,
without limitation, controls and procedures designed to ensure that information required to be
disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934 is
accumulated and communicated to our management, including our principal executive officer and
principal financial officer as appropriate, to allow timely decisions regarding required
disclosure. Based on this evaluation, our principal executive officer and principal financial
officer concluded that our disclosure controls and procedures were effective as of March 31, 2011.
Changes in Internal Control Over Financial Reporting
During the three months March 31, 2011, there were no significant changes in our internal control
over financial reporting that materially affected, or that are reasonably likely to materially
affect, our internal control over financial reporting.
25
Table of Contents
PART II – OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS.
Not applicable.
ITEM 1A. RISK FACTORS.
Please see
discussion in Part I, Note 11, “Commitments and
Contingencies.”
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS.
Not applicable.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES.
Not applicable.
ITEM 4. [Removed and Reserved.]
ITEM 5. OTHER INFORMATION.
Not applicable.
ITEM 6. EXHIBITS.
(a) Exhibits.
The following exhibits are
filed as a part of this
quarterly report on Form
10-Q or are incorporated
herein by
reference:
26
Table of Contents
| Exhibit | ||
| No. | Description | |
3.1
|
Fourth Amended and Restated Certificate of Incorporation of Wright Medical Group, Inc., (1) as amended by Certificate of Amendment of Fourth Amended and Restated Certificate of Incorporation of Wright Medical Group, Inc. (2) | |
3.2
|
Second Amended and Restated By-laws of Wright Medical Group, Inc. (3) | |
4.1
|
Form of Common Stock certificate. (1) | |
4.2
|
Indenture, dated as of November 26, 2007, between Wright Medical Group, Inc. and The Bank of New York, as trustee (including form of 2.625% Convertible Senior Notes due 2014). (4) | |
4.3
|
Underwriting Agreement, dated as of November 19, 2007, among Wright Medical Group, Inc. and J.P. Morgan Securities Inc., Piper Jaffray & Co., and Wachovia Capital Markets, LLC. (4) | |
10.1
|
Credit Agreement dated as of February 10, 2011, among Wright Medical Group, Inc., as the Borrower; the U.S. subsidiaries of the Borrower, as the Guarantors; the Lenders named therein; Bank of America, N.A., as Administrative Agent, Swing Line Lender and L/C Issuer; SunTrust Bank and Wells Fargo Bank, N.A., as Co-Syndication Agents; and US Bank National Association, as Documentation Agent.(19) | |
10.2
|
Fifth Amended and Restated 1999 Equity Incentive Plan (1999 Plan), (5) as amended by First Amendment to 1999 Plan.(6) | |
10.3
|
Amended and Restated 2009 Equity Incentive Plan (2009 Plan) (7) | |
10.4*
|
Form of Executive Stock Option Agreement pursuant to the 2009 Plan.(8) | |
10.5*
|
Form of Non-Employee Director Stock Option Agreement (one year vesting) pursuant to the 2009 Plan. (8) | |
10.6*
|
Form of Non-Employee Director Stock Option Agreement (four year vesting) pursuant to the 2009 Plan. (8) | |
10.7*
|
Form of Executive Restricted Stock Grant Agreement pursuant to the 2009 Plan. (8) | |
10.8*
|
Form of Non-Employee Director Restricted Stock Grant Agreement (one year vesting) pursuant to the 2009 Plan. (8) | |
10.9*
|
Form of Non-Employee Director Restricted Stock Grant Agreement (four year vesting) pursuant to the 2009 Plan. (8) | |
10.10*
|
Form of Executive Stock Option Agreement pursuant to the 1999 Plan. (8) | |
10.11*
|
Form of Non-Employee Director Stock Option Agreement (one year vesting) pursuant to the 1999 Plan. (8) | |
10.12*
|
Form of Non-Employee Director Stock Option Agreement (four year vesting) pursuant to the 1999 Plan. (8) | |
10.13*
|
Form of Executive Restricted Stock Grant Agreement pursuant to the 1999 Plan. (8) | |
10.14*
|
Form of Non-Employee Director Restricted Stock Grant Agreement (four year vesting) pursuant to the 1999 Plan. (9) | |
10.15*
|
Wright Medical Group, Inc. Executive Performance Incentive Plan. (10) | |
10.16*
|
Wright Medical Group, Inc. 2010 Executive Performance Incentive Plan (11) | |
10.17*
|
Form of Indemnification Agreement between Wright Medical Group, Inc. and its directors and executive officers. (12) | |
10.18*
|
Employment Agreement dated as of April 2, 2009, between Wright Medical Technology, Inc. and Gary D. Henley (12) as amended by Employment Contract Amendment dated as of August 2, 2010. (17) | |
10.19*
|
Separation Pay Agreement dated as of April 1, 2009 between Wright Medical Technology, Inc. and Lance A. Berry. (14) | |
10.20*
|
Separation Pay Agreement dated as of April 1, 2009 between Wright Medical Technology, Inc. and William L. Griffin, Jr. (15) | |
10.21*
|
Separation Pay Agreement dated as of April 1, 2009 between Wright Medical Technology, Inc. and Eric A. Stookey.(12) | |
10.22*
|
Separation Pay Agreement dated as of April 1, 2009 between Wright Medical Technology, Inc. and Edward A. Steiger. (15) | |
10.23*
|
Separation Pay Agreement dated as of April 1, 2009 between Wright Medical Technology, Inc. and Frank S. Bono. (13) | |
10.24*
|
Inducement Stock Option Grant Agreement between the Registrant and Raymond C. Kolls dated May 31, 2010 (16) |
27
Table of Contents
| Exhibit | ||
| No. | Description | |
10.25†
|
Amended and Restated Supply and Development Agreement dated January 28, 2011 between Wright Medical Technology, Inc. and LifeCell Corporation.(20) | |
10.26†
|
Trademark License Agreement dated January 28, 2011 between Wright Medical Technology, Inc. and KCI Medical Records. (20) | |
10.27
|
Settlement Agreement dated September 29, 2010, among the United States of America, acting through the United States Department of Justice and on behalf of the Office of Inspector General of the Department of Health and Human Services, and Wright Medical Technology, Inc. (18) | |
10.28
|
Corporate Integrity Agreement dated September 29, 2010, between Wright Medical Technology, Inc. and the Office of Inspector General of the Department of Health and Human Services (18) | |
10.29
|
Deferred Prosecution Agreement dated September 29, 2010, between Wright Medical Technology, Inc. and the United States Attorney’s Office for the District of New Jersey (18) | |
10.30*
|
Employment Agreement dated as of May 3, 2011, between Wright Medical Technology, Inc. and David D. Stevens effective April 4, 2011. (21) | |
11
|
Computation of earnings per share (included in Note 9 of the Notes to Condensed Consolidated Financial Statements in “Financial Statements and Supplementary Data”). | |
31.1
|
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) Under the Securities Exchange Act of 1934. | |
31.2
|
Certification of Chief Financial Officer Pursuant to Rule 13a-14(a) Under the Securities Exchange Act of 1934. | |
32
|
Certification of Chief Executive Officer and Chief Financial Officer Pursuant to Rule 13a-14(b) Under the Securities Exchange Act of 1934 and Section 1350 of Chapter 63 of Title 18 of the United States Code. | |
101
|
The following materials from Wright Medical Group, Inc. Quarterly Report on Form 10-Q for the quarter ended March 31, 2011 formatted in XBRL (Extensible Business Reporting Language): (1) the Condensed Consolidated Balance Sheets, (2) Parenthetical Data to the Condensed Consolidated Balance Sheets, (3) the Condensed Consolidated Statements of Operations, (4) Parenthetical Data to the Condensed Consolidated Statements of Operations, (5) the Condensed Consolidated Statements of Cash Flows and (6) Notes to Condensed Consolidated Financial Statements, tagged as blocks of text. |
| (1) | Incorporated by reference to our Registration Statement on Form S-1 (Registration No. 333-59732), as amended. | |
| (2) | Incorporated by reference to our Registration Statement on Form S-8 filed on May 14, 2004. | |
| (3) | Incorporated by reference to our current report on Form 8-K filed on February 19, 2008. | |
| (4) | Incorporated by reference to our current report on Form 8-K filed on November 26, 2007. | |
| (5) | Incorporated by reference to our definitive Proxy Statement filed on April 14, 2008. | |
| (6) | Incorporated by reference to our quarterly report on Form 10-Q for the quarter ended September 30, 2008. | |
| (7) | Incorporated by reference to our definitive Proxy Statement filed on April 15, 2010. | |
| (8) | Incorporated by reference to our quarterly report on Form 10-Q for the quarter ended June 30, 2009. | |
| (9) | Incorporated by reference to our Registration Statement on Form S-8 filed on June 18, 2008. | |
| (10) | Incorporated by reference to our current report on Form 8-K filed on February 10, 2005. | |
| (11) | Incorporated by reference to our current report on Form 8-K filed on March 25, 2010. | |
| (12) | Incorporated by reference to our current report on Form 8-K filed on April 7, 2009. | |
| (13) | Incorporated by reference to our quarterly report on Form 10-Q for the quarter ended March 31, 2009. | |
| (14) | Incorporated by reference to our current report on Form 8-K filed on November 16, 2009. | |
| (15) | Incorporated by reference to our quarterly report on Form 10-Q for the quarter ended March 31, 2010. |
28
Table of Contents
| (16) | Incorporated by reference to our Registration Statement on Form S-8 filed June 22, 2010. | |
| (17) | Incorporated by reference to our current report on Form 8-K filed August 2, 2010. | |
| (18) | Incorporated by reference to our current report on Form 8-K filed on September 30, 2010. | |
| (19) | Incorporated by reference to our annual report on Form 10-K for the fiscal year ended December 31, 2010. | |
| (20) | Incorporated by reference to our current report on Form 8-K filed on February 3, 2011. | |
| (21) | Incorporated by reference to our current report on Form 8-K filed on May 5, 2011. | |
| * | Denotes management contract or compensatory plan or arrangement. | |
| † | Confidential treatment requested under 17 CFR 24b-2. The confidential portions of this exhibit have been omitted and are marked accordingly. The confidential portions have been filed separately with the Securities and Exchange Commission pursuant to the Confidential Treatment Request. |
29
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly
caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Date: May 11, 2011 | ||||
| WRIGHT MEDICAL GROUP, INC. |
||||
| By: | /s/ David D. Stevens | |||
| David D. Stevens | ||||
| Interim Chief Executive Officer (Principal Executive Officer) |
||||
| By: | /s/ Lance A. Berry | |||
| Lance A. Berry | ||||
| Senior Vice President and Chief Financial Officer (Principal Financial Officer and Chief Accounting Officer) |
||||
30
Table of Contents
EXHIBIT INDEX
| Exhibit | ||
| Number | DESCRIPTION | |
31.1
|
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) Under the Securities Exchange Act of 1934. | |
31.2
|
Certification of Chief Financial Officer Pursuant to Rule 13a-14(a) Under the Securities Exchange Act of 1934. | |
32
|
Certification of Chief Executive Officer and Chief Financial Officer Pursuant to Rule 13a-14(b) Under the Securities Exchange Act of 1934 and Section 1350 of Chapter 63 of Title 18 of the United States Code. | |
101
|
The following materials from Wright Medical Group, Inc. Quarterly Report on Form 10-Q for the quarter ended March 31, 2011 formatted in XBRL (Extensible Business Reporting Language): (1) the Condensed Consolidated Balance Sheets, (2) Parenthetical Data to the Condensed Consolidated Balance Sheets, (3) the Condensed Consolidated Statements of Operations, (4) Parenthetical Data to the Condensed Consolidated Statements of Operations, (5) the Condensed Consolidated Statements of Cash Flows and (6) Notes to Condensed Consolidated Financial Statements, tagged as blocks of text. |
