Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CADENCE PHARMACEUTICALS INC | d8k.htm |
 Improving the lives of hospitalized patients
Cadence Pharmaceuticals, Inc.
Corporate Presentation
May 11, 2011
Exhibit 99.1 |
 2
This presentation includes forward-looking statements, which are based on our
current beliefs and expectations. Such statements include, without
limitation, statements regarding: the anticipated U.S. market opportunity for OFIRMEV; our
projections regarding the number of formulary approvals of OFIRMEV; our belief that
we can rapidly accelerate sales of OFIRMEV; the potential for us to
ultimately acquire Incline Therapeutics, Inc. or other product candidates; and our strategy for
building a long-term hospital pain franchise.
You are cautioned not to place undue reliance on these forward-looking
statements, which speak only as of the date hereof. Our
actual
future
results
may
differ
materially
from
our
current
expectations
due
to
the
risks
and
uncertainties
inherent
in
our
business. These risks include our dependence on the successful commercialization of
OFIRMEV; the potential that delays in achieving formulary acceptance for
OFIRMEV at a substantial number of targeted accounts may decrease the market
potential for OFIRMEV; our ability to generate revenues from OFIRMEV; our ability
to ensure an adequate and continued supply of OFIRMEV; our ability to
successfully enforce our marketing exclusivities and intellectual property rights, and to
defend our patents; the potential product liability exposure associated with
OFIRMEV; the risk that we may not be able to raise
sufficient
capital
when
needed,
or
at
all;
and
other
risks
detailed
under
“Risk
Factors”
and
elsewhere
in
our
Annual
Report on Form 10-K for the period ended December 31, 2010, and our other
filings made with the Securities and Exchange Commission from time to time.
All forward-looking statements are qualified in their entirety by this
cautionary statement, which is made under the safe harbor provisions of
Section 21E of the Private Securities Litigation Reform Act of 1995 and we undertake no obligation to revise or
update this presentation to reflect events or circumstances after the date hereof.
Forward-looking statements
CADENCE®
and
OFIRMEV®
are
trademarks
of
Cadence
Pharmaceuticals,
Inc..
IONSYS™
is a trademark of Incline Therapeutics, Inc.
©
2011 Cadence Pharmaceuticals, Inc. All rights reserved.
|
 3
Cadence:
investment highlights
Hospital-focused specialty pharmaceutical company
OFIRMEV
®
launched with broad pain and fever indication in
January 2011
–
Rapid formulary adoption demonstrated
–
Established hospital sales team provides a core platform for the
acquisition of additional products
–
Management team with significant hospital commercial experience
Option to acquire Incline Therapeutics
–
IONSYS™
transdermal PCA system
–
Opportunity to leverage OFIRMEV sales force
Strong balance sheet |
 4
OFIRMEV
®
: product overview
OFIRMEV (acetaminophen) injection
–
Proprietary IV acetaminophen formulation
–
First and only IV formulation of acetaminophen
approved in the United States
–
New class of IV medication
•
non-narcotic / opioid
•
non-NSAID
–
Same formulation of IV acetaminophen marketed
by
BMS
in
Europe
since
2002
as
Perfalgan
™
U.S. Commercial launch : January 17, 2011
PERFALGAN™
is
a
trademark
of
Bristol
Myers
Squibb
Company. |
 5
OFIRMEV
®
: indication supports message
Broad Indication
•
Mild to moderate pain
•
Moderate to severe pain with adjunctive opioids
•
Reduction of fever
•
Adults and children 2 and older
Message
•
Significant pain relief
•
Reduced opioid consumption*
•
Improved patient satisfaction
•
Established safety profile
* Clinical benefit of opioid reduction not demonstrated
|
 6
OFIRMEV
®
:
strong foundation for commercial success
Effective Pain
Control
•
Sales force average >10 years hospital selling experience
•
Extensive relationships, significant overlap with prior
territory
•
Substantial hospital commercial experience throughout
management
–
CEO > 25 years, CCO > 15 years, VP of Sales > 25 years
•
$10.75/ vial
•
General Surgery DRG $23,000
•
OFIRMEV may help reduce post surgical ambulation
time, and time to extubation in the ICU
•
Significant pain relief
•
Reduced opioid consumption*
•
Improved patient satisfaction
Experienced
Hospital Sales
Force
Economic
Value
* Clinical benefit of opioid reduction not demonstrated
|
 7
OFIRMEV
®
:
strong early launch indicators*
Rapid formulary adoption
–
On formulary in 675 hospitals in first 15 weeks of launch
–
Mix of formulary wins is representative of overall target market
–
Includes major academic medical centers and large community
healthcare systems
Most hospitals approved OFIRMEV without restriction
–
Allows access across the hospital by range of physicians
Physician support and early experience positive
–
Physician support strong driver of formulary success
–
Physician feedback:
•
Significant pain relief
•
Utilization of less opioids
•
Improved patient experience
* Launch through April 30, 2011 |
 8
OFIRMEV
®
: vial sales off to strong start
Vial* Sales of Comparable New Hospital Product Launches Over Last 5 Years
0
20,000
40,000
60,000
80,000
100,000
120,000
1
2
3
4
5
6
7
8
9
10
11
12
Month Post Launch
OFIRMEV
(1-11)
TEFLARO
(1-11)
DORIBAX
(10-07)
CLEVIPREX
(9-08)
VAPRISOL
(3-06)
VIBATIV
(10-09)
LUSEDRA
(11-09)
ENTEREG (doses)
(06-08)
CALDOLOR
(9-09)
Average
(Excl OFIRMEV)
* # of doses are shown for Entereg, which is an oral product
OFIRMEV vs Avg:
546%
OFIRMEV Rank:
1
Source: Wolters Kluwer Pharma Solutions, Source® PHAST Institution. Based on Cadence comparison
to other selected product launches in hospital market over period March 2006 – March 2011
|
 9
Limitations of other IV pain therapies
Sedation
Nausea
Vomiting
Constipation
Headache
Cognitive impairment
Respiratory depression
Opioids
NSAIDs
Black Box Warning
Bleeding
GI complications
Kidney complications
Cardiovascular risks
Prolonged recovery
Increased length of stay
Higher costs to the institution
Limited use |
 10
Pain Intensity
Current US Approach
Current EU Approach
Severe
Opioids
IV
acetaminophen
+ opioids
Moderate
Opioids
IV
acetaminophen
+/-
opioids*
Mild
Opioids
IV
acetaminophen
Multi-modal analgesia: the norms
* First post operative analgesic drug, then add opioids if necessary
|
 11
Sinatra, et al. Anesthesiology, V 102, No. 4, April 2005.
IV acetaminophen
placebo
p-value
Weighted sums of pain relief over 6hrs
6.6
2.2
<0.05
Patient
Satisfaction
(Good/excellent
–
24
hrs)
41%
23%
<0.01
Morphine consumption over 24 hrs**
38.3 mg (33%
)
57.4 mg
<0.001
Safety
placebo
IV acetaminophen
Placebo-controlled, total hip or total knee replacement
(n=49/52)
p<0.001
.
.
Sinatra Study: pivotal acute pain clinical
trial IV acetaminophen
placebo
p-value
Sum of pain intensity differences over 24hrs*
-.28
-242.3
<0.001
6.6
2.2
<0.05
41%
23%
<0.01
38.3 mg (33%
)
57.4 mg
IV acetaminophen comparable to placebo
* Post hoc analysis based on currently acceptable regulatory
endpoint ** Clinical benefit of opioid reduction was not demonstrated
|
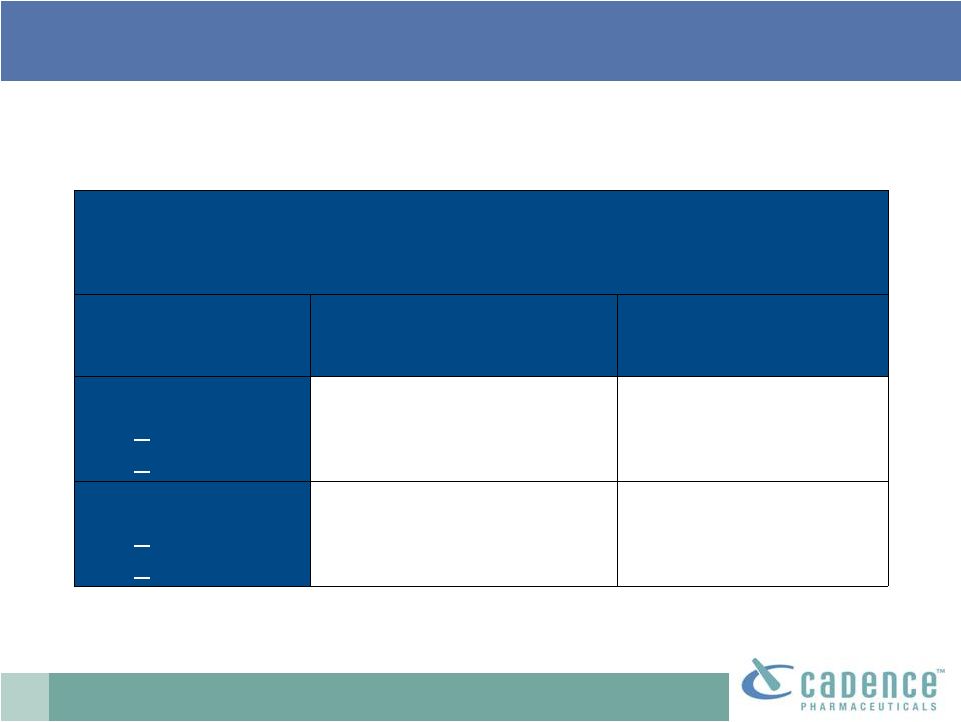 12
OFIRMEV
®
: established safety profile
% of adult patients with AST/ALT elevations
in five repeat-dose clinical trials
IV APAP (n=402)
Placebo (n=379)
ALT
>
3x ULN
>
5x ULN
1.1% (n=4)
0.3% (n=1)
1.7% (n=6)
0.6% (n=2)
AST
>
3x ULN
>
5x ULN
1.0% (n=4)
0.5% (n=2)
1.1% (n=4)
0.8% (n=3)
* Data from a pooled analysis of 5 multiple –dose clinical studies involving
adult patients. P value is based on Fisher’s Exact
Test. Established safety profile; well tolerated in clinical trials
|
 13
Pain Intensity
Opioid Reduction*
Time
p Value
Severe
33%
24h
<0.01
61%
24h
<0.05
Moderate
53%
0-6h
0.016
Mild
86%
24h
<0.001
78%
24h
<0.01
Consistent opioid reduction across studies
*Reduction
in
number
of
patients
requiring
analgesic
rescue
with
ketorolac
and
fentanyl
1)
Sinatra, et al, 2005;
2)
Memis, et al, 2005;
3)
Viscusi, et al, 2005;
4)
Hong, et al, 2005;
5)
Atef, Fawaz, 2007
1
2
3
4
5 |
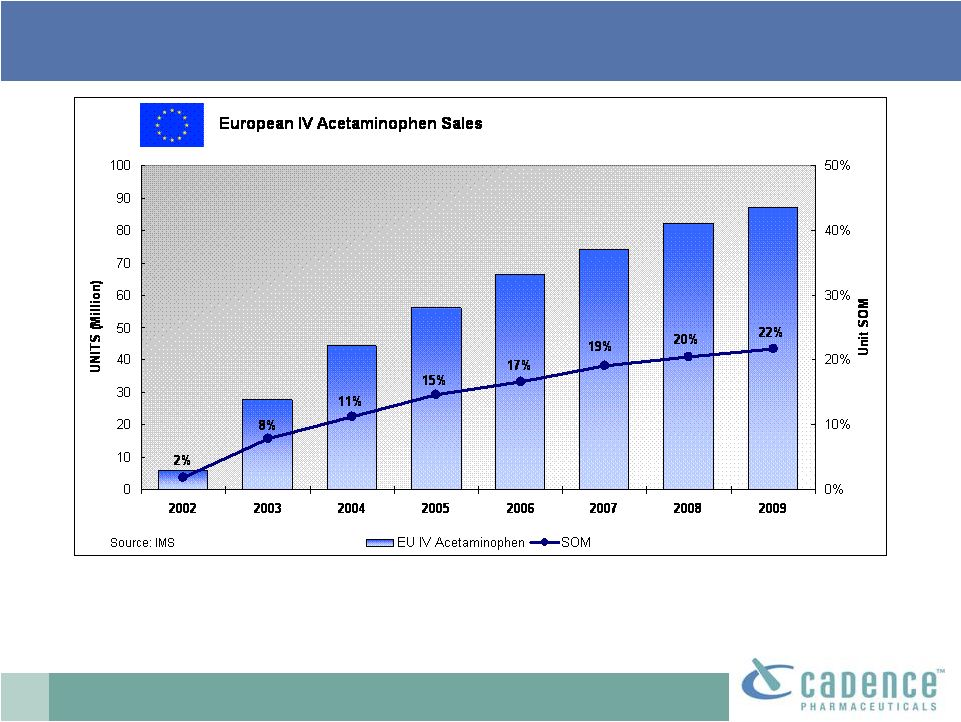 14
Source: IMS Data
*(SOM –
Share of market)
•
>20% unit share of injectable analgesic market
IV acetaminophen: EU market leader
|
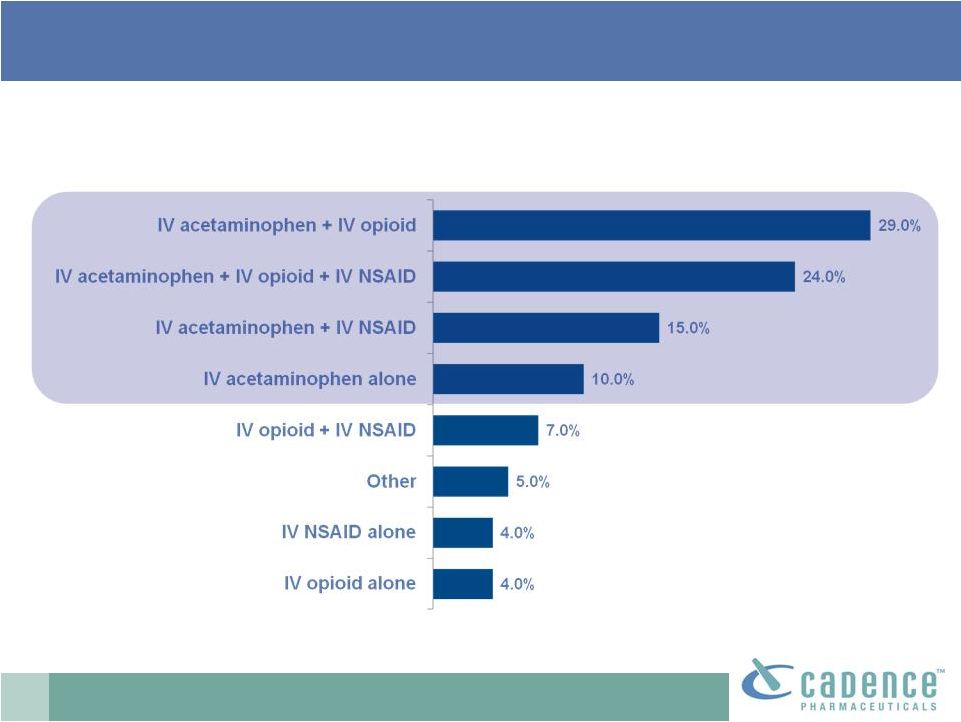 15
Europe: 78% receive IV acetaminophen post-op % of
Inpatients Given Each of the Following Treatments Post-operatively
PharmaSavvy market research 2009. n=60 anesthetists (commissioned by Cadence
Pharmaceuticals) |
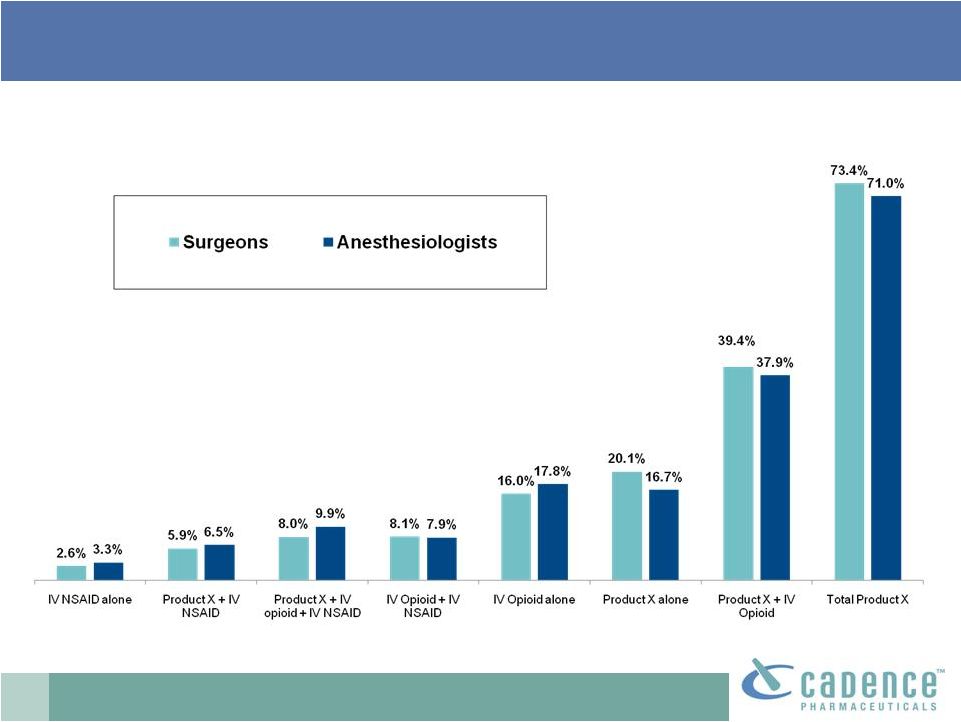 16
US: >70% may receive IV acetaminophen
post-op
Post-op inpatient treatment assignments assuming
availability of IV acetaminophen* (Product X)
PharmaSavvy market research 2009. n=102 (52 surgeons, 50 anesthesiologists)
* Based
on
target
product
profile
similar
to
OFIRMEV™
approved
indications |
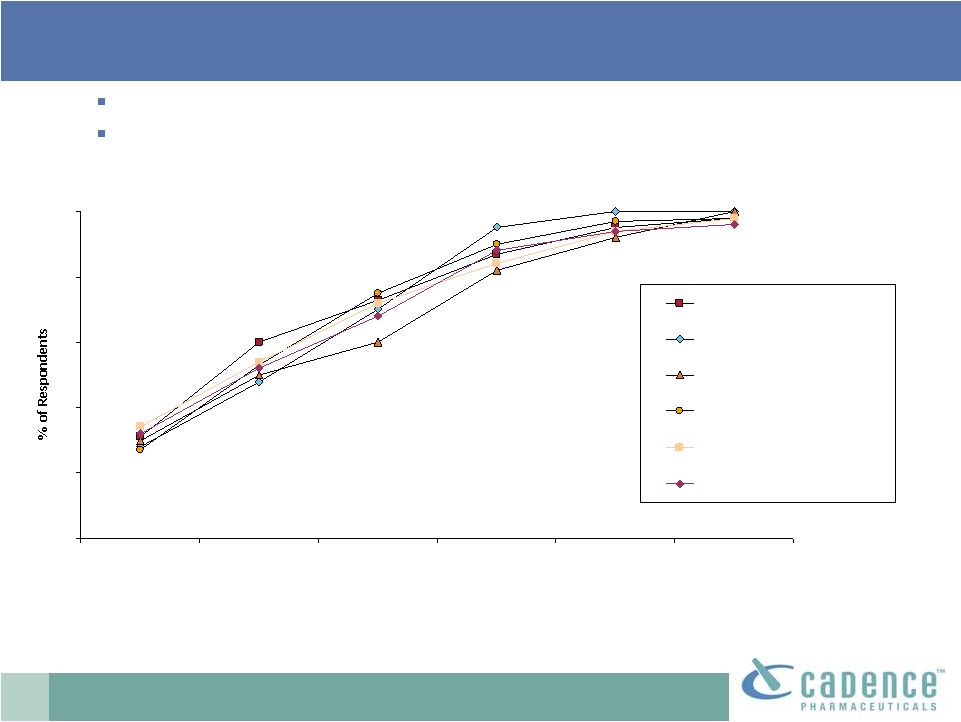 17
ATU Research 10/2010
Base: Anesthesiologists and surgeons
Q55. How soon after FDA approval would you expect to first use Product X (IV
acetaminophen)? Virtually all respondents expect to use OFIRMEV at some
point. About half of respondents across specialties would expect to
try OFIRMEV within one month after it is approved.
0
20
40
60
80
100
As soon as it is
available
1 month
3 months
6 months
Within one year
After more than
1 year
Anesthesiologist
General Surgeon
Orthopedic Surgeon
OB-GYN
Cardiothoracic Surgeon
Neurosurgeon
OFIRMEV
®
: early adoption |
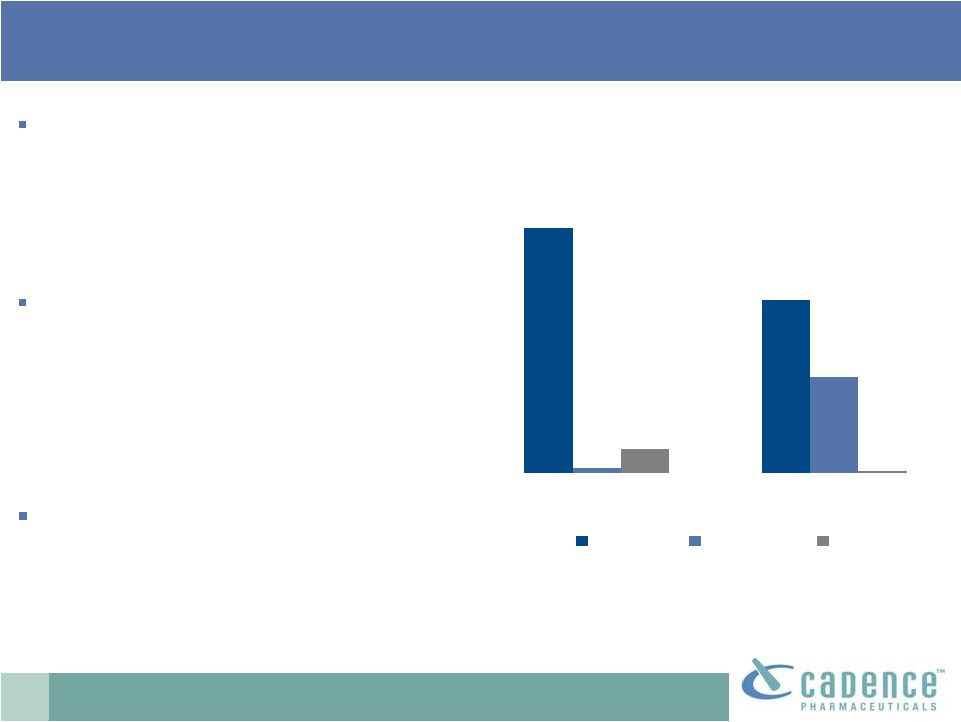 18
The UK experience:
an appropriate model
UK treatment paradigms for moderate
and acute pain are similar to those in
the US
–
Higher opioid than NSAID usage
–
Multimodal pain therapy
Perfalgan, BMY’s IV acetaminophen
–
Launched in 2004
–
Has taken approximately 35% market
share
–
Most share taken from opioids, some
from NSAIDS
Used alone and in combination
with opioids
–
Multimodal therapy provides broader
market opportunity
UK Injectable Analgesics
Source: IMS data, 2009
2004
2009
Opioids
Perfalgan
NSAIDS
89%
2%
35%
1%
9%
63% |
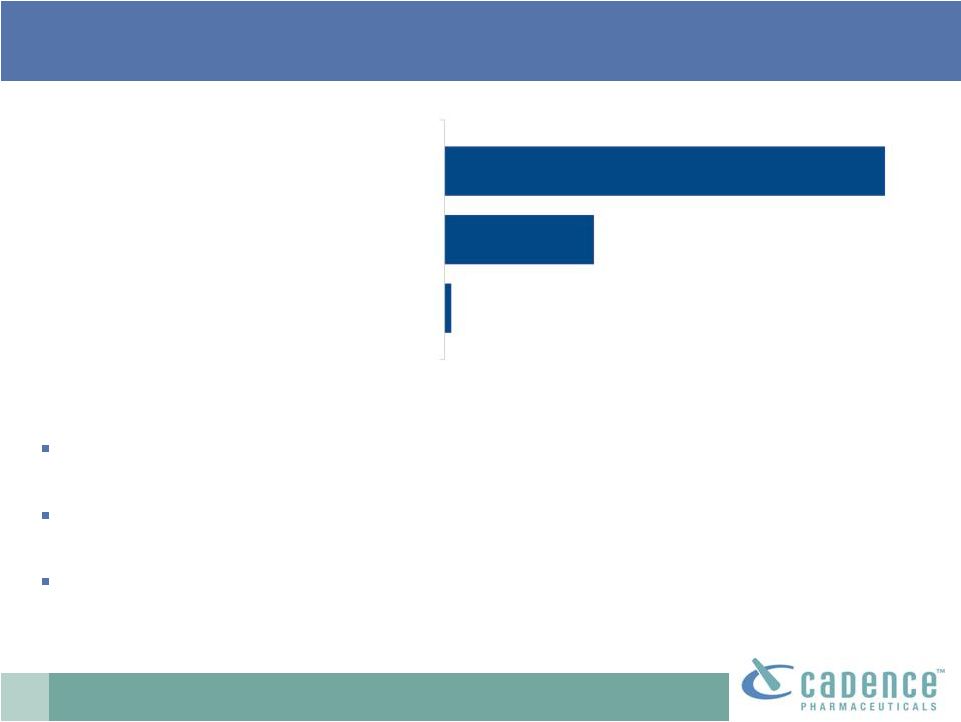 19
Oral
opioids:
acetaminophen
combinations
dominate
Opioid-acetaminophen combinations
Opioid-only products
Opioid-NSAID combinations
73%
25%
1%
Source: IMS data, 2008.
73% of oral opioid doses sold in U.S. contain acetaminophen
Approximately 14.4 billion total doses sold in 2008
Acetaminophen + hydrocodone is the most frequently dispensed Rx
drug in the US (FDA, 2009) |
 20
OFIRMEV
®
: US market opportunity
US and EU injectable analgesics markets are similarly sized
Approximately 87 million IV acetaminophen units sold in
Europe in 2009
Continued EU market expansion after introduction of IV
acetaminophen
List price $10.75 per vial; net price approximately $10.05 per
vial
Sources: IMS data, company estimates |
 21
Hospital products:
multi-step launch process
Regulatory
Approval
Doctor
Access
Formulary
Access |
 22
Injectable analgesic market: highly
concentrated Source: WoltersKluwer Non-Retail Outlet Data,
June 2009 MAT *Injectable analgesics consist of USCs 02221, 02211, 02131,
02231 0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
0
1,000
2,000
3,000
4,000
5,000
6,000
Number of Hospitals
The top 1,874 (28%)
hospitals generated
80% of the total
market volume
Less than 750 (11%)
hospitals generated
50% of the total
market volume |
 23
Experienced, Seasoned, Tested Sales Organization
147 Hospital Sales Specialists
–
>10 years hospital selling experience
–
90% resigned to join Cadence
–
Extensive relationships, 70% overlap with prior territory
Deep hospital commercial experience
–
CEO > 25 years
–
CCO > 15 years
–
VP Sales > 25 years
–
Regional Business Directors > 20 years
–
District Sales Managers > 9 years |
 24
OFIRMEV
®
: economic value
Placebo-controlled studies using IV acetaminophen demonstrated
results that may be associated with possible hospital cost savings:
–
Decreased opioid consumption *
•
Total hip/knee replacement
(1)
•
Total hip replacement
(2, 3)
•
Adult tonsillectomy
(4)
•
Endoscopic thyroidectomy
(5)
–
Decreased
time
in
PACU
(post-anesthesia
care
unit)
(6)
–
Decreased time to ambulation
(7)
–
Decreased
time
to
extubation
in
ICU
(8)
* Note: (1-5) Clinical benefit
of opioid reduction was not demonstrated References: (1) Sinatra, 2005; (2)
Viscusi, 2008; (3) Gimbel, 2008; (4) Atef, 2007; (5) Hong, 2010a; (6) Salihoglu, 2009; (7) Ohnesorge, 2009;
(8) Memis, 2010 |
 25
Cadence: financial position
12 Months
Ended
12/31/10
(MM)
3 Months
Ended
3/31/11
(MM)
Operating expenses
$ 54.9
$ 22.7
Cash, cash equivalents &
short-term investments
$134.1
$109.0
(1)
Shares outstanding
63.1
63.3
(1)
Does not include a $5.3 million upfront payment received in April 2011 under a data
license agreement with Terumo |
 26
OFIRMEV
®
: product overview
OFIRMEV (acetaminophen) injection
–
Proprietary IV acetaminophen formulation
–
First and only IV formulation of acetaminophen
approved in the United States
–
New Class of IV Analgesic
OFIRMEV launched with broad pain and fever
indication in January 2011
–
Rapid formulary adoption demonstrated; 675
approvals by April 30
–
Management team with significant hospital
commercial experience
–
Established hospital sales team provides a core
platform for the acquisition of additional products |
