As filed with the Securities and Exchange
Commission on April 20, 2011
Registration No. 333-169720
UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C.
20549
Amendment No. 6
to
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ARBORGEN INC.
(Exact Name of Registrant As
Specified in Its Charter)
| |
|
|
|
|
|
Delaware
|
|
0800
|
|
58-2521259
|
(State or Other Jurisdiction
of
Incorporation or Organization)
|
|
(Primary Standard Industrial
Classification Code Number)
|
|
(I.R.S. Employer
Identification Number)
|
180 Westvaco Road
Summerville, South Carolina 29483
(843) 851-4129
(Address, Including Zip Code,
and Telephone Number,
Including Area Code, of
Registrant’s Principal Executive Offices)
Barbara H. Wells, Ph.D.
President and Chief Executive Officer
ArborGen Inc.
180 Westvaco Road
Summerville, South Carolina 29483
(843) 851-4129
(Name, address, including zip
code, and telephone number,
including area code, of agent
for service)
Copies to:
| |
|
|
Mark T. Bettencourt, Esq.
Michael J. Minahan, Esq.
Goodwin Procter LLP
Exchange Place
Boston, Massachusetts 02109
Telephone:
(617) 570-1000
|
|
Leslie N. Silverman, Esq.
Sandra L. Flow, Esq.
Cleary Gottlieb Steen & Hamilton LLP
One Liberty Plaza
New York, New York 10006
Telephone: (212) 225-2000
|
Approximate date of commencement of proposed sale to the
public: As soon as practicable after the
effective date of this registration statement.
If any of the securities being registered on this Form are to be
offered on a delayed or continuous basis pursuant to
Rule 415 under the Securities Act of 1933, check the
following
box. o
If this Form is filed to register additional securities for an
offering pursuant to Rule 462(b) under the Securities Act,
please check the following box and list the Securities Act
registration statement number of the earlier effective
registration statement for the same
offering. o
If this Form is a post-effective amendment filed pursuant to
Rule 462(c) under the Securities Act, check the following
box and list the Securities Act registration statement number of
the earlier effective registration statement for the same
offering. o
If this Form is a post-effective amendment filed pursuant to
Rule 462(d) under the Securities Act, check the following
box and list the Securities Act registration statement number of
the earlier effective registration statement for the same
offering. o
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in Rule
12b-2 of the Exchange Act. (Check one):
| |
|
|
|
|
|
|
|
Large accelerated filer o
|
|
Accelerated filer o
|
|
Non-accelerated filer þ
|
|
Smaller reporting company o
|
|
|
|
|
|
(Do not check if a smaller reporting company)
|
|
|
The Registrant hereby amends this Registration Statement on
such date or dates as may be necessary to delay its effective
date until the Registrant shall file a further amendment which
specifically states that this Registration Statement shall
thereafter become effective in accordance with Section 8(a)
of the Securities Act of 1933 or until the Registration
Statement shall become effective on such date as the Commission,
acting pursuant to such Section 8(a), may determine.
The
information in this prospectus is not complete and may be
changed. We may not sell these securities until the registration
statement filed with the Securities and Exchange Commission is
effective. This prospectus is not an offer to sell these
securities and we are not soliciting offers to buy these
securities in any jurisdiction where the offer or sale is not
permitted.
|
SUBJECT TO COMPLETION,
PRELIMINARY PROSPECTUS DATED APRIL 20, 2011
PROSPECTUS
Shares
ArborGen Inc.
Common Stock
$ per
share
This is ArborGen Inc.’s initial public offering. We are
selling shares
of our common stock.
We currently expect the public offering price to be between
$ and
$ per share. Currently, no public
market exists for our shares. We have applied to list our common
stock on the Nasdaq Global Market under the symbol
“ARBR.”
Investing in our common stock involves risks that are
described under “Risk Factors” beginning on
page 14 of this prospectus.
Neither the Securities and Exchange Commission nor any state
securities commission has approved or disapproved of these
securities or determined if this prospectus is truthful or
complete. Any representation to the contrary is a criminal
offense.
| |
|
|
|
|
|
|
|
|
|
|
|
Per Share
|
|
Total
|
|
|
|
Public offering price
|
|
$
|
|
|
|
$
|
|
|
|
Underwriting discount
|
|
$
|
|
|
|
$
|
|
|
|
Proceeds, before expenses, to us
|
|
$
|
|
|
|
$
|
|
|
The underwriters may also purchase up to an
additional shares
from us, at the public offering price, less the underwriting
discount, within 30 days from the date of this prospectus.
The underwriters expect to deliver the shares to purchasers on
or
about .
|
|
| Goldman,
Sachs & Co. |
Citi |
|
|
| Piper
Jaffray |
RBC Capital Markets |
The date of this prospectus
is ,
2011
TABLE OF
CONTENTS
| |
|
|
|
|
|
|
|
|
1
|
|
|
|
|
|
14
|
|
|
|
|
|
35
|
|
|
|
|
|
36
|
|
|
|
|
|
37
|
|
|
|
|
|
38
|
|
|
|
|
|
39
|
|
|
|
|
|
40
|
|
|
|
|
|
41
|
|
|
|
|
|
45
|
|
|
|
|
|
69
|
|
|
|
|
|
109
|
|
|
|
|
|
117
|
|
|
|
|
|
134
|
|
|
|
|
|
139
|
|
|
|
|
|
141
|
|
|
|
|
|
145
|
|
|
|
|
|
147
|
|
|
|
|
|
149
|
|
|
|
|
|
155
|
|
|
|
|
|
155
|
|
|
|
|
|
155
|
|
|
|
|
|
F-1
|
|
| Ex-23.1 |
We are responsible for the information contained in this
prospectus and in any related free-writing prospectus we prepare
or authorize. Neither we nor the underwriters have authorized
anyone to give you any other information, and neither we nor the
underwriters take responsibility for any other information that
others may give you. We are offering to sell, and seeking offers
to buy, shares of our common stock only in jurisdictions where
offers and sales are permitted. The information contained in
this prospectus is accurate only as of the date on the front
cover of this prospectus, or other earlier date stated in this
prospectus, regardless of the time of delivery of this
prospectus or of any sale of our common stock.
We own, have rights to, or have applied for the trademarks and
trade names that we use in conjunction with our business,
including
ArborGen®,
Mass Control
Pollinated®,
MCP®,
More Wood Less
Land®,
ArborGenabled®,
FlexStand®,
SuperTree
Seedlings®
and our logo. All other trademarks and trade names appearing in
this prospectus are the property of their respective holders.
For investors outside of the United States: Neither we nor any
of the underwriters has done anything that would permit this
offering outside the United States or to permit the possession
or distribution of this prospectus outside the United States.
Persons outside the United States who come into possession of
this prospectus must inform themselves about, and observe any
restrictions relating to, the offering of the shares of common
stock and the distribution of this prospectus outside of the
United States.
PROSPECTUS
SUMMARY
This summary highlights information contained elsewhere in
this prospectus and does not contain all of the information that
you should consider in making your investment decision. Before
investing in our common stock, you should carefully read this
entire prospectus, including our consolidated financial
statements and the related notes included elsewhere in this
prospectus. You should also consider, among other things, the
matters described under “Risk Factors” and
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations,” in each case
appearing elsewhere in this prospectus. Unless otherwise stated,
all references to “us,” “our,”
“ArborGen,” “we,” the “Company”
and similar designations refer to ArborGen Inc. and its
subsidiaries.
Our
Company
We believe we are the world’s leading developer of
biotechnology tree seedling products, one of the largest
providers of conventional and technology-enhanced seedlings to
the forestry industry and the only integrated global commercial
seedling company. We are focused on improving and selling the
most widely grown commercial forestry species in some of the
largest markets in the world. Our products are designed for use
by our customers in the traditional pulp and paper and wood
products markets, the growing biopower and charcoal markets and
the emerging biofuels market. We have a base of over 5,000
customers, including some of the largest land owners and
managers in the United States, New Zealand and Australia, and in
the year ended March 31, 2010, we sold 240 million
seedlings in these markets. We also have a growing presence in
Brazil through collaborations with the country’s leading
pulp producers. Based on our research and estimates, we believe
that our high-value, technology-enhanced seedling products,
including our pipeline of advanced and biotechnology products,
improve the productivity of a given acre of land by enabling our
customers to grow trees that yield more wood per acre with
greater consistency and quality in a shorter period of time. The
combination of our fully integrated business model, proprietary
technology and established customer base creates a scalable
platform that we are using to develop and commercialize the next
generation of seedling products. We do not currently sell any
biotechnology products and, prior to any commercial sales, our
biotechnology products are subject to a multi-year deregulation
process. We believe, but cannot guarantee, our biotechnology
products will revolutionize productivity standards in the
forestry industry and have an impact on that industry similar to
the impact that biotechnology crops have had on the agricultural
industry.
We are currently the only commercial seedling company with
products spanning the entire technology spectrum, from
conventional and advanced seedlings, which we currently offer,
to biotechnology seedlings, which are currently in development.
As a result, no single entity competes with us in commercial
sales across the full range of our business. In the year ended
March 31, 2010, sales of our open-pollinated, or
conventional, seedling products represented approximately 72% of
our revenue. We also sell advanced seedling products, which
consist of our mass-control pollinated, or MCP, and varietal
products. These advanced products are designed to improve the
growth rates, yields, stress-tolerance, uniformity, wood quality
and processing efficiency of trees. In the year ended
March 31, 2010, sales of our advanced products represented
approximately 28% of our revenue. Our product pipeline includes
six advanced and 15 biotechnology products in various stages of
development. While we do not currently sell any biotechnology
seedling products, our freeze-tolerant tropical eucalyptus
product is the first and only biotechnology forestry product
under review for deregulation by the United States Department of
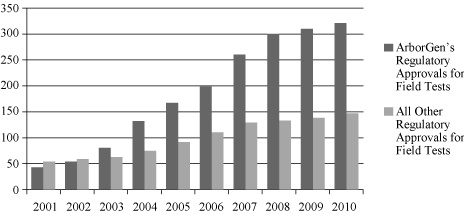
Agriculture, or USDA. In addition, we have the largest number of
regulatory approvals for field tests of biotechnology forestry
products in the United States and Brazil.
We have developed our products by utilizing our leading
technology platform, which is built on over 100 years, in
the aggregate, of tree improvement research. Our technology
platform combines one of the largest and most diverse
repositories of tree genetic resources, or germplasm, and
substantially all of the commercial seed orchard and nursery
businesses and the related research and development activities
of three industry leaders: International Paper Company,
MeadWestvaco Corporation and Rubicon Limited. As a result of our
technology leadership, our portfolio of owned and exclusively
licensed patents and the inherent tree growth time associated
with tree improvement research, we believe we are decades ahead
of any new market entrants seeking to develop and commercialize
a product portfolio and technology platform comparable to ours;
however, there is no guarantee that we will be able to
commercialize our biotechnology products in the near future.
1
We believe we are the largest provider of tree seedlings to the
commercial forestry industry in the world. Based on our annual
seedling sales and management estimates, we believe we have an
approximately 27% share of the total seedling market in the
Southeastern United States, an approximately 36% share of the
total seedling market in New Zealand and a more than 10%
share of the Australian pine seedling market. In addition, our
customers include 13 of the 20 largest land owners and managers
in the United States as reported in 2008 by RISI, the leading
information provider for the global forest products industry,
and our customers include six of the ten largest land owners and
managers in New Zealand as reported in 2009 by the
New Zealand Forest Owners Association and the New Zealand
Ministry of Agriculture and Forestry. The geographies in which
we currently operate represent some of the largest commercial
forestry markets in the world, and we believe our existing
market presence and our pipeline of advanced and biotechnology
seedling products position us well to expand into other large
and fast-growing forestry markets, including China and markets
throughout South America.
Our technology platform and our tree improvement expertise
gained over multiple generations of tree breeding have allowed
us to develop products that increase the productivity of land
and address the evolving needs of the global commercial forestry
industry, including the increased demand for wood as a source of
energy. Our broad portfolio of seedling products, which we sell
under the
ArborGen®
and SuperTree
Seedlings®
brand names, includes the most widely grown tree species in the
commercial forestry industry, such as loblolly pine, radiata
pine and eucalyptus. We continue to enhance traits of these
species, including growth rates, yields, stress-tolerance,
uniformity, wood quality and processing efficiency, through a
variety of conventional and advanced production processes,
including open pollination, mass-control pollination and
varietal manufacturing, and through the application of
biotechnology. As a result, we are able to offer a broad
portfolio of seedling products with a range of traits, which
allows us to address different potential
end-users at
different price points and returns on investment. For example,
in the United States, our products currently range in price from
approximately $45 per 1,000 seedlings for our first-generation
conventional open-pollinated loblolly pine seedlings to
approximately $400 per 1,000 seedlings for a varietal product of
the same species that exhibits enhanced traits selected for
suitability in a specific end market and that we believe
provides our customers with higher returns on investment.
To accelerate adoption of our higher-value products, we intend
to leverage our longstanding customer relationships and the
improved returns that we believe will be provided by these
products. In some of our geographic markets, the transition to
higher-value seedling products is already well-established. In
the year ended March 31, 2010, we generated approximately
70% of our revenue in New Zealand and Australia from sales of
our advanced products. The shift to advanced seedling products
and higher average selling price that has already occurred in
New Zealand is now beginning to occur with our customer sales in
the significantly larger U.S. market. For example, as our
customers in the United States have transitioned to our
higher-value products, which consist of our “elite”
open-pollinated conventional seedlings, our MCP seedlings and
our varietal seedlings, sales of these products have grown from
5.7% of U.S. revenue in the year ended March 31, 2008
to 24.2% in the year ended March 31, 2010, with increasing
sales of MCP and varietal seedlings each year. We use the term
“elite” to refer to the latest and most improved
generation of a particular open-pollinated product.
Our advanced and biotechnology products are designed to provide
significant additional value, which will be shared by us and our
customers. Agricultural biotechnology companies have
successfully employed this value-sharing model to establish
premium pricing for their higher-value products. However, unlike
agricultural biotechnology companies, which typically sell
through distributors, we expect to continue to sell our products
directly to our customers. As a result, we expect to capture a
significant portion of the value created by our advanced and
biotechnology products. Over the three-year period ended
March 31, 2010, the shift in sales to our higher-value
products drove a 15.5% increase in our U.S. average selling
price and we expect this trend to continue.
We expect to be the first company in our markets to introduce
biotechnology products for sale to the commercial forestry
industry. Prior to any commercial sales, our biotechnology
products are subject to a multi-year deregulation process. We
submitted our initial petition for the deregulation of our
freeze-tolerant tropical eucalyptus product to the USDA in
December 2008 and resubmitted that petition in January 2011 to
include additional data. We expect to submit petitions for the
deregulation of our short rotation loblolly pine and short
2
rotation populus products to the USDA for review in the next two
to four years. We expect to make regulatory submissions to
Comissão Técnica Nacional de Biossegurança, or
CTNBio, the governmental agency in Brazil that regulates
biotechnology products, for our short rotation tropical
eucalyptus product in the next three to four years and for our
improved pulping tropical eucalyptus product in the next four to
five years.
For the year ended March 31, 2010, we generated
$21.6 million of revenue, of which 76% was generated from
customers located in the United States, 19% from customers
located in New Zealand and 5% from customers located in
Australia. Of the 240 million seedlings we sold in the year
ended March 31, 2010, 91% were sold in the United States.
We generated a gross profit of $5.2 million for the year
ended March 31, 2010. After incurring research and
development expenses of $11.2 million, or 51.9% of revenue,
in the year to enhance and expand our pipeline of future
advanced and biotechnology products, we recorded a net loss of
$(14.7) million. For the year ended March 31, 2009, we
generated $23.7 million of revenue and recorded a net loss
of $(15.3) million, and for the year ended March 31,
2008, we generated $18.2 million of revenue and recorded a
net loss of $(18.1) million. For the nine months ended
December 31, 2010, we generated $11.1 million of
revenue and recorded a net loss of $(14.2) million. We
expect to continue to incur net losses over the next several
years, including the year ending March 31, 2011, primarily
as a result of our continuing investment in the research and
development of advanced and biotechnology seedling products. As
of March 31, 2011, we had 181 employees and operated
13 nurseries, 15 seed orchards, 20 distribution centers and
three research and development facilities located throughout the
Southeastern United States, New Zealand and Australia, as well
as an office in Brazil.
Our
Market Opportunity
Historically, the global commercial forestry industry has relied
heavily on the harvesting of native forests. However, the use of
wood from native forests is now under increasing pressure from
competing uses of land, such as conversion to agricultural uses
and the continued expansion of commercial and residential
development, and from the limited accessibility to remaining
unharvested forests, including as a result of conservation
efforts. According to the Food and Agriculture Organization of
the United Nations, or the FAO, from 1990 to 2005, the total
area of deforestation was approximately 395 million acres,
which is more than twice the size of Texas. In addition, over
the past ten years, 150 million acres of the remaining
global forests have been set aside as protected forests.
Further, the FAO expects increases in the global consumption of
wood to be driven by economic growth in China and other emerging
markets, population growth, environmental policies and
regulations, and increased global demand for energy from
renewable sources. In its 2011 State of the World’s Forest
report, the FAO also noted that current wood production levels
in certain regions that are becoming major consumers of wood
products, such as Asia, will not be sufficient to meet this
increased demand.
Due to these increasing supply and demand pressures on global
wood availability, the FAO expects that purpose-grown trees will
meet a larger proportion of the demand for wood in the future,
thereby alleviating some of the pressure on native forests.
Purpose-grown trees, which are planted, maintained and harvested
on plantations for commercial purposes, deliver improved
per-acre
productivity as compared to harvesting native forests. In 1992,
the United Nations Conference on Environment and Development
issued an Authoritative Statement stating that purpose-grown
trees are a sustainable and environmentally sound source of
renewable energy and industrial raw material. Based on the
FAO’s 2010 Global Forest Resource Assessment, purpose-grown
trees currently account for approximately 7% of the global
forest area. Purpose-grown trees, however, represent a larger
proportion of the wood supply for industrial use. According to a
2005 FAO report, due to the increased productivity of planted
forests, these trees contributed approximately 35% of industrial
roundwood supply in 2000 and the FAO anticipated that this
percentage would rise to 40-44% by 2020.
According to the FAO, purpose-grown trees are more productive
than native forests as a result of the cumulative impact of
improved planting material, nursery practices, the planting of
species that are best-suited to a particular end-market or
geography, and intensive site management. We believe additional
productivity gains result from reduced harvesting costs and the
placement of a plantation within close proximity to a paper
mill, biopower facility or export hub. In addition, unlike
native forests, plantations can be planted withuniform
3
high-value seedlings that exhibit more predictable traits, which
enable land owners or managers to make more informed planting
and harvesting decisions.
Additionally, the shift in ownership of commercial timberland
from integrated corporate owners to investment companies is
driving the demand for purpose-grown trees. Land owners and
managers who actively manage timberland to achieve improvements
in the financial returns of their investors have helped to drive
the transition from harvesting native forests to planting and
harvesting purpose-grown trees on plantations. In addition,
based on our experience, these land owners and managers have
purchased higher-value seedling products to improve their
returns. We believe this shift will continue to be an important
catalyst for broad industry adoption of our advanced and
biotechnology seedling products.
As a result of these supply and demand dynamics, the proven
productivity benefits of purpose-grown trees and the shifts in
land ownership, we believe there is an opportunity for our
high-value advanced and biotechnology seedling products to
improve the
per-acre
productivity of land used for commercial forestry. We have
designed our advanced and biotechnology products to suit
particular geographies and end-markets by modifying certain
targeted genetic traits, such as freeze tolerance for certain
areas of the Southeastern United States and other geographies
with similar climates, including parts of China, and improved
lignin content to reduce costs for pulp and biofuels processors
in Brazil. As a result, we believe these products will provide a
significant increase in value to our customers compared to the
conventional products that we currently offer.
Our five primary end-markets are the traditional pulp and paper
and wood products markets, the growing biopower and charcoal
markets, and the emerging biofuels market. Biopower refers to
the conversion of wood and other biomass into energy through
combustion or gasification, and biofuels are liquid fuels for
transportation derived from wood and other organic materials.
The demand for high-value, purpose-grown trees is driven by
industry-specific dynamics, growth of developing economies and
the increasing competition for available land. In addition, we
expect growth in the biopower and biofuels markets to be driven
by government standards and policies for energy from renewable
sources, such as purpose-grown trees.
We are applying a business model similar to the model that has
been successfully used by the agricultural biotechnology
industry; however, there are differences between these two
industries. For example, the rotation length of agricultural
crops is shorter than the rotation length of trees and there are
additional regulations that govern biotechnology products
intended for human and animal consumption that do not apply to
our products. In particular, we are applying biotechnology
techniques to develop new, higher value genetic products. As
occurred in the agriculture industry in the 1990s, we intend to
share in the value that these products create through the prices
we will charge for them. We believe our profitability will
increase over time as we transition our customers from
conventional seedling products to our biotechnology products
without having to significantly increase our fixed costs. In the
agricultural industry, companies such as Monsanto Company, the
first mover in that industry, introduced biotechnology crops
that redefined productivity, which dramatically improved the
economics of farmed crops as compared to their conventional seed
competitors. According to the 2010 ISAAA Brief No. 42, issued by
the International Service for the Acquisition of Agri-Biotech
Applications, the number of acres planted with
biotechnology-enhanced crops grew from approximately
4.3 million acres in 1996 to approximately 370 million
acres in 2010, representing a compound annual growth rate of
38%. We believe, but cannot guarantee, that our biotechnology
seedlings will have an impact on the productivity and economics
of the commercial forestry industry similar to the impact that
biotechnology crops have had on the agricultural industry.
Our
Technology Platform
Our technology platform provides us with a broad portfolio of
intellectual property, ownership of a large and diverse
repository of germplasm that includes the most widely used tree
species in the global commercial forestry industry, and
proprietary production processes. We produce tree seedlings
using a variety of processes, ranging from open pollination to
biotechnology and other laboratory-based techniques that are
designed to enhance desired traits in the seedlings. In our pine
seed orchards, we produce seeds through naturally occurring open
pollination, where we do not control the pollen source but do
select the seed source, and through
mass-control
pollination, where we select both the pollen source and the seed
source to produce seeds with desirable traits. We also produce
varietal seedling products through several proprietary,
laboratory-based processes whereby we identically replicate
selected seedlings that best meet the needs of our customers.
4
We believe our technology platform is a critical component of
our integrated business model and is at the core of our
continued success, and we intend to continue investing in this
platform. Our technology platform consists of the following key
elements:
|
|
|
| |
•
|
Germplasm Repository. We own one of the
largest and most diverse repositories of germplasm in the world,
including over 50 distinct commercial tree species and hybrids,
which provides us with the resources to develop a wide variety
of improved trees and is the base material into which we
integrate specific genetic traits to produce our biotechnology
products.
|
| |
| |
•
|
Gene Discovery, Research and Licensing
Program. In connection with the development of
our biotechnology seedling products, we have patented
approximately 25 distinct genes and promoters in the
United States through our in-house research programs and
have licensed the rights to use over 50 genes and promoters
through licensing arrangements with several leading academic and
commercial institutions.
|
| |
| |
•
|
Proprietary Development and Production
Processes. We have developed a number of
proprietary techniques for use in various stages of our
development and production processes, including the
cost-effective,
large-scale
commercial manufacturing of varietal and biotechnology seedlings
and mass production of bioengineered genetic material for use in
biotechnology seedlings.
|
| |
| |
•
|
Silviculture and Tree Improvement
Expertise. Our ability to consistently develop
improved generations of conventionally grown trees, which
depends on our
well-established
expertise in silviculture (forestry management) and tree
improvement, is the foundation for the proprietary varietal and
biotechnology techniques we use to develop our high-value
products.
|
Our
Integrated Business Model
We are currently the only integrated global commercial tree
seedling company. As a result, no single entity competes with us
in commercial sales across the full range of our business. Our
integrated business model combines our research and product
development program, tree improvement program, field testing,
product production, sales and distribution infrastructure with
our established customer base. This business model facilitates
product innovation and commercialization and the transition of
our customers to our advanced products. We intend to continue to
leverage this business model to drive the future development,
production and commercialization of our new products, including
our biotechnology products. We believe that this makes us an
attractive partner for companies and other entities seeking to
commercialize new technologies and products for the commercial
forestry industry, particularly those related to biotechnology.
Our
Competitive Strengths
We believe we possess a number of competitive strengths and a
first mover advantage that position us for significant growth
and make it difficult for existing or potential competitors to
replicate our technology and products. Some of these key
strengths are as follows:
|
|
|
| |
•
|
Broad Product Portfolio that Delivers Significant Value for
Our Customers. Our portfolio of seedling products
includes the most widely grown tree species in the commercial
forestry industry and range from conventional to
technology-enhanced seedlings, including MCP and varietal
seedlings. Our advanced products offer significant value to our
customers through a range of enhanced traits including improved
uniformity, higher growth rates, higher yields, improved wood
quality, increased stress-tolerance and better processing
efficiency relative to conventional products. Based on our
research and estimates, this improved product performance offers
our customers significantly higher returns on their land, which
justifies premium pricing relative to conventional products. For
example, our containerized MCP pine seedling products in the
United States currently sell for more than four times the price
of our best-selling conventional open-pollinated, or OP, pine
seedling product.
|
| |
| |
•
|
Pipeline of Advanced and Biotechnology
Products. In addition to our ongoing advanced
tree improvement programs, we currently have six advanced and 15
biotechnology products under development, including several in
the advanced stages of development. We have designed our
advanced
|
5
|
|
|
| |
|
and biotechnology products to suit particular geographies and
end-markets by modifying certain targeted genetic traits, such
as freeze tolerance for certain areas of the Southeastern
United States and other geographies with similar climates,
including parts of China, and improved lignin content to reduce
costs for pulp and biofuels processors in Brazil. We do not
currently sell any biotechnology products and, prior to any
commercial sales, our biotechnology products are subject to a
multi-year deregulation process. Based on our research and
estimates, we believe these products will provide a
significantly higher value to our customers compared to the
conventional products that we currently offer. We have made
significant investments in the research and development of these
advanced and biotechnology products, which contributed to our
lack of profitability. Our research and development expense
represented 52%, 58% and 56% of our revenue in the years ended
March 31, 2010, 2009 and 2008, respectively.
|
|
|
|
| |
•
|
Established Existing Business and Expansive Customer
Base. We believe we are the largest provider of
tree seedlings to the commercial forestry industry in the world.
Our base of over 5,000 customers includes some of the largest
land owners and managers in the United States, New Zealand and
Australia. We expect that our established customer base will
facilitate the adoption of our biotechnology products and that
as a result the average selling price of our products will
continue to increase.
|
| |
| |
•
|
Technology Leadership Position. Our technology
leadership position is built on over 100 years, in the
aggregate, of tree improvement research. We own a portfolio of
over 230 patents and patent applications, with license rights to
more than 200 additional patents and patent applications. We
also own one of the largest and most diverse repositories of
tree genetic resources, or germplasm, in the world. To produce
our advanced and biotechnology products on a commercial scale,
we have developed numerous proprietary processes and techniques
related to the biotechnology transformation process and the
large-scale manufacturing of advanced seedlings. Our patent
portfolio, trade secrets, license agreements and unique
germplasm provide us with a solid technology foundation that we
believe is difficult to replicate and upon which we continue to
build. We believe our technology leadership will enable us to
continue setting the technology standard in our industry.
|
| |
| |
•
|
Demonstrated and Scalable Development, Production and
Commercialization Platform. We believe that our
fully integrated business model, with capabilities spanning
research, product development, testing, production, and sales
and distribution, enables us to better match our products to the
changing needs of the market, reduce the time and cost of
commercializing new products, and transition customers to
higher-value products. The scalability of our technology enables
us to leverage our research and development programs to develop
and commercialize new products and expand into new markets at
relatively low marginal cost.
|
| |
| |
•
|
Experienced Management Team with a Strong Track Record and
Biotechnology Expertise. With an average of
26 years of industry experience, our management team brings
expertise in forestry and biotechnology, as well as a track
record of successfully navigating biotechnology products through
the USDA regulatory process to commercialize them. Several
members of our senior management team have held important
regulatory, product development and product launch positions at
Monsanto Company, which successfully introduced biotechnology
products to the agricultural industry.
|
| |
| |
•
|
First Mover Advantage. We believe that the
combination of our scalable technology platform, intellectual
property and patents, large and diverse germplasm repository,
global production capability, established customer base and
pipeline of advanced and biotechnology products, as well as the
time and investment associated with tree improvement research
and regulatory approval for biotechnology products, places us
decades ahead of any new market entrants seeking to develop and
commercialize a product portfolio and technology platform
comparable to ours; however, there is no guarantee that we will
be able to commercialize our biotechnology products in the near
future.
|
Our
Strategy
Our goal is to be the leader in developing and selling
proprietary seedling products by revolutionizing the
productivity of the global commercial forestry industry. We
believe that by significantly improving the
6
productivity of a given acre of timberland for our customers
around the world, we will be able to rapidly grow our business,
deliver superior financial performance and help conserve the
world’s native forests. We are focused on achieving these
goals by employing the following business strategies:
|
|
|
| |
•
|
Develop and Commercialize New Advanced and Biotechnology
Products. We intend to revolutionize the
productivity of, and expand our market position in, the
commercial forestry industry by developing and commercializing
new advanced and biotechnology products that build upon the
improved growth rates, yields, stress-tolerance, uniformity,
wood quality and processing efficiency of our existing products.
Our goal is to develop and commercialize biotechnology seedling
products that have an impact on the commercial forestry industry
similar to the impact that biotechnology crops have had on the
agricultural industry.
|
| |
| |
•
|
Transition Our Customer Base to Higher-Value
Products. We intend to accelerate the adoption of
our higher-value products by leveraging our customer
relationships and the improved returns that we believe these
products will provide. The adoption of advanced products is
already well established in New Zealand and Australia where we
generated approximately 70% of our revenue from sales of our
advanced products in the year ended March 31, 2010. We have
demonstrated our ability to transition our customers to our
higher-value products in the United States, where sales of our
higher-value products, which consist of our elite
open-pollinated conventional seedlings, our MCP seedlings and
our varietal seedlings, have increased as a percentage of
revenue, and have driven the 15.5% increase in our
U.S. average selling price over the three-year period ended
March 31, 2010.
|
| |
| |
•
|
Grow Through Customer Expansion into Emerging
End-Markets. We intend to grow our business by
increasing the number of our seedling products used by customers
for the biopower, charcoal and biofuels end-markets. Our
customers are actively seeking strategies to help them establish
a position in these markets given their forecasted growth rates,
and we believe our advanced and biotechnology seedling products
have the potential to significantly broaden our customers’
access to these end-markets.
|
| |
| |
•
|
Expand into New High Growth Geographies. We
intend to grow our business around the world to capitalize on
opportunities in the large and growing global commercial
forestry market. Specifically, we intend to grow and further
strengthen our competitive position in the United States, New
Zealand and Australia, grow our presence in Brazil and expand
our sales and operations into additional high growth markets,
such as China and markets throughout South America. We believe
our ability to develop biotechnology seedlings with traits
suited to specific geographies, growing conditions and
end-markets provides us with a significant competitive advantage
as we enter new markets.
|
| |
| |
•
|
Extend Our Technology Leadership. We intend to
extend our technology leadership, grow our broad product
portfolio and commercialize our pipeline of advanced and
biotechnology products by continuing to make significant
investments in research and development and expanding our
current base of research collaborations. Our pipeline of
products currently consists of six advanced and 15 biotechnology
products in various stages of development.
|
| |
| |
•
|
Leverage Our Technology and Business Model to Drive
Profitability. We intend to leverage our leading
technology platform, established customer base and business
model to drive our revenue growth and increased profitability.
Although our revenue decreased from $23.7 million for the
year ended March 31, 2009, to $21.6 million for the
year ended March 31, 2010 due to the economic downturn, our
net loss also decreased from $(15.3) million to
$(14.7) million. We expect our revenues to grow, and our
average selling prices and gross margins to increase, as we
transition our customers to our higher-value products without
having to significantly increase our fixed costs. While we
expect to continue to incur net losses for the next several
years, we believe our scalable business model will enable us to
drive profitability through the introduction of new products and
expansion into new markets with relatively low marginal costs.
|
7
Recent
Developments
We estimate that as of and for the year ended March 31,
2011:
|
|
|
| |
•
|
revenue will be between $25.0 million and
$25.75 million, compared to $21.6 million for the year
ended March 31, 2010;
|
| |
| |
•
|
total seedlings shipped will be approximately 255 million,
compared to 240 million as of March 31, 2010;
|
| |
| |
•
|
the average selling price per 1,000 seedlings will be between
approximately $98 and $100, compared to $90 as of March 31,
2010; and
|
|
|
|
| |
•
|
sales of our open-pollinated, or conventional, seedling products
will represent approximately 56.5% of our revenue, compared to
65.0% as of March 31, 2010, and sales of our elite,
mass-control pollinated, or MCP, and varietal products will
represent approximately 43.5% of our revenue, compared to 35.0%
as of March 31, 2010.
|
The preliminary financial results presented above are subject to
the completion of our financial closing procedures for the year
ended March 31, 2011. Those procedures have not been
completed and we do not anticipate completion of those
procedures until late May 2011. Accordingly, these results may
change and those changes may be material. In particular, we have
provided ranges for the preliminary revenue and average selling
price estimates presented above because we expect that our final
results upon completion of our financial closing procedures may
vary from our preliminary estimates within these ranges. This
preliminary financial data has been prepared by and is the
responsibility of management. PricewaterhouseCoopers LLP has not
audited, reviewed, compiled or performed any procedures with
respect to this preliminary financial data. Accordingly,
PricewaterhouseCoopers LLP does not express an opinion or any
other form of assurance with respect thereto.
Risk
Factors
Our business is subject to many risks and uncertainties, as more
fully described under “Risk Factors” in this
prospectus, which you should be aware before investing in our
common stock. For example:
|
|
|
| |
•
|
We have a limited operating history, which may make it difficult
to evaluate our current business and predict our future
performance.
|
| |
| |
•
|
We may not achieve or sustain positive cash flow in the future.
|
| |
| |
•
|
We have a history of net losses and may not achieve or sustain
profitability in the future.
|
| |
| |
•
|
Our growth depends on customer acceptance of our
technology-enhanced products, particularly our pipeline of
biotechnology products.
|
| |
| |
•
|
Lower than expected product prices may adversely affect our
financial results.
|
| |
| |
•
|
Commercialization of our biotechnology products is dependent on
regulatory approval.
|
| |
| |
•
|
Negative public perceptions relating to the use of biotechnology
for purpose-grown trees may adversely affect customer acceptance
of our biotechnology products.
|
Our
History
We were formed in February 2000 by combining all of the
biotechnology forestry research and development programs of
three leading forest products companies: Fletcher Challenge
Limited (now Rubicon Limited, a New Zealand-based company),
International Paper Company and Westvaco Corporation (now
MeadWestvaco Corporation). Each company independently developed
its own biotechnology forestry research program over decades of
significant investment, and the combination of these programs
provided us with a broad portfolio of intellectual property. The
combination also provided us with a long-term financial
commitment from our stockholders. Following this initial
combination, in October 2007, our stockholders further
contributed to us substantially all of their commercial tree
improvement research programs and seed orchard and nursery
businesses in the United States, New Zealand and Australia.
Our stockholders’ aggregate
8
investment over the past ten years, including the fair value of
the assets contributed, has totaled more than $200 million.
The October 2007 transaction significantly changed our
competitive position, as it provided us with the platform from
which to commercialize the products we develop by giving us
ownership of one of the largest and most diverse repositories of
germplasm in the world, demonstrated production and distribution
capabilities and an established customer base of land owners and
managers. As a result, we immediately transitioned from a
research-based business to a fully integrated commercial
developer and provider of conventional and technology-enhanced
tree seedlings, and began selling conventional seedlings to many
of the same customers previously served by our stockholders. In
our first full fiscal year following the contribution, we
generated $23.7 million in revenue from the global
commercial sales of approximately 278 million seedlings and
also recorded a net loss of $(15.3) million.
Our
Corporate Information
Our predecessor company, ArborGen, LLC, was formed as a Delaware
limited liability company in February 2000. On June 1,
2010, we reincorporated in Delaware as a corporation in
preparation for the initial public offering of our common stock.
Our principal executive office is located at 180 Westvaco
Road, Summerville, South Carolina 29483, and our telephone
number is
(843) 851-4129.
Our website address is www.arborgen.com. We do not incorporate
the information on or accessible through our website into this
prospectus, and you should not consider any information on, or
that can be accessed through, our website as part of this
prospectus.
9
THE
OFFERING
|
|
|
|
Common stock offered by us |
|
shares |
| |
|
|
|
|
| |
|
Common stock to be outstanding after this offering |
|
shares
( shares
if the option to
purchase
additional shares of common stock is exercised in full) |
| |
|
Option to purchase additional shares |
|
We have granted the underwriters an option to purchase up
to additional
shares of our common stock from us. |
|
|
|
|
Use of proceeds |
|
We intend to use the net proceeds from this offering to expand
container production capacity, repay amounts due under our
revolving credit facility and pursuant to loans from
stockholders, potentially exercise our option to purchase a new
manufacturing, research and development laboratory and
headquarters facility and construct related infrastructure, fund
research and development programs and product launches, and for
working capital and other general corporate purposes including
capital expenditures, expansion of other production capabilities
and possibly acquisitions. See the section entitled “Use of
Proceeds.” |
|
|
|
|
Proposed Nasdaq Global Market symbol |
|
“ARBR” |
| |
|
Risk factors |
|
You should read carefully the section entitled “Risk
Factors” for a discussion of factors that you should
consider before deciding to invest in shares of our common stock. |
The number of shares of common stock to be outstanding after
this offering is based
on shares
of our common stock outstanding as of March 31, 2011 and
excludes 1,800,000 shares of our common stock reserved for
future issuance as of March 31, 2011 under our equity
incentive plan.
Except as otherwise indicated, all information in this
prospectus reflects or assumes:
|
|
|
| |
•
|
the filing of our amended and restated certificate of
incorporation and the adoption of our amended and restated
by-laws, which will be in effect upon completion of this
offering; and
|
| |
| |
•
|
no exercise by the underwriters of their option to purchase up
to an
additional shares
of our common stock in this offering.
|
10
SUMMARY
CONSOLIDATED FINANCIAL DATA
The summary consolidated financial data for the years ended
March 31, 2008, 2009 and 2010 have been derived from our
consolidated financial statements audited by
PricewaterhouseCoopers LLP, an independent registered public
accounting firm, and included elsewhere in this prospectus. The
summary consolidated financial data for the nine months ended
December 31, 2009 and 2010 have been derived from our
unaudited consolidated financial statements included elsewhere
in this prospectus. In our opinion, these unaudited consolidated
financial statements have been prepared on a basis consistent
with our audited consolidated financial statements and contain
all adjustments, consisting only of normal and recurring
adjustments, necessary for a fair presentation of such
consolidated financial data. Results for interim periods are not
necessarily indicative of results for a full fiscal year. The
financial data listed under “Other Selected Data”
below has been derived from the audited and unaudited
consolidated financial statements noted above, as well as from
our internal records of our operations. Historical results are
not necessarily indicative of results for future periods. This
summary consolidated financial data should be read in
conjunction with the section entitled “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations” and our consolidated financial statements and
the related notes included elsewhere in this prospectus.
Prior to October 2007, our business was focused on research and
development with no product revenue. In October 2007, our
current stockholders contributed to us substantially all of
their commercial tree improvement research programs and seed
orchard and nursery businesses in the United States, New Zealand
and Australia, which enabled us to start generating operating
revenue. As a result, only the U.S. operations seedling
season (December 2007 to March 2008) was reflected in
our revenue for the year ended March 31, 2008. Sales made
during the New Zealand and Australian operations seedling season
(June 2007 to September 2007) were not part of our revenue
for the year ended March 31, 2008 because they occurred
prior to the October 2007 contribution.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
|
Year Ended March 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(In thousands)
|
|
|
|
|
Consolidated Statement of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
18,182
|
|
|
$
|
23,680
|
|
|
$
|
21,576
|
|
|
$
|
9,583
|
|
|
$
|
11,083
|
|
|
Cost of revenue
|
|
|
15,667
|
|
|
|
15,840
|
|
|
|
16,381
|
|
|
|
6,927
|
|
|
|
7,055
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
2,515
|
|
|
|
7,840
|
|
|
|
5,195
|
|
|
|
2,656
|
|
|
|
4,028
|
|
|
Operating expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development(1)
|
|
|
10,212
|
|
|
|
13,833
|
|
|
|
11,206
|
|
|
|
7,836
|
|
|
|
9,171
|
|
|
Selling, general and administrative(2)(3)
|
|
|
9,709
|
|
|
|
7,599
|
|
|
|
7,139
|
|
|
|
5,194
|
|
|
|
7,866
|
|
|
Depreciation and amortization
|
|
|
679
|
|
|
|
813
|
|
|
|
780
|
|
|
|
665
|
|
|
|
444
|
|
|
Loss on impairment of intangible assets(4)
|
|
|
203
|
|
|
|
643
|
|
|
|
213
|
|
|
|
200
|
|
|
|
154
|
|
|
Restructuring charge(5)
|
|
|
183
|
|
|
|
493
|
|
|
|
101
|
|
|
|
—
|
|
|
|
—
|
|
|
Other operating expense (income)
|
|
|
(387
|
)
|
|
|
(464
|
)
|
|
|
(234
|
)
|
|
|
(185
|
)
|
|
|
(185
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expense
|
|
|
20,599
|
|
|
|
22,917
|
|
|
|
19,205
|
|
|
|
13,710
|
|
|
|
17,450
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(18,084
|
)
|
|
|
(15,077
|
)
|
|
|
(14,010
|
)
|
|
|
(11,054
|
)
|
|
|
(13,422
|
)
|
|
Total interest income (expense), net
|
|
|
3
|
|
|
|
(269
|
)
|
|
|
(646
|
)
|
|
|
(435
|
)
|
|
|
(765
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss(6)
|
|
$
|
(18,081
|
)
|
|
$
|
(15,346
|
)
|
|
$
|
(14,656
|
)
|
|
$
|
(11,489
|
)
|
|
$
|
(14,187
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11
| |
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2010
|
|
|
|
|
Actual
|
|
|
As Adjusted(7)
|
|
|
|
|
(In thousands)
|
|
|
|
|
Consolidated Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
2,133
|
|
|
$
|
|
|
|
Total assets
|
|
|
50,878
|
|
|
|
|
|
|
Long-term debt, net of current
maturities(8)
|
|
|
4,356
|
|
|
|
|
|
|
Total liabilities
|
|
|
32,275
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
18,603
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
|
Year Ended March 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(In thousands, except average selling price and
|
|
|
|
|
per share data)
|
|
|
|
|
Other Selected Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total seedlings shipped
|
|
|
278,537
|
(9)
|
|
|
286,147
|
|
|
|
239,958
|
|
|
|
71,386
|
|
|
|
61,880
|
|
|
Average selling price per 1,000 seedlings(10)
|
|
$
|
65
|
|
|
$
|
83
|
|
|
$
|
90
|
|
|
$
|
134
|
|
|
$
|
179
|
|
|
EBITDA(11)
|
|
$
|
(17,165
|
)
|
|
$
|
(13,676
|
)
|
|
$
|
(12,560
|
)
|
|
$
|
(9,932
|
)
|
|
$
|
(12,423
|
)
|
|
Loss per share(12)
|
|
$
|
(0.92
|
)
|
|
$
|
(0.62
|
)
|
|
$
|
(0.54
|
)
|
|
$
|
(0.43
|
)
|
|
$
|
(0.48
|
)
|
|
Pro forma loss per share(12)
|
|
|
|
|
|
|
|
|
|
$
|
|
|
|
|
|
|
|
$
|
|
|
|
|
|
|
(1)
|
|
The decrease in research and
development expense for the year ended March 31, 2010 as
compared to the year ended March 31, 2009 was attributable
to our cost mitigation program in light of the impact of the
worldwide economic downturn on our primary end-markets. For the
year ending March 31, 2011, we expect that our research and
development spending will return to levels consistent with the
year ended March 31, 2009, and may increase further.
|
| |
|
(2)
|
|
Our selling, general and
administrative expense for the year ended March 31, 2008
included significant legal and accounting fees in connection
with the asset contribution in October 2007.
|
| |
|
(3)
|
|
The decrease in selling, general
and administrative expense for the year ended March 31,
2010 as compared to the year ended March 31, 2009 was
attributable to our cost mitigation program in light of the
impact of the worldwide economic downturn on our primary
end-markets. We expect that our selling, general and
administrative expense will increase as we focus on increasing
our global market share and continuing to build brand awareness
and in preparation for becoming and operating as a public
company.
|
| |
|
(4)
|
|
Impairment of intangible assets is
attributable to the abandonment of patents and
patents-in-progress that are no longer of strategic interest.
The amounts recorded for the years ended March 31, 2008,
2009 and 2010 and the nine months ended December 31, 2009
and 2010 represent impairment charges for the abandonment of
patents-in-progress.
|
| |
|
(5)
|
|
During the years ended
March 31, 2008 and 2009, we undertook several restructuring
actions in connection with the integration of the operations
contributed in 2007. In the year ended March 31, 2010, due
to the economic downturn, we suspended operations at one of our
U.S. nurseries.
|
| |
|
(6)
|
|
On June 1, 2010, we converted
from a non-taxable limited liability company to a taxable
corporation. From our inception to May 31, 2010, we were
treated as a partnership for U.S. income tax purposes and as a
result our income (loss) was passed through to our limited
liability company members. Consequently, income tax expense is
not reflected in our consolidated statement of operations, nor
does the consolidated balance sheet contain an amount for
deferred tax expense in recognition of tax effects of temporary
differences prior to our conversion. Subsequent to our
conversion to a taxable corporation, no income tax benefit has
been reflected due to recording of a full valuation allowance on
our net deferred tax assets.
|
| |
|
(7)
|
|
The as adjusted column in the
consolidated balance sheet data table reflects the effect of our
receipt of estimated net proceeds from the sale
of shares
of our common stock in this offering, at an assumed initial
public offering price of $ per
share, which is the midpoint of the estimated price range set
forth on the cover page of this prospectus, and after deducting
estimated underwriting discounts and offering expenses payable
by us, and the anticipated use of net proceeds from this
offering.
|
| |
|
(8)
|
|
Includes $0.2 million of capital
lease obligations. Does not include $14.1 million of current
portion of obligations or $1.2 million of debt owing to our
stockholders, which was incurred in January 2011.
|
|
|
|
|
(9)
|
|
This amount excludes approximately
26 million seedlings shipped in Australia and New Zealand
during the year ended March 31, 2008 by certain entities
prior to our acquisition thereof in the October 2007 combination
transaction described in the section entitled “Certain
Relationships and Related Party Transactions — Asset
Contribution.”
|
|
|
|
|
(10)
|
|
Average selling price reflects
revenue divided by thousands of seedlings sold. Revenue includes
both revenue from seedling sales and other revenue, including
revenue from government grants of $0, $0 and $1.1 million
for the years ended March 31, 2008, 2009 and 2010,
respectively, and $0.8 and $0.8 million for the nine
months ended December 31, 2009 and 2010, respectively.
|
12
|
|
|
|
(11)
|
|
EBITDA is defined as earnings
before interest, taxes, depreciation and amortization. We use
EBITDA to plan, forecast and monitor our
day-to-day
operating performance and capital structure. We further believe
that providing this information allows investors greater
transparency and a better understanding of our ability to plan
our operating cash requirements and make long-term capital
spending commitments. EBITDA does not represent net loss or cash
flow from operations as those terms are defined by GAAP and does
not necessarily indicate whether cash flows will be sufficient
to fund cash requirements. EBITDA is not a recognized
measurement under GAAP, and investors should not consider EBITDA
as a substitute for measures of our financial performance and
liquidity as determined in accordance with GAAP, such as net
loss, operating income or net cash provided by operating
activities. EBITDA has other limitations as an analytical tool,
when compared to the use of net loss, which is the most directly
comparable GAAP financial measure, including: (i) EBITDA
does not reflect the interest expense we incur as a result of
our debt leverage and (ii) EBITDA does not reflect any
attribution of costs to our operations related to our capital
expenditures through depreciation and amortization charges.
|
The following is a reconciliation of net income to EBITDA:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
|
Year Ended March 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(In thousands)
|
|
|
|
|
Net loss(a)
|
|
$
|
(18,081
|
)
|
|
$
|
(15,346
|
)
|
|
$
|
(14,656
|
)
|
|
$
|
(11,489
|
)
|
|
$
|
(14,187
|
)
|
|
Added back:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation included in cost of revenue
|
|
|
240
|
|
|
|
588
|
|
|
|
670
|
|
|
|
457
|
|
|
|
555
|
|
|
Depreciation and amortization
|
|
|
679
|
|
|
|
813
|
|
|
|
780
|
|
|
|
665
|
|
|
|
444
|
|
|
Interest expense (income), net
|
|
|
(3
|
)
|
|
|
269
|
|
|
|
646
|
|
|
|
435
|
|
|
|
765
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EBITDA
|
|
$
|
(17,165
|
)
|
|
$
|
(13,676
|
)
|
|
$
|
(12,560
|
)
|
|
$
|
(9,932
|
)
|
|
$
|
(12,423
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
|
|
On June 1, 2010, we converted
from a non-taxable limited liability company to a taxable
corporation. From our inception to May 31, 2010, for U.S.
tax purposes, we were treated as a partnership and as a result
our income (loss) was passed through to our limited liability
company members. Consequently, income tax expense is not
reflected on the statements of operations. Subsequent to our
conversion to a taxable corporation, no income tax benefit has
been reflected due to recording of a full valuation allowance on
our net deferred tax assets.
|
|
|
|
|
(12)
|
|
Upon our conversion from a limited
liability company to a corporation on June 1, 2010, our
limited liability company members received a total of
30 million shares of stock for their members’ equity
interests. For the periods prior to conversion, for purposes of
determining the weighted average number of common stock shares
outstanding, we computed the equivalent number of shares based
on the conversion ratio we used on June 1, 2010.
|
|
|
|
|
|
|
The following table presents the
calculation of historical basic and diluted net loss per common
share:
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
|
Year Ended March 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(In thousands, except per share data)
|
|
|
|
|
Net loss(a)
|
|
$
|
(18,081
|
)
|
|
$
|
(15,346
|
)
|
|
$
|
(14,656
|
)
|
|
$
|
(11,489
|
)
|
|
$
|
(14,187
|
)
|
|
Weighted average shares outstanding
|
|
|
19,558
|
|
|
|
24,677
|
|
|
|
27,078
|
|
|
|
26,864
|
|
|
|
29,824
|
|
|
Loss per share, basic and diluted
|
|
$
|
(0.92
|
)
|
|
$
|
(0.62
|
)
|
|
$
|
(0.54
|
)
|
|
$
|
(0.43
|
)
|
|
$
|
(0.48
|
)
|
|
|
|
|
|
|
As discussed under “Use of
Proceeds”, we intend to use approximately
$ of the net proceeds of this
offering to repay debt outstanding under our credit facility.
The pro forma loss per share, basic and diluted, represents loss
per share assuming repayment of this debt as of April 1,
2009. Had this debt been repaid as of April 1, 2009, we
would have avoided interest expense of approximately
$ and
$ for the year ended
March 31, 2010 and the nine months ended December 31,
2010, respectively. At an assumed initial public offering price
of $ per share, which is the
midpoint of the estimated price range set forth on the cover
page of this
prospectus, shares
are required to pay down this amount of debt. These shares have
been considered outstanding for all periods in the calculation
of pro forma loss per share.
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended March 31, 2010
|
|
|
Nine Months Ended December 31, 2010
|
|
|
|
|
Actual
|
|
|
Adjustment
|
|
|
Pro forma
|
|
|
Actual
|
|
|
Adjustment
|
|
|
Pro forma
|
|
|
|
|
(In thousands, except per share data)
|
|
|
|
|
Interest income(expense), net
|
|
$
|
(646
|
)
|
|
|
|
|
|
|
|
|
|
$
|
(765
|
)
|
|
|
|
|
|
|
|
|
|
Net loss(a)
|
|
$
|
(14,656
|
)
|
|
|
|
|
|
|
|
|
|
$
|
(14,187
|
)
|
|
|
|
|
|
|
|
|
|
Net loss per common share, basic and diluted
|
|
$
|
(0.54
|
)
|
|
|
|
|
|
|
|
|
|
$
|
(0.48
|
)
|
|
|
|
|
|
|
|
|
|
Shares used in computing net loss per common share, basic and
diluted
|
|
|
27,078
|
|
|
|
|
|
|
|
|
|
|
|
29,824
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
|
|
On June 1, 2010, we converted
from a non-taxable limited liability company to a taxable
corporation. From our inception to May 31, 2010, for U.S.
tax purposes, we were treated as a partnership and as a result
our income (loss) was passed through to our limited liability
company members. Consequently, income tax expense is not
reflected on the statements of operations. Subsequent to our
conversion to a taxable corporation, no income tax benefit has
been reflected due to recording of a full valuation allowance on
our net deferred tax assets.
|
13
RISK
FACTORS
Investing in our common stock involves a high degree of risk.
You should carefully consider the following risks and
uncertainties, together with all other information in this
prospectus, including our consolidated financial statements and
related notes, before investing in our common stock. Any of the
risk factors we describe below could adversely affect our
business, financial condition or results of operations. The
market price of our common stock could decline if one or more of
these risks or uncertainties actually occurs, causing you to
lose all or part of your investment. Certain statements below
are forward-looking statements. See “Forward-Looking
Statements” in this prospectus.
Risks
Related to Our Business and Our Industry
We
have a limited operating history, which may make it difficult to
evaluate our current business and predict our future
performance.
From February 2000 to October 2007, our operations focused on
research and development. Following the contribution in October
2007 of substantially all of our stockholders’ commercial
tree improvement research programs and seed orchard and nursery
businesses in the United States, New Zealand and Australia, we
immediately transitioned from a research-based business to a
fully integrated commercial developer and provider of
conventional and technology-enhanced tree seedlings, and began
selling conventional seedlings to many of the same customers
previously served by our stockholders. Our limited history as an
operating company may make it difficult for you to evaluate our
current business, our future performance or the viability of our
business model or products. Any assessments you make about our
future success or viability may not be as accurate as they might
be if we had a longer operating history. To date, we have
derived our revenues principally from sales of conventional
open-pollinated seedlings. During the three-year period ended
March 31, 2010, we have increased the percentage of revenue
derived from sales of our higher-value products, which consist
of our “elite” open-pollinated conventional seedlings,
our MCP seedlings and our varietal seedlings, which are produced
using a proprietary lab-based process that identically
replicates selected superior performing individual trees. Sales
of our higher-value products have grown from 5.7% of U.S.
revenue in the year ended March 31, 2008 to 24.2% in the
year ended March 31, 2010. In addition, we have not yet
commercialized any of our biotechnology products, and our
efforts to expand the commercial forestry market with advanced
and biotechnology products may not be successful. Even if we are
able to successfully commercialize these products, it is
difficult to predict the impact of these products on the
industry or our business and results of operations. As a result,
recent historical growth may not provide an accurate
representation of the growth we may experience in the future,
which will make it difficult to evaluate our future prospects.
We may
not achieve or sustain positive cash flow in the
future.
To develop our advanced and biotechnology products, we have made
significant investments in research and development. We have had
negative cash flow before financing activities of
$27.9 million, $14.4 million and $13.5 million in
the years ended March 31, 2008, 2009 and 2010,
respectively, and $17.0 million in the nine months ended
December 31, 2010. The aggregate investment of our
stockholders over the past 10 years, including the fair
value of the assets contributed, totaled more than
$200 million. We anticipate that we will continue to have
negative cash flow for the next several years as we continue to
incur substantial research and development and selling, general
and administrative expenses. Our business will also require
significant amounts of working capital to support our growth and
our fluctuating capital requirements across the planting and
harvesting cycles of our tree seedlings. Our inability to
generate positive cash flow or raise additional capital on
reasonable terms would materially and adversely affect our
business, financial condition and results of operations.
We
have a history of net losses and may not achieve or sustain
profitability in the future.
We have not been profitable in any fiscal period since we were
formed and we may never achieve profitability. Even if we
achieve profitability in the future, we may not be able to
maintain or increase our level of profitability. We incurred net
losses of $(18.1) million, $(15.3) million and
$(14.7) million in the years ended
14
March 31, 2008, 2009 and 2010, respectively, and a net loss
of $(14.2) million in the nine months ended
December 31, 2010. From our inception through
December 31, 2010, we have recorded annual net losses
aggregating $(155.2) million. As a result of our
expectation that we will continue to incur net losses for the
next several years, we have recorded a full valuation allowance
on our U.S. net deferred tax assets. We intend to continue
to incur significant research and product development expenses
and selling, general and administrative expenses to grow our
business. Our efforts to grow our business may be more costly
than we expect, and we may not be able to increase our revenue
enough to offset our operating expenses. We will need to
generate and sustain increased revenue levels in future periods
in order to become profitable. We expect to incur net losses for
the next several years, and net losses may increase in the year
ending March 31, 2011 as we continue to research, develop
and commercialize our pipeline of advanced and biotechnology
products and increase our general and administrative functions
to support our growing operations.
Our
growth depends on customer acceptance of our technology-enhanced
products, particularly our pipeline of biotechnology
products.
Our growth is highly dependent on the adoption by our customers
of our technology-enhanced tree seedling products, particularly
our pipeline of biotechnology products. In the year ended
March 31, 2010, our higher-value products, which consist of
our elite open-pollinated conventional seedlings, our MCP
seedlings and our varietal seedlings, comprised 24.2% of
U.S. revenue. We do not currently sell any biotechnology
products. We expect to be the first company in our markets to
introduce biotechnology products for sale to the commercial
forestry industry. There can be no assurance that any of our new
advanced and biotechnology products will achieve improved
performance levels relative to our existing product offerings.
Each customer’s actual return on investment will depend on
a number of variables, including that customer’s land
management strategy, weather conditions and fluctuations in the
market price of timber or pulp, and we can not guarantee that
each customer will achieve improved returns. We expect to sell
our advanced products and, in particular, our biotechnology
seedling products at significantly higher prices per seedling
than our customers currently pay for our seedling products. To
be successful, we will need to convince our customers that the
higher price of these products are justified by the greater
returns on investment that these seedling products provide to
our customers. We cannot guarantee that our efforts to educate
our customers about the benefits of our technology-enhanced
products will be effective. Even if effective, these efforts may
take a significant amount of time and customers may delay large
purchases of these products until the benefits are demonstrated
at additional test sites.
Factors that may influence our customers’ adoption of our
products include:
|
|
|
| |
•
|
the value that our customers attribute to our products, which
may differ from our own for many reasons, including willingness
to sacrifice higher long-term value for lower product prices in
the short term;
|
| |
| |
•
|
the willingness of customers in the Southeastern United States
to purchase eucalyptus, which has not been widely grown in that
region;
|
| |
| |
•
|
the desire of potential purchasers of biotechnology products to
secure certification requirements that are only granted to land
owners and managers that produce forestry products that do not
include biotechnology enhancements;
|
| |
| |
•
|
the willingness of our customers to pay higher prices for
seedling products;
|
| |
| |
•
|
the value that third parties, such as land appraisers, assign to
land planted with our advanced and biotechnology products;
|
| |
| |
•
|
perceptions about the use of biotechnology plants; and
|
| |
| |
•
|
the impact of economic and industry conditions on the purchasing
decisions of and rate of adoption by our customers.
|
15
If our customers do not accept our new technology-enhanced
seedling products, we may fail to recognize any expected
increases in our profit margin through our technology-enhanced
products or in the volume of our technology-enhanced products
sold and our business and results of operations may be adversely
affected.
Lower
than expected product prices may adversely affect our financial
results.
Prices for our future advanced and biotechnology products are
projected to be significantly higher than prices for seedling
products that we currently sell. Customers may be unwilling to
purchase these seedling products because of their higher selling
prices. We base the pricing of our products on our assessment of
the value that these products provide to our customers. If our
customers attribute a lower value to our products than we do,
they may not be willing to pay the premium prices we charge.
Pricing levels may be negatively affected if our advanced and
biotechnology products are less successful than we expect. In
addition, if our competitors are able to develop competitive
products and sell them at lower prices, we may be forced to
lower our prices. Lower than expected prices would adversely
impact our financial condition and results of operations.
Commercialization
of our biotechnology products is dependent on regulatory
approval.
There are certain regulatory requirements affecting the testing,
planting and commercialization of our biotechnology tree
seedlings in each of the markets in which we operate. Currently,
there are no similar regulatory requirements materially
affecting our open-pollinated, mass-control pollinated and
varietal seedling products. In the United States, the USDA must
first review and deregulate our biotechnology seedling products.
The USDA deregulation process for biotechnology products is a
costly,
multi-year
process, with no guarantee of success. The length of the
deregulation process varies based on a number of factors,
including the extent of the supporting information required, the
nature and extent of review by the USDA, including the type and
scope of the environmental review conducted, and the number and
types of public comments received. For example, after the
initial filing of a petition for deregulation or the
resubmission of a petition, in response to science-based
concerns, the USDA may ask for additional data, including data
on new areas of inquiry that might require us to conduct
additional field tests or analyses, which may cause delays in
the deregulation process. Furthermore, in granting a petition
for deregulation, the USDA may limit the total acres planted or
the areas in which our biotechnology tree seedlings can be
planted. Alternatively, the USDA or other regulators may impose
costly monitoring requirements on the planting of our
biotechnology tree seedlings. In addition, following
deregulation of any of our biotechnology products, subsequent
litigation may result in a court ordering the USDA to revoke
such product’s deregulated status and conduct further
environmental analysis prior to issuing a new decision on
deregulation. In New Zealand, Australia and Brazil, the
commercialization of biotechnology products is subject to
approval by the regulatory authorities with approval processes
that are subject to similar risks. Any delays in obtaining or
failure to obtain deregulation or regulatory approval, as the
case may be, for any of the biotechnology products in our
pipeline could delay or prevent the commercialization of our
biotechnology products.
Before the USDA will review and deregulate our biotechnology
tree seedlings, the USDA requires us to obtain permits to plant
and test our biotechnology products, and there are similar
permitting requirements in New Zealand, Australia and Brazil. In
determining whether to grant a field test permit and what
conditions to impose, regulators consider any significant
impacts that field tests may have on the environment and on
endangered or threatened species. In the United States, the
permitting process for the initial field tests typically ranges
from two to four months, but, as we have experienced, this time
period can be significantly longer for novel products or
circumstances. If we are not able to obtain the necessary field
test permits or if there are significant delays in the
permitting process, the commercialization of our biotechnology
tree products may be delayed or prevented and our business and
results of operations may be adversely affected. A prolonged
delay in the regulatory process could adversely impact our
expected financial performance.
On July 1, 2010, the Center for Biological Diversity and
several other parties filed suit against the
U.S. Department of Agriculture, or USDA, and the
USDA’s Animal and Plant Health Inspection Service, or
APHIS, in the U.S. District Court, Southern District of
Florida, challenging the issuance by APHIS of several field test
permits for our freeze-tolerant tropical eucalyptus product. In
the complaint, which was amended on
16
August 10, 2010 and again on October 11, 2010, the
plaintiffs allege that APHIS did not follow appropriate
processes and procedures when issuing certain permits to us. The
plaintiffs seek relief through a variety of remedies, including
judicial declaration that APHIS violated the National
Environmental Policy Act of 1969, the Endangered Species Act of
1973 and other federal statutes, revocation of certain field
test permits for our freeze-tolerant tropical eucalyptus
product, enjoining the flowering of our freeze-tolerant tropical
eucalyptus product in current field tests and requiring APHIS to
prepare an environmental impact statement that addresses the
overall cumulative impacts of all permits and petitions covering
our freeze-tolerant tropical eucalyptus product. On
September 7, 2010 and October 25, 2010, the U.S.
federal government filed answers to the amended complaints. On
September 7, 2010, we filed a motion to intervene in this
case in order to protect our interests and support the
government’s position that the field test permits were
properly issued, which the court granted on October 19,
2010. We are now a party to this litigation, and we filed our
answer to the amended complaint on October 27, 2010. This
litigation may result in increased costs, delay or denial of our
request for field test permits for our freeze-tolerant tropical
eucalyptus product or revocation of our existing permits. There
may be additional claims filed by third parties against the USDA
or other regulatory agencies relating to the permitting or
deregulation of this or our other biotechnology products.
Changes
to existing regulations, or the need to adapt to new regulations
in the jurisdictions into which we expand, may delay, increase
the cost of or prevent the commercialization of our
biotechnology products.
The development and commercialization of any biotechnology
seedling products is subject to regulation in each of the
jurisdictions in which we expect to sell these products. We
cannot predict whether any such jurisdiction will modify its
regulations with respect to biotechnology products. Problems
with any biotechnology product, forestry or otherwise, could
lead to increased scrutiny or regulation for our biotechnology
tree seedling products. Limitations on the development of our
biotechnology seedling products could be imposed, including
restrictions on flowering or field testing that could delay,
prevent or make more costly the development and eventual
commercialization of such products. Public concern with
biotechnology products may also lead to increased regulation.
Any modifications or limitations may adversely affect our
ability to introduce our biotechnology tree seedlings to
commercial markets. We anticipate expanding into other large and
fast-growing forestry markets, including China. If we expand
into these markets, we may face additional regulatory barriers
and requirements that are different than those in the
jurisdictions in which we currently operate. New regulations or
changes to existing regulations may delay, increase the cost of
or prevent the commercialization of our biotechnology products,
and our inability to adapt to regulatory changes may have a
material adverse effect on our biotechnology business and
results of operations.
Negative
public perceptions relating to the use of biotechnology for
purpose-grown trees may adversely affect customer acceptance of
our biotechnology products.
Our freeze-tolerant tropical eucalyptus and other biotechnology
products in our pipeline are not created using traditional
breeding methods but are developed in a laboratory by altering
the genetic material of trees. Some individuals and groups,
including environmental non-governmental organizations, have
expressed concerns relating to all biotechnology-enhanced
plants, including trees. The commercial success of our
biotechnology products may be adversely affected by claims that
genetically modified products pose risks of damage to the
environment. On occasion, there has been vandalism and
destruction of property of companies in the biotechnology
industry. In the event that public attitudes adversely impact
public or customer acceptance of our products, we may be forced
to incur increased expenses, delays or other impediments to our
product development and commercialization programs. In addition,
public concern may lead to increased regulation or legislation
or litigation against government regulators concerning prior
regulatory decisions, which could lead to greater regulation of
biotechnology products or delays in customer acceptance.
17
The
successful commercialization of our advanced and biotechnology
products depends on our ability to produce high-quality products
cost-effectively on a large scale.
The production of our MCP products requires the ability to
produce seed from our MCP orchards in sufficient quantity to
meet seedling demand. As MCP seed production from year to year
is dependent upon factors beyond our control (i.e. changes in
seasonal weather and environmental conditions), we cannot
guarantee that we will be able to produce sufficient MCP seed in
any given year to fully meet demand for this product. We
estimate that during the year ended March 31, 2011, sales
of our MCP products will account for approximately 68.8% of our
revenue in Australia and New Zealand and approximately 16.0% of
our revenue in the United States, and we expect the percentage
of revenue we derive from our MCP products to continue to
increase over time. As a result, a failure to meet customer
demand could result in lost sales and could harm our reputation
and customer relationships.
The cost-effective production of high-volume quantities of some
of our varietal and biotechnology products depends on our
ability to scale our existing or subsequent complex production
processes. We cannot assure you that our existing or subsequent
production processes will enable us to meet our large-scale
production goals cost-effectively for the varietal and
biotechnology products in our pipeline. Even if we are
successful in developing our high volume manufacturing
capability and processes, we may not be able to do so in a
manner that avoids significant delays and cost overruns. If we
are unable to increase our production capacity in a timely or
cost-effective manner, our ability to achieve profitability in
these products will be severely constrained. If we are unable to
maintain the quality of our products as we increase our
production capacity, including through the expected use of
third-party nursery growers, we may experience reductions in
customer demand, higher costs and increased seedling write-offs.
Additionally, if our production process results in the presence
of unintended biotechnology material in our seedling products,
we may be subject to potential liability or regulatory
compliance actions such as seedling destruction or product
recalls, which could negatively affect our business and results
of operations.
The
development of products through advanced and biotechnology
processes is complex and uncertain, and we may not continue to
succeed in developing tree seedlings with improved genetic
traits, or the rate of the improvement may be slower than we
expect.
The complex and uncertain nature of our advanced and
biotechnology tree seedling products creates significant
challenges to product development and manufacturing. For
example, in the course of developing our advanced and
biotechnology seedling products, we may not be successful in
developing tree seedlings with improved genetic traits. Any
product candidate may fail to enter field tests, grow
successfully during field tests or exhibit the intended traits
necessary to advance to the next stage of our product
development process. No company in our markets currently offers
a biotechnology-enhanced forestry product. The development
process is long and may be slower than we expect. There is no
guarantee that our product candidates will be commercialized or,
following commercialization, will successfully exhibit the
improved traits. Our inability to continue to develop advanced
and biotechnology seedling products could negatively affect our
business and results of operations.
If we
fail to continue to develop a pipeline of and commercialize new
products, our future financial performance would be materially
adversely affected.
Our business model is premised upon our ability to continue to
develop and commercialize new seedling products in a timely
manner with demonstrable improvements in growth rates, yields,
stress-tolerance, wood quality and processing efficiency. The
processes of tree improvement, biotechnology trait discovery,
field testing and product development are lengthy. Only a very
small percentage of the genes and germplasm that we test are
selected for field testing, and only a very small percentage of
seedlings tested are selected for commercialization.
Additionally, we may be forced to delay or abandon development
of pipeline products in the event of greater than anticipated
development costs, technical difficulties, inability to prove
the original concept, regulatory obstacles, competition or lack
of demand. In addition, to the extent we introduce seedling
products that we have not previously grown on a large scale, we
may experience production difficulties as we establish best
practices for the planting, cultivation and harvesting of these
new products. Delays or the failure to offer new products at
18
competitive prices will adversely affect our financial
performance. As new products enter the market, our existing
products may become obsolete and customer preference for new
products may result in short product life cycles as we begin to
commercialize second and third generations of our advanced and
biotechnology products. Decreases in demand for our conventional
products may require that we write down our inventory of these
seeds.
The development of new products has required, and will require,
that we expend significant financial and management resources.
We have incurred, and we expect to continue to incur,
significant research and development expenses. If we are unable
to devote adequate resources to develop new products or cannot
otherwise successfully develop new products or enhancements that
meet customer requirements on a timely basis, our products could
lose market share, our revenue could decline and we could
experience operating losses. In addition, we believe that we
will benefit from being the first company to commercialize
biotechnology forestry products in our markets. Consequently, if
we are not able to fund extensive research and development
activities or successfully commercialize our products on a
timely basis, the growth of our business and results of
operations could be harmed by competing products entering the
market.
We may
not generate significant revenue as a result of our current
research and development efforts for several years, if at
all.
The development of advanced and biotechnology seedling products
requires significant investment. Our research and development
expense was $10.2 million, or 56% of revenue, in the year
ended March 31, 2008, $13.8 million, or 58% of
revenue, in the year ended March 31, 2009, and
$11.2 million, or 52% of revenue, in the year ended
March 31, 2010. Our future plans include significant
investments in research and development and related product
opportunities. However, we may not receive significant revenue
from these investments for several years, if at all.
The
growing biopower market may not develop as anticipated and may
not generate the demand for
purpose-grown
trees that we anticipate.
We intend to market our products as a potential source of
biomass for the growing biopower market. However, our ability to
fully enter into and participate in the biopower market may be
hindered by a variety of factors outside our control. For
example, the number and capacity of biopower processing plants
may not reach anticipated levels, resulting in less demand for
biomass sources such as our products than anticipated. In
addition, biopower plants may rely on biomass sources other than
purpose-grown trees more heavily than expected. There is no
guarantee that government policies will encourage the use of
purpose-grown trees as a source of biomass. If demand for
purpose-grown trees for the biopower industry is less than
anticipated for these reasons or for any other reasons, we may
not achieve our anticipated growth in the biopower industry and
our business and results of operations would be adversely
affected.
Our
business is impacted by changes in general economic conditions
in the countries and end-markets in which we sell our products,
and a prolonged downturn could affect the demand for our
products and our financial performance.
Economic conditions in the United States, New Zealand, Australia
and Brazil could adversely affect our efforts to achieve
profitability. The purchasing decisions of our customers and
their willingness to pay premium prices for our higher-value
products are impacted by their economic health, the availability
of credit and the conditions in the end-markets that they
currently serve. For example, during the year ended
March 31, 2010, each of the end-markets our customers serve
was impacted by the worldwide economic downturn, which resulted
in lower prices for pulp and wood products, leading to reduced
seedling planting by our customers as they chose to harvest
fewer of their existing trees. These markets may not recover
quickly, fully or at all. We expect growth in several of these
end-markets to be driven by economic growth in emerging markets,
such as China. However, this growth may not be as high as
expected. In addition, we expect to charge premium prices for
our higher-value products. A prolonged downturn may cause our
customers to reduce spending and discourage them from investing
in our higher-value products.
19
Our business and results of operations may be impacted by
changes in market dynamics over which we have no control,
including:
|
|
|
| |
•
|
with respect to the pulp and paper market, a continued reduction
in the demand for paper in developed economies, particularly if
this decline is not offset by growth in emerging economies;
|
| |
| |
•
|
with respect to the wood products market, a prolonged downturn
in U.S. housing starts, home renovations and industrial
production, particularly if this downturn is not offset by
growth in emerging economies;
|
| |
| |
•
|
with respect to the biopower market, fluctuations in market
conditions that reduce the amount of investment in alternative
energy sources or lead to the adoption of biomass sources other
than purpose-grown trees for biopower generation;
|
| |
| |
•
|
with respect to the charcoal market in Brazil, changes in
regulations that impact the use of purpose-grown trees for
charcoal production or the use of charcoal for steel production;
|
|
|
|
| |
•
|
with respect to the biofuels market, a downturn in the economy
or significant decreases in the cost of oil, either of which may
lead to decreased investment in alternative energy research and
production; and
|
|
|
|
| |
•
|
with respect to all end-markets in which we sell our products,
changes to regulations designed to address climate change and
promote the recycling of carbon dioxide into oxygen and other
byproducts.
|
Given the inherent time required to plant, grow and harvest
trees, our customers make purchasing decisions based on their
perception of future market conditions. As a result, there may
be a delay in the impact to us of an improvement in general
economic conditions. We cannot predict the timing or duration of
any downturns in the general economy or in the end-markets that
our customers serve.
The
emerging cellulosic biofuels market may not develop as
anticipated and may not generate the demand for purpose-grown
trees that we anticipate.
We intend to market our products as a potential source of
biomass for the emerging cellulosic biofuels market. However,
the production and commercialization of woody biomass for
biofuels may not be feasible for a variety of reasons. For
example, the market for advanced biofuels, including cellulosic
biofuels, is heavily influenced by foreign, federal, state and
local government regulations and policies. If the cost of oil
decreased significantly, the outlook for the long-term supply of
oil to the United States improved or gasoline prices declined
over extended periods of time, the perception in government and
the private sector that cheaper, more readily available energy
alternatives should be developed or produced could be reduced
and could reduce the demand for biofuels. Changes to existing or
adoption of new domestic or foreign federal, state and local
legislative initiatives that impact the production, distribution
or sale of biofuels may reduce the demand for our products. The
cellulosic biofuels market is still at an early stage of
development, and to date, cost-effective processes for the use
of woody biomass for biofuels are still under development. These
processes may never be developed or commercialized, and future
research may determine that a source other than purpose-grown
trees is the most productive source of biomass for the
production of biofuels. There can be no assurance that anyone
will be able or willing to develop and operate biofuel
production plants at commercial scale or that any biofuel
facilities can be profitable. Increased production,
transportation or other costs may reduce the cost-effectiveness
of woody biomass as an alternative to fossil fuels. In addition,
current development of the biofuels industry is heavily
dependent on government targets and incentives, and there is no
guarantee this support will continue.
Because
of the lead time required to grow trees, we base our production
of tree seedlings and other expenses on anticipated levels of
customer demand. If actual demand is significantly different
than expected, our business and operating results will
suffer.
To fulfill the anticipated demand for our products, we invest in
capital expenditures in advance of actual customer orders, based
on estimates of future demand. We experience 12- to
24-month
lead times for delivery of our seedlings depending on the type
of seedling. Our conventional and MCP products must grow from
seeds to seedlings, typically over a period of approximately
nine months, followed by a harvesting period of approximately
three months. In the case of varietal pine seedlings, this lead
time typically increases to a total of up to 24 months due
to the longer production time required for the varietal
manufacturing process, and this will also typically be the
20
case for our future biotechnology products. We base the number
of seedlings we plant on our expectations of customer demand,
which take into account customer input, purchasing history,
existing long-term contracts, feedback from our sales force and
market conditions. In New Zealand, we receive deposits from most
of our customers for their seedling orders. Our financial
performance will be impacted by our ability to accurately
predict demand for our products in future years, as well as our
ability to budget appropriately for anticipated operating
expenses, which do not vary directly with sales and are
difficult to adjust in the short term. Most of our tree seedling
products are sold pursuant to annual sales contracts or purchase
orders, which may not be renewed, affecting our ability to
accurately predict product sales from year to year.
Unanticipated changes in demand or economic conditions that
affect our customers’ end-markets could adversely affect
our results due in part to the limitations on our ability to
make adjustments to the size of our harvest and our ability to
adjust operating expenses in tandem with reductions in sales. As
a result, increases in customer demand following our planting
season can lead to lost sales and could harm our customer
relationships and overall reputation. Also, the demand for our
products may be lower than we estimated. For example, during the
year ended March 31, 2010, our end-markets were impacted by
the worldwide economic downturn, which resulted in reduced
seedling planting by our customers as they chose to harvest
fewer of their existing trees. Such decreases in customer demand
can lead to unsold inventory at year-end that must be written
off. In addition, a sales shortfall during a fiscal quarter, and
in particular the fourth quarter of a fiscal year, could have a
disproportionate effect on our operating results and cause us to
fail to meet the expectations of equity research analysts and
investors, potentially causing the trading price of our common
stock to decline.
We are
party to an ongoing lawsuit, and may in the future be named in
additional litigation, which could require significant
management time and attention, and could result in significant
expense and an unfavorable outcome, either or both of which
could have a material adverse effect on our business, financial
condition, results of operations and cash flows.
From time to time, we may be subject to litigation. In
connection with any litigation in which we are involved, we may
incur costs and expenses in connection with defending ourselves
and the payment of any settlement or judgment in connection
therewith if there is an unfavorable outcome. The expense of
defending litigation may be significant, the amount of time to
resolve lawsuits is unpredictable and defending ourselves may
divert management’s attention from the
day-to-day
operations of our business, any of which could materially
adversely affect our business, results of operations and cash
flows. In addition, an unfavorable outcome in any such
litigation could have a material adverse effect on our business,
results of operations and cash flows.
In November 2010, several of our employees filed a complaint in
South Carolina state court. The suit named as defendants us, our
current directors, certain of our current officers, certain of
our former directors and officers and a number of other parties.
The suit alleges that the plaintiffs and certain of our current
employees have rights to future potential payouts based on a
document that the plaintiffs allege provides for larger
potential payouts to employees upon a sale of the Company or of
all or substantially all of its assets than the incentive plan
that we actually adopted. The plaintiffs seek relief through a
declaratory judgment, specific performance, an accounting and
monetary damages. This proceeding is in its early stages. While
we intend to defend ourselves vigorously against the claims made
in this dispute, we are unable to predict the outcome of this
proceeding. We cannot estimate the amount or range of possible
losses that we may incur in connection with this proceeding. In
connection with this proceeding, we will incur costs and
expenses defending ourselves and those other defendants which we
had obligated ourselves to indemnify. These costs and expenses
could materially and adversely affect our financial condition,
results of operations and cash flows. If this proceeding is
adversely decided, it could have a material adverse effect on
our business, financial condition and results of operations.
Weather
conditions, natural disasters or other catastrophes could result
in a disruption of our operations and negatively impact our
results of operations.
Weather conditions, natural disasters or other catastrophes can
affect the timing of planting and harvesting of our seedlings,
as well as the quality, cost and volumes of seedlings that we
are able to produce and sell, which will affect our results of
operations. The occurrence of heavy rain storms, hail storms,
droughts, freezing conditions, hurricanes, fires or other
natural disasters could severely damage our seed orchards, the
setting and growing of our
21
seedlings, the timing of our annual harvest of seedlings, our
field tests or our product development facilities as well as our
headquarters. Weather conditions and natural disasters also
affect decisions by our customers about the types and amounts of
tree seedlings to plant and the yields of our seedlings once
they have been planted by our customers. In addition,
disruptions that cause delays by our customers in harvesting or
planting can result in movement of orders to a future quarter,
disrupting our operations and negatively impacting our results
in the affected quarter. We do not insure our seedlings, seed
orchards or field tests, and therefore any large-scale loss of
seedlings, or trees in our field tests, would disrupt our
operations and adversely affect our results of operations and
our future product development. In addition, we do not carry
liability insurance coverage on product performance, therefore,
if we were subject to claims for failure of performance, we
would have to bear the costs of our defense and our results of
operations would be adversely affected.
Our
business is highly seasonal, which typically results in losses
and net use of cash in the first three quarters of the fiscal
year.
Our seedling revenue is highly seasonal. In the year ended
March 31, 2010, approximately 56% of our revenue was
recorded in the fourth quarter, which corresponds to the
harvesting season in the United States. Planting for our
U.S. operations occurs primarily in April and May and
harvesting and delivery to customers occurs between December and
March. This seasonality is partially balanced by the growing
cycle in our operations in New Zealand and Australia, where
planting occurs primarily in October and November and harvesting
and delivery to customers occurs between June and September. We
expect this balancing effect to increase as our international
operations grow and new products, such as our tropical
eucalyptus seedling product, which has a different growing and
sales cycle, are introduced. This seasonality tends to result in
higher production expenses, a net loss and a net use of cash
during the first three quarters of our fiscal year and
significant generation of revenue and net cash generated in our
fourth fiscal quarter. In addition, quarter to quarter, we may
experience fluctuation in the mix of our products resulting in
variations in average selling prices, revenue and gross margins.
As a result of this seasonality, we believe that
quarter-to-quarter
comparisons of our operating results are not necessarily
meaningful and that these comparisons cannot be relied upon as
indicators of future performance.
Our
results of operations may fluctuate and could be difficult to
predict.
Our results of operations may fluctuate from period to period or
within certain periods as a result of a number of factors, many
of which are outside of our control. You should not rely on our
past results as an indication of future performance. The
following factors, as well as other factors described in this
prospectus, could affect our quarterly results:
|
|
|
| |
•
|
the volume and timing of seedlings harvested, which may be
impacted by weather conditions;
|
| |
| |
•
|
the volume of seedlings sold, which may be impacted by economic
conditions in the market;
|
| |
| |
•
|
the timing of the launch of new products;
|
| |
| |
•
|
the timing of recognizing revenue as a result of revenue
recognition rules;
|
| |
| |
•
|
the timing of customer acceptance of our new products;
|
| |
| |
•
|
rapid price increases of raw materials;
|
| |
| |
•
|
employee compensation expense in the fiscal quarter immediately
following this offering; and
|
| |
| |
•
|
fluctuations in interest rates generally.
|
In addition, any of these factors could negatively impact our
results of operations and cause us to fail to meet the financial
performance expectations of securities industry research
analysts or investors in future periods, which would likely
cause the market price of our common stock to decline.
We
depend on a limited number of customers for a significant
portion of our revenue and cannot be certain they will
consistently purchase our tree seedling products in the
future.
We depend, and expect to continue to depend, on a limited number
of customers for a significant portion of our revenue. In the
year ended March 31, 2010, we generated 10.4% of our
revenue from sales to Hancock
22
Natural Resource Group Inc. and its affiliates, and our top ten
customers accounted for approximately 36% of our total revenue.
In the future, a small number of customers may continue to
represent a significant portion of our total revenue in any
given period. We cannot be certain that such customers will
consistently purchase our products at any particular rate over
any subsequent period. A loss of, or any credit issues related
to, any of these customers could adversely affect our financial
performance.
We
have experienced a period of significant growth in recent years
and manage operations in multiple geographies, and if we were
unable to manage this growth and breadth in the future it could
have a material adverse effect on our business, the quality of
our products and services, and on our ability to retain key
personnel.
We have experienced a period of significant growth in recent
years, which has placed increased demands on our management and
other resources and will continue to do so in the future. We may
not be able to maintain or accelerate our current growth rate,
manage our expanding operations effectively or achieve planned
growth on a timely or profitable basis. We also conduct our
business across several countries, including the United States,
New Zealand, Australia and Brazil, and may expand geographically
in the future. These diversified, global operations place
increased demands on our resources and require us to
substantially expand the capabilities of our administrative and
operational resources. As our operations expand domestically and
internationally, we will need to continue to manage multiple
locations and additional relationships with various customers,
collaborators and other third parties. Managing our growth
effectively will involve, among other things:
|
|
|
| |
•
|
continuing to retain, motivate and manage our existing employees
and attract and integrate new employees;
|
| |
| |
•
|
developing, implementing and improving our operational,
financial, accounting and other internal systems and controls on
a timely basis; and
|
| |
| |
•
|
maintaining and developing our various support functions
including human resources, information technology, safety, legal
and corporate communications.
|
If we are unable to manage our growth effectively, there could
be a material adverse effect on our ability to maintain or
increase revenue and profitability, the quality of our products
and our ability to retain key personnel.
Loss
of or damage to our germplasm repository would significantly
impair our research and development efforts.
We own one of the largest and most diverse repositories of
germplasm in the world. Our research and development activities
are based on the various germplasm to which we have access. Loss
of or damage to our germplasm repository, whether through
failure of our systems or otherwise, would significantly impair
our operations. Although we carefully restrict access to and
cryopreserve our germplasm at our research facilities and in
back-up
facilities to protect this valuable resource, we cannot
guarantee that our efforts to protect our germplasm repositories
will be sufficient. The destruction of a significant portion of
our germplasm repository would adversely affect our business and
results of operations.
Our
patents and other protective measures may not adequately protect
our technology, and any loss of our intellectual property or
unauthorized use of our technology by others could materially
adversely affect our sales and results of
operations.
Our intellectual property portfolio is a valuable asset of our
business. To protect our proprietary rights, we rely on a
combination of patent (including plant patent), plant
breeders’ rights, copyright, trademark and trade secret
laws, confidentiality procedures and contractual provisions, and
physical security measures in jurisdictions in which our
products are produced. In the United States and in other
countries, we have received a number of patents and have filed
additional patent applications for various aspects of our
technologies, processes and products. We have also sought
patent-like protection through plant breeders’ rights or
similar rights in other countries. In addition, we generally
enter into confidentiality agreements with our employees,
23
consultants, contractors and collaboration partners. Such
patents and agreements and various other measures we take to
protect our intellectual property from use by others may not be
effective for various reasons, including the following:
|
|
|
| |
•
|
we may fail to apply for patents on important technologies or
product candidates in a timely fashion, or at all;
|
| |
| |
•
|
for various reasons, including the existence of conflicting
patents or defects in our applications, we cannot predict which
of our pending patent applications, if any, will result in
issued patents;
|
| |
| |
•
|
we may not be able to protect some of our innovations, and even
if we receive patent or similar protection the scope of our
intellectual property rights may offer insufficient protection
from competition or unauthorized use;
|
| |
| |
•
|
our products may rely on the technology of others and,
therefore, require us to obtain intellectual property licenses
from other parties in order for us to develop or commercialize
our products;
|
| |
| |
•
|
the patents we have been granted may not include claims covering
our products and services, may lapse or expire, be challenged,
narrowed, invalidated or circumvented because of the
pre-existence of similar patented or unpatented intellectual
property rights or for other reasons, or deemed unenforceable or
abandoned;
|
| |
| |
•
|
our confidentiality agreements may not effectively prevent
disclosure or use of confidential information and may not
provide an adequate remedy in the event of unauthorized
disclosure or use;
|
| |
| |
•
|
competitors may be issued patents from applications that were
unknown to us prior to their publication or issuance,
potentially impacting the value of our intellectual property,
our ability to operate our business or the value of our
commercial or pipeline products;
|
| |
| |
•
|
the costs associated with enforcing patents, confidentiality and
invention agreements or other intellectual property rights may
make enforcement prohibitive;
|
| |
| |
•
|
even if we enforce our rights aggressively, injunctions, fines
and other penalties may be insufficient to deter violations of
our intellectual property rights; and
|
| |
| |
•
|
other persons may independently develop proprietary technology,
information and techniques that are functionally equivalent or
superior to our proprietary intellectual property and techniques
but do not infringe or conflict with our patented or unpatented
proprietary rights, or may use their own proprietary
intellectual property rights to block us from taking full
advantage of the market.
|
Effective intellectual property rights protection may be
unavailable, limited, or subject to change in some countries
where we operate or plan to do business. In addition, our
efforts to protect our proprietary rights in foreign
jurisdictions may not be adequate. Many companies have
encountered significant problems in protecting and defending
intellectual property rights in certain foreign jurisdictions.
The legal systems of certain countries, particularly certain
developing countries, do not favor the enforcement of patents
and other intellectual property protection, particularly those
relating to biotechnology. This could make it difficult for us
to stop the infringement of our patents or misappropriation of
our other intellectual property rights in some countries.
We rely in part on trade secret protection and contractual
restrictions to protect our confidential and proprietary
information and processes. We have taken various measures to
protect our trade secrets and other confidential or proprietary
information, including requiring new employees and consultants
to execute confidentiality agreements upon the commencement of
employment or consulting engagements with us. However, trade
secrets are difficult to protect, and our security measures may
not be effective or sufficient to prevent circumvention or
disclosure. The individuals who signed our agreements may
intentionally or unintentionally breach their confidentiality
and/or
non-use obligations. Such agreements may be deemed
unenforceable, not provide adequate remedies, or be the subject
of disputes that may not be resolved in our favor. Third parties
could reverse engineer our agricultural biotechnology or
otherwise gain access to our trade secrets, and others may
independently develop substantially similar, analogous or
alternative information and technologies to ours, for example,
through the isolation and development of homologous genes with
the same function or different genes with similar function to
those we use, in which case we may not have the ability to stop
them from using or commercializing our technology. Costly and
time-consuming litigation could be
24
necessary to enforce and determine the scope of our proprietary
rights. Courts outside the United States may be less willing to
protect trade secrets, and it may be difficult to prove or
enforce a claim that a third party had or has illegally obtained
and used our trade secrets. Failure to obtain or maintain trade
secret protection could adversely affect our competitive
business position.
Proceedings to enforce our intellectual property domestically
and in foreign jurisdictions could result in substantial costs
and divert our efforts and attention from other aspects of our
business. Moreover, in countries where we do not have adequate
patent or other intellectual property protection, third parties
could use our inventions or commercialize our products or
technologies. For example, while to date we are not aware of
misappropriation of our products, in the future some land owners
or managers may use cuttings containing our biotechnology
traits, which could prevent us from realizing the full value of
our intellectual property, particularly outside the United
States. Accordingly, our efforts to protect our intellectual
property rights in such countries may be inadequate.
From time to time, we may seek to protect and enforce our
intellectual property and proprietary rights against third
parties. Policing unauthorized use of intellectual property can
be difficult and expensive. The fact that we have intellectual
property rights, including registered intellectual property, may
not guarantee success in our attempts to enforce these rights
against third parties. We may not be aware of infringement or
misappropriation, or elect not to seek to prevent it. When we
seek to enforce our rights, we may be subject to claims that our
intellectual property rights are invalid, otherwise
unenforceable, or are licensed to the party against whom we are
asserting the claim. In addition, our assertions of intellectual
property rights may result in the other party seeking to assert
various claims against us, including its own alleged
intellectual property rights, claims of unfair competition, or
others.
We
rely in part on licenses from third parties for certain advanced
technologies, and changes to or loss of these licenses could
curtail access to certain technology.
In the past, we have established license agreements with several
collaborators and third parties in the application of forestry
technology. We have received these licenses from our
stockholders, academic institutions and other companies in this
industry. Some of our licensors and collaborators have the right
to control the filing, prosecution, maintenance and defense of
all licensed intellectual property and, if a third party
infringes on any of the licensed intellectual property, to
control any legal or other proceedings instituted against that
third party for infringement. As a result, our licensors or
collaborators may take actions or make decisions relating to
these matters with which we do not agree or which could harm our
business. In addition, we may disagree with our licensors or
collaborators as to rights to intellectual property we develop,
or their research programs or commercialization activities, or
our licensors or collaborators might become our competitors or
enter into agreements with our competitors, which could
adversely affect our competitive business position. If we are
unable to maintain or renew these license agreements in the
future on commercially reasonable terms, or if we are unable to
obtain additional licenses to add to or replace them as the
technology advances, or if such rights are licensed to
competitors, our access to certain advanced technology and
intellectual property may be curtailed, and our business,
financial condition and results of operations may be adversely
affected.
If we
infringe, or are alleged to infringe, the intellectual property
rights of third parties, it may adversely affect our business,
financial condition and results of operations.
The biotechnology industry is characterized by frequent and
extensive litigation regarding patents and other intellectual
property rights. Many biotechnology companies have employed
intellectual property litigation as a way to gain a competitive
advantage. Third parties could claim that the making, using,
selling, offering for sale or importation of our products,
processes or technologies infringe on their proprietary rights.
It is possible that infringement claims may be asserted as the
number of products and competitors in our market increases. In
addition, to the extent that we gain greater visibility and
market exposure as a public company, we face a greater risk of
being the target of intellectual property infringement claims.
We cannot be certain that the conduct of our business does not
and will not infringe intellectual property or other proprietary
rights of others in the United States or in foreign
jurisdictions. Any claim of infringement by a third party,
25
even those without merit, could cause us to incur substantial
costs defending against the claim and could distract our
management from our business. Furthermore, a party making such a
claim, if successful, could secure a judgment that requires us
to pay substantial damages. A judgment could also include an
injunction or other court order that could prevent us from
offering our products or using our processes and technologies.
In addition, we might be required to seek a license and pay
royalties for the use of such intellectual property, which may
not be available on commercially reasonable terms or at all.
Alternatively, we may be required to develop non-infringing
technology, which could require significant effort and expense
and ultimately may not be successful. Any of these events could
seriously harm our business, operating results and financial
condition.
Our
future success depends on our ability to retain key
personnel.
Our future success will depend to a significant extent on the
continued service and performance of our senior management team.
With an average of 26 years of industry experience, our
management team brings expertise in forestry and biotechnology,
as well as a track record of successfully navigating
biotechnology products through the USDA regulatory process to
commercialize them. The loss or unavailability of key members of
our senior management team could impact the execution of our
business plan and harm our ability to maintain important
business relationships. The replacement of key members of our
senior management team likely would involve significant time and
costs.
If we
are unable to recruit or retain qualified scientific personnel,
we may be significantly delayed in developing our conventional
and biotechnology products.
Competition for qualified scientific personnel is extremely
intense among agricultural biotechnology and other
technology-based businesses. This competition will intensify if
other companies emerge to develop biotechnology products for the
commercial forestry industry. We may not be able to recruit and
retain such personnel at compensation levels consistent with our
existing compensation and salary structure. In addition, in
making employment decisions, job candidates often consider the
value of the equity they are to receive in connection with their
employment. Therefore, significant volatility in the price of
our stock after this offering may adversely affect our ability
to attract or retain personnel. If we fail to identify, attract,
train, integrate and retain highly qualified and motivated
personnel, our reputation could suffer, we may be unable to
develop and commercialize our products and our business,
financial condition and results of operations could be adversely
affected.
Our
international operations expose us to additional business risks,
and failure to manage these risks may adversely affect our
overall operating results.
Approximately 24% of our revenue in the year ended
March 31, 2010 was generated from operations outside the
United States, and we have significant operations in New
Zealand, Australia and Brazil. We plan to expand our existing
international operations in the future, and we may enter
additional geographic regions as well, including emerging
markets such as China. Our international operations are subject
to risks related to the differing legal, political, social and
regulatory requirements and economic conditions of many
countries, including:
|
|
|
| |
•
|
difficulties and costs of staffing and managing our
international operations and the increased travel,
infrastructure and legal compliance costs associated with
multiple international locations;
|
| |
| |
•
|
potential imposition by foreign countries of additional
withholding taxes or other taxes on our foreign income, tariffs
or other restrictions on foreign trade or investment, including
currency exchange controls and restrictions on our ability to
repatriate funds;
|
| |
| |
•
|
general economic conditions in the countries in which we
operate, which could have an adverse effect on our earnings from
operations in those countries;
|
| |
| |
•
|
change in government policies that are currently in favor of our
products and end-markets, limiting our development and
commercialization programs or customer demand;
|
| |
| |
•
|
reduced protection for intellectual property rights in some
countries;
|
| |
| |
•
|
imposition of, or unexpected adverse changes in, foreign laws or
regulatory requirements, including those pertaining to import
and export duties and quotas, trade and employment restrictions;
|
26
|
|
|
| |
•
|
risks associated with expansion into developing economies;
|
| |
| |
•
|
increased risk of fraud, including in emerging markets;
|
| |
| |
•
|
fluctuations in currency exchange rates; and
|
| |
| |
•
|
political and economic instability, war or acts of terrorism.
|
Our failure to manage additional business risks associated with
international sales and operations may have a material adverse
effect on our business and results of operations.
Our
presence in Brazil depends in large part on our development
collaborations with the country’s leading pulp producers
and exporters, and our failure to successfully manage these
relationships could prevent us from developing and
commercializing our products in Brazil.
We have product development collaborations with the leading pulp
producers and exporters in Brazil representing more than 50% of
the pulp production in Brazil. Maintaining and further
developing our relationships with these companies is key to the
continued development of our presence in the Brazilian market.
The loss of these relationships would adversely affect our
business and results of operations. Furthermore, we may have
limited or no control over the amount or timing of resources
that either collaborator is able or willing to devote to our
collaborative efforts. Any of these collaborators may fail to
perform their obligations as expected. In addition, our current
collaboration agreements terminate in 2011 unless they are
amended or otherwise extended, and we cannot guarantee that we
will be able to extend them. Furthermore, our collaborators may
breach or terminate their agreements with us or otherwise fail
to conduct their collaborative activities successfully and in a
timely manner. Further, our collaborators may not utilize
products arising out of our collaborative arrangements or devote
sufficient resources to the development, manufacturing,
marketing or sale of these products. Disagreements with our
collaborators could develop and any conflict with a collaborator
could reduce our ability to enter into future collaboration
agreements and negatively impact our relationships with one or
more existing collaborators. We may disagree with our
collaborators as to rights to intellectual property we develop,
or their research programs or commercialization activities.
Moreover, our collaborators might become our competitors or
enter into agreements with our competitors.
Foreign
currency exchange rate fluctuations could adversely affect our
results.
We own and operate production
and/or
research facilities in New Zealand and Australia and have
development collaborations in Brazil. For the years ended
March 31, 2008, 2009 and 2010, 2%, 25% and 24% of our
revenue was derived from customers located in New Zealand and
Australia and denominated in local currencies. In addition, we
have a significant amount of assets and liabilities that are
denominated in New Zealand dollars and are exposed to foreign
currency movements. As of December 31, 2010, 27% and 20% of
our total assets and liabilities were denominated in New Zealand
dollars. As a result of these transactions, we may be exposed to
foreign currency fluctuations when we convert the results of our
non-U.S. operations
from their functional currency into U.S. dollars for
inclusion in our consolidated financial results. As our
collaborations in Brazil develop, we expect our exposure to the
Brazilian real to increase. During the past few decades,
the exchange rate between the U.S. dollar and the Brazilian
real has fluctuated significantly. There can be no
assurance that the Brazilian real, the New Zealand dollar
or the Australian dollar will not significantly appreciate or
depreciate against the U.S. dollar in the future. Changes
in the rate of exchange of foreign currencies in relation to the
U.S. dollar and other currencies may adversely impact our
U.S. dollar denominated results.
Competitors
and potential competitors may gain market share at our expense
or may develop products and technologies that are superior to
ours.
Our conventional tree seedling products compete with products
from private and state-owned nurseries and seedling companies
that primarily produce seedlings for external sales, including
White City Nursery, LLC and Bell Brothers Nurseries Ltd. in the
Southeastern United States. Our competitors in New Zealand and
Australia are primarily owner-operators, rather than large
commercial nurseries. For example, in New Zealand, we compete
with Rangiora Nursery Limited and Cambridge Forest and Native
Nursery Limited. In addition, in the United States,
27
large forestry companies, such as Weyerhaeuser Company and Plum
Creek Timber Company, Inc. produce tree seedlings primarily for
their own consumption. Similarly in Australia, large companies
and state organizations such as Gunns Limited, Hancock Victoria
Plantations and Forestry New South Wales produce tree
seedlings primarily for their own consumption. As a result, we
compete with these companies to meet their internal demand and
to the extent they sell seedlings to third parties. In the case
of varietal loblolly and radiata pine, we compete with CellFor
in the United States and Forest Genetics Cellfor Limited in New
Zealand. Weyerhaeuser Company and Plum Creek Timber Company,
Inc. are active in the development of MCP tree seedlings in the
United States, which we believe will be used largely for their
internal consumption, and International Forest Company also
produces MCP tree seedlings for sale in the market. In New
Zealand, we compete with PF Olsen Group for the sale of MCP
seedlings. In addition, independent nurseries can purchase
mass-pollinated seed from Proseed New Zealand Limited, a tree
seed supplier to the New Zealand forest industry.
There are other companies in geographies in which we do not
currently operate that are developing and selling advanced and
biotechnology seedling products. There are also other
biotechnology companies and other research-based organizations
that are developing technology that could in the future be
applied to trees. We can provide no assurance that these
companies will not enter our markets or start developing
products that compete with ours. Some of these companies may be
substantially larger than us and may have substantially greater
resources than we do, and there can be no assurance that our
competitors will not consolidate in the industry to gain greater
resources. Our advanced products may be rendered less
competitive or uneconomical by technological advances or
entirely different approaches developed by our current and
future competitors.
We
rely on third parties to produce some of our tree seedlings, and
these third parties may not perform
satisfactorily.
For certain of our varietal and containerized products in the
United States, which currently account for a relatively small
percentage of total seedlings sold, we outsource seedling
production to an independent contractor. As we expand our
operations generally and our sale of containerized products in
particular, we expect to outsource a larger percentage of our
seedling production to third parties, although we may encounter
difficulty finding third-party providers that will be able to
meet our seedling production requirements. Our use of third
parties for seedling production reduces our control over
planting, cultivation and harvesting of the seedlings. These
independent contractors may not complete activities on schedule,
or may not produce our seedlings in accordance with our strict
guidelines intended to ensure the high quality of our products.
If these independent contractors do not successfully carry out
their contractual duties, meet expected deadlines or produce
quality seedlings, a portion of our harvest may be delayed or
otherwise adversely affected. This would hamper our ability to
deliver seedling products to our customers, which could result
in loss of sales and damage to our reputation. In addition, if
these independent contractors do not successfully carry out
their contractual duties or meet expected deadlines, we may need
to replace them, either with another third-party provider or
with our internal resources, either of which would cause us to
incur additional costs and adversely affect our business and
results of operations. We may also find that using third parties
for the production of our tree seedlings is not cost-effective.
Our
estimates of the number of replacement seedlings required for
customers may be materially incorrect and our actual costs may
be higher, causing revenue to vary from period to
period.
Occasionally, our customers may receive damaged seedlings. We
may replace those seedlings depending on the specific facts and
circumstances. Accordingly, we record an allowance for estimated
replacement costs at the time of sale. We estimate our future
replacement costs using historical experience and input from our
customers. However, actual replacement costs could vary from our
allowance estimates for a number of reasons, including, among
others, unusually severe weather patterns, particularly those
occurring during our harvest season. If actual costs are
significantly higher, or we adjust the estimates we use to
establish these allowances, our cost of revenue could vary from
period to period.
28
We
receive part of our funding through government grants, and a
decrease in government support could negatively affect our
financial results.
In the year ended March 31, 2010, we received
$1.0 million in government grant funding. While we expect
to secure government grant funding in the future, based solely
on currently secured grants, government funding will decrease
over the next several years. Although we may in the future
secure additional grants, there is no guarantee that we will be
able to do so. The level of government funding is unpredictable
and subject to a variety of factors, including the political
process. In addition, as a recipient of government funding, we
are subject to government audits, employment standards and
diversity requirements, which may require management attention
and expenditures to ensure compliance.
Our
business could be harmed by systems failures or other
unanticipated business interruptions.
Telecommunications failures or other unanticipated catastrophes,
such as computer viruses or other cyber-attacks or terrorist
attacks, at any of the locations in which we do business, could
cause interruptions in our operations. System failures could
cause disruption in our laboratory processes and damage to our
valuable germplasm repositories. Disruption in transportation
infrastructure could cause delays in shipments to our customers,
which could result in the loss of sales and damage to our
reputation. In addition, even in the absence of direct damage to
our operations, these events could have a significant impact on
our customers’ businesses, which in turn could result in a
negative impact on our results of operations. Extensive or
multiple disruptions in our operations or our customers’
businesses due to unanticipated catastrophes could have a
material adverse effect on our results of operations.
We
have had significant deficiencies in our internal control over
financial reporting. Any future material weaknesses or
significant deficiencies in our internal controls could result
in a material misstatement in our financial statements as well
as result in our inability to timely file periodic reports as
required by federal securities laws, which could have a material
adverse effect on our business and stock price.
We have had significant deficiencies in our internal control
over financial reporting that related to the adequacy of our
financial and accounting organization support for our financial
accounting and reporting needs. These deficiencies mainly
resulted from insufficient disbursement oversight and other
accounting controls in overseas operations and in bank covenant
reporting. While we have implemented procedures designed to
remediate these deficiencies, we cannot be certain that we will
not in the future have material weaknesses or significant
deficiencies in our internal control over financial reporting,
or that we will successfully remediate any that we find. Future
weaknesses or deficiencies in our internal controls could result
in a material misstatement in our annual or interim consolidated
financial statements or cause us to fail to meet our obligations
to file periodic financial reports with the SEC. We also may not
be able to conclude on an ongoing basis that we have effective
internal control over financial reporting as contemplated by
Section 404 of the Sarbanes-Oxley Act of 2002, as amended,
or our independent registered public accounting firm may issue
an adverse opinion on the effectiveness of our internal control
over financial reporting. Any of these failures could result in
adverse consequences that could materially and adversely affect
our business, including potential action by the SEC against us,
possible defaults under our debt agreements, stockholder
lawsuits, delisting of our stock and general damage to our
reputation.
Covenants
in our credit agreements may significantly restrict our
business.
We currently maintain a working capital line of credit in the
United States and a term loan in New Zealand through our New
Zealand subsidiary. These credit facilities are described in the
section entitled “Management’s Discussion and Analysis
of Financial Condition and Results of Operations-Liquidity and
Capital Resources-Credit Facilities.” Under these credit
facilities, we are subject to a variety of business and
financial covenants that may significantly restrict our
business, including restrictions on incurring additional debt,
effecting a merger or consolidation with a third party and
creating liens. We are also required to maintain certain annual
net worth, tangible net worth, fixed charge coverage and
loan-to-value
ratios. We were not in compliance with our annual net worth and
tangible net worth covenants under our U.S. credit facility as
of December 31, 2010. We have obtained a waiver from the
bank as of February 4, 2011, which waives any
29
remedies available to the bank for lack of compliance with these
covenants as of December 31, 2010 and for the five month
period commencing on January 1, 2011 and ending on the
expiration of this line of credit on May 31, 2011. Any
future credit facilities may have similar, or more burdensome,
covenants and restrictions. If we default on either of our
credit agreements, our business and results of operations will
be adversely affected.
We may
be unable to obtain additional financing to grow our business,
develop or enhance our products or respond to competitive
pressures.
We may need to raise additional funds in the future in order to
grow our business. Any required additional financing may not be
available on terms acceptable to us, or at all. If we raise
additional funds by issuing equity securities, you may
experience significant dilution of your ownership interest, and
the newly-issued securities may have rights senior to those of
the holders of our common stock. If we raise additional funds by
obtaining loans from third parties, our interest expense would
increase. If additional financing is not available when required
or is not available on acceptable terms, we may be unable to
successfully develop or enhance our offerings through
acquisitions or otherwise in order to take advantage of business
opportunities or respond to competitive pressures, which could
have a material adverse effect on our offerings, revenue,
results of operations and financial condition. We have no plans,
nor are we currently considering any proposals or arrangements,
written or otherwise, to acquire a business, technology, product
or service. In the past, we have relied in large part on funding
from our stockholders, including through capital contributions
and stockholder loans. Following this offering, we do not expect
our stockholders to continue these financing activities and we
may be more dependent on accessing financing in the market.
To the
extent that we acquire complementary technologies, products or
businesses in the future, we may experience difficulty
integrating those additional acquisitions.
As a public company, we believe we have greater opportunities to
make acquisitions of, or significant investments in,
complementary companies, products or technologies, although no
acquisitions or investments are currently pending or planned. We
cannot guarantee you that we will be able to successfully
complete or integrate any business, products, technologies or
personnel that we might acquire or seek to acquire in the
future, and our failure to do so could harm our business.
Furthermore, any future acquisitions, if completed, would
subject us to many risks, including:
|
|
|
| |
•
|
diversion of management’s attention during the acquisition
and integration process;
|
| |
| |
•
|
costs, delays and difficulties of integrating the acquired
company’s operations, products, technologies, systems and
personnel into our existing operations and organization;
|
| |
| |
•
|
difficulties in maintaining uniform standards, controls,
procedures and policies;
|
| |
| |
•
|
adverse impact on earnings as a result of amortizing the
acquired company’s intangible assets or impairment charges
related to write-downs of goodwill related to acquisitions;
|
| |
| |
•
|
potential issuances of equity securities to pay for
acquisitions, which may be dilutive to existing stockholders;
|
| |
| |
•
|
potential loss of customers or key employees of acquired
companies;
|
| |
| |
•
|
impact on financial condition due to the cost or timing of the
acquisition or failure to meet operating expectations for
acquired businesses; and
|
| |
| |
•
|
assumption of unknown liabilities of the acquired company.
|
Any acquisitions of businesses, technologies, products or
services may not generate sufficient revenue to offset the
associated costs of the acquisitions or may result in other
adverse effects.
30
Risks
Related to Our Common Stock
Our
stock price may fluctuate significantly.
Prior to this offering, you could not buy or sell our common
stock publicly. An active public market for our common stock may
not develop or be sustained after the completion of this
offering. We will negotiate and determine the initial public
offering price with the underwriters based on several factors.
This price may vary from the market price of our common stock
after this offering. You may be unable to sell your shares of
common stock at or above the initial offering price. The market
price of our common stock could fluctuate significantly after
this offering. Factors that could cause volatility in the market
price of our common stock include the other risks in this
section, and in particular:
|
|
|
| |
•
|
market conditions affecting our customers’ businesses,
including general conditions in the global pulp and paper, wood
products, biopower, charcoal and biofuels end-markets;
|
| |
| |
•
|
developments concerning intellectual property rights;
|
| |
| |
•
|
severe storms and natural disasters affecting our ability to
conduct our business;
|
| |
| |
•
|
comments by securities analysts, including the publication of
their estimates of our operating results;
|
| |
| |
•
|
actual and anticipated fluctuations in our operating results;
|
| |
| |
•
|
changes in existing laws, and government regulations and
policies;
|
| |
| |
•
|
actions of stockholders, including sales of shares by our
directors and executive officers;
|
| |
| |
•
|
additions or departures of key personnel; and
|
| |
| |
•
|
developments concerning current or future strategic alliances or
acquisitions.
|
In recent years, the stock market has experienced significant
volatility, particularly with respect to technology stocks. The
volatility of technology stocks often does not relate to the
operating performance of the companies represented by the stock.
These and other factors may cause the market price and demand
for our common stock to fluctuate substantially, which may limit
or prevent investors from readily selling their shares of common
stock and may otherwise negatively affect the liquidity of our
common stock. In addition, in the past, when the market price of
a stock has been volatile, holders of that stock have instituted
securities class action litigation against the company that
issued the stock. If any of our stockholders brought a lawsuit
against us, we could incur substantial costs defending the
lawsuit. Such a lawsuit could also divert the time and attention
of our management from other business concerns.
Our
principal stockholders will exercise significant control over
our company.
After this offering, our stockholders, International Paper
Company, MeadWestvaco Corporation and Rubicon Limited, will
beneficially own, in the aggregate, shares representing
approximately % of our outstanding
capital stock. Although we are not aware of any voting
arrangements that will be in place among these stockholders
following this offering, if these stockholders were to choose to
act together, as a result of their stock ownership, they would
be able to influence our management and affairs and control all
matters submitted to our stockholders for approval, including
the election of directors and approval of any merger,
consolidation or sale of all or substantially all of our assets.
This concentration of ownership may have the effect of delaying
or preventing a change in control of our company and might
affect the market price of our common stock.
Conflicts
of interest may arise because some of our directors are
representatives of our principal stockholders.
Messrs. Burton, Hundley, Liebetreu, Moriarty, Munson and
Watkins, who are representatives of our stockholders, serve on
our board of directors. As representatives of our stockholders,
these directors are subject to the non-competition provisions of
our stockholders agreement, which provide that, subject to the
provisions of the license agreements between us and our three
stockholders described below under “Certain Relationships
and Related Party Transactions,” none of our stockholders
may, during the time that such
31
stockholder remains a stockholder of our company or for five
years thereafter, engage in the business of genetically
engineered trees anywhere in the world or engage in other
tree-related business in any of the United States, New
Zealand or Australia. Notwithstanding the foregoing, our
stockholders may acquire other entities that participate in the
restricted businesses, as long as they are limited to certain
geographies and are incidental to the principal business
and/or
entity being acquired, and our stockholders may conduct research
and development activities relating to tree breeding, production
and related technologies, including genetic engineering of
trees, but may not engage in related commercialization or sale
activities. Although our directors and officers have a duty of
loyalty to us under Delaware law and our amended and restated
certificate of incorporation, transactions that we enter into in
which a director or officer has a conflict of interest are
generally permissible so long as (1) the material facts
relating to the director’s or officer’s relationship
or interest as to the transaction are disclosed to our board of
directors and a majority of our disinterested directors, or a
committee consisting solely of disinterested directors, approves
the transaction, (2) the material facts relating to the
director’s or officer’s relationship or interest as to
the transaction are disclosed to our stockholders and a majority
of our disinterested stockholders approves the transaction or
(3) the transaction is otherwise fair to us. Under our
certificate of incorporation, representatives of our
stockholders are not required to offer to us any transaction
opportunity of which they become aware and could take any such
opportunity for themselves or offer it to other companies in
which they have an investment, unless such opportunity is
expressly offered to them solely in their capacity as a director
of ours.
Future
sales of shares by existing stockholders could cause our stock
price to decline.
If our existing stockholders sell, or indicate an intent to
sell, substantial amounts of our common stock in the public
market after the
180-day
contractual
lock-up and
other legal restrictions on resale discussed in this prospectus
lapse, the trading price of our common stock could decline
significantly and could decline below the initial public
offering price. Moreover, three of our stockholders own large
blocks of shares. We cannot predict the effect, if any, that
future public sales of these shares or the availability of these
shares for sale will have on the market price of our common
stock. Based
on
on shares outstanding as of March 31, 2011, upon the
completion of this offering, we will have
outstanding shares
of common stock, assuming no exercise of outstanding stock
appreciation rights. Of these
shares, shares
of common stock, plus any shares sold pursuant to the
underwriters’ option to purchase additional shares, will be
immediately freely tradable, without restriction, in the public
market. Our officers, directors and current stockholders have
executed lock-up agreements preventing them from selling any
stock they hold for a period of 180 days from the date of
this prospectus, subject to certain limited exceptions and
extensions described under the section entitled
“Underwriting.” The representatives of the
underwriters may, in their sole discretion, permit our officers,
directors and current stockholders to sell shares prior to the
expiration of these
lock-up
agreements.
After the
lock-up
agreements pertaining to this offering expire, an
additional shares
will be eligible for sale in the public market. In addition,
shares subject to outstanding stock appreciation rights under
our equity incentive plans and shares reserved for future
issuance under our equity incentive plans will become eligible
for sale in the public market in the future, subject to certain
legal and contractual limitations. Moreover, 180 days after
the completion of this offering, holders of
approximately shares
of our common stock will have the right to require us to
register these shares under the Securities Act of 1933, as
amended, pursuant to a registration rights agreement. If our
existing stockholders sell substantial amounts of our common
stock in the public market, or if the public perceives that such
sales could occur, this could have an adverse impact on the
market price of our common stock, even if there is no
relationship between such sales and the performance of our
business.
We
will have broad discretion in how we use the net proceeds of
this offering. We may not use these proceeds effectively, which
could affect our results of operations and cause our stock price
to decline.
We will have considerable discretion in the application of the
net proceeds of this offering. We currently intend to use the
net proceeds from this offering to expand container production
capacity, repay amounts due under our revolving credit facility
and pursuant to loans from stockholders, potentially exercise
our option to purchase a new manufacturing, research and
development laboratory and headquarters facility and construct
32
related infrastructure, fund research and development programs
and product launches, and for working capital and other general
corporate purposes including capital expenditures, expansion of
other production capabilities and possibly acquisitions. We
expect to continue to expend significant funds for research and
product development and funds for selling, general and
administrative expenses. As a result, investors will be relying
upon management’s judgment with only limited information
about our specific intentions for the use of the balance of the
net proceeds of this offering. We may use the net proceeds for
purposes that do not yield a significant return or any return at
all for our stockholders. In addition, pending their use, we may
invest the net proceeds from this offering in a manner that does
not produce income or that loses value.
Provisions
of Delaware law, our charter documents and our loan agreements
could delay or prevent an acquisition of our company, even if
the acquisition would be beneficial to our stockholders, and
could make it more difficult for you to change
management.
Provisions of Delaware law and our amended and restated
certificate of incorporation and amended and restated by-laws,
which will be effective upon the completion of this offering,
may discourage, delay or prevent a merger, acquisition or other
change in control that stockholders may consider favorable,
including transactions in which stockholders might otherwise
receive a premium for their shares. These provisions may also
prevent or delay attempts by stockholders to replace or remove
our current management or members of our board of directors.
These provisions include:
|
|
|
| |
•
|
a classified board of directors;
|
| |
| |
•
|
limitations on the removal of directors;
|
| |
| |
•
|
advance notice requirements for stockholder proposals and
nominations;
|
| |
| |
•
|
the inability of stockholders to act by written consent or to
call special meetings;
|
| |
| |
•
|
the ability of our board of directors to make, alter or repeal
our amended and restated by-laws; and
|
| |
| |
•
|
the authority of our board of directors to issue preferred stock
with such terms as our board of directors may determine.
|
The affirmative vote of the holders of at least 75% of our
shares of capital stock entitled to vote, and not less than 75%
of the outstanding shares of each class entitled to vote thereon
as a class, is generally necessary to amend or repeal the above
provisions that are contained in our amended and restated
certificate of incorporation. Also, absent approval of our board
of directors, our amended and restated by-laws may only be
amended or repealed by the affirmative vote of the holders of at
least 75% of our shares of capital stock entitled to vote.
In addition, upon the closing of this offering, we will be
subject to the provisions of Section 203 of the Delaware
General Corporation Law, which limits business combination
transactions with stockholders of 15% or more of our outstanding
voting stock that our board of directors has not approved. These
provisions and other similar provisions make it more difficult
for stockholders or potential acquirers to acquire us without
negotiation. These provisions may apply even if some
stockholders may consider the transaction beneficial to them.
As a result, these provisions could limit the price that
investors are willing to pay in the future for shares of our
common stock. These provisions might also discourage a potential
acquisition proposal or tender offer, even if the acquisition
proposal or tender offer is at a premium over the then current
market price for our common stock.
We do
not intend to pay cash dividends. We have never paid dividends
on our capital stock and we do not anticipate paying any
dividends in the foreseeable future. Consequently, any gains
from an investment in our common stock will likely depend on
whether the price of our common stock increases.
We have not paid dividends on any of our capital stock to date
and we currently intend to retain our future earnings, if any,
to fund the development and growth of our business. In certain
circumstances, we are prohibited by our loan agreements from
paying cash dividends without the prior written consent of our
lenders. In addition, any future indebtedness that we may incur
could preclude us from paying dividends. As a result, capital
appreciation, if any, of our common stock will be your sole
source of gain for the foreseeable
33
future. Consequently, in the foreseeable future, you will likely
only experience a gain from your investment in our common stock
if the price of our common stock increases.
An
active trading market for our common stock may not develop, and
you may not be able to resell your shares at or above the
initial public offering price.
Prior to this offering, there has been no public market for
shares of our common stock. Although we have applied to have our
shares of common stock listed on the Nasdaq Global Market in
connection with this offering, an active trading market for our
shares may never develop or be sustained following this
offering. The initial public offering price of our common stock
will be determined through negotiations between us and the
underwriters. This initial public offering price may not be
indicative of the market price of our common stock after this
offering. In the absence of an active trading market for our
common stock, investors may not be able to sell their common
stock at or above the initial public offering price or at the
time that they would like to sell.
We
will incur significant increased costs as a result of operating
as a public company, and our management will be required to
devote substantial time to new compliance
initiatives.
We have never operated as a stand-alone public company. As a
public company, we will incur significant legal, accounting and
other expenses that we did not incur as a private company. As a
public company, we will be subject to rules and regulations that
regulate corporate governance practices of public companies,
including the Securities Exchange Act of 1934, as amended, the
Sarbanes-Oxley Act of 2002, as amended, and rules promulgated by
Nasdaq. We expect that compliance with these public company
requirements will make some activities more time consuming and
may result in a diversion of management’s time and
attention from revenue-generating activities. For example, we
will create new board committees, adopt new internal controls
and disclosure controls and procedures, and devote significant
management resources to our SEC reporting requirements. A number
of those requirements will require us to carry out activities we
have not done previously. For example, beginning with our Annual
Report on Form 10-K filed after our fiscal year ended
March 31, 2012, we will need to document and test our
internal control procedures, our management will need to assess
and report on our internal control over financial reporting and
our registered public accounting firm will need to issue an
opinion on that assessment and the effectiveness of those
controls. Furthermore, if we identify any issues in complying
with those requirements (for example, if we or our registered
public accounting firm identify a material weakness or
significant deficiency in our internal control over financial
reporting), we may be required to devote additional management
attention to rectify those issues, and the existence of those
issues could adversely affect us, our reputation or investor
perceptions of us.
Investors
in this offering will pay a much higher price than the book
value of our common stock.
If you purchase common stock in this offering, you will pay more
for your shares than the amounts paid by existing stockholders
for their shares. You will incur immediate and substantial
dilution of $ per share,
representing the difference between our pro forma net tangible
book value per share after giving effect to this offering and an
assumed initial public offering price of
$ per share, which is the midpoint
of the estimated price range set forth on the cover page of this
prospectus.
If
equity research analysts do not publish research or reports
about our business or if they issue unfavorable commentary or
downgrade our common stock, the price of our common stock could
decline.
The trading market for our common stock will rely in part on the
research and reports that equity research analysts publish about
us and our business. We do not control these analysts. The price
of our common stock could decline if one or more equity analysts
downgrade our common stock or if analysts issue other
unfavorable commentary or cease publishing reports about us or
our business.
34
FORWARD-LOOKING
STATEMENTS
This prospectus contains forward-looking statements. In some
cases, you can identify forward-looking statements by
terminology such as “may,” “will,”
“should,” “expect,” “intend,”
“plan,” “anticipate,” “believe,”
“estimate,” “predict,”
“potential,” “continue” or the plural or
negative of these terms or other comparable terminology. These
statements are only predictions. We have based these
forward-looking statements largely on our current expectations
and projections about future events and financial trends that we
believe may affect our business, results of operations and
financial condition.
You should not place undue reliance on forward-looking
statements because they involve known and unknown risks,
uncertainties and other factors, which are, in some cases,
beyond our control and which could materially affect results.
Factors that may cause actual results to differ materially from
current expectations include those listed under “Risk
Factors” and elsewhere in this prospectus. If one or more
of these risks or uncertainties occur, or if our underlying
assumptions prove to be incorrect, actual events or results may
vary significantly from those implied or projected by the
forward-looking statements. No forward-looking statement is a
guarantee of future performance. You should read this prospectus
and the documents that we reference in this prospectus and have
filed with the Securities and Exchange Commission as exhibits to
the registration statement, of which this prospectus is a part,
completely and with the understanding that our actual future
results may be materially different from those expressed or
implied by any forward-looking statements. Forward-looking
statements in this prospectus include, among others, statements
regarding:
|
|
|
| |
•
|
preliminary results for the year ended March 31, 2011;
|
| |
| |
•
|
the impact of our biotechnology seedlings on the commercial
forestry industry and on native forests;
|
| |
| |
•
|
our ability to expand our business and develop and commercialize
new advanced and biotechnology seeding products;
|
| |
| |
•
|
the timing of the completion of the construction of our new
manufacturing, research and development laboratory and
headquarters facility in Summerville, South Carolina and the
timing of our exercise of the option to purchase that facility;
|
| |
| |
•
|
our occupation of our current headquarters facility until
completion of our new headquarters facility;
|
| |
| |
•
|
our customers’ adoption of our advanced and biotechnology
products, the anticipated prices of each of those products, and
the average selling price of our products;
|
| |
| |
•
|
our ability to have biotechnology products deregulated and our
expectations as to timing of our submissions of petitions for
deregulation;
|
| |
| |
•
|
our expectation that we will be the first company to introduce
biotechnology forestry products in our markets;
|
| |
| |
•
|
the increases in productivity that we expect our advanced and
biotechnology products will provide;
|
| |
| |
•
|
our projections, expectations and assumptions related to
consumption of wood globally and in our target markets;
|
| |
| |
•
|
the development of, and the consumption of wood by, the biopower
and biofuels markets;
|
| |
| |
•
|
our ability to maintain relationships with existing customers
and collaborators and attract new ones; and
|
| |
| |
•
|
our ability to protect our intellectual property rights.
|
The forward-looking statements in this prospectus represent our
views as of the date of this prospectus. We anticipate that
subsequent events and developments will cause our views to
change. However, while we may elect to update these
forward-looking statements at some point in the future, we have
no current intention of doing so except to the extent required
by applicable law. You should, therefore, not rely on these
forward-looking statements as representing our views as of any
date subsequent to the date of this prospectus.
35
INDUSTRY
AND MARKET DATA
Market data and certain industry data and forecasts included in
this prospectus were obtained from internal company surveys,
market research, consultant surveys, publicly available
information, reports of governmental agencies or industry
publications and surveys. We have relied upon certain
third-party publications as our primary sources for third-party
industry data and forecasts. Industry surveys, publications,
consultant surveys and forecasts generally state that the
information contained therein has been obtained from sources
believed to be reliable, but that the accuracy and completeness
of such information is not guaranteed. While we believe the
third-party market and industry data and forecasts included in
this prospectus is generally reliable, we have not independently
verified any of the data from third-party sources, nor have we
ascertained the underlying economic assumptions relied upon
therein. Similarly, internal surveys, industry forecasts and
market research, which we believe to be reliable based upon our
management’s knowledge of the industry, have not been
independently verified. Forecasts and projections are
particularly likely to be inaccurate, especially over long
periods of time. In addition, we do not know what assumptions
regarding general economic growth were used in preparing the
forecasts and projections we cite. Statements as to our market
position are based on recently available data. While we are not
aware of any misstatements regarding industry data presented
herein, our estimates involve risks and uncertainties and are
subject to change based on various factors, including those
discussed under “Risk Factors” in this prospectus.
While we believe our internal business research is reliable and
market definitions are appropriate, neither such research nor
definitions have been verified by any independent source.
36
USE OF
PROCEEDS
We estimate that our net proceeds from the sale of shares of our
common stock in this offering will be approximately
$ , after deducting the
underwriting discounts and estimated expenses of this offering
and assuming we sell shares for $
per share, which is the midpoint of the estimated price range
set forth on the cover page of this prospectus. If the
underwriters exercise their option to purchase additional shares
of common stock in full, we estimate that our net proceeds from
this offering will be approximately
$ .
A $1.00 increase or decrease in the assumed initial public
offering price of $ per share
would increase or decrease the net proceeds to us from this
offering by approximately
$ million, assuming the
number of shares offered by us, as listed on the cover page of
this prospectus, remains the same.
We intend to use the net proceeds from this offering as follows:
|
|
|
| |
•
|
to expand our container production capacity;
|
|
|
|
| |
•
|
approximately $ for the repayment
of all or a portion of outstanding debt under our revolving
credit facility bearing interest at a rate of 5.0% and having a
maturity date of May 31, 2011;
|
|
|
|
| |
•
|
approximately $1,200,000 for the repayment of loans from our
stockholders made in January 2011 bearing interest at a rate of
LIBOR plus 3.0% (unless repaid by the maturity date, in which
case all accrued interest will be forgiven) and having a
maturity date of the earlier of July 15, 2011 or seven
business days following either the closing of this offering or
our receipt of proceeds from other sales of capital stock
sufficient to repay the note;
|
|
|
|
| |
•
|
up to $15,000,000 for the potential exercise of our option to
purchase our new manufacturing, research and development
laboratory and headquarters facility during the year ended
March 31, 2012, plus additional amounts for the
construction of related infrastructure; and
|
|
|
|
| |
•
|
the balance for funding research and development programs and
product launches, and for working capital and general corporate
purposes, which may include capital expenditures, expansion of
other production capabilities and possible investments in, or
acquisitions of, complementary businesses, services or
technologies.
|
As of the date of this prospectus, we cannot estimate the amount
of net proceeds that will be used for any of the general
corporate purposes described above. The amount of what, and
timing of when, we actually spend for these purposes may vary
significantly and will depend on a number of factors, including
the amount of net proceeds we receive in this offering, our
future revenue and cash generated by operations and the other
factors described in the section entitled “Risk
Factors.” In addition, we currently have no agreements or
commitments with respect to any investment or acquisition and we
are not currently engaged in negotiations with respect to any
investment or acquisition. Accordingly, our management will have
significant discretion in applying the net proceeds of this
offering. Pending specific application of our net proceeds, we
intend to invest the net proceeds in short-term,
interest-bearing, investment-grade securities.
37
DIVIDEND
POLICY
We have never declared or paid dividends on our capital stock.
In addition, in certain circumstances, we are prohibited by our
loan agreements from paying cash dividends without the prior
written consent of the lender. Subject to the foregoing, our
board of directors will have discretion in determining whether
to declare or pay dividends, which will depend upon our results
of operations, financial condition, future prospects and any
other factors deemed relevant by our board of directors. We
currently intend to retain all available funds and any future
earnings to fund the development and growth of our business and
do not anticipate paying any dividends in the foreseeable
future. Investors should not purchase our common stock with the
expectation of receiving cash dividends.
38
CAPITALIZATION
The following table sets forth our cash and cash equivalents and
our capitalization as of December 31, 2010:
|
|
|
| |
•
|
on an actual basis; and
|
|
|
|
| |
•
|
on an as-adjusted basis to give effect to our sale in this
offering
of shares
of our common stock at an assumed initial public offering price
of $ per share, which is the
midpoint of the estimated price range set forth on the cover
page of this prospectus, after deducting the estimated
underwriting discounts and estimated offering expenses payable
by us, and after the application of the estimated net proceeds
of this offering as described in the section entitled “Use
of Proceeds.”
|
You should read the following table in conjunction with the
sections entitled “Selected Consolidated Financial
Data” and “Management’s Discussion and Analysis
of Financial Condition and Results of Operations” and our
consolidated financial statements and related notes included
elsewhere in this prospectus. Effective June 1, 2010, we
converted from a limited liability company to a corporation.
| |
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2010
|
|
|
|
|
Actual
|
|
|
As Adjusted
|
|
|
|
|
(Unaudited)
|
|
|
|
|
(In thousands, except share data)
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
2,133
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term debt, including current maturities(1)
|
|
$
|
18,464
|
|
|
$
|
|
|
|
Stockholders’ equity (deficit)
|
|
|
|
|
|
|
|
|
|
Common stock, $0.001 par value, 50,000,000 shares
authorized, 30,529,866 shares issued and outstanding,
actual, shares issued and outstanding, as adjusted
|
|
|
31
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
29,828
|
|
|
|
|
|
|
Accumulated deficit
|
|
|
(10,958
|
)
|
|
|
|
|
|
Accumulated other comprehensive loss
|
|
|
(298
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
18,603
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total capitalization
|
|
$
|
37,067
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
|
Does not include $1.2 million
of debt owing to our stockholders, which was incurred in January
2011.
|
The above table does not include 1,800,000 shares of common
stock reserved as
of
for future issuance under our 2010 Stock Option and Incentive
Plan.
39
DILUTION
If you invest in our common stock, your interest will be diluted
to the extent of the difference between the initial public
offering price per share and the as adjusted net tangible book
value per share of our common stock immediately after this
offering. Our historical net tangible book value as
of ,
was $ , or approximately
$ per share
(assuming shares
outstanding as
of ).
Net tangible book value per share is determined by dividing our
total tangible assets less total liabilities by the number of
outstanding shares of our common stock.
After giving effect to our sale
of shares
of common stock in this offering at an assumed initial public
offering price of $ per share,
which is the midpoint of the estimated price range set forth on
the cover page of this prospectus, after deducting estimated
underwriting discounts and estimated offering expenses payable
by us, our as adjusted net tangible book value as
of
would have been approximately $ ,
or approximately $ per share. This
represents an immediate increase in net tangible book value per
share of $ to our existing
stockholders and an immediate dilution in net tangible book
value of approximately $ , or
approximately $ per share to new
investors purchasing shares of our common stock in this offering
at the assumed initial public offering price. Dilution per share
to new investors is determined by subtracting as adjusted net
tangible book value per share after this offering from the
assumed initial public offering price per share paid by a new
investor. The following table illustrates the per share dilution
without giving effect to the option to purchase additional
shares of common stock granted to the underwriters or the use of
net proceeds from this offering:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Assumed initial public offering price per share
|
|
|
|
|
|
|
|
|
|
$
|
|
|
|
Net tangible book value per share as of
|
|
|
|
|
|
$
|
|
|
|
|
|
|
|
Increase per share attributable to this offering
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As adjusted net tangible book value per share after this offering
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dilution in net tangible book value per share to new investors
|
|
|
|
|
|
|
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A $1.00 increase or decrease in the assumed initial public
offering price of $ , which is the
midpoint of the estimated price range set forth on the cover
page of this prospectus, would increase or decrease our net
tangible book value per share after this offering by
$ per share and the dilution in
net tangible book value to new investors by
$ per share, assuming the number
of shares offered by us, as set forth on the cover page of this
prospectus, remains the same.
The following table summarizes, as
of ,
the number and percentage of shares of our common stock
purchased from us, the total cash consideration paid to us and
the average price per share paid to us by existing stockholders
and by new investors in this offering. The calculation below is
based on an assumed initial public offering price of
$ per share, which is the midpoint
of the estimated price range set forth on the cover page of this
prospectus, and before deducting underwriting discounts and
estimated offering expenses payable to us:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
Shares Purchased
|
|
|
Total Consideration
|
|
|
Price per
|
|
|
|
|
Number
|
|
|
Percent
|
|
|
Amount
|
|
|
Percent
|
|
|
Share
|
|
|
|
|
Existing stockholders
|
|
|
|
|
|
|
|
%
|
|
$
|
|
|
|
|
|
%
|
|
$
|
|
|
|
New investors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
%
|
|
$
|
|
|
|
|
|
%
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If the underwriters exercise their option to purchase additional
shares of common stock in full, the number of shares of common
stock held by new investors will increase
to ,
or percent of the total number of shares of our
common stock outstanding after this offering.
We may choose to raise additional capital due to market
conditions or strategic considerations even if we believe we
have sufficient funds for our current or future operating plans.
To the extent that additional capital is raised through the sale
of equity or securities convertible into equity, the issuance of
these securities may result in further dilution to our
stockholders.
40
SELECTED
CONSOLIDATED FINANCIAL DATA
The selected consolidated statement of operations data for the
years ended March 31, 2008, 2009 and 2010 and the
consolidated balance sheet data as of March 31, 2010 and
2009 have been derived from our consolidated financial
statements audited by PricewaterhouseCoopers LLP, an independent
registered public accounting firm, and included elsewhere in
this prospectus. The selected consolidated statement of
operations data presented below for the years ended
December 31, 2005 and 2006 and the selected consolidated
balance sheet data presented below as of March 31, 2007 and
2008 and December 31, 2005 and 2006 have been derived from
our audited consolidated financial statements not included in
this prospectus. Our consolidated statement of operations data
for the quarter ended March 31, 2007 has been derived from
unaudited consolidated financial statements not included in this
prospectus. Our consolidated statement of operations data for
the nine months ended December 31, 2009 and 2010 and the
selected consolidated balance sheet data presented below as of
December 31, 2010 have been derived from unaudited
consolidated financial statements included elsewhere in this
prospectus. In our opinion, these unaudited consolidated
financial statements have been prepared on a basis consistent
with our audited consolidated financial statements and contain
all adjustments, consisting only of normal and recurring
adjustments, necessary for a fair presentation of such
consolidated financial data. Results for interim periods are not
necessarily indicative of results for a full fiscal year. The
financial data listed under “Other Selected Data”
below has been derived from the audited and unaudited
consolidated financial statements noted above, as well as from
our internal records of our operations. Historical results are
not necessarily indicative of results for future periods. This
selected consolidated financial data should be read in
conjunction with the section entitled “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations” and our consolidated financial statements and
the related notes included elsewhere in this prospectus.
For the past three years, our fiscal year has ended on
March 31. Prior to the year ended March 31, 2008, our
fiscal years ended on December 31. Therefore, there is a
stub period for the quarter ended March 31, 2007.
Prior to October 2007, our business was focused on research and
development with no product revenue. In October 2007, our
current stockholders contributed to us substantially all of
their commercial tree improvement research programs and seed
orchard and nursery businesses in the United States, New Zealand
and Australia, which enabled us to start generating operating
revenue. As a result, only the U.S. operations seedling
season (December 2007 to March 2008) was reflected in our
revenue for the year ended March 31, 2008. Sales made
during the New Zealand and Australian operations seedling season
(June 2007 to September 2007) were not part of our revenue
for the year ended March 31, 2008 because they occurred
prior to the October 2007 contribution.
41
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
Quarter Ended
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
|
December 31,
|
|
|
March 31,
|
|
|
Year Ended March 31,
|
|
|
December 31,
|
|
|
|
|
2005
|
|
|
2006
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(In thousands)
|
|
|
|
|
Consolidated Statement of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
1,173
|
|
|
$
|
880
|
|
|
$
|
0
|
|
|
$
|
18,182
|
|
|
$
|
23,680
|
|
|
$
|
21,576
|
|
|
$
|
9,583
|
|
|
$
|
11,083
|
|
|
Cost of revenue
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
15,667
|
|
|
|
15,840
|
|
|
|
16,381
|
|
|
|
6,927
|
|
|
|
7,055
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
1,173
|
|
|
|
880
|
|
|
|
0
|
|
|
|
2,515
|
|
|
|
7,840
|
|
|
|
5,195
|
|
|
|
2,656
|
|
|
|
4,028
|
|
|
Operating expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development(1)
|
|
|
13,528
|
|
|
|
9,264
|
|
|
|
2,793
|
|
|
|
10,212
|
|
|
|
13,833
|
|
|
|
11,206
|
|
|
|
7,836
|
|
|
|
9,171
|
|
|
Selling, general and administrative(2)(3)
|
|
|
5,931
|
|
|
|
5,158
|
|
|
|
1,085
|
|
|
|
9,709
|
|
|
|
7,599
|
|
|
|
7,139
|
|
|
|
5,194
|
|
|
|
7,866
|
|
|
Depreciation and amortization
|
|
|
343
|
|
|
|
398
|
|
|
|
157
|
|
|
|
679
|
|
|
|
813
|
|
|
|
780
|
|
|
|
665
|
|
|
|
444
|
|
|
Loss on impairment of intangible assets(4)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
203
|
|
|
|
643
|
|
|
|
213
|
|
|
|
200
|
|
|
|
154
|
|
|
Restructuring charge(5)
|
|
|
310
|
|
|
|
—
|
|
|
|
—
|
|
|
|
183
|
|
|
|
493
|
|
|
|
101
|
|
|
|
—
|
|
|
|
—
|
|
|
Other operating expense (income)
|
|
|
4
|
|
|
|
(233
|
)
|
|
|
(74
|
)
|
|
|
(387
|
)
|
|
|
(464
|
)
|
|
|
(234
|
)
|
|
|
(185
|
)
|
|
|
(185
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expense
|
|
|
20,116
|
|
|
|
14,587
|
|
|
|
3,961
|
|
|
|
20,599
|
|
|
|
22,917
|
|
|
|
19,205
|
|
|
|
13,710
|
|
|
|
17,450
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(18,943
|
)
|
|
|
(13,707
|
)
|
|
|
(3,961
|
)
|
|
|
(18,084
|
)
|
|
|
(15,077
|
)
|
|
|
(14,010
|
)
|
|
|
(11,054
|
)
|
|
|
(13,422
|
)
|
|
Total interest income (expense), net
|
|
|
77
|
|
|
|
136
|
|
|
|
24
|
|
|
|
3
|
|
|
|
(269
|
)
|
|
|
(646
|
)
|
|
|
(435
|
)
|
|
|
(765
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss(6)
|
|
$
|
(18,866
|
)
|
|
$
|
(13,571
|
)
|
|
$
|
(3,937
|
)
|
|
$
|
(18,081
|
)
|
|
$
|
(15,346
|
)
|
|
$
|
(14,656
|
)
|
|
$
|
(11,489
|
)
|
|
$
|
(14,187
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of
|
|
As of
|
|
|
|
As of
|
|
|
|
December 31,
|
|
March 31,
|
|
As of March 31,
|
|
December 31,
|
|
|
|
2005
|
|
2006
|
|
2007
|
|
2008
|
|
2009
|
|
2010
|
|
2010
|
|
|
|
(In thousands)
|
|
|
|
Consolidated Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
3,220
|
|
|
$
|
2,532
|
|
|
$
|
1,943
|
|
|
$
|
2,054
|
|
|
$
|
1,624
|
|
|
$
|
3,742
|
|
|
$
|
2,133
|
|
|
Total assets
|
|
|
8,409
|
|
|
|
8,299
|
|
|
|
7,868
|
|
|
|
40,805
|
|
|
|
36,644
|
|
|
|
42,345
|
|
|
|
50,878
|
|
|
Long-term debt, net of current maturities(7)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
168
|
|
|
|
11,675
|
|
|
|
4,356
|
|
|
Total liabilities
|
|
|
7,459
|
|
|
|
1,362
|
|
|
|
1,221
|
|
|
|
7,996
|
|
|
|
14,215
|
|
|
|
23,199
|
|
|
|
32,275
|
|
|
Total members’/stockholders’ equity
|
|
|
950
|
|
|
|
6,937
|
|
|
|
6,647
|
|
|
|
32,809
|
|
|
|
22,429
|
|
|
|
19,146
|
|
|
|
18,603
|
|
42
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
Quarter Ended
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
|
December 31,
|
|
|
March 31,
|
|
|
Year Ended March 31,
|
|
|
December 31,
|
|
|
|
|
2005
|
|
|
2006
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(In thousands, except average selling price and per share
data)
|
|
|
|
|
Other Selected Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total seedlings shipped
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
278,537
|
(8)
|
|
|
286,147
|
|
|
|
239,958
|
|
|
|
71,386
|
|
|
|
61,880
|
|
|
Average selling price per 1,000 seedlings(9)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
65
|
|
|
$
|
83
|
|
|
$
|
90
|
|
|
$
|
134
|
|
|
$
|
179
|
|
|
EBITDA(10)
|
|
$
|
(18,600
|
)
|
|
$
|
(13,309
|
)
|
|
$
|
(3,804
|
)
|
|
$
|
(17,165
|
)
|
|
$
|
(13,676
|
)
|
|
$
|
(12,560
|
)
|
|
$
|
(9,932
|
)
|
|
$
|
(12,423
|
)
|
|
Loss per share(11)
|
|
$
|
(1.58
|
)
|
|
$
|
(0.91
|
)
|
|
$
|
(0.23
|
)
|
|
$
|
(0.92
|
)
|
|
$
|
(0.62
|
)
|
|
$
|
(0.54
|
)
|
|
$
|
(0.43
|
)
|
|
$
|
(0.48
|
)
|
|
Pro forma loss per share(11)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
|
|
|
|
|
|
|
$
|
|
|
|
|
|
|
(1)
|
|
The decrease in research and
development expense for the year ended March 31, 2010 as
compared to the year ended March 31, 2009 was attributable
to our cost mitigation program in light of the impact of the
worldwide economic downturn on our primary end-markets. For the
year ending March 31, 2011, we expect that our research and
development spending will return to levels consistent with the
year ended March 31, 2009, and may increase further.
|
| |
|
(2)
|
|
Our selling, general and
administrative expense for the year ended March 31, 2008
included significant legal and accounting fees in connection
with the asset contribution in October 2007.
|
| |
|
(3)
|
|
The decrease in selling, general
and administrative expense for the year ended March 31,
2010 as compared to the year ended March 31, 2009 was
attributable to our cost mitigation program in light of the
impact of the worldwide economic downturn on our primary
end-markets. We expect that our selling, general and
administrative expense will increase as we focus on increasing
our global market share and continuing to build brand awareness
and in preparation for becoming and operating as a public
company.
|
| |
|
(4)
|
|
Impairment of intangible assets is
attributable to the abandonment of patents and
patents-in-progress that are no longer of strategic interest.
The amounts recorded for the years ended March 31, 2008,
2009 and 2010 and the nine months ended December 31, 2009
and 2010 represent impairment charges for the abandonment of
patents-in-progress.
|
| |
|
(5)
|
|
During the years ended
March 31, 2008 and 2009, we undertook several restructuring
actions in connection with the integration of the operations
contributed in 2007. In the year ended March 31, 2010, due
to the economic downturn, we suspended operations at one of our
U.S. nurseries.
|
| |
|
(6)
|
|
On June 1, 2010, we converted
from a non-taxable limited liability company to a taxable
corporation. From our inception to May 31, 2010, we were
treated as a partnership for U.S. income tax purposes and as a
result our income (loss) was passed through to our limited
liability company members. Consequently, income tax expense is
not reflected in our consolidated statement of operations, nor
does the consolidated balance sheet contain an amount for
deferred tax expense in recognition of tax effects of temporary
differences prior to our conversion. Subsequent to our
conversion to a taxable corporation, no income tax benefit has
been reflected due to recording of a full valuation allowance on
our net deferred tax assets.
|
| |
|
(7)
|
|
Includes $0.2, $0.2 and
$0.2 million of capital lease obligations as of
March 31, 2009 and 2010 and December 31, 2010,
respectively. Does not include $8.0, $3.2 and $14.1 million
of current portion of obligations as of March 31, 2009 and
2010 and December 31, 2010, respectively. Also does not
include $1.2 million of debt owing to our stockholders,
which was incurred in January 2011.
|
|
|
|
|
(8)
|
|
This amount excludes approximately
26 million seedlings shipped in Australia and New Zealand
during the year ended March 31, 2008 by certain entities
prior to our acquisition thereof in the October 2007 combination
transaction described in the section entitled “Certain
Relationships and Related Party Transactions — Asset
Contribution.”
|
|
|
|
|
(9)
|
|
Average selling price reflects
revenue divided by thousands of seedlings sold. Revenue includes
both revenue from seedling sales and other revenue, including
revenue from government grants of $0, $0 and $1.1 million
for the years ended March 31, 2008, 2009 and 2010,
respectively, and $0.8 and $0.8 million for the nine months
ended December 31, 2009 and 2010, respectively.
|
|
|
|
|
(10)
|
|
EBITDA is defined as earnings
before interest, taxes, depreciation and amortization. We use
EBITDA to plan, forecast and monitor our
day-to-day
operating performance and capital structure. We further believe
that providing this information allows investors greater
transparency and a better understanding of our ability to plan
our operating cash requirements and make long-term capital
spending commitments. EBITDA does not represent net loss or cash
flow from operations as those terms are defined by GAAP and does
not necessarily indicate whether cash flows will be sufficient
to fund cash requirements. EBITDA is not a recognized
measurement under GAAP, and investors should not consider EBITDA
as a substitute for measures of our financial performance and
liquidity as determined in accordance with GAAP, such as net
loss, operating income or net cash provided by operating
activities. EBITDA has other limitations as an analytical tool,
when compared to the use of net loss, which is the most directly
comparable GAAP financial measure, including: (i) EBITDA
does not reflect the interest expense we incur as a result of
our debt leverage and (ii) EBITDA does not reflect any
attribution of costs to our operations related to our capital
expenditures through depreciation and amortization charges.
|
43
|
|
|
|
|
|
The following is a reconciliation
of net income to EBITDA:
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
Quarter Ended
|
|
|
|
Nine Months Ended
|
|
|
|
December 31,
|
|
March 31,
|
|
Year Ended March 31,
|
|
December 31,
|
|
|
|
2005
|
|
2006
|
|
2007
|
|
2008
|
|
2009
|
|
2010
|
|
2009
|
|
2010
|
|
|
|
(In thousands)
|
|
|
|
Net loss(a)
|
|
$
|
(18,866
|
)
|
|
$
|
(13,571
|
)
|
|
$
|
(3,937
|
)
|
|
$
|
(18,081
|
)
|
|
$
|
(15,346
|
)
|
|
$
|
(14,656
|
)
|
|
$
|
(11,489
|
)
|
|
$
|
(14,187
|
)
|
|
Added back:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation included in cost of revenue
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
240
|
|
|
|
588
|
|
|
|
670
|
|
|
|
457
|
|
|
|
555
|
|
|
Depreciation and amortization
|
|
|
343
|
|
|
|
398
|
|
|
|
157
|
|
|
|
679
|
|
|
|
813
|
|
|
|
780
|
|
|
|
665
|
|
|
|
444
|
|
|
Interest expense (income), net
|
|
|
(77
|
)
|
|
|
(136
|
)
|
|
|
(24
|
)
|
|
|
(3
|
)
|
|
|
269
|
|
|
|
646
|
|
|
|
435
|
|
|
|
765
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EBITDA
|
|
$
|
(18,600
|
)
|
|
$
|
(13,309
|
)
|
|
$
|
(3,804
|
)
|
|
$
|
(17,165
|
)
|
|
$
|
(13,676
|
)
|
|
$
|
(12,560
|
)
|
|
$
|
(9,932
|
)
|
|
$
|
(12,423
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
|
|
On June 1, 2010, we converted
from a non-taxable limited liability company to a taxable
corporation. From our inception to May 31, 2010, for U.S.
tax purposes, we were treated as a partnership and as a result
our income (loss) was passed through to our limited liability
company members. Consequently, income tax expense is not
reflected on the statements of operations. Subsequent to our
conversion to a taxable corporation, no income tax benefit has
been reflected due to recording of a full valuation allowance on
our net deferred tax assets.
|
|
|
|
|
(11)
|
|
Upon our conversion from a limited
liability company to a corporation on June 1, 2010, our
limited liability company members received a total of
30 million shares of stock for their members’ equity
interests. For the periods prior to conversion, for purposes of
determining the weighted average number of common stock shares
outstanding, we computed the equivalent number of shares based
on the conversion ratio we used on June 1, 2010.
|
|
|
|
|
|
|
The following table presents the
calculation of historical basic and diluted net loss per common
share:
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
Quarter Ended
|
|
|
|
Nine Months Ended
|
|
|
|
December 31,
|
|
March 31,
|
|
Year Ended March 31,
|
|
December 31,
|
|
|
|
2005
|
|
2006
|
|
2007
|
|
2008
|
|
2009
|
|
2010
|
|
2009
|
|
2010
|
|
|
|
(In thousands)
|
|
|
|
Net loss(a)
|
|
$
|
(18,866
|
)
|
|
$
|
(13,571
|
)
|
|
$
|
(3,937
|
)
|
|
$
|
(18,081
|
)
|
|
$
|
(15,346
|
)
|
|
$
|
(14,656
|
)
|
|
$
|
(11,489
|
)
|
|
$
|
(14,187
|
)
|
|
Weighted average shares outstanding
|
|
|
11,905
|
|
|
|
14,941
|
|
|
|
17,475
|
|
|
|
19,558
|
|
|
|
24,677
|
|
|
|
27,078
|
|
|
|
26,864
|
|
|
|
29,824
|
|
|
Loss per share, basic and diluted
|
|
$
|
(1.58
|
)
|
|
$
|
(0.91
|
)
|
|
$
|
(0.23
|
)
|
|
$
|
(0.92
|
)
|
|
$
|
(0.62
|
)
|
|
$
|
(0.54
|
)
|
|
$
|
(0.43
|
)
|
|
$
|
(0.48
|
)
|
|
|
|
|
|
|
As discussed under “Use of
Proceeds”, we intend to use approximately
$ of the net proceeds of this
offering to repay debt outstanding under our credit facility.
The pro forma loss per share, basic and diluted, represents loss
per share assuming repayment of this debt as of April 1,
2009. Had this debt been repaid as of April 1, 2009, we
would have avoided interest expense of approximately
$ and
$ for the year ended
March 31, 2010 and the nine months ended December 31,
2010, respectively. At an assumed initial public offering price
of $ per share, which is the
midpoint of the estimated price range set forth on the cover
page of this
prospectus,
shares are required to pay down this amount of debt. These
shares have been considered outstanding for all periods in the
calculation of pro forma loss per share.
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended March 31, 2010
|
|
|
Nine Months Ended December 31, 2010
|
|
|
|
|
Actual
|
|
|
Adjustment
|
|
|
Pro forma
|
|
|
Actual
|
|
|
Adjustment
|
|
|
Pro forma
|
|
|
|
|
(In thousands, except per share data)
|
|
|
|
|
Interest income(expense), net
|
|
$
|
(646
|
)
|
|
|
|
|
|
|
|
|
|
$
|
(765
|
)
|
|
|
|
|
|
|
|
|
|
Net loss(a)
|
|
$
|
(14,656
|
)
|
|
|
|
|
|
|
|
|
|
$
|
(14,187
|
)
|
|
|
|
|
|
|
|
|
|
Net loss per common share, basic and diluted
|
|
$
|
(0.54
|
)
|
|
|
|
|
|
|
|
|
|
$
|
(0.48
|
)
|
|
|
|
|
|
|
|
|
|
Shares used in computing net loss per common share, basic and
diluted
|
|
|
27,078
|
|
|
|
|
|
|
|
|
|
|
|
29,824
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
|
|
On June 1, 2010, we converted
from a non-taxable limited liability company to a taxable
corporation. From our inception to May 31, 2010, for U.S.
tax purposes, we were treated as a partnership and as a result
our income (loss) was passed through to our limited liability
company members. Consequently, income tax expense is not
reflected on the statements of operations. Subsequent to our
conversion to a taxable corporation, no income tax benefit has
been reflected due to recording of a full valuation allowance on
our net deferred tax assets.
|
44
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial
condition and results of operations should be read in
conjunction with our consolidated financial statements and
related notes that appear elsewhere in this prospectus. In
addition to historical consolidated financial information, the
following discussion contains forward-looking statements that
reflect our plans, estimates and beliefs. Our actual results
could differ materially from those discussed in the
forward-looking statements. Factors that could cause or
contribute to these differences include those discussed below
and elsewhere in this prospectus, particularly in “Risk
Factors.” Our fiscal year ends on March 31 of each year.
All currency translations from New Zealand dollars to U.S.
dollars specifically identified in this section are based on the
rate set forth in the H.10 statistical release of the Federal
Reserve Board as of the date indicated.
Overview
We believe we are the world’s leading developer of
biotechnology tree seedling products, one of the largest
providers of conventional and technology-enhanced seedlings to
the forestry industry and the only integrated global commercial
seedling company. We are focused on improving and selling the
most widely grown commercial forestry species in some of the
largest markets in the world. Our products are designed for use
by our customers in the traditional pulp and paper and wood
products markets, the growing biopower and charcoal markets and
the emerging biofuels market. We have a base of over 5,000
customers, including some of the largest land owners and
managers in the United States, New Zealand and Australia, and in
the year ended March 31, 2010, we sold 240 million
seedlings in these markets. We also have a growing presence in
Brazil through collaborations with the country’s leading
pulp producers. Based on our research and estimates, we believe
that our high-value, technology-enhanced seedling products,
including our pipeline of advanced and biotechnology products,
improve the productivity of a given acre of land by enabling our
customers to grow trees that yield more wood per acre with
greater consistency and quality in a shorter period of time. The
combination of our fully integrated business model, proprietary
technology and established customer base creates a scalable
platform that we are using to develop and commercialize the next
generation of seedling products. We do not currently sell any
biotechnology products and, prior to any commercial sales, our
biotechnology products are subject to a multi-year deregulation
process. We believe, but cannot guarantee, our biotechnology
products will revolutionize productivity standards in the
forestry industry and have an impact on that industry similar to
the impact that biotechnology crops have had on the agricultural
industry.
We are currently the only commercial seedling company with
products spanning the entire technology spectrum, from
conventional and advanced seedlings, which we currently offer,
to biotechnology seedlings, which are currently in
development. As a result, no single entity competes with us in
commercial sales across the full range of our business. We have
a pipeline of six advanced and 15 biotechnology products in
development. These technology-enhanced products are designed to
improve the growth rates, yields, stress-tolerance, uniformity,
wood quality and processing efficiency of trees. Our
freeze-tolerant tropical eucalyptus product is the first and
only biotechnology forestry product under review for
deregulation by the United States Department of Agriculture, or
USDA. In addition, we have over 140 active field tests for
biotechnology products and the largest number of regulatory
approvals for field tests of biotechnology forestry products in
the United States and Brazil. As of March 31, 2011, we
operated 13 nurseries, 15 seed orchards, 20 distribution centers
and three research and development facilities located throughout
the Southeastern United States, New Zealand and Australia, as
well as an office in Brazil.
We operate as a single operating segment. For the year ended
March 31, 2010, 76% of our revenue was generated from
customers located in the United States, 19% from customers
located in New Zealand and 5% from customers located in
Australia. While the majority of our revenue and expense is
currently denominated in U.S. dollars, most of our revenue
and expense outside of the United States is denominated in local
currencies. As a result, we may be exposed to foreign currency
fluctuations when we convert the results of our
non-U.S. operations
from their functional currency into U.S. dollars for
inclusion in our consolidated results. Historically, the foreign
exchange impact on our results has been immaterial; however, it
may increase in the future, particularly as our revenue and
gross margin increases in our
non-U.S. operations.
45
Our results are highly seasonal, with planting for our
U.S. operations occurring primarily in April and May and
harvesting and delivery to customers occurring between December
and March. This seasonality is partially balanced by the growing
cycle in our New Zealand and Australia operations, where
planting occurs primarily in October and November and harvesting
and delivery to customers occurs between June and September. We
expect this balancing effect to increase as our international
operations grow and new products, such as our tropical
eucalyptus seedling product, which has a different growing and
sales cycle, are introduced. Currently, our seasonality tends to
result in higher operational expenses, a net loss and a net use
of cash during the first three quarters of our fiscal year and
significant generation of revenue and net cash in our fourth
fiscal quarter. In addition, quarter to quarter, we may
experience fluctuations in the mix of our products resulting in
variations in revenue, average selling price and gross margins.
We experience 12 to 24 month lead times on delivery of our
seedlings depending on the type of seedling. Our conventional
products must grow from seeds to seedlings, typically over a
period of approximately nine months, followed by a harvesting
period of approximately three months. In the case of varietal
pine seedlings, this lead time typically increases to a total of
up to 24 months due to the longer production time required
for the varietal manufacturing process, and this will also
typically be the case for our future pine biotechnology
products. We base the number of seedlings we plant on our
expectations of customer demand, which take into account
customer input, purchasing history, existing long-term
contracts, feedback from our sales force and market conditions.
We typically set our pricing for products sold in the United
States in March, and our pricing for products sold in New
Zealand and Australia in September, in each case, just before we
plant our seedlings. A significant majority of our
U.S. customers place their orders between April and
October. In New Zealand and Australia, where we have long-term
contracts with a number of our customers, we often have
meaningful indications of customer demand as early as October
and November. Our yield from planting may vary as a result of
conditions during the growing season, particularly the weather.
Our five primary end-markets are the traditional pulp and paper
and wood products markets, the growing biopower and charcoal
markets, and the emerging biofuels market. As a result, our
customer demand may be impacted by factors affecting these
end-markets. During the year ended March 31, 2010, each of
these end-markets, and particularly the traditional end-markets,
was impacted by the worldwide economic downturn, which resulted
in reduced seedling planting by our customers as they chose to
harvest fewer of their existing trees. In response, we reduced
our production volumes and implemented various cost mitigation
programs during the year ended March 31, 2010, which
impacted our overall expenditure levels and also resulted in a
restructuring charge of $0.1 million as we temporarily
suspended operations at one of our U.S. nurseries. As part
of our cost mitigation program, we elected not to pay employee
bonuses for the year ended March 31, 2009. In addition, for
the year ended March 31, 2010, we reduced our annual
investment in research and development by 19% to
$11.2 million from $13.8 million for the prior year.
We anticipate that our spending in the year ending
March 31, 2011, and in particular, our investment in
research and development spending, will return to levels
consistent with the year ended March 31, 2009, and may
increase further. In November 2010, we signed a build-to-suit
and lease agreement with Forestry Research Holdings, LLC for a
new manufacturing, research and development laboratory and
headquarters facility in Summerville, South Carolina, which we
intend to occupy upon its anticipated completion by the end of
2011. The agreement includes an option for us to purchase the
facility, which we may exercise during the year ended
March 31, 2012 using net proceeds from this offering.
Our pine products range in price from approximately $45 to $70
per 1,000 seedlings for our conventional open-pollinated
seedlings to over $400 per 1,000 seedlings for a varietal
product of the same species that has been designed to provide
customers with higher returns on their investment. As our
customers have transitioned to our higher-value products, which
consist of our “elite” open-pollinated seedlings, our
mass-control pollinated seedlings and our varietal seedlings,
sales of these products have grown from 5.7% of
U.S. revenue in the year ended March 31, 2008 to 24.2%
in the year ended March 31, 2010, with increasing sales of
MCP and varietal seedlings each year. This shift in sales to our
higher-value products also drove the 15.5% increase in our
U.S. average selling price over the same period. We expect
our average selling prices and gross margins to increase further
as we introduce our biotechnology products. As we introduce new
advanced and biotechnology products, it is likely that demand
for our conventional products will decrease and
46
that we may need to write down our inventory of conventional
seeds. We have a pipeline of technology-enhanced products in
field tests in the United States, New Zealand, Australia and
Brazil.
We expect that the percentage of our revenue from sales of
advanced products will continue to grow. Prior to any commercial
sales, our biotechnology products are subject to a multi-year
deregulation process. We will not generate revenue from our
biotechnology products unless and until one of our biotechnology
products completes the applicable regulatory processes, as
described in “Business—Government Regulation” on
page 104. We submitted our initial petition for the
deregulation of our freeze-tolerant tropical eucalyptus product
to the USDA in December 2008 and resubmitted that petition in
January 2011 to include additional data. We expect to submit
petitions for the deregulation of our short rotation loblolly
pine and short rotation populus products to the USDA for review
in the next two to four years. We expect to make regulatory
submissions to Comissão Técnica Nacional de
Biossegurança, or CTNBio, the governmental agency in Brazil
that regulates biotechnology products, for our short rotation
tropical eucalyptus product in the next three to four years and
for our improved pulping tropical eucalyptus product in the next
four to five years.
Historically, we have incurred significant losses, largely
attributable to our investment in research and development. For
the years ended March 31, 2008, 2009 and 2010, we generated
revenue of $18.2 million, $23.7 million and
$21.6 million, respectively, and had net losses of
$(18.1) million, $(15.3) million and
$(14.7) million, respectively. We expect that we will
continue to incur net losses for the next several years.
History
We were formed in February 2000 by combining all of the
biotechnology forestry research and development programs of
three leading forest products companies: Fletcher Challenge
Limited (now Rubicon Limited, a New Zealand-based company),
International Paper Company and Westvaco Corporation (now
MeadWestvaco Corporation). Each company independently developed
its own biotechnology forestry research programs over decades of
significant investment, and the combination provided us with a
broad portfolio of intellectual property and a strong
biotechnology base. Due to our resulting technology leadership
position and the inherent tree growth time associated with this
tree improvement research, we believe we are decades ahead of
any new market entrants seeking to develop and commercialize
products and technology comparable to ours; however, there is no
guarantee that we will be able to commercialize our
biotechnology products in the near future. The combination also
provided us with a long-term financial commitment from our
stockholders. Following this initial combination, in October
2007, our stockholders further contributed to us substantially
all of their commercial tree improvement research programs and
seed orchard and nursery businesses in the United States, New
Zealand and Australia. Our stockholders’ aggregate
investment over the past ten years, including the fair value of
the assets contributed, has totaled more than $200 million.
The October 2007 transaction significantly changed our
competitive position, as it provided us with the platform from
which to commercialize the products we develop by giving us
ownership of one of the largest and most diverse repositories of
germplasm in the world, demonstrated production and distribution
capabilities and an established customer base of land owners and
managers. As a result, we immediately transitioned from a
research-based business to a fully integrated commercial
developer and provider of conventional and technology-enhanced
tree seedlings, and began selling conventional seedlings to many
of the same customers previously served by our stockholders. In
our first full fiscal year following the contribution, we
generated $23.7 million in revenue from the global
commercial sales of approximately 278 million seedlings and
also recorded a net loss of $(15.3) million.
In anticipation of this asset contribution, we changed our
fiscal year-end from December 31 to March 31 to match more
closely the growing, harvesting and selling cycle of our
U.S. products. As a result, there is a stub period for the
quarter ended March 31, 2007.
Our revenue for the year ended March 31, 2008 reflects only
our U.S. operations, for which seedling harvesting and
sales occur between December and March. Harvesting and sales
related to our operations in New Zealand and Australia, which
occur primarily between June and September, are not included in
our revenues for the year ended March 31, 2008 because they
occurred prior to the October 2007 contribution. Related to the
contribution transaction, we recorded charges of
$1.6 million in cost of revenue and
47
$2.3 million in selling, depreciation and amortization for
the year ended March 31, 2008, as well as restructuring
costs of $0.2 million and $0.5 million in the year
ended March 31, 2008 and 2009, respectively, as we
integrated the contributed businesses.
As of June 1, 2010, we converted from a limited liability
company to a corporation, changing our name from “ArborGen,
LLC” to “ArborGen Inc.”
Statement
of Operations
Revenue
We currently derive revenue primarily from the sale of our
seedling products. As described above, our revenue is highly
seasonal, with substantially all of our U.S. revenue
generated between December and March, and substantially all of
our New Zealand and Australian revenue generated between June
and September. The timing of our harvest and seedling shipments
in the United States depends upon weather conditions in December
and January, which could shift our sales between the third and
fourth quarter in any particular fiscal year. A similar shift
may occur in the first and second quarters related to the timing
of our harvest and seedling shipments in New Zealand and
Australia. In addition, our revenue
year-to-year
can be affected by the timing of the launch of new products.
Revenue from sales of our products is recognized upon transfer
of title to our customers. Some of our customers enter into
agreements to pay for products or services in advance of
delivery, and we record those prepaid amounts as deferred
revenue until the product is shipped to the customer. Under our
standard purchase order for sales in the United States, which
establishes the terms and conditions for the majority of our
sales, our customers pay the full purchase price not later than
30 days after the delivery date. A majority of our customer
contracts in New Zealand and Australia are for more than one
year and typically contain deposit requirements. In most cases,
our customers in New Zealand and Australia pay 20% of the
purchase price upon signing, an additional 20% of the purchase
price not later than 120 days prior to the targeted
delivery dates and the remaining 60% of the purchase price not
later than 30 days after the actual delivery dates. We have
also entered into, and may continue to enter into, long-term
seedling production contracts with several of our largest
U.S. customers. For example, we have entered into a
long-term seedling contract with one of our largest customers to
supply at least 85% of its required seedlings for its properties
in Arkansas, Louisiana, Oklahoma and Texas, subject to certain
offsets, over the next ten years. In the future, we anticipate
that some of our long-term customer contracts for our
higher-value products will incorporate terms for advance
deposits prior to the shipment of seedlings. These advance
deposits would be accounted for as deferred revenue until the
product is shipped to the customer.
We also receive revenue from grant contracts awarded by
U.S. federal and state governments. In the year ended
March 31, 2010, we received grants of $1.0 million,
which we recorded as revenue. Prior to the year ended
March 31, 2010, the grants received were not material and
the grants and related expenses were recorded in research and
development expense. Revenue from our grant contracts is
influenced by the timing of various contract progress milestones
but is not otherwise significantly seasonal.
Cost
of Revenue
Cost of product revenue comprises the cost of labor, raw
materials and third-party services related to growing,
harvesting, packaging and shipping our seedlings, as well as
charges related to the value of our inventory and depreciation
expense for assets directly related to the production of our
products. Labor costs for our employees involved in the
production of our products are a significant portion of our cost
of revenue. For the years ended March 31, 2008, 2009 and
2010, labor costs were 11%, 27% and 20% of cost of revenue,
respectively. These costs are comprised of the direct costs of
our employees at our global nurseries and orchards, as well as
seasonal labor costs to augment our resources during planting
and harvest times. Raw materials costs include costs for seeds,
as well as fumigation and pesticide, and generally have
increased in line with global inflation rates. Third-party
services include contract labor, other professional services
related to our field operations and consulting services and cost
of outsourcing. To satisfy increased customer demand, we also
outsource the production of some of our containerized and
varietal products to independent contractors. We
48
currently outsource only a small percentage of seedlings sold,
but as we expand our operations generally and our sale of
containerized products in particular, we expect to outsource a
larger percentage of our seedling production to third parties.
Charges related to the value of our inventory consist primarily
of write-offs of our seedling inventory and charges to increase
our seed inventory valuation reserve. Our seedling inventory
generally must be sold within 12 months of being planted.
Accordingly, we generally destroy and write off any remaining
seedling product at the end of each growing season to allow for
the planting of the next season’s crop. Unsold seedling
inventory that must be written off usually results from changes
in customer demand. The largest portion of our inventory is in
the United States, for which sales and delivery to customers
generally occurs between December and March. As a result, any
unsold seedling inventory in the United States typically results
in a charge in the fourth fiscal quarter. For our seed
inventory, we consider inventory levels and product life cycles,
and we generally write down inventory to net realizable value,
as a result of obsolescence or slow movement, based on
forecasted product demand. Actual demand and market conditions
may differ from our projections, which could result in further
write-offs.
Occasionally, our customers receive damaged seedlings. We may
replace those seedlings depending on the specific facts and
circumstances. For example, during the year ended March 31,
2008, our seedlings experienced a higher than usual mortality
rate in certain areas in the United States following an
unusually cold and dry harvest season. To maintain customer
relationships, customers were offered replacement seedling
agreements to offset planting losses. Accordingly, we recorded a
product replacement reserve of $0.6 million in cost of
revenue. We have since modified certain of our harvesting
procedures and subsequently have not experienced these issues.
We may incur charges for product replacement reserves from time
to time in the future; however, based on historical experience,
we expect these amounts, if any, to be immaterial. We estimate
the need for additional reserves at each period end using
historical experience and input from our customers. Other than
the reserve we recorded in the March 31, 2008 fiscal year,
additional reserves have been insignificant. Actual replacement
costs could vary from our allowance estimates for a number of
reasons, including, among others, unusually severe weather
patterns, particularly those occurring during our harvest
season. If actual costs are significantly higher, or we adjust
the estimates we use to establish these allowances, our cost of
revenue could vary from period to period. We have historically
not allowed for product returns other than for these replacement
situations.
In connection with the revenue we receive from our grant
contracts, cost of revenue also includes the direct labor costs
of engineering resources committed to research and development
contracts, third-party consulting, travel and associated direct
material costs, as well as overhead expenses such as indirect
engineering labor, occupancy costs associated with the project
resources, engineering tools and supplies and program management
expenses.
Gross
Profit/Gross Margin
Gross margin is the percentage derived by dividing gross profit
by revenue. Our gross margin varies according to the mix of
products sold. We expect that our gross margin will increase
over time as we introduce new products and transition our
customers to our higher-value, and, in the future, biotechnology
products. Our introduction of new advanced and biotechnology
products could also result in lower demand for conventional
seedlings, which may lead to an increased write-down of our seed
inventory for these products during those periods. Due to the
seasonal nature of our business, which tends to result in higher
expenses during the first three quarters of our fiscal year and
significant generation of revenue in our fourth fiscal quarter,
gross margin for a fiscal quarter is not a meaningful indication
of full-year gross margin and is therefore not comparable with
gross margin for prior annual periods.
Research
and Development Expense
Research and development expense consists primarily of:
|
|
|
| |
•
|
salaries and related costs for our scientists;
|
| |
| |
•
|
costs for materials used in product development;
|
49
|
|
|
| |
•
|
costs for third-party licensing, academic programs and
intellectual property;
|
| |
| |
•
|
costs for initiating and managing field tests; and
|
| |
| |
•
|
allocated facility costs.
|
Over the past three years, we have significantly expanded our
research and development activities and expect to continue this
expansion in the future. Substantially all of our in-house
research and development is performed in the United States,
although we maintain small product development staffs in both
New Zealand and Brazil. We are committed to increasing the pace
of new product development as we strive to enhance our ability
to create new and innovative seedling products for use by our
customers in existing forestry end-markets as well as new and
emerging end-markets. Accordingly, we anticipate that our
research and development expense will increase for the
foreseeable future.
In addition, we incur research and development expenses under
grant contracts with both U.S. federal and state
governments. Starting in the year ended March 31, 2010,
these expenses under grant contracts have been classified as
cost of revenue rather than research and development expense.
Prior to the year ended March 31, 2010, the grants and
related expenses were not material and were recorded in research
and development expense. In addition, some of our research and
development programs are partially funded by collaborations with
commercial entities. The expenses and related funding for these
programs were not material and were recorded in research and
development expense.
Selling,
General and Administrative Expense
Our selling, general and administrative, or SG&A, expense
consists primarily of:
|
|
|
| |
•
|
salaries and related costs for executives and administrative
personnel;
|
| |
| |
•
|
professional services costs;
|
| |
| |
•
|
salaries and related costs for sales and marketing personnel;
|
| |
| |
•
|
occupancy and other overhead costs;
|
| |
| |
•
|
advertising, marketing and other brand-building costs; and
|
| |
| |
•
|
allocated facility costs.
|
As we focus on increasing our global share of the commercial
forestry tree seeding market and on continuing to build brand
awareness, we anticipate that SG&A expense will continue to
increase for the foreseeable future. As a percentage of revenue,
however, we generally expect SG&A costs to gradually
decline over the next several years as our revenue grows. In the
short term, however, we expect that our general and
administrative expenses will increase in preparation for
becoming and operating as a public company, including costs
associated with compliance with Section 404 of the
Sarbanes-Oxley Act, directors’ and officers’ liability
insurance, increased professional services and a new investor
relations function.
Depreciation
and Amortization Expense
We capitalize equipment and buildings that we own and depreciate
the costs of those assets over their estimated useful lives.
Depreciation of assets directly related to the production of our
products is charged to cost of revenue. The remaining
depreciation expense is recorded as an operating expense. We
also capitalize certain intangible assets, primarily patents,
and we amortize the costs of those intangibles over their useful
lives.
Loss
on Impairment of Intangible Assets
Impairment of intangible assets occurs primarily when we abandon
patents and patents-in-progress that are no longer of strategic
interest. We perform a review of our intangible assets on a
quarterly basis, and we record loss on impairment of assets if
the review indicates that the fair market value of the
intangibles is less than the recorded value.
50
Restructuring
Expense
In the years ended March 31, 2008 and 2009, we undertook
several restructuring actions in connection with the integration
of the operations contributed to us in October 2007. The costs
relating to those restructurings were $0.2 million and
$0.5 million, respectively. In the year ended
March 31, 2010, due to the worldwide economic downturn, we
suspended operations at one of our U.S. nurseries at a cost
of $0.1 million, as described above.
Other
Operating Expense (Income)
Other operating expense (income) includes job development
credits granted by the State of South Carolina in
connection with increased in-state employment.
Stock-Based
Compensation Expense
To date, we have not incurred stock-based compensation expense
because the appreciation rights held by our employees entitle
them to payment in cash or stock, at the discretion of our board
of directors, upon a sale event (including a sale of our
business or an initial public offering) that has not yet
occurred. In connection with this offering, the holders of
appreciation rights will become entitled to receive a payment
for each appreciation right they hold equal to the difference
between 0.00001% of our value, based on the offering price, the
number of shares issued prior to the event and the adjusted
initial base price of the appreciation right. For additional
information refer to the section below entitled
“—Critical Accounting Policies and
Estimates—Stock-Based Compensation.” Our board of
directors may settle these amounts in cash or shares of our
common stock net of tax withholding obligations. We expect to
incur $ in stock-based
compensation expense upon the closing of this offering, based
upon an assumed initial public offering price of
$ , which is the midpoint of the
estimated price range set forth on the cover page of this
prospectus. In the future, we also expect to incur stock-based
compensation expense in connection with stock awards to be
granted.
Income
Tax Benefit (Expense)
Since we have operated as a limited liability company from
inception to May 31, 2010, any U.S. federal or state
income tax liability or benefit had, prior to our conversion to
a corporation, passed through to our members. As a result, we
have not recorded any income tax expense or benefit in our
consolidated statements of operations, or any tax assets or
liabilities on our consolidated balance sheets relating to our
operations in the United States. In the future, we will record
tax benefits or expense in conjunction with our annual operating
results, although we do not expect to do so in the short term
because we expect that the deferred tax assets resulting from
anticipated losses will be fully offset by a valuation
allowance. As a result, we expect that those losses will not
give rise to a deferred tax benefit in our consolidated
statements of operations in the short term.
51
Results
of Operations
The table below sets forth our results of operations for the
periods shown, including as a percentage of revenue, with all
percentages calculated based on non-rounded numbers:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended March 31,
|
|
|
Nine Months Ended December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(In millions, except %)
|
|
|
|
|
Revenue
|
|
$
|
18.2
|
|
|
|
100.0
|
%
|
|
$
|
23.7
|
|
|
|
100.0
|
%
|
|
$
|
21.6
|
|
|
|
100.0
|
%
|
|
$
|
9.6
|
|
|
|
100.0
|
%
|
|
$
|
11.1
|
|
|
|
100.0
|
%
|
|
Cost of revenue
|
|
|
15.7
|
|
|
|
86.2
|
|
|
|
15.9
|
|
|
|
66.9
|
|
|
|
16.4
|
|
|
|
75.9
|
|
|
|
6.9
|
|
|
|
72.3
|
|
|
|
7.1
|
|
|
|
63.7
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
2.5
|
|
|
|
13.8
|
|
|
|
7.8
|
|
|
|
33.1
|
|
|
|
5.2
|
|
|
|
24.1
|
|
|
|
2.7
|
|
|
|
27.7
|
|
|
|
4.0
|
|
|
|
36.3
|
|
|
Operating expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
10.2
|
|
|
|
56.2
|
|
|
|
13.8
|
|
|
|
58.4
|
|
|
|
11.2
|
|
|
|
51.9
|
|
|
|
7.8
|
|
|
|
81.8
|
|
|
|
9.2
|
|
|
|
82.7
|
|
|
Selling, general and administrative
|
|
|
9.7
|
|
|
|
53.4
|
|
|
|
7.6
|
|
|
|
32.1
|
|
|
|
7.1
|
|
|
|
33.1
|
|
|
|
5.2
|
|
|
|
54.2
|
|
|
|
7.9
|
|
|
|
71.0
|
|
|
Depreciation and amortization
|
|
|
0.7
|
|
|
|
3.7
|
|
|
|
0.8
|
|
|
|
3.4
|
|
|
|
0.8
|
|
|
|
3.6
|
|
|
|
0.7
|
|
|
|
6.9
|
|
|
|
0.4
|
|
|
|
4.0
|
|
|
Loss on impairment of intangible assets
|
|
|
0.2
|
|
|
|
1.1
|
|
|
|
0.6
|
|
|
|
2.7
|
|
|
|
0.2
|
|
|
|
1.0
|
|
|
|
0.2
|
|
|
|
2.1
|
|
|
|
0.1
|
|
|
|
1.4
|
|
|
Restructuring charge
|
|
|
0.2
|
|
|
|
1.0
|
|
|
|
0.5
|
|
|
|
2.1
|
|
|
|
0.1
|
|
|
|
0.5
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Other operating (income), net
|
|
|
(0.4
|
)
|
|
|
(2.1
|
)
|
|
|
(0.5
|
)
|
|
|
(2.0
|
)
|
|
|
(0.2
|
)
|
|
|
(1.1
|
)
|
|
|
(0.2
|
)
|
|
|
(1.9
|
)
|
|
|
(0.2
|
)
|
|
|
(1.7
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expense
|
|
|
20.6
|
|
|
|
113.3
|
|
|
|
22.8
|
|
|
|
96.8
|
|
|
|
19.2
|
|
|
|
89.0
|
|
|
|
13.7
|
|
|
|
143.1
|
|
|
|
17.4
|
|
|
|
157.4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(18.1
|
)
|
|
|
(99.5
|
)
|
|
|
(15.0
|
)
|
|
|
(63.7
|
)
|
|
|
(14.0
|
)
|
|
|
(64.9
|
)
|
|
|
(11.0
|
)
|
|
|
(115.3
|
)
|
|
|
(13.4
|
)
|
|
|
(121.1
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
0.1
|
|
|
|
0.6
|
|
|
|
0
|
|
|
|
0.1
|
|
|
|
0
|
|
|
|
0.1
|
|
|
|
0
|
|
|
|
0.1
|
|
|
|
0
|
|
|
|
0.2
|
|
|
Interest expense
|
|
|
(0.1
|
)
|
|
|
(0.6
|
)
|
|
|
(0.3
|
)
|
|
|
(1.3
|
)
|
|
|
(0.7
|
)
|
|
|
(3.0
|
)
|
|
|
(0.5
|
)
|
|
|
(4.6
|
)
|
|
|
(0.8
|
)
|
|
|
(7.1
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(18.1
|
)
|
|
|
(99.4
|
)%
|
|
$
|
(15.3
|
)
|
|
|
(64.8
|
)%
|
|
$
|
(14.7
|
)
|
|
|
(67.9
|
)%
|
|
$
|
(11.5
|
)
|
|
|
(119.9
|
)%
|
|
$
|
(14.2
|
)
|
|
|
(128.0
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The table below sets forth a reconciliation of adjusted gross
profit and adjusted gross margin to the most directly comparable
GAAP measures. Adjusted gross profit and adjusted gross margin
are our gross profit and gross margin, respectively, less
certain costs. We believe that adjusted gross profit and
adjusted gross margin, when viewed with our GAAP results and the
accompanying reconciliations, provide useful information for
investors and for evaluating our operating performance because
these measures adjust for items that we believe are not
representative of our operating performance. Adjusted gross
profit and adjusted gross margin, however, are not recognized
measurements under GAAP and should not be considered as an
alternative to any measure of financial performance calculated
in accordance with GAAP. The table does not include data for the
nine months ended December 31, 2010 and 2009. Due to the
seasonal nature of our business, which tends to result in higher
expenses during the first three quarters of our fiscal year and
significant generation of revenue in our fourth fiscal quarter,
gross margin for a fiscal quarter or portion of the fiscal year
is not a meaningful
52
indication of full-year gross margin and is therefore not
comparable with gross margin for prior annual periods.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended March 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
|
|
Gross
|
|
|
Gross
|
|
|
Gross
|
|
|
Gross
|
|
|
Gross
|
|
|
Gross
|
|
|
|
|
Profit
|
|
|
Margin
|
|
|
Profit
|
|
|
Margin
|
|
|
Profit
|
|
|
Margin
|
|
|
|
|
(In millions, except %)
|
|
|
|
|
Gross profit/Gross margin
|
|
$
|
2.5
|
|
|
|
13.8
|
%
|
|
$
|
7.8
|
|
|
|
33.1
|
%
|
|
$
|
5.2
|
|
|
|
24.1
|
%
|
|
October 2007 asset contribution-related items:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserve for older generation seed(1)
|
|
|
0.8
|
|
|
|
4.4
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0.9
|
|
|
|
4.2
|
|
|
Initial vacation accrual(2)
|
|
|
0.2
|
|
|
|
1.1
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Product replacement reserve(3)
|
|
|
0.6
|
|
|
|
3.3
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
1.6
|
|
|
|
8.8
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0.9
|
|
|
|
4.2
|
|
|
Bonus accrual variance(4)
|
|
|
—
|
|
|
|
—
|
|
|
|
(0.2
|
)
|
|
|
(0.8
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Restructuring of nursery operations(5)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0.1
|
|
|
|
0.5
|
|
|
Grant contracts(6)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0.3
|
|
|
|
1.4
|
|
|
Seedling write-off(7)
|
|
|
0.2
|
|
|
|
1.1
|
|
|
|
0.9
|
|
|
|
3.8
|
|
|
|
0.9
|
|
|
|
4.2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjusted gross profit/Adjusted gross margin
|
|
|
4.3
|
|
|
|
23.7
|
(8)
|
|
|
8.5
|
|
|
|
36.1
|
(6)(7)
|
|
|
7.4
|
|
|
|
34.4
|
(6)(7)
|
|
|
|
|
(1)
|
|
Following our acquisition of seed
inventory as part of the October 2007 asset contribution, we
established an initial reserve for the oldest generation seed in
the inventory. As we developed our understanding of customer
demand for our products following the asset contribution, we
refined our standards for aged inventory reserves and added an
additional reserve for older seed in the year ended
March 31, 2010.
|
| |
|
(2)
|
|
Following the asset contribution,
we established an initial vacation accrual for employees
involved in the nursery and orchard operations that were
contributed to us.
|
| |
|
(3)
|
|
In the initial period following the
asset contribution, as we took over the operations that were
contributed to us, our seedlings experienced a higher than usual
mortality rate in certain areas in the United States following
an unusually cold and dry harvest season. To maintain customer
relationships, some customers were offered replacement seedling
agreements to offset planting losses, for which we established a
reserve. We have since modified certain of our harvesting
procedures and subsequently have not experienced these issues.
We may incur charges for product replacement reserves from time
to time in the future; however, based on historical experience,
we expect these amounts, if any, to be immaterial.
|
| |
|
(4)
|
|
As part of our cost mitigation
program, we elected not to pay employee bonuses for the year
ended March 31, 2009. We normally accrue for bonuses on an
annual basis. Accordingly, for greater comparability between
years, we have included an adjustment to reflect bonuses at the
level originally accrued for the year ended March 31, 2009.
|
| |
|
(5)
|
|
In connection with the suspension
of operations at one of our U.S. nurseries as part of our cost
mitigation plan, we wrote off deferred costs associated with
fumigation in the year ended March 31, 2010.
|
| |
|
(6)
|
|
As described above, in the year
ended March 31, 2010, we began including grant contract
revenue and associated costs in revenue and cost of revenue,
respectively. In prior years, grants and expense related to
these grant contracts were recorded as a net offset to research
and development expense. In general, contract margins are lower
than product margins. In the future, we expect to record grant
contracts and associated costs in revenues and cost of revenues,
respectively, and this could impact annual gross margins by one
to two percentage points.
|
| |
|
(7)
|
|
Seedling write-off amounts
represent the charge in each fiscal year required to write off
the remaining unsold seedlings at the end of the growing season.
As a result of the worldwide economic downturn, which resulted
in reduced customer demand, we recorded higher charges to
write-off unsold inventory in the fourth quarter of the years
ended March 31, 2009 and 2010. We expect these charges to
return to levels similar to those for the year ended
March 31, 2008. Due to the timing of our growing and sales
cycle, expected customer demand and expected market conditions,
future results may reflect a seedling write-off that could have
a one to four percentage point impact on our annual gross margin.
|
| |
|
(8)
|
|
We acquired
work-in-process
inventory as part of the asset contribution from our
stockholders, which included certain overhead expense that we
estimate reduced our gross margin in the year ended
March 31, 2008 by six to seven percentage points.
|
Comparison
of the Nine-Month Periods ended December 31, 2010 and
2009
Revenue
Revenue increased $1.5 million, or 16%, to
$11.1 million for the nine months ended December 31,
2010 from $9.6 million for the nine months ended
December 31, 2009. During the nine months ended
December 31, 2010, we sold 61.9 million seedlings in
New Zealand, Australia and the United States, at an
53
average selling price of $179 per 1,000 seedlings. During the
nine months ended December 31, 2009, we sold
71.4 million seedlings in New Zealand, Australia and the
United States at an average selling price of $134 per 1,000
seedlings. The increase in the average selling price of $45 per
1,000 seedlings, or 34%, contributed an increase in revenue of
$2.8 million. The increase in the average selling price was
due to increased sales to customers in the United States of our
elite OP, MCP and varietal seedling products, which range in
list price from $70 to $400 per 1,000 seedlings, and increased
sales to New Zealand and Australia customers of our higher
priced MCP and varietal radiata seedling products, which range
in list price from $140 to $550 per 1,000 seedlings. The
increase in average selling price was partially offset by a
decrease in sales volume of 9.5 million seedlings, or 13%,
representing a $1.3 million decrease in revenue, which was
primarily attributable to the timing of the beginning of our
seedling harvest season in December 2010, as compared to a
slightly earlier harvest initiation in December 2009. As in
prior years, we expect that the average selling price of our
products will decline for the three-month period ending on
March 31, 2011, as compared to the average selling price as
of December 31, 2010, primarily due to an increase in sales
during this quarter of OP seedlings which are priced at levels
below the prices of our higher-value products.
Cost of
Revenue
Our cost of revenue increased $0.2 million, or 2%, to
$7.1 million for the nine months ended December 31,
2010 from $6.9 million for the nine months ended
December 31, 2009. This increase was primarily due to the
increased sales volume associated with our higher-value
products, which in general have a higher cost of revenue. The
increase was partially offset by a $0.3 million decrease in
cost of revenue related to our largest research and development
grant contract, and by the decline in sales volume described
above, due to the timing of the beginning of our seedling
harvest season.
Gross
Profit/Gross Margin
Gross profit increased $1.4 million, or 52%, to
$4.0 million for the nine months ended December 31,
2010 from $2.7 million for the nine months ended
December 31, 2009. Gross margin increased
8.6 percentage points to 36.3% of revenue for the nine
months ended December 31, 2010 from 27.7% of revenue for
the nine months ended December 31, 2009. This increase was
primarily the result of the higher average selling prices for
products sold during the nine months ended December 31,
2010 as compared to the nine months ended December 31, 2009.
Research
and Development Expense
Research and development expense increased to $9.2 million
for the nine months ended December 31, 2010, as compared to
$7.8 million for the nine months ended December 31,
2009. The increase of $1.4 million is consistent with our
plan to return to more normal levels of research and development
spending following our cost mitigation program in the year ended
March 31, 2010. We expect research and development expense
to increase over the next 12 months.
Selling,
General and Administrative Expense
Selling, general and administrative expense increased
$2.7 million, or 51%, to $7.9 million for the nine
months ended December 31, 2010 from $5.2 million for
the nine months ended December 31, 2009. The increase was
primarily due to an increase of $1.2 million for the hiring
of new personnel, the accrual of bonuses for retention of
management, and other payroll related expenses, the costs
related to preparation for becoming and operating as a public
company of $0.4 million, an increase of $0.6 million
for litigation expenses, and $0.3 million in sales and
marketing expenses for the promotion of our varietal and
eucalyptus seedling products.
Depreciation
and Amortization Expense
Our depreciation and amortization expense decreased by
$0.3 million, or 33%, to $0.4 million for the nine
months ended December 31, 2010 from $0.7 million for
the nine months ended December 31, 2009. The
54
decrease was due to the full depreciation of certain depreciable
assets as of March 31, 2010, resulting in a lower base for
the calculation of annual depreciation. The amount of annual
depreciation is expected to increase in the future due to
several capital expenditure projects being placed in service in
the current fiscal year, and due to the expected purchase of a
new manufacturing, research and development laboratory and
headquarters facility.
Loss on
Impairment of Intangible Assets
Loss on impairment of intangible assets was $0.1 million
for the nine months ended December 31, 2010, a decrease of
$0.1 million as compared to the $0.2 million for the
nine months ended December 31, 2009, and represented the
abandonment of several
patents-in-progress.
Other
Operating (Income), Net
Other operating income was $0.2 million for both the nine
months ended December 31, 2010 and December 31, 2009,
and represented state job development credits.
Interest
Income (Expense), Net
Net interest expense increased $0.3 million to
$0.8 million for the nine months ended December 31,
2010 from $0.5 million for the nine months ended
December 31, 2009. This increase was due primarily to the
renewal and increased drawdown of our U.S. line of credit,
and the establishment of a credit facility in New Zealand in
November 2009 and borrowings under that facility.
Comparison
of Years Ended March 31, 2010 and 2009
Revenue
Revenue decreased $2.1 million, or 8.9%, to
$21.6 million for the year ended March 31, 2010 from
$23.7 million for the year ended March 31, 2009.
During the year ended March 31, 2010, we sold
240 million seedlings in the United States, New Zealand and
Australia at an average selling price of $90 per 1,000
seedlings. During the year ended March 31, 2009, we sold
286 million seedlings in the United States,
New Zealand and Australia at an average selling price of
$83 per 1,000 seedlings. The decrease in volume of
46 million seedlings, or 16%, representing a
$3.8 million decline in revenue, was primarily attributable
to reduced customer demand in the United States due primarily to
the worldwide economic downturn, the slowdown in housing starts,
and the reduction in demand for paper products, all of which led
to a reduction in forest harvesting levels. In addition,
weather-related delays in land preparation by our customers in
some areas of the Southeastern United States led to reduced
seedling planting. The volume decrease was partially offset by
an increase in the average selling price of $7 per 1,000
seedlings, or 8.4%, which contributed an increase in revenue of
$1.7 million. The increase in average selling price was due
primarily to an increase in our elite OP, MCP and varietal
loblolly pine seedling products as a percentage of our sales,
which range in list price from $70 to $400 per 1,000 seedlings.
In comparison, there was a decrease in our other OP loblolly
pine seedling products as a percentage of our sales for the year
ended March 31, 2010, which range in list price from $45 to
$63 per 1,000 seedlings. The average selling price increase for
the year ended March 31, 2010 was augmented by the
inclusion in revenue of contract revenue. During the year ended
March 31, 2009, grants and related expenses were not
material and were recorded in research and development expense.
Cost of
Revenue
Our cost of revenue increased $0.5 million, or 3.4%, to
$16.4 million for the year ended March 31, 2010 from
$15.9 million for the year ended March 31, 2009. As we
developed our understanding of customer demand for our products
following the asset contribution, we refined our standards for
aged inventory reserves and added an additional reserve of
$0.9 million for older generation seed that is sought less
frequently by our customers due to higher demand for our
higher-value seedling products. In addition, for the year ended
March 31, 2010, we recorded costs associated with grant
contract revenue of $0.9 million. For the year ended
March 31, 2009, these costs were recorded in research and
development expense. Cost of revenue also
55
increased in the year ended March 31, 2010 because, as part
of our cost mitigation program, we elected not to pay employee
bonuses for the year ended March 31, 2009. The increase in
cost of revenue was partially offset by the decline in sales
volume described above, which decreased cost of revenue by
almost $1.4 million.
Gross
Profit/Gross Margin
Gross profit decreased $2.6 million, or 33.7%, to
$5.2 million for the year ended March 31, 2010 from
$7.8 million for the year ended March 31, 2009. Gross
margin decreased 9.0 percentage points from 33.1% of
revenue for the year ended March 31, 2009 to 24.1% of
revenue for the year ended March 31, 2010. Of this decline
in gross margin, 4.2 percentage points resulted from the
charge of $0.9 million to reserve for older generation
seed. Gross margin further declined during the year ended
March 31, 2010, due to changes in our freight fee structure
that resulted in a 1.9 percentage point impact to gross
margin. The addition of grant contract revenue and costs also
lowered gross margin by 1.4 percentage points. For the year
ended March 31, 2009, the gross margin was also higher by
0.8 percentage points because we did not pay bonuses. In
both periods, gross margin included the impact of a
$0.9 million write-off for unsold seedlings at the end of
each year. The impact in the year ended March 31, 2010 was
an incremental decline in gross margin of 0.4 percentage
points. These write-offs reflected reduced customer demand as a
result of the worldwide economic downturn, and we expect this
charge to decrease for the year ending March 31, 2011.
Research
and Development Expense
Research and development expense decreased $2.6 million, or
19.0%, to $11.2 million for the year ended March 31,
2010 from $13.8 million for the year ended March 31,
2009. The decrease was primarily attributable to a reduction in
research and development spending of $3.0 million due to
our cost mitigation program, which we put in place in light of
the impact of the worldwide economic downturn. Offsetting this
decrease is the absence of grant contract funding and expenses,
which were a net credit of $0.1 million for the year ended
March 31, 2009, but are recorded in revenue and cost of
revenue, respectively, beginning in the year ended
March 31, 2010.
Selling,
General and Administrative Expense
Selling, general and administrative expense decreased
$0.5 million, or 6.1%, to $7.1 million for the year
ended March 31, 2010 from $7.6 million for the year
ended March 31, 2009. The decrease was due to our cost
mitigation program, which included deferring new hires, reducing
advertising and marketing expenses and delaying technology
projects.
Depreciation
and Amortization Expense
Our depreciation and amortization expense was $0.8 million
for the years ended March 31, 2010 and 2009, and
represented capital additions of furniture and equipment,
buildings and building improvements during the year ended
March 31, 2010.
Loss on
Impairment of Intangible Assets
Impairment of intangible assets decreased $0.4 million, or
66.9%, to $0.2 million for the year ended March 31,
2010 from $0.6 million for the year ended March 31,
2009. The decrease was primarily due to the abandonment of fewer
patents-in-progress as compared to the year ended March 31,
2009.
Restructuring
Expense
Restructuring expense decreased $0.4 million, or 79.5%, to
$0.1 million for the year ended March 31, 2010 from
$0.5 million for the year ended March 31, 2009.
Restructuring expense for the year ended March 31, 2009
resulted from the integration of the operations contributed to
us in the asset contribution in October 2007, while the
restructuring expense for the year ended March 31, 2010 was
primarily due to the suspension of operations at one of our
U.S. nurseries as part of our cost mitigation program.
56
Other
Operating (Income), Net
Other operating income, net decreased $0.3 million, or
49.8%, to $0.2 million for the year ended March 31,
2010 from $0.5 million for the year ended March 31,
2009. The decrease was primarily due to a $0.1 million
decrease in state job development credits and $0.1 million
decrease in gains on sales of property, plant and equipment.
Interest
Income (Expense), Net
Net interest expense increased $0.4 million, or 139.3%, to
$0.7 million for the year ended March 31, 2010 from
$0.3 million for the year ended March 31, 2009. This
increase was due primarily to the renewal and increased drawdown
of our U.S. line of credit, and the establishment of a
credit facility in New Zealand in November 2009 and
borrowings under such facility.
Comparison
of Years Ended March 31, 2009 and 2008
Revenue
Revenue increased $5.5 million, or 30.2%, to
$23.7 million for the year ended March 31, 2009 from
$18.2 million for the year ended March 31, 2008. As
described above, as a result of the asset contribution in
October 2007, our revenue for the year ended March 31, 2008
reflects only our U.S. operations and does not include our
operations in New Zealand and Australia. During the year ended
March 31, 2009, we sold 286 million seedlings in the
United States, New Zealand and Australia at an average selling
price of $83 per 1,000 seedlings. During the year ended
March 31, 2008, we sold 278 million seedlings in the
United States at an average selling price of $65 per 1,000
seedlings. The average selling price increase of $18 per 1,000
seedlings, or 27.7%, accounted for $5.0 million of the
revenue increase and was due primarily to an increase in the
sales volume and average selling price of our elite OP, MCP and
varietal seedling products along with increases in the average
selling prices of our other conventional products. The increase
in sales volume of 8 million seedlings, or 2.9%,
represented $0.7 million of the revenue increase and was
due to the inclusion of 22 million seedlings sold in New
Zealand and Australia in the year ended March 31, 2009,
partially offset by a decrease in U.S. sales volume of
14 million seedlings due to the worldwide economic
downturn, which led to reduced seedling planting by our
customers as they chose to harvest fewer of their existing trees.
Cost of
Revenue
Our cost of revenue increased $0.2 million, or 1.1%, to
$15.9 million for the year ended March 31, 2009 from
$15.7 million for the year ended March 31, 2008. Cost
of revenue increased by $0.8 million as a result of
increased sales volume associated with our higher-value
products, which in general have a higher cost of revenue, and by
$0.7 million as a result of an increase in the seedling
write-off for unsold inventory for the year ended March 31,
2009 in light of the worldwide economic downturn. These
increases in cost of revenue were offset by the absence of
$1.6 million of charges in the year ended March 31,
2008 related to the asset contribution in October 2007. These
charges included $0.8 million to reserve for older
generation seed, $0.6 million to reserve for replacement
seedlings and $0.2 million to establish an initial vacation
accrual.
Gross
Profit/Gross Margin
Gross profit increased $5.3 million, or 211.7%, to
$7.8 million for the year ended March 31, 2009 from
$2.5 million for the year ended March 31, 2008. Gross
margin increased from 13.8% in the year ended March 31,
2008 to 33.1% in the year ended March 31, 2009, an
improvement of 19.3 percentage points. The increase in
sales volume and the improvement in average selling price during
the year ended March 31, 2009 contributed 9 percentage
points to the higher gross margin. A further 8.8 percentage
points was the result of the absence of $1.6 million of
charges for the year ended March 31, 2008 related to the
October 2007 asset contribution described above. Gross margin
improved by approximately 6 to 7 percentage points due to
higher cost of revenue for the year ended March 31, 2008
related to certain overhead expense that was included in the
cost of
work-in-process
inventory contributed to us in October 2007. Gross margin was
reduced by 2.7 percentage points due to higher seedling
write-offs associated with unsold inventory reflecting reduced
57
customer demand as a result of the worldwide economic downturn.
As part of our cost mitigation plan, we did not accrue for
bonuses for the year ended March 31, 2009, although we
normally accrue for bonuses on an annual basis. The lack of a
bonus accrual for the year ended March 31, 2009 resulted in
a gross margin improvement of 0.8 percentage points.
Research
and Development Expense
Research and development expense increased $3.6 million, or
35.5%, to $13.8 million for the year ended March 31,
2009 from $10.2 million for the year ended March 31,
2008. For the years ended March 31, 2009 and 2008, we
incurred the majority of our research and development expense to
support the development of various advanced seedling products
and increased regulatory staffing to support the submission of
petitions for deregulation of our new biotechnology products to
the USDA.
Selling,
General and Administrative Expense
Selling, general and administrative expense decreased
$2.1 million, or 21.7%, to $7.6 million for the year
ended March 31, 2009 from $9.7 million for the year
ended March 31, 2008. Our selling, general and
administrative expense in the year ended March 31, 2008
included $2.3 million of legal and accounting expenses we
incurred in connection with the October 2007 asset contribution.
Partially offsetting this $2.3 million decrease is the
$0.2 million impact of nursery and orchard selling expenses
for a full year in the year ended March 31, 2009 as
compared to only five months in the year ended March 31,
2008.
Depreciation
and Amortization Expense
Our depreciation and amortization expense increased
$0.1 million, or 19.7%, to $0.8 million for the year
ended March 31, 2009 from $0.7 million for the year
ended March 31, 2008. The increase was primarily due to the
inclusion of a full year of depreciation and amortization on
property, plant and equipment contributed in the October 2007
asset contribution for the year ended March 31, 2009, as
compared to only five months of depreciation for the year ended
March 31, 2008.
Loss on
Impairment of Intangible Assets
Impairment of intangible assets increased $0.4 million, or
217.8%, to $0.6 million for the year ended March 31,
2009 from $0.2 million for the year ended March 31,
2008. The increase was primarily due to the abandonment of fewer
patents-in-progress. Furthermore, we recorded an impairment of
$0.1 million for a non-compete agreement during the year
ended March 31, 2009.
Restructuring
Expense
Restructuring expense increased $0.3 million, or 169.1%, to
$0.5 million for the year ended March 31, 2009 from
$0.2 million for the year ended March 31, 2008.
Restructuring expense in both years related to the integration
of the operations contributed to us in the asset contribution in
October 2007.
Other
Operating (Income), Net
Other operating (income), net increased $0.1 million, or
20.4%, to $0.5 million for the year ended March 31,
2009 from $0.4 million for the year ended March 31,
2008. The increase was primarily due to gains on the sale of
equipment as a result of consolidating nursery and orchard
activities after the asset contribution in October 2007.
Interest
Income (Expense), Net
Interest income (expense), net increased $0.3 million to a
net expense of $0.3 million for the year ended
March 31, 2009 from net income of less than
$0.1 million for the year ended March 31, 2008. The
increase was due to the renewal and increased drawdown of our
U.S. line of credit during the year ended March 31,
2009.
58
Liquidity
and Capital Resources
As of December 31, 2010, in addition to
cash-on-hand
and cash provided by operations, our external sources of
liquidity were comprised of a revolving line of credit, a
multi-option credit facility and cash contributions from our
stockholders. We have historically funded our growth primarily
through capital contributions from our stockholders,
International Paper Company, MeadWestvaco Corporation and
Rubicon Limited and borrowings under our lines of credit. We
believe that the net proceeds of this offering, existing cash
and cash equivalents on hand and funds available through our
bank lines of credit will be sufficient to meet our working
capital and capital expenditure needs for at least the next
12 months.
Cash
Flows
The table below sets forth a summary of cash flows for the years
ended March 31, 2008, 2009, 2010 and for the nine months
ended December 31, 2009 and 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
|
Year Ended March 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(In millions)
|
|
|
|
|
Net cash (used in) operating activities
|
|
$
|
(26.1
|
)
|
|
$
|
(12.3
|
)
|
|
$
|
(12.0
|
)
|
|
$
|
(12.6
|
)
|
|
$
|
(15.3
|
)
|
|
Net cash (used in) investing activities
|
|
|
(1.7
|
)
|
|
|
(2.1
|
)
|
|
|
(1.5
|
)
|
|
|
(1.1
|
)
|
|
|
(1.7
|
)
|
|
Net cash provided by financing activities
|
|
|
27.9
|
|
|
|
14.3
|
|
|
|
15.3
|
|
|
|
15.1
|
|
|
|
15.3
|
|
|
Effect in exchange rate changes on cash
|
|
|
—
|
|
|
|
(0.3
|
)
|
|
|
0.3
|
|
|
|
0.1
|
|
|
|
0.1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
$
|
0.1
|
|
|
$
|
(0.4
|
)
|
|
$
|
2.1
|
|
|
$
|
1.5
|
|
|
$
|
(1.6
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Our seedling inventory levels in the United States tend to be
minimal at the end of each fiscal year, since almost all of our
U.S. products are delivered to customers by the end of
March. New seedlings are then planted primarily in April and May
for the next production year. New Zealand and Australian
seedlings are planted primarily in October and November, with
customer shipments occurring between June and September. Seed
inventory is long-term in nature and does not vary substantially
on a
quarter-to-quarter
basis, except when seed inventory is written down.
Our seedling product sales are, and are expected to continue to
be, highly seasonal. This seasonality, especially in the United
States, typically results in a net use of cash to support our
operating needs during the first three quarters of the fiscal
year with the most significant use of cash occurring during the
months of July through September, and a favorable cash flow
during the fourth quarter of the fiscal year as we recognize the
sales of a large portion of our products. We have relied on
contributions from our stockholders and our revolving line of
credit to cover the short-term cash needs resulting from the
seasonality of our seedling business. The negative net working
capital as of December 31, 2010 was primarily the result of
the classification of borrowings under our U.S. revolving line
of credit from long-term debt to a current liability, as well as
costs incurred in connection with this offering that had not
been paid as of December 31, 2010. We intend to reduce or
extinguish our outstanding borrowings and pay offering costs
with the net proceeds from this offering.
Cash Used
in Operating Activities
Our cash used in operating activities for the nine months ended
December 31, 2010 was $15.3 million, compared to
$12.6 million during the nine months ended
December 31, 2009. The $2.7 million increase in cash
used in our operating activities in the nine months ended
December 31, 2010 was primarily due to increased research
and development spending and to working capital needs primarily
related to an increase in inventories for the current seedling
season in the United States. Our allowance for doubtful accounts
increased to $0.4 million as of December 31, 2010 from
$0.1 million as of March 31, 2010. This increase was
due primarily to the bankruptcy filing of one of our large
Australian customers, and to a lesser extent to our ongoing
evaluations of our allowance for doubtful accounts, during the
nine months ended December 31, 2010. Historically, our
allowance for doubtful accounts has fluctuated in line with
fluctuations in our accounts receivables, which are generally
higher in the second and third quarter of our fiscal year due to
the seasonality of our business.
59
Our cash used in operating activities for the year ended
March 31, 2010 was $12.0 million, compared to
$12.3 million during the year ended March 31, 2009.
The $0.3 million decrease in cash used in our operating
activities in the year ended March 31, 2010 was primarily
due to a decrease in working capital needs resulting from the
implementation of cost mitigation programs in response to the
worldwide economic downturn.
Cash used in operating activities decreased to
$12.3 million during the year ended March 31, 2009
from $26.1 million for the year ended March 31, 2008.
This decrease in cash used in operating activities was primarily
the result of decreased working capital spending during the year
ended March 31, 2009. This decrease in spending was
primarily due to the October 2007 contribution of the nursery
and orchard operations by our stockholders, which resulted in
unusual spending in the year ended March 31, 2008,
including for legal and accounting services.
Net Cash
Used in Investing Activities
Net cash used in our investing activities was $1.7 million
in the nine months ended December 31, 2010, and
$1.1 million for the nine months ended December 31,
2009. Investment activities for both periods were primarily for
the purchase of capital equipment to support our growth,
especially to expand our seedling production capacity, and to
add engineering and test equipment. The increase in the
investing activity for the nine months ended December 31,
2010 was primarily the result of a significant expansion in our
capacity to grow containerized seedlings at one of our
U.S. nursery operations.
Net cash used in our investing activities was $1.5 million
in the year ended March 31, 2010, and $2.1 million for
the year ended March 31, 2009. Investment activities
throughout the periods represent the purchase of capital
equipment to support our growth, including materials and labor
to construct production facilities for increased capacity to
grow containerized seedlings, computer equipment, internal use
software, furniture and fixtures, and engineering and test
equipment. The decrease in cash used in investing activities of
$0.6 million was primarily due to a decrease in investment
in intangible assets as a result of a management decision to
focus on core technologies and established research and
development programs due to the implementation of cost
mitigation programs in response to the worldwide economic
downturn. Investments in intangible assets for the year ending
March 31, 2011 are expected to return to levels consistent
with the year ended March 31, 2009, and may increase
further.
Cash used in investing activities increased to $2.1 million
during the year ended March 31, 2009 from $1.7 million
for the year ended March 31, 2008. This increase in cash
used in investing activities was primarily the result of
increased spending during the year ended March 31, 2009 to
expand our intangible asset portfolio.
Net Cash
Provided by Our Financing Activities
Net cash provided by our financing activities was
$15.3 million in the nine months ended December 31,
2010, and $15.1 million for the nine months ended
December 31, 2009. Net cash provided by our financing
activities in the nine months ended December 31, 2010
consisted primarily of $12.8 million received from our
stockholders and borrowings on our U.S. revolving line of
credit of $6.0 million, partially offset by repayments of
$2.8 million on our U.S. revolving line of credit and
payment of $0.8 million of public offering costs. Net cash
provided by our financing activities for the nine months ended
December 31, 2009 consisted primarily of $7.3 million
received from our stockholders and $8.3 million of
borrowings on our U.S. revolving line of credit. These
borrowings were partially offset by repayments of
$0.5 million on our U.S. revolving line of credit.
Net cash provided by our financing activities was
$15.3 million in the year ended March 31, 2010, and
$14.3 million for the year ended March 31, 2009. The
increase in net cash provided by financing activities was due to
increased borrowings on our credit facilities in the year ended
March 31, 2010. Net cash provided by our financing
activities in the year ended March 31, 2010 consisted
primarily of $8.8 million received from our stockholders,
$4.3 million of borrowings under our New Zealand
multi-option credit facility and $4.1 million of borrowings
under our U.S. revolving line of credit. These borrowings
were partially offset by repayments on our U.S. revolving
line of credit of $1.7 million during the year ended
March 31, 2010. Net
60
cash provided by our financing activities in the year ended
March 31, 2009 consisted primarily of $8.7 million
received from our stockholders and $10.0 million of
borrowings under our U.S. revolving line of credit. These
borrowings were partially offset by repayments on our
U.S. revolving line of credit of $4.5 million during
the year ended March 31, 2009.
Net cash provided by our financing activities was
$14.3 million in the year ended March 31, 2009, and
$27.9 million for the year ended March 31, 2008. The
decrease in net cash provided by financing activities in the
year ended March 31, 2009 compared to the year ended
March 31, 2008 was primarily due to the decrease in our
stockholders’ capital contributions of $16.8 million
from $25.5 million in the year ended March 31, 2008 to
$8.7 million in the year ended March 31, 2009. The
decrease in the stockholders’ capital contributions between
the comparative periods is primarily the result of the
contribution of the nursery and orchard operations by our
stockholders in October 2007. Net cash provided by our financing
activities in the year ended March 31, 2008 consisted
primarily of $25.5 million received from our stockholders
and $5.0 million of borrowings under our
U.S. revolving line of credit. These borrowings were
partially offset by repayments to our U.S. revolving line
of credit of $2.5 million during the year ended
March 31, 2008.
Capital
Requirements
We currently have no material cash commitments, except for
normal recurring trade payables, expense accruals and operating
leases, all of which we anticipate funding through the net
proceeds we receive from this offering. We expect that capital
spending will rise in the future as we expand our greenhouse
facilities and container production capacity, increase sales of
our varietal seedling products and then begin to introduce
biotechnology seedling products into the market. In November
2010, we signed a lease for a new manufacturing, research and
development laboratory and headquarters facility in Summerville,
South Carolina. The lease includes an option for us to purchase
the facility, which we may exercise during the year ended
March 31, 2012 using net proceeds from this offering. We
believe that our existing cash, cash equivalents, funds
available through our working capital lines of credit, and the
net proceeds from this offering will be sufficient to meet our
working capital and capital expenditure needs over at least the
next 12 months. In the event that our revenue plan does not
meet our expectations, we may eliminate or curtail expenditures
to mitigate the impact on our use of cash.
Our future capital requirements will depend on many factors,
including our rate of revenue growth, the expansion of our
marketing and sales activities, the timing and extent of
spending to support product development efforts, the timing of
introductions of new products and enhancements to existing
products, the acquisition of new capabilities or technologies,
and the rate of market acceptance of our products and services.
Moreover, to the extent that existing cash, cash equivalents,
cash from short-term borrowing and the net proceeds from this
offering are insufficient to fund our future activities, we may
need to raise additional funds through public or private equity
or debt financing. Although we are currently not a party to any
agreement or letter of intent with respect to potential
investments in, or acquisitions of, businesses, services or
technologies, we may enter into these types of arrangements in
the future, which could also require us to seek additional
equity or debt financing. Additional funds may not be available
on terms favorable to us or at all.
Our capital spending is generally limited to leasehold
improvements, computers, office furniture, test equipment and
the expansion of our production capacity at our nurseries,
especially for increased capacity to grow containerized
seedlings. To satisfy increased customer demand, we also
outsource the production of some of our containerized and
varietal products to independent contractors. We currently
outsource only a small percentage of seedlings sold, but as we
expand our operations generally and our sale of containerized
products in particular, we expect to outsource a larger
percentage of our seedling production to third parties. This
outsourcing will reduce the amount of cash needed for expansion.
New
Lease
In November 2010, we signed a build-to-suit and lease agreement
with Forestry Research Holdings, LLC for a new manufacturing,
research and development laboratory and headquarters facility in
Summerville, South Carolina, which we expect will be completed
by the end of 2011. Under this
20-year
lease, we are obligated
61
to pay annual rent of the greater of $1.0 million or 10% of
the landlord’s investment in the property; provided that
the landlord’s investment will not exceed
$14.3 million. The agreement includes an option for us to
purchase the facility beginning on the date of rent
commencement, which is the earlier of commencement of business
activities on the premises or 30 days after substantial
completion of the facility. We may use net proceeds from this
offering to exercise our option to purchase the new facility
during the year ended March 31, 2012. At the time we expect
to exercise the option, the purchase price will be the
landlord’s investment in the property (which will not
exceed $14.3 million) plus five percent and any tax liability
incurred by the landlord due to sale of the property within one
year of development.
Credit
Facilities
Revolving
Line of Credit
On March 4, 2010, we renewed a working capital line of
credit with a
U.S.-based
bank under which we can borrow up to $18.5 million,
including a $1.5 million
sub-limit
for equipment financing. As of December 31, 2010, we had
$13.6 million outstanding and $4.9 million available
under the revolving line of credit. Interest accrues at a
variable rate based on prime rates. The line expires on
May 31, 2011, at which time all advances will be
immediately due and payable. Borrowings are collateralized by
substantially all of our U.S. assets, including property,
plant, and equipment, inventory and accounts receivable
(carrying value of $25.9 million). The credit facility
restricts our ability to:
|
|
|
| |
•
|
make distributions, loans or advances to our affiliates;
|
| |
| |
•
|
incur or guaranty additional debt;
|
| |
| |
•
|
create liens;
|
| |
| |
•
|
sell or encumber assets;
|
| |
| |
•
|
lease real property; or
|
| |
| |
•
|
merge or consolidate with other entities.
|
In addition, we are required, among other covenants, to maintain
an annual net worth of not less than $20 million and a
tangible net worth of not less than $16 million. Annual net
worth is defined as preferred interest of member companies plus
members’ equity interest, without deducting or adding
accumulated other comprehensive loss. Tangible net worth is
defined as annual net worth less intangible assets, net. In
addition, pursuant to the credit facility, for the year ended
March 31, 2010, our stockholders were required to make cash
equity contributions of up to $8.9 million to support
research and development expenditures of up to
$11.9 million. These operating and financial covenants may
restrict our ability to finance our operations, engage in
business activities or expand our business.
We were not in compliance with our annual net worth and tangible
net worth covenants under this credit facility as of
December 31, 2010. Our annual net worth and tangible net
worth as of December 31, 2010 were $18.9 million and
$14.8 million, respectively. We have obtained a waiver from
the bank as of February 4, 2011, which waives any remedies
available to the bank for lack of compliance with these
covenants as of December 31, 2010 and for the five month
period commencing on January 1, 2011 and ending on the
expiration of the line on May 31, 2011. This waiver does
not restrict our ability to borrow additional funds under this
line of credit prior to its expiration. We expect to continue to
borrow under this line of credit as needed until the completion
of this offering. We expect to extinguish amounts payable under
this line using a portion of the net proceeds of this offering.
Multi-Option
Credit Facility
On November 4, 2009, our New Zealand subsidiary obtained a
term loan with a New Zealand-based bank under which we can
borrow up to NZ$6.0 million (equivalent to
US$4.6 million as of December 31, 2010). Interest
accrues at a variable rate based on prime rates. The line
expires on November 4, 2012, at which time all advances
will be immediately due and payable. As of December 31,
2010, our New Zealand subsidiary had
62
NZ$6.0 million outstanding (equivalent to
US$4.6 million as of December 31, 2010) and the credit
facility was fully drawn. The credit facility requires our New
Zealand subsidiary to make annual principal repayments of
NZ$0.5 million (equivalent to US$0.4 million as of
December 31, 2010). The multi-option credit facility is
collateralized by mortgages on land and buildings that are
located in New Zealand and owned by our New Zealand subsidiary
and by assets located in Australia and owned by one of our
Australian subsidiaries. Our Australian subsidiaries, ArborGen
Australia Pty. Limited and ArborGen Australia Holdings Pty.
Limited are guarantors of the multi-option credit facility.
The credit facility restricts our New Zealand subsidiary’s
ability to:
|
|
|
| |
•
|
incur additional debt;
|
| |
| |
•
|
create liens;
|
| |
| |
•
|
materially change the nature or scope of its business;
|
| |
| |
•
|
redeem its shares;
|
| |
| |
•
|
make distributions;
|
| |
| |
•
|
acquire interests in real estate;
|
| |
| |
•
|
dispose of assets;
|
| |
| |
•
|
apply funds drawn down under the credit facility toward capital
reductions, interest repayments, dividends
and/or
management fees; or
|
| |
| |
•
|
consolidate or merge with other entities.
|
In addition, our New Zealand subsidiary is required, among other
covenants, to maintain certain fixed charge coverage and equity
and
loan-to-value
ratios under the credit facility. These operating and financial
covenants may restrict our ability to finance our operations,
engage in business activities or expand our business. As of
December 31, 2010, we were in compliance with all covenants
under the credit facility.
Stockholder
Loans
In January 2011, we issued promissory notes to our three
stockholders in the aggregate amount of $1,200,000. Pursuant to
the terms of each $400,000 note to each stockholder, interest
accrues at a rate of LIBOR plus 3%, and each note matures on the
earlier of July 15, 2011 or seven business days following
either the closing of this offering or our receipt of proceeds
from other sales of capital stock sufficient to repay the note.
All accrued interest under each note will be forgiven in its
entirety if the note balance is repaid by the maturity date. We
expect to use net proceeds from this offering to repay the
indebtedness under these notes on or prior to the maturity date.
Contractual
Obligations
We generally do not enter into binding purchase commitments. Our
principal commitments consist of obligations under our lines of
credit, leases for office and lab space, leases for agricultural
and automotive vehicles, and minimum contractual obligations for
services. The following table describes our commitments to
settle contractual obligations in cash as of December 31,
2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments Due by Period
|
|
|
|
|
|
|
|
Less than
|
|
|
1 to
|
|
|
3 to 5
|
|
|
|
|
Total
|
|
|
1 Year
|
|
|
3 Years
|
|
|
Years
|
|
|
|
|
(In thousands)
|
|
|
|
|
Capital leases and other obligations(1)
|
|
$
|
225
|
|
|
$
|
96
|
|
|
$
|
118
|
|
|
$
|
11
|
|
|
Credit facilities(2)
|
|
|
18,239
|
|
|
|
14,011
|
|
|
|
4,228
|
|
|
|
—
|
|
|
Credit facilities — interest
|
|
|
687
|
|
|
|
398
|
|
|
|
289
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
19,151
|
|
|
$
|
14,505
|
|
|
$
|
4,635
|
|
|
$
|
11
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
|
In November 2010, we signed a
build-to-suit
and lease agreement to construct a new manufacturing, research
and development and headquarters facility in Summerville, South
Carolina. The lease has a term of 20 years with rental
payments due annually at the greater of $1.0 million or 10%
of the landlord’s investment in the property, provided that
the landlord’s investment will not exceed
|
63
|
|
|
|
|
|
$14.3 million. We may use net
proceeds from this offering to exercise our option to purchase
the new facility during the year ended March 31, 2012.
Accordingly, we have not included lease payments in capital
leases and other obligations. At the time we expect to exercise
the option, the purchase price will be the landlord’s
investment in the property (which will not exceed $14.3 million)
plus five percent and any tax liability incurred by the landlord
due to sale of the property within one year of development.
|
| |
|
(2)
|
|
Does not include $1.2 million
of debt owing to our stockholders, which was incurred in
January 2011.
|
Off-Balance
Sheet Arrangements
As of March 31, 2010, we had no off-balance sheet
arrangements as defined in Item 303(a)(4) of
Regulation S-K
under the Securities Exchange Act of 1934, as amended.
Related
Party Transactions
The following is a discussion of transactions with related
parties that we believe are necessary for an understanding of
our current and prospective financial position and operating
results. Additional detail about these transactions can be found
in the section entitled “Certain Relationships and Related
Party Transactions.”
Capital
Contributions and Stockholder Loans
From our inception through our conversion to a corporation on
June 1, 2010, our stockholders made capital contributions
to us in an aggregate of $166.2 million in cash and assets.
This amount reflects contributed assets in 2007 at the
stockholders’ respective historical costs. Including the
contributed assets at their fair value, which was determined at
the time of the contribution, the aggregate contributions of our
stockholders have been more than $200 million. During the
three-year period ended March 31, 2010, excluding the
assets contributed in the transaction described below under
“—Asset Contribution,” MeadWestvaco and Rubicon
each made cash contributions to us of $13.6 million and
International Paper made cash contributions of $2.4 million
and reduced its preferred interest by $11.1 million in lieu
of making cash contributions. Since our conversion to a
corporation on June 1, 2010, our stockholders have made
aggregate capital contributions of $7.9 million pursuant to
the terms of our Stockholders Agreement. In addition, as
described under “Liquidity and Capital
Resources—Stockholder Loans” above, in
January 2011, we issued promissory notes to our three
stockholders in the aggregate amount of $1,200,000.
Asset
Contribution
On October 31, 2007, the commercial tree improvement,
nursery and orchard businesses of International Paper,
MeadWestvaco and Rubicon were contributed to us. Pursuant to
this transaction, we acquired cash and various non-cash assets
from our stockholders. International Paper, MeadWestvaco, and
Rubicon added contributed assets, recorded at the
stockholders’ historical costs, of $9.0 million,
$13.4 million and $9.6 million, respectively. These
contributions were determined by our board of directors to have
a fair value of $28 million above their historical cost;
however, between the three stockholders, the contributed assets
were determined to have equal fair market value, such that they
remain equal stockholders. In connection with this transaction,
our board of directors created a $13.3 million preferred
interest to account for the excess of the fair value of the
assets contributed by International Paper in the asset
contribution transaction over the fair value of the assets
contributed by each of MeadWestvaco and Rubicon. This preferred
interest (which was subsequently reduced to $0 by offsetting
periodic contribution obligations) had various rights and
preferences, including the ability to reduce the amount of the
preferred interest in lieu of making a cash payment when our
board of directors requested capital contributions by all three
stockholders.
Leases
We currently lease our global headquarters in Summerville, South
Carolina from MeadWestvaco Forestry, LLC, an affiliate of
MeadWestvaco Corporation. We currently pay annual rent of
$0.8 million under this lease. We expect to occupy these
premises until construction on our new headquarters facility is
completed, which is expected by the end of 2011. We lease two
seed orchards, a greenhouse and associated outdoor growing areas
adjacent to our headquarters and multiple field testing sites
from MeadWestvaco Corporation or its affiliates, including
several properties leased as a result of the transaction
described above under “—Asset Contribution.”
During the three years ended March 31, 2010 and the nine
months ended December 31, 2010, we paid an
64
aggregate of $3.1 million and $0.7 million in rent and
related fees to MeadWestvaco and its affiliates pursuant to
these leases.
In November 2010, we signed a build-to-suit and lease agreement
with Forestry Research Holdings, LLC for a new manufacturing,
research and development laboratory and headquarters facility in
Summerville, South Carolina, which we expect will be completed
by the end of 2011. Under this
20-year
lease, we are obligated to pay annual rent of the greater of
$1.0 million or 10% of the landlord’s investment in
the property; provided that the landlord’s investment will
not exceed $14.3 million. The agreement includes an option
for us to purchase the facility beginning on the date of rent
commencement, which is the earlier of commencement of business
activities on the premises or 30 days after substantial
completion of the facility. We intend to use net proceeds from
this offering to exercise our option to purchase the new
facility during the year ended March 31, 2012. At the time
we expect to exercise the option, the purchase price will be the
landlord’s investment in the property (which will not
exceed $14.3 million) plus five percent and any tax liability
incurred by the landlord due to sale of the property within one
year of development. The property developer purchased the land
on which our new facility is being built from MeadWestvaco, and
a portion of the property developer’s financing for this
purchase and the related building development costs comes from a
mortgage granted by an affiliate of MeadWestvaco. In addition,
each of MeadWestvaco, International Paper and Rubicon are
guarantors of our obligations under the lease. See also the
description under “Certain Relationships and Related Party
Transactions—Leases.”
Seedling
Sales
During the three-year period ended March 31, 2010 and the
nine month period ended December 31, 2010, we sold an
aggregate of $0.1 million and $7,000 of our seedling
products to International Paper and an aggregate of
$2.4 million and $0.1 million of our seedling products
to MeadWestvaco. These sales were made on terms and conditions,
including price, that were substantially equivalent to the terms
and conditions upon which our customers generally purchased our
seedling products.
License
Agreements
In connection with the formation of our business in 2000, we
entered into license agreements with each of MeadWestvaco
(formerly Westvaco Corporation), International Paper and Rubicon
(then Fletcher Challenge Limited). In addition, in 2004, we
entered into a nonexclusive license agreement with MeadWestvaco
that allowed us to use somatic embryogenesis for the development
of non-biotechnology trees. Effective June 1, 2010, we
replaced the non-exclusive license agreement with MeadWestvaco
for use of somatic embryogenesis for non-biotechnology trees
with a new, exclusive license agreement covering the same
technology. Pursuant to this agreement, we are obligated to pay
royalties and milestone payments to MeadWestvaco based on sales
of our products containing the licensed technology, subject to
annual minimum royalty payments, as well as an annual fee to
cover patent maintenance. The exclusive license agreement is for
an initial term of two years, with successive two-year terms
upon written agreement of the parties.
The terms of these license agreements are described in the
section entitled “Certain Relationships and Related Party
Transactions—License Agreements.” During the three
years ended March 31, 2010, the royalties and milestone
payments paid to our stockholders pursuant to these license
agreements were not material to our financial position and
operating results; however, they may be material in the future.
Critical
Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance
with accounting principles generally accepted in the United
States. The preparation of these consolidated financial
statements requires us to make estimates and assumptions that
reflect the reported amounts of assets, liabilities, revenue,
costs and expenses, and related disclosures. We evaluate our
estimates and assumptions on an ongoing basis. Estimates and
assumptions are by nature uncertain, and our actual results may
differ. We believe that the accounting policies and estimates
described below are the most critical to aid in fully
understanding and evaluating our consolidated financial
condition and results of operations, both because they are
important to the portrayal of
65
our financial condition and results of operations and because
their application involves a significant amount of management
judgment.
Revenue
Recognition
The majority of our revenue is recognized based on sales of our
seedling products. We recognize revenue from sales of our
seedlings products, as well as other contract revenue, upon
transfer of title to the customer, provided persuasive evidence
of an arrangement exists, the price is fixed and determinable,
collection is determined to be reasonably assured and no
significant obligations remain. During the year ended
March 31, 2010, we recognized revenue related to funding we
received as a subcontractor under a research and development
grant. We recognize revenue from this grant in an amount equal
to the lesser of the amount due under the contract or the amount
earned based on proportional performance to date. Use of this
method requires judgment in estimating the level of completion
of the various tasks required under the contract and changes in
these estimates would impact the amount revenue recognized.
During the year ended March 31, 2010, approximately 5% of
our revenue was recognized under this method.
To the extent that our customers are billed or pay for products
or services in advance of delivery, we record these amounts as
deferred revenue.
Allowance
for Doubtful Accounts
We record an allowance for doubtful accounts for any customer
receivables that we estimate are likely to be uncollectible.
Actual collections may differ from our estimates. We perform
ongoing evaluations of our allowance for doubtful accounts, and
we adjust the allowances accordingly. In determining the
adequacy of the allowance, we consider our historical bad-debt
experience, customer creditworthiness, the age of outstanding
receivables and current economic conditions. Any significant
changes in any of these criteria would affect our estimates for
the allowance.
Intangible
Assets
We capitalize certain intangible assets, primarily patents, that
we estimate are likely to have a long-term life in our business.
We amortize these intangible assets over their estimated useful
lives. We review our amortizable intangible assets for
impairment periodically when specific circumstances or events
indicate that the carrying amount may exceed fair value or
warrant a revision to the estimated useful lives. Significant
changes in key assumptions about our business, the activities
for which the asset is currently used or expected to be used and
economic conditions could result in additional impairment
charges. We recorded asset impairment charges of
$0.2 million, $0.6 million and $0.2 million for
the years ended March 31, 2008, 2009 and 2010,
respectively, for patents and patents-in-progress that are no
longer of strategic interest to us.
Inventory
Valuation
We value our inventory at the lower of its actual cost and its
current estimated market value. Our seedling inventory generally
must be sold within 12 months of being planted.
Accordingly, we generally destroy and write off any remaining
seedling product at the end of each growing season to allow for
the planting of the next season’s crop. Unsold seedling
inventory that must be written off usually results from changes
in customer demand for our seed inventory. We consider inventory
levels and product life cycles and we generally write down
inventory to net realizable value based on forecasted product
demand. Actual demand and market conditions may differ from our
projections, which could result in further write-offs. For
example, we recorded charges for unsold inventory write offs at
year-end of $0.9 million for each of the years ended
March 31, 2009 and 2010, as compared to $0.2 million
for the year ended March 31, 2008. These charges were
higher than expected, as a result of the worldwide economic
downturn and the resulting reduction in customer demand.
Stock-Based
Compensation
In connection with our conversion from a limited liability
company to a corporation in June 2010, we granted appreciation
rights, or ARs, to certain employees to replace the net value
added units that had been
66
previously granted to employees under our New Value Added Plan.
ARs allow the employee the opportunity to share in the growth of
our equity value over time. Employees with ARs will, upon a sale
event (including a sale of our business or an initial public
offering of our common stock), and subject to the terms and
conditions of the ARs, share in the increase in our value
through the date of the sale event.
The ARs are granted unvested and only become vested upon a sale
event as long as the recipient has remained employed by us
through that date. The ARs are required to be settled (in cash
or stock at our election) on or within 60 days following
the vesting date. The amount of the settlement payment per AR
shall be equal to the difference between the final value of each
AR, defined by the value of the sale event, and the adjusted
initial value with respect to that AR. The ARs are considered
cash-settled stock-appreciation rights, which, when triggered,
would require us to record a liability for the fair market value
of the units. As the units would not be paid until there is a
sale event, this is deemed the triggering event. As no such
event has occurred as of December 31, 2010, no compensation
expense relating to the ARs, or the net value added units that
preceded the ARs, has been recorded.
In July 2010, we adopted our 2010 Stock Option and Incentive
Plan, or the 2010 Plan. We anticipate issuing various forms of
stock-based compensation pursuant to the 2010 Plan in the
future, such as stock options and stock appreciation rights. The
accounting for stock-based compensation involves significant
management judgment. Stock-based compensation cost is measured
at the grant date based on the fair value of the award and is
recognized as expense over the requisite service period, which
is the vesting period. The fair value of the stock-based awards
will be determined using an option pricing model, which requires
us to use significant judgment to make estimates regarding the
life of the award, as well as the expected volatility of the
common stock, the risk-free interest rate and the dividend yield
of the common stock over the life of the award. Changes to these
estimates would result in different fair values of awards.
Management judgment is also required in estimating the number of
awards that will be forfeited in order to recognize expense only
for those awards that ultimately vest. Compensation expense in a
period could be impacted, favorably or unfavorably, by
differences between forfeiture estimates and actual forfeitures.
Recent
Accounting Pronouncements
In October 2009, the FASB issued authoritative guidance
regarding revenue arrangements with multiple deliverables. The
guidance requires entities to allocate revenue using estimated
selling prices of the delivered goods and services based on a
selling price hierarchy. The guidance further eliminates the
residual method of revenue allocation and requires revenue to be
allocated using the relative selling price method. The new
guidance should be applied on a prospective basis for revenue
arrangements entered into or materially modified in fiscal years
beginning on or after June 15, 2010, with early adoption
permitted. We are currently assessing the impact of adopting
this new guidance.
Quantitative
and Qualitative Disclosures about Market Risk
Foreign
Currency Risk
We have business operations in the United States, New Zealand
and Australia. During the three-year period ended March 31,
2010, 11.7% of our revenue was derived from New Zealand and 6.2%
of our revenue was derived from Australia. This revenue was
denominated in local currencies. In addition, we have a
significant amount of assets and liabilities that are
denominated in New Zealand dollars and are exposed to foreign
currency movements. As a result, we may be exposed to foreign
currency fluctuations when we convert the results of our
non-U.S. operations
from their functional currency into U.S. dollars for
inclusion in our consolidated financial results. Historically,
the foreign exchange impact on our results has been immaterial;
however, it may increase in the future as our revenues and gross
margins increase in our
non-U.S. operations.
We expect that the impact of exchange rate fluctuations between
the United States and the New Zealand dollar on our New
Zealand-based assets and liabilities will continue to have a
significant impact on our “Accumulated Other Comprehensive
Income (Loss)” in our consolidated balance sheet. During
our last two fiscal years, the relative value of the New Zealand
dollar ranged from a low of $0.49 to a high of $0.80. We do not
engage in any hedging activities related to this exchange risk.
A hypothetical 10% change in
67
the foreign currency exchange rate between the New Zealand
dollar and the U.S. dollar would have changed our
consolidated net loss by $0.8 million for the year ended
March 31, 2010.
Interest
Rate Sensitivity
We had cash and cash equivalents totaling $3.7 million at
March 31, 2010. The cash and cash equivalents are held for
working capital purposes. We do not enter into investments for
trading or speculative purposes. None of the cash and cash
equivalents in the past has been invested in securities which
were subject to market risk. As of March 31, 2010, all of
our investments were held in bank deposit accounts or money
market accounts. We are exposed to interest rate risk on our
variable-rate long-term and short-term credit facilities. Our
objective is to manage the impact of interest rate changes on
earnings and cash flows. As of March 31, 2008, 2009 and
2010, we had $2.5 million, $8.0 million and
$14.6 million, respectively, outstanding under our credit
facilities. The advances under our lines of credit bear a
variable rate of interest determined as a function of the prime
rate or the published LIBOR rate at the time of the borrowing.
At March 31, 2010, we had $10.4 million outstanding
under our U.S. line of credit and NZ$6.0 million
(equivalent to US$4.3 million as of March 31, 2010)
outstanding under our New Zealand line of credit. The interest
rate on our U.S. line of credit was 5.0% as of
March 31, 2010, which represented the bank’s prime
rate per annum, subject to a minimum rate of 5%. Our wholly
owned subsidiary, ArborGen New Zealand Unlimited, entered into
interest rate swap agreements having a notional value of
$3.5 million that effectively fixes our interest rate on
the New Zealand facility at approximately 6.25% and expires on
various dates between December 31, 2010 and
September 30, 2012. A hypothetical 10% increase in interest
rates would result in an increase to our interest expense of
approximately $0.1 million and a decrease to our earnings
of approximately $0.1 million.
68
BUSINESS
Our
Company
We believe we are the world’s leading developer of
biotechnology tree seedling products, one of the largest
providers of conventional and technology-enhanced seedlings to
the forestry industry and the only integrated global commercial
seedling company. We are focused on improving and selling the
most widely grown commercial forestry species in some of the
largest markets in the world. Our products are designed for use
by our customers in the traditional pulp and paper and wood
products markets, the growing biopower and charcoal markets and
the emerging biofuels market. We have a base of over 5,000
customers, including some of the largest land owners and
managers in the United States, New Zealand and Australia, and in
the year ended March 31, 2010, we sold 240 million
seedlings in these markets. We also have a growing presence in
Brazil through collaborations with the country’s leading
pulp producers. Based on our research and estimates, we believe
that our high-value, technology-enhanced seedling products,
including our pipeline of advanced and biotechnology products,
improve the productivity of a given acre of land by enabling our
customers to grow trees that yield more wood per acre with
greater consistency and quality in a shorter period of time. The
combination of our fully integrated business model, proprietary
technology and established customer base creates a scalable
platform that we are using to develop and commercialize the next
generation of seedling products. We do not currently sell any
biotechnology products and, prior to any commercial sales, our
biotechnology products are subject to a multi-year deregulation
process. We believe, but cannot guarantee, that our
biotechnology products will revolutionize productivity standards
in the forestry industry and have an impact on that industry
similar to the impact that biotechnology crops have had on the
agricultural industry.
We are currently the only commercial seedling company with
products spanning the entire technology spectrum, from
conventional and advanced seedlings, which we currently offer,
to biotechnology seedlings, which are currently in development.
As a result, no single entity competes with us in commercial
sales across the full range of our business. In the year ended
March 31, 2010, sales of our open-pollinated, or
conventional, seedling products represented approximately 72% of
our revenue. We also sell advanced seedling products, which
consist of our mass-control pollinated, or MCP, and varietal
products. These advanced products are designed to improve the
growth rates, yields, stress-tolerance, uniformity, wood quality
and processing efficiency of trees. In the year ended
March 31, 2010, sales of our advanced products represented
approximately 28% of our revenue. Our product pipeline includes
six advanced and 15 biotechnology products in various stages of
development. While we do not currently sell any biotechnology
seedling products, our freeze-tolerant tropical eucalyptus
product is the first and only biotechnology forestry product
under review for deregulation by the United States Department of
Agriculture, or USDA. In addition, we have over 140 active field
tests for biotechnology products and the largest number of
regulatory approvals for field tests of biotechnology forestry
products in the United States and Brazil.
We have developed our products by utilizing our leading
technology platform, which is built on over 100 years, in
the aggregate, of tree improvement research. Our technology
platform combines substantially all of the commercial seed
orchard and nursery businesses and the related research and
development activities of three industry leaders: International
Paper Company, MeadWestvaco Corporation and Rubicon Limited. We
own a portfolio of over 230 patents and patent applications,
with license rights to more than 200 additional patents and
patent applications. We also own one of the largest and most
diverse repositories of tree genetic resources, or germplasm, in
the world. To produce our advanced and biotechnology products on
a commercial scale, we have developed numerous proprietary
processes and techniques related to the biotechnology
transformation process and the large-scale manufacturing of
advanced seedlings. As a result of our technology leadership and
the inherent tree growth time associated with tree improvement
research, we believe we are decades ahead of any new market
entrants seeking to develop and commercialize a product
portfolio and technology platform comparable to ours; however,
there is no guarantee that we will be able to commercialize our
biotechnology products in the near future.
We believe we are the largest provider of tree seedlings to the
commercial forestry industry in the world. We focus on the most
important species to the commercial forestry industry, including
loblolly pine, which is the most widely grown commercial tree
species in the Southeastern United States, radiata pine, which
is the
69
most widely grown commercial tree species in New Zealand, and
widely grown hardwoods such as populus (which includes aspen and
cottonwood) and eucalyptus. Based on management estimates, we
believe we currently have an approximately 27% share of the
total seedling market in the Southeastern United States,
including an approximately 31% share of the loblolly pine
market. In addition, based on a report prepared by the New
Zealand Ministry of Agriculture and Forestry, we believe we
currently have an approximately 36% share of the total seedling
market in New Zealand, including an approximately 41% share of
the radiata pine market. Based on management estimates, we
believe we currently have a more than 10% share of the
Australian pine seedling market. In addition, our customers
include 13 of the 20 largest land owners and managers in the
United States as reported in 2008 by RISI, the leading
information provider for the global forest products industry,
and our customers include six of the ten largest land owners and
managers in New Zealand as reported in 2009 by the
New Zealand Forest Owners Association and the New Zealand
Ministry of Agriculture and Forestry. We also have a growing
presence in Brazil through our product development
collaborations with the country’s leading pulp producers
and exporters. The geographies in which we currently operate
represent some of the largest commercial forestry markets in the
world, and we believe our existing market presence and our
pipeline of advanced and biotechnology seedling products
position us well to expand into other large and fast-growing
forestry markets, including China and markets throughout South
America.
We believe there are increasing supply and demand pressures on
global wood availability that will require the forestry industry
to move to purpose-grown trees, which deliver higher per-acre
productivity as compared to harvesting native forests. According
to the Food and Agriculture Organization of the United Nations,
or the FAO, global industrial wood production in 2009 was
approximately 1.4 billion cubic meters or the equivalent of
approximately 11 billion trees, assuming that 1.25 green
tons of wood yield one cubic meter of wood and that an acre of
land with 600 trees planted on it produces 100 green tons at
harvest. The FAO expects the increases in global consumption of
wood to be driven by economic growth in China and other emerging
markets, population growth, environmental policies and
regulations, and increased global demand for energy from
renewable sources to reduce dependence on fossil fuels. Given
the pressures on the availability of native forests, including
the growing focus on conservation, the increased development of
land for other uses and the reduced accessibility to unharvested
tracts of forest, we anticipate that traditional sources of wood
supply will not be sufficient to meet this increased demand. As
a result of this supply and demand dynamic, we believe there is
a large market opportunity for high-value purpose-grown trees
that improve the productivity of land already used for
commercial forestry and that our advanced and biotechnology
seedling products enable land owners and managers to meet these
challenges.
Our technology platform and our tree improvement expertise
gained over multiple generations of tree breeding have allowed
us to develop products that increase the productivity of land
and address the evolving needs of the global commercial forestry
industry, including the increased demand for wood as a source of
energy. Our broad portfolio of seedling products, which we sell
under the ArborGen and SuperTree Seedlings brand names, includes
the most widely grown tree species in the commercial forestry
industry, such as loblolly pine, radiata pine and eucalyptus. We
continue to enhance traits of these species, including growth
rates, yields, stress-tolerance, uniformity, wood quality and
processing efficiency, through a variety of conventional and
advanced production processes, including open pollination,
mass-control pollination, varietal manufacturing and through the
application of biotechnology. As a result, we are able to offer
a broad portfolio of seedling products with a range of traits,
which allows us to address different potential end-users at
different price points and returns on investment. For example,
in the United States, our products currently range in price
from approximately $45 per 1,000 seedlings for our
first-generation conventional open-pollinated loblolly pine
seedlings to approximately $400 per 1,000 seedlings for a
varietal product of the same species that exhibits enhanced
traits selected for suitability in a specific end market and
that we believe provides our customers with higher returns on
investment. In New Zealand, our radiata pine seedlings range in
price from approximately $110 per 1,000 seedlings for
open-pollinated seedlings to $550 per 1,000 seedlings for
varietal seedlings.
To accelerate adoption of our higher-value products, we intend
to leverage our longstanding customer relationships and the
improved returns that we believe will be provided by these
products. In some of our geographic markets, the transition to
higher-value seedling products is already well-established. In
the year ended March 31, 2010, we generated approximately
70% of our revenue in New Zealand and Australia from sales of
our advanced products. The shift to advanced seedling products
and higher average selling price that
70
has already occurred in New Zealand is now beginning to occur
with our customer sales in the significantly larger
U.S. market. For example, as our customers in the United
States have transitioned to our higher-value products, which
consist of our “elite” open-pollinated conventional
seedlings, our MCP seedlings and our varietal seedlings, sales
of these products have grown from 5.7% of U.S. revenue in
the year ended March 31, 2008 to 24.2% in the year ended
March 31, 2010, with increasing sales of MCP and varietal
seedlings each year. This shift in sales to our higher-value
products also drove the 15.5% increase in our U.S. average
selling price over the same period and we expect this trend to
continue.
For the year ended March 31, 2010, we generated
$21.6 million of revenue, of which 76% was generated from
customers located in the United States, 19% from customers
located in New Zealand and 5% from customers located in
Australia. Our top ten customers represented approximately 36%
of this revenue. Of the 240 million seedlings we sold in
the year ended March 31, 2010, 91% were sold in the United
States. We generated a gross profit of $5.2 million for the
year ended March 31, 2010. After incurring research and
development expenses of $11.2 million, or 51.9% of revenue,
in the year to enhance and expand our pipeline of future
advanced and biotechnology products, we recorded a net loss of
$(14.7) million. For the year ended March 31, 2009, we
generated $23.7 million of revenue and recorded a net loss
of $(15.3) million, and for the year ended March 31,
2008, we generated $18.2 million of revenue and recorded a
net loss of $(18.1) million. For the nine months ended
December 31, 2010, we generated $11.1 million of
revenue and recorded a net loss of $(14.2) million. We
expect to continue to incur net losses over the next several
years, including the year ended March 31, 2011, primarily
as a result of our continuing investment in the research and
development of advanced and biotechnology seedling products. As
of March 31, 2011, we had 181 employees and operated
13 nurseries, 15 seed orchards, 20 distribution centers and
three research and development facilities located throughout the
Southeastern United States, New Zealand and Australia, as well
as an office in Brazil. In November 2010, we signed a
build-to-suit and lease agreement for a new manufacturing,
research and development laboratory and headquarters facility in
Summerville, South Carolina, which we intend to occupy upon its
anticipated completion by the end of 2011. The agreement
includes an option for us to purchase the facility, which we
intend to exercise during the year ended March 31, 2012
using net proceeds from this offering.
Our
Market Opportunity
We believe that there is a large market opportunity for our
advanced and biotechnology seedling products. Due to the
increasing supply and demand pressures on global wood
availability, we expect the commercial forestry industry to rely
more heavily on purpose-grown trees. Purpose-grown trees are
trees that are planted, maintained and harvested on plantations
for commercial purposes. Purpose-grown trees deliver improved
per-acre productivity as compared to harvesting native forests
and are a more sustainable source of wood. Historically, the
global commercial forestry industry has relied heavily on the
harvesting of native forests. The use of wood from native
forests is under increasing pressure from competing uses of
land, such as conversion to agricultural uses and the continued
expansion of commercial and residential development, and from
the limited accessibility to remaining unharvested forests,
including as a result of conservation efforts. According to the
FAO, from 1990 to 2005, the total area of deforestation was
approximately 395 million acres, which is more than twice
the size of Texas. In addition, over the past ten years,
150 million acres of the remaining global forests have been
set aside as protected forests.
The historic and ongoing reliance on native forests is primarily
the result of the relatively low costs associated with native
forests as a source of wood. Purpose-grown trees must be planted
and maintained, and the land utilized for purpose-grown trees
will not be available for other uses during the growing cycle,
which is often 20 years or more. As a result, land owners
and managers incur higher costs with the use of purpose-grown
trees. For purpose-grown trees to be cost-effective for the
commercial forestry industry, the trees must be of sufficiently
high value to justify their increased cost. Trees are considered
“high-value” when they grow more quickly, are taller
and wider, have a high tolerance to stresses like cold, insects
or disease, exhibit uniform height, thickness and branching, can
be converted more efficiently to pulp or sawtimber, or have
certain other wood quality characteristics.
Based on the FAO’s 2010 Global Forest Resource Assessment,
purpose-grown trees currently account for approximately 7% of
the global forest area. Purpose-grown trees, however, represent
a larger proportion of the wood supply for industrial use.
According to a 2005 FAO report, due to the increased
productivity of planted
71
forests, these trees contributed approximately 35% of industrial
roundwood supply in 2000 and the FAO anticipated that this
percentage would rise to
40-44% by
2020. According to the FAO, the total area of planted forests
increased by an aggregate of 60 million acres from 2005 to
2010. By growing trees on plantations, land owners and managers
are able to plant species that are best-suited to a particular
end-market or geography, and reduce transportation and other
costs by locating the plantation within close proximity to a
paper mill, biopower facility or export hub. Purpose-grown trees
also enable biopower and biofuels facility developers to plant
their own trees, providing them with a captive, stable source of
low-cost biomass. Unlike native forests, plantations can be
planted with uniform high-value seedlings that exhibit more
predictable traits, which enable land owners or managers to make
more informed planting and harvesting decisions. In addition,
purpose-grown trees are planted in rows and managed to minimize
underbrush and competing vegetation, which improves the growth
of the trees and reduces the cost of harvesting trees. By
reforesting land that was previously clear, land owners and
managers may also be able to capitalize on the carbon offset
markets, if and when those markets develop.
We believe that the demand for high-value, purpose-grown trees
is driven by the shift in ownership of commercial timberland.
According to The Campbell Group, LLC, a timberland investment
advisory firm, over the past 15 years, ownership of
U.S. industrial timberland has gradually shifted from
integrated corporate owners such as forest products companies,
which owned 95% of the industrial timberland in 1996 and 32% in
2009, to investment companies such as TIMOs and REITs, which
owned 5% of the industrial timberland in 1996 and 63% in 2009. A
similar shift in ownership has occurred in New Zealand and
Australia. Land owners and managers who actively manage
timberland to achieve improvements in the financial returns of
their investors have helped to drive the transition from
harvesting native forests to planting and harvesting
purpose-grown trees on plantations. In addition, based on our
experience, these land owners and managers have purchased
high-value seedling products to improve their returns. We
believe this shift will continue to be an important catalyst for
broad industry adoption of our advanced and biotechnology
seedling products.
We are applying a business model similar to the model that has
been successfully used by the agricultural biotechnology
industry; however, there are differences between these two
industries. For example, the rotation length of agricultural
crops is shorter than the rotation length of trees and there are
additional regulations that govern biotechnology products
intended for human and animal consumption that do not apply to
our products. In particular, we are applying biotechnology
techniques to develop new, higher value genetic products. As
occurred in the agriculture industry in the 1990s, we intend to
share in the value that these products create through the prices
we will charge for them. We believe our profitability will
increase over time as we transition our customers from
conventional seedling products to our biotechnology products
without having to significantly increase our fixed costs. In the
agricultural industry, companies such as Monsanto Company, the
first mover in that industry, introduced biotechnology crops
that redefined productivity, which dramatically improved the
economics of farmed crops as compared to their conventional seed
competitors. According to the 2010 ISAAA Brief No. 42, the
number of acres planted with biotechnology-enhanced crops grew
from approximately 4.3 million acres in 1996 to
approximately 370 million acres in 2010, representing a
compound annual growth rate of 38%. In 2010,
biotechnology-enhanced crops represent approximately 81% of
soybean, 64% of cotton, 23% of canola and 29% of maize planted
globally. We believe, but cannot guarantee, that our
biotechnology seedlings will have an impact on the productivity
and economics of the commercial forestry industry similar to the
impact that biotechnology crops have had on the agricultural
industry.
Our
Primary End-Markets
Our five primary end-markets are the traditional pulp and paper
and wood products markets, the growing biopower and charcoal
markets, and the emerging biofuels market. The demand for
high-value, purpose-grown trees in each of these end-markets
will continue to be driven by industry-specific dynamics, growth
of developing economies and the increasing competition for
available land.
Pulp and
Paper
The global pulp and paper industry produces printing and writing
paper, newsprint, tissue paper, packaging paper and other
pulp-based products. The pulp and paper market has historically
been relatively stable, with a
72
global compound annual growth rate of 3% between 1995 and 2007,
according to RISI. The U.S. paper grade pulp market
contracted by approximately 4% in 2008 and by approximately 10%
in 2009 primarily as a result of the worldwide economic
downturn. In contrast, wood pulp production in Brazil, China and
other emerging economies increased during this period. Emerging
economies are expected to drive future growth in this market.
According to RISI, China is the second largest consumer of
paper-grade wood pulp and is projected to account for more than
45% of the growth in this market from 2009 to 2020. Due to the
relative scarcity of wood fiber in China, it is expected that
China will need to continue to increase its imports from Brazil,
New Zealand, Australia and other major commercial forestry
markets to meet this growing demand. For example, according to
the New Zealand Ministry of Agriculture and Forestry, the
percentage of New Zealand’s wood chips exported to China
increased from 0% in 2008 to 8% in 2009 and, according to the
Australian Bureau of Agriculture and Resource Economics, the
percentage of Australia’s wood chips exported to China
increased from 5% in 2008 to 9% in 2009.
We believe our existing presence in these wood-consuming and
exporting markets and our pipeline of advanced and biotechnology
seedling products position us well to expand into China and
other fast-growing forestry markets. Our product pipeline
includes products that are specifically designed to improve
returns to integrated pulp producers and to land owners and
managers growing trees for use in the pulp industry. Our
freeze-tolerant tropical eucalyptus product, which we intend to
sell initially in the Southeastern United States, may also be
sold in the future to other geographies with similar climates,
including parts of China. In addition, we are currently
developing a short rotation eucalyptus product for use primarily
in Brazil and a short rotation populus product for use primarily
in the United States. In addition, we believe that our
“stacked” products, which are products that combine
multiple improved product traits, such as introducing fast
growth traits to our freeze-tolerant tropical eucalyptus
product, will provide an even higher level of performance.
In addition to these global drivers, the dynamics of the pulp
and paper industry in the Southeastern United States and Brazil
are an important factor driving demand for our products.
Hardwood pulp in the Southeastern United States is used to
produce high-quality printing and writing paper, and the
manufacturers of hardwood pulp in this region currently consume
wood primarily from slow-growing, naturally regenerated forests.
The supply of these trees in the Southeastern United States is
declining due to a higher rate of consumption relative to their
growth rate, the conversion of land away from forestry uses and
the reduced accessibility of remaining forest acres, all of
which has resulted in increased prices and reduced reliability
of wood supply. We believe that the use of purpose-grown
hardwood trees with faster growth rates and greater wood yields,
such as our conventional subtropical eucalyptus and our pipeline
of biotechnology products, including our freeze-tolerant and
short rotation products, has the potential to improve the
competitiveness of pulp and paper producers in the Southeastern
United States as compared to other markets.
In addition, we expect Brazil to continue to increase its global
share of hardwood pulp exports. Pulp producers and exporters in
Brazil are actively pursuing advanced seedling products to
improve growth rates and wood quality. We are developing a
growing presence in Brazil through collaborations with the
country’s leading pulp producers and exporters. According
to RISI, Brazil’s share of global hardwood market pulp,
which excludes pulp produced for internal consumption, increased
from 18% in 1992 to 38% in 2009 and is expected to increase to
44% by 2014.
Wood
Products
The global wood products industry produces plywood, lumber and
other wood products for construction, furniture and other
industrial uses. Lumber is produced from sawtimber, which
commands significantly higher prices than pulpwood and other
lower value products. This superior revenue potential makes
sawtimber particularly important to land owners and managers
seeking to maximize their
per-acre
financial returns. We believe that the use of high-value
purpose-grown trees improves the quality of the sawtimber and
the customer’s return on investment. For example, the pine
products we produce using advanced methods, such as mass-control
pollination and varietal manufacturing, are designed to be more
uniform and to exhibit height, width and branching
characteristics that result in superior timber for the wood
products end-market. Growing uniform, high-value trees in
plantations results in trees with improved and more predictable
wood quality characteristics, which enhance the ability of the
land owner or manager to better predict yields and make
harvesting decisions in response to changing market conditions.
Our product pipeline includes products that
73
are specifically designed to improve returns to the wood
products end-market. We are currently developing a short
rotation loblolly pine product and a short rotation radiata pine
product that focus on improved wood quality and significantly
shorter rotations. As a result, we expect land owners and
managers to benefit from improved
per-acre
returns.
From 1995 to 2005, the size of the U.S. wood products
market remained relatively constant, according to RISI. From
2007 to 2009, this market contracted by approximately 33.4%
primarily as a result of the decline in the U.S. housing
market. According to RISI, the anticipated recovery in the
U.S. housing market is expected to drive a recovery in pine
and other softwood lumber demand, with U.S. housing starts
expected to more than triple over 2009 levels by 2014.
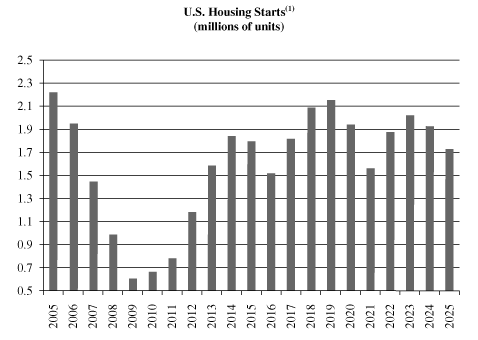
We also expect the global wood products market to benefit from
the growing demand for wood products in China and other emerging
economies. According to the New Zealand Ministry of Agriculture
and Forestry, China is emerging as a major export market for New
Zealand logs, with a 133% increase in log export volumes to
China in 2009, making it the largest importer of New Zealand
logs on a volume basis. China is also the largest importer of
logs from Australia representing 55% of log exports in 2009,
according to the Australian Bureau of Agricultural and Resource
Economics.
Biopower
Biopower refers to the conversion of wood and other biomass into
energy through combustion or gasification, and is an
increasingly common source of renewable energy. Biomass can
either be directly burned for electricity or blended with coal
in existing coal-powered generation plants. Sources of biomass
for electricity generation, which are referred to as feedstocks,
currently include wood, wood residue, other agricultural waste,
municipal waste, industrial waste and biogas. High-density wood
pellets, which are often manufactured from wood residue such as
shavings or sawdust, are also a significant source of biomass
for electricity generation. We believe that growth of the
biopower market will generate a shortage of feedstock, creating
an opportunity for increased use of purpose-grown trees as a
source of biomass. In addition, we expect that in order to
secure funding to build new biopower plants, developers will
need to demonstrate ready access to feedstocks, and in some
cases the execution of long-term supply contracts may be
required. Purpose-
74
grown trees have the potential to become a more economical
feedstock than waste materials and other alternatives due to
their higher and more consistent yields, improved supply
reliability and reduced transportation costs. According to
Forisk Consulting, the annual consumption of wood for biopower
in the United States has the potential to increase to
105.8 million green tons by 2020 based solely on currently
announced and operating bioenergy projects in the United States.
Biomass is a more reliable source of electricity generation and
available on demand as compared to wind and solar energy, the
availability of which varies based on weather conditions and
geography. For example, in the Southeastern United States, wind
and solar resources are not as plentiful as they are in other
parts of the United States while biomass is abundant. In
addition, biomass does not require as much additional
infrastructure investment as wind or solar energy. According to
the U.S. Energy Information Administration, or the EIA, by
2030, biomass electricity generation in the United States is
projected to represent approximately 40% of total
non-hydroelectric renewable energy generation. The following
table shows the projected increase in British thermal units, or
BTUs, generated from biopower between 2009 and 2020, as reported
by the EIA.
Projected
BTUs Generated from Biopower
The United States, Europe, China and Brazil are the most
important markets for renewable energy sources, together
representing more than 75% of total energy generation from
non-hydroelectric renewable and waste sources in 2008, according
to the International Energy Agency, or IEA. The growth of the
biopower market is being driven by government renewable energy
standards and policies and the availability of low-cost sources
of biomass, such as waste material. For example, the Chinese
government is developing a plan designed to encourage the
planting of approximately 50 million acres of purpose-grown
trees by 2020, which would significantly increase the use of
purpose-grown trees as a biopower feedstock in China.
The growth of the biopower market is also facilitated by the
ability to repurpose commercially available power generation
technology and existing coal-power infrastructure for biopower
use, either by converting a coal power plant to a biomass power
plant or by blending biomass with coal, which reduces the amount
of coal burned by the power plant, a process referred to as
co-firing. According to the EIA, biomass electricity generation
in the United States is projected to grow from 38.3 billion
kilowatt hours in 2008 to 95.6 billion kilowatt hours in
2020, representing a compound annual growth rate of 8%, a large
portion of which is expected to result from increased co-firing.
In the Southeastern United States, we expect the demand for
biomass to increase as new power generation and wood pellet
manufacturing facilities currently proposed or under
construction are completed. In addition, according to the
European Biomass Association, the amount of wood pellets
exported from North America to Europe for energy production has
more than doubled from 2007 to 2009, and growth in this market
is expected to continue.
75
Charcoal
Charcoal is produced by applying extreme pressure and heat to
wood and other substances. Unlike other countries that primarily
use coke in steel production, Brazil also uses charcoal in steel
production. In 2009, 35 million cubic meters of wood were
consumed for charcoal production according to the Brazilian
Association of Forest Plantation Producers. From 2008 to 2009,
charcoal production decreased by 38.5% as a result of the
worldwide economic downturn. Historically, the wood used in
charcoal production came primarily from native forests. The use
of purpose-grown trees began increasing in the 1940s, primarily
in response to concerns over forest conservation in Brazil, and
in 2009, 55% of the wood used in charcoal production came from
purpose-grown eucalyptus.
Minas Gerais, Brazil’s largest charcoal-producing state,
producing 60% of the charcoal consumed in that country according
to the Brazilian Association of Mining Forestry, recently
adopted a law requiring that purpose-grown trees account for 85%
of total charcoal production in that state from 2009 to 2013,
90% from 2014 to 2017 and 95% starting in 2018, as compared to
only 72% in 2009. In addition, the Brazilian Ministry of
Environment has recently proposed that the Brazilian iron and
steel industries use more charcoal produced from purpose-grown
trees, such as planted eucalyptus, in their pig iron production
processes. The emphasis on using purpose-grown trees is intended
to preserve Brazil’s native forests and to reduce
greenhouse gas emissions. These two regulatory developments will
require increased plantings of purpose-grown trees, creating an
increase in demand for fast-growing eucalyptus, the most widely
used purpose-grown tree in Brazil. Any increase in charcoal
consumption as a result of stronger demand and improved economic
conditions would further add to that growth.
Biofuels
Biofuels are liquid fuels for transportation derived from wood
and other organic materials. The Energy Policy Act of 2005 and
the Energy Independence and Security Act of 2007 established
renewable fuel standards and set target production and import
levels of renewable fuels in the United States. Currently,
biofuels are primarily produced from corn, wheat, sugarcane,
vegetable oils and other organic materials. Current
Environmental Protection Agency, or EPA, renewable fuel
standards for the United States require increases in the
production of advanced biofuels. Advanced biofuels include,
among others, those produced using biodiesel, sugar and
cellulose (found in organic materials such as wood, switchgrass
and other perennial grasses). The EPA has targets of
600 million, 950 million, 1.35 billion,
2 billion and 15 billion gallons of advanced biofuels
by 2009, 2010, 2011, 2012 and 2020, respectively. We believe
that wood as a source of biomass for the production of advanced
biofuels has significant benefits as compared to switchgrass and
other perennial grasses. For example, unlike trees, switchgrass
and other perennial grasses cannot be grown and harvested
year-round and cannot be stored as efficiently as wood. Trees
also do not need to be harvested on an annual basis, unlike
grasses, which die if not harvested each year. As a result, land
owners and managers have flexibility in the timing of their
harvests. In addition, most other advanced biofuel feedstocks
are not currently grown for commercial purposes and the
large-scale use of these sources of biomass for biofuels
production would require substantial infrastructure investment.
76
The following table shows the projected increase in BTUs
generated from advanced biofuels between 2009 and 2020, as
reported by the EIA.
Projected
BTUs Generated from Advanced Biofuels
As the technology for producing advanced biofuels on a
commercial scale develops, we believe there will be a
significant increase in the consumption of wood for biofuels
production. In addition to the emerging opportunity for biofuels
production and use in the United States, we believe that
government mandates for the production and use of biofuels in
Europe and other large energy-consuming countries such as China
will drive global demand for alternatives to fossil fuels. There
has also been increased focus on developing the technology
required to produce advanced biofuels from wood and other
non-food based sources that do not impact food prices. In
addition, there is an emerging market for bioproducts, which are
chemicals or other materials produced as co-products in the
manufacturing of biofuels. These bioproducts are used to
supplement or replace traditional industrial petroleum-based
products.
77
Our
End-Market Growth Opportunity
Except as otherwise noted, the graphic below illustrates the
potential growth opportunity presented by our five primary
end-markets (as well as pellets used for biopower) using
third-party industry data on wood production adjusted in the
manner described in the table below. This data is presented for
the geographies in which we currently operate as well as China,
in which we do not currently operate but believe will be a key
element of our future expansion strategy. To put this end-market
size and growth data in context to show the potential
opportunity for our business of selling seedlings, we have also
converted the data to an estimated equivalent number of trees
that would be needed to satisfy the reported or projected
demand, using a number of assumptions described in the table
below. As described in more detail below, this data does not
represent the number of trees actually consumed or projected to
be consumed, nor does it represent the number of seedlings
purchased or planted by land owners and managers.
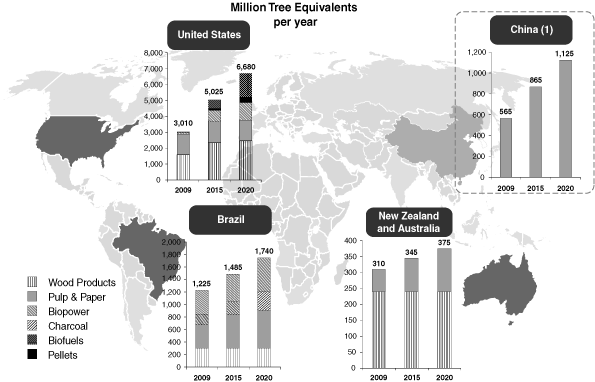
|
|
|
|
(1)
|
|
This data represents RISI’s
estimates of current and projected consumption of pulp by the
Chinese market. We expect a portion of this consumption to be
supplied by imports of pulp produced in other geographies,
including the United States, Brazil, New Zealand and Australia.
This table does not include any wood consumed in China for the
wood products, biopower and biofuels end-markets.
|
The table below sets forth in greater detail the estimated size
and projected growth of the markets illustrated above. Although
the demand in each of these end-markets is global, third-party
industry data is readily available only for the selected
geographies that we have presented in the table. Although these
are our target markets, we do not currently sell our seedling
products in all of the geographies presented. Market size is
generally reported as wood harvested for use in that end-market.
For the biofuels market, however, the data represents fuels
produced. The biopower and biofuels market size data is also
based on certain other assumptions described below. We have also
presented the approximate compound annual growth rate (CAGR)
that the projected future market sizes represent. Actual growth
in these end-markets may vary significantly from the projections
for the reasons described in the section entitled “Risk
Factors,” including as a result of shifts in underlying
market trends, such as demand in developing economies,
regulatory initiatives related to renewable energy and
mitigation of greenhouse gas emissions, and general economic
conditions.
78
To put this end-market size and growth data in context to show
the potential opportunity for our business of selling seedlings,
we have also converted the data to an estimated equivalent
number of trees that would be needed to satisfy the reported or
projected demand, using a number of assumptions described below.
This data does not represent the number of trees actually
consumed or projected to be consumed, nor does it represent the
number of seedlings purchased or planted by land owners and
managers. For the U.S. biofuels market we have assumed that
40% of advanced biofuels production comes from wood. We believe
this is a reasonable assumption based on an aggregation of
regional projections as to the amount of wood used in the
production of biofuels. As described above, the projected demand
for biofuels includes sources other than wood, such as
switchgrass, agricultural waste or other organic material.
Furthermore, even if wood from trees were used to satisfy demand
in all of the end-markets presented, it may have been and in the
future may be sourced in part by depleting existing forests
rather than requiring the planting of new tree seedlings.
Finally, the assumptions we used to convert the market data into
an estimated equivalent number of trees consumed are based on
our estimates of general industry standards for each end-market,
which vary based on the particular types of trees planted, the
size of trees when harvested and the growing conditions.
Accordingly, the actual number of trees consumed will vary
significantly from the estimated equivalent number of trees set
forth below.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
Projected 2015
|
|
|
Projected 2020
|
|
|
|
|
|
|
Approximate
|
|
|
|
|
Approximate
|
|
|
Approx.
|
|
|
|
|
Approximate
|
|
|
Approx.
|
|
|
|
|
Reported
|
|
Number of
|
|
|
Reported
|
|
Number of
|
|
|
CAGR
|
|
|
Reported
|
|
Number of
|
|
|
CAGR
|
|
|
|
|
Metric
|
|
Trees(1)
|
|
|
Metric
|
|
Trees(1)
|
|
|
(‘09-‘15)
|
|
|
Metric
|
|
Trees(1)
|
|
|
(‘09-‘20)
|
|
|
Pulp and
Paper(2)
|
|
Global
|
|
163 million metric tons of pulp
|
|
|
4,300 million
|
|
|
190 million metric tons of pulp
|
|
|
5,020 million
|
|
|
|
3
|
%
|
|
208 million metric tons of pulp
|
|
|
5,510 million
|
|
|
|
2
|
%
|
|
United States
|
|
48 million metric tons of pulp
|
|
|
1,280 million
|
|
|
50 million metric tons of pulp
|
|
|
1,310 million
|
|
|
|
0.4
|
%
|
|
49 million metric tons of pulp
|
|
|
1,295 million
|
|
|
|
0.1
|
%
|
|
New Zealand and Australia
|
|
3 million metric tons of pulp
|
|
|
70 million
|
|
|
4 million metric tons of pulp
|
|
|
105 million
|
|
|
|
6
|
%
|
|
5 million metric tons of pulp
|
|
|
135 million
|
|
|
|
6
|
%
|
|
Brazil
|
|
14 million metric tons of pulp
|
|
|
370 million
|
|
|
20 million metric tons of pulp
|
|
|
535 million
|
|
|
|
6
|
%
|
|
23 million metric tons of pulp
|
|
|
605 million
|
|
|
|
5
|
%
|
|
China
|
|
21 million metric tons of pulp
|
|
|
565 million
|
|
|
33 million metric tons of pulp
|
|
|
865 million
|
|
|
|
7
|
%
|
|
43 million metric tons of pulp
|
|
|
1,125 million
|
|
|
|
6
|
%
|
|
Wood
Products(3)
|
|
United States
|
|
212 million cubic meters of wood
|
|
|
1,590 million
|
|
|
312 million cubic meters of wood
|
|
|
2,340 million
|
|
|
|
7
|
%
|
|
328 million cubic meters of wood
|
|
|
2,455 million
|
|
|
|
4
|
%
|
|
New Zealand and Australia
|
|
32 million cubic meters of wood
|
|
|
240 million
|
|
|
32 million cubic meters of wood
|
|
|
240 million
|
|
|
|
0
|
%
|
|
32 million cubic meters of wood
|
|
|
240 million
|
|
|
|
0
|
%
|
|
Brazil
|
|
40 million cubic meters of wood
|
|
|
300 million
|
|
|
40 million cubic meters of wood
|
|
|
300 million
|
|
|
|
0
|
%
|
|
40 million cubic meters of wood
|
|
|
300 million
|
|
|
|
0
|
%
|
|
Biopower(4)(5)
|
|
United States (Electricity)
|
|
11 million green tons wood
|
|
|
110 million
|
|
|
71 million green tons wood
|
|
|
710 million
|
|
|
|
36
|
%
|
|
109 million green tons wood
|
|
|
1,095 million
|
|
|
|
23
|
%
|
|
United States (Pellets)
|
|
3 million green tons wood
|
|
|
30 million
|
|
|
12 million green tons wood
|
|
|
115 million
|
|
|
|
26
|
%
|
|
33 million green tons wood
|
|
|
335 million
|
|
|
|
25
|
%
|
|
Brazil
|
|
42 million cubic meters wood
|
|
|
380 million
|
|
|
49 million cubic meters wood
|
|
|
445 million
|
|
|
|
3
|
%
|
|
59 million cubic meters wood
|
|
|
540 million
|
|
|
|
3
|
%
|
|
Charcoal(6)
|
|
Brazil
|
|
19 million cubic meters of wood from plantations
|
|
|
175 million
|
|
|
23 million cubic meters of wood from plantations
|
|
|
205 million
|
|
|
|
3
|
%
|
|
32 million cubic meters of wood from plantations
|
|
|
295 million
|
|
|
|
5
|
%
|
|
Biofuels(7)
|
|
United States
|
|
—
|
|
|
—
|
|
|
2 billion gallons of advanced biofuels
|
|
|
550 million
|
|
|
|
—
|
|
|
6 billion gallons of advanced biofuels
|
|
|
1,500 million
|
|
|
|
—
|
|
|
|
|
|
(1)
|
|
The number of trees is rounded to
the nearest five million.
|
| |
|
(2)
|
|
Production of paper-grade wood pulp
for 2009 and projections for 2015 and 2020 were obtained from
the RISI
15-Year
World Pulp and Recovered Paper Forecast, July 2010. Our
conversion from metric tons of pulp to an estimated equivalent
number of trees harvested assumes that (a) four tons of
green, or “wet,” wood yield one ton of pulp, as the
pulp must be separated from water and
|
79
|
|
|
|
|
|
other components, and (b) one
acre of land with 600 trees planted on it, most of which are
high-quality tree species grown to maximize pulp volume,
produces 100 green tons of wood at harvest.
|
| |
|
(3)
|
|
Wood harvested in the United States
for use by the wood products industry for 2009 and projections
for 2015 and 2020 were obtained from the RISI
15-year
North American Timber Forecast, September 2010. Use of roundwood
(or logs) harvested from purpose-grown forests in Brazil for use
by the wood products industry for 2009 was obtained from the
Brazilian Association of Forest Plantation Producers (ABRAF)
Statistical Yearbook, 2010. Wood harvested for saw, veneer and
other logs in Australia for use by the wood products industry
for 2009 (12 million cubic meters) was obtained from the
Australian Forest and Wood Products Statistics report by the
Australian Bureau of Agricultural and Resource Economics and
Sciences (ABARES), November 2010. Saw logs, peeler logs and logs
exported harvested in New Zealand for use by the wood products
industry for 2010 (20 million cubic meters) was obtained
from the Forestry Production and Trade Statistical Release,
September 2010, by the New Zealand Ministry of Agriculture and
Forestry. Third-party projections of harvest for wood products
in 2015 and 2020 in Brazil, Australia and New Zealand are not
available. As a result, we have assumed that the wood products
harvest levels in 2015 and 2020 in these three countries will be
identical to 2009 levels. Our conversion from cubic meters of
wood to an estimated equivalent number of trees consumed assumes
that (a) 1.25 green tons of wood yield one cubic meter of
wood for wood products and (b) one acre of land with 600
trees planted on it, most of which are high-quality tree species
grown in spacings that maximize thickness and straightness,
produces 100 green tons of wood at harvest.
|
| |
|
(4)
|
|
United States: Roundwood and chip
capacity for operating and announced electricity generation and
pellet production projects in the United States for 2009 (11 and
3 million green tons, respectively) and projections for
2015 (71 and 12 million green tons, respectively) were
obtained from Wood Bioenergy US published by Forisk Consulting
in August, 2010. To estimate roundwood and chip consumption
capacity for electricity generation projects in 2020, we applied
the projected compound annual growth rate for U.S. electricity
generation from wood and other biomass from 2015 to 2020 (9%)
obtained from the EIA Annual Energy Outlook 2011 to the 2015
electricity generation projection (71 million green tons).
To estimate roundwood and chip utilization capacity for pellet
production projects in 2020, we applied the projected compound
annual growth rate in European pellet use from 2008 to 2020
(24%) obtained from the European Biomass Association European
Biomass Statistics, 2010 to the 2015 pellet production capacity
projection (12 million green tons). Our conversion from
green tons to an estimated equivalent number of trees assumes
that one acre of land with 1,000 trees planted on it, most of
which are high volume trees species grown in dense stands,
produces 100 green tons of wood at harvest. Our biopower market
projections differ significantly from those put forward by RISI
in the
15-year
North American Timber Forecast which shows 10 million
green tons in 2009, 31 million green tons in 2015 and
45 million green tons in 2020 of wood harvested for wood
pellets and electricity produced by private power companies.
RISI states that its assessment of bioenergy capacity
development is conservative and it has assumed a decelerated
investment cycle, delay in
start-up
operations from announced projects and a slowdown in the pace of
development from 2015 to 2020. We believe RISI’s underlying
assumptions also fail to incorporate the substantial benefits of
improved genetics and changes in forest management to maximize
biomass production for this emerging market.
|
| |
|
(5)
|
|
Brazil: Utilization of wood from
purpose-grown forests for industrial fuelwood in Brazil for 2009
(42 million cubic meters) was obtained from the ABRAF
Statistical Yearbook, 2010. To estimate wood use for industrial
fuelwood in 2015 we applied the projected compound annual growth
rate for biomass and waste electricity generation in Latin
America from 2008 to 2015 (3%) obtained from the IEA World
Energy Outlook for 2010 (WEO 2010) to the 2009 usage
(42 million cubic meters) for an estimate of
49 million cubic meters of wood use for industrial fuelwood
in 2015. To estimate wood use for industrial fuelwood in 2020 we
applied the projected compound annual growth rate for biomass
and waste electricity generation in Latin America from 2015 to
2020 (4%) obtained from the WEO 2010 to the 2015 projection
estimate (49 million cubic meters). Our conversion from
cubic meters of wood to an estimated equivalent number of trees
assumes that one acre of land with 650 trees planted on it, most
of which are lower-value trees, produces 71 cubic meters of wood
at harvest.
|
| |
|
(6)
|
|
The amount of purpose-grown
eucalyptus used for charcoal in 2009 was obtained from ABRAF
Statistical Yearbook, 2010. We estimated projected growth to
2020 assuming the implementation of a new law adopted by Minas
Gerais, Brazil’s largest charcoal-producing state
representing 68% of charcoal consumption from eucalyptus
plantations in Brazil, requiring that purpose-grown trees
account for 95% of total charcoal production in that state by
2018, compared to approximately 72% in 2009, together with
projected growth of charcoal consumption in Minas Gerais of
2.2 million cubic meters per year starting in 2017, based
on the expected completion date for projects, which was obtained
from ABRAF Statistical Yearbook, 2010. The charcoal industry in
the rest of Brazil was assumed to remain flat. We assumed that
purpose-grown trees account for 90% of charcoal production in
Minas Gerais by 2015 and 95% by 2020, in accordance with the new
law’s annual requirements. Our conversion from cubic meters
of wood to an estimated equivalent number of trees consumed
assumes that (a) one acre of land with 650 trees planted on
it, most of which are lower-value trees, produces 71 cubic
meters of wood at harvest.
|
| |
|
(7)
|
|
Advanced biofuels production
requirements for 2015 and 2020 were obtained from the EPA
Renewable Fuels Standards, March 2010. We have assumed that wood
was not a source for advanced biofuels production in 2009. In
addition, for 2015 and 2020, we have assumed that 40% of
advanced biofuels production comes from wood. Our conversion
from gallons of advanced biofuels to an estimated equivalent
number of trees needed to satisfy this demand assumes that
(a) one green ton of wood yields 40 gallons of ethanol and
(b) one acre of land with 1,000 trees planted, most of
which are high-volume tree species grown in dense stands,
produces 100 green tons of wood at harvest. Data for bioproducts
is not readily available and therefore not included.
|
80
Our
Technology Platform
Our leading technology platform is built on over 100 years,
in the aggregate, of tree improvement research, combining
substantially all of the research and development activities,
commercial tree improvement, seed orchard and nursery businesses
of three industry leaders: International Paper, MeadWestvaco and
Rubicon. The tree improvement activities of these companies were
formed from the prior consolidation of numerous genetics
programs, including Champion International Corporation, Union
Camp Corporation, Fletcher Challenge Limited, Hammermill Paper
Company, Carter Holt Harvey Limited and Federal Paper Board
Company. This combination of outstanding expertise in tree
breeding, genetics, seedling production and sales and
distribution channels that these three companies have developed
independently over decades of significant investment, and now
owned by us, provides us with a broad portfolio of intellectual
property, ownership of a large and diverse repository of
germplasm that includes the most widely used tree species in the
global commercial forestry industry, and the production
capabilities and channel to market to commercialize our products.
We produce tree seedlings using a variety of processes, ranging
from open pollination to biotechnology and other
laboratory-based techniques that are designed to enhance desired
traits in the seedlings. In our pine seed orchards, we produce
seeds through naturally occurring open pollination where we do
not control the pollen source but do select the seed source, and
through mass-control pollination where we select both the pollen
source and the seed source to produce seeds with desirable
traits. We also produce varietal seedling products through
several proprietary, laboratory-based processes whereby we
identically replicate selected seedlings that best meet the
needs of our customers. In addition, we have a pipeline of 15
biotechnology products in development, where we use genetic
technology to introduce new traits, such as freeze tolerance or
accelerated growth, to our varietal seedling products. Our
freeze-tolerant tropical eucalyptus product is the first and
only biotechnology forestry product under review for
deregulation by the USDA, and we have the largest number of
regulatory approvals for field tests of biotechnology forestry
products in the United States and Brazil.
We believe our technology platform is a critical component of
our integrated business model and is at the core of our
continued success, and we intend to continue investing in this
platform. Our technology platform consists of the following key
elements:
|
|
|
| |
•
|
Germplasm Repository. We own one of the
largest and most diverse repositories of germplasm in the world,
including over 50 distinct commercial tree species and hybrids,
which have been selected and developed since the 1950s for their
desirable traits. Our portfolio of germplasm includes the most
widely used pine and hardwood species in the United States, New
Zealand and Australian commercial forestry markets. Through our
product development collaborations in Brazil, we also work with
leading eucalyptus germplasm, which is used by our
collaborators. For two of our largest commercial pine species,
loblolly and radiata, we have catalogued over 13,500 unique
varieties. Our high-quality germplasm provides us with the
resources to develop a wide variety of improved trees and is the
base material into which we integrate specific genetic traits to
produce our biotechnology products. To protect this valuable
resource, we carefully restrict access to and cryopreserve our
germplasm at our research facilities and in
back-up
locations.
|
| |
| |
•
|
Gene Discovery, Research and Licensing
Program. In connection with the development of
our biotechnology seedling products, we have patented
approximately 25 distinct genes and promoters in the
United States through our in-house research programs and
have licensed the rights to use over 50 genes and promoters
through licensing arrangements with several leading academic and
commercial institutions, many of which provide us with exclusive
rights to commercially valuable traits in certain fields of use
and/or
geographies. We also collaborate with the New Zealand Forest
Research Institute Limited on the development of new genetic
traits. We focus in particular on developing or licensing genes
responsible for traits that could improve the commercial
usefulness of a particular tree species, such as improved growth
rates, yields, stress-tolerance, uniformity, wood quality and
processing efficiency, as well as genes responsible for pollen
and flower control, which can be used to restrict the
reproduction of our products to address regulatory and
environmental considerations related to biotechnology products.
|
81
|
|
|
| |
•
|
Proprietary Development and Production
Processes. We have developed a number of
proprietary techniques for use in various stages of our
development and production processes, including the
cost-effective, large-scale commercial manufacturing of varietal
and biotechnology seedlings and mass production of bioengineered
genetic material for use in biotechnology seedlings. For
example, we have developed automated embryo harvesting
techniques for use in somatic embryogenesis, which is a critical
technology for pine varietal and biotechnology seedling
production. In addition, through our biotechnology
transformation processes, we have the capability to introduce
gene traits into the germplasm of many tree species, providing
us with thousands of new transformed tree varieties that can be
tested and refined into the best performing candidates for our
biotechnology products. In addition to trade secret protection,
as of March 31, 2011, we held 11 patents and had 13 patent
applications pending relating to our proprietary development and
production techniques.
|
| |
| |
•
|
Silviculture and Tree Improvement
Expertise. Silviculture is the practice of
managing the establishment, growth, composition, health and
quality of forests to accomplish specified targets set by land
owners and managers. Tree improvement refers to the
identification and breeding of trees with highly desirable
combinations of traits. Our ability to consistently develop
improved generations of conventionally grown trees, which in the
year ended March 31, 2010 represented approximately 72% of
our revenue, depends on our well-established expertise in
silviculture and tree improvement. This expertise is built on
the over 100 years, in the aggregate, of tree improvement
research as well as the extensive know-how of our orchard,
nursery and product development employees who are experienced
commercial foresters with an average industry experience of
approximately 20 years. Our ability to grow, select, breed
and cultivate high-quality parent trees is also the foundation
for the proprietary varietal and biotechnology techniques we use
to develop our high-value products. Furthermore, our
silviculture expertise is the basis for our
FlexStand®
System, which allows our customers to optimize production of
multiple products on the same tract of land by using carefully
designed land use strategies.
|
82
Our
Integrated Business Model
We are currently the only integrated global commercial tree
seedling company. As a result, no single entity competes with us
in commercial sales across the full range of our business. Our
integrated business model combines our research and product
development program, tree improvement program, field testing,
product production, sales and distribution infrastructure with
our established customer base. This business model facilitates
both product innovation and commercialization, and the
transition of our customers to our advanced products. We believe
our integrated business model plays a critical role in our
success. Our business model enables us to work together with our
customers to understand their needs and to facilitate the
adoption of our advanced products. We intend to continue to
leverage this business model to drive the future development,
production and commercialization of our new products, including
our biotechnology products. We believe that this makes us an
attractive partner for companies and other entities seeking to
commercialize new technologies and products for the commercial
forestry industry, particularly those related to biotechnology.
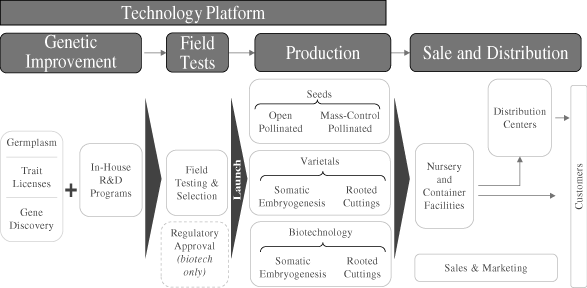
Our
Competitive Strengths
We believe we possess a number of competitive strengths and a
first mover advantage that position us for significant growth
and make it difficult for existing or potential competitors to
replicate our technology and products. Some of these key
strengths are as follows:
|
|
|
| |
•
|
Broad Product Portfolio that Delivers Significant Value for
Our Customers. Our portfolio of seedling products
includes the most widely grown tree species in the commercial
forestry industry and range from conventional to
technology-enhanced seedlings, including MCP and varietal
seedlings. This portfolio enables us to meet the needs of more
customers in a greater number of geographies while also helping
them access a larger number of end-markets. For example, we
supply multiple generations of pine seedlings that enable us to
address the needs of multiple types of land owners and managers
(including private foresters, TIMOs, REITs and integrated forest
products companies), that can be planted in multiple geographies
(including the United States, New Zealand, Australia and Brazil)
and that can be harvested to serve multiple end-markets
(including pulp and paper, wood products, biopower, charcoal and
biofuels). Our advanced products offer significant value to our
customers through a range of enhanced traits including improved
uniformity, higher growth rates, higher yields, improved wood
quality, increased stress-tolerance and better processing
efficiency relative to conventional products. Based on our
research and estimates, this improved product performance offers
our customers significantly higher returns on their land, which
justifies premium pricing relative to conventional products. For
example, our containerized MCP pine seedling products in the
United States
|
83
|
|
|
| |
|
currently sell for more than four times the price of our
best-selling conventional OP pine seedling product.
|
|
|
|
| |
•
|
Pipeline of Advanced and Biotechnology
Products. In addition to our ongoing advanced
tree improvement programs, we currently have six advanced and
15 biotechnology products under development, including
several in the advanced stages of development. These include
freeze-tolerant tropical eucalyptus, short rotation populus,
short rotation loblolly pine, short rotation eucalyptus and
improved pulping tropical eucalyptus, as well as next
generations of our MCP and varietal products. We are the first
and only company with a biotechnology forestry product currently
under review for deregulation by the USDA and have the largest
number of regulatory approvals for field tests of biotechnology
forestry products in the United States and Brazil. We have
designed our advanced and biotechnology products to suit
particular geographies and end-markets by modifying certain
targeted genetic traits, such as freeze tolerance for certain
areas of the Southeastern United States and other geographies
with similar climates, including parts of China, and improved
lignin content to reduce costs for pulp and biofuels processors
in Brazil. As a result, we believe these products will provide a
significantly higher value to our customers compared to the
conventional products that we currently offer. Our technology
platform also gives us ability to “stack” product
traits at marginal additional cost to us. For example, we can
combine a short rotation product with a product with superior
wood quality, or a fast-growing eucalyptus product with a
product that exhibits freeze tolerance. In addition, by
leveraging their use into multiple geographies and end-markets,
we are able to significantly increase the value of the products
we are developing at marginal cost. For example, we expect that
our short rotation eucalyptus product, which is designed for the
Brazilian pulp and paper market, will also have application to
the Brazilian charcoal market. Similarly, we expect that our
freeze-tolerant tropical eucalyptus product, which is targeted
for the Southeastern United States, may also have application to
other geographies with similar climates, including parts of
China. As a result, we are able to expand the breadth of our
portfolio and maximize the return on our research and
development spending.
|
| |
| |
•
|
Established Existing Business and Expansive Customer
Base. We believe we are the largest provider of
tree seedlings to the commercial forestry industry in the world.
Based on our annual seedling sales and management estimates, we
believe we currently have an approximately 27% share of the
total seedling market in the Southeastern United States,
including an approximately 31% share of the loblolly pine
market. In addition, we believe we currently have an
approximately 36% share of the total seedling market in New
Zealand, including an approximately 41% share of the radiata
pine market, and a significant share of the Australian pine
seedling market. Our base of over 5,000 customers includes some
of the largest land owners and managers in the United States,
New Zealand and Australia. Our customers include 13 of the 20
largest land owners and managers in the United States as
reported by RISI in 2008, and six of the ten largest land owners
and managers in New Zealand as reported in 2009 by the New
Zealand Forest Owners Association and the New Zealand Ministry
of Agriculture and Forestry. Many of our large customers
actively manage timberland to improve the financial returns of
their investors and have been among the first to transition to
our higher-value products. As our customers have transitioned to
our
higher-value
products, which consist of our elite open-pollinated seedlings,
our mass-control pollinated seedlings and our varietal
seedlings, sales of these products have grown from 5.7% of
U.S. revenue in the year ended March 31, 2008 to 24.2%
in the year ended March 31, 2010. This shift in sales to
our higher-value products also drove the 15.5% increase in our
U.S. average selling price over the same period. In
addition, in the year ended March 31, 2010, we generated
approximately 70% of our revenue in New Zealand and Australia
from sales of our advanced products. We expect that our
established customer base will facilitate the adoption of our
biotechnology products and that as a result the average selling
price of our products will continue to increase.
|
| |
| |
•
|
Technology Leadership Position. Our technology
leadership position is built on over 100 years, in the
aggregate, of tree improvement research. We own a portfolio of
over 230 patents and patent applications, with license rights to
more than 200 additional patents and patent applications. We
also own one of the largest and most diverse repositories of
tree genetic resources, or germplasm, in the world. To produce
our advanced and biotechnology products on a commercial scale,
we have
|
84
|
|
|
| |
|
developed numerous proprietary processes and techniques related
to the biotechnology transformation process and the large-scale
manufacturing of advanced seedlings. Our patent portfolio, trade
secrets, license agreements and unique germplasm provide us with
a solid technology foundation that we believe is difficult to
replicate and upon which we continue to build. Our in-house
discovery program and numerous license arrangements provide us
with many distinct genes that we use in the development of our
biotechnology seedling products. In addition, we have entered
into over 30 product and research collaborations to enhance our
technology platform, including collaborations with Brazil’s
largest integrated forest companies and research partnerships in
the United States with biofuels research organizations. We have
also developed numerous proprietary processes and techniques for
large-scale seedling production and for commercializing
biotechnology products. We believe our technology leadership
will enable us to continue setting the technology standard in
our industry.
|
|
|
|
| |
•
|
Demonstrated and Scalable Development, Production and
Commercialization Platform. We believe that our
fully integrated business model, with capabilities spanning
research, product development, testing, production, and sales
and distribution, enables us to better match our products to the
changing needs of the market, reduce the time and cost of
commercializing new products and transition customers to
higher-value products. Our business model is also scalable. The
scalability of our technology enables us to leverage our
research and development programs and expenses, and develop new
products at relatively low marginal cost. In addition, traits
enhanced in one tree species can be developed in other species
at marginal additional cost to us. For example, the wood quality
traits developed in our short rotation loblolly pine products in
the U.S. market can be used in radiata pine products for
the New Zealand and Australian markets. Moreover, we can apply
the expertise we have accumulated in developing, testing and
producing our advanced varietal products to the development and
commercial production of our biotechnology products at a
relatively limited incremental cost.
|
| |
| |
•
|
Experienced Management Team with a Strong Track Record and
Biotechnology Expertise. With an average of
26 years of industry experience, our management team brings
expertise in forestry and biotechnology, as well as a track
record of successfully navigating biotechnology products through
the USDA regulatory process to commercialize them. Barbara H.
Wells, Ph.D., our President and Chief Executive Officer, is
an internationally recognized expert in agriculture,
biotechnology and forestry. Dr. Wells is a member of the
Executive Committee and vice-chair of the Food and Agriculture
Section Governing Board of the Biotechnology Industry
Organization (BIO). Geoffrey P. Clear, our Senior Vice President
and Chief Financial Officer, has successfully guided three
technology companies through periods of rapid growth, including
their initial public offerings. Wayne A. Barfield, our Vice
President and General Manager of U.S. Operations, has
decades of silviculture and operational forestry experience, and
Leslie Pearson, Ph.D., our Director of Regulatory Affairs,
has more than 12 years of regulatory experience in the
forestry industry. In addition, Dr. Wells, as well as David
M. Nothmann, our Vice President of Business and Product
Development, and Maud A. W. Hinchee, Ph.D., our Chief
Science Officer, have each held important regulatory, product
development and product launch positions at Monsanto Company,
which successfully introduced biotechnology products to the
agricultural industry.
|
| |
| |
•
|
First Mover Advantage. We believe that the
combination of our scalable technology platform, intellectual
property and patents, large and diverse germplasm repository,
global production capability, established customer base and
pipeline of advanced and biotechnology products, places us in a
strong position to penetrate emerging market opportunities as a
first mover in the production of advanced and biotechnology
seedling products. Given the inherent tree growth time
associated with tree improvement research and the considerable
time and investment required to develop and secure regulatory
approval for biotechnology products, we believe our first mover
advantage places us decades ahead of any new market entrants
seeking to develop and commercialize a product portfolio and
technology platform comparable to ours; however, there is no
guarantee that we will be able to commercialize our
biotechnology products in the near future.
|
85
Our
Strategy
Our goal is to be the leader in developing and selling
proprietary seedling products by revolutionizing the
productivity of the global commercial forestry industry. We
believe that by significantly improving the productivity of a
given acre of timberland for our customers around the world, we
will be able to rapidly grow our business, deliver superior
financial performance and help conserve the world’s native
forests. We are focused on achieving these goals by employing
the following business strategies:
|
|
|
| |
•
|
Develop and Commercialize New Advanced and Biotechnology
Products. We intend to revolutionize the
productivity of, and expand our market position in, the
commercial forestry industry by developing and commercializing
new advanced and biotechnology products. For example, our MCP
seedlings and advanced varietal loblolly pine products are
already setting higher standards for growth rates, yields,
stress-tolerance, uniformity and wood quality as compared to the
conventional products we currently offer, and we believe that
our next-generation varietal products and our first-generation
biotechnology pine products will offer significant value over
our existing offerings. We believe that the productivity
improvements of our next-generation products will significantly
increase the value of our customers’ land. We believe this
value proposition will accelerate the adoption of our high-value
products and help us achieve significant market share gains in
growing markets. We believe there are several similarities
between the commercial forestry industry today and the
agricultural industry in the early 1990s. In the agricultural
industry, companies such as Monsanto Company, the first mover in
that industry, introduced biotechnology crops that redefined
productivity, which dramatically improved the economics of
farmed crops as compared to their conventional seed competitors.
According to the 2010 ISAAA Brief No. 42, the number of
acres planted with biotechnology-enhanced crops grew from
approximately 4.3 million acres in 1996 to approximately
370 million acres in 2010, representing a compound annual
growth rate of 38%. In 2010, biotechnology-enhanced crops
represent approximately 81% of soybean, 64% of cotton, 23% of
canola and 29% of maize planted globally. Our goal is to develop
and commercialize biotechnology seedling products that have an
impact on the productivity and economics of the commercial
forestry industry similar to the impact that biotechnology crops
have had on the agricultural industry.
|
| |
| |
•
|
Transition Our Customer Base to Higher-Value
Products. We intend to accelerate the adoption of
our higher-value products by leveraging our customer
relationships and the improved returns that we believe these
products will provide. For example, we are engaged in detailed
discussions with MeadWestvaco about arrangements to supply them
with our freeze-tolerant tropical eucalyptus product when
deregulated and commercialized. Our advanced and biotechnology
products are designed to provide significant additional value,
which will be shared by us and our customers. Agricultural
biotechnology companies have successfully employed this
value-sharing model to establish premium pricing for their
higher-value products. We utilize field test sites to
demonstrate to our customers the value of our advanced and
biotechnology products. In addition, we recently introduced the
FlexStand System, which expands our customers’ ability to
serve multiple end-markets by optimizing production of multiple
products on the same tract of land by alternating rows of trees
planted for sawtimber and biomass. The adoption of advanced
products is already well established in New Zealand and
Australia where we generated approximately 70% of our revenue
from sales of our advanced products in the year ended
March 31, 2010. In the United States, we have demonstrated
our ability to transition our customers to our higher-value
products with increased sales of our higher-value products,
which consist of our elite open-pollinated seedlings, our
mass-control pollinated seedlings and our varietal seedlings,
from 5.7% of U.S. revenue in the year ended March 31, 2008
to 24.2% in the year ended March 31, 2010, with increasing
sales of MCP and varietal seedlings each year. This shift in
sales to our higher-value products also drove the 15.5% increase
in our U.S. average selling price over the same period.
|
| |
| |
•
|
Grow Through Customer Expansion into Emerging
End-Markets. We intend to grow our business by
increasing the number of our seedling products used by customers
for the biopower, charcoal and biofuels end-markets. Our
customers are actively seeking strategies to help them establish
a position in these markets given their forecasted growth rates,
and we believe our advanced and biotechnology seedling products
have the potential to significantly broaden our customers’
access to these end-
|
86
|
|
|
| |
|
markets. To be profitable in these markets, our customers need
cost-effective means to increase yields and quickly grow trees
with specific traits and characteristics, and we believe our
current and future products meet this need. We are currently
working with a number of land owners and managers on strategies
to help them meet the increased demand for new renewable energy
sources for specific biopower facilities. In addition, we are
also advising biopower processors and suppliers on meeting their
long-term biomass needs by planting our purpose-grown trees.
|
|
|
|
| |
•
|
Expand into New High-Growth Geographies. We
intend to grow our business around the world to capitalize on
opportunities in the large and growing global commercial
forestry market. Specifically, we intend to grow and further
strengthen our competitive position in the United States, New
Zealand and Australia, grow our presence in Brazil and expand
our sales and operations into additional high-growth markets,
such as China and markets throughout South America. We believe
our ability to develop biotechnology seedlings with traits
suited to specific geographies, growing conditions and
end-markets provides us with a significant competitive advantage
as we enter new markets. In addition, our existing relationships
with large exporters of wood fiber in the United States, New
Zealand, Australia and Brazil provide us with the opportunity to
benefit from the growth of emerging markets to which they export
without physical expansion of our operations geographically.
|
| |
| |
•
|
Extend Our Technology Leadership. We intend to
extend our technology leadership, grow our broad product
portfolio and commercialize our pipeline of advanced and
biotechnology products by continuing to make significant
investments in research and development and expanding our
current base of research collaborations. Our pipeline of
products currently consists of six advanced and 15 biotechnology
products in various stages of development, including
freeze-tolerant tropical eucalyptus, short rotation loblolly
pine, short rotation populus, short rotation eucalyptus and
improved pulping tropical eucalyptus. Additionally, we have the
largest number of regulatory approvals for field tests of
biotechnology forestry products in the United States and Brazil.
We plan to continue to make significant investments in research
and development for the foreseeable future. We also intend to
expand our current base of over 30 product and research
collaboration arrangements to further enhance our technology
platform. As a result of our technology leadership position and
the inherent tree growth time associated with tree improvement
research, we believe we are decades ahead of any new market
entrants seeking to develop a germplasm base, technology
platform and product portfolio comparable to ours; however,
there is no guarantee that we will be able to commercialize our
biotechnology products in the near future.
|
| |
| |
•
|
Leverage Our Technology and Business Model to Drive
Profitability. We intend to leverage our leading
technology platform, established customer base and business
model to drive our revenue growth and increased profitability.
Although our revenue decreased from $23.7 million for the
year ended March 31, 2009, to $21.6 million for the
year ended March 31, 2010 due to the economic downturn, our
net loss also decreased from $(15.3) million to
$(14.7) million. We expect our revenues to grow, and our
average selling prices and gross margins to increase, as we
transition our customers to our high-value products without
having to significantly increase our fixed costs. While we
expect to continue to incur net losses for the next several
years, we believe our scalable business model will enable us to
drive profitability through the introduction of new products and
expansion into new markets with relatively low marginal costs.
|
Our
History
We were formed in February 2000 by combining all of the
biotechnology forestry research and development programs of
three leading forest products companies: Fletcher Challenge
Limited (now Rubicon Limited, a New Zealand-based company),
International Paper Company and Westvaco Corporation (now
MeadWestvaco Corporation). Each company independently developed
its own biotechnology forestry research program over decades of
significant investment, and the combination of these programs
provided us with a broad portfolio of intellectual property. The
combination also provided us with a long-term financial
commitment from our stockholders. Following this initial
combination, in October 2007, our stockholders further
contributed to us substantially all of their commercial tree
improvement research programs and seed orchard and nursery
businesses in the United States, New Zealand and Australia. Our
stockholders’ aggregate
87
investment over the past ten years, including the fair value of
the assets contributed, has totaled more than $200 million.
The October 2007 transaction significantly changed our
competitive position, as it provided us with the platform from
which to commercialize the products we develop by giving us
ownership of one of the largest and most diverse repositories of
germplasm in the world, demonstrated production and distribution
capabilities and an established customer base of land owners and
managers. As a result, we immediately transitioned from a
research-based business to a fully integrated commercial
developer and provider of conventional and technology-enhanced
tree seedlings, and began selling conventional seedlings to many
of the same customers previously served by our stockholders,
generating $23.7 million in revenue from the global
commercial sales of approximately 278 million seedlings in
our first full fiscal year following the contribution, during
which we also recorded a net loss of $(15.3) million. As of
June 1, 2010, we converted from a limited liability company
to a corporation, changing our name from “ArborGen,
LLC” to “ArborGen Inc.”
Our
Products
Our current portfolio of seedling products includes nine species
of pine, including most widely used commercial species such as
loblolly, radiata and slash pine, as well as 85 species of
hardwoods, including eucalyptus, populus (which includes both
aspen and cottonwood) and numerous specialty hardwoods. Our
seedling products are currently sold to land owners and managers
in the United States, New Zealand and Australia, with the
largest part of our sales coming from the Southeastern United
States, where we sold 218 million seedlings, or 91% of our
volume in the year ended March 31, 2010. Of those seedlings
sold in the Southeastern United States during that period,
209 million were pine seedlings and 9 million were
hardwood seedlings. Of the 15.6 million seedlings sold in
New Zealand during that period, 15.4 million were pine
seedlings and 0.2 million were hardwood seedlings. Of the
6.3 million seedlings sold in Australia, 5.0 million
were pine seedlings and 1.3 million were hardwood
seedlings. We sell multiple generations of our most commonly
sold trees, such as loblolly and radiata pine, to provide our
customers with a broad portfolio of seedling products with a
range of traits, which allows us to address different potential
end-uses at different price points and returns on investment. We
use the term “OP” to refer to those products produced
using an open pollination process, “elite” to refer to
the latest and most improved generation of a particular OP
product, “MCP” to refer to those products produced
using a mass-control pollination process, and
“varietal” to refer to those products produced using a
varietal manufacturing process. In addition, we refer to
seedlings that are planted directly into the soil at our
nurseries as our “bare root” seedlings, and we refer
to seedlings that are planted in containers in our greenhouses
as our “containerized” seedlings. We typically use the
container method for our high-value seedling products to
improve planting survival and to expand the planting window for
our customers.
Pine
Pine trees are one of the most valuable and versatile commercial
trees because they are a source of wood, fiber and energy and
they are grown across a wide range of soil types and
geographies. We sell loblolly, slash and longleaf pine products
in the Southeastern United States and radiata pine products in
New Zealand and Australia. Our pine products are currently
harvested by our customers primarily for use in the pulp and
paper and wood products markets. Pine products are also being
used in the growing biopower market, and are beginning to be
used in the emerging biofuels markets. Loblolly pine is the most
widely grown tree in the Southeastern United States, with a
geographic scope extending across 14 states from Texas to
Florida to New Jersey. Radiata pine is the most widely
grown tree in New Zealand. Variations of pine species similar to
our products are also grown in Argentina, Brazil, Chile,
Uruguay, China and other major forestry markets throughout the
world.
Hardwood
Hardwood trees play an important role in the commercial forestry
industry because they have a diverse set of traits that are
valuable for the pulp and paper and wood products markets and,
increasingly, the biopower and biofuels markets. Hardwood is
used for many different purposes including the production of
timber and composite materials for the wood products market, the
manufacture of pulp and paper and the
88
production of charcoal and wood pellets. The wood and fiber from
hardwood species has unique properties that provide specific
benefits not available from pine or other softwoods. We sell a
wide array of hardwood species, including eucalyptus, populus
and numerous specialty hardwoods. While not currently widely
grown in plantations in the United States, we believe eucalyptus
and populus have the potential to be widely grown purpose-grown
hardwoods in the Southeastern United States for the pulp and
paper and biopower markets.
Eucalyptus is the world’s most widely grown purpose-grown
hardwood species. Eucalyptus trees are grown for commercial
purposes throughout the world, with a particular concentration
in tropical and temperate climates such as Australia, China,
India, Brazil, Argentina, Uruguay, Chile, South Africa, Spain
and Portugal. There has been increased demand for eucalyptus in
the Southeastern United States because of its fast growth rates
and high quality fibers as well as the declining availability of
hardwood fiber harvested from naturally regenerated forests,
making it valuable for the pulp and paper, biopower and biofuels
markets. Historically, the use of eucalyptus for commercial
purposes in the United States has been limited because of its
general inability to withstand sudden drops in temperature. We
have, however, identified and recently introduced subtropical
eucalyptus seedling products that can be grown as far north as
South Carolina. We expect pulp and paper companies and biomass
producers with operations in the United States to have an
increasing interest in purpose-grown eucalyptus as customer
awareness of the benefits of eucalyptus increases. We have also
developed a biotechnology freeze-tolerant tropical eucalyptus
product, currently under USDA review for deregulation, that
combines the fast-growing characteristics of the tropical
eucalyptus grown extensively in Brazil with the ability to grow
in geographic areas farther north than conventional tropical
eucalyptus can grow.
Product
Valuation Methodologies
In developing new products, we use two different methods for
calculating the value that these products are expected to create
for our customers: bare land value and delivered cost savings.
The method we use for a particular product is based on the
target customer and end-market for that product.
|
|
|
| |
•
|
Bare Land Value (BLV). We typically use BLV
for new products that will be sold to land owners and managers
who are primarily focused on the wood products market. BLV is a
metric commonly used in forestry to assess the returns from
planting and harvesting trees relative to other land-use
alternatives. BLV refers to the net present value, or NPV, of
estimated revenues and costs associated with planting, growing
and harvesting trees on a given tract of land without
interruption into perpetuity, using a consistent land management
strategy. We have selected BLV as a valuation metric due to its
ability to more accurately calculate the value that is generated
by a product that shortens the planting and harvesting cycle and
makes the land available sooner for replanting. BLV is also
useful for comparing the relative values of species with
different rotation lengths. Other valuation metrics, such as
NPV, do not account for the value of multiple planting and
harvesting cycles over the same period of time. Key assumptions
that we have used in our BLV calculations, which are described
in more detail below, include the discount rate, the market
price for wood, planting density, yields, the costs associated
with site preparation, planting, fertilization, competing
vegetation control and thinning, the rotation length, timing and
percentage of thinning and product prices. The calculation of
BLV may vary based on changes to any of these assumptions.
|
| |
| |
•
|
Delivered Cost Savings. We typically use
delivered cost savings for new products expected to be sold to
processors who consume wood for the production of pulp and paper
and the generation of biopower. These processors base their
purchasing decisions on the total “delivered cost” of
the purpose-grown wood relative to wood and wood waste that can
be purchased on the open market and the additional value to the
finished product that a particular feedstock delivers. Delivered
cost consists primarily of the cost of purchasing and planting
seedlings, the cost of managing the land until the trees reach
harvest age, the cost of harvesting the wood and the cost of
delivering the wood to the processing facility. Delivered cost
savings from purpose-grown wood are primarily the result of
reduced harvesting and transportation costs, improved access and
increased availability of hardwoods. In addition to delivered
cost savings, a processor may realize value from increases in
feedstock supply reliability, improvements in fiber quality,
reductions in processing costs and higher yields.
|
89
Product
Pipeline
We currently have 21 new advanced and biotechnology products in
development, including our freeze-tolerant tropical eucalyptus,
short rotation populus, short rotation loblolly pine, short
rotation eucalyptus and improved pulping tropical eucalyptus
products. We are also developing “stacked”
biotechnology products, which are products that combine multiple
improved product traits at marginal additional cost to us.
Freeze-Tolerant Tropical Eucalyptus. Our
freeze-tolerant tropical eucalyptus product combines the
fast-growing and high-fiber quality characteristics of Brazilian
eucalyptus with the ability to withstand freezing temperatures.
Brazilian eucalyptus is widely regarded as the fastest-growing
hardwood fiber and is particularly well-suited for the
production of high-quality pulp and paper. Brazilian eucalyptus
is typically harvested after seven years of growth, yielding an
average of 18 green tons per acre per year, as compared to
a hardwood tree from a naturally regenerated forest in the
United States, which is typically harvested after 40 to
50 years of growth and yields an average of two green tons
per acre per year. Our first-generation freeze-tolerant tropical
eucalyptus, targeted at the Southeastern United States, is
designed to have growth and quality traits comparable to
Brazilian eucalyptus and to grow in areas significantly farther
north than the areas in which conventional tropical eucalyptus
is currently grown. We believe our freeze-tolerant tropical
eucalyptus will be able to be grown in all of Florida, southern
parts of Alabama, Mississippi, Georgia, Louisiana and the
southern half of Texas, expanding the potential geographical
scope of tropical eucalyptus in the United States by nearly four
times, from 15 million to 56 million acres. While our
subtropical eucalyptus products can also be grown in these
areas, based on our field tests, the yields for our
freeze-tolerant tropical eucalyptus are 15% to 36% higher than
our best-performing subtropical eucalyptus.
We believe the commercialization of our freeze-tolerant tropical
eucalyptus product will have a very large and positive impact on
the U.S. commercial forestry industry since it would allow
tropical eucalyptus to be planted over a much greater area in
the United States, enabling more land owners and managers to
benefit from its beneficial growth and yield characteristics. We
believe our purpose-grown freeze-tolerant tropical eucalyptus
has the potential to significantly reduce a pulp and paper
manufacturer’s delivered cost. We believe our
freeze-tolerant tropical eucalyptus product also has potential
commercial application outside the United States, including in
Southern Brazil, China, Chile, Uruguay, Argentina, New Zealand
and Australia. In December 2008, we filed our initial petition
for the deregulation of our freeze-tolerant tropical eucalyptus
product and resubmitted that petition in January 2011.
Short Rotation Products. We have three short
rotation products in the field testing stage: populus, loblolly
pine and tropical eucalyptus. These products are designed to
enable our customers to shorten the rotation cycle of trees of
comparable wood quality and yield by several years. By
shortening the rotation cycle of a tree, our customers are able
to harvest earlier, allowing them to receive an earlier return
on their invested capital, reduce their costs per rotation and
make the land available sooner for replanting or for alternative
uses. As a result, they are able to improve their returns. As
compared to the rotation cycles for the conventional version of
each species, our first-generation biotechnology products are
designed to shorten the rotation cycle of loblolly pine from 23
to 20 years, of populus from 12 to 8 years, and of
tropical eucalyptus from 7 to 5 years, without changing
wood quality or yield. We expect rotation cycles of subsequent
generations of our short rotation products to be shorter. For
example, we anticipate our fourth generation short rotation
loblolly pine product will reduce the rotation age of loblolly
pine to only 15 years. We believe that our short rotation
populus products may have the potential to significantly reduce
a pulp and paper manufacturer’s delivered cost. We
currently expect to submit deregulation petitions for short
rotation loblolly pine and short rotation populus to the USDA
for review in the next two to four years. Our short rotation
tropical eucalyptus product is designed specifically for the
Brazilian market, and we currently expect to submit a regulatory
submission for this product to the Comissão Técnica
Nacional de Biossegurança, or CTNBio, the governmental
agency in Brazil that regulates biotechnology products, in the
next three to four years.
Improved Pulping Tropical Eucalyptus. Our
improved pulping tropical eucalyptus product is designed to grow
trees with a particular lignin composition to increase the value
of those trees to pulp and paper processors. Lignins are wood
components that are removed by pulp mills as part of the pulping
and bleaching processes. There are two types of lignin: S-lignin
and G-lignin. S-lignin is more easily removed during
90
pulping than G-lignin. We are designing our improved pulping
tropical eucalyptus product to have an increased ratio of
S-lignin to G-lignin, so that more pulp can be extracted from
the trees grown from these seedlings for lower cost. In
addition, mills typically burn lignin removed as part of the
pulping process to generate energy to power the mill. By
improving the mix of S-lignin and G-lignin, rather than reducing
the total amount of lignin, we aim to enable mills to benefit
from the improved processing efficiency of our product without
reducing the availability of lignin for energy generation. We
have initially developed our improved pulping tropical
eucalyptus for use in the Brazilian commercial forestry market.
Our improved pulping tropical eucalyptus is designed to improve
pulp yield by 1.5%, reduce chemical consumption by 5% and reduce
energy consumption. We currently expect to submit a regulatory
submission for our improved pulping tropical eucalyptus for
review by CTNBio in the next four to five years.
“Stacked” Biotechnology
Products. Our “stacked” biotechnology
products combine two or more product traits developed for the
biotechnology products described above into a single product. We
are in the proof of concept stage for two “stacked”
biotechnology products: our freeze-tolerant tropical eucalyptus
plus growth product, which introduces fast growth traits to our
already-developed freeze-tolerant tropical eucalyptus product,
and our improved pulping tropical eucalyptus plus growth
product, which will introduce fast growth traits to our improved
pulping tropical eucalyptus product currently in the advanced
stages of development. These products will enable us to target
specific geographies and end-markets and expand the breadth of
our portfolio, significantly increasing the value of the
underlying product traits. We currently expect to submit a
regulatory submission for freeze-tolerant tropical eucalyptus
plus growth for review by the USDA in the next six to eight
years and a regulatory submission for improved pulping tropical
eucalyptus plus growth products for review by CTNBio in the next
nine to eleven years.
91
The following table illustrates the production processes and
primary benefits for selected seedling products as well as
pipeline products that are in development.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Production
|
|
|
|
|
|
|
|
|
Product
|
|
|
Process
|
|
|
Primary Benefits
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selected Current
Product Offerings:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.5 Loblolly Pine
|
|
|
OP
|
|
|
•
•
|
|
Multiple generations with returns on investment that increase
with each generation
Improved growth rates, wood quality and stress-tolerance over
earlier generations
|
|
|
|
|
|
|
|
|
|
|
2.0 Loblolly Pine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.0 Loblolly Pine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Elite Loblolly Pine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Radiata Pine (Multiple Generations)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MCP Loblolly Pine
|
|
|
MCP
|
|
|
•
|
|
Improved uniformity, growth rate, yield, stress-tolerance and
other wood quality traits as compared to OP products
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MCP Radiata Pine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Varietal Loblolly Pine
|
|
|
Varietal
|
|
|
•
•
|
|
Genetically identical seedlings lower forest management costs
Improved uniformity, growth rate, yield, stress-tolerance and
other wood quality traits as compared to OP and MCP products
|
|
|
|
|
|
|
|
|
|
|
Varietal Radiata Pine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Eucalyptus macarthurii (Subtropical)
|
|
|
Conventional
|
|
|
•
•
•
|
|
High-volume, fast-growing trees well-suited for use as pulp or
biomass
Larger geographic scope as compared to tropical eucalyptus
Cost-effective alternative to pine in certain end-markets
|
|
|
|
|
|
|
|
|
|
|
Eucalyptus benthamii (Subtropical)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product
Pipeline:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Freeze-Tolerant Eucalyptus (Tropical)
|
|
|
Biotechnology
|
|
|
•
|
|
Designed to have comparable growth and quality traits to
tropical eucalyptus, which is faster growing than subtropical
eucalyptus
|
|
|
|
|
|
|
|
|
|
•
|
|
Can be grown significantly farther north than tropical eucalyptus
|
|
|
|
|
|
|
|
|
|
•
|
|
Genetically identical seedlings lower forest management costs
|
|
|
|
|
|
|
|
|
|
•
|
|
Cost-effective alternative to pine in certain end-markets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short Rotation Populus
|
|
|
Biotechnology
|
|
|
•
•
|
|
Designed to shorten rotation cycle to eight years for the
1st
generation
product and six years for the
2nd
generation product as compared to
12-year standard rotation cycle for populus
Genetically identical seedlings lower forest management costs
|
|
|
|
|
|
|
|
|
|
|
2nd Gen
Short Rotation Populus
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short Rotation Loblolly Pine
|
|
|
Biotechnology
|
|
|
•
•
|
|
Designed to shorten rotation cycle to 20 years for the
1st
generation
product and 18 years for the
2nd
generation product, 18 years for the
3rd
generation (but with an approximately 10% increase in yields)
and
16 years for the
4th
generation product, as compared to 23-year
standard rotation cycle for loblolly pine
Genetically identical seedlings lower forest management costs
|
|
|
|
|
|
|
|
|
|
|
2nd Gen
Short Rotation Loblolly Pine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3rd Gen
Short Rotation Loblolly Pine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4th Gen
Short Rotation Loblolly Pine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short Rotation Eucalyptus (Tropical)
|
|
|
Biotechnology
|
|
|
•
|
|
Designed to shorten rotation cycle to five years as compared to
seven-year standard rotation cycle for eucalyptus
|
|
|
|
|
|
|
|
|
|
•
|
|
Designed specifically for Brazilian forestry market
|
|
|
|
|
|
|
|
|
|
•
|
|
Genetically identical seedlings lower forest management costs
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Improved Pulping Eucalyptus (Tropical)
|
|
|
Biotechnology
|
|
|
•
|
|
Designed to adjust lignin composition to improve processing
efficiency
|
|
|
|
|
|
|
|
|
|
•
|
|
Higher-value product for pulp and paper industry
|
|
|
|
|
|
|
|
|
|
•
|
|
Designed for Brazilian forestry market
|
|
|
|
|
|
|
|
|
|
•
|
|
Genetically identical seedlings lower forest management costs
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short Rotation Loblolly Pine – Brazil
|
|
|
Biotechnology
|
|
|
•
•
•
|
|
Designed for Brazilian forestry market
Designed to shorten rotation cycle to 15 years for the
1st
generation
product and 10 years for the
2nd
generation product, as compared to
18-year standard rotation cycle for loblolly pine grown in
Brazil
Genetically identical seedlings lower forest management costs
|
|
|
|
|
|
|
|
|
|
|
2nd Gen
Short Rotation Loblolly Pine – Brazil
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short Rotation Radiata Pine
|
|
|
Biotechnology
|
|
|
•
•
|
|
Designed to shorten rotation cycle to 22 years for the
1st
generation
product and 18 years for the
2nd
generation product as compared to 28-
year standard rotation cycle for radiata pine
Genetically identical seedlings lower forest management costs
|
|
|
|
|
|
|
|
|
|
|
2nd Gen
Short Rotation Radiata Pine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Improved Pulping Eucalyptus (Tropical) Plus Growth
|
|
|
Biotechnology
|
|
|
•
|
|
Designed to shorten rotation cycle from seven to five years and
further improve lignin composition to improve pulping efficiency
compared to the
1st
generation improved pulping eucalyptus product
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Freeze-Tolerant Eucalyptus (Tropical) Plus Growth
|
|
|
Biotechnology
|
|
|
•
|
|
Designed to shorten rotation cycle from seven to four years and
further improve freeze tolerance compared to the
1st
generation freeze-tolerant tropical eucalyptus product
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
92
The following table illustrates the target end-markets and
geographies for selected seedling products as well as pipeline
products that are in development.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Target End-Markets and Geographies(1)
|
|
|
|
Pulp and Paper
|
|
|
Wood Products
|
|
|
Biofuels
|
|
|
Biopower
|
|
|
Charcoal
|
|
Product
|
|
U.S.
|
|
SA
|
|
ANZ
|
|
|
U.S.
|
|
SA
|
|
ANZ
|
|
|
U.S.
|
|
SA
|
|
ANZ
|
|
|
U.S.
|
|
SA
|
|
ANZ
|
|
|
SA
|
|
Selected Current
Product Offerings:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.5 Loblolly Pine
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.0 Loblolly Pine
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.0 Loblolly Pine
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Elite Loblolly Pine (our newest generation)
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Radiata Pine (OP)
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
MCP Loblolly Pine
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MCP Radiata Pine
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
Varietal Loblolly Pine
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
Varietal Radiata Pine
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
Conventional U.S. Hardwoods
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Eucalyptus macarthurii (Subtropical)
|
|
|
ü
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
Eucalyptus benthamii (Subtropical)
|
|
|
ü
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product
Pipeline:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Freeze-Tolerant Eucalyptus (Tropical)
|
|
|
ü
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
Short Rotation Populus
(1st and
2nd
Generation)
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
Short Rotation Loblolly Pine
(1st,
2nd,
3rd and
4th
Generation)
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short Rotation Eucalyptus (Tropical)
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
Improved Pulping Eucalyptus (Tropical)
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short Rotation Loblolly Pine – Brazil
(1st and
2nd
Generation)
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
Short Rotation Radiata Pine
(1st and
2nd
Generation)
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
Improved Pulping Eucalyptus (Tropical) Plus Growth
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ü
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Freeze-Tolerant Eucalyptus (Tropical) Plus Growth
|
|
|
ü
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
ü
|
|
|
|
|
ü
|
|
|
|
|
|
(1)
|
|
In this table “SA” refers
to South America and “ANZ” refers to Australia and New
Zealand.
|
The following graph illustrates what we believe, based on our
research and estimates, to be the potential increase in BLV from
the products in our pipeline. The graph expresses the potential
increase in BLV as a multiple relative to our best-selling
conventional OP pine seedling product, taking into account the
higher estimated price of those products. BLV refers to the net
present value of estimated revenues and costs associated with
planting, growing and harvesting trees on a given tract of land
without interruption into perpetuity, using a consistent land
management strategy. In this graph, “OP” refers to our
bare root 2.0 loblolly pine product, “MCP” refers to
our containerized MCP loblolly pine product, “Var-C”
refers to our current varietal loblolly pine product,
“SRL-1” refers to our short rotation loblolly pine
product and “SRL-2,” “SRL-3” and
“SRL-4” refer to our next-generation short rotation
loblolly pine products in development. Actual results may vary.
As described in greater detail below, there are a number of
assumptions used in the calculations of BLV set forth below,
including planting density, yields, the market price for wood,
the costs associated with site preparation, planting,
fertilization, competing vegetation control and thinning,
rotation
93
length, product prices, timing and percentage of thinning, and
the discount rate. The calculation of BLV may vary based on
changes to any of these assumptions.
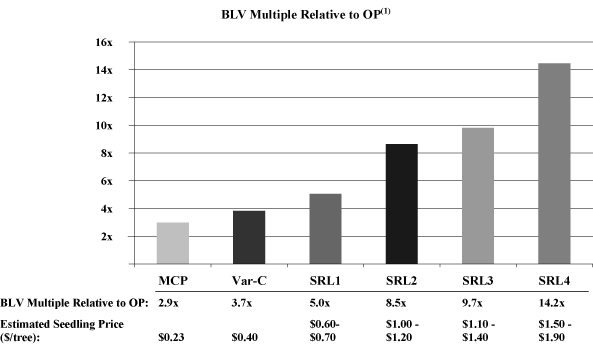
|
|
|
|
(1)
|
|
BLV refers to the net present
value, or NPV, of all revenues and costs associated with
planting, growing and harvesting trees on a given tract of land
without interruption into perpetuity, using a consistent land
management strategy. For purposes of calculating BLV in the
graph above for loblolly pine products in the United States, we
have made the following assumptions, which we believe are
reasonable estimates based on our expertise and available data:
|
| |
|
|
|
• A planting density of
400 to 550 trees per acre, which is lower than the
industry-standard planting density of 600 trees per acre to
account for the enhanced traits of our advanced and
biotechnology products. For products that yield more sawtimber,
such as our SRL products, we have used a planting density at the
lower end of this range.
|
| |
|
|
|
• Market prices of $39
per green ton for sawtimber, which assumes a recovery in the
sawtimber market to 2006 pricing, and $15 per green ton for
chip-n-saw and $8 per green ton for pulpwood, which are the
average 2009 prices in the Southeastern United States for
chip-n-saw and pulpwood.
|
| |
|
|
|
• Estimated costs of $700
to $850 per acre per rotation associated with site preparation,
planting, fertilization, competition control and thinning, which
assumes land management practices and expense levels designed to
maximize yields of high value seedling products.
|
| |
|
|
|
• The harvesting of a
total 200 to 250 green tons per acre, which is higher than the
industry average harvested green tons per acre. We anticipate
greater harvested green tons per acre due to the increased
productivity of our products and land management practices. Of
this amount, we assume approximately 25 to 55% of the total
yield will be used for sawtimber. The remaining yield is assumed
to be used approximately 20 to 40% for chip-n-saw and
approximately 25 to 35% for pulpwood. For our varietal and SRL
products, which have superior wood quality and yield more
sawtimber, the total yield and percentage of sawtimber are at
the higher end of the ranges, while our conventional
OP products have yields below the ranges.
|
| |
|
|
|
• Rotation lengths of
23 years for our OP, MCP and varietal seedlings,
20 years for our first-generation short rotation loblolly
pine product, 18 years for our second- and third-generation
short rotation loblolly pine products and 15 years for our
fourth-generation short rotation loblolly pine product.
|
| |
|
|
|
• The thinning of 13 to
20% of the trees between the sixth and tenth year of the growing
cycle.
|
| |
|
|
|
• Product prices set
forth above, which are based on management’s estimates of
list prices for our future products.
|
| |
|
|
|
• An 8% discount rate,
which we believe is well-established in the forestry industry.
|
| |
|
|
|
The calculation of BLV may vary
based on changes to any of these assumptions. For example, the
calculation of BLV could change materially with even slight
changes in the assumed discount rate.
|
Our bare root 2.0 loblolly pine product, which is our
best-selling conventional OP pine product, has a list price of
$53 per 1,000 seedlings and our elite loblolly pine product has
a list price of $70 per 1,000 seedlings.
94
We currently sell our bare root MCP loblolly pine product for
$140 per 1,000 seedlings, our containerized MCP loblolly pine
product for $230 per 1,000 seedlings and our varietal loblolly
pine product for $400 per 1,000 seedlings. Our advanced and
biotechnology products are designed to provide significant
additional value, which will be shared by us and our customers.
Agricultural biotechnology companies have successfully employed
this value-sharing model in their businesses to establish
premium pricing for their higher-value products. However, unlike
agricultural biotechnology companies, which typically sell
through distributors, we expect to continue to sell our products
directly to our customers. As a result, we expect to capture a
significant portion of the value created by our advanced and
biotechnology products. Upon deregulation, we currently
anticipate offering our freeze-tolerant tropical eucalyptus
product at a list price approximately 1.5 to 2 times the current
list price of our subtropical eucalyptus benthamii product of
$400 per 1,000 seedlings and our short rotation loblolly pine
products at list prices ranging from approximately 10 to 20
times (depending on the generation of the product) the current
list price of our bare root 2.0 loblolly pine product of $53 per
1,000 seedlings. We are also working on future generations of
our varietal and biotechnology products and expect the list
prices of these products to be higher than the first-generation
products given the estimated increased return on investment that
these products deliver to land owners and managers who plant and
grow them.
Production
Processes
We produce tree seedlings using a variety of processes, ranging
from natural pollination to advanced laboratory-based techniques
that are designed to enhance desired traits in the seedlings. We
do not internally produce the seeds used to grow our hardwood
products and nearly all of our hardwood seedlings are grown from
seeds that we purchase from third-parties.
Seed-Based Method — Open
Pollination. We use both open pollination and
mass-control pollination in the production of our pine products.
Open pollination refers to the conventional process of producing
seedlings from seeds collected from trees in orchards. These
trees are pollinated naturally, resulting in some variability of
genetic traits among these seeds since we know the flower source
but not the source of the pollen. We use our tree improvement
expertise to select higher-quality trees to plant in our
orchards, which increases the quality of our seedlings, while
the proximity of other high-quality trees in our orchards
increases the probability of high-quality pollen germinating
those flowers. For each of our OP pine products, we typically
sell multiple generations to provide our customers with a broad
portfolio of seedling products with a range of traits, which
allows us to address different potential end-users at different
price points and returns on investment.
Seed-Based Method — Mass-Control
Pollination. Mass-control pollination refers to a
more advanced process of producing pine seedlings from seeds
that have been pollinated by crossing the genetic material of
specific high-quality trees. In the MCP process, tree breeding
is controlled by individually bagging the flowers of the
selected trees before they are pollinated. At the appropriate
time, the selected pollen is applied directly to the bagged
flower. The resulting seeds are then harvested and processed. By
carefully selecting and controlling the trees used for both the
flower and pollen sources, we are able to generate seedlings
with greater uniformity and improved traits than can be achieved
under the OP process.
Varietal Manufacturing (Varietals). In
varietal manufacturing, we use a proprietary lab-based process
to identically replicate selected superior-performing individual
trees, such as those trees exhibiting the best combination of
growth and wood quality characteristics. We believe varietals
create significant value for land owners and managers by
providing seedlings with greater uniformity of seedling
genetics, which enables more efficient forest management and
enhances investment returns. We utilize our tree improvement
research process to identify through multiple field tests the
trees with desired traits to be used for the base genetic
material and then produce exact duplicates of this genetic
material using embryo replication to duplicate individual
plants. The traits we select include wood and fiber quality,
yield and disease resistance. For most hardwood species, the
replication process involves growing seedlings from stem
cuttings taken from the base tree using tissue culture. For our
pine species, we use our proprietary somatic embryogenesis
process, which is the process of splitting a cell into identical
cells, inducing those cells to change into embryos, and then
growing those embryos into seedlings by introducing certain
hormones. These advanced processes enable the
95
cost-effective production of millions of genetically identical
seedlings and are an important prerequisite for the production
of our future biotechnology seedling products.
Biotechnology. Biotechnology refers to the
process of identifying, isolating and modifying genes, and has
been used effectively in the improvement of plants for decades.
Our biotechnology seedling products are produced using a complex
process of identifying and selecting genes for traits that
improve the commercial usefulness of a particular tree species,
such as the growth rate, yield, stress-tolerance, uniformity,
wood quality and processing efficiency of trees, and introducing
those genes into the germplasm of our highest-quality trees. In
addition to selecting and introducing genes, we also develop and
license “promoters” to control the expression of a
particular gene and improve the overall efficacy of the genetic
transformation. For example, without the appropriate promoter,
the introduction of a freeze tolerance gene can result in slowed
growth, which would result in extremely slow-growing eucalyptus
trees. In developing our freeze-tolerant tropical eucalyptus
product, we used a promoter that interacts with the freeze
tolerance gene to ensure that the freeze tolerance trait is
expressed only in times of extreme cold, resulting in eucalyptus
trees that exhibit normal growth rates as well as the ability to
withstand freezing temperatures. We carefully consider the
environments into which we intend to introduce our biotechnology
seedling products and introduce genes for traits that would make
the species more suitable for introduction in different
geographies or habitats. We have developed techniques to reduce
or eliminate pollen dispersal in certain of our biotechnology
products. Once we have developed a new tree variety, we
manufacture our seedlings using our proprietary varietal
manufacturing processes and conduct extensive field tests to
evaluate and test the products and determine which to
commercialize. Biotechnology products also require regulatory
clearance for commercialization.
Nursery
Operations
We produce over 80% of the seeds for our products internally. We
supplement our supply of radiata pine seed, and we procure
subtropical eucalyptus and certain other hardwood seeds, from
third parties. Both the seeds produced in our orchards using
seed-based methods and the seeds and seedlings produced in our
laboratories using varietal manufacturing or biotechnology are
then planted and grown for one season. Some of our seedlings are
planted directly into the soil at our nurseries, which we refer
to as our “bare root” seedlings. While in production
at our nurseries, our bare root seedlings are grown using
techniques designed to maximize the number of seedlings
harvested and protect them, from being washed away by heavy rain
and winds. Some of our seedlings are planted in containers in
our greenhouses and then moved to specially designed nurseries
that preserve the protective container around the seedling (we
refer to these as “containerized” seedlings). We
typically use the container method for our high-value seedling
products to improve survival and expand the planting window for
our customers. Additionally, production of seedlings in
containers minimizes the number of seedlings damaged during the
harvesting, packaging and shipping process.
We package our seedlings at the nurseries and keep them in
climate-controlled conditions throughout the packaging, shipping
and delivery process. The seedlings are then either collected by
our customers at the nursery or sent to our distribution centers
for delivery to or collection by our customers. To satisfy
increased customer demand, we also outsource the production of
some of our containerized and varietal products to select
independent contractors. We currently outsource only a small
percentage of seedlings sold, but as we expand our operations
generally and our sale of containerized products in particular,
we expect to outsource a larger percentage of our seedling
production to third parties. We believe we have strong
relationships with our independent contractors. We expect the
additional capacity will be available as we increase production
of varietal products and introduce biotechnology products.
Product
Development and Commercialization Process
Our product development and commercialization process is broken
into three broad phases: product development, pre-launch and
launch, and spans the full product lifecycle from early product
“proof of concept” to commercial-scale production and
sales. Our advanced products, and our biotechnology products in
particular, require significantly longer development, testing
and commercial production times than our conventional seedling
products. In addition, as described below in the section
entitled “—Government
96
Regulation,” we are required to submit our biotechnology
products for regulatory review in the jurisdictions in which we
plan to sell those products, a step which is not required for
our non-biotechnology products.
OP and
MCP Seedling Products
|
|
|
| |
•
|
Product Development. During the product
development phase, our research team conducts continuous field
testing of the trees in our orchards to help us select the
highest-quality trees to breed using our OP or MCP production
methods. In addition to field testing, in the United States, we
also have access to a number of cooperatives at universities
that collect genetic information on and material for pine trees,
which we use in our tree breeding programs. We have extensive
records on the characteristics of every tree we grow or use as a
pollen source, which we use to increase the likelihood that the
next-generation trees will exhibit the desired traits.
|
| |
| |
•
|
Pre-Launch. During the pre-launch phase, we
provide our customers with product demonstrations and invite
them to visit our field test sites to introduce them to the
benefits of our products and to create demand for our products
once they are launched. We also continue to gather data on the
characteristics of the current generation of products for use in
the sales process.
|
| |
| |
•
|
Launch. During the launch phase, our sales and
marketing team regularly interacts with our customers to
demonstrate the value of our tree seedlings. Once a product line
is launched, our seedling production moves from field tests to
nursery production and harvesting.
|
Varietal
and Biotechnology Seedling Products
|
|
|
| |
•
|
Varietal Product Development. During the
product development phase of our varietal products, we leverage
the expertise of our product development team to identify the
best-performing trees to replicate. We have over
8,350 distinct types of varietal seedlings in trials in
multiple sites in the United States, New Zealand, Australia
and Brazil. Genetic tissue from these trials is then catalogued
and cryopreserved for future use. Once the trials are complete
and the best-performing trees have been selected for
replication, we use the cryopreserved genetic tissue to mass
produce chosen varieties through our proprietary varietal
manufacturing process. We are not required to go through a
regulatory approval process for our varietal products, which
means that they are much quicker to commercialize than our
biotechnology products.
|
| |
| |
•
|
Biotechnology Product Development. For our
biotechnology products, we use our internal discovery programs,
as well as the genetic resources from our licensed technology
and institutional collaborations, to select and test genes
responsible for high-performance traits. We then introduce the
selected genes to our highest-quality germplasm and in some
cases use promoters to activate genes or groups of genes to
enhance targeted traits, and produce multiple lines that are
tested for efficacy. We conduct continuous field testing to help
us select the most effective lines, which takes several years
given the inherent growth cycle of trees. We must obtain permits
from the relevant regulatory authority before conducting field
tests of our biotechnology products.
|
| |
| |
•
|
Pre-Launch. During the pre-launch phase of our
varietal and biotechnology products, in addition to the customer
efforts described above for our OP and MCP products, we begin to
implement our manufacturing strategy for the commercial-scale
production of our products. With respect to our biotechnology
products, once sufficient data is gathered through field
testing, we must obtain the required approvals from the
applicable regulatory authorities in the jurisdictions in which
we plan to sell those products.
|
| |
| |
•
|
Launch. During the launch phase of our
varietal and biotechnology products, we continue to engage in
extensive customer education efforts to demonstrate the value of
our tree seedlings and, to the extent necessary, engage in
public awareness and other efforts to address any concerns
relating to our biotechnology products. Once a product line is
launched, our varietal and biotechnology products move from our
research facility to nurseries for commercial-scale production
and harvesting.
|
97
The development of our advanced forestry products is driven by
the combined efforts of our product development team, which
focuses on tree improvement, orchard management and field
testing, and our research and development team, which focuses on
the discovery and development of commercially useful gene traits
and the introduction of those traits into our best-performing
varieties. We currently have a pipeline of six advanced and
15 biotechnology products in various stages of development
and currently expect the commercialization process for each of
our biotechnology seedling products to be completed in
approximately 10 to 12 years. As of March 31, 2011,
our product development team consisted of 31 employees, and
our research and development team consisted of
56 employees. Our research and development expense was
$10.2 million, $13.8 million and $11.2 million
for the years ended March 31, 2008, 2009 and 2010,
respectively, and we anticipate that our research and
development expense will increase for the foreseeable future as
we continue to develop and commercialize our existing portfolio
of advanced and biotechnology seedling products, and as we
expand the depth and breadth of our pipeline of future products.
Sales and
Marketing
In the United States, New Zealand and Australia, we sell our
products and promote our technologies through our 25 person
sales team, with 18 located in the United States and seven
located in New Zealand and Australia. Our sales team consists of
dedicated sales people as well as nursery managers and business
managers. Our sales and marketing efforts include the
distribution of promotional materials, including new product
overviews and our product catalogue; in-person visits with large
customers; trade journal advertisements; booths and displays at
state forestry association meetings; attendance at national
meetings for a variety of organizations; and attendance at local
and regional land owner meetings. We expect to increase our
sales and marketing expenses to help drive the broad adoption of
our advanced and biotechnology products and focus on increasing
our global market share and continuing to build brand awareness.
A significant portion of our sales effort is focused on
transitioning our existing customers to our higher-value
seedling products. Throughout the year, we conduct product
demonstrations and individual presentations for our customers to
educate them about the benefits of our high-value seedling
products and provide customer-specific analysis on silviculture
strategies, seedling selection and investment returns. We also
conduct field tests and site tours for our customers to
demonstrate the benefits of our products. In addition, we have
developed a number of marketing initiatives to help drive the
ongoing transition of our customers to our higher-value products
and to attract new customers.
One example of such an initiative is our FlexStand System.
Launched in late 2009, the FlexStand System is designed to
provide our customers with a planting system that helps them use
our products to increase their return on investment while
facilitating the transition to new products. The FlexStand
System optimizes production of multiple products on the same
acre by alternating rows of trees planted for sawtimber and
biomass. The FlexStand System gives land owners and managers the
potential for both an initial return from the early harvest of
the trees grown for pulp or biomass depending on market prices
at the time of harvest, and a larger total return on investment
from the subsequent harvest of trees grown for sawtimber, which
benefit from the increased spacing created by the earlier
biomass tree harvest. As a result, the FlexStand System creates
considerable option value for land owners and managers as to the
ultimate end-market for their trees, which we believe encourages
investment in advanced products.
We also recently launched a business development initiative to
establish relationships with biopower processors and suppliers
to meet their increasing demand for sources of biomass. We
believe that by directly serving this growing end-market, we
will be able to drive adoption of our faster-growing high-value
seedlings and demonstrate to our traditional customer base the
benefits of broadening their target end-markets to include
biopower.
In Brazil, we currently collaborate with the country’s
leading eucalyptus pulp producers and exporters to develop
biotechnology products for their internal consumption. For
additional information regarding these collaborations, see the
section below entitled “—Our Strategic
Alliances—Brazilian Eucalyptus Collaborations.” We
engage in marketing efforts to educate the Brazilian market on
the benefits of these products. These efforts are intended to
capitalize on the opportunity described above in the section
entitled
98
“—Our Market Opportunity,” which has been created
by the changing regulatory environment in Brazil, including new
requirements in Minas Gerais, Brazil’s largest charcoal
producing state, that a certain percentage of charcoal be
produced using purpose-grown trees.
Our business is seasonal. Our sales cycle has historically been
significantly concentrated with a significant majority of our
annual U.S. product orders placed between April and October
of each year (which is the six-month period following the
harvest season in the United States). As a result, we have
historically recognized approximately 75% of our revenue for
each fiscal year in the fourth quarter. We expect this
seasonality to continue for the foreseeable future. This
seasonality is partially balanced by the growing cycle in New
Zealand and Australia, where planting occurs primarily in
October and November and harvesting and delivery to customers
occurs between June and September. We expect this balancing
effect to increase as our international operations grow and as
we introduce new products, such as our tropical eucalyptus
seedlings, which have a different growing and sales cycle.
Our
Customers
With over 5,000 customers, we have an extensive and
well-established customer base that includes TIMOs, REITs,
integrated forest products companies, private land owners and
managers, forestry consultants and regeneration vendors.
Currently, our customers are located in the United States, New
Zealand and Australia. For the year ended March 31, 2010,
76% of our revenue was generated from customers located in the
United States, while the remaining 24% was generated from
customers located in New Zealand and Australia. For details on
geographic reporting related to our revenues and long-lived
assets, see note 13 to our consolidated financial
statements included elsewhere in this prospectus.
We believe we are the largest provider of tree seedlings to the
commercial forestry industry in the world. We focus on the most
important species to the commercial forestry industry, including
loblolly pine, which is the most widely grown commercial tree
species in the Southeastern United States, radiata pine, which
is the most widely grown commercial tree species in New Zealand,
as well as widely grown hardwoods such as populus and
eucalyptus. Based on management estimates, we believe we
currently have an approximately 27% share of the total seedling
market in the Southeastern United States, including an
approximately 31% share of the loblolly pine market. In
addition, we believe we currently have an approximately 36%
share of the total seedling market in New Zealand, including an
approximately 41% share of the radiata pine market. Based on
management estimates, we believe we currently have a more than
10% share of the Australian pine seedling market. Based on
management estimates, we believe that, in 2009, approximately
800 million total seedlings and 629 million loblolly
pine seedlings, were sold in the Southeastern United States.
According to the New Zealand Ministry of Agriculture and
Forestry, the total market size for New Zealand in 2009 was
approximately 43.2 million seedlings, including
37.7 million radiata pine seedlings. Based on management
estimates, we believe the total market size for Australia in
2009 was approximately 29 million pine seedlings.
Our customers include 13 of the 20 largest land owners and
managers in the United States as reported in 2008 by RISI, and
our customers include six of the ten largest land owners and
managers in New Zealand as reported in 2009 by the New Zealand
Forest Owners Association and the New Zealand Ministry of
Agriculture and Forestry. In addition, we believe that we supply
substantially all of the tree seedlings for a significant number
of our customers. For the year ended March 31, 2010, our
top ten customers accounted for approximately 36% of our total
sales. For the year ended March 31, 2010, we generated
10.4% of our revenue from sales in the United States, New
Zealand and Australia to Hancock Natural Resource Group, Inc.
and its affiliates. This customer is the only customer that
accounted for 10% or more of our revenue during this period. We
benefit from longstanding relationships with our customers based
on our consistent product quality, broad product portfolio,
focus on customer service and scalable production and reliable
distribution capability. We believe our strong relationships
with our customers provide us the opportunity to educate them
about the benefits of our advanced products, and that the
growing focus on financial returns in the forestry industry will
lead to greater adoption of these products.
For sales to customers in the United States, our customers buy
our products either through negotiated contracts or through
sales agreements that require a 30% deposit upon execution,
although in some cases we
99
waive the deposit for credit-approved customers. The sales
agreements require payment not later than 30 days after
delivery of the product. Payment terms vary among the negotiated
contracts. We have recently entered into a long-term seedling
contract and we may enter into additional long-term seedling
contracts in the future. Our long-term seedling contract was
entered into with one of our largest U.S. customers and
provides that we will supply at least 85% of the customer’s
required seedlings for its properties in Arkansas, Louisiana,
Oklahoma and Texas, subject to certain offsets, over the next
ten years. In the future, we anticipate that some of our
long-term customer contracts for our advanced products will
incorporate terms for advance deposits prior to the shipment of
seedlings. A significant majority of our U.S. customers
place their orders between April and October of each year, which
are the months in which we grow our seedlings in the United
States.
For sales to customers in New Zealand and Australia, the
majority of our customers enter into contracts for more than one
year and typically contain deposit requirements. In most cases,
the customer pays 20% of the purchase price upon signing, an
additional 20% of the purchase price not later than
120 days prior to the targeted delivery dates and the
remaining 60% of the purchase price not later than 30 days
after the actual delivery dates. In cases in which we have
long-term contracts with our customers, we often have meaningful
indications of customer demand as early as October and November,
which are the months in which we conduct our planting in New
Zealand and Australia.
Our
Strategic Alliances
To enhance our technology platform we have entered into over 30
product and research collaboration arrangements, including our
strategically important product development collaboration with
Brazil’s leading eucalyptus pulp producers and exporters
and our research partnership with BioEnergy Science Center, or
BESC, the biofuel research center funded by the Department of
Energy. We have also entered into exclusive and non-exclusive
license arrangements with companies and universities relating to
certain technologies, processes, and intellectual property
rights that support and enhance our internal research and
development program. See “—Intellectual
Property—Our License Agreements” below for more
information.
Brazilian
Eucalyptus Collaborations
We have product development collaborations with the leading
integrated forestry companies in Brazil, who in the aggregate
account for in excess of 50% of annual pulp production in
Brazil. Under these collaborations, we combine our biotechnology
processes with eucalyptus germplasm owned by those companies to
develop for their internal consumption advanced eucalyptus tree
seedling products that result in enhanced, higher-value trees.
We generally share the costs of this development work on a 50/50
basis, and we have the right to joint ownership of all
intellectual property and all products developed under these
collaborations. These collaborations provide us with an
important foothold in the large and growing Brazilian forestry
market and we plan to continue to develop these and similar
collaboration relationships and use this foothold to expand our
business in Brazil.
Other
Product Collaborations
We have partnered with New Zealand Forest Research Institute
Limited (Scion), a Crown Research Institute focused on forest
products and new biomaterials, to develop and apply valuable
genetic traits, such as improved growth and superior wood
quality in pine trees, for both commercial forestry and
biomaterials applications. Begun in 2006, our partnership has
been successful in identifying gene traits associated with wood
quality improvements in pine. Together, we have identified more
than one hundred new genes to test in radiata pine research
being conducted at our facilities. Costs of the collaboration
are borne equally by the parties, and the project is overseen by
a management committee consisting of representatives from both
parties. Under our agreement with Scion, we pay a royalty based
on commercial sales of products containing genetic traits
developed as part of our collaboration. We also have an option
to exclusively license on commercially reasonable terms any
intellectual property developed by Scion outside of our
collaboration, to the extent that it applies to biotechnology
tree products.
100
In January 2011, we entered into a strategic alliance with
GreenWood Resources Inc., or GreenWood, a developer of
high-quality hybrid poplar germplasm and optimized growing
systems for high yield, short rotation tree farms. Under this
alliance, we gained co-exclusive rights with GreenWood to
commercialize certain GreenWood proprietary germplasm and serve
as GreenWood’s exclusive supplier for certain plant
materials for tree farms managed by GreenWood within a fifteen
state region of the Southeastern and Mid-Western United States.
Under this agreement, we will pay GreenWood a royalty based on
our commercial sales of products containing materials licensed
from GreenWood. We have also agreed to pay commissions to
GreenWood based on commercial sales by GreenWood of our products
or of certain products we produce that contain material licensed
from GreenWood. We are not obligated to pay any amounts until
the products covered by the agreement are commercialized, which
we do not expect will occur for two to three years. The alliance
was formed with the intent of providing a significant increase
in the supply of hybrid and pure-species poplar varietals to
address the growing demand for woody biomass in the United
States. This collaboration is expected to leverage our regional
nurseries and extensive tree germplasm resources with
GreenWood’s proprietary hybridization and varietal
development program as well as its considerable expertise in
management and tree farm operations.
We are also working with several of our customers on projects to
assist them in accessing the emerging biopower market. For
example, we are conducting collaborative field tests with, among
others, Forest Investment Associates L.P., Resource Management
Service, LLC, Plum Creek Timberlands L.P., Mosaic Fertilizer,
LLC and Florida Crystals Corporation to evaluate the potential
of purpose-grown woody biomass as a source of biomass for
biofuels.
We also are working with a Brazilian company in the field of
plant-tissue-culture services, on a project relating to the
production of higher-value loblolly pine for use in Brazil.
Under our agreement, we provide our collaborator with tree
genetic material in exchange for seedling production and field
planting services.
External
Research Collaborations
In addition to our in-house research and development team that
uses our proprietary and licensed technology to develop new tree
seedling products, we participate in a variety of external
research collaborations throughout the forestry technology
industry that support our development of superior products,
including:
|
|
|
| |
•
|
DOE BioEnergy Science Center. We are involved
in the Department of Energy’s BioEnergy Science Center, a
government-funded research center that has as its goal the
development of technologies that will allow cellulosic ethanol
to be produced from biomass and become cost-competitive with
fossil-derived fuel and gasoline. The BESC is a partnership of
universities and industrial companies, including Mascoma
Corporation, that provides us with early access to technological
breakthroughs in wood trait development.
|
| |
| |
•
|
Biofuels Cooperatives. We have joined with
Clemson University to form a research cooperative focused on the
use of biomass for the biofuels industry. The research focuses
on development and conversion of biomass, such as switchgrass,
wood chips and other fibrous plant matter, into ethanol. Through
our partnership with Clemson University, we have joined the
Bioenergy Collaborative, an interdisciplinary team investigating
commercial bioethanol production in South Carolina. We are also
a member of the North Carolina State Wood to Ethanol Consortium,
which focuses on the understanding and development of technology
to enable the repurposing of pulp mills for biofuel generation.
These collaborations provide us with access to important
research into biofuel generation that we can use to determine
how best to develop our seedling products for use as woody
biomass.
|
| |
| |
•
|
DOE Joint Genome Institute. We are a partner
in this international project that brings together more than
twenty institutions from around the world. The project’s
fundamental goal is to decode the entire genome of eucalyptus in
order to maximize its potential in bioenergy applications. We
have provided access to our own database of more than 240,000
eucalyptus gene sequences, and we will undertake work to enable
functional genomics in the model eucalyptus clone that will be
the source of the genomic sequences.
|
101
|
|
|
| |
•
|
Tree Breeding and Silviculture
Cooperatives. In the United States, we are a
member of university tree breeding and silviculture cooperatives
at North Carolina State, Texas A&M, Virginia Tech and the
University of Florida. In New Zealand and Australia, we are a
member of the Radiata Pine Breeding Company, the Southern Tree
Breeding Association and the Future Forests Research Limited.
|
| |
| |
•
|
Biotechnology Cooperatives. We are a
participant in The Tree Genetics and Biotechnology Consortium at
Oregon State University and the Forest Biotechnology Research
Consortium at North Carolina University, and we are a member of
the Center for Advanced Forestry Systems.
|
Academic
Associations
We are a sponsor of academic research directed towards tree
improvement technology at the University of Georgia, University
of Florida, Michigan Technological University, Georgia Tech,
State University of New York (ESF), Oregon State University and
Michigan State University. These relationships not only provide
us with access to new and innovative technologies with direct
applications for potential future products, but they also
generate a strong academic network that supports us in our
commercialization, regulatory and public acceptance endeavors.
Intellectual
Property
Our success depends in part on continuing technological
innovation and on maintaining our proprietary technology
leadership position. In the United States and other countries,
we rely on a combination of patent, plant patent, plant
breeders’ rights, copyright, trademark, trade secret and
other intellectual property laws, as well as collaboration
agreements, licenses and various contractual and technical
measures to create and protect our intellectual property rights
and technology. See “Risk Factors” beginning on
page 14 for important limitations of and risks associated
with intellectual property.
Our
Patents
Where appropriate, we seek patent and other intellectual
property protection for our products and technologies. As of
March 31, 2011, our portfolio included a total of
117 patents in the United States, New Zealand, Australia,
Argentina, Canada, Chile, Portugal, South Africa and Sweden. As
of March 31, 2011, we had 38 patent families that are being
prosecuted, five patent families that are being prepared for
prosecution, and 114 pending patent applications in the
United States and many other countries. Our patents and patent
applications include claims directed to specific genetic traits
of our advanced and biotechnology seedlings, including, but not
limited to, freeze tolerance, increased growth, modified lignin
and pollen control. Our U.S. patent rights have terms that
expire between 2014 and 2030. Five of our U.S. patents will
expire prior to 2017. These patents relate to selectable marker
technology in plants, somatic embryogenesis of sweetgum,
starvation and storage of mature somatic embryos, and two
related patents covering modification of plant lignin in pine
and eucalyptus. We do not believe the expiration of these five
patents will be significant to our business, as none of the
technology covered by these five patents currently is used in
the development or production of our products. From time to
time, we also pursue plant patents in the United States and
plant breeders’ or similar rights in other countries. In
addition, we occasionally will abandon or allow certain patents
and patent applications to expire or be cancelled when we
determine further investment to not be in our strategic interest.
Our
Trade Secrets
We rely on trade secret laws to protect certain of our
technology that we determine to be better suited for protection
through trade secrets than through patent protection, or for
which we believe we may be unable to secure patent protection.
We seek to protect these trade secrets by entering into
confidentiality agreements with our employees, consultants, and
third parties with whom we collaborate, and by effecting
appropriate physical security measures.
102
Our
Trademarks
We have a number of unregistered, pending and registered
trademarks in various countries, including ten registered
trademarks in the United States:
ArborGen®,
ArborGen®
(words and design mark), SuperTree
Nursery®,
MCP®,
Mass Control
Pollinated®,
More Wood Less
Land®,
ArborGenabled®,
Flex
Stand®,
SuperTree
Seedlings®
and SuperTree
Seedlings®
(words and design mark).
Our
License Agreements
We have entered into and seek to enter into license agreements,
including exclusive licenses, with other parties relating to
certain technologies, processes, and intellectual property
rights. We currently have license agreements in place with
22 companies and universities that support and enhance our
internal research and development program, through which we
license rights to approximately 200 patents and patent
applications, both in the United States and throughout the
world. Generally these licenses may be terminated early for
material breaches that are not cured within a specified
timeframe, and for patent licenses, expire with the
last-to-expire
licensed patent or within a specified time frame.
As part of the founding of our company, we entered into license
agreements with International Paper, MeadWestvaco and Rubicon
pursuant to which we were granted rights, on a non-exclusive
basis, to certain technologies, research, scientific
information, biological materials and intellectual property.
Pursuant to these agreements, we owe royalties to each licensor
based on sales of products developed using certain of the
licensed technologies. Our obligation to pay royalties with
respect to a particular licensed product in a particular country
shall cease upon the later to occur of the expiration of all
relevant patents in such country, or ten years from the first
commercial sale of such product.
We have also entered into numerous additional agreements with
several leading academic and commercial institutions pursuant to
which we are granted rights to specific genetic traits that
support our biotechnology product development. Many of these
agreements provide us with exclusive license rights to certain
genes, including genes relating to freeze tolerance, shorter
rotation cycles and modified lignin composition, and certain
technological processes, including processes relating to somatic
embryogenesis, a key process in the production of our varietal
and biotechnology seedling products. Generally, the terms of
these agreements provide for royalty payments on the sale of
products developed using the licensed technology, and in some
cases additional scheduled or milestone payments. The rights we
license that provide us with access to the genetic traits we use
in the development of our biotechnology products include:
|
|
|
| |
•
|
Freeze Tolerance. We exclusively license
rights to genes relating to freeze tolerance traits that allow
us to produce high-quality tropical eucalyptus tree seedlings
that grow in colder climates. We use these genes in our
freeze-tolerant tropical eucalyptus product, which will allow
our customers to expand the eucalyptus growing area in the
Southeastern United States and take economic advantage of the
high-quality attributes of these trees. This product is
currently in the USDA deregulation process.
|
| |
| |
•
|
Short Rotation. We exclusively license rights
to genes to improve the productivity of our pine and hardwood
products by increasing wood yields to enable shorter rotation
cycles. We use these genes in our pipeline of short rotation
pine and hardwood products. By shortening the rotation cycles of
these trees, our customers will be able to improve their returns
by harvesting earlier and receive an earlier return on
investment, reduce their costs per rotation, and make the land
available sooner for replanting.
|
| |
| |
•
|
Modified Lignin Composition. We exclusively
license rights to genes to modify lignin composition of trees.
We use these genes in our improved pulping eucalyptus, which has
an altered mix of S-lignin and G-lignin that will allow our
customers to remove more pulp from purpose-grown trees for lower
cost.
|
| |
| |
•
|
Pollen Control. We license, on a non-exclusive
basis, a method of utilizing genes that control the ability of
our seedlings to produce and distribute pollen. Pollen control
can be important to the regulatory process for our biotechnology
seedling products, as regulators consider the potential spread
of our products when evaluating requests for field tests. By
including pollen control genes in some of our biotechnology
products, we believe we can increase the likelihood of approval
of our field tests by reducing the likelihood of our
biotechnology trees spreading beyond the planted area.
|
103
Competition
We compete on the basis of a number of factors, including the
total financial return of our products, customer service,
product quality and availability and range of products. Our
conventional tree seedling products compete with products from
private and state-owned nurseries and seedling companies that
primarily produce seedlings for external sales, including White
City Nursery, LLC and Bell Brothers Nurseries Ltd. in the
Southeastern United States. Our competitors in New Zealand and
Australia are primarily owner-operators, rather than large
commercial nurseries. For example, in New Zealand, we compete
with Rangiora Nursery Limited and Cambridge Forest and Native
Nursery Limited. In addition, in the United States, large
forestry companies, such as Weyerhaeuser Company and Plum Creek
Timber Company, Inc. produce tree seedlings primarily for their
own consumption. Similarly, in Australia, large companies and
state organizations such as Gunns Limited, Hancock Victoria
Plantations and Forestry New South Wales produce tree seedlings
primarily for their own consumption. As a result, we compete
with these companies to meet their internal demand and to the
extent they sell seedlings to third parties.
In the case of varietal loblolly and radiata pine, we compete
with CellFor in the United States and Forest Genetics Cellfor
Limited in New Zealand. Weyerhaeuser Company and Plum Creek
Timber Company, Inc. are active in the development of MCP tree
seedlings in the United States, which we believe will be used
largely for their internal consumption, and International Forest
Company also produces MCP tree seedlings for sale in the market.
In New Zealand, we compete with PF Olsen Group for the sale of
MCP seedlings. In addition, independent nurseries can purchase
mass-pollinated seed from Proseed New Zealand Limited, a tree
seed supplier to the New Zealand forest industry. There are
currently no biotechnology forestry products being sold
commercially in the geographies in which we operate. Several
companies in geographies in which we do not currently operate
are selling and developing advanced and biotechnology seedlings
products, and other biotechnology companies are developing
technology that could in the future be applied to trees. We can
provide no assurance that these companies will not enter our
markets or start developing products that compete with ours.
Government
Regulation
The production and sale of the conventional and advanced
seedling products that we currently offer are not subject to
material regulation in any of the jurisdictions in which we
currently operate. In general, our biotechnology seedling
products are subject to regulation. Our biotechnology seedling
products are currently being developed in two jurisdictions, the
United States and Brazil, and are regulated by the United States
Department of Agriculture, or USDA, and the Comissão
Técnica Nacional de Biossegurança, or CTNBio,
respectively. The regulatory system for plant biotechnology
products in the United States is well established, resulting in
81 plant biotechnology products granted deregulated status
since 1992, including two tree species (virus-resistant papaya
and virus-resistant plum), and 22 plant biotechnology product
deregulation petitions pending as of February 15, 2011. Our
freeze-tolerant tropical eucalyptus product is the first
forestry biotechnology product to be submitted for deregulation
in the United States. In Brazil, 21 biotechnology plant products
have been approved for commercial sale, all of which are
soybean, cotton or maize products. The regulatory processes for
biotechnology products in the United States and Brazil are
described below.
In New Zealand, the Environmental Risk Management Authority, or
ERMA, which was established under the Hazardous Substances and
New Organisms Act of 1996, regulates plant biotechnology
products and the export of our eucalyptus seedling products.
While Scion has recently received approval to establish field
tests using technology we have jointly developed, we do not
currently conduct any independent field tests of our
biotechnology products in New Zealand. However, our expectation
is to conduct field tests and commercialize biotechnology
products in New Zealand, which will result in our having to
comply with the regulatory process managed by ERMA. In addition,
although we currently have no immediate plans to conduct field
tests or commercialize biotechnology products in Australia, as
we implement our long-term plan to sell such products in
Australia we will need to comply with regulatory process managed
by Australia’s Office of the Gene Technology Regulator
under the Gene Technology Act of 2000.
104
U.S.
Regulatory Process
Under the Plant Protection Act of 2000, regulatory approval is
required before the introduction, including the environmental
release, interstate movement, and importation, of certain
genetically engineered organisms, including our biotechnology
products. The Coordinated Framework for the Regulation of
Biotechnology (1986) directs the USDA, the Environmental
Protection Agency, or EPA, and the Food and Drug Administration,
or FDA, to coordinate their efforts to regulate products
improved through biotechnology. The primary U.S. regulatory
agency overseeing field testing and deregulation for
commercialization of our biotechnology products is the USDA.
Currently, our products do not include pesticide or herbicide
tolerant traits and, consequently, do not require the
involvement of the EPA. Moreover, our products are not intended
for food or animal feed uses and, consequently, do not require
the involvement of the FDA. The Biotechnology Regulatory
Services, or BRS, within the USDA’s Animal and Plant Health
Inspection Service, or APHIS, has direct oversight of the field
testing and deregulation of our biotechnology products.
In the typical product development process for our biotechnology
products, approval by APHIS initially is required for a product
to move outside the laboratory for field testing. Our
biotechnology products are subject to a rigorous permit process
that typically results in authorization by APHIS for a
three-year field testing period. We are the leader in terms of
the size and number of field tests for biotechnology forestry
products. As of March 31, 2011, we have been granted 284
regulatory approvals for field trials in the U.S., with 3
permits pending approval. Our regulatory approvals represent
over 60% of all of the regulatory approvals granted by APHIS for
forestry biotechnology products.
Regulatory
Approvals for Field
Tests(1)
|
|
|
|
(1)
|
|
Based on published regulatory
approvals granted by APHIS for forestry biotechnology products.
|
The permit application must contain detailed information about
the product, including a description of the inserted genes,
their origin, the purpose of the test, how it will be conducted
and any actions taken to prevent the release of pollen or seed
from the test site. In determining whether to grant a permit and
what conditions to impose, APHIS considers any possible impacts
of the field test on the environment and any endangered or
threatened species. The permitting process for the establishment
of initial field tests typically ranges from two to four months,
but, as we have experienced, can be significantly longer for
novel products or circumstances. The process for obtaining
favorable action on petitions for non-regulated status, as well
as permits for field testing, has become more complex and time
consuming in recent years. In October 2008, APHIS issued
proposed regulations that would significantly revise the
permitting process; however, whether or when APHIS will issue
final regulations is not known.
A developer must petition APHIS to deregulate a biotechnology
product before being able to commercialize the product. The
petition process is a multi-year process that varies based on a
number of factors, including the extent of the supporting
information required, the nature and extent of review by the
105
USDA, including the type and scope of the environmental review
conducted, and the number and types of public comments received.
The process consists of the following steps:
(1) Once sufficient data is gathered through the field
testing and product development process, a petitioner prepares
and submits a petition for deregulation to APHIS that includes
information regarding the field testing, product genetics and
environmental impacts, accompanied by a detailed statistical
analysis and other data in support of the application.
(2) APHIS conducts a comprehensive review of the petition
for completeness and requests revisions to the petition or
additional data or testing, or other information as necessary
until APHIS deems the petition to be complete.
(3) As part of its review of a completed petition, APHIS
assesses plant pest risk, conducts an environmental review
pursuant to the National Environmental Policy Act of 1969, or
NEPA, and assesses the potential impact on endangered species.
As part of the NEPA environmental review, APHIS prepares an
environmental assessment, or EA, which either results in a
finding of no significant impact, or a FONSI, with respect to
the petition or determines that further study in the form of an
environmental impact statement, or EIS, is necessary. If APHIS
determines that deregulation may affect an endangered or
threatened species, APHIS will consult with the Fish and
Wildlife Service of the Department of the Interior as part of
the review.
(4) Following the initial review of the petition and
preliminary completion of the EA, APHIS publishes a Federal
Register notice announcing the availability of the petition and
EA for public comment. The public comment period typically
extends for 60 days, subject to extension. APHIS considers
and addresses each of the public comments and, in the course of
their consideration, APHIS typically consults with the
petitioner.
(5) If the EA does not result in a FONSI determination,
APHIS must undertake the additional step of preparing an EIS.
Under NEPA, the EIS process is designed to ensure that
environmental impacts are identified, disclosed and accounted
for in APHIS’ decision on the petition. The identification
of an environmental impact will not necessarily prevent APHIS
from granting the petition for deregulation. When an EIS is
required, APHIS makes a draft available for public comment
before it is finalized.
(6) If the EA does result in a FONSI determination, or upon
the completion of an EIS, if necessary, APHIS then grants (in
whole or in part) or denies the petition for deregulation,
taking into account the information received from the
petitioner, the public comments and the environmental review(s).
If APHIS grants the petition, the product is no longer regulated
and the petitioner may commercialize the product, subject to any
conditions set forth in the decision.
To date, no biotechnology product that has been submitted to
APHIS for deregulation in the United States has been denied
deregulated status; however, deregulation is not a guaranteed
outcome. There have been a limited number of cases where a
petitioner withdrew its petition during the review process. A
petitioner’s reasons for withdrawing its petition are not
made public. In some cases, a petition for deregulation that has
been withdrawn has been subsequently resubmitted by the
petitioner and granted by APHIS. Furthermore, it is possible
that APHIS may deny a petition for plant pest risk, whether
direct or indirect, or other impacts or deficiencies.
In December 2008, we filed our first petition for deregulation,
which related to our freeze-tolerant tropical eucalyptus
product. In January 2011, we withdrew and resubmitted the
petition to include additional data. The petition is currently
pending before APHIS. As part of the information-gathering
process, we conduct field tests for which we are required to
obtain permits from APHIS. On July 1, 2010, the Center for
Biological Diversity and several other parties filed suit
against the USDA and APHIS in the U.S. District Court,
Southern District of Florida, challenging the issuance by APHIS
of several field test permits for our freeze-tolerant tropical
eucalyptus product. In the complaint, which was amended on
August 10, 2010 and again on October 11, 2010, the
plaintiffs allege that APHIS did not follow appropriate
processes and procedures when issuing certain permits to us. The
plaintiffs seek relief through a variety of remedies, including
judicial declaration that APHIS violated federal statutes,
revocation of certain field test permits for our freeze-tolerant
tropical eucalyptus product, enjoining the flowering of our
freeze-tolerant tropical
106
eucalyptus product in current field tests, and requiring APHIS
to prepare an environmental impact statement that addresses the
overall cumulative impacts of all permits and petitions covering
our freeze-tolerant tropical eucalyptus product. On
September 7, 2010 and October 25, 2010, the
U.S. federal government filed answers to the amended
complaints. On September 7, 2010, we filed a motion to
intervene in this case in order to protect our interests and
support the government’s position that the field test
permits were properly issued, which the court granted on
October 19, 2010. We are now a party to this litigation,
and we filed our answer to the amended complaint on
October 27, 2010. We continue to closely monitor this case.
Brazilian
Regulatory Process
In Brazil, the approval of biotechnology products is regulated
by CTNBio. CTNBio consists of a full-time administrative staff
and a committee of experts and representatives of different
Ministries (including Science and Technology, Agriculture,
Environment, and Health) that meets once per month to review
applications. CTNBio has developed guidance describing the
information required as part of an application for commercial
approval of a biotechnology product. Once an application is
submitted it is analyzed by a small team of reviewers who then
present the application to the broader committee for a decision.
The review team or the committee can request additional
information from the applicant. The application process is
generally an iterative process with the applicant providing
additional data for review and consideration at subsequent
monthly meetings until all the reviewers’ and the
committee’s questions have been resolved. CTNBio also holds
public hearings on certain products to seek additional input.
CTNBio may refer applications to, among others, the National
Biosafety Council to review any socioeconomic aspects or
national interests that may be implicated.
Other
Regulation
In the ordinary course of business in our nurseries and
orchards, we use substances, including pesticides, which are
regulated or may be classified as hazardous under environmental
laws. Accordingly, we are subject to regulation by the EPA and
similar state and local agencies. We do not currently anticipate
that future expenditures for compliance with such environmental
laws and regulations will have a material adverse effect on our
financial position, results of operations or competitive
position.
Properties
Our global headquarters is in Summerville, South Carolina, where
we lease 49,500 square feet of laboratory, office and
greenhouse space that serves as our primary research,
development and production facility. We lease this space from
MeadWestvaco Forestry, LLC, an affiliate of one of our
stockholders. We currently pay annual rent of $0.8 million
under this lease, which expires on January 15, 2012. We
expect to occupy the premises until construction on our new
headquarters facility is completed by the end of 2011. As of
March 31, 2011, we owned or leased seven nurseries, 13 seed
orchards, 20 distribution centers and two research and
development facilities in the Southeastern United States, five
nurseries, two seed orchards and one research and development
facility in New Zealand and a nursery in Australia. In Brazil,
we lease approximately 4,000 square feet of office space.
In November 2010, we signed a build-to-suit and lease agreement
with Forestry Research Holdings, LLC for a new manufacturing,
research and development laboratory and headquarters facility in
Summerville, South Carolina, which includes an option for us to
purchase the facility beginning on the date of rent
commencement, which is the earlier of commencement of business
activities on the premises or 30 days after substantial
completion of the facility. We intend to occupy this new
facility, which consists of approximately 55,000 square
feet of office and laboratory space, upon its completion, which
is expected by the end of 2011. We may use net proceeds from
this offering to exercise our option to purchase the new
facility during the year ended March 31, 2012. The property
developer purchased the land on which our new facility is being
built from MeadWestvaco, and a portion of the property
developer’s financing for this purchase and the related
building development costs comes from a mortgage granted by an
affiliate of MeadWestvaco. In addition, each of MeadWestvaco,
International Paper and Rubicon are guarantors of our
obligations under the lease.
107
We believe that the nurseries, orchards, distribution centers
and other facilities that we currently own or
lease, including our planned new U.S. headquarters
facility, are adequate to meet our needs for the immediate
future, and that, should it be needed, additional space can be
acquired or leased to accommodate future growth.
Employees
As of March 31, 2011, we had 181 employees worldwide.
Of these employees, 145 employees are in the United States,
with 56 engaged in research and development, 75 in operations,
and 14 in general and administrative activities. In addition, as
of March 31, 2011, we had 24 employees in New Zealand,
six in Australia and six in Brazil. We plan to continue to
expand our workforce in the future, especially in the research
and development area. To support the growth in research and
development employees, we will need to expand managerial,
product development, operations, finance and other functions.
None of our employees are represented by a labor union, and we
consider our employee relations to be excellent.
Legal
Proceedings
From time to time, we may be involved in disputes or litigation
relating to claims arising out of our operations. In December
2008, we filed our first petition for deregulation, which
related to our freeze-tolerant tropical eucalyptus product,
which we resubmitted in January 2011. The petition is currently
pending before APHIS. APHIS has requested supplemental
information before our petition is deemed complete and we are in
the process of gathering that information. As part of the
information-gathering process, we conduct field tests for which
we are required to obtain permits from APHIS. On July 1,
2010, the Center for Biological Diversity and several other
parties filed suit against the USDA and APHIS in the
U.S. District Court, Southern District of Florida,
challenging the issuance by APHIS of several field test permits
for our freeze-tolerant tropical eucalyptus product. In the
complaint, which was amended on August 10, 2010 and again
on October 11, 2010, the plaintiffs allege that APHIS did
not follow appropriate processes and procedures when issuing
certain permits to us. The plaintiffs seek relief through a
variety of remedies, including judicial declaration that APHIS
violated federal statutes, revocation of certain field test
permits for our freeze-tolerant tropical eucalyptus product,
enjoining the flowering of our freeze-tolerant tropical
eucalyptus product in current field tests, and requiring APHIS
to prepare an environmental impact statement that addresses the
overall cumulative impacts of all permits and petitions covering
our freeze-tolerant tropical eucalyptus product. On
September 7, 2010 and October 25, 2010, the
U.S. federal government filed answers to the amended
complaints. On September 7, 2010, we filed a motion to
intervene in this case in order to protect our interests and
support the government’s position that the field test
permits were properly issued, which the court granted on
October 19, 2010. We are now a party to this litigation,
and we filed our answer to the amended complaint on
October 27, 2010. We continue to closely monitor this case.
On November 24, 2010, several of our employees filed a
Complaint in the First Judicial Circuit of the Court of Common
Pleas in South Carolina, which was replaced on November 29,
2010 with an Amended Complaint. The suit named as defendants us,
our current directors, certain of our current officers, certain
of our former directors and officers and a number of other
parties, including thirteen of our current employees. In January
2011, the thirteen current employees were dismissed from the
suit. The suit alleges that the plaintiffs and certain other of
our current employees have rights to future potential payouts
based on a document that the plaintiffs allege provides for
larger potential payouts to employees upon a sale of the Company
or of all or substantially all of its assets than the incentive
plan that we actually adopted. The plaintiffs seek relief
through a declaratory judgment, specific performance, an
accounting and monetary damages. This proceeding is in its early
stages. While we intend to defend ourselves vigorously against
the claims made in this dispute, we are unable to predict the
outcome of this proceeding. We cannot estimate the amount or
range of possible losses that we may incur in connection with
this proceeding.
108
MANAGEMENT
Executive
Officers, Key Employees, Directors and Director
Designees
The following table sets forth certain information about our
executive officers, key employees, directors and director
designees, including their ages as of March 31, 2011.
| |
|
|
|
|
|
|
|
Name
|
|
Age
|
|
Position(s)
|
|
|
|
Executive Officers:
|
|
|
|
|
|
|
|
Barbara H. Wells, Ph.D.
|
|
|
55
|
|
|
President and Chief Executive Officer, and Director Designee
|
|
Geoffrey P. Clear
|
|
|
61
|
|
|
Senior Vice President and Chief Financial Officer
|
|
Wayne A. Barfield
|
|
|
62
|
|
|
Vice President and General Manager of U.S. Operations
|
|
David M. Nothmann
|
|
|
39
|
|
|
Vice President of Business and Product Development
|
|
Key Employees:
|
|
|
|
|
|
|
|
Maud A. W. Hinchee, Ph.D.
|
|
|
58
|
|
|
Chief Science Officer
|
|
Gregory L. Mann
|
|
|
48
|
|
|
General Manager of Australasian Operations
|
|
Warren A. Banner III
|
|
|
55
|
|
|
Director of Varietal Manufacturing
|
|
Michael W. Cunningham, Ph.D.
|
|
|
54
|
|
|
Director of Product Development
|
|
Leslie Pearson, Ph.D.
|
|
|
52
|
|
|
Director of Regulatory Affairs
|
|
Directors and Director Designees:
|
|
|
|
|
|
|
|
Bruce G. Burton, Ph.D.(1)
|
|
|
55
|
|
|
Director
|
|
M. Eugene Hundley(1)
|
|
|
58
|
|
|
Director
|
|
David A. Liebetreu(2)(3)
|
|
|
52
|
|
|
Director
|
|
S. Luke Moriarty(2)(3)
|
|
|
51
|
|
|
Director
|
|
Kenneth R. Munson, Ph.D.(1)
|
|
|
58
|
|
|
Director
|
|
Mark T. Watkins, Sr.(2)(3)
|
|
|
57
|
|
|
Director
|
|
Edward T. Shonsey(1)(3)
|
|
|
65
|
|
|
Director Designee(4)
|
|
|
|
|
(1)
|
|
Member of the compensation committee
|
| |
|
(2)
|
|
Member of the audit committee
|
| |
|
(3)
|
|
Member of the nominating and
corporate governance committee
|
| |
|
(4)
|
|
Mr. Shonsey will become a
director and a member of the compensation and nominating and
corporate governance committees upon the closing of this offering
|
The following paragraphs provide information as of the date of
this prospectus about our executive officers, key employees,
directors and director designees. The information presented
includes information about each of our director’s specific
experience, qualifications, attributes and skills that led to
the conclusion that he or she should serve as a director.
Barbara H. Wells, Ph.D. has served as our President
and Chief Executive Officer since September 2002. Prior to
joining ArborGen, Dr. Wells served as vice president in
charge of Latin American growth initiatives and investments for
Emergent Genetics, an agricultural investment firm, from 2000 to
2002. From 1982 to 1999, Dr. Wells held various roles over
an 18-year
career at Monsanto Company, where she served as co-managing
director of Monsanto Brazil and leader of the Roundup Ready
soybean team. When she left Monsanto she was regional business
director for the Southeastern U.S. Region. Dr. Wells
is a board member and vice chair of the Biotechnology Industry
Association’s (BIO) Food and Agriculture Governing Body and
a member of the executive committee of the BIO board of
directors. She also serves as a board member and executive
committee member of the Institute of Forest Biotechnology and is
a member of the industry advisory committee of the DOE’s
Joint BioEnergy Institute. Dr. Wells received her Ph.D. in
agronomy from Oregon State University. She holds an M.S. in
plant pathology and a B.S. in horticulture from the University
of Arizona. Dr. Wells will be elected one of our directors
in connection with this offering.
Geoffrey P. Clear has served as our Chief Financial
Officer since September 2008 and as our Senior Vice President
since September 2010. Mr. Clear has over 35 years of
experience in finance. Prior to joining
109
ArborGen, Mr. Clear served as chief financial officer for
iRobot Corporation from 2002 to June 2008, chief financial
officer and vice president of finance for MicroTouch Systems,
Inc. from 1992 to 2001, and chief financial officer and vice
president of finance for T Cell Sciences, Inc. from 1986 to
1992, each of which became public companies during
Mr. Clear’s tenure. Prior to 1992, Mr. Clear
served in multiple financial roles at W.R. Grace & Co.
and as a public accountant for Arthur Andersen LLP.
Mr. Clear holds an M.B.A. and a B.S. in economics from
Dartmouth College.
Wayne A. Barfield has served as our Vice President and
General Manager of U.S. Operations since September 2010 and
as our General Manager of North American Operations from April
2008 to August 2010, and is responsible for our
U.S. nursery and seed orchard operations. Mr. Barfield
has over 35 years of experience in the forestry industry,
particularly with respect to pine and hardwood management in the
Southeastern United States. Prior to joining ArborGen,
Mr. Barfield served as vice president of forest research at
MeadWestvaco Corporation from 2002 to March 2008, and in various
other roles with MeadWestvaco and its predecessor, Westvaco
Corporation, starting in 1971. Mr. Barfield also served as
a director of ArborGen from 2002 to 2007, and currently serves
on the boards of several forestry-related organizations,
including the Institute of Forest Biotechnology.
Mr. Barfield holds an M.S. in environmental management from
Duke University and a B.S. in forestry from the University of
Georgia.
David M. Nothmann has served as Vice President of
Business and Product Development since April 2010 and is
responsible for all of our strategic business planning, product
development and regulatory activities. Prior to joining
ArborGen, Mr. Nothmann held various positions of increasing
responsibility from September 1997 to April 2010 at Monsanto
Company or its subsidiaries, including serving as Americas
product management lead from November 2008 to April 2010,
soybean agronomic trait lead from June 2006 to October 2008, and
strategic account and trait licensing lead for Corn States
Hybrid Services, a Monsanto subsidiary, from January 2004 to May
2006. Mr. Nothmann holds a M.B.A. and European management
certificate from New York University, Stern School of Business
and a B.A. in American studies and German area studies from
Tufts University.
Maud A. W. Hinchee, Ph.D. has served as our Chief
Science Officer since September 2000 and is responsible for our
research and intellectual property development programs.
Dr. Hinchee is responsible for constructing our technical
platforms and has overseen the development of our biotechnology
tree seedling products. Dr. Hinchee joined ArborGen
following an
18-year
career at Monsanto Company, where she led research in multiple
biotechnology crops. She has authored more than 40 scientific
research articles in the area of plant biotechnology, culture
and morphogenesis, and is credited as inventor on numerous
patent applications. She also serves as a member of several
industry-related boards and committees. Dr. Hinchee holds a
Ph.D. in botany from the University of California at Davis, an
M.S. in botany from the University of Washington, and a B.S. in
botany from the University of California–Davis.
Gregory L. Mann has served as our General Manager of
Australasian Operations since April 2010 and is responsible for
our operations in New Zealand and Australia. Mr. Mann has
more than 15 years of science and business experience.
Mr. Mann joined ArborGen from The New Zealand Institute for
Plant & Food Research Limited (a company formed from
the merger of HortResearch and Crop & Food Research),
where he held the positions of general manager –
commercial from December 2008 to March 2010 and general
manager – business development (at HortResearch) from
January 2003 to December 2008. In these positions, Mr. Mann
led the teams responsible for the commercialization of research
and intellectual property. He previously served as national
sales manager and general manager at Southward Engineering
Company Limited, and he began his career in sales management
with the specialty chemicals company W.R. Grace Limited in
Auckland, New Zealand. Mr. Mann holds a B.S. degree from
both Victoria University, Wellington, and Massey University,
Palmerston North, and a national diploma in business studies
from Open Polytechnic.
Warren A. Banner III has served as our Director of
Varietal Manufacturing since July 2008, and is responsible for
the manufacturing of our varietal seedlings. Mr. Banner has
extensive experience in vegetative tissue culture production,
the scale-up
of production systems and building large-scale supply chains to
serve broad market demand. From October 2006 to May 2008,
Mr. Banner served as director of operations for Northwest
Horticulture LLC, and from December 1984 to May 2006, he served
in a number of positions at
110
Ball Horticultural Company, most recently as president of Ball
FloraPlant, a provider of vegetative ornamental plants, from
June 2005 to May 2006. Mr. Banner holds an M.S. in plant
physiology and a B.S. in botany from the University of
Tennessee-Knoxville.
Michael W. Cunningham, Ph.D. has served as our
Director of Product Development since November 2007 and is
responsible for the development of conventional and
biotechnology tree seedling products for North America including
pine, eucalyptus and hardwood species. From January 2005 to
October 2007, Dr. Cunningham served as project leader and
manager of hardwood genetics for International Paper Company,
where he led the hardwood genetics program for both domestic and
European operations. He was previously a project leader and
regeneration group leader managing all activities, operational
and research, related to forest regeneration for Union Camp
Corporation. Dr. Cunningham started his career at North
Carolina State University as a postdoctoral research associate
focused on sweetgum macropropagation, micropropagation and
genetic transformation. Dr. Cunningham holds a Ph.D. in
forest genetics at North Carolina State University, an M.S. in
forest genetics from Texas A&M University and a B.S. in
forestry from Oklahoma State University.
Leslie Pearson, Ph.D. has served as our Director of
Regulatory Affairs since January 2003 and is responsible for the
regulatory oversight of, and commercial approvals for, our
biotechnology tree seedlings. From 1989 to 2003,
Dr. Pearson served in a number of positions at Westvaco
Corporation, including most recently as the leader of
Westvaco’s forest biotechnology group, where he was
responsible for managing research teams in growth genes,
flowering control, and stress resistance, as well as the
regulatory oversight of Westvaco’s biotechnology tree
research and development. He started his career at the
University of Georgia, where he worked as a post-doctoral
student in plant gene expression research. Dr. Pearson
holds a Ph.D. in plant molecular biology from the John Innes
Institute and a B.S. in biochemistry from the University of East
Anglia.
Bruce G. Burton, Ph.D. has served on our board of
directors since February 2000. Dr. Burton brings to our
board extensive experience with the global forestry,
biotechnology and energy industries. Dr. Burton is
currently vice president business development with New
Zealand-based Rubicon Ltd, which he joined in 2001. Prior to
joining Rubicon, Dr. Burton held a number of senior
business development and strategy roles with the Fletcher
Challenge Group including within Primary and Resources, Energy,
Methanol and Forests. Dr. Burton currently serves on the
board of directors of a number of private companies. Previous
directorships include Horizon2 Limited, the Australasian forest
biotechnology company, from May 2004 until October 2007, when it
was contributed to ArborGen. He has a Ph.D. and a M.S. in
development economics from Cornell University and a M.A. from
the University of Auckland, New Zealand.
M. Eugene Hundley has served on our board of
directors since January 2008. Mr. Hundley brings to the
board over 35 years of experience in the forestry industry
and the management of public companies. He has held various
positions of increasing responsibility at MeadWestvaco
Corporation (and its predecessor, Westvaco Corporation) since
1974, where he has served as president of the forestry division
of MeadWestvaco Corporation since May 2005. He has an M.A. in
business administration from the University of South Carolina
and a B.S. in forest management from the University of Tennessee.
David A. Liebetreu has served on our board of directors
since January 2005. Mr. Liebetreu brings to the board
extensive experience in the forestry and pulp and paper
industries. He has held various positions of increasing
responsibility at International Paper Company since 1992, where
he has served as vice president of global sourcing since
December 2007, and served as vice president of forest resources
from January 2005 to November 2007. He has a B.S. in Applied
Sciences and Engineering from the United States Military Academy
and an M.S.E. in Industrial and Operations Engineering from the
University of Michigan.
S. Luke Moriarty has served on our board of
directors since 2004. He is the chief executive officer and a
director of Rubicon Ltd. and brings extensive international
management and financial experience in both public and private
companies in the forestry, pulp and paper, building products and
energy industries. In addition to his role with Rubicon, he is
also currently the chairman of Tenon Limited, a New
Zealand-based wood products manufacturer and distributor. From
1998 to 2001, Mr. Moriarty was a member of the executive
office of Fletcher Challenge Limited, a New Zealand-based
conglomerate operating globally across a wide variety of
business sectors, and prior to that held a number of senior
roles within the forest products industry,
111
including chief financial officer of Fletcher Challenge Canada
and a director of TimberWest Forest Corp. He has also previously
served on the board of directors of Horizon2 Limited, a forest
biotechnology company, until its contribution to ArborGen in
2007. Mr. Moriarty holds a M.S. in Management from Stanford
University, and a LLB in Company and Commercial Law and BCA in
Economics and Accounting from Victoria University in New Zealand.
Kenneth R. Munson, Ph.D. has served on our board of
directors since February 2000. Dr. Munson brings to our
board extensive experience in forest research and nursery
development and forestry and wood supply. Dr. Munson has
served as director of forestry and wood supply for International
Paper Europe since July 2010. From October 2007 to June 2010, he
served as deputy chief executive officer and managing director
of global forestry and wood supply at Ilim Group, a Russian pulp
and paper company. From July 1984 until September 2007,
Dr. Munson held various roles of increasing responsibility
at International Paper Company, including most recently as
director of global forestry from August 2005 to September 2007.
Dr. Munson holds a Ph.D. in Soil Science and Forestry from
the University of Florida and M.A. and B.A. degrees in Soil
Science and Forestry from Oregon State University.
Mark T. Watkins, Sr. has served on our board of
directors since 2003. Mr. Watkins brings to our board over
20 years of experience in the forest products industry.
Mr. Watkins has served as senior vice president of
technology and forestry at MeadWestvaco Corporation since
January 2002, where he is responsible for corporate engineering,
stewardship, sustainability, safety, health and environmental
matters. Prior to that, Mr. Watkins held various positions
of increasing responsibility at Mead Corporation from August
1997 to January 2002, when he transitioned to MeadWestvaco
Corporation as part of a corporate merger. Mr. Watkins
holds a B.S. in Paper Science and Engineering from Syracuse
University and an A.S. from the State University of New York.
Edward T. Shonsey has been nominated to serve as one of
our directors and as chairman of our board of directors
effective upon consummation of this offering. Mr. Shonsey
brings to our board extensive experience in the agricultural
biotechnology and agribusiness industries. He has served as
president and chief executive officer and a member of the board
of directors of L1 Agrosciences Inc., an agricultural life
science company, since 2010. From 2008 to 2010, Mr. Shonsey
served as president and chief executive officer and a member of
the board of directors of HR BioPetroleum Inc., a renewable
energy technology company focused on utilizing marine microalgae
to produce biofuel feedstocks. Prior to that, from 2003 to 2007,
Mr. Shonsey served in several capacities (including as
chief executive officer from
2005-2007)
for Diversa Corporation, a public biotechnology company focused
on the discovery, development, large-scale manufacture, and
commercialization of industrial enzymes for a variety of
applications, including ethanol, biodiesel, and other biofuels.
Before Diversa, Mr. Shonsey was the president and chief
executive officer of Syngenta Seeds Inc. / Northrup
King, Inc., where he led the launch of the first agricultural
biotech product. While at Northrup King, he was part of the
leadership team that merged Sandoz and Ciba-Geigy (creating
Novartis) and, subsequently, Novartis and Astra-Zeneca
agricultural companies (creating Syngenta). Before that,
Mr. Shonsey spent ten years at Pioneer Hi-Bred
International, Inc. (now a subsidiary of DuPont Corporation),
where he served in various executive positions. Mr. Shonsey
began his career at Procter & Gamble, serving as an
Operations Manager. Mr. Shonsey is presently a member of
the boards of Agrivida Inc., RiceTec, Inc. and RiceTec AG, and
the Liechtenstein Foundation. Additionally, Mr. Shonsey has
served on the board of the Biotechnology Industry
Association’s (BIO) Food and Agriculture Governing Body and
Business Higher Education Council of Washington, DC, as chair of
the Personnel Commission for the state of Iowa, and was
decorated for combat operations in Vietnam as a Lieutenant,
U.S. Navy. Mr. Shonsey holds a B.S. in Electrical
Engineering from Marquette University and an M.B.A. from
Creighton University.
Composition
of Our Board of Directors
Our board of directors currently consists of six members, each
of whom were elected pursuant to the board composition
provisions of our stockholders agreement, which is described
under “Certain Relationships and Related Party
Transactions—Stockholders Agreement” in this
prospectus. These board composition provisions will terminate
immediately prior to the closing of this offering. Upon the
termination of these provisions, there will be no further
contractual obligations regarding the election of our directors.
Our board of
112
directors will be expanded in connection with this offering. We
intend to fill the vacancies created by this increase in the
number of directors by electing Dr. Wells and
Mr. Shonsey as directors.
Following the closing of this offering, our nominating and
corporate governance committee and board of directors may
consider a broad range of factors relating to the qualifications
and background of nominees, which may include diversity and is
not limited to race, gender or national origin. We have no
formal policy regarding board diversity. Our nominating and
corporate governance committee’s and board of
directors’ priority in selecting board members is
identification of persons who will further the interests of our
company through his or her established record of professional
accomplishment, the ability to contribute positively to the
collaborative culture among board members, and professional and
personal experiences and expertise relevant to our growth
strategy.
Director Independence. Our board of directors
has determined that all current members of the board of
directors are independent, as determined in accordance with the
rules of Nasdaq and the Securities and Exchange Commission.
Dr. Wells and Mr. Shonsey will be elected as directors
upon the closing of this offering, and Mr. Shonsey is
expected to be considered an independent member of our board of
directors. Upon the closing of this offering, we expect that the
composition and functioning of our board of directors and each
of our committees will comply with all applicable requirements
of Nasdaq and the rules and regulations of the Securities and
Exchange Commission. There are no family relationships among any
of our directors or executive officers.
Staggered Board. Immediately prior to the
closing of this offering, our board of directors will be divided
into three staggered classes of directors of the same or nearly
the same number and each will be assigned to one of the three
classes. At each annual meeting of the stockholders, a class of
directors will be elected for a three year term to succeed the
directors of the same class whose terms are then expiring. The
terms of the directors will expire upon the election and
qualification of successor directors at the annual meeting of
stockholders to be held in 2011 for Class I directors, 2012
for Class II directors and 2013 for Class III
directors.
|
|
|
| |
•
|
Our Class I directors will be Bruce G. Burton, Ph.D., M.
Eugene Hundley and Kenneth R. Munson, Ph.D.;
|
| |
| |
•
|
Our Class II directors will be David A. Liebetreu,
S. Luke Moriarty and Mark T. Watkins, Sr.; and
|
| |
| |
•
|
Our Class III directors will be Barbara H. Wells, Ph.D. and
Edward T. Shonsey.
|
Our amended and restated certificate of incorporation and
amended and restated by-laws, which will be effective upon the
completion of this offering, provide that the number of our
directors shall be fixed from time to time by a resolution of
the majority of our board of directors. Any additional
directorships resulting from an increase in the number of
directors will be distributed among the three classes so that,
as nearly as possible, each class shall consist of one third of
the board of directors. The division of our board of directors
into three classes with staggered three-year terms may delay or
prevent stockholder efforts to effect a change of our management
or a change in control.
Board
Leadership Structure and Board’s Role in Risk
Oversight
Historically, we have separated the positions of chairman of the
board and chief executive officer. We believe that separating
these positions allows our chief executive officer to focus on
our
day-to-day
business, while allowing the chairman of the board to lead the
board of directors in its fundamental role of providing advice
to and independent oversight of management. Our board of
directors recognizes the time, effort and energy that the chief
executive officer is required to devote to her position in the
current business environment, as well as the commitment required
to serve as our chairman, particularly as the board of
directors’ oversight responsibilities continue to grow.
While our amended and restated by-laws, which will be effective
upon the completion of this offering, and corporate governance
guidelines do not require that our chairman and chief executive
officer positions be separate, our board of directors believes
that having separate positions is the appropriate leadership
structure for us at this time and demonstrates our commitment to
good corporate governance.
113
Risk is inherent with every business, and how well a business
manages risk can ultimately determine its success. We face a
number of risks, including risks relating to our operations,
strategic direction and intellectual property as more fully
discussed under “Risk Factors” in this prospectus.
Management is responsible for the
day-to-day
management of risks we face, while our board of directors, as a
whole and through its committees, has responsibility for the
oversight of risk management. In its risk oversight role, our
board of directors has the responsibility to satisfy itself that
the risk management processes designed and implemented by
management are adequate and functioning as designed.
The board of directors’ role in overseeing the management
of our risks is conducted primarily through committees of the
board of directors, as disclosed in the descriptions of each of
the committees below and in the charters of each of the
committees. The full board of directors (or the appropriate
board committee in the case of risks that are under the purview
of a particular committee) discusses with management our major
risk exposures, their potential impact on our company, and the
steps we take to manage them. When a board committee is
responsible for evaluating and overseeing the management of a
particular risk or risks, the chairman of the relevant committee
reports on the discussion to the full board of directors during
the committee reports portion of the next board meeting. This
enables to the board of directors and its committees to
coordinate the risk oversight role, particularly with respect to
risk interrelationships.
Committees
of Our Board of Directors
Our board of directors has established an audit committee, a
compensation committee and a nominating and corporate governance
committee, each of which operates pursuant to a charter adopted
by our board of directors. Upon the closing of this offering,
the composition and functioning of all of our committees will
comply with all applicable requirements of Nasdaq, the
Sarbanes-Oxley Act of 2002 and the Securities and Exchange
Commission rules and regulations.
Audit Committee. Upon completion of this
offering, Messrs. Liebetreu, Moriarty and Watkins will serve on
the audit committee, which will be chaired by Mr. Moriarty. Our
board of directors has designated Mr. Moriarty as an
“audit committee financial expert” as defined under
the applicable rules of the Securities and Exchange Commission.
The composition of our audit committee meets the requirements
for independence under the listing standards of Nasdaq and the
applicable rules of the Securities and Exchange Commission,
including the applicable transition rules. Our board of
directors has determined that Messrs. Liebetreu and Watkins will
be considered “independent” for audit committee
purposes as that term is defined in the rules of the Securities
and Exchange Commission and the applicable Nasdaq rules.
Mr. Moriarty is the chief executive officer and director of
one of our significant stockholders. As a result, while Mr.
Moriarty satisfies the independence requirements under
Nasdaq’s rules, he does not satisfy the independence
requirements of the Securities and Exchange Commission
applicable to members of audit committees. The transition rules
of the Securities and Exchange Commission provide that one
member of the audit committee may be exempt from these more
stringent independence requirement for one year after the
effectiveness of this registration statement. Our board of
directors intends to cause our audit committee to be comprised
of only directors that are independent under the rules of both
Nasdaq and the Securities and Exchange Commission within one
year of effectiveness of this registration statement. The audit
committee’s responsibilities include:
|
|
|
| |
•
|
appointing and approving the compensation of our independent
registered public accounting firm;
|
| |
| |
•
|
assessing qualifications, performance and independence of our
independent registered public accounting firm;
|
| |
| |
•
|
approving auditing and permissible non-audit services, and the
terms of such services, to be provided by our independent
registered public accounting firm;
|
| |
| |
•
|
reviewing the audit plans with the independent registered public
accounting firm and members of management responsible for
preparing our financial statements;
|
| |
| |
•
|
reviewing and discussing with management and the independent
registered public accounting firm our annual and quarterly
financial statements and related disclosures as well as critical
accounting policies and practices used by us;
|
| |
| |
•
|
reviewing the adequacy of our internal control over financial
reporting;
|
114
|
|
|
| |
•
|
establishing policies and procedures for the receipt and
retention of accounting-related complaints and concerns;
|
| |
| |
•
|
recommending based upon the audit committee’s review and
discussions with management and the independent registered
public accounting firm whether our audited financial statements
shall be included in our Annual Report on
Form 10-K;
|
| |
| |
•
|
monitoring the integrity of our financial statements and our
compliance with legal and regulatory requirements as they relate
to our financial statements and accounting matters;
|
| |
| |
•
|
preparing the audit committee report required by Securities and
Exchange Commission rules to be included in our annual proxy
statement;
|
| |
| |
•
|
reviewing all related person transactions for potential conflict
of interest situations and approving all such
transactions; and
|
| |
| |
•
|
reviewing our earnings releases.
|
Compensation Committee. Upon completion of
this offering, Drs. Burton and Munson and Messrs. Hundley and
Shonsey will serve on the compensation committee, which will be
chaired by Dr. Burton. Our board of directors has determined
that each member of the compensation committee will be
considered “independent” as that term is defined in
the applicable Nasdaq rules. The compensation committee’s
responsibilities include:
|
|
|
| |
•
|
reviewing and approving corporate goals and objectives relevant
to the compensation of our executive officers and other key
employees;
|
| |
| |
•
|
evaluating the performance of our executive officers and other
key employees in light of such corporate goals and objectives;
|
| |
| |
•
|
reviewing and making recommendations to the board of directors
with respect to the compensation of our chief executive officer
and the goals and objectives related to such compensation;
|
| |
| |
•
|
reviewing and approving the compensation of our other executive
officers and other key employees;
|
| |
| |
•
|
reviewing and establishing our overall compensation structure,
policies and programs;
|
| |
| |
•
|
reviewing and making recommendations to the board of directors
with respect to the processes and procedures for the
consideration and determination of director and executive
compensation;
|
| |
| |
•
|
reviewing and making recommendations to the board of directors
with regard to incentive-based compensation plans and
equity-based plans;
|
| |
| |
•
|
reviewing and making recommendations to the board of directors
with respect to our policies and procedures for the grant of
equity-based awards;
|
| |
| |
•
|
reviewing and making recommendations to the board of directors
with regard to the compensation of our directors, including with
respect to any equity-based plans;
|
| |
| |
•
|
reviewing and discussing with management the compensation
discussion and analysis to be included in our annual proxy
statement or Annual Report on
Form 10-K;
|
| |
| |
•
|
making a recommendation to the board of directors that the
compensation discussion and analysis be included in our annual
proxy statement or Annual Report on
Form 10-K;
|
| |
| |
•
|
preparing the compensation committee report to be included in
our annual proxy statement or Annual Report on
Form 10-K;
|
| |
| |
•
|
engaging, retaining, managing and terminating compensation
consultants and other outside advisors with respect to
compensation matters;
|
| |
| |
•
|
reviewing and discussing with the board of directors corporate
succession plans for the chief executive officer and other key
officers;
|
115
|
|
|
| |
•
|
recommending to the board of directors the employment and
appointment of future key executives, as well as promotion and
changes in position of incumbent key executives upon evaluation
of their performance;
|
| |
| |
•
|
reviewing and approving employment agreements, severance
agreements and change in control agreements, and any additional
special or supplemental benefits for key executives;
|
| |
| |
•
|
reviewing our policies and programs for the development of
management personnel; and
|
| |
| |
•
|
reviewing and reassessing the adequacy of the compensation
committee charter annually and submitting any proposed changes
to the board of directors for approval.
|
Nominating and Corporate Governance
Committee. Upon completion of this offering,
Messrs. Liebetreu, Moriarty, Shonsey and Watkins will serve
on the nominating and corporate governance committee, which will
be chaired by Mr. Shonsey. Our board of directors has
determined that each member of the nominating and corporate
governance committee will be considered “independent”
as that term is defined in the applicable Nasdaq rules. The
nominating and corporate governance committee’s
responsibilities include:
|
|
|
| |
•
|
developing and recommending to the board of directors criteria
for board and committee membership;
|
| |
| |
•
|
establishing procedures for identifying and evaluating board of
director candidates, including nominees recommended by
stockholders;
|
| |
| |
•
|
identifying individuals qualified to become members of the board
of directors;
|
| |
| |
•
|
recommending to the board of directors the persons to be
nominated for election as directors and to each of the
board’s committees;
|
| |
| |
•
|
developing and recommending to the board of directors a set of
corporate governance guidelines; and
|
| |
| |
•
|
overseeing the evaluation of the board of directors and
management.
|
Our board of directors may from time to time establish other
committees.
Compensation
Committee Interlocks and Insider Participation
None of the members of our compensation committee has at any
time during the prior three years been one of our officers or
employees. None of our executive officers currently serves, or
in the past fiscal year has served, as a member of the board of
directors or compensation committee of any entity that has one
or more executive officers serving on our board of directors or
compensation committee.
Executive
Officers
Each of our executive officers has been elected by our board of
directors and serves until his or her successor is duly elected
and qualified.
Corporate
Governance
We have adopted a code of business conduct and ethics that
applies to all of our employees, officers and directors,
including those officers responsible for financial reporting.
Upon the closing of this offering, our code of business conduct
and ethics will be available on our website at
www.arborgen.com. We intend to disclose any amendments to
the code, or any waivers of its requirements, on our website.
116
COMPENSATION
DISCUSSION AND ANALYSIS
This section discusses our executive compensation policies
and arrangements as they relate to our named executive officers
who are listed in the compensation tables set forth below. The
following discussion should be read together with the
compensation tables and related disclosures set forth below.
Our executive compensation program is designed to attract
talented individuals to lead, manage and operate all aspects of
our business and reward and retain those individuals who
continue to meet our high expectations over time. Our executive
compensation program combines short and long-term components,
and fixed and contingent payments in amounts and proportions
that we believe are most appropriate to incentivize and reward
our executive officers for achieving our objectives. Our
executive compensation program is also intended to make us
competitive in the market places where we recruit in which there
is considerable competition for talented executives.
Our named executive officers for the year ended March 31,
2011 were Barbara H. Wells, Ph.D., our President and Chief
Executive Officer, Geoffrey P. Clear, our Senior Vice President
and Chief Financial Officer, Wayne A. Barfield, our Vice
President and General Manager of U.S. Operations, and David
M. Nothmann, our Vice President of Business and Product
Development.
Objectives
and Philosophy of Our Executive Compensation Program
The compensation program for our key executives, including our
named executive officers, is designed to achieve the following
objectives:
|
|
|
| |
•
|
attract, engage and retain individuals of superior ability,
experience and managerial talent, enabling us to be an employer
of choice in our highly competitive and dynamic industry;
|
| |
| |
•
|
motivate and reward executives whose knowledge, skills and
performance ensure our continued success;
|
| |
| |
•
|
encourage and inspire our executives to achieve key corporate
performance objectives by linking base salary increases and
incentive awards to the achievement of individual and
company-wide short and long-term goals; and
|
| |
| |
•
|
align the interests of our executives and stockholders by
motivating executives to increase stockholder value.
|
Use of
Compensation Consultants
Historically, our compensation committee has engaged a
compensation consultant in connection with the recruiting or
hiring of certain senior executives. In these instances, our
compensation committee sought market data to ensure that the
compensation paid to a new hire was not unnecessarily above
market, but still sufficient to recruit and retain a qualified
candidate. In the past, the compensation committee has engaged
Mercer (US) Inc. for this service. Mercer was engaged by the
compensation committee and does not provide any additional
services to us.
As part of our preparation to become a public company, we
retained DolmatConnell & Partners in 2010 to provide a
competitive review of compensation levels for our executive
team. DolmatConnell & Partners was engaged by the
compensation committee and does not provide any additional
services to us. DolmatConnell & Partners, with input
from our compensation committee, compiled a peer group of
companies against which to assess base salary, variable
incentive pay and equity compensation. This peer group is
discussed in further detail below.
Components
of Our Executive Compensation Program
The components of our executive compensation program consist
primarily of base salary, an annual cash incentive bonus, our
long-term value add program and broad-based benefits programs.
We combine short-term compensation components (such as base
salary and an annual cash incentive bonus) and long-term
compensation components (such as our value add program) to
provide an overall compensation structure that
117
is designed to both attract and retain key executives as well as
provide incentive for the achievement of short and long-term
corporate objectives.
Our compensation committee is responsible for evaluating and
administering our compensation programs and practices for our
key executives. Our compensation committee uses its judgment and
experience and the recommendations of our Chief Executive
Officer to determine the appropriate mix of short and long-term
compensation elements for each key executive, including our
named executive officers. Short and long-term compensation
elements are balanced to encourage each executive to use his or
her time and talents to accomplish our corporate objectives.
Both our Chief Executive Officer and Director of Human Resources
attend our compensation committee meetings to provide input on
factors that may influence our compensation committee’s
consideration of compensation programs and individual
compensation, including individual performance and compensation
parity considerations. An executive is not present at the
meetings at the time that his or her own compensation is being
reviewed by the compensation committee. Our compensation
committee analyzes each of the primary elements of our
compensation program to ensure internal compensation parity
among our executives and that our key executives’ overall
compensation, including the compensation of our named executive
officers, is competitive with executives in similar positions at
comparable companies in our labor market. In the past, our board
of directors made most compensation-related decisions, based on
recommendations of our compensation committee. In connection
with our preparation to become a public company, our
compensation committee’s authority and responsibility has
been increased.
Historically, our board of directors has determined —
and currently our compensation committee determines —
compensation for our key executives, including our named
executive officers, in large part based on our financial
resources, as well as competitive market data. In November 2010,
Mercer provided benchmarking data for all positions, including
our named executive officers. As a result, the compensation
committee increased Mr. Barfield’s base salary from
$162,000 to $170,000. No changes were made to the compensation
of our other named executive officers as a result of the Mercer
benchmarking data. With regard to annual base salaries and
annual cash incentive bonus opportunity targets for the year
ending March 31, 2011, a peer group drawn from publicly
listed companies was researched and recommended by
DolmatConnell & Partners. This task was challenging as
there are no direct publicly listed comparables for us.
Companies with similar current revenues and with strong growth
ambitions were selected from a mix of clean technology and
biotechnology companies; however, our compensation committee
determined that the selected companies will need to be
reassessed for applicability as we continue to grow. The group
included the following companies:
|
|
|
| |
•
|
Akeena Solar, Inc.
|
| |
•
|
Array BioPharma Inc.
|
| |
•
|
Cypress Bioscience, Inc.
|
| |
•
|
Dyax Corp.
|
| |
•
|
Energy Recovery, Inc.
|
| |
•
|
Inspire Pharmaceuticals, Inc.
|
| |
•
|
Metabolix, Inc.
|
| |
•
|
Momenta Pharmaceuticals, Inc.
|
| |
•
|
Ocean Power Technologies, Inc.
|
| |
•
|
Sangamo BioSciences, Inc.
|
| |
•
|
Targacept, Inc.
|
| |
•
|
Verenium Corporation (no longer expected to be a member of our
peer group after the fiscal year ended March 31, 2011)
|
We believe that the practices of the companies in our peer group
provide us with appropriate compensation benchmarks, in
particular for our named executive officers. As a result of this
peer group data the compensation committee in May 2010 increased
Dr. Wells’ target bonus for the year ending
March 31, 2011 from 50% to 65% of base salary. No changes
were made to the compensation of our other named executive
officers arising out of this peer group data review.
118
The compensation committee’s philosophy is to determine
each component of an executive’s compensation package based
on numerous factors, including:
|
|
|
| |
•
|
the demand for the particular skill sets we need within the
marketplace;
|
| |
| |
•
|
performance goals and other expectations for the position and
the individual;
|
| |
| |
•
|
the individual’s background and relevant expertise,
including training and prior relevant work experience;
|
| |
| |
•
|
the individual’s role with us and the compensation paid to
similar persons in the peer group of companies that we
review; and
|
| |
| |
•
|
comparison to other executives within our company having similar
levels of expertise and experience.
|
Each of the primary elements of our executive compensation
program is discussed in more detail below. While we have
identified particular compensation objectives that each element
of executive compensation serves, our compensation programs are
designed to be flexible and complementary and to collectively
serve all of the executive compensation objectives described
above. Accordingly, whether or not specifically mentioned below,
we believe that, as a part of our overall executive compensation
policy, each individual element of our executive compensation
program, to a greater or lesser extent, serves each of our
objectives as set forth above.
Annual
Cash Compensation
Base
Salary
During the fiscal year ended March 31, 2011, our board of
directors recognized that our compensation program would benefit
from an overall competitive salary benchmarking survey. This
survey was carried out during the third quarter of the fiscal
year and resulted in salary adjustments to a majority of our
employee work force. After reviewing the survey, the
compensation committee increased Mr. Barfield’s base
salary from $162,000 to $170,000. While our compensation
committee did not benchmark Mr. Barfield’s base salary to a
specific percentile, it determined that his base salary was
lower than the current market rate for his position, and
therefore made the upward adjustment.
Short-Term
Incentive Compensation
Our named executive officers participate in our Senior Executive
Incentive Bonus Plan. On an annual basis, each participating
executive is entitled to receive an annual cash bonus award that
is tied to attainment of corporate
and/or
individual performance targets. The compensation committee has
the authority to adjust bonuses payable under this plan based on
achievement of individual performance goals and to pay bonuses
(including discretionary bonuses) to eligible executives under
this plan based upon such other terms and conditions as the
compensation committee may in its discretion determine.
Each year, our compensation committee will select the applicable
corporate performance metrics for the fiscal year relating to
financial and operational metrics, including one or more of the
following: cash flow (including, but not limited to, operating
cash flow and free cash flow); revenue; earnings before
interest, taxes, depreciation and amortization; net income
(loss) (either before or after interest, taxes, depreciation
and/or
amortization); changes in the market price of our common stock;
economic value-added; acquisitions or strategic transactions;
operating income (loss); return on capital, assets, equity, or
investment; stockholder returns; return on sales; gross or net
profit levels; productivity; expense; margins; operating
efficiency; customer satisfaction; working capital; earnings
(loss) per share of our common stock; production and sales
volumes or market shares and number of customers; any of which
may be measured either in absolute terms or as compared to any
incremental increase; or the achievement of key business
development, marketing, research, product development,
regulatory, public and government affairs milestones. Each
performance goal has a target (100% attainment of the
performance goal).
Each eligible executive must be employed on the bonus payment
date to be entitled to receive any bonus under the Senior
Executive Incentive Bonus Plan; however, the compensation
committee will pay a pro rata bonus (to the extent the relevant
goals are met) to eligible executives (i) whose employment
is terminated without cause after completing nine or more months
of service in the fiscal year in which such termination
119
occurs or (ii) who first became eligible to participate in
the incentive plan after the beginning of the fiscal year but
who completed nine or more months of service in such fiscal year.
For our fiscal year ended March 31, 2011, the metrics used
under our Senior Executive Incentive Bonus Plan were comprised
of the following three target components:
1. Company goals
2. Regional/functional goals
3. Personal goals
The weightings of the three components of the incentive target
are set by our compensation committee. Different weightings
apply to each employee based on their role. For our named
executive officers, the weightings and target bonus percentage
for the year ended March 31, 2011 were:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Regional
|
|
|
|
|
|
Target Bonus
|
|
|
|
|
Company
|
|
|
/Functional
|
|
|
Personal
|
|
|
Percentage of
|
|
|
Executive
|
|
Weighting
|
|
|
Weighting
|
|
|
Weighting
|
|
|
Base Salary
|
|
|
|
|
Barbara H. Wells, Ph.D.
|
|
|
90
|
%
|
|
|
0
|
%
|
|
|
10
|
%
|
|
|
65
|
%
|
|
Geoffrey P. Clear
|
|
|
50
|
%
|
|
|
40
|
%
|
|
|
10
|
%
|
|
|
40
|
%
|
|
Wayne A. Barfield
|
|
|
40
|
%
|
|
|
50
|
%
|
|
|
10
|
%
|
|
|
40
|
%
|
|
David M. Nothmann
|
|
|
40
|
%
|
|
|
50
|
%
|
|
|
10
|
%
|
|
|
30
|
%
|
Company goals and their associated milestones are approved each
year as a part of the annual business planning and budgeting
process. Regional/functional and individual milestones for each
key employee including our named executive officers are approved
each year by our Chief Executive Officer and the compensation
committee, as are the respective weightings. This process starts
with a formal assessment to highlight the key issues facing the
company and to review progress against plan. The assessment
covers our markets, operations, business development programs,
product positioning and pricing, product pipeline and science
program, regulatory and public acceptance program and human
resources. A combination of planning and budgeting processes are
then used to derive our corporate plan and budget complete with
the key milestones that are then monitored through the year.
At fiscal year-end, a formal review process is undertaken across
the company. Each employee is scored on each applicable
milestone. Our Chief Executive Officer reviews the company and
regional/functional performance recommendations with the
compensation committee and the compensation committee makes
recommendations to the board of directors regarding the company
and regional/functional scores and the overall performance
scores for all executives, including our named executive
officers. Our Chief Executive Officer, in consultation with our
board of directors (or the board of directors alone, in the case
of awards applicable to our Chief Executive Officer), has the
discretion to provide additional bonus amounts to individuals
deemed to have made significant contributions to the
organization. Although we cannot always predict the different
events that will impact our business during an upcoming year, we
set our performance goals at levels that we believe will be very
challenging to achieve so in general will not be fully met a
majority of the time.
Five overall company goals were set for the year ended
March 31, 2011, focused on ensuring (1) that we have
the right capital base to support the business going forward;
(2) that we deliver or exceed our financial targets;
(3) that our
company-wide
operating performance is “world class”; (4) that
we continue to grow the value of the business at or above our
cost of capital; and (5) that we continue to develop our
public and customer profile. Regional or functional goals also
focused on financial and operating performance as well as the
achievement of key business and product development milestones.
Target weightings for these five goals were set at the start of
the fiscal year ended March 31, 2011. These weightings are
subject to adjustment during the assessment process to take into
account any changes in
120
priorities that occurred during the fiscal year. The process of
assessment of each of the five company goals and the expected
weightings are as follows:
|
|
|
| |
1.
|
The capital base assessment will include a review of both debt
management as well as the progress that was made in preparing
the business for this offering. This goal has an expected
weighting of 25%.
|
|
|
|
| |
2.
|
The financial goal will be measured based upon our achievement
of earnings before interest, tax, depreciation and amortization
as adjusted for certain special expenses (Adjusted EBITDA) and
total cashflow targets. The Adjusted EBITDA and year-end
cashflow targets for the year ended March 31, 2011 were
$(13.9) million and $(21.3) million respectively. The
special expenses that the compensation committee determined
should be excluded from the calculation of Adjusted EBITDA for
the fiscal year ended March 31, 2011 were costs related to
certain litigation matters disclosed under
“Business — Legal Proceedings” as well as
certain costs related to this offering. As a result, the
Adjusted EBITDA amount that the compensation committee expects
to use for purpose of determining bonuses will differ from our
reported EBITDA. This goal has an expected weighting of 20%.
|
|
|
|
| |
3.
|
The company-wide operating performance will be measured based
upon an assessment of actual results against a net sales target
of $25.3 million, a gross margin target of 35.4%, the
delivery of our products to our customers in full, on time and
meeting specifications more than 95% of the time, as well as
certain operational factors, including the development and
relationship management of our key customers, environmental and
regulatory permit compliance and the achievement of no
recordable injuries. This goal has an expected weighting of 20%.
|
|
|
|
| |
4.
|
The business growth and product development goals will be
measured through an assessment of new business and product
opportunities and the existing opportunities that were advanced.
This goal has an expected weighting of 20%.
|
|
|
|
| |
5.
|
The measurement of our public and customer acceptance profile
was measured from a review of our progress in achieving key
milestones in our public and government relations plan. This is
a qualitative goal that assesses our progress in communicating
the Company’s business and strategies to the public,
government organizations and non-governmental organizations.
This goal has an expected weighting of 15%.
|
Our compensation committee will assess the achievement of these
company goals during the
May/June
2011 timeframe and our compensation committee will make bonus
determinations for our named executive officers once that
process is complete. During this assessment, the compensation
committee will score each goal on a five point scale, with 5
meaning “achieved target”, 4 meaning “nearly met
target”, 3 meaning “made progress but some way from
meeting target”, 2 meaning “inadequate
performance” and 1-0 meaning “totally inadequate
performance.” Both qualitative and quantitative goals are
evaluated and scored in this manner. A score above 5 can be
given in the event that the achievement of an objective is truly
outstanding. These scores are then combined to provide a
weighted average overall company score.
For the fiscal year ended March 31, 2011,
Dr. Wells’ target cash bonus was $178,750, with 90%
being determined based on the achievement of company goals and
the remaining 10% being determined based on the achievement of
individual goals.
For the fiscal year ended March 31, 2011,
Mr. Clear’s target cash bonus was $120,000, with 50%
being determined based on the achievement of company goals, 40%
being determined based on the achievement of functional goals,
specifically the finance department’s performance, and the
remaining 10% being determined based on the achievement of
individual goals.
For the fiscal year ended March 31, 2011,
Mr. Barfield’s target cash bonus was $68,000, with 40%
being determined based on the achievement of company goals, 50%
being determined based on the achievement of functional goals,
specifically our U.S. operations’ performance, and the
remaining 10% being determined based on the achievement of
individual goals.
121
For the fiscal year ended March 31, 2011,
Mr. Nothmann’s target cash bonus was $61,500, with 40%
being determined based on the achievement of company goals, 50%
being determined based on the achievement of functional goals,
specifically our achievement of certain product development and
research program milestones, combined with certain business
development objectives, and the remaining 10% being determined
based on the achievement of individual goals.
The following discussion illustrates how the bonuses for the
fiscal year ended March 31, 2010 were determined by our
compensation committee based on the Senior Executive Incentive
Bonus Plan described above.
Dr. Wells’ target cash bonus was $137,500 for the
fiscal year ended March 31, 2010, with 90% being determined
based on the achievement of company goals and the remaining 10%
being determined based on the achievement of individual goals.
Based on the determination of our compensation committee that
each of the named executive officers was entitled to receive 66%
of the targeted payout for the achievement of company goals and
our compensation committee’s assessment of
Dr. Wells’ achievement of her individual goals, our
compensation committee determined that Dr. Wells was
entitled to receive $81,923 for company goals and $12,375 for
individual goals. Our compensation committee also determined to
pay Dr. Wells a special bonus of $25,000 to acknowledge the
leadership she showed during a challenging year.
Mr. Clear’s target cash bonus was $120,000 for the
fiscal year ended March 31, 2010, with 50% being determined
based on the achievement of company goals, 40% being determined
based on the achievement of functional goals, specifically the
finance department’s performance, and the remaining 10%
being determined based on the achievement of individual goals.
Based on the determination of our compensation committee that
each of the named executive officers was entitled to receive 66%
of the targeted payout for the achievement of company goals, the
determination that the finance department had achieved at the
92% level and our compensation committee’s assessment of
Mr. Clear’s achievement of his individual goals, our
compensation committee determined that Mr. Clear was
entitled to receive $39,720 for company goals, $43,920 for
functional goals and $9,660 for individual goals.
Mr. Barfield’s target cash bonus was $64,800 for the
fiscal year ended March 31, 2010, with 40% being determined
based on the achievement of company goals, 50% being determined
based on the achievement of functional goals, specifically our
U.S. operations’ performance, and the remaining 10%
being determined based on the achievement of individual goals.
Based on the determination of our compensation committee that
each of the named executive officers was entitled to receive 66%
of the targeted payout for the achievement of company goals, the
determination that our U.S. operations had achieved at the
72% level and our compensation committee’s assessment of
Mr. Barfield’s achievement of his individual goals,
our compensation committee determined that Mr. Barfield was
entitled to receive $17,159 for company goals, $23,328 for
functional goals and $5,119 for individual goals. Our
compensation committee also determined to pay Mr. Barfield
a special bonus of $10,000 to acknowledge the leadership he
showed during a challenging year and the impact of the worldwide
economic downturn on the performance of our U.S. operations
despite Mr. Barfield’s significant efforts.
Based on these assessments, we awarded cash bonuses to our named
executive officers in June 2010 in the following amounts:
| |
|
|
|
|
|
Executive
|
|
Bonus
|
|
|
|
|
Barbara H. Wells, Ph.D.
|
|
$
|
119,298
|
|
|
|
|
|
|
|
|
Geoffrey P. Clear
|
|
$
|
93,360
|
|
|
|
|
|
|
|
|
Wayne A. Barfield
|
|
$
|
55,606
|
|
|
|
|
|
|
|
122
Retention
Payment Program
In the final months of the year ended March 31, 2009, our
board of directors elected not to pay any cash bonuses to
executives for services rendered in the year ended
March 31, 2009. Instead, our board of directors implemented
a company-wide retention payment program, which provided that:
|
|
|
| |
•
|
Each full-time employee who was employed by us on
December 31, 2008 would receive a retention payment if he
or she was continuously employed by us on a full-time basis
through March 31, 2011, except as otherwise provided below.
|
| |
| |
•
|
Employees whose employment with us terminated or whose hours
were reduced to less than full-time prior to March 31, 2011
for any or no reason will not receive a retention payment.
|
The payments made to our named executive officers on
March 31, 2011 under our retention payment program were as
follows:
| |
|
|
|
|
|
Name
|
|
Retention Payment
|
|
|
|
|
Barbara H. Wells, Ph.D.
|
|
$
|
89,495
|
|
|
Geoffrey P. Clear
|
|
$
|
36,751
|
|
|
Wayne A. Barfield
|
|
$
|
33,522
|
|
As Mr. Nothmann was not employed by us on December 31,
2008, he was not eligible to receive a retention payment under
this program.
New
Employment Agreements
In January 2011, we entered into new employment agreements with
each of our named executive officers in order to foster our
goals of encouraging executive retention and focus on the
management and best interests of the company. We also prefer to
have certainty regarding the potential severance amounts payable
to the named executive officers under certain circumstances,
rather than negotiating severance at the time that a named
executive officer’s employment terminates.
The employment agreements set the executive’s annual base
salary and the target for annual incentive compensation as a
percentage of base salary. The agreements also provide that, in
the event that the executive terminates employment for good
reason or we terminate employment without cause, the executive
will receive severance payments in the form of salary
continuation, continued health insurance benefits and full
acceleration of all stock options and other stock-based awards
the executive holds at the time of termination. The agreements
also include a
12-month
post-employment non-competition and non-solicitation
restriction. See “New Employment Agreements” and
“Potential Payments Upon Termination” below for a
description of each employment agreement and the benefits to
which each named executive officer would be entitled in certain
circumstances.
ArborGen
Long-Term Incentivization Plan
In 2002, we introduced the ArborGen Net Value Added Plan, or the
NVA Plan, to promote the best interests of our company and our
stockholders by providing our employees the opportunity to share
in our enterprise value growth over time. Employees who
participated in the NVA Plan would, upon a sale event (including
a sale of our business or an initial public offering), and
subject to the terms and conditions of the NVA Plan, share in
the increase in our value once the investment contributions made
by our stockholders to the business, including a financial
return on that investment, had been satisfied.
The granting of NVA Units under the NVA Plan was consistent with
the goal of enabling us to attract and retain the services of
such individuals upon whose judgment, interest, skills, and
special effort the successful conduct of our operations would be
largely dependent. The NVA Units have been an important
long-term component of our overall compensation policy,
particularly for our named executive officers.
Upon our conversion from a limited liability company to a
corporation on June 1, 2010, the NVA Plan was terminated
pursuant to its terms as were all outstanding awards. Each
participant who remained our
123
employee immediately prior to such conversion became entitled to
receive an equitable award granted under a new incentive as soon
as practicable after such conversion. Our board of directors
agreed to issue to these former NVA holders new appreciation
rights in July 2010.
The key terms of the new appreciation rights are as follows:
|
|
|
| |
•
|
Each appreciation right allows the holder to share in 0.00001%
of the value difference between the “adjusted initial base
price” and the value at a sale event or initial public
offering, where the adjusted initial base price is the initial
base price each appreciation right is issued at, adjusted for
(a) all equity investments in our company that are made on
or after the grant date through the date immediately preceding
the sale event or public offering and (b) the accumulated
interest calculated daily on the initial base price minus $10.00
and the amount described in (a) from the date of each such
investment through the date immediately preceding the sale event
or initial public offering, both at an annual rate equal to 15%.
|
| |
| |
•
|
appreciation rights have been issued with an initial base price
of $42.43, $41.72 or $46.75 depending on the grant date of the
applicable NVAs or, in the case of Mr. Nothmann, the grant
date of the appreciation rights issued to him in connection with
his new employment with us.
|
| |
| |
•
|
In total, 582,250 appreciation rights have been issued to
29 employees.
|
| |
| |
•
|
Named executive officer grants of appreciation rights are
included in the following table:
|
| |
|
|
|
|
|
|
|
|
|
|
|
Appreciation Rights Granted
|
|
|
|
|
Fiscal 2010
|
|
|
|
|
Appreciation
|
|
|
Initial Base
|
|
|
Executive Officer
|
|
Rights
|
|
|
Price
|
|
|
|
|
Barbara H. Wells, Ph.D.
|
|
|
150,000
|
|
|
$
|
42.43
|
|
|
|
|
|
50,000
|
|
|
$
|
41.72
|
|
|
Geoffrey P. Clear
|
|
|
100,000
|
|
|
$
|
41.72
|
|
|
Wayne A. Barfield
|
|
|
50,000
|
|
|
$
|
41.72
|
|
|
David M. Nothmann
|
|
|
50,000
|
|
|
$
|
46.75
|
|
|
|
|
| |
•
|
Upon the completion of this offering, the appreciation rights
holders would become entitled to receive a payment equal to the
difference between 0.00001% of our value, based on the offering
price, and the adjusted initial base price. Our board of
directors may settle these amounts in cash or shares of our
common stock, net of tax withholding obligations.
|
The following table provides an indication of the total value of
the payout based on the midpoint of the estimated price range
set forth on the cover page of this prospectus:
| |
|
|
|
|
|
|
|
Estimated Appreciation
|
|
Executive Officer
|
|
Rights Payment
|
|
|
|
Barbara H. Wells, Ph.D.
|
|
|
|
|
|
Geoffrey P. Clear
|
|
|
|
|
|
Wayne A. Barfield
|
|
|
|
|
|
|
|
|
|
|
|
David M. Nothmann
|
|
|
|
|
Other
Executive Benefits and Perquisites
We provide the following benefits to our executive officers on
the same basis as other eligible employees:
|
|
|
| |
•
|
health insurance;
|
| |
| |
•
|
vacation, personal holidays and sick days; and
|
| |
| |
•
|
a 401(k) plan.
|
We believe these benefits are generally consistent with those
offered by other companies with which we compete for executive
talent.
124
Some of the executives whom we have hired, including
Mr. Clear and Mr. Nothmann, held positions in
locations outside of South Carolina at the time that they agreed
to join us. We have agreed in these instances to pay relocation
expenses to these executives.
Risk
Management Practices
When determining our compensation policies and practices, the
compensation committee considered various matters relative to
the development of a reasonable and prudent compensation
program, including whether the policies and practices were
reasonably likely to have a material adverse effect on the
Company. We believe that the mix and design of our executive
compensation plans and policies do not encourage management to
assume excessive risks and are not reasonably likely to have a
material adverse effect on the Company for the following
reasons: we offer an appropriate balance of short and long-term
incentives and fixed and variable amounts; our variable
compensation is based on a balanced mix of criteria; and our
board of directors and compensation committee have the authority
to adjust variable compensation as appropriate.
Impact of
Tax and Accounting Considerations
Under Section 162(m) of the Internal Revenue Code, or the
Code, subject to certain exceptions, a publicly held corporation
is denied a deduction for remuneration paid to a “covered
employee” for services performed by such employee to the
extent the remuneration exceeds $1 million for the taxable
year. An individual is a “covered employee” for
purposes of Section 162(m) if he or she is either the chief
executive officer or one of the other “named executive
officers” whose compensation must be reported to
stockholders by reason of being among the three most highly
compensated officers (other than the chief financial officer).
Section 162(m) excludes performance-based compensation from
the $1 million deduction limit. In order to meet such
exception, compensation must satisfy certain specific
requirements. Our compensation committee is likely to consider
the impact of Section 162(m) on future compensation
decisions if it appears that the deduction limit could apply.
Summary
Compensation Table — 2010
The following table shows the compensation earned by our named
executive officers during the year ended March 31, 2011.
SUMMARY
COMPENSATION TABLE
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Equity
|
|
|
|
|
|
|
|
Name and Principal
|
|
|
|
|
|
|
|
|
|
|
Incentive Plan
|
|
|
All Other
|
|
|
|
|
|
Position
|
|
Year
|
|
|
Salary
|
|
|
Bonus
|
|
|
Compensation(1)
|
|
|
Compensation
|
|
|
Total
|
|
|
|
|
Barbara H. Wells, Ph.D.
|
|
|
2010
|
|
|
$
|
275,000
|
|
|
$
|
89,495
|
(2)
|
|
$
|
—
|
|
|
$
|
10,295
|
(3)
|
|
$
|
374,790
|
|
|
President and Chief Executive Officer
|
|
|
2009
|
|
|
|
269,729
|
|
|
|
25,000
|
(4)
|
|
|
94,298
|
|
|
|
10,462
|
|
|
|
399,489
|
|
|
Geoffrey P. Clear
|
|
|
2010
|
|
|
|
300,000
|
|
|
|
36,751
|
(2)
|
|
|
—
|
|
|
|
43,416
|
(5)
|
|
|
380,167
|
|
|
Senior Vice President and Chief Financial Officer
|
|
|
2009
|
|
|
|
294,250
|
|
|
|
—
|
|
|
|
93,360
|
|
|
|
30,288
|
|
|
|
417,898
|
|
|
Wayne A. Barfield
|
|
|
2010
|
|
|
|
170,000
|
|
|
|
33,522
|
(2)
|
|
|
—
|
|
|
|
9,300
|
(6)
|
|
|
212,822
|
|
|
Vice President and General Manager of U.S. Operations
|
|
|
2009
|
|
|
|
157,746
|
|
|
|
10,000
|
(4)
|
|
|
45,606
|
|
|
|
6,885
|
|
|
|
220,237
|
|
|
David M. Nothmann
|
|
|
2010
|
|
|
|
197,247
|
|
|
|
—
|
|
|
|
—
|
|
|
|
101,476
|
(7)
|
|
|
298,723
|
|
|
Vice President of Business and Product Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
|
The amount of compensation payable
under our Senior Executive Incentive Bonus Plan for the year
ended March 31, 2011 has not yet been determined. Our
compensation committee will assess the achievement of these
company goals during the May/June 2011 timeframe and our
compensation committee will make bonus determinations for our
named executive officers once that process is complete. For a
description of our Senior Executive Incentive Bonus Plan, refer
to the discussion under the caption “Short-Term Incentive
Compensation” above.
|
125
|
|
|
|
(2)
|
|
These amounts represent payments
made on March 31, 2011 pursuant to our retention payment
program. For a description of our retention payment program,
refer to the discussion under the caption “Retention
Payment Program” above.
|
|
|
|
|
(3)
|
|
Includes a company matching
contribution to our 401(k) plan of $9,855 and company-paid life
insurance premiums paid of $440.
|
|
|
|
|
(4)
|
|
These amounts represent special
bonuses awarded by our compensation committee to acknowledge
leadership demonstrated by the named executive officer during a
challenging year and, in the case of Mr. Barfield, the
impact of the worldwide economic downturn on the performance of
our U.S. operations despite Mr. Barfield’s significant
efforts.
|
|
|
|
|
(5)
|
|
Includes a company matching
contribution to our 401(k) plan of $9,860, company-paid life
insurance premiums paid of $725 and company-provided relocation
benefits of $32,831.
|
|
|
|
|
(6)
|
|
Includes a company matching
contribution to our 401(k) plan of $9,025 and company-paid life
insurance premiums paid of $275.
|
|
|
|
|
(7)
|
|
Includes a company matching
contribution to our 401(k) plan of $6,150, company-paid life
insurance premiums paid of $392 and company-provided relocation
benefits of $94,934.
|
Grants of
Plan-Based Awards — 2010
The following table presents information on grants of plan-based
(non-equity) awards made during the fiscal year ended
March 31, 2011 to our named executive officers:
GRANTS OF
PLAN-BASED AWARDS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Appreciation Rights
|
|
|
Estimated Possible Payouts Under
|
|
|
|
|
|
|
|
Award(1)
|
|
|
Non-Equity Incentive Plan Awards
|
|
|
Name
|
|
Grant Date
|
|
|
(#)
|
|
|
Threshold(2)
|
|
|
Target
|
|
|
Maximum(2)
|
|
|
|
|
Barbara H. Wells, Ph.D.
|
|
|
|
|
|
|
|
|
|
$
|
—
|
|
|
$
|
178,750
|
|
|
$
|
—
|
|
|
President and Chief
|
|
|
7/23/10
|
|
|
|
150,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Executive Officer
|
|
|
7/23/10
|
|
|
|
50,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Geoffrey P. Clear
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
120,000
|
|
|
|
—
|
|
|
Senior Vice President and
|
|
|
7/23/10
|
|
|
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chief Financial Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wayne A. Barfield
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
68,000
|
|
|
|
—
|
|
|
Vice President and General
|
|
|
7/23/10
|
|
|
|
50,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Manager of U.S. Operations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
David M. Nothmann
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
61,500
|
|
|
|
—
|
|
|
Vice President of Business
|
|
|
2/20/11
|
|
|
|
50,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
and Product Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
The initial base price of the appreciation rights awarded to
Mr. Clear and Mr. Barfield and 50,000 of the
appreciation rights awarded to Dr. Wells is $41.72. The
initial base price of the remaining 150,000 appreciation rights
awarded to Dr. Wells is $42.43. The initial base price of
the appreciation rights awarded to Mr. Nothmann is $46.75.
For a description of the appreciation rights awards, refer to
the discussion under the caption “ArborGen Long-Term
Incentivization Plan” above. |
| |
|
(2) |
|
There are no “threshold” or “maximum”
amounts established under our Senior Executive Incentive Bonus
Plan. |
Outstanding
Equity Awards at Fiscal Year End
None of our named executive officers had any outstanding equity
awards at March 31, 2011. However, as described above, each
of our named executive officers did receive a grant of
appreciation rights in fiscal 2010 that our board of directors
may settle in cash or shares of our common stock, net of tax
withholding obligations, upon the completion of this offering.
Option
Exercises and Stock Vested
None of our named executive officers held any stock options or
other stock-based awards in the year ended March 31, 2011.
126
New
Employment Agreements
On January 20, 2011, we entered into an employment
agreement with Barbara H. Wells, Ph.D., our President and
Chief Executive Officer. Dr. Wells’ agreement calls
for the payment of $275,000 in annual base salary, subject to
periodic review and adjustment by the compensation committee.
The agreement entitles Dr. Wells to participate in our
Senior Executive Incentive Bonus Plan or any successor plan
adopted by the compensation committee, sets her target annual
incentive compensation at 65 percent of her annual base
salary and provides for a commuting allowance of up to $6,000
per year for travel between our headquarters and her home in
Colorado. In the case of termination of her employment by
Dr. Wells for good reason or by us without cause,
Dr. Wells is entitled to health insurance benefits for a
period of one year, full acceleration of all stock options and
other stock-based awards held by Dr. Wells at the time of
her termination and, subject to her execution of a general
release of claims in favor of us, severance compensation equal
to 100% of her annual base salary.
On January 31, 2011, we entered into an employment
agreement with Geoffrey P. Clear, our Senior Vice President and
Chief Financial Officer. Mr. Clear’s agreement calls
for the payment of $300,000 in annual base salary, subject to
periodic review by the compensation committee. The agreement
entitles Mr. Clear to participate in our Senior Executive
Incentive Bonus Plan or any successor plan adopted by the
compensation committee, and sets his target annual incentive
compensation at 40 percent of his annual base salary. In
the case of termination of his employment by Mr. Clear for
good reason or by us without cause, Mr. Clear is entitled
to health insurance benefits for a period of nine months, full
acceleration of all stock options and other stock-based awards
held by Mr. Clear at the time of his termination and,
subject to his execution of a general release of claims in favor
of us, severance compensation equal to 75% of his annual base
salary.
On January 20, 2011, we entered into an employment
agreement with Wayne A. Barfield, our Vice President and General
Manager of U.S. Operations. Mr. Barfield’s
agreement calls for the payment of $170,000 in annual base
salary, subject to periodic review by the compensation
committee. The agreement entitles Mr. Barfield to
participate in our Senior Executive Incentive Bonus Plan or any
successor plan adopted by the compensation committee, and sets
his target annual incentive compensation at 40 percent of
his annual base salary. In the case of termination of his
employment by Mr. Barfield for good reason or by us without
cause, Mr. Barfield is entitled to health insurance
benefits for a period of six months, full acceleration of all
stock options and other stock-based awards held by
Mr. Barfield at the time of his termination and, subject to
his execution of a general release of claims in favor of us,
severance compensation equal to 50% of his annual base salary.
On January 31, 2011, we entered into an employment
agreement with David M. Nothmann, our Vice President of Business
and Product Development. Mr. Nothmann’s agreement
calls for the payment of $205,000 in annual base salary, subject
to periodic review by the compensation committee. The agreement
entitles Mr. Nothmann to participate in our Senior
Executive Incentive Bonus Plan or any successor plan adopted by
the compensation committee, and sets his target annual incentive
compensation at 30 percent of his annual base salary. In
the case of termination of his employment by Mr. Nothmann
for good reason or by us without cause, Mr. Nothmann is
entitled to health insurance benefits for a period of six
months, full acceleration of all stock options and other
stock-based awards held by Mr. Nothmann at the time of his
termination and, subject to his execution of a general release
of claims in favor of us, severance compensation equal to 50% of
his annual base salary.
Each of the employment agreements provide for standard employee
benefits. In addition, each employment agreement provides that
the executive agrees not to compete with us, solicit or divert
our customers, or solicit or hire any of our employees for a
period ending one year following the termination of employment
with us for any reason.
The definition of good reason in the employment agreements
includes a material diminution in responsibilities, authority or
duties, a material diminution in base salary, except for
across-the-board
salary reductions based on our financial performance similarly
affecting all or substantially all of our senior management
employees, a material change in the geographic location at which
the officer provides services to us or a material breach of the
employment agreement by us.
The definition of cause as set forth in the employment
agreements includes conduct constituting an act of misconduct in
connection with the performance of the officer’s duties,
including misappropriation of our funds or
127
property other than the occasional, customary and de minimis use
of our property for personal purposes, the commission of any
felony or a misdemeanor involving moral turpitude, deceit,
dishonesty or fraud, or any conduct that would reasonably be
expected to result in injury or reputational harm to us or any
of our subsidiaries and affiliates if the officer were retained
in his or her position, continued non-performance of duties
under the employment agreement (other than by reason of physical
or mental illness, incapacity or disability) which has continued
for more than 30 days following written notice of such
non-performance, a breach of the confidentiality or
non-competition and non-solicitation provisions of the
employment agreement, a violation of our written employment
policies, or failure to cooperate with a bona fide internal
investigation or an investigation by regulatory or law
enforcement authorities, after being instructed by us to
cooperate, or the destruction or failure to preserve documents
or other materials known to be relevant to such investigation or
the inducement of others to fail to cooperate or to produce
documents or other materials in connection with such
investigation.
Potential
Payments Upon Termination
The following summaries set forth potential payments payable to
our named executive officers upon termination of employment by
us other than for cause or death or disability, or by the
executive for good reason.
Termination by us other than for cause or termination by
executive for good reason. Our employment
agreements with our named executive officers provide that if we
terminate such executive’s employment other than for cause,
or if the executive terminates his or her employment for good
reason, the executive is entitled to salary continuation and
continued health benefits for either twelve months (in the case
of Dr. Wells), nine months (in the case of Mr. Clear)
or six months (in the case of Mr. Barfield), as well as
full acceleration of all stock options and other stock-based
awards held by the executive at the time of termination. In
addition, pursuant to our Senior Executive Incentive Bonus Plan,
if we terminate such executive’s employment other than for
cause (but not if the executive terminates his or her employment
for good reason) after completing nine or more months of service
in the fiscal year in which such termination occurs, the
executive will be entitled to a prorated share of the annual
bonus, if any, which he would have earned for such fiscal year.
Termination by us for cause; death or disability; or
termination by executive for other than good
reason. We are not obligated to make any cash
payment or benefit to our executive officers if their employment
is terminated by us for cause or due to death or disability of
the executive, other than the payment of unpaid salary and
accrued and unused vacation pay.
The following tables describe the potential payments and
benefits upon employment termination or change in control for
our named executive officers, as if their respective employment
terminated as of March 31, 2011, the last business day of
our last fiscal year.
Barbara
H. Wells, Ph.D.
| |
|
|
|
|
|
|
|
|
|
|
|
Termination by the
|
|
|
Termination by the
|
|
Executive Benefits and
|
|
Company for Other
|
|
|
Executive for Good
|
|
|
Payments Upon Termination
|
|
than Cause
|
|
|
Reason
|
|
|
|
|
Base Salary Continuation
|
|
$
|
275,000
|
|
|
$
|
275,000
|
|
|
Bonus(1)
|
|
$
|
178,750
|
|
|
$
|
—
|
|
|
Continued Health Benefits
|
|
$
|
13,668
|
|
|
$
|
13,668
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
467,418
|
|
|
$
|
288,668
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
The bonus amount reflected is based on the target bonus for our
fiscal year ended March 31, 2011. |
128
Geoffrey
P. Clear
| |
|
|
|
|
|
|
|
|
|
|
|
Termination by the
|
|
|
Termination by the
|
|
Executive Benefits and
|
|
Company for Other
|
|
|
Executive for Good
|
|
|
Payments Upon Termination
|
|
than Cause
|
|
|
Reason
|
|
|
|
|
Base Salary Continuation
|
|
$
|
225,000
|
|
|
$
|
225,000
|
|
|
Bonus(1)
|
|
$
|
120,000
|
|
|
$
|
—
|
|
|
Continued Health Benefits
|
|
$
|
6,880
|
|
|
$
|
6,880
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
351,880
|
|
|
$
|
231,880
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
The bonus amount reflected is based on the target bonus for our
fiscal year ended March 31, 2011. |
Wayne A.
Barfield
| |
|
|
|
|
|
|
|
|
|
|
|
Termination by the
|
|
|
Termination by the
|
|
Executive Benefits and
|
|
Company for Other
|
|
|
Executive for Good
|
|
|
Payments Upon Termination
|
|
than Cause
|
|
|
Reason
|
|
|
|
|
Base Salary Continuation
|
|
$
|
85,000
|
|
|
$
|
85,000
|
|
|
Bonus(1)
|
|
$
|
68,000
|
|
|
$
|
—
|
|
|
Continued Health Benefits
|
|
$
|
2,352
|
|
|
$
|
2,352
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
155,352
|
|
|
$
|
87,352
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
The bonus amount reflected is based on the target bonus for our
fiscal year ended March 31, 2011. |
David M.
Nothmann
| |
|
|
|
|
|
|
|
|
|
|
|
Termination by the
|
|
|
Termination by the
|
|
Executive Benefits and
|
|
Company for Other
|
|
|
Executive for Good
|
|
|
Payments Upon Termination
|
|
than Cause
|
|
|
Reason
|
|
|
|
|
Base Salary Continuation
|
|
$
|
102,500
|
|
|
$
|
102,500
|
|
|
Bonus(1)
|
|
$
|
61,500
|
|
|
$
|
—
|
|
|
Continued Health Benefits
|
|
$
|
6,834
|
|
|
$
|
6,834
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
170,834
|
|
|
$
|
109,334
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
The bonus amount reflected is based on the target bonus for our
fiscal year ended March 31, 2011. |
2010
Stock Option and Incentive Plan
In July 2010, our board of directors, upon the recommendation of
our compensation committee, adopted our 2010 Stock Option and
Incentive Plan, or the 2010 Plan, which was subsequently
approved by our stockholders. Our 2010 Plan provides flexibility
to our compensation committee to use various equity-based
incentive awards as compensation tools to motivate our workforce.
We have initially reserved 1,800,000 shares of our common
stock for the issuance of awards under the 2010 Plan. The 2010
Plan provides that the number of shares reserved and available
for issuance under the plan will automatically increase each
January 1, beginning in 2011, by 5% of the outstanding
number of shares of our common stock on the immediately
preceding December 31 or such lesser number of shares as
determined by our compensation committee. This number is subject
to adjustment in the event of a stock split, stock dividend or
other change in our capitalization. Our compensation committee
determined not to increase the shares available for issuance in
2011 pursuant to the automatic increase feature.
The shares we issue under the 2010 Plan will be authorized but
unissued shares or shares that we reacquire. The shares of
common stock underlying any awards that are forfeited,
cancelled, held back upon exercise or settlement of an award to
satisfy the exercise price or tax withholding, reacquired by us
prior to vesting, satisfied without any issuance of stock,
expire or are otherwise terminated (other than by exercise)
under the 2010 Plan are added back to the shares of common stock
available for issuance under the 2010 Plan.
129
The 2010 Plan is administered by our compensation committee. Our
compensation committee has full power to select, from among the
individuals eligible for awards, the individuals to whom awards
will be granted, to make any combination of awards to
participants, and to determine the specific terms and conditions
of each award, subject to the provisions of the 2010 Plan.
Persons eligible to participate in the 2010 Plan will be those
full or part-time officers, employees, non-employee directors
and other key persons (including consultants and prospective
employees) as selected from time to time by our compensation
committee in its discretion.
The 2010 Plan permits the granting of both options to purchase
common stock intended to qualify as incentive stock options
under Section 422 of the Code and options that do not so
qualify. The option exercise price of each option will be
determined by our compensation committee but may not be less
than 100% of the fair market value of our common stock on the
date of grant. The term of each option will be fixed by our
compensation committee and may not exceed ten years from the
date of grant. Our compensation committee will determine at what
time or times each option may be exercised.
Our compensation committee may award stock appreciation rights
subject to such conditions and restrictions as we may determine.
Stock appreciation rights entitle the recipient to shares of
common stock, or cash, equal to the value of the appreciation in
our stock price over the exercise price. The exercise price is
the fair market value of the common stock on the date of grant.
Our compensation committee may award restricted shares of common
stock and restricted stock units to participants subject to such
conditions and restrictions as we may determine. These
conditions and restrictions may include the achievement of
certain performance goals
and/or
continued employment with us through a specified vesting period.
Our compensation committee may also grant shares of common stock
that are free from any restrictions under the 2010 Plan.
Unrestricted stock may be granted to participants in recognition
of past services or other valid consideration and may be issued
in lieu of cash compensation due to such participant.
Our compensation committee may grant performance share awards to
participants which entitle the recipient to receive shares of
common stock upon the achievement of certain performance goals
and such other conditions as our compensation committee shall
determine. Our compensation committee may grant dividend
equivalent rights to participants which entitle the recipient to
receive credits for dividends that would be paid if the
recipient had held a specified number of shares of common stock.
Our compensation committee may grant cash bonuses under the 2010
Plan to participants, subject to the achievement of certain
performance goals.
Our compensation committee may grant awards of restricted stock,
restricted stock units, performance shares or cash-based awards
under the 2010 Plan that are intended to qualify as
“performance-based compensation” under
Section 162(m) of the Code. Those awards would only vest or
become payable upon the attainment of performance goals that are
established by our compensation committee and related to one or
more performance criteria. The performance criteria that would
be used with respect to any such awards include: earnings before
interest, taxes, depreciation and amortization, net income
(loss) (either before or after interest, taxes, depreciation
and/or
amortization), changes in the market price of our common stock,
economic value-added, funds from operations or similar measure,
sales or revenue, acquisitions or strategic transactions,
operating income (loss), cash flow (including, but not limited
to, operating cash flow and free cash flow), return on capital,
assets, equity, or investment, stockholder returns, return on
sales, gross or net profit levels, productivity, expense,
margins, operating efficiency, customer satisfaction, working
capital, earnings (loss) per share of stock, sales or market
shares and number of customers, any of which may be measured
either in absolute terms or as compared to any incremental
increase or as compared to results of a peer group. From and
after the time that we become subject to Section 162(m) of
the Code, the maximum award that is intended to qualify as
“performance-based compensation” under
Section 162(m) of the Code that may be made to any one
employee during any one calendar year period is
900,000 shares of common stock with respect to a
stock-based award and $2,000,000 with respect to a cash-based
award.
The 2010 Plan provides that upon the effectiveness of a
“sale event” as defined in the 2010 Plan, all awards
will be assumed or continued by the successor entity. If the
employment of a holder of an award is terminated without cause
on or within 12 months after the sale event, then all
awards held by such holder will become fully exercisable
and/or
vested at that time. In addition, in connection with a sale
event, we may make
130
or provide for a cash payment to participants holding options
and stock appreciation rights equal to the difference between
the per share cash consideration payable to stockholders in the
sale event and the exercise price of the options or stock
appreciation rights.
Our board of directors may amend or discontinue the 2010 Plan
and our compensation committee may amend or cancel outstanding
awards for purposes of satisfying changes in law or any other
lawful purpose, but no such action may adversely affect rights
under an award without the holder’s consent. Certain
amendments to the 2010 Plan require the approval of our
stockholders.
No awards may be granted under the 2010 Plan after the date that
is 10 years from the date of stockholder approval. No
awards under the 2010 Plan have been made prior to the date
hereof, however, our compensation committee intends to make
grants upon completion of this offering to certain key
employees, including our named executive officers, as a special
acknowledgment to these individuals for the successful
completion of our initial public offering. Our compensation
committee expects these grants to total approximately $2,450,000
and to represent approximately 0.5% of our outstanding
securities. For most of our employees, this grant will consist
of restricted stock units that are settled in shares of our
common stock at vesting. The vesting will generally occur in
equal annual installments over four years. For certain of our
key employees, including our named executive officers, 25% of
their total equity grant will consist of restricted stock units
and 75% of such award will consist of stock options. The stock
options will have an exercise price equal to the fair market
value of our common stock at the time of the grant, will have a
term of seven years and will generally vest in equal annual
installments over four years. Our compensation committee has
approved, and in the case of our chief executive officer, our
board of directors has also approved, a dollar value for these
equity awards to be made to our named executive officers, as
follows:
| |
|
|
|
|
|
Name
|
|
IPO Equity Award
|
|
|
|
|
Barbara H. Wells, Ph.D.
|
|
$
|
250,000
|
|
|
Geoffrey P. Clear
|
|
$
|
200,000
|
|
|
Wayne A. Barfield
|
|
$
|
127,500
|
|
|
David M. Nothmann
|
|
$
|
153,750
|
|
25% of the above total grants will be made in the form of
restricted stock units, based on the fair market value of our
common stock at the time of our initial public offering. 75% of
the above total grants will be made in the form of stock
options, based on the Black-Scholes value of such options at the
time of our initial public offering.
Director
Compensation
Historically, we have not compensated our directors (none of
whom has been an employee of the company) for their service on
our board of directors or on any of its committees.
We have adopted a new non-employee director compensation policy
that will be effective immediately prior to consummation of this
offering. The new policy will provide that each non-employee
director will receive an annual cash retainer of $32,000. In
addition, the chair of each committee will receive an annual
cash fee as follows:
| |
|
|
|
|
|
Committee Chair
|
|
|
|
|
|
|
|
|
|
|
|
Audit Committee Chairperson
|
|
$
|
20,000
|
|
|
Compensation Committee Chairperson
|
|
$
|
10,000
|
|
|
Nominating and Corporate Governance Committee Chairperson
|
|
$
|
5,000
|
|
Each committee member, other than the chair, will receive an
annual cash fee as follows:
| |
|
|
|
|
|
Non-Chair Committee Members
|
|
|
|
|
|
|
|
|
|
|
|
Audit Committee
|
|
$
|
10,000
|
|
|
Compensation Committee
|
|
$
|
5,000
|
|
|
Nominating and Corporate Governance Committee
|
|
$
|
3,000
|
|
The chairman of our board of directors will receive an
additional annual cash fee of $28,000.
131
The new non-employee director compensation policy will also
provide that, upon initial election to our board of directors, a
non-employee director will receive an initial equity grant of
$120,000 of restricted stock units, issued at market price, that
vest quarterly over three years; provided, however, that all
vesting ceases if the director resigns from our board of
directors or otherwise ceases to serve as a director, unless the
board of directors determines that the circumstances warrant
continuation of vesting. Mr. Shonsey will receive an equity
grant of $120,000 of restricted stock units upon the
consummation of this offering, based on the price to the public
in this offering of one share of common stock. Non-employee
directors may not sell the shares of common stock that comprise
the initial grant of restricted stock units while he or she
remains a member of our board of directors.
In addition, on the date of each annual meeting of stockholders,
each continuing non-employee member of our board of directors
who has served as a director for the previous six months will
receive an option with a grant date fair value of $65,000 to
purchase shares of common stock that vests quarterly over the
subsequent 12 months; provided, however, that all vesting
ceases if the director resigns from our board of directors or
otherwise ceases to serve as a director, unless the board of
directors determines that the circumstances warrant continuation
of vesting.
We will reimburse all reasonable
out-of-pocket
expenses incurred by non-employee directors in attending
meetings of the board of directors or any committee.
Limitation
of Liability and Indemnification Arrangements
As permitted by the Delaware General Corporation Law, we intend
to adopt provisions in our amended and restated certificate of
incorporation and amended and restated by-laws, which will be
effective upon the completion of this offering, that limit or
eliminate the personal liability of our directors. Consequently,
a director will not be personally liable to us or our
stockholders for monetary damages for breach of fiduciary duty
as a director, except for liability for:
|
|
|
| |
•
|
any breach of the director’s duty of loyalty to us or our
stockholders;
|
| |
| |
•
|
any act or omission not in good faith or that involves
intentional misconduct or a knowing violation of law;
|
| |
| |
•
|
any unlawful payments related to dividends or unlawful stock
repurchases, redemptions or other distributions; or
|
| |
| |
•
|
any transaction from which the director derived an improper
personal benefit.
|
These limitations of liability do not alter director liability
under the federal securities laws and do not affect the
availability of equitable remedies such as an injunction or
rescission.
In addition, our amended and restated by-laws, which will be
effective upon the completion of this offering, provide that:
|
|
|
| |
•
|
we will indemnify our directors, officers and, in the discretion
of our board of directors, certain employees to the fullest
extent permitted by the Delaware General Corporation
Law; and
|
| |
| |
•
|
advance expenses, including attorneys’ fees, to our
directors and, in the discretion of our board of directors, to
our officers and certain employees, in connection with legal
proceedings, subject to limited exceptions.
|
We have also entered into indemnification agreements with each
of our executive officers and directors and certain other
employees. These agreements provide that we will indemnify each
of these individuals to the fullest extent permitted by the
Delaware General Corporation Law and advance expenses to each
indemnitee in connection with any proceeding in which
indemnification is available.
We also maintain general liability insurance to provide
insurance coverage to our directors and officers for losses
arising out of claims based on acts or omissions in their
capacities as directors or officers, including liabilities under
the Securities Act of 1933, as amended, or the Securities Act.
Insofar as indemnification for liabilities arising under the
Securities Act may be permitted to directors, officers, or
persons controlling the registrant pursuant to the foregoing
provisions, we have been informed that in the opinion of the
Securities and Exchange Commission such indemnification is
against public policy as expressed in the Securities Act and is
therefore unenforceable.
132
These provisions may discourage stockholders from bringing a
lawsuit against our directors in the future for any breach of
their fiduciary duty. These provisions may also have the effect
of reducing the likelihood of derivative litigation against
directors and officers, even though such an action, if
successful, might otherwise benefit us and our stockholders.
Furthermore, a stockholder’s investment may be adversely
affected to the extent we pay the costs of settlement and damage
awards against directors, officers and certain employees
pursuant to these indemnification provisions. We believe that
these provisions, the indemnification agreements and the
insurance are necessary to attract and retain talented and
experienced directors and officers.
Other than as described in “Business—Legal
Proceedings,” there is no pending litigation or proceeding
involving any of our directors, officers or employees in which
indemnification will be required or permitted. We are not aware
of any threatened litigation or proceeding that might result in
a claim for such indemnification.
133
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Since March 31, 2007, we have been party or will be party
to the following transactions in which (a) the amount
involved exceeded or will exceed $120,000 and (b) a
director, executive officer or holders of five percent or more
of our common stock or any member of their immediate family had
or will have a direct or indirect material interest. We believe
that we have executed all of the transactions set forth below on
terms no less favorable to us than we could have obtained from
unaffiliated third parties.
In accordance with its written charter, our audit committee is
responsible for reviewing all related party transactions for
potential conflict of interest situations on an ongoing basis,
and the approval of the audit committee is required for all such
transactions. The term “related party transactions”
refers to transactions required to be disclosed in our filings
with the SEC pursuant to Item 404 of
Regulation S-K.
Capital
Contributions
In February 2000, we were formed by combining all of the
biotechnology forestry research and development programs of
Fletcher Challenge Forests (now Rubicon Limited, a New
Zealand-based company), International Paper Company, and
Westvaco Corporation (now MeadWestvaco Corporation). Rubicon
Industries USA, LLC (an affiliate of Rubicon Limited),
International Paper Company and MeadWestvaco Corporation are our
three current stockholders, each of which own one-third of our
outstanding capital stock. Each of these stockholders have two
representatives on our board of directors.
Prior to our conversion from a limited liability company to a
corporation on June 1, 2010, we operated under the Amended
and Restated Limited Liability Company Agreement of ArborGen,
LLC, or the LLC Agreement. The LLC Agreement set forth the
mechanics for the operation and governance of our company, as
well as the guidelines for our board of directors to request
capital contributions from International Paper, MeadWestvaco and
Rubicon, which were the three members of ArborGen, LLC. From our
inception through our conversion to a corporation on
June 1, 2010, International Paper, MeadWestvaco and Rubicon
contributed to us an aggregate of $166.2 million in cash
and assets. This amount reflects contributed assets in 2007 at
cost. Including the contributed assets at fair value, which was
calculated at the time of the contribution, the aggregate
contributions of our stockholders are more than
$200 million. Since our conversion to a corporation on
June 1, 2010, our stockholders have made aggregate capital
contributions of $7.9 million pursuant to the terms of the
Stockholders Agreement described below under
“—Stockholders Agreement.”
During the three-year period ended March 31, 2010,
excluding the assets contributed in the transaction described
below under “—Asset Contribution,” MeadWestvaco
and Rubicon each made cash contributions to us of
$13.6 million. In connection with the transactions
described below under “—Asset Contribution,” our
board of directors created preferred interest to account for the
excess of the fair value of the assets contributed by
International Paper in the asset contribution transaction over
the fair value of the assets contributed by each of MeadWestvaco
and Rubicon. $13.3 million of International Paper’s
capital was designated as a preferred interest, having various
rights and preferences, including the ability to reduce the
amount of the preferred interest in lieu of making a cash
payment when our board of directors requested capital
contributions. As a result, during the three-year period ended
March 31, 2010, International Paper made cash contributions
of $2.4 million and reduced its preferred interest by
$11.1 million in lieu of making cash contributions.
Stockholder
Loans
In January 2011, we issued promissory notes to our three
stockholders in the aggregate amount of $1,200,000. Pursuant to
the terms of each $400,000 note to each stockholder, interest
accrues at a rate of LIBOR plus 3%, and each note matures on the
earlier of July 15, 2011 or seven business days following
either the closing of this offering or our receipt of proceeds
from other sales of capital stock sufficient to repay the note.
All accrued interest under each note will be forgiven in its
entirety if the note balance is repaid by the maturity date.
134
Asset
Contribution
On October 31, 2007, the commercial tree improvement,
nursery and orchard businesses of International Paper,
MeadWestvaco, and Rubicon were contributed to us. The
contribution of cash, property, plant and equipment from our
stockholders’ nursery and orchard businesses transformed us
from a research-based business to a fully integrated commercial
developer and provider of conventional and technology-enhanced
tree seedlings. Pursuant to this transaction, we acquired cash
and various non-cash assets from our stockholders. International
Paper, MeadWestvaco and Rubicon added contributed assets,
recorded at historical cost, of $9.0 million,
$13.4 million and $9.6 million, respectively. These
contributions were determined by our board of directors as equal
in fair market value. In connection with this transaction, our
board of directors created a $13.3 million preferred
interest to account for the excess of the fair value of the
assets contributed by International Paper in the asset
contribution transaction over the fair value of the assets
contributed by each of MeadWestvaco and Rubicon. This preferred
interest (which was subsequently reduced to $0 by offsetting
periodic contribution obligations) had various rights and
preferences, including the ability to reduce the amount of the
preferred interest in lieu of making a cash payment when our
board of directors requested capital contributions.
Pursuant to our contribution agreement with Rubicon,
substantially all of the nursery, orchard and tree improvement
businesses of Trees & Technology Limited in New
Zealand and Horizon2 (Australia) Pty Ltd in Australia were
contributed to us. The assets of these contributed businesses
included: real property located in New Zealand and Australia;
leases and leased property located in New Zealand and Australia;
various personal property, fixed assets and inventory;
regulatory permits and certifications; data and records;
contracts; intellectual property; germplasm and related
licenses; reserve seed inventory; the treestock crops
established in 2007 prior to the contribution and scheduled to
be harvested in 2008; and $100,000 in cash. Those assets that
were excluded included: the treestock crops established in 2006
and scheduled to be harvested in 2007; accounts receivable
(other than those related to the treestock crops included in
contributed assets); cash and cash equivalents (other than the
$100,000 included in contributed assets); and intellectual
property and personal property not related to the business. We
also assumed the obligations of the contributing entities
arising from and related to the business for periods after the
date of the contribution agreement. We agreed to offer
employment to all of the employees of the contributing entities.
The contribution agreement contained provisions pursuant to
which we and the contributing entities agreed to indemnify the
other for breaches of representations, warranties, covenants and
certain other claims.
Pursuant to our contribution agreement with MeadWestvaco,
substantially all of the nursery, orchard, tree improvement and
germplasm development business conducted by MeadWestvaco and
certain of its subsidiaries in the United States were
contributed to us. The assets contributed by MeadWestvaco
included: various personal property, fixed assets and inventory;
data and records; contracts; intellectual property; germplasm
and related licenses; reserve seed inventory; the treestock
crops established in 2007 prior to the contribution and
scheduled to be harvested in 2008; and $13.2 million in
cash. The assets that were excluded included:
MeadWestvaco’s real property; the treestock crops
established in 2006 and scheduled to be harvested in 2007;
accounts receivable (other than those related to the treestock
crops included in contributed assets); cash and cash equivalents
(other than the $13.2 million included in contributed
assets); and intellectual property and personal property not
related to the business. We also assumed the obligations of
MeadWestvaco arising from and related to the business for
periods after the date of the contribution agreement. Under the
agreement, we had the option to offer employment to any
MeadWestvaco employees whose job function was solely related to
the contributed business. The contribution agreement contained
provisions pursuant to which we and MeadWestvaco agreed to
indemnify the other for breaches of representations, warranties,
covenants and certain other claims. In connection with the
contribution, as described under “—Leases” below,
we also entered into several leases with MeadWestvaco as part of
the contribution transaction. In addition, we entered into a
license agreement with MeadWestvaco pursuant to which we license
back to MeadWestvaco, on a royalty-free, non-exclusive basis,
the contributed germplasm for use by MeadWestvaco in
non-biotechnology tree research, development, production and
commercialization anywhere in the world except the United
States, New Zealand and Australia and, only for internal
development and production by MeadWestvaco and its
collaborators, in Brazil.
Pursuant to our contribution agreement with International Paper,
substantially all of the nursery, orchard, tree improvement and
germplasm development business (with the exception of one type
of germplasm) conducted by International Paper and certain of
its subsidiaries in the United States were contributed to us.
The assets contributed
135
by International Paper included: real property in Texas and the
Southeastern United States and certain subsurface rights related
thereto; leases and leased property located in Texas and the
Southeastern United States; various personal property, fixed
assets and inventory; regulatory permits and certifications;
data and records; contracts; intellectual property; germplasm
and related licenses; reserve seed inventory; and the treestock
crops established in 2007 prior to the contribution and
scheduled to be harvested in 2008. Excluded assets included: the
treestock crops established in 2006 and scheduled to be
harvested in 2007; accounts receivable (other than those related
to the treestock crops included in contributed assets); cash and
cash equivalents; and intellectual property and personal
property not related to the business. We also assumed the
obligations of International Paper arising from and related to
the business for periods after the date of the contribution
agreement. Under the agreement, we had the option to offer
employment to any International Paper employees whose job
function was solely related to the contributed business. The
contribution agreement contained provisions pursuant to which we
and International Paper agreed to indemnify the other for
breaches of representations, warranties, covenants and certain
other claims. In addition, we entered into a license agreement
with International Paper pursuant to which we license back to
International Paper, on a royalty-free, non-exclusive basis, the
contributed germplasm and propagation technology for use by
International Paper in non-biotechnology tree research,
development, production and commercialization anywhere in the
world except the United States, New Zealand and Australia and,
only for internal development and production by International
Paper and its collaborators, in Brazil.
Stockholders
Agreement
In connection with our conversion from a limited liability
company to a corporation on June 1, 2010, we entered into a
Stockholders Agreement with our three stockholders that,
following this offering will continue to impose non-competition
restrictions on our three stockholders. Subject to the
provisions of the license agreements described below between us
and our three stockholders, the Stockholders Agreement provides
that none of our three stockholders may, during the time that
such stockholder remains a stockholder of our company or for
five years thereafter, engage in the business of genetically
engineered trees anywhere in the world or engage in other
tree-related business in either the United States, New Zealand
or Australia. Notwithstanding the foregoing, our three
stockholders may acquire other entities that participate in the
restricted businesses on a limited basis, and our three
stockholders may conduct research and development activities
relating to tree breeding, production and related technologies,
including genetic engineering of trees, but may not engage in
related commercialization or sale activities. The foregoing
summary of these non-competition provisions is qualified by
reference to the Stockholders Agreement, a copy of which is
attached as an exhibit to the registration statement of which
this prospectus is a part.
The Stockholders Agreement also provides registration rights to
our three stockholders following this offering. Subject to the
terms of the Stockholders Agreement, our three stockholders have
the right to demand that we file a registration statement or
request that their shares be covered by a registration statement
that we are otherwise filing. For a description of the
registration rights, see below under “Description of
Capital Stock—Registration Rights.”
Pursuant to the Stockholders Agreement, our board of directors
had the right to request pro rata capital contributions from our
three stockholders in exchange for shares of common stock. Our
stockholders were obligated to contribute a maximum of
$5.0 million per year, although they had the ability to
contribute additional funds in their sole discretion pursuant to
a request from our board of directors. These capital
contribution provisions will terminate immediately prior to the
closing of this offering.
Other than the non-competition and registration rights
provisions described above, the terms of the Stockholders
Agreement will terminate upon the completion of this offering,
including the obligations of the stockholders to purchase
additional shares and the requirement that two representatives
of each of the three stockholders be designated to our board of
directors.
Leases
We currently lease our global headquarters in Summerville, South
Carolina from MeadWestvaco Forestry, LLC, an affiliate of
MeadWestvaco Corporation. We currently pay annual rent of
$0.8 million under this lease, which expires on
January 15, 2012. We expect to continue to occupy the
premises until construction on our new headquarters facility is
completed, which is expected by the end of 2011. We lease two
seed orchards, a greenhouse and associated outdoor growing areas
adjacent to our headquarters and multiple field testing sites
from MeadWestvaco Corporation or its affiliates, including
several properties leased as a result of the
136
transaction described under “—Asset Contribution”
above. During the three years ended March 31, 2010 and the
nine months ended December 31, 2010, we paid an aggregate
of $3.1 million and $0.7 million in rent and related
fees to MeadWestvaco and its affiliates pursuant to these leases.
In November 2010, we signed a
build-to-suit
and lease agreement with Forestry Research Holdings, LLC for a
new manufacturing, research and development laboratory and
headquarters facility in Summerville, South Carolina, which we
expect will be completed in Fall 2011. The agreement includes an
option for us to purchase the facility beginning on the date of
rent commencement, which is the earlier of commencement of
business activities on the premises or 30 days after
substantial completion of the facility. Under this
20-year
lease, we are obligated to pay annual rent of the greater of
$1.0 million or 10% of the landlord’s investment in
the property; provided that the landlord’s investment will
not exceed $14.3 million. The landlord purchased the land
on which our new facility is being built from MeadWestvaco, and
$4.0 million of the landlord’s financing for this
purchase and the related building development costs comes from a
mortgage granted by an affiliate of MeadWestvaco. In addition,
each of MeadWestvaco, International Paper and Rubicon are
guarantors of our obligations under the lease. We may use net
proceeds from this offering to exercise our option to purchase
the new facility during the year ended March 31, 2012. At
the time we expect to exercise the option, the purchase price
will be the landlord’s investment in the property (which
will not exceed $14.3 million) plus five percent and any
tax liability incurred by the landlord due to sale of the
property within one year of development.
Seedling
Sales
During the
three-year
period ended March 31, 2010 and the nine month period ended
December 31, 2010, we sold an aggregate of
$0.1 million and $7,000 of our seedling products to
International Paper and an aggregate of $2.4 million and
$0.1 million of our seedling products to MeadWestvaco.
These sales were made on terms and conditions, including price,
that were substantially equivalent to the terms and conditions
upon which our customers generally purchased our seedling
products.
License
Agreements
In connection with the formation of our business in 2000, we
entered into license agreements with each of MeadWestvaco (then
Westvaco Corporation), International Paper and Rubicon (then
Fletcher Challenge Forests Limited). Pursuant to these
agreements, we received nonexclusive, worldwide licenses to use
the tree genetic material, patent rights and technology to make
and commercialize products in the field of biotechnology trees
for forestry applications. We also granted each licensor a
royalty-free, perpetual, nonexclusive license to any discoveries
or improvements derived from the licensed genetic material,
patent rights and technology. In addition, we were granted a
right of first refusal to license, on a nonexclusive basis, any
gene discovered or acquired by each licensor after the date of
the agreement that has the potential to improve a trait of a
biotechnology tree. Pursuant to these license agreements, we are
obligated to pay royalties based on sales of our products that
utilize certain of the licensed rights. Each license agreement
will remain in force until the expiration of our obligations to
pay royalties under the agreement. For a particular licensed
product, the royalty obligation lasts until the later of the
expiration of all relevant patents, or ten years from the first
commercial sale of such product. We have paid no royalties to
date under these license agreements, as we have not had
commercial sales of any of our biotechnology seedling products.
In 2004, we entered into a nonexclusive license agreement with
MeadWestvaco that allowed us to use somatic embryogenesis for
the development of non-biotechnology trees (as use of somatic
embryogenesis for biotechnology trees is encompassed by the
license agreement with MeadWestvaco described in the paragraph
above). During the three years ended March 31, 2010 and the
nine month period ended December 31, 2010, we paid
royalties of approximately $46,000 and $7,500, respectively, to
MeadWestvaco pursuant to this license agreement. Effective
June 1, 2010, we replaced the nonexclusive license
agreement with MeadWestvaco for use of somatic embryogenesis for
non-biotechnology trees with a new, exclusive license agreement
covering the same technology. Pursuant to this agreement, we are
obligated to pay royalties and milestone payments to
MeadWestvaco based on sales of our products containing the
licensed technology, subject to annual minimum royalty payments,
as well as an annual fee to cover patent maintenance. The
exclusive license agreement is for an initial term of two years,
with successive two-year terms upon written agreement of the
parties.
137
In addition, as described under “—Asset
Contribution” above, we are party to license agreements
with International Paper and MeadWestvaco pursuant to which we
license back to each party, on a royalty-free basis, rights to
use germplasm contributed to us by such party in 2007 for
geographically limited non-biotechnology tree research,
development, production and commercialization.
Indemnification
Arrangements
Our certificate of incorporation that will become effective upon
the closing of this offering provides that we will indemnify our
directors and executive officers to the fullest extent permitted
by Delaware law. In addition, we have entered into
indemnification agreements with each of our directors and
executive officers and certain other employees that may be
broader in scope than the specific indemnification provisions
contained in the Delaware General Corporation Law. For more
information regarding these agreements, see “Compensation
Discussion and Analysis—Limitation of Liability and
Indemnification Arrangements.”
138
PRINCIPAL
STOCKHOLDERS
The following table presents information concerning the
beneficial ownership of the shares of our common stock as of
March 31, 2011 by:
|
|
|
| |
•
|
each person we know to be the beneficial owner of 5% or more of
our outstanding shares of our common stock;
|
| |
| |
•
|
each of our directors and director designees;
|
| |
| |
•
|
each of our named executive officers; and
|
| |
| |
•
|
all of our executive officers and directors as a group.
|
We have determined beneficial ownership in accordance with
Securities and Exchange Commission rules. The information does
not necessarily indicate beneficial ownership for any other
purpose. Under these rules, a person is deemed to be a
beneficial owner of our common stock if that person has a right
to acquire ownership within 60 days. A person is also
deemed to be a beneficial holder of our common stock if that
person has or shares voting power, which includes the power to
vote or direct the voting of our common stock, or investment
power, which includes the power to dispose of or to direct the
disposition of such capital stock. Except in cases where
community property laws apply or as indicated in the footnotes
to this table, we believe that each stockholder identified in
the table possesses sole voting and investment power over all
shares of common stock shown as beneficially owned by the
stockholder.
Percentage of beneficial ownership in the table below is based
on shares
of common stock deemed to be outstanding as of March 31,
2011,
and shares
of common stock outstanding after the completion of this
offering. The table below assumes that the underwriters do not
exercise their option to purchase additional shares of common
stock. The address of each individual listed below is
c/o ArborGen
Inc., 180 Westvaco Road, Summerville, South Carolina 29483.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Percentage of Shares
|
|
Percentage of Shares
|
|
|
|
|
|
Beneficially Owned
|
|
Beneficially Owned
|
|
Name and Address of Beneficial Owner
|
|
Number of Shares
|
|
Before Offering
|
|
After Offering
|
|
|
|
5% Stockholders:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
International Paper Company(1)
|
|
|
10,176,622
|
|
|
|
33.33
|
%
|
|
|
|
|
|
MeadWestvaco Corporation(2)
|
|
|
10,176,622
|
|
|
|
33.33
|
%
|
|
|
|
|
|
Rubicon(3)
|
|
|
10,176,622
|
|
|
|
33.33
|
%
|
|
|
|
|
|
Directors, Director Designees and Named Executive
Officers:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bruce G. Burton, Ph.D.(4)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
M. Eugene Hundley(4)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
David A. Liebetreu(4)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
S. Luke Moriarty(4)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Kenneth R. Munson, Ph.D.(4)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Edward T. Shonsey
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Mark T. Watkins, Sr.(4)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Barbara H. Wells, Ph.D.(5)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Geoffrey P. Clear(5)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Wayne A. Barfield(5)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
David M. Nothmann(5)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
All directors and executive officers as a group
(10 persons)(5)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
(1)
|
|
The address of International Paper
Company is 6400 Poplar Avenue, Memphis, Tennessee 38197.
|
| |
|
(2)
|
|
The address of MeadWestvaco
Corporation is 501 South 5th Street, Richmond, Virginia 23219.
|
139
|
|
|
|
(3)
|
|
Consists of shares held by Rubicon
Industries USA, LLC (“Rubicon USA”), which is a wholly
owned subsidiary of Rubicon Forests Holdings Limited
(“Rubicon Forests”). Rubicon Forests is a wholly owned
subsidiary of Rubicon Limited. Each of Rubicon Forests and
Rubicon Limited may be deemed to share voting and investment
power with respect to all shares held by Rubicon USA. The
address for each of Rubicon USA, Rubicon Forests and Rubicon
Limited is Level 3,
7-9 Fanshawe
Street, Auckland 1010, New Zealand.
|
|
|
|
|
(4)
|
|
Although the director is a
representative of one of our stockholders, the director has
neither voting nor dispositive power over the shares held by
such stockholder.
|
| |
|
(5)
|
|
Each of our executive officers hold
appreciation rights, which entitle them to receive a payment,
based on the value of our company in this offering, no later
than 60 days following the completion of this offering.
These appreciation rights are described in greater detail under
“Compensation Discussion and Analysis—ArborGen
Long-Term Incentivization Plan.” The appreciation rights
may, at the discretion of our board of directors, be settled in
cash or shares of our common stock, net of tax withholding
obligations, and so are not included in the table above. If our
board of directors determines to settle the appreciation rights
in shares of common stock, Dr. Wells would
receive shares,
Mr. Clear would
receive shares,
Mr. Barfield would
receive shares,
Mr. Nothmann would
receive shares
and all directors and executive officers as a group would
receive shares,
based on an assumed initial public offering price of
$ per share, which is the midpoint
of the estimated price range set forth on the cover page of this
prospectus.
|
140
DESCRIPTION
OF CAPITAL STOCK
Upon the completion of this offering, our authorized capital
stock will consist of 200,000,000 shares of common stock,
par value $0.001 per share, and 10,000,000 shares of
preferred stock, par value $0.001 per share, and there will
be shares
of common stock outstanding and no shares of preferred stock
outstanding. As of March 31, 2011, we had three record
holders of our capital stock. Upon completion of this offering,
1,800,000 shares of our common stock will be reserved for
future grants under our stock option plans.
The following description of our capital stock and provisions of
our amended and restated certificate of incorporation and
amended and restated by-laws are summaries of material terms and
provisions and are qualified by reference to our amended and
restated certificate of incorporation and amended and restated
by-laws, copies of which have been filed with the Securities and
Exchange Commission as exhibits to the registration statement of
which this prospectus is a part. The descriptions of our common
stock and preferred stock reflect provisions of our amended and
restated certificate of incorporation and amended and restated
by-laws that will become effective immediately prior to the
completion of this offering.
Common
Stock
Upon the completion of this offering, we will be authorized to
issue one class of common stock. Holders of our common stock are
entitled to one vote for each share of common stock held of
record for the election of directors and on all matters
submitted to a vote of stockholders. Holders of our common stock
are entitled to receive dividends ratably, if any, as may be
declared by our board of directors out of legally available
funds, subject to any preferential dividend rights of any
preferred stock then outstanding. Upon our dissolution,
liquidation or winding up, holders of our common stock are
entitled to share ratably in our net assets legally available
after the payment of all our debts and other liabilities,
subject to the preferential rights of any preferred stock then
outstanding. Holders of our common stock have no preemptive,
subscription, redemption or conversion rights. The rights,
preferences and privileges of holders of common stock are
subject to, and may be adversely affected by, the rights of the
holders of shares of any series of preferred stock that we may
designate and issue in the future. Except as described under
“—Antitakeover Effects of Delaware Law and Provisions
of Our Amended and Restated Certificate of Incorporation and
Amended and Restated By-laws” below, a majority vote of the
holders of common stock is generally required to take action
under our amended and restated certificate of incorporation and
amended and restated by-laws.
Preferred
Stock
Upon the closing of this offering, our board of directors will
be authorized, without action by the stockholders, to designate
and issue up to an aggregate of 10,000,000 shares of
preferred stock in one or more series. Our board of directors
can designate the rights, preferences and privileges of the
shares of each series and any of its qualifications, limitations
or restrictions. Our board of directors may authorize the
issuance of preferred stock with voting or conversion rights
that could adversely affect the voting power or other rights of
the holders of common stock. The issuance of preferred stock,
while providing flexibility in connection with possible future
financings and acquisitions and other corporate purposes could,
under certain circumstances, have the effect of restricting
dividends on our common stock, diluting the voting power of our
common stock, impairing the liquidation rights of our common
stock, or delaying, deferring or preventing a change in control
of our company, which might harm the market price of our common
stock. See also “—Antitakeover Effects of Delaware Law
and Provisions of Our Amended and Restated Certificate of
Incorporation and Amended and Restated By-laws—Provisions
of Our Amended and Restated Certificate of Incorporation and
Amended and Restated By-laws—Undesignated Preferred
Stock” below.
Our board of directors will make any determination to issue such
shares based on its judgment as to our company’s best
interests and the best interests of our stockholders. Upon the
completion of this offering, we will have no shares of preferred
stock outstanding and we have no current plans to issue any
shares of preferred stock.
141
Registration
Rights
Pursuant to the Stockholders Agreement described under
“Certain Relationships and Related Party
Transactions—Stockholders Agreement” in this
prospectus, we have agreed to provide our three stockholders
with registration rights with respect to their shares of our
common stock. The summary of these registration rights below is
qualified by reference to the Stockholders Agreement, a copy of
which is attached as an exhibit to the registration statement of
which this prospectus is a part. Immediately following this
offering, our three stockholders will hold
approximately shares
of our common stock.
Demand
Registration Rights
After the expiration of the
180-day
period following the effectiveness of this offering, our three
stockholders will together have the right to make one request
that we register all or a portion of their shares of our common
stock on
Form S-1.
In addition, after the expiration of one year following the
completion of this offering, our three stockholders will have
the right to request that we register their shares on
Form S-3
if we are then eligible to file a registration statement on
Form S-3.
These stockholders may make an unlimited number of requests for
registration on
Form S-3;
however, we will not be required to effect a registration
pursuant to these
Form S-3
registration rights if we have previously effected one such
registration in the
90-day
period preceding the request for registration or two such
registrations in the
12-month
period preceding the request for registration.
Piggyback
Registration Rights
In the event that we propose to register any of our securities
under the Securities Act of 1933, as amended, either for our own
account or for the account of other security holders, our three
stockholders will be entitled to “piggyback”
registration rights allowing them to include their shares in
such registration, subject to certain marketing and other
limitations. As a result, whenever we propose to file a
registration statement under the Securities Act, other than with
respect to certain registrations (including those related to
employee benefit plans, stock purchase plans or convertible
securities), the holders of these shares of our common stock are
entitled to notice of the registration and have the right,
subject to limitations that the underwriters may impose on the
number of shares included in the registration, to include their
shares in the registration.
We will pay the registration expenses, other than underwriting
discounts and commissions, of the holders of the shares
registered pursuant to the demand and piggyback registrations
described above. In an underwritten offering, the underwriters,
if any, have the right, subject to specified conditions, to
limit the number of shares of our common stock such holders may
include.
Antitakeover
Effects of Delaware Law and Provisions of Our Amended and
Restated Certificate of Incorporation and Amended and Restated
By-laws
Certain provisions of the Delaware General Corporation Law and
of our amended and restated certificate of incorporation and
amended and restated by-laws that will become effective upon the
completion of this offering could have the effect of delaying,
deferring or discouraging another party from acquiring control
of us. These provisions, which are summarized below, are
expected to discourage certain types of coercive takeover
practices and inadequate takeover bids and, as a consequence,
they might also inhibit temporary fluctuations in the market
price of our common stock that often result from actual or
rumored hostile takeover attempts. These provisions are also
designed in part to encourage anyone seeking to acquire control
of us to first negotiate with our board of directors. These
provisions might also have the effect of preventing changes in
our management. It is possible that these provisions could make
it more difficult to accomplish transactions that stockholders
might otherwise deem to be in their best interests. However, we
believe that the advantages gained by protecting our ability to
negotiate with any unsolicited and potentially unfriendly
acquirer outweigh the disadvantages of discouraging such
proposals, including those priced above the then-current market
value of our common stock, because, among other reasons, the
negotiation of such proposals could improve their terms.
142
Delaware
Takeover Statute
We are subject to the provisions of Section 203 of the
Delaware General Corporation Law. In general, Section 203
prohibits a publicly held Delaware corporation from engaging in
a “business combination” with an “interested
stockholder” for a three-year period following the time
that this stockholder becomes an interested stockholder, unless
the business combination is approved in a prescribed manner. A
“business combination” includes, among other things, a
merger, asset or stock sale or other transaction resulting in a
financial benefit to the interested stockholder. An
“interested stockholder” is a person who, together
with affiliates and associates, owns, or did own within three
years prior to the time of determination of interested
stockholder status, 15% or more of the corporation’s
outstanding voting stock. Under Section 203, a business
combination between a corporation and an interested stockholder
is prohibited unless it satisfies one of the following
conditions:
|
|
|
| |
•
|
before the time the stockholder became interested, the board of
directors approved either the business combination or the
transaction which resulted in the stockholder becoming an
interested stockholder;
|
| |
| |
•
|
upon consummation of the transaction which resulted in the
stockholder becoming an interested stockholder, the interested
stockholder owned at least 85% of the voting stock of the
corporation outstanding at the time the transaction commenced,
excluding for purposes of determining the voting stock
outstanding, shares owned by persons who are directors and also
officers, and employee stock plans, in some instances; or
|
| |
| |
•
|
at or after the time the stockholder became interested, the
business combination was approved by the board of directors of
the corporation and authorized at an annual or special meeting
of the stockholders by the affirmative vote of at least
two-thirds of the outstanding voting stock which is not owned by
the interested stockholder.
|
Provisions
of Our Amended and Restated Certificate of Incorporation and
Amended and Restated
By-laws
Our amended and restated certificate of incorporation and
amended and restated by-laws to be in effect upon completion of
this offering will include a number of provisions that may have
the effect of delaying, deferring or discouraging another party
from acquiring control of us and encouraging persons considering
unsolicited tender offers or other unilateral takeover proposals
to negotiate with our board of directors rather than pursue
non-negotiated takeover attempts. These provisions include the
items described below.
Board Composition and Filling Vacancies. In
accordance with our amended and restated certificate of
incorporation, our board of directors is divided into three
classes serving staggered three-year terms, with one class being
elected each year. Our amended and restated certificate of
incorporation also provides that directors may be removed only
for cause and then only by the affirmative vote of the holders
of 75% or more of the shares then entitled to vote at an
election of directors. Furthermore, any vacancy on our board of
directors, however occurring, including a vacancy resulting from
an increase in the size of our board of directors, may only be
filled by the affirmative vote of a majority of our directors
then in office even if less than a quorum.
No Written Consent of Stockholders. Our
amended and restated certificate of incorporation provides that
all stockholder actions are required to be taken by a vote of
the stockholders at an annual or special meeting, and that
stockholders may not take any action by written consent in lieu
of a meeting. This limit may lengthen the amount of time
required to take stockholder actions and would prevent the
amendment of our by-laws or removal of directors by our
stockholder without holding a meeting of stockholders.
Meetings of Stockholders. Our amended and
restated by-laws provide that only a majority of the members of
our board of directors then in office may call special meetings
of stockholders and only those matters set forth in the notice
of the special meeting may be considered or acted upon at a
special meeting of stockholders. Our by-laws limit the business
that may be conducted at an annual meeting of stockholders to
those matters properly brought before the meeting.
143
Advance Notice Requirements. Our amended and
restated by-laws establish advance notice procedures with regard
to stockholder proposals relating to the nomination of
candidates for election as directors or new business to be
brought before meetings of our stockholders. These procedures
provide that notice of stockholder proposals must be timely
given in writing to our corporate secretary prior to the meeting
at which the action is to be taken. Generally, to be timely,
notice must be received at our principal executive offices not
less than 90 days or more than 120 days prior to the
first anniversary date of the annual meeting for the preceding
year. The notice must contain certain information specified in
our amended and restated by-laws.
Amendment to Certificate of Incorporation and
By-laws. As required by the Delaware General
Corporation Law, any amendment of our amended and restated
certificate of incorporation must first be approved by a
majority of our board of directors, and if required by law or
our amended and restated certificate of incorporation, must
thereafter be approved by a majority of the outstanding shares
entitled to vote on the amendment, and a majority of the
outstanding shares of each class entitled to vote thereon as a
class, except that the amendment of the provisions relating to
stockholder action, directors, limitation of liability and the
amendment of our amended and restated certificate of
incorporation must be approved by not less than 75% of the
outstanding shares entitled to vote on the amendment, and not
less than 75% of the outstanding shares of each class entitled
to vote thereon as a class. Our amended and restated by-laws may
be amended by the affirmative vote of a majority vote of the
directors then in office, subject to any limitations set forth
in the by-laws; and may also be amended by the affirmative vote
of at least 75% of the outstanding shares entitled to vote on
the amendment, or, if the board of directors recommends that the
stockholders approve the amendment, by the affirmative vote of
the majority of the outstanding shares entitled to vote on the
amendment, in each case voting together as a single class.
Undesignated Preferred Stock. Our amended and
restated certificate of incorporation
authorizes shares
of preferred stock. The existence of authorized but unissued
shares of preferred stock may enable our board of directors to
render more difficult or to discourage an attempt to obtain
control of us by means of a merger, tender offer, proxy contest
or otherwise. For example, if in the due exercise of its
fiduciary obligations, our board of directors were to determine
that a takeover proposal is not in the best interests of us or
our stockholders, our board of directors could cause shares of
preferred stock to be issued without stockholder approval in one
or more private offerings or other transactions that might
dilute the voting or other rights of the proposed acquirer or
insurgent stockholder or stockholder group. In this regard, our
amended and restated certificate of incorporation grants our
board of directors broad power to establish the rights and
preferences of authorized and unissued shares of preferred
stock. The issuance of shares of preferred stock could decrease
the amount of earnings and assets available for distribution to
holders of shares of common stock. The issuance may also
adversely affect the rights and powers, including voting rights,
of these holders and may have the effect of delaying, deterring
or preventing a change in control of us.
Transfer
Agent and Registrar
Upon completion of this offering, the transfer agent and
registrar for our common stock will be Computershare Trust
Company, N.A.
Listing
We have applied to list our common stock on the Nasdaq Global
Market under the symbol “ARBR.”
144
SHARES ELIGIBLE
FOR FUTURE SALE
Prior to this offering, there has been no public market for our
common stock. Future sales of our common stock in the public
market, or the availability of such shares for sale in the
public market, could adversely affect market prices prevailing
from time to time. As described below, only a limited number of
shares will be available for sale shortly after this offering
due to contractual and legal restrictions on resale.
Nevertheless, sales of our common stock in the public market
after such restrictions lapse, or the perception that those
sales may occur, could adversely affect the prevailing market
price at such time and our ability to raise equity capital in
the future.
Based
on shares
of common stock outstanding as of March 31, 2011, upon
completion of this
offering, shares
of common stock will be outstanding,
reflecting
shares of common stock sold in this offering and assuming no
exercise of the underwriters’ option to purchase additional
shares of common stock. All of the shares sold in this offering
will be freely tradable. The
remaining shares
of common stock outstanding after this offering will be
restricted as a result of securities laws or
lock-up
agreements as described below. Following the expiration of the
lock-up
period, all shares will be eligible for resale in compliance
with Rule 144 or Rule 701 under the Securities Act.
“Restricted securities” as defined under Rule 144
of the Securities Act were issued and sold by us in reliance on
exemptions from the registration requirements of the Securities
Act. These shares may be sold in the public market only if
registered or pursuant to an exemption from registration, such
as Rule 144 or Rule 701 under the Securities Act.
Rule 144
In general, under Rule 144 under the Securities Act of
1933, as in effect on the date of this prospectus, a person who
is one of our affiliates and has beneficially owned shares of
our common stock for at least six months would be entitled to
sell within any three-month period a number of shares that does
not exceed the greater of:
|
|
|
| |
•
|
one percent of the number of shares of common stock then
outstanding, which will equal
approximately shares
immediately after the completion of this offering; or
|
| |
| |
•
|
the average weekly trading volume of our common stock on the
Nasdaq Global Market during the four calendar weeks preceding
the filing of a notice on Form 144 with respect to the sale.
|
Sales under Rule 144 by our affiliates or persons selling
shares on behalf of our affiliates are also subject to manner of
sale provisions and notice requirements and to the availability
of current public information about us.
In general, under Rule 144 under the Securities Act, as in
effect on the date of this prospectus, a person who is not
deemed to have been one of our affiliates at any time during the
90 days preceding a sale, and who has beneficially owned
the shares proposed to be sold for at least six months,
including the holding period of any prior owner other than an
affiliate, is entitled to sell the shares without complying with
the manner of sale, volume limitation or notice provisions of
Rule 144, and will be subject only to the public
information requirements of Rule 144. If such a person has
beneficially owned the shares proposed to be sold for at least
one year, including the holding period of any prior owner other
than our affiliates, then such person is entitled to sell such
shares without complying with any of the requirements of
Rule 144. As of March 31,
2011,
shares of our common stock would qualify for resale under
Rule 144 within 180 days of the date of this
prospectus, subject to the
lock-up
agreements as described under
“—Lock-Up
Agreements” below and under “Underwriting” in
this prospectus.
Rule 701
Rule 701 under the Securities Act, or Rule 701, as in
effect on the date of this prospectus, permits resales of shares
in reliance upon Rule 144 but without compliance with
certain restrictions of Rule 144, including the holding
period requirement. Most of our employees, executive officers or
directors who purchased shares under a written compensatory plan
or contract may be entitled to rely on the resale provisions of
Rule 701, but all holders of Rule 701 shares are
required to wait until 90 days after the date of this
prospectus before selling
145
their shares. However, as of the date hereof, we have not issued
any shares of common stock to any of our employees, executive
officers or directors.
Lock-Up
Agreements
In connection with this offering, we and each of our officers,
directors and stockholders have agreed that, subject to certain
exceptions, without the prior written consent of Goldman, Sachs
& Co. and Citigroup Global Markets Inc., we and they will
not, for a period of 180 days after the date of this
prospectus (as such period may be extended under certain
circumstances), offer, sell, contract to sell, pledge, grant any
option to purchase, make any short sale or otherwise dispose of,
directly or indirectly, any shares of capital stock, any options
or warrants to purchase any shares of capital stock, or any
securities convertible into, or exercisable or exchangeable for,
such capital stock.
Registration
Rights
We are party to a Stockholders Agreement which provides that our
three stockholders have the right to demand that we file a
registration statement or request that their shares of our
common stock be covered by a registration statement that we are
otherwise filing. See “Description of Capital
Stock—Registration Rights” in this prospectus. Except
for shares purchased by affiliates, registration of their shares
under the Securities Act would result in these shares becoming
freely tradable without restriction under the Securities Act
immediately upon effectiveness of the registration, subject to
the expiration of the
lock-up
period described above and under “Underwriting” in
this prospectus.
Stock
Plans
As soon as practicable after the completion of this offering, we
intend to file a
Form S-8
registration statement under the Securities Act to register
shares of our common stock reserved for issuance under our stock
plan. This registration statement will become effective
immediately upon filing, and shares covered by this registration
statement will thereupon be eligible for sale in the public
markets, subject to Rule 144 limitations applicable to
affiliates and any
lock-up
agreements. For a more complete discussion of our stock plans,
see “Compensation Discussion and Analysis.”
146
UNITED
STATES TAX CONSEQUENCES FOR
NON-U.S.
STOCKHOLDERS
The following discussion is a summary of material
U.S. federal income and estate tax considerations with
respect to your acquisition, ownership and disposition of our
common stock if you are a
Non-U.S. Holder
(as defined below). This summary is based on current provisions
of the Internal Revenue Code of 1986, as amended (the
“Code”), U.S. Treasury regulations, judicial opinions,
published positions of the Internal Revenue Service (the
“IRS”), and all other applicable authorities. All of
these authorities are subject to change, possibly with
retroactive effect. This summary applies if you
(i) purchase our common stock in this offering,
(ii) will hold the common stock as a “capital
asset” within the meaning of Section 1221 of the Code
and (iii) will not hold our common stock in connection with
your conduct of a trade or business within the United States.
You are a
Non-U.S. Holder
if you are a beneficial owner of shares of our common stock
other than:
|
|
|
| |
•
|
an individual who is a citizen or resident, as defined for
U.S. federal income tax purposes, of the United States;
|
| |
| |
•
|
a corporation or other entity taxable as a corporation created
or organized in, or under the laws of, the United States, any
state thereof or the District of Columbia;
|
| |
| |
•
|
an estate whose income is subject to U.S. federal income
tax regardless of its source; or
|
| |
| |
•
|
a trust that (a) is subject to the primary supervision of a
court within the United States and one or more U.S. persons
have the authority to control all substantial decisions of the
trust or (b) has a valid election in effect under
applicable U.S. Treasury regulations to be treated as a
U.S. person.
|
Notwithstanding the foregoing, for purposes of this discussion,
a
Non-U.S. Holder
does not include any entity that is taxable as a partnership for
U.S. federal income tax purposes. The tax treatment of a
partnership owning our common stock will depend upon the
organization and activities of the partnership and the tax
status of its partners, among other factors. Partnerships
considering the purchase of our common stock should consult
their tax advisors regarding the tax consequences to the
partnership and its partners of acquiring, holding and disposing
of shares of our common stock.
In addition, this summary does not address all of the
U.S. federal income and estate tax considerations that may
be relevant to you in light of your particular circumstances or
if you are a beneficial owner subject to special treatment under
U.S. federal income tax laws (such as, but not limited to,
a tax-exempt organization, financial institution, broker or
dealer in securities, insurance company, person who holds our
common stock as part of a hedging or conversion transaction or
as part of a short-sale or straddle, or former U.S. citizen
or resident). This summary does not discuss non-income taxes
(except U.S. federal estate tax), any aspect of the
U.S. federal alternative minimum tax or state, local or
non-U.S. taxation.
Prospective
Non-U.S. Holders
should consult their tax advisors regarding the
U.S. federal, state, local and
non-U.S. income,
estate and other tax considerations of acquiring, holding and
disposing of shares of our common stock.
Dividends
In general, any distributions we make to you with respect to
your shares of common stock that constitute dividends for
U.S. federal income tax purposes will be subject to
U.S. withholding tax at a rate of 30% of the gross amount,
unless you are eligible for a reduced rate of withholding tax
under an applicable income tax treaty and you provide proper
certification of your eligibility for such reduced rate. A
distribution will constitute a dividend for U.S. federal
income tax purposes to the extent of our current or accumulated
earnings and profits as determined for U.S. federal income
tax purposes. Any distribution not constituting a dividend will
be treated first as a tax-free reduction of your adjusted tax
basis in your shares of common stock and, to the extent it
exceeds your adjusted tax basis, as capital gain, subject to the
tax treatment described below in “—Sale or Other
Disposition of Our Common Stock.”
147
Sale or
Other Disposition of Our Common Stock
You generally will not be subject to U.S. federal income or
withholding tax on any gain realized upon the sale or other
disposition of your shares of our common stock unless:
|
|
|
| |
•
|
you are an individual present in the United States for
183 days or more in the taxable year of disposition and
other conditions are met; or
|
| |
| |
•
|
we are or have been a “United States real property holding
corporation” (a “USRPHC”) for U.S. federal
income tax purposes at any time during the relevant statutory
period.
|
With respect to the second bullet point above, we believe we
currently are not, and we do not anticipate becoming, a USRPHC.
Because the determination of whether we are a USRPHC depends on
the fair market value of our interests in real property located
within the United States relative to the fair market value of
our interests in real property located outside the United States
and our other business assets, however, there can be no
assurance that we will not become a USRPHC in the future. In the
event we do become a USRPHC, so long as our common stock is
regularly traded on an established securities market, a
non-U.S. Holder
will not be subject to U.S. federal withholding tax on the
sale or other disposition of his, her or its shares of our
common stock and any gain realized on such sale or other
disposition would only be subject to U.S. federal income
tax if the selling
non-U.S. Holder
actually or constructively holds more than 5 percent of our
common stock at any time during the shorter of the five-year
period preceding the date of disposition or the holder’s
holding period.
Information
Reporting and Backup Withholding
Generally, we must report annually to the IRS and to each
Non-U.S. Holder
the amount of dividends paid to such holder and the amount of
any tax withheld with respect to those dividends. This
information also may be made available under a specific treaty
or agreement with the tax authorities of the country in which
the
Non-U.S. Holder
resides or is established. Under certain circumstances, the Code
imposes a backup withholding obligation on certain reportable
payments. Backup withholding generally will not, however, apply
to payments of dividends to a
Non-U.S. Holder
of our common stock provided the
Non-U.S. Holder
furnishes to us or our paying agent the required certification
as to its
Non-U.S. status,
such as by providing a valid
IRS Form W-8BEN
or W-8ECI,
or otherwise establishes an exemption.
U.S.
Federal Estate Tax
Common stock owned or treated as owned by an individual who is
not a citizen or resident (as defined for U.S. federal
estate tax purposes) of the United States at the time of his or
her death will be included in the individual’s gross estate
for U.S. federal estate tax purposes and therefore may be
subject to U.S. federal estate tax unless an applicable
treaty provides otherwise.
Recent
Legislation Relating to Foreign Accounts
Recently enacted rules require the reporting to the IRS of
direct and indirect ownership of foreign financial accounts and
certain foreign entities by U.S. persons. Failure to
provide this required information can result in a thirty percent
(30%) withholding tax on certain payments made after
December 31, 2012 including
U.S.-source
dividends and gross proceeds from the sale or other disposition
of stock issued by U.S. persons, such as the Company.
Because the IRS has not yet issued final regulations with
respect to these new rules, their scope remains unclear and
potentially subject to material change. Prospective
Non-U.S. Holders
are advised to consult a tax advisor regarding this new
reporting and withholding regime, in light of their particular
circumstances.
148
UNDERWRITING
Goldman, Sachs & Co. and Citigroup Global Markets Inc.
are acting as joint book-running managers of the offering and as
representatives of the underwriters named below. Subject to the
terms and conditions stated in the underwriting agreement dated
the date of this prospectus, each underwriter named below has
severally agreed to purchase, and we have agreed to sell to that
underwriter, the number of shares set forth opposite the
underwriter’s name.
| |
|
|
|
|
|
|
|
Number of
|
|
|
Underwriter
|
|
Shares
|
|
|
|
|
Goldman, Sachs & Co.
|
|
|
|
|
|
Citigroup Global Markets Inc.
|
|
|
|
|
|
Piper Jaffray & Co.
|
|
|
|
|
|
RBC Capital Markets LLC
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
The underwriting agreement provides that the obligations of the
underwriters to purchase the shares included in this offering
are subject to approval of legal matters by counsel and to other
conditions. The underwriters are obligated to purchase all the
shares (other than those covered by the option to purchase
additional shares described below) if they purchase any of the
shares.
Shares sold by the underwriters to the public will initially be
offered at the initial public offering price set forth on the
cover page of this prospectus. Any shares sold by the
underwriters to securities dealers may be sold at a discount
from the initial public offering price not to exceed
$ per share. If all the shares are
not sold at the initial offering price, the underwriters may
change the offering price and the other selling terms. The
underwriters do not expect sales to discretionary accounts to
exceed five percent of the total number of shares offered. The
offering of the shares by the underwriters is subject to receipt
and acceptance and subject to the underwriters’ right to
reject any order in whole or in part.
We have granted to the underwriters an option, exercisable for
30 days from the date of this prospectus, to purchase up
to
additional shares at the public offering price less the
underwriting discount. To the extent the option is exercised,
each underwriter must purchase a number of additional shares
approximately proportionate to that underwriter’s initial
purchase commitment. Any shares issued or sold under the option
will be issued and sold on the same terms and conditions as the
other shares that are the subject of this offering.
We and each of our officers, directors and stockholders have
agreed that, subject to certain exceptions, without the prior
written consent of Goldman, Sachs & Co. and Citigroup
Global Markets Inc., we and they will not for a period of
180 days after the date of this prospectus, offer, sell,
contract to sell, pledge, grant any option to purchase or
otherwise dispose of, directly or indirectly, any shares of
capital stock, any option or warrants to purchase any shares of
capital stock, or any securities convertible into, or
exercisable or exchangeable for, such capital stock.
Notwithstanding the foregoing, if (i) during the last
17 days of the
180-day
restricted period, we issue an earnings release or announce
material news or a material event, or a material event relating
to us occurs; or (ii) prior to the expiration of the
180-day
restricted period, we announce that we will release earnings
results during the
16-day
period beginning on the last day of the
180-day
restricted period, the restrictions described above shall
continue to apply until the expiration of the
18-day
period beginning on the issuance of the earnings release or the
announcement of the material news or the announcement or
occurrence of a material event.
Prior to this offering, there has been no public market for our
shares. Consequently, the initial public offering price for the
shares was determined by negotiations between us and the
representatives. Among the factors considered in determining the
initial public offering price were our results of operations,
our current financial condition, our future prospects, our
markets, the economic conditions in and future prospects for the
industry in which we compete, our management, and currently
prevailing general conditions in the equity securities markets,
including current market valuations of publicly traded companies
considered comparable to our company. We cannot assure you,
however, that the price at which the shares will sell in the
public market
149
after this offering will not be lower than the initial public
offering price or that an active trading market in our shares
will develop and continue after this offering.
We have applied to have our shares listed on the Nasdaq Global
Market under the symbol “ARBR.”
The following table shows the underwriting discounts that we are
to pay to the underwriters in connection with this offering.
These amounts are shown assuming both no exercise and full
exercise of the underwriters’ option to purchase additional
shares.
| |
|
|
|
|
|
|
|
|
|
|
|
Paid by ArborGen Inc.
|
|
|
|
No
|
|
Full
|
|
|
|
Exercise
|
|
Exercise
|
|
|
|
Per share
|
|
$
|
|
|
|
$
|
|
|
|
Total
|
|
$
|
|
|
|
$
|
|
|
We estimate that the total expenses of this offering will be
$ . We have agreed to pay the
reasonable fees and expenses of underwriters’ counsel
related to qualifying the offering with the Financial Industry
Regulatory Authority, Inc. under FINRA Rule 5110.
In connection with the offering, the underwriters may purchase
and sell shares in the open market. Purchases and sales in the
open market may include short sales, purchases to cover short
positions, which may include purchases pursuant to the option to
purchase additional shares, and stabilizing purchases.
|
|
|
| |
•
|
Short sales involve secondary market sales by the underwriters
of a greater number of shares than they are required to purchase
in the offering.
|
|
|
|
| |
•
|
“Covered” short sales are sales of shares in an amount
up to the number of shares represented by the underwriters’
option to purchase additional shares.
|
| |
| |
•
|
“Naked” short sales are sales of shares in an amount
in excess of the number of shares represented by the
underwriters’ option to purchase additional shares.
|
|
|
|
| |
•
|
Covering transactions involve purchases of shares either
pursuant to the option to purchase additional shares or in the
open market after the distribution has been completed in order
to cover short positions.
|
|
|
|
| |
•
|
To close a naked short position, the underwriters must purchase
shares in the open market after the distribution has been
completed. A naked short position is more likely to be created
if the underwriters are concerned that there may be downward
pressure on the price of the shares in the open market after
pricing that could adversely affect investors who purchase in
the offering.
|
| |
| |
•
|
To close a covered short position, the underwriters must
purchase shares in the open market after the distribution has
been completed or must exercise the option to purchase
additional shares. In determining the source of shares to close
the covered short position, the underwriters will consider,
among other things, the price of shares available for purchase
in the open market as compared to the price at which they may
purchase shares through the option to purchase additional shares.
|
| |
| |
•
|
Stabilizing transactions involve bids to purchase shares so long
as the stabilizing bids do not exceed a specified maximum.
|
Purchases to cover short positions and stabilizing purchases, as
well as other purchases by the underwriters for their own
accounts, may have the effect of preventing or retarding a
decline in the market price of the shares. They may also cause
the price of the shares to be higher than the price that would
otherwise exist in the open market in the absence of these
transactions. The underwriters may conduct these transactions on
the Nasdaq Global Market, in the
over-the-counter
market or otherwise. If the underwriters commence any of these
transactions, they may discontinue them at any time.
The underwriters may also impose a penalty bid. This occurs when
a particular underwriter repays to the underwriters a portion of
the underwriting discount received by it because the
representatives have repurchased shares sold by or for the
account of such underwriter in stabilizing or short covering
transactions.
150
The underwriters and their respective affiliates are
full-service financial institutions engaged in various
activities, which may include securities trading, commercial and
investment banking, financial advisory, investment management,
investment research, principal investment, hedging, financing
and brokerage activities. Certain of the underwriters and their
respective affiliates have, from time to time, performed, and
may in the future perform, various financial advisory and
investment banking services for us, for which they received or
will receive customary fees and reimbursement of expenses.
In the ordinary course of their various business activities, the
underwriters and their respective affiliates may make or hold a
broad array of instruments and actively trade debt and equity
securities (or related derivative securities) and financial
instruments (including bank loans) for their own account and for
the accounts of their customers and such investment and
securities activities may involve securities
and/or
instruments of the issuer. The underwriters and their respective
affiliates may also make investment recommendations
and/or
publish or express independent research views in respect of such
securities or instruments and may at any time hold, or recommend
to clients that they acquire, long
and/or short
positions in such securities and instruments.
We have agreed to indemnify the several underwriters against
certain liabilities, including liabilities under the Securities
Act, or to contribute to payments the underwriters may be
required to make because of any of those liabilities.
Notice to
Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area
which has implemented the Prospectus Directive (each, a Relevant
Member State), with effect from and including the date on which
the Prospectus Directive is implemented in that Relevant Member
State (the Relevant Implementation Date) an offer of the shares
which are the subject of the offering contemplated by this
Prospectus may not be made to the public in that Relevant Member
State other than an offer:
|
|
|
| |
•
|
to any legal entity which is a qualified investor as defined in
the Prospectus Directive;
|
| |
| |
•
|
to fewer than 100 or, if the Relevant Member State has
implemented the relevant provision of the 2010 PD Amending
Directive, 150, natural or legal persons (other than qualified
investors as defined in the Prospectus Directive), as permitted
under the Prospectus Directive, subject to obtaining the prior
consent of the relevant Dealer or Dealers nominated by the
Issuer for any such offer; or
|
| |
| |
•
|
in any other circumstances falling within Article 3(2) of
the Prospectus Directive,
|
provided that no such offer of the shares shall require the
Issuer or any Manager to publish a prospectus pursuant to
Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an offer of
the shares to the public in relation to any shares in any
Relevant Member State means the communication in any form and by
any means of sufficient information on the terms of the offer
and the shares to be offered so as to enable an investor to
decide to purchase or subscribe the shares, as the same may be
varied in that Member State by any measure implementing the
Prospectus Directive in that Member State, the expression
Prospectus Directive means Directive 2003/71/EC (and amendments
thereto, including the 2010 PD Amending Directive, to the extent
implemented in the Relevant Member State), and includes any
relevant implementing measure in the Relevant Member State and
the expression 2010 PD Amending Directive means Directive
2010/73/EU.
Notice to
Prospective Investors in the United Kingdom
This prospectus is only being distributed to, and is only
directed at, persons in the United Kingdom that are qualified
investors within the meaning of Article 2(1)(e) of the
Prospectus Directive that are also (i) investment
professionals falling within Article 19(5) of the Financial
Services and Markets Act 2000 (Financial Promotion) Order 2005
(the “Order”) or (ii) high net worth entities,
and other persons to whom it may lawfully be communicated,
falling within Article 49(2)(a) to (d) of the Order
(each such person being referred to as a “relevant
person”). This prospectus and its contents are confidential
and should not be distributed, published or reproduced (in whole
or in part) or disclosed by recipients to any other persons in
the
151
United Kingdom. Any person in the United Kingdom that is not a
relevant person should not act or rely on this document or any
of its contents.
Notice to
Prospective Investors in France
Neither this prospectus nor any other offering material relating
to the shares described in this prospectus has been submitted to
the clearance procedures of the Autorité des Marchés
Financiers or of the competent authority of another member state
of the European Economic Area and notified to the Autorité
des Marchés Financiers. The shares have not been offered or
sold and will not be offered or sold, directly or indirectly, to
the public in France. Neither this prospectus nor any other
offering material relating to the shares has been or will be:
|
|
|
| |
•
|
released, issued, distributed or caused to be released, issued
or distributed to the public in France; or
|
| |
| |
•
|
used in connection with any offer for subscription or sale of
the shares to the public in France.
|
Such offers, sales and distributions will be made in France only:
|
|
|
| |
•
|
to qualified investors (investisseurs qualifiés)
and/or to a
restricted circle of investors (cercle restreint
d’investisseurs), in each case investing for their own
account, all as defined in, and in accordance with
articles L.411-2,
D.411-1, D.411-2, D.734-1, D.744-1, D.754-1 and D.764-1 of the
French Code monétaire et financier;
|
| |
| |
•
|
to investment services providers authorized to engage in
portfolio management on behalf of third parties; or
|
| |
| |
•
|
in a transaction that, in accordance with
article L.411-2-II-1°-or-2°-or
3° of the French Code monétaire et financier
and
article 211-2
of the General Regulations (Règlement
Général) of the Autorité des Marchés
Financiers, does not constitute a public offer (appel
public à l’épargne).
|
The shares may be resold directly or indirectly, only in
compliance with
articles L.411-1,
L.411-2, L.412-1 and L.621-8 through L.621-8-3 of the French
Code monétaire et financier.
Notice to
Prospective Investors in Hong Kong
The shares may not be offered or sold in Hong Kong by means of
any document other than (i) in circumstances which do not
constitute an offer to the public within the meaning of the
Companies Ordinance (Cap. 32, Laws of Hong Kong), or
(ii) to “professional investors” within the
meaning of the Securities and Futures Ordinance (Cap. 571, Laws
of Hong Kong) and any rules made thereunder, or (iii) in
other circumstances which do not result in the document being a
“prospectus” within the meaning of the Companies
Ordinance (Cap. 32, Laws of Hong Kong) and no advertisement,
invitation or document relating to the shares may be issued or
may be in the possession of any person for the purpose of issue
(in each case whether in Hong Kong or elsewhere), which is
directed at, or the contents of which are likely to be accessed
or read by, the public in Hong Kong (except if permitted to do
so under the laws of Hong Kong) other than with respect to
shares which are or are intended to be disposed of only to
persons outside Hong Kong or only to “professional
investors” within the meaning of the Securities and Futures
Ordinance (Cap. 571, Laws of Hong Kong) and any rules made
thereunder.
Notice to
Prospective Investors in Japan
The shares offered in this prospectus have not been registered
under the Securities and Exchange Law of Japan. The shares have
not been offered or sold and will not be offered or sold,
directly or indirectly, in Japan or to or for the account of any
resident of Japan, except (i) pursuant to an exemption from
the registration requirements of the Securities and Exchange Law
and (ii) in compliance with any other applicable
requirements of Japanese law.
152
Notice to
Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the
Monetary Authority of Singapore. Accordingly, this prospectus
and any other document or material in connection with the offer
or sale, or invitation for subscription or purchase, of the
shares may not be circulated or distributed, nor may the shares
be offered or sold, or be made the subject of an invitation for
subscription or purchase, whether directly or indirectly, to
persons in Singapore other than (i) to an institutional
investor under Section 274 of the Securities and Futures
Act, Chapter 289 of Singapore (the “SFA”),
(ii) to a relevant person pursuant to Section 275(1),
or any person pursuant to Section 275(1A), and in
accordance with the conditions specified in Section 275 of
the SFA or (iii) otherwise pursuant to, and in accordance
with the conditions of, any other applicable provision of the
SFA, in each case subject to compliance with conditions set
forth in the SFA.
Where the shares are subscribed or purchased under
Section 275 of the SFA by a relevant person which is:
|
|
|
| |
•
|
a corporation (which is not an accredited investor (as defined
in Section 4A of the SFA)) the sole business of which is to
hold investments and the entire share capital of which is owned
by one or more individuals, each of whom is an accredited
investor; or
|
| |
| |
•
|
a trust (where the trustee is not an accredited investor) whose
sole purpose is to hold investments and each beneficiary of the
trust is an individual who is an accredited investor,
|
shares, debentures and units of shares and debentures of that
corporation or the beneficiaries’ rights and interest
(howsoever described) in that trust shall not be transferred
within six months after that corporation or that trust has
acquired the shares pursuant to an offer made under
Section 275 of the SFA except:
|
|
|
| |
•
|
to an institutional investor (for corporations, under
Section 274 of the SFA) or to a relevant person defined in
Section 275(2) of the SFA, or to any person pursuant to an
offer that is made on terms that such shares, debentures and
units of shares and debentures of that corporation or such
rights and interest in that trust are acquired at a
consideration of not less than S$200,000 (or its equivalent in a
foreign currency) for each transaction, whether such amount is
to be paid for in cash or by exchange of securities or other
assets, and further for corporations, in accordance with the
conditions specified in Section 275 of the SFA;
|
| |
| |
•
|
where no consideration is or will be given for the
transfer; or
|
| |
| |
•
|
where the transfer is by operation of law.
|
Notice to
Prospective Investors in Australia
This prospectus is not a formal disclosure document and has not
been lodged with the Australian Securities and Investments
Commission (“ASIC”). It does not purport to contain
all information that an investor or their professional advisers
would expect to find in a prospectus for the purposes of
Chapter 6D.2 of the Australian Corporations Act 2001
(“Act”) in relation to the Common Stock or ArborGen
Inc.
This prospectus is not an offer to retail investors in Australia
generally. Any offer of Common Stock in Australia is made on the
condition that the recipient is a “sophisticated
investor” within the meaning of section 708(8) of the
Act or a “professional investor” within the meaning of
section 708(11) of the Act. If any recipient does not
satisfy the criteria for these exemptions, no applications for
the Common Stock will be accepted from that recipient. Any offer
to a recipient in Australia, and any agreement arising from
acceptance of the offer, is personal and may only be accepted by
the recipient.
If a recipient on-sells their Common Stock within 12 months
of their issue, that person will be required to lodge a
disclosure document with ASIC unless either:
|
|
|
| |
•
|
the sale is pursuant to an offer received outside Australia or
is made to a “sophisticated investor” within the
meaning of 708(8) of the Act or a “professional
investor” within the meaning of section 708(11) of the
Act; or
|
153
|
|
|
| |
•
|
it can be established that ArborGen Inc. issued, and the
recipient subscribed for, the Common Stock without the purpose
of the recipient on-selling them or granting, issuing or
transferring interests in, or options or warrants over them.
|
Notice to
Prospective Investors in New Zealand
The shares offered hereby have not been offered or sold, and
will not be offered or sold, directly or indirectly in New
Zealand and no offering materials or advertisements have been or
will be distributed in relation to any offer of shares in New
Zealand, in each case other than:
• to persons whose principal business is the
investment of money or who, in the course of and for the
purposes of their business, habitually invest money; or
• to persons who in all the circumstances can properly
be regarded as having been selected otherwise than as members of
the public; or
• to persons who are each required to pay a minimum
subscription price of at least NZ$500,000 for the shares before
the allotment of those shares (disregarding any amounts payable,
or paid, out of money lent by the Issuer or any associated
person of the Issuer); or
• to persons who are eligible persons within the
meaning of section 5(2CC) of the Securities Act
1978; or
• in other circumstances where there is no
contravention of the Securities Act 1978 of New Zealand (or
any statutory modification or re-enactment of, or statutory
substitution for, the Securities Act 1978 of New Zealand).
154
LEGAL
MATTERS
The validity of the common stock offered hereby will be passed
upon for us by Goodwin Procter LLP, Boston, Massachusetts.
Certain legal matters relating to this offering will be passed
upon for the underwriters by Cleary Gottlieb Steen &
Hamilton LLP, New York, New York.
EXPERTS
The financial statements as of March 31, 2010 and 2009 and
for each of the three years in the period ended March 31,
2010 included in this prospectus have been so included in
reliance on the report of PricewaterhouseCoopers LLP, an
independent registered public accounting firm, given on the
authority of such firm as experts in auditing and accounting.
WHERE YOU
CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange Commission
(“SEC”) a registration statement on
Form S-1
under the Securities Act that registers the shares of our common
stock to be sold in this offering. This prospectus does not
contain all of the information set forth in the registration
statement and the exhibits and schedules filed as part of the
registration statement. For further information with respect to
us and our common stock, we refer you to the registration
statement and the exhibits and schedules filed as a part of the
registration statement. Statements contained in this prospectus
concerning the contents of any contract or any other document
are not necessarily complete. If a contract or document has been
filed as an exhibit to the registration statement, we refer you
to the copy of the contract or document that has been filed.
Each statement in this prospectus relating to a contract or
document filed as an exhibit is qualified in all respects by the
filed exhibit. The reports and other information we file with
the SEC can be read and copied at the SEC’s Public
Reference Room at 100 F Street, NE, Washington D.C.
20549. Copies of these materials can be obtained at prescribed
rates from the Public Reference Section of the SEC at the
principal offices of the SEC, 100 F Street, NE,
Washington D.C. 20549. You may obtain information regarding the
operation of the public reference room by calling 1(800)
SEC-0330. The SEC also maintains a web site (
http://www.sec.gov
) that contains reports, proxy and information statements and
other information regarding issuers like us that file
electronically with the SEC.
Upon completion of this offering, we will become subject to the
reporting and information requirements of the Securities
Exchange Act of 1934, as amended, and, as a result, will file
periodic reports, proxy statements and other information with
the SEC. These periodic reports, proxy statements and other
information will be available for inspection and copying at the
SEC’s public reference room and the web site of the SEC
referred to above.
155
ARBORGEN
INC.
| |
|
|
|
|
|
|
|
Page
|
|
|
|
|
|
|
F-2
|
|
|
|
|
|
F-3
|
|
|
|
|
|
F-4
|
|
|
|
|
|
F-5
|
|
|
|
|
|
F-6
|
|
|
|
|
|
F-7
|
|
|
Financial Statement Schedule
|
|
|
|
|
|
|
|
|
F-22
|
|
F-1
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors of
ArborGen Inc. and Subsidiaries
In our opinion, the consolidated financial statements listed in
the accompanying index present fairly, in all material respects,
the financial position of ArborGen, LLC and its subsidiaries
(the “Company”) at March 31, 2010 and 2009, and
the results of their operations and their cash flows for each of
the three years in the period ended March 31, 2010 in
conformity with accounting principles generally accepted in the
United States of America. In addition, in our opinion, the
financial statement schedule listed in the accompanying index
presents fairly, in all material respects, the information set
forth therein when read in conjunction with the related
consolidated financial statements. These financial statements
and financial statement schedule are the responsibility of the
Company’s management. Our responsibility is to express an
opinion on these financial statements and financial statement
schedule based on our audits. We conducted our audits of these
statements in accordance with the standards of the Public
Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. An audit includes examining, on a
test basis, evidence supporting the amounts and disclosures in
the financial statements, assessing the accounting principles
used and significant estimates made by management, and
evaluating the overall financial statement presentation. We
believe that our audits provide a reasonable basis for our
opinion.
As more fully discussed in Notes 4, 7 and 8 to the
consolidated financial statements, the Company has significant
related party transactions with member companies.
/s/ PricewaterhouseCoopers
LLP
Spartanburg, South Carolina
July 22, 2010, except for net loss per share information
discussed in Note 1 and segment information discussed in
Note 13, as to which the date is September 30, 2010.
F-2
ArborGen,
LLC
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31,
|
|
|
March 31,
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2010
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
ASSETS
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
1,624,330
|
|
|
$
|
3,742,048
|
|
|
$
|
2,133,421
|
|
|
Accounts receivable, net of allowance for doubtful accounts of
$200,000, $125,000 and $436,053 respectively
|
|
|
3,043,462
|
|
|
|
3,542,511
|
|
|
|
4,137,431
|
|
|
Accounts receivable — member companies
|
|
|
254,347
|
|
|
|
272,683
|
|
|
|
52,956
|
|
|
Inventories
|
|
|
11,185,209
|
|
|
|
12,201,897
|
|
|
|
18,015,996
|
|
|
Prepaid expenses and other assets
|
|
|
419,699
|
|
|
|
515,195
|
|
|
|
356,541
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
16,527,047
|
|
|
|
20,274,334
|
|
|
|
24,696,345
|
|
|
Property, plant and equipment, net
|
|
|
16,075,723
|
|
|
|
17,939,416
|
|
|
|
19,135,212
|
|
|
Intangible assets, net
|
|
|
4,041,053
|
|
|
|
3,937,622
|
|
|
|
4,123,841
|
|
|
Other assets
|
|
|
—
|
|
|
|
193,463
|
|
|
|
2,922,401
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
36,643,823
|
|
|
$
|
42,344,835
|
|
|
$
|
50,877,799
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND MEMBERS’/STOCKHOLDERS’ EQUITY
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
3,378,308
|
|
|
$
|
3,630,145
|
|
|
$
|
6,221,109
|
|
|
Accounts payable — member companies
|
|
|
133,787
|
|
|
|
332,139
|
|
|
|
389,290
|
|
|
Accrued expenses
|
|
|
840,545
|
|
|
|
773,152
|
|
|
|
1,037,396
|
|
|
Lines of Credit
|
|
|
8,000,000
|
|
|
|
2,750,000
|
|
|
|
14,011,174
|
|
|
Current maturities of long-term debt
|
|
|
—
|
|
|
|
403,472
|
|
|
|
96,302
|
|
|
Salaries and benefits payable
|
|
|
1,031,868
|
|
|
|
1,845,178
|
|
|
|
2,767,300
|
|
|
Deferred revenue
|
|
|
662,209
|
|
|
|
1,789,310
|
|
|
|
3,396,172
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
14,046,717
|
|
|
|
11,523,396
|
|
|
|
27,918,743
|
|
|
Long-term debt, net of current maturities
|
|
|
167,841
|
|
|
|
11,675,202
|
|
|
|
4,356,346
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
14,214,558
|
|
|
|
23,198,598
|
|
|
|
32,275,089
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred interest of member company
|
|
|
6,534,897
|
|
|
|
2,151,947
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Members’/Stockholders’ equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock — $0.001 par value,
50,000,000 shares authorized; 30,529,866 outstanding as of
December 31, 2010
|
|
|
—
|
|
|
|
—
|
|
|
|
30,530
|
|
|
Additional
paid-in-capital
|
|
|
—
|
|
|
|
—
|
|
|
|
29,828,268
|
|
|
Members’ equity interest
|
|
|
19,594,214
|
|
|
|
18,087,269
|
|
|
|
—
|
|
|
Accumulated deficit
|
|
|
—
|
|
|
|
—
|
|
|
|
(10,958,267
|
)
|
|
Accumulated other comprehensive loss
|
|
|
(3,699,846
|
)
|
|
|
(1,092,979
|
)
|
|
|
(297,821
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total members’/stockholders’ equity
|
|
|
15,894,368
|
|
|
|
16,994,290
|
|
|
|
18,602,710
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and members’/stockholders’ equity
|
|
$
|
36,643,823
|
|
|
$
|
42,344,835
|
|
|
$
|
50,877,799
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements
F-3
ArborGen,
LLC
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
|
Years Ended March 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
Revenue
|
|
$
|
17,720,923
|
|
|
$
|
22,936,365
|
|
|
$
|
20,670,497
|
|
|
$
|
9,224,503
|
|
|
$
|
10,903,582
|
|
|
Revenue — member companies
|
|
|
460,912
|
|
|
|
743,314
|
|
|
|
905,162
|
|
|
|
358,290
|
|
|
|
179,647
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue
|
|
$
|
18,181,835
|
|
|
$
|
23,679,679
|
|
|
$
|
21,575,659
|
|
|
$
|
9,582,793
|
|
|
$
|
11,083,229
|
|
|
Cost of revenue
|
|
|
15,348,862
|
|
|
|
15,729,967
|
|
|
|
16,375,654
|
|
|
|
6,921,388
|
|
|
|
7,036,663
|
|
|
Cost of revenue — member companies
|
|
|
317,890
|
|
|
|
109,675
|
|
|
|
5,150
|
|
|
|
5,000
|
|
|
|
18,860
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cost of revenue
|
|
|
15,666,752
|
|
|
|
15,839,649
|
|
|
|
16,380,804
|
|
|
|
6,926,388
|
|
|
|
7,055,523
|
|
|
Gross profit
|
|
|
2,515,083
|
|
|
|
7,840,037
|
|
|
|
5,194,855
|
|
|
|
2,656,405
|
|
|
|
4,027,706
|
|
|
Operating expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
9,658,778
|
|
|
|
13,294,957
|
|
|
|
10,694,145
|
|
|
|
7,377,745
|
|
|
|
9,160,236
|
|
|
Research and development — member companies
|
|
|
553,335
|
|
|
|
538,257
|
|
|
|
511,482
|
|
|
|
458,555
|
|
|
|
10,375
|
|
|
Selling, general and administrative
|
|
|
8,116,652
|
|
|
|
6,148,125
|
|
|
|
5,939,879
|
|
|
|
4,146,117
|
|
|
|
6,580,838
|
|
|
Selling, general and administrative — member companies
|
|
|
1,592,203
|
|
|
|
1,450,813
|
|
|
|
1,198,705
|
|
|
|
1,048,000
|
|
|
|
1,285,480
|
|
|
Depreciation and amortization
|
|
|
679,408
|
|
|
|
813,198
|
|
|
|
780,415
|
|
|
|
664,788
|
|
|
|
444,094
|
|
|
Loss on impairment of intangible assets
|
|
|
202,430
|
|
|
|
643,389
|
|
|
|
213,124
|
|
|
|
199,960
|
|
|
|
154,019
|
|
|
Restructuring charge
|
|
|
182,983
|
|
|
|
492,392
|
|
|
|
100,902
|
|
|
|
—
|
|
|
|
—
|
|
|
Other operating (income), net
|
|
|
(386,048
|
)
|
|
|
(464,680
|
)
|
|
|
(233,168
|
)
|
|
|
(185,299
|
)
|
|
|
(185,116
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(18,084,658
|
)
|
|
|
(15,076,414
|
)
|
|
|
(14,010,629
|
)
|
|
|
(11,053,461
|
)
|
|
|
(13,422,220
|
)
|
|
Other income (expense)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
106,631
|
|
|
|
29,865
|
|
|
|
12,213
|
|
|
|
6,410
|
|
|
|
22,536
|
|
|
Interest expense
|
|
|
(103,216
|
)
|
|
|
(299,438
|
)
|
|
|
(657,379
|
)
|
|
|
(441,887
|
)
|
|
|
(787,054
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(18,081,243
|
)
|
|
$
|
(15,345,987
|
)
|
|
$
|
(14,655,795
|
)
|
|
$
|
(11,488,938
|
)
|
|
$
|
(14,186,738
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share
|
|
$
|
(0.92
|
)
|
|
$
|
(0.62
|
)
|
|
$
|
(0.54
|
)
|
|
$
|
(0.43
|
)
|
|
$
|
(0.48
|
)
|
|
Weighted average shares used in computing net loss per common
share
|
|
|
19,558,223
|
|
|
|
24,676,724
|
|
|
|
27,078,203
|
|
|
|
26,864,061
|
|
|
|
29,824,486
|
|
The accompanying notes are an integral part of these
consolidated financial statements
F-4
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Members’ Equity Interest
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
International
|
|
|
|
|
|
Number of
|
|
|
Par Value of
|
|
|
Additional
|
|
|
|
|
|
Other
|
|
|
|
|
|
|
|
MeadWestvaco
|
|
|
Paper
|
|
|
Rubicon
|
|
|
Common
|
|
|
Common
|
|
|
Paid-
|
|
|
Accumulated
|
|
|
Comprehensive
|
|
|
|
|
|
|
|
Corporation
|
|
|
Company
|
|
|
Limited
|
|
|
Shares
|
|
|
Shares
|
|
|
in-Capital
|
|
|
Deficit
|
|
|
Loss
|
|
|
Total
|
|
|
|
|
Members’ equity, April 1, 2007
|
|
$
|
2,215,703
|
|
|
$
|
2,215,703
|
|
|
$
|
2,215,703
|
|
|
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
6,647,109
|
|
|
Capital contributions, cash
|
|
|
4,863,540
|
|
|
|
2,431,770
|
|
|
|
4,863,540
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
12,158,850
|
|
|
October 31, 2007 equity transactions (Notes 7 and 8)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash received from members
|
|
|
13,200,000
|
|
|
|
—
|
|
|
|
100,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
13,300,000
|
|
|
Noncash contributions from members
|
|
|
212,634
|
|
|
|
9,032,186
|
|
|
|
9,538,893
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
18,783,713
|
|
|
Preferred interest received by October 31, 2007
|
|
|
—
|
|
|
|
(13,300,000
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(13,300,000
|
)
|
|
Reduction of preferred interest in lieu of cash payment, from
November 1, 2007 through March 31, 2008
|
|
|
—
|
|
|
|
2,431,770
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,431,770
|
|
|
Net loss
|
|
|
(6,027,081
|
)
|
|
|
(6,027,081
|
)
|
|
|
(6,027,081
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(18,081,243
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Members’ equity (deficit), March 31, 2008
|
|
|
14,464,796
|
|
|
|
(3,215,652
|
)
|
|
|
10,691,055
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
21,940,199
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital contributions-cash
|
|
|
4,333,334
|
|
|
|
—
|
|
|
|
4,333,334
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8,666,668
|
|
|
Reduction of preferred interest in lieu of cash payment
April 1, 2008 to March 31, 2009
|
|
|
—
|
|
|
|
4,333,334
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
4,333,334
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total member contributions
|
|
|
4,333,334
|
|
|
|
4,333,334
|
|
|
|
4,333,334
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
13,000,002
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cumulative translation adjustment
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,699,846
|
)
|
|
|
(3,699,846
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(5,115,329
|
)
|
|
|
(5,115,329
|
)
|
|
|
(5,115,329
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(15,345,987
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
(19,045,833
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Members’ equity (deficit), March 31, 2009
|
|
|
13,682,801
|
|
|
|
(3,997,647
|
)
|
|
|
9,909,060
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(3,699,846
|
)
|
|
|
15,894,368
|
|
|
Capital contributions-cash
|
|
|
4,382,950
|
|
|
|
—
|
|
|
|
4,382,950
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
8,765,900
|
|
|
Reduction of preferred interest in lieu of cash payment from
April 1, 2009 to March 31, 2010
|
|
|
—
|
|
|
|
4,382,950
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
4,382,950
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total member contributions
|
|
|
4,382,950
|
|
|
|
4,382,950
|
|
|
|
4,382,950
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
13,148,850
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cumulative translation adjustment
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,606,867
|
|
|
|
2,606,867
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(4,885,265
|
)
|
|
|
(4,885,265
|
)
|
|
|
(4,885,265
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(14,655,795
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(12,048,928
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Members’ equity (deficit), March 31, 2010
|
|
|
13,180,486
|
|
|
|
(4,499,962
|
)
|
|
|
9,406,745
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,092,979
|
)
|
|
|
16,994,290
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital contributions-cash, unaudited
|
|
|
2,350,650
|
|
|
|
198,703
|
|
|
|
2,350,650
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
4,900,003
|
|
|
Reduction of preferred interest in lieu of cash payment from
April 1, 2010 to May 31, 2010, unaudited
|
|
|
—
|
|
|
|
2,151,947
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,151,947
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total member contributions, unaudited
|
|
|
2,350,650
|
|
|
|
2,350,650
|
|
|
|
2,350,650
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,051,950
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cumulative translation adjustment, through May 31, 2010,
unaudited
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(341,398
|
)
|
|
|
(341,398
|
)
|
|
Net loss through May 31, 2010 unaudited
|
|
|
(1,076,157
|
)
|
|
|
(1,076,157
|
)
|
|
|
(1,076,157
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,228,471
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total members’ equity (deficit) May 31, 2010, unaudited
|
|
|
14,454,979
|
|
|
|
(3,225,469
|
)
|
|
|
10,681,238
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,434,377
|
)
|
|
|
20,476,371
|
|
|
Conversion of Members’ equity interests to common stock on
June 1, 2010, unaudited
|
|
|
(14,454,979
|
)
|
|
|
3,225,469
|
|
|
|
(10,681,238
|
)
|
|
|
30,000,000
|
|
|
|
30,000
|
|
|
|
21,880,748
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Issuance of common stock, unaudited
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
529,866
|
|
|
|
530
|
|
|
|
7,947,520
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,948,050
|
|
|
Cumulative translation adjustment, unaudited
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,136,556
|
|
|
|
1,136,556
|
|
|
Net loss from June 1, to December 31, 2010, unaudited
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(10,958,267
|
)
|
|
|
—
|
|
|
|
(10,958,267
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ equity (deficit), December 31, 2010,
unaudited
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
30,529,866
|
|
|
$
|
30,530
|
|
|
$
|
29,828,268
|
|
|
$
|
(10,958,267
|
)
|
|
$
|
(297,821
|
)
|
|
$
|
18,602,710
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements
F-5
ArborGen,
LLC
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended March 31,
|
|
|
Nine Months Ended December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(18,081,243
|
)
|
|
$
|
(15,345,987
|
)
|
|
$
|
(14,655,795
|
)
|
|
$
|
(11,488,938
|
)
|
|
$
|
(14,186,738
|
)
|
|
Adjustment to reconcile net loss to net cash used in operating
activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
478,628
|
|
|
|
937,564
|
|
|
|
1,037,719
|
|
|
|
829,420
|
|
|
|
865,506
|
|
|
Amortization
|
|
|
441,022
|
|
|
|
463,831
|
|
|
|
412,854
|
|
|
|
347,263
|
|
|
|
133,710
|
|
|
Loss on impairment of intangible assets
|
|
|
202,430
|
|
|
|
643,389
|
|
|
|
213,124
|
|
|
|
199,960
|
|
|
|
154,019
|
|
|
Gain on sale of property, plant and equipment
|
|
|
—
|
|
|
|
—
|
|
|
|
(43,599
|
)
|
|
|
—
|
|
|
|
(23,318
|
)
|
|
Amortization of deferred financing costs
|
|
|
—
|
|
|
|
—
|
|
|
|
14,760
|
|
|
|
—
|
|
|
|
159,499
|
|
|
Change in operating assets and liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(4,568,490
|
)
|
|
|
1,238,541
|
|
|
|
(446,573
|
)
|
|
|
248,647
|
|
|
|
(282,681
|
)
|
|
Inventories
|
|
|
(8,873,287
|
)
|
|
|
(1,299,233
|
)
|
|
|
(314,453
|
)
|
|
|
(4,580,594
|
)
|
|
|
(5,481,223
|
)
|
|
Prepaid expenses and other assets
|
|
|
(5,603
|
)
|
|
|
142,791
|
|
|
|
(125,905
|
)
|
|
|
50,964
|
|
|
|
190,201
|
|
|
Accounts payable and accrued liabilities
|
|
|
4,266,986
|
|
|
|
957,600
|
|
|
|
1,944,861
|
|
|
|
1,766,243
|
|
|
|
3,174,413
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(26,139,557
|
)
|
|
|
(12,261,504
|
)
|
|
|
(11,963,007
|
)
|
|
|
(12,627,035
|
)
|
|
|
(15,296,612
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment
|
|
|
(1,053,103
|
)
|
|
|
(1,114,321
|
)
|
|
|
(1,032,356
|
)
|
|
|
(709,293
|
)
|
|
|
(1,280,539
|
)
|
|
Proceeds from the sale of property, plant and equipment
|
|
|
—
|
|
|
|
—
|
|
|
|
53,435
|
|
|
|
—
|
|
|
|
49,247
|
|
|
Purchases of intangible assets
|
|
|
(663,254
|
)
|
|
|
(1,025,000
|
)
|
|
|
(522,546
|
)
|
|
|
(400,820
|
)
|
|
|
(473,948
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(1,716,357
|
)
|
|
|
(2,139,321
|
)
|
|
|
(1,501,467
|
)
|
|
|
(1,110,113
|
)
|
|
|
(1,705,240
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital contributions
|
|
|
25,458,850
|
|
|
|
8,666,668
|
|
|
|
8,765,900
|
|
|
|
7,295,310
|
|
|
|
4,900,003
|
|
|
Proceeds from issuance of stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,948,050
|
|
|
Borrowings on line of credit
|
|
|
5,008,127
|
|
|
|
10,000,000
|
|
|
|
8,365,234
|
|
|
|
8,265,000
|
|
|
|
5,999,999
|
|
|
Borrowings on installment loans
|
|
|
|
|
|
|
167,841
|
|
|
|
32,853
|
|
|
|
16,522
|
|
|
|
24,109
|
|
|
Repayment of line of credit
|
|
|
(2,500,000
|
)
|
|
|
(4,500,000
|
)
|
|
|
(1,700,000
|
)
|
|
|
(500,000
|
)
|
|
|
(2,750,000
|
)
|
|
Payment of public offering costs
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(784,114
|
)
|
|
Payment of debt refinancing fees
|
|
|
—
|
|
|
|
—
|
|
|
|
(177,123
|
)
|
|
|
—
|
|
|
|
(31,784
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
27,966,977
|
|
|
|
14,334,509
|
|
|
|
15,286,864
|
|
|
|
15,076,832
|
|
|
|
15,306,263
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate changes on cash
|
|
|
—
|
|
|
|
(363,683
|
)
|
|
|
295,328
|
|
|
|
151,035
|
|
|
|
86,962
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash
|
|
|
111,063
|
|
|
|
(429,999
|
)
|
|
|
2,117,718
|
|
|
|
1,490,719
|
|
|
|
(1,608,627
|
)
|
|
Cash and cash equivalents
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning of year
|
|
|
1,943,266
|
|
|
|
2,054,329
|
|
|
|
1,624,330
|
|
|
|
1,624,330
|
|
|
|
3,742,048
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
End of year
|
|
$
|
2,054,329
|
|
|
$
|
1,624,330
|
|
|
$
|
3,742,048
|
|
|
$
|
3,115,049
|
|
|
$
|
2,133,421
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of noncash investing and financing
transactions
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contribution of seed inventories
|
|
$
|
1,865,367
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Contribution of property, plant and equipment
|
|
|
16,918,346
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Property, plant and equipment included in accounts payable and
accrued liabilities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
13,428
|
|
|
Deferred offering costs included in accounts payable and accrued
liabilities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,103,639
|
|
|
Supplemental disclosure of cash flow information
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest
|
|
$
|
103,216
|
|
|
$
|
284,438
|
|
|
$
|
648,120
|
|
|
$
|
450,153
|
|
|
$
|
661,866
|
|
The accompanying notes are an integral part of these
consolidated financial statements
F-6
ArborGen,
LLC
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
AS OF AND FOR THE YEARS ENDED MARCH 31, 2010, 2009, AND 2008 AND
AS OF AND FOR
THE NINE MONTHS ENDED DECEMBER 31, 2009 (UNAUDITED) AND DECEMBER
31, 2010 (UNAUDITED)
|
|
|
1.
|
Summary
of Significant Accounting Policies
|
General
ArborGen, LLC (the “Company”) is a Limited Liability
Company organized on February 7, 2000 pursuant to the
Delaware Limited Liability Company Act. The Company is a
partnership between MeadWestvaco Corporation (“MWV”),
International Paper Company (“IP”) and Rubicon Limited
(“Rubicon”) (“member companies”). The
Company is focused on the research, development and
commercialization of higher-value treestock products with
advanced genetic properties. As of June 1, 2010, the
Company converted from a Limited Liability Company to a
Corporation changing its name from “ArborGen, LLC” to
“ArborGen Inc.” References to the Company for
activities subsequent to June 1, 2010 refer to ArborGen Inc.
The Company has five subsidiaries, ArborGen Tecnologia Florestal
Ltda., ArborGen Australia Holdings Pty. Ltd., ArborGen Australia
Pty. Ltd., ArborGen New Zealand Unlimited and ArborGen New
Zealand Holdings, LLC. All subsidiaries are wholly owned and are
included in the consolidated financial statements.
Effective April 1, 2007, the Company changed its fiscal
year end from December 31 to March 31.
The Company obtained non-exclusive licenses from its member
companies for certain technologies, access to scientific
research information, biological materials and intellectual
property relating to forestry biotechnology. The member
companies are entitled to royalties on future sales utilizing
certain technologies. On October 31, 2007, member companies
contributed to the Company various seed orchards and nursery
businesses including physical operations, certain land holdings,
research and development, and intellectual property including
pine and hardwood germplasm.
ArborGen, LLC has incurred annual losses and negative cash flows
from operations since inception. Based on current operating
plans, the Company expects it has sufficient liquidity to
continue its planned operations beyond the year ending
March 31, 2011. The Company’s liquidity needs will
largely be determined by the commercial success of its products
and key development events. In order to continue its operations
substantially beyond March 31, 2011 it will need to:
(1) successfully increase revenues; (2) raise
additional capital through equity or debt financings, or from
continued member capital contributions;
and/or
(3) further restructure its operations. The Company will
continue to incur operating losses until revenues reach a level
sufficient to support ongoing operations. For the nine months
ended December 31, 2010, the Company has received cash
contributions from member companies totaling $12,848,053. In the
absence of continued member contributions, the Company would
further consider its restructuring options to avoid adverse
consequences.
Principles
of Consolidation
The Company’s consolidated financial statements include the
accounts of ArborGen, LLC and its wholly owned subsidiaries. All
intercompany accounts and transactions have been eliminated in
consolidation.
Unaudited
Interim Financial Information
The interim financial information as of December 31, 2010
and for the nine months ended December 31, 2009 and 2010 is
unaudited and has been prepared on the same basis as the audited
financial statements. In the opinion of management, such
unaudited information includes all adjustments, consisting of
normal recurring adjustments (unless otherwise noted), necessary
for fair presentation of the interim financial information.
Operating results for the interim periods are not necessarily
indicative of results for any subsequent periods. Certain
information in the footnote disclosure normally included in
annual financial
F-7
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
statements has been condensed or omitted for the interim periods
presented, in accordance with the rules and regulations of the
Securities and Exchange Commission for interim financial
statements.
Use of
Estimates
The preparation of the financial statements in conformity with
generally accepted accounting principles requires management to
make estimates and assumptions that affect the reported amounts
of assets and liabilities at the date of the financial
statements and the reported amount of revenues and expenses
during the period. Areas of the financial statements where
estimates may have the most significant effect include revenue
recognition, allowance for doubtful accounts, and the reserve
for slow moving seed. Changes in the facts or circumstances
underlying these estimates could result in material changes and
actual results could differ from these estimates.
Property,
Plant and Equipment
The Company records property, plant and equipment at cost and
depreciates them over their estimated useful life using the
straight-line method. Property and equipment subject to capital
leases and leasehold improvements are depreciated over the
lesser of the expected term of the lease or estimated useful
life of the asset. When property and equipment is sold or
otherwise disposed of, the cost and related accumulated
depreciation are removed from the accounts and the resulting
gains and losses are included in other operating income.
Expenditures for maintenance and repairs are charged to expense
as incurred, whereas major betterments are capitalized.
Inventories
Inventories are valued at the lower of cost or market and
consist principally of seed and seed cuttings. Cost is
determined using an average cost method.
Inventories are written down to net realizable value based on
regular evaluations for slow-moving seed product advancements
and production needs.
Cash
and Cash Equivalents
The Company classifies as cash and cash equivalents all highly
liquid investments with an original maturity of three months or
less at the acquisition date.
The Company maintains its cash in bank deposit accounts, which
at times exceed federally insured limits. The Company has not
experienced any losses in such accounts.
Intangible
Assets
Licenses to use certain intellectual property are amortized on a
straight-line basis over
10-17 years.
Financial Accounting Standards Board Accounting Standards
Codification Topic 350, Goodwill and Other Intangible Assets
requires that intangible assets that are subject to amortization
be reviewed for impairment upon adoption and thereafter when
specific circumstances indicate the carrying amount may exceed
its fair value.
The Company records licenses and patents at cost, and no
specific valuations are performed related to these licenses and
patents as no fair value estimations are applicable at this
stage in development. The Company evaluates the recoverability
of intangible assets periodically and takes into account events
or circumstances that warrant revised estimates of useful lives
or that indicate that impairment may exist. Impairment charges
of $202,430, $643,389, and $213,124 were recorded for the fiscal
years ended March 31, 2008, 2009, and 2010, and $199,960
and $154,019 for the nine months ended December 31, 2009
and 2010,
F-8
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
respectively, for
patents-in-progress
that were no longer in the Company’s strategic interests to
pursue. These charges are reflected as loss on impairment of
intangible assets on the accompanying consolidated statements of
operations.
Revenue
Recognition
The majority of the Company’s revenue is derived from the
sale of seedling products to its customers across multiple end
markets. Assuming persuasive evidence of an arrangement exists,
the fee is fixed or determinable, and collection of the
resulting receivable is reasonably assured, revenue from sales
of these seedlings is recognized upon transfer of title to the
Company’s customers. Transfer of title typically results
upon shipment of the seedlings.
To the extent that the Company’s customers agree to pay for
its seedling products in advance of delivery, the Company
records these amounts as deferred revenue. The Company does not
offer warranties on its products.
The Company recognizes contract revenue in an amount equal to
the lesser of the amount due under contract or the amount earned
based on proportional performance to date.
Research
and Development Cost
Research and development costs are expensed in the period
incurred. These costs include salaries and related cost for
scientists, cost for materials used in product development,
costs for third-party licensing, academic programs and
intellectual property, cost for initiating and management of
field tests and facility costs. Payments related to the
acquisition of technology rights, for which the development work
is in process, are expensed and considered a part of research
and development cost.
Income
Taxes
The Company reports as a partnership for income tax purposes
with income or losses passing directly to the limited liability
company members. Consequently, income tax expense (benefit) is
not reflected on the statements of operations, nor does the
balance sheet contain an amount for deferred tax assets or
liabilities resulting from the tax effects of temporary
differences. On June 1, 2010, the Company converted from a
limited liability company to a corporation changing the
Company’s tax status from nontaxable to taxable.
Concentration
of Credit Risk
The Company’s top three customers comprised 19%, 17%, and
22% of total product sales for the years ended March 31,
2008, 2009 and 2010, respectively, and 19% and 25% for the nine
months ended December 31, 2009 and December 31, 2010,
respectively. The loss of one or more of these customers could
negatively impact results of operations of the Company.
Foreign
Currency Transactions and Translation
The Company translates the operating results of its foreign
subsidiaries using average exchange rates during each period,
whereas monetary asset and liability accounts are translated
using exchange rates at the end of each period. For the fiscal
years ended March 31, 2008, 2009, and 2010 the Company
recognized translation gains (losses) of $0, ($3,699,846), and
$2,606,867, respectively, and $2,841,025 and $795,158 for the
nine months ended December 31, 2009 and 2010, respectively.
The translation gains (losses) are reflected as a component of
equity in accumulated other comprehensive loss.
Local currency is the functional currency in jurisdictions
outside of the U.S. Foreign currency transaction gains and
losses are recorded in the consolidated statements of operations
in other operating income, net and
F-9
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
were not material for the years ended March 31, 2008, 2009,
and 2010 or for the nine months ended December 31, 2009 and
2010.
Net
loss per share
Basic net loss per common share is computed by dividing net loss
by the weighted average number of common shares outstanding for
the period. Diluted EPS reflects the potential dilution from
securities that could share in the earnings of the Company.
Antidilutive securities are excluded from diluted earnings per
share. Upon conversion of the partnership into a corporation on
June 1, 2010, the members received a total of 30,000,000
common shares for their member interests. For the periods prior
to the conversion, for purposes of determining the weighted
average number of common shares outstanding, the Company
computed the historical equivalent shares based on the
conversion ratio used on June 1, 2010.
The following table presents the calculation of historical basic
and diluted net loss per common share:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended March 31,
|
|
Nine Months Ended December 31,
|
|
|
|
2008
|
|
2009
|
|
2010
|
|
2009
|
|
2010
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
Net loss attributable to common shareholders
|
|
$
|
(18,081,243
|
)
|
|
$
|
(15,345,987
|
)
|
|
$
|
(14,655,795
|
)
|
|
$
|
(11,488,938
|
)
|
|
$
|
(14,186,738
|
)
|
|
Net loss per common share, basic and diluted
|
|
$
|
(0.92
|
)
|
|
$
|
(0.62
|
)
|
|
$
|
(0.54
|
)
|
|
$
|
(0.43
|
)
|
|
$
|
(0.48
|
)
|
|
Shares used in computing net loss per common share, basic and
diluted
|
|
|
19,558,223
|
|
|
|
24,676,724
|
|
|
|
27,078,203
|
|
|
|
26,864,061
|
|
|
|
29,824,486
|
|
Recently
Issued Accounting Pronouncements
In October 2009, the FASB issued authoritative guidance
regarding revenue arrangements with multiple deliverables. The
guidance requires entities to allocate revenue in an arrangement
using estimated selling prices of the delivered goods and
services based on a selling price hierarchy. The guidance
eliminates the residual method of revenue allocation and
requires revenue to be allocated using the relative selling
price method. The new guidance should be applied on a
prospective basis for revenue arrangements entered into or
materially modified in fiscal years beginning on or after
June 15, 2010, with early adoption permitted. The Company
is currently assessing the impact of adopting this new guidance.
In January 2010, the FASB issued authoritative guidance for
improving disclosures about fair value measurements. This
guidance requires some new disclosures and clarifies some
existing disclosure requirements about fair value measurement.
New disclosures required include the amount of significant
transfers in and out of levels 1 and 2 fair value
measurements and the reasons for the transfers. In addition, the
reconciliation for level 3 activity will be required on a
gross rather than net basis. The new disclosure requirements are
effective for interim and annual reporting periods beginning
after December 15, 2009, except for the disclosures about
purchases, sales, issuances, and settlements in the roll forward
of activity in Level 3 fair value measurements. Those
disclosures are effective for fiscal years beginning after
December 15, 2010, and for interim periods within those
fiscal years. Since the new guidance only impacts financial
statement disclosures, there will be no impact to the
Company’s balance sheets or statements of operations upon
adoption.
F-10
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
|
|
|
2.
|
Property,
Plant and Equipment
|
Property, plant and equipment consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Useful
|
|
|
|
|
|
|
|
|
|
|
|
|
Lives in
|
|
As of March 31,
|
|
|
As of March 31,
|
|
|
As of December 31,
|
|
|
|
|
Years
|
|
2009
|
|
|
2010
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
Computer equipment and software
|
|
3 to 7
|
|
$
|
390,672
|
|
|
$
|
416,924
|
|
|
$
|
349,237
|
|
|
Furniture and equipment
|
|
5 to 15
|
|
|
3,844,945
|
|
|
|
4,650,164
|
|
|
|
5,399,463
|
|
|
Leasehold improvements
|
|
8
|
|
|
184,709
|
|
|
|
236,680
|
|
|
|
236,680
|
|
|
Buildings and building improvements
|
|
10 to 39
|
|
|
6,092,601
|
|
|
|
7,485,069
|
|
|
|
8,430,787
|
|
|
Land improvements
|
|
|
|
|
893,597
|
|
|
|
893,597
|
|
|
|
1,430,232
|
|
|
Land
|
|
|
|
|
6,073,163
|
|
|
|
7,001,920
|
|
|
|
6,873,002
|
|
|
Construction in process
|
|
|
|
|
515,139
|
|
|
|
262,258
|
|
|
|
349,123
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
17,994,826
|
|
|
|
20,946,612
|
|
|
|
23,068,524
|
|
|
Less: Accumulated depreciation
|
|
|
|
|
(1,919,103
|
)
|
|
|
(3,007,196
|
)
|
|
|
(3,933,312
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Property, plant and equipment, net
|
|
|
|
$
|
16,075,723
|
|
|
$
|
17,939,416
|
|
|
$
|
19,135,212
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation expense was $478,628, $937,564, $1,037,719 for the
years ended March 31, 2008, 2009, 2010, respectively.
Depreciation expense for the nine months ended December 31,
2009 and 2010 was $829,420 and $865,506, respectively. The
portions of depreciation expense that relate to the production
of the Company’s products have been recorded in cost of
revenue. Depreciation recorded in cost of revenue for the years
ended March 31, 2008, 2009 and 2010 was $240,242, $588,197,
and $670,158, respectively. Depreciation recorded in cost of
revenue for the nine months ended December 31, 2009 and
2010 was $456,713 and $555,427, respectively.
Inventory consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of March 31,
|
|
|
As of March 31,
|
|
|
As of December 31,
|
|
|
|
|
2009
|
|
|
2010
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
Finished goods, seed
|
|
$
|
7,099,147
|
|
|
$
|
7,421,939
|
|
|
$
|
5,611,409
|
|
|
Reserve for slow moving seed
|
|
|
(423,547
|
)
|
|
|
(1,276,422
|
)
|
|
|
(1,271,517
|
)
|
|
Finished goods, seedlings
|
|
|
64,595
|
|
|
|
164,037
|
|
|
|
—
|
|
|
Work in process, seed
|
|
|
1,072,712
|
|
|
|
1,191,395
|
|
|
|
1,110,380
|
|
|
Work in process, seedlings/cuttings
|
|
|
3,153,377
|
|
|
|
4,557,710
|
|
|
|
12,441,780
|
|
|
Mother plants
|
|
|
218,925
|
|
|
|
95,702
|
|
|
|
61,411
|
|
|
Raw materials
|
|
|
—
|
|
|
|
47,536
|
|
|
|
62,533
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total inventories
|
|
$
|
11,185,209
|
|
|
$
|
12,201,897
|
|
|
$
|
18,015,996
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company performs a review of the valuation of its
inventories on a quarterly basis. As a result of the reviews
during the periods ended March 31, 2008, 2009 and 2010, the
Company determined that due to the development of various
advanced products, a seed reserve provision of $771,723, $0, and
$850,000, respectively, should be recorded. For the nine months
ended December 31, 2009 and 2010 a seed provision of $2,874
and $0, respectively, was recorded. These charges are reflected
in cost of revenue in the Company’s consolidated statement
of operations.
F-11
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
The Company entered into a renewed lease with a member company
for greenhouse and office space and a field testing site during
the year ended March 31, 2009. The Company leased from a
member company office space at a rate of $64,524 per month,
greenhouse space at a rate of $18,329 per month, and field
testing sites and field testing and seed orchards at a rate of
$48,299 per year. The Company entered into a new lease with a
member company for seed orchard sites during the year ended
March 31, 2009.
Lease expense totaled $1,219,041, $1,266,744, and $2,120,567 for
the years ended March 31, 2008, 2009, and 2010,
respectively. Lease expense was $1,512,764 and $1,529,956 for
the nine months ended December 31, 2009 and 2010,
respectively. Of these amounts, $1,015,860, $1,089,187, and
$1,016,109 for the years ended March 31, 2008, 2009, and
2010, respectively, and $707,249 and $733,459 for the nine
months ended December 31, 2009 and 2010, respectively, were
paid to related parties.
As of March 31, 2010, the future minimum lease commitments
under non-cancelable lease agreements were as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Operating
|
|
|
Capital
|
|
|
|
|
2011
|
|
$
|
749,657
|
|
|
$
|
63,131
|
|
|
2012
|
|
|
192,594
|
|
|
|
63,131
|
|
|
2013
|
|
|
82,779
|
|
|
|
57,454
|
|
|
2014
|
|
|
63,245
|
|
|
|
43,009
|
|
|
2015
|
|
|
63,245
|
|
|
|
9,472
|
|
|
Thereafter
|
|
|
99,558
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,251,078
|
|
|
$
|
236,197
|
|
|
|
|
|
|
|
|
|
|
|
In November 2010, the Company signed a
build-to-suit
and lease agreement for a new manufacturing, research and
development laboratory and headquarters facility. The agreement
includes an option for the Company to purchase the facility
beginning on the date of rent commencement, which is the earlier
of commencement of business activities on the premises or
30 days after substantial completion of the facility. Under
this 20-year
lease, the Company is obligated to pay annual rent of the
greater of $1.0 million or 10% of the landlord’s
investment in the property; provided that the landlord’s
investment will not exceed $14.3 million. The landlord
purchased the land on which the new facility is being built from
MeadWestvaco, and a portion of the landlord’s financing for
this purchase and the related building development costs comes
from a mortgage granted by an affiliate of MeadWestvaco. In
addition, each of MeadWestvaco, International Paper and Rubicon
are guarantors of a portion of the Company’s obligations
under the lease.
Intangible assets consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization
|
|
As of
|
|
|
As of
|
|
|
As of
|
|
|
|
|
Basis of
|
|
Period
|
|
March 31,
|
|
|
March 31,
|
|
|
December 31,
|
|
|
|
|
Amortization
|
|
(in Years)
|
|
2009
|
|
|
2010
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
Licenses and patents
|
|
Straight-line
|
|
10-17
|
|
$
|
5,235,655
|
|
|
$
|
5,545,087
|
|
|
$
|
5,901,168
|
|
|
Less: Accumulated amortization
|
|
|
|
|
|
|
(3,209,353
|
)
|
|
|
(3,622,207
|
)
|
|
|
(3,767,623
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,026,302
|
|
|
|
1,922,880
|
|
|
|
2,133,545
|
|
|
Patent and license application costs
|
|
|
|
|
|
|
2,014,751
|
|
|
|
2,014,742
|
|
|
|
1,990,296
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
4,041,053
|
|
|
$
|
3,937,622
|
|
|
$
|
4,123,841
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-12
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
Amortization expense is as follows for the subsequent years
ending March 31:
| |
|
|
|
|
|
2011
|
|
$
|
162,323
|
|
|
2012
|
|
|
162,323
|
|
|
2013
|
|
|
162,323
|
|
|
2014
|
|
|
162,323
|
|
|
2015
|
|
|
162,323
|
|
|
Thereafter
|
|
|
1,111,265
|
|
|
|
|
|
|
|
|
|
|
$
|
1,922,880
|
|
|
|
|
|
|
|
Amortization expense for the years ending March 31, 2008,
2009, 2010 was $441,022, $463,831, and $412,854, respectively.
Amortization expense for the nine months ended December 31,
2009 and 2010 was $347,263 and $133,710, respectively.
|
|
|
6.
|
Long-term
Debt and Borrowings
|
The Company’s borrowings consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of March 31,
|
|
|
As of March 31,
|
|
|
As of December 31,
|
|
|
|
|
2009
|
|
|
2010
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
Revolving line of credit, due 2011
|
|
$
|
8,000,000
|
|
|
$
|
10,376,824
|
|
|
$
|
13,626,824
|
|
|
Multi-option credit facility, with foreign lender
|
|
|
—
|
|
|
|
4,251,156
|
|
|
|
4,612,200
|
|
|
Capitalized leases and other obligations
|
|
|
167,841
|
|
|
|
200,694
|
|
|
|
224,798
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total borrowings
|
|
|
8,167,841
|
|
|
|
14,828,674
|
|
|
|
18,463,822
|
|
|
Less current maturities
|
|
|
(8,000,000
|
)
|
|
|
(3,153,472
|
)
|
|
|
(14,107,476
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term debt
|
|
$
|
167,841
|
|
|
$
|
11,675,202
|
|
|
$
|
4,356,346
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following represents the schedule of maturities in the years
ending March 31:
| |
|
|
|
|
|
2011
|
|
$
|
3,153,472
|
|
|
2012
|
|
|
8,033,828
|
|
|
2013
|
|
|
3,593,473
|
|
|
2014
|
|
|
39,967
|
|
|
2015
|
|
|
7,528
|
|
|
Thereafter
|
|
|
406
|
|
|
|
|
|
|
|
|
|
|
$
|
14,828,674
|
|
|
|
|
|
|
|
The Company has a revolving line of credit (“LOC”)
with the National Bank of South Carolina bearing interest at the
prime rate, subject to a minimum annual rate of 5%. The LOC has
a maturity date of May 31, 2011. The line is collateralized
by all U.S. assets of the Company including property,
plant, and equipment, inventory and accounts receivable
(carrying value $20,757,797). The line of credit requires the
Company to maintain an annual net worth of at least $20,000,000
and a tangible net worth of at least $16,000,000. Also, for the
year ended March 31, 2010, the Company’s member owners
were required to make cash equity contributions of up to
$8,900,000 to support research and development expenditures up
to $11,900,000 for the twelve months ended March 31, 2010.
Required capital contributions are reduced to the extent
research and development expenditures are below the $11,900,000
limit. Total research and development for the relevant twelve
months amounted to $11,205,627. The maximum that can be borrowed
under the line is $18,500,000.
F-13
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
The LOC had an interest rate of 4.25%, 5% and 5% at
March 31, 2009 and 2010 and December 31, 2010,
respectively.
The Company was not in compliance with its annual net worth and
tangible net worth covenants under this LOC as of
December 31, 2010. As of December 31, 2010, the
Company’s annual net worth and tangible net worth were
$18,900,530 and $14,776,689, respectively. The Company has
obtained a waiver from the bank for lack of compliance with
these covenants as of February 4, 2011, which waives any
remedies available to the bank as of December 31, 2010 and
for the five month period commencing on January 1, 2011 and
ending on the expiration of this line of credit on May 31,
2011.
The Company, through its wholly owned subsidiary ArborGen New
Zealand Unlimited, has entered into an agreement with Westpac
New Zealand Limited for a multi-option credit facility
(“MOCF”) for an amount up to NZ$6,000,000. As of
March 31, 2010, the MOCF is fully drawn and the balance is
NZ$6,000,000 (US$4,251,156 as of March 31, 2010). This
facility is collateralized by mortgages over ArborGen New
Zealand Unlimited’s land and buildings with an aggregate
book value of $8,201,947 as of March 31, 2010. ArborGen
Australia Pty. Ltd. assets with an aggregate book value of
$1,063,777 are also collateral for mortgages to Westpac New
Zealand Ltd. ArborGen Australia Pty. Ltd. is a guarantor to the
ArborGen New Zealand Unlimited line of credit.
Under the MOCF, ArborGen New Zealand Unlimited is required to
make an annual loan payment of NZ$500,000 (US$345,450).
ArborGen New Zealand Unlimited has met all of the financial
covenants required by Westpac NZ Ltd. as of March 31, 2010
and December 31, 2010, including: EBIT/Interest coverage of
more than 1.75 times, equity ratio of not less than 60% of
adjusted tangible assets, and loan value ratio not to exceed 50%
of secured property.
Deferred
Financing Costs
As of March 31, 2010 and December 31, 2010, deferred
financing costs totaling $162,363 and $34,648, respectively, are
included in other assets on the consolidated balance sheet.
These costs are amortized as interest expense using the
straight-line method. Amortization expense for deferred
financing costs was $14,760 and $159,499 for the year ended
March 31, 2010 and nine months ended December 31,
2010, respectively, and includes the amortization of costs
related to the March 4, 2010 renewal of the line of credit.
Deferred public offering costs of $2,887,753 are included in
other assets as of December 31, 2010.
|
|
|
7.
|
Members’/Stockholders’
Equity and Agreements and Preferred Interest
|
The member companies have the following allocable shares in the
Company:
| |
|
|
|
|
|
MWV
|
|
|
33.33
|
%
|
|
IP
|
|
|
33.33
|
%
|
|
Rubicon
|
|
|
33.33
|
%
|
The Company’s profits and losses are allocated to member
accounts based on respective ownership interest with a stop loss
provision for deficit accounts.
On October 31, 2007, the member companies contributed to
the Company cash, property, plant and equipment and other assets
from their nursery and orchard businesses. The recording of
these contributions at the predecessor companies’ net book
value resulted in the member companies no longer showing equal
members’ equity accounts on the financial statements, even
though their legal ownership is equal as outlined above.
F-14
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
In conjunction with the contribution of the nursery and orchard
businesses from the Company’s members on October 31,
2007, the Board of Directors designated $13,300,000 of IP’s
capital as a Preferred Interest, having the terms, rights, and
privileges set forth in the Amended and Restated LLC Agreement.
The Preferred Interest instrument amount of $13,300,000 was
determined as the excess of the fair value, as determined by the
member companies, of the assets contributed by IP over the fair
value of the assets contributed by the other member companies,
MWV and Rubicon.
In the event of any liquidation event, IP shall be paid prior
and in preference to the holders of Common Interests. IP can, by
written request, redeem its Preferred Interest by payment in
cash, in full or in part, provided that redemption does not take
place before November 1, 2009. If the Company calls for
capital contributions and the Preferred Interest held by a
member at that time is greater than zero, that member can reduce
the amount of the Preferred Interest in lieu of cash payment. IP
exercised this clause for the capital contribution requests for
the years ended March 31, 2008, 2009, and 2010. The cash
contribution requests resulted in reductions of the Preferred
Interest of $2,431,770, $4,333,334, and $4,382,950 for the years
ended March 31, 2008, 2009, and 2010, respectively. The
balance of the Preferred Interest was $2,151,947 as of
March 31, 2010.
IP exercised its redemption of preferred interest in lieu of
cash for the April 2010 capital contribution of $1,100,000, and
for $1,051,947 of the capital contribution for May 2010. As of
May 2010, the preferred interest balance was reduced to zero. IP
made a cash contribution on May 17, 2010 of $198,703.
From April 1, 2010 through May 31, 2010, the Company
received cash contributions from member companies totaling
$4,900,003. A reduction of preferred interest in lieu of cash of
$2,151,947 as of May 31, 2010 was made for IP, reducing the
preferred interest to zero. Upon conversion of the limited
liability company into a corporation on June 1, 2010, the
members received a total of 30,000,000 common shares for their
member interests (10,000,000 shares per member), at $0.001
per share. Between June 1 and December 31, 2010 the
members had contributed an additional $7,948,050 in exchange for
529,866 shares of common stock at $0.001 per share.
MWV and Rubicon each made cash contributions of $4,382,950
during the year ended March 31, 2010. A reduction of
preferred interest in lieu of cash of $4,382,950 for the year
ended March 31, 2010 was made for IP.
MWV and Rubicon each made cash contributions of $4,333,334
during the year ended March 31, 2009. A reduction of
preferred interest in lieu of cash of $4,333,334 for the year
ended March 31, 2009 was made for IP.
MWV and Rubicon each made cash contributions of $4,863,540
during the year ending March 31, 2008. IP made cash
contributions of $2,431,770 and a reduction of preferred
interest in lieu of cash of $2,431,770 for the year ending
March 31, 2008.
|
|
|
8.
|
Transactions
With Related Parties
|
During the years ended March 31, 2008, 2009, 2010 and the
nine months ended December 31, 2009 and 2010, the Company
had various transactions with its member companies that had a
material impact on its consolidated financial results. These
transactions include sales to member companies, rental expense
paid to member companies, and licensing agreements with member
companies that require payment of royalties on
F-15
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
future revenues utilizing certain technologies. The impacts on
the consolidated financial results for related party
transactions are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of
|
|
|
As of
|
|
|
As of
|
|
|
|
|
March 31, 2009
|
|
|
March 31, 2010
|
|
|
December 31, 2010
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
Accounts receivable — member companies
|
|
$
|
254,347
|
|
|
$
|
272,683
|
|
|
$
|
52,956
|
|
|
Accounts payable — member companies
|
|
|
133,787
|
|
|
|
332,139
|
|
|
|
389,290
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
|
|
|
|
|
Years Ended March 31,
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
Revenue — member companies
|
|
$
|
460,912
|
|
|
$
|
743,314
|
|
|
$
|
905,162
|
|
|
$
|
358,290
|
|
|
$
|
179,647
|
|
|
Cost of revenue — member companies
|
|
|
317,890
|
|
|
|
109,675
|
|
|
|
5,150
|
|
|
|
5,000
|
|
|
|
18,860
|
|
|
Research and development — member companies
|
|
|
553,335
|
|
|
|
538,257
|
|
|
|
511,482
|
|
|
|
458,555
|
|
|
|
10,375
|
|
|
Selling, general and administrative — member companies
|
|
|
1,592,203
|
|
|
|
1,450,813
|
|
|
|
1,198,705
|
|
|
|
1,048,000
|
|
|
|
1,285,480
|
|
|
|
|
9.
|
Long-Term
Incentive Plans
|
As of March 31, 2010 the Company had two Long-Term
Incentive plans in place: the 2002 Value Added Plan, which
became effective January 1, 2002, and the 2003 Value Added
Plan, which became effective July 1, 2003. The two plans
(“NVA Plans”) provide employees the opportunity to
share in the growth of the equity value of the Company over
time. Employees who participate in the NVA Plans will, upon a
Sale Event, and subject to the terms and conditions of the Plan,
share in the increase in the value of the Company once the
investment contributions made by the members to the business
(including a financial return on that investment) have been
satisfied.
A Sale Event is defined in both plans as (i) the Company
and/or any
one or more of the holders of the equity interests of the
Company sell, in the aggregate, more than 10% of the aggregate
amount of the equity interest in the Company outstanding
immediately after such sale to Disinterested Persons in an
underwritten public offering of its equity securities registered
under the Securities Act of 1933, as amended, (ii) the
acquisition by any Disinterested Person(s) of more than 51% of
the outstanding voting interests of the Company held by the
members of the Company, or (iii) the sale of all or
substantially all of the assets of the Company.
The Company shall redeem a Participant’s Vested NVA Units
within sixty days, unless an underwriter specifies a longer term
(up to twelve months), following an underwritten public
offering. The amount of the redemption payment per NVA Unit
shall be equal to the difference between the Final Value of each
unit defined by the value of the Sale Event and the Adjusted
Initial Value with respect to that NVA Unit.
The Adjusted Initial Value formula differs between the 2002 and
2003 Plans, with the former requiring a 15% return on the
capital contributions made after the date the units are granted
(“Grant Date”) to the employee, whereas the 2003 Plan
also takes into account a 15% return on the capital contributed
by the members prior to the Grant Date.
Under the NVA Plans, a total of 10,000,000 units exist, of
which 85% of the units are to be distributed to the member
companies and 15% are available for award to employees at the
discretion of the Company’s Board of Directors. Initial
units awarded vest two years after their award, while subsequent
units vest one year following the award.
F-16
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
At March 31, 2010, a total of 685,500 units had been
awarded and are fully vested. These units consist of 658,750
under the 2002 Plan and 26,750 under the 2003 Plan.
Effective June 1, 2010, the Company replaced the awards
issued under the 2002 Value Added Plan and the 2003 Value Added
Plan with Appreciation Right Agreements (the “AR
Agreements”). The AR Agreements provide employees with
appreciation rights (“ARs”) that allow the employee
the opportunity to share in the growth of the equity value of
the Company over time. Employees who have ARs will, upon a Sale
Event or an IPO, as defined, and subject to the terms and
conditions of the AR Agreements, share in the increase in the
value of the Company once the equity investments in the Company
(including a specified financial return on that investment) have
been satisfied.
A Sale Event is defined in the AR Agreements as the consummation
of (i) the sale of all or substantially all of the assets
of the Company on a consolidated basis to an unrelated person or
entity, (ii) the acquisition of more than 51% of the
outstanding voting stock of the Company in a single transaction
or a series of related transactions by a person or group of
persons, or (iii) any other acquisition or merger of the
business of the Company, as determined by the Board of Directors
of the Company; provided, however, that any capital raising
event, or a merger effected solely to change the Company’s
domicile shall not constitute a Sale Event. An IPO is defined in
the AR Agreements as the consummation of the first firm
commitment underwritten public offering pursuant to an effective
registration statement under the Securities Act covering the
offer and sale by the Company of its equity securities and
relating to at least 10% of the Company’s equity
securities, as a result of or following which the Common Stock
shall be publicly held.
The ARs are granted unvested and only become vested upon the
first to occur of an IPO or Sale Event as long as the recipient
has remained employed by the Company through that date. The ARs
are required to be settled (in cash or stock at the
Company’s election) on or within 60 days following the
vesting date. The amount of the settlement payment per AR shall
be equal to the difference between the Final Value of each AR
defined by the value of the Sale Event or IPO and the Adjusted
Initial Value with respect to that AR.
At December 31, 2010, a total of 532,250 ARs had been
awarded and are unvested. The Adjusted Initial Value is $47.84
for 212,500 of the ARs and $45.97 for 319,750 of the ARs.
Under the provisions of ASC 718, the awards issued under
the NVA Plans and the AR Agreements are considered
stock-appreciation rights, which, when triggered, would require
the Company to record compensation expense for the fair market
value of the units. As the units would not be paid until there
is a Sale Event or IPO (as defined above), this is deemed the
triggering event. No such event has occurred as of
December 31, 2010; as such, no related compensation expense
has been recorded.
|
|
|
10.
|
Restructuring
Expense
|
During the year ended March 31, 2008, the Company received
the seed orchards and the nursery businesses as contributions
from its member companies including physical operations, certain
land holdings, research and development, and intellectual
property including pine and hardwood germplasm. As a result of
the contributions, the Company recorded restructuring costs of
$182,983 for employee separation costs.
During the year ended March 31, 2009, as a result of market
conditions and a strategic business assessment, the Company
recorded restructuring costs of $492,392, primarily the result
of employee related separation costs at a South Carolina and a
New Zealand facility.
During the year ended March 31, 2010, the Company continued
its review of the seed orchards and nursery businesses acquired
as a part of the October 31, 2007 contributions from its
member companies. As a result of this business assessment for
the year ended March 31, 2010, the Company recorded
restructuring costs of $100,902, primarily due to the temporary
suspension of operations at the Livingston, Texas facility and
resultant employee separation costs.
F-17
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
| |
|
|
|
|
|
|
|
Workforce
|
|
|
|
|
Reduction
|
|
|
|
|
Accrual balance April 1, 2007
|
|
$
|
—
|
|
|
Restructuring charges
|
|
|
182,983
|
|
|
Cash payments
|
|
|
—
|
|
|
|
|
|
|
|
|
Accrual balance March 31, 2008
|
|
|
182,983
|
|
|
Restructuring charges
|
|
|
492,392
|
|
|
Cash payments
|
|
|
(333,375
|
)
|
|
|
|
|
|
|
|
Accrual balance March 31, 2009
|
|
|
342,000
|
|
|
Restructuring charges
|
|
|
100,902
|
|
|
Cash payments
|
|
|
(340,685
|
)
|
|
|
|
|
|
|
|
Accrual balance March 31, 2010
|
|
|
102,217
|
|
|
Cash payments (unaudited)
|
|
|
(102,217
|
)
|
|
|
|
|
|
|
|
Accrual balance December 31, 2010 (unaudited)
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
11.
|
Commitments
and Contingencies
|
Included in prepaid expenses as of March 31, 2010 is
$302,093 of engineering costs related to a planned new global
headquarters facility in Summerville, South Carolina. As of
December 2010, the Company has been reimbursed for this amount
from the building contractor.
The Company is involved in certain claims arising in the normal
course of business. Management believes that the eventual
outcome of these claims will not have a material effect on the
Company’s operations, financial position or cash flows.
|
|
|
12.
|
Fair
Value Measurements
|
The following tables summarize the fair values of financial
instruments measured at fair value on a recurring basis at
March 31, 2010, 2009 and December 31, 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Items Measured at Fair Value on a Recurring Basis
|
|
|
|
|
Quoted Price in
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Active Markets for
|
|
|
Significant Other
|
|
|
Significant
|
|
|
|
|
|
|
|
Identical Assets
|
|
|
Observable Inputs
|
|
|
Unobservable Inputs
|
|
|
Balance
|
|
|
|
|
(Level 1)
|
|
|
(Level 2)
|
|
|
(Level 3)
|
|
|
|
|
|
|
|
Assets, December 31, 2010 (unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
2,133,421
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
2,133,421
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities, December 31, 2010 (unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest rate swap agreement
|
|
$
|
—
|
|
|
$
|
62,324
|
|
|
$
|
—
|
|
|
$
|
62,324
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets, March 31, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
3,742,048
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
3,742,048
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets, March 31, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
1,624,330
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
1,624,330
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-18
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
Cash and all highly liquid investments with a maturity of
90 days or less at date of purchase are classified as cash
and cash equivalents.
The Company periodically enters into interest-rate swap
agreements to moderate its exposure to interest-rate changes and
to lower its overall cost of borrowing. Fair value measurements
for the Company’s derivatives are classified under
Level 2 because such measurements are determined using
published market prices or estimated based on observable inputs
such as interest rates. Interest-rate swap agreements are
recorded in accrued expenses on the Company’s balance sheet.
In accordance with the provisions of the ASC 360,
Impairment or Disposal of Long-Lived Assets, intangible assets
with a carrying amount of $202,430, $643,389 and $213,124 were
written down to their fair value of $0 as of March 31,
2008, 2009 and 2010, respectively. For the nine months ended
December 31, 2009 and 2010 intangible assets with a
carrying amount of $199,960 and $154,019, respectively, were
written down to their fair value of $0. These non-recurring fair
value measurements were made utilizing significant unobservable
(level 3) inputs under ASC 820. This valuation
was based on the Company’s strategic decision to no longer
actively pursue these
patents-in-progress;
therefore, management implied a fair value of $0.
ASC 280, Disclosures About Segments of an Enterprise and Related
Information, or ASC 280, establishes standards for
reporting information about operating segments. It defines
operating segments as components of an enterprise about which
separate financial information is available that is evaluated
regularly by the chief operating decision-maker, or
decision-making group, in deciding how to allocate resources and
in assessing performance. The Company’s chief operating
decision-maker is the President and Chief Executive Officer, who
reviews financial information on a consolidated basis and
aggregates the enterprise into one operating segment for
purposes of allocating resources and evaluating financial
performance. Accordingly, the Company reports as a single
operating segment.
For geographic reporting, revenues are attributed to the
geographic location in which the customers’ facilities are
located. Long-lived assets consist primarily of property, plant,
and equipment attributed to the geographic location in which
they are located. Net revenues and long-lived assets by
geographic region are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Nine Months
|
|
|
|
|
For the Years Ended March 31,
|
|
|
Ended December 31,
|
|
|
Revenues:
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
United States
|
|
$
|
17,891,149
|
|
|
$
|
17,745,598
|
|
|
$
|
16,435,626
|
|
|
$
|
4,377,824
|
|
|
$
|
5,338,377
|
|
|
New Zealand and Australia
|
|
|
290,686
|
|
|
|
5,934,081
|
|
|
|
5,140,033
|
|
|
|
5,204,969
|
|
|
|
5,744,852
|
|
|
Brazil
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of March 31,
|
|
|
As of December 31,
|
|
|
Property, Plant and Equipment, Net:
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
United States
|
|
$
|
8,713,532
|
|
|
$
|
8,591,935
|
|
|
$
|
8,634,564
|
|
|
$
|
9,110,050
|
|
|
New Zealand and Australia
|
|
|
9,892,986
|
|
|
|
7,453,669
|
|
|
|
9,265,724
|
|
|
|
9,985,094
|
|
|
Brazil
|
|
|
36,183
|
|
|
|
30,119
|
|
|
|
39,128
|
|
|
|
40,068
|
|
|
|
|
14.
|
Unaudited
income taxes
|
Historically, the Company has operated as a limited liability
company for U.S. tax purposes. As such, any U.S. and
state income tax liability or benefit has passed through to its
members. Accordingly, the Company
F-19
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
has not historically recorded any domestic income tax expense or
benefit in its consolidated statements of operations or income
tax assets or liabilities on its consolidated balance sheets.
The Company converted to a corporation on June 1, 2010 and
will be liable for U.S. and state income tax prospectively.
Thus, the Company has provided for income taxes on its domestic
operations subsequent to the conversion. The Company generated
net losses for the period June 1 through December 31, 2010.
Due to the recording of a full valuation allowance, no domestic
income tax expense or benefit has been recorded in the current
year.
The Company has historically been subject to corporate tax in
foreign jurisdictions, but has never been profitable. Due to the
recording of a full valuation allowance, no foreign income tax
expense or benefit has ever been recorded historically or in the
current year.
Deferred income taxes reflect the net effects of temporary
differences between the carrying amounts of assets and
liabilities calculated for financial reporting purposes and the
amounts calculated for the Company’s income tax returns
prepared in accordance with tax regulations and the net tax
effects of operating loss and tax credit carry-forwards. The
items that give rise to the Company’s deferred tax assets
are as follows:
| |
|
|
|
|
|
|
|
As of
|
|
|
|
|
December 31, 2010
|
|
|
|
|
(Unaudited)
|
|
|
|
|
Deferred tax liabilities:
|
|
|
|
|
|
Property, plant and equipment
|
|
$
|
(2,059,105
|
)
|
|
Fumigation costs
|
|
|
(48,804
|
)
|
|
|
|
|
|
|
|
Total deferred tax liabilities
|
|
|
(2,107,909
|
)
|
|
Deferred tax assets:
|
|
|
|
|
|
Amortization of intangibles
|
|
|
2,585,277
|
|
|
Accrued vacation
|
|
|
208,424
|
|
|
Accrued bonuses
|
|
|
526,235
|
|
|
Deferred revenue
|
|
|
644,437
|
|
|
Allowance for doubtful accounts
|
|
|
242,967
|
|
|
Inventory reserve
|
|
|
450,275
|
|
|
Replacement seedling accrual
|
|
|
8,853
|
|
|
Net operating loss carry-forward
|
|
|
4,921,201
|
|
|
|
|
|
|
|
|
Total deferred tax assets
|
|
|
9,587,669
|
|
|
|
|
|
|
|
|
Net deferred tax assets before valuation allowance
|
|
|
7,479,760
|
|
|
Valuation allowance for deferred tax assets
|
|
|
(7,479,760
|
)
|
|
|
|
|
|
|
|
Net deferred taxes
|
|
$
|
—
|
|
|
|
|
|
|
|
The ultimate realization of deferred tax assets depends on the
generation of sufficient taxable income of the appropriate
character and in the appropriate taxing jurisdictions during the
future periods in which the related temporary differences become
deductible. The Company determined the valuation allowance on
its deferred tax assets in accordance with the provisions of ASC
740, which require weight of both positive and negative evidence
in order to ascertain whether it is more likely than not that
deferred tax assets would be realized. The Company evaluated all
significant available positive and negative evidence, including
the existence of cumulative net losses, benefits that could be
realized from available tax strategies and forecasts of future
taxable income, in determining the need for a valuation
allowance on its deferred tax assets. After applying the
evaluation guidance of ASC 740, the Company determined that
is was necessary to record a
F-20
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
valuation allowance against all of its net deferred tax assets
as the Company does not believe it is more likely than not that
the deferred tax assets will be realized.
As of December 31, 2010, the net operating loss
carry-forward available to the company subsequent to converting
to a corporation is approximately $8,136,000 in the US, and
$6,496,000 in the foreign jurisdictions. The
U.S. carry-forward will begin to expire in 2031 and the
foreign carry-forwards do not expire. A full valuation allowance
has been recorded against net deferred tax assets as of
December 31, 2010; accordingly, there is no income tax
benefit for the period ended December 31, 2010.
On a pro forma basis, no income tax benefit is recorded for any
period in any jurisdiction due to the recording of valuation
allowances on net deferred tax assets. Accordingly, there is no
difference between pro forma net loss and pro forma net loss per
share and the net loss and net loss per share as presented on
the consolidated statement of operations.
|
|
|
15.
|
Subsequent
Events (Unaudited)
|
The Company evaluated transactions occurring after
March 31, 2010 in accordance with ASC 855, Subsequent
Events, through July 22, 2010, which was the original date
of the issuance of the financial statements. The Company has
also evaluated transactions through September 30, 2010, the
date of reissuance of these financial statements and through
February 24, 2011, the date the unaudited interim financial
statements were issued, and noted no items requiring adjustment
of the financial statements. In January 2011, the Company issued
promissory notes to its three stockholders in the aggregate
amount of $1,200,000. Pursuant to the terms of the $400,000 note
to each stockholder, interest accrues at a rate of LIBOR plus
3%, and each note matures on the earlier of July 15, 2011
or seven business days following either the closing of this
offering or the Company’s receipt of proceeds from other
sales of capital stock sufficient to repay the note.
F-21
ArborGen,
LLC
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS — (Continued)
Financial
statement schedule
ArborGen,
LLC
Valuation
and qualifying accounts
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
|
|
|
Charges
|
|
|
|
|
|
|
|
|
|
|
Beginning
|
|
|
(Credited)
|
|
|
|
|
|
Balance at
|
|
|
Allowance for Doubtful Accounts
|
|
of Year
|
|
|
to Expenses
|
|
|
Deductions
|
|
|
End of Year
|
|
|
|
|
Year ended March 31, 2008
|
|
|
—
|
|
|
|
200,000
|
|
|
|
—
|
|
|
|
200,000
|
|
|
Year ended March 31, 2009
|
|
|
200,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
200,000
|
|
|
Year ended March 31, 2010
|
|
|
200,000
|
|
|
|
—
|
|
|
|
(75,000
|
)
|
|
|
125,000
|
|
|
Nine months ended December 31, 2010 (unaudited)
|
|
|
125,000
|
|
|
|
326,053
|
|
|
|
(15,000
|
)
|
|
|
436,053
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
|
|
|
Charges
|
|
|
|
|
|
|
|
|
|
|
Beginning
|
|
|
(Credited)
|
|
|
|
|
|
Balance at
|
|
|
Reserve for Slow Moving Seed
|
|
of Year
|
|
|
to Expenses
|
|
|
Deductions
|
|
|
End of Year
|
|
|
|
|
Year ended March 31, 2008
|
|
|
—
|
|
|
|
771,723
|
|
|
|
—
|
|
|
|
771,723
|
|
|
Year ended March 31, 2009
|
|
|
771,723
|
|
|
|
—
|
|
|
|
(348,176
|
)
|
|
|
423,547
|
|
|
Year ended March 31, 2010
|
|
|
423,547
|
|
|
|
852,875
|
|
|
|
—
|
|
|
|
1,276,422
|
|
|
Nine months ended December 31, 2010 (unaudited)
|
|
|
1,276,422
|
|
|
|
—
|
|
|
|
(4,905
|
)
|
|
|
1,271,517
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
|
|
|
Charges
|
|
|
|
|
|
|
|
|
|
|
Beginning
|
|
|
(Credited)
|
|
|
|
|
|
Balance at
|
|
|
Seedling Customer Allowance
|
|
of Year
|
|
|
to Expenses
|
|
|
Deductions
|
|
|
End of Year
|
|
|
|
|
Year ended March 31, 2008
|
|
|
—
|
|
|
|
572,000
|
|
|
|
—
|
|
|
|
572,000
|
|
|
Year ended March 31, 2009
|
|
|
572,000
|
|
|
|
—
|
|
|
|
(394,472
|
)
|
|
|
177,528
|
|
|
Year ended March 31, 2010
|
|
|
177,528
|
|
|
|
—
|
|
|
|
(152,528
|
)
|
|
|
25,000
|
|
|
Nine months ended December 31, 2010 (unaudited)
|
|
|
25,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
25,000
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
|
|
|
Charges
|
|
|
|
|
|
|
|
|
|
|
Beginning
|
|
|
(Credited)
|
|
|
|
|
|
Balance at
|
|
|
Deferred Tax Asset Valuation Allowance
|
|
of Year
|
|
|
to Expenses
|
|
|
Deductions
|
|
|
End of Year
|
|
|
|
|
Nine months ended December 31, 2010 (unaudited)
|
|
|
—
|
|
|
|
7,479,760
|
(1)
|
|
|
—
|
|
|
|
7,479,760
|
|
|
|
|
|
(1) |
|
Balance includes $5,078,837 recorded against net deferred tax
assets existing at June 1, 2010, the date of conversion
from a Limited Liability Company to a taxable Corporation. |
F-22
Shares
ArborGen Inc.
Common Stock
PROSPECTUS
|
|
| Goldman,
Sachs & Co. |
Citi |
|
|
| Piper
Jaffray |
RBC Capital Markets |
Through and
including ,
2011 (the 25th day after the date of this prospectus), all
dealers effecting transactions in these securities, whether or
not participating in this offering, may be required to deliver a
prospectus. This is an addition to a dealer’s obligation to
deliver a prospectus when acting as an underwriter and with
respect to an unsold allotment or subscription.
PART II
INFORMATION
NOT REQUIRED IN PROSPECTUS
|
|
|
Item 13.
|
Other
Expenses of Issuance and Distribution
|
The following table sets forth all expenses, other than the
underwriting discounts, payable by the registrant in connection
with the sale of the common stock being registered. All the
amounts shown are estimates except the SEC registration fee and
the FINRA filing fee.
| |
|
|
|
|
|
|
|
Total
|
|
|
|
|
SEC registration fee
|
|
$
|
5,348
|
|
|
FINRA filing fee
|
|
$
|
8,000
|
|
|
Nasdaq Global Market initial listing fee
|
|
$
|
*
|
|
|
Printing and engraving expenses
|
|
$
|
*
|
|
|
Legal fees and expenses
|
|
$
|
*
|
|
|
Accounting fees and expenses
|
|
$
|
*
|
|
|
Transfer agent and registrar fees
|
|
$
|
*
|
|
|
Miscellaneous
|
|
$
|
*
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
*
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
To be filed by amendment. |
|
|
|
Item 14.
|
Indemnification
of Directors and Officers
|
Section 145 of the Delaware General Corporation Law permits
a corporation to include in its charter documents, and in
agreements between the corporation and its directors and
officers, provisions expanding the scope of indemnification
beyond that specifically provided by the current law.
The Registrant’s amended and restated certificate of
incorporation provides for the indemnification of directors to
the fullest extent permissible under Delaware law.
The Registrant’s amended and restated by-laws, which will
be effective upon the completion of this offering, provide for
the indemnification of officers, directors and third parties
acting on the Registrant’s behalf if such persons act in
good faith and in a manner reasonably believed to be in and not
opposed to the Registrant’s best interest, and, with
respect to any criminal action or proceeding, such indemnified
party had no reason to believe his or her conduct was unlawful.
The Registrant is entering into indemnification agreements with
each of its directors and executive officers, in addition to the
indemnification provisions provided for in its charter
documents, and the Registrant intends to enter into
indemnification agreements with any new directors and executive
officers in the future.
The underwriting agreement (filed as Exhibit 1.1 hereto)
will provide for indemnification by the underwriters, severally
and not jointly, of the Registrant, its directors and its
officers who sign this Registration Statement with respect to
losses arising from misstatements or omissions in the
Registration Statement or prospectus with reference to
information relating to such underwriters furnished to the
Registrant in writing by such underwriters expressly for use
herein.
The Registrant intends to purchase and maintain insurance on
behalf of any person who is or was a director or officer against
any loss arising from any claim asserted against him or her and
incurred by him or her in that capacity, subject to certain
exclusions and limits of the amount of coverage.
II-1
|
|
|
Item 15.
|
Recent
Sales of Unregistered Securities
|
In the three years preceding the filing of this registration
statement, we have issued the following securities that were not
registered under the Securities Act:
Issuances
of Capital Stock
On June 1, 2010, ArborGen, LLC, a Delaware limited
liability company, converted into ArborGen Inc., a Delaware
corporation, and all of the outstanding membership interests of
ArborGen, LLC converted into 30,000,000 shares of common
stock of ArborGen Inc.
On June 15, 2010, we issued and sold an aggregate of
133,329 shares of common stock to our three stockholders
for an aggregate purchase price of $1,999,950. These shares were
sold pursuant to our Stockholders Agreement and in reliance upon
the exemption from the registration requirements of the
Securities Act available under Section 4(2) of the
Securities Act.
On July 15, 2010, we issued and sold an aggregate of
166,659 shares of common stock to our three stockholders
for an aggregate purchase price of $2,499,900. These shares were
sold pursuant to our Stockholders Agreement and in reliance upon
the exemption from the registration requirements of the
Securities Act available under Section 4(2) of the
Securities Act.
On August 15, 2010, we issued and sold an aggregate of
93,318 shares of common stock to our three stockholders for
an aggregate purchase price of $1,399,800. These shares were
sold pursuant to our Stockholders Agreement and in reliance upon
the exemption from the registration requirements of the
Securities Act available under Section 4(2) of the
Securities Act.
On September 15, 2010, we issued and sold an aggregate of
136,560 shares of common stock to our three stockholders
for an aggregate purchase price of $2,048,400. These shares were
sold pursuant to our Stockholders Agreement and in reliance upon
the exemption from the registration requirements of the
Securities Act available under Section 4(2) of the
Securities Act.
|
|
|
Item 16.
|
Exhibits
and Financial Statement Schedules
|
The exhibits to the registration statement are listed in the
Exhibit Index to this registration statement and are
incorporated herein by reference.
|
|
|
(b)
|
Financial
Statement Schedule.
|
The financial statement schedule can be found in the
consolidated financial statements section of this registration
statement under the heading “Schedule II —
Valuation and Qualifying Accounts” and is incorporated
herein by reference.
Insofar as indemnification for liabilities arising under the
Securities Act of 1933, as amended, may be permitted to
directors, officers and controlling persons of the Registrant
pursuant to the foregoing provisions, or otherwise, the
Registrant has been advised that in the opinion of the
Securities and Exchange Commission such indemnification is
against public policy as expressed in the Securities Act and is,
therefore, unenforceable. In the event that a claim for
indemnification against such liabilities (other than the payment
by the Registrant of expenses incurred or paid by a director,
officer or controlling person of the Registrant in the
successful defense of any action, suit or proceeding) is
asserted by such director, officer or controlling person in
connection with the securities being registered, the Registrant
will, unless in the opinion of its counsel the matter has been
settled by controlling precedent, submit to a court of
appropriate jurisdiction the question whether such
indemnification by it is against public policy as expressed in
the Securities Act of 1933 and will be governed by the final
adjudication of such issue.
II-2
The Registrant hereby undertakes that:
(a) The Registrant will provide to the underwriters at the
closing as specified in the underwriting agreement, certificates
in such denominations and registered in such names as required
by the underwriters to permit prompt delivery to each purchaser.
(b) For purposes of determining any liability under the
Securities Act, the information omitted from the form of
prospectus filed as part of this registration statement in
reliance upon Rule 430A and contained in a form of
prospectus filed by the Registrant pursuant to
Rule 424(b)(1) or (4) or 497(h) under the Securities
Act shall be deemed to be part of this registration statement as
of the time it was declared effective.
(c) For the purpose of determining any liability under the
Securities Act each post-effective amendment that contains a
form of prospectus shall be deemed to be a new registration
statement relating to the securities offered therein, and the
offering of such securities at that time shall be deemed to be
the initial bona fide offering thereof.
II-3
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, the Registrant has duly caused this Amendment
No. 6 to the Registration Statement on
Form S-1
to be signed on its behalf by the undersigned, thereunto duly
authorized, in the Town of Summerville, the State of South
Carolina, on the 20th day of April, 2011.
ARBORGEN INC.
|
|
|
| |
By:
|
/s/ Barbara
H. Wells, Ph.D.
|
Barbara H. Wells, Ph.D.
President and Chief Executive Officer
Pursuant to the requirements of the Securities Act of 1933, as
amended, this Amendment No. 6 to the Registration Statement
has been signed by the following persons in the capacities
indicated below on the 20th day of April, 2011.
| |
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
/s/ Barbara
H. Wells, Ph.D.
Barbara
H. Wells, Ph.D.
|
|
President and Chief Executive Officer
(Principal Executive Officer)
|
|
|
|
|
|
/s/ Geoffrey
P. Clear
Geoffrey
P. Clear
|
|
Senior Vice President and Chief Financial Officer
(Principal Financial and Accounting Officer)
|
|
|
|
|
|
*
Bruce
G. Burton, Ph.D.
|
|
Director
|
|
|
|
|
|
*
M.
Eugene Hundley
|
|
Director
|
|
|
|
|
|
*
David
A. Liebetreu
|
|
Director
|
|
|
|
|
|
*
S.
Luke Moriarty
|
|
Director
|
|
|
|
|
|
*
Kenneth
R. Munson, Ph.D.
|
|
Director
|
|
|
|
|
|
*
Mark
T. Watkins, Sr.
|
|
Director
|
|
|
|
|
|
|
|
*By
|
|
/s/ Geoffrey
P. Clear
Attorney-in-fact
|
|
|
II-4
EXHIBIT INDEX
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
1
|
.1*
|
|
Form of Underwriting Agreement
|
|
|
3
|
.1**
|
|
Form of Amended and Restated Certificate of Incorporation of the
Registrant
|
|
|
3
|
.2**
|
|
Amended and Restated By-laws of the Registrant
|
|
|
4
|
.1**
|
|
Specimen Common Stock Certificate
|
|
|
4
|
.2*
|
|
Stockholders Agreement by and among the Registrant, Rubicon
Industries USA, LLC, International Paper Company and
MeadWestvaco Corporation dated as of June 1, 2010
|
|
|
5
|
.1*
|
|
Opinion of Goodwin Procter LLP
|
|
|
10
|
.1*
|
|
Lease Agreement by and between MeadWestvaco Forestry, LLC and
ArborGen, LLC dated as of May 3, 2005, as amended
|
|
|
10
|
.2**
|
|
Build to Suit and Lease Agreement by and between Forestry
Research Holdings, LLC and the Registrant dated as of
November 24, 2010
|
|
|
10
|
.3**
|
|
2010 Amended and Restated Revolving and Non-Revolving Credit
Loan Agreement by and between The National Bank of South
Carolina and ArborGen, LLC dated as of March 4, 2010
|
|
|
10
|
.4**
|
|
Multi Option Credit Facility by and between Westpac New Zealand
Limited and ArborGen New Zealand Unlimited dated as of
October 28, 2009
|
|
|
10
|
.5**
|
|
Registrant’s 2010 Stock Option and Incentive Plan and forms
of agreements thereunder
|
|
|
10
|
.6**
|
|
Form of Appreciation Right Agreement
|
|
|
10
|
.7**
|
|
Form of Indemnification Agreement between the Registrant and its
Directors and Executive Officers
|
|
|
10
|
.8**
|
|
Registrant’s Senior Executive Incentive Bonus Plan
|
|
|
10
|
.9*
|
|
Executive Employment Agreement between the Registrant and
Barbara H. Wells, Ph.D. dated as of January 20, 2011
|
|
|
10
|
.10*
|
|
Executive Employment Agreement between the Registrant and
Geoffrey P. Clear dated as of January 31, 2011
|
|
|
10
|
.11*
|
|
Executive Employment Agreement between the Registrant and Wayne
A. Barfield dated as of January 20, 2011
|
|
|
10
|
.12*
|
|
Executive Employment Agreement between the Registrant and David
M. Nothmann dated as of January 31, 2011
|
|
|
10
|
.13*
|
|
Form of Promissory Note and Agreement executed by the Registrant
in favor of each of International Paper Company, MeadWestvaco
Corporation and Rubicon Industries USA, LLC dated
January 13, 2011
|
|
|
21
|
.1**
|
|
List of Subsidiaries of the Registrant
|
|
|
23
|
.1
|
|
Consent of PricewaterhouseCoopers LLP, Independent Registered
Public Accounting Firm
|
|
|
23
|
.2*
|
|
Consent of Goodwin Procter LLP (included in Exhibit 5.1)
|
|
|
23
|
.3**
|
|
Consent of Resource Information Systems, Inc.
|
|
|
23
|
.4**
|
|
Consent of Edward T. Shonsey
|
|
|
24
|
.1**
|
|
Power of Attorney (included on signature page)
|
|
|
|
|
* |
|
To be filed by amendment. |
** Previously filed.