Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HeartWare International, Inc. | c15651e8vk.htm |
| EX-99.2 - EXHIBIT 99.2 - HeartWare International, Inc. | c15651exv99w2.htm |
Exhibit 99.1

| Evaluation of the HeartWare HVAD Left Ventricular Assist System for the Treatment of Advanced Heart Failure: Results of the ADVANCE Bridge to Transplant Trial and Update with Continued Access Patients M Slaughter, K Aaronson, S Boyce, LW Miller, EC McGee, WG Cotts, M Acker, ML Jessup, ID Gregoric, P Loyalka, V Jeevanandam, AS Anderson, RL Kormos, JJ Teuteberg, FD Pagani, DR Hathaway for the HeartWare ADVANCE InvestigatorsISHLT April 2011 Data cut-off Feb 28, 2011 |
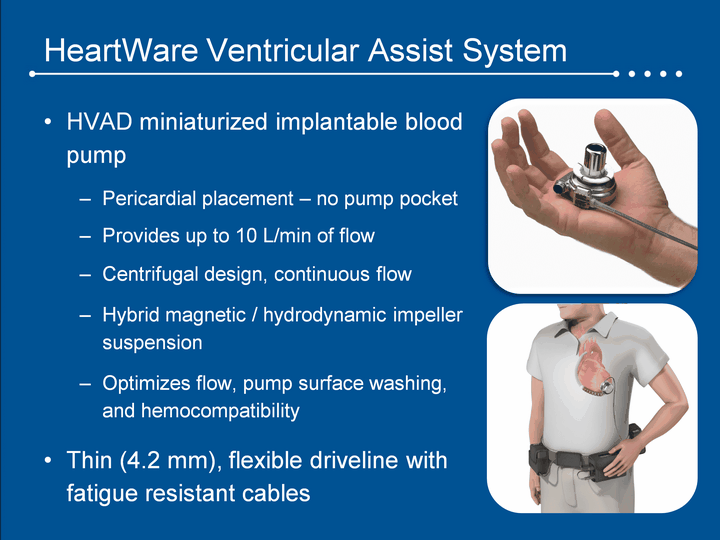
| HeartWare Ventricular Assist System HVAD miniaturized implantable blood pump Pericardial placement - no pump pocketProvides up to 10 L/min of flowCentrifugal design, continuous flowHybrid magnetic / hydrodynamic impeller suspensionOptimizes flow, pump surface washing, and hemocompatibilityThin (4.2 mm), flexible driveline with fatigue resistant cables |

| Multi-site (30), prospective trial to evaluate the HeartWare HVAD as a bridge-to-transplant in the USEnrollment period was August 2008 to February 2010 All patients were followed for ^ 180 days following implant or until cardiac transplantation, device explant for recovery or deathTreatment group was compared to a contemporaneous control group consisting of patients enrolled in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) who received a commercially available left ventricular assist device as a BTT ADVANCE Trial Design |

| "Success" was 92% - survival to 180 days on original device or transplantSurvival at 180 days was 94% Non-inferiority to INTERMACS control was established (p < 0.0001) Recap of Results of the ADVANCE Trial |
| Continued Access Protocol for ADVANCE Trial Continued access for the bridge-to-transplant indication was approved for the 30 sites that enrolled at least 1 patient in ADVANCEThe same enrollment protocol is in effect for CAP 3 allotments of additional patients (54, 54 and 94) have been granted by the FDA for a total of 202 patients |
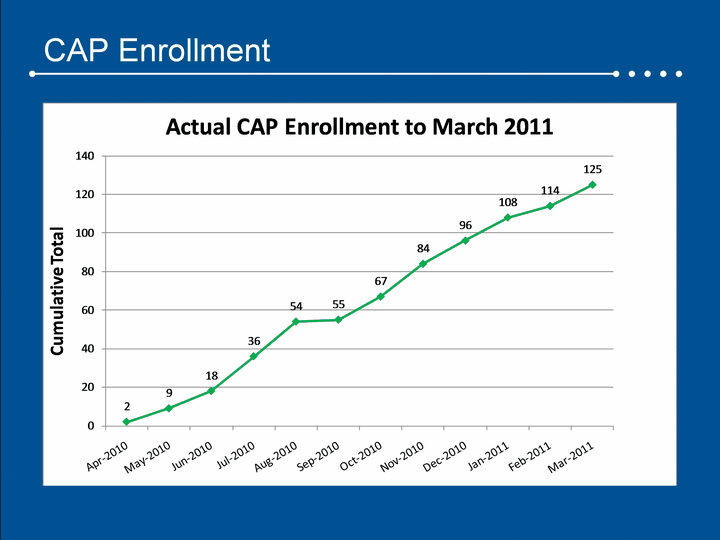
| CAP Enrollment |
| Baseline Characteristics ADVANCE (N = 140) CAP (N =110) Age (years) 53. 3 + 10.3 52 + 13.4 Male sex (%) 101 (72) 76 (66) Race [number (%)] White 102 (73) 76 (66) Black/African American 32 (23) 31 (27) Hispanic/Other 6 (3.3) 8 (7) Body-mass index (kg/m2) 28.6 + 6.1 28.8 + 6.2 Body surface area (m2) 2.1 + 0.3 2.0 + 0.3 Ischemic cause of heart failure (%) 41 34 250 patients; 174 patient-years |
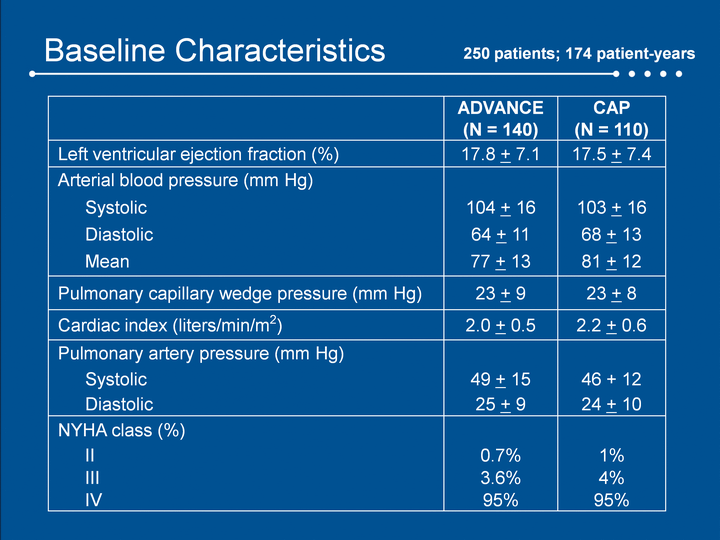
| Baseline Characteristics ADVANCE (N = 140) CAP (N = 110) Left ventricular ejection fraction (%) 17.8 + 7.1 17.5 + 7.4 Arterial blood pressure (mm Hg) Systolic 104 + 16 103 + 16 Diastolic 64 + 11 68 + 13 Mean 77 + 13 81 + 12 Pulmonary capillary wedge pressure (mm Hg) 23 + 9 23 + 8 Cardiac index (liters/min/m2) 2.0 + 0.5 2.2 + 0.6 Pulmonary artery pressure (mm Hg) Systolic 49 + 15 46 + 12 Diastolic 25 + 9 24 + 10 NYHA class (%) II 0.7% 1% III 3.6% 4% IV 95% 95% 250 patients; 174 patient-years |

| INTERMACS Classification ADVANCEPatients (%) CAPPatients (%) INTERMACS 1 7 (5.0) 4 (4.0) INTERMACS 2 39 (27.9) 35 (34.7) INTERMACS 3 62 (44.3) 48 (47.5) INTERMACS 4-7 32 (22.3) 14 (12.7) CAP patients appear to have more advanced disease by INTERMACS criteria. 250 patients; 174 patient-years |
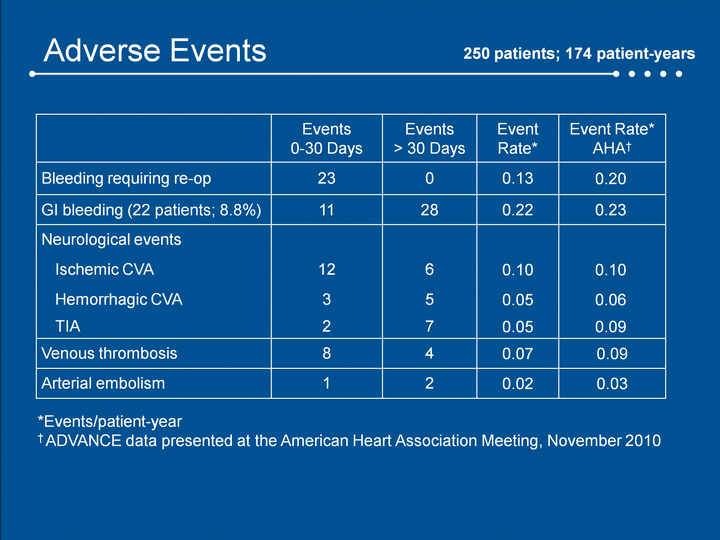
| Adverse Events Events0-30 Days Events> 30 Days Event Rate* Event Rate* AHA† Bleeding requiring re-op 23 0 0.13 0.20 GI bleeding (22 patients; 8.8%) 11 28 0.22 0.23 Neurological events Ischemic CVA 12 6 0.10 0.10 Hemorrhagic CVA 3 5 0.05 0.06 TIA 2 7 0.05 0.09 Venous thrombosis 8 4 0.07 0.09 Arterial embolism 1 2 0.02 0.03 250 patients; 174 patient-years *Events/patient-year† ADVANCE data presented at the American Heart Association Meeting, November 2010 |
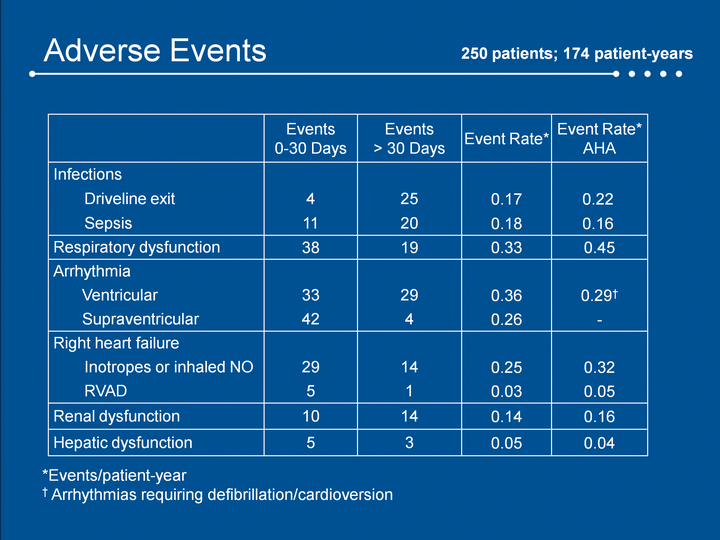
| Adverse Events 250 patients; 174 patient-years Events0-30 Days Events> 30 Days Event Rate* Event Rate* AHA Infections Driveline exit 4 25 0.17 0.22 Sepsis 11 20 0.18 0.16 Respiratory dysfunction 38 19 0.33 0.45 Arrhythmia Ventricular 33 29 0.36 0.29† Supraventricular 42 4 0.26 - Right heart failure Inotropes or inhaled NO 29 14 0.25 0.32 RVAD 5 1 0.03 0.05 Renal dysfunction 10 14 0.14 0.16 Hepatic dysfunction 5 3 0.05 0.04 *Events/patient-year† Arrhythmias requiring defibrillation/cardioversion |

| Device Exchange Reasons for ExchangeGraft Infection (1) BiVAD (1) Tissue in Connector (1) Iatrogenic (3) Thrombus (11) * 5 patients treated with LV tPA - not exchanged |
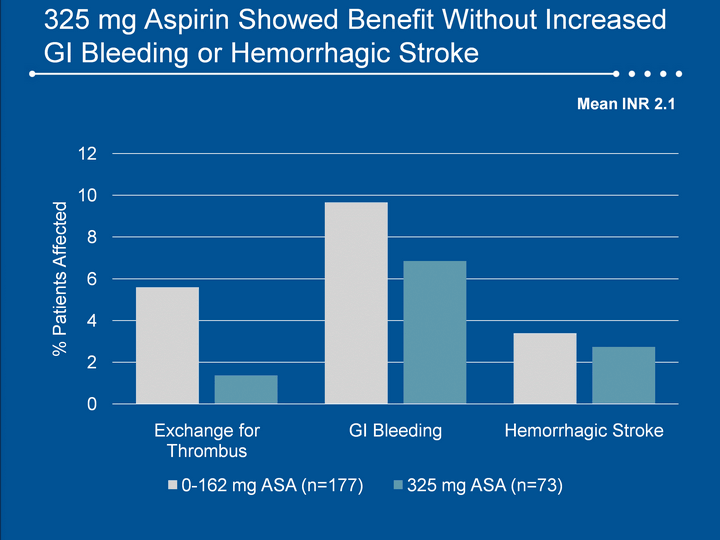
| 325 mg Aspirin Showed Benefit Without Increased GI Bleeding or Hemorrhagic Stroke GI Bleeding or Hemorrhagic Stroke GI Bleeding or Hemorrhagic Stroke Mean INR 2.1 |
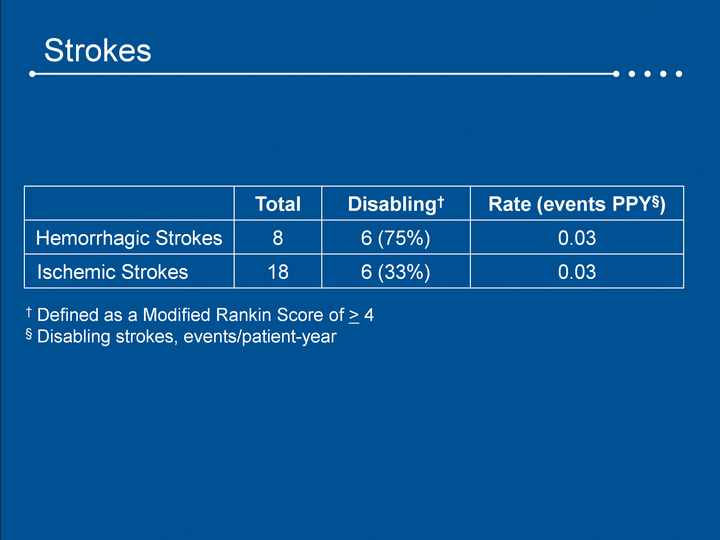
| Strokes Total Disabling† Rate (events PPY§) Hemorrhagic Strokes 8 6 (75%) 0.03 Ischemic Strokes 18 6 (33%) 0.03 † Defined as a Modified Rankin Score of > 4§ Disabling strokes, events/patient-year |
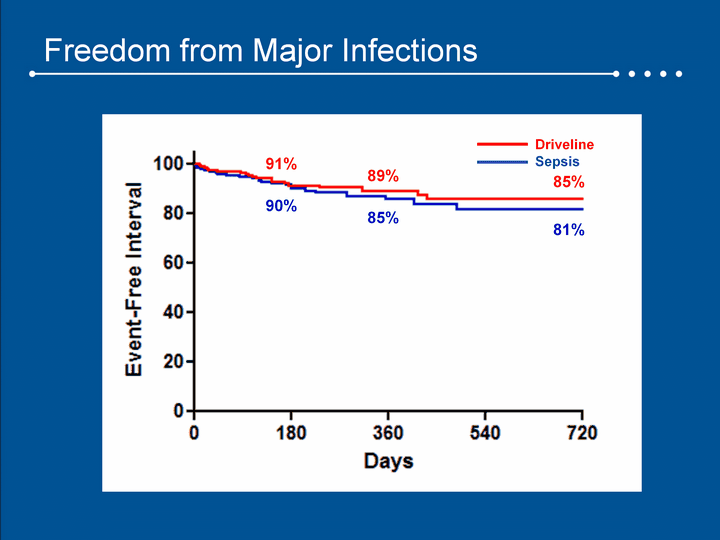
| Freedom from Major Infections DrivelineSepsis 91% 89% 85% 90% 85% 81% |

| Survival for ADVANCE and CAP CAPADVANCE (BTT) 98% 95% 92% 97% 94% 86% 98% 96% |
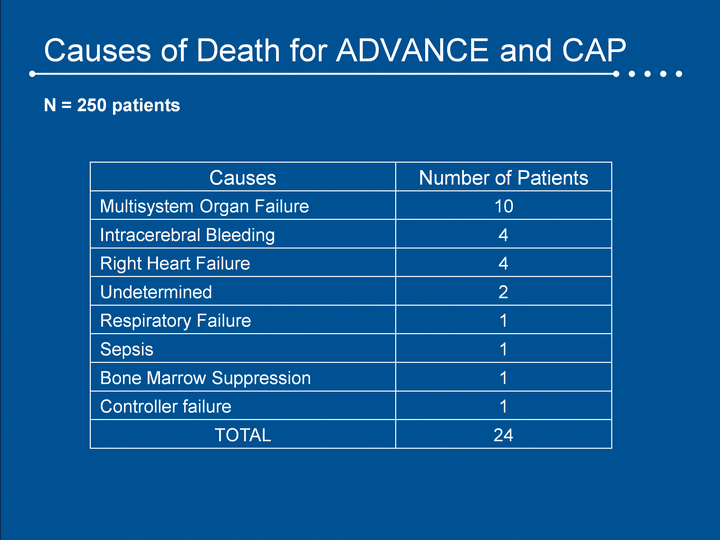
| Causes of Death for ADVANCE and CAP Causes Number of Patients Multisystem Organ Failure 10 Intracerebral Bleeding 4 Right Heart Failure 4 Undetermined 2 Respiratory Failure 1 Sepsis 1 Bone Marrow Suppression 1 Controller failure 1 TOTAL 24 N = 250 patients |
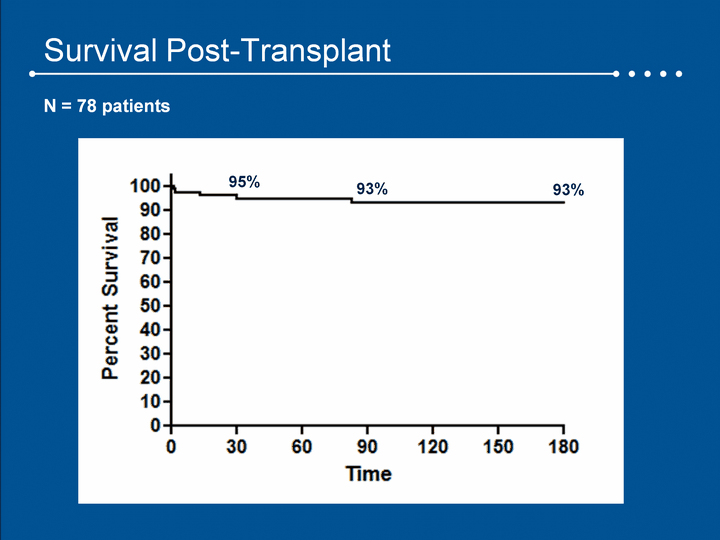
| Survival Post-Transplant 95% 93% 93% N = 78 patients |
| Summary 250 patients have been enrolled in ADVANCE and CAP for 174 patient-years of support.Survival remains high in CAP, confirming ADVANCE results.Sepsis and driveline infections were notably infrequent. Full dose aspirin (325 mg) was associated with low risk of pump thrombus without increased risk of bleeding or hemorrhagic stroke (vs. 0-162 mg). Device exchanges for system malfunction or pump failure were similar to previously reported rates. Post-transplant survival is excellent, showing no negative impact of mechanical support. |
