Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HUMAN GENOME SCIENCES INC | c15531e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - HUMAN GENOME SCIENCES INC | c15531exv99w1.htm |
Exhibit 99.2
| Analyst AND investor meeting April 14, 2011 |
| Analyst and Investor Meeting David Southwell Executive Vice President and Chief Financial Officer April 14, 2011 2 |

| Note Regarding Forward-Looking Statements 3 This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The forward-looking statements are based on Human Genome Sciences' current intent, belief and expectations. These statements are not guarantees of future performance and are subject to certain risks and uncertainties that are difficult to predict. Actual results may differ materially from these forward-looking statements because of Human Genome Sciences' unproven business model, its dependence on new technologies, the uncertainty and timing of clinical trials and regulatory approvals, Human Genome Sciences' ability to develop and commercialize products, its dependence on collaborators for services and revenue, its substantial indebtedness and lease obligations, its changing requirements and costs associated with facilities, intense competition, the uncertainty of patent and intellectual property protection, Human Genome Sciences' dependence on key management and key suppliers, the uncertainty of regulation of products, the impact of future alliances or transactions and other risks described in the Company's filings with the SEC. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. Human Genome Sciences undertakes no obligation to update or revise the information contained in this presentation whether as a result of new information, future events or circumstances or otherwise. HGS, Human Genome Sciences, and BENLYSTA are trademarks of Human Genome Sciences, Inc. Other trademarks referenced are the property of their respective owners. |

| Strategic Priorities BENLYSTA(r) Commercial Update BENLYSTA Clinical Update Mid/Early Stage Portfolio Financial Wrap Up and General Q & A TODAY'S AGENDA 4 |
| HGS PRESENTERS H. Thomas Watkins President and Chief Executive Officer Barry Labinger Executive Vice President and Chief Commercial Officer David Stump, M.D. Executive Vice President, Research and Development David Southwell Executive Vice President and Chief Financial Officer AVAILABLE FOR QUESTIONS Jim Davis, Ph.D., J.D. Executive Vice President, General Counsel and Secretary 5 Susan Bateson Senior Vice President, Human Resources Curran Simpson Senior Vice President, Operations William Freimuth, M.D., Ph.D. Vice President, Clinical Research - Immunology, Rheumatology and Infectious Disease Gilles Gallant, B. Pharm, Ph.D. Vice President, Clinical Research - Oncology Kevin McRaith Vice President, Sales and Marketing Scott Habig Vice President, Sales TODAY'S PRESENTERS |

| Analyst and Investor Meeting H. Thomas Watkins President and Chief Executive Officer 6 |

| Strategic prioritY 1 Successfully launch and commercialize BENLYSTA(r) 7 |

| Achieve the full therapeutic and commercial potential of BENLYSTA through further development in SLE and other B-cell mediated diseases Strategic prioritY 2 8 |
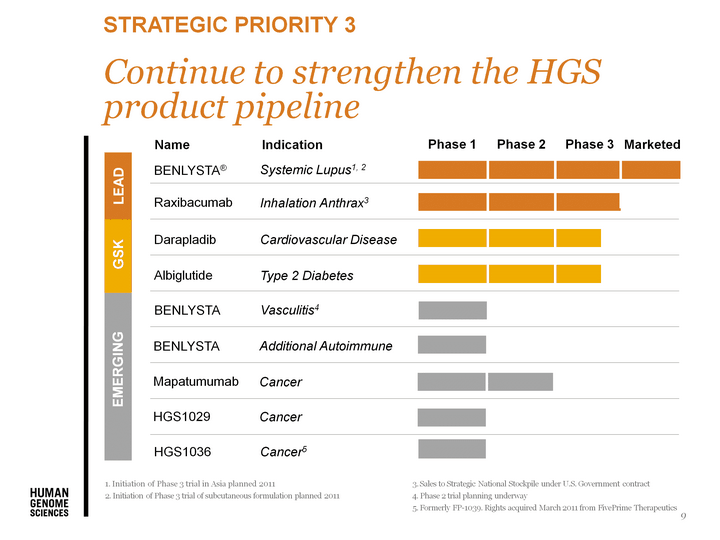
| 9 Initiation of Phase 3 trial in Asia planned 2011 Initiation of Phase 3 trial of subcutaneous formulation planned 2011 Sales to Strategic National Stockpile under U.S. Government contract Phase 2 trial planning underway Formerly FP-1039. Rights acquired March 2011 from FivePrime Therapeutics Strategic Priority 3 Raxibacumab Inhalation Anthrax3 BENLYSTA(r) Systemic Lupus1, 2 Type 2 Diabetes Albiglutide Darapladib Cardiovascular Disease Marketed EMERGING GSK LEAD Continue to strengthen the HGS product pipeline |
| Analyst and Investor Meeting Barry Labinger Executive Vice President and Chief Commercial Officer April 14, 2011 10 |

| BENLYSTA Launch update Market Opportunity Access and Reimbursement Marketing Plan Field organization Product positioning Consumer programs Performance Tracking Europe Progress 11 |
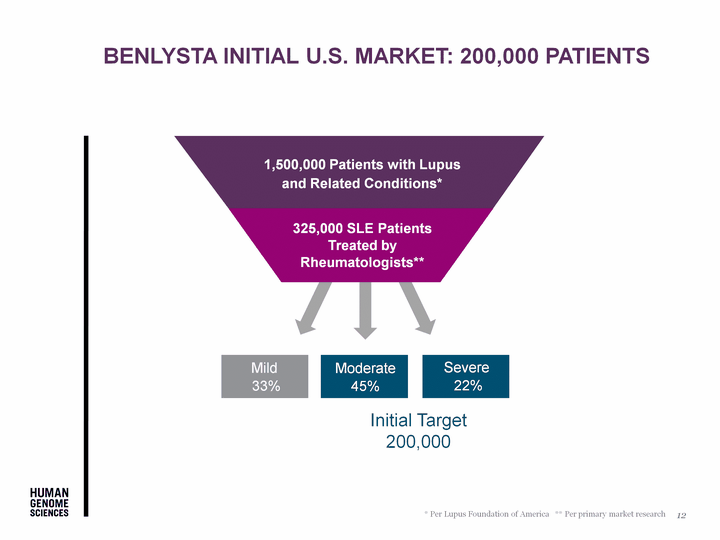
| Mild 33% Severe 22% Moderate 45% Initial Target 200,000 1,500,000 Patients with Lupus and Related Conditions* 325,000 SLE Patients Treated by Rheumatologists** * Per Lupus Foundation of America ** Per primary market research BENLYSTA INITIAL U.S. Market: 200,000 Patients 12 |

| Indications and Usage 13 BENLYSTA (belimumab) 10 mg/kg is indicated for the treatment of adult patients with active, autoantibody^positive, systemic lupus erythematosus who are receiving standard therapy. Limitations of Use: The efficacy of BENLYSTA has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus. BENLYSTA has not been studied in combination with other biologics or intravenous cyclophosphamide. Use of BENLYSTA is not recommended in these situations. Safety: See package insert and medication guide at www.BENLYSTA.com for important safety information Limitations of Use: The efficacy of BENLYSTA has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus. BENLYSTA has not been studied in combination with other biologics or intravenous cyclophosphamide. Use of BENLYSTA is not recommended in these situations. BENLYSTA (belimumab) 10 mg/kg is indicated for the treatment of adult patients with active, autoantibody^positive, systemic lupus erythematosus who are receiving standard therapy. |

| Labeled Indication treatment of sle Auto-antibody positive 72% in Phase 2 trial at baseline 100% in medical history Severe active lupus nephritis, severe active CNS lupus Acute manifestations Often requires cyclophosphamide As acute manifestations are treated, many patients continue with chronic active disease Combination with biologics or IV cyclophosphamide 14 |

| Use of Rituximab and Cyclophosphamide 15 Source: HGS and GSK market research Cyclophosphamide Rituxan Percent of SLE Patients 11% 2% 5% Ever used Currently using 2% |

| Effect on concomitant steroid treatment In BLISS-76 and BLISS-52, 46% and 69% of patients, respectively, were receiving prednisone at doses >7.5 mg/day at baseline. The proportion of patients able to reduce their average prednisone dose by at least 25% to ^7.5 mg/day during Weeks 40 through 52 was not consistently significantly different for BENLYSTA relative to placebo in both trials. In BLISS-76, 17% of patients receiving BENLYSTA 10 mg/kg and 19% of patients receiving BENLYSTA 1 mg/kg achieved this level of steroid reduction compared with 13% of patients receiving placebo. In BLISS-52, 19%, 21%, and 12% of patients receiving BENLYSTA 10 mg/kg, BENLYSTA 1 mg/kg, and placebo, respectively, achieved this level of steroid reduction. Effect on concomitant steroid treatment In BLISS-76 and BLISS-52, 46% and 69% of patients, respectively, were receiving prednisone at doses >7.5 mg/day at baseline. The proportion of patients able to reduce their average prednisone dose by at least 25% to ^7.5 mg/day during Weeks 40 through 52 was not consistently significantly different for BENLYSTA relative to placebo in both trials. In BLISS-76, 17% of patients receiving BENLYSTA 10 mg/kg and 19% of patients receiving BENLYSTA 1 mg/kg achieved this level of steroid reduction compared with 13% of patients receiving placebo. In BLISS-52, 19%, 21%, and 12% of patients receiving BENLYSTA 10 mg/kg, BENLYSTA 1 mg/kg, and placebo, respectively, achieved this level of steroid reduction. Effect on concomitant steroid treatment In BLISS-76 and BLISS-52, 46% and 69% of patients, respectively, were receiving prednisone at doses >7.5 mg/day at baseline. The proportion of patients able to reduce their average prednisone dose by at least 25% to ^7.5 mg/day during Weeks 40 through 52 was not consistently significantly different for BENLYSTA relative to placebo in both trials. In BLISS-76, 17% of patients receiving BENLYSTA 10 mg/kg and 19% of patients receiving BENLYSTA 1 mg/kg achieved this level of steroid reduction compared with 13% of patients receiving placebo. In BLISS-52, 19%, 21%, and 12% of patients receiving BENLYSTA 10 mg/kg, BENLYSTA 1 mg/kg, and placebo, respectively, achieved this level of steroid reduction. Benlysta labeling aligns with study design and clinical data Important clinical endpoints 16 |
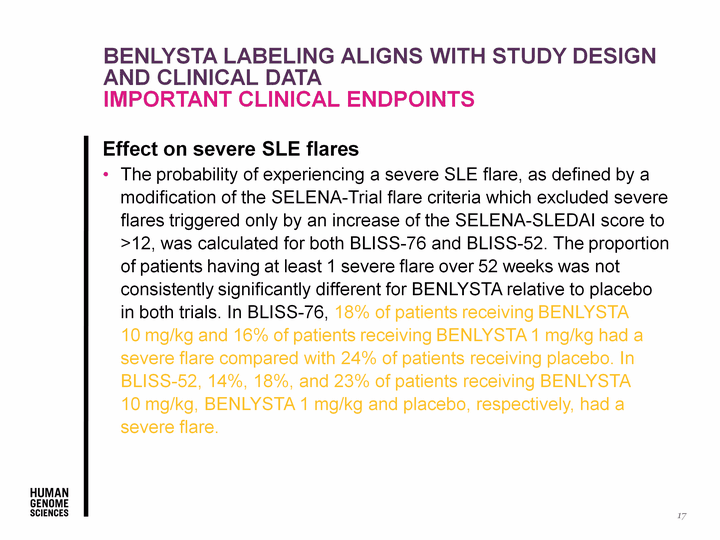
| Benlysta labeling aligns with study design and clinical data Important clinical endpoints Effect on severe SLE flares The probability of experiencing a severe SLE flare, as defined by a modification of the SELENA-Trial flare criteria which excluded severe flares triggered only by an increase of the SELENA-SLEDAI score to >12, was calculated for both BLISS-76 and BLISS-52. The proportion of patients having at least 1 severe flare over 52 weeks was not consistently significantly different for BENLYSTA relative to placebo in both trials. In BLISS-76, 18% of patients receiving BENLYSTA 10 mg/kg and 16% of patients receiving BENLYSTA 1 mg/kg had a severe flare compared with 24% of patients receiving placebo. In BLISS-52, 14%, 18%, and 23% of patients receiving BENLYSTA 10 mg/kg, BENLYSTA 1 mg/kg and placebo, respectively, had a severe flare. 17 Effect on severe SLE flares The probability of experiencing a severe SLE flare, as defined by a modification of the SELENA-Trial flare criteria which excluded severe flares triggered only by an increase of the SELENA-SLEDAI score to >12, was calculated for both BLISS-76 and BLISS-52. The proportion of patients having at least 1 severe flare over 52 weeks was not consistently significantly different for BENLYSTA relative to placebo in both trials. In BLISS-76, 18% of patients receiving BENLYSTA 10 mg/kg and 16% of patients receiving BENLYSTA 1 mg/kg had a severe flare compared with 24% of patients receiving placebo. In BLISS-52, 14%, 18%, and 23% of patients receiving BENLYSTA 10 mg/kg, BENLYSTA 1 mg/kg and placebo, respectively, had a severe flare. Effect on severe SLE flares The probability of experiencing a severe SLE flare, as defined by a modification of the SELENA-Trial flare criteria which excluded severe flares triggered only by an increase of the SELENA-SLEDAI score to >12, was calculated for both BLISS-76 and BLISS-52. The proportion of patients having at least 1 severe flare over 52 weeks was not consistently significantly different for BENLYSTA relative to placebo in both trials. In BLISS-76, 18% of patients receiving BENLYSTA 10 mg/kg and 16% of patients receiving BENLYSTA 1 mg/kg had a severe flare compared with 24% of patients receiving placebo. In BLISS-52, 14%, 18%, and 23% of patients receiving BENLYSTA 10 mg/kg, BENLYSTA 1 mg/kg and placebo, respectively, had a severe flare. |

| Benlysta safety Warnings and precautions Mortality Serious infections Malignancy Hypersensitivity reactions, including anaphylaxis Infusion reactions Depression Immunization Concomitant use with other biologic therapies or IV cyclophosphamide Most commonly reported adverse reactions: nausea, diarrhea, pyrexia, nasopharyngitis, bronchitis, insomnia, pain in extremity, depression, migraine, and pharyngitis REMS not required 18 |
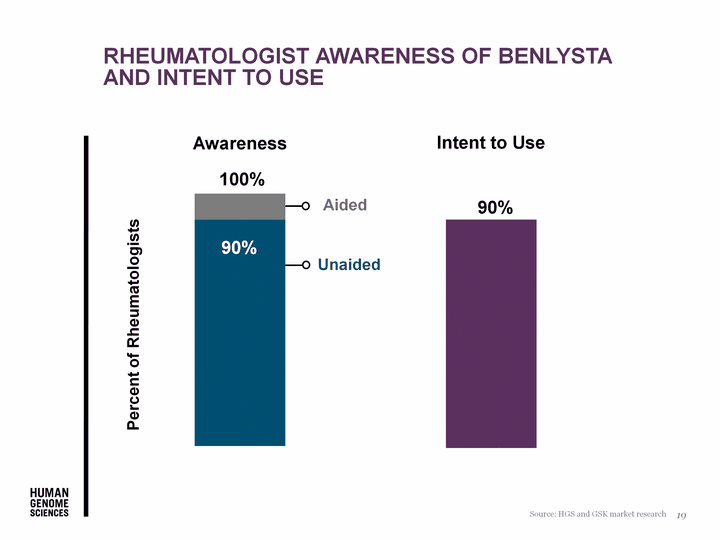
| Rheumatologist awareness of benlysta and intent to use 19 100% Source: HGS and GSK market research Awareness Aided Unaided 90% 90% Percent of Rheumatologists Intent to Use |

| 20 ACCESS & REIMBURSEMENT |

| BENLYSTA Pricing and Coverage ~$35,000 WAC price for average patient per year Varies by body weight 13 infusions Within range of biologics for other chronic autoimmune diseases Coverage under Medical Benefit Subject to prior authorization Typical for biologics Step therapy expected 21 |
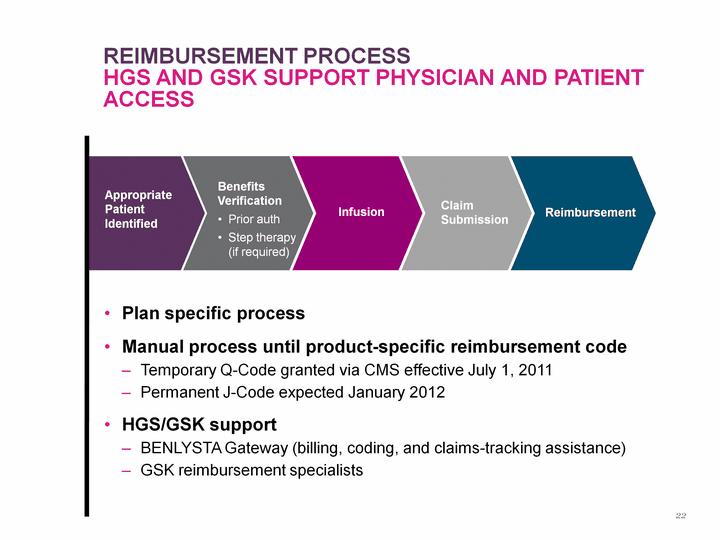
| Appropriate Patient Identified Benefits Verification Prior auth Step therapy (if required) Infusion Claim Submission Reimbursement Reimbursement process hgS and GSK Support Physician and Patient Access Plan specific process Manual process until product-specific reimbursement code Temporary Q-Code granted via CMS effective July 1, 2011 Permanent J-Code expected January 2012 HGS/GSK support BENLYSTA Gateway (billing, coding, and claims-tracking assistance) GSK reimbursement specialists 22 |
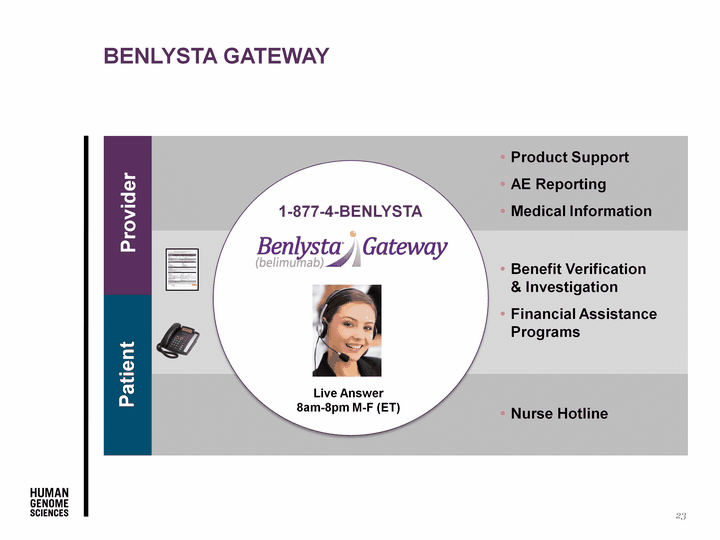
| Provider Patient Live Answer 8am-8pm M-F (ET) 1-877-4-BENLYSTA Benefit Verification & Investigation Financial Assistance Programs Product Support AE Reporting Medical Information Nurse Hotline Benlysta Gateway 23 |

| Payer distribution for sle 24 Note: "Other" = Workers' Compensation, no charge/charity care, blank or unknown Source: Years, 2004 to 2007: National Ambulatory/Medical Care Survey / National Hospital Ambulatory Medical Care Survey, survey conducted by the National Center for Health Statistics Self-Pay Commercial 57% Medicaid 16% Other Medicare 18% 3% 6% |

| Estimate of Out of Pocket DRUG Costs Commercially insured 25 (CHART) No OOP Cost Fixed copay ($50-100) Coinsurance (10-20%) with cap (up to $2500) Coinsurance (10-20%), no cap |

| BENLYSTA Financial Assistance Programs 26 Patient Assistance Program Free drug Uninsured ^ 500% Federal Poverty Level Copay Assistance Commercially insured ^ 500% FPL Full copay/co-ins $7,000 annual max Site of care payment >500%- ^ 750% FPL 50% copay/co-ins $3,500 annual max Reimbursed |

| 27 BENLYSTA MARKETING PLAN |
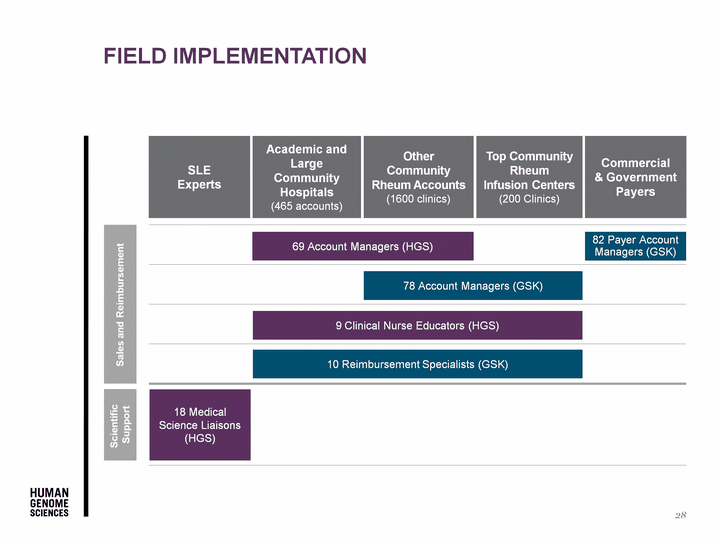
| Field Implementation 28 SLE Experts Academic and Large Community Hospitals (465 accounts) Top Community Rheum Infusion Centers (200 Clinics) Other Community Rheum Accounts (1600 clinics) Commercial & Government Payers 18 Medical Science Liaisons (HGS) 69 Account Managers (HGS) 78 Account Managers (GSK) 9 Clinical Nurse Educators (HGS) 10 Reimbursement Specialists (GSK) 82 Payer Account Managers (GSK) Scientific Support Sales and Reimbursement |
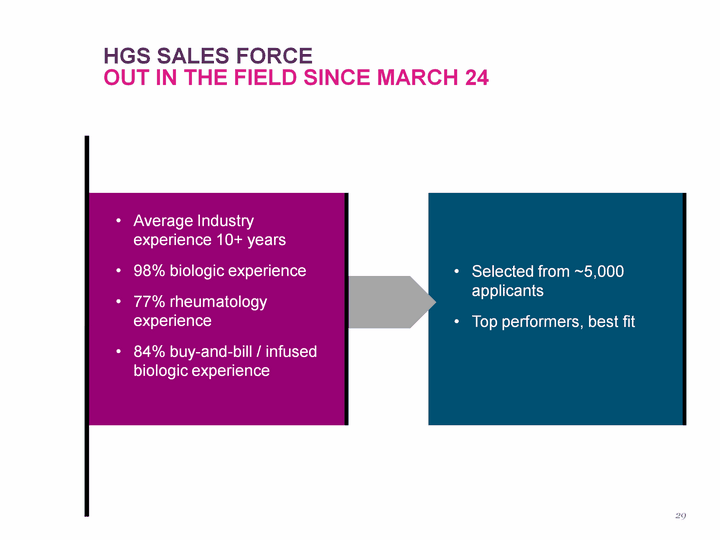
| Selected from ~5,000 applicants Top performers, best fit Average Industry experience 10+ years 98% biologic experience 77% rheumatology experience 84% buy-and-bill / infused biologic experience HGS Sales Force Out in the field since march 24 29 |

| Benlysta positioning in SLE treatment algorithm Initial Therapies (persistent symptoms) Low dose corticosteroids Anti-malarials 30 Acute (severe active)1 Chronic Intensified Therapy (persistent symptoms) High dose corticosteroids Immunosuppressants Methotrexate Mycophenolate mofetil Azathioprine Cyclophosphamide Other biologics 1Use of BENLYSTA is not recommended in these situations. |

| educated Lupus Patients are more likely to request new therapies 31 |
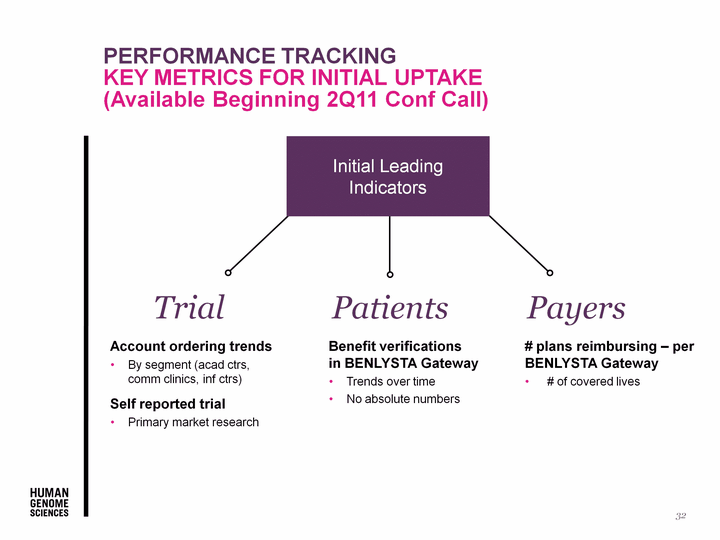
| Performance Tracking key metrics for initial uptake (Available Beginning 2Q11 Conf Call) 32 Account ordering trends By segment (acad ctrs, comm clinics, inf ctrs) Self reported trial Primary market research Benefit verifications in BENLYSTA Gateway Trends over time No absolute numbers # plans reimbursing - per BENLYSTA Gateway # of covered lives Trial Patients Payers Initial Leading Indicators |

| Europe Update 33 Marketing Authorization Application review ongoing EMA decision expected 2H 2011 CHMP opinion expected by mid-year Many markets require 9-12 months for pricing/reimbursement HGS senior team in place Headquarters near Geneva, Switzerland Close collaboration with GSK regional leadership Pan-European accountability Local HGS organizations in Germany, France, Spain Close collaboration with local GSK teams GSK organization established and growing |

| Benlysta Launch Summary 34 Large market opportunity and favorable label U.S. launch off to a good start Positive reception from rheumatologists/staff Outstanding team in place and well prepared Launch preparations in Europe on track |

| 35 QUESTIONS |

| Analyst and Investor Meeting David Stump, M.D. Executive Vice President, Research and Development April 14, 2011 36 |

| BENLYSTA life cycle plan progressing Post-marketing studies Long term continuation trials Subcutaneous formulation New indications Advance mid-stage development and expand early-stage pipeline Mapatumumab HGS1029 HGS10361, fibroblast growth factor ligand trap Research & Development priorities 37 1Formerly FP-1039; in-licensed from FivePrime Therapeutics |
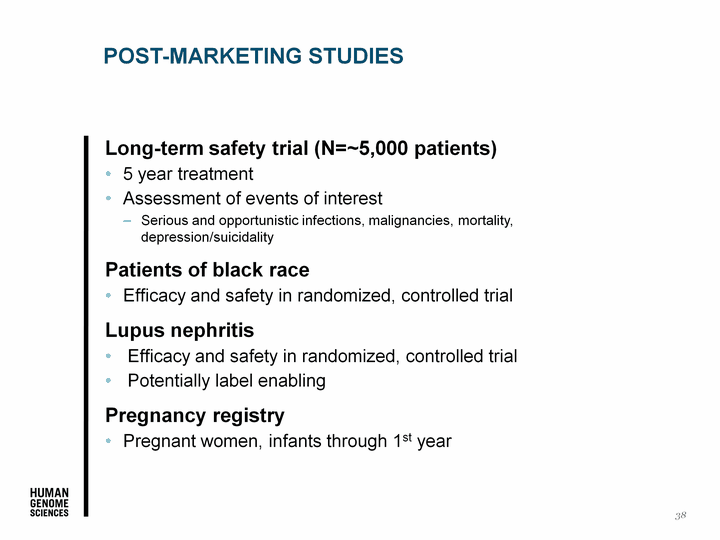
| Post-Marketing Studies Long-term safety trial (N=~5,000 patients) 5 year treatment Assessment of events of interest Serious and opportunistic infections, malignancies, mortality, depression/suicidality Patients of black race Efficacy and safety in randomized, controlled trial Lupus nephritis Efficacy and safety in randomized, controlled trial Potentially label enabling Pregnancy registry Pregnant women, infants through 1st year 38 |

| Post-Marketing Studies Continued Pediatric assessment ~100 patients, age 5-17, active SLE Safety, efficacy, pharmacokinetics Assess response to certain vaccines Randomized clinical trial No REMS required-med guide only Pregnancy registry open by the end of 2011 Plan to start post-marketing studies 2012 39 |
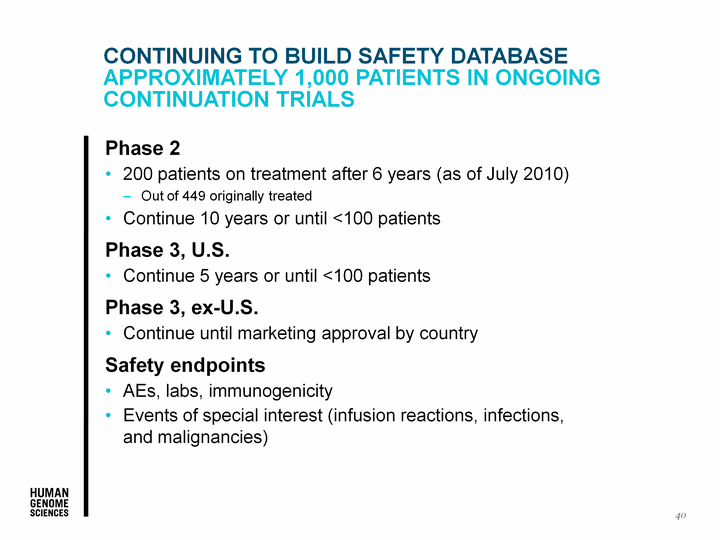
| CONTINUING TO BUILD SAFETY DATABASE Approximately 1,000 PATIENTS IN ONGOING CONTINUATION TRIALS Phase 2 200 patients on treatment after 6 years (as of July 2010) Out of 449 originally treated Continue 10 years or until <100 patients Phase 3, U.S. Continue 5 years or until <100 patients Phase 3, ex-U.S. Continue until marketing approval by country Safety endpoints AEs, labs, immunogenicity Events of special interest (infusion reactions, infections, and malignancies) 40 |
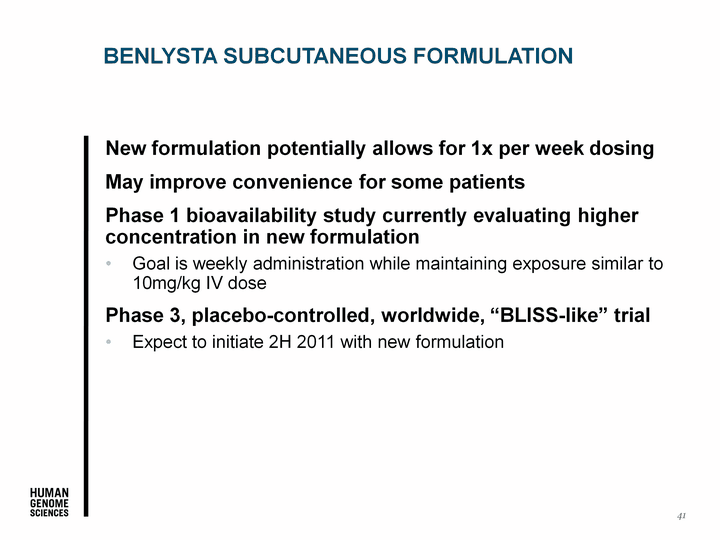
| 41 New formulation potentially allows for 1x per week dosing May improve convenience for some patients Phase 1 bioavailability study currently evaluating higher concentration in new formulation Goal is weekly administration while maintaining exposure similar to 10mg/kg IV dose Phase 3, placebo-controlled, worldwide, "BLISS-like" trial Expect to initiate 2H 2011 with new formulation BENLYSTA SUBCUTANEOUS FORMULATION |
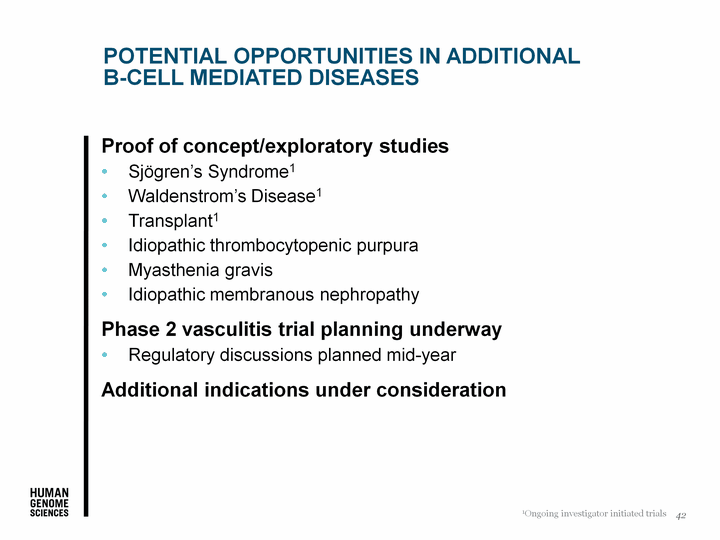
| Potential Opportunities in Additional B-CELL Mediated Diseases Proof of concept/exploratory studies Sjogren's Syndrome1 Waldenstrom's Disease1 Transplant1 Idiopathic thrombocytopenic purpura Myasthenia gravis Idiopathic membranous nephropathy Phase 2 vasculitis trial planning underway Regulatory discussions planned mid-year Additional indications under consideration 42 1Ongoing investigator initiated trials |
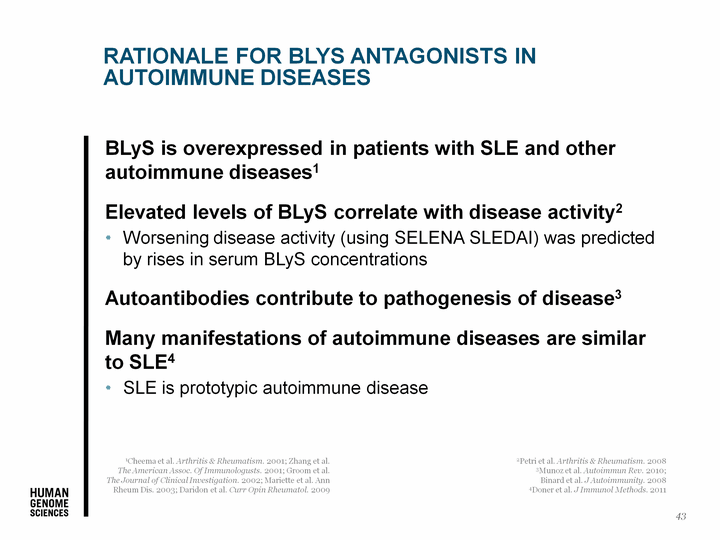
| Rationale for BLyS Antagonists in Autoimmune Diseases BLyS is overexpressed in patients with SLE and other autoimmune diseases1 Elevated levels of BLyS correlate with disease activity2 Worsening disease activity (using SELENA SLEDAI) was predicted by rises in serum BLyS concentrations Autoantibodies contribute to pathogenesis of disease3 Many manifestations of autoimmune diseases are similar to SLE4 SLE is prototypic autoimmune disease 43 1Cheema et al. Arthritis & Rheumatism. 2001; Zhang et al. The American Assoc. Of Immunologusts. 2001; Groom et al. The Journal of Clinical Investigation. 2002; Mariette et al. Ann Rheum Dis. 2003; Daridon et al. Curr Opin Rheumatol. 2009 2Petri et al. Arthritis & Rheumatism. 2008 3Munoz et al. Autoimmun Rev. 2010; Binard et al. J Autoimmunity. 2008 4Doner et al. J Immunol Methods. 2011 |

| ANCA-ASSOCIATED VASCULITIS RATIONALe FOR POTENTIAL EFFICACY OF BENLYSTA Activity on biomarkers Normalization of anti-dsDNA antibodies Reduction in immunoglobulins Increased complement (C3 and C4) levels Lymphocyte effects Reduced activated B cells and short-lived plasma cells Vascular system organ improvement SLEDAI improvements in SLE 44 |
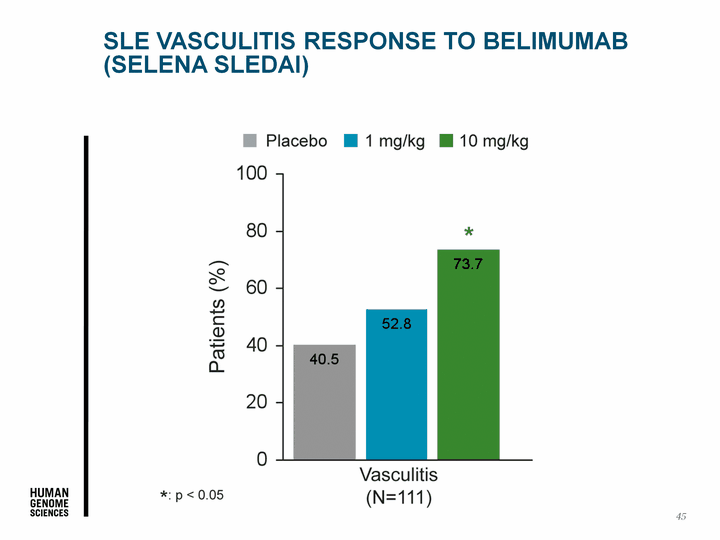
| 45 SLE Vasculitis Response to Belimumab (SELENA SLEDAI) |

| ANCA-ASSOCIATED VASCULITIS PHASE 2 PLANNED Autoimmune condition that involves inflammation in the blood vessels Autoantibodies Inflammation-increased immunoglobulins, low complement, elevated CRP Lymphocytic infiltration of the blood vessel-increased giant cells, plasma cells and T-cells Blood vessel damage Current standard of care-HD corticosteroids plus IV Cytoxan or IV rituximab; azathioprine Approximately 175,000 patients in the U.S.1 46 1HGS/GSK estimates |

| TRAIL Pathway Offers Therapeutic Opportunities Across a Broad Range of Cancers 47 Mapatumumab (HGS-ETR1) Targets TRAIL apoptosis pathway Proof of concept randomized Phase 2 chemotherapy combo trial underway in hepatocellular carcinoma HGS1029 (IAP inhibitor) Novel intracellular target in apoptosis pathway Synergistic preclinical activity demonstrated with mapatumumab and other therapeutic agents Currently in Phase 1 trials as monotherapy-advanced solid and lymphoid tumors Planning underway for Phase 1b combination oncology studies HGS1029 HGS-ETR1 |

| HGS10361 Affects Multiple, Pathogenic FGF Ligand-Dependent Signaling Pathways in Cancer Cells 48 Potentially impacted by HGS1036 Modified from Nature Reviews | Cancer Volume 10 | February 2010 | 123 1Formerly FP-1039; in-licensed from FivePrime Therapeutics |

| 2011 R&D DEVELOPMENT MILESTONES Finalize protocols and reach regulatory agreement on BENLYSTA post-marketing studies Initiate Phase 3 BENLYSTA sub-Q formulation study Complete regulatory discussions and begin site initiation activities for vasculitis Phase 2 study Continue exploratory work in additional indications for BENLYSTA Continue mapatumumab Phase 2 trial w/sorafenib in HCC Continue HGS1029 program towards initiating studies in combination with mapatumumab and other agents Continue HGS1036 Phase 2 trial in patients with mutant endometrial cancer 49 |

| 50 QUESTIONS |

| Analyst and Investor Meeting David Southwell Executive Vice President and Chief Financial Officer April 14, 2011 51 |

| Cash and investments at December 31, 2010: $933 million Expected cash and investments at year-end 2011: $550-$650 million Includes in-licensing of HGS10361 Expect SG&A and R&D expense to total $330-$390 million SG&A expense increase to $150-$170 million primarily reflects full-year impact of U.S. expansion, as well as the continued growth of Europe R&D expense of $180-$220 million reflects FivePrime Therapeutics in-licensing transaction and shift to capitalization of BENLYSTA manufacturing 75% to 80% BENLYSTA gross margin, steady state Raxibacumab and CMO business, which have lower GM vs. BENLYSTA, will represent a greater proportion of total revenue in 2011 than in 2012 or later Expect total company GM ~50% in 2011 Total company GM should increase as BENLYSTA share of revenues increases Anticipate GAAP net income profitability for 2013 Select financial data 52 1Formerly FP-1039; in-licensed from FivePrime Therapeutics |

| Drop-ship direct to customer U.S. Revenue Recognition At Launch Sell-Through Basis 53 2011 Revenue Recognition 2011 Revenue Recognition Drop-ship direct to customer HGS Fill/Finish GSK Distribution Centers Specialty Distributor Wholesaler Physician Hospital |

| Commercial collaboration accounting U.S. BENLYSTA SALES 54 Total U.S. BENLYSTA Product P&L Total U.S. BENLYSTA Product P&L Net Product Sales A Cost of Product Sales B Gross Profit C SG&A Expense D R&D Expense1 (global) E Operating Profit OP 100% Impact on HGS P&L Impact on HGS P&L A Net Product Sales B Cost of Product Sales C Gross Profit C/2 (50% of GM) Commercial Collaboration Expense2 (to GSK) D/2 SG&A Expense E/2 R&D Expense1 (global) OP/2 Operating Profit 1BENLYSTA-related R&D is budgeted on a global basis. 2Separate line item above Operating Income on P&L 50% 50% |

| Commercial collaboration accounting International BENLYSTA SALES 55 1If income: separate line item in revenue section of P&L-Commercial Collaboration Income. If expense: HGS' share of split will be in Commercial Collaboration Expense line above Operating Income on P&L. Intl BENLYSTA Product P&L Intl BENLYSTA Product P&L GSK reports all Product Sales (net)... A ...all Cost of Product Sales B ...and all Gross Margin C SG&A Expense D Intl Contribution (profit) or... E Intl Contribution (loss) E Impact to HGS P&L Impact to HGS P&L E/2 Commercial Collaboration Income1 E/2 Commercial Collaboration Expense1 |
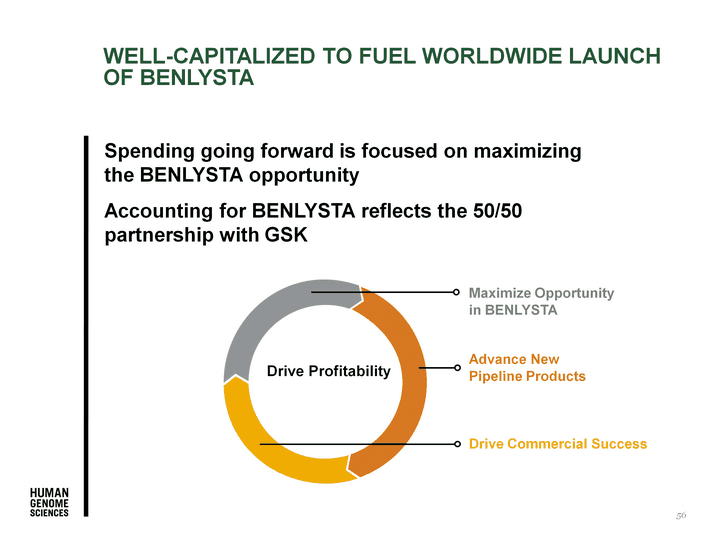
| Spending going forward is focused on maximizing the BENLYSTA opportunity Accounting for BENLYSTA reflects the 50/50 partnership with GSK Drive Commercial Success Maximize Opportunity in BENLYSTA WELL-CAPITALIZED to Fuel Worldwide LAUNCH OF BENLYSTA Advance New Pipeline Products 56 Drive Profitability |

| H. Thomas Watkins President and Chief Executive Officer Wrap Up and General Q&A |

| WELL-POSITIONED TO GENERATE SIGNIFICANT VALUE We are focused on maximizing the BENLYSTA franchise We are well capitalized to fund the BENLYSTA launch We expect to reach profitability in 2013 58 |
| Potential Blockbuster Product Pipeline to Fuel Future Growth Manufacturing Excellence Commercial Readiness World-Class Partner Seasoned Leadership A Formula for Sustainable Growth 59 Building Shareholder Value |
