UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 2
FORM 8-K/A
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 12, 2010
GLOBAL PHARM HOLDINGS GROUP, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
|
|
333-152286
|
|
20-8767223
|
|
(State or other jurisdiction of incorporation)
|
|
(Commission
File Number)
|
|
(IRS Employer
Identification No.)
|
|
25/F New World Center, No. 6009 Yitian Road, Futian District, Shenzhen, PRC
|
|
518026
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
Registrant’s telephone number, including area code: 86-755-83230226
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
¨ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
EXPLANATORY NOTE
We are filing this Amendment No. 2 to our Current Report on Form 8-K/A (this “Amendment”) to amend certain disclosures in our Current Report on Form 8-K originally filed with the Securities and Exchange Commission (the “SEC”) on August 12, 2010, as amended (”Report”). The primary changes to our Report in this Amendment are as follows:
The Company made amendments or additional disclosures with regard to the following under the Business section.
|
|
·
|
The Company amended and added disclosure on its corporate history.
|
|
|
·
|
The Company added a flowchart illustrating its corporate structure.
|
|
|
·
|
The Company updated its disclosure under “Our PRC Subsidiaries.”
|
|
|
·
|
The Company revised its scope of business and added more disclosure on its products.
|
|
|
·
|
The Company updated its disclosure under “Customers and Suppliers” and “Marketing and Sales.”
|
|
|
·
|
The Company added disclosure on its distribution process and quality control.
|
|
|
·
|
The Company added disclosure on its material supply agreements.
|
|
|
·
|
The Company updated its disclosure under “Overview of the PRC Pharmaceutical Industry.”
|
|
|
·
|
The Company revised the pharmaceutical distribution chart and its business strategy.
|
|
|
·
|
The Company revised the sections on its employees, insurance and intellectual property.
|
|
|
·
|
The Company revised its disclosure under the section “Compliance with Circular 106 and the Revised M&A Regulations.”
|
|
|
·
|
The Company updated information on its licenses, approvals and certificates.
|
The Company revised its disclosure on scope of business throughout the Amendment.
The Company revised the following risk factors:
|
|
·
|
“Failure to comply with the anti-corruption measures taken by the PRC government could subject us to penalties and other adverse consequences” and
|
|
|
·
|
“An active public market for our common stock may not develop or be sustained, which would adversely affect the ability of our investors to sell their securities in the public market.”
|
The Company made the following revision to its disclosure under “Management’s Discussion and Analysis of Financial Condition and Results of Operation.”
|
|
·
|
The Company revised its disclosure under company overview.
|
|
|
·
|
The Company amended its disclosure regarding net income and capital resources.
|
|
|
·
|
The Company revised its disclosure concerning contractual obligations.
|
The Company revised its disclosure in the section “Properties.”
The Company updated the table of security ownership of certain beneficial owners and management.
The Company made additional disclosure regarding its officers and directors and executive compensation.
The Company revised the section “Certain Relationships and Related Transactions” and “Description of Securities.”
The following amendments have been made to the Company’s financial statements for the years ended December 31, 2008 and 2009 and accompanying footnotes.
|
|
·
|
The Company revised its disclosure under “Note 1- Nature of Operations and Summary of Significant Accounting Policies.”
|
2
|
|
·
|
The Company revised the disclosure regarding inventories and revenue recognition under “Note 1- Nature of Operations and Summary of Significant Accounting Policies.”
|
|
|
·
|
The Company amended its disclosure under “Note 6- Short-term Loans.”
|
|
|
·
|
The Company revised its disclosure under “Note 8- Related Party Transactions.”
|
|
|
·
|
The Company added a note on segment reporting.
|
The following amendments have been made to the Company’s financial statements for the three and six months ended June 30, 2010 and 2009 and accompanying footnotes.
|
|
·
|
The Company restated its financial statements for the six months ended June 30, 2010.
|
|
|
·
|
The Company revised its disclosure regarding inventories under “Note 1- Nature of Operations and Summary of Significant Accounting Policies”
|
|
|
·
|
The Company amended its disclosure under “Note 5- Short-term Loans.”
|
|
|
·
|
The Company revised its disclosure under “Note 7- Related Party Transactions.”
|
|
|
·
|
The Company added a note on segment reporting.
|
The Company also updated its pro forma financial statements.
Other than the update described above, all other information in our original Form 8-K remains unchanged. For the convenience of the reader, this amendment includes, in their entirety, those items in our original filing not being amended. Except for this Amendment, this Form 8-K/A continues to describe conditions as of our original filing, and does not update disclosures contained herein to reflect events that occurred at a later date. Accordingly, this Form 8-K/A should be read in conjunction with our other filings made with the SEC subsequent to the filing of our Report, if any.
3
TABLE OF CONTENTS
|
Item No.
|
Description of Item
|
Page
No.
|
||
|
Item 1.01
|
Entry Into a Material Definitive Agreement
|
6
|
||
|
Item 2.01
|
Completion of Acquisition or Disposition of Assets
|
7
|
||
|
Item 3.02
|
Unregistered Sales of Equity Securities
|
75
|
||
|
Item 4.01
|
Changes in Registrant’s Certifying Accountant
|
75
|
||
|
Item 5.03
|
Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year
|
76
|
||
|
Item 5.06
|
Change in Shell Company Status
|
76
|
||
|
Item 9.01
|
Financial Statements and Exhibits
|
77
|
4
CONVENTIONS THAT APPLY TO THIS CURRENT REPORT ON FORM 8-K/A
Except where the context otherwise requires and for purposes of this Current Report on Form 8-K/A only:
|
|
·
|
“we,” “us,” “our company,” “our” and “Top Flight” refer to Top Flight Gamebirds, Inc., and its consolidated subsidiaries, namely Global Pharma Enterprise Group Limited, a British Virgin Islands limited liability company (“Global Pharma BVI”), Binomial Biopharm Group Limited (“Binomial”) and Hong Kong Wisdom Fortune Medicine Holding Group Limited (“Wisdom Fortune”), two wholly owned Hong Kong incorporated companies of Global Pharma and Anhui Xuelingxian Pharmaceutical Co., Ltd.(“Xuelingxian”), Tonghua Tongdetang Pharmaceutical and Medicinal Materials Co., Ltd.(“Tongdetang”) and Shandong Global Pharm Co., Ltd. (“Yaoyuan”) (Xuelingxian, Tongdetang and Yaoyuan collectively referred to as “PRC Subsidiaries”);
|
|
|
·
|
references to the “Bulletin Board” and the “OTC Bulletin Board” are to the Over-the-Counter Bulletin Board, a securities quotation service, which is accessible at the website www.otcbb.com.
|
|
|
·
|
references to PRC Subsidiaries’ “registered capital” are to the equity of PRC Subsidiaries, which under PRC law is measured not in terms of shares owned but in terms of the amount of capital that has been contributed to a company by a particular shareholder or all shareholders. The portion of a limited liability company’s total capital contributed by a particular shareholder represents that shareholder’s ownership of the company, and the total amount of capital contributed by all shareholders is the company’s total equity. Capital contributions are made to a company by deposits into a dedicated account in the company’s name, which the company may access in order to meet its financial needs. When a company’s accountant certifies to PRC authorities that a capital contribution has been made and the company has received the necessary government permission to increase its contributed capital, the capital contribution is registered with regulatory authorities and becomes a part of the company’s “registered capital.”
|
|
|
·
|
“China” or “PRC” refers to the People’s Republic of China, excluding Taiwan and the Special Administrative Regions of Hong Kong and Macau;
|
|
|
·
|
all references to “Renminbi” or “RMB” are to the legal currency of China; and
|
|
|
·
|
all references to “U.S. dollars,” “dollars,” or “$” are to the legal currency of the United States.
|
Amounts may not always add to the totals due to rounding.
5
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Current Report on Form 8-K/A, or Form 8-K/A, and other reports filed by us from time to time with the Securities and Exchange Commission (collectively the “Filings”) contain or may contain forward-looking statements and information that are based upon beliefs of, and information currently available to, our management as well as estimates and assumptions made by our management. When used in the filings the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan” or the negative of these terms and similar expressions as they relate to us or our management identify forward-looking statements. Such statements reflect the current view of our management with respect to future events and are subject to risks, uncertainties, assumptions and other factors (including the risks contained in the section of this report entitled “Risk Factors”) as they relate to our industry, our operations and results of operations, and any businesses that we may acquire. Should one or more of the events described in these risk factors materialize, or should our underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the U.S. federal securities laws, we do not intend to update any of the forward-looking statements to conform them to actual results. The following discussion should be read in conjunction with our pro forma financial statements and the related notes that will be filed herein.
Item 1.01. Entry into a Material Definitive Agreement
On August 12, 2010, Top Flight entered into and consummated a Share Exchange Agreement with Mei Li Tsai, the then sole shareholder of Global Pharma Enterprise Group Limited, a British Virgin Islands company (“Global Pharma BVI”), and Global Pharma BVI to acquire all the issued and outstanding capital stock of Global Pharma BVI, in consideration for 1,800,000 newly issued restricted shares of Top Flight (the “Reverse Merger”). The Reverse Merger was approved by the board of directors on August 12, 2010. Since Mei Li Tsai had previously purchased 19,094,000 shares of common stock from Rhonda Heskett and became our largest shareholder of 78.9% of our then total issued and outstanding shares (as described below in further detail) and since she was also the sole shareholder of Global Pharma BVI, both Global Pharma BVI and Top Flight were under common control of Mei Li Tsai just prior to the Reverse Merger.
Prior to the consummation of the Share Exchange Agreement, on August 6, 2010, our then sole director and officer, Rhonda Heskett, entered into a share purchase agreement with Mei Li Tsai, pursuant to which Ms. Tsai acquired 19,094,000 of Ms. Heskett’s shares of our common stock for cash consideration of $450,000. The $450,000 was expensed and was charged to additional paid-in capital. Subsequently, Mei Li Tsai became our largest stockholder of approximately 78.9% of our then total issued and outstanding shares of stock. In addition, on August 6, 2010, our board of directors approved the appointment of Yunlu Yin as our new Chief Executive Officer and sole director, An Fu as our new Chief Financial Officer and Dan Li as our new Secretary while accepting the resignation of Rhonda Heskett as our director, President, Chief Executive Officer and Chief Financial Officer. As a result of the foregoing, there was a change in control of the Company on August 6, 2010.
Immediately after the closing of the Reverse Merger, we had a total of 26,000,000 issued and outstanding shares of common stock. As a result of the Reverse Merger, Global Pharma BVI is now our wholly owned subsidiary. Global Pharma BVI is the holding company of all the shares of Binomial BioPharm Group Limited (“Binomial”) and Hong Kong Wisdom Fortune Medicine Holding Group Limited (“Wisdom Fortune”), two wholly owned Hong Kong-incorporated companies.
Binomial, in turn, holds all the equity interests in Anhui Xuelingxian Pharmaceutical Co., Ltd.(“Xuelingxian”), Tonghua Tongdetang Pharmaceutical and Medicinal Materials Co., Ltd.(“Tongdetang”), two PRC incorporated companies.
Wisdom Fortune holds all the equity interest in Shandong Global Pharm Co., Ltd. (“Yaoyuan”).
We are, through our indirect wholly owned PRC subsidiaries, namely Xuelingxian, Tongdetang and Yaoyuan (the “PRC Subsidiaries”) in the business of wholesale distribution of pharmaceutical-related products, Chinese herb cultivation and medicine raw materials preparations.
6
We claim an exemption from the registration requirements of the Securities Act of 1933 (the “Act”) for the private placement of these securities pursuant to Section 4(2) of the Act and/or Regulation D promulgated thereunder since, among other things, the transaction did not involve a public offering, the recipient is an accredited investor and had access to information about the Company and their investment, the recipient took the securities for investment and not resale, and the Company took appropriate measures to restrict the transfer of the securities.
Item 2.01. Completion of Acquisition or Disposition of Assets
As described in Item 1.01 above, on August 12, 2010, we acquired all the issued and outstanding shares of Global Pharma BVI pursuant to the Share Exchange Agreement.
As a result of the Reverse Merger, we are now, through our indirect wholly owned PRC subsidiaries, namely Xuelingxian, Tongdetang and Yaoyuan (the “PRC Subsidiaries”) in the business of wholesale distribution of pharmaceutical-related products, Chinese herb cultivation and medicine raw materials preparations.
7
DESCRIPTION OF OUR BUSINESS
Overview
Global Pharm Holdings Group, Inc. and its subsidiaries (collectively “the Company,” “we,” “our” or “us”) are engaged in the wholesale distribution of pharmaceuticals-related products, Chinese herb cultivation and preparations of raw materials for medicine. We conduct business in the People’s Republic of China, or the PRC, through our operating subsidiaries: Shandong Global Pharm Co., Ltd., formerly Shandong Yaoyuan Pharmaceutical Co., Ltd, (referred to as Yaoyuan), Tonghua Tongdetang Pharmaceutical and Medicinal Materials Co., Ltd. (referred to as Tongdetang), and Anhui Xuelingxian Pharmaceutical Co. Ltd. (referred to as Xuelingxian and, together with Yaoyuan and Tongdetang, referred to as the PRC Subsidiaries). We currently have four reportable segments, consisting of (i) pharmaceutical products distribution, (ii) Traditional Chinese Medicine, or TCM, processing and distribution, (iii) herbs cultivation and sales and production and (iv) sales of flower tea bags in China. Currently, we do not have intersegment sales.
Our principal executive offices are located at Room 2503-2505, New World Center, No.6009 Yitian Road, Futian District, Shenzhen, Guangdong 518026, People’s Republic of China.
Business History
We were incorporated in Delaware on February 9, 2007, under the name “Top Flight Gamebirds, Inc,” or Top Flight, to enter the commercial game bird industry and establish a large scale commercial game bird farm. We raised Bobwhite Quail to provide the needs of the commercial hunting preserves, dog trainers and national organizations such as the National Shoot to Retrieve Association. On September 20, 2010, we changed our name to Global Pharm Holdings Group, Inc. Prior to the Reverse Merger (as defined herein), our revenues had not been sufficient to cover our operating costs and to allow us to continue as a going concern.
On August 6, 2010, our sole director and officer, Rhonda Heskett, entered into a share purchase agreement with Mei Li Tsai, pursuant to which Ms. Tsai acquired 19,094,000 of Ms. Heskett’s shares of our common stock for cash consideration of $450,000. Subsequently, Mei Li Tsai became our largest stockholder of approximately 78.9% of our then total issued and outstanding shares of stock. In addition, on August 6, 2010, our board of directors approved the appointment of Yunlu Yin as our new Chief Executive Officer and sole director, An Fu as our new Chief Financial Officer and Dan Li as our new Secretary while accepting the resignation of Rhonda Heskett as our director, President, Chief Executive Officer and Chief Financial Officer. As a result of the foregoing, there was a change in control of the Company on August 6, 2010.
On August 12, 2010, Top Flight entered into and consummated a Share Exchange Agreement with Mei Li Tsai, the then sole shareholder of Global Pharma Enterprise Group Limited, a BVI company (referred to as Global Pharma BVI), and Global Pharma BVI to acquire all the issued and outstanding capital stock of Global Pharma BVI, in consideration for 1,800,000 newly issued restricted shares of Top Flight (referred to as the Reverse Merger). The Reverse Merger was approved by the board of directors on August 12, 2010. Immediately after the closing of the Reverse Merger, we had a total of 26,000,000 issued and outstanding shares of common stock and Mei Li Tsai was our then single largest shareholder of 20,894,000 shares of common stock, or approximately 80.36% of our total issued and outstanding 26,000,000 common shares. As a result of the Reverse Merger, Global Pharma BVI is now our wholly owned subsidiary. Since Mei Li Tsai had purchased 19,094,000 shares of common stock from Rhonda Heskett pursuant to the share purchase agreement as referenced above and became our largest shareholder of 78.9% of Top Flight’s then total issued and outstanding shares and was also the sole shareholder of Global Pharma BVI, both Global Pharma BVI and Top Flight were under common control of Mei Li Tsai just prior to the Reverse Merger.
After Top Flight entered into the Share Exchange Agreement with Global Pharma BVI on August 12, 2010, pursuant to the Earn-In Agreement, as thereafter amended as further described below, the shareholders, including the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan, and the key management of Global Pharma BVI were given the right to purchase 20,894,000 shares at four different occurrence dates, contingent on various targets for total consideration of $300,000. The $300,000 was expensed and was charged to additional paid-in capital. Targets include binding three-year employment contracts with various members of management within six months of these agreements and target after tax net income (the non-cash expenses is excluded from the calculation of net income) of Global Pharma BVI of $3.6 million, $3.8 million, and $15.2 million for the three months ended June 30, 2010 and September 30, 2010, and for the 12 months ended December 31, 2010, respectively. Pursuant to the Share Exchange Agreement, key management and the former shareholders, including beneficial owners of Xuelingxian, Tongdetang and Yaoyuan, acquired call rights to own 80.36% of Top Flight. On September 20, 2010, Top Flight changed its name to Global Pharm Holdings Group, Inc.
8
On March 29, 2011, we entered into an Agreement to Amend the Earn-In Agreement, or the Amendment, with the former shareholders and the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan and 20 individuals of its management who were parties to the original agreement. As of March 29, 2011, pursuant to the Amendment, all of the four earn-in targets have been achieved and the key management and former shareholders and the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan hold, in the aggregate, 20,894,000 shares, or 80.36%, of the Company’s shares of stock.
Prior to our acquisition of Global Pharma BVI on August 12, 2010, Global Pharma BVI entered into a series of agreements pursuant to which Global Pharma BVI acquired 100% of certain entities, which subsequently become wholly owned subsidiaries of Global Pharma BVI and, subsequent to the Reverse Merger, became our wholly owned indirect subsidiaries. On June 29, 2010, Mei Li Tsai and Global Pharma BVI entered into a share transfer agreement, pursuant to which Mei Li Tsai transferred all the 10,000 ordinary shares of Binomial Biopharm Group Limited, a Hong Kong company (referred to as Binomial), to Global Pharma BVI for a consideration of 10,000 Hong Kong dollars. On the same day, Mei Li Tsai and Global Pharma BVI entered into another share transfer agreement, pursuant to which Mei Li Tsai transferred all the 1,000,000 ordinary shares of Hong Kong Wisdom Fortune Medicine Holding Group Limited, a Hong Kong company (referred to as Wisdom Fortune), to Global Pharma BVI for a consideration of 1,000,000 Hong Kong dollars. As a result thereof, Global Pharma BVI is the holding company of all the shares of Binomial and Wisdom Fortune. Global Pharma BVI was incorporated on June 14, 2010 under the laws of the British Virgin Island and Binomial and Wisdom Fortune were each incorporated under the laws of Hong Kong on September 9, 2009 and July 25, 2008, respectively.
On May 6, 2010 and May 8, 2010, Wisdom Fortune and Binomial, respectively, entered into Equity Transfer Agreements, with the shareholders of Yaoyuan, Xuelingxian and Tongdetang. Global Pharma BVI paid RMB 5,180,000 (approximately US$ 700,418), RMB3,000,000 (approximately US $405,647), and RMB10,000,000 (approximately US$1,352,158) to the shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan, respectively, representing their registered capital at the time (referred to as the Equity Transfers).
As a result of the Equity Transfers, Binomial currently holds all the equity interests in Xuelingxian and Tongdetang. Tongdetang and Xuelingxian were incorporated on February 2, 2002 and on July 23, 2008, respectively, under the laws of the PRC. Prior to the Equity Transfer, the shareholders of Xuelingxian were Yunlu Yin, Dandan Wang, Shulan Li, and Hong Zhang. The shareholders of Tongdetang were Yunlu Yin, Qingdong Zeng, and Feng Jin. Mei Li Tsai was the sole shareholder of Binomial from its inception until Global Pharma BVI acquired Binomial on June 29, 2010.
In addition, as a result of the Equity Transfers, Wisdom Fortune currently holds all the equity interest in Yaoyuan. Yaoyuan was incorporated under the laws of the PRC on June 18, 2007. Prior to the Equity Transfer, the shareholders of Yaoyuan were Yunlu Yin, Shouqiang Han, Yanming Lv, Guojun Zhao, Junyan Su, Boliang Zhu, and Chaobo Song. Mei Li Tsai was the sole shareholder of Wisdom Fortune from its inception until Global Pharma BVI acquired Wisdom Fortune on June 29, 2010.
On May 25, 2006 and April 15,2010, Yunlu Yin signed the Trust Agreements with Qinghui Zeng and Qingdong Zeng, respectively, documenting Yunlu Yin as the 62% of owner of Tongdetang and documenting each of Qinghui Zeng and Qingdong Zeng to act as the nominee owner of Tongdetang on behalf of Yunlu Yin. On May 31, 2008 and March 20, 2010 Yunlu Yin signed the Trust Agreements with Yanliang Song and Shouqiang Han, respectively, documenting Yunlu Yin as 80% of owner of Yaoyuan and documenting each of Yanliang Song and Shouqing Han to act as the nominee owner of Yaoyuan on behalf of Yunlu Yin. On September 5, 2009 and April 10, 2010, Yunlu Yin signed the Trust Agreements with Shunli Wang and Shulan Li and Hong Zhang, respectively, documenting Yunlu Yin as 65% of owner of Xuelingxian and documenting each of Shunli Wang and Shulan Li and Hong Zhang to act as the nominee owner of Xuelingxian on behalf of Yunlu Yin.
On June 29, 2010, Mei Li Tsai, the then sole shareholder of Global Pharma BVI, entered into an Earn-In Agreement, as thereafter amended, with key management and the former shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan. Pursuant to the agreement, Global Pharma BVI agreed to enter into a share exchange agreement, at a date subsequent to the agreement, with a United States domiciled shell company and at that time the former shareholders, including beneficial owners, would be entitled to call rights to acquire the controlling interest in the publicly held company according to the following schedule:
9
a. At the time the Buyers enter into a binding employment agreement for a term of not less than three years with the public shell company and the operating companies to serve as the management team within six months after the date of this Agreement, the Buyers would have the call right to purchase 25% of the Seller’s shares, or 5,223,500 shares of common stock of the public shell company for a cash consideration of $75,000. On August 6, 2010, the Buyers, Yinlu Yin, An Fu and Dan Li entered into three-year employment agreements with the Company (as the public shell company) and Zhihao Pan, Zhengang Chi, Yanmin Song, Zhencheng Huang, Yiting Zhang, Yanming Lu, Yanliang Song, Yan Zhang, Xueye Jing, Xianming Zeng, Shunli Wang, Renyuan Su, Qingwei Meng, Nan Li, Naihua Hu, Li Li, Hong Li, Hanjun Liu, Fangyuan Song, Chaobo Song entered into three-year employment contracts with the Company’s operating companies. Because the employment agreements were entered into within six months after the date of the Agreement, the Buyers acquired the first call right to purchase 25% of the Seller’s shares.
b. At the time Global Pharma BVI and its subsidiaries achieve an after-tax net income of $3.6 million for the three months ended June 30, 2010, the Buyers shall have the call right to purchase another 25% of the Seller’s shares, or 5,223,500 shares of common stock of the Company, for a cash consideration of $75,000. The non-cash expenses would be excluded from the calculation of the net income. Global Pharma BVI and its subsidiaries achieved an after-tax, non-cash expenses-excluded net income of $3.8 million for the three months ended June 30, 2010, which was disclosed in the Company’s Current Report on Form 8-K/A filed with the SEC on August 25, 2010 and which has been reviewed and approved by our auditor. Accordingly, the Buyers acquired the second call right to purchase the second 25% tranche of the Seller’s shares.
c. At the time Global Pharma BVI and its subsidiaries achieve an after-tax net income of $3.8 million for the three months ended September 30, 2010, the Buyers would have the call right to purchase another 25% of the Seller’s shares, or 5,223,500 shares of common stock of the Company, for a cash consideration of $75,000. The non-cash expenses would be excluded from the calculation of the net income. Global Pharma BVI and its subsidiaries achieved an after-tax, non-cash expenses-excluded net income of $3.9 million for the three months ended September 30, 2010, which was disclosed in the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 15, 2010 and has been reviewed and approved by the Company’s auditors. Therefore, the Buyers acquired the third call right to purchase the third 25% tranche of the Seller’s shares.
d. At the time Global Pharma BVI and its subsidiaries achieve an after-tax net income of $15.2 million for the year ended December 31, 2010 according to the financial statement prepared by the management, the Buyers would have the call right to purchase the remaining 25% of the Seller’s shares. The non-cash expenses would be excluded from the calculation of the net income. Global Pharma BVI and its subsidiaries have achieved an after-tax, non-cash expenses-excluded net income of $15.2 million.
On November 19, 2010, all the Buyers exercised the first three call rights except that one of the Buyers, having acquired the call rights to purchase a total of 86,200 shares of common stock of Global Pharm, has not exercised her call right due to personal reasons. On March 29, 2011, all the Buyers exercised the fourth call rights and as of March 29, 2011, all call rights have been exercised. The aggregate price for exercising the four call rights is $300,000. As a result of the exercise of the call rights, the former shareholders and the beneficial owners currently hold 20,894,000 or 80.36% of the total outstanding shares of Global Pharm and Ms. Mei Li Tsai owns nil of the total outstanding shares of Global Pharm.
Our Corporate Structure
As set forth in the following diagram, following the Reverse Merger and change of control, we own all the issued and outstanding shares of Global Pharma BVI. Global Pharma BVI, in turn owns all the issued and outstanding shares of Binomial and Wisdom Fortune. Binomial holds all the equity interests in Xuelingxian and Tongdetang. Wisdom Fortune holds all the equity interest in Yaoyuan. A diagrammatic representation of our corporate structure, indicating the legal domicile, geographic location and ownership/control, is as follows:
10
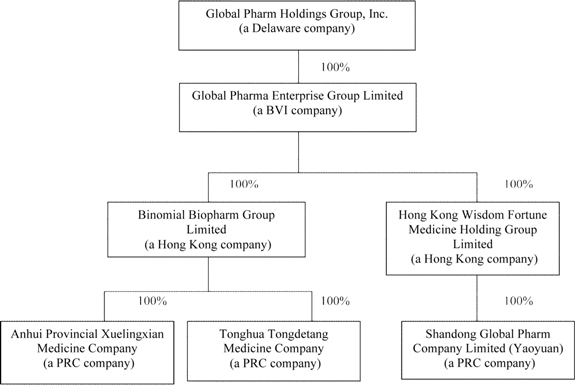
Our PRC Subsidiaries
Yaoyuan
Yaoyuan, was incorporated under the laws of the PRC with a registered capital of RMB10, 000,000 (approximately US$1,352,158). Its predecessor, Ji’nan Sanchao Pharmaceutical Co., Ltd., incorporated as a limited liability company under the PRC laws on November 1, 2005, changed its name to Ji’nan Tian’an Pharmaceutical Co., Ltd. on June 18, 2007 and then to Shandong Yaoyuan Pharmaceutical Co., Ltd. on June 4, 2008. The term of operation for Yaoyuan is from November 1, 2005 to June 28, 2030. On May 6, 2010, the shareholders of Yaoyuan and Wisdom Fortune entered into an agreement, whereby a 100% equity interest in Yaoyuan was transferred to Wisdom Fortune for a consideration of RMB10, 000,000 (approximately US$1,352,158) in cash. On May 21, 2010, Yaoyuan became a foreign wholly owned subsidiary of Wisdom Fortune under the PRC laws.
Yaoyuan primarily engages in the wholesale distribution and sales of pharmaceuticals and traditional Chinese medicine, or TCM, to customers in Shandong province, including hospitals, pharmaceutical companies and retail pharmacies. As a Good Supply Practice, or GSP, certified pharmaceutical company, Yaoyuan operates a 215,000 square feet modern distribution center comprising an advanced modern warehouse of 161,458 square feet. The facility has the ability to store its pharmaceutical inventory at various temperatures.
Yaoyuan currently purchases its products for resale from various suppliers in the PRC including pharmaceutical manufacturers and wholesalers. Yaoyuan makes its product selection based on customer preference and market demand. It also ensures that the manufacture date of the products it purchases does not exceed six months prior to the date of purchase.
Typically, Yaoyuan enters into annual master supplier or distribution contracts with its suppliers and customers at the beginning of each year, which provide the general terms and conditions for transactions in the supplier's products and customer sales over the year. It then enters into a separate order for each consignment of goods, which specifies the type, price and amount of the products it purchases or distributes. Yaoyuan sells or distributes the products to its customers, usually at a price predetermined by the suppliers. Depending on the terms of the contracts, Yaoyuan may or may not be responsible for the shipping cost.
11
Yaoyuan normally enters into annual delivery contracts with third party carriers for delivery of products to its customers. Pursuant to these contracts, Yaoyuan pays a delivery fee of RMB400 (approximately US $59) per vehicle per day, plus tolls, parking and fuel, and the carrier ships the products to designated places and will be responsible for the damages where the products are lost or damaged in transit. Yaoyuan also owns a small fleet of six trucks used for short distance delivery from its warehouse to third-party carriers.
Tongdetang
Tongdetang, a PRC company, was incorporated in Tonghua city, Jilin province on February 2, 2002 with a registered capital of RMB3, 000,000 (approximately US $405,647). Its term of operation is from February 2, 2002 to February 5, 2032. On May 8, 2010, the shareholders of Tongdetang and Binomial entered into an agreement, whereby Binomial acquired a 100% equity interest from Tongdetang’s shareholders for a consideration of RMB3, 000,000 (approximately US $405,647) in cash. On May 19, 2010, Tongdetang became a foreign wholly owned subsidiary of Binomial under the PRC laws.
Tongdetang engages in the wholesale distribution of pharmaceuticals and TCM to over 2,100 customers, including various local Class II hospitals and numerous county health centers/clinics and drugstores. Tongdetang’s core business centers on the major towns and cities in Jilin and Liaoning provinces and every county within Tonghua city and its nearby regions. Its area of operations covers 26,791 square feet, including a 22,486-square foot GSP-certified warehouse and 4,306 square feet office area. The 2,089 square meters GSP-certified warehouse has the ability to store Tongdetang’s pharmaceutical inventory at various temperatures.
Tongdetang purchases its products for resale based on the market demand. It is able to purchase the products at lower prices from local manufacturers and wholesalers because of its geographical advantage. It is situated in the hub of the northern PRC pharmaceutical manufacturing industry cluster and rich Chinese herb resource bank. Typically, Tongdetang enters into master supplier contracts with its suppliers at the beginning of each year, which provides the general terms and conditions for transactions in the supplier’s products over the year. It then enters into a separate order for each consignment of goods each time it actually purchases products from a supplier. Upon purchase, Tongdetang takes title to the products and books them as inventory. It then distributes the products to its customers at a price predetermined by the suppliers. The suppliers bear the delivery fee and guarantee products supply during the term of the contract. Typically, Tongdetang pays for these products within 60 days from the date of delivery. Tongdetang typically provides 30-day credit terms to its customers.
The suppliers normally deliver products to Tongdetang’s warehouse, but Tongdetang may arrange for the transportation of products to its warehouse if a particular supplier is unable to do so. In such a case, Tongdetang levies a fee on the supplier for reimbursement of the transportation costs. Tongdetang usually enters into annual delivery contracts with third party carriers for delivery of products to its customers. Tongdetang will pay an agreed upon delivery fee to its carriers for each delivery. The carriers assume the responsibility for any loss or damage to the products enroute to Tongdetang’s customers. There is no seasonality or cycles for its sales.
Xuelingxian
Xuelingxian, a PRC company, was incorporated in Anhui province on July 23, 2008 with a registered capital of RMB 5,180,000 (approximately US $700,418). Its term of operation is from July 23, 2008 to June 8, 2030. On May 8, 2010, the shareholders of Xuelingxian and Binomial entered into an agreement, whereby a 100% equity interest in Xuelingxian was transferred to Binomial in exchange for a consideration of RMB5, 180,000 (approximately US $700,418) in cash. On May 24, 2010, Xuelingxian became a foreign wholly owned subsidiary of Binomial under the PRC laws.
Xuelingxian engages primarily in the Chinese herb cultivation, TCM processing and distribution, sale of flower tea bags, OEM drugs (OEM drugs refer to generic drugs we purchase from pharmaceutical manufacturers that we rebrand and sell under our own brand names), and health products distribution business.
Xuelingxian rents two Chinese herbal planting bases comprising approximately 2,960 acres and has entered into independent contracts with local farmers, whereby the farmers tend to the cultivation of the Chinese herbs including seeding, conducting planting experiments, and harvesting. These farmers are independent contractors and are paid monthly. The planting base, as well as the warehouses and administrative offices of Xuelingxian, are located in Bozhou region, which is the largest domestic Chinese herb cultivation and trading center in the PRC. Bozhou has a history of 1,800 years in Chinese herb cultivation and trading. Every year, thousands of varieties of Chinese herbs are traded at the Bozhou TCM raw materials transaction market.
12
Besides the agribusiness, Xuelingxian also processes approximately 500 varieties of Chinese natural herbs that it purchases from the Bozhou TCM raw materials transaction market, and sells these processed herbs to either pharmaceutical distributors, hospitals or various types of medical institutions. Those treated herbs are often called “prepared slices” and can be immediately used as herbal medicine. The kind of treatment applied to the herbs varies according to the type of herbs. Xuelingxian now hires 104 skillful farmers working as independent contractors to carry out these processes such as crushing, powdering and slicing. Additionally, Xuelingxian engages in the business of sale and distribution of five varieties of OEM drugs. These drugs are originally manufactured by small pharmaceutical companies such as Jilin Bencaotang Pharmaceuticals Co. and Changchun Chenguang Pharmaceuticals LLC. Xuelingxian has contracted to obtain the right to affix its own trademark “Xuelingxian” on the drugs and to resell them. Besides OEM drugs, Xuelingxian also engages in the business of distributing 31 varieties of Chinese herbal drugs and health products. These drugs and health products are often distributed to local hospitals and clinics in Anhui province, individual distributors and downstream distribution companies. Xuelingxian normally contracts with third party carriers for delivery of products to its customers, and Xuelingxian bears the delivery fees.
Xuelingxian maintains a production line for the manufacture of flower tea bags in Bozhou. Xuelingxian entered into a 15-year contract with Bozhou Fengyi Institute of Traditional Chinese Medicine to rent a 107,639 square feet factory. (For more information about the lease, please see the subsection “Properties.”) Xuelingxian currently sells 13 different types of flower tea bags. The flower tea bags are sold to supermarkets, chain drug stores and small pharmacies in Anhui province.
In 2010, the revenue composition of Xuelingxian was 53%, 36% and 11% for TCM processing and distribution, herb planting and flower tea, respectively.
Xuelingxian’s herb cultivation and sales business is subject to seasonal fluctuations. Demand is lower in the first quarter of each year because our customers generally pay fewer visits to drugstores during the Chinese New Year, which occurs during that period. Sales also are lower in summer from July to August. Sales increase during the autumn period, starting from September until the winter, which is harvest season for herbal plants.
Products
Yaoyuan and Tongdetang
The major pharmaceutical products Yaoyuan and Tongdetang sell can be divided into two major groups: OTC and prescription drugs. Based on different usages, they can be broken down into 18 categories, including: Gynecologic, Anti-allergy, Pediatrics, Analgesia, Cardiovascular, Antibiotics, Diet, Rheumatology and Bone Disease, Neural, Gastrointestinal diseases, Urology, Hepatobiliary, ENT (Ear, Nose and Throat), Diabetes, Asthma, Flu, Kidney, and Ophthalmology.
In 2010, Yaoyuan sold over 4,800 types of products, of which approximately 680 are exclusively distributed by Yaoyuan in Shandong province. Yaoyuan’s primary products sold consist of prescription drugs and over-the-counter, or OTC, drugs. Sales for each category accounted for 72% and 27% of the total sales in 2010, respectively. There is no seasonality or cycles for its sales.
In 2010, Tongdetang sold over 7,200 types of products. Tongdetang’s primary products sold are OTC drugs, prescription drugs and nutritional supplements. Sales for each category accounted for 45%, 42% and 13% of the total sales, respectively, in 2010. There is no seasonality or cycles for its sales.
Xuelingxian
The major products of Xuelingxian are self-cultivated Chinese herbs and processed Chinese herbs, flower tea bags series and OEM drugs.
13
Xuelingxian has the license to sell five varieties of OEM drugs under the trademark of “Xuelingxian.” (For more information of the trademark, please see the “Intellectual Properties” subsection.) These drugs are Tenghuang Jianguwan, Yigan Jiedu (capsule), Fuke Zhixueling, Tianma (capsule) and Sanqi Shangyaopian. The ingredients and usages of these drugs are listed as follows:
|
Name
|
Ingredients
|
Usage
|
||
|
Tenghuang Jianguwan
|
Prepared rehmannia root, pyrola, drynaria rhizome (hot), desertliving cistanche, herba epimedii, caulis spatholobi and semen raphani (frying).
|
Tonifying the kidney, invigorating the circulation of blood and relieving pain. Treatment of Marie-Strumpell disease, cervical spondylosis, calcaneal spur, hypertrophic arthritis and kaschin beck disease.
|
||
|
Yigan Jiedu (capsule)
|
Golden cypress, rhizomabistortae, scutellariabaicalensis, rheum officinale, rhizome picrorhizae, rhizoma smilacis glabrae, black alum and cyrtomium fortune.
|
Clearing heat and detoxicating, soothing liver-gallbladder; treating hepatitis B and liver and gallbladder damp-heat.
|
||
|
Fuke Zhixueling
|
Prepared rehmannia root, schisandra chinensis, eucommia ulmoides (carbonized), teasel root, radix paeoniae alba, Chinese yam, oyster (calcining), cuttlebone, garden burnet (frying), cattail pollen (carbonized) and mistletoe.
|
Tonifying kidney, retaining yin with astringent, consolidating Chong Vessel and promoting bloodclotting ; used to treat uterine bleeding in women.
|
||
|
Tianma (capsule)
|
Rhizoma gastrodiae, notopterygium root, radix angelicae pubescentis, eucommia ulmoides (hot salt frying), the root of bidentate achyranthes, rhizoma dioscoreae hypoglaucae, monkshood (processed), angelica sinensis, rehmannia and radix scrophulariae.
|
Dispelling wind, eliminating dampness, relaxing tendons, and activating collaterals; relieving muscular constricture and back and leg ache, etc.
|
||
|
Sanqi Shangyaopian
|
Pseudo-ginseng, radix aconiti agrestis (steamed), short-pedicel aconite root, borneol, drynaria rhizome, safflower, blood-wort and root of common peony.
|
Relaxing muscles and stimulating blood circulation; used in the treatment of traumatic injury, rheumatism, joint ache, acute and chronic bruise and neurodynia.
|
In addition to the OEM drugs, Xuelingxian also grows several varieties of Chinese herbs in the herbal planting base, and sells them at the Bozhou TCM raw materials transaction market. These herbs are rhizoma dioscoreae, flos chrysanthemi, radix rehmanniae, herba menthae radix angelicae dahuricae, radix ophiopogonis, and radix glehniae. Depending on the nature of the plant, the growth period of these herbs are one year. There is a steady demand for our products at the Bozhou TCM raw materials transaction market. Since the Bozhou TCM raw materials transaction market attracts thousands of buyers across the country every year, and the varieties of herbs Xuelingxian grows are among the 1,200 varieties of the herbs which are highly sought after by the Chinese herbal medicine manufacturing market, Xuelingxian usually finds a ready market for its products. Xuelingxian does not maintain any long-term contracts with buyers, and such deals are often negotiated and consummated at the market.
14
Xuelingxian also processes over 40 varieties of herbs, making herbs into prepared slice of Chinese herbal medicine by different means such as drying, powdering, and slicing. Since these treatments applied to the herbs are very basic, Xuelingxian does not have to pay income tax on the income it earns for the processed herbs. (For more information, please see the subsection “Government Regulation.”)
Distribution Process
Our distribution process is set forth below:
|
|
1.
|
Certification/Management of supplier’s qualification: we review our suppliers’ qualifications and only purchase goods from certified suppliers;
|
|
|
2.
|
Procurement forecasting: we make medium to long-term procurement plans based on market demand and our forecast and then implement the procurement plan;
|
|
|
3.
|
Procurement agreement: we enter into procurement agreements with manufacturers and upper-level suppliers and establish sales terms such as sales/purchase price and term of payment;
|
|
|
4.
|
Procurement planning: we enter into short-term procurement plans to promptly react to the market demand and deal with the short-term emergencies;
|
|
|
5.
|
Procurement request: we receive purchase orders from our clients;
|
|
|
6.
|
Order: we order drugs from manufacturers or upper-level distributors based on the procurement request we received from our clients;
|
|
|
7.
|
Quality inspection: we conduct quality inspection according to our drug quality control standards;
|
|
|
8.
|
Storage: our purchased products are stored in the warehouse;
|
|
|
9.
|
Distribution approval: orders from our clients need to be approved by us before delivery;
|
|
|
10.
|
Distribution center: from our distribution center, we deliver goods to our clients and our branches (or regional offices).
|
Quality Control
In China, each pharmaceutical distributor is required to meet the GSP standards and obtain a Pharmaceutical Trading Permit before it engages in any pharmaceutical distribution. GSP standards regulate wholesale and retail pharmaceutical product distributors to ensure the quality of distribution of pharmaceutical products in China. The current applicable GSP standards require pharmaceutical product distributors to implement strict controls on the distribution of medicine products, including standards regarding staff qualifications, distribution premises, warehouses, inspection equipment and facilities, management and quality control. Pharmaceutical Trading Permits are granted by the State Food and Drug Administration to distributors that meet requirements such as legally qualified pharmaceutical experts, hygienic equipment and facilities, quality control on products distributed and records retention for at least three years.
We are GSP-certified and have obtained a Pharmaceutical Trading Permit. In order to prevent counterfeit products penetrating into our supply chain, we have implemented a series of quality control procedures in our procurement process. We strongly emphasize quality control for both merchandise sourcing and delivering services. Our quality control starts with procurement. In particular, we have screened numerous GMP-certified manufacturers in China and selected a core set of certain suppliers after reviewing product selection and quality, manufacturing, packaging, transportation and storage capabilities as well as cost competitiveness.
We conduct random quality inspections of each batch of products we procure. We replace any suppliers that fail to pass our quality inspections. We have established a quality control department and maintain quality inspectors at each of our subsidiaries.
Customers and Suppliers
Yaoyuan
None of Yaoyuan’s customers accounted for more than 10% of its annual sales for the past three fiscal years. Yaoyuan has a very diverse customer base and is not dependent on any one customer for a major portion of its sales. The top ten customers accounted for 32%, 35% and 42% of its annual sales for years 2010, 2009 and 2008, respectively.
15
For 2010, 2009 and 2008, the sales of Yaoyuan have been mainly concentrated in Shandong province, which accounts for 75%, 78% and 75%of the annual sales of Yaoyuan respectively.
Tongdetang
None of Tongdetang’s customers accounted for more than 10% of the total sales for the past three fiscal years. Tangdetang’s customers are very diverse. Tongdetang is not dependent on any one major customer for a material portion of its sales. The top ten customers accounted for only 13%, 16% and 17% of its total sales for the years ended December 31, 2010, 2009 and 2008, respectively.
The significant majority of sales for 2010, 2009 and 2008 are in the Tonghua and Baishan regions of Jilin province, and a small percentage of sales are in the Liaoning province. The percentage of sales for 2010 and 2009 in Jilin was 97% and 99%, respectively and 2% and 1% in Liaoning, respectively.
Xuelingxian
Xuelingxian’s main customers are downstream distributors, individual agents, individual vendors, hospitals, clinics, supermarkets and pharmacies. One customer, Anhui Wan An Medicine Company, Ltd, accounted for more than 10% of Xuelingxian’s sales in 2010 and 2009 and two customers accounted for more than 10% of its sales in 2008. Xuelingxian does not have any long-term contracts with any of its customers, and it distributes its products to various provinces in the PRC including Jilin, Shandong and Jiangxi.
Suppliers
Yaoyuan
For the year ended December 31, 2010, Yaoyuan had two major suppliers that provided approximately 56% and 10%, respectively, of its annual raw materials. For the years ended December 31, 2009 and 2008, Yaoyuan had only one major supplier that supplied more than over 10% of its annual raw materials. For the past three years, Xiuzheng Pharmaceutical Group/Marketing Co. Ltd. (“Xiuzheng”) has been Yaoyuan’s biggest supplier. The purchases from Xiuzheng have accounted for 9.7%, 24.5% and 46.1% of Yaoyuan’s total purchase for 2010, 2009 and 2008, respectively. Yaoyuan typically enters into annual distribution agreements with Xiuzheng, under which Yaoyuan distributes Xiuzheng’s products at a predetermined price within Shandong province and makes monthly payment to Xiuzheng for the products it distributes each month.
Tongdetang
Tongdetang had no supplier that accounted for more than 10% of its annual purchase for 2010 and two major suppliers that accounted for 13.5% and 11.6%, respectively, of its annual purchase in 2009, and 13.2% and 11.2%, respectively, of its annual purchase in 2008. Changchun Yongxin Dirui Drug Co., Ltd, (“Yongxin”) and Changchun Changheng (“Changheng”) have been Tongdetang’s biggest suppliers for the past three years. Tongdetang typically enters into annual distribution agreements with Yongxin and Changheng, under which Tongdetang distributes their products at a predetermined price and makes monthly payment to them for the products distributed each month.
Xuelingxian
Since Xuelingxian itself is an upstream supplier, supplying herb plants and roughly processed herbs which need to be furthered processed by downstream manufacturers, its suppliers of raw materials are mainly suppliers of drugs and health products for its distribution business.
Xuelingxian had one supplier, Bozhou Zhongzheng City Sliced Chinese Crude Drugs Company, Ltd (“Zhongzheng”). that accounted for 30% and 46% of its annual purchase in 2010 and 2009, respectively and two major suppliers that accounted for 63.2% and 16.5% of its annual purchase of raw materials in 2008. Xuelingxian entered into annual supply agreements, dated February 1, 2009 and January 15, 2011 respectively, with Zhongzheng.
16
We do not have long-term contracts with any of our suppliers.
Material Supply Agreement
On January 1, 2010, Yaoyuan and Tongyao Branch of Xiuzheng entered into a general distribution agreement, or the Xiuzheng Agreement. The term of the Xiuzheng Agreement is from January 1, 2010 to December 31, 2010. Pursuant to the Xiuzheng Agreement, Yaoyuan is obligated to distribute Xiuzheng’s products within Shandong province. Xiuzheng bears the costs for the delivery of its products to Yaoyuan while Yaoyuan is responsible for the expenses of delivery to its clients. If the products are defective, Yaoyuan will notify Xiuzheng within seven days upon receiving the products. Otherwise, Yaoyuan bears the loss incurred. Yaoyuan is also obligated to help market Xiuzheng’s products, protect Xiuzheng’s intellectual property rights and deal with public relation affairs. The Xiuzheng Agreement will be terminated if Yaoyuan distributes counterfeit products, does not distribute products at specified prices or distributes the products outside Shangdong province.
On January 1, 2010, Yaoyuan and Hainan Lingkang Pharmaceutical Co., Ltd., or Lingkang, entered into a provincial exclusive distribution agreement, or the Lingkang Agreement. The term of the Lingkang Agreement is from January 1, 2010 to December 31, 2010. According to the Lingkang Agreement, Yaoyuan has the right to distribute Lingkang’s products exclusively in Shandong province. Lingkang bears the costs for delivery of its products to Yaoyuan while Yaoyuan is responsible for the expenses of delivery to its clients. If the products are defective, Lingkang is responsible to recall such products and to pay for all the related costs. Yaoyuan is obligated to help market Lingkang’s products.
Both contracts have been renewed at the same terms for 2011.
Marketing and Sales
The success of our business largely depends on our marketing and sales efforts. We expanded our market reach by increasing the size of our sales force and increasing our marketing budget.
We have an extensive distribution network covering Shandong, Jilin and Anhui provinces with 140 marketing and sales employees dedicated to marketing and selling our products. We generate business by marketing directly to hospitals, pharmaceutical companies, retail drugstores and medical clinics in the PRC. Additionally, we promote our company and products by attending pharmaceutical exhibitions, such as Pharm China Exhibition and monthly provincial exhibitions. We also advertise our business and products to consumers through brochures and outdoor advertising.
Our sales staff is paid a commission based on the volume of sales they generate and the types of products they sell.
Set forth below is a breakdown of our marketing and sales staff among our PRC Subsidiaries and the annual marketing expense for the past three years. We anticipate that our marketing expense will grow as we expand our business organically and through future acquisitions.
|
Number
of Sales
|
Marketing Expense (US$)
|
|||||||||||||||
|
Company
|
Staff
|
2010
|
2009
|
2008
|
||||||||||||
|
|
|
|
|
|||||||||||||
|
Yaoyuan
|
75 | 775,296 | 309,586 | 187,627 | ||||||||||||
|
|
||||||||||||||||
|
Tongdetang
|
53 | 195,576 | 177,312 | 106,110 | ||||||||||||
|
|
||||||||||||||||
|
Xuelingxian
|
12 | 150,450 | 123,632 | 26,294 | ||||||||||||
17
Overview of the PRC Pharmaceutical Industry
We operate in the large and rapidly growing healthcare industry in the PRC. The PRC healthcare industry is supported by a combination of socio-economic factors, such as the growth of the PRC’s economy, size of its overall population and the proportion of its population over the age of 60, living standards, health consciousness, lifestyle related disorders and active PRC government support.
Primary Growth Drivers of the Healthcare Industry: Increased Spending and Active Governmental Support
According to the PRC Statistical Yearbook 2009, or the “Yearbook, from 2005 to 2009, the average per capita annual disposable income of the PRC’s urban residents increased from approximately RMB 3,225 (approximately $474) to RMB5,153 (approximately $1,356). According to the Yearbook, the PRC’s Gross Domestic Product, or GDP, grew at a compound annual growth rate, or CAGR, of 16.4% from 2005 to 2009, and its per capita GDP grew from RMB18, 494 (approximately $2,719) in 2005 to approximately RMB33, 535 (approximately $4,931) in 2009. During this period, national income and disposable income levels increased significantly.
With rising living standards and increasing disposable income, people in the PRC have naturally become more health conscious. These developments have resulted in both urban and rural residents spending more on healthcare. According to the PRC National Bureau of Statistics, consumer expenditure on healthcare in the PRC’s urban and rural areas increased from approximately RMB476 (approximately $70) and RMB118 (approximately $17) per person in 2003, respectively, to approximately RMB786 (approximately $115) and RMB246 (approximately $36) per person in 2007, respectively.
As part of its Eleventh Five-Year Plan (2006-2010), the PRC government has actively supported the PRC healthcare industry by creating a number of incentives and enacting programs, including increased funding for building additional hospitals, research centers and other healthcare facilities, enacting healthcare reforms and standards and subsidizing healthcare services for its citizens. The PRC government has announced it will spend an additional RMB850 billion on healthcare programs from 2009 to 2011, which will significantly bolster the PRC healthcare market.
Pharmaceutical Distribution in the PRC
The pharmaceutical distribution market connects pharmaceutical manufacturers with pharmaceutical retailers, including hospital pharmacies, drugstore chains and independent community drugstores, community clinics and other points of sale retail outlets. Distributors can increase the operating efficiencies of manufacturers by acting as the latter’s direct customers and relieving them from the burden of delivery to, and collecting payment from, numerous retailers. On the other hand, by using distributors as suppliers, retailers benefit from reduced transaction costs and administrative burdens as well as improved confidence in their product supply. The chart below illustrates the distribution value chain of pharmaceutical products in the PRC:
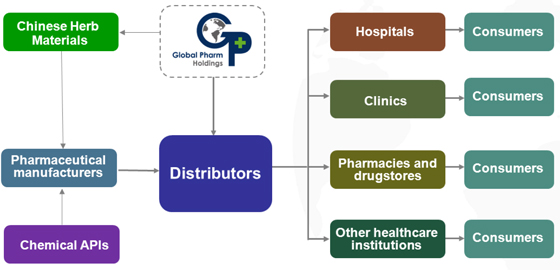
18
Typically, pharmaceutical distributors enter into agreements to purchase pharmaceutical products from manufacturers. In many cases, distributors also seek from manufacturers the right to be an exclusive distributor of a particular medicine or groups of medicines. They generate revenue by reselling these pharmaceutical products downstream and providing relevant services to customers in the retail market. In general, pharmaceutical distributors do not hire pharmaceutical sales representatives to promote medicines and healthcare products to physicians, pharmacists and other healthcare professionals. Promotional efforts are generally undertaken by the manufacturers or companies providing outsourced promotion services. However, distributors’ sales teams work with sales representatives of manufacturers to ensure that product demands are met.
In addition, large pharmaceutical distributors in the PRC offer complementary logistics and value-added services to manufacturers and retailers. In the PRC market, the ability to provide these services is highly valued and increasingly required by many larger customers, such as hospitals. As a result, a distributor’s ability to provide services constitutes a competitive advantage and enhances its relationships with customers and suppliers. These logistics and value-added services include electronic purchase orders confirmation, tailored packaging, repackaging and reprocessing services, product insurance brokerage, payment collection on behalf of manufacturers, product return, replacement or recall mechanisms, inventory tracking and management, delivery of specialty pharmaceutical products, technical support and sales assistance, import agency, customs clearance, free trade zone warehousing and other services.
Hospitals and Retail Pharmacies
In the PRC, people usually purchase medication from hospital pharmacies or retail pharmacies. Particularly, most prescribed drugs are filled in hospital pharmacies, because the drugs prescribed from doctors in a particular hospital are always reliably available. Retail pharmacies, though more convenient, do not maintain a large variety of prescribed drugs. With respect to OTC drugs, retail pharmacies are more preferable because of their proximity to customers.
Most hospitals in the PRC are owned and operated by the PRC government. In addition, the vast majority of hospitals are located in urban areas, with rural areas suffering from both a lack of hospitals and clinics as well as qualified medical staff and resources at the facilities they do have. PRC hospitals are classified under the Ministry of Health-administered hospital classification system into three classes based upon a number of factors, including reputation, number of doctors and nurses, total number of in-patient beds, equipment and expertise. The best and largest hospitals are designated as Class III hospitals, and the second and third tiers as Class II and Class I, respectively. In 2009, 1,233, 6,523, 5,110 and 7,425 were designated as Class III, Class II, Class I and upgrading hospitals, respectively, according to Ministry of Health statistics yearbook of 2010.
While outpatients in the PRC generally fill their prescriptions at hospital pharmacies, they primarily purchase OTC medicine from retail pharmacies. To the extent that a medical condition can be treated with an OTC medicine, many Chinese choose to purchase an OTC medicine instead of seeing a doctor in a hospital for a prescription medicine.
The retail pharmacy sector in the PRC is highly fragmented, including pharmacy chain stores, individual stores, retail chain stores with OTC counters, and OTC medicine counters in supermarkets. While they are expanding quickly, neither pharmacy chain stores nor retail chain stores with OTC medicine counters have developed a nationwide presence in the PRC. Retail pharmacies grew at a 16% CAGR, 2% higher than the growth rate of hospital pharmacies during the period of 2002 to 2007. As a result, the market share of retail drugstores increased from 27.3% in 2002 to 29.4% in 2007, as cited by Morgan Stanley Research dated February 6, 2009, China Pharmaceuticals (referred to as Morgan Stanley Research).
Future Development
Consolidation Needed to Survive in Fragmented Industry
|
|
·
|
Currently, the pharmaceutical distribution industry in China is highly fragmented. There were more than 7,000 GSP-certified pharmaceutical distributors as of 2009 according to the South Medicine Economics Research Institute, an affiliate of the State Food and Drug Administration, or the SFDA. According to China Association of Pharmaceutical Commerce (CAPC), the three largest pharmaceutical distributors in China accounted for only approximately 19.6% of the PRC pharmaceutical distribution industry in 2010, in terms of their share of the total revenues of the pharmaceutical distribution industry in China. Given the level of fragmentation in the pharmaceutical distribution industry, we believe that only large distributors with effective nationwide distribution capabilities, value-added supply chain services and large-scale operations will thrive.
|
19
|
|
·
|
In 2009, the top ten drugstore chains produced 38.9% of the sales from the top 100 drugstore chains. Retail drugstores can be categorized into three major types: individual stores, drugstore chains and OTC in supermarket or chain stores, as cited by China Drug Store Magazine.
|
Low Barriers to Entry for Start-ups
|
|
·
|
It is relatively easy to open a drugstore in the PRC. Although a GSP certification and registration with the local government are required, more than 100,000 drugstores were opened in 2009 and 2010. By the end of 2010, there were 388,581 drugstores including 244,934 individual stores.
|
Increasing Numbers of Chain Drugstores
|
|
·
|
According to the SFDA’s report, 2,130 chain drugstore companies managing approximately 143,647chain drugstores exist in the PRC. Facing the fierce market competition and changes in government policies, more and more individual drugstores are seeking to consolidate their operations, as cited from 2010 Deep Study Report of China Pharmaceutical Economics.
|
Government Policies Shifting the Balance to Drugstores
|
|
·
|
Government policy encourages drugstores to take more business from hospital pharmacies. We expect this trend to continue due to the: 1) government‘s intention to separate pharmacies from hospitals – an effort to avoid potential conflicts of interest between distributors and doctors, 2) use of drug names instead of brand names to enable fair selection by the patient and 3) intention to make prescription medicines that were only available in hospital pharmacies available in retail drugstores as well.
|
Offering Non-Drug Products and Services
|
|
·
|
Retail drugstores are now more diverse in terms of services and products. Some drugstores offer in-house doctors for quick consultations. Non-pharmaceutical health products such as nutritional supplements and personal healthcare consumables are also available. These new revenue streams complement the traditional sales of OTC-only drugs.
|
Competition
Competitive Environment
Through our PRC Subsidiaries, we are now engaged in pharmaceutical wholesale and distribution, Chinese herb cultivation and sales, preparation and sales of Chinese medicine raw materials and sales of health products. In the rapidly evolving and highly fragmented PRC pharmaceutical industry, we face fierce competition in each of our operations.
20
Yaoyuan
Relying on its modern warehouse management systems and high storage capacity, Yaoyuan maintains its competitive advantages in the market. Below is a list, to the best of our knowledge, of our five major competitors in the pharmaceutical business:
|
Competitors
|
Competing Business Line
|
|
|
Shandong Jointown Pharmaceutical Group
|
Distribution
|
|
|
Ji’nan Zhongxin Pharmaceutical Co., Ltd.
|
TCM raw materials, TCM products, chemical preparation, antibiotics, medical equipment
|
|
|
Ji’nan Pharmaceutical Group Co., Ltd.
|
TCM raw materials, TCM products, chemical preparation, antibiotics, medical equipment
|
|
|
Shandong Daxun Pharmaceutical Logistics Co.
|
Distribution
|
|
|
Ji’nan Hengfeng Weiye Pharmaceutical Co.
|
TCM raw materials, TCM products, chemical preparation, antibiotics, medical equipment
|
Tongdetang
Tongdetang faces fierce competition in local pharmaceutical distribution business in the Tonghua region. Below is a list, to the best of our knowledge, of its major competitors:
|
Competitors
|
Competing Business Line
|
|
|
Tonghua Tianxiang Pharmaceuticals Co.
|
Pharmaceutical wholesale and distribution
|
|
|
TonghuaXiuzhengtang Drug Wholesale Co.
|
Pharmaceutical wholesale and distribution
|
|
|
Tonghua Medicine Plaza Co.
|
Pharmaceutical wholesale and distribution
|
|
|
Tonghua Boxiang Mega Chain Drugstores Co.
|
Pharmaceutical wholesale and distribution
|
|
|
Tonghua Medicing & TCM Materials Co.
|
Pharmaceutical wholesale and distribution
|
Xuelingxian
There are 26 pharmaceutical companies including between three to five herb cultivation companies operating in Bozhou city of Anhui province. Xuelingxian faces direct competition from 25 local Chinese medicine manufacturers. Below is a list, to the best of our knowledge, of Xuelingxian’s major competitors:
|
Competitors
|
Competing Business Line
|
|
|
Bozhou Jianqiao Medicine Co.
|
Pharmaceutical wholesale
|
|
|
Anhui Bozhou Medicine & TCM Materials Co.
|
Pharmaceutical wholesale
|
|
|
Anhui Bozhou Drug Procurement Station
|
Pharmaceutical wholesale
|
|
|
Bozhou Hengcheng Medicine Co.
|
Pharmaceutical wholesale
|
|
|
Bozhou Rainbow GAP Herb Planting Co.
|
TCM cultivation, seed breeding and GAP planting base construction, herbs trading and agriculture products procurement
|
21
Our Competitive Strengths
We believe that by leveraging the following strengths, we can effectively compete and enhance our market position.
|
|
·
|
Extensive sales network and broad product portfolio. We have an extensive distribution network covering Shandong, Jilin and Anhui provinces. We also have a broad product portfolio of over 8,200 different types of pharmaceutical and healthcare products. We believe our distribution network and product portfolio is broader than those of our local competitors.
|
|
|
·
|
Strong relationships with customers and suppliers. We had entered contracts with numerous reputable domestic pharmaceutical and healthcare products manufacturers, and have maintained close business relationships with these leading manufacturers. We also have good long-time relationship with our customers, comprising of hospitals, other distributors, retail drug stores and other entities that sell pharmaceutical and healthcare products, located throughout the PRC. We believe that the breadth of our distribution network and product portfolio, and our advanced value added and logistics services, strengthens our existing supplier and customer relationships, allows us to respond quickly and efficiently to our customers’ pharmaceutical requirements and enables us to pursue new relationships with key pharmaceutical and healthcare product suppliers.
|
|
|
·
|
Advanced value-added supply chain services. We provide advanced value-added supply chain services, such as supplier solutions, online product ordering, inventory tracking and management, distribution center management as well as supply chain management for middle-small size distributors. Our value-added services benefit our customers by improving the delivery of pharmaceutical and healthcare products to patients, lowering their overall costs in the pharmaceutical supply chain, and benefit our suppliers by ensuring the quality and timely distribution of their products and meeting their needs for operational flexibility, efficiency and cost-effectiveness.
|
|
|
·
|
Comprehensive logistics arrangements. We provide comprehensive logistics arrangements, consisting of storage, warehousing, and long-distance, regional and local transportation and delivery services through our logistics infrastructure. Our logistics arrangements aim to manage the flow of products and information with high efficiency and precision, as well as minimize our inventory holding costs.
|
|
|
·
|
Integrated business units. We have achieved cost advantages and stability in upstream quality TCM supply through our herb-cultivating subsidiary, Xuelingxian. Through Yaoyuan and Tongdetang, we are able to directly deliver our aggregated TCM and healthy products from the herb plating bases, private label drugs from the manufacturing business and OEM drugs from the distribution business to either our downstream customers. The integration of our three business units provide significant operating and margin synergies. In this regard, we believe we can achieve a significant integrated value chain return from our vertically consolidated business and significant synergy which provides us with a competitive edge over our competitors.
|
|
|
·
|
Experienced management team. We are led by a team of highly experienced professionals in the fields of pharmaceutical manufacture, distribution and retail. The majority of our senior management team possesses an average of 15 years of related industry experience. Our strong management team has rich industry resources as well as extensive experience in mergers and acquisitions in the pharmaceutical industry, and has been active in capturing market opportunities, forming and implementing successful business strategies, assessing and managing risks, directing our expansion efforts to high growth areas and increasing our overall profitability. We believe that we have the requisite leadership to reinforce our core strengths and execute our business strategies.
|
Business Strategy
We intend to grow by developing new products and expanding our existing distribution and sales networks. We also intend to implement a growth strategy through acquisitions of companies or operations that complement our existing distribution networks, product lines, or other capabilities.
We intend to continue to improve our operations, exploit our competitive strengths, and expand our operations by extending our direct geographical reach in China through establishing or acquiring new distribution centers. Our goal is to have a facility in each of the target provinces through continuous acquisitions of local pharmaceutical distributors with GSP-certified facilities in the next three to five years. The acquisition cost will depend on our negotiations with the acquisition targets. We will use our retained earnings as well as proceeds from future financings to implement our acquisition strategy. We intend to bolster our direct selling efforts to hospitals, in order to build stronger relationships with these significant customers. We also expect to enhance our penetration into new customer categories and demographics. We will also seek to optimize our product portfolio to include more products with higher margins and expand our product offerings. Further, we intend to distinguish ourselves through the variety and depth of our products and value-added services, operational flexibility and rapid and responsive customer support. We also believe we can capitalize on significant market opportunities in the rapidly growing PRC pharmaceutical industry by expanding our existing market coverage and enhancing strategic cooperation with our suppliers and customers.
22
We plan to continue to build upon our integrated business platform in order to enhance the synergies that arise from our pharmaceutical operations spanning the distribution and manufacturing of pharmaceutical, healthcare products and TCM products. We plan to leverage our existing businesses to capitalize on particular opportunities that may arise and create efficiencies and cost savings in our business operations. In addition, we plan to utilize our extensive distribution network to provide reliable supply channels for our prospective retail drug stores customers which, in turn, will sell the pharmaceutical and healthcare products supplied by our distribution network. Further, we intend to utilize outside pharmaceutical manufacturing operations to produce OEM products for either our distribution operation or prospective retail pharmacy customers prior to the establishment of our own pharmaceutical manufacturing operations.
Research and Development
We currently do not conduct any research and development activities.
Intellectual Property
We regard our trademarks as a factor to our success. We do not own any trademarks. However, we own two exclusive trademark use rights - “Xuelingxian” and “Yaoyuan.” Trademark “Xuelingxian” is owned by a former shareholder of Xuelingxian, Jingsheng Wang, pursuant to a license agreement dated January 1, 2009 for a term from January 1, 2009 to January 1, 2014. “Yaoyuan” is owned by a former shareholder of Yaoyuan, Yanliang Song, pursuant to a license agreement dated April 7, 2010, from April 7, 2010 to April 6, 2020. We do not have to pay any license fee to Jingsheng Wang and Yanliang Song for the use of the trademark.
|
Mark
|
Registration
Number
|
Category
|
Effective
Date
|
Expiration
Date
|
Owner
|
|||||
|
|
|
|
|
|
|
|||||
 |
3836034
|
Class 5. Pharmaceutical and veterinary preparations; sanitary preparations for medical purposes; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
|
|
April 28, 2006
|
|
April 27, 2016
|
|
Jingsheng Wang
|
||
|
|
|
|
|
|
|
|||||
 |
6725571
|
Class 17. For drug packaging- PVC rigid sheet (semi processing); PVC plastic pipe; PVC plastic pipe; polychlorinated; Vinyl PVC plastic rod; PVC plastic articles; plastic pipe, plastic board; fill crack with chemical compounds; non-metallic hose
|
|
April 4, 2010
|
|
April 6, 2020
|
|
Yanliang Song
|
Insurance
We maintain property insurance policies covering our inventory in Yaoyuan’s warehouse for losses due to fire, earthquake, flood and a wide range of other disasters. We also maintain limited insurance coverage for our inventory in transit from our warehouse to our customers. The total insurance coverage for Yaoyuan’s inventory was RMB 30 million (approximately $4.4 million) from November 30, 2010 to November 30, 2011 and RMB 20 million (approximately $2.9 million) from November 2009 to November 2010. We paid insurance premiums of RMB 33,000 (approximately $4,870) and RMB 7,000 (approximately $1,030) in 2010 and 2009 , respectively. In addition, like other similar companies in the PRC, we do not carry product liability insurance, and we do not have any business interruption insurance due to the limited coverage of any business interruption insurance in the PRC.
23
Employees
Overview
We have approximately 500 employees, all of whom are full-time workers and are based in the PRC. Set forth below is a detailed description of employees employed by our three PRC Subsidiaries, respectively.
Yaoyuan
At December 31, 2010, Yaoyuan had approximately 250 employees, all of whom were full-time workers and are based in Shandong province, PRC. There are no collective bargaining contracts covering any of our employees. We believe our relationship with our employees is satisfactory.
Tongdetang
At December 31, 2010, Tongdetang had approximately 100 employees, all of whom were full-time workers and are based in Jilin province, PRC. There are no collective bargaining contracts covering any of our employees. We believe our relationship with our employees is satisfactory.
Xuelingxian
At December 31, 2010, Xuelingxian had approximately 150 employees, all of whom were full-time workers and are based in Anhui province, PRC. There are no collective bargaining contracts covering any of our employees. We believe our relationship with our employees is satisfactory.
We are required to contribute a portion of our employees’ total salaries to the PRC government’s social insurance funds, including pension insurance, medical insurance, unemployment insurance, work-related injury insurance and maternity insurance, in accordance with relevant regulations.
We have purchased work injury insurance, endowment insurance and medical insurance for management. Some of our other employees have asked to purchase such insurances and we have made the relevant deductions and accruals to account for such purchases.
Effective January 1, 2008, the PRC introduced a new labor contract law that enhances rights for the nation's workers, including open-ended work contracts and severance pay. The legislation requires employers to provide written contracts to their workers, restricts the use of temporary laborers and makes it harder to lay off employees. It also requires that employees with fixed-term contracts be entitled to an indefinite-term contract after a fixed-term contract is renewed twice. Although the new labor contract law will increase our labor costs, we do not anticipate there will be any significantly effects on our overall profitability in the near future since such amount was historically not material to our operating cost. Management anticipates this may be a step toward improving candidate retention for skilled workers.
24
Government Regulations
Below is a list of agencies which may have a jurisdiction over our business:
|
Agency
|
Functions
|
|
|
|
|
|
|
State Food and Drug Administration (“SFDA”)
|
|
Supervise the entire process from research and development, manufacturing, and distribution to utilization of drugs; supervise and coordinate the safety management of food, health food and cosmetics and organize investigations of serious accidents.
|
|
|
|
|
|
National Development and Reform Commission (“NDRC”)
|
|
Make strategic and mid- to long-term plans for the PRC healthcare industry; regulate drug prices; manage disaster relief funds and carry out healthcare development projects sponsored by the government.
|
|
|
|
|
|
Ministry of Commerce (“MOFCOM”)
|
|
Formulate regulations and policies on foreign trade, foreign direct investments, consumer protection, and market competition; negotiate bilateral and multilateral trade agreements.
|
|
|
|
|
|
State Administration of Traditional Chinese Medicine (“SATCM”)
|
|
Draft Traditional Chinese Medicine (“TCM”) industry development strategies, planning, policies, standards, related laws and regulations.
|
|
|
|
|
|
Ministry of Labor and Social Security (“MOLSS”)
|
|
Manage state medical insurance systems.
|
|
|
|
|
|
Ministry of Health (“MOH”)
|
|
Guide healthcare reform; compile the basic insurance drug list and draw up procurement regulations for non-profit hospitals (i.e., state-owned hospitals).
|
|
|
|
|
|
National Population and Family Planning Commission (“NPFPC”)
|
|
Propose guidelines and policies for the national family planning programs; draft laws and regulations related to population and family planning; assist the related departments to formulate social and economic policies so as to promote the implementation of comprehensive approaches for population and family planning programs.
|
|
|
|
|
|
Ministry of Science and Technology (“MST”)
|
|
Lay out science and technology development plans and policies; draft relevant regulations and rules and guarantee implementation of regulations and rules
|
|
|
|
|
|
General Administration of Quality Supervision, Inspection and Quarantine (“AQSIQ”)
|
|
Manage national quality, metrology, entry-exit commodity inspection, entry-exit health quarantine, entry-exit animal and plant quarantine, import-export food safety, certification, accreditation, and standardization, as well as enforce administrative laws
|
|
|
|
|
|
State Administration of Taxation (“SAT”)
|
|
Draft tax regulations and implementation rules and propose tax policies.
|
|
|
|
|
|
State Administration of Foreign Exchange (“SAFE”)
|
|
Make regulations and policies governing foreign exchange market activities and manage state foreign exchange reserves.
|
As a business operating in the PRC, we are subject to various regulations and permit systems by the PRC government. These regulations cover many of our products, including herbal products, OTC medicines and prescription medications.
25
Pharmaceutical Product Distribution
We are subject to the Drug Administration Law of the PRC, which governs the licensing, manufacturing, marketing and distribution of pharmaceutical products in the PRC and sets penalties for violations of the law. Distributors of pharmaceutical products are required to obtain permits from the appropriate provincial or county level SFDA where the pharmaceutical distribution enterprise is located. The grant of such permits is subject to an inspection of a distributor's facilities, warehouses, hygienic environment, quality control systems, personnel and equipment. Pharmaceutical distribution permits have five year-terms and distributors must apply for renewal no later than six months prior to the expiration date of the permit. Yaoyuan and Tongdetang have wholesale pharmaceutical distribution permits which expire on various dates.
Additionally, under the Supervision and Administration Rules on Pharmaceutical Product Distribution disseminated by the SFDA on January 31, 2007, and effective May 1, 2007, a pharmaceutical product distributor is accountable for its procurement and sales activities and is liable for the actions of its employees or agents in connection with their conduct of distribution on behalf of the distributor. Retail distributors may not sell prescription pharmaceutical products, or Part A (discussed under the heading “insurance catalogue) OTC pharmaceutical products, listed in the national or provincial medical insurance catalogs without a prescription from a certified in-store pharmacist.
Online Pharmaceutical Operation Permit
The Measures regarding the Administration of Drug Information Service over the Internet, effective on July 8, 2004, define the delivery of free publicly available drug information services over the internet as a non-profit online drug information service. This service requires a qualification certificate from the provincial food and drug administration. The provincial food and drug administration must file its approval with the SFDA for records and make a public announcement. The qualification certificate is valid for five years and may be renewed by filing for an extension at least six months prior to its expiration date and undergoing a reexamination by the relevant authority.
Yaoyuan obtained an internet drug information service qualification certificate from Shandong Food and Drug Administration on November 26, 2009 and the certificate is valid until November 16, 2013.
Good Supply Practice Standards
We are required to operate in accordance with GSP standards that regulate wholesale and retail pharmaceutical product distributors. The GSP standards ensure the quality of distribution of pharmaceutical products in the PRC. Pursuant to applicable GSP standards, we must implement strict controls on the distribution of our pharmaceutical products, including those concerning staff qualifications, distribution premises, warehouses, inspection equipment and facilities, management and quality control. Additionally, we are subject to inspections organized by the local drug regulatory department of the people's government of the province, autonomous region or municipality directly under the PRC central government. All our subsidiaries received GSP Certificates from various SFDA bureaus, which expire on various days, and are subject to periodic renewal.
Good Agricultural Practice Standards
The SFDA issued Good Agricultural Practice, or GAP, for Chinese Crude Drugs on June 1, 2002. Currently, GAP is not a compulsory practice applicable to all Chinese herb-planting entities.
Insurance Catalogue
Pursuant to the Decision of the State Council on the Establishment of the State Basic Medical Insurance System for Urban Employees and the Implementation Measures for the Administration of the Scope of Medical Insurance Coverage for Pharmaceuticals for Urban Employees, the Ministry of Labor and Social Security in the PRC has established a national Insurance Catalogue (referred to as the Insurance Catalog), in which the retail prices of certain pharmaceutical products are listed and subject to price controls in the form of fixed prices or price ceilings by the PRC government. Manufacturers and distributors are not permitted to set or change the retail price for any price-controlled product above the applicable price ceiling or deviate from the applicable fixed price imposed by the PRC government. The prices of other pharmaceuticals that are not subject to price controls are determined by the pharmaceutical manufacturers, subject, in certain cases, to providing notice to the provincial pricing authorities.
26
The Price Control Office of National Development and Reform Commission, or NDRC, as well as provincial and regional price control authorities, set the retail prices of products that are subject to price controls. The wholesale price of the pharmaceutical products subject to the price controls are generally determined by the set retail price. The maximum prices of such pharmaceutical products are published by the state and provincial administration authorities from time to time. Only the pharmaceutical product manufacturer can apply for an increase in the retail price of the product. All of our pharmaceutical products are subject to price controls.
The pharmaceuticals included in the Insurance Catalogue are selected by the PRC government authorities based on various factors including treatment requirements, frequency of use, effectiveness and price. Medicines included in the Insurance Catalogue are subject to price control by the PRC government. The Insurance Catalogue is revised every two years. In connection with each revision, the relevant provincial drug authority collects proposals from relevant enterprises before organizing a comprehensive appraisal. The SFDA then makes the final decision on any revisions based on the preliminary opinion suggested by the provincial drug administration.
The Insurance Catalogue is divided into Parts A and B. The pharmaceuticals included in Part A are designated by the Chinese governmental authorities for general application. Local governmental authorities may not adjust the content of pharmaceuticals in Part A. Although the pharmaceuticals included in Part B are designated by PRC governmental authorities in the first instance, provincial level authorities may make limited changes to the medicines included in Part B, resulting in some regional variations in the pharmaceuticals included in Part B from region to region.
Patients purchasing medicines included in Part A are entitled to reimbursement of the costs of such medicines from the social medical fund in accordance with relevant regulations in the PRC. Patients purchasing medicines included in Part B are required to pay a predetermined proportion of the costs of such medicines.
PRC National Medical Insurance Program
Eligible participants in the PRC national medical insurance program, mainly consisting of urban residents, can purchase pharmaceuticals in an authorized pharmacy by presenting their medical insurance cards if the pharmaceuticals purchased are included in the national or provincial medical insurance catalogues. Authorized pharmacies can generally either sell pharmaceuticals on credit and obtain reimbursement from relevant government social security bureaus on a monthly basis, or accept payments from the participants at the time of purchase, and the participants in turn obtain reimbursement from relevant government social security bureaus.
Purchases of Part A pharmaceutical products are generally fully reimbursable, except for certain Part A pharmaceutical products that are only reimbursable to the extent the medicine is used the purposes stated in the insurance catalogs. Only a portion of purchases of Part B pharmaceuticals are reimbursable; participants purchasing Part B pharmaceutical products must make a certain co-payment which is not reimbursable. Participants have varying amounts in their individual accounts, which vary based on the contributions made by the participants and his or her employer. Different regions in the PRC have different requirements regarding the caps of reimbursements in excess of the amounts in the individual accounts.
Pharmaceutical Product Advertisement
The Standards for Examination and Publication of Advertisements of Pharmaceutical Products and Rules for Examination of Advertisement of Pharmaceutical Products, promulgated by the PRC State Administration of Industry and Commerce, or SAIC, and SFDA, prevent the deceptive and misleading advertising of pharmaceutical products. These regulations prohibit the advertisement of certain pharmaceutical products and mandate that prescription pharmaceuticals only be advertised in certain authorized medical magazines upon obtaining proper approval from the provincial level food and drug administration.
27
Environmental Regulations
The major environmental regulations applicable to us include the PRC Environmental Protection Law, the PRC Law on the Prevention and Control of Water Pollution and its Implementation Rules, the PRC Law on the Prevention and Control of Air Pollution and its Implementation Rules, the PRC Law on the Prevention and Control of Solid Waste Pollution, and the PRC Law on the Prevention and Control of Noise Pollution.
We have not been named as a defendant in any legal proceedings alleging violation of environmental laws and have no reasonable basis to believe that there is any threatened claim, action or legal proceedings against us that would have a material adverse effect on our business, financial condition or results of operations due to any non-compliance with environmental laws.
Intellectual Property
Trademark
In the PRC, the Trademark Office administers the system of trademark law (Trademark Review and Adjudication Board and the courts administer the appeal function). The two principal pieces of legislation forming the trademark system are the Trademark Law and the Unfair Competition Law. The Trademark Law of the PRC stipulates that goods mark, service mark, collective mark and certification mark can be registered in the PRC and the holder of the marks can obtain exclusive trademark rights. Trademarks should be visible marks, including words, device, letters, digits, 3-D marks and combinations of colors, and combinations of any of the aforementioned. Sound or smell is not registrable yet in the PRC.
A trademark applicant must file an application with the PRC Trademark Office. Normally, the Trademark Office renders a decision within 18 months after it receives all supporting documents. If the Trademark Office approves the application, the mark will be published in the PRC Trademark Gazette. After the mark is published, there will be a three-month opposition period. If nobody files opposition within that period, the application will mature into registration. The Trademark Office will then issue the certificate and the Trademark Gazette will publish the mark again as a registered mark.
The term of trademark protection is ten years from the date the registration is granted. The registrant may renew the trademark for an additional ten-year term within six months before the expiration date of the mark's present term. Where no such application could be filed within the stated period, a grace period of six months may be allowed. If the registrant does not file for renewal within the grace period, the registered trademark will be canceled. Currently, the applicant does not need to prove the use of the trademark prior to renewal.
However, registration of a mark may be blocked by an unregistered famous mark in the PRC in accordance with the PRC’s Trademark Law and obligations under the Paris Convention and the 1995 United States-China Intellectual Property Protection Agreement (referred to as the IP Action Plan), provided that the owner of the famous mark can prove that the mark was well-known in the PRC before the filing date of the similar mark. Owners of unregistered famous marks may also bring oppositions or cancellations for previously registered marks, based on Articles 13, 30 and 41 of the Trademark Law.
Without authorization from the trademark owner, no one may use a mark identical or similar to the registered mark on identical or similar goods as the registered mark. Infringers will be subject to administrative, civil or criminal punishment. The damages awarded to the trademark owner will be calculated upon the illegal profits of the infringer and actual losses to the rights owner. In the event that the damages from infringement are difficult to calculate, the statutory maximum compensation will be RMB 500,000 (about US $61,000), which may be a real deterrent in some cases. In addition, the trademark owner may apply to a competent court for preliminary injunction against ongoing or threatened trademark infringement before a lawsuit is initiated.
Tax
Pursuant to the Provisional Regulation of the PRC on Value-Added Tax, or VAT, and their implementing rules, all entities and individuals that are engaged in the sale of goods, the provision of repairs and replacement services and the importation of goods in the PRC are generally required to pay VAT at a rate of 17% of the gross sales proceeds received, less any deductible VAT already paid or borne by the taxpayer. Further, when exporting goods, the exporter is entitled to a portion of or all the refund of VAT that it has already paid or borne.
28
Pursuant to the PRC Enterprise Income Tax Law and its Implementation Rules, companies engaged in the cultivation or basic processing of TCM are exempt from enterprise income tax on sales revenue of TCM they grow or process. In accordance with the PRC Value-Added Tax Tentative Provisions and its Implementation Rules, income generated from sales of self-cultivated TCM is also exempt from VAT. Xuelingxian grows TCM and also carries out basic treatments of TCM; therefore it does not pay any enterprise income tax or VAT on the income arising from sales of TCM it grows nor does it pay any enterprise income tax on the sales revenue from basic treated TCM.
Compliance with Circular 106 and the Revised M&A Regulations
On May 29, 2007, SAFE issued an official notice known as “Circular 106”, which requires the owners of any PRC companies to obtain approval from Ministry of Finance, or MOFCOM, before establishing any offshore holding company structure in so-called “round-trip” investment transactions (a round-trip investment refers to an investment made by a PRC resident in a PRC enterprise through an offshore special purpose vehicle) for foreign financing as well as subsequent acquisition matters in the PRC. Likewise, on August 8, 2006, MOFCOM joined by State-owned Assets Supervision and Administration Commission, State Administration of Taxation, SAIC, CSRC and SAFE, released a substantially amended version of the Provisions for Foreign Investors to Merge with or Acquire Domestic Enterprises (referred to as the Revised M&A Regulations) which imposed approval requirements from MOFCOM for “round-trip” investment transactions, including acquisitions in which equity was used as consideration. The offshore special purpose vehicle is defined as an offshore enterprise directly established by or under the indirect control of a PRC resident legal person or a PRC resident individual using his or her assets, or equity interest held, in a PRC enterprise, for the purpose of offshore equity financing (including convertible debt financing). Because our Company and intermediate “offshore” companies are not enterprises established by PRC residents for the purpose of offshore equity financing, we are not special purpose vehicle companies and as such the acquisition of our PRC Subsidiaries and the Reverse Merger are not “round-trip” investments as defined by the above-mentioned rules. Despite that we have PRC residents as shareholders after the exercise of certain call rights under the Amendment to the Earn In Agreement whereby some PRC residents acquired a total of 20,894,000 shares, or 80.36% of our common stock, Circular 106 and the Revised M&A are inapplicable to us.
Foreign investments in pharmaceutical industry are subject to certain restrictions. However, pursuant to Supplement VI to Mainland and Hong Kong Closer Economic Partnership Arrangement, PRC pharmaceutical distribution industry is open to Hong Kong invested companies. Therefore, the acquisition of Our PRC Subsidiaries by our two Hong Kong subsidiaries, Wisdom Fortune and Binomial, is not subject to any limitation.
Foreign Currency Exchange
Under the PRC foreign currency exchange regulations applicable to us, the RMB is convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions. Conversion of RMB for capital account items, such as direct investment, loan, security investment and repatriation of investment, however, is still subject to the approval of the PRC State Administration of Foreign Exchange, or SAFE. Foreign-invested enterprises may only buy, sell and/or remit foreign currencies at those banks authorized to conduct foreign exchange business after providing valid commercial documents and, in the case of capital account item transactions, obtaining approval from the SAFE. Capital investments by foreign-invested enterprises outside of the PRC are also subject to limitations, which include approvals by the Ministry of Commerce, the SAFE and the State Reform and Development Commission.
Dividend Distributions
Under applicable PRC regulations, foreign-invested enterprises in the PRC may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, a foreign-invested enterprise in the PRC are required to set aside at least 10% of their after-tax profit based on PRC accounting standards each year to its general reserves until the accumulative amount of such reserves reach 50% of its registered capital. These reserves are not distributable as cash dividends.
29
Approvals, Licenses and Certificates
We require a number of approvals, licenses and certificates in order to operate our business. Our principal approvals, licenses and certificates are set forth below.
Yaoyuan
|
|
·
|
Business License (No. 370100000030386) issued by Shandong Province Administration of Industry and Commerce, valid from November 1, 2005 to June 28, 2030.
|
|
|
·
|
Certificate for Drug Information Service over the Internet PRC issued by Shandong Province Food and Drug Administration (No.: (Lu) non-operational-2008-0009) with an expiration date of November 16, 2013.
|
|
|
·
|
License for Medical Device Operation Enterprise of PRC issued by Shandong Province Food and Drug Administration (No: Lu 012410 (T)), the valid period of which is from January 29, 2010 to January 28, 2015.
|
|
|
·
|
Social Insurance Register (No. 0010010239) issued by Ministry of Human Resources and Social Security of the PRC.
|
|
|
·
|
Tax Registration Certificate (Shandong Yaoyuan Pharmaceutical Co., Ltd., No. 370112780611311) issued by State Administration of Taxation and local administration of taxation in 2010.
|
|
|
·
|
Pharmaceutical Trading License (No. Lu AA5310492) issued by the Jinan City Food and Drug Administration with an expiration date of August 30, 2015.
|
|
|
·
|
Certificate for Goods Supply Practice (No: A-SD06-024) issued by Shandong Province Food and Drug Administration which is valid until February 27, 2011. We submitted an application to renew this certificate and such application was approved by Shandong Food and Drug Administration on January 13, 2011.
|
|
|
·
|
Organization Code Certificate (Code: 78061131–1) issued by Shandong Bureau of Quality and Technical Supervision, the valid period of which is from August 18, 2009 to August 18, 2013.
|
|
|
·
|
License for Road Transportation & Operation (No. 370102408281) issued by Ministry of Communication of the PRC, the valid period of which is from September 8, 2008 to September 7, 2012.
|
Tongdetang
|
|
·
|
Business License (Tonghua Tongdetang Pharmaceutical Co., Ltd. No. 220500000002444) issued by Tonghua City Administration of Industry and Commerce, valid from February 2, 2002 to February 5, 2030.
|
|
|
·
|
Organization Code Certificate (No. 73257943-3) issued by Tonghua Bureau of Quality and Technical Supervision, valid from May 23, 2007 to May 22, 2011.
|
|
|
·
|
Tax Registration Certificate (No: 220503732579439) issued by Tonghua City Administration of Industry and Commerce on April 12, 2007.
|
|
|
·
|
Certificate for Goods Supply Practice (No: A-JL09-154) issued by Jilin Province Food and Drug Administration, valid from May 12, 2009 to May 11, 2014.
|
Xuelingxian
|
|
·
|
Business License (No. 341600000022207 (1-1)) issued by Anhui Province Administration of Industry and Commerce, valid from June 8, 2010 to June 8, 2030.
|
|
|
·
|
Pharmaceutical Trading Enterprise License (No. Wan AA0300166) issued by Anhui Province Food and Drug Administration on June 27, 2008 and valid till June 26, 2013.
|
30
|
·
|
Organization Code Certificate (Code No. 67758291-1) issued by Bozhou City Bureau of Quality and Technical Supervision, valid from July 24, 2008 to July 24, 2012.
|
|
·
|
Certificate for Goods Supply Practice (No: A-AH09-040) issued by Anhui Province Food and Drug Administration, valid from February 1, 2009 to January 31, 2014.
|
|
·
|
Food Hygiene License (No. 341601-0469) issued by Public Health Bureau of Bozhou City, valid from May 31, 2009 to May 30, 2013.
|
|
·
|
Tax Registration Certificate (Anhui Xuelingxian Pharmaceutical Co., Ltd., No: 341600677582911) issued by Bozhou City Administration of Industry and Commerce on June 11, 2009.
|
|
·
|
Industrial Product Manufacture Permit (No. OS3416 1402 0136) issued by Anhui Province Bureau of Quality and Technical Supervision, valid from June 15, 2010 to June 24, 2013.
|
31
RISK FACTORS
The following statements describe the major risks to our business and should be considered carefully. Any of these factors could significantly and negatively affect our business, prospects, financial condition, operating results or credit ratings, which could cause the trading price of our common stock to decline. The risks described below are not the only risks we may face. Additional risks and uncertainties not presently known to us, or risks that we currently consider immaterial, could also negatively affect our business, our results and operations.
Risks Related To Our Business
The purchase of many of our products is discretionary, and may be particularly affected by adverse trends in the general economy; therefore challenging economic conditions may make it more difficult for us to generate revenue.
Our business is affected by global, national and local economic conditions since many of the products we sell are discretionary and we depend, to a significant extent, upon a number of factors relating to discretionary consumer spending in the PRC. These factors include economic conditions and perceptions of such conditions by consumers, employment rates, the level of consumers' disposable income, business conditions, interest rates, consumer debt levels, availability of credit and levels of taxation in regional and local markets in the PRC where we sell such products. There can be no assurance that consumer spending on the products we sell, will not be adversely affected by changes in general economic conditions in the PRC and globally.
The success of our business depends on our ability to market and advertise the products we sell effectively.
Our ability to establish effective marketing and advertising campaigns is key to our success. Our advertisements promote our corporate image, our merchandise and the pricing of such products. If we are unable to increase awareness of our brands and our products, we may not be able to attract new customers. Our marketing activities may not be successful in promoting the products we sell or pricing strategies or in retaining and increasing our customer base. We cannot assure you that our marketing programs will be adequate to support our future growth, which may result in a material adverse effect on our results of operations.
We may be unable to identify and respond effectively to shifting customer preferences, and we may fail to optimize our product offerings and inventory position.
Consumer preferences in the pharmaceutical industry change rapidly and are difficult to predict. The success of our business depends on our ability to predict accurately and respond to future changes in consumer preferences, carry the inventory demanded by customers, deliver the appropriate quality of products, price products correctly and implement effective purchasing procedures. We must optimize our product selection and inventory positions based on consumer preferences and sales trends. If we fail to anticipate, identify or react appropriately to changes in consumer preferences and adapt our product selection to these changing preferences, we could experience excess inventories, higher than normal markdowns or an inability to sell the products we sell, which, in turn, could significantly reduce our revenue and have a material adverse effect on our business, financial condition and results of operations.
If we fail to maintain optimal inventory levels, our inventory holding costs could increase or cause us to lose sales, either of which could have a material adverse effect on our business, financial condition and results of operations.
While we must maintain sufficient inventory levels to operate our business successfully and meet our customers' demands, we must be careful to avoid amassing excess inventory. Changing consumer demands, manufacturer backorders and uncertainty surrounding new product launches expose us to increased inventory risks. Demand for products can change rapidly and unexpectedly, including the time between when the product is ordered from the supplier to the time it is offered for sale. We carry a wide variety of products and must maintain sufficient inventory levels of the products we sell. We may be unable to sell certain products in the event that consumer demand changes. Our inventory holding costs will increase if we carry excess inventory. However, if we do not have a sufficient inventory of a product to fulfill customer orders, we may lose orders or customers, which may adversely affect our business, financial condition and results of operations. We cannot assure you that we can accurately predict consumer demand and events and avoid over-stocking or under-stocking products.
32
We may not be able to optimize the management of our distribution network or be successful in expanding our distribution network.
We sell substantially all of our products to our customers through our distribution network which comprises initial three distribution companies that are located in three provinces in the PRC. Our ability to meet customer demand may be significantly limited if we do not successfully operate our distribution centers and logistics facilities as well as efficiently conduct our distribution activities, or if one or more of our distribution centers or logistics facilities are destroyed or shut down for any reason, including as a result of a natural disaster. Any disruption in the operation of our distribution network could result in higher costs or longer lead times associated with distributing our products. In addition, as it is difficult to predict accurate sales volumes in our industry, we may be unable to optimize our distribution activities, which may result in excess or insufficient inventory, warehousing, fulfillment of logistics or value-added services, or distribution capacity. In addition, failure to effectively control product damage or spoilage during the distribution process could decrease our operating margins and reduce our profitability.
We intend to expand our distribution network to include additional cities and rural areas in the PRC to expand our geographic reach to customers. However, we may not be successful in expanding our distribution network. Our distribution, logistics and value-added services and products may face competition from similar services and products offered by our competitors. Therefore, the success of our proposed expansion will depend on many factors, including our ability to form relationships with, and manage an increasing number of, customers nationwide and optimize our distribution channels. We must also be able to anticipate and respond effectively to competition posed by other pharmaceutical distributors. If we fail to expand our distribution network in the PRC as planned or if we are unable to compete effectively with other distributors, our business, financial condition and results of operations may be materially and adversely affected.
If all or a significant portion of our customers with accounts receivables fail to pay all or part of the trade receivables or delay the repayment, our net income will decrease and our profitability will be adversely affected.
We had accounts receivables, net of allowance for doubtful accounts, of approximately $19,711,619 as of December 31, 2010. There is no assurance that our accounts receivables will be fully repaid on a timely basis. If all or a significant portion of our customers with accounts receivables fail to pay all or part of the accounts receivables or delay the payment due to us for whatever reason, our net profit will decrease and our profitability will be adversely affected.
A major failure of our information systems could harm our business.
We depend on information systems to process transactions, manage inventory, purchase, sell and ship goods on a timely basis, and maintain cost-efficient operations. Any material disruption or slowdown of our systems could cause information to be lost or delayed, which could have a negative effect on our business. We may experience operational problems with our information systems as a result of system failures, viruses, computer “hackers” or other causes, and our business, financial condition and results of operations will be adversely affected.
Our operations would be materially adversely affected if third-party carriers were unable to transport our products on a timely basis.
All of our products are shipped through third-party carriers. If a strike or other event prevented or disrupted these carriers from transporting our products, other carriers may be unavailable or may not have the capacity to deliver our products to our customers. If adequate third-party sources to ship our products were unavailable at any time, our business would be materially adversely affected.
Certain disruptions in supply of and changes in the competitive environment for our products may adversely affect our profitability.
We carry a broad range of merchandise. A significant disruption in the supply of these products could decrease inventory levels and sales, and materially adversely affect our business and financial results. Shortages of products or interruptions in transportation systems, labor strikes, work stoppages, war, acts of terrorism or other interruptions or difficulties in the employment of labor or transportation in the markets in which we purchase products may adversely affect our ability to maintain sufficient inventories of our products to meet consumer demand. If we were to experience a significant or prolonged shortage of products from any of our suppliers and could not procure the products from other sources, we would be unable to meet customer demand, which, in turn, would adversely affect our sales, margins and customer relations.
33
Adverse weather conditions, natural disasters, pestilences and other natural conditions can affect our Chinese herbal cultivation and the raw material costs of certain medications, which can adversely affect our operations and our results of operations.
The ingredients and raw materials that are used in certain medications are vulnerable to adverse weather conditions and natural disasters, such as floods, droughts, frosts, earthquakes and pestilences. Adverse weather conditions may be impacted by global warming and other factors. Adverse weather conditions and natural disasters can reduce crop size and crop quality and thus have an adverse effect on our production of Chinese herbal medicines. Adverse weather conditions could also in turn could reduce our supplies from third parties of raw materials, lower recoveries of usable raw materials, increase the prices of our raw materials, and increase our cost of storing raw materials if harvests are accelerated and processing capacity is unavailable. Our competitors may be affected differently by weather conditions and natural disasters depending on the location of their supplies or operations. If our supplies of raw materials are reduced, we may not be able to find enough supplemental supply sources on favorable terms, if at all, which could impact our ability to supply product to our customers and adversely affect our business, financial condition and results of operations.
The market for our products and services is very competitive and, if we cannot effectively compete, our business will be harmed.
The industries in which we operate are highly fragmented and very competitive. We compete with local pharmaceutical companies and with large foreign multinational companies that offer products that are similar to ours. Some of these competitors have larger local or regional customer bases, more locations, more brand equity, and substantially greater financial, marketing and other resources than we have. As a result, our competitors may be in a stronger position to respond quickly to potential acquisitions and other market opportunities, new or emerging technologies and changes in customer tastes. We cannot assure you that we will be able to maintain or increase our market share against the emergence of these or other sources of competition. Failure to maintain and enhance our competitive position could materially adversely affect our business and prospects.
We may not be able to maintain our supplier relationships in our pharmaceutical distribution operations.
In our pharmaceutical distribution operations, we depend on more than 1,840 suppliers for a steady supply of pharmaceutical and healthcare products. We typically distribute products pursuant to annual agency or distribution agreements entered into directly between us and our suppliers or upstream distributors, under which our suppliers provide us with a series of economic incentives and other support. Normally, the terms of our agreements with our suppliers are one year. We cannot assure you that manufacturers and other suppliers will continue to sell products to us on commercially reasonable terms, or at all. We also cannot assure you that we will be able to establish new manufacturer and other supplier relationships, or extend existing relationships with suppliers when our agreements with them expire. Our annual agency or distribution agreements with suppliers may be terminated from time to time due to various reasons beyond our control. Moreover, the annual agency or distribution agreements for some of our products are not exclusive, and we cannot assure you that our competitors will not obtain the distribution rights of certain of our products.
If we are unable to renew the leases of any of our property, our operations may be adversely affected.
We do not directly own any land use rights over the properties we rent. We may lose our rental properties or may not be able to renew them when they are due on terms that are reasonable or favorable to us. This may adversely impact our operations, including disrupting our operations or increasing our cost of operations.
34
We may not be successful in competing with other wholesalers and distributors of pharmaceutical products in the tender processes for the purchase of medicines by state-owned and state-controlled hospitals.
Our wholesale business sells various pharmaceutical products to hospitals owned and controlled by government authorities in the PRC. Government-owned hospitals purchase pharmaceutical products by using collective tender processes. During a collective tender process, a hospital establishes a committee of recognized pharmaceutical experts, which assesses bids submitted by pharmaceutical manufacturers. The hospitals may only purchase pharmaceuticals that win in collective tender processes. The collective tender process for pharmaceuticals with the same chemical composition must be conducted at least annually, and pharmaceuticals that have won in the collective tender processes previously must participate and win in the collective tender processes in the following period before hospitals may make new purchases. If we are unable to win purchase contracts through the collective tender processes in which we decide to participate, we will lose market share to our competitors, and our sales and profitability will be adversely affected.
Counterfeit products sold in the PRC could negatively impact our revenues, brand reputation, business and results of operations.
The products we sell are also subject to competition from counterfeit products, which are pharmaceuticals manufactured without proper licenses or approvals and are fraudulently mislabeled with respect to their content and/or manufacturer. Counterfeit products are generally sold at lower prices than authentic products due to their low production costs, and in some cases are very similar in appearance to authentic products. Counterfeit pharmaceuticals may or may not have the same chemical content as their authentic counterparts. Although the PRC government has recently been increasingly active in policing counterfeit products, including counterfeit pharmaceuticals, there is a lack of effective counterfeit product regulation control and enforcement systems in the PRC. The proliferation of counterfeit products has grown in recent years and may continue to grow in the future. Despite our implementation of quality controls, we cannot assure you that we would not be distributing or selling counterfeit products inadvertently. Any accidental sale or distribution of counterfeit products can subject our company to fines, administrative penalties, litigation and negative publicity, which could negatively impact our revenues, brand reputation, business and results of operations. Moreover, the continued proliferation of counterfeit products and other products in recent years may reinforce the negative image of retailers among consumers in the PRC. The continued proliferation of counterfeit products in the PRC could have a material adverse effect on our business, financial condition and results of operation.
The retail prices of some of our products are subject to price controls by the PRC government, which may affect both our revenues and net income.
The laws of the PRC permit the PRC government to fix and adjust prices of certain pharmaceutical products, including many of those listed in the Insurance Catalogue. Through these price controls, the government can fix retail prices and set retail price ceiling for certain of the pharmaceutical products we sell. Additionally, the PRC government may periodically adjust the retail prices of these products downward in order to make pharmaceuticals more affordable to the general PRC population. To the extent that we are subject to price controls, our revenue, gross profit, gross margin and net income will be affected because the revenue we derive from our sales will be limited and we may have limited ability to control our costs. Any future price controls or price reductions may reduce our revenue and profitability and have a material adverse effect on our financial condition and results of operations.
The required certificates, permits, and licenses related to our operations are subject to governmental control and renewal and failure to obtain renewal will cause all or part of our operations to be terminated.
We are subject to various PRC laws and regulations pertaining to our wholesale operations. We have attained certificates, permits and licenses required for the operation of a pharmaceutical distributor. We cannot assure you that we will have all necessary permits, certificates and authorizations for the operation of our business at all times. Additionally, our certifications, permits and authorizations are subject to periodic renewal by the relevant government authorities. We intend to apply for renewal of these certificates, permits and authorizations prior to their expiration. During the renewal process, we will be re-evaluated by the appropriate governmental authorities and must comply with the then prevailing standards and regulations which may change from time to time. In the event that we are not able to renew the certificates, permits and licenses, all or part of our operations may be terminated. Furthermore, if escalating compliance costs associated with governmental standards and regulations restrict or prohibit any part of our operations, it may adversely affect our operations and profitability.
35
If we become subject to product liability claims, personal injury claims or defective products our business may be harmed.
We will be exposed to risks inherent in the packaging and distribution of pharmaceutical and other healthcare products, such as the unintentional distribution of counterfeit drugs. Furthermore, we may sell products which inadvertently have an adverse effect on the health of individuals. Product liability claims may be asserted against us, although we may have the right under applicable PRC laws, rules and regulations to recover from the relevant manufacturer for compensation we paid to our customers in connection with a product liability claim. Any product liability claim, product recall, adverse side effects caused by improper use of the products we sell or manufacturing defects may result in adverse publicity regarding us and the products we sell, which would harm our reputation. If we are found liable for product liability claims, we could be required to pay substantial monetary damages. Furthermore, even if we successfully defend ourselves against this type of claim, we could be required to spend significant management, financial and other resources, which could disrupt our business, and our reputation and our brand name may also suffer. We, like many other similar companies in the PRC, do not carry product liability insurance. As a result, any imposition of product liability could materially harm our business, financial condition and results of operations. In addition, we do not have any business interruption insurance due to the limited coverage of any business interruption insurance in the PRC and, as a result, any business disruption or natural disaster could severely disrupt our business and operations and significantly decrease our revenue and profitability.
The failure to manage growth effectively could have an adverse effect on our employee efficiency, product quality, working capital levels and results of operations.
Any significant growth in the market for our products or our entry into new markets may require an expansion of our employee base for managerial, operational, financial and other purposes. As of the date of this Annual Report, we had 501 full-time employees. During any growth, we may face problems related to our operational and financial systems and controls, including quality control and delivery and service capacities. We would also need to continue to expand, train and manage our employee base. Continued future growth will impose significant added responsibilities upon the members of management to identify, recruit, maintain, integrate, and motivate new employees.
Aside from increased difficulties in the management of human resources, we may also encounter working capital issues, as we will need increased liquidity to finance the purchase of raw materials and supplies, development of new products, and the hiring of additional employees. For effective growth management, we will be required to continue improving our operations, management, and financial systems and controls. Our failure to manage growth effectively may lead to operational and financial inefficiencies that will have a negative effect on our profitability. We cannot assure investors that we will be able to timely and effectively meet that demand and maintain the quality standards required by our existing and potential customers.
If we need additional capital to fund our growing operations, we may not be able to obtain sufficient capital and may be forced to limit the scope of our operations.
If adequate additional financing is not available on reasonable terms, we may not be able to undertake our expansion plan, purchase additional equipment for our operations and we would have to modify our business plans accordingly. There is no assurance that additional financing will be available to us.
In connection with our growth strategies, we may experience increased capital needs and, accordingly, we may not have sufficient capital to fund our future operations without additional capital investments. Our capital needs will depend on numerous factors, including (i) our profitability; (ii) the release of competitive products by our competitors; (iii) the level of our investment in research and development; and (iv) the amount of our capital expenditures, including acquisitions. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
If we cannot obtain additional funding, we may be required to: (i) limit our investments in research and development; (ii) limit our marketing efforts; and (iii) decrease or eliminate capital expenditures. Such reductions could materially adversely affect our business and our ability to compete.
Even if we do find a source of additional capital, we may not be able to negotiate terms and conditions for receiving the additional capital that are acceptable to us. Any future capital investments could dilute or otherwise materially and adversely affect the holdings or rights of our existing shareholders. In addition, new equity or convertible debt securities issued by us to obtain financing could have rights, preferences and privileges senior to our common stock. We cannot give you any assurance that any additional financing will be available to us or, if available, will be on terms favorable to us.
36
We are dependent on certain key personnel and loss of these key personnel could have a material adverse effect on our business, financial condition and results of operations.
Our success is, to a certain extent, attributable to the management, sales and marketing, and operational and technical expertise of certain key personnel. In addition, we will require an increasing number of experienced and competent executives and other members of senior management to implement our growth plans. We do not maintain key-man insurance for members of our management team because it is not a customary practice in the PRC. If we lose the services of any member of our senior management, we may not be able to locate suitable or qualified replacements, and may incur additional expenses to recruit and train new personnel, which could severely disrupt our business and prospects.
We are dependent on a trained workforce and any inability to retain or effectively recruit such employees, particularly distribution personnel and regional managers for our business, could have a material adverse effect on our business, financial condition and results of operations.
We must attract, recruit and retain a sizeable workforce of qualified and trained staff to operate our business. Our ability to implement effectively our business strategy and expand our operations will depend upon, among other factors, the successful recruitment and retention of highly skilled and experienced distribution personnel, regional sales managers and other technical and marketing personnel. There is significant competition for qualified personnel in our business and we may not be successful in recruiting or retaining sufficient qualified personnel consistent with our current and future operational needs.
Our financial results may fluctuate because of many factors and, as a result, investors should not rely on our historical financial data as indicative of future results.
Fluctuations in operating results or the failure of operating results to meet the expectations of public market analysts and investors may negatively impact the market price of our securities. Operating results may fluctuate in the future due to a variety of factors that could affect revenues or expenses in any particular quarter. Fluctuations in operating results could cause the value of our securities to decline. Investors should not rely on comparisons of results of operations as an indication of future performance. As result of the factors listed below, it is possible that in future periods results of operations may be below the expectations of public market analysts and investors. This could cause the market price of our securities to decline. Factors that may affect our quarterly results include:
|
|
·
|
vulnerability of our business to a general economic downturn in the PRC;
|
|
|
·
|
fluctuation and unpredictability of the prices of the products we sell;
|
|
|
·
|
seasonality of our business;
|
|
|
·
|
changes in the laws of the PRC that affect our operations;
|
|
|
·
|
competition from other retailers and wholesalers; and
|
|
|
·
|
our ability to obtain necessary government certifications and/or licenses to conduct our business.
|
Our strategy to acquire companies may result in unsuitable acquisitions or failure to successfully integrate acquired companies, which could lead to reduced profitability.
We intend to expand our business through acquisitions of companies or operations that complement existing product lines, customers or other capabilities. We may be unsuccessful in identifying suitable acquisition candidates, or may be unable to consummate a desired acquisition. To the extent any future acquisitions are completed, we may be unsuccessful in integrating acquired companies or their operations, or if integration is more difficult than anticipated, we may experience disruptions that could have a material adverse impact on future profitability. Some of the risks that may affect our ability to integrate, or realize any anticipated benefits from, acquisitions include:
|
|
·
|
unexpected losses of key employees or customer of the acquired company;
|
|
|
·
|
difficulties integrating the acquired company's standards, processes, procedures and controls;
|
|
|
·
|
difficulties coordinating new product and process development;
|
|
|
·
|
difficulties hiring additional management and other critical personnel;
|
|
|
·
|
difficulties increasing the scope, geographic diversity and complexity of our operations;
|
|
|
·
|
difficulties consolidating facilities, transferring processes and know-how;
|
|
|
·
|
difficulties reducing costs of the acquired company's business;
|
|
|
·
|
diversion of management's attention from our management; and
|
|
|
·
|
adverse impacts on retaining existing business relationships with customers.
|
37
We are overly dependent of certain suppliers and a failure to continue to obtain our supplies from such suppliers may adversely affect our business.
We are dependent on certain suppliers for the supply of a significant portion of our products and/or raw materials. Although we believe that these products and/or raw materials are readily available in the market, there is no guarantee that this would be the case. Also, we are relying on our good relations with such suppliers to ensure the best prices for our products and/or raw materials since we do not typically enter into long term supply contracts with them. A failure to continue receiving these product and/or materials and/or to continue purchasing them on terms favorable to us would negatively affect our operations and profitability.
We do not own any trademarks we currently use in our business. Any failure to continue using this trademark may affect our business in that any goodwill and brand recognition may be lost.
We do not own any trademarks. However, we own two trademark use rights - “Xuelingxian” and “Yaoyuan.”
“Xuelingxian” trademark is owned by a former shareholder of Xuelingxian, Jingsheng Wang, pursuant to Mr. Wang’s consent dated January 1, 2009 for a term from January 1, 2009 to January 1, 2014. “Yaoyuan” is owned by a former shareholder of Yaoyuan, Yanliang Song, pursuant to a license agreement dated April 7, 2010 for a term from April 7, 2010 to April 6, 2020. We do not have any payable license fees to Jingsheng Wang and Yanliang Song for the use of the trademarks. There is no assurance that we may continue using these trademarks on the expiration of the license agreement or that we will be allowed to use them on terms favorable to use. Any failure to continue using these trademarks may affect our business in that any brand recognition may be lost.
Risks Related to Conducting Business in the PRC
Our operations are subject to PRC laws and regulations that are sometimes vague and uncertain. Any changes in such PRC laws and regulations, or the interpretations thereof, may have a material and adverse effect on our business.
The PRC's legal system is a civil law system based on written statutes. Unlike the common law system prevalent in the United States, decided legal cases have little value as precedent in the PRC. There are substantial uncertainties regarding the interpretation and application of PRC laws and regulations, including but not limited to, the laws and regulations governing our business, or the enforcement and performance of our arrangements with customers in the event of the imposition of statutory liens, death, bankruptcy or criminal proceedings. The PRC government has been developing a comprehensive system of commercial laws, and considerable progress has been made in introducing laws and regulations dealing with economic matters such as foreign investment, corporate organization and governance, commerce, taxation and trade. However, because these laws and regulations are relatively new, and because of the limited volume of published cases and judicial interpretation and their lack of authority as precedents, interpretation and enforcement of these laws and regulations involve significant uncertainties. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively.
Our principal operating subsidiaries are regarded as foreign invested enterprises, or FIEs, under PRC laws, and as a result are required to comply with PRC laws and regulations, including laws and regulations specifically governing the activities and conduct of FIEs. We cannot predict what effect the interpretation of existing or new PRC laws or regulations may have on our businesses. If the relevant authorities find us in violation of PRC laws or regulations, they would have broad discretion in dealing with such a violation, including, without limitation:
|
|
·
|
levying fines;
|
|
|
·
|
revoking our business license, other licenses or authorities;
|
|
|
·
|
requiring that we restructure our ownership or operations; and
|
|
|
·
|
requiring that we discontinue any portion or all of our business
|
38
New labor law in the PRC may adversely affect our results of operations.
On January 1, 2008, the PRC government promulgated the Labor Contract Law of the PRC, or the New Labor Contract Law. The New Labor Contract Law imposes greater liabilities on employers and significantly impacts the cost of an employer’s decision to reduce its workforce. Further, it may require certain terminations to be based upon seniority and not merit. In the event we decide to significantly change or decrease our workforce, the New Labor Contract Law could adversely affect our ability to enact such changes in a manner that is most advantageous to our business or in a timely and cost effective manner, thus materially and adversely affecting our financial condition and results of operations.
We may not be able to comply with applicable Good Manufacture Practice (“GMP”) requirements and other regulatory requirements, which could have a material adverse affect on our business, financial condition and results of operations.
We are required to comply with applicable GMP regulations, which include requirements relating to quality control and quality assurance as well as corresponding maintenance, record-keeping and documentation standards. Manufacturing facilities must be approved by governmental authorities before we use them to commercially manufacture our products and are subject to inspection by regulatory agencies. If we fail to comply with applicable regulatory requirements, including following any product approval, we may be subject to sanctions, including:
|
|
·
|
product recalls or seizure;
|
|
|
·
|
injunctions;
|
|
|
·
|
refusal of regulatory agencies to review pending market approval applications or supplements to approval applications;
|
|
|
·
|
total or partial suspension of production;
|
|
|
·
|
civil penalties;
|
|
|
·
|
withdrawals of previously approved marketing applications; or
|
|
|
·
|
criminal prosecution.
|
If we fail to protect our intellectual property rights, it could harm our business and competitive position.
Our business relies in part on intellectual properties to stay competitive in the market place. We rely on a combination of trademark laws, trade secrets, confidentiality procedures and contractual provisions to protect our intellectual property rights and the obligations we have to third parties from whom we license intellectual property rights. Nevertheless, these afford only limited protection and policing unauthorized use of proprietary technology can be difficult and expensive. In addition, intellectual property rights historically have not been enforced in the PRC to the same extent as in the United States, and intellectual property theft presents a serious risk in doing business in the PRC. We may not be able to detect unauthorized use of, or take appropriate steps to enforce, our intellectual property rights and this could have a material adverse effect on our business, operating results and financial condition.
Under the new EIT Law, we may be classified a “resident enterprise” for PRC tax purposes, which may subject us to PRC enterprise income tax for any dividends we receive from our PRC subsidiaries and to PRC income tax withholding for any dividends we pay to our non-PRC shareholders.
On March 16, 2007, the National People’s Congress, or NPC, promulgated the Law of the People’s Republic of China on Enterprise Income Tax, or the new EIT Law, which became effective on January 1, 2008. In accordance with the new EIT Law, the corporate income tax rate is set at 25% for all enterprises. However, certain industries and projects, such as FIEs, may enjoy favorable tax treatment pursuant to the new EIT Law and its implementing rules.
Under the new EIT Law, an enterprise established outside of the PRC whose “de facto management bodies” are located in the PRC is considered a “resident enterprise” and is subject to the 25% enterprise income tax rate on its worldwide income. The new EIT Law and its implementing rules are relatively new and, currently, no official interpretation or application of this new “resident enterprise” classification is available. Therefore, it is unclear how tax authorities will determine the tax residency of enterprises established outside of the PRC.
Most of our management is currently based in the PRC. If the PRC tax authorities determine that our U.S. holding company is a “resident enterprise” for PRC enterprise income tax purposes, we may be subject to an enterprise income tax rate of 25% on our worldwide taxable income. The “resident enterprise” classification also could subject us to a 10% withholding tax on any dividends we pay to our non-PRC shareholders if the relevant PRC authorities determine that such income is PRC-sourced income. In addition to the uncertainties regarding the interpretation and application of the new “resident enterprise” classification, the new EIT Law may change in the future, possibly with retroactive effect. If we are classified as a “resident enterprise” and we incur these tax liabilities, our net income will decrease accordingly.
39
Our ability to pay dividends is restricted by PRC laws.
Our ability to pay dividends is primarily dependent on receiving distributions of funds from our PRC Subsidiaries. Relevant PRC statutory laws and regulations permit payments of dividends by our PRC Subsidiaries only out of their retained earnings, if any, as determined in accordance with PRC accounting standards and regulations. The results of operations reflected in the financial statements prepared in accordance with United States Generally Accepted Accounting Principles, or U.S.GAAP, differ from those reflected in the statutory financial statements of our PRC Subsidiaries.
The principal laws, rules and regulations governing dividends paid by our PRC Subsidiaries include the Company Law of the PRC, Wholly Foreign Owned Enterprise Law and its Implementation Rules. Under these laws and regulations, our PRC Subsidiaries are required to set aside at least 10% of their after-tax profit based on PRC accounting standards each year to its statutory surplus reserve fund until the accumulative amount of such reserve reaches 50% of their respective registered capital. These reserve funds are recorded as part of shareholders' equity but are not available for distribution to shareholders other than in the case of liquidation. As a result of this requirement, the amount of net income available for distribution to shareholders will be limited.
The scope of our business license in the PRC is limited, and we may not expand or continue our business without government approval and renewal, respectively.
Our operating subsidiaries are FIEs located in the PRC. An FIE can only conduct business within its approved business scope, which is designated in its business license. Our licenses permit us to sell and market pharmaceutical products throughout the PRC. Any amendment to the scope of our business requires further application and government approval. In order for us to expand our business beyond the scope of our license, it will be required to enter into negotiations with the government authorities to obtain the approval that would be required to expand the scope of our business. We cannot assure investors that our subsidiaries will be able to obtain the necessary government approval for any change or expansion of its business.
Our business is subject to a variety of environmental laws and regulations. Our failure to comply with environmental laws and regulations may have a material adverse effect on our business and results of operations.
Since the beginning of the 1980s, the PRC has formulated and implemented a series of environmental protection laws and regulations. Our operations are subject to these environmental protection laws and regulations in the PRC. These laws and regulations impose fees for the discharge of waste substances, permit the levy of fines and claims for damages for serious environmental offences and allow the PRC government, at its discretion, to close any facility that fails to comply with orders requiring it to correct or stop operations causing environmental damage. Our operations are in compliance with PRC environmental regulations in all material aspects. The PRC government has taken steps and may take additional steps towards more rigorous enforcement of applicable environmental laws, and towards the adoption of more stringent environmental standards. If the PRC national or local authorities enact additional regulations or enforce current or new regulations in a more rigorous manner, we may be required to make additional expenditures on environmental matters, which could have an adverse impact on our financial condition and results of operations. In addition, environmental liability insurance is not common in the PRC. Therefore, any significant environmental liability claims successfully brought against us would adversely affect our business, financial condition and results of operations.
PRC regulations relating to acquisitions of PRC companies by foreign entities may create regulatory uncertainties that could restrict or limit our ability to operate, including our ability to pay dividends.
On August 8, 2006, the PRC MOFCOM joined by the State-owned Assets Supervision and Administration Commission of the State Council, the State Administration of Taxation, SAIC, the China Securities Regulatory Commission, or CSRC, and SAFE,, released a substantially amended version of the Provisions for Foreign Investors to Merge with or Acquire Domestic Enterprises, or the Revised M&A Regulations, which took effect on September 8, 2006. These new rules significantly revised the PRC's regulatory framework governing onshore-to-offshore restructurings and foreign acquisitions of domestic enterprises. These new rules signify greater PRC government attention to cross-border merger, acquisition and other investment activities, by confirming MOFCOM as a key regulator for issues related to mergers and acquisitions in the PRC and requiring MOFCOM approval of a broad range of merger, acquisition and investment transactions. Further, the new rules establish reporting requirements for acquisition of control by foreigners of companies in key industries, and reinforce the ability of the PRC government to monitor and prohibit foreign control transactions in key industries.
40
These rules may significantly affect the means by which offshore-onshore restructurings are undertaken in the PRC in connection with offshore private equity and venture capital financings, mergers and acquisitions. It is expected that such transactional activity in the PRC in the near future will require significant case-by-case guidance from MOFCOM and other government authorities as appropriate. It is anticipated that application of the new rules will be subject to significant administrative interpretation, and we will need to closely monitor how MOFCOM and other ministries apply the rules to ensure its domestic and offshore activities continue to comply with PRC laws. Given the uncertainties regarding interpretation and application of the new rules, we may need to expend significant time and resources to maintain compliance. It is uncertain how our business operations or future strategy will be affected by the interpretations and implementation of the SAFE notices and new rules. Our business operations or future strategy could be adversely affected by the SAFE notices and the new rules. For example, we may be subject to more stringent review and approval processes with respect to our foreign exchange activities.
The foreign currency exchange rate between U.S. dollars and Renminbi, or RMB, could adversely affect our reported financial results and condition.
To the extent that we need to convert U.S. dollars into RMB for our operational needs, our financial position and the price of our common stock may be adversely affected should RMB appreciate against U.S. dollar at that time. Conversely, if we decide to convert our RMB into U.S. dollars for the operational needs or paying dividends on our common stock, the dollar equivalent of our earnings from our subsidiaries in the PRC would be reduced should U.S. dollar appreciate against RMB.
Until 1994, RMB experienced a gradual but significant devaluation against most major currencies, including dollars, and there was a significant devaluation of RMB on January 1, 1994 in connection with the replacement of the dual exchange rate system with a unified managed floating rate foreign exchange system. Since 1994, the value of RMB relative to U.S. dollar has remained stable and has appreciated slightly against U.S. dollar. Countries, including the United States, have argued that RMB is artificially undervalued due to the PRC's current monetary policies and have pressured the PRC to allow RMB to float freely in world markets. In July 2005, the PRC government changed its policy of pegging the value of RMB to the U.S. dollar. Under the new policy, RMB is permitted to fluctuate within a narrow and managed band against a basket of designated foreign currencies. While the international reaction to RMB revaluation has generally been positive, there remains significant international pressure on the PRC government to adopt an even more flexible currency policy, which could result in further and more significant appreciation of RMB against the dollar.
Restrictions on currency exchange may limit our ability to utilize our revenues effectively and the ability of our PRC Subsidiaries to obtain financing.
Substantially all of our revenues and operating expenses are denominated in RMB . Restrictions on currency exchange imposed by the PRC government may limit our ability to utilize revenues generated in RMB to fund our business activities outside the PRC, if any, or expenditures denominated in foreign currencies. Under current PRC regulations, RMB may be freely converted into foreign currency for payments relating to “current account transactions,” which include among other things dividend payments and payments for the import of goods and services, by complying with certain procedural requirements. Our PRC Subsidiaries may also retain foreign exchange in their respective current account bank accounts, subject to a cap set by SAFE or its local counterpart, for use in payment of international current account transactions.
However, conversion of RMB into foreign currencies and of foreign currencies into RMB, for payments relating to “capital account transactions,” which principally includes investments and loans, generally requires the approval of SAFE and other relevant PRC governmental authorities. Restrictions on the convertibility of the RMB for capital account transactions could affect the ability of our PRC Subsidiary to make investments overseas or to obtain foreign exchange through debt or equity financing, including by means of loans or capital contributions from us.
41
In August 2008, SAFE promulgated Circular 142, a notice regulating the conversion by FIEs of foreign currencies into RMB by restricting how the converted RMB may be used. Circular 142 requires that RMB converted from the foreign currency-denominated capital of a FIE may only be used for purposes within the business scope approved by the applicable government authority and may not be used for equity investments within the PRC unless specifically provided for otherwise. In addition, SAFE strengthened its oversight over the flow and use of RMB funds converted from the foreign currency-denominated capital of a FIE. The use of such RMB may not be changed without approval from SAFE, and may not be used to repay RMB loans if the proceeds of such loans have not yet been used. Violations of Circular 142 may result in severe penalties, including substantial fines as set forth in the SAFE rules.
Any existing and future restrictions on currency exchange may affect the ability of our PRC Subsidiary or affiliated entity to obtain foreign currencies, limit our ability to utilize revenues generated in RMB to fund our business activities outside the PRC that are denominated in foreign currencies, or otherwise materially and adversely affect our business.
Failure to comply with the anti-corruption measures taken by the PRC government could subject us to penalties and other adverse consequences.
We face the risks in relations to actions taken by us, our employees or our subsidiaries that violate the anti-corruption measures taken by the PRC government to prevent fraud and abuse in the pharmaceutical industry. Our failure to comply with these measures, or effectively manage our employees and subsidiaries, could adversely affect our reputation, results of operations and business prospects.
In the pharmaceutical industry, corrupt practices include, among others, acceptance of kickbacks, bribes or other illegal gains or benefits by pharmacies, hospitals and medical practitioners from pharmaceutical manufacturers and distributors in connection with the prescription of certain pharmaceutical products. If we, our employees or subsidiaries violate these laws, rules or regulations, we could be required to pay damages or fines. In the case of our distribution and manufacturing operations, the products involved may be seized and our operations may be suspended, which could materially and adversely affect our business, financial condition and results of operations. Actions by PRC regulatory authorities or the courts to provide an interpretation of PRC laws and regulations that differs from our own or to adopt additional anti-corruption laws and regulations could also require us to make changes to our operations. Our reputation and our sales activities could be adversely affected if we become the target of any negative publicity as a result of actions taken by us, our employees or subsidiaries.
Failure to comply with the United States Foreign Corrupt Practices Act could subject us to penalties and other adverse consequences.
As our ultimate holding company is a Delaware corporation, we are subject to the United States Foreign Corrupt Practices Act, which generally prohibits U.S. companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Foreign companies, including some that may compete with us, are not subject to these prohibitions. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices may occur from time-to-time in the PRC. Although we specifically forbid our employees from engaging in such corrupt practices, we can make no assurance that our employees or other agents will not engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
If we make equity compensation grants to persons who are PRC citizens, they may be required to register with SAFE. We may also face regulatory uncertainties that could restrict our ability to adopt an equity compensation plan for our directors and employees and other parties under PRC law.
On April 6, 2007, SAFE issued the Operating Procedures for Administration of Domestic Individuals Participating in the Employee Stock Ownership Plan or Stock Option Plan of An Overseas Listed Company, also known as Circular 78. It is not clear whether Circular 78 covers all forms of equity compensation plans or only those which provide for the granting of stock options. For any plans which are so covered and are adopted by a non-PRC listed company after April 6, 2007, Circular 78 requires all participants who are PRC citizens to register with and obtain approvals from SAFE prior to their participation in the plan. In addition, Circular 78 also requires PRC citizens to register with SAFE and make the necessary applications and filings if they participated in an overseas listed company's covered equity compensation plan prior to April 6, 2007. We intend to adopt an equity compensation plan in the future and make option grants to our officers and directors, most of whom are PRC citizens. Circular 78 may require our officers and directors who receive option grants and are PRC citizens to register with SAFE. We believe that the registration and approval requirements contemplated in Circular 78 will be burdensome and time-consuming. If it is determined that any of our equity compensation plans is subject to Circular 78, failure to comply with such provisions may subject us and participants of our equity incentive plan who are PRC citizens to fines and legal sanctions and prevent us from being able to grant equity compensation to our PRC employees. In that case, our ability to compensate our employees and directors through equity compensation would be hindered and our business operations may be adversely affected.
42
Any recurrence of severe acute respiratory syndrome, or SARS, Avian Flu, or another widespread public health problem in the PRC could adversely affect our operations.
A renewed outbreak of SARS, Avian Flu or another widespread public health problem in the PRC, where all of our businesses are located and where all of our sales occur, could have a negative effect on our operations. Our businesses are dependent upon our ability to continue to efficiently distribute and sell our products. Such an outbreak could have an impact on our operations as a result of:
|
|
·
|
quarantines or closure of our distribution center, which would severely disrupt our operations,
|
|
|
·
|
the sickness or death of our key officers and employees, and
|
|
|
·
|
a general slowdown in the PRC economy.
|
Any of the foregoing events or other unforeseen consequences of public health problems could adversely affect our operations.
Adverse changes in political, economic and other policies of the PRC government could have a material adverse effect on the overall economic growth of the PRC, which could reduce the demand for our products and materially and adversely affect our competitive position.
All of our business operations are conducted in the PRC, and all of our sales are currently made in the PRC. Accordingly, our business, financial condition, results of operations and prospects are affected significantly by economic, political and legal developments in the PRC. The PRC economy differs from the economies of most developed countries in many respects, including:
|
|
·
|
the extent of government involvement;
|
|
|
·
|
the level of development;
|
|
|
·
|
the growth rate;
|
|
|
·
|
the control of foreign exchange;
|
|
|
·
|
the allocation of resources;
|
|
|
·
|
an evolving regulatory system; and
|
|
|
·
|
lack of sufficient transparency in the regulatory process.
|
While the PRC economy has experienced significant growth in the past 20 years, growth has been uneven, both geographically and among various sectors of the economy. The PRC government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall PRC economy, but may also have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us.
The PRC economy has been transitioning from a planned economy to a more market-oriented economy. Although in recent years the PRC government has implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of the productive assets in the PRC are still owned by the PRC government. The continued control of these assets and other aspects of the national economy by the PRC government could materially and adversely affect our business. The PRC government also exercises significant control over PRC economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Efforts by the PRC government to slow the pace of growth of the PRC economy could result in decreased expenditures by hospitals and other users of our products, which in turn could reduce demand for our products.
43
Moreover, the political relationship between the United States, Europe, or other Asian nations and the PRC is subject to sudden fluctuation and periodic tension. Changes in political conditions in the PRC and changes in the state of foreign relations are difficult to predict and could adversely affect our operations or cause our products to become less attractive. This could lead to a decline in our profitability.
Any adverse change in the economic conditions or government policies in the PRC could have a material adverse effect on overall economic growth and the level of healthcare investments and expenditures in the PRC, which in turn could lead to a reduction in demand for our products and consequently have a material adverse effect on our businesses.
We may not be guaranteed a continuance to receive the preferential tax treatment we currently enjoy, and revenue from our operations in the PRC may become subject to taxation.
The PRC government has provided various incentives to promote the development its TCM industry. These incentives include limited tax exemptions, reduced tax rates and other preferential treatments. Xuelingxian, as a TCM material producer, has been enjoying these preferential tax treatments. Pursuant to the EIT Law, its Implementation Rules, the PRC Value-Added Tax Tentative Provisions and its Implementation Rules, Xuelingxian is entitled to an enterprise income tax rate of 0% for revenue arising from sales of self-cultivated or basic treated TCM and a VAT rate of 0% for income from sales of self-cultivated TCM.
However, we cannot predict when the PRC will cease its preferential tax treatments. In the event of a change of preferential tax policy, Xuelingxian’s business income will be subject to enterprise income tax and VAT.
Because our business is located in the PRC, we may have difficulty establishing adequate management, legal and financial controls, which are required in order to comply with United States securities laws.
PRC companies have historically not adopted a Western style of management and financial reporting concepts and practices, which includes strong corporate governance, internal controls, and computer, financial and other control systems. In addition, we may have difficulty in hiring and retaining a sufficient number of qualified employees to work in the PRC. As a result of these factors, we may experience difficulty in establishing management, legal and financial controls, collecting financial data and preparing financial statements, books of account and corporate records and instituting business practices that meet Western standards. Therefore, we may, in turn, experience difficulties in maintaining adequate internal controls as required under Section 404 of the Sarbanes-Oxley Act of 2002, or SOX. This may result in significant deficiencies or material weaknesses in our internal controls which could impact the reliability of its financial statements and prevent us from complying with the rules and regulations promulgated by the Securities Exchange Commission, the SEC, and the requirements of SOX. Any such deficiencies, weaknesses or lack of compliance could have a materially adverse effect on our business.
Investors may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing original actions in the PRC based upon U.S. laws, including the federal securities laws or other foreign laws against us or our management.
All of our current business operations are conducted in the PRC. Moreover, our directors, two of our officers and two of independent directors are nationals and residents of the PRC. All the assets of these persons are located outside the United States and in the PRC. As a result, it may not be possible to effect service of process within the United States or elsewhere outside the PRC upon these persons. In addition, uncertainty exists as to whether the PRC courts would recognize or enforce judgments of United States courts obtained against us or such officers and/or directors predicated upon the civil liability provisions of the securities laws of the United States or any state thereof, or be competent to hear original actions brought in the PRC against us or such persons predicated upon the securities laws of the United States or any state thereof.
If we are found to be in violation of current or future PRC laws, rules or regulations regarding the legality of foreign investment in the PRC with respect to our ownership structure, we could be subject to severe penalties.
We currently conduct business operations solely in the PRC through our PRC Subsidiaries, in which we hold 100% equity ownership interest. We are a Delaware corporation and our direct and indirect subsidiaries are companies organized under the laws of British Virgin Islands and Hong Kong. As a result, our subsidiaries in the PRC are regarded as FIEs under PRC law and we are subject to PRC law limitations on foreign ownership of PRC companies. There are substantial uncertainties regarding the interpretation and application of PRC laws and regulations, including, but not limited to, the laws and regulations governing our pharmaceutical distribution and production businesses.
44
Accordingly, it is possible that the relevant PRC authorities could, at any time, assert that any portion of our existing or future ownership structure and businesses violate existing or future PRC laws, regulations or policies. It is also possible that the new laws or regulations governing our business operations in the PRC that have been adopted or may be adopted in the future will prohibit or restrict foreign investment in, or other aspects of, any of our PRC Subsidiaries' and our current or proposed businesses and operations. The effectiveness of newly enacted laws, regulations or amendments may be delayed, resulting in detrimental reliance by foreign investors. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively.
The PRC government has broad discretion in dealing with violations of laws and regulations, including:
|
|
·
|
levying fines;
|
|
|
·
|
confiscating our income;
|
|
|
·
|
revoking business and other licenses;
|
|
|
·
|
requiring us to discontinue any portion or all of our business;
|
|
|
·
|
requiring us to restructure our ownership structure or operations; and
|
|
|
·
|
requiring actions necessary for compliance.
|
In particular, licenses and permits issued or granted to us by relevant governmental bodies may be revoked at a later time by higher regulatory bodies. We cannot predict the effect of the interpretation of existing or new PRC laws or regulations on our businesses. We cannot assure you that our current ownership and operating structure would not be found in violation of any current or future PRC laws or regulations. As a result, we may be subject to sanctions, including fines, and could be required to restructure our operations or cease to provide certain services. Any of these or similar actions could significantly disrupt our business operations or restrict us from conducting a substantial portion of our business operations, which, in turn, could materially and adversely affect our business, financial condition and results of operations.
We may be adversely affected by complexity, uncertainties and changes in PRC regulation of pharmaceutical businesses, including limitations on our ability to own key assets.
The PRC government regulates the pharmaceutical industries including foreign ownership of, and the licensing and permit requirements pertaining to, companies operating in these industries. These laws and regulations are relatively new and evolving, and their interpretation and enforcement involve significant uncertainty. As a result, in certain circumstances it may be difficult to determine what actions or omissions may be deemed to be a violation of applicable laws and regulations. Issues, risks and uncertainties relating to PRC government regulation of the pharmaceutical industry include those relating evolving licensing practices. Permits, licenses or operations at our company are subject to government review and scrutiny, which may disrupt our business, or subject us to sanctions, requirements to increase capital or other conditions or enforcement, or compromise enforceability of related contractual arrangements, or have other harmful effects on us. Although we believe we comply with current PRC regulations, we cannot assure you that our ownership and operating structure comply with PRC licensing, registration or other regulatory requirements, with existing policies or with requirements or policies that may be adopted in the future. If the PRC government determines that we do not comply with applicable law, it could take other regulatory or enforcement actions against us that could be harmful to our business.
Failure to comply with PRC regulations regarding the registration requirements for employee stock ownership plans or share option plans may subject the PRC plan participants or us to fines and other legal or administrative sanctions.
In January 2007, SAFE issued implementing rules for the Administrative Measures of Foreign Exchange Matters for Individuals, which, among other things, specified approval requirements for certain capital account transactions such as a PRC citizen's participation in the employee stock ownership plans or stock option plans of an overseas publicly-listed company. In March 2007, SAFE promulgated the Application Procedures of Foreign Exchange Administration for Domestic Individuals Participating in Employee Stock Ownership Plan or Stock Option Plan of Overseas-Listed Company, or Circular 78.
Under Circular 78, PRC citizens who participate in an employee stock option plan in an overseas publicly-listed company are required to register with SAFE or its local office and complete certain other procedures. A PRC agent, which could be the PRC subsidiary of such overseas publicly-listed company, is required to retain a financial institution with stock brokerage qualification at the listing place or a qualified institution relating to the exercise or sale of share options. For participants who had already participated in an employee stock option plan before the date of Circular 78, their PRC employers or PRC agents are required to complete the relevant formalities within three months of the date of this Circular. We and our PRC employees who receive stock option plans will be subject to these regulations. If we or our PRC optionees fail to comply with these regulations, we or our PRC optionees may be subject to fines and other legal or administrative sanctions.
45
We have requested our other stockholders and beneficial owners who are PRC residents to make the necessary applications and filings as required under these regulations and under any implementing rules or approval practices that may be established under these regulations. However, as a result of the vagueness of these regulations and implementing rules, it remains unclear how these regulations, and any future legislation concerning offshore or cross-border transactions, will be interpreted, amended and implemented by SAFE and its local branch. Furthermore, there is a risk that not all of our stockholders and beneficial owners who are PRC residents will in the future comply with our request to make or obtain any applicable registration or approvals.
We face uncertainty from the Circular on Strengthening the Administration of Enterprise Income Tax on Non-resident Enterprises' Share Transfer ("Circular 698") released in December 2009 by China's State Administration of Taxation (SAT), Effective as of January 1, 2008.
Pursuant to the Notice on Strengthening Administration of Enterprise Income Tax for Share Transfers by Non-PRC Resident Enterprises (Circular 698) issued by the State Administration of Taxation on December 10, 2009, where a foreign investor transfers the equity interests of a PRC resident enterprise indirectly via disposing of the equity interests of an overseas holding company ("Indirect Transfer") and such overseas holding company is located in a tax jurisdiction that: (i) has an effective tax rate less than 12.5% or (ii) does not tax foreign income of its residents, the foreign investor shall report to the competent tax authority of the PRC resident enterprise this Indirect Transfer. The PRC tax authority will examine the true nature of the Indirect Transfer, and if the tax authority considers that the foreign investor has adopted an abusive arrangement in order to avoid PRC tax, they will disregard the existence of the overseas holding company and re-characterize the Indirect Transfer and as a result, gains derived from such Indirect Transfer may be subject to PRC withholding tax at the rate of up to 10%. Circular 698 also provides that, where a non-PRC resident enterprise transfers its equity interests in a PRC resident enterprise to its related parties at a price lower than the fair market value, the competent tax authority has the power to make a reasonable adjustment to the taxable income of the transaction.
Risks Relating to Investment in Our Securities
An active public market for our common stock may not develop or be sustained, which would adversely affect the ability of our investors to sell their securities in the public market.
Our shares of common stock currently trade on the OTCBB. We cannot predict the extent to which an active public market for our common stock will develop or be sustained.
Shares eligible for future sale may adversely affect the market price of our common stock, as the future sale of a substantial amount of outstanding stock in the public marketplace could reduce the price of our common stock.
Holders of a significant number of our shares and/or their designees may be eligible to sell our shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, promulgated under the Securities Act of 1933, as amended, or Rule 144, subject to certain limitations. In general, pursuant to Rule 144, a non-affiliate stockholder (or stockholders whose shares are aggregated) who has satisfied a six-month holding period, and provided that there is current public information available, may sell all of its securities. Rule 144 also permits the sale of securities, without any limitations, by a non-affiliate that has satisfied a one-year holding period. Any substantial sale of common stock pursuant to any resale prospectus or Rule 144 may have an adverse effect on the market price of our common stock by creating an excessive supply.
If we fail to maintain effective internal controls, we may not be able to accurately report our financial results or prevent fraud, and our business, financial condition, results of operations and reputation could be materially and adversely affected.
We are a public company and our internal control will be essential to the integrity of our business and financial results. Our public reporting obligations may place a strain on our management, operational and financial resources and systems. We have implemented measures to enhance our internal controls, and plan to take steps to further improve our internal controls on an ongoing basis. If we encounter difficulties in improving our internal controls and management information systems, we may incur additional costs and management time in meeting our improvement goals. In addition, we plan to grow further through acquisitions, which may involve the incurrence of costs and difficulties in integrating acquired businesses and centralizing our internal controls for our acquired businesses. We cannot assure you that the measures taken to improve our internal controls will be effective. If we fail to maintain effective internal controls in the future, our business, financial condition, results of operations and reputation may be materially and adversely affected.
46
Compliance with changing regulation of corporate governance and public disclosure will result in additional expenses.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including SOX and related SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the public markets and public reporting. Our management team must invest significant management time and financial resources to comply with both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities.
We do not foresee paying cash dividends in the near future.
We do not plan to declare or pay any cash dividends on our shares of common stock in the foreseeable future and currently intend to retain any future earnings for funding growth. As a result, investors should not rely on an investment in our securities if they require the investment to produce dividend income.
47
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATION
The following discussion should be read in conjunction with the Condensed Consolidated Financial Statements and Notes thereto appearing elsewhere in this Form 8-KA. The following discussion contains forward-looking statements. Our actual results may differ significantly from those projected in such statements. Factors that may cause future results to differ materially from those projected include but are not limited to those discussed in "Risk Factors" and elsewhere in this Form 8-K/A.
Critical Accounting Policies
Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements that have been prepared in accordance with accounting principles generally accepted in the United States of America, or U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported net sales and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and assumptions. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Our significant accounting policies are described in Note 1 to our consolidated financial statements. Our critical accounting policies are those where we have made the most difficult, subjective or complex judgments in making estimates, and/or where these estimates can significantly impact our financial results under different assumptions and conditions. Our critical accounting policies are:
Revenue Recognition
Our revenue recognition policies are in compliance with Staff Accounting Bulletin 104. Sales revenue is recognized when all of the following have occurred: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the price is fixed or determinable, and (iv) the ability to collect is reasonably assured. These criteria are generally satisfied at the time of shipment when risk of loss and title passes to the customer. Our four segments have the same revenue recognition policy. Revenues are recorded net of value-added taxes.
Accounts Receivable
Accounts receivable, which are unsecured, are stated at the amount we expect to collect. We continuously monitor collections and payments from our customers and maintain a provision for estimated credit losses based upon historical experience and any specific customer collection issues that have been identified. Our credit losses have historically not been significant. As of December 31, 2009 and 2008, all of the trade receivable balances were aged less than one year and have been collected in full subsequent to the balance sheet date. Therefore, management concluded that no allowance for uncollectible amounts was required.
Income Taxes
We are subject to income taxes, primarily in the PRC. We believe we have adequately provided for all taxes due but amounts asserted by tax authorities could be greater or less than the amounts we have accrued. We have concluded all PRC corporate income tax matters through 2010 and do not anticipate adjustments as a result of any tax audits within the next twelve months.
Segments
Segments are defined as components of our business for which separate financial information is available that is evaluated by our chief operating decision-maker (our CEO) in deciding how to allocate resources and assess performance. We presently have four operating segments, consisting of (i) pharmaceutical products distribution, (ii) Traditional Chinese Medicine, or TCM, processing and distribution, (iii) herbal cultivation and sales, and (iv) flower tea bags. Currently, we do not have intersegment sales.
48
Overview
We were incorporated in Delaware on February 9, 2007, under the name “Top Flight Gamebirds, Inc,” or Top Flight. Our principal executive offices are located at Room 2503-2505, New World Center, No.6009 Yitian Road, Futian District, Shenzhen, Guangdong 518026, People’s Republic of China. On September 20, 2010, we changed our name to Global Pharm Holdings Group, Inc. From the time of inception until August 6, 2010, we had entered the commercial game bird industry and had no significant operations. Prior to the Reverse Merger (as defined herein), our revenues had not been sufficient to cover our operating costs and to allow us to continue as a going concern.
On August 6, 2010, our then sole director and officer, Rhonda Heskett, entered into a share purchase agreement with Mei Li Tsai, pursuant to which Ms. Tsai acquired 19,094,000 of Ms. Heskett’s shares of our common stock for cash consideration of $450,000. The $450,000 was expensed and was charged to additional paid-in capital. Subsequently, Mei Li Tsai became our largest stockholder of approximately 78.9% of our then total issued and outstanding shares of stock. In addition, on August 6, 2010, our board of directors approved the appointment of Yunlu Yin as our new Chief Executive Officer and sole director, An Fu as our new Chief Financial Officer and Dan Li as our new Secretary while accepting the resignation of Rhonda Heskett as our director, President, Chief Executive Officer and Chief Financial Officer. As a result of the foregoing, there was a change in control of the Company on August 6, 2010.
On August 12, 2010, Top Flight entered into and consummated a Share Exchange Agreement with Mei Li Tsai, the then sole shareholder of Global Pharma Enterprise Group Limited, a BVI company (referred to as Global Pharma BVI), and Global Pharma BVI to acquire all the issued and outstanding capital stock of Global Pharma BVI, in consideration for 1,800,000 newly issued restricted shares of Top Flight (referred to as the Reverse Merger). The Reverse Merger was approved by the board of directors on August 12, 2010. Immediately after the closing of the Reverse Merger, we had a total of 26,000,000 issued and outstanding shares of common stock and Mei Li Tsai was our then single largest shareholder of 20,894,000 shares of common stock, or approximately 80.36% of our total issued and outstanding 26,000,000 common shares. As a result of the Reverse Merger, Global Pharma BVI is now our wholly owned subsidiary.
Global Pharma BVI is a holding company incorporated in British Virgin Islands and mainly conducts the wholesale and distribution business of pharmaceutical-related products in China through its three indirectly wholly owned Chinese subsidiaries including Tonghua Tongdetang Pharmaceutical Co., Ltd. (referred to as Tongdetang), Anhui Xuelingxian Pharmaceutical Co., Ltd. (referred to as Xuelingxian) and Shandong Global Pharm Co., Ltd. (referred to as Yaoyuan and, together with Xuelingxian and Tongdetang, referred to as the PRC Subsidiaries). Global Pharm holds all of the shares of Hong Kong Wisdom Fortune Medicine Holding Group Limited, a Hong Kong company (referred to as Wisdom Fortune) and Binomial Biopharm Group Limited, a Hong Kong company (referred to as Binomial). Binomial currently holds all the equity interests in Xuelingxian and Tongdetang, and Wisdom Fortune currently holds all the equity interest in Yaoyuan.
After Top Flight entered into the Share Exchange Agreement with Global Pharma BVI on August 12, 2010, pursuant to the Earn-In Agreement, as thereafter amended as described below, the shareholders, including the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan, and the key management of Global Pharma BVI were given the right to purchase 20,894,000 shares at four different occurrence dates, contingent on various targets for total consideration of $300,000. The $300,000 was expensed and was charged to additional paid-in capital. Targets include binding three-year employment contracts with various members of management within six months of this agreements and target after tax net income (the non-cash expenses need to be excluded from the calculation of net income) of $3.6 million, $3.8 million, and $15.2 million for the three months ended June 30, 2010 and September 30, 2010, and for the 12 months ended December 31, 2010, respectively. Pursuant to the Amendment to the Earn-In Agreement as discussed below, the key management and former shareholders, including beneficial owners of Xuelingxian, Tongdetang and Yaoyuan, acquired call rights to own 80.36% of Top Flight. On September 20, 2010, Top Flight changed its name to Global Pharm Holdings Group, Inc.
On March 29, 2011, we entered into an Agreement to Amend the Earn-In Agreement, or the Amendment, with the former shareholders and the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan and 20 individuals of its management who were parties to the original agreement. As of March 29, 2011, pursuant to the Amendment, all of the four earn-in targets have been achieved and the key management and former shareholders and the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan hold, in the aggregate, 20,894,000 shares, or 80.36%, of our common stock.
49
On the date of Reverse Merger, we changed our business plan to focus on pharmaceutical-related product distribution in China.
Pharmaceutical Products Distribution Segment
Our products include prescription drugs, over-the-counter, or OTC, drugs, and nutritional supplements. In 2009, we sold approximately 8,400 different types of products and we had approximately 2,600 clients that spanned from hospital, clinics, pharmacies and drugstores, and other healthcare institution
In 2008 and 2009, the sales from our distribution segment accounted for approximately 86% and 68% of total sales, respectively. Yaoyuan and Tongdetang focus primarily on the distribution business. They purchase pharmaceutical-related products from manufacturers and wholesalers and sell the products to hospitals, other distributors, health clinics, chain and individual drug stores, among others.
In January 2010, we entered into an exclusive distribution agreement with Xiuzheng Pharmaceutical Group Marketing Co., Ltd., or Xiuzheng, and obtained the exclusive distribution right to sell over 500 different types of its OTC drugs in Shandong province (referred to as the Xiuzheng Agreement). The term of the Xiuzheng Agreement is one year, commencing January 1, 2010, which we renewed on January 1, 2011. Xiuzheng is a well-known pharmaceutical manufacturer and its products are popular in China. The exclusive distribution right of Xiuzheng’s products has generated significant revenue in the first three quarters in 2010 of approximately 5%. We have expanded our effort to sell Xiuzheng’s products in 2010 and renewed the contact with Xiuzheng in 2011.
In addition, in January 2010, we entered into another provincial exclusive distribution agreement with Hainan Lingkang Pharmaceutical Co., Ltd., or Lingkang (referred to as the Lingkang Agreement), pursuant to which we obtained exclusive distribution rights to sell 40 types of its prescription drugs in Shandong province. We also obtained the right to price the drug according to the market. The term of the Lingkang Agreement is one year, commencing January 1, 2010, which we renewed on January 1, 2011. The exclusive distribution and pricing rights for Lingkang’s products has generated significant revenue and profit in 2010 of approximately 28%.
TCM Processing and Distribution Segment
The sales from our TCM processing and distribution segment accounted for 17% of the total sales in 2009. Xuelingxian focused on the TCM processing and distribution business.
Herbal Cultivation and Sales Segment
The sales from our herbal cultivation and sales segment accounted for 9% and nil of the total sales in 2009 and 2008, respectively. Xuelingxian focused on the herbal cultivation and sales business.
In January 2009, we rented a parcel of land from Mengwang Village Committee of Dayang County, Qiocheng District, Boxhou City in Anhui province. The land generated revenue in the third and fourth quarters of 2009, in the aggregate, of $7,500,814.
On each of January 1, 2010 and July 2, 2010, we rented parcels of land from Mengwang Village Committee of Dayang County, Qiocheng District, Boxhou City in Anhui province to expand our herbal cultivation business. Both parcels of land generated revenue in the third and fourth quarters of 2010, in the aggregate, of $15,390,594.
According to statistics of China Association of Traditional Chinese Medicine, as quoted by Ministry of Commerce of the People’s Republic of China (http://www.mofcom.gov.cn/aarticle/o/di/201011/20101107245413.html), the price of 84% of the 537 most commonly used herbal products increased in amounts ranging from 5% to 180%. Of these herbal products, 28% increased more than 50% in price. Our management believes this trend will continue until the spring of 2011 because the demand for herbal products in the winter is traditionally higher than other seasons. The profit of our herbal cultivation segment has increased 105.2% in 2010 compared to 2009.
50
Flower Tea Bags Segment
The sales from our flower tea bags segment accounted for 6% and nil of the total sales in 2009 and 2008, respectively. The current production of flower tea bags includes basic processing, screening, and bagging. In order to satisfy the increasingly sophisticated market trends and accommodate customers’ needs, management plans to restructure the flower tea segment and refine the current tea products to grinding tea drinks to meet the market demand.
Recent Developments
Joint Venture
On March 21, 2011, the Company obtained the Certificate of Approval from Anhui Provincial Government for the establishment of a Joint Venture, “Anhui Sino-Green TCM Tech Development Co., Ltd” (“Sino-Green TCM Tech”), with foreign investment in the People’s Republic of China. On April 6, 2011, we obtained the Business license for Sino-Green TCM Tech.
Wisdom Fortune, our Hong Kong subsidiary, together with Anhui Qianyi Pharmaceutical Co., Ltd. (referred to as Qianyi Pharmaceutical), a PRC company, established Anhui Sino-Green TCM Tech Development Co., Ltd. (referred to as Sino-Green TCM Tech), a Joint Venture in Bozhou City, Anhui Province. Wisdom Fortune will invest USD 9.7 million and Qianyi Pharmaceutical will invest USD 0.3 million, reflecting 97% and 3%, respectively, of the ownership of Sino-Green TCM Tech to the Joint-Venture. Qianyi Pharmaceutical will have a total investment of USD 20 million and registered capital of USD 10 million. Wisdom Fortune and Qianyi will invest an additional USD 10 million in proportional amounts in the future. The Company is required to pay $1,455,000 registered capital within three months and $8,245,000 registered capital within two years after April 6, 2011.
The Joint Venture with a local company in Bozhou City represents a new stage in the development of a TCM cultivation base in Bozhou over the next five years, and is based on the Letter of Intent recently signed with the Bozhou Municipal Government. The total project is comprised of the following key components: the rent of 100,000 mu (about 6,667 hectares) of planting base from 2011 to 2015; the construction of an integrated TCM facility for health-care product research and development (refer to as R&D), herbal processing, and TCM extraction during 2011 to 2013; and the establishment of a modern information platform for the collection, compilation and analysis of TCM industrial information during 2011 and 2012. We, together with the support of the Bozhou municipal government, plan to invest approximately RMB 500 million (about $76.1 million) in aggregate capital expenditures over the next five years to support the project.
In addition, based on the Letter of Intent with the Bozhou municipal government, we will have the right to sign contracts with local farmers to procure annual harvests at the current market price. These agreements cover 250,000 mu (about 16,667 hectares) of herbal cultivation land in Bozhou City.
We are engaging in the TCM cultivation, TCM herbal processing and related products R&D business to alleviate the impact on price inflation and maintain the supply stability of TCM raw materials. The cost-saving for TCM herbal-related raw materials obtained directly from the place of origin will significantly enhance our products portfolio, and create a significant synergy between our plantation base and distribution business units. With the goal of integrating production, distribution, wholesale and retail, we hope to establish a complete value chain in the pharmaceutical distribution industry.
Agreement to Amend the Earn-In Agreement
On August 12, 2010, Global Pharma BVI entered into and consummated a Share Exchange Agreement with the Company. Pursuant to the Earn-In Agreement, as thereafter amended as described below, the shareholders, including the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan, and the key management of Global Pharma BVI were given the right to purchase 20,894,000 shares at four different occurrence dates, contingent on various targets for total consideration of $300,000. On March 29, 2011, we entered into an Agreement to Amend the Earn-In Agreement, (the “Amendment”), with the former shareholders and the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan and 20 individuals of its management who were parties to the original agreement. As of March 29, 2011, pursuant to the Amendment, all of the four earn-in targets have been achieved and the key management and former shareholders and the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan hold, in the aggregate, 20,894,000 shares, or 80.36%, of the Company’s shares of stock.
51
Results of Operations
The following table sets forth a summary of our statements of operations for the periods indicated.
|
For the three months ended June 30,
|
||||||||||||||||||||||||
|
in USD except percentage
|
2010
|
2009
|
Change
|
|||||||||||||||||||||
|
|
% of revenue
|
% of
revenue |
Amount
|
Percentage
|
||||||||||||||||||||
|
Revenues, net
|
$ | 30,530,977 | 100.0 | $ | 19,189,756 | 100.0 | $ | 11,341,221 | 59.1 | |||||||||||||||
|
Cost of goods sold
|
24,989,792 | 81.9 | 15,524,554 | 80.9 | 9,465,238 | 61.0 | ||||||||||||||||||
|
Gross profit
|
5,541,185 | 18.1 | 3,665,202 | 19.1 | 1,875,983 | 51.2 | ||||||||||||||||||
|
Expenses
|
||||||||||||||||||||||||
|
Operating expenses
|
233,371 | 0.8 | 198,606 | 1.0 | 34,765 | 17.5 | ||||||||||||||||||
|
General and administration
|
1,794,759 | 5.9 | 259,179 | 1.4 | 1,535,580 | 592.5 | ||||||||||||||||||
|
Income from operations
|
3,513,055 | 11.5 | 3,207,417 | 16.7 | 305,638 | 9.5 | ||||||||||||||||||
|
Other incomes
|
13,226 | 0.0 | 6,450 | 0.0 | 6,776 | 105.1 | ||||||||||||||||||
|
Income before income taxes
|
3,526,281 | 11.5 | 3,213,867 | 16.7 | 312,414 | 9.7 | ||||||||||||||||||
|
Provision for income taxes
|
1,255,682 | 4.1 | 807,246 | 4.2 | 448,436 | 55.6 | ||||||||||||||||||
|
Net income
|
$ | 2,270,599 | 7.4 | $ | 2,406,621 | 12.5 | $ | (136,022 | ) | (5.7 | ) | |||||||||||||
|
For the six months ended June 30,
|
||||||||||||||||||||||||
|
in USD except percentage
|
2010
|
2009
|
Change
|
|||||||||||||||||||||
|
|
|
% of revenue
|
|
% of
revenue
|
Amount
|
Percentage
|
||||||||||||||||||
|
Revenues, net
|
$ | 59,259,637 | 100.0 | $ | 38,952,977 | 100.0 | $ | 20,306,660 | 52.1 | |||||||||||||||
|
Cost of goods sold
|
48,632,162 | 82.1 | 31,687,148 | 81.3 | 16,945,014 | 53.5 | ||||||||||||||||||
|
Gross profit
|
10,627,475 | 17.9 | 7,265,829 | 18.7 | 3,361,646 | 46.3 | ||||||||||||||||||
|
Expenses
|
||||||||||||||||||||||||
|
Operating expenses
|
364,999 | 0.6 | 298,644 | 0.8 | 66,355 | 22.2 | ||||||||||||||||||
|
General and administration
|
2,165,148 | 3.7 | 515,866 | 1.3 | 1,649,282 | 319.7 | ||||||||||||||||||
|
Income from operations
|
8,097,328 | 13.7 | 6,451,319 | 16.6 | 1,646,009 | 25.5 | ||||||||||||||||||
|
Other incomes
|
17,874 | 0.0 | 13,007 | 0.0 | 4,867 | 37.4 | ||||||||||||||||||
|
Income before income taxes
|
8,115,202 | 13.7 | 6,464,326 | 16.6 | 1,650,876 | 25.5 | ||||||||||||||||||
|
Provision for income taxes
|
2,419,451 | 4.1 | 1,619,857 | 4.2 | 799,594 | 49.4 | ||||||||||||||||||
|
Net income
|
$ | 5,695,751 | 9.6 | $ | 4,844,469 | 12.4 | $ | 851,282 | 17.6 | |||||||||||||||
Result of Operations – The three months ended June 30, 2010 as compared to three months ended June 30, 2009
Revenue. Revenue increased by $11,341,221 or 59.1% to $30,530,977 for the three months ended June 30, 2010 from $19,189,756 for the comparable period in 2009. 32.3% of the increase was attributable to our Yaoyuan subsidiary obtaining the exclusive rights to distribute more than 100 different types of drugs from Lingkang at the beginning of 2010. 12.8% of the increase was attributable to our Xuelingxian subsidiary, due to the growth of its TCM processing and distribution business. More specifically, revenue from the TCM processing and distribution segment increased $2,264,664 or 64.1% to $6,271,328 for the three months ended June 30, 2010 from $4,006,664 for the comparable period in 2009.
52
Cost of Goods Sold. The cost of goods sold increased by $9,465,238 or 61.0% to $24,989,792 for the three months ended June 30, 2010 from $15,524,554 for the comparable period in 2009, consistent with the increase of revenue. Cost of goods sold for our flower tea bags business increased $142,695 to $1,371,531 for the three months ended June 30, 2010 from $1,228,836 for the comparable period in 2009. Cost of goods sold for TCM processing and distribution increased $2,012,027 or 64.1% to $5,149,236 for the three months ended June 30, 2010 from $3,137,209 for the comparable period in 2009, consistent with the increase in revenue.
Gross Profit. Gross profit increased by $1,875,983 or 51.2% to $5,541,185 for the three months ended June 30, 2010 from $3,665,202 for the comparable period in 2009, consistent with the increase in revenue during the period.
Operating Expenses. Operating expenses increased by $34,765 to $233,371 for the three months ended June 30, 2010 from $198,606 for the comparable period in 2009. The increase in operating expense was primarily a result of increased revenue.
General and Administrative. Our general and administrative expenses increased by $1,535,580 to $1,531,447 for the three months ended June 30, 2010 from $259,179 for the comparable period in 2009 as a result of the increase of stock-based compensation expenses in 2010. On June 29, 2010, in connection with the Earn-In Agreement, as thereafter amended, 20 management team members obtained the call right to purchase 2,279,930 shares of our common stock and the fair value of these shares of stock was appropriately $6.0 million at the grant date. These stock-based compensation expenses had been fully amortized in 2010 and $1,492,100 has been amortized in the three months ended June 30, 2010. The increase of the general and administrative expenses is also consistent with the increase in net revenue and our becoming a public company, resulting in increased professional service fees, such as legal and accounting.
Income from Operations. As a result of the foregoing, income from operations increased to $3,513,055 for the three months ended June 30, 2010 from $3,207,417 for the comparable period in 2009, an increase of 17.5% due to the increase of revenue and offset by the increase of stock-based compensation expenses.
Income Taxes. Income tax expense increased to $1,255,682 in the three months ended June 30, 2010 from $807,246 in the comparable period in 2009. Our effective tax rate for our operating subsidiaries were 25.0% and 25.1% for the three months ended June 30, 2010 and 2009, respectively. The statutory rate under the laws of the PRC is 25% and the herb cultivation business is subject to zero income tax in China.
Net Income. Our net income decreased by $136,022 or 5.7% to $2,270,599 for the three months ended June 30, 2010 from $2,406,621 for the comparable period in 2009. Our profit margin decreased from 12.5% for the three months ended June 30, 2009 to 7.4% for the comparable period in 2010. The decrease of profit margin was due to the increase of stock-based compensations expenses and the cost of pharmaceutical products.
Result of Operations – The six months ended June 30, 2010 as compared to six months ended June 30, 2009
Revenue. Revenue increased by $20,306,660 or 52.1% to $59,259,637 for the six months ended June 30, 2010 from $38,952,977 for the comparable period in 2009. 27.1% of the increase was attributed to our Yaoyuan subsidiary obtaining the exclusive rights to distribute more than 100 different types of drugs from Lingkang at the beginning of 2010. 17.7% of the increase was attributable to our Xuelingxian subsidiary, due to the growth of its herbal tea sales and TCM processing and distribution business. More specifically, our flower tea bag sales increased $1,231,685 for the six months ended June 30, 2010 from $1,677,663 in the comparable period in 2009. Revenue from the TCM processing and distribution segment increased $5,689,049 or 74.7% to $13,305,270 for the six months ended June 30, 2010 from $7,616,221 for the comparable period in 2009.
53
Cost of Goods Sold. Cost of goods sold increased by $16,945,014 or 53.5% to $48,632,162 for the six months ended June 30, 2010 from $31,687,148 for the comparable period in 2009 consistent with increase of revenue. Cost of goods sold for our flower tea bags business increased $874,451 to $2,103,287 for the six months ended June 30, 2010 from $1,228,836 for the comparable period in 2009, due to the commencement of the manufacture and sale of flower tea in April 2009. Cost of goods sold for the TCM processing and distribution increased $4,873,360 or 82.0% to $10,815,961 for the six months ended June 30, 2010 from $5,942,601 for the comparable period in 2009, and this is in tandem with the increase in revenue.
Gross Profit. Gross profit increased by $3,361,646 or 46.3% to $10,627,475 for the six months ended June 30, 2010 from $7,265,829 for the comparable period in 2009. Our gross margin slightly decreased from 18.7% for the six months ended June 30, 2009 to 17.9% for the six months ended June 30, 2010 due to the price volatility of the herbal raw material.
Operating Expenses. Operating expenses increased by $66,355 to $364,999 for the six months ended June 30, 2010 from $298,644 for the six months ended June 30, 2009. The increase in operating expenses was primarily a result of increased revenue.
General and Administrative. General and administrative expenses increased by $1,649,282 to $2,165,148 as a result of increase of stock-based compensation expenses in 2010. On June 29, 2010, in connection with the Earn-In Agreement, as thereafter amended, 20 management team members obtained the call right to purchase 2,279,930 shares of our common stock and the fair value of these shares of stock was appropriately $6.0 million at the grant date. The employee-based stock expenses had been fully amortized in 2010 and $1,492,100 has been amortized in the six months ended June 30, 2010. Except for the stock-based compensation, the increase of the general and administrative expenses is consistent with the increase in net revenue.
Income from Operations. As a result of the foregoing, our income from operations increased to $8,097,328 for the six months ended June 30, 2010 from $6,451,319 for the comparable period in 2009, an increase of 25.5% due to the increase of revenue and offset by the increase of stock-based compensation expenses.
Income Taxes. Income tax expense increased to $2,419,451 in the six months ended June 30, 2010 from $1,619,857 in the comparable period in 2009. Our effective tax rate for our operating subsidiaries was 25.0% for the six months ended June 30, 2010 and 2009.
Net Income. Our net income increased by 17.6% to $5,695,751 for the six months ended June 30, 2010 from $4,844,469 for the comparable period in 2009. Our profit margin decreased from 12.4% for the six months ended June 30, 2009 to 9.6% for the comparable period in 2010. The decrease of profit margin was due to the increase of stock-based compensation expenses and the increase of cost of pharmaceutical products.
Result of Operations – The year ended December 31, 2009 as compared to the year ended December 31, 2008
|
For the years ended December 31,
|
||||||||||||||||||||||||
|
in USD except percentage
|
2009
|
2008
|
Change
|
|||||||||||||||||||||
|
|
|
% of revenue
|
|
% of
revenue
|
Amount
|
Percentage
|
||||||||||||||||||
|
Revenues, net
|
$ | 86,784,002 | 100.0 | $ | 54,475,666 | 100.0 | $ | 32,308,336 | 59.3 | |||||||||||||||
|
Cost of goods sold
|
70,725,013 | 81.5 | 45,362,590 | 83.3 | 25,362,423 | 55.9 | ||||||||||||||||||
|
Gross profit
|
16,058,989 | 18.5 | 9,113,076 | 16.7 | 6,945,913 | 76.2 | ||||||||||||||||||
|
Expenses
|
||||||||||||||||||||||||
|
Operating expenses
|
792,295 | 0.9 | 366,718 | 0.7 | 425,577 | 116.1 | ||||||||||||||||||
|
General and administration
|
1,096,333 | 1.3 | 648,520 | 1.2 | 447,813 | 69.1 | ||||||||||||||||||
|
Income from operations
|
14,170,361 | 16.3 | 8,097,838 | 14.9 | 6,072,523 | 75.0 | ||||||||||||||||||
|
Other incomes
|
29,318 | 0.0 | 21,085 | 0.0 | 8,233 | 39.0 | ||||||||||||||||||
|
Income before income taxes
|
14,199,679 | 16.4 | 8,118,923 | 14.9 | 6,080,756 | 74.9 | ||||||||||||||||||
|
Provision for income taxes
|
3,298,277 | 3.8 | 2,029,731 | 3.7 | 1,268,546 | 62.5 | ||||||||||||||||||
|
Net income
|
$ | 10,901,402 | 12.6 | $ | 6,089,192 | 11.2 | $ | 4,812,210 | 79.0 | |||||||||||||||
54
Revenue. Revenue increased by $32,308,336 or 59.3% to $86,784,002 for the year ended December 31, 2009 from $54,475,666 for the comparable period in 2008, primarily due to the growth of our Xuelingxian subsidiary. Xuelingxian was incorporated in July 2008 and had only five months of operations in 2008. More specifically, revenue from our herb cultivation business increased $7,525,649 from nil for the year ended December 31, 2008 to $7,525,649 for the comparable period in 2009, due to the commencement of our herb planting in 2009. Revenue from the TCM processing and distribution segment increased $7,106,217 or 91.3% to $14,893,522 for the year ended December 31, 2009 from $7,787,305 for the comparable period in 2008, due to the fact that the operating period for Xuelingxian in 2009 was12 months and only five months in 2008. The revenue from our flower tea bags segment increased $5,276,780 for the year ended December 31, 2009 from nil in 2008 because we started producing and selling flower tea bags in April 2009.
Cost of Goods Sold. Cost of goods sold increased by $25,362,423 or 55.9% to $70,725,013 for the year ended December 31, 2009 from $45,362,590 for the comparable period in 2008. The increase of cost of goods sold was consistent with the increase in revenues. Cost of goods sold for the herb cultivation and sales segment increased to $6,431,470 for the year ended December 31, 2009 from nil in the same period in 2008. This was due to the fact that Xuelingxian did not have any herb cultivation business in 2008. Cost of goods sold for our TCM processing and distribution segment increased $4,988,888 or 75.2% to $11,621,422 for the year ended December 31, 2009 from $6,632,534 for the year ended December 31, 2008, due to the fact that the operating period for Xuelingxian was 12 months in 2009 and only five months in 2008. Cost of goods sold for our flower tea bags segment increased to $3,734,882 for the year ended December 31, 2009 consistent with the increase in revenue.
Gross Profit. Gross profit increased by $6,945,913 or 76.2% to $16,058,989 for the year ended December 31, 2009 from $9,113,076 for the comparable period in 2008. Our gross margin increased from 16.7% for the year ended December 31, 2008 to 18.5% for the comparable period in 2008. We anticipate that our overall gross profit will continue to increase as our sales increase.
Operating Expenses. Sales and marketing expenses increased by 116.1% to $792,295 for the year ended December 31, 2009 from $366,718 for the comparable period in 2008 and was a primary driver of our increased revenues during 2009. The increase in sales and marketing expense was primarily a result of increased revenue.
General and Administrative. General and administrative expenses increased by 69.1% to $1,096,333 for the year ended December 31, 2009 from $648,520 for the comparable period in 2008 as a result of the increased revenue during the year. General and administrative expenses as a percentage of our revenue was 1.3% and 1.2% of the total sales for the years ended December 31, 2009 and 2008, respectively.
Income from Operations. As a result of the foregoing, our income from operations increased by 75.0% to $14,170,361 for the year ended December 31, 2009 from $8,097,838 for the comparable period in 2008.
Income Taxes. Our income tax expense increased to $3,298,277 for the year ended December 31, 2009 from $2,029,731 in the comparable period in 2008. Our effective tax rate was 23.2% and 25.0 for the year ended December 31, 2009 and 2008, respectively. The decrease of the effective tax was primarily a result of the decreased effective income tax rate of Xuelingxian, our herbal subsidiary, Xuelingxian’s herb cultivation business is subject to income tax exemption according to PRC tax law.
Net Income. Our net income increased by 79.0% to $10,901,402 for the year ended December 31, 2009 from $6,089,192 for the comparable period in 2008. Our profit margin slightly increased from 11.2% for the year ended December 31, 2008 to 12.6% for the comparable period in 2009. The increase of net income was consistent with the increase of revenue.
55
Liquidity and Capital Resources
The following table sets forth a summary of our net cash flow information for the periods indicated:
|
For the years ended December 31,
|
For the six months ended June 30,
|
|||||||||||||||||||||||||||||||
|
in USD except percentage
|
2009
|
2008
|
Change
|
2010
|
2009
|
Change
|
||||||||||||||||||||||||||
|
|
%
|
|
%
|
|||||||||||||||||||||||||||||
|
Net cash provided by operating activities
|
$ | 8,699,909 | $ | 5,600,586 | $ | 3,099,323 | 55.3 | $ | 5,641,687 | $ | 1,277,991 | $ | 4,363,696 | 341.4 | ||||||||||||||||||
|
Net cash used in investing activities
|
(155,971 | ) | (58,434 | ) | (97,537 | ) | 166.9 | (9,063 | ) | (119,219 | ) | 110,156 | (92.4 | ) | ||||||||||||||||||
|
Net cash provided by (used in) financing activities
|
6,286,912 | (2,954,052 | ) | 9,240,964 | (312.8 | ) | (2,905,096 | ) | (1,395,962 | ) | (1,509,134 | ) | 108.1 | |||||||||||||||||||
|
Net increase (decrease) in cash
|
2,257,026 | 2,588,100 | (331,074 | ) | (12.8 | ) | 2,727,528 | (237,190 | ) | 2,964,718 | (1,249.9 | ) | ||||||||||||||||||||
|
Cash at the end of the year
|
$ | 7,455,147 | $ | 5,188,587 | $ | 2,266,560 | 43.7 | $ | 10,169,823 | $ | 4,959,904 | $ | 5,209,919 | 105.0 | ||||||||||||||||||
For the six months ended June 30, 2010 as compared to the six months ended June 30, 2009
We had net working capital of $6,413,557 at June 30, 2010, a decrease of $3,566,680 over $9,980,237 at December 31, 2009. Cash for operations and liquidity needs are funded primarily through cash flows from operations. We believe that the funds and cash generated from operations available to us are adequate to meet our operating needs in 2010.
Our cash at December 31, 2009 was $7,455,147 and increased to $10,169,823 at June 30, 2010, an increase of $2,714,676 or 36.4% over the cash amount at December 31, 2009. The increase was primarily attributable to a number of factors, including the following:
For the six months ended June 30, 2010, we generated $5,641,687 from operating activities, as compared to $1,277,991 for the six months ended June 30, 2009. The increase of $4,363,696 is primarily a result of the increase of net income and the lesser amount of inventory purchased during the comparable periods. Our Xuelingxian subsidiary leased additional land for its herbal planting business in 2010 and paid appropriately $2.2 million in the first quarter as rent expense for 2010.
We used $9,063 in investing activities during the six months ended June 30, 2010 as compared to $119,219 during the six months ended June 30, 2009. This decrease of $110,156 in investing activities was primarily a result of purchasing less equipment.
Cash used in financing activities was $2,905,096 for the six months ended June 30, 2010 as compared to cash used in financing activities of $1,395,962 for the six months ended June 30, 2009. The cash financing activities in both periods were primarily the result of dividends paid to the former shareholders of our subsidiaries
For the year ended December 31, 2009 as compared to the year ended December 31, 2008
We had net working capital of $9,980,237 at December 31, 2009, an increase of $3,891,385 over $6,088,852 at December 31, 2008.
Cash and Cash Equivalents
Our cash and cash equivalents as at the beginning of the year ended December 31, 2009 was $5,188,587 and increased to $7,455,147 by the end of the year, an increase of $2,266,560 or 43.7% over the base amount at January 1, 2009. The increase was primarily attributable to a number of factors, including the following:
For the year ended December 31, 2009, we generated $8,699,909 from operating activities, as compared to $5,600,586 for the year ended December 31, 2008. The increase of $3,099,323 was a result of an increase in net income from the year ended December 31, 2008 to December 31, 2009 of $4,812,210.
We used $155,971 in investing activities during the year ended December 31, 2009 as compared to $58,434 during the year ended December 31, 2008. The increase in investing activities was primarily a result of purchasing additional equipment.
56
Cash used in financing activities was $6,286,912 for the year ended December 31, 2009 as compared to cash used in financing activities of $2,954,052 for the year ended December 31, 2008. The increase of $3,332,860 was primarily a result of an increase in the dividend paid to the former shareholders of our subsidiaries from the year ended December 31, 2008 to December 31, 2009 of $4,764,817.
Capital Resources
During the year ended December 31, 2009, we borrowed $219,751 from Shandong Qilu Bank. The loan bears an annual interest of 7.434% and is due on December 21, 2010. We entered an entrustment guarantee contract with Kexin Fengda Investment Guarantee Co, Ltd (“Kexin Fengda”) whereby Kexin Fengda guarantees the note payable to Shandong Qilu bank. Kexin Fengda received payment of approximately $6,000 in consideration for this guarantee. Yanliang Song, an officer and shareholder, provided a counter-guarantee to Kexin Fengda using all his personal/family assets.
During the six months ended June 30, 2010, we borrowed 219,000 from Shandong Qilu Bank. The loan bears an annual interest of 6.372% and is due on March 22, 2011. The principal and interest of the two loans will be paid monthly. The second loan is secured by our inventories, valued at RMB 6,000,000. The pledge is valid from March 22, 2010 to March 22, 2011. Qilu Bank has authorized Shandong Woerde Guarantee Company to supervise the pledged inventories.
On May 7, 2010, we entered into a credit agreement with Construction Bank of China in the amount of $151,704. As part of the loan agreement, RMB 4,000,000 or $606,815 of property and land of two shareholders and Bozhou City Herb Research Institute guarantee the outstanding principal balance to the bank over the term of the loan.
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
We lease various facilities under lease agreements ranging from month-to-month to 15-year terms. The following tables set forth the detail information of the lease obligations:
57
|
No.
|
Lessor
|
Term
|
Rent (RMB)
|
Rent (US$)
|
Description
|
|||||
|
1
|
General Tobacco Group Co., Ltd.
|
July 1, 2009 – June 30, 2010
|
1,417,295 per year
|
215,009 per year
|
Used as distribution center, totaling 15,533 square meters
|
|||||
|
2
|
General Tobacco Group Co., Ltd.
|
March 1, 2010 -February 28, 2011
|
180,000 for the first year and increase at a rate of 5% per year thereafter
|
27,307 per year and increase at a rate of 5% per year thereafter
|
Used for parking, totaling 156,020 square meters
|
|||||
|
3
|
General Trading Co., Ltd.
|
March 1, 2010-March 28, 2011
|
30,000 for the first year and increase at a rate of 5% per year thereafter
|
4,551 for the first year and increase at a rate of 5% per year thereafter
|
Used as offices, totaling 1,160.46 square meters
|
|||||
|
4
|
General Tobacco Group Co., Ltd.
|
July 1, 2010 -June 30, 2011
|
1,335,334 per year
|
202,575 per year
|
Used as distribution center, totaling 13,937 square meters
|
|||||
|
5
|
General Tobacco Group Co., Ltd.
|
July 1, 2010 -June 30, 2011
|
390,898 per year
|
59,301 per year
|
Used as warehouse, totaling 2,677 square meters
|
|||||
|
6
|
Shujun Xu
|
November 1, 2006-October 31, 2011,
|
360,000 for the first year; 420,000 for the second year; 480,000 for the third year; 540,000 for the fourth year; 600,000 for the fifth year.
|
54,613 for the first year; 63,716 for the second year; 72,818 for the third year; 81,920 for the fourth year; 91,022 for the fifth year.
|
Used as distribution center, totaling 2,449 square meters
|
|||||
|
7
|
Xiuying Hou
|
December 1, 2010-December 1, 2013
|
64,800 every year
|
9,830 every year
|
Used as distribution center, totaling 300 square meters
|
|||||
|
8
|
Anhui Province Bozhou City Fengyi Institute of Traditional Chinese Medicine
|
August 1 2008 –July 31, 2023 renewable annually
|
1,200,000 per year
|
182,045 per year
|
Used as warehouse, totaling 3,000 square meters
|
|||||
|
9
|
Mengwang Village Committee of Dayang County, Qiaocheng District, Bozhou City, Anhui Province, PRC
|
January 1, 2010-December 31, 2014 renewable annually
|
14,970,000 for 2010, 8,800,000 per year thereafter.
|
2,271,007 for 2010, 1,334,994 per year thereafter.
|
Used for herbal cultivation, totaling 1,318 acres
|
|||||
|
10
|
Mengwang Village Committee of Dayang County, Qiaocheng District, Bozhou City, Anhui Province, PRC
|
July 1, 2010 - December 31, 2015 renewable annually
|
31,450,367 for 2010, 16,939,939 for 2011, 10,468,500 per year thereafter.
|
4,771,142 for 2010, 2,569,854 for 2011, 1,588,112 per year thereafter
|
Used for herbal cultivation, totaling 1,642 acres.
|
|||||
|
11
|
25/F New World Center, 6009 Yitian Road, Futian District, Shenzhen, PRC
|
Dec 1, 2009 - Nov 30, 2012
|
606,000 per year
|
104,676 per year
|
Used as office
|
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity or capital expenditures or capital resources that is material to an investor in our securities.
58
Legal and Administrative Proceedings
We are not aware, to the best of our knowledge, of any material, active, pending or threatened proceeding against us or our subsidiaries, nor are we, or any subsidiary, involved as a plaintiff or defendant in any material proceeding or pending litigation.
PROPERTIES
Our main office is located on 25/F New World Center, No. 6009 Yitian Road, Futian District, Shenzhen, PRC. Our main manufacture, distribution and sales facilities are located in Tonghua city of Jilin province, Anhui province and Shandong province. We do not have any land use rights and we do not own any buildings. We rent all the land and buildings we use. We believe that our existing facilities are well maintained and in good operating condition and sufficient for our present needs.
Below is a list of land and premises we currently lease:
|
No.
|
Lessor
|
Term
|
Rent (RMB)
|
Rent (US$)
|
Description
|
|||||
|
1
|
General Tobacco Group Co., Ltd.
|
July 1, 2009 – June 30, 2010
|
1,417,295 per year
|
215,009 per year
|
Used as distribution center, totaling 15,533 square meters
|
|||||
|
2
|
General Tobacco Group Co., Ltd.
|
March 1, 2010 -February 28, 2011
|
180,000 for the first year and increase at a rate of 5% per year thereafter
|
27,307 per year and increase at a rate of 5% per year thereafter
|
Used for parking, totaling 156,020 square meters
|
|||||
|
3
|
General Trading Co., Ltd.
|
March 1, 2010-March 28, 2011
|
30,000 for the first year and increase at a rate of 5% per year thereafter
|
4,551 for the first year and increase at a rate of 5% per year thereafter
|
Used as offices, totaling 1,160.46 square meters
|
|||||
|
4
|
General Tobacco Group Co., Ltd.
|
July 1, 2010 -June 30, 2011
|
1,335,334 per year
|
202,575 per year
|
Used as distribution center, totaling 13,937 square meters
|
|||||
|
5
|
General Tobacco Group Co., Ltd.
|
July 1, 2010 -June 30, 2011
|
390,898 per year
|
59,301 per year
|
Used as warehouse, totaling 2,677 square meters
|
|||||
|
6
|
Shujun Xu
|
November 1, 2006-October 31, 2011,
|
360,000 for the first year; 420,000 for the second year; 480,000 for the third year; 540,000 for the fourth year; 600,000 for the fifth year.
|
54,613 for the first year; 63,716 for the second year; 72,818 for the third year; 81,920 for the fourth year; 91,022 for the fifth year.
|
Used as distribution center, totaling 2,449 square meters
|
|||||
|
7
|
Xiuying Hou
|
December 1, 2010-December 1, 2013
|
64,800 every year
|
9,830 every year
|
Used as distribution center, totaling 300 square meters
|
|||||
|
8
|
Anhui Province Bozhou City Fengyi Institute of Traditional Chinese Medicine
|
August 1 2008 –July 31, 2023 renewable annually
|
1,200,000 per year
|
182,045 per year
|
Used as warehouse, totaling 3,000 square meters
|
|||||
|
9
|
Mengwang Village Committee of Dayang County, Qiaocheng District, Bozhou City, Anhui Province, PRC
|
January 1, 2010-December 31, 2014 renewable annually
|
14,970,000 for 2010, 8,800,000 per year thereafter.
|
2,271,007 for 2010, 1,334,994 per year thereafter.
|
Used for herbal cultivation, totaling 1,318 acres
|
|||||
|
10
|
Mengwang Village Committee of Dayang County, Qiaocheng District, Bozhou City, Anhui Province, PRC
|
July 1, 2010 - December 31, 2015 renewable annually
|
31,450,367 for 2010, 16,939,939 for 2011, 10,468,500 per year thereafter.
|
4,771,142 for 2010, 2,569,854 for 2011, 1,588,112 per year thereafter
|
Used for herbal cultivation, totaling 1,642 acres.
|
|||||
|
11
|
25/F New World Center, 6009 Yitian Road, Futian District, Shenzhen, PRC
|
Dec 1, 2009 - Nov 30, 2012
|
606,000 per year
|
104,676 per year
|
Used as office
|
59
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL
OWNERS AND MANAGEMENT
The following table sets forth certain information with respect to the beneficial ownership of our voting securities by (i) any person or group owning more than 5% of any class of voting securities, (ii) each director, (iii) our named executive officers (pursuant to Regulation SK-402(a)(3)) and (iv) all of our named executive officers and directors as a group as of April 11, 2011.
|
Number of Shares of
|
Percentage Ownership
|
|||||||
|
Common Stock
|
of Shares of
|
|||||||
|
Name and Address
|
Beneficially Owned1
|
Common Stock
|
||||||
|
|
|
|||||||
|
Owner of More than 5% of Class
|
|
|
||||||
|
|
|
|||||||
|
Shouqiang, Han
|
1,623,930 | 6.25 | % | |||||
|
25/F New World Center
|
||||||||
|
No. 6009 Yitian Road
|
||||||||
|
Futian District, Shenzhen
|
||||||||
|
People’s Republic of China 518026
|
||||||||
|
Named Executive Officers and Directors
|
||||||||
|
Yunlu Yin2
|
13,145,000 | 50.56 | % | |||||
|
25/F New World Center
|
||||||||
|
No. 6009 Yitian Road
|
||||||||
|
Futian District, Shenzhen
|
||||||||
|
People’s Republic of China 518026
|
||||||||
|
An Fu 2,3
|
208,000 | * | ||||||
|
25/F New World Center
|
||||||||
|
No. 6009 Yitian Road
|
||||||||
|
Futian District, Shenzhen
|
||||||||
|
People’s Republic of China 518026
|
||||||||
|
Dan Li 2
|
156,000 | * | ||||||
|
25/F New World Center,
|
||||||||
|
No. 6009 Yitian Road,
|
||||||||
|
Futian District, Shenzhen,
|
||||||||
|
People’s Republic of China 518026
|
||||||||
|
Gene Michael Bennett 3
|
||||||||
|
J4-2-12 Diplomtic Compound,
|
||||||||
|
No.1 Xiushui Street, Jianguomen Wai
|
||||||||
|
ChaoYang District,Beijing
|
||||||||
|
People’s Republic of China 100600
|
||||||||
|
Zhixian Long 3
|
||||||||
|
Room 302, Unit 6, Block 9
|
||||||||
|
No.11, North Third Ring East Road,
Chao Yang District, Beijing
|
||||||||
|
People’s Republic of China 100600
|
||||||||
|
Peitong Yu3
|
||||||||
|
Room 2504,Tian An International Plaza,
Zhongshan District, Dalian City
|
||||||||
|
People’s Republic of China 116001
|
||||||||
|
All directors and exeutive officers (5 persons)
|
13,509,000 | 51.96 | % | |||||
*Less than 1% of the issued and outstanding shares as of April 11, 2011.
|
(1)
|
In determining beneficial ownership of our common stock as of a given date, the number of shares shown includes shares of common stock, if any, that may be acquired upon exercise of warrants or options or conversion of convertible securities within 60 days of that date. In determining the percent of common stock owned by a person or entity on April 11, 2011, (a) the numerator is the number of shares of the class beneficially owned by such person or entity, including shares which may be acquired within 60 days on exercise of warrants or options and conversion of convertible securities and (b) the denominator is the sum of (i) the total shares of common stock outstanding on April 11, 2011 (26,000,000) and (ii) the total number of shares, if any, that the beneficial owner may acquire upon conversion of convertible securities and the exercise of the warrants and options, subject to limitations on conversion and exercise. Unless otherwise stated, each beneficial owner has sole power to vote and dispose of its shares.
|
|
(2)
|
Yunlu Yin was appointed our new Chief Executive Officer, director and Chairman, An Fu was appointed our new Chief Financial Officer and Dan Li was appointed our new Secretary effective August 6, 2010. On November 19, 2010, pursuant to the Earn-In Agreement, as thereafter amended,Yunlu Yin, An Fu and Dan Li exercised their call rights and purchased 9,858,750, 156,000 and 117,000 shares of our common stock, respectively.
|
|
(3)
|
On February 18, 2011, our board of directors appointed An Fu, Gene Michael Bennett, Peitong Yu and Zhixian Long as members of the board of directors.
|
60
DIRECTORS AND EXECUTIVE OFFICERS, PROMOTERS
AND CONTROL PERSONS
Our Directors and Executive Officers
In connection with the change in control of the Company on August 6, 2010, Rhonda Heskett resigned as our President, Chief Executive Officer, Chief Financial Officer, sole director and Chairwoman and we appointed Yunlu Yin as our new Chief Executive Officer, sole director and chairman, An Fu as our new Chief Financial Officer and Dan Li as our new Secretary on the same date.
Yunlu Yin was appointed our new Chief Executive Officer, sole director and Chairman, An Fu was appointed our new Chief Financial Officer and Dan Li was appointed our new Secretary effective August 6, 2010. On February 18, 2011, our sole director increased the size of our board of directors from one to five and appointed An Fu, its Chief Financial Officer, Gene Michael Bennett, Peitong Yu and Zhixian Long (collectively, referred to as the New Directors) to serve as directors, along with Yunlu Yin. Along with the appointment of the New Directors, the board of directors also established the Audit Committee, the Compensation Committee and the Nominating Committee of the Company and adopted the respective committee charters and a code of ethics.
Each member of the Board of Directors serves for a term of one year, or until his or her successor has been duly elected and has been qualified. Each of our officers serves until they are replaced by the Board of Directors.
Other than An Fu and Gene Michael Bennett, all our officers and directors are residents of the PRC. As a result, it may be difficult for investors to effect service of process within the United States upon any of them or to enforce court judgments obtained against them in the United States courts.
The following table sets forth certain information concerning our directors and executive officers:
|
Name
|
Age
|
Position
|
||
|
Yunlu Yin
|
44
|
Chief Executive Officer, Director and Chairman
|
||
|
An Fu
|
36
|
Director, Chief Financial Officer
|
||
|
Dan Li
|
41
|
Vice President, Secretary
|
||
|
Gene Michael Bennett
|
62
|
Independent Director
|
||
|
Zhixian Long
|
76
|
Independent Director
|
||
|
Peitong Yu
|
35
|
Independent Director
|
The following is a summary of the biographical information of our current directors and executive officers:
Yunlu Yin, age 44, has served as Chief Executive Officer of Biopharm Asia, Inc. from May 7, 2009 to April 26, 2010. He also served as Chief Executive Officer of Huachen International Group Limited Company, general manager of Guangzhou Zhonghui Pharmaceutical Limited Company and general manager of Guangzhou Zhongshun Medicine Research Limited Company since 2006.From 2003 to 2006, Mr. Yin served as general manager of Jilin Province Changchun Hongli Pharmaceutical Limited Company, general manager of Jilin Province Changchun Zhongbo Medicine Marketing Planning Limited Company, general manager of China Academy of Traditional Chinese Medicine Research Institute Scientific and Technological Cooperation Center Tumor Expert Long-Distance Diagnosing and Treating Center, assistant director of World Chinese Medicine and Pharmaceutical Society Information Network Center and general manager of World Chinese Medicine and Pharmaceutical Society Tumor Expert Electronic Service System. From 2001 to 2003, Mr. Yin served as general manager of Jilin Province Changchun People's Pharmaceutical Limited Company and deputy general manager of Jilin Province Canye Group Guoli Pharmaceutical Limited Company.
An Fu, age 36, is a founding partner of eVisions Consulting, LLC, a U.S. based consulting firm that he founded in November 2009. Previously, he served as an auditor at the Davis Accounting Group P.C. in Cedar City, Utah from November 2007 to May 2010. Mr. Fu also worked as an engineer and a manager for China Financial Data Networks CO., Beijing, from 1998 to 2005. He received a Bachelor’s degree in Accounting from the Southern Utah University in the United States in 2007 and a Master of Accountancy from the Southern Utah University in 2008.
61
Dan Li, age 41, worked as assistant to the President in BioPharm Asia Inc. from May 2009 to November 2009.From June 2007 to May 2009, Mr. Li was the assistant director of China-America Capital Holdings. From December 2006 to June 2007, he worked as an independent financial and strategy advisor for a China beef project for US Stoney Point Agri Corp. From March 2005 to December 2006, he was a senior project manager in US Kotler Consulting Group (Shenzhen). From January 2004 to February 2005, he was the senior investment manager of Shenzhen Small & Medium Enterprise Venture Capital Company and a senior investment manager in Guangzhou Hongde Investment Company in Shenzhen. From June 2001 to January 2003, he was a senior investment manager of China S&T Cash Capital Limited in Shenzhen. From January 2001 to May 2001, Mr. Li was a project manager in Locux Company in Helsinki of Finland. From August 1994 to May 1997, he was a sales manager of Hunan Leader International Trade Company in Hunan province. Mr. Li received his Master in Business Administration in Finance from the Helsinki School of Economics and Business Administration in October 2000. He received his Bachelor degree in Facility Management from Hanzege school of the Netherlands in August 1998.
Gene Michael Bennett, age 62, currently serves as a member of the Board of Directors since February 18, 2011 and as chairman of the Audit Committee and member of the Compensation Committee and the Nominating Committee for a term of three years. Mr. Bennett is the CEO of American General Business Association, a non-governmental organization that assists Chinese companies to develop business overseas. He has recently been appointed the chairman of the Advisory Committee to Swiss Private Client Capital Partners Ltd. Since January 2010, he served as the managing partner for Beijing-based Nexis Investment Consulting Corporation, helping Chinese companies establish good corporate governance and in raising funds. From 2000 to 2004, he was a partner at ProCFO, a California-based contract-CFO consulting firm. From 1998 to 2000, Mr. Bennett was a professor and lecturer in accounting and tax for University of Hawaii, and Chaminade University of Honolulu. Prior to that, he was CFO and board member of Argonaut Computers; professor and lecturer in accounting and tax at California State University at Fullerton; and CPA with GerbelButzbaugh which became part of Grant Thornton. Mr. Bennett holds a B.A. and MBA from Michigan State University and is currently pursuing a doctorate of business administration at City University of Hong Kong in Corporate Governance. Mr. Bennett currently serves on the boards of China Agritech, Inc. (NasdaqGS: CAGC), China Pharma Holding, Inc. (AMEX:CPHI) and China Shen Zhou Mining & Resources, Inc. (AMEX: SHZ).
Zhixian Long, age 76, currently serves as a member of the Board of Directors since February 18, 2011 and as chairman of the Nominating Committee and member of the Audit Committee and the Compensation Committee for a term of two years. Mr.
Long was a professor and PhD supervisor in Chinese medicine at Beijing University of Chinese Medicine (“BUCM”) from 1997 to 2009. He served as Vice President of BUCM from 1985 to 1990 and President of BUCM from 1990 to 1997. Mr. Long was also a member of Chinese People’s Political Consultative Conference from 1998 to 2009. Since 2006, Mr. Long has served as Executive Vice President and Deputy Secretary General of Word Federation of Chinese Medicine Societies (“WFCMS”). He has also served as the Chairman of Chinese Medicine Committee of WFCMS since 2006. Mr. Long graduated from BUCM (formerly, Beijing College of Chinese Medicine) in 1964.
Peitong Yu, age 35, currently serves as a member of the Board of Directors since February 18, 2011 and as chairman of the Compensation Committee and member of the Audit Committee and the Nominating Committee for a term of two years. Mr. Yu currently serves as the Vice Chairman and Vice President of Dalian KaidaVenture Capital Co., Ltd., Vice Chairman and Vice President of KaidaJiarui (Tianjin) Equity Investment Funds Management Co., Ltd. and the director of Asia-Pacific Finance Group as well as the Vice Chairman of World Eminence Chinese Businessman Association. He served as the General Manager of Dalian Tianmu Investment Consultant Co., Ltd. from 2003 to 2005. He served as General Manager of Dalian Branch of Beijing Gold Tount Venture Capital Co., Ltd. in 2006. From 2006 to 2007, he was the deputy general manager of Dalian Huanyu Venture Capital Co., Ltd. From 2007 to 2008, he served as the CEO of Beijing Gold Tount Venture Capital Co., Ltd. Mr. Yu has significant experience in business operation and venture capital. Mr. Yu graduated from Jili University in 1997.
Except as otherwise reported above, none of our directors hold directorships in other reporting companies.
There are no family relationships among our directors or officers.
To our knowledge, during the last ten years, none of our directors and executive officers (including those of our subsidiaries) has:
|
·
|
Had a bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time.
|
|
·
|
Been convicted in a criminal proceeding or been subject to a pending criminal proceeding, excluding traffic violations and other minor offenses.
|
62
|
·
|
Been subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities.
|
|
·
|
Been found by a court of competent jurisdiction (in a civil action), the SEC, or the Commodities Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated.
|
|
·
|
Been the subject to, or a party to, any sanction or order, not subsequently reverse, suspended or vacated, of any self-regulatory organization, any registered entity, or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
|
Directors and Officers of the PRC Subsidiaries
Under each of the PRC Subsidiaries’ Articles of Association and PRC law, each company is managed by one executive director instead of a board of directors. The executive director is elected and appointed by the shareholders for a term of three years and can be re-elected for consecutive terms. The appointment and termination of the CEO (sometimes called the General Manager) is determined by the executive director. None of the officers of the PRC Subsidiaries are deemed to be executive officers of the Company.
In accordance with the PRC Subsidiaries’ Articles of Association and PRC law, each of the PRC Subsidiaries’ executive director is monitored by a supervisor, appointed by the shareholders for a term of three years.
The following table sets forth certain information as concerning executive officers of each of the PRC Subsidiaries:
Yaoyuan
|
Name
|
Age
|
Position
|
||
|
Yanliang Song
|
36
|
General Manager
|
||
|
Hong Li
|
51
|
Financial Manager
|
Yanliang Song, age 36, has significant experience in the pharmaceutical industry. He holds a Master of Business Adminsitration and Bachelor of TCM. Mr. Song has served as the section member of medical department of Binzhou Municipal Hospital of Traditional Chinese Medicine from 1995 to 1998; the Binzhou medical representative of China Academy of Chinese Medical Sciences Zhonghui Pharmaceutical Co., Ltd. from 1998 to 1999; the manager of Binzhou Office of Jilin Xiuzheng Pharmaceutical Group Co., Ltd. from 1999 to 2003; the manager of Shandong Office of Fujian Sanai Pharmaceutical Co., Ltd. from 2004 to 2006; and served as the chairman of Shandong Yaoyuan Pharmaceutical Company from 2006 to 2010.
Hong Li, age 50, has served as the chief management director of the First Motor Factory of Jinan City from November 1977 to November 1992; he has served as the manager of Jingbaweier Branch of Qilu Bank from November 1992 to January 2010; and as the Financial Director of Shandong Yaoyuan Pharmaceutical Co., Ltd. from January 2010 to present day.
Tongdetang
|
Name
|
Age
|
Position
|
||
|
Xianming Zeng
|
62
|
General Manager
|
||
|
Shuyan Liu
|
40
|
Financial Manager
|
63
Xianming Zeng, age 62, has over 40 years experience in Corporate Management. Mr.Zeng founded Tongdetang in 2003. Mr.Zeng entered the service in 1964 and served as an accountant for Tonghua Sankeyushu Branch of Agricultural Bank from 1964 to 1970. He also served as the financial section chief of Tonghua Coal and Silk Plant from 1970 to 1976; as the financial section chief of Tonghua Bureau of Township Enterprises from 1976 to 1980; as the administrator manager of Tonghua Silk Plant from 1980 to 1984; as the deputy director and director of Tonghua Economic Cooperation Committee from 1984 to 1988; as the secretary of the Party committee and factory director of Tonghua Silk Plant from 1988 to 1992; as the director of Tonghua Commodities Bureau from 1992 to 1994; and as the first deputy director of Tonghua County Government Office and the director of Tonghua County Commission for Economic Restructuring from 1994 to 2003. He retired in 2003 and served as the deputy general manager, general manager and chairman of Tongdetang from 2003 to present day.
Shuyan Liu, age 40, has almost 20 years’ accounting and auditing experience. He is a Certified Public Accountant and has Bachelor of Accountancy, and is a member of ACCA. Mr. Liu served as an accountant for the Tonghua textiles purchase and supply station from 1992 to 1994. He served as the chief accountant of Fuzhou Xindai Cultural Products Co., Ltd. from 1994 to 1996; as the project manager of Zhongqin Wanxin CPA (Beijing) from 1996 to 2004; as the manager of Integrated Business Department of Tonghua Tianwei CPA from 2004 to 2005; as the financial controller of Tonghua Limin Pharmaceutical Co., Ltd. from 2005 to 2008; and the financial controller of Tongdetang from 2008 to present day.
Xuelingxian
|
Name
|
Age
|
Position
|
||
|
Shunli Wang
|
59
|
General Manager
|
||
|
Fulan Li
|
56
|
Financial Manager
|
Shunli Wang, age 59, has over 30 years experience in the pharmaceutical industry. Mr. Wang founded Xuelingxian in 2008. Prior to founding the company, he was engaged in trading of traditional Chinese medicine from 1983 to 1988. He worked at the Bozhou Medicine Station from September 1988 to 2000 and obtained his chief pharmacist qualification in TCM in 1994. He obtained his pharmacist qualification in western medicine in 1997. He served as the General Manager of the sixth branch of Bozhou Pharmaceutical Group from 2000 to 2001; as the contractor of Changchun Renmin Pharmaceutical Co., Ltd. from 2001 to 2004; and as the General Manager of Changchun Pharmaceutical Co., Ltd, from 2005 to 2006. Mr.Wang has served as the Chairman of Xuelingxian from 2008 to present day.
Fulan Li, age 56, has over 28 years experience in the pharmaceutical industry. She was engaged in the operation of Chinese medicinal materials from 1980 to 1988. She worked as the financial manager in the 6th branch of Bozhou Pharmaceutical Group from June 1988 to 2001; as the financial department manager of Changchun Renmin Pharmaceutical Factory from 2001 to 2004; and the financial manager of Xuelingxian from 2008 to present day.
Audit Committee Financial Expert
Prior to the establishment of the audit committee on February 18, 2011, there was no audit committee or “audit committee financial expert” as defined by Item 401(h) in Regulation S-K as promulgated by the Securities and Exchange Commission. Upon the establishment our audit committee, the board of directors determined that our audit committee financial expert is Gene Michael Bennett.
Audit Committee
We did not have an audit committee in 2010. We believe that our board of directors is capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting. On February 18, 2011, our board of directors established the Audit Committee and adopted the Audit Committee Charter, which is available on our corporate website at http://www.globalpharmholdings.com/investor1.asp.
Gene Michael Bennett serves as chairman of the Audit Committee and Peitong Yu and Zhixian Long serve as members of the Audit Committee. Our board of directors has determined that all the Audit Committee members are independent, as that term is defined under the National Association of Securities Dealers.
64
Compensation Committee
Prior to February 18, 2011, we did not have a compensation committee, and the committee duties were performed by the board of directors.
On February 18, 2011, the board of directors established the Compensation Committee and adopted the Compensation Committee Charter, which is available on our corporate website at http://www.globalpharmholdings.com/investor1.asp. Peitong Yu serves as chairman of the Compensation Committee and Gene Michael Bennett and Zhixian Long serve as members.
Nominating Committee
Prior to February 18, 2011, we did not have a nominating committee, and the committee duties were performed by the board of directors.
On February 18, 2011, the board of directors established the Nominating Committee and adopted the Nominating Committee Charter, which is available on our corporate website at http://www.globalpharmholdings.com/investor1.asp. Zhixian Long serves as chairman of the Nominating Committee and Gene Michael Bennett and Peitong Yu serve as members.
The Nominating Committee currently does not have an express policy with regard to the consideration of any director candidates recommended by shareholders since the committee believes that it can adequately evaluate any such nominees on a case-by-case basis. The committee will evaluate shareholder-recommended candidates under the same criteria as internally generated candidates. Although the committee does not currently have any formal minimum criteria for nominees, substantial relevant business and industry experience would generally be considered important, as would the ability to attend and prepare for board, committee and shareholder meetings.
Code of Ethics
In 2010, we did not have a code of ethics. On February 18, 2011, the board of directors adopted a Code of Ethics that applies to all of our executive officers, including our principal executive, financial and accounting officers, our directors, our financial managers and all employees. The Code of Ethics is filed as Exhibit 11.1 to our Annual Report on Form 10-K for the year ended December 31, 2010, as filed with the SEC.
Board Leadership Structure and Role in Risk Oversight
In 2010, Yunlu Yin was the sole director, chairman and chief executive officer. There were no independent directors. Mr. Yin is most capable of identifying strategic priorities executing business strategy and overseeing daily operations.
On February 18, 2011, the board of directors increased the size of the Company’s board of directors from one to five and appointed An Fu, its Chief Financial Officer, Gene Michael Bennett, Peitong Yu and Zhixian Long (collectively, “New Directors”) to serve as directors of the Company, along with Yunlu Yin. We believe that this leadership structure has served the Company well.
Our board of directors has overall responsibility for risk oversight, and the directors will be well assisted by the aforementioned newly established committees since February 18, 2011 in performing the risk oversight duties.
The board’s role in the risk oversight of the Company includes, among other things:
|
·
|
appointing, retaining and overseeing the work of the independent auditors, including resolving disagreements between the management and the independent auditors relating to financial reporting;
|
|
·
|
approving all auditing and non-auditing services permitted to be performed by the independent auditors;
|
|
·
|
reviewing annually the independence and quality control procedures of the independent auditors;
|
|
·
|
reviewing and approving all proposed related party transactions;
|
|
·
|
discussing the annual audited financial statements with the management;
|
|
·
|
meeting separately with the independent auditors to discuss critical accounting policies, management letters, recommendations on internal controls, the auditor’s engagement letter and independence letter and other material written communications between the independent auditors and the management.
|
65
Director Qualifications
Directors are responsible for overseeing the Company’s business consistent with their fiduciary duty to stockholders. This significant responsibility requires highly skilled individuals with various qualities, attributes and professional experience. The board believes that there are general requirements for service on the Company’s board of directors that are applicable to all directors and that there are other skills and experience that should be represented on the board as a whole but not necessarily by each director. The board considers the qualifications of director and director candidates individually and in the broader context of the board’s overall composition and the Company’s current and future needs.
Qualifications for All Directors
In its assessment of each potential candidate, including those recommended by stockholders, the board considers the nominee’s judgment, integrity, experience, independence, understanding of the Company’s business or other related industries and such other factors the board determines are pertinent in light of the current needs of the board. The board also takes into account the ability of a director to devote the time and effort necessary to fulfill his or her responsibilities to the Company.
The board requires that each director be a recognized person of high integrity with a proven record of success in his or her field. Each director must demonstrate innovative thinking, familiarity with and respect for corporate governance requirements and practices, an appreciation of multiple cultures and a commitment to sustainability and to dealing responsibly with social issues. In addition to the qualifications required of all directors, the board conducts interviews of potential director candidates to assess intangible qualities including the individual’s ability to ask difficult questions and, simultaneously, to work collegially. The board does not have a specific diversity policy, but considers diversity of race, ethnicity, gender, age, cultural background and professional experiences in evaluating candidates for board membership. Diversity is important because a variety of points of view contribute to a more effective decision-making process.
Qualifications, Attributes, Skills and Experience to be Represented on the Board as a Whole
The board has identified particular qualifications, attributes, skills and experience that are important to be represented on the board as a whole, in light of the Company’s current needs and its business priorities. The board believes that it should include some directors with a high level of financial literacy and some directors who possess relevant business experience as a Chief Executive Officer or a President or like position. Marketing is the core focus of our business and the Company seeks to develop and deploy the world’s most innovative and effective marketing and technology. Therefore, the board believes that marketing and technology experience should be represented on the board. The Company is involved in the pharmaceutical business in the PRC. Therefore the Company’s business also requires compliance with a variety of regulatory requirements and relationships with various governmental entities. Therefore, the board believes that governmental, political or diplomatic expertise should be represented on the Board.
Set forth below are a chart and a narrative disclosure that summarize the specific qualifications, attributes, skills and experiences described above. An “X” in the chart below indicates that the item is a specific reason that the director has been nominated to serve on the Company’s Board. The lack of an “X” for a particular qualification does not mean that the director does not possess that qualification or skill. Rather, an “X” indicates a specific area of focus or expertise of a director on which the board currently relies.
|
High level of
financial literacy
|
Diversity of race,
ethnicity, gender,
age, cultural
background or
professional
experience
|
Extensive
knowledge of the
Company’s
Business
|
Marketing/Marketing
related technology
experience
|
Marketing/Marketing
related technology
experience
|
Governmental,
political or
diplomatic
expertise
|
|||||||
|
Yunlu Yin
|
|
X |
X
|
X
|
X
|
X
|
||||||
|
An Fu
|
X
|
X |
X
|
X
|
X
|
|||||||
|
Gene Michael Bennett
|
X
|
X |
|
X |
X
|
|||||||
|
Peitong Yu
|
X |
X
|
X
|
X
|
X
|
|||||||
|
Zhixian Long
|
|
|
X |
|
|
|
X |
|
X
|
|
X
|
66
EXECUTIVE COMPENSATION
The following is a summary of the compensation we paid to our former President, Chief Executive Officer, Chief Financial Officer, sole director and chairwoman, Rhonda Heskett, for the two years ended February 28, 2010 and 2009. No executive officer received compensation in excess of $100,000 for any of those years.
|
Name and
Principal
Position
|
Fiscal
Year |
Salary
($) |
Bonus
($) |
Stock
Awards ($) |
Option
Awards ($) |
Non-equity
Incentive Plan Compensation ($) |
Change in
Pension Value and Nonqualified Deferred Compensation Earnings ($) |
All Other
Compensation ($) |
Total
($) |
|||||||||||||||||||||||||
|
Rhonda Heskett,
|
2009
|
— | — | — | — | — | — | — | — | |||||||||||||||||||||||||
|
Former President, Chief Executive Officer, Chief Financial Officer and director
|
2010
|
— | — | — | — | — | — | — | — | |||||||||||||||||||||||||
Our current Chief Executive Officer, director and chairman, Yunlu Yin, and our current Chief Financial Officer, An Fu, only assumed their respective positions in the Company on August 6, 2010.On August 6, 2010, we entered into an employment agreement with each of Yunlu Yin, An Fu and Dan Li. Under their respective agreements, Yunlu Yin is employed as our new Chief Executive Officer for a term of three years and a monthly salary of $6,000, An Fu is employed as our Chief Financial Officer for a term of three years and a monthly salary of $5,000 and Dan Li is employed as our secretary and vice-president at a monthly salary of $2,500 for a term of three years. Pursuant to these agreements, we may terminate each officer’s employment for cause at any time and with thirty days’ notice without cause. Conversely, each officer may terminate his employment with us with no less than 90 days’ written notice. The officers serve as the pleasure of the board.
The following is a summary of the compensation paid by Yaoyuan to Yanliang Song, its General Manager, and Hong Li, its Finance Manager for the two years ended December 31, 2009 and 2008, respectively. No other executive officer of Yaoyuan received compensation in excess of $100,000 for any of those years. Executive officers of Yaoyuan are not deemed to be executive officers of the Company.
|
Name and
Principal
Position
|
Fiscal
Year |
Salary*
($) |
Bonus
($) |
Stock
Awards ($) |
Option
Awards ($) |
Non-equity
Incentive Plan Compensation ($) |
Change in
Pension Value and Nonqualified Deferred Compensation Earnings ($) |
All Other
Compensation ($) |
Total
($) |
|||||||||||||||||||||||||
|
Yanliang Song,
|
2008
|
21,834 | — | — | — | — | — | — | 21,834 | |||||||||||||||||||||||||
|
General Manager
|
2009
|
29,283 | — | — | — | — | — | — | 29,283 | |||||||||||||||||||||||||
|
Hong Li,
|
2008
|
— | — | — | — | — | — | — | — | |||||||||||||||||||||||||
|
Finance Manager
|
2009
|
15,373 | — | — | — | — | — | — | 15,373 | |||||||||||||||||||||||||
*The work injury insurance, endowment insurance and medical insurance we purchased for our management are part of their compensation and included in their salary.
The following is a summary of the compensation paid by Tongdetang to Xianming Zeng, its General Manager, and Shuyan Liu, its Finance Manager for the two years ended December 31, 2009 and 2008, respectively. No other executive officer of Tongdetang received compensation in excess of $100,000 for any of those years. Executive Officers of Tongdetang are not deemed to be executive officers of the Company.
67
|
Name and
Principal
Position
|
Fiscal
Year |
Salary*
($) |
Bonus
($) |
Stock
Awards ($) |
Option
Awards ($) |
Non-equity
Incentive Plan Compensation ($) |
Change in
Pension Value and Nonqualified Deferred Compensation Earnings ($) |
All Other
Compensation ($) |
Total($)
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
Xianming Zeng,
|
2008
|
7,278.02 | — | — | — | — | — | — | 7,278.02 | |||||||||||||||||||||||||
|
General Manager
|
2009
|
9,516.84 | — | — | — | — | — | — | 9,516.84 | |||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Shuyan Liu,
|
2008
|
6,113.54 | — | — | — | — | — | — | 6,113.54 | |||||||||||||||||||||||||
|
Finance Manager
|
2009
|
6,588.58 | — | — | — | — | — | — | 6,588.58 | |||||||||||||||||||||||||
*The work injury insurance, endowment insurance and medical insurance we purchased for our management are part of their compensation and included in their salary.
The following is a summary of the compensation paid by Xuelingxian to Shunli Wang, its General Manager, and Fulan Li, its Finance Manager for the two years ended December 31, 2009 and 2008, respectively. No other executive officer of Xuelingxian received compensation in excess of $100,000 for any of those years. Executive officers of Xuelingxian are not deemed to be executive officers of the Company.
|
Name and
Principal
Position
|
Fiscal
Year |
Salary*
($) |
Bonus
($) |
Stock
Awards ($) |
Option
Awards ($) |
Non-equity
Incentive Plan Compensation ($) |
Change in
Pension Value and Nonqualified Deferred Compensation Earnings ($)
|
All Other
Compensation ($) |
Total
($) |
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
Shunli Wang,
|
2008
|
7,994.19 | — | — | — | — | — | — | 7,994.19 | |||||||||||||||||||||||||
|
General Manager
|
2009
|
11,713.03 | — | — | — | — | — | — | 11,713.03 | |||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||
|
Fulan Li,
|
2008
|
4,949.05 | — | — | — | — | — | — | 4,949.05 | |||||||||||||||||||||||||
|
Finance Manager
|
2009
|
6,149.34 | — | — | — | — | — | — | 6,149.34 | |||||||||||||||||||||||||
*The work injury insurance, endowment insurance and medical insurance we purchased for our management are part of their compensation and included in their salary.
Compensation Discussion and Analysis
We strive to provide our named executive officers (as defined in Item 402 of Regulation S-K) with a competitive base salary that is in line with their roles and responsibilities when compared to peer companies of comparable size in similar locations.
It is not uncommon for PRC private companies in China to have base salaries as the sole form of compensation. The base salary level is established and reviewed based on the level of responsibilities, the experience and tenure of the individual and the current and potential contributions of the individual. The base salary is compared to the list of similar positions within comparable peer companies and consideration is given to the executive’s relative experience in his or her position. Base salaries are reviewed periodically and at the time of promotion or other changes in responsibilities.
We plan to implement a more comprehensive compensation program, which takes into account other elements of compensation, including, without limitation, short and long term compensation, cash and non-cash, and other equity-based compensation such as stock options. We expect that this compensation program will be comparable to the programs of our peer companies and aimed to retain and attract talented individuals.
We will also consider forming a compensation committee to oversee the compensation of our named executive officers. The majority of the members of the compensation committee would be independent directors.
68
Compensation of Directors
Directors are permitted to receive fixed fees and other compensation for their services as directors. The board of directors has the authority to fix the compensation of directors. No amounts have been paid to, or accrued to, directors in such capacity as of December 31, 2010.
Effective February 18, 2011, Gene Michael Bennett was appointed a director of the Company. Mr Bennett’s compensation is set forth in an appointment letter pursuant to which he will be paid an annual fee of $20,000, payable on a quarterly basis. He will also be granted options to purchase an aggregate of 37,000 shares of common stock of the Company. The option to purchase 4,000 shares shall vest on the date of grant and the rest shall vest, in equal quarterly installments of 3,000 shares each, on the first day of each quarter commencing from July 1, 2011 to January 1, 2014. The exercise price for all options is the fair market value of the stock on the date of grant. None of the above-referenced options have been granted yet to Mr. Bennett and are pending the adoption by the Company of a stock option plan, which the Company anticipates adopting in the near future.
Effective February 18, 2011, Peitong Yu will was appointed a director of the Company. Mr. Yu’s compensation is set forth in an appointment letter pursuant to which he will be paid an annual fee of 120,000 Hong Kong dollars (“HKD”), payable on a quarterly basis. He will also be granted options to purchase an aggregate of 18,000 shares of common stock of the Company. The option to purchase 4,000 shares shall vest on the date of grant and the rest shall vest, in equal quarterly installments of 2,000 shares each, on the first day of each quarter commencing from July 1, 2011 to January 1, 2013. The exercise price for all options is the fair market value of the stock on the date of grant. None of the above-referenced options have been granted yet to Mr. Yu and are pending the adoption by the Company of a stock option plan, which the Company anticipates adopting in the near future.
Effective February 18, 2011, Zhixian Long was appointed a director of the Company. Mr. Long’s compensation is set forth in an appointment letter pursuant to which he will be paid an annual fee of 120,000 HKD, payable on a quarterly basis. He will also be granted options to purchase an aggregate of 18,000 shares of common stock of the Company. The option to purchase 4,000 shares shall vest on the date of grant and the rest shall vest, in equal quarterly installments of 2,000 shares each, on the first day of each quarter commencing from July 1, 2011 to January 1, 2013. The exercise price for all options is the fair market value of the stock on the date of grant. None of the above-referenced options have been granted yet to Mr. Long and are pending the adoption by the Company of a stock option plan, which the Company anticipates adopting in the near future.
Mr. Yunlu Yin and Mr. An Fu, the Company’s Chief Executive Officer and Chief Financial Officer, will not be compensated for their services as a director of the Company.
Option Grants Table
There were no individual grants or stock options to purchase our common stock made to the executive officer named in the Executive Compensation Table through December 31, 2010.
Aggregated Option Exercises and Fiscal Year-End Option Value Table
There were no stock options exercised during the fiscal year ended December 31, 2010, by the executive officer named in the Executive Compensation Table.
Long-Term Incentive Plan (“LTIP”) Awards Table
There were no awards made to a named executive officer in the last completed fiscal year under any LTIP.
Employment Agreements
On August 6, 2010, we entered into an employment agreement with each of Yunlu Yin, An Fu and Dan Li. Under their respective agreements, Yunlu Yin is employed as our new Chief Executive Officer for a term of three years and a monthly salary of $6,000; An Fu is employed as our Chief Financial Officer for a term of three years and a monthly salary of $5,000; and Dan Li is employed as our secretary at a monthly salary of $2,500 for a term of three years. Pursuant to these agreements, we may terminate each officer’s employment for cause at any time and with thirty days’ notice without cause. Conversely, each officer may terminate his employment with us with no less than 90 days’ written notice. The officers serve as the pleasure of the board.
69
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Except for the ownership of our securities, and except as set forth below, none of the directors, executive officers, holders of more than five percent of the Company’s outstanding common stock, or any member of the immediate family of any such person have, to the knowledge of the Company, had a material interest, direct or indirect, in any transaction or proposed transaction which may materially affect the Company.
|
|
·
|
On May 25, 2006 and April 15,2010, Yunlu Yin signed the Trust Agreements with Qinghui Zeng and Qingdong Zeng, respectively, documenting Yunlu Yin as the 62% of owner of Tongdetang and documenting each of Qinghui Zeng and Qingdong Zeng to act as the nominee owner of Tongdetang on behalf of Yunlu Yin. On May 31, 2008 and March 20, 2010 Yunlu Yin signed the Trust Agreements with Yanliang Song and Shouqiang Han, respectively, documenting Yunlu Yin as 80% of owner of Yaoyuan and documenting each of Yanliang Song and Shouqing Han to act as the nominee owner of Yaoyuan on behalf of Yunlu Yin. On September 5, 2009 and April 10, 2010, Yunlu Yin signed the Trust Agreements with Shunli Wang and Shulan Li and Hong Zhang, respectively, documenting Yunlu Yin as 65% of owner of Xuelingxian and documenting each of Shunli Wang and Shulan Li and Hong Zhang to act as the nominee owner of Xuelingxian on behalf of Yunlu Yin.
|
|
|
·
|
During each of the years ended February 28, 2010 and February 28, 2009, our former President, Chief Executive Officer, Chief Financial Officer, sole director and chairwoman, Rhonda Heskett contributed services and brooding facilities with a fair value of $6,000 and $2,400, respectively. These non-cash expenses totaling $8,400 in each of the years ended February 28, 2010 and 2009 were treated as contributed capital.
|
|
|
·
|
On July 23 and September 24, 2010, our former shareholder, Shunli Wang, lentRMB 5,000,000 and RMB 600,000 (totally, $836,058) respectively, to Xuelingxian as working capital and the balance are non-interest bearing and payable on demand. The balance was paid off on December 25, 2010.
|
|
|
·
|
As of August 6, 2010, our former President, Chief Executive Officer, Chief Financial Officer, sole director and chairwoman, Rhonda Heskett loaned us a total of $24,879.53 in exchange for two 5% per annum promissory notes. Rhonda Heskett waived this payment on August 6, 2010 in full and the promissory notes were cancelled on that date.
|
|
|
·
|
As of December 31, 2009 and 2008, we had $24,568 and $1,465,738, respectively due from related parties. In 2009, we advanced $24,568 to Yanliang Song, an officer of Yaoyuan, and the balance was paid off in 2010. In 2008, Xianming Zeng and Qingdong Zeng, former stockholders of Tongdetang, borrowed RMB 6,000,000 and RMB 4,000,000 (totally, $1,465,738), respectively, from Tongdetang and such balance was offset in 2009 when Tongdetang declared dividends in the amount of RMB 6,000,000 and RMB 4,000,000, respectively, to Xianming Zeng and Qingdong Zeng.
|
|
|
·
|
We use a trademark for drug packaging that is owned by a director and former shareholder of Yaoyuan, Yanliang Song. The trademark was applied for on May 16, 2008 and approved April 7, 2010. It expires April 6, 2020. While Yanliang Song has paid for all associated costs, we use this trademark at no cost.
|
|
|
·
|
During 2009, Xuelingxian had sales of approximately $1.4 million to a number of related parties, and employees. The details of the transactions are set forth below:
|
|
Name
|
Relationship
|
Amount
|
||||
|
Fei Gao
|
General Manager
|
$ | 472,625 | |||
|
Wenhua Chou
|
General Manager
|
$ | 218,363 | |||
|
Jingzhong Wang
|
General Manager
|
$ | 445,381 | |||
|
Enhui Fan
|
General Manager
|
$ | 89,474 | |||
|
Fengyi Wang
|
General Manager
|
$ | 120,054 | |||
|
Min Xue
|
General Manager
|
$ | 74,458 | |||
|
Total
|
$ | 1,420,354 | ||||
70
|
|
·
|
For the years ended December 31, 2009 and 2008, and for the period ended June 30, 2010, we paid $8,190,085, $3,425,268, and $3,208,521 to the former shareholders of our subsidiaries, respectively. On June 29, 2010, as part of May 6 and May 8, 2010 Equity Transfer Agreements, Wisdom Fortune and Binomial declared a dividend of approximately RMB 57,522,347 (approximately $8,438, 816) to its then stockholders. The following table set forth the dividend payable as of June 30, 2010 and December 31, 2009 and 2008.
|
|
Related Party
|
Relationship
|
June 30, 2010
|
December 31, 2009
|
December 31, 2008
|
||||||||||
|
Shouqiang Han
|
Stockholder
|
$ | 2,090,395 | $ | 370,006 | $ | 158,624 | |||||||
|
Qinghui Zeng
|
Stockholder
|
1,244,115 | 1,427,408 | 2,525,968 | ||||||||||
|
Xianming Zeng
|
Stockholder
|
622,058 | 713,704 | 1,262,983 | ||||||||||
|
Shunli Wang
|
Stockholder
|
3,634,757 | 93,948 | - | ||||||||||
|
Jingsheng Wang
|
Stockholder
|
1,009,364 | 26,089 | - | ||||||||||
|
Fulan Li
|
Stockholder
|
585,745 | 15,140 | - | ||||||||||
|
Others
|
Stockholder
|
110,022 | 19,474 | 8,349 | ||||||||||
|
Total
|
Stockholder
|
$ | 9,296,455 | $ | 2,665,768 | $ | 3,955,924 | |||||||
|
·
|
On June 29, 2010 the then sole shareholder of Global Pharma BVI entered into an Earn-In Agreement, as thereafter amended, with the former shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan. Pursuant to the agreement, the Company would enter into a share exchange agreement, at a date after the Earn-In Agreement, with a United States domiciled shell company and at that time the former shareholders, including beneficial owners, would have call rights to own the controlling interest in the U.S. publicly held company.
|
|
·
|
On August 6, 2010, we were party to a share purchase agreement between our former President, Chief Executive Officer, Chief Financial Officer and sole director and Chairwoman, Rhonda Heskett and Mei Li Tsai involving the sale of 19,094,000 shares of common stock of the Company for a cash consideration of $450,000.
|
|
·
|
On August 12, 2010, we entered into and consummated a Share Exchange Agreement with Mei Li Tsai, the sole shareholder of Global Pharma BVI and Global Pharma BVI to acquire all the issued and outstanding capital stock of Global Pharma BVI, in consideration for our 1,800,000 newly issued restricted shares.
|
|
·
|
Pursuant to the Earn-In Agreement dated June 29, 2010, as thereafter amended, after Global Pharma BVI entered into and consummated a Share Exchange Agreement with a U.S. public company, the shareholders, including the beneficial owners of Xuelingxian, Tongdetang, and Yaoyuan, and the key management of the Company were given the right to purchase 20,894,000 shares at four different occurrence dates, contingent on various targets for total consideration of $300,000. Targets include binding three-year employment contracts with various members of management within six months of these agreements and target after tax net income of $3.6 million, $3.8 million, and $15.2 million for the three months ended June 30, 2010 and September 30, 2010, and for the 12 months ended December 31, 2010, respectively. Upon execution the former shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan would have call rights to own 80.36% of the publicly held company.
|
Except as disclosed above, no executive officer, director or any member of these individuals’ immediate families, any corporation or organization with whom any of these individuals is an affiliate or any trust or estate in which any of these individuals serve as a trustee or in a similar capacity or has a substantial beneficial interest in is or has been indebted to the Company at any time since the beginning of the Company’s last fiscal year.
Procedures for Approval of Related Party Transactions
Our board of directors is charged with reviewing and approving all potential related party transactions. All such related party transactions must then be reported under applicable SEC rules. We have not adopted other procedures for review, or standards for approval, of such transactions, but instead review them on a case-by-case basis.
71
LEGAL PROCEEDINGS
We know of no material, active, pending or threatened proceeding against us or our subsidiaries, nor are we, or any subsidiary, involved as a plaintiff or defendant in any material proceeding or pending litigation.
MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANTS
COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Our common stock has been traded over-the-counter on the OTC Bulletin Board since May 5, 2009 under the designation TOPG.OB. However, to date there has been no trading for our common stock.
The market price of our common stock is subject to significant fluctuations in response to variations in our quarterly operating results, general trends in the market, and other factors, over many of which we have little or no control. In addition, broad market fluctuations, as well as general economic, business and political conditions, may adversely affect the market for our common stock, regardless of our actual or projected performance.
Holders of Our Common Stock
As of August 12, 2010, we had 28 shareholders of our common stock, including the shares held in street name by brokerage firms. The holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. Holders of the common stock have no preemptive rights and no right to convert their common stock into any other securities. There are no redemption or sinking fund provisions applicable to the common stock
Dividends
Since we have been a public company, we have not paid cash dividends on our common stock. Holders of our common stock are entitled to receive dividends, if any, declared and paid from time to time by the Board of Directors out of funds legally available. We intend to retain any earnings for the operation and expansion of our business and do not anticipate paying cash dividends in the foreseeable future. Any future determination as to the payment of cash dividends will depend upon future earnings, results of operations, capital requirements, our financial condition and other factors that our Board of Directors may consider.
On June 29, 2010, as part of May 6 and May 8, 2010 Equity Transfer Agreements, Wisdom Fortune and Binomial declared a dividend of approximately RMB 57,522,347 (approximately $8,438, 816) to its then stockholders. Prior to the Reverse Merger, our subsidiaries PRC subsidiaries paid $8,190,085, $3,425,268, and $3,208,521 to the former shareholders for the years ended December 31, 2009 and 2008, and for the period ended June 30, 2010, respectively.
The following table set forth the due to related party payable as of June 30, 2010 and December 31, 2009 and 2008.
|
Related Party
|
Relationship
|
June 30, 2010
|
December 31, 2009
|
December 31, 2008
|
||||||||||||
|
Shouqiang Han
|
Stockholder
|
Dividend
|
$ | 2,090,395 | $ | 370,006 | $ | 158,624 | ||||||||
|
Qinghui Zeng
|
Stockholder
|
Dividend
|
1,244,115 | 1,427,408 | 2,525,968 | |||||||||||
|
Xianming Zeng
|
Stockholder
|
Dividend
|
622,058 | 713,704 | 1,262,983 | |||||||||||
|
Shunli Wang
|
Stockholder
|
Dividend
|
3,634,757 | 93,948 | - | |||||||||||
|
Jingsheng Wang
|
Stockholder
|
Dividend
|
1,009,364 | 26,089 | - | |||||||||||
|
Fulan Li
|
Stockholder
|
Dividend
|
585,745 | 15,140 | - | |||||||||||
|
Others
|
Stockholder
|
Dividend
|
110,022 | 19,474 | 8,349 | |||||||||||
|
Total
|
$ | 9,296,455 | $ | 2,665,768 | $ | 3,955,924 | ||||||||||
72
Stock Option Grants
To date, we have not granted any stock options.
Registration Rights
We have not granted registration rights to any person.
Securities authorized for issuance under equity compensation plans
As of the date of this Current Report, we do not have any securities authorized for issuance under any equity compensation plans and we do not have any equity compensation plans.
Penny Stock Regulations
Our shares of common stock are subject to the "penny stock" rules of the Securities Exchange Act of 1934 and various rules under this Act. In general terms, "penny stock" is defined as any equity security that has a market price less than $5.00 per share, subject to certain exceptions. The rules provide that any equity security is considered to be a penny stock unless that security is registered and traded on a national securities exchange meeting specified criteria set by the SEC, issued by a registered investment company, and excluded from the definition on the basis of price (at least $5.00 per share), or based on the issuer's net tangible assets or revenues. In the last case, the issuer's net tangible assets must exceed $3,000,000 if in continuous operation for at least three years or $5,000,000 if in operation for less than three years, or the issuer's average revenues for each of the past three years must exceed $6,000,000.
Trading in shares of penny stock is subject to additional sales practice requirements for broker-dealers who sell penny stocks to persons other than established customers and accredited investors. Accredited investors, in general, include individuals with assets in excess of $1,000,000 or annual income exceeding $200,000 (or $300,000 together with their spouse), and certain institutional investors. For transactions covered by these rules, broker-dealers must make a special suitability determination for the purchase of the security and must have received the purchaser's written consent to the transaction prior to the purchase. Additionally, for any transaction involving a penny stock, the rules require the delivery, prior to the first transaction, of a risk disclosure document relating to the penny stock. A broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, and current quotations for the security. Finally, monthly statements must be sent disclosing recent price information for the penny stocks. These rules may restrict the ability of broker-dealers to trade or maintain a market in our common stock, to the extent it is penny stock, and may affect the ability of shareholders to sell their shares.
RECENT SALES OF UNREGISTERED SECURITIES
On February 11, 2007, we issued 20,000,000 shares of common stock to our former President, Chief Executive Officer, Chief Financial Officer and sole director, Rhonda Heskett.
73
In February 2008, we issued 4,200,000 shares of common stock to 42 shareholders under an exemption from the registration requirements of the Securities Act of 1933(the “Act”) for the placement of these securities pursuant to Section 4(2) of the Act and/or Regulation D promulgated thereunder.
On August 12, 2010, we entered into and consummated a Share Exchange Agreement with the sole shareholder of Global Pharma Enterprise Group Limited (“Global Pharma BVI”) and Global Pharma BVI to acquire all the issued and outstanding capital stock of Global Pharma BVI, a British Virgin Islands company, in consideration for 1,800,000 newly issued restricted shares of the Company. We claim an exemption from the registration requirements of the Act for the private placement of these securities pursuant to Section 4(2) of the Act and/or Regulation D promulgated thereunder since, among other things, the transaction did not involve a public offering, the recipient is an accredited investor and had access to information about the Company and their investment, the recipient took the securities for investment and not resale, and the Company took appropriate measures to restrict the transfer of the securities
The following is a summary description of all material provisions of our certificate of incorporation and by-laws pertaining to our capital stock and certain provisions of our certificate of incorporation and by-laws, copies of which have been filed as exhibits to this report. The following discussion is qualified in its entirety by reference to such exhibits.
General
We are authorized to issue 100,000,000 shares of common stock, par value $.001 per share, and 10,000,000 shares of blank-check preferred stock, par value $.001 per share.
Common Stock
The holders of our common stock are entitled to one vote for each share held of record on all matters to be voted on by stockholders. There is no cumulative voting with respect to the election of directors, with the result that the holders of more than 50% of the shares voting for the election of directors can elect all of the directors then up for election. The holders of our common stock are entitled to receive dividends when, as and if declared by the board of directors out of funds legally available therefor. In the event of liquidation, dissolution or winding up of the Company, the holders of common stock are entitled to share ratably in all assets remaining which are available for distribution to them after payment of liabilities and after provision has been made for each class of stock, if any, having preference over the common stock. Holders of shares of our common stock, as such, have no conversion, preemptive or other subscription rights, and there are no redemption provisions applicable to the common stock.
Preferred Stock
In addition to the 100,000,000 shares of common stock, we are authorized to issue up to 10,000,000 shares of preferred stock. Shares of our preferred stock may be issued from time to time in one or more classes or series, each of which class or series shall have such distinctive designation or title as shall be fixed by the board of directors prior to the issuance any shares thereof.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Limitations on Liability
Under Delaware law, a corporation may indemnify its officers, directors, employees and agents under certain circumstances, including indemnification of such persons against liability under the Securities Act of 1933, as amended. Those circumstances include that an officer, director, employee or agent may be indemnified if the person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
74
We are also permitted to apply for insurance on behalf of any director, officer, employee or other agent for liability arising out of his actions, whether or not the Delaware General Corporation Law would permit indemnification.
Indemnification Against Public Policy
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling an issuer pursuant to the foregoing provisions, the opinion of the Commission is that such indemnification is against public policy as expressed in the Securities Act of 1933 and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The effect of indemnification may be to limit the rights of the Company and its stockholders (through stockholders’ derivative suits on behalf of the Company) to recover monetary damages and expenses against a director for breach of fiduciary duty.
Item 3.02 Unregistered Sales of Equity Securities.
On August 12, 2010, we entered into and consummated a Share Exchange Agreement with the then sole shareholder of Global Pharma Enterprise Group Limited (“Global Pharma BVI”) and Global Pharma BVI to acquire all the issued and outstanding capital stock of Global Pharma BVI, a British Virgin Islands company, in consideration for 1,800,000 newly issued restricted shares of Top Flight (the “Reverse Merger”). On September 20, 2010, Top Flight changed its name to Global Pharm Holdings Group, Inc.
Immediately after the closing of the Reverse Merger, we have a total of 26,000,000 issued and outstanding shares of common stock. As a result of the Reverse Merger, Global Pharma BVI is now our wholly owned subsidiary. Global Pharma BVI is the holding company of all the shares of Binomial Biopharm Group Limited (“Binomial”) and Hong Kong Wisdom Fortune Medicine Holding Group Limited (“Wisdom Fortune”), two wholly-owned Hong Kong-incorporated companies.
Binomial, in turn, holds all the equity interests in Anhui Xuelingxian Pharmaceutical Co., Ltd.(“Xuelingxian”), Tonghua Tongdetang Pharmaceutical and Medicinal Materials Co., Ltd.(“Tongdetang”), two PRC-incorporated companies.
Wisdom Fortune holds all the equity interest in Shandong Global Pharm Co., Ltd. (“Yaoyuan”).
We are, through our indirect wholly owned PRC subsidiaries, namely Xuelingxian, Tongdetang and Yaoyuan (the “PRC Subsidiaries”) in the business of wholesale distribution of pharmaceuticals-related products, Chinese herb cultivation and medicine raw materials preparations.
We claim an exemption from the registration requirements of the Securities Act of 1933 (the “Act”) for the private placement of these securities pursuant to Section 4(2) of the Act and/or Regulation D promulgated thereunder since, among other things, the transaction did not involve a public offering, the recipient is an accredited investor and had access to information about the Company and their investment, the recipient took the securities for investment and not resale, and the Company took appropriate measures to restrict the transfer of the securities.
Item 4.01Changes in Registrant’s Certifying Accountant.
On December 16, 2010, we dismissed Acqavella, Chiarelli, Shuster, Berkower & Co., LLP (“ACSB”), as our independent registered public accounting firm.
75
On August 12, 2010, the Company acquired Global Pharma Enterprise Group Limited (“Global Pharma BVI”), a British Virgin Islands company, pursuant to a Share Exchange Agreement in a Reverse Merger transaction previously reported in the Company’s Current Report on Form 8-K filed on August 13, 2010. Since prior to the merger the Company was a “shell company” (as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended), Global Pharma BVI is considered the predecessor of the Company and the historical financial statements of Global Pharma BVI are considered those of the Company. ACSB issued an audit report on the consolidated financial statements of Global Pharma BVI as of and for the years ended December 31, 2009, and 2008, which did not contain an adverse opinion or a disclaimer of opinion, and was not qualified or modified as to uncertainty, audit scope or accounting principles.
In connection with the audit of the consolidated financial statements of Global Pharma BVI as of and for the years ended December 31, 2009 and 2008 and through the date of this current report we have had no disagreements with ACSB on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of ACSB, would have caused ACSB to make reference to the subject matter of such disagreements in its report on the Company’s financial statements for such years or during the interim period through the date of the this Report.
During our two most recent fiscal years and through the date of this Report, there have been no reportable events as defined under Item 304(a)(1)(v) of Regulation S-K.
The Company has provided ACSB with a copy of this disclosure in the Form 8-K and has requested that ACSB provide us with a letter addressed to the SEC stating whether or not it agrees with the above statements, and we received a letter from ACSB stating that it agrees with the above statements.
New Independent Accountants
Our board of directors appointed Crowe Horwath LLP (“Crowe”) as our new independent registered public accounting firm effective as of December 16, 2010. During the two most recent fiscal years and through the date of our engagement, we did not consult with Crowe regarding either (1) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on our financial statements, or (2) any matter that was either the subject of a disagreement (as defined in Regulation S-K Item 304(a)(1)(v)), during the two most recent fiscal years.
Prior to engaging Crowe, Crowe did not provide our Company with either written or oral advice that was an important factor considered by our Company in reaching a decision to change our independent registered public accounting firm from ACSB to Crowe.
Item 5.03 Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year.
On August 12, 2010, concurrent with the Reverse Merger, we adopted the fiscal year end of our PRC Subsidiaries, thereby changing our fiscal year end from February 28 to December 31. The audited financial statements for the new fiscal year will be reflected in the Company’s Annual Report on Form 10-K for the year ended December 31, 2010.
Item 5.06 Change in Shell Company Status
We were a “shell company” (as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended) immediately before the closing of the Reverse Merger. As a result of the Reverse Merger, Global Pharma BVI is now our wholly owned subsidiary. Global Pharma BVI is the holding company of all the shares of Binomial BioPharm Group Limited (“Binomial”) and Hong Kong Wisdom Fortune Medicine Holding Group Limited (“Wisdom Fortune”), two wholly owned Hong Kong-incorporated companies.
Binomial, in turn, holds all the equity interests in Anhui Xuelingxian Pharmaceutical Co., Ltd.(“Xuelingxian”), Tonghua Tongdetang Pharmaceutical and Medicinal Materials Co., Ltd.(“Tongdetang”), two PRC-incorporated companies.
Wisdom Fortune holds all the equity interest in Shandong Global Pharm Co., Ltd. (“Yaoyuan”).
76
We are now, through our indirect wholly owned PRC Subsidiaries, namely Xuelingxian, Tongdetang and Yaoyuan in the business of wholesale distribution of pharmaceuticals-related products, Chinese herb cultivation and medicine raw materials preparations and, as a result of the Reverse Merger on August 12, 2010, have ceased to be a “shell” company.
Item 9.01 Financial Statements and Exhibits.
(a) Financial statements of businesses acquired.
The audited financial statements of Global Pharma BVI as of December 31, 2009 and 2008 and unaudited financial statements as for the six months ended June 30, 2010 and 2009 are appended to this report beginning on page F-1.
77
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009 and 2008
TABLE OF CONTENTS
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
Consolidated Balance Sheets
|
F-2
|
|
Consolidated Statements of Income and Comprehensive Income
|
F-3
|
|
Consolidated Statements of Stockholders’ Equity
|
F-4
|
|
Consolidated Statements of Cash Flows
|
F-5
|
|
Notes to Consolidated Financial Statements
|
F-6 - F-16
|
78
ACSB Acquavella, Chiarelli, Shuster, Berkower & Co., LLP
|
517 Route One
|
C e r t i f i e d P u b l i c A c c o u n t a n t s a n d A d v i s o r s
|
330 7th Avenue
|
|
Iselin, New Jersey 08830
|
Suite 202
|
|
|
732. 855.9600
|
New York, NY 10001
|
|
|
Fax:732.855.9559
|
212.867.1319
|
|
|
www.acsbco.com
|
To the Board of Directors and Stockholder of
Global Pharm Holdings Group, Inc.
(formally Global Pharma Enterprise Group Limited)
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the use of our audit report in this Form 8K-A of Global Pharm Holdings Group, Inc. (formally Global Pharm Holding Enterprise Group Limited), for the year ended December 31, 2009 and 2008 of our audit report dated August 2, 2010.
/S/ Acquavella, Chiarelli, Shuster, Berkower & Co., LLP
New York, New York
April 13, 2011
New York · New Jersey · San Francisco · Los Angeles · Cayman Islands
F-1
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31,
|
ASSETS
|
2009
|
2008
|
||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalent
|
$ | 7,455,147 | $ | 5,188,587 | ||||
|
Accounts receivable
|
11,707,848 | 8,564,025 | ||||||
|
Other receivable
|
- | 1,772 | ||||||
|
Due from related parties
|
24,568 | 1,465,738 | ||||||
|
Inventories
|
9,373,762 | 7,180,388 | ||||||
|
Total current assets
|
28,561,325 | 22,400,510 | ||||||
|
Property, plant and equipment, net
|
229,587 | 134,020 | ||||||
|
Total assets
|
$ | 28,790,912 | $ | 22,534,530 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Short-term loan
|
$ | 219,751 | $ | - | ||||
|
Bank acceptance
|
219,751 | - | ||||||
|
Accounts payable
|
13,598,744 | 11,075,591 | ||||||
|
Accrued salary
|
218,546 | 148,043 | ||||||
|
Income taxes payable
|
944,143 | 707,401 | ||||||
|
Other taxes payable
|
552,560 | 401,685 | ||||||
|
Other accrued liabilities
|
161,825 | 23,014 | ||||||
|
Due to related party
|
2,665,768 | 3,955,924 | ||||||
|
Total liabilities, all current
|
18,581,088 | 16,311,658 | ||||||
|
Stockholders' equity:
|
||||||||
|
Common stock, par value, $ 1 per share, 50,000
shares authorized, issued and outstanding.
|
50,000 | 50,000 | ||||||
|
Stock subscription receivable
|
(50,000 | ) | (50,000 | ) | ||||
|
Paid-in capital
|
2,458,223 | 2,458,223 | ||||||
|
Statutory surplus reserves
|
1,310,701 | 367,238 | ||||||
|
Retained earnings
|
6,206,903 | 3,121,166 | ||||||
|
Accumulated other comprehensive income
|
233,997 | 276,245 | ||||||
|
Total stockholders' equity
|
10,209,824 | 6,222,872 | ||||||
|
Total liabilities and stockholders' equity
|
$ | 28,790,912 | $ | 22,534,530 | ||||
See accompanying notes to consolidated financial statements.
F-2
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
FOR THE YEARS ENDED DECEMBER 31,
|
2009
|
2008
|
|||||||
|
Revenues, net
|
$ | 86,784,002 | $ | 54,475,666 | ||||
|
Cost of Goods Sold
|
70,725,013 | 45,362,590 | ||||||
|
Gross profit
|
16,058,989 | 9,113,076 | ||||||
|
Expenses:
|
||||||||
|
Operating expenses
|
792,295 | 366,718 | ||||||
|
General and administrative
|
1,096,333 | 648,520 | ||||||
|
Income from operations
|
14,170,361 | 8,097,838 | ||||||
|
Other income (expense)
|
||||||||
|
Interest income
|
21,052 | 24,379 | ||||||
|
Miscellaneous income (expense)
|
8,266 | (3,294 | ) | |||||
|
Income before income taxes
|
14,199,679 | 8,118,923 | ||||||
|
Provision for income taxes
|
3,298,277 | 2,029,731 | ||||||
|
Net income
|
10,901,402 | 6,089,192 | ||||||
|
Other comprehensive income
|
||||||||
|
Foreign currency translation adjustment
|
(42,248 | ) | 52,607 | |||||
|
Total comprehensive income
|
$ | 10,859,154 | $ | 6,141,799 | ||||
|
Earnings per share of common stock:
|
||||||||
|
Basic and diluted earnings per share
|
$ | 218 | $ | 122 | ||||
|
Basic and diluted weighted average shares
|
50,000 | 50,000 | ||||||
See accompanying notes to consolidated financial statements.
F-3
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDER'S EQUITY
YEARS ENDED DECEMBER 31, 2009
|
Accumulated
|
||||||||||||||||||||||||||||||||
|
Additional
|
other
|
Total
|
||||||||||||||||||||||||||||||
|
Common stock
|
paid-in
|
Retained
|
Statuary
|
comprehensive
|
Subscription
|
stockholders'
|
||||||||||||||||||||||||||
|
Shares
|
Amount
|
capital
|
earnings
|
reserve
|
income
|
receivable
|
equity
|
|||||||||||||||||||||||||
|
Balance, January 1, 2008
|
50,000 | $ | 50,000 | $ | 687,742 | $ | 2,526,413 | $ | 150,468 | $ | 223,638 | $ | (50,000 | ) | $ | 3,588,261 | ||||||||||||||||
|
Capital contribution
|
1,770,481 | 1,770,481 | ||||||||||||||||||||||||||||||
|
Net income
|
6,089,192 | 6,089,192 | ||||||||||||||||||||||||||||||
|
Dividends *
|
(5,277,669 | ) | (5,277,669 | ) | ||||||||||||||||||||||||||||
|
Transfer to statutory reserve
|
(216,770 | ) | 216,770 | - | ||||||||||||||||||||||||||||
|
Foreign currency translation
|
||||||||||||||||||||||||||||||||
|
adjustments
|
52,607 | 52,607 | ||||||||||||||||||||||||||||||
|
Balance, December 31, 2008
|
50,000 | 50,000 | 2,458,223 | 3,121,166 | 367,238 | 276,245 | (50,000 | ) | 6,222,872 | |||||||||||||||||||||||
|
Net income
|
10,901,402 | 10,901,402 | ||||||||||||||||||||||||||||||
|
Dividends *
|
(6,872,202 | ) | (6,872,202 | ) | ||||||||||||||||||||||||||||
|
Transfer to statutory reserve
|
(943,463 | ) | 943,463 | - | ||||||||||||||||||||||||||||
|
Foreign currency translation
|
||||||||||||||||||||||||||||||||
|
adjustments
|
(42,248 | ) | (42,248 | ) | ||||||||||||||||||||||||||||
|
Balance, December 31, 2009
|
50,000 | $ | 50,000 | $ | 2,458,223 | $ | 6,206,903 | $ | 1,310,701 | $ | 233,997 | $ | (50,000 | ) | $ | 10,209,824 | ||||||||||||||||
* dividend paid to former stockholders prior to Reverse Merger
See accompanying notes to consolidated financial statements.
F-4
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31,
|
2009
|
2008
|
|||||||
|
Cash flows from operating activities
|
||||||||
|
Net income
|
$ | 10,901,402 | $ | 6,089,192 | ||||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
||||||||
|
Depreciation and amortization
|
50,392 | 27,937 | ||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable
|
(3,145,877 | ) | (1,795,590 | ) | ||||
|
Other receivables
|
(22,781 | ) | (141 | ) | ||||
|
Inventories
|
(2,195,407 | ) | (2,804,776 | ) | ||||
|
Accounts payable
|
2,526,380 | 3,338,722 | ||||||
|
Accrued payroll and employee benefits
|
70,527 | 54,687 | ||||||
|
Other payables and accrued liabilities
|
138,725 | (662,809 | ) | |||||
|
Income taxes payable
|
236,928 | 58,799 | ||||||
|
Other taxes payable
|
139,620 | 1,294,565 | ||||||
|
Net cash provided by operating activities
|
8,699,909 | 5,600,586 | ||||||
|
Cash flows from investing activities
|
||||||||
|
Purchases of property, plant and equipment
|
(155,971 | ) | (58,434 | ) | ||||
|
Net cash used in investing activities
|
(155,971 | ) | (58,434 | ) | ||||
|
Cash flows from financing Aativities:
|
||||||||
|
Short-term loan
|
219,597 | - | ||||||
|
Bank acceptance
|
219,597 | - | ||||||
|
Due from shareholders
|
1,463,979 | (1,284,324 | ) | |||||
|
Due to related parties
|
- | 2,442 | ||||||
|
Paid-in Capital
|
- | 1,753,098 | ||||||
|
Dividend paid
|
(8,190,085 | ) | (3,425,268 | ) | ||||
|
Net cash used in financing activities
|
(6,286,912 | ) | (2,954,052 | ) | ||||
|
Net increase in cash and cash equivalents
|
2,257,026 | 2,588,100 | ||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
9,534 | 212,724 | ||||||
|
Cash and cash equivalents - Beginning of the year
|
5,188,587 | 2,387,763 | ||||||
|
Cash and cash equivalents - End of the year
|
$ | 7,455,147 | $ | 5,188,587 | ||||
|
Supplemental disclosure of cash flow information:
|
||||||||
|
Cash paid for interest
|
$ | 2,490 | $ | - | ||||
|
Cash paid for income taxes
|
$ | 3,061,337 | $ | 1,926,330 | ||||
See accompanying notes to consolidated financial statements.
F-5
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009 AND 2008
Note 1 – Organization and Summary of Significant Accounting Policies
Global Pharma Enterprise Group Limited (“Global Pharma BVI” or the “Company”) was incorporated on June 14, 2010 under the laws of the British Virgin Island and is a holding company. The Company conducts the wholesale and distribution of pharmaceuticals-related products, Chinese herb cultivation and medicine raw materials preparations through three operating subsidiaries located in China. On June 29, 2010 Global Pharma BVI acquired all of the outstanding shares of Bionmial Biopharm Group Limited (“Bionmial”), Incorporated under the laws of Hong Kong on September 9, 2009 and Hong Kong Wisdom Fortune Medicine Holding Group Limited, (“Wisdom Fortune”), formally Aecko Industrial Limited, incorporated under the laws of Hong Kong.
Tonghua Tongdetang Pharmaceutical Company (“Tongdetang”) was incorporated on February 2, 2002, under the laws of the People’s Republic of China (“PRC”). Anhui Xuelingxian Pharmaceutical (“Xuelingxian”) was incorporated on July 23, 2008, under the laws of the PRC. Shandong Yaoyuan Pharmaceutical Company (“Yaoyuan”) was incorporated on November 1, 2005, under the laws of the PRC.
On May 6, 2010 and May 8, 2010, Wisdom Fortune and Binomial, respectively, entered into Equity Transfer Agreements, with the shareholders of Yaoyuan, Xuelingxian and Tongdetang. Global Pharma BVI paid RMB 5,180,000 (approximately US$ 700,418), RMB 3,000,000 (approximately US $405,647), and RMB10,000,000 (approximately US$1,352,158) to the shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan, respectively, representing their registered capital at the time (referred to as the Equity Transfers). The Equity transfers by the PRC operating companies, pursuant to the Earn In Agreement, as thereafter amended, (and as further described below), was considered to be a transaction between entities under common control.
As a result of the Equity Transfers, Binomial currently holds all the equity interests in Xuelingxian and Tongdetang. Prior to the Equity Transfer, the shareholders of Xuelingxian were Yunlu Yin, Dandan Wang, Shulan Li, and Hong Zhang. The shareholders of Tongdetang were Yunlu Yin, Qingdong Zeng, and Feng Jin. Mei Li Tsai was the sole shareholder of Binomial from its inception until Global Pharma BVI acquired Binomial on June 29, 2010.
In addition, as a result of the Equity Transfers, Wisdom Fortune currently holds all the equity interest in Yaoyuan. Prior to the Equity Transfer, the shareholders of Yaoyuan were Yunlu Yin, Shouqiang Han, Yanming Lv, Guojun Zhao, Junyan Su, Boliang Zhu, and Chaobo Song. Mei Li Tsai was the sole shareholder of Wisdom Fortune from its inception until Global Pharma BVI acquired Wisdom Fortune on June 29, 2010.
On May 25, 2006 and April 15,2010, Yunlu Yin signed the Trust Agreements with Qinghui Zeng and Qingdong Zeng, respectively, documenting Yunlu Yin as the 62% of owner of Tongdetang and documenting each of Qinghui Zeng and Qingdong Zeng to act as the nominee owner of Tongdetang on behalf of Yunlu Yin. On May 31, 2008 and March 20, 2010 Yunlu Yin signed the Trust Agreements with Yanliang Song and Shouqiang Han, respectively, documenting Yunlu Yin as 80% of owner of Yaoyuan and documenting each of Yanliang Song and Shouqing Han to act as the nominee owner of Yaoyuan on behalf of Yunlu Yin. On September 5, 2009 and April 10, 2010, Yunlu Yin signed the Trust Agreements with Shunli Wang and Shulan Li and Hong Zhang, respectively, documenting Yunlu Yin as 65% of owner of Xuelingxian and documenting each of Shunli Wang and Shulan Li and Hong Zhang to act as the nominee owner of Xuelingxian on behalf of Yunlu Yin.
F-6
On June 29, 2010 the sole Shareholder of the Company entered into an Earn-in Agreement, as thereafter amended, (and as further described below), with the former shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan. Pursuant to the agreement, the Company shall entered into share exchange agreement, at a date after this agreement, with a United States domiciled shell company an at that time the former shareholders, including beneficial owners, will have call rights to own the controlling interest in a Publicly held Company.
After the Company entered into and consummated the Share Exchange Agreement with Top Flight GameBirds, Inc., a U.S. public company on August 6, 2010, pursuant to the Earn-In Agreement, as thereafter amended, the shareholders, including the beneficial owners of Xuelingxian, Tongdetang, and Yaoyuan, and the key management of the Company will have the right to purchase 20,894,000 shares at four different occurrence dates, contingent on various targets for total consideration of $300,000. Targets include binding three-year employment contracts with various members of management with six months of this agreements and target after tax net income of $3.6 Million, $3.8 Million, and $15.2 Million for the three months ended June 30, 2010 and September 30, 2010, and for the 12 months ended December 31, 2010, respectively. The non-cash expenses would be excluded from the calculation of the net income. Upon execution the former shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan will have call rights to own 80.36% of the publicly held Company.
As part of these restructuring transactions, no new capital was introduced. As a result, no new basis in the net assets of the PRC Subsidiaries was established. During this restructuring, management continued to serve and continued to direct both the day-to-day operating and management of the PRC Subsidiaries, as well as their strategic direction. Because of this operating and management control and because the restructuring plan effectively resulted in the Company continuing to bear the residual risks and rewards related to the PRC Subsidiaries, the Company consolidated the PRC Subsidiaries during the restructuring. The Company’s acquisition of the PRC Subsidiaries, which represented the return to legal ownership of the PRC Subsidiaries by the Company, represented a transaction between related parties under common control and did not establish a new basis in the assets and liabilities of the PRC Subsidiaries. The Earn-In Agreement, as thereafter amended, enables former shareholders to regain ownership of the Company’s shares originally transferred by them to the Company as part of the restructuring arrangements and, accordingly, the Company does not consider the reacquisition of those shares to represent compensation cost to the Company. The Company accounted for the shares earned by management as share-based compensation and recognized $5,968,400 of compensation expense.
Basis of Presentation
The consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America ("U.S. GAAP").
Principles of Consolidation
The consolidated financial statements include the accounts of Global Pharma Enterprise Group Limited and its direct and indirect wholly owned subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
Foreign Currency Translation
The Company has its local currency, Renminbi (“RMB”), as its functional currency. The consolidated financial statements of the Company are translated from RMB into US$. Accordingly, all assets and liabilities are translated at the exchange rates prevailing at the balance sheet dates, all income and expenditure items are translated at the average rates for each of the periods and equity accounts, except for retained earnings, are translated at the rate at the transaction date. Retained earnings reflect the cumulative net income (loss) translated at the average rates for the respective periods since inception less dividends translated at the rate at the transaction date.
RMB is not a fully convertible currency. All foreign exchange transactions involving RMB must take place either through the People's Bank of China (the "PBOC") or other institutions authorized to buy and sell foreign exchange. The exchange rates adopted for the foreign exchange transactions are the rates of exchange quoted by the PBOC, which are determined largely by supply and demand. Translation of amounts from RMB into US$ has been made at the following exchange rates for the respective years:
F-7
|
Year Ended December 31,
|
||||||
|
2009
|
2008
|
|||||
|
Assets and liabilities
|
1 US $ : 6.8259 RMB
|
1 US $ : 6.8225 RMB
|
||||
|
Statement of income
|
1 US $ : 6.8307 RMB
|
1 US $ : 6.9477 RMB
|
||||
The resulting translation adjustments are recorded as other comprehensive income in the consolidated statement of stockholders equity and comprehensive income and as a separate component of stockholders equity.
Commencing from July 21, 2005, China adopted a managed floating exchange rate regime based on market demand and supply with reference to a basket of currencies. Since then, the PBOC administers and regulates the exchange rate of US$ against RMB taking into account the demand and supply of RMB, as well as domestic and foreign economic and financial conditions.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements. The reported amounts of revenues and expenses during the reporting period may be affected by the estimates and assumptions we are required to make. Estimates that are critical to the accompanying consolidated financial statements relate primarily to returns, sales allowances and customer chargebacks, and the valuation of long-lived assets. Estimates and assumptions are reviewed periodically and the effects of revisions are reflected in the period that they are determined to be necessary. Actual results could differ from these estimates.
Comprehensive Income
The Company follows the Comprehensive Income Topic of the FASB Accounting Standard Codification (“ASC 220 Comprehensive Income”). Comprehensive income is a more inclusive financial reporting methodology that includes disclosure of certain financial information that historically has not been recognized in the calculation of net income.
The Company’s only component of other comprehensive income is foreign currency translation gains and losses. The foreign currency translation gains (losses) for the years ended December 31, 2009 and 2008 were $(42,248) were $52,607 respectively. Accumulated other comprehensive income is recorded as a separate component of stockholders’ equity.
Cash and Cash Equivalents
Cash and cash equivalents comprise cash in banks and on hand, demand deposits with banks and other financial institutions, and short-term, highly liquid investments which are readily convertible into known amounts of cash and which are subject to an insignificant risk of changes in value, having been within three months of maturity at acquisition.
Restricted Cash
Restricted cash represents amounts held by a bank as security for bank acceptance notes and loan collateral and therefore is not available for the Company’s use in operations.
Accounts Receivable
The Company records accounts receivable, net of an allowance for doubtful accounts. The Company maintains allowances for doubtful accounts for estimated losses. The Company reviews the accounts receivable on a quarterly basis and makes general and specific allowances when there is doubt as to the collectability of individual balances. In evaluating the collectability of individual receivable balances, the Company considers many factors, including the age of the balance, customer's historical payment history, its current credit-worthiness and current economic trends. The amount of the provision, if any, is recognized in the consolidated statement of income within "General and administrative." Accounts are written off after exhaustive efforts at collection. There are no allowances for doubtful accounts as of December 31, 2010 and 2009.
F-8
Inventories
Inventories are stated at the lower of cost (determined on a weighted average basis) or market value. Work-in-progress is composed of direct materials, direct labor, manufacturing overhead, and an attributable portion of land lease cost that has be capitalized as a component of inventory. The capitalized land lease cost will be recorded in cost of goods sold at such time the goods are sold. An allowance is established when management determines that certain inventories may not be saleable. If inventory costs exceed expected market value due to obsolescence or quantities in excess of expected demand, the Company will record reserves for the difference between the cost and the market value. These reserves are recorded based on estimates and reflected in cost of sales. There is no allowance as of December 31, 2009 and 2008.
Long-Lived Assets
The Company estimates the future undiscounted cash flows to be derived from an asset to assess whether or not a potential impairment exists when events or circumstances indicate the carrying value of a long-lived asset may be impaired. If the carrying value exceeds the Company’s estimate of future undiscounted cash flows, the Company then calculates the impairment as the excess of the carrying value of the asset over the Company’s estimate of its fair market value.
Lease Obligations
All non-cancellable leases with an initial term greater than one year are categorized as either capital leases or operating leases. Assets recorded under capital leases are amortized according to the methods employed for property and equipment or over the term of the related lease, if shorter.
Property, Plant and Equipment
Property and equipment are stated at cost. Expenditures for maintenance and repairs are charged to earnings as incurred; additions, renewals and betterments are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided using the straight-line method for substantially all assets with estimated lives of:
|
Buildings
|
30 years
|
|
Furniture, fixtures and equipment
|
5 years
|
|
Motor vehicles
|
5 years
|
|
Office equipment
|
5 years
|
|
Plant and machinery
|
5 to 15 years
|
Revenue Recognition
The Company’s revenue recognition policies are in compliance with Staff Accounting Bulletin 104. Sales revenue is recognized when all of the following have occurred: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the price is fixed or determinable, and (iv) the ability to collect is reasonably assured. These criteria are generally satisfied at the time of shipment when risk of loss and title passes to the customer. The Company’s four segments have the same revenue recognition policy. Revenues are recorded net of value-added taxes.
Shipping and Handling Expense
Shipping and handling costs are expenses as incurred and are included in cost of goods sold.
F-9
Advertising costs are expensed in the period in which the advertisements are first run or over the life of the endorsement contract. Advertising expense for the years ended December 31, 2009 and 2008 were approximately $4,146 and $8,888 respectively. Advertising costs include advertising subsidy expense which is accrued based on the terms in effect with distributors and paid when all attaching conditions have been completed.
Income Taxes
The Company is subject to the Income Tax Law of the People’s Republic of China. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases and tax loss carry forwards. Any deferred tax assets and liabilities would be measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The statutory rate under the laws of the PRC is 25%. The Company’s herbal cultivation segment is subjected to zero income taxes.
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates applicable to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
The Company has not been subjected to income tax examinations by taxing authorities for the years ended December 31, 2009 and 2008. The Company is open to tax examination in the PRC for all years, as tax returns remain open to examination until notified by the taxing authorities, and the Company has not received any notifications to date. The Company records interest and penalties as other expense on the consolidated statements of income and other comprehensive income. During the years ended December 31, 2009 and 2008, the Company did not recognize any amount in interest and penalties.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are cash and accounts receivable arising from its normal business activities. The Company places its cash in what it believes to be credit-worthy financial institutions. The Company has a diversified customer base. The Company controls credit risk related to accounts receivable through credit approvals, credit limits and monitoring procedures. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk, establishes an allowance, if required, for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is limited. As of December 31, 2009 and 2008, there was no allowance for uncollectible accounts as previously discussed.
Fair Value of Financial Instruments
The carrying amounts of the Company’s financial instruments, which principally include accounts receivable and accounts payable, approximate their fair values due to the relatively short maturity of such instruments.
The carrying amount of the Company’s short-term borrowings approximates their fair value based upon current rates and terms available to the Company for similar debt.
Basic and Diluted Earnings per Share
The Company reports earnings per share in accordance with FASB ASC-260, Earnings per Share. The Company’s basic earnings per share is computed using the weighted-average number of shares outstanding for the periods presented.
F-10
Diluted earnings per share is based on the assumption that any dilutive options, warrants or other instruments were converted or exercised. Dilution is computed by applying the treasury stock method. Under this method, the Company’s outstanding stock options are assumed to be exercised, and funds thus obtained were assumed to be used to purchase common stock at the average market price during the period. As of September 30, 2010 and December 31, 2009, the Company has not dilutive securities
Stock-Based Compensation
The Company uses the fair value recognition provision of ASC Topic 718, Compensation-Stock Compensation (formerly named as SFAS 123(R)), which requires the Company to expense the cost of employee services received in exchange for an award of equity instruments based on the grant date fair value of such instruments over the vesting period.
The Company records all share-based payments, including grants of common shares and stock options to employees, in the consolidated financial statements based on their fair values on date of grant. The grant is expensed over the required service period, which is generally equal to the vesting period.
Recent Accounting Pronouncements
In December 2010, the FASB issued ASU 2010-29 which addresses diversity in practice about the interpretation of the pro forma revenue and earnings disclosure requirements for business combinations (Topic 805). This ASU specifies that if a public entity presents comparative financial statements, the entity should disclose revenue and earnings of the combined entity as though the business combination(s) that occurred during the current year had occurred as of the beginning of the comparable prior annual reporting period only. This ASU also expands the supplemental pro forma disclosures under Topic 805 to include a description of the nature and amount of material, non-recurring pro forma adjustments directly attributable to the business combination included in the reported pro forma revenue and earnings. ASU 2010-29 is effective prospectively for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2010. Early adoption is permitted. The Company is currently evaluating the impact of this ASU and expected the adoption of this ASU will have an impact on its future business combinations.
Reclassification
Certain items have been reclassified in the accompanying consolidated Financial Statements and Notes for prior periods to be comparable with the classification for the year ended December 31, 2009. The reclassification had no effect on previously reported net income
|
2.
|
Inventories
|
As of December 31, 2009 and 2008, inventories consisted of the following:
|
December 31,2009
|
December 31,2008
|
|||||||
|
Raw materials (1)
|
$ | 1,401,865 | $ | 708,836 | ||||
|
Work-in-progress (2)
|
- | - | ||||||
|
Finished goods (3)
|
7,971,897 | 6,471,553 | ||||||
|
Total
|
$ | 9,373,762 | $ | 7,180,388 | ||||
(1) Raw materials include the herbal material that will be used to produce the flower tea and the OEM product.
(2) Work-in-process is composed of direct materials, direct labor, an attributable portion of land lease cost that has been capitalized, and an attributable portion of manufacturing overhead.
(3) Finished goods only consist of pharmaceutical products.
F-11
|
3.
|
Property, plant and equipment
|
As of December 31, 2009 and 2008, property, plant and equipment consist of the following:
|
|
December 31,
|
|||||||
|
|
2009
|
2008
|
||||||
|
Operating equipment
|
$
|
219,321
|
$
|
84,878
|
||||
|
Office equipment
|
73,382
|
51,811
|
||||||
|
Transportation equipment
|
10,267
|
10,272
|
||||||
|
Leasehold improvements
|
50,093
|
50,118
|
||||||
|
Sub-total
|
353,063
|
197,079
|
||||||
|
Less: Accumulated depreciation
|
(123,476
|
)
|
(63,059
|
)
|
||||
|
Property, plant and equipment, net
|
$
|
229,587
|
$
|
134,020
|
||||
Depreciation expenses totaled $50,392 and $27,937 for the years ended December 31, 2009 and 2008, respectively.
|
4.
|
Intangible Assets
|
The Company uses the “Yaoyuan” trademark for drug packaging that is owned by Yanliang Song, a former shareholder of Yaoyuan. The trademark was applied for on May 16, 2008 and approved on April 7, 2010 and was subsequently transferred to Yaoyuan without cost or value. The trademark will expire on April 6, 2020. The cost of the trademark for Yanliang Song is less than $200 and the Company is not required to pay Yanliang Song any license fee for the use of the trademark. The Company also believes this amount is immaterial.
The Company has the exclusive right to use the “Xuelingxian” trademark for pharmaceutical products, which is owned by Jingsheng Wang, a former shareholder of Xuelingxian, pursuant to a license agreement dated January 1, 2009 for a term from January 1, 2009 to January 1, 2014. The cost of the trademark for Jingsheng Wang is less than $200 and the Company does not have to pay Jingsheng Wang any license fee for the use of the trademark. The Company also believe this amount is immaterial.
|
5.
|
Due from related parties
|
During 2008 the Company advanced a director and former shareholder approximately $1,465,000. The advance bears no interest and was payable upon demand. This amount was repaid during 2009.
During 2009, the Company advanced related parties certain monies bearing no interest and payable upon demand. At December 31, 2009, $24,500 remained outstanding.
|
6.
|
Short-term Loans
|
The Company entered into a short-term loan on December 23, 2009 with Shandong Qilu bank. The loan amount is $219,751 and is due on December 21, 2010. Term of this loan calls for monthly principal repayment plus interest at the one-year commercial loan rate issued by the People’s Bank of China plus 40% (7.434% at December 31, 2009) each month. There was no outstanding note payable balance at December 31, 2008.
The Company entered an entrustment guarantee contract with Kexin Fengda Investment Guarantee Co, Ltd (“Kexin Fengda”) whereby Kexin Fengda guarantees the payment of the note payable in the amount of RMB1, 500,000 to Shandong Qilu bank from December 9, 2009 to December 9, 2010. Kexin Fengda received payment of approximately $6,000 in consideration for this guarantee.
Yanliang Song, the general manager of Yaoyuan, provided counter-guarantee to Kexin Fengda using all his personal/family assets. Pursuant to the counter-guarantee, Yanliang Song guarantees the payment of the principal, interests, penalty interests, damages, and all the costs incurred by Kexin Fengda in the event that the Company defaults on the loan. The counter-guarantee is valid for two years from December 9, 2010.
The interest expense was $3,684 and nil for the years ended December 31, 2009 and 2008, respectively.
F-12
|
7.
|
Bank Acceptance
|
The Company has a bank acceptance with Qilu bank in the amount of $219,751 as of December 31, 2009. The term is six-month and bearing non-interest. The balance outstanding at December 31, 2009 is $219,751. There was no outstanding bank acceptance balance at December 31, 2008.
|
8.
|
Related Party Transactions
|
|
|
·
|
On May 25, 2006 and April 15,2010, Yunlu Yin signed the Trust Agreements with Qinghui Zeng and Qingdong Zeng, respectively, documenting Yunlu Yin as the 62% of owner of Tongdetang and documenting each of Qinghui Zeng and Qingdong Zeng to act as the nominee owner of Tongdetang on behalf of Yunlu Yin. On May 31, 2008 and March 20, 2010 Yunlu Yin signed the Trust Agreements with Yanliang Song and Shouqiang Han, respectively, documenting Yunlu Yin as 80% of owner of Yaoyuan and documenting each of Yanliang Song and Shouqing Han to act as the nominee owner of Yaoyuan on behalf of Yunlu Yin. On September 5, 2009 and April 10, 2010, Yunlu Yin signed the Trust Agreements with Shunli Wang and Shulan Li and Hong Zhang, respectively, documenting Yunlu Yin as 65% of owner of Xuelingxian and documenting each of Shunli Wang and Shulan Li and Hong Zhang to act as the nominee owner of Xuelingxian on behalf of Yunlu Yin.
|
|
|
·
|
During each of the years ended February 28, 2010 and February 28, 2009, our former President, Chief Executive Officer, Chief Financial Officer, sole director and chairwoman, Rhonda Heskett contributed services and brooding facilities with a fair value of $6,000 and $2,400, respectively. These non-cash expenses totaling $8,400 in each of the years ended February 28, 2010 and 2009 were treated as contributed capital.
|
|
|
·
|
On July 23 and September 24, 2010, our former shareholder, Shunli Wang, lent RMB 5,000,000 and RMB 600,000 (totally, $836,058) respectively, to Xuelingxian as working capital and the balance are non-interest bearing and payable on demand. The balance was paid off on December 25, 2010.
|
|
|
·
|
As of December 31, 2009 and 2008, Global Pharma BVI had $24,568 and $1,465,738, respectively due from related parties. In 2009, Global Pharma BVI advanced $24,568 to Yanliang Song, an officer of Yaoyuan and the balance is nil at June 30, 2010. In 2008, Xianming Zeng and Qingdong Zeng, former stockholders of Tongdetang, borrowed RMB 6,000,000 and RMB 4,000,000 (totally, $1,465,738), respectively, from Tongdetang and such balance was offset in 2009 when Tongdetang declared dividends in the amount of RMB 6,000,000 and RMB 4,000,000, respectively, to Xianming Zeng and Qingdong Zeng.
|
|
|
·
|
Global Pharma BVI use a trademark for drug packaging that is owned by a director and former shareholder of Yaoyuan, Yanliang Song. The trademark was applied for on May 16, 2008 and approved April 7, 2010. It expires April 6, 2020. While Yanliang Song has paid for all associated costs, we use this trademark at no cost.
|
|
|
·
|
During 2009, Xuelingxian had sales of approximately $1.4 million to a number of related parties, and employees. The details of the transactions are set forth below:
|
|
Name
|
Relationship
|
Amount
|
||||
|
Fei Gao
|
General Manager
|
$ | 472,625 | |||
|
Wenhua Chou
|
General Manager
|
$ | 218,363 | |||
|
Jingzhong Wang
|
General Manager
|
$ | 445,381 | |||
|
Enhui Fan
|
General Manager
|
$ | 89,474 | |||
|
Fengyi Wang
|
General Manager
|
$ | 120,054 | |||
|
Min Xue
|
General Manager
|
$ | 74,458 | |||
|
Total
|
$ | 1,420,354 | ||||
|
|
·
|
For the years ended December 31, 2009 and 2008, and for the period ended June 30, 2010, we paid $8,190,085, $3,425,268, and $3,208,521 to the former shareholders of our subsidiaries, respectively. On June 29, 2010, as part of May 6 and May 8, 2010 Equity Transfer Agreements, Wisdom Fortune and Binomial declared a dividend of approximately RMB 57,522,347 (approximately $8,438, 816) to its then stockholders. The following table set forth the dividend payable as of December 31, 2009 and 2008.
|
F-13
|
Related Party
|
Relationship
|
December 31, 2009
|
December 31, 2008
|
|||||||
|
Shouqiang Han
|
Stockholder
|
$ | 370,006 | $ | 158,624 | |||||
|
Qinghui Zeng
|
Stockholder
|
1,427,408 | 2,525,968 | |||||||
|
Xianming Zeng
|
Stockholder
|
713,704 | 1,262,983 | |||||||
|
Shunli Wang
|
Stockholder
|
93,948 | - | |||||||
|
Jingsheng Wang
|
Stockholder
|
26,089 | - | |||||||
|
Fulan Li
|
Stockholder
|
15,140 | - | |||||||
|
Others
|
Stockholder
|
19,474 | 8,349 | |||||||
|
Total
|
Stockholder
|
$ | 2,665,768 | $ | 3,955,924 | |||||
|
|
·
|
On June 29, 2010 the sole shareholder of Global Pharma BVI entered into an Earn-In Agreement with the former shareholders, as amended on March 29, 2011, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan. Pursuant to the agreement, the Company shall entered into a share exchange agreement, at a date after this agreement, with a United States domiciled shell company an at that time the former shareholders, including beneficial owners, will have call rights town the controlling interest in the publicly held company.
|
|
|
·
|
On August 6, 2010, we were party to a share purchase agreement between our former President, Chief Executive Officer, Chief Financial Officer and sole director and Chairwoman, Rhonda Heskett and Mei Li Tsai involving the sale of 19,094,000 shares of common stock of the Company for a cash consideration of $450,000.
|
|
|
·
|
On August 12, 2010, Global Pharm entered into and consummated a Share Exchange Agreement with Mei Li Tsai, the sole shareholder of Global Pharma BVI and Global Pharma BVI to acquire all the issued and outstanding capital stock of Global Pharma BVI, in consideration for our 1,800,000 newly issued restricted shares.
|
|
|
·
|
Pursuant to the Earn-In Agreement dated June 29, 2010, amended on March 29, 2011, after Global Pharma BVI entered into and consummated a Share Exchange Agreement with a U.S. public company, the shareholders, including the beneficial owners of Xuelingxian, Tongdetang, and Yaoyuan, and the key management of the Company were given the right to purchase 20,894,000 shares at four different occurrence dates, contingent on various targets for total consideration of $300,000. Targets include binding three-year employment contracts with various members of management with six months of this agreements and target after tax net income of $3.6 million, $3.8 million, and $15.2 million for the three months ended June 30, 2010 and September 30, 2010, and for the 12 months ended December 31, 2010, respectively. Non-cash expenses are excluded from the calculation of the net income. Upon execution the former shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan will have call rights to own 80.36% of the publicly held company. As of March 29, 2011, all of the earn-in targets have been achieved and all buyers have exercised the call rights.
|
|
9.
|
Statutory Reserve
|
The laws and regulations of the PRC require that before a foreign invested enterprise can legally distribute profits, it must first satisfy all tax liabilities, provide for losses in previous years, and make allocations, in proportions determined at the discretion of the board of directors, to the statutory reserve. The statutory reserves include the surplus reserve fund.
The Company is required to transfer 10% of its net income, as determined in accordance with the PRC accounting rules and regulations, to a statutory surplus reserve fund until such reserve balance reaches 50% of the Company’s registered capital. The transfer to this reserve must be made before distribution of any dividends to shareholders. Transfers to the statutory surplus reserve fund were $943,363 and $216,770 for 2009 and 2008, respectively. As of December 31, 2009, the Company has fulfilled the reserve requirement.
The surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance after such issue is not less than 50% of the registered capital.
F-14
|
10.
|
Commitments
|
The Company leases various facilities under lease agreements ranging from month to month to fifteen-year terms. Rental expense for the years ended December 31, 2009 and 2008 was $315,255 and $171,461, respectively.
Additionally, subsequent to December 31, 2009, the Company entered into a five-year land lease expiring December 31, 2014. The future minimum obligations under the aforementioned agreements are as follows:
|
2010
|
$
|
2,962,874
|
||
|
2011
|
2,993,865
|
|||
|
2012
|
2,842,485
|
|||
|
2013
|
2,507,402
|
|||
|
2014
|
2,374,277
|
|||
|
Thereafter
|
$
|
1,509,849
|
During 2008, Yaoyuan entered into an employment contract with Zhao Guojun to act as deputy general manager. The annual salary is approximately $9,000 and the contract expires October 31, 2011.
|
11.
|
Concentrations
|
The Company had one customer who accounted for approximately 14% and 13% of accounts receivable at December 31, 2009 and 2008, respectively. There were no sales concentrations during either year.
Two vendors accounted for approximately 21% and 31% of the Company’s purchases for the years ended December 31, 2009 and 2008, respectively.
One vendor accounted for approximately 26% of the Company’s accounts payable at December 31, 2009 whereas two vendors accounted for approximately 45% of the Company’s accounts payable at December 31, 2008.
|
12.
|
Segment Report
|
FASB ASC 280-10 requires the use of the management approach model for segment reporting. The management approach model is based on how a company's management organizes segments within the company for making operating decisions and assessing performance. Reportable segments are based on products and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company. Based on this model, the Company has determined that it has four segments. The Company’s principal businesses are herbal cultivation, TCM processing and distribution, flower tea sales, and distribution. Based on the various operation activities, the Company’s reportable segments are as follows:
|
|
·
|
Herb cultivation – the planting, processing and selling herbs in China
|
|
|
·
|
TCM processing and distribution – rough processing and sale of TCM product
|
|
|
·
|
Distribution – the sale of healthcare products to hospitals and pharmacy shops
|
|
|
·
|
Flower tea sales – Manufacture and sale of flower tea bags
|
The segment reporting for each period ended December 31, 2009 and 2008 as follows:
|
For the year ended December 31, 2009
|
||||||||||||||||||||
|
Herb cultivation
|
TCM processing
and distribution
|
Flower tea
|
Distribution
|
Total
|
||||||||||||||||
|
Sales
|
7,500,814 | 14,844,372 | 5,259,366 | 59,179,450 | 86,784,002 | |||||||||||||||
|
Interest income
|
2,916 | 4,728 | 1,447 | 11,962 | 21,052 | |||||||||||||||
|
Interest expense
|
- | - | - | - | - | |||||||||||||||
|
Depreciation
|
8,330 | 11,485 | 4,728 | 25,849 | 50,392 | |||||||||||||||
|
Income tax
|
- | 774,598 | 274,440 | 2,249,238 | 3,298,277 | |||||||||||||||
|
Net income
|
908,629 | 2,130,292 | 1,137,355 | 6,725,126 | 10,901,402 | |||||||||||||||
|
Total assets
|
2,870,078 | 5,679,985 | 2,012,421 | 18,228,427 | 28,790,912 | |||||||||||||||
|
Expenditures for segment assets
|
- | 75,653 | 34,609 | 45,709 | 155,971 | |||||||||||||||
F-15
|
For the year ended December 31, 2008
|
||||||||||||||||||||
|
Herb cultivation
|
TCM processing
and distribution
|
Flower tea
|
Distribution
|
Total
|
||||||||||||||||
|
Sales
|
- | 7,779,375 | - | 46,696,291 | 54,475,666 | |||||||||||||||
|
Interest income
|
- | 3,969 | - | 20,409 | 24,379 | |||||||||||||||
|
Interest expense
|
- | - | - | - | - | |||||||||||||||
|
Depreciation
|
- | 3,201 | - | 24,736 | 27,937 | |||||||||||||||
|
Income tax
|
- | 245,938 | - | 1,783,793 | 2,029,731 | |||||||||||||||
|
Net income
|
- | 737,814 | - | 5,351,378 | 6,089,192 | |||||||||||||||
|
Total assets
|
- | 4,514,775 | - | 18,019,755 | 22,534,530 | |||||||||||||||
|
Expenditures for segment assets
|
- | 43,427 | - | 15,006 | 58,434 | |||||||||||||||
|
13.
|
Subsequent Events
|
The Company has evaluated all events or transactions that occurred from January 1, 2010 through the filing with the SEC. We have listed the material recognizable subsequent events during this period as below:
During May 2010, Yaoyuan was acquired by Wisdom Fortune Medicine Holding Group, Inc. (“Wisdom”) in a transaction treated as a capital re-organization.
During May 2010, Xuelingxian and Tongdetang were both acquired by Binomial BioPharm, Inc. (“Binomial”) in a transaction treated as a capital re-organization. The merger between Tongdetang and Binomial was approved and Tongdetang was granted a new business license under which it is authorized to conduct business for duration of 20 years. Upon the expiration of this 20 year period, Tongdetang may file for a renewal or extension of this business license.
On June 29, 2010, the outstanding stock of Binomial and Wisdom was acquired by Global Pharma Enterprise Group Limited (“Global”) in a transaction treated as a capital re-organization.
On August 12, 2010, Global Pharma BVI entered into and consummated a Share Exchange Agreement with the Global Pharm Holdings Group, Inc. (“Gobal Pharm”). Pursuant to the Earn-In Agreement, as thereafter amended as described below, the shareholders, including the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan, and the key management of Global Pharma BVI were given the right to purchase 20,894,000 shares at four different occurrence dates, contingent on various targets for total consideration of $300,000. On March 29, 2011, Global Pharma BVI entered into an Agreement to Amend the Earn-In Agreement, or the Amendment, with the former shareholders and the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan and 20 individuals of its management who were parties to the original agreement. As of March 29, 2011, pursuant to the Amendment, all of the four earn-in targets have been achieved and the key management and former shareholders and the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan hold, in the aggregate, 20,894,000 shares, or 80.36%, of the shares of stock of Global Pharm..
F-16
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONSOLIDATED FINANCIAL STATEMENTS
AND
INDEPENDENT AUDITORS' REPORT
JUNE 30, 2010
F-17
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONTENTS
|
Independent Auditors' Review Report
|
F-19
|
|
Consolidated Financial Statements
|
|
|
Balance Sheets
|
F-20
|
|
Statements of Income and Comprehensive Income
|
F-21
|
|
Statements of Cash Flows
|
F-22
|
|
Statements of Stockholders Equity
|
F-23
|
|
Notes to Financial Statements
|
F-24-F-39
|
F-18
ACSB Acquavella, Chiarelli, Shuster, Berkower & Co., LLP
|
517 Route One
|
Certified Public Accountants and Advisors
|
330 7th Avenue
|
|
Iselin, New Jersey 08830
|
Suite 202
|
|
|
732. 855.9600
|
New York, NY 10001
|
|
|
Fax:732.855.9559
|
212.867.1319
|
|
|
www.acsbco.com
|
INDEPENDENT AUDITORS’ REPORT
To the Board of Directors and Stockholder of
Global Pharm Holdings Group, Inc.
We have reviewed the accompanying consolidated balance sheet of Global Pharma Enterprise Group Limited (the “Company”) at June 30, 2010, and the related consolidated statements of income and comprehensive income for the three and six month periods ended June 30, 2010 and 2009 and consolidated statements of cash flows and stockholders’ equity for the six months then ended. These financial statements are the responsibility of the Company’s management.
We conducted our review in accordance with the standards of the Public Company Accounting Oversight Board (United States). A review consists principally of inquiries of Company personnel and analytical procedures applied to financial data. It is substantially less in scope than an audit in accordance with generally accepted auditing standards, the objective of which is the expression of an opinion regarding the financial statements taken as a whole. Accordingly, we do not express such an opinion.
Based on our review, we are not aware of any material modifications that should be made to the accompanying financial statements in order for them to be in conformity with generally accepted accounting principles.
We have previously audited, in accordance with auditing standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet of the Company as of December 31, 2009 and the related consolidated statements of income and comprehensive income, stockholders’ equity and cash flows for the year then ended; and in our report dated August 2, 2010, we expressed an unqualified opinion on those financial statements. In our opinion, the information set forth in the accompanying consolidated balance sheet as of December 31, 2009, is fairly stated, in all material respects, in relation to the consolidated balance sheet from which it has been derived.
/S/ ACQUAVELLA, CHIARELLI, SHUSTER, BERKOWER & CO., LLP
Iselin, New Jersey
August 18, 2010
Except for Note 13 as to which the date is April 13, 2011
|
New York
|
·
|
New Jersey
|
·
|
San Francisco
|
·
|
Los Angeles
|
·
|
Cayman Islands
|
F-19
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONSOLIDATED BALANCE SHEETS
June 30, 2010 and December 31, 2009
|
June 30, 2010
|
December 31,
2009
|
|||||||
|
(Restated)
|
(Audited)
|
|||||||
|
ASSETS
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$ | 10,169,823 | $ | 7,455,147 | ||||
|
Accounts receivable
|
13,099,650 | 11,707,848 | ||||||
|
Other current assets
|
1,304,508 | - | ||||||
|
Due from related parties
|
33,926 | 24,568 | ||||||
|
Inventories
|
14,750,986 | 9,373,762 | ||||||
|
Total current assets
|
39,358,893 | 28,561,325 | ||||||
|
Property, plant and equipment, net
|
203,705 | 229,587 | ||||||
|
Total assets
|
$ | 39,562,598 | $ | 28,790,912 | ||||
|
LIABILITIES AND STOCKHOLDER'S EQUITY
|
||||||||
|
Current liabilities
|
||||||||
|
Short-term loan
|
$ | 584,000 | $ | 219,751 | ||||
|
Bank acceptance
|
- | 219,751 | ||||||
|
Accounts payable
|
21,060,188 | 13,598,744 | ||||||
|
Accrued salary
|
85,265 | 218,546 | ||||||
|
Income taxes payable
|
1,267,758 | 944,143 | ||||||
|
Other taxes payable
|
397,149 | 552,560 | ||||||
|
Other accrued liabilities
|
166,459 | 161,825 | ||||||
|
Due to related parties
|
9,384,517 | 2,665,768 | ||||||
|
Total liabilities, all current
|
32,945,336 | 18,581,088 | ||||||
|
Stockholder's equity
|
||||||||
|
Common stock, par value, $ 1 per share, 50,000 shares authorized, issued and outstanding
|
50,000 | 50,000 | ||||||
|
Stock subscription receivable
|
(50,000 | ) | (50,000 | ) | ||||
|
Additional paid-in capital
|
3,950,323 | 2,458,223 | ||||||
|
Statutory surplus reserves
|
1,310,701 | 1,310,701 | ||||||
|
Retained earnings
|
1,090,015 | 6,206,903 | ||||||
|
Accumulated other comprehensive income
|
266,223 | 233,997 | ||||||
|
Total stockholders' equity
|
6,617,262 | 10,209,824 | ||||||
|
Total liabilities and stockholders' equity
|
$ | 39,562,598 | $ | 28,790,912 | ||||
See accompanying notes to consolidated financial statements.
F-20
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
Three and Six Months Ended June 30,
|
Three Months Ended June 30,
|
Six Months Ended June 30,
|
|||||||||||||||
|
2010
|
2009
|
2010
|
2009
|
|||||||||||||
|
(Restated)
|
(Restated)
|
|||||||||||||||
|
Revenues, net
|
$ | 30,530,977 | $ | 19,189,756 | $ | 59,259,637 | $ | 38,952,977 | ||||||||
|
Cost of goods sold
|
24,989,792 | 15,524,554 | 48,632,162 | 31,687,148 | ||||||||||||
|
Gross profit
|
5,541,185 | 3,665,202 | 10,627,475 | 7,265,829 | ||||||||||||
|
Expenses
|
||||||||||||||||
|
Operating expenses
|
233,371 | 198,606 | 364,999 | 298,644 | ||||||||||||
|
General and administrative (Include stock-based compensation expenses $1,492,100 for the three and six months ended June 30, 2010)
|
1,794,759 | 259,179 | 2,165,148 | 515,866 | ||||||||||||
|
Total expenses
|
2,028,130 | 457,785 | 2,530,147 | 814,510 | ||||||||||||
|
Income from operations
|
3,513,055 | 3,207,417 | 8,097,328 | 6,451,319 | ||||||||||||
|
Other income
|
||||||||||||||||
|
Interest income
|
13,226 | 6,450 | 17,874 | 13,007 | ||||||||||||
|
Income before income taxes
|
3,526,281 | 3,213,867 | 8,115,202 | 6,464,326 | ||||||||||||
|
Provision for income taxes
|
1,255,682 | 807,246 | 2,419,451 | 1,619,857 | ||||||||||||
|
Net income
|
2,270,599 | 2,406,621 | 5,695,751 | 4,844,469 | ||||||||||||
|
Other comprehensive income
|
||||||||||||||||
|
Foreign currency translation adjustment
|
33,092 | 90,802 | 32,226 | 240,720 | ||||||||||||
|
Total comprehensive income
|
$ | 2,303,691 | $ | 2,497,423 | $ | 5,727,977 | $ | 5,085,189 | ||||||||
|
Earnings per share of common stock
|
||||||||||||||||
|
Basic and diluted earnings per share
|
$ | 45.41 | $ | 48.13 | $ | 113.92 | $ | 96.89 | ||||||||
|
Basic and diluted weighted average shares
|
50,000 | 50,000 | 50,000 | 50,000 | ||||||||||||
See accompanying notes to consolidated financial statements.
F-21
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONSOLIDATED STATEMENTS OF CASH FLOWS
Six Months Periods Ended June 30,
|
2010
(unaudited)
|
2009
(unaudited)
|
|||||||
|
|
(Restated)
|
|||||||
|
Cash flows from operating activities
|
||||||||
|
Net income
|
$ | 5,959,063 | $ | 4,844,469 | ||||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
||||||||
|
Depreciation and amortization
|
29,169 | 23,007 | ||||||
|
Stock-based compensation
|
1,228,788 | |||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable
|
(1,431,800 | ) | (456,481 | ) | ||||
|
Due from related parties
|
(33,983 | ) | - | |||||
|
Other current assets
|
(1,304,551 | ) | (411 | ) | ||||
|
Inventories
|
(5,409,249 | ) | (8,345,108 | ) | ||||
|
Accounts payable
|
7,507,902 | 6,214,516 | ||||||
|
Accrued payroll and employee benefits
|
(132,534 | ) | (51,635 | ) | ||||
|
Other payables and accrued liabilities
|
5,187 | 31,006 | ||||||
|
Income taxes payable
|
326,841 | (398,520 | ) | |||||
|
Other taxes payable
|
(1,103,146 | ) | (582,852 | ) | ||||
|
Net cash provided by operating activities
|
5,641,687 | 1,277,991 | ||||||
|
Cash flows used in investing activities
|
||||||||
|
Purchases of property, plant and equipment
|
(9,063 | ) | (119,219 | ) | ||||
|
Net cash used in investing activities
|
(9,063 | ) | (119,219 | ) | ||||
|
Cash flows from financing activities
|
||||||||
|
Short-term loan
|
365,001 | 438,389 | ||||||
|
Bank acceptance
|
(219,000 | ) | - | |||||
|
Due from shareholders
|
24,484 | 1,607,428 | ||||||
|
Due to related parties
|
132,940 | - | ||||||
|
Dividend paid
|
(3,208,521 | ) | (3,441,779 | ) | ||||
|
Net cash used in financing activities
|
(2,905,096 | ) | (1,395,962 | ) | ||||
|
Net increase in cash and cash equivalents
|
2,727,528 | (237,190 | ) | |||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(12,852 | ) | 8,507 | |||||
|
Cash and cash equivalent, beginning of period
|
7,455,147 | 5,188,587 | ||||||
|
Cash and cash equivalent, end of period
|
$ | 10,169,823 | $ | 4,959,904 | ||||
|
Supplemental disclosure of cash flow information
|
||||||||
|
Cash paid for interest
|
$ | 12,908 | $ | - | ||||
|
Cash paid for income taxes
|
$ | 2,743,066 | $ | 1,219,972 | ||||
See accompanying notes to consolidated financial statements.
F-22
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDER'S EQUITY
Six Months Period Ended June 30, 2010 and Year Ended December 31, 2009 (Restated)
|
Common Stock
|
Additional
|
Retained
|
Statuary
|
Accumulated
other
|
Subscription
|
Total
stockholder's
|
||||||||||||||||||||||||||
|
Shares
|
Amount
|
paid-in
capital
|
earnings
|
reserve
|
comprehensive
income
|
receivable
|
equity
|
|||||||||||||||||||||||||
|
Balance, January 1, 2009
|
50,000 | $ | 50,000 | $ | 2,458,223 | $ | 3,121,166 | $ | 367,238 | $ | 276,245 | $ | (50,000 | ) | $ | 6,222,872 | ||||||||||||||||
|
Net income
|
- | - | - | 10,901,402 | - | - | - | 10,901,402 | ||||||||||||||||||||||||
|
Dividend *
|
- | - | - | (6,872,202 | ) | - | - | - | (6,872,202 | ) | ||||||||||||||||||||||
|
Transfer to statutory reserve
|
- | - | - | (943,463 | ) | 943,463 | - | - | - | |||||||||||||||||||||||
|
Foreign currency translation adjustments
|
(42,248 | ) | - | (42,248 | ) | |||||||||||||||||||||||||||
|
Balance, December 31, 2009
|
50,000 | 50,000 | 2,458,223 | 6,206,903 | 1,310,701 | 233,997 | (50,000 | ) | 10,209,824 | |||||||||||||||||||||||
|
Net income
|
- | - | - | 5,695,751 | - | - | - | 5,695,751 | ||||||||||||||||||||||||
|
Dividend *
|
- | - | - | (10,812,639 | ) | - | - | - | (10,752,051 | ) | ||||||||||||||||||||||
|
Stock based compensation
|
1,492,100 | - | 1,492,100 | |||||||||||||||||||||||||||||
|
Foreign currency translation adjustments
|
32,226 | - | (28,362 | ) | ||||||||||||||||||||||||||||
|
Balance, June 30, 2010
|
50,000 | $ | 50,000 | $ | 3,950,323 | $ | 1,090,015 | $ | 1,310,701 | $ | 266,223 | $ | (50,000 | ) | $ | 6,617,262 | ||||||||||||||||
* Dividend paid to former stockholders before Reverse Merger
See accompanying notes to consolidated financial statements.
F-23
GLOBAL PHARMA ENTERPRISE GROUP LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2010 and December 31, 2009
|
1.
|
Nature of Operations and Summary of Significant Accounting Policies
|
Global Pharma Enterprise Group Limited (“Global Pharma BVI” or the “Company”) was incorporated on June 14, 2010 under the laws of the British Virgin Island and is a holding company. The Company conducts the wholesale and distribution of pharmaceuticals-related products, Chinese herb cultivation and medicine raw materials preparations through three operating subsidiaries located in China. On June 29, 2010 Global Pharma BVI acquired all of the outstanding shares of Bionmial Biopharm Group Limited (“Bionmial”), Incorporated under the laws of Hong Kong on September 9, 2009 and Hong Kong Wisdom Fortune Medicine Holding Group Limited, (“”Wisdom Fortune”), formally Aecko Industrial Limited, incorporated under the laws of Hong Kong.
Tonghua Tongdetang Pharmaceutical Company (“Tongdetang”) was incorporated on February 2, 2002, under the laws of the People’s Republic of China (“PRC”). Anhui Xuelingxian Pharmaceutical (“Xuelingxian”) was incorporated on July 23, 2008, under the laws of the PRC. Shandong Yaoyuan Pharmaceutical Company (“Yaoyuan”) was incorporated on November 1, 2005, under the laws of the PRC.
On May 6, 2010 and May 8, 2010, Wisdom Fortune and Binomial, respectively, entered into Equity Transfer Agreements, with the shareholders of Yaoyuan, Xuelingxian and Tongdetang. Global Pharma BVI paid RMB 5,180,000 (approximately US$ 700,418), RMB 3,000,000 (approximately US $405,647), and RMB10,000,000 (approximately US$1,352,158) to the shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan, respectively, representing their registered capital at the time (referred to as the Equity Transfers). The Equity transfers by the PRC operating companies, pursuant to the Earn In Agreement, as thereafter amended, (and as further described below), was considered to be a transaction between entities under common control.
As a result of the Equity Transfers, Binomial currently holds all the equity interests in Xuelingxian and Tongdetang. Prior to the Equity Transfer, the shareholders of Xuelingxian were Yunlu Yin, Dandan Wang, Shulan Li, and Hong Zhang. The shareholders of Tongdetang were Yunlu Yin, Qingdong Zeng, and Feng Jin. Mei Li Tsai was the sole shareholder of Binomial from its inception until Global Pharma BVI acquired Binomial on June 29, 2010.
In addition, as a result of the Equity Transfers, Wisdom Fortune currently holds all the equity interest in Yaoyuan. Prior to the Equity Transfer, the shareholders of Yaoyuan were Yunlu Yin, Shouqiang Han, Yanming Lv, Guojun Zhao, Junyan Su, Boliang Zhu, and Chaobo Song. Mei Li Tsai was the sole shareholder of Wisdom Fortune from its inception until Global Pharma BVI acquired Wisdom Fortune on June 29, 2010.
On May 25, 2006 and April 15,2010, Yunlu Yin signed the Trust Agreements with Qinghui Zeng and Qingdong Zeng, respectively, documenting Yunlu Yin as the 62% of owner of Tongdetang and documenting each of Qinghui Zeng and Qingdong Zeng to act as the nominee owner of Tongdetang on behalf of Yunlu Yin. On May 31, 2008 and March 20, 2010 Yunlu Yin signed the Trust Agreements with Yanliang Song and Shouqiang Han, respectively, documenting Yunlu Yin as 80% of owner of Yaoyuan and documenting each of Yanliang Song and Shouqing Han to act as the nominee owner of Yaoyuan on behalf of Yunlu Yin. On September 5, 2009 and April 10, 2010, Yunlu Yin signed the Trust Agreements with Shunli Wang and Shulan Li and Hong Zhang, respectively, documenting Yunlu Yin as 65% of owner of Xuelingxian and documenting each of Shunli Wang and Shulan Li and Hong Zhang to act as the nominee owner of Xuelingxian on behalf of Yunlu Yin.
F-24
On June 29, 2010 the sole Shareholder of the Company entered into an Earn in Agreement, as thereafter amended, (and as further described below), with the former shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan. Pursuant to the agreement, the Company shall entered into share exchange agreement, at a date after this agreement, with a United States domiciled shell company an at that time the former shareholders, including beneficial owners, will have call rights to own the controlling interest in a Publicly held Company.
After the Company entered into and consummated the Share Exchange Agreement with Top Flight GameBirds, Inc., a U.S. public company on August 6, 2010, pursuant to the Earn-In Agreement, as thereafter amended, the shareholders, including the beneficial owners of Xuelingxian, Tongdetang, and Yaoyuan, and the key management of the Company will have the right to purchase 20,894,000 shares at four different occurrence dates, contingent on various targets for total consideration of $300,000. Targets include binding three-year employment contracts with various members of management with six months of this agreements and target after tax net income of $3.6 million, $3.8 million, and $15.2 million for the three months ended June 30, 2010 and September 30, 2010, and for the 12 months ended December 31, 2010, respectively. The non-cash expenses would be excluded from the calculation of the net income. Upon execution the former shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan will have call rights to own 80.36% of the publicly held Company.
As part of these restructuring transactions, no new capital was introduced. As a result, no new basis in the net assets of the PRC Subsidiaries was established. During this restructuring, management continued to serve and continued to direct both the day-to-day operating and management of the PRC Subsidiaries, as well as their strategic direction. Because of this operating and management control and because the restructuring plan effectively resulted in the Company continuing to bear the residual risks and rewards related to the PRC Subsidiaries, the Company consolidated the PRC Subsidiaries during the restructuring. The Company’s acquisition of the PRC Subsidiaries, which represented the return to legal ownership of the PRC Subsidiaries by the Company, represented a transaction between related parties under common control and did not establish a new basis in the assets and liabilities of the PRC Subsidiaries. The Earn-In Agreement, as thereafter amended, enables former shareholders to regain ownership of the Company’s shares originally transferred by them to the Company as part of the restructuring arrangements and, accordingly, the Company does not consider the reacquisition of those shares to represent compensation cost to the Company. The Company accounted for the shares earned by management as share-based compensation and recognized $5,968,400 of compensation expense.
Basis of Presentation
The consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America ("U.S. GAAP").
Principles of Consolidation
The consolidated financial statements include the accounts of Global Pharma Enterprise Group Limited and its direct and indirect wholly owned subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
Foreign Currency Translation
The Company has its local currency, Renminbi (“RMB”), as its functional currency. The consolidated financial statements of the Company are translated from RMB into US$. Accordingly, all assets and liabilities are translated at the exchange rates prevailing at the balance sheet dates, all income and expenditure items are translated at the average rates for each of the periods and equity accounts, except for retained earnings, are translated at the rate at the transaction date. Retained earnings reflect the cumulative net income (loss) translated at the average rates for the respective periods since inception less dividends translated at the rate at the transaction date.
RMB is not a fully convertible currency. All foreign exchange transactions involving RMB must take place either through the People's Bank of China (the "PBOC") or other institutions authorized to buy and sell foreign exchange. The exchange rates adopted for the foreign exchange transactions are the rates of exchange quoted by the PBOC, which are determined largely by supply and demand. Translation of amounts from RMB into US$ has been made at the following exchange rates for the respective years:
F-25
|
Six months ended June 30,
|
||||||
|
2010
|
2009
|
|||||
|
Assets and liabilities
|
1 US $ : 6.84930 RMB
|
1 US $ : 6.82590 RMB
|
||||
|
Statement of income
|
1 US $ : 6.84930 RMB
|
1 US $ : 6.83347 RMB
|
||||
The resulting translation adjustments are recorded as other comprehensive income in the consolidated statement of stockholders equity and comprehensive income and as a separate component of stockholders equity.
Commencing from July 21, 2005, China adopted a managed floating exchange rate regime based on market demand and supply with reference to a basket of currencies. Since then, the PBOC administers and regulates the exchange rate of US$ against RMB taking into account the demand and supply of RMB, as well as domestic and foreign economic and financial conditions.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements. The reported amounts of revenues and expenses during the reporting period may be affected by the estimates and assumptions we are required to make. Estimates that are critical to the accompanying consolidated financial statements relate primarily to returns, sales allowances and customer chargebacks, and the valuation of long-lived assets. Estimates and assumptions are reviewed periodically and the effects of revisions are reflected in the period that they are determined to be necessary. Actual results could differ from these estimates.
Comprehensive Income
The Company follows the Comprehensive Income Topic of the FASB Accounting Standard Codification (“ASC 220 Comprehensive Income”). Comprehensive income is a more inclusive financial reporting methodology that includes disclosure of certain financial information that historically has not been recognized in the calculation of net income.
The Company’s only component of other comprehensive income is foreign currency translation gains and losses. The foreign currency translation gains (losses) for the six months ended June 30, 2010 and 2009 were $32,226 and $240,720 respectively. Accumulated other comprehensive income is recorded as a separate component of stockholders’ equity.
Cash and Cash Equivalents
Cash and cash equivalents comprise cash in banks and on hand, demand deposits with banks and other financial institutions, and short-term, highly liquid investments which are readily convertible into known amounts of cash and which are subject to an insignificant risk of changes in value, having been within three months of maturity at acquisition.
Restricted Cash
Restricted cash represents amounts held by a bank as security for bank acceptance notes and loan collateral and therefore is not available for the Company’s use in operations.
Accounts Receivable
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. There are no allowances for doubtful accounts at June 30, 2010 and December 31, 2009.
F-26
Inventories
Inventories are stated at the lower of cost (determined on a weighted-average basis) or market value. Work-in-progress is composed of direct materials, direct labor, manufacturing overhead and an attributable portion of land rental cost that has been capitalized as a component of inventory. The capitalized land rental cost will be recorded in cost of goods sold at such time the goods are sold. An allowance is established when management determines that certain inventories may not be saleable. If inventory costs exceed expected market value due to obsolescence or quantities in excess of expected demand, the Company will record reserves for the difference between the cost and the market value. These reserves are recorded based on estimates and reflected in cost of sales. There are no inventory reserves as of June 30, 2010 and December 31, 2009, respectively.
Long-Lived Assets
The Company estimates the future undiscounted cash flows to be derived from an asset to assess whether or not a potential impairment exists when events or circumstances indicate the carrying value of a long-lived asset may be impaired. If the carrying value exceeds the Company’s estimate of future undiscounted cash flows, the Company then calculates the impairment as the excess of the carrying value of the asset over the Company’s estimate of its fair market value.
Lease Obligations
All non-cancellable leases with an initial term greater than one year are categorized as either capital leases or operating leases. Assets recorded under capital leases are amortized according to the methods employed for property and equipment or over the term of the related lease, if shorter.
Property, Plant and Equipment
Property and equipment are stated at cost. Expenditures for maintenance and repairs are charged to earnings as incurred; additions, renewals and betterments are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided using the straight-line method for substantially all assets with estimated lives of:
|
Buildings
|
30 years
|
|
|
Furniture, fixtures and equipment
|
5 years
|
|
|
Motor vehicles
|
5 years
|
|
|
Office equipment
|
5 years
|
|
|
Plant and machinery
|
|
5 to 15 years
|
Revenue Recognition
The Company’s revenue recognition policies are in compliance with Staff Accounting Bulletin 104. Sales revenue is recognized when all of the following have occurred: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the price is fixed or determinable and (iv) the ability to collect is reasonably assured. These criteria are generally satisfied at the time of shipment when risk of loss and title passes to the customer. The Company’s four segments have the same revenue recognition policy. Revenues are recorded net of value-added taxes.
Shipping and Handling Expense
Shipping and handling costs are expenses as incurred and are included in cost of goods sold.
Advertising Costs
Advertising costs are expensed in the period in which the advertisements are first run or over the life of the endorsement contract. Advertising expense for the six months ended June 30, 2010 and 2009 were approximately $20,834 and $1,092, respectively. Advertising costs include advertising subsidy expense which is accrued based on the terms in effect with distributors and paid when all attaching conditions have been completed.
F-27
Income Taxes
The Company is subject to the Income Tax Law of the People’s Republic of China. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases and tax loss carry forwards. Any deferred tax assets and liabilities would be measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The statutory rate under the laws of the PRC is 25%. The Company’s herbal cultivation segment is subjected to zero income taxes.
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates applicable to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
The Company has not been subjected to income tax examinations by taxing authorities for the six months ended June 30, 2010 and 2009. The Company is open to tax examination in the PRC for all years, as tax returns remain open to examination until notified by the taxing authorities, and the Company has not received any notifications to date. The Company records interest and penalties as other expense on the consolidated statements of income and other comprehensive income. During the years ended December 31, 2010 and 2009, the Company did not recognize any amount in interest and penalties.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are cash and accounts receivable arising from its normal business activities. The Company places its cash in what it believes to be creditworthy financial institutions. The Company has a diversified customer base. The Company controls credit risk related to accounts receivable through credit approvals, credit limits and monitoring procedures. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk, establishes an allowance, if required, for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is limited. As of June 30, 2010 and December 31, 2009, there was no allowance for uncollectible accounts as previously discussed.
Fair Value of Financial Instruments
The carrying amounts of the Company’s financial instruments, which principally include accounts receivable and accounts payable, approximate their fair values due to the relatively short maturity of such instruments.
The carrying amount of the Company’s short-term borrowings approximates their fair value based upon current rates and terms available to the Company for similar debt.
Basic and Diluted Earnings per Share
The Company reports earnings per share in accordance with FASB ASC-260, Earnings per Share. The Company’s basic earnings per share is computed using the weighted-average number of shares outstanding for the periods presented.
Diluted earnings per share is based on the assumption that any dilutive options, warrants or other instruments were converted or exercised. Dilution is computed by applying the treasury stock method. Under this method, the Company’s outstanding stock options are assumed to be exercised, and funds thus obtained were assumed to be used to purchase common stock at the average market price during the period. As of June 30, 2010 and December 31, 2009, the Company has not dilutive securities
F-28
Stock-Based Compensation
The Company uses the fair value recognition provision of ASC Topic 718, Compensation-Stock Compensation (formerly named as SFAS 123(R)), which requires the Company to expense the cost of employee services received in exchange for an award of equity instruments based on the grant date fair value of such instruments over the vesting period.
The Company records all share-based payments, including grants of common shares and stock options to employees, in the consolidated financial statements based on their fair values on date of grant. The grant is expensed over the required service period, which is generally equal to the vesting period.
Recent Accounting Pronouncements
In December 2010, the FASB issued ASU 2010-29 which addresses diversity in practice about the interpretation of the pro forma revenue and earnings disclosure requirements for business combinations (Topic 805). This ASU specifies that if a public entity presents comparative financial statements, the entity should disclose revenue and earnings of the combined entity as though the business combination(s) that occurred during the current year had occurred as of the beginning of the comparable prior annual reporting period only. This ASU also expands the supplemental pro forma disclosures under Topic 805 to include a description of the nature and amount of material, non-recurring pro forma adjustments directly attributable to the business combination included in the reported pro forma revenue and earnings. ASU 2010-29 is effective prospectively for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2010. Early adoption is permitted. The Company is currently evaluating the impact of this ASU and expected the adoption of this ASU will have an impact on its future business combinations.
|
2.
|
Inventories
|
At June 30, 2010 and December 31, 2009, inventories consist of the following:
|
June 30, 2010
|
December 31,
2009
|
|||||||
|
Raw materials (1)
|
$
|
1,484,114
|
$
|
1,401,865
|
||||
|
Work-in-progress (2)
|
3,676,593
|
-
|
||||||
|
Finished goods (3)
|
9,590,279
|
7,971,897
|
||||||
|
$
|
14,750,986
|
$
|
9,373,762
|
|||||
(1) Raw materials include the herbal material that will be used to produce the flower tea and the OEM product.
(2) Work-in-process is composed of direct materials, direct labor, an attributable portion of land lease cost that has been capitalized, and an attributable portion of manufacturing overhead.
(3) Finished goods only consist of pharmaceutical products.
|
3.
|
Property, plant and equipment
|
At June 30, 2010 and December 31, 2009, property, plant and equipment consist of the following:
|
June 30, 2010
|
December 31,
2009
|
|||||||
|
Operating equipment
|
$
|
221,640
|
$
|
219,321
|
||||
|
Office equipment
|
80,923
|
73,382
|
||||||
|
Transportation equipment
|
10,293
|
10,267
|
||||||
|
Leasehold improvements
|
50,220
|
50,093
|
||||||
|
Sub-total
|
363,076
|
353,063
|
||||||
|
Less: Accumulated depreciation
|
(156,935
|
)
|
(123,476
|
)
|
||||
|
Property, plant and equipment, net
|
$
|
203,705
|
$
|
229,587
|
||||
F-29
Depreciation expense was $29,169 and $23,007 for the six-month periods ended June 30, 2010 and 2009, respectively.
|
4.
|
Intangible Assets
|
The Company uses the “Yaoyuan” trademark for drug packaging that is owned by Yanliang Song, a former shareholder of Yaoyuan. The trademark was applied for on May 16, 2008 and approved on April 7, 2010 and was subsequently transferred to Yaoyuan without cost or value. The trademark will expire on April 6, 2020. The cost of the trademark for Yanliang Song is less than $200 and the Company is not required to pay Yanliang Song any license fee for the use of the trademark. The Company also believes this amount is immaterial.
The Company has the exclusive right to use the “Xuelingxian” trademark for pharmaceutical products, which is owned by Jingsheng Wang, a former shareholder of Xuelingxian, pursuant to a license agreement dated January 1, 2009 for a term from January 1, 2009 to January 1, 2014. The cost of the trademark for Jingsheng Wang is less than $200 and the Company does not have to pay Jingsheng Wang any license fee for the use of the trademark. The Company also believes this amount is immaterial.
|
5.
|
Short-term Loans
|
The Company entered into a short-term loan on December 23, 2009 with Shandong Qilu bank. The loan amount is $219,000 and $219,751 at June 30, 2010 and December 31, 2009, respectively, and is due on December 21, 2010. Term of this loan calls for monthly principal repayment plus interest at the one-year commercial loan rate issued by the People’s Bank of China plus 40% (7.434% at December 23, 2009) each month.
The Company entered an entrustment guarantee contract with Kexin Fengda Investment Guarantee Co, Ltd (“Kexin Fengda”) whereby Kexin Fengda guarantees the note payable to Shandong Qilu bank. Kexin Fengda received payment of approximately $6,000 in consideration for this guarantee.
The chairman of the Company provided counter-guarantee to Kexin Fengda using all his personal/family assets.
The Company entered into a short-term loan on March 22, 2010 with Shandong Qilu Bank. The loan amount is $219,000 at June 30, 2010 and is due on March 22, 2011. Term of this loan calls for monthly principal repayment plus interest at the one-year commercial loan rate issued by the People’s Bank of China plus 20% (6.372% at March 22, 2010) each month.
The Company entered a pledge agreement with Qilu Bank, which requested at least 6 million RMB of inventories to secure the loan. The pledge is valid from March 22, 2010 to March 22, 2011. Shandong Qilu Bank has authorized Shandong Woerde Guarantee Company to supervise the pledged inventories.
On May 7, 2010, the Company entered into a credit agreement with Construction Bank of China in the amount of $151,704. As part of the loan agreement, RMB 4,000,000 or $606,815 of property and land of two shareholders and Bozhou City Herb Research Institute guarantee the outstanding principal balance to the bank over the term of the loan.
The interest expense was $12,908 and nil for the periods ended June 30, 2010 and 2009, respectively.
|
6.
|
Bank Acceptance
|
The Company has a bank acceptance with Shandong Qilu bank in the amount of $219,751 as of December 31, 2009. The term is six-month and bearing non-interest. The balance outstanding at December 31, 2009 was $219,751.
The Company has a bank acceptance with Shandong Qilu Bank in the amount of $219,000 at June 30, 2010. The one-year line-of-credit commenced on March 22, 2010 and bears no interest and expires March 22, 2011. The Company does not have an outstanding balance at June 30, 2010.
F-30
|
7.
|
Related Party Transactions
|
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operational decisions. Parties are also considered to be related if they are subject to common control or common significant influence.
|
|
·
|
On May 25, 2006 and April 15,2010, Yunlu Yin signed the Trust Agreements with Qinghui Zeng and Qingdong Zeng, respectively, documenting Yunlu Yin as the 62% of owner of Tongdetang and documenting each of Qinghui Zeng and Qingdong Zeng to act as the nominee owner of Tongdetang on behalf of Yunlu Yin. On May 31, 2008 and March 20, 2010 Yunlu Yin signed the Trust Agreements with Yanliang Song and Shouqiang Han, respectively, documenting Yunlu Yin as 80% of owner of Yaoyuan and documenting each of Yanliang Song and Shouqing Han to act as the nominee owner of Yaoyuan on behalf of Yunlu Yin. On September 5, 2009 and April 10, 2010, Yunlu Yin signed the Trust Agreements with Shunli Wang and Shulan Li and Hong Zhang, respectively, documenting Yunlu Yin as 65% of owner of Xuelingxian and documenting each of Shunli Wang and Shulan Li and Hong Zhang to act as the nominee owner of Xuelingxian on behalf of Yunlu Yin.
|
|
|
·
|
During each of the years ended February 28, 2010 and February 28, 2009, our former President, Chief Executive Officer, Chief Financial Officer, sole director and chairwoman, Rhonda Heskett contributed services and brooding facilities with a fair value of $6,000 and $2,400, respectively. These non-cash expenses totaling $8,400 in each of the years ended February 28, 2010 and 2009 were treated as contributed capital.
|
|
|
·
|
On July 23 and September 24, 2010, our former shareholder, Shunli Wang, lent RMB 5,000,000 and RMB 600,000 (totally, $836,058) respectively, to Xuelingxian as working capital and the balance are non-interest bearing and payable on demand. The balance was paid off on December 25, 2010.
|
|
|
·
|
As of December 31, 2009 and 2008, Global Pharma BVI had $24,568 and $1,465,738, respectively due from related parties. In 2009, Global Pharma BVI advanced $24,568 to Yanliang Song, an officer of Yaoyuan and the balance is nil at June 30, 2010. In 2008, Xianming Zeng and Qingdong Zeng, former stockholders of Tongdetang, borrowed RMB 6,000,000 and RMB 4,000,000 (totally, $1,465,738), respectively, from Tongdetang and such balance was offset in 2009 when Tongdetang declared dividends in the amount of RMB 6,000,000 and RMB 4,000,000, respectively, to Xianming Zeng and Qingdong Zeng.
|
|
|
·
|
At June 30, 2010 and December 31, 2009, the Company had $33,926 and $24,568, respectively, due from related parties. These balances are non-interest bearing and payable upon demand.
|
|
|
·
|
At June 30, 2010, Yunlu Yin, our Chairman, Chief Executive Officer and director, made advances for business expenses in an amount of $132,717. The advances were non-interest bearing and payable on demand. . No monies were due at December 31, 2009.
|
|
|
·
|
Global Pharma BVI uses a trademark for drug packaging that is owned by a director and former shareholder of Yaoyuan, Yanliang Song. The trademark was applied for on May 16, 2008 and approved April 7, 2010. It expires April 6, 2020. While Yanliang Song has paid for all associated costs, we use this trademark at no cost.
|
|
|
·
|
During 2009, Xuelingxian had sales of approximately $1.4 million to a number of related parties, and employees. The details of the transactions are set forth below:
|
|
Name
|
Relationship
|
Amount
|
||||
|
Fei Gao
|
General Manager
|
$ | 472,625 | |||
|
Wenhua Chou
|
General Manager
|
$ | 218,363 | |||
|
Jingzhong Wang
|
General Manager
|
$ | 445,381 | |||
|
Enhui Fan
|
General Manager
|
$ | 89,474 | |||
|
Fengyi Wang
|
General Manager
|
$ | 120,054 | |||
|
Min Xue
|
General Manager
|
$ | 74,458 | |||
|
Total
|
$ | 1,420,354 | ||||
F-31
|
|
·
|
For the years ended December 31, 2009 and for the period ended June 30, 2010, we paid $8,190,085 and $3,208,521 to the former shareholders of our subsidiaries, respectively. On June 29, 2010, as part of May 6 and May 8, 2010 Equity Transfer Agreements, Wisdom Fortune and Binomial declared a dividend of approximately RMB 57,522,347 (approximately $8,438, 816) to its then stockholders.
|
|
|
·
|
The following table set forth the related party payable on the dividend announced to the former shareholders as of June 30, 2010 and December 31, 2009.
|
|
Related Party
|
Relationship
|
June 30, 2010
|
December 31, 2009
|
|||||||
|
Shouqiang Han
|
Stockholder
|
$ | 2,080,354 | $ | 370,006 | |||||
|
Qinghui Zeng
|
Stockholder
|
1,238,139 | 1,427,408 | |||||||
|
Xianming Zeng
|
Stockholder
|
619,070 | 713,704 | |||||||
|
Shunli Wang
|
Stockholder
|
3,617,298 | 93,948 | |||||||
|
Jingsheng Wang
|
Stockholder
|
1,004,516 | 26,089 | |||||||
|
Fulan Li
|
Stockholder
|
582,931 | 15,140 | |||||||
|
Others
|
Stockholder
|
109,492 | 19,474 | |||||||
|
Total
|
Stockholder
|
$ | 9,251,800 | $ | 2,665,768 | |||||
|
|
·
|
On June 29, 2010 the then sole shareholder of Global Pharma BVI entered into an Earn-In Agreement with the former shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan. Pursuant to the agreement, the Company shall entered into a share exchange agreement, at a date after this agreement, with a United States domiciled shell company an at that time the former shareholders, including beneficial owners, will have call rights town the controlling interest in the publicly held company.
|
|
|
·
|
On August 6, 2010, the Company was party to a share purchase agreement between our former President, Chief Executive Officer, Chief Financial Officer and sole director and Chairwoman, Rhonda Heskett and Mei Li Tsai involving the sale of 19,094,000 shares of common stock of the Company for a cash consideration of $450,000.
|
|
|
·
|
On August 12, 2010, Global Pharm Holdings Group, Inc. (f/k/a Top Flight Gamebirds, Inc. (“Top Flight”)) entered into and consummated a Share Exchange Agreement with Mei Li Tsai, the sole shareholder of Global Pharma BVI and Global Pharma BVI to acquire all the issued and outstanding capital stock of Global Pharma BVI, in consideration for 1,800,000 newly issued restricted shares of Top Flight (the “Reverse Merger”). On September 20, 2010, Top Flight changed its name to Global Pharm Holdings Group, Inc.
|
|
|
·
|
Pursuant to the Earn-In Agreement dated June 29, 2010, as thereafter amended, after Global Pharma BVI entered into and consummated a Share Exchange Agreement with Top Flight, the shareholders, including the beneficial owners of Xuelingxian, Tongdetang, and Yaoyuan, and the key management of the Company were given the right to purchase 20,894,000 shares at four different occurrence dates, contingent on various targets for total consideration of $300,000. Targets include binding three-year employment contracts with various members of management with six months of this agreements and target after tax net income of $3.6 million, $3.8 million, and $15.2 million for the three months ended June 30, 2010 and September 30, 2010, and for the 12 months ended December 31, 2010, respectively. Upon execution the former shareholders, including beneficial owners, of Xuelingxian, Tongdetang, and Yaoyuan would have call rights to own 80.36% of the publicly held company.
|
|
8.
|
Statutory Reserve
|
The laws and regulations of the PRC require that before a foreign invested enterprise can legally distribute profits, it must first satisfy all tax liabilities, provide for losses in previous years, and make allocations, in proportions determined at the discretion of the board of directors, to the statutory reserve. The statutory reserves include the surplus reserve fund.
F-32
The Company is required to transfer 10% of its net income, as determined in accordance with the PRC accounting rules and regulations, to a statutory surplus reserve fund until such reserve balance reaches 50% of the Company’s registered capital. The transfer to this reserve must be made before distribution of any dividends to shareholders. Transfers to the statutory surplus reserve fund were nil and $943,463 for the six months ended June 30, 2010 and year ended December 31, 2009, respectively. As of June 30, 2010, the Company has fulfilled the reserve requirement.
The surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance after such issue is not less than 50% of the registered capital.
|
9.
|
Stock-based Compensation
|
Pursuant to the Earn-In Agreement, as thereafter amended, the management team members have the rights to purchase 10.9% of ownership of the PRC subsidiaries as part of the restructuring arrangements. The Company treated the shares earned by the management as share-based compensation. The fair value of these shares granted to the management is valued at the fair market value of the PRC subsidiaries at the grant day, which accounted for $5,968,400 at June 29, 2010.
|
10.
|
Commitments
|
The Company leases various facilities under lease agreements ranging from month to month to fifteen-year terms. Rental expense for the periods ended June 30, 2010 and 2009 was approximately $654,000 and $590,000, respectively. Of these amounts, approximately $549,000 and $506,000 related to a land lease has been capitalized as a component of inventory and will be recorded in cost of sales at such time as goods are sold.
The future minimum obligations under the aforementioned agreements at June 30, 2010 are as follows:
During 2008, Yaoyuan entered into an employment contract with Zhao Guojun to act as deputy general manager. The annual salary is approximately $9,000 and the contract expires October 31, 2011.
|
11.
|
Concentrations
|
Two vendors accounted for approximately 43% and 46% of the Company’s purchases for the six and three months ended June 30, 2010, respectively, whereas one vendor accounted for approximately 16% and 12% of the Company’s purchases for the six and three months ended June 30, 2009, respectively.
Two vendors accounted for approximately 57% of the Company’s accounts payable at June 30, 2010 whereas one vendor accounted for approximately 26% of the Company’s accounts payable at December 31, 2009.
One customer accounted for approximately 12% and 14% of accounts receivable at June 30, 2010 and December 31, 2009, respectively. There were no customers who accounted for greater than 10% of accounts receivable at June 30, 2010.
|
12.
|
Segment Report
|
FASB ASC 280-10 requires the use of the management approach model for segment reporting. The management approach model is based on how a company's management organizes segments within the company for making operating decisions and assessing performance. Reportable segments are based on products and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company. Based on this model, the Company has determined that it has four segments. The Company’s principal businesses are herbal cultivation, TCM processing and distribution, flower tea sales, and distribution. Based on the various operation activities, the Company’s reportable segments are as follows:
F-33
|
¨
|
Pharmaceutical products distribution – the sale of healthcare products to hospitals and pharmacy shops.
|
|
¨
|
TCM processing and distribution – rough processing and sale of TCM product
|
|
¨
|
Herb cultivation and sales – the planting, processing and selling herbs in China.
|
|
¨
|
Flower tea bags – manufacture and sale of flower tea bags
|
We propose to add the segment reporting for the three and six months ended June 30, 2010, and 2009 as follows:
|
For the three months ended June 30, 2010
|
||||||||||||||||||||||||
|
Herb cultivation
and sales
|
TCM processing and
distribution
|
Flower tea
bags
|
Pharmaceutical
products
distribution
|
Corporate
|
Total
|
|||||||||||||||||||
|
Sales
|
- | 6,236,530 | 1,869,342 | 22,425,105 | - | 30,530,977 | ||||||||||||||||||
|
Interest income
|
- | 3,614 | 3,436 | 13,881 | - | 20,932 | ||||||||||||||||||
|
Interest expense
|
- | - | - | (7,706 | ) | - | (7,706 | ) | ||||||||||||||||
|
Depreciation
|
- | 6,293 | 2,216 | 8,771 | - | 17,281 | ||||||||||||||||||
|
Income tax
|
- | 283,717 | 85,751 | 886,214 | - | 1,255,682 | ||||||||||||||||||
|
Net income
|
- | 782,239 | 321,817 | 2,729,146 | (1,562,603 | ) | 2,270,599 | |||||||||||||||||
|
Total assets
|
3,676,593 | 7,490,662 | 1,605,968 | 26,727,557 | 61,818 | 39,562,598 | ||||||||||||||||||
|
Expenditures for segment assets
|
- | - | - | - | - | - | ||||||||||||||||||
|
For the three months ended June 30, 2009
|
||||||||||||||||||||||||
|
Herb cultivation
and sales
|
TCM processing and
distribution
|
Flower tea
bags
|
Pharmaceutical
products
distribution
|
Corporate
|
Total
|
|||||||||||||||||||
|
Sales
|
- | 4,000,529 | 1,675,868 | 13,513,359 | - | 19,189,756 | ||||||||||||||||||
|
Interest income
|
- | 2,148 | 1,198 | 3,103 | - | 6,450 | ||||||||||||||||||
|
Interest expense
|
- | - | - | - | - | - | ||||||||||||||||||
|
Depreciation
|
- | 3,613 | 995 | 7,553 | - | 12,162 | ||||||||||||||||||
|
Income tax
|
- | 204,712 | 82,549 | 519,985 | - | 807,246 | ||||||||||||||||||
|
Net income
|
- | 605,751 | 240,919 | 1,559,950 | - | 2,406,621 | ||||||||||||||||||
|
Total assets
|
3,843,288 | 7,399,903 | 1,630,014 | 18,253,611 | - | 31,126,816 | ||||||||||||||||||
|
Expenditures for segment assets
|
- | - | - | 7,756 | - | 7,756 | ||||||||||||||||||
|
For the six months ended June 30, 2010
|
||||||||||||||||||||||||
|
Herb cultivation
and sales
|
TCM processing
and distribution
|
Flower tea
bags
|
Pharmaceutical
Products
distribution
|
Corporate
|
Total
|
|||||||||||||||||||
|
Sales
|
- | 13,200,603 | 2,886,405 | 43,172,628 | - | 59,259,637 | ||||||||||||||||||
|
Interest income
|
- | 7,675 | 4,356 | 18,751 | - | 30,782 | ||||||||||||||||||
|
Interest expense
|
- | - | - | (12,908 | ) | - | (12,908 | ) | ||||||||||||||||
|
Depreciation
|
- | 7,543 | 4,415 | 17,211 | - | 29,169 | ||||||||||||||||||
|
Income tax
|
- | 610,316 | 133,450 | 1,675,684 | - | 2,419,451 | ||||||||||||||||||
|
Net income
|
- | 1,701,364 | 529,936 | 5,027,054 | (1,562,603 | ) | 5,695,751 | |||||||||||||||||
|
Total assets
|
3,676,593 | 7,490,662 | 1,605,968 | 26,727,557 | 61,818 | 39,562,598 | ||||||||||||||||||
|
Expenditures for segment assets
|
- | - | 1,752 | 7,311 | - | 9,063 | ||||||||||||||||||
F-34
|
For the six months ended June 30, 2009
|
||||||||||||||||||||||||
|
Herb cultivation
and sales
|
TCM processing
and distribution
|
Flower tea
bags
|
Pharmaceutical
products
Distribution
|
Corporate
|
Total
|
|||||||||||||||||||
|
Sales
|
- | 7,604,390 | 1,675,057 | 29,673,530 | - | 38,952,977 | ||||||||||||||||||
|
Interest income
|
- | 2,438 | 204 | 10,365 | - | 13,007 | ||||||||||||||||||
|
Interest expense
|
- | - | - | - | - | - | ||||||||||||||||||
|
Depreciation
|
- | 7,226 | 1,991 | 13,790 | - | 23,007 | ||||||||||||||||||
|
Income tax
|
- | 374,573 | 82,509 | 1,162,775 | - | 1,619,857 | ||||||||||||||||||
|
Net income
|
- | 1,111,342 | 244,801 | 3,488,326 | - | 4,844,469 | ||||||||||||||||||
|
Total assets
|
3,843,288 | 7,399,903 | 1,630,014 | 18,253,611 | - | 31,126,816 | ||||||||||||||||||
|
Expenditures for segment assets
|
- | 75,444 | 34,541 | 9,234 | - | 119,219 | ||||||||||||||||||
|
13.
|
Restatement
|
Subsequent to the issuance of Global Pharm Holdings Group, Inc.’s interim financial statements on Form 10-Q for the third quarter ended September 30, 2010, management discovered financial statement errors that caused an overstatement of the previously recorded net income for the Company’s three months and six months ended June 30, 2010 by $1,492,100. These errors have been corrected in this Form 8K/A. Global Pharma BVI is the wholly owned subsidiary of Global Pharm Holdings Group, Inc. (f/k/a Top Flight Gamebirds, Inc.) as a result of the Reverse Merger on August 12, 2010. The errors primarily related to the following:
|
|
·
|
Errors in the accounting for employee stock-based compensation and merger cost. These expenses had previously not been recorded in the Company’s financial statements. The fair value of the shares granted to the management team members was $5,968,400 at the grant date. The attributable portion of the stock-based compensation was $1,492,100 for the three months and six months ended June 30, 2010, respectively. The impact of this error had the effect of a increase in the general administrative expenses, decrease in net income.
|
|
|
·
|
Errors in the accounting for short-term loan. The $151,704 short-term loan had previously not been timely recorded in the Company’s financial statements. The impact of this error had no effect on net income and earning per common share.
|
The adjustment to net income (loss) for the three and nine months ended September 30, 2010 is summarized below:
|
Three months
|
Six months
|
|||||||||||||||
|
ended June 30, 2010
|
ended June 30, 2010
|
|||||||||||||||
|
Earning per
share
|
Earning per
share
|
|||||||||||||||
|
Net Income, as previously reported
|
$ | 3,762,699 | $ | 75 | $ | 7,187,851 | $ | 144 | ||||||||
|
Adjustment (pre-tax):
|
||||||||||||||||
|
Stock-based compensation
|
(1,492,100 | ) | (30 | ) | (1,492,100 | ) | (30 | ) | ||||||||
|
Tax effect of restatement adjustment
|
- | - | - | - | ||||||||||||
|
Net income, as restated
|
$ | 2,270,599 | $ | 45 | $ | 5,695,751 | $ | 114 | ||||||||
F-35
|
14.
|
Subsequent Events
|
On August 12, 2010, Global Pharma BVI entered into and consummated a Share Exchange Agreement with Global Pharm Holdings Group, Inc., f/k/a Top Flight Gamebirds, Inc. (“Global Pharm”). Pursuant to the Earn-In Agreement, as thereafter amended as described below, the shareholders, including the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan, and the key management of Global Pharma BVI were given the right to purchase 20,894,000 shares of Global Pharm at four different occurrence dates, contingent on various targets for total consideration of $300,000. As of March 29, 2011, Global Pharm entered into an Agreement to Amend the Earn-In Agreement (the “Amendment”) with the former shareholders and the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan and 20 individuals of its management who were parties to the original agreement. As of March 29, 2011, pursuant to the Amendment, all of the four earn-in targets have been achieved and the key management and former shareholders and the beneficial owners of Xuelingxian, Tongdetang and Yaoyuan hold, in the aggregate, 20,894,000 shares, or 80.36%, of Global Pharm’s shares of stock.
F-36
|
(b)
|
Pro forma financial information
|
The pro forma financial information concerning the acquisition of the business operations of our PRC Subsidiaries appears below.
|
Global Pharma
|
Top Flight
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Enterprise Group Limited
|
Game Birds, Inc.
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Consolidated Balance Sheets
|
Balance Sheets
|
Pro Forma
|
Pro Forma
|
|||||||||||||||||||||||||||||||||||||||||||||
|
12/31/09
|
12/31/08
|
06/30/10
|
02/28/10
|
02/28/09
|
06/30/10
|
Adjustments
|
Consolidated Balance Sheets
|
|||||||||||||||||||||||||||||||||||||||||
|
(Audited)
|
(Audited)
|
(Unaudited)
|
(Audited)
|
(Audited)
|
(Unaudited)
|
|||||||||||||||||||||||||||||||||||||||||||
|
ASSETS
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Current assets:
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Cash and cash equivalents
|
7,455,147 | 5,188,587 | 10,169,823 | 1,539 | 21,333 | 3,617 | (1,539 | ) | (21,333 | ) | (3,617 | ) | 7,455,147 | 5,188,587 | 10,169,823 | |||||||||||||||||||||||||||||||||
|
Accounts receivable
|
11,707,848 | 8,564,025 | 13,099,650 | - | - | - | - | - | - | 11,707,848 | 8,564,025 | 13,099,650 | ||||||||||||||||||||||||||||||||||||
|
Other current assets
|
- | 1,772 | 1,304,508 | - | - | - | - | - | - | - | 1,772 | 1,304,508 | ||||||||||||||||||||||||||||||||||||
|
Due from related parties
|
24,568 | 1,465,738 | 33,926 | - | - | - | 50,000 | 50,000 | 50,000 | 74,568 | 1,515,738 | 83,926 | ||||||||||||||||||||||||||||||||||||
|
Inventories
|
9,373,762 | 7,180,388 | 14,750,986 | 623 | - | - | (623 | ) | - | - | 9,373,762 | 7,180,388 | 14,750,986 | |||||||||||||||||||||||||||||||||||
|
Total current assets
|
28,561,325 | 22,400,510 | 39,358,893 | 2,162 | 21,333 | 3,617 | 47,838 | 28,667 | 46,383 | 28,611,325 | 22,450,510 | 39,408,893 | ||||||||||||||||||||||||||||||||||||
|
Property and equipment, net
|
229,587 | 134,020 | 203,705 | 173 | 597 | 67 | (173 | ) | (597 | ) | (67 | ) | 229,587 | 134,020 | 203,705 | |||||||||||||||||||||||||||||||||
|
Total assets
|
28,790,912 | 22,534,530 | 39,562,598 | 2,335 | 21,930 | 3,684 | 47,665 | 28,070 | 46,316 | 28,840,912 | 22,584,530 | 39,612,598 | ||||||||||||||||||||||||||||||||||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT)
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Current liabilities
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Short term loan
|
219,751 | - | 584,000 | - | - | 12,060 | - | - | (12,060 | ) | 219,751 | - | 584,000 | |||||||||||||||||||||||||||||||||||
|
Bank acceptance
|
219,751 | - | - | - | - | - | - | - | - | 219,751 | - | - | ||||||||||||||||||||||||||||||||||||
|
Accounts payable
|
13,598,744 | 11,075,591 | 21,060,188 | 22,212 | 21,316 | 19,859 | (22,212 | ) | (21,316 | ) | (19,859 | ) | 13,598,744 | 11,075,591 | 21,060,188 | |||||||||||||||||||||||||||||||||
|
Accrued salary
|
218,546 | 148,043 | 85,265 | - | - | - | - | - | - | 218,546 | 148,043 | 85,265 | ||||||||||||||||||||||||||||||||||||
|
Income taxes payable
|
944,143 | 707,401 | 1,267,758 | - | - | - | - | - | - | 944,143 | 707,401 | 1,267,758 | ||||||||||||||||||||||||||||||||||||
|
Other taxes payable
|
552,560 | 401,685 | 397,149 | - | - | - | - | - | - | 552,560 | 401,685 | 397,149 | ||||||||||||||||||||||||||||||||||||
|
Other accrued liabilities
|
161,825 | 23,014 | 166,459 | - | - | - | - | - | - | 161,825 | 23,014 | 166,459 | ||||||||||||||||||||||||||||||||||||
|
Due to related parties
|
2,665,768 | 3,955,924 | 9,384,517 | - | - | - | - | - | - | 2,665,768 | 3,955,924 | 9,384,517 | ||||||||||||||||||||||||||||||||||||
|
Total current liabilities
|
18,581,088 | 16,311,658 | 32,945,336 | 22,212 | 21,316 | 31,919 | (22,212 | ) | (21,316 | ) | (31,919 | ) | 18,581,088 | 16,311,658 | 32,945,336 | |||||||||||||||||||||||||||||||||
|
Stockholders' equity (deficit)
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Preferred stock, $0.001 par value per shares,10,000,000 shares authorized; none issued
|
- | - | - | - | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
|
Common stock, par value, $0.001 per share, 100,000,000 shares authorized; 24,200,000 shares issued and outstanding
|
- | - | - | 24,200 | 24,200 | 24,200 | (5,106 | ) | (5,106 | ) | 1,800 | 19,094 | 19,094 | 26,000 | ||||||||||||||||||||||||||||||||||
|
Common stock, par value, $1 per share, 50,000 shares authorized,
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
50,000 shares issued and outstanding
|
50,000 | 50,000 | 50,000 | (50,000 | ) | (50,000 | ) | (50,000 | ) | - | - | - | ||||||||||||||||||||||||||||||||||||
|
Stock subscription receivable
|
(50,000 | ) | (50,000 | ) | (50,000 | ) | 50,000 | 50,000 | 50,000 | - | - | - | ||||||||||||||||||||||||||||||||||||
|
Retistered capital
|
2,458,223 | 2,458,223 | 3,950,323 | (2,458,223 | ) | (2,458,223 | ) | (3,950,323 | ) | - | - | - | ||||||||||||||||||||||||||||||||||||
|
Additional paid-in capital
|
83,000 | 74,600 | 85,100 | 2,406,129 | 2,414,529 | 3,889,223 | 2,489,129 | 2,489,129 | 3,974,323 | |||||||||||||||||||||||||||||||||||||||
|
Statutory surplus reserves
|
1,310,701 | 367,238 | 1,310,701 | - | - | - | - | - | - | 1,310,701 | 367,238 | 1,310,701 | ||||||||||||||||||||||||||||||||||||
|
Retained earnings (Accumulated deficit)
|
6,206,903 | 3,121,166 | 1,090,015 | (127,077 | ) | (98,186 | ) | (137,535 | ) | 127,077 | 98,186 | 137,535 | 6,206,903 | 3,121,166 | 1,090,915 | |||||||||||||||||||||||||||||||||
|
Accumulated other comprehensive income
|
233,997 | 276,245 | 266,223 | - | - | - | - | - | - | 233,997 | 276,245 | 266,223 | ||||||||||||||||||||||||||||||||||||
|
Total stockholders' equity (deficit)
|
10,209,824 | 6,222,872 | 6,617,262 | (19,877 | ) | 614 | (28,235 | ) | 69,877 | 49,386 | 78,235 | 10,259,824 | 6,272,872 | 6,667,262 | ||||||||||||||||||||||||||||||||||
|
Total liabilities and stockholders' equity (deficit)
|
28,790,912 | 22,534,530 | 39,562,598 | 2,335 | 21,930 | 3,684 | 47,665 | 28,070 | 46,316 | 28,840,912 | 22,584,530 | 39,612,598 | ||||||||||||||||||||||||||||||||||||
F-37
|
Global Pharma
|
Top Flight
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Enterprise Group Limited
|
Game Birds, Inc.
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Statements of Income and Comprehensive
Income
|
Statements of Income and
Comprehensive Income
|
Pro Forma
|
||||||||||||||||||||||||||||||||||||||||||||||
|
For the year/period ended
|
For the year/period ended
|
Pro Forma
|
Statements of Income and
|
|||||||||||||||||||||||||||||||||||||||||||||
|
12/31/09
|
12/31/08
|
06/30/10
|
02/28/10
|
02/28/09
|
06/30/10
|
Adjustments
|
Comprehensive Income
|
|||||||||||||||||||||||||||||||||||||||||
|
(Audited)
|
(Audited)
|
(Unaudited)
|
(Audited)
|
(Audited)
|
(Unaudited)
|
(Unaudited)
|
(Unaudited)
|
(Unaudited)
|
(Unaudited)
|
(Unaudited)
|
(Unaudited)
|
|||||||||||||||||||||||||||||||||||||
|
Revenues, net
|
86,784,002 | 54,475,666 | 59,259,637 | 22,476 | 35,659 | 7,816 | (22,476 | ) | (35,659 | ) | (7,816 | ) | 86,784,002 | 54,475,666 | 59,259,637 | |||||||||||||||||||||||||||||||||
|
Cost of Goods Sold
|
70,725,013 | 45,362,590 | 48,632,162 | 3,151 | 29,566 | 623 | (3,151 | ) | (29,566 | ) | (623 | ) | 70,725,013 | 45,362,590 | 48,632,162 | |||||||||||||||||||||||||||||||||
|
Gross Profit
|
16,058,989 | 9,113,076 | 10,627,475 | 19,325 | 6,093 | 7,193 | (19,325 | ) | (6,093 | ) | (7,193 | ) | 16,058,989 | 9,113,076 | 10,627,475 | |||||||||||||||||||||||||||||||||
|
Expenses:
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Operating expenses
|
792,295 | 366,718 | 364,999 | - | - | - | - | - | - | 792,295 | 366,718 | 364,999 | ||||||||||||||||||||||||||||||||||||
|
General and administrative
|
1,096,333 | 648,520 | 2,165,148 | 48,216 | 82,658 | 17,516 | (48,216 | ) | (82,658 | ) | (17,516 | ) | 1,096,333 | 648,520 | 2,165,148 | |||||||||||||||||||||||||||||||||
|
Income (loss) from operations
|
14,170,361 | 8,097,838 | 8,097,328 | (28,891 | ) | (76,565 | ) | (10,323 | ) | 28,891 | 76,565 | 10,323 | 14,170,361 | 8,097,838 | 8,097,328 | |||||||||||||||||||||||||||||||||
|
Other Income (Expense)
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Interest income (Expense)
|
21,052 | 24,379 | 17,874 | - | - | (135 | ) | - | - | 135 | 21,052 | 24,379 | 17,874 | |||||||||||||||||||||||||||||||||||
|
Miscellaneous income (Expense)
|
8,266 | (3,294 | ) | - | - | - | - | - | - | - | 8,266 | (3,294 | ) | - | ||||||||||||||||||||||||||||||||||
|
Income before income taxes
|
14,199,679 | 8,118,923 | 8,115,202 | (28,891 | ) | (76,565 | ) | (10,458 | ) | 28,891 | 76,565 | 10,458 | 14,199,679 | 8,118,923 | 8,115,202 | |||||||||||||||||||||||||||||||||
|
Provision for Income Taxes
|
3,298,277 | 2,029,731 | 2,419,451 | - | - | - | - | - | - | 3,298,277 | 2,029,731 | 2,419,451 | ||||||||||||||||||||||||||||||||||||
|
Net Income (loss)
|
10,901,402 | 6,089,192 | 5,695,751 | (28,891 | ) | (76,565 | ) | (10,458 | ) | 28,891 | 76,565 | 10,458 | 10,901,402 | 6,089,192 | 5,695,751 | |||||||||||||||||||||||||||||||||
|
Other comprehensive income
|
- | - | - | |||||||||||||||||||||||||||||||||||||||||||||
|
Foreign currency translation adjustment
|
(42,248 | ) | 52,607 | 32,226 | - | - | - | - | - | - | (42,248 | ) | 52,607 | 32,226 | ||||||||||||||||||||||||||||||||||
|
Total comprehensive income
|
10,859,154 | 6,141,799 | 5,727,977 | (28,891 | ) | (76,565 | ) | (10,458 | ) | 28,891 | 76,565 | 10,458 | 10,859,154 | 6,141,799 | 5,727,977 | |||||||||||||||||||||||||||||||||
F-38
NOTES TO UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS
Note 1 – BASIS OF PRESENTATION
On August 6, 2010, Global Pharma Enterprise Group Limited (the “Company”) entered into a Share Exchange Agreement with Top Flight Game Birds, Inc. (“Top Flight”), whereby Top Flight acquired 100% of the issued and outstanding capital stock of the Company in exchange for 19,094,000 shares of the common stock of Top Flight. As a result of the reverse acquisition, the Company became Top Flight’s wholly owned subsidiary and the former shareholders of the Company became controlling stockholders of Top Flight. The share exchange transaction with Top Flight was treated as a reverse acquisition, with the Company as the accounting acquirer and Top Flight as the acquired party.
Consequently, the assets and liabilities and the historical operations that will be reflected in the consolidated financial statements for periods prior to the Share Exchange Agreement will be those of the Company and will be recorded at the historical cost basis. After the completion of the Share Exchange Agreement, the Company’s consolidated financial statements will include the assets and liabilities of the Company and Action, the historical operations of the Company and the operations of Top Flight from the closing date of the Share Exchange Agreement.
Top Flight had 24,200,000 common shares outstanding prior to the Share Exchange Agreement of which 19,094,000 were held by Mei Li Tsai, the sole shareholder of Global Pharma BVI prior to the Reverse Merger. For accounting purposes, the 19,094,000 shares held by Mei Li Tsai are assumed to have been outstanding on January 1, 2009 and the 5,106,000 held by existing shareholders of Top Flight prior to the execution of the Share Purchase Agreement on August 9, 2010 and the 1,800,000 shares issued in connection with the Share Exchange Agreement and Reverse Merger on August 12, 2010 are assumed to have been issued on those dates in exchange for the net assets of Top Flight.
These pro forma consolidated financial statements are prepared assuming the above transaction occurred on December 31, 2008 (as to the balance sheets) and on January 1, 2008 (as to the income statements).
Audited and unaudited financial statements of the Company and Top Flight have been used in the preparation of these pro forma consolidated financial statements. These pro forma consolidated financial statements should be read in conjunction with the historical financial statements of Top Flight and the Company.
F-39
|
Pro Forma Adjustments
|
Pro Forma Adjustments
|
Pro Forma Adjustments
|
|||||||||||||||||||||||
|
December 31, 2009
|
December 31, 2008
|
June 30, 2010
|
|||||||||||||||||||||||
|
1
|
Common stock
|
50,000 | 50,000 | 50,000 | |||||||||||||||||||||
|
Paid-in capital
|
2,458,223 | 2,458,223 | 3,950,323 | ||||||||||||||||||||||
|
Due from related party
|
50,000 | 50,000 | 50,000 | ||||||||||||||||||||||
|
Common stock
|
6,906 | 6,906 | - | ||||||||||||||||||||||
|
Common stock
|
1,800 | 1,800 | 1,800 | ||||||||||||||||||||||
|
Additional paid-in capital
|
2,513,329 | 2,513,329 | 3,998,523 | ||||||||||||||||||||||
|
Subscription receivable
|
50,000 | 50,000 | 50,000 | ||||||||||||||||||||||
|
2
|
Notes payable
|
- | - | 12,060 | |||||||||||||||||||||
|
Accounts payable
|
22,212 | 21,316 | 19,859 | ||||||||||||||||||||||
|
Additional paid-in capital
|
107,200 | 98,800 | 109,300 | ||||||||||||||||||||||
|
Cash
|
1,539 | 21,333 | 3,617 | ||||||||||||||||||||||
|
Inventory
|
623 | - | - | ||||||||||||||||||||||
|
Property and equipment
|
173 | 597 | 67 | ||||||||||||||||||||||
|
Accumulated deficit
|
127,077 | 98,186 | 137,535 | ||||||||||||||||||||||
|
1
|
To consolidate the equity of the Company through its elimination, into Top Flight GameBirds, Inc.
|
|
2
|
To recapitalize the Company, (or reverse merger), through the elimination of Top Flight GameBirds, Inc. cash, inventory, PPE, notes payable, and accounts payable.
|
|
(c)
|
Shell company transactions.
|
Reference is made to Items 9.01(a) and 9.01(b) above and the exhibits referred to therein, which are incorporated herein by reference.
|
(d)
|
The following exhibits are filed with this report:
|
|
Exhibit
Number
|
Description
|
|
|
3.1
|
Articles of Incorporation (1)
|
|
|
3.2
|
Bylaws (1)
|
|
|
3.3
|
Specimen of Common Stock Certificate(2)
|
|
|
10.1
|
Share Exchange Agreement, dated August 12, 2010, by and among Top Flight, Global Pharma BVI, and Mei Li Sai(2)
|
|
|
10.2
|
Loan Contract, dated December 23, 2009, by and between Yaoyuan and Qilu Bank(2)
|
|
|
10.3
|
Loan Contract, dated March 22, 2010, by and between Yaoyuan and Qilu Bank(2)
|
79
|
10.4
|
Lease Contract, by and between Xuelingxian and Bozhou Fengyi Chinese Medicine Development and Research Institute (2)
|
|
|
10.5
|
Lease Agreement, dated January 1, 2010, by and between Meng Wang Village Committee of Dayang Town of Qiaocheng District in Bozhou City and Xuelingxian(5)
|
|
|
10.6
|
Supplementary Lease Agreement, dated June 23, 2010, by and between Meng Wang Village Committee of Dayang Town of Qiaocheng District in Bozhou City and Xuelingxian(2)
|
|
|
10.7
|
Lease Agreement, dated July 2, 2010, by and between Meng Wang Village Committee of Dayang Town of Qiaocheng District in Bozhou City and Xuelingxian(5)
|
|
|
10.8
|
Lease Contract, dated May 27, 2009, by and between Yaoyuan and General Tobacco Group Co., Ltd.(5)
|
|
|
10.9
|
Lease Contract, dated March 1, 2010, by and between Yaoyuan and General Tobacco Group Co., Ltd.(2)
|
|
|
10.10
|
Lease Contract, dated June 19, 2010, by and between Yaoyuan and General Tobacco Group Co., Ltd.(5)
|
|
|
10.11
|
Lease Contract, dated July 1, 2010, by and between Yaoyuan and General Tobacco Group Co., Ltd. (5)
|
|
|
10.12
|
Lease Contract, dated October 25, 2008, by and between XuShujun and Tonghua Tongdetang Pharmaceutical Company(2)
|
|
|
10.13
|
Lease Agreement, dated November 28, 2010, by and between XiuyingHou and Tongdetang(5)
|
|
|
10.14
|
Lease Contract, dated November 17, 2010, by and between Xiangqing Zhao, Qiudong Yu and Global Pharma BVI(5)
|
|
|
10.15
|
Drug Purchase and Sales Contract, dated January 9, 2009, by and between Changchun YongxinDirui Drug Co., Ltd. and Tongdetang(2)
|
|
|
10.16
|
Drug Purchase and Sales Agreement, dated January 16, 2009, by and between Changchun Changheng Pharmaceutical Co., Ltd. and Tongdetang(2)
|
|
|
10.17
|
Distribution Agency Agreement, dated February 1, 2009, by and between Bozhou City ZhongzhengSliced Chinese Crude Drugs Co., Ltd and Xuelingxian(2)
|
80
|
10.18
|
Distribution Agency Agreement, dated February 11, 2010, by and between Bozhou City Zhongzheng Sliced Chinese Crude Drugs Co., Ltd and Xuelingxian(5)
|
|
|
10.18A
|
Distribution Agency Agreement, dated January 15, 2011, by and between Bozhou City Zhongzheng Sliced Chinese Crude Drugs Co., Ltd and Xuelingxian(5)
|
|
|
10.19
|
Sales Agreement, dated January 20, 2009, by and between Anhui Province Suzhou City Sliced Chinese Crude Drugs Co., Ltd and Xuelingxian(2)
|
|
|
10.20
|
Distribution Agreement, dated March 15, 2009, by and between Anhui Province Suzhou City Traditional Chinese Medicine Co., Ltd., and Xuelingxian(2)
|
|
|
10.21
|
Distribution Agreement, dated January 1, 2010 by and between Xiuzheng Pharmaceutical Group Marketing Co., Ltd., and Yaoyuan(5)
|
|
|
10.21A
|
Distribution Agreement, dated January 1, 2011 by and between Xiuzheng Pharmaceutical Group Marketing Co., Ltd., and Yaoyuan(5)
|
|
|
10.22
|
Distribution Agreement, dated January 1, 2010 by and between Hainan Lingkang Pharmaceutical Co., Ltd., and Yaoyuan(5)
|
|
|
10.22A
|
Distribution Agreement, dated January 1, 2011 by and between Hainan Lingkang Pharmaceutical Co., Ltd., and Yaoyuan(5)
|
|
|
10.23
|
Trademark Use Agreement, dated January 1, 2009, by and between Jingsheng Wang and Xuelingxian; Trademark Use Agreement, dated March 28, 2009, by and between Jingsheng Wang and Binomial Biopharm Group Limited(2)
|
|
|
10.24
|
Trademark License Declaration, dated April 7, 2010, by Yanliang Song(2)
|
|
|
10.25
|
Employment Agreement, dated August 6, 2010, by and between Yunlu Yin and Top Flight(2)
|
|
|
10.26
|
Employment Agreement, dated August 6, 2010, by and between An Fu and Top Flight(2)
|
|
|
10.27
|
Employment Agreement, dated August 6, 2010, by and between Dan Li and Top Flight(2)
|
|
|
10.28
|
Earn-in Agreement(5)
|
|
|
10.28A
|
Agreement to Amend Earn-In Agreement(4)
|
|
|
10.29
|
Equity Transfer Agreement of Shandong Global Pharm Company(5)
|
81
|
10.30
|
Equity Transfer Agreement of Tonghua Tongdetang Pharmaceutical Company(5)
|
|
|
10.31
|
Equity Transfer Agreement of Anhui Xuelingxian Pharmaceutical Company(5)
|
|
|
10.32
|
Loan Contract, dated May 7, 2010, by and between Xueling Xian and Construction Bank of China at Bozhou Branch(5)
|
|
|
11.1
|
Code of Business Conduct and Ethics (5)
|
|
|
16.1
|
Letter from the Company to GBH CPAs, CPA, dated as August 11, 2010(2)
|
|
|
16.2
|
Letter from GBH CPAs to the SEC, dated August 12, 2010(2)
|
|
|
16.3
|
Consent Letter of Acquavella, Chiarelli, Shuster, Berkower& Co., LLP dated August 25, 2010(2)
|
|
|
99.1
|
Audit Committee Charter(3)
|
|
|
99.2
|
Compensation Committee Charter(3)
|
|
|
99.3
|
Nominating Committee Charter(3)
|
(1) Incorporated by reference to the exhibit to our registration statement on Form S-1 filed with the SEC on July 11, 2008.
(2) Incorporated by reference to the exhibit to our current report on Form 8-K filed with the SEC on August 13, 2010.
(3) Incorporated by reference to the exhibit to our current report on Form 8-K filed with the SEC on February 18, 2011.
(4) Incorporated by reference to Exhibit 10.1 to our current report on Form 8-K filed with the SEC on March 29, 2011.
(5) Incorporated by reference to the exhibit to our annual report on Form 10-K f/y/e 12/31/10 filed with the SEC.
82
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
GLOBAL PHARM HOLDINGS GROUP, INC.
|
|
|
Date: April 13, 2011
|
|
|
/s/ Yunlu Yin
|
|
|
Name: Yunlu Yin
|
|
|
Title: Chief Executive Officer
|
83
