Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Kips Bay Medical, Inc. | a11-10144_18k.htm |
Exhibit 99.1
|
|
eSVS® MESH |
|
|
DISCLAIMER Certain statements and estimates in this presentation are “forward-looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These forward-looking statements are mainly based on our current expectations and estimates of future events and trends which affect or might affect our business and operations. Although we believe that these estimates and forward-looking statements are based on reasonable assumptions, they are subject to several risks and uncertainties including the risks set forth under the heading “Risk Factors” in our prospectus dated February 11, 2011 and annual report on Form 10-K dated March 31, 2011. These risks could cause results to differ materially from those projected or estimated. Any estimates or other forward-looking statements provided in this presentation speak only as of April 12, 2011, the date this presentation was furnished to the SEC as an exhibit on Form 8-K, and, except to the extent required by law, we undertake no obligation to update or review any estimate and/or forward-looking statement because of new information, future events or other factors. |
|
|
Co-Inventor of the first lithium pacemaker and Founder of CPI/Guidant Corporation Co-Inventor of the St. Jude heart valve and Founder of St. Jude Medical Inc. Co-Inventor the ATS heart valve and Founder of ATS Medical Co-Founder of the Lillehei Surgical Society Recipient “Living Legend of Medicine” Award USA Master Entrepreneur Award 2003 Minnesota Business Hall of Fame NY Ellis Island Medal of Honor Recipient Boys & Girls Club of America Hall of Fame Kips Bay Medical was his seventh IPO MANNY VILLAFAÑA |
|
|
EXPERIENCED MANAGEMENT TEAM Manny Villafaña – Founder, CEO and Chairman Founder, Chief Executive Officer and Chairman of the Board ATS Medical, Inc. Sold to Medtronic, Inc. for $370M Founder, Chief Executive Officer and Chairman of the Board St. Jude Medical, Inc. Current annual sales of approx. $5 billion Founder, Chief Executive Officer and Chairman of the Board of Cardiac Pacemakers, Inc. Sold as Guidant to Boston Scientific, Inc. for $27 billion Michael P. Winegar – Chief Operating Officer Vice President of Regulatory and Quality at Enpath Medical, Inc. Management positions in Regulatory Affairs, Clinical Research, and Quality Assurance at ev3 Inc. Vice President of Regulatory Affairs, Clinical Research, and Quality Assurance for Myocor, Inc. Management positions at Medical Incorporated, Medtronic, Inc., SciMed Life Systems Inc., and Boston Scientific Corporation Scott Kellen – Chief Financial Officer Chief Financial Officer at Transoma Medical, Inc. Corporate Controller at ev3 Inc. Senior Audit Manager at Deloitte & Touche, LLP. Management positions at Science Incorporated, Micronet Medical, Inc. and Spectranetics, Inc. |
|
|
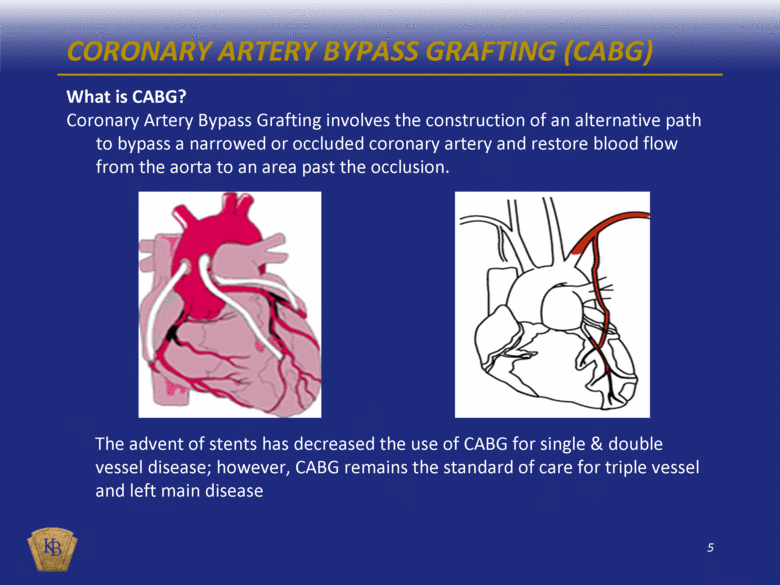
CORONARY ARTERY BYPASS GRAFTING (CABG) What is CABG? Coronary Artery Bypass Grafting involves the construction of an alternative path to bypass a narrowed or occluded coronary artery and restore blood flow from the aorta to an area past the occlusion. The advent of stents has decreased the use of CABG for single & double vessel disease; however, CABG remains the standard of care for triple vessel and left main disease |
|
|
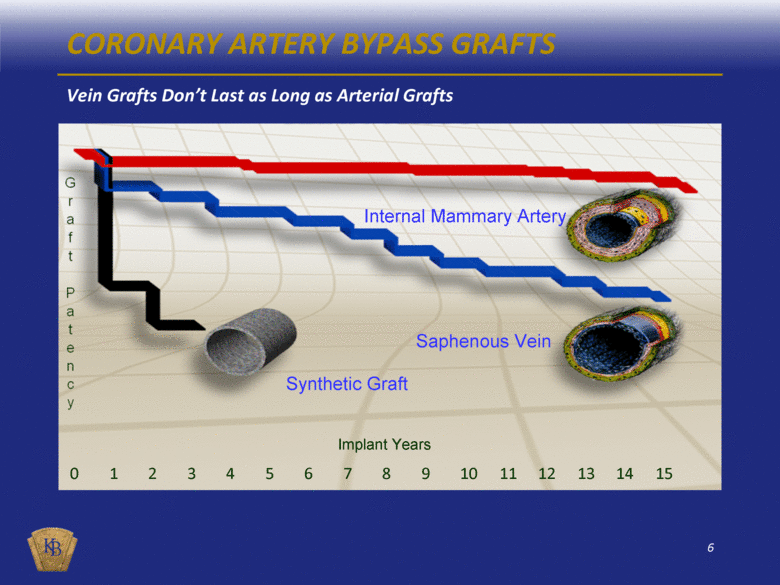
Internal Mammary Artery Saphenous Vein Synthetic Graft Implant Years CORONARY ARTERY BYPASS GRAFTS Vein Grafts Don’t Last as Long as Arterial Grafts 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 |
|
|
The eSVS MESH product is designed to give an implanted vein the physiological attributes of an artery by: Providing radial constriction (up to 35%), leading to arterial like flow characteristics within the graft Matching the typical compliance (~8%) of an artery KIPS BAY MEDICAL, INC. eSVS MESH |
|
|
CABG: STANDARD OF CARE FOR MULTI-VESSEL DISEASE January 2008 study compared drug-eluting stents with CABG in multi-vessel coronary disease New York study as Published in the New England Journal of Medicine Determined death rates and revascularization rates were higher in patients receiving drug-eluting stents than in patients receiving CABG CABG was superior in spite of patients receiving CABG being older and having more severe coronary disease Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery, or SYNTAX, study Published in the New England Journal of Medicine March 2009 Evaluated long-term patient outcomes based upon survival rate and need for re-intervention 12 months after surgery Concluded CABG is the more effective long-term treatment for coronary artery disease Practice Guidelines Recommend Bypass Surgery for Triple Vessel and Left Main Disease American Heart Association journal Circulation 2009 CABG is the only appropriate method of coronary revascularization for patients with triple vessel disease and left main disease |
|
|
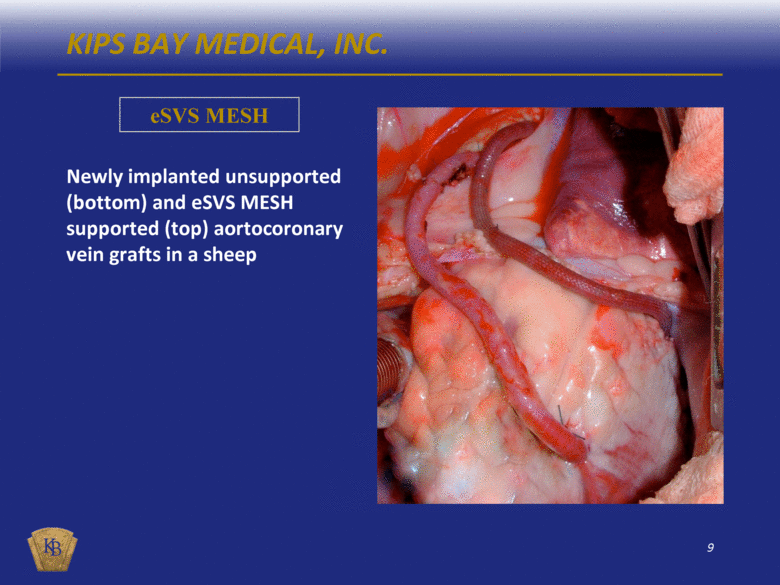
KIPS BAY MEDICAL, INC. eSVS MESH Newly implanted unsupported (bottom) and eSVS MESH supported (top) aortocoronary vein grafts in a sheep |
|
|
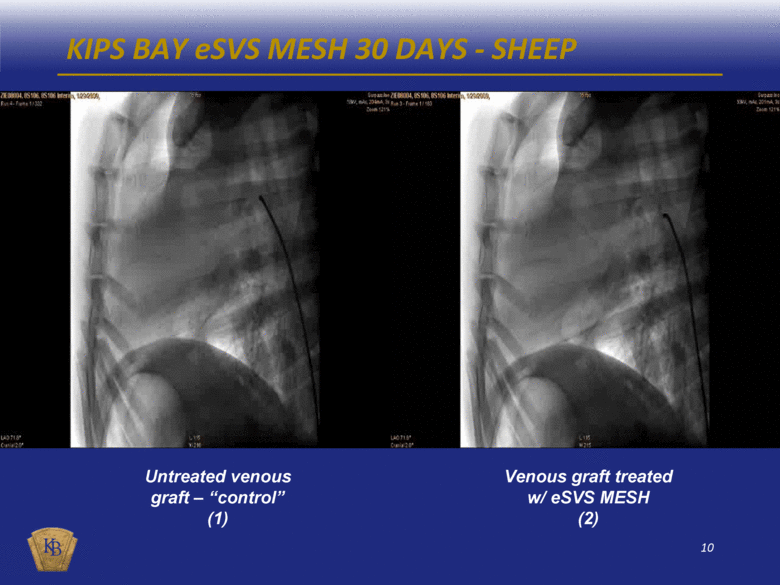
KIPS BAY eSVS MESH 30 DAYS - SHEEP Untreated venous graft – “control” (1) Venous graft treated w/ eSVS MESH (2) |
|
|
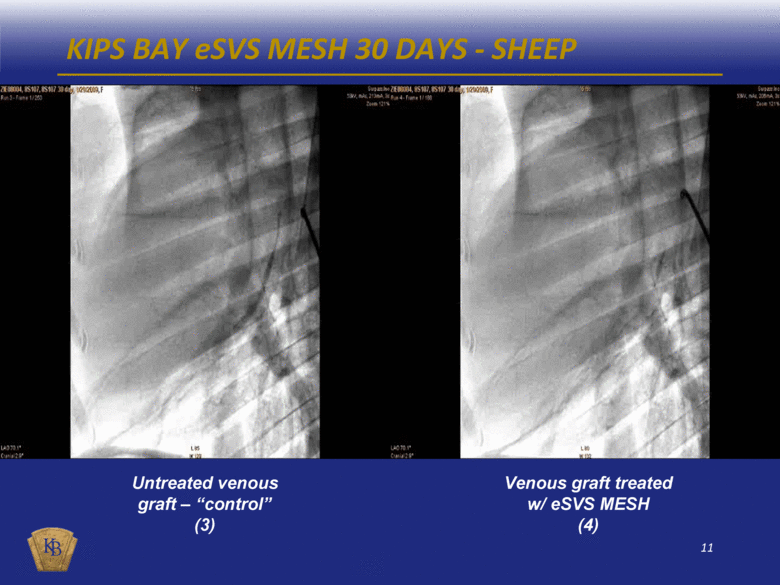
KIPS BAY eSVS MESH 30 DAYS - SHEEP Venous graft treated w/ eSVS MESH (4) Untreated venous graft – “control” (3) |
|
|
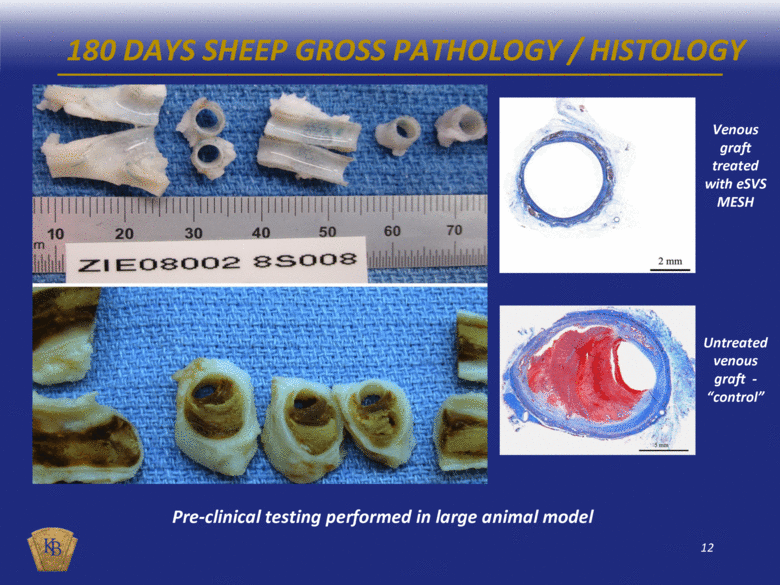
180 DAYS SHEEP GROSS PATHOLOGY / HISTOLOGY Pre-clinical testing performed in large animal model Venous graft treated with eSVS MESH Untreated venous graft - “control” |
|
|
CLINICAL STUDIES AND DATA International Clinical Trial A study designed to support a European CE Mark application with enrollment from August 2008 to July 2009 7 centers enrolled 90 patients 85 patients (94%) reported results for the Primary Safety Endpoint (30-day MACCE) 73 patients (81%) reported results for the Primary Efficacy Endpoint (9-12 months angiographic patency) This trial formed the basis for our CE Mark application, which we submitted in February 2010 and was approved in May 2010 Our EU product sales began June 21, 2010 |
|
|
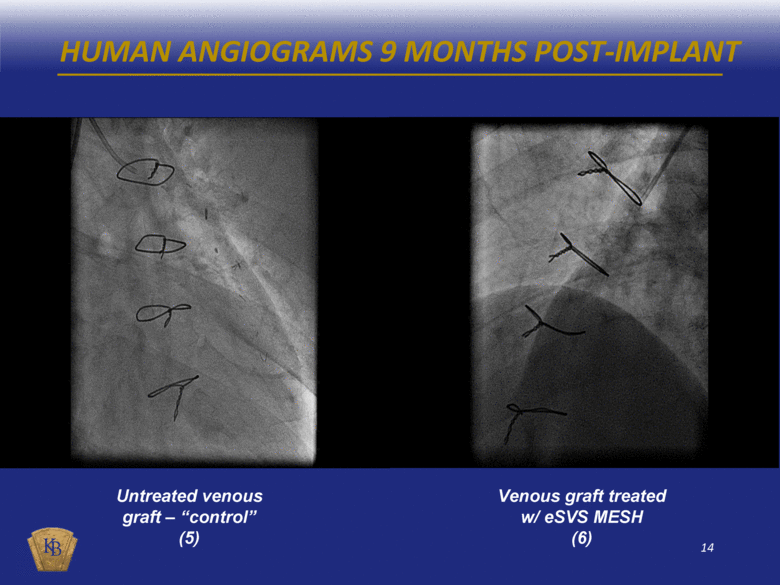
HUMAN ANGIOGRAMS 9 MONTHS POST-IMPLANT Untreated venous graft – “control” (5) Venous graft treated w/ eSVS MESH (6) |
|
|
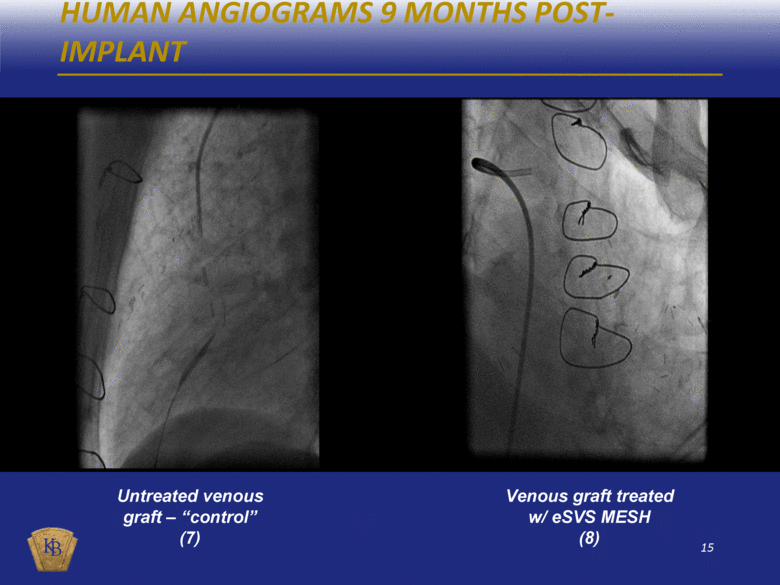
HUMAN ANGIOGRAMS 9 MONTHS POST-IMPLANT Untreated venous graft – “control” (7) Venous graft treated w/ eSVS MESH (8) |
|
|
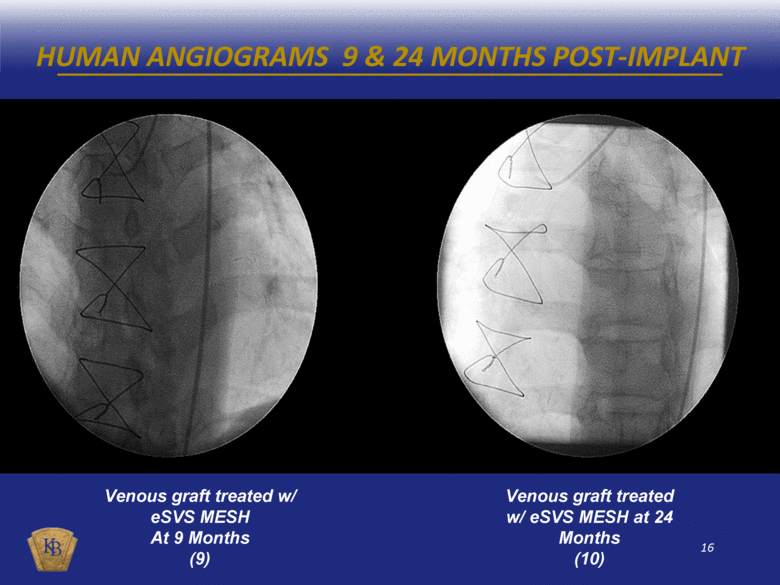
HUMAN ANGIOGRAMS 9 & 24 MONTHS POST-IMPLANT Venous graft treated w/ eSVS MESH At 9 Months (9) Venous graft treated w/ eSVS MESH at 24 Months (10) |
|
|
LEARNINGS FROM INTERNATIONAL TRIAL Device and implant procedure proven safe We recalibrated our sizing chart and discontinued the 3.0 millimeter eSVS MESH, our smallest sized device, as, in some cases, aggressive downsizing led to premature closure of the eSVS MESH treated venous grafts (we currently market the 3.5, 4.0 and 4.5 millimeter internationally) Proper anastomotic technique required for optimal results. We changed the product instructions for use to clearly explain how to prepare the anastomotic site with the eSVS MESH Insights from this study have guided the design of our US IDE trial |
|
|
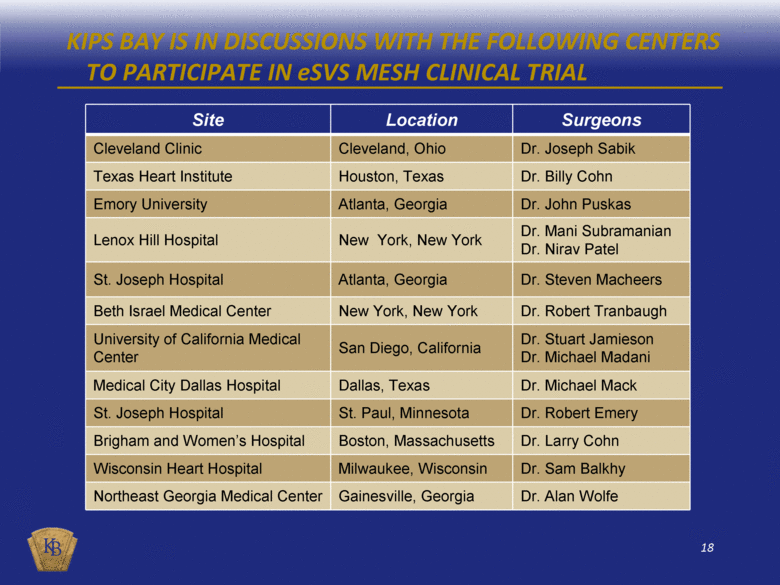
KIPS BAY IS IN DISCUSSIONS WITH THE FOLLOWING CENTERS TO PARTICIPATE IN eSVS MESH CLINICAL TRIAL Site Location Surgeons Cleveland Clinic Cleveland, Ohio Dr. Joseph Sabik Texas Heart Institute Houston, Texas Dr. Billy Cohn Emory University Atlanta, Georgia Dr. John Puskas Lenox Hill Hospital New York, New York Dr. Mani Subramanian Dr. Nirav Patel St. Joseph Hospital Atlanta, Georgia Dr. Steven Macheers Beth Israel Medical Center New York, New York Dr. Robert Tranbaugh University of California Medical Center San Diego, California Dr. Stuart Jamieson Dr. Michael Madani Medical City Dallas Hospital Dallas, Texas Dr. Michael Mack St. Joseph Hospital St. Paul, Minnesota Dr. Robert Emery Brigham and Women’s Hospital Boston, Massachusetts Dr. Larry Cohn Wisconsin Heart Hospital Milwaukee, Wisconsin Dr. Sam Balkhy Northeast Georgia Medical Center Gainesville, Georgia Dr. Alan Wolfe |
|
|
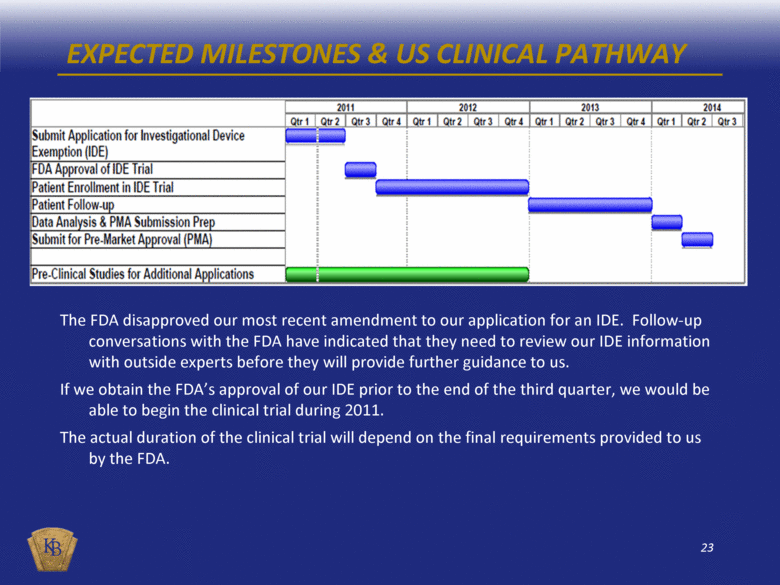
PROPOSED US CLINICAL TRIAL DESIGN Prospective, multi-center, randomized controlled trial (each patient receives both control and eSVS MESH treatments) Study to confirm the equivalent clinical safety and superior angiographic effectiveness At least 366 patients in two phases, feasibility rolling into pivotal A maximum of 20 US investigational sites Follow-up requirements: 30 days or hospital discharge, whichever is longer 9 months (angiography) Post-study: annual (year 2-5) phone follow-up to assess adverse events Subject to FDA granting our IDE by Q3 2011, we can begin enrollment by the end of 2011 |
|
|
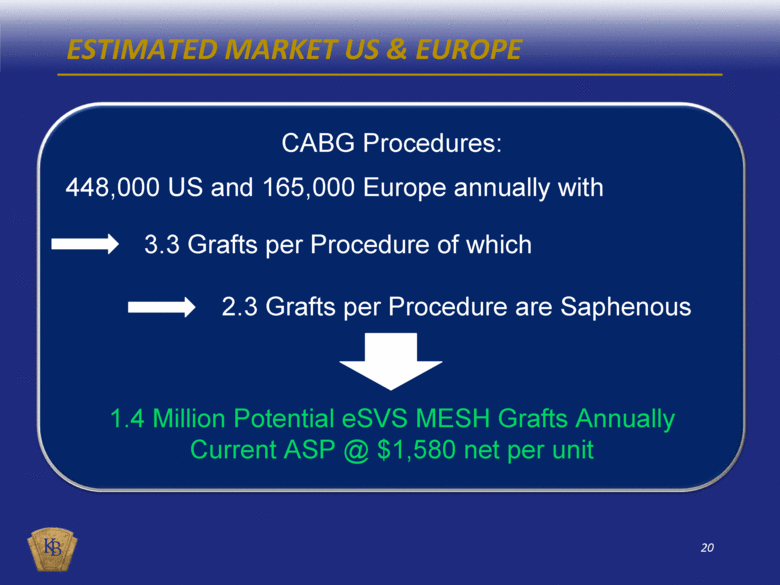
ESTIMATED MARKET US & EUROPE CABG Procedures: 448,000 US and 165,000 Europe annually with 3.3 Grafts per Procedure of which 2.3 Grafts per Procedure are Saphenous 1.4 Million Potential eSVS MESH Grafts Annually Current ASP @ $1,580 net per unit |
|
|
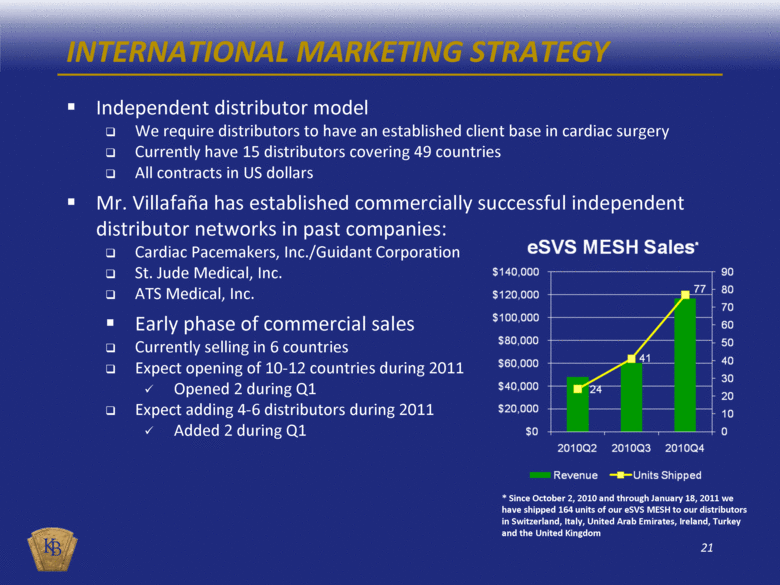
INTERNATIONAL MARKETING STRATEGY Independent distributor model We require distributors to have an established client base in cardiac surgery Currently have 15 distributors covering 49 countries All contracts in US dollars Mr. Villafaña has established commercially successful independent distributor networks in past companies: Cardiac Pacemakers, Inc./Guidant Corporation St. Jude Medical, Inc. ATS Medical, Inc. Early phase of commercial sales Currently selling in 6 countries Expect opening of 10-12 countries during 2011 Opened 2 during Q1 Expect adding 4-6 distributors during 2011 Added 2 during Q1 * Since October 2, 2010 and through January 18, 2011 we have shipped 164 units of our eSVS MESH to our distributors in Switzerland, Italy, United Arab Emirates, Ireland, Turkey and the United Kingdom |
|
|
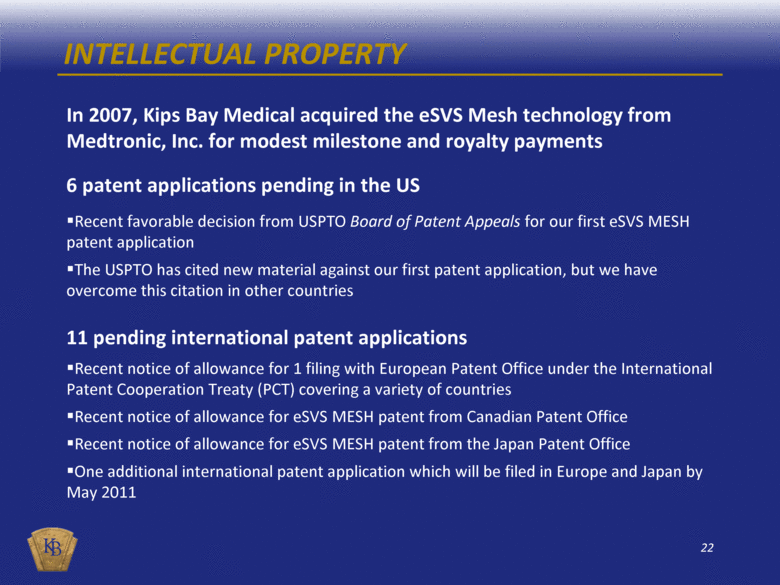
INTELLECTUAL PROPERTY In 2007, Kips Bay Medical acquired the eSVS Mesh technology from Medtronic, Inc. for modest milestone and royalty payments 6 patent applications pending in the US Recent favorable decision from USPTO Board of Patent Appeals for our first eSVS MESH patent application The USPTO has cited new material against our first patent application, but we have overcome this citation in other countries 11 pending international patent applications Recent notice of allowance for 1 filing with European Patent Office under the International Patent Cooperation Treaty (PCT) covering a variety of countries Recent notice of allowance for eSVS MESH patent from Canadian Patent Office Recent notice of allowance for eSVS MESH patent from the Japan Patent Office One additional international patent application which will be filed in Europe and Japan by May 2011 |
|
|
EXPECTED MILESTONES & US CLINICAL PATHWAY The FDA disapproved our most recent amendment to our application for an IDE. Follow-up conversations with the FDA have indicated that they need to review our IDE information with outside experts before they will provide further guidance to us. If we obtain the FDA’s approval of our IDE prior to the end of the third quarter, we would be able to begin the clinical trial during 2011. The actual duration of the clinical trial will depend on the final requirements provided to us by the FDA. |
|
|
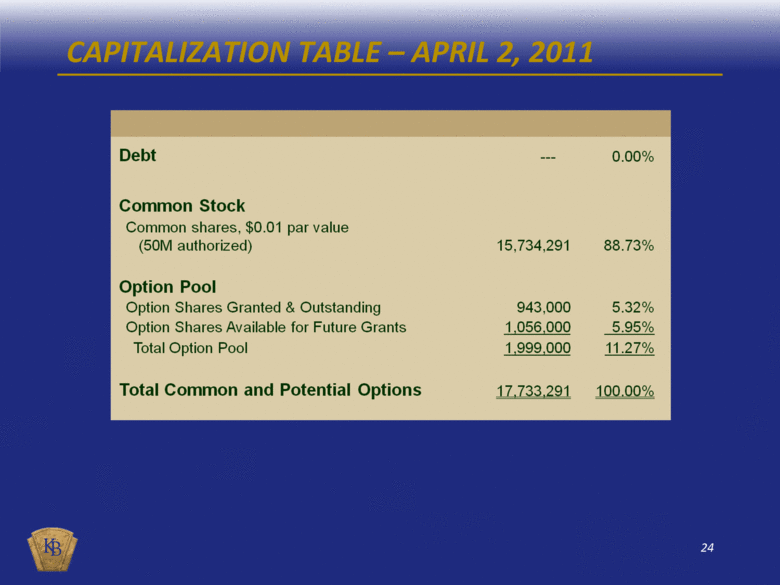
CAPITALIZATION TABLE – APRIL 2, 2011 Debt --- 0.00% Common Stock Common shares, $0.01 par value (50M authorized) 15,734,291 88.73% Option Pool Option Shares Granted & Outstanding 943,000 5.32% Option Shares Available for Future Grants 1,056,000 5.95% Total Option Pool 1,999,000 11.27% Total Common and Potential Options 17,733,291 100.00% |
|
|
INVESTMENT HIGHLIGHTS New medical technology in a company founded by Manny Villafaña Experienced management team with history of success Developing technology to address limitations in CABG surgery with potential for 1.4 million eSVS MESH grafts in the US and Europe annually Already approved and initiating sales in select international markets Clean capital structure - No debt / No preferred |
|
|
eSVS® MESH Thank you Not Available in the United States |
|
|
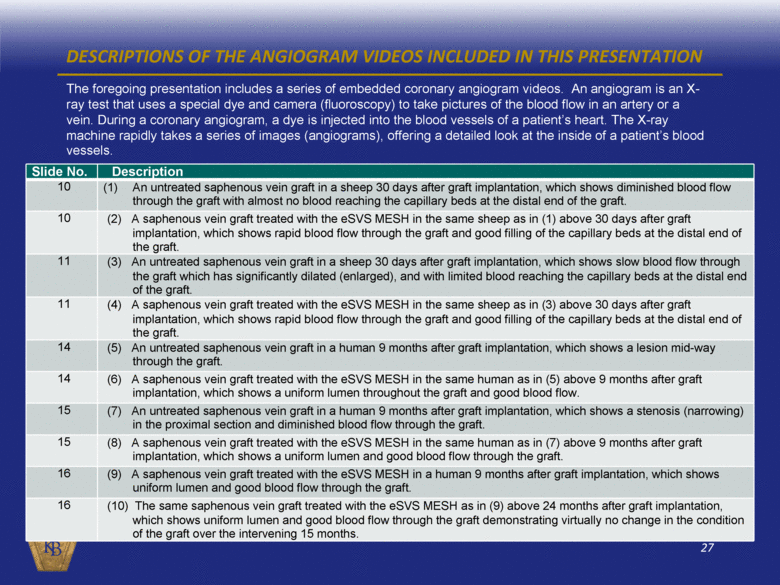
DESCRIPTIONS OF THE ANGIOGRAM VIDEOS INCLUDED IN THIS PRESENTATION The foregoing presentation includes a series of embedded coronary angiogram videos. An angiogram is an X-ray test that uses a special dye and camera (fluoroscopy) to take pictures of the blood flow in an artery or a vein. During a coronary angiogram, a dye is injected into the blood vessels of a patient’s heart. The X-ray machine rapidly takes a series of images (angiograms), offering a detailed look at the inside of a patient’s blood vessels. Slide No. Description 10 (1) An untreated saphenous vein graft in a sheep 30 days after graft implantation, which shows diminished blood flow through the graft with almost no blood reaching the capillary beds at the distal end of the graft. 10 (2) A saphenous vein graft treated with the eSVS MESH in the same sheep as in (1) above 30 days after graft implantation, which shows rapid blood flow through the graft and good filling of the capillary beds at the distal end of the graft. 11 (3) An untreated saphenous vein graft in a sheep 30 days after graft implantation, which shows slow blood flow through the graft which has significantly dilated (enlarged), and with limited blood reaching the capillary beds at the distal end of the graft. 11 (4) A saphenous vein graft treated with the eSVS MESH in the same sheep as in (3) above 30 days after graft implantation, which shows rapid blood flow through the graft and good filling of the capillary beds at the distal end of the graft. 14 (5) An untreated saphenous vein graft in a human 9 months after graft implantation, which shows a lesion mid-way through the graft. 14 (6) A saphenous vein graft treated with the eSVS MESH in the same human as in (5) above 9 months after graft implantation, which shows a uniform lumen throughout the graft and good blood flow. 15 (7) An untreated saphenous vein graft in a human 9 months after graft implantation, which shows a stenosis (narrowing) in the proximal section and diminished blood flow through the graft. 15 (8) A saphenous vein graft treated with the eSVS MESH in the same human as in (7) above 9 months after graft implantation, which shows a uniform lumen and good blood flow through the graft. 16 (9) A saphenous vein graft treated with the eSVS MESH in a human 9 months after graft implantation, which shows uniform lumen and good blood flow through the graft. 16 (10) The same saphenous vein graft treated with the eSVS MESH as in (9) above 24 months after graft implantation, which shows uniform lumen and good blood flow through the graft demonstrating virtually no change in the condition of the graft over the intervening 15 months. |