Attached files
| file | filename |
|---|---|
| EX-21.1 - TIENS BIOTECH GROUP USA INC | v216077_ex21-1.htm |
| EX-31.1 - TIENS BIOTECH GROUP USA INC | v216077_ex31-1.htm |
| EX-32.1 - TIENS BIOTECH GROUP USA INC | v216077_ex32-1.htm |
| EX-10.48 - TIENS BIOTECH GROUP USA INC | v216077_ex10-48.htm |
| EX-10.49 - TIENS BIOTECH GROUP USA INC | v216077_ex10-49.htm |
| EX-10.46 - TIENS BIOTECH GROUP USA INC | v216077_ex10-46.htm |
| EX-10.51 - TIENS BIOTECH GROUP USA INC | v216077_ex10-51.htm |
| EX-10.47 - TIENS BIOTECH GROUP USA INC | v216077_ex10-47.htm |
| EX-10.50 - TIENS BIOTECH GROUP USA INC | v216077_ex10-50.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark one)
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2010
or
¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _____ to_____
Commission File Number: 001-32477
TIENS BIOTECH GROUP (USA), INC.
(Exact name of registrant as specified in its charter)
|
Delaware
|
75-2926439
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
No. 17, Xinyuan Rd.
Wuqing New Tech Industrial Park
Tianjin, China 301700
(Address of principal executive offices) (Zip Code)
Registrant’s Telephone Number, including area code: (011) 86-22-8213-7914
Securities registered pursuant to Section 12(b) of the Exchange Act:
|
Title of Each Class
|
Name of Each Exchange on which Registered
|
|
|
Common Stock, par value $0.001
|
|
NYSE Amex
|
Securities registered pursuant to Section 12(g) of the Exchange Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ¨
|
Accelerated filer ¨
|
|
Non-accelerated filer ¨
(Do not check if a smaller reporting company)
|
Smaller reporting company x
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
Based upon the closing sale price of $1.84 per share of Common Stock on NYSE Amex on June 30, 2010, the aggregate market value of the 3,503,586 shares of voting stock held by non-affiliates of the registrant was approximately $6,446,598.
There were 71,333,586 shares of the registrant’s common stock outstanding on March 29, 2011.
DOCUMENTS INCORPORATED BY REFERENCE – None.
FORWARD-LOOKING INFORMATION
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, and Section 21E of the Securities Exchange Act of 1934. These statements relate to future events or our future financial performance. We have attempted to identify forward-looking statements by terminology including “anticipates”, “believes”, “expects”, “can”, “continue”, “could”, “estimates”, “expects”, “intends”, “may”, “plans”, “potential”, “predict”, “should” or “will” or the negative of these terms or other comparable terminology. These statements are only predictions; uncertainties and other factors may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels or activity, performance or achievements expressed or implied by these forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Our expectations are as of the date this Form 10-K is filed, and we do not intend to update any of the forward-looking statements after the date this Annual Report on Form 10-K is filed to confirm these statements to actual results, unless required by law.
ITEM 1. BUSINESS.
In this Annual Report on Form 10-K, references to “dollars” and “$” are to United States Dollars and references to “RMB” and “renminbi” are to Chinese Renminbi (RMB). References to “we”, “us”, “our”, the “Company” or “Tiens USA” include Tiens Biotech Group (USA), Inc. and its subsidiaries.
Overview
Tiens USA researches, develops, manufactures, and markets nutrition supplement products, including wellness products and dietary supplement products. Our operations are conducted from our headquarters in Tianjin, People’s Republic of China (“China” or the “PRC”) through our 80% owned subsidiary, Tianjin Tianshi Biological Development Co., Ltd. (“Biological”), and our wholly-owned subsidiary, Tianjin Tiens Life Resources Co., Ltd. (“Life Resources”). We sell our products for distribution in China to an affiliated company that in turn sells the products to consumers through its chain store and its Chinese affiliated companies. Outside of China, we sell our products to overseas affiliated companies located in 45 countries that in turn sell them to independent direct sales distributors.
Corporate History and Organization
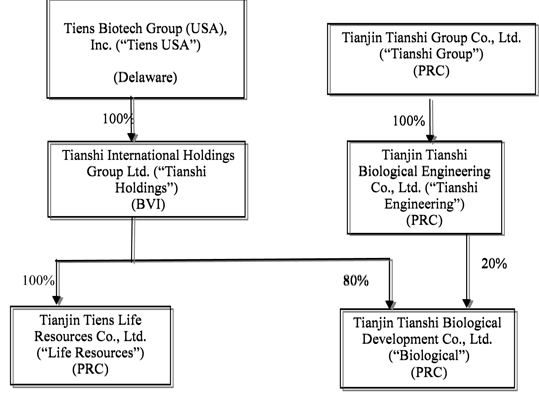
Tiens USA is a Delaware corporation. We own 100% of Tianshi International Holdings Group Ltd., a British Virgin Islands company (“Tianshi Holdings”) which owns all of the registered share capital of Tianjin Tiens Life Resources Co., Ltd., a Chinese Foreign Investment Enterprise (“Life Resources”) and 80% of Biological’s outstanding shares. Biological is a Chinese-foreign equity joint venture company established under Chinese laws on March 27, 1998, subject to the Law on Sino Foreign Equity Joint Ventures.
Tianjin Tianshi Biological Engineering Co., Ltd. (“Tianshi Engineering”), a Chinese company, owns the remaining 20% of Biological. Tianshi Engineering is 100% owned by Tianjin Tianshi Group Co., Ltd. (“Tianshi Group”), a Chinese company. Tianshi Group is 90% owned by Jinyuan Li, our Chairman, Chief Executive Officer, Acting Chief Financial Officer and President and owner of 95.1% of our outstanding stock, and 10% owned by Baolan Li, Jinyuan Li’s daughter, who is a member of our Board of Directors.
Life Resources is currently constructing research and development, manufacturing and logistic facilities, as well as administrative offices in Tianjin, China totaling approximately 420,000 square meters. We moved our administrative offices to the newly constructed Industrial Park on October 16, 2010. We expect to complete the move of our other facilities at the end of 2011.
The following chart shows the ownership interests in our operating subsidiaries.
Products and Manufacturing
We have developed and produce 37 nutrition supplement products, which include wellness products and dietary supplements.
Each of our wellness products includes at least one health function and has been issued a Certificate of Domestic Wellness Product by the State Food and Drug Administration (SFDA). This SFDA certificate is required for the production and sale of wellness products in China. Dietary supplements, which do not include any health functions, are considered to be “ordinary food,” and do not require a SFDA certificate. Each of our products has been issued a Product Standard Code by the Bureau of Technical Supervision.
We put great emphasis on product quality assurance. In 2002, we were awarded a Quality System Certificate for compliance with the standard “ISO9001: 2000” in the area of Design and Development, Production and Service of Food and Health Care Food in China. In addition, many of our products have received a certificate of Hazard Analysis Critical Control Point (“HACCP”). HACCP identifies and assesses hazards and risks associated with the manufacture, distribution and use of food-handling establishments. In 2009 and 2010, four of our products received a kosher certificate from the Kosher Supervision of America (“KSA”), which is recognized by rabbinical societies throughout the world. These products bear the KSA symbol, which tells consumers that the products are in compliance with kosher standards.
Our products are manufactured by Life Resources and Biological at our facilities in Tianjin, China. The manufacturing processes of our nutrition supplement products are categorized into six types depending on the different forms of the finished products: Powder, Tea, Capsules, Tablets, Granules and Soft Gel Capsules. All of our manufacturing complies with the product standards approved by the Bureau of Technical Supervision in China.
The following table lists our products.
|
Wellness Products *
|
Dietary Supplement Products *
|
|
|
Bone Treasure Tablets (b)
|
Tianshi Barley Green Tablets (b)
|
|
|
Chewable Calcium Tablets (b)
|
Tianshi Breast Beauty Capsules
|
|
|
Chewable Calcium Tablets with multi-flavor (b)
|
Tianshi Calcium-Treasure Tablets (b)
|
|
|
Grape Extract Capsules (a) (b)
|
Tianshi Double-cellulose Tablets (a) (b)
|
|
|
Tianshi Beauty Face Capsules (b) (c)
|
Tianshi Eel Oil Capsules
|
|
|
Tianshi Cell Rejuvenation Capsules (a) (b)
|
Tianshi Hemp Seed Oil Softgels
|
|
|
Tianshi Chitosan Capsules (a) (b)
|
Tianshi Lycopene Tablets (a) (b)
|
|
|
Tianshi Cordyceps Capsules (a) (b)
|
Tianshi Multi-Vit-Mine Coffee (b)
|
|
|
Tianshi Lipid Metabolic Management Tea (a) (b) (c)
|
Tianshi Natto Capsules (b)
|
|
|
Tianshi Metabolic Balance Capsules (a) (b) (c)
|
Tianshi Perilla Oil Softgels (b)
|
|
|
Tianshi Nutrient Super Calcium Powder (a) (b)
|
Tianshi Pine Pollen Powder Capsules
|
|
|
Tianshi Pressure Care Tea (b)
|
Tianshi Propeptide Polypeptide Albumen Powder (b)
|
|
|
Tianshi Slimming Tea (a) (b) (c)
|
Tianshi Rich Selenium Green Tea
|
|
|
Tianshi Spirulina Capsules (a) (b)
|
Tianshi Sea Buckthorn Oil Softgels (a) (b)
|
|
|
Tianshi Super Calcium Powder with Metabolic Factors (a) (b)
|
Tianshi Super Calcium Milk Powder (b)
|
|
|
Tianshi Super Calcium Powder for Children (a) (b)
|
Tianshi Tibet-Garlic Capsules (b)
|
|
|
Tianshi Super Calcium Capsules with Lecithin (a) (b)
|
||
|
Tianshi Sweet Dreams Granules (a) (b)
|
||
|
Tianshi Vitality Softgels (a) (b)
|
||
|
Tianshi Throat Care Granules (b)
|
||
|
Tianshi Zinc Capsules (a) (b)
|
|
|
*
|
These products are not intended to diagnose, treat, cure or prevent any disease.
|
|
(a)
|
This product has received Halal Approval, which certifies that our manufacturing processes comply with the requirements of Islamic dietary law.
|
|
(b)
|
This product has received an HACCP Certificate.
|
|
(c)
|
This product has received KSA Kosher Certificate.
|
For the years ended December 31, 2010 and 2009, almost all of our revenue was generated from related party customers. See note 17 to our consolidated financial statements, for a breakdown of domestic and international revenue, and revenue by product group, for the last two fiscal years. In 2010 and 2009, our Tianshi Cordyceps Capsules accounted for 17.4% and 10.6% of our revenue, respectively, and our Tianshi Nutrient Super Calcium Powder accounted for 15.7% and 19.0% of our revenue, respectively.
Trademarks and Patents
We consider the “Tiens” logo important to our business and have registered our products under the logo “Tiens” with the State Administration of Industry and Commerce in China. The registration is valid for a period of ten years from May 21, 2002 and can be renewed for further ten-year periods multiple times. We have conducted extensive research and developed Tianshi Super Calcium Powder with Metabolic Factors and Tianshi Super Calcium Powder for Children, which have each been awarded a patent from the State Intellectual Properties Office in China with respective patent numbers of ZL97115067.2 and ZL97115068.0. These two patents are effective for 20 years, commencing on January 13, 2001.
Suppliers
We have established long-term relationships with most of our suppliers. We believe that the raw materials required for manufacturing our products are relatively easy to find and alternative suppliers are convenient to locate. The following table lists our principal suppliers:
|
SUPPLIER
|
PRODUCT
|
|
|
Tianjin Xinhengyang Import and Export Trade Co.,Ltd
|
Whole and de-fatted milk powder, Polydextrose
|
|
|
Tianjin Xingsheng Import and Export Trade Co.,Ltd
|
Cordyceps mycelium powder
|
|
|
Shanghai Guangdeli Capsule Co.,Ltd
|
Capsules
|
|
|
Qinghai Kangpu Biological Technology Development Co.,Ltd
|
Seabuckthorn seed oil
|
|
|
Tianjin Tangchao Food Industry Co.,Ltd
|
Extraction of grape seed, yolk lecithin
|
|
|
Tianjin Xindayutong Trade Co., Ltd
|
Gelatin, Vitamin E powder
|
|
|
Shanxi Guangsheng Capsule Co., Ltd
|
Capsules
|
|
|
Baolingbao Biological Co., Ltd
|
Isomaltooligosaccharide, Anhydrous glucose
|
|
|
Hebei Jinmu Pharmaceutical Group Co.,Ltd
|
Folium nelumbinis, Herba gynostemma pentaphyllum
|
|
|
Shandong Aokang Biological Technology Co.,Ltd
|
|
Chitosan
|
Research and Development
We incurred research and development expenses of $1.8 million and $1.3 million in 2010 and 2009, respectively. As of December 31, 2010, we employed 91 staff members in research and development, which accounts for an increase of 12 employees in our research and development team during 2010.
Marketing and Distribution
In China, we sell our products to Tianshi Engineering, an affiliated Chinese company, through our subsidiaries Biological and Life Resources. Tianshi Engineering, in turn, sells the products to customers through its branches and affiliated companies and at chain stores, which are owned by individual distributors, who are not affiliates of the Company. During 2010 Tianshi Engineering closed five of its less profitable branches in China. As of December 31, 2010, Tianshi Engineering had 87 branches in China. Prior to 2006, Biological sold all of its products to Tianshi Engineering as finished products at a price equal to 25% of the Chinese market price for the products. This 25% figure was negotiated between the parties in 2003, before we acquired Tianshi Holdings, and we believe that it is a reasonable sales price for us to receive. We used this pricing formula in 2010, and currently continue to use the same pricing formula.
At the beginning of 2006, Biological also began selling semi-finished products to Tianshi Engineering. To qualify for a direct selling license in China, Tianshi Engineering is required to produce a part of the products that it sells in China. As a result, Biological began to sell semi-finished products to Tianshi Engineering, which jointly shares with us licenses to produce, manufacture and sell the products. The price of semi-finished goods sold to Tianshi Engineering was originally set at the beginning of 2006 to provide us with a 75% gross profit margin. However, based on fluctuations in the cost of raw materials and quantities produced, this percentage varied during the year. This 75% figure was negotiated between the parties, and we believe that it is reasonable. The goal of this new pricing policy was to try to maintain our gross margins on semi-finished goods at a similar level to historical gross margins for finished goods.
As of June 2008, Life Resources replaced Biological in the production of semi-finished products and began to produce and sell semi-finished products to Tianshi Engineering on the same pricing terms as Biological’s previous sales. In October 2009, Life Resources transferred part of its production and sale of semi-finished products to Biological.
Internationally, our strategy is to develop a strong direct sales force through our international affiliated companies. We sell our products to overseas affiliated companies located in 45 countries who in turn re-package the products to meet the needs of the local markets and sell them to independent direct sales distributors. Currently, the United States is not a significant part of our business. During 2010, we decreased the number of countries where we sell directly to overseas affiliates from 54 to 45. Our CEO, Jinyuan Li, owns or controls these overseas related companies. Due to the common ownership, there are no formal sales or administrative agreements among us and those overseas related parties. The business operations among these related entities have historically been, and continue to be, regulated through internal policies. In 2010 our highest sales outside of China were to the following eleven countries, in descending order: Russia, South Africa, Kazakhstan, Roumania, Ukraine, Lagos, India, Bengal, Ghana, Uganda and Columbia.
As operation costs vary from country to country, international market prices vary accordingly. We sell our products to overseas affiliates at the FOB (destination port) price, which consists of 25% of the Chinese retail price for similar products in Chinese market, including customs duty, value-added tax and other miscellaneous transportation cost. The overseas affiliates mark up the products to cover their expenses and realize profits of approximately 10%.
Competition
Internationally, we engage in the direct selling industry and compete with other direct selling organizations. Some of them have a longer operating history and higher visibility, name recognition and financial resources than we do. The leading direct selling companies in our existing markets are Avon and Alticor (Amway). Some of our competitors, including Avon and Alticor (Amway), have been granted a direct selling business license in certain parts of China pursuant to China’s regulations governing direct selling. In other cites and/or provinces of China, the selling models of Avon and Alticor (Amway) are similar to the model utilized by our company; therefore, Avon and Alticor (Amway) are our main competitors in our Chinese operations. The direct selling regulations require Tianshi Engineering, our affiliate who sells our products in China, to apply for approval to conduct a direct selling enterprise in China. On March 11, 2011, Tianshi Engineering received a direct selling license in Tianjin.
Regulatory Framework
Product Regulation
The central governing authority in China for wellness products is the SFDA, which is under the jurisdiction of the State Council. SFDA issues administrative rules. Provincial, city and town authorities implement the rules of the SFDA. Other than the SFDA, other ministries and administrations also have certain responsibility for the management of wellness or nutrition supplement products, such as the State Administration for Industry and Commerce.
We develop and manufacture products that are classified as nutrition supplement products, which include wellness products and dietary supplement products. Wellness products may not be sold in China without a wellness products certificate. The governmental approval process in China for a newly developed wellness product is as follows:
|
|
·
|
An application for a product certificate is filed with SFDA, which directs the applicant to send product samples to one of the government appointed research institutes;
|
|
|
·
|
The appointed research institute conducts clinical trials, stability tests, function tests and toxicity tests on the product, makes a report and sends the report back to SFDA within 6 months; and
|
|
|
·
|
The Expert Committee of SFDA makes a final decision on the application and issues a “wellness products certificate” or a refusal notice to the applicant.
|
This certificate authorizes the sales and marketing of the product in China. The certificate does not expire and does not require renewal. The approval process generally takes nine to twelve months. Dietary supplement products are not subject to SFDA regulation.
Sales and Marketing Regulations
In most countries, our products are usually considered general commodities, which do not require specific selling permits and are not subject to the strict regulations applied to drugs and medicine. In some countries, direct selling (or multi-level marketing) is highly regulated or prohibited. Since we sell our products to our affiliated companies for sale internationally, the local approval issues with respect to sales and distribution are addressed by our affiliates.
In China, we aim to expand our market share through the branches, chain stores, and affiliated companies of Tianshi Engineering, our affiliate that sells our products in China. The regulatory environment with respect to direct selling in this market remains fluid and the process for obtaining the necessary governmental approvals has been interpreted differently by different governmental authorities.
Tianshi Engineering has applied for a direct selling license in a number of provinces and must obtain a series of approvals from the Departments of Commerce in such provinces, as well as the Departments of Commerce in each city and district in which we plan to operate. Tianshi Engineering is also required to obtain the approval of the State Ministry of Commerce, which is the national government authority overseeing direct selling.
Tianshi Engineering has found that it is taking more time than anticipated to work through the approval process with the Chinese authorities. These authorities have broad discretion in interpreting the regulations and granting necessary approvals. A delay in obtaining approvals at one level can delay its ability to obtain approvals at the next level. The complexity of the approval process as well as the government’s continued cautious approach as direct selling develops in China makes it difficult to predict a timeline for obtaining these approvals.
Environmental Compliance
We are subject to China’s National Environmental Protection Law, as well as a number of other national and local laws and regulations regulating air, water and noise pollution and setting pollutant discharge standards. We believe that all our manufacturing operations are in material compliance with all applicable environmental laws. During 2010, we did not incur any costs to comply with environmental laws.
Employees
As of December 31, 2010, we had 1,380 full-time employees. We have no part-time employees. We believe that our relations with our employees are satisfactory.
Available Information
This Annual Report on Form 10-K for the fiscal year ended December 31, 2010 is available on our website at http://www.tiens-bio.com. The Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2010, excluding exhibits, and the Company’s complete audited financial statements for the fiscal year ended December 31, 2010, will be mailed without charge to any stockholder, upon written request to Secretary of the Company, c/o Tiens Biotech Group (USA), Inc., No. 17, Xinyuan Road, Wuqing New-Tech Industrial Park, Tianjin, People’s Republic of China 301700.
We file annual and quarterly reports, proxy statements and other information with the SEC. Stockholders may read and copy any reports, statements or other information that we file at the SEC’s public reference room in Washington, D.C. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. Our public filings are also available from commercial document retrieval services and at the Internet Web site maintained by the SEC at www.sec.gov.
Not applicable.
Not applicable.
We conduct our main business activities in Tianjin, China. Our primary facilities are located at No. 17, Xinyuan Road, Wuqing New-tech Industrial Park, Tianjin, PRC.
On January 1, 2009, Biological entered into an office and facilities lease agreement with Tianshi Group. Under the terms of the agreement, Biological’s annual rent is equal to 1% of its gross revenues. In addition, Biological is obligated to pay insurance, maintenance and other expenses related to the premises. This agreement expired on December 31, 2009. On January 1, 2010, Biological and Life Resources each entered an office and facilities lease agreement with Tianshi Group on the same terms as Biological’s lease agreement with Tianshi Group in 2009. These two leases cover the following real properties:
|
|
·
|
one office buildings with an area of 1,350 square meters; and
|
|
|
·
|
one dormitory with an area of 2,365 square meters.
|
The Company paid rent under these leases in the amount of $500,361 and $458,382 for the year ended December 31, 2010 and 2009, respectively.
On January 1, 2009, each of Biological and Life Resources entered a Lease Agreement with Tianshi Group pursuant to which Biological and Life Resources will have the right to use and occupy the workshop and warehouse spaces being transferred under the 2007 Transfer Agreement and the 2008 Transfer Agreement. The lease agreements cover the following workshop and warehouse spaces:
|
|
·
|
four workshops with an area of 8,549 square meters; and
|
|
|
·
|
one warehouse with an area of 3,870 square meters.
|
The leases are rent-free, except that, Biological and Life Resources are required to pay Tianshi Group for utility charges and maintenance costs on the buildings. The leases continue until the earlier of the date Biological and Life Resources acquire use of alternate facilities or the land use rights on the underlying property expire. For the year ended December 31, 2010, Biological and Life Resources recorded $330,708 of the rent expense, which is not paid to Tianshi Group, but recorded as paid in capital based upon market price.
Life Resources Property
Life Resources is currently constructing research and development, manufacturing and logistic facilities, as well as administrative offices totaling approximately 420,000 square meters. The facilities are located in our current headquarters in Tianjin, China.
As of December 31, 2010, the office buildings, dormitories, power center, boiler room, warehouse for wellness products had been transferred from construction in progress to fixed assets. The product exhibition center, quality control center and wash house were undergoing interior decoration and expect to be completed at the end of June 2011. The interior decoration of work plants and other warehouses has been nearly completed and these facilities will be put into use once the equipment is installed. We expect the equipment to be installed at the end of 2011. As of the end of 2010, the cost of the completed work of construction in progress and other facilities was $204,854,576 (based on an exchange rate of $1 = RMB 6.6120 as of December 31, 2010), which includes amounts paid for the underlying land use right. The office buildings, dormitories, power center, boiler room and warehouse for wellness products had been put into use from October 2010. We expect that other construction work and equipment installation will be completed in 2011.
We are not a party to any material pending legal proceedings.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASERS OF EQUITY SECURITIES.
Market Prices of Common Stock
Our common stock is listed on NYSE Amex under the symbol “TBV”. The following table sets forth the range of high and low sales prices for our common stock reported by NYSE Amex in each fiscal quarter from January 1, 2009 to December 31, 2010.
|
High
|
Low
|
|||||||
|
2010
|
||||||||
|
Quarter Ended December 31
|
$ | 1.75 | $ | 1.25 | ||||
|
Quarter Ended September 30
|
$ | 1.85 | $ | 1.33 | ||||
|
Quarter Ended June 30
|
$ | 2.62 | $ | 1.75 | ||||
|
Quarter Ended March 31
|
$ | 3.10 | $ | 2.16 | ||||
|
2009
|
||||||||
|
Quarter Ended December 31
|
$ | 5.35 | $ | 2.45 | ||||
|
Quarter Ended September 30
|
$ | 5.57 | $ | 2.26 | ||||
|
Quarter Ended June 30
|
$ | 3.02 | $ | 1.67 | ||||
|
Quarter Ended March 31
|
$ | 4.45 | $ | 1.11 | ||||
Stockholders
As of March 29, 2011, there were a total of 71,333,586 shares of our common stock outstanding, held by approximately 930 stockholders of record.
Dividend Policy
We have not declared any dividends on our common stock since inception and do not intend to pay dividends on our common stock in the foreseeable future. If we ever determine to pay a dividend, we may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency from the PRC for the payment of such dividends from the profits of Biological and Life Resources. Please see additional discussion under Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Condition, Liquidity and Capital Resources.”
Securities Authorized for Issuance Under Equity Compensation Plans
None.
Not applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
FORWARD-LOOKING STATEMENTS:
The following discussion of the financial condition and results of operations should be read in conjunction with the consolidated financial statements and related notes thereto. The words or phrases “would be,” “will allow,” “expect to”, “intends to,” “will likely result,” “are expected to,” “will continue,” “is anticipated,” “estimate,” “project,” or similar expressions are intended to identify “forward-looking statements”. Such statements include those concerning our expected financial performance, our corporate strategy and operational plans. Actual results could differ materially from those projected in the forward-looking statements as a result of a number of risks and uncertainties, including: (a) those risks and uncertainties related to general economic conditions in China, including regulatory factors that may affect such economic conditions; (b) whether we are able to manage our planned growth efficiently and operate profitable operations, including whether our management will be able to identify, hire, train, retain, motivate and manage required personnel or that management will be able to successfully manage and exploit existing and potential market opportunities; (c) whether we are able to generate sufficient revenues or obtain financing to sustain and grow our operations; (d) whether we are able to successfully fulfill our primary requirements for cash, which are explained below under “Liquidity and Capital Resources”; and (e) whether Tianshi Engineering, our affiliate who sells our products in China, obtains a direct selling license in China. Statements made herein are as of the date of the filing of this Form 10-K with the Securities and Exchange Commission and should not be relied upon as of any subsequent date.
Unless otherwise required by applicable law, we do not undertake, and we specifically disclaim any obligation, to update any forward-looking statements to reflect occurrences, developments, unanticipated events or circumstances after the date of such statement.
Overview
Tiens USA researches, develops, manufactures, and markets 37 nutrition supplement products, including wellness products and dietary supplement products. Our operations are conducted from our headquarters in Tianjin, China through our 80% owned subsidiary, Biological, and our wholly-owned subsidiary, Life Resources. We sell our products to affiliated companies in China and internationally.
We develop our products at our product research and development center, which employs highly qualified professionals in the fields of pharmacology, biology, chemistry and fine chemistry.
In China, we sell our products to Tianshi Engineering, an affiliated company. Tianshi Engineering, in turn, sells the products to customers through its branches and affiliated companies and at chain stores, which are owned by individual distributors. Internationally, we sell our products to overseas affiliates who in turn re-package the products to meet the needs of the local markets and sell to independent distributors who use the products themselves and/or resell them to other distributors or consumers.
Results of Operations
Year Ended December 31, 2010 Compared to Year Ended December 31, 2009
|
Year ended December 31,
|
||||||||||||
|
2010
|
2009
|
Change
|
||||||||||
|
REVENUE - RELATED PARTIES
|
$ | 41,021,135 | $ | 60,032,968 | -33.3 | % | ||||||
|
REVENUE - THIRD PARTIES
|
323,585 | 1,943,101 | ||||||||||
|
COST OF SALES-RELATED PARTIES
|
14,850,739 | 18,754,680 | -25.6 | % | ||||||||
|
COST OF SALES-THIRD PARTIES
|
158,638 | 1,412,812 | ||||||||||
|
GROSS PROFIT
|
26,335,343 | 41,808,577 | -37.0 | % | ||||||||
|
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES
|
19,530,501 | 16,009,382 | 22.0 | % | ||||||||
|
INCOME FROM OPERATIONS
|
6,804,842 | 25,799,195 | -73.6 | % | ||||||||
|
OTHER (EXPENSE) INCOME, NET
|
(176,578 | ) | (61,591 | ) | 186.7 | % | ||||||
|
INCOME BEFORE PROVISION FOR INCOME TAXES
|
6,628,264 | 25,737,604 | -74.2 | % | ||||||||
|
PROVISION FOR INCOME TAXES
|
1,469,548 | 930,703 | 57.9 | % | ||||||||
|
NET INCOME
|
5,158,716 | 24,806,901 | -79.2 | % | ||||||||
|
LESS: Net income attributable to the noncontrolling interest
|
(323,101 | ) | (965,557 | ) | -66.5 | % | ||||||
|
NET INCOME ATTRIBUTABLE TO TIENS BIOTECH GROUP
|
$ | 4,835,615 | $ | 23,841,344 | -79.7 | % | ||||||
|
WEIGHTED AVERAGE NUMBER OF SHARES, BASIC AND DILUTED
|
71,333,586 | 71,333,586 | ||||||||||
|
EARNINGS PER SHARE, BASIC AND DILUTED
|
$ | 0.07 | $ | 0.33 | ||||||||
Revenue. In 2010 revenue was $41.3 million, compared to $62.0 million in 2009, a decrease of 33.3%. The breakdown of revenue between Chinese and international sales is as follows:
Chinese and International Revenue
|
Revenue
|
Year 2010
|
Year 2009
|
% Change
|
|||||||||
|
China
|
$ | 24,894,472 | $ | 27,241,333 | -8.6 | % | ||||||
|
International
|
$ | 16,450,248 | $ | 34,734,736 | -52.6 | % | ||||||
For 2010, revenue in China was $24.9 million, a decrease of 8.6% compared to $27.2 million for 2009.
The decrease in revenue for the year of 2010 was mainly due to the decrease in international sales. For the year of 2010, international revenue was $16.5 million, a decrease of 52.6% compared to $34.7 million for the year of 2009. The reasons for the decrease in international revenue include, but are not limited to the following: (1) During 2008, China’s Administration of Quality Supervision, Inspection and Quarantine (“AQSIQ”) carried out a national campaign against unsafe food and substandard products, which brought on a general slow-down and backlog of export clearances for Chinese food products. Upon the lifting of the regulations, overseas affiliated companies began to purchase more products, thereby increasing sales of 2009; (2) Our Indonesia affiliated company has not purchased from us during the year of 2010, given they purchased more products in 2009 after the 2008 product scarcity for the reason noted above. During the first half of 2009, our Indonesia affiliated company purchased $9.2 million of finished and semi-finished product from us, which was 2.7 times their purchases for the first half of 2008. In addition, local Original Equipment Manufacturers (“OEM”) in Indonesia have been producing healthcare products with our semi-finished goods, which have a profit margin that is much lower than the profit margin of finished goods. The sales decline in Indonesia accounts for 43.56% of that in overseas. (3) The lasting global economic recession has generally reduced customers' buying power. During the early stages of the recession, the direct selling business is generally benefited with the new sales force joined by the unemployed population. This positive effect has been fading away during the later stages of the recession; (4) Our affiliated companies in many regions have made certain adjustments to their marketing programs and reorganizations at their branch and higher levels, which is expected to boost sales performance over the long-run but negatively affect sales in the short-run.
Cost of sales. Cost of sales were $15.0 million in 2010 compared to $20.2 million in 2009, a decrease of 25.6%. This decrease was primarily due to the corresponding decrease in sales. Cost of sales decreased at a lower rate than revenue, primarily due to fixed costs, which do not increase or decrease in line with sales.
Gross profit. Gross profit decreased by 37.0% to $26.3 million in 2010, compared to $41.8million in 2009. The gross profit margin for 2010 was 63.7% compared to 67.5% in 2009.
Selling, general and administrative expenses. Selling, general and administrative expenses increased by 22.0% to $19.5 million in 2010, compared to $16.0million in 2009. The increase was primarily due to increases in allowance for bad debt ($1.9 million), salaries expenses ($0.7 million) and Research & Development Expenses ($0.6 million).
Other (expense) income, net. Other income, net was $0.2 million of expense in 2010, compared to expense of $0.06 million in 2009. This was mainly due to the decrease in interest income and the increase in interest expense of 2010 compared to 2009. In the second half of 2010, the Company obtained a $9.1million loan from Tianshi Engineering with an interest rate of 5.31% per year and a $3.0 million loan from Construction Bank with the benchmark interest rate of one-year loan (The interest rate is 5.56% from October 20, 2010 to December 25, 2010 and 5.81% from December 26, 2010 to December 31, 2010.), which contributed to the increase of interest expense.
Provision for income tax. Provision for income tax increased by 57.9% to $1.5 million in 2010, compared to $0.9 million in 2009. The main reason of this increase was that Life Resources began to pay income tax at the rate of 12.5% from January 1, 2010.
Net income. For the above stated reasons, net income in 2010 was $4.8 million compared to $23.8 million in 2009, a decrease of 79.7%.
Financial Condition, Liquidity and Capital Resources
We have historically met our working capital and capital expenditure requirements, including funding for expansion of operations, through net cash flow provided by operating activities. Our principal source of liquidity is our operating cash flow. From the forth quarter of 2010, due to the large amount of cash required for construction in progress and materials purchase of Life Resources, we obtained bank loans.
On October 9, 2010, Life Resources entered into a loan agreement with Agricultural Bank of China (Agricultural Bank), pursuant to which Agricultural Bank loaned RMB200,000,000 (or US$30,248,000) with the benchmark interest rate of The People’s Bank of China to fund Life Resources’ construction in progress under the guarantee of Tiens Group. Under the agreement, Life Resources is permitted the full amount before October 8, 2011, and repay it before October 8, 2013. As of December 31, 2010, Life Resources has drawn RMB122,000,000 (or US$18,451,280) of the loan.
On October 20, 2010, we obtained a loan of RMB20,000,000 (US $3,024,800) from Construction Bank of China for purchasing raw materials and other auxiliary materials at the term of one year. The weighted-average interest rate of the short-term debt is the one-year benchmark lending rate (The interest rate is 5.56% from October 20, 2010 to December 25, and 5.81% from December 26, 2010 to December 31.).
Net cash provided by operating activities was $60.4 million in 2010, compared to $43.9million in 2009. Our net income in the year of 2010 was $5.2 million, a 79.2% decrease from 2009.
As of December 31, 2010, we had negative working capital of $10.3 million. Cash was $10.2 million as of December 31, 2010, compared to $1.8 million as of December 31, 2009.
Net cash used in investing activities was $73.2 million in 2010 compared to $83.0 million in 2009. In 2010, Life Resources paid $54.5 million on construction in progress.
Accounts receivable-related parties decreased to $10.0 million as of December 31, 2010 from $15.4 million as of December 31, 2009, which was mainly due to the decrease in sales in the year of 2010 compared to those of 2009. Other receivables-related parties decreased to $17.4 million as of December 31, 2010 from $44.6 million as of December 31, 2009, which was mainly due to our collection of a receivable from Tianshi Investment for the sale of Tiens Yihai Co., Ltd. (“Tiens Yihai”).
Going forward, our primary requirements for cash consist of:
|
|
·
|
completion of construction by Life Resources of new research and development, manufacturing and logistic facilities, and administrative offices;
|
|
|
·
|
the continued production of existing products and general overhead and personnel related expenses to support these activities;
|
|
|
·
|
the development costs of new products; and
|
|
|
·
|
expansion of production scale to meet the demands of our markets.
|
The Wholly Foreign Owned Enterprise Law (1986), as amended, and The Wholly Foreign Owned Enterprise Law Implementing Rules (1990), as amended, contain the principal regulations governing dividend distributions by wholly foreign owned enterprises. Under these regulations, wholly foreign owned enterprises, such as Life Resources, may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. Additionally, Life Resources are required to set aside a certain amount of any accumulated profits each year (a minimum of 10%, and up to an aggregate amount equal to half of its registered capital), to fund certain reserve funds. These reserves are not distributable as cash dividends, except in the event of liquidation, and cannot be used for working capital purposes. The PRC government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of the PRC. We have not declared any dividends on our common stock since inception and do not intend to pay dividends on our common stock in the foreseeable future. If we ever determine to pay a dividend, we may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of such dividends from the profits of Biological and Life Resources.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements.
Critical Accounting Estimates
Management’s discussion and analysis of its financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). Our financial statements reflect the selection and application of accounting policies which require management to make significant estimates and judgments. See note 2 to our consolidated financial statements, “Summary of Significant Accounting Policies.” Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions. We believe that the following reflect the more critical accounting policies that currently affect our financial condition and results of operations.
Revenue Recognition
During 2010, we sold both semi-finished products and finished products to Tianshi Engineering domestically. Revenue from semi-finished products was recognized at delivery point. Revenue from finished products was recognized only when the related party Chinese distributors recognized sales of our products to unaffiliated third parties. Revenues in both cases are net of taxes.
For overseas sales, we sell finished products. We recognize revenue from international sales (non-Chinese) to affiliated parties, net of taxes, as goods are shipped and clear review by the customs department of the Chinese government.
We are generally not contractually obligated to accept returns. However, on a case by case negotiated basis, we permit customers to return products. Revenue is recorded net of an allowance for estimated returns. Such reserves are based upon management’s evaluation of historical experience and estimated costs. The amount of the reserves ultimately required could differ materially in the near term from amounts included in the accompanying consolidated financial statements. As of December 31, 2010, Tianshi Engineering, an affiliated company, owned all of the related party distributors that sell our products in China.
Allowance for Doubtful Accounts
Our trade accounts receivables are mainly due from related companies. We have made full provision for accounts receivable-related parties aging over one year and a general allowance for doubtful debts of 0.5% of the remaining accounts receivable-related parties. Management reviews its accounts receivable on a regular basis to determine if the bad debt allowance is adequate at each year-end, paying particular attention to the age of receivables outstanding.
Inventories
Inventories are stated at the lower of cost or market using the moving average basis. Management reviews inventory annually for possible obsolete goods or to determine if any reserves are necessary for potential obsolescence.
Recent accounting pronouncements
In October 2009, the FASB issued Accounting Standards Update (ASU) 2009-13, Multiple-Deliverable Revenue Arrangements (ASU 2009-13) (formerly EITF 08-1, Revenue Arrangements with Multiple Deliverables) which amends ASC 605, Revenue Recognition. This accounting update establishes a hierarchy for determining the value of each element within a multiple deliverable arrangement. The amendments in this update are effective for revenue arrangement entered into or materially modified in fiscal years beginning on or after June 15, 2010. The adoption of ASU 2009-13 did not have an impact on the Company’s consolidated financial statements.
In January 2010, the FASB issued ASU 2010-02 — Consolidation (Topic 810): Accounting and Reporting for Decreases in Ownership of a Subsidiary. This update amends Subtopic 810-10 and related guidance to clarify that the scope of the decrease in ownership provisions of the Subtopic and related guidance applies to (i) a subsidiary or group of assets that is a business or nonprofit activity; (ii) a subsidiary that is a business or nonprofit activity that is transferred to an equity method investee or joint venture; and (iii) an exchange of a group of assets that constitutes a business or nonprofit activity for a noncontrolling interest in an entity, but does not apply to: (i) sales of in substance real estate; and (ii) conveyances of oil and gas mineral rights. The amendments in this update are effective beginning in the period that an entity adopts FAS 160 (now included in Subtopic 810-10). The adoption of the provisions in ASU 2010-02 did not have an impact on the Company’s consolidated financial statements.
The FASB has issued ASU 2010-06, Fair Value Measurements and Disclosures (Topic 820): Improving Disclosures about Fair Value Measurements. This ASU requires some new disclosures and clarifies some existing disclosure requirements about fair value measurement as set forth in Codification Subtopic 820-10. ASU 2010-06 amends Codification Subtopic 820-10 and now requires a reporting entity to use judgment in determining the appropriate classes of assets and liabilities and to provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements. ASU 2010-06 was effective for interim and annual reporting periods beginning after December 15, 2009. As this standard relates specifically to disclosures, the adoption did not have an impact on the Company’s consolidated financial position and results of operations.
In February 2010, the FASB issued ASU 2010-10, "Consolidation (Topic 810)." The amendments to the consolidation requirements of ASC Topic 810 resulting from the issuance of Statement 167 are deferred for a reporting entity's interest in an entity (1) that has all the attributes of an investment company or (2) for which it is industry practice to apply measurement principles for financial reporting purposes that are consistent with those followed by investment companies. An entity that qualifies for the deferral will continue to be assessed under the overall guidance on the consolidation of variable interest entities in ASC Subtopic 810-10 (before the Statement 167 amendments) or other applicable consolidation guidance, such as the guidance for the consolidation of partnerships in ASC Subtopic 810-20. The deferral is primarily the result of differing consolidation conclusions reached by the International Accounting Standards Board ("IASB") for certain investment funds when compared with the conclusions reached under Statement 167. The deferral is effective as of the beginning of a reporting entity's first annual period that begins after November 15, 2009, and for interim periods within that first annual reporting period, which coincides with the effective date of Statement 167. Early application is not permitted. The provisions of ASU 2010-10 are effective for the Company beginning in 2010. The adoption of ASU 2010-10 did not have an impact on the financial position, results of operations or cash flows of the Company.
In February 2010, the FASB issued an accounting standard that amended certain recognition and disclosure requirements related to subsequent events. The accounting standard requires an entity that is an SEC filer to evaluate subsequent events through the date that the financial statements are issued and removes the requirement that an SEC filer disclose the date through which subsequent events have been evaluated. This guidance was effective upon issuance. The adoption of this standard had no effect on the Company’s consolidated financial position or results of operations.
In May 2010, the FASB issued ASU 2010-19, “Foreign Currency (Topic 830): Foreign Currency Issues: Multiple Foreign Currency Exchange Rates”. The amendments in this ASU are effective as of the announcement date of March 18, 2010. The adoption of this update did not have an impact on the Company’s financial statements.
In July 2010, the FASB issued ASU 2010-20, Disclosures about the Credit Quality of Financing Receivables and the Allowance for Credit Losses, which amends ASC 310, Receivables. This ASU requires disclosures related to financing receivables and the allowance for credit losses by portfolio segment. The ASU also requires disclosures of information regarding the credit quality, aging, nonaccrual status and impairments by class of receivable. A portfolio segment is the level at which a creditor develops a systematic methodology for determining its credit allowance. A receivable class is a subdivision of a portfolio segment with similar measurement attributes, risk characteristics and common methods to monitor and assess credit risk. Trade accounts receivable with maturities of one year or less are excluded from the disclosure requirements. The effective date for disclosures as of the end of the reporting period is the first quarter of fiscal year 2011. The effective date for disclosures for activity during the reporting period is the second quarter of fiscal year 2011. The adoption will not have a material effect on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s consolidated financial statements upon adoption.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Not applicable.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
The financial statements required by this item can be found following the signature page of this Annual Report.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness, as of the end of the period covered by this report, of our disclosure controls and procedures, as such term is defined in Exchange Act Rule 13a-15(e). Based on this evaluation, our principal executive officer and principal financial officer concluded that, as of such date, the Company’s disclosure controls and procedures were effective.
Disclosure controls and procedures are designed to ensure that information required to be disclosed by us in our Exchange Act reports is recorded, processed, summarized, and reported, within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f).Our management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and concluded that as of the end of the period covered by this report, our internal controls over financial reporting were effective.
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Our management’s report was not subject to attestation by our registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit us to provide only our management’s report in this annual report.
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting during the fourth quarter of 2010 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Not applicable.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Set forth below are the names of our directors and executive officers as of March 29, 2011. Jinyuan Li has served on the Board since September 2003. Baolan Li was appointed to the Board in October 2010. All other directors have served on the Board since January 2004. Directors are elected annually by our shareholders at the annual meeting. Each director holds his or her office until his or her successor is elected and qualified or his or her earlier resignation or removal. Mr. Li’s and Ms. Li’s statutory employment agreements provide for an indefinite period.
|
NAME
|
AGE
|
POSITION
|
||
|
Jinyuan Li
|
53
|
Chairman, Chief Executive Officer, President and Acting Chief Financial Officer
|
||
|
Yupeng Yan
|
48
|
Executive Vice President and Director
|
||
|
Baolan Li
|
28
|
Director
|
||
|
Socorro Quintero
|
59
|
Director
|
||
|
Gilbert Raker
|
67
|
Director
|
None of our directors and officers was selected pursuant to any agreement or understanding with any other person. There is no family relationship between any director or executive officer and any other director or executive officer, except that Ms. Baolan Li is the daughter of Mr. Jinyuan Li.
Jinyuan Li
Jinyuan Li has served as the Chairman of the Board and a Director since September 2003. In June 2010, Jinyuan Li was appointed as Acting Chief Financial Officer. Jinyuan Li is also the President and founder of Tianshi Group and has held the position of President since 1995. Mr. Li has 14 years of experience in the petroleum and plastics industries. He holds a number of leadership positions in government and social associations, including as Standing Committee Member of Tianjin Political Consultative Conference; Vice-chairman of China Enterprise Confederation & China Enterprise; Executive of All-China Federation of Industry & Commerce; Vice Chairman of China Association for the Promotion of Industrial Development; and Vice President of Chinese Healthcare Association, etc. Mr. Li was elected as one of the Top Ten Most Outstanding Talents in the Greater China Area; one of the Ten Most Popular Personages Among the High-Ranking, by China Economic Forum; Excellent Entrepreneur, by the Organization Committee of the Second Chinese Entrepreneur Forum in 2003, and as the Most Creative Chinese Businessman of Asia in 2004. Mr. Li holds an MBA from Nankai University.
Yupeng Yan
Mr. Yan has served as our Executive Vice-President since September 2003. Mr. Yan has also served as Vice-President of Tianshi Group since March 1997, acting as general manager of its Global Information Technology Center from July 2007 to January 2009 and as head of its Global Marketing Center from June 2004 to June 2007. Since November 2008 Mr. Yan has served as the Vice General Manager of Global Marketing Center and Vice President of Tianshi Group’s China Region. Mr. Yan currently holds a number of leadership positions including Vice-Dean of Tianshi College (formerly Tianshi Occupational Technique Institute), and Vice-Chairman of Tianshi Science and Technique Association. Mr. Yan was elected as one of the Chinese Ten Outstanding Professional Managers in 2004. Mr. Yan received an Executive MBA from Nankai University in July 2004.
Baolan Li
On October 20, 2010, Baolan Li joined the Board of Directors of the Company. Ms. Li is the daughter of Jinyuan Li, the Chairman, Chief Executive Officer, President and Acting Chief Financial Officer of the Company. From August 2008 to October 2010, Ms. Li served as the Assistant of the Chief Financial Officer of the Company and as the Assistant of the Vice President – Marketing. Prior to her appointment to the Company, Ms Li attended the Royal Holloway University of London, London, UK and received a Bachelor’s Degree with Honors in Industrial and Financial Economics in 2006 and a Master’s Degree in Economics in 2008. Ms. Baolan Li held a short-term internship position with ICEA Investment Banking Division (Hong Kong) from September 2006 to November 2006.
Socorro Quintero
Dr. Socorro Quintero serves as our director since January 2004. She is Associate Professor of Finance at Oklahoma City University’s Meinders School of Business (“OCU”) where she has organized and managed the Corporate Directors Institute (“CDI”) in 2007 and 2009. The CDI brings together directors and governance professionals to share the latest issues and best practices in corporate board governance. In February 2011, Dr. Quintero joined the board of directors and became the chairperson of the audit committee of the board of directors of Joway Health Industries Group (GTVI.OB). Prior to joining OCU in 1993, she was an Assistant Professor of Finance at the University of South Florida in Tampa. Her teaching and research expertise covers varied areas in finance, including, but not limited to, investments, asset pricing, capital budgeting, and financial derivatives. She obtained her PhD in Finance from the University of Texas at Austin in 1989. Before her career in finance, her earlier work experiences were in various industrial engineering and management capacities while working for Abbott Laboratories, Atlantic Steel Company and Levi Strauss & Company. Dr. Quintero obtained a Bachelor of Science in Physics from the University of the Philippines in 1972, and Master of Science in Industrial Engineering from the Georgia Institute of Technology in Atlanta in 1977.
Gilbert Raker
Mr. Raker serves as our director since January 2004. Since February 2010 Mr. Raker has been the President of Wyngate Management Corporation,, a private consulting company that works in association with several IR / PR, investment and merchant banking firms in New York City that specialize in assisting middle market firms. From February 2010 to February 2011 he was also the Vice Chairman and a director of Electro-Comp Services, Inc., a private company engaged in the testing, verification and brokering of electronic components. From January 2009 to February 2010 he worked with a niche investment bank in New York that focused on financing middle market companies. From November 1988 to January 2009 he was the President, Chief Executive Officer and Chairman of the Board of SEMX Corporation, a multi-national company that manufactured materials and components used in microelectronic circuitry on a worldwide basis for the automotive, consumer electronics, defense, medical and aerospace industries. Prior to November 1988, Mr. Raker worked at two private equity investment firms, was employed as the Chief Financial Officer and in one case as the Chief Operating Officer of two New York Stock Exchange listed companies and served in a variety of capacities in numerous private and public companies. He began his career as a Management Consultant for Touche Ross. Mr. Raker received his B.S. in Chemistry from Eastern University, his MBA in Production Management from Syracuse University and completed all of the course work for a PhD in Finance and Economics at Syracuse University. Early in his career he taught Accounting and Production Management at Eastern University and Syracuse University, respectively.
Board Leadership Structure
The Board of Directors believes that Mr. Li’s service as both Chairman of the Board, Chief Executive Officer, President and Active Chief Financial Officer is in the best interest of the Company and its stockholders. Mr. Li possesses detailed and in-depth knowledge of the issues, opportunities and challenges facing the Company and its business and is thus best positioned to develop agendas that ensure that the Board’s time and attention are focused on the most critical matters. His combined role enables decisive leadership, ensures clear accountability, and enhances the Company’s ability to communicate its message and strategy clearly and consistently to the Company’s shareholders, employees and customers.
Each of the directors other than Jinyuan Li, Yupeng Yan and Baolan Li is independent (see “Director Independence” below), and the Board of Directors believes that the independent directors provide effective oversight of management. The Board of Directors has not designated a lead director. Our independent directors call and plan their executive sessions collaboratively and, between Board of Directors meetings, communicate with management and one another directly. In the circumstances, the directors believe that formalizing in a lead director functions in which they all participate might detract from rather than enhance performance of their responsibilities as directors.
Board’s Role In Risk Oversight
The Board of Directors is responsible for the overall risk oversight of the Company. The Board discusses, and receives updates from senior management on the identification, assessment, management and mitigation of the critical risks facing the Company. The Audit Committee requires the Company’s management to update the Audit Committee about the Company’s major financial risk exposure and the steps that management has taken to monitor and control such exposure and oversee the risks related to financial issues. The Audit Committee monitors risks associated with financial reporting and internal controls, receives annual reports on risk assessment from the Company’s auditors and regularly discusses financial and economic risks as well as financial implications of certain regulatory or legal risks with the Company’s Chief Executive Officer, Acting Chief Financial Officer, other members of senior management and outside counsel or consultants.
Director Qualifications
We seek directors with established strong professional reputations and experience in areas relevant to the strategy and operations of our businesses. We seek directors who possess the qualities of integrity and candor, who have strong analytical skills and who are willing to engage management and each other in a constructive and collaborative fashion. We also seek directors who have the ability and commitment to devote significant time and energy to service on the Board and its committees. We believe that all of our directors meet the foregoing qualifications.
The Board of Directors believes that the leadership skills and other experiences of its Board members, as described below, provide the Company with a range of perspectives and judgment necessary to guide our strategies and monitor their execution.
Jinyuan Li: Jinyuan Li has extensive PRC business experience and being a founder of the Company has enabled him to lead the Board throughout the development of the Company.
Yupeng Yan: Yupeng Yan has extensive experience in management and marketing which enables him to guide the Board through related decisions.
Baolan Li: Baolan Li has a strong academic background that enables her to be an effective director.
Socorro Quintero: Socorro Quintero has strong academic background and extensive experience in reviewing financial results of companies which enables her to effectively serve as the chairman of our Audit Committee since the Company’s listing in the United States.
Gilbert Raker: Gilbert Raker has extensive experience in finance and management, and his leadership experience gained while serving on the board of a multi-national company enable him to effectively serve as a member of our Audit Committee and Compensation Committee.
Board Practices
Our business and affairs are managed under the direction of our Board of Directors. The primary responsibilities of our Board of Directors are to provide oversight, strategic guidance, counseling and direction to our management.
The Board has an Audit Committee and does not have a Nominating Committee. The entire Board assumes the duties that would be delegated to a Nominating Committee. The Company believes that this practice focuses the attention of each director on the important task of selecting nominees, and a separate committee is unnecessary.
Nominations by Stockholders
There have been no changes to the procedures by which the stockholders of our company may recommend nominees to the Board of Directors since the filing of the Company’s Definitive Proxy Statement on April 29, 2010 for its Annual Meeting of Stockholders, which was held on May 28, 2010.
Audit Committee
The Board of Directors has established an audit committee in accordance with Section 3(a)(58)(A) of the Exchange Act. The members of the Audit Committee during the fiscal year ended December 31, 2010 were Socorro Quintero, Chairperson, Howard Balloch and Gilbert Raker, each of whom was independent as defined under Section 121(A) of NYSE Amex listing standards currently in effect. During the fiscal year ended December 31, 2010, none of the Audit Committee members was a current officer or employee of our company or any of its affiliates.
The Board of Directors has determined that Socorro Quintero and Gilbert Raker each qualifies as an “audit committee financial expert” under Item 407(d) of Regulation S-K.
Code of Ethics
The Board has adopted a Code of Ethics to promote its commitment to the legal and ethical conduct of our company’s business. The Chief Executive Officer, Chief Financial Officer, and other senior officers are required to abide by the Code of Ethics, which provides the foundation for compliance with all corporate policies and procedures, and best business practices.
The Code of Ethics was filed as an exhibit to our Annual Report on Form 10-KSB for the year ended December 31, 2004. A written copy of the Code of Ethics can be found on our website at www.tiens-bio.com and will be provided upon request at no charge by writing to Secretary, Tiens Biotech Group (USA), Inc., No. 17, Xinyuan Road, Wuqing New-Tech Industrial Park, Tianjin, China 301700.
Section 16(a) Beneficial Ownership Reporting Compliance
Based solely upon a review of Forms 3, 4 and 5 and amendments to these forms furnished to us, all parties subject to the reporting requirements of Section 16(a) of the Exchange Act filed all such required reports during and with respect to 2010 except that Ms. Li did not timely file one Form 3.
Compensation Discussion and Analysis
All compensation decisions for our executive officers, including the salary of our Chief Executive Officer, Acting Chief Financial Officer and President, Jinyuan Li, are made by Jinyuan Li. Because Jinyuan Li owns more than 50% of our voting stock, we are a “controlled company” pursuant to Rule 801(a) of NYSE Amex Rules (“NYSE Amex Rules”). Therefore, we are exempt from NYSE Amex Rule 805(a), which requires that the compensation of a CEO and all other executive officers be determined, or recommended to the Board for determination, by a compensation committee composed of independent directors, or the majority of independent directors on the Board.
The objectives of our compensation programs.
We seek to attract and retain executive officers of the highest caliber and motivate them to maximize the success of our business.
What our compensation program is designed to reward.
Our CEO believes that he is incentivized by his large equity ownership in our company. Therefore, he believes that a long-term employment contract providing a base salary is appropriate compensation for him. With respect to the other executive officers’ base salaries, our CEO bases his recommendations on past salary levels with our company and his perception of the quality of their respective performances and attempts to match their salaries with his perception of compensation levels at a small number of companies he considers comparable. The CEO also takes in to consideration the relatively low salaries provided to executive officers by companies in China compared to public companies in the United States. Our CEO assesses the normal responsibilities of each position, as well as the extra responsibilities and additional work related to special projects which such executive officers may be expected to perform. No relative weight was assigned to any of the foregoing factors.
Elements of compensation.
Each executive officer receives cash compensation as a base salary. Base salary for our executive officers is fixed by their respective employment agreements, as described under “Employment Agreements.” Jinyuan Li’s salary for 2010 was fixed pursuant to employment agreements with Tianjin Tianshi Biological Development Co. Ltd. (“Biological”) entered into in 2005. Manbo He was our principal financial officer from June 1, 2009 to June 1, 2010, and his salary for 2010 was fixed pursuant to an employment agreement with Biological, dated June 1, 2009. Their base salaries were based on our CEO’s subjective perceptions of salaries paid by comparable companies for comparable positions. Our executive officers did not receive any bonuses for 2010. Due to the fact that we do not currently and did not in 2010 give bonuses to any of our named executive officers, Jinyuan Li did not identify any individual or corporate goals when setting the remuneration of Mr. He for 2010.
Our strategy is to maintain compensation for employees at levels that are equal to or in excess of those offered by companies of comparable size, consistent with the individual employees’ capabilities and responsibilities. We do not currently have a stock option plan, but may consider adopting one in the future to further incentivize its employees.
Why we chose to pay each element.
We have entered into long-term employment agreements with Mr. Li, providing for his base salary.
The employment agreements with Mr. Li and Ms. Li provide for payments upon termination for specified reasons. These payments are required by local Chinese employment regulations. Additional information regarding applicable payments under such agreements is provided under the heading “Potential Payments Upon Termination or Change of Control.”
How we determine the amount for each element of pay.
With respect to the executive officers’ base salaries, our CEO bases his recommendations on past salary levels and his perception of the quality of the executive officers’ respective performances and attempts to match their salaries with his perception of compensation levels at a small number of companies he considers comparable, although not necessarily included in the NYSE Amex Composite Index or NASDAQ Biotechnology Index. Our CEO assesses the normal responsibilities of each position, as well as the extra responsibilities and additional work related to special projects which such executive officers may be expected to perform. Our CEO also takes in to consideration the relatively low salaries provided to executive officers by companies in China compared to public companies in the United States.
Compensation Committee Interlocks and Insider Participation
During 2010, the members of the Compensation Committee were Gilbert Raker and Yupeng Yan. The Compensation Committee did not deliberate on executive compensation for fiscal 2010. Yupeng Yan was an employee and officer of our company during 2010. No member of the Compensation Committee has a relationship that would constitute an interlocking relationship with Executive Officers or Directors of our company or another entity.
Compensation Committee Report
The Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K of the Exchange Act with management and the full Board. Based on that review and discussion, the Compensation Committee recommended to the Board that the Compensation Discussion and Analysis be included in our Annual Report on Form 10-K for 2010 .
The Compensation Committee
Gilbert Raker, Chairman
Yupeng Yan
Summary Compensation Table
The table below summarizes the total compensation paid or earned by each of the named executive officers for the fiscal years ended December 31, 2010 and 2009.
|
Name and
Principal Position (1)
|
Year
|
Salary
($)
|
Total
($)
|
|||||||
|
Jinyuan Li
|
2010
|
$
|
166,660
|
$
|
166,660
|
|||||
|
Chairman, Chief Executive
|
2009
|
$
|
166,660
|
$
|
166,660
|
|||||
|
Manbo He
|
2010
|
$
|
21,778
|
$
|
21,778
|
|||||
|
Chief Financial Officer (2)
|
2009
|
$
|
23,333
|
$
|
23,333
|
|||||
(1) Yupeng Yan was an employee Director of our company during 2010 and 2009 but is not identified as a “named executive officer” because his total compensation was less than $100,000.
(2) Manbo He became Chief Financial Officer and Director of the Company on June 1, 2009 and resigned effective June 17, 2010.
Grants of Plan Based Awards; Outstanding Equity Awards at Fiscal Year-End; Option Exercises and Stock Vested
We do not have any stock option plans.
Pension Benefits
Nonqualified Deferred Compensation
We do not provide any pension benefits or nonqualified deferred compensation to our employees.
Employment Agreements
Our subsidiary, Biological, has entered into a statutory employment agreement with each of the named executive officers of our company. Jinyuan Li’s contract is dated June 1, 2005 and has an indefinite period. The employment contract for Yupeng Yan is dated April 1, 2004 and provided for a term through March 31, 2009. On April 1, 2009, Yupeng Yan’s employment contract was renewed with an indefinite period.
Under each of these employment contracts, the employee receives vacation time and social insurance according to Chinese government regulations. Biological may rescind each agreement without notice if, among other events, the employee materially violates Biological’s rules and regulations or the employee grossly neglects his or her duties and discloses our confidential business information that harms us. Biological may rescind each agreement on 30 days’ notice and provide economic compensation if, among other events, the employee suffers from a disease or non-work related injury and after a recovery period is unable to work, or due to material changes, the performance of the agreement has become unpractical. Pursuant to the terms of each of the employment agreements, the employee may rescind his contract on 30 days’ written notice.
Potential Payments upon Termination
Mr. Li’s employment contract provides for a one-time lump sum payment equal to six months of his then current salary if we terminate his employment contract for one of the following reasons:
|
|
·
|
The employee has a non-work-related injury and is unable to perform his responsibilities; or
|
|
|
·
|
The employee is unable to perform his responsibilities for other reasons; or
|
|
|
·
|
The circumstances based on which the employment contract was entered into have materially changed, and the performance of the contract becomes impractical; or
|
|
|
·
|
We are contemplating bankruptcy and determine to reduce staff.
|
Assuming that Mr. Li was terminated for one of the above-stated reason, Mr. Li would receive $83,330 There are no other circumstances, including a change of control of our company, where we are required to make any additional payment to Mr. Li.
Director Compensation
On October 20, 2010, our subsidiary, Biological, entered into a statutory labor contract with Ms. Li to document the terms of her employment by Biological. Pursuant to the terms of the agreement, Biological shall pay Ms. Li an annual fee of $40,000. The agreement is for an indefinite period. The contract shall be terminated in compliance with the PRC Labor Contract Law, when unilaterally terminated by either party or when terminated pursuant to a mutual consent between the parties.
For the fiscal year ended December 31, 2010, the remaining members of the Board who are not our employees are entitled to receive an annual cash retainer of $30,000.
Director Summary Compensation Table
The table below summarizes the compensation we paid to non-employee Directors for the fiscal year ended December 31, 2010.
|
Name (1)
|
Fees Earned or
Paid in Cash
($)
|
Total
($)
|
||||||
|
Howard Balloch(2)
|
$
|
30,000
|
$
|
30,000
|
||||
|
Baolan Li(3)
|
$ |
7,889
|
$ |
7,889
|
||||
|
Gilbert Raker
|
$
|
30,000
|
$
|
30,000
|
||||
|
Socorro Quintero
|
$
|
30,000
|
$
|
30,000
|
||||
|
(1)
|
Jinyuan Li, and Manbo He are not included in this table as they were our employees during 2010 and received no compensation for their services as Directors. Their compensation is disclosed in the table in the “Summary Compensation Table”.
|
|
(2)
|
Effective as of December 31, 2010, Howard Balloch resigned from his position as Director of the Company.
|
|
(3)
|
On October 20, 2010, the Board of Directors appointed Baolan Li as a member of the Board of Directors of the Company.
|
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The following table sets forth information with respect to the beneficial ownership of shares of common stock as of March 29, 2011 by each person or entity who is known by us to beneficially own five percent or more of our common stock; each director and executive officer of our company; and all directors and executive officers of our company as a group.
|
Name of Beneficial Owner (1)
|
Number of Shares
|
Percent of Class
|
||||||
|
Jinyuan Li
|
67,830,000 | 95.1 | % | |||||
|
Baolan Li
|
— | — | ||||||
|
Yupeng Yan
|
— | — | ||||||
|
Socorro Quintero
|
— | — | ||||||
|
Gilbert Raker
|
— | — | ||||||
|
All Directors and Executive Officers as a Group (6 persons)
|
67,830,000 | 95.1 | % | |||||
|
TIENS (USA) Investment Holdings Group Overseas Limited (2)
|
67,830,000 | 95.1 | % | |||||
(1) Unless otherwise indicated, the address for each named individual or group is c/o Tiens Biotech Group (USA), Inc., No. 17, Xinyuan Road, Wuqing New-Tech Industrial Park, Tianjin, China 301700.
(2) The shares are owned by TIENS (USA) Investment Holding Group Overseas Limited (“TIH”). As sole director of TIH, Jinyuan Li has voting and dispositive power over the shares. The business address of TIH is c/o Tiens Biotech Group (USA), Inc., No. 17, Xinyuan Road, Wuqing New-Tech Industrial Park, Tianjin, China 301700.
Unless otherwise indicated, we believe that all persons named in the table have sole voting and investment power with respect to all shares of common stock beneficially owned by them.
Changes in Control
There were no arrangements, known to the Company, including any pledge by any person of securities of the Company the operation of which may at a subsequent date result in a change in control of the Company.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Director Independence
The Board has determined that during the fiscal year ended December 31, 2010, directors Socorro Quintero and Gilbert Raker are “independent” under Section 803(A) of the listing standards of NYSE Amex. The remaining members of the Board did not satisfy these “independence” definitions. The ownership by Jinyuan Li, the Company’s President, Chief Executive Officer and Acting Chief Financial Officer, of more than 50% of the Company’s voting stock makes it a “controlled company” to which NYSE Amex rules requiring a majority of the directors to be independent and relating to executive compensation and Board nominations need not apply. The Board has an Audit Committee and a Compensation Committee.
Transactions with Related Parties
We market our products through various domestic and international business entities that are related to us through common ownership. As a result, most of our total consolidated sales in 2010 were to related parties.
We have a sales contract with Tianshi Engineering which requires Tianshi Engineering to purchase all of our products to be sold in China. We sell our finished products to Tianshi Engineering at a price equal to 25% of the Chinese retail market price for the products. This 25% figure was negotiated between the parties in 2003, before we acquired Tianshi Holdings, and we believe that it is a reasonable sales price for us to receive. The price of semi-finished goods sold to Tianshi Engineering was originally set at the beginning of 2006 to provide us with a 75% gross profit margin. However, based on fluctuations in the cost of raw materials and quantities produced, the gross profit margin percentage varied during the year. This 75% figure was negotiated between the parties, and we believe that it is reasonable. The goal of this new pricing policy was to try to maintain our gross margins on semi-finished goods at a similar level to historical gross margins for finished goods. All of Tianshi Engineering’s Chinese affiliated companies are owned in whole or in part by Jinyuan Li’s immediate family members.
Internationally, we sell our products to overseas related companies located in 45 countries who in turn re-package and sell them to independent direct sales distributors. Our CEO, Jinyuan Li, owns or controls these overseas related companies. Due to the common ownership, there are no formal sales or administrative agreements among us and those overseas related parties. The business operations among these related entities are regulated through internal policies.
As operation costs vary from country to country, international market prices vary accordingly. We sell our products to overseas affiliates at the FOB (destination port) price, which consists of 25% of the Chinese retail price, including customs duty, value-added tax and other miscellaneous transportation cost. The overseas affiliates mark up the products to cover their expenses and realize profits of approximately 10%.
Our related party transactions are required to be reviewed and approved or ratified by a majority of our non-interested Board of Directors. The following tables are provided to facilitate your understanding of the transactions and outstanding balances between those related parties and us during 2010 and 2009.
|
December 31,
2010
|
December 31,
2009
|
|||||||
|
Accounts receivable, trade – related parties, net of allowance for doubtful accounts of $3,869,617 and $1,419,178 as of December 31, 2010 and 2009, respectively
|
$ | 10,012,861 | $ | 15,379,312 | ||||
|
Other receivables – related parties
|
$ | 17,376,522 | $ | 44,561,626 | ||||
|
Advances from customers – related parties
|
$ | 8,688,877 | $ | 4,426,751 | ||||
|
Other payables – related parties
|
$ | 1,417,516 | $ | 3,326,110 | ||||
Revenue-related Parties
The details of revenue-related parties are as follows:
|
2010
|
2009
|
|||||||
|
Tianshi Engineering
|
$ | 24,570,887 | $ | 25,298,232 | ||||
|
Overseas Related Companies
|
16,450,248 | 34,734,736 | ||||||
|
Total
|
$ | 41,021,135 | $ | 60,032,968 | ||||
In China, we sell our products to Tianshi Engineering, an affiliated company. Tianshi Engineering, in turn, sells the products to customers through its branches and affiliated companies and at chain stores which are owned by individual distributors. Internationally, we sell our products to overseas affiliates who in turn re-package the products to meet the needs of the local markets and sell to independent distributors who use the products themselves and/or resell them to other distributors or consumers.
Accounts Receivable-related Parties
The details of accounts receivables, trade-related parties are as follows:
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Tianshi Engineering
|
$ | 1,803,688 | $ | 5,035,320 | ||||
|
Overseas Related Companies
|
12,078,790 | 11,763,170 | ||||||
|
Allowance for Doubtful Accounts
|
(3,869,617 | ) | (1,419,178 | ) | ||||
|
Total
|
$ | 10,012,861 | $ | 15,379,312 | ||||
Other Receivables-related Parties
Other receivables - related parties are generated by our making various cash advances and short term loans, the allocation of various expenses to related parties, and amounts transferred from accounts receivable. The following table summarizes the other receivables- related parties balances:
The details of other receivables-related parties are as follows:
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Tiens Life science Co., Ltd.
|
11,525,219 | - | ||||||
|
Tianshi Engineering
|
5,603,197 | 5,688,926 | ||||||
|
All-Legend Property service (Tianjin) Co.,Ltd.
|
155,798 | 77,612 | ||||||
|
All-Legend Hotel Co.,Ltd.
|
37,856 | - | ||||||
|
Tianshi Yinshi Hotel
|
37,810 | 36,566 | ||||||
|
Tianshi Indonesia Logistic & Trade Co., Ltd.
|
9,902 | 9,873 | ||||||
|
Tianshi Taijisheng Health management Co., Ltd
|
5,459 | - | ||||||
|
Shengshi Real Estate Development
|
1,072 | 634 | ||||||
|
Tianshi Pharmaceuticals
|
139 | 1,588 | ||||||
|
All-Legend Hotel management Co.,Ltd.
|
70 | 2,730 | ||||||
|
Tianshi Group
|
- | 1,613,168 | ||||||
|
Tiens SmartFlow Logistics (International) Group Ltd.
|
- | 74,651 | ||||||
|
Tianjin Tianshi Life Science Co., Ltd.
|
- | 55,878 | ||||||
|
Tianshi investment
|
- | 37,000,000 | ||||||
|
Total
|
$ | 17,376,522 | $ | 44,561,626 | ||||
Historically, Tianshi Engineering remitted payment to us upon sales to third party customers. However, in order to support Tianshi Engineering’s marketing efforts in anticipation of receiving a direct selling license in China, we have agreed to allow Tianshi Engineering to defer payment to us. The credit terms provide an interest-free credit term of three months. Any amounts exceeding this term are transferred from accounts receivable-related parties to other receivable-related parties. The other receivables-related parties become interest bearing once a loan contract is adopted. The interest rate is the interest rate, on the date the loan commences, that is stipulated by the People’s Bank of China for a loan of the same level.
On April 21, 2009, the Company entered into a loan agreement with Tianshi Engineering. Pursuant to that agreement, effective as of April 1, 2009, $2,562,017 of other receivables-related parties, which originated from Tianshi Engineering as accounts receivable, became interest bearing. The loan was due on June 30, 2009 and the stated interest rate was 4.86%. Both the principal of $2,562,017 and interest on the loan of $12,624 were paid off on May 7, 2009. In 2010, Tianshi Engineering paid all accounts receivable within three months. For the years ended December 31, 2010 and 2009, the interest income from the other receivables-related parties amounted to $0 and $12,624, respectively.
During the years ended December 31, 2010 and 2009, we and Tianshi Group used common meters at our headquarters for electricity and water, and also used the same employee insurance account. When making payments to these outside parties, we usually pay the fees first and then are reimbursed by Tianshi Group. These pro-rated amounts relating to Tianshi Group are categorized as other receivables-related parties.
On December 25, 2008, Biological entered into a Property Transfer Agreement with Tianshi Group, pursuant to which Biological transferred four buildings at the price of RMB32,800,000 (US$4,785,520). As of March 31, 2010, the amount was paid in full by Tianshi Group.
On November 15, 2009, Tianshi Holdings and Tianshi Investment entered the Transfer Contract, pursuant to which Tianshi Holdings agreed to sell all of the registered share capital of Tiens Yihai it owned to Tianshi Investment for $37.0 million. Tiens Yihai holds land use rights for 50 acres of land located in Shanghai, China. Tiens Yihai was originally established to build a new research and development facility, but the Company suspended the proposed development in March 2007. Tianshi Holdings held 96% of the equity interest in Tiens Yihai. Tianjin Tianshi Pharmaceuticals Co., Ltd. owned the remaining 4% of Tiens Yihai’s share capital. The sale closed on November 15, 2009. Pursuant to the Transfer Contract, the purchase price of $37.0 million was paid in full by November 12, 2010. Payment of $3,700,000 was received in cash, $6,000,000 was offset against payables owed to Fuhong Development Co., Ltd, an entity that is 100% owned by Mr. Li, and $27,300,000 was offset against payables to Tianshi Engineering and Tianshi Group.
A portion of the construction in progress at an amount of RMB83,263,708 (US$12,592,803), which is recorded and paid for by Life Resources is built on the land owned by Tianshi Life Science. In addition, a portion of the construction in progress at an amount of RMB5,245,839 (US$793,381), which is recorded and paid for by Tianshi Life Science is built on the land owned by Life Resources. Tianshi Life Science and Life Resources intend to reimburse each other for the cost of the above construction in progress. The agreement is currently being drafted. The balance of RMB78,017,869 (US$11,799,422) has been reclassified as other receivable-related party as of December 31, 2010, accordingly.
Advances from Customers-related Parties
Advances from related party customers were $8.7 million and $4.4 million as of December 31, 2010 and 2009, respectively. These advances represented prepayments made to us to insure that overseas customers could obtain enough of our products to meet their market demands.
Other Payables-related Parties
These amounts arose primarily from previous cash advances from related parties such as management fees due to related parties and various non-operational transactions incurred with related parties. The details of other payable-related parties are as follows:
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Tiens Group
|
$ | 639,529 | $ | 0 | ||||
|
Tianshi Engineering
|
431,400 | 40,805 | ||||||
|
Tianshi Germany Co., Ltd.
|
99,759 | 107,326 | ||||||
|
Tianjin Tianshi Global International Trade Co., Ltd.
|
96,794 | 93,606 | ||||||
|
Tianyuan Capital Development Co. Ltd.
|
84,359 | 84,359 | ||||||
|
Bejing All-legeng travel Co., Ltd.
|
65,660 | 0 | ||||||
|
Tianshi Administrative Committee of Industrial Park
|
15 | 14 | ||||||
|
Fuhong Development Co.Ltd.
|
0 | 3,000,000 | ||||||
|
Total
|
$ | 1,417,516 | $ | 3,326,110 | ||||
On November 12, 2010, Tianshi Investment, Tianshi Engineering, Tianshi Group and the Company entered into an agreement, pursuant to which the Company’s other receivables from Tianshi Investment of $27,300,000 was offset against advances from Tianshi Engineering of $11,767,357, short-term debt from Tianshi Engineering of $9,077,156 and other payables to Tianshi Group of $6,455,487.
On November 10, 2009, Tianshi Holdings borrowed $3,000,000 from Fuhong Development Co., Ltd, a British Virgin Islands company, which is 100% owned by Jinyuan Li, to fund its capital contribution to Life Resources. On the February 10, 2010, the loan was paid in full by cancelling the same amount Tianshi Investment owned to us.
On March 3, 2010, Tianshi Holdings borrowed another $3,000,000 from Fuhong Development to fund its capital contribution to Life Resources. On June 11, 2010, the loan was paid in full by canceling the same amount Tianshi Investment owed to the Company.
During the third quarter of 2010, the Company received $7,614,300 and $6,270,600 from Tianshi Group and Tianshi Engineering, respectively, in order to fund a portion of the construction in progress. As of December 31, 2010, the amounts had been returned in full.
Transactions with Tianshi Group
On December 25, 2008, Biological and Tianshi Group entered into a Property Transfer Agreement (the “Property Transfer Agreement”). Under the Property Transfer Agreement, Biological transferred to Tianshi Group four buildings consisting of 9,974.31 square meters, including three workshops and a canteen, located at our headquarters in Tianjin China. Pursuant to the Property Transfer Agreement, Tianshi Group will pay Biological RMB32,800,000 (US$4,797,328). This transaction resulted in a loss of RMB1,912,983 (US$274,762) for Biological. We bore 80% of the loss, or $219,810.
On January 1, 2009, Biological entered into an office and facilities lease agreement with Tianshi Group. Under the terms of the agreement, Biological’s annual rent is equal to 1% of its gross revenues. In addition, Biological is obligated to pay insurance, maintenance and other expenses related to the premises. This agreement expires on December 31, 2010. On January 1, 2010, Life Resources entered an office and facilities lease agreement with Tianshi Group on the same terms as Biological’s lease agreement with Tianshi Group. The Company paid rent under these leases in the amount of $500,361 and $458,382 for the year ended December 31, 2010 and 2009, respectively.
On January 1, 2009, each of Biological and Life Resources entered a Lease Agreement with Tianshi Group pursuant to which Biological and Life Resources will have the right to use and occupy the workshop spaces being transferred under the Property Transfer Agreement. The leases are rent-free, except that Biological and Life Resources are required to pay Tianshi Group for utility charges and maintenance costs on the buildings. The leases continue until the earlier of the date that Biological and Life Resources acquire use of alternate facilities or the land use rights on the underlying property expire. For the year ended December 31, 2010, Biological and Life Resources recorded $330,708 of the rent expense, which is not paid to Tianshi Group, but recorded as paid in capital based upon market price.
Transactions with Tianshi Engineering
On October 31, 2007, Biological entered into two lease agreements with Tianshi Engineering that enable Tianshi Engineering to share the use of certain of Biological’s production equipment to manufacture products which Tianshi Engineering owns, or jointly owns, with Biological. Each of the two agreements was effective as of January 1, 2008 and expired on December 31, 2009. On November 20, 2009, the lease agreement for health products production equipment and the lease agreement for personal care product production equipment were renewed by Biological and Tianshi Engineering for 2010. Following is a summary of the monthly rent payable to Biological under the two leases Biological entered into on November 20, 2009.
|
Lease Agreement
|
Monthly rent
|
|||
|
Lease Agreement for Health Products Production Equipment
|
$ | 11,383 | ||
|
Lease Agreement for Personal Care Product Production Equipment
|
$ | 6,098 | ||
Rent revenue accrued from these leases amounted to $209,770 and $219,605 for the year ended December 31, 2010 and 2009, respectively.
On December 3, 2010, lease agreements for health products production equipment and household chemical products production equipment were renewed by Biological and Tianshi Engineering. Each of the two agreements is effective as of January 1, 2011 and expires on December 31, 2011.
Transactions with Tianshi Pharmaceuticals
On December 15, 2009, the Company entered into a one-year lease agreement with Tianshi Pharmaceutical. Under the terms of the lease agreement, the Company leased equipment for the fee of RMB25,383 (or US$3,755) per month. In addition, the Company is obligated to pay insurance, maintenance and other expenses related on the equipment. This agreement is effective from January 1, 2010 and expires on December 31, 2010. The company has paid $45,062 of rent expense for the twelve months ended December 31, 2010. The lease agreement was extended for an additional one year period.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
The Board appointed Crowe Horwath LLP (“Crowe”) as independent auditors to audit our financial statements for the fiscal years ended December 31, 2009 and 2010.
Public Accounting Fees
|
2010
|
2009
|
|||||||
|
Audit Fees
|
$ | 270,000 | $ | 248,000 | ||||
|
Audit Related Fees
|
$ | 50,000 | $ | 2,800 | ||||
|
Tax Fees
|
$ | 0 | $ | 0 | ||||
|
All Other Fees
|
$ | 7,766 | $ | 0 | ||||
Audit fees were for professional services rendered by Crowe during the 2010 and 2009 fiscal years for the audit of our annual financial statements and the review of the financial statements included in our quarterly reports on Form 10-Q, and services that are normally provided by Crowe in connection with statutory and regulatory filings or engagements for that fiscal year. Audit related fees include performing the interim audit procedures related to construction in progress in 2010 and discussions with management and SEC legal counsel regarding sale of Yihai in 2009. All other fees were travel expenses incurred for audit by auditors.
Pre-approval of Services
The Audit Committee has adopted pre-approval policies for all services, including both audit and non-audit services, provided by our independent auditors. For audit services, each year the independent auditor provides the Audit Committee with an engagement letter outlining the scope of the audit services proposed to be performed during the year, which must be formally accepted by the Audit Committee before the audit commences. The independent auditor also submits an audit services fee proposal, which also must be approved by the Committee before the audit commences. The audit, tax, and all other fees and services described above were pre-approved for 2009 and 2010.
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
(a)
|
The following are filed with this report:
|
(1) The financial statements listed on the Financial Statements Table of Contents.
(2) Not applicable.
(3) The exhibits referred to below, which include the following managerial contracts or compensatory plans or arrangements:
|
|
·
|
Form of Labor Contract.
|
|
|
·
|
Labor Contract dated June 1, 2009 between Tianjin Tianshi Biological Development Co., Ltd. and Manbo He.
|
|
|
·
|
Labor Contract dated as of October 20, 2010 between Tianjin Tianshi Biological Development Co., Ltd. and Baolan Li.
|
(b) The exhibits listed on the Exhibit Index are filed as part of this report.
(c) Not applicable.
EXHIBIT INDEX
|
Exhibit No.
|
Description
|
|
|
2.1
|
Agreement and Plan of Reorganization, as amended, dated as of August 22, 2003, by and among Strategika, Tianshi Holdings and the Stockholders of Tianshi Holdings. (1)
|
|
|
3.1
|
Certificate of Incorporation of Tiens Biotech Group (USA), Inc., as amended (11)
|
|
|
3.2
|
By-laws of Tiens Biotech Group (USA), Inc. (2)
|
|
|
4.1
|
Specimen Stock Certificate (2)
|
|
|
10.1
|
Product Purchase and Sales Agreement, dated June 25, 2003, by and between Tianjin Tianshi Biological Development Co., Ltd. And Tianjin Tianshi Biological Engineering Co. Ltd. (3)
|
|
|
10.2
|
Term Loan Agreement, dated March 29, 2007 by and between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd. (4)
|
|
|
10.3
|
Term Loan Agreement, dated April 24, 2007 by and between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd. (5)
|
|
|
10.4
|
Term Loan Extension Agreement, dated June 28, 2007 by and between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd. (5)
|
|
|
10.5
|
Term Loan Agreement, dated July 23, 2007 by and between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd. (11)
|
|
|
10.6
|
Term Loan Extension Agreement, dated September 27, 2007 by and between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd. (6)
|
|
|
10.7
|
Term Loan Agreement, dated October 15, 2007 by and between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd. (11)
|
|
|
10.8
|
Health Products Production Workshops Lease Agreement, dated October 31, 2007 by and between Tianjin Tianshi Group Co., Ltd. and Tianjin Tianshi Biological Development Co., Ltd. (11)
|
|
|
10.9
|
Personal Care Products Production Workshops Lease Agreement, dated October 31, 2007 by and between Tianjin Tianshi Group Co., Ltd. and Tianjin Tianshi Biological Development Co., Ltd. (11)
|
|
|
10.10
|
Health Products Production Equipment Lease Agreement, dated October 31, 2007 by and between Tianjin Tianshi Biological Engineering Co., Ltd.. and Tianjin Tianshi Biological Development Co., Ltd. (11)
|
|
|
10.11
|
Personal Care Products Production Equipment Lease Agreement, dated October 31, 2007 by and between Tianjin Tianshi Biological Engineering Co., Ltd. and Tianjin Tianshi Biological Development Co., Ltd. (11)
|
|
|
10.12
|
Real Property Transfer Agreement, dated December 14, 2007, by and between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Group Co. Ltd. (11)
|
|
|
10.13
|
Lease Agreement, dated December 14, 2007, by and between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Group Co. Ltd. (11)
|
|
|
10.14
|
Sale and Purchase Agreement dated December 20, 2007 by and among Tianshi International Investment Group Co., Ltd., Tianshi International Holdings Group Limited, Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd. (11)
|
|
|
10.15
|
Capital Contribution Agreement dated December 21, 2007 by and between Tianshi International Holdings Group Co., Ltd. and Tianshi International Investment Group Co. Ltd. (11)
|
|
|
10.16
|
Health Products Production Workshops Supplemental Agreement, dated December 31, 2007 by and between Tianjin Tianshi Group Co., Ltd. and Tianjin Tianshi Biological Development Co., Ltd. (11)
|
|
|
10.17
|
Personal Care Products Production Equipment Supplemental Agreement, dated December 31, 2007 by and between Tianjin Tianshi Group Co., Ltd. and Tianjin Tianshi Biological Development Co., Ltd. (11)
|
|
|
10.18
|
Loan Agreement dated January 14, 2008 by and between Tianshi International Holdings Group Co., Ltd. and Tianshi International Investment Group Co. Ltd. (11)
|
|
|
10.19
|
Lease Agreement, dated January 17, 2008, by and between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Group Co. Ltd. (11)
|
|
|
10.20
|
Form of Labor Contract (3)
|
|
|
10.21
|
Loan Agreement dated January 14, 2008 by and between Tianshi International Holdings Group Limited and Tianshi International Investment Group Co., Ltd. (7)
|
|
10.22
|
Loan Agreement dated January 21, 2008 by and between Tianshi International Investment Group Co., Ltd. and Tianjin Tiens Life Resources Co., Ltd. (7)
|
|
10.23
|
Agreement dated April 9, 2008 by and between Tianshi International Holdings Group Limited, Tianshi International Investment Group Co., Ltd., Tianjin Tianshi Biological Development Co., Ltd, and Tianjin Tianshi Biological Engineering Co., Ltd. (8)
|
|
10.24
|
Loan Agreement Amendment dated June 30, 2008 by and between Tianshi International Investment Group Co., Ltd. and Tianjin Tiens Life Resources Co., Ltd. (8)
|
|
10.25
|
Supplementary Agreement for Grant Contract of State-Owned Land Use Right dated August 25, 2008 by and among Tianjin Tiens Life Resources Co., Ltd., Wuqing Branch Bureau of Tianjin Municipal Land and Resources and Administrative Bureau and Tianjin Tiens Life Science Co., Ltd. (9)
|
|
10.26
|
Labor Contract dated November 3, 2008 between Tianjin Tianshi Biological Development Co., Ltd. and Zheng Wan (12)
|
|
10.27
|
Property Transfer Agreement dated December 25, 2008 between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Group Co. Ltd. (12)
|
|
10.28
|
Lease Agreement dated January 1, 2009 between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Group Co. Ltd. (12)
|
|
10.29
|
Equipment Lease Agreement dated January 1, 2009 between Tianjin Tiens Life Resources Co., Ltd. and Tianjin Tianshi Group Co. Ltd. (12)
|
|
10.30
|
Storage Service Agreement dated September 4, 2008 between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Baofeng Construction & Engineering Co., Ltd. (12)
|
|
10.31
|
Loan Agreement Amendment dated December 31, 2008 between Tianjin Tiens Life Resources Co., Ltd. and Tianshi International Investment Group Co., Ltd. (12)
|
|
10.32
|
Supplemental Agreement of Lease Agreement for Health Products Production Workshops dated December 31, 2007 between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd. (12)
|
|
10.33
|
Supplemental Agreement of Lease Agreement for Personal Care Products Production Equipment dated December 31, 2007 between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd. (12)
|
|
10.34
|
Term Loan Agreement, dated April 21, 2009 by and between Tianjin Tiens Life Resources Co., Ltd. and Tianjin Tianshi Biological Engineering Co, Ltd. (13)
|
|
10.35
|
Labor Contract dated June 1, 2009 between Tianjin Tianshi Biological Development Co., Ltd. and Manbo He. (14)
|
|
10.36
|
Agreement, dated June 5, 2009 by and among Tianjin Tianshi Biological Development Co, Ltd., Tianshi International Holdings Group Limited, Tianshi International Investment Group Co., Ltd. and Tianjin Tianshi Group Co., Ltd. (15)
|
|
10.37
|
Agreement on Debt Transfer and Offset, dated as of June 30, 2009, by and among Tianshi International Holdings Group Limited, Tianyuan Capital Development Co., and Tianjin Tianshi Biological Engineering Co., Ltd. (15)
|
|
10.38
|
Contract for the Transfer of Equity Interest, dated November 15, 2009, by and between Tianshi International Holdings Group Ltd. and Tianshi International Investment Group Co., Ltd. (16)
|
|
10.36
|
Health Products Production Equipment Lease Agreement, dated November 20, 2009 by and between Tianjin Tianshi Biological Engineering Co., Ltd.. and Tianjin Tianshi Biological Development Co., Ltd. (17)
|
|
10.37
|
Household Chemical Products Production Equipment Lease Agreement, dated November 20, 2009 by and between Tianjin Tianshi Biological Engineering Co., Ltd. and Tianjin Tianshi Biological Development Co., Ltd. (17)
|
|
10.38
|
Equipment Lease Agreement dated December 15, 2009 between Tianjin Tiens Life Resources Co., Ltd. and Tianjin Tianshi Pharmaceuticals Co., Ltd. (17)
|
|
10.39
|
Agreement on Debt Transfer and Offset, dated as of December 31, 2009, by and among Tianyuan Capital Development Co., Tianshi International Holdings Group Limited and Tianjin Tianshi Biological Engineering Co., Ltd. (17)
|
|
10.40
|
Agreement on Debt Transfer and Offset, dated as of February 10, 2010, by and among Xianggang Fuhong Development Co., Ltd., Tianshi International Holdings Group Co., Ltd. and Tianshi International Investment Group Co., Ltd. (17)
|
|
10.41
|
Agreement, dated January 1, 2010 by and between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Group Co. Ltd. (18)
|
|
10.42
|
Agreement, dated January 1, 2010 by and between Tianjin Tiens Life Resources Co., Ltd. and Tianjin Tianshi Group Co. Ltd. (18)
|
|
10.43
|
Agreement, dated June 11, 2010 by and between Tianshi International Holding Group Co., Ltd., Tianshi International Investment Group Co., Ltd. and Fuhong Development Co., Ltd. (19)
|
|
10.44
|
Labor Contract dated as of October 20, 2010 between Tianjin Tianshi Biological Development Co., Ltd. and Baolan Li (20)
|
|
10.45
|
Loan Agreement, dated August 24, 2010 by and between Tianjin Tiens Life Resources Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd. (21)
|
|
10.46*
|
Agreement on Debt Transfer and Offset, dated November 12, 2010 among Tianshi Group Co., Ltd., Tianshi International Holdings Group Co., Ltd., Tianshi International Investment Group Co., Ltd., Tianjin Tiens Life Resource Co., Ltd., Tianjin Tianshi Biological Development Co., Ltd., Tianjin Tianshi Biological Engineering Co., Ltd.
|
|
10.47*
|
Health Products Production Equipment Lease Agreement, dated December 3, 2010 between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd.
|
|
10.48*
|
Household Chemical Products Production Equipment Lease Agreement, dated December 3, 2010 between Tianjin Tianshi Biological Development Co., Ltd. and Tianjin Tianshi Biological Engineering Co., Ltd.
|
|
10.49*
|
Loan Agreement, dated August 24, 2010, by and between Tianjin Tianshi Biological Engineering Co., Ltd. and Tianjin Tiens Life Resource Co., Ltd.
|
|
10.50*
|
Summary of RMB Working Capital Loan Contract dated October 20, 2010 by and between Tianjin Tiens Life Resources Co., Ltd. and China Construction Bank Co. Ltd., Tianjin Wuqing Branch
|
|
10.51*
|
Fixes Assets Loan Contract dated October 9, 2010 by and between Tianjin Tiens Life Resources Co., Ltd. and Agricultural Bank of China Tianjin Wuqing Branch
|
|
14.1
|
Code of Ethics (10)
|
|
21.1*
|
Subsidiaries
|
|
31.1*
|
Certification of the Principal Executive Officer and Principal Financial and Accounting Officer pursuant to Rules 13a-14 and 15d-14 of the Securities Exchange Act of 1934.
|
|
32.1*
|
Certification of the Chief Executive Officer and Acting Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
* Filed herewith
(1) Filed as an exhibit to the Registrant’s Annual Report on Form 10-KSB filed with the Securities and Exchange Commission on March 31, 2005.
(2) Filed as an exhibit to the Registrant’s Form 10-KSB filed with the Securities and Exchange Commission on March 7, 2002.
(3) Filed as an exhibit to the Registrant’s Form 10-K filed with the Securities and Exchange Commission on March 31, 2006.
(4) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on May 15, 2007.
(5) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on August 14, 2007.
(6) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on November 14, 2007.
(7) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on May 15, 2008.
(8) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on August 14, 2008.
(9) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on November 14, 2008.
(10) Filed as an exhibit to the Registrant’s Form 10-KSB filed with the Securities and Exchange Commission on March 31, 2005.
(11) Filed as an exhibit to the Registrant’s Form 10-K filed with the Securities and Exchange Commission on March 31, 2008.
(12) Filed as an exhibit to the Registrant’s Form 10-K filed with the Securities and Exchange Commission on March 31, 2009.
(13) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on May 15, 2009.
(14) Filed as an exhibit to the Registrant’s Form 8-K filed with the Securities and Exchange Commission on June 4, 2009.
(15) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on August 14, 2009.
(16) Filed as an exhibit to the Registrant’s Form 8-K filed with the Securities and Exchange Commission on November 19, 2009.
(17) Filed as an exhibit to the Registrant’s Form 10-K filed with the Securities and Exchange Commission on April 2, 2010.
(18) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on May 17, 2010.
(19) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on August 19, 2010.
(20) Filed as an exhibit to the Registrant’s Form 8-K filed with the Securities and Exchange Commission on October 25, 2010.
(21) Filed as an exhibit to the Registrant’s Form 10-Q filed with the Securities and Exchange Commission on November 15, 2010.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
TIENS BIOTECH GROUP (USA), INC.
|
|
|
Date: March 31, 2011
|
|
|
/s/ Jinyuan Li
|
|
|
Jinyuan Li
|
|
|
Chief Executive Officer, President and Acting Chief
|
|
|
Financial Officer
|
|
|
(Principal Executive Officer, Principal Financial and
|
|
|
Accounting Officer)
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
||
|
/s/ Jinyuan Li
|
Chairman of the Board, Chief Executive
|
March 31, 2011
|
||
|
Jinyuan Li
|
Officer, Acting Chief Financial Officer and
|
|||
|
President (Principal Executive Officer,
|
||||
|
Principal Financial and Accounting Officer)
|
||||
|
/s/ Yupeng Yan
|
Executive Vice President and Director
|
March 31, 2011
|
||
|
Yupeng Yan
|
||||
|
/s/ Baolan Li
|
Director
|
March 31, 2011
|
||
|
Baolan Li
|
||||
|
/s/ Gilbert Raker
|
Director
|
March 31, 2011
|
||
|
Gilbert Raker
|
||||
|
/s/ Socorro Quintero
|
Director
|
March 31, 2011
|
||
|
Socorro Quintero
|
TIENS BIOTECH GROUP (USA), INC. AND SUBSIDIARIES
CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2010 AND 2009
TABLE OF CONTENTS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
To the Stockholders and Board of Directors of Tiens Biotech Group (USA), Inc.
We have audited the accompanying consolidated balance sheets of Tiens Biotech Group (USA), Inc. (the “Company”) as of December 31, 2010 and 2009, and the related consolidated statements of income and other comprehensive income, shareholders’ equity, and cash flows for each of the two years in the period ended December 31, 2010. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2010 and 2009, and the consolidated results of its operations and its cash flows for each of the two years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles.
As discussed in Notes 1, 3, 12, 13, 14 and 15 to the consolidated financial statements, the Company has had numerous significant transactions with entities that are under common control, including that most of the Company’s sales are currently and have historically been made to related parties controlled by the majority shareholder of the Company.
/s/ Crowe Horwath LLP
Indianapolis, Indiana
March 31, 2011
TIENS BIOTECH GROUP (USA), INC. AND SUBSIDIARIES
AS OF DECEMBER 31, 2010 AND DECEMBER 31, 2009
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
CURRENT ASSETS:
|
||||||||
|
Cash
|
$ | 10,155,522 | $ | 1,848,328 | ||||
|
Accounts receivable, trade - related parties, net of
|
||||||||
|
allowance for doubtful accounts of $3,869,617 and $1,419,178
|
||||||||
|
as of December 31, 2010 and December 31, 2009 , respectively
|
10,012,861 | 15,379,312 | ||||||
|
Inventories
|
5,703,349 | 5,328,052 | ||||||
|
Other receivables
|
1,045,952 | 995,657 | ||||||
|
Other receivables - related parties
|
17,376,522 | 44,561,626 | ||||||
|
Employee advances
|
170,842 | 115,673 | ||||||
|
Prepaid expenses, net
|
415,208 | 658,193 | ||||||
|
Prepaid taxes
|
3,646,140 | 407,534 | ||||||
|
Total current assets
|
48,526,396 | 69,294,375 | ||||||
|
PROPERTY, PLANT AND EQUIPMENT, net
|
72,037,542 | 10,124,483 | ||||||
|
OTHER ASSETS:
|
||||||||
|
Construction in progress
|
128,715,283 | 125,572,621 | ||||||
|
Construction deposits
|
12,490,855 | 1,405,997 | ||||||
|
Intangible assets, net
|
12,987,000 | 12,864,295 | ||||||
|
Other assets
|
10,721,040 | 11,847,937 | ||||||
|
Total other assets
|
164,914,178 | 151,690,850 | ||||||
|
Total assets
|
$ | 285,478,116 | $ | 231,109,708 | ||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES:
|
||||||||
|
Accounts payable
|
$ | 14,120,791 | $ | 5,012,157 | ||||
|
Advances from customers - related parties
|
8,688,877 | 4,426,751 | ||||||
|
Wages and benefits payable
|
1,613,782 | 1,484,852 | ||||||
|
Short-term debt
|
3,024,800 | - | ||||||
|
Income taxes payable
|
490,782 | - | ||||||
|
Contractor deposits
|
209,376 | 183,395 | ||||||
|
Contractor payables
|
28,134,711 | 18,513,216 | ||||||
|
Other payables
|
1,113,416 | 1,151,551 | ||||||
|
Other payables - related parties
|
1,417,516 | 3,326,110 | ||||||
|
Total current liabilities
|
58,814,051 | 34,098,032 | ||||||
|
NON-CURRENT LIABILITIES
|
||||||||
|
Long term debt
|
18,451,280 | - | ||||||
|
Deferred income
|
11,473,853 | 11,236,501 | ||||||
|
Total non current liabilities
|
29,925,133 | 11,236,501 | ||||||
|
Total liabilities
|
88,739,184 | 45,334,533 | ||||||
|
Commitments and Contingencies
|
||||||||
|
EQUITY:
|
||||||||
|
Shareholders' equity of the Company:
|
||||||||
|
Common stock, $0.001 par value, 250,000,000 shares authorized,
|
||||||||
|
71,333,586 issued and outstanding, respectively
|
71,334 | 71,334 | ||||||
|
Paid-in-capital
|
18,349,908 | 18,042,189 | ||||||
|
Statutory reserves
|
16,465,144 | 13,217,217 | ||||||
|
Retained earnings
|
127,957,951 | 126,370,263 | ||||||
|
Accumulated other comprehensive income
|
23,393,626 | 18,262,123 | ||||||
|
Total shareholders' equity of the Company
|
186,237,963 | 175,963,126 | ||||||
|
Noncontrolling interest
|
10,500,969 | 9,812,049 | ||||||
|
Total equity
|
196,738,932 | 185,775,175 | ||||||
|
Total liabilities and equity
|
$ | 285,478,116 | $ | 231,109,708 | ||||
The accompanying notes are an integral part of this statement.
TIENS BIOTECH GROUP (USA), INC. AND SUBSIDIARIES
FOR THE YEARS ENDED DECEMBER 31, 2010 and 2009
|
2010
|
2009
|
|||||||
|
REVENUE - RELATED PARTIES
|
$ | 41,021,135 | $ | 60,032,968 | ||||
|
REVENUE - THIRD PARTIES
|
323,585 | 1,943,101 | ||||||
|
COST OF SALES - RELATED PARTIES
|
14,850,739 | 18,754,680 | ||||||
|
COST OF SALES - THIRD PARTIES
|
158,638 | 1,412,812 | ||||||
|
GROSS PROFIT
|
26,335,343 | 41,808,577 | ||||||
|
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES
|
19,530,501 | 16,009,382 | ||||||
|
INCOME FROM OPERATIONS
|
6,804,842 | 25,799,195 | ||||||
|
Interest expense
|
(230,905 | ) | (186,543 | ) | ||||
|
Interest income
|
18,362 | 301,709 | ||||||
|
Other expense
|
35,965 | (176,757 | ) | |||||
|
OTHER EXPENSE, NET
|
(176,578 | ) | (61,591 | ) | ||||
|
INCOME BEFORE INCOME TAXES
|
6,628,264 | 25,737,604 | ||||||
|
INCOME TAXES
|
1,469,548 | 930,703 | ||||||
|
NET INCOME
|
5,158,716 | 24,806,901 | ||||||
|
LESS: Net income attributable to the noncontrolling interest
|
(323,101 | ) | (965,557 | ) | ||||
|
NET INCOME ATTRIBUTABLE TO THE COMPANY
|
4,835,615 | 23,841,344 | ||||||
|
OTHER COMPREHENSIVE INCOME:
|
||||||||
|
Foreign currency translation adjustment
|
5,131,503 | 441,140 | ||||||
|
Loss from the realization of foreign currency sale
|
- | (6,030,079 | ) | |||||
|
COMPREHENSIVE INCOME ATTRIBUTABLE TO THE COMPANY
|
9,967,118 | 18,252,405 | ||||||
|
COMPREHENSIVE INCOME ATTRIBUTABLE TO THE NONCONTROLLING INTEREST
|
664,854 | 993,504 | ||||||
|
COMPREHENSIVE INCOME
|
$ | 10,631,972 | $ | 19,245,909 | ||||
|
EARNINGS PER SHARE, BASIC AND DILUTED
|
$ | 0.07 | $ | 0.33 | ||||
|
WEIGHTED AVERAGE NUMBER OF SHARES, BASIC AND DILUTED
|
71,333,586 | 71,333,586 | ||||||
The accompanying notes are an integral part of this statement.
|
Accumulated
|
||||||||||||||||||||||||||||||||
|
other
|
||||||||||||||||||||||||||||||||
|
Number
|
Common
|
Paid-in
|
Statutory
|
Retained
|
comprehensive
|
Noncontrolling
|
||||||||||||||||||||||||||
|
of shares
|
stock
|
capital
|
reserves
|
earnings
|
income
|
interest
|
Totals
|
|||||||||||||||||||||||||
|
BALANCE, December 31, 2008
|
71,333,586 | $ | 71,334 | $ | 9,234,123 | $ | 9,420,783 | $ | 106,325,356 | $ | 23,851,062 | $ | 9,006,438 | $ | 157,909,096 | |||||||||||||||||
|
Net Income
|
3,796,434 | 20,044,907 | 965,560 | 24,806,901 | ||||||||||||||||||||||||||||
|
Foreign currency translation gain
|
441,140 | 27,947 | 469,087 | |||||||||||||||||||||||||||||
|
Free rent provided by related party
|
303,056 | 23,699 | 326,755 | |||||||||||||||||||||||||||||
|
Effect from sale of Yihai
|
2,474,931 | (211,595 | ) | 2,263,336 | ||||||||||||||||||||||||||||
|
Realization of foreign currency translation gain relating to the sale of Yihai
|
6,030,079 | (6,030,079 | ) | - | ||||||||||||||||||||||||||||
|
BALANCE, December 31, 2009
|
71,333,586 | $ | 71,334 | $ | 18,042,189 | $ | 13,217,217 | $ | 126,370,263 | $ | 18,262,123 | $ | 9,812,049 | $ | 185,775,175 | |||||||||||||||||
|
Net Income
|
3,247,927 | 1,587,688 | 323,101 | 5,158,716 | ||||||||||||||||||||||||||||
|
Foreign currency translation gain
|
5,131,503 | 341,753 | 5,473,256 | |||||||||||||||||||||||||||||
|
Free rent provided by related party
|
307,719 | 24,066 | 331,785 | |||||||||||||||||||||||||||||
|
BALANCE, December 31, 2010
|
71,333,586 | $ | 71,334 | $ | 18,349,908 | $ | 16,465,144 | $ | 127,957,951 | $ | 23,393,626 | $ | 10,500,969 | $ | 196,738,932 | |||||||||||||||||
The accompanying notes are an integral part of this statement.
TIENS BIOTECH GROUP (USA), INC. AND SUBSIDIARIES
FOR THE YEARS ENDED DECEMBER 31, 2010 AND 2009
|
2010
|
2009
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||
|
Net income
|
$ | 5,158,716 | $ | 24,806,901 | ||||
|
Adjustments to reconcile net income to cash provided by (used in) operating activities:
|
||||||||
|
Deferred income
|
(142,070 | ) | - | |||||
|
Provision for doubtful accounts
|
2,349,703 | 406,795 | ||||||
|
Increase (decrease) provision for obsolete inventory
|
(237,632 | ) | 309,343 | |||||
|
Depreciation
|
2,036,280 | 2,173,251 | ||||||
|
Amortization
|
338,019 | 381,742 | ||||||
|
Interest expense
|
167,586 | 4,761 | ||||||
|
(Gain) loss on sale of assets
|
(63,773 | ) | 47,054 | |||||
|
Loss on assets written off
|
40,982 | 5,876 | ||||||
|
Rental expense borne by a related party
|
330,708 | 326,774 | ||||||
|
(Increase) decrease in assets:
|
||||||||
|
Accounts receivable, trade - related parties
|
3,411,877 | 8,308,993 | ||||||
|
Other receivables
|
(16,036 | ) | (184,540 | ) | ||||
|
Other receivables - related parties
|
9,936,038 | 1,668,812 | ||||||
|
Inventories
|
93,315 | 2,761,335 | ||||||
|
Employee advances
|
(50,112 | ) | (38,359 | ) | ||||
|
Prepaid expense
|
258,945 | (419,419 | ) | |||||
|
Increase (decrease) in liabilities:
|
||||||||
|
Accounts payable
|
8,742,972 | (1,249,152 | ) | |||||
|
Advances from customers - related parties
|
21,603,664 | 1,178,463 | ||||||
|
Wages and benefits payable
|
76,914 | (43,279 | ) | |||||
|
Other taxes payable
|
(2,674,295 | ) | 968,294 | |||||
|
Other payables
|
(74,068 | ) | (650,296 | ) | ||||
|
Other payables - related parties
|
9,069,853 | 3,107,699 | ||||||
|
Net cash provided by operating activities
|
60,357,586 | 43,871,048 | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||
|
Cash outflow arising from disposal of a subsidiary
|
- | (23,975,473 | ) | |||||
|
Collections from loans to local government
|
- | 105,229 | ||||||
|
Construction deposits
|
(11,248,302 | ) | (2,664,741 | ) | ||||
|
Contractor deposits
|
19,306 | 19,734 | ||||||
|
Addition to construction in progress
|
(54,487,459 | ) | (42,734,161 | ) | ||||
|
Equipment deposits
|
(6,102,039 | ) | (11,782,984 | ) | ||||
|
Proceeds from sales of properties
|
73,650 | 29,131 | ||||||
|
Purchase of equipment and automobiles
|
(1,441,906 | ) | (2,009,536 | ) | ||||
|
Net cash used in investing activities
|
(73,186,750 | ) | (83,012,801 | ) | ||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||
|
Loan from (repayment to) related parties
|
- | (3,946,860 | ) | |||||
|
Proceed from short term debt
|
2,958,800 | - | ||||||
|
Proceeds from long term debt
|
18,048,680 | - | ||||||
|
Net cash provided by (used in) financing activities
|
21,007,480 | (3,946,860 | ) | |||||
|
EFFECT OF EXCHANGE RATE CHANGES ON CASH
|
128,878 | 82,430 | ||||||
|
INCREASE (DECREASE) IN CASH
|
8,307,194 | (43,006,183 | ) | |||||
|
CASH, beginning of period
|
1,848,328 | 44,854,511 | ||||||
|
CASH, end of period
|
$ | 10,155,522 | $ | 1,848,328 | ||||
|
Supplemental disclosures of cash flow information
|
||||||||
|
Cash paid during the period for:
|
||||||||
|
Interest
|
$ | 217,154 | $ | 105,817 | ||||
|
Income taxes
|
$ | 1,489,417 | $ | 3,287,531 | ||||
The accompanying notes are an integral part of this statement.
Tiens Biotech Group (USA). Inc.
Note 1 - Background
Tiens Biotech Group (USA), Inc. (the "Company" or "Tiens") was incorporated on July 13, 1990 as Super Shops, Inc. in the State of Michigan. In October 2000, Super Shops, Inc. reincorporated in Delaware and changed its name to MIA Acquisition Corp., and subsequently to Strategika, Inc. in February 2002. On December 31, 2003, the Company changed its name from Strategika, Inc. to Tiens Biotech Group (USA), Inc.
The Company is currently owned 4.91% by public stockholders and 95.09% by Jinyuan Li, the Company’s current Chairman, Chief Executive Officer, Acting Chief Financial Officer and President. The Company owns 100% of Tianshi International Holdings Group Limited ("Tianshi Holdings"). Tianshi Holdings owns 80% of Tianjin Tianshi Biological Development Co., Ltd. ("Biological") and 100% of Tianjin Tiens Life Resources Co., Ltd. (“Life Resources”).
Acquisition of Tianshi Holdings and Biological
On September 9, 2003, the Company received all of the issued and outstanding common stock of Tianshi Holdings in exchange for the issuance by the Company of 68,495,000 shares of its common stock to the original stockholders of Tianshi Holdings, representing 95.09% of the issued and outstanding common stock of the Company at such time, after giving effect to the issuance.
On June 18, 2003, Tianshi Holdings acquired 80% of Biological from Tianshi Hong Kong International Development Co., Ltd. ("Tianshi Hong Kong"), which was 100% owned by the Company's current Chairman, Chief Executive Officer, Acting Chief Financial Officer and President, Jinyuan Li. Tianjin Tianshi Biological Engineering Co., Ltd. (“Tianshi Engineering”) owned the remaining 20% of Biological.
Tianshi Engineering is 49% owned by Baolan Li, the daughter of Jinyuan Li and a director of the Company, and 51% by Tianjin Tianshi Group Co., Ltd. ("Tianshi Group"). In June 2003, Tianshi Engineering transferred its 20% interest in Biological for no consideration to Tianjin Tianshi Pharmaceuticals Co., Ltd (“Tianshi Pharmaceuticals”) and Tianshi Holdings acquired 80% of Biological from Tianshi Hong Kong for no consideration. On February 25, 2008, Tianshi Pharmaceuticals, which is 100% owned by Tianshi Group, transferred its 20% interest in Biological to Tianshi Engineering.
Acquisition and sale of Tiens Yihai
In April 2004, Tianshi Holdings entered into a joint venture contract with Tianshi Pharmaceuticals to establish Tiens Yihai Co. Ltd., a Chinese-Foreign Equity Joint Venture (“Tiens Yihai”). Tiens Yihai was 99.4% owned by Tianshi Holdings and 0.6% owned by Tianshi Pharmaceuticals. Tiens Yihai is located in Shanghai, China, and was established to build a new research and development facility in Shanghai, China. In March 2007, the Company decided to suspend the proposed development by Tiens Yihai.
In anticipation of this sale, the registered capital requirement of Tiens Yihai was decreased from $200 million to $29,989,361, equal to its then-current paid in, registered capital. This reduction was approved by the local government on October 14, 2008. Pursuant to the change of the registered capital requirement and the capital contributions from both investors, the equity interest held by Tianshi Holdings was decreased from 99.4% to 96% and the equity interest held by Tianshi Pharmaceuticals was increased from 0.6% to 4%. Tianshi Holdings, Tianshi Pharmaceuticals and Tianjin Tianshi Group Co., Ltd. (“Tianshi Group”) entered into a letter of intent on December 31, 2008, pursuant to which Tianshi Pharmaceuticals agreed to purchase the 96% equity interest in Tiens Yihai held by Tianshi Holdings, but this transaction was abandoned.
On November 15, 2009, Tianshi Holdings entered into a contract to transfer of its 96% Equity Interests of Tiens Yihai to Tianshi International Investment Group, Co., Ltd., a British Virgin Islands Company (“Tianshi Investment”). Jinyuan Li owns 100% of Tianshi Investment. Pursuant to the Sale and Purchase Agreement, Tianshi Holdings agreed to sell its 96% equity interest in Tiens Yihai for $37.0 million. As of the year ended 2010, the company had received the amount in full.
Acquisition of Life Resources
On December 20, 2007, Tianshi Holdings entered a Sale and Purchase Agreement with Tianshi Investment. Pursuant to the Sale and Purchase Agreement, Tianshi Holdings agreed to buy all of the registered share capital of Life Resources for $64.2 million. On March 13, 2008, the Chinese government approved the transfer and the Company became the 100% shareholder of Life Resources.
Life Resources was incorporated on April 29, 2005 as a Foreign Investment Enterprise (“FIE”) in Wuqing, Tianjin, PRC, with a registered share capital of $30,000,000. The Company is currently constructing research and development and manufacturing and logistic facilities, as well as administrative offices. Construction on the project began in July 2006. On March 13, 2008 and Jun 24, 2009, the Chinese government approved the increase of registered capital of Life Resources from $30,000,000 to $50,000,000 and from $50,000,000 to $65,000,000, respectively.
Nature of operations
The Company through its subsidiaries is primarily engaged in the manufacturing of nutritional supplement products, including wellness products and dietary supplement products. In the PRC, the Company sells its products to Tianshi Engineering. Tianshi Engineering, in turn, sells the products to customers through its branches and affiliated companies and at chain stores which are owned by individual distributors. Outside the PRC, the Company sells its products to overseas affiliated companies located in 45 countries who in turn re-package them and sell to independent direct sales distributors.
Note 2 – Summary of significant accounting policies
BASIS OF PRESENTATION
These consolidated financial statements of the Company and its subsidiaries are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
The reporting entity
The Company’s consolidated financial statements reflect the activities of the following Company subsidiaries:
|
Subsidiary
|
% Ownership
|
||||
|
Tianshi Holding
|
British Virgin Islands
|
100.0 | % | ||
|
Biological
|
P.R.C.
|
80.0 | % | ||
|
Life Resources
|
P.R.C.
|
100.0 | % | ||
Principles of consolidation
The consolidated financial statements include the financial statements of the Company and its subsidiaries. All significant inter-company transactions and balances are eliminated in consolidation.
Use of estimates
In preparing financial statements in conformity with accounting principles generally accepted in the United States of America, the Company makes estimates, judgments and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and sales and expenses during the reported periods. Significant estimates include useful life of long lives asset, provision for doubtful accounts and allowance for sales returns. Management bases its estimates on historical experience and on various other assumptions that they believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results could differ from those estimates.
Foreign currency translation
The reporting currency of the Company is the US dollar. Biological and Life Resources’ financial records are maintained and the statutory financial statements are stated in its local currency, Renminbi (RMB), as their functional currency. Results of operations and cash flows are translated at average exchange rates during the period, and assets and liabilities are translated at the unified exchange rate as quoted by OANDA.com at the end of each reporting period. Translation adjustments resulting from this process are included in other comprehensive income in the statements of income and other comprehensive income.
This quotation of the exchange rates does not imply free convertibility of RMB to other foreign currencies. All foreign exchange transactions continue to take place either through the People's Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rate quoted by the People's Bank of China. Approval of foreign currency payments by the People’s Bank of China or other institutions requires submitting a payment application form together with invoices, shipping documents and signed contracts.
Translation adjustments amounted to $5,131,503 and $441,140 for the years ended December 31, 2010 and 2009, respectively. Asset and liability accounts at December 31, 2010 were translated at RMB6.61 to US$1.00 compared to RMB6.84 at December 31, 2009. Equity accounts were stated at their historical rate. The average translation rates applied to income statement accounts for the years ended December 31, 2010 and 2009 were RMB6.76 and RMB6.84, respectively. Cash flows are also translated at average translation rates for the period. Therefore, amounts reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet and statement of shareholders’ equity.
Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred. These amounts are not material to the financial statements.
Translation adjustments are reported under comprehensive income as a component of stockholders’ equity.
Fair value of financial instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. Fair value hierarchy, prioritizes the inputs used in measuring fair value into three broad levels as follows:
• Level one — Quoted market prices in active markets for identical assets or liabilities;
• Level two — Inputs other than level one inputs that are either directly or indirectly observable; and
• Level three — Unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use.
Determining which category an asset or liability falls within the hierarchy requires significant judgment. The Company's financial instruments consist primarily of cash, trade accounts receivable, trade payables, advances, other receivables, and debt instruments. The carrying amounts of the Company's financial instruments generally approximate their fair values at December 31, 2010 and December 31, 2009.
Cash
Cash includes cash on hand and demand deposits in accounts maintained with banks of which no deposits are covered by insurance. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant risks on its cash in bank accounts.
Accounts receivable
The Company's trade accounts receivable are mainly due from related companies. The Company maintains a full provision for accounts receivable-related parties aged over one year based upon the related parties abilities to collect their receivables and a general allowance for doubtful debt of 0.5% of the remaining accounts receivable-related parties. Management reviews its accounts receivable on a regular basis to determine if the provision for doubtful accounts is adequate, paying particular attention to the age of receivables outstanding. At December 31, 2010 and December 31, 2009 receivables outstanding more than 180 days totaled $6,985,556 and $5,089,510, respectively. The Company did not charge-off any receivables for the periods reported and does not accrue interest on any of its accounts receivable. The following table represents the changes in the allowance for doubtful accounts:
|
Balance at
Beginning of
Period
|
Increase of provision for
Doubtful Accounts
|
Balance at End
of Period
|
||||||||||
|
Year ended December 31, 2010
|
||||||||||||
|
Allowance for doubtful accounts:
|
$ | 1,419,178 | $ | 2,450,439 | $ | 3,869,617 | ||||||
|
Year ended December 31, 2009
|
||||||||||||
|
Allowance for doubtful accounts:
|
$ | 1,108,789 | $ | 310,389 | $ | 1,419,178 | ||||||
Inventories
Inventories are stated at the lower of cost or market using the weighted average basis. The Company reviews its inventory annually for possible obsolete goods or to determine if any reserves are necessary for potential obsolescence.
Prepaid expenses
Prepaid expenses consist of advances to suppliers and short-term prepaid expenses. The Company reviews its advances to suppliers annually to determine whether provisions should be adjusted. The amount included in prepaid expense is net of any provisions. Provisions for prepaid expenses are as follows:.
|
Balance at
Beginning of
Period
|
Increase of provision for
Doubtful Accounts
|
Balance at End
of Period
|
||||||||||
|
Year ended December 31, 2010
|
||||||||||||
|
Provision for prepaid expenses:
|
$ | 1,018,474 | $ | 38,077 | $ | 1,056,551 | ||||||
|
Year ended December 31, 2009
|
||||||||||||
|
Provision for prepaid expenses:
|
$ | 952,071 | $ | 66,403 | $ | 1,018,474 | ||||||
Property, plant and equipment, net
Property, plant and equipment are stated at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Estimated useful lives of the assets are as follows:
|
Estimated Useful Life
|
|
|
Buildings and improvements
|
20 years
|
|
Machinery and equipment
|
10 years
|
|
Computer, office equipment and furniture
|
5 years
|
|
Automobiles
|
5 years
|
The cost and related accumulated depreciation of assets sold or otherwise retired are eliminated from the accounts, and any gain or loss is included in the consolidated statements of income and other comprehensive income. Maintenance, repairs and minor renewals are charged directly to expenses as incurred. Major additions and betterment to buildings and equipment are capitalized.
Construction in progress
Construction in progress represents the costs incurred in connection with the construction of buildings or new additions to the Company's plant facilities. No depreciation is provided for construction in progress until such time as the relevant assets are completed and are ready for their intended use.
Construction deposits
Construction deposits represent advances paid by the Company to contractors.
Intangible assets
Intangible assets mainly consist of land use rights. All land located in the PRC is owned by the government and cannot be sold to any individual or company. However, the government grants "land use rights" for a specified period of time. The Company amortizes its land use rights according to the actual useful life of 50 years. Other intangible assets include patents and trademarks and are amortized over their estimated useful life ranging from five to ten years.
Other assets
Other assets consist of deposits made to purchase equipment and a long-term prepaid expense. The Company will transfer the deposits made to purchase equipment from other assets to property, plant and equipment upon taking ownership. The Company amortizes its long-term prepaid expense according to the service period.
Impairment of long-lived assets
Long-lived assets, including intangible assets of the Company, are reviewed annually as to whether their carrying value has become impaired. The Company considers assets to be impaired if the carrying value exceeds the future projected cash flows from related operations. The Company also re-evaluates the periods of depreciation and amortization to determine whether subsequent events and circumstances warrant revised estimates of useful lives. For the years ended December 31, 2010 and 2009, the Company determined there to be no impairment of long-term assets.
Deferred income
Deferred income consists of government grants. On August 2, 2005 and November 20, 2006, the Company received two government grants related to the purchase of land use rights in the amount of RMB35,803,461 (or US$5,223,725). On August 28, 2008, Life Resources paid RMB41,022,061 (or US$5,985,119) for the zoning changes to one parcel of land on which the land use rights were changed from “industrial” to “educational”. On September 12, 2008, the Company received RMB41,022,061 (or US$5,985,119) from a government grant. The grants are treated as deferred income and amortized over the life of the buildings on the land from October 2010.
Noncontrolling interest
Noncontrolling interest represents the outside shareholder’s 20% ownership of Biological and is presented as a reduction of net income to arrive at net income attributable to the Company and as a component of equity.
Revenue recognition
The Company sells both semi-finished products and finished products to Tianshi Engineering domestically. Revenue from semi-finished products was recognized at delivery point. Revenue from finished products is recognized only when the related party Chinese distributors recognized sales of the Company's products to unaffiliated third parties. Revenues in both cases are net of taxes.
For overseas sales, the Company sells mostly finished products. The Company recognizes revenue from international sales (non-Chinese) to affiliated parties, net of taxes, as goods are shipped and clear review by the customs department of the Chinese government.
The Company is generally not contractually obligated to accept returns. However, on a case by case negotiated basis, the Company permits customers to return their products. Revenue is recorded net of an allowance for estimated returns. Such allowance is based upon management's evaluation of historical experience and estimated costs. The amount of the allowance ultimately required could differ materially in the near term from amounts included in the accompanying consolidated financial statements. As the Company did not receive any returns of products during the past two years, and management does not anticipate allowing any returns in 2011 related to revenues in 2010, no allowance for estimated returns has been recorded for the years ended December 31, 2010 and 2009.
Advertising costs
The Company sells all of its products to related parties, and these related parties are primarily responsible for marketing. Advertising costs of the Company for the years ended December 31, 2010 and 2009 amounted to $13,167 and $11,914, respectively, and were expensed as incurred.
Shipping and handling
Shipping and handling expense $390,197 and $340,275 for the years ended December 31, 2010 and 2009, respectively. The Company sells products on FOB condition, however, it usually prepays shipping and handling expenses to transportation companies on behalf of customers and collects these shipping and handling expenses when it receives payments from customers. The payments received from customers are included in revenue, and the related shipping and handling costs are included in selling, general and administrative expenses.
Research and development
Research and development expenses include salaries, supplies, and overhead such as depreciation, utilities and other costs. These costs are expensed as incurred. The Company expensed research and development costs of $1,832,360 and $1,262,623 for the years ended December 31, 2010 and 2009, respectively. These costs are included in selling, general and administrative expenses in the consolidated statements of income and other comprehensive income.
Income taxes
The Company accounts for income taxes under the liability method. Deferred income taxes are recognized for the estimated tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each year-end based on enacted tax laws and statutory rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established to reduce deferred tax assets to the amount expected to be realized when, in management’s opinion, it is more likely than not that some portion of the deferred tax assets will not be realized. The provision for income taxes represents current taxes payable net of the change during the period in deferred tax assets and liabilities.
The Company has not been subjected to income tax examinations by taxing authorities for the years ended December 31, 2010 and 2009. The Company is open to tax examination in the PRC for all years, as tax returns remain open to examination until notified by the taxing authorities, and the Company has not received any notifications to date. The Company records interest and penalties as other expense on the consolidated statements of income and other comprehensive income. During the years ended December 31, 2010 and 2009, the Company did not recognize any amount in interest and penalties.
Earnings per share
Basic earnings per share (“EPS”) excludes dilution and is computed by dividing net income (loss) attributable to common stockholders by the weighted average number of common shares outstanding for the period. There are no differences between Basic and Diluted EPS for the periods ended December 31, 2010 and 2009.
Recently issued accounting pronouncements
In October 2009, the FASB issued Accounting Standards Update (ASU) 2009-13, Multiple-Deliverable Revenue Arrangements (ASU 2009-13) (formerly EITF 08-1, Revenue Arrangements with Multiple Deliverables) which amends ASC 605, Revenue Recognition. This accounting update establishes a hierarchy for determining the value of each element within a multiple deliverable arrangement. The amendments in this update are effective for revenue arrangement entered into or materially modified in fiscal years beginning on or after June 15, 2010. The adoption of ASU 2009-13 did not have an impact on the Company’s consolidated financial statements.
In January 2010, the FASB issued ASU 2010-02 — Consolidation (Topic 810): Accounting and Reporting for Decreases in Ownership of a Subsidiary. This update amends Subtopic 810-10 and related guidance to clarify that the scope of the decrease in ownership provisions of the Subtopic and related guidance applies to (i) a subsidiary or group of assets that is a business or nonprofit activity; (ii) a subsidiary that is a business or nonprofit activity that is transferred to an equity method investee or joint venture; and (iii) an exchange of a group of assets that constitutes a business or nonprofit activity for a noncontrolling interest in an entity, but does not apply to: (i) sales of in substance real estate; and (ii) conveyances of oil and gas mineral rights. The amendments in this update are effective beginning in the period that an entity adopts FAS 160 (now included in Subtopic 810-10). The adoption of the provisions in ASU 2010-02 did not have an impact on the Company’s consolidated financial statements.
The FASB has issued ASU 2010-06, Fair Value Measurements and Disclosures (Topic 820): Improving Disclosures about Fair Value Measurements. This ASU requires some new disclosures and clarifies some existing disclosure requirements about fair value measurement as set forth in Codification Subtopic 820-10. ASU 2010-06 amends Codification Subtopic 820-10 and now requires a reporting entity to use judgment in determining the appropriate classes of assets and liabilities and to provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements. ASU 2010-06 was effective for interim and annual reporting periods beginning after December 15, 2009, As this standard relates specifically to disclosures, the adoption did not have an impact on the Company’s consolidated financial position and results of operations.
In February 2010, the FASB issued ASU 2010-10, "Consolidation (Topic 810)." The amendments to the consolidation requirements of ASC Topic 810 resulting from the issuance of Statement 167 are deferred for a reporting entity's interest in an entity (1) that has all the attributes of an investment company or (2) for which it is industry practice to apply measurement principles for financial reporting purposes that are consistent with those followed by investment companies. An entity that qualifies for the deferral will continue to be assessed under the overall guidance on the consolidation of variable interest entities in ASC Subtopic 810-10 (before the Statement 167 amendments) or other applicable consolidation guidance, such as the guidance for the consolidation of partnerships in ASC Subtopic 810-20. The deferral is primarily the result of differing consolidation conclusions reached by the International Accounting Standards Board ("IASB") for certain investment funds when compared with the conclusions reached under Statement 167. The deferral is effective as of the beginning of a reporting entity's first annual period that begins after November 15, 2009, and for interim periods within that first annual reporting period, which coincides with the effective date of Statement 167. Early application is not permitted. The provisions of ASU 2010-10 are effective for the Company beginning in 2010. The adoption of ASU 2010-10 did not have an impact on the financial position, results of operations or cash flows of the Company.
In February 2010, the FASB issued an accounting standard that amended certain recognition and disclosure requirements related to subsequent events. The accounting standard requires an entity that is an SEC filer to evaluate subsequent events through the date that the financial statements are issued and removes the requirement that an SEC filer disclose the date through which subsequent events have been evaluated. This guidance was effective upon issuance. The adoption of this standard had no effect on the Company’s consolidated financial position or results of operations.
In May 2010, the FASB issued ASU 2010-19, “Foreign Currency (Topic 830): Foreign Currency Issues: Multiple Foreign Currency Exchange Rates”. The amendments in this ASU are effective as of the announcement date of March 18, 2010. The adoption of this update did not have a material impact on the Company’s financial statements.
In July 2010, the FASB issued ASU 2010-20, Disclosures about the Credit Quality of Financing Receivables and the Allowance for Credit Losses, which amends ASC 310, Receivables. This ASU requires disclosures related to financing receivables and the allowance for credit losses by portfolio segment. The ASU also requires disclosures of information regarding the credit quality, aging, nonaccrual status and impairments by class of receivable. A portfolio segment is the level at which a creditor develops a systematic methodology for determining its credit allowance. A receivable class is a subdivision of a portfolio segment with similar measurement attributes, risk characteristics and common methods to monitor and assess credit risk. Trade accounts receivable with maturities of one year or less are excluded from the disclosure requirements. The effective date for disclosures as of the end of the reporting period is the first quarter of fiscal year 2011. The effective date for disclosures for activity during the reporting period is the second quarter of fiscal year 2011. The adoption will not have a material effect on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s consolidated financial statements upon adoption.
Note 3 – Supplemental disclosure of cash flow information
On February 10, 2010, Tianshi Holdings, Fuhong Development Co., Ltd. (“Fuhong Development”), and Tianshi Investment entered into an agreement, pursuant to which liabilities in the form of a loan receivable due to Fuhong Development from the Company in the amount of $3,000,000 were offset against assets in the form of a loan receivable due to the Company from Tianshi Investment. Each of Fuhong Development and Tianshi Investment is 100% owned by Jinyuan Li.
The right to offset existed because:
|
|
1.
|
Tianshi Investment had a determinable outstanding debt payable to the Company;
|
|
|
2.
|
The Company had a determinable outstanding debt payable to Fuhong Development;
|
|
|
3.
|
The Company had the right to offset the two amounts;
|
|
|
4.
|
The Company, Fuhong Development and Tianshi Investment agreed to offset the two amounts; and
|
|
|
5.
|
The agreement to offset is enforceable under Chinese contract law.
|
On June 10, 2010, Tianshi Holdings, Fuhong Development, and Tianshi Investment entered into another agreement, pursuant to which liabilities in the form of a loan receivable due to Fuhong Development from the Company in the amount of $3,000,000 were offset against assets in the form of a loan receivable due to the Company from Tianshi Investment.
The right to offset existed because:
|
|
1.
|
Tianshi Investment had a determinable outstanding debt payable to the Company;
|
|
|
2.
|
The Company had a determinable outstanding debt payable to Fuhong Development;
|
|
|
3.
|
The Company had the right to offset the two amounts;
|
|
|
4.
|
The Company, Fuhong Development and Tianshi Investment agreed to offset the two amounts; and
|
|
|
5.
|
The agreement to offset is enforceable under Chinese contract law.
|
On November 12, 2010, Tianshi Group, Tianshi Investment, Tianshi Engineering and the company entered into an agreement, pursuant to which the Company’s liabilities in the form of advance from Tianshi Engineering of $11,767,357, short-term debt from Tianshi Engineering of $9,077,156 and other payable to Tianshi Group of $6,455,487 were offset against assets in the form of other receivable from Tianshi Investment.
The right to offset existed because:
|
|
1.
|
Tianshi Investment had a determinable outstanding debt payable to the Company;
|
|
|
2.
|
The Company had a determinable outstanding debt payable to Tianshi Group and Tianshi Engineering;
|
|
|
3.
|
The Company had the right to offset the four amounts;
|
|
|
4.
|
The Company, Tianshi Engineering, Tianshi Group and Tianshi Investment agreed to offset the four amounts; and
|
|
|
5.
|
The agreement to offset is enforceable under Chinese contract law.
|
Note 4 – Inventories
Inventories consist of the following:
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Raw materials
|
$ | 1,392,141 | $ | 1,937,306 | ||||
|
Packing materials
|
479,651 | 729,517 | ||||||
|
Miscellaneous supplies
|
1,860,462 | 848,720 | ||||||
|
Work in process
|
640,664 | 476,529 | ||||||
|
Finished goods
|
1,330,431 | 1,335,980 | ||||||
|
Total
|
$ | 5,703,349 | $ | 5,328,052 | ||||
The above amount indicates an inventory obsolescence reserve of $76,942 and $309,343 for the years ended December 31, 2010 and 2009, respectively.
Note 5 – Employee advances
Employee advances represents cash advances to various employees of the Company. In the PRC, a majority of business transactions are completed in cash. These cash advances represent monies advanced to certain employees to pay for various expenses and purchases related to the Company's daily operations. Employee advances amounted to $170,842 and $115,673 at December 31, 2010 and 2009, respectively.
Note 6 – Prepaid expenses
Prepaid expenses consist of advances to suppliers and short-term prepaid expenses. The details of prepaid expenses net of the allowance as disclosed in Note 2 are as follows:
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Advance to suppliers
|
$ | 412,458 | $ | 655,443 | ||||
|
Short-term prepaid expenses
|
2,750 | 2,750 | ||||||
|
Total
|
$ | 415,208 | $ | 658,193 | ||||
Note 7 – Prepaid taxes
Prepaid taxes primarily represents prepaid value added tax and income tax, which will be used to deducted from the value added tax payables and income tax payables in future. The detail of prepaid taxes was as follows:
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Prepaid value added taxes
|
$ | 3,822,250 | $ | 542,433 | ||||
|
Prepaid income taxes
|
- | 81,600 | ||||||
|
Taxes payable (except income taxes payable)
|
(176,110 | ) | (216,499 | ) | ||||
|
Total
|
$ | 3,646,140 | $ | 407,534 | ||||
Note 8 – Property, plant and equipment, net
Property, plant and equipment consisted of the following:
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Buildings and improvements
|
$ | 63,687,629 | $ | 1,558,934 | ||||
|
Office equipment
|
487,179 | 348,284 | ||||||
|
Computer equipment and software
|
2,855,978 | 2,405,467 | ||||||
|
Machinery and equipment
|
15,325,589 | 14,211,726 | ||||||
|
Automobiles
|
4,998,126 | 4,470,909 | ||||||
|
Total
|
87,354,501 | 22,995,320 | ||||||
|
Less: accumulated depreciation
|
(15,316,959 | ) | (12,870,837 | ) | ||||
|
Property, plant and equipment, net
|
$ | 72,037,542 | $ | 10,124,483 | ||||
Depreciation expense for the years ended December 31, 2010 and 2009 amounted to $2,036,280 and $2,173,251, respectively.
Note 9 - Intangible assets
Intangible assets consisted of the following:
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Land use rights
|
$ | 13,992,186 | $ | 13,531,454 | ||||
|
Other intangible assets
|
355,996 | 344,274 | ||||||
|
Less accumulated amortization
|
(1,361,182 | ) | (1,011,433 | ) | ||||
|
Intangible assets, net
|
$ | 12,987,000 | $ | 12,864,295 | ||||
Amortization expense for the years ended December 31, 2010 and 2009 amounted to $338,019, and $381,742 respectively.
The estimated amortization expense for the next five years is as follows:
|
Estimated amortization expense for
|
||||
|
the year ending December 31,
|
Amount
|
|||
|
2011
|
$ | 308,577 | ||
|
2012
|
$ | 308,577 | ||
|
2013
|
$ | 297,105 | ||
|
2014
|
$ | 296,450 | ||
|
2015
|
$ | 294,747 | ||
|
2016 and thereafter
|
$ | 11,481,544 | ||
Note 10 -Short-term debt
On October 20, 2010, Life Resources entered into an agreement with Construction Bank of China to obtain the loan of RMB20,000,000 (US$3,024,800) for purchasing raw materials and other auxiliary materials at the term of one year. The weighted-average interest rate of the short-term debt is the one-year benchmark lending rate (The interest rate is 5.56% from October 20, 2010 to December 25, 2010 and 5.81% from December 26, 2010 to December 31, 2010). Under the agreement, Life Resources is required to take the following obligations:
|
1.
|
Submit quarterly financial statement within fifteen days after each quarter;
|
|
2.
|
Submit any information that significantly affect the repayment ability of the Company;
|
|
3.
|
The condition of using the fund should be fulfilled the environmental law;
|
|
4.
|
No utilization of bank loans in other than prescribed;
|
|
5.
|
To inform the bank for any related party transaction with an amount over 10% of net asset.
|
Note 11 -Long-term debt
On October 9, 2010, Life Resources entered into a loan agreement with Agricultural Bank of China (Agricultural Bank), pursuant to which Agricultural Bank loaned RMB200,000,000 (or US$30,248,000) with the benchmark lending interest rate of The People’s Bank of China to fund Life Resources’ construction in progress under the guarantee of Tiens Group. Under the agreement, Life Resources is required to take the following obligations:
1. The usage of the loan is in accordance with the laws and regulations or contracts;
2. Agree and actively cooperate with the lender and their agents on monitoring, inspecting of the construction of the project, financial activities, usage of loan and other related matters;
3. Submit information timely to the lender as the lender requested regarding the projects, usage of the loan, finance and other.
4. Submit any information that significantly affect the repayment ability of the Company;
Life Resources is permitted the full amount before October 8, 2011, and repay it before October 8, 2013. As of December 31, 2010, Life Resources has drawn RMB122,000,000 (or US$18,451,280) of the loan with three-year benchmark lending interest rate of The People’s Bank of China, which was adjusted twice, from 5.40% to 5.60% and to 5.85% since the Company received the first amount of the loan. The detail information of the obtained amount is as follows:
|
Obtained date
|
October 11, 2010
|
November 12, 2010
|
December 10, 2010
|
|||||||||
|
Amount
|
$ | 9,830,600 | $ | 6,049,600 | $ | 2,571,080 | ||||||
The amounts to be paid are as follows:
|
Date
|
October 8, 2011
|
October 8, 2012
|
October 8, 2013
|
|||||||||
|
Amount
|
$ | 4,537,200 | $ | 9,074,400 | $ | 16,636,400 | ||||||
Note 12 – Related party sale of property
On December 25, 2008, Biological and Tianshi Group entered into a Real Property Transfer Agreement (the “Transfer Agreement”). Under the Transfer Agreement, Biological transferred to Tianshi Group four buildings consisting of three workshops and a canteen, totaling 9,974.31 square meters, located at No. 6 Yuanquan Road, Wuqing Development Area, Tianjin New-Tech Industry Park, China. Pursuant to the Transfer Agreement, Tianshi Group paid Biological RMB32,800,000 (or US$4,797,328) to Biological. This transaction resulted in a loss of RMB1,912,983 (or US$274,762) for Biological.
On January 1, 2009, Biological and Life Resources entered two Lease Agreements with Tianshi Group, pursuant to which Biological and Life Resources had the right to use and occupy the workshop spaces being transferred under the Transfer Agreement. The leases are rent-free, but Biological and Life Resources are required to pay Tianshi Group for utility charges and maintenance costs on the buildings. The leases continue until the earlier of the date that Biological and Life Resources acquire use of alternate facilities or the land use rights on the underlying property expire. For the years ended December 31, 2010 and 2009, Biological and Life Resources recorded $330,708 and $303,056 of the rent expense, which is not paid to Tianshi Group, but recorded as paid in capital based upon market price.
Note 13 – Other related party transactions and balances
During the years 2010 and 2009, the Company mainly conducted related party transactions with Tianshi Group, Tianshi Engineering, Tianshi Investment and overseas related companies of Tianshi Group. Tianshi Group is owned 90% by Jinyuan Li and 10% by his daughter, Baolan Li, who is also a director of the Company. Tianshi Engineering is owned 51% by Tianshi Group and 49% by Baolan Li. Tianshi Investment is 100% owned by Jinyuan Li. Jinyuan Li owns or controls the overseas related companies of Tianshi Group.
The Company’s related party transactions are required to be reviewed and approved or ratified by Board of Directors. No director that is a related person in a related party transaction may participate in any discussion, approval or ratification of the related party transaction except to provide information concerning it. The following tables are provided to facilitate an understanding of the transactions and outstanding balances between those related parties and the Company during 2010 and 2009.
|
2010
|
2009
|
|||||||
|
Revenue-related parties
|
$ | 41,021,135 | $ | 60,032,968 | ||||
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Accounts receivable, trade – related parties, net of allowance for doubtful accounts of $3,869,617 and $1,419,178 as of December 31, 2010 and 2009, respectively
|
$ | 10,012,861 | $ | 15,379,312 | ||||
|
Other receivables – related parties
|
$ | 17,376,522 | $ | 44,561,626 | ||||
|
Advances from customers – related parties
|
$ | 8,688,877 | $ | 4,426,751 | ||||
|
Other payables – related parties
|
$ | 1,417,516 | $ | 3,326,110 | ||||
Revenue-related parties
The details of revenue-related parties are as follows:
|
2010
|
2009
|
|||||||
|
Tianshi Engineering
|
$ | 24,570,887 | $ | 25,298,232 | ||||
|
Overseas Related Companies
|
16,450,248 | 34,734,736 | ||||||
|
Total
|
$ | 41,021,135 | $ | 60,032,968 | ||||
The Company markets most of its products through various domestic and international business entities that are related to the Company through common ownership.
In China, the Company sells its products to Tianshi Engineering. Tianshi Engineering, in turn, markets and sells the products to customers through its branches and affiliated companies and at chain stores which are owned by individual distributors. Tianshi Engineering is solely responsible for all marketing and payments of sales commissions to independent distributors.
The Company has a sales contract with Tianshi Engineering which requires Tianshi Engineering to purchase all of the Company’s products to be sold in China. The Company sells its finished products to Tianshi Engineering at a price equal to 25% of the Chinese market price for the products. This 25% figure was negotiated between the parties in 2003, before the Company acquired Tianshi Holdings. The price of semi-finished goods sold to Tianshi Engineering was originally set at the beginning of 2006 to provide the Company with a 75% gross profit margin. However, based on fluctuations in the cost of raw materials and quantities produced, the gross profit margin percentage varies. The goal of this pricing policy was to try to maintain the Company’s gross margins on semi-finished goods at a similar level to historical gross margins for finished goods. All of Tianshi Engineering’s Chinese affiliated companies are owned in whole or in part by Jinyuan Li’s immediate family members. For the year ended 2010, the average gross profit margin of finished goods and semi-finished goods, which were sold to Tianshi Engineering, were approximately 72% and 69%, respectively.
Internationally, the Company sells its products directly to overseas affiliates. These overseas related companies re-package the Company’s products and then sell to overseas independent distributors or end users of the products. Due to the common ownership, there are no formal sales or administrative agreements among Biological and those overseas related companies. The business operations among these related entities are regulated through internal ordinances.
Accounts receivable, trade-related parties
The details of accounts receivable, trade-related parties are as follows:
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Tianshi Engineering
|
$ | 1,803,688 | $ | 5,035,320 | ||||
|
Overseas Related Companies
|
12,078,790 | 11,763,170 | ||||||
|
Allowance for Doubtful Accounts
|
(3,869,617 | ) | (1,419,178 | ) | ||||
|
Total
|
$ | 10,012,861 | $ | 15,379,312 | ||||
On December 31, 2010, there are accounts receivables due from some overseas related companies outstanding over one year. The Company maintains a provision for the year end balance of accounts receivable due from those overseas related companies.
Other receivables-related parties
Other receivables - related parties are generated by the Company making various cash advances and short term loans, the allocation of various expenses to related parties, and amounts transferred from accounts receivable. The following table summarizes the other receivables-related parties balances:
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Tiens Life Science Co., Ltd.
|
$ | 11,525,219 | $ | - | ||||
|
Tianshi Engineering
|
5,603,197 | 5,688,926 | ||||||
|
All-Legend Property Service (Tianjin) Co., Ltd.
|
155,798 | 77,612 | ||||||
|
All-Legend Hotel Co., Ltd.
|
37,856 | - | ||||||
|
Tianshi Yinshi Hotel
|
37,810 | 36,566 | ||||||
|
Tianshi Indonesia Logistic & Trade Co., Ltd.
|
9,902 | 9,873 | ||||||
|
Tianshi Taijisheng Health management Co., Ltd
|
5,459 | - | ||||||
|
Shengshi Real Estate Development
|
1,072 | 634 | ||||||
|
Tianshi Pharmaceuticals
|
139 | 1,588 | ||||||
|
All-Legend Hotel Management Co., Ltd.
|
70 | 2,730 | ||||||
|
Tianshi Group
|
- | 1,613,168 | ||||||
|
Tiens SmartFlow Logistics (International) Group Ltd.
|
- | 74,651 | ||||||
|
Tianjin Tianshi Life Science Co., Ltd.
|
- | 55,878 | ||||||
|
Tianshi Investment
|
- | 37,000,000 | ||||||
|
Total
|
$ | 17,376,522 | $ | 44,561,626 | ||||
On November 15, 2009, Tianshi Holdings entered into a contract for the transfer of its 96% Equity Interests to sell all of the registered share capital of Tiens Yihai it owned to Tianshi Investment for $37.0 million. Pursuant to the Transfer Contract, the purchase price of $37.0 million was paid in full by November 12, 2010. Payment of $3,700,000 was received in cash, $6,000,000 was offset against payables owed to Fuhong Development, and $27,300,000 was offset against payables to Tianshi Engineering and Tianshi Group.
Historically, Tianshi Engineering remitted payment to the Company upon sales to third party customers. However, to support Tianshi Engineering’s marketing efforts in anticipation of receiving a direct selling license in China, the Company agreed to allow Tianshi Engineering to defer payment. Balances not remitted to the Company within 90 days are converted to other receivables-related parties.The other receivables-related parties become interest bearing. The stated interest rate is the interest rate for the same level of loan stipulated by the People’s Bank of China. On April 21, 2009, the Company entered into a loan agreement with Tianshi Engineering. Pursuant to that agreement, effective as of April 1, 2009, $2,562,017 of other receivables-related parties, which originated from Tianshi Engineering as accounts receivable, became interest bearing. The loan was due on June 30, 2009 and the stated interest rate was 4.86%. Both the principal of $2,562,017 and interest on the loan of $12,624 were paid off on May 7, 2009. For the year ended December 31, 2010 and 2009, the interest income from the other receivables-related parties amounted to $0 and $12,624, respectively.
The Company and Tianshi Group use common meters at the company’s headquarters for electricity and water, and also use the same employee insurance account. When making payments to these outside parties, the Company usually pays the fees first and then is reimbursed by Tianshi Group. These pro-rated amounts relating to Tianshi Group are categorized as other receivables-related parties.
On December 25, 2008, Biological entered into a Real Property Transfer Agreement with Tianshi Group, pursuant to which Biological transferred four buildings at the price of $4,797,328 . As of March 31, 2010, the amount was paid in full by Tianshi Group.
A portion of the construction in progress at an amount of RMB83,263,708 (US$12,592,803), which is recorded and paid for by Life Resources is built on the land owned by Tianshi Life Science. In addition, a portion of the construction in progress at an amount of RMB5,245,839 (US$793,381), which is recorded and paid for by Tianshi Life Science is built on the land owned by Life Resources. Tianshi Life Science and Life Resources intend to reimburse each other for the cost of the above construction in progress. The agreement is currently being drafted. The balance of RMB78,017,869 (US$11,799,422) has been reclassified as other receivable-related party as of December 31, 2010, accordingly.
Advances from customers-related parties
These advances represented prepayments made to the Company to insure that overseas related companies could obtain enough of the Company’s products to meet their market demands. As of December 31, 2010 and December 31, 2009, advances from related party customers amounted to $8,688,877 and $4,426,751, respectively.
Other payables-related parties
The details of other payables-related parties are as follows:
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Tiens Group
|
$ | 639,529 | $ | 0 | ||||
|
Tianshi Engineering
|
431,400 | 40,805 | ||||||
|
Tianshi Germany Co., Ltd.
|
99,759 | 107,326 | ||||||
|
Tianjin Tianshi Global International Trade Co., Ltd.
|
96,794 | 93,606 | ||||||
|
Tianyuan Capital Development Co. Ltd.
|
84,359 | 84,359 | ||||||
|
Bejing All-legeng travel Co., lTd
|
65,660 | 0 | ||||||
|
Tianshi Administrative Committee of Industrial Park
|
15 | 14 | ||||||
|
Fuhong Development Co.Ltd.
|
0 | 3,000,000 | ||||||
|
Total
|
$ | 1,417,516 | $ | 3,326,110 | ||||
These amounts arose primarily from previous cash advances from related parties such as management fees due to related parties and various non-operational transactions incurred with related parties.
On November 10, 2009, Tianshi Holdings borrowed $3,000,000 from Fuhong Development Co.,Ltd., a British Virgin Islands Company which is 100% owned by Jinyuan Li, to fund its capital contribution to Life Resources. On the February 10, 2010, the loan was paid in full by cancelling the same amount Tianshi Investment owed to the Company.
On March 3, 2010, Tianshi Holdings borrowed another $3,000,000 from Fuhong Development to fund its capital contribution to Life Resources. On June 11, 2010, the loan was paid in full by canceling the same amount Tianshi Investment owed to the Company.
During the third quarter of 2010, the Company received $7,614,300 and $6,270,600 from Tianshi Group and Tianshi Engineering, respectively, in order to fund a portion of the construction in progress. As of December 31, 2010, the amounts had been returned in full.
On November 12, 2010, Tianshi Investment, Tianshi Engineering, Tianshi Group and the Company entered into an agreement, pursuant to which the Company’s other receivables from Tianshi Investment of $27,300,000 were offset against advances from Tianshi Engineering of $11,767,357, short-term debt from Tianshi Engineering of $9,077,156 and other payable to Tianshi Group of $6,455,487.
Other transactions with Tianshi Engineering
On October 31, 2007, Biological entered into two lease agreements with Tianshi Engineering that enable Tianshi Engineering to share the use of certain of Biological’s production equipment to manufacture products which Tianshi Engineering owns, or jointly owns, with Biological. Each of the two agreements was effective as of January 1, 2008 and expired on December 31, 2009. On November 20, 2009, the lease agreement for health products production equipment and the lease agreement for personal care product production equipment were renewed by Biological and Tianshi Engineering for 2010. Following is a summary of the monthly rent payable to Biological under the two leases Biological entered into on November 20, 2009. Rent revenue accrued from these leases amounted to $209,770 and $219,605 for the year ended December 31, 2010 and 2009, respectively.
Other transactions with Tianshi Group
On January 1, 2009, Biological entered into an office and facilities lease agreement with Tianshi Group. Under the terms of the agreement, Biological’s annual rent is equal to 1% of its gross revenues. In addition, Biological is obligated to pay insurance, maintenance and other expenses related to the premises. This agreement expires on December 31, 2010. On January 1, 2010, Life Resources entered an office and facilities lease agreement with Tianshi Group on the same terms as Biological’s lease agreement with Tianshi Group. The Company paid rent under these leases in the amount of $500,361 and $458,382 for the year ended December 31, 2010 and 2009, respectively.
On January 1, 2009, each of Biological and Life Resources entered a Lease Agreement with Tianshi Group pursuant to which Biological and Life Resources will have the right to use and occupy the workshop spaces being transferred under the Property Transfer Agreement. The leases are rent-free, except that Biological and Life Resources are required to pay Tianshi Group for utility charges and maintenance costs on the buildings. The leases continues until the earlier of the date that Biological and Life Resources acquire use of alternate facilities or the land use rights on the underlying property expire. For the year ended December 31, 2010, Biological and Life Resources recorded $330,708 of the rent expense, which is not paid to Tianshi Group, but recorded as paid in capital based upon market price.
Other transactions with Tianshi Pharmaceutical
On December 15, 2009, the Company entered into a one-year lease agreement with Tianshi Pharmaceutical. Under the terms of the lease agreement, the Company leased equipment for the fee of RMB25,383 (or US$3,755) per month. In addition, the Company is obligated to pay insurance, maintenance and other expenses related on the equipment. This agreement is effective from January 1, 2010 and expires on December 31, 2010. The Company has rent expense of $45,062 for the twelve months ended December 31, 2010. The lease agreement was extended for an additional one year period.
Note 14 – Commitments and contingencies
Capital commitments
Capital commitments not provided for in the consolidated financial statements include the followings:
|
2010
|
2009
|
|||||||
|
Purchases of machinery and equipment
|
$ | 10,338,915 | $ | 28,134,098 | ||||
|
Construction of premises
|
$ | 36,851,228 | $ | 33,627,022 | ||||
|
Total
|
$ | 47,190,143 | $ | 61,761,120 | ||||
Operating lease commitments
The following table sets forth payments due by period for fixed contractual obligations as of December 31, 2010.
|
Payments due by period
|
||||||||||||||||||||
|
Less than 1 year
|
1-3 years
|
3-5 years
|
More than 5
years
|
Total
|
||||||||||||||||
|
Operating Lease Obligations
|
$ | 46,067 | $ | - | $ | - | $ | - | $ | 46,067 | ||||||||||
|
Total fixed contractual obligations
|
$ | 46,067 | $ | - | $ | - | $ | - | $ | 46,067 | ||||||||||
The Company leases its office building and manufacturing facilities in Tianjin, China from Tianshi Group, a related party, through common ownership. On June 30, 2002, the Company entered into a written lease agreement with Tianshi Group to pay annual rent at 1% of its total gross revenues. This agreement expired on December 31, 2007. The Company entered into a new one-year lease agreement with Tianshi Group for the fiscal year of 2008 and renewed in January 2009 and 2010 for the fiscal year of 2009 and 2010 with the same terms. Because the rent is based upon a percentage of its gross total revenues, the Company is not able to include a fixed figure in the table relating to this obligation. The total amount paid in 2010 and 2009 under this lease was $500,361 and $458,382.
On December 15, 2009, the Company entered into a one-year lease agreement with Tianshi Pharmaceutical. Under the terms of the lease agreement, the Company leased equipments for RMB25,383 (US$3,755) per month from January 1, 2010. In addition, the Company is obligated to pay insurance, maintenance and other expenses related to the premises. This lease agreement expired on December 31, 2010 and was extended for an additional one year period.
Note 15 –Noncontrolling interest and distribution
Effective from January 1, 2009, the share of Tiens Yihai held by minority shareholder increased from 0.6% to 4%. On November 15, 2009, Tianshi Holdings entered a Contract for the Transfer of Equity Interests to sell all of the registered share capital in Tiens Yihai it owned to Tianshi Investment. The share of Tiens Yihai held by noncontrolling shareholder decreased from 4% to 0% effective from November 16, 2009.
Dividends declared by Biological are split pro rata between the shareholders according to their ownership interests. The payment of the dividends to the shareholders may occur at different times, resulting in distributions which do not appear to be reflective of the noncontrolling ownership percentages. The table below shows the outstanding dividends payable of Biological which are eliminated upon consolidation, as well as the allocation of dividends between Tianshi Holdings and the noncontrolling shareholder.
|
Tianshi
|
||||||||
|
Date
|
Holding
|
Totals
|
||||||
|
Dividends outstanding, December 31, 2008 Balance
|
$ | 6,873,995 | $ | 6,873,995 | ||||
|
Dividends declared
|
- | - | ||||||
|
Dividends paid
|
(3,945,510 | ) | (3,945,510 | ) | ||||
|
Accumulated Other Comprehensive Income (loss)
|
13,452 | 13,452 | ||||||
|
Dividends outstanding, December 31, 2009 Balance
|
$ | 2,941,937 | $ | 2,941,937 | ||||
|
Dividends declared
|
- | - | ||||||
|
Dividends paid
|
- | - | ||||||
|
Accumulated Other Comprehensive Income (loss)
|
100,170 | 100,170 | ||||||
|
Dividends outstanding, December 31, 2010 Balance
|
$ | 3,042,107 | $ | 3,042,107 | ||||
As Biological declares dividends in RMB, and the RMB has appreciated against the dollar since 2005, dividends outstanding generated comprehensive income to Tianshi Holdings. Comprehensive income to Tianshi Holdings for the years ended December 31, 2010 and 2009 amounted to $100,170 and $13,452 respectively.
Note 16 – Accumulated other comprehensive income (loss)
|
Life
|
Tianshi
|
|||||||||||||||||||
|
Biological
|
Tiens Yihai
|
Resources
|
Holdings
|
Total
|
||||||||||||||||
|
Balance as of January 1, 2008,
|
$ | 4,467,207 | $ | 3,734,903 | $ | 1,979,698 | $ | 5,778,236 | $ | 15,960,044 | ||||||||||
|
Increase during the year
|
1,744,406 | 2,158,515 | 3,445,181 | 542,916 | 7,891,018 | |||||||||||||||
|
Balance as of December 31, 2008
|
$ | 6,211,613 | $ | 5,893,418 | $ | 5,424,879 | $ | 6,321,152 | $ | 23,851,062 | ||||||||||
|
Increase during the year
|
89,014 | (5,893,418 | ) | 202,022 | 13,443 | (5,588,939 | ) | |||||||||||||
|
Balance as of December 31, 2009
|
$ | 6,300,627 | $ | - | $ | 5,626,901 | $ | 6,334,595 | $ | 18,262,123 | ||||||||||
|
Increase during the year
|
1,367,015 | - | 3,664,396 | 100,092 | 5,131,503 | |||||||||||||||
|
Balance as of December 31, 2010
|
7,667,642 | $ | - | $ | 9,291,297 | $ | 6,434,687 | $ | 23,393,626 | |||||||||||
Accumulated other comprehensive income recorded in Biological, Tiens Yihai and Life Resources are due to foreign currency translation adjustments. Accumulated other comprehensive income recorded in Tianshi Holdings is due to the effect of foreign exchange rates on dividend receivables from Biological.
Note 17 – Statutory reserves
The laws and regulations of the People’s Republic of China require that before a Sino-foreign cooperative joint venture enterprise distributes profits to its partners, it must first satisfy all tax liabilities, provide for losses in previous years, and make allocations, in proportions determined at the discretion of the board of directors, after the statutory reserve. The statutory reserves include the surplus reserve fund, the common welfare fund, and the enterprise fund.
Statutory reserve fund
Each of the Company’s Chinese subsidiaries is required to transfer 10% of its net income, as determined in accordance with the PRC accounting rules and regulations, to a statutory surplus reserve fund until such reserve balance reaches 50% of that entity’s registered capital. During 2005, Biological’s statutory reserve fund had reached 50% of its registered capital, so no statutory reserve was required thereafter. Life Resources, as a newer operating subsidiary which has not reached its statutory reserve fund level, is currently required to make this transfer. On June 21, 2010, Life Resources transferred $2,165,285, representing 10% of its 2009 net income, as determined in accordance with PRC accounting rules and regulations, to this reserve.
The transfer to this reserve must be made before distribution of any dividend to shareholders. For the years ended December 31, 2010 and 2009, the amounts of the statutory reserve fund were $7,151,510 and $4,986,225, respectively.
The surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance after such issue is not less than 25% of the registered capital.
Common welfare fund
Each of the Company’s Chinese subsidiaries is required to transfer part of its net income in accordance with the PRC accounting rules and regulations, which is determined by its board of directors, to a statutory common welfare fund until the statutory reserve fund reaches 50% of that entity’s registered capital. Beginning 2005, Biological was not required to transfer any additional net income to the statutory reserve fund, so no transfer to the common welfare fund was required thereafter. As a newer operating subsidiary which has not reached its statutory reserve fund level, Life Resources is still required to make this transfer accompanying the transfer of statutory reserve. On June 21, 2010, Life Resources transferred $1,082,642, representing 5% of its 2009 net income, as determined by its board of directors, to this fund.
This fund can only be utilized on capital items for the collective benefit of the Company’s employees, such as construction of dormitories, cafeteria facilities, and other staff welfare facilities. This fund is non-distributable other than upon liquidation. The transfer to this fund must be made before distribution of any dividend to shareholders. For the years ended December 31, 2010 and 2009, the amounts of the Company’s common welfare fund were $7,057,619 and $5,974,977, respectively.
Enterprise fund
The enterprise fund may be used to acquire fixed assets or to increase the working capital to expend on production and operation of the business. No minimum contribution is required. For the years ended December 31, 2010 and 2009, the board of directors determined, subject to shareholders’ approval, that no funds be transferred to this reserve. The amount of the enterprise fund reserve at December 31, 2010 was $2,256,015.
The Chinese government restricts distributions of registered capital and the additional investment amounts required by the Chinese joint ventures. Approval by the Chinese government must be obtained before distributions of these amounts can be returned to the shareholders.
Note18 – Additional product sales information
The Company has a single operating segment. Most of the Company's revenues were generated from related parties for the years ended December 31, 2010 and 2009. Summarized enterprise-wide financial information concerning the Company’s revenues based on geographic area and product groups is shown in the following tables:
Revenue by Geographic Area:
|
2010
|
2009
|
|||||||
|
China
|
$ | 24,894,472 | $ | 27,241,333 | ||||
|
Europe-Asia
|
8,053,906 | 10,054,640 | ||||||
|
Africa
|
4,339,087 | 6,838,377 | ||||||
|
Asia-Pacific
|
2,741,258 | 11,805,899 | ||||||
|
America
|
1,248,965 | 4,728,925 | ||||||
|
Europe
|
67,032 | 1,306,895 | ||||||
|
Total
|
$ | 41,344,720 | $ | 61,976,069 | ||||
Revenue by Product Group:
|
2010
|
2009
|
|||||||
|
Wellness products
|
$ | 37,357,998 | $ | 57,474,154 | ||||
|
Dietary supplement products
|
3,986,722 | 4,478,222 | ||||||
|
Personal care products
|
0 | 23,693 | ||||||
|
Total
|
$ | 41,344,720 | $ | 61,976,069 | ||||
Note 19 - Income taxes
The Company is subject to income taxes on an entity basis on income arising in or derived from the tax jurisdiction in which each entity is domiciled. The Company's subsidiary, Tianshi Holdings, was incorporated in the British Virgin Islands and is not liable for income taxes.
The Company's subsidiaries, Biological and Life Resources, are Sino-Foreign Joint Ventures incorporated in the PRC. According to US GAAP, the following are the income tax credits granted by the Chinese government, which are significant components of income taxes associated with continuing operations required to be disclosed.
Beginning January 1, 2008, the new Enterprise Income Tax (“EIT”) law replaced the laws for Domestic Enterprises (“DES”) and Foreign Invested Enterprises (“FIEs”). The new standard EIT rate of 25% will replace the 33% rate currently applicable to both DES and FIEs. According to the new EIT, high-tech companies could be subject to a special reduced tax rate of 15%. The qualification of a high-tech company is to be reviewed annually. Biological currently qualifies as a high-tech company. However, as there is no detailed regulation regarding the implementation of the new EIT, for the year of 2009 and 2010, Biological was required by the local tax authority to prepay income tax at a tax rate of 25%. In June, 2010, the prepaid income tax $582,480 for the year of 2009 was fully refunded.
According to the new EIT law, Life Resources could still be fully exempt from PRC income taxes for two years starting from January 1, 2008, followed by a 12.5% reduced tax rate for the next three years.
Provisions for income taxes were all current income tax expenses and for the years ended December, 2010 and 2009 were $1,469,548 and $930,703, respectively.
The following table reconciles the U.S. statutory rates to the Company's effective tax rate:
|
2010
|
2009
|
|||||||
|
U.S. Statutory rate
|
34.0 | % | 34.0 | % | ||||
|
Foreign income not recognized in USA
|
(34.0 | ) | (34.0 | ) | ||||
|
China income taxes
|
25.0 | 25.0 | ||||||
|
Effect of reduced tax rate
|
(2.8 | ) | (20.60 | ) | ||||
|
Total provision for income taxes
|
22.2 | % | 4.4 | % | ||||
The estimated tax savings due to the reduced tax rate for the years ended December 31, 2010 and 2009 amounted to $1,228,667 and $4,311,490, respectively. The net effect on earnings per share if the income tax had been applied would decrease earnings per share for the years ended December 31, 2010 and 2009 by $0.02 and $0.06, respectively
Note 20 – Retirement plan
Regulations in the PRC require the Company to contribute to a defined contribution retirement plan for all employees. All employees are entitled to a retirement pension amount calculated based upon their salaries at their dates of retirement and their length of service in accordance with a government managed pension plan. The PRC government is responsible for the pension liability to the retired staff.
The company is required to make contributions to the state retirement plan at 20% of the employees' monthly salary. Employees are required to contribute 8% of their salary to the plan. Total pension expense incurred by the Company amounted to $1,085,547 and $1,505.954 for the years ended December 31, 2010 and 2009, respectively.
The Company also has an unemployment insurance plan for its employees. The plan requires each employee to contribute 1% of his or her salary to the plan. The Company matches the contributions in an amount equal to two times the contribution of each participant. The Company made contributions to the unemployment insurance plan of $104,840 and $149,206 for the years ended December 31, 2010 and 2009, respectively. All contributions are paid to a PRC insurance company, which in turn, is responsible for the unemployment liability. On January 1, 2002, the Company introduced a basic medical insurance plan for its employees. Pursuant to that medical insurance plan, the Company is required to pay an amount equal to 10% of its employees' salary to a PRC insurance company, which amounted to $545,600 and $761,901 for the years ended December 31, 2010 and 2009, respectively.
F-20
