Attached files
| file | filename |
|---|---|
| EX-31.1 - EX-31.1 - HELICOS BIOSCIENCES CORP | a2203068zex-31_1.htm |
| EX-23.1 - EX-23.1 - HELICOS BIOSCIENCES CORP | a2203068zex-23_1.htm |
| EX-31.2 - EX-31.2 - HELICOS BIOSCIENCES CORP | a2203068zex-31_2.htm |
| EX-32.1 - EX-32.1 - HELICOS BIOSCIENCES CORP | a2203068zex-32_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| (Mark One) | ||
| ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the fiscal year ended December 31, 2010 |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to |
||
COMMISSION FILE NUMBER 001-33484 |
||
HELICOS BIOSCIENCES CORPORATION
(Exact name of registrant as specified in its charter)
| DELAWARE | 05-0587367 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
One Kendall Square Building 200 Cambridge, Massachusetts (Address of principal executive offices) |
02139 (Zip Code) |
|
Registrant's telephone number, including area code: (617) 264-1800 |
||
Securities registered pursuant to Section 12(b) of the Act: |
||
Title of each class |
Name of each exchange on which registered |
|
| Common Stock, $0.001 par value | OTC Bulletin Board | |
Securities registered pursuant to Section 12(g) of the Act: None |
||
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company ý |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the registrant's Common Stock held beneficially or of record by stockholders who are not affiliates of the registrant, based upon the closing price of the Common Stock on June 30, 2010, as reported by the NASDAQ Global Market, was approximately $13,900,000. For the purposes hereof, "affiliates" include all executive officers and directors of the registrant.
As of March 24, 2011, the Company had 87,624,912 shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None
HELICOS BIOSCIENCES CORPORATION (a development stage company)
FORM 10-K
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2010
TABLE OF CONTENTS
2
Helicos BioSciences Corporation ("Helicos" or the "Company") is a life sciences company focused on innovative genetic analysis technologies. Helicos has developed a proprietary technology to enable the rapid analysis of large volumes of genetic material by directly sequencing single molecules of DNA or RNA. Our tSMS approach differs from current methods of sequencing DNA or RNA because it analyzes individual molecules of DNA directly instead of analyzing a large number of copies of the molecule which must be produced through complex sample preparation techniques. Our tSMS technology eliminates the need for costly, labor-intensive and time-consuming sample preparation techniques, such as amplification or cloning, which are required by other methods to produce a sufficient quantity of genetic material for analysis.
Our Helicos® Genetic Analysis Platform is designed to obtain sequencing information by repetitively performing a cycle of biochemical reactions on individual DNA or RNA molecules and imaging the results after each cycle. The platform consists of an instrument called the HeliScope™ Single Molecule Sequencer, an image analysis computer tower called the HeliScope™ Analysis Engine, associated reagents, which are chemicals used in the sequencing process, and disposable supplies. The information generated from using our tSMS products may lead to improved drug therapies, personalized medical treatments and more accurate diagnostics for cancer and other diseases.
As previously disclosed in our quarterly and periodic reports with the Securities and Exchange Commission, in the first quarter of 2010, we began a process of considering alternatives to our long-term strategic focus, including a repositioning of the Company in the genetic analysis markets. As a result of this process, on June 25, 2010, we announced that we had launched our diagnostics business and that we were in early stages of validating molecular diagnostic (MDx) tests that utilize our HeliScope® Single Molecule Sequencer. To date, our research and development team has made progress in developing two prototype tests. The first test is for the detection of gene mutations indicative of a woman's increased risk of developing hereditary breast or ovarian cancer. The second prototype is a non-invasive prenatal diagnostic test. Nevertheless, despite this scientific progress, and a lengthy and comprehensive financing initiative supported by a nationally recognized investment bank during the second and third quarters of 2010, we were unable to secure any long-term equity financings or financing arrangements with strategic partners during the third quarter of 2010. We had numerous meetings with prospective investors and corporate partners but were not able to secure a bona-fide offer for financing or a strategic transaction. In addition, we had very limited commercial activity during fiscal 2010. Furthermore, as of December 31, 2010 and March 24, 2011, we only had $2.5 million and $2.9 million, respectively, in cash and cash equivalents.
As previously disclosed, on May 11, 2010, our Board of Directors (the "Board") approved a reduction in headcount and restructuring plan (the "May Restructuring Plan") which involved the consolidation and reorganization of our operations in order to reduce operating costs. The May Restructuring Plan resulted in the elimination of approximately 40 positions during the second fiscal quarter of 2010. In order to further reduce operating costs and preserve cash, on September 15, 2010, our Board approved a subsequent reduction in headcount and restructuring plan (the "September Restructuring Plan") which involved the consolidation and reorganization of our operations. The September Restructuring Plan eliminated approximately 14 positions during the third quarter of 2010. We entered the first quarter of 2011 with 22 employees. In addition, during the third quarter of 2010, as a result of our inability to complete the financing referenced above and the subsequent change in business direction, we recorded charges of $2.6 million in connection with a write-off of certain of our inventory that is no longer used in the business and $1.9 million in connection with the impairment of certain long-lived assets.
3
Going forward, we plan to deploy our limited resources by focusing on intellectual property monetization and concentrating our research and development efforts in targeted areas designed to achieve tangible proof-of-concept goals, which may enable us to seek partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives. As of the date of this filing, we do not have adequate financial resources and will continue to need to generate additional funding from various sources including contract sequencing service projects, government grants, strategic collaborations and licensing activities. On October 14, 2010, we announced the launch of a technology licensing program for our Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. We plan to defend our intellectual property rights through licensing and enforcement strategies. In this regard, we took several actions. First, on August 27, 2010, we filed a lawsuit against Pacific Biosciences of California, Inc. ("Pacific Biosciences") for patent infringement. The lawsuit, filed in the United States District Court for the District of Delaware, accuses Pacific Biosciences of infringing four patents and seeks injunctive relief and monetary damages. Second, on October 14, 2010, we announced the launch of a technology licensing program for our Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. Third, on October 22, 2010, we filed an amended complaint against Pacific Biosciences by naming Illumina, Inc. and Life Technologies Corporation as additional defendants. The amended lawsuit alleges that the defendants willfully infringe our patents in suit by using their respective technologies, sequencing systems and products which we allege are within the scope of one or more claims of our patents in suit. We are seeking a permanent injunction enjoining the defendants from further infringement as well as monetary and punitive damages, costs and attorneys' fees, interests and other relief as determined by the Court. See Part I, Item 3, Legal Proceedings, for more information regarding the litigation. We believe that the proceeds generated by these litigations, if we are successful, could be substantial. We continue to pursue all of the above-mentioned opportunities in the IP and technology platform areas.
We entered the fourth quarter of 2010 without any prospects of securing long-term financing from outside investors. An independent committee (the "Independent Committee") of our Board evaluated the alternatives available to us and determined that a bridge financing led by certain inside investors was in the best interests of our stakeholders in view of our current financial and operating condition, and would best position us to continue operations for the remainder of 2010 and into 2011. The Independent Committee met multiple times to consider the alternatives and concluded that the bridge financing was the best alternative for generating value for stakeholders. As a result, we entered into an insider bridge loan facility with two of the four venture capital firms whose representatives were and are members of our Board. In connection with this transaction, we entered into a Subordinated Secured Note Purchase Agreement (the "Note Purchase Agreement") with investment funds affiliated with Atlas Venture and Flagship Ventures (the "Purchasers"), pursuant to which we have agreed to sell to the Purchasers, and the Purchasers have agreed to purchase, up to an aggregate of $4,000,000 of convertible promissory notes (the "Notes"), a portion of which is committed and a portion of which is optional (the "Bridge Debt Financing"). On November 16, 2010, the Purchasers purchased Notes having an aggregate principal amount of $333,333. Subject to our continued compliance with the terms of the Note Purchase Agreement, including that there not be an event of default thereunder, upon notice from us the Purchasers are committed to purchase an additional $333,333 of Notes in each of five subsequent closings, for a total aggregate committed amount of $2,000,000. The Purchasers also have the right, but not the obligation, upon our request to purchase up to an additional $2,000,000 of Notes on or before December 31, 2012. As of December 31, 2010 and as of the date of this filing, we have borrowed $666,667 against the Bridge Debt Financing facility.
The Notes will accrue interest at a rate of 10% per annum plus a risk premium as described below. The outstanding principal and any unpaid accrued interest on all of the Notes shall be due and payable in full (i) upon demand by the Purchasers, which demand shall be no earlier than December 31, 2012, (ii) upon our receiving at least $10,000,000 in proceeds from a subsequent equity
4
financing, (iii) upon a change of control of the Company or (iv) upon an event of default. A Purchaser may elect to convert principal and interest outstanding under any of the Notes upon the closing of any future round of equity or debt financing of the Company into shares, and at the per share price, of the securities issued at such financing. Simultaneously with execution of the Note Purchase Agreement, we and the requisite parties entered into an Amendment to the Amended and Restated Investor Rights Agreement, dated as of March 1, 2006, by and among us and the parties specified therein, including the Purchasers (the "IRA Amendment"), pursuant to which the Purchasers will be entitled to demand and piggyback registration rights consistent with the terms of the Purchasers' existing registration rights for any securities issued upon conversion of the Notes.
Our obligations under the Notes are subordinated to the indebtedness incurred by us under the Loan and Security Agreement among us, certain lenders (the "Lenders") and General Electric Capital Corporation ("GE"), as agent, dated as of December 31, 2007, as amended (the "Loan Agreement"). Our obligations under the Notes are secured by a security interest on all of our assets, including our intellectual property, that, with regard to all of our assets other than the cause of action that is the subject of the intellectual property litigation against Pacific Biosciences, Illumina and Life Technologies (and any recovery therefrom) (the "Cause of Action"), is junior to the Lenders' first priority security interest under the Loan Agreement, and with regard to the Cause of Action, is junior to the Lenders' first priority security interest under the Loan Agreement and the second priority security interest of our outside legal counsel that is representing us in the Cause of Action ("Outside IP Litigation Counsel").
As an inducement for the Purchasers to purchase the Notes, we entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement") simultaneous with the execution of, and as required by, the Note Purchase Agreement. Under the terms of the Risk Premium Agreement, we have agreed to pay the Purchasers the following portions of the consideration we receive (the "Payment Consideration") in Liquidity Transactions, as defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30% of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). For purposes of determining amounts payable under the Risk Premium Agreement, the Payment Consideration will not include amounts we use to first satisfy specified existing obligations, including obligations under the Loan Agreement, under professional contingency arrangements (including the contingency fee arrangement between us and Outside IP Litigation Counsel relating to the Cause of Action) and obligations that may arise under existing technology license agreements (the "Existing IP Licensing Agreements") between us and each of Arizona Technology Enterprises, Roche Diagnostics GMBH and Roche Diagnostics Corporation, and California Institute of Technology (as previously disclosed by us, under the Existing IP Licensing Agreements, we are obligated to pay license fees in connection with the licensing, sublicensing, legal settlements or sales of specified intellectual property or services provided to third parties ranging from a single digit percentage up to one-half of the income from those activities). For purposes of the Risk Premium Agreement and subject to various provisions therein, the following shall constitute Liquidity Transactions: (A) the receipt by us of any payments from third parties relating to our intellectual property portfolio of the Company, including payments received as a license or other settlement in patent infringement litigation brought by us ("IP Licensing Events"); (B) a merger of the Company in which there is a change of control; and (C) the sale or exclusive license by us of all or substantially all of our assets or intellectual property.
In connection with the Bridge Debt Financing, on November 16, 2010, our company, GE and the Lenders executed the Fourth Amendment to the Loan and Security Agreement, dated November 16, 2010 (the "Fourth Amendment"). Pursuant to the Fourth Amendment, GE and the Lenders waived
5
various existing events of default and consented to our incurrence of additional indebtedness and the grant of a junior security interest to the Purchasers under the Note Purchase Agreement. We amended warrants previously issued to the Lenders to re-price the Warrants from $4.80 to $0.01 and GE and the Lenders agreed to defer a $200,000 payment due January 31, 2011 until to the January 1, 2012 term loan maturity date. In addition, subject to our continued compliance with the terms of the Loan Agreement, including that there not be an event of default thereunder, GE and the Lenders agreed to defer up to five additional principal monthly payments with respect to the term loan. Also, pursuant to the Fourth Amendment, we agreed to grant the Lenders a first priority security interest in all of our intellectual property and to pay the Lenders an additional $50,000 to $200,000 (depending upon how many principal payments are deferred) upon the full repayment of all outstanding amounts due with respect to the term loans. Under the terms of the Loan and Security Agreement prior to its amendment by the Fourth Amendment, the scope of the Lenders' first priority security interest included all of our assets other than intellectual property. As of the date of this filing, the Lenders have agreed to defer the additional five monthly principal payments referenced above.
In connection with the Bridge Debt Financing and the Fourth Amendment and as security for our obligations under our contingency fee arrangement with Outside IP Litigation Counsel relating to the Cause of Action, we entered into a Subordinated Security Agreement with Outside IP Litigation Counsel pursuant to which we agreed to grant Outside IP Litigation Counsel a second priority security interest in the Cause of Action. Under the terms of the contingency fee arrangement, we are obligated to pay Outside IP Litigation Counsel up to 40% of the proceeds paid to us in connection with the settlement, judgment or other resolution of the Cause of Action or certain business transactions resulting from or relating to the Cause of Action.
In approving the transactions relating to the Bridge Debt Financing and the Fourth Amendment, the Board approved a management incentive plan (the "Management Incentive Plan") pursuant to which participants in the Management Incentive Plan, including Dr. Ivan Trifunovich and Mr. Jeffrey Moore, will be entitled to receive, in the aggregate, an amount equal to 7.5% of certain payment consideration resulting from intellectual property licensing events. The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the Board.
The agreements and arrangements described above and all of the transactions contemplated by those agreements were approved by an independent committee of the Board. In addition, Noubar B. Afeyan, PhD, who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, Ph.D., who is a Partner of Atlas Venture, abstained from the vote of the Board approving the transactions.
Although we believe that the HeliScope Sequencer has unique utility across a broad array of MDx tests, due to significant financial and resource constraints, we have deferred our plan to develop our own genetic testing laboratory certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA). Nevertheless, we still believe that diagnostic applications may benefit from the specific features of the Helicos System, including the platform's ability to sequence non-amplified natural DNA and RNA directly, quantitative accuracy, high throughput, ability to use small sample quantities in simple and cost effective preparation methods, as well as lack of biases typically seen with sample amplification. We will continue to evaluate the best modality of bringing high-value MDx tests based on our technology to the market through a CLIA laboratory, either in partnership or through our own efforts should the financial outlook for us improve however there can be no assurance that we will be successful in deploying MDx tests or our technology through a CLIA laboratory.
Notwithstanding recent workforce reductions, restructurings, and the Bridge Debt Financing, we may not have sufficient funds to pursue our business priorities. We will require significant additional capital in the second half of 2011 to continue our operations. As a result, we are no longer able to
6
remain current in making payments to trade vendors and other unsecured creditors when such payments are due and, despite the restructuring of our credit facility with GE discussed above, this inability to remain current increases the likelihood that GE could declare a default under the credit facility in the near-term, including with regard to solvency. These workforce reductions, as well as curtailed financial resources, will further impact our ability to implement our business priorities. In addition, these constraints have caused us to significantly scale back service support and reagent supply to our current installed base. We have also significantly curtailed collaborative activities with other parties. Nevertheless, we believe that these actions will preserve our viability and provide additional time to execute our business priorities.
On June 28, 2010, we received a letter from The NASDAQ Stock Market LLC ("NASDAQ") advising that for the previous 30 consecutive business days, we failed to comply with the minimum market value of listed securities ("MVLS") requirement for continued listing on the NASDAQ Global Market pursuant to NASDAQ Marketplace Rule 5450(b)(2)(A) and that we did not otherwise satisfy the criteria for continued listing pursuant to NASDAQ Marketplace Rule 5450(b). NASDAQ stated in its letter that in accordance with NASDAQ Marketplace Rule 5810(c)(3)(C), we would be provided 180 calendar days, or until December 27, 2010, to regain compliance with the MVLS continued listing requirement. MVLS is calculated by multiplying the total shares outstanding by the daily closing bid price. On October 12, 2010, we received a delisting determination letter from NASDAQ due to our failure to regain compliance with the NASDAQ Global Market's minimum bid price requirement for continued listing as set forth in Listing Rule 5450(a)(1). In addition, we did not hold our annual meeting of stockholders for fiscal year 2010 which is an additional NASDAQ listing requirement. Based on the unlikely ability for us to meet the NASDAQ MLVS requirements due to the state of our finances and near-term business prospects, on November 12, 2010, we withdrew our appeal to NASDAQ regarding the delisting notice. As a result, our securities were delisted from the NASDAQ Global Market and the trading of our common stock was suspended before the opening of business on November 16, 2010, and a Form 25-NSE was subsequently filed with the Securities and Exchange Commission on January 21, 2011 which removed our securities from listing and registration on The NASDAQ Stock Market. We were advised by Pink OTC Markets Inc., which operates an electronic quotation service for securities traded over-the-counter ("OTC"), that our securities are eligible for quotation on the OTCQB. The OTCQB is a market tier for OTC traded companies that are registered and reporting with the Securities and Exchange Commission. Our shares continue to trade under the symbol HLCS on the OTCQB. Investors can now view real time stock quotes for HLCS at http://www.otcmarkets.com.
Please see Part I, Item 1A, in this Form 10-K, for a discussion of the risks related to ownership of our common stock.
Our future capital requirements will depend on many factors and we may require additional capital beyond our currently anticipated amounts. Any such required additional capital may not be available on reasonable terms, if at all, given our prospects, the current economic environment and restricted access to capital markets. The continued depletion of our resources may make future funding more difficult or expensive to attain. Additional equity financing may be dilutive to our stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict our ability to operate as a business; and strategic partnerships may result in royalties or other terms which reduce our economic potential from any adjustments to our existing long-term strategy. If we are unable to execute our operations according to our plans or to obtain additional financing, we may be forced to cease operations. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets or the amount of reclassification of liabilities, or any adjustment that might be necessary should we be unable to continue as a going concern.
Since our inception, we have incurred losses and have not generated positive cash flows from operations. We expect our losses to continue for a considerable period of time. These losses, among
7
other things, have had and will continue to have an adverse effect on our working capital, total assets and equity/(deficit). As of December 31, 2010 and March 24, 2011, we had $2.5 million and $2.9 million, respectively, in cash and cash equivalents. We will, during the second half of 2011, require significant additional capital to continue our operations. Because our present capital resources are not sufficient to fund our planned operations for a twelve month period as of the date of this Annual Report on Form 10-K for the year ended December 31, 2010, our current financial resources raise substantial doubt about our ability to continue as a going concern.
BACKGROUND ON DNA STRUCTURE AND FUNCTION
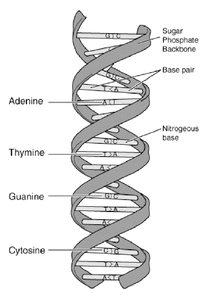
The genetic program that controls a living cell is encoded in its DNA. The diagram below shows the typical double-helix structure of DNA. The two strands are made of subunits called nucleotides, each of which contains a phosphate, a sugar and a side-chain called a base. The phosphates and sugars form the backbone of the polymer, and the bases face each other. The letters A, G, T and C represent the four types of nucleotide bases: adenine, guanine, thymine and cytosine.
The bases align with each other in a complementary structure held together by hydrogen bonds. A "T" on one strand always bonds with an "A" on the other strand, and a "G" on one strand always bonds with a "C" on the other strand. This bonding between DNA strands is called hybridization, and the resulting structure is called a base pair. The genome of an organism is a complete DNA sequence of that organism. The human genome contains about three billion base pairs of DNA, which is represented twice in each cell. In a human, the individual acquires one version of the genome from the mother and one version from the father. |
|
The human genome includes approximately 30,000 genes. Genes are segments of DNA that contain the information needed for a cell to make proteins. Each gene has one or more parts called exons including coding regions that specify the sequence of amino acids for that protein. Genes also contain regulatory elements that determine when, where and how much protein is made. While it is currently understood that approximately 97% of the human genome does not code for proteins, recent research suggests that this non-coding DNA also contains important regulatory elements which play important roles in controlling when and how much genes are expressed. In addition, new knowledge reveals a wealth of information contained within the transcribed regions of the genome that do not make up protein coding genes but yet these non-coding RNAs are becoming increasingly important in the study of biology.
The process of making proteins using the information contained with in DNA involves a process called gene expression. To express a gene, enzymes called RNA polymerases transcribe the coding region into molecules of messenger RNA, or mRNA. The mRNA moves from the nucleus into the cytoplasm, where the cell's protein synthesis machinery translates the genetic sequence information and assembles a chain of amino acids into a protein.
8
GENETIC ANALYSIS INDUSTRY OVERVIEW
Genomic information has become a critical tool to understand the mechanics of life, the environmental effect on biological systems, as well as diagnosis and treatment of disease. Life science tools that analyze genomic material have provided tremendous insights into the complexity and variability of the genome and have changed the methods and strategies by which scientists conduct their research. Genomic information enables the possibility and promise of personalized medicine and should bring forth a new era in patient knowledge whereby individuals now can have access to their own genetic information to make informed decisions concerning the prevention and treatment of disease in partnership with their physician.
Since the development of genetic engineering techniques in the 1970s, the analysis of genetic material has become a mainstay of biological research. The first automated DNA sequencer was invented in 1986, based on technology developed by Frederick Sanger and his colleagues in 1975, which is commonly referred to as Sanger sequencing. Subsequent versions of commercial DNA sequencers have increased the speed of DNA sequencing by 3,000 fold, making possible the Human Genome Project. In 1996 the first commercial microarray was introduced and enabled a new era of RNA analysis by measuring gene expression across many genes in a single experiment. Subsequent versions of the commercial microarrays including DNA and RNA have significantly increased the amount of information per experiment and provided selected single nucleotide polymorphisms, or SNPs of the whole human genome on a single chip, enabled large scale genome-wide SNP association studies and have been commercialized for several diagnostic applications.
Since 2007, there has been significant replacement of both of these existing genetic analysis technologies with Next Generation Sequencers (NGS) and recently, Single-Molecule Sequencing technologies, because the limitations of Sanger sequencing technologies, such as low throughput, high cost and complexity of use restricted their use in large-scale studies. Today, next generation DNA and cDNA sequencing have emerged to provide the most comprehensive genome-wide information without any prior knowledge of the sequence or sequence variation contained within experimental samples.
Nevertheless, as next generation sequencing technologies continue to improve the speed and reduce the per base cost of DNA sequencing, theses instruments continue to be limited by their detection sensitivity, thereby requiring DNA amplification to obtain sufficient material to adequately read the sequence. As with Sanger-based sequencing technologies, this requirement for amplification adds to the cost and complexity of these sequencing methods, and presents limits on the scalability of sample preparation and may limit the accuracy of the data produced. Moreover, many NGS technologies appear to possess biases and are hampered by their lack of absolute quantitative accuracy which may limit their applicability to the broader genetic analysis space. Additionally, the practical application of sequence data for molecular diagnostics is still heavily burdened by the costs associated with NGS sample preparation workflows.
In the past, the prohibitive cost of high-volume sequencing at the genome scale has caused scientists to use other genetic analysis technologies to examine discrete aspects of gene structure or function. For example, researchers use gene expression analysis to compare amounts of mRNA made from different genes, and genotyping to examine specific gene segments known to contain sequence variations, called single nucleotide polymorphisms, or SNPs. Technologies available for gene expression analysis and genotyping include:
- •
- chip- or bead-based microarrays, in which collections of short DNA molecules are attached to the
surface of a glass chip or to beads and used to determine the identity and abundance of particular DNA or RNA molecules in a sample; and
- •
- real-time PCR, also called RT-PCR, which is the method of biochemically copying or amplifying the DNA in a sample through a process called PCR in which the identity and quantity of amplified DNA from the sample is measured as the analysis is performed.
9
While these other genetic analysis technologies address the cost limitations of DNA and RNA sequencing, they generally provide only limited information and suffer from a range of technical limitations, the most important of which is the high cost of replacement as new sequence information is added and products are updated. NGS technologies that enable a broad range of applications with a relatively similar sample preparation methodology and data analysis format would greatly enable the ability to perform both broad and deep studies of important biological samples. NGS technologies that do not require significant investments in up front automation and infrastructure would also allow large scale studies to be performed in mid to small sized laboratories as well as translational and diagnostic laboratories by allowing continuous use of the same capital equipment for each of the different genetic analysis applications.
The scope and pace of much important research, and the routine application of genomic information in clinical medicine, had been limited by the shear cost of data acquisition however today we have begun to see hope for patients. Dramatic NGS sequencing performance improvements have resulted in the declining cost of whole genome sequencing and now enable large scale experimentation not previously possible. Efforts to overcome this barrier have been supported by the National Institutes of Health, whose National Human Genome Research Institute established the "Revolutionary Genome Sequencing Technologies—The $1,000 Genome," grant program to fund researchers' efforts to develop technology to enable the complete sequencing of an individual human genome at a cost of approximately $1,000 along with the more recent Sequencing Technology Development Program representing NHGRI's Signature Project for the American Recovery and Reinvestment Act. This project was specifically designed to support large-scale, high impact research projects that are expected to accelerate critical scientific breakthroughs and enable growth and investment in biomedical research. We have been supported by grants, from both of these funding initiatives, which have been applied directly to the development of our single molecule sequencing technology. In April 2010, we were awarded an R01 Research Project Grant from the NHGRI for Direct RNA Sequencing and are eligible to receive reimbursement for our research expenses of up to $1.6 million through February 2013. In June 2010, we were awarded a Fast Track Phase 1 Small Business Innovation Research grant to pursue research related to the development of methods to sequence patient DNA and RNA samples at an attomole level. The phase 1 portion of the grant provides funding for eligible research expenses of up to $146,000 through November 2010. Subsequent to December 31, 2010 we received approval for Phase II Fast Track funding for an additional two years totaling approximately $1.5 million. Additionally, we received a $150,000 subcontract award for a Department of Homeland Security grant funded to the Midwest Research Institute to interrogate rare variant detection in bacterial samples using our single molecule sequencing technology. In October 2010, we were awarded three federal grants totaling approximately $722,000 under the Patient Protection and Affordable Care Act of 2010, of which $472,000 was received during the fourth quarter of 2010 and the balance was received during the first quarter of 2011. The grants were awarded under the Qualified Therapeutic Discovery Project (QDTP) aimed towards producing products that diagnose diseases or determine molecular factors related to diseases by developing molecular diagnostics to guide therapeutic decisions.
Scientists have long realized that many of the disadvantages of ensemble based sequencing could be addressed through the direct sequencing of single molecules. The ability to directly measure individual sequences has the potential to reduce the cost and complexity of large scale experiments while increasing sensitivity. The simplicity of the sample preparation and detection would also provide the capability to combine multiple application techniques in order to get the most comprehensive view of each sample. For nearly 20 years, researchers have attempted without success to develop such a single molecule sequencing technology. Past efforts fell short largely due to complexity or technological hurdles in signal detection, surface materials, biochemistry, enzymology, bioinformatics, automation or engineering. In 2003, one of our co-founders, Stephen R. Quake, DPhil, demonstrated, we believe for the first time, that sequence information could be obtained from single molecules of DNA. We have replicated and improved upon Professor Quake's approach to develop our True Single Molecule
10
Sequencing (tSMS)™ technology. (Harris, T., et al., Single molecule DNA sequencing of a viral genome. Science 2008, 320:106-109; Bowers, J., et al. Virtual terminator nucleotides for next-generation DNA sequencing. Nat Methods 2009, 6:593-595.)
HELICOS TECHNOLOGY
Our True Single Molecule Sequencing (tSMS)™ technology is a powerful approach that directly measures single molecules. This novel approach allows our system to directly analyze billions of individual sequences in parallel and avoids the need for complex sample preparation techniques, amplification or cloning required by other methods. Our products utilizing our tSMS technology benefit from simple, scalable sample preparation techniques and automated high-throughput sequencing processes that enable sequencing with speed and provide unprecedented quantitative accuracy. This technology provides scientists and clinicians with extensive capabilities for basic and translational research, for pharmaceutical research and development, and for the development and clinical application of genomic diagnostics.
Our Helicos® Genetic Analysis System is designed to provide the following benefits:
- •
- Enhanced single-molecule throughput. Scientists measure
the throughput of a DNA sequencing technology based on the number of bases analyzed per unit of time. The HeliScope™ Single Molecule Sequencer has achieved throughput rates of
approximately 180 million analyzable bases per hour, depending on the application. The HeliScope Sequencer remains the highest throughput single molecule platform providing sequence information
on upwards of a billion nucleic acid molecules per run. In addition, we have designed the imaging capability of the HeliScope Sequencer to accommodate a maximum throughput approaching one billion
bases per hour. To achieve this additional increase in throughput, we would need to improve the efficiency and accuracy of the system's sequencing chemistry, increase the density of strands of DNA
that bind to the surface of the flow cell in which the sequencing reactions take place and make corresponding enhancements to the image processing software.
- •
- Simplicity of sampling preparation. Because the sample
preparation process for genome sequencing using our HeliScope Sequencer involves only small quantities of reagents and a few simple steps, we believe that it will be less costly, less
time-consuming, less error prone, and require lower amounts of starting material (without amplification) than the sample preparation processes used in current NGS technologies.
- •
- Scalability. The sample preparation process is highly scalable because it does not require the need for the costly automation of complex sample preparation techniques, amplification or cloning required by existing NGS methods and thus makes genomic scale studies possible in small to mid-sized research organizations.
We believe that our Helicos System can be used as a universal method of genetic analysis potentially replacing existing methods of gene expression analysis and genotyping. Based on the quantitative performance exhibited by the HeliScope Sequencer, we believe that the Helicos System is capable of performing applications of gene expression analysis at a comparable cost per sample, and in the case of high volume analyses, at lower cost, in comparison with current technologies. Moreover, we believe that performing expression analysis using the tSMS approach provides a more unbiased and accurate measurement of expression and reveals new insight into the complexity of the transcriptome (Kapranov et al 2010. New class of gene-termini-associated human RNAs suggests a novel RNA copying mechanism. Nature. 2010, 29: 642-6). In addition, we believe that Helicos' technology is uniquely suited for applications in molecular diagnostics. In particular, we believe that our platform's quantitative accuracy, the use of small sample quantities in simple preparation methods, and high throughput, as well as lack of biases typically seen with sample amplification, can be specially useful in the field of molecular diagnostics.
11
Our True Single Molecule Sequencing (tSMS)™ Technology
Our True Single Molecule Sequencing (tSMS)™ technology enables the simultaneous sequencing of large numbers of strands of single DNA or RNA molecules. The first step of our single molecule sequencing approach is to cut, or shear, a sample of DNA or RNA into relatively small fragments. The double helix of each fragment is then separated into its two complementary strands. Each strand is used as a template for synthesis of a new complementary strand. This is accomplished through a series of biochemical reactions in which each of the four bases are successively introduced. If the introduced base is complementary to the next base in the template, it will be added to the new strand. Each of the added bases is tagged with a fluorescent dye, which is illuminated, imaged and then removed. The sequence of each new DNA or RNA strand is determined by collating the images of the illuminated bases from each cycle of highly specific incorporation and imaging. The raw sequencing data is then analyzed by computer algorithms. In 2010, our scientists authored or co-authored ten scientific manuscripts based on our tSMS technology in top-tier peer-reviewed journals.
The series of figures below outlines an example of how our tSMS technology operates to sequence single molecules from genomic DNA. The actual process our HeliScope™ Single Molecule Sequencer will utilize to sequence DNA molecules will depend on the application. In September 2009 we published the first demonstration of the direct sequencing of RNA molecules which enables additional potentially significant areas of research and in 2010 published an extension of our work on direct RNA sequencing demonstrating genome surveys of polyadenylation maps in yeast and human RNAs.
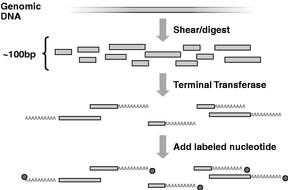
Figure 1 To prepare the sample for sequencing, the genomic DNA is first cut into small pieces of about 100 bases. The enzyme called terminal transferase is then used to add a string of "A" nucleotides to one end of each strand. Then, a nucleotide labeled with a single fluorescent dye molecule is added to the end of the strand. |
|
|
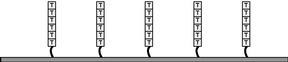
Figure 2 Inside the flow cell, short strands of "T" nucleotides, called primers, have already been attached to the surface. |
|
|
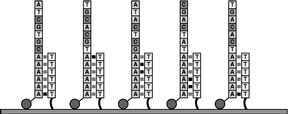
Figure 3 When the DNA sample is added, the strings of "As" on each DNA strand hybridize with the strands of "Ts" on the surface, anchoring the sample strands to be sequenced. The sample strands will act as a template and the strand of Ts as a "primer" for DNA synthesis. A laser subsystem illuminates the flow cell and the camera records the location of each captured sample strand. A mechanical stage moves the flow cell in sequential steps to allow the camera to image the entire active area of the flow cell. The dye molecules are then cleaved and washed away. |
|
12
Figure 4 An enzyme called DNA polymerase and the first of the four types of our proprietary fluorescently labeled nucleotides are added. If the nucleotide is complementary to the next base in the template strand, the polymerase will add it to the primer strand. The nucleotides are designed to inhibit the polymerase from incorporating more than one base at a time on the same strand. Excess polymerase and unincorporated nucleotides are then washed away. |
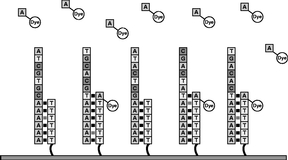
|
|
Figure 5 The laser subsystem illuminates the flow cell and the camera records the locations where fluorescently labeled nucleotides were added. The fluorescent dye molecules are then cleaved from the labeled nucleotides and washed away. |
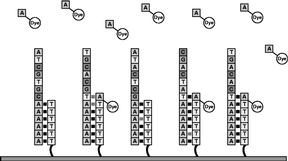
|
|
Figure 6 The process outlined in Figures 4 and 5 is repeated with each of the four types of labeled nucleotides. Repeating this cycle for a total of 120 times adds an average of more than 33-35 nucleotides to the primer strand. The number of bases added to a primer is the "read length." |
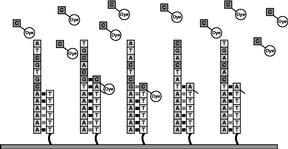
|
|
Figure 7 The system's computer analyzes the series of images from each cycle and determines the sequence of bases in the template strand. The sequence is "read" by correlating the position of a fluorescent molecule in its vertical track with the knowledge of which base was added at that cycle. Finally, the sequence data is exported to another computer system for further analysis depending on the application. |
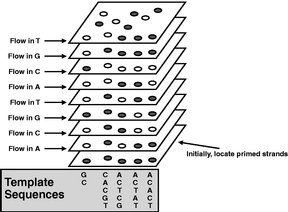
|
13
The Helicos® Genetic Analysis Platform
The Helicos® Genetic Analysis Platform consists of the following components:
- •
- Helicos® Genetic Analysis System—The instrument
component of the Helicos Genetic Analysis Platform consists of three major components:
- •
- The HeliScope™ Single Molecule Sequencer which performs the
True Single Molecule Sequencing (tSMS)™ chemistry and directly analyzes images of single molecules, producing accurate sequences of billions of templates at a time. The HeliScope Sequencer
consists of a high-speed mechanical stage and a laser illumination subsystem, an image acquisition subsystem, a fluid handling subsystem and computer subsystems that control and analyze
the sequencing reactions. To operate the instrument, a user loads a prepared sample of DNA, complementary DNA (cDNA) or RNA onto our flow cell using the HeliScope™ Sample Loader, places
the flow cell on the mechanical stage and inserts our consumable reagent pack into the fluid handling system. From that point onward, all sequencing reactions are conducted automatically by the
instrument. After each base is added, the mechanical stage moves the flow cells under a microscope lens. Four lasers illuminate the fluorescent tags of the bases, and a camera images the flow cells
through the microscope lens.
- •
- HeliScope™ Analysis Engine provides computing power for near
real-time image analysis and on-board data storage. The on-board data storage is appropriately sized to support two complete runs, enabling flexibility of operation
and maximizing uptime. The Analysis Engine operates downstream from the HeliScope™ Sequencer in the data pipeline. It consists of the System Server, Object Finders, and an uninterruptible
power supply (UPS). Components are mounted in a single enclosure for locating convenience and installation ease. Data communication between the HeliScope Sequencer and Analysis Engine is accomplished
across Gigabit Ethernet (GigE) lines, providing high reliability and allowing for considerable physical distance between components.
- •
- HeliScope™ Sample Loader facilitates and speeds the loading of
samples into the Helicos® flow cells. It provides 25 discreet loading ports to ensure proper separation of samples and ease of loading.
- •
- Helicos® True Single Molecule Sequencing (tSMS)™ Reagent Kits. Reagent kits for sequencing which consist of proprietary formulations of a DNA polymerase enzyme, our proprietary fluorescently tagged bases, our proprietary imaging reagent, a proprietary formulation of a cleavage reagent and our proprietary application specific flow cells that have a proprietary surface coating with the chemical and optical properties needed for single molecule sequencing.
Consumable reagents. The biochemical sequencing reactions that occur in the HeliScope Sequencer involve the use of a proprietary formulation of a DNA polymerase enzyme, proprietary fluorescently tagged bases and proprietary imaging reagents. We have developed proprietary nucleotide triphosphates, called Virtual Terminator™ Nucleotides that allow us to add only one base at a time to each DNA strand. Our proprietary imaging reagents improve the stability of our fluorescent tags and increase their brightness. Our cleavage reagents are used to remove the fluorescent tags from the Virtual Terminators nucleotides.
Disposable supplies. The HeliScope™ Single Molecule Sequencer is designed to perform sequencing reactions inside one or two glass flow cells. When two flow cells are used, the system alternates between the flow cells, performing sequencing reactions in one flow cell while recording images from the other. Each flow cell has an active area of about 16 square centimeters and contains 25 separate channels. Our flow cells are designed to allow researchers to sequence separate samples in each channel, which will enable the simultaneous sequencing of at least 50 different DNA, cDNA or RNA samples. Our flow cell is designed to permit binding of DNA strands at an average density of
14
approximately 100 million strands of DNA per square centimeter, equaling an average of approximately 2.8 billion strands of DNA for both flow cells. In addition, we have other reagent kit and run configurations that allow customers to choose the use of 1 or 2 flow cells and between 1 and 50 channels, adjust the number of strands of DNA imaged, and select run times between 2.5 and 8 days.
SCIENTIFIC APPLICATIONS
Our ability to build genomic knowledge from patient samples to discover and inform healthcare options will require novel, simplified methods for gene sequencing derived from patient natural DNA as well as methods for the analysis of limiting quantities of nucleic acids derived from clinical samples, including, but not limited to, fine needle aspirates, circulating tumor cells and body fluids. Further, many patient specimens with linked clinical information have been collected and stored as formalin-fixed paraffin embedded tissues. The Helicos® Genetic Analysis System provides new opportunities for genomic studies which utilize limiting nucleic acid quantities and encompass many areas of research, development and diagnostic use. The areas where we believe Helicos' technology offers significant opportunities include:
- •
- DirectGene™ Sequencing: Helicos has developed
new technology to perform targeted sequencing without upfront enrichment or amplification using our newly developed DirectGene™ Sequencing capability. This approach relies on our ability
to deposit locus specific capture oligonucleotides directly on the sequencing substrate, hybridizing fragmented genomic DNA to this modified substrate, and initiating the
sequencing-by-synthesis reaction, without any amplification providing a readout of gene sequencing for genetic tests.
- •
- Analysis of Small Sample Quantities: With funding from a
Phase I SBIR Grant, Helicos has demonstrated the ability to perform experimentation not possible with other commercially available next-gen sequencing
platforms—maintaining the basic principles of simple sample preparation involving no amplification and minimal sample manipulation enabling experiments never before possible. The ability
to work with attomole level nucleic acid as well as new technologies emerging from this work remain core to our business and allow competitive distinction for Helicos at this time. By enabling the
analysis of small amounts of nucleic acid material, without the requirement for amplification, a number of commercial and scientific applications can be realized including: 1) the detection and
analysis of small amounts of circulating tumor cells from bodily fluids will enable diagnostics for the early detection of cancer onset, 2) the ability to perform molecular analyses on rare and
precious biomedical samples without consuming large amounts of the sample and without skewing the results by amplification will enable heretofore impossible retrospective clinical research,
3) the ability to manipulate and analyze degraded and damaged nucleic acid material will facilitate forensic studies, and 4) the potential for direct analysis of unculturable
microorganisms, which represent over 80% of the earth's microbiome and are available in limited quantities, can be analyzed for the discovery and scientific insight into novel biological functions and
the elucidation of pathogenic molecular pathways.
- •
- Direct RNA Sequencing: The traditional approaches to
transcriptome analyses have been typically dependent on the creation of a cDNA intermediate, an indirect measurement of the RNA in the cell. A unique feature of the Helicos System is our ability to
sequence RNA directly without amplification or cDNA conversion. This method can also be adapted for experiments where sample material is limiting. We currently focus on demonstrating the unique
attributes of the data derived from this technology with collaborators across the globe.
- •
- Accurate Quantitation for RNA and DNA: Digital gene expression and RNA Sequencing provides a hypothesis free, global, and quantitative analysis of the transcriptome. Helicos single molecule sequencing method allows for the quantitative measurement of virtually all genes in a sample by
15
- •
- Ancient DNA Sequencing: Ancient DNA sequencing benefits from the unique attributes of the Helicos single molecule direct sequencing methods. DNA derived from tissue abstracts obtained from ancient remains may be degraded, heavily modified and contaminated with larger fragments of modern DNA. Traditional sequencing methods depend on ligation and amplification steps and typically enrich for the larger, modern DNA leading to low sequence yields of the ancient DNA of interest. With SMS methods, degraded ancient DNA is simply tailed and sequenced in the presence of the modern DNA and provides significant benefit in yield and sequence output for researchers.
counting the number of individual mRNA molecules produced from each gene. This allows one to examine all the genes present in a cell or tissue in a hypothesis independent manner with no bias toward the genes believed to be expressed. We believe the end result is a highly sensitive and quantitative measurement which will allow not only for the detection of highly expressed transcripts but also for the detection of very rare transcripts represented by only a few molecules of RNA per cell. Our DNA quantitation has focused on the application of quantitative analysis of the human genome for detection of copy number differences across the genome.
BIOMEDICAL RELEVANCE
The applications described above provide opportunity for biomedical research and molecular diagnostics as described below.
- •
- Cancer research. Cancer genetics involves understanding
the effects of the inherited genome as well as the tumor genome including acquired mutations and other genetic alterations. Diagnosing and treating cancer therefore requires a more comprehensive
understanding of the individual patient tumor genome to better-predict responses to drug therapy. We believe the availability of low-cost genomic analysis including quantitative Digital
Gene Expression, RNA Sequencing and microRNA profiling on small samples, circulating tumor cells, tumor cell biopsies or formalin-fixed paraffin embedded tissue specimens to characterize acquired
changes of the genome that contribute to cancer would enable improved diagnosis and treatment of cancer.
- •
- Molecular diagnostics. Patients who present with the same
disease symptoms often have different prognoses and drug responses based on their underlying genetic differences. We believe that delivering patient-specific genetic and genomic information at a
reasonable cost represents a multi-billion dollar potential market waiting to be fully realized. Commercial markets for molecular diagnostics include gene- or expression-based diagnostic
kits and services and targeted gene resequencing for companion diagnostic products offer the opportunity for selecting and monitoring particular therapies, as well as patient screening for early
disease detection and disease monitoring. Creating more effective and targeted molecular diagnostics and screening tests requires a better understanding of genes, regulatory factors and other disease-
or drug-related factors. We believe that our platform's quantitative accuracy, the use of small sample quantities in simple preparation methods, and high throughput, as well as lack of
biases typically seen with sample amplification, can be particularly useful in the field of molecular diagnostics.
- •
- Infectious disease. All viruses, bacteria and fungi contain DNA or RNA. The detection and sequencing of DNA or RNA from pathogens at the single molecule level would provide medically and environmentally useful information for the diagnosis, treatment and monitoring of infections and to predict potential drug resistance. Such sequencing would not require the growth or purification of organisms that can be difficult to culture or work with.
16
RESEARCH AND DEVELOPMENT
We advanced the development of our True Single Molecule Sequencing (tSMS)™ technology since we began operations in 2003. In 2004, we began to produce sequence data from single molecules of DNA and in 2005, we sequenced genomic DNA from a small virus called M13 using our tSMS technology. Also in 2005, we began to design the Helicos® Genetic Analysis System. In 2006, we received a $2 million grant from the National Human Genome Research Institute and completed the design of the critical components of the Helicos System. In 2007, we substantially finished the assembly of five commercial grade Helicos Systems and began shipping our Helicos Systems to initial customers in 2008. Our initial shipment to our first customer, Expression Analysis, Inc. ("EA"), was made on March 5, 2008. This system was an early version and did not consistently achieve our commercial specification levels at EA and, as a result, on January 27, 2009, we agreed to have EA return the Helicos System that was installed at EA. Following EA's return of this system, our commercial relationship with EA was suspended. EA did not make any payments for this Helicos System that was ultimately returned.
In 2008, we introduced a second generation Virtual Terminator™ Nucleotides with improved performance and shelf-life and second generation image analysis software with improved performance. During 2010, we invested in limited research and development activities to maintain the performance of our Helicos System and develop new applications with the existing system. The pace of these research and development activities was significantly curtailed during 2010, as demonstrated by the decline in our spending rate from approximately $4 million in the three month period ended March 31, 2010 to approximately $1 million in the three month period ended December 31, 2010. Going forward, we do not plan to allocate a significant amount of resources to research and development activities beyond activities related to grant-funded initiatives and the development of assays to support our limited efforts in molecular diagnostics.
In the years ended December 31, 2008, 2009, and 2010 we incurred $24.6 million, $18.3 million and $14.0 million respectively, of research and development expenses.
MANUFACTURING AND RAW MATERIALS
We have manufactured our products using a combination of outsourced components and subassemblies. In addition to our in-house production capability, we have utilized subcontractors for parts of the manufacturing process where we have determined it is in our best interest to do so. As a result of our efforts to reposition the Company in the genetic analysis markets, we do not plan to allocate substantial resources to increasing our manufacturing process capability or capacity, and may choose to outsource the manufacturing and repair and maintenance of our instrument and consumables entirely.
MARKETING, SALES, SERVICE AND SUPPORT
As a result of our limited resources, we have significantly curtailed our sales, marketing and service efforts. We are nevertheless continuing our business development activities to identify and pursue partnership opportunities in the molecular diagnostics market. We expect that any revenues in the foreseeable future will be derived primarily from conducting research under grants that have been awarded to us, the sale of sequencing services to research institutions in the academic, clinical, governmental, pharmaceutical, biotechnology and agriculture biology segments, and from the sale of consumables to our installed base of customers. To date, we have instruments in operation at several research institutions including two academic centers, a genome center, a cancer center and two commercial locations. As of the date of this filing, we do not foresee commencing any efforts to sell new instruments.
17
COMPETITION IN THE GENETIC ANALYSIS MARKET
Competition among entities developing or commercializing instruments, research tools or services for genetic analysis is intense. A number of companies offer DNA sequencing equipment or consumables, including Life Technologies, Inc., Beckman Coulter, Inc., the Life Sciences Division of GE Healthcare, Illumina, Inc., Complete Genomics, Inc. and Roche Applied Science. Furthermore, a number of other companies and academic groups are in the process of developing novel techniques for DNA sequencing. These companies include, among others, Ion Torrent, Genizon BioSciences, Intelligent Bio-Systems, Lucigen, Microchip Biotechnologies, Pacific Biosciences, Shimadzu Biotech, ZS Genetics, Oxford Nanopore, NabSys and IBM. For RNA analysis and/or genotyping there are a number of companies that offer equipment and supplies including Affymetrix, Inc., Agilent Technologies, Life Technologies Corporation (formerly Applera Corporation), Bio-Rad Laboratories, Luminex and Nanostring. Three companies provide a wide range of products that span both DNA and RNA analysis—Life Technologies, Inc., Affymetrix, Inc. and Illumina, Inc. However, the solutions that are provided by these competitors are separate applications that require different sample preparation techniques, consumables, analysis software and instrumentation with limited correlation between platforms.
Many of these companies have substantially greater capital resources, research and product development capabilities and greater financial, scientific, manufacturing, marketing, and distribution experience and resources, including human resources, than we do. These companies may develop or commercialize genetic analysis technologies before us or that are more effective than those we are developing, and may obtain patent protection or other intellectual property rights that could limit our rights to offer genetic analysis products or services.
As we consider the repositioning of the Company in the genetic analysis markets, we will need to continue to assess our ability to successfully compete against existing and future technologies. Moreover, we will need to demonstrate that the price and performance of our technologies and platform applied to our target markets are competitive with those of competing technologies.
As we redirect our efforts towards the molecular diagnostics market, and if we ultimately begin to market CLIA-certified diagnostic tests, we may compete with a number of companies that commercialize services for molecular diagnostics and genetic testing. Companies offering CLIA-certified testing services include LabCorp/Correlagen Diagnostics, Bio-Reference Laboratories/GeneDx, Genzyme Genetics, Myriad Genetics, Gen-Probe, Transgenomic/PGxHealth, PerkinElmer, Signature Genomics, Quest Diagnostics/Athena Diagnostics, Celera, Genomic Health and many others. Certain hospitals and academic institutions also offer CLIA-certified genetic testing services. Direct-to-Consumer (DTC) genetic testing companies are also emerging, offering whole genome or gene sequence information to consumers without a physician's prescription. Some of these companies use assays that have not been validated according to CLIA standards, and it is unclear what impact these companies will have on the CLIA-certified testing market. Companies offering DTC tests include Illumina, 23andMe, deCODE Genetics, Knome and Navigenics.
INTELLECTUAL PROPERTY
Developing and maintaining a strong intellectual property position is an important element of our business strategy. In light of our intensified focus on intellectual property monetization through licensing and enforcement strategies, this aspect of our business has become even more central to our strategy and operations. See Part I, Item 3, Legal Proceedings for further discussion relating to our patent infringement enforcement actions. To that end, we have increased the resources of outside legal counsel, and established a disciplined process of growing and broadening our intellectual property assets, which involves substantial management attention. Our patent portfolio relating to our proprietary technology is comprised, on a worldwide basis, of various patents and pending patent
18
applications, which, in either case, we own directly or for which we are the exclusive or semi-exclusive licensee. A number of these patents and patent applications are foreign counterparts of U.S. patents or patent applications. Among other things, our patent estate includes patents and/or patent applications having claims directed to:
- •
- a broad array of next generation sequencing technologies based on sequencing-by-synthesis methods;
- •
- the overall True Single Molecule Sequencing (tSMS)™ method;
- •
- certain components of the Helicos® Genetic Analysis Platform, including our laser illumination subassembly,
our flow cells and various methods for using our HeliScope Sequencer;
- •
- methods for focusing our lasers and imaging our flow cell surfaces, and our use of combinations of laser optical paths;
- •
- our Virtual Terminator™ Nucleotides and other nucleotides;
- •
- various aspects of our sample preparation processes;
- •
- algorithms for analysis of our data;
- •
- reagent formulations for imaging and for sequencing; and
- •
- methods that can be used in molecular diagnostic testing for making inferences about disease state and for improving accuracy.
Within broad technological categories, our patent portfolio can be broken down as follows: most of our issued (81%) and pending (60%) patents are directed to sequencing by synthesis, primarily at the single molecule level; the smallest portion of the patent portfolio (3% of issued patents and 3% of pending applications) involves bioinformatics and data processing; the remainder of the portfolio is roughly evenly split among sample preparation (6% of issued patents and 12% of pending applications), instrumentation (10% of issued patents and 11% of pending applications), and commercial applications (14% of pending patent applications). The last-mentioned of these groups—commercial applications—encompasses wide areas of scientific and commercial interest including direct RNA sequencing, single-cell analysis, high-throughput screening, digital gene expression, and candidate region re-sequencing.
Our patents generally have terms of 20 years from their respective non-provisional priority filing dates. The first non-provisional patent applications prosecuted by Helicos were filed in 2004 and thus our issued patents are not scheduled to expire until 2024 or later. We have filed terminal disclaimers in certain later-filed patents, which means that such later-filed patents are scheduled to expire earlier than the twentieth anniversary of their respective non-provisional priority filing dates, although not earlier than 2024 with respect to patent applications prosecuted by Helicos. We have also in-licensed patents and patent applications. All of the material patents and patent applications to which we have licensed rights are scheduled to expire in 2017 or later.
Patent law relating to the scope of claims in the technology field in which we operate is still evolving. The degree to which we will be able to protect our technology with patents, therefore, is uncertain. Others may independently develop similar or alternative technologies, duplicate any of our technologies and, if patents are licensed or issued to us, design around the patented technologies licensed to or developed by us. In addition, we could incur substantial costs in litigation if we are required to defend ourselves in patent suits brought by third parties or if we initiate such suits.
We regard as proprietary any technology that we or our exclusive licensors have developed or discovered, including technologies disclosed in our patent estate, and that was not previously in the public domain. Aspects of our technology that we consider proprietary may be placed into the public
19
domain by us or by our licensors, either through publication or as a result of the patent process. We may choose for strategic business reasons to make some of our proprietary technology publicly available whether or not it is protected by any patent or patent application.
With respect to proprietary know-how that is not patentable and for processes for which patents are difficult to obtain or enforce, we may rely on trade secret protection and/or confidentiality agreements to protect our interests. While we require all employees, consultants, collaborators, customers and licensees to enter into confidentiality agreements, we cannot be certain that proprietary information will not be disclosed or that others will not independently develop substantially equivalent proprietary information.
In addition to our patents, patent applications, confidential know-how, and potential trade secrets, we license technology that we consider to be material to our business.
Roche License Agreement. In June 2004, we entered into a license agreement with Roche Diagnostics (the "Roche License Agreement") which granted us a worldwide, semi-exclusive royalty-bearing license, with the right to grant sublicenses under a patent relating to sequencing methods. In connection with the Roche License Agreement, we paid an upfront fee of 175,000 Euros and committed to pay an annual license fee ranging from 10,000 to 40,000 Euros. There are no milestone payments potentially payable by us under the Roche License Agreement in addition to those described above. We have an option to convert the license to non-exclusive beginning in 2008, in which case the annual license fees would be reduced to 10,000 Euros beginning in 2008. We have the right to terminate the Roche License Agreement at any time for convenience upon 90 days prior written notice to Roche Diagnostics. We both have the right to terminate the Roche License Agreement upon breach by the other party, subject to notice and an opportunity to cure. The Roche License Agreement also terminates upon the occurrence of specified bankruptcy events. As part of the Roche License Agreement, we agreed to pay single digit royalties based on a percentage of defined net sales. We also agreed to pay half of our income amounts that we receive based on sublicenses that we grant to third parties. Our royalty obligation, if any, extends until the expiration of the last-to-expire of the licensed patents. Through December 31, 2010, no royalty payments have been made. All license fee amounts paid to date have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. The total expense recognized under the Roche License Agreement for the years ended December 31, 2008, 2009 and 2010, and the period from May 9, 2003 (date of inception) through December 31, 2010 was $62,000, $54,000, $86,000 and $507,000, respectively.
AZTE License Agreement. In March 2005, we entered into a license agreement with Arizona Technology Enterprises (the "AZTE License Agreement") that granted us a worldwide, exclusive, irrevocable, royalty-bearing license, with the right to grant sublicenses, under specified patents and patent applications exclusively licensed by AZTE from Arizona State University and the University of Alberta. In connection with the AZTE License Agreement, we paid an upfront fee of $350,000, committed to an annual license fee of $50,000, which has increased to $100,000 upon the successful issuance of a U.S. patent, committed to pay a three-year maintenance fee of $50,000, payable in equal annual installments beginning in March 2006, and issued 88,888 shares of restricted common stock, which vest in two equal installments upon the achievement of separate milestones. There are no milestone payments potentially payable by us under the AZTE License Agreement in addition to those described above. We are obligated to use reasonable commercial efforts to develop, manufacture and commercialize licensed products. In addition, if we fail to use commercially-reasonable efforts to develop, manufacture and sell licensed products, the AZTE License Agreement converts from exclusive to non-exclusive. The AZTE License Agreement will remain in force until terminated. We have the right to terminate the AZTE License agreement at any time for convenience upon 60 days prior written notice to Arizona Technology Enterprises. We both have the right to terminate the agreement
20
upon breach by the other party, subject to notice and an opportunity to cure. The AZTE License Agreement also terminates upon the occurrence of specified bankruptcy events.
As part of the AZTE License Agreement, we agreed to pay a single digit percentage royalty based on defined net sales. We also agreed to pay a mid-teens percentage of specified sublicense income amounts that are received based on sublicenses granted to third parties which increases to 30 percent after we receive an aggregate of $50,000 of such amounts. Our royalty obligation, if any, extends until the expiration of the last-to-expire of the licensed patents. Through December 31, 2010, no royalty payments have been made. All license fee amounts paid to date have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. In May 2006, in accordance with the license agreement, due to the successful issuance of a U.S. patent, the committed annual license fee increased from $50,000 to $100,000 and 44,444 shares of the restricted common stock vested. The vesting of 44,444 shares of restricted common stock resulted in a charge to research and development expense of $127,000 based on the fair value of our common stock at the time the milestone was achieved. The remaining 44,444 shares of restricted common stock vested immediately upon the successful issuance of a second U.S. patent on January 12, 2010 and we recorded an expense of $54,000 associated with the vesting of these shares. The total expense recognized under the AZTE License Agreement for the years ended December 31, 2008, 2009 and 2010, and the period from May 9, 2003 (date of inception) through December 31, 2010 was $103,000, $141,000, $154,000 and $1,144,000, respectively.
Caltech License Agreement. In November 2003, we entered into a license agreement with California Institute of Technology (the "Caltech License Agreement") that granted us a worldwide, exclusive, royalty-bearing license, with the right to grant sublicenses, under specified patents and patent applications, and a worldwide, non-exclusive royalty bearing license, with the right to grant sublicenses, under specified technology outside the scope of the licensed patents. In connection with the Caltech License Agreement, we issued 46,514 shares of common stock, and recorded a charge of $20,000. In addition, we pay an annual license fee of $10,000 per year. The license fee payments are creditable against single digit royalties calculated upon sales of products covered by patents licensed under the agreement. We are also obligated to pay California Institute of Technology a single digit percentage of specified license and sublicense income, a single digit percentage of proceeds from sales of specified intellectual property and a single digit percentage of service revenue amounts that we receive based on licenses and sublicenses that we grant, sales of intellectual property and services that we provide to third parties. The royalty obligation with respect to any licensed product extends until the later of the expiration of the last-to-expire of the licensed patents covering the licensed product and three years after the first commercial sale of the licensed product in any country for non-patented technology covered under the agreement. Through December 31, 2010, no royalty payments have been made. In March 2007, we amended the Caltech License Agreement to provide rights under an additional patent application under the terms of the existing license in exchange for a one-time payment of $50,000 to the California Institute of Technology. There are no milestone payments potentially payable by us under the Caltech License Agreement in addition to those described above. All license fee amounts paid to date and the value of the common stock issued have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. The total expense recognized under the Caltech License Agreement for the years ended December 31, 2008, 2009 and 2010, and the period from May 9, 2003 (date of inception) through December 31, 2010 was $10,000, $10,000, $10,000 and $133,000, respectively.
21
PerkinElmer License Agreement. In April 2007, we entered into an agreement with PerkinElmer LAS, Inc. ("PerkinElmer"), in which PerkinElmer granted us a worldwide, non-exclusive, non-transferable, non-sublicensable, royalty bearing license under specified patents. Our license from PerkinElmer grants us rights under certain patents to produce and commercialize certain of the reagents used in some applications on the Helicos System, which contain chemicals purchased from PerkinElmer. In exchange for rights licensed from PerkinElmer, we are obligated to pay PerkinElmer a single digit percentage of our net revenue from the sale of reagents that contain chemicals covered by the patents licensed under the PerkinElmer agreement. There are no milestone payments potentially payable by us under this agreement with PerkinElmer. We have the right to terminate the agreement at any time upon 90 days written notice to PerkinElmer. Each party has the right to terminate the agreement upon breach by the other party subject to notice and an opportunity to cure. The agreement also terminates upon the occurrence of specified bankruptcy events. PerkinElmer has the sole right under the agreement to enforce the licensed patents. There has been no expense recorded for this agreement for any period from May 9, 2003 (inception) through December 31, 2010.
See Note 7 to the Consolidated Financial Statements contained in this Form 10-K for additional information on license agreements.
COMPLIANCE WITH ENVIRONMENTAL PROVISIONS
We are not materially affected by compliance with federal, state, and local environmental provisions which have been enacted or adopted to regulate the distribution of materials into the environment.
CORPORATE INFORMATION
We were incorporated in Delaware in May 2003. In 2003, one of our co-founders, Professor Stephen R. Quake, who was then at the California Institute of Technology, demonstrated, we believe for the first time, that sequence information could be obtained from a single strand of DNA. Shortly thereafter, Noubar Afeyan, Chief Executive Officer of Flagship Ventures, and Stanley Lapidus, then a Venture Partner at Flagship Ventures, met with Professor Quake and agreed to found a company to develop and commercialize technology based on Professor Quake's single molecule approach. Combining the experience of Professor Quake in single molecule methods, Dr. Afeyan in the sequencing technology and life sciences businesses, and Mr. Lapidus in diagnostics and entrepreneurship, we focused exclusively on the technical and commercial development of technology based on Professor Quake's approach. Professor Eric Lander, Director of the Broad Institute of MIT and Harvard, and a leader in the DNA sequencing field, provided helpful guidance and advice during our founding stages.
EMPLOYEES
We had 22 full-time employees at December 31, 2010. We have never had a work stoppage and none of our employees are covered by collective bargaining agreements. We believe our employee relations are good. Our success depends in large part on our ability to attract and retain skilled and experienced employees.
AVAILABLE INFORMATION
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, definitive proxy statements on Form 14A, current reports on Form 8-K, and any amendments to those reports are made available free of charge on our website, www.helicosbio.com, as soon as reasonably practicable after such reports are electronically filed with or furnished to the Securities and Exchange Commission (the "SEC"). Statements of changes in beneficial ownership of our securities on Form 4 by our executive officers and
22
directors are made available on our website by the end of the business day following the submission to the SEC of such filings. In addition, the SEC's website, www.sec.gov, contains reports, proxy statements, and other information regarding reports that we file electronically with the SEC.
The following important factors could cause our actual business, prospects, financial results or financial condition to differ materially from those contained in forward-looking statements made in this Annual Report on Form 10-K or elsewhere by management from time to time.
RISKS RELATED TO OUR BUSINESS
We are implementing a new strategic focus. If we are unsuccessful in pursuing our new business initiatives, our business, financial condition, results of operations and prospects will be materially adversely affected and we may have to cease operations.
We are implementing a new strategic focus and plan to deploy our limited resources by focusing on intellectual property monetization and concentrating our research and development efforts in targeted areas designed to achieve tangible proof-of-concept goals, which may enable us to seek partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives. In addition, on October 14, 2010, we announced the launch of a technology licensing program for our Single Molecule Sequencing Platform. While our new business opportunities will rely upon the capabilities of our proprietary Helicos® Genetic Analysis Platform and the strength of our intellectual property portfolio, the business model will be materially different than that which we have engaged in before. We are unable to give any assurance we will be commercially successful in our new business strategy. Our failure to be commercially successful in any such new business opportunity would materially adversely impact our business, financial condition, results of operations and prospects and could ultimately require that we cease operations.
A successful shift in our strategic focus will require significant organizational changes. Given the potential uncertainty about the success of our new business priorities and long term roles within our organization, morale may be lowered, key employees may leave or be distracted and our business may experience a loss of continuity while we implement our business priorities. Our inability to retain existing personnel would prevent us from fully exploiting our new business priorities and thereby cause a material adverse effect on our business, financial condition, results of operations and prospects.
During the second half of 2011, our business will require additional funding, which may not be available on favorable terms, if at all, or without potentially very substantial dilution to our stockholders. If we do not raise the necessary funds, we will need to dramatically reduce, or even terminate all of, our operations.
Our business will require additional financial investment that we have not yet secured and we will need to raise additional capital during the second half of 2011 in order to sustain our business and to pursue our new business priorities. The amount of additional capital we will need to raise depends on many factors, including:
- •
- our current or any future litigation related to defending our intellectual property or defending lawsuits that allege our
actions infringe the intellectual property of third parties;
- •
- the success of our research and development efforts;
- •
- the expenses we incur in marketing and selling our products or services;
- •
- the revenue generated by future sales of our products or services;
23
- •
- the potential significant shortfall in expected revenues from reagent kit consumable purchases by our customer in Japan as
a result of the recent natural disasters in Japan;
- •
- the timeliness of payments from our customers;
- •
- the ability to structure arrangements with, and make payments to, our unsecured creditors;
- •
- the continued deferrals by our senior lenders of our monthly principal payments under the Fourth Amendment and the ability
to further restructure this lending arrangement in the future;
- •
- the continued funding to the Bridge Debt Financing facility under the November, 2010 Note Purchase Agreement;
- •
- the emergence of competing or complementary technological developments;
- •
- the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
- •
- regulatory changes in our markets; and
- •
- the terms and timing of any collaborative, licensing or other arrangements that we may establish.
We do not know whether the additional capital that we will require will be available when and as needed, on favorable terms or if at all, or that our actual cash requirements will not be greater than anticipated. We may seek additional capital through a combination of private and public equity offerings, debt financings and collaboration, strategic and licensing arrangements. To the extent we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of such sales may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, would involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt or making capital expenditures or that require early repayment, even with penalties, in connection with a change of control. If we raise additional funds through collaboration, strategic alliance and licensing arrangements with third parties, we would have to relinquish valuable rights to our technologies or products, or grant licenses on terms that are not favorable to us. We may not be able to complete any such capital raising transactions on acceptable terms, or at all. If adequate additional funds are not available to us when required, we will be required to terminate some or all aspects of our operations.
The audit report contained in our Annual Report on Form 10-K for the year ended December 31, 2010 contains an explanatory paragraph to the effect that there is substantial doubt about our ability to continue as a going concern.
This "going concern" statement may discourage some third parties from contracting with us and some investors from purchasing our stock or providing alternative capital financing. The resulting failure to establish, or the loss of existing, favorable commercial relationships or to satisfy our capital requirements would adversely affect our business, financial condition, results of operations and prospects. Unless we raise additional funds, either through public or private sales of equity, from borrowings or from strategic partners, we will likely have to cease operations. Even if we can continue operating by reducing expenses, the requisite significant reduction in our workforce and operating expenses will adversely affect our business and prospects.
We may not be able to meet our debt service obligations, or may otherwise default, under our senior and junior secured loan and security agreements, which would materially adversely affect our business, financial condition, results of operations and prospects.
Our senior and junior secured loan and security agreements impose restrictions and obligations on us with which we may not be able to comply. If we fail to comply with such restrictions, our lenders
24
may declare us in default. Also, we have debt service obligations under our senior and junior secured loan and security agreements. We may be unable to generate sufficient cash flow from operations or other sources to service this debt. Failure to service our debt could result in an event of default. The occurrence of an event of default could lead to acceleration of such debt or any instruments evidencing indebtedness that contain cross-acceleration or cross-default provisions. In such event, we may be required to sell assets, to refinance, or to obtain additional financing. Such refinancing may not be possible and additional financing may not be available on commercially acceptable terms or at all. The borrowings under the loan agreement are collateralized by essentially all of our assets and include account control agreements for each of our cash and investment accounts. These account control agreements permit the lenders to control our cash and investment accounts in the event of a default.
We have a history of operating losses, expect to continue to incur substantial losses, and might never achieve or maintain profitability.
We are a development-stage company with a limited operating history. We have incurred significant losses in each fiscal year since our inception, including net losses attributable to common stockholders of $45.7 million, $28.0 million and $18.8 million in the years ended December 31, 2008, 2009 and 2010, respectively. As of December 31, 2010, we had an accumulated deficit of $186.4 million. These losses have resulted principally from costs incurred in our research and development programs and from our selling, general and administrative expenses. In the year ended December 31, 2010, we used cash in operating activities of $12.0 million. As of December 31, 2010 and March 24, 2011, we had $2.5 million and $2.9 million, respectively, in cash and cash equivalents.
We expect our losses to continue for a considerable period of time as we pursue our new business priorities. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and stockholders' equity/(deficit). Because of the numerous risks and uncertainties associated with our new business priorities, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we are unable to achieve and then maintain profitability, the market value of our common stock will decline.
Following our reductions in force in May 2010 and September 2010, which resulted in the elimination of approximately two-thirds of the Company's headcount, we may not be able to retain our remaining employees while we pursue our business priorities. As a result, we may be unable to achieve our goals, in which case, our business, financial conditions, results of operations and prospects will be materially adversely affected and we may have to cease operations.
In connection with our efforts to consider alternatives to our long-term strategic focus, in May 2010 and September 2010, we committed to reductions in headcount and restructuring plans (the "Restructuring Plans") which involved the consolidation and reorganization of our operations in order to reduce operating costs. The Restructuring Plans involved a reduction in headcount of approximately 54 employees or two-thirds of the organization. As a result, while we begin to pursue our new business priorities, we will rely heavily on our ability to retain our remaining employees. The loss of the service of any number of our remaining employees may significantly delay or prevent our ability to pursue our business objectives. For example, because of the complex and technical nature of our Helicos System, any failure to retain a sufficient number of our remaining, qualified employees could materially harm our ability to maintain our existing know-how and proprietary technology which would severely limit any ability to pursue our business priorities.
Given the magnitude of our Restructuring Plans' reductions in force and the potential uncertainty about our strategic direction and long-term roles within our organization, the morale of our remaining employees may be lowered, key employees may be distracted and our business may experience a loss of continuity while we pursue our business priorities. Each of our remaining employees could terminate
25
his or her relationship with us at any time. These persons' expertise would be difficult to replace and the failure to do so would have a material adverse effect on our ability to achieve our business goals. There can be no assurance that we will have the financial resources or otherwise be successful in retaining our remaining personnel and our failure to do so would have a material adverse effect on our business, financial condition and results of operations. Our inability to retain existing personnel during this period of uncertainty or our inability to attract new personnel that fit within a new strategic focus would prevent our exploiting fully our business priorities and thereby cause a material adverse effect on our business, financial condition, results of operations and prospects.
We have limited resources in selling and marketing and, as a result, may be unable to successfully market and sell our products and services.
We have a small sales and marketing team. Our ability to sustain our operations depends on attracting customers for our products and services and taking advantage of the capabilities of our proprietary technology for business opportunities that are materially different than that which we have engaged in before. To successfully perform sales, marketing and customer support functions ourselves, we will face a number of risks, including:
- •
- our ability to successfully implement our new business priorities;
- •
- the ability of our remaining limited sales and marketing team to achieve our near term goals following our recent
workforce reductions; and
- •
- our ability to support our current installed base with limited resources.
We have limited experience.
You should evaluate us in the context of the uncertainties and complexities affecting an early stage company in the life science industry which is experiencing the challenges associated with entering into new, highly competitive markets. Based on our limited experience, we may not:
- •
- effectively execute on or focus our research and development efforts;
- •
- properly model new opportunities to ensure appropriate resource allocation; or
- •
- create product or service offerings that are appropriately developed to meet customer needs.
If we are unable to retain key executives, scientists and other employees, we will be unable to achieve our goals.
We are substantially dependent on the performance of our senior management and key scientific and technical personnel. We do not maintain employment contracts with any of our employees. We have implemented a number of workforce reductions to reduce our operating costs, conserve cash and direct our resources to continue advancing towards our near term goals. Depending on our circumstances, we may need to implement additional workforce reductions in the future. The loss of the services of any member of our senior management or our scientific or technical staff may significantly delay or prevent the development of our products and other business objectives by diverting management's attention to transition matters and identification of suitable replacements, if any, and could have a material adverse effect on our business, prospects, operating results and financial condition.
Because of the complex and technical nature of our Helicos System and the dynamic market in which we compete, any failure to retain a sufficient number of qualified employees could materially harm our ability to implement our new business priorities. Competition for these people is intense. Each of our executive officers and other key employees could terminate his or her relationship with us at any time. These persons' expertise would be difficult to replace and could have a material adverse
26
effect on our ability to achieve our business goals. There can be no assurance that we will have the financial resources or otherwise be successful in retaining qualified personnel and our failure to do so could have a material adverse effect on our business, financial condition and results of operations.
We use hazardous chemicals and biological materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research and development activities involve the controlled use of hazardous materials, including chemicals and biological materials. Our operations produce hazardous waste products. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials. We do not currently maintain separate environmental liability coverage. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of hazardous materials. Compliance with environmental laws and regulations may be expensive, and current or future environmental regulations may impair our research, development and production efforts.
We rely on a single laboratory facility for our research and development activities and are occupying our space without a long term lease agreement as tenants-at-will, operating on a month to month basis with our landlord.
We rely on a single laboratory facility in Cambridge, Massachusetts for our research and development activities. This facility and certain pieces of laboratory equipment would be difficult to replace and may require significant replacement lead-time. This facility may be affected by natural disasters such as earthquakes, floods and fires. In the event our laboratory facility or equipment is affected by man-made or natural disasters, we would be unable to develop products for a significant period of time. Although we maintain insurance on this facility, it may not be adequate to protect us from all potential losses if this facility were damaged or destroyed. In addition, the lease for our Cambridge facility expired on December 31, 2010 and, as a result, we continue to occupy our space on a month to month basis as tenants-at-will. We are unable to enter into a longer term lease arrangement due to our limited resources and financial condition. Our landlord may notify us at any time to vacate our leased premises with little or no notice and, as a result, any such action by our landlord could cause a significant disruption to our operations.
Because we are subject to existing and potential additional governmental regulation, we may become subject to burdens on our operations, and the markets for our products may be narrowed.
We are subject, both directly and indirectly, to the adverse impact of existing and potential future government regulation of our operations and markets. The life sciences industry, which is the market for our technology, has historically been heavily regulated. Given the evolving nature of this industry, legislative bodies or regulatory authorities may adopt additional regulation that adversely affects our market opportunities. Our business is also directly affected by a wide variety of government regulations applicable to business enterprises generally and to companies operating in the life science industry in particular. Failure to comply with these regulations or obtain or maintain necessary permits and licenses could result in a variety of fines or other censures or an interruption in our business operations which may have a negative impact on our ability to generate revenues and could increase the cost of operating our business. In particular, if we decide to market our diagnostic assays as laboratory developed tests ("LDTs") under Clinical Laboratory Improvement Amendments ("CLIA"), we will need to seek CLIA certification, and be subject to the requirements and oversight of the Centers for Medicare and Medicaid Services. We may not receive such certification in a timely fashion or at all. Furthermore, CLIA regulations may change over time and the U.S. Food and Drug Administration ("FDA") may ultimately decide to regulate the devices and methods we will use in the delivery of our
27
LDTs. Although we can closely monitor the initiatives and announcements and policy changes coming from the regulatory agencies, we cannot predict the regulatory changes that may arise and we may not be able to meet the requirements of new laws or regulations enacted by the FDA pertaining to the performance and marketing of our testing services.
We have incurred, and will continue to incur, significant increased costs as a result of operating as a public company, and our management is and will continue to be required to devote substantial time to new compliance initiatives.
The Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the Securities and Exchange Commission have imposed various requirements on public companies, including requiring changes in corporate governance practices. Our management and other personnel devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly.
In addition, the Sarbanes-Oxley Act requires, among other things, that we maintain effective internal control over financial reporting and disclosure controls and procedures. In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting, as required by Section 404(a) of the Sarbanes-Oxley Act. However, our testing may reveal deficiencies in our internal control over financial reporting that are deemed to be material weaknesses. Our compliance with Section 404 will require that we incur substantial accounting expense and expend significant management time on compliance-related issues. We currently do not have an internal audit group and we will evaluate the need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. Moreover, if we are not able to comply with the requirements of Section 404 in a timely manner, or if we identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by the Securities and Exchange Commission or other regulatory authorities, which would require additional financial and management resources.
If we make acquisitions in the future, we may encounter a range of problems that could harm our business.
We may acquire technologies, products or companies that we feel could accelerate our ability to compete in our core markets. Acquisitions involve numerous risks, including:
- •
- difficulties in integrating operations, technologies, accounting and personnel;
- •
- difficulties in supporting and transitioning customers of our acquired companies;
- •
- diversion of financial and management resources from existing operations;
- •
- risks of entering new markets;
- •
- potential loss of key employees; and
- •
- inability to generate sufficient revenue to offset acquisition costs.
Acquisitions also frequently result in the recording of goodwill and other intangible assets which are subject to potential impairments in the future that could harm our financial results. In addition, if we finance acquisitions by issuing convertible debt or equity securities, our existing stockholders may be diluted, which could affect the market price of our stock. As a result, if we fail to properly evaluate acquisitions or investments, we may not achieve the anticipated benefits of any such acquisitions, and we may incur costs in excess of what we anticipate.
28
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
Our failure to establish a strong intellectual property position and enforce our intellectual property rights against others would enable competitors to develop similar or alternative technologies.
Our success depends in part on our ability to obtain and maintain intellectual property protection for our technologies. Our policy is to seek to protect our intellectual property by, among other methods, filing U.S. patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business.
Our patent portfolio relating to our proprietary technology is comprised of issued patents and pending patent applications which, in either case, we own directly or for which we are the exclusive or semi-exclusive licensee. Some of these patents and patent applications are foreign counterparts of U.S. patents or patent applications. We may not be able to maintain and enforce existing patents or obtain further patents for our products, processes and technologies. Even if we are able to maintain our existing patents or obtain further patents, these patents may not provide us with substantial protection or be commercially beneficial. The issuance of a patent is not conclusive as to its validity or enforceability, nor does it provide the patent holder with freedom to operate unimpeded by the patent rights of others. Patent law relating to the scope of claims in the technology fields in which we operate is still evolving and the extent of future protection is highly uncertain, so there can be no assurance that the patent rights that we have or may obtain will be valuable. Others have filed patent applications that are similar in scope to ours, and in the future are likely to file patent applications that are similar or identical in scope to ours or those of our licensors. We cannot predict whether any of our competitors' pending patent applications will result in the issuance of valid patents. Moreover, we cannot assure investors that any such patent applications will not have priority or dominate over our patents or patent applications. The invalidation of key patents owned by or licensed to us or non-approval of pending patent applications could increase competition, and materially adversely affect our business, financial condition and results of operations. Furthermore, there can be no assurance that others will not independently develop similar or alternative technologies, duplicate any of our technologies, or, if patents are issued to us, design around the patented technologies developed by us.
We are involved in lawsuits and administrative proceedings to protect or enforce our patents and proprietary rights and to determine the scope and validity of others' proprietary rights, which will result in substantial costs and diversion of resources and which, if unsuccessful, could harm our competitive position and our results of operations.
Litigation and administrative proceedings are and may continue to be necessary to enforce our patent and proprietary rights and/or to determine the scope and validity of others' proprietary rights. Litigation on these matters has been prevalent in our industry and we expect that this will continue. To determine the priority of inventions, we may have to initiate and participate in interference proceedings declared by the U.S. Patent and Trademark Office that could result in substantial costs in legal fees and could substantially affect the scope of our patent protection. Also, our intellectual property is and may contimue to be subject to significant administrative and litigation proceedings such as invalidity, opposition, reexamination, or reissue proceedings against our patents. The outcome of any litigation or administrative proceeding might not be favorable to us, and, in that case, we might be required to develop alternative technological approaches that we may not be able to complete successfully or require licenses from others that we may not be able to obtain. Even if such licenses are obtainable, they may not be available at a reasonable cost. We may also be held liable for money damages to third parties and could be enjoined from using technologies. In addition, legal proceedings to enforce our intellectual property rights or to determine the validity and scope of the intellectual property or other proprietary rights of others, the proceedings will be burdensome and expensive, even if we were to prevail. These types of administrative proceedings and any additional litigation that may be necessary in the future could result in our patent protection being significantly modified or reduced, and could
29
result in substantial costs and diversion of resources and could have a material adverse effect on our business, operating results or financial condition. In August 2006, we filed an opposition against EP 1 105 529 B1 in the European Patent Office, with respect to which we received a preliminary non-binding opinion upholding the patent. In October 2008, EP 1 105 529 B1 was maintained in amended form and in January 2009 we filed a notice of appeal of the European Patent Office's decision. The patent owner filed a response brief on October 19, 2009, and we filed a reply brief on March 19, 2010.
On August 27, 2010, we filed a lawsuit against Pacific Biosciences of California, Inc. ("Pacific Biosciences") in the United States District Court for the District of Delaware (CA. No. 10-735-SLR) alleging that Pacific Biosciences infringes U.S. Patent Nos. 7,645,596 ("'596 Patent"), 7,037,687 ("'687 Patent"), 7,169,560 ("'560 Patent"), and 7,767,400 ("'400 Patent"). On October 22, 2010, we filed an amended complaint naming Illumina, Inc. ("Illumina") and Life Technologies Corporation ("Life Technologies") as additional defendants. In the amended complaint, we further allege that Life Technologies infringes the '596 Patent, the '687 Patent, and the '560 Patent, and that Illumina infringes the '687 Patent, the '560 Patent, and U.S. Patent No. 7,593,109 ("'109 Patent). We accuse Pacific Biosciences of willful patent infringement based at least on its manufacture, use, and sale of its Single Molecule Real Time ("SMRT™") DNA sequencing technology. We accuse Life Technologies of willful patent infringement based at least on its manufacture, use, and offer for sale of its Single Molecule Real-Time DNA Sequencing of a Quantum-dot Nanocrystal technology for single molecule sequencing of DNA. We accuse Illumina of willful patent infringement based at least on its manufacture, use, offer for sale, sale, and importation of its sequencing technology for single molecule sequencing of DNA including its Genome Analyzer, HiSeq 2000, and its Sq Module in combination with its iScan and HiScan platforms. We are seeking a permanent injunction enjoining each of the defendants from further infringement as well as compensatory and punitive damages, costs, and attorneys' fees, interest, and other relief as determined by the Court. On January 27, 2011, Pacific Biosciences submitted requests to the United States Patent and Trademark Office (the "Patent Office") for inter parties reexamination of the '687 Patent, the '560 Patent, the '596 Patent and the '400 Patent and are seeking a determination that one or more of the claims of those patents are invalid. The Patent Office granted the requests for reexamination of the '400 Patent and the '596 Patent on March 10, 2011, and issued office actions rejecting all claims of the patents. The Patent Office has not yet acted on the reexamination requests for the '687 Patent and the '560 Patent. Discovery opened in the matter on January 12, 2011. The Court conducted a scheduling conference on February 9, 2011 and set a trial date of September 10, 2012.
We depend upon our ability to license technologies, and the failure to license or otherwise acquire necessary technologies could harm our ability to sell our products or defend our intellectual property position.
We hold various licenses to use certain technologies that we consider to be material to our business. Each of these licenses imposes a range of obligations on us and may be terminated if we breach the terms of any of the respective agreements. We may also be required to enter into additional licenses with third parties for other technologies that we consider to be necessary for our business. If we are unable to maintain our existing licenses or obtain additional technologies on acceptable terms, we could be required to develop alternative technologies, either alone or with others, in order to avoid infringing the intellectual property to which we no longer hold a license. This could require the tests we are developing to be re-configured, which could negatively impact the availability of our tests, once developed, for commercial sale and increase our development costs. Failure to license or otherwise acquire necessary technologies would harm our ability to market and sell our diagnostic products, once developed, which could materially adversely affect our business, financial condition and results of operations. In addition, any licenses we obtain from federally-funded institutions are subject to the march-in rights of the U.S. government.
30
We may be the subject of costly and time-consuming lawsuits brought by third parties for alleged infringement of their proprietary rights, which could limit our ability to use certain technologies in the future, force us to redesign or discontinue our products, or pay royalties to continue to sell our products.
Our success depends, in part, on us neither infringing patents or other proprietary rights of third parties nor breaching any licenses to which we are a party. We may be the subject of legal claims by third-parties that our technology or products infringe their patents or otherwise violate their intellectual property rights. In addition, the technology that we license from third parties for use in our Helicos System could become subject to similar infringement claims. Infringement claims asserted against us or our licensors may have a material adverse effect on our business, results of operations or financial condition. Any claims, either with or without merit, could be time-consuming and expensive to defend, and could divert our management's attention away from the execution of our business plan. Moreover, any settlement or adverse judgment resulting from the claim could require us to pay substantial amounts of money or obtain a license to continue to use the technology that is the subject of the claim, or otherwise restrict or prohibit our use of the technology. There can be no assurance that we would be able to obtain a license on commercially reasonable terms, if at all, from third parties asserting an infringement claim; that we would be able to develop alternative technology on a timely basis, if at all; or that we would be able to obtain a license to use a suitable alternative technology to permit us to continue offering, and our customers to continue using, our affected products. We believe that there may be significant litigation in the industry regarding patent and other intellectual property rights. If we become involved in such litigation, it could consume a substantial portion of our managerial and financial resources.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
The measures that we use to protect the security of our intellectual property and other proprietary rights may not be adequate, which could result in the loss of legal protection for, and thereby diminish the value of, such intellectual property and other rights.
Our success depends in part on our ability to protect our intellectual property and other proprietary rights. In addition to patent protection, we also rely upon a combination of trademark, trade secret, copyright and unfair competition laws, as well as license agreements and other contractual provisions, to protect our intellectual property and other proprietary rights. In addition, we attempt to protect our intellectual property and proprietary information by requiring our employees, consultants and certain academic collaborators to enter into confidentiality and assignment of inventions agreements. There can be no assurance, however, that such measures will provide adequate protection for our patents, copyrights, trade secrets or other proprietary information. In addition, there can be no assurance that trade secrets and other proprietary information will not be disclosed, that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to or disclose our trade secrets and other proprietary information. To the extent that our intellectual property and other proprietary rights are not adequately protected, third parties might gain access to our proprietary information, develop and market genetic analysis systems similar to our tSMS technology, or use trademarks similar to ours, each of which could materially harm our business. Existing U.S. federal and state intellectual property laws offer only limited protection. Moreover, the laws of other countries in which we may market our technology may afford little or no effective
31
protection of our intellectual property. The failure to adequately protect our intellectual property and other proprietary rights could materially harm our business.
RISKS RELATED TO OWNERSHIP OF OUR COMMON STOCK
Our common stock has been delisted from The NASDAQ Global Market, which negatively impacts the price of our common stock and our ability to access the capital markets.
On June 28, 2010, we received a letter from The NASDAQ Stock Market LLC ("NASDAQ") advising that for the previous 30 consecutive business days, we failed to comply with the minimum market value of listed securities ("MVLS") requirement for continued listing on the NASDAQ Global Market pursuant to NASDAQ Marketplace Rule 5450(b)(2)(A) and that we did not otherwise satisfy the criteria for continued listing pursuant to NASDAQ Marketplace Rule 5450(b). NASDAQ stated in its letter that in accordance with NASDAQ Marketplace Rule 5810(c)(3)(C), we would be provided 180 calendar days, or until December 27, 2010, to regain compliance with the MVLS continued listing requirement. MVLS is calculated by multiplying the total shares outstanding by the daily closing bid price. On October 12, 2010, we received a delisting determination letter from NASDAQ due to our failure to regain compliance with the NASDAQ Global Market's minimum bid price requirement for continued listing as set forth in Listing Rule 5450(a)(1). In addition, we did not hold our annual meeting of stockholders for fiscal 2010 which is an additional NASDAQ continued listing requirement. Based on the unlikely ability for us to meet the NASDAQ MVLS requirements due to the state of our finances and near-term business prospects, on November 12, 2010, we withdrew our appeal to NASDAQ regarding this delisting notice. Therefore, our securities were delisted from the NASDAQ Global Market and the trading of our common stock was suspended before the opening of business on November 16, 2010, and a Form 25-NSE was subsequently filed with the Securities and Exchange Commission on January 21, 2011 which removed our securities from listing and registration on The NASDAQ Stock Market. The delisting of our common stock will significantly affect the ability of investors to trade our securities and will significantly negatively affect the value and liquidity of our common stock. In addition, the delisting of our common stock will materially adversely affect our ability to raise capital on terms acceptable to us or at all. Delisting from the NASDAQ Global Market will also have other negative results, including the potential loss of confidence by suppliers and employees, the loss of institutional investor interest and fewer business development opportunities.
As a result of our most recent financing activities, we are subject to various obligations to creditors and others that are likely to materially reduce the total return on equity for our common stockholders.
Our obligations to make future Risk Premium Payments under the terms of the Risk Premium Agreement, together with our obligations under the credit facility with GE and the Lenders (including the Fourth Amendment), the Note Purchase Agreement, the contingency fee arrangement with Outside IP Litigation Counsel, the Existing IP Licensing Agreements and the Management Incentive Plan, are highly dilutive to our common shareholders and materially reduce their share of any future earnings of our company. The agreements and obligations referenced in this risk factor are more fully described in Note 8 of the Notes to Consolidated Financial Statements, under the caption Bridge Financing and Senior Debt Restructuring, in this Form 10-K.
Our directors and management will exercise significant control over our company, which will limit your ability to influence corporate matters.
Certain of our directors and executive officers and their affiliates collectively control a majority of our outstanding common stock as of December 31, 2010. As a result, these stockholders, if they act together, will be able to influence our management and affairs and all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This
32
concentration of ownership may have the effect of delaying or preventing a change in control of our company and might negatively affect the market price of our common stock.
The market price of our common stock may be volatile, which could result in substantial losses for our stockholders and subject us to securities class action litigation.
Market prices of technology and healthcare companies have been particularly volatile recently. Some of the factors that may cause the market price of our common stock to fluctuate include:
- •
- investors' perception of our ability to successfully implement our new business priorities;
- •
- fluctuations in our quarterly operating results or the operating results of companies perceived to be similar to us;
- •
- changes in estimates of our financial results;
- •
- failure of our technology to achieve or maintain market acceptance or commercial success;
- •
- changes in market valuations of similar companies;
- •
- success of competitive products and services;
- •
- changes in our capital structure, such as future issuances of securities or the incurrence of additional debt;
- •
- announcements by us or our competitors of significant products, contracts, acquisitions or strategic alliances;
- •
- regulatory developments in the United States, foreign countries or both;
- •
- litigation involving our company, our general industry or both;
- •
- additions or departures of key personnel;
- •
- investors' general perception of us; and
- •
- changes in general economic, industry and market conditions.
In addition, if the market for biotechnology and life sciences stocks or the stock market in general experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition or results of operations. If any of the foregoing occurs, it could cause our stock price to fall and may expose us to class action lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
A significant portion of our total outstanding shares may be sold into the public market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
A majority of the shares of our common stock outstanding as of December 31, 2010 may be offered and sold by selling stockholders pursuant to effective registration statements on Forms S-3 declared effective by the SEC. In addition, up to 23,917,179 additional shares of our common stock may be offered and sold pursuant to effective registration statements by selling stockholders upon exercise of warrants. In addition, a majority of the other outstanding shares of our common stock and other warrants are eligible for resale by the holders of those shares pursuant to other effective registration statements or in exempt private transactions.
If our existing stockholders sell substantial amounts of our common stock in the public market, the market price of our common stock could decrease significantly. The perception in the public market that our stockholders might sell shares of common stock could also depress the market price of our
33
common stock. A decline in the price of shares of our common stock might impede our ability to raise capital through the issuance of additional shares of our common stock or other equity securities, and may cause you to lose part or all of your investment in our shares of common stock.
We have registered the issuance of all shares of common stock that we have issued and may issue under our employee option plans. Having registered the issuance of these shares, they can be freely sold in the public market upon issuance. In addition, as of December 31, 2010, there were 277,777 shares of common stock reserved for future issuance as charitable contribution to the Broad Institute, Inc. that will become eligible for sale in the public market to the extent permitted by Rule 144 under the Securities Act of 1933, as amended.
Due to these factors, sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock.
Provisions in our certificate of incorporation and by-laws or Delaware law might discourage, delay or prevent a change of control of our company or changes in our management and, therefore, depress the trading price of our common stock.
Provisions of our certificate of incorporation and by-laws and Delaware law may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares of our common stock. These provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions include:
- •
- a staggered board of directors;
- •
- limitations on the removal of directors;
- •
- advance notice requirements for stockholder proposals and nominations;
- •
- the inability of stockholders to act by written consent or to call special meetings; and
- •
- the ability of our board of directors to make, alter or repeal our by-laws.
The affirmative vote of the holders of at least 75% of our shares of capital stock entitled to vote is necessary to amend or repeal the above provisions of our certificate of incorporation. In addition, our board of directors has the ability to designate the terms of and issue new series of preferred stock without stockholder approval. Also, absent approval of our board of directors, our by-laws may only be amended or repealed by the affirmative vote of the holders of at least 75% of our shares of capital stock entitled to vote. Accordingly, given that our executive officers, directors and their affiliates collectively own a majority of our outstanding common stock, certain of these persons acting together will have the ability to block any such amendment.
In addition, Section 203 of the Delaware General Corporation Law prohibits a publicly-held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person which together with its affiliates owns, or within the last three years has owned, 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner.
The existence of the foregoing provisions and anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock. They could also deter potential acquirers of our company, thereby reducing the likelihood that you could receive a premium for your common stock in an acquisition.
34
We do not currently intend to pay dividends on our common stock and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have never declared or paid any cash dividends on our common stock and do not currently intend to do so for the foreseeable future. We currently intend to invest our future earnings, if any, to fund our growth. Therefore, you are not likely to receive any dividends on your common stock for the foreseeable future and the success of an investment in shares of our common stock will depend upon any future appreciation in its value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
We leased a facility in Cambridge, Massachusetts under a lease arrangement that expired on December 31, 2010. Our current 19,000 square foot facility in Cambridge, Massachusetts houses our headquarters and scientific laboratory. We currently occupy and lease the Cambridge, Massachusetts facility on a tenant-at-will basis. As a result of our process of considering alternatives to our existing long-term strategic focus and limited resources, we are currently evaluating our facility requirements and are working with our landlord in order to avoid any disruption to our occupancy of our facilities.
For additional information regarding obligations under operating leases see Note 7 of the Notes to the Consolidated Financial Statements contained in this Form 10-K.
On August 27, 2010, we filed a lawsuit against Pacific Biosciences of California, Inc. ("Pacific Biosciences") in the United States District Court for the District of Delaware (CA. No. 10-735-SLR) alleging that Pacific Biosciences infringes U.S. Patent Nos. 7,645,596 ("'596 Patent"), 7,037,687 ("'687 Patent"), 7,169,560 ("'560 Patent"), and 7,767,400 ("'400 Patent"). On October 22, 2010, we filed an amended complaint naming Illumina, Inc. ("Illumina") and Life Technologies Corporation ("Life Technologies") as additional defendants. In the amended complaint, we further allege that Life Technologies infringes the '596 Patent, the '687 Patent, and the '560 Patent, and that Illumina infringes the '687 Patent, the '560 Patent, and U.S. Patent No. 7,593,109 ("'109 Patent). We accuse Pacific Biosciences of willful patent infringement based at least on its manufacture, use, and sale of its Single Molecule Real Time ("SMRT™") DNA sequencing technology. We accuse Life Technologies of willful patent infringement based at least on its manufacture, use, and offer for sale of its Single Molecule Real-Time DNA Sequencing of a Quantum-dot Nanocrystal technology for single molecule sequencing of DNA. We accuse Illumina of willful patent infringement based at least on its manufacture, use, offer for sale, sale, and importation of its sequencing technology for single molecule sequencing of DNA including its Genome Analyzer, HiSeq 2000, and its Sq Module in combination with its iScan and HiScan platforms. We are seeking a permanent injunction enjoining each of the defendants from further infringement as well as compensatory and punitive damages, costs, and attorneys' fees, interest, and other relief as determined by the Court. On January 27, 2011, Pacific Biosciences submitted requests to the United States Patent and Trademark Office (the "Patent Office") for inter parties reexamination of the '687 Patent, the '560 Patent, the '596 Patent and the '400 Patent and are seeking a determination that one or more of the claims of those patents are invalid. The Patent Office granted the requests for reexamination of the '400 Patent and the '596 Patent on March 10, 2011, and issued office actions rejecting all claims of the patents. The Patent Office has not yet acted on the reexamination requests for the '687 Patent and the '560 Patent. Discovery opened in the matter on
35
January 12, 2011. The Court conducted a scheduling conference on February 9, 2011 and set a trial date of September 10, 2012.
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock has traded publicly under the symbol "HLCS" since our initial public offering in May 2007. Our securities were delisted from the NASDAQ Global Market and the trading of our common stock was suspended on November 16, 2010, and a Form 25-NSE was subsequently filed with the Securities and Exchange Commission on January 21, 2011 which removed our securities from listing and registration on The NASDAQ Stock Market. Our securities are currently traded under the symbol "HLCS" on the OTCQB which is a market tier for OTC traded companies that are registered and reporting with the SEC. The following table sets forth the range of quarterly high and low sales prices for our common stock in 2010 and 2009 on the NASDAQ Global Market and the OTCQB, as applicable.
| |
2009 | 2010 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
High | Low | High | Low | |||||||||
First quarter |
$ | 1.80 | $ | 0.39 | $ | 1.43 | $ | 0.72 | |||||
Second quarter |
$ | 0.81 | $ | 0.38 | $ | 0.98 | $ | 0.38 | |||||
Third quarter |
$ | 3.54 | $ | 0.40 | $ | 0.85 | $ | 0.38 | |||||
Fourth quarter |
$ | 2.99 | $ | 0.88 | $ | 0.58 | $ | 0.11 | |||||
Holders
As of March 28, 2011, there were approximately 59 holders of record of our common stock, one of which is Cede & Co., a nominee for Depository Trust Company ("DTC"). All of the shares of common stock held by brokerage firms, banks and other financial institutions as nominees for beneficial owners are deposited into participant accounts at DTC and therefore are considered to be held of record by Cede & Co. as one shareholder.
Dividends
We have never declared or paid any cash dividends on our capital stock and do not expect to pay any cash dividends for the foreseeable future. We intend to use future earnings, if any, in the operation and expansion of our business. Any future determination relating to our dividend policy will be made at the discretion of our board of directors, based on our financial condition, results of operations, contractual restrictions, capital requirements, business properties, restrictions imposed by applicable law and other factors our board of directors may deem relevant.
36
Issuer Purchases of Equity Securities
During 2010, we purchased 608,151 restricted shares from employees to cover withholding taxes due from the employees at the time the shares vested. The following table provides information about these purchases of restricted shares for the year ended December 31, 2010:
Period
|
Total Number of Shares Purchased |
Average Price Paid Per Share ($) |
||||||
|---|---|---|---|---|---|---|---|---|
January 1 to 31, 2010 |
128,340 | $ | 1.05 | |||||
February 1 to 28, 2010 |
7,452 | $ | 1.02 | |||||
March 1 to 31, 2010 |
133,254 | $ | 0.79 | |||||
April 1 to 30, 2010 |
62,674 | $ | 0.79 | |||||
May 1 to 31, 2010 |
39,102 | $ | 0.65 | |||||
June 1 to 30, 2010 |
70,491 | $ | 0.44 | |||||
July 1 to 31, 2010 |
104,307 | $ | 0.45 | |||||
August 1 to 31, 2010 |
— | — | ||||||
September 1 to 30, 2010 |
— | — | ||||||
October 1 to 31, 2010 |
62,531 | $ | 0.48 | |||||
November 1 to 30, 2010 |
— | — | ||||||
December 1 to 31, 2010 |
— | — | ||||||
Total |
608,151 | |||||||
Upon the termination of employees during the year ended December 31, 2010, 1,282,223 unvested restricted shares were forfeited. The following table provides information about our forfeited restricted shares for the year ended December 31, 2010:
Period
|
Total Number of Shares Forfeited |
||||
|---|---|---|---|---|---|
January 1 to 31, 2010 |
4,861 | ||||
February 1 to 28, 2010 |
25,070 | ||||
March 1 to 31, 2010 |
— | ||||
April 1 to 30, 2010 |
2,501 | ||||
May 1 to 31, 2010 |
109,441 | ||||
June 1 to 30, 2010 |
5,784 | ||||
July 1 to 31, 2010 |
391 | ||||
August 1 to 31, 2010 |
1,094 | ||||
September 1 to 30, 2010 |
679,406 | ||||
October 1 to 31, 2010 |
302,659 | ||||
November 1 to 30, 2010 |
— | ||||
December 1 to 31, 2010 |
151,016 | ||||
Total |
1,282,223 | ||||
ITEM 6. SELECTED FINANCIAL DATA
Smaller reporting companies are not required to provide disclosure pursuant to this Item.
37
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following "Management's Discussion and Analysis of Financial Condition and Results of Operations", as well as disclosures included elsewhere in this Form 10-K, include "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. This Act provides a safe harbor for forward-looking statements to encourage companies to provide prospective information about themselves so long as they identify these statements as forward-looking and provide meaningful cautionary statements identifying important factors that could cause actual results to differ from the projected results. All statements other than statements of historical fact we make in this Form 10-K are forward-looking. In particular, the statements herein regarding future sales and operating results; our ability to raise capital or finance our operations; Company and industry growth and trends; growth of the markets in which we participate; international events; product performance; the generation, protection and acquisition of intellectual property, and litigation related to such intellectual property; new product introductions; development of new products, technologies and markets; the acquisition of or investment in other entities; the construction of new or refurbishment of existing facilities by us; and statements preceded by, followed by or that include the words "intends", "estimates", "plans", "believes", "expects", "anticipates", "should", "could" or similar expressions, are forward-looking statements. Forward-looking statements reflect our current expectations and are inherently uncertain. Our actual results may differ significantly from our expectations. We assume no obligation to update this forward-looking information. The section entitled "Risk Factors" describes some, but not all, of the factors that could cause these differences.
The following discussion and analysis should be read in conjunction with our historical financial statements and the notes to those financial statements which are included in Item 8 of Part II of this Form 10-K.
BUSINESS OVERVIEW
Helicos BioSciences Corporation ("Helicos" or the "Company") is a life sciences company focused on innovative genetic analysis technologies. Helicos has developed a proprietary technology to enable the rapid analysis of large volumes of genetic material by directly sequencing single molecules of DNA or RNA. Our tSMS approach differs from current methods of sequencing DNA or RNA because it analyzes individual molecules of DNA directly instead of analyzing a large number of copies of the molecule which must be produced through complex sample preparation techniques. Our tSMS technology eliminates the need for costly, labor-intensive and time-consuming sample preparation techniques, such as amplification or cloning, which are required by other methods to produce a sufficient quantity of genetic material for analysis.
Our Helicos® Genetic Analysis Platform is designed to obtain sequencing information by repetitively performing a cycle of biochemical reactions on individual DNA or RNA molecules and imaging the results after each cycle. The platform consists of an instrument called the HeliScope™ Single Molecule Sequencer, an image analysis computer tower called the HeliScope™ Analysis Engine, associated reagents, which are chemicals used in the sequencing process, and disposable supplies. The information generated from using our tSMS products may lead to improved drug therapies, personalized medical treatments and more accurate diagnostics for cancer and other diseases.
As previously disclosed in our quarterly and periodic reports with the Securities and Exchange Commission, in the first quarter of 2010, we began a process of considering alternatives to our long-term strategic focus, including a repositioning of the Company in the genetic analysis markets. As a result of this process, on June 25, 2010, we announced that we had launched our diagnostics business and that we were in early stages of validating molecular diagnostic (MDx) tests that utilize our HeliScope® Single Molecule Sequencer. To date, our research and development team has made
38
progress in developing two prototype tests. The first test is for the detection of gene mutations indicative of a woman's increased risk of developing hereditary breast or ovarian cancer. The second prototype is a non-invasive prenatal diagnostic test. Nevertheless, despite this scientific progress, and a lengthy and comprehensive financing initiative supported by a nationally recognized investment bank during the second and third quarters of 2010, we were unable to secure any long-term equity financings or financing arrangements with strategic partners during the third quarter of 2010. We have had numerous meetings with prospective investors and corporate partners but have not been able to secure a bona-fide offer for financing or a strategic transaction. In addition, we have had very limited commercial activity to date during fiscal year 2010. Further, as of December 31, 2010 and March 24, 2011, we only had $2.5 million and $2.9 million, respectively, in cash and cash equivalents.
As previously disclosed, on May 11, 2010, our Board of Directors (the "Board") approved a reduction in headcount and restructuring plan (the "May Restructuring Plan") which involved the consolidation and reorganization of our operations in order to reduce operating costs. The May Restructuring Plan resulted in the elimination of approximately 40 positions during the second fiscal quarter of 2010. In order to further reduce operating costs and preserve cash, on September 15, 2010, our Board approved a subsequent reduction in headcount and restructuring plan (the "September Restructuring Plan") which involved the consolidation and reorganization of our operations. The September Restructuring Plan eliminated approximately 14 positions during the third quarter of 2010. We entered the first quarter of 2011 with 22 employees. In addition, during the third quarter of 2010, as a result of our inability to complete the financing referenced above and the subsequent change in business direction, we recorded charges of $2.6 million in connection with a write-off of certain of our inventory that is no longer used in the business and $1.9 million in connection with the impairment of certain long-lived assets.
Going forward, we plan to deploy our limited resources by focusing on intellectual property monetization and concentrating our research and development efforts in targeted areas designed to achieve tangible proof-of-concept goals, which may enable us to seek partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives. As of the date of this filing, we do not have adequate resources and will continue to need to generate additional funding from various sources including contract sequencing service projects, government grants, strategic collaborations and licensing activities. On October 14, 2010, we announced the launch of a technology licensing program for our Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. We plan to defend our intellectual property rights through licensing and enforcement strategies. In this regard, we took several actions. First, on September 8, 2010, we filed a lawsuit against Pacific Biosciences of California, Inc. ("Pacific Biosciences") for patent infringement. The lawsuit, filed in the United States District Court for the District of Delaware, accuses Pacific Biosciences of infringing four patents and seeks injunctive relief and monetary damages. Second, on October 14, 2010, we announced the launch of a technology licensing program for our Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. Third, on October 22, 2010, we filed an amended complaint against Pacific Biosciences by naming Illumina, Inc. and Life Technologies Corporation as additional defendants. The amended lawsuit alleges that the defendants willfully infringe our patents in suit by using their respective technologies, sequencing systems and products which we allege are within the scope of one or more claims of our patents in suit. We are seeking a permanent injunction enjoining the defendants from further infringement as well as monetary and punitive damages, costs and attorneys' fees, interests and other relief as determined by the Court. See Part I, Item 3, Legal Proceedings, for more information regarding the litigation. We believe that the proceeds generated by these litigations, if we are successful, could be substantial. We continue to pursue all the above-mentioned opportunities in the IP and technology platform areas.
39
We entered the fourth quarter of 2010 without any prospects of securing long-term financing from outside investors. An independent committee (the "Independent Committee") of our Board evaluated the alternatives available to us and determined that a bridge financing led by certain inside investors was in the best interests of our stakeholders in view of our current financial and operating condition, and would best position us to continue operations for the remainder of 2010 and into 2011. The Independent Committee met multiple times to consider the alternatives and concluded that the bridge financing was the best alternative for generating value for stakeholders. As a result, we entered into an insider bridge loan facility with two of the four venture capital firms who whose representatives are members of the Board. In connection with this transaction, we entered into a Subordinated Secured Note Purchase Agreement (the "Note Purchase Agreement") with investment funds affiliated with Atlas Venture and Flagship Ventures (the "Purchasers"), pursuant to which we have agreed to sell to the Purchasers, and the Purchasers have agreed to purchase, up to an aggregate of $4,000,000 of convertible promissory notes (the "Notes"), a portion of which is committed and a portion of which is optional (the "Bridge Debt Financing"). On November 16, 2010, the Purchasers purchased Notes having an aggregate principal amount of $333,333. Subject to our continued compliance with the terms of the Note Purchase Agreement, including that there not be an event of default thereunder, upon notice from us the Purchasers are committed to purchase an aggregate of an additional $333,333 of Notes in each of five subsequent closings, for a total aggregate committed amount of $2,000,000. The Purchasers also have the right, but not the obligation, upon our request to purchase up to an additional $2,000,000 of Notes on or before December 31, 2012. As of December 31, 2010 and as of the date of this filing, we have borrowed $666,667 against the Bridge Debt Financing facility.
The Notes will accrue interest at a rate of 10% per annum plus a risk premium as described below. The outstanding principal and any unpaid accrued interest on all of the Notes shall be due and payable in full (i) upon demand by the Purchasers, which demand shall be no earlier than December 31, 2012, (ii) upon our receiving at least $10,000,000 in proceeds from a subsequent equity financing, (iii) upon a change of control of the Company or (iv) upon an event of default. A Purchaser may elect to convert principal and interest outstanding under any of the Notes upon the closing of any future round of equity or debt financing of the Company into shares, and at the per share price, of the securities issued at such financing. Simultaneously with the execution of the Note Purchase Agreement, we and the requisite parties entered into an Amendment to the Amended and Restated Investor Rights Agreement, dated as of March 1, 2006, by and among us and the parties specified therein, including the Purchasers (the "IRA Amendment"), pursuant to which the Purchasers will be entitled to demand and piggyback registration rights consistent with the terms of the Purchasers' existing registration rights for any securities issued upon conversion of the Notes.
Our obligations under the Notes are subordinated to the indebtedness incurred by us under the Loan and Security Agreement among us, certain lenders (the "Lenders") and General Electric Capital Corporation ("GE"), as agent, dated as of December 31, 2007, as amended (the "Loan Agreement"). Our obligations under the Notes are secured by a security interest on all of our assets, including our intellectual property, that, with regard to all of our assets other than the cause of action that is the subject of the intellectual property litigation against Pacific Biosciences, Illumina and Life Technologies (and any recovery therefrom) (the "Cause of Action"), is junior to the Lenders' first priority security interest under the Loan Agreement, and with regard to the Cause of Action, is junior to the Lenders' first priority security interest under the Loan Agreement and the second priority security interest of our outside legal counsel that is representing us in the Cause of Action ("Outside IP Litigation Counsel").
As an inducement for the Purchasers to purchase the Notes, we entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement") simultaneously with the execution of, and as required by, the Note Purchase Agreement. Under the terms of the Risk Premium Agreement, we have agreed to pay the Purchasers the following portions of the consideration we receive (the "Payment Consideration") in Liquidity Transactions, as
40
defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30% of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). In connection with the Bridge Debt Financing, on November 16, 2010, our company, GE and the Lenders executed the Fourth Amendment to the Loan and Security Agreement, dated November 16, 2010 (the "Fourth Amendment"). Pursuant to the Fourth Amendment, GE and the Lenders waived various existing events of default and consented to our incurrence of additional indebtedness and the grant of a junior security interest to the Purchasers under the Note Purchase Agreement. We amended warrants previously issued to the Lenders to re-price the Warrants from $4.80 to $0.01 and GE and the Lenders agreed to defer a $200,000 payment due January 31, 2011 to the January 1, 2012 term loan maturity date. In addition, subject to our continued compliance with the terms of the Loan Agreement, including that there not be an event of default thereunder, GE and the Lenders agreed to defer up to five additional principal monthly payments with respect to the term loan such that repayments of principal will re-commence in May 2011. Also, pursuant to the Fourth Amendment, we agreed to grant the Lenders a first priority security interest in all of our intellectual property and to pay the Lenders an additional $50,000 to $200,000 (depending upon how many principal payments are deferred) upon the full repayment of all outstanding amounts due with respect to the term loan. Under the terms of the Loan and Security Agreement prior to its amendment by the Fourth Amendment, the scope of the Lenders' first priority security interest included all of our assets other than intellectual property. As of the date of this filing, the Lenders have agreed to defer the additional five monthly principal payments referenced above.
In connection with the Bridge Debt Financing and the Fourth Amendment and as security for our obligations under our contingency fee arrangement with Outside IP Litigation Counsel relating to the Cause of Action, we entered into a Subordinated Security Agreement with Outside IP Litigation Counsel pursuant to which we agreed to grant Outside IP Litigation Counsel a second priority security interest in the Cause of Action. Under the terms of the contingency fee arrangement, we are obligated to pay Outside IP Litigation Counsel up to 40% of the proceeds paid to us in connection with the settlement, judgment or other resolution of the Cause of Action or certain business transactions resulting from or relating to the Cause of Action.
In approving the transactions relating to the Bridge Debt Financing and the Fourth Amendment, our Board of Directors approved a management incentive plan pursuant to which participants in the plan will be entitled to receive, in the aggregate, an amount equal to 7.5% of the Payment Consideration resulting from IP Licensing Events (which, as noted above, represents gross proceeds from IP Licensing Events less various contractual and contingent payments described above) (the "Management Incentive Plan"). The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the and direction of our Board.
The agreements and arrangements described above and all of the transactions contemplated by those agreements were approved by an independent committee of our Board. In addition, Noubar B. Afeyan, PhD, who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, Ph.D., who is a Partner of Atlas Venture, abstained from the vote of our Board approving the transactions.
Although we believe that the HeliScope Sequencer has unique utility across a broad array of MDx tests, due to significant financial and resource constraints, we have deferred our plan to develop our own genetic testing laboratory certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA). Nevertheless, we still believe that diagnostic applications may benefit from the specific
41
features of the Helicos System, including the platform's ability to sequence non-amplified natural DNA and RNA directly, quantitative accuracy, high throughput, ability to use small sample quantities in simple and cost effective preparation methods, as well as lack of biases typically seen with sample amplification. We will continue to evaluate the best modality of bringing high-value MDx tests based on its technology to the market through a CLIA laboratory, either in partnership or through our own efforts should the financial outlook for us improve, however, there can be no assurance that we will be successful in deploying MDx tests or our technology through a CLIA laboratory.
Notwithstanding recent workforce reductions, restructurings, and the Bridge Debt Financing, we may not have sufficient funds to pursue our business priorities. We will require significant additional capital in the second half of 2011 to continue our operations. As a result, we are no longer able to remain current in making payments to trade vendors and other unsecured creditors when such payments are due and, despite the restructuring of our credit facility with GE discussed above, this inability to remain current increases the likelihood that GE could declare a default under the credit facility in the near-term, including with regard to solvency. These workforce reductions, as well as curtailed financial resources, will further impact our ability to implement our business priorities. In addition, these constraints have caused us to significantly scale back service support and reagent supply to our current installed base. We have also significantly curtailed collaborative activities with other parties. Nevertheless, we believe that these actions will preserve our viability and provide additional time to execute our business priorities.
Our future capital requirements will depend on many factors and we may require additional capital beyond our currently anticipated amounts. Any such required additional capital may not be available on reasonable terms, if at all, given our prospects, the current economic environment and restricted access to capital markets. The continued depletion of our resources may make future funding more difficult or expensive to attain. Additional equity financing may be dilutive to our stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict our ability to operate as a business; and strategic partnerships may result in royalties or other terms which reduce our economic potential from any adjustments to our existing long-term strategy. If we are unable to execute our operations according to our plans or to obtain additional financing, we may be forced to cease operations. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets or the amount of reclassification of liabilities, or any adjustment that might be necessary should we be unable to continue as a going concern.
Since inception, we have incurred losses and have not generated positive cash flows from operations. We expect our losses to continue for a considerable period of time. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and stockholders' equity/(deficit). As of December 31, 2010 and March 24, 2011, we had $2.5 million and $2.9 million, respectively, in cash and cash equivalents. We will, during the second half of 2011, require significant additional capital to continue our operations. Because our present capital resources are not sufficient to fund our planned operations for a twelve month period as of the date of this Annual Report on Form 10-K for the year ended December 31, 2010, our current financial resources raise substantial doubt about our ability to continue as a going concern.
We were incorporated in Delaware in May 2003 under the name RareEvent Medical Corporation, renamed Newco LS6, Inc. in September 2003 and ultimately renamed Helicos BioSciences Corporation in November 2003. Our activities to date have consisted primarily of conducting research and development. Accordingly, we are considered to be in the development stage at December 31, 2010, as defined by the Financial Accounting Standards Board ("FASB"). Our fiscal year ends on December 31, and we operate as one reportable segment. Our corporate offices are located at One Kendall Square, Building 200, Cambridge, Massachusetts 02139.
42
FINANCIAL OVERVIEW
Product revenue
Product revenue for the year ended December 31, 2010 consists primarily of $750,000 of revenue recognized from the sale of one Helicos System for which all acceptance criteria were satisfied during the period and approximately $550,000 of revenue recognized from the sale of proprietary reagents to customers. Product revenue for the year ended December 31, 2009 consists of $1.8 million of revenue recognized from the sale of two instrument systems for which all acceptance criteria were satisfied during the period and $0.5 million of revenue recognized from the sale of proprietary reagents to customers for the year ended December 31, 2009. We recognized of product revenue for the year ended December 31, 2008 of $36,000 related to the sale of proprietary reagents to a customer.
Grant revenue
In September 2006, we were awarded a grant from the National Human Genome Research Institute ("NHGRI"), a branch of the National Institutes of Health ("NIH"), pursuant to which we are eligible to receive reimbursement of our research expenses of up to $2.0 million through August 2009. We recognized revenue during the years ended December 31, 2008 and 2009 of $772,000 and $454,000, respectively, in connection with this award. We have fully expended all available funds under this grant as of December 31, 2009.
In September 2009, we were awarded another grant from the NHGRI, pursuant to which we are eligible to receive reimbursement of our research expenses of up to $2.9 million through August 2011. The two year grant is part of the Sequencing Technology Development Program, representing one of NHGRI's signature projects under the American Recovery and Reinvestment Act's effort to jumpstart the economy and create or save millions of jobs. This project, part of NIH's "Grand Opportunities" program, is designed to support large-scale, high impact research projects that are expected to accelerate critical scientific breakthroughs and enable growth and investment in biomedical research and development. In connection with this award we recognized $247,000 and $ 2.2 million in revenue in the years ended December 31, 2009, and 2010, respectively.
In April 2010, we were awarded an RO1 Research Project Grant from the NHGRI, pursuant to which we are eligible to receive reimbursement for our research expenses of up to $1.6 million through February 2013. We are pursuing research related to direct RNA sequencing under this grant. In connection with this award, we recognized $346,000 as revenue in the year ended December 31, 2010.
In April 2010, we completed a collaborative study that was sponsored by The Children's Oncology Group, under which we were reimbursed for our research expenses and recognized grant revenue of $199,000 in the year ended December 31, 2010. The purpose of the study was to generate large-scale data sets and obtain novel information that would allow deeper insights into cancer genomes with a focus on Ewing's Sarcoma.
In June 2010, we were awarded a Phase 1 Small Business Innovation Research grant from the NHGRI under which we will be pursuing research related to the development of methods to sequence patient DNA and RNA samples at an attomole level. The phase 1 portion of the grant provides funding for eligible research expenses of up to $146,000 through November 2010. Subsequent to December 31, 2010, we were awarded approximately $1.5 million in additional funding under this grant. In connection with this award we recognized $141,000 in grant revenue in the year ended December 31, 2010.
In October 2010, we were awarded three federal grants totaling approximately $722,000 under the Patient Protection and Affordable Care Act of 2010, of which $472,000 was received during the fourth quarter of 2010 and reflected in other income and the balance was received during the first quarter of 2011. The grants were awarded under the Qualified Therapeutic Discovery Project (QDTP) which is
43
aimed towards producing products that diagnose diseases or determine molecular factors related to diseases by developing molecular diagnostics to guide therapeutic decisions.
Cost of product revenue
Cost of product revenue for the years ended December 31, 2008, 2009 and 2010 was $10,000, $1.2 million, and $0.7 million, respectively, and is related to the sale of instruments and proprietary reagents.
Research and development expenses
Research and development expenses consist of costs associated with scientific research activities, and engineering development efforts. Such costs primarily include salaries, benefits and stock-based compensation; lab and engineering supplies; investment in equipment; consulting fees; and facility related costs, including rent and depreciation. During the years ended December 31, 2008, 2009 and 2010, research and development expenses included $4.2 million, $1.9 million, and $0.7 million, respectively, of labor and overhead costs associated with the under-utilization of our manufacturing facility. Research and development expenses for the years ended December 31, 2008, 2009 and 2010 were $24.6 million, $18.3 million and $14.0 million, respectively. In December 2008, we implemented a 30% reduction in our workforce and took other measures to reduce our future operating costs. From 2008 to 2009, our expenses decreased in connection with the reduction in force plan implemented in December 2008, as we focused more of our efforts on manufacturing activities and spent less time and resources on research and development activities. In 2010, we had two additional reductions in force, the economic benefits from which started to be realized in the third quarter of fiscal year 2010 and will be fully realized in fiscal 2011. Additionally, in fiscal years 2008, 2009 and 2010, we recorded charges of $1.6 million, $3.1 million and $2.6 million, respectively, in connection with write-offs of certain inventory or accrual of non-cancellable purchase commitments for inventory items that are no longer used in the business and we included this charge in research and development expense.
In addition to our ongoing research and development efforts, we have incurred start-up manufacturing costs related to the assembly, testing and performance validation of the Helicos System. These costs were accounted for as research and development expenses in our pre-commercialization phase as we prepared to ship the first Helicos System, which occurred on March 5, 2008. We reached technological feasibility of the Helicos System in December 2007 and, as a result, we began to record the cost of the Helicos System in inventory.
We believe that the Helicos System can potentially access a wide range of genetic analysis tests useful to the basic, pharmaceutical, and biomedical research and diagnostic markets. In addition, we have envisioned a series of performance enhancements to the Helicos System and chemistries and consumables used on the initial Helicos System which potentially serve to greatly enhance the sequencing throughput. Each of these research and development projects is dependent upon achieving technical objectives, which are inherently uncertain. As a result of these uncertainties, we are unable to predict to what extent we will receive cash inflows from the sale of future tests or from the future enhanced throughput. As of the date of this Annual Report on Form 10-K, we do not have adequate resources to pursue the further development of these tests or enhanced throughput initiatives.
Impairment of Long-Lived Assets
During the third quarter of 2010, we altered our business direction as a result of our inability to raise funding to support the repositioning of our business in the genetic analysis markets. As a result, we redirected our efforts to focus on a technology licensing and enforcement program to monetize the value of our intellectual property portfolio and to deploy our limited resources by concentrating our research and development efforts in targeted areas designed to achieve tangible proof-of-concept goals,
44
which may enable us to seek partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives.
As a result of this altered business direction, we undertook an analysis of the ongoing carrying value of our long-lived assets. Based on this analysis, during the twelve months ended December 31, 2010, we recorded an impairment charge related to long-lived assets of approximately $1.9 million, of which approximately $1.8 million was related to assets used in research and development and approximately $0.1 million was related to assets used for selling, general and administrative purposes. These charges were derived from the net book value of the assets.
Selling, general and administrative expenses
Selling, general and administrative expenses consist principally of salaries, benefits and stock-based compensation, consulting and professional fees, including patent related costs, general corporate costs and facility costs not otherwise included in research and development expenses or cost of product revenue.
Selling, general and administrative expenses for the years ended December 31, 2008, 2009 and 2010 were $20.1 million, $12.5 million and $10.5 million, respectively. The decrease in fiscal year 2009 was due to the reduction in force plan implemented in December 2008, as well as a significant reduction in costs associated with ERP system enhancements, public company expenses, investor relations programs, and outside consultant costs related to Sarbanes-Oxley compliance. The reduction in fiscal year 2010 was due primarily to the reductions in force implemented during that fiscal year.
Restructuring
In December 2008, we implemented a reduction in force plan that resulted in the reduction of approximately 30% of our workforce and restructuring charges relating to one-time termination benefits of approximately $433,000. These charges represent employee severance and termination costs which were paid out during the fourth quarter of 2008 and the first quarter of 2009.
In fiscal year 2010, we implemented two additional reductions in force as part of our repositioning strategy in the diagnostics market. In May 2010, we eliminated approximately 40 positions and incurred restructuring charges relating to employee severance and termination costs as well as facility consolidation costs of approximately $549,000 in fiscal year 2010, of which $543,000 was recorded in research and development expense and $6,000 was recorded in selling, general and administrative expense. As of December 31, 2010, there were no remaining payments due pursuant to this restructuring.
In September 2010, we implemented a reduction in force of approximately 14 positions and incurred restructuring charges relating to one-time termination benefits of approximately $172,000 all of which was recorded in research and development expense. As of December 31, 2010, $11,000 of such restructurings costs remains to be paid. We estimate the annualized savings from these 2010 restructurings to be approximately $8.2 million.
45
RESULTS OF OPERATIONS
Year ended December 31, 2009 compared to year ended December 31, 2010
Revenues. Revenue for the years ended December 31, 2009 and 2010 were as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 | 2010 | Change | ||||||||||
Product revenue |
$ | 2,325 | $ | 1,301 | (1,024 | ) | -44 | % | |||||
Grant revenue |
701 | 3,096 | 2,395 | 342 | % | ||||||||
|
$ | 3,026 | $ | 4,397 | $ | 1,371 | 45 | % | |||||
Product revenue. We recognized $2.3 million of product revenue during the year ended December 31, 2009, and $1.3 million of product revenue during the year ended December 31, 2010. The $1.0 million decrease from the year ended December 31, 2009 to the year ended December 31, 2010 is due primarily to the recognition of revenue related to the sale of one Helicos System in fiscal year 2010 compared to the recognition of the sales of two Helicos Systems in fiscal year 2009 while sale of proprietary reagents to customers remained essentially constant in both years. As of December 31, 2010, we are no longer actively engaged in the sale of Helicos Systems. As of December 31, 2010, we had $7.2 million in deferred revenue which relates to six Helicos Systems for which all revenue recognition criteria have not been met.
Grant revenue. We recognized $0.7 million of grant revenue during the year ended December 31, 2009, and $3.1 million of grant revenue during the year ended December 31, 2010. We worked on four grants in fiscal year 2010 compared to two grants in fiscal year 2009. Grant revenue is related to the reimbursement of expenses in connection with our government research grants.
Cost of product revenue. Cost of product revenue for the years ended December 31, 2009 and 2010 was as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 | 2010 | Change | ||||||||||
Cost of product revenue |
$ | 1,239 | $ | 713 | $ | (526 | ) | -42 | % | ||||
Cost of product revenue for the year ended December 31, 2009 was $1.2 million compared to $713,000 for the year ended December 31, 2010. In fiscal year 2009, cost of product revenue included the net production cost of two Helicos Systems recognized as sales, the net cost of a system donated to the Broad Institute, Inc., as well as the cost of proprietary reagents shipped to customers and recognized as revenue during this period. In fiscal year 2010, cost of product revenue included the net production cost of one Helicos System recognized as sales as well as the cost of proprietary reagents shipped to customers and recognized as revenue during this period. In March 2011, the Broad Institute, Inc. returned their Helicos System to us. There are no costs to be reflected in the financial statements of the Company related to the return of the Helicos System from the Broad Institute, Inc.
Research and development expenses. Research and development expenses during the years ended December 31, 2009 and 2010 were as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 | 2010 | Change | ||||||||||
Research and development |
$ | 18,256 | $ | 14,047 | $ | (4,209 | ) | -23 | % | ||||
46
Research and development expenses decreased $4.3 million from the year ended December 31, 2009 to the year ended December 31, 2010. This decrease was due primarily to a number of cost reductions implemented during fiscal year 2010 as we shifted our strategic focus toward the molecular diagnostic market. During fiscal year 2010, we significantly reduced research and development activities as we focused our efforts on developing molecular diagnostic tests resulting in a reduction of labor costs of approximately $2.3 million and a related reduction in research support costs of approximately $1.2 million. In connection with the change in our strategic focus, we discontinued the production of Helicos Systems in the first half of fiscal year 2010 which resulted in a reduction of pre-production variances of $1.2 million and the recognition of a further $2.6 million charge for excess and obsolete inventories during the third quarter of 2010, resulting in a decrease of $500,000 in excess and obsolete inventory charges compared to fiscal year 2009. Lastly, as a result of curtailing and ceasing our Helicos System manufacturing operations in early 2010, these costs were classified as research and development expenses for the period.
Selling, general and administrative expenses. Selling, general and administrative expenses during the years ended December 31, 2009 and 2010 were as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 | 2010 | Change | ||||||||||
Selling, general and administrative |
$ | 12,481 | $ | 10,506 | $ | (1,975 | ) | -16 | % | ||||
Selling, general and administrative expenses decreased $2.0 million from the year ended December 31, 2009 to the year ended December 31, 2010. This decrease was due primarily to a significant decrease in expenses related to the field application support group for fiscal year 2010 as a result of the curtailment of our commercial sales activity in early 2010 as we focus on developing a strategy to pursue the molecular diagnostic market. In addition during fiscal year 2010, cost reduction measures of approximately $300,000 related to the 2010 reduction in force activities were implemented and a reduction in depreciation expense of $600,000 was realized due to the expiration of useful lives of a significant portion of the fixed assets utilized by this function during the current fiscal period.
Impairment of long-lived assets.
| |
Year ended December 31, |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 | 2010 | Change | |||||||
Impairment of long-lived assets |
$ | — | $ | 1,894 | $ | 1,894 | ||||
During the third quarter of 2010, we altered our business direction as a result of our inability to raise funding to support the repositioning of our business in the genetic analysis markets. As a result of this altered business direction, we undertook an analysis of the ongoing carrying value of our long lived assets. We determined that the ongoing value of certain laboratory equipment was equivalent to our liquidation value, which we estimated was approximately ten percent of the original purchase price based on the value realized by other similarly situated life science companies that have been forced to realize value through a liquidation process. Based on this analysis, during the twelve months ended December 31, 2010, we recorded an impairment charge related to long lived assets of approximately $1.9 million, of which approximately $1.8 million was related to assets used in research and development and approximately $0.1 million was related to assets used for selling, general and administrative purposes.
47
Interest income. Interest income for the years ended December 31, 2009 and 2010 was as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 | 2010 | Change | ||||||||||
Interest income |
$ | 75 | $ | 19 | $ | (56 | ) | -75 | % | ||||
The decrease in interest income from the year ended December 31, 2009 compared to the year ended December 31, 2010 was due primarily to significantly lower cash balances.
Interest expense. Interest expense for the years ended December 31, 2009 and 2010 was as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 | 2010 | Change | ||||||||||
Interest expense |
$ | 1,044 | $ | 614 | $ | (430 | ) | -41 | % | ||||
The decrease in interest expense from the year ended December 31, 2009 compared to the year ended December 31, 2010 is attributable to the year over year decrease in our outstanding senior debt obligations.
Other income. Other income for the years ended December 31, 2009 and 2010 was as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 | 2010 | Change | |
|||||||||
Other income |
$ | — | $ | 472 | $ | 472 | |||||||
In the year ended December 31, 2010, we received $472,000 from the federal government related to the Therapeutic Discovery Tax Credit pursuant to grants we received under the Patient Protection and Affordable Care Act of 2010.
Change in fair value of warrant liability. Change in fair value of warrant liability for the years ended December 31, 2009 and 2010 was as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2009 | 2010 | Change | ||||||||||
Change in fair value of warrant liability |
$ | 1,959 | $ | 4,118 | $ | 2,159 | 110 | % | |||||
In connection with the September and December 2009 equity offerings, we issued warrants to purchase an aggregate of 6,482,509 shares of common stock. The fair value of the warrants is recorded in the liability section of the balance sheet and was estimated at $5.3 million and $0.2 million at December 31, 2009 and 2010, respectively. The fair value of the warrant liability is determined at the end of each reporting period with the resulting gains and losses recorded as the change in fair value of warrant liability on the income statement. For the years ended December 31, 2009 and 2010, we realized a gain of $2.0 million and $4.1 million, respectively, due to the change in the fair value of the warrant liability. The gain in fiscal year 2009 was principally a result of the decrease of our stock price between the closing dates of the respective equity offerings and December 31, 2009 and the gain in fiscal year 2010 was a result of the decrease of our stock price between December 31, 2009 and December 31, 2010. Future changes in the fair value of the warrant liability will be due primarily to fluctuations in the value of our common stock.
48
Year ended December 31, 2008 compared to year ended December 31, 2009
Revenues. Revenue for the years ended December 31, 2008 and 2009 were as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2008 | 2009 | Change | |
|||||||||
Product revenue |
$ | 36 | $ | 2,325 | 2,289 | ||||||||
Grant revenue |
772 | 701 | (71 | ) | |||||||||
|
$ | 808 | $ | 3,026 | $ | 2,218 | |||||||
Product revenue. We recognized $36,000 of product revenue during the year ended December 31, 2008, and $2.3 million of product revenue during the year ended December 31, 2009. The $2.3 million increase from the year ended December 31, 2008 to the year ended December 31, 2009 is due to the recognition of revenue from the sale of two Helicos Systems for which all acceptance criteria were satisfied during the period. We recognized our first commercial revenue from the sale of Helicos Systems in 2009. No system sales occurred in 2008. Included in product revenue recognized during the year ended December 31, 2009 was $0.5 million related to the sale of proprietary reagents to customers. Total product revenue recognized during the year ended December 31, 2008 was related to the sale of proprietary reagents to a customer.
Grant revenue. We recognized $772,000 of grant revenue during the year ended December 31, 2008, and $701,000 of grant revenue during the year ended December 31, 2009. Grant revenue recognized during the years ended December 31, 2008 and 2009 related to the reimbursement of expenses in connection with our government research grant.
Cost of product revenue. Cost of product revenue for the years ended December 31, 2008 and 2009 was as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2008 | 2009 | Change | |
|||||||||
Cost of product revenue |
$ | 10 | $ | 1,239 | $ | 1,229 | |||||||
Cost of product revenue for the year ended December 31, 2008 was $10,000, compared to $1.2 million for the year ended December 31, 2009. During 2009 we recognized our first commercial revenue from the sale of two Helicos Systems and reagents. Cost of product revenue includes the net production cost of these systems, the net cost of the system donated to the Broad Institute, Inc., as well as the cost of proprietary reagents shipped to customers and recognized as revenue during this period.
Research and development expenses. Research and development expenses during the years ended December 31, 2008 and 2009 were as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2008 | 2009 | Change | ||||||||||
Research and development |
$ | 24,615 | $ | 18,256 | $ | (6,359 | ) | -26 | % | ||||
Research and development expenses decreased by $6.4 million from the year ended December 31, 2008 to the year ended December 31, 2009. This decrease was due primarily to a number of cost reductions associated with the reduction in force plan implemented in late 2008 as well as reductions related to lower pre-production activities as commercial production activities increased in 2009. The 2008 reduction in force plan resulted in 2009 salary and benefit expense savings of $2.2 million and
49
related support cost savings of $3.6 million that included lower lab expenses, materials, supplies, temporary help and prototype expenses. Our product development costs also decreased as we increased manufacturing and production activities related to a higher rate of system shipments in 2009. This improved activity level resulted in a decrease in research and development expenses of $2.0 million associated with the under-utilization of the manufacturing facility. In addition, we incurred a $3.1 million charge for excess and obsolete inventories during 2009, resulting in an increase of $1.5 million in excess and obsolete inventories compared the prior period. This $3.1 million charge was recorded as a research and development expense and resulted in a $1.7 million decrease to inventory and a $1.4 million increase to accrued expenses for excess non-cancelable purchase commitments.
Selling, general and administrative expenses. Selling, general and administrative expenses during the years ended December 31, 2008 and 2009 were as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2008 | 2009 | Change | ||||||||||
Selling, general and administrative |
$ | 20,139 | $ | 12,481 | $ | (7,658 | ) | -38 | % | ||||
Selling, general and administrative expenses decreased by $7.7 million from the year ended December 31, 2008 to the year ended December 31, 2009. This decrease was principally due to the reduction in force plan implemented in late 2008. Under this plan, our salary and benefit expenses decreased by $2.9 million and other related support costs were reduced by $4.5 million. These reduced support costs related to lower spending on the following activities: investor relations programs, business insurance, outside consulting fees associated with Sarbanes-Oxley compliance, certain outside legal expenses, marketing programs, and travel. These decreases were slightly offset by a $443,000 increase in stock-based compensation expense from the year ended December 31, 2008 to the year ended December 31, 2009.
Interest income. Interest income for the years ended December 31, 2008 and 2009 was as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2008 | 2009 | Change | ||||||||||
Interest income |
$ | 670 | $ | 75 | $ | (595 | ) | -89 | % | ||||
The decrease in interest income from the year ended December 31, 2008 compared to the year ended December 31, 2009 was due primarily to lower interest rates, and to a lesser extent, our lower cash balances.
Interest expense. Interest expense for the years ended December 31, 2008 and 2009 was as follows:
| |
Year ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2008 | 2009 | Change | ||||||||||
Interest expense |
$ | 2,365 | $ | 1,044 | $ | (1,321 | ) | -56 | % | ||||
The decrease in interest expense from the year ended December 31, 2008 compared to the year ended December 31, 2009 is attributable to the year over year decrease in our long-term debt obligations.
50
Change in fair value of warrant liability. Change in fair value of warrant liability for the years ended December 31, 2008 and 2009 was as follows:
| |
Year ended December 31, |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ($ in thousands) |
2008 | 2009 | Change | |||||||
Change in fair value of warrant liability |
$ | — | $ | 1,959 | $ | 1,959 | ||||
In connection with the September and December 2009 equity offerings, we issued warrants to purchase an aggregate of 6,482,509 shares of common stock. The fair value of the warrants is recorded in the liability section of the balance sheet and at December 31, 2009 was estimated at $5.3 million. The fair value of the warrant liability will be determined at the end of each reporting period with the resulting gains and losses recorded as the change in fair value of warrant liability on the income statement. For the year ended December 31, 2009, we realized a gain of $2.0 million due to the change in the fair value of the warrant liability. This gain is principally a result of the decrease of our stock price between the closing dates of the respective equity offerings and December 31, 2009. Future changes in the fair value of the warrant liability will be due primarily to fluctuations in the value of our common stock.
LIQUIDITY AND CAPITAL RESOURCES
We have incurred losses since our inception in May 2003 and, as of December 31, 2010, we have an accumulated deficit of $186.4 million. We have financed our operations to date principally through the sale of common stock and preferred stock, including our IPO, two private placements of common stock and warrants, an underwritten offering of common stock and warrants, debt financings and interest earned on investments and revenue. Through December 31, 2010, we have received net proceeds of $80.3 million through the issuance of common stock, including our IPO, private placements and an underwritten offering of common stock and warrants, $66.4 million from the issuance of preferred stock, $2.5 million in debt financing from a lender to finance equipment purchases, $20.3 million in debt financing from lenders for working capital, capital expenditures and general corporate purposes and $9.0 million of revenue. Working capital as of December 31, 2009 was $11.4 million, consisting of $24.5 million in current assets and $13.1 million in current liabilities. Working capital deficit as of December 31, 2010 was $(5.6) million, consisting of $6.7 million in current assets and $12.3 million in current liabilities. Our cash and cash equivalents are held in interest-bearing cash accounts. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily to achieve liquidity and capital preservation.
The following table summarizes our net increase (decrease) in cash and cash equivalents for the years ended December 31, 2008, 2009, 2010 and for the period from May 9, 2003 (date of inception) through December 31, 2010:
| |
|
|
|
Period from May 9, 2003 (date of inception) through December 31, 2010 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31, | |||||||||||||
| ($ in thousands) |
2008 | 2009 | 2010 | |||||||||||
Net cash provided by (used in): |
||||||||||||||
Operating activities |
$ | (44,301 | ) | $ | (15,488 | ) | (11,967 | ) | $ | (137,841 | ) | |||
Investing activities |
(2,664 | ) | (22 | ) | 231 | (9,508 | ) | |||||||
Financing activities |
13,995 | 11,727 | (1,681 | ) | 149,862 | |||||||||
Net increase (decrease) in cash and cash equivalents |
$ | (32,970 | ) | $ | (3,783 | ) | $ | (13,417 | ) | $ | 2,513 | |||
51
Net cash used in operating activities was $15.5 million for the twelve months ended December 31, 2009 compared to $12.0 million for the twelve months ended December 31, 2010. The $3.5 million decrease was primarily due to a decrease in the net loss of $9.2 million and the $1.9 million impairment of long-lived assets in fiscal year 2010 partially offset by a $1.3 million decrease in depreciation and amortization and a $2.2 million increase related to the change in the fair value of the warrant liability. Additionally offsetting the decrease in cash used by operating activities was $4.8 million of net changes in operating assets and liabilities, primarily comprised of $3.6 million from deferred revenue and $3.0 million from accrued expenses and other current liabilities.
Net cash provided by investing activities of $231,000 is primarily due to a $225,000 reduction of restricted cash.
Net cash provided from financing activities was $11.7 million for the twelve months ended December 31, 2009 compared to net cash usage of $1.7 million for the twelve months ended December 31, 2010. The $13.4 million decrease was primarily due to $15.0 million of proceeds from the issuance of common stock and warrants in fiscal year 2009, partially offset by a decrease in debt payments of $1.0 million and the Bridge Debt Financing of $667,000 in fiscal year 2010.
Operating capital and capital expenditure requirements
Since inception, we have incurred losses and have not generated positive cash flows from operations. We expect our losses to continue for a considerable period of time. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and stockholders' equity/(deficit). As of December 31, 2010 and March 24, 2011, we had $2.5 million and $2.9 million, respectively, in cash and cash equivalents. We will, during the second half of 2011, require significant additional capital to continue our operations. Because our present capital resources are not sufficient to fund our planned operations for a twelve month period as of the date of this Annual Report on Form 10-K for the year ended December 31, 2010, our current financial resources raise substantial doubt about our ability to continue as a going concern.
Notwithstanding recent workforce reductions, restructurings, and the Bridge Debt Financing, we may not have sufficient funds to pursue our business priorities. We will require significant additional capital in the second half of 2011 to continue our operations. As a result, we are no longer able to remain current in making payments to trade vendors and other unsecured creditors when such payments are due and, despite the restructuring of our credit facility with GE discussed above, this inability to remain current increases the likelihood that GE could declare a default under the credit facility in the near-term, including with regard to solvency. These workforce reductions, as well as curtailed financial resources, will further impact our ability to implement our business priorities. In addition, these constraints have caused us to significantly scale back service support and reagent supply to our current installed base. We have also significantly curtailed collaborative activities with other parties. Nevertheless, we believe that these actions will preserve our viability and provide additional time to execute our business priorities.
On June 28, 2010, we received a letter from The NASDAQ Stock Market LLC ("NASDAQ") advising that for the previous 30 consecutive business days, we failed to comply with the minimum market value of listed securities ("MVLS") requirement for continued listing on the NASDAQ Global Market pursuant to NASDAQ Marketplace Rule 5450(b)(2)(A) and that we did not otherwise satisfy the criteria for continued listing pursuant to NASDAQ Marketplace Rule 5450(b). NASDAQ stated in its letter that in accordance with NASDAQ Marketplace Rule 5810(c)(3)(C), we would be provided 180 calendar days, or until December 27, 2010, to regain compliance with the MVLS continued listing requirement. MVLS is calculated by multiplying the total shares outstanding by the daily closing bid price. On October 12, 2010, we received a delisting determination letter from NASDAQ due to our failure to regain compliance with the NASDAQ Global Market's minimum bid price requirement for
52
continued listing as set forth in Listing Rule 5450(a)(1). In addition, we had not yet held our annual meeting of stockholders for fiscal 2010 which is an additional requirement for us to comply with in order to maintain its continued listing on NASDAQ. Based on the unlikely ability of us to meet the NASDAQ MLVS requirements due to the state of our finances and near-term business prospects, on November 12, 2010, we withdrew our appeal to NASDAQ regarding this delisting notice. As a result, our securities were delisted from the NASDAQ Global Market and the trading of our common stock was suspended before the opening of business on November 16, 2010, and a Form 25-NSE was subsequently filed with the Securities and Exchange Commission on January 21, 2011 which removed our securities from listing and registration on The NASDAQ Stock Market. We were was advised by Pink OTC Markets Inc., which operates an electronic quotation service for securities traded over-the-counter ("OTC"), that our securities were immediately eligible for quotation on the OTCQB. The OTCQB is a market tier for OTC traded companies that are registered and reporting with the Securities and Exchange Commission.
Our future capital requirements will depend on many factors and we may require additional capital beyond our currently anticipated amounts. Any such required additional capital may not be available on reasonable terms, if at all, given our prospects, the current economic environment and restricted access to capital markets. The continued depletion of our resources may make future funding more difficult or expensive to attain. Additional equity financing may be dilutive to our stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict our ability to operate as a business; and strategic partnerships may result in royalties or other terms which reduce our economic potential from any adjustments to our existing long-term strategy. If we are unable to execute our operations according to our plans or to obtain additional financing, we may be forced to cease operations. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets or the amount of reclassification of liabilities, or any adjustment that might be necessary should we be unable to continue as a going concern.
Failure to satisfy our capital requirements or any delay in doing so would have a material negative impact on our ability to continue as a going concern.
Through March 22, 2011, we have received cumulative sales orders for ten Helicos Systems. During 2010 and from our inception to date, we recognized revenue on three of the ten sales orders, respectively. We have shipped six units that have not met our revenue recognition criteria and one sales order unit was returned. A system that was installed at The Broad Institute, Inc. on a no-cost basis was returned to us in March 2011. In addition, we had one Helicos System at a leading academic institution for scientific and commercial evaluation which was returned to us in March 2011.
The continued depletion of our resources make future funding more difficult or expensive to attain, if at all. Additional equity financing would be dilutive to our stockholders; debt financing, if available, would involve significant cash payment obligations and covenants that restrict our ability to operate as a business; and strategic partnerships would result in royalties or other terms which reduce our economic potential. If we are unable to execute our operations according to our plans or to obtain additional financing, we will be forced to cease operations.
Our forecast of the period of time through which our financial resources will be adequate to support our operations, and the costs associated with our new business priorities are forward-looking statements and involve risks and uncertainties, and actual results could vary materially and negatively as a result of a number of factors, including the factors discussed in the "Risk Factors" section of this Annual Report on Form 10-K. We have based these estimates on assumptions that may prove to be wrong, and we could consume our available capital resources sooner than we currently expect.
Because of the numerous risks and uncertainties associated with our business we are unable to estimate the exact amounts of capital outlays and operating expenditures necessary to sustain our
53
operations. Our future capital requirements will depend on many factors, including, but not limited to, the following:
- •
- any litigation related to defending our intellectual property or defending lawsuits that allege our actions infringe
intellectual property of third parties;
- •
- the success of our research and development efforts;
- •
- the expenses we incur in marketing and selling our products or services;
- •
- the revenue generated by future sales of our products or services;
- •
- the timeliness of payments from our customers;
- •
- the ability to structure arrangements with and make payments to our unsecured creditors;
- •
- the continued deferrals by our senior lendors of our monthly principal payments under the Fourth Amendment which are
currently scheduled to resume in May 2011 and the ability to further restructure this lending arrangement in the future;
- •
- the continued funding to the Bridge Debt Financing facility under the Note Purchase Agreement;
- •
- the emergence of competing or complementary technological developments;
- •
- the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
- •
- regulatory changes in our markets; and
- •
- the terms and timing of any collaborative, licensing or other arrangements that we may establish.
Contractual Obligations
The following table summarizes our outstanding obligations as of December 31, 2010 and the effect those obligations are expected to have on our liquidity and cash flows in future periods:
Contractual obligations
|
Total | Less than 1 year |
1-3 years | 3-5 years | More than 5 years |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
($ in thousands) |
||||||||||||||||
Purchase commitments |
$ | 539 | $ | 539 | $ | — | $ | — | $ | — | |||||||
Debt (including interest) |
3,600 | 2,779 | 821 | — | — | ||||||||||||
License agreements(1) |
1,346 | 187 | 360 | 345 | 455 | ||||||||||||
Total |
$ | 5,485 | $ | 3,505 | $ | 1,181 | $ | 345 | $ | 455 | |||||||
- (1)
- Consists of fixed payments that we believe we are reasonably likely to make under the license agreements over the lives of the underlying existing patents.
The table above does not include possible royalties payable under our license agreements or contingent payments which may become due in connection with Risk Premium Payments due under the Risk Premium Agreement or proceeds derived from the current pending intellectual property litigation. We currently occupy and lease our corporate facilities on a tenant-at-will basis.
License agreements and patents
We have fixed annual costs associated with license agreements into which we have entered. In addition, we may have to make contingent payments in the future upon the realization of certain milestones or royalties payable under these agreements.
54
Loan and security agreement
In December 2007, we entered into a loan and security agreement with two lenders including GE Capital Corporation, which is serving as agent. The loan agreement provided that we may borrow up to $20.0 million at an interest rate equal to the sum of (i) the greater of (A) an interest rate based on the Federal Reserve's three year Treasury Constant Maturities Rate and (B) 3.84% plus (ii) 6.11%. The initial term loan was made on the closing date in an aggregate principal amount equal to $10.0 million.
In June 2008, we entered into an amendment to the loan and security agreement with two lenders including GE Capital Corporation. A subsequent term loan was made upon execution of the amendment in an aggregate principal amount equal to $10.0 million. The loan amendment provided that the interest rate for the subsequent term loan is equal to the sum of (i) the greater of (A) an interest rate based on the Federal Reserve's three year Treasury Constant Maturities Rate and (B) 3.17% plus (ii) 8.33%. The loan agreement, as amended, contained affirmative and negative covenants to which we and our subsidiaries were required to adhere. Pursuant to the amendment, we were required to maintain, at all times, unrestricted cash in our bank account equal to at least $10.0 million. The borrowings under the loan agreement were collateralized by essentially all of our assets. Payments are required to be made on a monthly basis. For the initial term loan, interest-only payments were required for the first five months. Thereafter, for the following 31 months, payments of principal and interest will be due. For the subsequent term loan, principal and interest payments are required for the 36 month term of the loan.
In connection with the execution of the June 2008 amendment to the loan and security agreement with two lenders including GE Capital Corporation, we issued warrants to the two lenders to purchase an aggregate of 110,000 shares of common stock. The warrants have an exercise price of $4.80 per share and expire in June 2014. The fair value of the warrants was estimated at $337,000 using a Black-Scholes model with the following assumptions: expected volatility of 65.4%, risk free interest rate of 3.4%, expected life of six years and no dividends. Expected volatility was based on the volatility of similar entities in the life sciences industry of comparable size of market capitalization and financial position that completed initial public offerings within the last ten years. The fair value of the warrants was recorded as equity and a debt discount and is being amortized to interest expense over the term of the loan.
In December 2008, we entered into an additional amendment to the loan and security agreement with certain lenders including GE Capital Corporation. The amendment amended the prepayment provisions of the loan and security agreement to allow us to make a prepayment of $10.0 million (the "Pay Down Amount") without incurring any prepayment penalties. Pursuant to the amendment, we made a prepayment, equal to the Pay Down Amount, before December 31, 2008. In connection with such prepayment, and in lieu of the 4% final payment fee with respect to the Pay Down Amount, the amendment provides that we will pay a fee equal to 2% of the initial $10.0 million term loan, payable on the earlier of (a) January 31, 2011 and (b) the maturity date for the subsequent term loan.
The amendment further provides that our obligations under the loan agreement, as amended, are no longer secured by a cash amount of $10.0 million. Such obligations continue to be secured under various collateral documents by interests in substantially all of our personal property, including the pledge of the stock of our wholly-owned subsidiary, and proceeds of any intellectual property, but not by our intellectual property.
As further discussed below under the caption "Bridge Financing and Senior Debt Restructuring (November 2010)," we restructured the loan and security agreement during the fourth quarter of 2010.
The loan and security agreement contains a subjective material adverse clause, which allows the debt holders to call the loans upon a material adverse event. Based on an updated assessment of the
55
terms of the loan and security agreement, the entire balance due is classified in the current liabilities section in the accompanying balance sheet at December 31, 2010.
As of December 31, 2009 and 2010, the outstanding balance on the loan agreement was $4.9 million and $2.8 million, respectively. Repayment of principal under the loan is scheduled to resume in May 2011.
Bridge Financing and Senior Debt Restructuring (November 2010)
We entered the fourth quarter of 2010 without any prospects of securing long-term financing from outside investors. An independent committee (the "Independent Committee") of our Board evaluated the alternatives available to us and and concluded that the bridge financing led by certain inside investors was in the best interests of our stakeholders in view of our current financial and operating condition, and would best position us to continue operations for the remainder of 2010 and into 2011. The Independent Committee met multiple times to consider the alternatives and reached the conclusion that the bridge financing was the best alternative for generating value for stakeholders. As a result, we entered into an insider bridge loan facility with two of the four venture capital firms whose representatives are members of our Board. In connection with this transaction, we entered into a Subordinated Secured Note Purchase Agreement (the "Note Purchase Agreement") with investment funds affiliated with Atlas Venture and Flagship Ventures (the "Purchasers"), pursuant to which we have agreed to sell to the Purchasers, and the Purchasers have agreed to purchase, up to an aggregate of $4,000,000 of convertible promissory notes (the "Notes"), a portion of which is committed and a portion of which is optional (the "Bridge Debt Financing"). On November 16, 2010, the Purchasers purchased Notes having an aggregate principal amount of $333,333. Subject to our continued compliance with the terms of the Note Purchase Agreement, including that there not be an event of default thereunder, upon notice from us the Purchasers are committed to purchase an aggregate of an additional $333,333 of Notes in each of five subsequent closings, for a total aggregate committed amount of $2,000,000. The Purchasers also have the right, but not the obligation, upon our request to purchase up to an additional $2,000,000 of Notes on or before December 31, 2012. As of December 31, 2010 and as of the date of this filing, we have borrowed $666,667 against the Bridge Debt Financing facility.
The Notes will accrue interest at a rate of 10% per annum plus a risk premium as described below. The outstanding principal and any unpaid accrued interest on all of the Notes shall be due and payable in full (i) upon demand by the Purchasers, which demand shall be no earlier than December 31, 2012, (ii) upon our receiving at least $10,000,000 in proceeds from a subsequent equity financing, (iii) upon a change of control of the Company or (iv) upon an event of default. A Purchaser may elect to convert principal and interest outstanding under any of the Notes upon the closing of any future round of equity or debt financing of the Company into shares, and at the per share price, of the securities issued at such financing. Simultaneous with execution of the Note Purchase Agreement, we and the requisite parties entered into an Amendment to the Amended and Restated Investor Rights Agreement, dated as of March 1, 2006, by and among us and the parties specified therein, including the Purchasers (the "IRA Amendment"), pursuant to which the Purchasers will be entitled to demand and piggyback registration rights consistent with the terms of the Purchasers' existing registration rights for any securities issued upon conversion of the Notes.
Our obligations under the Notes are subordinated to the indebtedness incurred by us under the Loan and Security Agreement among us, certain lenders (the "Lenders") and General Electric Capital Corporation ("GE"), as agent, dated as of December 31, 2007, as amended (the "Loan Agreement"). Our obligations under the Notes are secured by a security interest on all of our assets, including our intellectual property, that, with regard to all of our assets other than the cause of action that is the subject of the intellectual property litigation against Pacific Biosciences, Illumina and Life Technologies (and any recovery therefrom) (the "Cause of Action"), is junior to the Lenders' first priority security
56
interest under the Loan Agreement, and with regard to the Cause of Action, is junior to the Lenders' first priority security interest under the Loan Agreement and the second priority security interest of our outside legal counsel that is representing us in the Cause of Action ("Outside IP Litigation Counsel").
As an inducement for the Purchasers to purchase the Notes, we entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement") simultaneously with the execution of, and as required by, the Note Purchase Agreement. Under the terms of the Risk Premium Agreement, we have agreed to pay the Purchasers the following portions of the consideration we receive (the "Payment Consideration") in Liquidity Transactions, as defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30% of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). For purposes of determining amounts payable under the Risk Premium Agreement, the Payment Consideration will not include amounts we use to first satisfy specified existing obligations, including obligations under the Loan Agreement, under professional contingency arrangements (including the contingency fee arrangement between us and Outside IP Litigation Counsel relating to the Cause of Action) and obligations that may arise under existing technology license agreements (the "Existing IP Licensing Agreements") between us and each of Arizona Technology Enterprises, Roche Diagnostics GMBH and Roche Diagnostics Corporation, and California Institute of Technology (as previously disclosed by us, under the Existing IP Licensing Agreements, we are obligated to pay license fees in connection with the licensing, sublicensing, legal settlements or sales of specified intellectual property or services provided to third parties ranging from a single digit percentage up to one-half of the income from those activities). For purposes of the Risk Premium Agreement and subject to various provisions therein, the following shall constitute Liquidity Transactions: (A) the receipt by us of any payments from third parties relating to our intellectual property portfolio of the Company, including payments received as a license or other settlement in patent infringement litigation brought by us ("IP Licensing Events"); (B) a merger of the Company in which there is a change of control; and (C) the sale or exclusive license by us of all or substantially all of our assets or intellectual property.
In connection with the Bridge Debt Financing, on November 16, 2010, our company, GE and the Lenders executed the Fourth Amendment to the Loan and Security Agreement, dated November 16, 2010 (the "Fourth Amendment"). Pursuant to the Fourth Amendment, GE and the Lenders waived various existing events of default and consented to our incurrence of additional indebtedness and the grant of a junior security interest to the Purchasers under the Note Purchase Agreement. We amended warrants previously issued to the Lenders to re-price the Warrants from $4.80 to $0.01 and GE and the Lenders agreed to defer a $200,000 payment due January 31, 2011 to the January 1, 2012 term loan maturity date. In addition, subject to our continued compliance with the terms of the Loan Agreement, including that there not be an event of default thereunder, GE and the Lenders agreed to defer up to five additional principal monthly payments with respect to the term loan such that principal payments will resume in May 2011. Also pursuant to the Fourth Amendment, we agreed to grant the Lenders a first priority security interest in all of our intellectual property and to pay the Lenders an additional $50,000 to $200,000 (depending upon how many principal payments are deferred) upon the full repayment of all outstanding amounts due with respect to the term loans. Under the terms of the Loan and Security Agreement prior to its amendment by the Fourth Amendment, the scope of the Lenders' first priority security interest included all of our assets other than intellectual property. As of the date of this filing, the Lenders have agreed to defer the additional five monthly principal payments referenced above.
57
In connection with the Bridge Debt Financing and the Fourth Amendment and as security for our obligations under our contingency fee arrangement with Outside IP Litigation Counsel relating to the Cause of Action, we entered into a Subordinated Security Agreement with Outside IP Litigation Counsel pursuant to which we agreed to grant Outside IP Litigation Counsel a second priority security interest in the Cause of Action. Under the terms of the contingency fee arrangement, we are obligated to pay Outside IP Litigation Counsel up to 40% of the proceeds paid to us in connection with the settlement, judgment or other resolution of the Cause of Action or certain business transactions resulting from or relating to the Cause of Action.
In approving the transactions relating to the Bridge Debt Financing and the Fourth Amendment, our Board of Directors approved a management incentive plan pursuant to which participants in the plan, including Dr. Ivan Trifunovich and Mr. Jeffrey Moore, will be entitled to receive, in the aggregate, an amount equal to 7.5% of the Payment Consideration resulting from IP Licensing Events (which, as noted above, represents gross proceeds from IP Licensing Events less various contractual and contingent payments described above) (the "Management Incentive Plan"). The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the Board.
The agreements and arrangements described above and all of the transactions contemplated by those agreements were approved by an independent committee of our Board. In addition, Noubar B. Afeyan, PhD, who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, Ph.D., who is a Partner of Atlas Venture, abstained from the vote of our Boardapproving the transactions.
OFF-BALANCE SHEET ARRANGEMENTS
During the twelve month periods ended December 31, 2009 and 2010, we did not have any special purpose entities or off-balance sheet financing arrangements.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our management's discussion and analysis of our financial condition and results of operations is based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported expenses during the reporting periods. We evaluate our estimates and judgments on an ongoing basis. Actual results may differ materially from these estimates under different assumptions or conditions.
Inventory
Prior to reaching technological feasibility, start-up manufacturing costs, such as those relating to the assembly, testing and performance validation of the Helicos® Genetic Analysis System, were expensed to research and development as the costs were incurred. When management determined that the Helicos System was ready for commercial launch during December 2007, we began capitalizing our manufacturing costs to inventory.
We value our inventory at the lower of cost or market on a first-in, first-out basis. Our policy is to capitalize inventory costs associated with its products when, based on management's judgment, future commercialization is considered probable and the future economic benefit is expected to be realized.
We periodically review our inventories for excess or obsolete inventory and writes-down obsolete or otherwise unmarketable inventory to its estimated net realizable value. If the actual net realizable
58
value is less than that estimated by us, or if there are any further determinations that inventory will not be marketable based on estimates of demand, additional inventory write-downs will be required.
We value inventory in accordance with FASB guidance on inventory costs and inventory pricing to clarify that abnormal amounts of idle facility expense, freight, handling costs, and wasted material (spoilage) should be recognized as current-period charges regardless of whether they meet the criterion of "so abnormal." In addition, guidance requires that allocation of fixed production overheads to the costs of conversion be based on the normal capacity of the production facilities.
We concluded that because we are not yet functioning under normal capacity, our production level is abnormally low. As such, abnormal costs such as the unfavorable labor, overhead and absorption variances were recognized as current period charges, rather than as a portion of the inventory cost. During the years ended December 31, 2008, 2009 and 2010, research and development expenses included $4.2 million, $1.9 million, and $0.7 million, respectively, of labor and overhead costs associated with the under-utilization of the manufacturing facility. During fiscal year 2010, we ceased manufacturing Helicos Systems. During the year ended December 31, 2008, we recorded a $1.6 million charge to research and development expense to write-off excess and obsolete inventory. During the year ended December 31, 2009, we recorded a $3.1 million charge to write-off excess and obsolete inventory. This charge was recorded as a research and development expense resulted in a $1.7 million decrease to inventory and a $1.4 million increase to accrued expenses for excess non-cancellable purchase commitments. During the year ended December 31, 2010, we recorded a $2.6 million charge research and development expense to write-off excess and obsolete inventory.
Revenue recognition
Government research grants that provide for payments to us for work performed are recognized as revenue when the related expenses are incurred.
We recognize revenue in accordance with FASB accounting guidance on revenue recognition in financial statements and accounting for multiple element revenue arrangements. This guidance requires that persuasive evidence of a sales arrangement exists; delivery of goods occurs through transfer of title and risk and rewards of ownership, the selling price is fixed or determinable and collectability is reasonably assured. We also follow FASB guidance on how to account for arrangements that involve the delivery or performance of multiple products, services and/or rights to use assets.
In instances where we sell instruments with a related installation obligation, we will allocate the revenue between the instrument and the installation based on relative fair value at the time of the sale. The instrument revenue will be recognized when title and risk of loss passes. The installation revenue will be recognized when the installation is performed. If fair value is not available for any undelivered element, revenue for all elements is deferred until delivery and installation are complete.
In instances where we sell an instrument with specified acceptance criteria, we will defer revenue recognition until such acceptance has been obtained.
Revenue from service contracts sold in conjunction with the sale of equipment is recognized ratably over the service period.
Impairment of long-lived assets
Long-lived assets primarily include property and equipment. In accordance with FASB accounting guidance for the impairment or disposal of long-lived assets, we periodically review long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives are no longer appropriate. Each impairment test is based on a comparison of the undiscounted cash flows expected to result from the use and eventual disposition of the asset to the carrying amount of the asset. If impairment is
59
indicated, the asset is written down to its estimated fair value based on a discounted cash flow analysis. Determining the fair value of long-lived assets includes significant judgment by management, and different judgments could yield different results. In fiscal year 2010, we incurred a charge for impairment of property and equipment of $1.9 million (see Note 5 of Notes to Consolidated Financial Statements).
Allowance for doubtful accounts
We plan to perform ongoing evaluations of our customers and continuously monitor collections and payments to estimate an allowance for doubtful accounts based on the aging of the underlying receivables and our experiences of specific collection issues.
Stock-based compensation
We account for stock-based compensation issued to non-employees in accordance with FASB accounting guidance accounting for equity instruments that are "Issued to Other Than Employees for Acquiring, or in Conjunction with, Selling Goods or Services." We record the expense of such services based on the estimated fair value of the equity instrument using the Black-Scholes option pricing model. The value of the equity instrument is charged to earnings over the term of the service agreement.
For stock-based compensation awards granted to both employees and non-employees, we use the fair value method of calculating stock-based compensation. Calculating the fair value of stock-based awards requires the input of highly subjective assumptions. We use the Black-Scholes option pricing model to value our stock option awards. Stock-based compensation expense is significant to our financial statements and is calculated using our best estimates which involve inherent uncertainties and the application of management's judgment. Significant estimates include the expected life of the stock option, stock price volatility, risk-free interest rate and forfeiture rates.
During the year ended December 31, 2010, we recognized approximately $5.1 million of stock-based compensation expense related to equity awards granted to employees and non-employees. Total unrecognized stock-based compensation expense for all stock-based awards was approximately $1.4 million at December 31, 2010, of which $1.0 million will be recognized in 2011, $200,000 in 2012 and $200,000 thereafter. This results in these amounts being recognized over a weighted-average period of 1.1 years.
Fair value of financial instruments
Effective January 1, 2008, we implemented new accounting guidance related to fair value measurement for our financial assets and liabilities that are re-measured and reported at fair value at each reporting period, and non-financial assets and liabilities that are re-measured and reported at fair value at least annually. Effective January 1, 2009, we implemented new accounting guidance related to fair value measurement as it relates to our non-financial assets and non-financial liabilities that are recognized and disclosed at fair value in the financial statements. The adoption of this new accounting guidance to our financial and non-financial assets and liabilities and non-financial assets and liabilities that are re-measured and reported at fair value at least annually did not have an impact on our financial results in any period.
RECENT ACCOUNTING PRONOUNCEMENTS
In October 2009, the FASB issued an amendment to the accounting guidance for revenue arrangements with multiple deliverables. This guidance provides principles for allocation of consideration among its multiple-elements, allowing more flexibility in identifying and accounting for separate deliverables under an arrangement. The guidance introduces an estimated selling price method
60
for valuing the elements of a bundled arrangement if vendor-specific objective evidence or third-party evidence of selling price is not available, and significantly expands related disclosure requirements. This guidance is effective on a prospective basis for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Alternatively, adoption may be on a retrospective basis, and early application is permitted. We are planning on adopting this accounting guidance in the first quarter of 2011, prospectively, for any new revenue arrangement.
In October 2009, the FASB issued an amendment to the accounting standards related to certain revenue arrangements that include software elements. This standard clarifies the existing accounting guidance such that tangible products that contain both software and non-software components that function together to deliver the product's essential functionality, shall be excluded from the scope of the software revenue recognition accounting standards. Accordingly, sales of these products may fall within the scope of other revenue recognition accounting standards or may now be within the scope of this standard and may require an allocation of the arrangement consideration for each element of the arrangement. This standard, for which we are currently assessing the impact, became effective for us on January 1, 2011. The adoption of this new accounting guidance is not expected to have a material impact on our consolidated financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Our exposure to market risk is limited to our cash. The goals of our investment policy are preservation of capital, fulfillment of liquidity needs and fiduciary control of cash and investments. We also seek to maximize income from our investments without assuming significant risk. To achieve our goals, we maintain our cash in interest-bearing cash accounts. As all of our investments are cash deposits in a global bank, it is subject to minimal interest rate risk.
EFFECT OF CURRENCY EXCHANGE RATES AND EXCHANGE RATE RISK MANAGEMENT
We conduct business operations outside of the United States primarily in Japan and to a lesser extent in certain European countries. The foreign denominated financial obligations that we are exposed to at this time in Japan are relatively short-term. Our practice in Europe has been to enter into US-dollar-denominated transactions with our customers. Therefore, any currency fluctuations will not have a material impact on our financial position, results of operations or cash flows.
61
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Helicos BioSciences Corporation (A development stage company)
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
62
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Board of Directors and Stockholders of
Helicos BioSciences Corporation:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of redeemable convertible preferred stock and stockholders' equity (deficit) and of cash flows present fairly, in all material respects, the financial position of Helicos BioSciences Corporation and its subsidiary (a development stage enterprise) at December 31, 2009 and December 31, 2010, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2010 and, cumulatively, for the period from May 9, 2003 (date of inception) to December 31, 2010, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations and has limited available funds as of December 31, 2010, which raises substantial doubt about its ability to continue as a going concern. Management's plans in regard to this matter are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/
PricewaterhouseCoopers LLP
Boston, Massachusetts
March 31, 2011
63
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| |
December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | |||||||
ASSETS |
|||||||||
Current assets |
|||||||||
Cash and cash equivalents |
$ | 15,930 | $ | 2,513 | |||||
Accounts receivable |
848 | 267 | |||||||
Unbilled receivables |
496 | 704 | |||||||
Inventory |
6,827 | 2,935 | |||||||
Prepaid expenses and other current assets |
424 | 306 | |||||||
Total current assets |
24,525 | 6,725 | |||||||
Property and equipment, net |
1,706 | 126 | |||||||
Restricted cash |
225 | — | |||||||
Other assets |
79 | 152 | |||||||
Total assets |
$ | 26,535 | $ | 7,003 | |||||
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) |
|||||||||
Current liabilities |
|||||||||
Accounts payable |
$ | 1,005 | $ | 794 | |||||
Accrued expenses and other current liabilities |
3,632 | 1,388 | |||||||
Deferred revenue |
5,705 | 7,314 | |||||||
Current portion of long-term debt |
2,773 | 2,779 | |||||||
Total current liabilities |
13,115 | 12,275 | |||||||
Long-term debt, net of current portion |
2,081 | 674 | |||||||
Warrants |
5,253 | 220 | |||||||
Other long-term liabilities |
400 | — | |||||||
Total liabilities |
20,849 | 13,169 | |||||||
Commitments and contingencies (Notes 7 and 8) |
|||||||||
Stockholders' equity (deficit) |
|||||||||
Preferred stock: par value $0.001 per share; 5,000,000 shares authorized at December 31, 2009 and 2010; no shares issued and outstanding at December 31, 2009 and 2010 |
— | — | |||||||
Common stock: par value $0.001 per share; 120,000,000 shares authorized at December 31, 2009 and 2010; 79,370,610 and 86,557,606 shares issued and outstanding at December 31, 2009 and December 31, 2010, respectively |
79 | 87 | |||||||
Additional paid-in capital |
173,272 | 180,180 | |||||||
Deficit accumulated during the development stage |
(167,665 | ) | (186,433 | ) | |||||
Total stockholders' equity (deficit) |
5,686 | (6,166 | ) | ||||||
Total liabilities and stockholders' equity (deficit) |
$ | 26,535 | $ | 7,003 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
64
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
| |
|
|
|
Period from May 9, 2003 (date of inception) through December 31, 2010 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | ||||||||||||||
| |
2008 | 2009 | 2010 | ||||||||||||
Product revenue |
$ | 36 | $ | 2,325 | $ | 1,301 | $ | 3,662 | |||||||
Grant revenue |
772 | 701 | 3,096 | 5,310 | |||||||||||
Total revenue |
808 | 3,026 | 4,397 | 8,972 | |||||||||||
Costs and expenses |
|||||||||||||||
Cost of product revenue |
10 | 1,239 | 713 | 1,962 | |||||||||||
Research and development |
24,615 | 18,256 | 14,047 | 108,663 | |||||||||||
Selling, general and administrative |
20,139 | 12,481 | 10,506 | 70,942 | |||||||||||
Impairment of long-lived assets |
— | — | 1,894 | 1,894 | |||||||||||
Total costs and expenses |
44,764 | 31,976 | 27,160 | 183,461 | |||||||||||
Operating loss |
(43,956 | ) | (28,950 | ) | (22,763 | ) | (174,489 | ) | |||||||
Interest income |
670 | 75 | 19 | 4,153 | |||||||||||
Interest expense |
(2,365 | ) | (1,044 | ) | (614 | ) | (4,506 | ) | |||||||
Other income |
— | — | 472 | 472 | |||||||||||
Change in fair value of warrant liability |
— | 1,959 | 4,118 | 6,077 | |||||||||||
Net loss |
(45,651 | ) | (27,960 | ) | (18,768 | ) | (168,293 | ) | |||||||
Beneficial conversion feature related to Series B redeemable convertible preferred stock |
— | — | — | (18,140 | ) | ||||||||||
Net loss attributable to common stockholders |
$ | (45,651 | ) | $ | (27,960 | ) | $ | (18,768 | ) | $ | (186,433 | ) | |||
Net loss per share attributable to common stockholders—basic and diluted |
$ | (2.10 | ) | $ | (0.42 | ) | $ | (0.24 | ) | ||||||
Weighted average number of common shares used in computation—basic and diluted |
21,773,394 | 65,833,252 | 78,440,311 | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
65
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT)
Period from May 9, 2003 (date of inception) to December 31, 2010
(in thousands, except share and per share data)
| |
Series A redeemable convertible preferred stock |
Series B redeemable convertible preferred stock |
|
|
|
|
Deficit accumulated during development stage |
|
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common stock | |
|
|
|
|||||||||||||||||||||||||||||
| |
Additional paid-in capital |
Subscription receivable |
Other accumulated income (loss) |
Total stockholders' equity (deficit) |
||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||||
Balance at inception |
— | $ | — | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||
Issuance of Series A redeemable convertible preferred stock in December 2003 for cash at $0.9555 per share, net of issuance costs of $59 |
27,815,946 | 26,469 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||
Conversion of promissory note for shares of Series A redeemable convertible preferred stock in December 2003 at $0.9555 per share |
366,300 | 350 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||
Issuance of restricted common stock in October 2003 to a founder for cash |
— | — | — | — | 444,444 | 2 | — | — | — | 2 | ||||||||||||||||||||||||
Issuance of restricted common stock in November and December 2003 to nonemployees |
— | — | — | — | 618,126 | 1 | 2 | (3 | ) | — | — | — | ||||||||||||||||||||||
Issuance of common stock in December 2003 at $0.45 per share in exchange for intellectual property |
— | — | — | — | 46,514 | — | 20 | — | — | — | 20 | |||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 23 | — | — | — | 23 | |||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (547 | ) | — | (547 | ) | |||||||||||||||||||||
Balance at December 31, 2003 |
28,182,246 | 26,819 | — | — | 1,109,084 | 1 | 47 | (3 | ) | (547 | ) | — | (502 | ) | ||||||||||||||||||||
Exercise of a stock warrant to purchase shares of common stock in January 2004 |
— | — | — | — | 120,123 | — | — | — | — | — | — | |||||||||||||||||||||||
Cash received from investors in January 2004 for previously issued shares of Series A redeemable convertible preferred stock |
— | 50 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||
Cash received from nonemployee in January 2004 for previously issued shares of restricted common stock |
— | — | — | — | — | — | — | 3 | — | — | 3 | |||||||||||||||||||||||
Issuance of restricted common stock in February, March and April 2004 to employees for cash at $0.45 per share |
— | — | — | — | 155,555 | 1 | — | — | — | 1 | ||||||||||||||||||||||||
Issuance of restricted common stock in September 2004 to nonemployees for cash at $0.45 per share |
— | — | — | — | 11,888 | — | — | — | — | — | — | |||||||||||||||||||||||
Exercise of nonemployee stock options in December 2004 for cash of $0.45 per share |
— | — | — | — | 15,200 | 6 | — | — | — | 6 | ||||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 138 | — | — | — | 138 | |||||||||||||||||||||||
Unrealized short-term loss |
— | — | — | — | — | — | — | — | — | (17 | ) | (17 | ) | |||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (7,064 | ) | — | (7,064 | ) | |||||||||||||||||||||
Balance at December 31, 2004 |
28,182,246 | 26,869 | — | — | 1,411,850 | 1 | 192 | — | (7,611 | ) | (17 | ) | (7,435 | ) | ||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
66
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2010
(in thousands, except share and per share data)
| |
Series A redeemable convertible preferred stock |
Series B redeemable convertible preferred stock |
|
|
|
|
Deficit accumulated during development stage |
|
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common stock | |
|
|
|
|||||||||||||||||||||||||||||
| |
Additional paid-in capital |
Subscription receivable |
Other accumulated income (loss) |
Total stockholders' equity (deficit) |
||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||||
Balance at December 31, 2004 |
28,182,246 | $ | 26,869 | — | $ | — | 1,411,850 | $ | 1 | $ | 192 | $ | — | $ | (7,611 | ) | $ | (17 | ) | $ | (7,435 | ) | ||||||||||||
Issuance of restricted common stock in March 2005 in exchange for intellectual property |
— | — | — | — | 88,888 | — | — | — | — | — | — | |||||||||||||||||||||||
Issuance of restricted common stock in July 2005 to employees for cash at $0.45 per share |
— | — | — | — | 55,555 | 1 | — | — | — | — | 1 | |||||||||||||||||||||||
Issuance of restricted common stock in April and December 2005 to nonemployees for cash at $0.45 per share |
— | — | — | — | 1,666 | — | 1 | — | — | — | 1 | |||||||||||||||||||||||
Exercise of employee stock options in June 2005 for cash at $0.45 per share |
— | — | — | — | 277 | — | — | — | — | — | — | |||||||||||||||||||||||
Exercise of nonemployee stock options in September 2005 for cash at $0.45 per share |
— | — | — | — | 444 | — | — | — | — | — | — | |||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 55 | — | — | — | 55 | |||||||||||||||||||||||
Vesting of previously issued shares of restricted common stock |
— | — | — | — | — | — | 36 | — | — | — | 36 | |||||||||||||||||||||||
Change in unrealized short-term loss |
— | — | — | — | — | — | — | — | — | 17 | 17 | |||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (10,918 | ) | — | (10,918 | ) | |||||||||||||||||||||
Balance at December 31, 2005 |
28,182,246 | 26,869 | — | — | 1,558,680 | 2 | 284 | — | (18,529 | ) | — | (18,243 | ) | |||||||||||||||||||||
Issuance of restricted common stock in January 2006 to nonemployees for cash at $0.45 per share |
— | — | — | — | 1,111 | — | — | — | — | — | — | |||||||||||||||||||||||
Issuances of Series B redeemable convertible preferred stock in March 2006 for cash at $1.29 per share, net of issuance costs of $108 |
— | — | 15,503,876 | 19,892 | — | — | — | — | — | — | — | |||||||||||||||||||||||
Issuance of common stock in July 2006 to employees for cash at $0.585 per share |
— | — | — | — | 44,444 | — | 247 | — | — | — | 247 | |||||||||||||||||||||||
Issuance of restricted common stock in September, November and December 2006 to employees for cash at $0.585 per share |
— | — | — | — | 394,444 | — | 2 | (4 | ) | — | — | (2 | ) | |||||||||||||||||||||
Exercise of nonemployee stock options in November 2006 for cash at $0.585 per share |
— | — | — | — | 4,444 | — | 2 | — | — | — | 2 | |||||||||||||||||||||||
Exercise of employee stock options in January and December 2006 for cash at $0.45 per share |
— | — | — | — | 48,146 | — | 22 | — | — | — | 22 | |||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 1,058 | — | — | — | 1,058 | |||||||||||||||||||||||
Vesting of previously issued shares of restricted common stock |
— | — | — | — | — | — | 157 | — | — | — | 157 | |||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (20,580 | ) | — | (20,580 | ) | |||||||||||||||||||||
Balance at December 31, 2006 |
28,182,246 | $ | 26,869 | 15,503,876 | $ | 19,892 | 2,051,269 | $ | 2 | $ | 1,772 | $ | (4 | ) | $ | (39,109 | ) | $ | — | $ | (37,339 | ) | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
67
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2010
(in thousands, except share and per share data)
| |
Series A redeemable convertible preferred stock |
Series B redeemable convertible preferred stock |
|
|
|
|
Deficit accumulated during development stage |
|
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common stock | |
|
|
|
|||||||||||||||||||||||||||||
| |
Additional paid-in capital |
Subscription receivable |
Other accumulated income (loss) |
Total stockholders' equity (deficit) |
||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||||
Balance at December 31, 2006 |
28,182,246 | $ | 26,869 | 15,503,876 | $ | 19,892 | 2,051,269 | $ | 2 | $ | 1,772 | $ | (4 | ) | $ | (39,109 | ) | $ | — | $ | (37,339 | ) | ||||||||||||
Issuances of Series B redeemable convertible preferred stock in January 2007 for cash at $1.29 per share, net of issuance costs of $6 |
— | — | 15,503,876 | 19,994 | — | — | — | — | — | — | — | |||||||||||||||||||||||
Exercise of employee stock options in January 2007 for cash at $0.585 per share |
— | — | — | — | 4,311 | — | 3 | — | — | — | 3 | |||||||||||||||||||||||
Exercise of employee stock options in June, August and December 2007 for cash at $0.45 per share |
— | — | — | — | 1,866 | — | — | — | — | — | — | |||||||||||||||||||||||
Exercise of employee stock options in June, July and November 2007 for cash at $1.80 per share |
— | — | — | — | 1,079 | — | 2 | — | — | — | 2 | |||||||||||||||||||||||
Exercise of non-employee stock options in June, October and November 2007 for cash at $0.45 per share |
— | — | — | — | 6,500 | — | 3 | — | — | — | 3 | |||||||||||||||||||||||
Issuance of restricted common stock in July and August 2007 to employees |
— | — | — | — | 56,757 | — | — | — | — | — | — | |||||||||||||||||||||||
Issuance of restricted common stock in April 2007 to a non-employee |
— | — | — | — | 2,222 | — | — | — | — | — | — | |||||||||||||||||||||||
Cash received from employee in January 2007 for previously issued shares of restricted common stock |
— | — | — | — | — | — | — | 4 | — | — | 4 | |||||||||||||||||||||||
Cancellation of shares of restricted common stock |
— | — | — | — | (88,888 | ) | — | — | — | — | — | — | ||||||||||||||||||||||
Forfeiture of shares of unvested restricted common stock |
— | — | — | — | (11,111 | ) | — | — | — | — | — | — | ||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 3,396 | — | — | — | 3,396 | |||||||||||||||||||||||
Vesting of previously issued shares of restricted common stock |
— | — | — | — | — | — | 85 | — | — | — | 85 | |||||||||||||||||||||||
Beneficial conversion feature related to Series B redeemable convertible preferred stock |
— | — | — | — | — | — | 18,140 | — | (18,140 | ) | — | — | ||||||||||||||||||||||
Reclassification of amounts due to stockholders for fractional shares upon reverse stock split |
— | — | — | — | (10 | ) | — | — | — | — | — | — | ||||||||||||||||||||||
Issuance of common stock in initial public offering ("IPO"), net of discounts, commissions and issuance costs of $4,750 |
— | — | — | — | 5,400,000 | 5 | 43,845 | — | — | — | 43,850 | |||||||||||||||||||||||
Issuance of common stock in over-allotment to underwriters, net of discounts and commissions of $250 |
— | — | — | — | 397,000 | 1 | 3,322 | 3,323 | ||||||||||||||||||||||||||
Conversion of preferred stock |
(28,182,246 | ) | (26,869 | ) | (31,007,752 | ) | (39,886 | ) | 13,153,293 | 13 | 66,742 | — | — | — | 66,755 | |||||||||||||||||||
Exercise of warrants to purchase common stock |
— | — | — | — | 9,350 | — | 162 | — | — | — | 162 | |||||||||||||||||||||||
Net loss |
— | — | — | — | (36,805 | ) | (36,805 | ) | ||||||||||||||||||||||||||
Balance at December 31, 2007 |
— | $ | — | — | $ | — | 20,983,638 | $ | 21 | $ | 137,472 | $ | — | $ | (94,054 | ) | $ | — | $ | 43,439 | ||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
68
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2010
(in thousands, except share and per share data)
| |
Series A redeemable convertible preferred stock |
Series B redeemable convertible preferred stock |
|
|
|
|
Deficit accumulated during development stage |
|
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common stock | |
|
|
|
|||||||||||||||||||||||||||||
| |
Additional paid-in capital |
Subscription receivable |
Other accumulated income (loss) |
Total stockholders' equity (deficit) |
||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||||
Balance at December 31, 2007 |
— | $ | — | — | $ | — | 20,983,638 | $ | 21 | $ | 137,472 | $ | — | $ | (94,054 | ) | $ | — | $ | 43,439 | ||||||||||||||
Exercise of employee stock options in January, February and July 2008 for cash at $0.45 per share |
— | — | — | — | 15,861 | — | 7 | — | — | — | 7 | |||||||||||||||||||||||
Exercise of employee stock options in January, July and August 2008 for cash at $1.80 per share |
— | — | — | — | 1,201 | — | 2 | — | — | — | 2 | |||||||||||||||||||||||
Exercise of non-employee stock options in January and April 2008 for cash at $0.45 per share |
— | — | — | — | 5,790 | — | 3 | — | — | — | 3 | |||||||||||||||||||||||
Issuance of restricted common stock in June and July 2008 to employees |
— | — | — | — | 190,000 | — | — | — | — | — | — | |||||||||||||||||||||||
Forfeiture of shares of unvested restricted common stock |
— | — | — | — | (142,077 | ) | — | — | — | — | — | — | ||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 4,538 | — | — | — | 4,538 | |||||||||||||||||||||||
Vesting of previously issued shares of restricted common stock |
— | — | — | — | — | — | 43 | — | — | — | 43 | |||||||||||||||||||||||
Issuance of common stock warrants in June 2008 in connection with debt issuance |
— | — | — | — | — | — | 337 | — | — | — | 337 | |||||||||||||||||||||||
Issuance of common stock and common stock warrants in securities offering, net of discounts, commissions and issuance costs of $813 |
— | — | — | — | 42,753,869 | 43 | 17,742 | 17,785 | ||||||||||||||||||||||||||
Net loss |
— | — | — | — | (45,651 | ) | (45,651 | ) | ||||||||||||||||||||||||||
Balance at December 31, 2008 |
— | $ | — | — | $ | — | 63,808,282 | $ | 64 | $ | 160,144 | $ | — | $ | (139,705 | ) | $ | — | $ | 20,503 | ||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
69
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2010
(in thousands, except share and per share data)
| |
Series A redeemable convertible preferred stock |
Series B redeemable convertible preferred stock |
|
|
|
|
Deficit accumulated during development stage |
|
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common stock | |
|
|
|
|||||||||||||||||||||||||||||
| |
Additional paid-in capital |
Subscription receivable |
Other accumulated income (loss) |
Total stockholders' equity (deficit) |
||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||||
Balance at December 31, 2008 |
— | $ | — | — | $ | — | 63,808,282 | $ | 64 | $ | 160,144 | $ | — | $ | (139,705 | ) | $ | — | $ | 20,503 | ||||||||||||||
Exercise of employee stock options in January 2009 for cash at $0.45 per share |
— | — | — | — | 5,555 | — | 2 | — | — | — | 2 | |||||||||||||||||||||||
Exercise of employee stock options in September and November 2009 for cash at $1.04 per share |
— | — | — | — | 50,000 | — | 52 | — | — | — | 52 | |||||||||||||||||||||||
Issuance of common stock in April and October 2009 to employees |
— | — | — | — | 35,633 | — | — | — | — | — | — | |||||||||||||||||||||||
Issuance of restricted common stock in January, February, April, August, September and October 2009 to employees |
— | — | — | — | 1,662,091 | 2 | (2 | ) | — | — | — | — | ||||||||||||||||||||||
Issuance of restricted common stock in April and June 2009 to non-employees |
— | — | — | — | 95,000 | — | — | — | — | — | — | |||||||||||||||||||||||
Forfeiture of shares of unvested restricted common stock |
— | — | — | — | (107,415 | ) | — | — | — | — | — | — | ||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 5,281 | — | — | — | 5,281 | |||||||||||||||||||||||
Vesting of previously issued shares of restricted common stock |
— | — | — | — | — | — | 25 | — | — | — | 25 | |||||||||||||||||||||||
Issuance of common stock and common stock warrants in securities offering, net of discounts, commissions and issuance costs of $1,364 |
— | — | — | — | 10,711,280 | 10 | 7,773 | — | — | — | 7,783 | |||||||||||||||||||||||
Exercise of warrants to purchase common stock |
— | — | — | — | 3,110,184 | 3 | (3 | ) | — | — | — | — | ||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (27,960 | ) | — | (27,960 | ) | |||||||||||||||||||||
Balance at December 31, 2009 |
— | $ | — | — | $ | — | 79,370,610 | $ | 79 | $ | 173,272 | $ | — | $ | (167,665 | ) | $ | — | $ | 5,686 | ||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
70
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2010
(in thousands, except share and per share data)
| |
Series A redeemable convertible preferred stock |
Series B redeemable convertible preferred stock |
|
|
|
|
Deficit accumulated during development stage |
|
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common stock | |
|
|
|
||||||||||||||||||||||||||||||
| |
Additional paid-in capital |
Subscription receivable |
Other accumulated income (loss) |
Total stockholders' equity (deficit) |
|||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||
Balance, December 31, 2009 |
— | $ | — | — | $ | — | 79,370,610 | $ | 79 | $ | 173,272 | $ | — | $ | (167,665 | ) | $ | — | $ | 5,686 | |||||||||||||||
Forfeiture of shares of vested restricted stock in lieu of cash paid for taxes in January, April, June, July, and October 2010 |
— | — | — | — | (608,151 | ) | — | — | — | — | — | — | |||||||||||||||||||||||
Issuance of common stock to Directors for fees in lieu of cash in January and April 2010 |
— | — | — | — | 48,356 | — | — | — | — | — | — | ||||||||||||||||||||||||
Forfeiture of shares of unvested restricted stock in January, |
— | — | — | — | (1,282,223 | ) | (1 | ) | 1 | — | — | — | — | ||||||||||||||||||||||
April, June, July and October 2010 |
— | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 5,104 | — | — | — | 5,104 | ||||||||||||||||||||||||
Issuance of restricted common stock to employees in January, April, July, August and October 2010 |
— | — | — | — | 3,831,381 | 4 | (4 | ) | — | — | — | — | |||||||||||||||||||||||
Issuance of restricted common stock in March, May and June 2010 to employees in lieu of cash for 2009 bonuses |
— | — | — | — | 655,871 | — | 584 | — | — | — | 584 | ||||||||||||||||||||||||
Re-pricing of warrants in connection with November 2010 Debt Amendment |
— | — | — | — | — | — | 47 | — | — | — | 47 | ||||||||||||||||||||||||
Exercise of employee stock options in August 2010 for cash at $0.71 per share |
— | — | — | — | 4,352 | — | 2 | — | — | — | 2 | ||||||||||||||||||||||||
Exercise of warrants to purchase common stock |
— | — | — | — | 4,537,410 | 5 | 1,107 | — | — | — | 1,112 | ||||||||||||||||||||||||
Vesting of previously issued shares of restricted common stock |
— | — | — | — | — | — | 67 | — | — | — | 67 | ||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (18,768 | ) | — | (18,768 | ) | ||||||||||||||||||||||
Balance, December 31, 2010 |
— | $ | — | — | $ | — | 86,557,606 | 87 | 180,180 | $ | — | $ | (186,433 | ) | $ | — | $ | (6,166 | ) | ||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
71
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| |
Year Ended December 31, | Period from May 9, 2003 (date of inception) through December 31, 2010 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | ||||||||||||
Cash flows from operating activities: |
|||||||||||||||
Net loss |
$ | (45,651 | ) | $ | (27,960 | ) | $ | (18,768 | ) | $ | (168,293 | ) | |||
Adjustments to reconcile net loss to cash used in operating activities: |
|||||||||||||||
Depreciation and amortization |
2,528 | 2,308 | 1,019 | 9,058 | |||||||||||
Amortization of lease incentive |
(149 | ) | (105 | ) | (4 | ) | (474 | ) | |||||||
Common stock issued for licenses |
— | — | 147 | ||||||||||||
Stock-based compensation expense |
4,538 | 5,281 | 5,104 | 19,814 | |||||||||||
Noncash interest expense related to debt and warrants |
404 | 325 | 260 | 1,137 | |||||||||||
Noncash license fee |
— | — | 54 | 54 | |||||||||||
Noncash income related to change in fair value of warrant liability |
— | (1,959 | ) | (4,118 | ) | (6,077 | ) | ||||||||
Provisions on inventory |
1,577 | 1,699 | 2,570 | 5,846 | |||||||||||
Gain (Loss) on disposal of property and equipment |
— | 26 | (33 | ) | (7 | ) | |||||||||
Impairment of long-lived assets |
1,894 | 1,894 | |||||||||||||
Changes in operating assets and liabilities: |
|||||||||||||||
Accounts receivable |
(223 | ) | (625 | ) | 581 | (267 | ) | ||||||||
Unbilled receivables |
(88 | ) | (41 | ) | (208 | ) | (454 | ) | |||||||
Inventory |
(7,050 | ) | (1,696 | ) | (868 | ) | (11,226 | ) | |||||||
Prepaid expenses and other current assets |
471 | (514 | ) | 117 | (632 | ) | |||||||||
Deferred revenue |
623 | 5,082 | 1,522 | 7,227 | |||||||||||
Accounts payable |
(902 | ) | 216 | (211 | ) | 794 | |||||||||
Accrued expenses and other current liabilities |
(207 | ) | 2,110 | (878 | ) | 3,061 | |||||||||
Other long-term liabilities |
(172 | ) | 365 | — | 557 | ||||||||||
Net cash used in operating activities |
(44,301 | ) | (15,488 | ) | (11,967 | ) | (137,841 | ) | |||||||
Cash flows from investing activities: |
|||||||||||||||
Purchases of property and equipment |
(2,889 | ) | (22 | ) | (27 | ) | (9,541 | ) | |||||||
Proceeds from sale of property and equipment |
— | — | 33 | 33 | |||||||||||
Decrease (Increase) in restricted cash |
225 | — | 225 | — | |||||||||||
Purchases of short-term investments |
— | — | (34,709 | ) | |||||||||||
Maturities of short-term investments |
— | — | 34,709 | ||||||||||||
Net cash (used in) provided by investing activities |
(2,664 | ) | (22 | ) | 231 | (9,508 | ) | ||||||||
Cash flows from financing activities: |
|||||||||||||||
Proceeds from debt issuances |
9,850 | — | — | 22,256 | |||||||||||
Payments on debt |
(13,585 | ) | (3,322 | ) | (2,288 | ) | (19,880 | ) | |||||||
Payments of debt issuance costs |
(20 | ) | — | (259 | ) | (453 | ) | ||||||||
Proceeds from initial public offering |
— | — | 49,011 | ||||||||||||
Deferred initial public offering costs |
— | — | (1,838 | ) | |||||||||||
Proceeds from issuance of redeemable convertible preferred stock, net of issuance costs |
— | — | 66,405 | ||||||||||||
Proceeds from bridge loan |
— | — | 667 | 1,017 | |||||||||||
Proceeds from issuance of common stock and common stock warrants |
17,785 | 14,996 | 32,807 | ||||||||||||
Proceeds from issuance of restricted common stock |
— | (1 | ) | — | 338 | ||||||||||
Payments to employees for cancelled restricted common stock |
(47 | ) | — | (104 | ) | ||||||||||
Proceeds from exercise of warrants |
197 | 197 | |||||||||||||
Proceeds from exercise of stock options |
12 | 54 | 2 | 106 | |||||||||||
Net cash (used in) provided by financing activities |
13,995 | 11,727 | (1,681 | ) | 149,862 | ||||||||||
Net increase (decrease) in cash and cash equivalents |
(32,970 | ) | (3,783 | ) | (13,417 | ) | 2,513 | ||||||||
Cash and cash equivalents, beginning of period |
52,683 | 19,713 | 15,930 | — | |||||||||||
Cash and cash equivalents, end of period |
$ | 19,713 | $ | 15,930 | $ | 2,513 | $ | 2,513 | |||||||
Supplemental disclosure of cash flow information |
|||||||||||||||
Cash paid during the year for interest |
$ | 1,478 | $ | 820 | $ | 360 | $ | 3,007 | |||||||
Transfers from inventory to property and equipment |
$ | 255 | $ | — | $ | 1,297 | $ | 1,552 | |||||||
Noncash financing activities: |
|||||||||||||||
Issuance of redeemable convertible preferred stock warrants |
$ | — | $ | — | $ | — | $ | 95 | |||||||
Issuance of common stock warrants |
$ | 6,021 | $ | 7,212 | $ | — | $ | 13,233 | |||||||
Number of common stock shares issued upon exercise of warrants |
— | 3,110,184 | 4,537,410 | 7,647,594 | |||||||||||
Change in warrant valuation |
$ | — | $ | (1,959 | ) | $ | (4,118 | ) | $ | (6,077 | ) | ||||
Conversion of bridge loan to equity |
$ | — | $ | — | $ | — | $ | 350 | |||||||
Beneficial conversion feature related to Series B redeemable convertible preferred stock |
$ | — | $ | — | $ | — | $ | 18,140 | |||||||
Conversion of preferred stock to common stock |
$ | — | $ | — | $ | — | $ | 66,755 | |||||||
Reclassification of preferred stock warrants to common stock warrants |
$ | — | $ | — | $ | — | $ | 162 | |||||||
The accompanying notes are an integral part of these consolidated financial statements.
72
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Business Description
Helicos BioSciences Corporation ("Helicos" or the "Company") is a life sciences company focused on innovative genetic analysis technologies. Helicos has developed a proprietary technology to enable the rapid analysis of large volumes of genetic material by directly sequencing single molecules of DNA or RNA. The Company's tSMS approach differs from current methods of sequencing DNA or RNA because it analyzes individual molecules of DNA directly instead of analyzing a large number of copies of the molecule which must be produced through complex sample preparation techniques. The Company's tSMS technology eliminates the need for costly, labor-intensive and time-consuming sample preparation techniques, such as amplification or cloning, which are required by other methods to produce a sufficient quantity of genetic material for analysis.
The Company's Helicos® Genetic Analysis Platform is designed to obtain sequencing information by repetitively performing a cycle of biochemical reactions on individual DNA or RNA molecules and imaging the results after each cycle. The platform consists of an instrument called the HeliScope™ Single Molecule Sequencer, an image analysis computer tower called the HeliScope™ Analysis Engine, associated reagents, which are chemicals used in the sequencing process, and disposable supplies. The information generated from using our tSMS products may lead to improved drug therapies, personalized medical treatments and more accurate diagnostics for cancer and other diseases.
The Company has had limited operations to date and its activities have consisted primarily of raising capital, conducting research and development and recruiting personnel. Accordingly, the Company is considered to be in the development stage at December 31, 2010, as defined by the Financial Accounting Standards Board ("FASB"). The Company's fiscal year ends on December 31. The Company operates as one reportable segment. Helicos is a Delaware corporation and was incorporated on May 9, 2003.
As previously disclosed in the Company's quarterly and periodic reports with the Securities and Exchange Commission, in the first quarter of 2010, the Company began a process of considering alternatives to its long-term strategic focus, including a repositioning of the Company in the genetic analysis markets. As a result of this process, on June 25, 2010, the Company announced that it had launched its diagnostics business and that the Company was in early stages of validating molecular diagnostic (MDx) tests that utilize its HeliScope® Single Molecule Sequencer. To date, the Company's research and development team has made progress in developing two prototype tests. The first test is for the detection of gene mutations indicative of a woman's increased risk of developing hereditary breast or ovarian cancer. The second prototype is a non-invasive prenatal diagnostic test. Nevertheless, despite this scientific progress, and a lengthy and comprehensive financing initiative supported by a nationally recognized investment bank during the second and third quarter of 2010, the Company was unable to secure any long-term equity financings or financing arrangements with strategic partners during the third quarter of 2010. The Company has had numerous meetings with prospective investors and corporate partners and has not been able to secure a bona-fide offer for financing or a strategic transaction. In addition, the Company had very limited commercial activity during fiscal year 2010. Furthermore, as of December 31, 2010 and March 24, 2011, the Company only had $2.5 million and $2.9 million, respectively, in cash and cash equivalents.
As previously disclosed, on May 11, 2010, the Company's Board of Directors (the "Board") approved a reduction in headcount and restructuring plan (the "May Restructuring Plan") which involved the consolidation and reorganization of its operations in order to reduce operating costs. The May Restructuring Plan resulted in the elimination of approximately 40 positions during the second fiscal quarter of 2010. In order to further reduce operating costs and preserve cash, on September 15,
73
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Business Description (Continued)
2010, the Board approved a subsequent reduction in headcount and restructuring plan (the "September Restructuring Plan") which involved the consolidation and reorganization of the Company's operations. The September Restructuring Plan eliminated approximately 14 positions during the third quarter of 2010. The Company entered the first quarter of 2011 with 22 employees. In addition, during the third quarter of 2010, as a result of the inability to complete the financing referenced above and the subsequent change in business direction, the Company recorded charges of $2.6 million in connection with a write-off of certain of its inventory that is no longer used in the business and $1.9 million in connection with the impairment of certain long-lived assets.
Going forward, the Company plans to deploy its limited resources by focusing on intellectual property monetization and concentrating our research and development efforts in targeted areas designed to achieve tangible proof-of-concept goals, which may enable the Company to seek partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives. As of the date of this filing, the Company does not have adequate resources and will continue to need to generate additional funding from various sources including contract sequencing service projects, government grants, strategic collaborations and licensing activities. On October 14, 2010, the Company announced the launch of a technology licensing program for its Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. The Company plans to defend its intellectual property rights through licensing and enforcement strategies. In this regard, the Company took several actions. First, on September 8, 2010, Helicos filed a lawsuit against Pacific Biosciences of California, Inc. ("Pacific Biosciences") for patent infringement. The lawsuit, filed in the United States District Court for the District of Delaware, accuses Pacific Biosciences of infringing four patents and seeks injunctive relief and monetary damages. Second, on October 14, 2010, Helicos announced the launch of a technology licensing program for its Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. Third, on October 22, 2010, Helicos filed an amended complaint against Pacific Biosciences by naming Illumina, Inc. and Life Technologies Corporation as additional defendants. The amended lawsuit alleges that the defendants willfully infringe its patents in suit by using their respective technologies, sequencing systems and products which the Company alleges are within the scope of one or more claims of its patents in suit. The Company is seeking a permanent injunction enjoining the defendants from further infringement as well as monetary and punitive damages, costs and attorneys' fees, interests and other relief as determined by the Court. The Company believes that the proceeds generated by these litigations, if the Company is successful, could be substantial. The Company continues to pursue all the above-mentioned opportunities in the IP and technology platform areas.
The Company entered the fourth quarter of 2010 without any prospects of securing long-term financing from outside investors. An independent committee ("Independent Committee") of the Board evaluated the alternatives available to the Company and determined that a bridge financing led by certain inside investors was in the best interests of its stakeholders in view of its current financial and operating condition, and would best position the Company to continue operations for the remainder of 2010 and into 2011. The Independent Committee met multiple times to consider the alternatives and concluded that the bridge financing was the best alternative for generating value for stakeholders. As a result, the Company entered into an insider bridge loan facility with two of the four venture capital firms who decided to participate in the bridge financing and whose representatives are members of the Board. In connection with this transaction, the Company entered into a Subordinated Secured Note Purchase Agreement (the "Note Purchase Agreement") with investment funds affiliated with Atlas Venture and Flagship Ventures (the "Purchasers"), pursuant to which the Company had agreed to sell
74
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Business Description (Continued)
to the Purchasers, and the Purchasers had agreed to purchase, up to an aggregate of $4,000,000 of convertible promissory notes (the "Notes"), a portion of which is committed and a portion of which is optional (the "Bridge Debt Financing"). On November 16, 2010, the Purchasers purchased Notes having an aggregate principal amount of $333,333. Subject to the Company's continued compliance with the terms of the Note Purchase Agreement, including that there not be an event of default thereunder, upon notice from the Company the Purchasers are committed to purchase an aggregate of an additional $333,333 of Notes in each of five subsequent closings, for a total aggregate committed amount of $2,000,000. The Purchasers also have the right, but not the obligation, upon the Company's request to purchase up to an additional $2,000,000 of Notes on or before December 31, 2012. As of December 31, 2010 and as of the date of this filing, the Company has borrowed $666,667 against the Bridge Debt Financing facility. See Note 8 of Notes to Consolidated Financial Statements for additional terms of the Notes.
As an inducement for the Purchasers to purchase the Notes, the Company entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement") simultaneous with the execution of, and as required by, the Note Purchase Agreement. Under the terms of the Risk Premium Agreement, the Company agreed to pay the Purchasers the following portions of the consideration it receives (the "Payment Consideration") in Liquidity Transactions, as defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30% of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). See Note 8 of Notes to Consolidated Financial Statements for additional terms of the Risk Premium Agreement.
In connection with the Bridge Debt Financing, on November 16, 2010, the Company, GE and the Lenders executed the Fourth Amendment to the Loan and Security Agreement, dated November 16, 2010 (the "Fourth Amendment"). Pursuant to the Fourth Amendment, GE and the Lenders waived various existing events of default and consented to the Company's incurrence of additional indebtedness and the grant of a junior security interest to the Purchasers under the Note Purchase Agreement. The Company amended warrants previously issued to the Lenders to re-price the Warrants from $4.80 to $0.01 and GE and the Lenders agreed to defer a $200,000 payment due January 31, 2011 to the January 1, 2012 extended term loan maturity date. In addition, subject to the Company's continued compliance with the terms of the Loan Agreement, including that there not be an event of default thereunder, GE and the Lenders agreed to defer up to five additional principal monthly payments with respect to the term loan. Also pursuant to the Fourth Amendment, the Company agreed to grant the Lenders a first priority security interest in all of its intellectual property and to pay the Lenders an additional $50,000 to $200,000 (depending upon how many principal payments are deferred) upon the full repayment of all outstanding amounts due with respect to the term loans. Under the terms of the Loan and Security Agreement prior to its amendment by the Fourth Amendment, the scope of the Lenders' first priority security interest included all of the Company's assets other than intellectual property. As of the date of this filing, the Lenders have agreed to defer the additional five monthly principal payments referenced above.
75
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Business Description (Continued)
In connection with the Bridge Debt Financing and the Fourth Amendment and as security for the Company's obligations under our contingency fee arrangement with Outside IP Litigation Counsel relating to the Cause of Action, the Company and Outside IP Litigation Counsel entered into a Subordinated Security Agreement pursuant to which the Company agreed to grant Outside IP Litigation Counsel a second priority security interest in the Cause of Action. Under the terms of the contingency fee arrangement, the Company is obligated to pay Outside IP Litigation Counsel up to 40% of the proceeds paid to the Company in connection with the settlement, judgment or other resolution of the Cause of Action or certain business transactions resulting from or relating to the Cause of Action.
In approving the transactions relating to the Bridge Debt Financing and the Fourth Amendment, the Board approved a management incentive plan (the "Management Incentive Plan") pursuant to which participants in the Management Incentive Plan, including Dr. Ivan Trifunovich and Mr. Jeffrey Moore, will be entitled to receive in the aggregate, an amount equal to 7.5% of certain payment consideration resulting from intellectual property licensing events. The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the Board.
The agreements and arrangements described above and all of the transactions contemplated by those agreements were approved by an independent committee of the Board. In addition, Noubar B. Afeyan, PhD, who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, Ph.D., who is a Partner of Atlas Venture, abstained from the vote of the Board approving the transactions.
Although the Company believes that the HeliScope Sequencer has unique utility across a broad array of MDx tests, due to significant financial and resource constraints, the Company has deferred the plan to develop its own genetic testing laboratory certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA). Nevertheless, the Company still believes that diagnostic applications may benefit from the specific features of the Helicos System, including the platform's ability to sequence non-amplified natural DNA and RNA directly, quantitative accuracy, high throughput, ability to use small sample quantities in simple and cost effective preparation methods, as well as lack of biases typically seen with sample amplification. The Company will continue to evaluate the best modality of bringing high-value MDx tests based on its technology to the market through a CLIA laboratory, either in partnership or through its own efforts should the financial outlook for the Company improve, however there can be no assurance that the Company will be successful in deploying MDx tests or its technology through a CLIA laboratory.
Notwithstanding recent workforce reductions, restructurings, and the Bridge Debt Financing, the Company may not have sufficient funds to pursue its business priorities. The Company will require significant additional capital in the second half of 2011 to continue its operations. As a result, the Company is no longer able to remain current in making payments to trade vendors and other unsecured creditors when such payments are due and, despite the restructuring of itscredit facility with GE Capital discussed above, this inability to remain current increases the likelihood that GE Capital could declare a default under the credit facility in the near-term, including with regard to solvency. These workforce reductions, as well as curtailed financial resources, will further impact the Company's ability to implement its business priorities. In addition, these constraints have caused the Company to significantly scale back service support and reagent supply to its current installed base. The Company has also significantly curtailed collaborative activities with other parties. Nevertheless, the Company
76
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Business Description (Continued)
believes that these actions will preserve its viability and provide additional time to execute its business priorities.
On June 28, 2010, the Company received a letter from The NASDAQ Stock Market LLC ("NASDAQ") advising that for the previous 30 consecutive business days, the Company failed to comply with the minimum market value of listed securities ("MVLS") requirement for continued listing on the NASDAQ Global Market pursuant to NASDAQ Marketplace Rule 5450(b)(2)(A) and that the Company did not otherwise satisfy the criteria for continued listing pursuant to NASDAQ Marketplace Rule 5450(b). NASDAQ stated in its letter that in accordance with NASDAQ Marketplace Rule 5810(c)(3)(C), the Company would be provided 180 calendar days, or until December 27, 2010, to regain compliance with the MVLS continued listing requirement. MVLS is calculated by multiplying the total shares outstanding by the daily closing bid price. On October 12, 2010, the Company received a delisting determination letter from NASDAQ due to its failure to regain compliance with the NASDAQ Global Market's minimum bid price requirement for continued listing as set forth in Listing Rule 5450(a)(1). In addition, the Company did not hold its annual meeting of stockholders for fiscal 2010 which is an additional requirement for the Company to comply with in order to maintain its continued listing on NASDAQ. Based on the unlikely ability of the Company to meet the NASDAQ MLVS requirements due to the state of its finances and near-term business prospects, on November 12, 2010, the Company withdrew its appeal to NASDAQ regarding this delisting notice. As a result, the Company's securities were delisted from the NASDAQ Global Market and the trading of its common stock was suspended before the opening of business on November 16, 2010, and a Form 25-NSE was subsequently filed with the Securities and Exchange Commission on January 21, 2011 which removed the Company's securities from listing and registration on The NASDAQ Stock Market. The Company was advised by Pink OTC Markets Inc., which operates an electronic quotation service for securities traded over-the-counter ("OTC"), that its securities were immediately eligible for quotation on the OTCQB. The OTCQB is a market tier for OTC traded companies that are registered and reporting with the Securities and Exchange Commission. The Company has also been advised that its shares will continue to trade under the symbol HLCS.
The Company's future capital requirements will depend on many factors and it may require additional capital beyond its currently anticipated amounts. Any such required additional capital may not be available on reasonable terms, if at all, given our prospects, the current economic environment and restricted access to capital markets. The continued depletion of its resources may make future funding more difficult or expensive to attain. Additional equity financing may be dilutive to the Company's stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict its ability to operate as a business; and strategic partnerships may result in royalties or other terms which reduce its economic potential from any adjustments to its existing long-term strategy. If the Company is unable to execute its operations according to its plans or to obtain additional financing, the Company may be forced to cease operations. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets or the amount of reclassification of liabilities, or any adjustment that might be necessary should the Company be unable to continue as a going concern.
Since inception, the Company has incurred losses and has not generated positive cash flows from operations. The Company expects is losses to continue for a considerable period of time. These losses, among other things, have had and will continue to have an adverse effect on its working capital, total assets and stockholders' equity/(deficit). As of December 31, 2010 and March 24, 2011, the Company
77
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Business Description (Continued)
had $2.5 million and $2.9 million, respectively, in cash and cash equivalents. The Company will, during the second half of 2011, require significant additional capital to continue its operations. The Company's present capital resources are not sufficient to fund its planned operations for a twelve month period as of the date of the Annual Report on Form 10-K for the year ended December 31, 2010, accordingly the Company's current financial resources raise substantial doubt about its ability to continue as a going concern.
The preparation of financial statements in conformity with GAAP requires the Company to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent liabilities at the dates of the financial statements. Actual amounts may differ from these estimates because of different assumptions or conditions. The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary. All intercompany accounts and transactions have been eliminated.
2. Initial Public Offering
On May 24, 2007, the Company completed its initial public offering ("IPO") of 5,400,000 shares of common stock at an initial public offering price of $9.00 per share. Net proceeds were approximately $43.9 million after deducting underwriting discounts and commissions and offering expenses paid by the Company. Total fees and expenses paid by the Company, excluding underwriting discounts and commissions were approximately $1.8 million which includes legal, accounting and printing costs and various other fees associated with registration and listing of the Company's common stock.
On May 24, 2007, upon completion of the Company's IPO, all of the Company's 59,189,998 shares of redeemable convertible preferred stock outstanding on that date were automatically converted into 13,153,293 shares of common stock. In addition, the outstanding warrants to purchase 81,184 shares of Series B redeemable convertible preferred stock were converted into warrants to purchase 18,040 shares of common stock. From January 1, 2007 through the date of the Company's IPO, the estimated fair value of the warrants to purchase 81,184 shares of Series B redeemable convertible preferred stock decreased by $42,000 to $162,000. Upon conversion on the date of the Company's IPO, the warrants to purchase 18,040 shares of the Company's common stock were reclassified to additional paid-in capital.
On June 27, 2007, the underwriters exercised their over-allotment option and purchased an additional 397,000 shares of the Company's common stock, and the net proceeds after deducting underwriters' discounts and commissions related to the offering were approximately $3.3 million.
3. Summary of Significant Accounting Policies
Basis of presentation and consolidation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary. All intercompany accounts and transactions have been eliminated.
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the
78
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
date of the financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, the Company evaluates its estimates, including those related to revenue recognition, inventory valuation, income taxes, contingencies, and stock-based compensation. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual amounts may differ from these estimates under different assumptions or conditions. Changes in estimates are recorded in the period in which they become known.
Cash and cash equivalents
The Company considers all highly liquid investments with original maturities of generally three months or less at the time of acquisition to be cash equivalents. Cash equivalents are stated at cost, which approximates fair market value.
Short-term investments
The Company classifies marketable securities as available-for-sale. These securities are carried at fair market value with unrealized gains and losses reported, if material, as a component of other comprehensive gain or loss in stockholders' equity/(deficit). There were no gross unrealized gains and losses at December 31, 2009 or 2010. Gains or losses on securities sold are based on the specific identification method.
Concentration of credit risk
The Company has no significant off-balance sheet concentrations of credit risk such as foreign currency exchange contracts, option contracts or other hedging arrangements. Financial instruments that subject the Company to credit risk consist primarily of cash and cash equivalents. The Company maintains all of its cash in two accredited financial institutions. Management believes these accounts are subject to minimal credit and market risk and are of high credit quality.
Fair value of financial instruments
Effective January 1, 2008, the Company implemented new accounting guidance related to fair value measurement for its financial assets and liabilities that are re-measured and reported at fair value at each reporting period, and non-financial assets and liabilities that are re-measured and reported at fair value at least annually. Effective January 1, 2009, the Company implemented new accounting guidance related to fair value measurement as it relates to the Company's non-financial assets and non-financial liabilities that are recognized and disclosed at fair value in the financial statements. The adoption of this new accounting guidance to the Company's financial and non-financial assets and liabilities and non-financial assets and liabilities that are re-measured and reported at fair value at least annually did not have an impact on the Company's financial results in any period.
79
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
The following table summarizes the number of trade customers that individually comprise greater than 10% of product revenues and their respective percentage of the Company's total product revenues on a gross basis:
| |
|
Percentage of Total Product Revenues by Customer |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of Significant Customers |
|||||||||
Year ended:
|
A | B | ||||||||
December 31, 2009 |
2 | 46 | % | 45 | % | |||||
December 31, 2010 |
2 | 58 | % | 15 | % | |||||
The table above excludes grant revenues.
The following table summarizes the number of customers that individually comprise greater than 10% of total accounts receivable and their respective percentage of the Company's total accounts receivable:
| |
|
Percentage of Total Product Revenues by Customer |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of Significant Customers |
|||||||||
As of:
|
A | B | ||||||||
December 31, 2009 |
2 | 23 | % | 62 | % | |||||
December 31, 2010 |
2 | 28 | % | 19 | % | |||||
The table above excludes unbilled receivables.
Inventory
Prior to reaching technological feasibility, start-up manufacturing costs, such as those relating to the assembly, testing and performance validation of the Helicos® Genetic Analysis System, were expensed to research and development as the costs were incurred. When management determined that the Helicos System was ready for commercial launch during December 2007, the Company began capitalizing its manufacturing costs to inventory.
The Company values its inventory at the lower of cost or market on a first-in, first-out basis. The Company's policy is to capitalize inventory costs associated with its products when, based on management's judgment, future commercialization is considered probable and the future economic benefit is expected to be realized.
The Company periodically reviews its inventories for excess or obsolete inventory and writes-down obsolete or otherwise unmarketable inventory to its estimated net realizable value. If the actual net realizable value is less than that estimated by the Company, or if there are any further determinations that inventory will not be marketable based on estimates of demand, additional inventory write-downs will be required.
The Company values inventory in accordance with FASB guidance on inventory costs and inventory pricing to clarify that abnormal amounts of idle facility expense, freight, handling costs, and wasted material (spoilage) should be recognized as current-period charges regardless of whether they meet the
80
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
criterion of "so abnormal." In addition, guidance requires that allocation of fixed production overheads to the costs of conversion be based on the normal capacity of the production facilities.
The Company concluded that because it is not yet functioning under normal capacity, its production level is abnormally low. As such, abnormal costs such as the unfavorable labor, overhead and absorption variances were recognized as current period charges, rather than as a portion of the inventory cost. During the years ended December 31, 2008, 2009 and 2010, research and development expense included $4.2 million, $1.9 million, and $0.7 million, respectively, of labor and overhead costs associated with the under-utilization of the manufacturing facility. In fiscal year 2010 the Company ceased production of Helicos Systems. During the year ended December 31, 2008, the Company recorded a $1.6 million charge to research and development expenses to write-off excess and obsolete inventory. During the year ended December 31, 2009, the Company recorded a $3.1 million charge to write-off excess and obsolete inventory. This charge was recorded as a research and development expense which resulted in a $1.7 million decrease to inventory and a $1.4 million increase to accrued expenses for excess non-cancellable purchase commitments. During the year ended December 31, 2010, the Company recorded a $2.6 million charge to research and development expense to write-off excess and obsolete inventory.
Property and equipment
Property and equipment are recorded at cost. Expenditures for maintenance and repairs are charged to expense while the costs of significant improvements are capitalized. Depreciation is provided using the straight-line method over the following estimated useful lives: Machinery and equipment—three to five years, office furniture and equipment—three years, leasehold improvements—the shorter of three years or the life of lease. Upon retirement or sale, the cost of the assets disposed of and the related accumulated depreciation are eliminated from the consolidated balance sheets and related gains or losses are reflected in the consolidated statements of operations. There have been no material retirements or sale of assets since May 9, 2003 (date of inception).
Long-lived assets
The Company evaluates the recoverability of property and equipment and other long-lived assets when circumstances indicate that an event of impairment may have occurred in accordance with FASB guidance. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written-down to their estimated fair values. Long-lived assets and certain identifiable intangible assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell. The Company recorded an impairment charge related to property and equipment of approximately $1.9 million. (See Note 5.)
Revenue recognition
Government research grants that provide for payments to the Company for work performed are recognized as revenue when the related expenses are incurred.
The Company recognizes revenue in accordance with FASB accounting guidance on revenue recognition in financial statements and accounting for multiple element revenue arrangements. This guidance requires that persuasive evidence of a sales arrangement exists; delivery of goods occurs through transfer of title and risk and rewards of ownership, the selling price is fixed or determinable
81
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
and collectability is reasonably assured. The Company also follows FASB guidance on how to account for arrangements that involve the delivery or performance of multiple products, services and/or rights to use assets.
In instances where the Company sells instruments with a related installation obligation, the Company will allocate the revenue between the instrument and the installation based on relative fair value at the time of the sale. The instrument revenue will be recognized when title and risk of loss passes. The installation revenue will be recognized when the installation is performed. If fair value is not available for any undelivered element, revenue for all elements is deferred until delivery and installation are complete.
In instances where the Company sells an instrument with specified acceptance criteria, the Company will defer revenue recognition until such acceptance has been obtained.
Revenue from service contracts sold in conjunction with the sale of equipment is recognized ratably over the service period.
Research and development
Research and development expenditures are charged to the consolidated statement of operations as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, clinical trial and related supply costs, contract services, depreciation and amortization expense and other related costs. During the years ended December 31, 2008, 2009 and 2010 research and development expenses also included $4.2 million, $1.9 million, and $0.7 million, respectively, of labor and overhead costs associated with the under-utilization of the manufacturing facility.
Income taxes
The Company records income taxes using the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases, and operating loss and tax credit carryforwards. The Company's consolidated financial statements contain certain deferred tax assets, which have arisen primarily as a result of operating losses, as well as other temporary differences between financial and tax accounting. FASB guidance on accounting for income taxes requires the Company to establish a valuation allowance if the likelihood of realization of the deferred tax assets is reduced based on an evaluation of objective verifiable evidence.
Significant management judgment is required in determining the Company's provision for income taxes, the Company's deferred tax assets and liabilities and any valuation allowance recorded against those net deferred tax assets. The Company evaluates the weight of all available evidence to determine whether it is more likely than not that some portion or all of the net deferred income tax assets will not be realized.
Effective January 1, 2007, the Company adopted FASB guidance on accounting for uncertainty in income taxes. This guidance prescribes the methodology by which a company must measure, report, present and disclose in its financial statements the effects of any uncertain tax return reporting positions that a company has taken or expects to take. See Note 11, "Income Taxes" for additional disclosure.
82
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
Stock-based compensation
Effective January 1, 2006, the Company adopted the FASB accounting guidance on fair value recognition provisions of share-based payment, using the modified prospective transition method. In accordance with the modified prospective transition method, results for prior periods have not been restated, and the impact of adopting this guidance was not material to the net loss or cash flows. For all grants, the amount of share-based compensation expense recognized has been adjusted for estimated forfeitures of awards for which the requisite service is not expected to be provided. Estimated forfeiture rates are developed based on the Company's analysis of historical forfeiture data.
The Company accounts for stock-based compensation issued to non-employees in accordance with FASB guidance on accounting for equity instruments that are issued to other than employees for acquiring, or in conjunction with, selling goods or services. The Company records the expense of such services based on the estimated fair value of the equity instrument using the Black-Scholes option pricing model. The value of the equity instrument is charged to earnings over the term of the service agreement.
Other Income
Other income in 2010 includes $472,000 of cash grants for qualifying therapeutic discovery projects that were awarded under the Patient Protection and Affordable Care Act of 2010.
Net loss per share
Basic net loss per share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. The Company's potential dilutive shares, which include outstanding common stock options, unvested restricted stock, and common stock warrants, have not been included in the computation of diluted net loss per share for all periods as the result would be antidilutive. Such potentially dilutive shares are excluded when the effect would be to reduce a net loss per share.
Other comprehensive income (loss)
FASB guidance on reporting comprehensive income establishes standards for reporting and displaying comprehensive income and its components in a full set of general-purpose financial statements. For each of the years ended December 31, 2008, 2009 and 2010, and the period from May 9, 2003 (date of inception) to December 31, 2010, there was no material difference between the net loss and comprehensive loss.
Segment reporting
FASB guidance on disclosure about segments of an enterprise and related information establishes standards for reporting information about operating segments in annual financial statements and in interim financial reports issued to stockholders. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated on a regular basis by the chief operating decision-maker, or decision making group, in deciding how to allocate resources to an individual segment and in assessing performance of the segment. The Company believes that it operates in one segment.
83
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
Recent accounting pronouncements
In October 2009, the FASB issued an amendment to the accounting guidance for revenue arrangements with multiple deliverables. This guidance provides principles for allocation of consideration among its multiple-elements, allowing more flexibility in identifying and accounting for separate deliverables under an arrangement. The guidance introduces an estimated selling price method for valuing the elements of a bundled arrangement if vendor-specific objective evidence or third-party evidence of selling price is not available, and significantly expands related disclosure requirements. This guidance is effective on a prospective basis for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Alternatively, adoption may be on a retrospective basis, and early application is permitted. The Company is planning on adopting this accounting guidance in the first quarter of 2011, prospectively, for any new revenue arrangement.
In October 2009, the FASB issued an amendment to the accounting standards related to certain revenue arrangements that include software elements. This standard clarifies the existing accounting guidance such that tangible products that contain both software and non-software components that function together to deliver the product's essential functionality, shall be excluded from the scope of the software revenue recognition accounting standards. Accordingly, sales of these products may fall within the scope of other revenue recognition accounting standards or may now be within the scope of this standard and may require an allocation of the arrangement consideration for each element of the arrangement. This standard, for which the Company is currently assessing the impact, will become effective for the Company on January 1, 2011. The adoption of this new accounting guidance is not expected to have a material impact on the Company's consolidated financial statements.
4. Inventory
The components of inventory are as follows (in thousands):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | |||||
Raw materials |
$ | 372 | $ | — | |||
Work in process |
2,462 | — | |||||
Finished goods |
3,993 | 2,935 | |||||
Inventory |
$ | 6,827 | $ | 2,935 | |||
The Company periodically reviews its inventories for excess or obsolete inventory and writes-down obsolete or otherwise unmarketable inventory to its estimated net realizable value. If the actual net realizable value is less than that estimated by the Company, or if there are any further determinations that inventory will not be marketable based on estimates of demand, additional inventory write-downs will be required. Based on management's estimates of demand, the Company determined that some of its inventory was in excess of its estimated net realizable value. During the year ended December 31, 2008, the Company recorded a $1.6 million charge to research and development expense to write-off excess and obsolete inventory. During the year ended December 31, 2009, the Company recorded a $3.1 million charge to write-off excess and obsolete inventory. This charge was recorded as a research and development expense resulted in a $1.7 million decrease to inventory and a $1.4 million increase to accrued expenses for excess non-cancellable purchase commitments. During the year ended
84
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Inventory (Continued)
December 31, 2010, the Company recorded a $2.6 million charge research and development expense to write-off excess and obsolete inventory.
5. Property and Equipment, net
Property and equipment, net consist of the following (in thousands):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | |||||
Machinery and equipment |
$ | 6,608 | $ | 126 | |||
Leasehold improvements |
1,871 | — | |||||
Software |
759 | — | |||||
Office furniture |
213 | — | |||||
|
9,451 | 126 | |||||
Less accumulated depreciation and amortization |
(7,745 | ) | — | ||||
Property and equipment, net |
$ | 1,706 | $ | 126 | |||
Depreciation and amortization charged to the consolidated statements of operations for the years ended December 31, 2008, 2009, and 2010 and from May 9, 2003 (date of inception) to December 31, 2010 was $2.5 million, $2.3 million, $1.0 million and $9.0 million, respectively.
The Company assesses its long-lived assets for impairment whenever events or changes in circumstances (a "triggering event") indicate that the carrying value of a group of long-lived assets may not be recoverable in accordance with FASB accounting guidance on accounting for the impairment or disposal of long-lived assets. During the third quarter of 2010, the Company altered its business direction as a result of its inability to raise funding to support the repositioning of the Company in the genetic analysis markets. As a result, the Company redirected its efforts to focus on a technology licensing and enforcement program to monetize the value of its intellectual property portfolio and to deploy its limited resources by concentrating its research and development efforts in targeted areas designed to achieve tangible proof-of-concept goals, which may enable the Company to seek partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives.
As a result of this altered business direction, the Company undertook an analysis of the ongoing carrying value of its long-lived assets. Based on this analysis, in fiscal year 2010 the Company recorded an impairment charge related to property and equipment of approximately $1.9 million, of which approximately $1.8 million was related to assets used in research and development activities and approximately $0.1 million was related to assets used for selling, general and administrative purposes. These charges were derived from the net book value of the assets.
85
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following (in thousands):
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | |||||
Professional fees |
$ | 684 | $ | 396 | |||
Compensation and benefits |
884 | 130 | |||||
Purchase commitments |
1,431 | 539 | |||||
Accrued interest |
86 | 22 | |||||
License fees |
87 | 90 | |||||
Other |
460 | 211 | |||||
Accrued expenses and other current liabilities |
$ | 3,632 | $ | 1,388 | |||
7. Commitments and Contingencies
License agreements and patents
In November 2003, the Company entered into a license agreement with California Institute of Technology (the "Caltech License Agreement") that granted the Company a worldwide, exclusive, royalty-bearing license, with the right to grant sublicenses, under specified patents and patent applications, and a worldwide, non-exclusive royalty bearing license, with the right to grant sublicenses, under specified technology outside the scope of the licensed patents. In connection with the Caltech License Agreement, the Company issued 46,514 shares of common stock, and recorded a charge of $20,000. In addition, the Company pays an annual license fee of $10,000 per year. The license fee payments are creditable against royalties based upon sales of products covered by patents licensed under the agreement. The up to single digit royalties are calculated based on a percentage of defined net sales. The Company is also obligated to pay California Institute of Technology up to a single digit percentage of specified license and sublicense income, a single digit percentage of proceeds from sales of specified intellectual property and a single digit percentage of service revenue amounts that it receives based on licenses and sublicenses that the Company grants, sales of intellectual property and services that are provided to third parties. The royalty obligation with respect to any licensed product extends until the later of the expiration of the last-to-expire of the licensed patents covering the licensed product and three years after the first commercial sale of the licensed product in any country for non-patented technology covered under the agreement. Through December 31, 2008, no royalty payments were made. In March 2007, the Company amended the Caltech License Agreement to provide rights under an additional patent application under the terms of the existing license in exchange for a one-time payment of $50,000 to the California Institute of Technology. There are no milestone payments potentially payable by the Company under the Caltech License Agreement in addition to those described above. All license fee amounts paid to date and the value of the common stock issued have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. The total expense recognized under the Caltech License Agreement for the years ended December 31, 2008, 2009 and 2010, and the period from May 9, 2003 (date of inception) through December 31, 2010 was $10,000, $10,000, $10,000 and $133,000, respectively.
In June 2004, the Company entered into a license agreement with Roche Diagnostics (the "Roche License Agreement") that granted the Company a worldwide, semi-exclusive royalty-bearing license,
86
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Commitments and Contingencies (Continued)
with the right to grant sublicenses under a patent relating to sequencing methods. In connection with the Roche License Agreement, the Company paid an upfront fee of 175,000 Euros and committed to pay an annual license fee ranging from 10,000 to 40,000 Euros. There are no milestone payments potentially payable by the Company under the Roche License Agreement in addition to those described above. The Company has an option to convert the license to non-exclusive beginning in 2008, in which case the annual license fees would be reduced to 10,000 Euros beginning in 2008. The Company has the right to terminate the Roche License Agreement at any time for convenience upon 90 days prior written notice to Roche Diagnostics. Both the Company and Roche Diagnostics have the right to terminate the Roche License Agreement upon breach by the other party, subject to notice and an opportunity to cure. The Roche License Agreement also terminates upon the occurrence of specified bankruptcy events. As part of the Roche License Agreement, the Company agrees to pay single digit royalties based on a percentage of defined net sales. The Company also agrees to pay half of income amounts that are received based on sublicenses that the Company grants to third parties. The Company's royalty obligation, if any, extends until the expiration of the last-to-expire of the licensed patents. Through December 31, 2008, no royalty payments were made. All license fee amounts paid to date have been expensed to research and development expense as technological feasibility had not established and the technology had no alternative future use. The total expense recognized under the Roche License Agreement for the years ended December 31, 2008, 2009 and 2010, and the period from May 9, 2003 (date of inception) through December 31, 2010 was $62,000, $54,000, $59,000 and $480,000, respectively.
In March 2005, the Company entered into a license agreement with Arizona Technology Enterprises (the "AZTE License Agreement") that granted the Company a worldwide, exclusive, irrevocable, royalty-bearing license, with the right to grant sublicenses, under specified patents and patent applications exclusively licensed by AZTE from Arizona State University and the University of Alberta. In connection with the AZTE License Agreement, the Company paid an upfront fee of $350,000, committed to an annual license fee of $50,000, which has increased to $100,000 upon the successful issuance of a U.S. patent, committed to pay a three-year maintenance fee of $50,000, payable in equal annual installments beginning in March 2006, and issued 88,888 shares of restricted common stock, which vested in two equal installments upon the achievement of separate milestones. There are no milestone payments potentially payable by the Company under the AZTE License Agreement in addition to those described above. The Company is obligated to use reasonable commercial efforts to develop, manufacture and commercialize licensed products. In addition, if the Company fails to use commercially-reasonable efforts to develop, manufacture and sell licensed products, the AZTE License Agreement converts from exclusive to non-exclusive. The AZTE License Agreement will remain in force until terminated. The Company has the right to terminate the AZTE License Agreement at any time for convenience upon 60 days prior written notice to Arizona Technology Enterprises. Both the Company and Arizona Technology Enterprises have the right to terminate the agreement upon breach by the other party, subject to notice and an opportunity to cure. The AZTE License Agreement also terminates upon the occurrence of specified bankruptcy events.
As part of the AZTE License Agreement, the Company agreed to pay a single digit percentage royalty based on defined net sales. The Company also agrees to pay a mid-teens percentage of specified sublicense income amounts that are received based on sublicenses granted to third parties which increases to 30 percent after the Company receives an aggregate of $50,000 of such amounts. The Company's royalty obligation, if any, extends until the expiration of the last-to-expire of the licensed
87
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Commitments and Contingencies (Continued)
patents. Through December 31, 2010, no royalty payments have been made. All license fee amounts paid to date have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. In May 2006, in accordance with the license agreement, due to the successful issuance of a U.S. patent, the committed annual license fee increased from $50,000 to $100,000 and 44,444 shares of the restricted common stock vested. The vesting of 44,444 shares of restricted common stock resulted in a charge to research and development expense of $127,000 based on the fair value of the Company's common stock at the time the milestone was achieved. The remaining 44,444 shares of restricted common stock vested immediately upon the successful issuance of a second U.S. patent on January 12, 2010 and resulted in a charge to research and development expense in fiscal 2010 of $54,000. The total expense recognized under the AZTE License Agreement for the years ended December 31, 2008, 2009 and 2010, and the period from May 9, 2003 (date of inception) through December 31, 2010 was $103,000, $141,000, $154,000 and $1,144,000, respectively.
In April 2007, the Company entered into an agreement with PerkinElmer LAS, Inc. ("PerkinElmer"), in which PerkinElmer granted the Company a worldwide, non-exclusive, non-transferable, non-sublicensable, royalty bearing license under specified patents. The license from PerkinElmer grants the Company rights under certain patents to produce and commercialize certain of the reagents used in some applications on the Helicos System, which contain chemicals purchased from PerkinElmer. In exchange for rights licensed from PerkinElmer, the Company is obligated to pay PerkinElmer a single digit percentage of the Company's net revenue from the sale of reagents that contain chemicals covered by the patents licensed under the PerkinElmer agreement. There are no milestone payments potentially payable by the Company under this agreement with PerkinElmer. The Company has the right to terminate the agreement at any time upon 90 days written notice to PerkinElmer. Each party has the right to terminate the agreement upon breach by the other party subject to notice and an opportunity to cure. The agreement also terminates upon the occurrence of specified bankruptcy events. PerkinElmer has the sole right under the agreement to enforce the licensed patents. There has been no expense recorded for this agreement for any period from May 9, 2003 (inception) through December 31, 2010.
Operating leases
In December 2005, the Company entered into an operating lease for new office and laboratory space. The lease expired in March 2010. In connection with this lease agreement, the Company entered into a letter of credit in the amount of $450,000, naming the Company's landlord as beneficiary. In October 2008, the letter of credit was decreased to $225,000 pursuant to the lease agreement which allows for a fifty percent reduction provided that the Company was not in default and that the lease was in full force and effect. As of December 31, 2009, the Company classified the $225,000 letter of credit as restricted cash on the consolidated balance sheet. As of December 31, 2010, the landlord fully utilized the letter of credit in lieu of rent payments from the Company. Additionally, in connection with the lease agreement, the Company received lease incentives from the landlord of certain leasehold improvements. The Company recorded the lease incentives as a liability and is amortizing them over the lease term as a reduction in rent expense. For the years ended December 31, 2008, 2009 and 2010, the Company recorded $149,000, $105,000 and $17,000, respectively, as a reduction in rent expense for this amortization. In March 2006, February and December 2007, July 2008, October 2009 and June 2010, the Company amended its operating lease for office and laboratory space to include additional
88
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Commitments and Contingencies (Continued)
office space in the same building. This lease expired as of December 31, 2010. In fiscal year 2010, under the terms of the lease agreement, the Company realized a rent abatement of $115,000 from the lessor in connection with a casualty loss. Effective December 31, 2010, the Company consolidated its operations into a single space which is leased on a tenant-at-will basis. Total rent expense was $1.9 million, $2.0 million, $1.3 million and $8.0 million for the years ended December 31, 2008, 2009, and 2010, and the period from May 9, 2003 (date of inception) through December 31, 2010, respectively.
The Company records rent expense on a straight-line basis over the term. Accordingly, the Company recorded a liability for deferred rent in accrued expenses and other current liabilities at December 31, 2009 and 2010 of $17,000 and $0, respectively.
Debt facilities
Pursuant to the bridge financing and senior debt restructuring which occurred in November 2010, the Company may be liable for certain contingent payments based upon potential future events. (See "Bridge Financing and Senior Debt Restructuring" in Note 8.)
Management Incentive Plan
In approving the transactions relating to the Bridge Debt Financing and the Fourth Amendment (see Note 8), the Board approved a management incentive plan (the "Management Incentive Plan") pursuant to which participants in the plan will be entitled to receive, in the aggregate, an amount equal to 7.5% of the Payment Consideration resulting from IP Licensing Events (gross proceeds from IP Licensing Events less various contractual and contingent payments). The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the Board. As of December 31, 2010, no amounts have been accrued and no payments have been made pursuant to the Management Incentive Plan.
Litigation
On October 14, 2010, Helicos announced the launch of a technology licensing program for its Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. The Company plans to defend its intellectual property rights through licensing and enforcement strategies. In this regard, on October 22, 2010, the Company filed an amended complaint against Pacific Biosciences by naming Illumina, Inc. and Life Technologies Corporation as additional defendants. The amended lawsuit alleges that the defendants willfully infringe the Company's patents in suit by using their respective technologies, sequencing systems and products which the Company alleges are within the scope of one or more claims of the Company's patents in suit. (See Note 1 of Notes to Consolidated Financial Statements.) Helicos is seeking a permanent injunction enjoining the defendants from further infringement as well as monetary and punitive damages, costs and attorneys' fees, interest and other relief as determined by the Court. A portion of any proceeds received by the Company pursuant to this amended lawsuit is due to counsel representing the Company in the action as counsel's only fees relating to their representation and to the Purchasers on the Notes issued pursuant to the Risk Premium Agreement entered into between the Company and the Purchasers of the Notes in connection with the Bridge Debt Financing. (See Note 8.)
89
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Debt
Loan and security agreement
In December 2007, the Company entered into a loan and security agreement with two lenders including GE Capital Corporation, which is serving as agent. The loan agreement provided that the Company may borrow up to $20.0 million at an interest rate equal to the sum of (i) the greater of (A) an interest rate based on the Federal Reserve's three year Treasury Constant Maturities Rate and (B) 3.84% plus (ii) 6.11%. The initial term loan was made in December 2007 in an aggregate principal amount equal to $10.0 million.
In June 2008, the Company entered into a second amendment to the loan and security agreement with two lenders including GE Capital Corporation. A subsequent term loan was made upon execution of the amendment in an aggregate principal amount equal to $10.0 million. The loan amendment provided that the interest rate for the subsequent term loan is equal to the sum of (i) the greater of (A) an interest rate based on the Federal Reserve's three year Treasury Constant Maturities Rate and (B) 3.17% plus (ii) 8.33%. The loan agreement, as amended, contained affirmative and negative covenants to which the Company and its subsidiaries must adhere. Pursuant to the amendment, the Company was required to maintain, at all times, unrestricted cash in its bank account equal to at least $10.0 million. The proceeds of the loan agreement were collateralized by essentially all of the Company's assets. Payments are required to be made on a monthly basis. For the initial term loan, interest-only payments were required for the first five months. Thereafter, for the following 31 months, payments of principal and interest will be due. For the subsequent term loan, principal and interest payments are required for the 36 month term of the loan. In connection with the execution of the June 2008 amendment to the loan and security agreement with two lenders including GE Capital Corporation, the Company issued warrants to the two lenders to purchase an aggregate of 110,000 shares of common stock. The warrants have an exercise price of $4.80 per share and expire in June 2014. The fair value of the warrants was estimated at $337,000 using a Black-Scholes model with the following assumptions: expected volatility of 65.4%, risk free interest rate of 3.4%, expected life of six years and no dividends. Expected volatility was based on the volatility of similar entities in the life sciences industry of comparable size of market capitalization and financial position that completed initial public offerings within the last ten years. The fair value of the warrants was recorded as equity and a debt discount and is being amortized to interest expense over the term of the loan. In connection with the senior debt restructuring in November 2010, the exercise price of the warrants was reduced to $0.01 per share and the Company recorded $47,000 of additional debt discount to reflect the change in the value of the warrants and is amortizing this amount over the term of the loan.
In December 2008, the Company entered into an additional amendment to the loan and security agreement with certain lenders including GE Capital Corporation. The amendment amended the prepayment provisions of the loan and security agreement to allow the Company to make a prepayment of $10.0 million (the "Pay Down Amount") without incurring any prepayment penalties. Pursuant to the amendment, the Company made a prepayment, equal to the Pay Down Amount, in December 2008. In connection with such prepayment, and in lieu of the 4% final payment fee with respect to the Pay Down Amount, the amendment provides that the Company will pay a fee equal to 2% of the initial $10.0 million term loan, payable on the earlier of (a) January 31, 2011 and (b) the maturity date for the subsequent term loan. In connection with the senior debt restructuring in November 2010, the due date for this fee was deferred to the amended maturity date for the subsequent term loan.
90
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Debt (Continued)
The amendment further provides that the Company's obligations under the loan agreement, as amended, are no longer secured by a cash amount of $10.0 million. As such, this amount is no longer classified as restricted cash as of December 31, 2008. Such obligations continue to be secured under various collateral documents by interests in substantially all of the Company's personal property, including the pledge of the stock of its wholly-owned subsidiary, and proceeds of any intellectual property, but not by its intellectual property. In connection with the Senior Debt Restructuring in November 2010, the collateral securing this facility was expanded to include the Company's intellectual property.
The loan and security agreement contains a subjective material adverse clause, which allows the debt holders to call the loans upon a material adverse event. Based on an updated assessment of the terms of the loan and security agreement, the entire balance due is classified in the current liabilities section in the accompanying balance sheet at December 31, 2010.
As of December 31, 2009, the loan agreement did not require the Company to comply with any financial covenants. Through December 31, 2010, advances on the loan agreement, as amended, were $20.0 million at an interest rate of 11.5%. As of December 31, 2009 and 2010, the outstanding balance on the loan agreement was $4.9 million and $2.8 million, respectively. This loan agreement was amended in November 2010 (see "Bridge Financing and Senior Debt Restructuring" below).
Bridge Financing and Senior Debt Restructuring
The Company entered the fourth quarter of 2010 without any prospects of securing long-term financing from outside investors. An independent committee ("Independent Committee") of the Board evaluated the alternatives available to the Company and determined that a bridge financing led by certain inside investors was in the best interests of its stakeholders in view of its current financial and operating condition, and would best position the Company to continue operations for the remainder of 2010 and into 2011. The Independent Committee met multiple times to consider the alternatives and concluded that the bridge financing was the best alternative for generating value for stakeholders. As a result, the Company entered into an insider bridge loan facility with two of the four venture capital firms whose representatives are members of the Board. In connection with this transaction, the Company entered into a Subordinated Secured Note Purchase Agreement (the "Note Purchase Agreement") with investment funds affiliated with Atlas Venture and Flagship Ventures (the "Purchasers"), pursuant to which the Company agreed to sell to the Purchasers, and the Purchasers have agreed to purchase, up to an aggregate of $4,000,000 of convertible promissory notes (the "Notes"), a portion of which is committed and a portion of which is optional (the "Bridge Debt Financing"). On November 16, 2010, the Purchasers purchased Notes having an aggregate principal amount of $333,333. Subject to the Company's continued compliance with the terms of the Note Purchase Agreement, including that there not be an event of default thereunder, upon notice from the Company the Purchasers are committed to purchase an aggregate of an additional $333,333 of Notes in each of five subsequent closings, for a total aggregate committed amount of $2,000,000. The Purchasers also have the right, but not the obligation, upon the Company's request to purchase up to an additional $2,000,000 of Notes on or before December 31, 2012. As of December 31, 2010 and as of the date of this filing, the Company has borrowed $666,667 against the Bridge Debt Financing facility.
The Notes will accrue interest at a rate of 10% per annum plus a risk premium as described below. The outstanding principal and any unpaid accrued interest on all of the Notes shall be due and
91
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Debt (Continued)
payable in full (i) upon demand by the Purchasers, which demand shall be no earlier than December 31, 2012, (ii) upon the Company receiving at least $10,000,000 in proceeds from a subsequent equity financing, (iii) upon a change of control of the Company or (iv) upon an event of default. A Purchaser may elect to convert principal and interest outstanding under any of the Notes upon the closing of any future round of equity or debt financing of the Company into shares, and at the per share price, of the securities issued at such financing. Simultaneous with execution of the Note Purchase Agreement, the Company and the requisite parties entered into an Amendment to the Amended and Restated Investor Rights Agreement, dated as of March 1, 2006, by and among the Company and the parties specified therein, including the Purchasers (the "IRA Amendment"), pursuant to which the Purchasers will be entitled to demand and piggyback registration rights consistent with the terms of the Purchasers' existing registration rights for any securities issued upon conversion of the Notes.
The Company's obligations under the Notes are subordinated to the indebtedness incurred by the Company under the Loan and Security Agreement among the Company, certain lenders (the "Lenders") and General Electric Capital Corporation ("GE"), as agent, dated as of December 31, 2007, as amended (the "Loan Agreement"). The Company's obligations under the Notes are secured by a security interest on all of the Company's assets, including its intellectual property, that, with regard to all of the Company's assets other than the cause of action that is the subject of the intellectual property litigation against Pacific Biosciences, Illumina and Life Technologies (and any recovery therefrom) (the "Cause of Action"), is junior to the Lenders' first priority security interest under the Loan Agreement, and with regard to the Cause of Action, is junior to the Lenders' first priority security interest under the Loan Agreement and the second priority security interest of its outside legal counsel that is representing the Company in the Cause of Action (the "Outside IP Litigation Counsel").
As an inducement for the Purchasers to purchase the Notes, the Company entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement") simultaneous with the execution of, and as required by, the Note Purchase Agreement. Under the terms of the Risk Premium Agreement, the Company agreed to pay the Purchasers the following portions of the consideration it receives (the "Payment Consideration") in Liquidity Transactions, as defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30% of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). For purposes of determining amounts payable under the Risk Premium Agreement, the Payment Consideration will not include amounts the Company uses to first satisfy specified existing obligations, including obligations under the Loan Agreement, under professional contingency arrangements (including the contingency fee arrangement between the Company and Outside IP Litigation Counsel relating to the Cause of Action) and obligations that may arise under existing technology license agreements (the "Existing IP Licensing Agreements") between the Company and each of Arizona Technology Enterprises, Roche Diagnostics GMBH and Roche Diagnostics Corporation, and California Institute of Technology (as previously disclosed by the Company, under the Existing IP Licensing Agreements, the Company is obligated to pay license fees in connection with the licensing, sublicensing, legal settlements or sales of specified intellectual property
92
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Debt (Continued)
or services provided to third parties ranging from a single digit percentage up to one-half of the income from those activities). For purposes of the Risk Premium Agreement and subject to various provisions therein, the following shall constitute Liquidity Transactions: (A) the receipt by the Company of any payments from third parties relating to its intellectual property portfolio of the Company, including payments received as a license or other settlement in patent infringement litigation brought by the Company ("IP Licensing Events"); (B) a merger of the Company in which there is a change of control; and (C) the sale or exclusive license by the Company of all or substantially all of its assets or intellectual property.
In connection with the Bridge Debt Financing, on November 16, 2010, the Company, GE and the Lenders executed the Fourth Amendment to the Loan and Security Agreement, dated November 16, 2010 (the "Fourth Amendment"). Pursuant to the Fourth Amendment, GE and the Lenders waived various existing events of default and consented to the Company's incurrence of additional indebtedness and the grant of a junior security interest to the Purchasers under the Note Purchase Agreement. The Company amended warrants previously issued to the Lenders to re-price the Warrants from $4.80 to $0.01 and GE and the Lenders agreed to defer a $200,000 payment originally due January 31, 2011 to the January 1, 2012 term loan maturity date. In addition, subject to the Company's continued compliance with the terms of the Loan Agreement, including that there not be an event of default thereunder, GE and the Lenders agreed to defer up to five additional principal monthly payments with respect to the term loan. Also pursuant to the Fourth Amendment, the Company agreed to grant the Lenders a first priority security interest in all of its intellectual property and to pay the Lenders an additional $50,000 to $200,000 (depending upon how many principal payments are deferred) upon the full repayment of all outstanding amounts due with respect to the term loans. Under the terms of the Loan and Security Agreement prior to its amendment by the Fourth Amendment, the scope of the Lenders' first priority security interest included all of its assets other than intellectual property. As of the date of this filing, the Lenders have agreed to defer the additional five monthly principal payments referenced above.
In connection with the Bridge Debt Financing and the Fourth Amendment and as security for the Company's obligations under its contingency fee arrangement with Outside IP Litigation Counsel relating to the Cause of Action, the Company and Outside IP Litigation Counsel entered into a Subordinated Security Agreement pursuant to which the Company agreed to grant Outside IP Litigation Counsel a second priority security interest in the Cause of Action. Under the terms of the contingency fee arrangement, the Company is obligated to pay Outside IP Litigation Counsel up to 40% of the proceeds paid to the Company in connection with the settlement, judgment or other resolution of the Cause of Action or certain business transactions resulting from or relating to the Cause of Action.
The agreements and arrangements described above and all of the transactions contemplated by those agreements were approved by an independent committee of the Board of Directors of the Company. In addition, Noubar B. Afeyan, PhD, who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, who is a Partner of Atlas Venture, abstained from the vote of the Board of Directors of the Company approving the transactions.
93
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Debt (Continued)
As of December 31, 2010, payments relating to the senior debt and the Notes are due as follows (in thousands):
| |
Senior Debt |
Notes | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
2011 |
$ | 3,250 | $ | — | $ | 3,250 | |||||
2012 |
— | 821 | 821 | ||||||||
Thereafter |
— | — | — | ||||||||
Total future minimum payments |
3,250 | 821 | 4,071 | ||||||||
Less: amount representing interest |
(195 | ) | (154 | ) | (349 | ) | |||||
Less: debt discount |
(800 | ) | — | (800 | ) | ||||||
Add: interest accrued |
— | 7 | 7 | ||||||||
Add: amortization of debt discount |
524 | — | 524 | ||||||||
Carrying value of debt |
2,779 | 674 | 3,453 | ||||||||
Less: current portion |
(2,779 | ) | — | (2,779 | ) | ||||||
Long-term obligations |
$ | — | $ | 674 | $ | 674 | |||||
The above table does not reflect any Risk Premium Payments potentially due pursuant to the Risk Premium Agreement.
9. Corporate Restructuring
In December 2008, the Company implemented a reduction in force plan that resulted in the reduction of approximately 30% of its workforce and restructuring charges relating to one-time termination benefits of approximately $433,000. These charges represent employee severance and termination costs which were paid out during the fourth quarter of 2008 and the first quarter of 2009. As of December 31, 2009 and 2010, $133,000 and $0, respectively, of such restructuring costs remained to be paid.
On May 11, 2010, the Board of Directors of the Company approved a reduction in headcount and restructuring plan (the "May 2010 Restructuring Plan") which involved the consolidation and reorganization of its operations in order to reduce operating costs. The May 2010 Restructuring Plan resulted in the elimination of approximately 40 positions during the second fiscal quarter of 2010. Employees directly affected by the May 2010 Restructuring Plan were notified on May 13, 2010, and were provided with severance arrangements including outplacement assistance. These actions were approved as part of the Company's repositioning strategy in the diagnostics market. As of December 31, 2010, there were no remaining payments due pursuant to this restructuring.
The Company incurred restructuring charges relating to one-time termination benefits of approximately $549,000 in fiscal year 2010, of which $543,000 was recorded in research and development expense and $6,000 was recorded in selling, general and administrative expense. These charges represent employee severance and termination costs as well as facility consolidation costs, which were paid during 2010. As of December 31, 2010, there were no remaining payments due pursuant to this restructuring.
94
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. Corporate Restructuring (Continued)
On September 15, 2010, the Board of Directors of the Company approved a reduction in headcount and restructuring plan (the "September 2010 Restructuring Plan") which involved the consolidation and reorganization of its operations in order to reduce operating costs. The September 2010 Restructuring Plan resulted in the elimination of approximately 14 positions during the third fiscal quarter of 2010. Employees directly affected by the September 2010 Restructuring Plan were notified on September 16, 2010, and have been provided with severance arrangements including outplacement assistance. These actions were approved as part of the Company's repositioning strategy in the diagnostics market.
The Company incurred restructuring charges relating to one-time termination benefits of approximately $172,000 in fiscal year 2010, all of which was recorded in research and development expense. These charges represent employee severance and termination costs. As of December 31, 2010, $11,000 of such restructurings costs remained to be paid.
10. Common Stock and Warrant Liability
As of December 31, 2009 and 2010, the Company had 120,000,000 shares of common stock authorized. As of December 31, 2009 and 2010, the Company had 79,370,610 and 86,557,606 shares issued and outstanding, respectively. As of December 31, 2010, the Company had reserved 23,917,179 shares of common stock for issuance to common stockholders upon exercise of common stock warrants. As of December 31, 2009 and 2010, the Company has reserved 4,761,135 and 5,757,926 shares of common stock, respectively, for future issuance upon exercise of outstanding and future grants of common stock options and future issuances of restricted stock awards pursuant to the Company's 2003 and 2007 stock option plans.
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company's stockholders. Common stockholders are not entitled to receive dividends unless declared by the Company's Board of Directors.
December 2008 Financing
In December 2008, the Company entered into a securities purchase agreement with certain investors pursuant to which it sold a total of 42,753,869 units (the "Units"), each Unit consisting of (i) one share of common stock (collectively, the "Shares") and (ii) one warrant (collectively, the "Warrants") to purchase 0.6 shares of common stock at an exercise price of $0.45 per share, for a purchase price of $0.435 per unit (representing the closing bid price plus an additional amount for the warrants) (the "Offering"). The closing of the transaction occurred on December 23, 2008. In connection with the Offering, the Company raised approximately $18.6 million in gross proceeds. After paying $813,000 in placement agent fees and offering expenses, the net proceeds were $17.8 million.
In connection with the Offering, the Company issued warrants to purchase an aggregate of 25,652,333 shares of common stock which are exercisable immediately. The warrants have an exercise price of $0.45 per share and have a five year term. The relative fair value of the warrants was estimated at $4,449,397 using a Black-Scholes model with the following assumptions: expected volatility of 66.15%, risk free interest rate of 1.53%, expected life of five years and no dividends. Expected volatility was based on the volatility of similar entities in the life sciences industry of comparable size of market capitalization and financial position that completed initial public offerings within the last ten years. The relative fair value of the warrants was recorded in the equity section of the balance sheet.
95
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. Common Stock and Warrant Liability (Continued)
In connection with the Offering, the Company entered into a registration rights agreement (the "Registration Rights Agreement") with each of the investors. The Registration Rights Agreement provides that the Company file a "resale" registration statement (the "Registration Statement") covering all of the Shares and the shares issuable upon exercise of the Warrants (the "Warrant Shares"), up to the maximum number of shares able to be registered pursuant to applicable Securities and Exchange Commission ("SEC") regulations, within 30 days of the closing of the Offering. The Company filed the Registration Statement with the SEC on January 22, 2009 (File No. 333-156885), which was amended on February 13, 2009. Under the terms of the Registration Rights Agreement, the Company is obligated to maintain the effectiveness of the "resale" registration statement until all securities therein are sold or can otherwise be sold pursuant to Rule 144, without any restrictions.
A cash penalty at the rate of 2% per month will be triggered for any filing or effectiveness failures or if, at any time after six months following the closing of the Offering, the Company ceases to be current in periodic reports with the SEC. The aggregate penalty accrued with respect to each investor may not exceed 12% of the original purchase price paid by that investor.
In December 2006, the FASB issued guidance on accounting for registration payment arrangements, which addresses an issuer's accounting for registration payment arrangements. This guidance specifies that the contingent obligation to make future payments or otherwise transfer consideration under a registration payment arrangement, whether issued as a separate agreement or included as a provision of a financial instrument or other agreement, should be separately recognized and measured in accordance with FASB guidance on accounting for contingencies. This guidance further clarifies that a financial instrument subject to a registration payment arrangement should be accounted for in accordance with US GAAP without regard to the contingent obligation to transfer consideration pursuant to the registration payment arrangement. The Company applied the recognition and measurement provisions of the FASB guidance to the registration rights associated with the Registration Rights Agreement. As result, the Company believes that the contingent obligation to make future payments is not probable under the guidance and as such has recorded no liability associated with these registration rights.
September 2009 Financing
In September 2009, the Company entered into a securities purchase agreement with certain investors pursuant to which it (i) sold to certain existing investors a total of 1,030,028 units, each unit consisting of (a) one share of common stock (collectively, the "Shares") and (b) one warrant (collectively, the "Warrants") to purchase 0.662 shares of common stock at an exercise price equal to $2.61 per share (105% of the closing bid price of the common stock on September 15, 2009), at a purchase price equal to $2.57 per unit, and (ii) sold to certain new investors a total of 3,281,252 units, each unit consisting of (a) one share of common stock and (b) one warrant to purchase 0.50 shares of common stock at an exercise price equal to $2.61 per share, at a purchase price equal to $2.24 per unit (together, the "September 2009 Offering"). The closing of the transaction occurred on September 18, 2009. In connection with the September 2009 Offering, the Company raised approximately $10.0 million in gross proceeds. After paying $646,000 in placement agent fees and offering expenses, the net proceeds were $9.4 million.
In connection with the September 2009 Offering, the Company issued warrants to purchase an aggregate of 2,322,504 shares of common stock which become exercisable on or after the six month
96
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. Common Stock and Warrant Liability (Continued)
anniversary following the closing of the transaction. The warrants had an exercise price of $2.61 per share and have a five and a half year term. The fair value of the warrants was estimated at $4.5 million using a Black-Scholes model with the following assumptions: expected volatility of 100%, risk free interest rate of 2.49%, expected life of five and a half years and no dividends. Expected volatility was based on the volatility contractually specified in the warrant agreements. Due to a price adjustment clause included in the warrant agreements, the warrants were deemed to be a liability and, therefore, the fair value of the warrants was recorded in the liability section of the balance sheet. As such, the warrants will be revalued each reporting period, with the resulting gains and losses recorded as the change in fair value of warrant liability on the statement of operations. In connection with the December 2009 Offering, the exercise price for the warrants issued in this transaction was subsequently reduced to $0.31 per share in accordance with the terms of the warrant. The Company recorded a gain in the amount of $2.4 million for the change in the fair value of the warrant liability from the closing date of September 18, 2009 through the end of the year December 31, 2009. For the twelve month period ended December 31, 2010 the Company recorded a gain in the amount of $1.9 million for the change in the fair value of the warrant liability. In connection with the Bridge Debt Financing and senior debt restructuring (see Note 8), the exercise price for the warrants held by non-related parties was further reduced to $0.01 per share. At December 31, 2010, related parties held 681,882 warrants at an exercise of $2.61 per share. Such warrant holders are entitled to a reduction in the exercise price to $0.01 per share following an affirmative approval from the Company's Stockholders. During the twelve months ended December 31, 2010, 1,417,410 warrant shares from the September 2009 Offering were exercised into common stock at an exercise price ranging from $0.01 to $0.3124 per share.
In connection with the September 2009 Offering, the Company entered into a registration rights agreement (the "September 2009 Registration Rights Agreement") with each of the investors. The Registration Rights Agreement provides that the Company will file a "resale" registration statement covering all of the shares of common stock and the shares of common stock issuable upon exercise of the warrants, up to the maximum number of shares able to be registered pursuant to applicable SEC regulations, within 15 days of the closing of the September 2009 Offering. The Company currently maintains an effective registration statement relating to these shares (File No. 333-162240). Under the terms of the September 2009 Registration Rights Agreement, the Company is obligated to maintain the effectiveness of the "resale" registration statement until all securities therein are sold or can otherwise be sold pursuant to Rule 144, without any restrictions. A cash penalty at the rate of 2% per month will be triggered for any filing or effectiveness failures or 1% in the event of suspensions of prospectus delivery or if, at any time after six months following the closing of the September 2009 Offering, the Company ceases to be current in its periodic reports with the SEC. The aggregate penalty accrued with respect to each investor may not exceed 12% of the original purchase price paid by that investor. The Company has not recorded an amount associated with the contingent obligation regarding the cash penalties as of December 31, 2010 as the Company believes that the contingent obligation to make future payments is not probable.
December 2009 Financing
In December 2009, the Company entered into an Underwriting Agreement (the "Underwriting Agreement") with Thomas Weisel Partners LLC (the "Underwriter"), pursuant to which it sold to the Underwriter 6,400,000 shares of common stock and warrants to purchase up to 4,160,000 shares of common stock sold in units, at a price of $1.00 per unit ("December 2009 Offering"). Each unit
97
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. Common Stock and Warrant Liability (Continued)
consists of one share of common stock and a warrant to buy 0.65 of a share of common stock. The warrants will be exercisable for a period of five years, beginning six months after issuance, at an exercise price of $1.4385 per share. The closing of the transaction occurred on December 15, 2009. The Company received approximately $6.4 million in gross proceeds from the December 2009 Offering. After paying $718,000 in underwriting discounts, commissions and offering expenses, the net proceeds were $5.6 million.
In connection with the December 2009 Offering, the Company issued warrants to purchase an aggregate of 4,160,000 shares of common stock. The warrants had an exercise price of $1.44 per share and have a five and a half year term. The fair value of the warrants was estimated at $2.7 million using a Black-Scholes model with the following assumptions: expected volatility of 100%, risk free interest rate of 2.43%, expected life of five and a half years and no dividends. Expected volatility was based on the volatility contractually specified in the warrant agreements. Due to a price adjustment clause included in the warrant agreements, the warrants were deemed to be a liability and, therefore, the fair value of the warrants was recorded in the liability section of the balance sheet. As such, the warrants will be revalued each reporting period, with the resulting gains and losses recorded as the change in fair value of warrant liability on the income statement. The Company recorded a gain in the amount of $0.4 million for the change in the fair value of the warrant liability from the closing date of September 18, 2009 through the end of the year December 31, 2009. For the twelve month period ended December 31, 2010 the Company recorded a gain in the amount of $2.2 million for the change in the fair value of the warrant liability. In connection with the Bridge Debt Financing and senior debt restructuring (see Note 8), the exercise price for the warrants was reduced to $0.01 per share. During the twelve months ended December 31, 2010, 3,120,000 warrant shares from the December 2009 Offering were exercised into common stock at an exercise price of $0.01 per share.
11. Income Taxes
There is no provision for income taxes because the Company has incurred operating losses since inception. The reported amount of income tax expense for the years differs from the amount that would result from applying domestic federal statutory tax rates to pretax losses primarily because of the changes in the valuation allowance. Significant components of the Company's deferred tax assets at December 31, 2009 and 2010 are as follows (in thousands):
| |
December 31, | |||||||
|---|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | ||||||
Deferred tax assets: |
||||||||
Net operating loss carryforwards |
$ | 42,989 | $ | 47,695 | ||||
Research and development credit carryforwards |
4,873 | 5,082 | ||||||
Depreciation and amortization |
8,743 | 11,164 | ||||||
Allowances and reserves |
3,243 | 2,490 | ||||||
|
59,848 | 66,431 | ||||||
Less: Valuation allowance |
(59,848 | ) | (66,431 | ) | ||||
Net deferred tax assets |
$ | — | $ | — | ||||
98
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Income Taxes (Continued)
As of December 31, 2010, the Company has federal and state net operating losses ("NOL") of approximately $121.8 million and $109.7 million, respectively, as well as federal and state research and development credits of approximately $3.4 million and $2.6 million, respectively, which may be available to reduce future taxable income and taxes. Federal NOLs and research and development credits each begin to expire in 2024. State NOLs and research and development credits begin to expire in 2010 and 2019, respectively. As required by FASB accounting guidance on accounting for income taxes, the Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets, which are comprised principally of NOLs. Management has determined that is it more likely than not that the Company will not recognize the benefits of the federal and state deferred tax assets and, as a result, a valuation allowance of $59.8 million and $66.4 million has been established at December 31, 2009 and 2010, respectively.
A reconciliation of income tax expense (benefit) at the statutory federal income tax rate and income taxes as reflected in the financial statements is as follows:
| |
Year ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | ||||||||
Federal income tax at statutory rate |
34.0 | % | 34.0 | % | 34.0 | % | |||||
State income tax, net of federal tax benefit |
5.9 | % | 4.2 | % | 2.5 | % | |||||
Research and development credits |
1.9 | % | 1.9 | % | 1.1 | % | |||||
Warrant revaluation |
— | % | 2.4 | % | 7.5 | % | |||||
Book compensation related to stock options |
(2.1 | )% | (4.1 | )% | (9.6 | )% | |||||
Other |
(0.1 | )% | — | % | (0.3 | )% | |||||
Increase in valuation allowance |
(39.6 | )% | (38.4 | )% | (35.2 | )% | |||||
Effective tax rate |
— | % | — | % | — | % | |||||
On January 1, 2007, the Company adopted the FASB accounting guidance on uncertain tax provisions. The Company has no amounts recorded for any unrecognized tax benefits as of December 31, 2009 and December 31, 2010. In addition, the Company did not record any amount for the implementation of this guidance. The Company's policy is to record estimated interest and penalties related to the underpayment of income taxes as a component of its income tax provision. As of December 31, 2009 and December 31, 2010, the Company had no accrued interest or tax penalties recorded. Each of the Company's income tax return reporting periods since May 9, 2003 (date of inception) are open to income tax audit examination by the federal and state tax authorities.
Utilization of NOL and research and development credit carryforwards may be subject to a substantial annual limitation due to ownership changes that have occurred previously or that could occur in the future as provided by Section 382 of the Internal Revenue Code of 1986, as well as similar state provisions. These ownership changes may limit the amount of NOL and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period. Since the Company's formation, the Company has raised capital through the issuance of common stock and preferred stock, which, combined with the purchasing shareholders' subsequent disposition of those shares, may have resulted in a change of control, as defined by Section 382, or could result in a change of control in the future upon subsequent
99
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Income Taxes (Continued)
disposition. The Company has not currently completed a study to assess whether a change of control has occurred or whether there have been multiple changes of control since the Company's formation due to the significant complexity and cost associated with such study because there could be additional changes in control in the future. If the Company has experienced a change of control at any time since the Company's formation, utilization of NOL or research and development credit carryforwards would be subject to an annual limitation under Section 382. Any limitation may result in expiration of a portion of the NOL or research and development credit carryforwards before utilization. Further, until a study is completed and any limitation known, no amounts are being presented as an uncertain tax position.
The change in the valuation allowance against the deferred tax assets in the three years ended December 31, 2008, 2009 and 2010 was as follows ($ in thousands):
Fiscal Year
|
Balance at Beginning of Period |
Additions | Balance at End of Period |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
2008 |
$ | 32,298 | $ | 18,022 | $ | 50,320 | ||||
2009 |
50,320 | 9,528 | 59,848 | |||||||
2010 |
59,848 | 6,583 | 66,431 | |||||||
12. Stock-Based Compensation
In 2003, the Company's Board of Directors adopted the 2003 Stock Option and Incentive Plan (the "2003 Stock Plan"). The 2003 Stock Plan provides for the granting of incentive and non-qualified stock options, restricted stock and other equity awards to employees, officers, directors, consultants and advisors of the Company. Provisions such as vesting, repurchase and exercise conditions and limitations are determined by the Board of Directors on the grant date. The Company's 2007 Stock Option and Incentive Plan (the "2007 Stock Plan") was adopted by the Company's Board of Directors in April 2007 and approved by the Company's stockholders in May 2007. The 2007 Stock Plan permits the Company to make grants of incentive stock options, non-qualified stock options, stock appreciation rights, deferred stock awards, restricted stock awards, unrestricted stock awards and dividend equivalent rights. The 2007 Stock Plan provides that the number of shares reserved and available for issuance under the plan will be automatically increased each January 1, beginning in 2008, by 4.5% of the outstanding number of shares of common stock on the immediately preceding December 31 or such lower number of shares of common stock as determined by the Board of Directors. This number is subject to adjustment in the event of a stock split, stock dividend or other change in the Company's capitalization. Generally, shares that are forfeited or canceled from awards under the 2007 Stock Plan also will be available for future awards. In addition, available shares under the Company's 2003 Stock Plan, including as a result of the forfeiture, expiration, cancellation, termination or net issuances of awards, are automatically made available for issuance under the 2007 Stock Plan. The total maximum number of shares of common stock that may be issued pursuant to the 2007 Stock Plan after December 31, 2010 is 5,757,926. As of December 31, 2010, 3,637,865 shares of common stock are available for issuance under the 2007 Stock Plan.
Stock options
During the years ended December 31, 2008, 2009, 2010 and the period from May 9, 2003 (date of inception) to December 31, 2010, the Company granted 1,163,091, 2,633,807, 10,750 and 6,120,821 stock
100
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Stock-Based Compensation (Continued)
options, respectively to certain employees and directors. The vesting of these awards is time-based and the restrictions typically lapse 25% after one year and monthly thereafter for the next 36 months.
During the years ended December 31, 2008, 2009, 2010 and the period from May 9, 2003 (date of inception) to December 31, 2010, the Company granted 0, 0, 0 and 97,530 stock options, respectively to certain nonemployees in exchange for certain consulting services. Vesting on these awards is time-based and the vesting periods range from immediate vesting on the grant date to a four-year period.
The exercise price of each stock option shall be specified by the Board of Directors at the time of grant. The vesting period for each stock option is specified by the Board of Directors at the time of grant and is generally over a four-year period. The stock options expire ten years after the grant date.
The fair value of each stock option grant was estimated on the date of grant using the Black-Scholes option-pricing model. The expected life assumption is based on the expected life assumptions of similar entities. Expected volatility is based on a blend of the volatility of Helicos and similar entities in the life sciences industry of comparable market capitalization and financial position that have completed initial public offerings within the last ten years. The risk-free interest rate is the yield currently available on U.S. Treasury zero-coupon issues with a remaining term approximating the expected term used as the input to the Black-Scholes model. FASB accounting guidance requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods as options vest, if actual forfeitures differ from those estimates. During the years ended December 31, 2008, 2009 and 2010, because substantially all of the Company's stock option grants vest monthly, stock-based employee compensation expense includes the actual impact of forfeitures. The relevant data used to determine the value of the stock option grants is as follows:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Weighted average risk-free interest rate |
2.7 | % | 2.2 | % | 2.5 | % | ||||
Expected life in years |
6.0 | 6.0 | 6.0 | |||||||
Expected volatility |
65.6 | % | 63.1 | % | 92.5 | % | ||||
Expected dividends |
0.0 | % | 0.0 | % | 0.0 | % | ||||
101
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Stock-Based Compensation (Continued)
A summary of stock option activity for the year ended December 31, 2010 is as follows:
| |
Number of Shares |
Weighted average exercise price |
Weighted average remaining contractual term (in years) |
Aggregate intrinsic value) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at December 31, 2009 |
4,761,135 | $ | 4.05 | ||||||||||
Granted |
10,750 | $ | 0.96 | ||||||||||
Exercised |
(4,352 | ) | $ | 0.72 | |||||||||
Cancelled |
(2,647,472 | ) | $ | 4.53 | |||||||||
Expired |
— | ||||||||||||
Balance at December 31, 2010 |
2,120,061 | $ | 3.45 | 7.9 | $ | — | |||||||
Exercisable at December 31, 2010 |
1,873,263 | $ | 3.56 | 7.9 | $ | — | |||||||
Vested and unvested expected to vest at December 31, 2010 |
2,095,998 | $ | 3.46 | 7.9 | $ | — | |||||||
From May 9, 2003 (date of inception) through December 31, 2010, there were 6,218,298 stock options granted, of which 165,022 were exercised and 3,933,215 were cancelled. The weighted average exercise prices of stock option grants, exercises, and forfeitures from May 9, 2003 (date of inception) through December 31, 2010 was $4.67 per share, $0.66 per share, $5.50 per share, respectively.
The aggregate intrinsic value is calculated based on the positive difference between the fair value of the Company's common stock on December 31, 2010 of $0.123 per share and the exercise price of the underlying options. Because all outstanding option exercise prices exceeded the fair value of the Company's common stock on December 31, 2010, there was no intrinsic value of the Company's options outstanding at December 31, 2010.
The weighted-average grant-date fair value of grants of stock options was $3.60 per share, $0.89 and $0.96 per share for the years ended December 31, 2008, 2009 and 2010, respectively.
The total intrinsic value of stock options exercised was $283,000, $76,000 and $1,000 for the years ended December 31, 2008, 2009 and 2010, respectively.
The following table summarizes information about stock options outstanding at December 31, 2010:
| Options outstanding | Options exercisable | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range of exercise prices |
Number of stock options |
Weighted average remaining life (years) |
Number of stock options |
Weighted average exercise price |
Weighted average remaining life (years) |
|||||||||||
| $ 0.45–$ 0.79 | 326,418 | 8.3 | 226,018 | $ | 0.77 | 8.2 | ||||||||||
| $ 1.00–$ 1.80 | 513,265 | 7.7 | 430,810 | $ | 1.05 | 7.7 | ||||||||||
| $ 1.80–$ 2.72 | 799,696 | 8.7 | 791,507 | $ | 2.72 | 8.7 | ||||||||||
| $ 2.72–$ 6.99 | 97,555 | 6.8 | 77,222 | $ | 5.12 | 6.8 | ||||||||||
| $ 6.99–$12.36 | 383,127 | 6.3 | 347,706 | $ | 10.05 | 6.3 | ||||||||||
| 2,120,061 | 7.9 | 1,873,263 | $ | 3.56 | 7.9 | |||||||||||
102
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Stock-Based Compensation (Continued)
Restricted stock
During the years ended December 31, 2008, 2009 and 2010, and the period from May 9, 2003 (date of inception) to December 31, 2010, the Company granted 190,000, 1,697,724, 4,535,608 and 7,441,195 shares of restricted stock, respectively to certain employees. The vesting of these awards is time-based. For restricted stock granted prior to December 31, 2007, the restrictions typically lapse 25% after one year and quarterly thereafter for the next 3 years. For restricted stock granted during 2010, the vesting periods range from immediate vesting to quarterly vesting over six fiscal quarters to a two-year vesting period, with 50% vesting on each anniversary of the grant date.
During the years ended December 31, 2008, 2009 and 2010, and the period from May 9, 2003 (date of inception) to December 31, 2010, the Company granted 0, 95,000, 0 and 730,009 shares of restricted stock, respectively to certain nonemployees in exchange for certain consulting services. Vesting on these awards is time-based and the vesting periods range from immediate vesting on the grant date to a four-year period. The Company recorded a stock-based compensation charge on these awards following the FASB accounting guidance for accounting for stock appreciation rights and other variable stock option or award plans. In fiscal year 2010, the Company awarded 48,356 shares to directors in lieu of cash for board fees.
For employee and nonemployee restricted stock awards granted before January 1, 2007, the employee or nonemployee paid the Company cash in an amount up to the fair market value of the award. If the employee ceases employment with the Company, or if the nonemployee terminates the service arrangement, the employee or nonemployee is automatically entitled to be refunded the cash paid for any unvested awards. At the time the cash is received, the Company records the cash as subscription payable in the consolidated balance sheet, and the amount is reclassified to additional paid-in capital over the vesting period. At December 31, 2009 and 2010, the Company had $12,000 and $0 respectively, recorded as subscription payable. The balance at December 31, 2009 of $12,000 was recorded in accrued expenses and other current liabilities.
A summary of restricted stock activity during the year ended December 31, 2010 is as follows:
| |
Nunber of Shares |
Grant date Fair value |
Weighted Average remaining contractual term in years |
Aggregate intrinsic value (in $000's) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance of unvested restricted stock at December 31, 2009 |
1,451,089 | $ | 2.22 | |||||||||||
Granted |
4,535,608 | $ | 0.47 | |||||||||||
Vested |
(1,887,886 | ) | $ | 1.77 | ||||||||||
Forfeited |
(1,282,223 | ) | $ | 0.58 | ||||||||||
Balance of unvested restricted stock at December 31, 2010 |
2,816,588 | $ | 0.45 | 2.3 | $ | 338 | ||||||||
From May 9, 2003 (date of inception) through December 31, 2010, there were 8,171,204 shares of restricted stock granted, at a weighted average grant date fair value of $0.96, of which 3,883,327 shares had fully vested at December 31, 2010, with a weighted average grant date fair value of $1.38.
103
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Stock-Based Compensation (Continued)
The aggregate intrinsic value is calculated based on the positive difference between the fair value of the Company's common stock on December 31, 2010 of $0.12 per share and the estimated fair value of the Company's common stock at the date of grant. The total intrinsic value of restricted stock vested was $0.6 million, $2.1 million and $1.3 million for the years ended December 31, 2008, 2009 and 2010, respectively.
In March 2005, in connection with the AZTE License Agreement, the Company issued 88,888 shares of restricted common stock, which vested in two equal installments upon the achievement of separate milestones. In May 2006, due to the successful issuance of a U.S. patent, 44,444 shares of the restricted common stock vested. The vesting of 44,444 shares of restricted common stock resulted in a charge to research and development expense of $127,000 based on the fair value of the Company's common stock at the time the milestone was achieved. The remaining 44,444 shares of restricted common stock vested immediately upon the successful issuance of a second U.S. patent on January 12, 2010 and resulted in a charge to research and development expense of $54,000 based on the fair value of the Company's common stock at the time the milestone was achieved.
The Company recorded stock-based compensation expense to the extent that the fair value of the Company's common stock at the date of the grant exceeded the exercise price of the equity awards.
The Company recognized stock-based compensation expense on all employee and nonemployee awards as follows (in thousands):
| |
|
|
|
Period from May 9, 2003 (date of inception) through December 31, 2010 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | ||||||||||||
| |
2008 | 2009 | 2010 | ||||||||||
Selling, general and administrative |
$ | 3,217 | $ | 3,660 | $ | 3,796 | $ | 14,427 | |||||
Research and development |
1,321 | 1,621 | 1,308 | 5,387 | |||||||||
Total |
$ | 4,538 | $ | 5,281 | $ | 5,104 | $ | 19,814 | |||||
Total unrecognized stock-based compensation expense for all stock-based awards was approximately $1.4 million at December 31, 2010, of which $1.0 million will be recognized in 2011, $200,000 in 2012 and $200,000 thereafter. This results in these amounts being recognized over a weighted-average period of 1.1 years.
13. Fair Value Measurement
The Company considers all highly liquid investments with original maturities of generally three months or less at the time of acquisition to be cash equivalents. Cash equivalents are stated at cost, which approximates fair market value. The Company classifies marketable securities as available-for-sale. These securities are carried at fair market value with unrealized gains and, to the extent deemed temporary, unrealized losses, reported as a component of other comprehensive gain or loss in stockholders' equity/(deficit). Realized gains or losses on securities sold are based on the specific identification method.
Marketable securities are considered to be impaired when a decline in fair value below cost basis is determined to be other-than-temporary. The Company evaluates whether a decline in fair value
104
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
13. Fair Value Measurement (Continued)
below cost basis is other-than-temporary using available evidence regarding its investments. In the event that the cost basis of a security significantly exceeds its fair value, the Company evaluates, among other factors, the duration of the period that, and extent to which, the fair value is less than cost basis, the financial health of and business outlook for the issuer, including industry and sector performance, and operational and financing cash flow factors, overall market conditions and trends, the Company's intent to sell the investment and if it is more likely than not that the Company would be required to sell the investment before its anticipated recovery. Once a decline in fair value is determined to be other-than-temporary, a write-down is recorded in the consolidated statements of operations and a new cost basis in the security is established. In April 2009, the Company adopted new accounting guidance which provides additional guidance in assessing the credit and noncredit component of an other-than-temporary impairment event for debt securities and modifies the presentation and disclosures when an other-than-temporary impairment event for debt securities has occurred. The adoption of this new accounting guidance did not have a significant impact on the Company's financial statements.
The Company's assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2009 and December 31, 2010 are measured in accordance with FASB standards. Fair values determined by Level 1 inputs utilize observable data such as quoted prices in active markets. Fair values determined by Level 2 inputs utilize data points other than quoted prices in active markets that are observable either directly or indirectly. Fair values determined by Level 3 inputs utilize unobservable data points in which there is little or no market data, which require the reporting entity to develop its own assumptions.
The following table represents the Company's assets and liabilities measured at fair value on a recurring basis at December 31, 2009:
| ($ in thousands) |
Quoted Prices in Active Markets (Level 1) |
Other Observable Inputs (Level 2) |
Unobservable Inputs (Level 3) |
Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Money market funds |
$ | — | $ | 3,053 | $ | — | $ | 3,053 | |||||
Warrant liability |
— | — | 5,253 | 5,253 | |||||||||
|
$ | — | $ | 3,053 | $ | 5,253 | $ | 8,306 | |||||
The following table represents the Company's assets and liabilities measured at fair value on a recurring basis at December 31, 2010:
| ($ in thousands) |
Quoted Prices in Active Markets (Level 1) |
Other Observable Inputs (Level 2) |
Unobservable Inputs (Level 3) |
Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Money market funds |
$ | — | $ | 53 | $ | — | $ | 53 | |||||
Warrant liability |
— | — | 220 | 220 | |||||||||
|
$ | — | $ | 53 | $ | 220 | $ | 273 | |||||
105
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
13. Fair Value Measurement (Continued)
The following table represents the Company's assets and liabilities measured at fair value on a non-recurring basis at December 31, 2010:
| |
Quoted Prices in Active Markets (Level 1) |
Other Observable Inputs (Level 2) |
Unobservakle Inputs (Level 3) |
Total Gains (Loses) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Long-lived assest held and used |
$ | — | $ | 126 | $ | — | $ | (1,894 | ) | ||||
The Company's warrant liability was valued at December 31, 2009 and 2010 using a Black-Scholes model and is therefore classified as Level 3. The valuation of the warrant liability is discussed further in Note 10.
The following tables roll forward the fair value of the warrant liability, whose fair value is determined by Level 3:
Balance at December 31, 2008 |
$ | — | |||
Issuance of new warrants in 2009 |
7,212 | ||||
Change in fair value in 2009 |
(1,959 | ) | |||
Balance at December 31, 2009 |
5,253 | ||||
Warrant exercises in 2010 |
(915 | ) | |||
Change in fair value in 2010 |
(4,118 | ) | |||
Balance at December 31, 2010 |
$ | 220 | |||
The carrying amount of the Company's debt approximates its fair value at December 31, 2009 and December 31, 2010.
14. Net Loss per Share
Basic net loss per share is computed by dividing net loss by the weighted average number of common shares outstanding for the period. The Company's potential dilutive shares, which include outstanding common stock options, unvested restricted stock, common stock warrants have not been included in the computation of diluted net loss per share for all periods as the result would be antidilutive. Such potentially dilutive shares are excluded when the effect would be to reduce net loss per share. Because the Company reported a net loss for the years ended December 31, 2008, 2009 and 2010, all potential common shares have been excluded from the computation of the dilutive net loss per share for all periods presented because the effect would have been antidilutive. Such potential common shares consist of the following:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Stock options |
2,679,614 | 4,761,135 | 2,120,061 | |||||||
Unvested restricted stock |
273,144 | 1,495,533 | 2,816,588 | |||||||
Warrants |
25,762,333 | 28,454,589 | 23,917,179 | |||||||
|
28,715,091 | 34,711,257 | 28,853,828 | |||||||
106
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
