Attached files
| file | filename |
|---|---|
| EX-21 - EX-21 - ALSERES PHARMACEUTICALS INC /DE | b84090exv21.htm |
| EX-31.2 - EX-31.2 - ALSERES PHARMACEUTICALS INC /DE | b84090exv31w2.htm |
| EX-32.2 - EX-32.2 - ALSERES PHARMACEUTICALS INC /DE | b84090exv32w2.htm |
| EX-32.1 - EX-32.1 - ALSERES PHARMACEUTICALS INC /DE | b84090exv32w1.htm |
| EX-31.1 - EX-31.1 - ALSERES PHARMACEUTICALS INC /DE | b84090exv31w1.htm |
| EX-23.1 - EX-23.1 - ALSERES PHARMACEUTICALS INC /DE | b84090exv23w1.htm |
| EX-10.61 - EX-10.61 - ALSERES PHARMACEUTICALS INC /DE | b84090exv10w61.htm |
| EX-10.54 - EX-10.54 - ALSERES PHARMACEUTICALS INC /DE | b84090exv10w54.htm |
| EX-10.60 - EX-10.60 - ALSERES PHARMACEUTICALS INC /DE | b84090exv10w60.htm |
Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Form 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2010
OR
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 0-6533
ALSERES PHARMACEUTICALS, INC.
(Exact Name of Registrant as Specified in Its Charter)
| DELAWARE (State or other jurisdiction of incorporation or organization) |
87-0277826 (I.R.S. Employer Identification No.) |
|
| 239 SOUTH STREET HOPKINTON, MASSACHUSETTS (Address of principal executive offices) |
01748 (Zip Code) |
Registrant’s telephone number, including area code (508) 497-2360
Securities registered pursuant to Section 12(b) of the Act:
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on Which Registered | |
| Common Stock, $.01 par value (Excluding Rights to Purchase Preferred Stock) |
Securities registered pursuant to Section 12(g) of the Act:
None
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as
defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant
to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant: (1) has filed all reports required
to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required to file
such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and
posted on its corporate Website, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter)
during the preceding 12 months (or for such shorter period that the registrant was required
to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be
contained, to the best of registrant’s knowledge, in definitive proxy or information
statements incorporated by reference in Part III of this Form 10-K or any amendment to this
Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting company. See the
definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting
company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller reporting company þ |
Indicate by check mark whether the registrant is a shell company (as defined in
Rule 12b-2 of the Exchange Act). Yes o No þ
Based on the last sales price of the registrant’s Common Stock as reported on the
Pink Sheets OTC Market on December 31, 2010 (the last business day of our most recently
completed fiscal quarter), the aggregate market value of the shares of voting stock held by
non-affiliates of the registrant was $1,289,729.
As of March 21, 2011, there were 26,785,645 shares of the registrant’s Common
Stock issued and outstanding.
TABLE OF CONTENTS
2
Table of Contents
PART I
Item 1. Business.
Overview
We are a biotechnology company focused on the development of diagnostic and
therapeutic products primarily for disorders in the central nervous system, or CNS.
Our clinical and preclinical product candidates are based on the following
proprietary technology platform:
| • | Molecular imaging program focused on the diagnosis of i) Parkinsonian Syndromes, or PS, including Parkinson’s Disease, or PD, and ii) Dementia with Lewy Bodies, or DLB; | ||
| • | Regenerative therapeutics program primarily focused on nerve repair in patients who have had significant loss of CNS function resulting from CNS trauma. |
At December 31, 2010, we were considered a “development stage enterprise” as
defined in Accounting Standards Codification (ASC) 915-10. “Development Stage
Entities” and will continue to be so until we commence commercial operations. A
development stage enterprise is one in which planned principal operations have not
commenced or, if its operations have commenced, there has been no significant
revenue there from. Development stage companies report cumulative costs from the
inception of the enterprise. Our development stage started on October 16, 1992 and
continued through December 31, 2010.
As of December 31, 2010, we have experienced total net losses since inception of
approximately $203,456,000, stockholders’ deficit of approximately $47,286,000 and
a net working capital deficit of approximately $42,079,000. The cash and cash
equivalents available at December 31, 2010 will not provide sufficient working
capital to meet our anticipated expenditures for the next twelve months. At March
21, 2011, we had cash and cash equivalents of approximately $100,000 which combined
with our ability to control administrative expenses will enable us to meet our
anticipated cash expenditures into April, 2011.
In order to continue as a going concern and fund the approximately $30 million of
investment required to complete the Altropane U.S. clinical trial program, we will
need to raise additional capital through one or more of the following: a debt
financing, an equity offering, or a collaboration, merger, acquisition or other
transaction with one or more pharmaceutical or biotechnology companies. We are
currently engaged in fundraising efforts. There can be no assurance that we will be
successful in our fundraising efforts or that additional funds will be available on
acceptable terms, if at all. We also cannot be sure that we will be able to obtain
additional credit from, or effect additional sales of debt or equity securities to
certain of our existing investors described below in Liquidity and Capital
Resources. If we are unable to raise additional or sufficient capital, we will need
to cease operations or reduce, cease or delay one or more of our research or
development programs, adjust our current business plan and may not be able to
continue as a going concern. Additionally, our common stock was delisted from
trading on the NASDAQ Capital Market as a result of our failure to meet continued
listing requirements of NASDAQ. On May 8, 2009 we began trading on the Pink Sheets
OTC Market. This delisting has had an adverse affect on our ability to obtain
future financing and has adversely impacted our stock price and the liquidity of
our common stock.
Our ability to advance our clinical programs, including the development of
Altropane, is affected by the availability of financial resources. Financial
considerations have caused us to suspend planned development activities for our
clinical and preclinical programs until we are able to secure additional working
capital. If we are not able to raise additional capital, we will not have
sufficient funds to complete the clinical trial programs for Altropane.
We were organized in 1992 and are incorporated in Delaware. Our principal executive
offices are located at 239 South Street, Hopkinton, Massachusetts 01748, and our
telephone number is (508) 497-2360. In this Annual Report on Form 10-K, the terms
“Alseres Pharmaceuticals”, the “Company”, “we”, “us” and “our” include Alseres
Pharmaceuticals, Inc. and its subsidiaries. The following are trademarks of ours
that are mentioned in this Annual Report on Form 10-K: Alseres
® , Altropane ® , and Fluoratec
tm . Other trademarks used in this Annual Report on Form 10-K
are the property of their respective owners.
3
Table of Contents
Product Development — Molecular Imaging Program
Altropane Molecular Imaging Agent
The Altropane molecular imaging agent is being developed for the differential
diagnosis of PS, including PD, and non-PS in patients with an upper extremity
tremor. In July 2007, we completed enrollment in a clinical study that optimized
Altropane’s image acquisition protocol which we believe will enhance Altropane’s
commercial use. After a series of discussions with the U.S. Food and Drug
Administration, or FDA, and our expert advisors, the POET-2 program was designed as
a two-part Phase III program using the optimized Altropane image acquisition
protocol. The first part of the program enrolled 54 subjects in a multi-center
clinical study to acquire a set of Altropane images which will be used to train the
expert readers, as is the customary process for clinical trials of molecular
imaging agents. Enrollment in the first part of POET-2 was completed in January
2009. In April, 2009 we reached agreement with the FDA under the Special Protocol
Assessment, or SPA, process for the second part of the Phase III clinical trial
program of Altropane. A SPA is a process by which sponsors and the FDA reach an
agreement on the size, design and analysis of clinical trials that will form the
primary basis of approval. The Phase III program is designed to confirm the
diagnostic utility of the agent in anticipation of drug registration. The second
portion of the Phase III, POET-2 program consists of two clinical trials in up to
480 subjects in total to be conducted in parallel at up to 40 medical facilities
throughout the US. The subjects to be tested will be 40-80 years of age and have
had a tremor in their hand(s) or arm(s) for less than three years. Each subject
will be assessed by a general neurologist, an Altropane imaging procedure and a
Movement Disorder Specialist considered the “gold standard”. The success of the
trial will be determined by measuring the diagnostic efficacy of the neurologist
diagnosis compared with the diagnosis determined by the Altropane scan versus the
MDS gold standard diagnosis. Based on the trial design and scope covered by the
Special Protocol Assessment Agreement for POET-2, we estimate that the total costs
to complete the POET-2 program and prepare and submit an NDA for Altropane in the
U.S. will be approximately $30 million. This funding is not available at present
and there can be no assurance that such funds will be available on acceptable terms
if at all.
We believe in the current environment that, due to their proximity to
commercialization and return on investment, late stage development programs may
continue to be of significant interest to potential partners and investors. To
maximize the value of our molecular imaging program, we are focusing on obtaining
the funding necessary to execute the Altropane Phase III registration program. We
are pursuing the capital necessary to enable us to advance the Altropane program
through our own means. In parallel, we are seeking to partner our molecular imaging
program with a firm or firms with the resources necessary for the completion of the
Phase III clinical program, for the manufacturing and supply of Altropane, and for
the launch and commercialization of Altropane. During 2010 we engaged in numerous
discussions with potential development and commercialization partners for Altropane
with no term sheets resulting from those discussions. Late in 2010 and early this
year, a number of events occurred which have stimulated new interest in the
Altropane program by potential partners. These included the purchase of Avid
Radiopharmaceuticals by Eli Lilly for up to $800 million, the US approval of GE’s
DaTscan product, a favorable FDA Advisory Panel review of Avid’s imaging product
and EU guidance recommending imaging as first line diagnosis in dementias. We
believe that these events could assist with our partnering efforts for Altropane.
We can provide no assurances that a partnership transaction will occur. We believe
that the expansion of the program into other indications such as DLB and other
countries including those in Europe could increase the value of the program for the
partner and us. All of these activities require additional funding not presently
available and as such have been suspended. There can be no assurance that the
required funding to advance the Altropane program will be available on acceptable
terms if at all.
The Altropane molecular imaging agent is a radiolabeled imaging agent that contains
the radioactive element iodine isotope 123 I and binds with extremely
high affinity and specificity to the dopamine transporter, or DAT. The DAT is a
protein that is on the surface membrane of specialized neurons in the brain that
produce dopamine, a key neurotransmitter. We believe that
the amount of Altropane taken up by the brain is directly proportional to the
number of DATs that are present in any given area of the brain. Since DATs are on
the membrane of dopamine-producing neurons, death of these neurons results in
decreased numbers of DATs. Therefore, PD, which is caused by a decreased number of
dopamine producing cells, is associated with a marked decrease in the number of
DATs. As a result, when Altropane is administered to patients with PD, its binding
is substantially diminished as compared to patients without PD. This decrease in
Altropane binding in patients with PD is the theoretical basis for using Altropane
imaging as a diagnostic test for PS, including PD.
4
Table of Contents
Altropane is administered by intravenous injection. Since Altropane contains
radioactive 123 I, it can be used as a nuclear imaging agent that can be
detected using a specialized nuclear medicine instrument known as a Single Photon
Emission Computed Tomography, or SPECT, camera. The strength of the SPECT signal
generated by Altropane is proportional to the number of DATs present and produces
images that distinguish PS and non-PS patients. SPECT cameras are widely available
in both community and academic medical centers. The scanning procedure using
Altropane takes less than one hour to complete. Results of these tests are usually
available the same day as the scanning procedure.
We have licensed worldwide exclusive rights to develop Altropane from Harvard
University and its affiliated hospitals, which we refer to as Harvard and its
Affiliates, including the Massachusetts General Hospital. The license agreement
provides for milestone payments and royalties based on product sales that are
consistent with industry averages for such products.
Altropane Diagnostic for Parkinsonian Syndromes (PS)
PS is characterized by loss of dopamine-producing neurons resulting in a variety of
movement disorders, especially tremors and gait problems. The most prevalent form
of PS is PD which is a chronic, irreversible, neurodegenerative disease that
generally affects people over 50 years old. PD is caused by a significant decrease
in the number of dopamine producing neurons in specific areas of the brain.
Inadequate production of dopamine causes, at least in part, the PD symptoms of
tremor, muscle retardation and rigidity. PD can be difficult to diagnose using
subjective analyses and can be confused with Essential Tremor, or ET. ET manifests
with clinical symptoms very similar to those of PD. However, ET patients do not
need the drugs routinely prescribed to PD patients.
Need for an Objective Diagnosis
According to published data, clinical criteria used to diagnose PS is prone to high
error rates, especially in early stages of PD. This highlights the critical need
for an effective diagnostic. Presently, patients who have experienced tremors and
other evidence of a movement disorder may pursue diagnosis and treatment with a
number of medical professionals. These include an internist or general
practitioner, also known as a primary care physician, or PCP, a neurologist, or a
movement disorder specialist, or MDS, whose practice is focused on movement
disorders.
Patients can exhibit symptoms and/or have clinical histories that are inconclusive.
A primary tool utilized to diagnose PD or PS is a clinical history and a physical
exam. However, studies in the literature have reported error rates in diagnosing PD
or PS from a low of 10% for MDSs to as high as 40 to 50% for PCPs.
This high error rate is driving the need for a diagnostic test that provides
physicians with additional clinical information to help them make a definitive
diagnosis when clinical symptoms and the patient’s history are inconclusive.
Further, while the accuracy of MDSs is reported to be higher, the number of MDSs in
the United States is limited with current estimates between 300 and 500. The
limited availability of MDSs underscores the potential utility of a widely
available diagnostic tool such as Altropane.
There are a number of important and potentially harmful results associated with
misdiagnosis. These include:
| • | Patients who are improperly diagnosed as having PD but actually do not (false positive) may be administered medications for PD. These drugs can have damaging effects on individuals who do not actually have PD. | ||
| • | Patients who are improperly diagnosed as not having PD but actually do (false negative), may not benefit from available treatments, thereby suffering further worsening of symptoms and progression of their disease. |
Market Opportunity
It has been estimated that approximately 180,000 individuals in the United States
per year present to their physician with new, undiagnosed cases of PD and ET, and
are therefore candidates for a scan using the Altropane molecular imaging agent to
diagnose or rule out early PS. It has also been estimated by the National Institute
of Neurological Disorders and Stroke and the National Parkinson’s Foundation that
the number of people in the United States with PD is between 500,000 and 1,500,000.
It has been estimated by the Tremor Action Network that there are approximately
10,000,000 people in the United States with ET. In addition, a study done by the
World Health Organization claims that approximately 2,000,000 individuals suffer
from PD in Europe. We expect the number of individuals affected by PD to grow
substantially as people continue to live longer and the overall population ages.
5
Table of Contents
Altropane Diagnostic for Dementia with Lewy Bodies (DLB)
DLB is a progressive brain disease and the second most common cause of
neurodegenerative dementia. The symptoms of DLB are caused by the build-up of Lewy
bodies inside the section of the brain that controls particular aspects of memory
and motor control. The similarity of symptoms between DLB and PD, and between DLB
and Alzheimer’s disease, can make it difficult to accurately diagnose. As with PD,
there is no objective diagnostic tool available in the United States. We believe
that the underlying basis for DLB coupled with our existing preclinical and
clinical data supports the potential development of Altropane as a diagnostic for
DLB. We also believe the potential use of our molecular imaging agents for the
diagnosis of DLB could be strategic in our partnering efforts for our molecular
imaging program.
It has been estimated by the Alzheimer’s Association that there are approximately
7,000,000 to 10,000,000 people in the United States with dementia of which the
journal Age and Ageing estimates that up to approximately 3,000,000 people have
DLB. According to Alzheimer Europe, there were approximately 1,800,000 people in
Europe with DLB.
Regenerative Therapeutics Program — Nerve Repair
Our nerve repair program is focused on restoring movement and sensory function in
patients who have had significant loss of CNS function resulting from traumas such
as SCI, stroke, traumatic brain injury, or TBI, and optic nerve damage. Our efforts
are aimed at the use of proprietary regenerative drugs and/or methods to induce
nerve fibers to regenerate and form new connections that restore compromised
abilities. Licensing or acquiring the rights to the technologies of complementary
approaches for nerve repair is part of our strategy of creating competitive
advantages by assembling a broad portfolio of related technologies and intellectual
property.
Resource constraints have severely restricted our ability to progress our
regenerative therapeutics program. On May 11, 2010, the Company was notified by
CMCC of the termination of its license agreements with the Company as a result of
the Company’s lack of resources and resulting inability to comply with the
performance conditions of the licenses. At December 31, 2010 CMCC was owed a total
of approximately $77,000 in license fees and legal costs associated with the
licenses. Since we were unable to pay the overdue amounts within the cure periods
specified in the CMCC licenses, we no longer have the rights to further develop
and/or partner the axon regeneration technologies licensed from CMCC.
Research and Development
We rely on licensing from third parties as our source for new technologies and
product candidates. Research and development expenses were approximately $465,000
and $3.7 million for the years ended December 31, 2010 and 2009, respectively.
Licensing Agreements, Patents and Intellectual Property
We have obtained exclusive licenses to patent portfolios related to our product
candidates in development. However, as to one or more of the patents and patent
applications of the patent portfolios, which we have licensed from a university or
academic institution, the United States government holds a nonexclusive,
royalty-free, license in exchange for providing research funding.
Our intellectual property strategy is to vigorously pursue patent protection for
our technologies in the United States and major developed countries. Generally,
each license agreement is effective until the last patent licensed relating to the
technology expires or a fixed and determined date.. The patents for the Altropane
molecular imaging agent expire beginning in 2013.
In the event that a third party has a patent or patent application overlapping an
invention claimed in one of our patents or patent applications, we may be required
to participate in a patent interference proceeding declared by the United States
Patent and Trademark Office, or USPTO, to determine priority of invention. A patent
interference could result in substantial uncertainties and cost for us, even if the
eventual outcome is favorable to us. We cannot provide assurance that
our patents and patent applications, if issued, would be held valid by a court of
competent jurisdiction.
We also rely on trade secrets and proprietary know-how. We seek to protect this
information through confidentiality agreements with our collaborators and
consultants. There can be no guarantee that these procedures and agreements will
not be breached or that we will have adequate remedies for such breach. In
addition, if consultants, scientific advisors, or other third parties apply
technological information which they have developed separate from us to our
technologies, there may be disputes as to the ownership of such information which
may not be resolved in our favor.
6
Table of Contents
Competition
To our knowledge, there is only one company, GE Healthcare (formerly
Nycomed/Amersham), that has marketed a diagnostic imaging agent for PD and DLB,
DaTSCAN ® . GE Healthcare has obtained marketing approval in Europe. In
January, 2011, GE Healthcare obtained approval to market DaTSCAN in the U.S. as
well. GE Healthcare has significantly greater infrastructure and financial
resources than us. Marketing approval s in the US and Europe combined with their
established market presence, and greater financial strength could position GE to
make it difficult for us to successfully market Altropane if it is approved for
sale.
Regulatory Considerations
Our technologies must undergo a rigorous regulatory approval process, which
includes extensive preclinical and clinical testing, to demonstrate safety and
efficacy before any resulting product can be marketed. To date, neither the FDA nor
any of its international equivalents has approved any of our technologies for
marketing. In the biotechnology industry, it has been estimated that less than five
percent of the technologies for which clinical efforts are initiated ultimately
result in an approved product. The clinical trial and regulatory approval process
can require many years and substantial cost, and there can be no guarantee that our
efforts will result in an approved product.
Our activities are regulated by a number of government authorities in the United
States and other countries, including the FDA pursuant to the Federal Food, Drug,
and Cosmetic Act. The FDA regulates drugs, including their research, development,
testing, manufacturing, labeling, packaging, storage, advertising and promotion,
and distribution. Data obtained from testing is subject to varying interpretations
which can delay, limit or prevent FDA approval. In addition, changes in existing
regulatory requirements could prevent or affect the timing of our ability to
achieve regulatory compliance. Federal and state laws, regulations and policies may
be changed with possible retroactive effect, and how these rules actually operate
can depend heavily on administrative policies and interpretations over which we
have no control.
Obtaining FDA approvals is time-consuming and expensive. The steps required before
any of our product candidates may be marketed in the United States include:
| • | Development of suitable manufacturing processes for preclinical, clinical and commercial drug supply; | ||
| • | Preclinical laboratory tests, animal studies and formulation studies according to good laboratory practice regulations; | ||
| • | Submission to the FDA of an IND application, which must become effective before United States human clinical trials may commence; | ||
| • | Adequate and well-controlled human clinical trials according to good clinical practice regulations, or GCP, to establish the safety and efficacy of the product for its intended use; | ||
| • | Submission to the FDA of an NDA; | ||
| • | Satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and | ||
| • | FDA review and approval of the application(s) prior to any commercial sale or shipment of the drug. |
Once a drug candidate is identified for development it enters the preclinical
testing stage. Preclinical tests include laboratory evaluations of product
chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must
submit the results of the preclinical tests, together with manufacturing
information and analytical data, to the FDA as part of the IND. Some preclinical or
non-clinical testing may continue even after the IND is submitted. In addition to
including the results of the preclinical studies, the IND will also include a
protocol detailing,
among other things, the objectives of the first phase of the clinical trial, the
parameters to be used in monitoring safety, and the effectiveness criteria to be
evaluated if the first phase lends itself to an efficacy determination. The IND
automatically becomes effective 30 days after receipt by the FDA, unless the FDA
places the clinical trial on clinical hold. In such a case, the IND sponsor and the
FDA must resolve any outstanding concerns before the clinical trial can begin.
7
Table of Contents
All clinical trials must be conducted under the supervision of one or more
qualified investigators in accordance with GCP. These regulations include the
requirement that all research subjects provide informed consent. Further, an
Institutional Review Board, or IRB, at each institution participating in the
clinical trial must review and approve the protocol before a clinical trial
commences at that institution. Each new clinical protocol must be submitted to the
FDA as part of the IND. Progress reports detailing the results of the clinical
trials must be submitted at least annually to the FDA and more frequently if
serious adverse events occur.
There is no guarantee that approvals will be granted for any of our product
candidates, or that the FDA review process will not involve delays that
significantly and negatively affect our product candidates. We also may encounter
similar delays in foreign countries. In addition, even if we receive regulatory
approvals, they may have significant limitations on the uses for which any approved
products may be marketed. After approval, some types of changes to the approved
product are subject to further FDA review and approval. Any marketed product and
its manufacturer are subject to periodic review, and any discovery of previously
unrecognized problems with a product or manufacturer could result in suspension or
limitation of approvals. Failure to comply with the applicable FDA requirements at
any time during the product development process, approval process, or after
approval, may subject an applicant to administrative or judicial sanctions,
including the FDA’s refusal to approve pending applications, withdrawal of
approval, a clinical hold, warning letters, product recalls and seizures, total or
partial suspension of production or distribution, or injunctions, fines, civil
penalties or criminal prosecution.
Pharmaceutical Pricing and Reimbursement
In both domestic and foreign markets, sales of any products for which we receive
regulatory approval for commercial sales will depend in part on the availability of
reimbursement for third party payors. Third party payors include government health
care program administrative authorities, managed care providers, private health
insurers and other organizations. These third party payors are increasingly
challenging the price and examining the cost-effectiveness of medical products and
services. In addition, significant uncertainty exists as to the scope of coverage
and payment amounts for newly approved heath care products. We may need to conduct
expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness
of our products. Our products may not be considered medically necessary or
cost-effective. Adequate third party reimbursement may not be available to enable
us to maintain price levels sufficient to realize an appropriate return on our
investment in product development.
Manufacturing
We currently outsource manufacturing for all of our product candidates and expect
to continue to outsource manufacturing in the future. We believe our current
suppliers of Altropane will be able to manufacture our products efficiently in
sufficient quantities and on a timely basis, while maintaining product quality. We
seek to maintain quality control over manufacturing through ongoing inspections,
rigorous review, control over documented operating procedures and thorough
analytical testing by outside laboratories. We believe that our current strategy of
primarily outsourcing manufacturing is cost-effective since we avoid the high fixed
costs of plant, equipment and large manufacturing staffs. FDA regulations require
that we establish manufacturing sources for each of our product candidates under
the cGMP regulations established by the FDA.
MDS, Inc., (previously MDS Nordion, Inc.), a Canadian corporation and
well-recognized manufacturer of 123 I and nuclear medicine labeled
imaging agents, has supplied Altropane to us since 2001. We are highly dependent
upon MDS Nordion. Under the terms of our agreement, which expired on December 31,
2010, MDS Nordion manufactured the Altropane molecular imaging agent for our
clinical trials in exchange for minimum monthly cash payments. We do not presently
have arrangements with any other suppliers in the event that MDS Nordion is unable
or unwilling to manufacture Altropane for us. We could encounter a significant
delay before another
supplier could manufacture Altropane for us due to the time required to establish a
cGMP manufacturing process for Altropane. We hope to enter into a new agreement
with MDS but there can be no assurance that we will be able to or that the terms
will be acceptable. We do not have a manufacturing agreement relating to the
commercial production of Altropane with MDS or any other manufacturer. We can
provide no assurances that such an agreement will be executed on acceptable terms
or that if we are unable to satisfy our monthly minimum payment obligations to MDS
that our existing agreement will remain in force.
Marketing and Sales
Although we currently sell no products, our strategy is to pursue partnering
opportunities in order to accelerate and maximize commercialization of our clinical
product candidates and strategic collaborations for development of our preclinical
product candidates. These collaborators may provide financial and other resources,
including capabilities in research, development, manufacturing, marketing and
sales. There can be no assurances that we will be successful in our collaboration
efforts.
8
Table of Contents
Employees
As of December 31, 2010, we employed 4 full-time employees. None of our employees
are covered by a collective bargaining agreement.
Other Information
We are subject to the informational requirements of the Securities Exchange Act of
1934, as amended, or the Exchange Act, and, accordingly, file reports, proxy
statements and other information with the Securities and Exchange Commission. Such
reports, proxy statements and other information can be read and copied at the
public reference facilities maintained by the Securities and Exchange Commission at
the Public Reference Room, 100 F Street, N.E., Room 1580, Washington, D.C. 20549.
Information regarding the operation of the Public Reference Room may be obtained by
calling the Securities and Exchange Commission at 1-800-SEC-0330. The Securities
and Exchange Commission maintains a web site (http://www.sec.gov) that contains
material regarding issuers that file electronically with the Securities and
Exchange Commission.
Our Internet address is www.alseres.com. We are not including the information
contained on our web site as a part of, or incorporating it by reference into, this
Annual Report on Form 10-K. We make available free of charge on our website our
Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on
Form 8-K and amendments to those reports filed or furnished pursuant to Section
13(a) or 15(d) of the Exchange Act, as soon as reasonably practicable after we
electronically file such material with, or furnish it to, the Securities and
Exchange Commission.
Additional financial information is contained in Management’s Discussion and
Analysis of Financial Condition and Results of Operations in Item 7 of Part II, and
in Item 8 of Part II of this Annual Report on Form 10-K.
| Item 1A. | Risk Factors. |
Statements contained or incorporated by reference in this Annual Report on Form
10-K that are not based on historical fact are “forward-looking statements” within
the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of
the Securities Act of 1933, as amended, and Section 21E of the Exchange Act. These
forward-looking statements regarding future events and our future results are based
on current expectations, estimates, forecasts, and projections, and the beliefs and
assumptions of our management including, without limitation, our expectations
regarding our product candidates, including the success and timing of our
preclinical, clinical and development programs, the submission of regulatory
filings and proposed partnering arrangements, the outcome of any litigation,
collaboration, merger, acquisition and fund raising efforts, results of operations,
selling, general and administrative expenses, research and development expenses and
the sufficiency of our cash for future operations. Forward-looking statements may
be identified by the use of forward-looking terminology such as “may,” “could,”
“will,” “expect,” “estimate,” “anticipate,” “continue,” or similar terms,
variations of such terms or the negative of those terms.
We cannot assure investors that our assumptions and expectations will prove to have
been correct. Important factors could cause our actual results to differ materially
from those indicated or implied by forward-looking statements. Such factors that
could cause or contribute to such differences include those factors discussed
below. We undertake no intention or obligation to update or revise any
forward-looking statements, whether as a result of new information, future events
or otherwise. If any of the following risks actually occur, our business, financial
condition or results of operations would likely suffer.
Risks Related to our Financial Results and Need for Additional Financing
WE ARE A DEVELOPMENT STAGE COMPANY. WE HAVE INCURRED LOSSES FROM OUR OPERATIONS
SINCE INCEPTION AND ANTICIPATE LOSSES FOR THE FORESEEABLE FUTURE. WE WILL NOT BE
ABLE TO ACHIEVE PROFITABILITY UNLESS WE OBTAIN REGULATORY APPROVAL AND MARKET
ACCEPTANCE OF OUR PRODUCT CANDIDATES. WE WILL NEED SUBSTANTIAL ADDITIONAL FUNDING
IN ORDER TO CONTINUE OUR BUSINESS AND OPERATIONS. IF WE ARE UNABLE TO SECURE SUCH
FUNDING ON ACCEPTABLE TERMS, WE WILL NEED TO CEASE OPERATIONS, SIGNIFICANTLY
REDUCE, DELAY OR CEASE ONE OR MORE OF OUR RESEARCH OR DEVELOPMENT PROGRAMS, OR
SURRENDER RIGHTS TO SOME OR ALL OF OUR TECHNOLOGIES. IF WE VIOLATE A DEBT COVENANT
OR DEFAULT UNDER OUR DEBT AGREEMENTS, WE MAY NEED TO CEASE OPERATIONS OR REDUCE,
CEASE OR DELAY ONE ORMORE OF OUR RESEARCH OR DEVELOPMENT PROGRAMS, ADJUST OUR
CURRENT BUSINESS PLAN AND MAY NOT BE ABLE TO CONTINUE AS A GOING CONCERN.
9
Table of Contents
Biotechnology companies that have no approved products or other sources of revenue
are generally referred to as development stage companies. We have never generated
revenues from product sales and we do not currently expect to generate revenues
from product sales for at least the next three years. If we do generate revenues
and operating profits in the future, our ability to continue to do so in the long
term could be affected by the introduction of competitors’ products and other
market factors. We expect to incur significant operating losses for at least the
next three years. The level of our operating losses may increase in the future if
more of our product candidates begin human clinical trials. We will never generate
revenues or achieve profitability unless we obtain regulatory approval and market
acceptance of our product candidates. This will require us to be successful in a
range of challenging activities, including clinical trial stages of development,
obtaining regulatory approval for our product candidates, and manufacturing,
marketing and selling them. We may never succeed in these activities, and may never
generate revenues that are significant enough to achieve profitability. Even if we
do achieve profitability, we may not be able to sustain or increase profitability
on a quarterly or annual basis.
We require significant funds to conduct research and development activities,
including preclinical studies and clinical trials of our technologies, and to
commercialize our product candidates. Because the successful development of our
product candidates is uncertain, we are unable to estimate the actual funds we will
require to develop and commercialize them. Our funding requirements depend on many
factors, including:
| • | The scope, rate of progress and cost of our clinical trials and other research and development activities; | ||
| • | Future clinical trial results; | ||
| • | The terms and timing of any collaborative, licensing and other arrangements that we may establish; | ||
| • | The cost and timing of regulatory approvals and of establishing sales, marketing and distribution capabilities; | ||
| • | The cost of establishing clinical and commercial supplies of our product candidates and any products that we may develop; | ||
| • | The cost of obtaining and maintaining licenses to use patented technologies; | ||
| • | The effect of competing technological and market developments; and | ||
| • | The cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights and other patent-related costs, including litigation costs and the results of such litigation. |
Until such time, if ever, as we can generate substantial revenue from product sales
or through collaborative arrangements with third parties, we will need to raise
additional capital. To date, we have experienced negative cash flows from
operations and have funded our operations primarily from equity and debt
financings.
As of December 31, 2010, we have experienced total net losses since inception of
approximately $203,456,000, stockholders’ deficit of approximately $47,286,000 and
a net working capital deficit of approximately $42,079,000. The cash and cash
equivalents available at December 31, 2010 will not provide sufficient working
capital to meet our anticipated expenditures for the next twelve months. At March
21, 2011, we had cash and cash equivalents of approximately $100,000 which combined
with our ability to control administrative expenses will enable us to meet our
anticipated cash expenditures into April, 2011.
In order to continue as a going concern and fund the approximately $30 million of
investment required to complete the Altropane U.S. clinical trial program, we will
need to raise additional capital through one or more of the following: a debt
financing, an equity offering, or a collaboration, merger, acquisition or other
transaction with one or more pharmaceutical or biotechnology companies. We are
currently engaged in fundraising efforts. There can be no assurance that we will be
successful in our fundraising efforts or that additional funds will be available on
acceptable terms, if at all. We also cannot be sure that we will be able to obtain
additional credit from, or effect additional sales of debt or equity securities to
certain of our existing investors described below in Liquidity and Capital
Resources. If we are unable to raise additional or sufficient capital, we will need
to cease operations or reduce, cease or delay one or more of our research or
development programs, adjust our current business plan and may not be able to
continue as a going concern. Additionally, our common stock was delisted from
trading on the NASDAQ Capital Market as a result of our failure to meet continued
listing requirements of NASDAQ. On May 8, 2009 we began trading on the Pink Sheets
OTC Market. This delisting has had an adverse affect on our ability to obtain
future financing and has adversely impacted our stock price and the liquidity of
our common stock. Our ability to advance our clinical programs, including the
development of Altropane, is affected by the availability of financial resources.
Financial considerations have caused us to suspend planned development activities
for our clinical and preclinical programs until we are able to secure additional
working capital. If we are not able to raise additional capital, we will not have
sufficient funds to complete the clinical trial programs for Altropane.
10
Table of Contents
Risks Related to Commercialization
OUR SUCCESS DEPENDS ON OUR ABILITY TO SUCCESSFULLY DEVELOP OUR PRODUCT CANDIDATES
INTO COMMERCIAL PRODUCTS.
To date, we have not marketed, distributed or sold any products and, with the
exception of Altropane, all of our technologies and early-stage product candidates
are in preclinical development. The success of our business depends primarily upon
our ability to successfully develop and commercialize our product candidates.
Successful research and product development in the biotechnology industry is highly
uncertain, and very few research and development projects produce a commercial
product. In the biotechnology industry, it has been estimated that less than five
percent of the technologies for which research and development efforts are
initiated ultimately result in an approved product. If we are unable to
successfully commercialize Altropane or any of our other product candidates, our
business would be materially harmed.
EVEN IF WE RECEIVE APPROVAL TO MARKET OUR PRODUCT CANDIDATES, THE MARKET MAY NOT BE
RECEPTIVE TO OUR PRODUCT CANDIDATES UPON THEIR COMMERCIAL INTRODUCTION, WHICH COULD
PREVENT US FROM SUCCESSFULLY COMMERCIALIZING OUR PRODUCTS AND FROM BEING
PROFITABLE.
Even if our drug candidates are successfully developed, our success and growth will
also depend upon the acceptance of these product candidates by physicians and
third-party payors. Acceptance of our product development candidates will be a
function of our products being clinically useful, being cost effective and
demonstrating superior diagnostic or therapeutic effect with an acceptable side
effect profile as compared to existing or future treatments. In addition, even if
our products achieve market acceptance, we may not be able to maintain that market
acceptance over time. Factors that we believe will materially affect market
acceptance of our product candidates under development include:
| • | The timing of our receipt of any marketing approvals, the terms of any approval and the countries in which approvals are obtained; | ||
| • | The safety, efficacy and ease of administration of our products; | ||
| • | The competitive pricing of our products; | ||
| • | The success of our education and marketing programs; | ||
| • | The sales and marketing efforts of competitors; and | ||
| • | The availability and amount of government and third-party reimbursement. |
If our products do not achieve market acceptance, we will not be able to generate
sufficient revenues from product sales to maintain or grow our business.
Risks Related to Regulation
IF OUR PRECLINICAL TESTING AND CLINICAL TRIALS ARE NOT SUCCESSFUL, WE WILL NOT
OBTAIN REGULATORY APPROVAL FOR COMMERCIAL SALE OF OUR PRODUCT CANDIDATES.
We will be required to demonstrate, through preclinical testing and clinical
trials, that our product candidates are safe and effective before we can obtain
regulatory approval for the commercial sale of our product candidates. Preclinical
testing and clinical trials are lengthy and expensive and the historical rate of
failure for product candidates is high. Product candidates that appear promising in
the early phases of development, such as in preclinical study or in early human
clinical trials, may fail to demonstrate safety and efficacy in clinical trials.
There is no assurance that the results obtained to date and/or any further work
completed in the future will be sufficient to achieve the approvability of
Altropane.
Clinical trials require sufficient patient enrollment which is a function of many
factors, including the size of the potential patient population, the nature of the
protocol, the availability of existing treatments for the indicated disease and the
eligibility criteria for enrolling in the clinical trial. Delays or difficulties in
completing patient enrollment can result in increased costs and longer development
times.
11
Table of Contents
OUR PRODUCT CANDIDATES ARE SUBJECT TO RIGOROUS REGULATORY REVIEW AND, EVEN IF
APPROVED, REMAIN SUBJECT TO EXTENSIVE REGULATION.
Our technologies and product candidates must undergo a rigorous regulatory approval
process which includes extensive preclinical and clinical testing to demonstrate
safety and efficacy before any resulting product can be marketed. Our research and
development activities are regulated by a number of government authorities in the
United States and other countries, including the FDA pursuant to the Federal Food,
Drug, and Cosmetic Act. The clinical trial and regulatory approval process usually
requires many years and substantial cost. To date, neither the FDA nor any of its
international equivalents has approved any of our product candidates for marketing.
The FDA regulates drugs in the United States, including their testing,
manufacturing and marketing. Data obtained from testing is subject to varying
interpretations which can delay, limit or prevent FDA approval. The FDA has
stringent laboratory and manufacturing standards which must be complied with before
we can test our product candidates in people or make them commercially available.
Examples of these standards include Good Laboratory Practices and current Good
Manufacturing Practices, or cGMP. Our compliance with these standards is subject to
initial certification by independent inspectors and continuing audits thereafter.
In addition, manufacturers of our product candidates are subject to the FDA’s cGMP
regulations and similar foreign standards and we do not have control over
compliance with these regulations by our manufacturers. If any third-party
manufacturer fails to perform as required, this could impair our ability to deliver
our products on a timely basis or cause delays in our clinical trials and
applications for regulatory approval.
Obtaining FDA approval to sell our product candidates is time-consuming and
expensive. The FDA usually takes at least 12 to 18 months to review an NDA which
must be submitted before the FDA will consider granting approval to sell a product.
If the FDA requests additional information, it may take even longer for the FDA to
make a decision especially if the additional information that they request requires
us to complete additional studies. We may encounter similar delays in foreign
countries. After reviewing any NDA we submit, the FDA or its foreign equivalents
may decide not to approve our products. Failure to obtain regulatory approval for a
product candidate will prevent us from commercializing our product candidates.
Other risks associated with the regulatory approval process include:
| • | Regulatory approvals may impose significant limitations on the uses for which any approved products may be marketed; | ||
| • | Any marketed product and its manufacturer are subject to periodic reviews and audits, and any discovery of previously unrecognized problems with a product or manufacturer could result in suspension or limitation of approvals; | ||
| • | Changes in existing regulatory requirements, or the enactment of additional regulations or statutes, could prevent or affect the timing of our ability to achieve regulatory compliance. Federal and state laws, regulations and policies may be changed with possible retroactive effect, and how these rules actually operate can depend heavily on administrative policies and interpretation over which we have no control, and we may possess inadequate experience to assess their full impact upon our business; and | ||
| • | The approval may impose significant restrictions on the indicated uses, conditions for use, labeling, advertising, promotion, marketing and/or production of such product, and may impose ongoing requirements for post-approval studies, including additional research and development and clinical trials. |
OUR PRODUCTS COULD BE SUBJECT TO RESTRICTIONS OR WITHDRAWAL FROM THE MARKET AND WE
MAY BE SUBJECT TO PENALTIES IF WE FAIL TO COMPLY WITH REGULATORY REQUIREMENTS, OR
IF WE EXPERIENCE UNANTICIPATED PROBLEMS WITH OUR PRODUCTS, WHEN AND IF ANY OF THEM
ARE APPROVED.
12
Table of Contents
Any product for which we obtain marketing approval, along with the manufacturing
processes, post-approval clinical data, labeling, advertising and promotional
activities for such product, will be subject to continual requirements of and
review by the FDA and other regulatory bodies. These requirements include
submissions of safety and other post-marketing information and reports,
registration requirements, quality assurance and corresponding maintenance of
records and documents, requirements regarding the distribution of samples to
physicians and recordkeeping. The manufacturer and the manufacturing facilities we
use to make any of our product candidates will also be subject to periodic review
and inspection by the FDA. The subsequent discovery of previously unknown problems
with a product, manufacturer or facility may result in restrictions on the product
or manufacturer or facility, including withdrawal of the product from the market.
Even if regulatory approval of a product is granted, the approval may be subject to
limitations on the indicated uses for which the product may be marketed or to the
conditions of approval, or contain requirements for costly post-marketing testing
and surveillance to monitor the safety or efficacy of the product. Later discovery
of previously unknown problems with our products, manufacturers or manufacturing
processes, or failure to comply with regulatory requirements, may result in:
| • | Restrictions on such products, manufacturers or manufacturing processes; | ||
| • | Warning letters; | ||
| • | Withdrawal of the products from the market; | ||
| • | Refusal to approve pending applications or supplements to approved applications that we submit; | ||
| • | Recall; | ||
| • | Fines; | ||
| • | Suspension or withdrawal of regulatory approvals; | ||
| • | Refusal to permit the import or export of our products; | ||
| • | Product seizure; and | ||
| • | Injunctions or the imposition of civil or criminal penalties. |
FAILURE TO OBTAIN REGULATORY APPROVAL IN FOREIGN JURISDICTIONS WOULD PREVENT US
FROM MARKETING OUR PRODUCTS ABROAD.
Although we have not initiated any marketing efforts in foreign jurisdictions, we
intend in the future to market our products outside the United States. In order to
market our products in the European Union and many other foreign jurisdictions, we
must obtain separate regulatory approvals and comply with numerous and varying
regulatory requirements. The approval procedure varies among countries and can
involve additional testing. The time required to obtain approval abroad may differ
from that required to obtain FDA approval. The foreign regulatory approval process
may include all of the risks associated with obtaining FDA approval and we may not
obtain foreign regulatory approvals on a timely basis, if at all. Approval by the
FDA does not ensure approval by regulatory authorities in other countries, and
approval by one foreign regulatory authority does not ensure approval by regulatory
authorities in other foreign countries or approval by the FDA. We may not be able
to file for regulatory approvals and may not receive necessary approvals to
commercialize our
products in any market outside the United States. The failure to obtain these
approvals could materially adversely affect our business, financial condition and
results of operations.
FOREIGN GOVERNMENTS TEND TO IMPOSE STRICT PRICE CONTROLS WHICH MAY ADVERSELY AFFECT
OUR REVENUES, IF ANY.
The pricing of prescription pharmaceuticals is subject to governmental control in
some foreign countries. In these countries, pricing negotiations with governmental
authorities can take considerable time after the receipt of marketing approval for
a product. To obtain reimbursement or pricing approval in some countries, we may be
required to conduct a clinical trial that compares the cost-effectiveness of our
product candidate to other available therapies. If reimbursement of our products is
unavailable or limited in scope or amount, or if pricing is set at unsatisfactory
levels, our business could be adversely affected.
13
Table of Contents
Risks Related to our Intellectual Property
IF WE ARE UNABLE TO SECURE ADEQUATE PATENT PROTECTION FOR OUR TECHNOLOGIES, THEN WE
MAY NOT BE ABLE TO COMPETE EFFECTIVELY AS A BIOTECHNOLOGY COMPANY.
At the present time, we do not have patent protection for all uses of our
technologies. There is significant competition in the field of CNS diseases, our
primary scientific area of research and development. Our competitors may seek
patent protection for their technologies, and such patent applications or rights
might conflict with the patent protection that we are seeking for our technologies.
If we do not obtain patent protection for our technologies, or if others obtain
patent rights that block our ability to develop and market our technologies, our
business prospects may be significantly and negatively affected. Further, even if
patents can be obtained, these patents may not provide us with any competitive
advantage if our competitors have stronger patent positions or if their product
candidates work better in clinical trials than our product candidates. Our patents
may also be challenged, narrowed, invalidated or circumvented, which could limit
our ability to stop competitors from marketing similar products or limit the length
of term of patent protection we may have for our products.
Our patent strategy is to obtain broad patent protection, in the United States
and in major developed countries, for our technologies and their related medical
indications. Risks associated with protecting our patent and proprietary rights
include the following:
| • | Our ability to protect our technologies could be delayed or negatively affected if the United States Patent and Trademark Office, or USPTO, requires additional experimental evidence that our technologies work; | ||
| • | Our competitors may develop similar technologies or products, or duplicate any technology developed by us; | ||
| • | Our competitors may develop products which are similar to ours but which do not infringe our patents or products; | ||
| • | Our competitors may successfully challenge one or more of our patents in an interference or litigation proceeding; | ||
| • | Our technologies may infringe the patents or rights of other parties who may decide not to grant a license to us. We may have to change our products or processes, pay licensing fees or stop certain activities because of the patent rights of third parties which could cause additional unexpected costs and delays; | ||
| • | Patent law in the fields of healthcare and biotechnology is still evolving and future changes in such laws might conflict with our existing and future patent rights, or the rights of others; | ||
| • | Our collaborators, employees and consultants may breach the confidentiality agreements that we enter into to protect our trade secrets and proprietary know-how. We may not have adequate remedies for such breach; and | ||
| • | There may be disputes as to the ownership of technological information developed by consultants, scientific advisors or other third parties which may not be resolved in our favor. |
WE IN-LICENSE A SIGNIFICANT PORTION OF OUR INTELLECTUAL PROPERTY AND IF WE FAIL TO
COMPLY WITH OUR OBLIGATIONS UNDER ANY OF THE RELATED AGREEMENTS, WE COULD LOSE
LICENSE RIGHTS THAT ARE NECESSARY TO DEVELOP OUR PRODUCT CANDIDATES.
We have entered into license agreements with Harvard University and its affiliated
hospitals, or Harvard and its Affiliates, that give us rights to intellectual
property that is necessary for our business. These license arrangements impose
various development, royalty and other obligations on us. If we breach these
obligations and fail to cure such breach in a timely manner, these exclusive
licenses could be converted to non-exclusive licenses or the agreements could be
terminated, which would result in our being unable to develop, manufacture and sell
products that are covered by the licensed technology.
In order to continue to expand our business we may need to acquire additional
product candidates including those in clinical development through in-licensing
that we believe will be a strategic fit with us. We may not be able to in-license
suitable product candidates at an acceptable price or at all. Engaging in any
in-license will incur a variety of costs, and we may never realize the anticipated
benefits of any such in-license.
IF WE BECOME INVOLVED IN PATENT LITIGATION OR OTHER PROCEEDINGS RELATED TO A
DETERMINATION OF RIGHTS, WE COULD INCUR SUBSTANTIAL COSTS AND EXPENSES, SUBSTANTIAL
LIABILITY FOR DAMAGES OR BE REQUIRED TO STOP OUR PRODUCT DEVELOPMENT AND
COMMERCIALIZATION EFFORTS.
14
Table of Contents
A third party may sue us for infringing its patent rights. Likewise, we may need to
resort to litigation to enforce a patent issued or licensed to us or to determine
the scope and validity of third-party proprietary rights. In addition, a third
party may claim that we have improperly obtained or used its confidential or
proprietary information. There has been substantial litigation and other
proceedings regarding patent and other intellectual property rights in the
pharmaceutical and biotechnology industries. In addition to infringement claims
against us, we may become a party to other patent litigation and other proceedings,
including interference proceedings declared against us by the USPTO, regarding
intellectual property rights with respect to our products and technology. The cost
to us of any litigation or other proceeding relating to intellectual property
rights, even if resolved in our favor, could be substantial, and the litigation
would divert our management’s efforts. Some of our competitors may be able to
sustain the costs of complex patent litigation more effectively than we can because
they have substantially greater resources. Uncertainties resulting from the
initiation and continuation of any litigation could limit our ability to continue
our operations.
If any parties successfully claim that our creation or use of proprietary
technologies infringes upon their intellectual property rights, we might be forced
to pay damages, potentially including treble damages, if we are found to have
willfully infringed on such parties’ patent rights. In addition to any damages we
might have to pay, a court could require us to stop the infringing activity or
obtain a license. Any license required under any patent may not be made available
on commercially acceptable terms, if at all. In addition, such licenses are likely
to be non-exclusive and, therefore, our competitors may have access to the same
technology licensed to us. If we fail to obtain a required license and are unable
to design around a patent, we may be unable to effectively market some of our
technology and products, which could limit our ability to generate revenues or
achieve profitability and possibly prevent us from generating revenue sufficient to
sustain our operations. We might be required to redesign the formulation of a
product candidate so that it does not infringe, which may not be possible or could
require substantial funds and time. Ultimately, we could be prevented from
commercializing a product or be forced to cease some aspect of our business
operations if we are unable to enter into license agreements that are acceptable to
us.
Moreover, we expect that a number of our collaborations will provide that royalties
payable to us for licenses to our intellectual property may be offset by amounts
paid by our collaborators to third parties who have competing or superior
intellectual property positions in the relevant fields, which could result in
significant reductions in our revenues from products developed through
collaborations.
IF WE ARE UNABLE TO MAINTAIN OUR KEY WORKING RELATIONSHIPS WITH OUR LICENSORS,
INCLUDING HARVARD AND ITS AFFILIATES, WE MAY NOT BE SUCCESSFUL SINCE SUBSTANTIALLY
ALL OF OUR CURRENT TECHNOLOGIES WERE LICENSED FROM SUCH LICENSORS.
We maintain relationships with our licensors, Harvard and its
Affiliates. Substantially all of our technologies are licensed from these
licensors. Under the terms of our license agreements with Harvard and its
Affiliates, we acquired the exclusive, worldwide license to make, use, and sell the
technology covered by each respective agreement
Generally, each of these license agreements is effective until the last patent
licensed relating to the technology expires or a fixed and determined date. The
patents on the Altropane molecular imaging agent expire beginning in 2013.
Our license agreements with Harvard and its Affiliates generally provide for
royalty payments equal to specified percentages of product sales, annual license
maintenance fees and continuing patent prosecution costs. Universities and other
not-for-profit research institutions are becoming increasingly aware of the
commercial value of their findings and are becoming more active in seeking patent
protection and licensing arrangements to collect royalties for the use of
technology that they have developed. The loss of our relationship with our key
licensor could adversely affect
our ongoing development programs and could make it more costly and difficult for us
to obtain the licensing rights to new scientific discoveries.
Risks Related to Competition
WE ARE ENGAGED IN HIGHLY COMPETITIVE INDUSTRIES DOMINATED BY LARGER, MORE
EXPERIENCED AND BETTER CAPITALIZED COMPANIES.
The biotechnology and pharmaceutical industries are highly competitive, rapidly
changing, and are dominated by larger, more experienced and better capitalized
companies. Such greater experience and financial strength may enable them to bring
their products to market sooner than us, thereby gaining the competitive advantage
of being the first to market. Research on the causes of, and possible treatments
for, diseases for which we are trying to develop therapeutic or diagnostic products
are developing rapidly and there is a potential for extensive technological
innovation in relatively short periods of time.
15
Table of Contents
To our knowledge, there is only one company, GE Healthcare (formerly
Nycomed/Amersham), that has marketed a diagnostic imaging agent for PD and DLB,
DaTSCAN ® . GE Healthcare has obtained marketing approval in Europe. In
January, 2011, GE Healthcare obtained approval to market DaTSCAN in the U.S. as
well. GE Healthcare has significantly greater infrastructure and financial
resources than us. Marketing approval s in the US and Europe combined with their
established market presence, and greater financial strength could position GE to
make it difficult for us to successfully market Altropane if it is approved for
sale.
IF THIRD-PARTY PAYORS DO NOT ADEQUATELY REIMBURSE OUR CUSTOMERS FOR ANY OF OUR
PRODUCTS THAT ARE APPROVED FOR MARKETING, THEY MIGHT NOT BE ACCEPTED BY PHYSICIANS
AND PATIENTS OR PURCHASED OR USED, AND OUR REVENUES AND PROFITS WILL NOT DEVELOP OR
INCREASE.
Substantially all biotechnology products are distributed to patients by physicians
and hospitals, and in most cases, such patients rely on insurance coverage and
reimbursement to pay for some or all of the cost of the product. In recent years,
the continuing efforts of government and third party payors to contain or reduce
health care costs have limited, and in certain cases prevented, physicians and
patients from receiving insurance coverage and reimbursement for medical products,
especially newer technologies. We believe that the efforts of governments and
third-party payors to contain or reduce the cost of healthcare will continue to
affect the business and financial condition of pharmaceutical and biopharmaceutical
companies. Obtaining reimbursement approval for a product from each governmental or
other third-party payor is a time-consuming and costly process that could require
us to provide to each prospective payor scientific, clinical and cost-effectiveness
data for the use of our products. If we succeed in bringing any of our product
candidates to market and third-party payors determine that the product is eligible
for coverage; the third-party payors may nonetheless establish and maintain price
levels insufficient for us to realize a sufficient return on our investment in
product development. Moreover, eligibility for coverage does not imply that any
product will be reimbursed in all cases.
MEDICARE PRESCRIPTION DRUG COVERAGE LEGISLATION AND FUTURE LEGISLATIVE OR
REGULATORY REFORM OF THE HEALTH CARE SYSTEM MAY AFFECT OUR ABILITY TO SELL OUR
PRODUCT CANDIDATES PROFITABLY.
A number of legislative and regulatory proposals to change the healthcare system in
the United States and other major healthcare markets have been passed or proposed
in recent years including but not limited to the major reform of the US healthcare
system passed by the United States congress in 2010. It is unclear today what
effect the rules and regulations anticipated as a result of this new legislation
will have on our business, our ability to promote and sell Altropane and our
ability to obtain appropriate reimbursement for our product candidates. In
addition, ongoing initiatives in the United States have exerted and will continue
to exert pressure on drug pricing. In some foreign countries, particularly
countries of the European Union, the pricing of prescription pharmaceuticals is
subject to governmental control. Significant changes in the healthcare system in
the United States or elsewhere, including changes resulting from the implementation
of the Medicare prescription drug coverage legislation and adverse trends in
third-party reimbursement programs, could limit our ability to raise capital and
successfully commercialize our product candidates.
Further federal, state and foreign healthcare proposals and reforms are likely.
While we cannot predict the legislative or regulatory proposals that will be
adopted or what effect those proposals may have on our business, including the
future reimbursement status of any of our product candidates, the announcement or
adoption of such proposals could have an adverse effect on potential revenues from
product candidates that we may successfully develop.
WE HAVE NO MANUFACTURING CAPACITY AND LIMITED MARKETING INFRASTRUCTURE AND EXPECT
TO BE HEAVILY DEPENDENT UPON THIRD PARTIES TO MANUFACTURE AND MARKET APPROVED
PRODUCTS.
We currently have no manufacturing facilities for either clinical trial or
commercial quantities of any of our product candidates and currently have no plans
to obtain additional facilities. To date, we have obtained the limited quantities
of drug product required for preclinical and clinical trials from contract
manufacturing companies. We intend to continue using contract manufacturing
arrangements with experienced firms for the supply of material for both clinical
trials and any eventual commercial sale.
16
Table of Contents
We will depend upon third parties to produce and deliver products in accordance
with all FDA and other governmental regulations. We may not be able to contract
with manufacturers who can fulfill our requirements for quality, quantity and
timeliness, or be able to find substitute manufacturers, if necessary. The failure
by any third party to perform their obligations in a timely fashion and in
accordance with the applicable regulations may delay clinical trials, the
commercialization of products, and the ability to supply product for sale. In
addition, any change in manufacturers could be costly because the commercial terms
of any new arrangement could be less favorable and because the expenses relating to
the transfer of necessary technology and processes could be significant.
Risks Related to our Stock
OUR STOCK PRICE MAY CONTINUE TO BE VOLATILE AND CAN BE AFFECTED BY FACTORS
UNRELATED TO OUR BUSINESS AND OPERATING PERFORMANCE.
The market price of our common stock has in the past, and may continue to fluctuate
significantly in response to factors that are beyond our control. The stock market
in general periodically experiences significant price and volume fluctuations. The
market prices of securities of pharmaceutical and biotechnology companies have been
volatile, and have experienced fluctuations that often have been unrelated or
disproportionate to the operating performance of these companies. These broad
market fluctuations could result in significant fluctuations in the price of our
common stock, which could cause a decline in the value of your investment.
| Item 1B. | Unresolved Staff Comments. |
Not applicable.
| Item 2. | Properties. |
Our corporate office is located in Hopkinton, Massachusetts. We lease
approximately 16,000 square feet of office space which expires in 2011. We also
lease office space in Boston, Massachusetts pursuant to a lease that expires in
2012. We do not occupy this space. We have entered into two sublease agreements
covering all 6,600 square feet under our lease through the expiration of such
lease. We believe that our existing facilities are adequate for their present and
anticipated purposes, except that additional facilities will be needed if we elect
to expand our space to include laboratory and/or manufacturing activities.
| Item 3. | Legal Proceedings. |
We are subject to legal proceedings in the normal course of business. We are
not currently a party to any material legal proceedings.
| Item 4. | Removed and Reserved. |
17
Table of Contents
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities. |
Market Information
Our common stock trades on the Pink Sheets OTC Market under the symbol ALSE.PK.
Prior to May 8, 2009, our common stock was traded on the NASDAQ Capital Market
under the symbol ALSE.
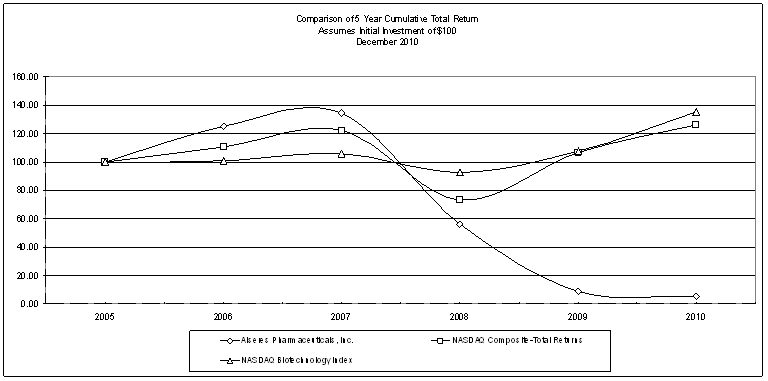
The following table sets forth the high and low per share sales prices for our
common stock for each of the quarters in the period beginning January 1, 2009
through December 31, 2010 as reported on the Pink Sheets OTC Market or the NASDAQ
Capital Market.
| Quarter Ended | High | Low | ||||||
March 31, 2009 |
$ | 1.88 | $ | 0.49 | ||||
June 30, 2009 |
$ | 1.18 | $ | 0.55 | ||||
September 30, 2009 |
$ | 1.01 | $ | 0.51 | ||||
December 31, 2009 |
$ | 0.75 | $ | 0.19 | ||||
March 31, 2010 |
$ | 0.40 | $ | 0.20 | ||||
June 30, 2010 |
$ | 0.39 | $ | 0.18 | ||||
September 30, 2010 |
$ | 0.30 | $ | 0.18 | ||||
December 31, 2010 |
$ | 0.35 | $ | 0.12 | ||||
As of March 21, 2011, there were approximately 2,800 holders of record of our
common stock. As of March 21, 2011, there were approximately 8,100 beneficial
holders of our common stock.
We have not paid or declared any cash dividends on our common stock and do not
expect to pay cash dividends on our common stock in the foreseeable future.
| Item 6. | Selected Financial Data. |
Not required.
18
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
Our management’s discussion and analysis of our financial condition and results of
operations include the identification of certain trends and other statements that
may predict or anticipate future business or financial results that are subject to
important factors that could cause our actual results to differ materially from
those indicated. See Item 1A, “Risk Factors.”
Overview
We are a biotechnology company focused on the development of therapeutic and
diagnostic products primarily for disorders in the central nervous system, or CNS.
Our clinical and preclinical product candidate is based on a proprietary technology
platform:
| • | Molecular imaging program focused on the diagnosis of i) Parkinsonian Syndromes, or PS, including Parkinson’s Disease, or PD, and ii) Dementia with Lewy Bodies, or DLB. |
As of December 31, 2010, we have experienced total net losses since inception of
approximately $203,456,000, stockholders’ deficit of approximately $47,286,000 and
a net working capital deficit of approximately $42,079,000. The cash and cash
equivalents available at December 31, 2010 will not provide sufficient working
capital to meet our anticipated expenditures for the next twelve months. At March
21, 2011, we had cash and cash equivalents of approximately $100,000 which combined
with our ability to control certain costs, including those related to clinical
trial programs, preclinical activities, and certain general and administrative
expenses will enable us to meet our anticipated cash expenditures into April, 2011.
We must immediately raise additional funds in order to continue operations and to
fund the estimated $30 million of development costs related to the Altropane POET-2
program. This funding is not available at present and there can be no assurance
that such funds will be available on acceptable terms if at all.
Product Development-Molecular Imaging Program
The Altropane molecular imaging agent is being developed for the differential
diagnosis of PS, including PD, and non-PS in patients with an upper extremity
tremor.
We believe in the current environment that, due to their proximity to
commercialization and return on investment, late stage development programs may
continue to be of significant interest to potential partners and investors. To
maximize the value of our molecular imaging program, we are focusing on obtaining
the funding necessary to execute the Altropane Phase III registration program. We
are pursuing financing necessary to enable us to advance the Altropane program
through our own means. In parallel, we are seeking to partner our molecular imaging
program with a firm or firms with the resources necessary for the completion of the
Phase III clinical program, for the manufacturing and supply of Altropane, and for
the launch and commercialization of Altropane. We can provide no assurances that a
partnership transaction will occur. We believe that the expansion of the program
into other indications such as DLB and other countries including those in Europe
could increase the value of the program for the partner and us. All of these
activities require additional funding and as such are proceeding, if at all, only
as rapidly as available resources permit. There can be no assurance that the
required funding to advance the Altropane program will be available on acceptable
terms if at all.
Results of Operations
Years Ended December 31, 2010 and 2009
Our net income was $512,419 during the year ended December 31, 2010 as compared
with a net loss of $10,776,937 during the year ended December 31, 2009. Net income
totaled $0.01 per share during 2010 as compared with a net loss of $0.46 per share
during 2009. The decrease in net loss in 2010 was primarily attributable to the
transaction covered by the Note Purchase Agreement dated September 10, 2010 which
resulted in the recording of a gain on the early extinguishment of debt of
$6,277,100 (Note 6).
Research and development expenses were $465,449 during the year ended December 31,
2010 as compared with $3,702,781 during the year ended December 31, 2009. The
decrease of $3,237,332, or 87% in 2010 was attributable to our decision in 2009 to
scale back operations which resulted in lower compensation and related costs of
approximately $2,482,000 and a reduction of $448,000 in stock compensation expense.
19
Table of Contents
General and administrative expenses were $2,657,488 during the year ended December
31, 2010 as compared with $4,623,201 during the year ended December 31, 2009. The
decrease of $1,965,713 or 43% in 2010 was primarily related to lower compensation
and related costs of approximately $608,000 which resulted from additional
reductions in headcount, lower stock-compensation expense of $764,000, lower legal
costs of approximately $666,000 and a reduction of $220,000 in directors’ fees as a
result of the January 2010 restructuring of our board of directors resulting in the
elimination of four board seats. The decrease was partially offset due to an
increase of $282,000 in occupancy expenses now being allocated to general and
administrative expense and a net increase in temporary and consulting services of
$70,000.
Interest expense totaled $2,643,630 during the year ended December 31, 2010 as
compared to $2,523,095 during the year ended December 31, 2009. The net increase of
$120,535 in the 2010 period was primarily attributable to the issuance of a
$350,000 promissory note in December 2009 and the issuance of $3,310,000 in
promissory notes during the twelve months ended December 31, 2010. The notes all
bear interest at the rate of 7% per annum.
Investment income was $1,886 during the year ended December 31, 2010 as compared
with investment income of $7,140 during the year ended December 31, 2009. The
decrease in 2010 was attributable to lower cash and cash equivalent balances in
2010 than in 2009.
Liquidity and Capital Resources
Net cash used for operating activities totaled $2,950,667 in 2010 as compared to
$8,060,298 in 2009. The decrease in cash used during the 2010 period reflects our
continued efforts to lower overall spending on staff and related overhead costs.
Net cash provided by investing activities totaled $35,017 in 2010 as compared to
cash provided by investing activities of $76,476 in 2009. The change in investing
activities reflects the scheduled refund of security deposits held by our landlord
on the Boston leased property. Net cash provided by financing activities totaled
$2,699,200 in 2010 as compared to $8,224,812 in 2009. The decrease in 2010
primarily reflects the lack of funding from the issuance of additional common or
preferred stock and our continued reliance for funding from one lead investor in
2010.
To date, we have dedicated most of our financial resources to the research and
development of our product candidates, general and administrative expenses
(including costs related to obtaining and protecting patents). Since inception, we
have primarily satisfied our working capital requirements from the sale of our
securities through private placements. These private placements have included the
sale and issuance of preferred stock, common stock, promissory notes and
convertible debentures.
A summary of financings completed through December 31, 2010 can be found in Note 5
of these Consolidated Financial Statements. A summary of financing activities
through March 21, 2011 can be found in Item 9B Other Information.
In the future, our working capital and capital requirements will depend on numerous
factors, including the progress of our research and development activities, the
level of resources that we devote to the developmental, clinical, and regulatory
aspects of our technologies, and the extent to which we enter into collaborative
relationships with pharmaceutical and biotechnology companies. At March 21, 2011,
we had cash and cash equivalents of approximately $100,000 which combined with our
ability to control administrative expenses will enable us to meet our anticipated
cash expenditures into April, 2011.
20
Table of Contents
Impact of Recently Issued Accounting Pronouncements
See Note 1 to the Notes to Consolidated Financial Statements — “Recent Accounting
Pronouncements.
Off-Balance Sheet Arrangements
We had no “off balance sheet arrangements” (as defined in Item 303(a)(4) of
Regulation S-K) during the year ended December 31, 2010.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results
of operations are based upon our consolidated financial statements which have been
prepared by us in accordance with accounting principles generally accepted in the
United States of America. The preparation of these financial statements requires us
to make estimates and judgments that affect the reported amounts of assets,
liabilities, revenues and expenses, and related disclosure of contingent assets and
liabilities. Our estimates include those related to marketable securities, research
contracts, the fair value and classification of financial instruments, our lease
accrual and stock-based compensation. We base our estimates on historical
experience and on various other assumptions that we believed to be reasonable under
the circumstances. Actual results may differ from these estimates under different
assumptions or conditions.
| Item 7A. | Quantitative Qualitative Disclosures About Market Risk |
Not required.
| Item 8. | Financial Statements and Supplementary Data. |
The
information required by Item 8 is included on pages 31 - 49.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
None
| Item 9A. | Controls and Procedures. |
As of the end of the period covered by this annual report on Form 10-K, we
evaluated the effectiveness of the design and operation of our disclosure controls
and procedures (as defined in the Securities Exchange Act of 1934 Rules 13a-15(e)
and 15d-15(e)). That evaluation was performed under the supervision and with the
participation of its management, including its Chief Executive Officer and its
Chief Financial Officer. Based on that evaluation, our Chief Executive Officer and
Chief Financial Officer concluded that the Company’s disclosure controls and
procedures are effective to ensure that information required to be disclosed in the
reports we file or submit under the Exchange Act is recorded, processed, summarized
and reported within the time periods specified by the SEC rules and forms, and that
such information is accumulated and communicated to our management, including our
certifying officer, to allow timely decisions regarding the required disclosure.
Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal
control over financial reporting, as such term is defined in Exchange Act Rule
13a-15(f). Under the supervision and with the participation of our management,
including our principal executive officer and principal financial officer, we
conducted an evaluation of the effectiveness of our internal control over financial
reporting based on the framework in Internal Control-Integrated Framework issued by
the Committee of Sponsoring Organizations of the Treadway Commission.
Based on our evaluation under the framework in Internal Control — Integrated
Framework, our management concluded that our internal control over financial
reporting was effective for the year ended December 31, 2010.
This annual report does not include an
attestation report of the Company’s independent public accounting firm regarding
internal control over financial reporting. Management’s report was not subject to
attestation by the Company’s independent public accounting firm pursuant to
rules of the Securities and Exchange Commission that call for the Company
to provide only management’s report in this annual report.
Changes in internal controls over financial reporting
There were no changes in our internal control over financial reporting that
occurred during the period covered by this Annual Report on Form 10-K that have
materially affected, or are reasonably likely to materially affect, our internal
control over financial reporting.
21
Table of Contents
| Item 9B. | Other Information |
In each of January, February and March 2011 the Company issued Promissory
Notes to Mr. Robert Gipson totaling $600,000. The Notes bear interest at 7% per
annum and are due and payable on demand.
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
At December 31, 2010 our Board of Directors (the “Board”) consisted of three directors.
| First Year Elected | ||||||||||
| Name | Age | as a Director | Position(s) with the Company (6) | |||||||
Peter G. Savas
|
62 | 2004 | Chairman of the Board of Directors, Chief Executive Officer and Director | |||||||
Michael J. Mullen, CPA
|
52 | 2004 | Director | |||||||
William Guinness
|
71 | 2006 | Director | |||||||
Peter G. Savas . Mr. Savas has been the Chairman of the Board and our Chief
Executive Officer since September 2004. From March 2004 to September 2004, Mr.
Savas was the Managing Partner of Tughill Partners, a life sciences consulting
firm. From September 2000 to March 2004, Mr. Savas served as Chief Executive
Officer and President and, from April 2001 to March 2004, as Chairman, of Aderis
Pharmaceuticals, Inc., a privately-held biopharmaceutical company. From 1992 to
2000, Mr. Savas served as President of Unisyn, Inc., a contract manufacturer of
biologics, and was also Unisyn’s Chief Executive Officer from 1995 to 2000. Mr.
Savas serves on the board of directors of pSivida Corp., a leading drug delivery
company.
Michael J. Mullen, C.P.A. Mr. Mullen has been a member of our Board since June
2004. Mr. Mullen is currently an independent consultant. From December 2009 to June
2010, Mr Mullen was Interim Chief Financial Officer of Valeritas, Inc., a medical
technology company. From July 2006 to October 2009, Mr. Mullen was the Chief
Financial Officer of Magellan Biosciences, Inc., a clinical diagnostics company
From March 2006 to July 2006, Mr. Mullen was an independent consultant. From
February 2003 to March 2006, Mr. Mullen was the Chief Financial Officer of JMH
Capital, a private equity firm..
William Guinness. Mr. Guinness has been a member of our Board since July 2006. Mr.
Guinness has been Chairman of Sibir Energy plc, a UK independent oil and gas
production company, since March 1999, having previously been a Non-Executive
Director of Pentex Energy plc and Pentex Oil plc. Since 1988, Mr. Guinness has been
involved with various private venture capital operations, which cover areas as
diverse as metal manufacturing, general aviation and fine art consultancy. Mr.
Guinness is also a director of a number of private companies involved in a wide
range of commercial activities. Mr. Guinness previously served on our Board of
Directors from June 30, 2003 to September 20, 2003.
Code of Business Conduct and Ethics
We have adopted a written Code of Business Conduct and Ethics that applies to our
directors, officers and employees, including our principal executive officer,
principal financial officer, principal accounting officer or controller, or persons
performing similar functions.
| Executive Officers | Position | In Current Position Since | ||
Peter G. Savas
|
Chairman of the Board of Directors and Chief Executive Officer | September 2004 | ||
Kenneth L. Rice,
Jr., JD, LLM, MBA
|
Executive Vice President, Finance and Administration, Chief Financial Officer and Secretary | September 2005 (Executive Vice President, Finance and Administration and Chief Financial Officer since July 2005) |
22
Table of Contents
Kenneth L. Rice, Jr., J.D., LL.M., MBA. Mr. Rice was appointed Executive Vice
President, Finance and Administration and Chief Financial Officer in July 2005. Mr.
Rice was appointed Secretary in September 2005. In June 2005, Mr. Rice served as a
part-time consultant to the Company. From April 2001 to June 2005, Mr. Rice served
as Vice President, Chief Financial Officer, Chief Commercial Officer and Secretary
of Aderis Pharmaceuticals, Inc., a privately-held biopharmaceutical company. From
August 1999 through March 2001, Mr. Rice served as Vice President and Chief
Financial Officer of MacroChem Corporation, a publicly-traded drug delivery
company.
No family relationships exist between any of our executive officers and our
directors. Our executive officers are elected annually by the board of directors
and serve until their successors are duly elected and qualified.
Audit Committee
Our Board has a standing Audit Committee that currently consists of Messrs. Mullen
and Guinness. Our Board has determined that each of the members of the Audit
Committee are independent as defined under the independence requirements
contemplated by Rule 10A-3 under the Exchange Act. The Board has determined that
Mr. Mullen is an “audit committee financial expert” as defined in Item 407(d)(5) of
Regulation S-K.
| Item 11. | Executive Compensation |
| Other | ||||||||||||||||||||
| Name and Principal Position | Year | Salary | Option Awards(1) | Compensation (2) | Total | |||||||||||||||
Peter G. Savas |
2010 | $ | 401,625 | $ | — | $ | 3,258 | $ | 404,883 | |||||||||||
Chairman and CEO (3) |
2009 | $ | 408,085 | $ | 334,373 | $ | 8,688 | $ | 751,146 | |||||||||||
Kenneth L. Rice, Jr., JD, LLM, MBA |
2010 | $ | 267,750 | $ | — | $ | — | $ | 267,750 | |||||||||||
Executive Vice President and Chief
Financial Officer |
2009 | $ | 267,750 | $ | 184,828 | $ | — | $ | 452,578 | |||||||||||
| (1) | Valuation based on the dollar amount recognized for financial statement reporting purposes pursuant to FASB ASC Topic 820 with respect to fiscal 2009, except that (i) such amounts do not reflect an estimate of forfeitures related to service-based vesting conditions and (ii) the amounts reported in these columns reflect additional expense, if any, resulting from the requirements of the SEC to report option grants made prior to 2009 using the modified prospective transition method pursuant to FASB ASC Topic 820. The assumptions used by us with respect to the valuation of option grants are set forth in Note 8. | |
| (2) | Other Compensation includes disability insurance for 2010 and 2009. | |
| (3) | Mr. Savas is also a member of our board of directors, but does not receive any additional compensation in his capacity as a director. |
Outstanding Equity Awards
Outstanding option awards held by our Named Executive Officers as of December 31,
2010:
| Number of Securities | Option | |||||||||||
| Underlying Unexercised | Exercise | Option | ||||||||||
| Options Exercisable | Price ($) | Expiration Date | ||||||||||
Peter G. Savas |
200,000 | (1) | $ | 2.31 | 3/11/2015 | |||||||
| 950,000 | (2) | $ | 1.15 | 1/31/2014 | ||||||||
Kenneth L. Rice, Jr., JD, LLM, MBA |
375,000 | (2) | $ | 1.15 | 1/31/2014 | |||||||
| (1) | The options are 33% exercisable initially; and thereafter vest in 36 equal installments | |
| (2) | The options are fully vested and exercisable at any time. |
23
Table of Contents
Potential Payments Upon Termination or Change-in-Control
On March 31, 2006, we entered into employment agreements with each of Messrs. Savas
and Rice effective January 1, 2006 for a term of one year. These agreements are
collectively referred to as the Employment Agreements. Each Employment Agreement
automatically renews for an additional 12 month period, unless either party
notifies the other party in writing not less than 90 days prior to expiration.
In general, in the event of termination without “cause” (as defined in the
Employment Agreements) or voluntarily by an executive within one year following a
“change in control” (as defined below), the Employment Agreements provide for (i) a
cash severance payment equal to the sum of 75% — 100% of the sum of an executive’s
highest base salary in effect during the preceding 12 month period and the average
annual cash bonus paid during the preceding twenty-four month period, (ii) the
continuation of health care benefits for a period of 9 to 12 months following
termination of employment, and (iii) full acceleration of the vesting of all of the
executive’s unvested equity awards. In the event of termination of employment by us
for “disability” (as defined in the Employment Agreements), the Employment
Agreements provide for a cash severance payment equal to the sum of 75% — 100% of
the sum of an executive’s highest base salary in effect during the preceding 12
month period and the average annual cash bonus paid during the preceding
twenty-four month period.
A “change in control” means:
| (1) | an acquisition of any of our voting securities by any person immediately after which such person has beneficial ownership of 45% or more of the combined voting power of our then outstanding voting securities; or | |
| (2) | approval by our stockholders of: | |
| (a) our merger, consolidation, share exchange or reorganization, unless our stockholders, immediately before such merger, consolidation, share exchange or reorganization, own, directly or indirectly immediately following such merger, consolidation, share exchange or reorganization, at least 51% of the combined voting power of the outstanding voting securities of the corporation that is the successor in such merger, consolidation, share exchange or in substantially the same proportion as their ownership of the voting securities immediately before such merger, consolidation, share exchange or reorganization; or | ||
| (b) our complete liquidation or dissolution; or | ||
| (c) an agreement for the sale or other disposition of all or substantially all of our assets. |
If the employment of any Named Executive Officer is terminated, unless employment
is terminated without cause or after the occurrence of a change in control, such
Named Executive Officer will remain subject to certain conditions regarding
non-competition, non-solicitation and confidentiality, for a period of one year
following the date of termination of employment.
Compensation of Directors
In 2010, our non-employee directors consisted of Michael J. Mullen and William
Guinness. Their compensation included an annual retainer of $25,000 and a $2,500
fee per meeting attended. Fees earned in 2010 but unpaid for these two directors
totaled $75,000. Fees earned for 2009 and a portion of 2008 for these two directors
and 4 former directors totaled approximately $445,000 and have not been paid as of
March 31, 2011.
As of December 31, 2010, the number of shares underlying options held by Michael J.
Mullen and William Guinness was 125,417 and 80,000, respectively.
| Item 12. | Security ownership of certain beneficial owners and management and related stockholder matters |
Security Ownership
The following table sets forth information, as of December 31, 2010, regarding the
beneficial ownership of our common stock by:
| • | each person or “group,” as that term is defined in Section 13(d)(3) of the Exchange Act, that beneficially owns more than 5% of our outstanding common stock based on currently available Schedules 13D and 13G filed with the Securities and Exchange Commission; | ||
| • | each of our directors; | ||
| • | each of the Named Executive Officers; and | ||
| • | all of our directors and executive officers as a group. |
24
Table of Contents
Unless otherwise indicated below, the address for each listed director and
executive officer is c/o Alseres Pharmaceuticals, Inc., 239 South Street,
Hopkinton, Massachusetts 01748. Beneficial ownership shown is determined in
accordance with the rules of the Securities and Exchange Commission and, as a
result, includes voting and investment power with respect to shares.
| 5% Beneficial Owners of Common Stock: | Amount of | Percent of | ||||||
| Name and Address | Beneficial Ownership | Class (2) | ||||||
Robert L. Gipson (3) c/o Ingalls & Snyder LLC, 61 Broadway, New York, NY 10006 |
21,692,226 | 49.2 | ||||||
Thomas L. Gipson (4) c/o Ingalls & Snyder LLC, 61 Broadway, New York, NY 10006 |
6,612,504 | 15.0 | ||||||
Ingalls & Snyder Value Partners, LP (5) 61 Broadway, New York, NY 10006 |
7,568,341 | 17.2 | ||||||
Ingalls & Snyder LLC (6) 61 Broadway, New York, NY 10006 |
2,891,674 | 6.6 | ||||||
Arthur Koenig (7) c/o Duferco Steel Inc., Matawan Rd., Suite 400, Matawan, NJ 07747 |
3,561,500 | 8.1 | ||||||
| Amount of | ||||||||
| Beneficial | Percent of | |||||||
| Directors and Named Executive Officers: | Ownership | Class (2) | ||||||
Peter G. Savas (8) Chairman of the Board and Chief Executive Officer |
1,161,212 | 2.6 | ||||||
Kenneth L. Rice, Jr., JD, LLM, MBA (9) Executive Vice President Finance and Administration, Chief Financial Officer and Secretary |
379,485 | * | ||||||
Michael J. Mullen, CPA (10) Director |
117,084 | * | ||||||
William Guinness (11) Director |
71,667 | * | ||||||
All directors and executive officers as a group (4 persons) |
1,729,448 | 3.9 | ||||||
| Represents less than 1% of the outstanding shares. | ||
| (1) | Except as set forth in the footnotes to this table and subject to applicable community property law, the persons and entities named in the table have sole voting and investment power with respect to all shares. | |
| (2) | Applicable percentage ownership for each holder is based on 26,785,645 shares of common stock outstanding on December 31, 2010, plus common stock issuable upon conversion of any outstanding convertible promissory notes, Series F Convertible Preferred Stock, and any common stock equivalents and presently exercisable stock options or warrants held by each such holder. | |
| (3) | Information is based on a Schedule 13 G/A filed on November 29, 2010 with the SEC. Consists of (i) 10,836,004 shares of common stock currently issued and outstanding, (adjusted for the December 2010 sale of 270,000 shares) (ii) 4,900,000 shares of common stock into which 196,000 shares of Series F Convertible Preferred Stock, $0.01 par value per share, (iii) 5,956,222 shares of common stock into which our promissory notes in the aggregate principal and interest amount of $14,890,556 were convertible as of December 31, 2010. As of December 31, 2010, Mr. Gipson held 100% of the issued and outstanding Series F Preferred. | |
| (4) | Information is based on a Schedule 13 G/A filed on January 8, 2010 with the SEC. Consists of (i) 5,186,004 shares of common stock currently issued and outstanding and (ii) 1,426,500 shares of common stock into which our promissory notes in the aggregate principal and interest amount of $3,566,250 were convertible as of December 31, 2010. | |
| (5) | Information is based on a Schedule 13 G/A filed on February 9, 2011with the SEC. Consists of (i) 2,891,674 shares of common stock currently issued and outstanding and (ii) 4,676,667 shares of common stock into which our promissory notes in the aggregate principal and interest amount of $11,691,668 were convertible as of December 31, 2010. |
25
Table of Contents
| (6) | Information is based on a Schedule 13G/A filed February 10, 2011 with the SEC. Ingalls & Snyder LLC beneficially owns 2,891,674 shares of common stock and has shared power to dispose or direct the disposition of 2,891,674 shares. Securities reported under shared dispositive power include securities owned by clients of Ingalls & Snyder LLC, a registered broker dealer and a registered investment advisor, in accounts managed under investment advisory contracts. Such clients include Ingalls & Snyder Value Partners, LP. | |
| (7) | Information is based on a Schedule 13G/A filed February 9, 2011 with the SEC. Consists of 2,135,000 shares of our common stock currently issued and outstanding and (ii) 1,426.500 shares of common stock into which our promissory notes in the aggregate principal and interest amount of $3,566,250 were convertible as of December 31, 2010. | |
| (8) | Includes 11,212 shares of common stock owned outright and 1,150,000 shares of common stock issuable upon exercise of options. | |
| (9) | Includes 4,485 shares of common stock owned outright and 375,000 shares of common stock issuable upon exercise of options. | |
| (10) | Consists of 117,084 shares of common stock issuable upon exercise of options and 200 shares of common stock held by a revocable trust of which Mr. Mullen is the trustee. | |
| (11) | Consists of 71,667 shares of common stock issuable upon exercise of options. | |
| (12) | See notes 8 through 11. |
26
Table of Contents
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
Certain Relationships and Related Transactions
We have entered into indemnity agreements with each of our directors and executive
officers containing provisions that may require us, among other things, to
indemnify those directors and officers against liabilities that may arise by reason
of their status or service as directors and officers. The agreements also provide
for us to advance to the directors and officers expenses that they expect to incur
as a result of any proceeding against them related to their service as directors
and officers.
For information regarding related party transactions see Note 12 of these
Consolidated Financial Statements. For information relating to our employment and
severance arrangements with our Named Executive Officers, see “Executive
Compensation — Potential Payments Upon Termination or Change-in-Control.”
| Item 14. | Principal Accountant Fees and Services |
Independent Registered Public Accounting Firm’s Fees and Other Matters
The following table summarizes the fees billed to us for professional services
rendered by McGladrey & Pullen, LLP and PricewaterhouseCoopers LLP, our prior
independent registered public accounting firm, for each of the last two years:
| Fee Category | 2010 | 2009 | ||||||
Audit Fees |
$ | 89,060 | $ | 131,000 | ||||
Audit-Related Fees |
7,800 | 7,500 | ||||||
Tax Fees |
30,000 | 30,000 | ||||||
All Other Fees |
— | 2,445 | ||||||
Total Fees |
$ | 126,860 | $ | 170,945 | ||||
Audit Fees
Audit fees consist of fees for the audit of our consolidated annual financial
statements, the review of the interim consolidated financial statements included in
our quarterly reports on Form 10-Q and other professional services provided in
connection with statutory and regulatory filings or engagements.
Audit-Related Fees
Audit-related fees consist of fees for assurance and related services that are
reasonably related to the performance of the audit or review of our consolidated
financial statements and are not reported under “Audit Fees.” These services
include consultations concerning financial accounting and reporting matters not
classified as audits.
Tax Fees
Tax fees consist of fees for tax compliance, tax advice and tax planning services.
All Other Fees
All other fees for 2009 consisted of fees relating to an accounting research tool.
27
Table of Contents
Policy on Audit Committee Pre-approval of Audit and Permissible Non-audit Services
of Independent Registered Public Accounting Firm
Consistent with policies of the SEC regarding independent registered public
accounting firm independence and our Audit Committee Charter, our Audit Committee
has the responsibility for appointing, retaining, setting compensation and
overseeing the work of the independent registered public accounting firm. Our Audit
Committee’s policy is to pre-approve all audit and permissible non-audit services
provided by the independent registered public accounting firm. Our Audit Committee
presently pre-approves particular services on a case-by-case basis. In assessing
requests for services by the independent registered public accounting firm, our
Audit Committee considers whether such services are consistent with the independent
registered public accounting firm’s independence, whether the independent
registered public accounting firm is likely to provide the most effective and
efficient service based upon their familiarity with us, and whether the service
could enhance our ability to manage or control risk or improve audit quality.
All of the audit-related, tax and other services provided by McGladrey & Pullen,
LLP in fiscal year 2010 and related fees were
approved in advance by our Audit Committee. None of the services and fees were
approved using the “de-minimis” exception under SEC rules.
Our Audit Committee believes that the provision of the non-audit services above is
compatible with maintaining the independent registered public accounting firm’s
independence.
28
Table of Contents
PART IV
| Item 15. | Exhibits and Financial Statement Schedules. |
The following documents are included as part of this Annual Report on Form 10-K.
| 1. | Financial Statements: |
Consolidated Financial Statements of the Company: |
||||
| 31 | ||||
| 32 | ||||
| 34 | ||||
| 36 | ||||
| 37 |
2. Financial Statement Schedules:
Schedules are omitted since the required information is not applicable or is not
present in amounts sufficient to require submission of the schedule, or because the
information required is included in the Consolidated Financial Statements or Notes
thereto.
3. Exhibits:
The Exhibits listed in the Exhibit Index immediately preceding the Exhibits are
filed as a part of this Annual Report on Form 10-K.
29
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholder
Alseres Pharmaceuticals, Inc.
Alseres Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheets of Alseres
Pharmaceuticals, Inc. and Subsidiaries (the “Company”) as of December 31, 2010 and
2009, and the related consolidated statements of operations, comprehensive loss and
stockholders’ (deficit) equity and cash flows for the years then ended and for the
period from October 16, 1992 (Inception) to December 31, 2010. These financial
statements are the responsibility of the Company’s management. Our responsibility
is to express an opinion on these financial statements based on our audits. The
financial statements for the period from October 16, 1992 (Inception) to December
31, 2006 were audited by other auditors and our opinion, insofar as it relates to
cumulative amounts include for such prior periods, is based solely on the reports
of such other auditors.
We conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement. The Company is not required to have,
nor were we engaged to perform an audit of its internal control over financial
reporting. Our audit included consideration of internal control over financial
reporting as a basis for designing audit procedures that are appropriate in the
circumstances, but not for the purpose of expressing an opinion on the
effectiveness of the Company’s internal control over financial reporting.
Accordingly, we express no such opinion. An audit also includes examining, on a
test basis, evidence supporting the amounts and disclosures in the financial
statements, assessing the accounting principles used and significant estimates made
by management, as well as evaluating the overall financial statement presentation.
We believe that our audits provide a reasonable basis for our opinion.
In our opinion, based on our audits and the reports of other auditors, the
consolidated financial statements referred to above present fairly, in all material
respects, the financial position of Alseres Pharmaceuticals, Inc. and Subsidiaries
as of December 31, 2010 and 2009, and the results of their operations and their
cash flows for the years then ended and for the period from October 16, 1992
(Inception) to December 31, 2010, in conformity with U.S. generally accepted
accounting principles.
The accompanying consolidated financial statements have been prepared assuming that
the Company will continue as a going concern. As discussed in Note 1 to the
consolidated financial statements, the Company has suffered recurring losses from
operations, its total liabilities exceed its total assets, and it has determined
that it will need to raise additional capital. This raises substantial doubt about
the Company’s ability to continue as a going concern. Management’s plans in regard
to these matters are also described in Note 1. The financial statements do not
include any adjustments that might result from the outcome of this uncertainty.
As discussed in Note 1 to the financial statements, the 2009 consolidated
financial statement have been restated to correct a misstatement.
/s/ McGladrey & Pullen, LLP
Boston, Massachusetts
March 31, 2011
Boston, Massachusetts
March 31, 2011
30
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
CONSOLIDATED BALANCE SHEETS
| (Restated) | ||||||||
| December 31, 2010 | December 31, 2009 | |||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 98,514 | $ | 314,964 | ||||
Security deposits |
96,163 | 38,928 | ||||||
Prepaid expenses and other current assets |
14,073 | 34,231 | ||||||
Total current assets |
208,750 | 388,123 | ||||||
Property and Equipment, net (Note 2) |
47,674 | 106,272 | ||||||
Indemnity fund (Note 11) |
115,568 | 115,720 | ||||||
Security deposits and other assets (Note 7) |
88,600 | 180,700 | ||||||
Total assets |
$ | 460,592 | $ | 790,815 | ||||
Current liabilities: |
||||||||
Accounts payable and accrued expenses (Note 4) |
$ | 3,847,568 | $ | 3,522,581 | ||||
Convertible notes payable (Note 5) |
29,000,000 | 34,155,632 | ||||||
Notes payable (Note 5) |
4,660,000 | 1,350,000 | ||||||
Accrued interest payable on convertible notes (Note 5) |
4,714,722 | 4,057,086 | ||||||
Accrued lease (Note 7) |
65,922 | 51,493 | ||||||
Total current liabilities |
42,288,212 | 43,136,792 | ||||||
Accrued lease, excluding current portion (Note 7) |
30,503 | 96,426 | ||||||
Total liabilities |
42,318,715 | 43,233,218 | ||||||
Commitments and contingencies (Note 11) |
||||||||
Series F convertible redeemable preferred stock,
$.01 par value; 200,000 shares designated; 196,000
shares issued and outstanding at December 31, 2010
and 2009, (liquidation preference of $4,900,000 at
December 31, 2010) |
5,428,158 | 5,066,919 | ||||||
Stockholders’ deficit: |
||||||||
Preferred stock, $.01 par value; 1,000,000 shares
authorized; 25,000 shares designated Convertible
Series A, 500,000 shares designated Convertible
Series D, and 800 shares designated Convertible
Series E; no shares issued and outstanding at
December 31, 2010 and 2009 |
— | — | ||||||
Common stock, $.01 par value; 79,730,000 and
80,000,000 shares authorized at December 31, 2010
and 2009, respectively; 26,785,645 and 25,555,645
shares issued and outstanding at December 31, 2010
and 2009, respectively |
267,856 | 255,556 | ||||||
Additional paid-in capital |
146,611,717 | 146,913,395 | ||||||
Deficit accumulated during development stage |
(194,165,854 | ) | (194,678,273 | ) | ||||
Total stockholders’ deficit |
(47,286,281 | ) | (47,509,322 | ) | ||||
Total liabilities and stockholders’ deficit |
$ | 460,592 | $ | 790,815 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
31
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
CONSOLIDATED STATEMENTS OF OPERATIONS
| From Inception | ||||||||||||
| For the Year Ended | (October 16, 1992) to | |||||||||||
| December 31, 2010 | December 31, 2009 | December 31, 2010 | ||||||||||
Revenues |
$ | — | $ | — | $ | 900,000 | ||||||
Operating expenses: |
||||||||||||
Research and development |
465,449 | 3,702,781 | 115,948,279 | |||||||||
General and administrative |
2,657,488 | 4,623,201 | 66,781,942 | |||||||||
Purchased in-process research and development |
— | — | 12,146,544 | |||||||||
Total operating expenses |
3,122,937 | 8,325,982 | 194,876,765 | |||||||||
Loss from operations |
(3,122,937 | ) | (8,325,982 | ) | (193,976,765 | ) | ||||||
Gain on early extinguishment of debt (Note 6) |
6,277,100 | — | 6,277,100 | |||||||||
Other income
(expense), net |
— | 65,000 | (1,517,878 | ) | ||||||||
Interest expense, net |
(2,643,630 | ) | (2,523,095 | ) | (12,650,626 | ) | ||||||
Investment income |
1,886 | 7,140 | 7,704,315 | |||||||||
Net income (loss) |
512,419 | (10,776,937 | ) | (194,163,854 | ) | |||||||
Preferred stock beneficial conversion feature |
— | — | (8,062,712 | ) | ||||||||
Accrual of preferred stock dividends and
modification of warrants held by preferred stock
stockholders (Note 8) |
— | — | (1,229,589 | ) | ||||||||
Net income (loss) |
$ | 512,419 | $ | (10,776,937 | ) | $ | (203,456,155 | ) | ||||
Net income
(loss) attributable to common stockholders, basic and diluted |
$ | 143,137 | $ | (10,776,937 | ) | |||||||
Net income (loss) per share, basic |
$ | 0.01 | $ | (0.46 | ) | |||||||
Net income (loss) per share, diluted |
$ | 0.00 | $ | (0.46 | ) | |||||||
Weighted average common shares, basic |
26,686,933 | 23,361,458 | ||||||||||
Weighted average common shares, diluted |
31,586,933 | 23,361,458 | ||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
32
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS AND STOCKHOLDERS’ (DEFICIT) EQUITY
For the Period from inception (October 16, 1992) to December 31, 2010
For the Period from inception (October 16, 1992) to December 31, 2010
| Deficit | ||||||||||||||||||||||||||||||||||||
| Accumulated | Accumulated | |||||||||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Other | During | Total | ||||||||||||||||||||||||||||||||
| Number of | Number of | Additional Paid- | Deferred | Comprehensive | Development | Stockholders’ | ||||||||||||||||||||||||||||||
| shares | Amount | shares | Par Value | In Capital | Compensation | Income (Loss) | Stage | (Deficit) Equity | ||||||||||||||||||||||||||||
Issuance of common
stock to founders |
304,009 | $ | 3,040 | $ | 45,685 | $ | 48,725 | |||||||||||||||||||||||||||||
Issuance of common
stock- exercise of
warrants and
options |
1,185,039 | 11,850 | 7,584,589 | 7,596,439 | ||||||||||||||||||||||||||||||||
Issuance of common
stock and warrants,
net of issuance
costs of $1,928,421 |
11,516,790 | 115,168 | 56,343,378 | 56,458,546 | ||||||||||||||||||||||||||||||||
Issuance of common
stock and warrants
upon Merger |
723,947 | 7,239 | 14,596,709 | 14,603,948 | ||||||||||||||||||||||||||||||||
Issuance of common
stock upon
conversion of
convertible
debentures |
31,321 | 313 | 988,278 | 988,591 | ||||||||||||||||||||||||||||||||
Issuance of
warrants in
connection with
debentures, net of
issuance costs of |
||||||||||||||||||||||||||||||||||||
$392,958 |
3,632,632 | 3,632,632 | ||||||||||||||||||||||||||||||||||
Issuance of
warrants in
connection with
preferred series C
stock issuance and
related beneficial
conversion feature,
net of issuance
costs of $590,890 |
3,736,789 | 3,736,789 | ||||||||||||||||||||||||||||||||||
Accretion of
preferred series C
stock |
(4,327,679 | ) | (4,327,679 | ) | ||||||||||||||||||||||||||||||||
Issuance of
preferred stock,
net of issuance
costs of $4,078,821 |
240,711 | $ | 2,296,355 | 23,288,101 | 25,584,456 | |||||||||||||||||||||||||||||||
Conversion of
preferred stock
into common stock
and payment of
interest in common
stock, net of
issuance costs of $27,664 |
(240,149.7 | ) | (1,491,474 | ) | 1,553,749 | 15,538 | 7,655,122 | 6,179,186 | ||||||||||||||||||||||||||||
Conversion of
debentures and
payment of interest
in common stock,
net of issuance
costs of $307,265 |
317,083 | 3,171 | 4,844,249 | 4,847,420 | ||||||||||||||||||||||||||||||||
33
Table of Contents
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS AND STOCKHOLDERS’ (DEFICIT) EQUITY-
(Continued)
For the Period from inception (October 16, 1992) to December 31, 2010
(Continued)
For the Period from inception (October 16, 1992) to December 31, 2010
| Deficit | |||||||||||||||||||||||||||||||||||||
| Accumulated | Accumulated | Total | |||||||||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Other | During | Stockholders’ | |||||||||||||||||||||||||||||||||
| Number of | Number of | Additional Paid- | Deferred | Comprehensive | Development | (Deficit) | |||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Par Value | In Capital | Compensation | Income (Loss) | Stage | Equity | |||||||||||||||||||||||||||||
Conversion of preferred stock into
common stock and modification of
warrants |
(561.3 | ) | (3,501,539 | ) | 900,646 | 9,006 | 3,492,533 | — | |||||||||||||||||||||||||||||
Preferred stock conversion
inducement |
(600,564 | ) | (600,564 | ) | |||||||||||||||||||||||||||||||||
Amortization of preferred stock
Series E beneficial conversion
feature |
2,696,658 | (2,696,658 | ) | — | |||||||||||||||||||||||||||||||||
Issuance of warrants in connection
with Series E Stock, net of
issuance costs of $278,426 |
2,049,297 | 2,049,297 | |||||||||||||||||||||||||||||||||||
Issuance of common stock in
connection with cancellation of
warrants |
42,667 | 427 | (427 | ) | — | ||||||||||||||||||||||||||||||||
Accrual of dividends on preferred
Series E stock |
(573,597 | ) | (573,597 | ) | |||||||||||||||||||||||||||||||||
Beneficial conversion feature on
10% convertible secured promissory
notes |
558,000 | 558,000 | |||||||||||||||||||||||||||||||||||
Deferred compensation related to
stock options and warrants granted |
804,607 | $ | (804,607 | ) | — | ||||||||||||||||||||||||||||||||
Compensation expense related to
stock options and warrants |
3,470,199 | 804,607 | 4,274,806 | ||||||||||||||||||||||||||||||||||
Modification of options and warrants |
1,804,694 | 1,804,694 | |||||||||||||||||||||||||||||||||||
Other |
783 | 8 | 69,925 | 69,933 | |||||||||||||||||||||||||||||||||
Comprehensive loss: |
|||||||||||||||||||||||||||||||||||||
Net loss from inception (October
16, 1992) to December 31, 2006 |
$ | (143,503,529 | ) | (143,503,529 | ) | ||||||||||||||||||||||||||||||||
Issuance of common stock upon
exercise of warrants and options |
202,183 | 2,022 | 143,683 | 145,705 | |||||||||||||||||||||||||||||||||
Issuance of common stock upon
conversion of notes payable |
4,000,000 | 40,000 | 9,960,000 | 10,000,000 | |||||||||||||||||||||||||||||||||
BCF on convertible notes
payable |
1,880,000 | 1,880,000 | |||||||||||||||||||||||||||||||||||
Compensation expense — stock options |
1,665,155 | 1,665,155 | |||||||||||||||||||||||||||||||||||
Modification of stock options |
5,614 | 5,614 | |||||||||||||||||||||||||||||||||||
Unrealized gain on marketable
securities |
9,310 | 9,310 | |||||||||||||||||||||||||||||||||||
Net loss |
(19,548,348 | ) | (19,548,348 | ) | |||||||||||||||||||||||||||||||||
Comprehensive loss |
(19,539,038 | ) | |||||||||||||||||||||||||||||||||||
Balance at December 31, 2007 |
— | — | 20,778,217 | 207,782 | 140,420,314 | — | 9,310 | (163,051,877 | ) | (22,414,471 | ) | ||||||||||||||||||||||||||
34
Table of Contents
CONSOLIDATED
STATEMENTS OF COMPREHENSIVE LOSS AND STOCKHOLDERS’ (DEFICIT) EQUITY-
(Continued)
(Continued)
For the Period from inception (October 16, 1992) to December 31, 2010
| Deficit | ||||||||||||||||||||||||||||||||||||
| Accumulated | Accumulated | Total | ||||||||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Other | During | Stockholders’ | ||||||||||||||||||||||||||||||||
| Number of | Number of | Additional Paid- | Deferred | Comprehensive | Development | (Deficit) | ||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Par Value | In Capital | Compensation | Income (Loss) | Stage | Equity | ||||||||||||||||||||||||||||
Issuance of common
stock upon exercise
of options |
1,100 | 11 | 2,530 | 2,541 | ||||||||||||||||||||||||||||||||
Issuance of common
stock upon
conversion of notes
payable |
48,000 | 480 | 119,520 | 120,000 | ||||||||||||||||||||||||||||||||
Beneficial conversion feature on
convertible notes payable |
380,000 | 380,000 | ||||||||||||||||||||||||||||||||||
Compensation expense- stock options |
1,670,063 | 1,670,063 | ||||||||||||||||||||||||||||||||||
Issuance of common stock |
571,806 | 5,718 | 1,079,557 | 1,085,275 | ||||||||||||||||||||||||||||||||
Comprehensive loss: |
||||||||||||||||||||||||||||||||||||
Unrealized loss on marketable
securities |
(9,310 | ) | (9,310 | ) | ||||||||||||||||||||||||||||||||
Net loss |
(20,847,459 | ) | (20,847,459 | ) | ||||||||||||||||||||||||||||||||
Comprehensive loss |
(20,856,769 | ) | ||||||||||||||||||||||||||||||||||
Balance at December 31, 2008 |
— | $ | — | 21,399,123 | $ | 213,991 | $ | 143,671,984 | $ | — | $ | — | $ | (183,899,336 | ) | $ | (40,013,361 | ) | ||||||||||||||||||
Compensation expense- stock options |
1,473,083 | 1,473,083 | ||||||||||||||||||||||||||||||||||
Issuance of common stock |
4,156,522 | 41,565 | 1,960,435 | 2,002,000 | ||||||||||||||||||||||||||||||||
Issuance of preferred stock |
196,000 | $ | 5,066,919 | (192,107 | ) | (2,000 | ) | 4,872,812 | ||||||||||||||||||||||||||||
Net Loss |
(10,776,937 | ) | (10,776,937 | ) | ||||||||||||||||||||||||||||||||
Comprehensive loss |
(10,776,937 | ) | ||||||||||||||||||||||||||||||||||
Balance at December 31, 2009 |
196,000 | $ | 5,066,919 | 25,555,645 | $ | 255,556 | $ | 146,913,395 | $ | — | $ | — | $ | (194,678,273 | ) | $ | (42,442,403 | ) | ||||||||||||||||||
Compensation expense- stock options |
61,722 | 61,722 | ||||||||||||||||||||||||||||||||||
Issuance of common stock |
1,500,000 | 15,000 | (15,000 | ) | ||||||||||||||||||||||||||||||||
Buyback of common stock |
(270,000 | ) | (2,700 | ) | (5,400 | ) | (8,100 | ) | ||||||||||||||||||||||||||||
Amortization of legal expenses |
18,239 | 18,239 | ||||||||||||||||||||||||||||||||||
Accretion of preferred stock |
343,000 | (343,000 | ) | |||||||||||||||||||||||||||||||||
Net income (loss) |
512,419 | 512,419 | ||||||||||||||||||||||||||||||||||
Comprehensive income (loss) |
512,419 | |||||||||||||||||||||||||||||||||||
Balance at December 31, 2010 |
196,000 | $ | 5,428,158 | 26,785,645 | $ | 267,856 | $ | 146,611,717 | $ | — | $ | — | $ | (194,165,854 | ) | $ | (41,858,123 | ) | ||||||||||||||||||
35
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Year Ended | From Inception | |||||||||||
| December 31, | December 31, | (October 16, 1992) to | ||||||||||
| 2010 | 2009 | December 31, 2010 | ||||||||||
Cash flows from operating activities: |
||||||||||||
Net income (loss) |
$ | 512,419 | $ | (10,776,937 | ) | $ | (194,163,854 | ) | ||||
Adjustments to reconcile net loss to net cash used for
operating activities: |
||||||||||||
Purchased in-process research and development |
— | — | 12,146,544 | |||||||||
Write-off of acquired technology |
— | — | 3,500,000 | |||||||||
Interest expense settled through issuance of notes payable |
— | — | 350,500 | |||||||||
Net gain on early extinguishment of debt (Note 6) |
(5,277,300 | ) | (5,277,300 | ) | ||||||||
Non-cash interest expense |
741,471 | 716,358 | 3,966,394 | |||||||||
Non-cash charges for options, warrants and common stock |
61,722 | 1,473,086 | 11,114,603 | |||||||||
Amortization of financing costs |
18,239 | 18,239 | ||||||||||
Amortization and depreciation |
58,598 | 72,371 | 2,849,915 | |||||||||
Changes in current assets and liabilities: |
||||||||||||
Decrease in prepaid expenses and other current assets |
3,055 | 129,475 | 691,390 | |||||||||
Increase (decrease) in accounts payable and accrued
expenses |
116,966 | (1,435,956 | ) | 2,804,144 | ||||||||
Increase in accrued interest payable |
865,657 | 1,806,734 | 4,985,481 | |||||||||
(Decrease) increase in accrued lease |
(51,494 | ) | (45,429 | ) | 96,425 | |||||||
Net cash used for operating activities |
(2,950,667 | ) | (8,060,298 | ) | (156,917,519 | ) | ||||||
Cash flows from investing activities: |
||||||||||||
Cash acquired through merger |
— | — | 1,758,037 | |||||||||
Purchases of fixed assets |
— | — | (1,652,114 | ) | ||||||||
Decrease (increase) in security deposits and other assets |
34,865 | 76,734 | (384,898 | ) | ||||||||
Decrease (increase) in indemnity fund |
152 | (258 | ) | (115,568 | ) | |||||||
Purchases of marketable securities |
— | — | (132,004,923 | ) | ||||||||
Sales and maturities of marketable securities |
— | — | 132,004,923 | |||||||||
Net cash provided by (used for) investing activities |
35,017 | 76,476 | (394,543 | ) | ||||||||
Cash flows from financing activities: |
||||||||||||
Proceeds from issuance of common stock |
— | 2,000,000 | 66,731,339 | |||||||||
Funds used to buyback common stock |
(8,100 | ) | — | (8,100 | ) | |||||||
Proceeds from issuance of preferred stock |
— | 4,900,000 | 39,922,170 | |||||||||
Preferred stock conversion inducement |
— | — | (600,564 | ) | ||||||||
Proceeds from issuance of promissory notes |
3,310,000 | 1,350,000 | 56,245,000 | |||||||||
Proceeds from issuance of convertible debentures |
— | — | 9,000,000 | |||||||||
Principal payments of notes payable/repurchase of debt |
(602,700 | ) | — | (7,749,667 | ) | |||||||
Dividend payments Series E Cumulative Convertible
Preferred Stock |
— | — | (516,747 | ) | ||||||||
Payments related to financing costs |
— | (25,188 | ) | (5,612,855 | ) | |||||||
Net cash provided by financing activities |
2,699,200 | 8,224,812 | 157,410,576 | |||||||||
Net (decrease) increase in cash and cash equivalents |
(216,450 | ) | 240,990 | 98,514 | ||||||||
Cash and cash equivalents, beginning of period |
314,964 | 73,974 | — | |||||||||
Cash and cash equivalents, end of period |
$ | 98,514 | $ | 314,964 | $ | 98,514 | ||||||
Supplemental cash flow disclosures: |
||||||||||||
Cash paid for interest |
$ | — | $ | — | $ | 628,406 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
36
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. The Company and Summary of Significant Accounting Policies
Alseres Pharmaceuticals, Inc. and its subsidiaries (the “Company”) is a biotechnology
company engaged in the development of therapeutic and diagnostic products primarily for
disorders in the central nervous system. The Company was founded in 1992 and merged
with a publicly held company in 1995 (the “Merger”) whereby the Company changed its
name to Boston Life Sciences, Inc. Effective June 7, 2007, the Company changed its name
to Alseres Pharmaceuticals, Inc. During the period from inception through December 31,
2010, the Company has devoted substantially all of its efforts to business planning,
raising financing, furthering the research and development of its technologies, and
corporate partnering efforts. Accordingly, the Company is considered to be a
“development stage enterprise” as defined in ASC 915, Development Stage Entities and
will continue to be so until the commencement of commercial operations. The
development stage is from October 16, 1992 (inception) through December 31, 2010.
The accompanying consolidated financial statements have been prepared on a basis which
assumes that the Company will continue as a going concern which contemplates the
realization of assets and the satisfaction of liabilities in the normal course of
business. The uncertainty inherent in the need to raise additional capital and the
Company’s recurring losses from operations raise substantial doubt about the Company’s
ability to continue as a going concern. The consolidated financial statements do not
include any adjustments that might result from the outcome of this uncertainty.
As of December 31, 2010, the Company has experienced total net losses since inception
of approximately $203,456,000, stockholders’ deficit of approximately $47,286,000 and a
net working capital deficit of approximately $42,079,000. For the foreseeable future,
the Company expects to experience continuing operating losses and negative cash flows.
The cash and cash equivalents available at December 31, 2010 will not provide
sufficient working capital to meet our anticipated expenditures for the next twelve
months. At March 21, 2011, we had cash and cash equivalents of approximately $100,000
which combined with our ability to control certain costs, including those related to
clinical trial programs, preclinical activities, and certain general and administrative
expenses will enable us to meet our anticipated cash expenditures
into April 2011. Should we fail to raise additional capital we
may have to suspend or cease our operations.
Basis of Presentation
The Company’s consolidated financial statements include the accounts of its six
subsidiaries where all of the Company’s operations are conducted. At December 31, 2010,
all of the subsidiaries were wholly-owned. All significant intercompany transactions
and balances have been eliminated.
Restatement
The Convertible Notes Payable at December 31, 2009 of $34.1million and the related
accrued interest of $4.0 million have been Restated as current
liabilities as of December 31, 2009 in the consolidated Balance Sheets.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted
accounting principles requires management to make estimates and assumptions that affect
the reported amounts of assets and liabilities and disclosure of contingent assets and
liabilities at the date of the financial statements and the reported amounts of
revenues and expenses during the reporting period. Actual results could differ from
those estimates. Changes in estimates are reflected in reported results in the period
in which they become known.
Cash and Cash Equivalents
The Company considers all highly liquid marketable securities purchased with an
original maturity of three months or less to be cash equivalents. As of December 31,
2010 and 2009, cash equivalents consisted of money market funds.
Fair Value Measurements
The carrying amounts of the Company’s financial instruments, which include cash and
cash equivalents, accounts payable and accrued expenses approximate their fair values
as of December 31, 2010 and 2009 due to their short-term maturity. It is not
practicable to estimate the fair value of the Company’s convertible debt. However, it
is likely that the fair value of the debt would be materially less than the carrying
value of the debt because the conversion price of $2.50 is higher than the Company’s
stock price of $0.12 as of December 31, 2010.
37
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Property and Equipment
Property and equipment are recorded at cost and depreciated using the straight-line
method over the estimated useful lives of the assets, ranging from three to five years.
Leasehold improvements are recorded at cost and amortized using the straight-line
method over the term of the lease or the estimated useful lives of the assets,
whichever is shorter.
Impairment of Long Lived Assets
The Company periodically assesses the impairment of long-lived assets in accordance
with ASC Topic 360, Property, Plant, and Equipment. The Company reviews long-lived
assets, including property and equipment, for impairment whenever changes in business
circumstances indicate that the carrying amount of the asset may not be fully
recoverable. During the years ended December 31, 2010 and 2009 the Company did not
record any impairment losses.
Reporting Comprehensive Income (loss)
Accounting Standards Codification 220, Comprehensive Income (ASC 220), establishes
standards for the reporting and display of comprehensive income (loss) and its
components in the financial statements. Comprehensive income (loss) is the total of net
income (loss) and all other non-owner changes in equity including such items as
unrealized holding gains/losses on securities, foreign currency translation adjustments
and minimum pension liability adjustments. The Company had no such items for the years
ended December 31, 2010 and 2009 and as a result, comprehensive income (loss) is the
same as reported net income (loss) for all periods presented.
Research and Development Expenses
Research and development expenses are expensed as incurred. Research and
development expenses include costs incurred in performing research and development
activities such as salary and benefits, stock-based compensation expense, facility
costs, license fees, contractual services, sponsored research and development, and
clinical trial costs.
Stock-Based Compensation Expense
The Company measures share based compensation at grant date, based on the fair value of
the award using the Black-Scholes Option Pricing Model, and recognizes such
compensation as an expense over the employee’s requisite service period (generally the
vesting period of the equity grant).
Income Taxes
The Company accounts for income taxes under the liability method. Under the liability
method, deferred income taxes are determined based on differences between the financial
reporting and tax bases of assets and liabilities. They are measured using the enacted
tax rates and laws that will be in effect when the differences are expected to reverse.
The Company is required to adjust its deferred tax liabilities in the period when tax
rates or the provisions of the income tax laws change. Valuation allowances are
established to reduce deferred tax assets to the amounts expected to be realized.
Beneficial Conversion Feature
The Company issued preferred stock and notes which are convertible into common stock at
a discount from the common stock market price at the date of issuance. The amount of
the discount associated with such conversion rights represents an incremental yield,
i.e. a “beneficial conversion feature”, that is recorded when the consideration
allocated to the convertible security, divided by the number of common shares into
which the security converts, is below the fair value of the common stock at the date of
issuance of the convertible instrument.
A beneficial conversion feature associated with preferred stock is recognized as a
return to the preferred stockholders and represents a non-cash charge in the
determination of net loss attributable to common stockholders. The beneficial
conversion feature is recognized in full immediately if there is no redemption date for
the preferred stock, or over the period of issuance through the redemption date, if
applicable. A beneficial conversion feature associated with debentures, notes or other
debt instruments is recognized as discount to the debt and is amortized as additional
interest expense using the effective interest method over the remaining term of the
debt instrument.
Convertible Redeemable Shares
In accordance with ASC 480-10-S99 the Company determined that due to the fact that the
Series F shares are mandatorily redeemable for cash or for a variable, uncapped, number
of common shares, they are more akin to a liability and as such are classified outside
of permanent equity.
38
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Recent Accounting Pronouncements
In January 2010, the Financial Accounting Standards Board (“FASB”) issued Accounting
Standards Update (“ASU”) No. 2010-06, Improving Disclosures about Fair Value
Measurements, which amends Accounting Standards Topic 820 Fair Value Measurements and
Disclosures (ASC 820) by adding required disclosures about items transferring into and
out of levels 1 and 2 in the fair value hierarchy; adding separate disclosures about
purchase, sales, issuances, and settlements relative to level 3 measurements; and
clarifying the existing fair value disclosures about the level of disaggregation. The
amendments in ASU 2010-6 were effective for fiscal years beginning after December 15,
2009. The adoption of this ASU has not had an impact on our consolidated financial
statements.
2. Property and Equipment, net
| 2010 | 2009 | |||||||
Leasehold improvements |
$ | 132,170 | $ | 132,170 | ||||
Computer equipment |
88,985 | 88,985 | ||||||
Office furniture and equipment |
78,403 | 78,403 | ||||||
| 299,558 | 299,558 | |||||||
Less accumulated amortization and depreciation |
251,884 | 193,286 | ||||||
| $ | 47,674 | $ | 106,272 | |||||
Amortization and depreciation expense for the years ended December 31, 2010 and 2009
was approximately $59,000 and $72,000, respectively, and $1,612,000 for the period from
inception through December 31, 2010.
3. Net Income (loss) per share
The Company reports earnings per share in accordance with Accounting Standards
Codification 260, Earnings Per Share (ASC 260), which establishes standards for
computing and presenting earnings per share. Basic and diluted net income (loss) per
share is presented in conformity with the two-class method required for participating
securities. Under the two-class method, basic net income (loss) per share is computed
by dividing the net income (loss) attributable to common stockholders by the
weighted-average number of common shares outstanding during the period. Net income
(loss) attributable to common stockholders is determined by allocating undistributed
earnings between holders of common and convertible preferred stock, based on the
contractual dividend rights contained in our preferred stock agreement. No preferred
dividends have been declared and, as such, preferred dividends have not affected our
computation of net income available to common stockholders. Diluted net income (loss)
per share is computed by dividing net income (loss) by the sum of the weighted average
number of common shares and the number of dilutive potential common share equivalents
outstanding during the period. Dilutive common share equivalents consist of the
incremental common shares issuable upon the conversion of the Series F convertible
preferred stock to common shares.
In computing diluted earnings per share, common stock equivalents are not considered in
periods in which a net loss is reported, as the inclusion of the common stock
equivalents would be anti-dilutive. As a result, there is no difference between the
Company’s basic and diluted loss per share for the year ended December 31, 2009.
| December 31, | ||||||||
| Basic and diluted net income (loss) per share | 2010 | 2009 | ||||||
Net income (loss) |
$ | 512,419 | $ | (10,776,937 | ) | |||
Less: Accretion of Preferred stock |
(343,000 | ) | — | |||||
Less: Income allocated to covertible preferred shares |
(26,282 | ) | — | |||||
Income (loss) available to common stockholders for the
purpose of calculating basic & diluted EPS |
143,137 | (10,776,937 | ) | |||||
Weighted average common shares, basic |
26,686,933 | 23,361,458 | ||||||
Dilutive effect of Series F preferred share conversion |
4,900,000 | — | ||||||
Weighted average common shares, diluted |
31,586,933 | 23,361,458 | ||||||
Net income (loss) per share, basic |
$ | 0.01 | $ | (0.46 | ) | |||
Net income (loss) per share, diluted |
$ | 0.00 | $ | (0.46 | ) | |||
39
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Stock options and warrants to purchase 3,652,443 and 3,697,745 shares of common stock
were outstanding at December 31, 2010 and 2009, respectively, but were not included in
the computation of diluted net income (loss) per common share because the exercise
prices were greater than the average market price for the period, making them
anti-dilutive. The exercise of those stock options and warrants outstanding at December
31, 2010 could potentially dilute earnings per share in the future
Shares of common stock reserved for issuance upon conversion of the 5% Convertible
Promissory Notes were 13,485,889 and 15,285,088 at December 31, 2010 and 2009,
respectively, but were not included in the computation of diluted net income (loss) per
share because they would have been anti-dilutive. The conversion of the 5% Convertible
Promissory Notes outstanding at December 31, 2010 could potentially dilute earnings per
share in the future.
4. Accounts Payable and Accrued Expenses
| Accounts payable and accrued expenses consist of the following at December 31, | 2010 | 2009 | ||||||
Research and development |
$ | 2,058,782 | $ | 1,774,943 | ||||
Accrued professional fees |
1,140,044 | 1,231,550 | ||||||
General and administrative |
270,920 | 328,435 | ||||||
Accrued interest expense-demand notes |
270,759 | 62,738 | ||||||
Accrued compensation |
107,063 | 124,915 | ||||||
| $ | 3,847,568 | $ | 3,522,581 | |||||
5. Notes Payable and Debt
| Convertible Notes Payable to Significant Stockholders | December 31, 2010 | December 31, 2009 | ||||||||||
Unsecured convertible promissory note; interest rate
of 5%; due December 31, 2010 |
$ | 9,000,000 | $ | 9,000,000 | ||||||||
Unsecured convertible promissory note; interest rate of 5%;
due December 31, 2010 |
BCF | 10,000,000 | 9,562,956 | |||||||||
Unsecured convertible promissory note; interest rate of 5%;
due December 31, 2010 |
BCF | 5,000,000 | 4,854,008 | |||||||||
Unsecured convertible promissory note; interest rate of 5%;
due December 31, 2010 |
5,000,000 | 5,000,000 | ||||||||||
Unsecured convertible promissory note; interest rate of 5%;
due December 31, 2010 |
BCF | — | 5,738,668 | |||||||||
Aggregate carrying value |
$ | 29,000,000 | $ | 34,155,632 | ||||||||
Interest expense of $1,657,432 and $1,743,999 was recorded related to the convertible
notes payable for the years ended December 31, 2010 and 2009, respectively.
The above unsecured convertible promissory notes may be converted, at the option of the
Purchasers, into (i) shares of the Company’s common stock at a conversion price per
share of $2.50, (ii) the right to receive future payments related to the Company’s
molecular imaging products (including Altropane and FLUORATEC) in amounts equal to 2%
of the Company’s pre-commercial revenue related to such products plus 0.5% of future
net sales of such products for each $1,000,000 of outstanding principal and interest
that a Purchaser elects to convert into future payments, or (iii) a combination of (i)
and (ii). Any outstanding notes that are not converted into the Company’s common stock
or into the right to receive future payments became due and payable on December 31,
2010. However, each Purchaser is prohibited from effecting a conversion into common
stock if at the time of such conversion the common stock issuable to such Purchaser,
when taken together with all shares of common stock then held or otherwise beneficially
owned by such Purchaser exceeds 19.9%, or 9.99% ISVP, of the total number of issued and
outstanding shares of the Company’s common stock immediately prior to such conversion
unless and until the Company’s stockholders approve the conversion of all of the shares
of common stock issuable there under.
The Company is subject to certain debt covenants pursuant to the March 2008 Amended
Purchase Agreement and the June 2008 Purchase Agreement (the “Purchase Agreements”). If
the Company (i) fails to pay the principal or interest due under the Purchase
Agreements, (ii) files a petition for action for relief under any bankruptcy or similar
law or (iii) an involuntary petition is filed against the Company, all amounts borrowed
under the Purchase Agreements may become immediately due and payable by the Company. In
addition, without the consent of the Purchasers, the Company may not (i) create, incur
or otherwise, permit to be outstanding any indebtedness for money borrowed, (ii)
declare or pay any cash dividend, or make a
40
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
distribution on, repurchase, or redeem, any class of the Company’s stock, subject to
certain exceptions or sell, lease, transfer or otherwise dispose of any of the
Company’s material assets or property or (iii) dissolve or liquidate.
Beneficial Conversion Feature (BCF)
Three of the unsecured promissory notes were issued with a conversion price of $2.50
which was below the market price of the Company’s common stock on the dates the
agreements were entered into and resulted in the recording of a beneficial conversion
feature. The Company recorded a BCF of $1,400,000 on (the “ISVP”) note and a
BCF of $380,000 on (the “2008 RG”) note which was recognized as a decrease in the
carrying value and an increase to additional paid-in capital. The BCF is being
recognized as interest expense using the effective interest method through December 31,
2010. The Company recorded interest expense related to the BCF of approximately
$724,000 and $699,000 for the years ended December 31, 2010 and 2009, respectively.
Promissory Notes
| Demand Notes Payable to Significant Stockholder | December 31, 2010 | December 31, 2009 | ||||||
Unsecured demand note payable; interest
rate of 7% to Neurobiologics, Inc. (a
subsidiary of the Company) |
$ | 1,000,000 | $ | 1,000,000 | ||||
Unsecured demand note payable; interest rate
of 7%: issued December 2009 |
350,000 | 350,000 | ||||||
Unsecured demand notes payable; interest rate
of 7%: issued January 2010 - December 2010 |
3,310,000 | — | ||||||
| 4,660,000 | 1,350,000 | |||||||
Accrued interest |
270,759 | 62,738 | ||||||
Aggregate carrying value |
$ | 4,830,759 | $ | 1,412,738 | ||||
Interest expense of $208,018 and $62,741 was recorded related to the demand notes
payable for the years ended December 31, 2010 and 2009, respectively
6. Gain on early extinguishment of debt
On September 10, 2010, we entered into a Note Purchase Agreement (the “Agreement”) with
Highbridge International LLC (the “Seller”) and Robert Gipson pursuant to which we
agreed to purchase from the Seller, effective September 14, 2010, a Convertible
Promissory Note issued on May 2, 2007 to the Seller in the principal amount of
$5,880,000. The purchase price of $602,700 was payable in immediately available federal
funds by us to the Seller. A gain on early extinguishment of debt was $6,277,100 was
recorded. There was no intrinsic value of the conversion feature at the extinguishment
date, therefore, we recorded additional interest expense of approximately $48,300
related to the unamortized portion of the Highbridge beneficial conversion feature
(“BCF”). The gain from the early extinguishment of debt is the difference between the
reacquisition (purchase) price of the debt of $602,700 and the carrying value of the
debt of $5,880,000 along with accrued interest of $999,800.
In a related transaction, on September 13, 2010 we issued to Robert L. Gipson (the
“Holder”) an unsecured promissory note, pursuant to which we borrowed an aggregate
principal amount of $700,000 (the “Note”). Interest on the Note shall accrue at the
rate of 7% per annum and all principal and accrued interest shall be due and payable on
demand of the Holder.
41
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
7. Exit Activities
In September 2005, the Company relocated its headquarters from Boston to Hopkinton,
Massachusetts. The Company amended its lease dated January 28, 2002 with Brentwood
Properties, Inc. (the “Landlord”) for the property located in Boston. At December 31,
2010, the security deposit under the Lease Agreement was approximately $88,600 and not
refundable to the Company until the termination of the lease in May 2012.
As a result of the Company’s relocation, an expense was recorded for the cost
associated with the exit activity at its fair value in the period in which the
liability was incurred. The liability recorded was calculated by discounting the
estimated cash flows for the two sublease agreements and the Lease Agreement using an
estimated credit-adjusted risk-free rate of 15%.
| Cash Payments, net | ||||||||||||
| December 31, 2009 | of sublease receipts | December 31, 2010 | ||||||||||
Lease Amendment |
$ | 147,919 | $ | 51,493 | $ | 96,426 | ||||||
Short-term portion of lease accrual |
51,493 | 65,922 | ||||||||||
Long-term portion of lease accrual |
$ | 96,426 | $ | 30,503 | ||||||||
For the years ended December 31, 2010 and 2009 the Company recorded approximately
$19,000 and $26,000, respectively of expense related to the imputed cost of the lease
expense accrual included in general and administrative expense in the accompanying Consolidated Statements of Operations.
8. Stockholders’ Deficit
Common Stock
In November 2008, the Company completed a private placement with Robert Gipson of
543,478 shares of its common stock which raised $1,000,000 in gross proceeds. In
connection with the November 2008 private placement, the Company also issued warrants
(the “November 2008 Warrants”) to purchase 543,478 additional shares of common stock
that were exercisable at $1.84 per share between six months and two years after the
closing. In connection with the private placement, the Company agreed with Mr. Gipson
(the “Letter Agreement”) that if the Company sold shares of its common stock at a price
below $1.84, subject to certain exceptions, prior to December 31, 2009, Mr. Gipson
would be entitled to receive, for no additional consideration, additional shares of
common stock and warrants in accordance with a pre-determined formula. In addition,
Dawson James Securities, Inc., (“Dawson James”) in its capacity as agent for the
private placement, was entitled to a warrant to purchase 38,043 shares of common stock
(the “Agent Warrant”). The Agent Warrant had a term of five years and was exercisable
at a price equal to $1.84. In February 2009, Dawson James gave up its right to the
Agent Warrant.
In January 2009, the Company completed a private placement with Robert Gipson of
1,000,000 shares of its common stock which raised $1,000,000 in gross proceeds. In
addition, the Company issued an additional 456,522 shares of its common stock to Mr.
Gipson pursuant to a Letter Agreement. In connection with the January 2009 private
placement, Mr. Gipson agreed to the cancellation of the November 2008 Warrants.
In February 2009, the Company entered into a private placement with Cato Holding
Company (“Cato”) of 200,000 shares of its common stock at a purchase price of $1.00 per
share. In connection with the February 2009 private placement, the Company agreed with
Cato that if the Company sells shares of its common stock, or securities convertible
into common stock, prior to September 30, 2009, and the purchaser of such securities
receives warrants to purchase additional shares of common stock (a “Qualified
Financing”), subject to certain exceptions, Cato shall be entitled to receive, for no
additional consideration, a warrant to purchase shares of common stock with the same
terms and conditions as those provided to a purchaser in a Qualified
Financing. No such additional warrants were issued pursuant to this
agreement.
In November 2009, the Company entered into a private placement with Robert Gipson of
2,500,000 shares of its common stock which raised $1,000,000 in gross proceeds. In
addition, in March 2010 the Company issued an additional 1,500,000 shares of its common
stock to Mr. Gipson pursuant to a Letter Agreement.
In December 2010, the Company purchased 270,000 common shares from Robert Gipson at
$.03 per share for a total of $8,125. The closing price for the Company’s stock on
December 28 was $0.15 per share. The price per share paid by the Company to Mr. Gipson
represented an 80% discount to the market price for the shares.
42
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Preferred Stock
The Company has authorized 1,000,000 shares of preferred stock of which 25,000 shares
have been designated as Series A Convertible Preferred Stock, 500,000 shares have been
designated as Series D Convertible Preferred Stock, and 800 shares have been designated
as Series E Cumulative Convertible Preferred Stock (the “Series E Stock”). In March
2009, the Company designated 200,000 shares as Series F Convertible Preferred Stock
(“Series F Stock”). The remaining authorized shares have not been designated.
Convertible Preferred Stock
In 2009 the Company issued a total of 196,000 shares of Series F convertible preferred
stock to Robert Gipson and received gross proceeds of $4,900,000. The key terms of the
Series F stock are summarized below:
Dividend — The Series F Stock is entitled to receive any dividend that is paid to
holders of our common stock. Any subdivisions, combinations, consolidations or
reclassifications to the common stock must also be made accordingly to Series F Stock,
respectively.
Liquidation Preference — In the event of our liquidation, dissolution or winding up,
before any payments are made to holders of our common stock or any other class or
series of our capital stock ranking junior as to liquidation rights to the Series F
Stock, the holders of the Series F Stock will be entitled to receive the greater of (i)
$25.00 per share (subject to adjustment in the event of any stock dividend, stock
split, combination or other similar recapitalization affecting such shares) plus any
outstanding and unpaid dividends thereon and (ii) such amount per share as would have
been payable had each share been converted into common stock. After such payment to the
holders of Series F Stock and the holders of shares of any other series of our
preferred stock ranking senior to the common stock as to distributions upon
liquidation, the remaining our assets will be distributed pro rata to the holders of
our common stock.
Voting Rights — Each share of Series F Stock shall entitle its holder to a number of
votes equal to the number of shares of our common stock into which such share of Series
F Stock is convertible.
Conversion — Each share of Series F Stock is convertible at the option of the holder
thereof at any time. Each share of Series F Stock is initially convertible into 25
shares of common stock, subject to adjustment in the event of certain dividends, stock
splits or stock combinations affecting the Series F Stock or the common stock, and
subject to adjustment on a weighted-average basis in the event of certain issuances by
us of securities for a price less than the then-current price at which the Series F
Stock converts into common stock.
Redemption — At any time after September 1, 2011, any holder of Series F Stock may
elect to have some or all of such shares redeemed by us at a price equal to the
aggregate of (i) $25 per share (subject to appropriate adjustment in the event of any
stock dividend, stock split, combination or other similar recapitalization affecting
such shares), or the Original Issue Price, plus (ii) all declared but unpaid dividends
thereon, plus (iii) an amount computed at a rate per annum of 7% of the Original Issue
Price from March 19, 2009 until the redemption date.
Accretion — The terms of the Series F Stock contain provisions that may require
redemption in circumstances that are beyond the Company’s control. Therefore, the shares have been recorded, net of issuance costs of approximately $25,000, as
convertible, redeemable stock outside of permanent equity. The Series F Stock was
recorded at fair value on the date of issuance. As of December 31, 2010, the Company
recorded approximately $343,000 in accretion on the Series F Stock.
Stock Options and Warrants
Stock Option Plans
The Company can issue both nonqualified and incentive stock options to employees,
officers, consultants and scientific advisors of the Company under the Amended and
Restated 2005 Stock Incentive Plan (the “2005 Plan”). At December 31, 2009, the 2005
Plan provided for the issuance of options, restricted stock, restricted stock units,
stock appreciation rights or other stock-based awards to purchase 3,050,000 shares of
the Company’s common stock. The 2005 Plan contains a provision that allows for an
annual increase in the number of shares available for issuance under the 2005 Plan on
the first day of each of the Company’s fiscal years during the period beginning in
fiscal year 2006 and ending on the second day of fiscal year 2014. The annual increase
in the number of shares shall be equal to the lowest of 400,000 shares; 4% of the
Company’s outstanding shares on the first day of the fiscal year; and an amount
determined by the Board of Directors. No adjustment to the 2005 Plan was made on
January 1, 2010.
43
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Company also has outstanding stock options in three other stock option plans, the
1998 Omnibus Plan, the Amended and Restated Omnibus Stock Option Plan and the Amended
and Restated 1990 Non-Employee Directors’ Non-Qualified Stock Option Plan. Both of
these plans have expired and no future issuance of awards is permissible.
Stock-based employee compensation expense recorded during the years ended December 31,
2010 and 2009 are as follows:
| 2010 | 2009 | |||||||
Research and development |
$ | — | $ | 447,612 | ||||
General and administrative |
61,722 | 825,471 | ||||||
| $ | 61,722 | $ | 1,273,083 | |||||
Impact on basic and diluted
net income (loss)
attributable to common
stockholders per share |
$ | (0.00 | ) | $ | (0.05 | ) | ||
The Company uses the Black-Scholes option-pricing model to calculate the fair value of
each option grant on the date of grant. The fair value of stock options granted during
the years ended December 31, 2010 and 2009 was calculated using the following estimated
weighted-average assumptions:
| 2010 | 2009 | |||||||
Expected term |
— | 5 years | ||||||
Risk-free interest rate |
0 | % | 1.36 | % | ||||
Stock volatility |
0 | % | 90 | % | ||||
Dividend yield |
0 | % | 0 | % | ||||
Expected term — The Company determined the weighted-average expected term assumption
for “plain vanilla” and performance-based option grants based on historical data on
exercise behavior.
Risk-free interest rate — The risk-free interest rate used for each grant is equal to
the U.S. Treasury yield curve in effect at the time of grant for instruments with a
similar expected term.
Expected
volatility — The Company’s expected stock-price volatility assumption is based
on historical volatilities of the underlying stock which is obtained from public data
sources.
Expected dividend yield — The Company has never declared or paid any cash dividends on
its common stock and does not expect to do so in the foreseeable future. Accordingly,
the Company uses an expected dividend yield of zero to calculate the grant-date fair
value of a stock option.
Stock Options
Outstanding stock options for the year ended December 31, 2010:
| Weighted-Average | ||||||||
| Shares | Exercise Price | |||||||
Outstanding at beginning of year |
3,695,745 | $ | 1.63 | |||||
Granted |
— | — | ||||||
Exercised |
— | — | ||||||
Forfeited and expired |
(45,302 | ) | 4.58 | |||||
Outstanding at end of year |
3,650,443 | 1.59 | ||||||
Options exercisable at end of year |
3,558,361 | 1.60 | ||||||
Summary information regarding stock options outstanding at December 31, 2010:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||||
| Weighted- | Weighted- | |||||||||||||||||||||||
| Average | Weighted- | Average | Weighted- | |||||||||||||||||||||
| Remaining | Average | Remaining | Average | |||||||||||||||||||||
| Number | Contractual | Exercise | Number | Contractual | Exercise | |||||||||||||||||||
| Range of Exercise Prices | Outstanding | Life | Price | Exercisable | Life | Price | ||||||||||||||||||
$1.15 — $1.36 |
2,713,000 | 3.4 years | $ | 1.15 | 2,620,918 | 3.4 years | $ | 1.15 | ||||||||||||||||
$2.00 — $3.00 |
639,980 | 4.7 years | 2.33 | 639,980 | 4.7 years | 2.33 | ||||||||||||||||||
$3.10 — $4.65 |
255,363 | 5.7 years | 3.40 | 255,363 | 5.7 years | 3.40 | ||||||||||||||||||
$4.99 — $6.96 |
28,500 | 3.0 years | 5.47 | 28,500 | 3.0 years | 5.47 | ||||||||||||||||||
$8.95 — $13.06 |
8,600 | 0.7 years | 10.57 | 8,600 | 0.7 years | 10.57 | ||||||||||||||||||
$15.62 — $22.36 |
5,000 | 0.0 years | 15.63 | 5,000 | 0.0 years | 15.63 | ||||||||||||||||||
| 3,650,443 | 3.7 years | $ | 1.59 | 3,558,361 | 3.8 years | $ | 1.60 | |||||||||||||||||
44
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
There was no intrinsic value for the outstanding and exercisable options as of December
31, 2010. There was no intrinsic value for the options vested during the year ended
December 31, 2010. No options were exercised during the years ended December 31, 2010
and 2009. There were no options granted during 2010 and 2009.
As of December 31, 2010, 384,172 shares are available for grant under the
Company’s option plans.
As of December 31, 2010, there remained approximately $1,000 of compensation costs
related to non-vested stock options to be recognized as expense over a weighted-average
period of approximately .08 years.
Warrants
As of December 31, 2010, the Company had 2,000 warrants outstanding to purchase common
stock at an exercise price of $9.50, with an expiration date of October 2011.
Each warrant is exercisable into one share of common stock. At December 31, 2010,
the Company has reserved 4,036,615 shares of common stock to meet its option and
warrant obligations.
Rights Agreement
On September 11, 2001, the Company entered into a Rights Agreement (the “Rights Plan”)
dated as of September 11, 2001, with Continental Stock Transfer & Trust Company, as
rights agent (the “Rights Agent”), and declared a dividend of one right (a “Right”) to
purchase from the Company one-thousandth of a share of its Series D Preferred Stock at
an exercise price of $25 for each outstanding share of the Company’s common stock at
the close of business on September 13, 2001. The Rights will expire on September 11,
2011.
In general, the Rights will be exercisable only if a person or group acquires 15% or
more of the Company’s common stock or announces a tender offer, the consummation of
which would result in ownership by a person or group of 15% or more of the Company’s
common stock. If, after the Rights become exercisable, the Company is acquired in a
merger or other business combination transaction, or sells 25% or more of its assets or
earning power, each unexercised Right will entitle its holder to purchase a number of
the acquiring company’s common shares as defined in the Rights Plan. At any time after
any person or group has acquired beneficial ownership of 15% or more of the Company’s
common stock, the Board, in its sole discretion, may exchange all or part of the then
outstanding and exercisable Rights for shares of the Company’s common stock at an
exchange ratio of one share of common stock per Right.
In November 2001, the Company and the Rights Agent amended the Rights Plan to provide
that the Rights Plan will be governed by the laws of the State of Delaware.
In November 2002, the Company and the Rights Agent amended the Rights Plan to provide
that, for purposes of any calculation under the Rights Plan of the percentage of
outstanding shares of the Company’s common stock beneficially owned by a person, any shares of the Company’s common stock such person beneficially owns that are not
outstanding (such as shares underlying options, warrants, rights or convertible
securities) shall be deemed to be outstanding. The amendment also exempted each of I&S,
ISVP and Robert Gipson (the “Ingalls Parties”) from being an “Acquiring Person” under
the Rights Plan so long as such persons, collectively, together with all affiliates of
such persons, shall beneficially own less than 20% of the shares of the Company’s
common stock then outstanding.
On March 12, 2003, the Company and the Rights Agent amended the Rights Plan to provide
that prior to June 1, 2005, the Ingalls Parties and their affiliates will be deemed not
to beneficially own certain convertible notes and warrants of the Company and any
common stock issued or issuable upon their conversion or exercise for purposes of
determining whether such person is an “Exempt Person” under the Rights Plan.
On December 23, 2003, the Company and the Rights Agent amended the Rights Plan to add
Boucher to the list of persons included in the definition of Ingalls Parties who are
exempt from being an “Acquiring Person” so long as such persons, collectively, together
with all affiliates of such persons, shall beneficially own less than 20% of the shares
of the Company’s common stock then outstanding. In addition, the amendment provides
that a person shall not be deemed to beneficially own securities held by another person
solely by reason of an agreement, arrangement or understanding among such persons to
vote such securities, if such agreement, arrangement or understanding is for the
purpose of (i) soliciting revocable proxies or consents to elect or remove directors of
the Company pursuant to a proxy or consent solicitation made or to be made pursuant to,
and in accordance with, the applicable proxy solicitation rules and regulations
promulgated under the Securities Exchange Act of 1934, as amended, and/or (ii)
nominating one or more individuals (or being nominated) for election to the Company’s
Board of Directors or serving as a director of the Company.
45
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
On March 14, 2005, the Company and the Rights Agent amended the Rights Plan to amend
the definition of Exempt Person to include all purchasers of shares of the Company’s
common stock in connection with the Company’s private placement completed in March
2005.
9. Fair Value Measurements
The fair value hierarchy prioritizes observable and unobservable inputs used to measure
fair value into three broad levels:
Level 1 — quoted prices in active markets for identical securities;
Level 2 — other significant observable inputs, including quoted prices for similar securities,
interest rates and credit risk,
Level 3 — significant unobservable inputs, including our own assumptions in determining fair value.
The following table summarizes the financial assets that we measured at fair value at
December 31, 2010 and 2009. We did not have any non-financial assets or liabilities
that were measured or disclosed at fair value for the years ended December 31, 2010 and
2009. No transfers occurred between Level 1and Level 2 assets for the years ended
December 31, 2010 and 2009.
| 2010 | 2009 | |||||||||||||||
| Fair Value | Input Level | Fair Value | Input Level | |||||||||||||
Cash and money market funds — current assets |
$ | 98,514 | Level 1 | $ | 314,964 | Level 1 | ||||||||||
Indemnity fund-restricted money market funds |
115,568 | Level 1 | 115,720 | Level 1 | ||||||||||||
| $ | 214,082 | $ | 430,684 | |||||||||||||
10. Income Taxes
Income tax provision (benefit) consists of the following for the years ended December
31:
| 2010 | 2009 | |||||||
Federal |
$ | (1,560,000 | ) | $ | (3,913,000 | ) | ||
State |
(389,000 | ) | (1,052,000 | ) | ||||
| (1,949,000 | ) | (4,965,000 | ) | |||||
Valuation allowance |
1,949,000 | 4,965,000 | ||||||
| $ | — | $ | — | |||||
Deferred tax assets consist of the following at December 31:
| 2010 | 2009 | |||||||
Net operating loss carry forwards |
$ | 31,534,000 | $ | 29,834,000 | ||||
Capitalized research and development expenses |
12,977,000 | 15,539,000 | ||||||
Research and development credit carry forwards |
— | — | ||||||
License fees |
617,000 | 525,000 | ||||||
Stock-based compensation expense |
2,264,000 | 2,232,000 | ||||||
Other |
2,754,000 | 2,408,000 | ||||||
Gross deferred tax assets |
50,146,000 | 50,538,000 | ||||||
Valuation allowance |
(50,146,000 | ) | (50,538,000 | ) | ||||
Net deferred tax assets |
$ | — | $ | — | ||||
A reconciliation between the amount of reported tax benefit and the amount computed
using the U.S. federal statutory rate of 35% for the year ended December 31 is as
follows:
| 2010 | 2009 | |||||||
Provision (benefit) at statutory rate |
$ | 179,000 | $ | (3,772,000 | ) | |||
State taxes, net of federal benefit |
32,000 | (638,000 | ) | |||||
Expiring state net operating loss carry forwards |
178,000 | 734,000 | ||||||
Permanent items |
3,000 | 10,000 | ||||||
(Decrease) increase in valuation allowance |
(392,,000 | ) | 3,581,000 | |||||
Other |
— | 85,000 | ||||||
| $ | — | $ | — | |||||
46
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
For the years ended December 31, 2010 and 2009, the Company did not record any federal
or state tax expense given its continued net operating loss position. The Company has
evaluated the positive and negative evidence bearing upon its ability to realize the
deferred tax assets, which are comprised principally of net operating losses (“NOL”)
and capitalized research and development expenditures. Management has determined that
it is more likely than not that the Company will not recognize the benefits of federal
and state deferred tax assets and, as a result, a full valuation allowance was
established at December 31, 2010.
A reconciliation of the unrecognized tax benefits recorded for the year ended December
31 is as follows:
| 2010 | 2009 | |||||||
Balance at January 1 |
$ | 2,798,199 | $ | 2,545,000 | ||||
Additions based on tax positions related to the current year |
24,332 | 253,199 | ||||||
Additions (reductions) for tax positions of prior years |
— | — | ||||||
Settlements |
— | — | ||||||
Lapse of applicable statute of limitations |
— | — | ||||||
Balance at December 31 |
$ | 2,822,531 | $ | 2,798,199 | ||||
The balance of unrecognized tax benefits at December 31, 2010 of approximately
$2,822,531 are tax benefits that, if recognized, would not affect the Company’s
effective tax rate since they are subject to a full valuation allowance.
As of December 31, 2010, the Company had federal and state NOL carryforwards of
approximately $82,362,000 and $52,071,000 respectively and federal and state research
and development (“R&D”) credit carryforwards of
approximately $1,578,000 and $1,110,000,
respectively, which may be available to offset future federal and state income tax
liabilities which expire at various dates starting in 2011 and going
through 2031.
Utilization of the NOL and R&D credit carryforwards may be subject to a substantial
annual limitation due to ownership change limitations that have occurred previously or
that could occur in the future provided by Section 382 of the Internal Revenue Code of
1986, as well as similar state and foreign provisions. These ownership changes may
limit the amount of NOL and R&D credit carryforwards that can be utilized annually to
offset future taxable income and tax, respectively. In fiscal year 1995 and in fiscal
year 2005, the Company experienced a change in ownership as defined by Section 382 of
the Internal Revenue Code. In general, an ownership change, as defined by Section 382,
results from transactions increasing the ownership of certain stockholders or public
groups in the stock of a corporation by more than 50 percentage points over a
three-year period. Since the Company’s formation, it has raised capital through the
issuance of capital stock on several occasions which, combined with stockholders’
subsequent disposition of those shares, has resulted in two changes of control, as
defined by Section 382. As a result of the 2005 ownership change, utilization of the
Company’s NOLs is subject to an annual limitation under Section 382 determined by
multiplying the value of our stock at the time of the ownership change by the
applicable long-term tax-exempt rate resulting in an annual limitation amount of
approximately $1,000,000. Any unused annual limitation may be carried over to later
years, and the amount of the limitation may, under certain circumstances, be subject to
adjustment if the fair value of the Company’s net assets are determined to be below or
in excess of the tax basis of such assets at the time of the ownership change, and such
unrealized loss or gain is recognized during the five-year period after the ownership
change. Federal research and development tax credits were also impaired by the
ownership change and were reduced accordingly. The Company does not expect to have any
taxable income for the foreseeable future. Subsequent ownership changes, as defined in
Section 382, could further limit the amount of net operating loss carryforwards and
research and development credits that can be utilized annually to offset future taxable
income.
The Company recognizes interest and penalties related to income tax matters in income
tax expense. The Company has no accrual for interest and penalties as of December 31,
2010.
The Company is subject to both federal and state income tax for the
jurisdiction within which
it operates, which are primarily focused in Massachusetts. Within these jurisdictions,
the Company is open to examination for tax years ended December 31,
2006 through December 31, 2009. However, because
we are carrying forward income tax attributes such as NOLs from 2005 and earlier tax years,
these attributes can still be audited when utilized on returns filed in the future. The U.S.
Internal Revenue Service (IRS) has completed an audit of tax years 2007
and 2008 and has informed us that no adjustments to the federal tax returns as filed will
be proposed as a result of the audit.
11. Commitments and Contingencies
The Company leases office space under non-cancelable operating leases. The Company’s
corporate office lease expires in September 2011. The Company’s former corporate
headquarters expires in May 2012 and has been amended for two sublease agreements.
(Note 7)
47
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The approximate future minimum commitments related to the above lease obligations are
as follows:
| Year Ended December 31, | Operating Leases | Sublease Income (Note 7) | Operating Leases, net | |||||||||
2011 |
$ | 529,000 | $ | (231,000 | ) | $ | 298,000 | |||||
2012 |
127,000 | (96,000 | ) | 31,000 | ||||||||
Thereafter |
— | — | — | |||||||||
| $ | 656,000 | $ | (327,000 | ) | $ | 329,000 | ||||||
Total rent expense was approximately $270,000 and $277,000 for the years ended December
31, 2010 and 2009, respectively, and approximately $3,824,000 for the period from
inception (October 16, 1992) through December 31, 2010. The Company received
approximately $244,000 and $211,000 for the years ended December 31, 2010 and 2009,
respectively, related to the sublease of the premises.
License Agreements
The Company has entered into license agreements (the “Harvard License Agreements”) with
Harvard University and its affiliated hospitals (“Harvard and its Affiliates”) to
acquire the exclusive worldwide rights to certain technologies within its molecular
imaging and neurodegenerative disease programs. The Harvard License Agreements obligate
the Company to pay up to an aggregate of approximately $2,520,000 in milestone payments
in the future. The future milestone payments are generally payable only upon
achievement of certain regulatory milestones.
The Company’s license agreements with Harvard and its Affiliates generally provide for
royalty payments equal to specified percentages of product sales, annual license
maintenance fees and continuing patent prosecution costs.
Guarantor Arrangements
The Company has entered into agreements to indemnify its executive officers and
directors for certain events or occurrences while the officer or director is, or was
serving, at the Company’s request in such capacity. The indemnification period is for
the officer’s or director’s lifetime. The maximum potential amount of future payments
the Company could be required to make under these indemnification agreements is
unlimited; however, the Company has a director and officer insurance policy that limits
the Company’s exposure and enables the Company to recover a portion of any future
amounts paid. As a result of the Company’s insurance policy coverage, the Company
believes the estimated fair value of these indemnification agreements is minimal.
S. David Hillson, our former Chairman of the Board and Chief Executive Officer,
requested that the Company create an indemnity trust for his benefit and fund the trust
in the amount of $100,000. On June 15, 2004, the Company entered into a directors and
officers indemnity trust agreement with Mr. Hillson and Boston Private Bank & Trust
Company, as trustee (the “Indemnity Trust Agreement”), and funded the trust with
$100,000. Mr. Hillson may request withdrawals of funds in the event that he becomes
entitled to receive indemnification payments or advances from the Company. Amounts not
disbursed from the trust will become unrestricted at such time as the Company and Mr.
Hillson agree that the indemnity trust is no longer required. The Company has
evaluated its obligations under this Indemnity Trust Agreement and has determined that
the fair value of this obligation is immaterial at December 31, 2010.
The Company enters into arrangements with service providers to perform research,
development, and clinical services. The Company enters into standard indemnification
agreements with those service providers, whereby the Company indemnifies them for any
liability associated with their use of the Company’s technologies. The maximum
potential amount of future payments the Company would be required to make under these
indemnification agreements is unlimited; however, the Company has product liability and
general liability policies that enable the Company to recover a portion of any amounts
paid. As a result of the Company’s insurance policy coverage, the Company believes the
estimated fair value of these indemnification agreements is minimal.
12. Related Party Transactions
In September 2006, the Company entered into a consulting agreement with Dr. Langer, a
member of the Company’s board of directors, for scientific and business consulting
services. This Agreement terminated in December 2009. The Company recorded consulting
fees totaling approximately
$159,000 under the consulting agreement, of which approximately
$80,000 remains unpaid as of December 31, 2010.
48
Table of Contents
ALSERES PHARMACEUTICALS, INC.
(A Development Stage Enterprise)
(A Development Stage Enterprise)
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Robert L. Gipson, Thomas L. Gipson & Arthur Koenig
Robert Gipson was a director of the Company from June 2004 through October 2004.
Robert Gipson is a Senior Director of I&S. Boucher is a Managing Director of I&S. ISVP
is an investment partnership managed under an investment advisory contract with I&S.
Robert Gipson and Boucher are the general partners of ISVP and share the power to vote
securities of the Company held by ISVP.
The Company amended its Rights Plan in connection with agreements with Robert Gipson,
Boucher, I&S and ISVP (Note 5).
In March 2007, the Company issued the Amended Notes to Robert Gipson and Thomas Gipson
(Note 5).
In March 2008, the Company entered into the March 2008 Amended Purchase Agreement with
Robert Gipson, Thomas Gipson, Arthur Koenig, Highbridge and ISVP (Note 5).
In June 2008, the Company entered into the June 2008 Purchase Agreement with Robert
Gipson (Note 5).
In November 2008, the Company issued and sold an aggregate of 543,478 shares of its
common stock at a purchase price of $1.84 per share in a private placement (Note 6) to
Robert Gipson.
In January 2009, the Company sold an aggregate of 1,000,000 shares of its common stock
at a purchase price of $1.00 per share in a private placement (Note 8) to Robert
Gipson.
In February 2009, the Subsidiary issued to Robert Gipson the Subsidiary Note (Note 5).
In March through September 2009, the Company sold Series F Stock to Robert Gipson (Note
8).
In November 2009, the Company entered into a private placement with Robert Gipson of
2,500,000 shares of its common stock which raised $1,000,000 in gross proceeds. In
addition, in March 2010 the Company issued an additional 1,500,000 shares of its common
stock to Mr. Gipson pursuant to a Letter Agreement (Note 8).
In December 2010, the Company purchased 270,000 common shares from Robert Gipson at
$.03 per share for a total of $8,125. The closing price for the Company’s stock on
December 28 was $0.15 per share. The price per share paid by the Company to Mr. Gipson
represented an 80% discount to the market price for the shares (Note 8).
From December 2009 through December 2010, Company issued promissory notes to Robert
Gipson in the aggregate of $3,660,000, payable on demand. The Notes bear interest at
the rate of 7% per annum. (Note 5)
13. Employee Benefit Plan
The company maintains a 401(k) savings plan (the “Plan”) but discontinued the employer
matching provision in the first quarter of 2009. Our matching contribution for the
savings plan was $25,000 in 2009.
14. Subsequent Events
In each of January, February and March 2011 the Company issued Promissory Notes to
Mr. Robert Gipson totaling $600,000. The Notes bear interest at 7% per annum and are
due and payable on demand.
49
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934,
the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized this 31st day of March, 2011.
| Alseres Pharmaceuticals, Inc. |
||||
| By: | /s/ Peter G. Savas | |||
| Peter G. Savas | ||||
| Chairman and Chief Executive Officer | ||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been
signed below by the following persons on behalf of the registrant and in the capacities and on the
dates indicated.
| Signature | Title | Date | ||
| /s/ Peter G. Savas
|
Chairman and Chief Executive Officer (Principal Executive Officer) |
March 31, 2011 | ||
| /s/ Kenneth L. Rice, Jr.
|
Executive Vice President Finance and Administration and Chief Financial Officer (Principal Financial and Accounting Officer) |
March 31, 2011 | ||
| /s/ William L.S. Guinness
|
Director | March 31, 2011 | ||
| /s/ Michael J. Mullen
|
Director | March 31, 2011 |
50
Table of Contents
EXHIBIT INDEX
| Incorporated by Reference to | ||||||||||||
| Exhibit | Exhibit | Filing | SEC File | |||||||||
| Number | Description | Form | Number | Date | Number | |||||||
| Articles of Incorporation and By-laws | ||||||||||||
3.1
|
Amended and Restated Certificate of Incorporation, dated March 28, 1996 | 10-K/A for 12/31/1998 |
3.1 | 3/19/1999 | 000-6533 | |||||||
3.2
|
Certificate of Amendment of Certificate of Incorporation, dated June 6, 1997 | 10-K/A for 12/31/1998 |
3.1 | 3/19/1999 | 000-6533 | |||||||
3.3
|
Certificate of Amendment of Amended and Restated Certificate of Incorporation, dated June 28, 1999 | 10-Q for 9/30/1999 | 3.5 | 11/15/1999 | 000-6533 | |||||||
3.4
|
Certificate of Amendment of Amended and Restated Certificate of Incorporation, dated June 14, 2000 | 10-K for 12/31/2000 | 3.3 | 3/29/2001 | 000-6533 | |||||||
3.5
|
Certificate of Correction to the Amended and Restated Certificate of Incorporation, dated March 14, 2001 | 10-K for 12/31/2000 | 3.3 | 3/29/2001 | 000-6533 | |||||||
3.6
|
Form of Certificate of Amendment of Amended and Restated Certificate of Incorporation dated June 11, 2002 | Proxy Statement | App. A | 5/1/2002 | 000-6533 | |||||||
3.7
|
Certificate of Amendment of Amended and Restated Certificate of Incorporation of the Company, dated as of July 9, 2003 | 10-Q for 6/30/2003 | 3.1 | 8/13/2003 | 000-6533 | |||||||
3.8
|
Certificate of Amendment of Amended and Restated Certificate of Incorporation of the Company, dated as of August 5, 2004 | 10-Q for 6/30/2004 | 3.1 | 8/13/2004 | 000-6533 | |||||||
3.9
|
Certificate of Amendment of Amended and Restated Certificate of Incorporation of the Company, dated as of February 4, 2005 | 8-K | 3.1 | 2/7/2005 | 000-6533 | |||||||
3.10
|
Certificate of Amendment of Amended and | 8-K | 3.1 | 6/8/2007 | 000-6533 | |||||||
| Restated Certificate of Incorporation of the Company, dated as of June 7, 2007 | ||||||||||||
3.11
|
Amended and Restated By-Laws, effective as of December 6, 2007 | 8-K | 3.1 | 12/7/2007 | 000-6533 | |||||||
| Instruments Defining the Rights of Security Holders | ||||||||||||
4.1
|
Specimen certificate evidencing shares of common stock, par value $.01 per share | 10-Q for 6/30/2007 | 4.1 | 8/14/2007 | 000-6533 | |||||||
| series D | ||||||||||||
4.2
|
Restated Certificate of Designations, Preferences, and Rights of Series D Preferred Stock | 8-A/A | Ex. A to 3.3 | 9/13/2001 | 000-6533 | |||||||
| series F | ||||||||||||
4.3
|
Certificate of Designations, Preferences, and Rights of Series F Convertible Preferred Stock | 8-K | 4.1 | 3/25/2009 | 000-6533 | |||||||
| Rights Agreement | ||||||||||||
4.4
|
Rights Agreement, dated as of September 11, 2001, including the form of Certificate of Designation with Respect to the Series D Preferred Stock and the form of Rights Certificate, between the Company and Continental Stock Transfer & Trust Company, as Rights Agent (the “Rights Agreement”) | 8-A/A | 1 | 9/13/2001 | 000-6533 | |||||||
Table of Contents
| Incorporated by Reference to | ||||||||||||
| Exhibit | Exhibit | Filing | SEC File | |||||||||
| Number | Description | Form | Number | Date | Number | |||||||
4.5
|
Amendment No. 1 to the Rights Agreement, dated November 13, 2001 | 8-A/A | 2 | 11/25/2002 | 000-6533 | |||||||
4.6
|
Amendment No. 2 to the Rights Agreement, dated November 22, 2002 | 8-A/A | 3 | 11/25/2002 | 000-6533 | |||||||
4.7
|
Amendment No. 3 to the Rights Agreement, dated March 12, 2003 | 8-K | 99.6 | 3/13/2003 | 000-6533 | |||||||
4.8
|
Amendment No. 4 to the Rights Agreement, dated December 23, 2003 | 8-A/A | 5 | 12/29/2003 | 000-6533 | |||||||
4.9
|
Amendment No. 5 to the Rights Agreement, dated March 14, 2005 | 8-K | 4.1 | 3/15/2005 | 000-6533 | |||||||
| Miscellaneous | ||||||||||||
4.10
|
Form of Warrant issued by the Company under the Securities Purchase Agreement dated November 20, 2008 | 8-K | 10.2 | 11/25/2008 | 000-6533 | |||||||
4.11
|
Promissory Note dated February 11, 2009 issued by Neurobiologics, Inc. to Robert L. Gipson | 8-K | 10.1 | 2/18/2009 | 000-6533 | |||||||
| Ingalls | ||||||||||||
4.12
|
Amended and Restated Registration Rights Agreement, dated as of March 9, 2005, by and among the Company and Ingalls, Robert L. Gipson and Nickolaos D. Monoyios and other Investors | 10-K for 12/31/2004 | 10.42 | 3/31/2005 | 000-6533 | |||||||
4.13
|
Amendment No. 1, dated August 30, 2005, to the Amended and Restated Registration Rights Agreement, dated as of March 9, 2005, by and among the Company and Ingalls, Robert L. Gipson and Nickolaos D. Monoyios and other Investors | 10-Q for 9/30/2005 | 10.6 | 11/14/2005 | 000-6533 | |||||||
4.14
|
Common Stock Purchase Agreement, dated March 9, 2005, by and among the Company, Ingalls and other Investors | 10-K for 12/31/2004 | 10.41 | 3/31/2005 | 000-6533 | |||||||
4.15
|
Common Stock Purchase Agreement, dated August 30, 2005, by and among the Company, Ingalls and other Investors | 10-Q for 9/30/2005 | 10.5 | 11/14/2005 | 000-6533 | |||||||
4.16
|
Mutual Release of Claims, dated as of June 15, 2004, by and among the Company, S. David Hillson, Marc E. Lanser, Robert L. Gipson, Thomas O. Boucher, Jr., Ingalls & Snyder, LLC and Ingalls | 8-K | 99.3 | 6/17/2004 | 000-6533 | |||||||
| Material Contracts — Supply, License, Distribution | ||||||||||||
CMCC |
||||||||||||
10.1+
|
License Agreement between CMCC and the Company dated as of May 10, 2006 (Dr. Larry Benowitz) (relating to INOSINE) | 10-Q for 6/30/2006 | 10.1 | 8/14/2006 | 000-6533 | |||||||
10.2+
|
License Agreement between CMCC and the Company dated as of May 10, 2006 (Dr. Zhigang He) (relating to Oncomodulin) | 10-Q for 6/30/2006 | 10.2 | 8/14/2006 | 000-6533 | |||||||
Table of Contents
Table of Contents
| Incorporated by Reference to | ||||||||||||
| Exhibit | Exhibit | Filing | SEC File | |||||||||
| Number | Description | Form | Number | Date | Number | |||||||
| Harvard | ||||||||||||
10.3
|
License Agreement between President and Fellows of Harvard College (“Harvard”) and NeuroBiologics, Inc. (a subsidiary of the Company) dated as of December 10, 1993 (relating to ALTROPANE) | S-4 | 10.16 | 4/12/1995 | 333-91106 | |||||||
10.4
|
Amendment, dated May 7, 2004, to License Agreement between Harvard and the Company dated as of December 10, 1993 (relating to ALTROPANE) | 10-Q for 6/30/2005 | 10.6 | 8/15/2005 | 000-6533 | |||||||
10.5
|
License Agreement between Harvard and the Company dated as of March 15, 2000 (relating to ALTROPANE) | S-3/A | 10.11 | 9/3/2002 | 333-88726 | |||||||
10.6
|
Amendment, dated May 11, 2004, to License Agreement between Harvard and the Company dated as of March 15, 2000 (relating to ALTROPANE) | 10-Q for 6/30/2005 | 10.4 | 8/15/2005 | 000-6533 | |||||||
10.7
|
License Agreement, effective as of October 15, 1996, between Harvard and the Company; as amended on August 22, 2001 and on May 4, 2004 (relating to FLUORATEC) | 10-Q for 9/30/2005 | 10.8 | 11/14/2005 | 000-6533 | |||||||
10.8
|
Third Amendment, dated April 1, 2007, to License Agreement between Harvard and the Company dated as of October 15, 1996, as amended on August 22, 2001 and on May 4, 2004 (relating to FLUORATEC) | 10-Q for 3/31/2007 | 10.2 | 5/15/2007 | 000-6533 | |||||||
| Nordion | ||||||||||||
10.9+
|
Manufacturing Agreement dated August 9, 2000 between the Company and MDS Nordion, Inc. (“Nordion Agreement”) | 10-K for 12/31/2001 | 10.15 | 3/29/2002 | 000-6533 | |||||||
10.10+
|
Amendment dated August 23, 2001 to Nordion Agreement | 10-K for 12/31/2001 | 10.16 | 3/29/2002 | 000-6533 | |||||||
10.11
|
Amendment No. 2 dated as of September 18, 2002 to Nordion Agreement | 10-K for 12/31/2002 | 10.16 | 3/31/2003 | 000-6533 | |||||||
10.12
|
Amendment No. 3 dated as of November 22, 2003 to Nordion Agreement | 10-K for 12/31/2003 | 10.17 | 3/30/2004 | 000-6533 | |||||||
10.13+
|
Amendment No. 4 dated as of December 22, 2004 to Nordion Agreement | 10-K for 12/31/2004 | 10.48 | 3/31/2005 | 000-6533 | |||||||
10.14+
|
Amendment No. 5 dated as of January 24, 2005 to Nordion Agreement | 10-K for 12/31/2004 | 10.48 | 3/31/2005 | 000-6533 | |||||||
10.15+
|
Amendment No. 6 dated as of December 19, 2005 to Nordion Agreement | 8-K | 99.1 | 12/19/2005 | 000-6533 | |||||||
10.16+
|
Amendment No. 7 dated as of December 7, 2006 to Nordion Agreement | 8-K | 10.1 | 12/8/2006 | 000-6533 | |||||||
10.17+
|
Amendment No. 8 dated as of December 4, 2007 to Nordion Agreement | 8-K | 10.1 | 12/7/2007 | 000-6533 | |||||||
10.18+
|
Amendment No. 9 dated as of December 3, 2008 to Nordion Agreement | 8-K | 10.1 | 12/8/2008 | 000-6533 | |||||||
Table of Contents
| Incorporated by Reference to | ||||||||||||
| Exhibit | Exhibit | Filing | SEC File | |||||||||
| Number | Description | Form | Number | Date | Number | |||||||
10.19+
|
Amendment No. 10 dated as of January 4 , 2010 to Nordion Agreement | 10-K for 12/31/2009 |
10.19 | 3/31/2010 | 000-6533 | |||||||
| Organix | ||||||||||||
10.20
|
License Agreement, effective as of July 1, 2000, between Organix, Inc. and the Company (“Organix Agreement”) (relating to 0-1369) | 10-Q for 9/30/2005 | 10.7 | 11/14/2005 | 000-6533 | |||||||
10.21
|
Amendment, dated May 11, 2004, to Organix Agreement (relating to 0-1369) | 10-Q for 9/30/2005 | 10.7 | 11/14/2005 | 000-6533 | |||||||
10.22
|
Second Amendment, dated April 1, 2007, to Organix Agreement (relating to 0-1369) | 10-Q for 3/31/2007 | 10.3 | 5/15/2007 | 000-6533 | |||||||
| BioAxone | ||||||||||||
10.23+
|
License Agreement, dated December 28, 2006, by and between the Company and BioAxone Therapeutic Inc. (“BioAxone Agreement) (relating to CETHRIN) | 8-K | 10.1 | 1/4/2007 | 000-6533 | |||||||
10.24+
|
First Amendment, dated March 23, 2007, to BioAxone Agreement (relating to CETHRIN) | 10-Q for 3/31/2007 | 10.1 | 5/15/2007 | 000-6533 | |||||||
10.25
|
Amendment to BioAxone License Agreement dated as of April 23, 2009 | 10-K for 12/31/2009 |
10.25 | 3/31/2010 | 000-6533 | |||||||
| Material Contracts — Leases | ||||||||||||
10.24
|
Lease Agreement, dated as of January 28, 2002, between the Company and Brentwood Properties, Inc. (“Brentwood”) | 10-K for 12/31/2004 | 10.47 | 3/31/2005 | 000-6533 | |||||||
10.25
|
Amendment of Lease, dated September 9, 2005, by and between Brentwood and the Company | 10-Q for 9/30/2005 | 10.1 | 11/14/2005 | 000-6533 | |||||||
10.26
|
Lease Agreement, dated as of June 9, 2005, by and between Straly Corporation and the Company | 10-Q for 6/30/2005 | 10.3 | 8/15/2005 | 000-6533 | |||||||
10.27
|
Sublease, dated September 9, 2005, by and between Small Army, Inc. and the Company | 10-Q for 9/30/2005 | 10.2 | 11/14/2005 | 000-6533 | |||||||
10.28
|
Sublease, dated September 9, 2005, by and between Dell Mitchell Architects, Inc. and the Company | 10-Q for 9/30/2005 | 10.3 | 11/14/2005 | 000-6533 | |||||||
| Material Contracts — Stock Purchase, Financing and Credit Agreements | ||||||||||||
10.29
|
Third Amended and Restated Convertible Promissory Note Purchase Agreement (unsecured), dated March 18, 2008 by and among the Company and the purchasers listed therein | 8-K | 10.1 | 3/20/2008 | 000-6533 | |||||||
10.30
|
Convertible Promissory Note Purchase Agreement (unsecured) dated June 25, 2008, by and between the Company and Robert L. Gipson | 8-K | 10.1 | 6/30/2008 | 000-6533 | |||||||
10.31
|
Securities Purchase Agreement, dated November 20, 2008, by and between the Company and Robert L. Gipson | 8-K | 10.1 | 11/25/2008 | 000-6533 | |||||||
10.32
|
Letter Agreement, dated November 20, 2008, by and between the Company and Robert L. Gipson | 8-K | 10.3 | 11/25/2008 | 000-6533 | |||||||
10.33
|
Securities Purchase Agreement, dated January 8, 2009, by and between the Company and Robert L. Gipson | 10-K for 12/31/2009 |
10.33 | 3/31/2010 | 000-6533 | |||||||
Table of Contents
| Incorporated by Reference to | ||||||||||||
| Exhibit | Exhibit | Filing | SEC File | |||||||||
| Number | Description | Form | Number | Date | Number | |||||||
10.34
|
Securities Purchase Agreement, dated February 24, 2009, by and between the Company and Cato Holding Company | 8-K | 10.1 | 2/27/2009 | 000-6533 | |||||||
10.35
|
Letter Agreement, dated February 24, 2009, by and between the Company and Cato BioVentures | 8-K | 10.2 | 2/27/2009 | 000-6533 | |||||||
10.36
|
Securities Purchase Agreement, dated March 19, 2009, by and between the Company and Robert L. Gipson | 8-K | 10.1 | 3/25/2009 | 000-6533 | |||||||
10.37
|
Securities Purchase Agreement, dated April 16, 2009, by and between the Company and Robert L. Gipson | 8-K | 10.7 | 4/22/2009 | 000-6533 | |||||||
10.38
|
Securities Purchase Agreement, dated May 12, 2009, by and between the Company and Robert L. Gipson | 10-Q | 10.2 | 8/14/2009 | 000-6533 | |||||||
10.39
|
Securities Purchase Agreement, dated June 10, 2009, by and between the Company and Robert L. Gipson | 8-K | 10.7 | 6/22/2009 | 000-6533 | |||||||
10.40
|
Securities Purchase Agreement, dated July 9, 2009, by and between the Company and Robert L. Gipson | 8-K | 10.7 | 7/13/2009 | 000-6533 | |||||||
10.41
|
Securities Purchase Agreement, dated July 23, 2009, by and between the Company and Robert L. Gipson | 8-K | 10.7 | 7/29/2009 | 000-6533 | |||||||
10.42
|
Securities Purchase Agreement, dated August 11, 2009, by and between the Company and Robert L. Gipson | 10-K for 12/31/2009 |
10.42 | 3/31/2010 | 000-6533 | |||||||
10.43
|
Securities Purchase Agreement, dated August 26, 2009, by and between the Company and Robert L. Gipson | 8-K | 10.7 | 9/1/2009 | 000-6533 | |||||||
10.44
|
Securities Purchase Agreement, dated September 10, 2009, by and between the Company and Robert L. Gipson | 8-K | 10.7 | 9/16/2009 | 000-6533 | |||||||
10.45
|
Securities Purchase Agreement, dated September 28, 2009, by and between the Company and Robert L. Gipson | 8-K | 10.7 | 10/2/2009 | 000-6533 | |||||||
10.46
|
Securities Purchase Agreement, dated November 4, 2009, by and between the Company and Robert L. Gipson | 8-K | 10.7 | 11/10/2009 | 000-6533 | |||||||
10.47
|
Promissory Note issued by the Company to Robert Gipson dated December 23, 2009 | 8-K | 10.7 | 12/28/2009 | 000-6533 | |||||||
10.48
|
Promissory Note issued by the Company to Robert Gipson dated January 28, 2010 | 8-K | 10.7 | 1/28/2010 | 000-6533 | |||||||
10.49
|
Promissory Note issued by the Company to Robert Gipson dated February 10, 2010 | 10-K for 12/31/2009 |
10.49 | 3/31/2010 | 000-6533 | |||||||
10.50
|
Promissory Note issued by the Company to Robert Gipson dated February 26, 2010 | 8-K | 10.7 | 3/4/2010 | 000-6533 | |||||||
10.51
|
Promissory Note issued by the Company to Robert Gipson dated March 9, 2010 | 10-K for 12/31/2009 |
10.51 | 3/31/2010 | 000-6533 | |||||||
Table of Contents
| Incorporated by Reference to | ||||||||||||
| Exhibit | Exhibit | Filing | SEC File | |||||||||
| Number | Description | Form | Number | Date | Number | |||||||
10.52
|
Promissory Note issued by the Company to Robert Gipson dated March 25, 2010 | 10-K for 12/31/2009 |
10.52 | 3/31/2010 | 000-6533 | |||||||
10.53
|
Promissory Note issued by the Company to Robert Gipson dated April 20, 2010 | 8-K | 10.7 | 4/21/2010 | 000-6533 | |||||||
10.54
|
Promissory Note issued by the Company to Robert Gipson dated May 24, 2010 | * | ||||||||||
10.55
|
Promissory Note issued by the Company to Robert Gipson dated June 10, 2010 | 8-K | 10.7 | 6/11/2010 | 000-6533 | |||||||
10.56
|
Promissory Note issued by the Company to Robert Gipson dated July 1, 2010 | 8-K | 10.7 | 7/8/2010 | 000-6533 | |||||||
10.57
|
Promissory Note issued by the Company to Robert Gipson dated August 2, 2010 | 8-K | 10.7 | 8/3/2010 | 000-6533 | |||||||
10.58
|
Promissory Note issued by the Company to Robert Gipson dated September 7, 2010 | 8-K | 10.7 | 9/8/2010 | 000-6533 | |||||||
10.59
|
Promissory Note issued by the Company to Robert Gipson dated September 13, 2010 | 8-K | 10.8 | 9/16/2010 | 000-6533 | |||||||
10.60
|
Promissory Note issued by the Company to Robert Gipson dated September 20, 2010 | * | ||||||||||
10.61
|
Promissory Note issued by the Company to Robert Gipson dated October 18, 2010 | * | ||||||||||
10.62
|
Note purchase Agreement with Highbridge International LLC dated September 10, 2010 | 8-K | 10.7 | 9/16/2010 | 000-6533 | |||||||
10.63
|
Promissory Note issued by the Company to Robert Gipson dated November 4, 2010 | 8-K | 10.7 | 11/5/2010 | 000-6533 | |||||||
10.64
|
Promissory Note issued by the Company to Robert Gipson dated December 9, 2010 | 8-K | 10.7 | 12/10/2010 | 000-6533 | |||||||
10.65
|
Promissory Note issued by the Company to Robert Gipson dated January 7, 2011 | 8-K | 10.7 | 1/13/2011 | 000-6533 | |||||||
10.66
|
Stock Repurchase Agreement with Robert Gipson dated December 28, 2010 | 8-K | 1.01 | 1/3/2011 | 000-6533 | |||||||
10.67
|
Promissory Note issued by the Company to Robert Gipson dated February 8, 2011 | 8-K | 10.7 | 2/9/2011 | 000-6533 | |||||||
10.68
|
Promissory Note issued by the Company to Robert Gipson dated March 4, 2011 | 8-K | 10.7 | 3/10/2011 | 000-6533 | |||||||
10.69
|
Promissory Note issued by the Company to Robert Gipson dated March 16, 2011 | 8-K | 10.7 | 3/22/2011 | 000-6533 | |||||||
Table of Contents
| Incorporated by Reference to | ||||||||||||||
| Exhibit | Exhibit | Filing | SEC File | |||||||||||
| Number | Description | Form | Number | Date | Number | |||||||||
| Management Contract or Compensatory Plan or Arrangement | ||||||||||||||
10.70#
|
Form of Indemnity for Directors and Executive Officers | 10-K for 12/31/2003 | 10.32 | 3/30/2004 | 000-6533 | |||||||||
10.71#
|
Form of Incentive Stock Option Agreement, as amended | 10-Q for 3/31/2005 | 10.1 | 5/16/2005 | 000-6533 | |||||||||
10.72#
|
Form of Non-Statutory Stock Option Agreement, as amended | 10-Q for 3/31/2005 | 10.2 | 5/16/2005 | 000-6533 | |||||||||
10.73#
|
Form of Incentive Stock Option Agreement for 2005 Stock Incentive Plan | 10-K for 12/31/2005 | 10.54 | 3/31/2006 | 000-6533 | |||||||||
10.74#
|
Form of Non-Statutory Stock Option Agreement for 2005 Stock Incentive Plan | 10-K for 12/31/2005 | 10.55 | 3/31/2006 | 000-6533 | |||||||||
10.75#
|
Amended and Restated 1990 Non-Employee Directors’ Non Qualified Stock Option Plan, as amended | 10-K for 12/31/2008 | 10.44 | 3/31/2009 | 000.6533 | |||||||||
10.76#
|
Amended and Restated Omnibus Stock Option Plan | 10-K for 12/31/2008 | 10.45 | 3/31/2009 | 000.6533 | |||||||||
10.77#
|
Amended and Restated 1998 Omnibus Stock Option Plan | 10-K for 12/31/2008 | 10.46 | 3/31/2009 | 000.6533 | |||||||||
10.78#
|
Amended and Restated 2005 Stock Incentive Plan | 10-K for 12/31/2008 | 10.47 | 3/31/2009 | 000.6533 | |||||||||
10.79#
|
Director and Officer Indemnity Trust Agreement, dated June 15, 2004, between S. David Hillson, Boston Private Bank & Trust Company and the Company | 8-K | 99.6 | 6/17/2004 | 000-6533 | |||||||||
10.80#
|
Amended and Restated Employment Agreement, dated December 31, 2008, between the Company and Peter G. Savas | 8-K | 10.1 | 1/6/2009 | 000-6533 | |||||||||
10.81#
|
Amended and Restated Employment Agreement, dated December 31, 2008, between the Company and Mark J. Pykett | 8-K | 10.2 | 1/6/2009 | 000-6533 | |||||||||
10.82#
|
Amended and Restated Employment Agreement, dated December 31, 2008, between the Company and Kenneth L. Rice, Jr. | 8-K | 10.3 | 1/6/2009 | 000-6533 | |||||||||
10.83#
|
Consulting Agreement dated September 29, 2006, by and between the Company and Robert S. Langer, Jr. | 8-K | 10.1 | 10/4/2006 | 000-6533 | |||||||||
Table of Contents
Additional Exhibits
21
|
Subsidiaries of the Registrant | * | ||
23.1
|
Consent of McGladrey & Pullen, LLP | * | ||
31.1
|
Certification of Chief Executive Officer pursuant to Rule 13a- 14(a)/Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended | * |
| Incorporated by Reference to | ||||||||||
| Exhibit | Exhibit | Filing | SEC File | |||||||
| Number | Description | Form | Number | Date | Number | |||||
31.2
|
Certification of Chief Financial Officer pursuant to Rule 13a- 14(a)/Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended | * | ||||||||
32.1
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | * | ||||||||
32.2
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | * | ||||||||
| * | Filed herewith | |
| (#) | Management contract or compensatory plan or arrangement filed as an exhibit to this Form pursuant to Item 15(b) of Form 10-K. | |
| (+) | Confidential treatment has been requested as to certain portions, which portions have been filed separately with the Securities and Exchange Commission. |
