Attached files
| file | filename |
|---|---|
| EX-23 - EX-23 - MEDQUIST INC | w81804aexv23.htm |
| EX-32.1 - EX-32.1 - MEDQUIST INC | w81804aexv32w1.htm |
| EX-31.1 - EX-31.1 - MEDQUIST INC | w81804aexv31w1.htm |
| EX-32.2 - EX-32.2 - MEDQUIST INC | w81804aexv32w2.htm |
| EX-31.2 - EX-31.2 - MEDQUIST INC | w81804aexv31w2.htm |
| EX-10.42.2 - EX-10.42.2 - MEDQUIST INC | w81804aexv10w42w2.htm |
| EX-10.42.1 - EX-10.42.1 - MEDQUIST INC | w81804aexv10w42w1.htm |
| EX-10.42.3 - EX-10.42.3 - MEDQUIST INC | w81804aexv10w42w3.htm |
Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2010
Commission file number 0-19941
MEDQUIST INC.
(Exact name of registrant as specified in its charter)
| New Jersey | 22-2531298 | |
| (State or other jurisdiction of | (I.R.S. Employer Identification No.) | |
| incorporation or organization) |
9009 Carothers Parkway, Franklin, TN 37067
(Address of principal executive offices)
(Address of principal executive offices)
Registrant’s telephone number, including area code:
(866) 295-4600
(866) 295-4600
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common Stock, no par value per share | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
None
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule
405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section
13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed
by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or
for such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days. Yes þ
No o
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate Web site, if any, every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period
that the registrant was required to submit and post such files). Yes o
No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation
S-K (§ 229.405) is not contained herein and will not be contained, to the best of registrant’s
knowledge, in definite proxy or information statements incorporated by reference in Part III of
this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated
filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large
accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2
of the Exchange Act. (Check One):
| Large Accelerated Filer o | Accelerated Filer þ | Non-Accelerated Filer o | Smaller reporting company o | |||
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of
the Exchange Act). Yes o No þ
The aggregate market value of the outstanding common stock held by non-affiliates of the
registrant as of June 30, 2010, was $90,519,912. Such aggregate market value was computed by
reference to the closing price of the common stock as reported on the Global Market of The NASDAQ
Stock Market LLC .
The number of registrant’s shares of common stock, no par value, outstanding as of March 14,
2011 was 37,555,893.
Documents incorporated by reference
Portions of the definitive Proxy Statement for the 2011 Annual Meeting of Shareholders are
incorporated by reference into Part III of this Annual Report on Form 10-K.
MedQuist Inc.
Annual Report on Form 10-K
Table of Contents
Annual Report on Form 10-K
Table of Contents
| Page | ||||||||
| 3 | ||||||||
| 13 | ||||||||
| 21 | ||||||||
| 21 | ||||||||
| 21 | ||||||||
| 22 | ||||||||
| 23 | ||||||||
| 24 | ||||||||
| 25 | ||||||||
| 37 | ||||||||
| 37 | ||||||||
| 37 | ||||||||
| 38 | ||||||||
| 38 | ||||||||
| 39 | ||||||||
| 39 | ||||||||
| 39 | ||||||||
| 39 | ||||||||
| 39 | ||||||||
| 39 | ||||||||
Consent of KPMG LLP |
||||||||
Certification of Chief Executive Officer |
||||||||
Certification of Chief Financial Officer |
||||||||
Certification of Chief Executive Officer, pursuant to Section 906 |
||||||||
Certification of Chief Financial Officer, pursuant to Section 906 |
||||||||
| EX-10.42.1 | ||||||||
| EX-10.42.2 | ||||||||
| EX-10.42.3 | ||||||||
| EX-23 | ||||||||
| EX-31.1 | ||||||||
| EX-31.2 | ||||||||
| EX-32.1 | ||||||||
| EX-32.2 | ||||||||
2
Table of Contents
PART I
| Item 1. | Business |
Overview
We are a leading provider of integrated clinical documentation solutions for the U.S. healthcare
system. Our end-to-end solutions convert physicians’ dictation of patient interactions, or the
physician narrative, into a high quality and customized electronic record. These solutions
integrate technologies and services for voice capture and transmission, automated speech
recognition (ASR), medical transcription and editing, workflow automation, and document management
and distribution to deliver a complete managed service for our customers. Our solutions enable
hospitals, clinics, and physician practices to improve the quality of clinical data as well as
accelerate and automate the documentation process, and we believe our solutions improve physician
productivity and satisfaction, enhance revenue cycle performance, and facilitate the adoption and
use of electronic health records.
We are the largest provider by revenue of clinical documentation solutions based on the physician
narrative in the United States. The majority of lines we process are edited or transcribed by our
approximately 5,600 medical transcriptionists (MTs) and medical editors (MEs). For the three months
ended December 31, 2010, 76% was processed using ASR technology and 34% was produced offshore. Our
size allows us to handle the clinical documentation requirements of many of the largest and most
complex healthcare delivery networks in the United States, provides us with economies of scale, and
enables us to devote significantly more resources to enhancing our solutions through research and
development than most of our competitors.
We serve a large number of hospital and physician practices and the majority of the work is
recurring.
We have realized significant increases in both revenue and profitability as the result of the
acquisition of Spheris Inc. (Spheris), which we acquired in April 2010 as well as benefits realized
from our scalable business model. From 2008 to 2010, our net revenue increased from $326.9 million
to $375.2 million.
Our industry
Growth of clinical documentation in the United States
Over the past several decades, our industry has evolved from almost exclusively in-house production
to outsourced services and from labor-intensive services to technologically-enabled solutions. The
market opportunity for our solutions is driven by overall healthcare utilization and cost
containment efforts in the United States. Numerous factors are driving increases in the demand for
healthcare services including population growth, longer life expectancy, the increasing prevalence
of chronic illnesses, and expanded coverage from healthcare reform. According to a September 2010
report by the U.S. Centers for Medicare and Medicaid Services, spending on healthcare grew from
$1.2 trillion in 1998 to $2.3 trillion in 2008, representing a compound annual growth rate of 7.0%.
It also projects that healthcare spending will grow to reach $4.2 trillion, or 19.3% of U.S. gross
domestic product, by 2018, representing a compound annual growth rate of 6.3%. At the same time,
U.S. healthcare providers remain under substantial pressure to reduce costs while maintaining or
improving the quality of care.
Accurate and timely clinical documentation has become a critical requirement of the growing U.S.
healthcare system. Medicare, Medicaid, and insurance companies demand extensive patient care
documentation. The Health Information Technology for Economic and Clinical Health Act (HITECH Act),
which was enacted into law on February 17, 2009 as part of the American Recovery and Reinvestment
Act of 2009 (ARRA), includes numerous incentives to promote the adoption and meaningful use of
electronic health records, or EHRs, across the healthcare industry. Consequently, healthcare
providers are increasingly using EHRs to input, store, and manage their clinical data in a digital
format. Healthcare providers that use EHRs require accurate, easy-to-use, and cost-effective means
to input clinical data that are not disruptive to the physician workflow.
Importance of the physician narrative
3
Table of Contents
Physicians generally use one of two methods to capture clinical data in a digital format: dictation
and on-screen data entry. Dictation allows a physician to use his or her voice to document patient
interactions, which is then converted into a text format or EHR record. On-screen data entry
enables a physician to populate templates or drop-down menus in an EHR system, typically with a
handheld or other hardware device.
In many hospital settings, dictation is the most popular method for capturing clinical data because
of the many advantages it provides over on-screen data entry. From an efficiency standpoint, a
physician’s time is typically the most expensive labor component of a clinical documentation
process, and reducing the time required for data capture lowers costs. It is generally faster to
dictate than enter data on-screen, and dictation frees up the physician to do other tasks in
parallel. From a documentation standpoint, dictation allows for a flexible narration of patient
interactions. Templates and drop-down menus typically restrict input to a structured format. While
dictation can be converted into structured format later, it provides a more flexible method for
data capture.
Market opportunity
The need to convert and manage the physician narrative represents a substantial market opportunity.
Historically, in-house hospital labor was used to transcribe clinical reports using analog
recordings from physicians. Later, healthcare providers began to outsource production to domestic
providers and use digital formats. Today, advanced automation technologies, such as ASR and
workflow platforms, and low-cost offshore resources are available to drive substantial improvements
in productivity and cost.
Market segmentation and trends
While outsourcing provides many benefits, the landscape for outsourced service providers is highly
fragmented with varying degrees of technological automation and offshore capabilities amongst
providers. Thousands of local and regional providers offer limited services without technology
offerings. A small set of national providers offer a combination of technology and services, but
have varying degrees of technological sophistication and production capacity. Some vendors also
focus more on pure technology, offering ASR software, with partnerships for third-party services,
though most of these vendors lack production scale.
Over the last five years, technological automation and a rise in offshore capabilities have
substantially decreased the cost of production and have further differentiated outsourcing
providers. ASR has been a key technological driver of productivity gains. ASR converts the
physician narrative into a text form which is available for editing. The effective use of this
technology lowers the cost of production relative to conventional transcription services. Another
key driver for cost reductions has been the increased use of offshore infrastructure and resources.
Historically, most U.S. healthcare providers that outsourced their
production did so to domestic service providers. With the advent of internet-based technologies and
improvements in the quality and training of offshore personnel, the clinical documentation industry
has seen a shift towards offshore resources to reduce costs. India is by far the most popular
destination for outsourcing given relatively low wages and a highly educated English-speaking
workforce.
As the industry’s cost of production has declined through increases in technological automation and
offshore capabilities, the average market price for medical transcription services has also
declined. This has allowed healthcare providers to participate in these economic gains. However, we
believe that participants in our industry must expand their technology platforms and offshore
capabilities to remain competitive.
Our competitive strengths
Our competitive strengths include:
| n | Leader in a large, fragmented market — We are the largest provider by revenue of clinical documentation solutions based on the physician narrative in the United States. Our size enables us to meet the needs of large, sophisticated healthcare customers, provides economies of scale, and enables us to devote significantly more resources to research and development and quality assurance than many other providers. |
4
Table of Contents
| n | Integrated solutions delivered as a complete managed service — We offer fully-integrated end-to-end managed services that capture and convert the physician narrative into a high quality customized electronic record. We integrate technologies and services for voice capture and transmission, ASR, medical transcription and editing, workflow automation, and document management and distribution. The end result is value-added clinical documentation with high accuracy and quick turn-around times. | ||
| n | Large and diversified customer base with long-term relationships — We serve a large number of hospital and physician practices and the majority of the work is recurring. | ||
| n | Highly-efficient operating model — Over the past two years, we have driven down our cost structure through leveraging our scalable infrastructure, standardizing processes, and increased utilization of ASR. Our use of ASR, which has grown from 39% of our volume in the fourth quarter of 2008 to 76% in the fourth quarter of 2010, has increased our productivity. Additionally, our expanding footprint in India through our relationship with MedQuist Holdings Inc.’s wholly owned subsidiary, CBay Systems and Services, Inc., our principal offshore labor provider has enabled us to increase our offshore production from 28% of our volume to 34% over this same period. The financial impact of these measures has been an improvement in gross margins during this timeframe from 34% to 36%. | ||
| n | Proven management team — We have assembled an outstanding senior leadership team with significant industry experience and domain expertise in both domestic and offshore operations. Our management team has delivered substantial results and brings an entrepreneurial spirit with proven experience in managing growth, driving operational improvements, and successfully integrating acquisitions. |
Our strategy
Key elements of our strategy include:
| n | Expand our customer base and increase existing customer penetration — We intend to grow our customer base by targeting three market segments: large healthcare providers still using in-house services, large healthcare providers currently using competing outsourced alternatives, and small to medium sized hospitals and physician practices. Given our market leadership, strong solution offerings, and low cost structure, we believe we are well positioned to both replace in-house solutions as well as displace competing outsourced alternatives for large healthcare providers. In order to increase penetration within our existing customer base, we intend to continue targeting additional healthcare clinical areas and facilities of our current customers. Additionally, as healthcare providers centralize their purchasing decisions, we believe that our ability to deliver outstanding services for large, complex requirements provides us with increasing access to new sales opportunities within our existing customer base and through existing customer relationships. | ||
| n | Continue to develop and enhance our integrated solutions — We seek to differentiate our integrated solutions through sophisticated technology and process improvement. We have approximately 90 employees dedicated to research and development. Over the last year, we launched numerous enhancements, including a front end speech platform for general medicine, additional EHR system integration, and advanced performance monitoring. | ||
| n | Enhance profitability through technical and operational expertise — We have made significant improvements in productivity through business process and infrastructure improvements. Notwithstanding reductions in customer pricing, our gross margins have expanded from 32% in the fourth quarter of 2008 to 36% in the fourth quarter of 2010. Our management team has proven its ability to implement continuous process improvements and we intend to further increase offshore production and our use of technological automation, including ASR, to lower costs and enhance our profitability. | ||
| n | Facilitate the adoption and promote meaningful use of EHR systems — Our integrated solutions provide a comprehensive, accurate and effective method to incorporate physician narrative into an EHR system. We interface with substantially all of the leading EHR vendors to integrate our clinical documentation solutions and to help our customers realize the full potential of their EHR systems through the use of the physician narrative. In our experience, when EHR is adopted, customers tend to consolidate their purchase decisions, which benefit us as a leading provider of clinical documentation solutions. |
5
Table of Contents
| n | Pursue strategic acquisitions — We believe that there are significant opportunities available to create value through strategic acquisitions. We intend to seek appropriate opportunities to grow our customer base, enhance or expand our solutions, incorporate synergy opportunities, and expand our value proposition to our customers. |
Our solutions
Clinical documentation solutions for healthcare providers
We provide enterprise-class solutions for healthcare providers ranging from fully-integrated
end-to-end managed services to stand-alone offerings. These solutions represent the large majority
of our revenues. Our solutions enable our customers to easily access advanced technologies with
confidence that their clinical documentation requirements will be completed accurately and quickly.
Our industry-leading solutions integrate voice capture and transmission, ASR, transcription/editing
services, workflow
management, and document management and distribution capabilities. In addition, we have coding
technology and services to complement our clinical documentation offerings which enhance and
improve the revenue cycle.
With proprietary and licensed technologies, we enable our customers to efficiently manage their
narrative-based documentation through customizable workflows. A typical workflow includes the
following steps:
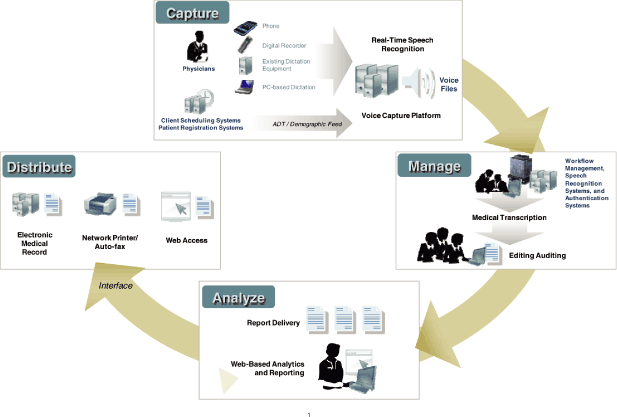
| n | Capture — As the first step in a workflow process, users can dictate into one of several input devices, including a variety of handheld dictation devices, Smartphone applications, proprietary handsets, standard telephones, or PC-based dictation stations. Users can also use PC-based speech recognition applications for those who prefer to edit their own files in real-time. By supporting a wide array of capture methods, we provide true choice to our customers to decide which workflow best suits their needs. Users can change as needed from real-time to batch-based workflows to maximize their productivity. |
6
Table of Contents
| n | Manage — Captured voice files are merged with patient information from our customers’ information systems and EHRs where they are loaded into our enterprise platform for processing. The platform balances production resources across both in-house and outsourced personnel, and its web-based management capabilities allow administrators to easily manage workflows from anywhere at any time. We generate draft reports using speech recognition technology which are reviewed by our Medical Editors (MEs). We can also use conventional transcription services from our Medical Transcriptionists (MTs). To maintain high quality and efficiency, our platform automatically matches voice files from various specialties and acuity levels to the MTs or MEs with the appropriate skill sets. It also includes random quality checks to give timely feedback to our personnel. Turn-around time is an important metric for our customers, and so the system optimizes processing to ensure we fulfill our contracted service level agreements, which typically range from one hour to 48 hours. In addition, the platform provides real-time access for our customers to not only see into their work moving through the system, but provides dashboard metrics and reporting to help manage their enterprises. | ||
| n | Analyze — Completed reports are routed back to physicians or other healthcare professionals for review, final editing (as required), and authentication. These reports are then available to drive additional value added services, such as coding, data abstraction for analytics, measures reporting, and billing services. We provide customers with sophisticated reporting capabilities and integrated electronic signature solutions to simplify and accelerate the review of their clinical reports. We use Quantify™, our patent-pending natural language processing technology, to convert final reports into structured documentation formats. This technology allows us to scan health records for information needed by the customer to fulfill their regulatory and local reporting requirements. Healthcare entities are required to report the meaningful use of their EHRs by reporting on defined quality measures set down by the government. Using the CAA tool, customers can extract this information for these quality measures to make sure the reporting is complete and reduce the time and expense that associated with a manual abstraction process. It can take as much as 40 minutes to manually abstract a single medical record. CAA technology can dramatically reduce this time and improve the accuracy of the information reported. CAA can also be used, based on a set of rules, to deliver information to the customer for Clinical Documentation Improvement initiatives (CDI). In this case, the tool can tell the customer when a healthcare worker has not documented something needed to support measures reporting and reimbursement. Finally, this technology can also be used to structure documentation in the health record for use in populating EHRs and for data analysis. In this case, the unstructured transcribed text can be structured and parsed into the information that can be populated into the customer’s EHR systems at a discrete level. This allows the vital information located in the narrative part of the physician’s documentation to be used in the EHR to support continuity of medical care and provide again for measures reporting and reimbursement needs. | ||
| n | Distribute — After being approved by the physician, electronic records are distributed. We provide a fully featured distribution solution for printing, faxing and electronic distribution to referring physicians. We have developed thousands of interfaces with major, mid-level and proprietary hospital information systems, radiology information systems, health information repositories and EHR systems. Our solution supports HL7 and XML-based formats which further allows us to meet the needs of each individual customer. Throughout the entire workflow, our managed service platform maintains security measures and audit trails in full compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) privacy and security standards and regulations and the protection of the confidentiality of patient information. |
In delivering these customized workflows, we offer a variety of software products. These can work
either as stand-alone solutions or as integrated solutions with our other managed services. These
solutions include:
| n | DocQment Enterprise Platform (DEP) — Our core platform provides a powerful and flexible transcription solution that integrates the process of dictation, transcription, speech recognition, editing, and document delivery into a unified clinical information management workflow. We offer the platform typically as a managed service. For those customers that prefer to use their own services, we also offer it on a license basis. Our platform provides a high performance and highly customizable clinical documentation workflow. It integrates with every major hospital system vendor, such as Epic, Cerner, and Meditech, and we developed thousands of interfaces with customer systems. |
7
Table of Contents
| n | SpeechQ — Our front-end speech recognition family of solutions enables physicians to dictate, edit, and sign their reports in real-time. With workflows customized for numerous medical practices, such as radiology and general medicine, SpeechQ offers end-to-end workflows that combine voice commands and dictation. SpeechQ integrates with our enterprise platform in scenarios where a physician prefers to send text to our editors for review. It interfaces with EHRs and other healthcare systems to allow patient demographic information to be automatically populated, updated, and distributed in real-time. Our newest SpeechQ offering, SpeechQDirect can voice-enable customer EHRs to drive greater adoption, which will assist them in realizing incentive funds from the government for that adoption. Healthcare providers can now use their voice in addition to keyboard and mouse to seamlessly interact with their desktop clinical applications, including the dictation of medical narrative and speech-based navigation within 3rd party applications. Using this solution, providers can improve documentation efficiency and avoid the trap of template-based point-and-click systems, augmenting discretely captured data with a narrative of the patient’s story. |
| n | CodeRunnerCAC — Our web-based workflow and workforce management system manages the entire coding process for our customers. Health records are fed into the system where natural language processing technology scans the information and delivers suggested codes to the hospital coding staff. This allows them to become more accurate, reduce turnaround time and increase productivity. All of these factors contribute to increased revenue and improved cash flow by shortening the revenue cycle and making fewer errors that can result in denials by payors. |
| n | DocQVoice — Our web-based enterprise digital voice capture and transport solution is deployed at the customer’s location and integrates with both our enterprise platform and legacy dictation systems. |
Selling and marketing
We employ
approximately 100 personnel in our sales force and account management organization. Our sales
force is focused on new customer sales opportunities including both the conversion of customers
that are using in-house solutions as well as the displacement of competitive offerings. This sales
organization employs consultative sales techniques to deliver customized programs and solutions
that respond to the customer’s unique requirements. Our account management organization is
responsible for continuity of our current customer relationships and the expansion of those
relationships to include additional services, facilities, or work types.
We complement our sales efforts with numerous marketing initiatives, including:
| n | attending and sponsoring industry trade shows of national organizations, such as the American Health Information Management Association, Healthcare Information and Management Systems Society, Association for Healthcare Documentation Integrity, Radiological Society of North America, Society for Imaging Informatics in Medicine, and Medical Transcription Industry Alliance; |
| n | participating in work groups and leadership committees of the industry associations; and | ||
| n | advertising in trade journals related to our industry. |
Operations
We serve our customers 24 hours a day, seven days a week with our integrated clinical documentation
solutions. We use ASR in most of our production, which we complement with skilled, English-speaking
MTs and MEs.
Technology
Technology development
We devote substantial resources to research and development to ensure that our solutions meet both
current and future customer requirements. As of December 31, 2010, we employed a development staff
of approximately 90 employees. Our development staff has expertise in multiple disciplines,
including service oriented architectures, web-based clients, high volume transactional databases,
data warehouses, web services and integration with third-party systems. We also utilize third party
resources for some specific technologies, such as ASR, capture-assisted codes, encoders, databases,
portal technologies and reporting. Much of the technology in our integrated solutions is
proprietary. Our development personnel follow a rigorous development methodology that ensures
repeatable, high quality and timely delivery of solutions.
8
Table of Contents
ASR is a key component of our narrative-based solutions, and we license software for a portion of
our ASR capabilities. We dual source some components of our ASR technologies.
Technology operations
Our clinical documentation solutions are hosted by us and accessed using high-speed internet
connections or private network connections. We have devoted significant resources to producing
software applications and managed services to meet the functionality and performance expectations
of our customers. We use commercially available hardware and a combination of proprietary and
commercially-available licensed software to provide our clinical documentation solutions.
Competition
Because we integrate technologies and services, we compete with companies in a number of different
sectors. These competitors include:
| n | in-house service departments of healthcare providers, which we believe produce the majority of clinical documentation today based on the physician narrative; | ||
| n | national medical transcription service providers, such as Focus Informatics, Inc. (a subsidiary of Nuance Communications, Inc. (Nuance)), Transcend Services, Inc., and Webmedex, Inc.; | ||
| n | local or regional medical transcription service organizations; | ||
| n | ASR software vendors, such as Nuance and Multimodal Technologies, Inc. (Multimodal), which market ASR as a means to reduce clinical documentation labor; and | ||
| n | EHR software vendors which promote their systems as a replacement to narrative-based input by using on-screen templates and drop-down boxes for data entry. |
Competition for our integrated clinical documentation solutions is based primarily on the following
factors:
| n | accuracy and timeliness of documentation produced; | ||
| n | capacity to handle large volumes and complex workflows; | ||
| n | ability to provide fully-integrated end-to-end solutions; | ||
| n | ease of upgrades and ability to add complementary offerings; | ||
| n | physician acceptance and productivity; | ||
| n | pricing; | ||
| n | physician acceptance and productivity; | ||
| n | analytics provided to customers; | ||
| n | domestic or offshore production capabilities; | ||
| n | time to implement for new customers; and | ||
| n | financial stability. |
9
Table of Contents
We believe we compete effectively on all of the above criteria. We provide fully integrated
end-to-end managed services that translate the physician narrative into a customized electronic
record with high accuracy and low turn-around time. We believe that our production cost structure
allows us to offer competitive prices while continuing to invest in the development of new
technologies and services. We have the largest production capacity in our industry, which we
believe strengthens our operational capabilities and assists us in meeting customer demands for
timely implementation of our solutions for new accounts.
Government regulation
The provision of clinical documentation solutions is heavily regulated by federal and state
statutes and regulations. We and our healthcare customers must comply with a variety of
requirements, including HIPAA and other restrictions regarding privacy, confidentiality, and
security of health information.
We have structured our operations to comply with HIPAA and other regulatory and contractual
requirements. We have implemented appropriate safeguards related to the access, use, or disclosure
of protected health information (PHI), to address the privacy and security of PHI consistent with
our regulatory and contractual requirements. We also train our personnel regarding HIPAA and other
requirements. We have made and continue to make investments in systems to support customer
operations that are regulated by HIPAA and other regulations. Because these standards are subject
to interpretation and change, we cannot predict the future impact of HIPAA or other regulations on
our business and operations.
HIPAA and HITECH Act
HIPAA establishes a set of national privacy and security standards for protecting the privacy,
confidentiality and security of PHI. Under HIPAA, health plans, healthcare clearinghouses, and
healthcare providers, together referred to as covered entities for purposes of HIPAA, and their
business associates must meet certain standards in order to protect individually identifiable
health information. The HITECH Act which was enacted into law on February 17, 2009 as part of the
ARRA, enhances and strengthens the
HIPAA privacy and security standards and makes certain provisions of HIPAA applicable to business
associates of covered entities.
As part of the operation of our business, our customers provide us with certain PHI, and we are
considered to be a business associate of most of our customers for purposes of HIPAA. The
provisions of HIPAA require our customers to have agreements in place with us whereby we are
required to appropriately safeguard the PHI we create or receive on their behalf. As a business
associate, we also have statutory and regulatory obligations under HIPAA. We are bound by our
business associate agreements to use and disclose PHI in a manner consistent with HIPAA in
providing services to those covered entities.
We and our customers are also subject to HIPAA security regulations that require the implementation
of certain administrative, physical and technical safeguards to ensure the confidentiality,
integrity and availability of electronic protected health information (EPHI). We are required by
regulation and contract to protect the security of EPHI that we create, receive, maintain or
transmit for our customers consistent with these regulations. These requirements include
implementing administrative, physical and technical safeguards that reasonably and appropriately
protect the confidentiality, integrity and availability of such EPHI. To comply with our regulatory
and contractual obligations, we may have to reorganize processes and invest in new technologies. On
February 17, 2010, we became directly subject to HIPAA’s criminal and civil penalties for any
breaches of our privacy and security obligations.
Other restrictions regarding privacy, confidentiality, and security of health information
In addition to HIPAA, numerous other state and federal laws govern the collection, dissemination,
use, access to, confidentiality and security of PHI. In addition, Congress and some states are
considering new laws and regulations that further protect the privacy and security of medical
records or medical information. In many cases, these state laws are not preempted by the HIPAA
privacy and security standards.
Intellectual property
We rely on a combination of copyright, patent, trademark, trade secret and other intellectual
property laws, nondisclosure agreements, license agreements, contractual provisions and other
measures to protect our proprietary rights. We have a number of registered
10
Table of Contents
trademarks in the United
States and abroad, including MedQuist® and SpeechQ®. We have common law
rights over a number of unregistered trademarks. We also own a limited number of United States and
foreign patents and patent applications that relate to our products, processes and technologies.
We dual source some components of our ASR technologies.
We license speech recognition and processing software from Nuance, pursuant to a licensing
agreement entered into in November 2009. Under our agreement with Nuance, we pay a licensing fee
based upon a per line charge for each transcribed line of text processed using the software
licensed from Nuance. Our licensing agreement with Nuance expires in June 2015. Thereafter, upon
written notice to Nuance, we have the right to renew the licensing agreement for two successive
periods of five years each on the same terms (except pricing) and conditions of the licensing
agreement then in effect.
Nuance granted us co-ownership rights to and interests in its SpeechQ product in exchange for a
fixed sum, pursuant to a supply agreement entered into in November 2009. The supply agreement also
provides that we receive, in exchange for periodic fees, the exclusive right in the United States,
Canada and certain Caribbean islands to sell, service and deliver SpeechQ. Our supply agreement
with Nuance expires in June 2015. Upon written notice to Nuance, we have the right to renew the
agreement for two successive terms of five years each on the same terms (except pricing) and
conditions of the agreement then in effect.
We also license the speech recognition and processing software used for SpeechQ from Nuance, under
a separate licensing agreement entered into in November 2009. Under this agreement, we pay a
licensing fee based on total number of individual users or named-user licenses per customer order.
This agreement expires in June 2015. Thereafter, upon written notice to Nuance, we have the right
to renew the licensing agreement for two successive periods of five years each on the same terms
(except pricing) and conditions of the licensing agreement then in effect.
We also license speech recognition and processing software from Multimodal. Our principal license
agreement with Multimodal was entered into in March 2010. Under that licensing agreement, we pay
Multimodal a monthly fee in exchange for a fixed number of minutes of recording. Each minute of
recording that exceeds the fixed number is charged at a specified rate per minute. Our agreement
with Multimodal expires in April 2013. Thereafter, the agreement automatically renews and is
extended for up to seven additional successive one-year periods, unless we notify Multimodal in
writing of our election not to extend at least sixty days prior to the last day of the term. We are
in discussions with Multimodal regarding an amendment to the license agreement that would modify
the structure of the term of the agreement. As part of that modified structure, we would have the
ability to use the software licensed under the agreement through April 2021. In the event of a
change of control that results in a direct competitor of Multimodal having, directly or indirectly,
a 50% or greater ownership interest in us, or 50% or more of the voting control of us, or in the
event we, through any acquisition of a direct competitor of Multimodal, begin selling or licensing
a software product other than Multimodal’s that is directly competitive with such technology,
Multimodal shall have the right to terminate its agreement with us.
Employees
As of December 31, 2010, we had approximately 6,500 employees in the United States. Most of our
employees are MTs and MEs involved in the production and quality assurance of clinical
documentation.
We believe we have good relationships with our employees. Our employees are not subject to
collective bargaining agreements or union representation.
Corporate developments
Recapitalization transactions
On October 14, 2010, we incurred $85.0 million of indebtedness through the issuance of
13% senior subordinated notes due 2016, or the Senior Subordinated Notes, under a note purchase
agreement, or the Note Purchase Agreement, and incurred $200.0 million of indebtedness under a
term loan, or the Term Loan, under a $225.0 million credit facility, or the Senior Secured
11
Table of Contents
Credit
Facility. MedQuist Holdings Inc. (MedQuist Holdings) is a guarantor of both the Senior Subordinated Notes and the
Senior Secured Credit Facility. We used the proceeds to repay $80.0 million of indebtedness
under its prior credit facility, or the Acquisition Credit Facility, to repay $13.6 million of
indebtedness under a subordinated promissory note, or the Acquisition Subordinated Promissory
Notes, each issued in connection with the Spheris Acquisition, and to pay a $176.5 million
special dividend to our stockholders. MedQuist Holdings received $122.6 million of this special
dividend.
Majority Owner
On August 6, 2008, MedQuist Holdings, formerly CBaySystems Holdings Limited (CBaySystems Holdings),
a company that is now publicly traded on The NASDAQ Global Market with a portfolio of investments
in medical transcription, which includes a company that competes in the medical transcription
market, healthcare technology, and healthcare financial services, acquired a large interest in us
from Koninklijke Philips Electronics N.V. (Philips).
MedQuist Holdings — U.S. initial public offering
On January 27, 2011, MedQuist Holdings changed its name from CBaySystems Holdings Limited to
MedQuist Holdings Inc. and re-domiciled from a British Virgin Islands company to a Delaware
corporation and authorized 300 million shares of common stock par value at $0.10 per share and 25
million shares of preferred stock at $0.10 par value per share. In connection with MedQuist
Holdings’ re-domiciliation, MedQuist Holdings adjusted the number of its shares outstanding through
a reverse share split pursuant to which every 4.5 shares of its common stock outstanding prior to
its re-domiciliation was converted into one share of MedQuist Holdings common stock upon its
re-domiciliation. MedQuist Holdings’ re-domiciliation and reverse share split resulted in no change
to its common stockholders’ relative ownership interests in MedQuist Holdings.
In February 2011, MedQuist Holdings completed its U.S. initial public offering of common stock
selling 3.0 million of its shares of common stock and 1.5 million shares of its common stock owned
by selling shareholders at an offer price of $8.00 per share, resulting in gross proceeds to
MedQuist Holdings of $24.0 million and net proceeds to MedQuist Holdings after underwriting fees of
$22.3 million. MedQuist Holdings’ common stock is listed on The NASDAQ Global Market under the
symbol “MEDH.”
Private Exchange
Certain of our noncontrolling stockholders entered into an exchange agreement, or the Exchange
Agreement, with MedQuist Holdings, whereby MedQuist Holdings issued 4.8 million shares of MedQuist
Holdings’ common stock in exchange for their 4.8 million shares of MedQuist Inc. common stock. We
refer to this transaction as the Private Exchange. The Private Exchange was completed on
February 11, 2011 and increased MedQuist Holdings’ ownership in us from 69.5% to 82.2%.
Registered Exchange Offer
In addition to the Private Exchange referred to above, in February 2011, MedQuist Holdings
commenced its public exchange offer, or Registered Exchange Offer, to those of our noncontrolling
stockholders who did not participate in the Private Exchange to exchange shares of MedQuist
Holdings’ common stock for shares of MedQuist Inc. common stock. The Registered Exchange Offer
expired on March 11, 2011. MedQuist Holdings accepted for, and consummated the exchange of, all
MedQuist Inc. shares of common stock that were validly tendered in the Registered Exchange Offer.
As a result of the Registered Exchange Offer, MedQuist Holdings increased its ownership interest in
us from 82.2% to approximately 97%.
Sale of A-Life Investment
During the three months ended December 31, 2010, we sold our approximately 32% interest in
A-Life Medical, Inc., or A-Life, an equity method investment. The consideration to us for the
sale of our A-Life investment was $23.6 million, of which $19.5 million was paid to us in cash
and $4.1 million was paid into escrow, to be released in March 2012, subject to the
satisfaction of indemnification obligations under the related merger agreement.
Available Information
All periodic and current reports, registration statements, and other filings that we are
required to file with the SEC, including our annual reports on Form 10-K, quarterly reports on Form
10-Q, current reports on Form 8-K, and amendments to those reports filed or
12
Table of Contents
furnished pursuant to
Section 13(a) of the Securities Exchange Act of 1934 (Exchange Act), are available free of charge
from the SEC’s website (www.sec.gov) or public reference room at 100 F Street N.E., Washington, DC
20549 (1-800-SEC-0330) or through our website at www.medquist.com. Such documents are available as
soon as reasonably practicable after electronic filing of the material with the SEC. Copies of
these reports (excluding exhibits) may also be obtained free of charge, upon written request to:
Investor Relations, MedQuist Inc., 9009 Carothers Parkway Franklin, TN 37067. The website addresses
included in this report are for identification purposes. The information contained therein or
connected thereto are not intended to be incorporated into this report.
Item 1A. Risk Factors
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements that are based on current expectations,
estimates, forecasts and projections about us, the industry in which we operate and other matters,
as well as management’s beliefs and assumptions and other statements regarding matters that are not
historical facts. These statements include, in particular, statements about our plans, strategies
and prospects. For example, when we use words such as “projects,” “expects,” “anticipates,”
“intends,” “plans,” “believes,” “seeks,” “estimates,” “should,” “would,” “could,” “will,”
“opportunity,” “potential” or “may,” variations of such words or other words that convey
uncertainty of future events or outcomes, we are making forward-looking statements within the
meaning of Section 27A of the Securities Act of 1933 (Securities Act) and Section 21E of the
Exchange Act. Our forward-looking statements are subject to risks and uncertainties. Actual events
or results may differ materially from the results anticipated in these forward-looking statements
as a result of a variety of factors. While it is impossible to identify all such factors, factors
that could cause actual results to differ materially from those estimated by us include:
| • | each of the factors discussed in this Item 1A, Risk Factors as well as risks discussed elsewhere in this report; | ||
| • | each of the matters discussed in Item 3, Legal Proceedings; | ||
| • | our ability to recruit and retain qualified MTs, MEs and other employees; | ||
| • | changes in law, including, without limitation, the impact HIPAA will have on our business; | ||
| • | the impact of our new services and products on the demand for our existing services and products; |
Risks related to our business
We compete with many others in the market for clinical documentation solutions which may result in
lower prices for our services, reduced operating margins and an inability to maintain or increase
our market share.
We compete with other outsourced clinical documentation solutions companies in a highly
fragmented market that includes national, regional and local service providers, as well as service
providers with global operations. These companies have services that are similar to ours, and
certain of these companies have substantially larger or have significantly greater financial
resources than we do. We also compete with the in-house medical transcription staffs of our
customers and potential customers. There can be no assurance that we will be able to compete
effectively against our competitors or timely implement new products and services. Many of our
competitors attempt to differentiate themselves by offering lower priced alternatives to our
outsourced medical transcription services and customers could elect to utilize less comprehensive
solutions than the ones we offer due to the lower costs of those competitive products. Some
competition may even be willing to accept less profitable business in order to grow revenue.
Increased competition and cost pressures affecting the healthcare markets in general may result in
lower prices for our services, reduced operating margins and the inability to maintain or increase
our market share.
Our business is dependent upon the continued demand for transcription services. If EHR companies
produce alternatives to medical transcription that reduce the need for transcription, the demand
for our solutions could be reduced.
13
Table of Contents
EHR companies’ solutions for the collection of clinical data typically require physicians to
directly enter and organize patient information through “point-and-click” templates which attempt
to reduce or eliminate the need for transcription. A second alternative to conventional
transcription involves a physician dictating a record of patient encounters and receiving a
speech-recognized draft of their dictation, which the physician can self-edit. There is significant
uncertainty and risk as to the demand for, and market acceptance of, these solutions for the
creation of electronic clinical documentation. In the event that these and other solutions are
successful and gain wide acceptance, the demand for our solutions could be reduced and our
business, financial condition and results of operations could be adversely affected.
Our growth is dependent on the willingness of new customers to outsource and adopt our technology
platforms.
We plan to grow, in part, by capitalizing on perceived market opportunities to provide our
services to new customers. These new customers must be willing to outsource functions which may
otherwise have been performed within their organizations, adopt new technologies and incur the time
and expense needed to integrate those technologies into their existing systems. For example, the
up-front cost and time involved in changing medical transcription providers or in converting from
an in-house medical transcription department to an outsourced provider may be significant. Many
customers may prefer to remain with their current provider or keep their transcription in-house
rather than invest the time and resources required for the implementation of a new system. Also, as
the maintenance of accurate medical records is a critical element of a healthcare provider’s
ability to deliver quality care to its patients and to receive proper and timely reimbursement for
the services it renders, potential customers may be reluctant to outsource or change providers of
such an important function.
Our success will depend on our ability to support existing technologies as well as to adopt and
integrate new technology into our workflow platforms.
Our ability to remain competitive in the clinical documentation industry is based, in part, on
our ability to develop, utilize and support technology in the services and solutions that we
provide to our customers. As our customers advance technologically, we must be able to effectively
integrate our solutions with their systems and provide advanced data collection technology. We also
may need to develop technologies to provide service systems comparable to those of our competitors
as they develop new technology. If we are unable to effectively develop and integrate new
technologies, we may not be able to compete effectively with our competitors. In addition, if the
cost of developing and integrating new technologies is high, we may not realize our expected return
on investment.
Technology innovations in the markets that we serve may create alternatives to our products and
result in reduced sales.
Technology innovations to which our current and potential customers might have access could
reduce or eliminate their need for our products. A new or other disruptive technology that reduces
or eliminates the use of one or more of our products could negatively impact the sale of these
products. Our failure to develop, introduce or enhance products able to compete with new
technologies in a timely manner could have an adverse effect on our business, results of operation
and financial condition.
Many of our customer contracts are terminable at will by our customers, and our ability to sustain
and grow profitable operations is dependent upon the ability to retain customers.
Many of our contracts can be terminated at will by our customers. If a significant number of
our customers were to cancel or materially change their commitments with us, we could have
significantly decreased revenue, which would harm our business, operating results and financial
condition. We must, therefore, engage in continual operational support and sales efforts to
maintain revenue stability and future growth with these customers. If a significant number of our
customers terminate or fail to renew their contracts with us, our business could be negatively
impacted if additional business is not obtained to replace the business which was lost.
Customer retention is largely dependent on providing quality service at competitive prices.
Customer retention may be impacted by events outside of our control, such as changes in customer
ownership, management, financial condition and competitors’ sales efforts. If we experience a
higher than expected rate of customer attrition the resulting loss of business could adversely
affect results of operations and financial condition.
14
Table of Contents
Our indebtedness could adversely affect our ability to raise additional capital to fund our
operations and limit our ability to pursue our growth strategy or to react to changes in the
economy or our industry, and our debt obligations include restrictive covenants which may restrict
our operations or otherwise adversely affect us.
As of December 31, 2010 we had approximately $285.0 million of indebtedness outstanding,
consisting of $200.0 million of Term Loan debt under our Senior Secured Credit Facility and
$85.0 million of Senior Subordinated Notes. We may incur additional indebtedness in the future.
For each of the years 2011 through 2014, assuming no change in our indebtedness following this
offering, we will have scheduled payment obligations of approximately $20.0 million for the
principal amount of our indebtedness. Our net interest expense for the year ended December 31,
2010 was $13.4 million. Our variable rate indebtedness bears interest at LIBOR plus 5.50% with a
LIBOR floor of 1.75%. Because the LIBOR floor is currently in effect, a 1.25% increase in LIBOR
above current LIBOR levels would not increase our effective interest rate. A 1.0% increase in the
interest rate above this floor would impact our interest expense by approximately $2.0 million.
This indebtedness could have important negative consequences to our business, including:
| n | increasing the difficulty of our ability to make payments on our outstanding debt; | ||
| n | increasing our vulnerability to general economic and industry conditions because our debt payment obligations may limit our ability to use our cash to respond to or defend against changes in the industry or the economy; | ||
| n | requiring a substantial portion of our cash flow from operations to be dedicated to the payment of principal and interest on our indebtedness, therefore reducing our ability to use our cash flow to fund our operations, capital expenditures and future business opportunities; | ||
| n | limiting our ability to obtain additional financing for working capital, capital expenditures, debt service requirements, acquisitions and general corporate or other purposes; | ||
| n | limiting our ability to pursue our growth strategy; and | ||
| n | placing us at a disadvantage compared to our competitors who are less leveraged and may be better able to use their cash flow to fund competitive responses to changing industry, market or economic conditions. |
In addition, under our debt financing agreements, we must abide by certain financial and other
restrictive covenants that, among other things, require us to maintain a minimum consolidated
interest coverage ratio, a maximum total leverage ratio and a maximum consolidated senior leverage
ratio. Upon a breach of any of the covenants in our debt financing agreements, the lenders could
declare us to be in default and could further require any outstanding borrowings to be immediately
due and payable, and terminate all commitments to extend further credit.
We are dependent on third party speech recognition software incorporated in certain of our
technologies, and the inability to maintain, support or enhance such third party software over time
could harm our business.
We license speech recognition software from third parties, both of which are competitors that
we incorporate into several of our key products and solutions. Our ability to continue to sell and
support these products and solutions depends on continued support from these licensors. If we were
to experience the loss of one of these licenses, the portion of our business that relies on this
software would be adversely affected while we transitioned it to the software provided under our
other license. If we were to experience the loss of both of these licenses at any one time, our
business would be adversely affected until we identify, license and integrate, or develop and
integrate equivalent software, which we may be unable to do. There can be no assurance that such
third party licensors will continue to invest the appropriate levels of resources in the software
to maintain and enhance the capabilities of the software and if such third party licensors do not
continue to develop their products, the development of our solutions to meet the requirements of
our customers and potential customers could be adversely affected.
15
Table of Contents
Our use of open source and third-party software could impose unanticipated conditions or
restrictions on our ability to commercialize our solutions.
We incorporate open source software into our workflow solutions platforms and other software
solutions. Open source software is accessible, usable and modifiable by anyone, provided that users
and modifiers abide by certain licensing requirements. Under certain conditions, the use of some
open source code to create derivative code may obligate us to make the resulting derivative code
available to others at no cost. The circumstances under which our use of open source code would
compel us to offer derivative code at no cost are subject to varying judicial interpretations, and
we cannot guarantee that a court would not require certain of our core technology be made available
as open source code. The use of such open source code may also ultimately require us to take
remedial action, such as replacing certain code used in our products, paying a royalty to use some
open source code, making certain proprietary source code available to others or discontinuing
certain products, any of which may divert resources away from our development efforts.
We may also find that we need to incorporate certain proprietary third-party technologies,
including software programs, into our products in the future. Licenses to relevant third-party
technologies may not be available to us on commercially reasonable terms, or at all. Therefore, we
could face delays in product releases until equivalent technology can be identified, licensed or
developed and integrated into our current products. Such delays could materially adversely affect
our business, operating results and financial condition.
If we fail to comply with contractual obligations and applicable laws and regulations governing the
handling of patient identifiable medical information, we could suffer material losses or be
adversely affected by exposure to material penalties and liabilities.
As part of the operation of our business, our customers provide us with certain patient
identifiable medical information. Although many regulatory and governmental requirements do not
directly apply to our operations, we and our hospital and other healthcare provider customers must
comply with a variety of requirements related to the handling of patient information, including
laws and regulations protecting the privacy, confidentiality and security of protected health
information, or PHI. Most of our customers are covered entities under the Health Insurance
Portability and Accountability Act of 1996, or HIPAA, and, in many of our relationships, we
function as a business associate. The provisions of HIPAA require our customers to have business
associate agreements with us under which we are required to appropriately safeguard the PHI we
create or receive on their behalf. Further, we and our customers are required to comply with HIPAA
security regulations that require us and them to implement certain administrative, physical and
technical safeguards to ensure the confidentiality, integrity and availability of electronic PHI,
or EPHI. We are required by regulation and contract to protect the security of EPHI that we create,
receive, maintain or transmit for our customers consistent with these regulations. To comply with
our regulatory and contractual obligations, we may have to reorganize processes and invest in new
technologies. We also are required to train personnel regarding HIPAA requirements. If we, or any
of our MTs, MEs or subcontractors, are unable to maintain the privacy, confidentiality and security
of the PHI that is entrusted to us, we and/or our customers could be subject to civil and criminal
fines and sanctions and we could be found to have breached our contracts with our customers.
We are bound by business associate agreements with covered entities that require us to use and
disclose PHI in a manner consistent with HIPAA in providing services to those covered entities. The
HITECH Act, which was enacted into law on February 17, 2009 as part of the American Recovery and
Reinvestment Act of 2009, or ARRA, enhances and strengthens the HIPAA privacy and security
standards and makes certain provisions applicable to “business associates” of covered entities. As
of February 17, 2010, some provisions of HIPAA apply directly to us. In addition, the HITECH Act
creates new security breach notification requirements. The direct applicability of the new HIPAA
Privacy and Security provisions will require us to incur additional costs and may restrict our
business operations. In addition, these new provisions will result in additional regulations and
guidance issued by the United States Department of Health and Human Services and will be subject to
interpretation by various courts and other governmental authorities, thus creating potentially
complex compliance issues for us and our customers.
As of February 17, 2010, we are directly subject to HIPAA’s criminal and civil penalties for
breaches of our privacy and security obligations.
16
Table of Contents
Security and privacy breaches in our systems may damage customer relations and inhibit our growth.
The uninterrupted operation of our hosted solutions and the confidentiality and security of
third-party information is critical to our business. Any failures or perceived failures in our
security and privacy measures could have a material adverse effect on our financial position and
results of operations. If we are unable to protect, or our customers perceive that we are unable to
protect, the security and privacy of our electronic information, our growth could be materially
adversely affected. A security or privacy breach may:
| n | cause our customers to lose confidence in our solutions; | ||
| n | harm our reputation; | ||
| n | expose us to liability; and | ||
| n | increase our expenses from potential remediation costs. |
While we believe that we use proven applications designed for data security and integrity to
process electronic transactions, there can be no assurance that our use of these applications will
be sufficient to address changing market conditions or the security and privacy concerns of
existing and potential customers.
Our business depends on the reliable and secure operation of our computer hardware, software,
Internet applications and data centers.
A substantial portion of our business involves the transfer of large amounts of data to and
from our workflow platforms. These workflow platforms, and their underlying technologies, are
designed to operate and to be accessible by our customers 24 hours a day, seven days a week.
Network and information systems, the Internet and other technologies are critical to our business
activities. We have periodically experienced short term outages with our workflow platforms that
have not significantly disrupted our business. However, a long term outage could adversely affect
our ability to provide service to our customers.
We also perform data center and/or hosting services for certain customers, including the
storage of critical patient and administrative data. Failure of public power and backup generators,
impairment of telecommunications lines, a “concerted denial of service cyber attack,” damage
(environmental, accidental, intentional or pandemic) to the buildings, the equipment inside the
buildings housing our data centers, the customer data contained therein and/or the personnel
trained to operate such facilities could cause a disruption in operations and negatively impact
customers who depend on us for data center and system support services. Any interruption in
operations at our data centers and/or customer support facilities could damage our reputation,
cause us to lose existing clients, hurt our ability to obtain new customers, result in revenue
loss, create potential liabilities for our customers and us and increase insurance and other
operating costs.
Recent and proposed legislation and possible negative publicity may impede our ability to utilize
offshore production capabilities.
Certain state laws that have recently been enacted and bills introduced in recent sessions of
the U.S. Congress seek to restrict the transmission of personally identifiable information
regarding a U.S. resident to any foreign affiliate, subcontractor or unaffiliated third party
without adequate privacy protections or without providing notice of the transmission and an
opportunity to opt out. Some of the proposals would require patient consent. If enacted, these
proposed laws would impose liability on healthcare businesses arising from the improper sharing or
other misuse of personally identifiable information. Some proposals would create a private civil
cause of action that would allow an injured party to recover damages sustained as a result of a
violation of the new law. A number of states have also considered, or are in the process of
considering, prohibitions or limitations on the disclosure of medical or other information to
individuals or entities located outside of the U.S. Further, as a result of concerns regarding the
possible misuse of personally identifiable information, some of our customers have contractually
limited our ability to use MTs and MEs located outside of the U.S. The effect of these proposals
would be to limit our ability to utilize our lower-cost offshore production facilities for affected
customers, which could adversely affect our operating margins.
17
Table of Contents
Any change in legislation, regulation or market practices in the United States affecting healthcare
or healthcare insurance may materially adversely affect our business and results of operations.
Over the past twenty years the U.S. healthcare industry has experienced a variety of
regulatory and market driven changes to how it is operated and funded. Further changes, whether by
government policy shift, insurance company changes or otherwise, may happen, and any such changes
may adversely affect the U.S. healthcare information and services market. As business process
outsourcing and “off-shoring” have grown in recent years, concerns have also grown about the impact
of these phenomena on jobs in the United States. These concerns could drive government policy in a
way which is disadvantageous to us. Further, if government regulation or market practices leads to
fewer individuals seeking medical treatment, we could experience a decline in our processed
volumes.
Our business, financial condition and results of operations could be adversely affected by the
political and economic conditions in India.
A significant portion of our operations are performed in India through our arrangements with
MedQuist Holdings’ wholly-owned subsidiary, CBay Systems & Services, Inc. Multiple factors
relating to our Indian operations could have a material adverse effect on our business, financial
condition and results of operations. These factors include:
| n | changes in political, regulatory, legal or economic conditions; | ||
| n | governmental actions, such as restrictions on the transfer or repatriation of funds and foreign investments; | ||
| n | civil disturbances, including terrorism or war; | ||
| n | political instability; | ||
| n | public health emergencies; | ||
| n | changes in employment practices and labor standards; | ||
| n | local business and cultural factors that differ from our customary standards and practices; and | ||
| n | changes in tax laws. |
In addition, the Indian economy may differ favorably or unfavorably from other economies in
several respects, including the growth rate of GDP, the rate of inflation, resource
self-sufficiency and balance of payments position. The Indian government has traditionally
exercised and continues to exercise a significant influence over many aspects of the Indian
economy. Further actions or changes in policy, including taxation, of the Indian central government
or the respective Indian state governments could have a significant effect on the Indian economy,
which could adversely affect private sector companies, market conditions and the success of our
operations.
We are highly dependent on certain key personnel and the loss of any or all of these key personnel
may have an adverse impact upon future performance.
Our operations and future success are dependent upon the existence and expertise in this
sector of certain key personnel. The loss of services of any of these individuals for any reason or
our inability to attract suitable replacements would have a material adverse effect on the
financial condition of our business and operations.
We have grown, and may continue to grow, through acquisitions, which could dilute existing
stockholders and could involve substantial integration risks.
As part of our business strategy, we have in the past acquired, and expect to continue to
acquire, other businesses and technologies. We may issue equity securities for future acquisitions,
which would dilute existing stockholders, perhaps significantly depending on the terms of the
acquisition. We may also incur additional debt in connection with future acquisitions, which may
place
18
Table of Contents
additional restrictions on the ability to operate the business. Furthermore, prior
acquisitions have required substantial integration and management efforts. Acquisitions involve a
number of risks, including:
| n | difficulty in integrating the operations and personnel of the acquired businesses, including different and complex accounting and financial reporting systems; | ||
| n | potential disruption of ongoing business and distraction of management; | ||
| n | potential difficulty in successfully implementing, upgrading and deploying in a timely and effective manner new operational information systems and upgrades of finance and accounting systems; | ||
| n | difficulty in incorporating acquired technology and rights into products and technology; | ||
| n | unanticipated expenses and delays in completing acquired development projects and technology integration; | ||
| n | management of geographically remote offices and operations; | ||
| n | impairment of relationships with partners and customers; | ||
| n | customers delaying purchases or seeking concessions pending resolution of integration between existing and newly acquired services or technology platforms; | ||
| n | entering markets or types of businesses in which management has limited experience; and | ||
| n | potential loss of customers or key employees of the acquired company. |
As a result of these and other risks, we may not realize anticipated benefits from
acquisitions. Any failure to achieve these benefits or failure to successfully integrate acquired
businesses and technologies could materially and adversely affect our business and results of
operations.
Our ability to use our net operating loss carryforwards may be limited.
As of December 31, 2010, we had approximately $78 million of federal net operating loss, or
NOL, carryforwards to offset future taxable income, which will begin to expire in 2026 if not
utilized, and approximately $216 million of state NOLs. Section 382 of the Internal Revenue Code of
1986, as amended, or the Code, imposes limitations on a company’s ability to use NOL carryforwards
if such company experiences a more-than-50-percent ownership change, or an ownership shift, over a
three-year testing period. We believe that, as a result of the initial public offering or as a
result of future issuances of capital stock related to our principal shareholder, MedQuist
Holdings, it is possible that such an ownership change may occur. If we experience an ownership
change, our ability to use our United States federal and state NOL carryforwards in any future
periods may be restricted. If we are limited in our ability to use our NOL carryforwards, we will
pay more taxes than if we were able to utilize such NOL carryforwards fully. As a result, any
inability to use our NOL carryforwards could adversely affect our financial condition and results
of operations.
Our largest stockholder will exercise significant control over our company.
Following
the Registered Exchange Offer, MedQuist Holdings owns approximately
97% of our
outstanding common stock. MedQuist Holdings has the ability to cause the election of all of the
members of our board of directors, the appointment of new management and the approval of actions
requiring the approval of our shareholders, including amendments to our certificate of
incorporation and mergers or sales of substantially all of our assets. The directors elected by
MedQuist Holdings will be able to make decisions affecting our capital structure, including
decisions to issue additional capital stock, implement stock repurchase programs and declare
dividends. The interests of MedQuist Holdings could conflict with our interests and the interests
of our other shareholders.
19
Table of Contents
Our ability to expand our business and properly service our customers depends on our ability to
effectively manage our domestic production capacity and our only offshore transcription labor
partner (CBay Systems & Services, Inc.), including our ability to recruit, train and retain
qualified MTs and MEs and maintain high standards of quality service in our operations, which we
may not be able to do.
Our success depends, in part, upon our ability to effectively manage our domestic production
capacity including our ability to attract and retain qualified MTs and MEs who can provide accurate
medical transcription. There is currently a shortage of qualified MTs and MEs in the U.S., and an
increasing need for more real-time turnaround of transcribed reports has created industry-wide
demand for quality MTs and MEs. As a result, competition for skilled MTs and MEs is intense. We
have active programs in place to attract domestic MTs and MEs. We must also effectively manage our
only current offshore transcription labor partner, CBay Systems & Services, Inc. However, this
strategy may not alleviate any issues caused by the shortage. Because medical transcription is a
skilled position in which experience is valuable, we require that our MTs and MEs have substantial
experience or receive substantial training before being hired. Competition may force us to increase
the compensation and benefits paid to our MTs and MEs, which could reduce our operating margins and
profitability. In addition, failure to recruit and retain qualified MTs and MEs may have an adverse
effect on our ability to service our customers, manage our production capacity and maintain our
high standards of quality service. An inability to hire and retain a sufficient number of qualified
MTs and MEs in the U.S. could have a negative impact on our ability to grow.
Shares of our common stock will be subject to different risks as a result of the Registered
Exchange Offer.
As a result of the completion of the Registered Exchange Offer, an active, liquid trading
market for our common stock no longer exists and shares of our common stock may be subject to
delisting from NASDAQ, which may adversely affect the liquidity and value of shares our common stock for an indefinite
period of time. If our common stock is delisted from NASDAQ, then equity research
coverage of our common stock may be discontinued. In addition, we may deregister under the Exchange
Act.
Section 14A:10A of the New Jersey Business Corporation Act, or the NJBCA, prohibits certain
business combinations involving New Jersey corporations and an interested stockholder. An
“interested stockholder” is defined generally as a stockholder who is the beneficial owner,
directly or indirectly, of 10% or more of the voting power of the outstanding stock of the
corporation. The NJBCA prohibits business combinations subject to the NJBCA for a period of five
years after the date the interested stockholder acquired its stock, unless the transaction was
approved by the corporation’s board of directors prior to the time the interested stockholder
acquired its shares. After the five year period expires, the prohibition on business combinations
with an interested stockholder continues unless: (i) the business combination is approved by the
board of directors of the target corporation; (ii) the business combination is approved by a vote
of two-thirds of the voting stock not owned by the interested stockholder; or (iii) the
stockholders of the corporation receive a price in accordance with a fair price formula set forth
in the NJBCA.
In August 2008, MedQuist Holdings, through its subsidiary, CBay Inc., a Delaware corporation,
acquired over 10% of the outstanding shares of MedQuist, Inc. from Royal Philips Electronics. Our
board of directors of did not approve future business combinations with MedQuist Inc. or CBay Inc.
prior to that acquisition for purposes of the provisions of NJBCA Section 14A:10A.
MedQuist Holdings may be able to utilize a short-form back-end merger through
Section 267 of the Delaware General Corporation Law, or the DGCL. Under Section 267 of the DGCL, if
(i) at least 90% of the outstanding shares of each class of stock of a corporation is owned by an
entity, (ii) one of the entities is a Delaware corporation and (ii) the entity that is not a
Delaware corporation is an entity of a state, the laws of which do not forbid such merger, the
entity having such stock ownership may either merge the entity into itself and assume all of its
obligations, or merge itself into the other entity.
In addition, Section 267 of the DGCL would permit MedQuist Holdings to merge us into MedQuist
Holdings or CBay Inc. without our shareholder approval if such merger is not forbidden by the laws
of New Jersey. Section 14A:10-7(4) of the NJBCA governs short-form mergers involving a New Jersey
corporation. This provision allows a non-New Jersey corporation owning at least 90% of the
outstanding shares of each class and series of a New Jersey corporation to merge the other
corporation into itself, or merge itself into any subsidiary corporation, without approval of the
shareholders of either corporation, though the board of the parent corporation must approve a plan
of merger. However, the New Jersey courts have not interpreted Section 14A: 10-7(4) in the context
of Section 14A:10A.
In connection with the proposed settlement of the Shareholder Litigation described in Item 3.
Legal proceedings, MedQuist Holdings has agreed with plaintiffs that MedQuist Holdings will conduct
a short-form merger under the NJBCA to acquire the remaining shares of our common stock that
MedQuist Holdings does not currently own at the same exchange ratio applicable under the Registered
Exchange Offer. The settlement of the Shareholder Litigation is conditioned upon, among other
things, execution of a final Stipulation of Settlement, notice to all class members, a fairness
hearing and final approval of the settlement by the court. The proposed settlement in this matter
will be a class settlement that will require notice to all class members, an opportunity to object
and a fairness hearing to review the terms of the settlement. There is a risk that a shareholder
could object to the settlement and the court could ultimately approve the settlement, approve it in
part and disapprove it in part, or disapprove the settlement altogether.
20
Table of Contents
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We lease 34 facilities in the U.S. and India representing approximately
677,550 square feet including our administrative headquarters for our United States operations, which is located in an approximately
48,000 square foot facility in Franklin, Tennessee and our sales, administrative and research and development office, which is
located in an approximately 19,500 square foot facility in Norcross, Georgia.
Item 3. Legal proceedings
We have been involved in a number of legal matters, including customer and stockholder issues and
regulatory investigations. Substantially all of these legal matters have been resolved. As of
December 31, 2010, one legal matter remains open related to our billing practices. The SEC is
pursuing civil litigation against our former chief financial officer, whose employment with us ended with
in July 2004. Pursuant to our by-laws, we have been providing indemnification for the legal fees of
this individual. In February 2011, we entered into a settlement agreement with our former chief financial officer and such settlement agreement is dependent on the individual settling with the SEC.
Shareholder Litigation
On February 8, 2011 and February 10, 2011, plaintiffs Victor N. Metallo and Joseph F.
Lawrence, respectively, filed purported shareholder class action complaints in the Superior Court
of New Jersey, Burlington County (Chancery Division) (the Shareholder Litigation). In their
complaints, the plaintiffs purported to be shareholders of MedQuist Inc. and sought to represent a
class of our minority shareholders in pursuit of claims against us, the members of our board of
directors and MedQuist Holdings.
Plaintiffs alleged that the defendants breached certain fiduciary duties they owed to minority
shareholders of MedQuist Inc. in connection with the structuring and disclosure of the Registered
Exchange Offer. Among other things, the plaintiffs alleged that (a) the Registered Exchange Offer
is procedurally and financially unfair, (b) the January 21, 2011 and February 16, 2011 Schedules
14D-9 that we filed with the SEC and the February 3, 2011 Prospectus that MedQuist Holdings filed
with the SEC are materially misleading and incomplete, and (c) the Registered Exchange Offer was
structured by the defendants in order to circumvent the provisions of the New Jersey Shareholders’
Protection Act. Plaintiffs sought, among other things, preliminary and permanent injunctive relief
enjoining consummation of the Registered Exchange Offer, unspecified damages, pre- and
post-judgment interest and attorneys’ fees and costs. The two Plaintiff actions were consolidated
on February 22, 2011 under the caption In Re: MedQuist Inc. Shareholder Litigation, Docket Number
C-018-11.
On March 4, 2011, the parties entered into a memorandum of understanding (the MOU) that
outlined the material terms of the Shareholder Litigation. Under the terms of the MOU, we agreed to
extend the expiration of the Registered Exchange Offer until 5:00 p.m., New York City time, on
Friday, March 11, 2011 and further agreed that if, as a result of the Registered Exchange Offer,
MedQuist Holdings obtained ownership of at least 90% of the outstanding common stock of MedQuist
Inc., MedQuist Holdings will conduct a short-form merger under applicable law to acquire the
remaining shares of MedQuist Inc. common stock that MedQuist Holdings does not currently own at
the same exchange ratio applicable under the Registered Exchange Offer. We agreed to make certain
supplemental disclosures concerning the Registered Exchange Offer, which were contained in an
amendment to Schedule 14D-9 that we filed with the SEC on March 7, 2011. The settlement of the
Shareholder Litigation is conditioned upon, among other things, execution of a Stipulation of
Settlement, notice to all class members, a fairness hearing and final approval of the settlement by
the court.
From time to time, we are involved in legal proceedings or regulatory investigations arising in the
ordinary course of our business. We are not currently a party to any material legal proceedings
that we believe would likely have a material adverse effect on our financial condition, results of
operations or cash flows.
21
Table of Contents
Table of Contents
PART II
Item 5. Market For Registrant’s Common Equity, Related Stockholder Matters And Issuer Purchases Of
Equity Securities
Our common stock began trading on the Global Market of The NASDAQ Stock Market LLC under the
ticker symbol “MEDQ” effective on July 17, 2008. Prior to that, our common stock traded on the Pink
Sheets under the symbol “MEDQ.PK”. Set forth below are the high and low closing bid quotations for
those periods our stock was traded on the Pink Sheets (as reported by the Pink Sheets LLC) and the
high and low sales prices for those periods our stock was quoted on NASDAQ (as reported by NASDAQ)
for each quarter of 2008, 2009, 2010 and the first quarter through March 14, 2011. The
over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down
or commission, and may not necessarily reflect the prices for actual transactions.
| High | Low | |||||||
2008 |
||||||||
First Quarter |
$ | 10.75 | $ | 8.05 | ||||
Second Quarter |
$ | 9.50 | $ | 6.60 | ||||
Third Quarter |
$ | 8.00 | $ | 4.35 | ||||
Fourth Quarter |
$ | 4.97 | $ | 1.92 | ||||
2009 |
||||||||
First Quarter |
$ | 2.75 | $ | 0.87 | ||||
Second Quarter |
$ | 6.88 | $ | 2.12 | ||||
Third Quarter |
$ | 9.32 | $ | 5.04 | ||||
Fourth Quarter |
$ | 7.89 | $ | 5.74 | ||||
2010 |
||||||||
First Quarter |
$ | 9.00 | $ | 6.61 | ||||
Second Quarter |
$ | 9.77 | $ | 7.46 | ||||
Third Quarter |
$ | 8.76 | $ | 7.31 | ||||
Fourth Quarter |
$ | 12.04 | $ | 8.14 | ||||
2011 |
||||||||
First Quarter (through
March 14, 2011) |
$ | 10.04 | $ | 8.19 | ||||
Holders
On
March 14, 2011, the closing price of our common stock (as reported by NASDAQ) was $8.73 and
we had 438 shareholders of record.
Dividends
In October 2010, we announced a special dividend of $4.70 per share of our common stock which
was paid on October 14, 2010. This dividend resulted in the use of $176.5 million of cash. On
September 2, 2009, we announced a dividend of $1.33 per share of our common stock which was paid on
September 15, 2009 to shareholders of record as of the close of business on September 9, 2009. This
resulted in the use of approximately $49.9 million of cash. On July 14, 2008, we announced a
dividend of $2.75 per share of our common stock which was paid on August 4, 2008 to shareholders of
record as of the close of business on July 25, 2008. This resulted in the use of approximately
$103.3 million of cash. Any future determination to pay dividends will be at the discretion of our
board of directors and will depend on our financial condition, results of operations, capital
requirements, and other factors that our board of directors may deem relevant.
23
Table of Contents
Item 6. Selected Financial Data
| For the Year Ended December 31, | ||||||||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||||||
| ($ in thousands, except per share data) | ||||||||||||||||||||
Statement of
Operations Data: |
||||||||||||||||||||
Net revenues |
$ | 375,240 | (7) | $ | 307,200 | $ | 326,853 | $ | 340,342 | $ | 358,091 | (1) | ||||||||
Net income (loss) |
$ | 31,051 | (2)(3)(4) | $ | 23,291 | (2)(3)(4) | $ | (68,795 | )(2)(4)(5) | $ | (15,206 | )(2)(4) | $ | (16,942 | )(2)(4) | |||||
Net income
(loss) per share —
Basic |
$ | 0.83 | $ | 0.62 | $ | (1.83 | ) | $ | (0.41 | ) | $ | (0.45 | ) | |||||||
Net income
(loss) per share —
Diluted |
$ | 0.83 | $ | 0.62 | $ | (1.83 | ) | $ | (0.41 | ) | $ | (0.45 | ) | |||||||
| As of December 31, | ||||||||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||||||
Balance Sheet Data: |
||||||||||||||||||||
Total assets |
$ | 323,918 | (7) | $ | 174,661 | $ | 202,205 | (5) | $ | 417,772 | $ | 441,139 | ||||||||
Total non-current liabilities |
$ | 272,508 | (7) | $ | 5,088 | $ | 2,832 | $ | 17,294 | $ | 18,492 | |||||||||
Total shareholder’s
(deficit) equity |
$ | (30,601 | )(6) | $ | 114,748 | (6) | $ | 140,352 | (6) | $ | 314,644 | $ | 327,519 | |||||||
| (1) | Reflects a reduction in net revenues of $10,402 in 2006, related to accommodation offers made to certain of our customers. | |
| (2) | In 2010, 2009, 2008, 2007, and 2006, we recorded a charge, net of insurance recoveries, of $3,603, $14,843, $19,738, $6,083, and $13,001, respectively, for costs associated with our review of certain of our historical line billings, our review of past allegations of improper billing practices, as well as legal fees, settlement costs and other costs associated with these matters. | |
| (3) | In 2010 and 2009, we recorded charges of $7,007 and $1,263, respectively, related to the Spheris acquisition and related integration charges. | |
| (4) | In 2010, 2009, 2008, 2007 and 2006, we recorded restructuring charges of $2,829, $2,727, $2,055, $2,756 and $3,422, respectively. | |
| (5) | In 2008, we recorded a goodwill impairment charge of $82,233 and recognized a deferred tax benefit of $18,470 primarily related to the reversal of deferred tax liabilities associated with indefinite life intangible assets. | |
| (6) | In 2010, 2009 and 2008, we paid cash dividends of $4.70, $1.33 and $2.75 per share, respectively, of our common stock which resulted in the use of approximately $176,513, $49,949 and $103,279, respectively, of cash. | |
| (7) | In April 2010, we acquired Spheris for $112.4 million, financed principally through debt. |
24
Table of Contents
Item 7. Management’s Discussion And Analysis Of Financial Condition And Results Of Operations
You should read the following discussion and analysis of our financial condition and
results of operations in conjunction with our audited consolidated financial statements and related
notes appearing elsewhere in this report. In addition to historical information, this discussion
and analysis contains forward-looking statements based on current expectations that involve risks,
uncertainties and assumptions, such as our plans, objectives, expectations and intentions set forth
in the “Cautionary Statement Regarding Forward-Looking Statements,” which can be found in Item 1A,
Risk Factors. Our actual results and the timing of events may differ materially from those
anticipated in these forward-looking statements as a result of various factors, including those set
forth in the “Risk Factors” section and elsewhere in this report.
Executive Overview
Overview
We are a leading provider of integrated clinical documentation solutions for the U.S.
healthcare system. Our end-to-end solutions convert physicians’ dictation of patient
interactions, or the physician narrative, into a high quality and customized electronic record.
These solutions integrate technologies and services for voice capture and transmission, ASR,
medical transcription and editing, workflow automation, and document management and distribution
to deliver a complete managed service for our customers. Our solutions enable hospitals, clinics,
and physician practices to improve the quality of clinical data as well as accelerate and
automate the documentation process, and we believe our solutions improve physician productivity
and satisfaction, enhance revenue cycle performance, and facilitate the adoption and use of
electronic health records.
Key factors affecting our performance
In 2010, we completed the acquisition of Spheris which materially impacted our financial
results. In addition our results have also been impacted by volume changes and pricing impacts as
we move to ASR and offshore production, as well as operating improvements and selling, general and
administrative expense savings leveraging from our scalable platform. These are discussed below.
Volume and pricing trends
The vast majority of our revenue is generated by providing clinical documentation services to
our customers. Medical transcription and medical editing by our MTs and MEs, respectively,
accounted for 89.5% of our net revenues in 2010. Product sales and related maintenance contracts
and other made up the balance of our net revenues. Our customers are generally charged a rate per
character multiplied by the number of characters that we process. Our transcription volume had been
declining prior to 2008, and we have been able to reverse this trend through the Spheris
acquisition, new accounts and additional work types from existing customers and decreasing our
losses of existing customers. We have reduced losses of our customers primarily by improving our
quality and improving our account management efforts.
We base our pricing on various factors, principally market forces, the extent to which we can
utilize our offshore production facilities, the extent to which customers utilize the ASR
technology available in our solutions, the scope of services provided, and turn-around times
requested by a particular customer. We work with our customers to evaluate how different solutions
affect pricing and to determine what for them is an optimal mix of service level and price. Higher
utilization of offshore production and ASR leads to lower costs for us, which permits us to offer
better pricing to our customers while at the same time contributing to margin growth. We have
successfully migrated a significant portion of our volume offshore and we will continue these
efforts.
As technological advances and increased use of offshore resources have driven down industry
costs, the average price per character has also declined as healthcare providers have sought to
participate in the economic gains. We intend to monitor and adjust our pricing accordingly to
remain competitive as these industry trends continue.
25
Table of Contents
Operating improvements
We have executed significant operational improvements since 2008. Cost of revenues on a per
unit basis has declined due to the increased percentage of volume produced offshore and the
increased utilization of ASR technology. Our use of ASR technology has increased from 47% to 76%
over the eight quarters ended December 31, 2010. We have increased our offshore production as a
percentage of our volume from 11% to 34% for the same period. As we continue to increase the use
of ASR technology and move volume offshore, we expect to continue to reduce costs.
Some of our contracts specify lower prices for work performed offshore or using speech
recognition technology. Therefore, our operating income will not increase by the full amount of
the savings we realize. Additionally, management has been reducing support staff headcount as we
shift volumes to India in order to further reduce operating costs.
Selling, general and administrative expense savings
We have made significant reductions in selling, general and administrative expenses since
2008. Such expenses were 14.5% of net revenues in 2008 compared to 9.9% of net revenues for 2010.
These savings were achieved primarily through headcount reductions and aggressive efforts to
reduce other administrative expenses.
In connection with the Spheris acquisition we have identified potential specific savings in
the sales and marketing and general and administrative areas. We also expect to consolidate
facilities in 2011. We anticipate that these savings will be implemented throughout 2011.
Significant developments in the clinical documentation industry:
Although we remain one of the leading providers of medical transcription services in the
U.S., we experience competition from many other providers at the local, regional and national
level. The medical transcription industry remains highly fragmented, with hundreds of small
companies in the U.S. performing medical transcription services.
We believe the outsourced portion of the medical transcription services market will increase
due in part to the majority of healthcare providers seeking the following:
| • | Reductions in overhead and other administrative costs; | ||
| • | Improvements in the quality and delivery speed of transcribed medical reports; | ||
| • | Access to leading technologies, such as speech recognition technology, without development and investment risk; | ||
| • | Implementation and management of a medical transcription system tailored to the providers’ specific requirements; | ||
| • | Access to skilled MTs and MEs; | ||
| • | Solutions for compliance with governmental and industry mandated privacy and security requirements; and | ||
| • | Product offerings that interface with EHR initiatives. |
We evaluate our operating results based upon the following factors:
| • | Production volumes; | ||
| • | adjusted EBITA (Adjusted earnings before interest, depreciation, amortization taxes, and other items) | ||
| • | Net cash provided by operating activities. |
26
Table of Contents
Our goal is to execute our strategy to yield growth in net revenues, operating income,
and net cash flow.
Network and information systems, the Internet and other technologies are critical to our
business activities. Substantially all of our transcription services are dependent upon the use of
network and information systems, including the use of our DEP and our license to use speech
recognition secured from a third party. If information systems including the Internet or our DEP
are disrupted, we could face a significant disruption of services provided to our customers. We
have periodically experienced short term outages with our DEP, which have not significantly
disrupted our business and we have a disaster recovery program in place for our information systems
and our DEP.
Critical Accounting Policies, Judgments and Estimates
Management’s Discussion and Analysis (MD&A) is based in part upon our consolidated financial
statements, which have been prepared in accordance with generally accepted accounting principles in
the U.S. (GAAP). We believe there are several accounting policies that are critical to
understanding our historical and future performance, as these policies affect the reported amounts
of revenues and other significant areas that involve management’s judgments and estimates. These
critical accounting policies and estimates have been discussed with our audit committee.
The preparation of our consolidated financial statements requires us to make estimates and
judgments that affect our reported amounts of assets, liabilities, expenses and related disclosure
of contingent liabilities. On an ongoing basis, we evaluate these estimates and judgments. We base
these estimates on historical experience and on various other assumptions that are believed to be
reasonable at such time, the results of which form the basis for making judgments about the
carrying values of assets and liabilities that are not readily apparent from other independent
sources. Actual results may ultimately differ from these estimates. A critical accounting estimate
must meet two criteria: (1) it requires assumptions about highly uncertain matters, and (2) there
would be a material effect on the financial statements from either using a different, although
reasonable, amount within the range of the estimate in the current period or from reasonably likely
period-to-period changes in the estimate. While there are a number of accounting policies, methods
and estimates affecting our consolidated financial statements as addressed in Note 2 to our
consolidated financial statements, areas that are particularly significant and critical include:
Valuation of Long-Lived and Other Intangible Assets and Goodwill: In connection with
acquisitions, we allocate portions of the purchase price to tangible and intangible assets,
consisting primarily of acquired technologies, and customer relationships, with the remainder
allocated to goodwill. We assess the realizability of goodwill and intangible assets with
indefinite useful lives at least annually, or sooner if events or changes in circumstances indicate
that the carrying amount may not be recoverable. We have determined that the reporting unit level
is our sole operating segment.
We review our long-lived assets, including amortizable intangibles, for impairment when events
indicate that their carrying amount may not be recoverable. When we determine that one or more
impairment indicators are present for an asset, we compare the carrying amount of the asset to net
future undiscounted cash flows that the asset is expected to generate. If the carrying amount of
the asset is greater than the net future undiscounted cash flows that the asset is expected to
generate, we then compare the fair value to the book value of the asset. If the fair value is less
than the book value, we recognize an impairment loss. The impairment loss is the excess of the
carrying amount of the asset over its fair value.
Some of the events that we consider as impairment indicators for our long-lived assets,
including goodwill, are:
| • | our net book value compared to our fair value; | ||
| • | significant adverse economic and industry trends; | ||
| • | significant decrease in the market value of the asset; | ||
| • | the extent that we use an asset or changes in the manner that we use it; | ||
| • | significant changes to the asset since we acquired it; and | ||
| • | other changes in circumstances that potentially indicate all or a portion of the company will be sold. |
27
Table of Contents
Deferred income taxes. Deferred tax assets represent future tax benefits that we expect to be
able to apply against future taxable income or that will result in future net operating losses that
we can carry forward to use against future taxable earnings. Our ability to utilize the deferred
tax assets is dependent upon our ability to generate future taxable income. To the extent that we
believe it is more likely than not that all or a portion of the deferred tax asset will not be
utilized, we record a valuation allowance against that asset. In making that determination we
consider all positive and negative evidence and give stronger consideration to evidence that is
objective in nature.
Commitments and contingencies. We routinely evaluate claims and other potential litigation to
determine if a liability should be recorded in the event it is probable that we will incur a loss
and can estimate the amount of such loss.
Revenue recognition. We recognize clinical documentation services revenues when there is
persuasive evidence that an arrangement exists, the price is fixed or determinable, services have
been rendered and collectability is reasonably assured. These services are recorded using
contracted rates and are net of estimates for customer credits. Historically, our estimates have
been adequate. If actual results are higher or lower than our estimates, we would have to adjust
our estimates and financial statements in future periods.
Accounts receivable and allowance for doubtful accounts. Accounts receivable are recorded at
the invoiced amount and do not bear interest. The carrying value of accounts receivable
approximates fair value. The allowance for doubtful accounts is our best estimate of potential
losses resulting from the inability of our customers to make required payments due. This allowance
is used to state trade receivables at estimated net realizable value.
We estimate uncollectible amounts based upon our historical write-off experience, current
customer receivable balances, aging of customer receivable balances, the customer’s financial
condition and current economic conditions. Historically, our estimates have been adequate to
provide for our accounts receivable exposure.
Customer Accommodation Program. In response to customers’ concerns regarding historical
billing matters, we established a plan to offer financial accommodations to certain of our
customers during 2005 and 2006 and recorded the related liability. In 2008 we reached an agreement
on customer litigation resolving all claims by the named parties. Since then we have not made
additional offers.
We are unable to predict how many customers, if any, may accept the outstanding accommodation
offers on the terms proposed by us, nor are we able to predict the timing of the acceptance (or
rejection) of any outstanding accommodation offers. Until any offers are accepted, we may withdraw
or modify the terms of the accommodation program or any outstanding offers at any time. In
addition, we are unable to predict how many future offers, if made, will be accepted on the terms
proposed by us. We regularly evaluate whether to proceed with, modify or withdraw the accommodation
program or any outstanding offers.
Basis of Presentation
Net revenues
We derive revenues primarily from providing clinical documentation services to integrated
delivery networks, academic centers, group practices and community hospital. Our customers are
generally charged a rate times the volume of work that we transcribe or edit. In the clinical
documentation workflow, we provide, in addition to medical transcription technology and services,
maintenance services, digital dictation, speech recognition and electronic signature services.
Net revenues from customers in the U.S. were $368.9 million, $301.1 million, and
$321.0 million for the years ended December 31, 2010, 2009 and 2008, respectively. Net revenues
from customers outside the U.S. were $6.3 million, $6.1 million, and $5.8 million for the years
ended December 31, 2010, 2009, and 2008, respectively.
Cost of revenues
Cost of revenues includes compensation of our U.S. based employee MTs and MEs and our
subcontractor MTs and MEs, other production costs (primarily related to operational and production
management, quality assurance, quality control and customer and field service personnel), and
telecommunication and facility costs. Cost of revenues also includes the direct cost of technology
products sold to customers. MT and ME costs are directly related to medical transcription and
medical editing, respectively, revenues and are based on lines transcribed or edited multiplied by
a specific rate.
28
Table of Contents
Selling, general and administrative (SG&A)
Our SG&A expenses include marketing and sales costs, accounting costs, information technology
costs, professional fees, corporate facility costs, corporate payroll and benefits expenses.
Research and development (R&D)
Our R&D expenses consist primarily of personnel and related costs, including salaries and
employee benefits for software engineers and consulting fees paid to independent consultants who
provide software engineering services to us. Our R&D efforts have been devoted to new products and
services offerings and increases in features and functionality of our existing products and
services.
Depreciation and amortization
Depreciation is calculated on a straight-line basis over the estimated useful lives of the
assets, which range from two to seven years for furniture, equipment and software, and the lesser
of the lease term or estimated useful life for leasehold improvements. Intangible assets are being
amortized using the straight-line method over their estimated useful lives which range from three
to 20 years.
Cost of legal proceedings and settlements
Cost of legal proceedings and settlements includes settlement of claims, ongoing
litigation, and associated legal and other professional fees incurred.
Consolidated Results of Operations
The following tables set forth our consolidated results of operations for the periods
indicated below:
29
Table of Contents
Comparison of Years Ended December 31, 2010 and 2009
| Years Ended December 31, | ||||||||||||||||||||||||
| 2010 | 2009 | Change in | ||||||||||||||||||||||
| % of Net | % of Net | % of Net | ||||||||||||||||||||||
| ($ in thousands) | Amount | Revenues | Amount | Revenues | $ Change | Revenues | ||||||||||||||||||
Net revenues |
$ | 375,240 | 100.0 | % | $ | 307,200 | 100.0 | % | $ | 68,040 | — | |||||||||||||
Operating costs and expenses: |
||||||||||||||||||||||||
Cost of revenues |
249,571 | 66.5 | % | 206,265 | 67.1 | % | 43,306 | (0.6 | %) | |||||||||||||||
Selling, general and administrative |
37,070 | 9.9 | % | 33,441 | 10.9 | % | 3,629 | (1.0 | %) | |||||||||||||||
Research and development |
12,813 | 3.4 | % | 9,604 | 3.1 | % | 3,209 | 0.3 | % | |||||||||||||||
Depreciation and amortization |
21,989 | 5.9 | % | 15,672 | 5.1 | % | 6,317 | 0.8 | % | |||||||||||||||
Cost of legal proceedings and settlements |
3,603 | 1.0 | % | 14,843 | 4.8 | % | (11,240 | ) | (3.8 | %) | ||||||||||||||
Acquisition and integration related charges |
7,007 | 1.9 | % | 1,263 | 0.4 | % | 5,744 | 1.5 | % | |||||||||||||||
Restructuring charges |
2,829 | 0.8 | % | 2,727 | 0.9 | % | 102 | (0.1 | %) | |||||||||||||||
Total operating costs and expenses |
334,882 | 89.2 | % | 283,815 | 92.4 | % | 51,067 | (3.2 | %) | |||||||||||||||
Operating income |
40,358 | 10.8 | % | 23,385 | 7.6 | % | 16,973 | 3.2 | % | |||||||||||||||
Gain on sale of investment |
9,911 | 2.6 | % | — | — | 9,911 | 2.6 | % | ||||||||||||||||
Equity in income of affiliated company |
693 | 0.2 | % | 2,015 | 0.7 | % | (1,322 | ) | (0.5 | %) | ||||||||||||||
Loss on extinguishment of debt |
(5,811 | ) | (1.5 | %) | — | — | (5,811 | ) | (1.5 | %) | ||||||||||||||
Interest expense, net |
(13,429 | ) | (3.6 | %) | (134 | ) | 0.0 | % | (13,295 | ) | (3.6 | %) | ||||||||||||
Income before income taxes |
31,722 | 8.5 | % | 25,266 | 8.2 | % | 6,456 | 0.3 | % | |||||||||||||||
Income tax provision |
671 | 0.2 | % | 1,975 | 0.6 | % | (1,304 | ) | (0.4 | %) | ||||||||||||||
Net income |
$ | 31,051 | 8.3 | % | $ | 23,291 | 7.6 | % | $ | 7,760 | 0.7 | % | ||||||||||||
Net revenues
Net revenues recorded for the year ended December 31, 2010 were $375.2 million, an increase of
$68.0 million or 22.2% when compared to the prior year net revenue amount of $307.2 million. The
Spheris acquisition contributed $88.1 million in incremental revenue in 2010, which was partially
offset by a decrease in legacy maintenance services revenues from $22.3 million in 2009 to $17.7
million in 2010. Current year net revenues were also unfavorably impacted by effects of lower
average pricing realized for our transcription services.
Cost of revenues
As a percentage of net revenues, cost of revenues decreased to 66.5% for the year ended
December 31, 2010 compared to 67.1% in 2009 primarily due to increased utilization of offshore
resources, increased utilization of automated speech recognition technologies, and other
operating cost reduction initiatives. The increase in total cost versus the prior year period was
primarily due to direct incremental costs associated with the Spheris acquisition as well as a
nonrecurring $1.2 million credit during 2009 related to medical claim costs.
Selling, general and administrative
SG&A expenses as a percentage of net revenues, improved to 9.9% of net revenues in 2010
compared with 10.9% for the 2009, due to synergies and other cost reduction initiatives.
30
Table of Contents
Research and development
R&D expenses as a percentage of net revenues were 3.4% for the year ended December 31, 2010
compared with 3.1% for the same period in 2009. This increase was attributable to costs associated
with the historical Spheris research and development activities partially offset by synergies
realized.
Depreciation and amortization
Depreciation and amortization expense as a percentage of net revenues was 5.9% for the year
ended December 31, 2010 compared with 5.1% for the same period in 2009. The increase was primarily
due to the amortization of acquired intangible assets associated with the Spheris acquisition.
Cost of legal proceedings and settlements
In 2010 we settled the Kaiser litigation which resulted in us recording a charge of $0.9
million. Costs in 2009 related to the Anthurium settlement of $5.9 million, related legal fees of
$3.8 million and other legal fees of $1.2 million.
| 2010 | 2009 | |||||||
Legal and professional fees |
$ | 2,693 | $ | 8,593 | ||||
Settlements |
910 | 6,250 | ||||||
Total |
$ | 3,603 | $ | 14,843 | ||||
Restructuring charges
For the years ended December 31, 2010 and 2009, we recorded restructuring charges of $2.8
million and $2.7 million for employee severance obligations. We expect that restructuring
activities will continue in 2011 as management identifies opportunities for synergies related to
the Spheris acquisition, including the elimination of redundant functions and locations.
Gain
on sale of investment
In October 2010, we sold our investment in A-Life and recognized a pre-tax gain of $9.9
million.
Loss
on extinguishment of debt
In October 2010, we refinanced our credit facilities and incurred $5.8 million in debt
extinguishment costs.
Interest expense, net
Interest expense, net increased $13.3 million to $13.4 million for the year ended December 31,
2010 compared with $0.1 million for the year ended December 31, 2009. The increase was due to the
debt incurred in connection with the Spheris acquisition and the refinancing in October 2010, which
increased our debt to $285 million.
Income tax provision
Income taxes were $0.7 million in 2010 and $2.0 million in 2009. The effective tax rates were
2.1% and 7.8% for years ended 2010 and 2009. The decrease in the 2010 effective tax rate from prior
year is due primarily to the reduction of the valuation allowance associated with foreign source
NOL’s based on management’s assessment of future earnings available to utilize these deferred tax
assets, along with the reduction in various tax reserves related to settlements in certain state
jurisdictions and the reduction in the deferred tax liability related
to the sale of our investment
in A-Life, offset by an increase in the tax reserve for various uncertain tax
positions taken in 2010. The low effective tax rate relative to statutory rates for the two
years is primarily due to the release of valuation allowances established against net operating
losses in past years that were used to offset current earnings. The 2010 tax expense includes an
increase in the deferred tax liabilities associated with indefinite life intangible assets related
to goodwill.
31
Table of Contents
Comparison of Years Ended December 31, 2009 and 2008
| Years Ended December 31, | ||||||||||||||||||||||||
| 2009 | 2008 | |||||||||||||||||||||||
| Change in | ||||||||||||||||||||||||
| % of Net | % of Net | % of Net | ||||||||||||||||||||||
| ($ in thousands) | Amount | Revenues | Amount | Revenues | $ Change | Revenues | ||||||||||||||||||
Net revenues |
$ | 307,200 | 100.0 | % | $ | 326,853 | 100.0 | % | $ | (19,653 | ) | — | ||||||||||||
Operating costs and expenses: |
||||||||||||||||||||||||
Cost of revenues |
206,265 | 67.1 | % | 230,375 | 70.5 | % | (24,110 | ) | (3.4 | %) | ||||||||||||||
Selling, general and administrative |
33,441 | 10.9 | % | 47,520 | 14.5 | % | (14,079 | ) | (3.6 | %) | ||||||||||||||
Research and development |
9,604 | 3.1 | % | 15,848 | 4.8 | % | (6,244 | ) | (1.7 | %) | ||||||||||||||
Depreciation and amortization |
15,672 | 5.1 | % | 17,504 | 5.4 | % | (1,832 | ) | (0.3 | %) | ||||||||||||||
Cost of legal proceedings and settlements |
14,843 | 4.8 | % | 19,738 | 6.0 | % | (4,895 | ) | (1.2 | %) | ||||||||||||||
Acquisition and integration related charges |
1,263 | 0.4 | % | — | — | 1,263 | 0.4 | % | ||||||||||||||||
Goodwill impairment charge |
— | — | 82,233 | 25.2 | % | (82,233 | ) | (25.2 | %) | |||||||||||||||
Restructuring charges |
2,727 | 0.9 | % | 2,055 | 0.6 | % | 672 | 0.3 | % | |||||||||||||||
Total operating costs and expenses |
283,815 | 92.4 | % | 415,273 | 127.1 | % | (131,458 | ) | (34.7 | %) | ||||||||||||||
Operating income (loss) |
23,385 | 7.6 | % | (88,420 | ) | (27.1 | %) | 111,805 | 34.7 | % | ||||||||||||||
Equity in income of affiliated company |
2,015 | 0.7 | % | 236 | 0.1 | % | 1,779 | 0.6 | % | |||||||||||||||
Other income |
— | — | 438 | 0.1 | % | (438 | ) | (0.1 | %) | |||||||||||||||
Interest income (expense), net |
(134 | ) | 0.0 | % | 2,438 | 0.7 | % | (2,572 | ) | (0.7 | %) | |||||||||||||
Income (loss) before income taxes |
25,266 | 8.2 | % | (85,308 | ) | (26.1 | %) | 110,574 | 34.3 | % | ||||||||||||||
Income tax provision (benefit) |
1,975 | 0.6 | % | (16,513 | ) | (5.1 | %) | 18,488 | 5.7 | % | ||||||||||||||
Net income (loss) |
$ | 23,291 | 7.6 | % | $ | (68,795 | ) | (21.0 | %) | $ | 92,086 | 28.6 | % | |||||||||||
Net revenues
Net revenues recorded for the year ended December 31, 2009 were $307.2 million, a decline of
$19.7 million or 6.0% when compared to the prior year net revenue amount of $326.9 million. The
revenue decline was primarily due to:
| • | lower prices for our transcription service related revenues, net of revenues realized from higher year over year transcription volume; | ||
| • | declining product and legacy maintenance revenues totaling $6.8 million largely related to customers not renewing maintenance contracts for legacy systems. |
Pricing for our transcription services remains under pressure as many customers seek to reduce
their costs by using more offshore labor and increasing productivity with expanded use of speech
recognition.
Cost of revenues
Cost of revenues as a percentage of net revenues improved to 67.1% in 2009, compared to 70.5%
in 2008. The improvement was attributable to the following:
| • | staffing reductions that reduced costs by $7.6 million resulting from restructuring actions taken by management to align our operating costs to better compete in a lower price environment for our services; | ||
| • | a $13.5 million reduction in medical transcription costs related to our increased use of speech recognition technology, and our increased use of offshore labor to supplement our domestic workforce capacity; | ||
| • | lower product costs of $1.5 million as a result of lower product and maintenance related sales and services; | ||
| • | other reductions of $0.3 million, net; and | ||
| • | a $1.2 million reversal of an accrual due to the lapsing of the statute of limitations. |
32
Table of Contents
Selling, general and administrative
SG&A expenses in total as a percentage of net revenues were 10.9% for the year ended December
31, 2009 compared with 14.5% for in the year 2008. This improvement was the result of company-wide
cost reduction initiatives that included a $3.7 million decrease in compensation costs due to
reductions in workforce and a $6.6 million decrease in legal and other professional fees. The
remaining cost savings covered employee stock option related expenses of $0.8 million, a reduction
in employee retention costs resulting from the change in control of our majority shareholder of
$0.5 million, lower advertising and marketing costs of $0.6 million, lower rent expenses of $0.4
million, reduced bad debt expense of $0.4 million, lower travel and entertainment of $0.3 million,
and a decrease in all other SG&A categories of $0.8 million.
Research and development
R&D expenses as a percentage of net revenues were 3.1% for the year ended December 31, 2009
compared with 4.8% for the same period in 2008. This improvement was attributable to reduced
compensation expense of $3.3 million; a decrease in consulting expense of $1.1 million; an increase
in the amount capitalized for software development of $0.6 million; a decrease of $0.4 million in
stock option compensation as a result of immediate vesting of previously unvested stock options due
to the change in control of our majority shareholder; a decrease in retention bonus for certain key
employees during the change in control of $0.4 million; and a decrease in all other R&D expenses of
$0.4 million.
Depreciation and amortization
Depreciation and amortization expense as a percentage of net revenues was 5.1% for the year
ended December 31, 2009 compared with 5.4% for the same period in 2008. The higher expenses in
2009 were the result of software capitalization in 2009 and 2008.
Cost
of legal proceedings and settlements
The 2009 settlements related to the Anthurium litigation and reseller arbitration. For the
year ended December 31, 2008, legal and professional fees were $12.3 million and settlement costs
were $7.4 million. The settlement was for the Department of Justice investigation related to our
historical billing practices.
| 2009 | 2008 | |||||||
Legal and professional fees |
$ | 8,593 | $ | 12,313 | ||||
Settlements |
6,250 | 7,425 | ||||||
Total |
$ | 14,843 | $ | 19,738 | ||||
Goodwill
impairment charge
In
2008 we recorded a goodwill impairment charge of $82.2 million. In 2009 the fair value substantially exceeded carrying value.
Restructuring charges
For the year ended December 31, 2009, restructuring charges included $2.4 million for employee
related severance obligations and $0.3 million for non-cancelable leases related to the closure or
consolidation of offices. For the year ended December 31, 2008, we had a restructuring charge of
$2.1 million for employee severance obligations.
Interest income (expense), net
Interest income (expense), net decreased $2.6 million, or 105.5%, to ($0.1) million for the
year ended December 31, 2009 compared with $2.4 million for the year ended December 31, 2008. This
decrease was due to the amortization of deferred financing costs and lower interest rates in the
2009 period as compared to 2008, and lower average cash balance in the 2009 period ($37.3 million)
as compared to 2008 ($110.5 million).
33
Table of Contents
Income tax provision (benefit)
The effective income tax rate for the year ended December 31, 2009 was 7.8% compared with an
effective income tax benefit rate of 19.4% for the year ended December 31, 2008. The 2009 tax
expense includes an increase in the deferred tax liabilities associated with indefinite life
intangible assets related to goodwill and an increase in the deferred tax liability associated with
our investment in A-Life. After consideration of all evidence, both positive and negative,
management concluded again in 2009, that it was more likely than not that a significant portion of
the domestic deferred income tax assets would not be realized. In addition, various adjustments
were recorded for the year ended December 31, 2009 including the reduction of the foreign valuation
allowance and various adjustments related to state tax exposures.
Liquidity and Capital Resources
Our principal sources of liquidity include cash generated from operations, available cash on
hand, and availability under our Senior Secured Credit Facility, as described below.
Available cash at December 31, 2010 was $41.3 million compared to $25.2 million at December
31, 2009. During 2010, we received $100.0 million in cash inflow from our Acquisition Credit
Facility which was utilized to fund the Spheris acquisition. In October 2010 we refinanced our
debt and we received $285.0 million in cash inflow which was used to pay off $93.6 million in
debt, and pay a dividend of $4.70 per share ($176.5 million). Additionally, several other items
impacted cash flows for the year ended December 31, 2010, resulting in a net increase of $16.0
million, including:
| • | addition of cash flows provided by Spheris operations; | ||
| • | cash used to pay financing costs associated with the Acquisition Credit Facility (as defined below); | ||
| • | cash payments related to debt | ||
| • | acquisition-related charges associated with the Spheris acquisition; | ||
| • | cash received from the sale of A-Life | ||
| • | restructuring payments; and | ||
| • | other working capital changes. |
We believe our existing cash, cash equivalents, cash to be generated from operations and
available borrowings under our revolving credit facility will be sufficient to finance our
operations for the next twelve months. However, if we fail to generate adequate cash flows from
operations in the future, due to an unexpected decline in our net revenues, or due to increased
cash expenditures in excess of the net revenues generated, then our cash balances may not be
sufficient to fund our continuing operations without obtaining additional debt or equity. There
are no assurances that sufficient funding from external sources will be available to us on
acceptable terms, if at all.
Prior to the Corporate Refinancing in October 2010
| In connection with the Spheris acquisition, in April 2010, we and certain of our subsidiaries entered into a credit agreement, or the Acquisition Credit Facility, with General Electric Capital Corporation, CapitalSource Bank, and Fifth Third Bank. The Acquisition Credit Facility provided for up to $100.0 million in senior secured credit facilities, consisting of a $50.0 million term loan, and a revolving credit facility of up to $50.0 million. The credit facilities were secured by a first priority lien on substantially all of the property of the borrowers. Borrowings under the revolving credit facility were able to be made from time to time, subject to availability under such facility, until the fourth anniversary of the closing date. Amounts borrowed under the Acquisition Credit Facility bore interest at a rate we selected equal to the Base Rate or the Eurodollar Rate (each as defined in the Acquisition Credit Facility agreement) plus a margin. The Acquisition Credit Facility was repaid in full on October 14, 2010. |
In connection with the Spheris acquisition, we also issued a promissory note, or the
Acquisition Subordinated Promissory Note, to Spheris. The Acquisition Promissory Note was to
mature in five years from the date of the Spheris acquisition. The Acquisition Subordinated
Promissory Note had a principal amount of $17.5 million with provisions for prepayment at
discounted amounts, ranging from 77.5% of the principal if paid within six months, 87.5% from six
to nine months, 97.5% from nine to twelve months, 102.0% between the first and second year, 101.0%
between the second and third
34
Table of Contents
year and 100.0% thereafter. The Acquisition Subordinated Promissory
Note bore interest at 8.0% for the first six months. The Acquisition Subordinated Promissory Note
was repaid at 77.5% of the face amount on October 14, 2010 in connection with our October 2010
refinancing described below.
Subsequent to the Corporate Refinancing in October 2010
In October 2010, we, MedQuist Transcriptions, Ltd., our wholly owned subsidiary, and MedQuist
Holdings, as co-borrowers and guarantors, entered into the Senior Secured
Credit Facility, with General Electric Capital Corporation, as administrative agent, and the
parties thereto, consisting of (i) a $200.0 million term loan and (ii) a $25.0 million revolving
credit facility. The Senior Secured Credit Facility is secured by a first priority lien on
substantially all existing and after-acquired property of the borrowers and the guarantors. The
term loan is repayable in equal quarterly installments of $5.0 million commencing on the first
fiscal quarter after the closing date, with the balance payable 5 years from the closing date. The
term loan interest rate is LIBOR plus 5.50% with a LIBOR floor of 1.75% and is payable monthly.
We may structure borrowing using the prime rate in the United States
or as Eurodollar loans based upon LIBOR .
Currently, the LIBOR floor is in effect. We may prepay the term loan without significant
penalties. Mandatory prepayments are required when we generate excess cash flows as defined under
the Senior Secured Credit Facility. Under the Senior Secured Credit Facility, we are required to
maintain (i) a minimum consolidated interest coverage ratio, initially, of 2.75x and increasing
over the term of the facility to 4.00x, (ii) a maximum total leverage ratio, initially of 4.00x
and declining over the term of the facility to 1.50x and (iii) a maximum consolidated senior
leverage ratio, initially of 3.00x and declining over the term of the facility to 1.00x. At
December 31, 2010 the interest rate on the term loan was 7.25%.
In addition to the Senior Secured Credit Facility, in September 2010, we, as issuer, MedQuist
Transcriptions, Ltd. and MedQuist Holdings as co-issuers and guarantors, and certain of our other
subsidiaries, as guarantors, entered into a Note Purchase Agreement, or the Senior Subordinated
Notes, for the issuance of $85.0 million aggregate principal amount of 13% Senior Subordinated
Notes due 2016 to BlackRock Kelso Capital Corporation, PennantPark Investment Corporation,
Citibank, N.A., and THL Credit, Inc. Interest on the notes is payable in quarterly installments at
the issuers’ option at either (i) 13% in cash or (ii) 12% in cash plus 2% in the form of
additional senior subordinated notes.
Closing and funding of the Senior Secured Credit Facility and the Senior Subordinated Notes
occurred on October 14, 2010.
Proceeds from the Senior Secured Credit Facility and the Senior Subordinated Notes were used
to repay the Acquisition Credit Facility in full, to pay the Acquisition Subordinated Promissory
Note in full and to pay a $176.5 million special dividend to our shareholders.
Operating activities
Cash flow provided by operating activities was $34.0 million for the year ended December 31,
2010 and $44.5 million for the same period in 2009. In 2008 cash used for operating activities was
$8.8 million. Net income was $31.1 million in 2010 and $23.3
million in 2009 and a net loss of $68.8 million in 2008. The
significant non-cash
adjustments to reconcile net income to cash provided by operating activities include $22.0
million, $15.7 million, and $17.5 million of depreciation and amortization in 2010, 2009, and
2008, respectively; $5.8 million non-cash loss on debt extinguishment in 2010, gain on sale and
equity in income of affiliated company (A-Life) of $10.6 million
and $2.0 million in 2010 and 2009,
respectively; non-cash interest expense of $4.1 million in 2010, and a non-cash impairment
charge of $82.2 million in 2008. Deferred taxes were a benefit of $0.2 million in 2010, a charge
of $1.9 million in 2009 and a benefit of $17.1 million in 2008.
Working capital changes that impacted cash flow from operations in 2010 included (a) $11.4
million higher accounts receivable balance due to the timing of collections, (b) $5.1 million
lower accrued compensation balance due to a change in timing of payroll payments, and (c) $3.0
million reduction in accrued expenses including a $4.6 million payment to a related party under
the acquisition, $2.0 million settlement of Kaiser litigation, additional expenditures related to
the acquisition, offset by an increase in interest accrued of $5.6 million.
35
Table of Contents
Investing activities
Cash used in investing activities was $90.2 million and $8.4 million in 2010 and 2009,
respectively. In 2010 $98.7 million of cash was used for the Spheris acquisition, $11.0 million
for capital spending and capitalized software offset by $19.5 million in proceeds related to the
sale of our investment in A-Life. During 2009 we spent $7.5 million for capital spending and
capitalized software and $0.9 million as an additional investment in A-Life. In 2008 investments
of $9.2 million were made for property and equipment and capitalized software.
Financing activities
Cash provided by financing activities in 2010 included $385.0 million in borrowings, offset
by $113.6 million in debt repayments, a use of $176.5 million for dividends, and $21.6 million
used for debt issuance costs. In 2009, cash used in financing activities were principally due to
$49.9 million of dividends paid. In 2008 we paid a dividend of $103.3 million.
Off-Balance Sheet Arrangements
We are not involved in any off-balance sheet arrangements that have or are reasonably likely
to have a current or future effect on our financial condition, changes in financial condition,
revenues or expenses, results of operations, liquidity, capital expenditures or capital resources
that is material to investors except as follows. In 2010 we sold our investment in A-Life and the
amounts held in escrow through April 2012, $4.1 million, have not been recorded on our balance
sheet.
Quantitative and qualitative disclosures about market risk
Market risk represents the risk of loss that may impact our financial position due to adverse
changes in financial market prices and rates. Our market risk exposure is primarily a result of
fluctuations in interest rates. We do not hold or issue financial instruments for trading
purposes.
Contractual Obligations
The following table summarizes our obligations to make future payments under current
contracts as of December 31, 2010 (in thousands):
| Payment Due By Period | ||||||||||||||||||||
| Less | ||||||||||||||||||||
| than | After | |||||||||||||||||||
| Total | 1 Year | 1-3 Years | 3-5 Years | 5 Years | ||||||||||||||||
Operating Lease Obligations |
$ | 19,023 | $ | 4,947 | $ | 9,345 | $ | 3,586 | $ | 1,145 | ||||||||||
Purchase Obligations(1) |
21,311 | 8,567 | 11,820 | 924 | — | |||||||||||||||
Severance and Other Guaranteed
Payment Obligations |
3,944 | 3,207 | 471 | 266 | — | |||||||||||||||
Long Term Debt including
current maturities |
285,000 | 20,000 | 40,000 | 140,000 | 85,000 | |||||||||||||||
Total Contractual Obligations |
$ | 329,278 | $ | 36,721 | $ | 61,636 | $ | 144,776 | $ | 86,145 | ||||||||||
| (1) | Purchase obligations are for ASR agreements ($12,150), telecommunication contracts ($8,636), software development ($275) and other recurring purchase obligations ($250). |
We have agreements with certain of our named executive officers that provide for
severance payments to the employee in the event the employee is terminated without cause. The
maximum cash exposure under these agreements was approximately $2.5 million as of December 31,
2010.
36
Table of Contents
Recent Accounting Pronouncements
In September 2009, the Financial Accounting Standards Board ratified two consensuses affecting
revenue recognition:
The first consensus, Revenue Recognition—Multiple-Element Arrangements, sets forth
requirements that must be met for an entity to recognize revenue from the sale of a delivered item
that is part of a multiple-element arrangement when other items have not yet been delivered. One of
those current requirements is that there be objective and reliable evidence of the standalone
selling price of the undelivered items, which must be supported by either vendor-specific objective
evidence (VSOE) or third-party evidence (TPE).
This consensus eliminates the requirement that all undelivered elements have VSOE or TPE
before an entity can recognize the portion of an overall arrangement fee that is attributable to
items that already have been delivered. In the absence of VSOE or TPE of the standalone selling
price for one or more delivered or undelivered elements in a multiple-element arrangement, entities
will be required to estimate the selling prices of those elements. The overall arrangement fee will
be allocated to each element (both delivered and undelivered items) based on their relative selling
prices, regardless of whether those selling prices are evidenced by VSOE or TPE or are based on the
entity’s estimated selling price. Application of the “residual method” of allocating an overall
arrangement fee between delivered and undelivered elements will no longer be permitted.
The second consensus, Software-Revenue Recognition addresses the accounting for transaction
involving software to exclude from its scope tangible products that contain both software and
non-software and not-software components that function together to deliver a products
functionality.
The Consensuses are effective prospectively for revenue arrangements entered into or
materially modified in fiscal years beginning on or after June 15, 2010. The standards were adopted
in the first quarter of 2011 and did not have a material impact on our results of operations or our
financial position.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Market risk represents the risk of loss that may impact our financial position due to adverse
changes in financial market prices and rates. Our market risk exposure is primarily a result of
fluctuations in interest rates. We do not hold or issue financial instruments for trading purposes.
Interest rate sensitivity
We earn interest income from our balances of cash and cash equivalents. This interest income
is subject to market risk related to changes in interest rates, which affects primarily our
investment portfolio. We invest in instruments that meet high credit quality standards, as
specified in our investment policy.
The Term Loan of our Senior Secured Credit Facility bears interest at LIBOR plus 5.50% with a
LIBOR floor of 1.75%. Our interest expense associated with this loan will increase if LIBOR
increases. Because the LIBOR floor is currently in effect, a 1.25% increase in LIBOR above current
LIBOR levels would not increase our effective interest rate. A 1% increase in LIBOR would result
in an approximate $2.0 million annual increase in our interest expense.
In January 2011, as required under our Credit Agreement, we entered into Interest Rate Cap
Contracts (for $60.0 million notional amounts which will amortize over time) to provide coverage
for fluctuation in interest rates.
Item 8. Financial Statements And Supplementary Data
Our consolidated financial statements and supplementary data required by this item are
attached to this report beginning on page F-1.
Item 9. Changes in And Disagreements With Accountants On Accounting And Financial Disclosure
None.
37
Table of Contents
Item 9A. Controls And Procedures
(a) Evaluation of Disclosure Controls and Procedures
Our management team, under the supervision and with the participation of our principal
executive officer and our principal financial officer, evaluated the effectiveness of the design
and operation of our disclosure controls and procedures as such term is defined under Rule
13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended (Exchange Act), as of
the last day of the fiscal period covered by this report, December 31, 2010. The term disclosure
controls and procedures means our controls and other procedures that are designed to ensure that
information required to be disclosed by us in the reports that we file or submit under the Exchange
Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s
rules and forms. Disclosure controls and procedures include, without limitation, controls and
procedures designed to ensure that information required to be disclosed by us in the reports that
we file or submit under the Exchange Act is accumulated and communicated to management, including
our principal executive and principal financial officer, or persons performing similar functions,
as appropriate to allow timely decisions regarding required disclosure. Based on this evaluation,
our principal executive officer and our principal financial officer concluded that, our disclosure
controls and procedures were effective as of December 31, 2010.
(b) Management’s Report on Internal Control over Financial Reporting as of December 31, 2010
Management is responsible for establishing and maintaining adequate internal control over
financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and
Rule 15d-15(f) under the Exchange Act as a process designed by, or under the supervision of, the
issuer’s principal executive and principal financial officers, or persons performing similar
functions, and effected by the issuer’s board of directors, management and other personnel, to
provide reasonable assurance regarding the reliability of financial reporting and the preparation
of financial statements for external purposes in accordance with GAAP and includes those policies
and procedures that:
| • | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the issuer; | ||
| • | provide reasonable assurance that transactions are recorded as necessary to permit the preparation of financial statements in accordance with GAAP, and that receipts and expenditures of the issuer are being made only in accordance with authorizations of management and directors of the issuer; and | ||
| • | provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use or disposition of the issuer’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent
or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are
subject to the risk that controls may become inadequate because of changes in conditions or that
the degree of compliance with existing policies or procedures may deteriorate.
In accordance with the internal control reporting requirements of the SEC, management
completed an assessment of the adequacy of our internal control over financial reporting as of
December 31, 2010. In making this assessment, management used the criteria set
forth in Internal Control — Integrated Framework by the Committee of Sponsoring Organizations
of the Treadway Commission (COSO). As a result of this assessment and based on the criteria in the
COSO framework, management has concluded that, as of December 31, 2010, our internal control over
financial reporting was effective. Our independent registered public accounting firm has issued an
audit report on the effectiveness of our internal control over financial reporting as of December
31, 2010. This report is included on page F-2 of our consolidated financial statements included as
part of this Annual Report on Form 10-K.
(c) Changes in Internal Control Over Financial Reporting
There have been no changes in internal control over financial reporting for the quarter ended
December 31, 2010 that have materially affected, or are reasonably likely to materially affect, our
internal control over financial reporting.
Item 9B. Other Information
None.
38
Table of Contents
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information called for by this item is incorporated by reference to the portions of our
definitive proxy statement entitled, “Election of Directors,” “Governance of the Company,” “Report
of the Audit Committee” and “Section 16 (a) Beneficial Ownership Reporting Compliance.”
Item 11. Executive Compensation
The information called for by this item is incorporated by reference to the portions of our
definitive proxy statement entitled “Compensation Discussion and Analysis,” “Report of the
Compensation Committee,” and “Compensation of our Named Executive Officers.”
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder
Matters
The information called for by this item is incorporated by reference to the portions of our
definitive proxy statement entitled “Stock Ownership of Our Directors, Executive Officers and 5%
Beneficial Owners,” “Compensation of Directors,” “Securities Authorized For Issuance Under Equity
Compensation Plans” and “Compensation of our Named Executive Officers.”
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information called for by this item is incorporated by reference to the portion of our
definitive proxy statement entitled “Certain Relationships and Related Transactions.”
Item 14. Principal Accountant Fees and Services
The information called for by this item is incorporated by reference to the portion of our
definitive proxy statement entitled “Fees Paid to Independent Registered Public Accounting Firm.”
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a) Documents filed as part of this report:
(1) Financial Statements. The consolidated financial statements filed as part of this report
are listed on the Index to Consolidated Financial Statements on page F-1.
(2) Financial Statement Schedules. All financial statement schedules have been omitted here
because they are not applicable, not required, or the information is shown in the consolidated
financial statements or notes thereto.
(3) Exhibits. See (b) below.
(b) Exhibits:
| No. | Description | |
3.1(1)
|
Certificate of Incorporation of MedQuist Inc. (as amended) | |
3.2(6)
|
Second Amended and Restated By-Laws, as amended, of MedQuist Inc. | |
4.1(1)
|
Specimen Stock Certificate | |
10.1*(1)
|
1992 Stock Option Plan of MedQuist Inc., as amended | |
10.2*(1)
|
Nonstatutory Stock Option Plan for Non-Employee Directors of MedQuist Inc. |
39
Table of Contents
| No. | Description | |
10.3*(1)
|
MedQuist Inc. 2002 Stock Option Plan | |
10.4*(1)
|
Form of Award Agreement under the MedQuist Inc. 2002 Stock Option Plan | |
10.5*(1)
|
1996 Employee Stock Purchase Plan | |
10.6*(1)
|
MedQuist Inc. Executive Deferred Compensation Plan | |
10.7*(1)
|
Letter Agreement, dated as of April 21, 2005, between MedQuist Inc. and Michael Clark | |
10.8*(1)
|
Letter Agreement, dated as of April 21, 2005, between MedQuist Inc. and Mark Sullivan | |
10.10*(1)
|
Letter Agreement, dated as of November 10, 2006, by and between MedQuist Inc. and James Brennan | |
10.11(1)
|
Licensing Agreement, dated as of May 22, 2000, between MedQuist Inc. and Philips Speech Processing GmbH | |
10.11.1(1)
|
Amendment No. 1 to Licensing Agreement, dated as of January 1, 2002, between MedQuist Inc. and Philips Speech Processing GmbH | |
10.11.2#(1)
|
Amendment No. 2 to Licensing Agreement, dated as of December 10, 2002, between MedQuist Inc. and Philips Speech Processing GmbH | |
10.11.3#(1)
|
Amendment No. 3 to Licensing Agreement, dated as of August 10, 2003, between MedQuist Inc. and Philips Speech Processing GmbH | |
10.11.4#(1)
|
Amendment No. 4 to Licensing Agreement, dated as of September 1, 2004, between MedQuist Inc. and Philips Speech Processing GmbH | |
10.11.5#(1)
|
Amendment No. 5 to Licensing Agreement, dated as of December 30, 2005, between MedQuist Transcriptions, Ltd. and Philips Speech Recognition Systems GmbH f/k/a Philips Speech Processing GmbH | |
10.11.6#(1)
|
Amendment No. 6 to Licensing Agreement, dated as of February 13, 2007, between MedQuist Inc. and Philips Speech Recognition Systems GmbH f/k/a Philips Speech Processing GmbH | |
10.11.7(17)
|
Amendment No. 7 to Licensing Agreement, dated as of November 10, 2009, between MedQuist Inc. and Nuance Communications, Inc. as the successor-in-interest to Philips Speech Recognition Systems GmbH | |
10.12##(17)
|
Third Amended and Restated OEM Supply Agreement dated November 10, 2009, between MedQuist Inc. and Nuance Communications, Inc. as the successor-in-interest to Philips Speech Recognition Systems GmbH | |
10.13*(1)
|
MedQuist Inc. Board of Directors Deferred Compensation Plan | |
10.14*(2)
|
Form of Management Indemnification Agreement by and between MedQuist Inc. and Certain Officers | |
10.14.1*(7)
|
First Amendment to the Form of Management Indemnification Agreement by and between MedQuist Inc. and Certain Officers | |
10.15*(4)
|
Indemnification Agreement, dated as of February 21, 2008 between MedQuist Inc. and Warren Pinckert | |
10.16*(8)
|
Employment Agreement by and between Peter Masanotti and MedQuist Inc., dated September 3, 2008 | |
10.17#(9)
|
Transcription Services Agreement by and between MedQuist Transcriptions, Ltd. and CBay Systems & Services, Inc. dated April 3, 2009 |
40
Table of Contents
| No. | Description | |
10.18*(10)
|
Indemnification Agreement dated November 21, 2008 between MedQuist Inc. and Peter Masanotti | |
10.19*(11)
|
Amended and Restated Stock Option Agreement by and between Peter Masanotti and MedQuist Inc., dated March 2, 2009 | |
10.21*(12)
|
Employment Agreement by and between Dominick Golio and MedQuist Inc. dated April 9, 2009 | |
10.22*(9)
|
Employment Agreement by and between Kevin Piltz and MedQuist Inc. dated May 18, 2009 | |
10.23(9)
|
Settlement and License Agreement by and between Anthurium Solutions, Inc. and MedQuist Inc. dated June 19, 2009 | |
10.24*(13)
|
MedQuist Inc. Long-Term Incentive Plan adopted on August 27, 2009 | |
10.25(13)
|
Credit Agreement by and among MedQuist Inc. and its subsidiaries, and Wells Fargo Foothill, LLC as the arranger and administrative agent and lender dated August 31, 2009 | |
10.26(14)
|
Services Agreement by and between MedQuist Inc. and CBay Inc. dated September 19, 2009 | |
10.27##(17)
|
Licensing Agreement by and between Nuance Communications, Inc. and MedQuist Inc. dated November 10, 2009 | |
10.28(15)
|
Transcription Services Subcontracting Agreement by and between MedQuist Inc. and CBay Systems & Services, Inc. dated March 31, 2009 | |
10.29(19)
|
Credit Agreement dated as April 22, 2010 among MedQuist Transcriptions, Ltd. as Borrower, MedQuist Inc. as Holdings, the Lenders and L/C Issuers party thereto, and General Electric Capital Corporation as Administrative Agent and Collateral Agent, CapitalSource Bank as Syndication Agent, and Fifth Third Bank as Documentation Agent. | |
10.30(20)
|
MedQuist Transcriptions, Ltd. Subordinated Promissory Note dated April 22, 2010 | |
10.31##(20)
|
Sales & Services Agreement dated March 9, 2010 between MedQuist, Inc. and CBay Systems & Services, Inc. | |
10.32(21)
|
Employment Agreement between Anthony D. James and MedQuist, Inc. for the position of Co-Chief Operating Officer dated June 24, 2010 | |
10.33##(24)
|
Amendment No. 1 to the Sales and Services Agreement, dated July 26, 2010 between MedQuist, Inc. and CBay Systems & Services, Inc. | |
10.34##(24)
|
Amendment No. 1 to the Subcontracting Agreement dated July 26, 2010, between MedQuist, Inc. and CBay Systems & Services, Inc. | |
10.35(22)
|
Settlement Agreement and Release dated August 12, 2010 between MedQuist Inc. and Kaiser Foundation Health Plan, Inc. |
41
Table of Contents
| No. | Description | |
10.36(24)
|
First Amendment to Norcross, Georgia Office Lease Agreement dated as of March 1, 2009 | |
10.37(24)
|
Second Amendment to Norcross, Georgia Office Lease Agreement dated as of August 1, 2009 | |
10.38(23)
|
Senior Subordinated Note Purchase Agreement, dated September 30, 2010, between CBay Inc., CBay, the Company, MedQuist Transcriptions, Ltd., Blackrock Kelso Capital Corporation, PennantPark Investment Corporation, Citibank, N.A. and THL Credit, Inc. as Purchasers. | |
10.39(23)
|
Credit Agreement, dated October 1, 2010, between CBay Inc., the Company and MedQuist Transcriptions, Ltd., CBay, the lenders and L/C issuers, General Electric Capital Corporation, as administrative agent and collateral agent | |
10.40(25)
|
Appointed Anthony D. James as Chief Financial Officer of MedQuist Inc., dated November 22, 2010 | |
10.41(26)
|
Licensing Agreement of Trade Name, dated as of Nov. 23, 2010, between MedQuist Inc. and CBaySystems Holdings Limited | |
10.42.1
|
Office Lease, dated June 2006, between Ford Motor Land Development Corporation and Spheris Operations Inc. | |
10.42.2
|
Amendment to Office Lease Agreement, dated March 27, 2009, between Carothers Office Acquisition LLC and Spheris Operations, Inc. | |
10.42.3
|
Assignment, Assumption and Agreement to Relinquish Office Space and Amendment to Office Lease Agreement, dated April 22, 2010 between Carothers Office Acquisition LLC and MedQuist Transcriptions, Ltd. | |
10.43(24)
|
First Amendment to Lease Agreement, dated March 1, 2009, by and between Atlanta Lakeside Real Estate, L.P. and MedQuist Transcriptions, Ltd. | |
10.44(24)
|
Second Amendment to Lease Agreement, effective August 1, 2009, by and between Atlanta Lakeside Real Estate, L.P. and MedQuist Transcriptions, Ltd. | |
21(1)
|
Subsidiaries of MedQuist Inc. | |
23
|
Consent of KPMG LLP | |
24
|
Power of Attorney (included on the signature page hereto) | |
31.1
|
Certification of Chief Executive Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
31.2
|
Certification of Chief Financial Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
32.1
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
32.2
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| * | Management contract or compensatory plan or arrangement. |
42
Table of Contents
| # | Portions of this Exhibit were omitted and filed separately with the Secretary of the SEC pursuant to an order for confidential treatment from SEC. | |
| ## | Portions of this Exhibit were omitted and filed separately with the Secretary of the SEC pursuant to a request for confidential treatment that has been filed with the SEC. | |
| (1) | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2005 filed on July 5, 2007 | |
| (2) | Incorporated by reference to our Current Report on Form 8-K filed on August 28, 2007 | |
| (3) | Incorporated by reference to our Current Report on Form 8-K filed on September 25, 2007 | |
| (4) | Incorporated by reference to our Current Report on Form 8-K filed on February 22, 2008 | |
| (5) | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2007 filed on March 17, 2008 | |
| (6) | Incorporated by reference to our Current Report on Form 8-K filed on July 15, 2008 | |
| (7) | Incorporated by reference to our Current Report on Form 8-K filed on August 25, 2008 | |
| (8) | Incorporated by reference to our Current Report on Form 8-K filed on September 9, 2008 | |
| (9) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2009 filed on July 30, 2009 | |
| (10) | Incorporated by reference to our Current Report on Form 8-K filed on November 28, 2008 | |
| (11) | Incorporated by reference to our Current Report on Form 8-K filed on March 6, 2009 | |
| (12) | Incorporated by reference to our Current Report on Form 8-K filed with the SEC on April 15, 2009 | |
| (13) | Incorporated by reference to our Quarterly Report on Form 10-Q filed on November 9, 2009 | |
| (14) | Incorporated by reference to our Current Report on Form 8-K filed with the SEC on September 24, 2009 | |
| (15) | Incorporated by reference to our Current Report on Form 8-K filed on April 6, 2009 | |
| (16) | Incorporated by reference to our Current Report on Form 8-K filed on February 4, 2010 | |
| (17) | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2009 filed on March 12, 2010 | |
| (18) | Incorporated by reference to our Current Report on Form 8-K filed on April 21, 2010 | |
| (19) | Incorporated by reference to our Current Report on Form 8-K filed on April 28, 2010 | |
| (20) | Incorporated by reference to our Quarterly Report on Form 10-Q filed on May 10, 2010 | |
| (21) | Incorporated by reference to our Current Report on Form 8-K filed on June 30, 2010 | |
| (22) | Incorporated by reference to our Current Report on Form 8-K filed on August 18, 2010 | |
| (23) | Incorporated by reference to our Current Report on Form 8-K filed on October 6, 2010 | |
| (24) | Incorporated by reference to our Quarterly Report on Form 8-Q filed on November 9, 2010 | |
| (25) | Incorporated by reference to our Current Report on Form 8-K filed on November 29, 2010 | |
| (26) | Incorporated by reference to our Current Report on Form 8-K filed on December 1, 2010 |
43
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| MedQuist Inc. |
||||
| By: | /s/ PETER MASANOTTI | |||
| Peter Masanotti | ||||
| President and Chief Executive Officer | ||||
Date: March 16, 2011
Pursuant to the requirements of the Exchange Act, this report has been signed below by the
following persons on behalf of the registrant and in the capacities and on the dates indicated.
Each person, in so signing also makes, constitutes, and appoints Peter Masanotti and Anthony
James, and each of them acting alone, as his or her true and lawful attorneys-in-fact, with full
power of substitution, in his or her name, place, and stead, to execute and cause to be filed with
the SEC any or all amendments to this report.
| Signature | Capacity | Date | ||
/s/ PETER MASANOTTI
|
President and Chief Executive Officer (Principal Executive Officer) | March 16. 2011 | ||
/s/ ANTHONY JAMES
|
Chief Financial Officer (Principal Financial Officer) | March 16. 2011 | ||
/s/ JAMES BRENNAN
|
Principal Accounting Officer | March 16. 2011 | ||
/s/ ROBERT AQUILINA
|
Non-Executive Chairman of the Board of Directors | March 16. 2011 | ||
/s/ FRANK BAKER
|
Director | March 16. 2011 | ||
/s/ PETER E. BERGER
|
Director | March 16. 2011 | ||
/s/ JOHN F. JASTREM
|
Director | March 16. 2011 | ||
/s/ COLIN J. O’BRIEN
|
Director | March 16. 2011 | ||
/s/ WARREN E. PINCKERT, II
|
Director | March 16. 2011 | ||
/s/ MICHAEL SEEDMAN
|
Director | March 16. 2011 | ||
/s/ ANDREW E. VOGEL
|
Director | March 16. 2011 |
44
Table of Contents
Index to Consolidated Financial Statements
| F-2 | ||||
| F-3 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-8 |
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm on Internal Control Over Financial Reporting
The Board of Directors and Shareholders of MedQuist Inc.:
We have audited MedQuist Inc.’s internal control over financial reporting as of December 31, 2010
based on criteria established in Internal Control — Integrated Framework issued by the Committee
of Sponsoring Organizations of the Treadway Commission (COSO). MedQuist Inc.’s management is
responsible for maintaining effective internal control over financial reporting and for its
assessment of the effectiveness of internal control over financial reporting, included in the
accompanying Management’s Report on Internal Control over Financial Reporting as of December 31,
2010. Our responsibility is to express an opinion on the Company’s internal control over financial
reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether effective internal control over financial reporting was
maintained in all material respects. Our audit included obtaining an understanding of internal
control over financial reporting, assessing the risk that a material weakness exists, and testing
and evaluating the design and operating effectiveness of internal control based on the assessed
risk. Our audit also included performing such other procedures as we considered necessary in the
circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable
assurance regarding the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally accepted accounting principles. A
company’s internal control over financial reporting includes those policies and procedures that (1)
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the
transactions and dispositions of the assets of the company; (2) provide reasonable assurance that
transactions are recorded as necessary to permit preparation of financial statements in accordance
with generally accepted accounting principles, and that receipts and expenditures of the company
are being made only in accordance with authorizations of management and directors of the company;
and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized
acquisition, use, or disposition of the company’s assets that could have a material effect on the
financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or
detect misstatements. Also, projections of any evaluation of effectiveness to future periods are
subject to the risk that controls may become inadequate because of changes in conditions, or that
the degree of compliance with the policies or procedures may deteriorate.
In our opinion, MedQuist Inc. maintained, in all material respects, effective internal control over
financial reporting as of December 31, 2010, based on criteria established in Internal Control —
Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission
(COSO).
We also have audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), the consolidated balance sheets of MedQuist Inc. and subsidiaries as of
December 31, 2010 and 2009 and the related consolidated statements of operations, shareholders’
(deficit) equity and other comprehensive income (loss), and cash flows for each of the years in the
three-year period ended December 31, 2010, and our report dated March 16, 2011 expressed an
unqualified opinion on those consolidated financial statements.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 16, 2011
March 16, 2011
F-2
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of MedQuist Inc.:
We have audited the accompanying consolidated balance sheets of MedQuist Inc. and subsidiaries (the
Company) as of December 31, 2010 and 2009, and the related consolidated statements of operations,
shareholders’ (deficit) equity and other comprehensive income (loss), and cash flows for each of
the years in the three-year period ended December 31, 2010. These consolidated financial statements
are the responsibility of the Company’s management. Our responsibility is to express an opinion on
these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement. An
audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles used and
significant estimates made by management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all
material respects, the financial position of MedQuist Inc. and subsidiaries as of December 31, 2010
and 2009, and the results of their operations and their cash flows for each of the years in the
three-year period ended December 31, 2010, in conformity with U.S. generally accepted accounting
principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), MedQuist Inc.’s internal control over financial reporting as of December 31,
2010, based on criteria established in Internal Control — Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March
16, 2011 expressed an unqualified opinion on the effectiveness of the Company’s internal control
over financial reporting.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 16, 2011
March 16, 2011
F-3
Table of Contents
MedQuist Inc. and Subsidiaries
Consolidated Statements of Operations
(In thousands, except per share amounts)
| Years Ended December 31 , | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
Net revenues |
$ | 375,240 | $ | 307,200 | $ | 326,853 | ||||||
Operating costs and expenses: |
||||||||||||
Cost of revenues |
249,571 | 206,265 | 230,375 | |||||||||
Selling, general and administrative |
37,070 | 33,441 | 47,520 | |||||||||
Research and development |
12,813 | 9,604 | 15,848 | |||||||||
Depreciation and amortization |
21,989 | 15,672 | 17,504 | |||||||||
Cost of legal proceedings and settlements |
3,603 | 14,843 | 19,738 | |||||||||
Acquistion and integration related charges |
7,007 | 1,263 | — | |||||||||
Goodwill impairment charge |
— | — | 82,233 | |||||||||
Restructuring charges |
2,829 | 2,727 | 2,055 | |||||||||
Total operating costs and expenses |
334,882 | 283,815 | 415,273 | |||||||||
Operating income (loss) |
40,358 | 23,385 | (88,420 | ) | ||||||||
Gain on sale of investment |
9,911 | — | — | |||||||||
Equity in income of affiliated company |
693 | 2,015 | 236 | |||||||||
Other income |
— | — | 438 | |||||||||
Loss on extinguishment of debt |
(5,811 | ) | — | — | ||||||||
Interest income (expense), net |
(13,429 | ) | (134 | ) | 2,438 | |||||||
Income (loss) before income taxes |
31,722 | 25,266 | (85,308 | ) | ||||||||
Income tax provision (benefit) |
671 | 1,975 | (16,513 | ) | ||||||||
Net income (loss) |
$ | 31,051 | $ | 23,291 | $ | (68,795 | ) | |||||
Net income (loss) per share: |
||||||||||||
Basic |
$ | 0.83 | $ | 0.62 | $ | (1.83 | ) | |||||
Diluted |
$ | 0.83 | $ | 0.62 | $ | (1.83 | ) | |||||
Weighted average shares outstanding: |
||||||||||||
Basic |
37,556 | 37,556 | 37,549 | |||||||||
Diluted |
37,556 | 37,556 | 37,549 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
MedQuist Inc. and Subsidiaries
Consolidated Balance Sheets
(In thousands)
| December 31, | December 31, | |||||||
| 2010 | 2009 | |||||||
Assets |
||||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 41,265 | $ | 25,216 | ||||
Accounts receivable, net of allowance of $3,142 and $3,159, respectively |
76,155 | 43,627 | ||||||
Income tax receivable |
— | 772 | ||||||
Other current assets |
9,780 | 4,940 | ||||||
Total current assets |
127,200 | 74,555 | ||||||
Property and equipment, net |
14,135 | 11,772 | ||||||
Goodwill |
88,982 | 40,813 | ||||||
Other intangible assets, net |
79,860 | 36,307 | ||||||
Deferred income taxes |
3,000 | 1,396 | ||||||
Other assets |
10,741 | 9,818 | ||||||
Total assets |
$ | 323,918 | $ | 174,661 | ||||
Liabilities and Shareholders’ (Deficit) Equity |
||||||||
Current liabilities: |
||||||||
Current portion of long term debt |
$ | 20,000 | $ | — | ||||
Accounts payable |
6,785 | 8,687 | ||||||
Accrued expenses |
27,106 | 21,490 | ||||||
Accrued compensation |
11,136 | 12,432 | ||||||
Current portion of lease obligations |
758 | — | ||||||
Related party payable |
5,386 | 1,362 | ||||||
Deferred revenue |
10,840 | 10,854 | ||||||
Total current liabilities |
82,011 | 54,825 | ||||||
Long term debt |
265,000 | — | ||||||
Deferred income taxes |
6,066 | 3,240 | ||||||
Other non-current liabilities |
1,442 | 1,848 | ||||||
Commitments and contingencies (Note 10) |
||||||||
Shareholders’ (deficit) equity: |
||||||||
Common stock — no par value; authorized 60,000 shares;
37,556 and 37,556 shares issued and outstanding, respectively |
238,042 | 237,848 | ||||||
Accumulated deficit |
(271,316 | ) | (125,854 | ) | ||||
Accumulated other comprehensive income |
2,673 | 2,754 | ||||||
Total shareholders’ (deficit) equity |
(30,601 | ) | 114,748 | |||||
Total liabilities and shareholders’ (deficit) equity |
$ | 323,918 | $ | 174,661 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
MedQuist Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(In thousands)
| Years ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
Operating activities: |
||||||||||||
Net income (loss) |
$ | 31,051 | $ | 23,291 | $ | (68,795 | ) | |||||
Adjustments to reconcile net income (loss) to cash provided by (used in) operating activities: |
||||||||||||
Depreciation and amortization |
21,989 | 15,672 | 17,504 | |||||||||
Loss on extinguishment of debt |
5,811 | — | — | |||||||||
Gain on sale and equity in income of affiliated company |
(10,604 | ) | (2,015 | ) | (236 | ) | ||||||
Goodwill impairment charge |
— | — | 82,233 | |||||||||
Deferred income taxes |
(189 | ) | 1,857 | (17,091 | ) | |||||||
Stock option expense |
194 | 193 | 1,427 | |||||||||
Non-cash interest expense |
4,132 | — | — | |||||||||
Provision for doubtful accounts |
1,326 | 2,306 | 3,073 | |||||||||
Loss on disposal of property and equipment |
5 | 133 | 571 | |||||||||
Changes in operating assets and liabilities: |
||||||||||||
Accounts receivable |
(11,421 | ) | 4,529 | (5,781 | ) | |||||||
Income tax receivable |
769 | (616 | ) | 661 | ||||||||
Other current assets |
(815 | ) | 3,391 | (154 | ) | |||||||
Other non-current assets |
(326 | ) | 25 | 134 | ||||||||
Accounts payable |
760 | 1,038 | (5,557 | ) | ||||||||
Accrued expenses |
(3,014 | ) | (1,198 | ) | (12,701 | ) | ||||||
Accrued compensation |
(5,126 | ) | 1,192 | (3,559 | ) | |||||||
Deferred revenue |
(91 | ) | (4,939 | ) | (272 | ) | ||||||
Other non-current liabilities |
(493 | ) | (307 | ) | (211 | ) | ||||||
Net cash provided by (used in) operating activities |
33,958 | 44,552 | (8,754 | ) | ||||||||
Investing activities: |
||||||||||||
Purchase of property and equipment |
(5,824 | ) | (4,932 | ) | (6,574 | ) | ||||||
Proceeds from sale of investments |
— | — | 692 | |||||||||
Capitalized software |
(5,208 | ) | (2,582 | ) | (3,411 | ) | ||||||
Proceeds from sale of invest in affiliated company |
19,469 | — | — | |||||||||
Investment in affiliated company |
— | (852 | ) | — | ||||||||
Acquisitions, net of cash acquired |
(98,661 | ) | — | — | ||||||||
Net cash used in investing activities |
(90,224 | ) | (8,366 | ) | (9,293 | ) | ||||||
Financing activities: |
||||||||||||
Net borrowings |
385,000 | — | — | |||||||||
Repayment of debt |
(113,570 | ) | — | — | ||||||||
Dividends paid |
(176,513 | ) | (49,949 | ) | (103,279 | ) | ||||||
Debt issuance costs |
(21,607 | ) | (1,201 | ) | — | |||||||
Payments on lease obligations |
(1,097 | ) | — | 68 | ||||||||
Net cash provided by (used in) financing activities |
72,213 | (51,150 | ) | (103,211 | ) | |||||||
Effect of exchange rate changes |
102 | 262 | (406 | ) | ||||||||
Net increase (decrease) in cash and cash equivalents |
16,049 | (14,702 | ) | (121,664 | ) | |||||||
Cash and cash equivalents — beginning of period |
25,216 | 39,918 | 161,582 | |||||||||
Cash and cash equivalents — end of period |
$ | 41,265 | $ | 25,216 | $ | 39,918 | ||||||
Supplemental cash flow information: |
||||||||||||
Cash paid for interest |
$ | 9,297 | $ | — | $ | — | ||||||
Cash paid for income taxes |
$ | 290 | $ | 234 | $ | 210 | ||||||
Accommodation payments paid with credits |
$ | — | $ | 103 | $ | 740 | ||||||
Non-cash debt incurred in connection with the Spheris acquisition |
$ | 13,570 | $ | — | $ | — | ||||||
Assets acquired using debt |
$ | 1,152 | $ | — | $ | — | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
MedQuist Inc. and Subsidiaries
Consolidated Statements of Shareholders’ (Deficit) Equity and Other Comprehensive Income (Loss)
Years ended December 31, 2010, 2009 and 2008
(In thousands)
| Accumulated | ||||||||||||||||||||
| Retained | Other | Total | ||||||||||||||||||
| Common Stock | Earnings | Comprehensive | Shareholders’ | |||||||||||||||||
| Shares | Amount | (Deficit) | Income | (Deficit) Equity | ||||||||||||||||
Balance, January 1, 2008 |
37,544 | $ | 236,412 | $ | 72,876 | $ | 5,356 | $ | 314,644 | |||||||||||
Comprehensive loss: |
||||||||||||||||||||
Net loss |
— | — | (68,795 | ) | — | (68,795 | ) | |||||||||||||
Foreign currency translation adjustments |
— | — | — | (3,713 | ) | (3,713 | ) | |||||||||||||
Total comprehensive loss |
(72,508 | ) | ||||||||||||||||||
Stock-based compensation expense |
— | 1,427 | — | — | 1,427 | |||||||||||||||
Cash dividend |
— | — | (103,279 | ) | — | (103,279 | ) | |||||||||||||
Exercise of stock options |
12 | 68 | — | — | 68 | |||||||||||||||
Balance, December 31, 2008 |
37,556 | $ | 237,907 | $ | (99,198 | ) | $ | 1,643 | $ | 140,352 | ||||||||||
Comprehensive income: |
||||||||||||||||||||
Net income |
— | — | 23,291 | — | 23,291 | |||||||||||||||
Foreign currency translation adjustments |
— | — | 2 | 1,111 | 1,113 | |||||||||||||||
Total comprehensive income |
24,404 | |||||||||||||||||||
Stock-based compensation expense |
— | 193 | — | — | 193 | |||||||||||||||
Cash dividend |
— | — | (49,949 | ) | — | (49,949 | ) | |||||||||||||
Dilution of affiliated company |
— | (252 | ) | — | — | (252 | ) | |||||||||||||
Balance, December 31, 2009 |
37,556 | $ | 237,848 | $ | (125,854 | ) | $ | 2,754 | $ | 114,748 | ||||||||||
Comprehensive income: |
||||||||||||||||||||
Net income |
— | — | 31,051 | — | 31,051 | |||||||||||||||
Foreign currency translation adjustments |
— | — | (81 | ) | (81 | ) | ||||||||||||||
Total comprehensive income |
30,970 | |||||||||||||||||||
Stock-based compensation expense |
— | 194 | — | — | 194 | |||||||||||||||
Cash dividend |
— | — | (176,513 | ) | — | (176,513 | ) | |||||||||||||
Balance, December 31, 2010 |
37,556 | $ | 238,042 | $ | (271,316 | ) | $ | 2,673 | $ | (30,601 | ) | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Notes to Consolidated Financial Statements
1. Description of the Business and Recent Developments
We are a leading provider of integrated clinical documentation solutions for the U.S.
healthcare system. Our end-to-end solutions convert physicians’ dictation of patient
interactions, or the physician narrative, into a high quality and customized electronic record.
These solutions integrate technologies and services for voice capture and transmission, automated
speech recognition (ASR), medical transcription and editing, workflow automation, and document
management and distribution to deliver a complete managed service for our customers. Our
solutions enable hospitals, clinics, and physician practices to improve the quality of clinical
data as well as accelerate and automate the documentation process, and we believe our solutions
improve physician productivity and satisfaction, enhance revenue cycle performance, and
facilitate the adoption and use of electronic health records.
Majority Owner
On August 6, 2008, MedQuist Holdings Inc. (MedQuist Holdings), formerly CBaySystems Holdings Limited (CBaySystems
Holdings), a company that is now publicly traded on The NASDAQ market with a portfolio of
investments in medical transcription, which includes a company that competes in the medical
transcription market, healthcare technology, and healthcare financial services, acquired a large
interest in us from Koninklijke Philips Electronics N.V. (Philips).
The Company’s consolidated financial statements do not include any components of purchase
accounting, which was recorded at the MedQuist Holdings reporting level, and are presented on the
historical basis of accounting.
We paid cash dividends of $4.70 per share in 2010 and $1.33 per share in 2009.
Recent developments of Our Majority Owner
U.S. initial public offering
On January 27, 2011, it changed its name from CBaySystems Holdings Limited to MedQuist
Holdings Inc. and re-domiciled from a British Virgin Islands company to a Delaware corporation and
authorized 300 million shares of common stock par value at $0.10 per share and 25 million shares of
preferred stock at $0.10 par value per share. In connection with MedQuist Holdings’
re-domiciliation, MedQuist Holdings adjusted the number of its shares outstanding through a reverse
share split pursuant to which every 4.5 shares of its common stock outstanding prior to its
re-domiciliation was converted into one share of MedQuist Holdings’ common stock upon its
re-domiciliation.
In February 2011, MedQuist Holdings completed its U.S. initial public offering of common stock
selling 3.0 million of its shares of common stock and 1.5 million shares of its common stock owned
by selling shareholders at an offer price of $8.00 per share, resulting in gross proceeds to
MedQuist Holdings of $24.0 million and net proceeds to MedQuist Holdings after underwriting fees of
$22.3 million. MedQuist Holdings’ common stock is listed on The NASDAQ Global Market under the
symbol “MEDH.”
Private Exchange
Certain of our noncontrolling stockholders entered into an exchange agreement with MedQuist
Holdings, whereby MedQuist Holdings issued 4.8 million shares of its common stock in exchange for
4.8 million shares of MedQuist Inc. common stock. This transaction is referred to as the Private
Exchange. The Private Exchange was completed on February 11, 2011 and increased MedQuist Holdings’
ownership in MedQuist Inc. from 69.5% to 82.2%.
Registered Exchange Offer
In addition to the Private Exchange referred to above, in February 2011, MedQuist Holdings
commenced its public exchange offer, or Registered Exchange Offer, to those of our noncontrolling
stockholders who did not participate in the Private Exchange to exchange shares of MedQuist
Holdings’ common stock for shares of MedQuist Inc. common stock. The Registered Exchange Offer
expired on March 11, 2011. MedQuist Holdings accepted for, and consummated the exchange of, all
MedQuist Inc. shares of common stock that were validly tendered in the Registered Exchange Offer. As a result of the Registered Exchange
Offer, MedQuist Holdings increased its ownership interest in us from 82.2% to approximately 97%.
2. Significant Accounting Policies
Principles of Consolidation
Our consolidated financial statements include the accounts of MedQuist Inc. and its subsidiary
companies. All intercompany balances and transactions have been eliminated in consolidation.
F-8
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
Use of Estimates and Assumptions in the Preparation of Consolidated Financial Statements
The preparation of our consolidated financial statements in conformity with accounting
principles generally accepted in the United States of America requires management to make estimates
and assumptions that affect amounts reported in our consolidated financial statements. Significant
items subject to such estimates and assumptions include the carrying amount of property and
equipment, valuation of long-lived and intangible assets and goodwill, valuation allowances for
receivables and deferred income taxes, revenue recognition, stock-based compensation and
commitments and contingencies. Actual results could differ from those estimates.
Revenue Recognition
The majority of our revenues are derived from providing medical transcription and editing
services. Revenues for medical transcription and editing services are recognized when the services
are rendered. These services are based on contracted rates. The remainder of our revenues is
derived from the sale of voice-capture and document management products including software,
hardware and implementation, training and maintenance service related to these legacy products.
We recognize software-related revenues using the residual method when vendor-specific
objective evidence (VSOE) of fair value exists for all of the undelivered elements in the
arrangement, but does not exist for one or more delivered elements. We allocate revenues to each
undelivered element based on its respective fair value determined by the price charged when that
element is sold separately or, for elements not yet sold separately, the price established by
management if it is probable that the price will not change before the element is sold separately.
We defer revenues for the undelivered elements including maintenance which is recognized over the contract period. Provided that the arrangement does not involve
significant production, modification, or customization of the software, revenues are recognized
when all of the following four criteria have been met: persuasive evidence of an arrangement
exists, delivery has occurred, the fee is fixed or determinable and collectability is probable.
Sales Taxes
We present taxes assessed by a governmental authority including sales, use, value added and
excise taxes on a net basis and therefore the presentation of these taxes is excluded from our
revenues and is included in accrued expenses in the accompanying consolidated balance sheets until
such amounts are remitted to the taxing authorities.
Litigation and Settlement Costs
From time to time, we are involved in litigation, claims, contingencies and other legal
matters. We record a charge equal to at least the minimum estimated liability for a loss
contingency when both of the following conditions are met: (i) information available prior to
issuance of the financial statements indicates that it is probable that an asset had been impaired
or a liability had been incurred at the date of the financial statements and (ii) the range of the
loss can be reasonably estimated. We expense legal costs, including those legal costs expected to
be incurred in connection with a loss contingency, as incurred.
Restructuring Costs
A liability for restructuring costs associated with an exit or disposal activity is recognized
and measured initially at fair value when the liability is incurred. We record a liability for
severance costs when the obligation is attributable to employee service already rendered, the
employees’ rights to those benefits accumulate or vest, payment of the compensation is probable and
the amount can be reasonably estimated. We record a liability for future, non-cancellable operating
lease costs when we vacate a facility.
Our estimates of future liabilities may change, requiring us to record additional
restructuring charges or reduce the amount of liabilities recorded. At the end of each reporting
period, we evaluate the remaining accrued restructuring charges to ensure their adequacy, that no
excess accruals are retained and the utilization of the provisions are for their intended purposes
in accordance with developed exit plans.
We periodically evaluate currently available information and adjust our accrued restructuring
reserve as necessary. Changes in estimates are accounted for as restructuring costs or credits in
the period identified.
Research and Development Costs
Research and development costs are expensed as incurred.
F-9
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
Income Taxes
Deferred tax assets and liabilities are recorded for temporary differences between the tax
basis of assets and liabilities and their reported amounts in the consolidated financial
statements, using statutory tax rates in effect for the year in which the differences are expected
to reverse. The effect on deferred tax assets and liabilities of a change in tax rates is
recognized in our statements of operations in the period that includes the enactment date. A
valuation allowance is recorded to reduce the carrying amounts of deferred tax assets unless it is
more likely than not that such assets will be realized. Management considers various sources of
future taxable income including projected book earnings, the reversal of deferred tax liabilities,
and prudent and feasible tax planning strategies in determining the need for a valuation allowance.
Stock-Based Compensation
We estimate the fair value of stock options on the date of grant using an option pricing
model. We use the Black-Scholes option pricing model to determine the fair value of our options.
The determination of the fair value of stock based awards using an option pricing model is affected
by a number of assumptions including expected volatility of the common stock over the expected
term, the expected term, the risk free interest rate during the expected term and the expected
dividends to be paid. The value of the portion of the award that is ultimately expected to vest is
recognized as compensation expense over the requisite service periods.
As of December 31, 2010, total unamortized stock-based compensation cost related to non-vested
stock options, net of expected forfeitures, was $145 which is expected to be recognized over a
weighted-average period of 1 year.
We
did not grant any stock options for years ended December 31, 2010 or 2009. In accordance
with the terms of our Chief Executive Officer’s option agreement, in December 2010, the
Compensation Committee approved an adjustment to the exercise price of our Chief Executive
Officer’s options to account for the payment of extraordinary dividends in 2010 and 2009 to our
shareholder’s.
Net Income (Loss) per Share
Basic net income (loss) per share is computed by dividing net income (loss) by the weighted
average number of shares outstanding during each period. Diluted net income (loss) per share is
computed by dividing net income (loss) by the weighted average shares outstanding, as adjusted for
the dilutive effect of common stock equivalents, which consist only of stock options, using the
treasury stock method.
The table below reflects basic and diluted net income (loss) per share for the years ended
December 31:
| 2010 | 2009 | 2008 | ||||||||||
Net income (loss) |
$ | 31,051 | $ | 23,291 | $ | (68,795 | ) | |||||
Weighted average shares outstanding: |
||||||||||||
Basic |
37,556 | 37,556 | 37,549 | |||||||||
Effect of dilutive stock |
— | — | — | |||||||||
Diluted |
37,556 | 37,556 | 37,549 | |||||||||
Net income (loss) per share: |
||||||||||||
Basic |
$ | 0.83 | $ | 0.62 | $ | (1.83 | ) | |||||
Diluted |
$ | 0.83 | $ | 0.62 | $ | (1.83 | ) | |||||
The computation of diluted net income (loss) per share does not assume conversion, exercise or
issuance of shares that would have an anti-dilutive effect on diluted net loss per share. During
2008 we had a net loss. As a result, any assumed conversions would result in reducing the net loss
per share and, therefore, are not included in the calculation. Shares having an anti-dilutive
effect on net loss per share and, therefore, excluded from the calculation of diluted net loss per
share, totaled 1,501 for the year ended December 31, 2008. For
the years ended December 31. 2010, and
2009, 692, and 1,208 options, respectively, were excluded from the computation of
diluted earnings per share as the options exercise price was greater than the average market price
of the common stock during the respective period.
Advertising Costs
Advertising costs are expensed as incurred and for the years ended December 31, 2010, 2009 and
2008 were $918, $613 and $1,256, respectively.
F-10
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
Cash and Cash Equivalents
We consider all highly liquid instruments with original maturities of three months or less to
be cash equivalents. Our cash management and investment policies dictate that cash equivalents be
limited to investment grade, highly liquid securities. We place our temporary cash investments with
high-credit rated, quality financial institutions. Deposits held with banks may exceed the amount
of insurance provided on such deposits. Consequently, our cash equivalents are subject to potential
credit risk. As of December 31, 2010 and 2009, cash equivalents consisted of money market
investments. The carrying value of cash and cash equivalents approximates fair value.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are recorded at the invoiced amount and do not bear interest. The carrying
value of accounts receivable approximates fair value. The allowance for doubtful accounts is our
best estimate for losses inherent in our accounts receivable portfolio.
We estimate uncollectible amounts based upon our historical write-off experience, current
customer receivable balances, age of customer receivable balances, the customer’s financial
condition and current economic conditions. Historically, these estimates have been adequate to
cover our accounts receivable exposure. We change the allowance if circumstances or economic
conditions change.
Product revenues for sales to end-user customers and resellers are recognized upon passage of
title if all other revenue recognition criteria have been met. End-user customers generally do not
have a right of return. We provide certain of our resellers and distributors with limited rights of
return of our products. We reduce revenues for rights to return our products based upon our
historical experience and have included an estimate of such credits in our allowance for doubtful
accounts.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Depreciation is
calculated on a straight-line basis over the estimated useful lives of the assets which range from
two to seven years for furniture, equipment and software, and the lesser of the lease term or
estimated useful life for leasehold improvements. Repairs and maintenance costs are charged to
expense as incurred while additions and betterments are capitalized. Gains or losses on disposals
are charged to operations. Upon retirement, sale or other disposition, the related cost and
accumulated depreciation are eliminated from the accounts and any gain or loss is included in
operations.
Valuation of Long-Lived and Other Intangible Assets and Goodwill.
In connection with acquisitions, we allocate portions of the purchase price to tangible and
intangible assets, consisting primarily of acquired technologies, and customer relationships,
agreements, with the remainder allocated to goodwill. We assess the realizability of goodwill and
intangible assets with indefinite useful lives at least annually, or sooner if events or changes in
circumstances indicate that the carrying amount may not be recoverable. We have determined that the
reporting unit level is our sole operating segment.
Software Development Costs
Software costs incurred in creating a computer software product are charged to expense when
incurred as research and development until technical feasibility has been established. Technical
feasibility is established upon completion of a detail program design or, in its absence,
completion of a working model. The time between attaining technological feasibility and completion
of software development has been short.
Software costs for internal use software incurred in the preliminary project stage are
expensed as incurred. Capitalization of costs begins when the preliminary project stage is
completed and management, with the relevant authority, authorizes and commits funding of the
project and it is probable that the project will be completed and the software will be used to
perform the function intended. Capitalization ceases no later than the point at which the project
is substantially complete and ready for its intended use.
Long-Lived and Other Intangible Assets
Long-lived assets, including property and equipment and purchased intangible assets subject to
amortization, are reviewed for impairment whenever events or changes in circumstances indicate that
the carrying amount of an asset may not be recoverable. To determine the recoverability of
long-lived assets, the estimated future undiscounted cash flows expected to be generated by an
asset is compared to the carrying value of the asset. If the carrying value of the long-lived asset
exceeds its estimated future undiscounted cash flows, an impairment charge is recognized in the
amount by which the carrying value of the asset exceeds its fair value. Annually we evaluate the
reasonableness of the useful lives of these assets.
F-11
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
Intangible assets include certain assets (primarily customer lists) obtained from business
acquisitions and are being amortized using the straight-line method over their estimated useful
lives which range from three to 20 years.
Foreign Currency
Our operating subsidiaries in the United Kingdom and Canada use the local currency as their
functional currency. We translate the assets and liabilities of those entities into U.S. dollars
using the month-end exchange rate. We translate revenues and expenses using the average exchange
rates prevailing during the reporting period. The resulting translation adjustments are recorded in
accumulated other comprehensive income within shareholders’ equity. Gains and losses from foreign
currency transactions are included as a component of selling, general and administrative expenses
in the accompanying consolidated statements of operations, and were not material for the years
ended December 31, 2010, 2009 and 2008, respectively.
Business Enterprise Segments
We operate in one reportable operating segment which is clinical documentation technology and
services.
Concentration of Risk, Geographic Data and Enterprise-wide Disclosures
No single customer accounted for more than 10% of our net revenues in any period. There is no
single geographic area of significant concentration other than the United States.
The following table summarizes the net revenues by the categories of our products and services
as a percentage of our total net revenues.
| 2010 | 2009 | 2008 | ||||||||||
Medical transcription |
89.5 | % | 85.7 | % | 84.9 | % | ||||||
Other |
10.5 | % | 14.3 | % | 15.1 | % | ||||||
Total |
100.0 | % | 100.0 | % | 100.0 | % | ||||||
Other includes product, maintenance, medical coding, application service provider
and other miscellaneous revenues.
Fair Value of Financial Instruments
Cash and cash equivalents, accounts receivable, accounts payable, accrued expenses, and debt
are reflected in the accompanying consolidated balance sheets at carrying values which approximate
fair value.
Comprehensive Income (Loss)
Comprehensive income (loss) is comprised of Net income (loss) and Other comprehensive income
(loss). Other comprehensive income (loss) consists of foreign currency translation adjustments.
Other comprehensive income (loss) and comprehensive income (loss) are displayed separately in the
Consolidated Statements of Shareholders’ (Deficit) Equity and Other Comprehensive Income (Loss).
Acquisition and Integration related charges
We expense costs incurred for acquisitions and related integration activities in the period
incurred. This includes legal, investment banking, accounting, tax and other consulting, as well as
any internal costs.
Recent Accounting Pronouncements
In September 2009, the Financial Accounting Standards Board ratified two consensuses affecting
revenue recognition:
The first consensus, Revenue Recognition—Multiple-Element Arrangements, sets forth
requirements that must be met for an entity to recognize revenue from the sale of a delivered item
that is part of a multiple-element arrangement when other items have not yet been delivered. One of
those current requirements is that there be objective and reliable evidence of the standalone
selling price of the undelivered items, which must be supported by either vendor-specific objective
evidence (VSOE) or third-party evidence (TPE).
This consensus eliminates the requirement that all undelivered elements have VSOE or TPE
before an entity can recognize the portion of an overall arrangement fee that is attributable to
items that already have been delivered. In the absence of VSOE or TPE of
F-12
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
the standalone selling price for one or more delivered or undelivered elements in a
multiple-element arrangement, entities will be required to estimate the selling prices of those
elements. The overall arrangement fee will be allocated to each element (both delivered and
undelivered items) based on their relative selling prices, regardless of whether those selling
prices are evidenced by VSOE or TPE or are based on the entity’s estimated selling price.
Application of the “residual method” of allocating an overall arrangement fee between delivered and
undelivered elements will no longer be permitted.
The second consensus, Software-Revenue Recognition addresses the accounting for transactions
involving software to exclude from its scope tangible products that contain both software and
non-software and not-software components that function together to deliver a products
functionality.
The consensuses are effective prospectively for revenue arrangements entered into or
materially modified in fiscal years beginning on or after
June 15, 2010. The standards were adopted
in the first quarter of 2011 and will not have a material impact on our results of operations or
our financial position.
3. Acquisition of Spheris Assets in the United States
On April 22, 2010, we and our majority shareholder, MedQuist Holdings, completed the
acquisition of substantially all of the assets of Spheris and certain of its affiliates, pursuant
to the terms of a Stock and Asset Purchase Agreement. This acquisition provided substantial
incremental volume growth and also provided opportunities for operating efficiencies and operating
margin expansion. See Note 9, Accrued Expenses for further discussion of our 2010 restructuring
plan approved by management to realize some of these savings. The acquisition was funded from the
proceeds of credit facilities entered into in connection with the acquisition.
The following unaudited pro forma summary presents the consolidated information of the Company
as if the business combination had occurred at the beginning of each period.
| Pro Forma Year Ended | ||||||||
| December 31, | ||||||||
| 2010 | 2009 | |||||||
Net revenues |
$ | 418,611 | $ | 463,796 | ||||
Net income |
$ | 35,594 | $ | 24,369 | ||||
Net income per share (Basic) |
$ | 0.95 | $ | 0.65 | ||||
Net income per share (Diluted) |
$ | 0.95 | $ | 0.65 | ||||
These amounts have been calculated after applying our accounting policies and adjusting the
results of Spheris to reflect the additional amortization of intangibles that would have been
charged assuming the fair value adjustments to intangible assets had been applied from the
beginning of the annual period being reported on, and the additional interest expense assuming the
acquisition related debt had been incurred at the beginning of the period being reported on and
including the related tax effects. The acquired business contributed net revenues of $88.1 million
for the period April 22, 2010 to December 31, 2010.
The following table summarizes the consideration transferred by us to acquire the domestic
assets of Spheris, and the amounts of identified assets acquired and liabilities assumed at the
acquisition date.
Cash consideration paid |
$ | 98,834 | ||
Fair value of unsecured Subordinated Promissory Note |
13,570 | |||
Total consideration transferred |
$ | 112,404 | ||
Recognized amounts of identifiable assets acquired and liabilities assumed: |
||||
Fair value of Spheris net assets acquired |
||||
Working capital |
$ | 7,373 | ||
Property, plant and equipment |
6,626 | |||
Developed technology (included in intangibles) |
11,390 | |||
Customer relationships (included in intangibles) |
37,210 | |||
Trademarks and trade names (included in intangibles) |
1,640 | |||
Goodwill |
48,165 | |||
Identifiable assets acquired and liabilities assumed |
$ | 112,404 | ||
F-13
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
The related amortization period is shown below:
| Amortization | ||
| Period | ||
Developed technology
|
9 years | |
Customer relationships
|
8 years | |
Trademarks and trade names
|
4 years | |
Goodwill
|
indefinite |
The amounts and lives of the identified intangibles other than goodwill were valued at fair
value. The analysis included a combination of the cost approach and an income approach. We used
discount rates from 15% to 17%. The goodwill is attributable to the workforce of the acquired
business and the significant synergies expected to occur after the Spheris acquisition. The
goodwill and intangible assets are deductible for tax purposes. We have performed a review of
Spheris’s accounting policies and procedures. As a result of that review, we did not identify any
differences between the accounting policies and procedures of the two companies that, when
conformed, would have a material impact on the future operating results.
Fair value is defined as the price that would be received to sell an asset or paid to transfer
a liability in an orderly transaction between market participants at the measurement date. There is
a hierarchy of valuation techniques based on the nature of the inputs used to develop the fair
value measures. This is an exit price concept for the valuation of the asset or liability. In
addition, market participants are assumed to be unrelated buyers and sellers in the principal or
the most advantageous market for the asset or liability. Fair value measurements for an asset
assume the highest and best use by these market participants. Many of these fair value measurements
can be highly subjective and it is also possible that other professionals, applying reasonable
judgment to the same facts and circumstances, could develop and support a range of alternative
estimated amounts.
4. Debt
Debt consisted of the following:
| December 31, | ||||
| 2010 | ||||
Senior Secured Credit Facility consisting of: |
||||
Term loan |
$ | 200,000 | ||
Revolving credit facility |
— | |||
Senior Subordinated Notes |
85,000 | |||
| 285,000 | ||||
Less current portion |
(20,000 | ) | ||
Long term debt |
$ | 265,000 | ||
There was no debt outstanding at December 31, 2009.
On October 1, 2010, we entered into a senior secured credit facility (the Senior Secured
Credit Facility), with certain lenders and General Electric Capital Corporation, as Administrative
Agent. The Senior Secured Credit Facility contains a number of significant covenants and consists
of $225.0 million in senior secured credit facilities comprised of:
F-14
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
| • | a $200.0 million term loan, advanced in one drawing on October 14, 2010 (the Closing Date), with a term of five years, repayable in equal quarterly installments of $5.0 million, commencing on the first day of the first fiscal quarter beginning after the Closing Date, with the balance payable at maturity; and | ||
| • | a $25.0 million revolving credit facility under which borrowings may be made from time to time during the period from the Closing Date until the fifth anniversary of the Closing Date. The revolving facility includes a $5.0 million letter of credit sub-facility and a $5.0 million swing line loan sub-facility. |
The borrowings under the Senior Secured Credit Facility bear interest at a rate equal to an
applicable margin plus, at the co-borrowers’ option, either (a) a base rate determined by reference
to the highest of (1) the rate last quoted by the Wall Street Journal as the “Prime Rate” in the
United States, (2) the federal funds rate plus 1/2 of 1% and (3) the LIBOR rate for a one-month
interest period plus 1.00% or (b) the higher of (1) a LIBOR rate determined by reference to the
costs of funds for deposits in the currency of such borrowing for the interest period relevant to
such borrowing adjusted for certain additional costs and (2) 1.75%. The applicable margin is 4.50%
with respect to base rate borrowings and 5.50% with respect to LIBOR borrowings. At December 31,
2010 the borrowings had an interest rate of 7.25%.
The loans are secured by substantially all of our assets and are guaranteed by MedQuist
Holdings. The agreements contain customary covenants, including reporting and notification and
acquisitions. The financial covenants are calculated on a consolidated basis for MedQuist Holdings.
and its subsidiaries (including us), and include a Senior Leverage Ratio, Total Leverage Ratio, and
an Interest Coverage Ratio. As of December 31, 2010 we believe we were in compliance. The loans
have customary cross default provisions.
In addition to the Senior Secured Credit Facility we issued $85.0 million aggregate principal
amount of 13% Senior Subordinated Notes due 2016 (the Senior Subordinated Notes), pursuant to a
purchase agreement. Interest on the notes is payable in quarterly installments at the issuers’
option at either (i) 13% in cash or (ii) 12% in cash payment plus 2% in the form of additional
senior subordinated notes. The Senior Subordinated Notes are non-callable for two years after the
closing date after which they are redeemable at 105.0% declining ratably until four years after the
closing date. The Senior Subordinated Notes contain a number of significant covenants that, among
other things, restrict our ability to dispose of assets, repay other indebtedness, incur additional
indebtedness, pay dividends, prepay subordinated indebtedness, incur liens, make capital
expenditures, investments or acquisitions, engage in mergers of consolidations, engage in certain
types of transactions with affiliates and otherwise restrict our activities.
Proceeds from the Senior Secured Credit Facility and the Senior Subordinated Notes were used
to repay $80.0 million of our indebtedness under the GE Credit Agreement, as defined below, plus interest, to repay
$13.6 million of our indebtedness under the Subordinated Promissory Note, as defined below, plus interest, and to pay
a $176.5 million special cash dividend to our shareholders.
We incurred $15.5 million of costs in connection with the financing, and there were remaining
unamortized costs from the prior financing. In connection with the refinancing, we evaluated
whether the prior facilities had been modified or extinguished. We recorded an extinguishment loss
of $5.8 million. At December 31, 2010, we had remaining deferred costs of $10.2 million recorded
in other noncurrent assets and $3.1 million recorded in other current assets.
In connection with the Spheris acquisition in April 2010, MedQuist Transcriptions, Ltd., a
subsidiary of MedQuist Inc. (MedQuist Transcriptions), and certain other subsidiaries of MedQuist
Inc. (collectively, the Loan Parties) entered into a credit agreement (the GE Credit Agreement)
with General Electric Capital Corporation, CapitalSource Bank, and Fifth Third Bank. The GE Credit
Agreement provided for up to $100.0 million in senior secured credit facilities, consisting of a
$50.0 million term loan, and a revolving credit facility of up to $50.0 million. The credit
facilities were secured by a first priority lien on substantially all of the property of the Loan
Parties. Amounts borrowed under the GE Credit Agreement incurred interest at a rate selected by
MedQuist Transcriptions equal to the Base Rate or the Eurodollar Rate (each as defined in the GE
Credit Agreement) plus a margin, all as more fully set forth in the GE Credit Agreement. We
incurred $6.1 million in costs in connection with the GE Credit Agreement of which $4.8 million had
not been amortized by the time of the refinance in October 2010.
When we entered into the GE Credit Agreement, we terminated the five-year $25.0 million
revolving credit agreement with Wells Fargo Foothill, LLC (the Wells Credit Agreement) that we
entered into on August 31, 2009. We never borrowed under the Wells
F-15
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
Credit Agreement. In 2010, we
wrote off deferred financing fees of $1.1 million and incurred termination fees of $0.6 million in
connection with the termination of this facility. Such costs are included in interest expense in
the accompanying consolidated statements of operations.
In connection with the Spheris acquisition, we entered into a subordinated promissory note
with Spheris (the Spheris Subordinated Promissory Note). The loan was scheduled to mature in five
years from the date of the acquisition. The face amount of the Spheris Subordinated Promissory Note
totaled $17.5 million with provisions for prepayment at discounted amounts, ranging from 77.5% of
the principal if paid within six months, 87.5% from six to nine months, 97.5% from nine to twelve
months, 102.0% by year two, 101.0% by year three and 100.0% thereafter. For purposes of the
purchase price allocation, the note was discounted at 77.5% of the principal ($13.6 million). This
note was a non-cash transaction. The fair value of the note was determined through the use of a
Monte Carlo model which is Level 3 in the Fair Value hierarchy based upon significant unobservable
inputs. The Subordinated Promissory Note was paid in full as noted above.
The Spheris Subordinated Promissory Note had stated interest rates of 8.0% for the first six
months, 9.0% from six to nine months, and 12.5% thereafter of which 2.5% may be paid by increasing
the principal amount. Payments of interest are made semi-annually on each six month anniversary of
the acquisition. For financial statement purposes the interest has been calculated using the
average interest rates over the term of the Subordinated Promissory Note. As discussed above, we
paid off all amounts outstanding, at 77.5% of the face value, plus accrued interest under the
Subordinated Promissory Note thus extinguishing the note.
The aggregate maturities of long-term obligations are as follows:
2011 |
$ | 20,000 | ||
2012 |
$ | 20,000 | ||
2013 |
$ | 20,000 | ||
2014 |
$ | 20,000 | ||
2015 |
$ | 120,000 | ||
2016 |
$ | 85,000 | ||
| $ | 285,000 | |||
In January 2011 we made an optional prepayment of $20.0 million in addition to the $5.0
million due under the Senior Secured Credit Facility.
5. Allowance for Doubtful Accounts
| Balance at | Charges to | Doubtful | Balance at | |||||||||||||
| Beginning | Revenues and | Accounts | End of | |||||||||||||
| of Period | and Expenses | Written Off | Period | |||||||||||||
December 31, 2008 |
$ | 4,359 | 3,073 | (2,630 | ) | $ | 4,802 | |||||||||
December 31, 2009 |
$ | 4,802 | 2,306 | (3,949 | ) | $ | 3,159 | |||||||||
December 31, 2010 |
$ | 3,159 | 1,326 | (1,343 | ) | $ | 3,142 | |||||||||
Substantially
all of the charges relate to revenues.
6. Property and Equipment
Property and equipment consisted of the following as of December 31:
| 2010 | 2009 | |||||||
Computer equipment |
$ | 37,729 | $ | 32,404 | ||||
Communication equipment |
5,914 | 5,447 | ||||||
Software |
20,994 | 18,743 | ||||||
Furniture and office equipment |
1,674 | 1,357 | ||||||
Leasehold improvements |
5,368 | 2,703 | ||||||
Total property and equipment |
71,679 | 60,654 | ||||||
Less: accumulated depreciation |
(57,544 | ) | (48,882 | ) | ||||
Property and equipment, net |
$ | 14,135 | $ | 11,772 | ||||
Depreciation expense was $10.1 million in 2010, $9.5 million in 2009 and $12.0 million in
2008.
F-16
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
7. Goodwill and Other Intangible Assets
| Goodwill |
The changes in the carrying amount of goodwill for the year ended December 31, 2010 and 2009
are as follows:
| 2010 | 2009 | |||||||
Balance as of January 1, |
||||||||
Goodwill |
$ | 123,046 | $ | 122,778 | ||||
Accumulated impairment losses |
(82,233 | ) | (82,233 | ) | ||||
| 40,813 | 40,545 | |||||||
Goodwill from Spheris acquisition |
48,165 | |||||||
Foreign currency adjustments |
4 | 268 | ||||||
Balance at December 31, |
||||||||
Goodwill |
171,215 | 123,046 | ||||||
Accumulated impairment losses |
(82,233 | ) | (82,233 | ) | ||||
Goodwill at December 31, |
$ | 88,982 | $ | 40,813 | ||||
We tested goodwill for impairment during the fourth quarter of 2010 and 2009 and determined
that the fair value of the reporting unit substantially exceeded the carrying value based upon our
market capitalization. Determining fair value requires the exercise of significant judgment,
including judgment about appropriate discount rates, perpetual growth rates, the amount and timing
of expected future cash flows, as well as relevant comparable company earnings multiples for the
market-based approach. The cash flows employed in the discounted cash flow analyses were based on
our internal business model for 2010 and, for years beyond 2010 the growth rates we used were an
estimate of the future growth in the industry in which we participate. The discount rates used in
the discounted cash flow analyses are intended to reflect the risks inherent in the future cash
flows of the reporting unit and are based on an estimated cost of capital, which we determined
based on our estimated cost of capital relative to our capital structure. In addition, the
market-based approach utilizes comparable company public trading values, research analyst estimates
and, where available, values observed in private market transactions. In 2008, our analysis
indicated that the reporting unit fair value was below our book value. The test for impairment of
goodwill is a two-step process:
| • | First, we compare the carrying amount of our reporting unit, which is the book value of our entire company, to the fair value of our reporting unit. If the carrying amount of our reporting unit exceeds its fair value, we have to perform the second step of the process. If not, no further testing is needed. In the fourth quarter of 2008 we determined that the carrying amount of our reporting unit exceeded the fair value and accordingly performed the second step in the analysis. |
F-17
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
| • | If the second part of the analysis is required, we allocate the fair value of our reporting unit to all assets and liabilities as if the reporting unit had been acquired in a business combination at the date of the impairment test. We then compare the implied fair value of our reporting unit’s goodwill to its carrying amount. If the carrying amount of our goodwill exceeds its implied fair value, we recognize an impairment loss in an amount equal to that excess. In the fourth quarter of 2008, the carrying value of goodwill exceeded its implied fair value and accordingly we recorded a non-cash, pre-tax impairment charge of $82.2 million. |
| Other Intangible Assets |
Some of the events that we consider as impairment indicators for our long-lived assets are:
| • | our net book value compared to our fair value; | ||
| • | significant adverse economic and industry trends; | ||
| • | significant decrease in the market value of the asset; | ||
| • | the extent that we use an asset or changes in the manner that we use it; | ||
| • | significant changes to the asset since we acquired it; and | ||
| • | other changes in circumstances that potentially indicate all or a portion of the company will be sold. |
During 2010, 2009 and 2008 we reviewed the carrying value of our long lived assets other than
goodwill and determined that the carrying amounts of such assets was less than the undiscounted
cash flows and accordingly no impairment charge was recorded.
As of December 31, other intangible asset balances were:
| 2010 | ||||||||||||
| Accumulated | Net book | |||||||||||
| Cost | Amortization | Value | ||||||||||
Customer lists |
$ | 114,525 | $ | (54,780 | ) | $ | 59,745 | |||||
Trademarks and trade names |
1,640 | (283 | ) | 1,357 | ||||||||
Internal use software |
15,839 | (7,598 | ) | 8,241 | ||||||||
Acquired technology |
11,390 | (873 | ) | 10,517 | ||||||||
Total |
$ | 143,394 | $ | (63,534 | ) | $ | 79,860 | |||||
| 2009 | ||||||||||||
| Accumulated | Net book | |||||||||||
| Cost | Amortization | Value | ||||||||||
Customer lists |
$ | 77,277 | $ | (46,836 | ) | $ | 30,441 | |||||
Internal use software |
10,625 | (4,759 | ) | 5,866 | ||||||||
Total |
$ | 87,902 | $ | (51,595 | ) | $ | 36,307 | |||||
F-18
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
The estimated useful life and the weighted average remaining lives of the intangible assets as
of December 31, 2010 were as
follows:
| Estimated | Weighted Average | |||
| Useful Life | Remaining Lives | |||
Customer lists
|
10-20 years | 8 years | ||
Trademarks and trade names
|
4 years | 3 years | ||
Capitalized software
|
3 years | 2 years |
Estimated annual amortization expense for intangible assets is as follows:
2011 |
$ | 14,780 | ||
2012 |
12,713 | |||
2013 |
10,447 | |||
2014 |
9,268 | |||
2015 |
8,768 | |||
Thereafter |
23,884 | |||
Total |
$ | 79,860 | ||
Amortization of intangible assets was $11.9 million in 2010, $6.2 million in 2009 and
$5.6 million in 2008.
8. Contractual Obligations
Leases
Minimum rental payments under operating leases are recognized on a straight-line basis over
the term of the lease, including any periods of free rent and landlord incentives. Rental expense
for operating leases for the years ended December 31, 2010, 2009 and 2008 was $3,645, $2,991 and
$3,201, respectively. Future minimum lease payments under non-cancelable operating leases (with
initial or remaining lease terms in excess of one year) as of December 31, 2010 were:
| Total | ||||
2011 |
$ | 4,947 | ||
2012 |
4,848 | |||
2013 |
4,497 | |||
2014 |
2,464 | |||
2015 and thereafter |
2,267 | |||
Total minimum lease payments |
$ | 19,023 | ||
Other Contractual Obligations
The following summarizes our other contractual obligations as of December 31, 2010:
| Severance and Other | ||||||||||||
| Total | Purchase | Obligations | ||||||||||
2011 |
$ | 11,774 | $ | 8,567 | $ | 3,207 | ||||||
2012 |
8,248 | 8,013 | 235 | |||||||||
2013 |
4,042 | 3,807 | 235 | |||||||||
2014 |
1,170 | 924 | 246 | |||||||||
2015 and thereafter |
21 | — | 21 | |||||||||
Total |
$ | 25,255 | $ | 21,311 | $ | 3,944 | ||||||
Purchase obligations represent telecommunication contracts, software development and other
recurring purchase obligations.
F-19
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
We have agreements with certain of our executive officers that provide for severance payments
to the employee in the event the employee is terminated without cause. The maximum cash exposure
under these agreements was approximately $2.5 million at December 31, 2010.
9. Accrued Expenses
Accrued expenses consisted of the following as of December 31:
| 2010 | 2009 | |||||||
Customer Accommodations |
$ | 10,387 | $ | 11,635 | ||||
Accrued Interest |
5,593 | — | ||||||
Restructure |
2,214 | 2,063 | ||||||
Other (No item exceeds 5% of current liabilities) |
8,912 | 7,792 | ||||||
Total accrued expenses |
$ | 27,106 | $ | 21,490 | ||||
In November 2003, one of our employees raised allegations that we had engaged in improper
billing practices. In response, our board of directors undertook an independent review of these
allegations (Review). In response to our customers’ concern over the public disclosure of certain
findings from the Review, we took action in the fourth quarter of 2005 to avoid unnecessary
litigation, which preserved and solidified our customer business relationships by offering a
financial accommodation to certain of our customers
In connection with our decision to offer financial accommodations to certain of our customers
(Accommodation Customers), we analyzed our historical billing information and the available
report-level data (Management’s Billing Assessment) to develop individualized accommodation offers
to be made to Accommodation Customers (Accommodation Analysis). Based on the Accommodation
Analysis, our board of directors authorized management to make cash or credit accommodation offers
to
Accommodation Customers. By accepting our accommodation offer, the customer agreed, among
other things, to release us from any and all claims and liability regarding the billing related
issues.
We are unable to predict how many customers, if any, may accept the outstanding accommodation
offers on the terms proposed by us, nor are we able to predict the timing of the acceptance (or
rejection) of any outstanding accommodation offers. Until any offers are accepted, we may withdraw
or modify the terms of the accommodation program or any outstanding offers at any time. In
addition, we are unable to predict how many future offers, if made, will be accepted on the terms
proposed by us. We regularly evaluate whether to proceed with, modify or withdraw the accommodation
program or any outstanding offers.
The following is a summary of the financial statement activity related to the customer
accommodation.
| 2010 | 2009 | |||||||
Beginning balance |
$ | 11,635 | $ | 12,055 | ||||
Payments and other adjustments |
(1,248 | ) | (317 | ) | ||||
Credits |
— | (103 | ) | |||||
Ending balance |
$ | 10,387 | $ | 11,635 | ||||
10. Commitments and Contingencies
Kaiser Litigation
On June 6, 2008, plaintiffs Kaiser Foundation Health Plan, Inc. and affiliates (collectively,
Kaiser) filed suit against us in the Superior Court of the State of California related to our
billing practices.
F-20
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
In July 2010, the parties reached a settlement of the litigation whereby we made a payment of $2.0 million to resolve all
of Kaiser’s claims. Neither we, nor Kaiser, admitted to any liability or wrongdoing in connection with the settlement. Of this amount, $1.1
million was included in accrued expenses at December 31, 2009 and we expensed an additional $0.9 million during 2010.
Kahn Putative Class Action
In 2008, one of our shareholders filed a shareholder putative class action lawsuit against us,
Koninklijke Philips Electronics N.V. (Philips), our former majority shareholder and four of our
former non-independent directors. We are no longer a defendant in this matter, but we are
monitoring the matter since it involves claims against our former directors.
Reseller Arbitration Demand
On October 1, 2007, we received from counsel to nine current and former resellers of our
products (Claimants), a copy of an arbitration demand filed by the Claimants, initiating an
arbitration proceeding.
On March 31, 2010, the parties entered into a Settlement Agreement and Release pursuant to
which we paid the Claimants $500 on April 1, 2010 to resolve all claims. Under the Settlement
Agreement and Release, (i) the parties exchanged mutual releases, (ii) the arbitration and related
state court litigation were dismissed with prejudice and (iii) we did not admit to any liability or
wrongdoing. We accrued the entire amount of this settlement as of December 31, 2009.
Shareholder Litigation
On February 8, 2011 and February 10, 2011, plaintiffs Victor N. Metallo and Joseph F.
Lawrence, respectively, filed purported shareholder class action complaints in the Superior Court
of New Jersey, Burlington County (Chancery Division) (the Shareholder Litigation). In their
complaints, the plaintiffs purported to be shareholders of MedQuist Inc. and sought to represent a
class of our minority shareholders in pursuit of claims against us, the members of our board of
directors and MedQuist Holdings.
Plaintiffs alleged that the defendants breached certain fiduciary duties they owed to minority
shareholders of MedQuist Inc. in connection with the structuring and disclosure of the Registered
Exchange Offer. Among other things, the plaintiffs alleged that (a) the Registered Exchange Offer
is procedurally and financially unfair, (b) the January 21, 2011 and February 16, 2011 Schedules
14D-9 that we filed with the SEC and the February 3, 2011 Prospectus that MedQuist Holdings filed
with the SEC are materially misleading and incomplete, and (c) the Registered Exchange Offer was
structured by the defendants in order to circumvent the provisions of the New Jersey Shareholders’
Protection Act. Plaintiffs sought, among other things, preliminary and permanent injunctive relief
enjoining consummation of the Registered Exchange Offer, unspecified damages, pre- and
post-judgment interest and attorneys’ fees and costs. The two Plaintiff actions were consolidated
on February 22, 2011 under the caption In Re: MedQuist Inc. Shareholder Litigation, Docket Number
C-018-11.
On March 4, 2011, the parties entered into a memorandum of understanding (the MOU) that
outlined the material terms of the Shareholder Litigation. Under the terms of the MOU, we agreed to
extend the expiration of the Registered Exchange Offer until 5:00 p.m., New York City time, on
Friday, March 11, 2011 and further agreed that if, as a result of the Registered Exchange Offer,
MedQuist Holdings obtained ownership of at least 90% of the outstanding common stock of MedQuist
Inc., MedQuist Holdings will conduct a short-form merger under applicable law to acquire the
remaining shares of MedQuist Inc. common stock that MedQuist Holdings does not currently own at
the same exchange ratio applicable under the Registered Exchange Offer. We agreed to make certain
supplemental disclosures concerning the Registered Exchange Offer, which were contained in an
amendment to Schedule 14D-9 that we filed with the SEC on March 7, 2011. The settlement of the
Shareholder Litigation is conditioned upon, among other things, execution of a Stipulation of
Settlement, notice to all class members, a fairness hearing and final approval of the settlement by
the
court.
F-21
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
SEC Investigation of Former Officer
With respect to our historical billing practices, the SEC is pursuing civil litigation against
our former chief financial officer, whose employment with us ended in July 2004. Pursuant to our
bylaws, we have been providing indemnification for the legal fees for our former chief financial
officer. In February 2011, we entered into a settlement agreement with our former chief financial officer and such
settlement agreement is dependent on the individual settling with the SEC.
In February 2010, one of our current employees, who was our former Controller but who does
not currently serve in a senior management or financial reporting oversight role, reached a
settlement agreement with the SEC in connection with the civil litigation that the SEC had brought
against him in connection with our historical billing practices.
11. Stock Option Plans
Our stock option plans provide for the granting of options to purchase shares of common stock
to eligible employees (including officers) as well as to our non-employee directors. Options may be
issued with the exercise prices equal to the fair market value of the common stock on the date of
grant or at a price determined by a committee of our board of directors. Stock options vest and are
exercisable over periods determined by the committee, generally five years, and expire no more than
10 years after the grant.
Information with respect to our common stock options is as follows:
| Weighted | Weighted Average |
|||||||||||||||
| Shares | Average | Remaining | Aggregate | |||||||||||||
| Subject to | Exercise | Contractual | Intrinsic | |||||||||||||
| Options | Price | Life in Years | Value | |||||||||||||
Outstanding, January 1, 2008 |
2,359 | $ | 31.08 | |||||||||||||
Granted |
296 | $ | 11.20 | |||||||||||||
Exercised |
(12 | ) | $ | 2.71 | ||||||||||||
Forfeited |
(827 | ) | $ | 29.10 | ||||||||||||
Outstanding, December 31, 2008 |
1,816 | $ | 23.34 | |||||||||||||
Granted |
— | $ | — | |||||||||||||
Exercised |
— | $ | — | |||||||||||||
Forfeited |
(553 | ) | $ | 22.57 | ||||||||||||
Outstanding, December 31, 2009 |
1,263 | $ | 24.47 | 3.3 | $ | — | ||||||||||
Granted |
— | $ | — | |||||||||||||
Exercised |
— | $ | — | |||||||||||||
Forfeited |
(261 | ) | $ | 44.84 | ||||||||||||
Outstanding, December 31, 2010 |
1,002 | $ | 17.39 | 3.0 | $ | 1,902 | ||||||||||
Exercisable, December 31, 2010 |
903 | $ | 19.05 | 2.5 | $ | 1,268 | ||||||||||
Options vested and expected
to vest as of December 31, 2010 |
1,002 | $ | 17.39 | 3.0 | $ | 1,902 | ||||||||||
The computation of diluted net income (loss) per share does not assume conversion, exercise or
issuance of shares that would have an anti-dilutive effect on diluted net income (loss) per share.
For the years ended December 31. 2010, and 2009, 692 and 1,262 options, respectively, were
excluded from the computation of diluted earnings per share as the options exercise price was
greater than the average market price of the common stock during the respective period. During 2008
we had a net loss. As a result, any assumed conversions would result in reducing the net loss per
share and, therefore, are not included in the calculation. Shares having an anti-dilutive effect on
net loss per share and, therefore, excluded from the calculation of diluted net loss per share,
totaled 1,501 for the year ended December 31, 2008.
The aggregate intrinsic value is calculated using the difference between the closing stock
price on the last trading day of 2010 and the option exercise price, multiplied by the number of
in-the-money options. As of December 31, 2010, 296 options were in the money.
There were no options granted or exercised in 2010 or 2009. There were 296 options granted and
12 options exercised in 2008. In the first quarter of 2009, we modified the options previously
granted in the third quarter of 2008 to our Chief Executive Officer. The grant price was increased
from $4.85 per share to $8.25 per share by a committee of our Board of Directors. There was no
incremental cost of the modification. We estimated fair value for the modified option grant as of
the date of the modification by applying the Black-Scholes option pricing valuation model. The
application of this model involves assumptions that are judgmental and sensitive in the
determination of compensation expense. The key assumptions used in determining the fair value of
the options modified in the
F-22
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
first quarter of 2009 were:
Expected term (years) |
5.92 | |||
Expected volatility |
54.5 | % | ||
Dividend yield |
0 | % | ||
Expected risk free interest rate |
3.25 | % |
Significant assumptions required to estimate the fair value of stock options include the
following:
| • | Expected term: The SEC Staff Accounting Bulletin No. 107 “Simplified” method has been used to determine a weighted average expected term of options granted. | ||
| • | Expected volatility: We have estimated expected volatility based on the historical stock price volatility of a group of similar publicly traded companies. We believe that our historical volatility is not indicative of future volatility. |
The weighted average grant date fair value of options modified in the first quarter of 2009
was $1.97 per share.
In the fourth quarter of 2010, in accordance with the terms of the option, the Compensation
Committee approved an adjustment to the exercise price of the Chief Executive Officer’s option from
$8.25 per share to $2.22 per share to account for the payment of an extraordinary dividend of $1.33
per share in September 2009 and $4.70 per share in October 2010 to our shareholders. There was no
incremental cost of the adjustment.
The weighted average grant date fair value of options modified in the fourth quarter of 2010
was $7.28 per share.
A summary of outstanding and exercisable options as of December 31, 2010 is as follows:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Weighted | ||||||||||||||||||||
| Average | Weighted | Weighted | ||||||||||||||||||
| Remaining | Average | Average | ||||||||||||||||||
| Range of | Number | Contractual Life | Exercise | Number | Exercise | |||||||||||||||
| Exercise Prices | of Shares | (in years) | Price | of Shares | Price | |||||||||||||||
$2.22-$10.00 |
296 | 7.8 | $ | 2.22 | 197 | $ | 2.22 | |||||||||||||
$10.01-$20.00 |
222 | 1.6 | $ | 17.16 | 222 | $ | 17.16 | |||||||||||||
$20.01-$30.00 |
452 | 0.8 | $ | 26.29 | 452 | $ | 26.29 | |||||||||||||
$30.01-$40.00 |
29 | 0.0 | $ | 32.36 | 29 | $ | 32.36 | |||||||||||||
$40.01-$70.00 |
3 | 0.1 | $ | 44.21 | 3 | $ | 44.21 | |||||||||||||
| 1,002 | 3.0 | $ | 17.39 | 903 | $ | 19.05 | ||||||||||||||
No
options were granted or exercised in 2010 or 2009. There were 12 options exercised in 2008.
The total fair value of shares vested during 2010 and 2009 was $193 and $193, respectively.
The change in ownership which occurred on August 6, 2008 was a change in control as defined in the
employment agreements for certain option holders. This resulted in the immediate vesting of
previously unvested stock options. All previously unamortized stock option compensation expense
related to such stock options was recognized as of August 6, 2008 resulting in a charge of
approximately $1,060. Also recorded in 2008 was stock option compensation expense of $367, not
related to the change in control.
As
of December 31, 2010, there were 994 additional options available for grant under our stock
option plans.
For the years ended December 31, 2010, 2009 and 2008, $194, $193 and $937, respectively, was
included as selling, general and administrative expenses and $0, $0 and $402 was included in
research and development expenses in the accompanying consolidated statements of operations related
specifically to these options granted to certain executive officers.
On August 27, 2009, our board of directors, upon the recommendation of its compensation
committee, approved the MedQuist Inc. Long-Term Incentive Plan. The Incentive Plan is designed to
encourage and reward the creation of long-term equity value by certain members of our senior
management team. The executives and key employees will be selected by the Compensation Committee
F-23
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
and will be eligible to participate in the Incentive Plan. During the third quarter of 2010, the
Compensation Committee awarded grants under the Long Term Incentive Plan. In December 2010, the
Compensation Committee approved the terms and conditions of the plan. As of December 2010 no
amounts have been recorded as compensation expense under the Long Term Incentive Plan as the
amounts were not material.
12. Income Taxes
The sources of income (loss) before income taxes and the income tax provision (benefit) for
the years ended December 31, 2010, 2009 and 2008 are as follows:
| 2010 | 2009 | 2008 | ||||||||||
Income (loss) before income taxes: |
||||||||||||
Domestic |
$ | 30,278 | $ | 24,314 | $ | (83,876 | ) | |||||
Foreign |
1,444 | 952 | (1,432 | ) | ||||||||
Income (loss) before income taxes |
$ | 31,722 | $ | 25,266 | $ | (85,308 | ) | |||||
Current income tax provision: |
||||||||||||
Federal |
$ | (12 | ) | $ | (660 | ) | $ | 107 | ||||
State and local |
296 | 131 | 216 | |||||||||
Foreign |
576 | 647 | 255 | |||||||||
Current income tax provision |
860 | 118 | 578 | |||||||||
Deferred income tax provision (benefit): |
||||||||||||
Federal |
858 | 1,724 | (15,694 | ) | ||||||||
State and local |
630 | 235 | (2,379 | ) | ||||||||
Foreign |
(1,677 | ) | (102 | ) | 982 | |||||||
Deferred income tax provision (benefit) |
(189 | ) | 1,857 | (17,091 | ) | |||||||
Income tax provision (benefit) |
$ | 671 | $ | 1,975 | $ | (16,513 | ) | |||||
The reconciliation of the statutory federal income tax rate to our effective income tax rate
is as follows:
| 2010 | 2009 | 2008 | ||||||||||
Statutory federal income tax rate |
35.0 | % | 35.0 | % | 35.0 | % | ||||||
State income taxes, net of federal tax effect |
1.7 | 0.9 | 3.9 | |||||||||
Valuation allowance |
(35.9 | ) | (29.8 | ) | (10.4 | ) | ||||||
Goodwill impairment |
— | — | (7.6 | ) | ||||||||
Impact of foreign operations |
2.5 | 3.2 | (0.4 | ) | ||||||||
Adjustments to tax reserves |
(1.3 | ) | (0.3 | ) | 0.2 | |||||||
Permanent differences |
0.1 | (0.9 | ) | (0.3 | ) | |||||||
Other |
— | (0.3 | ) | (1.0 | ) | |||||||
Effective income tax rate |
2.1 | % | 7.8 | % | 19.4 | % | ||||||
Deferred income taxes reflect the net tax effect of temporary differences between the
carrying amounts of assets and liabilities for financial reporting purposes and the amounts used
for income tax purposes. Significant components of our deferred tax assets and liabilities as of
December 31, 2010, 2009 and 2008 were as follows:
F-24
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
| 2010 | 2009 | 2008 | ||||||||||
Deferred tax assets: |
||||||||||||
Foreign net operating loss carry forwards |
$ | 1,659 | $ | 1,957 | $ | 1,892 | ||||||
Domestic net operating loss carry forwards |
38,336 | 48,397 | 47,694 | |||||||||
Accounts receivable |
1,227 | 1,226 | 1,859 | |||||||||
Property and equipment |
2,093 | 2,020 | 1,563 | |||||||||
Intangibles |
11,308 | 12,784 | 20,976 | |||||||||
Employee compensation and benefit plans |
2,823 | 1,555 | 1,149 | |||||||||
Deferred compensation |
— | — | 168 | |||||||||
Customer accommodation |
3,470 | 4,515 | 4,668 | |||||||||
Accruals and reserves |
2,250 | 2,805 | 3,613 | |||||||||
Other |
5,107 | 2,554 | 2,129 | |||||||||
Total gross deferred tax assets |
68,273 | 77,813 | 85,711 | |||||||||
Less: Valuation allowance |
(63,268 | ) | (74,641 | ) | (81,785 | ) | ||||||
Total deferred tax assets |
5,005 | 3,172 | 3,926 | |||||||||
Deferred tax liabilities: |
||||||||||||
Intangibles |
(5,731 | ) | (3,925 | ) | (2,907 | ) | ||||||
Other |
(1,189 | ) | (1,095 | ) | (1,265 | ) | ||||||
Total deferred tax liabilities |
(6,920 | ) | (5,020 | ) | (4,172 | ) | ||||||
Net deferred tax liability |
$ | (1,915 | ) | $ | (1,848 | ) | $ | (246 | ) | |||
As of December 31, 2010, we had federal net operating loss carry forwards of
approximately $78 million which will begin to expire in 2026 and 2028.
As of December 31, 2010 and 2009, we had state net operating loss (NOL) carry forwards of
approximately $216 million and $225 million, respectively, which will expire between 2011 and 2029.
In addition, we have foreign NOL carry forwards of approximately $10 million, which do not expire.
Utilization of the NOL carry forwards will be subject to an annual limitation in future years as a
result of the change in ownership as defined by Section 382 of the Internal Revenue Code and
similar state provisions. We performed an analysis on the annual limitation as a result of the
ownership change that occurred in 2008. As a result of this analysis, the annual limitation in
future years would not prevent us from using the federal NOL carry forwards before their respective
expiration periods.
During 2010, we reduced a portion of our valuation allowance against our deferred tax assets
generated in foreign tax jurisdictions based on management’s assessment of future earnings
available to utilize these deferred tax assets. As a result of the release of the valuation
allowance, we recognized a foreign deferred tax benefit of $1,310.
After consideration of all evidence, both positive and negative, management concluded that it
was more likely than not that a majority of the domestic deferred tax assets would not be realized.
As of December 31, 2010, this valuation allowance has been decreased from $73.3 million to $63.3
million. Although management currently believes that a valuation allowance is required at December
31, 2010, it is at least reasonably possible that this belief could change in the near term,
resulting in the release of all or a substantial portion of the valuation allowance.
Domestic net deferred tax assets were recognized to the extent that objective positive
evidence existed with respect to their future
utilization. The objective positive evidence included the potential to carry back any losses
generated by the deferred tax assets in the future as well as income expected to be recognized due
to the reversal of deferred tax liabilities as of December 31, 2010. In analyzing deferred tax
liabilities as a source for potential income for purposes of recognizing deferred tax assets, the
deferred tax liabilities related to excess book basis in goodwill over tax basis in goodwill were
considered a source of future income for benefiting deferred tax assets with indefinite lives only
due to the indefinite life and uncertainty of reversal of these liabilities during the same period
as the non-indefinite life deferred tax assets.
F-25
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
Our consolidated income tax expense for the years ended December 31, 2010 and December 31,
2009 consists principally of an increase in deferred tax liabilities related to goodwill
amortization deductions for income tax purposes during the applicable year as well as state and
foreign income taxes. Our consolidated income tax benefit for the year ended December 31, 2008
consists primarily of the reversal of $18.5 million of deferred tax liabilities associated with
indefinite life intangible assets related to goodwill which was impaired in 2008, offset by state
and foreign income tax expense.
We provide reserves for income taxes in accordance with the guidance contained in ASC 740 for
uncertainty in taxes. ASC 740 prescribes a recognition threshold and measurement attributes for
the financial statement recognition and measurement of uncertain tax positions taken or expected to
be taken in a company’s income tax return and utilizes a two-step approach for evaluating uncertain
tax positions. Step one, Recognition, requires a company to determine if the weight of available
evidence indicates that a tax position is more likely than not to be sustained upon audit,
including resolution of related appeals or litigation processes, if any. Step two, Measurement, is
based on the largest amount of benefit, which is more likely than not to be realized on settlement
with the taxing authority.
The total amount of unrecognized tax benefits as of December 31, 2010 was $4,657 which
includes $116 of accrued interest related to unrecognized income tax benefits which we recognize as
a component of the provision for income taxes. Of the $4,657 unrecognized tax benefits, $4,152
relates to tax positions which if recognized would impact the effective tax rate, not considering
the impact of any valuation allowance. Of the $4,152, $3,503 is attributable to uncertain tax
positions with respect to certain deferred tax assets which if recognized would currently be offset
by a full valuation allowance due to the fact that at the current time it is more likely than not
that these assets would not be recognized due to a lack of sufficient projected income in the
future.
We file income tax returns in the U.S. federal jurisdiction, all U.S. states which require
income tax returns, and foreign jurisdictions. Due to the nature of our operations, no state or
foreign jurisdiction is individually significant. With limited exceptions we are no longer subject
to examination by U.S. federal or states jurisdictions for years prior to 2007. The Internal
Revenue Service concluded its federal tax audit for years 2003 through 2006 with no material
adjustments. We are no longer subject to examination by the UK federal jurisdiction for years
prior to 2008. We do have various state tax audits and appeals in process at any given time.
The following is a roll-forward of the changes in our unrecognized tax benefits:
Total unrecognized tax benefits as of January 1, 2010 |
$ | 4,831 | ||
Gross amount of decreases in unrecognized tax benefits as a result of tax positions taken during the prior period |
(2 | ) | ||
Gross amount of increases in unrecognized tax benefits as a result of tax positions taken during the prior period |
— | |||
Gross amount of increases in unrecognized tax benefits as a result of tax positions taken during the current period |
575 | |||
Amount of decreases in the unrecognized tax benefits relating to settlements with taxing authorities |
(826 | ) | ||
Reduction to unrecognized tax benefits as a result of a lapse of applicable statute of limitations |
(38 | ) | ||
Total unrecognized tax benefits as of December 31, 2010 |
$ | 4,540 | ||
Total unrecognized tax benefits that would impact the effective tax rate if recognized |
$ | 4,152 | ||
Total amount of interest and penalties recognized in the accompanying consolidated statement of operations
for the year ended December 31, 2010 |
$ | (239 | ) | |
Total amount of interest and penalties recognized in the accompanying consolidated balance sheet as of
December 31, 2010 |
$ | 116 | ||
We
anticipate decreases in unrecognized tax benefits of approximately $118 related to
state statutes of limitations expiring during 2011. Our unrecognized tax benefits are expected to
change in 2011. However, we do not anticipate any significant increases or decreases within the
next twelve months.
F-26
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
13. Employee Benefit Plans
401(k) Plan
We maintain a tax-qualified retirement plan named the MedQuist 401(k) Plan (401(k) Plan) that
provides eligible employees with an opportunity to save for retirement on a tax advantaged basis.
Our 401(k) Plan allows eligible employees to contribute up to 25% of their annual eligible
compensation on a pre-tax basis, subject to applicable Internal Revenue Code limits. Elective
deferral contributions are allocated to each participant’s individual account and are then invested
in selected investment alternatives according to the participant’s directives. Employee elective
deferrals are 100% vested at all times. Our 401(k) Plan provides that we may make a discretionary
matching contribution to the participants in the 401(k) Plan. We did not match the employee
contributions for the years ended December 31, 2010, 2009 and 2008.
14. Related-Party Transactions
MedQuist Holdings had an approximately 69.5% ownership interest in us at December 31, 2010.
We have an agreement with a wholly-owned subsidiary of MedQuist Holdings, CBay Systems &
Services, Inc. (CBay Services), under which we outsource medical transcription services. We
incurred expenses of $34,038, $6,798 and $283 for the years ended December 31, 2010, 2009 and
2008. All medical transcription expenses were recorded in Cost of revenues in the accompanying
consolidated statements of operations. We recorded other expense of $541 in 2010 recorded in
research and development.
We also have a subcontracting agreement (Subcontracting Agreement) with CBay Services,
pursuant to which CBay Services subcontracts medical transcription, editing and related services to
us. For the years ended December 31, 2010, 2009 and 2008, we recorded revenue of $2,292, $1,483,
and $0, respectively.
We have a Management Services Agreement with CBay Inc, pursuant to which certain senior
executives and directors of CBay Inc. provide certain advisory and consulting services. The
Management Services Agreement provides that, in consideration of the management services rendered
by CBay Inc. to us since July 1, 2009 we pay CBay Inc. a quarterly services fee equal to $350,
payable in arrears. For the years ended December 31, 2010 and 2009, and 2008, we incurred $1.4
million, $1.4 million, and $0 in services expenses with CBay Inc. All Management Services Agreement
costs incurred have been recorded as selling, general and administrative expenses in the
accompanying consolidated statements of operations.
The Related party payable of $5.4 million and $1.4 million at December 31, 2010 and 2009,
respectively, primarily represent amounts payable to MedQuist Holdings for transcription and
other services provided. We paid MedQuist Holdings $4.6 million for a related party liability
assumed in the Spheris acquisition. MedQuist Holdings provided guarantees related to our Senior
Secured Credit Facility.
As of December 31, 2010 and 2009, Accounts receivable in the accompanying consolidated balance
sheets included $753 and $710, respectively, for amounts due from MedQuist Holdings.
On May 4, 2010 we recorded a $1.5 million success-based transaction fee, which was included in
acquisition related charges, to SAC Private Capital Group, LLC (SAC) for work performed on the
Spheris acquisition. SAC owns a majority interest in MedQuist Holdings.
Prior to August 6, 2008, Koninklijke Philips Electronics N.V. (Philips) had approximately a
69.5% ownership interest in us. There were no amounts due to or from Philips on our balance sheets
at December 31, 2010 or 2009. Prior to August 6, 2008 we incurred $4,479 in expenses to Philips,
which were recorded primarily in cost of revenues.
15. Cost of Legal Proceedings and Settlements
For the years ended December 31, 2010, 2009 and 2008, we recorded charges of $3,603, $14,843
and $19,738, respectively, for costs associated with legal proceedings and settlements. The
following is a summary of the amounts recorded in the accompanying consolidated statements of
operations:
F-27
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
| 2010 | 2009 | 2008 | ||||||||||
Legal fees |
$ | 2,693 | $ | 8,593 | $ | 12,313 | ||||||
Settlements |
910 | 6,250 | 7,425 | |||||||||
Total |
$ | 3,603 | $ | 14,843 | $ | 19,738 | ||||||
The amounts included in settlements for 2010 represent an additional charge of $0.9 related to
our settlement with Kaiser Foundation Health Plan, Inc., and affiliates. The amounts included in
settlements for 2009 represent the settlement of a patent litigation matter. The 2008 amount of
$7,425 was for the settlements of all claims related to the consolidated medical transcriptionists
putative class action and the Department of Justice (DOJ) investigation.
16. Restructuring Charges
2010 Restructuring Plan
Management’s ongoing cost reduction initiatives, including process improvement, combined with
the acquisition of Spheris, resulted in a restructuring plan involving staff reductions and other
actions designed to maximize operating efficiencies. The affected employees are entitled to receive
severance benefits under existing established severance policies. The employees affected were
primarily in the operations and administrative functions. Management approved the initial actions
under the plan during 2010.
Beginning balance |
$ | — | ||
Charge |
3,460 | |||
Cash paid |
(1,421 | ) | ||
Ending balance |
$ | 2,039 | ||
The Company expects that restructuring activities may continue in 2011 as management
identifies opportunities for synergies resulting from the acquisition of Spheris including the
elimination of redundant functions and locations.
2009 Restructuring Plan
During the third and fourth quarters of 2009, as a result of management’s continued planned
process improvement and technology development investments we committed to an exit and disposal
plan which includes projected employee severance for planned reduction in headcount. Because of
plan development in late 2009 and execution of the plan over multiple quarters in 2009 and 2010,
not all personnel affected by the plan know of the plan or its impact. The plan includes costs of
$2.5 million for employee severance and $0.3 million for vacating operating leases. The table below
reflects the financial statement activity related to the 2009 restructuring plan and is included in
accrued expenses in the accompanying consolidated balance sheets:
| For the twelve months ended | ||||||||
| December 31, | ||||||||
| 2010 | 2009 | |||||||
Beginning balance |
$ | 2,064 | $ | — | ||||
Charge (reversal) |
(631 | ) | 2,810 | |||||
Cash paid |
(1,258 | ) | (746 | ) | ||||
Ending balance |
$ | 175 | $ | 2,064 | ||||
We expect the remaining balance to be paid in 2011.
17. Investment in A-Life Medical, Inc. (A-Life)
We previously had an investment of $8,864 in A-Life, a privately held entity which provides
advanced natural language processing technology for the medical industry that was recorded under
the equity method of accounting. In October 2010 we sold our shares in A-Life for cash
consideration of $23.6 million, of which $4.1 million will be held in escrow until March 2012. We
recorded a pretax gain of $9.9 million. For the year ended December 31, 2009, our investment
increased by $2,015 related to our share
F-28
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Notes to Consolidated Financial Statements — (Continued)
of A-Life’s net income, primarily related to a gain
resulting from an acquisition, additional cash investments in A-Life of $852 offset by $255 for a
dilution, which was recorded in shareholders’ equity in our consolidated balance sheet. Our
investment in A-Life had been recorded in Other assets in the accompanying consolidated balance
sheets. We will record the remaining gain, if any, in 2012 when escrow is released.
18. Quarterly Data
(unaudited)
| 1st | 2nd | 3rd | 4th | |||||||||||||
| Quarter | Quarter | Quarter | Quarter | |||||||||||||
2010 |
||||||||||||||||
Net revenues |
$ | 73,981 | $ | 97,528 | (4) | $ | 102,933 | $ | 100,798 | |||||||
Cost of revenues |
$ | 49,833 | $ | 67,090 | $ | 68,427 | $ | 64,221 | ||||||||
Net income |
$ | 7,344 | $ | 880 | (3) | $ | 8,971 | $ | 13,856 | (5) | ||||||
Net income per share: |
||||||||||||||||
Basic |
$ | 0.20 | $ | 0.02 | $ | 0.24 | $ | 0.37 | ||||||||
Diluted |
$ | 0.20 | $ | 0.02 | $ | 0.24 | $ | 0.37 | ||||||||
Weighted average shares outstanding: |
||||||||||||||||
Basic |
37,556 | 37,556 | 37,556 | 37,556 | ||||||||||||
Diluted |
37,556 | 37,556 | 37,556 | 37,590 | ||||||||||||
2009 |
||||||||||||||||
Net revenues |
$ | 78,944 | $ | 77,471 | $ | 76,836 | $ | 73,949 | ||||||||
Cost of revenues |
$ | 53,868 | $ | 51,357 | $ | 52,768 | $ | 48,272 | ||||||||
Net income |
$ | 6,854 | $ | 836 | (1) | $ | 9,704 | $ | 5,897 | (2) | ||||||
Net income per share: |
||||||||||||||||
Basic |
$ | 0.18 | $ | 0.02 | $ | 0.26 | $ | 0.16 | ||||||||
Diluted |
$ | 0.18 | $ | 0.02 | $ | 0.26 | $ | 0.16 | ||||||||
Weighted average shares outstanding: |
||||||||||||||||
Basic |
37,556 | 37,556 | 37,556 | 37,556 | ||||||||||||
Diluted |
37,556 | 37,556 | 37,560 | 37,556 | ||||||||||||
| (1) | Includes $5,750 recorded in cost of legal proceedings and settlements, related to the settlement of all claims related a patent litigation settlement. | |
| (2) | Includes $500 recorded in cost of legal proceedings and settlements, related to the settlement of all claims related to a reseller arbitration settlement. | |
| (3) | Includes $4.8 million of acquisition and related integration charges, and $1.1 million in cost of legal proceedings and settlements. | |
| (4) | Includes the results of operations of Spheris from the date of acquisition in April 2010. | |
| (5) | In October 2010, we recorded a pre-tax gain of $9.9 million on the sale of our investment in A-Life and we recorded a loss on extinguishment of debt of $5.8 million. |
F-29
Table of Contents
EXHIBIT INDEX
| No. | Description | |
23
|
Consent of KPMG LLP | |
24
|
Power of Attorney (included on the signature page hereto) | |
31.1
|
Certification of Chief Executive Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
31.2
|
Certification of Chief Financial Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
32.1
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
32.2
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| # | Portions of this Exhibit were omitted and filed separately with the Secretary of the SEC pursuant to a request for confidential treatment that has been filed with the SEC. |
