Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ENDO HEALTH SOLUTIONS INC. | d8k.htm |
 ENDO
PHARMACEUTICALS Barclays Capital 2011 Global Healthcare Conference
March 15, 2011
grow. collaborate. innovate.
thrive. Exhibit
99.1 |
 grow.
collaborate. innovate. thrive. 2
FORWARD LOOKING STATEMENTS
This presentation contains forward-looking statements within the meaning of the Private
Securities Litigation
Reform
Act
of
1995.
Statements
including
words
such
as
“believes,”
“expects,”
“anticipates,”
“intends,”
“estimates,”
“plan,”
“will,”
“may,”
“look forward,”
“intend,”
“guidance,”
“future”
or similar
expressions are forward-looking statements. Because these statements reflect our
current views, expectations and beliefs concerning future events, these
forward-looking statements involve risks and uncertainties. Investors should note
that many factors, as more fully described under the caption “Risk
Factors”
in our Form 10-K, Form 10-Q and Form 8-K filings with the Securities and Exchange
Commission and as otherwise enumerated herein or therein, could affect our future
financial results and could cause our actual results to differ materially from those
expressed in forward-looking statements contained in our Annual Report on Form
10-K. The forward-looking statements in this presentation are qualified by
these risk factors. These are factors that, individually or in the aggregate, could cause our
actual results to differ materially from expected and historical results. We assume no
obligation to publicly update any forward-looking statements, whether as a result
of new information, future developments or otherwise. ©2011 Endo Pharmaceuticals
Inc. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
PRESENTATION OVERVIEW
3
I.
Sustaining Growth
II.
Our Business Model:
Branded Pharmaceuticals
Devices and Services
Generic Pharmaceuticals
III.
Financial Outlook |
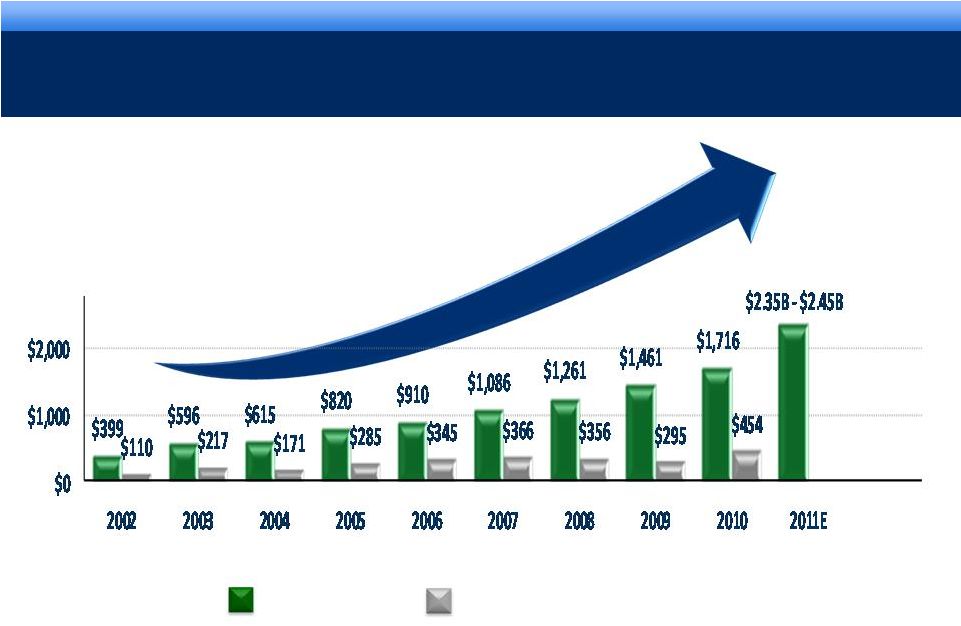 STRONG
OPERATING PERFORMANCE 16% 3-YEAR CAGR FOR REVENUE*
* Revenue CAGR 2007-2010.
$mm
4
Revenue
Cash Flow from Operations
grow. collaborate. innovate. thrive.
©2011 Endo Pharmaceuticals Inc.
Sustaining our Growth |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
5
Through Organic Growth
•
Diversified business lines to maximize growth
•
Enhanced commercial model driving growth via
strategic resource deployment
•
Invested in R&D portfolio yielding a diversified
pipeline of products and a recent FDA approval
•
Bolstered management team by adding
expertise and experience in managing and
growing a larger enterprise
5
ENDO’S TRANSFORMATION
Through Strategic Growth
•
With Indevus, we secured a position in urology
•
HealthTronics gave us an established presence
in Devices & Services and critical mass in
urology
•
Penwest strengthened our pain business
enhancing profitability & flexibility in the opioid
franchise
•
Qualitest brings critical mass to our generics
business & strengthens our pain portfolio |
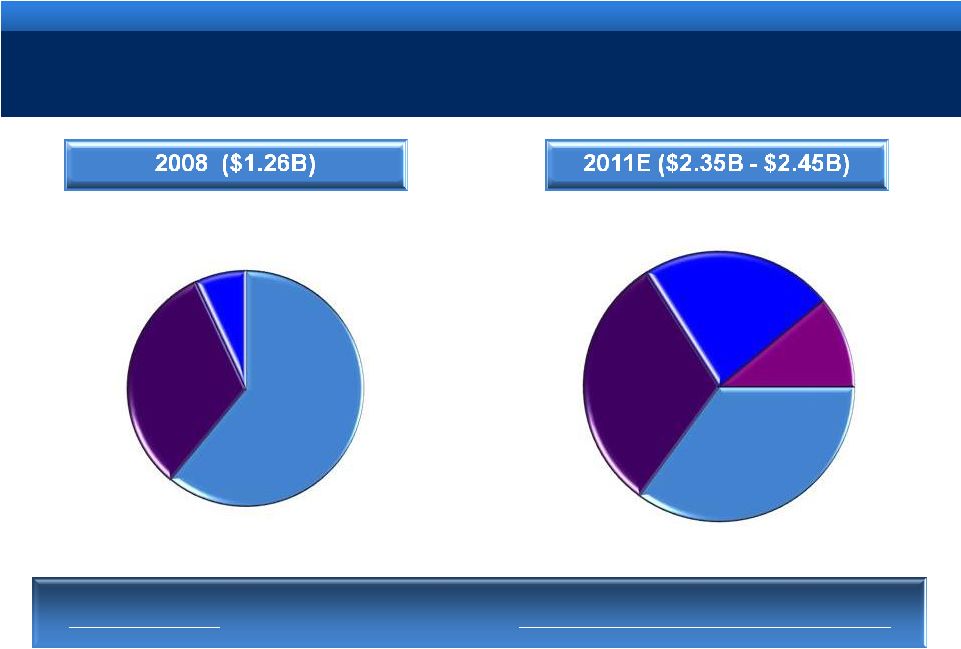 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
6
DIVERSIFICATION OF BUSINESS
Diversifying
our revenues through organic and strategic growth
Lidoderm
Other
Brands
Generics
Devices &
Services
Lidoderm
Other
Brands
Generics |
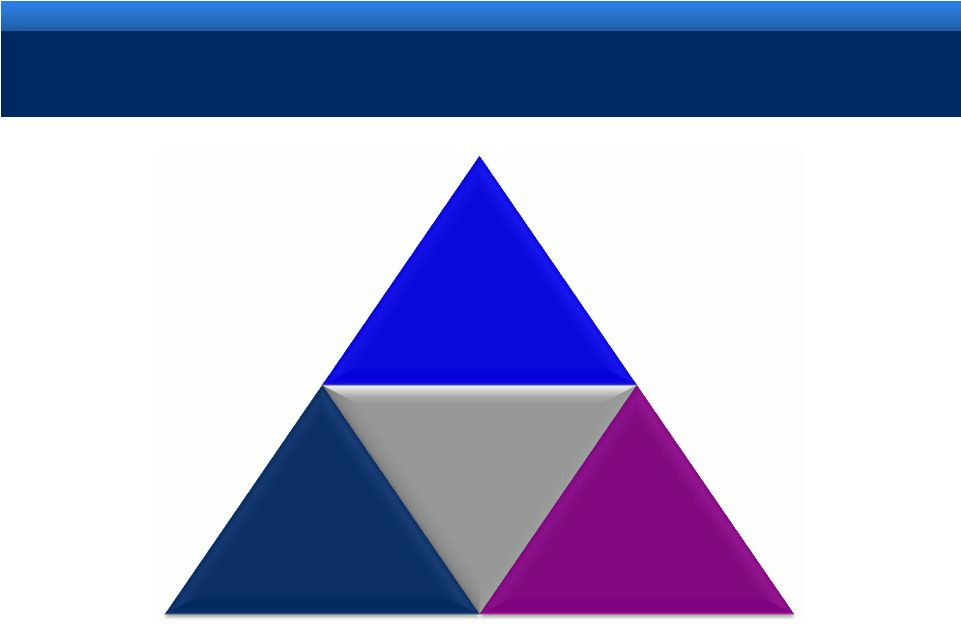 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
7
ENDO’S INTEGRATED BUSINESS MODEL
Branded
Pharmaceuticals
Generics
Pain
Urology
Oncology
Endocrinology
Devices
& Services |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
PRESENTATION OVERVIEW
8
I.
Sustaining Growth
II.
Our Business Model:
Branded Pharmaceuticals
Devices and Services
Generic Pharmaceuticals
III.
Financial Outlook |
 grow.
collaborate. innovate. thrive. NEW TESTOSTERONE REPLACEMENT THERAPY
14 million men suffer with hypogonadism in US
9% are treated
Market TRx Net Sales: >$1.2B
Growth Rate: approximately 20% CAGR last 5 years
Gels have the greatest utilization in the TRT market: ~72%
PCPs account for majority of TRT prescriptions:
More than 60% through Primary Care Physicians
Approximately 30% through specialty (Urologists/Endocrinologists)
Method of Payment for TRT class:
Approximately 80% Commercial
Approximately 12% Government
Remainder from Cash Payment
9
©2011 Endo Pharmaceuticals Inc. |
 STRONG CORE
BUSINESS SUPPORTING GROWTH 10
grow. collaborate. innovate. thrive.
©2011 Endo Pharmaceuticals Inc. |
 grow.
collaborate. innovate. thrive. STRONG CORE BUSINESS SUPPORTING GROWTH
11
LIDODERM®
key component of our core business
Strong source of operating cash flow
Expect low single digit sales growth in 2011
Stable TRx trends and solid managed care positioning
10-year Commercial launch anniversary in September 2009
Differentiated
product
profile
provides
unique
offering
for
HCPs
and
patients suffering from PHN
Improved PHN physician targeting for more efficient utilization
of resources
Paragraph IV filings update:
Filed suit on March 14, 2011 in response to
Mylan
Technologies’
Paragraph IV Notice
©2011 Endo Pharmaceuticals Inc. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
©2011 Endo Pharmaceuticals Inc.
LONG-ACTING OPIOID FRANCHISE
OPANA®
ER
Settlement with generic companies provides for 2013 entry on primary dosage forms
>35% YOY TRx growth for full year 2010
Gaining share; Growing source of cash flow
Completed Acquisition of Penwest
Advances our leadership and growth in pain management
New formulation of OPANA ER designed to be crush resistant
Complete Response Letter from FDA on January 7, 2011
Letter did not require additional clinical trials
Confident that we can address the issue set forth
Currently anticipate responding to the FDA by mid-2011
Expect a 6-month review cycle once our response is filed
12 |
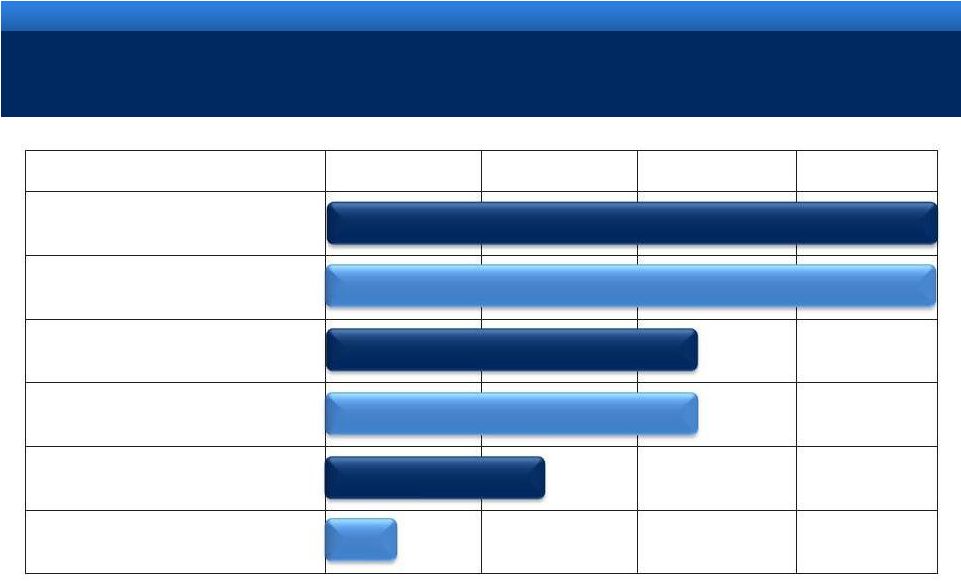 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
©2011 Endo Pharmaceuticals Inc.
DEVELOPMENT PIPELINE*
* There can be no assurance that any of these development programs
will be successful or if successful, the products will ultimately be approved by FDA.
** Granted orphan drug designation
*** Licensed from Orion Corporation for joint development and
commercialization 13
Phase I
Phase II
Phase III
NDA
OPANA®
ER
Formulation designed to be crush-resistant
AVEED
TM
Long Acting Injectable Testosterone
Urocidin
TM
Bladder Cancer
Octreotide Implant
Acromegaly**
Axomadol
Moderate to moderately severe chronic pain
Androgen Receptor Agonist***
Castration Resistant Prostate Cancer
Pending
Update Pending |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
©2011 Endo Pharmaceuticals Inc.
EXPANDING OUR RESEARCH CAPABILITIES
14
Building capabilities and investing for organic growth
Creating virtual discovery networks at multiple stages of
discovery and early development
Select partnerships:
Discovery: Aurigene and Jubilant
Early development: Orion
Flexible, efficient relationships focused on value creation |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
PRESENTATION OVERVIEW
15
I.
Sustaining Growth
II.
Our Business Model:
Branded Pharmaceuticals
Devices and Services
Generic Pharmaceuticals
III.
Financial Outlook |
 ©2011
Endo Pharmaceuticals Inc. HEALTHTRONICS SERVICES
16
grow. collaborate. innovate. thrive. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
Established business channel with 1/3 of urologists in U.S.
Furthers our commitment to the urologist community
Opportunities for a complete suite of Endo products in this specialty:
Branded Drugs
Generic Drugs
Devices and Services
Total solution for the urology marketplace
Improve patient care
Enhance practice economics
HEALTHTRONICS OVERVIEW
17 |
 grow.
collaborate. innovate. thrive. PRESENTATION OVERVIEW
18
I.
Sustaining Growth
II.
Our Business Model:
Branded Pharmaceuticals
Devices and Services
Generic Pharmaceuticals
III.
Financial Outlook
©2011 Endo Pharmaceuticals Inc. |
 grow.
collaborate. innovate. thrive. 19
QUALITEST -
A STRATEGIC FIT
Furthers our stated strategy to build a healthcare company better able
to respond to the changing economics that drive the U.S. healthcare
environment
Adds critical mass to our current generics business, alongside
Branded Pharmaceuticals and Devices & Services
Enhances our core pain franchise
As a high-growth asset, will significantly diversify and accelerate the
growth of Endo’s revenue and earnings’
streams
©2011 Endo Pharmaceuticals Inc. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
20
A STRONGER GENERICS BUSINESS WITH ROBUST
PIPELINE
Combined Generics Business
•
Solid commercial base entering 2011
•
Expect to file 14 ANDAs in 2011
•
45 ANDAs currently under review by FDA
•
Expect 14 ANDA product launches in 2011
We expect our combined generics business to grow over 15% over the
2010-12 period driven by launches from the development portfolio
2011 Generic Development
14 ANDA
Filings
45 Current
ANDA
Reviews
14 ANDA
Product
Launches |
 grow.
collaborate. innovate. thrive. PRESENTATION OVERVIEW
21
I.
Sustaining Growth
II.
Our Business Model:
Branded Pharmaceuticals
Devices and Services
Generic Pharmaceuticals
III.
Financial Outlook
©2011 Endo Pharmaceuticals Inc. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
22
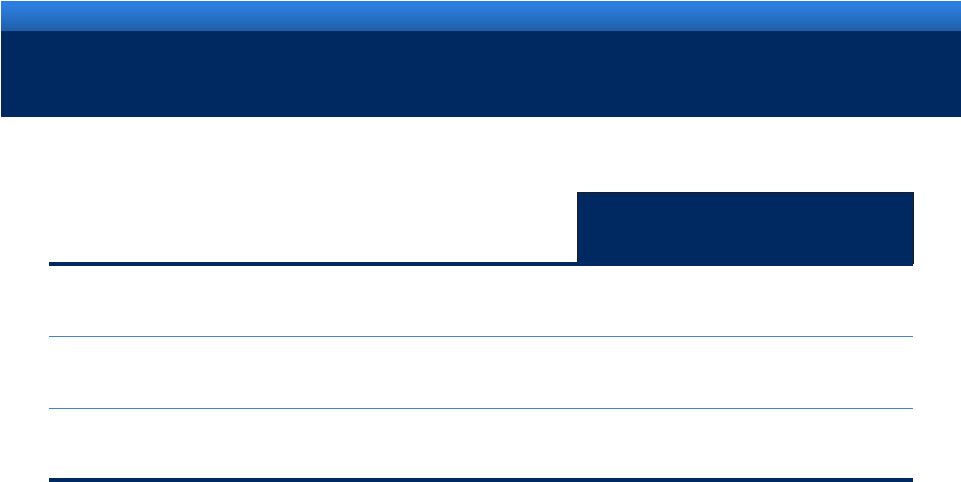
2011 ENDO GUIDANCE
Guidance
Revenue range
$2.35BN -
$2.45BN
Adjusted diluted EPS range
$4.20 -
$4.30
Reported (GAAP) diluted EPS range
$2.43 -
$2.53 |
 ENDO
PHARMACEUTICALS grow. collaborate. innovate. thrive. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals Inc.
24
24
RECONCILIATION OF NON-GAAP MEASURES
24
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s
Current Report on Form 8-K filed today with the Securities and Exchange Commission
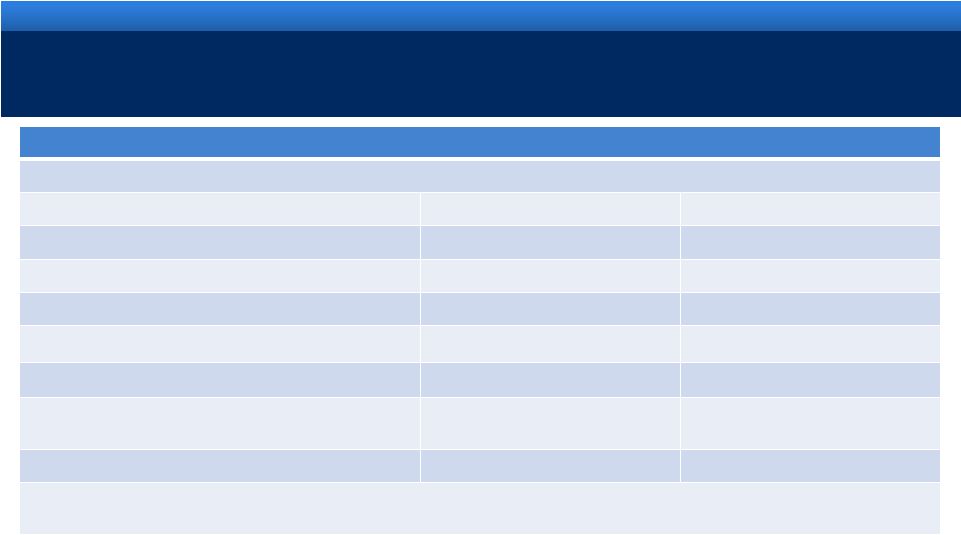
Reconciliation of Projected GAAP Diluted Earnings Per Share to Adjusted Diluted Earnings Per
Share Guidance for the Year Ending December 31, 2011 Lower End of Range
Upper End of Range
Projected GAAP diluted income per common share
$2.43
$2.53
Upfront and milestone-related payments to partners
$0.35
$0.35
Amortization of commercial intangible assets and inventory step-up
$1.33
$1.33
Acquisition and integration costs related to recent acquisitions.
$0.26
$0.26
Interest expense adjustment for ASC 470-20 and the amortization of
the premium on debt acquired from Indevus
$0.16
$0.16
Tax effect of pre-tax adjustments at the applicable tax rates and
certain other expected cash tax savings as a result of recent
acquisitions
($0.33)
($0.33)
Diluted adjusted income per common share guidance
$4.20
$4.30
The company's guidance is being issued based on certain assumptions including:
•Certain of the above amounts are based on estimates and there
can be no assurance that Endo will achieve these results
•Includes all completed business development transactions as of
February 28, 2011 |
 grow.
collaborate. innovate. thrive.
Barclays Capital 2011 Global Healthcare Conference
March 15, 2011 |
