UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, DC
20549
Form 10-K
| |
|
|
|
þ
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the fiscal
year ended December 31, 2010
|
|
o
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the transition
period to
|
Commission File Number
001-33744
TRANS1 INC.
(Exact name of Registrant as
specified in its charter)
| |
|
|
DELAWARE
(State or other jurisdiction
of
incorporation or organization)
|
|
33-0909022
(I.R.S. employer
identification no.)
|
301 GOVERNMENT CENTER DRIVE, WILMINGTON, NC 28403
(Address of principal
executive office) (Zip code)
(910) 332-1700
(Registrant’s telephone
number, including area code)
Securities registered pursuant to Section 12(b) of the
Act
| |
|
|
|
Title of Each Class:
|
|
Name of Each Exchange on which Registered:
|
|
|
|
Common Stock, par value $0.0001 per share
|
|
The NASDAQ Global Stock Market
|
Securities registered pursuant to Section 12(g) of the
Act:
None
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Securities
Act. YES o NO þ
Indicate by check mark if the registrant is not required to file
reports pursuant to Section 13 or Section 15(d) of the
Act. YES o NO þ
Indicate by check mark whether the registrant (1) has filed
all reports required to be filed by Section 13 or 15(d) of
the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant
was required to file such reports), and (2) has been
subject to such filing requirements for the past
90 days. YES þ NO o
Indicate by check mark whether the registrant has submitted
electronically and posted on its corporate Web site, if any,
every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of Regulations S-T
(§ 232.405 of this chapter) during the preceding
12 months (or for such shorter period that the registrant
was required to submit and post such
files). YES o NO o
Indicate by check mark if disclosure of delinquent filers
pursuant to Item 405 of
Regulation S-K
is not contained herein, and will not be contained, to the best
of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in
Part III of this
Form 10-K
or any amendment to this
Form 10-K. þ
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in
Rule 12b-2
of the Exchange Act. (Check one):
| |
|
|
|
|
|
|
|
Large accelerated filer o
|
|
Accelerated filer o
|
|
Non-accelerated filer o
|
|
Smaller reporting company þ
|
|
|
|
|
|
(Do not check if a smaller reporting company)
|
|
|
Indicate by check mark whether the registrant is a shell company
(as defined in
Rule 12b-2
of the Exchange
Act). YES o NO þ
As of June 30, 2010, the last business day of our most
recently completed second fiscal quarter, the aggregate market
value of the voting stock held by non-affiliates was
approximately $26.1 million, based on the number of shares
held by non-affiliates of the registrant and based on the
reported last sale price of registrant’s common stock on
June 30, 2010. This calculation does not reflect a
determination that persons are affiliates for any other purposes.
The number of shares of the registrant’s common stock
outstanding as of March 9, 2011 was 20,877,594 shares.
DOCUMENTS
INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement on
Schedule 14A to be filed with the Securities and Exchange
Commission within 120 days after the close of the fiscal
year covered by this annual report, relating to the
registrant’s 2011 annual meeting of stockholders, are
incorporated by reference into Part III of this annual
report. With the exception of the portions of the Proxy
Statement specifically incorporated herein by reference, the
Proxy Statement is not deemed to be filed as part of this annual
report.
TRANS1
INC.
FORM 10-K
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2010
TABLE OF CONTENTS
2
Cautionary
Note Regarding Forward-Looking Statements
In addition to historical financial information, this report
contains forward-looking statements within the meaning of
Section 21E of the Securities Exchange Act of 1934 that
concern matters that involve risks and uncertainties that could
cause actual results to differ materially from those projected
in the forward-looking statements. These forward-looking
statements are intended to qualify for the safe harbor from
liability established by the Private Securities Litigation
Reform Act of 1995. All statements other than statements of
historical fact contained in this report, including statements
regarding future events, our future financial performance, our
future business strategy and the plans and objectives of
management for future operations, are forward-looking
statements. We have attempted to identify forward-looking
statements by terminology including “anticipates,”
“believes,” “can,” “continue,”
“could,” “estimates,” “expects,”
“intends,” “may,” “plans,”
“potential,” “predicts,” “should”
or “will” or the negative of these terms or other
comparable terminology. Although we do not make forward-looking
statements unless we believe we have a reasonable basis for
doing so, we cannot guarantee their accuracy. Such
forward-looking statements are subject to risks, uncertainties
and other factors that could cause actual results and the timing
of certain events to differ materially from future results
expressed or implied by such forward-looking statements. Actual
results could differ materially from those projected in
forward-looking statements as a result of the following factors,
among others: acceptance and continued use of our products by
surgeons, the lack of clinical data about the efficacy of our
products, uncertainty of reimbursement from third-party payors,
our historical lack of profitability, cost pressures in the
healthcare industry, competitive pressures from substitute
products and larger companies, our dependence on key employees,
regulatory approval and market acceptance for new products, our
reliance on a limited number of suppliers to provide our
products, our ability to effectively manage a sales force to
meet our objectives and our ability to conduct successful
clinical studies. Readers are urged to carefully review and
consider the various disclosures made by us, which attempt to
advise interested parties of the risks, uncertainties, and other
factors that affect our business, operating results, financial
condition and stock price, including without limitation the
disclosures made under the captions “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations” and “Risk Factors” in this report and
in the consolidated financial statements and notes thereto
included elsewhere in this report, as well as in other filings
we make with the Securities and Exchange Commission, or the SEC.
Furthermore, such forward-looking statements speak only as of
the date of this report and in other filings we make with the
SEC. We expressly disclaim any intent or obligation to update
any forward-looking statements after the date hereof to conform
such statements to actual results or to changes in our opinions
or expectations, except as required by applicable law or the
rules of the NASDAQ Stock Market.
References in this report to “TranS1”, “we”,
“our”, “us”, or the “Company”
refer to TranS1 Inc.
3
PART I
Overview
We are a medical device company focused on designing, developing
and marketing products that implement our proprietary approach
to treat degenerative conditions of the spine affecting the
lower lumbar region. Using our pre-sacral approach, a surgeon
can access discs in the lower lumbar region of the spine through
a small incision adjacent to the tailbone and can perform an
entire interbody fusion procedure through a small tube that
provides direct access to the intervertebral space. We developed
our pre-sacral approach to allow spine surgeons to access and
treat intervertebral spaces without compromising important
surrounding soft tissue. We believe this approach enables fusion
procedures to be performed with low complication rates, low
blood loss, short hospital stays, fast recovery times and
reduced pain. We currently market our
AxiaLIF®
family of products for single and multilevel lumbar fusion, the
Vectretm
and
Avatartm
lumbar posterior fixation systems and
Bi-Ostetictm
bone void filler, a biologics product.
From our incorporation in 2000 through 2004, we devoted
substantially all of our resources to research and development
and start-up
activities, consisting primarily of product design and
development, clinical trials, manufacturing, recruiting
qualified personnel and raising capital. We received 510(k)
clearance from the U.S. Food and Drug Administration, or
FDA, for our AxiaLIF 1L product in the fourth quarter of 2004,
and commercially introduced our AxiaLIF 1L product in the United
States in the first quarter of 2005. We received a CE mark to
market our AxiaLIF 1L product in the European market in the
first quarter of 2005 and began commercialization in the first
quarter of 2006. We received a CE mark for our AxiaLIF 2L
product in the third quarter of 2006 and began commercialization
in the European market in the fourth quarter of 2006. We
received FDA 510(k) clearance for our AxiaLIF 2L product and
began marketing this product in the United States in the second
quarter of 2008. The AxiaLIF 2L product was discontinued in 2010
after we launched our AxiaLIF
2L+tm
product in July 2010, for which we had received FDA 510(k)
clearance in January 2010. We commercially launched our next
generation Vectre facet screw system in April 2010. In January
2010, we entered into an agreement to distribute Avatar, a
pedicle screw system, and in February 2010, we entered into an
agreement to distribute Bi-Ostetic bone void filler, a biologics
product. As of December 31, 2010, over 11,000 fusion
procedures have been performed globally using our AxiaLIF
products. At December 31, 2010, we sold our AxiaLIF
products through 46 direct sales personnel and 14 independent
sales agents in the United States and 3 direct sales personnel
and 16 independent distributors internationally. For the year
ended December 31, 2010, our revenues were
$26.2 million and our net loss was $19.5 million.
Spine
Anatomy
The human spine is the core of the human skeleton and provides
important structural support while remaining flexible to allow
movement. It consists of 33 separate interlocking bones called
vertebrae that are connected by soft tissue and provide
stability while facilitating motion. Vertebrae are paired into
motion segments that move by means of two facet joints and one
disc. The facet joints provide stability and enable the spine to
bend and twist while the discs absorb pressures and shocks to
the vertebrae. Nerves are contained in the spinal column and run
through the foramen openings to the rest of the body.
The vertebrae are categorized into five regions: cervical,
thoracic, lumbar, sacral and coccyx. The lumbar region, which is
at the bottom of the spine and consists of five vertebrae, is
capable of limited movement, and primarily functions as support
for the body’s weight. The sacrum consists of five fused
vertebrae labeled S1 through S5 directly below the lumbar region
and that provide attachment for the hipbones as well as
protection to organs in the pelvic area. The coccyx, also known
as the tailbone, is at the end of the spine.
Medical
Conditions Affecting the Lower Lumbar Spine and Traditional
Treatment Alternatives
Degenerative disc disease is a common medical condition
affecting the lower lumbar spine and refers to the degeneration
of the disc from aging and repetitive stresses resulting in a
loss of flexibility, elasticity and shock-absorbing properties.
As degenerative disc disease progresses, the space between the
vertebrae narrows,
4
which can pinch the nerves exiting the spine and result in back
pain, leg pain, numbness and loss of motor function. This lower
back pain can be overwhelming for patients as the resulting pain
can have significant physical, psychological and financial
implications.
Treatment alternatives for lower lumbar spine conditions range
from non-operative conservative therapies to highly invasive
surgical interventions. Conservative therapies are typically the
initial treatments selected by patients and physicians and they
include rest, bracing, physical therapy, chiropractics,
electrical stimulation and medication. When conservative
therapies fail to provide adequate pain relief, surgical
interventions, including fusion procedures, may be used to
address the pain. If a patient’s disc degeneration has not
progressed to a stage requiring fusion, but has progressed
beyond the stage where conservative therapies provide pain
relief, physicians may use non-fusion surgical procedures.
Non-fusion surgical procedures utilize implants that are
designed to restore disc height and allow limited movement of
the vertebrae similar to a healthy spine in order to avoid
increased pressures on adjacent vertebrae. These procedures and
implants include dynamic stabilization devices, artificial discs
and prosthetic disc nucleuses.
Fusion procedures attempt to alleviate lower back pain by
removing problematic disc material and permanently joining
together two or more opposing vertebrae. This is done in a
manner that restores the appropriate space between the vertebrae
surrounding the degenerative disc and eliminates mobility of the
affected vertebrae. By restoring disc height and eliminating
motion, fusion attempts to prevent the pinching of the nerves
exiting the spine and thereby reducing pain.
Traditional fusion procedures typically involve an incision in
the skin, and cutting muscle or moving organs to gain access to
the spine. The degenerated disc is then removed, referred to as
a discectomy, and a rigid implant is inserted, such as a bone
graft or cage, to stabilize the diseased vertebrae. This process
is referred to as fixation. The bone graft or cage promotes the
growth of bone between the vertebrae. Surgeons often also affix
supplementary rods and screws along the spine to provide
additional stabilization while the vertebrae fuse together
during the six to eighteen months following surgery. The primary
surgical fusion procedures performed in the lower lumbar region
include: ALIF, PLIF, TLIF and direct lateral.
Anterior Lumbar Interbody Fusion, or ALIF. To
perform an ALIF procedure, surgeons access the spine through an
incision on the patient’s abdomen which provides them with
optimal access to the vertebral space for performing a
discectomy and inserting bone grafts for fusion. Supporting soft
tissue and nerves are manipulated or removed to accommodate the
anterior access required by the ALIF procedure. Surgeons
commonly perform ALIF procedures in conjunction with a general
or vascular surgeon because critical vasculature and organs must
be retracted to gain access to the spine. The assistance of a
second surgeon can reduce the economics for the spine surgeon
and increase the difficulty in scheduling the surgery.
Complications associated with ALIF procedures include vascular
damage to the vena cava and aorta.
Posterior Lumbar Interbody Fusion, or PLIF. To
perform a PLIF procedure, surgeons access the spine through an
incision on the center of the patient’s back. The surgeon
then navigates through muscles and nerves to gain access to the
spine. Once at the spine, the surgeon removes bone from the
lamina to gain access to the affected disc space where a
discectomy is performed and a bone graft is placed. When
compared to ALIF procedures, PLIF procedures are generally
considered easier to perform and can achieve better nerve root
decompression in certain cases. However, the anatomy of the
spine prevents surgeons from removing the entire degenerated
disc and obtaining optimal access for insertion of an implant or
bone graft. Complications associated with PLIF procedures
include nerve damage, soft tissue damage and implant migration.
Transforaminal Lumbar Interbody Fusion, or
TLIF. TLIF procedures are performed in a similar
manner to PLIF procedures, except the surgeon accesses the spine
through a small incision slightly to the left or right of the
center of the patient’s back. After reaching the spine, the
surgeon removes a portion of the facet joint and navigates
through the foramen which provides better visualization and disc
removal capabilities than PLIF. Complications associated with
TLIF procedures are similar to those found in PLIF procedures
including nerve damage, soft tissue damage and implant migration.
Direct Lateral Interbody Fusion. To perform a
direct lateral procedure, the surgeon accesses the spine from
the patient’s side through one or two small incisions. The
direct lateral procedure is not appropriate for
5
fusions in the L5/S1 segment because the pelvis interferes with
access. Complications associated with direct lateral procedures
are similar to those found in PLIF and TLIF procedures,
including nerve damage and implant migration, but with less soft
tissue damage.
360° Lumbar Fusion Procedure. Currently,
the most common and structurally rigid lumbar fusion surgery is
referred to as a 360° fusion and requires a second surgical
procedure immediately following an ALIF, PLIF, TLIF or direct
lateral procedure. The additional procedure involves the
permanent placement of screws and rods in the back to provide
additional support while the vertebrae fuse together during the
six to eighteen months following surgery.
Limitations
of Traditional Lower Lumbar Spine Procedures
While traditional and minimally invasive fusion procedures can
be effective at treating medical conditions affecting the lower
lumbar spine, common drawbacks of these procedures include:
|
|
|
| |
•
|
Disruption to Soft Tissue and Support
Structures. Current lumbar fusion procedures
require creating a pathway from the skin to the degenerative
disc that is large enough to allow direct visualization and work
by the surgeon. It is common to cut through healthy muscle or
move critical organs, arteries, nerves and soft tissue, which
can lead to bleeding, scarring, nerve damage and bowel
disruption.
|
| |
| |
•
|
Significant Blood Loss. As a result of
undergoing current lumbar fusion procedures, patients typically
experience significant blood loss of between 100 and 1,400 cc of
blood. As a result, it is common for patients to use the
hospital’s blood supply or donate units of their own blood
before a lumbar fusion procedure to replenish any significant
blood loss.
|
| |
| |
•
|
Lengthy Operative Procedure Times. It is
common for current lumbar fusion procedures to take between 90
minutes and 4 hours to complete. With some procedures a
second surgeon may be required. Long procedure times increase
the risks of complications and blood loss. Also, hospital and
physician resources are consumed for lengthy periods of time,
which can reduce productivity and increase costs.
|
| |
| |
•
|
Lengthy Patient Hospital Stays. Patients
remain in the hospital for an average of three days following a
lumbar fusion procedure which consumes hospital and physician
resources.
|
| |
| |
•
|
Significant Patient Recovery Time. Patients
require three to six months to recover and rehabilitate after
undergoing a lumbar fusion procedure before resuming normal
activities.
|
| |
| |
•
|
Unresolved Patient Pain. Patients may continue
to experience symptoms after undergoing lumbar fusion procedures
even though x-rays show successful fusion has been achieved
through the growth of new bone. We believe this may be caused by
muscle dissection, implant irritation and scar tissue that
develops around the site of the surgery.
|
Our
Solution
We have developed the least invasive approach for surgeons to
perform fusion and motion preserving surgeries in the L4/L5/S1
region without many of the drawbacks associated with current
lumbar fusion procedures. We refer to this unique proprietary
approach as our pre-sacral approach. We have developed and are
marketing two fusion products that are delivered using our
pre-sacral approach: AxiaLIF 1L and AxiaLIF 2L+.
To access the spine using our pre-sacral approach, the surgeon
creates a small incision adjacent to the tailbone while the
patient is lying on their stomach. The surgeon then navigates a
blunt dissecting tool a short distance along the sacrum using
x-ray imaging technologies. As the dissecting tool is advanced,
it moves aside soft tissue structures such as fat, loose
connective tissue and bowel. When the tool reaches an access
point near the junction of the S1 and S2 vertebral bodies, a
guide pin is inserted through the bone into the disc of the
lowest lumbar motion segment (the L5/S1 disc). A tubular
dissector is inserted over the guide pin to create a
tissue-protecting working channel between the surgical access
site and the L5/S1 disc. This protected working channel provides
access to the interior of the disc, where rotating cutters and
brushes are used to remove disc material. This is followed by
the introduction of bone graft material with special
instrumentation and finally, insertion of our AxiaLIF implants.
The implant immediately provides rigid fixation and can
6
provide restoration of disc height based on the clinical
pathology of the patient. The AxiaLIF 2L+ procedure uses the
same approach to provide access to both the L5/S1 and L4/L5
discs. The AxiaLIF 1L and AxiaLIF 2L+ procedure must be
supplemented with facet or pedicle screws, which provide
fixation for the back of the spine and are supplied by TranS1.
We believe our pre-sacral approach and its associated products
provide the following benefits for patients, providers and
payors:
|
|
|
| |
•
|
Least Invasive Approach Minimizes
Complications. Our AxiaLIF products are delivered
using our unique pre-sacral approach, which is the least
invasive solution for delivering fusion products to the L4/L5/S1
region. Procedures performed utilizing our pre-sacral approach
have been documented to have favorable clinical safety profiles
with complication rates of approximately 1%.
|
| |
| |
•
|
Spinal Stability. Our approach does not
violate or cut through any muscles or ligaments that control the
stability of the spine. The axial approach to the spine also
creates inherent spinal stability that cannot be achieved with
traditional approaches to interbody fusion.
|
| |
| |
•
|
Short Learning Curve. The ease of use of our
pre-sacral approach enables reduced physician training as
compared to alternative lower lumbar spine procedures.
|
| |
| |
•
|
Low Blood Loss. The least invasive nature of
our pre-sacral approach results in the average patient losing
approximately 25 to 125 cc of blood during an AxiaLIF 1L or
AxiaLIF 2L+ procedure, which is much lower than current
techniques and correlates to reduced pain and faster recoveries.
In addition, patients generally do not need to donate blood
prior to undergoing procedures that utilize our pre-sacral
approach.
|
| |
| |
•
|
Short Patient Hospital Stays. Patients
typically stay only one to two nights in the hospital after
receiving a procedure performed using our pre-sacral approach.
In a small percentage of cases, patients are able to return home
the same day.
|
| |
| |
•
|
Reduction in Patient Pain. Our pre-sacral
approach can be effective at reducing pain symptoms because
surrounding soft tissue is not violated, which prevents the
creation of scar tissue, a leading cause of pain.
|
Our
Strategy
Our goal is to become a global leader in the treatment of
conditions affecting the lower lumbar region of the spine. To
achieve this goal, we are pursuing the following strategies:
|
|
|
| |
•
|
Establish our Pre-Sacral Approach as a Standard of Care for
Lower Lumbar Spine Surgery. We believe patients
commonly avoid back surgery due to its invasive nature and other
drawbacks associated with current surgical treatment options. We
expend significant resources promoting our pre-sacral approach
as the least invasive approach to lower back surgery and we
believe the advantages of our technique will enable our AxiaLIF
products to become a standard of care for the lower lumbar
region of the spine. We also make substantial research and
development expenditures to enhance our AxiaLIF products and
develop new products to expand our technological advantage in
minimally invasive spine surgeries.
|
| |
| |
•
|
Drive Reimbursement for Our Procedure. We will
continue to work with our surgeon customers to generate
published, peer reviewed clinical literature that demonstrates
our procedure’s clinical efficacy and safety. We will also
work to leverage this data along with our AxiaLIF surgeon
advocates, with payors to secure positive coverage decisions for
the reimbursement of the AxiaLIF procedure.
|
| |
| |
•
|
Focus our Sales and Marketing Infrastructure to Drive Surgeon
Adoption. We intend to continue expending
significant resources targeting spine surgeons through our sales
and marketing efforts in the United States and internationally
in order to drive the adoption of our pre-sacral approach. We
believe the ease of use, short hospital stays and reduction in
patient pain are compelling reasons for surgeon adoption of our
technologies.
|
7
|
|
|
| |
•
|
Opportunistically Pursue Acquisitions of Complementary
Businesses and Technologies. In addition to
building our internal product development efforts, we intend to
selectively license or acquire complementary products and
technologies that we believe will enable us to leverage our
growing distribution platform.
|
Our
Products
Our products include surgical instruments for creating a safe
and reproducible access route to the L4/L5/S1 vertebral bodies,
fusion implants, as well as supplemental stabilization products.
We believe our AxiaLIF implants and instruments, combined with
facet screws or pedicle screws, provide surgeons with the tools
necessary to perform a lumbar fusion in the least invasive
manner available. We sell these products to our customers in
procedure kits that include all the instruments and implants
needed to complete a lumbar fusion.
AxiaLIF Lumbar Fusion Implants. Our AxiaLIF 1L
and AxiaLIF 2L+ implants are threaded titanium rods, that come
in varying lengths to enable one-level L5/S1 fusions and
two-level L4/L5/S1 fusions. As they are implanted, our
proprietary design can allow for the separation of the vertebrae
to restore disc height based on the clinical pathology of the
patient. The increased disc height relieves pressure on the
nerve, while the rod itself provides immediate rigid fixation.
TranS1 Access and Disc Preparation
Instruments. Our pre-sacral approach requires the
use of a sterile set of surgical instruments that are used to
create a safe and reproducible working channel and to prepare
the disc and vertebrae for our implant. The instrumentation
contained in the set includes stainless steel navigation tools
and tubular dissectors to create the working channel, as well as
nitinol cutters and brushes to cut and remove the degenerated
disc material and prepare the disc space for our implant and the
bone graft material.
Vectre Facet Screw System. Our Vectre facet
screw system offers a cannulated facet screw inserted over a
guidewire to provide stability while reducing the muscle and
tissue trauma associated with conventional pedicle screws. The
Vectre system features offer a safe, reproducible posterior
fixation option in select patients.
AVATAR Pedicle Screw System. In January 2010,
we entered into an agreement to distribute Avatar, a pedicle
screw system. Avatar can be used with or without our implants to
provide lumbar posterior fixation. The AVATAR MIS System offers
cannulated pedicle screws inserted over a guidewire to reduce
muscle and tissue trauma. Extended tabs integrated to the screws
provide a pathway for secure implantation of the rod while
minimizing tissue dissection. Reduction, compression and
distraction are achieved simply and effectively with intuitive
instrumentation.
Bi-Ostetic Bone Void Filler. In February 2010,
we entered into an agreement to sell Bi-Ostetic, an
osteoconductive bone substitute that is intended to be used to
fill voids and gaps that are not intrinsic to the stability of
bone structure. Bi-Ostetic is an alternative to allografts or
cadaver bone. The spongy granules are bioceramics with
interconnected porosity that mimic the cancellous bone structure
in order to enhance osteo-conduction and ensure complete and
rapid bone in-growth.
Product
Pipeline
We have prioritized our product development efforts and are
pursuing products that enhance our existing product line and
those that that have a shorter pathway to regulatory clearance
and commercialization. We intend to launch three products in
2011, a bowel retractor in the second quarter, which is in
limited market release, an MIS bone graft harvesting system in
the third quarter and the AxiaLIF 1L+ in the second half of the
year. Additionally, we are working on our minimally invasive
direct lateral and TLIF systems which we anticipate launching in
2012.
In the future, we expect our product offerings will be expanded
to address additional clinical applications in the surgical
treatment of conditions affecting the lumbar spine. Such
applications could require FDA 510(k) clearance or PMA approval,
most likely supported by safety and efficacy data from clinical
trials. We also have an active program aimed at developing tools
that will improve outcomes and lower complications for our
procedures.
8
Sales and
Marketing
Our sales and marketing effort primarily targets spine surgeons.
We also market our products at various industry conferences and
through industry organized surgical training courses.
In the United States, we market and sell our products through a
combination of direct sales representatives and independent
sales agents. At December 31, 2010, our U.S. sales
team included regional managers, direct sales representatives,
case coverage specialists and independent sales agents covering
specific geographic regions. Our sales representatives receive a
base salary and a percentage of the net sales that they
generate. As of December 31, 2010, we had 46 direct sales
representatives and 11 regional managers. The independent sales
agents are compensated based on a percentage of the net sales
that they generate. We have agreements with our independent
sales agents that provide them with an exclusive right to sell
our products in their territories, which are generally
terminable upon 90 days’ written notice.
Outside of the United States, we utilize our own direct sales
representatives, independent sales agents and third-party
distributors. Through December 31, 2010, the majority of
our international sales have been in Europe. In 2008, we hired
direct sales representatives in Germany, and in 2009 we began
direct sales through our own sales representatives and agents in
Germany, Switzerland, Netherlands and Belgium. In 2010, 56% of
our international revenues were through our direct sales efforts
and agents and 44% were through third-party distributors.
In 2010, no customer accounted for 10% or more of revenues.
Surgeon
Training
We devote significant resources to training and educating
surgeons on the specialized skills involved in the proper use of
our instruments and implants. We believe that the most effective
way to introduce and build market demand for our products is by
training spine surgeons in the use of our products. We
accomplish our training objectives primarily through cadaver and
surrogate models and live case observations with surgeons
experienced in our pre-sacral approach. We supplement this
training with online didactic tutorials. After this training,
surgeons are generally able to perform unsupervised surgeries
using our pre-sacral approach. As of December 31, 2010, we
had trained over 1,290 U.S. spine surgeons and over 275
surgeons outside of the U.S. in the use of our single-level
product. Of the U.S. surgeons trained on our pre-sacral
approach, approximately 269 have performed a procedure in the
12 months ended December 31, 2010 using our pre-sacral
approach. In addition, we have trained over 380 U.S. spine
surgeons in the use of our AxiaLIF 2L and 2L+ products. We
believe we have the necessary capacity to train a sufficient
number of surgeons to meet our current goals.
Third-Party
Reimbursement
In the United States, healthcare providers generally rely on
third-party payors, principally private insurers and
governmental payors such as Medicare and Medicaid, to cover and
reimburse all or part of the cost of a spine fusion surgery in
which our medical device is used. Surgeons are reimbursed for
performing the surgical procedure, while hospitals are
reimbursed for the cost of the device, all patient care related
to the fusion procedure and the overhead associated with
maintaining the facility.
Most payors follow Medicare’s Diagnosis-Related Group, or
DRG, based payment system for reimbursing facilities. Under this
model, hospitals are paid a set amount to cover the costs
associated with a fusion patient, including the cost of the
device used in the procedure. The most commonly associated DRGs
for spinal fusion are
453/454/455
(“Combined Anterior/Posterior Spinal Fusion with major
complications and comorbidities (MCC), with complications and
comorbidities (CC) or without MCC/CC”) and 459/460
(“Spinal Fusion Except Cervical with or without MCC”).
Private payors typically use Medicare DRGs as a benchmark when
setting their own reimbursement rates for facilities but
typically pay above Medicare established rates.
Surgeons use the American Medical Association’s Current
Procedural Terminology, or CPT, system to bill payors for the
AxiaLIF procedures. CPT codes describe the services and
procedures provided for patients to third-party payors so that
physicians may be reimbursed. Effective January 1, 2009,
the AMA implemented
9
a Category III code which may describe the work involved in
treating some AxiaLIF patients. Unlike Category I CPT codes,
Category III codes do not have a set value which physicians
use as a benchmark for setting their fee. Additionally, some
payors view Category III codes as
“investigational” or “experimental” and may
not reimburse them. However, unlike many new or novel
procedures, AxiaLIF is an access variation on the current
standard of care (interbody spinal fusion) and has been
performed in over 10,000 U.S. procedures. In December 2010,
Humana Inc. made a decision to cover the AxiaLIF procedures and
reimburse physicians for use of the Category III Code. The
reimbursement rates are consistent with reimbursement levels for
performing other interbody fusion procedures. We intend to gain
further positive reimbursement coverage decisions with other
payors in the coming quarters by utilizing published clinical
literature and leveraging the support of physicians that perform
our procedures. Discussions are normally held with medical
directors representing the payors to inform them that our
approach to interbody fusion is not “investigational”
or “experimental.” Under our current timetable, we
anticipate graduating to a Category 1 CPT code for our AxiaLIF
procedures on January 1, 2013. In order to meet this
objective, we will have to have a successful outcome from any of
the June 2011, October 2011 or February 2012 American Medical
Association CPT meetings.
As the breadth and depth of peer-reviewed clinical research
regarding AxiaLIF continues to grow, we will continue to work to
ensure that patients have continued access to AxiaLIF should
their doctors determine this is the preferred clinical solution
for their condition.
Internationally, reimbursement and healthcare payment systems
vary substantially from country to country and include
single-payor, government-managed systems as well as systems in
which private payors and government-managed systems exist
side-by-side.
Our ability to achieve market acceptance or significant sales
volume in international markets we enter will depend in large
part on the availability of reimbursement for procedures
performed using our products under the healthcare payment
systems in such markets. There is favorable reimbursement in
Germany for the AxiaLIF 1L procedure. We also received a
favorable ruling from the British United Provident Association,
the leading provider of private health insurance and healthcare
services in the United Kingdom. A small number of countries may
require us to gather additional clinical data before recognizing
coverage and reimbursement for our products. It is our intent to
complete the requisite clinical studies and obtain coverage and
reimbursement approval in countries where it makes economic and
strategic sense to do so.
We believe that the overall escalating cost of medical products
and services has led to, and will continue to lead to, increased
pressures on the healthcare industry to reduce the costs of
products and services. We cannot assure you that government or
other third-party payors will cover and reimburse our procedures
in whole or in part in the future or that payment rates will be
adequate. In addition, it is possible that future legislation,
regulation, or reimbursement policies of third-party payors will
adversely affect the demand for our procedures and products or
our ability to sell them on a profitable basis. The
unavailability or inadequacy of third-party payor coverage or
reimbursement could have a material adverse effect on our
business, operating results and financial condition.
Competition
The medical device industry is highly competitive, subject to
rapid technological change and significantly affected by new
product introductions and market activities of other
participants. Our currently marketed products are, and any
future products we commercialize will be, subject to intense
competition. Our competitors include providers of conservative,
non-operative therapies for lower lumbar spine conditions, as
well as a number of major medical device companies that have
developed or plan to develop products for minimally invasive
spine surgery in each of our current and future product
categories. We believe that the principal competitive factors in
our markets include:
|
|
|
| |
•
|
improved outcomes for medical conditions affecting the lower
lumbar spine;
|
| |
| |
•
|
acceptance by spine surgeons;
|
| |
| |
•
|
ease of use and reliability;
|
| |
| |
•
|
product price and qualification for reimbursement;
|
10
|
|
|
| |
•
|
technical leadership and superiority;
|
| |
| |
•
|
effective marketing and distribution; and
|
| |
| |
•
|
speed to market.
|
We are aware of several companies that compete or are developing
technologies in our current and future product areas. As a
result, we expect competition to remain intense. Our most
significant competitors are Medtronic Sofamor Danek,
Johnson & Johnson DePuy Spine, Stryker Spine,
NuVasive, Zimmer Spine, Synthes, Orthofix International, Globus
Medical and Alphatec Spine, many of which have substantially
greater sales and financial resources than we do. In addition,
these companies may have more established distribution networks,
entrenched relationships with physicians, and greater experience
in launching, marketing, distributing and selling products.
Additional competition also comes from physician-owned spine
specific medical device distribution companies, which have
expanded in recent years. These companies, which are partially
owned or completely owned by spine surgeons, have significant
market knowledge and access to the physicians who use our
products and the hospitals that purchase our products. These
surgeons may have an incentive to direct implant use toward
implants that are distributed by these organizations.
Our ability to compete successfully will depend on our ability
to develop proprietary products that reach the market in a
timely manner, receive adequate reimbursement and demonstrate
that they are safer and less invasive than alternatives
available for the same purpose. Because of the size of the
potential market, we anticipate that companies will dedicate
significant resources to developing competing products.
Research
and Development
As of December 31, 2010, our research and development team
was comprised of 12 employees who have extensive experience
in developing products to treat medical conditions affecting the
lower lumbar spine. These employees work closely with our
clinical advisors and spine surgeon customers to design and
enhance our products and approach. Our R&D spending was
$4.2 million, $6.4 million and $4.1 million for
the years ending December 31, 2010, 2009 and 2008,
respectively. Since inception, we have devoted significant
resources to develop and enhance our AxiaLIF products utilizing
the pre-sacral approach.
Manufacturing
and Supply
We rely on third parties to manufacture all of our products and
their components, except for our nitinol nucleus cutter blades,
which we manufacture at our facility in Wilmington, North
Carolina. Our outsourcing partners are manufacturers that meet
FDA, International Organization for Standardization, or ISO, and
other internal quality standards, where applicable. We believe
these manufacturing relationships allow us to work with
suppliers who have the best specific competencies while we
minimize our capital investment, control costs and shorten cycle
times, all of which we believe allows us to compete with
larger-volume manufacturers of spine surgery products.
All of our products and components are assembled, packaged,
labeled and sterilized at third-party facilities in the United
States under our existing contracts requiring compliance with
Good Manufacturing Processes, or GMPs. Following receipt of
products or components from our third-party manufacturers, we
inspect, warehouse and ship the products and components at our
facilities in Wilmington or at a third-party distribution
facility in Memphis, Tennessee. We reserve the exclusive right
to inspect and assure conformance of each product and component
to our specifications. In addition, FDA or other regulatory
authorities may inspect our facilities and those of our
suppliers to ensure compliance with quality system regulations.
The majority of our instruments and implants are produced by
third-party manufacturers on precision, high-speed machine shop
equipment. However, for a certain number of our products, the
components and materials used to manufacture such products and
the manufacturing operations are performed or supplied by
third-party specialty vendors due to their proprietary or
non-conventional nature. For example, the blades for our nucleus
cutters are made from a metal called nitinol, which is converted
into strip form by three
11
manufacturers in the United States known to us. We have sourced
nitinol strip from two of these vendors. The nitinol strip is
then further converted for us into cutter blanks by a scalpel
blade specialty vendor. Other vendors are available to
manufacture the cutter blanks, as we may deem desirable or
necessary. We convert the cutter blanks into cutter blades at
our facilities. Our tissue extractor product is produced for us
by a supplier that specializes in wire forming and coiling
specifically for the medical device industry. A limited number
of similar vendors exist that could be used to produce the
tissue extractor product, and we believe we could replace this
supplier on reasonable terms without substantial delay, if
necessary.
We are currently working with our third-party manufacturers to
plan for our future manufacturing requirements. In most cases,
we have redundant manufacturing capability with multiple vendors
and enjoy the significant capacity this arrangement provides to
us. We may consider manufacturing certain products or product
components internally, if and when demand or quality
requirements make it appropriate to do so. We believe the
manufacturing capacity available to us is sufficient to meet our
demands into the foreseeable future.
Patents
and Proprietary Technology
We rely on a combination of patent, trademark, copyright, trade
secret and other intellectual property laws, nondisclosure
agreements and other measures to protect our intellectual
property rights. We believe that in order to have a competitive
advantage, we must develop and maintain the proprietary aspects
of our technologies. We require our employees, consultants and
advisors to execute confidentiality agreements in connection
with their employment, consulting or advisory relationships with
us. We also require our employees, consultants and advisors who
we expect to work on our products to agree to disclose and
assign to us all inventions conceived during the work day, using
our property or which relate to our business. We cannot provide
any assurance that employees and consultants will abide by the
confidentiality or assignment terms of these agreements. Despite
any measures taken to protect our intellectual property,
unauthorized parties may attempt to copy aspects of our products
or to obtain and use information that we regard as proprietary.
Patents
As of December 31, 2010, we had 35 issued United States
patents, 36 pending patent applications in the United States, 4
issued European patents, 4 issued Japanese patents and 66
foreign patent applications as counterparts of U.S. cases.
The issued and pending patents cover, among other things:
|
|
|
| |
•
|
our method for performing trans-sacral procedures in the spine,
including diagnostic or therapeutic procedures, and trans-sacral
introduction of instrumentation or implants;
|
| |
| |
•
|
apparatus for conducting these procedures including access, disc
preparation and implantation including the current TranS1
instruments individually and in kit form; and
|
| |
| |
•
|
implants for fusion and motion preservation in the spine.
|
Our issued patents begin to expire in 2021 assuming timely
payment of all maintenance fees. We have multiple patents
covering unique aspects and improvements for many of our methods
and products. We do not believe that the expiration of any
single patent is likely to significantly affect our business,
operating results or prospects.
Trademarks
We own 7 trademark registrations in the United States and 8
trademark registrations in the European Union. We also own 4
pending trademark applications in the United States, 2 pending
trademark applications in the European Union and 9 pending
trademark applications in Canada.
Government
Regulation
Our products are medical devices subject to extensive regulation
by the FDA and other U.S. federal and state regulatory
bodies and comparable authorities in other countries. To ensure
that medical products
12
distributed domestically and internationally are safe and
effective for their intended use, FDA and comparable authorities
in other countries have imposed regulations that govern, among
other things, the following activities that we or our partners
perform and will continue to perform:
|
|
|
| |
•
|
product design and development;
|
| |
| |
•
|
registration and listing;
|
| |
| |
•
|
product testing (preclinical and clinical);
|
| |
| |
•
|
product manufacturing;
|
| |
| |
•
|
product labeling;
|
| |
| |
•
|
product storage;
|
| |
| |
•
|
premarket clearance or approval;
|
| |
| |
•
|
advertising and promotion;
|
| |
| |
•
|
product marketing, sales and distribution; and
|
| |
| |
•
|
post-market surveillance, including reporting deaths or serious
injuries related to products and certain product malfunctions.
|
FDA’s
Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device we wish to
commercially distribute in the United States will require either
prior 510(k) clearance or prior premarket approval from the FDA.
The FDA classifies medical devices into one of three classes.
Devices deemed to pose lower risk are placed in either
class I or II, which in many cases requires the
manufacturer to submit to the FDA a premarket notification or
510(k) submission requesting permission for commercial
distribution. This process is known as requesting 510(k)
clearance. Some low risk devices are exempt from this
requirement. Devices deemed by the FDA to pose the greatest
risk, such as many life-sustaining, life-supporting or
implantable devices, or devices deemed not substantially
equivalent to a legally marketable device, are placed in
class III, requiring a PMA. Our current commercial products
are class II devices marketed under FDA 510(k) premarket
clearance. Premarket clearance applications are subject to the
payment of user fees, paid at the time of submission for FDA
review.
510(k)
Clearance Pathway
To obtain 510(k) clearance, we must submit a premarket
notification demonstrating that the proposed device is
substantially equivalent to a legally marketable device not
requiring a PMA. Although statutorily mandated to clear or deny
a 510(k) premarket notification within 90 days of
submission of the application, FDA’s 510(k) clearance
pathway usually takes from three to twelve months, or even
longer, depending on the extent of requests for additional
information by FDA. Additional information can include clinical
data to make a determination regarding substantial equivalence.
After a device receives 510(k) clearance, any modification that
could significantly affect its safety or effectiveness, or that
would constitute a new or major change in its intended use, will
require a new 510(k) clearance or, depending on the
modification, require a PMA. The FDA requires each manufacturer
to determine whether the proposed change requires submission of
a 510(k), or a PMA, but the FDA can review any such decision and
can disagree with a manufacturer’s determination. If the
FDA disagrees with a manufacturer’s determination, the FDA
can require the manufacturer to cease marketing
and/or
recall the modified device until 510(k) clearance or a PMA is
obtained. If the FDA requires us to seek 510(k) clearance or a
PMA for any modifications to a previously cleared product, we
may be required to cease marketing or recall the modified device
until we obtain this clearance or approval. Also, in these
circumstances, we may be subject to significant regulatory fines
or penalties. We have made and plan to continue to make
additional product enhancements to our products.
13
Clinical
Trials
Clinical trials are sometimes required for a 510(k) premarket
notification. In the U.S., these trials require submission of an
application for an investigational device exemption, or IDE. The
IDE application must be supported by appropriate data, such as
animal and laboratory testing results, showing that it is safe
to test the device in humans and that the testing protocol is
scientifically sound. The IDE application must be approved in
advance by the FDA for a specified number of patients, unless
the product is deemed a non-significant risk device and eligible
for more abbreviated IDE requirements. Clinical trials for a
significant risk device may begin once the IDE application is
approved by the FDA and the appropriate institutional review
boards at the clinical trial sites. Clinical trials must be
conducted in accordance with FDA regulations and federal and
state regulations concerning human subject protection, including
informed consent and healthcare privacy and financial disclosure
by clinical investigators. A clinical trial may be suspended by
FDA or the investigational review board at any time for various
reasons, including a belief that the risks to the study
participants outweigh the benefits of participation in the
study. Even if a study is completed, the results of our clinical
testing may not demonstrate the safety and efficacy of the
device, or may be equivocal or otherwise not be sufficient to
obtain clearance or approval. Similarly, in Europe the clinical
study must be approved by the local ethics committee and in some
cases, including studies of high-risk devices, by the Competent
Authority in the applicable country.
Pervasive
and Continuing FDA Regulation
After a device is placed on the market, numerous FDA and other
regulatory requirements continue to apply. These include:
|
|
|
| |
•
|
quality system regulation, which requires manufacturers,
including third-party contract manufacturers, to follow
stringent design, testing, control, documentation, and other
quality assurance controls, during all aspects of the
manufacturing process;
|
| |
| |
•
|
establishment registration and listing;
|
| |
| |
•
|
labeling regulations, and FDA prohibitions against the promotion
of products for uncleared or unapproved “off-label”
uses;
|
| |
| |
•
|
medical device reporting obligations, which require that
manufacturers submit reports to the FDA if information
reasonably suggests that their device (i) may have caused
or contributed to a death or serious injury, or
(ii) malfunctioned and the device or a similar company
device would likely cause or contribute to a death or serious
injury if the malfunction were to recur; and
|
| |
| |
•
|
other post-market surveillance requirements, which apply when
necessary to protect the public health or to provide additional
safety and effectiveness data for the device.
|
We must register and list with FDA as a medical device
manufacturer and must obtain all necessary state permits or
licenses to operate our business. As manufacturers, we are
subject to announced and unannounced inspections by FDA to
determine our compliance with quality system regulation and
other regulations. We were inspected by the FDA in May 2010 with
no Form 483 observations. We believe that we are in
substantial compliance with quality system regulation and other
regulations.
We are subject to announced and unannounced inspections by the
FDA and these inspections may include the manufacturing
facilities of our subcontractors.
Fraud
and Abuse
We may directly or indirectly be subject to various federal and
state laws pertaining to healthcare fraud and abuse, including
anti-kickback laws. In particular, the federal healthcare
program anti-kickback statute prohibits persons from knowingly
and willfully soliciting, offering, receiving or providing
remuneration, directly or indirectly, in exchange for or to
induce either the referral of an individual, or the furnishing,
arranging for or recommending a good or service, for which
payment may be made in whole or part under federal healthcare
programs, such as the Medicare and Medicaid programs. The
anti-kickback statute is broad
14
and prohibits many arrangements and practices that are lawful in
businesses outside of the healthcare industry. In implementing
the statute, the Office of Inspector General, or OIG, has issued
a series of regulations, known as the “safe harbors,”
which began in July 1991. These safe harbors set forth
provisions that, if all their applicable requirements are met,
will assure healthcare providers and other parties that they
will not be prosecuted under the anti-kickback statute. The
failure of a transaction or arrangement to fit precisely within
one or more safe harbors does not necessarily mean that it is
illegal or that prosecution will be pursued. However, conduct
and business arrangements that do not fully satisfy all
requirements of an applicable safe harbor may result in
increased scrutiny by government enforcement authorities such as
the OIG. Penalties for violations of the federal anti-kickback
statute include criminal penalties and civil sanctions such as
fines, imprisonment and possible exclusion from Medicare,
Medicaid and other federal healthcare programs.
The federal False Claims Act prohibits persons from knowingly
filing or causing to be filed a false claim to, or the knowing
use of false statements to obtain payment from, the federal
government. Suits filed under the False Claims Act, known as
“qui tam” actions, can be brought by any individual on
behalf of the government. These individuals, sometimes known as
“relators” or, more commonly, as
“whistleblowers,” may share in any amounts paid by the
entity to the government in fines or settlement. The number of
filings of qui tam actions has increased significantly in recent
years, causing more healthcare companies to have to defend a
False Claim action. If an entity is determined to have violated
the federal False Claims Act, it may be required to pay up to
three times the actual damages sustained by the government, plus
civil penalties of between $5,500 and $11,000 for each separate
false claim. Various states have also enacted similar laws
modeled after the federal False Claims Act which apply to items
and services reimbursed under Medicaid and other state programs,
or, in several states, apply regardless of the payor.
The Health Insurance Portability and Accountability Act of 1996,
or HIPAA, created two new federal crimes: healthcare fraud and
false statements relating to healthcare matters. The healthcare
fraud statute prohibits knowingly and willfully executing a
scheme to defraud any healthcare benefit program, including
private payors. A violation of this statute is a felony and may
result in fines, imprisonment or exclusion from government
sponsored programs. The false statements statute prohibits
knowingly and willfully falsifying, concealing or covering up a
material fact or making any materially false, fictitious or
fraudulent statement in connection with the delivery of or
payment for healthcare benefits, items, or services. A violation
of this statute is a felony and may result in fines or
imprisonment.
We are also subject to certain state laws that are analogous to
each of the federal laws summarized above, such as anti-kickback
and false claims laws that may apply to items or services
reimbursed by any third-party payor, including commercial
insurers, and state laws governing the privacy of certain health
information, many of which differ from each other in significant
ways and often are not preempted by HIPAA, thus complicating
compliance efforts.
While we have adopted comprehensive compliance programs to
attempt to comply with these laws and regulations, if any of our
operations are found to have violated or be in violation of any
of the laws described above and other applicable state and
federal fraud and abuse laws, we may be subject to penalties,
among them being civil and criminal penalties, damages, fines,
exclusion from government healthcare programs, and the
curtailment or restructuring of our operations.
International
International sales of medical devices are subject to foreign
government regulations, which vary substantially from country to
country. The time required to obtain approval by a foreign
country may be longer or shorter than that required for FDA
clearance or approval, and the requirements may differ.
The European Union, which consists of 27 of the major countries
in Europe, has adopted numerous directives and standards
regulating the design, manufacture, clinical trials, labeling,
and adverse event reporting for medical devices. Other
countries, such as Switzerland, have voluntarily adopted laws
and regulations that mirror those of the European Union with
respect to medical devices. Devices that comply with the
requirements of a relevant directive will be entitled to bear CE
conformity marking and, accordingly, can be commercially
distributed throughout the member states of the European Union,
and other countries that
15
comply with or mirror these directives. The method of assessing
conformity varies depending on the type and class of the
product, but normally involves a combination of self-assessment
by the manufacturer and a third-party assessment by a
“Notified Body,” an independent and neutral
institution appointed to conduct conformity assessments. This
third-party assessment consists of audits of the
manufacturer’s quality system. An assessment by a Notified
Body in one country within the European Union is required for
each product in order for a manufacturer to commercially
distribute the product throughout the European Union. Compliance
with voluntary harmonizing standards ISO 9001 and ISO 13845
issued by the ISO establishes the presumption of conformity with
the essential requirements for a CE mark. In August 2004, our
quality system was initially certified by Intertek ETL-Semko, a
Notified Body, under the European Union Medical Device Directive
to be in compliance with the Canadian Medical Device Conformity
Assessment System, or CMDCAS, and ISO standards 9001:2000 and
ISO 13485:2003. The system was recertified in September 2010 to
CMDCAS, ISO 9001:2008 and ISO 13485:2003 and is due for further
recertification in September of 2013.
Employees
As of December 31, 2010, we had 129 employees, most of
whom were full-time employees, with 78 employees in
U.S. sales, marketing, professional affairs, customer
service, training and reimbursement, 8 employees in
international sales, marketing and training, 7 employees in
manufacturing, 12 employees in research and development,
16 employees in general and administrative and
8 employees in clinical, regulatory and quality assurance.
We believe that our future success will depend in part on our
continued ability to attract, hire and retain qualified
personnel. None of our employees are represented by a labor
union, and we believe that we maintain good relations with our
employees.
General
Information
We were incorporated in Delaware in May 2000 under the name
“aXiaMed, Inc.” and changed our name to “TranS1
Inc.” in February 2003. Our principal executive office is
located at 301 Government Center Drive, Wilmington, North
Carolina 28403 and our telephone number is
(910) 332-1700.
Our website is www.trans1.com. The information on, or
that can be accessed through, our website is not incorporated by
reference into this report and should not be considered to be a
part of this report.
We make our annual reports on
Form 10-K,
quarterly reports on
Form 10-Q,
current reports on
Form 8-K
and amendments to these reports available on our website, at
www.trans1.com, free of charge as soon as practicable
after filing with the SEC.
All such reports are also available free of charge via EDGAR
through the SEC website at www.sec.gov. In addition, the public
may read and copy materials filed by us with the SEC at the
SEC’s public reference room located at 100 F St., NE,
Washington, D.C., 20549. Information regarding operation of
the SEC’s public reference room can be obtained by calling
the SEC at
1-800-SEC-0330.
16
Investing in our common stock involves a high degree of risk.
You should carefully consider the following risk factors, as
well as the other information contained in this annual report,
before deciding whether to invest in shares of our common stock.
If any of the following risks actually occur, our business,
financial condition, operating results and prospects would
suffer. In that case, the trading price of our common stock
would likely decline and you might lose all or part of your
investment in our common stock. The risks described below are
not the only ones we face. Additional risks that we currently do
not know about or that we currently believe to be immaterial may
also impair our operations and business results.
Risks
Related to Our Business
To be
commercially successful, spine surgeons must accept that our
products are a safe and effective alternative to other surgical
treatments of certain spine disorders.
Our revenue is primarily derived from sales of our AxiaLIF
products and related surgical instruments. We expect that sales
of our AxiaLIF products will continue to account for a
substantial portion of our revenues for the foreseeable future.
We believe spine surgeons may not widely adopt our products
unless they determine, based on experience, long-term clinical
data and published peer reviewed journal articles, that our
products provide a safe and effective alternative to
conventional procedures used to treat certain spine disorders or
other non-conventional procedures offered by our competitors.
Spine surgeons may be slow to adopt our technology for the
following reasons, among others:
|
|
|
| |
•
|
lack of long-term clinical data supporting additional patient
benefits;
|
| |
| |
•
|
lack of experience with our products;
|
| |
| |
•
|
lack of evidence supporting cost savings of our procedure over
existing surgical alternatives;
|
| |
| |
•
|
perceived liability risks generally associated with the use of
new products and procedures;
|
| |
| |
•
|
training time required to use a new product; and
|
| |
| |
•
|
availability of adequate coverage and reimbursement for
hospitals and surgeons.
|
If we are unable to effectively demonstrate to spine surgeons
the benefits of our products as compared to other surgical
treatments of spine disorders that are available to them and our
products fail to achieve market acceptance, our future revenues
will be adversely impacted. In addition, we believe
recommendations and support of our products by influential spine
surgeons are essential for market acceptance and adoption. If we
do not receive support from these spine surgeons or do not have
favorable long-term clinical data, spine surgeons may not use
our products and our future revenues will be harmed and our
stock price would likely decline.
The
efficacy of our products is not yet supported by long-term
clinical data and may therefore prove to be less effective than
initially thought.
We obtained 510(k) clearance to manufacture, market and sell all
of our currently U.S. marketed products from the FDA. The
FDA’s 510(k) clearance process is less costly and rigorous
than the PMA process and requires less supporting clinical data.
As a result, we currently lack the breadth of published
long-term clinical data supporting the efficacy of our AxiaLIF
products and the benefits they offer that might have been
generated in connection with the PMA process.
The
demand for our products and the prices which customers and
patients are willing to pay for our products depend upon the
ability of our customers to obtain adequate third-party coverage
and reimbursement for their purchases of our
products.
Sales of our products depend in part on the availability of
adequate coverage and reimbursement from governmental and
private payors. In the United States, healthcare providers that
purchase our products generally rely on third-party payors,
principally Medicare, Medicaid and private health insurance
plans, to pay
17
for all or a portion of the costs and fees associated with our
AxiaLIF procedures. The challenge in reimbursement for our
procedure has centered around the CPT code that the physician
uses to get paid for performing the AxiaLIF surgery. In 2008
most of our surgeons billed their payor based on an existing
ALIF access code. In January 2009, physicians transitioned
billing for our procedure to a Category 3 tracking code. This
change made it more difficult for physicians to obtain
reimbursement for our procedure because many commercial payors
view a Category 3 code as “experimental” and thus will
not pre-approve the procedure or will decline to pay for that
code until they see additional clinical evidence. This
uncertainty around the availability and amount of reimbursement
has caused some physicians to revert to other fusion surgeries
where reimbursement is more certain.
Under our current timetable, we anticipate graduating to a
Category 1 CPT code for our AxiaLIF procedures on
January 1, 2013. In order to meet this objective, we will
have to have a successful outcome from any of the June 2011,
October 2011 or February 2012 American Medical Association CPT
meetings. Any delays in obtaining, or an inability to obtain,
adequate coverage or reimbursement for procedures using our
products could significantly affect the acceptance of our
products and have a material adverse effect on our business.
Third-party payors continue to review their coverage policies
carefully for existing and new therapies and can, without
notice, deny coverage for treatments that include the use of our
products. In early January 2011, we filed a Current Report on
Form 8-K
reporting a recent positive coverage decision for AxiaLIF by
Humana Inc. While we are currently engaged in discussions with
other payors, it is difficult to predict the timing of receiving
any additional positive coverage decisions and there is no
guarantee that payors will make a positive coverage decision.
Our business would be negatively impacted to the extent any such
coverage decisions reduce reimbursement for our products. In
addition, our stock price could be negatively affected to the
extent that coverage decisions by payors do not reflect market
expectations for such decisions.
With respect to coverage and reimbursement outside of the United
States, reimbursement systems in international markets vary
significantly by country, and by region within some countries,
and reimbursement approvals must be obtained on a
country-by-country
basis and can take up to 18 months, or longer. Many
international markets have government-managed healthcare systems
that govern reimbursement for new devices and procedures. In
most markets, there are private insurance systems as well as
government-managed systems. Additionally, some foreign
reimbursement systems provide for limited payments in a given
period and therefore result in extended payment periods.
Reimbursement in international markets may require us to
undertake country-specific reimbursement activities, including
additional clinical studies, which could be time consuming,
expensive and may not yield acceptable reimbursement rates.
Furthermore, healthcare costs have risen significantly over the
past decade. There have been and may continue to be proposals by
legislators, regulators and third-party payors to contain these
costs. These cost-control methods include prospective payment
systems, capitated rates, group purchasing, redesign of
benefits, pre-authorizations or second opinions prior to major
surgery, encouragement of healthier lifestyles and exploration
of more cost-effective methods of delivering healthcare. Some
healthcare providers in the United States have adopted or are
considering a managed care system in which the providers
contract to provide comprehensive healthcare for a fixed cost
per person. Healthcare providers may also attempt to control
costs by authorizing fewer elective surgical procedures or by
requiring the use of the least expensive devices possible. These
cost-control methods also potentially limit the amount which
healthcare providers may be willing to pay for certain types of
medical procedures.
In addition, in the United States, no uniform policy of coverage
and reimbursement for medical technology exists among payors.
Therefore, coverage of and reimbursement for medical technology
can differ significantly for each payor. The continuing efforts
of third-party payors, whether governmental or commercial,
whether inside the United States or outside, to contain or
reduce these costs, combined with closer scrutiny of such costs,
could restrict our customers’ ability to obtain adequate
coverage and reimbursement from these third-party payors. The
cost containment measures that healthcare providers are
instituting both in the United States and internationally could
harm our business by adversely affecting the demand for our
products or the price at which we can sell our products.
18
We
have incurred losses since inception and we expect to continue
to incur losses for the foreseeable future. We may never achieve
or sustain profitability.
We have incurred net losses since our inception in May 2000 and
we have an accumulated deficit of $90.6 million through
December 31, 2010. To date, we have financed our operations
primarily through sales of our equity securities and have
devoted substantially all of our resources to research and
development of our products, the commercial launch of our
AxiaLIF products and the development of a sales team to market
our products. We expect our expenses to increase significantly
in connection with our clinical trials and research and
development activities, as well as to support our sales and
marketing efforts. As a result, we expect to continue to incur
operating losses for the foreseeable future. These losses will
continue to have an adverse effect on our stockholders’
equity and we may never achieve or sustain profitability.
We are
in a highly competitive market segment, which is subject to
rapid technological change.
The market for treatment of spine disorders is highly
competitive and subject to rapid and profound technological
change. Our success depends, in part, upon our ability to
maintain a competitive position in the development of
technologies and products for use in the treatment of spine
disorders. We face competition from both established and
development stage companies. Many of these companies have
competitive advantages, including:
|
|
|
| |
•
|
greater financial and human resources for product development,
sales and marketing and patent litigation;
|
| |
| |
•
|
significantly greater name recognition;
|
| |
| |
•
|
established relationships with spine surgeons, customers and
third-party payors;
|
| |
| |
•
|
additional lines of products, and the ability to offer rebates
or bundle products to offer greater discounts or incentives to
gain a competitive advantage;
|
| |
| |
•
|
established sales and marketing, and distribution
networks; and
|
| |
| |
•
|
greater experience in developing new products, conducting
clinical trials, preparing regulatory submissions and obtaining
regulatory clearance or approval for products and marketing
approved products.
|
Our
failure to continue building effective sales and marketing
capabilities for our products could significantly impair our
ability to increase sales of our products.
We utilize a hybrid model of independent sales agents and direct
sales representatives for product sales in the United States and
rely on third-party distributors, direct sales representatives
and independent sales agents for international sales. As of
December 31, 2010, we employed 46 direct sales
representatives in the United States and 3 direct sales
representatives in Europe. If we are unable to efficiently
manage those individuals, our sales will suffer. We also rely on
marketing arrangements with independent sales agents in the
United States and independent distributors in Europe. We do not
control, nor monitor the marketing practices of our independent
sales agents or distributors and they may not be successful in
implementing our marketing plans or complying with applicable
laws regarding marketing practices. Independent distributors and
sales agents may terminate their relationship with us, or devote
insufficient sales efforts to our products, which could have an
adverse effect on our operations.
Our
future success depends on our ability to develop, receive
regulatory clearance or approval, and introduce new products or
product enhancements that will be accepted by the market in a
timely manner.
It is important to our business that we continue to build a more
complete product offering for treatment of spine disorders. As
such, our success will depend in part on our ability to develop
and introduce new products and enhancements to our existing
products to keep pace with the rapidly changing spine market.
However, we may not be able to successfully develop and obtain
regulatory clearance or approval for product enhancements, or
new products, or these products may not be accepted by spine
surgeons or payors who financially support many of the
procedures performed with our products.
19
The success of any new product offering or enhancement to an
existing product will depend on several factors, including our
ability to:
|
|
|
| |
•
|
properly identify and anticipate spine surgeon and patient needs;
|
| |
| |
•
|
develop and introduce new products or product enhancements in a
timely manner;
|
| |
| |
•
|
avoid infringing upon the intellectual property rights of third
parties;
|
| |
| |
•
|
demonstrate, if required, the safety and efficacy of new
products with data from preclinical studies and clinical trials;
|
| |
| |
•
|
obtain the necessary regulatory clearances or approvals for new
products or product enhancements;
|
| |
| |
•
|
be fully FDA-compliant with marketing of new devices or modified
products;
|
| |
| |
•
|
provide adequate training to potential users of our products;
|
| |
| |
•
|
receive adequate coverage and reimbursement for procedures
performed with our products; and
|
| |
| |
•
|
develop an effective and FDA-compliant, dedicated marketing and
distribution network.
|
If we do not develop new products or product enhancements in
time to meet market demand or if there is insufficient demand
for these products or enhancements, our results of operations
will suffer.
If
clinical trials of our current or future product candidates do
not produce results necessary to support regulatory clearance or
approval in the United States or elsewhere, we will be unable to
commercialize these products.
Clinical failure can occur at any stage of the testing. Our
clinical trials may produce negative or inconclusive results,
and we may decide, or regulators may require us, to conduct
additional clinical
and/or
non-clinical testing in addition to those we have planned. Our
failure to adequately demonstrate the efficacy and safety of any
of our devices would prevent receipt of regulatory clearance or
approval and, ultimately, the commercialization of that device.
Our
international operations subject us to certain operating risks,
which could adversely impact our net revenues, results of
operations and financial condition.
Sales of our products outside the United States represented 9.0%
of our revenue in 2010. The sale and shipment of our products
across international borders, as well as the purchase of
components and products from international sources, subject us
to extensive U.S. and foreign governmental trade, import
and export, and custom regulations and laws. Compliance with
these regulations is costly and exposes us to penalties for
non-compliance. Other laws and regulations that can
significantly impact us include various anti-bribery laws,
including the U.S. Foreign Corrupt Practices Act and
anti-boycott laws. Any failure to comply with applicable legal
and regulatory obligations could impact us in a variety of ways
that include, but are not limited to, civil and administrative
penalties, denial of export privileges, seizure of shipments and
restrictions on certain business activities.
Our international operations expose us and our distributors to
risks inherent in operating in foreign jurisdictions. These
risks include:
|
|
|
| |
•
|
the imposition of additional U.S. and foreign governmental
controls or regulations;
|
| |
| |
•
|
the imposition of costly and lengthy new export licensing
requirements;
|
| |
| |
•
|
the imposition of U.S. or international sanctions against a
country, company, person or entity with whom we do business that
would restrict or prohibit continued business with the
sanctioned country, company, person or entity;
|
| |
| |
•
|
economic or political instability;
|
20
|
|
|
| |
•
|
changes in third-party reimbursement policies that may require
some of the patients who receive our products to directly absorb
medical costs or that may necessitate the reduction of the
selling prices of our products;
|
| |
| |
•
|
changes in duties and tariffs, license obligations and other
non-tariff barriers to trade;
|
| |
| |
•
|
the imposition of new trade restrictions;
|
| |
| |
•
|
the imposition of restrictions on the activities of foreign
agents, representatives and distributors;
|
| |
| |
•
|
difficulties in maintaining consistency with our internal
guidelines;
|
| |
| |
•
|
difficulties in enforcing agreements and collecting receivables
through certain foreign legal systems; and
|
| |
| |
•
|
difficulties in enforcing or defending intellectual property
rights.
|
The
use, misuse or off-label use of our products may harm our image
in the marketplace or result in injuries that lead to product
liability suits, which could be costly to our business or result
in FDA sanctions if we are deemed to have engaged in such
promotion.
Our currently marketed products have been cleared by the
FDA’s 510(k) clearance process for use under specific
circumstances for the treatment of certain lower lumbar spine
conditions. We cannot, however, prevent a physician from using
our products or procedure outside of those indications cleared
for use, known as off-label use. There may be increased risk of
injury if physicians attempt to use our products off-label. We
train our sales force not to promote our products for off-label
uses. Furthermore, the use of our products for indications other
than those indications for which our products have been cleared
by the FDA may not effectively treat such conditions, which
could harm our reputation in the marketplace among physicians
and patients. Physicians may also misuse our products or use
improper techniques if they are not adequately trained,
potentially leading to injury and an increased risk of product
liability. If our products are misused or used with improper
technique, we may become subject to costly litigation by our
customers or their patients. Product liability claims could
divert management’s attention from our core business, be
expensive to defend and result in sizable damage awards against
us that may not be covered by insurance. If we are deemed by the
FDA to have engaged in the promotion of any our products for
off-label use, we could be subject to FDA prohibitions on the
sale or marketing of our products or significant fines and
penalties, and the imposition of these sanctions could also
affect our reputation and position within the industry. Any of
these events could harm our business and results of operations
and cause our stock price to decline.
We
purchase some of the key components of our products from single
suppliers. The loss of these suppliers could prevent or delay
shipments of our products or delay our clinical trials or
otherwise adversely affect our business.
Some of the key components of our products and related services
are currently purchased from only single suppliers with which we
do not have long-term contracts. Some of these suppliers may be
located outside of the United States, which could make us
subject to foreign export laws and U.S. import and customs
statutes and regulations, thus complicating and delaying
shipments of components. If necessary or desirable, we could
source our product components and related services from other
suppliers. However, establishing additional or replacement
suppliers for these components, and obtaining any necessary
regulatory clearances or approvals, could take a substantial
amount of time and could result in increased costs and impair
our ability to produce our products, which would adversely
impact our business, operating results and prospects.
If our
independent contract manufacturers fail to timely deliver to us
sufficient quantities of some of our products and components in
a timely manner, our operations may be harmed.
Our reliance on independent contract manufacturers to
manufacture most of our products and components involves several
risks, including:
|
|
|
| |
•
|
inadequate capacity of the manufacturer’s facilities;
|
21
|
|
|
| |
•
|
financial difficulties experienced by manufacturers due to the
recent economic recession and continuing economic uncertainty;
|
| |
| |
•
|
interruptions in access to certain process technologies; and
|
| |
| |
•
|
reduced control over product availability, quality, delivery
schedules, manufacturing yields and costs.
|
Shortages of raw materials, production capacity or financial
constraints, or delays by our contract manufacturers could
negatively affect our ability to meet our production obligations
and result in increased prices for affected parts. Any such
reduction, constraint or delay may result in delays in shipments
of our products or increases in the prices of components, either
of which could have a material adverse effect on our business.
We
depend on the specialized knowledge and skills of our officers
and other key employees, and if we are unable to retain and
motivate them or recruit additional qualified personnel, our
business will suffer.
We are highly dependent on our officers and other key employees.
Due to the specialized knowledge and skills each of our officers
and other key employees possesses with respect to the treatment
of spine disorders and our operations, the loss of service of
any of our officers and other key employees could delay or
prevent the successful completion of our clinical trials, the
growth of revenue from existing products and the
commercialization of our new products. This risk may be
exacerbated by the fact that our principal offices are not
located in a large, metropolitan area, which could make it more
difficult for us to retain employees or attract new employees
with the required set of skills. Each of our officers and key
employees may terminate his or her employment without notice and
without cause or good reason.
If we
are unable to raise capital, it could force us to delay, reduce,
eliminate or abandon our commercialization efforts or product
development programs.
We believe that our existing cash and cash equivalent balances
and cash receipts generated from sales of our products will be
sufficient to meet our anticipated cash requirements for at
least the next two years. However, our future funding
requirements will depend on many factors, including:
|
|
|
| |
•
|
market acceptance of our products and the revenues we are able
to generate as a result;
|
| |
| |
•
|
the availability of adequate coverage and reimbursement for
hospitals and surgeons;
|
| |
| |
•
|
the scope, rate of progress and cost of our clinical trials;
|
| |
| |
•
|
the cost of our research and development activities;
|
| |
| |
•
|
the cost of defending, in litigation or otherwise, any claims
that we infringe third-party patent or other intellectual
property rights;
|
| |
| |
•
|
the cost and timing of additional regulatory clearances or
approvals;
|
| |
| |
•
|
the cost and timing of establishing additional sales, marketing
and distribution capabilities;
|
As a result of the recent economic recession, and the continuing
economic uncertainty, it has been difficult for companies,
particularly small, medical device companies, to obtain equity
or debt financing. If we raise additional funds by issuing
equity or convertible debt securities, our stockholders may
experience dilution and the securities may contain terms that
are preferential to the new investors as compared to the holders
of our common stock. If we raise additional funds by issuing
debt securities, the securities may have rights senior to those
associated with our common stock and contain covenants
restricting our operations, our ability to pay dividends, the
amount of capital expenditures we can make, or our ability to
incur additional debt. Any debt financing or additional equity
that we raise may contain terms that are not favorable to us or
our stockholders. In addition, if we raise additional funds
through collaboration and licensing arrangements with third
parties, it may be necessary to relinquish some rights to our
technologies or our products, or grant licenses on terms that
are not favorable to us. If we are unable to raise adequate
funds, we may have to delay, reduce the scope of or eliminate
some or all of our planned product development and marketing
efforts. Additionally, if we do not
22
have, or are not able to obtain, sufficient funds, we may have
to delay development or commercialization of our products or
license to third parties the rights to commercialize products or
technologies that we would otherwise seek to commercialize. We
also may have to reduce marketing, customer support or other
resources devoted to our products or cease operations.
Pricing
pressures in the healthcare industry could lead to further
demands for price concessions and restricted access to spinal
fusions, which could have an adverse effect on our business,
financial condition or results of operations.
Because healthcare costs have risen significantly over the past
decade, numerous initiatives and reforms initiated by
legislators, regulators and third-party payors to curb these
costs have resulted in difficulty in raising or maintaining
prices and procedure volumes. The increase in pricing pressure
is driven by the competitive environment in the spine market as
many larger companies cut prices as they struggle to retain
market share as total spine volume growth began to subside.
Decreasing healthcare utilization as a result of the economic
downturn, high unemployment rates, a reduction in the ranks of
the full-time employed and the expiration of COBRA benefits has
limited the number of patients that are covered for procedures
and patients with coverage are faced with increasing
deductibles. Additionally, potential patients are increasingly
concerned about an extended absence from work, which would be
typical of major surgery like lumbar fusion. The spine market
generally, and lumbar fusion market in particular, has seen
increasing push back from payors with regard to coverage. Spine
surgery is a major cost area for payors, and from 2001 to 2008,
lumbar fusions for lower back pain grew more than twice as fast
as other lumbar fusions. As a result, private payors have
increased the criteria that a patient must meet in order to be
pre-authorized for lumbar fusion procedures and have put more
effort into the preauthorization process. Payors have
specifically stopped covering lumbar spine procedures involving
degenerative disc disease patients that have no other symptoms
such as leg pain or general spinal instability. We expect that
market demand, government regulation, third-party reimbursement
policies and societal pressures will continue to exert downward
pressure on the prices of our products and may adversely impact
our business, financial condition or results of operations.
We
face the risk of product liability or other claims and may not
be able to obtain sufficient insurance coverage, if at
all.
Our business exposes us to the risk of product liability claims
that is inherent in the testing, manufacturing and marketing of
implantable medical devices. We may be subject to product
liability claims if our products cause, or merely appear to have
caused, an injury or death. Claims may be made by patients,
consumers or healthcare providers. Although we have product
liability and clinical trial liability insurance that we believe
is appropriate for our current level of operations, this
insurance is subject to deductibles and coverage limitations.
Our current product liability insurance may not continue to be
available to us on acceptable terms, if at all, and, if
available, these coverages may not be adequate to protect us
against any future product liability claims.
Further, we may be subject to claims against us even if the
apparent injury is due to the actions of others. For example, we
rely on the expertise of spine surgeons, nurses and other
associated medical personnel to perform the medical procedure
and related processes for our products. If these medical
personnel are not properly trained or are negligent in their
provision of care, the therapeutic effect of our products may be
diminished or the patient may suffer critical injury, which may
subject us to liability. In addition, an injury that is caused
by the activities of our suppliers may be the basis for a claim
against us.
These liabilities could prevent, delay or otherwise adversely
interfere with our product commercialization efforts, and result
in judgments, fines, damages and other financial liabilities
which have adverse effects on our business, operating results
and prospects.
23
We
operate at a single location. Any disruption in this facility or
any inability to ship a sufficient number of our products to
meet demand could adversely affect our business and results of
operations.
We operate at a single location in Wilmington, North Carolina.
Our facility may be affected by man-made or natural disasters,
such as a hurricane. Our facility, if damaged or destroyed,
could require substantial lead-time to repair or replace.
Although we believe we possess adequate insurance for damage to
our property and the disruption of our business from casualties,
this insurance may not be sufficient to cover all of our
potential losses and may not continue to be available to us on
acceptable terms, or at all.
Risks
Related to the Regulatory Environment
If we
fail to maintain regulatory approvals and clearances, or are
unable to obtain, or experience significant delays in obtaining,
FDA clearances or approvals for our future products or product
modifications, our ability to commercially distribute and market
our products could suffer.
Our products are subject to rigorous regulation by the FDA and
numerous other federal, state and foreign governmental
authorities. The process of obtaining regulatory clearances or
approvals to market a medical device can be costly and time
consuming, and we may not be able to obtain these clearances or
approvals on a timely basis, if at all. In particular, the FDA
permits commercial distribution of most new medical devices only
after the device has received clearance under
Section 510(k) of the Federal Food, Drug and Cosmetic Act,
or is the subject of an approved PMA. The FDA will clear
marketing of a non-exempt lower risk medical device through the
510(k) process if the manufacturer demonstrates that the new
product is substantially equivalent to other legally marketed
products not requiring PMA approval. Our currently
commercialized products have been cleared through the 510(k)
process. However, we may need to submit a PMA for future
products we develop. The PMA process is more costly, lengthy and
uncertain than the 510(k) clearance process. A PMA application
must be supported by extensive data, including, but not limited
to, technical, preclinical, clinical trial, manufacturing and
labeling data, to demonstrate to the FDA’s satisfaction the
safety and efficacy of the device for its intended use.
Any modification to our currently marketed 510(k)-cleared
devices that could significantly affect their safety or
efficacy, or that would constitute a change in their intended
use, requires a new 510(k) clearance or, possibly, a PMA. The
FDA requires every manufacturer to make this determination in
the first instance, but the FDA may review the
manufacturer’s decision. The FDA may not agree with our
decisions regarding whether new clearances or approvals are
necessary. If the FDA requires us to seek 510(k) clearance or a
PMA for any modification to a previously cleared product, we may
be required to cease marketing and distributing, or to recall
the modified product until we obtain such clearance or approval,
and we may be subject to significant regulatory fines or
penalties.
Our failure to comply with U.S. federal, state and foreign
governmental regulations could lead to the imposition of
injunctions, suspensions or loss of regulatory clearance or
approvals, product recalls, termination of distribution, product
seizures or civil penalties, among other things. In the most
extreme cases, criminal sanctions or closure of our
manufacturing facility are possible.
Further, foreign governmental authorities that regulate the
manufacture and sale of medical devices have become increasingly
stringent and, to the extent we market and sell our products
internationally, we may be subject to rigorous international
regulation in the future. In these circumstances, we rely
significantly on our foreign independent distributors to comply
with the varying regulations, and any failures on their part
could result in restrictions on the sale of our products in
foreign countries.
Conducting successful clinical studies may require the
enrollment of large numbers of patients, and suitable patients
may be difficult to identify and recruit. Patient enrollment in
clinical trials and completion of patient participation and
follow-up
depends on many factors, including the size of the patient
population, the nature of the trial protocol, the attractiveness
of, or the discomforts and risks associated with, the treatments
received by enrolled subjects, the availability of appropriate
clinical trial investigators, support staff, and proximity of
patients to clinical sites.
24
Development of sufficient and appropriate clinical protocols and
data to demonstrate safety and efficacy are required and we may
not adequately develop such protocols to support clearance and
approval. Further, the FDA may require us to submit data on a
greater number of patients than we originally anticipated
and/or for a
longer
follow-up
period or change the data collection requirements or data
analysis applicable to our clinical trials. Delays in patient
enrollment or failure of patients to continue to participate in
a clinical trial may cause an increase in costs and delays in
the clearance or approval and attempted commercialization of our
products or result in the failure of the clinical trial. In
addition, despite considerable time and expense invested in our
clinical trials, FDA may not consider our data adequate to
demonstrate safety and efficacy. Such increased costs and delays
or failures could adversely affect our business, operating
results and prospects.
Even
if our products are approved by regulatory authorities, if we or
our suppliers fail to comply with ongoing FDA or other foreign
regulatory authority requirements, or if we experience
unanticipated problems with our products, these products could
be subject to restrictions or withdrawal from the
market.
Any product for which we obtain clearance or approval, and the
manufacturing processes, reporting requirements, post-approval
clinical data and labeling and promotional activities for such
product, will be subject to continued regulatory review,
oversight and periodic inspections by the FDA and other domestic
and foreign regulatory bodies. In particular, we and our
suppliers are required to comply with the Quality System
Regulations, or QSR, and Medical Device Directive regulations,
which may include the International Organization for
Standardization, or ISO, standards, for the manufacture of our
products and other regulations which cover the methods and
documentation of the design, testing, production, control,
quality assurance, labeling, packaging, storage and shipping of
any product for which we obtain clearance or approval.
Regulatory bodies enforce the QSR and ISO regulations through
inspections. The failure by us or one of our suppliers to comply
with applicable statutes and regulations administered by the FDA
and other regulatory bodies, or the failure to timely and
adequately respond to any adverse inspectional observations or
product safety issues, could result in, among other things,
warning letters, fines and civil penalties, delays in approving
products, withdrawal or suspension of the approval of products,
product recalls, operating restrictions, or unanticipated
expenditures to address or defend regulatory actions.
Even if regulatory clearance or approval of a product is
granted, such clearance or approval may be subject to
limitations on the intended uses for which the product may be
marketed and reduce our potential to successfully commercialize
the product and generate revenue from the product. If the FDA
determines that our promotional materials, labeling, training or
other marketing or educational activities constitute promotion
of an unapproved use, it could request that we cease or modify
our training educational, labeling or promotional materials or
subject us to regulatory enforcement actions. It is also
possible that other federal, state or foreign enforcement
authorities might take action if they consider our training
educational, labeling or other promotional materials to
constitute promotion of an unapproved use, which could result in
significant fines or penalties under other statutory
authorities, such as laws prohibiting false claims for
reimbursement.
We may
be subject to or otherwise affected by federal and state
healthcare laws, including fraud and abuse and health
information privacy and security laws, and could face
substantial penalties if we are unable to fully comply with such
laws.
Although we do not provide healthcare services, submit claims
for third-party reimbursement, or receive payments directly from
Medicare, Medicaid, or other third-party payors for our products
or the procedures in which our products are used, healthcare
regulation by federal and state governments could significantly
impact our business.
We have adopted comprehensive compliance programs to attempt to
comply with these regulations. However, because of the breadth
of these laws and regulations and the sometimes subjective
nature of their application, it is possible that some of our
business activities could be subject to challenge under one or
more of such laws. If our past or present operations, or those
of our independent sales agents and distributors, are found to
be in violation of any of such laws or any other applicable
governmental regulations, we may be subject to penalties,
including civil and criminal penalties, damages, fines,
exclusion from federal healthcare
25
programs
and/or the
curtailment or restructuring of our operations. Similarly, if
the healthcare providers or entities with whom we do business
are found to be non-compliant with applicable laws, they may be
subject to sanctions, which could also have a negative impact on
us. The risk of being found to have violated such laws is
increased by the fact that many of them have not been fully
interpreted by the regulatory authorities or the courts, and
their provisions are open to a variety of interpretations. Any
action against us for violation of these laws, even if we
successfully defend against them, could cause us to incur
significant legal expenses and divert our management’s
attention from the operation of our business.
Risks
Related to Our Intellectual Property
Our
ability to protect our intellectual property and proprietary
technology through patents and other means is
uncertain.
Our success depends significantly on our ability to protect our
proprietary rights to the procedures created with, and the
technologies used in, our products. We rely on patent
protection, as well as a combination of copyright, trade secret
and trademark laws, and nondisclosure, confidentiality and other
contractual restrictions to protect our proprietary technology.
However, these legal means afford only limited protection and
may not adequately protect our rights or permit us to gain or
keep any competitive advantage. For example, our pending United
States and foreign patent applications may not be approved, may
not issue as patents in a form that will be advantageous to us,
or may issue patents which may be subsequently successfully
challenged by others and invalidated. In addition, our pending
patent applications include claims to material aspects of our
products and procedures that are not currently protected by
issued patents. Both the patent application process and the
process of managing patent disputes can be time consuming and
expensive. The patents we own may not be of sufficient scope or
strength to provide us with any meaningful protection or
commercial advantage, and competitors may be able to design
around our patents or develop products which provide outcomes
which are comparable to ours. Although we have taken steps to
protect our intellectual property and proprietary technology,
including entering into confidentiality agreements and
intellectual property assignment agreements with our officers,
employees, consultants and advisors, such agreements may not be
enforceable or may not provide meaningful protection for our
trade secrets or other proprietary information in the event of
unauthorized use or disclosure or other breaches of such
agreements. Furthermore, the laws of some foreign countries do
not protect our intellectual property rights to the same extent
as do the laws of the United States.
We rely on our trademarks, trade names, and brand names to
distinguish our products from the products of our competitors,
and have registered or applied to register many of these
trademarks. However, our trademark applications may not be
approved. Third parties may also oppose our trademark
applications, or otherwise challenge our use of the trademarks.
In the event that our trademarks are successfully challenged, we
could be forced to rebrand our products, which could result in
loss of brand recognition, and could require us to devote
resources to advertising and marketing new brands. Further, our
competitors may infringe our trademarks, or we may not have
adequate resources to enforce our trademarks.
In the event a competitor infringes upon our intellectual
property rights, enforcing our rights may be costly, difficult
and time consuming and we may not have sufficient resources to
enforce such rights.
Any
lawsuit, whether initiated by us to enforce our intellectual
property rights or by a third party against us alleging
infringement, may cause us to expend significant financial and
other resources, and may divert our attention from our business
and adversely affect our business, operating results and
prospects.
The medical device industry is characterized by the existence of
a large number of patents and frequent litigation based on
allegations of patent infringement. Patent litigation can
involve complex factual and legal questions and its outcome is
uncertain. Any claim relating to infringement of patents that is
successfully asserted against us may require us to pay
substantial damages. Even if we were to prevail, any litigation
could be costly and time-consuming and would divert the
attention of our management and key personnel from our business
operations.
Our success will also depend in part on our not infringing
patents issued to others, including our competitors and
potential competitors. If our products are found to infringe the
patents of others, our
26
development, manufacture and sale of such products could be
severely restricted or prohibited. In addition, our competitors
may independently develop similar technologies. Because of the
importance of our patent portfolio and unpatented proprietary
technology to our business, we may lose market share to our
competitors if we fail to protect our patent rights.
A patent infringement suit or other infringement or
misappropriation claim brought against us or any of our
strategic partners or licensees may force us or any of our
strategic partners or licensees to stop or delay developing,
manufacturing or selling potential products that are claimed to
infringe a third party’s intellectual property, unless that
party grants us or any strategic partners or licensees rights to
use its intellectual property. In such cases, we may be required
to obtain licenses to patents or proprietary rights of others in
order to continue to commercialize our products. However, even
if we, our strategic partners or our licensees are able to
obtain rights to the third party’s intellectual property,
these rights may be non-exclusive, thereby giving our
competitors access to the same intellectual property. Also,
there is the possibility that we may not be able to obtain any
licenses required under any patents or proprietary rights of
third parties on acceptable terms. Ultimately, we may be unable
to commercialize some of our potential products or may have to
cease some of our business operations as a result of patent
infringement claims, which could severely harm our business.
Risks
Related to our Common Stock
Our
directors, officers and principal stockholders have significant
voting power and may take actions that may not be in the best
interests of our other stockholders.
At December 31, 2010 our officers, directors and principal
stockholders, each holding more than 5% of our common stock,
collectively controlled approximately 59% of our outstanding
common stock. As a result, these stockholders, if they act
together, could potentially control the management and affairs
of our company and most matters requiring stockholder approval,
including the election of directors and approval of significant
corporate transactions. This concentration of ownership may not
be in the best interests of our other stockholders.
The
price of our common stock has been, and may continue to be,
volatile and our stockholders may not be able to resell shares
of our common stock at or above the price paid for such
shares.
The price for shares of our common stock on the Nasdaq Global
Market has exhibited high levels of volatility with significant
price fluctuations, which makes our common stock unsuitable for
many investors. For example, for the three years ended
December 31, 2010, the closing price of our common stock
ranged from a high of $17.24 per share to a low of $1.76 per
share. At times, the fluctuations in the price of our common
stock may have been unrelated to our operating performance.
These broad fluctuations may negatively impact the market price
of shares of our common stock.
Trading
in our stock has historically been limited, so investors may not
be able to sell as much stock as they want at prevailing
prices.
The average daily trading volume in our common stock for the
year ended December 31, 2010 was approximately
45,000 shares. If limited trading in our stock continues,
it may be difficult for investors to sell their shares in the
public market at any given time at prevailing prices. Moreover,
the market price for shares of our common stock may be made more
volatile because of the relatively low volume of trading in our
common stock. When trading volume is low, significant price
movement can be caused by the trading in a relatively small
number of shares. Volatility in our common stock could cause
stockholders to incur substantial losses.
Our
amended and restated certificate of incorporation, amended and
restated bylaws and Delaware law contain provisions that could
have the effect of deterring or delaying changes in incumbent
management, proxy contests or changes in control.
Anti-takeover provisions of our amended and restated certificate
of incorporation, amended and restated bylaws and Delaware law
may have the effect of deterring or delaying attempts by our
stockholders to remove
27
or replace management, engage in proxy contests and effect
changes in control. The provisions of our charter documents
include:
|
|
|
| |
•
|
a classified board so that only one of the three classes of
directors on our board of directors is elected each year;
|
| |
| |
•
|
procedures for advance notification of stockholder director
nominations and proposals;
|
| |
| |
•
|
the ability of our board of directors to amend our bylaws
without stockholder approval;
|
| |
| |
•
|
a supermajority stockholder vote requirement for amending
certain provisions of our amended and restated certificate of
incorporation and our amended and restated bylaws; and
|
| |
| |
•
|
the ability of our board of directors to issue up to
5,000,000 shares of preferred stock without stockholder
approval upon the terms and conditions and with the rights,
privileges and preferences as our board of directors may
determine.
|
In addition, as a Delaware corporation, we are subject to
Delaware law, including Section 203 of the Delaware General
Corporation Law, or DGCL. In general, Section 203 prohibits
a Delaware corporation from engaging in any business combination
with any interested stockholder for a period of three years
following the date that the stockholder became an interested
stockholder unless certain specific requirements are met as set
forth in Section 203. These provisions, alone or together,
could have the effect of deterring or delaying changes in
incumbent management, proxy contests or changes in control.
|
|
|
Item 1B.
|
Unresolved
Staff Comments.
|
None.
In February 2010, we moved into a new facility of approximately
31,000 square feet, located in Wilmington, North Carolina.
Of that amount, approximately 5,000 square feet is used for
manufacturing and warehousing, 19,000 square feet for
office space and 7,000 square feet for research and
development activities. This lease expires in December 2014,
with an option to extend the lease through December 2019. We
believe that our current facility will be sufficient to meet our
needs through that time.
|
|
|
Item 3.
|
Legal
Proceedings.
|
We are not currently party to any material legal proceedings. We
may be subject to various claims and legal actions arising in
the ordinary course of business from time to time.
|
|
|
Item 4.
|
(Removed
and Reserved).
|
28
|
|
|
Item 5.
|
Market
for the Registrant’s Common Equity, Related Stockholder
Matters and Issuer Purchases of Equity Securities
|
Price of
Common Stock
Our common stock is traded on the NASDAQ Global Market under the
symbol “TSON.” The following table sets forth the high
and low sales prices of our common stock as quoted on the NASDAQ
Global Market for the periods indicated.
| |
|
|
|
|
|
|
|
|
|
|
|
Price Range
|
|
|
|
High
|
|
Low
|
|
|
|
Fiscal 2010:
|
|
|
|
|
|
|
|
|
|
Fourth quarter
|
|
$
|
2.63
|
|
|
$
|
1.76
|
|
|
Third quarter
|
|
|
2.98
|
|
|
|
2.21
|
|
|
Second quarter
|
|
|
3.93
|
|
|
|
2.55
|
|
|
First quarter
|
|
|
4.11
|
|
|
|
3.01
|
|
|
Fiscal 2009:
|
|
|
|
|
|
|
|
|
|
Fourth quarter
|
|
$
|
5.00
|
|
|
$
|
3.26
|
|
|
Third quarter
|
|
|
6.83
|
|
|
|
4.08
|
|
|
Second quarter
|
|
|
8.74
|
|
|
|
5.65
|
|
|
First quarter
|
|
|
7.66
|
|
|
|
4.55
|
|
The closing price for our common stock as reported by the NASDAQ
Global Market on March 9, 2011 was $3.69 per share.
As of March 9, 2011, we had approximately 34 stockholders
of record based upon the records of our transfer agent, which
does not include beneficial owners of our common stock whose
shares are held in the names of various securities brokers,
dealers and registered clearing agencies.
Uses of
Proceeds from Sale of Registered Securities
On October 22, 2007, we completed our initial public
offering of 6,325,000 shares of common stock at the initial
public offering price of $15.00 per share. We effected the
offering through a Registration Statement on
Form S-1
(Registration
No. 333-144802),
which was declared effective by the SEC on October 16,
2007, and through a Registration Statement on
Form S-1
filed pursuant to Rule 462(b) under the Securities Act
(Registration
No. 333-146753),
which became effective upon filing on October 17, 2007
pursuant to Rule 462(b) (collectively, the
“Registration Statement”).
As of December 31, 2010, we had used all of the net
proceeds for sales, marketing, general administrative and
research and development activities.
Dividend
Policy
We have never declared or paid any cash dividends on our capital
stock. We currently intend to retain future earnings, if any,
for development of our business and do not anticipate that we
will declare or pay cash dividends on our capital stock in the
foreseeable future.
Securities
Authorized For Issuance Under Equity Compensation
Plans
The information regarding securities authorized for issuance
under our equity compensation plans is set forth in Item 12
of this annual report on
10-K and is
incorporated herein by reference.
29
Stock
Price Performance Graph
The following graph compares the cumulative total stockholder
return on our common stock from October 17, 2007 (the date
our common stock began trading on the NASDAQ Global Market)
through December 31, 2010 to that of the cumulative return
over such period for (i) The NASDAQ Stock Market Composite
Index, and (ii) NASDAQ Medical Equipment Index. Total
stockholder return assumes $100.00 invested at the beginning of
the period in our common stock and in each of the comparative
indices. The graph further assumes that such amount was
initially invested in our common stock at the closing market
price on the first day of trading, and that any dividends have
been reinvested. We have not paid any dividends on our common
stock. The stock price performance on the following graph is not
necessarily indicative of future stock price performance.
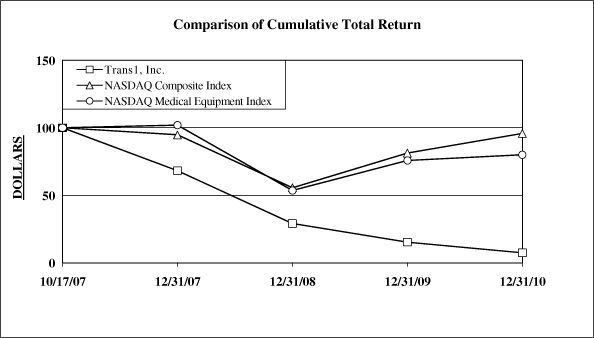
The material in the above performance graph does not
constitute soliciting material and should not be deemed filed or
incorporated by reference into any other Company filing, whether
under the Securities Act of 1933, as amended, or the Securities
Exchange Act of 1934, as amended, whether made on, before or
after the date of this report and irrespective of any general
incorporation language in such filing, except to the extent we
specifically incorporate this performance graph by reference
therein.
30
|
|
|
Item 6.
|
Selected
Financial Data.
|
The following selected financial data has been derived from our
audited consolidated financial statements. You should read the
following financial information together with the information
under “Management’s Discussion and Analysis of
Financial Condition and Results of Operations” and our
consolidated financial statements and the notes included
elsewhere in this report.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
(In thousands, except share data)
|
|
|
|
|
Consolidated Statements of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
26,154
|
|
|
$
|
29,807
|
|
|
$
|
25,304
|
|
|
$
|
16,473
|
|
|
$
|
5,812
|
|
|
Cost of revenue
|
|
|
7,104
|
|
|
|
5,687
|
|
|
|
4,315
|
|
|
|
3,042
|
|
|
|
1,580
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
19,050
|
|
|
|
24,120
|
|
|
|
20,989
|
|
|
|
13,431
|
|
|
|
4,232
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
4,223
|
|
|
|
6,439
|
|
|
|
4,081
|
|
|
|
3,885
|
|
|
|
3,816
|
|
|
Sales and marketing
|
|
|
26,275
|
|
|
|
34,098
|
|
|
|
29,375
|
|
|
|
15,706
|
|
|
|
9,288
|
|
|
General and administrative
|
|
|
8,565
|
|
|
|
7,184
|
|
|
|
7,116
|
|
|
|
3,801
|
|
|
|
1,596
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
39,063
|
|
|
|
47,721
|
|
|
|
40,572
|
|
|
|
23,392
|
|
|
|
14,700
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(20,013
|
)
|
|
|
(23,601
|
)
|
|
|
(19,583
|
)
|
|
|
(9,961
|
)
|
|
|
(10,468
|
)
|
|
Other income, net
|
|
|
486
|
|
|
|
405
|
|
|
|
2,548
|
|
|
|
1,384
|
|
|
|
989
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(19,527
|
)
|
|
$
|
(23,196
|
)
|
|
$
|
(17,035
|
)
|
|
$
|
(8,577
|
)
|
|
$
|
(9,479
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share - basic and diluted
|
|
$
|
(0.94
|
)
|
|
$
|
(1.13
|
)
|
|
$
|
(0.84
|
)
|
|
$
|
(1.46
|
)
|
|
$
|
(3.91
|
)
|
|
Weighted average common shares outstanding — basic and
diluted
|
|
|
20,738,433
|
|
|
|
20,603,600
|
|
|
|
20,288,711
|
|
|
|
5,872,008
|
|
|
|
2,423,223
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
(In thousands)
|
|
|
|
|
Consolidated Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and short-term investments
|
|
$
|
42,536
|
|
|
$
|
55,251
|
|
|
$
|
77,266
|
|
|
$
|
93,921
|
|
|
$
|
14,962
|
|
|
Working capital
|
|
|
46,166
|
|
|
|
63,467
|
|
|
|
84,174
|
|
|
|
98,351
|
|
|
|
17,448
|
|
|
Total assets
|
|
|
52,019
|
|
|
|
68,991
|
|
|
|
90,491
|
|
|
|
102,856
|
|
|
|
20,004
|
|
|
Preferred stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
40,089
|
|
|
Common stock
|
|
|
2
|
|
|
|
2
|
|
|
|
2
|
|
|
|
2
|
|
|
|
—
|
|
|
Additional paid in capital
|
|
|
138,401
|
|
|
|
136,402
|
|
|
|
133,507
|
|
|
|
130,325
|
|
|
|
820
|
|
|
Total stockholders’ equity/(deficit)
|
|
|
47,728
|
|
|
|
65,280
|
|
|
|
85,586
|
|
|
|
99,439
|
|
|
|
(21,529
|
)
|
31
|
|
|
Item 7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
The following discussion of our financial condition and results
of operations should be read in conjunction with our “Risk
Factors” and our consolidated financial statements and the
related notes to our consolidated financial statements included
in this annual report. The following discussion contains
forward-looking statements. See “Cautionary Note Regarding
Forward-Looking Statements” at the beginning of this report.
Overview
We are a medical device company focused on designing, developing
and marketing products that implement our proprietary approach
to treat degenerative conditions of the spine affecting the
lower lumbar region. Using our pre-sacral approach, a surgeon
can access discs in the lower lumbar region of the spine through
a small incision adjacent to the tailbone and can perform an
entire fusion procedure through a small tube that provides
direct access to the intervertebral space. We developed our
pre-sacral approach to allow spine surgeons to access and treat
intervertebral spaces without compromising important surrounding
soft tissue. We believe this approach enables fusion procedures
to be performed with low complication rates, low blood loss,
short hospital stays, fast recovery times and reduced pain. We
currently market our
AxiaLIF®
family of products for single and multilevel lumbar fusion, the
Vectretm
and
Avatartm
lumbar posterior fixation systems and
Bi-Ostetictm
bone void filler, a biologics product.
From our incorporation in 2000 through 2004, we devoted
substantially all of our resources to research and development
and start-up
activities, consisting primarily of product design and
development, clinical trials, manufacturing, recruiting
qualified personnel and raising capital. We received 510(k)
clearance from the U.S. Food and Drug Administration, or
FDA, for our AxiaLIF 1L product in the fourth quarter of 2004,
and commercially introduced our AxiaLIF 1L product in the United
States in the first quarter of 2005. We received a CE mark to
market our AxiaLIF 1L product in the European market in the
first quarter of 2005 and began commercialization in the first
quarter of 2006. We received a CE mark for our AxiaLIF 2L
product in the third quarter of 2006 and began commercialization
in the European market in the fourth quarter of 2006. We
received FDA 510(k) clearance for our AxiaLIF 2L product and
began marketing this product in the United States in the second
quarter of 2008. The AxiaLIF 2L product was discontinued in 2010
after we launched our AxiaLIF
2L+tm
product in July 2010, for which we had received FDA 510(k)
clearance in January 2010. We commercially launched our next
generation Vectre facet screw system in April 2010. In January
2010, we entered into an agreement to distribute Avatar, a
pedicle screw system, and in February 2010, we entered into an
agreement to distribute and Bi-Ostetic bone void filler, a
biologics product. We currently sell our products through a
direct sales force, independent sales agents and international
distributors.
We rely on third parties to manufacture most of our products and
their components. We believe these manufacturing relationships
allow us to work with suppliers who have the best specific
competencies while we minimize our capital investment, control
costs and shorten cycle times, all of which allow us to compete
with larger volume manufacturers of spine surgery products.
Since inception, we have been unprofitable. As of
December 31, 2010, we had an accumulated deficit of
$90.6 million.
We expect to continue to invest in creating a sales and
marketing infrastructure for our AxiaLIF family of products in
order to gain wider acceptance for them. We also expect to
continue to invest in research and development and related
clinical trials, and increase general and administrative
expenses as we grow. As a result, we will need to generate
significant revenue in order to achieve profitability.
32
Results
of Operations
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
% change
|
|
|
2008
|
|
|
% change
|
|
|
|
|
(In thousands, except gross margin percentage)
|
|
|
|
|
Revenue
|
|
$
|
26,154
|
|
|
$
|
29,807
|
|
|
|
(12.3
|
)%
|
|
$
|
25,304
|
|
|
|
17.8
|
%
|
|
Cost of revenue
|
|
|
7,104
|
|
|
|
5,687
|
|
|
|
24.9
|
%
|
|
|
4,315
|
|
|
|
31.8
|
%
|
|
Gross margin %
|
|
|
72.8
|
%
|
|
|
80.9
|
%
|
|
|
(10.0
|
)%
|
|
|
82.9
|
%
|
|
|
(2.4
|
)%
|
|
Total operating expenses
|
|
|
39,063
|
|
|
|
47,721
|
|
|
|
(18.1
|
)%
|
|
|
40,572
|
|
|
|
17.6
|
%
|
|
Net loss
|
|
|
(19,527
|
)
|
|
|
(23,196
|
)
|
|
|
15.8
|
%
|
|
|
(17,035
|
)
|
|
|
(36.2
|
)%
|
Revenue
We generate revenue from the sales of our implants and
disposable surgical instruments. We have two distinct sales
methods. The first method is when implants
and/or
disposable surgical instruments are sold directly to hospitals
or surgical centers for the purpose of conducting a scheduled
surgery. Our sales representatives or independent sales agents
hand deliver the products to the customer on or before the day
of the surgery. The sales representative or independent agent is
then responsible for reporting the delivery of the products and
the date of the operation to the corporate office for proper
revenue recognition. We recognize revenue upon the confirmation
that the products have been used in a surgical procedure. The
other sales method is for sales to hospitals for consumption
during future surgeries or to distributors outside the United
States. These transactions require the customer to send in a
purchase order before shipment will be made to the customer.
Under the distributor agreements currently in place, a
distributor only has the right of return for defective products.
We expect that a substantial amount of our revenues will
continue to be generated in the United States in future periods.
Cost
of Revenue
Cost of revenue consists primarily of material and overhead
costs related to our products. Cost of revenue also includes
facilities-related costs, such as rent, utilities and
depreciation.
Research
and Development
Research and development expenses consist primarily of personnel
costs within our product development, regulatory and clinical
functions and the costs of clinical studies and product
development projects. In future periods, we expect research and
development expenses to grow as we continue to invest in basic
research, clinical trials, product development and in our
intellectual property.
Sales
and Marketing
Sales and marketing expenses consist of personnel costs, sales
commissions paid to our direct sales representatives and
independent sales agents, and costs associated with physician
training programs, promotional activities, and participation in
medical conferences. In future periods, we expect sales and
marketing expenses to increase as we expand our sales and
marketing efforts.
General
and Administrative
General and administrative expenses consist of personnel costs
related to the executive, finance, business development,
information technology and human resource functions, as well as
professional service fees, legal fees, accounting fees,
insurance costs and general corporate expenses. We expect
general and administrative expenses to increase as we grow our
business.
33
Other
Income, Net
Other income, net, is primarily composed of government grants,
interest earned on our cash, cash equivalents and
available-for-sale
securities and the gain or loss on disposal of fixed assets. In
2010, we received a grant from the U.S. Treasury under the
Qualifying Therapeutic Discovery Program of $0.5 million.
Comparison
of the Years Ended December 31, 2010, 2009 and
2008
Revenue. Revenue was $26.2 million in
2010, $29.8 million in 2009 and $25.3 million in 2008.
The $3.6 million decrease in revenue from 2009 to 2010 was
primarily a result of a lower number of AxiaLIF cases performed
due primarily to physician reimbursement limitations and
insurance denials for lumbar fusion surgery due to medical
necessity, partially offset by a price increase in April 2010
and revenues from new products. The $4.5 million increase
in revenue from 2008 to 2009 was primarily attributable to an
increase in the number of products sold, which we believe
resulted from the continued market acceptance of our AxiaLIF
products. None of the revenue increase in 2009 was attributable
to price increases. Domestically, sales of our AxiaLIF 1L
products decreased to $12.9 million in 2010 from
$17.1 million in 2009 and $17.2 million in 2008. Sales
of our AxiaLIF 2L products were $7.8 million in 2010,
$7.8 million in 2009 and $3.0 million in 2008. New
products accounted for revenue of $1.2 million in 2010. In
2010, average revenue per AxiaLIF case continued to increase
from 2009 and 2008, due to a price increase effective
April 1, 2010, the full market release of our AxiaLIF 2L+
product, and penetration into existing cases by our new
products. In 2010, 2009 and 2008, we recorded 1,985, 2,578 and
2,280 domestic AxiaLIF cases, respectively, including 555
AxiaLIF 2L and 2L+ cases in 2010, 596 AxiaLIF 2L cases in 2009
and 229 AxiaLIF 2L cases in 2008. Additionally, during 2010,
2009 and 2008, we generated $1.9 million, $3.1 million
and $3.1 million, respectively, in revenues from sales of
our facet screw systems. Revenue generated outside the United
States increased to $2.4 million in 2010 from
$1.8 million in 2009, which was a decrease from
$2.0 million in 2008. In 2010, 2009 and 2008, initial
stocking shipments to new distributors were $110,000, $122,000
and $382,000, respectively. In 2009, we began direct sales
through our own sales representatives and independent sales
agents in Europe and generated revenue of $1.3 million in
2010 and $543,000 in 2009. In 2010, 2009 and 2008, 91%, 94% and
92%, respectively, of our revenues were generated in the United
States.
Cost of Revenue. Cost of revenue increased to
$7.1 million in 2010 from $5.7 million in 2009 and
$4.3 million in 2008. The $1.4 million increase from
2009 to 2010 was primarily related to inventory obsolescence
reserves of $2.0 million taken in 2010 for existing
products that were being replaced, or were obsolete and excess,
partially offset by lower material and overhead costs associated
with decreased sales volume for our products. This included
reserves for the AxiaLIF 1L, AxiaLIF 2L and facet products. The
$1.4 million increase from 2008 to 2009 was primarily the
result of higher material and overhead costs associated with
increased sales volumes. Gross margin decreased to 72.8% in
2010, from 80.9% in 2009 and 82.9% in 2008. The decrease in
gross margin from 2009 to 2010 was primarily related to the
inventory reserves of $2.0 million taken in 2010, as
discussed above. The decrease in gross margin from 2008 to 2009
was primarily related to specific inventory reserves of
$0.4 million taken in 2009 for excess inventory and the
under-absorption of manufacturing overhead of $0.2 million
as we reduced inventory purchases in response to lower than
anticipated business volume.
Research and Development. Research and
development expenses decreased to $4.2 million in 2010 from
$6.4 million in 2009, which was an increase from
$4.1 million in 2008. The $2.2 million decrease in
expense from 2009 to 2010 was primarily the result of a
$1.0 million expense in 2009 to acquire the rights to
develop a technology for future use and a decrease in project
spending in 2010. The $2.3 million increase in expense from
2008 to 2009 was primarily the result of the $1.0 million
technology acquisition previously discussed and increased
research and development and clinical project-related spending.
Sales and Marketing. Sales and marketing
expenses decreased to $26.3 million in 2010 from
$34.1 million in 2009, which was an increase from
$29.4 million in 2008. The decrease in expense from 2009 to
2010 of $7.8 million was primarily due to decreased
personnel related costs of $2.6 million as we reduced our
U.S. sales force, decreased travel and entertainment
expenses of $2.2 million, lower commissions of
$1.7 million related to the lower revenue, decreased
surgeon training costs of $1.6 million and a decrease in
promotional costs of
34
$0.4 million. These lower expenses were partially offset by
severance costs of $0.5 million for employees affected by
the sales force reduction. The increase in expense from 2008 to
2009 of $4.7 million was primarily attributable to
increased personnel related costs, including commission expense,
of $3.6 million, as we built out our sales and marketing
organization, increased training costs of $0.6 million and
increased promotional activities of $0.5 million.
General and Administrative. General and
administrative expenses increased to $8.6 million in 2010
from $7.2 million in 2009 and $7.1 million in 2008.
The increase in expenses from 2009 to 2010 of $1.4 million
was primarily attributable to an increase in personnel-related
costs related to the management transition that incurred in
2010, including severance, recruiting and other
personnel-related expenses of $1.1 million. The increase in
expenses from 2008 to 2009 of $0.1 million was primarily
attributable to increased personnel related costs of
$0.5 million, partially offset by a decrease in consulting
expenses, of $0.4 million.
Other Income, Net. Other income, net,
increased to $0.5 million in 2010 from $0.4 million in
2009, which was a decrease from $2.5 million in 2008. The
increase of $0.1 million in other income, net from 2009 to
2010 was primarily the result of a grant we received from the
U.S. Treasury under the Qualifying Therapeutic Discovery
Program in 2010 for $0.5 million, partially offset by a
decrease of $0.3 million in interest income and a loss on
disposal of fixed assets of $0.1 million. The decrease of
$2.1 million in other income from 2008 to 2009 was
primarily the result of historically low interest rates, the
conservative and liquid nature of the investment portfolio and
lower investment balances.
Liquidity
and Capital Resources
Sources
of Liquidity
Since our inception in 2000, we have incurred significant losses
and, as of December 31, 2010, we had an accumulated deficit
of $90.6 million. We have not yet achieved profitability,
and anticipate that we will continue to incur losses in the near
term. We expect that research and development, sales and
marketing and general and administrative expenses will continue
to grow and, as a result, we will need to generate significant
revenues to achieve profitability. Prior to our October 2007
initial public offering, our operations were funded primarily
with the gross proceeds from the sale of preferred stock of
$40.5 million. The net proceeds from our October 2007
initial public offering of $86.7 million have funded our
operations since then.
As of December 31, 2010, we did not have any significant
outstanding debt financing arrangements, we had working capital
of $46.2 million and our primary source of liquidity was
$42.5 million in cash, cash equivalents and short-term
investments. We currently invest our cash and cash equivalents
primarily in money market treasury funds and our short-term
investments primarily in U.S. agency backed debt
instruments.
Cash, cash equivalents and short-term investments
decreased from $55.3 million at December 31, 2009 to
$42.5 million at December 31, 2010. The decrease of
$12.8 million was primarily the result of net cash used in
operating activities of $12.3 million and purchases of
property and equipment of $0.6 million offset by net cash
provided from issuance of our common stock upon the exercise of
stock options of $0.1 million.
Cash, cash equivalents and short-term investments
decreased from $77.3 million at December 31, 2008 to
$55.3 million at December 31, 2009. The decrease of
$22.0 million was primarily the result of net cash used in
operating activities of $20.8 million and purchases of
property and equipment of $1.3 million.
Cash
Flows
Net Cash Used in Operating Activities. Net
cash used in operating activities was $12.3 million in
2010, $20.8 million in 2009 and $15.7 million in 2008.
For each of these periods, net cash used in operating activities
was attributable primarily to net losses after adjustment for
non-cash items, such as depreciation, stock-based compensation
expense, inventory reserves, bad debt reserves and changes in
working capital requirements to support the market acceptance of
our AxiaLIF products. The decrease in working capital
requirements for 2010 was primarily related to the decrease in
inventories associated with decreased sales volume for our
product. The increase in working capital requirements for 2009
and 2008 was driven by the growth in inventories, which in 2008
was partially offset by the increase to payables. Working
capital
35
requirements in all years were also affected by smaller changes
in prepaid assets and accrued liabilities due to the timing of
activities in those accounts.
Net Cash Provided by (Used In) Investing
Activities. Net cash provided by investing
activities was $7.3 million in 2010 and $8.0 million
in 2009. Net cash used in investing activities was
$7.1 million in 2008. For each of these periods, this
amount reflected purchases or sales and maturities of
investments and purchases of property and equipment.
Net Cash Provided by Financing Activities. Net
cash provided by financing activities was $0.1 million in
2010 and 2009, and $0.2 million in 2008. Net cash provided
by financing activities represents proceeds from the issuance of
shares of our common stock upon the exercise of stock options.
Operating
Capital and Capital Expenditure Requirements
We believe that our existing cash and cash equivalents, together
with cash received from sales of our products, will be
sufficient to meet our cash needs for at least the next two
years. We intend to spend substantial sums on sales and
marketing initiatives to support the ongoing commercialization
of our products and on research and development activities,
including product development, regulatory and compliance,
clinical studies in support of our currently marketed products
and future product offerings, and the enhancement and protection
of our intellectual property. We may need to obtain additional
financing to pursue our business strategy, to respond to new
competitive pressures or to take advantage of opportunities that
may arise. The sale of additional equity or convertible debt
securities could result in dilution to our stockholders. If
additional funds are raised through the issuance of debt
securities, these securities could have rights senior to those
associated with our common stock and could contain covenants
that would restrict our operations. Any additional financing may
not be available in amounts or on terms acceptable to us, if at
all. If we are unable to obtain this additional financing, we
may be required to reduce the scope of our planned product
development and marketing efforts.
Contractual
Obligations
The following table discloses information about our contractual
obligations by the year in which payments are due as of
December 31, 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments Due by Year
|
|
|
|
|
|
Less Than
|
|
|
|
|
|
After
|
|
Contractual Obligations
|
|
Total
|
|
1 Year
|
|
1-3 Years
|
|
3-5 Years
|
|
5 Years
|
|
|
|
(In thousands)
|
|
|
|
Operating leases(1)
|
|
$
|
1,413
|
|
|
$
|
354
|
|
|
$
|
726
|
|
|
$
|
333
|
|
|
$
|
—
|
|
|
|
|
|
(1) |
|
We rent office space under an operating lease which expires in
2014, with an option to extend the lease through 2019. |
Off-Balance
Sheet Arrangements
As of December 31, 2010, we did not have any outstanding
debt or available debt financing arrangements or off-balance
sheet liabilities.
Critical
Accounting Policies and Estimates
Our discussion and analysis of our financial condition and
results of operations is based upon our consolidated financial
statements, which have been prepared in accordance with
accounting principles generally accepted in the United States.
The preparation of financial statements requires management to
make estimates and judgments that affect the reported amounts of
assets and liabilities, revenue and expenses, and disclosures of
contingent assets and liabilities at the date of the financial
statements. On an on-going basis, we evaluate our estimates,
including those related to revenue recognition, accounts
receivable reserves, inventory reserves, accrued expenses income
tax valuations and stock-based compensation. We base our
estimates on historical experience and various other assumptions
that we believe to be reasonable, the results of which form
36
the basis for making judgments about the carrying value of
assets and liabilities that are not apparent from other sources.
Actual results could differ from those estimates under different
assumptions or conditions.
We believe the following critical accounting policies affect our
more significant judgments and estimates used in the preparation
of our consolidated financial statements.
Revenue Recognition. We recognize revenue
based on the following criteria: (i) persuasive evidence
that an arrangement exists with the customer; (ii) delivery
of the products
and/or
services has occurred; (iii) the selling price has been
fixed for the products or services delivered; and
(iv) collection is reasonably assured. Revenue is generated
from the sale of our implants and disposable surgical
instruments. We have two distinct sales methods. The first
method is when implants
and/or
disposable surgical instruments are sold directly to hospitals
or surgical centers for the purpose of conducting a scheduled
surgery. Our sales representatives or independent sales agents
hand deliver the procedure kit to the customer on or before the
day of the surgery. The sales representative or independent
agent is then responsible for reporting the delivery of the
products and the date of the surgery to our corporate office for
proper revenue recognition. We recognize revenue upon the
confirmation that the products have been used in a surgical
procedure. The other sales method is for sales to hospitals for
consumption during future surgeries or to distributors outside
the United States. These distributors order multiple procedure
kits at one time to have on hand. These transactions require the
customer to send in a purchase order before shipment will be
made to the customer. Under the distributor agreements currently
in place, a distributor only has the right of return for
defective products.
Accounts Receivable and Allowances. We monitor
collections and payments from our customers and maintain an
allowance for doubtful accounts based upon our historical
experience and any specific customer collection issues that we
have identified. While our credit losses have historically been
within our expectations and the allowance established, we may
not continue to experience the same credit loss rates that we
have in the past. We make estimates on the collectability of
customer accounts based primarily on analysis of historical
trends and experience and changes in customers’ financial
condition. Management uses its best judgment, based on the best
available facts and circumstances, and records a reserve against
the amounts due to reduce the receivable to the amount that is
expected to be collected. These reserves are reevaluated and
adjusted as additional information is received that impacts the
amount reserved.
Inventory. We state our inventories at the
lower of cost or market, computed on a standard cost basis,
which approximates actual cost on a
first-in,
first-out basis and market being determined as the lower of
replacement cost or net realizable value. Costs are monitored on
an annual basis and updated as necessary to reflect changes in
supplier costs and the rate of our overhead absorption is
adjusted based on projections of our manufacturing costs and
production plan. The components of inventory are reviewed on a
periodic basis for excess, obsolete and impaired inventory, and
a reserve is recorded for the identified items. An inventory
reserve is calculated for estimated excess and obsolete
inventory based upon historical turnover and assumptions about
future demand for the products and market conditions.
Accounting for Income Taxes. Significant
management judgment is required in determining our provision for
income taxes, our deferred tax assets and liabilities and any
valuation allowance recorded against our net deferred tax
assets. We account for income taxes using the liability method
which requires the recognition of deferred tax assets or
liabilities for the temporary differences between financial
reporting and tax bases of the Company’s assets and
liabilities and for tax carryforwards at enacted statutory tax
rates in effect for the years in which the differences are
expected to reverse. The effect on deferred taxes of a change in
tax rates is recognized in the period that includes the
enactment date. In addition, valuation allowances are
established when necessary to reduce deferred tax assets to the
amounts expected to be realized. We have recorded a full
valuation allowance on our net deferred tax assets as of
December 31, 2010 due to uncertainties related to our
ability to utilize our deferred tax assets in the foreseeable
future.
Stock-Based Compensation. The fair value of
stock options is estimated using a Black-Scholes option pricing
model. This model requires the input of subjective assumptions,
including expected stock price volatility, expected life and
estimated forfeitures of each award. The fair value of
equity-based awards is amortized over the vesting period of the
award, and we have elected to use the straight-line method of
amortization. Due to the limited amount of historical data
available to us, particularly with respect to stock-
37
price volatility, employee exercise patterns and forfeitures,
actual results could differ materially from our expectations.
Recent
Accounting Pronouncements
There have been no recently issued accounting standards that
have an impact on our financial statements.
|
|
|
Item 7A.
|
Quantitative
and Qualitative Disclosures About Market Risk
|
Not required.
|
|
|
Item 8.
|
Financial
Statements and Supplementary Data.
|
The consolidated financial statements and supplementary data
required by this item are set forth under Item 15.
|
|
|
Item 9.
|
Changes
in and Disagreements With Accountants on Accounting and
Financial Disclosure.
|
None.
|
|
|
Item 9A.
|
Controls
and Procedures
|
Evaluation
of Disclosure Controls and Procedures
Our management, with the participation of our principal
executive officer and principal financial officer, evaluated the
effectiveness of our disclosure controls and procedures (as such
term is defined in
Rules 13a-15(e)
and
15d-15(e)
under the Securities Exchange Act of 1934, as amended, or the
Exchange Act) as of December 31, 2010. We maintain
disclosure controls and procedures that are designed to provide
a reasonable assurance level that information required to be
disclosed in our reports filed or submitted under the Exchange
Act is recorded, processed, summarized and reported within the
time periods specified in the SEC’s rules and forms and
that such information is accumulated and communicated to our
management, including our principal executive officer and
principal financial officer, as appropriate, to allow for timely
decisions regarding required disclosure. Our management
recognizes that any controls and procedures, no matter how well
designed and operated, can provide only reasonable assurance of
achieving their objectives and management necessarily applies
its judgment in evaluating the cost-benefit relationship of
possible controls and procedures. Based on the evaluation of our
disclosure controls and procedures as of December 31, 2010,
our principal executive officer and principal financial officer
concluded that, as of such date, our disclosure controls and
procedures were effective at the reasonable assurance level.
Internal
Control Over Financial Reporting
Management’s annual report on internal control over
financial reporting. Management’s report on
our internal control over financial reporting is included on
page 43 hereof.
Changes in Internal Control over Financial
Reporting. There has been no change in our
internal control over financial reporting (as such term is
defined in
Rules 13a-15(f)
and
15d-15(f)
under the Exchange Act) during our most recent fiscal quarter
that has materially affected, or is reasonably likely to
materially affect, our internal control over financial reporting.
|
|
|
Item 9B.
|
Other
Information.
|
None.
38
|
|
|
Item 10.
|
Directors,
Executive Officers and Corporate Governance.
|
The information required by this Item is incorporated herein by
reference to our definitive Proxy Statement, which will be filed
within 120 days of December 31, 2010, and delivered to
stockholders in connection with our annual meeting of
stockholders.
|
|
|
Item 11.
|
Executive
Compensation.
|
The information required by this Item is incorporated herein by
reference to our definitive Proxy Statement, which will be filed
within 120 days of December 31, 2010, and delivered to
stockholders in connection with our annual meeting of
stockholders.
The material incorporated herein by reference to the material
under the caption “Compensation Committee Report” in
the Proxy Statement shall be deemed furnished, and not filed, in
this report and shall not be deemed incorporated by reference
into any filing under the Securities Act of 1933, as amended, or
the Securities Exchange Act of 1934, as amended, as a result of
this furnishing, except to the extent that we specifically
incorporate it by reference.
|
|
|
Item 12.
|
Security
Ownership of Certain Beneficial Owners and Management and
Related Stockholder Matters.
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(c)
|
|
|
|
|
|
|
|
|
|
|
Number of Securities
|
|
|
|
|
(a)
|
|
|
|
|
|
Remaining Available
|
|
|
|
|
Number of
|
|
|
(b)
|
|
|
for Future Issuance
|
|
|
|
|
Securities to be
|
|
|
Weighted-Average
|
|
|
Under Equity
|
|
|
|
|
Issued Upon Exercise
|
|
|
Exercise Price of
|
|
|
Compensation Plans
|
|
|
|
|
of Outstanding
|
|
|
Outstanding
|
|
|
(Excluding Securities
|
|
|
|
|
Options, Warrants
|
|
|
Options, Warrants
|
|
|
Reflected in Column
|
|
|
Plan category
|
|
and Rights
|
|
|
and Rights
|
|
|
(a))
|
|
|
|
|
Equity compensation plans approved by security holders
|
|
|
2,491,799
|
|
|
$
|
5.43
|
|
|
|
610,051
|
|
|
Equity compensation plans not approved by security holders
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
2,491,799
|
|
|
$
|
5.43
|
|
|
|
610,051
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The number of securities remaining available for future issuance
includes 250,000 shares available for purchase under the
Company’s 2007 Employee Stock Purchase Plan. The number of
shares subject to purchase under this plan during the current
six-month purchase period is approximately 30,000.
With the exception of the information regarding securities
authorized for issuance under our equity compensation plans set
forth above, the information required by this Item is
incorporated herein by reference to our definitive Proxy
Statement, which will be filed within 120 days of
December 31, 2010, and delivered to stockholders in
connection with our annual meeting of stockholders.
|
|
|
Item 13.
|
Certain
Relationships and Related Transactions, and Director
Independence.
|
The information required by this Item is incorporated herein by
reference to our definitive Proxy Statement, which will be filed
within 120 days of December 31, 2010, and delivered to
stockholders in connection with our annual meeting of
stockholders.
|
|
|
Item 14.
|
Principal
Accountant Fees and Services.
|
The information required by this Item is incorporated herein by
reference to our definitive Proxy Statement, which will be filed
within 120 days of December 31, 2010, and delivered to
stockholders in connection with our annual meeting of
stockholders.
39
|
|
|
Item 15.
|
Exhibits,
Financial Statement Schedules.
|
(a) Financial Statements
(1) Index to Financial Statements
| |
|
|
|
|
|
|
|
Page
|
|
|
|
Management Report on Internal Control Over Financial Reporting
|
|
|
43
|
|
|
Report of Independent Registered Public Accounting Firm
|
|
|
44
|
|
|
Consolidated Balance Sheets
|
|
|
45
|
|
|
Consolidated Statements of Operations
|
|
|
46
|
|
|
Consolidated Statements of Stockholders’ Equity
|
|
|
47
|
|
|
Statements of Cash Flows
|
|
|
48
|
|
|
Notes to Consolidated Financial Statements
|
|
|
49
|
|
|
(2) Financial Statement Schedule: Schedule II —
Valuation Accounts
|
|
|
62
|
|
All other financial statement schedules have been omitted
because they are not applicable, not required or the information
required is shown in the financial statements or the notes
thereto.
The exhibits filed with this Annual Report on
Form 10-K
are listed in the Exhibit Index immediately following the
financial statement schedules, which Exhibit Index is
incorporated herein by reference.
40
SIGNATURES
Pursuant to the requirements of Section 13(a) or 15(d) of
the Securities Exchange Act of 1934, the registrant has duly
caused this report to be signed on its behalf by the undersigned
thereunto duly authorized.
TranS1 Inc.
Ken Reali
President and Chief Executive Officer
Date: March 14, 2011
POWER OF
ATTORNEY
We, the undersigned directors and officers of TranS1 Inc., do
hereby constitute and appoint Ken Reali and Joseph Slattery, and
each of them, as our true and lawful attorneys-in-fact and
agents with power of substitution, to do any and all acts and
things in our name and behalf in our capacities as directors and
officers and to execute any and all instruments for us and in
our names in the capacities indicated below, which said
attorney-in-fact and agent may deem necessary or advisable to
enable said corporation to comply with the Securities Exchange
Act of 1934, as amended, and any rules, regulations and
requirements of the Securities and Exchange Commission, in
connection with this Annual Report on
Form 10-K,
including specifically but without limitation, power and
authority to sign for us or any of us in our names in the
capacities indicated below, any and all amendments hereto; and
we do hereby ratify and confirm all that said attorney-in-fact
and agent, shall do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of
1934, this report has been signed below by the following persons
on behalf of the registrant and in the capacities and on the
dates indicated.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
/s/ Ken
Reali
Ken
Reali
|
|
President, Chief Executive Officer and Director
(Principal Executive Officer)
|
|
March 14, 2011
|
|
|
|
|
|
|
|
/s/ Joseph
Slattery
Joseph
Slattery
|
|
Executive Vice President and
Chief Financial Officer
(Principal Financial and Accounting Officer)
|
|
March 14, 2011
|
|
|
|
|
|
|
|
/s/ Michael
Carusi
Michael
Carusi
|
|
Director
|
|
March 14, 2011
|
|
|
|
|
|
|
|
/s/ Mitchell
Dann
Mitchell
Dann
|
|
Director
|
|
March 14, 2011
|
|
|
|
|
|
|
|
/s/ Paul
LaViolette
Paul
LaViolette
|
|
Director
|
|
March 14, 2011
|
|
|
|
|
|
|
|
/s/ Jonathan
Osgood
Jonathan
Osgood
|
|
Director
|
|
March 14, 2011
|
|
|
|
|
|
|
|
/s/ Richard
Randall
Richard
Randall
|
|
Executive Chairman and Director
|
|
March 14, 2011
|
|
|
|
|
|
|
|
/s/ James
Shapiro
James
Shapiro
|
|
Director
|
|
March 14, 2011
|
|
|
|
|
|
|
|
/s/ David
Simpson
David
Simpson
|
|
Director
|
|
March 14, 2011
|
41
TranS1
Inc.
| |
|
|
|
|
|
|
|
Page
|
|
|
|
|
|
|
43
|
|
|
|
|
|
44
|
|
|
|
|
|
45
|
|
|
|
|
|
46
|
|
|
|
|
|
47
|
|
|
|
|
|
48
|
|
|
|
|
|
49
|
|
42
Report of
Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of TranS1 Inc.
In our opinion, the consolidated financial statements listed in
the index appearing under Item 15(a)(1) present fairly, in
all material respects, the financial position of TranS1, Inc.
and its subsidiaries at December 31, 2010 and 2009, and the
results of their operations and their cash flows for each of the
three years in the period ended December 31, 2010 in
conformity with accounting principles generally accepted in the
United States of America. In addition, in our opinion, the
financial statement schedule listed in the index appearing under
Item 15(a)(2) presents fairly, in all material respects,
the information set forth therein when read in conjunction with
the related consolidated financial statements. These financial
statements and financial statement schedule are the
responsibility of the Company’s management. Our
responsibility is to express an opinion on these financial
statements and financial statement schedule based on our audits.
We conducted our audits of these statements in accordance with
the standards of the Public Company Accounting Oversight Board
(United States). Those standards require that we plan and
perform the audit to obtain reasonable assurance about whether
the financial statements are free of material misstatement. An
audit includes examining, on a test basis, evidence supporting
the amounts and disclosures in the financial statements,
assessing the accounting principles used and significant
estimates made by management, and evaluating the overall
financial statement presentation. We believe that our audits
provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers
LLP
Raleigh, NC
March 14, 2011
44
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(In thousands, except share amounts)
|
|
|
|
|
ASSETS
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
24,461
|
|
|
$
|
29,298
|
|
|
Short-term investments
|
|
|
18,075
|
|
|
|
25,953
|
|
|
Accounts receivable, net
|
|
|
3,654
|
|
|
|
3,926
|
|
|
Inventory
|
|
|
3,878
|
|
|
|
7,325
|
|
|
Prepaid expenses and other assets
|
|
|
389
|
|
|
|
676
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
50,457
|
|
|
|
67,178
|
|
|
Property and equipment, net
|
|
|
1,562
|
|
|
|
1,813
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
52,019
|
|
|
$
|
68,991
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
2,214
|
|
|
$
|
2,442
|
|
|
Accrued expenses
|
|
|
2,077
|
|
|
|
1,269
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
4,291
|
|
|
|
3,711
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments (Note 5)
|
|
|
|
|
|
|
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
Common stock, $0.0001 par value; 75,000,000 shares
authorized, 20,877,171 and 20,648,447 shares issued and
outstanding at December 31, 2010 and 2009, respectively
|
|
|
2
|
|
|
|
2
|
|
|
Preferred stock, $0.0001 par value; 5,000,000 shares
authorized, none issued and outstanding at December 31,
2010 and 2009
|
|
|
—
|
|
|
|
—
|
|
|
Additional paid-in capital
|
|
|
138,401
|
|
|
|
136,402
|
|
|
Accumulated other comprehensive loss
|
|
|
(29
|
)
|
|
|
(5
|
)
|
|
Accumulated deficit
|
|
|
(90,646
|
)
|
|
|
(71,119
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
47,728
|
|
|
|
65,280
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
52,019
|
|
|
$
|
68,991
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
45
TranS1
Inc.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands, except per share amounts)
|
|
|
|
|
Revenue
|
|
$
|
26,154
|
|
|
$
|
29,807
|
|
|
$
|
25,304
|
|
|
Cost of revenue
|
|
|
7,104
|
|
|
|
5,687
|
|
|
|
4,315
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
19,050
|
|
|
|
24,120
|
|
|
|
20,989
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
4,223
|
|
|
|
6,439
|
|
|
|
4,081
|
|
|
Sales and marketing
|
|
|
26,275
|
|
|
|
34,098
|
|
|
|
29,375
|
|
|
General and administrative
|
|
|
8,565
|
|
|
|
7,184
|
|
|
|
7,116
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
39,063
|
|
|
|
47,721
|
|
|
|
40,572
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(20,013
|
)
|
|
|
(23,601
|
)
|
|
|
(19,583
|
)
|
|
Other income, net
|
|
|
486
|
|
|
|
405
|
|
|
|
2,548
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(19,527
|
)
|
|
$
|
(23,196
|
)
|
|
$
|
(17,035
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share — basic and diluted
|
|
$
|
(0.94
|
)
|
|
$
|
(1.13
|
)
|
|
$
|
(0.84
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding — basic and
diluted
|
|
|
20,738
|
|
|
|
20,604
|
|
|
|
20,289
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
46
TranS1
Inc.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
Other
|
|
|
|
|
|
Total
|
|
|
|
|
Common Stock
|
|
|
Paid - In
|
|
|
Comprehensive
|
|
|
Accumulated
|
|
|
Stockholders’
|
|
|
|
|
Number
|
|
|
Amount
|
|
|
Capital
|
|
|
Loss
|
|
|
Deficit
|
|
|
Equity
|
|
|
|
|
(In thousands, except share data)
|
|
|
|
|
Balance at December 31, 2007
|
|
|
19,843,941
|
|
|
|
2
|
|
|
|
130,325
|
|
|
|
—
|
|
|
|
(30,888
|
)
|
|
|
99,439
|
|
|
Issuance of common stock from exercised options
|
|
|
694,392
|
|
|
|
|
|
|
|
203
|
|
|
|
|
|
|
|
|
|
|
|
203
|
|
|
Stock based compensation
|
|
|
|
|
|
|
|
|
|
|
2,979
|
|
|
|
|
|
|
|
|
|
|
|
2,979
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(17,035
|
)
|
|
|
(17,035
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
20,538,333
|
|
|
|
2
|
|
|
|
133,507
|
|
|
|
—
|
|
|
|
(47,923
|
)
|
|
|
85,586
|
|
|
Issuance of common stock from exercised options
|
|
|
110,114
|
|
|
|
|
|
|
|
96
|
|
|
|
|
|
|
|
|
|
|
|
96
|
|
|
Stock based compensation
|
|
|
|
|
|
|
|
|
|
|
2,799
|
|
|
|
|
|
|
|
|
|
|
|
2,799
|
|
|
Other comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(5
|
)
|
|
|
|
|
|
|
(5
|
)
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(23,196
|
)
|
|
|
(23,196
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
|
20,648,447
|
|
|
|
2
|
|
|
|
136,402
|
|
|
|
(5
|
)
|
|
|
(71,119
|
)
|
|
|
65,280
|
|
|
Issuance of common stock from exercised options
|
|
|
228,724
|
|
|
|
|
|
|
|
148
|
|
|
|
|
|
|
|
|
|
|
|
148
|
|
|
Stock based compensation
|
|
|
|
|
|
|
|
|
|
|
1,851
|
|
|
|
|
|
|
|
|
|
|
|
1,851
|
|
|
Other comprehensive loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(24
|
)
|
|
|
|
|
|
|
(24
|
)
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(19,527
|
)
|
|
|
(19,527
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2010
|
|
|
20,877,171
|
|
|
$
|
2
|
|
|
$
|
138,401
|
|
|
$
|
(29
|
)
|
|
$
|
(90,646
|
)
|
|
$
|
47,728
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
47
TranS1
Inc.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(19,527
|
)
|
|
$
|
(23,196
|
)
|
|
$
|
(17,035
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating
activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
746
|
|
|
|
909
|
|
|
|
804
|
|
|
Stock-based compensation
|
|
|
1,851
|
|
|
|
2,799
|
|
|
|
2,979
|
|
|
Allowance for excess and obsolete inventory
|
|
|
2,004
|
|
|
|
505
|
|
|
|
400
|
|
|
Provision for bad debts
|
|
|
226
|
|
|
|
80
|
|
|
|
101
|
|
|
Loss on sale of fixed assets
|
|
|
70
|
|
|
|
—
|
|
|
|
—
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Increase) decrease in accounts receivable
|
|
|
46
|
|
|
|
806
|
|
|
|
(1,688
|
)
|
|
(Increase) decrease in inventory
|
|
|
1,443
|
|
|
|
(1,461
|
)
|
|
|
(2,744
|
)
|
|
(Increase) decrease in prepaid expenses
|
|
|
287
|
|
|
|
(44
|
)
|
|
|
(35
|
)
|
|
Increase (decrease) in accounts payable
|
|
|
(228
|
)
|
|
|
(454
|
)
|
|
|
1,265
|
|
|
Increase (decrease) in accrued liabilities
|
|
|
808
|
|
|
|
(745
|
)
|
|
|
223
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(12,274
|
)
|
|
|
(20,801
|
)
|
|
|
(15,730
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment
|
|
|
(565
|
)
|
|
|
(1,310
|
)
|
|
|
(1,128
|
)
|
|
Purchases of investments
|
|
|
(18,050
|
)
|
|
|
(50,872
|
)
|
|
|
(55,761
|
)
|
|
Sales and maturities of investments
|
|
|
25,928
|
|
|
|
60,134
|
|
|
|
49,791
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities
|
|
|
7,313
|
|
|
|
7,952
|
|
|
|
(7,098
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock
|
|
|
148
|
|
|
|
96
|
|
|
|
203
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
148
|
|
|
|
96
|
|
|
|
203
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate change on cash and cash equivalents
|
|
|
(24
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net decrease in cash and cash equivalents
|
|
|
(4,837
|
)
|
|
|
(12,753
|
)
|
|
|
(22,625
|
)
|
|
Cash and cash equivalents, beginning of period
|
|
|
29,298
|
|
|
|
42,051
|
|
|
|
64,676
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
24,461
|
|
|
$
|
29,298
|
|
|
$
|
42,051
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these
consolidated financial statements.
48
TranS1
Inc.
TranS1 Inc., a Delaware corporation (the “Company”),
was incorporated in May 2000 and is headquartered in Wilmington,
North Carolina. The Company is a medical device company focused
on designing, developing and marketing products that implement
its minimally invasive surgical approach to treat degenerative
conditions of the spine affecting the lower lumbar region. The
Company operates in one business segment. The Company currently
markets the
AxiaLIF®
family of products for single and multilevel lumbar fusion, the
Vectretm
and
Avatartm
lumbar posterior fixation systems and
Bi-Ostetictm
bone void filler, a biologics product. All of the Company’s
AxiaLIF products are delivered using its pre-sacral approach.
The AxiaLIF 1L product was commercially released in January
2005. The AxiaLIF
2Ltm
product was commercially released in Europe in the fourth
quarter of 2006 and in the United States in the second quarter
of 2008. The AxiaLIF 2L product was discontinued in 2010 after
the Company launched its AxiaLIF
2L+tm
product in July 2010. The Company commercially launched its next
generation Vectre facet screw system in April 2010. In January
2010, the Company entered into an agreement to distribute
Avatar, a pedicle screw system, and in February 2010, the
Company entered into an agreement to distribute Bi-Ostetic bone
void filler, a biologics product. The Company sells its products
directly to hospitals and surgical centers in the United States
and certain European countries, and to independent distributors
elsewhere.
The Company is subject to a number of risks similar to other
similarly-sized companies in the medical device industry. These
risks include, without limitation, acceptance and continued use
of our products by surgeons, the lack of clinical data about the
efficacy of our products, uncertainty of reimbursement from
third-party payors, cost pressures in the healthcare industry,
competitive pressures from substitute products and larger
companies, our historical lack of profitability, our dependence
on key employees, regulatory approval and market acceptance for
new products, our reliance on a limited number of suppliers to
provide our products, changes in economic conditions, our
ability to effectively manage a sales force to meet our
objectives and our ability to conduct successful clinical
studies.
|
|
|
2.
|
Summary
of Significant Accounting Policies
|
Basis
of Presentation
The Company has prepared the accompanying consolidated financial
statements in conformity with accounting principles generally
accepted in the United States of America. The Company’s
fiscal year ends on December 31.
Principles
of Consolidation:
These consolidated financial statements include the accounts of
the Company and its subsidiaries. All intercompany transactions
and accounts have been eliminated in consolidation.
For the Company’s international operations, local
currencies have been determined to be the functional currencies.
We translate the financial statements of international
subsidiaries to their U.S. dollar equivalents at
end-of-period
currency exchange rates for assets and liabilities and at
average exchange rates for revenues and expenses. We record
these translation adjustments as a component of other
comprehensive loss within stockholders’ equity. The Company
recognizes transaction gains and losses arising from
fluctuations in currency exchange rates on transactions
denominated in currencies other than the functional currency in
the consolidated income statements. The Company incurred foreign
currency exchange losses of $24,000, $5,000 and zero in the
years ended December 31, 2010, 2009 and 2008, respectively.
Use of
Estimates
The consolidated financial statements have been prepared in
conformity with accounting principles generally accepted in the
United States of America. These principles require management to
make estimates
49
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities
at the dates of the consolidated financial statements and the
reported amounts of revenues and expenses during the reporting
periods. Actual results could differ from those estimates. The
principal estimates relate to accounts receivable reserves,
inventory reserves, stock-based compensation, accrued expenses
and income tax valuations.
Cash
and Cash Equivalents
The Company considers all highly liquid investments with an
original maturity of three months or less at the date of
purchase to be cash equivalents. Cash and cash equivalents
include money market treasury funds. Cash equivalents are
carried at fair market value. Related unrealized gains and
losses were not material as of December 31, 2010 and 2009.
Investments
All marketable investments are classified as
available-for-sale
and therefore are carried at fair market value. Related
unrealized gains and losses were not material as of
December 31, 2010 and 2009. Realized gains and losses on
the sale of all such investments are reported in earnings and
computed using the specific identification cost method and were
not material for the years ended December 31, 2010 and
2009. All of the Company’s investments as of
December 31, 2010 have maturities of one year or less.
Short-term investments at December 31, 2010 and 2009
consisted of U.S. agency backed debt instruments.
Fair
Value of Financial Instruments
The carrying values of cash equivalents, short-term investments,
accounts receivable, and accounts payable at December 31,
2010 and 2009 approximated their fair values due to the
short-term nature of these items.
At December 31, 2010, the Company held certain assets that
are required to be measured at fair value on a recurring basis.
These assets included available for sale securities classified
as cash equivalents and short-term investments. Accounting
Standards Codification (“ASC”)
820-10
requires the valuation of investments using a three-tiered
approach, which requires that fair value measurements be
classified and disclosed in one of three tiers. These tiers are:
Level 1, defined as quoted prices in active markets for
identical assets or liabilities; Level 2, defined as
valuations based on observable inputs other than those included
in Level 1, such as quoted prices for similar assets and
liabilities in active markets, or other inputs that are
observable or can be corroborated by observable input data; and
Level 3, defined as valuations based on unobservable inputs
reflecting the Company’s own assumptions, consistent with
reasonably available assumptions made by other market
participants.
At December 31, 2010, all available for sale securities are
classified as Level 1 assets with a fair value of
$42.1 million, which included money market funds of
$24.0 million and short-term investments of
$18.1 million. At December 31, 2009, all available for
sale securities are classified as Level 1 assets with a
fair value of $54.9 million, which included money market
funds of $28.9 million and short-term investments of
$26.0 million. The Company had no Level 2 or
Level 3 assets or liabilities at December 31, 2010 or
2009.
50
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
Accounts
Receivable
Accounts receivable are presented net of an allowance for
uncollectible accounts. Estimates on the collectability of
customer accounts are based primarily on analysis of historical
trends and experience and changes in customers’ financial
condition. The following table presents the components of
accounts receivable:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(In thousands)
|
|
|
|
|
Gross accounts receivable
|
|
$
|
4,001
|
|
|
$
|
4,119
|
|
|
Allowance for uncollectible accounts
|
|
|
(347
|
)
|
|
|
(193
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total accounts receivable, net
|
|
$
|
3,654
|
|
|
$
|
3,926
|
|
|
|
|
|
|
|
|
|
|
|
Concentration
of Credit Risk and Significant Customers
Financial instruments which potentially subject the Company to
concentrations of credit risk consist principally of cash and
cash equivalents.
The Company places cash deposits with a federally insured
financial institution, in amounts that at times exceed the
federally insured limit, which was $250,000 at December 31,
2010 and 2009. The total amount of deposits in excess of
federally insured limits was $179,335 and $150,580 at
December 31, 2010 and 2009, respectively.
In 2010, 2009 and 2008 no customer accounted for 10% or more of
revenues. As of December 31, 2010 and 2009, no single
customer accounted for 10% or more of the accounts receivable
balance.
Inventories
Inventories consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(In thousands)
|
|
|
|
|
Raw materials
|
|
$
|
146
|
|
|
$
|
220
|
|
|
Work-in-process
|
|
|
2,005
|
|
|
|
3,993
|
|
|
Finished goods
|
|
|
1,727
|
|
|
|
3,112
|
|
|
|
|
|
|
|
|
|
|
|
|
Total inventories, net
|
|
$
|
3,878
|
|
|
$
|
7,325
|
|
|
|
|
|
|
|
|
|
|
|
Inventories are stated at the lower of cost or market, computed
on a standard cost basis, which approximates actual cost on a
first-in,
first-out basis and market being determined as the lower of
replacement cost or net realizable value. Costs are monitored on
an annual basis and updated as necessary to reflect changes in
supplier costs and the rate of our overhead absorption is
adjusted based on projections of our manufacturing costs and
production plan. The components of inventory are reviewed on a
periodic basis for excess, obsolete and impaired inventory, and
a reserve is recorded for the identified items. An inventory
reserve is calculated for estimated excess and obsolete
inventory based upon historical turnover and assumptions about
future demand for the products and market conditions. At
December 31, 2010 and 2009, the allowance for excess and
obsolete inventory was $2.2 million and $0.7 million,
respectively.
Property
and Equipment
Property and equipment are recorded at cost and depreciated over
their estimated useful lives primarily using the straight-line
method. Leasehold improvements are amortized over the shorter of
the lease term or the
51
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
estimated useful life of the related asset. Maintenance and
repairs are charged to expense as incurred. Upon retirement or
sale, the cost of assets disposed of and the related accumulated
depreciation and amortization are removed from the accounts and
any resulting gain or loss is credited or charged to income.
The estimated useful lives are:
| |
|
|
|
Furniture and fixtures
|
|
5-10 years
|
|
Equipment
|
|
3-10 years
|
|
Other depreciable assets
|
|
2-10 years
|
|
Leasehold improvements
|
|
Lesser of estimated useful life or lease term
|
Revenue
Recognition
Revenue is recognized based on the following criteria:
(i) persuasive evidence that an arrangement exists with the
customer; (ii) delivery of the products
and/or
services has occurred; (iii) the selling price has been
fixed for the products or services delivered; and
(iv) collection is reasonably assured. Revenue is generated
from the sale of implants and disposable surgical instruments.
The Company has two distinct sales methods. The first method is
when implants
and/or
disposable surgical instruments are sold directly to hospitals
or surgical centers for the purpose of conducting a scheduled
surgery. The Company’s sales representatives or independent
sales agents hand deliver the products to the customer on or
before the day of the surgery. The sales representative or
independent agent is then responsible for reporting the delivery
of the products and the date of the surgery to the corporate
office for proper revenue recognition. The Company recognizes
revenue upon the confirmation that the products have been used
in a surgical procedure. The other sales method is for sales to
hospitals for consumption during future surgeries or to
distributors outside the United States. These transactions
require the customer to send in a purchase order before shipment
will be made to the customer. Under the distributor agreements
currently in place, a distributor only has the right of return
for defective products.
Shipping
and Handling Costs
Shipping and handling costs in the United States are expensed as
incurred and are included in the cost of revenue. These costs
are generally not reimbursed by the Company’s customers.
Shipping costs relating to sales to distributors outside the
United States are either paid directly by the distributor to the
freight carrier or charged to the distributor and reimbursed to
the Company.
Sales
and Marketing Expenses
Sales and marketing expenses consist primarily of personnel
costs, sales commissions paid to the Company’s direct sales
representatives and independent sales agents, and costs
associated with physician training programs, promotional
activities, and participation in medical conferences. All costs
of advertising and promotional activities are expensed as
incurred. Advertising expenses were $0.7 million,
$0.8 million and $0.7 million for the years ending
December 31, 2010, 2009 and 2008, respectively.
Research
and Development Expenses
Research and development expenses consist primarily of personnel
costs within the Company’s product development, regulatory
and clinical functions, and the costs of clinical studies and
product development projects. Research and development expenses
are expensed as incurred.
52
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
General
and Administrative Expenses
General and administrative expenses consist of personnel costs
related to the executive, finance, business development,
information technology and human resource functions, as well as
professional service fees, legal fees, accounting fees,
insurance costs and general corporate expenses.
Patent
Costs
Costs associated with the submission of a patent application are
expensed as incurred given the uncertainty of the patents
resulting in probable future economic benefits to the Company.
Other
Income, Net
Other income, net, is composed of government grants, interest
earned on our cash, cash equivalents and
available-for-sale
securities and the gain or loss on disposal of fixed assets. In
November 2010, the Company received grants of $0.5 million
from the U.S. Treasury under the Qualifying Therapeutic
Discovery Program. These grants have been included in other
income, net, for the year ended December 31, 2010.
Income
Taxes
The Company accounts for income taxes using the liability method
which requires the recognition of deferred tax assets or
liabilities for the temporary differences between financial
reporting and tax bases of the Company’s assets and
liabilities and for tax carryforwards at enacted statutory tax
rates in effect for the years in which the differences are
expected to reverse. The effect on deferred taxes of a change in
tax rates is recognized in the period that includes the
enactment date. In addition, valuation allowances are
established when necessary to reduce deferred tax assets to the
amounts expected to be realized.
Stock-Based
Compensation
The Company accounts for stock-based compensation under the fair
value provisions of ASC 718. ASC 718 requires the
recognition of compensation expense, using a fair-value-based
method, for costs related to all share-based payments including
stock options. ASC 718 requires companies to estimate the
fair value of share-based payment awards on the date of grant
using an option-pricing model. All options granted after
January 1, 2006 are expensed on a straight-line basis over
the vesting period.
The Company accounts for stock-based compensation arrangements
with nonemployees in accordance with
ASC 505-50.
The Company records the expense of such services based on the
estimated fair value of the equity instrument using the
Black-Scholes pricing model. The value of the equity instruments
is charged to earnings over the term of the service agreement.
Net
Loss Per Common Share
Basic net loss per common share (“Basic EPS”) is
computed by dividing net loss by the weighted average number of
common shares outstanding. Diluted net loss available to common
stockholders per common share (“Diluted EPS”) is
computed by dividing net loss by the weighted average number of
common shares and dilutive potential common share equivalents
then outstanding. The Company’s potential dilutive common
shares, which consist of shares issuable upon the exercise of
stock options, have not been included in the computation of
diluted net loss per share for all periods as the result would
be anti-dilutive.
53
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
The following table sets forth the potential shares of common
stock that are not included in the calculation of diluted net
loss per share as the result would be anti-dilutive as of the
end of each period presented:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2010
|
|
2009
|
|
2008
|
|
|
|
Weighted average stock options outstanding
|
|
|
2,166,657
|
|
|
|
2,110,689
|
|
|
|
1,915,687
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment
and Geographic Reporting
The Company applies ASC 280 which establishes standards for
the reporting by business enterprises of information about
operating segments, products and services, geographic areas, and
major customers. The Company has determined that it did not have
any separately reportable segments as of December 31, 2010,
2009 or 2008.
Revenue by geographic area was:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
United States
|
|
$
|
23,798
|
|
|
$
|
28,045
|
|
|
$
|
23,322
|
|
|
Europe
|
|
|
2,138
|
|
|
|
1,602
|
|
|
|
1,854
|
|
|
Other
|
|
|
218
|
|
|
|
160
|
|
|
|
128
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
26,154
|
|
|
$
|
29,807
|
|
|
$
|
25,304
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-lived assets are primarily located in the United States.
Recently
Issued Accounting Standards
There have been no recently issued accounting standards that
have an impact on the Company’s financial statements.
|
|
|
3.
|
Property
and Equipment
|
Property and equipment consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(In thousands)
|
|
|
|
|
Furniture and fixtures
|
|
$
|
241
|
|
|
$
|
210
|
|
|
Equipment
|
|
|
2,955
|
|
|
|
2,620
|
|
|
Computer software
|
|
|
481
|
|
|
|
410
|
|
|
Leasehold improvements
|
|
|
682
|
|
|
|
380
|
|
|
Tools
|
|
|
—
|
|
|
|
73
|
|
|
Construction in process
|
|
|
—
|
|
|
|
588
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,359
|
|
|
|
4,281
|
|
|
Less: accumulated depreciation and amortization
|
|
|
(2,797
|
)
|
|
|
(2,468
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,562
|
|
|
$
|
1,813
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization expense for the years ended
December 31, 2010, 2009 and 2008 was $746,000, $909,000,
and $804,000, respectively.
54
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
Accrued expenses consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(In thousands)
|
|
|
|
|
Bonus
|
|
$
|
770
|
|
|
$
|
10
|
|
|
Commissions
|
|
|
589
|
|
|
|
597
|
|
|
Legal and professional fees
|
|
|
300
|
|
|
|
90
|
|
|
Vacation
|
|
|
175
|
|
|
|
186
|
|
|
Franchise taxes
|
|
|
116
|
|
|
|
122
|
|
|
Travel and entertainment
|
|
|
53
|
|
|
|
50
|
|
|
Consulting
|
|
|
5
|
|
|
|
120
|
|
|
Other
|
|
|
69
|
|
|
|
94
|
|
|
|
|
|
|
|
|
|
|
|
|
Total accrued expenses
|
|
$
|
2,077
|
|
|
$
|
1,269
|
|
|
|
|
|
|
|
|
|
|
|
The Company rents office space under the terms of an operating
lease, which expires in 2014, with an option to extend the lease
through 2019.
Future minimum lease payments under operating lease obligations
at December 31, 2010 are as follows (in thousands):
| |
|
|
|
|
|
Year ending December 31,
|
|
|
|
|
|
2011
|
|
$
|
354
|
|
|
2012
|
|
|
363
|
|
|
2013
|
|
|
363
|
|
|
2014
|
|
|
333
|
|
|
2015 and after
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
$
|
1,413
|
|
|
|
|
|
|
|
Rent expense related to operating leases for the years ended
December 31, 2010, 2009 and 2008 was $401,000, $204,000 and
$157,000, respectively.
At December 31, 2010 and 2009, the Company’s Amended
and Restated Certificate of Incorporation, which was adopted in
connection with the Company’s initial public offering,
authorized up to 80,000,000 shares of capital stock, of
which 75,000,000 shares were designated as common stock
with a par value of $0.0001 and up to 5,000,000 shares were
designated as preferred stock with a par value of $0.0001. At
December 31, 2010 and 2009, there were 20,877,171 and
20,648,447 shares of common stock issued and outstanding,
respectively, and there were no shares of preferred stock issued
and outstanding.
In 2010, the Company issued 228,724 shares of the common
stock to employees and consultants for $148,000 pursuant to the
exercise of stock options. In 2009, the Company issued
110,114 shares of common stock to employees and consultants
for $96,000 pursuant to the exercise of stock options. In 2008,
the Company issued 694,392 shares of common stock to
employees and consultants for $203,000 pursuant to the exercise
of stock options.
55
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
|
|
|
7.
|
Stock
Incentive Plans and Stock-Based Compensation
|
2007
Employee Stock Purchase Plan
The Company’s board of directors adopted the 2007 Employee
Stock Purchase Plan (as amended, the “ESPP”) in July
2007. The ESPP became effective upon the completion of the
Company’s initial public offering. A total of
250,000 shares of common stock are available for sale. In
addition, the ESPP provides for annual increases in the number
of shares available for issuance under the ESPP on the first day
of each fiscal year beginning in 2008, equal to the lesser of
(i) 2.0% of the outstanding shares of common stock on the
first day of such fiscal year or (ii) an amount determined
by the administrator of the ESPP. The Company’s
Compensation Committee administers the ESPP.
Shares shall be offered pursuant to the ESPP in six-month
periods commencing on the first trading day on or after June 1
and December 1 of each year, or on such other date as the
administrator may determine.
Our ESPP permits participants to purchase common stock through
payroll deductions of up to 10% of their eligible compensation,
which includes a participant’s base straight time gross
earnings, certain commissions, overtime and shift premium, but
exclusive of payments for incentive compensation, bonuses and
other compensation. A participant may purchase no more than
10,000 shares during a six-month purchase period. Amounts
deducted and accumulated by the participant are used to purchase
shares of the Company’s common stock at the end of each
six-month purchase period. The purchase price of the shares will
be 85% of the fair market value of Company common stock on the
exercise date. Participants may end their participation at any
time during an offering period, and will be paid their accrued
payroll deductions that have not yet been used to purchase
shares of common stock. Participation ends automatically upon
termination of employment with the Company. Pursuant to
ASC 718, the Company is required to recognize compensation
expense in connection with purchases under the ESPP.
During fiscal years ended December 31, 2010, 2009 and 2008,
no shares were issued to participants under the ESPP.
2000
and 2007 Stock Incentive Plans
The Company established the TranS1 Inc. Stock Incentive Plan in
2000, (as amended, the “2000 Plan”) and the 2007 Stock
Incentive Plan (the “2007 Plan”) in October 2007
(collectively, the “Plans”) . Under the 2000 Plan, the
Company could have granted options to employees, directors or
service providers and contractors for a maximum of
3,159,108 shares of the Company’s common stock. The
2000 Plan remains active, but no new options may be granted.
Under the 2007 Plan, the Company may grant options to employees,
directors or service providers and contractors for a maximum of
2,000,000 shares of the Company’s common stock.
Options granted under the Plans may be incentive stock options
or non-qualified stock options. Incentive stock options may only
be granted to employees. The exercise periods may not exceed ten
years for options. However, in the case of an incentive stock
option granted to an optionee who, at the time of the option
grant owns stock representing more than 10% of the outstanding
shares, the term of the option shall be five years from the date
of the grant. The exercise price of incentive stock options
cannot be less than 100% of the fair market value per share of
the Company’s common stock on the grant date. The exercise
price of a nonqualified option under the 2000 Plan and the 2007
Plan shall not be less than 85% and 100%, respectively, of the
fair market value per share on the date the option is granted.
If an optionee owns more than 10% of the outstanding shares, the
exercise price cannot be less than 110% of the fair market value
of the stock on the date of the grant. Options granted under the
Plans generally vest over periods ranging from three to four
years.
56
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
The following table summarizes the activity of the
Company’s 2000 Plan and 2007 Plan, including the number of
shares under options (“Number”) and the weighted
average exercise price (“Price”):
| |
|
|
|
|
|
|
|
|
|
|
|
Number
|
|
|
Price
|
|
|
|
|
Outstanding as of December 31, 2009
|
|
|
2,216,026
|
|
|
$
|
6.96
|
|
|
Options granted
|
|
|
1,297,000
|
|
|
|
3.31
|
|
|
Options exercised
|
|
|
(228,724
|
)
|
|
|
0.65
|
|
|
Options forfeited
|
|
|
(792,503
|
)
|
|
|
7.63
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding as of December 31, 2010
|
|
|
2,491,799
|
|
|
$
|
5.43
|
|
|
|
|
|
|
|
|
|
|
|
The following table summarizes information about the
Company’s stock options at December 31, 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Average
|
|
|
Number of
|
|
|
|
|
Number
|
|
|
Contractual
|
|
|
Exercise
|
|
|
Options
|
|
|
Range of Exercise Prices
|
|
Outstanding
|
|
|
Life (Years)
|
|
|
Price
|
|
|
Exercisable
|
|
|
|
|
$ 0.11 - $2.00
|
|
|
353,080
|
|
|
|
5.4
|
|
|
$
|
1.01
|
|
|
|
322,760
|
|
|
$ 2.01 - $3.50
|
|
|
614,000
|
|
|
|
9.2
|
|
|
|
3.12
|
|
|
|
59,000
|
|
|
$ 3.51 - $6.00
|
|
|
864,597
|
|
|
|
8.6
|
|
|
|
4.38
|
|
|
|
184,014
|
|
|
$ 6.01 - $10.00
|
|
|
327,249
|
|
|
|
7.5
|
|
|
|
8.65
|
|
|
|
239,077
|
|
|
$12.00 - $20.00
|
|
|
332,873
|
|
|
|
6.9
|
|
|
|
13.91
|
|
|
|
263,075
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,491,799
|
|
|
|
7.9
|
|
|
|
5.43
|
|
|
|
1,067,926
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The aggregate intrinsic value of outstanding stock options at
December 31, 2010 was $0.4 million. At
December 31, 2010, exercisable stock options had an
aggregate intrinsic value of $0.4 million, a weighted
average contractual life of 6.5 years and a weighted
average exercise price of $6.78.
Stock-Based
Compensation for Non-employees
During 2010, the Company did not issue any options to purchase
common stock to consultants. During 2009, the Company issued
options to purchase 2,500 shares of common stock with an
exercise price of $6.00 per share to consultants. These options
vest over 3 years and expire 10 years from the date of
issuance. During 2008, the Company issued options to purchase
35,000 shares of common stock with an average exercise
price of $12.43 per share to consultants. These options vest
over a range of 1 to 3 years and expire 10 years from
the date of issuance. The Company determined the estimated fair
value of the options issued to the consultants using the
Black-Scholes pricing model. The Company used the following
assumptions in the Black-Scholes pricing model for 2009 grants:
60% volatility, 0% dividend yield, 2.65% risk-free rate and a
6 year expected legal life. The Company used the following
assumptions in the Black-Scholes pricing model for 2008 grants:
54% volatility, 0% dividend yield, 1.54% risk-free rate and
6 year expected life.
There was no stock-based compensation expense charged to
operations on options granted to non-employees for the year
ended December 31, 2010. Stock-based compensation expense
charged to operations on options granted to non-employees for
years ended December 31, 2009 and 2008 was $8,000 and
$118,000, respectively. As of December 31, 2010, there was
less than $1,000 of total unrecognized compensation costs
related to non-vested stock option awards which is expected to
be recognized over a weighted-average period of 0.4 years.
Employee
Stock-Based Compensation
Under ASC 718, compensation cost for employee stock-based
awards is based on the estimated grant-date fair value and is
recognized over the vesting period of the applicable award on a
straight-line basis. For the
57
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
period from January 1, 2006 to December 31, 2010, the
Company issued employee stock-based awards in the form of stock
options. The Company recorded stock-based compensation expense
of $1.9 million, $2.8 million and $2.9 million
for the years ended December 31, 2010, 2009 and 2008,
respectively. The weighted average grant-date fair value of the
employee stock options granted for the years ended
December 31, 2010, 2009 and 2008 was $3.31, $6.53 and
$11.77 per share, respectively. The aggregate intrinsic value of
stock options (the amount by which the market price of the stock
on the date of exercise exceeded the exercise price of the
option) exercised for the years ended December 31, 2010,
2009 and 2008, was $0.5 million, $0.6 million, and
$5.4 million, respectively.
The Company uses the Black-Scholes pricing model to determine
the fair value of stock options. The determination of the fair
value of stock-based payment awards on the date of grant is
affected by our stock price as well as assumptions regarding a
number of complex and subjective variables. These variables
include our expected stock price volatility over the term of the
awards, actual and projected employee stock option exercise
behaviors, risk-free interest rates and expected dividends.
Prior to the Company’s initial public offering, the Board
of Directors, with the assistance of management, performed
contemporaneous fair value analyses to determine the fair value
of the common stock at the time of the stock option grants. The
estimated grant-date fair values of the employee stock options
were calculated using the Black-Scholes valuation model, based
on the following assumptions for the years ended
December 31, 2010, 2009 and 2008:
Expected Life. The expected life of six years
is based on the “simplified” method described in the
SEC Staff Accounting Bulletin No. 110, which provides
guidance regarding the application of ASC 718.
Volatility. Prior to 2008, the Company was a
private entity with no historical data regarding the volatility
of its common stock. Accordingly, the expected volatility was
based on a weighted average of the actual volatility of the
Company and the volatility of similar entities for prior
periods. In evaluating similarity, the Company considered
factors such as industry, stage of life cycle and size. The
Company utilized an expected volatility range of 59.5% to 62.0%
for 2010, 54% to 58% for 2009 and 45% to 54% for 2008.
Risk-Free Interest Rate. The risk-free rate is
based on U.S. Treasury zero-coupon issues with remaining
terms similar to the expected term on the options. The risk-free
rates were:
| |
|
|
|
Option Grant Year
|
|
Risk-Free Rate Range
|
|
|
|
2010
|
|
1.26% to 2.61%
|
|
2009
|
|
1.79% to 2.52%
|
|
2008
|
|
2.68% to 3.21%
|
Dividend Yield. The Company has never declared
or paid any cash dividends and does not plan to pay cash
dividends in the foreseeable future, and, therefore, used an
expected dividend yield of zero in the valuation model.
Forfeitures. ASC 718 also requires the Company
to estimate forfeitures at the time of grant, and revise those
estimates in subsequent periods if actual forfeitures differ
from those estimates. The Company uses historical data to
estimate pre-vesting option forfeitures and record stock-based
compensation expense only for those awards that are expected to
vest. All stock-based payment awards are amortized on a
straight-line basis over the requisite service periods of the
awards, which are generally the vesting periods.
As of December 31, 2010, there was $2.9 million of
total unrecognized compensation cost related to non-vested
employee stock option awards granted after January 1, 2006,
which is expected to be recognized over a weighted-average
period of 2.3 years.
58
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
No provision for federal or state income taxes has been recorded
as the Company has incurred net operating losses since inception.
Significant components of the Company’s deferred tax assets
and liabilities consist of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(In thousands)
|
|
|
|
|
Deferred tax assets
|
|
|
|
|
|
|
|
|
|
Domestic net operating loss carryforwards
|
|
$
|
25,417
|
|
|
$
|
20,707
|
|
|
Inventory
|
|
|
839
|
|
|
|
272
|
|
|
Fixed assets
|
|
|
592
|
|
|
|
532
|
|
|
Other
|
|
|
1,437
|
|
|
|
638
|
|
|
Research and development credit
|
|
|
1,207
|
|
|
|
1,031
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax assets
|
|
|
29,492
|
|
|
|
23,180
|
|
|
Valuation allowance for deferred assets
|
|
|
(29,492
|
)
|
|
|
(23,180
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax assets
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax liabilities
|
|
|
|
|
|
|
|
|
|
Fixed assets
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax liabilities
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Net deferred tax assets (liabilities)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
The Company provided a full valuation allowance against its net
deferred tax assets. A valuation allowance must be established
for deferred tax assets when it is more likely than not (a
probability level of more than 50 percent) that they will
not be realized. The increase in valuation allowance resulted
primarily from the additional net operating loss carryforward
generated.
As of December 31, 2010, the Company had federal and state
net operating loss carryforwards of approximately
$67.6 million and $62.0 million, respectively. These
net operating loss carryforwards begin to expire in 2021 and
2016 for federal and state tax purposes, respectively.
Additionally, as of December 31, 2010, the Company had
research credit carryforwards of $1.2 million for federal
tax purposes. These credit carryforwards begin to expire in
2021. The utilization of the federal net operating loss
carryforwards may be subject to limitations under the rules
regarding a change in stock ownership as determined by the
Internal Revenue Code.
59
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
A reconciliation of differences between the U.S. federal
income tax rate and the Company’s effective tax rate for
the years ended December 31 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
|
|
|
% of
|
|
|
|
|
|
% of
|
|
|
|
|
|
% of
|
|
|
|
|
Amount
|
|
|
Net Loss
|
|
|
Amount
|
|
|
Net Loss
|
|
|
Amount
|
|
|
Net Loss
|
|
|
|
|
(In thousands)
|
|
|
|
|
Tax at statutory rate
|
|
$
|
(6,834
|
)
|
|
|
35.0
|
%
|
|
$
|
(8,119
|
)
|
|
|
35.0
|
%
|
|
$
|
(5,962
|
)
|
|
|
35.0
|
%
|
|
State taxes
|
|
|
(303
|
)
|
|
|
1.5
|
%
|
|
|
(59
|
)
|
|
|
0.3
|
%
|
|
|
(447
|
)
|
|
|
2.6
|
%
|
|
Non deductible items
|
|
|
746
|
|
|
|
(3.8
|
)%
|
|
|
1,129
|
|
|
|
(4.9
|
)%
|
|
|
1,168
|
|
|
|
(6.9
|
)%
|
|
Other
|
|
|
258
|
|
|
|
(1.3
|
)%
|
|
|
215
|
|
|
|
(0.9
|
)%
|
|
|
(470
|
)
|
|
|
2.8
|
%
|
|
R&D credits
|
|
|
(251
|
)
|
|
|
1.3
|
%
|
|
|
(486
|
)
|
|
|
2.1
|
%
|
|
|
(148
|
)
|
|
|
0.9
|
%
|
|
Change in valuation allowance
|
|
|
6,384
|
|
|
|
(32.7
|
)%
|
|
|
7,320
|
|
|
|
(31.6
|
)%
|
|
|
5,859
|
|
|
|
(34.4
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
—
|
|
|
|
0.0
|
%
|
|
$
|
—
|
|
|
|
0.0
|
%
|
|
$
|
—
|
|
|
|
0.0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of January 1, 2007, the Company adopted the provisions
of ASC 740, which clarifies the accounting for uncertainty
in tax positions. As of that date, the Company had
$1.1 million of unrecognized tax benefits related to the
adoption of ASC 740. This would be recorded as a component
of income tax expense once the valuation allowance is released.
For the year ended December 31, 2010, the Company increased
its unrecognized tax benefits by $75,000. The change was
recorded as a reduction to the respective deferred tax asset
which was reflected as an increase in the valuation allowance.
As of December 31, 2010, the Company had $1.0 million
of unrecognized tax benefits which, if recognized, would be
recorded as a component of income tax expense. The
Company’s policy is to record estimated interest and
penalties related to the underpayment of income taxes as a
component of its income tax provision. As of December 31,
2008, 2009 and 2010, the Company had no accrued interest or tax
penalties recorded. A reconciliation of the beginning and ending
uncertain tax positions is as follows (in thousands):
| |
|
|
|
|
|
Balance at December 31, 2008
|
|
$
|
887
|
|
|
Gross increases related to current period tax positions
|
|
|
13
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
|
900
|
|
|
Gross increases related to current period tax positions
|
|
|
75
|
|
|
|
|
|
|
|
|
Balance at December 31, 2010
|
|
$
|
975
|
|
|
|
|
|
|
|
In many cases, uncertain tax positions are related to tax years
that remain subject to examination by the relevant tax
authorities. Given the losses accumulated to date, periods open
for examination are 2003 to 2010 for the primary taxing
jurisdictions of the United States and North Carolina. The
Company currently does not expect a significant change in the
liability for uncertain tax provisions in the next
12 months.
The following table presents the components of other
comprehensive loss:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
Net loss
|
|
$
|
(19,527
|
)
|
|
$
|
(23,196
|
)
|
|
$
|
(17,035
|
)
|
|
Other comprehensive loss:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation adjustments
|
|
|
(24
|
)
|
|
|
(5
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive loss
|
|
$
|
(19,551
|
)
|
|
$
|
(23,201
|
)
|
|
$
|
(17,035
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60
TranS1
Inc.
Notes to
Consolidated Financial
Statements — (Continued)
|
|
|
10.
|
Quarterly
Data (Unaudited)
|
The following unaudited quarterly financial data, in the opinion
of management, reflects all adjustments, consisting of normal
recurring adjustments, necessary to a fair statement of the
results for the periods presented (in thousands, except per
share data):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, 2010
|
|
|
|
|
First
|
|
|
Second
|
|
|
Third
|
|
|
Fourth
|
|
|
|
|
Quarter
|
|
|
Quarter
|
|
|
Quarter
|
|
|
Quarter
|
|
|
|
|
Total revenues
|
|
$
|
6,713
|
|
|
$
|
7,244
|
|
|
$
|
6,339
|
|
|
$
|
5,858
|
|
|
Gross profit
|
|
|
5,284
|
|
|
|
5,880
|
|
|
|
5,134
|
|
|
|
2,752
|
|
|
Total operating expenses
|
|
|
11,591
|
|
|
|
9,541
|
|
|
|
8,945
|
|
|
|
8,986
|
|
|
Net loss
|
|
$
|
(6,356
|
)
|
|
$
|
(3,646
|
)
|
|
$
|
(3,791
|
)
|
|
$
|
(5,734
|
)
|
|
Basic and diluted net loss per common share
|
|
$
|
(0.31
|
)
|
|
$
|
(0.18
|
)
|
|
$
|
(0.18
|
)
|
|
$
|
(0.27
|
)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, 2009
|
|
|
|
|
First
|
|
|
Second
|
|
|
Third
|
|
|
Fourth
|
|
|
|
|
Quarter
|
|
|
Quarter
|
|
|
Quarter
|
|
|
Quarter
|
|
|
|
|
Total revenues
|
|
$
|
8,678
|
|
|
$
|
7,938
|
|
|
$
|
6,912
|
|
|
$
|
6,279
|
|
|
Gross profit
|
|
|
7,136
|
|
|
|
6,423
|
|
|
|
5,546
|
|
|
|
5,015
|
|
|
Total operating expenses
|
|
|
12,517
|
|
|
|
13,293
|
|
|
|
11,171
|
|
|
|
10,740
|
|
|
Net loss
|
|
$
|
(5,164
|
)
|
|
$
|
(6,759
|
)
|
|
$
|
(5,571
|
)
|
|
$
|
(5,702
|
)
|
|
Basic and diluted net loss per common share
|
|
$
|
(0.25
|
)
|
|
$
|
(0.33
|
)
|
|
$
|
(0.27
|
)
|
|
$
|
(0.28
|
)
|
61
TRANS1
INC.
SCHEDULE II
VALUATION
AND QUALIFYING ACCOUNTS
FOR THE
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
|
|
|
|
|
|
Balance at
|
|
|
|
Beginning of
|
|
|
|
|
|
End of
|
|
|
|
Period
|
|
Additions(1)
|
|
Deductions(2)
|
|
Period
|
|
|
|
(In thousands)
|
|
|
|
Accounts Receivable Reserve:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2010
|
|
$
|
193
|
|
|
$
|
226
|
|
|
$
|
72
|
|
|
$
|
347
|
|
|
Year ended December 31, 2009
|
|
|
193
|
|
|
|
80
|
|
|
|
80
|
|
|
|
193
|
|
|
Year ended December 31, 2008
|
|
|
110
|
|
|
|
101
|
|
|
|
18
|
|
|
|
193
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
|
|
|
|
|
|
Balance at
|
|
|
|
Beginning of
|
|
|
|
|
|
End of
|
|
|
|
Period
|
|
Additions(3)
|
|
Deductions(4)
|
|
Period
|
|
|
|
Inventory Reserve:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2010
|
|
$
|
581
|
|
|
$
|
2,004
|
|
|
$
|
430
|
|
|
$
|
2,155
|
|
|
Year ended December 31, 2009
|
|
|
397
|
|
|
|
505
|
|
|
|
321
|
|
|
|
581
|
|
|
Year ended December 31, 2008
|
|
|
295
|
|
|
|
400
|
|
|
|
298
|
|
|
|
397
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
|
|
|
|
|
|
Balance at
|
|
|
|
Beginning of
|
|
|
|
|
|
End of
|
|
|
|
Period
|
|
Additions
|
|
Deductions
|
|
Period
|
|
|
|
Valuation Allowance for Deferred Tax Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2010
|
|
$
|
23,180
|
|
|
$
|
6,354
|
|
|
$
|
42
|
|
|
$
|
29,492
|
|
|
Year ended December 31, 2009
|
|
|
15,993
|
|
|
|
7,559
|
|
|
|
372
|
|
|
|
23,180
|
|
|
Year ended December 31, 2008
|
|
|
9,524
|
|
|
|
6,469
|
|
|
|
—
|
|
|
|
15,993
|
|
|
|
|
|
(1) |
|
Amount represents customer balances deemed uncollectible. |
| |
|
(2) |
|
Uncollectible accounts written-off. |
| |
|
(3) |
|
Amount represents excess and obsolete reserve recorded to cost
of sales. |
| |
|
(4) |
|
Excess and obsolete inventory written-off against reserve. |
62
EXHIBIT INDEX
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
3
|
.1
|
|
Amended and Restated Certificate of Incorporation of TranS1 Inc.
(incorporated by reference to Exhibit 3.2 to the
Registration Statement on
Form S-1,
as amended (File
No. 333-144802),
and as declared effective on October 16, 2007).
|
|
|
3
|
.2
|
|
Amended and Restated Bylaws of TranS1 Inc. (incorporated by
reference to Exhibit 3.4 to the Registration Statement on
Form S-1,
as amended (File
No. 333-144802),
and as declared effective on October 16, 2007).
|
|
|
4
|
.1
|
|
Specimen common stock certificate (incorporated by reference to
Exhibit 4.1 to the Registration Statement on
Form S-1,
as amended (File
No. 333-144802),
and as declared effective on October 16, 2007).
|
|
|
10
|
.1
|
|
Amended and Restated 2000 Stock Incentive Plan (incorporated by
reference to Exhibit 10.1 to the Registration Statement on
Form S-1,
as amended (File
No. 333-144802),
and as declared effective on October 16, 2007).*
|
|
|
10
|
.2
|
|
Form of Stock Option Agreement under Amended and Restated 2000
Stock Incentive Plan (incorporated by reference to
Exhibit 10.2 to the Registration Statement on
Form S-1,
as amended (File
No. 333-144802),
and as declared effective on October 16, 2007).*
|
|
|
10
|
.3
|
|
2007 Stock Incentive Plan, as amended (incorporated by reference
to Appendix A to the Definitive Proxy Statement on
Schedule 14A, as filed with the Commission on
April 30, 2009.*
|
|
|
10
|
.4
|
|
Form of Stock Option Agreement under 2007 Stock Incentive Plan
(incorporated by reference to Exhibit 10.4 to the
Registration Statement on
Form S-1,
as amended (File
No. 333-144802),
and as declared effective on October 16, 2007).*
|
|
|
10
|
.5
|
|
2007 Employee Stock Purchase Plan, as amended on
December 16, 2010 and as declared effective on
October 16, 2007).**
|
|
|
10
|
.6
|
|
Lease, dated July 30, 2009, between TranS1 Inc. and Market
Place Group, LLC (incorporated by reference to Exhibit 99.1
of TranS1’s Current Report on
Form 8-K
filed with the Commission on March 11, 2010).
|
|
|
10
|
.7
|
|
Form of Indemnification Agreement (incorporated by reference to
Exhibit 10.7 to the Registration Statement on
Form S-1,
as amended (File
No. 333-144802),
and as declared effective on October 16, 2007).
|
|
|
10
|
.7.1
|
|
Schedule of Parties to Indemnification Agreement.**
|
|
|
10
|
.8
|
|
Offer Letter, dated December 11, 2009, between TranS1 Inc.
and Kenneth Reali (incorporated by reference to
Exhibit 10.1 of TranS1’s Current Report on
Form 8-K
filed with the Commission on January 12, 2010).*
|
|
|
10
|
.9
|
|
Separation Agreement, dated February 23, 2010, between
TranS1 Inc. and Michael Luetkemeyer (incorporated by reference
to Exhibit 10.1 of TranS1’s Current Report on
Form 8-K
filed with the Commission on February 25, 2010).*
|
|
|
10
|
.10
|
|
Offer Letter, dated February 22, 2010, between TranS1 Inc.
and Dwayne Montgomery (incorporated by reference to
Exhibit 10.1 of TranS1’s Current Report on
Form 8-K
filed with the Commission on March 18, 2010).*
|
|
|
10
|
.11
|
|
Offer Letter, dated April 13, 2010, between TranS1 Inc. and
Joseph Slattery (incorporated by reference to Exhibit 10.1
of TranS1’s Current Report on
Form 8-K
filed with the Commission on April 21, 2010).*
|
|
|
10
|
.12
|
|
Offer Letter, dated July 30, 2010, between TranS1 Inc. and
Rick Feiler (incorporated by reference to Exhibit 10.1 of
TranS1’s Current Report on
Form 8-K
filed with the Commission on August 4, 2010).*
|
|
|
10
|
.13
|
|
Offer Letter, dated September 28, 2010, between TranS1 Inc.
and Steve Ainsworth (incorporated by reference to
Exhibit 10.1 of TranS1’s Current Report on
Form 8-K
filed with the Commission on October 4, 2010).*
|
|
|
23
|
.1
|
|
Consent of Independent Registered Public Accounting Firm.**
|
|
|
24
|
.1
|
|
Power of Attorney (included in the signature page).
|
63
| |
|
|
|
|
Exhibit
|
|
|
|
No.
|
|
Description
|
|
|
|
|
31
|
.1
|
|
Certification of Chief Executive Officer Pursuant to
Rule 13a-14(a)
/ 15d-14(a) of the Securities Exchange Act of 1934.**
|
|
|
31
|
.2
|
|
Certification of Chief Financial Officer Pursuant to
Rule 13a-14(a)
/ 15d-14(a) of the Securities Exchange Act of 1934.**
|
|
|
32
|
.1
|
|
Certification of Chief Executive Officer Pursuant to
Rule 13a-14(b)
/ 15d-14(b) of the Securities Exchange Act of 1934 and
18 U.S.C. Section 1350.**
|
|
|
32
|
.2
|
|
Certification of Chief Financial Officer Pursuant to
Rule 13a-14(b)
/ 15d-14(b) of the Securities Exchange Act of 1934 and
18 U.S.C. Section 1350.**
|
|
|
|
|
* |
|
These exhibits are identified as management contracts or
compensatory plans or arrangements of the Registrant pursuant to
Item 15 of
Form 10-K. |
64

