Attached files
| file | filename |
|---|---|
| EX-32 - EX-32 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex32.htm |
| EX-31.1 - EX-31.1 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex31d1.htm |
| EX-23.1 - EX-23.1 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex23d1.htm |
| EX-21.1 - EX-21.1 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex21d1.htm |
| EX-10.32 - EX-10.32 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex10d32.htm |
| EX-10.33 - EX-10.33 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex10d33.htm |
| EX-10.38 - EX-10.38 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex10d38.htm |
| EX-10.28 - EX-10.28 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex10d28.htm |
| EX-10.27 - EX-10.27 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex10d27.htm |
| EX-10.37 - EX-10.37 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex10d37.htm |
| EX-31.2 - EX-31.2 - OPTIMER PHARMACEUTICALS INC | a11-2421_1ex31d2.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
Form 10-K
FOR ANNUAL AND TRANSITION REPORTS
PURSUANT TO SECTIONS 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
(Mark One)
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2010
or
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 001-33291
Optimer Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in its Charter)
|
Delaware |
|
33-0830300 |
|
(State or Other Jurisdiction of |
|
(I.R.S. Employer |
10110 Sorrento Valley Road, Suite C, San Diego, California, 92121
(Address of principal executive offices and zip code)
Registrant’s telephone number, including area code: (858) 909-0736
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Name of Each Exchange on Which Registered |
|
Common Stock, par value $0.001 per share |
|
Nasdaq Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x.
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files): Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer o |
|
Accelerated filer x |
|
|
|
|
|
Non-accelerated filer o |
|
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x.
The aggregate market value of the registrant’s common stock held by non-affiliates as of June 30, 2010 (without admitting that any person whose shares are not included in such calculation is an affiliate), computed by reference to the price at which the common stock was last sold as of such date on the Nasdaq Global Market, was $345,839,000.
The number of outstanding shares of the registrant’s common stock, par value $0.001 per share, as of February 28, 2011 was 46,179,902.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Definitive Proxy Statement for our 2011 Annual Meeting of Stockholders are incorporated by reference in Part III of this report.
OPTIMER PHARMACEUTICALS
FORM 10-K—ANNUAL REPORT
For the Fiscal Year Ended December 31, 2010
Cautionary Note Regarding Forward-Looking Statements
This report and other documents we file with the SEC contain forward looking statements that are based on current expectations, estimates, forecasts and projections about us, our future performance, our business or others on our behalf, our beliefs and our management’s assumptions. In addition, we, or others on our behalf, may make forward looking statements in press releases or written statements, or in our communications and discussions with investors and analysts in the normal course of business through meetings, webcasts, phone calls and conference calls. Words such as “expect,” “anticipate,” “will,” “could,” “would” “project,” “intend,” “plan,” “believe,” “predict,” “estimate,” “should,” “may,” “potential,” “continue,” “ongoing”, or variations of such words and similar expressions are intended to identify such forward looking statements. These statements are not guarantees of future performance and involve certain risks, uncertainties and assumptions that are difficult to predict. We describe our respective risks, uncertainties and assumptions that could affect the outcome or results of operations in “Item 1A. Risk Factors”. We have based our forward looking statements on our management’s beliefs and assumptions based on information available to our management at the time the statements are made. We caution you that actual outcomes and results may differ materially from what is expressed, implied or forecast by our forward looking statements. Except as required under the federal securities laws and the rules and regulations of the SEC, we do not have any intention or obligation to update publicly any forward looking statements after the filing of this report, whether as a result of new information, future events, changes in assumptions or otherwise.
Overview
We are a biopharmaceutical company focused on discovering, developing and commercializing innovative hospital specialty products. Our current development efforts are focused on products that treat gastrointestinal infections and related diseases where current therapies have limitations, including limited efficacy, serious adverse side effects, drug-to-drug interactions, difficult patient compliance and bacterial resistance. We currently have two late-stage anti-infective product candidates, fidaxomicin and Pruvel™ (prulifloxacin).
Fidaxomicin, our lead product candidate, is a narrow spectrum antibiotic with minimal systemic absorption. We are developing fidaxomicin as a treatment for Clostridium difficile-infection, or CDI, the most common nosocomial, or hospital acquired, diarrhea. We currently hold rights to fidaxomicin in all regions of the world except for Europe and certain other countries in the Middle East, Africa and the Commonwealth of Independent States, or CIS.
In February 2011, we entered into an exclusive collaboration and license agreement with Astellas Pharma Europe Ltd., or Astellas, to develop and commercialize fidaxomicin in Europe and certain other countries in the Middle East, Africa and the CIS, which we refer to as the Astellas territory. In return for an exclusive license to fidaxomicin in the Astellas territory, Astellas paid to us an upfront cash payment of 50 million Euros, or $69.2 million, and we are eligible to receive additional cash payments totaling up to 115 million Euros upon the achievement of certain regulatory and commercial milestones. Furthermore, we will be entitled to receive escalating double-digit royalties ranging from the high teens to low twenties on net sales of fidaxomicin in the Astellas territory, if approved. Astellas will be responsible for all future costs associated with the development and commercialization of fidaxomicin in the Astellas territory including the costs associated with the Marketing Authorization Application, or MAA, for fidaxomicin in Europe. In connection with the collaboration and license agreement, we also entered into a supply agreement with Astellas pursuant to which we will be the exclusive supplier of fidaxomicin to Astellas in the Astellas territory and Astellas is obligated to pay us an amount equal to cost plus an agreed mark-up for such supply.
In July 2010, we filed, and in August 2010 the European Medicines Agency, or EMA, accepted for review an MAA to permit marketing of fidaxomicin in Europe. In September 2010, we began a submission to the United States Food and Drug Administration, or FDA, of a rolling New Drug Application, or NDA, and in November 2010 we completed the submission of our NDA. In January 2011, the FDA accepted our NDA filing for the treatment of CDI and for reducing the risk of recurrence when used for treatment of initial CDI. The FDA has also granted our request for six-month priority review, and has assigned a Prescription Drug User Fee Act, or PDUFA, goal date of May 30, 2011 for its review of the NDA. The FDA also informed us that it plans to discuss the NDA at a meeting of its Anti-Infective Drugs Advisory Committee currently scheduled for April 5, 2011.
We have completed two fidaxomicin Phase 3 trials which showed that fidaxomicin achieved the primary endpoint of clinical cure and demonstrated a significantly lower recurrence rate and significantly higher global cure rate (defined as cure with no recurrence within four weeks of completing therapy) compared to Vancocin, the only FDA-approved antibiotic for the treatment of CDI. In the two Phase 3 trials, among subjects who had experienced a prior CDI episode and recurred within three months of entering
the study, treatment with fidaxomicin resulted in a 47% reduction in repeat CDI recurrence compared to Vancocin (p=0.045). The data also indicated that treatment with fidaxomicin significantly improved the recurrence rate and global cure rate in CDI patients requiring concomitant antibiotics compared to Vancocin. Fidaxomicin was also well-tolerated in the trials. In February 2011, the New England Journal of Medicine published results from the first Phase 3 trial. We have also reported additional data from the second Phase 3 trial showing a clinically meaningful reduction in recurrence rates and higher global cure rates compared to Vancocin in both the hyper-virulent BI/NAP1/027 and the non-BI strain type subgroups. Clinical cure rates for fidaxomicin and Vancocin were similar in these two strain type subgroups.
Pruvel is a prodrug in the fluoroquinolone class of antibiotics, a widely-used class of broad-spectrum antibiotics. We are developing Pruvel as a treatment for infectious diarrhea. In July 2008, we reported positive top-line data from the first Phase 3 trial conducted in Mexico and Peru and in February 2009, we reported positive top-line data from the second Phase 3 trial conducted in India, Guatemala and Mexico. The top-line analysis of data from these studies showed that Pruvel met the primary endpoint of time to last unformed stool, or TLUS, compared to placebo. On November 9, 2010, due to a higher than expected incidence of cutaneous rash during the course of a study of the possible interaction between Pruvel and antacids, we informed the FDA that we were voluntarily terminating the research study. All reported events of cutaneous rash from the drug interaction study were mild or moderate in severity and required little or no treatment and all resolved completely. We are currently conducting an investigation into the cause of the rash prior to initiating any new study with Pruvel. Rashes are a known and infrequent side effect of fluoroquinolone antibiotics, such as Pruvel, and rashes occurred at or below the rate generally expected for other fluoroquinolones in both of our previous Pruvel Phase 3 clinical trials. Pending the results of our investigation, we are not currently able to estimate when we will initiate any new study or the extent of the delay in our planned submission of an NDA for Pruvel. We currently hold rights to commercialize Pruvel in the United States, and it is sold by other parties in Japan, Italy and certain other European countries.
We are developing additional product candidates using our proprietary technology, including our Optimer One-Pot Synthesis, or OPopS™ drug discovery platform. OPopS is a computer-aided technology that allows the development of potential drug candidates through carbohydrate mediated medicinal chemistry and enables the rapid synthesis of a wide variety of proprietary molecules. It includes GlycoOptimization, which enables the modification of a carbohydrate group on an existing drug to improve its properties, and De Novo Glycosylation, which introduces new carbohydrate groups on existing drugs to create new patentable compounds with improvement of pharmacokinetics.
We previously acquired exclusive rights to OPT-822/821, a combination of a novel carbohydrate-based cancer immunotherapy together with an adjuvant, which we licensed from Memorial Sloan-Kettering Cancer Center, or MSKCC. In October 2009, we assigned to our subsidiary, Optimer Biotechnology, Inc., or OBI, certain of our patent rights and know-how related to OPT-822/821 and also assigned to OBI our rights and obligations under a related license agreement with MSKCC. In April 2010, OBI filed an investigational new drug application, or IND, in Taiwan for OPT-822/821, and in January 2011, OBI initiated a Phase 2/3 clinical trial for the treatment of metastatic breast cancer which it intends to conduct in Taiwan, South Korea, Hong-Kong and Singapore. Other potential indications for OPT-822/821 are being evaluated. We have the right to receive up to $10 million from OBI in future milestone payments related to the development of OPT-822/821 as well as royalties on net sales of this product candidate. In February 2011, pursuant to an amendment to an October 2009 financing agreement, OBI sold newly-issued shares of its common stock for gross proceeds of approximately 462.0 million New Taiwan Dollars (approximately $15.5 million based on then- current exchange rates). We purchased 277.2 million New Taiwan Dollars (approximately $9.3 million based on then-current exchange rates) of the shares issued in the financing, such that we currently maintain a 60% equity interest in OBI.
We were incorporated in November 1998. We have one majority-owned subsidiary, OBI, which is located and incorporated in Taiwan. In this disclosure, “Optimer Pharmaceuticals,” “Optimer,” “we,” “us” and “our” refer to Optimer Pharmaceuticals, Inc. and OBI on a consolidated basis, unless the context otherwise provides. Our principal offices are in San Diego, California and we recently expanded our operations to include additional office space in Jersey City, New Jersey. We maintain an internet site at www.optimerpharma.com, which includes links to reports we have filed with the Securities and Exchange Commission, or SEC. Any information that is included on or linked to our Internet site is not a part of this report or any registration statement that incorporates this report by reference. Our filings may also be read and copied at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-732-0330. The SEC also maintains a website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is www.sec.gov.
Antibiotic Market Background
Infectious diseases can be caused by bacteria present in the environment that enter the body through the skin or mucous membranes of the lungs, nasal passages and gastrointestinal tract, or GI tract. These bacteria can be pathogenic, or disease-causing, and can overwhelm the body’s immune system by establishing themselves throughout the body in various tissues and organs where they proliferate. This can cause a number of serious and, in some cases, fatal infections, including those of the GI tract, urinary tract, respiratory tract, bloodstream, skin and heart.
Bacteria can be classified as either gram-positive or gram-negative. The difference in classification is largely based on a difference in bacteria cell wall structure in that gram-positive bacteria have exposed thick peptidoglycan, a polymer consisting of sugars and amino acids, cell walls, while gram-negative bacteria do not. Antibiotics that treat bacterial infections can be classified as either broad-spectrum or narrow-spectrum. Most antibiotics in use today are generally considered broad-spectrum, meaning they target a wide variety of bacteria. In contrast, narrow-spectrum antibiotics target a select group of bacteria such as gram-positive or gram-negative bacteria. Current research is increasingly focused on antibiotics that target specific bacteria, which may be beneficial for the treatment of certain infections.
Antibiotics used to treat bacterial infections work by interfering with bacterial cellular activities, such as cell wall synthesis or protein synthesis. Antibiotics may be bacteriostatic or bactericidal. Bacteriostatic antibiotics stop the growth of bacteria, which prevents the infecting bacteria from multiplying and allows the patient’s own immune system to eradicate the infecting bacteria. Bactericidal antibiotics work by directly killing the bacteria, which is particularly important for patients with weakened immune systems that cannot effectively eradicate the infecting bacteria on their own.
The anti-infective market is one of the largest therapeutic categories worldwide. According to IMS Health, the combined market for prescription antibacterial drugs in 2009 was reported to be $39.7 billion worldwide. The largest antibacterial sales per region were: North America ($11.1 billion), Europe ($10.5 billion), Asia Pacific ($12.7 billion), and others ($5.4 billion). The market for anti-infective products is generally divided into two categories, nosocomial infections and community-acquired infections, which represent approximately 30% and 70% of the anti-infectives market, respectively. According to the U.S. Centers for Disease Control and Prevention, or CDC, approximately two million nosocomial infections occur annually in the United States and these infections can increase the average length of hospital stays by seven to nine days. Approximately four million nosocomial infections occur annually in Europe, three million in North America, two million in South America and two million in East Asia (excluding China). Nosocomial infections are costly to address, with an estimated annual aggregate healthcare cost in the United States and the United Kingdom of approximately $4.5 billion and $1.9 billion, respectively. In addition, in the United States, nosocomial infections cause approximately 100,000 deaths annually, making them one of the five leading causes of death in the United States. We believe that bacterial infections, especially infections caused by difficult-to-treat, drug resistant bacteria, cause or contribute to a majority of these deaths.
Our Market Opportunity
Many marketed antibiotics used to treat infections have well-documented shortcomings. For example, current antibiotics often fail to reach sufficient concentrations at the site of infection to adequately eliminate harmful bacteria. Certain of these antibiotics have also been associated with serious adverse side effects, including renal toxicities, heart rhythm abnormalities, phototoxicity, rashes and central nervous effects, such as seizures. These side effects limit the use of antibiotics for certain patients. In addition, certain antibiotics have interaction issues with prescribed drugs, such as cholesterol lowering agents. Safety problems can arise when increased doses of these antibiotics are needed to treat resistant bacteria. If bacteria develop resistance to currently available antibiotics, the underlying infection can become difficult or impossible to treat, and may even lead to death. Patients also often fail to comply with treatment regimens due to many factors including the inability to tolerate an antibiotic due to its side effects, inconvenient method of dosing and undesirable frequency and length of dosing. Because of these shortcomings associated with marketed antibiotics, we believe an opportunity exists to improve upon existing treatments.
Our Product Candidates
We believe that our product candidates may offer advantages over existing antibiotics in terms of efficacy, safety, bacterial resistance and dosing convenience. We also believe that the markets for these product candidates present us with significant commercial opportunities. Our product candidates are in various stages of development and none have been approved for sale. Our ability or our licensees’ ability to obtain regulatory approval of any of our product candidates requires us or our licensees to successfully complete the clinical development of each such product candidate and demonstrate through data submissions the safety and efficacy of the product candidate to the satisfaction of the FDA and comparable foreign regulatory authorities. Clinical trials involve a lengthy and expensive process with an uncertain outcome, and efficacy and safety data of earlier studies and trials may not be predictive of future trial results.
Our current product candidate portfolio consists of the following:
|
Product Candidate |
|
Target Indications |
|
Development Status |
|
Commercial Rights |
|
Anti-Infectives |
|
|
|
|
|
|
|
Fidaxomicin |
|
CDI treatment |
|
NDA and MAA submitted; PDUFA goal date of May 30, 2011 |
|
Optimer — worldwide except Astellas territory; Astellas — Astellas territory (all fidaxomicin product candidates and indications) |
|
|
|
CDI prophylaxis |
|
Proof of concept trial (1) |
|
|
|
Fidaxomicin (oral suspension) |
|
Pediatric CDI treatment |
|
Pre-clinical |
|
|
|
Pruvel™ (prulifloxacin) |
|
Infectious diarrhea |
|
Investigating interaction with antacids; NDA preparation; Two Phase 3 trials completed |
|
Optimer U.S. |
|
CEM-101 (OP-1068) |
|
Respiratory tract infections |
|
Phase 2 |
|
Cempra worldwide (2) |
|
|
|
|
|
|
|
|
|
Other Therapeutic Areas |
|
|
|
|
|
|
|
OPT-822/821 |
|
Breast cancer |
|
Phase 2/3 |
|
Licensed to OBI (3) |
(1) A proof-of concept trial is an exploratory clinical trial to provide or establish evidence that a product candidate is efficacious for a target indication.
(2) We granted to Cempra an exclusive worldwide license, except in countries in the Association of Southeast Asian Nations, or ASEAN. We have the right to receive royalties from Cempra on any sales of CEM-101 (OP-1068) outside of ASEAN countries.
(3) We have the right to receive royalties from OBI on any sales of OPT-822/821.
Anti-Infective Product Candidates
Fidaxomicin
Overview. We are initially developing fidaxomicin for the treatment of infections caused by Clostridium difficile, or C. difficile, bacteria. Fidaxomicin is a differentiated antibiotic for the treatment of CDI, the most common nosocomial diarrhea. Specifically, fidaxomicin has a narrow spectrum of activity against certain gram-positive bacteria. Pre-clinical data indicates that fidaxomicin is bactericidal and acts by inhibiting RNA polymerase, a bacterial enzyme. Fidaxomicin has also been shown to inhibit the growth of other potentially harmful bacteria such as Staphylococci, common bacteria that reside on the skin and in the GI tract, and Enterococci, common bacteria that reside in the GI tract.
Clostridium Difficile Infections. CDI has become a significant medical problem in hospitals, long-term care facilities, and in the community and is estimated to afflict more than 700,000 people each year in the United States. CDI is a serious illness resulting from infection of the inner lining of the colon by C. difficile bacteria, that produce toxins that cause inflammation of the colon, severe diarrhea and, in serious cases, death. Patients typically develop CDI from the use of broad-spectrum antibiotics that disrupt normal gastrointestinal (gut) flora, thus allowing C. difficile bacteria to flourish and produce toxins.
Current therapeutic options for CDI include the off-label use of metronidazole and oral vancomycin, the latter being the only FDA-approved treatment for CDI. However, approximately 20% to 30% of CDI patients who initially respond to these treatments experience a clinical recurrence following cessation of the CDI treatment.
Primary risk factors for CDI include broad-spectrum antibiotic use (such as cephalosporins and fluoroquinolones), older age (over 65) and exposure to emerging hyper-virulent strains (BI/NAP1/027, 078, 001) of C. difficile. The increasing incidence of CDI, along with high rates of both treatment failures and recurrences with current therapies have resulted in greater awareness and concern about CDI among medical professionals and public health officials.
Outbreaks and illness related to C. difficile generally occur during or after therapy with broad-spectrum antibiotics. Broad-spectrum antibiotics can cause CDI by disrupting normally present gastrointestinal bacteria, or gut flora, thereby allowing C. difficile to proliferate. Studies have suggested that the use of proton pump inhibitors, or PPIs, a widely used group of heartburn drugs, may also be linked to C. difficile infections. CDI accounts for up to 33% of antibiotic-associated diarrhea incidences as well as many cases of antibiotic-associated colitis, or inflammation of the colon. C. difficile can be transmitted by direct or indirect contact with infected patients via spores that can live for months on dry surfaces.
We believe that the incidence of CDI may be higher than what is currently being reported because many hospitals are not required to and do not report incidents of CDI. For example, a survey conducted in May 2008 through August 2008 by the Association for Professionals in Infection Control and Epidemiology, or APIC, showed that 13 out of every 1,000 inpatients were either infected or colonized with C. difficile (94.4% infected) which is 6.5 to 20 times higher than previous incidence estimates.
Additionally, recent reports indicate that the incidence of community-acquired CDI cases may be increasing. For example, a study conducted in one major U.S. city and cited at the 2006 Interscience Conference on antimicrobial Agents and Chemotherapy, or ICAAC, reported that the percentage of CDI cases found to be community-acquired increased from 12% in 2003 to 22% in 2004 and to 29% in 2005. A recently published survey conducted by a Veterans Affairs hospital in North Carolina showed that 35% patients with CDI experienced onset of the disease in the community. In the community, exposure to CDI is believed to occur through household contacts of persons with diarrhea, or ingestion of contaminated food or soil. Some animals, including domesticated pets, may also carry C. difficile.
According to a study cited in the New England Journal of Medicine, the increased rates of CDI and severity of the disease may be caused by a combination of factors, including the excessive use of antibiotics, a growing population over the age of 65 and the impact of new hyper-virulent strains of C. difficile. Recent CDI studies have demonstrated increasing frequency of new hyper-virulent strains, which can increase the risk of clinical failure, recurrence and mortality. The analysis of C. difficile isolates from our first Phase 3 trial presented at the 2009 ICAAC meeting demonstrated that even though the BI hyper-virulent group continues to be dominant in North America, 60% of the strains analyzed belonged to non-BI groups including G, J, K and Y groups. Hyper-virulent strains are also a growing concern in Europe.
Generally, CDI results in longer hospital stays and increases average patient cost which is often not reimbursed to the hospital. In more complicated cases of CDI, hospitalization may be prolonged by up to two weeks. A recent analysis suggests that patients with CDI have their hospital stay extended by at least 3 days compared with patients without the infection, with the incremental cost of approximately $13,700 per patient. The total annual costs associated with hospital cases of CDI in United States are estimated at $3.2 billion. According to the data presented at the 2006 ICAAC, CDI results in an estimated increase in average patient cost of over $6,000 per patient in the United Kingdom and the total projected annual cost for treating the disease in Europe is approximately $3.8 billion.
Physicians often care for patients with CDI by discontinuing previously administered broad-spectrum antibiotics, if possible, and providing supportive care such as fluid and electrolyte replacement. If these measures fail, the standard therapy for CDI includes the administration of metronidazole and/or oral vancomycin.
Current Treatments and Limitations. Metronidazole is generally used for patients in the United States and Europe experiencing their first episode or first recurrent episode of CDI. Metronidazole is a generic drug that is used off-label to treat CDI due to its low cost and historical efficacy. The typical treatment regimen for metronidazole is 250 mg every six hours, for a minimum of ten days. Metronidazole can be associated with numerous adverse side effects such as seizures, toxic reactions to alcohol, leukopenia, or reduction of white blood cells, neuropathy, a disease affecting one or more nerves, unpleasant taste and dry mouth.
Oral vancomycin is used in the United States and also in Europe and Japan for the treatment of CDI. As a result of its broad antibacterial activity, intravenously administered vancomycin is frequently used for certain other life-threatening infections caused by multi-drug resistant bacteria such as vancomycin-resistant Enterococci, or VRE. In an effort to slow the continuing emergence of vancomycin-resistant bacteria, the medical community discourages the use of the drug for the treatment of CDI except for patients who are not responding to metronidazole, for patients with severe, life-threatening colitis or for patients with risk factors that are predictive of negative treatment outcomes. Despite these recommendations, based on our survey of 131 U.S. hospital physicians, hospital physicians would intend to prescribe oral vancomycin approximately 37% of the time, compared to our estimate of an approximately 27% expected prescription rate per the treatment guidelines. In addition, our internal market research suggests that hospitals in the U.S. are increasing oral use of generic reconstituted intravenous vancomycin relative to the use of branded oral vancomycin capsules, or Vancocin. Oral vancomycin’s recommended treatment protocol is 125 mg or 250 mg doses every six hours, for approximately ten days.
Both metronidazole and oral vancomycin have shortcomings as treatments for CDI including:
· Limited Efficacy. A controlled study conducted in North America and reported in 2007 showed that approximately 19% of CDI patients treated with oral vancomycin and 28% of CDI patients treated with metronidazole do not respond to therapy, and these patients are at risk of developing more severe CDI.
· High Recurrence Rate. Approximately 25% of CDI patients who initially respond to oral vancomycin and approximately 27% of CDI patients who initially respond to metronidazole experience a clinical recurrence following the cessation of antibiotic administration.
· Bacterial Resistance. Widespread use of oral vancomycin is discouraged for the treatment of CDI in some hospitals due to concerns over the development of cross-resistant bacteria, including VRE, and vancomycin-resistant Staphylococcus, which can also cause other serious nosocomial infections. Furthermore, C. difficile resistance to metronidazole has been reported in at least one study.
· Adverse Side Effects. Metronidazole, which is systemically absorbed and must be administered in high doses to treat CDI, may result in serious adverse side effects and complications, including seizures, toxic reactions to alcohol, leukopenia, neuropathy, unpleasant taste and dry mouth.
· Inducement of CDI. Oral vancomycin and metronidazole are both broad-spectrum antibiotics that disrupt the normal gut flora. Because normal and healthy gut flora generally suppresses the growth of C. difficile, administration of oral vancomycin or metronidazole may actually induce the development of CDI.
· Inconvenient Dosing and Difficult Compliance. The current treatment regimen for both oral vancomycin and metronidazole is inconvenient as both must be administered every six hours for a minimum of seven days, which may result in lower levels of patient compliance.
Potential Fidaxomicin Advantages. Fidaxomicin is a differentiated macrocycle antibiotic consisting of an 18-member ring structure. Fidaxomicin has significant differentiating features, including a narrow antimicrobial spectrum, fast-acting bactericidal activity against C. difficile, minimal systemic exposure and an enduring clinical effect. Based on our clinical and pre-clinical studies of fidaxomicin for the treatment of CDI, we believe fidaxomicin may offer the following advantages:
· Demonstrated high potency activity against C. difficile, including hypervirulent strains such as B1/NAP1/027, with low rates of treatment failures and recurrences. Our two Phase 3 studies provided evidence of a high cure rate and substantially improved recurrence rates and global cure rates compared to Vancocin;
· Superiority to vancomycin in treating a CDI recurrence and in reducing the chance of another relapse, based on data from our two Phase 3 trials presented in September 2010 at ICAAC;
· Cost effectiveness as a result of lower rates of recurrence, VRE colonization and complications in high risk sub-populations. For example, in an analysis of Medicare claims during 2007 and 2008, the cost of a CDI recurrence was estimated at between $10,100 to $34,000 and thus fidaxomicin has the potential to provide a significant cost advantage based upon the 47% reduction in recurrence versus oral vancomycin in our two Phase 3 studies;
· Limited disruption of normal gut flora resulting in a lower likelihood of inducement of CDI recurrence and decreased severity of disease. In November 2010, a peer-review journal the Society for General Microbiology published research which highlighted fidaxomicin’s narrow spectrum activity and minimal disruption of microflora in patients with CDI compared to vancomycin;
· Minimal systemic exposure resulting in a favorable safety profile;
· Improved outcomes compared to oral vancomycin for CDI patients requiring concomitant antibiotics;
· Lower propensity to cause VRE colonization compared to vancomycin;
· Faster time to resolution of diarrhea;
· Evidence of low C. difficile resistance, including hypervirulent strains such as B1/NAP1/027; and
· Convenient, twice daily dosing regimen.
Clinical Development
Phase 3 Pivotal Trials.
Second Phase 3 trial. In February 2010, we reported positive top-line results from our second of two Phase 3 clinical trials of fidaxomicin for the treatment of CDI. This trial was a multi-center, randomized, double-blind trial of fidaxomicin (200 mg q12h) against vancomycin (125 mg q6h) in adult subjects suffering from CDI. We enrolled 535 adult subjects at more than 100 sites throughout North America and Europe. The primary endpoint of the study was clinical cure defined as patients requiring no further CDI therapy two days after completion of study medication, as determined by the treating physicians. The secondary endpoint evaluated CDI recurrence up to four weeks post therapy with recurrence defined as the return of diarrhea associated with CDI confirmed by a positive toxin test. Global cure, also a secondary endpoint, was defined as patients who were cured and did not have a recurrence during the subsequent four-week period. We believe that global cure is the most significant outcome in CDI treatment as it indicates a complete cure without the need for further medical treatment related to CDI. Patients were dosed with either fidaxomicin at 200 mg twice daily (400 mg/day) or Vancocin at its recommended dosing regimen of 125 mg every six hours (500 mg/day) for ten days.
In the second Phase 3 study, 91.7% of patients treated with fidaxomicin in the per protocol population achieved clinical cure versus 90.6% for Vancocin. In addition, only 12.8% of per protocol patients treated with fidaxomicin experienced a recurrence versus 25.3% for Vancocin (p = 0.002). Per protocol patients treated with fidaxomicin had a global cure of 79.6% which was greater than Vancocin at 65.5% (p = <0.001). Fidaxomicin was also well-tolerated in the study. The top-line results from the second Phase 3 trial are summarized in the table below.
|
Per Protocol (microbiologically evaluable) |
|
Fidaxomicin (200 mg bid) |
|
Vancocin ® capsules |
|
P-Value |
|
95% Confidence |
|
Clinical cure |
|
91.7% (198/216 patients) |
|
90.6% (212/224 patients) |
|
NA |
|
(-4.3, 6.3) |
|
Recurrence |
|
12.8% (23/180) |
|
25.3% (46/182) |
|
0.002 |
|
(-20.3, -4.4) |
|
Global Cure |
|
79.6% (172/216) |
|
65.5% (154/235) |
|
<0.001 |
|
(5.9, 22.1) |
|
modified Intent-to-Treat (mITT) |
|
Fidaxomicin (200 mg bid) |
|
Vancocin ® capsules |
|
P-Value |
|
95% Confidence |
|
Clinical cure |
|
87.7% (221/252 patients) |
|
86.8% (223/257 patients) |
|
NA |
|
(-4.9, 6.7) |
|
|
|
|
|
|
|
|
|
|
|
Recurrence |
|
12.7% (28/221) |
|
26.9% (60/223) |
|
<0.001 |
|
(-21.4, -6.8) |
|
Global Cure |
|
76.6% (193/252) |
|
63.4% (163/257) |
|
0.001 |
|
(5.2, 20.9) |
NA = Not Applicable (trial met non-inferiority endpoint)
The Per Protocol (microbiologically evaluable) population is the patient group with CDI confirmed by diarrhea with a positive toxin assay, met all inclusion/exclusion criteria, and that received at least 3 days of therapy if deemed a failure or at least 8 days of therapy if deemed a cure.
The modified Intent-to-Treat population is the patient group that had CDI confirmed by diarrhea with a positive toxin assay and received at least one dose of study medication.
In September 2010, we reported additional data from our two fidaxomicin Phase 3 trials at the Interscience Conference on Antimicrobial Agents and Chemotherapy, or ICAAC, which indicated that the use on concomitant antibiotics is a significant risk factor for a negative CDI treatment outcome. Many patients with CDI have persistent infections or develop new infections during the course of CDI treatment making concomitant antibiotics clinically necessary and common. Overall, 28% of patients from the Phase 3 trials received concomitant antibiotic exposure in addition to fidaxomicin and vancomycin for intervening infections during the trials. An analysis of the impact of concomitant antibiotic exposure in these patients on clinical outcomes indicated fidaxomicin was a superior therapy to vancomycin in preventing recurrence and in promoting global cure in patients requiring concomitant antibiotics. The analysis from the Phase 3 trials indicated that in patients receiving concomitant antibiotics, those treated with fidaxomicin versus vancomycin had a significantly higher clinical cure rate (90% vs. 79%, p=0.044), significantly reduced recurrence rate (17% vs 29%, p=0.048) and improved global cure rate (73% vs. 59%, p=0.020). At the same time, among patients who received no concomitant antibiotics, recurrence in vancomycin-treated patients was double that of fidaxomicin-treated patients (23% vs. 12%, p<0.001).
First Phase 3 trial. In November 2008, we reported positive top-line results from our Phase 2b/3 clinical trial of fidaxomicin for the treatment of CDI. This trial was a multi-center, double-blind, controlled Phase 3 trial and was the largest single comparative study conducted against Vancocin in CDI. We enrolled 629 adult subjects at more than 100 sites throughout North America. The primary endpoint of the study was clinical cure defined as patients requiring no further CDI therapy two days after completion of study medication, as determined by the treating physicians. The secondary endpoint evaluated CDI recurrence up to four weeks post
therapy with recurrence defined as the return of diarrhea associated with CDI confirmed by a positive toxin test. Global cure, an exploratory endpoint in the first Phase 3 trial, was defined as patients who were cured and did not have a recurrence during the four week post-therapy period. Patients were dosed with either fidaxomicin at 200 mg twice daily (400 mg/day) or Vancocin at its recommended dosing regimen of 125 mg every six hours (500 mg/day) for ten days. In the initial Phase 2b portion of the trial, we enrolled a total of 93 CDI patients at 32 sites. Following an interim blinded safety analysis by an independent data safety monitoring board, we transitioned into a Phase 3 clinical trial in March 2007.
In the first Phase 3 study, 92.1% of patients treated with fidaxomicin in the per protocol population achieved clinical cure versus 89.8% for Vancocin. In addition, only 13.3% of per protocol patients treated with fidaxomicin experienced a recurrence versus 24.0% for Vancocin (p = 0.004). Per protocol patients treated with fidaxomicin had a global cure of 77.7% which was greater than Vancocin at 67.1% (p = 0.006). Fidaxomicin was well-tolerated. The top-line results from the first Phase 3 trial are summarized in the table below.
|
Per Protocol (microbiologically evaluable) |
|
Fidaxomicin (200 mg bid) |
|
Vancocin ® capsules |
|
P-Value |
|
95% Confidence |
|
Clinical cure |
|
92.1% (244/265 patients) |
|
89.8% (254/283 patients) |
|
NA |
|
(-2.6, 7.1) |
|
Recurrence |
|
13.3% (28/211) |
|
24.0% (53/221) |
|
0.004 |
|
(-17.9, -3.3) |
|
Global Cure |
|
77.7% (206/265) |
|
67.1% (190/283) |
|
0.006 |
|
(3.1, 17.9) |
|
modified Intent-to-Treat (mITT) |
|
Fidaxomicin (200 mg bid) |
|
Vancocin ® capsules |
|
P-Value |
|
95% Confidence |
|
Clinical cure |
|
88.2% (253/287 patients) |
|
85.8% (265/309 patients) |
|
NA |
|
(-3.1, 7.8) |
|
Recurrence |
|
15.4% (39/253) |
|
25.3% (67/265) |
|
0.005 |
|
(-16.6, -2.9) |
|
Global Cure |
|
74.6% (214/287) |
|
64.1% (198/309) |
|
0.006 |
|
(3.1, 17.7) |
NA = Not Applicable (trial met non-inferiority endpoint)
The Per Protocol (microbiologically evaluable) population is the patient group that had CDI confirmed by diarrhea with a positive toxin assay, met all inclusion/exclusion criteria, and received at least 3 days of therapy and were considered a failure or received at least 8 days of therapy and were considered a cure.
The modified Intent-to-Treat population is the patient group that had CDI confirmed by diarrhea with a positive toxin assay and received at least one dose of study medication.
Further analysis of the results from the first Phase 3 trial demonstrated other potential advantages of fidaxomicin. Specifically, data from the first Phase 3 trial demonstrated that:
· in patients with more pronounced diarrhea (diarrhea that did not resolve in the first 24 hours of therapy), fidaxomicin was associated with a faster time to resolution of diarrhea than vancomycin (79 hours versus 105 hours, p=0.056);
· in patients who did not receive either vancomycin or metronidazole in the 24-hour pre-trial enrollment period, the difference in recurrence rate between fidaxomicin and vancomycin was more pronounced than in the overall patient population at 10.9% versus 24.3%, respectively;
· CDI recurrence occurred significantly later in patients treated with fidaxomicin with only 3% and 9% recurrence rates versus 14% and 20% recurrence rates for vancomycin, at 10 and 20 days post-treatment, respectively; and
· fidaxomicin was less likely than vancomycin to promote VRE colonization in patients treated for CDI, which we believe may be due to inhibitory activity of fidaxomicin against many VRE strains and fidaxomicin’s relative sparing of the intestinal flora including Bacteroides bacteria. The analysis showed that only 7% of patients treated with fidaxomicin acquired VRE versus 31% of vancomycin-treated patients (p<0.001) from among a subset of 302 CDI patients, 248 of whom had a negative VRE stool sample upon entering the Phase 3 trial.
Phase 2a Study. In July 2005, we completed an open-label, dose-ranging, randomized safety and clinical evaluation study of fidaxomicin in patients with CDI at five sites. Fidaxomicin was administered to 48 patients. Three patients withdrew from the trial for reasons unrelated to the administration of fidaxomicin, resulting in 45 patients eligible for evaluation. Forty-one of these patients completed a ten-day therapy regimen consisting of twice daily doses of 50 mg (100 mg/day), 100 mg (200 mg/day) or 200 mg (400 mg/day). A primary endpoint of the trial was clinical cure of CDI, as determined by the treating physician for each patient on the tenth day of administration. Additional endpoints investigated were time-to-resolution of diarrhea, recurrence rate through six weeks post-treatment and total relief of CDI symptoms, defined as complete relief of diarrhea, fever and abdominal pain, and normalized white blood cell counts by the end of the ten-day therapy.
Among the 45 evaluated patients, only four patients failed to achieve clinical cure by the end of ten days of therapy, two of whom were in the 100 mg/day dose group and two of whom were in the 200 mg/day dose group. None of the patients in the 400 mg/day dose group failed to achieve clinical cure. All 41 cured subjects were subsequently monitored for six weeks following therapy for recurrence. CDI recurred in two of the 41 cured subjects, one in the 100 mg/day dose group and one in the 400 mg/day dose group. The median cure times, or time-to-resolution of diarrhea, were as follows: 5.5 days for the 100 mg/day dose group, 3.5 days for the 200 mg/day dose group and 3.0 days for the 400 mg/day dose group.
A summary of the results of the Phase 2a clinical trial for fidaxomicin is presented below:
|
|
|
Dose Group | ||||
|
Parameter |
|
100 mg/day |
|
200 mg/day |
|
400 mg/day |
|
Clinical Cures |
|
86% (12/14) |
|
87% (13/15) |
|
100% (16/16) |
|
Total Symptom Relief |
|
43% (6/14) |
|
53% (8/15) |
|
81% (13/16) |
|
Recurrence |
|
8% (1/12) |
|
0% (0/13) |
|
6% (1/16) |
|
Median Time to Cure of Diarrhea |
|
5.5 Days |
|
3.5 Days |
|
3.0 Days |
Pharmacokinetic analyses were performed on all patients. Fidaxomicin was not detectable in the blood in half of the patients and only three subjects had levels exceeding 0.02 mg/mL. Stool concentrations of fidaxomicin averaged over 1,400 mg/g of stool at the 400 mg/day dose level at day ten. As C. difficile is present mainly in the gut, high stool concentrations suggest that fidaxomicin is present where needed to treat CDI and low concentrations in the blood indicate fidaxomicin is minimally absorbed in the system, thus reducing the risk of side effects. There were no adverse events determined by the physicians to be related to fidaxomicin. At one site in this Phase 2a trial, we performed a microbiologic analysis of the stool of 29 patients. This analysis showed that fidaxomicin did not cause any unusual disruptions of normal gut flora for patients in any of the three dose groups.
In this study there was a 42% presence of the super toxin-producing strain of C. difficile, the BI/NAP1/027 strain, and there was no significant difference in the overall cure rates with fidaxomicin between subjects with the BI/NAP1/027 strain and subjects without the hypervirulent strain. The distribution of the BI/NAP1/027 strain was nearly equal in all three dosing groups.
The bacterial isolates from the study were also tested for susceptibility to fidaxomicin, vancomycin, and metronidazole. Overall antibiotic susceptibilities were consistent with the previously reported MIC90 values for the three antibiotics. However, fidaxomicin showed no difference in MIC values between the BI/NAP1/027 strains and the non-BI/NAP1/027 strains, whereas currently available antibiotics, metronidazole and vancomycin, showed slightly higher MIC values for the hypervirulent strains.
Phase 1 Studies. We have completed two double-blind, oral, dose-escalating, placebo-controlled Phase 1 trials, one of which was a Phase 1a single-dose trial, and one of which was a Phase 1b multiple-dose trial. The trials were designed to determine the safety, tolerability, and pharmacokinetic characteristics of fidaxomicin in healthy volunteers. Each Phase 1a patient received two single oral administrations of either a 100 mg dose followed by a 300 mg dose, or a 200 mg dose followed by a 450 mg dose of fidaxomicin. Each Phase 1b patient received daily oral administrations of 150, 300, or 450 mg doses of fidaxomicin for ten consecutive days. In both trials, there were eight subjects for each dose level, six of whom were randomly selected to receive fidaxomicin and two of whom received placebo. We collected blood, urine and stool samples for pharmacokinetic analysis. Vital signs including blood pressure, pulse, body temperature and electrocardiograms were measured following each dosing and on a regular basis throughout the study. In both studies, fidaxomicin was well tolerated by all subjects and no drug-related adverse events were observed.
Fidaxomicin also exhibited a favorable pharmacokinetic profile for CDI treatment. After oral administration at either single dose or multiple doses, all blood samples had low, usually lower than 0.02 mg/mL, or undetectable levels of fidaxomicin which indicates very low systemic absorption. In contrast, fidaxomicin was found to be present in high concentrations in the stool. For example, at day ten for the 450 mg per day multiple-dose group, the mean fidaxomicin stool concentration exceeded 10,000 times the MIC90, or minimum concentration of a drug needed to inhibit growth of 90% of microorganisms, of C. difficile.
Commercialization Rights
With the exception of the rights we granted to Astellas to develop and commercialize fidaxomicin in the Astellas territory, we hold worldwide rights to fidaxomicin. In February 2007, we regained worldwide rights to fidaxomicin from Par Pharmaceuticals, Inc., or Par, under a prospective buy-back agreement to develop and commercialize fidaxomicin in North America and Israel. We paid Par a one-time $5.0 million milestone payment in June 2010 for the successful completion of our second pivotal Phase 3 trial for fidaxomicin. We may be obligated to pay Par a 5% royalty on net sales by us or our affiliates of fidaxomicin in North America and Israel, and a 1.5% royalty on net sales by us or our affiliates of fidaxomicin in the rest of the world. In addition, we are required to pay Par a 6.25% royalty on net revenues we receive from licensees of our right to market fidaxomicin in the rest of the world. We are obligated to pay each of these royalties, if any, on a country-by-country basis for seven years commencing on the applicable commercial launch in each such country.
Fidaxomicin — Label Expansion
Oral Suspension Formulation Development. We are developing an oral suspension formulation for use in pediatric CDI patients as required by regulatory agencies. We are also evaluating the development of an oral formulation for patients in intensive care units and elderly patients who cannot swallow tablets. In December 2010, the FDA granted orphan drug designation for the treatment of pediatric CDI. This orphan drug designation applies to fidaxomicin as the active pharmaceutical ingredient and covers all formulations of fidaxomicin used to treat CDI in children 16 years of age or younger.
CDI Prophylaxis. We believe fidaxomicin may be effective not only for treating CDI but also for preventing CDI. Patients at high risk of developing CDI may benefit from prophylactic protection from the disease. There is currently no therapeutic drug approved for the prevention of CDI. We believe fidaxomicin may provide safe, potent and narrow-spectrum bactericidal activity against C. difficile, thereby protecting high-risk patients while limiting disruption to normal gut flora. We are evaluating the possibility of conducting a proof-of-concept trial in high risk patients.
Pruvel (prulifloxacin)
Overview. We are developing Pruvel for the treatment of infectious diarrhea, including travelers’ diarrhea, a community-acquired infection which can be caused by a broad range of bacteria. We plan to initially seek approval for Pruvel for the treatment of infectious diarrhea. Pruvel is a prodrug in the fluoroquinolone class of antibiotics, a widely-used class of broad-spectrum antibiotics. A prodrug is an inactive form of a compound that is converted in the body to an active drug either by spontaneous chemical reaction or through the enzymatic process. Following oral administration, Pruvel is converted to ulifloxacin, which is rapidly bactericidal by killing susceptible bacterial pathogens through inhibition of DNA replication. Ulifloxacin has demonstrated potent broad-spectrum activity against gram-positive and gram-negative bacteria. We have completed two Phase 3 clinical trials of Pruvel for the treatment of travelers’ diarrhea and reported positive top-line data from each study. In July 2008, we reported positive top-line data from the first Phase 3 trial conducted in Mexico and Peru and in February 2009, we reported positive top-line data from the second Phase 3 trial conducted in India, Guatemala and Mexico. The top-line analysis of data from these studies showed that Pruvel met the primary endpoint of TLUS compared to placebo. Pruvel was generally well tolerated and had a similar safety profile compared to placebo.
On November 9, 2010, due to a higher than expected incidence of cutaneous rash during the course of a study of the possible interaction between Pruvel and antacids, we informed the FDA that we were voluntarily terminating the research study. All reported events of cutaneous rash from the drug interaction study were mild or moderate in severity and required little or no treatment and all resolved completely. We are conducting an investigation into the cause of the rash prior to initiating any new study with Pruvel. Rashes are a known and infrequent side effect of fluoroquinolone antibiotics, such as Pruvel, and, in both of our previous Pruvel Phase 3 clinical trials, rashes occurred at or below the rate generally expected for other fluoroquinolones. Pending the results of our investigation, we are not currently able to estimate when we will initiate any new study or the extent of the delay in our planned submission of an NDA for Pruvel. We currently hold rights to commercialize Pruvel in the United States.
We believe that Pruvel will be a differentiated therapeutic option for infectious diarrhea due to its broad and potent activity against gastrointestinal pathogens, favorable safety profile, clinical efficacy and convenient dosing regimen.
In June 2004, we acquired from Nippon Shinyaku Co., Ltd., or Nippon Shinyaku, the exclusive rights to develop and commercialize Pruvel for all indications in the United States. Pruvel has been marketed by other companies in Japan since 2002 to treat a wide range of bacterial infections, including infectious diarrhea, and in Italy since 2004 to treat urinary tract infections, or UTIs, and respiratory tract infections, or RTIs. Other parties have found that prulifloxacin is well-tolerated, as demonstrated by its use in the treatment of more than four million patients. A 1996 investigator-initiated clinical study of prulifloxacin in Japan by a third party for the treatment of infectious diarrhea evaluated the safety and efficacy of prulifloxacin in 122 subjects, with an endpoint of clinical cure, as evidenced by eradication of bacterial pathogens. Prulifloxacin was considered effective in approximately 98% of the 54 subjects evaluated for clinical cure. Prulifloxacin also eradicated the bacterial pathogen in approximately 95% of the 77 subjects evaluated for bacteriological effect. One hundred eight of the 109 subjects evaluated for safety had no adverse effects while one subject experienced a mild rash that was possibly related to prulifloxacin administration, but quickly recovered and continued to receive all scheduled therapy.
Infectious Diarrhea. Infectious diarrhea is associated with an infection caused by bacteria, viruses or parasites. Its symptoms include stomach cramps, vomiting, nausea, fever and headache. Infectious diarrhea is the world’s second-leading cause of morbidity and mortality. It is a significant problem even in the United States where it is often found in otherwise healthy individuals.
Travelers’ diarrhea is infectious diarrhea contracted by the ingestion of contaminated food or water. The CDC estimates that there are approximately 50,000 cases of travelers’ diarrhea each day among the 50 million worldwide annual travelers to developing countries. We estimate that approximately 23 million patients are treated with antibiotics for infectious diarrhea annually in the United States. Bacteria cause approximately 85% of travelers’ diarrhea in most localities, and the majority of these cases involve E. coli, Shigella or Salmonella. Severe infections can cause large fluid loss and result in dehydration and hospitalization. The CDC estimates that 20% to 50% of travelers to high-risk regions (including most of Asia, the Middle East, Africa, Central America and South America) will develop travelers’ diarrhea during a one- to two-week visit. The risk of infection increases with the duration of travel, and infection is possible throughout the world. A study of Americans visiting developing countries found that 46% acquired diarrhea.
Current Treatments and Limitations. Authorities such as the Infectious Disease Society of America and the CDC recommend treatment for travelers’ diarrhea with an antibiotic that has an appropriate spectrum of activity against typical pathogens related to travelers’ diarrhea. These antibiotics include fluoroquinolones such as ciprofloxacin, macrolides such as azithromycin, sulfonamides such as trimethoprim-sulfamethoxazole, or TMP/SMX, tetracyclines such as doxycycline, and rifamycins such as rifaximin. Fluoroquinolones remain the first-line treatment for infectious diarrhea because of their bactericidal nature, broad spectrum of activity and generally well-tolerated profile.
Many of the treatments for travelers’ diarrhea have significant limitations. Limitations of ciprofloxacin, rifaximin and TMP/SMX, three of the most commonly prescribed treatments for infectious diarrhea, include one or more of the following:
· Limited Spectrum of Activity and Antimicrobial Resistance. Rifaximin is approved only for the treatment of travelers’ diarrhea caused by noninvasive strains of E. coli. Rifaximin is not recommended for the treatment of diarrhea caused by other pathogens commonly associated with travelers’ diarrhea such as Shigella, Salmonella, Aeromonas, Campylobacter, Plesiomonas, and Yersinia. In addition, our studies with a panel of 582 infectious diarrhea-associated bacteria have shown that 25% of E. coli and 67% of Shigella strains associated with travelers’ diarrhea are resistant to TMP/SMX.
· Possible Side Effects. Ciprofloxacin has been associated with phototoxicity and QT interval prolongation, a condition that is associated with potentially life-threatening cardiac arrhythmias. Rifaximin has been linked to allergic reactions to the drug. TMP/SMX has been associated with both frequent mild allergic reactions and rare but serious adverse effects including bone marrow suppression, severe liver damage, severe renal impairment and agranulocytosis, an acute condition related to leukopenia.
· Convenience and Compliance. Ciprofloxacin is approved as therapy for infectious diarrhea with a dosing regimen of twice daily administrations for five to seven days. Rifaximin is approved as a therapy for diarrhea caused by noninvasive E. coli and is typically given three times daily for three days. These treatment regimens may be inconvenient for traveling patients.
Pruvel Advantages. We believe that Pruvel will be a differentiated and better therapeutic course for bacterial infectious diarrhea for several reasons, including:
· Efficacy. In October 2008, positive top-line data was presented at ICAAC/IDSA from the first of two Phase 3 trials. The first trial which was conducted in Mexico and Peru, and was a randomized, double-blind placebo-controlled clinical trial in which two-thirds of the patients received prulifloxacin in 600 mg doses and one-third of the patients received a placebo. Data from this study showed that prulifloxacin met the primary endpoint of Time to Last Unformed Stool, or TLUS, in both the modified intent-to treat, or mITT (n=187) and microbiologically evaluable (per protocol; n=165) populations compared to placebo. The median TLUS for patients treated with prulifloxacin was approximately 24 hours; this was significantly different from the TLUS for placebo with a p-value of <0.0001.
In February 2009, we reported the top-line analysis of data from the second of two Phase 3 trials. The second trial was also a randomized, double-blind placebo-controlled clinical trial in which half of the patients received prulifloxacin in 600 mg doses and half of the patients received placebo. The second trial was conducted in India, Guatemala and Mexico. Data from this study showed that prulifloxacin met the objective of superiority to placebo in the resolution of diarrhea, measured by TLUS in Both the mITT (n=200) and microbiologically evaluable (per protocol; n=173 populations compared to placebo. The median TLUS for patients treated with prulifloxacin was 32.8 hours; this was significantly different from the TLUS for placebo with a p-value of <0.0001.
· Unique Pharmacokinetic Profile. Ulifloxacin, the active metabolite of Pruvel, quickly reached effective concentration levels following a single administration of the drug in patients with profuse diarrhea. Approximately two-thirds of the oral dose of Pruvel remains in the stool after being converted to ulifloxacin while approximately one-third is absorbed and accumulated in tissues and in phagocytes, the white blood cells which engulf bacterial pathogens. Ulifloxacin
remains active in these tissues and available in the body to eliminate invasive and intracellular pathogens such as Shigella and Salmonella and invasive forms of E. coli.
· Spectrum of Activity. Ulifloxacin has more potent antibacterial activity relative to other antibacterial agents against infectious diarrhea pathogens. In a study published in Antimicrobial Agents and Chemotherapy, ulifloxacin was the most active of nine antibacterial agents tested against a panel of 582 international infectious diarrhea-associated bacteria. The potency of six of these agents against common bacterial pathogens that cause diarrhea is shown below, normalized to the potency of rifaximin:
|
|
|
Comparative Potency Against Bacteria(1) |
| ||||
|
Antibacterial |
|
E. coli |
|
Salmonella |
|
Shigella |
|
|
Ulifloxacin |
|
2,000 |
|
533 |
|
2,000 |
|
|
Ciprofloxacin |
|
1,000 |
|
133 |
|
1,000 |
|
|
Azithromycin |
|
4 |
|
4 |
|
4 |
|
|
Rifaximin |
|
1 |
|
1 |
|
1 |
|
|
Doxycycline |
|
1 |
|
0.5 |
|
0.5 |
|
|
TMP/SMX |
|
<0.25 |
|
>32 |
|
<0.25 |
|
(1) The potency of an antibiotic normalized to rifaximin is expressed as the quotient obtained by dividing the MIC90 concentration of rifaximin by the MIC90 concentration of that antibiotic.
· Side Effects. Prulifloxacin has an established and favorable safety profile with minimal potential to produce adverse side effects associated with other treatments for travelers’ diarrhea, such as QT interval prolongation, phototoxicity, or central nervous system effects. In the first phase 3 trial, Pruvel was generally well tolerated and had a similar safety profile compared to placebo.
· Convenience and Compliance. If approved, we expect Pruvel will be marketed as therapy for infectious diarrhea with a convenient dosing regimen of one tablet daily for three days.
On-Going Clinical Development and Next Steps. We conducted two Phase 3 clinical trials for the registration of Pruvel in the United States for the treatment of bacterial infectious diarrhea.
· Phase 3 Trials. In July 2008, we reported positive top-line data from the first of two Phase 3 trials. The first trial which was conducted in the United States, Mexico and Peru, was initiated in July 2006 and was a randomized, double-blind placebo-controlled clinical trial in which two-thirds of the patients received prulifloxacin in 600 mg doses and one-third of the patients received a placebo. The top-line analysis of data from this study showed that prulifloxacin met the primary endpoint of Time to Last Unformed Stool, or TLUS, in both the modified intent-to treat, or mITT (n=187) and microbiologically evaluable (per protocol; n=165) populations compared to placebo. The median TLUS for patients treated with Pruvel was approximately 24 hours; this was significantly different from the TLUS for placebo with a p-value of <0.0001. Pruvel was generally well tolerated and had a similar safety profile compared to placebo.
In February 2009, we reported the top-line analysis of data from the second of two Phase 3 trials. The second trial was also a randomized, double-blind placebo-controlled clinical trial in which half of the patients received prulifloxacin in 600 mg doses and half of the patients received placebo. The primary endpoint of both trials is TLUS. Secondary endpoints include microbiological eradication of the disease pathogen and relief of other disease symptoms. Data from this study showed that Pruvel met the objective of superiority to placebo in the resolution of diarrhea, measured by TLUS in both the mITT (n=200) and microbiologically evaluable (per protocol; n=173 populations compared to placebo. The median TLUS for patients treated with Pruvel was 32.8 hours; this was significantly different from the TLUS for placebo with a p-value of <0.0001.
On November 9, 2010, due to a higher than expected incidence of cutaneous rash during the course of a study of the possible interaction between Pruvel and antacids, we informed the FDA that we were voluntarily terminating the research study. All reported events of cutaneous rash from the drug interaction study were mild or moderate in severity and required little or no treatment and all resolved completely. We are conducting an investigation into the cause of the rash prior to initiating any new study with Pruvel. Rashes are a known and infrequent side effect of fluoroquinolone antibiotics, such as Pruvel, and, in both of our previous Pruvel Phase 3 clinical trials, rashes occurred at or below the rate generally expected for other fluoroquinolones. Pending the results of our investigation, we are not currently able to estimate when we will initiate any new study or the extent of the delay in our planned submission of an NDA for Pruvel. We currently hold rights to commercialize Pruvel in the United States.
OPopS Drug Discovery
Our OPopS drug discovery platform enables the rapid synthesis of a wide array of carbohydrate containing compounds, from small molecules, to peptides, to complex oligosaccharides, large molecules containing a small number of simple sugars. We acquired worldwide rights to this technology from the Scripps Research Institute, or TSRI, in July 1999. We have built approximately 500 carbohydrate building blocks, and are able to rapidly and reliably produce a wide variety of carbohydrate-based molecules. Key components of our OPopS technology are:
· GlycoOptimization. This process enables the modification of a carbohydrate group on an existing drug to improve its therapeutic properties such as potency and pharmacokinetics.
· De Novo Glycosylation. This process introduces new carbohydrate groups to drug candidates and create new, patentable compounds through improvement of pharmacokinetics.
We intend to continue using our OPopS technology to develop a pipeline of promising new drug candidates for the treatment of various diseases, including infectious disease.
Other Pipeline Product Candidates
In addition to fidaxomicin and Pruvel which we are developing internally, we have also licensed rights to others to develop and commercialize our other product candidates in order to maximize their potential. These product candidates are as follows:
CEM-101 (OP-1068): Macrolide and Ketolide Antibiotics
Macrolide antibiotics have been marketed for the treatment of upper and lower respiratory tract infections. Macrolides such as erythromycin and azithromycin, and ketolides, such as telithromycin, are related classes of antibiotics which have strong gram-positive activity and inhibit bacterial growth. However, an increasing number of pathogens are now resistant to currently available macrolides and ketolides. The leading product candidate developed with our discovery technology, including glycooptimization, CEM-101 (OP-1068), was effective against these resistant bacterial strains according to a pre-clinical study conducted by the Institute for Medical Microbiology. This product candidate has shown to possess potent activity against multi-drug resistant Streptococcus pneumoniae and Streptococcus pyogenes, common RTI pathogens. The pre-clinical study also showed that CEM-101 was orally active with potent efficacy in animal models after once-a-day administration. Cempra Pharmaceuticals, Inc. or Cempra, has licensed from us a library of approximately 500 macrolides related to this product candidate. Cempra has completed a Phase 1 clinical trial with CEM-101 and has announced preparations for a Phase 2 trial in moderate to severe community-acquired bacterial pneumonia.
OPT-822/821: A Cancer Immunotherapy.
Overview. In October 2009, we entered into a number of transactions involving OBI, in which we sold 40% of our existing equity in OBI. We assigned to OBI certain of our patent rights and know-how related to OPT-822/821, a novel carbohydrate-based cancer immunotherapy which we licensed from MSKCC. We also assigned to OBI our rights and obligations under the related license agreement with MSKCC.
OBI is developing OPT-822/821 for the treatment of metastatic breast cancer. According to the American Cancer Society, breast cancer was the second most common form of cancer among women in the United States, with more than 250,000 new cases and more than 40,000 deaths in 2008. The survival rate for patients with metastatic breast cancer remains limited, with a median survival of two to three years and a five-year survival rate of 21% for those patients diagnosed with late-stage cancer that has metastasized to other parts of the body. In July 2002, we acquired exclusive rights from MSKCC to develop and commercialize OPT-822/821 worldwide. Carbohydrate antigens are known to stimulate the immune response against cancer cells in the body. We have applied our OPopS technology to manufacture effectively complex carbohydrate cancer antigens, including Globo-H, a prominent antigen in breast cancer cells, and sialyl Lewis a, an antigen in breast and small lung cancer cells. OPT-822/821 is a novel cancer immunotherapy and is composed of Globo H linked to a protein carrier.
MSKCC completed Phase 1 safety studies of OPT-822/821 in prostate cancer patients and breast cancer patients in 1999 and 2001, respectively. In these studies, OPT-822/821 appeared to be well tolerated and to stimulate responses to tumor antigens. Twenty-one of 27 metastatic breast cancer patients treated with OPT-822/821 in the study survived after four years, with 48% of patients surviving more than nine years following completion of the Phase 1 safety study.
In January 2011, OBI initiated a Phase 2/3 clinical trial of OPT-822/821. This study, referenced as OPT-822-001, is designed as a multi-center trial with sites in Taiwan, South Korea, Hong Kong and Singapore. The Phase 2/3 clinical trial is expected to enroll up to 342 patients. The primary endpoint is the progression free survival rate from the time of randomization until disease progression. The secondary endpoint is the overall survival rate from the time of enrollment. Patients will receive a total of up to nine injections of OPT-822/821 plus Cyclophosphamide, or placebo plus Cyclophosphamide (2:1 randomization) over a treatment period of nine months with study follow-up at eight week intervals until disease progression or up to two years, as well as survival follow-up until five years from randomization.
In February 2011, pursuant to an amendment to an October 2009 financing agreement, OBI sold newly-issued shares of its common stock for gross proceeds of approximately 462.0 million New Taiwan Dollars (approximately $15.5 million based on then-current exchange rates). We purchased 277.2 million New Taiwan Dollars (approximately $9.3 million based on then-current exchange rates) of the shares issued in the financing, such that we currently maintain a 60% equity interest in OBI.
Our Strategy
Our principal objective is to become a leading biopharmaceutical company focused on the discovery, development and commercialization of innovative hospital specialty products, with an initial focus on gastrointestinal infections and related diseases. To achieve these objectives, our strategy includes the following key elements:
· Build a branded anti-infective franchise through current and in-licensed product candidates. We currently have two late-stage antibiotic product candidates, fidaxomicin for the treatment of CDI, and Pruvel for the treatment of infectious diarrhea. We also intend to develop fidaxomicin for additional indications and selectively in-license additional hospital specialty products for development and/or commercialization. In addition, in order to maximize the value of our franchise, we intend to opportunistically seek partners to commercialize our product candidates outside of our core markets, such as our recent collaboration with Astellas for fidaxomicin. We believe our management’s industry knowledge and contacts will be a significant advantage in executing this part of our strategy.
· Develop our lead product candidates for clinical and regulatory approval. We are currently focusing our resources on developing fidaxomicin and preparing for its potential commercial launch in the United States if approved by the FDA, as well as completing the development of Pruvel and submitting an NDA to the FDA. We have filed our NDA for fidaxomicin and have received a priority review with a PDUFA goal date of May 30, 2011. We are currently evaluating our strategic alternatives regarding Pruvel.
· Build marketing and sales capabilities in our core markets. Our objective is to market innovative hospital specialty products in areas of unmet medical needs. Specifically, we initially plan to develop and commercialize fidaxomicin in the United States, if approved. In order to achieve this goal, we intend to develop our own marketing organization and sales force in the United States. We also plan to evaluate partnering alternatives to commercialize our product candidates where appropriate.
· Leverage our internal discovery capabilities and expertise in carbohydrate chemistry to expand our portfolio of product candidates. We intend to expand our product portfolio by exploiting our internal expertise to discover and develop additional product candidates. We believe our proprietary technology and our capabilities and expertise in carbohydrate chemistry will enable us to more rapidly identify and develop successful product candidates. We may opportunistically seek partners for the development and commercialization of product candidates in order to maximize value and maintain our strategic focus.
Marketing and Sales
We recently hired key personnel to prepare for our potential commercialization of fidaxomicin but currently have a limited marketing organization and do not have a sales organization for the sale and distribution of pharmaceutical products. We plan to develop a domestic sales organization for fidaxomicin internally and/or through collaborations with third parties. We have entered into a collaboration and license agreement with Astellas for the Astellas territory. We hold rights to fidaxomicin in all other regions of the world. We also plan to seek collaborations with one or more third parties for the commercialization of fidaxomicin in other regions of the world such as Japan and China. In the United States, we plan to target our marketing and sales of fidaxomicin to hospital-based and long-term care physicians, including gastroenterologists, infectious disease specialists and internists. We plan to evaluate our commercialization options for Pruvel, for which we hold commercialization rights in the United States. We expect that if approved, Pruvel would be marketed to high-prescribing physicians of antibiotics for infectious diarrhea.
We have also established a medical affairs group to introduce our product candidates to key opinion leaders in CDI and healthcare professionals focusing on infectious diseases and gastroenterology.
Collaborations, Commercial and License Agreements and Grants
Astellas Pharma Europe Ltd. In February 2011, we entered into a collaboration and license agreement with Astellas pursuant to which we granted to Astellas an exclusive, royalty-bearing license under certain of our know-how and intellectual property to develop and commercialize fidaxomicin in the Astellas territory. Under the terms of the agreement, Astellas has agreed to use commercially reasonable efforts to develop and commercialize fidaxomicin in the Astellas territory at its expense, and is obligated to achieve certain additional regulatory and commercial diligence milestones with respect to fidaxomicin in the Astellas territory. We and Astellas may also agree to collaborate in, and share data resulting from, global development activities with respect to fidaxomicin, in which case we and Astellas will be obligated to co-fund such activities. In addition, under the terms of the agreement, Astellas granted us an exclusive, royalty-free license under know-how and intellectual property generated by Astellas and its sublicensees in the course of developing fidaxomicin and controlled by Astellas or its affiliates for use by us and any of our sublicensees in the development and commercialization of fidaxomicin outside the Astellas territory and, following termination of the agreement and subject to payment by us of single-digit royalties, in the Astellas territory. In addition, under the terms of a supply agreement entered into by us and Astellas on the same date, we will be the exclusive supplier of fidaxomicin to Astellas for Astellas’ development and commercialization activities in the Astellas territory during the term of the supply agreement, and Astellas is obligated to pay us an amount equal to cost plus an agreed mark-up for such supply.
Under the terms of the license agreement with Astellas, Astellas paid to us an upfront fee equal to 50.0 million Euros, or approximately $69.2 million, and we are eligible to receive additional cash payments totaling up to 115.0 million Euros upon the achievement by Astellas of specified regulatory and commercial milestones. In addition, we will be entitled to receive escalating double-digit royalties ranging from the high teens to low twenties on net sales of fidaxomicin products in the Astellas territory, which royalties are subject to reduction in certain, limited circumstances. Such royalties will be payable by Astellas on a product-by-product and country-by-country basis until a generic product accounts for a specified market share of the applicable fidaxomicin product in the applicable country.
The agreements with Astellas will continue in effect on a product-by-product and country-by-country basis until expiration of Astellas’ obligation to pay royalties with respect to each fidaxomicin product in each country in the Astellas territory, unless terminated early by either party as more fully described below. Following expiration, Astellas’ license to develop and commercialize the applicable fidaxomicin product in the applicable country will become non-exclusive. We and Astellas may each terminate either of the agreements prior to expiration upon the material breach of such agreement by the other party, or upon the bankruptcy or insolvency of the other party. In addition, we may terminate the agreements prior to expiration in the event Astellas or any of its affiliates or sublicensees commences an interference or opposition proceeding with respect to, challenges the validity or enforceability of, or opposes any extension of or the grant of a supplementary protection certificate with respect to, any patent licensed to it, and Astellas may terminate the agreements prior to expiration for any reason on a product-by-product and country-by-country basis upon 180 days’ prior written notice to us. Upon any such termination, the license granted to Astellas (in total or with respect to the terminated product or terminated country, as applicable) will terminate and revert to us.
Par Pharmaceutical, Inc. In February 2007, we repurchased the right to develop and commercialize fidaxomicin in North America and Israel from Par under a prospective buy-back agreement. As a result of the prospective buy-back agreement, we hold worldwide rights to fidaxomicin. We paid Par a one-time $5.0 million milestone payment in June 2010 for the successful completion of our second pivotal Phase 3 trial for fidaxomicin. We may be obligated to pay Par a 5% royalty on net sales by us or our affiliates of fidaxomicin in North America and Israel, and a 1.5% royalty on net sales by us or our affiliates of fidaxomicin in the rest of the world. In addition, we are required to pay Par a 6.25% royalty on net revenues we receive from licensees of our right to market fidaxomicin in the rest of the world. We are obligated to pay each of these royalties, if any, on a country-by-country basis for seven years commencing on the applicable commercial launch in each such country. The agreement also includes a mutual release between the parties and our indemnification of Par for actions related to fidaxomicin, the agreements assigned to us by Par and certain other matters.
Biocon Limited. In May 2010, we entered into a long-term supply agreement with Biocon, for the commercial manufacture of fidaxomicin’s active pharmaceutical ingredient, or API. Pursuant to the agreement, Biocon agreed to manufacture and supply to us, up to certain limits, fidaxomicin API and, subject to certain conditions, we agreed to purchase from Biocon at least a portion of its requirements for fidaxomicin API in the United States and Canada. We previously paid to Biocon $2.5 million for certain equipment purchases and manufacturing scale-up activities, and we may be entitled to recover up to $1.5 million of this amount under the supply agreement in the form of discounted prices for fidaxomicin API. We expensed the entire $2.5 million in the second quarter of 2009 because at the time of payment the recovery of up to $1.5 million could not be assured. We may be obligated to make additional payments to Biocon if it fails to meet the minimum purchase requirements after Biocon has dedicated certain manufacturing capacity to the production of fidaxomicin API and if Biocon is unable to manufacture alternative products with the dedicated capacity. Unless both we and Biocon agree to extend the term of the supply agreement, it will terminate seven and a half years from the date we obtain
marketing authorization for fidaxomicin in the United States or Canada. The supply agreement will be terminated earlier in the event that we do not obtain marketing authorization for fidaxomicin in the United States or Canada prior to December 31, 2013. In addition, the supply agreement may be earlier terminated (i) by either party by giving two and a half years notice after the fifth anniversary of the Effective Date or upon a material breach of the supply agreement by the other party, (ii) by us upon the occurrence of certain events, including Biocon’s failure to supply requested amounts of fidaxomicin API, or (iii) by Biocon upon the occurrence of certain events, including our failure to purchase amounts of fidaxomicin API indicated in binding forecasts. The supply agreement terminated and superseded the prior supply agreement with Biocon, dated August 29, 2005, which was assigned to the Company by Par in February 2007.
Nippon Shinyaku, Co., Ltd. In June 2004, we entered into a license agreement with Nippon Shinyaku. Under the terms of the agreement, we acquired the non-exclusive right to import and purchase Pruvel, and the exclusive right (with the right to sublicense) within the United States to develop, make, use, offer to sell, sell and license products suitable for consumption by humans containing Pruvel. Under this agreement, we paid Nippon Shinyaku a $1.0 million upfront licensing fee and will be required to pay Nippon Shinyaku a milestone payment in the amount of $1.0 million upon the submission of a NDA for Pruvel in the United States. We also agreed to exclusively purchase Pruvel from Nippon Shinyaku and to purchase a certain amount of Pruvel annually that is to be mutually agreed upon by us and Nippon Shinyaku, commencing in the year of the first commercial sale of Pruvel in the United States. If Nippon Shinyaku is unable to supply us with the required amount of Pruvel, then Nippon Shinyaku is obligated to grant us a non-exclusive, worldwide license to make or have made Pruvel, in which event we will owe Nippon Shinyaku a royalty based on the amount of net sales of Pruvel generated by us and our sublicensees. Additionally, we will owe Nippon Shinyaku certain royalties based on the amount of net sales of Pruvel less the amount of Pruvel we buy from Nippon Shinyaku.
Either party may terminate the agreement 60 days after giving notice of a material breach which remains uncured 60 days after written notice. If not terminated earlier, the agreement will terminate upon the later of ten years from the date of the first commercial sale of Pruvel in the United States or the date on which the last valid patent claim relating to Pruvel expires in the United States.
Cempra Pharmaceuticals, Inc. In March 2006, we entered into a collaborative research and development and license agreement with Cempra, a biotechnology company focused on anti-infectives. We are collaborating with Cempra to discover, develop and commercialize drugs based on macrolide and ketolide compounds. We granted to Cempra an exclusive worldwide license, except in Association of Southeast Asian Nations, or ASEAN, countries as of the effective date of the agreement, with the right to sublicense, our patent and know-how related to our macrolide and ketolide antibacterial program, several other pre-clinical compounds and our related proprietary technology. Cempra is responsible for many of the costs associated with the development and commercialization of product candidates arising under the agreement, including manufacturing, marketing and sales costs. As partial consideration for granting Cempra the license, we obtained equity of Cempra and we assigned no value to such equity. Pursuant to the terms of the agreement, in July 2010, we received a $500,000 milestone payment form Cempra for the continuing development of a next generation macrolide, CEM 101, for the treatment of respiratory infections. We may receive additional milestone payments as product candidates are developed and/or co-developed by Cempra, in addition to milestone payments based on certain sublicense revenue. The aggregate potential amount is not capped and, based in part on the number of products developed under the agreement may exceed $24.5 million. We will also be entitled to receive royalty payments based on a percentage of net sales of licensed products. The milestone payments will be triggered upon the completion of certain clinical development milestones and in certain instances, regulatory approval of products. In consideration of the foregoing, Cempra will be entitled to receive milestone payments of $1.0 million from us for each of the first two products we develop which receive regulatory approval in ASEAN countries as well as royalty payments on the net sales of such products.
The research term of this agreement was completed on March 31, 2008. Subject to certain exceptions, on a country-by-country basis, the general terms of this agreement continue until the later of: (i) the expiration of the last to expire patent rights related to a covered product in the applicable country or (ii) ten years from the first commercial sale of a covered product in the applicable country. Either party may terminate the agreement in the event of a material breach by the other party, subject to prior notice and the opportunity to cure. Either party may also terminate the agreement for any reason upon 30 days’ prior written notice provided that all licenses granted by the terminating party to the non-terminating party will survive upon the express election of the non-terminating party.
OBI. In October 2009, we entered into certain transactions involving OBI, then our wholly-owned subsidiary, to provide funding for the development of two of our early-stage, non-core programs. The transactions with OBI included an Intellectual Property Assignment and License Agreement, pursuant to which we assigned to OBI certain patent rights, information and know-how related to OPT-88 and OPT-822/821. In anticipation of these transactions, we also assigned, and OBI assumed, our rights and obligations under related license agreements with MSKCC and TSRI. Under this agreement, we are eligible to receive up to $10 million in milestone payments for each product developed under the development programs and are also eligible to receive royalties on net sales of any product which is commercialized under the programs. The term of the Intellectual Property Assignment and License Agreement continues until the last to expire of the patents assigned by us to OBI and the patents licensed to OBI under the TSRI and MSKCC agreements. After further evaluation, OBI determined not to pursue additional development of OPT-88 and in February 2011, OBI and TSRI agreed to terminate the license agreement and OBI returned the related OPT-88 patents to TSRI.
To provide capital for OBI’s product development efforts, we and OBI also entered into a financing agreement with a group of new investors. Simultaneously, we sold 40 percent of our existing OBI shares to the same group of new investors, and we and the new investors also purchased new OBI shares. The financing agreement also contemplated an additional financing pursuant to which we and the new investors would invest approximately an additional $184.8 million New Taiwan Dollars and $277.2 million New Taiwan Dollars, respectively, in exchange for new OBI shares. In February 2011, pursuant to an amendment to the October 2009 financing agreement, OBI completed the second financing and sold newly-issued shares of its common stock for gross proceeds of approximately 462.0 million New Taiwan Dollars (approximately $15.5 million based on then-current exchange rates). We purchased 277.2 million New Taiwan Dollars (approximately $9.3 million based on then-current exchange rates) of the shares issued in the second financing, such that we currently maintain a 60% equity interest in OBI.
NIH Small Business Innovation Research Award. We have one active grant from the National Institute of Allergy and Infectious Diseases, or NIAID. This $3 million grant was awarded in September 2007 for three years, and was subsequently extended to August 2011. The award is used to conduct supplementary studies with fidaxomicin to confirm a narrow spectrum activity and potency of fidaxomicin against hyper-virulent epidemic strains, to support additional toxicology and microbiological studies to demonstrate the safety and efficacy of the fidaxomicin compound and its major metabolite in CDI patients and to support a surveillance study of C. difficile isolates across North America to compare the activity of fidaxomicin with existing CDI treatments.
Qualifying Therapeutic Discovery Project Grant. In November 2010, we received $244,000 in the form of a cash grant from the Qualifying Therapeutic Discovery Project program of the Internal Revenue Service (IRS) / the U.S. Treasury Department for expenditures related to our fidaxomicin development program. We had to meet eligible criteria defined by the guidelines of the Patient Protection and Affordable Care Act signed into law March 23, 2010 to qualify for the cash grant.
Memorial Sloan-Kettering Cancer Center. In July 2002, we entered into a license agreement with MSKCC, to acquire, together with certain non-exclusive licenses, exclusive, worldwide licensing and sublicensing rights to certain patented and patent-pending carbohydrate-based cancer immunotherapies, which includes OPT-822. As partial consideration for the licensing rights, we paid to MSKCC a one-time fee consisting of both cash and 55,383 shares of our common stock. In anticipation of the various transactions involving OBI which we completed in October 2009, we assigned our rights and obligations under this agreement to OBI. Under the agreement, which was amended in June 2005, OBI may owe MSKCC milestone payments in the following amounts for each licensed product: (i) $500,000 upon the commencement of Phase 3 clinical studies, (ii) $750,000 upon the submission of the first NDA, (iii) $1.5 million upon marketing approval in the United States and (iv) $1.0 million upon marketing approval in each and any of Japan and certain European countries, but only to the extent that we, and not a sublicensee, achieve such milestones. OBI may owe MSKCC royalties based on net sales generated from the licensed products and income OBI sources from its sublicensing activities, which royalty payments are credited against a minimum annual royalty payment OBI owes to MSKCC during the term of the agreement.
The Scripps Research Institute. In July 1999, we acquired exclusive, worldwide rights to our OPopS technology from TSRI. This agreement includes the license to us of patents, patent applications and copyrights related to our OPopS technology. We also acquired, pursuant to three separate license agreements with TSRI, exclusive, worldwide rights to over 20 TSRI patents and patent applications related to other potential drug compounds and technologies, including HIV/FIV protease inhibitors, aminoglycoside antibiotics, polysialytransferase, selectin inhibitors, nucleic acid binders, carbohydrate mimetics and osteoarthritis.
Under the four agreements with TSRI, we paid TSRI license fees consisting of an aggregate of 239,996 shares of our common stock with a deemed aggregate fair market value of $46,400, as determined on the dates of each such payment. In October 2009, we assigned to OBI one of the agreements with TSRI related to OPT-88 in which after further evaluation, OBI decided not to pursue. In February 2011, the license agreement related to OPT-88 was terminated and OBI returned the patents related to OPT-88. Under each of the three remaining agreements, we owe TSRI royalties based on net sales by us, our affiliates and sublicensees of the covered products and royalties based on revenue we generate from sublicenses granted pursuant to the agreements. For the first licensed product under each of the three agreements, we also will owe TSRI payments upon achievement of certain milestones. In two of the three TSRI agreements, the milestones are the successful completion of a Phase 2 trial or its foreign equivalent, the submission of an NDA or its foreign equivalent and government marketing and distribution approval. In the remaining TSRI agreement, the milestones are the initiation of a Phase 3 trial or its foreign equivalent, the submission of an NDA or its foreign equivalent and government marketing and distribution approval. The aggregate potential amount of milestone payments we may be required to pay TSRI under the remaining TSRI agreements is approximately $11.1 million.
Manufacturing
We rely on third parties to manufacture our product candidates and currently have no plans to develop our own manufacturing facility. We require in our manufacturing and processing agreements that all third-party contract manufacturers and processors produce APIs and finished products in accordance with current Good Manufacturing Practices, or cGMP, and all other applicable laws and regulations. We maintain confidentiality agreements with potential and existing manufacturers in order to protect our proprietary rights related to our drug candidates.
In May 2010, we entered into a long-term supply agreement with Biocon for the commercial manufacture of fidaxomicin’s API. We contracted with Patheon Inc. to manufacture the drug product supplies used to complete our Phase 3 clinical trials for fidaxomicin. The manufacturing facilities of Biocon and Patheon have been approved by the FDA for other companies’ drug products, however, neither Biocon’s nor Patheon’s facilities have yet been approved by the FDA for the manufacture of our fidaxomicin drug supplies. We may contract with other third-party contract manufacturers for additional commercial supply of fidaxomicin API and fidaxomicin drug product for commercial sale.
In June 2004, as part of our license agreement for exclusive rights to develop and commercialize Pruvel in the United States, we entered into a supply agreement with Nippon Shinyaku for the manufacture and supply of the API for Pruvel. In turn, Nippon Shinyaku contracts with Juzen Chemical Co. for the manufacture of the API for Pruvel. The tablets used in our Phase 3 clinical trials for Pruvel were manufactured by Angelini ACRAF, or Angelini. We have also contracted with Patheon for development services related to Pruvel tablet manufacturing. We are in discussion with Angelini, Patheon and other contract manufacturers for the manufacturing, packaging and labeling of Pruvel for commercial sale in the United States. The manufacturing facilities of Juzen and of Patheon have been approved by the FDA for other companies’ drug products, however, neither Juzen’s nor Patheon’s facilities have been approved for the manufacture of our Pruvel drug supplies. Angelini’s facilities have not been inspected or approved by the FDA for the manufacture of any drug.
We have used both in-house capabilities and outside third-party cGMP manufacturers for the preparation of compounds for pre-clinical development and for the manufacture of limited quantities of finished products for clinical development. We have developed a proprietary synthetic process in our laboratories for Globo-H, the carbohydrate portion of OPT-822. Third parties with cGMP facilities have manufactured OPT-822 for clinical trials. We also expect that OBI will use third-party cGMP manufacturers for the production of the adjuvant, OPT-821.
Intellectual Property
The proprietary nature of, and protection for, our products, product candidates, processes and know-how are important to our business. We seek patent protection in the United States and internationally for our product candidates and other technology where available and when appropriate. Our policy is to patent or in-license the technology, inventions and improvements that we consider important to the development of our business. In addition, we use license agreements to selectively convey to others, rights to our own intellectual property. We also rely on trade secrets, know-how and continuing innovation to develop and maintain our competitive position. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our technology. For this and more comprehensive risks related to our intellectual property, please see “Risk Factors — Risks Related to Our Intellectual Property.”
We have established and continue to build proprietary positions for our pipeline product candidates and technology in the United States and abroad. We have built a portfolio of more than 100 patents and patent applications that we either own or have licensed around our key products and technologies. As of February 1, 2011, this portfolio included four issued U.S. patents and 14 pending U.S. patent applications. Foreign counterparts to these included seven issued patents and 102 pending patent applications. Of the more than 100 patents and patent applications that we own or license, as of February 1, 2011, 39 were owned or licensed by OBI. In addition to the patents and patent applications mentioned above, we have licensed U.S. and foreign patents and patent applications related to our proprietary OPopS drug discovery platform.
For our lead product candidate fidaxomicin, we have three issued U.S. patents and ten U.S. pending patent applications, one of which is allowed. We also have five issued foreign patents and 69 pending foreign counterparts in Australia, Canada, China, Europe, Hong Kong, Israel, Japan, South Korea, India, New Zealand, Taiwan, South Africa, Russian Federation, Mexico and Brazil. The issued patents cover a specific polymorphic form and specific manufacturing methods and expire in 2027 and 2023, respectively. The pending patent applications, if issued, may cover the composition of matter, an additional polymorphic form, methods of use and pharmaceutical formulations containing the various components and would expire between 2023 and 2029. For our other product candidate Pruvel, we have licensed one issued U.S. patent from Nippon Shinyaku which covers the compound prulifloxacin; however, this patent expired in February 2009. Another U.S. patent covering the novel polymorphic form of the key intermediate in the manufacturing process was issued in 2009 and will expire in 2023. The remainder of our patents and patent applications, and licensed patents and patent applications, relate to our other products and technology, and expire between 2015 and 2023.
Government Regulation and Product Approval
FDA Approval Process
Regulation by governmental authorities in the United States and other countries is a significant factor in the development, manufacture and marketing of pharmaceuticals and antibiotics. All of our products will require regulatory approval by governmental agencies prior to commercialization. In particular, pharmaceutical drugs are subject to rigorous pre-clinical testing and clinical trials and other premarketing approval requirements by the FDA and regulatory authorities in other countries. In the United States, various federal, and, in some cases, state statutes and regulations, also govern or impact the manufacturing, safety, labeling, storage, record-keeping and marketing of pharmaceutical products. The lengthy process of seeking required approvals and the continuing need for compliance with applicable statutes and regulations require the expenditure of substantial resources. Regulatory approval, if and when obtained for any of our product candidates, may be limited in scope, which may significantly limit the indicated uses for which our product candidates may be marketed. Furthermore, approved drugs and manufacturers are subject to ongoing review and discovery of previously unknown problems may result in restrictions on their manufacture, sale or use or in their withdrawal from the market.
Before testing any compounds with potential therapeutic value in human subjects in the United States, we must satisfy stringent government requirements for pre-clinical studies. Pre-clinical testing includes both in vitro and in vivo laboratory evaluation and characterization of the safety and efficacy of a drug and its formulation. Pre-clinical testing results obtained from studies in several animal species, as well as data from in vitro studies, are submitted to the FDA as part of an IND and are reviewed by the FDA prior to the commencement of human clinical trials. These pre-clinical data must provide an adequate basis for evaluating both the safety and the scientific rationale for the initial trials in human volunteers. In order to test a new drug in humans in the United States, an IND must be submitted to the FDA. The IND will become effective automatically 30 days after receipt by the FDA, unless the FDA raises concern or questions significant enough to merit a clinical hold, in which case, the IND sponsor and the FDA must resolve any outstanding concerns before a hold is lifted and clinical trials can proceed.
Clinical trials are typically conducted in three sequential phases, Phases 1, 2 and 3, with Phase 4 trials potentially conducted after initial marketing approval. These phases may be compressed, may overlap or may be omitted in some circumstances. Certain clinical trials are required to be publicly registered, as with www.clinicaltrials.com, and their results made publicly available.
· Phase 1. Phase 1 human clinical trials evaluate a drug’s safety profile and the range of safe dosages that can be administered to healthy volunteers and/or patients, including the maximum tolerated dose that can be given to a trial subject with the target disease or condition. Phase 1 trials also determine how a drug is absorbed, distributed, metabolized and excreted by the body and the duration of its action. In some cases, we may decide to run what is referred to as a “Phase 1a” evaluation in which we administer single doses of a new drug candidate in a small group of people to evaluate its pharmacokinetic properties, safety, dose range and side effects. We may also decide to run what is referred to as a “Phase 1b” evaluation, which is a second safety-focused Phase 1 trial in which we administer a new drug candidate at its targeted dosing regimen in a small group of people to evaluate its pharmacokinetic properties, safety, dose range and side effects.
· Phase 2. Phase 2 clinical trials are typically designed to evaluate the potential effectiveness of the drug in patients and to further ascertain the safety of the drug at the dosage given in a larger patient population. In some cases, we may decide to run what is referred to as a “Phase 2a” evaluation, which is a trial to determine the ideal dosing regimen and length of treatment and to evaluate effectiveness and safety. We may also decide to conduct what is referred to as a “Phase 2b” evaluation, which is a second, confirmatory Phase 2 clinical trial in which we collect more efficacy and safety data prior to initiation of a Phase 3 clinical trial. If positive and accepted by the FDA, results from Phase 2b study can serve as a part of pivotal clinical trial in the approval of a drug candidate.
· Phase 3. In Phase 3 clinical trials, often referred to as pivotal or registration clinical trials, the drug is usually tested in one or more controlled, randomized trials comparing the investigational new drug to an approved form of therapy or placebo in an expanded and well-defined patient population at multiple clinical sites. The goal of these trials is to obtain definitive statistical evidence of safety and effectiveness of the investigational new drug regimen as compared to a placebo or an approved standard therapy in defined patient populations with a given disease and stage of illness.
· Phase 4. Phase 4 clinical trials are studies required of or agreed to by a sponsor that are conducted after the FDA has approved a product for marketing. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication. Failure to promptly conduct any mandatory Phase 4 clinical trials that are committed to as part of an NDA’s approval could result in withdrawal of approval or other legal sanction.
After completion of Phase 1, 2 and 3 clinical trials, if there is substantial evidence that the drug is safe and effective, an NDA is prepared and submitted for the FDA to review. The NDA must contain all of the essential information on the drug gathered to that date, including data from pre-clinical and clinical trials, and the content and format of an NDA must conform to all FDA regulations and guidelines. Accordingly, the preparation and submission of an NDA is a significant undertaking for a company. The FDA reviews all submitted NDAs before it accepts them for filing and may request additional information from the sponsor rather than accepting an NDA for filing. In this case, the NDA must be re-submitted with the additional information and, again, is subject to review before filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. Most NDAs are reviewed by the FDA within ten months of submission. The review process is often significantly extended by the FDA through requests for additional information and clarification. The FDA may refer the application to an appropriate advisory committee, typically a panel of clinicians, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation but typically gives it great weight. If the FDA evaluations of both the NDA and the manufacturing facilities are favorable, the FDA may issue either an approval letter or a complete response, the latter of which usually contains a number of conditions that must be satisfied in order to secure final approval.
Any products we manufacture or distribute under FDA approvals are subject to pervasive and continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the products. Drug manufacturers and their subcontractors are required to register with the FDA and, where appropriate, state agencies, and are subject to periodic unannounced inspections by the FDA and state agencies for compliance with cGMP regulations which impose procedural and documentation requirements upon us and any third party manufacturers we utilize.
The FDA closely regulates the marketing and promotion of drugs. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available drugs for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturer’s communications on the subject of off-label use and healthcare payors, including the federal government, can use the False Claims Act and related statutes to pursue drug companies for off-label promotions that result in the submission of claims for payment for uses that have not been approved by the FDA as safe and effective.
The FDA’s policies may change and additional government regulations may be enacted that could prevent or delay regulatory approval of our product candidates or approval of new indications after the initial approval of our existing product candidates. We cannot predict the likelihood, nature or extent of adverse governmental regulations that might arise from future legislative or administrative action, either in the United States or abroad.
We will also be subject to a wide variety of foreign regulations governing the development, manufacture and marketing of our products. Whether or not FDA approval has been obtained, approval of a product by the comparable regulatory authorities of foreign countries must still be obtained prior to manufacturing or marketing the product in those countries. The approval process varies from country to country and the time needed to secure approval may be longer or shorter than that required for FDA approval. We cannot assure you that clinical trials conducted in one country will be accepted by other countries or that approval in one country will result in approval in any other country.
Fast Track Product Designation, NDA Submission and PDUFA Goal Date for Fidaxomicin
The FDA has granted Fast Track status for fidaxomicin in the treatment of CDI. The FDA’s Fast Track program is intended to facilitate the development and expedite the review of drugs that are intended for the treatment of a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for their condition. Under Fast Track designation, the FDA may initiate review of sections of an NDA before the application is complete. This rolling review is available if the applicant provides a schedule for the submission of the remaining information and pays applicable user fees. However, the time period specified in the Prescription Drug User Fee Act, which governs the time period goals the FDA has committed to for reviewing an application, does not begin until the complete application has been submitted. The Fast Track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process. Participation in the Fast Track program does not eliminate any phase of clinical studies. Additionally, in some cases, a Fast Track designated product may also qualify for priority review, or review within a six-month target time frame from the time an NDA is accepted for filing. A Fast Track designated product would ordinarily meet the FDA’s criteria for priority review. We cannot guarantee any of our products will obtain priority review designation, or if a priority designation is received, that review or approval will be faster than conventional FDA procedures.
In September 2010, we began a submission to the FDA of a rolling NDA and in November 2010 we completed the submission of our NDA. In January 2011, the FDA accepted our NDA filing for the treatment of CDI and for reducing the risk of recurrence when used for treatment of initial CDI. The FDA has also granted our request for six-month priority review, and has assigned a PDUFA goal date of May 30, 2011 for its review of the NDA. The FDA also informed us that it plans to discuss the NDA at a meeting of its Anti-Infective Drugs Advisory Committee currently scheduled for April 5, 2011.
Competition
The pharmaceutical industry is highly competitive. We face significant competition from pharmaceutical companies and biotechnology companies that are researching and selling products designed to treat infectious disease. Many of these companies have significantly greater financial, manufacturing, marketing and product development resources than us. Additionally, many of these companies have substantially greater experience developing, manufacturing and commercializing drugs which may allow them to bring their products to market quicker than we can. Several pharmaceutical and biotechnology companies have already established themselves in the markets for the treatment of CDI and/or infectious diarrhea and many additional companies are currently developing products for the treatment of CDI and/or infectious diarrhea, which we expect will compete with fidaxomicin and Pruvel if approved for marketing. Potentially significant competitors to fidaxomicin and Pruvel, both currently marketed and in clinical development, include the following:
|
Product |
|
Stage of Development |
|
Company |
|
|
Fidaxomicin Competitors |
|
|
|
|
|
|
Flagyl™/metronidazole |
|
Marketed |
|
Pfizer, Sanofi-Aventis and generics |
|
|
Vancocin™/oral vancomycin |
|
Marketed |
|
Viropharma and generics |
|
|
Xifaxan™/rifaximin |
|
Phase 3 trial stopped |
|
Salix and generics |
|
|
Ramoplanin |
|
Phase 2 completed |
|
Nanotherapeutics |
|
|
ACAM-Cdiff (vaccine) |
|
Phase 2 |
|
Sanofi-Aventis |
|
|
MDX-066/ MDX-1388 (antibodies combination) |
|
Phase 2 completed |
|
Merck |
|
|
CB-183,315 |
|
Phase 2 |
|
Cubist |
|
|
|
|
|
|
|
|
|
Pruvel Competitors |
|
|
|
|
|
|
Cipro™/ciprofloxacin |
|
Marketed |
|
Bayer and generics |
|
|
Zithromax™/azithromycin |
|
Marketed |
|
Pfizer |
|
|
Xifaxan™/rifaximin |
|
Marketed |
|
Salix and generics |
|
|
Bactrim™/Septra™/TMP/SMX |
|
Marketed |
|
Roche and generics |
|
|
Vibramycin™/doxycycline |
|
Marketed |
|
Pfizer and generics |
|
|
Levaquin™/levofloxacin |
|
Marketed |
|
Johnson & Johnson |
|
|
Campy jejuni vaccine |
|
Phase 2 completed |
|
ACE BioSciences |
|
|
Rifamycin SV MMX® |
|
Phase 3 |
|
Santarus |
|
Research and Development
Our research and development efforts are primarily focused on developing fidaxomicin and Pruvel and our other product candidates. Our research and development expense was approximately $32.8 million, $33.7 million and $29.0 million in years 2010, 2009 and 2008, respectively.
Employees
As of February 28, 2011, we employed 87 persons, 23 of whom hold Ph.D., M.D. or JD degrees. Sixteen employees were engaged in discovery research, 42 in clinical research and regulatory affairs, nine in commercial and corporate development and 20 in support administration, including finance, information systems, facilities and human resources. None of our employees is subject to a collective bargaining agreement. We consider our relations with our employees to be good.
Long-Lived Assets
Information regarding long-lived assets by geographic area is as follows:
|
|
|
As of December 31, |
| |||||||
|
|
|
2010 |
|
2009 |
|
2008 |
| |||
|
|
|
(in thousands) |
| |||||||
|
United States |
|
$ |
590 |
|
$ |
644 |
|
$ |
656 |
|
|
Taiwan |
|
108 |
|
29 |
|
38 |
| |||
|
Total |
|
$ |
698 |
|
$ |
673 |
|
$ |
694 |
|
Risks Related to Our Business
We are a company with limited sources of revenue, and we are largely dependent on the success of our lead product candidate fidaxomicin and, to a lesser degree, our other lead product candidate Pruvel.
We are a biopharmaceutical company with no products approved for commercial sale and, to date, we have not generated any revenues from product sales. Our ability to generate future revenues depends heavily on our and our collaborators’ success in:
· developing and securing U.S. and foreign regulatory approvals for fidaxomicin and, to a lesser extent, Pruvel and our other product candidates; and
· commercializing, alone or with a partner, any product candidates for which we or our collaborators receive approval from the FDA and/or comparable foreign regulatory authorities.
Our ability to generate and sustain revenues in the long term will also depend on our success in generating a pipeline of innovative product candidates utilizing our drug discovery platform or through licensing strategies.
Our product candidates will generally require extensive clinical study and evaluation, regulatory approval in multiple jurisdictions, substantial investment and significant marketing efforts before we generate any revenues from product sales. We and our collaborators are not permitted to market or promote our product candidates before receiving regulatory approval from the FDA or comparable foreign regulatory authorities. We recently submitted an NDA for fidaxomicin to the FDA and submitted a MAA for fidaxomicin to the EMA. We have not previously submitted an NDA or MAA, nor have we or any collaborator received marketing approval for either fidaxomicin or Pruvel. We cannot be certain that either of these product candidates will receive regulatory approval in a timely fashion, or at all. If we and/or any collaborators do not receive regulatory approval for and successfully commercialize fidaxomicin and Pruvel, we will not generate any revenues from product sales for several years, if at all, and we may not be able to continue our operations.
We believe our initial success will be more dependent on fidaxomicin than Pruvel, because we believe that the market for the treatment of CDI is larger than the market for the treatment of infectious diarrhea. Even if we or any collaborators successfully obtain regulatory approval to market fidaxomicin or Pruvel, our revenues for either drug candidate will be dependent upon the size of the markets in the territories for which we or any collaborators have commercial rights and our and our collaborators’ ability to successfully commercialize our drug candidates in those markets, either alone or with a partner. If the markets for the treatment of CDI or infectious diarrhea are not as significant as we estimate or we or our collaborators are otherwise unsuccessful in commercializing our drug candidates, our business and prospects will be harmed.
If we are unable to obtain FDA approval of our product candidates, we will not be able to commercialize them in the United States.
We need FDA approval prior to marketing our product candidates in the United States. If we fail to obtain FDA approval to market our product candidates, we will be unable to sell our product candidates in the United States, which will significantly impair our ability to generate any revenues.
The FDA’s regulatory review and approval process, which includes evaluation of pre-clinical studies and clinical trials of our product candidates as well as the evaluation of our manufacturing processes and our third-party contract manufacturers’ facilities, is lengthy, expensive and inherently uncertain. To receive approval for a product candidate, we must, among other things, demonstrate to the FDA’s satisfaction with substantial evidence from well-controlled clinical trials that the product candidate is both safe and effective for each indication for which approval is sought. Satisfaction of the approval requirements typically takes several years and
the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the pharmaceutical product candidate. We cannot predict if or when we might receive regulatory approvals for any of our product candidates currently under development.
We may experience delays in the FDA’s review process of our recently submitted NDA filing for fidaxomicin and may experience delays if we submit an NDA filing for Pruvel. We completed the submission of our NDA for fidaxomicin in November 2010. The FDA accepted our NDA for filing in January 2011 and granted our request for a six-month priority review. As part of the Prescription Drug User Fee Act, or PDUFA, the FDA has a goal to review and act on submissions in a given time frame. Accordingly, the FDA assigned our fidaxomicin NDA a PDUFA goal date of May 30, 2011 as the date by which the FDA intends to complete its review and issue a determination. The FDA is not bound by, and has in the past missed, its PDUFA goals, and it is unknown whether the review of our NDA filing for fidaxomicin, any future NDA filing for Pruvel, or for any of our other drug candidates, will be completed within the FDA review goals or will be delayed. Moreover, the duration of the FDA’s review may depend on the number and type of other NDAs that are submitted with the FDA during the same time period.
With the exception of our recently submitted fidaxomicin NDA, we have not previously submitted NDAs to the FDA. In addition, we have never obtained FDA approval for any drug. This lack of experience may impede our ability to obtain FDA approval in a timely manner, if at all, for fidaxomicin, Pruvel or our other product candidates. We have also experienced delays in submitting our NDA for Pruvel to the FDA due to a pending investigation into an unexpectedly high incidence of cutaneous rash observed during a safety study. We currently cannot predict if or when we will be able to initiate any new study for Pruvel or the ultimate extent of the delay in our planned submission of an NDA for Pruvel. Any further impairment or delay in our ability to complete and submit an NDA for a product candidate could further impair our or a collaborator’s ability to successfully commercialize Pruvel and our ability to pursue a potential collaboration with respect to Pruvel.
Even if our product candidates receive regulatory approval from the FDA, any approvals that we obtain may not cover all of the clinical indications for which we are seeking approval, or could contain significant limitations in the form of narrow indications, warnings, precautions or contra-indications with respect to conditions of use, or the requirement that we implement a risk evaluation and mitigation strategy, or REMS. In such an event, our ability to generate revenues from such products would be greatly reduced and our business would be harmed.
The FDA has substantial discretion in the approval process and may either refuse to consider our applications for substantive review or may form the opinion after review of our data that our applications are insufficient to allow approval of our product candidates. Even if we believe that data collected from our preclinical studies and clinical trials of our product candidates are promising, our data may not be sufficient to support marketing approval by the FDA, or regulatory interpretation of these data and procedures may be unfavorable. In addition, the FDA’s regulatory review of NDAs for product candidates intended for widespread use by a large proportion of the general population is becoming increasingly focused on safety. If the FDA does not consider or approve an application that we submit, it may require that we conduct additional clinical, pre-clinical or manufacturing validation studies and submit that data before it will reconsider our application. Depending on the extent of these or any other studies, approval of any applications that we submit may be delayed by several years, or may require us to expend more resources than we have available. For example, we intend to rely in part on certain legacy data from a third party to support an NDA for Pruvel. If the FDA deems this data to be insufficient, it may delay our submission of an NDA for Pruvel and could require us to complete additional studies. It is also possible that additional studies, if performed and completed, may not be successful or considered sufficient by the FDA for approval or even to make our applications approvable. If any of these outcomes occur, we may be forced to abandon one or more of our future applications for approval, which might significantly harm our business and prospects. As a result, we cannot predict when or whether regulatory approval will be obtained for any product candidate we develop.
Even after completing clinical trials and other studies, our product candidates could fail to receive regulatory approval for many reasons, including the following:
· the results of our clinical trials and other studies may not demonstrate to the satisfaction of or meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for any indication;
· the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials or other studies;
· the results of our clinical trials or other studies may not demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks;
· the results of our clinical trials or other studies may not demonstrate that a product candidate presents an advantage over existing therapies, or over placebo in any indications for which the FDA requires a placebo-controlled trial;
· the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from clinical trials or other studies;
· the data collected from clinical trials and other studies of our product candidates may not be sufficient to support the submission of an NDA or other submission or to obtain regulatory approval in the United States or elsewhere;
· the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical and other study data insufficient for approval; and
· FDA or comparable foreign regulatory authorities may not approve the proposed manufacturing processes and facilities for a product candidate.
Although we were granted priority review by the FDA of our NDA for fidaxomicin, there is no guarantee that the FDA will complete its review by its PDUFA goal date of May 30, 2011. In addition, although the FDA has granted Fast Track status to fidaxomicin and selected it for participation in a CMA Pilot 2 Program, we cannot be certain that we will receive any benefits from these designations or that the designations will expedite regulatory review or approval of fidaxomicin. Participation in these programs has not eliminated any phase of clinical development. Moreover, our participation in the CMA Pilot 2 Program has involved and will continue to involve frequent discussions and other interactions with the staff of the FDA. These frequent discussions could subject fidaxomicin to a greater level of scrutiny than it might otherwise have received or require us to make more frequent submissions and endure other burdens that would have been avoided if we had not participated in the program. Therefore, despite any potential benefits of fidaxomicin’s Fast Track and CMA Pilot 2 Program designations, significant uncertainty remains regarding the regulatory approval process for fidaxomicin. In addition, the FDA has informed us that it plans to discuss the NDA at a meeting of its Anti-Infective Drugs Advisory Committee currently scheduled for April 5, 2011. We cannot ensure a positive outcome from the Advisory Committee meeting and the views of the Advisory Committee could directly impact the FDA’s decision on whether to approve our NDA for fidaxomicin. In addition, even if the Advisory Committee were to recommend approval of our NDA, there can be no assurance that the FDA would follow such recommendation.
If we or our collaborators fail to gain and/or maintain marketing approvals from regulatory authorities in international markets for fidaxomicin and any future product candidates for which we have or license rights in international markets, our market opportunities will be limited.
Sales of our product candidates outside of the United States will be subject to foreign regulatory requirements governing clinical trials and marketing approval. Even if the FDA grants marketing approval for a product candidate, comparable regulatory authorities of foreign countries must also approve the marketing of the product candidate in those countries. This is important for the commercialization of fidaxomicin for which we have granted an exclusive license to Astellas in the Astellas territory and for which we retain commercialization rights in the rest of the world. We could experience significant delays and difficulties and incur significant costs in obtaining foreign regulatory approvals in the territories for which we retain commercialization rights. Regulatory requirements can vary widely from country to country and could delay the introduction of our products in those countries. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not guarantee regulatory approval will be obtained in any other country. In addition, our or our collaborators’ failure to obtain regulatory approval in any country may delay or have negative effects on the process for regulatory approval in others. For example, if unanticipated concerns are raised by the EMA or the FDA in their review of the MAA or NDA for fidaxomicin, respectively, these concerns could negatively impact applications for marketing approval of fidaxomicin in a different country or territory. We have received correspondence from the EMA accepting our MMA for filing, as well as a list of questions setting forth the EMA’s requests for additional information pursuant to its review of our MMA submission. We have 120 days, or until approximately mid-April 2011, to respond to the EMA’s requests and recommendations. If our response fails to satisfy the EMA, approval of the fidaxomicin MMA may be delayed or ultimately withheld.
Other than Pruvel, which is sold by other parties in Japan, Italy and certain other European countries, none of our product candidates is approved for sale in any international market for which we have or have licensed rights. If we or our collaborators fail to comply with regulatory requirements with respect to our product candidates in international markets or to obtain and maintain required approvals, our market opportunities and ability to generate revenues will be diminished, which would significantly harm our business, results of operations and prospects.
Even if we and our collaborators receive regulatory approval for our product candidates, we and our collaborators will be subject to ongoing significant regulatory obligations and oversight.
Even if we and our collaborators receive regulatory approval to sell our product candidates, the FDA and foreign regulatory authorities will likely impose significant restrictions on the indicated uses or marketing of such products, or impose ongoing requirements for potentially costly post-approval studies. In addition, following any regulatory approval of our product candidates, we and our collaborators will be subject to continuing regulatory obligations, such as requirements for testing, storage, recordkeeping and safety reporting, and additional post-marketing obligations, including regulatory oversight of the labeling, packaging, advertising, promotion and marketing of our products. If we or our collaborators become aware of previously unknown problems with any of our product candidates in the United States or overseas or at our third-party manufacturers’ facilities, a regulatory agency may impose restrictions on our products, our third-party manufacturers or on us, including requiring us to reformulate our products, conduct additional clinical trials, make changes in the labeling of our products, implement changes to, or obtain re-approvals of, our third-party manufacturers’ facilities, or withdraw the product from the market. In addition, we or our collaborators may experience a significant drop in the sales of the affected products and our product revenues will be reduced, our reputation in the marketplace may suffer and we may become the target of lawsuits, including class action suits. Moreover, if we or our collaborators or third-party manufacturers fail to comply with applicable regulatory requirements, we may be subject to warning letters, civil or criminal fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, costly new manufacturing requirements and criminal prosecution. Any of these events could harm or prevent sales of the affected products and reduce our related revenues or could substantially increase the costs and expenses of commercializing and marketing these products, which would significantly harm our business, financial condition and prospects.
We have incurred significant operating losses since inception and anticipate that we will incur continued losses for the foreseeable future.
We have experienced significant operating losses since our inception in 1998. As of December 31, 2010, we had an accumulated deficit of approximately $222.8 million. We have generated no revenues from product sales to date and we expect our expenses to increase substantially in the near term as we prepare for a potential commercial launch of fidaxomicin, including due to recent and planned increases to our head count and our pursuit of additional research and development activities, including potential additional indications for fidaxomicin. We have funded our operations through December 31, 2010 from the sale of approximately $256.2 million of our securities and through research funding pursuant to collaborations with partners or government grants. We expect to continue to incur substantial additional operating losses for the next several years as we build our marketing and sales capabilities, prepare for the potential commercial launch of fidaxomicin, continue developing Pruvel and preparing a potential submission of an NDA for Pruvel, and continue our clinical trial and research and development initiatives. Because of the numerous risks and uncertainties associated with developing, obtaining regulatory approval for and commercializing our product candidates, we are unable to predict the extent of any future losses. We or our collaborators may never successfully commercialize our product candidates and thus we may never have any significant future revenues or achieve and sustain profitability.
We are dependent on our collaboration agreement with Astellas to commercialize and further develop fidaxomicin in the Astellas territory. The failure to maintain this agreement or the failure of Astellas to perform its obligations under such agreement, could negatively impact our business.
Pursuant to the terms of our collaboration agreement with Astellas, we granted to Astellas exclusive rights to develop and commercialize fidaxomicin in the Astellas territory, and pursuant to the terms of our supply agreement with Astellas, we are obligated to supply to Astellas all of its requirements of fidaxomicin for such development and commercialization activities. Consequently, our ability to generate any revenues from fidaxomicin in the Astellas territory depends on Astellas’ ability to obtain regulatory approvals for and successfully commercialize fidaxomicin in the Astellas territory. We have limited control over the amount and timing of resources that Astellas will dedicate to these efforts.
We are subject to a number of other risks associated with our dependence on our collaboration agreement with Astellas, including:
· Astellas may not comply with applicable regulatory guidelines with respect to developing or commercializing fidaxomicin, which could adversely impact sales or future development of fidaxomicin in the Astellas territory;
· we and Astellas could disagree as to future development plans and Astellas may delay future clinical trials or stop a future clinical trial;
· there may be disputes between us and Astellas, including disagreements regarding the collaboration agreement, that may result in (1) the delay of or failure to achieve regulatory and commercial objectives that would result in milestone or
royalty payments, (2) the delay or termination of any future development or commercialization of fidaxomicin, and/or (3) costly litigation or arbitration that diverts our management’s attention and resources;
· because the milestone and royalty payments in the collaboration agreement are stated in terms of Euros but paid to us in U.S. Dollars, the amounts of any milestone or royalty payments that may be paid to us under the collaboration agreement could be less than what we expect, depending on the applicable exchange rate at the time of such payments;
· Astellas may not provide us with timely and accurate information regarding sales and marketing activities and supply forecasts, which could adversely impact our ability to comply with our supply obligations to Astellas and manage our own inventory of fidaxomicin, as well as our ability to generate accurate financial forecasts;
· business combinations or significant changes in Astellas’ business strategy may adversely affect Astellas’ ability or willingness to perform its obligations under our collaboration and supply agreements;
· Astellas may not properly maintain or defend our intellectual property rights in the Astellas territory or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property rights or expose us to potential litigation;
· the royalties we are eligible to receive from Astellas may be reduced or eliminated based upon Astellas’ and our ability to maintain or defend our intellectual property rights and the presence of generic competitors in the Astellas territory;
· limitations on our or an acquiror’s ability to maintain or pursue development or commercialization of products that are competitive with fidaxomicin could deter a potential acquisition of us that our stockholders may otherwise view as beneficial; and
· if Astellas is unsuccessful in obtaining regulatory approvals for or commercializing fidaxomicin in the Astellas territory, we may not receive any additional milestone or royalty payments under the collaboration agreement and our business prospects and financial results may be materially harmed.
The collaboration and supply agreements are subject to early termination, including through Astellas’ right to terminate without cause upon advance notice to us. If the agreements are terminated early, we may not be able to find another collaborator for the commercialization and further development of fidaxomicin in the Astellas territory on acceptable terms, or at all, and we may be unable to pursue continued commercialization or development of fidaxomicin in the Astellas territory on our own.
We may enter into additional agreements for the commercialization of fidaxomicin or other of our drug candidates, and may be similarly dependent on the performance of third parties with similar risk.
If we fail to obtain additional financing, we may be unable to commercialize fidaxomicin and Pruvel or develop and commercialize other product candidates, or continue our other research and development programs.
We will require additional capital to commercialize our current lead product candidates, fidaxomicin and Pruvel. We cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Any debt financing, if available, may require us to pledge our assets as collateral or involve restrictive covenants, such as limitations on our ability to incur additional indebtedness, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could negatively impact our ability to conduct our business. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates or one or more of our other research and development initiatives. We also could be required to:
· seek collaborators for one or more of our current or future product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available; or
· relinquish or license on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves.
Any of the above events could significantly harm our business and prospects and could cause our stock price to decline.
We do not currently have sufficient resources to commercialize fidaxomicin and Pruvel on our own. If we are unable to raise additional capital or are unable to effectively collaborate with additional partners for the commercialization of fidaxomicin or Pruvel, we will not generate significant revenues from sales of these products and our business will be materially harmed.
Our management team has recently undergone significant change and expansion and we may experience difficulties or delays integrating our new executive level employees into our organization.
Our success as an organization depends in part on our ability to successfully integrate new employees into our organization. We recently hired Kurt Hartman to the position of General Counsel and Access, Gregory Papaz as our Senior Vice President of Commercial Operations, Linda Amper as our Senior Vice President of Human Resources, Hemal Shah as our Senior Vice President of Health, Economics & Outcomes Research, and John Womelsdorf as our Vice President of Business Development. In addition, Francois-Xavier Frapaise, our former Chief Scientific Officer and Senior Vice President, recently ceased employment with us. We may experience delays in the execution of our business strategy as our new management team members become familiar with our company and integrate with our longer-term employees. This process may be further complicated by the fact that certain of our newly-hired management team members will reside on the East Coast, while the substantial majority of our personnel are located in San Diego, California.
If we fail to attract and retain senior management and key scientific personnel, we may be unable to successfully develop or commercialize our product candidates.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, sales and marketing, clinical and scientific personnel and on our ability to develop and maintain important relationships with leading academic institutions, clinicians and scientists. We are highly dependent on our chief executive officer, and the other principal members of our executive and scientific teams. The unexpected loss of the service of any of these persons may significantly delay or prevent the achievement of research, development, commercialization and other business objectives. Replacing key employees may be difficult and costly and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop and commercialize pharmaceutical products successfully. We do not maintain “key person” insurance policies on the lives of these individuals or the lives of any of our other employees. We employ these individuals on an at-will basis and their employment can be terminated by us or them at any time, for any reason and with or without notice.
We will need to hire additional personnel as we expand our commercial activities. We may not be able to attract or retain qualified management, sales and marketing and scientific personnel on acceptable terms in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses, particularly in the San Diego, California and New Jersey areas. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that will impede significantly the achievement of our commercialization and research and development objectives, our ability to raise additional capital and our ability to implement our business strategy. In particular, if we lose any members of our senior management team, we may not be able to find suitable replacements, and our business and prospects may be harmed as a result.
We currently have no sales organization and have no experience as a company in marketing drug products. If we are unable to expand our marketing capabilities and establish sales capabilities or enter into agreements with third parties to market and sell our product candidates in the territories in which we retain commercialization rights, we may not be able to generate product revenues.
Our strategy is to build a fully-integrated U.S.-focused biopharmaceutical company to successfully execute the commercial launch of fidaxomicin in the U.S. market following regulatory approval. We currently have only a limited marketing organization and do not have a sales organization for sales and distribution of pharmaceutical products and, as a company, we do not have any experience commercializing pharmaceutical products on our own. In order to commercialize any products, we must build our marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services. Our strategy within the United States includes a plan to establish a commercial organization based primarily in New Jersey, and our bicoastal organizational structure could create management challenges. We own exclusive rights to commercialize Pruvel in the United States, and are evaluating our commercialization options for Pruvel in the United States. The establishment and development of our own sales force to market any products we may develop will be expensive and time consuming and could delay any product launch, and we cannot be certain that we will be able to successfully develop this capability. We do not currently have sufficient funds to develop a sales force and other resources that we believe would be necessary to adequately market fidaxomicin and Pruvel in the United States. We will also have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train and retain marketing and sales personnel. To the extent we rely on third parties to commercialize our products, if any, we may receive less revenues than if we commercialized these products ourselves. In addition, we may have little or no control over the sales efforts of any third parties involved in commercializing our products. In the event we are unable to develop our own marketing and sales force or collaborate with a third-party marketing and sales organization, we would not be able to commercialize our product candidates which would negatively impact our ability to generate product revenues.
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
We are a small company with 87 employees as of February 28, 2011. To commercialize our product candidates or commence new clinical trials, we will need to expand our employee base for managerial, operational, marketing, sales, financial and other resources. Future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees and may take time away from running other aspects of our business, including development and commercialization of our product candidates. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. In particular, as our commercialization plans and strategies develop, we will need to recruit and train a substantial number of sales and marketing personnel and expect to need to expand the size of our employee base for managerial, operational, financial and other resources. To that end, we must be able to:
· manage our development efforts effectively;
· integrate additional management, administrative and manufacturing personnel;
· build a marketing and sales organization; and
· maintain sufficient administrative, accounting and management information systems and controls.
We may not be able to accomplish these tasks, and accordingly, may not achieve our research, development and commercialization goals. Our failure to accomplish any of these goals could harm our financial results and prospects.
We currently depend, and will in the future continue to depend, on third parties to manufacture our product candidates, including fidaxomicin and Pruvel. If these manufacturers fail to provide us and our collaborators with adequate supplies of clinical trial materials and commercial product or fail to comply with the requirements of regulatory authorities, we may be unable to develop or commercialize our products.
We have outsourced all manufacturing of supplies of our product candidates to third parties. We seek to establish long-term supply arrangements with third-party contract manufacturers. For example, in May 2010, we entered into a long-term supply agreement with Biocon for the commercial manufacturing of the active pharmaceutical ingredient, or API, for fidaxomicin. We intend to continue outsourcing the manufacture of our product candidates to third parties for any future clinical trials and large-scale commercialization of any product candidates that receive regulatory approval and become commercial drugs.
Our ability and that of our collaborators to develop and commercialize fidaxomicin and Pruvel and any other product candidates will depend in part on our ability and that of our collaborators to arrange for third parties to manufacture our products at a competitive cost, in accordance with strictly enforced regulatory requirements and in sufficient quantities for regulatory approval, commercialization and any future clinical trials. We have not yet manufactured commercial batches of fidaxomicin, Pruvel or any of our other product candidates. Third-party manufacturers that we select to manufacture our product candidates for clinical testing or on a commercial scale may encounter difficulties with the small- and large-scale formulation and manufacturing processes required for such manufacture. Such difficulties could result in delays in clinical trials, regulatory submissions and approvals, or commercialization of our product candidates. Our inability or that of our collaborators to enter into and maintain agreements with third-party manufacturers on acceptable terms would cause shortages of clinical trial supplies of our product candidates, thereby delaying or preventing regulatory approval and/or commercialization of the affected product candidate, and adversely affecting our ability to generate revenues. Specifically, Biocon is currently our sole supplier of fidaxomicin API. While we work closely with Biocon to try to ensure continuity of supply while maintaining high quality and reliability, we cannot guarantee that these efforts will be successful. A reduction or interruption in our supply of fidaxomicin API from Biocon, and an inability to develop alternative sources for such supply, could adversely affect our ability to manufacture fidaxomicin in a timely or cost effective manner to make our related product sales, and could result in a breach of our supply agreement with Astellas. Further, development of large-scale manufacturing processes will require additional validation studies, which the FDA must review and approve, and the time it will take us or a third party manufacturer to develop such large-scale processes and capabilities may delay any product launch following regulatory approval. Even if we are able to establish additional or replacement manufacturers, identifying these sources and entering into definitive supply agreements and obtaining regulatory approvals may involve a substantial amount of time and cost and such supply arrangements may not be available on acceptable economic terms.
In addition, we, our collaborators and other third-party manufacturers of our products must comply with strictly enforced current good manufacturing practices, or cGMP, requirements enforced by the FDA through its facilities inspection program. These requirements include quality control, quality assurance and the maintenance of records and documentation. We currently rely on Biocon to manufacture fidaxomicin API and rely on Patheon, Inc. to manufacture the drug product supplies. As such, Biocon and Patheon will be subject to ongoing periodic unannounced inspections by the FDA and other agencies for compliance with current cGMP, and similar foreign standards. We also rely on Nippon Shinyaku, which contracts with Juzen Chemical Corporation, or Juzen,
as well as Angelini Francesco Acraf SpA, or Angelini, and Patheon, to manufacture Pruvel drug supplies. The manufacturing facilities of Biocon, Juzen and Patheon have been inspected and approved by the FDA for other companies’ drug products; however, none of Biocon’s, Juzen’s nor Patheon’s facilities have been inspected by the FDA for the manufacture of our drug supplies. Angelini’s facilities have not been inspected or approved by the FDA. We or other third-party manufacturers of our products may be unable to comply with cGMP requirements and with other FDA, state, local and foreign regulatory requirements. We and our collaborators have little control over third-party manufacturers’ compliance with these regulations and standards. A failure to comply with these requirements by our third-party manufacturers, including Biocon, Juzen, Angelini, and Patheon could result in the issuance of untitled letters and/or warning letters from authorities, as well as sanctions being imposed on us, including fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall or withdrawal of product approval. In addition, we have no control over these manufacturers’ ability to maintain adequate quality control, quality assurance and qualified personnel. If the safety of any quantities supplied by third parties is compromised due to their failure to adhere to applicable laws or for other reasons, we and our collaborators may not be able to obtain or maintain regulatory approval for or successfully commercialize one or more of our product candidates, which would significantly harm our business and prospects.
Other than our collaboration agreement with Astellas, we may not be able to enter into acceptable agreements to commercialize fidaxomicin outside of the United States or if, needed, adequately build our own marketing and sales capabilities.
We intend to pursue the development and potential commercialize fidaxomicin outside of the United States through collaboration arrangements with third parties, such as our collaboration with Astellas. We may be unable to enter into additional collaboration arrangements in international markets outside of the Astellas territory. In addition, there can be no guarantee that Astellas or any other parties that we may enter into collaboration arrangements with will be successful or result in more revenues than we could obtain by marketing fidaxomicin on our own. If we are unable to enter into additional collaboration arrangements for our products or develop an effective international sales force, our ability to generate product revenues would be limited, which would adversely affect our business, financial condition, results of operations and prospects. If we are unable to enter into such collaboration arrangements for development of fidaxomicin in areas outside of the United States and outside of the Astellas territory, we may need to develop our own marketing and sales force to market fidaxomicin in these territories to hospital-based and long-term care physicians. These efforts may not be successful as we have no relationships among such hospital-based and long-term care physicians and do not currently have sufficient funds to develop an adequate sales force in these regions. There is no guarantee that we will be able to develop an effective international sales force to successfully commercialize our products in these international markets. If we cannot commercialize fidaxomicin in any territory that represents a significant market opportunity, our ability to achieve and sustain profitability will be substantially limited.
If our product candidates are unable to compete effectively with branded and generic antibiotics, our commercial opportunity would be reduced or eliminated.
If approved, our lead product candidates will compete against both branded antibiotic therapies, such as Vancocin Pulvules with respect to fidaxomicin and Xifaxan®/rifaxamin with respect to Pruvel, and generic antibiotics such as metronidazole and oral vancomycin with respect to fidaxomicin and ciprofloxacin with respect to Pruvel. In addition, we anticipate that fidaxomicin will compete with other antibiotic and anti-infective product candidates currently in development for the treatment of CDI. Many of these products have been or will be developed and marketed by major pharmaceutical companies, who have significantly greater financial resources and expertise in research and development, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals, manufacturing and marketing approved products than we do. As a result, these companies may obtain regulatory approval more rapidly than we are able to and may be more effective in selling and marketing their products as well. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established pharmaceutical or other companies.
We anticipate that, if approved, fidaxomicin and Pruvel will face increasing competition in the form of generic versions of branded products of competitors that will lose their patent exclusivity. For example, fidaxomicin, if approved, will immediately face steep competition from an inexpensive generic form of metronidazole. Fidaxomicin would currently face generic oral vancomycin competition in Europe and in the future may face competition from generic oral vancomycin in the United States as well. In addition, our internal market research suggests that there is increasing use of oral reconstituted intravenous vancomycin “slurry” in the hospital setting. Generic antibiotic therapies typically are sold at lower prices than branded antibiotics and are generally preferred by managed care providers of health services. For example, because metronidazole and generic vancomycin “slurry” are available at such a low price, we believe it may be difficult to sell fidaxomicin as a first-line therapy for the treatment of CDI other than in certain limited circumstances, such as in patients at high risk of recurrence. If we or our collaborators are unable to demonstrate to physicians and patients that, based on experience, clinical data, side-effect profiles and other factors, our products are preferable to these generic antibiotic therapies, we may never generate meaningful product revenues. In addition, many antibiotics experience bacterial resistance over time because of their continued use. There can be no guarantee that bacteria would not develop resistance to
fidaxomicin, Pruvel or any of our other product candidates. Our commercial opportunity would also be reduced or eliminated if our competitors develop and commercialize generic or branded antibiotics that are safer, more effective, have lower recurrence rates, have fewer side effects or are less expensive than our product candidates.
The commercial success of our product candidates will depend upon attaining significant market acceptance of these product candidates among physicians, patients, healthcare payors and the medical community.
Even if our product candidates are approved by the appropriate regulatory authorities for marketing and sale, physicians may not prescribe our product candidates, which would prevent us from generating revenues or becoming profitable. Market acceptance of fidaxomicin, Pruvel and any of our future product candidates by physicians, patients and healthcare payors will depend on a number of factors, many of which are beyond our control, including:
· timing of market introduction of our product candidates as well as of competitive drugs;
· the clinical indications for which the product candidate is approved;
· acceptance by physicians and patients of each product candidate as a safe and effective treatment;
· perceived advantages over alternative treatments;
· the cost of treatment in relation to alternative treatments, including numerous generic antibiotics;
· the extent to which the product candidate is approved for inclusion on formularies of hospitals and managed care organizations;
· the extent to which bacteria develops resistance to the product candidate, thereby limiting its efficacy in treating or managing infections;
· whether the product candidate is designated under physician treatment guidelines as a first-line therapy or as a second- or third-line therapy for particular infections;
· the availability of adequate reimbursement by third parties, such as insurance companies and other healthcare payors;
· limitations or warnings contained in a product’s FDA-approved labeling;
· relative convenience and ease of administration; and
· prevalence and severity of adverse side effects.
Because fidaxomicin is a differentiated antibiotic for the treatment of CDI, it may encounter additional hurdles to market acceptance by physicians, who may be skeptical about its clinical benefits, or healthcare payors, who may resist reimbursing a premium-priced therapeutic particularly in light of the availability of generic alternatives.
If approved, we plan to target our marketing of Pruvel primarily to high-prescribing physicians of antibiotics for infectious diarrhea, including those at travel clinics. Because of the number of these physicians in the United States, we will be required to expend significant time and resources to obtain broad market acceptance of Pruvel among these physicians. We do not have experience in marketing to this population of physicians and do not currently have the resources to be able to conduct such marketing efforts on our own. As such, we may not be successful in any of these marketing efforts which would limit the commercial success of Pruvel.
In addition, in July 2008 the FDA notified makers of fluoroquinolone antimicrobial drugs for systemic use that a boxed warning is necessary for those products due to the risk of tendonitis and tendon rapture. As prulifloxacin, the generic name for Pruvel, is a fluoroquinolone antibiotic it may be required to carry a black box warning. Although these risks have been described for years on the product label of many fluoroquinolones, the increased awareness of this risk may impact the market potential for the fluoroquinolone class of antibiotics, including prulifloxacin. Furthermore, because prulifloxacin has already been marketed by other companies outside the United States to treat a wide range of bacterial infections, including infectious diarrhea, urinary tract infections and respiratory tract infections, patients may be able to obtain prulifloxacin from these other companies, and not from us, if
prulifloxacin is approved in the market where the patient is located. We have rights to Pruvel only in the United States. These patients may obtain prulifloxacin in these other markets from other companies even if these patients are from the United States.
Third-party payor coverage and reimbursement may be insufficient or unavailable altogether for our product candidates, which could diminish our or our collaborators’ sales or affect our or our collaborators’ ability to sell our products profitably.
Market acceptance and sales of our product candidates will depend on reimbursement policies and may be affected by future healthcare reform measures. Government third-party payors, such as the Medicare and Medicaid programs, and private payors, including health maintenance organizations, decide which drugs they will pay for and establish reimbursement levels for these drugs. Because third-party payors increasingly are challenging prices charged and the cost-effectiveness of medical products, significant uncertainty exists as to the ability of our product candidates to receive adequate coverage and reimbursement. We cannot be sure that third-party payors will place our product candidates on approved formularies or that reimbursement will be available in whole or in part for any of our product candidates. Also, we cannot be sure that insufficient reimbursement amounts will not reduce the demand for, or the price of, our products, if approved.
Many healthcare providers, such as hospitals, receive a fixed reimbursement amount per procedure or other treatment therapy based on a prospective payment system, and these amounts are not necessarily based on the actual costs incurred. As a result, these healthcare providers may be inclined to choose the least expensive therapies. We cannot guarantee that our potential customers will find the reimbursement amounts sufficient to cover the costs of our product candidates or that our product candidates will be cost competitive compared to alternatives.
We have not commenced efforts to have our product candidates covered and reimbursed by government or third-party payors. If reimbursement is not available or is available only to limited levels, we may not be able to commercialize our products successfully or at all, which would harm our business and prospects.
If we fail to develop and commercialize other products or product candidates, we may be unable to grow our business.
A key element of our strategy is to commercialize a portfolio of innovative hospital specialty products in addition to fidaxomicin and Pruvel. As a significant part of our growth strategy, we intend to develop and commercialize, independently and/or through collaboration partners, additional products and product candidates through our discovery research program using our proprietary technology, including OPopS. The success of this strategy depends upon our ability to identify, select and acquire pharmaceutical product candidates and products that fit into our development plans on terms that are acceptable to us. To supplement this strategy, we may also obtain rights to additional product candidates from third parties through acquisition or in-licensing transactions.
Any product candidate we identify or to which we acquire rights will likely require additional development efforts prior to commercial sale, including pre-clinical studies, extensive clinical testing and approval by the FDA and applicable foreign regulatory authorities. All product candidates are prone to the risks of failure that are inherent in pharmaceutical product development, including the possibility that the product candidate will not be shown to be sufficiently safe and/or effective for approval by regulatory authorities. In addition, we cannot assure you that any such products that are approved will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace or be more effective than other commercially available alternatives.
A significant portion of the research that we are conducting involves new and unproven technologies. Research programs to identify new disease targets and product candidates require substantial technical, financial and human resources whether or not we ultimately identify any candidates. Our research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development.
If we are unable to develop suitable potential product candidates through internal research programs or by obtaining rights to novel therapeutics from third parties, our business and prospects will suffer.
Our focus on drug discovery and development using our technology platform, including our patented proprietary OPopS drug discovery platform, is novel and unique. As a result, we cannot be certain that our product candidates will produce commercially viable drugs that safely and effectively treat infectious diseases or other diseases. To date, our technology platform has yielded only a small number of anti-infective product candidates. In addition, we do not have significant clinical data with respect to any of these potential product candidates. Even if we or our collaborators are successful in completing clinical development and receiving regulatory approval for one commercially viable drug for the treatment of one disease using our technology platform and carbohydrate chemistry focus, we cannot be certain that we or our collaborators will also be able to develop and receive regulatory approval for other drug candidates for the treatment of other forms of that disease or other diseases. If we fail to develop and commercialize,
independently and/or through collaborators, viable drugs using our platform and specialized focus, we will not be successful in developing a pipeline of potential product candidates to follow fidaxomicin and Pruvel, and our business prospects would be significantly harmed.
Our future growth depends on our ability to identify and acquire or in-license products. If we do not successfully identify and acquire or in-license related product candidates or integrate them into our operations, we may have limited growth opportunities.
We in-licensed the U.S. rights to Pruvel from Nippon Shinyaku who, along with Meiji-Seika Kaisha Ltd., conducted the initial development of this product candidate. An important part of our business strategy is to continue to develop a pipeline of product candidates by acquiring or in-licensing products, businesses or technologies that we believe are a strategic fit for our business. Future in-licenses or acquisitions, however, may entail numerous operational and financial risks, including:
· exposure to unknown liabilities;
· disruption of our business and diversion of our management’s time and attention to develop acquired products or technologies;
· incurrence of substantial debt or dilutive issuances of securities to pay for acquisitions;
· higher than expected acquisition and integration costs;
· increased amortization expenses;
· difficulty and cost in combining the operations and personnel of any acquired businesses with our operations and personnel;
· impairment of relationships with key suppliers or customers of any acquired businesses due to changes in management and ownership; and
· inability to retain key employees of any acquired businesses.
We have limited resources to identify and execute the acquisition or in-licensing of third-party products, businesses and technologies and integrate them into our current infrastructure. In particular, we may compete with larger pharmaceutical companies and other competitors in our efforts to establish new collaborations and in-licensing opportunities. These competitors likely will have access to greater financial resources than us and may have greater expertise in identifying and evaluating new opportunities. Moreover, we may devote resources to potential acquisitions or in-licensing opportunities that are never completed, or we may fail to realize the anticipated benefits of such efforts.
Our ability to pursue the development and commercialization of Pruvel, our other product candidates and our future product candidates depends upon the continuation of our licenses from third parties.
Our license agreement with Nippon Shinyaku provides us with an exclusive license to develop and commercialize Pruvel for any indication in the United States, with a right to sublicense to third parties. In the event Nippon Shinyaku is not able to supply us with Pruvel, the license agreement provides us with a non-exclusive, worldwide right and license to manufacture or have Pruvel manufactured for us. Either we or Nippon Shinyaku may terminate the license agreement immediately upon the bankruptcy or dissolution of the other party or upon a breach of any material provision of the agreement if the breach is not cured within 60 days following written notice. In addition, we are entitled to terminate the agreement in the event that the FDA compels us to cease sales of Pruvel in the United States. If our license agreement with Nippon Shinyaku terminates, we will lose our rights to develop, manufacture and commercialize Pruvel and our potential revenues would be limited. Similarly, if our agreement with TSRI, for the license of our OPopS technology is terminated, we will not be able to further develop future product candidates using our OPopS technology, and our third party licensees may be unable to continue development of our existing out-licensed product candidates.
We rely on our majority-owned subsidiary, OBI, for the development of one of our product candidates.
In October 2009, we completed a number of transactions involving our subsidiary, OBI, including the sale of 40% of our ownership interest in OBI to various third party investors. In connection with these transactions, we assigned to OBI, and OBI assumed from us, our rights and obligations under our license agreement with MSKCC related to our OPT-822/821 product candidate. We also assigned to OBI certain of our intellectual property and know-how related to this product candidate. In exchange for these assignments, we have the right to receive certain milestone or royalty payments relating to OPT-822/821.
We cannot assure you that OBI will successfully advance the development of OPT-822/821. In addition, if OBI does not comply with its obligations under the agreement with MSKCC, the agreement may be terminated and we may not be able to re-assume our rights under the agreement. If the agreement with MSKCC was terminated and we were unable to re-assume our rights, we would not be able to pursue further development of OPT-822/821. Moreover, the addition of third party investors in OBI has also diminished our ability to control OBI, which is now subject in some respects to the rights of the third party minority stockholders. In the future, additional equity financings or other issuances of equity securities or securities convertible into equity by OBI may further reduce our ownership position in OBI and we may not maintain a controlling interest in OBI. To the extent that we do not maintain a controlling equity interest in OBI in the future, we would have to rely on OBI’s contractual obligations, including under our Intellectual Property Assignment and License Agreement, to ensure that OBI continues development of OPT-822/821 and complies with its obligations under the MSKCC agreement. Finally, OBI will need additional funds to further develop and commercialize OPT-822/821, and OBI may not be able to secure adequate funding or be able to do so on terms you or we believe are favorable. If OBI is unable to raise additional funds to continue operations, or otherwise fails to advance the development of OPT-822/821, we will not receive milestone or royalty payments with respect to this product candidate, and the value of our OBI equity position would likely diminish. To the extent we provide funds to OBI through additional equity investments or otherwise, we may need to divert funds away from our other product candidate programs which could adversely affect the development and/or commercialization of those other product candidates.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability lawsuits related to the testing of our product candidates, and will face an even greater risk if product candidates are introduced commercially. An individual may bring a liability claim against us if one of our product candidates causes, or merely appears to have caused, an injury. If we cannot successfully defend ourselves against the product liability claim, we may incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
· decreased demand for our product candidates;
· injury to our reputation;
· termination of clinical trial sites or entire clinical trial programs;
· withdrawal of clinical trial participants;
· significant litigation costs;
· substantial monetary awards to or costly settlement with patients;
· product recalls;
· loss of revenues; and
· the inability to commercialize our product candidates.
We may become dependent upon consumer perceptions of us and the safety and quality of our product candidates. We could be adversely affected if we or our product candidates are subject to negative publicity. We could also be adversely affected if any of our potential products or any similar products distributed by other companies prove to be, or are asserted to be, harmful to consumers. Also, because of our dependence upon consumer perceptions, any adverse publicity associated with illness or other adverse effects resulting from consumers’ use or misuse of our potential products or any similar products distributed by other companies could have a material adverse impact on our results of operations.
We have global clinical trial liability insurance that covers our clinical trials up to a $10.0 million annual aggregate limit. Our current or future insurance coverage may prove insufficient to cover any liability claims brought against us. We intend to expand our insurance coverage to include the sale of commercial products if marketing approval is obtained for our product candidates, which would increase our insurance premiums. Because of the increasing costs of insurance coverage, we may not be able to maintain insurance coverage at a reasonable cost or obtain insurance coverage that will be adequate to satisfy any liability that may arise.
Clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of pre-clinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through pre-clinical studies and initial clinical testing. In addition, sub-analysis of clinical trial data may reveal limitations of our product candidates even though top-line results are positive. The type and amount of clinical data necessary to gain regulatory approval for our product candidates may also change during or after completion of our clinical trials or we may inaccurately characterize such requirements. Moreover, we cannot guarantee that the FDA or comparable foreign regulatory authorities will agree with our interpretation of clinical trial data, or find such data sufficient to grant product approval.
Delays in clinical trials are common and have many causes, and any such delays could result in increased costs to us and jeopardize or delay our ability to achieve regulatory approval and commence product sales as currently contemplated.
We have in the past experienced delays in clinical trials of our product candidates and we may experience delays in future clinical trials. We do not know whether planned clinical trials will begin on time, will need to be redesigned or will be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including delays in obtaining regulatory approval to commence a trial, in reaching agreement on acceptable terms with prospective clinical research organizations, or CROs, and clinical trial sites, in obtaining institutional review board approval at each site, in recruiting suitable patients to participate in a trial, in having patients complete a trial or return for post-treatment follow-up, in adding new sites or in obtaining sufficient supplies of clinical trial materials. Many factors affect patient enrollment, including the size and nature of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, competing clinical trials, clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating and whether the clinical trial design involves comparison to placebo.
We could encounter delays if prescribing physicians encounter unresolved ethical issues associated with enrolling patients in clinical trials of our product candidates in lieu of prescribing existing antibiotics that have established safety and efficacy profiles or with administering placebo to patients in our placebo-controlled trials. Further, a clinical trial may be suspended or terminated by us, our collaborators, the FDA or other regulatory authorities due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates may be harmed, and our ability to generate product revenues from any of these product candidates will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our product development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may significantly harm our business, financial condition and prospects.
We may be required to suspend or discontinue clinical trials due to adverse events, adverse side effects or other safety risks that could preclude approval of our product candidates or negatively affect sales of any marketed product.
Our clinical trials may be suspended at any time for a number of reasons. We may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to participants. For example, in November 2010, due to a higher than expected incidence of cutaneous rash during the course of a study of the possible interaction between Pruvel and antacids, we informed the FDA that we were voluntarily terminating the research study. In addition, regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to participants. In our Phase 3 clinical trials of fidaxomicin, the most common drug-related side effects reported were nausea, vomiting, constipation, anorexia, headache and dizziness. Patients treated with Pruvel have experienced drug-related side effects including abdominal pain, diarrhea, nausea, renal toxicities, cardiac arrhythmias, photosensitivity, rash, excessive flushing of the skin and central nervous system effects, such as seizures. If adverse, drug-related events are encountered or suspected, our trials would be interrupted, delayed or halted and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all targeted indications. Adverse events encountered in any post-approval studies may also harm our efforts and those of our collaborators to market our product candidates or could result in withdrawal of regulatory approvals. Even if we believe our product candidates are safe, our data is subject to review by the FDA, which may disagree with our conclusions and delay or deny approval of our product candidates which would significantly harm the commercial prospects of such product candidates. Moreover,
we could be subject to significant liability if any volunteer or patient suffers, or appears to suffer, adverse side effects as a result of participating in our clinical trials. Any of these occurrences may significantly harm our business and prospects.
We have relied and in the future may rely on third parties to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we and our collaborators may not be able to obtain regulatory approval for or commercialize our product candidates.
We have in the past entered into agreements with third-party CROs, such as INC Research, to provide monitors for and to manage data for our clinical programs.
We and any CROs conducting clinical trials for our product candidates are required to comply with current good clinical practices, or GCPs, regulations and guidelines enforced by the FDA for all of our products in clinical development. The FDA enforces GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we or the CROs that conduct clinical trials of our product candidates fail to comply with applicable GCPs, the clinical data generated in the clinical trials may be deemed unreliable and the FDA may require additional clinical trials before approving any marketing applications. We cannot assure you that, upon inspection, the FDA will determine that any clinical trials of our product candidates comply with GCPs. In addition, our clinical trials must be conducted with product produced under cGMP regulations, and require a large number of test subjects. Our failure to comply with these regulations may require us to repeat clinical trials, which would be costly and delay the regulatory approval process and commercialization of our product candidates.
In addition, these third-party CROs are not our employees, and we cannot control whether or not they devote sufficient time and resources to our clinical programs. These CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical studies or other drug development activities, which could harm our competitive position. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements, or for other reasons, our clinical trials may be extended, delayed or terminated or may have to be repeated, and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our financial results and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
Current healthcare laws and regulations and future legislative or regulatory reforms to the healthcare system may affect our ability to sell our product candidates profitably.
The U.S. and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our products profitably. Among policy makers and payors in the U.S. and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the U.S., the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively, PPACA, became law in the U.S. PPACA substantially changes the way healthcare is financed by both governmental and private insurers and significantly affects the pharmaceutical industry. Among the provisions of PPACA of greatest importance to the pharmaceutical industry are the following:
· an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, beginning in 2011;
· an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program, retroactive to January 1, 2010, to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively;
· a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their overage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D, beginning in 2011;
· extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations, effective March 23, 2010;
· expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals beginning in April 2010 and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the Federal Poverty Level beginning in 2014, thereby potentially increasing manufacturers’ Medicaid rebate liability;
· expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program, effective in January 2010;
· new requirements to report certain financial arrangements with physicians, including reporting any “transfer of value” made or distributed to prescribers and other healthcare providers, effective March 30, 2013, and reporting any investment interests held by physicians and their immediate family members during the preceding calendar year;
· a new requirement to annually report drug samples that manufacturers and distributors provide to physicians, effective April 1, 2012;
· a licensure framework for follow-on biologic products; and
· a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
We anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and an additional downward pressure on the price that we receive for any approved product, and could seriously harm our business. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors.
We also cannot be certain that fidaxomicin and Pruvel or other current or future drug candidates will successfully be placed on the list of drugs covered by particular health plan formularies, nor can we predict the negotiated price for our drug candidates, which will be determined by market factors. Many states have also created preferred drug lists and include drugs on those lists only when the manufacturers agree to pay a supplemental rebate. If fidaxomicin and Pruvel or other current or future drug candidates are not included on these preferred drug lists, physicians may not be inclined to prescribe them to their Medicaid patients, thereby diminishing the potential market for our products.
As a result of the PPACA and the trend towards cost-effectiveness criteria and managed healthcare in the United States, third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement of new drugs. They may also refuse to provide any coverage of uses of approved products for medical indications other than those for which the FDA has granted market approvals. As a result, significant uncertainty exists as to whether and how much third-party payors will reimburse for newly-approved drugs, which in turn will put pressure on the pricing of drugs. Further, we do not have experience in ensuring approval by applicable third-party payors outside of the United States for coverage and reimbursement of our products. The availability of numerous generic antibiotics at lower prices than branded antibiotics can also be expected to substantially reduce the likelihood of reimbursement for fidaxomicin and Pruvel. We also anticipate pricing pressures in connection with the sale of our products due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative proposals.
We must comply with federal and state “fraud and abuse” laws, and, if we are unable to fully comply with such laws, we could face substantial penalties, which may adversely affect our business, financial condition and results of operations.
In the United States, we are also subject to healthcare fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
· the federal healthcare programs’ Anti-Kickback Law, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs;
· federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent;
· the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created federal criminal laws that prohibit executing a scheme to defraud any health care benefit program or making false statements relating to health care matters;
· federal “sunshine” laws that require transparency regarding financial arrangements with health care providers, such as the reporting and disclosure requirements imposed by PPACA on drug manufacturers regarding any “transfer of value” made or distributed to prescribers and other health care providers; and
· state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers.
Some states, such as California, Massachusetts and Vermont, mandate implementation of comprehensive compliance programs to ensure compliance with these laws.
The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by applicable regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Moreover, recent healthcare reform legislation has strengthened these laws. For example, the recently enacted PPACA, among other things, amends the intent requirement of the federal anti-kickback and criminal health care fraud statutes; a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. We also expect there will continue to be federal and state laws and/or regulations, proposed and implemented, that could impact our operations and business. The extent to which future legislation or regulations, if any, relating to healthcare fraud abuse laws and/or enforcement, may be enacted or what effect such legislation or regulation would have on our business remains uncertain. Violations of these laws are punishable by criminal and civil sanctions, including, in some instances, exclusion from participation in federal and state healthcare programs, including Medicare and Medicaid, and the curtailment or restructuring of operations. We believe that our operations are in material compliance with such laws and we are aware of the need to increase our compliance resources if we begin marketing products. However, because of the far-reaching nature of these laws, there can be no assurance that we would not be required to alter one or more of our practices to be in compliance with these laws. In addition, there can be no assurance that the occurrence of one or more violations of these laws or regulations would not result in a material adverse effect on our financial condition and results of operations.
Our business involves the use of hazardous materials and we and our third-party manufacturers must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our third-party manufacturers’ activities and, to a lesser extent, our own activities involve the controlled storage, use and disposal of hazardous materials, including the components of our product candidates and other hazardous compounds. We and our manufacturers are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. Although we believe that the safety procedures for handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials. We currently have insurance coverage in the amount of approximately $250,000 for damage claims arising from contamination on our property. These amounts may not be sufficient to adequately protect us from liability for damage claims relating to contamination. If we are subject to liability exceeding our insurance coverage amounts, our business and prospects would be harmed. In the event of an accident, state or federal authorities may also curtail our use of these materials and interrupt our business operations.
Our business and operations would suffer in the event of computer, telecommunications or other system failure.
Despite the implementation of security measures, our internal computer systems are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Any system failure, accident or security breach that causes interruptions in our operations could result in a material disruption of our drug development programs. For example, the loss of clinical trial information from completed or ongoing clinical trials for fidaxomicin or Pruvel, which is maintained by our third-party CRO, could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we may incur liability and the further development of our product candidates may be delayed.
Risks Related to Our Intellectual Property
It is difficult and costly to protect our intellectual property and our proprietary technologies, and we may not be able to ensure their protection.
Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection of the use, formulation and structure of our product candidates, and the methods used to manufacture them, as well as successfully defending these patents against third-party challenges, including those from generic drug manufacturers. Our ability to protect our product candidates from unauthorized making, using, selling, offering to sell or importing by third parties is dependent upon the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities.
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date in the United States. The biotechnology patent environment outside the United States is even more uncertain. Changes in either the patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our licensed patents, our patents or in third-party patents.
The degree of future protection for our proprietary rights is uncertain, because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
· others may be able to make compounds that are similar to our product candidates but that are not covered by the claims of our issued patents and pending patent applications or licensed patents and pending patent applications, or for which we are not licensed under our license agreements;
· others may be able to make competing pharmaceutical formulations containing our product candidates or components of our product formulations that are either not covered by the claims of our issued patents or licensed patents, not licensed to us under our license agreements or are subject to patents that expire;
· we or our licensors might not have been the first to make the inventions covered by our issued patents and pending patent applications or the pending patent applications and issued patents of our licensors;
· we or our licensors might not have been the first to file patent applications for these inventions;
· others may independently develop similar or alternative technologies or duplicate any of our technologies;
· it is possible that our pending patent applications or our licensed patent applications will not result in issued patents;
· our issued patents and pending patent applications or the pending patent applications and issued patents of our licensors may not provide us with any competitive advantages, may be designed around by our competitors, including generic drug companies, or may be held invalid or unenforceable as a result of legal challenges by third parties;
· we may not develop additional proprietary technologies that are patentable; or
· the patents of others may have an adverse effect on our business.
In addition, to the extent we are unable to obtain and maintain patent protection for our product candidates or in the event such patent protection expires, it may no longer be cost effective to extend our portfolio by pursuing additional development of a product candidate for follow-on indications.
We have three issued patents and ten pending patent applications related to fidaxomicin in the U.S, one of which is allowed. We also have five issued foreign patents related to fidaxomicin.
The patent and patent applications related to fidaxomicin encompass various topics relating to:
· composition of matter;
· pharmaceutical composition and methods of use;
· polymorphic forms and pharmaceutical compositions thereof;
· manufacturing processes;
· treatment of diseases;
· formulation;
· selected indications in CDI patients; and
· primary metabolites.
If we are unable to obtain sufficient patent protection encompassing fidaxomicin, our competitors, including generic drug companies, may be able to design other similar formulations of the active ingredient of fidaxomicin. Furthermore, even though the manufacturing process patent has issued, and even if the formulation patent application results in an issued patent, our competitors, including generic drug companies, may be able to design around our manufacturing processes or formulation for fidaxomicin. As a result, our competitors may be able to develop competing products without infringing our patents.
We plan to evaluate our commercialization options for Pruvel which we expect would be marketed to high-prescribing physicians of antibiotics for infectious diarrhea. The primary patent covering Pruvel expired in February 2009. In 2009, our licensor, Nippon Shinyaku, received an additional patent in the U.S. which relates to a key intermediate in the manufacturing process for Pruvel. Despite its issuance, this patent may later be challenged by a third party or fail to prevent a third party from producing and marketing a generic form of Pruvel in the United States. If the available protection afforded by this patent is insufficient, we will likely rely on one or more regulatory marketing exclusivities for Pruvel in the United States. Specifically, if our NDA for Pruvel is approved, we expect to receive a five-year period of marketing exclusivity under the Hatch-Waxman Act. This exclusivity period is available to the first applicant to gain approval of an NDA for a new chemical entity, or NCE, that has not previously been approved as an active ingredient under Section 505(b) of the Food Drug and Cosmetic Act, or FDCA. While we expect that the NCE exclusivity period will be available for Pruvel, if the recent patent is insufficient to maintain exclusivity in the United States with respect to Pruvel, we may face generic competition in the United States five years after our NDA for Pruvel is approved by the FDA. If we are unable to obtain marketing exclusivity beyond five years from the approval of our NDA, our potential revenues from Pruvel sales in the U.S. will be limited.
We depend, in part, on our licensors and collaborators to protect a portion of our proprietary rights. In such cases, our licensors and collaborators may be primarily or wholly responsible for the maintenance of patents and prosecution of patent applications relating to important areas of our business. For example, Nippon Shinyaku is responsible for the maintenance of patents and prosecution of patent applications relating to Pruvel. We may also be dependent on Par to provide technical support for patent applications relating to fidaxomicin. If any of these parties fail to adequately protect these product candidates with issued patents, our business and prospects would be significantly harmed.
Under our agreement with Nippon Shinyaku, in the event Nippon Shinyaku fails to take all steps necessary to seek extension of the patents licensed to us in the United States 180 days after we request such action be taken, then we have the right to take all necessary actions to extend the licensed patents. Our agreement with Par does not have explicit provisions regarding our rights to take necessary action with respect to maintenance of patents and prosecution of patent applications nor do such agreements provide us with any legal recourse in the event such parties do not so maintain and/or prosecute. If any of these parties or others on which we rely for patent maintenance and prosecution fail to adequately maintain patents and prosecute patent applications relating to technology licensed to or from us, we may be required to take further action on our own to protect this technology. However, we may not be successful in maintaining such patents or prosecuting such patent applications and if so, our business and prospects would be significantly harmed.
We also rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, outside scientific collaborators and other advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third-party entity illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how.
If we or our licensors fail to obtain or maintain patent protection or trade secret protection for our product candidates or our technologies, third parties could use our proprietary information, which could impair our ability to compete in the market and adversely affect our ability to generate revenues and attain profitability.
We may incur substantial costs as a result of litigation or other proceedings relating to our patent, trademark and other intellectual property rights, and we may be unable to protect our rights to, or use, our technology.
If we or, as applicable, our commercialization partners, including Astellas pursuant to its first right to enforce the patents licensed to it in the Astellas territory, choose to go to court to stop someone else from using our inventions, that individual or company has the right to ask the court to rule that the underlying patents are invalid and/or should not be enforced against that third party. These lawsuits are expensive and would consume time and other resources even if we or our commercialization partner were successful in stopping the infringement of these patents. There is also the risk that, even if the validity of these patents is upheld, the court will refuse to stop the other party on the ground that such other party’s activities do not infringe our rights to these patents.
Furthermore, a third party may claim that we or our manufacturing or commercialization partners are using inventions covered by the third party’s patent rights and may go to court to stop us from engaging in our normal operations and activities, including making, using or selling our product candidates. These lawsuits are costly and could affect our results of operations and divert the attention of managerial and technical personnel. There is a risk that a court would decide that we or our commercialization partners are infringing the third party’s patents and would order us or our partners to stop the activities covered by the patents. In addition, there is a risk that a court will order us or our partners to pay the other party damages for having violated the other party’s patents. We have indemnified our commercialization partners, including Astellas, against patent infringement claims and thus would be responsible for any of their costs associated with such claims and actions. The biotechnology industry has produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our products or methods of use either do not infringe the patent claims of the relevant patent and/or that the patent claims are invalid, and we may not be able to do this. Proving invalidity, in particular, is difficult since it requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents.
Although we have conducted searches of third-party patents with respect to fidaxomicin and Pruvel, these searches may not have identified all third-party patents relevant to those products and we have not conducted an extensive search of patents issued to third parties with respect to our other product candidates. Consequently, no assurance can be given that third-party patents containing claims covering our products, technology or methods do not exist, have not been filed, or could not be filed or issued. Because of the number of patents issued and patent applications filed in our technical areas or fields, we believe there is a risk that third parties may allege they have patent rights encompassing our products, technology or methods. In addition, we have not conducted an extensive search of third-party trademarks, so no assurance can be given that such third-party trademarks do not exist, have not been filed, could not be filed or issued, or could not exist under common trademark law. While we have filed a trademark application for the names “Optimer”, “Optimer Pharmaceuticals” and “Pruvel”, we are aware that the name “Optimer” has been registered as a trademark with the U.S. PTO by more than one third party, including one in the biotechnology space. As such, we believe there is a significant risk that third parties may allege they have trademark rights encompassing the names for which we have applied for protection.
Because some patent applications in the United States may be maintained in secrecy until the patents are issued, because patent applications in the United States and many foreign jurisdictions are typically not published until eighteen months after filing, and because publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered by our licensors’ issued patents or our pending applications or our licensors’ pending applications, or that we or our licensors were the first to invent the technology. Our competitors may have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent application may have priority over our or our licensors’ patent applications and could further require us to obtain rights to issued patents covering such technologies. If another party has filed a U.S. patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the U.S. PTO to determine priority of invention in the United States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in a loss of our U.S. patent position with respect to such inventions.
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
Risks Related to the Securities Market and Ownership of Our Common Stock
The market price of our common stock may be highly volatile.
Before our initial public offering in February 2007, there was no public market for our common stock. We cannot assure you that an active trading market will exist for our common stock. You may not be able to sell your shares quickly or at the market price if trading in our common stock is not active.
The trading price of our common stock is likely to be highly volatile and could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
· general economic and market conditions and other factors that may be unrelated to our operating performance or the operating performance of our competitors;
· announcement of FDA or comparable foreign regulatory agency approval or non-approval of our or our competitors’ product candidates, or specific label indications for their use, or delays in the FDA or comparable foreign regulatory agency review process;
· actions taken by the FDA or other regulatory agencies with respect to our product candidates, clinical trials, manufacturing process or marketing and sales activities;
· any adverse development or perceived adverse development with respect to the FDA’s review of our NDA for fidaxomicin or the EMA’s review of our MAA for fidaxomicin, including a request for additional information;
· any delays in the FDA’s review of our NDA for fidaxomicin, including the FDA’s inability to complete its review by the PDUFA goal date of May 30, 2011;
· developments our ability to submit an NDA for Pruvel to the FDA, including as a result of our pending investigation of the safety of Pruvel;
· changes in laws or regulations applicable to our products, including but not limited to clinical trial requirements for approvals;
· the success of our development efforts and clinical trials, particularly with respect to fidaxomicin and Pruvel;
· announcements by our collaborators with respect to clinical trial results, regulatory submissions and communications from the FDA or comparable foreign regulatory agencies;
· the success of our efforts to acquire or in-license additional products or product candidates;
· developments concerning our collaborations and partnerships, including but not limited to those with our sources of manufacturing supply and our development and commercialization partners;
· our dependence on our collaborators, such as Astellas, to commercialize and further develop our products in foreign countries in compliance with foreign regulatory schemes;
· our failure to successfully execute our commercialization strategy with respect to our products following marketing approval thereof;
· the success of our continuing efforts to establish and build marketing and sales capabilities;
· inability to obtain adequate commercial supply for any product following marketing approval thereof, or inability to do so at acceptable prices;
· actual or anticipated variations in our quarterly operating results;
· announcements of technological innovations by us, our collaborators or our competitors;
· new products or services introduced or announced by us or our commercialization partners, or our competitors and the timing of these introductions or announcements;
· the development of generic product alternatives to our or our competitors’ products;
· third-party coverage or reimbursement policies;
· actual or anticipated changes in earnings estimates or recommendations by securities analysts;
· conditions or trends in the biotechnology and biopharmaceutical industries;
· announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
· changes in the market valuations of similar companies;
· sales of common stock or other securities by us or our stockholders in the future;
· additions or departures of key scientific or management personnel;
· our ability to successfully integrate our new executive personnel into our organization;
· difficulties associated with the expansion of our domestic operations into a bicoastal organization;
· disputes or other developments relating to intellectual property, proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; and
· trading volume of our common stock.
In addition, the stock market in general and the market for biotechnology and biopharmaceutical companies in particular have experienced extreme price and volume fluctuations that have often been unrelated and/or disproportionate to the operating performance of those companies. These broad market and industry factors may significantly harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could significantly harm our business, financial condition and prospects.
Future sales of our common stock in the public market could cause our stock price to decline.
Persons who were our stockholders prior to our initial public offering continue to hold a substantial number of shares of our common stock. Many of these stockholders are able to sell their shares in the public market. Significant portions of these shares are held by a small number of stockholders. Sales by such stockholders of a substantial number of shares, or the expectation that such sales may occur, could significantly reduce the market price of our common stock. Moreover, the holders of a substantial number of shares of our common stock have rights, subject to certain conditions, to require us to file registration statements to permit the resale of their shares in the public market or to include their shares in registration statements that we may file for ourselves or other stockholders.
We have also registered all common stock that we have issued under our employee benefits plans. As a result, these shares can be freely sold in the public market upon issuance, subject to any applicable restrictions under the securities laws. In addition, our directors and executive officers may in the future establish programmed selling plans under Rule 10b5-1 of the Securities Exchange Act of 1934, as amended, for the purpose of effecting sales of our common stock. If any of these events cause a large number of our shares to be sold in the public market, the sales could reduce the trading price of our common stock and impede our ability to raise future capital.
We may not be able to liquidate our holding in an auction rate security.
Our investment securities consist of money market funds, corporate debt securities and government agency securities. As of December 31, 2010, we held one auction rate preferred security valued at $882,000 with perpetual maturity dates that reset every 28 days. The negative conditions in the global credit markets have prevented some investors from liquidating their holdings, including their holdings of auction rate securities. There was insufficient demand at auction for our auction rate preferred security. As a result, such security is currently not liquid, and we could be required to hold it until it is redeemed by the issuer or to maturity. In the event
we need to access the funds that are in an illiquid state, we will not be able to do so without a loss of principal, until a future auction on this investment is successful, the security is redeemed by the issuer or it matures. We have reduced the carrying value of our auction rate security by $118,000 to reflect an estimated change in fair market value due primarily to a lack of liquidity. Although the auction rate security continues to pay interest according to its stated terms, based on valuation models, we have recorded an unrealized loss for an other-than-temporary change in valuation of $118,000. If the credit ratings of the security issuer deteriorates or if uncertainties in these markets continue and any decline in market value is determined to be other-than-temporary, we would be required to adjust the carrying value of the investment through an impairment charge, which could negatively affect our financial condition, cash flow and reported earnings.
We will continue to incur significant costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we will continue to incur significant legal, accounting and other expenses. In addition, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, as well as rules subsequently implemented by the Securities and Exchange Commission, or SEC, and the Nasdaq Stock Market, or Nasdaq, impose various requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Our management and other personnel need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations result in increased legal and financial compliance costs and will make some activities more time-consuming and costly. For example, these rules and regulations make it more difficult and more expensive for us to maintain director and officer liability insurance, and we may be required to incur substantial costs in the future to maintain the same or similar coverage.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. We are required to perform an evaluation of our internal controls over financial reporting to allow management to report on the effectiveness of those controls, as required by Section 404 of the Sarbanes-Oxley Act. Additionally, our independent auditors were required to perform a similar evaluation and report on the effectiveness of our internal controls over financial reporting. At December 31, 2010, management and our independent auditors did not identify any material weaknesses in our internal controls over financial reporting. Our efforts to comply with Section 404 and related regulations have required, and continue to require, the commitment of significant financial and managerial resources. While we anticipate maintaining the integrity of our internal controls over financial reporting and all other aspects of Section 404, we cannot be certain that a material weakness will not be identified when we test the effectiveness of our control systems in the future. If a material weakness is identified, we could be subject to sanctions or investigations by Nasdaq, the SEC or other regulatory authorities, which would require additional financial and management resources, costly litigation or a loss of public confidence in our internal controls, which could have an adverse effect on the market price of our stock.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders, or remove our current management. These provisions include:
· a board of directors divided into three classes serving staggered three-year terms, such that not all members of the board will be elected at one time;
· authorizing the issuance of “blank check” preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval;
· limiting the removal of directors by the stockholders;
· prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders;
· eliminating the ability of stockholders to call a special meeting of stockholders; and
· establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings.
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management. In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with a stockholder owning 15% or more of our outstanding voting stock for a period of three years following the date on which the stockholder became an interested stockholder, unless such transactions are approved by our board of directors. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders. Such a delay or prevention of a change of control transaction could cause the market price of our stock to decline.
Item 1B. Unresolved Staff Comments
None.
Our facilities consist of approximately 40,000 square feet of laboratory and office space in two facilities in San Diego, California and one facility in Jersey City, New Jersey. The leases for both San Diego facilities expire in November 2011, with one lease subject to one five-year renewal option and the other lease subject to two five-year renewal options. The lease for the facility in Jersey City expires in February 2016, subject to one five year renewal option.
We believe these facilities are adequate to meet our current needs and that additional space will be available on commercially reasonable terms as needed.
We are not currently a party to any legal proceeding.
Item 4. (Removed and Reserved)
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock has been traded on the Nasdaq Global Market under the symbol “OPTR” since February 9, 2007. Prior to that time, there was no public market for our common stock. The following table sets forth the range of high and low sale prices for our common stock reported by Nasdaq Global Market.
|
2010 |
|
High |
|
Low |
| ||
|
First Quarter |
|
$ |
13.86 |
|
$ |
11.10 |
|
|
Second Quarter |
|
$ |
12.98 |
|
$ |
8.85 |
|
|
Third Quarter |
|
$ |
10.00 |
|
$ |
7.68 |
|
|
Fourth Quarter |
|
$ |
11.87 |
|
$ |
8.78 |
|
|
2009 |
|
High |
|
Low |
| ||
|
First Quarter |
|
$ |
14.00 |
|
$ |
9.12 |
|
|
Second Quarter |
|
$ |
15.00 |
|
$ |
9.54 |
|
|
Third Quarter |
|
$ |
15.17 |
|
$ |
12.49 |
|
|
Fourth Quarter |
|
$ |
14.03 |
|
$ |
10.64 |
|
As of February 28, 2011, there were approximately 35 holders of record of our common stock.
Dividends
We have never paid or declared cash dividends on our capital stock. We currently intend to retain future earnings, if any, for use in the expansion and operation of our business and do not anticipate paying any cash dividends in the foreseeable future.
Performance Measurement Comparison
The following stock performance graph illustrates a comparison of the total stockholder return on our common stock since February 9, 2007, which is the date our common stock first began trading on the Nasdaq Global Market, to two indices: the Nasdaq Composite Index and the Nasdaq Biotechnology Index. The graph assumes an initial investment of $100 on February 9, 2007, and that all dividends were reinvested.
COMPARISON OF 47 MONTH CUMULATIVE TOTAL RETURN*
Among Optimer Pharmaceuticals Inc, the NASDAQ Composite Index
and the NASDAQ Biotechnology Index
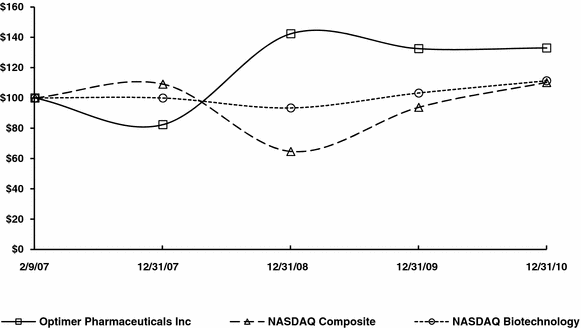
*$100 invested on 2/9/07 in stock or 1/31/07 in index, including reinvestment of dividends.
Fiscal year ending December 31.
Item 6. Selected Consolidated Financial Data
You should read the following selected consolidated financial and operating information together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and notes thereto included elsewhere in this report. Historical results for any prior period are not necessarily indicative of the results to be expected for any future period.
|
|
|
Years Ended December 31, |
| |||||||||||||
|
|
|
2010 |
|
2009 |
|
2008 |
|
2007 |
|
2006 |
| |||||
|
|
|
(in thousands, except per share amounts) |
| |||||||||||||
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Collaboration and grant revenues |
|
$ |
1,480 |
|
$ |
893 |
|
$ |
1,023 |
|
$ |
767 |
|
$ |
933 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Research and development |
|
32,797 |
|
34,417 |
|
30,206 |
|
41,569 |
|
10,481 |
| |||||
|
Marketing |
|
5,051 |
|
1,050 |
|
1,281 |
|
2,048 |
|
— |
| |||||
|
General and administrative |
|
12,500 |
|
8,024 |
|
6,683 |
|
5,351 |
|
3,523 |
| |||||
|
Total operating expenses |
|
50,348 |
|
43,491 |
|
38,170 |
|
48,968 |
|
14,004 |
| |||||
|
Loss from operations |
|
(48,868 |
) |
(42,598 |
) |
(37,147 |
) |
(48,201 |
) |
(13,071 |
) | |||||
|
Interest income and other, net |
|
329 |
|
364 |
|
1,562 |
|
2,062 |
|
1,169 |
| |||||
|
Net loss before accretion to redemption amount of redeemable convertible preferred stock |
|
(48,539 |
) |
(42,234 |
) |
(35,585 |
) |
(46,139 |
) |
(11,902 |
) | |||||
|
Accretion to redemption amount of redeemable convertible preferred stock |
|
— |
|
— |
|
— |
|
— |
|
(329 |
) | |||||
|
Net loss attributable to noncontrolling interest, net of tax |
|
1,199 |
|
142 |
|
— |
|
— |
|
— |
| |||||
|
Net loss attributable to Optimer Pharmaceuticals, Inc. |
|
$ |
(47,340 |
) |
$ |
(42,092 |
) |
$ |
(35,585 |
) |
$ |
(46,139 |
) |
$ |
(12,231 |
) |
|
Basic and diluted net loss per share attributable to common stockholders |
|
$ |
(1.25 |
) |
$ |
(1.30 |
) |
$ |
(1.24 |
) |
$ |
(2.12 |
) |
$ |
(4.81 |
) |
|
Weighted average shares outstanding |
|
37,830 |
|
32,469 |
|
28,683 |
|
21,715 |
|
2,543 |
| |||||
|
|
|
As of December 31, |
| |||||||||||||
|
|
|
2010 |
|
2009 |
|
2008 |
|
2007 |
|
2006 |
| |||||
|
|
|
(in thousands) |
| |||||||||||||
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Cash, cash equivalents and short-term investments |
|
$ |
49,415 |
|
$ |
38,185 |
|
$ |
39,326 |
|
$ |
58,806 |
|
$ |
21,341 |
|
|
Working capital |
|
45,239 |
|
30,951 |
|
32,258 |
|
52,173 |
|
17,990 |
| |||||
|
Total assets |
|
52,020 |
|
40,656 |
|
42,295 |
|
60,786 |
|
24,114 |
| |||||
|
Redeemable convertible preferred stock |
|
— |
|
— |
|
— |
|
— |
|
65,460 |
| |||||
|
Accumulated deficit |
|
(222,814 |
) |
(175,475 |
) |
(133,382 |
) |
(97,698 |
) |
(51,558 |
) | |||||
|
Noncontrolling interest |
|
1,997 |
|
3,040 |
|
— |
|
— |
|
— |
| |||||
|
Total stockholders’ equity (deficit) |
|
47,186 |
|
32,752 |
|
34,231 |
|
52,903 |
|
(46,702 |
) | |||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation
The following discussion and analysis should be read in conjunction with our “Selected Consolidated Financial Data” and consolidated financial statements and accompanying notes appearing elsewhere in this report. This discussion and other parts of this report may contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under “Risk Factors” and elsewhere in this report.
Overview
We are a biopharmaceutical company focused on discovering, developing and commercializing innovative hospital specialty products. Our current development efforts are focused on products that treat gastrointestinal infections and related diseases where current therapies have limitations, including limited efficacy, serious adverse side effects, drug-to-drug interactions, difficult patient compliance and bacterial resistance. We currently have two late-stage anti-infective product candidates, fidaxomicin and Pruvel™ (prulifloxacin).
Fidaxomicin, our lead product candidate, is a narrow spectrum antibiotic with minimal systemic absorption. We are developing fidaxomicin as a treatment for Clostridium difficile-infection, or CDI, the most common nosocomial, or hospital acquired, diarrhea. We currently hold rights to fidaxomicin in all regions of the world except for Europe and certain other countries in the Middle East, Africa and the Commonwealth of Independent States, or CIS.
In February 2011, we entered into an exclusive collaboration and license agreement with Astellas Pharma Europe Ltd., or Astellas, to develop and commercialize fidaxomicin in Europe and certain other countries in the Middle East, Africa and the CIS, which we refer to as the Astellas territory. In return for an exclusive license to fidaxomicin in the Astellas territory, Astellas paid to us an upfront cash payment of 50 million Euros, or $69.2 million, and we are eligible to receive additional cash payments totaling up to 115 million Euros upon the achievement of certain regulatory and commercial milestones. Furthermore, we will be entitled to receive escalating double-digit royalties ranging from the high teens to low twenties on net sales of fidaxomicin in the Astellas territory, if approved. Astellas will be responsible for all future costs associated with the development and commercialization of fidaxomicin in the Astellas territory including the costs associated with the Marketing Authorization Application, or MAA, for fidaxomicin in Europe. In connection with the collaboration and license agreement, we also entered into a supply agreement with Astellas pursuant to which we will be the exclusive supplier of fidaxomicin to Astellas in the Astellas territory and Astellas is obligated to pay us an amount equal to cost plus an agreed mark-up for such supply.
In July 2010, we filed, and in August 2010 the European Medicines Agency, or EMA, accepted for review an MAA to permit marketing of fidaxomicin in Europe. In September 2010, we began a submission to the United States Food and Drug Administration, or FDA, of a rolling New Drug Application, or NDA, and in November 2010 we completed the submission of our NDA. In January 2011, the FDA accepted our NDA filing for the treatment of CDI and for reducing the risk of recurrence when used for treatment of initial CDI. The FDA has also granted our request for six-month priority review, and has assigned a Prescription Drug User Fee Act, or PDUFA, goal date of May 30, 2011 for its review of the NDA. The FDA also informed us that it plans to discuss the NDA at a meeting of its Anti-Infective Drugs Advisory Committee currently scheduled for April 5, 2011.
We have completed two fidaxomicin Phase 3 trials which showed that fidaxomicin achieved the primary endpoint of clinical cure and demonstrated a significantly lower recurrence rate and significantly higher global cure rate (defined as cure with no recurrence within four weeks of completing therapy) compared to Vancocin, the only FDA-approved antibiotic for the treatment of CDI. In the two Phase 3 trials, among subjects who had experienced a prior CDI episode and recurred within three months of entering the study, treatment with fidaxomicin resulted in a 47% reduction in repeat CDI recurrence compared to Vancocin (p=0.045). The data also indicated that treatment with fidaxomicin significantly improved the recurrence rate and global cure rate in CDI patients requiring concomitant antibiotics compared to Vancocin. Fidaxomicin was also well-tolerated in the trials. In February 2011, the New England Journal of Medicine published results from the first Phase 3 trial. We have also reported additional data from the second Phase 3 trial showing a clinically meaningful reduction in recurrence rates and higher global cure rates compared to Vancocin in both the hyper-virulent BI/NAP1/027 and the non-BI strain type subgroups. Clinical cure rates for fidaxomicin and Vancocin were similar in these two strain type subgroups.
Pruvel is a prodrug in the fluoroquinolone class of antibiotics, a widely-used class of broad-spectrum antibiotics. We are developing Pruvel as a treatment for infectious diarrhea. In July 2008, we reported positive top-line data from the first Phase 3 trial conducted in Mexico and Peru and in February 2009, we reported positive top-line data from the second Phase 3 trial conducted in India, Guatemala and Mexico. The top-line analysis of data from these studies showed that Pruvel met the primary endpoint of TLUS compared to placebo. On November 9, 2010, due to a higher than expected incidence of cutaneous rash during the course of a study of the possible interaction between Pruvel and antacids, we informed the FDA that we were voluntarily terminating the research study. All reported events of cutaneous rash from the drug interaction study were mild or moderate in severity and required little or no treatment and all resolved completely. We are currently conducting an investigation into the cause of the rash prior to initiating any new study with Pruvel. Rashes are a known and infrequent side effect of fluoroquinolone antibiotics, such as Pruvel, and rashes occurred at or below the rate generally expected for other fluoroquinolones in both of our previous Pruvel Phase 3 clinical trials. Pending the results of our investigation, we are not currently able to estimate when we will initiate any new study or the extent of the delay in our planned submission of an NDA for Pruvel. We currently hold rights to commercialize Pruvel in the United States, and it is sold by other parties in Japan, Italy and certain other European countries.
We are developing additional product candidates using our proprietary technology, including our OPopS™. OPopS is a computer-aided technology that allows the development of potential drug candidates through carbohydrate mediated medicinal chemistry and enables the rapid synthesis of a wide variety of proprietary molecules. It includes GlycoOptimization, which enables the modification of a carbohydrate group on an existing drug to improve its properties, and De Novo Glycosylation, which introduces new carbohydrate groups on existing drugs to create new patentable compounds with improvement of pharmacokinetics.
We previously acquired exclusive rights to OPT-822/821, a combination of a novel carbohydrate-based cancer immunotherapy together with an adjuvant, which we licensed from Memorial Sloan-Kettering Cancer Center, or MSKCC. In October 2009, we assigned to OBI certain of our patent rights and know-how related to OPT-822/821 and also assigned to OBI our rights and obligations under a related license agreement with MSKCC. In April 2010, OBI filed an investigational new drug application, or IND, in Taiwan for OPT-822/821, and in January 2011, OBI initiated a Phase 2/3 clinical trial for the treatment of metastatic breast cancer which it intends to conduct in Taiwan, South Korea, Hong-Kong and Singapore. Other potential indications for OPT-822/821 are being evaluated. We have the right to receive up to $10 million from OBI in future milestone payments related to the development of OPT-822/821 as well as royalties on net sales of this product candidate. In February 2011, pursuant to an amendment to an October 2009 financing agreement, OBI sold newly-issued shares of its common stock for gross proceeds of approximately 462.0 million New Taiwan Dollars (approximately $15.5 million based on then-current exchange rates). We purchased 277.2 million New Taiwan Dollars (approximately $9.3 million based on then-current exchange rates) of the shares issued in the financing, such that we currently maintain a 60% equity interest in OBI.
We were incorporated in November 1998. Since inception, we have focused on developing our product candidates, including fidaxomicin and Pruvel. We have never been profitable and have incurred significant net losses since our inception. As of December 31, 2010, we had an accumulated deficit of $222.8 million. These losses have resulted principally from costs incurred in connection with research and development activities, including the costs of clinical trial activities associated with our current lead product candidates, license fees and general and administrative expenses. We expect to continue to incur operating losses for the next several years as we pursue the regulatory approval and commercialization of our product candidates, as well as acquire or in-license additional products or product candidates, technologies or businesses that are complementary to our own.
Financial Operations Overview
Collaboration and Grant Revenues
We have not generated any revenues from sales of commercial products. Since inception, we have generated revenues primarily as a result of various collaborations with pharmaceutical and biotechnology companies and grants from government agencies. We may also periodically recognize as revenues non-refundable payments for achieving certain milestones during the term of our collaboration agreements.
Research and Development Expense
Research and development expense consists of expenses incurred in connection with identifying and developing our drug candidates and developing and advancing our drug discovery technology. Our research and development expenses consist primarily of salaries and related employee benefits, costs associated with clinical trials managed by our CROs and costs associated with non-clinical research activities and regulatory approvals. Our most significant costs are for clinical trials, including payments to vendors such as CROs, investigators, manufacturers of clinical supplies and related consultants. Our historical research and development expenses have resulted predominantly from our clinical trials of fidaxomicin and Pruvel, the development of our carbohydrate technology platforms, including OPopS, in-licensing fees and general research activities. We charge all research and development expenses to operations as they are incurred because the underlying technology associated with these expenditures relates to our research and development efforts and has no alternative future uses. From inception through December 31, 2010, we incurred total research and development expenses of approximately $181.8 million.
We use our internal research and development resources across several projects, and much of this use is not allocable to a specific project. Accordingly, we do not account for all of our internal research and development costs on a project basis. In addition to our internal resources, we use external service providers and vendors to conduct our clinical trials, to manufacture our product candidates to be used in clinical trials and to provide various other research and development related products and services. These external costs are allocable to specific projects.
We incurred $22.5 million, $21.9 million and $110.2 million of research and development expenses directly related to the development of fidaxomicin for the years ended December 31, 2010 and 2009, and cumulatively through December 31, 2010, respectively. We incurred $4.1 million, $9.1 million, and $29.5 million of research and development expenses directly related to the development of Pruvel for the years ended December 31, 2010 and 2009, and cumulatively through December 31, 2010, respectively. All other research and development expenses were for other clinical programs.
General and Administrative Expense
General and administrative expense consists primarily of compensation, including stock-based compensation, and other expenses related to an allocated portion of facility cost, legal fees and other professional services expenses, our corporate administrative employees and insurance costs. We anticipate that we will maintain our existing level of general and administrative expenditures. However, we will make determinations as to the necessary levels of general and administrative expenditures on an on-going basis in response to our research and development activities and regulatory obligations.
Interest Income (Expense) and Other, Net
Interest income (expense) and other, net consists of interest earned on our cash, cash equivalents and short-term investments and other-than-temporary declines in the market value of available-for-sale securities and cash and non-cash interest charges related to bridge financings.
Net Operating Losses and Tax Credit Carryforwards
As of December 31, 2010 we had federal, state and foreign net operating loss carryforwards of approximately $183.7 million, $184.3 million and $2.9 million, respectively. If not utilized, the net operating loss carryforwards will begin expiring in 2020 for federal purposes and 2012 for state purposes. The foreign losses originate from our subsidiary in Taiwan. The losses from our subsidiary in Taiwan expire ten years after origination. As of December 31, 2010, we had both federal and state research and development tax credit carryforwards of approximately $5.5 million and $3.0 million, respectively. The federal tax credits will begin expiring in 2020 unless previously utilized and the state tax credits carryforward indefinitely. As of December 31, 2010, we had a state manufacturer’s investment tax credit carryforward of approximately $103,000 which will begin to expire in 2011, unless previously utilized. Under Section 382 of the Internal Revenue Code of 1986, as amended, substantial changes in our ownership may limit the amount of net operating loss and tax credit carryforwards that could be utilized annually in the future to offset taxable income. Any such annual limitation may significantly reduce the utilization of the net operating losses and tax credits before they expire.
As of December 31, 2010, we have completed a Section 382/383 analysis regarding the limitation of the net operating losses and credit carryovers and determined that the entire amount of U.S. federal and state net operating losses and credit carryovers are available for utilization, subject to the annual limitation. Any carryforwards that will expire prior to utilization as a result of future limitation will be removed from deferred tax assets with a corresponding reduction of the valuation allowance. Due to the existence of the valuation allowance, future changes in the unrecognized tax benefits will not impact the effective tax rate.
As of December 31, 2010, we have completed a Section 382/383 analysis regarding the limitation of the net operating losses and credit carryovers and had determined that the maximum amount of U.S. federal and state NOL and credit carryovers are available for utilization, subject to the annual limitation. Based on the analysis, the related deferred tax assets were reinstated in 2008 and the corresponding valuation allowance was increased. Any carryforwards that will expire prior to utilization as a result of future limitations will be removed from deferred tax assets with a corresponding reduction of the valuation allowance. Due to the existence of the valuation allowance, future changes in the unrecognized tax benefits will not impact the effective tax rate.
Critical Accounting Policies and Estimates
Our Management’s Discussion and Analysis of our Financial Condition and Results of Operations is based on our consolidated financial statements, which have been prepared in conformity with generally accepted accounting principles in the United States. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, expenses and related disclosures. Actual results could differ from those estimates. While our significant accounting policies are described in more detail in Note 1 of the Notes to Consolidated Financial Statements appearing elsewhere in this report, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
Our license and collaboration agreements contain multiple elements, including non-refundable upfront fees, payments for reimbursement of third-party research costs, payments for ongoing research, payments associated with achieving specific development milestones and royalties based on specified percentages of net product sales, if any. We consider a variety of factors in determining the appropriate method of revenue recognition under these arrangements, such as whether the elements are separable, whether there are determinable fair values and whether there is a unique earnings process associated with each element of a contract.
Cash received in advance of services being performed is recorded as deferred revenue and recognized as revenue as services are performed over the applicable term of the agreement.
When a payment is specifically tied to a separate earnings process, revenues are recognized when the specific performance obligation associated with the payment is completed. Performance obligations typically consist of significant and substantive milestones. Revenues from milestone payments may be considered separable from funding for research services because of the uncertainty surrounding the achievement of milestones for products in early stages of development. Accordingly, these milestone payments are allowed to be recognized as revenue if and when the performance milestone is achieved if they represent a substantive earnings process.
In connection with certain research collaboration agreements, revenues are recognized from non-refundable upfront fees, which we do not believe are specifically tied to a separate earnings process, ratably over the term of the agreement or the period over which we have significant involvement or perform services. Research fees are recognized as revenue as the related research activities are performed.
With respect to revenues derived from reimbursement of direct out-of-pocket expenses for research costs associated with grants, in transactions where we act as a principal with discretion to choose suppliers, bear credit risk and perform part of the services required in the transaction, we record revenue for the gross amount of the reimbursement. The costs associated with these reimbursements are reflected as a component of research and development expense in the consolidated statements of operations.
None of the payments that we have received from collaborators to date, whether recognized as revenue or deferred, are refundable even if the related program is not successful.
Research and Development
Research and development costs are expensed as incurred and consist primarily of costs associated with clinical trials, compensation, including stock-based compensation, and other expenses related to research and development, including personnel costs, facilities costs and depreciation.
When nonrefundable payments for goods or services to be received in the future for use in research and development activities are made, we defer and capitalize these types of payments. The capitalized amounts are expensed when the related goods are delivered or the services are performed.
Accrued Expenses
A substantial portion of our on-going research and development activities are performed under agreements we enter into with external service providers, including CROs, who conduct many of our research and development activities. We accrue the costs incurred under these contracts based on factors such as estimates of work performed, milestones achieved, patient enrollment and costs historically incurred for similar contracts. As actual costs become known, we adjust our accruals. To date, our accruals have been within management’s estimates, and no material adjustments to research and development expenses have been recognized. Subsequent changes in estimates may result in a material change in our accruals, which could also materially affect our results of operations.
Stock-Based Compensation
Authoritative guidance requires that share-based payment transactions with employees be recognized in the financial statements based on their fair value and recognized as compensation expense over the vesting period. We used the modified prospective method and accordingly we did not restate the results of operations for the prior periods. Compensation expense of $6.4 million, $2.8 million and $1.8 million was recognized in the years ended December 31, 2010, 2009 and 2008, respectively.
Stock-based compensation expense is estimated as of the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which generally represents the vesting period. We estimate the fair value of our stock options using the Black-Scholes option-pricing model and the fair value of our stock awards based on the quoted market price of our common stock.
Estimating the fair value for stock options requires judgment, including estimating stock-price volatility, expected term, expected dividends and risk-free interest rates. Due to limited historical date, the expected volatility rate incorporates the historical volatility of comparable companies whose share prices are publicly available. The average expected term is calculated using the Simplified Method for Estimating the Expected Term. Expected dividends are estimated based on our dividend history as well as our current projections. Optimer’s risk-free interest rate for periods approximating the expected terms of the options is based on the U.S. Treasury zero-coupon bonds with maturities similar to the periods approximating the expected terms of the options while OBI’s the risk free interest rate is based on Central Bank of China. These assumptions are updated on an annual basis or sooner if there is a significant change in circumstances that could affect these assumptions.
Equity instruments issued to non-employees are recorded at their fair value and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period.
Results of Operations
Comparison of Years Ended December 31, 2010 and 2009
Collaboration and Grant Revenues. Collaboration and grant revenues for the years ended December 31, 2010 and 2009 were $1,480,000 and $893,000, respectively. The increase of $587,000, or 66%, was primarily due to a milestone payment received from Cempra Pharmaceuticals for the completion of its Phase 1 study for the treatment of respiratory infections.
Research and Development Expense. Research and development expense for the years ended December 31, 2010 and 2009 was $32,798,000 and $34,417,000, respectively. The decrease of $1,619,000, or 5%, was due primarily to a decrease in fidaxomicin and Pruvel development expenses and manufacturing set-up expenses reimbursable to Biocon incurred in the prior year. The decrease was offset by a $5.0 million milestone to Par for the successful completion of the second fidaxomicin Phase 3 trial and the regulatory filing fee for the fidaxomicin NDA.
Marketing Expense. Marketing expense for the years ended December 31, 2010 and 2009 was $5,051,000 and $1,050,000, respectively. The increase of $4,001,000, or 381%, was primarily due to increased market research and pre-commercial launch commercialization efforts related to our fidaxomicin program.
General and Administrative Expense. General and administrative expense for the years ended December 31, 2010 and 2009 was $12,500,000 and $8,024,000, respectively. The increase of $4,476,000, or 56%, was primarily due to $4,360,000 of stock compensation expense, an increase of $3,086,000 over the same period in the prior year, as well as an increase in consulting, recruitment and compensation expenses.
Interest Income and Other, net. Net interest income and other of $329,000 for the year ended December 31, 2010 was relatively consistent with the $364,000 for the year ended December 31, 2009.
Comparison of Years Ended December 31, 2009 and 2008
Collaboration and Grant Revenues. Collaboration and grant revenues for the years ended December 31, 2009 and 2008 were $892,000 and $1,023,000, respectively. The decrease of $131,000, or 13%, was primarily due to the conclusion of one of two National Institutes of Health grants as well as a decrease in revenue from the remaining grant.
Research and Development Expense. Research and development expense for the years ended December 31, 2009 and 2008 was $34,417,000 and $30,206,000, respectively. The increase of $4,211,000, or 14%, was due primarily to an increase in development expenses, manufacturing scale-up expenses and expenses to prepare regulatory filings related to fidaxomicin and Pruvel.
Marketing Expense. Marketing expense for the years ended December 31, 2009 and 2008 was $1,050,000 and $1,281,000, respectively. The decrease of $231,000, or 18%, was primarily due to lower medical education, tradeshow and consulting expenses.
General and Administrative Expense. General and administrative expense for the years ended December 31, 2009 and 2008 was $8,024,000 and $6,683,000, respectively. The increase of $1,341,000, or 20%, was due to higher compensation expenses, including $1,274,000 of stock compensation expense, an increase of $350,000 over the same period in the prior year, as well as an increase in patent and consulting expenses.
Interest Income and Other, net. Net interest income and other for the year ended December 31, 2009 and 2008 were $364,000 and $1,562,000, respectively. The decrease was primarily due to lower interest rates on cash, cash equivalents and investments.
Liquidity and Capital Resources
Sources of Liquidity
Since inception, our operations have been financed primarily through the sale of equity securities. Through March 3, 2011, we received gross proceeds of approximately $333.8 million from the sale of shares of our preferred and common stock as follows:
· in May 2000, we sold a total of 1.6 million shares of Series A preferred stock for proceeds of $3.4 million;
· from March 2001 to December 2001, we sold a total of 4.1 million shares of Series B preferred stock for proceeds of $32.2 million;
· in April 2005, we sold a total of 1.5 million shares of Series C preferred stock for proceeds of $12.0 million;
· from April 2005 to November 2005, we sold a total of 2.9 million shares of Series D preferred stock for proceeds of $22.3 million;
· in February 2007, we sold a total of 7.0 million shares of our common stock in connection with our initial public offering for proceeds of $49.0 million;
· in October 2007, we sold a total of 4.6 million shares of our common stock in connection with a private placement offering for proceeds of $35.9 million;
· in July 2008, we sold a total of 1.7 million shares of our common stock in a registered direct offering for proceeds of $14.7 million;
· in March 2009, we sold a total of 3.3 million shares of our common stock and warrants to purchase up to an aggregate of 91,533 shares of our common stock in a registered direct offering for proceeds of $32.9 million;
· in March 2010, we sold a total of 4.9 million shares of our common stock in a public offering for proceeds of $53.8 million;
· in February 2011, we sold a total of 6.9 million shares of our common stock in a public offering for proceeds of $77.6 million.
In addition, in March 2011, pursuant to our collaboration and license agreement with Astellas, we received 50 million Euros, or $69.2 million, as an upfront payment from Astellas.
Until required for operations, we invest a substantial portion of our available funds in money market funds, United States government instruments and other readily marketable debt instruments, all of which are investment-grade quality. We have established guidelines relating to diversification and maturities of our investments to preserve principal and maintain liquidity.
Cash Flows
As of December 31, 2010, cash, cash equivalents and short-term investments totaled approximately $49.4 million as compared to $38.2 million as of December 31, 2009, an increase of approximately $11.2 million. The increase in our cash, cash equivalents and short-term investments was primarily due to the net $51.2 million raised in a follow-on offering of our common stock in March 2010, offset by the use of cash for our operating expenses. Of the $49.4 million, $3.7 million was held by OBI as of December 31, 2010.
We cannot be certain if, when or to what extent we will receive cash inflows from the commercialization of our product candidates. We expect our development expenses to be substantial and to increase over the next few years as we advance the development of our product candidates and prepare for regulatory submissions and commercialization.
In February 2011, we entered into a collaboration and license agreement with Astellas pursuant to which we granted to Astellas an exclusive, royalty-bearing license under certain of our know-how and intellectual property to develop and commercialize fidaxomicin in the Astellas territory. Under the terms of the license agreement with Astellas, Astellas paid to us an upfront fee equal to 50.0 million Euros, or approximately $69.2 million, and we are eligible to receive additional cash payments totaling up to 115.0 million Euros upon the achievement by Astellas of specified regulatory and commercial milestones. In addition, we will be entitled to receive escalating double-digit royalties ranging from the high teens to low twenties on net sales of fidaxomicin products in the Astellas territory, which royalties are subject to reduction in certain, limited circumstances. Such royalties will be payable by Astellas on a product-by-product and country-by-country basis until a generic product accounts for a specified market share of the applicable fidaxomicin product in the applicable country.
In February 2007, we regained worldwide rights to fidaxomicin from Par under a prospective buy-back agreement. We paid Par a one-time $5.0 million milestone payment in June 2010 for our successful completion of the second pivotal Phase 3 trial for fidaxomicin. We are obligated to pay Par a 5% royalty on net sales by us or our affiliates of fidaxomicin in North America and Israel, and a 1.5% royalty on net sales by us or our affiliates of fidaxomicin in the rest of the world. In addition, we are required to pay Par a 6.25% royalty on net revenues we receive from licensees of our right to market fidaxomicin in the rest of the world. We are obligated to pay each of these royalties, if any, on a country-by-country basis for seven years commencing on the applicable commercial launch in each such country.
In May 2010, we entered into a long-term supply agreement with Biocon for the commercial manufacture of fidaxomicin’s active pharmaceutical ingredient, or API. Pursuant to the agreement, Biocon agreed to manufacture and supply to us, up to certain limits, fidaxomicin API and, subject to certain conditions, we agreed to purchase from Biocon at least a portion of our requirements for fidaxomicin API in the United States and Canada. We previously paid to Biocon $2.5 million for certain equipment purchases and manufacturing scale-up activities, and we may be entitled to recover up to $1.5 million of this amount under the supply agreement in the form of discounted prices for fidaxomicin API. We may be obligated to make additional payments to Biocon if we fail to meet minimum purchase requirements after Biocon has dedicated certain manufacturing capacity to the production of fidaxomicin API and if Biocon is unable to manufacture alternative products with the dedicated capacity.
In June 2004, we entered into a license agreement with Nippon Shinyaku pursuant to which we acquired the non-exclusive right to import and purchase Pruvel, and the exclusive right (with the right to sublicense), within the United States, to develop, make, use, offer to sell, sell and license products suitable for consumption by humans containing Pruvel. Under the terms of the agreement, we will be required to pay Nippon Shinyaku a milestone payment in the amount of $1.0 million upon the filing, if any, of a NDA for Pruvel in the United States.
In October 2008, our Compensation Committee adopted a Severance Benefit Plan covering certain eligible employees of the Company, including executive officers. In May 2010, the Severance Benefit Plan was amended and restated. Pursuant to the plan, upon an involuntary termination other than for cause, an eligible employee may be entitled to receive specified severance benefits. The benefits may include cash severance payments and acceleration of stock award vesting. The level of benefits provided under the plan depends upon an eligible employee’s position and years of service, and whether the termination is related to a change in control.
In October 2009, we entered into certain transactions with our subsidiary, OBI, pursuant to which we assigned certain intellectual property rights and license agreements to OBI related to our OPT-822/821 product candidate. In connection with these transactions, we entered into a financing agreement with OBI and a group of new investors. Under the terms of the financing agreement, if OBI achieves certain development milestones with respect to OPT-822/821, we and the group of new investors may be required to purchase approximately an additional $8.6 million and $5.7 million, respectively, of new OBI common shares. In February 2011, pursuant to an amendment to an October 2009 financing agreement, OBI sold newly-issued shares of its common stock for gross proceeds of approximately 462.0 million New Taiwan Dollars (approximately $15.5 million based on then-current exchange rates). We purchased 277.2 million New Taiwan Dollars (approximately $9.3 million based on then-current exchange rates) of the shares issued in the financing, such that we currently maintain a 60% equity interest in OBI.
In July 2010, we received a $500,000 milestone payment from Cempra under the terms of a licensing agreement between the companies. The milestone payment was made as a result of Cempra’s continuing development of a next-generation macrolide (CEM-101) for the treatment of respiratory infections. Cempra licensed CEM-101 from Optimer and has successfully completed a Phase 1 study.
Funding Requirements
Our future capital uses and requirements depend on numerous factors including, but not limited to, the following:
· the costs of preparing and pursuing applications for regulatory approvals and the timing of such approvals;
· the costs of establishing sales or distribution capabilities and the timing of such efforts;
· our decision to conduct future clinical trials, including the timing and progress of such clinical trials;
· our ability to establish and maintain strategic collaborations, including licensing and other arrangements;
· the costs involved in prosecuting, enforcing or defending patent claims or other intellectual property rights;
· the success of the commercialization of our products; and
· the extent to which we in-license, acquire or invest in other indications, products, technologies and businesses.
We believe that our existing cash and cash equivalents will be sufficient to meet our capital requirements for at least the next 18 months.
Until we can generate significant cash from our operations, we expect to continue to fund our operations with existing cash resources that were primarily generated from the proceeds of offerings of our equity securities and collaborations and government grants. In addition, we may finance future cash needs through the sale of additional equity securities, strategic collaboration agreements and debt financing. However, we may not be successful in completing future equity financings, in entering into additional collaboration agreements, in receiving milestone or royalty payments under new or existing collaboration agreements, in obtaining new government grants or in obtaining debt financing. In addition, we cannot be sure that our existing cash and investment resources will be adequate, that financing will be available when needed or that, if available, financing will be obtained on terms favorable to us or our stockholders. The credit markets and the financial services industry have been experiencing a period of unprecedented turmoil and upheaval characterized by the government take-over, bankruptcy, failure, collapse or sale of various financial institutions. These events have generally made equity and debt financing more difficult to obtain, and may negatively impact our ability to complete financing transactions. Having insufficient funds may require us to delay, scale-back or eliminate some or all of our development programs, relinquish some or even all of our rights to product candidates at an earlier stage of development or renegotiate less favorable terms than we would otherwise choose. Failure to obtain adequate financing also may adversely affect our ability to operate as a going concern. If we raise funds by issuing equity securities, substantial dilution to existing stockholders would likely result. If we raise funds by incurring additional debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Contractual Obligations
The following table describes our long-term contractual obligations and commitments as of December 31, 2010:
|
|
|
Payments Due by Period |
| |||||||||||||
|
|
|
Total |
|
Less than 1 year |
|
1-2 years |
|
3-5 years |
|
After 5 years |
| |||||
|
|
|
(in thousands) |
| |||||||||||||
|
Operating lease obligations |
|
$ |
1,283 |
|
$ |
1,172 |
|
$ |
111 |
|
$ |
— |
|
$ |
— |
|
Our facilities currently consist of laboratory and office space in two facilities in San Diego, California. Our operating leases for these facilities expire on November 2011 with one of our leases subject to a one five-year renewal option and the other lease subject to a two-year renewal option.
The contractual obligations table does not include (a) a potential future milestone payment in the amount of $1.0 million to Nippon Shinyaku due upon filing our first NDA in the United States for Pruvel, (b) potential future milestone payments to Cempra in the amount of $1.0 million due upon the regulatory approval of each of the first two products we develop under our licensing agreement with Cempra in any country which is a member of the Association of Southeast Asian Nations, or ASEAN, (c) potential future milestone payments of up to $11.1 million to TSRI due upon achievement of certain clinical milestones, the filing of NDAs or their foreign equivalents and government marketing and distribution approval, or (d) the payments for newly signed lease agreement for office space in New Jersey in February 2011. We may also be required to pay royalties on any net sales of Pruvel, fidaxomicin and other licensed product candidates. The milestone and royalty payments under our license agreements are not included in the table above because we cannot, at this time, determine when or if the related milestones will be achieved or the events triggering the commencement of payment obligations will occur.
Recently Issued Accounting Pronouncements
FASB issued the following accounting amendments:
In April 2010, the FASB issued amendments related to the revenue recognition method for milestone payments in research and development agreements. Under these amendments, entities can make an accounting policy election to recognize a payment that is contingent upon the achievement of a substantive milestone in its entirety in the period in which the milestone is achieved. The amendments are effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010, which for us means such amendments will take effect beginning with the fiscal year starting on January 1, 2011. The adoption of this standard is not expected to have a material impact on our financial position, cash flow or results of operations.
In October 2009, the FASB issued authoritative guidance for arrangements with multiple deliveries. The guidance will allow companies to allocate consideration from contractual arrangements in multiple deliverables arrangements in a manner that better reflects the economics of the transaction. The new guidance requires expanded qualitative and quantitative disclosures and is effective for fiscal years beginning on or after June 15, 2010. Early adoption of the auidance is permitted. The Company is currently evaluating the impact of adopting the guidance on the Company’s future financial statements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Cash Equivalents and Marketable Securities Risk
Our cash and cash equivalents and short-term investments as of December 31, 2010 consisted primarily of money market funds and United States government instruments and other readily marketable debt instruments. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates. The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive from our investments without significantly increasing risk. A hypothetical ten percent change in interest rates during the year ended December 31, 2010 would have resulted in approximately a $13,000 change in net loss. Accordingly, we would not expect our operating results or cash flows to be affected to any significant degree by a sudden change in market interest rates applicable to our securities portfolio. In general, money market funds are not subject to market risk because the interest paid on such funds fluctuates with the prevailing interest rate.
Our cash and cash equivalents and short-term investments as of December 31, 2009 consisted primarily of money market funds and United States government instruments and other readily marketable debt instruments. A hypothetical ten percent change in interest rates during the year ended December 31, 2009 would have resulted in approximately a $36,000 change in net loss.
Fair Value measurements
All of our investment securities are available-for-sale securities and are reported on the consolidated balance sheet at market value except for one auction rate preferred securities or ARPS with a par value of approximately $1.0 million. As a result of the negative conditions in the global credit markets, our ARPS is currently not liquid. In the event we need to access the funds that are in an illiquid state, we will not be able to do so without a loss of principal, until the securities are redeemed by the issuer or they mature.
Foreign Currency Risk
We have operated primarily in the United States of America and most transactions during the year ended December 31, 2010, have been made in U.S. dollars. Accordingly, we have not had any material exposure to foreign currency rate fluctuations. We do not have any foreign currency hedging instruments in place.
Certain transactions related to our Company and our subsidiary, OBI, are denominated primarily in Taiwan dollars. As a result, our financial results could be affected by factors such as changes in foreign currency exchange rates or weak economic conditions in foreign markets where Optimer conducts business, including the impact of the existing crisis in the global financial markets in such countries and the impact on both the U.S. dollar and the Taiwan dollars.
We do not use derivative financial instruments for speculative purposes. We do not engage in exchange rate hedging or hold or issue foreign exchange contracts for trading purposes. Currently, we do not expect the impact of fluctuations in the relative fair value of other currencies to be material.
Item 8. Financial Statements and Supplementary Data
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
60 | |
|
61 | |
|
62 | |
|
63 | |
|
64 | |
|
65 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and
Stockholders of Optimer Pharmaceuticals Inc.
We have audited the accompanying consolidated balance sheets of Optimer Pharmaceuticals, Inc. as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Optimer Pharmaceuticals, Inc. at December 31, 2010 and 2009, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Optimer Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 10, 2011 expressed an unqualified opinion thereon.
|
|
/s/ ERNST & YOUNG LLP |
|
|
|
|
San Diego, California |
|
|
March 10, 2011 |
|
Optimer Pharmaceuticals, Inc.
|
|
|
December 31, |
| ||||
|
|
|
2010 |
|
2009 |
| ||
|
ASSETS |
|
|
|
|
| ||
|
Current assets: |
|
|
|
|
| ||
|
Cash and cash equivalents |
|
$ |
19,861,924 |
|
$ |
17,054,328 |
|
|
Short-term investments |
|
29,553,506 |
|
21,131,145 |
| ||
|
Research grant, contract, and other receivables |
|
53,552 |
|
84,164 |
| ||
|
Prepaid expenses and other current assets |
|
463,307 |
|
332,695 |
| ||
|
Total current assets |
|
49,932,289 |
|
38,602,332 |
| ||
|
Property and equipment, net |
|
697,683 |
|
672,896 |
| ||
|
Long-term investments |
|
882,000 |
|
882,000 |
| ||
|
Other assets |
|
508,190 |
|
498,762 |
| ||
|
Total assets |
|
$ |
52,020,162 |
|
$ |
40,655,990 |
|
|
|
|
|
|
|
| ||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
| ||
|
Current liabilities: |
|
|
|
|
| ||
|
Accounts payable |
|
$ |
2,307,820 |
|
$ |
2,625,240 |
|
|
Accrued expenses |
|
2,385,046 |
|
5,025,669 |
| ||
|
Total current liabilities |
|
4,692,866 |
|
7,650,909 |
| ||
|
Deferred rent |
|
141,138 |
|
253,474 |
| ||
|
Commitments and contingencies |
|
— |
|
— |
| ||
|
Stockholders’ equity: |
|
|
|
|
| ||
|
Preferred stock, par value $0.001, 10,000,000 shares authorized and no shares issued and outstanding at December 31, 2010 and 2009, respectively |
|
— |
|
— |
| ||
|
Common stock, $0.001 par value, 75,000,000 shares authorized, 39,278,965 shares and 33,139,373 shares issued and outstanding at December 31, 2010 and 2009, respectively |
|
39,279 |
|
33,139 |
| ||
|
Additional paid-in capital |
|
267,665,732 |
|
205,114,914 |
| ||
|
Accumulated other comprehensive gain |
|
298,850 |
|
38,063 |
| ||
|
Accumulated deficit |
|
(222,814,407 |
) |
(175,474,665 |
) | ||
|
Total Optimer Pharmaceuticals, Inc. stockholders’ equity |
|
45,189,454 |
|
29,711,451 |
| ||
|
Noncontrolling interest |
|
1,996,704 |
|
3,040,156 |
| ||
|
Total stockholders’ equity |
|
47,186,158 |
|
32,751,607 |
| ||
|
Total liabilities and stockholders’ equity |
|
$ |
52,020,162 |
|
$ |
40,655,990 |
|
See accompanying notes.
Optimer Pharmaceuticals, Inc.
Consolidated Statements of Operations
|
|
|
Years Ended December 31, |
| |||||||
|
|
|
2010 |
|
2009 |
|
2008 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Revenues: |
|
|
|
|
|
|
| |||
|
Research grants |
|
$ |
980,362 |
|
$ |
792,644 |
|
$ |
973,370 |
|
|
Collaborative research agreements |
|
500,000 |
|
100,000 |
|
50,000 |
| |||
|
Total revenues |
|
1,480,362 |
|
892,644 |
|
1,023,370 |
| |||
|
Operating expenses: |
|
|
|
|
|
|
| |||
|
Research and development |
|
32,797,672 |
|
34,416,983 |
|
30,206,068 |
| |||
|
Marketing |
|
5,051,006 |
|
1,049,760 |
|
1,280,951 |
| |||
|
General and administrative |
|
12,499,877 |
|
8,024,138 |
|
6,682,881 |
| |||
|
Total operating expenses |
|
50,348,555 |
|
43,490,881 |
|
38,169,900 |
| |||
|
Loss from operations |
|
(48,868,193 |
) |
(42,598,237 |
) |
(37,146,530 |
) | |||
|
Interest income and other, net |
|
329,290 |
|
363,998 |
|
1,561,934 |
| |||
|
Consolidated net loss |
|
(48,538,903 |
) |
(42,234,239 |
) |
(35,584,596 |
) | |||
|
Net loss attributable to noncontrolling interest, |
|
1,199,161 |
|
141,682 |
|
— |
| |||
|
Net loss attributable to Optimer Pharmaceuticals, Inc. |
|
$ |
(47,339,742 |
) |
$ |
(42,092,557 |
) |
$ |
(35,584,596 |
) |
|
Basic and diluted net loss per share attributable to Optimer Pharmaceuticals, Inc. common stockholders |
|
$ |
(1.25 |
) |
$ |
(1.30 |
) |
$ |
(1.24 |
) |
|
Shares used to compute basic and diluted net loss per share attributable to common stockholders |
|
37,830,452 |
|
32,468,702 |
|
28,682,542 |
| |||
See accompanying notes.
Optimer Pharmaceuticals, Inc.
Consolidated Statements of Stockholders’ Equity
|
|
|
Common Stock |
|
Additional paid- |
|
Accumulated |
|
Accumulated |
|
Noncontrolling |
|
|
| ||||||||
|
|
|
Shares |
|
Amount |
|
in capital |
|
income (loss) |
|
deficit |
|
Interest |
|
Total |
| ||||||
|
Balance at December 31, 2007 |
|
27,903,705 |
|
$ |
27,904 |
|
$ |
150,681,519 |
|
$ |
(8,396 |
) |
$ |
(97,697,558 |
) |
$ |
— |
|
$ |
53,003,469 |
|
|
Issuance of common stock upon exercise of options |
|
68,077 |
|
68 |
|
69,694 |
|
— |
|
— |
|
— |
|
69,762 |
| ||||||
|
Issuance of common stock during the registered direct offerings, net |
|
1,743,396 |
|
1,743 |
|
14,738,332 |
|
— |
|
— |
|
— |
|
14,740,075 |
| ||||||
|
Issuance of common stock pursuant to employee stock purchase plan |
|
47,726 |
|
48 |
|
291,185 |
|
— |
|
— |
|
— |
|
291,233 |
| ||||||
|
Compensation expense related to grants of consultant stock options |
|
— |
|
— |
|
25,637 |
|
— |
|
— |
|
— |
|
25,637 |
| ||||||
|
Employee stock based compensation |
|
— |
|
— |
|
1,738,439 |
|
— |
|
— |
|
— |
|
1,738,439 |
| ||||||
|
Retirement of treasury stock |
|
(46,153 |
) |
(46 |
) |
— |
|
— |
|
(99,954 |
) |
— |
|
(100,000 |
) | ||||||
|
Comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Unrealized loss on short-term investment |
|
— |
|
— |
|
— |
|
(5,374 |
) |
— |
|
— |
|
(5,374 |
) | ||||||
|
Foreign currency translation adjustment |
|
— |
|
— |
|
— |
|
51,858 |
|
— |
|
— |
|
51,858 |
| ||||||
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
(35,584,596 |
) |
— |
|
(35,584,596 |
) | ||||||
|
Comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
(35,538,112 |
) | ||||||
|
Balance at December 31, 2008 |
|
29,716,751 |
|
29,717 |
|
167,544,806 |
|
38,088 |
|
(133,382,108 |
) |
— |
|
34,230,503 |
| ||||||
|
Issuance of common stock upon exercise of options |
|
125,430 |
|
125 |
|
145,050 |
|
— |
|
— |
|
— |
|
145,175 |
| ||||||
|
Issuance of common stock during the registered direct offerings, net |
|
3,252,366 |
|
3,252 |
|
32,865,715 |
|
— |
|
— |
|
— |
|
32,868,967 |
| ||||||
|
Issuance of common stock pursuant to employee stock purchase plan |
|
44,826 |
|
45 |
|
382,246 |
|
— |
|
— |
|
— |
|
382,291 |
| ||||||
|
Compensation expense related to grants of consultant stock options |
|
— |
|
— |
|
89,395 |
|
— |
|
— |
|
— |
|
89,395 |
| ||||||
|
Employee stock based compensation |
|
— |
|
— |
|
2,718,597 |
|
— |
|
— |
|
— |
|
2,718,597 |
| ||||||
|
Sale of investment in subsidiary to non-controlling interest |
|
|
|
|
|
1,369,105 |
|
|
|
|
|
3,165,906 |
|
4,535,011 |
| ||||||
|
Comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Unrealized gain on short-term investment |
|
— |
|
— |
|
— |
|
13,959 |
|
— |
|
— |
|
13,959 |
| ||||||
|
Foreign currency translation adjustment |
|
— |
|
— |
|
— |
|
(13,984 |
) |
— |
|
15,932 |
|
1,948 |
| ||||||
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
(42,092,557 |
) |
(141,682 |
) |
(42,234,239 |
) | ||||||
|
Comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
(125,750 |
) |
(42,218,332 |
) | ||||||
|
Balance at December 31, 2009 |
|
33,139,373 |
|
33,139 |
|
205,114,914 |
|
38,063 |
|
(175,474,665 |
) |
3,040,156 |
|
32,751,607 |
| ||||||
|
Issuance of common stock upon exercise of options |
|
552,253 |
|
552 |
|
1,171,550 |
|
— |
|
— |
|
— |
|
1,172,102 |
| ||||||
|
Issuance of common stock during the public offerings, net |
|
4,887,500 |
|
4,888 |
|
51,203,837 |
|
— |
|
— |
|
— |
|
51,208,725 |
| ||||||
|
Issuance of common stock pursuant to employee stock purchase plan |
|
49,077 |
|
49 |
|
422,481 |
|
— |
|
— |
|
— |
|
422,530 |
| ||||||
|
Issuance of common stock for consulting services |
|
585,762 |
|
586 |
|
3,377,331 |
|
— |
|
— |
|
— |
|
3,377,917 |
| ||||||
|
Compensation expense related to grants of consultant stock options and awards |
|
65,000 |
|
65 |
|
2,121,984 |
|
— |
|
— |
|
— |
|
2,122,049 |
| ||||||
|
Employee stock based compensation |
|
— |
|
— |
|
4,253,635 |
|
— |
|
— |
|
— |
|
4,253,635 |
| ||||||
|
Comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Unrealized loss on short-term investment |
|
— |
|
— |
|
— |
|
(3,020 |
) |
— |
|
— |
|
(3,020 |
) | ||||||
|
Foreign currency translation adjustment |
|
— |
|
— |
|
— |
|
263,807 |
|
— |
|
155,709 |
|
419,516 |
| ||||||
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
(47,339,742 |
) |
(1,199,161 |
) |
(48,538,903 |
) | ||||||
|
Comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
(1,043,452 |
) |
(48,122,407 |
) | ||||||
|
Balance at December 31, 2010 |
|
39,278,965 |
|
$ |
39,279 |
|
$ |
267,665,732 |
|
$ |
298,850 |
|
$ |
(222,814,407 |
) |
$ |
1,996,704 |
|
$ |
47,186,158 |
|
Optimer Pharmaceuticals, Inc.
Consolidated Statements of Cash Flows
|
|
|
Years Ended December 31, |
| |||||||
|
|
|
2010 |
|
2009 |
|
2008 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Operating activities |
|
|
|
|
|
|
| |||
|
Consolidated net loss |
|
$ |
(48,538,903 |
) |
$ |
(42,234,239 |
) |
$ |
(35,584,596 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
| |||
|
Depreciation and amortization |
|
306,718 |
|
216,783 |
|
219,878 |
| |||
|
Stock based compensation |
|
6,375,684 |
|
2,807,992 |
|
1,764,076 |
| |||
|
Issuance of common stock for consulting services |
|
3,377,917 |
|
— |
|
— |
| |||
|
(Gain) Loss on disposal of assets |
|
(25,511 |
) |
20,267 |
|
— |
| |||
|
Fair value adjustment for auction rate securities |
|
— |
|
— |
|
118,000 |
| |||
|
Deferred rent |
|
(112,336 |
) |
1,970 |
|
(30,390 |
) | |||
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
| |||
|
Prepaid expenses and other current assets |
|
(130,612 |
) |
200,676 |
|
339,439 |
| |||
|
Research grant and contract receivables |
|
30,612 |
|
127,135 |
|
(116,115 |
) | |||
|
Restricted cash |
|
— |
|
— |
|
(170,000 |
) | |||
|
Other assets |
|
(9,428 |
) |
(512 |
) |
(21,677 |
) | |||
|
Accounts payable and accrued expenses |
|
(2,958,043 |
) |
(162,582 |
) |
213,314 |
| |||
|
Net cash used in operating activities |
|
(41,683,902 |
) |
(39,022,510 |
) |
(33,268,071 |
) | |||
|
|
|
|
|
|
|
|
| |||
|
Investing activities |
|
|
|
|
|
|
| |||
|
Purchases of short-term investments |
|
(55,284,340 |
) |
(28,567,298 |
) |
(31,306,561 |
) | |||
|
Sales or maturity of short-term investments |
|
46,845,000 |
|
30,153,000 |
|
63,250,000 |
| |||
|
Purchase of property and equipment |
|
(305,992 |
) |
(215,763 |
) |
(208,686 |
) | |||
|
Net cash provided (used) in investing activities |
|
(8,745,332 |
) |
1,369,939 |
|
31,734,753 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Financing activities |
|
|
|
|
|
|
| |||
|
Proceeds from sale of common stock |
|
52,803,357 |
|
33,396,433 |
|
15,101,070 |
| |||
|
Proceeds from sale of subsidiary common stock |
|
— |
|
4,535,011 |
|
— |
| |||
|
Net cash provided by financing activities |
|
52,803,357 |
|
37,931,444 |
|
15,101,070 |
| |||
|
Effect of exchange rate changes on cash and cash equivalents |
|
433,473 |
|
(3,425 |
) |
19,314 |
| |||
|
Net increase in cash and cash equivalents |
|
2,807,596 |
|
275,448 |
|
13,587,066 |
| |||
|
Cash and cash equivalents at beginning of year |
|
17,054,328 |
|
16,778,880 |
|
3,191,814 |
| |||
|
Cash and cash equivalents at end of year |
|
$ |
19,861,924 |
|
$ |
17,054,328 |
|
$ |
16,778,880 |
|
|
|
|
|
|
|
|
|
| |||
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
| |||
|
Retirement of treasury stock |
|
$ |
— |
|
$ |
— |
|
$ |
100,000 |
|
See accompanying notes.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Summary of Significant Accounting Policies
Optimer Pharmaceuticals, Inc. (“Optimer” or the “Company”) was incorporated in Delaware on November 18, 1998. The Company has one majority-owned subsidiary, Optimer Biotechnology, Inc. (“OBI”), which is incorporated and located in Taiwan. In October 2009, Optimer sold 40% of its equity interest in OBI. Prior to the sale, OBI was a wholly owned subsidiary of Optimer.
Optimer is a biopharmaceutical company focused on discovering, developing and commercializing innovative hospital specialty products. The Company currently has two late-stage anti-infective product candidates, fidaxomicin, for the treatment of Clostridium difficile-infection, and Pruvel™ (prulifloxacin), for the treatment of infectious diarrhea. The Company is developing additional product candidates using its proprietary technology, including its OPopS™ drug discovery platform.
Principles of Consolidation
The consolidated financial statements include all the accounts of the Company and its majority owned subsidiary. All intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Cash, Cash Equivalents and Short-Term Investments
Investments with original maturities of less than 90 days at the date of purchase are considered to be cash equivalents. All other investments are classified as short-term investments which are deemed by management to be available-for-sale and are reported at fair value with net unrealized gains or losses reported in the consolidated statement stockholders’ equity (deficit). Realized gains and losses, and declines in value judged to be other than temporary, are included in investment income or interest expense. The cost of securities sold is computed using the specific identification method.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to a significant concentration of credit risk consist primarily of cash and cash equivalents and short-term investments. The Company maintains deposits in federally insured financial institutions in excess of federally insured limits. However, management believes the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. Additionally, the Company has established guidelines regarding diversification of its investments and their maturities, which are designed to maintain safety and liquidity.
Property and Equipment
Property and equipment, including leasehold improvements, are stated at cost. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets, generally five years. Leasehold improvements are amortized over the shorter of their useful lives or the terms of the related leases.
Deferred Rent
Rent expense is recorded on a straight-line basis over the term of the lease. The difference between rent expense accrued and amounts paid under the lease agreement is recorded as deferred rent in the accompanying consolidated balance sheets. Deferred rent is amortized over the lease term as a reduction of rent expense.
Foreign Currency Translation
The financial statements of foreign subsidiaries having the U.S. dollar as the functional currency, with certain transactions denominated in a local currency, are remeasured into U.S. dollars at their historical rates. The remeasurement of local currency amounts into U.S. dollars creates transaction adjustments that are included in net loss. Transaction and translation gains or losses were not material to the Company’s financial statements for any periods presented.
Fair Value of Financial Instruments
The carrying amount of cash and cash equivalents, short-term investments and accounts payable and accrued liabilities are considered to be representative of their respective fair values because of the short-term nature of those instruments. The fair value of available-for-sale securities is based upon market prices quoted on the last day of the fiscal period.
Impairment of Long-Lived Assets
Long-lived assets, such as property and equipment, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of would be separately presented in the balance sheet and reported at the lower of the carrying amount or the fair value less costs to sell, and are no longer depreciated. Assets and liabilities that are part of a disposed group and classified as held for sale would be presented separately in the appropriate asset and liability sections of the balance sheet. The Company has not recognized any impairment losses through December 31, 2010.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company provides a valuation allowance against net deferred tax assets unless, based upon the available evidence, it is more likely than not that the deferred tax assets will be realized.
Revenue Recognition
The Company’s license and collaboration agreements contain multiple elements, including non-refundable upfront fees, payments for reimbursement of third-party research costs, payments for ongoing research, payments associated with achieving specific development milestones and royalties based on specified percentages of net product sales, if any. The Company considers a variety of factors in determining the appropriate method of revenue recognition under these arrangements, such as whether the elements are separable, whether there are determinable fair values and whether there is a unique earnings process associated with each element of a contract.
Cash received in advance of services being performed is recorded as deferred revenue and recognized as revenue as services are performed over the applicable term of the agreement.
When a payment is specifically tied to a separate earnings process, revenues are recognized when the specific performance obligation associated with the payment is completed. Performance obligations typically consist of significant and substantive milestones pursuant to the related agreement. Revenues from milestone payments may be considered separable from funding for research services because of the uncertainty surrounding the achievement of milestones for products in early stages of development. Accordingly, these payments are allowed to be recognized as revenue if and when the performance milestone is achieved if they represent a separate earnings process.
In connection with certain research collaboration agreements, revenues are recognized from non-refundable upfront fees, which the Company does not believe are specifically tied to a separate earnings process, ratably over the term of the agreement. Research fees are recognized as revenue as the related research activities are performed.
With respect to revenues derived from reimbursement of direct out-of-pocket expenses for research costs associated with grants, where the Company acts as a principal, with discretion to choose suppliers, bears credit risk and performs part of the services required in the transaction, the Company records revenue for the gross amount of the reimbursement. The costs associated with these reimbursements are reflected as a component of research and development expense in the consolidated statements of operations.
None of the payments that the Company has received from collaborators to date, whether recognized as revenue or deferred, are refundable even if the related program is not successful.
Research and Development Expenses
The Company expenses costs related to research and development until technological feasibility has been established for the product. Once technological feasibility is established, all product costs are generally capitalized until the product is available for general release to customers. The Company has determined that technological feasibility for its product candidates will be reached when the requisite regulatory approvals are obtained to make the product available for sale, which, in the United States, generally occurs upon the approval of the NDA for such product. In November 2010, the Company paid the FDA $1.5 million for the NDA filing for fidaxomicin. The regulatory fee is potentially refundable if the Company can establish that it qualifies as a small business which is defined by the Small Business Association (“SBA”) as a company with 250 employees or less. The Company has asserted that it qualifies as a small business, has submitted data to the SBA to support its assertion and continues in discussions with the SBA. As of December 31, 2010, the Company has recorded the $1.5 million NDA fee as research and development expense. The Company will continue its pursuit of the refund and, if obtained, the Company will record a reduction to research and development expense in the quarter received.
The Company’s research and development expenses consist primarily of license fees, salaries and related employee benefits, costs associated with clinical trials managed by the Company’s contract research organizations and costs associated with non-clinical activities and regulatory approvals. The Company uses external service providers and vendors to conduct clinical trials, to manufacture supplies of product candidates to be used in clinical trials and to provide various other research and development-related products and services.
When nonrefundable payments for goods or services to be received in the future for use in research and development activities are made, the Company defers and capitalizes these types of payments. The capitalized amounts are expensed when the related goods are delivered or the services are performed.
In the consolidated statements of operations for the years ended December 31, 2009 and 2008 included in this filing, the Company reclassified $702,000 and $1,170,000, respectively, of medical affairs department expenses from marketing expenses to research and development expenses to be consistent with the classification in the year ended December 31, 2010.
Stock-Based Compensation
The Company recognizes in its financial statements the share-based payment transactions with employees and consultants based on their fair value and recognized as compensation expense over the vesting period. Compensation expense of $6.4 million, $2.8 million and $1.8 million was recognized in the years ended December 31, 2010, 2009 and 2008, respectively.
Stock-based compensation expense is estimated as of the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which generally represents the vesting period. The Company estimates the fair value of its stock options using the Black-Scholes option-pricing model and the fair value of its stock awards based on the quoted market price of its common stock.
Estimating the fair value for stock options requires judgment, including estimating stock-price volatility, expected term, expected dividends and risk-free interest rates. The expected volatility rates are based on the historical fluctuation in the stock price since inception. The average expected term is calculated using the simplified method. Expected dividends are estimated based on the Company’s dividend history as well as the Company’s current projections. The risk-free interest rate for periods approximating the expected terms of the options is based on the U.S. Treasury yield curve in effect at the time of grant. These assumptions are updated on an annual basis or sooner if there is a significant change in circumstances that could affect these assumptions.
Equity instruments issued to non-employees are recorded at their fair value, are periodically revalued as the equity instruments vest and are recognized as expense over the related service period.
Comprehensive Income (Loss)
Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Net income (loss) and other comprehensive income (loss), including foreign currency translation adjustments and unrealized gains and losses on investments, is required to be reported, net of their related tax effect, to arrive at comprehensive income (loss). As of December 31, 2010, the cumulative unrealized loss on investments and the cumulative gain on foreign currency translation adjustment was $3,020 and $301,870, respectively. As of December 31, 2009, the cumulative unrealized gain on investments and the cumulative loss on foreign currency adjustment was $13,959 and $52,022, respectively.
Net Loss Per Share Attributable to Common Stockholders
Basic net loss per share attributable to common stockholders is calculated by dividing the net loss attributable to common stockholders by the weighted average number of shares of common stock outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share attributable to common stockholders is computed by dividing the net loss attributable to common stockholders by the weighted average number of common stock equivalents outstanding for the period determined using the treasury-stock method. For purposes of this calculation stock options and warrants are considered to be common stock equivalents and are only included in the calculation of diluted net loss per share attributable to common stockholders when their effect is dilutive.
|
|
|
Years Ended December 31, |
| |||||||
|
|
|
2010 |
|
2009 |
|
2008 |
| |||
|
Historical |
|
|
|
|
|
|
| |||
|
Numerator: |
|
|
|
|
|
|
| |||
|
Net loss attributable to Optimer Pharmaceuticals, Inc. |
|
$ |
(47,339,742 |
) |
$ |
(42,092,557 |
) |
$ |
(35,584,596 |
) |
|
Denominator: |
|
|
|
|
|
|
| |||
|
Weighted average common shares outstanding |
|
37,830,452 |
|
32,468,702 |
|
28,682,542 |
| |||
|
Net loss attributable to common stockholders per share — basic and diluted |
|
$ |
(1.25 |
) |
$ |
(1.30 |
) |
$ |
(1.24 |
) |
|
Historical outstanding anti-dilutive securities not included in diluted net loss per share calculation |
|
|
|
|
|
|
| |||
|
Common stock options |
|
3,589,626 |
|
2,466,751 |
|
1,979,660 |
| |||
|
Common stock warrants |
|
91,533 |
|
91,533 |
|
— |
| |||
|
Total |
|
3,681,159 |
|
2,558,284 |
|
1,979,660 |
| |||
Recently Issued Accounting Pronouncements
The FASB issued the following accounting amendments:
In April 2010, the FASB issued amendments related to the revenue recognition method for milestone payments in research and development agreements. Under these amendments, entities can make an accounting policy election to recognize a payment that is contingent upon the achievement of a substantive milestone in its entirety in the period in which the milestone is achieved. The amendments are effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010, which means such amendments will take effect for the Company beginning with the fiscal year starting January 1, 2011. The adoption of this standard is not expected to have a material impact on the Company’s financial position, cash flow or results of operations.
In October 2009, the FASB issued authoritative guidance for arrangements with multiple deliverables. The guidance will allow companies to allocate consideration from contractual arrangements in multiple deliverables arrangements in a manner that better reflects the economics of the transaction. The new guidance requires expanded qualitative and quantitative disclosures and is effective for fiscal years beginning on or after June 15, 2010. Early adoption of the guidance is permitted. The Company is currently evaluating the impact of adopting this guidance on the Company’s future financial statements.
2. Fair Value of Financial Instruments
The following table summarizes the Company’s financial assets measured at fair value on a recurring basis as of December 31, 2010:
|
|
|
Quoted Prices in |
|
Other |
|
Unobservable |
|
Total |
| ||||
|
Cash equivalents |
|
$ |
19,861,924 |
|
$ |
— |
|
$ |
— |
|
$ |
19,861,924 |
|
|
Government agency bonds |
|
26,539,597 |
|
— |
|
— |
|
26,539,597 |
| ||||
|
Corporate bonds |
|
3,013,909 |
|
— |
|
— |
|
3,013,909 |
| ||||
|
Auction rate preferred securities |
|
— |
|
— |
|
882,000 |
|
882,000 |
| ||||
|
Level 1: |
|
Quoted prices in active markets for identical assets and liabilities; or |
|
|
Level 2: |
|
Quoted prices for identical or similar assets and liabilities in markets that are not active, or observable inputs other than quoted prices in active markets for identical assets and liabilities; or |
|
|
Level 3: |
|
Unobservable inputs. |
|
A reconciliation of the beginning and ending balances of assets measured at fair value on a recurring basis using Level 3 inputs is as follows:
|
|
|
Auction Rate |
| |
|
Beginning balance at January 1, 2010 |
|
$ |
882,000 |
|
|
Total gains and losses: |
|
|
| |
|
Realized net income |
|
— |
| |
|
Unrealized in accumulated other comprehensive income |
|
— |
| |
|
Purchases, sales, issuances and settlements |
|
— |
| |
|
Transfers in (out) of Level 3 |
|
— |
| |
|
Ending balance at December 31, 2010 |
|
$ |
882,000 |
|
|
|
|
|
| |
|
Change in unrealized gains (losses) included in net loss related to assets still held |
|
$ |
— |
|
All of the Company’s investments in available-for-sale securities are recorded at fair value based on quoted market prices. As of December 31, 2010, the Company held one auction rate preferred security (“ARPS”) valued at $882,000 with a perpetual maturity date that resets every 28 days. Although as of December 31, 2010, this ARPS continued to pay interest according to its stated terms, the market in these securities continues to be illiquid. Based on a discounted cash flow model used to determine the estimated fair value of its investment in ARPS, the Company has previously recognized in the consolidated statement of operations an unrealized loss of approximately $118,000 in investment income since the Company had determined that the decline in value was other than temporary. The assumptions used for the discounted cash flow model include estimates for interest rates, timing and amount of cash flows and expected holding period of the ARPS. The Company’s ARPS is classified as a long-term investment on the consolidated balance sheets, as the Company does not believe it could liquidate the security in the near term.
3. Short-Term Investments
The following is a summary of the Company’s investment securities, all of which are classified as available-for-sale. Determination of estimated fair value is based upon quoted market prices.
|
|
|
December 31, 2010 |
| ||||||||||
|
|
|
Gross |
|
Gross |
|
Gross |
|
Market Value |
| ||||
|
Government agency bonds |
|
$ |
26,542,210 |
|
$ |
2,311 |
|
$ |
(4,924 |
) |
$ |
26,539,597 |
|
|
Corporate bonds |
|
3,014,319 |
|
23 |
|
(433 |
) |
3,013,909 |
| ||||
|
|
|
$ |
29,556,529 |
|
$ |
2,334 |
|
$ |
(5,357 |
) |
$ |
29,553,506 |
|
|
|
|
December 31, 2009 |
| ||||||||||
|
|
|
Gross |
|
Gross |
|
Gross |
|
Market Value |
| ||||
|
Government agency bonds |
|
$ |
21,117,189 |
|
$ |
14,235 |
|
$ |
(279 |
) |
$ |
21,131,145 |
|
Investments in net unrealized loss positions as of December 31, 2010 are as follows:
|
|
|
|
|
Less Than 12 Months of |
|
Greater Than 12 Months of |
|
Total Temporary |
| ||||||||||||
|
|
|
Number of |
|
Fair Value |
|
Unrealized |
|
Fair Value |
|
Unrealized |
|
Fair Value |
|
Unrealized |
| ||||||
|
Government agency bonds |
|
14 |
|
$ |
18,578,023 |
|
$ |
(4,924 |
) |
$ |
— |
|
$ |
— |
|
$ |
18,578,023 |
|
$ |
(4,924 |
) |
|
Corporate bonds |
|
2 |
|
2,010,993 |
|
(433 |
) |
— |
|
— |
|
2,010,993 |
|
(433 |
) | ||||||
|
|
|
16 |
|
$ |
20,589,016 |
|
$ |
(5,357 |
) |
$ |
— |
|
$ |
— |
|
$ |
20,589,016 |
|
$ |
(5,357 |
) |
The amortized cost and estimated fair value of securities available-for-sale at December 31, 2010, by contractual maturity, are as follows:
|
|
|
Amortized Cost |
|
Estimated Fair Value |
| ||
|
Due in one year or less |
|
$ |
27,394,219 |
|
$ |
27,391,700 |
|
|
Due in one year to two years |
|
2,162,310 |
|
2,161,806 |
| ||
|
Total |
|
$ |
29,556,529 |
|
$ |
29,553,506 |
|
The weighted average maturity of short-term investments as of December 31, 2010 and 2009, was approximately four months and three months, respectively.
Evaluating Investments for Other-than Temporay Impairments
The Company considered a number of factors to determine whether the decline in value in its investments is other than temporary, including the length of time and the extent of which the market value has been less than cost, the financial condition of the issuer and the Company’s intent to hold and ability to retain these short-term investments. Based on these factors, excepts for the ARPS which the Company recorded as an other temporary impairment in 2008, the Company believes that the decline in value is temporary and primarily related to the change in market interest rates since purchase. The Company determined that it does not intend to sell its short-term investments and it is not more likely than not that the Company will be required to sell its short-term investment before recovery of their amortized cost bases, which may not occur until maturity. The Company anticipates full recovery of amortized cost with respect to these securities at maturity or sooner in the event of a change in the market interest rate environment.
4. Property and Equipment
Property and equipment is stated at cost and consists of the following:
|
|
|
December 31, |
| ||||
|
|
|
2010 |
|
2009 |
| ||
|
Equipment |
|
$ |
2,294,784 |
|
$ |
2,475,135 |
|
|
Furniture and fixtures |
|
211,295 |
|
198,716 |
| ||
|
Leasehold improvements |
|
1,288,714 |
|
1,217,694 |
| ||
|
Computer equipment and software |
|
495,971 |
|
364,155 |
| ||
|
|
|
4,290,764 |
|
4,255,700 |
| ||
|
Less accumulated depreciation and amortization |
|
(3,593,081 |
) |
(3,582,804 |
) | ||
|
Total property and equipment, net |
|
$ |
697,683 |
|
$ |
672,896 |
|
Depreciation of property and equipment is computed using the straight-line method over the estimated useful lives of the assets, which typically is five years. Leasehold improvements and assets acquired under capital leases are amortized over their estimated useful life or the related lease term, whichever is shorter.
5. Accrued Expenses
Accrued expenses consisted of the following:
|
|
|
December 31, |
| ||||
|
|
|
2010 |
|
2009 |
| ||
|
Accrued preclinical and clinical expenses |
|
$ |
262,399 |
|
$ |
3,443,275 |
|
|
Accrued research services |
|
151,751 |
|
165,705 |
| ||
|
Accrued legal fees |
|
61,967 |
|
73,663 |
| ||
|
Accrued salaries, wages and benefits |
|
1,872,191 |
|
1,313,145 |
| ||
|
Other accrued liabilities |
|
36,738 |
|
29,881 |
| ||
|
Total accrued expenses |
|
$ |
2,385,046 |
|
$ |
5,025,669 |
|
6. Revenue and Other Collaborative Agreements
Revenues from Research Grants
The Company has one active grant from National Institute of Allergy and Infectious Diseases (“NIAID”). This $3 million grant was awarded in September 2007 for three years and was extended to August 2011. The award is being used to conduct supplementary studies to the ongoing fidaxomicin trials to confirm narrow spectrum activity and potency of fidaxomicin against hypervirulent epidemic strains, to support additional toxicology and microbiological studies to demonstrate the safety and efficacy of the fidaxomicin compound and its major metabolite in CDI patients and to support a surveillance study of C. difficile isolates across North America to compare activity of fidaxomicin with existing CDI treatments. For the years ended December 31, 2010, 2009 and 2008, the Company recognized revenues related to research grants of $980,362, $792,644 and $973,370, respectively.
In November 2010, the Company received $244,000 in the form of a cash grant from the Qualifying Therapeutic Discovery Project program of the Internal Revenue Service (IRS) / the U.S. Treasury Department for expenditures related to its fidaxomicin development program. The Company had to meet eligible criteria defined by the guidelines of the Patient Protection and Affordable Care Act signed into law March 23, 2010 to qualify for the cash grant. The Company recorded the receipt of the cash grant as interest income and other, net in the Consolidated Statements of Operations.
Other Collaborative Agreements
Par Pharmaceuticals, Inc.
The Company holds worldwide rights to fidaxomicin. In February 2007, the Company repurchased the rights to develop and commercialize fidaxomicin in North America and Israel from Par under a prospective buy-back agreement. The Company paid Par a one-time $5.0 million milestone payment in June 2010 for the successful completion by the Company of its second pivotal Phase 3 trial for fidaxomicin. The Company may be obligated to pay Par a 5% royalty on net sales by the Company, its affiliates or its licensees of fidaxomicin in North America and Israel, and a 1.5% royalty on net sales by the Company or its affiliates of fidaxomicin in the rest of the world. In addition, in the event the Company licenses its right to market fidaxomicin in the rest of the world, the Company will be required to pay Par a 6.25% royalty on net revenues received by it related to fidaxomicin. The Company is obligated to pay each of these royalties, if any, on a country-by-country basis for seven years commencing on the applicable commercial launch in each such country.
Biocon Limited
In May 2010, the Company entered into a long-term supply agreement with Biocon, for the commercial manufacture of fidaxomicin API. Pursuant to the agreement, Biocon agreed to manufacture and supply the Company, up to certain limits, fidaxomicin API and subject to certain conditions, the Company agreed to purchase from Biocon at least a portion of its requirements for fidaxomicin API in the United States and Canada. The Company previously paid to Biocon $2.5 million for certain equipment purchases and manufacturing scale-up activities, and it may be entitled to recover up to $1.5 million of this amount under the supply agreement in the form of discounted prices for fidaxomicin API. The Company expensed the entire $2.5 million in the second quarter of 2009 because at the time of payment the recovery of up to $1.5 million could not be assured. The Company may be obligated to make additional payments to Biocon if it fails to meet the minimum purchase requirements after Biocon has dedicated certain manufacturing capacity to the production of fidaxomicin API and if Biocon is unable to manufacture alternative products with the dedicated capacity. Unless both the Company and Biocon agree to extend the term of the supply agreement, it will terminate seven and a half years from the date the Company obtains marketing authorization for fidaxomicin in the United States or Canada. The supply agreement will be terminated earlier in the event that the Company does not obtain marketing authorization for fidaxomicin in the United States or Canada prior to December 31, 2013. In addition, the supply agreement may be earlier terminated (i) by either party by giving two and a half years notice after the fifth anniversary of the Effective Date or upon a material breach of the supply agreement by the other party, (ii) by the Company upon the occurrence of certain events, including Biocon’s failure to supply requested amounts of fidaxomicin API, or (iii) by Biocon upon the occurrence of certain events, including our failure to purchase amounts of fidaxomicin API that it indicates in binding forecasts. The supply agreement terminated and superseded the prior supply agreement with Biocon, dated August 29, 2005, which was assigned to the Company by Par in February 2007.
Nippon Shinyaku, Co., Ltd.
In June 2004, the Company entered into a license agreement with Nippon Shinyaku. Under the terms of the agreement, the Company acquired the non-exclusive right to import and purchase Pruvel, and the exclusive right (with the right to sublicense), within the United States, to develop, make, use, offer to sell, sell and license products suitable for consumption by humans containing Pruvel. Under this agreement, the Company paid Nippon Shinyaku an up-front fee in the amount of $1.0 million and will be required to make one future milestone payment in the amount of $1.0 million upon filing, if any, its first NDA for Pruvel in the United States. Under the agreement, the Company pays Nippon Shinyaku for certain materials. If Nippon Shinyaku is unable to supply the Company with the contracted amount of Pruvel, then Nippon Shinyaku is obligated to grant the Company a non-exclusive, worldwide license to make or have made Pruvel, in which event the Company will owe Nippon Shinyaku a royalty based on the amount of net sales of Pruvel generated by the Company and the Company’s subsidiary. Additionally, the Company will owe Nippon Shinyaku certain royalties based on the amount of net sales of Pruvel less the amount of Pruvel the Company buys from Nippon Shinyaku. Either party may
terminate the agreement 60 days after giving notice of a material breach which remains uncured 60 days after written notice. If not terminated earlier, the agreement will terminate upon the later of ten years from the date of the first commercial sale of Pruvel in the United States or the date on which the last valid patent claim relating to Pruvel expires in the United States.
Cempra Pharmaceuticals, Inc.
In March 2006, the Company entered into a collaborative research and development and license agreement with Cempra. The Company granted to Cempra an exclusive worldwide license, except in ASEAN countries, with the right to sublicense, the Company’s patent and know-how related to the Company’s macrolide and ketolide antibacterial program. As partial consideration for granting Cempra the licenses, the Company obtained equity of Cempra and the Company assigned no value to such equity. The Company may receive milestone payments as product candidates are developed and/or co-developed by Cempra, in addition to milestone payments based on certain sublicense revenue. The aggregate potential amount of such milestone payments is not capped and, based in part on the number of products developed under the agreement, may exceed $24.5 million. The Company will also receive royalty payments based on a percentage of net sales of licensed products. The milestone payments will be triggered upon the completion of certain clinical development milestones and in certain instances, regulatory approval of products. In consideration of the foregoing, Cempra may receive milestone payments from the Company in the amount of $1.0 million for each of the first two products the Company develops which receive regulatory approval in ASEAN countries, as well as royalty payments on the net sales of such products. The research term of the agreement was completed in March 2008. Subject to certain exceptions, on a country-by-country basis, the general terms of this agreement continue until the later of: (i) the expiration of the last to expire patent rights of a covered product in the applicable country or (ii) ten years from the first commercial sale of a covered product in the applicable country. Either party may terminate the agreement in the event of a material breach by the other party, subject to prior notice and the opportunity to cure. Either party may also terminate the agreement for any reason upon 30 days’ prior written notice provided that all licenses granted by the terminating party to the non-terminating party will survive upon the express election of the non-terminating party.
Memorial Sloan-Kettering Cancer Center
In July 2002, the Company entered into a license agreement with MSKCC to acquire, together with certain nonexclusive licenses, exclusive, worldwide licensing and sublicensing rights to certain patented and patent-pending carbohydrate-based cancer immunotherapies. As partial consideration for the licensing rights, the Company paid to MSKCC a one-time fee consisting of both cash and 55,383 shares of its common stock. In anticipation of the various transactions involving OBI which the Company completed in October 2009, the Company assigned its rights and obligations under this agreement with OBI. Under the agreement, which was amended in June 2005, the Company owes MSKCC milestone payments in the following amounts for each licensed product: (i) $500,000 upon the commencement of Phase 3 clinical studies, (ii) $750,000 upon the filing of the first NDA, (iii) $1.5 million upon obtaining marketing approval in the United States and (iv) $1.0 million upon obtaining marketing approval in each and any of Japan and certain European countries, but only to the extent that the Company, and not a sublicensee, achieves such milestones. OBI may owe MSKCC royalties based on net sales generated from the licensed products and income OBI sources from its sublicensing activities, which royalty payments are credited against a minimum annual royalty payment OBI owes to MSKCC during the term of the agreement.
Scripps Research Institute
In July 1999, the Company acquired exclusive, worldwide rights to its OPopS technology from TSRI. This agreement includes the license to the Company of patents, patent applications and copyrights related to OPopS technology. The Company also acquired, pursuant to three separate license agreements with TSRI, exclusive, worldwide rights to over 20 TSRI patents and patent applications related to other potential drug compounds and technologies, including HIV/FIV protease inhibitors, aminoglycoside antibiotics, polysialytransferase, selectin inhibitors, nucleic acid binders, carbohydrate mimetics and osteoarthritis. Under the four agreements with TSRI, the Company paid TSRI license fees consisting of an aggregate of 239,996 shares of the Company’s common stock with a deemed aggregate fair market value of $46,400, as determined on the dates of each such payment. In October 2009, the Company assigned to OBI one of the agreements with TSRI related to OPT-88 which, after further evaluation, OBI decided not to pursue. In February 2011, OBI and TSRI agreed to terminate the agreement and OBI returned the patents related to OPT-88. Under each of the three remaining agreements, the Company owes TSRI royalties based on net sales by the Company, the Company’s affiliates and sublicensees of the covered products and royalties based on revenue the Company generates from sublicenses granted pursuant to the agreements. For the first licensed product under each of the three remaining agreements, the Company also will owe TSRI payments upon achievement of certain milestones. In two of the three TSRI agreements, the milestones are the successful completion of a Phase 2 trial or its foreign equivalent, the submission of an NDA or its foreign equivalent and government marketing and distribution approval. In the remaining TSRI agreement, the milestones are the initiation of a Phase 3 trial or its foreign equivalent, the submission of an NDA or its foreign equivalent and government marketing and distribution approval. The aggregate potential amount of milestone payments the Company may be required to pay TSRI under the three remaining TSRI agreements is approximately $11.1 million.
Optimer Biotechnology, Inc.
In October 2009, the Company entered into certain transactions involving OBI, then its wholly-owned subsidiary, to provide funding for the development of two of its early-stage, non-core programs. The transactions with OBI included an Intellectual Property Assignment and License Agreement, pursuant to which the Company assigned to OBI certain patent rights, information and know-how related to OPT-88 and OPT-822/821. In anticipation of these transactions, the Company also assigned, and OBI assumed, its rights and obligations under related license agreements with MSKCC and TSRI. Under this agreement, the Company is eligible to receive up to $10 million in milestone payments for each product developed under the development programs and is also eligible to receive royalties on net sales of any product which is commercialized under the programs. The term of the Intellectual Property Assignment and License Agreement continues until the last to expire of the patents assigned by the Company to OBI and the patents licensed to OBI under the TSRI and MSKCC agreements.
To provide capital for OBI’s product development efforts, the Company and OBI also entered into a financing agreement with a group of new investors. Simultaneously, the Company sold 40 percent of its existing OBI shares to the same group of new investors, and the Company and the new investors also purchased new OBI shares. The Company anticipates, pursuant to an amendment to an October 2009 financing agreement, that OBI will sell newly-issued shares of its common stock for gross proceeds of approximately 462.0 million New Taiwan dollars. The Company also anticipates that it will purchase 277.2 million New Taiwan Dollars of the shares expected to be issued in the financing such that the Company will maintain a 60% equity interest in OBI after the financing. The OBI financing occured in February 2011. See Subsequent Event footnote 11 for further disclosure.
7. Commitments
Leases
The Company leases office and research facilities under operating lease agreements that extend through November 2011 with one lease subject to one five-year renewal option and the other lease subject to two five-year renewal options. The Company has recorded deferred rent of $141,138 and $253,474 as of December 31, 2010 and 2009, respectively, in conjunction with the lease agreements.
At December 31, 2010, annual minimum rental payments due under the Company’s operating leases are as follows:
|
Years ending December 31, |
|
|
| |
|
2011 |
|
$ |
1,171,865 |
|
|
2012 |
|
111,204 |
| |
|
Total minimum lease payments |
|
$ |
1,283,069 |
|
Rent expense was $1,057,565, $1,068,542 and $831,137, for the years ended December 31, 2010, 2009, and 2008, respectively.
8. Stockholders’ Equity
Public Offerings
In July 2008, the Company received approximately $14.8 million in gross proceeds from the sale of 1,743,396 million shares of its common stock at $8.48 per share in a registered direct offering to institutional investors. These shares were sold pursuant to a shelf registration statement relating to $100 million worth of common or preferred stock, debt securities, warrants or any combination of these securities.
In March 2009, the Company received approximately $32.9 million in gross proceeds from the sale of its securities in a registered direct offering to institutional investors. The Company sold 3,252,366 million shares and warrants to purchase up to an aggregate of 91,533 shares of its common stock. The warrants are exercisable at an exercise price of $10.93 per share and will expire on March 9, 2014.
In March 2010, the Company completed the sale of 4,887,500 shares of its common stock in a public offering which included 637,500 shares sold pursuant to the full exercise of an overallotment option previously granted to the underwriter. The net proceeds to the Company from the sale of shares in the offering were approximately $51.2 million.
In July 2010, the Company issued 585,762 shares of common stock to AFOS, LLC as consideration for the engagement of an affiliate of AFOS to provide certain services to the Company. The shares are subject to various lock-up periods during which AFOS may not transfer such shares, except under certain exceptions.
Warrants
In connection with the registered direct offering which occurred in March 2009, the Company sold warrants to purchase up to an aggregate of 91,533 shares of its common stock. The warrants are exercisable at an exercise price of $10.93 per share and will expire on March 9, 2014.
Noncontrolling Interest
In October 2009, the Company sold 40% of its equity interest in OBI. Pursuant to authoritative guidance, the Company accounts and reports for minority interests, the portion of OBI not owned by the Company, as noncontrolling interests and classifies them as a component of stockholders’ equity on the consolidated balance sheet of the Company. The Company includes the net loss attributable to noncontrolling interests as part of its consolidated net loss.
The following table reconciles equity attributable to noncontrolling interest:
|
|
|
For the Year Ended |
| |
|
Noncontrolling interest, January 1, 2010 |
|
$ |
3,040,156 |
|
|
Net loss attributable to noncontrolling interest |
|
(1,199,161 |
) | |
|
Translation adjustments |
|
155,709 |
| |
|
Noncontrolling interest, December 31, 2010 |
|
$ |
1,996,704 |
|
Equity Compensation Plans
Optimer Pharmaceuticals, Inc.
Stock Options
In November 1998, the Company adopted the 1998 Stock Plan (the “1998 Plan”). The Company terminated and ceased granting options under the 1998 Plan upon the closing of the Company’s initial public offering in February 2007.
In December 2006, the Company’s board of directors approved the 2006 Equity Incentive Plan (“2006 Plan”). The 2006 Plan became effective upon the closing of the Company’s initial public offering. A total of 2,000,000 shares of the Company’s common stock were initially made available for sale under the plan. The 2006 Plan provides for annual increases in the number of shares available for issuance thereunder on the first day of each fiscal year, beginning with the Company’s 2008 fiscal year, equal to the lesser of (i) 5% of the outstanding shares of the Company’s common stock on the last day of the immediately preceding fiscal year; (ii) 750,000 shares; or (iii) such other amount as the board of directors may determine. Pursuant to this provision, 750,000 additional shares of the Company’s common stock were reserved for issuance under the 2006 Plan on January 1, 2010 and 2009. Under the 2006 Plan, the exercise price of options granted must at least be equal to the fair market value of the Company’s common stock on the date of grant.
Options granted under both the 1998 Plan and the 2006 Plan generally expire 10 years from the date of grant (five years for a 10% stockholder) and vest over a period of four years. The exercise price of options granted must at least be equal to the fair market value of the Company’s common stock on the date of grant. The 2006 Plan is administered by the compensation committee of the Company’s board of directors. In September 2008, the Company’s board of directors established a New Hire Stock Option Committee consisting of the Chief Executive Officer and the Chief Financial Officer. The New Hire Stock Option has authority to grant stock options under specified parameters to newly-hired, non-executive employees.
Performance-Based Stock Options, Performance-Based Restricted Stock Units, and Stock Awards
On May 5, 2010, the Company’s Board of Directors appointed Pedro Lichtinger as its President and CEO and as a member of its Board of Directors. Pursuant to Mr. Lichtinger offer letter, he received performance-based stock options to purchase up to an aggregate of 480,000 shares of common stock and performance-based restricted stock units covering up to an aggregate of 120,000 shares of common stock, which vest over time beginning on the dates the Company achieves specified development and commercialization goals.
Simultaneously with Mr. Lichtinger’s appointment, Michael Chang resigned as the Company’s President and CEO. The Company entered into a consulting agreement with Dr. Chang to provide general consulting services. Pursuant to his consulting agreement and as part of his compensation, Dr. Chang received performance-based stock options to purchase up to an aggregate of 400,000 shares of common stock which vest over time beginning on the dates certain regulatory filings are accepted and approved. Dr. Chang has continued to serve as a member of the Company’s Board of Directors as Chairman and following Dr. Chang’s appointment as Chairman, Dr. Chang received a grant of 65,000 fully-vested shares of common stock.
The performance-based stock options, performance-based restricted stock units and stock grant were made under the 2006 Plan.
Following is a summary of stock option activity, including performance-based stock options:
|
|
|
Options |
|
Weighted- |
| |
|
Balance as of December 31, 2007 |
|
1,615,317 |
|
$ |
2.65 |
|
|
Granted |
|
452,250 |
|
$ |
6.95 |
|
|
Exercised |
|
(68,078 |
) |
$ |
1.02 |
|
|
Canceled |
|
(19,829 |
) |
$ |
7.66 |
|
|
Balance as of December 31, 2008 |
|
1,979,660 |
|
$ |
3.64 |
|
|
Granted |
|
637,750 |
|
$ |
11.24 |
|
|
Exercised |
|
(125,430 |
) |
$ |
1.16 |
|
|
Canceled |
|
(25,229 |
) |
$ |
7.60 |
|
|
Balance as of December 31, 2009 |
|
2,466,751 |
|
$ |
5.69 |
|
|
Granted |
|
1,815,450 |
|
$ |
11.88 |
|
|
Exercised |
|
(552,253 |
) |
$ |
2.12 |
|
|
Canceled |
|
(140,322 |
) |
$ |
11.39 |
|
|
Balance as of December 31, 2010 |
|
3,589,626 |
|
$ |
9.15 |
|
The aggregate intrinsic value of options exercised during the year ended December 31, 2010, 2009 and 2008 was approximately $4,680,334, $1,294,667 and $452,296, respectively. The aggregate intrinsic value of options outstanding and options exercisable as of December 31, 2010 was approximately $9,329,018 and $8,256,849, respectively.
The following table summarizes information concerning outstanding and exercisable stock options as of December 31, 2010:
|
|
|
December 31, 2010 |
| ||||||||||
|
|
|
Options Outstanding |
|
Options Exercisable |
| ||||||||
|
Exercise Price |
|
Number of Shares |
|
Weighted Average |
|
Weighted |
|
Number of Shares |
|
Weighted |
| ||
|
$0.65 - $9.56 |
|
1,271,687 |
|
5.86 |
|
$ |
4.44 |
|
950,540 |
|
$ |
3.07 |
|
|
$9.70 - $12.34 |
|
2,234,439 |
|
8.15 |
|
$ |
11.66 |
|
529,764 |
|
$ |
10.68 |
|
|
$12.42 - $14.86 |
|
83,500 |
|
8.53 |
|
$ |
13.68 |
|
28,102 |
|
$ |
13.80 |
|
|
$0.65 - $14.86 |
|
3,589,626 |
|
7.35 |
|
$ |
9.15 |
|
1,508,406 |
|
$ |
5.94 |
|
Of the options outstanding, options to purchase 1,508,406 shares were vested as of December 31, 2010, with a weighted average remaining contractual life of 5.6 years and a weighted average exercise price of $5.94 per share, while options to purchase 2,081,220 shares were unvested.
Share-Based Compensation
The Black-Scholes option-pricing model was developed for use in estimating the fair value of traded options, which have no vesting restrictions and are fully transferable. In addition, the Black-Scholes option-pricing model requires the input of subjective assumptions, including the expected stock price volatility. The following table shows the assumptions used to compute stock-based compensation expense for the stock options granted during the years ended December 31, 2010, 2009 and 2008 using the Black-Scholes option pricing model:
|
Stock Options including performance-based stock options |
|
2010 |
|
2009 |
|
2008 |
|
|
Risk-free interest rate |
|
2.27-3.53 |
% |
2.00-2.56 |
% |
2.41-3.00 |
% |
|
Dividend yield |
|
0.00 |
% |
0.00 |
% |
0.00 |
% |
|
Expected life of options (years) |
|
5.02-10.00 |
|
5.27-6.08 |
|
6.02-6.08 |
|
|
Volatility |
|
69.30-79.07 |
% |
69.93-71.39 |
% |
64.95-67.00 |
% |
The risk-free interest rate assumption was based on the United States Treasury’s rates for U.S. Treasury zero-coupon bonds with maturities similar to those of the expected term of the award being valued. The assumed dividend yield was based on the Optimer’s expectation of not paying dividends in the foreseeable future. The weighted average expected life of options was calculated using the simplified method. This decision was based on the lack of relevant historical data due to Optimer’s limited history. In addition, due to Optimer’s limited historical data, the estimated volatility incorporates the historical volatility of comparable companies whose share prices are publicly available.
Based on these assumptions, the weighted average grant-date fair values of stock options granted during the years ended December 31, 2010, 2009 and 2008 was $7.35, $7.11 and $4.01 per share, respectively.
As of December 31, 2010, the total unrecognized compensation expense related to stock options was approximately $8,401,921 and the related weighted-average period over which it is expected to be recognized is approximately 3.6 years.
Employee Stock Purchase Plan
Concurrent with the Company’s initial public offering in February 2007, the Company’s board of directors adopted the employee stock purchase plan (“ESPP”) in December 2006, and the stockholders approved the plan in January 2007. A total of 200,000 shares of the Company’s common stock were initially made available for sale under the plan. In addition, the employee stock purchase plan provides for annual increases in the number of shares available for issuance under the purchase plan on the first day of each fiscal year, beginning with the Company’s 2008 fiscal year, equal to the lesser of (i) 3% of the outstanding shares of the Company’s common stock on the last day of the immediately preceding fiscal year; (ii) 300,000 shares; or (iii) such other amount as may be determined by the Company’s board of directors. Pursuant to this provision, 300,000 additional shares of the Company’s common stock were reserved for issuance under the ESPP on January 1, 2008. The Company’s board of directors determined to reserve zero additional shares under the ESPP as of January 1, 2011 and 2010.
As of December 31, 2010, there were 164,312 shares of common stock issued and 335,688 shares remained available for issuance under the ESPP.
The following table shows the assumptions used to compute stock-based compensation expense for the stock purchased under the ESPP during the year ended December 31, 2010, 2009 and 2008 using the Black-Scholes option pricing model:
|
Employee Stock Options |
|
2010 |
|
2009 |
|
2008 |
|
|
Risk-free interest rate |
|
0.17%-0.20 |
% |
0.21-0.43 |
% |
1.30-2.19 |
% |
|
Dividend yield |
|
0.00 |
% |
0.00 |
% |
0.00 |
% |
|
Expected life (years) |
|
6 months |
|
6 months |
|
6 months |
|
|
Volatility |
|
34.08%-40.82 |
% |
35.53-74.94 |
% |
45.05-143.12 |
% |
For the years ended December 31, 2010, 2009 and 2008, the Company recorded stock-based compensation expense related to the ESPP of $119,281, $146,777 and $147,712, respectively.
Total stock-based compensation expense, related to all of Optimer’s stock options, restricted stock units, stock awards issued to employees and consultants and employee stock purchases, recognized for the years ended December 31, 2010, 2009 and 2008 was comprised as follows:
|
|
|
December 31, |
| |||||||
|
|
|
2010 |
|
2009 |
|
2008 |
| |||
|
Research and development |
|
$ |
1,596,515 |
|
$ |
1,162,274 |
|
$ |
527,874 |
|
|
Marketing |
|
375,429 |
|
371,738 |
|
309,635 |
| |||
|
General and administrative |
|
4,246,903 |
|
1,273,980 |
|
926,567 |
| |||
|
Total stock-based compensation expense |
|
$ |
6,218,847 |
|
$ |
2,807,992 |
|
$ |
1,764,076 |
|
Optimer Biotechnology, Inc.
Stock Options
In March 2010, OBI’s board of directors approved a Stock Option Plan and reserved 8.0 million shares of OBI common stock for issuance of equity awards thereunder. The Stock Option Plan provides for the issuance of stock options, restricted stock awards and stock appreciation rights to employees, directors and consultants of OBI. The options generally vest over four years and have a maximum contractual term of ten years.
During 2010, OBI granted 2,664,000 option shares to its employees and 50,000 option shares to a consultant. There were no options exercised or canceled during the year.
Valuations
The following table shows the assumptions used to compute stock-based compensation expense for the stock options granted by OBI during the year ended December 31, 2010, using the Black-Scholes option pricing model:
|
Risk-free interest rate |
|
1.25%-1.63 |
% |
|
Dividend yield |
|
0.00 |
% |
|
Expected life of options (years) |
|
6.08-10.00 |
|
|
Volatility |
|
88.10%-92.14 |
% |
The risk-free interest rate assumption was based on the Central Bank of China interest rates. The assumed dividend yield was based on OBI’s expectation of not paying dividends in the foreseeable future. The weighted-average expected life of options was calculated using the simplified method. This decision was based on the lack of relevant historical data due to OBI’s limited history. Due to OBI’s limited historical data, OBI used the historical volatility of OBI’s peers whose share prices are publicly available to estimate the volatility rate of OBI options.
The following table summarizes the stock-based compensation expense for OBI included in each operating expense line item in Optimer’s consolidated statements of operations as of December 31, 2010:
|
Research and development |
|
$ |
43,450 |
|
|
General and administrative |
|
113,387 |
| |
|
Stock-based compensation expense |
|
$ |
156,837 |
|
At December 31, 2010, the total unrecognized stock-based compensation expense relating to OBI’s unvested stock-based awards granted to employees, net of forfeitures, was $511,041, which OBI anticipates recognizing as a charge against income over a weighted average period of 2.9 years.
9. Income Taxes
There were no unrecognized tax benefits as of the date of adoption and there were no unrecognized tax benefits included in the balance sheet at December 31, 2010 that would, if recognized, affect the effective tax rate.
The Company’s practice is to recognize interest and/or penalties related to income tax matters in income tax expense. The Company had no accrual for interest or penalties on the Company’s balance sheets at December 31, 2010 and 2009 and has not recognized interest and/or penalties in the statement of operations for the year ended December 31, 2010.
The Company is subject to taxation in the United States, California and various foreign jurisdictions. The Company’s tax years for 1999 and forward are subject to examination by the Federal and California tax authorities due to the carryforward of unutilized net operating losses and research and development credits. The Company’s treatment of certain expenses in 2007 as currently deductible was audited by the IRS. The IRS determined that these expenses were related to a license fee and should have been capitalized and amortized over 15 years. The Company has agreed to this change and adjusted the deferred tax asset related to the net operating loss (“NOL”) carryover and fixed assets accordingly.
As of December 31, 2010, the Company completed a Section 382/383 analysis regarding the limitation of net operating loss and research and development credit carryforwards and determined that the entire amount of federal and state NOL and credit carryovers are available for utilization, subject to the annual limitation. Any carryforwards that will expire prior to utilization as a result of future limitations will be removed from deferred tax assets with a corresponding reduction of the valuation allowance. Due to the existence of the valuation allowance, future changes in the unrecognized tax benefits will not impact the effective tax rate.
At December 31, 2010, the Company had Federal, California and foreign income tax net operating loss carryforwards of approximately $183.7 million, $184.3 million and $2.9 million, respectively. The Federal and California tax loss carryforwards will begin expiring in 2020 and 2012 respectively, unless previously utilized. The foreign losses originate from the Company’s Taiwan subsidiary and expire ten years after origination. In addition, the Company has Federal and California research tax credit carryforwards of approximately $5.5 million and $3.0 million, respectively. The Federal research and development credit carryforwards will begin to expire in 2020 unless previously utilized. The California research and development credit carryforwards will carry forward indefinitely until utilized. The Company also has California state manufacturer’s investment tax credit carryforwards of $103,000 which will begin to expire in 2011 unless previously utilized. Under the Section 382 of the Internal Revenue Code of 1986, as amended, substantial changes in our ownership may limit the amount of net operating loss and tax credit carryforwards that could be utilized annually in the future to offset taxable income. Any such annual limitation may significantly reduce the utilization of the net operating losses and tax credits before they expire.
The Company has net operating loss carryforwards related to stock option exercises, which will result in an increase to additional paid-in capital and a decrease in income taxes payable when the tax loss carryforwards are utilized.
Significant components of the Company’s deferred tax assets as of December 31, 2010 and 2009 are listed below. A valuation allowance of $91.0 million and $71.4 million at December 31, 2010 and 2009, respectively, has been recognized to offset the net deferred tax assets as realization of such assets is uncertain. Amounts are shown as of December 31, of the respective years:
|
|
|
2010 |
|
2009 |
| ||
|
Deferred tax assets: |
|
|
|
|
| ||
|
Net operating loss carryforwards |
|
$ |
72,499,000 |
|
$ |
61,743,000 |
|
|
Tax credits |
|
7,575,000 |
|
6,303,000 |
| ||
|
Capitalized license, net |
|
6,477,000 |
|
— |
| ||
|
Other, net |
|
4,440,000 |
|
3,340,000 |
| ||
|
Total deferred tax assets |
|
90,991,000 |
|
71,386,000 |
| ||
|
Valuation allowance for deferred tax assets |
|
(90,991,000 |
) |
(71,386,000 |
) | ||
|
|
|
$ |
— |
|
$ |
— |
|
Income taxes computed by applying the U.S. Federal Statutory rates to income from continuing operations before income taxes are reconciled to the provision for income taxes set forth in the statement of earnings as follows:
|
|
|
2010 |
|
2009 |
|
2008 |
| |||
|
Tax benefit at statutory federal rate |
|
$ |
16,085,000 |
|
$ |
14,308,000 |
|
$ |
12,099,000 |
|
|
State tax benefit, net of federal |
|
2,758,000 |
|
2,453,000 |
|
2,075,000 |
| |||
|
Foreign subsidiary transactions |
|
(77,000 |
) |
115,000 |
|
(223,000 |
) | |||
|
Generation of research and development credits |
|
1,273,000 |
|
3,020,000 |
|
524,000 |
| |||
|
Stock compensation expense |
|
14,000 |
|
(248,000 |
) |
(207,000 |
) | |||
|
Permanent difference for R&D credit expense addback |
|
(356,000 |
) |
(862,000 |
) |
(143,000 |
) | |||
|
Other |
|
(92,000 |
) |
(33,000 |
) |
94,000 |
| |||
|
Change in valuation allowance |
|
(19,605,000 |
) |
(18,753,000 |
) |
(14,219,000 |
) | |||
|
|
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
10. Employee Benefit Plans
Effective January 1, 2000, the Company established a 401(k) plan covering substantially all employees. Employees may contribute up to 100% of their compensation per year (subject to a maximum limit prescribed by federal tax law). The Company may elect to make a discretionary contribution or match a discretionary percentage of employee contributions. As of December 31, 2010, 2009 and 2008, the Company had not elected to make any contributions to the 401(k) plan.
11. Subsequent Event
In February 2011, the Company entered into a collaboration and license agreement with Astellas Pharma Europe Ltd. (“Astellas”) pursuant to which the Company granted to Astellas an exclusive, royalty-bearing license under certain of the Company’s know-how and intellectual property to develop and commercialize fidaxomicin in the Astellas territory. Under the terms of the agreement, Astellas has agreed to use commercially reasonable efforts to develop and commercialize fidaxomicin in the Astellas territory at its expense, and is obligated to achieve certain additional regulatory and commercial diligence milestones with respect to fidaxomicin in the Astellas territory. The Company and Astellas may also agree to collaborate in, and share data resulting from, global development activities with respect to fidaxomicin, in which case we and Astellas will be obligated to co-fund such activities. In addition, under the terms of the agreement, Astellas granted the Company an exclusive, royalty-free license under know-how and intellectual property generated by Astellas and its sublicensees in the course of developing fidaxomicin and controlled by Astellas or its affiliates for use by the Company and any of the Company’s sublicensees in the development and commercialization of fidaxomicin outside the Astellas territory and, following termination of the agreement and subject to payment by the Company of single-digit royalties, in the Astellas territory. In addition, under the terms of a supply agreement entered into by the Company and Astellas on the same date, the Company will be the exclusive supplier of fidaxomicin to Astellas for Astellas’ development and commercialization activities in the Astellas territory during the term of the supply agreement, and Astellas is obligated to pay the Company an amount equal to cost plus an agreed mark-up for such supply.
Under the terms of the license agreement with Astellas, Astellas paid the Company an upfront fee equal to 50.0 million Euros, or approximately $69.2 million, and the Company is eligible to receive additional cash payments totaling up to 115.0 million Euros upon the achievement by Astellas of specified regulatory and commercial milestones. In addition, the Company will be entitled to receive escalating double-digit royalties ranging from the high teens to low twenties on net sales of fidaxomicin products in the Astellas territory, which royalties are subject to reduction in certain, limited circumstances. Such royalties will be payable by Astellas on a product-by-product and country-by-country basis until a generic product accounts for a specified market share of the applicable fidaxomicin product in the applicable country.
The agreements with Astellas will continue in effect on a product-by-product and country-by-country basis until expiration of Astellas’ obligation to pay royalties with respect to each fidaxomicin product in each country in the Astellas territory, unless terminated early by either party as more fully described below. Following expiration, Astellas’ license to develop and commercialize the applicable fidaxomicin product in the applicable country will become non-exclusive. The Company and Astellas may each terminate either of the agreements prior to expiration upon the material breach of such agreement by the other party, or upon the bankruptcy or insolvency of the other party. In addition, the Company may terminate the agreements prior to expiration in the event Astellas or any of its affiliates or sublicensees commences an interference or opposition proceeding with respect to, challenges the validity or enforceability of, or opposes any extension of or the grant of a supplementary protection certificate with respect to, any patent licensed to it, and Astellas may terminate the agreements prior to expiration for any reason on a product-by-product and country-by-country basis upon 180 days’ prior written notice to the Company. Upon any such termination, the license granted to Astellas (in total or with respect to the terminated product or terminated country, as applicable) will terminate and revert to the Company.
On February 9, 2011, the Company entered into an office lease agreement with 101 Hudson Leasing Associates for approximately 14,000 square feet of office space in Jersey City, New Jersey. The initial lease will expire approximately February 2016 with minimum rent payable of approximately $39,000 per month during the first 27 months of the initial term, will increase to approximately $41,000 per month thereafter through the 39th month of the initial term and will increase to approximately $44,000 per month thereafter through the expiration of the initial term. In addition, we have the option to extend the lease for an additional five-year term, which would commence upon the expiration of the initial five-year term.
On February 16, 2011, the Company completed the sale of 6,900,000 shares of its common stock in a public offering which included 900,000 shares sold pursuant to the full exercise of an overallotment option previously granted to the underwriter. The net proceeds to the Company from the sale of shares in the offering were approximately $73.1 million.
On February 16, 2011, OBI terminated its license agreement with TSRI and returned patents related to OPT-88 to TSRI.
On February 28, 2011, pursuant to an amendment to an October 2009 financing agreement, OBI sold newly-issued shares of its common stock for gross proceeds of approximately 462.0 million New Taiwan Dollars (approximately $15.5 million based on then-current exchange rates). The Company purchased 277.2 million New Taiwan Dollars (approximately $9.3 million based on then-current exchange rates) of the shares issued in the financing, such that the Company currently maintains a 60% equity interest in OBI.
12. Quarterly Financial Date (Unaudited)
Selected quarterly consolidated financial data is shown below (in thousands, except per share data).
|
|
|
First |
|
Second |
|
Third |
|
Fourth |
| ||||
|
2010 Quarters |
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ |
298 |
|
$ |
357 |
|
$ |
669 |
|
$ |
156 |
|
|
Operating expenses |
|
14,019 |
|
10,762 |
|
12,920 |
|
12,647 |
| ||||
|
Loss from operations |
|
(13,721 |
) |
(10,405 |
) |
(12,251 |
) |
(12,491 |
) | ||||
|
Net loss |
|
(13,697 |
) |
(10,351 |
) |
(12,228 |
) |
(12,263 |
) | ||||
|
Net loss attributable to Optimer Pharmaceuticals, Inc. common stockholders |
|
$ |
(13,496 |
) |
$ |
(10,061 |
) |
$ |
(11,837 |
) |
$ |
(11,946 |
) |
|
Basic and diluted net loss attributable to Optimer Pharmaceuticals, Inc. common stockholders |
|
$ |
(0.39 |
) |
$ |
(0.26 |
) |
$ |
(0.30 |
) |
$ |
(0.31 |
) |
|
|
|
First |
|
Second |
|
Third |
|
Fourth |
| ||||
|
2009 Quarters |
|
|
|
|
|
|
|
|
| ||||
|
Revenue |
|
$ |
83 |
|
$ |
425 |
|
$ |
176 |
|
$ |
209 |
|
|
Operating expenses |
|
11,158 |
|
12,875 |
|
9,616 |
|
9,842 |
| ||||
|
Loss from operations |
|
(11,074 |
) |
(12,450 |
) |
(9,440 |
) |
(9,634 |
) | ||||
|
Net loss |
|
(10,887 |
) |
(12,362 |
) |
(9,406 |
) |
(9,579 |
) | ||||
|
Net loss attributable to Optimer Pharmaceuticals, Inc. common stockholders |
|
$ |
(10,887 |
) |
$ |
(12,362 |
) |
$ |
(9,406 |
) |
$ |
(9,437 |
) |
|
Basic and diluted net loss attributable to Optimer Pharmaceuticals, Inc. common stockholders |
|
$ |
(0.36 |
) |
$ |
(0.37 |
) |
$ |
(0.28 |
) |
$ |
(0.28 |
) |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this report, an evaluation was performed under the supervision and with the participation of our management, including our chief executive officer and chief financial officer (collectively, our “certifying officers”), of the effectiveness of the design and operation of our disclosure controls and procedures. Based on their evaluation, our certifying officers concluded that these disclosure controls and procedures are effective in providing reasonable assurance that the information required to be disclosed by us in our periodic reports filed with the SEC is recorded, processed, summarized and reported within the time periods specified by the SEC’s rules and SEC reports.
We believe that a controls system, no matter how well designed and operated, is based in part upon certain assumptions about the likelihood of future events, and therefore can only provide reasonable, not absolute, assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
In addition, we have reviewed our internal controls over financial reporting and have made no changes during the quarter ended December 31, 2010, that our certifying officers concluded materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Internal Control Over Financial Reporting
(a) Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
Our internal control over financial reporting is supported by written policies and procedures that pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and disposition of our assets’ provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles and that our receipts and expenditures are being made only in accordance with authorizations of our management and our Board or Directors; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2010. We based this assessment on criteria for effective internal control over financial reporting described in “Internal Control-Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Our assessment included an evaluation of the design of our internal control over financial reporting and testing of the operational effectiveness of its internal control over financial reporting. We reviewed the results of our assessment with the Audit Committee.
Based on this assessment, management determined that, as of December 31, 2010, our internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
Ernst & Young LLP, the independent registered public accounting firm that audited our consolidated financial statements included in this annual report, has issued its report on the effectiveness of our internal control over financial reporting as of December 31, 2010.
(b) Report of Independent Registered Public Accounting Firm
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The Board of Directors and
Stockholders of Optimer Pharmaceuticals, Inc.
We have audited Optimer Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“the COSO criteria”). Optimer Pharmaceuticals, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Optimer Pharmaceuticals, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Optimer Pharmaceuticals, Inc. as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010 of Optimer Pharmaceuticals, Inc. and our report dated March 10, 2011 expressed an unqualified opinion thereon.
|
|
/s/ Ernst & Young LLP |
|
San Diego, California |
|
|
March 10, 2011 |
|
None.
Certain information required by Part III is omitted from this report because we will file a definitive proxy statement within 120 days after the end of our fiscal year ended December 31, 2010 pursuant to Regulation 14A (the “Proxy Statement”) for our annual meeting of stockholders to be held on April 25, 2011, and certain information included in the Proxy Statement is incorporated herein by reference.
Item 10. Directors, Executive Officers and Corporate Governance
We have adopted a Code of Business Conduct and Ethics that applies to all officers, directors and employees. The Code of Business Conduct and Ethics is available on our website at www.optimerpharma.com. If we make any substantive amendments to the Code of Business Conduct and Ethics or grant any waiver from a provision of the Code to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our website. We will promptly disclose on our website (i) the nature of any amendment to the policy that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and (ii) the nature of any waiver, including an implicit waiver, from a provision of the policy that is granted to one of these specified individuals, the name of such person who is granted the waiver and the date of the waiver.
The other information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
Item 11. Executive Compensation
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
Item 14. Principal Accounting Fees and Services
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
Item 15. Exhibits, Financial Statement Schedules
1. Financial Statements
See Index to Consolidated Financial Statements in Item 8 of this report.
2. Financial Statement Schedules
None
3. Exhibits
|
Exhibit No. |
|
|
|
Description of Document |
|
3.1 |
|
(2) |
|
Certificate of Incorporation of Optimer Pharmaceuticals, Inc., as amended and restated. |
|
3.2 |
|
(5) |
|
Bylaws of Optimer Pharmaceuticals, Inc., as amended. |
|
4.1 |
|
(3) |
|
Common Stock Certificate of Optimer Pharmaceuticals, Inc. |
|
4.2 |
|
(1) |
|
Investors’ Rights Agreement by and among Optimer Pharmaceuticals, Inc. and certain investors listed therein dated November 30, 2005, as amended and restated. |
|
4.3 |
|
(6) |
|
Registration Rights Agreement, dated October 23, 2007, by and between Optimer Pharmaceuticals, Inc. and the purchasers listed on the signature pages thereto. |
|
4.4 |
|
(9) |
|
Form of Warrant to Purchase Common Stock. |
|
10.1 |
|
(1)* |
|
Master Services Agreement between Optimer Pharmaceuticals, Inc. and Advanced Biologics, LLC (subsequently INC Research, Inc.), dated November 16, 2005, as amended. |
|
10.2 |
|
(3)* |
|
License Agreement between Optimer Pharmaceuticals, Inc. and Sloan-Kettering Institute for Cancer Research dated July 31, 2002, as amended. |
|
10.3 |
|
(2)* |
|
License Agreement between Optimer Pharmaceuticals, Inc. and Nippon Shinyaku Co., Ltd., dated June 10, 2004. |
|
10.4 |
|
(1)* |
|
License Agreement between Optimer Pharmaceuticals, Inc. and The Scripps Research Institute dated July 23, 1999. |
|
10.5 |
|
(1)* |
|
License Agreement between Optimer Pharmaceuticals, Inc. and The Scripps Research Institute dated May 30, 2001. |
|
10.6 |
|
(1)* |
|
License Agreement between Optimer Pharmaceuticals, Inc. and The Scripps Research Institute dated June 1, 2004. |
|
10.7 |
|
(2) |
|
Building Lease between Optimer Pharmaceuticals, Inc. and Pacific Sorrento Technology Park dated May 1, 2001, as amended. |
|
10.8 |
|
(1)+ |
|
Form of Employee Proprietary Information Agreement of Optimer Pharmaceuticals, Inc. |
|
10.9 |
|
(1)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Sherwood L. Gorbach dated October 6, 2005. |
|
10.10 |
|
(10)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and John D. Prunty dated May 10, 2006, as amended. |
|
10.11 |
|
(1)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Tessie M. Che dated August 30, 2001. |
|
10.12 |
|
(1)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Youe-Kong Shue dated February 18, 2000. |
|
10.13 |
|
(1)+ |
|
Form of Indemnification Agreement between Optimer Pharmaceuticals, Inc. and its directors and officers. |
|
10.14 |
|
(1)+ |
|
1998 Stock Plan of Optimer Pharmaceuticals, Inc. |
|
10.15 |
|
(1)+ |
|
Stock Plan Stock Option Agreement of Optimer Pharmaceuticals, Inc. |
|
10.16 |
|
(2)+ |
|
2006 Equity Incentive Plan of Optimer Pharmaceuticals, Inc. |
|
10.17 |
|
(5)+ |
|
Employee Stock Purchase Plan of Optimer Pharmaceuticals, Inc., as amended. |
|
10.18 |
|
(2) |
|
Prospective Buy-Back Agreement between Optimer Pharmaceuticals, Inc. and Par Pharmaceutical, Inc. dated January 19, 2007. |
|
10.19 |
|
(12)+ |
|
Summary of Optimer Pharmaceuticals, Inc. 2010 Incentive Compensation Plan. |
|
10.20 |
|
(7) |
|
Office Lease, dated August 18, 2008, by and between Optimer Pharmaceuticals, Inc. and Trizec Sorrento Towers, LLC, as amended. |
|
10.21 |
|
(8)+ |
|
Optimer Pharmaceuticals, Inc. Severance Benefit Plan. |
|
10.22 |
|
(11)* |
|
Collaboration Research and Development and License Agreement between Optimer Pharmaceuticals, Inc. and Cempra Pharmaceuticals, Inc., dated March 31, 2006, as amended. |
|
10.23 |
|
(11)* |
|
Intellectual Property Assignment and License Agreement between Optimer Pharmaceuticals, Inc. and Optimer Biotechnology, Inc., dated October 30, 2009. |
|
10.24 |
|
(11) |
|
Financing Agreement between Optimer Pharmaceuticals, Inc. Optimer Biotechnology, Inc. and certain investors named therein, dated October 30, 2009, as amended. |
|
10.25 |
|
(17)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Francois-Xavier Frapaise dated January 27, 2009. |
|
10.26 |
|
(13)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Pedro Lichtinger dated May 5, 2010. |
|
10.27 |
|
+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Kurt Hartman dated November 12, 2010. |
|
10.28 |
|
+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Gregory Papaz dated December 20, 2010. |
|
10.29 |
|
(13)+ |
|
Separation and Consulting Agreement between Optimer Pharmaceuticals, Inc. and Michael N. Change dated May 5, 2010. |
|
10.30 |
|
(4) |
|
Compensation Agreement between Optimer Pharmaceuticals, Inc. and AFOS, LLC dated July 22, 2010. |
|
10.31 |
|
(15)* |
|
API Manufacturing and Supply Agreement between Optimer Pharmaceuticals, Inc. and Biocon Limited dated May 18, 2010. |
|
10.32 |
|
+ |
|
Separation Agreement between Optimer Pharmaceuticals, Inc. and Xavier Francois Frapaise dated January 20, 2011. |
|
10.33 |
|
+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Linda Amper dated January 18, 2011. |
|
10.34 |
|
(14)+ |
|
Amended and Restated Severance Benefit Plan of Optimer Pharmaceuticals,Inc. |
|
10.35 |
|
(15) |
|
First Amendment to Office Lease between Optimer Pharmaceuticals, Inc. and Trizec Sorrento Towers, LLC, dated May 3, 2010. |
|
10.36 |
|
(12)+ |
|
Summary of Optimer Pharmaceuticals, Inc. 2010 Incentive Compensation Plan. |
|
10.37 |
|
** |
|
Amendment to API Manufacturing and Supply Aggreement between Optimer Pharmaceuticals, Inc. and Biocon Limited, dated December 21, 2010. |
|
10.38 |
|
|
|
Office Lease between Optimer Pharmaceuticals, Inc. and 101 Hudson Leasing Associates dated February 9, 2011. |
|
10.39 |
|
(16) |
|
First Amendment to Financing Agreement between Optimer Pharmaceuticals, Inc. and Optimer Biotechnology, |
|
|
|
|
|
Inc. dated February 28, 2011. |
|
10.40 |
|
*** |
|
Collaboration and License Agreement between Optimer Pharmaceuticals, Inc. and Astellas Pharma Europe Ltd. dated February 2, 2011. |
|
10.41 |
|
*** |
|
Supply Agreement between Optimer Pharmaceuticals, Inc. and Astellas Pharma Europe Ltd. dated February 2, 2011. |
|
21.1 |
|
|
|
Subsidiary of Optimer Pharmaceuticals, Inc. |
|
23.1 |
|
|
|
Consent of Independent Registered Public Accounting Firm. |
|
31.1 |
|
|
|
Certification of principal executive officer required by Rule 13a-14(a) or Rule 15d-14(a). |
|
31.2 |
|
|
|
Certification of principal financial officer required by Rule 13a-14(a) or Rule 15d-14(a). |
|
32 |
|
|
|
Certification by the Chief Executive Officer and the Chief Financial Officer of Optimer Pharmaceuticals, Inc., as required by Rule 13a-14(b) or 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350). |
|
+ |
|
Indicates management contract or compensatory plan. |
|
* |
|
Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
|
** |
|
Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
|
*** |
|
Exhibit will be filed with the Securities and Exchange Commission with the Periodic Report filed by the Company for the three-month period ending March 31, 2011. |
|
(1) |
|
Filed with Registrant’s Registration Statement on Form S-1 on November 9, 2006. |
|
(2) |
|
Filed with Registrant’s Amendment No. 3 to Registration Statement on Form S-1 on January 22, 2007. |
|
(3) |
|
Filed with Registrant’s Amendment No. 4 to Registration Statement on Form S-1 on February 5, 2007. |
|
(4) |
|
Filed with the Registrant’s Current Report on Form 8-K on July 22, 2010. |
|
(5) |
|
Filed with the Registrant’s Current Report on Form 8-K on September 18, 2007. |
|
(6) |
|
Filed with the Registrant’s Current Report on Form 8-K on October 29, 2007. |
|
(7) |
|
Filed with the Registrant’s Current Report on Form 8-K on August 21, 2008. |
|
(8) |
|
Filed with the Registrant’s Current Report on Form 8-K on October 8, 2008. |
|
(9) |
|
Filed with the Registrant’s Current Report on Form 8-K on March 5, 2009. |
|
(10) |
|
Filed with the Registrant’s Annual Report on Form 10-K on March 12, 2009. |
|
(11) |
|
Filed with the Registrant’s Quarterly Report on Form 10-Q on November 3, 2009. |
|
(12) |
|
Filed with the Registrant’s Current Report on Form 8-K on March 11, 2010. |
|
(13) |
|
Filed with the Registrant’s Current Report on Form 8-K on May 6, 2010. |
|
(14) |
|
Filed with the Registrant’s Quarterly Report on Form 10-Q on August 4, 2010. |
|
(15) |
|
Filed with the Registrant’s Current Report on Form 8-K on February 10, 2011. |
|
(16) |
|
Filed with the Registrant’s Current Report on Form 8-K on March 2, 2011. |
|
(17) |
|
Filed with the Registrant’s Annual Report on Form 10-K on March 11, 2010. |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
OPTIMER PHARMACEUTICALS, INC. | |
|
|
|
|
|
Dated: March 10, 2011 |
By: |
/s/ Pedro Lichtinger |
|
|
Name: |
Pedro Lichtinger |
|
|
Title: |
President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ PEDRO LICHTINGER |
|
President, Chief Executive Officer and Director (Principal Executive Officer) |
|
March 10, 2011 |
|
Pedro Lichtinger |
|
|
|
|
|
|
|
|
|
|
|
/s/ JOHN D. PRUNTY |
|
Chief Financial Officer (Principal Accounting and Financial Officer) |
|
March 10, 2011 |
|
John D. Prunty |
|
|
|
|
|
|
|
|
|
|
|
/s/ MICHAEL N. CHANG |
|
Chairman |
|
March 10, 2011 |
|
Michael N. Chang |
|
|
|
|
|
|
|
|
|
|
|
/s/ ANTHONY E. ALTIG |
|
Director |
|
March 10, 2011 |
|
Anthony E. Altig |
|
|
|
|
|
|
|
|
|
|
|
/s/ MARK AUERBACH |
|
Director |
|
March 10, 2011 |
|
Mark Auerbach |
|
|
|
|
|
|
|
|
|
|
|
/s/ JOSEPH Y. CHANG |
|
Director |
|
March 10, 2011 |
|
Joseph Y. Chang |
|
|
|
|
|
|
|
|
|
|
|
/s/ PETER E. GREBOW |
|
Director |
|
March 10, 2011 |
|
PETER E. GREBOW |
|
|
|
|
|
|
|
|
|
|
|
/s/ HENRY A. MCKINNELL |
|
Director |
|
March 10, 2011 |
|
Henry A. McKinnell |
|
|
|
|
|
|
|
|
|
|
|
/s/ ROBERT L. ZERBE |
|
Director |
|
March 10, 2011 |
|
Robert L. Zerbe |
|
|
|
|
EXHIBIT INDEX
|
Exhibit No. |
|
|
|
Description of Document |
|
3.1 |
|
(2) |
|
Certificate of Incorporation of Optimer Pharmaceuticals, Inc., as amended and restated. |
|
3.2 |
|
(5) |
|
Bylaws of Optimer Pharmaceuticals, Inc., as amended. |
|
4.1 |
|
(3) |
|
Common Stock Certificate of Optimer Pharmaceuticals, Inc. |
|
4.2 |
|
(1) |
|
Investors’ Rights Agreement by and among Optimer Pharmaceuticals, Inc. and certain investors listed therein dated November 30, 2005, as amended and restated. |
|
4.3 |
|
(6) |
|
Registration Rights Agreement, dated October 23, 2007, by and between Optimer Pharmaceuticals, Inc. and the purchasers listed on the signature pages thereto. |
|
4.4 |
|
(9) |
|
Form of Warrant to Purchase Common Stock. |
|
10.1 |
|
(1)* |
|
Master Services Agreement between Optimer Pharmaceuticals, Inc. and Advanced Biologics, LLC (subsequently INC Research, Inc.), dated November 16, 2005, as amended. |
|
10.2 |
|
(3)* |
|
License Agreement between Optimer Pharmaceuticals, Inc. and Sloan-Kettering Institute for Cancer Research dated July 31, 2002, as amended. |
|
10.3 |
|
(2)* |
|
License Agreement between Optimer Pharmaceuticals, Inc. and Nippon Shinyaku Co., Ltd., dated June 10, 2004. |
|
10.4 |
|
(1)* |
|
License Agreement between Optimer Pharmaceuticals, Inc. and The Scripps Research Institute dated July 23, 1999. |
|
10.5 |
|
(1)* |
|
License Agreement between Optimer Pharmaceuticals, Inc. and The Scripps Research Institute dated May 30, 2001. |
|
10.6 |
|
(1)* |
|
License Agreement between Optimer Pharmaceuticals, Inc. and The Scripps Research Institute dated June 1, 2004. |
|
10.7 |
|
(2) |
|
Building Lease between Optimer Pharmaceuticals, Inc. and Pacific Sorrento Technology Park dated May 1, 2001, as amended. |
|
10.8 |
|
(1)+ |
|
Form of Employee Proprietary Information Agreement of Optimer Pharmaceuticals, Inc. |
|
10.9 |
|
(1)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Sherwood L. Gorbach dated October 6, 2005. |
|
10.10 |
|
(10)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and John D. Prunty dated May 10, 2006, as amended. |
|
10.11 |
|
(1)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Tessie M. Che dated August 30, 2001. |
|
10.12 |
|
(1)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Youe-Kong Shue dated February 18, 2000. |
|
10.13 |
|
(1)+ |
|
Form of Indemnification Agreement between Optimer Pharmaceuticals, Inc. and its directors and officers. |
|
10.14 |
|
(1)+ |
|
1998 Stock Plan of Optimer Pharmaceuticals, Inc. |
|
10.15 |
|
(1)+ |
|
Stock Plan Stock Option Agreement of Optimer Pharmaceuticals, Inc. |
|
10.16 |
|
(2)+ |
|
2006 Equity Incentive Plan of Optimer Pharmaceuticals, Inc. |
|
10.17 |
|
(5)+ |
|
Employee Stock Purchase Plan of Optimer Pharmaceuticals, Inc., as amended. |
|
10.18 |
|
(2) |
|
Prospective Buy-Back Agreement between Optimer Pharmaceuticals, Inc. and Par Pharmaceutical, Inc. dated January 19, 2007. |
|
10.19 |
|
(12)+ |
|
Summary of Optimer Pharmaceuticals, Inc. 2010 Incentive Compensation Plan. |
|
10.20 |
|
(7) |
|
Office Lease, dated August 18, 2008, by and between Optimer Pharmaceuticals, Inc. and Trizec Sorrento Towers, LLC, as amended. |
|
10.21 |
|
(8)+ |
|
Optimer Pharmaceuticals, Inc. Severance Benefit Plan. |
|
10.22 |
|
(11)* |
|
Collaboration Research and Development and License Agreement between Optimer Pharmaceuticals, Inc. and Cempra Pharmaceuticals, Inc., dated March 31, 2006, as amended. |
|
10.23 |
|
(11)* |
|
Intellectual Property Assignment and License Agreement between Optimer Pharmaceuticals, Inc. and Optimer Biotechnology, Inc., dated October 30, 2009. |
|
10.24 |
|
(11) |
|
Financing Agreement between Optimer Pharmaceuticals, Inc. Optimer Biotechnology, Inc. and certain investors named therein, dated October 30, 2009, as amended. |
|
10.25 |
|
(17)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Francois-Xavier Frapaise dated January 27, 2009. |
|
10.26 |
|
(13)+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Pedro Lichtinger dated May 5, 2010. |
|
10.27 |
|
+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Kurt Hartman dated November 12, 2010. |
|
10.28 |
|
+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Gregory Papaz dated December 20, 2010. |
|
10.29 |
|
(13)+ |
|
Separation and Consulting Agreement between Optimer Pharmaceuticals, Inc. and Michael N. Change dated May 5, 2010. |
|
10.30 |
|
(4) |
|
Compensation Agreement between Optimer Pharmaceuticals, Inc. and AFOS, LLC dated July 22, 2010. |
|
10.31 |
|
(15)* |
|
API Manufacturing and Supply Agreement between Optimer Pharmaceuticals, Inc. and Biocon Limited dated May 18, 2010. |
|
10.32 |
|
+ |
|
Separation Agreement between Optimer Pharmaceuticals, Inc. and Xavier Francois Frapaise dated January 20, 2011. |
|
10.33 |
|
+ |
|
Offer letter between Optimer Pharmaceuticals, Inc. and Linda Amper dated January 18, 2011. |
|
10.34 |
|
(14)+ |
|
Amended and Restated Severance Benefit Plan of Optimer Pharmaceuticals,Inc. |
|
10.35 |
|
(15) |
|
First Amendment to Office Lease between Optimer Pharmaceuticals, Inc. and Trizec Sorrento Towers, LLC, dated May 3, 2010. |
|
10.36 |
|
(12)+ |
|
Summary of Optimer Pharmaceuticals, Inc. 2010 Incentive Compensation Plan. |
|
10.37 |
|
** |
|
Amendment to API Manufacturing and Supply Aggreement between Optimer Pharmaceuticals, Inc. and Biocon Limited, dated December 21, 2010. |
|
10.38 |
|
|
|
Office Lease between Optimer Pharmaceuticals, Inc. and 101 Hudson Leasing Associates dated February 9, 2011. |
|
10.39 |
|
(16) |
|
First Amendment to Financing Agreement between Optimer Pharmaceuticals, Inc. and Optimer Biotechnology, Inc. dated February 28, 2011. |
|
10.40 |
|
*** |
|
Collaboration and License Agreement between Optimer Pharmaceuticals, Inc. and Astellas Pharma Europe Ltd. dated February 2, 2011. |
|
10.41 |
|
*** |
|
Supply Agreement between Optimer Pharmaceuticals, Inc. and Astellas Pharma Europe Ltd. dated February 2, 2011. |
|
21.1 |
|
|
|
Subsidiary of Optimer Pharmaceuticals, Inc. |
|
23.1 |
|
|
|
Consent of Independent Registered Public Accounting Firm. |
|
31.1 |
|
|
|
Certification of principal executive officer required by Rule 13a-14(a) or Rule 15d-14(a). |
|
31.2 |
|
|
|
Certification of principal financial officer required by Rule 13a-14(a) or Rule 15d-14(a). |
|
32 |
|
|
|
Certification by the Chief Executive Officer and the Chief Financial Officer of Optimer Pharmaceuticals, Inc., as required by Rule 13a-14(b) or 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350). |
|
+ |
|
Indicates management contract or compensatory plan. |
|
* |
|
Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
|
** |
|
Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
|
*** |
|
Exhibit will be filed with the Securities and Exchange Commission with the Periodic Report filed by the Company for the three-month period ending March 31, 2011. |
|
(1) |
|
Filed with Registrant’s Registration Statement on Form S-1 on November 9, 2006. |
|
(2) |
|
Filed with Registrant’s Amendment No. 3 to Registration Statement on Form S-1 on January 22, 2007. |
|
(3) |
|
Filed with Registrant’s Amendment No. 4 to Registration Statement on Form S-1 on February 5, 2007. |
|
(4) |
|
Filed with the Registrant’s Current Report on Form 8-K on July 22, 2010. |
|
(5) |
|
Filed with the Registrant’s Current Report on Form 8-K on September 18, 2007. |
|
(6) |
|
Filed with the Registrant’s Current Report on Form 8-K on October 29, 2007. |
|
(7) |
|
Filed with the Registrant’s Current Report on Form 8-K on August 21, 2008. |
|
(8) |
|
Filed with the Registrant’s Current Report on Form 8-K on October 8, 2008. |
|
(9) |
|
Filed with the Registrant’s Current Report on Form 8-K on March 5, 2009. |
|
(10) |
|
Filed with the Registrant’s Annual Report on Form 10-K on March 12, 2009. |
|
(11) |
|
Filed with the Registrant’s Quarterly Report on Form 10-Q on November 3, 2009. |
|
(12) |
|
Filed with the Registrant’s Current Report on Form 8-K on March 11, 2010. |
|
(13) |
|
Filed with the Registrant’s Current Report on Form 8-K on May 6, 2010. |
|
(14) |
|
Filed with the Registrant’s Quarterly Report on Form 10-Q on August 4, 2010. |
|
(15) |
|
Filed with the Registrant’s Current Report on Form 8-K on February 10, 2011. |
|
(16) |
|
Filed with the Registrant’s Current Report on Form 8-K on March 2, 2011. |
|
(17) |
|
Filed with the Registrant’s Annual Report on Form 10-K on March 11, 2010. |
