Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2010
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 000-51623
Cynosure, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 04-3125110 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 5 Carlisle Road Westford, MA |
01886 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code
(978) 256-4200
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Name of each exchange on which registered | |
| Class A Common Stock, $0.001 par value | The Nasdaq Global Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ¨ Yes ¨ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer | x | Non-accelerated filer | ¨ | Smaller reporting company | ¨ | ||||||
| (Do not check if a smaller reporting company) | ||||||||||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
Aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant, based on the last sale price for such stock on June 30, 2010: $103,789,553.
The number of shares outstanding of each of the registrant’s classes of common stock, as of March 7, 2011:
| Class | Number of Shares | |
| Class A Common Stock, $0.001 par value |
9,639,018 | |
| Class B Common Stock, $0.001 par value |
2,939,161 | |
Portions of the registrant’s definitive Proxy Statement for its 2010 Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report.
Table of Contents
| PART I | ||||||
| Item 1. |
4 | |||||
| Item 1A. |
22 | |||||
| Item 1B. |
37 | |||||
| Item 2. |
37 | |||||
| Item 3. |
37 | |||||
| Item 4. |
38 | |||||
| PART II | ||||||
| Item 5. |
39 | |||||
| Item 6. |
42 | |||||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
43 | ||||
| Item 7A. |
57 | |||||
| Item 8. |
58 | |||||
| Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
58 | ||||
| Item 9A. |
58 | |||||
| Item 9B. |
61 | |||||
| PART III | ||||||
| Item 10. |
62 | |||||
| Item 11. |
62 | |||||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
62 | ||||
| Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
62 | ||||
| Item 14. |
62 | |||||
| PART IV | ||||||
| Item 15. |
63 | |||||
| 64 | ||||||
2
Table of Contents
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this Annual Report regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans, objectives of management and expected market growth are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
| • | our ability to identify and penetrate new markets for our products and technology; |
| • | our ability to innovate, develop and commercialize new products; |
| • | our ability to obtain and maintain regulatory clearances; |
| • | our sales and marketing capabilities and strategy in the United States and internationally; |
| • | our intellectual property portfolio; and |
| • | our estimates regarding expenses, future revenues, capital requirements and needs for additional financing. |
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this Annual Report, particularly in Item 1A of this Annual Report, and in our other public filings with the Securities and Exchange Commission that could cause actual results or events to differ materially from the forward-looking statements that we make.
You should read this Annual Report and the documents that we have filed as exhibits to the Annual Report completely and with the understanding that our actual future results may be materially different from what we expect. It is routine for internal projections and expectations to change as the year or each quarter in the year progresses, and therefore it should be clearly understood that the internal projections and beliefs upon which we base our expectations are made as of the date of this Annual Report and may change prior to the end of each quarter or the year. While we may elect to update forward-looking statements at some point in the future, we do not undertake any obligation to update any forward-looking statements whether as a result of new information, future events or otherwise, except as required by law.
3
Table of Contents
PART I
| Item 1. | Business |
Overview
We develop and market aesthetic treatment systems that are used by physicians and other practitioners to perform non-invasive and minimally invasive procedures to remove hair, treat vascular and pigmented lesions, rejuvenate the skin, liquefy and remove unwanted fat through laser lypolysis and reduce the appearance of cellulite. We are also developing in conjunction with our development agreement with Unilever Ltd., (Unilever) a laser treatment system for the home use market. This product is targeted for commercialization in 2012. Our systems incorporate a broad range of laser and other light-based energy sources, including Alexandrite, pulse dye, Nd:Yag and diode lasers, as well as intense pulsed light. We believe that we are one of only a few companies that currently offer aesthetic treatment systems utilizing Alexandrite and pulse dye lasers, which are particularly well suited for some applications and skin types. We offer single energy source systems as well as workstations that incorporate two or more different types of lasers or pulsed light technologies. We offer multiple technologies and system alternatives at a variety of price points depending primarily on the number and type of energy sources included in the system. Our newer products are designed to be easily upgradeable to add additional energy sources and handpieces, which provides our customers with technological flexibility as they expand their practices. As the aesthetic treatment market evolves to include new customers, such as aesthetic spas and additional physician specialties, we believe that our broad technology base and tailored solutions will provide us with a competitive advantage.
We focus our development and marketing efforts on offering leading, or flagship, products for the following high volume applications:
| • | the Elite product line for hair removal, treatment of facial and leg veins and pigmentations; |
| • | the Smartlipo product line for LaserBodySculptingSM for the removal of unwanted fat; |
| • | the Cellulaze,SmoothShapes XV and TriActive product line for the temporary and long-term reduction in the appearance of cellulite; |
| • | the Affirm/SmartSkin product line for anti-aging applications, including treatments for wrinkles, skin texture, skin discoloration and skin tightening; |
| • | the Cynergy product line for the treatment of vascular lesions; and |
| • | the Accolade product line for the removal of benign pigmented lesions, as well as multi-colored tattoos. |
We sell our products through a direct sales force in North America, France, Spain, the United Kingdom, Germany, Korea, China and Japan and through international distributors in 78 other countries. As of December 31, 2010, we had sold more than 10,300 aesthetic treatment systems worldwide. See Note 6 to our Consolidated Financial Statements for revenue, net asset and long-lived asset information by geographic region.
Corporate Information
We were incorporated under the laws of the State of Delaware in July 1991. Our principal executive offices are located at 5 Carlisle Road, Westford, Massachusetts 01886, and our telephone number is (978) 256-4200.
We are subject to the informational requirements of the Securities Exchange Act of 1934, as amended, and, accordingly, file reports, proxy statements and other information with the Securities and Exchange Commission. Such reports, proxy statements and other information can be read and copied at the public reference facilities
4
Table of Contents
maintained by the Securities and Exchange Commission at the Public Reference Room, 100 F Street, NE, Room 1580, Washington, D.C. 20549. Information regarding the operation of the Public Reference Room may be obtained by calling the Securities and Exchange Commission at 1-800-SEC-0330. The Securities and Exchange Commission maintains a website (http://www.sec.gov) that contains material regarding issuers that file electronically with the Securities and Exchange Commission.
Our website address is www.cynosure.com. The information on our website is not a part of this Annual Report. We make available free of charge on our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission.
Industry
Aesthetic Market Opportunity
Medical Insight, Inc., an independent industry research and analysis firm, reported that in 2009, total sales of products in the global aesthetic market reached $3.8 billion and that they would expand 12.3% per year to more than $6.8 billion by 2014. Global sales of disposable components of professional aesthetic equipment would also increase by 13.3% per year while disposables for skin tightening and body shaping systems would increase 12.7% annually from $72.9 million to $132.7 million. Additionally Medical Insight, Inc. noted that, in 2009, the body shaping and skin tightening global markets reached $325.1 million and that the overall platform would expand 12.4% annually through 2014.
Key factors affecting growth rates in the markets for aesthetic treatment procedures and aesthetic laser equipment include:
| • | improvements in overall economic conditions and an expanding physician base; |
| • | the aging population of industrialized countries and the amount of discretionary income of the “baby boomer” demographic segment; |
| • | the desire of many individuals to improve their appearance; |
| • | the development of technology that allows for safe and effective aesthetic treatment procedures as well as advances in treatable conditions; |
| • | the impact of managed care and reimbursement on physician economics, which has motivated physicians to establish or seek to expand their elective aesthetic practices with procedures that are paid for directly by patients; and |
| • | reductions in cost per procedure, which has attracted a broader base of clients and patients for aesthetic treatment procedures. |
Expansion Into Non-Traditional Physician Customer and Medi-Spa Markets
Aesthetic treatment procedures that use lasers and other light-based equipment have traditionally been performed by dermatologists and plastic surgeons. Based on published membership information from professional medical organizations, there are approximately 18,000 dermatologists and plastic surgeons in the United States. A broader group of physicians in the United States, including primary care physicians, obstetricians and gynecologists, have incorporated aesthetic treatment procedures into their practices. These non-traditional physician customers are largely motivated to offer aesthetic procedures by the potential for a reliable revenue stream that is unaffected by managed care and government payor reimbursement economics. We believe that there are approximately 100,000 of these potential non-traditional physician customers in the United States and Canada, representing a significant market opportunity that is only beginning to be addressed by
5
Table of Contents
suppliers of lasers and other light-based aesthetic equipment. Some physicians are electing to open medical spas, often adjacent to their conventional office space, where they perform aesthetic procedures in an environment designed to feel less like a health care facility.
The Structure of Skin and Conditions that Affect Appearance
The human skin consists of several layers. The epidermis is the outer layer and contains the cells that determine pigmentation, or skin color. The dermis is a thicker inner layer that contains hair follicles and large and small blood vessels. Beneath the dermis is a layer that contains subdermal fat. The dermis is composed of mostly collagen, which provides strength and flexibility to the skin.
The appearance of the skin may change over time due to a variety of factors, including age, sun damage, circulatory changes, deterioration of collagen and the human body’s diminished ability to repair and renew itself. These changes include:
| • | unwanted hair growth; |
| • | uneven pigmentation; |
| • | wrinkles; |
| • | blood vessels and veins that are visible at the skin’s surface; and |
| • | the appearance of cellulite. |
Changes to the skin caused by pigmentation are called pigmented lesions and are the result of the accumulation of excess melanin, the substance that gives skin its color. Pigmented lesions are characterized by the brown color of melanin and include freckles, solar lentigines, also known as sun spots or age spots, and café au lait birthmarks. Changes to the skin caused by abnormally large or numerous blood vessels located under the surface of the skin are called vascular lesions. Vascular lesions are characterized by blood vessels that are visible through the skin or that result in a red appearance of the skin. Vascular lesions may be superficial and shallow in the skin or deep in the skin. Shallow vascular lesions include small spider veins, port wine birthmarks, facial veins and rosacea, a chronic skin condition that causes rosy coloration and acne-like pimples on the face. Deep vascular lesions include large spider veins and leg veins.
People with undesirable skin conditions or unwanted hair growth often seek aesthetic treatments, including treatments using non-invasive laser and light-based technologies.
Laser and Light-Based Aesthetic Treatments
A laser is a device that creates and amplifies a narrow, intense beam of light. Lasers have been used for medical and aesthetic applications since the 1960s. Intense pulsed light technology was introduced in the 1990s and uses flashlamps, rather than lasers, to generate multiple wavelengths of light with varying pulse durations, or time intervals, over which the energy is delivered.
By producing intense bursts of highly focused light, lasers and other light-based technologies selectively target hair follicles, veins or collagen in or below the dermis, as well as cells responsible for pigmentation in the epidermis. When the target absorbs sufficient energy, it is destroyed. The degree to which energy is absorbed in the skin depends upon the skin structure targeted—e.g., hair follicle or blood vessel—and the skin type—e.g., light or dark. Different types of lasers and other light-based technologies are needed to effectively treat the spectrum of skin types and conditions. As a result, an active aesthetic practice may require multiple laser or other light-based systems in order to offer treatments to its entire client base.
6
Table of Contents
Different types of lasers are currently used for a wide range of aesthetic treatments. Each type of laser operates at its own wavelength, measured in nanometers, which corresponds to a particular emission and color in the light spectrum. The most common lasers used for non-invasive aesthetic treatments are:
| • | Pulse dye lasers—produce a yellow light that functions at a shallow penetration depth. |
| • | Alexandrite lasers—produce a near infrared invisible light that functions with high power at a deep penetration depth. |
| • | Diode lasers—produce a near infrared invisible light that functions at a deep penetration depth. |
| • | Nd:Yag lasers—produce a near infrared invisible light that functions over a wide range of penetration depths. |
In addition to selecting the appropriate wavelength for a particular application, laser and other light-based treatments require an appropriate balance among three other parameters to optimize safety and effectiveness for aesthetic treatments:
| • | energy level—the amount of light emitted to heat the target; |
| • | pulse duration—the time interval over which the energy is delivered; and |
| • | spot size—the diameter of the energy beam. |
As a result of the wide spectrum of aesthetic applications, patient skin types and users of technology, customer purchasing objectives for aesthetic treatment systems are diverse. We believe that as aesthetic spas and non-traditional physician customers play increasingly important roles as purchasers of aesthetic treatment systems, the market for these products will become even more diverse. Specifically, we expect that owners of different types of aesthetic treatment practices will place different emphases on various system attributes, such as breadth of treatment applications, return on investment, upgradeability and price. Accordingly, we believe that there is significant market opportunity for a company that tailors its product offerings to meet the needs of a wide range of market segments.
Our Solution
We offer tailored customer solutions to address the market for non-invasive light-based aesthetic treatment applications, including hair removal, treatment of vascular lesions, skin rejuvenation through the treatment of shallow vascular lesions and pigmented lesions, as well as multi-colored tattoos, treatment of wrinkles, skin texture, skin discoloration, skin tightening through tissue coagulation and the treatment of cellulite through temporary and long-term methods. We also offer products for a minimally invasive procedure for LaserBodySculpting for the removal of unwanted fat. We believe our laser and other light-based systems are reliable, user friendly and easily incorporated into both physician practices and medi-spas. We complement our product offerings with comprehensive and responsive service offerings, including assistance with training, aesthetic practice development consultation and product maintenance. As of December 31, 2010, we had sold more than 10,300 aesthetic treatment systems worldwide.
We believe that the following factors enhance our market position:
| • | Broad Technology Base. Our products are based on a broad range of technologies and incorporate different types of lasers, such as Alexandrite, pulse dye, Nd:Yag and diode, as well as intense pulsed light devices. We believe we are one of a few companies that currently offer aesthetic treatment systems using Alexandrite and pulse dye lasers, which are particularly well suited for some applications and skin types. The following table provides information regarding the principal energy sources used in laser and other light-based aesthetic treatments that we offer and the primary application of each of these energy sources. |
7
Table of Contents
| Energy Source |
Type of Light/Wavelength |
Principal Applications | ||
| Pulse Dye Laser |
Visible light (Yellow)(585/595 nm) |
Vascular lesions, including shallow and deep lesions | ||
| Alexandrite Laser |
Near infrared invisible light (755 nm) |
Hair removal, particularly for light skin types | ||
| Diode Laser |
Near infrared invisible light (805/940/980 nm) |
Hair removal, particularly for light skin types Vascular lesions, particularly shallow lesions Temporary reduction in the appearance of cellulite | ||
| Nd:Yag Laser |
Near infrared invisible light (1064 nm) |
Hair removal, particularly for medium and dark skin types Vascular lesions, particularly deep lesions | ||
| Intense Pulsed Light |
Visible/Near infrared invisible light (400-950 nm) |
Hair removal, all skin types Vascular lesions, particularly shallow lesions and pigmented lesions Temporary reduction in the appearance of cellulite | ||
| Multiple Energy Source Workstations |
Multiple | Multiple | ||
| (incorporating two or more energy sources) |
||||
| • | Expansive Portfolio of Aesthetic Treatment Systems. We offer a variety of individual workstations tailored to specific high volume cosmetic applications to enable our customers to select the aesthetic treatment system best suited to their practice, business or clinical need. Our product portfolio includes single energy source systems as well as workstations that incorporate two or more different types of lasers or light-based technologies. By offering multiple technologies and system alternatives at a variety of price points, we seek to provide customers with tailored solutions that meet the specific needs of their practices while providing significant flexibility in their level of investment. |
| • | Upgrade Paths Within Product Families. We design our products to facilitate upgrading within product families. These upgrade paths provide our customers with the opportunity to add additional energy sources and handpieces, which provides our customers with technological flexibility as they expand their practices. |
| • | Global Presence. We have offered our products in international markets for over 19 years, with approximately 59% of our revenue generated from product sales outside of North America in 2010. We target international markets through a direct sales force in France, Spain, the United Kingdom, Germany, Korea, China, Japan and Mexico and through international distributors in 78 other countries. |
| • | Strong Reputation Established Over 19-Year History. We have been in the business of developing and marketing aesthetic treatment systems for over 19 years. As a result of this history, we believe the Cynosure brand name is associated with a tradition of technological leadership. |
8
Table of Contents
Our Business Strategy
Our goal is to become the worldwide leader in providing non-invasive and minimally invasive aesthetic treatment systems. The key elements of our business strategy to achieve this goal are to:
| • | Offer a Full Range of Tailored Aesthetic Solutions. We believe that we have one of the broadest product portfolios in the industry, with multiple product offerings incorporating a range of laser and light sources at various price points across many aesthetic applications. Our approach is designed to allow our customers to select products that best suit their client base, practice size and the types of treatments that they wish to offer. This allows us to address the needs of the traditional physician customer market as well as the growing non-traditional physician customer market. Many of our newer products can be upgraded to systems with greater functionality as our customers’ practices expand. |
| • | Launch Innovative New Products and Technologies into High-Growth Aesthetic Applications. Our research and development team builds on our existing broad range of laser and light-based technologies to develop new solutions and products to target unmet needs in significant aesthetic treatment markets. Innovation continues to be a strong contributor to our strength. Since 2002, we have introduced 21 new products. In February 2011, we launched Cellulaze, the world’s first aesthetic laser device for the long-term reduction of cellulite into the European community. Also in February 2011, we expanded our body shaping treatment platform by acquiring substantially all of the assets of Eleme Medical and the introduction of SmoothShapes® XV. |
| • | Provide Comprehensive, Ongoing Customer Service. We support our customers with a worldwide service organization that includes 26 field service engineers in North America and 29 field service engineers outside of North America. The field service engineers install our products and respond rapidly to service calls to minimize disruption to our customers’ businesses. Most of our new products are modular in design to enable quick and efficient service and support. We maintain our service infrastructure with training and inventory hubs in Europe and the Asia/Pacific region. |
| • | Generate Additional Revenue from Existing Customer Base. We believe that there are opportunities for us to generate additional revenue from existing customers who are already familiar with our products. |
| • | Develop Home Use Application for our Technology. In June, 2009, we entered into a cooperative development agreement with Unilever to develop and commercialize light-based devices for the emerging home use personal care market for commercialization in 2012. |
Many of our existing traditional and non-traditional customers may be purchasers of additional aesthetic treatment systems to address increasing treatment volumes or new treatment applications. We also expect that customers purchasing our new modular products will be candidates for technology upgrades to enhance the capabilities of their systems. In addition, three of our flagship products, our Affirm, Smartlipo and Cellulaze systems, contain consumable parts and we generate additional revenue on sales of these consumable parts to our existing customers. As we continue to grow our service organization, we are seeking to increase the percentage of our customers that enter into service contracts, which would provide additional recurring customer revenue.
Products
We offer a broad portfolio of aesthetic treatment systems that address a wide variety of applications.
9
Table of Contents
The following table provides information concerning our flagship products and their applications. We use the flagship designation for our products that are our leading products for a particular application.
| Hair Removal |
Vascular Lesions |
Skin Rejuve- nation(1) |
Pigmented Lesions |
Treatment of Cellulite |
Acne | Tattoo Removal |
Anti- Aging |
LaserBody Sculpting and Removal of Unwanted Fat |
||||||||||||||||||||||||||||
| Flagship Products: |
||||||||||||||||||||||||||||||||||||
| Elite Family |
Flagship | X | X | X | ||||||||||||||||||||||||||||||||
| Smartlipo Family(2) |
Flagship | |||||||||||||||||||||||||||||||||||
| Cellulaze(2)/SmoothShapes XV |
Flagship | |||||||||||||||||||||||||||||||||||
| Affirm / SmartSkin |
Flagship | |||||||||||||||||||||||||||||||||||
| Cynergy |
X | Flagship | X | X | X | |||||||||||||||||||||||||||||||
| Accolade |
Flagship | X | ||||||||||||||||||||||||||||||||||
| (1) | We consider skin rejuvenation to be the treatment of shallow vascular lesions and pigmented lesions to rejuvenate the skin’s appearance. |
| (2) | We distribute the TriActive LaserDermology and Smartlipo systems in North America pursuant to a distribution agreement with El.En. S.p.A, which we refer to as El.En. We distribute the Smartlipo MPX/Triplex and Cellulaze systems internationally pursuant to a distribution agreement with El.En. |
System Components
Each of our systems consists of a control console and one or more handpieces. Our control consoles are each comprised of a graphical user interface, a laser or other light source, control system software and high voltage electronics. The graphical user interface allows the practitioner to set the appropriate laser or flashlamp parameters to meet the requirements of a particular application and patient. The laser or other light source consists of electronics, a visible aiming beam, a focusing lens and a laser or flashlamp. Using the graphical user interface, the practitioner can independently adjust the system’s power level and pulse duration to optimize the desired treatment’s safety and effectiveness. The graphical user interface on our multiple energy workstations also allows the practitioner to change energy sources with the press of a button. The graphical user interfaces on our intense pulsed light systems offer practitioners a choice between using programmed preset treatment settings that address a variety of skin types and treatment options or manually adjusting the energy level and pulse duration settings. The control system software communicates the operator’s instructions from the graphical user interface to the system’s components and manages system performance and calibration.
The handpieces on our laser systems deliver the laser energy through a maneuverable optical fiber to the treatment area. These handpieces weigh approximately eight ounces and are ergonomically designed to allow the practitioner to use the system with one hand and without becoming fatigued. Other features of our laser system handpieces include:
| • | interchangeable components that permit the practitioner to easily adjust the spot size; and |
| • | an integrated aiming beam of harmless visible light that allows the practitioner to verify the treatment area, thereby reducing the risk of unintended skin damage and potentially reducing treatment time. |
The handpieces for our intense pulsed light systems consist of the flashlamp, a wavelength filter and, on some models, an integrated flashlamp cooling system. These handpieces weigh approximately two pounds and also are ergonomically designed to be operated with one hand.
Three of our flagship products, our Affirm, Smartlipo and Cellulaze systems, contain consumable parts. The Affirm system contains a highly durable micro lens array tip, which delivers the laser energy employed that can
10
Table of Contents
treat an average of ten treatment areas. We currently offer three different micro lens array tips, which cover a variety of treatment areas. The Smartlipo systems contain a consumable laser fiber that delivers the laser energy directly to subcutaneous fat cells-causing them to rupture. The Smartlipo and Cellulaze systems also contain the ThermaGuide Intelligent delivery system which allows the physician the ability to accurately monitor temperature and determine the treatment doses that will provide safe and more effective tissue tightening through tissue coagulation and maintan an even, controlled flow of laser energy. Additionally, Cellulaze contains a consumable SideLight 3DTM fiber which delivers laser thermal energy and increases the elasticity and thickness of the skin.
Practitioners generally use our laser systems in combination with a cooling system. We offer our customers our SmartCool treatment cooling system, which we purchase from a third party supplier and sell as a private label product under the Cynosure SmartCool brand. Our SmartCool product has nine variable settings and allows the practitioner to provide a continuous flow of chilled air before, during and after treatment to cool and comfort the patient’s skin. The SmartCool handpiece, which is specially designed for use with our laser systems, interlocks with the laser handpiece. In contrast to some competitive cooling systems, there are no disposable supplies required to use our SmartCool system. In North America, our SmartCool system is generally packaged and sold with our laser aesthetic treatment systems, and nearly all of our North American customers purchase a SmartCool system when they purchase one of our laser aesthetic treatment systems. Outside of North America, our customers either purchase our SmartCool system when they purchase one of our aesthetic treatment systems or they purchase another cooling system from a third party supplier.
Applications
Practitioners use our products to perform a variety of non-invasive procedures to remove hair, treat vascular and pigmented lesions, rejuvenate skin through the treatment of shallow vascular lesions and pigmented lesions, treat wrinkles, skin texture, skin discoloration, skin tightening through tissue coagulation and to treat cellulite through both temporary and long-term methods. Practitioners also use our products to perform minimally invasive procedures for LaserBodySculpting for the removal of unwanted fat. The applications of our products are described below.
Hair Removal. In a typical laser or pulsed light hair removal treatment the practitioner selects appropriate laser or pulsed light parameters based on the patient’s skin and hair types and pre-cools the treatment area. Next, the practitioner applies the handpiece to the target area and delivers laser or pulsed light energy to the target melanin pigment of the hair follicle, destroying the hair follicle without harming the surrounding skin. Our Elite workstation is our flagship product for hair removal. In March 2009, we launched the Elite MPX system, a multi-wavelength workstation that combines vascular treatment, hair removal and skin rejuvenation in a single system. The workstation features a built-in Zimmer SmartCool® skin cooling system which is integrated into a single compact model saving office space and reducing treatment time. Our Elite MPX and Apogee Elite products include two energy sources in one laser system; an Alexandrite laser, which is best suited for patients with light skin types, and an Nd:Yag laser, which is best suited for hair removal for patients with medium and dark skin types or tanned skin. The practitioner can switch between these two energy sources simply by pushing a button on the system console. Our Elite MPX allows the practitioner to blend the two energy sources for a customized treatment protocol. The Apogee 5500 and Acclaim 7000 systems can also be used for hair removal.
LaserBodySculpting for the Removal of Unwanted Fat. The Smartlipo system was the first laser lipolysis system to offer a minimally invasive procedure for the removal of unwanted fat. The Smartlipo LaserBodySculpting(SM) procedure enables aesthetic surgeons to remove localized deposits of fat. The Smartlipo LaserBodySculpting procedure is performed by inserting a small cannula, or metal tube, containing a laser fiber and placing it under the skin in direct contact with the treatment area. The laser’s energy causes the fat cells to rupture and melt. In addition, the laser’s energy promotes collagen shrinkage and causes a tissue tightening effect. LaserBodySculpting is a minimally invasive procedure; therefore, it can be performed under local anesthesia with minimal trauma in comparison to alternative liposuction procedures. Since we launched the
11
Table of Contents
Smartlipo system in 2006, we have enhanced the product by increasing the energy levels from 6 watts to 18 watts therefore increasing the speed of the procedure. In 2008, we introduced Smartlipo MPX, which added a second wavelength in a new platform and includes our patented MultiPlex technology that enables the energy from two lasers to be blended and increased the energy levels up to 46 watts. In March 2009, we introduced SmartSense with ThermaGuide and ThermaView, our proprietary intelligent delivery systems for our Smartlipo laser lipolysis workstations. In October 2009, we introduced Smartlipo Triplex which added a third wavelength to the system.
Treatment of Cellulite Cellulite is a deposit of fat that causes a dimple or other uneven appearance of the skin on women, typically around the thighs, hips and buttocks. According to published reports, an estimated 80% of women have some degree of cellulite.
Long-Term Reduction of Cellulite. In February 2011, we introduced our CellulazeTM Cellulite Laser Workstation into the European community. Cellulaze is the world’s first minimally invasive surgical device, designed to reduce cellulite by restoring the normal structure of the skin and underlying connective tissue. In the Cellulaze procedure, which is performed under a local anesthetic, the physician inserts a small cannula under the skin. Our SideLight 3DTM side-firing technology directs controlled, laser thermal energy to the treatment zones. The laser is designed to diminish the lumpy pockets of fat, release the areas of skin depression and increase the elasticity and thickness of the skin. Patients require just one treatment. Like the Smartlipo systems, Cellulaze incorporates the ThermaGuide Intelligent delivery system which allows the physician the ability to accurately monitor temperature and determine the treatment doses that will provide safe and more effective tissue tightening through tissue coagulation and maintain an even, controlled flow of laser energy. The Cellulaze workstation is not FDA cleared. A 510k application was submitted in 2010 and we expect action from the FDA in June 2011.
Temporary Reduction of Appearance of Cellulite. The TriActive system contains six low-power diode lasers, mechanical massage and suction features and localized cooling. In addition, the FDA has cleared TriActive system as an over-the-counter device, for sale and use without physician supervision, because its diode lasers are sealed and do not pose a risk of exposure to operators’ eyes. In a treatment for the temporary reduction of the appearance of cellulite, the practitioner applies the multifunction handpiece to deliver diode laser energy, as well as suction and manipulation therapy, to the treatment area. The laser energy and suction and manipulation therapy enhance micro-circulation in the area of the cellulite. Treatment for the temporary reduction in the appearance of cellulite requires a series of treatments of approximately 30 to 45 minutes each, depending on the treatment area and patient response.
Also in February 2011, we expanded our body shaping treatment platform by acquiring substantially all of the assets of Eleme Medical, including Eleme’s non-invasive SmoothShapes® XV system. The SmoothShapes XV system treats cellulite through a proprietary process known as Photomology, which combines laser and light energy with mechanical manipulation (vacuum and massage) to produce tighter, smoother-looking skin. The system is FDA cleared for marketing in the United States and CE marked for sale in the European Union. The device is also marketed for circumferential reduction outside the United States.
Anti-Aging. We believe the marketplace has moved to a less invasive approach toward treating the indications of anti-aging, including wrinkle reduction, pigmentation, redness and overall skin rejuvenation. Anti-aging treatments were historically performed by physicians who could only target one condition and one skin layer during each treatment. Previously, patients often faced longer, more painful procedures that penetrated deep into the dermal layers and could potentially damage healthy skin.
Our Affirm and SmartSkin workstations provide a non-ablative and micro-ablative treatment approach for wrinkles, skin texture, skin discoloration and skin tightening through tissue coagulation. The Affirm system was the first non ablative micro-rejuvenation system, which included our patent pending mid-infrared Combined Apex Pulse(TM), or CAP, delivery system and Xenon Pulsed Light, or XPL, technology in one system. Our proprietary CAP technology stimulates collagen production throughout the entire treatment area. It remodels
12
Table of Contents
collagen through the papillary dermis to promote collagen production and skin tightening through precise thermal manipulation of the epidermal and dermal tissue. The laser energy is delivered through a durable and disposable tip that can treat an average of ten treatment areas. The XPL portion of the system effectively eradicates dyschromia, a common condition associated with aging skin. The XPL provides enhanced outcomes by targeting superficial pigments, veins and the blush of rosacea associated with sun damaged skin. Our SmartSkin micro-ablative workstation includes a proprietary scanning delivery system that combines ablative CO2 resurfacing and rejuvenation in a single laser system. The SmartSkin system offers a range of settings that enable physicians to customize the treatment based on the aesthetic goals and downtime expectations of the individual patient.
Treatment of Vascular Lesions. To treat vascular lesions the practitioner generally first pre-cools the target area and then applies the system handpiece to deliver laser energy to the treatment area. Depending on the size of the treatment area, procedures last between 20 and 30 minutes. In some cases, a topical anesthetic is applied to the treatment area to minimize pain. For spider veins, redness and rosacea, patients generally receive between two and four treatments spaced over two to three weeks. For port wine birthmarks, patients may receive ten or more treatments.
Our Cynergy system is used for the treatment of vascular lesions. The Cynergy system combines a pulse dye laser, which is best suited for treating shallow vascular lesions, such as port wine birthmarks, facial veins and rosacea, and an Nd:Yag laser, which is best suited for treating large or deep veins, such as leg veins. The practitioner can switch between these two energy sources simply by pressing a button on the system console. The Cynergy system also includes our patented MultiPlex technology that enables the energy from the two lasers to be blended during delivery by quickly following a pulse of energy from the pulse dye laser with a pulse of energy from the Nd:Yag laser. Clinical studies that we have conducted have shown that Multiplex delivery allows for more efficient treatment of vascular lesions by reducing the amount of laser energy required and allowing the laser energy to penetrate deeper into the target area.
In addition to the Cynergy system, each of our Apogee Elite, Acclaim 7000, and VStar systems can be used for the treatment of vascular lesions.
Skin Rejuvenation. Skin rejuvenation involves the treatment of shallow vascular lesions and pigmented lesions to rejuvenate the skin’s appearance. In a skin rejuvenation procedure, the practitioner applies the system handpiece to the target area and delivers laser or pulsed light energy. The energy destroys the shallow vascular lesions and pigmented lesions and rejuvenates the skin’s appearance without damage to the treated or surrounding area through the improvement in skin texture and reduction or elimination of skin irregularities. Cooling is generally not required. Patients typically receive between four to six treatments of approximately 30 minutes each. Treatments are spaced two to four weeks apart.
Each of our Elite, Acclaim 7000, and Cynergy systems can be used for skin rejuvenation through the treatment of shallow vascular lesions and pigmented lesions.
Our Accolade system is our flagship solution for the removal of pigmented lesions. The Accolade is a high powered 755 nm, Q-switched Alexandrite laser. The combination of various spot sizes and the laser’s high repetition rates allow for rapid treatment. The target markets for the Accolade include Korea, China and Japan, where dermal lesions such as Nevus of Ota and Nevus of Ito are common.
Sales and Marketing
We sell our aesthetic treatment systems to the traditional physician customer base of dermatologists and plastic surgeons as well as to the increasing number of non-traditional physician customers who are providing aesthetic services using laser and light-based technology. Non-traditional physician customers can include primary care physicians, obstetricians and gynecologists.
13
Table of Contents
We target potential customers through office visits, trade shows and trade journals. We also conduct clinical workshops and webinars featuring recognized expert panelists and opinion leaders to promote existing and new treatment techniques using our products. We believe that these workshops and webinars enhance customer loyalty and provide us with new sales opportunities. We also use direct mail programs to target specific segments of the market that we seek to access, such as members of medical societies and attendees at meetings sponsored by medical societies or associations. In addition, we maintain a public relations program that has resulted in sales opportunities based on our products being featured in several popular publications around the world.
We do not provide financing to our customers to purchase our products. If a potential customer requests financing, we refer the customer to third party financing sources.
Physician Sales
We sell our products to physicians in North America through a direct sales force. Outside of North America, we sell our products to physicians through a direct sales force in France, Spain, the United Kingdom, Germany, Korea, China, Japan and Mexico and through independent distributors in 78 other countries.
We conduct our own international sales and service operations through wholly-owned subsidiaries in the United Kingdom, France, Germany, Spain, Korea, China, Japan, and Mexico. We seek distributors in international markets where we do not believe that a direct sales presence is warranted or feasible. In those markets, we select distributors that have extensive knowledge of our industry and their local markets. Our distributors sell, install and service our products. We require our distributors to invest in service training and equipment, to stock and supply maintenance and service parts for our systems, to attend exhibitions and industry meetings and, in some instances, to commit to minimum sales amounts to gain or retain exclusivity. Currently, we have written distribution agreements with 33 of our 34 third party distributors. Generally, the written agreements with our distributors have terms of between one and two years.
Service and Support
We support our customers with a range of services, including installation and product training, business and practice development consulting and product service and maintenance. In North America, our field service organization has 26 field service engineers. Outside of North America, we employ 29 field service engineers.
In connection with direct sales of our aesthetic treatment systems, we arrange for the installation of the system and initial product training. Generally, installation and initial training takes less than three hours. The installation is conducted by our field service engineers. We offer a service that is particularly appealing to the non-traditional physician customer and aesthetic spa segments of the market, which have less familiarity with the business aspects of laser and light-based aesthetic treatments than dermatologists and cosmetic surgeons. The cost of installation and initial training for North American purchasers are all included in the purchase price of our systems. We also offer for an additional charge a more comprehensive package of services from pre-qualified third party consultants.
We strive to respond to all service calls within 24 hours to minimize disruption of our customers’ businesses. We have designed our products in a modular fashion to enable quick and efficient service and support. Specifically, we build these products with several separate components that can easily be removed and replaced when the product is being serviced. We provide initial warranties on our products to cover parts and service, and we offer extended warranty packages that vary by type of product and level of service desired. Our base warranty covers parts and service for one year. We offer extended warranty arrangements through service plans. We believe that we have a significant opportunity to increase our recurring customer revenues by increasing the percentage of our customers that enter into service contracts for our systems.
14
Table of Contents
Research and Development
Our research and development team consists of 33 employees, including three physicists, with a broad base of experience in lasers and optoelectronics. Our research and development team works closely with opinion leaders and customers, both individually and through our sponsored seminars, to understand unmet needs and emerging applications in the field of aesthetic skin treatments and to innovate and develop new products and improvements to our existing products. They also conduct and coordinate clinical trials of our products. Our research and development team builds on the significant base of patented and proprietary intellectual property that we have developed in the fields of laser and other light-based technologies since our inception in 1991. From time to time, we may enter into collaborative research and development agreements to enhance our technology and develop new products.
Our research and development expenses were approximately $7.3 million in 2010, $6.7 million in 2009 and $7.5 million in 2008. We expect our research and development expenditures to increase in absolute dollars in 2011.
Manufacturing and Raw Materials
We manufacture all of our products, other than the Smartlipo, SmartSkin, Cellulaze, Performa and TriActive systems, which are manufactured by El.En. and which we sell and market under our distribution agreement with El.En. We manufacture our products with components and subassemblies purchased from third party suppliers. Accordingly, our manufacturing operations consist principally of assembly and testing of our systems and integration of our proprietary optics and software.
We design our products, including our Elite, Affirm, Accolade and Cynergy product families, so that they are built in a modular fashion using fewer components. This approach enables us to manufacture our products more efficiently.
We purchase many of our components and subassemblies from third party manufacturers on an outsourced basis. We use one third party to assemble and test many of the components and subassemblies for our Elite, Affirm, Accolade and Cynergy product families, as well as complete manufacturing and test our SmoothShapes XV products. We also depend exclusively on sole source suppliers for Alexandrite rods, which we use in the manufacturing of our Elite products, and for our SmartCool treatment cooling systems.
We do not have long-term contracts with our third party manufacturers or sole source suppliers. We generally purchase components and subassemblies as well as our other supplies on a purchase order basis. If for any reason, our third party manufacturers or sole source suppliers are not willing or able to provide us with components, subassemblies or supplies in a timely fashion, or at all, our ability to manufacture and sell many of our products could be impaired. To date, we have been able to obtain adequate outsourced manufacturing services and supplies of Alexandrite rods and air cooling systems from our third party manufacturers and suppliers in a timely manner. We believe that over time alternative component and subassembly manufacturers and suppliers can be identified if our current third party manufacturers and suppliers fail to fulfill our requirements.
El.En. Commercial Relationship
The Smartlipo systems and SmartSkin, Performa and TriActive LaserDermology products sold by us were developed, and associated intellectual property rights are owned by El.En. El.En. manufactures, and we distribute, these products pursuant to distribution agreements between us and El.En. These agreements provide us with exclusive distribution rights in the United States and Canada for the single wavelength Smartlipo systems, SmartSkin and TriActive LaserDermology. These agreements also provide us with exclusive distribution rights worldwide for the Smartlipo MPX and Triplex systems and distribution rights outside the United States and
15
Table of Contents
Canada for the Performa system. The transfer prices for products that we currently distribute under the agreements are specified in the agreement; however, they may be changed by El.En. at its discretion upon 30 days’ notice.
El.En. is required to provide us with training, marketing and other sales support for the products we distribute under these agreements. We are required to use best efforts to sell and promote these products, and we are responsible for obtaining and maintaining regulatory approvals for them. If El.En. wishes to discontinue producing products that we distribute, it must make reasonable efforts to provide us with one year’s notice of its plan to do so.
The distribution agreement for the Smartlipo systems expires in 2014. The distribution agreement for the Smartlipo MPX and Triplex systems expires in 2016. The distribution agreement relating to the TriActive LaserDermology system will automatically renew for additional one-year terms unless either party provides notice of termination at least six months prior to the expiration of any subsequent renewal term. We or El.En. may terminate the distribution agreements at any time based upon material uncured breaches by, or the insolvency of, the other party. In addition, El.En. may terminate the distribution agreement for the Smartlipo systems and TriActive LaserDermology if we do not meet annual minimum purchase obligations specified in the agreements.
Patents, Proprietary Technology and Trademarks
Our success depends in part on our ability to obtain and maintain proprietary protection for our products, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position.
As of December 31, 2010, we owned a total of 38 United States patents, as well as foreign counterparts to 17 of these patents. Our patent portfolio includes patents and patent applications with claims directed to:
| • | the design and method of use and operation of our pulse dye laser systems; |
| • | the design and method of use and operation of our Alexandrite laser systems for hair removal; |
| • | our Multiplex energy delivery system for our pulse dye lasers; and |
| • | the design of endoscopic laser and light delivery systems. |
The expiration dates for our issued United States patents and patent application range from 2013 to 2030. Additionally, El.En. has applied for a patent covering the methods of use and operation of the TriActive LaserDermology system. We do not consider any single patent or patent application that we hold to be material to our business.
The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. Our ability to maintain and solidify our proprietary position for our technology will depend on our success in obtaining effective patent claims and enforcing those claims once granted.
We do not know whether any of our patent applications or those patent applications that we license will result in the issuance of any patents. Our issued patents and those that may issue in the future, or those licensed to us, may be challenged, invalidated or circumvented, which could limit our ability to stop competitors from marketing related products or shorten the term of patent protection that we may have for our products. In addition, the rights granted under any issued patents may not provide us with competitive advantages against
16
Table of Contents
competitors with similar technology. Furthermore, our competitors may independently develop similar technologies or duplicate any technology developed by us. Because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that, before any of our products under development can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent.
In 2006, we entered into a patent cross-license agreement with Palomar Medical Technologies, Inc., which we refer to as Palomar. Under the cross-license agreement, we obtained a non-exclusive license to integrate into our products for certain hair removal technology covered by specified U.S. and foreign patents held by Palomar and Palomar obtained a non-exclusive license under certain U.S. and foreign patents held by us. In connection with this agreement, we agreed to pay royalties to Palomar on future sales of certain hair removal-only products. The royalty rate for sales of hair removal products ranges from 3.75% to 7.5% of net sales, depending upon product configuration and the number of energy sources. Our revenues from systems that do not include hair removal capabilities and revenues from service are not subject to any past or future royalties under this agreement.
We rely, in some circumstances, on trade secrets to protect our technology. Trade secrets, however, are difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements with our employees, consultants, scientific advisors and other contractors. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our employees, consultants or contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
We use trademarks on nearly all of our products and believe that having distinctive marks is an important factor in marketing our products. We have registered our Cynosure®, Apogee®, Apogee Elite®, Smartlipo® , Triplex®, Affirm®and SmartCool®marks, among others, in the United States. We have also registered some of our marks in a number of foreign countries. In addition, El.En. has registered the TriActive®and Smartlipo®mark in the United States. Although we have a foreign trademark registration program for selected marks, we may not be able to register or use such marks in each foreign country in which we seek registration.
Competition
Our industry is subject to intense competition. Our products compete against laser and other light-based products offered by public companies, such as Syneron Medical Ltd., Cutera, Inc., Palomar Medical Technologies,Inc. and Solta Medical, Inc., as well as several smaller specialized private companies, such as Alma Lasers, Ltd. Some of these competitors have greater financial and human resources than we do and have established reputations, as well as worldwide distribution channels and sales and marketing capabilities that are larger and more established than ours. Additional competitors may enter the market, and we are likely to compete with new companies in the future. Our products also compete against non-light-based medical products, such as BOTOX® and collagen injections, and surgical and non-surgical aesthetic procedures, such as face lifts, chemical peels, abdominoplasty, liposuction, microdermabrasion, sclerotherapy and electrolysis.
Competition among providers of aesthetic laser and other light-based products is characterized by significant research and development efforts and rapid technological progress. There are few barriers that would prevent new entrants or existing competitors from developing products that would compete directly with ours. There are many companies, both public and private, that are developing innovative devices that use both light-based and alternative technologies for aesthetic and medical applications. Accordingly, our success depends in part on developing and commercializing new and innovative applications of laser and other light-based technology and identifying new markets for and applications of existing products and technology.
To compete effectively, we have to demonstrate that our products are attractive alternatives to other devices and treatments by differentiating our products on the basis of performance, reputation, quality of customer
17
Table of Contents
support and price. Breadth of product offering is also important. We believe that we perform favorably with respect to each of these factors. However, we have encountered and expect to continue to encounter potential customers who, due to pre-existing relationships with our competitors, are committed to, or prefer the products offered by these competitors. Potential customers also may decide not to purchase our products, or to delay such purchases, based on a decision to recoup the cost of expensive products that they may have already purchased from our competitors. In addition, we expect that competitive pressures may result in price reductions and reduced margins over time for our products.
Government Regulation
Our products are medical devices subject to extensive and rigorous regulation by the U.S. Food and Drug Administration, or FDA, as well as other regulatory bodies. FDA regulations govern the following activities that we perform and will continue to perform to ensure that medical devices distributed domestically are safe and effective for their intended uses.
FDA’s Regulation of Manufacturing
The FDA requires that we manufacture our products in accordance with its Quality System Regulation, or QSR. The QSR covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of our products. The FDA enforces the QSR through periodic announced and unannounced inspections. Our last such inspection was in May 2008.
Our failure to maintain compliance with the QSR requirements could result in the shut down of, or restrictions on, our manufacturing operations and the recall or seizure of our products, which would have a material adverse effect on our business. In the event that one of our suppliers fails to maintain compliance with our quality requirements, we may have to qualify a new supplier and could experience manufacturing delays as a result.
We maintain quality assurance and quality management certifications to enable us to market our products in the member states of the European Union, the European Free Trade Association and some countries that have entered into Mutual Recognition Agreements with the European Union. In October 2003, we received our ISO 9001 updated certification as well as our certification for ISO 13485, which replaced our EN 46001 certification.
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device we wish to distribute commercially in the United States requires either prior 510(k) clearance or premarket approval from the FDA. The FDA classifies medical devices into one of three classes. Devices deemed to pose lower risks are placed in either class I or II, which requires the manufacturer to submit to the FDA a premarket notification requesting permission to distribute the device commercially. This process is generally known as 510(k) clearance. Class I devices are subject to general controls such as labeling and adherence to FDA’s QSR. Class II devices are subject to special controls such as performance standards and FDA guidelines as well as general controls. The FDA exempts some low risk devices from premarket notification requirements and the requirement of compliance with certain provisions of the QSR. The FDA places devices in class III, requiring premarket approval, if insufficient information exists to determine that the application of general controls or special controls are sufficient to provide reasonable assurance of safety and effectiveness and they are life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously cleared 510(k) device or to a “preamendment” class III device in commercial distribution before May 28, 1976, for which premarket approval applications have not been required. All of our current products are class II devices. Both premarket notifications and premarket approval applications when submitted to FDA must be accompanied by a user fee, unless exempt.
18
Table of Contents
510(k) Clearance
When a 510(k) clearance is required, we must submit a premarket notification to the FDA demonstrating that our proposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of premarket approval applications, or premarket approval. By regulation, the FDA must clear or deny a 510(k) premarket notification within 90 days of submission of the application. As a practical matter, clearance often takes significantly longer. The FDA may require further information, including clinical data, to make a determination regarding substantial equivalence.
Laser devices used for aesthetic procedures, such as hair removal, have generally qualified for clearance under 510(k) procedures. The FDA has determined that clearance of Cellulaze in the United States requires a 510(k) submission, which we filed in late 2010.
Premarket Approval
If the device cannot be cleared through the 510(k) process, the sponsor must submit a premarket approval application, which is known as a PMA. The sponsor must support the PMA with extensive data, including but not limited to, technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device.
No device that we have developed has required premarket approval, nor do we currently expect that any future device or indication will require premarket approval.
Product Modifications
After a device receives 510(k) clearance or a PMA approval, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, will require a new clearance or approval. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. We have modified aspects of various products since receiving regulatory clearance and believe that new 510(k) clearances are not required for these modifications. If the FDA disagrees with our determination not to seek a new 510(k) clearance or PMA approval, the FDA may retroactively require us to seek 510(k) clearance or premarket approval. The FDA could also require us to cease marketing and distributing the modified device, and to recall any sold devices, until 510(k) clearance or premarket approval is obtained. Also, in these circumstances, we may be subject to significant regulatory fines or penalties.
Clinical Trials
We perform clinical trials to provide data to support the FDA clearance process for our products and for use in our sales and marketing efforts. Human clinical studies are generally required in connection with approval of class III devices and may be required for clearance of class I and II devices. When FDA clearance or approval of a device requires human clinical trials, and if the device presents a “significant risk,” as defined by the FDA, to human health, the FDA requires the device sponsor to file an investigational device exemption, or IDE, application with the FDA and obtain IDE approval prior to commencing the human clinical trials. The sponsor must support the IDE application with appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The sponsor also must obtain approval from the Institutional Review Board overseeing the clinical trial.
While we believe that a majority of our devices present only “non-significant” risks and, therefore, do not require IDE submission to the FDA, we have obtained an IDE approval for the Cellulaze laser workstation to have concurrence from the FDA for the study protocol. Future clinical trials of our products may require that we
19
Table of Contents
submit and obtain approval of an IDE from the FDA prior to commencing clinical trials. The FDA, and the institutional review board at each institution at which a clinical trial is being performed, may suspend a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable health risk.
Our clinical trials may not generate favorable data to support any PMA or 510(k), and we may not be able to obtain such approvals or clearances on a timely basis, or at all. Delays in receipt of or failure to receive such approvals or clearances or failure to comply with existing or future regulatory requirements would have a material adverse effect on our business, financial condition and results of operations. Even if granted, the approvals or clearances may include significant limitations on the intended use and indications for use for which our products may be marketed.
Clinical studies conducted on 510(k) cleared devices, when used or investigated in accordance with the devices’ labeled instructions, are exempt from most of the FDA’s IDE requirements.
Pervasive and Continuing Regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
| • | establishment registration and device listing; |
| • | the quality system regulation, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
| • | labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or “off-label” uses, and other requirements related to promotional activities; |
| • | medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; |
| • | corrections and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug, and Cosmetic Act that may present a risk to health; and |
| • | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
The FDA may require us to maintain a system for tracking our products through the chain of distribution to the patient level. The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA to determine our compliance with the QSR and other regulations. These inspections may include the manufacturing facilities of our subcontractors. Thus, we must continue to spend time, money and effort to maintain compliance. The FDA inspected our current manufacturing facility in May 2008 and we believe that we are in substantial compliance with the QSR. Since 1994, we have received five untitled letters from the FDA regarding alleged violations caused by our promotional activities. We have responded to these letters and the FDA found our responses acceptable.
We are also regulated under the Radiation Control for Health and Safety Act, which requires laser products to comply with performance standards, including design and operation requirements. The law also requires manufacturers to certify in product labeling and in reports to the FDA that their products comply with all such standards. The law and applicable federal regulations also require laser manufacturers to file new product and annual reports, maintain manufacturing, testing and sales records, and report product defects. Various warning labels must be affixed and certain protective devices installed, depending on the class of the product.
20
Table of Contents
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | repair, replacement, refunds, recall or seizure of our products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | refusing or delaying our requests for 510(k) clearance or premarket approval of new products or new intended uses; |
| • | withdrawing 510(k) clearance or premarket approvals that are already granted; and |
| • | criminal prosecution. |
The FDA also has the authority to require us to repair, replace or refund the cost of any medical device that we have manufactured or distributed. If any of these events were to occur, they could have a material adverse effect on our business.
We are also subject to a wide range of federal, state and local laws and regulations, including those related to the environment, health and safety, land use and quality assurance. We believe that compliance with these laws and regulations as currently in effect will not have a material adverse effect on our capital expenditures, earnings and competitive and financial position.
International
International sales of medical devices are subject to foreign governmental regulations, which vary substantially from country to country. The time required to obtain clearance or approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may be different.
The primary regulatory environment in Europe is that of the European Union, which consists of 27 countries encompassing most of the major countries in Europe. The European Union has adopted numerous directives, and European Standardization Committees have promulgated voluntary standards, regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear CE conformity marking, indicating that the device conforms to the essential requirements of the applicable directives and, accordingly, can be commercially distributed throughout the member states of the European Union and the member states of the European Free Trade Association, including Switzerland.
The method of assessing conformity varies depending on the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third party assessment by a Notified Body, an independent and neutral institution appointed by a country to conduct the conformity assessment. This third party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s device. An assessment by a Notified Body in one member state of the European Union or the European Free Trade Association is required in order for a manufacturer to distribute the product commercially throughout these countries. ISO 9001 and ISO 13845 certification are voluntary harmonized standards. Compliance establishes the presumption of conformity with the essential requirements for a CE marking. In October 2003, we received our ISO 9001 updated certification as well as our certification for ISO 13485, which replaced our EN 46001 certification.
Employees
As of December 31, 2010, we had 259 employees, including 94 employees in sales and marketing functions, 33 employees in research, development and engineering functions, 89 employees in manufacturing and service functions and 43 employees in general and administrative functions. We believe that our future success will depend in part on our continued ability to attract, hire and retain qualified personnel. None of our employees are represented by a labor union, and we believe our employee relations are good.
21
Table of Contents
| ITEM 1A. | Risk Factors. |
The following important factors, among others, could cause our actual operating results to differ materially from those indicated or suggested by forward-looking statements made in this Annual Report or presented elsewhere by management from time to time.
Risks Related to Our Business and Industry
We have a history of net losses, and we may not regain profitability.
Although we were profitable in 2007 and 2008, we incurred net losses of approximately $5.5 million in 2010, $22.8 million in 2009 and $0.7 million in 2006. During the fourth quarter of 2008, due to the decrease in our revenue, we began to reduce our worldwide headcount, and as of December 31, 2009, we had reduced it by approximately 25% from the third quarter of 2008. Our headcount remained relatively stable during 2010. We also decreased spending on various programs and implemented general cost-control initiatives intended to reduce our 2009 and 2010 operating expenses. Since the end of 2008, we have reduced our operating expenses by $27.0 million, or 34%. We may not be able to regain or realize profitability on a quarterly or annual basis. If we are unable to regain profitability, the market value of our stock may decline, and an investor could lose all or a part of their investment.
Macroeconomic conditions have led, and may continue to lead to decreased demand for our products.
The aesthetic laser and light-based treatment system industry in which we operate is particularly vulnerable to economic trends. Most treatments performed using our products are elective procedures, the cost of which must be borne by the patient and are not reimbursable through government or private health insurance. Accordingly, the decision to undergo a treatment in which one of our products is used is affected by the willingness of an individual to pay for such a treatment.
Consumer demand, and therefore our business, is sensitive to a number of factors that affect consumer spending, including political and economic conditions, health of credit markets, disposable consumer income levels, consumer debt levels, interest rates and consumer confidence. Practitioners’ demand for our products decreased during 2009. However, demand increased slightly during 2010, but not to levels realized in historical periods. We believe that consumer demand for discretionary aesthetic laser treatments remains uncertain and may continue to adversely affect our operating results.
We may be exposed to credit risk of customers that have been adversely affected by weakened markets.
Due to adverse general business conditions, lack of available credit or favorable terms, the creditworthiness of our customers and potential customers may deteriorate over time, which may cause them to cancel or delay their purchase of our products. In addition, we may be subject to increased risk of non-payment of our accounts receivables. We may also be adversely affected by bankruptcies or other business failures of our customers and potential customers. A significant delay in the collection of funds or a reduction of funds collected may impact our liquidity or result in bad debts.
Our competition may prevent us from achieving further market penetration or improving operating results.
Competition in the aesthetic laser industry is intense. Our products compete against products offered by public companies, such as Syneron Medical Ltd., Cutera, Inc., Palomar Medical Technologies, Inc., and Solta Medical, Inc., as well as several smaller specialized private companies, such as Alma Lasers, Ltd. Some of these competitors have greater financial and human resources than we do and have established reputations, as well as worldwide distribution channels and sales and marketing capabilities that are larger and more established than ours. Additional competitors may enter the market, and we are likely to compete with new companies in the future.
22
Table of Contents
We also face competition from medical products, such as BOTOX(R) and collagen injections, and surgical and non-surgical aesthetic procedures, such as face lifts, sclerotherapy, electrolysis, microdermabrasion and chemical peels. We may also face competition from manufacturers of pharmaceutical and other products that have not yet been developed. As a result of competition with these companies, products and procedures, we could experience loss of market share and decreasing revenue as well as reduced prices and profit margins, any of which would harm our business and operating results.
Our ability to compete effectively depends upon our ability to distinguish our company and our products from our competitors and their products. Factors affecting our competitive position include:
| • | product performance and design; |
| • | ability to sell products tailored to meet the applications needs of clients and patients; |
| • | quality of customer support; |
| • | product pricing; |
| • | product safety; |
| • | sales, marketing and distribution capabilities; |
| • | success and timing of new product development and introductions; and |
| • | intellectual property protection. |
If we fail to obtain Alexandrite rods or our air cooling system from our sole suppliers, our ability to manufacture and sell our products and components would be impaired.
We use Alexandrite rods to manufacture the lasers for our Elite products. We depend exclusively on Northrop Grumman SYNOPTICS to supply Alexandrite rods to us, and we are aware of no alternative supplier meeting our quality standards. We offer our SmartCool(R) treatment cooling systems for use with our laser aesthetic treatment systems, and we depend exclusively on Zimmer Elektromedizin GmbH to supply SmartCool systems to us. Both Alexandrite lasers and our SmartCool systems are important to our business.
We do not have long-term arrangements with Northrop Grumman SYNOPTICS or Zimmer Elektromedizin for the supply of Alexandrite rods or SmartCool systems, but instead purchase from them on a purchase order basis. Northrop Grumman SYNOPTICS and Zimmer Elektromedizin are not required, and may not be able or willing, to meet our future requirements at current prices, or at all. Any extended interruption in our supplies of Alexandrite rods or our SmartCool treatment cooling systems could materially harm our business.
We rely upon third party suppliers for the components and subassemblies of many of our products, making us vulnerable to supply shortages and price fluctuations, which could harm our business.
Many of the components and subassemblies that comprise our aesthetic treatment systems are currently manufactured for us by a limited number of suppliers. In addition, one third party supplier assembles and tests many of the components and subassemblies for our Elite, Cynergy, Affirm and Accolade product families. We do not have long-term contracts with any of these third parties, including the third party supplier that assembles many of our components and subassemblies, for the supply of parts or services. Any interruption in the supply of components or subassemblies, or our inability to obtain substitute components or subassemblies from alternate sources at acceptable prices in a timely manner, or our inability to obtain assembly and testing services, could impair our ability to meet the demand of our customers, which would have an adverse effect on our business and operating results.
23
Table of Contents
If we do not continue to develop and commercialize new products and identify new markets for our products and technology, we may not remain competitive, and our revenues and operating results could suffer.
The aesthetic laser and light-based treatment system industry is subject to continuous technological development and product innovation. If we do not continue to be innovative in the development of new products and applications, our competitive position will likely deteriorate as other companies successfully design and commercialize new products and applications. Accordingly, our success depends in part on developing new and innovative applications of laser and other light-based technology and identifying new markets for and applications of existing products and technology. If we are unable to develop and commercialize new products and identify new markets for our products and technology, our products and technology could become obsolete and our revenues and operating results could be adversely affected.
To remain competitive, we must:
| • | develop or acquire new technologies that either add to or significantly improve our current products; |
| • | convince our target customers that our new products or product upgrades would be attractive revenue-generating additions to their practices; |
| • | sell our products to non-traditional customers, including primary care physicians, gynecologists and other specialists; |
| • | identify new markets and emerging technological trends in our target markets and react effectively to technological changes; and |
| • | maintain effective sales and marketing strategies. |
If our new products do not gain market acceptance, our revenues and operating results could suffer, and our newer generation product sales could cause earlier generation product sales to suffer.
The commercial success of the products and technology we develop will depend upon the acceptance of these products by providers of aesthetic procedures and their patients and clients. It is difficult for us to predict how successful recently introduced products, or products we are currently developing, will be over the long term. If the products we develop do not gain market acceptance, our revenues and operating results could suffer.
We expect that many of the products we develop will be based upon new technologies or new applications of existing technologies. It may be difficult for us to achieve market acceptance of some of our products, particularly the first products that we introduce to the market based on new technologies or new applications of existing technologies.
As we introduce new technologies to the market, our earlier generation product sales could suffer, which may result in write-offs of those earlier generation products. For example, in 2009, we recorded a $2.1 million charge to cost of product revenues related to the write-down of an earlier generation product. The write-down resulted, in part, from customers adopting our newer generation products more quickly than we anticipated, coupled with the downturn in the overall aesthetic laser market.
If demand for our aesthetic treatment systems by non-traditional physician customers and spas does not increase, our revenues will suffer and our business will be harmed.
The percentage of revenues from non-traditional physician customers and spa purchasers of our products has increased significantly since 2005. We believe, and our growth expectations assume, that we and other companies selling lasers and other light-based aesthetic treatment systems have not fully penetrated these markets and that we will continue to receive a significant percentage of our revenues from selling to these markets. If our expectations as to the size of these markets and our ability to sell our products to participants in these markets are not correct, our revenues will continue to suffer and our business will be harmed.
24
Table of Contents
We sell our products and services through subsidiaries and distributors in numerous international markets. Our operating results may suffer if we are unable to manage our international operations effectively.
We sell our products and services through subsidiaries and distributors in 85 foreign countries, and we therefore are subject to risks associated with having international operations. We derived 59%, 54% and 34% of our revenues from sales outside North America for the years ended December 31, 2010, 2009 and 2008, respectively. Our gross margin for 2010 and 2009 declined from 2008, primarily as a result of the higher percentage of laser revenue from international markets where our products tend to have lower average selling prices than in North America.
Our international sales are subject to a number of risks, including:
| • | foreign certification and regulatory requirements; |
| • | difficulties in staffing and managing our foreign operations; |
| • | import and export controls; and |
| • | political and economic instability. |
We may incur foreign currency translation charges as a result of changes in currency exchange rates, which could cause our operating results to suffer.
We face risks associated with changes in foreign currency exchange rates. Revenues outside of North America that were recorded in U.S. dollars represented approximately 38% of our total 2010 revenues outside of North America. Substantially all of the remaining 62% of our total 2010 revenues outside of North America were sales in euros, British pounds, Japanese yen, Chinese yuan and South Korean won. Since we conduct our business in U.S. dollars, our most significant foreign currency exposure, results from changes in the exchange rate between these currencies and the U.S. dollar. Our functional currency is the U.S. dollar. Our policy is to reduce exposure to exchange rate fluctuations by having most of our assets and liabilities, as well as most of our revenues and expenditures, in U.S. dollars, or U.S. dollar linked. We have not historically engaged in hedging activities relating to our non-U.S. dollar operations. We sell inventory to our subsidiaries in U.S. dollars. These amounts are recorded at our local subsidiaries in local currency rates in effect on the transaction date. Therefore, we may be exposed to exchange rate fluctuations that occur while the debt is outstanding which we recognize as unrealized gains and losses in our statements of operations. Upon settlement of these debts, we may record realized foreign exchange gains and losses in our statement of operations. We may incur negative foreign currency translation charges as a result of changes in currency exchange rates, which could cause our operating results to suffer.
We rely on third party distributors to market, sell and service a significant portion of our products. If these distributors do not commit the necessary resources to effectively market, sell and service our products or if our relationships with these distributors are disrupted, our business and operating results may be harmed.
In North America, France, Spain the United Kingdom, Germany, Korea, China and Japan, we sell our products through our internal sales organization. Outside of these markets, we sell our products through third party distributors. Our sales and marketing success in these other markets depends on these distributors, in particular their sales and service expertise and relationships with the customers in the marketplace. Sales of our aesthetic treatment systems by third party distributors represented 18% of our product revenue in 2010, 16% of our product revenue in 2009 and 16% of our product revenue in 2008. We do not control these distributors, and they may not be successful in marketing our products. Third party distributors may terminate their relationships with us, or fail to commit the necessary resources to market and sell our products to the level of our expectations. Currently, we have written distributor agreements in place with 33 of our 34 third party distributors. The third party distributors with which we do not have written distributor agreements may terminate their relationships with us and stop selling and servicing our products with little or no notice. If current or future third party
25
Table of Contents
distributors do not perform adequately, or if we fail to maintain our existing relationships with these distributors or fail to recruit and retain distributors in particular geographic areas, our revenue from international sales may be adversely affected and our operating results could suffer.
We may not receive revenues from our current research and development efforts for several years, if at all.
Investment in product development often involves a long payback cycle. We have made and expect to continue making significant investments in research and development and related product opportunities. Accelerated product introductions and short product life cycles require high levels of expenditures for research and development that could adversely affect our operating results if not offset by revenue increases. We believe that we must continue to dedicate a significant amount of resources to our research and development efforts to maintain our competitive position. However, we may not generate anticipated revenues from these investments for several years, if at all.
Because we do not require training for users of our non-invasive products, and sell these products to non-physicians, there exists an increased potential for misuse of these products, which could harm our reputation and our business.
Federal regulations allow us to sell our products to or on the order of practitioners licensed by law to use or order the use of a prescription device. The definition of “licensed practitioners” varies from state to state. As a result, our products may be purchased or operated by physicians with varying levels of training and, in many states, by non-physicians, including nurse practitioners, chiropractors and technicians. Outside the United States, many jurisdictions do not require specific qualifications or training for purchasers or operators of our products. We do not supervise the procedures performed with our non-invasive products, nor do we require that direct medical supervision occur. We and our distributors offer product training sessions, but neither we nor our distributors require purchasers or operators of our non-invasive products to attend training sessions. The lack of required training and the purchase and use of our non-invasive products by non-physicians may result in product misuse and adverse treatment outcomes, which could harm our reputation and expose us to costly product liability litigation.
Product liability suits could be brought against us due to defective design, material or workmanship or due to misuse of our products. These lawsuits could be expensive and time consuming and result in substantial damages to us and increases in our insurance rates.
If our products are defectively designed, manufactured or labeled, contain defective components or are misused, we may become subject to substantial and costly litigation by our customers or their patients or clients. Misusing our products or failing to adhere to operating guidelines for our products can cause severe burns or other damage to the eyes, skin or other tissue. We are routinely involved in claims related to the use of our products. Product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizable damage awards against us. Our current insurance coverage may not be sufficient to cover these claims. Moreover, in the future, we may not be able to obtain insurance in amount or scope sufficient to provide us with adequate coverage against potential liabilities. Any product liability claims brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing continuing coverage, could harm our reputation in the industry and reduce product sales. We would need to pay any product losses in excess of our insurance coverage out of cash reserves, harming our financial condition and adversely affecting our operating results.
We may incur substantial expenses if our past practices are shown to have violated the Telephone Consumer Protection Act.
We previously used facsimiles to disseminate information about our clinical workshops to large numbers of customers and potential customers. These facsimiles were transmitted by third parties retained by us, and were
26
Table of Contents
sent to recipients whose facsimile numbers were supplied by us as well as other recipients whose facsimile numbers we purchased from other sources. In May 2005, we stopped sending unsolicited facsimiles to customers and potential customers.
Under the federal Telephone Consumer Protection Act, or TCPA, recipients of unsolicited facsimile “advertisements” may be entitled to damages of up to $500 per facsimile for inadvertent violations and up to $1,500 per facsimile for knowing or willful violations. Recipients of unsolicited facsimile advertisements may seek enforcement of the TCPA in state courts. The TCPA also permits states to initiate a civil action in a federal district court to enforce the TCPA against a party who engages in a pattern or practice of violations of the TCPA. In addition, complaints may be filed with the Federal Communications Commission, which has the power to assess penalties against parties for violations of the TCPA. In 2005, Dr. Ari Weitzner, individually and as putative representative of a purported class, filed a complaint against us under the TCPA in Massachusetts Superior Court in Middlesex County seeking monetary damages, injunctive relief, costs and attorneys fees. The complaint alleges that we violated the TCPA by sending unsolicited advertisements by facsimile to the plaintiff and other recipients without the prior express invitation or permission of the recipients. Under the TCPA, recipients of unsolicited facsimile advertisements are entitled to damages of up to $500 per facsimile for inadvertent violations and up to $1,500 per facsimile for knowing or willful violations. Based on discovery in this matter, the plaintiff alleges that approximately three million facsimiles were sent on our behalf by a third party to approximately 100,000 individuals. In February 2008, several months after the close of discovery, the plaintiff served a motion for class certification, which we opposed on numerous factual and legal grounds. The Court held a hearing on the plaintiff’s class certification motion in June 2008, but no decision on the motion has been rendered. In July 2010, the Court issued an order dismissing this matter without prejudice for Dr. Weitzner’s failure to prosecute the case. In August 2010, Dr. Weitzner filed a motion for relief from the dismissal order, which the Court allowed. At a status conference held in November 2010, the Court confirmed that the class certification motion was still under advisement. We are not currently able to estimate the amount or range of loss that could result from an unfavorable outcome of this lawsuit.
In July 2008, we commenced a declaratory judgment action in the U.S. District Court for the District of Massachusetts requesting a declaration that Dr. Weitzner’s and the putative class claims are covered under our general liability insurance policies. In August 2008, our insurance company filed an Answer and Counterclaim against us seeking a declaration that our policy does not provide coverage for Dr. Weitzner’s claims, and we filed a reply to the Counterclaim. The insurance company filed a Motion for Summary Judgment in December 2008, and we cross moved for Summary Judgment in January 2009. The court held a hearing on the motions in February 2009, and in April 2009 rendered a decision that our liability insurer is obligated to provide us with a defense to the Weitzner action and, if necessary, indemnify us for the putative class claims. Thereafter, our liability insurer filed a motion for reconsideration, which we opposed. The court denied the insurer’s motion in May 2009. In January 2010, the court entered an Order for Judgment consistent with its April 2009 decision that the insurer is obligated to defend us against the putative class claims and to indemnify us for any single damages, attorneys’ fees or costs. Per agreement of the parties, we were awarded $0.4 million in fees and costs for the period through July 1, 2009. The insurer filed a Notice of Appeal of the judgment in January 2010. The matter has been fully briefed and the U.S. First Circuit Court of Appeals heard oral arguments in January 2011.
We are vigorously defending the Weitzner lawsuit, but litigation is subject to numerous uncertainties and we are unable to predict the ultimate outcome of this matter. Even if we prevail in this lawsuit, other individual or class action claims may be brought against us alleging past violations of the TCPA. Moreover, the amount of any potential liability in connection with this lawsuit or other possible lawsuits will depend, to a large extent, on whether a class in a class action lawsuit is certified and, if one is certified, on the scope of the class, neither of which we can predict at this time.
We have not recorded a liability related to this lawsuit or other possible future lawsuits. However, we may determine in the future that an accrual is required, and we may be required to pay damages in respect of this lawsuit or other possible future lawsuits arising out of our past transmission of facsimiles, any of which could
27
Table of Contents
materially and adversely affect our results of operations, cash flows and financial condition. Regardless of the outcome, this lawsuit or other possible future lawsuits may cause us to incur significant expenses and divert the attention of our management and key personnel from our business operations.
Our financial results may fluctuate from quarter to quarter, which makes our results difficult to predict and could cause our results to fall short of expectations.
Our financial results may fluctuate as a result of a number of factors, many of which are outside of our control. For these reasons, comparing our financial results on a period-to-period basis may not be meaningful, and you should not rely on our past results as an indication of our future performance. Our future quarterly and annual expenses as a percentage of our revenues may be significantly different from those we have recorded in the past or which we expect for the future. Our financial results in some quarters may fall below our expectations or the expectations of market analysts or investors. Any of these events could cause our stock price to fall. Each of the risk factors listed in this “Risk Factors” section, and the following factors, may adversely affect our financial results:
| • | continued availability of attractive equipment leasing terms for our customers, which may be negatively influenced by interest rate increases or lack of available credit; |
| • | increases in the length of our sales cycle; and |
| • | reductions in the efficiency of our manufacturing processes. |
If there is not sufficient demand for the procedures performed with our products, practitioner demand for our products could decline, which would adversely affect our operating results.
Most procedures performed using our aesthetic treatment systems are elective procedures that are not reimbursable through government or private health insurance. The cost of these elective procedures must be borne by the patient. As a result, the decision to undergo a procedure that utilizes our products may be influenced by a number of factors, including:
| • | patient awareness of procedures and treatments; |
| • | the cost, safety and effectiveness of the procedure and of alternative treatments; |
| • | the success of our and our customers’ sales and marketing efforts to purchasers of these procedures; and |
| • | consumer confidence, which may be affected by economic and other conditions. |
If there is not sufficient demand for the procedures performed with our products, practitioner demand for our products would be reduced, which would adversely affect our operating results.
We may be unable to attract and retain management and other personnel we need to succeed.
Our success depends on the services of our senior management and other key research and development, manufacturing, sales and marketing employees. The loss of the services of one or more of these employees could have a material adverse effect on our business. We consider retaining Michael R. Davin, our president and chief executive officer, to be key to our efforts to develop, sell and market our products and remain competitive. We have entered into an employment agreement with Mr. Davin; however, the employment agreement is terminable by him on short notice and may not ensure his continued service with our company. Our future success will depend in large part upon our ability to attract, retain and motivate highly skilled employees. We cannot be certain that we will be able to do so.
28
Table of Contents
As a result of the death of George J. Vojta,who was an independent director and served as Chairman of our Audit Committee, we are not in compliance with certain Nasdaq listing requirements, and if we do not regain compliance, we could face delisting from the Nasdaq.
George J. Vojta, who was a member of our Board of Directors, died in December 2010. Mr. Vojta was Chairman of our Audit Committee and an “audit committee financial expert,” as defined by applicable rules of the Securities and Exchange Commission, Chairman of the Company’s Nominating and Corporate Governance Committee, and a member of the Company’s Compensation Committee.
As a result of Mr. Vojta’s death, we are no longer in compliance with NASDAQ Marketplace Rule 5605(b)(1), because a majority of our Board is no longer comprised of independent directors, and Rule 5605(c)(2)(A), because our Audit Committee no longer has at least three members and has no member whom our Board has determined meets the financial sophistication requirements set out in Rule 5605(c)(2)(A). We notified the Nasdaq that we intend to rely on the cure provisions of Rules 5605(b)(1)(A) and 5605(c)(4)(B), which each provide that we have until the earlier of our next annual shareholders meeting or one year from Mr. Vojta’s death to comply with the above-referenced listing requirements; provided, however, that our annual shareholders meeting occurs no later than June 20, 2011, 180 days after the death of Mr. Vojta.
If we are not able to regain compliance with these listing requirements by June 20, 2011, we could face delisting from the Nasdaq.
Any acquisitions that we make could disrupt our business and harm our financial condition.
From time to time, we evaluate potential strategic acquisitions of complementary businesses, products or technologies, as well as consider joint ventures and other collaborative projects. We may not be able to identify appropriate acquisition candidates or strategic partners, or successfully negotiate, finance or integrate any businesses, products or technologies that we acquire. We have minimal experience acquiring companies or products. Any acquisition we pursue could diminish our cash available to us for other uses or be dilutive to our stockholders, and could divert management’s time and resources from our core operations.
El.En. has substantial control over us. In addition, El.En. and our executive officers and directors have the ability to control all matters submitted to stockholders for approval.
In addition to the factors discussed below regarding El.En.’s ability to control the election of a majority of the members of our board of directors, El.En. and our executive officers and directors, in the aggregate, beneficially own approximately 23% of our outstanding common stock. As a result, if these stockholders were to act together, they would be able to control all matters submitted to our stockholders for approval. For example, these persons could control any amendment of our certificate of incorporation and bylaws and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of our company on terms that other stockholders may desire. Please also see the discussion under “Risks Related to Our Relationship with El.En.—El.En. has substantial control over us and could delay or prevent a change of control.”
Provisions in our corporate charter documents and under Delaware law may delay or prevent attempts by our stockholders to change our management and hinder efforts to acquire a controlling interest in us.
Provisions of our certificate of incorporation and bylaws may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions include:
| • | a dual class capital structure that allows El.En. to control the election of a majority of the members of our board of directors; |
29
Table of Contents
| • | the classification of the members of our directors who are elected by holders of our Class A common stock and Class B common stock, voting together as a single class; |
| • | limitations on the removal of directors who are elected by holders of our Class A common stock and Class B common stock, voting together as a single class; |
| • | advance notice requirements for stockholder proposals and nominations; |
| • | the inability of Class A stockholders to act by written consent or to call special meetings; and |
| • | the ability of our board of directors to designate the terms of and issue new series of preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors. |
The affirmative vote of the holders of at least 75% of our shares of capital stock entitled to vote is necessary to amend or repeal the above provisions of our certificate of incorporation, and the right of the holders of shares of our Class B common stock to elect a majority of the members of our board of directors may not be modified without the approval of the holders of at least a majority of the shares of our Class B common stock outstanding. In addition, absent approval of our board of directors, our bylaws may only be amended or repealed by the affirmative vote of the holders of at least 75% of the voting power of our shares of capital stock entitled to vote and the affirmative vote of holders of at least a majority of the shares of Class B common stock outstanding. In addition, Section 203 of the Delaware General Corporation Law prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person which together with its affiliates owns or within the last three years has owned 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. Accordingly, Section 203 may discourage, delay or prevent a change in control of our company.
Our stock price may be volatile.
Our Class A common stock price may be volatile. The stock market in general has experienced extreme volatility that has often been unrelated to the operating performance of particular companies. The market price for our Class A common stock may be influenced by many factors, including:
| • | the success of competitive products or technologies; |
| • | regulatory developments in the United States and foreign countries; |
| • | developments or disputes concerning patents or other proprietary rights; |
| • | the recruitment or departure of key personnel; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | market conditions in the our industry and issuance of new or changed securities analysts’ reports or recommendations; and |
| • | general economic, industry and market conditions. |
30
Table of Contents
Risks Related to Our Relationship with El.En.
El.En. has substantial control over us and could delay or prevent a change of control.
El.En., our largest stockholder, is able to control the election of a majority of the members of our board of directors. El.En. owns approximately 100% of our outstanding Class B common stock, which comprises 23% of our aggregate outstanding common stock. Until El.En. beneficially owns less than 20% of the aggregate number of shares of our Class A common stock and Class B common stock outstanding or less than 50% of the number of shares of our class B common stock outstanding, El.En., as holder of a majority of the shares of our Class B common stock, will have the right:
| • | to elect a majority of the members of our board of directors; |
| • | to approve amendments to our bylaws adopted by our Class A and Class B stockholders, voting as a single class; and |
| • | to approve amendments to any provisions of our restated certificate of incorporation relating to the rights of holders of common stock, the powers, election and classification of the board of directors, corporate opportunities and the rights of holders of Class A common stock and Class B common stock to elect and remove directors, act by written consent and call special meetings of stockholders. |
In addition, the holders of shares of our Class B common stock will vote with our Class A stockholders for the election of the remaining directors.
Because El.En. is the holder of a majority of the shares of our Class B common stock, El.En.’s approval will be required for any of the actions described above. In addition, because El.En. will be able to control the election of a majority of our board, and because of its substantial holdings of our capital stock, El.En. will likely have the ability to delay or prevent a change of control of our company that may be favored by other directors or stockholders and otherwise exercise substantial control over all corporate actions requiring board or stockholder approval.
El.En. and its subsidiaries market and sell products that compete with our products, and any competition by El.En. could have a material adverse effect on our business.
El.En. is a leading laser manufacturer in Europe and a leading light-based medical device manufacturer worldwide. El.En. and its subsidiaries develop and produce laser systems with scientific, industrial, commercial and medical applications. Although we have exclusive North American distribution rights for our single wavelength Smartlipo systems, SmartSkin and TriActive LaserDermology products, exclusive worldwide distribution rights for our Smartlipo MPX and Triplex products and distribution rights outside the United States and Canada for the Performa system, El.En. competes with us in North America with its other products. In the event that our distribution agreements with El.En. terminate, El.En. may compete with us in North America with these products. El.En. markets, sells, promotes and licenses products that compete with our products worldwide. Our business could be materially and adversely affected by competition from El.En.
Conflicts of interest may arise between us and El.En., and these conflicts might ultimately be resolved in a manner unfavorable to us.
For financial reporting purposes, our financial results are included in El.En.’s consolidated financial statements. Two of our directors, Andrea Cangioli and Leonardo Masotti, and the spouse of Leonardo Masotti, are also officers or directors of El.En. and certain of its subsidiaries and affiliates that compete with us in the worldwide market. These two directors own or have an interest in substantial amounts of El.En. stock. Ownership interests of our directors in El.En. stock, or service as a director of our company while at the same time serving as, or being the spouse of, a director or officer of El.En., could give rise to conflicts of interest when a director or officer is faced with a decision that could have different implications for the two companies.
31
Table of Contents
Conflicts may arise with respect to possible future distribution and research and development arrangements with El.En. or another El.En. affiliated company in which the terms and conditions of the arrangements are subject to negotiation between us and El.En. or such other El.En. affiliated company. These potential conflicts could also arise, for example, over matters such as:
| • | the nature, timing, marketing, distribution and price of our products and El.En.’s products that compete with each other; |
| • | intellectual property matters; and |
| • | business opportunities that may be attractive to both El.En. and us. |
In order to address potential conflicts of interest between us and El.En., our restated certificate of incorporation contains provisions regulating and defining the conduct of our affairs as they may involve El.En. and El.En. affiliated companies and El.En.’s officers and directors who serve as our directors. These provisions recognize that we and El.En. and El.En. affiliated companies engage and may continue to engage in the same or similar business activities and lines of business and will continue to have contractual and business relations with each other. These provisions expressly permit El.En. and its affiliated companies to compete against us and narrowly limit corporate opportunities that El.En. or its directors or officers who serve as our directors must make available to us.
Our Class A share price may decline because of future sales of our shares by El.En.
El.En. may sell all or part of the shares of our Class B common stock that it owns, at which time those shares would automatically convert into shares of our Class A common stock. El.En. is not subject to any contractual obligation to maintain its ownership position in our shares. Consequently, El.En. may not maintain its ownership of our common stock. Sales by El.En. of substantial amounts of our common stock in the public market could adversely affect prevailing market prices for our Class A common stock.
If El.En. sells the shares of our stock held by it and no longer has control over us, our commercial relationship with El.En. may be adversely affected.
El.En. has advised us that it currently does not intend to sell its shares of our common stock in the foreseeable future. However, El.En.’s plans and intentions may change at any time. El.En. is not subject to any contractual obligation to maintain an ownership position in our shares.
If El.En. sells our shares and no longer has control over us, El.En. will cease to include our financial results in its consolidated financial statements, and El.En.’s interests may differ significantly from ours. If this occurs, our commercial relationship with El.En. may be adversely affected, which, in turn, could have a material adverse effect on our business. For example, if El.En. does not have a continuing interest in our financial success, it may be more inclined to compete with us in North America and in other markets, not to enter into future commercial agreements with us or to terminate or not renew our existing distribution agreements. If any of these events were to occur, it could harm our business.
Risks Related to Intellectual Property
If we infringe or are alleged to infringe intellectual property rights of third parties, our business could be adversely affected.
Our products may infringe or be claimed to infringe patents or patent applications under which we do not hold licenses or other rights. Third parties may own or control these patents and patent applications in the United States and abroad. These third parties could bring claims against us that would cause us to incur substantial expenses and, if successfully asserted against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay manufacturing or sales of the product that is the subject of the suit.
32
Table of Contents
As a result of patent infringement claims, or in order to avoid potential claims, we may choose or be required to seek a license from the third party and be required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be forced to cease some aspect of our business operations if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms. This could harm our business significantly.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in our industry. In addition to infringement claims against us, we may become a party to other types of patent litigation and other proceedings, including interference proceedings declared by the United States Patent and Trademark Office and opposition proceedings in the European Patent Office, regarding intellectual property rights with respect to our products and technology. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time.
If we are unable to obtain or maintain intellectual property rights relating to our technology and products, the commercial value of our technology and products will be adversely affected and our competitive position could be harmed.
Our success and ability to compete depends in part upon our ability to obtain protection in the United States and other countries for our products by establishing and maintaining intellectual property rights relating to or incorporated into our technology and products. We own a variety of patents and patent applications in the United States and corresponding patents and patent applications in many foreign jurisdictions. Although we have reached a patent infringement settlement agreement with CoolTouch Inc., we do not know how successful we would be should we choose to assert our patents against suspected infringers. Our pending and future patent applications may not issue as patents or, if issued, may not issue in a form that will be advantageous to us. Even if issued, patents may be challenged, narrowed, invalidated or circumvented, which could limit our ability to stop competitors from marketing similar products or limit the length of term of patent protection we may have for our products. Changes in either patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection.
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely upon unpatented proprietary technology, processes and know-how, particularly with respect to our Alexandrite and pulse dye lasers. We generally seek to protect this information in part by confidentiality agreements with our employees, consultants and third parties. These agreements may be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently developed by competitors.
Risks Related to Government Regulation
If we fail to obtain and maintain necessary U.S. Food and Drug Administration clearances for our products and indications or if clearances for future products and indications are delayed or not issued, our business would be harmed.
Our products are classified as medical devices and are subject to extensive regulation by the FDA and other federal, state and local authorities. These regulations relate to manufacturing, labeling, sale, promotion,
33
Table of Contents
distribution, importing and exporting and shipping of our products. In the United States, before we can market a new medical device, or a new use of, or claim for, an existing product, we must first receive either 510(k) clearance or premarket approval from the FDA, unless an exemption applies. Both of these processes can be expensive and lengthy and entail significant user fees, unless exempt. The FDA’s 510(k) clearance process usually takes from three to 12 months, but it can last longer. The process of obtaining premarket approval is much more costly and uncertain than the 510(k) clearance process. It generally takes from one to three years, or even longer, from the time the premarket approval application is submitted to the FDA until an approval is obtained.
In order to obtain premarket approval and, in some cases, a 510(k) clearance, a product sponsor must conduct well controlled clinical trials designed to test the safety and effectiveness of the product. Conducting clinical trials generally entails a long, expensive and uncertain process that is subject to delays and failure at any stage. The data obtained from clinical trials may be inadequate to support approval or clearance of a submission. In addition, the occurrence of unexpected findings in connection with clinical trials may prevent or delay obtaining approval or clearance. If we conduct clinical trials, they may be delayed or halted, or be inadequate to support approval or clearance, for numerous reasons, including:
| • | FDA, other regulatory authorities or an institutional review board may place a clinical trial on hold; |
| • | patients may not enroll in clinical trials, or patient follow-up may not occur, at the rate we expect; |
| • | patients may not comply with trial protocols; |
| • | institutional review boards and third party clinical investigators may delay or reject our trial protocol; |
| • | third party clinical investigators may decline to participate in a trial or may not perform a trial on our anticipated schedule or consistent with the clinical trial protocol, good clinical practices, or other FDA requirements; |
| • | third party organizations may not perform data collection and analysis in a timely or accurate manner; |
| • | regulatory inspections of our clinical trials or manufacturing facilities may, among other things, require us to undertake corrective action or suspend or terminate our clinical trials, or invalidate our clinical trials; |
| • | changes in governmental regulations or administrative actions; and |
| • | the interim or final results of the clinical trials may be inconclusive or unfavorable as to safety or effectiveness. |
Medical devices may be marketed only for the indications for which they are approved or cleared. The FDA may not approve or clear indications that are necessary or desirable for successful commercialization. Indeed, the FDA may refuse our requests for 510(k) clearance or premarket approval of new products, new intended uses or modifications to existing products. Our clearances can be revoked if safety or effectiveness problems develop.
After clearance or approval of our products, we are subject to continuing regulation by the FDA, and if we fail to comply with FDA regulations, our business could suffer.
Even after clearance or approval of a product, we are subject to continuing regulation by the FDA, including the requirements that our facility be registered and our devices listed with the agency. We are subject to Medical Device Reporting regulations, which require us to report to the FDA if our products may have caused or contributed to a death or serious injury or malfunction in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur. We must report corrections and removals to the FDA where the correction or removal was initiated to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug, and Cosmetic Act caused by the device that may present a risk to health, and maintain records of other corrections or removals. The FDA closely regulates promotion and advertising and our
34
Table of Contents
promotional and advertising activities could come under scrutiny. Since 1994, we have received five untitled letters from the FDA regarding alleged violations caused by our promotional activities. We have responded to these letters and the FDA has found our responses acceptable. If the FDA objects to our promotional and advertising activities or finds that we failed to submit reports under the Medical Device Reporting regulations, for example, the FDA may allege our activities resulted in violations.
The FDA and state authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA or state agencies, which may include any of the following sanctions:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | repair, replacement, refunds, recall or seizure of our products; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusing or delaying our requests for 510(k) clearance or premarket approval of new products or new intended uses; |
| • | withdrawing 510(k) clearance or premarket approvals that have already been granted; and |
| • | criminal prosecution. |
If any of these events were to occur, they could harm our business.
Federal regulatory reforms may adversely affect our ability to sell our products profitably.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the clearance or approval, manufacture and marketing of a device. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be.
We have modified some of our products without FDA clearance. The FDA could retroactively determine that the modifications were improper and require us to stop marketing and recall the modified products.
Any modifications to one of our FDA-cleared devices that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or a premarket approval. We may be required to submit extensive pre-clinical and clinical data depending on the nature of the changes. We may not be able to obtain additional 510(k) clearances or premarket approvals for modifications to, or additional indications for, our existing products in a timely fashion, or at all. Delays in obtaining future clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our revenue and operating results. We have made modifications to our devices in the past and may make additional modifications in the future that we believe do not or will not require additional clearances or approvals. If the FDA disagrees, and requires new clearances or approvals for the modifications, we may be required to recall and to stop marketing the modified devices, which could harm our operating results and require us to redesign our products.
If we fail to comply with the FDA’s Quality System Regulation and laser performance standards, our manufacturing operations could be halted, and our business would suffer.
We are currently required to demonstrate and maintain compliance with the FDA’s QSR. The QSR is a complex regulatory scheme that covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of our products. Because our products involve the use of lasers, our products also are covered by a performance standard for lasers set forth in
35
Table of Contents
FDA regulations. The laser performance standard imposes specific record keeping, reporting, product testing and product labeling requirements. These requirements include affixing warning labels to laser products as well as incorporating certain safety features in the design of laser products. The FDA enforces the QSR and laser performance standards through periodic unannounced inspections. We have been, and anticipate in the future being, subject to such inspections. Our failure to comply with the QSR or to take satisfactory corrective action in response to an adverse QSR inspection or our failure to comply with applicable laser performance standards could result in enforcement actions, including a public warning letter, a shutdown of or restrictions on our manufacturing operations, delays in approving or clearing a product, refusal to permit the import or export of our products, a recall or seizure of our products, fines, injunctions, civil or criminal penalties, or other sanctions, such as those described in the preceding paragraphs, any of which could cause our business and operating results to suffer.
If we fail to comply with state laws and regulations, or if state laws or regulations change, our business could suffer.
In addition to FDA regulations, most of our products are also subject to state regulations relating to their sale and use. These regulations are complex and vary from state to state, which complicates monitoring compliance. In addition, these regulations are in many instances in flux. For example, federal regulations allow our prescription products to be sold to or on the order of “licensed practitioners,” that is, practitioners licensed by law to use or order the use of a prescription device. Licensed practitioners are defined on a state-by-state basis. As a result, some states permit non-physicians to purchase and operate our products, while other states do not. Additionally, a state could change its regulations at any time to prohibit sales to particular types of customers. We believe that, to date, we have sold our prescription products only to licensed practitioners. However, our failure to comply with state laws or regulations and changes in state laws or regulations may adversely affect our business.
We or our distributors may be unable to obtain or maintain international regulatory qualifications or approvals for our current or future products and indications, which could harm our business.
Sales of our products outside the United States are subject to foreign regulatory requirements that vary widely from country to country. In many countries, our third party distributors are responsible for obtaining and maintaining regulatory approvals for our products. We do not control our third party distributors, and they may not be successful in obtaining or maintaining these regulatory approvals. In addition, the FDA regulates exports of medical devices from the United States.
Complying with international regulatory requirements can be an expensive and time consuming process, and approval is not certain. The time required to obtain foreign clearances or approvals may be longer than that required for FDA clearance or approval, and requirements for such clearances or approvals may differ significantly from FDA requirements. Foreign regulatory authorities may not clear or approve our products for the same indications cleared or approved by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA clearance or approval in addition to other risks. Although we or our distributors have obtained regulatory approvals in the European Union and other countries outside the United States for many of our products, we or our distributors may be unable to maintain regulatory qualifications, clearances or approvals in these countries or obtain qualifications, clearances or approvals in other countries. For example, we are in the process of seeking regulatory approvals from the Japanese Ministry of Health, Labour and Welfare for the direct sale of our products into that country. If we are not successful in doing so, our business will be harmed. We may also incur significant costs in attempting to obtain and in maintaining foreign regulatory clearances, approvals or qualifications.
Foreign regulatory agencies, as well as the FDA, periodically inspect manufacturing facilities both in the United States and abroad. If we experience delays in receiving necessary qualifications, clearances or approvals to market our products outside the United States, or if we fail to receive those qualifications, clearances or
36
Table of Contents
approvals, or if we fail to comply with other foreign regulatory requirements, we and our distributors may be unable to market our products or enhancements in international markets effectively, or at all. Additionally, the imposition of new requirements may significantly affect our business and our products. We may not be able to adjust to such new requirements.
New regulations may limit our ability to sell to non-physicians, which could harm our business.
Currently, we sell our products primarily to physicians and, outside the United States, to aestheticians. In addition, we recently began marketing our products to the growing aesthetic spa market, where non-physicians under physician supervision perform aesthetic procedures at dedicated facilities. However, federal, state and international regulations could change at any time, disallowing sales of our products to aestheticians, and limiting the ability of aestheticians and non-physicians to operate our products. Any limitations on our ability to sell our products to non-physicians or on the ability of aestheticians and non-physicians to operate our products could cause our business and operating results to suffer.
| Item 1B. | Unresolved Staff Comments |
None.
| Item 2. | Properties |
We lease a 67,500 square foot facility in Westford, Massachusetts which houses our executive offices and our manufacturing, research and development and warehouse operations. The lease on this facility expires in December 2012. In addition, we lease an aggregate of approximately 29,000 square feet of space at eight other locations in Europe and the Asia/Pacific region that we use for sales and service purposes.
| Item 3. | Legal Proceedings |
In 2005, Dr. Ari Weitzner, individually and as putative representative of a purported class, filed a complaint against us under the federal Telephone Consumer Protection Act, or the TCPA in Massachusetts Superior Court in Middlesex County seeking monetary damages, injunctive relief, costs and attorneys fees. The complaint alleges that we violated the TCPA by sending unsolicited advertisements by facsimile to the plaintiff and other recipients without the prior express invitation or permission of the recipients. Under the TCPA, recipients of unsolicited facsimile advertisements are entitled to damages of up to $500 per facsimile for inadvertent violations and up to $1,500 per facsimile for knowing or willful violations. Based on discovery in this matter, the plaintiff alleges that approximately three million facsimiles were sent on our behalf by a third party to approximately 100,000 individuals. On February 6, 2008, several months after the close of discovery, the plaintiff served a motion for class certification, which we opposed on numerous factual and legal grounds, including that a nationwide class action may not be maintained in a Massachusetts state court by Dr. Weitzner, a New York resident; individual issues predominate over common issues; a class action is not superior to other methods of resolving TCPA claims; and Dr. Weitzner is an inadequate class representative. We also believe we have many merits defenses, including that the faxes in question do not constitute “advertising” within the meaning of the TCPA and many recipients had an established business relationship with us and are thereby deemed to have consented to the receipt of facsimile communications. The Court held a hearing on the plaintiff’s class certification motion in June 2008, but no decision on the motion has been rendered. In July 2010, the Court issued an order dismissing this matter without prejudice for Dr. Weitzner’s failure to prosecute the case in August 2010, Dr. Weitzner filed a motion for relief from the dismissal order, which the Court allowed. At a status conference held in November 2010, the Court confirmed that the class certification motion was still under advisement. We are not currently able to estimate the amount or range of loss that could result from an unfavorable outcome of this lawsuit.
In July 2008, we commenced a declaratory judgment action in the U.S. District Court for the District of Massachusetts requesting a declaration that Dr. Weitzner’s and the putative class claims are covered under our
37
Table of Contents
general liability insurance policies. In August 2008, our insurance company filed an Answer and Counterclaim against us seeking a declaration that our policy does not provide coverage for Dr. Weitzner’s claims. In August 2008, we filed a reply to the Counterclaim. The insurance company filed a Motion for Summary Judgment in December 2008, and we cross moved for Summary Judgment in January 2009. The Court held a hearing on the motions in February 2009, and in April 2009 rendered a decision that our liability insurer is obligated to provide us with a defense to the Weitzner action and, if necessary, indemnify us for the putative class claims. Thereafter, our liability insurer filed a motion for reconsideration, which we opposed. The court denied the insurer’s motion in May 2009. In January 2010, the court entered an Order for Judgment consistent with its April 8, 2009 decision that the insurer is obligated to defend us against the putative class claims and to indemnify us for any single damages, attorneys’ fees or costs. Per agreement of the parties, we were awarded $0.4 million in fees and costs for the period through July 1, 2009. The insurer filed a Notice of Appeal of the judgment in January 2010. The matter has been fully briefed and the U.S. First Circuit Court of Appeals heard oral arguments in January 2011.
In addition to the matters discussed above, from time to time, we are subject to various claims, lawsuits, disputes with third parties, investigations and pending actions involving various allegations against us incident to the operation of its business, principally product liability. Each of these other matters is subject to various uncertainties, and it is possible that some of these other matters may be resolved unfavorably to us. We establish accruals for losses that management deems to be probable and subject to reasonable estimate. We believe that the ultimate outcome of these matters will not have a material adverse impact on our consolidated financial position, results of operations or cash flows.
| Item 4. | Reserved |
38
Table of Contents
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuers Purchases of Equity Securities |
Market Price of and Dividends on Our Common Stock and Related Stockholder Matters.
Our Class A common stock trades on The Nasdaq Global Market under the symbol “CYNO” The following table sets forth, for the periods indicated, the high and low sales prices of our Class A common stock on The Nasdaq Global Market.
| High | Low | |||||||
| Fiscal Year Ended December 31, 2009 |
||||||||
| First quarter |
$ | 9.70 | $ | 4.50 | ||||
| Second quarter |
$ | 8.70 | $ | 5.57 | ||||
| Third quarter |
$ | 11.85 | $ | 6.74 | ||||
| Fourth quarter |
$ | 12.62 | $ | 9.65 | ||||
| Fiscal Year Ended December 31, 2010 |
||||||||
| First quarter |
$ | 12.18 | $ | 9.62 | ||||
| Second quarter |
$ | 14.06 | $ | 10.16 | ||||
| Third quarter |
$ | 10.75 | $ | 8.80 | ||||
| Fourth quarter |
$ | 11.00 | $ | 9.48 | ||||
There is no established public trading market for our Class B common stock because, under the terms of our restated certificate of incorporation, shares of our Class B common stock will convert automatically into Class A common stock upon any transfer of such shares, whether or not for value. Additionally, shares of our Class B common stock are also convertible into Class A common stock upon the occurrence of events specified in our restated certificate of incorporation. Each share of our Class B common stock is convertible into one share of Class A common stock.
On March 7, 2011, the closing price per share of our Class A common stock was $12.58, as reported on The Nasdaq Global Market. The number of record holders of our Class A common stock as of March 7, 2011 was 11. The number of record holders of our Class B common stock as of March 7, 2011 was three.
On July 28, 2009, our Board of Directors authorized the repurchase of up to $10 million of our Class A common stock, from time to time, on the open market or in privately negotiated transactions under a stock repurchase program. The program will terminate upon the purchase of $10 million in common stock, unless our Board of Directors discontinues it sooner. During the year ended December 31, 2010, we repurchased 145,621 shares of our common stock at an aggregate cost of approximately $1.4 million and at a weighted average price of $9.54 per share under this program. As of December 31, 2010, we have repurchased an aggregate of 148,935 shares under this program at an aggregate cost of $1.4 million.
39
Table of Contents
The following table provides information about purchases by the Company of equity securities that are registered by the Company pursuant to Section 12 of the Exchange Act during the quarter ended December 31, 2010:
| Period |
Total number of shares purchased |
Average price paid per share |
Total number of shares purchased as part of publicly announced plans or programs |
Maximum number (or appropriate dollar value) of shares that may yet be purchased under the plans or programs |
||||||||||||
| October 1, 2010—October 31, 2010 |
— | $ | — | — | $ | 8,821,795 | ||||||||||
| November 1, 2010—November 30, 2010 |
9,469 | 9.99 | 9,469 | 8,727,215 | ||||||||||||
| December 1, 2010—December 31, 2010 |
15,000 | 9.90 | 15,000 | 8,578,749 | ||||||||||||
| Total |
24,469 | $ | 9.93 | 24,469 | $ | 8,578,749 | ||||||||||
We have never paid or declared any cash dividends on our common stock. We currently intend to retain our earnings, if any, to finance the growth and development of our business. Payment of future dividends, if any, will be at the discretion of our board of directors.
At December 31, 2010, our total cash, cash equivalents and short and long-term marketable securities balance was $96.8 million.
We did not sell any unregistered securities during the period covered by this Annual Report filed on Form 10-K.
40
Table of Contents
The graph below shows the cumulative total stockholder return of an investment of $100 (and the reinvestment of any dividends thereafter) on December 30, 2005 (the last trading day for the year ended December 31, 2005) in our Class A common stock, the Nasdaq U.S. Index and the Nasdaq Medical Devices, Instruments, Supplies, Manufacturers and Distributors Index. Our stock price performance shown in the graph below is not indicative of future stock price performance.
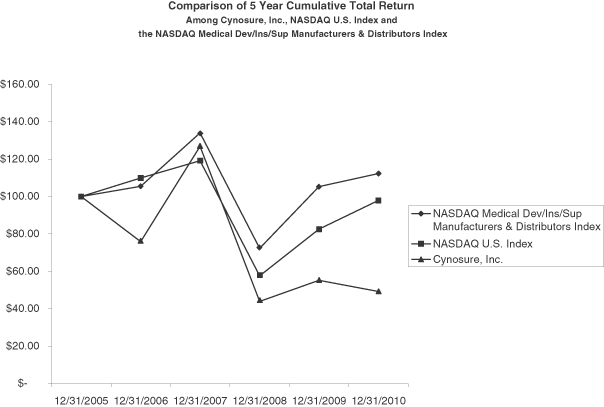
| Name |
12/31/2005 | 12/31/2006 | 12/31/2007 | 12/31/2008 | 12/31/2009 | 12/31/2010 | ||||||||||||||||||
| NASDAQ Medical Dev/Ins/Sup Manufacturers & Distributors Index |
$ | 100.00 | $ | 105.40 | $ | 134.02 | $ | 72.17 | $ | 105.25 | $ | 112.23 | ||||||||||||
| NASDAQ U.S. Index |
100.00 | 109.84 | 119.14 | 57.41 | 82.53 | 97.95 | ||||||||||||||||||
| Cynosure, Inc. |
100.00 | 75.89 | 126.85 | 43.77 | 55.08 | 49.04 | ||||||||||||||||||
41
Table of Contents
| Item 6. | Selected Consolidated Financial Data |
You should read the following selected consolidated financial data in conjunction with our consolidated financial statements and the related notes which are included elsewhere in this Annual Report and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this Annual Report. We have derived the consolidated statement of operations data for the years ended December 31, 2010, 2009 and 2008 and the consolidated balance sheet data as of December 31, 2010 and 2009 from our audited consolidated financial statements, which are included elsewhere in this Annual Report. We have derived the consolidated statement of operations data for the years ended December 31, 2007 and 2006 and the consolidated balance sheet data as of December 31, 2008, 2007 and 2006 from our audited consolidated financial statements, which are not included in this Annual Report. Our historical results for any prior period are not necessarily indicative of results to be expected for any future period.
| Year Ended December 31, | ||||||||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||||||
| (In thousands, except per share data) | ||||||||||||||||||||
| Consolidated Statement of Operations Data: |
||||||||||||||||||||
| Revenues |
$ | 81,775 | $ | 72,825 | $ | 139,662 | $ | 124,315 | $ | 78,401 | ||||||||||
| Cost of revenues |
35,388 | 32,808 | 48,705 | 44,507 | 32,920 | |||||||||||||||
| Gross profit |
46,387 | 40,017 | 90,957 | 79,808 | 45,481 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Sales and marketing |
32,818 | 39,098 | 53,062 | 42,058 | 26,213 | |||||||||||||||
| Research and development |
7,300 | 6,679 | 7,497 | 6,827 | 4,673 | |||||||||||||||
| General and administrative |
11,312 | 14,556 | 17,837 | 11,346 | 8,975 | |||||||||||||||
| Royalty settlement |
— | — | — | — | 10,000 | |||||||||||||||
| Total operating expenses |
51,430 | 60,333 | 78,396 | 60,231 | 49,861 | |||||||||||||||
| (Loss) income from operations |
(5,043 | ) | (20,316 | ) | 12,561 | 19,577 | (4,380 | ) | ||||||||||||
| Interest income, net |
163 | 523 | 2,498 | 2,516 | 2,579 | |||||||||||||||
| Gain (loss) on investments |
23 | 22 | (46 | ) | (171 | ) | 118 | |||||||||||||
| Other (expense) income, net |
(247 | ) | 672 | (43 | ) | 866 | 813 | |||||||||||||
| (Loss) income before provision for (benefit from) income taxes and minority interest |
(5,104 | ) | (19,099 | ) | 14,970 | 22,788 | (870 | ) | ||||||||||||
| Provision for (benefit from) income taxes |
442 | 3,659 | 4,771 | 8,276 | (266 | ) | ||||||||||||||
| Minority interest in net income of subsidiary |
— | — | — | — | 46 | |||||||||||||||
| Net (loss) income |
$ | (5,546 | )_ | $ | (22,758 | ) | $ | 10,199 | $ | 14,512 | $ | (650 | ) | |||||||
| Basic net (loss) income per share |
$ | (0.44 | ) | $ | (1.79 | ) | $ | 0.81 | $ | 1.21 | $ | (0.06 | ) | |||||||
| Diluted net (loss) income per share |
$ | (0.44 | ) | $ | (1.79 | ) | $ | 0.80 | $ | 1.15 | $ | (0.06 | ) | |||||||
| Basic weighted average common shares outstanding |
12,666 | 12,709 | 12,581 | 11,993 | 11,084 | |||||||||||||||
| Diluted weighted average common shares outstanding |
12,666 | 12,709 | 12,806 | 12,654 | 11,084 | |||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents, marketable securities, investments and related financial instruments |
$ | 96,826 | $ | 91,967 | $ | 95,451 | $ | 86,097 | $ | 57,246 | ||||||||||
| Working capital |
99,687 | 106,908 | 109,495 | 113,732 | 80,460 | |||||||||||||||
| Total assets |
141,812 | 145,201 | 173,122 | 149,844 | 109,566 | |||||||||||||||
| Capital lease obligation, net of current portion |
40 | 171 | 436 | 794 | 1,069 | |||||||||||||||
| Retained earnings |
2,227 | 7,773 | 30,531 | 20,332 | 5,820 | |||||||||||||||
| Total stockholders’ equity |
120,300 | 123,830 | 140,354 | 120,878 | 85,870 | |||||||||||||||
42
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Company Overview
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the related notes and other financial data included elsewhere in this Annual Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should review Item 1A of this Annual Report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Company Overview
We develop and market aesthetic treatment systems that are used by physicians and other practitioners to perform non-invasive and minimally invasive procedures to remove hair, treat vascular and pigmented lesions, rejuvenate the skin, liquefy and remove unwanted fat through laser lypolysis and reduce the appearance of cellulite. As of December 31, 2010, we had sold more than 10,300 aesthetic treatment systems worldwide.
We were incorporated in July 1991. In 2002, El.En. S.p.A., an Italian company that itself and through subsidiaries develops and markets laser systems for medical and industrial applications, acquired a majority of our capital stock. In 2005, we completed our initial public offering of our Class A common stock. As of December 31, 2010, El.En. owns approximately 100% of our Class B common stock, which comprises 23% of our aggregate outstanding common stock. El.En., through its ownership of our Class B common stock, has the right to elect and remove a majority of our board of directors and to approve stockholder-proposed amendments to our bylaws and amendments to specified provisions of our certificate of incorporation.
We focus our development and marketing efforts on offering leading, or flagship, products for the following high volume applications:
| • | the Elite product line for hair removal, treatment of facial and leg veins and pigmentations; |
| • | the Smartlipo product line for LaserBodySculptingSM for the removal of unwanted fat; |
| • | the Cellulaze, SmoothShapes XV and TriActive product line for the temporary and long-term reduction in the appearance of cellulite; |
| • | the Affirm/SmartSkin product line for anti-aging applications, including treatments for wrinkles, skin texture, skin discoloration and skin tightening; |
| • | the Cynergy product line for the treatment of vascular lesions; and |
| • | the Accolade product line for the removal of benign pigmented lesions as well as multi-colored tattoos. |
A key element of our business strategy is to launch innovative new products and technologies into high-growth aesthetic applications. Our research and development team builds on our existing broad range of laser and light-based technologies to develop new solutions and products to target unmet needs in significant aesthetic treatment markets. Innovation continues to be a strong contributor to our strength. Since 2002, we have introduced 21 new products. In February 2011, we launched Cellulaze, the world’s first aesthetic laser device for the long-term reduction of cellulite. Also in February 2011, we expanded our body shaping treatment platform by acquiring substantially all of the assets of Eleme Medical, including Eleme’s non-invasive SmoothShapes® XV system.
43
Table of Contents
Revenues
We generate revenues primarily from sales of our products, parts and accessories and from services, including product warranty revenues. In 2010, we derived approximately 76% of our revenues from sales of our products and 24% of our revenues from parts, accessories and service revenues. In 2009, we derived approximately 77% of our revenues from sales of our products and 23% of our revenues from parts, accessories and service revenues. In 2008, we derived approximately 88% of our revenues from sales of our products and 12% of parts, accessories and service revenues. Generally, we recognize revenues from the sales of our products upon delivery to our customers, revenues from service contracts and extended product warranties ratably over the coverage period and revenues from service in the period in which the service occurs.
We sell directly in North America, France, Spain, the United Kingdom, Germany, Korea, China, Japan and Mexico, and use distributors to sell our products in other countries where we do not have a direct presence. We derived 59%, 54% and 34% of our revenues from sales outside North America for the years ended December 31, 2010, 2009 and 2008, respectively. As of December 31, 2010, we had 32 sales employees in North America, 29 sales employees in France, Spain, the United Kingdom, Germany, Korea, China and Japan and distributors that cover 78 countries. The following table provides revenue data by geographical region for the years ended December 31, 2010, 2009 and 2008:
| Percentage of Revenues | ||||||||||||
| Year Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Region |
||||||||||||
| North America |
41 | % | 46 | % | 66 | % | ||||||
| Europe |
29 | 27 | 20 | |||||||||
| Asia/Pacific |
24 | 21 | 11 | |||||||||
| Other |
6 | 6 | 3 | |||||||||
| Total |
100 | % | 100 | % | 100 | % | ||||||
See Note 6 to our consolidated financial statements included in this Annual Report for revenues and asset data by geographic region.
Cost of Revenues
Our cost of revenues consists primarily of material, labor and manufacturing overhead expenses and includes the cost of components and subassemblies supplied by third party suppliers. Cost of revenues also includes royalties incurred on certain products sold, service and warranty expenses, as well as salaries and personnel-related expenses, including stock-based compensation, for our operations management team, purchasing and quality control. In 2009, we recorded a $2.1 million charge to cost of product revenues related to the write-down of an earlier generation product. The write-down resulted in part from customers adopting our newer generation products more quickly than we anticipated, coupled with the downturn in the overall aesthetic laser market.
Sales and Marketing Expenses
Our sales and marketing expenses consist primarily of salaries, commissions and other personnel-related expenses, including stock-based compensation, for employees engaged in sales, marketing and support of our products, trade show, promotional and public relations expenses and management and administration expenses in support of sales and marketing. We expect our sales and marketing expenses to increase in absolute dollars but decrease as a percentage of revenues in 2011.
44
Table of Contents
Research and Development Expenses
Our research and development expenses consist of salaries and other personnel-related expenses, including stock-based compensation, for employees primarily engaged in research, development and engineering activities, materials used and other overhead expenses incurred in connection with the design and development of our products and, from time to time, expenses associated with collaborative research and development agreements that we may enter into. We expense all of our research and development costs as incurred. We expect our research and development expenditures to increase in absolute dollars in 2011 but remain consistent as a percentage of revenues with 2010.
General and Administrative Expenses
Our general and administrative expenses consist primarily of salaries and other personnel-related expenses, including stock-based compensation for executives, accounting and administrative personnel, professional fees and other general corporate expenses. We expect our general and administrative expenses to remain relatively unchanged in absolute dollars, but decrease as a percentage of revenues in 2011.
Interest Income (Expense), net
Interest income consists primarily of interest earned on our short and long-term marketable securities consisting of state and municipal bonds and U.S. government agencies and treasuries. Interest expense consists primarily of interest due on capitalized leases.
Gain (Loss) on Investments and Other Income (Expense), net
Gain (loss) on investments consists primarily of recoveries and losses related to the fair value of auction rate securities. Other income (expense), net consists primarily of foreign currency remeasurement gains or losses and other miscellaneous income and expense items.
Provision for Income Taxes
As of December 31, 2010, we maintain a full valuation allowance on the net deferred tax assets in the United States, Germany, Japan and Mexico. During the fourth quarter of 2009, we determined that our net domestic deferred tax assets were no longer more-likely-than-not realizable. As a result, we recorded a deferred tax provision charge of $10.4 million to establish a full valuation allowance on our net domestic deferred tax assets.
Valuation allowances are provided if, based on the weight of available evidence, it is more-likely-than-not that some or all of the deferred tax assets will not be realized. We will continue to monitor the need for valuation allowances in each jurisdiction, and may adjust our positions in the future based on actual results.
45
Table of Contents
Results of Operations
Year Ended December 31, 2010 and 2009
The following table contains selected statement of operations data, which serves as the basis of the discussion of our results of operations for the years ended December 31, 2010 and 2009:
| Year Ended December 31, 2010 |
Year Ended December 31, 2009 |
Change 2009 to 2010 |
||||||||||||||||||||||
| Amount | As a % of Total Revenues |
Amount | As a % of Total Revenues |
$ Change | % Change | |||||||||||||||||||
| (Dollars in thousands) | ||||||||||||||||||||||||
| Product revenues |
$ | 62,357 | 76 | % | $ | 55,869 | 77 | % | 6,488 | 12 | % | |||||||||||||
| Parts, accessories and service revenues |
19,418 | 24 | 16,956 | 23 | 2,462 | 15 | ||||||||||||||||||
| Total revenues |
81,775 | 100 | 72,825 | 100 | 8,950 | 12 | ||||||||||||||||||
| Cost of revenues |
35,388 | 43 | 32,808 | 45 | 2,580 | 8 | ||||||||||||||||||
| Gross profit |
46,387 | 57 | 40,017 | 55 | 6,370 | 16 | ||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Sales and marketing |
32,818 | 40 | 39,098 | 54 | (6,280 | ) | (16 | ) | ||||||||||||||||
| Research and development |
7,300 | 9 | 6,679 | 9 | 621 | 9 | ||||||||||||||||||
| General and administrative |
11,312 | 14 | 14,556 | 20 | (3,244 | ) | (22 | ) | ||||||||||||||||
| Total operating expenses |
51,430 | 63 | 60,333 | 83 | (8,903 | ) | (15 | ) | ||||||||||||||||
| Loss from operations |
(5,043 | ) | (6 | ) | (20,316 | ) | (28 | ) | 15,273 | 75 | ||||||||||||||
| Interest income, net |
163 | — | 523 | 1 | (360 | ) | (69 | ) | ||||||||||||||||
| Gain on investments |
23 | — | 22 | — | 1 | 5 | ||||||||||||||||||
| Other (expense) income, net |
(247 | ) | — | 672 | 1 | (919 | ) | (137 | ) | |||||||||||||||
| Loss before provision for income taxes |
(5,104 | ) | (6 | ) | (19,099 | ) | (26 | ) | 13,995 | 73 | ||||||||||||||
| Provision for income taxes |
442 | (1 | ) | 3,659 | 5 | (3,217 | ) | (88 | ) | |||||||||||||||
| Net loss |
$ | (5,546 | ) | (7 | )% | $ | (22,758 | ) | (31 | )% | $ | (17,212 | ) | (76 | )% | |||||||||
Revenues
Total revenue for the year ended December 31, 2010 increased by $9.0 million, or 12%, to $81.8 million as compared to the year ended December 31, 2009 revenues of $72.8 million (in thousands, except for percentages):
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2010 | 2009 | |||||||||||||||
| Product sales in North America |
$ | 27,788 | $ | 27,582 | $ | 206 | 1 | % | ||||||||
| Product sales outside North America |
34,569 | 28,287 | 6,282 | 22 | ||||||||||||
| Global parts, accessories and service sales |
19,418 | 16,956 | 2,462 | 15 | ||||||||||||
| Total Revenues |
$ | 81,775 | $ | 72,825 | $ | 8,950 | 12 | % | ||||||||
| • | Revenues from the sale of products in North America increased 1% from the 2009 period. We believe that the availability of credit remains limited and demand for discretionary aesthetic laser treatments remains uncertain and as a result our North American revenues may continue to be adversely affected. We develop and market aesthetic treatment systems (capital equipment) that are used by physicians and other practitioners to perform non-invasive and minimally invasive procedures in the aesthetic marketplace. These procedures are discretionary and not reimbursed by third-party insurers. The |
46
Table of Contents
| significant majority of our business each year is derived from new customers. A portion of our customers finance the purchase of these lasers through third party finance companies or banks. During the year ended December 31, 2010, credit continued to be difficult to obtain similar to the year ended December 31, 2009, and as a result, our potential customers were not as able to expand their practices or commit to purchasing equipment from us. |
| • | Revenues from sales of products outside of North America increased by approximately $6.3 million, or 22%, from the 2009 period, due to a increase in the number of units sold by our European distributors and our Asian subsidiaries. We attribute this to improved global economic business conditions compared with the year ended December 31, 2009. |
| • | Revenues from the global sale of parts, accessories and services increased $2.5 million, or 15%, from the 2009 period, which includes an increase in revenues generated from the sale of our service contracts as well as an increase in sales of certain parts and accessories. |
Cost of Revenues
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2010 | 2009 | |||||||||||||||
| Cost of revenues (in thousands) |
$ | 35,388 | $ | 32,808 | $ | 2,580 | 8 | % | ||||||||
| Cost of revenues (as a percentage of total revenues) |
43 | % | 45 | % | ||||||||||||
Total cost of revenues increased $2.6 million, or 8%, to $35.4 million in 2010, as compared to $32.8 million in 2009. The increase was primarily associated with a 12% increase in total revenues. Our total cost of revenues decreased as a percentage of total revenues to 43% for the year ended December 31, 2010, from 45% for the year ended December 31, 2009 partially due to a $2.1 million charge to cost of product revenues in the 2009 period related to the write-down of an earlier generation product. The write-down resulted in part from customers adopting our newer generation products more quickly than we anticipated. Excluding the effect of the write-down, our total cost of revenues as a percentage of total revenues increased to 43% from 42% due to a higher percentage of laser revenue from our international distribution where our products tend to have lower average selling prices than in North America.
Sales and Marketing
| Year Ended December 31, |
$ Change |
% Change | ||||||||||||||
| 2010 | 2009 | |||||||||||||||
| Sales and marketing (in thousands) |
$ | 32,818 | $ | 39,098 | $ | (6,280 | ) | (16 | )% | |||||||
| Sales and marketing (as a percentage of total revenues) |
40 | % | 54 | % | ||||||||||||
Sales and marketing expenses decreased $6.3 million, or 16%, to $32.8 million in 2010, as compared to $39.1 million in 2009. The decline was associated with a decrease of $3.2 million in personnel, administrative costs and travel expenses due to the overall reduction of our worldwide direct sales organization, promotional costs of $2.1 million due to the reduction in clinical workshops, tradeshows and other promotional efforts, and stock compensation expense of $1.0 million. Our sales and marketing expenses for the year ended December 31, 2010 decreased as a percentage of total revenues to 40% due to the reduction of these expenses, as well as a 12% increase in total revenues for the 2010 period as compared to the 2009 period.
Research and Development
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2010 | 2009 | |||||||||||||||
| Research and development (in thousands) |
$ | 7,300 | $ | 6,679 | $ | 621 | 9 | % | ||||||||
| Research and development (as a percentage of total revenues) |
9 | % | 9 | % | ||||||||||||
47
Table of Contents
Research and development expenses increased by $0.6 million, or 9%, for the year ended December 31, 2010, as compared with the year ended December 31, 2009 due to an increase in professional fees and consulting associated with clinical studies and increased research and development efforts.
General and Administrative
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2010 | 2009 | |||||||||||||||
| General and administrative (in thousands) |
$ | 11,312 | $ | 14,556 | $ | (3,244 | ) | (22 | )% | |||||||
| General and administrative (as a percentage of total revenues) |
14 | % | 20 | % | ||||||||||||
General and administrative expenses decreased by $3.2 million, or 22%, due to a decrease of $1.7 million in legal and professional services costs associated with the patent infringement case against CoolTouch, which was settled in January 2010, and a $1.6 million decrease in bad debt expense.
Interest Income, net
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2010 | 2009 | |||||||||||||||
| Interest income, net (in thousands) |
$ | 163 | $ | 523 | $ | (360 | ) | (69 | )% | |||||||
The decrease in interest income, net is primarily due to investing in securities that bear less risk and lower interest rates than in 2009.
Gain on Investment and Other (Expense) Income, net
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2010 | 2009 | |||||||||||||||
| Gain on investment (in thousands) |
23 | 22 | 1 | 5 | % | |||||||||||
| Other (expense) income, net (in thousands) |
(247 | ) | 672 | (919 | ) | (137 | )% | |||||||||
The decrease in other (expense) income is primarily a result of net foreign currency remeasurement losses in the year ended December 31, 2010 compared to the net foreign currency remeasurement gains for the year ended December 31, 2009 due to the strengthening of the U.S. dollar during 2010, primarily against the euro.
Provision for Income Taxes
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2010 | 2009 | |||||||||||||||
| Provision for income taxes (in thousands) |
$ | 442 | $ | 3,659 | $ | (3,217 | ) | (88 | )% | |||||||
| Provision as a percentage of income before provision for income taxes |
9 | % | 19 | % | ||||||||||||
The provision for income taxes results from a combination of the activities of our domestic and foreign subsidiaries. In 2010, we recorded an income tax provision of $0.4 million, representing an effective tax rate of 9%. We continue to maintain a valuation allowance against our net domestic deferred tax assets, and therefore we did not record a benefit for our current year loss in the U.S. for the year ended December 31, 2010, with the exception of the portion that can be carried back to recover federal income taxes paid in prior years. Therefore, we have recorded a benefit of $0.5 million for the federal refund as a result of the federal carryback claim. At December 31, 2010, we have no additional carryback capacity for future U.S. tax losses. In 2009, we recorded an
48
Table of Contents
income tax provision of $3.7 million, representing an effective tax rate of 19%. Our 2010 effective tax rate decreased from 2009 primarily due to the valuation allowance that was established against our net domestic deferred tax assets during the fourth quarter of 2009.
Year Ended December 31, 2009 and 2008
The following table contains selected statement of operations data, which serves as the basis of the discussion of our results of operations for the years ended December 31, 2009 and 2008:
| Year Ended December 31, 2009 |
Year Ended December 31, 2008 |
Change 2008 to 2009 |
||||||||||||||||||||||
| Amount | As a % of Total Revenues |
Amount | As a % of Total Revenues |
$ Change | % Change | |||||||||||||||||||
| (Dollars in thousands) | ||||||||||||||||||||||||
| Product revenues |
$ | 55,869 | 77 | % | $ | 123,208 | 88 | % | (67,339 | ) | (55 | )% | ||||||||||||
| Parts, accessories and service revenues |
16,956 | 23 | 16,454 | 12 | 502 | 3 | ||||||||||||||||||
| Total revenues |
72,825 | 100 | 139,662 | 100 | (66,837 | ) | (48 | ) | ||||||||||||||||
| Cost of revenues |
32,808 | 45 | 48,705 | 35 | (15,897 | ) | (33 | ) | ||||||||||||||||
| Gross profit |
40,017 | 55 | 90,957 | 65 | (50,940 | ) | (56 | ) | ||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Sales and marketing |
39,098 | 54 | 53,062 | 38 | (13,964 | ) | (26 | ) | ||||||||||||||||
| Research and development |
6,679 | 9 | 7,497 | 5 | (818 | ) | (11 | ) | ||||||||||||||||
| General and administrative |
14,556 | 20 | 17,837 | 13 | (3,281 | ) | (18 | ) | ||||||||||||||||
| Total operating expenses |
60,333 | 83 | 78,396 | 56 | (18,063 | ) | (23 | ) | ||||||||||||||||
| (Loss) income from operations |
(20,316 | ) | (28 | ) | 12,561 | 9 | (32,877 | ) | (262 | ) | ||||||||||||||
| Interest income, net |
523 | 1 | 2,498 | 2 | (1,975 | ) | (79 | ) | ||||||||||||||||
| Gain (loss) on investments |
22 | — | (46 | ) | — | 68 | 148 | |||||||||||||||||
| Other income (expense), net |
672 | 1 | (43 | ) | — | 715 | 1,663 | |||||||||||||||||
| (Loss) income before provision for income taxes |
(19,099 | ) | (26 | ) | 14,970 | 11 | (34,069 | ) | (228 | ) | ||||||||||||||
| Provision for income taxes |
3,659 | 5 | 4,771 | 3 | (1,112 | ) | (23 | ) | ||||||||||||||||
| Net (loss) income |
$ | (22,758 | ) | (31 | )% | $ | 10,199 | 7 | % | $ | (32,957 | ) | (323 | )% | ||||||||||
Revenues
Total revenue for the year ended December 31, 2009 decreased by $66.8 million, or 48%, to $72.8 million as compared to the year ended December 31, 2008 revenues of $139.7 million (in thousands, except for percentages):
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2009 | 2008 | |||||||||||||||
| Product sales in North America |
$ | 27,582 | $ | 85,188 | $ | (57,606 | ) | (68 | )% | |||||||
| Product sales outside North America |
28,287 | 38,020 | (9,733 | ) | (26 | ) | ||||||||||
| Global parts, accessories and service sales |
16,956 | 16,454 | 502 | 3 | ||||||||||||
| Total Revenues |
$ | 72,825 | $ | 139,662 | $ | (66,837 | ) | (48 | )% | |||||||
| • | Revenues from the sale of products in North America decreased by approximately $57.6 million, or 68%, from the 2008 period, primarily due to a decrease in the number of product units sold. We develop and market aesthetic treatment systems (capital equipment) that are used by physicians and |
49
Table of Contents
| other practitioners to perform non-invasive and minimally invasive procedures in the aesthetic marketplace. These procedures are discretionary and not reimbursed by third-party insurers. The significant majority of our business each year is derived from new customers. A portion of our customers finance the purchase of these lasers through third party finance companies or banks. During the year ended December 31, 2009, credit was more difficult to obtain and our potential customers were not as able to expand their practices or commit to purchasing equipment from us as compared to the year ended December 31, 2008. Additionally, we believe that due to the uncertain and adverse business conditions, some of our customers and potential customers anticipated a decline in the number of patients seeking discretionary aesthetic laser treatments and therefore, decided not to purchase new systems during the year. |
| • | Revenues from sales of products outside of North America decreased by approximately $9.7 million, or 26%, from the 2008 period, due to a decrease in the number of units sold by our Asian distributors and our European subsidiaries, which we attribute to the adverse global business conditions. This decrease was partially offset by an increase in product units sold at our subsidiaries in Asia, including our recently established subsidiary in Korea. |
| • | Revenues from the global sale of parts, accessories and services increased $0.5 million, or 3%, from the 2008 period, which includes an increase in revenues generated from the sale of our service contracts. |
Cost of Revenues
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2009 | 2008 | |||||||||||||||
| Cost of revenues (in thousands) |
$ | 32,808 | $ | 48,705 | $ | (15,897 | ) | (33 | )% | |||||||
| Cost of revenues (as a percentage of total revenues) |
45 | % | 35 | % | ||||||||||||
Total cost of revenues decreased $15.9 million, or 33%, to $32.8 million in 2009, as compared to $48.7 million in 2008. Our total cost of revenues increased as a percentage of total revenues to 45% for the year ended December 31, 2009, from 35% for the year ended December 31, 2008 primarily due to a higher percentage of laser revenue from our international distribution where our products tend to have lower average selling prices than in North America. Also, in 2009, we recorded a $2.1 million charge to cost of product revenues related to the write-down of an earlier generation product. The write-down resulted in part from customers adopting our newer generation products more quickly than we anticipated, coupled with the downturn in the overall aesthetic laser market.
Sales and Marketing
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2009 | 2008 | |||||||||||||||
| Sales and marketing (in thousands) |
$ | 39,098 | $ | 53,062 | $ | (13,964 | ) | (26 | )% | |||||||
| Sales and marketing (as a percentage of total revenues) |
54 | % | 38 | % | ||||||||||||
Sales and marketing expenses decreased $14.0 million, or 26%, to $39.1 million in 2009, as compared to $53.1 million in 2008. The decrease was primarily attributable to a decrease of $5.2 million in commission expense associated with the 55% decrease in laser revenue, as well as a decrease of $6.9 million in personnel costs, consulting costs and travel expenses associated with the reduction of our North American and international direct sales organization and a decrease of $1.9 million in promotional costs primarily due to a decreased number of clinical workshops, trade shows and other promotional efforts. Although we reduced sales and marketing expenses in the year ended December 31, 2009, as compared to the year ended December 31, 2008, our sales and marketing expenses for the year ended December 31, 2009 increased as a percentage of total revenues to 54% as a result of the 48% decrease in total revenues.
50
Table of Contents
Research and Development
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2009 | 2008 | |||||||||||||||
| Research and development (in thousands) |
$ | 6,679 | $ | 7,497 | $ | (818 | ) | (11 | )% | |||||||
| Research and development (as a percentage of total revenues) |
9 | % | 5 | % | ||||||||||||
Research and development expenses decreased by $0.8 million for the year ended December 31, 2009, as compared with the year ended December 31, 2008 due to a reduction in professional services of $0.3 million and a reduction in personnel and other travel related expenses of $0.5 million associated with the overall reduction of our workforce.
General and Administrative
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2009 | 2008 | |||||||||||||||
| General and administrative (in thousands) |
$ | 14,556 | $ | 17,837 | $ | (3,281 | ) | (18 | )% | |||||||
| General and administrative (as a percentage of total revenues) |
20 | % | 13 | % | ||||||||||||
General and administrative expenses decreased by $3.3 million primarily due to a decrease in bad debt expense of $1.5 million and a decrease of $1.2 million related to the reduction in personnel costs, travel related expenses and other administrative costs associated with the overall reduction of our workforce, as well as reduced stock compensation expense of $0.6 million.
Interest Income, net
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2009 | 2008 | |||||||||||||||
| Interest income, net (in thousands) |
$ | 523 | $ | 2,498 | $ | (1,975 | ) | (79 | )% | |||||||
The decrease in interest income, net is primarily due to investing in securities that carry less risk and lower interest rates than in 2008.
Gain (Loss) on Investment and Other Income (Expense), net
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2009 | 2008 | |||||||||||||||
| Gain (loss) on investment (in thousands) |
22 | (46 | ) | 68 | 148 | % | ||||||||||
| Other income (expense), net (in thousands) |
672 | (43 | ) | 715 | 1,663 | % | ||||||||||
The gain (loss) on investment during the year ended December 31, 2009 relates to the recovery of $2.9 million for the fair value of the ARS and an offsetting loss of $2.9 million in the fair value of the Rights, netting to a gain of $22,000 for the year ended December 31, 2009. For the year ended December 31, 2008, an other-than-temporary impairment charge of ARS of $4.3 million was realized and we established the value of the Rights of $4.3 million, netting to a loss of $46,000. The increase in other income (expense) is primarily a result of net foreign currency remeasurement gains in the year ended December 31, 2009 compared to the net foreign currency remeasurement loss for the year ended December 31, 2008 due to the weakening of the U.S. dollar during 2009, primarily against the euro.
51
Table of Contents
Provision for Income Taxes
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2009 | 2008 | |||||||||||||||
| Provision for income taxes (in thousands) |
$ | 3,659 | $ | 4,771 | $ | (1,112 | ) | (23 | )% | |||||||
| Provision as a percentage of income before provision for income taxes |
(19 | )% | 32 | % | ||||||||||||
The provision for income taxes results from a combination of the activities of our domestic and foreign subsidiaries. In 2009, we recorded an income tax provision of $3.7 million, representing an effective tax rate of (19%). In 2008, we recorded an income tax provision of $4.8 million, representing an effective tax rate of 32%. Our 2009 effective tax rate changed from 2008 primarily due to the valuation allowance that was established against our $10.4 million of net domestic deferred tax assets.
Liquidity and Capital Resources
We require cash to pay our operating expenses, make capital expenditures and pay our long-term liabilities. Since our inception, we have funded our operations through our 2005 initial public offering, private placements of equity securities, short-term borrowings and funds generated from our operations.
At December 31, 2010, our cash, cash equivalents and short and long-term marketable securities were $96.8 million, as compared to $92.0 million at December 31, 2009. Our cash and cash equivalents of $27.4 million are highly liquid investments with maturities of 90 days or less at date of purchase and consist of cash in operating accounts, investments in money market funds, various state and municipal governments, U.S. government agencies and treasuries. Our short-term marketable securities of $59.4 million consist of investments in various state and municipal governments, U.S. government agencies and treasuries all of which mature by December 15, 2011. Our long-term marketable securities of $10.0 million consist of investments in various state and municipal governments, U.S. government agencies and treasuries all of which mature by September 17, 2012.
Our future capital requirements depend on a number of factors, including the rate of market acceptance of our current and future products, the resources we devote to developing and supporting our products and continued progress of our research and development of new products. We incurred minimal capital expenditures during the year ended December 31, 2010, and expect that capital expenditures during the next 12 months will be relatively consistent. During the year ended December 31, 2010 and 2009, respectively, we transferred $4.8 million and $7.4 million of demonstration equipment to fixed assets.
On July 28, 2009, our Board of Directors authorized the repurchase of up to $10 million of our Class A common stock, from time to time, on the open market or in privately negotiated transactions under a stock repurchase program. The program will terminate upon the purchase of $10 million in common stock, unless our Board of Directors discontinues it sooner. During the year ended December 31, 2010, we repurchased 145,621 shares of our common stock at an aggregate cost of approximately $1.4 million and at a weighted average price of $9.54 per share under this program. As of December 31, 2010, we have repurchased an aggregate of 148,935 shares under this program at an aggregate cost of $1.4 million.
We believe that our current cash, cash equivalents and short and long-term marketable securities, as well as cash generated from operations, will be sufficient to meet our anticipated cash needs for working capital and capital expenditures for the foreseeable future.
Cash Flows
Net cash provided by operating activities was $7.8 million for the year ended December 31, 2010. This resulted primarily from net loss for the period of $5.5 million, increased by approximately $9.3 million in
52
Table of Contents
depreciation and amortization and stock-based compensation expense and approximately $0.9 million in accretion of discounts on marketable securities. Net changes in working capital items increased cash from operating activities by approximately $3.4 million principally related to a decrease in prepaid expenses and other assets of $2.5 million associated with our income tax refund, a decrease in accounts receivable of $0.9 million due to increased collection efforts, and an increase in amounts due to related parties and accrued expenses of $1.6 million. This was offset by an increase in inventory of $2.0 million net of demonstration inventory transfers of $4.8 million. Net cash used in investing activities was $23.7 million for the year ended December 31, 2010, which consisted primarily of the net purchases of $23.1 million of marketable securities and $0.7 million used for fixed asset purchases. Net cash used in financing activities during the year ended December 31, 2010 was $1.5 million, principally relating to $1.4 million in the repurchase of our common stock and $0.2 million for payments on capital lease obligations.
Net cash used in operating activities was $1.5 million for the year ended December 31, 2009. This resulted primarily from net loss for the period of $22.8 million, decreased by approximately $11.6 million in depreciation and amortization and stock-based compensation expense, $6.8 million in deferred tax assets and approximately $0.4 million in accretion of discounts on marketable securities. Net changes in working capital items increased cash from operating activities by approximately $2.4 million principally related to a decrease in accounts receivable of $13.6 million due to reduced sales and increased collection efforts, and a decrease in inventory of $1.3 million primarily related to less purchases made during 2009 and the sale of inventory on hand at December 31, 2008, net of a $2.1 million charge to write-down inventory. This was offset by an increase in prepaid expenses and other assets related to our tax receivable position, and a decrease in amounts due to related party, accrued expenses and accounts payable of $11.3 million. Net cash used in investing activities was $2.3 million for the year ended December 31, 2009, which consisted primarily of net purchases of $1.5 million of marketable securities and fixed asset purchases of $0.7 million. Net cash used in financing activities during the year ended December 31, 2009 was $0.4 million, principally relating to payments on capital lease obligations.
Net cash provided by operating activities was $10.1 million for the year ended December 31, 2008. This resulted primarily from net income for the period of $10.2 million, approximately $11.4 million in depreciation and stock-based compensation expense, partially offset by approximately $2.8 million in deferred income tax benefits. Net changes in working capital items reduced cash from operating activities by approximately $9.1 million, principally due to an increase in inventory for anticipated future sales and lower-than-expected sales for the fourth quarter, as well as an increase in accounts receivable reflecting the record sales during 2008, and an overall slowdown in collections as certain customers were affected by the overall economic environment and the tightening of the credit markets and an increase in accounts payable and amounts due to a related party of $6.1 million. These were partially offset by a decrease in accrued expenses. Net cash used in investing activities was $2.3 million for the year ended December 31, 2008, which consisted primarily of $47.5 million used to purchase marketable securities and $1.9 million used for fixed asset purchases, offset by $48.1 million in proceeds generated from sales and maturities of securities. Net cash provided by financing activities during the year ended December 31, 2008 was $2.9 million, principally relating to proceeds from option exercises of $1.6 million, tax benefits related to stock options of $1.8 million and partially offset by $0.5 million in payments on capital lease obligations.
53
Table of Contents
Contractual Obligations
Our significant outstanding contractual obligations relate to our capital leases from equipment financings and our facilities leases. Our facility leases are non-cancellable and typically contain renewal options. Certain leases contain rent escalation clauses for which we recognize the expense on a straight-line basis. In addition, we guaranteed the lease obligations for two facilities that are operated by Sona MedSpa and will be obligated to pay these leases if Sona MedSpa cannot or does not make the required lease payments. We have summarized in the table below our fixed contractual cash obligations as of December 31, 2010.
| Total | Less Than One Year |
One to Three Years |
Three to Five Years |
More than Five Years |
||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Capital lease obligations, including interest |
$ | 183 | $ | 143 | $ | 38 | $ | 2 | $ | — | ||||||||||
| Operating leases |
3,032 | 1,492 | 1,540 | — | — | |||||||||||||||
| Lease guarantees |
18 | 18 | — | — | — | |||||||||||||||
| Total contractual obligations |
$ | 3,233 | $ | 1,653 | $ | 1,578 | $ | 2 | $ | — | ||||||||||
Off Balance Sheet Arrangements
Since inception, we have not engaged in any off balance sheet financing activities.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations set forth above are based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. On an ongoing basis, we evaluate our estimates and judgments, including those described below. We base our estimates on historical experience and on various assumptions that we believe to be reasonable under the circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities, and the reported amounts of revenues and expenses, that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies require significant judgment and estimates by us in the preparation of our financial statements.
Revenue Recognition and Deferred Revenue
In accordance with the Revenue Recognition Topic ASC 605-10-S99, we recognize revenue from sales of aesthetic treatment systems and parts and accessories when each of the following four criteria are met:
| • | delivery has occurred; |
| • | there is persuasive evidence of an agreement; |
| • | the fee is fixed or determinable; and |
| • | collection is reasonably assured. |
Revenue from the sale of service contracts is deferred and recognized on a straight-line basis over the contract period as services are provided.
We defer, until earned, payments that we receive in advance of product delivery or performance of services. When we enter into arrangements with multiple elements, which may include sales of products together with
54
Table of Contents
service contracts and warranties, we allocate revenue among the elements based on each element’s relative fair value in accordance with the principles of Accounting Standards Update (“ASU”) 2009-13, Revenue Recognition Topic—Multiple Element Arrangements. This allocation requires us to make estimates of fair value for each element. We adopted ASU 2009-13 during the second quarter of 2010 and applied it retrospectively beginning January 1, 2010. The adoption of ASU 2009-13 did not have a material impact on our financial position or statements of operations.
Accounts Receivable and Concentration of Credit Risk
Our accounts receivable balance, net of allowance for doubtful accounts, was $10.6 million as of December 31, 2010, compared with $11.7 million as of December 31, 2009. The allowance for doubtful accounts as of December 31, 2010 was $2.2 million and as of December 31, 2009 was $3.0 million. We maintain an allowance for doubtful accounts based upon the aging of our receivable balances, known collectability issues and our historical experience with losses. We work to mitigate bad debt exposure through our credit evaluation policies, reasonably short payment terms and geographical dispersion of sales. Our revenues include export sales to foreign companies located principally in Europe, the Asia/Pacific region and the Middle East. We obtain letters of credit for foreign sales that we consider to be at risk.
Inventories and Allowance for Excess and Obsolescence
We state all inventories at the lower of cost or market value, determined on a first-in, first-out method. We monitor standard costs on a monthly basis and update them annually and as necessary to reflect changes in raw material costs and labor and overhead rates. Our inventory balance was $18.7 million as of December 31, 2010 compared to $21.8 million as of December 31, 2009. The decrease in inventory primarily relates to the transfer of demonstration equipment to fixed assets of $4.8 million partially offset by increased purchases for the year ended December 31, 2010 when compared with the year ended December 31, 2009. Our inventory allowance was $2.7 million and $3.9 million as of December 31, 2010 and 2009, respectively. The decrease in our inventory allowance primarily relates to the disposition of inventory reserved for in prior periods.
We provide inventory allowances when conditions indicate that the selling price could be less than cost due to physical deterioration, usage, obsolescence, reductions in estimated future demand and reductions in selling prices. We balance the need to maintain strategic inventory levels with the risk of obsolescence due to changing technology and customer demand levels. Unfavorable changes in market conditions may result in a need for additional inventory reserves that could adversely impact our gross margins. Conversely, favorable changes in demand could result in higher gross margins when we sell products.
Product Warranty Costs and Provisions
We provide a one-year parts and labor warranty on end-user sales of our aesthetic treatment systems. Distributor sales generally include a warranty on parts only. We estimate and provide for future costs for initial product warranties at the time revenue is recognized. We base product warranty costs on related material costs, technical support labor costs and overhead. We provide for the estimated cost of product warranties by considering historical material, labor and overhead expenses and applying the experience rates to the outstanding warranty period for products sold. As we sell new products to our customers, we must exercise considerable judgment in estimating the expected failure rates and warranty costs. If actual product failure rates, material usage, service delivery costs or overhead costs differ from our estimates, we would be required to revise our estimated warranty liability.
Fair Value of Financial Instruments
Effective January 1, 2008, we adopted the Fair Value Measurements Topic ASC 820. ASC 820 defines fair value, establishes a framework for measuring fair value under U.S. GAAP and enhances disclosures about fair
55
Table of Contents
value measurements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes the following fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value:
| • | Level 1—Quoted prices in active markets for identical assets or liabilities. |
| • | Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable markets data for substantially the full term of the assets or liabilities. |
| • | Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
At December 31, 2009, our short-term investments consisted of $18.5 million, at par value, of auction rate securities (ARS) investments. The ARS were managed by UBS Financial Services, Inc. (UBS). On November 3, 2008, we agreed to accept Auction Rate Securities Rights (the Rights) from UBS. On June 30, 2010, we exercised the Rights and sold our ARS to UBS at full par value. At December 31, 2010, we held no short-term investments.
Stock-Based Compensation
We follow the fair value recognition provisions of ASC 718, Stock Compensation Topic (ASC 718). ASC 718 requires companies to utilize an estimated forfeiture rate when calculating the expense for the period. Accordingly, we review our actual forfeiture rates periodically and align our stock compensation expense with the options that are vesting.
The fair value of each stock option we granted is estimated using the Black-Scholes option pricing model. This option-pricing model requires the input of various subjective assumptions, including the option’s expected life and the price volatility of the underlying stock. Our estimated expected stock price volatility is based on our own historic volatility for the 2010 period and based on a weighted average of our own historical volatility and of the average volatilities of other guideline companies in the same industry for the 2009 and 2008 periods. We believe this is more reflective and a better indicator of the expected future volatility, than using an average of a comparable market index or of a comparable company in the same industry. Our expected term of options granted since adoption of ASC 718 was derived from the short-cut method described in SEC’s Staff ASC 718. The risk-free rate for the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The dividend yield of zero is based on the fact that we have never paid cash dividends and have no present intention to pay cash dividends.
We account for transactions in which services are received from non-employees in exchange for equity instruments based on the fair value of such services received or of the equity instruments issued, whichever is more reliably measured, in accordance with ASC 718 and the Equity Topic, ASC 505.
Income Taxes
We provide for income taxes in accordance with ASC 740, Accounting for Income Taxes. ASC 740 recognizes tax assets and liabilities for the cumulative effect of all temporary differences between the financial statement carrying amounts and the tax basis of assets and liabilities, and are measured using the enacted tax rates that will be in effect when these differences are expected to reverse. Valuation allowances are provided if, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
56
Table of Contents
We account for uncertain tax positions following the provisions of ASC 740. ASC 740 clarifies the accounting for income taxes, by prescribing a minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. ASC 740 also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition.
Recent Accounting Pronouncements
In December 2010, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2010-28, “Intangibles—Goodwill and Other (Topic 350) When to Perform Step 2 of the Goodwill Impairment Test for Reporting Units with Zero or Negative Carrying Amounts”. The objective of this ASU is to address diversity in practice in the application of goodwill impairment testing by entities with reporting units with zero or negative carrying amounts, eliminating an entity’s ability to assert that a reporting unit is not required to perform Step 2 because the carrying amount of the reporting unit is zero or negative despite the existence of qualitative factors that indicate the goodwill is more likely than not impaired. This ASU is effective for interim periods after January 1, 2011. The adoption of this guidance is not expected to have a material impact on our financial position or statements of operations.
In December, 2010, the FASB issued ASU 2010-29, “Business Combinations (Topic 805), Disclosure of Supplementary Pro Forma Information for Business Combinations”. The objective of this ASU is to address diversity in practice about the presentation of pro forma revenue and earnings disclosure requirements for business combinations, and specifies that a public entity that presents comparative financial statements should disclose revenue and earnings of the combined entity as though the business combination(s) that occurred during the current year had occurred as of the beginning of the comparable prior annual reporting period. This ASU is effective prospectively for business combinations on or after January 1, 2011. We are currently evaluating the impact of adopting ASU 2010-29 on our financial statements.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
The following discussion about our market risk disclosures involves forward-looking statements. Actual results could differ materially from those projected in the forward-looking statements. We are exposed to market risk related to changes in interest rates and foreign currency exchange rates. We do not use derivative financial instruments.
Interest Rate Sensitivity. We maintain an investment portfolio consisting mainly of money market funds, state and municipal bonds, and U.S. government agencies and treasuries. The securities, other than money market funds, are classified as available-for-sale and consequently are recorded on the balance sheet at fair value with unrealized gains and losses reported as a separate component of accumulated other comprehensive (loss) income. All investments mature by September 17, 2012. These available-for-sale securities are subject to interest rate risk and will fall in value if market interest rates increase, which could result in a realized loss if we are forced to sell an investment before its scheduled maturity. We currently have the ability and intent to hold our fixed income investments until maturity. We do not utilize derivative financial instruments to manage our interest rate risks.
The following table provides information about our investment portfolio in available-for-sale debt securities. For investment securities, the table presents principal cash flows (in thousands) and weighted average interest rates by expected maturity dates.
| December 31, 2010 | 2011 | 2012 | ||||||||||
| Investments (at fair value) |
$ | 75,497 | $ | 65,507 | $ | 9,990 | ||||||
| Weighted average interest rate |
0.38 | % | 0.36 | % | 0.53 | % | ||||||
Foreign Currency Exchange. A significant portion of our operations is conducted through operations in countries other than the United States. Revenues from our international operations that were recorded in U.S.
57
Table of Contents
dollars represented approximately 38% of our total international revenues during the year ended December 31, 2010. Substantially all of the remaining 62% were sales in euros, British pounds, Japanese yen, Chinese yuan and South Korean won. Since we conduct our business in U.S. dollars, our main exposure, if any, results from changes in the exchange rate between these currencies and the U.S. dollar. Our functional currency is the U.S. dollar. Our policy is to reduce exposure to exchange rate fluctuations by having most of our assets and liabilities, as well as most of our revenues and expenditures, in U.S. dollars, or U.S. dollar linked. We have not historically engaged in hedging activities relating to our non-U.S. dollar operations. We sell inventory to our subsidiaries in U.S. dollars. These amounts are recorded at our local subsidiaries in local currency rates in effect on the transaction date. Therefore, we may be exposed to exchange rate fluctuations that occur while the debt is outstanding which we recognize as unrealized gains and losses in our statements of operations. Upon settlement of these debts, we may record realized foreign exchange gains and losses in our statements of operations. We may incur negative foreign currency translation charges as a result of changes in currency exchange rates.
| Item 8. | Financial Statements and Supplementary Data |
All financial statements and schedules required to be filed hereunder are included beginning on page F-1 and are incorporated in this report by reference
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2010. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2010, our chief executive officer and chief financial officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
No change in our internal control over financial reporting occurred during the fiscal quarter ended December 31, 2010 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management’s Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate control over financial reporting as defined in Rule 13(a)-15(f) and 15(d)-15(f) under the Exchange Act. Internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of
58
Table of Contents
financial statements for external purposes in accordance with GAAP. Internal control over financial reporting includes those policies and procedures that: 1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; 2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and 3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, a system of internal control over financial reporting can provide only reasonable assurance and may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2010. In making its assessment, management used the criteria set forth in Internal Control–Integrated Framework issued by the Committee of Sponsoring Organizations (“COSO”) of the Treadway Commission. A “material weakness” is a control deficiency (within the meaning of Public Company Accounting Oversight Board Auditing Standard No. 5), or combination of control deficiencies, that result in there being more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis by employees in the normal course of their assigned functions. Based on management’s assessment, management determined that the Company maintained effective internal control over financial reporting as of December 31, 2010 based on the COSO criteria.
Our internal control over financial reporting as of December 31, 2010 has been audited by Ernst & Young, LLP, an independent registered public accounting firm, as stated in its report below.
59
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of
Cynosure, Inc.:
We have audited Cynosure Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Cynosure, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Cynosure, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders’ equity and comprehensive (loss) income and cash flows for each of the three years in the period ended December 31, 2010 of Cynosure, Inc. and our report dated March 10, 2011 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston, Massachusetts
March 10, 2011
60
Table of Contents
| Item 9B. | Other Information |
None.
61
Table of Contents
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
The information required by this item with respect to our directors and executive officers will be contained in our 2011 Proxy Statement under the caption “INFORMATION ABOUT OUR DIRECTORS, OFFICERS AND 5% STOCKHOLDERS” and is incorporated in this report by reference.
The information required by this item with respect to Section 16(a) beneficial ownership reporting compliance will be contained in our 2011 Proxy Statement under the caption “SECTION 16(A) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE” and is incorporated in this report by reference.
The information required by this item with respect to corporate governance matters will be contained in our 2011 Proxy Statement under the caption “CORPORATE GOVERNANCE” and is incorporated in this report by reference.
| Item 11. | Executive Compensation |
The information required by this item will be contained in our 2011 Proxy Statement under the captions “DIRECTOR COMPENSATION,” “COMPENSATION DISCUSSION AND ANALYSIS” and “EXECUTIVE COMPENSATION” and is incorporated in this report by reference.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this item with regard to security ownership of certain beneficial owners and management will be contained in our 2011 Proxy Statement under the caption “INFORMATION ABOUT OUR DIRECTORS, OFFICERS AND 5% STOCKHOLDERS—Security Ownership of Certain Beneficial Owners and Management” and is incorporated in this report by reference.
The information required by this item with regard to securities authorized for issuance under equity compensation plans will be contained in our 2011 Proxy Statement under the caption “EXECUTIVE COMPENSATION—Securities Authorized for Issuance under our Equity Compensation Plans” and is incorporated in this report by reference.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this item will be contained in our 2011 Proxy Statement under the captions “RELATED-PARTY TRANSACTIONS” and “CORPORATE GOVERNANCE” and is incorporated in this report by reference.
| Item 14. | Principal Accountant Fees and Services |
The information required by this item will be contained in our 2011 Proxy Statement under the caption “PROPOSAL 3—RATIFICATION OF THE SELECTION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM” and is incorporated in this report by reference.
62
Table of Contents
PART IV
| Item 15. | Exhibits and Financial Statement Schedules |
| (a) |
1. Financial Statements. The financial statements and notes thereto annexed to this report begin on page F-1. | |
| 2. Financial Statement Schedules. All other supplemental schedules are omitted because of the absence of conditions under which they are required or because the required information is given in the financial statements or notes thereto. | ||
| 3. Exhibits. The Exhibit Index annexed to this report, and immediately preceding the exhibits, is incorporated by reference. | ||
63
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| CYNOSURE, INC. | ||
| By: |
/s/ MICHAEL R. DAVIN | |
| Michael R. Davin | ||
| President, Chief Executive Officer and Chairman of the Board of Directors | ||
Pursuant to the requirements of the Securities Act of 1934, this report has been signed by the following persons on behalf of the registrant in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ MICHAEL R. DAVIN Michael R. Davin |
President, Chief Executive Officer and Chairman of the Board of Directors (Principal Executive Officer) |
March 10, 2011 | ||
| /s/ TIMOTHY W. BAKER Timothy W. Baker |
Executive Vice President and Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
March 10, 2011 | ||
| /s/ ETTORE V. BIAGIONI Ettore V. Biagioni |
Director |
March 10, 2011 | ||
| /s/ ANDREA CANGIOLI Andrea Cangioli |
Director |
March 10, 2011 | ||
| /s/ MARINA HATSOPOULOS Marina Hatsopoulos |
Director |
March 10, 2011 | ||
| /s/ LEONARDO MASOTTI Leonardo Masotti |
Director |
March 10, 2011 | ||
| /s/ THOMAS H. ROBINSON Thomas H. Robinson |
Director |
March 10, 2011 | ||
64
Table of Contents
INDEX TO FINANCIAL STATEMENTS
| Consolidated Financial Statements of Cynosure, Inc. |
||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| Consolidated Statements of Stockholders’ Equity and Comprehensive (Loss) Income |
F-5 | |||
| F-6 | ||||
| F-7 |
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Cynosure, Inc.:
We have audited the accompanying consolidated balance sheets of Cynosure, Inc. as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders’ equity and comprehensive (loss) income and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Cynosure, Inc. at December 31, 2010 and 2009, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Cynosure, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 10, 2011 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston, Massachusetts
March 10, 2011
F-2
Table of Contents
CONSOLIDATED BALANCE SHEETS
(In thousands, except per share data)
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| ASSETS | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 27,434 | $ | 44,797 | ||||
| Short-term marketable securities |
59,402 | 23,708 | ||||||
| Short-term investments and related financial instruments |
— | 18,454 | ||||||
| Accounts receivable, net of allowance of $2,207 and $2,983 in 2010 and 2009, respectively |
10,621 | 11,741 | ||||||
| Inventories |
18,684 | 21,815 | ||||||
| Prepaid expenses and other current assets |
3,902 | 6,441 | ||||||
| Deferred income taxes |
489 | 160 | ||||||
| Total current assets |
120,532 | 127,116 | ||||||
| Property and equipment, net |
8,892 | 10,567 | ||||||
| Long-term marketable securities |
9,990 | 5,008 | ||||||
| Other assets |
2,398 | 2,510 | ||||||
| Total assets |
$ | 141,812 | $ | 145,201 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 4,840 | $ | 4,822 | ||||
| Amounts due to related party |
1,785 | 1,350 | ||||||
| Accrued expenses |
10,427 | 9,535 | ||||||
| Deferred revenue |
3,660 | 4,237 | ||||||
| Capital lease obligations |
133 | 264 | ||||||
| Total current liabilities |
20,845 | 20,208 | ||||||
| Capital lease obligations, net of current portion |
40 | 171 | ||||||
| Deferred revenue, net of current portion |
348 | 620 | ||||||
| Other noncurrent liability |
279 | 372 | ||||||
| Commitments and Contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.001 par value Authorized—5,000 shares as of December 31, 2010 and 2009 Issued—no shares as of December 31, 2010 and 2009 |
— | — | ||||||
| Class A and Class B common stock, $0.001 par value Authorized—70,000 shares as of December 31, 2010 and 2009 Issued—9,786 Class A shares and 2,975 Class B shares at December 31, 2010; Issued—9,775 Class A shares and 2,975 Class B Shares at December 31, 2009 |
13 | 13 | ||||||
| Additional paid-in capital |
121,706 | 117,814 | ||||||
| Retained earnings |
2,227 | 7,773 | ||||||
| Accumulated other comprehensive loss |
(1,938 | ) | (1,451 | ) | ||||
| Treasury stock, 149 Class A shares and 36 Class B shares, at cost, at December 31, 2010; 3 Class A and 36 Class B shares, at cost, at December 31, 2009 |
(1,708 | ) | (319 | ) | ||||
| Total stockholders’ equity |
120,300 | 123,830 | ||||||
| Total liabilities and stockholders’ equity |
$ | 141,812 | $ | 145,201 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| Year Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Product revenues |
$ | 62,357 | $ | 55,869 | $ | 123,208 | ||||||
| Parts, accessories and service revenues |
19,418 | 16,956 | 16,454 | |||||||||
| Total revenues |
81,775 | 72,825 | 139,662 | |||||||||
| Cost of revenues |
35,388 | 32,808 | 48,705 | |||||||||
| Gross profit |
46,387 | 40,017 | 90,957 | |||||||||
| Operating expenses: |
||||||||||||
| Sales and marketing |
32,818 | 39,098 | 53,062 | |||||||||
| Research and development |
7,300 | 6,679 | 7,497 | |||||||||
| General and administrative |
11,312 | 14,556 | 17,837 | |||||||||
| Total operating expenses |
51,430 | 60,333 | 78,396 | |||||||||
| (Loss) income from operations |
(5,043 | ) | (20,316 | ) | 12,561 | |||||||
| Interest income, net |
163 | 523 | 2,498 | |||||||||
| Gain (loss) on investments (Note 3) |
23 | 22 | (46 | ) | ||||||||
| Other (expense) income, net |
(247 | ) | 672 | (43 | ) | |||||||
| (Loss) income before provision for income taxes |
(5,104 | ) | (19,099 | ) | 14,970 | |||||||
| Provision for income taxes |
442 | 3,659 | 4,771 | |||||||||
| Net (loss) income |
$ | (5,546 | ) | $ | (22,758 | ) | $ | 10,199 | ||||
| Basic net (loss) income per share |
$ | (0.44 | ) | $ | (1.79 | ) | $ | 0.81 | ||||
| Diluted net (loss) income per share |
$ | (0.44 | ) | $ | (1.79 | ) | $ | 0.80 | ||||
| Basic weighted average common shares outstanding |
12,666 | 12,709 | 12,581 | |||||||||
| Diluted weighted average common shares outstanding |
12,666 | 12,709 | 12,806 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY AND COMPREHENSIVE
(LOSS) INCOME
(In thousands)
| Class A and B Common Stock |
Additional Paid-In Capital |
Retained Earnings |
Accumulated Other Comprehensive (Loss) Income |
Class A &
B Treasury Stock |
Total Stockholders Equity |
Comprehensive Income (Loss) |
||||||||||||||||||||||||||||||
| Shares | $0.001 Par Value |
|||||||||||||||||||||||||||||||||||
| Shares | Cost | |||||||||||||||||||||||||||||||||||
| Balance at December 31, 2007 |
12,448 | $ | 12 | $ | 101,298 | $ | 20,332 | $ | (477 | ) | (36 | ) | $ | (287 | ) | $ | 120,878 | |||||||||||||||||||
| Stock-based compensation expense |
— | — | 7,408 | — | — | — | — | 7,408 | ||||||||||||||||||||||||||||
| Tax benefit from stock-based compensation expense in excess of book deductions |
— | — | 1,570 | — | — | — | — | 1,570 | ||||||||||||||||||||||||||||
| Exercise of stock options |
286 | 1 | 1,616 | — | — | — | — | 1,617 | ||||||||||||||||||||||||||||
| Net income |
— | — | — | 10,199 | — | — | — | 10,199 | $ | 10,199 | ||||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | (1,376 | ) | — | — | (1,376 | ) | (1,376 | ) | ||||||||||||||||||||||||
| Unrealized gain on marketable securities, net of tax provision |
— | — | — | — | 58 | — | — | 58 | 58 | |||||||||||||||||||||||||||
| Balance at December 31, 2008 |
12,734 | $ | 13 | $ | 111,892 | $ | 30,531 | $ | (1,795 | ) | (36 | ) | $ | (287 | ) | $ | 140,354 | $ | 8,881 | |||||||||||||||||
| Stock-based compensation expense |
— | — | 6,184 | — | — | — | — | 6,184 | ||||||||||||||||||||||||||||
| Tax deficiency from stock-based compensation expense in excess of book deductions |
— | — | (320 | ) | — | — | — | — | (320 | ) | ||||||||||||||||||||||||||
| Exercise of stock options |
16 | — | 58 | — | — | — | — | 58 | ||||||||||||||||||||||||||||
| Repurchase of common stock |
— | — | — | — | (3 | ) | (32 | ) | (32 | ) | ||||||||||||||||||||||||||
| Net loss |
— | — | — | (22,758 | ) | — | — | — | (22,758 | ) | $ | (22,758 | ) | |||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | 422 | — | — | 422 | 422 | |||||||||||||||||||||||||||
| Unrealized gain on marketable securities, net of tax provision |
— | — | — | — | (78 | ) | — | — | (78 | ) | (78 | ) | ||||||||||||||||||||||||
| Balance at December 31, 2009 |
12,750 | $ | 13 | $ | 117,814 | $ | 7,773 | $ | (1,451 | ) | (39 | ) | $ | (319 | ) | $ | 123,830 | $ | (22,414 | ) | ||||||||||||||||
| Stock-based compensation expense |
— | — | 3,807 | — | — | — | — | 3.807 | ||||||||||||||||||||||||||||
| Exercise of stock options |
11 | — | 85 | — | — | — | — | 85 | ||||||||||||||||||||||||||||
| Repurchase of common stock |
— | — | — | — | (146 | ) | (1,389 | ) | (1,389 | ) | ||||||||||||||||||||||||||
| Net loss |
— | — | — | (5,546 | ) | — | — | — | (5,546 | ) | $ | (5,546 | ) | |||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | (510 | ) | — | — | (510 | ) | (510 | ) | ||||||||||||||||||||||||
| Unrealized gain on marketable securities, net of tax provision |
— | — | — | — | 23 | — | — | 23 | 23 | |||||||||||||||||||||||||||
| Balance at December 31, 2010 |
12,761 | $ | 13 | $ | 121,706 | $ | 2,227 | $ | (1,938 | ) | (185 | ) | $ | (1,708 | ) | $ | 120,300 | $ | (6,033 | ) | ||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Operating activities: |
||||||||||||
| Net (loss) income |
$ | (5,546 | ) | $ | (22,758 | ) | $ | 10,199 | ||||
| Reconciliation of net (loss) income to net cash provided by (used in) operating activities: |
||||||||||||
| Depreciation and amortization |
5,431 | 5,444 | 3,994 | |||||||||
| (Gain) loss on investments |
(21 | ) | (22 | ) | 46 | |||||||
| Stock-based compensation |
3,843 | 6,184 | 7,422 | |||||||||
| Deferred income taxes |
(221 | ) | 6,775 | (2,819 | ) | |||||||
| Loss on disposal of fixed assets |
62 | 24 | — | |||||||||
| Accretion of discounts on marketable securities |
877 | 407 | 267 | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
883 | 13,649 | (1,665 | ) | ||||||||
| Due from related party |
— | 40 | (34 | ) | ||||||||
| Inventories |
(1,988 | ) | 1,255 | (12,327 | ) | |||||||
| Net book value of demonstration inventory sold |
1,244 | 734 | 730 | |||||||||
| Prepaid expenses and other current assets |
2,480 | (2,082 | ) | (214 | ) | |||||||
| Accounts payable |
71 | (280 | ) | 2,318 | ||||||||
| Due to related party |
448 | (4,723 | ) | 3,826 | ||||||||
| Tax benefit from stock option exercises |
(2 | ) | (3 | ) | (1,774 | ) | ||||||
| Accrued expenses |
1,116 | (6,313 | ) | (506 | ) | |||||||
| Deferred revenue |
(883 | ) | 140 | 327 | ||||||||
| Other noncurrent liability |
— | (5 | ) | 264 | ||||||||
| Net cash provided by (used in) operating activities |
7,794 | (1,534 | ) | 10,054 | ||||||||
| Investing activities: |
||||||||||||
| Purchases of property and equipment |
(663 | ) | (656 | ) | (1,889 | ) | ||||||
| Proceeds from the sales and maturities of securities |
72,718 | 37,145 | 48,121 | |||||||||
| Purchases of marketable securities |
(95,768 | ) | (38,625 | ) | (47,452 | ) | ||||||
| Acquisition of Orient MG |
— | — | (512 | ) | ||||||||
| Increase in other assets |
— | (192 | ) | (555 | ) | |||||||
| Net cash used in investing activities |
(23,713 | ) | (2,328 | ) | (2,287 | ) | ||||||
| Financing activities: |
||||||||||||
| Excess tax benefit on options exercised |
2 | 3 | 1,774 | |||||||||
| Repurchases of common stock |
(1,389 | ) | (32 | ) | — | |||||||
| Proceeds from stock option exercises |
85 | 58 | 1,617 | |||||||||
| Payments on capital lease obligation |
(235 | ) | (390 | ) | (481 | ) | ||||||
| Net cash (used in) provided by financing activities |
(1,537 | ) | (361 | ) | 2,910 | |||||||
| Effect of exchange rate changes on cash and cash equivalents |
93 | (237 | ) | (431 | ) | |||||||
| Net increase (decrease) in cash and cash equivalents |
(17,363 | ) | (4,460 | ) | 10,246 | |||||||
| Cash and cash equivalents, beginning of year |
44,797 | 49,257 | 39,011 | |||||||||
| Cash and cash equivalents, end of year |
$ | 27,434 | $ | 44,797 | $ | 49,257 | ||||||
| Supplemental cash flow information: |
||||||||||||
| Cash paid for interest |
$ | 57 | $ | 81 | $ | 123 | ||||||
| Cash paid for income taxes |
$ | 1,021 | $ | 1,304 | $ | 6,383 | ||||||
| Supplemental noncash investing and financing activities: |
||||||||||||
| Transfer of demonstration equipment from inventory to fixed assets |
$ | 4,802 | $ | 7,359 | $ | 4,048 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of the Business
Cynosure, Inc. (Cynosure or the Company) develops, manufactures and markets aesthetic treatment systems that are used by physicians and other practitioners to perform non-invasive to remove hair, treat vascular lesions, rejuvenate skin through the treatment of shallow vascular lesions and pigmented lesions, as well as multi-colored tattoos, treat wrinkles, skin texture, skin discoloration and skin tightening, perform minimally invasive procedures for LaserBodySculpting for the removal of unwanted fat and temporary and long-term reduction in the appearance of cellulite. Cynosure markets and sells its products primarily to the dermatology, plastic surgery and general medical markets, both domestically and internationally. Cynosure is a Delaware corporation, incorporated on July 10, 1991, located in Westford, Massachusetts.
2. Summary of Significant Accounting Policies
Significant accounting policies followed in the preparation of these consolidated financial statements are as follows:
Management Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and the related disclosures at the date of the financial statements and during the reporting period. Components particularly subject to estimation include the allowance for doubtful accounts, inventory reserves, fair value of stock options and investments and accrued warranties. On an ongoing basis, management evaluates its estimates. Actual results could differ from these estimates.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Cynosure, Inc. and its wholly owned subsidiaries: Cynosure GmbH, Cynosure S.A.R.L., Cynosure UK Limited, Cynosure Spain, S.L., Cynosure KK, Suzhou Cynosure Medical Devices, Co., Cynosure Mexico and Cynosure Korea Limited (See Note 5). All significant intercompany balances and transactions have been eliminated.
Reclassifications
Certain amounts in the prior year’s financial statements have been reclassified to conform to the current year’s presentation within the consolidated balance sheet and the consolidated statements of cash flows.
Cash, Cash Equivalents, Short and Long-Term Marketable Securities
Cynosure considers all short-term, highly liquid investments with original maturities at the time of purchase of 90 days or less to be cash equivalents. Cynosure accounts for short and long-term marketable securities as available-for-sale in accordance with Accounting Standards Codification (ASC) 320, Investments—Debt and Equity Securities Topic. Under ASC 320, securities purchased to be held for indefinite periods of time and not intended at the time of purchase to be held until maturity are classified as available-for-sale securities. ASC 320 requires Cynosure to recognize all marketable securities on the consolidated balance sheets at fair value. Cynosure’s marketable securities are stated at fair value based on quoted market prices. Adjustments to the fair value of marketable securities that are classified as available-for-sale are recorded as increases or decreases, net of income taxes, within accumulated other comprehensive gain (loss) in shareholders’ equity. The amortized cost of marketable debt securities is adjusted for amortization of premiums and discounts to maturity computed
F-7
Table of Contents
under the effective interest method. The cost of securities sold is determined by the specific identification method. Cynosure continually evaluates whether any marketable investments have been impaired and, if so, whether such impairment is temporary or other than temporary.
Fair Value of Financial Instruments
Cynosure’s financial instruments consist of cash, cash equivalents, short and long-term marketable securities, accounts receivable and capital leases. Cynosure’s estimate of fair value for financial instruments, other than marketable securities, approximates their carrying value at December 31, 2010 and 2009.
Cynosure adopted the provisions of ASC 820, Fair Value Measurements Topic, for financial assets and liabilities measured on a recurring basis on January 1, 2008. ASC 820 applies to all financial assets and financial liabilities that are being measured and reported on a fair value basis, establishes a framework for measuring fair value of assets and liabilities and expands disclosures about fair value measurements. There was no impact to the consolidated financial statements as a result of the adoption of this Topic. The additional disclosure requirements regarding fair value measurements are included in Note 3 to the consolidated financial statements.
Accounts Receivable and Concentration of Credit Risk
Cynosure’s accounts receivable balance, net of allowance for doubtful accounts, was $10.6 million as of December 31, 2010, compared with $11.7 million as of December 31, 2009. The allowance for doubtful accounts as of December 31, 2010 was $2.2 million and as of December 31, 2009 was $3.0 million. Cynosure maintains an allowance for doubtful accounts based upon the aging of its receivable balances, known collectibility issues and Cynosure’s historical experience with losses. Cynosure works to mitigate bad debt exposure through its credit evaluation policies, reasonably short payment terms and geographical dispersion of sales. Cynosure’s revenue includes export sales to foreign companies located principally in Europe, the Asia/Pacific region and the Middle East. Cynosure obtains letters of credit for foreign sales that the Company considers to be at risk.
No customer accounted for 10% or greater of revenue during 2010, 2009 or 2008. No customer accounted for 10% or greater of accounts receivable as of December 31, 2010 or 2009. Accounts receivable allowance activity consisted of the following for the years ended December 31:
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands) | ||||||||||||
| Balance at beginning of year |
$ | 2,983 | $ | 2,861 | $ | 1,451 | ||||||
| Additions |
933 | 2,485 | 4,200 | |||||||||
| Deductions |
(1,709 | ) | (2,363 | ) | (2,790 | ) | ||||||
| Balance at end of year |
$ | 2,207 | $ | 2,983 | $ | 2,861 | ||||||
Inventory
Cynosure states all inventories at the lower of cost or market, determined on a first-in, first-out method. Inventory includes material, labor and overhead and consists of the following:
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| (In thousands) | ||||||||
| Raw materials |
$ | 3,980 | $ | 3,700 | ||||
| Work in process |
711 | 1,302 | ||||||
| Finished goods |
13,993 | 16,813 | ||||||
| $ | 18,684 | $ | 21,815 | |||||
F-8
Table of Contents
Included in finished goods are lasers used for demonstration purposes. Cynosure’s policy is to include demonstration lasers as inventory for a period of up to one year after production at which time the demonstration lasers are either sold or transferred to fixed assets at the lower of cost or market and depreciated over their estimated useful life of three years. Similar to any other finished goods in inventory, Cynosure accounts for such demonstration inventory in accordance with the policy for excess and obsolescence review of Cynosure’s entire inventory.
Cynosure’s policy is to establish inventory reserves when conditions exist that suggest that inventory may be in excess of anticipated demand or is obsolete based upon assumptions about future demand for products and market conditions. Cynosure regularly evaluates the ability to realize the value of inventory based on a combination of factors including the following: historical usage rates, forecasted sales or usage, product end of life dates, estimated current and future market values and new product introductions. Assumptions used in determining management’s estimates of future product demand may prove to be incorrect; in which case the provision required for excess and obsolete inventory would have to be adjusted in the future. If inventory is determined to be overvalued, Cynosure recognizes such costs as cost of goods sold at the time of such determination. Although Cynosure performs a detailed review of its forecasts of future product demand, any significant unanticipated changes in demand could have a significant impact on the value of Cynosure’s inventory and reported operating results. Cynosure’s inventory allowance was $2.7 million and $3.9 million as of December 31, 2010 and 2009, respectively. The decrease in the inventory allowance primarily relates to the disposition of inventory reserved for in prior periods.
In 2009, Cynosure recorded a $2.1 million charge to cost of product revenues related to the write-down of an earlier generation product. The write-down resulted in part from customers adopting Cynosure’s newer generation products more quickly than the Company anticipated, coupled with the downturn in the overall aesthetic laser market.
Cynosure purchases a significant raw material component from one vendor, who is the sole manufacturer of this component. A delay in the production capabilities of this vendor could cause a delay in Cynosure’s manufacturing, and a possible loss of revenues, which would adversely affect operating results.
Property and Equipment
Property and equipment are recorded at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Assets under capital leases and leasehold improvements are amortized using the straight-line method over the shorter of the estimated useful life of the asset or the respective lease term. Included in property and equipment are certain lasers that are used for demonstration purposes. Maintenance and repairs are charged to expense as incurred. Cynosure continually evaluates whether events or circumstances have occurred that indicate that the estimated remaining useful life of its long-lived assets may warrant revision or that the carrying value of these assets may be impaired. Cynosure evaluates the realizability of its long-lived assets based on profitability and cash flow expectations for the related asset. Any write-downs are treated as permanent reductions in the carrying amount of the assets. Based on this evaluation, Cynosure believes that, as of each of the balance sheet dates presented, none of Cynosure’s long-lived assets were impaired.
Revenue Recognition and Deferred Revenue
Cynosure generates revenue from the sale of aesthetic treatment systems that are used by physicians and other practitioners to perform various non-invasive and minimally invasive aesthetic procedures. These systems incorporate a broad range of laser and other light-based energy sources. Cynosure offers service and warranty contracts in connection with these sales.
Cynosure recognizes revenue from sales of aesthetic treatment systems and parts and accessories in accordance with the Revenue Recognition Topic ASC 605-10-S99. Cynosure recognizes revenue from sales of its
F-9
Table of Contents
treatment systems and parts and accessories upon delivery, provided there are no uncertainties regarding customer acceptance, there is persuasive evidence of an arrangement, the fee is fixed or determinable, and collectibility of the related receivable is reasonably assured. Revenues from the sales of service and warranty contracts are deferred and recognized on a straight-line basis over the contract period as services are provided. Payments received by Cynosure in advance of product delivery or performance of services are deferred until earned.
Multiple-element arrangements are evaluated in accordance with the principles of Accounting Standards Update (“ASU”) 2009-13, Revenue Recognition Topic—Multiple Element Arrangements and Cynosure allocates revenue among the elements based upon each element’s relative fair value. Cynosure adopted ASU 2009-13 during the second quarter of 2010 and applied it retrospectively beginning January 1, 2010. The adoption of ASU 2009-13 did not have a material impact on Cynosure’s financial position or statements of operations.
In accordance with the provisions of ASC 605-45, Revenue Recognitions Topic—Principal Agent Considerations, Cynosure records shipping and handling costs billed to its customers as a component of revenue, and the underlying expense as a component of cost of revenue. Shipping and handling costs included as a component of revenue totaled approximately $0.3 million, $0.3 million, and $0.7 million for the years ended December 31, 2010, 2009 and 2008, respectively. Shipping and handling costs included as a component of cost of revenue totaled $0.3 million, $0.3 million and $0.8 million for the years ended December 31, 2010, 2009 and 2008, respectively.
Cynosure collects sales tax from its customers on product sales for which the customer is not tax exempt and remits such taxes to the appropriate governmental authorities. Cynosure presents its sales taxes on a net basis; therefore, these taxes are excluded from revenues.
Product Warranty Costs
Cynosure typically provides a one-year parts and labor warranty on end-user sales of lasers. Distributor sales generally include a one-year warranty on parts only. Estimated future costs for initial product warranties are provided for at the time of revenue recognition. The following table sets forth activity in the accrued warranty account:
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands) | ||||||||||||
| Balance at beginning of year |
$ | 2,440 | $ | 3,052 | $ | 3,094 | ||||||
| Warranty provision related to new sales |
3,550 | 4,422 | 4,378 | |||||||||
| Costs incurred |
(3,878 | ) | (5,034 | ) | (4,420 | ) | ||||||
| Balance at end of year |
$ | 2,112 | $ | 2,440 | $ | 3,052 | ||||||
Royalty Costs
Under a cross-license agreement with Palomar Medical Technologies, Inc. (Palomar), Cynosure has a non-exclusive license to integrate its products for certain hair removal technology covered by specified U.S. and foreign patents held by Palomar and Palomar has a non-exclusive license under certain U.S. and foreign patents held by Cynosure. In connection with this agreement, Cynosure has agreed to pay royalties to Palomar on future sales of certain hair removal-only products. The royalty rate for sales of hair removal products ranges from 3.75% to 7.5% of net sales, depending upon product configuration and the number of energy sources. Cynosure’s revenues from systems that do not include hair removal capabilities and revenues from service are not subject to any royalties under this agreement.
F-10
Table of Contents
Research and Development
Research and development costs consist of salaries and other personnel-related expenses, including stock-based compensation, of employees primarily engaged in research, development and engineering activities and materials used and other overhead expenses incurred in connection with the design and development of Cynosure’s products and from time to time expenses associated with collaborative research agreements that the Company may enter into. These costs are expensed as incurred.
Advertising Costs
Cynosure expenses advertising costs as incurred. Advertising costs totaled $0.4 million, $0.6 million and $0.9 million for the years ended December 31, 2010, 2009 and 2008, respectively.
Foreign Currency Translation
The financial statements of Cynosure’s foreign subsidiaries are translated from local currency into U.S. dollars using the current exchange rate at the balance sheet date for assets and liabilities, and the average exchange rate prevailing during the period for revenue and expenses. The functional currency for Cynosure’s foreign subsidiaries is considered to be the local currency for each entity and, accordingly, translation adjustments for these subsidiaries are included in accumulated other comprehensive income within stockholders’ equity. Certain intercompany and third party foreign currency-denominated transactions generated foreign currency remeasurement (losses) gains of approximately $(274,000), $573,000 and $17,000 during 2010, 2009 and 2008, respectively, which are included in other (expense) income, net, in the consolidated statements of operations.
Comprehensive (Loss) Income and Accumulated Other Comprehensive Loss
Comprehensive loss is the change in equity of a company during a period from transactions and other events and circumstances, excluding transactions resulting from investments by owners and distributions to owners.
The components of accumulated other comprehensive loss as of December 31, 2010 and 2009 are as follows:
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| (In thousands) | ||||||||
| Unrealized gain on marketable securities, net of $11 and $3 income taxes |
$ | 29 | $ | 6 | ||||
| Cumulative translation adjustment |
(1,967 | ) | (1,457 | ) | ||||
| Total accumulated other comprehensive loss |
$ | (1,938 | ) | $ | (1,451 | ) | ||
The components of total other comprehensive income (loss) for the years ended December 31, 2010 and 2009 are as follows:
| Years Ended December 31, |
||||||||
| 2010 | 2009 | |||||||
| (In thousands) | ||||||||
| Cumulative translation adjustment |
$ | (510 | ) | $ | 422 | |||
| Unrealized gain (loss) on marketable securities |
23 | (78 | ) | |||||
| Total other comprehensive (loss) income |
(487 | ) | 344 | |||||
| Reported net loss |
(5,546 | ) | (22,758 | ) | ||||
| Total comprehensive loss |
$ | (6,033 | ) | $ | (22,414 | ) | ||
F-11
Table of Contents
Stock-Based Compensation
Cynosure follows the fair value recognition provisions of ASC 718, Stock Compensation Topic (ASC 718). ASC 718 requires companies to utilize an estimated forfeiture rate when calculating the expense for the period. Accordingly, Cynosure reviews its actual forfeiture rates and periodically aligns its stock compensation expense with the options that are vesting.
Cynosure recorded stock-based compensation expense of $3.8 million, $6.2 million and $7.4 million. Cynosure capitalized $17,000 and $53,000, respectively, of stock-based compensation expense as a part of inventory as of December 31, 2010 and 2009.
Total stock-based compensation expense was recorded to cost of revenues and operating expenses based upon the functional responsibilities of the individual holding the respective options, as follows:
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands) | ||||||||||||
| Cost of revenues |
$ | 289 | $ | 447 | $ | 538 | ||||||
| Sales and marketing |
1,715 | 2,685 | 2,987 | |||||||||
| Research and development |
538 | 899 | 1,046 | |||||||||
| General and administrative |
1,301 | 2,153 | 2,708 | |||||||||
| Other Expenses |
— | — | 143 | |||||||||
| Total stock-based compensation expense |
$ | 3,843 | $ | 6,184 | $ | 7,422 | ||||||
As of December 31, 2010, there was $3.3 million of unrecognized compensation expense related to non-vested share awards that is expected to be recognized on a straight-line basis over a weighted average period of 1.67 years. Cash received from option exercises was $0.1 million, $0.1 million and $1.6 million during the years ended December 31, 2010, 2009 and 2008, respectively.
Cynosure granted 440,711, 506,225 and 372,090 stock options during the years ended December 31, 2010, 2009 and 2008, respectively. Cynosure uses the Black-Scholes option pricing model to determine the weighted average fair value of options. The weighted average fair value of the options granted during the years ended December 31, 2010, 2009 and 2008 was $5.79, $4.38 and $13.15, respectively, using the following assumptions:
| Years Ended December 31, | ||||||
| 2010 | 2009 | 2008 | ||||
| Risk-free interest rate |
1.49% - 2.50% | 1.87% - 2.66% | 2.19% - 3.30% | |||
| Expected dividend yield |
— | — | — | |||
| Expected term |
5.8 years | 5.8 years | 5.8 years | |||
| Expected volatility |
57% - 59% | 63% - 64% | 64% - 66% | |||
Option-pricing models require the input of various subjective assumptions, including the option’s expected life and the price volatility of the underlying stock. Cynosure’s estimated expected stock price volatility is based on its own historical volatility for the 2010 period and based on a weighted average of its own historical volatility and of the average volatilities of other guideline companies in the same industry for the 2009 and 2008 periods. Cynosure’s expected term of options granted during the years ended December 31, 2010, 2009 and 2008 was derived from the simplified method described in ASC 718-10-S99. The risk-free rate for the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The dividend yield of zero is based on the fact that Cynosure has never paid cash dividends and has no present intention to pay cash dividends.
Cynosure accounts for transactions in which services are received from non-employees in exchange for equity instruments based on the fair value of such services received or of the equity instruments issued, whichever is more reliably measured, in accordance with ASC 718 and the Equity Topic, ASC 505.
F-12
Table of Contents
During the year ended December 31, 2008, Cynosure recorded approximately $143,000 of stock compensation expense for an option grant to a former employee in connection with a legal settlement. This option grant was issued during the fourth quarter of 2008. Cynosure calculated the value of this option grant using the Black-Scholes and using the following assumptions: (1) risk-free interest rate of 3.82%; (2) expected life of 10 years, which is the equivalent of the contractual life of the options; and (3) expected volatility of 63%. This option grant became immediately exercisable upon issuance.
Income Taxes
Cynosure provides for income taxes in accordance with ASC 740, Accounting for Income Taxes (ASC 740). ASC 740 recognizes tax assets and liabilities for the cumulative effect of all temporary differences between the financial statement carrying amounts and the tax basis of assets and liabilities, and are measured using the enacted tax rates that will be in effect when these differences are expected to reverse. Valuation allowances are provided if, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
Cynosure accounts for uncertain tax positions following the provisions of ASC 740. ASC 740 clarifies the accounting for income taxes, by prescribing a minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. ASC 740 also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition.
Net (Loss) Income per Common Share
Basic net (loss) income per share is determined by dividing net (loss) income by the weighted average common shares outstanding during the period. Diluted net (loss) income per share is determined by dividing net (loss) income by the diluted weighted average shares outstanding during the period. Diluted weighted average shares reflect the dilutive effect, if any, of common stock options based on the treasury stock method. Common shares outstanding includes both Class A and Class B as each share participates equally in earnings. Class B shares are convertible at any time into shares of Class A on a one-for-one basis at the option of the holder.
The reconciliation of basic and diluted weighted average shares outstanding for the years ended December 31, 2010, 2009 and 2008 is as follows:
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Net (loss) income |
$ | (5,546 | ) | $ | (22,758 | ) | 10,199 | |||||
| Basic weighted average common shares outstanding |
12,666 | 12,709 | 12,581 | |||||||||
| Weighted average common stock equivalents |
— | — | 225 | |||||||||
| Diluted weighted average common shares outstanding |
12,666 | 12,709 | 12,806 | |||||||||
| Basic net (loss) income per share |
$ | (0.44 | ) | $ | (1.79 | ) | $ | 0.81 | ||||
| Diluted net (loss) income per share |
$ | (0.44 | ) | $ | (1.79 | ) | $ | 0.80 | ||||
For the years ended December 31, 2010 and 2009, the number of basic and diluted weighted average shares outstanding was the same because any increase in the number of shares of common stock equivalents for those periods would be antidilutive based on the net loss for the period. During the years ended December 31, 2010, 2009 and 2008, respectively, outstanding options to purchase 1.6 million, 1.1 million and 1.1 million shares were excluded from the computation of diluted earnings per share because their inclusion would have been antidilutive.
F-13
Table of Contents
Recent Accounting Pronouncements
In December 2010, the Financial Accounting Standards Board (“FASB”) issued ASU 2010-28, “Intangibles—Goodwill and Other (Topic 350) When to Perform Step 2 of the Goodwill Impairment Test for Reporting Units with Zero or Negative Carrying Amounts”. The objective of this ASU is to address diversity in practice in the application of goodwill impairment testing by entities with reporting units with zero or negative carrying amounts, eliminating an entity’s ability to assert that a reporting unit is not required to perform Step 2 because the carrying amount of the reporting unit is zero or negative despite the existence of qualitative factors that indicate the goodwill is more likely than not impaired. This ASU is effective for interim periods after January 1, 2011. Cynosure does not expect the adoption of ASU 2010-28 will have a material impact on its financial position or statements of operations.
In December, 2010, the FASB issued ASU 2010-29, “Business Combinations (Topic 805), Disclosure of Supplementary Pro Forma Information for Business Combinations”. The objective of this ASU is to address diversity in practice about the presentation of pro forma revenue and earnings disclosure requirements for business combinations, and specifies that a public entity that presents comparative financial statements should disclose revenue and earnings of the combined entity as though the business combination(s) that occurred during the current year had occurred as of the beginning of the comparable prior annual reporting period. This ASU is effective prospectively for business combinations on or after January 1, 2011. Cynosure is currently evaluating the impact of adopting ASU 2010-29 on its financial statements.
3. Fair Value
Effective January 1, 2008, Cynosure adopted the Fair Value Measurements Topic ASC 820. ASC 820 establishes a framework for measuring fair value under generally accepted accounting principles and enhances disclosures about fair value measurements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes the following fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value:
| • | Level 1 – Quoted prices in active markets for identical assets or liabilities. |
| • | Level 2 – Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable markets data for substantially the full term of the assets or liabilities. |
| • | Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
At December 31, 2009, Cynosure’s short-term investments consisted of $18.5 million, at par value, of auction rate securities (ARS) investments. The ARS were managed by UBS Financial Services, Inc. (UBS). On November 3, 2008, Cynosure agreed to accept Auction Rate Securities Rights (the Rights) from UBS. On June 30, 2010, Cynosure exercised the Rights and sold its ARS to UBS at full par value. At December 31, 2010, Cynosure held no short-term investments.
F-14
Table of Contents
In accordance with ASC 820, the following table represents Cynosure’s fair value hierarchy for its financial assets (cash equivalents, marketable securities, long-term investments and related financial instruments) measured at fair value as of December 31, 2010 (in thousands):
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Money market funds(1) |
$ | 5,466 | $ | — | $ | — | $ | 5,466 | ||||||||
| State and municipal bonds(2) |
— | 19,716 | — | 19,716 | ||||||||||||
| Treasuries and government agencies(3) |
— | 55,781 | — | 55,781 | ||||||||||||
| Equity securities |
12 | — | — | 12 | ||||||||||||
| Total |
$ | 5,478 | $ | 75,497 | $ | — | $ | 80,975 | ||||||||
| (1) | Included in cash and cash equivalents at December 31, 2010. |
| (2) | $1.1 million included in cash and cash equivalents at December 31, 2010. |
| (3) | $5.0 million included in cash and cash equivalents at December 31, 2010 |
The following table provides a summary of changes in fair value of Cynosure’s Level 3 financial assets for the year ended December 31, 2010 (in thousands):
| Auction Rate Securities |
||||
| Balance at December 31, 2009 |
$ | 18,454 | ||
| Recovery on ARS included in gain on investments |
1,212 | |||
| Loss related to value of ARS Rights included in (loss) on investments |
(1,191 | ) | ||
| Net settlements |
(18,475 | ) | ||
| Balance at December 31, 2010 |
$ | — | ||
4. Short and Long-Term Marketable Securities
Cynosure’s available-for-sale securities at December 31, 2010 consist of approximately $75.5 million of investments in debt securities consisting of state and municipal bonds, treasuries and government agencies and approximately $12,000 in equity securities. All investments in available-for-sale securities are recorded at fair market value, with any unrealized gains and losses reported as a separate component of accumulated other comprehensive loss.
F-15
Table of Contents
As of December 31, 2010, Cynosure’s marketable securities consist of the following (in thousands):
| Market Value | Amortized Cost |
Unrealized Gains |
Unrealized Losses |
|||||||||||||
| Available-for-sale Securities: |
||||||||||||||||
| Cash equivalents: |
||||||||||||||||
| State and municipal bonds |
$ | 1,109 | $ | 1,109 | $ | — | $ | — | ||||||||
| Treasuries and government agencies |
5,008 | 5,011 | — | (3 | ) | |||||||||||
| Total cash equivalents |
$ | 6,117 | $ | 6,120 | $ | — | $ | (3 | ) | |||||||
| Short-term marketable securities: |
||||||||||||||||
| State and municipal bonds |
$ | 13,416 | $ | 13,420 | $ | — | $ | (4 | ) | |||||||
| Treasuries and government agencies |
45,974 | 45,953 | 31 | (10 | ) | |||||||||||
| Equity securities |
12 | 15 | — | (3 | ) | |||||||||||
| Total short-term marketable securities |
$ | 59,402 | $ | 59,388 | $ | 31 | $ | (17 | ) | |||||||
| Long-term marketable securities: |
||||||||||||||||
| State and municipal bonds |
$ | _5,191 | $ | 5,200 | $ | — | $ | (9 | ) | |||||||
| Treasuries and government agencies |
4,799 | 4,798 | 1 | — | ||||||||||||
| Total long-term marketable securities |
$ | 9,990 | $ | 9,998 | $ | 1 | $ | (9 | ) | |||||||
| Total available-for-sale securities |
$ | 75,509 | $ | 75,506 | $ | 32 | $ | (29 | ) | |||||||
| Total marketable securities |
$ | 69,392 | ||||||||||||||
As of December 31, 2009, Cynosure’s marketable securities consisted of the following (in thousands):
| Market Value | Amortized Cost |
Unrealized Gains |
Unrealized Losses |
|||||||||||||
| Available-for-sale Securities: |
||||||||||||||||
| Cash equivalents: |
||||||||||||||||
| State and municipal bonds |
$ | 1,612 | $ | 1,612 | $ | — | $ | — | ||||||||
| Total cash equivalents |
$ | 1,612 | $ | 1,612 | $ | — | $ | — | ||||||||
| Short-term marketable securities: |
||||||||||||||||
| State and municipal bonds |
$ | 11,673 | $ | 11,671 | $ | 2 | $ | — | ||||||||
| Corporate obligations |
1,503 | 1,502 | 1 | — | ||||||||||||
| U.S. government sponsored enterprises |
10,525 | 10,515 | 10 | — | ||||||||||||
| Equity securities |
7 | 8 | — | (1 | ) | |||||||||||
| Total short-term marketable securities |
$ | 23,708 | $ | 23,696 | $ | 13 | $ | (1 | ) | |||||||
| Long-term marketable securities: |
||||||||||||||||
| U.S. government agencies and treasuries |
5,008 | 5,011 | (3 | ) | ||||||||||||
| Total long-term marketable securities |
$ | 5,008 | $ | 5,011 | $ | — | — | |||||||||
| Total available-for-sale securities |
$ | 30,328 | $ | 30,319 | $ | 13 | $ | (4 | ) | |||||||
| Trading Securities: |
||||||||||||||||
| Short-term investments: |
||||||||||||||||
| Auction rate securities |
$ | 16,387 | * | |||||||||||||
| Total marketable securities and short-term investments |
$ | 46,715 | ||||||||||||||
| * | Excludes $2.1 million for fair value of ARS Rights |
F-16
Table of Contents
As of December 31, 2010, Cynosure’s available-for-sale debt securities mature as follows (in thousands):
| Total | Maturities | |||||||||||||||
| Less Than One Year | One to Five Years | More than five years | ||||||||||||||
| State and municipal bonds |
$ | 19,716 | $ | 14,525 | $ | 5,191 | $ | — | ||||||||
| U.S. government sponsored enterprises |
55,781 | 50,982 | 4,799 | — | ||||||||||||
| Total available-for-sale debt securities |
$ | 75,497 | $ | 65,507 | $ | 9,990 | $ | — | ||||||||
5. Acquisitions
On February 3, 2011, Cynosure announced that it acquired substantially all of the assets of Eleme Medical. The business purpose of this transaction was to acquire and incorporate into Cynosure’s product portfolio Eleme’s non-invasive SmoothShapes® XV system for the temporary reduction in the appearance of cellulite. Cynosure paid $2.5 million in cash for the Eleme Medical assets, which include licensing rights to intellectual property related to the SmoothShapes technology. Cynosure is currently evaluating the fair value of assets acquired and plans on filing required pro forma financial information with the SEC within the applicable time period.
The following table presents comparative financial information on an unaudited pro forma basis as if the acquisition of Eleme Medical had taken place at the beginning of the period presented:
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| (In thousands) | ||||||||
| Revenue |
$ | 88,078 | $ | 77,384 | ||||
| Pre-tax loss |
(11,461 | ) | (28,979 | ) | ||||
In December 2008, Cynosure established a wholly-owned subsidiary in Korea, Cynosure, Korea Limited (Cynosure Korea) in order to acquire the aesthetic laser sales division of Orient MG CO, Ltd. (Orient MG), an independent distributor of Cynosure’s products and services in the Korean market. On December 3, 2008, Cynosure Korea acquired the aesthetic laser business and assets of Orient MG through an asset purchase agreement for $1.0 million, which consisted of a cash payment of $464,000 by Cynosure and Cynosure’s forgiveness of $536,000 in accounts payable due to Cynosure and $50,000 of direct legal costs. The business purpose of this acquisition was to gain the ability to sell directly in Korea by acquiring the product registration licenses, customer lists and hire the employees operating this division from Orient MG. The assets acquired from Orient MG over which the purchase price was allocated in accordance with ASC 805, Business Combinations, included $164,000 of intangible assets, related to product registration rights and customer lists, with the remaining purchase price of $886,000 allocated to goodwill. Cynosure assigned an estimated useful life of five years to the product registration licenses, which was based on product lifecycles, and assigned an estimated useful life of two years to the customer lists.
In accordance with ASC 350, Cynosure evaluated the goodwill at the Korea reporting unit for impairment at December 31, 2010. Impairment is a condition that exists when the carrying amount of the goodwill exceeds its implied fair value. In accordance with ASC 350, step one of the evaluation process includes determining the fair value of the reporting unit, and then comparing the fair value of the reporting unit to its carrying value. If the fair value exceeds the carrying value, no impairment exists and no loss is recognized. However, if the carrying value exceeds the fair value, the goodwill of the reporting unit is potentially impaired and the company would move to step two to further evaluate the goodwill.
At December 31, 2010, Cynosure calculated the fair value of the goodwill at the Korea reporting units using a discounted cash flow model resulting in a fair value of the reporting unit in excess of its carrying value. Therefore, Cynosure concluded that no impairment condition existed. Cynosure will continue to monitor the goodwill for impairment annually, unless events occur that would more likely than not reduce the fair value of the reporting unit below its carrying value.
F-17
Table of Contents
6. Segment and Geographic Information
In accordance with ASC 280, Segment Reporting Topic, operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions how to allocate resources and assess performance. Cynosure’s chief decision-maker, as defined under ASC 280, is a combination of the Chief Executive Officer and the Chief Financial Officer. Cynosure views its operations and manages its business as one segment, aesthetic treatment products and services.
The following table represents total revenue by geographic destination:
| Year Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands) | ||||||||||||
| United States |
$ | 29,292 | $ | 30,736 | $ | 85,736 | ||||||
| Europe |
24,201 | 19,554 | 27,716 | |||||||||
| Asia/Pacific |
19,373 | 15,113 | 16,237 | |||||||||
| Other |
8,909 | 7,422 | 9,973 | |||||||||
| $ | 81,775 | $ | 72,825 | $ | 139,662 | |||||||
Total assets by geographic area are as follows:
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| (In thousands) | ||||||||
| United States |
$ | 123,211 | $ | 127,973 | ||||
| Europe |
12,119 | 12,870 | ||||||
| Asia/Pacific |
8,279 | 6,346 | ||||||
| Eliminations |
(1,797 | ) | (1,988 | ) | ||||
| $ | 141,812 | $ | 145,201 | |||||
Long-lived assets by geographic area are as follows:
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| (In thousands) | ||||||||
| United States |
$ | 7,571 | $ | 8,398 | ||||
| Europe |
1,833 | 2,867 | ||||||
| Asia/Pacific |
1,886 | 1,812 | ||||||
| $ | 11,290 | $ | 13,077 | |||||
No individual country within Europe or Asia/Pacific represented greater than 10% of total revenue for any period presented.
Cynosure Korea has long-lived assets of $1.5 million, or 13% of the Company’s total long-lived assets, as of December 31, 2010. These long-lived assets consist primarily of goodwill and intangibles associated with Cynosure’s acquisition of the aesthetic division of Orient MG in December 2008.
F-18
Table of Contents
7. Balance Sheet Accounts
Property and Equipment
Property and equipment consists of the following at December 31:
| Estimated Useful Life (Years) |
2010 Cost |
2009 Cost |
||||||||||
| (In thousands) | ||||||||||||
| Equipment |
3-5 | $ | 3,701 | $ | 3,486 | |||||||
| Furniture and fixtures |
5 | 1,905 | 1,979 | |||||||||
| Computer equipment and software |
3 | 3,273 | 3,137 | |||||||||
| Leased Equipment |
5 | 2,118 | 2,594 | |||||||||
| Leasehold improvements |
5 | 1,476 | 1,463 | |||||||||
| Demonstration equipment |
3 | 18,558 | 16,988 | |||||||||
| Construction in-progress |
14 | 25 | ||||||||||
| 31,045 | 29,672 | |||||||||||
| Less: Accumulated depreciation and amortization |
(22,153 | ) | (19,105 | ) | ||||||||
| $ | 8,892 | $ | 10,567 | |||||||||
Depreciation expense relating to property and equipment was $5.3 million, $5.4 million and $3.6 million for the years ended December 31, 2010, 2009 and 2008, respectively. As of December 31, 2010 and 2009, the cost of assets recorded under capitalized leases was approximately $2.1 million and $2.4 million, respectively, and the related accumulated amortization was approximately $1.9 million and $2.1 million, respectively. Amortization expense of assets recorded under capitalized leases is included as a component of depreciation expense.
Accrued Expenses
Accrued expenses consist of the following at December 31:
| 2010 | 2009 | |||||||
| (In thousands) | ||||||||
| Accrued payroll and taxes |
$ | 1,440 | $ | 501 | ||||
| Accrued employee benefits |
709 | 721 | ||||||
| Accrued warranty costs |
2,112 | 2,440 | ||||||
| Accrued commissions |
1,153 | 1,027 | ||||||
| Accrued other |
5,013 | 4,846 | ||||||
| $ | 10,427 | $ | 9,535 | |||||
8. Related Party Transactions
Purchases of inventory from El.En. S.p.A, (“El.En.”) during the years ended December 31, 2010, 2009 and 2008 were approximately $5.2 million, $4.9 million and $15.8 million, respectively. As of December 31, 2010 and 2009, amounts due to related party for these purchases were approximately $1.8 million and $1.4 million, respectively. There were no amounts due from El.En. as of December 31, 2010 or 2009.
9. Stockholders’ Equity
Common Stock Authorized
Cynosure has a dual class capital structure consisting of $0.001 par value Class A and Class B common stock. Cynosure has authorized 61,500,000 shares of $0.001 par value Class A common stock and 8,500,000
F-19
Table of Contents
shares of $0.001 par value Class B common stock. As of December 31, 2010, there were 9,785,848 shares of Class A common stock issued and 2,975,297 shares of Class B common stock issued. The rights, preferences and privileges of each class of common stock are as follows:
Voting Rights
The holders of Class A common stock and Class B common stock have identical rights and will be entitled to one vote per share with respect to each matter presented to Cynosure stockholders on which the holders of common stock are entitled to vote, except for the approval rights of the holders of the Class B common stock applicable to specified amendments to Cynosure’s certificate of incorporation and amendments of Cynosure’s bylaws by stockholders and except with respect to the election and removal of directors. El.En., Cynosure’s largest stockholder, is able to control the election of a majority of the members of Cynosure’s board of directors. El.En. owns 99.98% of Cynosure’s outstanding Class B common stock, which comprises 23% of Cynosure’s aggregate outstanding common stock. Until El.En. beneficially owns less than 20% of the aggregate number of shares of Cynosure’s Class A common stock and Class B common stock outstanding or less than 50% of the number of shares of Cynosure’s Class B common stock outstanding, El.En., as holder of a majority of the shares of Cynosure’s Class B common stock, will have the right:
| • | to elect a majority of the members of Cynosure’s board of directors; |
| • | to approve amendments to the bylaws adopted by Cynosure’s Class A and Class B stockholders, voting as a single class; and |
| • | to approve amendments to any provisions of Cynosure’s restated certificate of incorporation relating to the rights of holders of common stock, the powers, election and classification of the board of directors, corporate opportunities and the rights of holders of Class A common stock and Class B common stock to elect and remove directors, act by written consent and call special meetings of stockholders. |
In addition, the holders of shares of Cynosure’s Class B common stock will vote with Cynosure’s Class A stockholders for the election of the remaining directors.
Conversion
Cynosure’s Class A common stock is not convertible into any other shares of Cynosure’s capital stock.
Each share of Class B common stock is convertible into one share of class A common stock at any time at the option of the holder. In addition, each share of Class B common stock shall convert automatically into one share of Class A common stock upon any transfer of such share of Class B common stock, whether or not for value.
Dividends
Subject to preferences that may apply to any shares of preferred stock outstanding at the time, the holders of Class A common stock and Class B common stock shall be entitled to share equally, on a per share basis, in any dividends that Cynosure’s board of directors may determine to issue from time to time.
Liquidation Rights
In the event of Cynosure’s liquidation or dissolution, the holders of Class A common stock and Class B common stock shall be entitled to share equally, on a per share basis, in all assets remaining after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock.
F-20
Table of Contents
Preferred Stock
Cynosure has authorized 5,000,000 shares of $0.001 par value preferred stock. The Board of Directors has full authority to issue this stock and to fix the voting powers, preference rights, qualifications, limitations, or restrictions thereof, including dividend rights, conversion rights, redemption privileges and liquidation preferences and the number of shares constituting any series or designation of such series.
Treasury Stock
On July 28, 2009, Cynosure’s Board of Directors authorized the repurchase of up to $10 million of its Class A common stock, from time to time, on the open market or in privately negotiated transactions under a stock repurchase program. The program will terminate upon the purchase of $10 million in common stock, unless Cynosure’s Board of Directors discontinues it sooner. During the year ended December 31, 2010, Cynosure repurchased 145,621 shares of its common stock at an aggregate cost of approximately $1.4 million and at a weighted average price of $9.54 per share under this program. As of December 31, 2010, Cynosure has repurchased an aggregate of 148,935 shares under this program at an aggregate cost of $1.4 million.
10. Stock-Based Compensation
2004 Stock Option Plan
In October 2004, the Board of Directors adopted and the stockholders approved the 2004 Stock Option Plan (the 2004 Plan). The 2004 Plan provided for the grant of ISOs, as well as nonstatutory options. The Board of Directors administers the 2004 Plan and had sole discretion to grant options to purchase shares of Cynosure’s common stock.
The Board of Directors determines the term of each option, option price, number of shares for which each option is granted, whether restrictions would be imposed on the shares subject to options and the rate at which each option is exercisable. The exercise price for options granted is determined by the Board of Directors, except that for ISOs, the exercise price could not be less than the fair market value per share of the underlying common stock on the date granted (110% of fair market value for ISOs granted to holders of more than 10% of the voting stock of Cynosure). The term of the options is set forth in the applicable option agreement, except that in the case of ISOs, the option term cannot exceed ten years. Options granted under the Plan vested either (i) over a 48-month period at the rate of 25% after the first year and 6.25% each quarter thereafter until fully vested or (ii) over a vesting period determined by the Board of Directors. As of December 31, 2010, there were no shares available for future grant under the 2004 Plan.
2005 Stock Incentive Plan
In August 2005, the Board of Directors adopted the 2005 Stock Incentive Plan (the 2005 Plan), which was approved by Cynosure’s stockholders in December 2005. The 2005 Plan provided for the grant of ISOs, as well as nonstatutory options. The Board of Directors administers the 2005 Plan and had sole discretion to grant options to purchase shares of Cynosure’s common stock.
The Board of Directors determines the term of each option, option price, number of shares for which each option is granted, whether restrictions would be imposed on the shares subject to options and the rate at which each option is exercisable. The exercise price for options granted is determined by the Board of Directors, except that for ISOs, the exercise price could not be less than the fair market value per share of the underlying common stock on the date granted (110% of fair market value for ISOs granted to holders of more than 10% of the voting stock of Cynosure). The term of the options is set forth in the applicable option agreement, except that in the case of ISOs, the option term cannot exceed ten years. At December 31, 2010 the number of shares of Class A common stock reserved for issuance under the 2005 Plan is 2,188,369 shares. Options granted under the Plan vest either (i) over a 36-month period at the rate of 8.33% after each quarter thereafter until fully vested or
F-21
Table of Contents
(ii) over a vesting period determined by the Board of Directors. As of December 31, 2010, there are 92,274 shares available for future grant under the 2005 Plan. In January 2011, the number of shares of Class A common stock reserved for issuance under the 2005 Plan increased by 300,000 shares in accordance with the terms of the plan.
Stock option activity under the 1992 Plan, the 2004 Plan and the 2005 Plan is as follows:
| Number of Options |
Exercise Price Range |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Life |
Aggregate Intrinsic Value (in thousands) |
||||||||||||||||
| Vested |
1,139,742 | $ 3.00 - $39.39 | $ | 18.13 | $ | 2,050 | ||||||||||||||
| Unvested |
585,985 | 3.00 - 39.39 | 13.57 | 1,551 | ||||||||||||||||
| Outstanding, December 31, 2009 |
1,725,727 | 3.00 - 39.39 | 16.58 | 7.54 years | 3,601 | |||||||||||||||
| Granted |
440,711 | 9.97 - 13.60 | 10.47 | 29 | ||||||||||||||||
| Exercised |
(10,615 | ) | 3.00 - 12.21 | 7.88 | 37 | |||||||||||||||
| Forfeited |
(58,073 | ) | 4.50 - 39.39 | 22.61 | 21 | |||||||||||||||
| Outstanding, December 31, 2010 |
2,097,750 | $ 3.00 - $36.94 | $ | 15.18 | 7.03 years | $ | 2,691 | |||||||||||||
| Vested |
1,523,495 | 3.00 - 36.94 | $ | 17.09 | 6.39 years | $ | 2,117 | |||||||||||||
| Unvested |
574,255 | 5.07 - 25.48 | 10.09 | 8.74 years | 574 | |||||||||||||||
| Vested or expected to vest, December 31, 2010 |
2,073,740 | $ 3.00 - $36.94 | $ | 15.21 | 7.02 years | $ | 2,663 | |||||||||||||
| Exercisable, December 31, 2010 |
1,523,495 | $ 3.00 - $36.94 | $ | 17.09 | 6.39 years | $ | 2,117 | |||||||||||||
11. Income Taxes
(Loss) income before income tax provision consists of the following:
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands) | ||||||||||||
| Domestic |
$ | (7,455 | ) | $ | (18,446 | ) | $ | 11,735 | ||||
| Foreign |
2,351 | (653 | ) | 3,235 | ||||||||
| Total |
$ | (5,104 | ) | $ | (19,099 | ) | $ | 14,970 | ||||
The provision for income taxes consists of:
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands) | ||||||||||||
| Current: |
||||||||||||
| Federal |
$ | (368 | ) | $ | (3,422 | ) | $ | 5,691 | ||||
| State |
46 | 122 | 754 | |||||||||
| Foreign |
985 | 184 | 1,145 | |||||||||
| Total current |
663 | (3,116 | ) | 7,590 | ||||||||
| Deferred: |
||||||||||||
| Federal |
— | 6,187 | (2,360 | ) | ||||||||
| State |
— | 512 | (245 | ) | ||||||||
| Foreign |
(221 | ) | 76 | (214 | ) | |||||||
| Total deferred |
(221 | ) | 6,775 | (2,819 | ) | |||||||
| $ | 442 | $ | 3,659 | $ | 4,771 | |||||||
F-22
Table of Contents
A reconciliation of the federal statutory rate to Cynosure’s effective tax rate is as follows for the years ended December 31:
| 2010 | 2009 | 2008 | ||||||||||
| Income tax provision at federal statutory rate: |
35.0 | % | 35.0 | % | 35.0 | % | ||||||
| (Decrease) increase in tax resulting from - |
||||||||||||
| State taxes, net of federal benefit |
0.8 | 3.7 | 1.8 | |||||||||
| Nondeductible expenses |
(3.5 | ) | (1.4 | ) | 1.9 | |||||||
| Tax-exempt interest income |
0.7 | 0.6 | (4.5 | ) | ||||||||
| Effect of foreign taxes |
6.9 | 0.8 | (1.3 | ) | ||||||||
| Stock-based compensation |
(2.3 | ) | (1.3 | ) | 1.9 | |||||||
| Research and development credit |
4.3 | 1.1 | (3.0 | ) | ||||||||
| Change in valuation allowance |
(54.3 | ) | (56.6 | ) | — | |||||||
| Other |
3.7 | (1.1 | ) | 0.1 | ||||||||
| Effective income tax rate |
(8.7 | )% | (19.2 | )% | 31.9 | % | ||||||
Significant components of Cynosure’s net deferred tax assets as of December 31, 2010 and 2009 are as follows:
| 2010 | 2009 | |||||||
| (In thousands) | ||||||||
| Deferred tax assets: |
||||||||
| Foreign net operating loss carryforwards and temporary differences |
$ | 1,319 | $ | 1,210 | ||||
| Domestic net operating loss and credit carryforwards |
3,400 | 1,247 | ||||||
| Reserves and allowances |
2,126 | 2,943 | ||||||
| Depreciation |
602 | 498 | ||||||
| Stock-based compensation |
6,132 | 5,072 | ||||||
| Other temporary differences |
741 | 660 | ||||||
| Gross deferred tax assets |
14,320 | 11,630 | ||||||
| Valuation allowance for deferred tax assets |
(13,708 | ) | (11,210 | ) | ||||
| Net deferred tax assets |
$ | 612 | $ | 420 | ||||
During the fourth quarter of 2009, Cynosure determined that its net domestic deferred tax assets were no longer more-likely-than-not realizable. As a result, Cynosure recorded a deferred tax provision of $10.4 million to establish a full valuation allowance on its net domestic deferred tax assets. At December 31, 2010, Cynosure maintains a full valuation allowance against its net domestic deferred tax assets. Cynosure’s domestic tax group is in a cumulative three-year domestic pre-tax book loss. The utilization of Cynosure’s net domestic deferred tax assets is dependent on future earnings, which cannot be projected with certainty at this time. As of December 31, 2010, Cynosure has recorded a receivable of $0.5 million related to the benefit of the current year federal tax net operating loss that will be carried back to prior years to recover previously paid income taxes. At December 31, 2010, Cynosure has no additional carryback capacity for future U.S. tax losses. In addition, at December 31, 2010, Cynosure has domestic federal net operating loss carryforwards of approximately $3.5 million, state net operating loss carryforwards of $8.0 million and federal tax credit carryforwards of $1.6 million that are available to reduce future taxable income. Cynosure’s U.S. federal net operating loss carryforwards and credits will begin to expire in 2030.
At December 31, 2010, Cynosure has foreign net operating losses of approximately $2.9 million in various foreign jurisdictions that are available to reduce future foreign income. Foreign net operating losses in Germany and France do not expire. Other foreign net operating loss carryforwards begin to expire in 2016. For the year
F-23
Table of Contents
ended December 31, 2010, Cynosure recorded a deferred tax benefit of $0.2 million related to its foreign deferred tax assets. As of December 31, 2010, Cynosure continues to maintain a full valuation allowance on all of its net foreign deferred tax assets in Germany, Japan and Mexico due to the uncertain realization of the deferred tax assets in these jurisdictions.
Income taxes have not been provided on certain undistributed earnings of foreign subsidiaries of approximately $10.1 million, because such earnings are considered to be indefinitely reinvested in the business. Cynosure does not believe it is practical to estimate the amount of income taxes payable on the earnings that are indefinitely reinvested in foreign operations.
In July 2006, the guidance within ASC 740 related to accounting for income taxes was issued which clarified a company’s accounting for uncertain income tax positions that are recognized in financial statements. Cynosure adopted the accounting pronouncements for uncertain tax positions as of January 1, 2007. ASC 740 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements by prescribing a minimum recognition threshold and measurement of a tax position taken or expected to be taken in a tax return. At December 31, 2010 and 2009, Cynosure had no material unrecognized tax benefits.
Cynosure files income tax returns in the U.S. federal jurisdiction, and in various state and foreign jurisdictions. With few exceptions, Cynosure is no longer subject to U.S. federal, state and local income tax examinations by tax authorities for years before 2007. Additionally, certain non-U.S. jurisdictions are no longer subject for income tax examinations by tax authorities for years before 2006.
Cynosure classifies interest and penalties related to income taxes as a component of its provision for income taxes, and the amount of interest and penalties recorded as of December 31, 2010 and 2009 in the statements of operations and balance sheet was immaterial.
No significant changes or material settlements for uncertain tax positions are expected in the next reporting year.
12. 401(k) Plan
Cynosure sponsors the Cynosure 401(k) defined contribution plan. Participation in the plan is available to all employees of Cynosure who meet certain eligibility requirements. The Plan is qualified under Section 401(k) of the Internal Revenue Code, and is subject to contribution limitations as set annually by the Internal Revenue Service. Employer matching contributions are at Cynosure’s discretion. Cynosure’s contributions to this plan totaled approximately $265,000, $286,000 and $366,000 for the years ended December 31, 2010, 2009 and 2008, respectively.
13. Commitments and Contingencies
Lease Commitments
Cynosure leases its U.S. operating facility and certain foreign facilities under noncancelable operating lease agreements expiring through December 2012. These leases are non-cancellable and typically contain renewal options. Certain leases contain rent escalation clauses for which Cynosure recognizes the expense on a straight-line basis. Rent expense for the years ended December 31, 2010, 2009 and 2008 was approximately $1.7 million, $1.8 million and $1.7 million, respectively.
F-24
Table of Contents
Cynosure leases certain equipment and vehicles under operating and capital lease agreements with payments due through June 2014. Commitments under Cynosure’s lease arrangements are as follows, in thousands:
| Operating Leases |
Capital Leases |
|||||||
| 2011 |
$ | 1,492 | $ | 143 | ||||
| 2012 |
1,454 | 32 | ||||||
| 2013 |
86 | 6 | ||||||
| 2014 |
— | 2 | ||||||
| 2015 and thereafter |
— | — | ||||||
| Total minimum lease payments |
$ | 3,032 | $ | 183 | ||||
| Less amount representing interest |
(10 | ) | ||||||
| Present value of obligations under capital leases |
$ | 173 | ||||||
| Current portion of capital lease obligations |
133 | |||||||
| Capital lease obligations, net of current portion |
$ | 40 | ||||||
Lease Guarantees
Cynosure has guarantees for lease obligations for two locations that are operated by Sona MedSpa, and will be obligated to pay approximately $18,000 for these leases during 2011 if Sona MedSpa cannot make the required lease payments.
Litigation
In 2005, Dr. Ari Weitzner, individually and as putative representative of a purported class, filed a complaint against Cynosure under the federal Telephone Consumer Protection Act (TCPA) in Massachusetts Superior Court in Middlesex County seeking monetary damages, injunctive relief, costs and attorneys fees. The complaint alleges that Cynosure violated the TCPA by sending unsolicited advertisements by facsimile to the plaintiff and other recipients without the prior express invitation or permission of the recipients. Under the TCPA, recipients of unsolicited facsimile advertisements are entitled to damages of up to $500 per facsimile for inadvertent violations and up to $1,500 per facsimile for knowing or willful violations. Based on discovery in this matter, the plaintiff alleges that approximately three million facsimiles were sent on Cynosure’s behalf by a third party to approximately 100,000 individuals. In February 2008, several months after the close of discovery, the plaintiff served a motion for class certification, which Cynosure opposed on numerous factual and legal grounds, including that a nationwide class action may not be maintained in a Massachusetts state court by Dr. Weitzner, a New York resident; individual issues predominate over common issues; a class action is not superior to other methods of resolving TCPA claims; and Dr. Weitzner is an inadequate class representative. Cynosure also believes it has many merits defenses, including that the faxes in question do not constitute “advertising” within the meaning of the TCPA and many recipients had an established business relationship with Cynosure and are thereby deemed to have consented to the receipt of facsimile communications. The Court held a hearing on the plaintiff’s class certification motion in June 2008, but no decision on the motion was rendered. In July 2010, the Court issued an order dismissing this matter without prejudice for Dr. Weitzner’s failure to prosecute the case. In August 2010, Dr. Weitzner filed a motion for relief from the dismissal order, which the Court allowed. At a status conference held in November 2010, the Court confirmed that the class certification motion was still under advisement. Cynosure is not currently able to estimate the amount of range of loss that could result from an unfavorable outcome of this lawsuit.
In July 2008, Cynosure commenced a declaratory judgment action in the U.S. District Court for the District of Massachusetts requesting a declaration that Dr. Weitzner’s and the putative class claims are covered under the Company’s general liability insurance policies. In August 2008, Cynosure’s insurance company filed an Answer
F-25
Table of Contents
and Counterclaim against Cynosure seeking a declaration that the Company’s policy does not provide coverage for Dr. Weitzner’s claims. In August 2008, Cynosure filed a reply to the Counterclaim. The insurance company filed a Motion for Summary Judgment in December 2008, and Cynosure cross moved for Summary Judgment in January 2009. The court held a hearing on the motions in February 2009, and in April 2009 rendered a decision that Cynosure’s liability insurer is obligated to provide Cynosure with a defense to the Weitzner action and, if necessary, indemnify Cynosure for the putative class claims. Thereafter, Cynosure’s liability insurer filed a motion for reconsideration, which Cynosure opposed. The court denied the insurer’s motion in May 2009. In January 2010, the court entered an Order for Judgment consistent with its April 2009 decision that the insurer is obligated to defend Cynosure against the putative class claims and to indemnify Cynosure for any single damages, attorneys’ fees or costs. Per agreement of the parties, Cynosure was awarded $0.4 million in fees and costs for the period through July 1, 2009. The insurer filed a Notice of Appeal of the judgment in January 2010. The matter has been fully briefed and the U.S. First Circuit Court of Appeals heard oral arguments in January 2011.
In addition to the matters discussed above, from time to time, Cynosure is subject to various claims, lawsuits, disputes with third parties, investigations and pending actions involving various allegations against Cynosure incident to the operation of its business, principally product liability. Each of these other matters is subject to various uncertainties, and it is possible that some of these other matters may be resolved unfavorably to Cynosure. Cynosure establishes accruals for losses that management deems to be probable and subject to reasonable estimate. Cynosure believes that the ultimate outcome of these matters will not have a material adverse impact on its consolidated financial position, results of operations or cash flows.
14. Subsequent Events
On February 3, 2011, Cynosure announced that it acquired substantially all of the assets of Eleme Medical. Cynosure paid $2.5 million in cash for the Eleme Medical assets, which include licensing rights to intellectual property related to the SmoothShapes technology. See footnote 5 for further information.
15. Summary Selected Quarterly Financial Data (Unaudited)
During the fourth quarter of 2009, Cynosure recorded a $2.1 million charge to cost of product revenues related to the write-down of an earlier generation product. The write-down resulted in part from customers adopting Cynosure’s newer generation products more quickly than the Company anticipated, coupled with the downturn in the overall aesthetic laser market.
During the fourth quarter of 2009, Cynosure determined that its net domestic deferred tax assets were no longer more-likely-than-not realizable. As a result, Cynosure recorded a deferred tax provision of $10.4 million to establish a full valuation allowance on certain net domestic deferred tax assets.
F-26
Table of Contents
The following table sets forth certain unaudited consolidated quarterly statement of operations data for the eight quarters ended December 31, 2010. This information is unaudited, but in the opinion of management, it has been prepared on the same basis as the audited consolidated financial statements and all necessary adjustments, consisting only of normal recurring adjustments, have been included in the amounts stated below to state fairly the unaudited consolidated quarterly results of operations. The results of operations for any quarter are not necessarily indicative of the results of operations for any future period.
| Quarter Ended | ||||||||||||||||
| March 31, 2010 |
June 30, 2010 |
Sept. 30, 2010 |
Dec. 31, 20010 |
|||||||||||||
| (In thousands, except per share data) | ||||||||||||||||
| Revenues |
$ | 18,893 | $ | 21,489 | $ | 19,051 | $ | 22,342 | ||||||||
| Gross profit |
$ | 10,800 | $ | 12,420 | $ | 10,684 | $ | 12,483 | ||||||||
| Loss from operations |
$ | (2,542 | ) | $ | (903 | ) | $ | (972 | ) | $ | (626 | ) | ||||
| Net loss |
$ | (2,812 | ) | $ | (1,477 | ) | $ | (460 | ) | $ | (797 | ) | ||||
| Basic net loss per share |
$ | (0.22 | ) | $ | (0.12 | ) | $ | (0.04 | ) | $ | (0.06 | ) | ||||
| Diluted net loss per share |
$ | (0.22 | ) | $ | (0.12 | ) | $ | (0.04 | ) | $ | (0.06 | ) | ||||
| Quarter Ended | ||||||||||||||||
| March 31, 2009 |
June 30, 2009 |
Sept. 30, 2009 |
Dec. 31, 2009 |
|||||||||||||
| (In thousands, except per share data) | ||||||||||||||||
| Revenues |
$ | 14,816 | $ | 20,813 | $ | 17,937 | $ | 19,259 | ||||||||
| Gross profit |
$ | 9,016 | $ | 12,076 | $ | 10,477 | $ | 8,448 | ||||||||
| Income (loss) from operations |
$ | (7,135 | ) | $ | (4,092 | ) | $ | (3,341 | ) | $ | (5,748 | ) | ||||
| Net income (loss) |
$ | (4,021 | ) | $ | (2,324 | ) | $ | (1,915 | ) | $ | (14,498 | ) | ||||
| Basic net income (loss) per share |
$ | (0.32 | ) | $ | (0.18 | ) | $ | (0.15 | ) | $ | (1.14 | ) | ||||
| Diluted net income (loss) per share |
$ | (0.32 | ) | $ | (0.18 | ) | $ | (0.15 | ) | $ | (1.14 | ) | ||||
F-27
Table of Contents
EXHIBIT INDEX
| Exhibit Number |
Description | |
| 3.1 | Restated Certificate of Incorporation of the Company (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 3.4 | Amended and Restated Bylaws of the Company (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 4.1 | Specimen certificate evidencing shares of Class A common stock (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.1* | 1992 Stock Option Plan (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.2* | 2004 Stock Option Plan, as amended (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.3* | 2005 Stock Incentive Plan (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.4* | Employment Agreement, dated December 15, 2008, between the Company and Michael Davin (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed December 19, 2008) | |
| 10.5* | First Amendment to Employment Agreement, dated December 20, 2010, between the Company and Michael Davin (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed December 21, 2010) | |
| 10.6* | Employment Agreement, dated January 1, 2003, between the Company and George Cho (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.7* | Employment Agreement, dated December 15, 2008, between the Company and Douglas Delaney (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed December 19, 2008) | |
| 10.8† | Distribution Agreement, effective as of January 1, 2005, between the Company and El.En. S.p.A. (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.9† | Distribution Agreement, effective as of January 1, 2005, between the Company and El.En. S.p.A. (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.10 | Promissory Note, dated October 1, 2004, between the Company and El.En. S.p.A. (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.11 | Lease, dated January 31, 2005, between Glenborough Fund V, Limited Partnership and the Company, as amended (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.12 | Reimbursement Agreement among the Company, El.En. S.p.A. and BRCT, Inc. (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.13* | Option Agreement, dated December 17, 2003, between El.En. and Michael Davin (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
| 10.14* | Option Agreement, dated May 13, 2005, between El.En. and Michael Davin (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 (333-127463)) | |
Table of Contents
| Exhibit Number |
Description | |
| 10.15 | Non-Exclusive Patent License, dated November 6, 2006, between Palomar Medical Technologies, Inc. and the Company (Incorporated by reference to the exhibit to the Company’s Current Report on Form 8-K filed November 7, 2006) | |
| 10.16* | Employment Agreement, dated December 15, 2008, between the Company and Timothy W. Baker (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed December 19, 2008) | |
| 21.1 | Subsidiaries of the Company | |
| 23.1 | Consent of Ernst & Young LLP | |
| 31.1 | Certification of the Principal Executive Officer | |
| 31.2 | Certification of the Principal Financial Officer | |
| 32.1 | Certification of the Chief Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2 | Certification of the Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| * | Management contract or compensation plan or arrangement required to be filed as an exhibit pursuant to Item 15(b) of Form 10-K. |
| † | Confidential treatment granted as to certain portions, which portions have been omitted and filed separately with the Securities and Exchange Commission. |
