Attached files
| file | filename |
|---|---|
| EX-31.2 - EX-31.2 - Astex Pharmaceuticals, Inc | a2202506zex-31_2.htm |
| EX-23.1 - EX-23.1 - Astex Pharmaceuticals, Inc | a2202506zex-23_1.htm |
| EX-31.1 - EX-31.1 - Astex Pharmaceuticals, Inc | a2202506zex-31_1.htm |
| EX-32.1 - EX-32.1 - Astex Pharmaceuticals, Inc | a2202506zex-32_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the Fiscal Year Ended December 31, 2010 |
||
OR |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to |
||
Commission file number 0-27628
SUPERGEN, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
91-1841574 (IRS Employer Identification Number) |
|
4140 Dublin Blvd., Suite 200, Dublin, CA (Address of principal executive offices) |
94568 (Zip Code) |
|
Registrant's telephone number, including area code: (925) 560-0100 |
||
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class: | Name of each exchange on which registered: | |
|---|---|---|
| Common Stock, $0.001 par value per share | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer ý | Non-accelerated filer o (do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý.
The aggregate market value of the voting stock held by non-affiliates of the Registrant (based on the closing sale price of the Common Stock as reported on the Nasdaq Stock Market on June 30, 2010, the last business day of the Registrant's most recently completed second fiscal quarter) was approximately $120,551,325. This determination of affiliate status is not necessarily a conclusive determination for other purposes. The number of outstanding shares of the Registrant's Common Stock as of the close of business on March 1, 2011 was 60,367,997.
DOCUMENTS INCORPORATED BY REFERENCE
Items 10, 11, 12, 13 and 14 of Part III incorporate by reference information from the definitive proxy statement for the Registrant's 2011 Annual Meeting of Stockholders. The proxy statement will be filed within 120 days of the Registrant's fiscal year ended December 31, 2010.
SUPERGEN, INC.
2010 ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
Special Note Regarding Forward-Looking Statements
Our disclosure and analysis in this report contain forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act, and within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements provide our current expectations or forecasts of future events. When we use the words "anticipate," "estimate," "project," "intend," "expect," "plan," "believe," "should," "likely" and similar expressions, we are making forward-looking statements. In particular, these statements include statements such as: our estimates about profitability; our forecasts regarding our revenues and research and development expenses; and our statements regarding the sufficiency of our cash to meet our operating needs. Our actual results could differ materially from those predicted in the forward-looking statements as a result of risks and uncertainties including, but not limited to, delays and risks associated with conducting and managing our clinical trials; the commercial success of Dacogen; developing products and obtaining regulatory approval; our ability to establish and maintain collaboration relationships; competition; our ability to protect our intellectual property; our expectations about the joint development program with GSK; our dependence on third party suppliers; risks associated with the hiring and loss of key personnel; adverse changes in the specific markets for our products; and our ability to launch and commercialize our products. Certain unknown or immaterial risks and uncertainties can also affect our forward-looking statements. Consequently, no forward-looking statement can be guaranteed and you should not rely on these forward-looking statements.
The forward-looking statements reflect our position as of the date of this report, and we undertake no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. You are advised, however, to consult any further disclosures we make on related subjects in our Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, or other filings. Also note that we provide a cautionary discussion of risks and uncertainties relevant to our business under Item 1A—Risk Factors in this report. These are currently known and material risks that we believe could cause our actual results to differ materially from expected and historical results. Other unknown and immaterial risks besides those listed in this report could also adversely affect us.
We incorporated in March 1991 as a California corporation and changed our state of incorporation to Delaware in May 1997. Our executive offices are located at 4140 Dublin Blvd., Suite 200, Dublin, CA, 94568 and our telephone number at that address is (925) 560-0100. We maintain a website on the internet at www.supergen.com. This is a textual reference only. We do not incorporate the information on our website into this annual report on Form 10-K, and you should not consider any information on, or that can be accessed through, our website as part of this annual report on Form 10-K.
Overview
We are a pharmaceutical company dedicated primarily to the discovery and development of novel cancer therapeutics in epigenetic and cell signaling modulation. We develop products through biochemical and clinical proof of concept to partner for further development and commercialization. We have Tyrosine Kinase and DNA methyltransferase inhibitors in pre-clinical and clinical development.
Our primary developmental efforts revolve around the products progressing out of our small-molecule drug discovery programs. We commenced Phase I clinical trials for amuvatinib (MP-470), our multi-targeted kinase inhibitor and DNA repair suppressor in June 2007, and we are anticipating the commencement of a Phase II trial in small cell lung cancer with this product in the first half of 2011. In early 2009, we initiated clinical trials for a second internally developed product, SGI-1776, a PIM kinase inhibitor. This clinical program was terminated in 2010 due to specific cardiac toxicity. We
1
intend to continue the larger discovery effort targeted at PIM kinases with alternative product candidates. In 2010, SGI-110, our small molecule DNA hypomethylating agent, received clearance from the United States Food and Drug Administration ("FDA") to advance into Phase I trials. We announced the dosing of the first patients in the Phase I trial in January 2011.
We currently receive royalty revenues relating to sales of Dacogen® (decitabine) for Injection, a product approved by the FDA for treatment of patients with myelodysplastic syndromes ("MDS"), which we licensed to MGI PHARMA Inc. ("MGI") in 2004.
In October 2009, we entered into a multi-year collaboration agreement with GlaxoSmithKline ("GSK") to discover and develop cancer therapeutics based on epigenetic targets. Pursuant to the agreement, GSK may exercise an option to license from us the compounds that are the result of the research effort. Upon execution of the agreement, we received an upfront payment of $2 million from GSK, as well as a $3 million investment in shares of our common stock, sold at a 10% premium to market price. Total potential development and commercialization milestones payable to us could exceed $375 million, and we may also receive tiered royalties into double digit magnitudes, payable on net sales of any resulting products.
Strategy
Our founding strategy was to in-license late-stage clinical products and commercialize these products by executing selective developmental and commercialization strategies that might allow these products to come into the market and be utilized by the widest possible patient populations. However, the competition for late-stage compounds that can be obtained through licensure or acquisition, that have shown initial efficacy in humans, has increased significantly with most major pharmaceutical companies taking positions in this market. Our current strategy mitigates the competitive risk of in-licensure and positions us to out-license selective products to our licensing competitors or other pharmaceutical companies. Our primary objective is to become a leading developer and seller or licensor of therapies for patients suffering from cancer. Key elements of our strategy include the following:
Discover and advance into clinical trials at least one product about every twelve to eighteen months. Our drug discovery group has been optimizing our proprietary process called CLIMB® that allows a small team of chemists and biologists to model difficult or previously unknown cancer targets for computerized drug creation and development. The flexibility and relative efficiency of CLIMB is a strategic advantage for SuperGen. Our drug discovery capabilities allow us to control access to innovative new chemical entities which we believe are important to the creation of value over the long run.
Focus on oncology molecular targets that are not readily tractable by traditional drug discovery methods. Most established pharmaceutical companies use some version of high throughput screening for potential drug candidates. This methodology does not work well for many complex molecular targets. CLIMB enables us to create an advantage by designing inhibitors of difficult oncology targets that are not tractable by standard drug discovery methods.
Focus discovery research on three areas. Our discovery research is currently focusing on three distinct areas. We believe that innovations in the discovery of epigenetic therapeutics as well as cancer metabolism are important. First, we have a particular strategic interest in the field of epigenetics which started with the development of Dacogen and SGI-110. Our discovery efforts are aimed at leveraging this institutional knowledge in this important area of research. Second, we are engaging in discovery research in the area of cancer metabolism. We believe that the emerging science in this area will lead to significant innovations in cancer treatment. Third, we are focused on the discovery of novel inhibitors of signal transduction.
2
Capitalize on our existing drug development expertise to maximize the commercial value of our products. Computer and animal models are only modestly predictive of how effective a product may be in humans. We have developed significant expertise in planning and managing clinical trials as well as regulatory filings in both the United States and Europe. Proving the concept that a specific drug will translate into an approvable, commercially viable product in humans is a difficult task. Some drug candidates demonstrate this "proof of concept" very early in non-clinical development, while other drug candidates will need to be compared clinically to existing therapies to achieve such a proof of concept. Typically, this proof of concept comes in Phase II trials where it is demonstrated that drug treatment leads to a desired pharmacologic effect and a safe dose. As product candidates move from non-clinical into Phase I and Phase II clinical studies, their potential value increases once proof of concept is established. We believe our clinical and regulatory expertise facilitates efficient use of our resources to achieve appropriate proof of concept.
CLIMB Discovery Process
Traditional drug discovery processes may require five or more years before presenting a candidate suitable for clinical development. This lengthy timeline is a major contributor to the ineffectiveness of pharmaceutical research and development. Utilizing our CLIMB platform, we are streamlining the discovery process in order to get potentially life-saving therapeutics to the clinical testing stage of development more effectively. CLIMB is SuperGen's approach to small molecule drug discovery, which integrates the rapid screening of compound libraries with computational chemistry and systems biology to design novel inhibitors of important pathways involved in cancer.
We have continued to improve our CLIMB process to increase its effectiveness by augmenting our computational analysis capabilities as well as other methods. We believe that it is important to continue to invest in drug discovery innovations over the long run. In traditional small molecule screening, very large physical libraries of millions of compounds may be created and screened in order to identify the few that interact selectively with a disease-related protein target. This approach has worked fairly well for simple or very well-characterized molecular targets; however, traditional drug discovery processes are long and resource intensive. In comparison, CLIMB incorporates proprietary algorithms to evaluate virtual libraries of compounds and compound fragments to screen against target models, and is less resource intensive and less time consuming than traditional approaches.
CLIMB has been used to create models and identify compounds with considerable potency while physically screening as few as several hundred rationally-selected compounds. This may reduce the duration from target selection to clinical candidate by several years, and may decrease the cost of drug development. As part of CLIMB, our software development team is actively involved in the creation of proprietary algorithms to integrate computation, biochemistry, medicinal chemistry and systems biology to improve the predictive properties of our models and streamline the drug discovery process even further.
3
Products in Research and Development
The chart below lists our current products or projects in development:
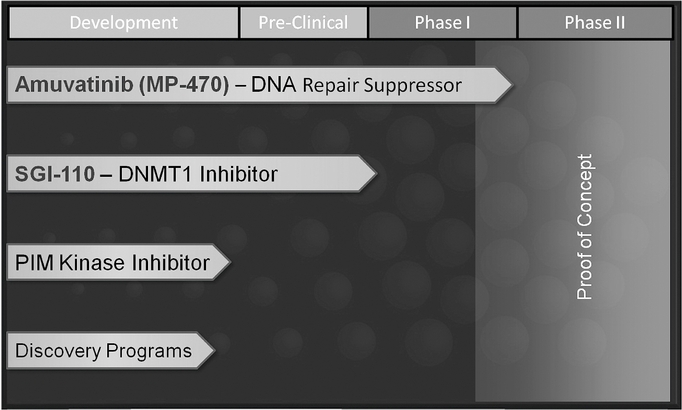
Amuvatinib—Multi-targeted Kinase Inhibitor and DNA Repair Suppressor
Amuvatinib is an oral multi-targeted Tyrosine Kinase Inhibitor that is particularly specific for mutant forms of protein kinase targets called c-kit and PDGFRa. These protein kinase targets are involved in the growth and proliferation of cancer cells. Amuvatinib is also a suppressor of Rad51, a DNA repair protein which is involved in resistance to a variety of chemotherapy agents and radiation. We submitted an Investigational New Drug Application ("IND") to the FDA in March 2007, and initiated a first-in-human Phase I single agent amuvatinib trial in June 2007, and Phase Ib trials in late 2007. Non-clinical experimentation has shown that amuvatinib in combination with etoposide has a synergistic effect on human small cell lung cancer cell lines in vitro. In addition, better tumor growth inhibition has been observed in animal studies in the group treated with a combination of etoposide and amuvatinib when compared to etoposide as a single agent in tumor-bearing nude mice.
Amuvatinib has a wide therapeutic window and shows minimal toxicity in the expected therapeutic dose range, despite suppressing several signaling pathways within cells. We have evaluated amuvatinib as a dry powder mix and as a lipid suspension formulation in multiple Phase I studies as a single agent in healthy volunteers and in cancer patients, as well as in combination with five standard of care chemotherapy regimens in different tumor types. Across these studies, over 180 patients and healthy volunteers received at least one dose of amuvatinib. As a single agent in cancer patients, gastrointestinal toxicity was the major adverse event noted at doses up to 1500 mg/day with the dry powder formulation. In the combination Phase Ib trial, preliminary data indicated twelve partial responses and numerous durable stable disease per response evaluation criteria in solid tumors, or RECIST, including responses with the paclitaxel/carboplatin and carboplatin/etoposide standard of care chemotherapy regimens in combination with oral amuvatinib. Tumor types demonstrating clinical benefit include small cell lung cancer, neuroendocrine, non-small cell lung, breast, and endometrial
4
carcinoma. The safety profile of amuvatinib in combination with standard of care was consistent with historical published data for each chemotherapeutic with no apparent increase in severity or prolongation of reported events.
We conducted additional clinical safety and oral pharmacokinetic studies with both the dry powder and the lipid suspension formulations of amuvatinib. The studies confirmed that the lipid suspension capsule formulation provided better overall exposure and that 300 mg three times a day was safe and achieved blood levels of the drug within the therapeutic range expected from non-clinical studies.
Given the above clinical data, a Phase II, multi-center, open-label, single-arm study of amuvatinib in combination with platinum-etoposide in up to 50 subjects with small cell lung cancer who are not responding to standard treatment or relapsed shortly after standard treatment is anticipated to commence in the first half of 2011. Eligible subjects will receive amuvatinib lipid suspension capsules at the dose of 300 mg orally three times a day on a continuous basis in 21 day cycles together with their platinum-etoposide treatment. The primary endpoint will be tumor objective response.
SGI-110—DNMT1 Inhibitor
In normal cells, silencing of unnecessary genes is commonly carried out by DNA methylation through the action of DNA Methyltransferase enzymes (DNMT). However, this machinery can be usurped during the process of tumorigenesis, resulting in the inactivation of tumor suppressor genes and ultimately, cancer. Inhibition of DNMT activity in cancer cells can cause the silenced genes to become unmethylated and re-expressed. These re-expressed tumor suppressor genes can interfere with the cancer cells proliferative pathways and lead to cell death. We have developed a compound called SGI-110 which targets and blocks the mechanism by which methylation is copied to newly forming cells, thus allowing re-expression of tumor suppressor genes in tumors. We received clearance from the FDA in 2010 to commence Phase I clinical trials for SGI-110, and the first patients were dosed in early January 2011. The initial Phase I/II study will evaluate multiple schedules in patients with intermediate-2 or high-risk MDS or acute myeloid leukemia ("AML"). We are developing SGI-110 in collaboration with the Epigenetics Dream Team from Stand Up to Cancer, the charitable program established by the Entertainment Industry Foundation to raise funds for cancer research.
PIM Kinase Inhibitor Program
PIM kinases are proteins that play a pivotal survival role in cancer cells. Over-expression of PIM kinases in cells prevents programmed cell death that normally occurs when cells malfunction and can lead to unchecked cell survival, or cancer. The most advanced drug candidate in our PIM kinase inhibitor program was SGI-1776, a novel, orally administered, small molecule anti-cancer compound that effectively blocks the pro-survival activity of PIM kinases, allowing these potentially malignant cells to self-abort. Our IND for SGI-1776 received clearance from the FDA in November 2008, and we initiated a Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetic profile of SGI-1776 in the first half of 2009. The first in-human clinical trial program enrolled patients with solid tumors with specific emphasis on hormone and docetaxel refractory prostate cancer and refractory non-Hodgkin's lymphomas. These tumor types have been reported to over-express the PIM kinase family of proteins at a high frequency. Over-expression of PIM-1 kinase has been shown to be a marker of poor prognosis in these tumors. In November 2010, after careful evaluation of the first 14 patients enrolled in the first in human study, we decided to discontinue the clinical development of SGI-1776 due to cardiac toxicity observed in the expected therapeutic range of the drug. A backup program is in progress with the objective to ultimately advance into the clinic a new kinase inhibitor of the PIM target with a more favorable safety profile.
5
Discovery Programs
Axl Kinase Inhibitor
Axl kinase is a receptor tyrosine kinase implicated in tumorigenesis and metastases. Over-expression of Axl is associated with increased cellular transformation, cell survival, proliferation, migration, angiogenesis, and adhesion. The oncogenic potential of Axl was first discovered in chronic myelogenous leukemia ("CML") and has been shown to play a role in the development of other cancers. Axl kinase has been shown to be an important resistance mechanism for other targeted anti-cancer compounds as well as traditional chemotherapy. Axl kinase may be an exciting target for small molecule drug discovery. A series of small molecule inhibitors were discovered and are being developed for potency and selectivity against Axl kinase.
Epigenetics
Epigenetics describes the processes by which the activity of genes is controlled without altering the genetic sequence of the cell. Extensive research over the last twenty years indicates that epigenetics is central to how cancers develop and progress. Cancer cells are able to epigenetically alter how genes such as tumor suppressor genes are controlled. There is emerging evidence to suggest that advances in epigenetics will have a significant impact on the treatment of cancer. We have ongoing discovery programs in epigenetics and this area will continue to be a key strategic focus for us.
Cancer Metabolism
Unlike normal cells, cancer cells have differentiated requirements for biosynthetic intermediates to sustain their uncontrolled growth. Although this differentiation has been known since the middle of the twentieth century, a better understanding around the molecular rationale of how cancer cells undergo a differentiated metabolism can be exploited to develop drugs that have the potential to have a significant impact in the treatment of cancer. We have active discovery programs in cancer metabolism.
Signal Transduction Inhibitors
In addition to epigenetics and cancer metabolism, we are also focusing our discovery research in the area of signal transduction inhibitors. In general, this area of research is dedicated to protein kinases as these are well known to be involved in aberrant signaling in cancer cells that leads to many of the classic hallmarks of cancer. Although this has been an active area of research for many years, there continues to be emerging data indicating that there are still significant opportunities for the development of new therapies that inhibit signal transduction in oncology.
Dacogen License and Sublicense
In September 2004, we executed a license agreement granting MGI exclusive worldwide rights to the development, manufacture, commercialization and distribution of Dacogen. Under the terms of the agreement, MGI made a $40 million equity investment in us and agreed to pay up to $45 million in connection with the achievement of specific regulatory and commercialization milestones. To date, we have received $32.5 million in payments related to the achievement of these milestones.
In July 2006, MGI entered into an agreement to sublicense Dacogen to Cilag GmbH International ("Cilag"), a Johnson & Johnson company, granting exclusive development and commercialization rights in all territories outside North America. In accordance with our license agreement with MGI, we are entitled to receive 50% of certain payments MGI receives as a result of any sublicenses. We received $5 million, or 50% of the $10 million upfront payment MGI received, and, as a result of both the original agreement with MGI and this sublicense with Cilag, we may receive up to $17.5 million in future milestone payments as they are achieved for Dacogen globally. Cilag is responsible for
6
conducting regulatory and commercial activities related to Dacogen in all territories outside North America, while MGI retains all commercialization rights and responsibility for all activities in the United States, Canada and Mexico. MGI was acquired by Eisai Corporation of North America ("Eisai") in January 2008.
Dacogen was approved by the FDA in May 2006 for the treatment of patients with MDS. The Dacogen license to Eisai has created for us a royalty income stream on worldwide net sales starting at 20% and escalating to a maximum of 30%. Outside of the United States, Dacogen is approved for sale in 29 countries. These international territories are managed by Cilag.
Acquisition of New Products and Technologies
We are continually reviewing new product development opportunities in an effort to enhance and create a broader product pipeline for future development.
In 2006, we acquired Montigen Pharmaceuticals, Inc., a privately held, oncology-focused drug discovery and development company headquartered in Salt Lake City, Utah. Montigen's assets included its research and development team, their proprietary drug discovery technology platform and optimization process called CLIMB, and late-stage non-clinical compounds, including amuvatinib. Pursuant to the terms of the merger agreement, we paid the Montigen stockholders a total of $17.9 million upon the closing of the transaction, consisting of $9.0 million in cash and $8.9 million in shares of our common stock. In April 2007, we paid the former Montigen stockholders a milestone payment of approximately $10.0 million, which was paid in shares of our common stock. In November 2008, we paid the former Montigen stockholders another milestone payment of approximately $5.2 million, which was paid in a combination of approximately $2.8 million in cash and 1.5 million shares of our common stock. We have an obligation to pay the former Montigen stockholders an additional $6.8 million in shares of our stock, contingent upon achievement of one additional regulatory milestone.
Research and Development
Because of the stage of our development and the nature of our business, we expend significant resources on research and development activities. We spent $28.4 million in 2010, $29.7 million in 2009, and $32.7 million in 2008 on research and development activities. We conduct research internally and also through collaborations with third parties, and we intend to maintain a strong commitment to research and development efforts in the future. Our major research and development projects have been focused on drug discovery, non-clinical activities, and Phase I and Phase Ib clinical trials for amuvatinib, SGI-110, and SGI-1776.
Sales and Marketing
We currently have no employees focused on sales, marketing, and sales support. Our marketing efforts are handled by our Corporate Communications and Business Development group.
Manufacturing
We currently outsource manufacturing of all our drug compounds to qualified United States and foreign suppliers. We expect to continue to outsource manufacturing in the near term. We believe our current suppliers will be able to efficiently manufacture our proprietary compounds in sufficient quantities and on a timely basis, maintaining product quality and compliance with FDA and foreign regulations. We maintain oversight of the quality of our third-party manufacturers through ongoing audits, rigorous review, control over documented operating procedures, and thorough analytical testing by qualified, contracted laboratories. We believe that our current strategy of outsourcing manufacturing is cost-effective because we avoid the high costs for plant, equipment, and large manufacturing staffs.
7
The FDA and the Competent Authorities outside the United States must approve our drug manufacturing sites and deem a manufacturer acceptable under current good manufacturing practices ("GMPs") before release of active pharmaceutical ingredients ("API") and finished dosage forms for clinical testing.
We intend to continue evaluating our manufacturing requirements and may establish or acquire our own facilities to manufacture our products for distribution if doing so would be cost effective or improve control and flexibility of product supply.
Government Regulation: New Drug Development and Approval Process
Regulation by governmental authorities in the United States and other countries is a significant factor in the manufacture and marketing of pharmaceuticals and in our ongoing research and development activities. All of our drug products will require regulatory approval by governmental agencies prior to commercialization. In particular, human therapeutic products are subject to rigorous non-clinical testing, clinical trials, and other pre-marketing approval requirements by the FDA and regulatory authorities in other countries. In the United States, various federal, and in some cases state statutes and regulations, also govern or have an impact upon the manufacturing, safety, labeling, storage, record-keeping and marketing of such products. The lengthy process of seeking required approvals and the continuing need for compliance with applicable statutes and regulations require the expenditure of substantial resources. Regulatory approval, when and if obtained, may be limited in scope which may significantly limit the indicated uses for which a product may be marketed. Further, approved drugs, as well as their manufacturers, are subject to ongoing review and inspections which could reveal previously unknown problems with such products, which may result in restrictions on their manufacture, sale or use or in their withdrawal from the market.
The process for new drug development and approval has three major stages: discovery, non-clinical testing and clinical testing.
Drug discovery. In the initial stages of small molecule drug discovery, potential biological targets are identified, these targets are characterized, and then large numbers of potential compounds are screened for activity. This drug discovery process can take several years. Once a company defines a lead compound, the next steps are to conduct further preliminary studies on the mechanism of action, in vitro (test tube) screening against particular disease targets and some in vivo (animal) screening. If results are satisfactory, the compound progresses from discovery to non-clinical development.
Non-clinical development and testing. During the non-clinical testing stage, laboratory and animal studies are conducted to show biological activity of the compound against the disease target and the compound is evaluated for safety. These tests can take several years to complete and must be conducted in compliance with Good Laboratory Practice ("GLP") regulations. If the compound passes these hurdles, animal toxicology studies are initiated. If the results demonstrate acceptable levels of toxicity, the compound emerges from non-clinical testing and moves into the clinical phase.
Clinical testing—The Investigational New Drug Application. After appropriate animal testing is evaluated and the candidate molecule is found to have an acceptable safety profile, a company may decide to expand the development programs to a clinical setting. To accomplish this in the United States, an IND is submitted to the FDA. IND applications include the known chemistry of the compound, how the compound is manufactured, the results of animal studies and other previous experiments, the method by which the drug is expected to work in the human body, a proposed clinical development plan and how, where and by whom the proposed new clinical studies will be conducted. Health authorities in Europe and the rest of the world require a similar clinical trial application. If the controlling authority does not object, a company may initiate human testing. All clinical trials must be conducted in accordance with globally-accepted standards of good clinical practices ("GCPs"). This
8
means there are specific obligations to protect trial subjects and potential patients, monitor the study, collect the data and prepare a report of the study. Clinical trial applications must be updated with new information obtained during the course of the trials.
Clinical protocols must be approved by independent reviewers, referred to as Institutional Review Boards ("IRB") in the United States and Ethics Committees ("EC") in Europe. The IRB/EC is charged with providing an independent assessment of the appropriateness of the study, particularly focusing on the safety of the patients that might enroll in the study. The IRB's/EC's responsibilities continue while the study is ongoing, focusing on protecting the rights and safety of those enrolled in the study.
Companies have an obligation to provide progress reports on clinical trials at least annually to the FDA and foreign authorities. The FDA may, at any time during a clinical trial, impose a "clinical hold" if it has serious safety concerns about a trial. If this occurs, the clinical trial cannot continue until the FDA is satisfied that it is appropriate to proceed.
Clinical Development Plan. Clinical trials are typically conducted in three sequential phases, but the phases may overlap.
- •
- Phase I clinical trials. After an IND becomes
effective, Phase I human clinical trials can begin. These trials generally involve 20 to 40 heavily pre-treated cancer patients who may have a wide variety of cancers and typically
take approximately one year to complete. These trials are designed to evaluate a drug's safety profile and may include studies to assess the optimal safe dosage range. Phase I clinical studies
may evaluate how a drug is absorbed, distributed, metabolized and excreted from the body. Phase I studies may be expanded to Phase Ib trials that test the research compound in combination with
other agents to define the combined safety and dosing parameters.
- •
- Phase II clinical trials. In Phase II
clinical trials, studies are conducted in patients who have the specific targeted disease. The primary purpose of these trials is to demonstrate preliminary efficacy of the drug in the target patient
population. These studies typically take a few years to complete. Once trial data is obtained that a specific dose and schedule is creating clinical efficacy that appears to be superior to other
treatments, advancement to Phase III can begin.
- •
- Phase III clinical trials. These trials are typically large, involving several hundred or even thousands of patients and can take several years to complete. Phase III trials typically compare an investigational agent against a control product or the standard of care, which could be a product or treatment already approved for use in that disease. The data generated in these studies are monitored regularly by clinical monitors as well as the participating physician. There are specific requirements for the reporting of any adverse reactions that may result from the use of the drug. Clinical monitors visit the sites regularly and transmit the data back to the company for analysis and ultimately for presentation to the FDA.
Marketing application. Companies have the opportunity to interact with health authorities during the course of a drug development program. Most companies take advantage of this access to gain further insights about the kind of data that will be expected in their marketing application. After completion of the clinical trial phase, a company must compile all of the chemistry, manufacturing, non-clinical and clinical data into a marketing application. In the United States, this is called a New Drug Application ("NDA"); in the EU it is called a Marketing Authorization Application ("MAA"). These applications involve a significant amount of information, often in excess of 100,000 pages, and are independently reviewed by the health authorities to which they are submitted.
Both the FDA and the European Medicines Agency ("EMA") review these submissions for overall content and completeness before accepting them for review and may request additional information. Once an application is accepted for filing, each agency independently begins its in-depth review. In
9
both the United States and Europe, there are specified timeframes for the completion of review. Under certain circumstances, the review period may be extended frequently to evaluate new data submitted during the review.
In the United States, the FDA may refer the application to an appropriate advisory committee to consider specific aspects of the application. The FDA is not bound by the advice that may be derived from this meeting. The review process concludes with the issuance of a "complete response" letter from the FDA. If FDA evaluations of the NDA, the manufacturing facilities, and non-clinical and clinical sites are favorable, the FDA will approve the application. If the FDA's evaluation of the NDA submission or manufacturing facilities is not favorable, the FDA will reject the application in the complete response. This complete response will describe specific deficiencies, and, when possible, will outline recommended actions the applicant might take to get the application ready for approval. When and if any deficiencies are corrected, and actions are completed to the FDA's satisfaction, the FDA will review new information and may issue an approval letter authorizing commercialization of the drug for specific indications. The review and approval process in Europe has substantial similarities to that outlined for the United States.
Marketing approval. Once a health authority grants marketing approval for a drug, it can then be made available in that country or region. Periodic safety reports must be submitted to health authorities as a way to monitor the use of new drugs introduced to the market. Regulatory agencies around the world place great emphasis on pharmacovigilance, the process of monitoring the safety of a drug when it is released for general use, as the real world setting can be different from the controlled environment of clinical trials.
Phase IV clinical trials and post marketing studies. In addition to studies that might have been requested by health authorities as a condition of approval, clinical trials may be conducted to generate more information about the drug after initial approval of the product, including use for additional indications, the use of new dosage forms, or new dosing regimens. These studies may generate approved label changes and publications that provide further information to patients and the medical community. More recently, targeted clinical safety studies and analyses are being required to address specific issues that are derived from the original application database.
Fast Track. The FDA Modernization Act of 1997 specifies that the FDA can assign a fast track designation to a new drug or biologic product that is intended for the treatment of a serious or life-threatening condition and has the potential to address unmet medical needs for such a condition. Under this program, the sponsoring company may request this designation at any time during the development of the product. The FDA must determine whether the product qualifies within 60 days of receipt of the sponsoring company's request. For a product designated as fast track, the FDA has the ability to define a faster review, which includes allowing the sponsor to provide the NDA in discrete sections. This process is called a "rolling" NDA and is intended to accelerate the review and approval process.
Priority Review. This is a designation by the FDA for a review period of six months, instead of the standard ten months defined by federal regulation.
Accelerated Approval. This is a program intended to make promising products for life-threatening diseases available on the market on the basis of preliminary evidence prior to formal demonstration of patient benefit.
Approvals in the European Union. In 1993, the EU established a system for the registration of medicinal products in the EU whereby marketing authorization may be submitted at either a centralized or decentralized level. The centralized procedure is administered by the EMA and is mandatory for the approval of biotechnology products and is available, at the applicant's option, for
10
other innovative products. The centralized procedure provides for the granting of a single marketing authorization that is valid in all EU member states. A mutual recognition procedure is available at the request of the applicant for all medicinal products that are not subject to the mandatory centralized procedure, but fall under a decentralized procedure.
Approvals outside of the United States and European Union. Applications to market a new drug product must be made to all countries prior to marketing. The approval procedure and the time required for approval vary and may involve additional testing and cost. There can be no assurance that approvals will be granted on a timely basis or at all. In addition, reimbursement and pricing approval is required in many countries and there can be no assurance that the reimbursement decisions or resulting prices would be sufficient to generate an acceptable return on investment.
Off-Label Use. Drugs are approved for a specific use ("label use") that is then set forth in the document ("label") accompanying the dispensed drug. Physicians may prescribe drugs for uses that are not approved in the product's label. Such "off-label" prescribing may be used by physicians across medical specialties. The FDA does not regulate the behavior of physicians in their choice of treatments but it does limit a manufacturer's communications on the subject of off-label use. Companies cannot promote FDA approved drugs for off-label uses, nor can companies promote specifics of the use of a drug before it is approved.
Other Government Regulations
As a United States-based company, in addition to laws and regulations enforced by the FDA, we are also subject to regulation by other agencies under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other present and potential future federal, state or local laws and regulations. These agencies have specialized responsibilities to monitor the controlled use of hazardous materials such as chemicals, viruses and various radioactive compounds.
Market Exclusivity
The commercial success of a product, once it is approved for marketing, will depend primarily on a company's ability to create and sustain market share and exclusivity. Market exclusivity can be gained and maintained by a number of methods, including, but not limited to: patents, trade secrets, know-how, trademarks, branding and special market exclusivity provided by regulations.
Orphan Drug Designation
The United States, European Union, Japan and Australia have all enacted regulations to encourage the development of drugs intended to treat rare diseases. Orphan drug designation must be requested before submitting an application for marketing approval. After the granting of an orphan drug designation, the chemical identity of the therapeutic agent and its potential treatment use are disclosed publicly. If and when a product with orphan drug status receives marketing approval for the orphan indication, the product is entitled to marketing exclusivity, which means the regulatory authority may not approve any other applications to market the same drug for the same indication for seven years in the United States, ten years in Europe and Japan, and four years in Australia.
Data Exclusivity and Generic Copies
There is an abbreviated regulatory review and approval process for a generic copy of an approved innovator drug product. The generic copy can be approved on the basis of an application that is usually limited to manufacturing and biologic equivalence data. The copy can be approved after expiration of relevant patents and any regulatory exclusivity afforded the innovator by special circumstances. A new chemical entity has five and ten years of regulatory exclusivity in the United States and European
11
Union, respectively, precluding approval of a generic copy. Additional exclusivity can be afforded in the United States by approval of a product or use that has orphan drug status (seven years), or that requires review of new clinical data (three years), or that is an expansion of use to a pediatric population (six months). These exclusivities are independent, and could run sequentially, effectively extending the period of regulatory exclusivity. There is no assurance that such special regulatory exclusivities are applicable for our compounds. Separate from regulatory data exclusivity is the exclusivity conferred by the Hatch Waxman Act based on patent protection of the drug. A company seeking to market a generic might, after the lapse of regulatory data exclusivity, successfully challenge the patent protection of the marketed drug, thereby shortening its exclusive marketing period.
Patents and Proprietary Technology
Patents are very important to us in establishing proprietary rights to the products we develop or license. The patent positions of pharmaceutical and biotechnology companies, including ours, can be uncertain and involve complex legal, scientific, and factual questions. See "Risk Factors—Our ability to protect our intellectual property rights will be critically important to the success of our business, and we may not be able to protect these rights in the United States or abroad."
We actively pursue patent protection when applicable for our proprietary products and technologies, whether they are developed in-house or acquired from third parties. We attempt to protect our intellectual property position by filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. Importantly, we are prosecuting a number of patent applications directed to various compounds in our pipeline, including those from our discovery group. Additionally, we have been granted patents and have received patent licenses relating to our proprietary formulation technology, non-oncology and non-core technologies.
There can be no assurance that the patents granted or licensed to us will afford adequate legal protection against competitors or provide significant proprietary protection or competitive advantage. The patents granted or licensed to us could be held invalid or unenforceable by a court, or infringed or circumvented by others. In addition, third parties could also obtain patents that we would need to license or circumvent. Competitors or potential competitors may have filed patent applications or received patents, and may obtain additional patents and proprietary rights relating to proteins, small molecules, compounds, or processes that are competitive with the products we are developing.
In general, we obtain licenses from various parties we deem necessary or desirable for the development, manufacture, use, or sale of our products or product candidates. Some of our proprietary products are dependent upon compliance with other licenses and agreements. These licenses and agreements may require us to make royalty and other payments, to reasonably exploit the underlying technology of applicable patents, and to comply with regulatory filings. If we fail to comply with these and other terms in these licenses and agreements, we could lose the underlying rights to one or more of these potential products, which would adversely affect our product development and harm our business.
We also have patents, licenses to patents and pending patent applications outside of the United States, such as in Europe, Australia, Japan, Canada, China, Israel and India. Limitations on patent protection in these countries, and the differences in what constitutes patentable subject matter in these countries outside the United States, may limit the protection we have on patents issued or licensed to us outside the United States. In addition, laws of foreign countries may not protect our intellectual property to the same extent as those laws in the United States. To minimize our costs and expenses and maintain effective protection, we focus our foreign patent and licensing activities primarily in the European Union, Canada, Australia and Japan. In determining whether or not to seek a patent or to license any patent in other specific foreign countries, we weigh the relevant costs and benefits, and
12
consider, among other things, the market potential and profitability, the scope of patent protection afforded by the law of the jurisdiction and its enforceability, and the nature of terms with any potential licensees. Failure to obtain adequate patent protection for our proprietary drugs and technology would impair our ability to be commercially competitive in these markets.
SuperGen has 110 issued patents and 164 pending patent applications. Of the total patents and pending patent applications those that relate to each of our material commercial products or product candidates are described in further detail below:
- •
- Dacogen (exclusively licensed to Eisai)—eleven issued patents and 30 patent applications, having projected
expiration dates ranging from June 5, 2022 to September 27, 2024, granted or pending in the jurisdictions of the U.S., Australia, Canada, Europe, Japan, China, India and Hong Kong.
- •
- Amuvatinib—four issued patents and eight patent applications, having projected expiration dates ranging from
October 14, 2024 to March 1, 2027, granted or pending in the jurisdictions of the U.S., the European Patent Office ("EPO"), Australia and Canada.
- •
- SGI-110—one issued patent and seven patent applications, each having a projected expiration date
of September 29, 2025, granted or pending in the jurisdictions of the U.S., the EPO, Israel, China, South Africa, Malaysia, Vietnam and New Zealand.
- •
- SGI-1776—one issued patent and eleven patent applications, each having a projected expiration date
of November 6, 2027, granted or pending in the jurisdictions of the U.S., the EPO, Hong Kong, India, Malaysia, Brazil, Canada, Japan, Russia, Vietnam, and China.
- •
- AXL inhibitor (in discovery)—thirteen patent applications, having projected expiration dates ranging from
April 11, 2028 to February 8, 2030, pending in the jurisdictions of the U.S., the EPO, Australia, Canada, China, Japan, Korea, New Zealand, and Taiwan.
- •
- GSK discovery collaboration—three patent applications, having projected expiration dates of February 26, 2030, pending in the jurisdictions of the U.S., the Patent Cooperation Treaty participants and Taiwan.
Trade Secrets and Trademarks
We also rely on trade secret protection for certain proprietary technology. To protect our trade secrets and our other confidential information, we pursue a policy of having our employees and consultants execute proprietary information agreements upon commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the relationship is confidential except in specified circumstances. Further, we minimize the dissemination of our trade secrets by limiting the knowledge of staff only to the specific knowledge of a trade secret to what they need to know, and protective sequestering of trade secrets behind, for example, locks and passwords.
Competition
The pharmaceutical industry in general and the oncology sector in particular is highly competitive and subject to significant and rapid technological change. There are many companies, both public and private, including well-known pharmaceutical companies that are engaged in the discovery and development of products for some of the applications that we are pursuing. Some of our competitors and probable competitors include ArQule, Array BioPharma, Astex Therapeutics, Crystal Genomics, Exelixis, Infinity, Plexxikon, Vertex, Sanofi-Aventis, Bristol-Myers Squibb Company, Celgene, Eli Lilly & Co., GSK, Novartis AG, Pfizer, and others.
Many of our competitors have substantially greater financial, research and development, and manufacturing resources than we do and may represent substantial long-term competition for us. Some
13
of our competitors have received regulatory approval for products or are developing or testing product candidates that compete directly with our product candidates. For example, amuvatinib faces competition from a multitude of other investigational drugs which are multi-targeted tyrosine kinase inhibitors and inhibitors of the DNA repair pathway. We also expect that there will be other inhibitors of PIM kinases that will emerge as competition to our backup program as well as other investigational drugs progressing through our discovery pipeline. In addition, Dacogen faces competition from 5-aza-cytidine and other drugs in development to treat MDS.
Many of these competitors, either alone or together with their customers and partners, have significantly greater experience than we do in discovering products, undertaking non-clinical testing and clinical trials, obtaining FDA and other regulatory approvals, and manufacturing and marketing products. Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA or other foreign marketing approval or commercializing products before we do. If we elect to commence commercial product sales of our product candidates, we could be at a disadvantage relative to many companies with greater marketing and manufacturing capabilities, areas in which we have limited or no experience.
Factors affecting competition in the pharmaceutical industry vary depending on the extent to which competitors are able to achieve an advantage based on superior differentiation of their products' greater institutional knowledge or depth of resources. If we are able to establish and maintain a competitive advantage based on the ability of CLIMB to discover new drug candidates more quickly and against targets not accessible by many competitors, our advantage will likely depend primarily on the ability of our CLIMB technology to make accurate predictions about the effectiveness and safety of our drug candidates as well as our ability to effectively and rapidly develop investigational drugs.
Extensive research and development efforts and rapid technological progress characterize the industry in which we compete. Although we believe that our proprietary drug discovery capabilities afford us certain advantages relative to other discovery and development companies competing in oncology, we expect competitive intensity in this pharmaceutical segment to continue and increase over time. Discoveries by others may render CLIMB and our current and potential products noncompetitive. Our competitive position also depends on our ability to attract and retain qualified scientific and other personnel at all our geographic locations, develop effective proprietary products, implement development plans, obtain patent protection and secure adequate capital resources.
Employees
As of December 31, 2010, we had 97 full-time employees, consisting of 74 employees in research and development and 23 employees in general and administrative functions. We use consultants and temporary employees to complement our staffing. Our employees are not subject to any collective bargaining agreements, and we consider our relations with employees to be good.
Executive Officers
Name
|
Age | Position | |||
|---|---|---|---|---|---|
James S. J. Manuso, Ph.D. |
62 | President, Chief Executive Officer and Director | |||
Mohammad Azab, M.D., M.Sc., MBA |
55 | Chief Medical Officer | |||
Michael Molkentin |
56 | Chief Financial Officer | |||
James S.J. Manuso, Ph.D., has served as our president and chief executive officer since January 1, 2004, as our chief executive officer-elect from September 2003 to December 2003 and as a director since February 2001. Dr. Manuso is co-founder and immediate past president and chief executive officer of Galenica Pharmaceuticals, Inc. Dr. Manuso co-founded and was general partner of PrimeTech Partners, a biotechnology venture management partnership, from 1998 to 2002, and co-founder and
14
managing general partner of The Channel Group LLC, an international life sciences corporate advisory firm. He was also president of Manuso, Alexander & Associates, Inc., management consultants and financial advisors to pharmaceutical and biotechnology companies. Dr. Manuso was a vice president and director of Health Care Planning and Development for The Equitable Companies (now Group Axa), where he also served as acting medical director. He currently serves on the boards of Novelos Therapeutics, Inc. (NVLT:OB) and privately-held KineMed, Inc. Previously, he served on the boards of Merrion Pharmaceuticals Ltd. (MERR:IEX; Dublin, Ireland), Inflazyme Pharmaceuticals, Inc., Symbiontics, Inc., Quark Biotech, Inc., Galenica Pharmaceuticals, Inc., and Supratek Pharma, Inc. Dr. Manuso earned a BA with Honors in Economics and Chemistry from New York University, a Ph.D. in Experimental Psychophysiology from the Graduate Faculty of The New School University, a Certificate in Health Systems Management from Harvard Business School, and an Executive MBA from Columbia Business School. Dr. Manuso is the author of over 30 chapters, articles and books on topics including health care cost containment and biotechnology company management. He has taught and lectured at Columbia, New York University, Georgetown, Polytechnic University, and Waseda University (Japan). He has delivered invited addresses at meetings of the American Management Association, the American Medical Association, the Securities Industry Association, the Biotechnology Industry Organization, and many other professional associations. Dr. Manuso previously served as vice president and a member of the Board of Trustees of the Greater San Francisco Bay Area Leukemia & Lymphoma Society.
Mohammad Azab, M.D., M.Sc., MBA, joined SuperGen as chief medical officer in July 2009. He possesses more than 20 years of experience in worldwide drug development, clinical research, and medical affairs, resulting in eight approved drugs, including six in oncology. Most recently, he was president and chief executive officer of Intradigm Corporation, a privately held Palo Alto, CA company developing siRNA cancer therapeutics. Previously, Dr. Azab served as executive vice president of research and development, and chief medical officer, of Vancouver, British Columbia-based QLT Inc., where he led clinical development for now-approved drugs in oncology, gastro-intestinal, and ophthalmologic indications. Prior to this, he served as oncology drug team leader at UK-based Zeneca Pharmaceuticals, now Astra Zeneca, where he held responsibilities in global clinical development and regulatory submissions. In this capacity, he managed the development of drugs for prostate, breast, colorectal, and lung cancer indications. Before Zeneca, Dr. Azab was an international medical manager in oncology at Sanofi Pharmaceuticals, now Sanofi-Aventis, in Gentilly, France. Dr. Azab received his medical degree in 1979 from Cairo University. He practiced as a medical oncologist and received post-graduate training and degrees in oncology research and statistics from the University of Paris-Sud and the University of Pierre and Marie Curie in France. He has published more than 100 medical papers and abstracts. He is an active member of the American Society of Clinical Oncology, the American Association of Cancer Research, the European Society of Medical Oncology, and the American Society of Hematology. Dr. Azab received an MBA, with Distinction, from the Richard Ivey School of Business, University of Western Ontario.
Michael Molkentin has served as chief financial officer and corporate secretary since October 2003. Prior to joining us, Mr. Molkentin served as interim chief financial officer at Aradigm Corporation from May 2000 to September 2002. From January 1995 to April 2000, Mr. Molkentin served as division controller for Thermo Finnigan Corporation, a subsidiary of Thermo Electron. Mr. Molkentin served in a variety of financial management positions with technology companies, including field controller of Vanstar Corporation, controller of Republic Telcom Systems, Inc. and corporate controller of Computer Automation, Inc. Mr. Molkentin is a CPA and received a BBA in accounting from Bernard M. Baruch College in New York City, New York.
15
Segment and Geographic Area Financial Information
We operate in one business segment—human therapeutics. We had no product revenue in 2010, 2009, or 2008.
Available Information
We are subject to the information requirements of the Securities Exchange Act of 1934 (the "Exchange Act"). Therefore, we file periodic reports, proxy statements, and other information with the Securities and Exchange Commission ("SEC"). Such reports, proxy statements, and other information may be obtained by visiting the Public Reference Room of the SEC at 100 F Street, NE, Washington, DC 20549 or by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains a website (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically.
Financial and other information about us is available on our website at www.supergen.com. We make available on our website, free of charge, copies of our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after filing such material electronically or otherwise furnishing it to the SEC. Information on our website does not constitute a part of this annual report on Form 10-K.
16
The following section lists some, but not all, of the risks and uncertainties that may have a material adverse effect on our business, financial condition and results of operations. You should carefully consider these risks in evaluating our company and business. Our business operations may be impaired if any of the following risks actually occur, and by additional risks and uncertainties that we do not know of or that we currently consider immaterial. In such case, the trading price of our common stock could decline.
This report also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of certain factors, including the risks described below and elsewhere in this report.
Risks Related to Our Financial Condition and Common Stock
If Dacogen is not commercially successful, our future revenues would be limited and our business would be harmed.
Dacogen is approved in the United States and has been granted Orphan Drug exclusivity by the FDA through May 2013, with potential extension to November 2013 with additional regulatory filings by Eisai, but there is no guarantee that new patients and physicians will continue to use it for the treatment of patients. Once the Orphan Drug exclusivity period ends, Dacogen may be susceptible to generic entry by other pharmaceutical companies. This type of generic market entry typically causes sales of the trade name drug to decline. If Eisai's sales of Dacogen decrease, our royalty revenue will decrease commensurately, and we cannot be assured that Eisai will commit the resources to expand sales of Dacogen. Currently, the royalty revenue we receive from Eisai is our primary source of revenue, and we are dependent on Dacogen royalty revenue to fund our operations.
Dacogen is approved for the treatment of MDS in the United States and 29 smaller countries globally, but is not yet approved in Europe or Japan. In July 2006, Eisai sublicensed Dacogen to Cilag, giving Cilag responsibility for conducting regulatory activities related to Dacogen and granting it exclusive development and commercialization rights in Europe and all territories outside North America. We received 50% of the $10 million upfront payment and, as a result of both the original agreement with Eisai and the sublicense with Cilag, may receive up to $17.5 million in future milestone payments upon achievement of global regulatory and sales targets. During 2010, Eisai completed a randomized Phase III clinical trial of Dacogen in elderly patients with AML and although the primary endpoint of the study was not met, a supplemental marketing application in the U.S is planned for the first quarter of 2011. Cilag is also planning to submit a corresponding marketing application for Dacogen in Europe in 2011. However, if Dacogen is not approved for additional indications in the U.S. or is never approved in Europe or Japan, we will receive decreasing, and ultimately no, royalty payments from commercial sales by Cilag or Eisai for these territories and our future revenues and business will be harmed.
Our license agreement with Eisai may not produce the full financial benefits that we are anticipating, which could cause our business to suffer.
We expect to record development and license revenue from payments made to us by Eisai upon the achievement of regulatory and commercialization milestones. However, we may never receive such payments because the milestones may never be achieved, either because of failure to secure regulatory approval of Dacogen in Europe or Japan, or due to Eisai's or Cilag's inability to expend the resources to grow or commence sales of Dacogen as prescribed by the license agreement. In addition, the license agreement provides that Eisai will pay us (i) a certain portion of revenues payable to Eisai as a result of Eisai sublicensing the rights to market, sell and/or distribute Dacogen, to the extent such revenues are in excess of the milestone payments already due to us under our agreement with Eisai, and (ii) a 20% royalty increasing to a maximum of 30% on annual worldwide net sales of Dacogen. We cannot
17
guarantee that we will receive these payments, and we cannot be assured that Eisai will commit the resources to expand sales of Dacogen in North America, or that Cilag will commit the resources to sell it in Europe, Japan, and elsewhere, or that either company will be successful in doing so. Because we are heavily reliant on royalties and milestone payments relating to Dacogen to fund our operations, the failure to achieve the milestones and/or receive royalty revenue from sales of Dacogen would cause our business to suffer.
Our collaborative relationship with GSK may not produce the financial benefits that we are anticipating, which could cause our business to suffer.
Part of our strategy is to partner with, or out-license selective products to, other pharmaceutical companies in order to mitigate the cost of developing a drug through clinical trials to commercialization. The agreement with GSK is an example of this strategy, providing for the joint development of compounds that we will discover using our CLIMB technology, followed by the option for GSK to take one or more of the jointly developed compounds and further develop, commercialize, and sell the resulting product worldwide. The agreement provides for milestone payments to be paid to us during the development process, but the majority of the payments will not occur unless and until GSK exercises its option to license one or more compounds from us. We will spend our own cash and other resources during the joint development process, and we cannot guarantee that any successful compounds will result from our joint development efforts. Further, even if we discover and develop one or more viable compounds, we cannot guarantee that GSK will exercise its option to license any such compounds from us. If GSK chooses not to exercise its license option, we may continue to develop the compounds on our own, but the post-option exercise developmental and sales milestones described in the agreement, which we have estimated to be approximately $300 million, plus additional royalty revenues, will never be realized. If our joint development program with GSK is not successful, and if we cannot earn revenue from collaborative arrangements such as this agreement, our future revenues and business will be harmed.
We have a history of operating losses and we may incur losses for the foreseeable future.
Since inception, we have funded our research and development activities primarily from private placements and public offerings of our securities, milestone and other payments from collaborators, sales of our products, royalty revenue, and product revenues primarily from sales of Nipent. The North American rights to Nipent were sold in August 2006 and we sold the remaining worldwide rights in April 2007. Our substantial research and development expenditures and limited revenues have resulted in significant net losses. We have incurred cumulative losses of $340.3 million from inception through December 31, 2010, and we have not generated sufficient revenues to support our business during that time. We expect to be close to break-even or have modest operating income over the next few years and, although we were profitable in the years ended December 31, 2009 and 2010, we may never achieve sustained profitability.
Whether we achieve sustained profitability depends primarily on the following factors:
- •
- successful sales of Dacogen in North America by Eisai;
- •
- obtaining regulatory approval in Europe and Asia and the successful commercialization of Dacogen outside of North America
by Cilag;
- •
- limiting or preventing delays in production of Dacogen;
- •
- the success of our joint development program with GSK and whether GSK exercises its option to further develop and commercialize any of the compounds resulting from the joint development effort;
18
- •
- our ability to discover and develop additional novel therapeutics that might advance through our internal clinical
development infrastructure;
- •
- our research and development efforts, including the timing and costs of clinical trials;
- •
- our competition's ability to develop and bring to market competing products;
- •
- our ability to control costs and expenses associated with the discovery, development, and manufacturing of our novel
compounds, as well as general and administrative costs related to conducting our business; and
- •
- costs and expenses associated with entering into and performing under licensing, joint development, and other collaborative agreements.
Our products and product candidates, even if successfully developed and approved, may not generate sufficient or sustainable revenues to enable us to achieve or sustain profitability.
We will require additional funding to expand our product pipeline and commercialize new drugs, and if we are unable to raise the necessary capital or to do so on acceptable terms, our planned expansion and continued chances of survival could be harmed.
We will continue to spend substantial resources on expanding our product pipeline, developing future products, and conducting research and development, including clinical trials for our product candidates. Based on our currently forecasted product development activities, we anticipate that our capital resources will be adequate to fund operations and capital expenditures at least through 2012. However, if we experience unanticipated cash requirements during this period, we could require additional funds much sooner. In February 2009 we filed a $100 million shelf registration statement on Form S-3 with the SEC, which gives us the flexibility to raise funds through the sale of a variety of securities. We may raise money by the sale of our equity securities or debt, or the exercise of outstanding stock options by the holders of such options. However, given uncertain market conditions and the volatility of our stock price, we may not be able to sell our securities in public offerings or private placements at prices and/or on terms that are favorable to us, if at all. Also, the dilutive effect of additional financings could adversely affect our per share results. We may also choose to obtain funding through licensing and other contractual agreements. For example, we licensed the worldwide rights to the development, commercialization and distribution of Dacogen to Eisai. Such arrangements may require us to relinquish our rights to our technologies, products or marketing territories, or to grant licenses on terms that are not favorable to us. If we fail to obtain adequate funding in a timely manner, or at all, we will be forced to scale back our product development activities, or be forced to cease our operations.
Our equity investment in AVI BioPharma Inc. ("AVI") exposes us to equity price risk and any impairment charge would affect our results of operations.
Our investments in marketable securities are carried at fair value with unrealized gains and losses included in accumulated other comprehensive gain or loss in stockholders' equity. However, we are exposed to equity price risk on our equity investment in AVI. The public trading prices of the AVI shares have fluctuated significantly since we purchased them and could continue to do so. If the public trading prices of these shares trade below their adjusted cost basis in future periods, we may incur additional impairment charges relating to this investment, which in turn will affect our results of operations.
Currently we own 2.4 million shares of AVI and recorded an other-than-temporary decline in value of $3.1 million related to this investment during the year ended December 31, 2008. We evaluate investments with unrealized losses to determine if the losses are other than temporary. In making these determinations, we consider the financial condition and near-term prospects of the issuers, the
19
magnitude of the losses compared to the investments' cost, the length of time the investments have been in an unrealized loss position, and our ability and intent to hold the investments for a reasonable period of time sufficient for a recovery of fair value. It is possible that we may record another other than temporary decline in value related to AVI in the future.
Product Development and Regulatory Risks
Our product candidates will require significant additional development.
Many of our product candidates are in the development, rather than the clinical trial stage. However, we must significantly develop all of our product candidates before we can market them, or before they will become desirable for partnering or licensing. Although we believe that our preclinical and pilot clinical studies support further development of these product candidates, the results we have obtained to date do not necessarily indicate what the results of further testing would be, including controlled human clinical testing. All of the product candidates that we are currently developing will require extensive clinical testing before we can submit any regulatory application for their commercial use.
Our product development efforts may ultimately fail.
Our product candidates are subject to the risks of failure inherent in the development of pharmaceutical products. These risks include the following:
- •
- some of our product candidates may be found to be unsafe or ineffective, or may fail to receive the necessary regulatory
clearances in a timely manner, if at all;
- •
- even if safe and effective, our product candidates may be difficult to manufacture on a large scale or may be uneconomical
to market;
- •
- the proprietary rights of third parties may preclude us from marketing such products; and
- •
- third parties may market more effective or less costly products for treatment of the same diseases.
As a result, we cannot be certain that any of our products will be successfully developed, receive required governmental approvals on a timely basis, become commercially viable or achieve market acceptance.
Before we can seek regulatory approval of any of our product candidates, we must complete clinical trials, which are expensive and have uncertain outcomes.
All of our product candidates will require the commitment of substantial resources and regulatory approval. Before obtaining regulatory approvals for the commercial sale of any of our product candidates, we must demonstrate through non-clinical testing and clinical trials that our product candidates are safe and effective for use in humans.
We have a portfolio of cancer drugs in various stages of development. We are currently conducting clinical trials on our products amuvatinib and SGI-110. We also expect to commence other new clinical trials from time to time in the course of our business as our product development work continues. Conducting clinical trials is a lengthy, time consuming and expensive process and the results are inherently uncertain. We have incurred and will continue to incur substantial expense for, and we have devoted and expect to continue to devote a significant amount of time to, non-clinical testing and clinical trials. However, regulatory authorities may not permit us to undertake any additional clinical trials for our product candidates. If we are unable to complete our clinical trials, our business will be severely harmed and the price of our stock will likely decline.
20
We also have ongoing research and non-clinical projects that may lead to product candidates, but we have not begun clinical trials for these projects. If we do not successfully complete our non-clinical trials, we might not be able to commence clinical trials as planned.
Our clinical trials may be delayed or terminated, which would prevent us from seeking necessary regulatory approvals.
Completion of clinical trials may take several years or more. The length of a clinical trial varies substantially according to the type, complexity, novelty and intended use of the product candidate. The length of time and complexity of these studies make statistical analysis difficult and regulatory approval unpredictable. The commencement and rate of completion of our clinical trials may be delayed by many factors, including:
- •
- ineffectiveness of the study compound, or perceptions by physicians that the compound is not effective for a particular
indication;
- •
- inability to manufacture sufficient quantities of compounds for use in clinical trials;
- •
- inability to obtain FDA approval of our clinical trial protocols;
- •
- slower than expected rate of patient recruitment;
- •
- inability to adequately follow patients after treatment;
- •
- difficulty in managing multiple clinical sites;
- •
- unforeseen safety issues;
- •
- lack of efficacy demonstrated during the clinical trials; or
- •
- governmental or regulatory delays.
If we are unable to achieve a satisfactory rate of completion of our clinical trials, our business will be significantly harmed.
We may be required to suspend, repeat or terminate our clinical trials if they are not conducted in compliance with regulatory requirements.
Our clinical trials must be conducted in accordance with the requirements of the FDA and other regulatory authorities, and are subject to continuous oversight by these authorities, and institutional review boards and ethical committees. We outsource certain aspects of our research and development activities to contract research organizations ("CROs"). We have agreements with these CROs for certain of our clinical programs. We and our CROs are required to comply with GCP regulations and guidelines for all of our products in clinical development. GCPs are enforced through periodic inspections of study sponsors, principal investigators, and study sites. If our CROs or we fail to comply with applicable GCPs, the clinical data generated in our studies may be deemed unreliable and regulatory authorities may require us to perform additional studies before approving our applications. Our non-clinical safety studies must be conducted according to the principles of GLP regulations. In addition, our clinical trials must be conducted with product candidates produced under current GMPs, and may require a large number of test subjects. Our failure to comply with these regulations may require us to repeat clinical studies, which would delay the regulatory approval process.
We may be required to suspend, repeat or terminate our clinical trials if later trial results fail to demonstrate safety and efficacy, or if the results are negative or inconclusive.
Our clinical trials may be suspended at any time if we or the FDA believe the patients participating in our studies are exposed to unacceptable health risks or if we or the FDA find
21
deficiencies in the conduct of these trials. Adverse medical events during a clinical trial could cause us to terminate or repeat a clinical trial. In 2010, we terminated clinical trials for SGI-1776 due to safety concerns.
We may encounter other problems and failures in our studies that would cause us or the FDA to delay or suspend the studies. Even if we achieve positive interim results in clinical trials, these results do not necessarily predict final results, and acceptable results in early trials may not be repeated in later trials. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials, even after achieving promising results in earlier trials.
Negative or inconclusive results during a clinical trial could cause us to terminate or repeat a clinical trial. The potential failures would delay development of our product candidates, hinder our ability to conduct related non-clinical testing and clinical trials and further delay the commencement of the regulatory approval process. Further, the failures or perceived failures in our clinical trials would delay our product development and the regulatory approval process, damage our business prospects, make it difficult for us to establish collaboration and partnership relationships and negatively affect our reputation and competitive position in the pharmaceutical industry. Finally, if we are required to conduct other clinical trials for the product candidates, the additional trials would require substantial funding and time, and we may be unable to obtain funding to conduct such clinical trials.
Our failure to obtain regulatory approvals to market our product candidates in foreign countries and delays caused by government regulation would adversely affect our anticipated revenues.
Sales of our products in foreign jurisdictions will be subject to separate regulatory requirements and marketing approvals. Approval in the United States, or in any one foreign jurisdiction, does not ensure approval in any other jurisdiction. The process of obtaining foreign approvals may result in significant delays, difficulties and expenses for us, and may require additional clinical trials. Although many of the regulations applicable to our products in these foreign countries are similar to those promulgated by the FDA, many of these requirements also vary widely from country to country, which could delay the introduction of our products in those countries. Failure to comply with these regulatory requirements or to obtain required approvals would impair our ability to commercialize our products in foreign markets.
Even if regulatory approval of our products is obtained, later discovery of previously unknown problems may result in restrictions of a product, including withdrawal of that product from the market. Further, governmental approval may subject us to ongoing requirements for post-marketing studies. For example, despite receipt of governmental approval, the facilities of our third-party manufacturers are still subject to unannounced inspections by the FDA and must continue to comply with GMPs and other regulations. These regulations govern all areas of production, record keeping, personnel and quality control. If we or our third-party manufacturers fail to comply with any of the manufacturing regulations, we may be subject to, among other things, product seizures, recalls, fines, injunctions, suspensions or revocations of marketing licenses, operating restrictions and criminal prosecution.
If we are unable to comply with environmental laws and regulations, our business may be harmed.
We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous materials and waste products. We currently maintain a supply of biohazardous materials at some of our facilities. We believe our safety procedures for these materials comply with all applicable environmental laws and regulations, and we carry insurance coverage we believe is adequate for the size of our business. However, we cannot entirely eliminate the risk of accidental contamination or injury from these materials. If an accident or environmental discharge occurs, we could be held liable for any resulting damages, which could exceed our insurance coverage and financial resources.
22
We currently outsource certain of our research and development programs involving the controlled use of biohazardous materials. We believe our collaborators have in place safety procedures for these materials that comply with governmental standards. Nevertheless, if an accident does occur, our research and product development will be negatively affected.
Additional Risks Associated with Our Business
If the third-party manufacturers upon whom we rely fail to produce our products in the volumes that we require on a timely basis, or fail to comply with stringent regulations applicable to pharmaceutical drug manufacturers, we may face delays in the delivery of, or be unable to meet demand for, our products.
Because we have no manufacturing facilities, we rely on third parties for manufacturing activities related to all of our product candidates. As we develop new products, we must establish and maintain relationships with manufacturers to produce and package sufficient supplies of our finished pharmaceutical products. Reliance on third party manufacturing presents the following risks:
- •
- delays in scale-up to quantities needed for multiple clinical trials, or failure to (a) manufacture
such quantities to our specifications or (b) deliver such quantities on the dates we require, which could cause delay or suspension of clinical trials, regulatory submissions and
commercialization of our products;
- •
- potential relinquishment or sharing of intellectual property rights to any improvements in the manufacturing processes or
new manufacturing processes for our products; and
- •
- unannounced ongoing inspections by the FDA and corresponding state agencies for compliance with GMPs, regulations and foreign standards, and failure to comply with any of these regulations and standards may subject us to, among other things, product seizures, recalls, fines, injunctions, suspensions or revocations of marketing licenses, operating restrictions and criminal prosecution.
Any of these factors could delay clinical trials or commercialization of our product candidates under development, and entail higher costs.
Our business may be harmed if the manufacture of our products is interrupted or discontinued.
We may be unable to maintain our relationships with our third-party manufacturers. If we need to replace or seek new manufacturing arrangements, we may have difficulty locating and entering into arrangements with qualified contract manufacturers on acceptable terms, if at all. We are aware of only a limited number of companies on a worldwide basis who operate manufacturing facilities in which our products can be manufactured to our specifications and in compliance with GMPs. It could take several months, or significantly longer, for a new contract manufacturing facility to obtain FDA approval and to develop substantially equivalent processes for the production of our product candidates. We may not be able to contract with any of these companies on acceptable terms, if at all.
If our suppliers cannot provide the components we require, our future product sales and revenue could be harmed.
We rely on third-party suppliers to provide us with numerous components used in our products under development. Relying on third-party suppliers makes us vulnerable to component failures and interruptions in supply, either of which could impair our ability to conduct clinical trials on a timely basis. Using third-party suppliers makes it difficult and sometimes impossible for us to maintain quality control, manage inventory and production schedules and control production costs. Vendor lead times to supply us with ordered components vary significantly and can exceed six months or more. Both now and as we expand our need for manufacturing capacity, we cannot be sure that our suppliers will furnish us with required components when we need them. These factors could make it difficult for us
23
to effectively and efficiently manufacture our products, and could adversely impact our clinical trials, product development and future sales of our products.
Some suppliers may be our only source for a particular component, which would make us vulnerable to cost increases and supply interruptions. We generally rely on one manufacturer for each product.
Vendors may decide to limit or eliminate sales of certain products to the medical industry due to product liability or other concerns. In the event one of our sole source suppliers decides not to manufacture the component, goes out of business, or decides to cut off our supply, we may be unable to locate replacement supply sources, or the sources that we may locate may not provide us with similar reliability or pricing and our business could suffer. If we cannot obtain a necessary component, we may need to find, test and obtain regulatory approval for a replacement component, produce the component or redesign the related product, which would cause significant delay and could increase our manufacturing costs. Any of these events could adversely impact our future sales and results of operations.
If we are not able to maintain and successfully establish new collaborative and licensing arrangements with third parties, our product development and business will be harmed.
Our business model is based on establishing collaborative relationships with other parties both to license compounds upon which our products and technologies are based and to manufacture our products or our collaborators' products. It is critical that we gain access to compounds and technologies to license for further development. Due to the expense of the drug approval process we must have relationships with established pharmaceutical companies to offset some of our development costs in exchange for a combination of development, marketing and distribution rights. For example, in our collaborative relationship with GSK, we expect to offset the costs of further development of the drugs we jointly develop with GSK, if and when GSK exercises its option to license such jointly developed drugs.
From time to time we enter into discussions with various companies regarding the establishment of new collaborations. If we are not successful in establishing new partners for our product candidates, we may not be able to pursue further development of such product candidates and/or may have to reduce or cease our current development programs, which would materially harm our business. Even if we are successful in establishing new collaborations, they are subject to numerous risks and uncertainties including:
- •
- our ability to negotiate acceptable collaborative arrangements;
- •
- the collaboration making us less attractive to potential acquirers;
- •
- freedom of our collaborative partners to pursue alternative technologies either on their own or with others, including our
competitors, for the diseases targeted by our programs and products;
- •
- the potential failure of our partners to fulfill their contractual obligations or their decision to terminate our
relationships, in which event we may be required to seek other partners, or expend substantial resources to pursue these activities independently; and
- •
- our ability to manage, interact and coordinate our timelines and objectives with our collaborative partners may not be successful.
In addition, our collaborators may undergo business combinations, which could have the effect of making the collaboration with us less attractive to them for a number of reasons. For example, if an existing collaborator purchases a company that is one of our competitors, that company may be less willing to continue its collaboration with us. A company that has a strategy of purchasing companies with attractive technologies might have less incentive to enter into a collaboration agreement with us. Moreover, disputes may arise with respect to the ownership of rights to any technology or products developed with any current or future collaborator. Lengthy negotiations with potential collaborators or disagreements between us and our collaborators may lead to delays in or termination of the research, development or commercialization of product candidates or result in time consuming and expensive litigation or arbitration.
24
Our collaborative relationships with third parties could cause us to expend significant funds on development costs with no assurance of financial return.
From time to time we enter into collaborative relationships with third parties to co-develop and market products, such as our relationship with GSK. These relationships require substantial financial commitments from us, and at the same time the product developments are subject to the same regulatory requirements, risks and uncertainties associated with the development of our other product candidates. The compounds that are the subject of these collaborative agreements may prove to be ineffective, may fail to receive regulatory approvals, may be unprotectable by patents or other intellectual property rights, or may not be otherwise commercially viable. If these collaborative relationships are not successful, our product developments will be adversely affected, and our investments and efforts devoted to the product developments will be wasted.
Our ability to protect our intellectual property rights will be critically important to the success of our business, and we may not be able to protect these rights in the United States or abroad.
The success of our operations depends in part on our ability to obtain patents, protect trade secrets, operate without infringing the proprietary rights of others and enforce our proprietary rights against accused infringers.
We actively pursue a policy of seeking patent protection when applicable for our proprietary products and technologies, whether they are developed in-house or acquired from third parties. We attempt to protect our intellectual property position by filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. To date, we have ownership of or acquired licenses to numerous patents covering various aspects of our proprietary drugs and technologies. In addition, we are prosecuting a number of patent applications for new drug candidates that we are actively developing at this time.
We also have patents, licenses to patents, and pending patent applications in Europe, Australia, Japan, Canada, China and Israel among other countries. Limitations on patent protection, and the differences in what constitutes patentable subject matter, may limit the protection we have on patents issued or licensed to us in these countries. In addition, laws of foreign countries may not protect our intellectual property to the same extent as would laws in the United States. In determining whether or not to seek patent protection or to license any patent in a foreign country, we weigh the relevant costs and benefits, and consider, among other things, the market potential and profitability, the scope of patent protection afforded by the law of the jurisdiction and its enforceability, and the nature of terms with any potential licensees. Failure to obtain adequate patent protection for our proprietary drugs and technology would impair our ability to be commercially competitive in these markets.
The pharmaceutical industry is characterized by a large number of patent filings involving complex legal and factual questions, and therefore we cannot predict with certainty whether our patents will be enforced effectively. Competitors may have filed applications for, or been issued patents on, products or processes that compete with or are similar to ours. We may not be aware of all of the patents potentially adverse to our interests which may have been issued to others. In addition, third parties may challenge, invalidate or circumvent any of our patents. Thus, any patents that we own or license from third parties may not provide adequate protection against competitors, if at all. Our pending patent applications and those we may file in the future, or those we may license from third parties, may not result in patents being issued with adequate claim scope, if at all.
In addition to pursuing patent protection in appropriate instances, we also rely on trade secret protection or regulatory marketing exclusivity for unpatented proprietary technology. However, trade secrets are difficult to protect. Our trade secrets or those of our collaborators may become known or
25
may be independently discovered by others. Furthermore, regulatory marketing exclusivity is for a limited time period, which may not be an adequate period for our business interests.
In the pharmaceutical industry there has been, and we believe that there will continue to be, significant litigation regarding patent and other intellectual property rights. Claims may be brought against us in the future based on patents held by others. These persons could bring legal actions against us claiming damages and seeking to enjoin clinical testing, manufacturing and marketing of the affected product. If we become involved in litigation, it could consume a substantial portion of our resources, regardless of the outcome of the litigation. If a lawsuit against us is successful, in addition to any potential liability for damages, we could be required to obtain a license to continue to manufacture or market the affected product. We cannot assure you that we would prevail in a lawsuit filed against us or that we could obtain any licenses required under any patents on acceptable terms, if at all.
Our proprietary products are dependent upon compliance with other licenses and agreements. These licenses and agreements require us to make royalty and other payments, reasonably exploit the underlying technology of the applicable patents, and comply with regulatory filings. If we fail to comply with these licenses and agreements, we could lose the underlying rights to one or more of these potential products, which would adversely affect our product development and harm our business.
If we fail to compete effectively against other pharmaceutical companies, our business will suffer.
The pharmaceutical industry in general and the oncology sector in particular is highly competitive and subject to significant and rapid technological change. There are many companies, both public and private, including well-known pharmaceutical companies that are engaged in the discovery and development of products for some of the applications that we are pursuing. Some of our competitors and probable competitors include ArQule, Array BioPharma, Astex Tx, Crystal Genomics, Exelixis, Infinity, Plexxikon, Vertex, Sanofi-Aventis, Bristol-Myers Squibb Company, Celgene, Eli Lilly & Co., GSK, Novartis AG, Pfizer, and others.
Many of our competitors have substantially greater financial, research and development, and manufacturing resources than we do and may represent substantial long-term competition for us. Some of our competitors have received regulatory approval for products or are developing or testing product candidates that compete directly with our product candidates. For example, amuvatinib faces competition from a multitude of other investigational drugs which are multi-targeted tyrosine kinase inhibitors and inhibitors of the DNA repair pathway. We also expect that there will be other inhibitors of PIM kinases that will emerge as competition for investigational drugs progressing through our discovery pipeline. In addition, Dacogen faces competition from 5-aza-cytidine and other drugs in development to treat MDS.
Many of these competitors, either alone or together with their customers and partners, have significantly greater experience than we do in discovering products, undertaking non-clinical testing and clinical trials, obtaining FDA and other regulatory approvals, and manufacturing and marketing products. Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA or foreign marketing approval or commercializing products before we do. If we elect to commence commercial product sales of our product candidates, we could be at a disadvantage relative to many companies with greater marketing and manufacturing capabilities, in areas that we may have limited or no experience.
Factors affecting competition in the pharmaceutical industry vary depending on the extent to which competitors are able to achieve an advantage based on superior differentiation of their products, greater institutional knowledge, or depth of resources. If we are able to establish and maintain a competitive advantage based on the ability of CLIMB to discover new drug candidates more quickly and against targets not accessible by many competitors, our advantage will likely depend primarily on
26
the ability of our CLIMB technology to make accurate predictions about the effectiveness and safety of our drug candidates as well as our ability to effectively and rapidly develop investigational drugs.
Extensive research and development efforts and rapid technological progress characterize the industry in which we compete. Although we believe that our proprietary drug discovery capabilities afford us a competitive advantage relative to other discovery and development companies competing in oncology, we expect competitive intensity in this pharmaceutical segment to continue and will increase over time. Discoveries by others may render CLIMB and our current and potential products noncompetitive. Our competitive position also depends on our ability to attract and retain qualified scientific and other personnel at all our geographic locations, develop effective proprietary products, implement development plans, obtain patent protection and secure adequate capital resources.
The pharmaceutical industry in general and the oncology sector in particular is subject to significant and rapid technological change. Developments by competitors may render our product candidates or technologies obsolete or non-competitive.
Our competitors may succeed in developing technologies or products that are more effective than ours. Additionally, our products that are under patent protection face intense competition from competitors' proprietary products. This competition may increase as new products enter the market.
A number of our competitors have substantially more capital, research and development, regulatory, manufacturing, marketing, human and other resources and experience than we have. As a result, our competitors may:
- •
- develop products that are more effective or less costly than any of our current or future products or that render our
products obsolete;
- •
- produce and market their products more successfully than we do;
- •
- establish superior proprietary positions; or
- •
- obtain FDA or foreign regulatory approval for labeling claims that are more favorable than those for our products.
We will also face increasing competition from lower-cost generic products after patents on our proprietary products expire. Loss of patent protection typically leads to a rapid decline in sales for that product and could affect our future results. As new products enter the market, our products may become obsolete or our competitors' products may be more effective or more effectively marketed and sold than our products. Technological advances, competitive forces and loss of intellectual property protection rights for our products may render our products obsolete.
We may be subject to product liability lawsuits and our insurance may be inadequate to cover damages.
Clinical trials and commercial use of our current and potential products may expose us to liability claims from the use or sale of these products. Consumers, healthcare providers, pharmaceutical companies and others selling such products might make claims of this kind. We may experience financial losses in the future due to product liability claims. We have obtained limited product liability insurance coverage for our products and clinical trials, under which the coverage limits are $10 million per occurrence and $10 million in the aggregate. We do not know whether this coverage will be adequate to protect us in the event of a claim. We may not be able to obtain or maintain insurance coverage in the future at a reasonable cost or in sufficient amounts to protect us against losses. If third parties bring a successful product liability claim or series of claims against us for uninsured liabilities or in excess of insured liabilities, we may not have sufficient financial resources to complete development or commercialization of any of our product candidates and our business and results of operations will be adversely affected.
27
If we are unable to attract and retain additional, highly skilled personnel required for the expansion of our activities, our business will suffer.
Our success is dependent on key personnel, including members of our senior management and scientific staff at all our geographic locations. If any of our executive officers decides to leave and we cannot locate a qualified replacement in time to allow a smooth transition, our business may be adversely affected. To successfully expand our operations, we will need to attract and retain additional highly skilled individuals, particularly in the areas of clinical administration, non-clinical and development research, manufacturing and finance. We compete with other companies for the services of existing and potential employees, however to the extent these employees favor larger, more established employers, we may be at a disadvantage.
Earthquake or other natural or man-made disasters and business interruptions could adversely affect our business.
Our operations are vulnerable to interruption by fire, power loss, floods, telecommunications failure and other events beyond our control. In addition, our operations are susceptible to disruption as a result of natural disasters such as earthquakes. So far we have never experienced any significant disruption of our operations as a result of earthquakes, other natural disasters, or any man-made disasters. Although we have a contingency recovery plan, any significant business interruption could cause delays in our drug development and future sales and harm our business.
Provisions in our certificate of incorporation, bylaws and applicable Delaware law may prevent or discourage third parties or stockholders from attempting to replace our management.
Anti-takeover provisions of our certificate of incorporation and bylaws make it more difficult for a third party to acquire us, even if doing so would be beneficial to our stockholders. These provisions include:
- •
- authorization of the issuance of up to 2,000,000 shares of our preferred stock;
- •
- elimination of cumulative voting; and
- •
- elimination of stockholder action by written consent.
Our bylaws establish procedures, including notice procedures, with regard to the nomination, other than by or at the direction of our board of directors, of candidates for election as directors or for stockholder proposals to be submitted at stockholder meetings.
We are also subject to Section 203 of the Delaware General Corporation Law, an anti-takeover provision. In general, Section 203 of the Delaware General Corporation Law prevents a stockholder owning 15% or more of a corporation's outstanding voting stock from engaging in business combinations with a Delaware corporation for three years following the date the stockholder acquired 15% or more of a corporation's outstanding voting stock. This restriction is subject to exceptions, including the approval of the board of directors and of the holders of at least two-thirds of the outstanding shares of voting stock not owned by the interested stockholder.
We believe that the benefits of increased protection of our potential ability to negotiate with the proponents of unfriendly or unsolicited proposals to acquire or restructure us outweigh the disadvantages of discouraging those proposals because, among other things, negotiation of those proposals could result in an improvement of their terms. Nevertheless, these provisions are expected to discourage different types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of our company to first negotiate with us, and may have the effect of preventing or discouraging third parties or stockholders from attempting to replace our management.
28
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None
Our principal administrative facility is currently located in leased general office space, containing approximately 37,000 square feet, in Dublin, California, under a lease that expires in November 2015. Our drug formulation laboratory operations are located in a 10,000 square foot industrial building that we own in Pleasanton, California. We are currently leasing 11,700 square feet of space for our drug discovery laboratory operations in Salt Lake City, Utah. The lease on this space expires in May 2012. We believe the above properties are adequate and suitable for our operations in the foreseeable future.
We are not currently subject to any pending material legal proceedings.
29
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market for Common Stock
Our common stock trades on the Nasdaq Global Market under the symbol "SUPG." The following table sets forth the high and low trading price information for our common stock for each quarterly period in the two most recent fiscal years as reported on the Nasdaq Global Market:
| |
High | Low | |||||
|---|---|---|---|---|---|---|---|
2010 |
|||||||
Quarter ended March 31, 2010 |
$ | 3.50 | $ | 2.57 | |||
Quarter ended June 30, 2010 |
3.80 | 1.86 | |||||
Quarter ended September 30, 2010 |
2.18 | 1.70 | |||||
Quarter ended December 31, 2010 |
3.08 | 2.07 | |||||
2009 |
|||||||
Quarter ended March 31, 2009 |
$ | 2.56 | $ | 1.54 | |||
Quarter ended June 30, 2009 |
2.23 | 1.69 | |||||
Quarter ended September 30, 2009 |
3.30 | 1.98 | |||||
Quarter ended December 31, 2009 |
3.17 | 2.31 | |||||
Holders of Record
As of March 1, 2011, there were 533 holders of record of our common stock and approximately 14,400 beneficial stockholders.
Dividends
We have never paid cash dividends on our capital stock and do not expect to pay any dividends in the foreseeable future. We intend to retain future earnings, if any, for use in our business.
Equity Securities
During the fiscal year ended December 31, 2010, we did not repurchase our common stock or issue unregistered securities.
30
Company Stock Price Performance Graph
The performance graph below is required by the SEC and shall not be deemed to be incorporated by reference by any general statement incorporating by reference this annual report on Form 10-K into any filing under the Securities Act or the Securities Exchange Act except to the extent we specifically incorporate this information by reference and shall not otherwise be deemed soliciting material or filed under such Acts.
The graph compares our cumulative total stockholder return with those of the Nasdaq Composite Index and the Nasdaq Pharmaceutical Index. The graph assumes that $100 was invested on December 31, 2005 in the Company's common stock and in the Nasdaq Composite Index and the Nasdaq Pharmaceutical Index, including reinvestment of dividends. Note that historic stock price performance should not be considered indicative of future stock price performance.
| |
12/05 | 12/06 | 12/07 | 12/08 | 12/09 | 12/10 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
SuperGen, Inc |
100.00 | 100.59 | 72.28 | 37.82 | 51.88 | 51.88 | |||||||||||||
NASDAQ Composite |
100.00 | 111.74 | 124.67 | 73.77 | 107.12 | 125.93 | |||||||||||||
NASDAQ Pharmaceutical |
100.00 | 101.61 | 94.58 | 87.40 | 95.29 | 101.44 | |||||||||||||
31
ITEM 6. SELECTED FINANCIAL DATA.
The information set forth below is not necessarily indicative of results of future operations and should be read in conjunction with the financial statements and notes thereto appearing in Item 15 of Part IV of this report.
| |
Year ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Consolidated Statement of Operations Data:
|
2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||
| |
(In thousands, except per share data) |
|||||||||||||||
Total revenues |
$ | 52,972 | $ | 41,253 | $ | 38,422 | $ | 22,954 | $ | 38,083 | ||||||
Cost of product revenue |
— | — | — | 221 | 2,003 | |||||||||||
Research and development expenses |
28,394 | 29,689 | 32,685 | 23,423 | 16,544 | |||||||||||
Selling, general and administrative expenses |
9,442 | 8,994 | 11,119 | 13,520 | 24,714 | |||||||||||
Acquired in-process research and development |
— | — | 5,185 | 9,967 | 16,318 | |||||||||||
Gain on sale of products |
(750 | ) | (595 | ) | (2,236 | ) | (33,677 | ) | — | |||||||
Income (loss) from operations |
15,886 | 3,165 | (8,331 | ) | 9,500 | (21,496 | ) | |||||||||
Other income (expense) and income tax benefit (provision) |
387 | 1,572 | (780 | ) | 3,581 | 5,009 | ||||||||||
Net income (loss) |
$ | 16,273 | $ | 4,737 | $ | (9,111 | ) | $ | 13,081 | $ | (16,487 | ) | ||||
Basic net income (loss) per common share |
$ | 0.27 | $ | 0.08 | $ | (0.16 | ) | $ | 0.23 | $ | (0.31 | ) | ||||
Diluted net income (loss) per common share |
$ | 0.27 | $ | 0.08 | $ | (0.16 | ) | $ | 0.23 | $ | (0.31 | ) | ||||
Shares used to compute basic net income (loss) per common share |
60,287 | 59,316 | 57,721 | 56,868 | 53,439 | |||||||||||
Shares used to compute diluted net income (loss) per common share |
60,635 | 59,340 | 57,721 | 57,301 | 53,439 | |||||||||||
| |
As of December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Consolidated Balance Sheet Data:
|
2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||
| |
(In thousands) |
|||||||||||||||
Cash, cash equivalents, marketable securities, and restricted cash |
$ | 122,511 | $ | 103,022 | $ | 90,679 | $ | 93,385 | $ | 78,585 | ||||||
Other current assets |
1,370 | 2,054 | 1,307 | 857 | 3,984 | |||||||||||
Property, plant and equipment, net |
3,932 | 4,205 | 4,437 | 4,435 | 3,752 | |||||||||||
Other assets |
1,285 | 1,236 | 1,342 | 1,771 | 1,725 | |||||||||||
Total assets |
$ | 129,098 | $ | 110,517 | $ | 97,765 | $ | 100,448 | $ | 88,046 | ||||||
Current liabilities |
$ | 6,048 | $ | 6,573 | $ | 6,629 | $ | 6,961 | $ | 21,276 | ||||||
Non-current liabilities |
1,438 | 1,958 | 645 | 832 | 938 | |||||||||||
Total stockholders' equity |
121,612 | 101,986 | 90,491 | 92,655 | 65,832 | |||||||||||
Total liabilities and stockholders' equity |
$ | 129,098 | $ | 110,517 | $ | 97,765 | $ | 100,448 | $ | 88,046 | ||||||
32
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
You should read the following discussion together with our consolidated financial statements and related notes included elsewhere in this report. The results discussed below are not necessarily indicative of the results to be expected in any future periods. Our disclosure and analysis in this section of the report also contain forward-looking statements. When we use the words "anticipate," "estimate," "project," "intend," "expect," "plan," "believe," "should," "likely" and similar expressions, we are making forward-looking statements. Forward-looking statements provide our current expectations or forecasts of future events. In particular, these statements include statements such as: our estimates about profitability; the percentage of royalties we expect to earn on Dacogen sales under our agreement with Eisai; our forecasts regarding our research and development and general and administrative expenses; our expectations about the joint development program with GSK; and our statements regarding the sufficiency of our cash to meet our operating needs. Our actual results could differ materially from those predicted in the forward-looking statements as a result of risks and uncertainties including, but not limited to: the commercial success of Dacogen; delays and risks associated with conducting and managing our clinical trials; developing products and obtaining regulatory approval; ability to establish and maintain collaborative relationships; competition; ability to obtain funding; ability to protect our intellectual property; our dependence on third party suppliers; risks associated with the hiring and loss of key personnel; adverse changes in the specific markets for our products; and our ability to launch and commercialize products. Certain unknown or immaterial risks and uncertainties can also affect our forward-looking statements. Consequently, no forward-looking statement can be guaranteed and you should not rely on these forward-looking statements. For a discussion of the known and material risks that could affect our actual results, please see the "Risk Factors" section of this report. We undertake no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. Readers should carefully review the Risk Factors section as well as other reports or documents we file from time to time with the Securities and Exchange Commission.
Overview
We are a pharmaceutical company dedicated primarily to the discovery and development of novel cancer therapeutics in epigenetic and cell signaling modulation. We develop products through biochemical and clinical proof of concept to partner for further development and commercialization. Our primary developmental efforts revolve around the products progressing out of our small-molecule drug discovery programs. We commenced Phase I clinical trials for amuvatinib, a multi-targeted kinase inhibitor and DNA repair suppressor in June 2007, and we are anticipating the commencement of a Phase II trial in small cell lung cancer with this product in the first half of 2011. In 2010, SGI-110, our small molecule, DNA hypomethylating agent, received clearance from the FDA to advance into Phase I trials. We announced the dosing of the first patients in the Phase I trial in early 2011. In addition, we initiated clinical trials for a second internally developed product, SGI-1776, a PIM kinase inhibitor. This clinical program was terminated last year due to specific cardiac toxicity. We intend to continue the larger discovery effort targeted at PIM kinases to ultimately identify alternative product candidates with more favorable safety profiles.
We currently receive royalty revenues relating to sales of Dacogen for treatment of patients with MDS, which is licensed to Eisai.
Dacogen. Dacogen was approved by the FDA in 2006 for the treatment of patients with MDS. In 2004, we executed an agreement granting Eisai exclusive worldwide rights to the development, manufacture, commercialization and distribution of Dacogen. We are entitled to receive a royalty on worldwide net sales of Dacogen starting at 20% and escalating to a maximum of 30%. Our royalty revenues have increased from $38.4 million in 2008, to $41.2 million in 2009 and $52.5 million in 2010. We recognize royalty revenue when the royalty statement is received from Eisai because we do not have sufficient ability to accurately estimate Dacogen sales prior to that time.
33
In 2006, Eisai executed an agreement to sublicense Dacogen to Cilag, a Johnson & Johnson company, granting exclusive development and commercialization rights in all territories outside North America. Cilag is responsible for conducting regulatory and commercial activities related to Dacogen in all territories outside North America, while Eisai retains all commercialization rights and responsibility for all activities in the United States, Canada and Mexico. As a result of both the original agreement with Eisai and the sublicense with Cilag, we may receive up to $17.5 million in future milestone payments as they are achieved for Dacogen globally.
Nipent. Nipent is approved by the FDA and EMA for the treatment of hairy cell leukemia. Nipent was marketed by us in the United States until August 2006, and distributed in Europe through March 2007.
In 2006, we sold the North American rights to Nipent and our SurfaceSafe cleaning system to Hospira. In 2007, we closed another transaction with Hospira, completing the sale of the remaining worldwide rights for Nipent. The balance of the purchase price relating to the sale of the worldwide rights was payable in five annual installments on the anniversary of the closing date, totaling $3 million. Through December 31, 2010, we have received three of the annual installments totaling $1.6 million. We expect to receive the remaining balance of $1.4 million in two annual installments of $700,000 each in 2011 and 2012.
Montigen Acquisition. In April 2006, we acquired Montigen, a privately-held oncology-focused drug discovery and development company headquartered in Salt Lake City, Utah. Montigen's assets included its research and development team, a proprietary drug discovery technology platform and optimization process known as CLIMB, and late-stage non-clinical compounds targeting Aurora-A Kinase and members of the Tyrosine Kinase receptor family.
In addition to the consideration paid at the closing of the transaction, the merger agreement specified $22 million due to the former Montigen stockholders, payable in shares of our common stock, contingent upon achievement of specific regulatory milestones. In April 2007, we paid the first contingent milestone payment of $10 million, and in November 2008, we paid the second contingent milestone payment of $5.2 million, leaving one remaining future contingent regulatory milestone payment of $6.8 million that is payable in shares of our common stock upon notification by the FDA of the first filing of an NDA for a product containing as the active ingredient a compound identified using the CLIMB technology.
GSK Collaboration. In October 2009, we entered into a multi-year collaboration with GSK to discover and develop cancer therapeutics based on epigenetic targets. Epigenetics refer to the regulation of genes with mechanisms other than changes to the underlying DNA sequence. Epigenetic processes are widely believed to play a central role in the development and progression of almost all cancers. Pursuant to the terms of the transaction, we will collaborate with GSK over a period of five years to discover and develop specific epigenetic therapeutics. At the end of the research term, or earlier if GSK elects, GSK may exercise its option to license from us the compounds that are the result of the joint research effort, in order to continue the development and ultimately commercialize and sell the products worldwide.
In connection with the transaction, we received $5 million upfront, inclusive of a $3 million purchase by GSK of shares of our common stock, priced at a premium to market. In addition, GSK is obligated to make certain payments to us if and when the compounds reach specified developmental milestones, as well as payments to us if and when the compounds that GSK has licensed achieve certain regulatory milestones. The agreement further provides that, if the licensed compounds derived from the joint research team become products, GSK will pay us sales milestone payments as well as royalties on annual net sales of such products. The royalties will be paid on a country-by-country and product-by-product basis. Total potential development and sales milestones payable to us could exceed
34
$375 million. In addition, we may receive tiered royalties into double digit magnitudes, payable on net sales of any resulting products.
All of our current products are in the development or clinical trial stage, and will require substantial additional investments in research and development, clinical trials, regulatory and sales and marketing activities to commercialize these product candidates. Conducting clinical trials is a lengthy, time-consuming, and expensive process involving inherent uncertainties and risks, and our studies may be insufficient to demonstrate safety and efficacy to support FDA approval of any of our product candidates.
As a result of our substantial research and development expenditures and minimal product revenues, we have incurred cumulative losses of $340.3 million through December 31, 2010, and have not consistently generated enough funds through our operations to support our business. We expect to be close to break-even or have modest operating income over the next few years and, although we were profitable in the years ended December 31, 2009 and 2010, we may never achieve sustained profitability.
Ultimately, our ability to sustain profitability will depend upon a variety of factors, including regulatory approvals of our products, the timing of the introduction and market acceptance of our products and competing products, Eisai's success in selling Dacogen, the success of our joint development program with GSK, the launch of new products and our ability to control our ongoing costs and operating expenses. If our drug discovery and research efforts are not successful, or if the results from our clinical trials are not positive, we may not be able to get sufficient funding to continue our trials or conduct new trials, and we would be forced to scale down or cease our business operations. Moreover, if our products are not approved or commercially accepted we will remain unprofitable for longer than we currently anticipate. Additionally, we might be forced to substantially scale down our operations or sell certain of our assets, and it is likely the price of our stock would decline precipitously.
Critical Accounting Policies
Our management discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and reported disclosures. On an on-going basis, we evaluate our estimates, including those related to revenue and gain recognition, the valuation of investments, goodwill and deferred tax assets, and stock-based compensation. We base our estimates on historical experience and on various other assumptions that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Our significant accounting policies are more fully disclosed in Note 1 to our consolidated financial statements. However, some of our accounting policies are particularly important to the portrayal of our financial position and results of operations and require the application of significant judgment by our management. We believe the following critical accounting policies, among others, affect our more significant judgments and estimates used in the preparation of our consolidated financial statements.
Stock-Based Compensation
We account for stock-based compensation based upon the fair value estimated on the measurement date of our stock awards using the Black-Scholes option-pricing model based on assumptions for volatility, risk-free interest rates, expected life of the award, and dividends (if any).
35
Expected volatility is determined based on a blend of historical volatility and implied volatility of our common stock based on the period of time corresponding to the expected life of the stock options. The expected life of our stock options is based on our historical data and represents the period of time that stock options granted are expected to be outstanding. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant commensurate with the expected life assumption.
We are using the straight-line attribution method to recognize stock-based compensation expense. The amount of stock-based compensation recognized during a period is based on the value of the portion of the awards that are ultimately expected to vest, including awards that vest based on certain performance criteria. For the awards that vest based on certain performance criteria we estimate the probability that the awards will vest as well as the time period over which they are expected to vest and refine these estimates as necessary each reporting period. We estimate forfeitures at the time of grant and revise them, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term "forfeitures" is distinct from "cancellations" or "expirations" and represents only the unvested portion of the surrendered option. This analysis is re-evaluated annually and the forfeiture rate is adjusted as necessary. Ultimately, the actual expense recognized over the vesting period will only be for those shares that vest.
As of December 31, 2010, there was $3.2 million of total unrecognized compensation cost related to unvested stock-based awards. This cost is expected to be recognized over a weighted average period of 2.77 years.
Revenue and Gain Recognition
Eisai is required to pay us royalties starting at 20% and escalating to a maximum of 30% of net worldwide Dacogen sales within 45 days after the end of each calendar quarter. Because we do not have sufficient ability to accurately estimate Dacogen sales, we recognize royalty revenue when we receive the royalty statement from Eisai. In accordance with our license agreement with Eisai, we are entitled to receive 50% of any payments Eisai receives as a result of any sublicenses.
We received initial cash proceeds from the North American transaction with Hospira of $13.4 million. From the initial cash proceeds from the agreement, we deferred an amount for price protection exposure for a period of three years from November 1, 2007. This three year period ended in 2010, and the remaining balance of the deferred gain on sale of products of $50,000 was recognized as gain on sale of products during the year ended December 31, 2010.
Development and license revenue relates to the agreements we entered into with GSK in October 2009. In connection with the agreements, we received an upfront payment of $2 million, in addition to a $3 million equity investment by GSK at above-market price. As our substantive performance obligations under the agreements are estimated to be completed over a five year period, the $2 million upfront payment and the premium paid on the $3 million equity investment of $0.5 million are being recognized ratably over 60 months. We assess the substantive performance period on a quarterly basis and will change it if appropriate based upon our latest expectations.
Goodwill
As of December 31, 2010, we have intangible assets related to goodwill of $731,000. The determination of whether or not this asset is impaired involves judgment. We review goodwill for impairment whenever there is an indicator that the asset may have been impaired as indicated by our market capitalization or otherwise and at least annually. In considering our market capitalization, the ability of a controlling shareholder to benefit from synergies and other intangible assets that arise from control might cause the fair value of a company as a whole to exceed its market capitalization. Therefore, the fair value of a company need not be based solely on the quoted market price of an individual share of that company. Thus, consideration of the impact of a control premium when control
36
is known to exist in measuring the fair value of our Company may be appropriate. In addition, the Company determines its market capitalization using a point-in-time market price as of the date of its goodwill impairment assessment. However, the Company also considers recent trends in its stock price over a relatively short period. Given occasional stock price volatility, we do not believe that the enterprise value of our Company should be estimated solely based on anomalous stock price fluctuations on or around the goodwill impairment assessment date. Nonetheless, the Company would not simply ignore, but would consider, a recent decline in market capitalization in this assessment.
Impairment of Investments in Financial Instruments
Investments in financial instruments are carried at fair value based on quoted market prices, with unrealized gains and losses included in accumulated other comprehensive gain or loss in stockholders' equity. Our investment portfolio includes equity securities that could subject us to material equity market risk and corporate and U.S. government or U.S. governmental agency obligations that subject us to varying levels of credit risk. An other than temporary decline in fair value of a financial instrument may be subject to a write-down resulting in a charge against earnings. The determination of whether a decline in fair value is other than temporary requires significant judgment, and could have a material impact on our balance sheet and results of operations. Our management reviews the securities within our portfolio for other than temporary declines in value on a regular basis. As of December 31, 2010, the gross unrealized losses on available for sale investments was $24,000 (less than 0.1% of our portfolio value) and such losses were not attributed to changes in credit risk. The prices of some of our marketable equity securities are subject to considerable volatility. Currently we own 2.4 million shares of AVI and recorded an other-than-temporary decline in value of $3.1 million related to this investment during the year ended December 31, 2008. However, as of December 31, 2010, the gross unrealized gain on our investment in AVI was approximately $2.4 million. Decreases in the fair value of our securities may continue to significantly impact our results of operations.
Investments in equity securities without readily determinable fair value are carried at cost. We periodically review those carried costs, amounting to $500,000 as of December 31, 2010, and evaluate whether an impairment has occurred. The determination of whether an impairment has occurred requires significant judgment, as each investment has unique market and development opportunities.
Deferred Tax Assets
We had total deferred tax assets of $130 million and $136 million as of December 31, 2010 and 2009, respectively, which have been fully offset by a valuation allowance. We record a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. We consider future taxable income, ongoing tax planning strategies and our historical financial performance in assessing the need for a valuation allowance. If it were determined that we would be able to realize all or part of our deferred tax assets in the future, a decrease in the valuation allowance would increase income in the period in which such determination was made. Likewise, if we determine that we would not be able to realize all or part of our deferred tax assets in the future, an increase in the valuation allowance would be charged to income in the period in which such determination was made. We evaluate the ability to realize our deferred tax assets on a quarterly basis.
Recent Accounting Pronouncements
In October 2009, the FASB issued ASU No. 2009-13, which addresses the accounting for multiple-deliverable arrangements to potentially enable vendors to account for products or services separately rather than as a combined unit, and modifies the manner in which the transaction consideration is allocated across the separately identified deliverables. The ASU also significantly expands the disclosure requirements for multiple-deliverable revenue arrangements and may be adopted prospectively or retrospectively. The Company will adopt the ASU as of January 1, 2011 as required, and apply it
37
prospectively to arrangements entered into or materially modified after the adoption date. The Company does not expect the adoption of ASU No. 2009-13 to have any material effect on its financial statements upon its required adoption on January 1, 2011.
In March 2010, Accounting Standards Codification Topic 605, Revenue Recognition ("ASC 605") was amended to define a milestone and clarify that the milestone method of revenue recognition is a valid application of the proportional performance model when applied to research or development arrangements. Accordingly, a company can make an accounting policy election to recognize a payment that is contingent upon the achievement of a substantive milestone in its entirety in the period in which the milestone is achieved. This guidance is effective for the Company as of January 1, 2011 and may be applied on a prospective or retrospective basis. The Company will adopt this guidance prospectively and does not expect the adoption to have any effect on its financial statements upon its required adoption on January 1, 2011.
Results of Operations
Revenues (in thousands)
|
2010 | 2009 | 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Royalty revenue |
$ | 52,463 | $ | 41,156 | $ | 38,422 | |||||
Development and license revenue |
509 | 97 | — | ||||||||
Total revenues |
$ | 52,972 | $ | 41,253 | $ | 38,422 | |||||
The increases in royalty revenue are due to higher worldwide Dacogen product sales by Eisai. Eisai is required to pay us royalties starting at 20% and escalating to a maximum of 30% of net Dacogen sales within 45 days after the end of each calendar quarter. We recognize royalty revenue when we receive the royalty statements from Eisai because we do not have sufficient ability to accurately estimate Dacogen sales prior to that time. For example, the royalty revenues recorded in 2010 represent Eisai's Dacogen sales for the fourth quarter of 2009 and the first three quarters of 2010.
Development and license revenue relates to the agreements we entered into with GSK in October 2009. In connection with the agreements, we received an upfront payment of $2 million, in addition to a $3 million equity investment by GSK at above market price. As our substantive performance obligations under the agreements are estimated to be completed over a five year period, the $2 million upfront payment and the premium paid on the $3 million equity investment of approximately $500,000 are being recognized ratably over 60 months to development and license revenue.
Operating expenses (in thousands)
|
2010 | 2009 | 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Research and development |
$ | 28,394 | $ | 29,689 | $ | 32,685 | |||||
General and administrative |
9,442 | 8,994 | 11,119 | ||||||||
Acquired in-process research and development |
— | — | 5,185 | ||||||||
Gain on sale of products |
(750 | ) | (595 | ) | (2,236 | ) | |||||
Total operating expenses |
$ | 37,086 | $ | 38,088 | $ | 46,753 | |||||
The decrease in research and development expenses from 2009 to 2010 was primarily due to lower clinical and laboratory supply costs and outside consulting expenses relating to amuvatinib and SGI-1776. The decrease in research and development expenses from 2008 to 2009 was primarily due to lower contracted outside research and development services for several of our drug candidates and lower clinical trial costs related to our Phase I and Phase Ib clinical trials for amuvatinib.
38
We conduct research internally and through collaborations with third parties as we continue to maintain a strong commitment to our research and development efforts. Our research and development activities consist primarily of drug discovery, drug development, and pre-clinical and clinical development, as we advance our existing product candidates through clinical trials. Our research and development expenses consist primarily of salaries, employee benefits and other personnel related costs; stock-based compensation expense; laboratory equipment and supplies; third-party consultant fees and contract labor; costs for pre-clinical and clinical trials, including clinical research organizations; other outsourced research; depreciation expense; corporate overhead; and allocated facility costs.
We do not allocate certain of our internal research and development costs such as salaries and other personnel related costs, corporate overhead and facility costs to our development programs on a project-by-project basis. These costs are incurred across and contribute to many discovery, research and development programs including a broad range of scientific research projects, many of which fail in the early stages of discovery and development. We do allocate direct salaries and third party costs to certain major development programs that are generally in later stages of pre-clinical or clinical development. Our scientists record their time to such specific projects when appropriate; however, many activities simultaneously benefit multiple projects and cannot be readily attributed to a specific program. Accordingly, the accurate assignment of time and costs to a specific project is difficult and does not give a true indication of the actual costs of a particular project on a fully burdened basis. Below is a summary of direct salaries and third party costs that are identifiable for our major drug programs:
| Third Party Costs and Direct Salaries Identifiable by Project (in thousands) | 2010 | 2009 | 2008 | Cumulative To Date |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Amuvatinib |
$ | 2,821 | $ | 4,601 | $ | 6,786 | $ | 18,860 | |||||
SGI-1776 |
2,277 | 3,243 | 4,094 | 9,980 | |||||||||
SGI-110 |
2,566 | 2,088 | 1,688 | 6,513 | |||||||||
All other projects |
4,383 | 3,216 | 3,530 | 20,683 | |||||||||
Conducting clinical trials is a lengthy, expensive and uncertain process. Completion of clinical trials may take several years or more. The length of time generally varies substantially according to the type, complexity, novelty and intended use of the product candidate. Our clinical trials may be suspended at any time if we or the FDA believe the patients participating in our studies are exposed to unacceptable health risks. We may encounter problems in our studies which will cause us or the FDA to delay or suspend the studies. Because of these uncertainties, we cannot predict when or whether we will successfully complete the development of our product candidates or the ultimate product development cost for any of our product candidates.
The increase in general and administrative expenses from 2009 to 2010 relates primarily to higher costs for accounting and tax consultation fees, additional mailing and consulting costs relating to our annual stockholder meeting and related proxy solicitation, and additional investor relations and business development consulting expenses, offset in part by lower stock-based compensation expenses. The decrease in general and administrative expenses from 2008 to 2009 relates primarily to lower stock-based compensation and legal expenses, as well as the elimination of administrative costs related to our European operations, which were liquidated in October 2008.
Acquired in-process research and development expenses relate to our acquisition of Montigen Pharmaceuticals in 2006. In November 2008, a second Montigen compound received clearance from the FDA to begin Phase I clinical trials, triggering a milestone payment of $5,185,000, which was paid through a combination of cash and the issuance of common stock and recorded as acquired in-process research and development expense in the year ended December 31, 2008. We had no similar transactions in 2009 or 2010.
39
Gain on sale of products relates to the sale of our North American and worldwide rights to Nipent to Hospira. During 2008 we received a $1 million indemnification holdback relating to the sale of the North American Nipent rights, and a $250,000 indemnification holdback and $400,000 annual payment relating to the sale of the remaining worldwide Nipent rights. In addition, in 2008 we reduced our price protection reserve by $426,000 and reversed $160,000 of residual products return reserve for Nipent as the reserve was no longer required due to the expiration of the contractual return period. The sum of these 2008 transactions, $2,236,000, was recorded as a gain on sale of products during the year ended December 31. 2008. Gain on sale of products for the year ended December 31, 2009 represented the receipt of a $500,000 annual payment from Hospira relating to the sale of the worldwide rights for Nipent, a $75,000 reduction of our price protection reserve, and $20,000 relating to the reversal of a residual products return reserve that was no longer required due to the expiration of the contractual return period. Gain on sale of products for the year ended December 31, 2010 represented the receipt of a $700,000 annual payment from Hospira relating to the sale of the worldwide rights for Nipent and a $50,000 reduction, representing the entire remaining balance, of our price protection reserve as the price protection period expired in 2010. We expect to receive additional $700,000 annual payments in both 2011 and 2012, representing the final two payments due under the sale of the worldwide rights for Nipent.
| Other income (expense) and income tax benefit (provision) (in thousands) | 2010 | 2009 | 2008 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Interest income |
$ | 182 | $ | 686 | $ | 2,193 | ||||
Other than temporary decline in value of investments |
— | — | (3,055 | ) | ||||||
Other income |
244 | — | 34 | |||||||
Income tax benefit (provision) |
(39 | ) | 886 | 48 | ||||||
The declines in interest income have been due primarily to significant declines in interest rates.
During the year ended December 31, 2008, we recorded a write-downs of $3,055,000 related to other than temporary declines in the values of two of our equity investments, primarily related to our AVI stock holdings. We had no similar write-downs in 2009 or 2010.
Other income in the year ended December 31, 2010 represents receipt of proceeds under the Qualifying Therapeutic Discovery Project program relating to our qualifying research and development programs. Other income in 2008 represented transaction gains on foreign currency exchange relating to activities of our subsidiary EuroGen, which was liquidated as of October 1, 2008.
In 2010, we recorded a tax provision of $39,000, which represented state tax provisions, taking into account estimated net operating loss carrybacks and research and development tax credits. In 2009, we recorded a tax benefit of $886,000, which was primarily due to the Worker, Home Ownership and Business Assistance Act of 2009, signed into law on November 6, 2009, that allowed for certain net operating losses to be used to eliminate or refund alternative minimum tax, as well as monetization of research credits and other state tax benefits. In 2008, we recorded a tax benefit of $48,000 due to estimated refundable research and development tax credits under a 2008 Housing Rescue bill.
Liquidity and Capital Resources
Our cash, cash equivalents, and current and non-current marketable securities totaled $120.4 million at December 31, 2010, compared to $100.8 million at December 31, 2009.
Net cash provided by operating activities was $18.0 million in 2010 and consisted primarily of the net income of $16.3 million plus non-cash depreciation of $1.3 million, and non-cash stock based compensation expense of $1.4 million. Net cash provided by operating activities was $8.7 million in 2009, and consisted primarily of the net income of $4.7 million plus non-cash depreciation of $1.2 million, non-cash stock based compensation expense of $2.5 million, and an increase in deferred revenue from entering into the GSK agreements in 2009 of $2.4 million, offset in part by an $818,000
40
increase in income tax receivable and a $1.1 million decrease in accounts payable and other liabilities. Net cash used in operating activities was $1.7 million in 2008, and consisted primarily of the net loss of $9.1 million and recognition of gain on sale of products of $1.6 million, offset in part by non-cash depreciation and amortization of $1.6 million, other than temporary declines in the value of investments of $3.1 million, non-cash stock based compensation expense of $2.8 million, and non-cash acquired in-process research and development expenses of $2.4 million.
Net cash used in investing activities was $0.5 million in 2010 and consisted primarily of $169.1 million for purchases of marketable securities and $1.0 million for purchases of property and equipment, offset in part by $169.0 million in maturities of marketable securities. Net cash used in investing activities was $52.6 million in 2009, and consisted primarily of $133.3 million for the purchase of marketable securities and $1 million for purchases of property and equipment, offset in part by $81.2 million in maturities of marketable securities. Net cash used in investing activities was $27.6 million in 2008, and consisted primarily of purchases of marketable securities of $46 million and property and equipment of $1.1 million, offset in part by proceeds from maturities of marketable securities of $17.9 million and proceeds from the sale of products of $1.6 million.
Net cash provided by financing activities was $336,000 in 2010 and related to proceeds from the issuance of common stock upon exercise of stock options and issuances under our employee stock purchase plan. Net cash provided by financing activities was $2.7 million in 2009, and related primarily to proceeds from the issuance of common stock to GSK, as well as proceeds from the issuance of common stock upon exercise of stock options and issuances under our employee stock purchase plan. Net cash provided by financing activities was $142,000 in 2008, and consisted of proceeds from the issuance of common stock upon exercise of stock options and warrants as well as issuances under our employee stock purchase plan.
Our contractual obligations as of December 31, 2010 are as follows (in thousands):
| |
Payments Due by Period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Total | < 1 year | 1-3 years | 4-5 years | After 5 years | |||||||||||
Operating leases |
$ | 3,691 | $ | 935 | $ | 2,146 | $ | 610 | $ | — | ||||||
Total contractual cash obligations |
$ | 3,691 | $ | 935 | $ | 2,146 | $ | 610 | $ | — | ||||||
Our principal administrative facility is currently located in leased general office space, containing approximately 37,000 square feet, in Dublin, California, under a lease that expires in November 2015. Our drug formulation laboratory operations are located in a 10,000 square foot industrial building that we own in Pleasanton, California. We are currently leasing 11,700 square feet of space for our drug discovery laboratory operations in Salt Lake City, Utah. The lease on this space expires in May 2012.
Avicine will require significant additional expenditures to complete the clinical development necessary to gain marketing approval from the FDA and equivalent foreign regulatory agencies. As part of our agreement with AVI BioPharma, we are obligated to make additional payments to AVI based on successful achievement of developmental, regulatory approval, and commercialization milestones over the next several years that could total $80 million. However, no significant development efforts have been incurred for Avicine since 2003 and none are anticipated in the near future. We are unable to determine precisely when and if our payment obligations under our agreement with AVI will become due as these obligations are based on milestone events, the achievement of which is subject to a significant number of risks and uncertainties. Because some of the milestone events are revenue-related and the payment obligation would not be triggered absent our receipt of revenues, we may be able to use funds generated from revenues to make the milestone payments if they become due. There is a $6.8 million remaining future contingent regulatory milestone payment due to the former Montigen
41
stockholders when and if the related milestone is achieved, which is payable in shares of our common stock.
We have financed our operations primarily through the issuance of equity and debt securities, the receipt of milestone, royalty and other payments in connection with collaborative agreements, and the sale of non-core assets. Based on our current forecasted product development activities, we believe that our current cash, cash equivalents, marketable securities and other investments will satisfy our cash requirements through at least December 31, 2012. We may pursue additional financing options, including the selling of additional shares of stock in public or private offerings.
We believe that our need for additional funding will increase in the future, especially if we acquire new product technologies for development and sale, and our ability to continue raising funds from external sources will be critical to our success. We continue to actively consider future contractual arrangements that would require significant financial commitments. If we experience currently unanticipated cash requirements, we could require additional capital much sooner than presently anticipated. We may raise money by the sale of our equity securities or debt, or the exercise of outstanding stock options by the holders of such options. However, given uncertain market conditions and the volatility of our stock price, we may not be able to sell our securities in public offerings or private placements at prices and on terms that are favorable to us, if at all. We may also choose to obtain funding through licensing and other contractual agreements. Such arrangements may require us to relinquish our rights to our technologies, products or marketing territories, or to grant licenses on terms that are not favorable to us. If we fail to obtain adequate funding in a timely manner, or at all, we will be forced to scale back our product development activities or our operations in a manner that will ensure we can discharge our obligations as they come due in the ordinary course of business.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that are currently material or reasonably likely to be material to our consolidated financial position or results of operations.
Liquidation of EuroGen Pharmaceuticals
We established our European subsidiary, EuroGen Pharmaceuticals, Ltd. in 2001 to expand our commercial presence in Europe. Since that time, we changed our strategic focus due to the sublicense of Dacogen to Eisai in 2004 and the sale of Nipent to Hospira in 2006 and 2007. As a result, in May 2008, we decided to discontinue our European operations and liquidate EuroGen. The liquidation was substantially completed by October 1, 2008. During the year ended December 31, 2008, we recorded total severance costs of $420,000, which were recorded in general and administrative expenses. We do not expect to incur any further severance costs related to this liquidation.
Income Taxes
As of December 31, 2010, we have net operating loss carryforwards for federal income tax purposes of approximately $248 million which expire in the years 2019 through 2028, net operating loss carryforwards for state income tax purposes of approximately $136 million which expire in the years 2011 through 2029, federal research and development credit carryforwards of approximately $12 million, which expire in the years 2011 through 2030, and state research and development carryforwards of approximately $10 million, which have no expiration. The realization of these future tax benefits is dependent upon our ability to generate sufficient taxable income within the carryforward period. Because of our history of operating losses, we believe that these benefits are not currently likely to be realized, and, accordingly, the net deferred tax assets have been fully offset by a valuation allowance.
42
Utilization of our net operating loss carryforwards may be subject to substantial annual limitations due to ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such an annual limitation could result in the expiration of the net operating losses before utilization.
We have no unrecognized tax benefits as of December 31, 2010. We do not anticipate that total unrecognized tax benefits will significantly change prior to December 31, 2011. No interest and penalties expenses were recognized in the statements of operations for the years ended December 31, 2008, 2009, and 2010. We are subject to income tax examinations for U.S. Federal income taxes and state income taxes from 1995 forward due to net operating losses in tax years 1995 through 2009. We are subject to tax examinations in the United Kingdom from 2001 through 2008.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Interest Rate Risk
Due to the short-term nature of our interest bearing assets, which consist primarily of certificates of deposit, United States corporate obligations, and United States government obligations, we believe that our exposure to interest rate risk would not significantly affect our operations.
Our investment policy is to manage our marketable securities portfolio to preserve principal and liquidity while maximizing the return on the investment portfolio. Our marketable securities portfolio is primarily invested in corporate debt securities and debt securities issued by U.S. government agencies with an average maturity of less than one year and a minimum investment grade rating of A, A-1 or better to minimize credit risk. Although changes in interest rates may affect the fair value of the marketable securities portfolio and cause unrealized gains or losses, such gains or losses would not be realized unless the investments were to be sold prior to maturity.
Equity Securities Market Price Risk
As of December 31, 2010 and 2009, we owned 2.4 million shares of AVI common stock that is traded on the NASDAQ exchange. The fair market value of this investment as of December 31, 2010 is $5.1 million. We recorded an other-than-temporary decline in value of $3.1 million related to this investment during the year ended December 31, 2008. Decreases in the market price of the AVI stock may generate additional impairment charges in the future. Increases in the market price of the AVI stock will only generate gains in our statement of operations if the stock can be sold above its written-down cost per share of $1.12.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
All information required by this item is included on pages F-1 to F-21 in Item 15 of Part IV of this Report and is incorporated into this item by reference.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures.
We carried out an evaluation, under the supervision of and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e)
43
under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this report. Based on the evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that as of December 31, 2010, our disclosure controls and procedures were effective.
Management's Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining an adequate internal control structure and procedures for financial reporting as defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Our internal control over financial reporting is a process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that:
- •
- pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and
dispositions of assets;
- •
- provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in
accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and our board of directors; and
- •
- provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision of and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, our management has assessed the effectiveness of internal control over financial reporting as of December 31, 2010. Our assessment was based on criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO") Internal Control-Integrated Framework. Based on using the COSO criteria, we believe our internal control over financial reporting as of December 31, 2010 was effective.
Ernst & Young LLP, our independent registered public accounting firm, has issued an attestation report on the Company's internal control over financial reporting as of December 31, 2010 as included below.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the fourth quarter of 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
44
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The
Board of Directors and Stockholders
of SuperGen, Inc.
We have audited SuperGen, Inc.'s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). SuperGen, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, SuperGen, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of SuperGen, Inc. as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2010 of SuperGen, Inc. and our report dated March 9, 2011 expressed an unqualified opinion thereon.
| /s/ ERNST & YOUNG LLP | ||
| Palo Alto, California March 9, 2011 |
45
None.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Information regarding our Board of Directors is incorporated by reference to the section entitled "Election of Directors" appearing in our definitive Proxy Statement for the 2011 Annual Meeting of Stockholders to be filed with the Commission within 120 days after the end of our fiscal year pursuant to Regulation 14A (the "Proxy Statement").
Audit Committee
Information regarding the Audit Committee is incorporated by reference to the Proxy Statement.
Audit Committee Financial Expert
Information regarding the financial expert(s) on the Audit Committee is incorporated by reference to the Proxy Statement.
Code of Ethics
Information regarding the Code of Ethics is incorporated by reference to the Proxy Statement.
Corporate Governance
Information regarding Corporate Governance is incorporated by reference to the Proxy Statement.
Section 16(a) Beneficial Ownership Reporting Compliance
Information regarding Section 16(a) beneficial ownership reporting compliance is set forth under "Voting Securities of Principal Stockholders and Management" in the Proxy Statement, which information is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION.
Information regarding executive compensation is incorporated by reference to the information set forth under the caption "Compensation of Directors and Executive Officers," including Compensation Committee Interlocks and Insider Participation, in the Proxy Statement. The information included under the heading "Compensation Committee Report" in the Proxy Statement is incorporated herein by reference; however, this information shall not be deemed to be "soliciting material" or to be "filed" with the Commission or subject to Regulation 14A or 14C, or to the liabilities of Section 18 of the Exchange Act.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Information regarding security ownership of certain beneficial owners and management is incorporated by reference to the information set forth under the caption "Voting Securities of Principal Stockholders and Management" in the Proxy Statement. Information regarding our Equity Compensation Plans is incorporated by reference to the Proxy Statement.
46
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE.
Information regarding certain relationships and related transactions is incorporated by reference to the information set forth under the caption "Certain Transactions" in the Proxy Statement. Certain of our relationships and related transactions are addressed in the "Management's Discussion and Analysis of Financial Condition and Results of Operations" section of this report. The information regarding director independence is incorporated herein by reference to the Proxy Statement.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
Information regarding principal auditor fees and services is set forth under "Principal Accounting Fees and Services" in the Proxy Statement, which information is incorporated herein by reference.
47
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
- (a)
- The
following documents are filed as part of this report:
- 1.
- All Financial Statements:
The following financial statements and report of Ernst & Young LLP, Independent Registered Public Accounting Firm, are included in Part IV of this Report on the pages indicated:
- 2.
- Financial Statement Schedules:
All schedules are omitted because they are not applicable or the required information is shown in the consolidated financial statements or the notes thereto.
- 3.
- Exhibits:
| Exhibit Number |
Description of Document | |
|---|---|---|
| (d)3.1 | Amended and Restated Certificate of Incorporation of the Registrant. | |
| (l)3.2 | Bylaws of the Registrant, as amended and restated through September 11, 2008. | |
| (g)4.1 | Specimen Common Stock Certificate. | |
| (f)10.1 | Form of Indemnification Agreement between the Registrant and each of its directors and officers. | |
| (**)(q)10.2 | 2003 Stock Plan, as amended effective March 11, 2010. | |
| (**)(i)10.3 | 2008 Employee Stock Purchase Plan. | |
| (**)(b)10.4 | Executive Employment and Confidential Information and Invention Assignment Agreement effective October 1, 2010 between Registrant and James S.J. Manuso. | |
| (**)(c)10.5 | Severance Benefit Plan for Officers. | |
| (d)10.6 | Office Building Lease dated June 23, 2000 between the Registrant and Koll Dublin Corporate Center, L.P. | |
| (p)10.7 | First Amendment to Lease between SuperGen, Inc. and Dublin Corporate Center Two, L.P. made as of August 2, 2010. | |
| (k)10.8 | Common Stock and Warrant Purchase Agreement dated April 4, 2000 between the Registrant and AVI BioPharma, Inc. | |
| (k)10.9 | United States of America Sales, Distribution, and Development Agreement dated April 4, 2000 between the Registrant and AVI BioPharma, Inc. | |
| (j)10.10 | Registration Rights Agreement dated April 4, 2000 between the registrant and AVI BioPharma, Inc. | |
| (*)(h)10.11 | Amended and Restated License Agreement effective September 21, 2004 between the Registrant and MGI PHARMA, Inc. | |
| (h)10.12 | Common Stock Purchase Agreement dated August 31, 2004 between the Registrant and MGI PHARMA, Inc. | |
| (h)10.13 | Investor Rights Agreement dated August 31, 2004 between the Registrant and MGI PHARMA, Inc. |
48
| Exhibit Number |
Description of Document | |
|---|---|---|
| (n)10.14 | Amended and Restated Agreement and Plan of Merger and Reorganization, dated March 30, 2006, by and among SuperGen, Inc., King's Peak Acquisition Corporation, Montigen Pharmaceuticals, Inc., James Clarke, as Stockholder Representative and U.S. Bank National Association, as Escrow Agent. | |
| (o)10.15 | Asset Acquisition Agreement, dated June 21, 2006, between SuperGen, Inc. and Mayne Pharma (USA), Inc. | |
| (m)10.16 | Asset Acquisition Agreement Amendment dated August 22, 2006 between SuperGen, Inc. and Mayne Pharma (USA), Inc. | |
| (a)10.17 | Asset Acquisition Agreement, dated November 25, 2006, between SuperGen, Inc. and Mayne Pharma plc. | |
| (*)(e)10.18 | Amended and Restated Commercial Research and License Agreement dated November 6, 2009 between SuperGen, Inc. and GlaxoSmithKline. | |
| (e)10.19 | Common Stock Purchase Agreement dated October 22, 2009 between SuperGen, Inc. and GlaxoSmithKline. | |
| 23.1 | Consent of Independent Registered Public Accounting Firm. | |
| 31.1 | Certification of Chief Executive Officer under Section 302(a) of the Sarbanes-Oxley Act of 2002. | |
| 31.2 | Certification of Chief Financial Officer under Section 302(a) of the Sarbanes-Oxley Act of 2002. | |
| 32.1 | Certifications of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
- (*)
- Confidential
treatment has been previously granted for certain portions of these exhibits.
- (**)
- Indicates
a management contract or compensatory plan or arrangement.
- (a)
- Incorporated
by reference from the Registrant's Report on Form 8-K dated November 25, 2006 filed with the Securities and Exchange
Commission on November 28, 2006.
- (b)
- Incorporated
by reference from the Registrant's Report on Form 8-K dated October 1, 2010 filed with the Securities and Exchange
Commission on October 4, 2010.
- (c)
- Incorporated
by reference from the Registrant's Report on Form 8-K dated October 28, 2008 filed with the Securities and Exchange
Commission on October 31, 2008.
- (d)
- Incorporated
by reference from the Registrant's Report on Form 10-Q filed with the Securities and Exchange Commission on
August 11, 2000.
- (e)
- Incorporated
by reference form the Registrant's Report on Form 10-Q filed with the Securities and Exchange Commission on
November 9, 2009.
- (f)
- Incorporated
by reference from the Registrant's Report on Form 10-K filed with the Securities and Exchange Commission on March 31,
2003.
- (g)
- Incorporated
by reference from the Registrant's Report on Form 10-K filed with the Securities and Exchange Commission on March 19,
1998.
- (h)
- Incorporated
by reference from the Registrant's Report on Form 10-Q filed with the Securities and Exchange Commission on
November 9, 2004.
- (i)
- Incorporated by reference from the Registrant's Registration Statement on Form S-8 (Reg. No. 333-152811) filed with the Securities and Exchange Commission on August 6, 2008.
49
- (j)
- Incorporated
by reference from the Registrant's Registration Statement on Form S-3 (Reg. No. 333-52326) filed with the
Securities and Exchange Commission on December 20, 2000.
- (k)
- Incorporated
by reference from the Registrant's Report on Form 10-K filed with the Securities and Exchange Commission on March 23,
2001.
- (l)
- Incorporated
by reference from the Registrant's Report on Form 8-K dated September 11, 2008 filed with the Securities and Exchange
Commission on September 16, 2008.
- (m)
- Incorporated
by reference from the Registrant's Report on Form 8-K dated August 22, 2006 filed with the Securities and Exchange
Commission on August 28, 2006.
- (n)
- Incorporated
by reference from the Registrant's Report on Form 8-K dated April 4, 2006 filed with the Securities and Exchange
Commission on April 7, 2006.
- (o)
- Incorporated
by reference from the Registrant's Report on Form 8-K dated June 21, 2006 filed with the Securities and Exchange
Commission on June 27, 2006.
- (p)
- Incorporated
by reference from the Registrant's Report on Form 8-K dated August 2, 2010 filed with the Securities and Exchange
Commission on August 6, 2010.
- (q)
- Incorporated
by reference from the Registrant's Registration Statement on Form S-8 (Reg. No. 333-169473) filed with
the Securities and Exchange Commission on September 17, 2010.
- (b)
- Exhibits. See Item 15(a) above.
- (c)
- Financial Statement Schedules. See Item 15(a) above.
50
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of SuperGen, Inc.
We have audited the accompanying consolidated balance sheets of SuperGen, Inc. as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of SuperGen, Inc. at December 31, 2010 and 2009, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), SuperGen, Inc.'s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 9, 2011 expressed an unqualified opinion thereon.
| /s/ ERNST & YOUNG LLP | ||
| Palo Alto, California March 9, 2011 |
F-1
SUPERGEN, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
| |
December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||||
ASSETS |
|||||||||
Current assets: |
|||||||||
Cash and cash equivalents |
$ | 25,554 | $ | 7,682 | |||||
Marketable securities |
89,699 | 89,515 | |||||||
Income tax receivable |
40 | 904 | |||||||
Prepaid expenses and other current assets |
1,330 | 1,150 | |||||||
Total current assets |
116,623 | 99,251 | |||||||
Marketable securities, non-current |
5,124 | 3,570 | |||||||
Property, plant and equipment, net |
3,932 | 4,205 | |||||||
Goodwill |
731 | 731 | |||||||
Restricted cash |
2,134 | 2,255 | |||||||
Other assets |
554 | 505 | |||||||
Total assets |
$ | 129,098 | $ | 110,517 | |||||
LIABILITIES AND STOCKHOLDERS' EQUITY |
|||||||||
Current liabilities: |
|||||||||
Accounts payable |
$ | 1,198 | $ | 2,011 | |||||
Accrued compensation |
3,556 | 2,861 | |||||||
Other accrued liabilities |
208 | 234 | |||||||
Payable to AVI BioPharma |
565 | 565 | |||||||
Deferred gain on sale of products to Hospira, Inc. |
— | 50 | |||||||
Deferred revenue |
509 | 509 | |||||||
Deferred rent |
12 | 343 | |||||||
Total current liabilities |
6,048 | 6,573 | |||||||
Deferred rent, non-current |
9 | 19 | |||||||
Deferred revenue, non-current |
1,429 | 1,939 | |||||||
Total liabilities |
7,486 | 8,531 | |||||||
Commitments and contingencies |
|||||||||
Stockholders' equity: |
|||||||||
Preferred stock, $.001 par value; 2,000,000 shares authorized; none outstanding |
— | — | |||||||
Common stock, $.001 par value; 150,000,000 shares authorized; 60,357,593 and 60,198,707 shares issued and outstanding at December 31, 2010 and December 31, 2009, respectively |
60 | 60 | |||||||
Additional paid in capital |
459,482 | 457,714 | |||||||
Accumulated other comprehensive income |
2,382 | 797 | |||||||
Accumulated deficit |
(340,312 | ) | (356,585 | ) | |||||
Total stockholders' equity |
121,612 | 101,986 | |||||||
Total liabilities and stockholders' equity |
$ | 129,098 | $ | 110,517 | |||||
See accompanying notes to consolidated financial statements
F-2
SUPERGEN, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| |
Year ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||||
Revenues: |
||||||||||||
Royalty revenue |
$ | 52,463 | $ | 41,156 | $ | 38,422 | ||||||
Development and license revenue |
509 | 97 | — | |||||||||
Total revenues |
52,972 | 41,253 | 38,422 | |||||||||
Operating expenses: |
||||||||||||
Research and development |
28,394 | 29,689 | 32,685 | |||||||||
General and administrative |
9,442 | 8,994 | 11,119 | |||||||||
Acquired in-process research and development |
— | — | 5,185 | |||||||||
Gain on sale of products |
(750 | ) | (595 | ) | (2,236 | ) | ||||||
Total operating expenses |
37,086 | 38,088 | 46,753 | |||||||||
Income (loss) from operations |
15,886 | 3,165 | (8,331 | ) | ||||||||
Interest income |
182 | 686 | 2,193 | |||||||||
Other than temporary decline in value of investments |
— | — | (3,055 | ) | ||||||||
Other income |
244 | — | 34 | |||||||||
Income (loss) before income tax benefit (provision) |
16,312 | 3,851 | (9,159 | ) | ||||||||
Income tax benefit (provision) |
(39 | ) | 886 | 48 | ||||||||
Net income (loss) |
$ | 16,273 | $ | 4,737 | $ | (9,111 | ) | |||||
Net income (loss) per common share: |
||||||||||||
Basic |
$ | 0.27 | $ | 0.08 | $ | (0.16 | ) | |||||
Diluted |
$ | 0.27 | $ | 0.08 | $ | (0.16 | ) | |||||
Weighted average shares outstanding: |
||||||||||||
Basic |
60,287 | 59,316 | 57,721 | |||||||||
Diluted |
60,635 | 59,340 | 57,721 | |||||||||
See accompanying notes to consolidated financial statements
F-3
SUPERGEN, INC.
CONSOLIDATED STATEMENT OF STOCKHOLDERS' EQUITY
(In thousands)
| |
Common Stock | |
Accumulated Other Comprehensive Income (Loss) |
|
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Additional Paid in Capital |
Accumulated Deficit |
|
||||||||||||||||||
| |
Shares | Amount | Total | ||||||||||||||||||
Balances at January 1, 2008 |
57,519 | $ | 58 | $ | 447,151 | $ | (2,343 | ) | $ | (352,211 | ) | $ | 92,655 | ||||||||
Comprehensive loss: |
|||||||||||||||||||||
Net loss |
— | — | — | — | (9,111 | ) | (9,111 | ) | |||||||||||||
Other than temporary decline in value of investments |
— | — | — | 3,055 | — | 3,055 | |||||||||||||||
Other comprehensive income (loss)—Change in unrealized gain (loss) on investments |
— | — | — | (1,482 | ) | — | (1,482 | ) | |||||||||||||
Comprehensive loss |
(7,538 | ) | |||||||||||||||||||
Issuance of common stock for milestone payment |
1,481 | 1 | 2,414 | — | — | 2,415 | |||||||||||||||
Issuance of common stock upon exercise of stock options |
6 | — | 10 | — | — | 10 | |||||||||||||||
Issuance of common stock in connection with employee stock purchase plan |
76 | — | 132 | — | — | 132 | |||||||||||||||
Compensation expense from stock option grants |
— | — | 2,817 | — | — | 2,817 | |||||||||||||||
Balances at December 31, 2008 |
59,082 | 59 | 452,524 | (770 | ) | (361,322 | ) | 90,491 | |||||||||||||
Comprehensive income: |
|||||||||||||||||||||
Net income |
— | — | — | — | 4,737 | 4,737 | |||||||||||||||
Other comprehensive income (loss)—Change in unrealized gain (loss) on investments |
— | — | — | 1,567 | — | 1,567 | |||||||||||||||
Comprehensive income |
6,304 | ||||||||||||||||||||
Issuance of common stock to GSK |
990 | 1 | 2,454 | — | — | 2,455 | |||||||||||||||
Issuance of common stock upon exercise of stock options |
60 | — | 128 | — | — | 128 | |||||||||||||||
Issuance of common stock in connection with employee stock purchase plan |
67 | — | 101 | — | — | 101 | |||||||||||||||
Compensation expense from stock option grants |
— | — | 2,507 | — | — | 2,507 | |||||||||||||||
Balances at December 31, 2009 |
60,199 | 60 | 457,714 | 797 | (356,585 | ) | 101,986 | ||||||||||||||
Comprehensive income: |
|||||||||||||||||||||
Net income |
— | — | — | — | 16,273 | 16,273 | |||||||||||||||
Other comprehensive income (loss)—Change in unrealized gain (loss) on investments |
— | — | — | 1,585 | — | 1,585 | |||||||||||||||
Comprehensive income |
17,858 | ||||||||||||||||||||
Issuance of common stock upon exercise of stock options |
92 | — | 182 | — | — | 182 | |||||||||||||||
Issuance of common stock in connection with employee stock purchase plan |
67 | — | 154 | — | — | 154 | |||||||||||||||
Compensation expense from stock option grants |
— | — | 1,432 | — | — | 1,432 | |||||||||||||||
Balances at December 31, 2010 |
60,358 | $ | 60 | $ | 459,482 | $ | 2,382 | $ | (340,312 | ) | $ | 121,612 | |||||||||
See accompanying notes to consolidated financial statements
F-4
SUPERGEN, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| |
Year ended December 31, | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||||
Operating activities: |
|||||||||||||
Net income (loss) |
$ | 16,273 | $ | 4,737 | $ | (9,111 | ) | ||||||
Adjustments to reconcile net income (loss) to net cash provided by operating activities: |
|||||||||||||
Depreciation |
1,280 | 1,221 | 1,134 | ||||||||||
Amortization of intangibles |
— | 106 | 426 | ||||||||||
Other than temporary decline in value of investments |
— | — | 3,055 | ||||||||||
Stock-based compensation expense |
1,432 | 2,507 | 2,817 | ||||||||||
Recognition of gain on sale of products |
(700 | ) | (500 | ) | (1,650 | ) | |||||||
Acquired in-process research and development |
— | — | 2,415 | ||||||||||
Changes in operating assets and liabilities: |
|||||||||||||
Accounts receivable |
— | — | 129 | ||||||||||
Income tax receivable |
864 | (818 | ) | (86 | ) | ||||||||
Prepaid expenses and other assets |
(229 | ) | 71 | (490 | ) | ||||||||
Restricted cash |
121 | 112 | 169 | ||||||||||
Accounts payable and other liabilities |
(485 | ) | (1,116 | ) | (44 | ) | |||||||
Deferred gain on sale of products |
(50 | ) | (75 | ) | (426 | ) | |||||||
Deferred revenue |
(510 | ) | 2,448 | — | |||||||||
Net cash provided by (used in) operating activities |
17,996 | 8,693 | (1,662 | ) | |||||||||
Investing activities: |
|||||||||||||
Purchases of marketable securities |
(169,107 | ) | (133,310 | ) | (45,958 | ) | |||||||
Sales and maturities of marketable securities |
168,954 | 81,196 | 17,866 | ||||||||||
Purchases of property and equipment |
(1,007 | ) | (989 | ) | (1,136 | ) | |||||||
Proceeds from sale of products |
700 | 500 | 1,601 | ||||||||||
Net cash used in investing activities |
(460 | ) | (52,603 | ) | (27,627 | ) | |||||||
Financing activities: |
|||||||||||||
Proceeds from issuances of common stock |
336 | 2,684 | 142 | ||||||||||
Net cash provided by financing activities |
336 | 2,684 | 142 | ||||||||||
Net increase (decrease) in cash and cash equivalents |
17,872 | (41,226 | ) | (29,147 | ) | ||||||||
Cash and cash equivalents at beginning of period |
7,682 | 48,908 | 78,055 | ||||||||||
Cash and cash equivalents at end of period |
$ | 25,554 | $ | 7,682 | $ | 48,908 | |||||||
Supplemental Disclosure of Non-Cash Financing Activities: |
|||||||||||||
Common stock issued in connection with Montigen acquisition |
$ | — | $ | — | $ | 2,415 | |||||||
Supplemental Disclosure of Cash Flow Information: |
|||||||||||||
Income taxes paid in cash during the year |
$ | 4 | $ | — | $ | 118 | |||||||
See accompanying notes to consolidated financial statements
F-5
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Summary of Significant Accounting Policies
Description of Business
SuperGen, Inc. ("SuperGen," "we," "us" or the "Company") was incorporated in California in March 1991. We changed our state of incorporation to Delaware in 1997. We are a pharmaceutical company dedicated to the discovery and development of therapies to treat patients with cancer. We operate in one reportable segment—human therapeutics. All of our operations are located in the U.S.
Principles of Consolidation
Our consolidated financial statements include the accounts of SuperGen, Inc. and its wholly-owned subsidiaries. Intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires us to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results may differ from those estimates.
Revenue Recognition
We recognize royalty revenue when we receive the related royalty statement because we do not have sufficient ability to accurately estimate the underlying sales prior to that time. We recognize milestone fees upon completion of specified substantive at-risk milestones according to the related contract terms.
We enter into revenue arrangements with multiple deliverables, such as intellectual property rights and research and development services. For these arrangements, we generally have not met the criteria to separate the deliverables for revenue recognition purposes and we have treated the deliverables as a combined unit of accounting. As such, non-refundable up-front payments received in connection with research and license agreements have been deferred and recognized on a straight-line basis over the relevant estimated periods of continuing involvement, generally the research term. We re-evaluate the period of continuing involvement each reporting period and adjust our estimates accordingly. Advance payments in excess of amounts earned are classified as deferred revenue until earned.
Other Income
Other income of $244,000 recorded in the year ended December 31, 2010 represents receipt of proceeds under the Qualifying Therapeutic Discovery Project program relating to our qualifying research and development programs.
Research and Development
Research and development expenditures, including direct and allocated expenses, are charged to expense as incurred. These expenditures include salaries and employee-related expenses; fees paid to physicians, hospitals, or other research institutions for clinical and non-clinical studies; fees paid to outside contractors for monitoring of clinical sites or collection and analysis of data; costs associated with the research and manufacture of clinical drug supplies; and payments made under technology license agreements prior to regulatory approval of drug candidates.
F-6
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Summary of Significant Accounting Policies (Continued)
Cash, Cash Equivalents and Marketable Securities
Cash and cash equivalents include bank demand deposits, commercial paper, marketable securities with maturities of three months or less when purchased, and money market funds which invest primarily in U.S. government and U.S. government agency obligations. These instruments are highly liquid and market risk is minimized by investing in highly rated securities. Cash equivalents are reported at fair value.
Marketable securities consist of corporate or government agency debt securities and equity securities that have a readily ascertainable market value based on quoted market prices or other observable inputs and are readily marketable. These investments are reported at fair value. All cash equivalents and marketable securities are designated as available-for-sale, with unrealized gains and losses included in accumulated other comprehensive gain or loss in stockholders' equity. A decline in the market value of a security below its cost that is deemed to be other than temporary is charged to earnings, and results in the establishment of a new cost basis for the security.
During the year ended December 31, 2008, we recorded a write-down of $3,055,000 related to other than temporary declines in the values of two of our equity investments. We had no such write-downs in 2010 or 2009. The cost of securities sold and the amount reclassified out of accumulated other comprehensive gain or loss into earnings is based on the specific identification method.
Equity Investments
Equity investments in securities without readily determinable fair value, which consist of investments in privately held companies, are carried at cost. As of December 31, 2010 and 2009 we held one such investment with a carrying amount of $500,000. This investment is included in other assets on the consolidated balance sheets. We periodically review this investment carried at cost and evaluate whether an impairment has occurred. We believe this equity investment continues to be realizable.
Fair Value of Financial Instruments
Fair value is defined as the exchange price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The following three levels of inputs that may be used to measure fair value under the fair value hierarchy:
- •
- Level 1—Quoted prices in active markets for identical
assets or liabilities that can be accessed at the measurement date.
- •
- Level 2—Observable inputs other than quoted prices
included within Level 1, such as quoted prices for similar assets or liabilities in active markets or other inputs that are observable or can be corroborated by observable market data for
substantially the full term of the assets or liabilities.
- •
- Level 3—Unobservable inputs that are supported by little or no market activity.
If the inputs used to measure the financial assets and liabilities fall within more than one of the different levels described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
F-7
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Summary of Significant Accounting Policies (Continued)
As of December 31, 2010, we held $118,738,000 of cash equivalents and marketable securities consisting of equity securities, high quality marketable debt instruments of the U.S. government and U.S. government agencies, commercial paper, and money market funds. We have adopted an investment policy and established guidelines relating to credit quality, diversification and maturities of our investments to preserve principal and maintain liquidity. All investment securities are issued by or guaranteed by the U.S. government and its Federal Agencies or have a credit rating of at least long-term of A or short-term of A1/P1 as determined by Moody's Investors Service and/or Standard & Poor's. We do not have any direct investments in auction-rate securities or securities that are collateralized by assets that include mortgages or subprime debt.
The fair value measurements of our cash equivalents and available-for-sale marketable securities are identified at the following levels within the fair value hierarchy (in thousands):
| |
Fair Value Measurements Using | |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||
At December 31, 2010 |
|||||||||||||
Money market funds |
$ | — | $ | 1,044 | — | $ | 1,044 | ||||||
Commercial paper |
— | 35,413 | — | 35,413 | |||||||||
U.S. government and U.S. government agency notes |
— | 77,157 | — | 77,157 | |||||||||
Equity securities |
5,124 | — | — | 5,124 | |||||||||
|
$ | 5,124 | $ | 113,614 | — | $ | 118,738 | ||||||
At December 31, 2009 |
|||||||||||||
Money market funds |
$ | — | $ | 4,713 | — | $ | 4,713 | ||||||
Commercial paper |
— | 29,986 | — | 29,986 | |||||||||
U.S. government and U.S. government agency notes |
— | 59,529 | — | 59,529 | |||||||||
Equity securities |
3,570 | — | — | 3,570 | |||||||||
|
$ | 3,570 | $ | 94,228 | — | $ | 97,798 | ||||||
Restricted Cash and Investments
Under one of our operating lease agreements as noted in Note 11 below, we were required to set aside cash and/or investments as collateral for two letters of credit. At December 31, 2010 and 2009, we had $2,134,000 and $2,255,000, respectively, of restricted cash related to this agreement.
Property, Plant and Equipment
Property, plant and equipment are stated at cost. Depreciation of building, office and manufacturing equipment and furniture and fixtures is provided on a straight-line basis over the estimated original useful lives of the respective assets, as noted below. Leasehold improvements are amortized over the shorter of the life of the lease or their estimated useful lives using the straight-line method.
F-8
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Summary of Significant Accounting Policies (Continued)
Property, plant and equipment consist of the following at December 31 (in thousands):
| |
2010 | 2009 | Estimated Useful Lives |
|||||
|---|---|---|---|---|---|---|---|---|
Land |
$ | 324 | $ | 324 | N/A | |||
Building |
2,671 | 2,671 | 31 years | |||||
Leasehold improvements |
2,778 | 2,757 | 5-10 years | |||||
Equipment |
5,109 | 4,367 | 5 years | |||||
Furniture and fixtures |
3,017 | 3,018 | 3-5 years | |||||
Construction in process |
95 | — | N/A | |||||
Total property and equipment |
13,994 | 13,137 | ||||||
Less accumulated depreciation and amortization |
(10,062 | ) | (8,932 | ) | ||||
Property, plant and equipment, net |
$ | 3,932 | $ | 4,205 | ||||
Goodwill
Goodwill is reviewed annually, or more frequently if impairment indicators arise, for impairment.
Major Customers
During 2010, 2009, and 2008, all of our royalty revenue was received from Eisai Corporation related to Dacogen sales (see Note 5 below). All of our development and license revenue recognized in 2010 and 2009 resulted from our agreement with GlaxoSmithKline (see Note 6 below).
Net Income (Loss) per Common Share
Basic net income (loss) per share is calculated by dividing the net income (loss) by the weighted-average number of common shares outstanding for the period, without consideration of potential common shares. Diluted net income (loss) per share is computed by dividing the net income (loss) by the weighted-average number of common shares outstanding for the period and potential dilutive common shares for the period determined using the treasury-stock method. For purposes of this calculation, options to purchase stock and warrants are considered to be potential common shares and are only included in the calculation of diluted net income (loss) per share when their effect is dilutive.
F-9
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Summary of Significant Accounting Policies (Continued)
The following table is a reconciliation of the denominator used in the calculation of basic and diluted net income (loss) per common share (in thousands):
| |
Year ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
Weighted-average common shares outstanding used in calculation of basic net income (loss) per share |
60,287 | 59,316 | 57,721 | |||||||
Dilutive stock options |
348 | 24 | — | |||||||
Weighted-average common shares outstanding used in calculation of diluted net income (loss) per share |
60,635 | 59,340 | 57,721 | |||||||
Stock options and warrants outstanding at year-end that are not included in dilutive net income (loss) per share as they had an antidilutive effect |
8,322 | 8,760 | 9,140 | |||||||
Long-lived Assets
We evaluate long-lived assets, other than goodwill, for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable based on expected undiscounted cash flows attributable to that asset. The amount of any impairment is measured as the difference between the carrying value and the fair value of the impaired asset. No such impairment has been recorded through December 31, 2010. As of both December 31, 2010 and 2009, all of our long-lived assets were located in the U.S.
Recent Accounting Pronouncements
In October 2009, the FASB issued ASU No. 2009-13, which addresses the accounting for multiple-deliverable arrangements to potentially enable vendors to account for products or services separately rather than as a combined unit, and modifies the manner in which the transaction consideration is allocated across the separately identified deliverables. The ASU also significantly expands the disclosure requirements for multiple-deliverable revenue arrangements and may be adopted prospectively or retrospectively. The Company will adopt the ASU as of January 1, 2011 as required, and apply it prospectively to arrangements entered into or materially modified after the adoption date. The Company does not expect the adoption of ASU No. 2009-13 to have any material effect on its financial statements upon its required adoption on January 1, 2011.
In March 2010, Accounting Standards Codification Topic 605, Revenue Recognition ("ASC 605") was amended to define a milestone and clarify that the milestone method of revenue recognition is a valid application of the proportional performance model when applied to research or development arrangements. Accordingly, a company can make an accounting policy election to recognize a payment that is contingent upon the achievement of a substantive milestone in its entirety in the period in which the milestone is achieved. This guidance is effective for the Company as of January 1, 2011 and may be applied on a prospective or retrospective basis. The Company will adopt this guidance prospectively and does not expect the adoption to have any effect on its financial statements upon its required adoption on January 1, 2011.
F-10
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Available-for-Sale Securities
The following is a summary of available-for-sale securities (in thousands):
| |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
At December 31, 2010 |
||||||||||||||
Money market funds |
$ | 1,044 | $ | — | $ | — | $ | 1,044 | ||||||
U.S. corporate debt securities |
35,413 | — | — | 35,413 | ||||||||||
Debt securities issued by U.S. government and U.S. government agencies |
77,173 | 8 | (24 | ) | 77,157 | |||||||||
Marketable equity securities |
2,726 | 2,398 | — | 5,124 | ||||||||||
Total |
$ | 116,356 | $ | 2,406 | $ | (24 | ) | $ | 118,738 | |||||
At December 31, 2009 |
||||||||||||||
Money market funds |
$ | 4,713 | $ | — | $ | — | $ | 4,713 | ||||||
U.S. corporate debt securities |
29,987 | — | (1 | ) | 29,986 | |||||||||
Debt securities issued by U.S. government and U.S. government agencies |
59,575 | 12 | (58 | ) | 59,529 | |||||||||
Marketable equity securities |
2,726 | 844 | — | 3,570 | ||||||||||
Total |
$ | 97,001 | $ | 856 | $ | (59 | ) | $ | 97,798 | |||||
The available-for-sale securities are classified on the balance sheet as follows (in thousands):
| |
Fair Value at December 31, | |||||||
|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | ||||||
Amounts included in cash and cash equivalents |
$ | 23,915 | $ | 4,713 | ||||
Marketable securities, current |
89,699 | 89,515 | ||||||
Marketable securities, non-current |
5,124 | 3,570 | ||||||
Total |
$ | 118,738 | $ | 97,798 | ||||
At December 31, 2010, all of our debt securities were due in one year or less based on their contractual maturities.
Realized gains and losses on the sale of available-for-sale securities for the years ended December 31, 2010, 2009, and 2008 were not material.
We evaluate investments with unrealized losses to determine if the losses are other than temporary. In making this determination, we consider changes in the credit risk of debt securities, the financial condition and near-term prospects of the issuers, the magnitude of the losses compared to the investments' cost, the length of time the investments have been in a continuous unrealized loss position, and whether it is more-likely-than-not that we will hold the investments for a reasonable period of time sufficient for a recovery of the cost basis. During the year ended December 31, 2008 we recorded write-downs totaling $3,055,000 related to other than temporary declines in the values of two of our equity investments. We had no such write-downs in 2009 or 2010. At December 31, 2010, all of our unrealized losses relate to 15 individual debt securities with a fair value of $59,743,000 that have been in an
F-11
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Available-for-Sale Securities (Continued)
unrealized loss position for less than a year. Such losses were not related to changes in credit risk and were deemed to be temporary.
3. Stockholders' Equity
Warrants
At December 31, 2009 and 2010, we had no outstanding warrants to purchase shares of our common stock. Warrants to purchase 1,135,000 shares of our common stock at $10.00 per share expired during 2009.
Stock Reserved for Future Issuance
At December 31, 2010, we have reserved shares of common stock for future issuance as follows:
Stock options outstanding |
11,131,949 | |||
Stock options available for grant |
3,524,446 | |||
Shares available for Employee Stock Purchase Plan |
78,430 | |||
|
14,734,825 | |||
4. Stock-Based Compensation
Stock Option Plans. We have 18,263,000 shares of common stock authorized for issuance upon the grant of incentive stock options or nonstatutory stock options to employees, directors, and consultants under our 2003 Stock Plan. The number of shares to be purchased, their price, and the terms of payment are determined by our Board of Directors, provided that the exercise price for incentive stock options cannot be less than the fair market value on the date of grant. The options granted generally expire ten years after the date of grant and become exercisable at such times and under such conditions as determined by the Board of Directors (generally over a four or five year period). Options that have performance-based vesting criteria become exercisable in accordance with the milestones determined by the Board of Directors.
Employee Stock Purchase Plan. We also have an employee stock purchase plan ("ESPP") that allows eligible employees to purchase common stock at a price per share equal to 85% of the lower of the fair market value of the common stock at the beginning or end of each six month period during the term of the ESPP. The current offering period began November 15, 2010 and is scheduled to end on May 14, 2011.
We recognized $1,432,000, $2,507,000, and $2,817,000 in stock-based compensation expense for the years ended December 31, 2010, 2009 and 2008, respectively. These amounts have been recorded in research and development expenses or general and administrative expenses, based on the home department of our employees.
F-12
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Stock-Based Compensation (Continued)
The fair value of each stock award is estimated on the grant date using the Black-Scholes option-pricing model based on the weighted average assumptions noted in the following table:
| |
Year ended December 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
Expected volatility |
61.0 | % | 65.9 | % | 74.8 | % | ||||
Expected life (in years) |
6.32 | 5.91 | 5.33 | |||||||
Risk-free interest rate |
2.63 | % | 2.36 | % | 3.23 | % | ||||
Dividend yield |
— | — | — | |||||||
The fair value of ESPP shares is estimated also using the Black-Scholes option-pricing model based on the weighted average assumptions noted in the following table:
| |
Year ended December 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
Expected volatility |
60.2 | % | 61.3 | % | 75.3 | % | ||||
Expected life (in years) |
0.50 | 0.50 | 0.50 | |||||||
Risk-free interest rate |
2.43 | % | 2.90 | % | 2.32 | % | ||||
Dividend yield |
— | — | — | |||||||
We compute expected volatility using a blend of historical volatility and implied volatility of our common stock based on the period of time corresponding to the expected life of the stock options. We do not rely exclusively on implied volatility because options on SuperGen stock with remaining terms of greater than one year are not regularly traded in the market. The expected life of stock options granted is based exclusively on historical data and represents the weighted average period of time that stock options granted are expected to be outstanding. The expected life is applied to one group as a whole as we do not expect substantially different exercise or post-vesting termination behavior among our employee and director population. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant commensurate with the expected life assumption. The dividend yield is zero as we do not expect to pay any dividends in the foreseeable future. We currently estimate when and if performance-based options will be earned. If the awards are not considered probable of achievement, no amount of stock-based compensation is recognized. If we consider the award to be probable of achievement, expense is recorded over the estimated service period.
We are using the straight-line attribution method to recognize stock-based compensation expense. The amount of stock-based compensation recognized during a period is based on the value of the portion of the awards that are ultimately expected to vest. We estimate forfeitures at the time of grant and revise them, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We used an estimated forfeiture rate of 8.6% in 2010, 6.91% in 2009, and 7.40% in 2008. The term "forfeitures" is distinct from "cancellations" or "expirations" and represents only the unvested portion of the surrendered option. The forfeiture rate is re-evaluated annually and is adjusted as necessary. Ultimately, the actual expense recognized over the vesting period will only be for those shares that vest.
F-13
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Stock-Based Compensation (Continued)
A summary of the Company's stock options as of December 31, 2010 and activity during the three years then ended is presented below:
| |
Options Outstanding |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (in years) |
Aggregate Intrinsic Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at January 1, 2008 |
7,286,806 | $ | 7.52 | ||||||||||
Granted |
1,275,694 | 2.69 | |||||||||||
Exercised |
(6,100 | ) | 1.75 | ||||||||||
Forfeited |
(88,053 | ) | 3.53 | ||||||||||
Expired |
(463,371 | ) | 8.42 | ||||||||||
Balance at December 31, 2008 |
8,004,976 | 6.74 | |||||||||||
Granted |
2,273,704 | 1.95 | |||||||||||
Exercised |
(59,802 | ) | 2.14 | ||||||||||
Forfeited |
(521,253 | ) | 3.41 | ||||||||||
Expired |
(327,720 | ) | 10.54 | ||||||||||
Balance at December 31, 2009 |
9,369,905 | 5.66 | |||||||||||
Granted |
2,374,897 | 2.82 | |||||||||||
Exercised |
(91,518 | ) | 1.98 | ||||||||||
Forfeited |
(88,686 | ) | 2.49 | ||||||||||
Expired |
(432,649 | ) | 18.32 | ||||||||||
Balance at December 31, 2010 |
11,131,949 | $ | 4.62 | 6.27 | $ | 2,119,005 | |||||||
Vested or expected to vest at December 31, 2010 |
10,398,063 | $ | 4.63 | 6.19 | $ | 1,940,797 | |||||||
Exercisable at December 31, 2010 |
7,094,995 | $ | 4.92 | 5.44 | $ | 1,012,584 | |||||||
| |
Year ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
Weighted average grant-date fair value of options granted |
$ | 1.66 | $ | 1.18 | $ | 1.70 | ||||
Intrinsic value of options exercised (i.e. difference between the market price at exercise and the price paid to exercise the options) |
106,000 | 35,567 | 4,860 | |||||||
Cash received from exercise of options |
182,000 | 127,788 | 10,675 | |||||||
As of December 31, 2010, there was $3,163,000 of total unrecognized compensation expense related to unvested stock-based awards. This expense is expected to be recognized over a weighted average period of 2.77 years.
5. License and Stock Purchase Agreements with MGI PHARMA, Inc./Eisai Corporation
In August 2004, we entered into a license agreement with MGI PHARMA, Inc., a Minnesota corporation ("MGI") relating to Dacogen® (decitabine) for Injection, an anti-cancer therapeutic which has been approved by the United States Food and Drug Administration ("FDA") for the treatment of patients with myelodysplastic syndrome ("MDS"). Pursuant to the terms of the license agreement, MGI received exclusive worldwide rights to the development, commercialization and distribution of Dacogen
F-14
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. License and Stock Purchase Agreements with MGI PHARMA, Inc./Eisai Corporation (Continued)
for all indications. We are entitled to receive royalties from MGI on all sales of licensed product worldwide. The license agreement will expire, on a country-by-country and licensed product-by-licensed product basis on the later to occur of (a) twenty years after the first commercial sale of the applicable licensed product in the respective country or (b) the expiration, termination, invalidation or abandonment of the patent rights covering the respective licensed product, or the manufacture or use thereof, in the respective country. Either we or MGI may terminate the license agreement for the non-payment by the other of any payment obligations under the agreement, or for any uncured material breach of the agreement. In addition, we have the right to terminate the agreement if (i) MGI is acquired by an entity that is not deemed an "equivalent" pharmaceutical company or (ii) MGI becomes insolvent. MGI became a wholly-owned subsidiary of Eisai Corporation of North America ("Eisai") in January 2008.
In May 2006, the FDA approved Dacogen for the treatment of patients with MDS and MGI commenced commercial sales of Dacogen in the United States. Eisai is required to pay us royalties starting at 20% and escalating to a maximum of 30% of net Dacogen sales within 45 days after the end of each calendar quarter. We recognize royalty revenue when we receive the royalty statement from Eisai because we do not have sufficient ability to accurately estimate Dacogen sales. During the years ended December 31, 2010, 2009, and 2008, we recorded royalty revenue of $52,463,000, $41,156,000, and $38,422,000, respectively.
In July 2006, Eisai executed an agreement to sublicense Dacogen to Cilag GmbH International, a Johnson & Johnson company ("Cilag"), granting exclusive development and commercialization rights in all territories outside North America. In accordance with our license agreement with Eisai, we are entitled to receive 50% of certain payments Eisai receives as a result of any sublicenses. As a result of both the original agreement with Eisai and this sublicense with Cilag, we may receive up to $17.5 million in future milestone payments as they are achieved for Dacogen sales globally. Cilag companies will be responsible for conducting regulatory and commercial activities related to Dacogen in all territories outside North America, while Eisai retains all commercialization rights and responsibility for all activities in the United States, Canada and Mexico.
6. Agreements with GlaxoSmithKline
In October 2009, we entered into two agreements with GlaxoSmithKline ("GSK"): (1) a Commercial Research and License Agreement (the "License Agreement") and (2) a Common Stock Purchase Agreement (the "Purchase Agreement"). These agreements have been combined and accounted for as one arrangement with one unit of accounting for revenue recognition purposes.
Pursuant to the terms of the License Agreement, we will collaborate with GSK over a period of five years to discover and develop specific epigenetic therapeutics. At the end of the research term, or earlier if GSK elects, GSK may exercise its option to license from us the compounds that are the result of the joint research effort in order to continue the development and ultimately commercialize and sell the products worldwide.
Upon execution of the License Agreement, we received an upfront payment of $2 million from GSK, which was initially recorded as deferred revenue. GSK is obligated to make certain additional payments to us if and when the compounds reach specified developmental milestones, as well as payments to us if and when the compounds that GSK has licensed achieve certain regulatory milestones. The License Agreement further provides that if the licensed compounds derived from the
F-15
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. Agreements with GlaxoSmithKline (Continued)
joint research team become products, GSK will pay us sales milestone payments as well as royalties on annual net sales of such products. Total potential development and commercialization milestones payable to us could exceed $375 million. The tiered royalties, into double digit magnitudes, will be paid on a country-by-country and product-by-product basis.
Pursuant to the Purchase Agreement, we also received $3 million from GSK for the purchase of shares of our common stock. The purchase price per share was based on 110% of the average closing price of our common stock for the thirty day period preceding the closing date. This resulted in the issuance of 990,099 shares of our common stock. The fair market value of the shares issued was $2,455,000, based upon the market value of our common stock on the date the transaction was executed and the number of shares to be issued was fixed, and the premium of $545,000 was recorded as additional deferred revenue. The total initial deferred revenue related to GSK of $2,545,000 is being recognized ratably over five years, the expected term of our substantive performance obligations under the License Agreement. For the years ended December 31, 2010 and 2009, we recognized $509,000 and $97,000, respectively, of the deferred revenue as development and license revenue. All deferred revenue recorded on our balance sheets as of December 31, 2010 and 2009 relates to this collaboration with GSK.
7. Acquisition of Montigen Pharmaceuticals, Inc.
In 2006, we acquired Montigen Pharmaceuticals, Inc. ("Montigen"), a privately-held oncology-focused drug discovery and development company headquartered in Salt Lake City, Utah. Montigen's assets included its research and development team, a proprietary drug discovery technology platform and optimization process, CLIMB, and late-stage pre-clinical compounds.
Upon closing of the acquisition, we paid the Montigen stockholders a total of $17.9 million, consisting of $9.0 million in cash and $8.9 million in shares of SuperGen common stock. The acquisition agreement required us to pay the former Montigen stockholders an additional $22 million in shares of SuperGen common stock, contingent upon the achievement of specific regulatory milestones. In April 2007, the first Montigen compound, amuvatinib, received clearance from the FDA to begin Phase I clinical trials, triggering the first contingent milestone payment to the former Montigen stockholders of $10 million, which we paid through the issuance of our common stock. In November 2008, a second Montigen compound, SGI-1776, received clearance from the FDA to begin Phase I clinical trials, triggering a second contingent milestone payment to the former Montigen stockholders of $5.2 million. This milestone was paid through the combination of a cash payment of $2,770,000 and the issuance of 1,481,000 shares of our common stock. The calculation of the number of shares used for the common stock portion of the payment was based on the average closing price of our stock on the five days preceding the payment. This payment was recorded as acquired in-process research and development expense in 2008. There is a $6.8 million remaining future contingent regulatory milestone payment due to the former Montigen stockholders when and if the related milestone is achieved, which will be recorded as additional acquired in-process research and development expense.
F-16
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Nipent Sale Transactions
In August 2006, we executed an Asset Acquisition Agreement with Mayne Pharma (USA), Inc. ("Mayne"), pursuant to which Mayne acquired the North American rights to our products Nipent and SurfaceSafe® cleaning system. We received cash proceeds of $13.4 million, which represented the purchase price per the agreement, reduced by a number of adjustments and holdbacks. Mayne was acquired by Hospira, Inc. ("Hospira") in February 2007.
In April 2007, we closed another transaction with Hospira completing the sale of the remaining worldwide rights for Nipent for total consideration of up to $8.3 million. We received an initial up-front payment of $3.75 million. The balance of the purchase price was guaranteed and payable in five installments over a five year period on the anniversary of the closing date, except for $1.25 million in holdbacks.
Under the terms of the North American Asset Acquisition agreement, we were obligated to reimburse Hospira for three years from the date of the agreement for amounts paid to a new supplier of Nipent in excess of the amounts referenced in a related commercial supply agreement. Hospira negotiated a manufacturing supply agreement with an FDA approved manufacturing site, and based on historical sales trends and manufacturing yields and forecasts of future sales and manufacturing yields, during 2007 we initially estimated our price protection exposure over the three year period to be $600,000. During 2008, we paid Hospira $49,000 relating to this obligation for the first of the three years covered under this agreement. At December 31, 2008, based on the updated estimates of manufacturing yields and sales trends for Nipent, we reduced the price protection reserve by $426,000 and computed our remaining potential liability for the remaining two years at $125,000. At December 31, 2009, we reduced the deferred gain on sale related to this obligation to $50,000, based on updated estimates of manufacturing yields and requirements and sales trends for the remaining year of the agreement. The three year obligation period expired in 2010.
During 2008, we received a $1 million indemnification holdback relating to the sale of the North American rights, a $400,000 annual installment payment relating to the sale of the remaining worldwide rights, and a $250,000 indemnification holdback relating to the sale of the remaining worldwide rights. We also reduced our price protection reserve by $426,000 and reversed $160,000 of residual products return reserve for Nipent as the reserve was no longer required due to the expiration of the contractual return period. The sum of these 2008 transactions, $2,236,000, was recorded as a gain on sale of products during the year ended December 31, 2008. During 2009, we received a $500,000 installment payment relating to the sale of the remaining worldwide rights, reversed $20,000 of a residual products return reserve that was no longer required due to the expiration of the contractual return period, and reduced our price protection reserve by $75,000, resulting in $595,000 which was recorded as gain on sale of products during the year ended December 31, 2009. During 2010, we received a $700,000 installment payment relating to the sale of the worldwide rights to Nipent and wrote off the remaining balance of our price protection reserve of $50,000, resulting in $750,000 which was recorded as gain on sale of products during the year ended December 31, 2010.
Due to the Company's determination that the Nipent operations sold to Hospira did not represent a separate component of the Company and the Company's continuing involvement with the Nipent operations, resulting from entering into the related agreements and certain additional obligations including the price protection obligation as described above, we have reflected activities related to the Nipent and SurfaceSafe businesses in operating activities for all periods presented.
F-17
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. AVI BioPharma, Inc.
In December 1999, we entered into an agreement with AVI BioPharma, Inc. At the time, the chief executive officer of AVI was a member of our Board of Directors. The former president and chief executive officer of SuperGen was a member of the Board of Directors of AVI. Under the terms of the agreement, we acquired one million shares of AVI common stock, which amounted to approximately 7.5% of AVI's outstanding common stock, for $2.5 million cash and 100,000 shares of our common stock at $28.25 per share. We also acquired exclusive negotiating rights for the United States market for Avicine, AVI's proprietary cancer vaccine.
In July 2000, we finalized an agreement with AVI to obtain the U.S. marketing rights for Avicine. We issued 347,826 shares of our common stock along with $5 million in cash to AVI, in exchange for 1,684,211 shares of AVI common stock. As part of this agreement, we obtained the right of first discussion to all of AVI's oncology compounds and an option to acquire up to 1,665,478 shares of AVI's common stock, subject to anti-dilution provisions, for $35.625 per share. This option is exercisable for a three-year period commencing on the earlier of the date the FDA accepts the NDA submitted for Avicine or the date on which the closing price of AVI's common stock exceeds the option exercise price. We have accounted for the investment in AVI as an investment in equity securities that are available-for-sale as our ownership is less than 20% of AVI's outstanding shares. As of December 31, 2010, we held 2,384,211 shares of AVI common stock with a fair market value of $5,055,000.
Avicine will require significant additional expenditures to complete the clinical development necessary to gain marketing approval from the FDA and equivalent foreign regulatory agencies. As part of this agreement, we are obligated to make additional payments to AVI based on successful achievement of developmental, regulatory approval, and commercialization milestones over the next several years that could total $80 million. However, no significant development efforts have been incurred for Avicine since 2003 and none are anticipated in the near future. In 2003 and 2002, we recorded a total of $565,000 in research and development expenses for Avicine. At December 31, 2010 and 2009, this amount was still payable and is presented on the balance sheet as Payable to AVI BioPharma, Inc.
10. Liquidation of EuroGen Pharmaceuticals
We established our European subsidiary, EuroGen Pharmaceuticals, Ltd. ("EuroGen") in 2001 to expand our commercial presence in Europe. Since that time, we changed our strategic focus due to the sublicense of Dacogen to Eisai in 2004 and the sale of Nipent to Hospira in 2006 and 2007. As a result, in May 2008, we decided to discontinue our European operations and commence liquidation of EuroGen. The liquidation was substantially completed by October 1, 2008. As part of this liquidation, we terminated the two employees at EuroGen as of September 30, 2008. As part of the employment agreements with these two employees, we were required to provide them with a twelve month notification period prior to termination. They continued to provide services from the May 2008 notification date through September 30, 2008, during which time they were compensated with their normal salary and benefits. At their termination date, we were obligated to pay these employees severance through the remainder of the twelve month notification period. During the year ended December 31, 2008, we recorded total severance costs of $420,000, which were recorded in general and administrative expenses. We do not expect to incur any further severance costs related to this liquidation.
F-18
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Commitments and Contingencies
In August 2010, we executed an amendment to the lease for our corporate headquarters in Dublin, California. The previous lease, which expired on November 30, 2010, called for base rent payments of approximately $197,000 per month. Under the new lease amendment, which took effect on December 1, 2010 for a five year term with a five year renewal option, we reduced the amount of rented square feet with base monthly rent payments of $49,500 in the first year. At the end of each year, the rent will increase by $1,500 per month.
The terms of our old corporate headquarters lease required us to establish and maintain two irrevocable and unconditional letters of credit to secure our obligations under the lease. The domestic financial institution issuing the letters of credit required us to collateralize our potential obligations under the lease by assigning to the institution approximately $2.1 million in certificates of deposit. Although the lease expired on November 30, 2010, the letters of credit did not expire until January 31, 2011. As a result, the certificates of deposit are included in the balance sheet under Restricted cash at December 31, 2010 and 2009.
Future minimum rentals under all operating leases with terms greater than one year as of December 31, 2010 are as follows (in thousands):
Year ending December 31,
|
Minimum rental obligations |
|||
|---|---|---|---|---|
2011 |
$ | 935 | ||
2012 |
796 | |||
2013 |
700 | |||
2014 |
650 | |||
2015 |
610 | |||
|
$ | 3,691 | ||
Rent expense was $2,091,000 in 2010, $2,194,000 in 2009, $2,256,000 in 2008. These amounts are net of sublease income of $219,000 in 2010, $232,000 in 2009, and $224,000 in 2008. From August 2007 through November 2010, we subleased a portion of our primary administrative facility under a non-cancellable lease.
As noted in Note 7 above, we will pay the former Montigen stockholders an additional $6.8 million in shares of SuperGen common stock, contingent upon achievement of specific regulatory milestones.
We have entered into technology license agreements allowing us access to certain technologies. These agreements generally require royalty payments based upon the sale of approved products incorporating the technology under license. No sales of such products have occurred as of December 31, 2010.
We have also entered into manufacturing and service agreements for certain manufacturing services, the supply of research materials and the performance of specified research studies. These agreements require payments based upon the performance of the manufacturing entity, delivery of the research materials or the completion of the studies. There are no material commitments for such payments as of December 31, 2010.
F-19
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Income Taxes
For financial reporting purposes, our net income (loss) included the following components (in thousands):
| |
Year ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
Pre-tax income (loss): |
|||||||||||
United States |
$ | 16,312 | $ | 3,851 | $ | (17,903 | ) | ||||
Foreign |
— | — | 8,744 | ||||||||
|
$ | 16,312 | $ | 3,851 | $ | (9,159 | ) | ||||
Income tax benefit (provision) consisted of the following components (in thousands):
| |
Year ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
Current: |
|||||||||||
Federal |
$ | — | $ | 835 | $ | 42 | |||||
State |
(39 | ) | 51 | 6 | |||||||
Total current |
(39 | ) | 886 | 48 | |||||||
Deferred |
— | — | — | ||||||||
Total income tax benefit (provision) |
$ | (39 | ) | $ | 886 | $ | 48 | ||||
The difference between the income tax benefit (provision) and the amount computed by applying the federal statutory income tax rate to income (loss) from continuing operations before income taxes is as follows (in thousands):
| |
Year ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
Income tax benefit (expense) at federal statutory rate |
$ | (5,709 | ) | $ | (1,348 | ) | $ | 3,206 | ||
State taxes (net of federal) |
(568 | ) | (178 | ) | (205 | ) | ||||
Acquired in-process research and development |
— | — | (1,815 | ) | ||||||
Non-deductible deferred compensation |
(411 | ) | (638 | ) | (335 | ) | ||||
Credits |
998 | 890 | 1,626 | |||||||
Expired losses |
— | — | (1,255 | ) | ||||||
Unrealized loss on investments |
(644 | ) | (636 | ) | (636 | ) | ||||
Nontaxable liquidation of foreign subsidiaries |
— | — | 3,061 | |||||||
Other |
205 | (65 | ) | (197 | ) | |||||
Change in valuation allowance |
6,090 | 2,861 | (3,402 | ) | ||||||
Total |
$ | (39 | ) | $ | 886 | $ | 48 | |||
Deferred income taxes reflect the net tax effects of (a) temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax
F-20
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Income Taxes (Continued)
purposes, and (b) operating losses and tax credit carryforwards. Significant components of our deferred tax assets are as follows (in thousands):
| |
December 31, | |||||||
|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | ||||||
Deferred tax assets: |
||||||||
Net operating loss carryforwards |
$ | 93,936 | $ | 99,755 | ||||
Purchased in-process technology |
2,722 | 3,285 | ||||||
Research and development credit carryforwards |
18,678 | 17,535 | ||||||
Capitalized research and development |
623 | 1,196 | ||||||
Investments |
6,062 | 6,706 | ||||||
Deferred revenue |
787 | 1,014 | ||||||
Other |
7,276 | 6,683 | ||||||
|
130,084 | 136,174 | ||||||
Valuation allowance |
(130,084 | ) | (136,174 | ) | ||||
Net deferred tax assets |
$ | — | $ | — | ||||
The tax benefit of operating losses, temporary differences, and credit carryforwards is recorded as an asset to the extent that management assesses that realization is "more likely than not." Realization of the future tax benefits is dependent upon our ability to generate sufficient taxable income within the carryforward period. Because limited history of operating income and our projected expenditures on development programs, management believes that recognition of the deferred tax assets arising from the above-mentioned tax benefits is not currently likely to be realized, and, accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance decreased by $6,090,000 during the year ended December 31, 2010, decreased by $2,861,000 during the year ended December 31, 2009, and increased by $3,402,000 during the year ended December 31, 2008. Approximately $7,377,000 of the valuation allowance for deferred tax assets relates to excess tax benefits of stock option deductions that, when recognized, will be allocated directly to additional paid-in capital.
Net operating losses and tax credit carryforwards as of December 31, 2010 are as follows (in thousands):
| |
Amount | Expiration Years | |||
|---|---|---|---|---|---|
Net operating losses, federal |
$ | 248,025 | 2019-2028 | ||
Net operating losses, state |
135,659 | 2011-2029 | |||
Tax credits, federal |
12,037 | 2011-2030 | |||
Tax credits, state |
10,217 | No expiration | |||
Utilization of our net operating loss carryforwards may be subject to substantial annual limitations due to ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such an annual limitation could result in the expiration of the net operating losses before utilization.
We had no unrecognized tax benefits as of December 31, 2010 and 2009. Also, there are no accrued amounts for interest and penalties.
F-21
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Income Taxes (Continued)
Our policy is to recognize interest and penalties related to income taxes as a component of income tax expense. No interest and penalty expenses have been recognized in the statements of operations for the years ended December 31, 2010, 2009 and 2008. We are subject to income tax examinations for U.S. Federal income taxes and state income taxes from 1995 forward due to net operating losses in tax years 1995 through 2009. We are subject to tax examinations in the United Kingdom from 2001 forward. We do not anticipate that total unrecognized tax benefits will significantly change prior to December 31, 2011.
13. Employee Benefit Plans
401(k) Profit Sharing Plan
We have adopted a 401(k) Profit Sharing Plan (the "401(k) Plan") for all eligible employees with a minimum of two months of service. We may be obligated to make contributions to the plan to comply with statutory requirements. Voluntary employee contributions to the 401(k) Plan may be matched by the Company, up to 3% of each participant's annual compensation, up to $6,000 maximum per participant. Our expense relating to contributions made to employee accounts under the 401(k) Plan was approximately $212,000 in 2010, $214,000 in 2009, and $161,000 in 2008.
Employee Stock Purchase Plan
In 2008 we established the 2008 Employee Stock Purchase Plan ("ESPP"), and a total of 250,000 shares of Common Stock were reserved for issuance under the plan. This ESPP replaced the 1998 Employee Stock Purchase Plan, which expired in 2008. Employees participating in the ESPP are granted the right to purchase shares of common stock at a price per share that is the lower of 85% of the fair market value of a share of Common Stock on the first day of an offering period, or 85% of the fair market value of a share of Common Stock on the last day of that offering period.
In 2010, we issued 29,185 and 38,183 shares through the ESPP at $2.30 and $2.27 per share, respectively. In 2009, we issued 37,342 and 29,455 shares through the ESPP at $1.34 and $1.73 per share, respectively. In 2008, we issued 37,965 and 37,405 shares through the ESPP at $2.13 and $1.36 per share, respectively. As of December 31, 2010, 78,430 shares are reserved for future issuance under the ESPP.
F-22
SUPERGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
14. Quarterly Financial Data (Unaudited)
Following is a summary of the quarterly results of operations for the years ended December 31, 2010 and 2009:
| |
Quarter Ended | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
March 31 | June 30 | September 30 | December 31 | |||||||||
| |
(Amounts in thousands, except per share data) |
||||||||||||
2010 |
|||||||||||||
Royalty revenue |
$ | 14,293 | $ | 9,764 | $ | 13,249 | $ | 15,157 | |||||
Development and license revenue |
127 | 127 | 127 | 127 | |||||||||
Net income |
4,674 | 961 | 3,892 | 6,746 | |||||||||
Basic and diluted net income per share |
0.08 | 0.02 | 0.06 | 0.11 | |||||||||
2009 |
|||||||||||||
Royalty revenue |
$ | 12,913 | $ | 6,011 | $ | 10,357 | $ | 11,873 | |||||
Development and license revenue |
— | — | — | 97 | |||||||||
Net income (loss) |
3,994 | (2,427 | ) | 833 | 2,336 | ||||||||
Basic and diluted net income (loss) per share. |
0.07 | (0.04 | ) | 0.01 | 0.04 | ||||||||
F-23
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized, on this 9th day of March 2011.
| SUPERGEN, INC. | ||||
By: |
/s/ JAMES S. J. MANUSO James S.J. Manuso Chief Executive Officer, President and Director |
|||
We, the undersigned officers and directors of SuperGen, Inc. hereby constitute and appoint James S.J. Manuso and Michael Molkentin, and each of them individually, our true and lawful attorney-in-fact, with full power of substitution, to sign for us and in our names in the capacities indicated below any amendments to this Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that said attorney-in-fact, or their substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report on Form 10-K has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ JAMES S.J. MANUSO (James S.J. Manuso) |
Chief Executive Officer, President and Director (Principal Executive Officer) |
March 9, 2011 | ||
/s/ MICHAEL MOLKENTIN (Michael Molkentin) |
Chief Financial Officer (Principal Financial and Accounting Officer) |
March 9, 2011 |
||
/s/ CHARLES J. CASAMENTO (Charles J. Casamento) |
Director |
March 9, 2011 |
||
/s/ THOMAS V. GIRARDI (Thomas V. Girardi) |
Director |
March 9, 2011 |
||
/s/ ALLAN R. GOLDBERG (Allan R. Goldberg) |
Director |
March 9, 2011 |
||
/s/ WALTER J. LACK (Walter J. Lack) |
Director |
March 9, 2011 |
||
/s/ MICHAEL D. YOUNG (Michael D. Young) |
Director |
March 9, 2011 |
S-1
