UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (date of earliest event reported): March 8, 2011
K-V PHARMACEUTICAL COMPANY
(Exact name of registrant as specified in its charter)
Commission File Number 1-9601
| Delaware | 1-9601 | 43-0618919 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| One Corporate Woods Drive Bridgeton, MO | 63044 | |
| (Address of principal executive offices) | (Zip Code) |
(314) 645-6600
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act. |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act. |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. |
Explanatory Note
This Current Report on Form 8-K is being filed by the Registrant solely for the purpose of fulfilling the Registrant’s obligations under the Second Amended and Restated Registration Rights Agreement described below to disclose certain information provided to the Registrants’s Lenders that has not previously been publicly disclosed and which the Registrant reasonably believes constitutes material non-public information.
| Item 2.02 | Results of Operations and Financial Condition |
Item 7.01 below discloses results of operations and financial condition of the Company for prior periods that were previously unreported. Item 7.01 is hereby incorporated by reference.
| Item 7.01 | Regulation FD Disclosure |
As previously disclosed, K-V Pharmaceutical Company (the “Company” or the “Registrant”), certain of the Company’s subsidiaries, and each of U.S. Healthcare I, L.L.C. and U.S. Healthcare II, L.L.C. (collectively, the “Lenders”), are parties to a Credit and Guaranty Agreement, dated November 17, 2010, as amended by that certain Amended and Restated Amendment No. 1 to Credit Agreement dated as of January 6, 2011, as amended by that Amendment No. 2 to Credit Agreement dated as of March 2, 2011 (as amended, the “Credit Agreement”). Pursuant to the Credit Agreement, at various times prior to the date hereof the Company has provided to the Lenders certain information regarding the Company and its business, including various consolidated financial projections and liquidity forecasts and internal financial information and results (collectively, the “Provided Information”). The Company and the Lenders are also parties to that certain Second Amended and Restated Registration Rights Agreement, dated as of March 2, 2011 (the “Second Amended RRA”), pursuant to which the Company has agreed to register for resale under the Securities Act of 1933, as amended, shares of the Company’s Class A Common Stock which are issuable upon the exercise of warrants issued by the Company to the Lenders in connection with the Credit Agreement. Under the Second Amended RRA, the Company is obligated under certain circumstances to publicly disclose all of the previously publicly undisclosed confidential information provided to the Lender or its affiliates that, in the Company’s reasonable judgment, constitutes material non-public information. This Current Report on Form 8-K is being filed by the Company solely for the purpose of fulfilling the Company’s obligation under the Second Amended RRA to disclose the portions of the Provided Information that have not previously been publicly disclosed and which the Company reasonably believes constitute material non-public information.
The Provided Information includes certain consolidated financial projections and liquidity forecasts for the Company set forth below in this Current Report on Form 8-K (collectively, the “Projections”). The Projections set forth below reflect information that was prepared at various times in connection with the Company’s exploration of potential financing alternatives, and were not prepared with a view toward public disclosure or compliance with the published guidelines of the Securities and Exchange Commission (the “SEC”) or with the standards for projections promulgated by the American Institute of Certified Public Accountants or the Financial Accounting Standards Board. The Projections do not purport to present financial condition in accordance with accounting principles generally accepted in the United States (“GAAP”). The Company’s independent accountants have not audited, examined, reviewed, compiled or otherwise applied procedures to the Projections and, accordingly, do not express an opinion or any other form of assurance with respect to the Projections.
The Projections also were prepared by the Company’s management solely for internal use, capital budgeting, discussions with financing sources under confidentiality agreements, and other management decisions and are subjective in many respects. The Projections reflect numerous assumptions made by management of the Company with respect to financial condition, business and industry performance, general economic, market and financial conditions, regulatory matters, loan compliance, and other matters. Some of the assumptions used have not been achieved to date and may not be realized in the future, and are subject to significant business, litigation, economic, regulatory and competitive uncertainties and contingencies, many of which are beyond the control of the Company. The Projections also contain information that is, in many cases, obsolete, superseded or no longer applicable due to subsequent developments and events occurring since the Projections were made. Unanticipated events and circumstances may also affect the actual financial results of the Company in the future.
Principal assumptions underlying the Projections include those relating to the launch of our recently approved product Makena™. In addition to the various material assumptions to the Projections set forth below, the following information is material to an understanding of uncertainties regarding the launch of Makena™ and could cause actual results to vary materially from the forecasts reflected in the Projections:
| • | The Projections assume that the average course of treatment will involve three vials (each vial consisting of five doses) of Makena™ per patient. The actual per patient usage of Makena™ could vary significantly from this assumption based on a variety of factors. |
| • | The Projections have been prepared on the basis of certain assumptions regarding gross and net pricing to be recognized from sales of Makena™, including estimates of adjustments to net sales reflecting discounts, rebates and other customary allowances. The Company’s ability to achieve these assumptions regarding gross and net pricing is subject to substantial uncertainty. |
| • | Although Makena™ is the first and only FDA-approved treatment indicated to reduce the risk of preterm birth in women with a singleton pregnancy that have a history of singleton spontaneous preterm birth, the Company’s sales of Makena™ could be negatively affected by treatment of this condition by unapproved drug therapies. |
| • | Subsequent to the preparation of the Projections, the Company received preliminary initial orders from distributors for approximately 6,000 vials of Makena™. The amount of these orders reflects various factors such as special discounts made available by the Company in connection with the product launch and should not be considered as indicative of expectations for future sales of Makena™. Actual sales volumes for Makena™ will depend on a number of factors, including market acceptance. |
With respect to the Company’s efforts to secure approval for additional products, in February 2011, the FDA conducted an inspection with respect to the Company’s Clindesse® product and issued a Form 483 with certain observations. Also in February 2011, the Company filed its responses with the FDA with respect to such observations
In view of the foregoing, the Projections should not be regarded as a representation or warranty by the Company, or any other person or entity, that the Projections will be realized. It is expected that there will be differences between actual and projected results, and the differences may be material. The inclusion of the Projections herein should not be regarded as an indication that the Company or its affiliates or representatives consider the Projections to be a reliable prediction of future events, and the Projections should not be relied upon as such. Neither the Company nor any of its affiliates or representatives make any representation or warranty regarding the accuracy, completeness, current relevance or applicability of any data or computations contained in the Projections, and neither the Company nor any of its affiliates or representatives undertake any obligation to publicly update the Projections or the information reflected in them to reflect circumstances or factual information existing or arising after the date when the Projections were made or to reflect the occurrence of future events, even in the event that any or all of the assumptions underlying the Projections are shown to be in error. The Projections should be read in conjunction with the information set forth in the Company’s Annual Report on Form 10-K for the fiscal year ended March 31, 2010 (the “2010 Form 10-K”), including the risk factors that are included under the caption “Item 1A—“Risk Factors”” in the 2010 Form 10-K, as supplemented by the Company’s subsequent SEC filings.
The Provided Information also included certain consolidated internal financial information and results derived from the Company’s internally prepared financial statements (collectively, “Internal Financial Results”). Internal Financial Results included in the materials set forth below that relate to periods for which the Company has not yet filed a Quarterly Report on Form 10-Q or an Annual Report on Form 10-K are identified by columns marked “Preliminary.” The Company’s independent accountants have not audited, examined, reviewed, compiled or otherwise applied procedures to the Internal Financial Information and, accordingly, do not express an opinion or any other form of assurance with respect to the Internal Financial Information. The Internal Financial Information is subject to adjustment in connection with the preparation of the Company’s periodic reports for the applicable periods, and such adjustments may be material. The Company’s actual financial results reported in its future filings with the SEC or otherwise publicly disclosed may vary from the Internal Financial Information presented below, and such variances may be material. Accordingly, neither the Company nor any of its affiliates or representatives make any representation or warranty regarding the accuracy of any data or computations contained in the Internal Financial Information, and the Internal Financial Information should not be regarded as a representation or warranty by the Company, or any other person or entity, that the Internal Financial Information set forth below is accurate or will be realized.
The Internal Financial Information should be read in conjunction with the consolidated financial statements and other information contained in the 2010 Form 10-K. As previously disclosed, the Company has not filed with the SEC its Quarterly Reports on
Form 10-Q for the quarters ended June 30, 2010, September 30, 2010 or December 31, 2010. Certain of the information contained in the Internal Financial Information may differ materially from the information in these reports when filed by the Company with the SEC. As discussed in the 2010 Form 10-K, the Company believes that there is substantial doubt regarding its ability to continue as a going concern and, as a result, the report of its independent registered public accounting firm accompanying its annual consolidated financial statements for the fiscal year ended March 31, 2010, included an explanatory paragraph disclosing the existence of substantial doubt regarding the Company’s ability to continue as a going concern. The Company does not expect that the substantial doubt will be resolved as of the end of the period covered by the Form 10-Q for the quarters ended June 30, 2010, September 30, 2010, December 31, 2010 or possibly the year ended March 31, 2011. The Internal Financial Information has been prepared using GAAP applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The historical consolidated financial statements included in the Internal Financial Information does not include any adjustments that might be necessary if the Company was unable to continue as a going concern.
On March 1, 2011, the Company announced that it intends to offer $200 million of senior secured notes due 2015 (the “Notes”) in a private placement, subject to market conditions, and that the Company intends to use the net proceeds from the private placement of the Notes to repay in full its obligations under the Credit Agreement with the Lenders (including the payment of related premiums) and terminate the related future loan commitments, to establish an escrow reserve for one year of interest payments on the Notes and for general corporate purposes. The information regarding the Projections and the Internal Financial Information included below does not include any adjustment for the issuance of the Notes or the application of the proceeds from the sale of the Notes. The information regarding the Projections and the Internal Financial Information also assumes the obligation of the Company to comply with the covenants contained in the Credit Facility, which covenants would no longer be applicable in the event that the Company completes the sale of the Notes.
 CONFIDENTIAL
CONFIDENTIAL
Makena™
Revenue Assumptions
1
The Projections included herein represent the Company’s projections provided
to the Lenders in connection with negotiating the amendment to the
Company’s Bridge Loan and the related Multi-draw Facility and have not been
updated
to
reflect
the
recently
announced
efforts
by
the
Company
to
refinance
the Credit Facility with secured debt.
•
Financing Case
–
This scenario was prepared for purposes of negotiating the Credit
Agreement and related covenants with the Lenders. Assumes ~47% of Research
Case by March 2012 and ~82% by March 2013
•
Internal Case –
This scenario was developed by the Company to be utilized for
financial and business planning purposes. Assumes ~71% of Research Case by
March 2012 and ~94% by March 2013
•
Research Case
–
Estimate of Makena™
market and potential penetration based on
a Company proprietary market study
•
List Price -
Makena™
list price per vial at launch in March 2011 $7,500
The information set forth herein is not intended to be relied upon by
investors. Please see the important explanatory information set forth
above in this Current Report on Form 8-K |
 CONFIDENTIAL
CONFIDENTIAL
Makena™
Revenue Assumptions
(Continued)
2
The information set forth herein is not intended to be relied upon by
investors. Please see the important explanatory information set forth
above in this Current Report on Form 8-K
Market
Quarter
Quarter End
Market Penetration at Quarter End
Estimated Quarterly Unit
2
Sales (000s)
End
(Patients)
1
Financing
Internal
Research
Financing
Internal
Research
Mar-11
8,755
0.6%
1.4%
2.1%
2.5
2.5
5.0
Jun-11
8,773
2.7%
6.3%
9.0%
0.0
1.0
0.0
Sep-11
8,791
6.1%
12.6%
18.0%
1.3
4.6
6.6
Dec-11
8,809
10.4%
19.8%
28.2%
4.7
9.7
13.5
Mar-12
8,827
16.0%
23.9%
34.1%
8.3
14.4
20.5
Market
Fiscal Year
Fiscal Year 2013
Market Penetration at Fiscal Year End
Estimated Fiscal Year Unit
2
Sales (000s)
End
(Patients)
1
Financing
Internal
Research
Financing
Internal
Research
Mar-13
105,000
29.6%
33.8%
36.2%
69.0
84.4
104.6
Notes:
2) One unit = 5 injections
1) Company's estimate of insured (or covered) lives who receive
prenatal care. Company assumes
that approximately 15% of market is uninsured. |
  CONFIDENTIAL
CONFIDENTIAL
Financing Case Key Assumptions
3
•
Expand to 150 sales force footprint upon approval of Makena™
•
Advertising & Promotion spend will increase in FY 2012 and
beyond, predominantly supporting Makena™
•
Evamist was assumed to be divested in March 2011 and Generics in
June 2011 (per requirements of existing loan with the Lenders)
The information set forth herein is not intended to be relied upon by investors. Please see the
important explanatory information set forth above in this Current Report on Form 8-K
$000s
Branded
Anti-Infectives
1
Generics
Grand
Description
Makena
& Evamist
Total
KCl
Total
Approval Date
Q4 FY2011
N/A
N/A
Q3 FY2011
N/A
Q4 FY 2011 Net Revenues
15,469
$
2,826
$
18,295
$
5,829
$
24,124
$
FY 2012 Net Revenues
92,892
$
26,626
$
119,518
$
8,967
$
128,485
$
FY 2013 Net Revenues
420,405
$
32,359
$
452,764
$
-
$
452,764
$
Notes:
1) Assumes Anti-Infectives are approved prior to June 30, 2011
Company is not required to divest Evamist or Generics if the Mutli-draw
facility is not closed and will evaluate strategic alternatives with Evamist and
Generics |
 CONFIDENTIAL
CONFIDENTIAL
Financing Case Key Assumptions
(Continued)
4
The information set forth herein is not intended to be relied upon by
investors. Please see the important explanatory information set forth
above in this Current Report on Form 8-K |
 CONFIDENTIAL
CONFIDENTIAL
5
The information set forth herein is not intended to be relied upon by
investors. Please see the important explanatory information set forth
above in this Current Report on Form 8-K |
 CONFIDENTIAL
CONFIDENTIAL
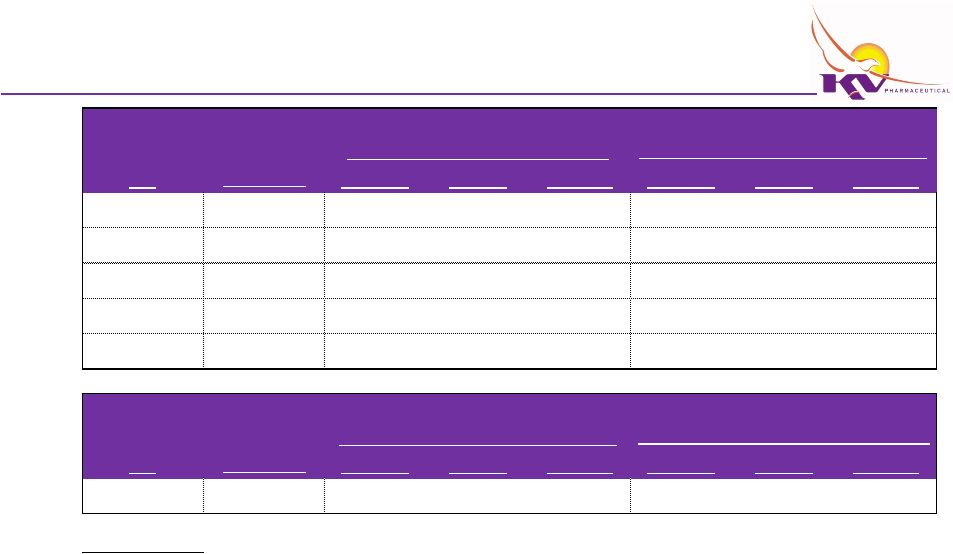
Financing Case Financial Projections
FY 2011 –
FY 2013
6
The information set forth herein is not intended to be relied upon by
investors. Please see the important explanatory information set forth
above in this Current Report on Form 8-K
Q1
Q2
Q3
Net Loss from continuing operations
(40,451)
$
(34,518)
$
(38,397)
$
Amortization and Depreciation
5,085
4,100
3,924
Interest expense, net
1,848
2,014
3,931
Taxes
(679)
1,923
2,560
EBITDA from continuing operations
(34,197)
$
(26,481)
$
(27,982)
$
Preliminary
$000s
FY 2011
FY 2012
FY 2013
Preliminary
Preliminary
Preliminary
Projected
Projected
Projected
Projected
Projected
Projected
Projected
Projected
Q1
Q2
Q3
Q4
Total
Q1
Q2
Q3
Q4
Total
Total
Income Statement
Net Sales
(1)
3,376
$
3,307
$
5,566
$
24,124
$
36,373
$
14,010
$
13,017
$
38,646
$
62,813
$
128,485
$
452,764
$
Gross Profit
(2)
(6,732)
(5,520)
(1,748)
14,686
686
5,129
10,055
35,518
59,662
110,363
441,158
Gross Margin
-199.4%
-166.9%
-31.4%
60.9%
1.9%
36.6%
77.2%
91.9%
95.0%
85.9%
97.4%
Operating Expenses
(2) (3)
32,003
25,069
31,668
38,553
127,293
30,974
27,680
26,888
25,912
111,453
117,921
EBITDA
(5)
(34,197)
$
(26,481)
$
(27,982)
$
(20,244)
$
(108,904)
$
(22,271)
$
(14,689)
$
13,330
$
34,627
$
10,997
$
329,517
$
-1012.9%
-800.8%
-502.7%
-83.9%
-299.4%
-159.0%
-112.8%
34.5%
55.1%
8.6%
72.8%
Cash Flows
(4)
Operating Activities
(50,874)
$
(37,276)
$
(32,564)
$
(72,597)
$
(193,311)
$
(30,932)
$
(31,681)
$
(7,001)
$
12,440
$
(57,174)
$
190,577
$
Investing Activities
33,212
2,150
144
2,939
38,445
60,019
306
306
306
60,937
-
Financing Activities
(1,508)
19,265
38,560
45,791
102,108
(33,358)
30,058
10,400
436
7,536
1,811
Net Increase (Decrease)
(19,170)
$
(15,861)
$
6,140
$
(23,866)
$
(52,757)
$
(4,270)
$
(1,318)
$
3,705
$
13,182
$
11,299
$
192,387
$
Beginning Cash
60,693
41,523
25,662
31,802
60,693
7,936
3,666
2,348
6,053
7,936
19,235
Ending Cash
41,523
$
25,662
$
31,802
$
7,936
$
7,936
$
3,666
$
2,348
$
6,053
$
19,235
$
19,235
$
211,623
$
6 |
 CONFIDENTIAL
CONFIDENTIAL
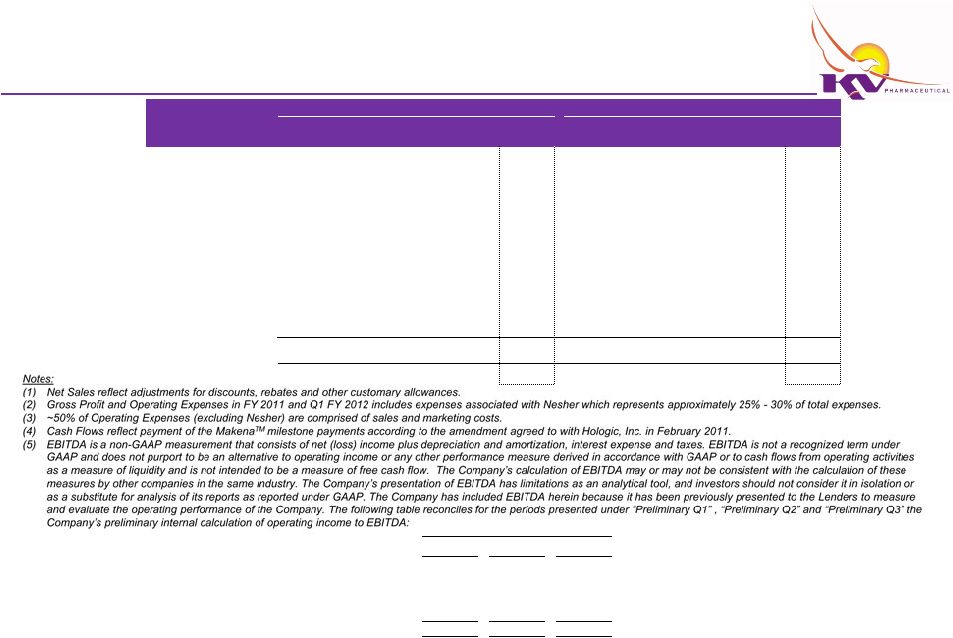
Internal Case Financial Projections
FY 2011 –
FY 2012
7
The information set forth herein is not intended to be relied upon by
investors. Please see the important explanatory information set forth
above in this Current Report on Form 8-K
Q1
Q2
Q3
Net Loss from continuing operations
(40,451)
$
(34,518)
$
(38,397)
$
Amortization and Depreciation
5,085
4,100
3,924
Interest expense, net
1,848
2,014
3,931
Taxes
(679)
1,923
2,560
EBITDA from continuing operations
(34,197)
$
(26,481)
$
(27,982)
$
Preliminary
$000s
FY 2011
FY 2012
Preliminary
Preliminary
Preliminary
Projected
Projected
Projected
Projected
Projected
Projected
Projected
Q1
Q2
Q3
Q4
Total
Q1
Q2
Q3
Q4
Total
Income Statement
Net Sales
(1)
3,376
$
3,307
$
5,566
$
24,124
$
36,373
$
20,657
$
34,462
$
70,572
$
102,546
$
228,236
$
Gross Profit
(2)
(6,732)
(5,520)
(1,748)
14,686
686
11,775
31,500
67,444
99,395
210,115
Gross Margin
-199.4%
-166.9%
-31.4%
60.9%
1.9%
57.0%
91.4%
95.6%
96.9%
92.1%
Operating Expenses
(2) (3)
32,003
25,069
31,668
38,553
127,293
30,974
27,681
26,889
25,913
111,457
EBITDA
(5)
(34,197)
$
(26,481)
$
(27,982)
$
(20,244)
$
(108,904)
$
(15,625)
$
6,755
$
45,255
$
72,981
$
109,366
$
-1012.9%
-800.8%
-502.7%
-83.9%
-299.4%
-75.6%
19.6%
64.1%
71.2%
47.9%
Cash Flows
(4)
Operating Activities
(50,874)
$
(37,276)
$
(32,564)
$
(72,597)
$
(193,311)
$
(29,166)
$
(15,026)
$
22,765
$
50,819
$
29,392
$
Investing Activities
33,212
2,150
144
2,939
38,445
60,019
306
306
306
60,937
Financing Activities
(1,508)
19,265
38,560
45,791
102,108
(33,358)
30,058
10,400
436
7,536
Net Increase (Decrease)
(19,170)
$
(15,861)
$
6,140
$
(23,866)
$
(52,757)
$
(2,505)
$
15,337
$
33,471
$
51,561
$
97,865
$
Beginning Cash
60,693
41,523
25,662
31,802
60,693
7,936
5,431
20,768
54,239
7,936
Ending Cash
41,523
$
25,662
$
31,802
$
7,936
$
7,936
$
5,431
$
20,768
$
54,239
$
105,801
$
105,801
$ |
 CONFIDENTIAL
CONFIDENTIAL
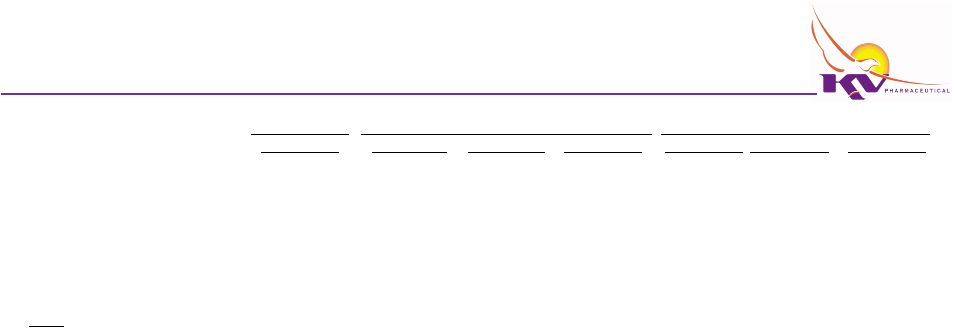
Financing Case
Selected Balance Sheet Information
8
The information set forth herein is not intended to be relied upon by
investors. Please see the important explanatory information set forth
above in this Current Report on Form 8-K
Audited
(1)
($ in thousands)
31-Mar-2010
30-Jun-2010
30-Sep-2010
31-Dec-2010
31-Mar-2011
31-Mar-2012
31-Mar-2013
Cash and cash equivalents
$60,693
$41,523
$25,662
$31,802
$7,936
$19,235
$211,623
Other current assets
(4)
31,020
20,813
21,627
28,577
60,596
88,082
121,043
Property and equipment, net
(5)
122,910
117,661
112,755
110,182
105,266
53,299
41,625
Total Assets
(6)
358,557
315,480
294,209
303,447
357,210
322,804
535,253
Current liabilities
(7)
172,794
152,527
166,521
206,223
271,415
267,171
187,087
Long-term debt
(8)
233,174
232,441
231,828
231,219
230,350
227,454
224,981
Notes:
(7)
Current
liabilities
includes
the
remaining
scheduled
Makena
TM
milestone
payments
and
remaining
outstanding
amounts
owed
under
the
Company's
loan
obligations to the Lenders.
(6)
The
increase
in
total
assets
beginning
in
March
2011
is
primarily
due
to
the
capitalization
of
$120,000
of
future
Makena
TM
milestone payments due uopn FDA
approval offset by a planned liquidation of the Company's remaining basis in its Auction Rate
Securities. (8) Long term debt is comprised primarily of $200,000 of convertible
notes, which have a next scheduled put date of May 2013, and the remaining balance of a
mortgage on four of the Company's properties. The long term debt balances shown do not
include a proposed $200,000 senior secured financing announced by the Company on March
1, 2011. Preliminary
(2)
Projected
(3)
(1) Per the Company's Form 10-K for Fiscal Year Ended March 31, 2010.
(2) Based on the Company's internally prepared financial statements, as provided to the
Lenders and required to be disclosed pursuant to covenants with the Lenders, and may
differ materially from actual results reported in Form 10-Q for each of the periods presented.
(3) Based on the Company's internal projections, as provided the
Lenders, and required to be disclosed pursuant to covenants with the Lenders and may
differ materially from actual results reported in future SEC filings for each of the
periods presented. (4)
Increase
in
current
assets
beginning
in
March
2011
is
primarily
due
to
receivables
associated
with
sales
launch
of
Makena
TM
.
(5) Decrease in property and equipment, net is primarily due to
the planned divestiture of the generics business in June 2011. |
 CONFIDENTIAL
CONFIDENTIAL
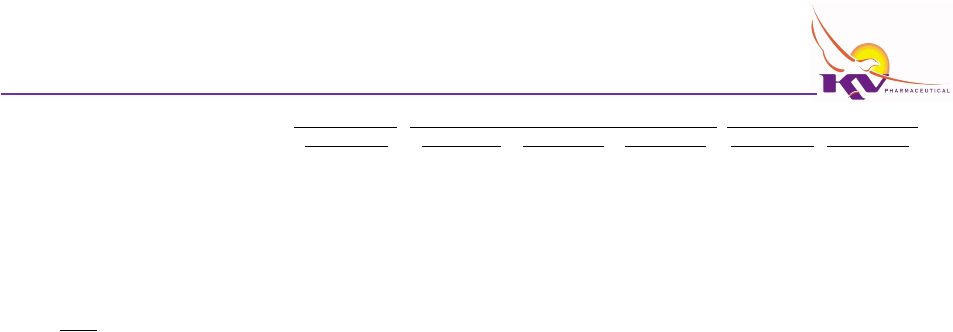
Internal Case
Selected Balance Sheet Information
9
The information set forth herein is not intended to be relied upon by
investors. Please see the important explanatory information set forth
above in this Current Report on Form 8-K
Audited
(1)
($ in thousands)
31-Mar-2010
30-Jun-2010
30-Sep-2010
31-Dec-2010
31-Mar-2011
31-Mar-2012
Cash and cash equivalents
$60,693
$41,523
$25,662
$31,802
$7,936
105,801
Other current assets
(4)
31,020
20,813
21,627
28,577
60,596
109,645
Property and equipment, net
(5)
122,910
117,661
112,755
110,182
105,266
53,299
Total Assets
(6)
358,557
315,480
294,209
303,447
357,210
430,931
Current liabilities
(7)
172,794
152,527
166,521
206,223
271,415
276,930
Long-term debt
(8)
233,174
232,441
231,828
231,219
230,350
227,454
Notes:
Preliminary
(2)
Projected
(3)
(1)
Per
the
Company's
Form
10-K
for
Fiscal
Year
Ended
March
31,
2010.
(2)
Based
on
the
Company's
internally
prepared
financial
statements,
as
provided
to
the
Lenders
and
required
to
be
disclosed
pursuant
to
covenants
with
the
Lenders,
and
may
differ
materially
from
actual
results
reported
in
Form
10-Q
for
each
of
the
periods
presented.
(3)
Based
on
the
Company's
internal
projections,
as
provided
to
the
Lenders,
and
required
to
be
disclosed
pursuant
to
covenants
with
the
Lenders
and
may
differ
materially
from
actual
results
reported
in
future
SEC
filings
for
each
of
the
periods
presented.
(4)
Increase
in
current
assets
beginning
in
March
2011
is
primarily
due
to
receivables
associated
with
sales
launch
of
Makena
TM
.
(
5)
Decrease
in
property
and
equipment,
net
is
primarily
due
to
the
planned
divestiture
of
the
generics
business
in
June
2011.
(6)
The
increase
in
total
assets
beginning
in
March
2011
is
primarily
due
to
the
capitalization
of
$120,000
of
future
Makena
TM
milestone
payments
due
upon
FDA
approval
offset
by
a
planned
liquidation
of
the
Company's
remaining
basis
in
its
Auction
Rate
Securities.
(7)
Current
liabilities
includes
the
remaining
scheduled
Makena
TM
milestone
payments
and
remaining
outstanding
amounts
owed
under
the
Company's loan obligations to the Lenders.
(8)
Long
term
debt
is
comprised
primarily
of
$200,000
of
convertible
notes,
which
have
a
next
scheduled
put
date
of
May
2013,
and
the
remaining
balance
of
a
mortgage
on
four
of
the
Company's
properties.
The
long
term
debt
balances
shown
do
not
include
a
proposed
$200,000
senior
secured
financing
announced
by
the
Company
on
March
1,
2011. |
The information contained herein shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended, nor shall they be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, except as shall be expressly set forth by specific reference in such filing.
The information contained in this Current Report on Form 8-K includes forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995. All statements (other than statements of historical facts) that address projected or estimated results, or events, developments or results that we intend, expect, believe, anticipate, plan, forecast or project, will or may occur in the future are forward-looking statements. The words “possible,” “propose,” “might,” “could,” “would,” “projects,” “plan,” “forecasts,” “anticipates,” “expect,” “intend,” “believe,” “seek” or “may,” and similar expressions, are intended to identify forward-looking statements, but are not the exclusive means of identifying them. Forward-looking statements are subject to a number of risks, contingencies and uncertainties, some of which our management has not yet identified. Forward-looking statements are not guarantees of future performance; subsequent developments may cause forward-looking statements to become outdated; and actual results, developments and business decisions may differ materially from those contemplated by such forward-looking statements as a result of various factors, certain (but not all) of which are discussed in the risk factors included in the Company’s reports filed with the SEC including, but not limited to, their Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q. Important factors that could cause actual results to differ from those contemplated by forward-looking statements include, but are not limited to, the terms of the restructuring or reorganization plan ultimately implemented, the timing thereof, the related costs and expenses, and the ability of the Company to maintain normal relationships with their vendors, service providers and customers. The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or circumstances, or otherwise.
The Company will post this Form 8-K on its Internet website at www.kvpharmaceutical.com. References to the Company’s website address are included in this Form 8-K only as inactive textual references and the Company does not intend them to be active links to its website. Information contained on the Company’s website does not constitute part of this Form 8-K.
* * *
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Dated: March 8, 2011
| K-V PHARMACEUTICAL COMPANY | ||
| By: | /s/ GREGORY J. DIVIS, JR. | |
| Gregory J. Divis, Jr. | ||
| President and Chief Executive Officer | ||
