Attached files
| file | filename |
|---|---|
| EX-31.2 - EX-31.2 - Redpoint Bio CORP | a2202484zex-31_2.htm |
| EX-23.1 - EX-23.1 - Redpoint Bio CORP | a2202484zex-23_1.htm |
| EX-32.2 - EX-32.2 - Redpoint Bio CORP | a2202484zex-32_2.htm |
| EX-32.1 - EX-32.1 - Redpoint Bio CORP | a2202484zex-32_1.htm |
| EX-31.1 - EX-31.1 - Redpoint Bio CORP | a2202484zex-31_1.htm |
| EX-21.1 - EX-21.1 - Redpoint Bio CORP | a2202484zex-21_1.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
Index to Financial Statements
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
| (Mark One) | ||
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended December 31, 2010 |
||
or |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to |
||
Commission File Number 000-51708
REDPOINT BIO CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
22-3393959 (I.R.S. Employer Identification No.) |
|
7 Graphics Drive |
||
| Ewing, New Jersey | 08628 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant's telephone number, including area code: (609) 637-9700
Securities registered pursuant to Section 12(b) of the Act: None.
Securities registered pursuant to Section 12(g) of the Act:
| |
Title of Class | |
||
|---|---|---|---|---|
| Common Stock, $.0001 par value per share |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. o Yes ý No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. o Yes ý No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ý Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). o Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company ý |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). o Yes ý No
The aggregate market value of the registrant's voting shares of common stock held by non-affiliates of the registrant on June 30, 2010, based on a $0.20 per share, the last reported sale price on the OTC Bulletin Board on that date, was approximately $11,094,854.
The number of shares of common stock, $0.0001 par value, of the registrant outstanding as of February 28, 2011 was 79,914,789 shares.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents are incorporated by reference into the Annual Report on Form 10-K: Portions of the registrant's definitive Proxy Statement for its 2011 Annual Meeting of Stockholders are incorporated by reference into Part III of this Report.
i
On March 12, 2007, Redpoint Bio Corporation, or Redpoint, entered into the Agreement and Plan of Merger, dated as of March 12, 2007, by and among Redpoint, on the one hand, and Robcor Properties, Inc., a Delaware corporation, or Robcor, Robcor Acquisition Corp., a Delaware corporation and wholly-owned subsidiary of Robcor, or Merger Sub, Robcor, LLC, a Kentucky limited liability company and wholly-owned subsidiary of Robcor, or Robcor, LLC, and Halter Financial Investments, L.P., a Texas limited partnership, and Michael Heitz, as stockholders of Robcor, on the other hand, referred to herein as the Reverse Merger Agreement. The transactions contemplated by the Reverse Merger Agreement (the "Closing") were consummated on March 12, 2007 (the "Closing Date").
Pursuant to the Reverse Merger Agreement, on the Closing Date, Merger Sub merged with and into Redpoint (the "Reverse Merger"), with Redpoint being the surviving corporation and becoming a wholly-owned subsidiary of Robcor, and the outstanding shares of capital stock, convertible notes and certain warrants to purchase capital stock of Redpoint were converted into shares of common stock of Robcor in accordance with the Delaware General Corporation Law (the "DGCL") on the terms and conditions as set forth in the Reverse Merger Agreement. On May 3, 2007, Redpoint and Robcor entered into an Agreement and Plan of Merger in which Robcor, the former parent company of Redpoint after consummation of the Reverse Merger, merged with and into Redpoint. Such merger, referred to herein as the Reincorporation Merger, was deemed effective on June 15, 2007.
In this Annual Report on Form 10-K, unless the context specifically indicates otherwise:
- •
- "the Company," "we," "us," "our," "Redpoint," and "Redpoint Bio" refer to Redpoint Bio Corporation;
- •
- "Robcor" refers to Robcor Properties, Inc. prior to the Reincorporation Merger effective on June 15, 2007;
and
- •
- "Original Redpoint" refers to Redpoint Bio Corporation, the privately held entity, prior to the Reincorporation Merger effective on June 15, 2007.
Overview
Redpoint Bio is a development stage biotechnology company focused on the development of healthier foods and beverages and new approaches to the treatment of diabetes and obesity.
Redpoint is leveraging recent discoveries in the molecular biology of taste to discover and develop novel taste modulators for the food and beverage industries. Our food and beverage program has been focused on identifying novel flavor modifiers that improve the taste of existing ingredients, enabling the development of better-tasting and more healthful foods and beverages. In June 2009, we announced that we had identified an all-natural sweetness enhancer, RP44. In June 2010, we entered into a license and commercialization agreement with IFF, a global leader in the food and beverage industry, covering the commercialization of RP44. In October 2010, IFF received notification by the Flavor and Extract Manufacturers Association (FEMA) that RP44 had been determined to be Generally Recognized As Safe (GRAS). We believe that our unique technology platform could lead to additional opportunities for discovering novel natural flavorings and flavor enhancers, to help meet increasing consumer demand for more healthful foods and beverages.
We are also pursuing a pharmaceutical discovery program for the treatment of diabetes and obesity. Our research in diabetes and obesity stems from the observation that taste signaling circuits responsible for sensing sweet, savory, and bitter compounds on the tongue are also found in the
1
pancreas and gastrointestinal tract, suggesting a potential role in the regulation of metabolism and satiety. We are actively seeking a research and development collaboration with major pharmaceutical companies for the further development of this discovery program. However, there can be no assurance that we will enter into any collaboration or license agreement.
We currently own or have exclusive licenses to 15 issued U.S. patents and three issued foreign patents. In addition, we have over 94 U.S. and foreign patent applications pending. We have not yet developed any products that are commercially available.
We believe that our current capital resources are only sufficient to meet our operating and capital requirements through April 2011, which raises substantial doubt about our ability to continue as a going concern. We are actively seeking additional corporate collaborations, equity investments from third parties and license agreements from various strategic partners to enhance our liquidity position. However, there can be no assurance that we will enter into any future corporate collaborations, equity investments or license agreements. Without additional funding, we will be unable to satisfy our obligations and will cease operations.
At December 31, 2010, we had cash and cash equivalents of $1.1 million. In addition, in January 2011, we sold a portion of our New Jersey State net operating loss carryforwards and received cash proceeds of approximately $0.4 million. As of the date of the filing of this annual report on Form 10-K, we have approximately $0.7 million of cash and cash equivalents. Since inception, we have used $42.4 million of cash to fund our operating activities and $3.4 million for capital expenditures. Through December 31, 2010, we have funded substantially all of our operations and capital expenditures through private placements of equity and convertible debt securities totaling $45.8 million, cash received from corporate collaborations totaling $11.4 million, government grants totaling $2.0 million, and capital equipment and working capital financing totaling $3.6 million.
Scientific Background and Our Technology Platform
The five basic taste senses include sweet, savory, bitter, salt, and sour. Savory is also sometimes referred to as "umami". The sweet, savory, and salt tastes are appetitive, while the bitter and sour tastes are aversive. The five basic tastes are sensed primarily by the taste buds on the tongue. Sequencing of the human genome has led to the identification of the molecular receptors and signal transduction components responsible for the sense of taste. This in turn has allowed discovery strategies originally developed for new pharmaceutical discovery to be applied to the identification of new taste modulators.
In the case of the sweet, savory and bitter taste sensations, taste cells on the tongue initially sense tastants like sugars (sweet flavor), amino acids (savory or umami flavor), and bitter substances (bitter flavor) through binding of the tastant molecules to specific receptors on the taste cell surface called G-Protein Coupled Receptors, or GPCRs. Generally, it is thought that individual taste cells express specific GPCR receptors, and sense only one kind of tastant. Binding of the tastant to the GPCR activates a signaling cascade that ultimately triggers a nerve signal that is sensed by the brain as taste.
The signaling cascade downstream of GPCR activation provides several targets for discovery of modulators of the sweet, savory and bitter taste sensations. Redpoint Bio's discovery programs initially focused on finding potent and specific compounds that bind to the TRPm5 ion channel that is involved in amplification of the GPCR-linked taste signals. We were motivated to follow this approach since biological studies showed that mice in which TRPm5 had been inactivated or "knocked out" were unable to taste sweet, savory, or bitter tastants. This in turn suggested that enhancers of TRPm5 channel conductance could function as taste enhancers in sweet or savory taste contexts, and as blockers that could work as bitter blockers in bitter taste contexts. Although we are not currently pursuing our TRPm5 discovery program for taste modulators, we believe that the TRPm5 ion channel may also be an attractive target for the identification and development of a new diabetes or obesity
2
therapeutic compound. Recent scientific research suggests that in addition to taste cells on the tongue, TRPm5 is expressed as part of a nutrient-sensing signal transduction chain in the gastrointestinal tract and also in the pancreas. We initiated a program designed to leverage the research we have already conducted on the discovery of modulators of the TRPm5 ion channel to further explore potential opportunities for the discovery of new diabetes or obesity therapeutics.
In addition to the TRPm5 ion channel, our technology base encompasses additional approaches to identifying taste enhancers. Recognizing the attractiveness of "natural" compounds for food applications, we have developed a novel strategy to find natural substances that may be effective as taste enhancers. This strategy led to our discovery of the sweetness enhancement properties of RP44. The basic strategy involves sourcing both natural compounds and natural product compound libraries, and then screening these compounds using our proprietary, computer controlled, operant animal model system termed the Microtiter Operant Gustometer, or MOG. In this system rodents are trained to identify novel tastants through discrimination taste tests. The MOG methodology is based on classical animal models of operant conditioning that have been used in the academic community for 50 years. Redpoint's unique application of this classical method has been to develop novel, computerized protocols for training the rodents in taste discrimination tests, coupled with a new apparatus allowing testing of taste samples in 96 well microtitre plates of the kind used for high throughput screening in pharmaceutical discovery. The key advantages to this approach include:
- •
- Ability to find taste-active compounds using very small initial quantities of test materials (typically less than 1 mg.),
thus allowing cost-effective screening of purified natural products.
- •
- Ability to find taste-active compounds in partially purified natural product samples.
- •
- Ability to rapidly perform dose-ranging studies to directly determine in vivo potency.
Compounds determined as active using this approach are then subsequently scaled up and evaluated for taste properties in human taste tests and, potentially for regulatory approval. This strategy may provide additional opportunities for the discovery of new all natural sweetness enhancers, sweeteners and salt enhancers. The use of "natural" ingredient statements for labeling and promotion of food products is regulated by the USDA and other governing bodies, and there is a risk that they may limit use of such terms.
Sweetness Enhancer Program
We first announced that we had identified RP44, an all-natural sweetness enhancer, in June 2009. RP44 is Reb-C (rebaudioside C), a component of the stevia plant. Native to South America, extracts of the leaves of the stevia plant have been used as sweeteners around the world for hundreds of years. More recently, a purified component of stevia known as Reb A has received regulatory approval in the United States and elsewhere and Redpoint believes its popularity as an all-natural non-caloric sweetener is increasing.
In June 2010, we entered into a License and Commercialization Agreement with IFF for the development, manufacture, use and commercialization of RP44. IFF has exclusive rights, for five years, to develop, manufacture and commercialize RP44 in virtually all food and beverage product categories. In return, we received an upfront payment of $0.5 million and we became eligible to receive two milestone payments of $0.5 million each based upon certain criteria regarding regulatory approval and supply. In October 2010, IFF received notification by the Flavor and Extract Manufacturers Association (FEMA) that RP44 had been determined to be Generally Recognized As Safe (GRAS), triggering $0.5 million regulatory approval milestone payment. We have not yet achieved the supply milestone. In addition, we are eligible to receive royalties based on the amount of RP44 purchased by IFF for use in products. IFF will continue to be responsible for the regulatory process and for costs associated with prosecuting and maintaining our intellectual property covering the sweetness enhancer.
3
Unlike Reb A, RP44 has a very low intrinsic level of sweetness and therefore is not useful as a sweetener. Instead, we discovered that RP44 acts as a potent sweetness enhancer. A sweetness enhancer imparts no sweet taste of its own when used in a product. Instead, sweetness enhancers act by amplifying the existing sweet taste of caloric sweeteners such as sugar or high fructose corn syrup. We believe this will enable the development of food and beverage products that require reduced amounts of caloric sweeteners while still retaining the "clean sweet taste" associated with a fully sugared product. Taste tests demonstrate that RP44 enables the reduction of up to 25% of the caloric sweetener content in various product prototypes, while still maintaining the taste quality of the fully sweetened product. These results have been demonstrated by using RP44 in combination with several common sweeteners including sucrose (sugar), fructose, glucose and high-fructose corn syrup (HFCS). The worldwide sweetener market is estimated to be in excess of $50 billion with sugar (including sucrose, HFCS, and fructose) being the second most common ingredient used in food and beverages after water.
In October 2010, IFF informed us that they had received notification by the Flavor and Extract Manufacturers Association (FEMA) that RP44 had been determined to be Generally Recognized As Safe (GRAS) under the provisions of the Federal Food, Drug and Cosmetic Act, administered by the United States Food and Drug Administration (FDA). This GRAS determination allows RP44 to be incorporated into specified products in the United State and potentially aids regulatory acceptance in numerous other countries. Although RP44 is currently derived from material found in the side stream of the Reb A production process there can be no assurance that it can be produced in large scale on an economically viable basis.
We believe there is an important potential role for RP44 in all-natural reduced-calorie products. Numerous scientific studies suggest a compelling link between the high levels of refined sugar found in common food and beverage products and the worsening epidemic of obesity and diabetes worldwide. Health and wellness trends continue to be major market drivers for the food and beverage industry, creating consumer demand for natural solutions that can preserve the clean sweet taste of sugar while reducing calories.
It is increasingly recognized that excess consumption of sugar is a major contributor to disease. Both government and consumer groups have become alarmed at the rate of obesity in the U.S. population, and its related negative health consequences. The Center for Disease Control reports in its 2003-2006 National Health and Nutrition Examination Survey that there are more than 145 million overweight and obese adults and more than 23 million overweight and obese children and adolescents in the United States. Moreover, this phenomenon can be linked to certain chronic diseases including type-2 diabetes, high blood pressure, cardiovascular disease and certain cancers. This provides additional impetus for the development of products that can deliver the taste impact of sugar without the harmful effects of excess dietary calories.
Nutritive sweeteners such as cane sugar, beet sugar, and HFCS are among the most commonly used food ingredients. According to Packaged Facts, the U.S. total sweetener market was approximately $3.08 billion in 2007. The United States Department of Agriculture shows that the U.S. consumption of sweeteners, natural and nutritive, represents approximately 6% of the market, making the global sweetener market approximately $51.4 billion in 2007. In some of the largest food categories such as carbonated soft drinks and confectionary products, sugar or HFCS are the major ingredients and contributors to product cost of goods. The worldwide rise in consumption of sweet foods and beverages has been linked to many health problems, particularly obesity and diabetes.
Artificial sweeteners provide one means to reduce caloric intake and cost, particularly in sweetened beverages like soft drinks, confectionary products, and dessert foods. A report published by Global Industry Analysts shows the worldwide artificial sweetener market at $3.5 billion and is likely to be stimulated by a worldwide effort to combat obesity. Although there are several artificial sweeteners on
4
the market, there is a continued desire to develop new products that can preserve the clean sweet taste and temporal profile of nutritive sweeteners (e.g. sugar and HFCS) while also reducing the caloric content.
Currently used non-nutritive, artificial sweeteners include saccharin, aspartame, sucralose and acesulfame potassium. These sweeteners are also marketed under branded tabletop products such as Sweet 'N Low® (saccharin), Equal®, Nutrasweet® or Canderel® (aspartame) and Splenda® (sucralose). Food and beverage companies commonly mix artificial sweeteners to achieve specific taste profiles. Despite the wide use of artificial sweeteners, they are generally viewed to have inferior taste properties relative to caloric sweeteners like sugar and HFCS. Specifically, these include off-tastes (e.g. sweeteners like saccharin are bitter) and altered temporal characteristics of the sweet sensation. For example, the sweet taste of many artificial sweeteners comes on more slowly and/or lingers longer than the sweet taste from sugar or HFCS.
Growing consumer preference for all-natural products, together with the increasing rates of obesity and diabetes, have created significant demand for all-natural, low calorie sweetener alternatives. Reb A, a purified component of stevia recently received regulatory approval in the United States and elsewhere. Despite some consumers detecting bitter or licorice aftertaste in certain applications, according to a report by Rabobank, U.S. sales of Reb A could reach $700 million by 2014.
Salt Reduction Program
The objective of our salt enhancer program is to identify natural flavor ingredients that can provide a significant reduction in the amount of sodium in food and beverage products, yet maintain the salty taste that consumers desire.
We are using our proprietary MOG assay technology for the salt enhancer discovery program. The MOG technology was developed specifically to deal with the complex sensory and experimental issues associated with the discovery of all-natural compounds, which are often derived from plant or fermentation sources containing complex mixtures of taste ingredients. Historically, natural tastants or enhancers have been discovered by trial and error using human tasters. The MOG is an enabling technology that facilitates high throughput screens of tastant libraries and natural product extracts by rodents trained to discriminate specific taste standards. MOG-trained rodents are "expert" taste testers capable of rapidly identifying taste from small samples with a high level of accuracy, providing an effective means of evaluating the taste properties of complex natural product extracts.
We originally developed our MOG technology to discover natural high-potency sweeteners and sweetener enhancers. More recently, Redpoint scientists have developed and optimized a high-throughput salt-taste detection system, in which MOG-trained rodents discriminate salt from other essential tastes (savory, sweet, sour, and bitter). The MOG approach will be used to evaluate the salt-tasting and salt-enhancing potential of natural sources such as fermentation products and edible plant extracts, with a focus on isolating active components from ingredients already used in the food industry.
The goal of salty taste enhancement is to allow the use of reduced quantities of common salt (sodium chloride) in food and beverage products while preserving the salty taste level desirable to consumers. The Dietary Guidelines for Americans, 2010 published by the department of Health and Human Services (DHHS) and the Department of Agriculture (USDA) recommends an intake of less than 1,500 mg of sodium per day for most Americans, although the average American consumes more than 4,000 mg of sodium per day. Reducing the intake of sodium can be beneficial in reducing the incidence of hypertension and heart attacks in a susceptible, mainly aging, population. Current solutions for salt replacement have not fully succeeded in maintaining both the taste and functionality of sodium chloride.
5
Diabetes and Obesity Drug Discovery Program
Diabetes is a chronic disease afflicting over 24 million Americans and its poor control is the principle cause of blindness and kidney failure. Consequently, there is a continuing need for new and improved diabetes therapies, especially in an aging and increasingly obese population, which both correlate with an increased incidence of adult-onset diabetes.
Our initial programs focused on the modulation of the TRPm5 ion channel, a key signaling element in taste sensation, in order to discover novel compounds that modulate the taste of food and beverage products. The TRPm5 ion channel is a member of a larger class of related TRP (Transient Receptor Potential) ion channels that are involved in the sensation of heat, cold, and spicy compounds. The TRPm5 ion channel was originally identified as an important component of taste signaling circuits responsible for sensing sweet, savory, and bitter compounds on the tongue. Recently, an emerging body of scientific evidence has demonstrated that the TRPm5 channel is also found in the pancreas and gastrointestinal tract, suggesting a potential role in the regulation of metabolism and satiety. Specifically, TRPm5 may be involved in the secretion of important hormones like GLP1 and insulin that control sugar uptake and metabolism. Consequently, modulators of TRPm5 could potentially find application as a new therapy for adult-onset diabetes and obesity. We initiated a program designed to leverage the research we have already conducted on the discovery of modulators of the TRPm5 ion channel to further explore potential opportunities for the discovery of new diabetes or obesity therapeutics. In November 2010, we received notification that our diabetes program had been approved for a grant in the amount of approximately $0.25 million in connection with the Qualifying Therapeutic Discovery Project Program under section 48D of the Internal Revenue Code. This amount was received in November 2010.
TRPm5 modulators discovered by Redpoint have been shown to elicit the secretion of hormones known to play important roles in metabolism in relevant model systems. We believe this discovery may serve as the foundation for a new method of action in the treatment of obesity and diabetes. Our therapeutic objective is the development of an orally acting incretin and insulin secretogogue for the treatment of Type 2 diabetes. Through an ongoing discovery program involving the synthesis of approximately 1,000 novel small molecules, we have identified a lead series of orally acting hormone secretogogues that have demonstrated positive results in a number of in vitro and in vivo experiments. We are seeking collaborations with major pharmaceutical companies for the further development and optimization of our TRPm5 modulators for therapeutic applications in diabetes and obesity.
Diabetes mellitus is a chronic metabolic disorder characterized by the body's inability to produce or properly utilize insulin. Insulin is a hormone that is needed to convert sugar, starches and other food into energy stores needed for daily life. Nearly 90% of diabetics have Type 2 (non-insulin dependent) diabetes, which is often associated with obesity, and about 10% have Type 1 (insulin dependent) diabetes. It is one of the most prevalent chronic diseases, afflicting 8% of the U.S. population, and is a growing global health problem, particularly in industrialized countries. Diabetes and its complications impose significant economic consequences on individuals, families, health systems and countries. The World Health Organization (WHO) estimated that in 2008, more than 200 million people worldwide have diabetes. This number is likely to more than double by 2030. The total annual economic cost of diabetes in 2008 was estimated to be $175 billion in the United States and more than $350 billion worldwide. The global diabetes prescription market was more than $24 billion in 2008, and is expected to grow to more than $34 billion in 2012. The key market segments for diabetes treatments include injectables such as insulin and GLP-1 receptor agonists, and several classes of oral anti-diabetics (OADs), which include sulfonylureas, DPP-IV inhibitors, PPAR agonists, and biguanides.
In 2008, the leading products for treatment of diabetes, Actos® (Takeda Pharmaceutical Company Limited) and Avandia® (GlaxoSmithKline), recorded over $5 billion in annual sales, collectively. The most recent treatment advances for diabetes include Byetta®, a GLP-1 receptor agonist launched by
6
Eli Lilly and Company/Amylin Pharmaceuticals and Januvia™, a DPP-IV inhibitor launched by Merck & Co., Inc. Collectively, these new products reported 2008 sales in excess of $2 billion. Despite the range of current treatment options, diabetes remains a poorly managed disease, causing serious ancillary pathologies (e.g. neuropathy, nephropathy, retinopathy). We believe that TRPm5 modulators may represent a new class of therapeutics with a novel mode of action, useful in addressing this unmet medical need.
Competition and Competing Technologies
We believe our taste technology approach is applicable to a wide range of food and beverage products representing many and diverse competitive companies and approaches. The life sciences and other technology industries are characterized by rapid technological change, and the area of sensory or taste research is a rapidly evolving field. Our future success will depend on our ability to maintain a competitive position with respect to technological advances. Technological developments by others may result in our taste modulators and technologies, as well as our pharmaceutical products, becoming obsolete.
We face substantial competition from companies pursuing the commercialization of products and services relevant to taste using various methods for the discovery of taste modulators. These competitors include leading flavor companies, such as International Flavors & Fragrances Inc. ("IFF"), Givaudan SA ("Givaudan"), Symrise and Firmenich. Senomyx is principally focused in the area of taste enhancement for food products and uses a high throughput screening technology and synthetic chemistry to discover novel flavor enhancers, which it licenses to end users in the food industry. Nutrinova, a German ingredients company, is also developing a biotechnology approach to finding novel taste enhancers in collaboration with the German Biotechnology Research and Information Network. In certain instances we may also compete with companies who are commercializing Reb A. These companies include PureCircle Ltd and GLG Life Tech Corporation. We currently compete and will continue to compete in the future with these companies in collaborating with and selling flavor products and technologies to manufacturers of packaged food and beverage products.
We also face substantial competition with respect to our diabetes program. Type 2 diabetes is a disease characterized by persistent hyperglycemia due to the body's inability to produce enough or respond to insulin, a hormone regulating the absorption of sugar. Most treatments, outside of insulin replacement, focus on correcting high blood sugar by either stimulating the pancreas to produce more insulin, enhancing tissue sensitivity to insulin, or by inhibiting the production and release of glucose from the liver. There are many drugs currently available for treating diabetes and metabolic disease, either alone or in combination with other drugs. However, many patients are still inadequately treated with poorly controlled blood sugar levels, and the unmet medical need remains a concern.
There are several classes of anti-diabetic treatments, either orally available or injectable, including insulin, GLP-1 receptor agonists, sulfonylureas, DPP-IV inhibitors, PPAR agonists, and biguanides. Many of these drugs exhibit not only limited efficacy, but are also associated with less than desired tolerability and significant mechanism-based side effects. Several new classes of anti-diabetic treatments have recently been developed, including incretin mimetics such as Exendin-4 (Byetta®), a GLP-1 mimetic, which enhance glucose-dependent insulin secretion by the pancreatic beta-cell and slows gastric emptying, and DPP-IV inhibitors which work by preventing the inactivation of endogenous GLP-1. Additionally, new mechanisms such as GPR119 receptor agonists are being developed as glucose-dependent insulin and GLP-1 secretagogues expected to lower postprandial glucose and reduce weight gain.
If successful, our core diabetes program will face competition. The major competitors to TPRM5 modulators would likely include several new classes of anti-diabetic treatments, including the incretin mimetic Byetta® (marketed by Eli Lilly and Company and Amylin Pharmaceuticals), DPP-IV inhibitors
7
(including Januvia™ marketed by Merck & Co., Inc.), and GPR119 receptor agonists, currently in development by Metabolex, Inc., OSI Pharmaceuticals, GlaxoSmithKline, and Arena Pharmaceuticals, Inc.
All of the above mentioned companies have substantially greater capital resources, research and development resources and experience, manufacturing capabilities, regulatory expertise, sales and marketing resources, established relationships with consumer products companies and production facilities than us.
We may in the future face competition from life sciences and other technology companies and other commercial enterprises. These entities engage as we do in biotechnology, biology or chemistry and could apply this technology to the discovery and development of taste modulators and new pharmaceutical products. We cannot guarantee that products developed as a result of our competitors' existing or future collaborations will not compete with our taste modifiers and new pharmaceutical products.
Universities and public and private research institutions are also potential competitors. While these organizations primarily have educational objectives, they may develop proprietary technologies related to the sense of taste or secure patent protection that we may need for the development of our technologies and products. We may attempt to license these proprietary technologies, but these licenses may not be available to us on acceptable terms, if at all, or we may not have the cash to pay for them.
Although there are several options for treating diabetes currently on the market with new drugs in development, we believe that because of the failure of current therapies to adequately manage the disease, the diabetes market is receptive to new treatment options. We believe our TRPm5 modulators represent a first-in-class mechanism for the treatment of diabetes that could be used in combination with current and/or emerging drugs, and have the potential to experience rapid clinical acceptance.
Our competitors, either alone or with their collaborative partners, may succeed in developing technologies or discovering taste enhancers and aversive taste blockers and pharmaceutical products that are more effective, safer, more affordable or more easily commercialized than ours, and our competitors may obtain intellectual property protection or commercialize products sooner than we do. Developments by others may render our product candidates or our technologies obsolete. In addition, it is likely that any of our future product discovery and development collaborators would not be prohibited from entering into research and development collaboration agreements with third parties in any product field. Our failure to compete effectively would have a significant adverse effect on our business, financial condition and results of operations.
Regulatory Process
Regulatory Approval for New Food Flavors and Flavor Modifiers
We intend to utilize the GRAS determination process to market our taste enhancers. In the United States, novel flavoring compounds, flavor enhancers and taste modulators intended for use in foods are regulated under provisions of the Federal Food, Drug, and Cosmetic Act administered by the FDA. In situations where compounds to be introduced as flavorings into food products are to be administered at low levels and in defined quantities, safety review can first be obtained through the GRAS process, which can involve FEMA, other independent panels of experts, and/or FDA. Redpoint Bio joined FEMA as a participating member in May 2005 and has experience with the GRAS determination process.
The GRAS process was established by the 1958 Food Additive Amendments to the Federal Food, Drug, and Cosmetic Act. GRAS ingredients are those which have been found to be generally recognized as safe by "experts qualified by scientific training and experience to evaluate the safety of substances directly or indirectly added to food" (21 CFR 570.30). These experts can be convened by
8
any source, including FDA, industry (individual or corporation), or a trade association such as FEMA. The FEMA GRAS Panel, which has existed since 1960, has recognized many ingredients as GRAS. This process is the predominant process used to determine the GRAS status of synthetic and natural compounds that modify taste and/or flavor in the United States.
In the FEMA GRAS review program, an expert panel evaluates published literature, together with experimental toxicology, metabolism, and safety data and determines whether a compound is generally recognized as safe under the conditions and amounts of its intended use. The safety studies required for GRAS approval of a new chemical compound are extensive. GRAS safety studies typically involve the following: pharmacological activity profiling, genotoxicity studies, in vivo reproductive toxicology, ADME (absorption, distribution, metabolism, excretion), acute toxicology, and 90-day chronic dosing, including metabolism and histopathology. We estimate that the required tests can be performed and reported in 12 to 18 months at an estimated cost of $1-2 million. However, if there are prior safety data on the ingredient or an ingredient with a related structure, then fewer safety studies may be required for the GRAS review and the GRAS review process can be shorter than 12 to 18 months.
A FEMA GRAS determination process for new flavoring agents and taste modifiers confers some key advantages:
- •
- Rapid commercialization: A novel compound approved through
the FEMA GRAS process can reach the U.S. marketplace in two to three years after initiation of safety studies.
- •
- Relatively low development costs: Studies required to
obtain FEMA GRAS approval typically cost about $1 to $2 million per new compound.
- •
- Facilitation of the European Union, or EU, and other major market approval: Although new flavoring agents are approved in Europe and the rest of the world on a country by country basis, FEMA is coordinating with the International Organization of the Flavor Industry, or IOFI, and the EU Regulatory body JECFA (Joint FAO/WHO Expert Committee on Food Additives) to facilitate the approval of FEMA GRAS compounds for sale in the EU and worldwide.
In October 2010, IFF received notification by the Flavor and Extract Manufacturers Association (FEMA) that RP44 had been determined to be Generally Recognized As Safe (GRAS).
While there is ample historic precedent, there can be no assurance that regulatory requirements will remain stable and that the GRAS pathway will be available in its current or modified form.
Regulatory Approval for New Therapeutics
Any products we may develop as new therapeutics (e.g. the use of our compounds as drugs to treat diabetes or obesity) are subject to stringent government regulation in the United States by the FDA and, in many instances, by corresponding foreign and state regulatory agencies. The European Union, or EU, has vested centralized authority in the European Medicines Evaluation Agency and Committee on Proprietary Medicinal Products to standardize review and approval across EU member nations.
These regulatory agencies enforce comprehensive statutes, regulations, and guidelines governing the drug development process. This process involves several steps. Initially, the company must generate preclinical data to show safety before human testing may be initiated. In the United States, the drug company must submit an IND to the FDA prior to securing authorization for human testing. The IND must contain adequate data on product candidate chemistry, toxicology and metabolism and, where appropriate, animal research testing to support initial safety.
A CTA is the European equivalent of the U.S. IND. CTA requirements are issued by the Medicines and Healthcare Products Regulatory Agency, the United Kingdom's health authority and were enacted through the U.K. Medicines for Human Use (Clinical Trials) Regulations 2004, which implemented the EU Clinical Trials Directive in the United Kingdom.
9
Any of our product candidates will require regulatory approval and compliance with regulations made by the United States and foreign government agencies prior to commercialization in such countries. The process of obtaining FDA or foreign regulatory agency approval has historically been extremely costly and time consuming. The FDA regulates, among other things, the development, testing, manufacture, safety, efficacy, record keeping, labeling, storage, approval, advertising, promotion, sale, and distribution of biologics and new drugs.
The standard process required by the FDA before a pharmaceutical agent may be marketed in the United States includes:
- •
- preclinical tests in animals that demonstrate a reasonable likelihood of safety and effectiveness in human patients;
- •
- submission to the FDA of an IND, which must become effective before clinical trials in humans can commence. If
Phase I clinical trials are to be conducted initially outside the United States, a different regulatory filing is required, depending on the location of the study;
- •
- adequate and well controlled human clinical trials to establish the safety and efficacy of the drug or biologic in the
intended disease indication;
- •
- for drugs, submission of a New Drug Application, or NDA with the FDA; and
- •
- FDA approval of the NDA before any commercial sale or shipment of the drug.
Preclinical studies can take several years to complete, and there is no guarantee that an IND based on those studies will become effective to permit clinical trials to begin. Once clinical trials are initiated, they generally take five to seven years, or longer, to complete. After completion of clinical trials of a new drug or biologic product, FDA approval of the NDA must be obtained. This process requires substantial time and effort and there is no assurance that the FDA will accept the NDA for filing and, even if filed, that the FDA will grant approval. In the past, the FDA's approval of an NDA has taken, on average, one to two years, but in some instances may take substantially longer. If questions regarding safety or efficacy arise, additional studies may be required, followed by a resubmission of the NDA. Review and approval of an NDA can take several years.
In addition to obtaining FDA approval for each product, each drug manufacturing facility must be inspected and approved by the FDA. All manufacturing establishments are subject to inspections by the FDA and by other federal, state, and local agencies, and must comply with GMP requirements.
We must also obtain regulatory approval in other countries in which we intend to market any drug. The requirements governing conduct of clinical trials, product licensing, pricing, and reimbursement vary widely from country to country. FDA approval does not ensure regulatory approval in other countries. The current approval process varies from country to country, and the time spent in gaining approval varies from that required for FDA approval. In some countries, the sale price of the drug must also be approved. The pricing review period often begins after market approval is granted. Even if a foreign regulatory authority approves a drug product, it may not approve satisfactory prices for the product.
In addition to regulations enforced by the FDA, we are also subject to regulation under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act, and other present and potential future federal, state, or local regulations. Our research and development involves the controlled use of hazardous materials, chemicals, biological materials, and various radioactive compounds. Although we believe that our safety procedures for handling and disposing of such materials currently comply in all material respects with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result and any such liability could exceed our available resources.
10
Marketing and Sales
As a development stage company, we currently have no sales, marketing or distribution capabilities. In general, the Company will be dependent on our future collaborators for the production, marketing, sales and other aspects of the commercialization of our product candidates when such products are developed through collaborative discovery and development programs with major food ingredient suppliers, food producers, or pharmaceutical companies. For example, with respect to RP44, pursuant to our license agreement, IFF will be responsible for establishing a supply chain and commercializing the product.
Intellectual Property
We currently own or have exclusive licenses to 15 issued U.S. patents and three issued foreign patents. Also, we own or have exclusive licenses to over 94 U.S. and foreign patent applications pending. In addition, we have assembled significant expertise and know-how in the biology, molecular biology, cell biology and chemistry of taste and taste modifiers. Redpoint Bio's founding intellectual property portfolio was licensed from the Mount Sinai School of Medicine. In addition to intellectual property in-licensed from Mount Sinai, scientists at Redpoint Bio have extended the portfolio with several new patent families. These include:
- •
- New assays and assay technology for taste modulation in food and pharmaceutical products.
- •
- New methods and compositions for blocking bitterness.
- •
- New methods and compositions for enhancing sweet and savory/umami flavors.
- •
- New methods and compositions for enhancing the sweetness of carbohydrate sugars and high intensity sweeteners.
- •
- New methods and apparatus for operant models of taste selection using in
vivo models.
- •
- New methods and compositions for enhancing insulin release, GLP-1 release, and insulin sensitivity, and for treating diabetes, insulin resistance syndrome, hyperglycemia and obesity.
Our primary intellectual property strategy is to obtain composition-of-matter and utility patents on new compounds that can be approved through the GRAS regulatory or FDA drug approval processes.
Members of our scientific and management team have been named as inventors on over 60 issued U.S. patents, covering various areas of drug discovery, and have a strong track record of successful management of intellectual property covering technology tools, new compositions of matter with pharmaceutical utility, and uses thereof.
Collaborative and Licensing Arrangements
International Flavor & Fragrances Inc.
In June 2010, we entered into a License and Commercialization Agreement with IFF for the development, manufacture, use and commercialization of RP44. IFF has exclusive rights, for five years, to develop, manufacture and commercialize RP44 in virtually all food and beverage product categories. In return, we received an upfront payment of $0.5 million and we became eligible to receive two milestone payments of $0.5 million each based upon certain criteria regarding regulatory approval and supply. In October 2010, IFF received notification by the Flavor and Extract Manufacturers Association (FEMA) that RP44 had been determined to be Generally Recognized As Safe (GRAS), triggering the $0.5 million regulatory approval milestone payment. We have not yet achieved the supply milestone. In addition, we are eligible to receive royalties based on the amount of RP44 purchased by IFF for use in products. IFF will continue to be responsible for the regulatory process and for costs associated with prosecuting and maintaining our intellectual property covering the sweetness enhancer.
11
Strategy
In addition to our agreement with IFF for the development, manufacture, use and commercialization of RP44, our strategy includes partnering with other major ingredient suppliers, or food and beverage companies, to utilize our technology to discover new all natural sweetness enhancers, sweeteners, and salt enhancers in exchange for technology access fees, research funding, product development milestones, and product royalties. We are also seeking collaborations with major pharmaceutical companies for the further development and optimization of our compounds for therapeutic applications in diabetes and obesity. Any failure to maintain or establish licensing or collaboration arrangements on favorable terms could adversely affect our business prospects, financial condition or ability to develop and commercialize our product candidates.
Employees
During the year ended December 31, 2009, we announced two restructurings which reduced our workforce by approximately 60%. As of December 31, 2010, we employed ten persons on a full-time basis, of whom seven were in research and development departments, and three of whom were engaged in finance, business development and administration. Of these employees, nine hold Ph.D. or other advanced degrees. We also retain outside consultants. We believe that while we have been successful to date in attracting skilled and experienced scientific personnel, competition for such personnel continues to be intense and there can be no assurance that we will continue to be able to attract and retain the professionals we will need to grow our business.
Our Executive Officers and Directors
The following table identifies our current executive officers and directors:
Name
|
Age | Capacities in Which Served | In Current Position Since |
||||||
|---|---|---|---|---|---|---|---|---|---|
F. Raymond Salemme, Ph.D.(1) |
65 | President, Chief Executive Officer and Director | 2007 | ||||||
Scott M. Horvitz(2) |
52 | Chief Financial Officer, Treasurer and Secretary | 2007 | ||||||
Richard P. Shanley, CPA(3) |
63 | Chairman of the Board | 2007 | ||||||
Robert Chefitz(4) |
51 | Director | 2007 | ||||||
Leif Kjaergaard, Ph.D.(5) |
64 | Director | 2007 | ||||||
Allen Bloom, Ph.D.(6) |
67 | Director | 2007 | ||||||
Irwin Scher, M.D.(7) |
71 | Director | 2007 | ||||||
- (1)
- F. Raymond Salemme, Ph.D. became Robcor's President on April 20, 2007 and Robcor's Chief Executive Officer and director on March 12, 2007. Dr. Salemme has been the Chief Executive Officer of Original Redpoint since June 2004. From April 2003 to December 2003, Dr. Salemme was engaged by Johnson & Johnson Pharmaceuticals as a consultant to assist management with the acquisition of 3-Dimensional Pharmaceuticals, Inc., or 3DP, a publicly traded biotechnology company founded in 1993, and acquired by Johnson & Johnson in 2003. Dr. Salemme was the founder of 3DP, and from February 1993 to April 2003, Dr. Salemme served in various positions at 3DP, including President and Chief Scientific Officer. Dr. Salemme established and directed the technical, operating, business and financing strategies leading to 3DP's initial public offering in 2000, and was co-inventor on 24 of 3DP's key technology patents. Prior to 3DP, Dr. Salemme directed research groups for biophysics, computational chemistry, and structure-based drug design at Sterling Winthrop Pharmaceuticals and DuPont Merck Pharmaceuticals. Prior to DuPont Merck, he worked at DuPont Central Research, where he directed molecular design teams involved in the discovery of new pharmaceuticals, agrochemicals, and engineered biopolymers. Dr. Salemme joined DuPont from Genex Corporation, one of the first biotechnology companies, where he pioneered the use of X-ray crystallography and computer-aided molecular design for engineering proteins
12
with novel or enhanced functional properties. Prior to Genex, Dr. Salemme was Professor of Chemistry and Biochemistry at the University of Arizona, where he worked in the area of structural proteomics and determined the 3D X-ray structures of several proteins involved in biological electron transfer. Dr. Salemme holds a B.A. in Molecular Biophysics from Yale University and a Ph.D. in Chemistry from the University of California at San Diego. Dr. Salemme participates in various advisory committees, is a founder at Imiplex LLC, a nanotechnology company, and a Board member at Via, a healthcare data analysis company and ExSar, a protein analysis company.
- (2)
- Scott M. Horvitz became Robcor's Chief Financial Officer, Treasurer and Secretary on March 12, 2007.
Mr. Horvitz has been Original Redpoint's Chief Financial Officer, Corporate Secretary and Treasurer since July 2004. Mr. Horvitz has approximately 25 years of experience in
accounting and financial management of biotechnology companies, including the management of initial public offerings with his two previous companies. Prior to becoming Chief Financial Officer of
Original Redpoint, Mr. Horvitz was a consultant to Original Redpoint from August 2003 to June 2004. Previously, Mr. Horvitz co-founded 3-Dimensional
Pharmaceuticals, Inc. where he worked from September 1993 until its acquisition by Johnson & Johnson in July 2003, most recently serving as its Chief Financial Officer and Corporate
Secretary and Treasurer. Prior to 3DP, Mr. Horvitz was the Executive Director of Finance and Human Resources with Magainin Pharmaceuticals. Mr. Horvitz holds a B.S. in Accounting from
the University at Albany and is a Certified Public Accountant. Mr. Horvitz served on the Board of Directors of Protez Pharmaceuticals, Inc. and was the Chairman of its Audit Committee
from 2004 until its acquisition by Novartis in 2008.
- (3)
- Richard P. Shanley, CPA was appointed as a director on September 12, 2007, and as Chairman of the
Board on February 23, 2010. Mr. Shanley, a Certified Public Accountant in New York, retired from public accounting at the end of 2006, having completed a 31 year career at
Deloitte & Touche LLP and Eisner LLP, with a combined total of 25 years as a Partner at the two firms. During his career, Mr. Shanley served as lead audit partner on
numerous engagements for both public and private companies, including those requiring application of Sarbanes-Oxley Section 404, as well as public stock offerings, primarily for biotechnology,
pharmaceutical and high-tech companies. Mr. Shanley is a recognized authority on financing for emerging growth companies and is a published author of Financing Technology's Frontier. He has spoken
at industry meetings and is actively involved in organizations including the Biotech Council of New
Jersey, the New York Biotechnology Association, the Connecticut Venture Group, the Biotechnology Industry Organization, and others. Mr. Shanley is currently serving as the chair of the New York
State Society of CPA's Professional Ethics Committee. Mr. Shanley currently serves on the Board of Harris & Harris Group, Inc. He earned his B.S. degree from Fordham University
and his M.B.A. in Accounting from Long Island University.
- (4)
- Robert Chefitz has been a General Partner of NJTC Venture Fund, SBIC, L.P., a private venture capital
firm based in New Jersey that is a lead investor in seed and early stage companies in the region since April 2002. Prior to joining the NJTC Venture Fund, from February 1987 to October 2001,
Mr. Chefitz was a partner at Apax Partners based in New York. Prior to Apax Partners, Mr. Chefitz was employed as an investment professional with Golder Thoma Cressey in Chicago.
Mr. Chefitz is a past president of the New York Venture Association. Mr. Chefitz holds a B.A. in History from Northwestern University and a M.B.A. from Columbia University. In addition
to Redpoint, Mr. Chefitz serves as a director on the boards at Hycrete Technologies, LLC, Achieve 3000, Point Blank Solutions, Inc., Westec Intelligent Surveillance, Alarm.com,
The Ladders.com, and RightAnswers, Inc. Mr. Chefitz has been a director of Original Redpoint since 2003.
- (5)
- Leif Kjaergaard, Ph.D., is currently the President of Leif and Food Science, a consulting firm to the food industry. Prior to that he was the Chief Technology Officer and a member of the executive committee at Danisco A/S, an international food ingredients and biotechnology company
13
based in Denmark from 2006 to 2008. Dr. Kjaergaard was responsible for new technology ventures and business development throughout Danisco. From 2002 to 2006, Dr. Kjaergaard was Senior Vice President of Danisco, and, from 1993 to 2002, he served as Executive Vice President and Department President of Danisco Cultor (formerly Danisco Ingredients). Dr. Kjaergaard has a M.Sc. in Chemical Engineering and a Ph.D. in Biotechnology from the Technical University of Denmark. Dr. Kjaergaard is a member of the European Research Advisory Board, past President of the Board of European Industrial Research Managers Association, and deputy chair of the Advisory Council on Research Policy in Denmark. He also serves as deputy chairman of the independent company BHJ in Denmark, and a member of the board of the Danish Investment Fund (Dansk Innovationsinvestering), a board member of Direvo Biotech (Germany) and a senior advisor to the American Investment bank AgriCapital Inc. Dr. Kjaergaard has been a director of Original Redpoint since 2006.
- (6)
- Allen Bloom, Ph.D. was appointed as a director on September 12, 2007. From 1994 until 2003,
Dr. Bloom, a patent attorney, was a partner at the law firm of Dechert LLP, where he served as Co-Chair of the Intellectual Property Group and headed a patent practice group
focusing on biotechnology, pharmaceuticals and medical devices. From 1985 until mid-1994, Dr. Bloom was Vice President, General Counsel and Secretary of The Liposome
Company, Inc., where he managed patent, regulatory and licensing activities. Before that, Dr. Bloom served as Patent Attorney and Patent Counsel for Pfizer, Inc. and RCA
Corporation, respectively. Dr. Bloom currently serves on the Board of the biopharmaceutical company Unigene Laboratories, Inc., where he is lead director. He earned his Ph.D. in organic
chemistry from Iowa State University, a J.D. degree from New York Law School and a B.S. in Chemistry from Brooklyn College.
- (7)
- Irwin Scher, M.D. was appointed as a director on September 12, 2007. Since 2005, Dr. Scher has held the position of President and Chief Executive Officer of Biosynexus, a biotechnology company focused on treating and preventing Staphylococcal infections. Prior to joining Biosynexus, Dr. Scher spent 22 years in various positions at Merck, Astra/Merck, Astra Zeneca and until 2005 as Consultant to the Chief Executive Officer at Merck KGaA. Before joining Merck, Dr. Scher enjoyed a long career in academic medicine. He held the rank of Captain USN Medical Corps and was stationed for 12 years at the Bethesda Naval Medical Complex. While there, Dr. Scher served as Professor of Medicine, Uniformed Services University, and Head, Immunology Branch, Naval Medical Research Institute. Dr. Scher is certified by the American Board of Rheumatology and American Board of Internal Medicine. He received his M.D. from Albert Einstein College of Medicine in New York and completed residencies at Presbyterian Hospital, University of Pittsburgh Medical Center, and Albert Einstein College of Medicine. Dr. Scher holds a B.S. in Chemistry from the State University of New York at Albany.
None of our executive officers is related to any other executive officer or to any of our Directors. Our executive officers are elected annually by the Board of Directors and serve until their successors are duly elected and qualified.
Corporate History
Redpoint was incorporated in August 1995 under the name of Linguagen Corp., based on the pioneering work of its scientific founder Robert Margolskee, M.D., Ph.D. Dr. Margolskee's laboratory has made major discoveries in the molecular biology of taste signaling pathways. We have obtained exclusive licenses to certain technology patents, covering discoveries in his laboratory, from the Mount Sinai School of Medicine.
In March 2007, we entered into the Agreement and Plan of Merger, dated as of March 12, 2007, by and among Redpoint, on the one hand, and Robcor, Merger Sub, Robcor, LLC, and Halter
14
Financial Investments, L.P. and Michael Heitz, as stockholders of Robcor, on the other hand. The transactions contemplated by the Reverse Merger Agreement were consummated on March 12, 2007.
Pursuant to the Reverse Merger Agreement, on the Closing Date, Merger Sub merged with and into Redpoint, with Redpoint being the surviving corporation and becoming a wholly-owned subsidiary of Robcor, and the outstanding shares of capital stock, convertible notes and certain warrants to purchase capital stock of Redpoint were converted into shares of common stock of Robcor in accordance with the Delaware General Corporation Law on the terms and conditions as set forth in the Reverse Merger Agreement. On May 3, 2007, Redpoint and Robcor entered into an Agreement and Plan of Merger in which Robcor, the former parent company of Redpoint after consummation of the Reverse Merger, merged with and into Redpoint. Such merger, referred to herein as the Reincorporation Merger, was deemed effective on June 15, 2007.
Redpoint was deemed to have been the accounting acquirer in the Reverse Merger. Accordingly, the financial statements of the Company presented reflect the historical results of Redpoint prior to the Reverse Merger, and of the combined entities following the Reverse Merger, and do not include the historical financial results of Robcor prior to the consummation of the Reverse Merger. For analysis of the accounting treatment of the Reverse Merger and the Reincorporation Merger as well as our business, please see the accompanying financial statements.
Our principal executive offices are located at 7 Graphics Drive, Ewing, New Jersey, 08628. Our telephone number is (609) 637-9700. Our website address is www.redpointbio.com. The information contained on our website is not incorporated by reference into, and does not form any part of, this annual report.
Available Information
We make available the following public filings with the Securities and Exchange Commission, or the SEC, free of charge through our Web site at www.redpointbio.com as soon as reasonably practicable after we electronically file such material with, or furnish such material to, the SEC:
- •
- our Annual Reports on Form 10-K and any amendments thereto;
- •
- our Quarterly Reports on Form 10-Q and any amendments thereto; and
- •
- our Current Reports on Form 8-K and any amendments thereto.
In addition, we make available our code of conduct free of charge through our web site. We intend to disclose any amendments to, or waivers from, our code of conduct that are required to be publicly disclosed pursuant to rules of the SEC and NASDAQ by filing such amendment or waiver with the SEC and posting it on our web site.
No information on our internet web site is incorporated by reference into this Annual Report on Form 10-K or any other public filing made by us with the SEC.
You should carefully consider the following risks and all of the other information set forth in this Annual Report on Form 10-K before deciding to invest in shares of our common stock. The risks described below are not the only ones facing us. Additional risks not presently known to us or that we currently deem immaterial may also impair our business operations.
If any of the following risks actually occurs, our business, financial condition or results of operations would likely suffer. In such case, the market price of our common stock would likely decline due to the occurrence of any of these risks, and you may lose all or part of your investment.
15
Risks Related To Our Business and Our Industry
As of December 31, 2010, we had approximately $1.1 million of cash and cash equivalents. As of the date of filing this annual report on Form 10-K, we have approximately $0.7 million of cash and cash equivalents. We will need to raise substantial additional capital before April 2011 to fund our future operations or we will be unable to satisfy our obligations and continue our business.
We cannot be certain that additional financing will be available on acceptable terms, or at all. In recent years, it has been difficult for companies to raise capital due to a variety of factors, which may or may not continue. To the extent we raise additional capital through the sale of equity securities, the ownership position of our existing stockholders could be substantially diluted. If additional funds are raised through the issuance of preferred stock or debt securities, these securities are likely to have rights, preferences and privileges senior to our common stock. Fluctuating interest rates could also increase the costs of any debt financing we may obtain. Failure to successfully address ongoing liquidity requirements will have a material adverse effect on our business. If we are unable to obtain additional capital on acceptable terms when needed, we may be required to take actions that harm our business and our ability to achieve cash flow in the future, including possibly the surrender of our rights to some technologies or product opportunities, delaying our clinical trials or curtailing or ceasing operations.
We will need substantial additional funding to develop our products and for our future operations. If we are unable to obtain the funds necessary to do so in the near term, we may be required to delay, scale back or eliminate our product development or may be unable to continue our business.
The development of our product candidates will require a commitment of substantial funds to conduct the costly and time-consuming research, which may include pre-clinical and clinical testing, necessary to obtain regulatory approvals and bring our products to market. We believe that our current capital resources are only sufficient to meet our operating and capital requirements through April 2011. Our future requirements will depend on many factors, including:
- •
- the progress of our research and development programs, including our ability to discover and develop new taste modifiers;
- •
- our ability, or our partners' ability and willingness, to formulate these taste modifiers, including RP44, into food and
beverage products;
- •
- our ability to discover and develop new diabetes or obesity therapeutic compounds;
- •
- the cost of prosecuting, defending and enforcing patent claims and other intellectual property rights;
- •
- the progress, scope and results of our pre-clinical and clinical testing of any future pharmaceutical
products;
- •
- the time and cost involved in obtaining regulatory approvals;
- •
- the cost of manufacturing our product candidates;
- •
- competing technological and market developments; and
- •
- our ability to establish and maintain collaborative and other arrangements with third parties to assist in bringing our products to market and the cost of such arrangements.
There can be no assurance that we will not need additional capital sooner than currently anticipated.
16
Redpoint is a development-stage company. We have incurred losses since inception and expect to incur additional net losses for at least the next several years, even if we are able to raise additional funds necessary to continue our business.
Since our inception we have incurred significant losses and negative cash flows from operations. As of December 31, 2010, we had an accumulated deficit of $56.8 million, and anticipate incurring additional losses for the foreseeable future. We will need to spend significant resources over the next several years to enhance our technologies and to fund research and development of our pipeline of potential products. In order to advance our programs, we will need to raise additional capital or fund operations through collaborations with third parties. We have not generated significant revenues in any particular year since our inception. To date, substantially all of our revenue has been derived from corporate collaborations, license agreements, and government grants. In order to achieve profitability, we must develop products and technologies that can be commercialized by us or through future collaborations. Our ability to generate revenues and become profitable will depend on our ability, alone or with potential collaborators, to timely, efficiently and successfully complete the development of our product candidates, which may include conducting pre-clinical and clinical tests, obtaining necessary regulatory approvals, and manufacturing and marketing our product candidates. There can be no assurance that any such events will occur or that we will ever become profitable. Even if we do achieve profitability, we cannot predict the level of such profitability. If we sustain losses over an extended period of time, we may be unable to continue our business.
We are an early stage company and currently have no products available for sale. Our product candidates require additional research, development, testing, expert reviews and/or regulatory approvals before marketing. We may be unable to develop, obtain regulatory approval or market any of our product candidates. If our product candidates are delayed or fail, our financial condition will be negatively affected, and we may have to curtail or cease our operations.
We are in the early stage of product development and we are dependent on new discoveries. We currently do not sell any products to third parties and do not expect to have any products commercially available in the immediate future, if at all. You must evaluate us in light of the uncertainties and complexities affecting an early stage biotechnology company. Our product candidates require additional research and development, pre-clinical testing, clinical testing (for our pharmaceutical products) and regulatory review and/or approvals clearances before marketing. There are many reasons that our product candidates may fail or not advance to commercialization, including the possibility that:
- •
- our product candidates may be ineffective, unsafe or associated with unacceptable side effects;
- •
- our product candidates may fail to receive the necessary regulatory approvals or otherwise fail to meet applicable
regulatory standards;
- •
- experts may not agree that our food product candidates are generally recognized as safe;
- •
- our product candidates, including RP44, may be too expensive to develop, manufacture or market;
- •
- other parties may hold or acquire proprietary rights that could prevent us or our potential collaborators from developing
or marketing our product candidates;
- •
- physicians, patients, third-party payers or the medical community in general may not accept or use our contemplated
pharmaceutical products;
- •
- our current and potential collaborators may withdraw support for or otherwise impair the development and commercialization
of our product candidates; or
- •
- others may develop equivalent or superior products.
17
In addition, we, or our collaborators, may not succeed in developing taste modulators, including RP44, with the appropriate attributes required for use in successful commercial products. Successful taste modulators require, among other things, appropriate biological activity, including the correct properties for the product application, an acceptable safety profile, including lack of toxicity or allergenicity, and appropriate physical or chemical properties, including relative levels of stability, volatility and resistance to heat. Successful taste enhancers must also be cost-efficient. We may not be able to develop taste modulators that meet these criteria.
If our product candidates are delayed or we fail to successfully develop and commercialize our product candidates, our financial condition may be negatively affected, and we may have to curtail or cease our operations.
We may not successfully establish and maintain collaborative and licensing arrangements, which could adversely affect our ability to develop and commercialize our product candidates.
A key element of our food and beverage strategy is to commercialize taste modulators through licensing arrangements with major ingredient suppliers and/or end users, such as the IFF Agreement. Our pharmaceutical strategy depends on establishing collaborations and licensing agreements with major pharmaceutical companies. We may not be able to maintain or expand these licenses and collaborations or establish additional licensing and collaboration arrangements necessary to develop and commercialize our product candidates. For example, we were unable to renew our one year research agreement with The Coca-Cola Company in 2008 and our agreement with Givaudan ended on May 1, 2009. Even if we are able to maintain or establish licensing or collaboration arrangements, these arrangements may not be on favorable terms and may contain provisions that will restrict our ability to develop, test and market our product candidates. Any failure to maintain or establish licensing or collaboration arrangements on favorable terms could adversely affect our business prospects, financial condition or ability to develop and commercialize our product candidates.
We expect to rely at least in part on third party collaborators to perform a number of activities relating to the development and commercialization of our product candidates, including the manufacturing of product materials, the design and conduct of clinical trials for our pharmaceutical candidates, and potentially the obtaining of regulatory approvals and marketing and distribution of any successfully developed products. Our collaborative partners may also have or acquire rights to control aspects of our product development and clinical programs. As a result, we may not be able to conduct these programs in the manner or on the time schedule we currently contemplate. In addition, if any of these collaborative partners withdraw support for our programs or product candidates or otherwise impair their development, our business could be negatively affected. To the extent we undertake any of these activities internally, our expenses may increase.
In addition, our success depends on the performance of our collaborators of their responsibilities under these arrangements. Some potential collaborators may not perform their obligations in a timely fashion or in a manner satisfactory to us. Because such agreements may be exclusive, we may not be able to enter into a collaboration agreement with any other company covering the same product field during the applicable collaborative period. In addition, our collaborators' competitors may not wish to do business with us at all due to our relationship with our collaborators. If we are unable to enter into additional product discovery and development collaborations or maintain our existing arrangements, our ability to sustain or expand our business will be significantly diminished.
18
If we or our collaborators are unable to obtain and/or maintain the GRAS determination or regulatory approval required before any taste modulator, including RP44, can be incorporated into products that are sold, we would be unable to commercialize our taste modulators and our business would be adversely affected.
In the United States, the development, sale and incorporation of our taste modulators into products are subject to regulation by the FDA, and in some instances, other government bodies. Obtaining and maintaining a GRAS determination or regulatory approval is typically costly and can take many years.
Depending on the amount or intended use of a particular taste modulator added to a product and the number of product categories in which the flavor or flavor enhancer will be incorporated, specific testing, safety assessment protocols, and regulatory processes must be satisfied before we or our collaborators can commercially market and sell products containing any taste modulators that we may discover. A key element of our strategy is to develop flavors and flavor enhancers that will be evaluated by the FEMA (Flavor and Extracts Manufacturers Association) GRAS Panel, which we expect will take 18 to 24 months and which is less expensive than the alternative of filing a food additive petition with the FDA, which can take eight years or more. The FEMA GRAS review process may take longer than 24 months and cost more than we currently anticipate if additional safety studies are requested by the FEMA GRAS Panel or are necessary to explain unexpected safety study findings. There is a risk that one or more of our product candidates may not qualify for a FEMA GRAS determination or that RP44 may not maintain FEMA GRAS status. This may occur for a variety of reasons, including the taste modulator's intended use, the amount of the taste modulator intended to be added to packaged foods and beverages, the number of product categories in which the taste modulator will be incorporated, whether the taste enhancer imparts sweetness, the safety profile of the taste enhancers and the FEMA GRAS Panel's interpretation of the safety data. Even if we obtain a GRAS determination with respect to a taste enhancer, the FDA has the ability to challenge such determination, which could materially adversely affect our ability to market products on schedule or at all. In the event that a particular taste modulator does not qualify for a FEMA GRAS determination, we may be required to pursue a lengthy FDA approval process to reach the U.S. market, or dedicate our development efforts to alternative compounds, which would further delay commercialization. In addition, laws, regulations or FDA practice governing the regulatory approval process, the availability of the GRAS determination process or the manufacture or labeling of such products, may change in a manner that could adversely affect our ability to commercialize products on schedule or at all.
Sales of our taste modulators, including RP44, outside of the United States will be subject to foreign regulatory requirements. In most cases, whether or not a GRAS determination or FDA approval has been obtained, approval of a product by the comparable regulatory authorities of foreign countries must still be obtained prior to manufacturing or marketing the product in those countries. A GRAS determination or FDA approval in the United States or in any other jurisdiction does not ensure approval in other jurisdictions because the requirements from jurisdiction to jurisdiction may vary widely. Obtaining foreign approvals could result in significant delays, difficulties and costs for us and require additional safety studies and additional expenses. If we fail to comply with these regulatory requirements or to obtain and maintain required approvals, our ability to generate revenue will be diminished.
We and our collaborators may not be successful in overcoming these regulatory hurdles, which could result in product launch delays, unanticipated expenses, termination of collaborations, and flavors and flavor enhancers not being approved for incorporation into consumer products. These consequences would have a material adverse effect on our business financial condition and results of operations.
19
Even if we or our collaborators receive a GRAS determination or regulatory approval and incorporate our taste modulators, including RP44, into products, those products may never be commercially successful.
Even if we discover and develop modulators, including RP44, that obtain the necessary GRAS determination or regulatory approval, our success depends to a significant degree upon the commercial success of packaged food and beverage products incorporating those modulators. If these products fail to achieve or subsequently maintain market acceptance or commercial viability, our business would be significantly harmed because our royalty revenue is dependent upon consumer sales of these products. In addition we could be unable to maintain our existing collaborations or attract new product discovery and development collaborators. Many factors may affect the market acceptance and commercial success of any potential products incorporating taste modulators that we may discover, including:
- •
- health concerns, whether actual or perceived, or unfavorable publicity regarding our taste modulators, including RP44, or
those of our competitors;
- •
- the timing of market entry as compared to competitive products;
- •
- the rate of adoption of products by our collaborators and other companies in the flavor industry; and
- •
- any product labeling that may be required by the FDA or other United States or foreign regulatory agencies for products incorporating our taste modulators, including RP44.
We may experience delays in clinical trials and regulatory approval relating to our products that could adversely affect our financial results and our commercial prospects for our pharmaceutical product candidates.
Any uses of our compounds that we develop as drugs acting to modulate incretin secretion with potential utility as therapeutic agents for diabetes or obesity, will be subject to full FDA 505(b)(1) safety testing and clinical trials demonstrating therapeutic efficacy as required to develop an NDA. We will be required to conduct clinical trials to demonstrate safety and efficacy of our product candidates for each intended use. For product candidates that advance to clinical testing, we cannot be certain that we or a collaborator will successfully complete the clinical trials, and will obtain the results necessary to receive regulatory approvals for the drug. These processes are lengthy, expensive and can have uncertain outcomes. Regulatory authorities may disagree with our interpretations of the clinical trial results. There can be no assurance that clinical trials will be deemed by regulatory authorities as demonstrating that our product candidates are safe and effective to obtain approval for commercial distribution.
We do not know when clinical trials for our products will commence or whether we will complete any of our clinical trials on schedule or at all. We may not be able to identify and successfully contract with an adequate number of clinical trial sites and clinical investigators. We may not be able to find a sufficient number of study subjects or may experience delays in enrolling patients for our clinical trials. Trial sites may not be able to meet the targets for recruiting study subjects, because of limited patient pools, or strict criteria for enrolling in the trial.
Additionally, the FDA, the IRB or we may suspend our clinical trials at any time if there is reason to believe that we are exposing the subjects participating in the trials to unacceptable health risks. The FDA or institutional review boards and/or institutional biosafety committees at the medical institutions and healthcare facilities where we seek to sponsor clinical trials may not permit a trial to proceed or may suspend any trial indefinitely if they find deficiencies in the conduct of the trials or patients develop an unacceptable number or severity of adverse events.
Product development costs to us and our potential collaborators will increase if we have delays in testing or approvals or if we need to perform more or larger clinical trials than planned. We expect to
20
continue to rely on third party clinical investigators at medical institutions and healthcare facilities to conduct our clinical trials, and, as a result, we may face additional delaying factors outside our control. Significant delays may adversely affect our financial results and the commercial prospects for our product candidates and delay our ability to become profitable.
If our pharmaceutical product candidates, including any product candidates we may develop under our early stage diabetes program, do not successfully complete the clinical trial process, we will not be able to market them. Even successful clinical trials may not result in a marketable product and may not be entirely indicative of a product's safety or efficacy.
Many factors, known and unknown, can adversely affect clinical trials and the ability to evaluate a product's efficacy. During the course of treatment, patients can die or suffer other adverse events for reasons that may or may not be related to the proposed product being tested. Even if unrelated to our product, certain events can nevertheless adversely impact our clinical trials. As a result, our ability to ultimately develop and market the products and obtain revenues would suffer.
Even promising results in pre-clinical studies and initial clinical trials do not ensure successful results in later clinical trials, which test broader human use of our products. Many companies in our industry have suffered significant setbacks in advanced clinical trials, despite promising results in earlier trials. Even successful clinical trials may not result in a marketable product or be indicative of the efficacy or safety of a product. Many factors or variables could affect the results of clinical trials and cause them to appear more promising than they may otherwise be. Product candidates that successfully complete clinical trials could ultimately be found to be unsafe or ineffective.
In addition, our ability to complete clinical trials depends on many factors, including obtaining adequate clinical supplies and having a sufficient rate of patient recruitment. For example, patient recruitment is a function of many factors, including:
- •
- the size of the patient population;
- •
- the proximity of patients to clinical sites;
- •
- the eligibility criteria for the trial;
- •
- the perceptions of investigators and patients regarding safety; and
- •
- the availability of other treatment options.
Even if patients are successfully recruited, we cannot be sure that they will complete the treatment process. Delays in patient enrollment or treatment in clinical trials may result in increased costs, program delays or both.
With respect to markets in other countries, we or a partner will also be subject to regulatory requirements governing clinical trials in those countries. Even if we complete clinical trials, we may not be able to submit a marketing application. If we submit an application, the regulatory authorities may not review or approve it in a timely manner, if at all.
Because we cannot predict whether or when we will obtain regulatory approval to commercialize our pharmaceutical product candidates, we cannot predict the timing of any future revenue from these product candidates.
We cannot commercialize any of our pharmaceutical product candidates to generate revenue until the appropriate regulatory authorities have reviewed and approved the applications for our product candidates. We cannot assure you that the regulatory agencies will complete their review processes in a timely manner or that we will obtain regulatory approval for any product candidate we or our potential collaborators develop. Satisfaction of regulatory requirements typically takes many years, is dependent
21
upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. Regulatory approval processes outside the United States include all or many of the risks associated with the FDA approval process and potentially others as well. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action or changes in FDA policy, or the policies of other relevant governmental or nongovernmental entities during the period of product development, pre-clinical and clinical trials and FDA regulatory review.
We will rely on third parties to manufacture taste modulators, including RP44, and pharmaceutical product candidates. There can be no guarantee that we can obtain sufficient and acceptable quantities of our taste modulators or pharmaceutical product candidates on acceptable terms, which may delay or impair our ability to develop, test and market such products.
Our business strategy relies on third parties to manufacture and produce our taste modulators, including RP44, and pharmaceutical product candidates in accordance with good manufacturing practices established by the FDA, or similar regulations in other countries. Our taste modulators, including RP44, and pharmaceutical product candidates may be in competition with other products for access to these facilities and may be subject to delays in manufacture if third parties give other products greater priority than our product candidates. These third parties also may not deliver sufficient quantities of our taste modulators, including RP44, and pharmaceutical product candidates, manufacture our taste modulators, including RP44, and pharmaceutical product candidates in accordance with specifications, or comply with applicable government regulations. Additionally, if the manufactured products fail to perform as specified, our business and reputation could be severely impacted.
We, or our collaborators, may need to enter into manufacturing agreements for the production of product materials. If any manufacturing agreement is terminated or any third party collaborator experiences a significant problem that could result in a delay or interruption in the supply of product materials to us, there are very few contract manufacturers who currently have the capability to produce our taste modulators, including RP44, and pharmaceutical product candidates on acceptable terms, or on a timely and cost-effective basis. There can be no assurance that manufacturers on whom we will depend will be able to successfully produce our taste modulators and pharmaceutical product candidates on acceptable terms, or on a timely or cost-effective basis. There can also be no assurance that manufacturers will be able to manufacture our products in accordance with our product specifications, or will meet FDA or other requirements. We must have sufficient and acceptable quantities of our product materials to conduct our clinical trials and to market our product candidates, if and when such products have been approved by the FDA for marketing. If we are unable to obtain sufficient and acceptable quantities of our product material, we may be required to delay the clinical testing and marketing of our products.
If we do not comply with applicable regulatory requirements in the manufacture and distribution of our product candidates, we may incur penalties that may inhibit our ability to commercialize our products and adversely affect our revenue.
Our failure or the failure of our potential collaborators or third party manufacturers to comply with applicable FDA or other regulatory requirements including manufacturing, quality control, labeling, safety surveillance, promoting and reporting may result in criminal prosecution, civil penalties, recall or seizure of our products, total or partial suspension of production or an injunction, as well as other regulatory action against our product candidates or us. Discovery of previously unknown problems with a product, supplier, manufacturer or facility may result in restrictions on the sale of our products, including a withdrawal of such products from the market. The occurrence of any of these events would negatively impact our business and results of operations.
22
If we are unable to create and maintain sales, marketing and distribution capabilities or enter into agreements with third parties to perform those functions, we will not be able to commercialize our product candidates.
We currently have no sales, marketing or distribution capabilities. Therefore, to commercialize our product candidates, if and when such products have been approved and are ready for marketing, we expect to collaborate with third parties to perform these functions. We have no experience in developing, training or managing a sales force and will incur substantial additional expenses if we decide to market any of our future products directly. Developing a marketing and sales force is also time consuming and could delay launch of our future products. In addition, we will compete with many companies that currently have extensive and well-funded marketing and sales operations. Our marketing and sales efforts may be unable to compete successfully against these companies.
Many potential competitors, including those who have greater resources and experience than we do, may develop products or technologies that make ours obsolete or noncompetitive.
We believe our taste technology approach is applicable to a wide range of food and beverage products representing many and diverse competitive companies and approaches. The life sciences and other technology industries are characterized by rapid technological change, and the area of sensory or taste research is a rapidly evolving field. Our future success will depend on our ability to maintain a competitive position with respect to technological advances. Technological developments by others may result in our taste modulators and technologies, as well as our pharmaceutical products, becoming obsolete.
We face substantial competition from companies pursuing the commercialization of products and services relevant to taste using various methods for the discovery of taste modulators. These competitors include leading flavor companies, such as International Flavors & Fragrances Inc., Givaudan SA, Symrise and Firmenich. Senomyx is principally focused in the area of taste enhancement for food products and uses a high throughput screening technology and synthetic chemistry to discover novel flavor enhancers, which it licenses to end users in the food industry. Nutrinova, a German ingredients company, is also developing a biotechnology approach to finding novel taste enhancers in collaboration with the German Biotechnology Research and Information Network. In certain instances we may also compete with companies who are commercializing Reb A. These companies include PureCircle Ltd and GLG Life Tech Corporation. We currently compete and will continue to compete in the future with these companies in collaborating with and selling flavor products and technologies to manufacturers of packaged food and beverage products.
We also face substantial competition with respect to our diabetes program. Type 2 diabetes is a disease characterized by persistent hyperglycemia due to the body's inability to produce enough or respond to insulin, a hormone regulating the absorption of sugar. Most treatments, outside of insulin replacement, focus on correcting high blood sugar by either stimulating the pancreas to produce more insulin, enhancing tissue sensitivity to insulin, or by inhibiting the production and release of glucose from the liver. There are many drugs currently available for treating diabetes and metabolic disease, either alone or in combination with other drugs. However, many patients are still inadequately treated with poorly controlled blood sugar levels, and the unmet medical need remains a concern.
There are several classes of anti-diabetic treatments, either orally available or injectable, including insulin, GLP-1 receptor agonists, sulfonylureas, DPP-IV inhibitors, PPAR agonists, and biguanides. Many of these drugs exhibit not only limited efficacy, but are also associated with less than desired tolerability and significant mechanism-based side effects. Several new classes of anti-diabetic treatments have recently been developed, including incretin mimetics such as Exendin-4 (Byetta®), a GLP-1 mimetic, which enhance glucose-dependent insulin secretion by the pancreatic beta-cell and slows gastric emptying, and DPP-IV inhibitors which work by preventing the inactivation of endogenous GLP-1. Additionally, new mechanisms such as GPR119 receptor agonists are being developed as glucose-dependent insulin and GLP-1 secretagogues expected to lower postprandial glucose and reduce weight gain.
23
If successful, our core diabetes program will face competition. The major competitors to TPRM5 modulators would likely include several new classes of anti-diabetic treatments, including the incretin mimetic Byetta® (marketed by Eli Lilly and Company and Amylin Pharmaceuticals), DPP-IV inhibitors (including Januvia™ marketed by Merck & Co., Inc.), and GPR119 receptor agonists, currently in development by Metabolex, Inc., OSI Pharmaceuticals, GlaxoSmithKline, and Arena Pharmaceuticals, Inc.
Many of the abovementioned companies have substantially greater capital resources, research and development resources and experience, manufacturing capabilities, regulatory expertise, sales and marketing resources, established relationships with consumer products companies and production facilities than us.
We may in the future face competition from life sciences and other technology companies and other commercial enterprises. These entities engage as we do in biotechnology, biology or chemistry and could apply this technology to the discovery and development of taste modulators and new pharmaceutical products. We cannot guarantee that products developed as a result of our competitors' existing or future collaborations will not compete with our taste modifiers and new pharmaceutical products.
Universities and public and private research institutions are also potential competitors. While these organizations primarily have educational objectives, they may develop proprietary technologies related to the sense of taste or secure patent protection that we may need for the development of our technologies and products. We may attempt to license these proprietary technologies, but these licenses may not be available to us on acceptable terms, if at all.
If we are unable to attract and retain key personnel and advisors, it may adversely affect our ability to obtain financing, pursue collaborations or develop our product candidates.
We are highly dependent on F. Raymond Salemme, Ph.D., our Chief Executive Officer and President, as well as Scott Horvitz, our Chief Financial Officer.
Our future success depends on our ability to attract, retain and motivate highly qualified management and scientific, development and commercial personnel and advisors. To pursue our business strategy, we may need to hire or otherwise engage qualified personnel and managers, including personnel with expertise in discovery, development, clinical trials, government regulation, manufacturing, marketing and sales. Competition for qualified personnel is intense among companies, academic institutions and other organizations. If we are unable to attract and retain key personnel and advisors, it may negatively affect our ability to successfully develop, test and commercialize our product candidates.
We use hazardous and biological materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our products and processes will involve the controlled storage, use and disposal of certain hazardous and biological materials and waste products. We and our suppliers and other collaborators are subject to federal, state and local regulations governing the use, manufacture, storage, handling and disposal of materials and waste products. Even if we and these suppliers and collaborators comply with the standards prescribed by law and regulation, the risk of accidental contamination or injury from hazardous materials cannot be completely eliminated. In the event of an accident, we could be held liable for any damages that result, and any liability could exceed the limits or fall outside the coverage of any insurance we may obtain and exceed our financial resources. We may not be able to maintain insurance on acceptable terms, or at all. We may incur significant costs to comply with current or future environmental laws and regulations.
24
We may be sued for product liability, which could adversely affect our business.
Because our business strategy involves the development and sale by either us or our collaborators of commercial products incorporating our taste modulators, including RP44, and our pharmaceutical products, we may be sued for product liability. We may be held liable if any product we develop and commercialize, or any product our collaborators commercialize that incorporates any of our taste modulators or pharmaceutical products, causes injury or is found otherwise unsuitable during product testing, manufacturing, marketing, sale or consumer use. In addition, the safety studies we must perform and the FEMA GRAS determination we must obtain prior to incorporating our taste modulators, including RP44, into a commercial product, and the regulatory approvals required to commercialize our pharmaceutical products, will not protect us from any such liability.
If we and our collaborators commence sale of commercial products we will need to obtain product liability insurance, and this insurance may be prohibitively expensive, or may not fully cover our potential liabilities. Inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of products developed by us or our product discovery and development collaborators. We may be obligated to indemnify our product discovery and development collaborators for product liability or other losses they incur as a result of our modulators. Any indemnification we receive from such collaborators for product liability that does not arise from our taste modulators or pharmaceutical products may not be sufficient to satisfy our liability to injured parties. If we are sued for any injury caused by our modulators or products incorporating our taste modulators or any other products we develop, our liability could exceed our total assets.
Risks Related To Intellectual Property
If our product candidates are not effectively protected by valid, issued patents or if we are not otherwise able to protect our proprietary information and technologies, it could harm our business.
The success of our operations will depend in part on our ability and that of our licensors to:
- •
- obtain patent protection for our taste screening technology and our taste enhancers and aversive taste blockers both in
the United States and in other countries with substantial markets;
- •
- obtain patent protection for our diabetes and obesity therapeutic compounds;
- •
- maintain patents once obtained;
- •
- maintain trade secrets and operate without infringing upon the intellectual property rights of others; and
- •
- obtain appropriate licenses upon reasonable terms to patents or proprietary rights held by others that are necessary or useful to us in commercializing our technology, both in the United States and in other countries with substantial markets.
In the event we are not adequately able to protect our intellectual property and proprietary information, our business will be materially harmed.
We may not have adequate protection for our unpatented proprietary information, which could adversely affect our competitive position.
In addition to patents, we will substantially rely on trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position. However, it is conceivable that others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. To protect our trade secrets, we may enter into confidentiality agreements with employees, consultants and
25
potential collaborators. However, these agreements may not provide meaningful protection of our trade secrets or adequate remedies in the event of unauthorized use or disclosure of such information. Likewise, our trade secrets or know-how may become known through other means or be independently discovered by our competitors. Any of these events could hinder us from developing or commercializing our product candidates.
Our ability to compete in the ingredient, food and beverage market and the pharmaceutical market may decline if we do not adequately protect our proprietary technologies, including RP44.
Because of the substantial length of time and expense associated with the development of new products, we, along with the rest of the ingredient, food and beverage industry and pharmaceutical industry, place considerable importance on obtaining and maintaining patent protection for new technologies, products and processes. Our success depends in part on our ability to obtain and maintain intellectual property that protects our technologies, taste enhancers or aversive taste blockers and our pharmaceutical products. Patent positions may be highly uncertain and may involve complex legal and factual questions, including the ability to establish patentability of sequences and or structures relating to taste receptors, proteins, chemical synthesis techniques, compounds and methods for using them to modulate taste for which we seek patent protection. No consistent pattern regarding the allowability or scope of claims in many of our pending patent applications has emerged to date. As a result, we cannot predict the breadth of claims that will ultimately be allowed in our patent applications, if any, including those we have in-licensed or the extent to which we may enforce these claims against our competitors. The degree of future protection for our proprietary rights is therefore highly uncertain and we cannot assure you that:
- •
- we, or our licensors, were the first to file patent applications or to invent the subject matter claimed in patent
applications relating to the technologies upon which we rely;
- •
- others will not independently develop similar or alternative technologies or duplicate any of our technologies;
- •
- others did not publicly disclose our claimed technology before we conceived the subject matter included in any of our
patent applications;
- •
- any of our patent applications will result in issued patents;
- •
- any of our patent applications will not result in interferences or disputes with third parties regarding priority of
invention;
- •
- any patents that may be issued to us, our collaborators or our licensors will provide a basis for commercially viable
products or will provide us with any competitive advantages or will not be challenged by third parties;
- •
- we will develop additional proprietary technologies that are patentable;
- •
- the patents of others will not have an adverse effect on our ability to do business; or
- •
- new proprietary technologies from third parties, including existing licensors, will be available for licensing to us on reasonable commercial terms, if at all.
In addition, the legal standards for patents outside the United States vary greatly and in many countries intellectual property laws are undergoing review and revision. The laws of some countries do not protect intellectual property rights to the same extent as domestic laws. It may be necessary or useful for us to participate in opposition proceedings to determine the validity of our competitors' patents or to defend the validity of any of our or our licensor's future patents, which could result in substantial costs and would divert our efforts and attention from other aspects of our business.
26
Technologies licensed to us by others, or in-licensed technologies, are important to our business. In particular, we depend on certain technologies relating to taste biology licensed from the Mount Sinai School of Medicine. In addition, we may in the future acquire rights to additional technologies by licensing such rights from existing licensors or from third parties. Such in-licenses may be costly. Also, we do not always control the patent prosecution, maintenance or enforcement of in-licensed technologies. Accordingly, we are unable to exercise the same degree of control over this intellectual property as we do over our internally developed technologies. Moreover, some of our academic institution licensors, collaborators and scientific advisors have rights to publish data and information to which we have rights. If we cannot maintain the confidentiality of our technologies and other confidential information in connection with our collaborations, our ability to protect our proprietary information or obtain patent protection in the future may be impaired, which could have a significant adverse effect on our business, financial condition and results of operations.
Many of the patent applications we and our licensors have filed have not yet been substantively examined and may not result in patents being issued.
Many of the patent applications filed by us and our licensors were filed recently with the United States Patent and Trademark Office and many have not been substantively examined and may not result in patents being issued. It is difficult to predict whether any of our or our licensors' applications will ultimately be found to be patentable or, if so, to predict the scope of any allowed claims. In addition, the disclosure in our or our licensors' patent applications, particularly in respect of the utility of our claimed inventions, may not be sufficient to meet the statutory requirements for patentability in all cases. As a result, it is difficult to predict whether any of our or our licensors' applications will be allowed, or, if so, to predict the scope of any allowed claims or the enforceability of the patents. Even if enforceable, others may be able to design around any patents or develop similar technologies that are not within the scope of such patents. Our and our licensors' patent applications may not issue as patents that will provide us with any protection or competitive advantage.
Disputes concerning the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be time consuming and extremely costly and could delay our research and development efforts.
Our commercial success, if any, will be significantly harmed if we infringe the patent rights of third parties or if we breach any license or other agreements that we have entered into with regard to our technology or business.
We are aware of other companies and academic institutions that have been performing research in the areas of taste modulation. In particular, other companies and academic institutions have announced that they have conducted taste-receptor research and have published data on taste receptor sequence information and taste receptors or filed patent applications or obtained patent protection on taste modulation or taste receptors and their uses. To the extent any of these companies or academic institutions currently have, or obtain in the future, broad patent claims, such patents could block our ability to use various aspects of our discovery and development process and might prevent us from developing or commercializing newly discovered taste enhancers and aversive taste blockers or otherwise conducting our business. In addition, it is possible that some of the taste enhancers or aversive taste blockers that are discovered using our technology may not be patentable or may be covered by intellectual property of third parties. Additionally, we may be subject to disputes with our collaborative partners relating to the scope of our research, our related agreements and the resulting rights or ownership of any discoveries.
With respect to third-party patent rights in the area of the TRPm5 receptor specifically, we are aware of pending U.S. and foreign patent applications by competitors and others that have claims directed to the TRPm5 receptor and/or methods of using the TRPm5 receptor to identify novel taste
27
modulating compounds. Although these patent applications are currently undergoing examination by the U.S. Patent and Trademark Office, and it is possible that they will not be granted or will not be granted with the current claims, it is also possible that patents will be granted on these applications with claims that are relevant to our current or future commercialization plans. To the extent we determine that we need a license to these or other patents relating to the TRPm5 receptor and are unable to obtain such licenses on commercially reasonable terms or at all, we may be restricted in our ability to commercialize our own TRPm5 technologies.
We are not currently a party to any litigation, interference, opposition, protest, reexamination or any other potentially adverse governmental, ex parte or inter-party proceeding with regard to our patent or trademark positions. However, the life sciences and other technology industries are characterized by extensive litigation regarding patents and other intellectual property rights. Many life sciences and other technology companies have employed intellectual property litigation as a way to gain a competitive advantage. If we become involved in litigation, interference proceedings, oppositions, reexaminations, protests or other potentially adverse intellectual property proceedings as a result, for example, of alleged infringement by us of the rights of others or as a result of priority of invention disputes with third parties, we might have to spend significant amounts of money, time and effort defending our position and we may not be successful. In addition, any claims relating to the infringement of third-party proprietary rights or proprietary determinations, even if not meritorious, could result in costly litigation, lengthy governmental proceedings, diversion of management's attention and resources, or require us to enter into royalty or license agreements that are not advantageous to us.
Should any person have filed patent applications or obtained patents in the United States that claim inventions also claimed by us, we may have to participate in an interference proceeding declared by U.S. Patent and Trademark Office to determine priority of invention and, thus, the right to a patent for these inventions in the United States. Such a proceeding could result in substantial cost to us even if the outcome is favorable. Even if successful on priority grounds, an interference action may result in loss of claims based on patentability grounds raised in the interference action. Litigation, interference proceedings or other proceedings could divert management's time and efforts. Even unsuccessful claims could result in significant legal fees and other expenses, diversion of management's time and disruption in our business. Uncertainties resulting from initiation and continuation of any patent proceeding or related litigation could harm our ability to compete and could have a significant adverse effect on our business, financial condition and results of operations.
An adverse ruling arising out of any intellectual property dispute, including an adverse decision as to the priority of our inventions, could undercut or invalidate our intellectual property position. An adverse ruling could also subject us to significant liability for damages, including possible treble damages, prevent us from using technologies or developing products, or require us to negotiate licenses to disputed rights from third parties. Although patent and intellectual property disputes in the technology area are often settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include license fees and ongoing royalties. Furthermore, necessary licenses may not be available to us on satisfactory terms, if at all. Failure to obtain a license in such a case could have a significant adverse effect on our business, financial condition and results of operations.
Risks Related to Our Fluctuating Operating Results, Possible Acquisitions and Management of Growth
We expect that our results of operations will fluctuate from period to period, and this fluctuation could cause our stock price to decline, causing investor losses.
Our operating results have fluctuated in the past and are likely to vary significantly in the future based upon a number of factors, many of which we have little or no control over. We operate in a
28
highly dynamic industry and future results could be subject to significant fluctuations. These fluctuations could cause us to fail to meet or exceed financial expectations of securities analysts or investors, which could cause our stock price to decline rapidly and significantly. Revenue and expenses in future periods may be greater or less than revenue and expenses in the immediately preceding period or in the comparable period of the prior year. Therefore, period-to-period comparisons of our operating results are not necessarily a good indication of our future performance. Some of the factors that could cause our operating results to fluctuate include:
- •
- our ability to discover and develop taste modulators;
- •
- our ability to discover and develop new diabetes or obesity therapeutic compounds;
- •
- our ability or the ability of our product discovery and development collaborators, to incorporate our taste modulators,
including RP44, into packaged food and beverage products;
- •
- our receipt of milestone payments in any particular period;
- •
- the ability and willingness of collaborators to commercialize products incorporating our taste modulators, including RP44,
on expected timelines, or at all;
- •
- our ability to enter into product discovery and development collaborations and technology collaborations, or to extend the
terms of any existing collaboration agreements, and our payment obligations, expected revenue and other terms of any other agreements of this type;
- •
- our ability, or our collaborators' ability, to successfully satisfy all pertinent regulatory requirements;
- •
- the demand for our future products and our collaborators' products containing our taste modulators, including RP44; and
- •
- general and industry specific economic conditions, which may affect our collaborators' research and development expenditures.
If we acquire products, technologies or other businesses, we will incur a variety of costs, may have integration difficulties and may experience numerous other risks that could adversely affect our business.
To remain competitive, we may decide to acquire additional businesses, products and technologies. We currently have no commitments or agreements with respect to, and are not actively seeking, any material acquisitions. We have limited experience in identifying acquisition targets, successfully acquiring them and integrating them into our current infrastructure. We may not be able to successfully integrate any businesses, products, technologies or personnel that we might acquire in the future without a significant expenditure of operating, financial and management resources, if at all. In addition, future acquisitions could require significant capital infusions and could involve many risks, including, but not limited to, the following:
- •
- we may have to issue convertible debt or equity securities to complete an acquisition, which would dilute our stockholders
and could adversely affect the market price of our common stock;
- •
- an acquisition may negatively impact our results of operations because it may require us to incur large
one-time charges to earnings, amortize or write down amounts related to goodwill and
- •
- other intangible assets, or incur or assume substantial debt or liabilities, or it may cause adverse tax consequences,
substantial depreciation or deferred compensation charges;
- •
- we may encounter difficulties in assimilating and integrating the business, technologies, products, personnel or operations of companies that we acquire;
29
- •
- certain acquisitions may disrupt our relationship with existing collaborators who are competitive to the acquired
business;
- •
- acquisitions may require significant capital infusions and the acquired businesses, products or technologies may not
generate sufficient revenue to offset acquisition costs;
- •
- an acquisition may disrupt our ongoing business, divert resources, increase our expenses and distract our management;
- •
- acquisitions may involve the entry into a geographic or business market in which we have little or no prior experience;
and
- •
- key personnel of an acquired company may decide not to work for us.
Any of the foregoing risks could have a significant adverse effect on our business, financial condition and results of operations.
To the extent we enter markets outside of the United States, our business will be subject to political, economic, legal and social risks in those markets, which could adversely affect our business.
There are significant regulatory and legal barriers in markets outside the United States that we must overcome to the extent we enter or attempt to enter markets in countries other than the United States. We will be subject to the burden of complying with a wide variety of national and local laws, including multiple and possibly overlapping and conflicting laws. We also may experience difficulties adapting to new cultures, business customs and legal systems. Any sales and operations outside the United States would be subject to political, economic and social uncertainties including, among others:
- •
- changes and limits in import and export controls;
- •
- increases in custom duties and tariffs;
- •
- changes in currency exchange rates;
- •
- economic and political instability;
- •
- changes in government regulations and laws;
- •
- absence in some jurisdictions of effective laws to protect our intellectual property rights; and
- •
- currency transfer and other restrictions and regulations that may limit our ability to sell certain products or repatriate profits to the United States.
Any changes related to these and other factors could adversely affect our business to the extent we enter markets outside the United States.
We may encounter difficulties managing our growth, which could adversely affect our business.
Our strategy includes entering into and working on simultaneous discovery and development programs across multiple markets that include both the food and beverage industry and the pharmaceutical industry. We expect to grow to meet our strategic objectives. If we grow, it will place a strain on us, our management and our resources. Our ability to effectively manage our operations, growth and various projects requires us to continue to improve our operational, financial and management controls, reporting systems and procedures and to attract and retain sufficient numbers of talented employees. We may not be able to successfully implement these tasks on a larger scale and, accordingly, we may not achieve our research, development and commercialization goals. If we fail to improve our operational, financial and management information systems, or fail to effectively monitor or manage our new and future employees or our growth, our business would suffer significantly. In
30
addition, no assurance can be made that we will be able to secure adequate facilities to house our staff, conduct our research or achieve our business objectives.
An economic downturn and adverse economic conditions may harm our business.
The recent economic downturn and adverse conditions in the national and global markets may negatively affect our operations in the future. Our revenues are contingent upon the expenditures of the pharmaceutical, biotech, and food and beverage industries, and as these industries continue to cut costs in response to the economic downturn, our revenues may be similarly decreased. Furthermore, while our revenues may decrease, our costs may remain fixed, resulting in decreased earnings.
Risks Related to our Common Stock; Liquidity Risks
The sale of a significant number of our shares of common stock in the public market, as well as substantial future issuances of our common stock, could depress the market price of common stock.
The sale of a significant number of shares of our common stock in the public market could harm the market price of our common stock. The number of shares that may be sold into the marketplace pursuant to the registration statement is significant. We believe that such sales may severely depress the market price of our common stock. In addition, some or all of the shares of common stock may be offered from time to time in the open market pursuant to Rule 144 (or pursuant to an additional registration statement, if one is effective), and these sales may also have a depressive effect on the market for the shares of common stock. In general, a person who is deemed to be an affiliate who has held shares for a period of six months may, upon filing of a notification on Form 144 with the SEC, sell into the market common stock in an amount up to the greater of 1% of the outstanding shares or the average weekly number of shares sold in the last four weeks before such sale. Such sales may be repeated once each three months. A non-affiliate holding restricted shares may sell such shares without restrictions after they have been held six months, subject only to the current public information requirement. After an additional six months have lapsed, a non-affiliate may sell such shares without any restrictions.
The market price for our common stock could also decline, perhaps significantly, as a result of issuances of a large number of shares of our common stock in the public market or even the perception that such issuances could occur. Under our Registration Rights Agreement, certain holders of our outstanding shares of our common stock and other securities have demand and Form S-3 registration rights. The existence of such registration rights could also make it more difficult for us to raise funds through future offerings of our equity securities.
The price of our common stock is expected to continue to be volatile.
The market price of our common stock, and the market prices for securities of biotechnology companies in general, have been, and are expected to be, highly volatile. The following factors, in addition to other risk factors described in this Annual Report on Form 10-K, and the potentially low volume of trades in our common stock, may have a significant impact on the market price of our common stock, some of which are beyond our control:
- •
- announcements of technological innovations and discoveries by the Company or its competitors;
- •
- developments concerning any research and development, clinical trials, manufacturing, and marketing collaborations;
- •
- new products or services that the Company or its competitors offer;
- •
- actual or anticipated variations in operating results;
- •
- the initiation, conduct and/or outcome of intellectual property and/or litigation matters;
31
- •
- changes in financial estimates by securities analysts;
- •
- conditions or trends in bio-pharmaceutical or other healthcare industries;
- •
- regulatory developments in the United States and other countries;
- •
- changes in the economic performance and/or market valuations of other biotechnology and flavor companies;
- •
- the Company's announcement of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
- •
- additions or departures of key personnel;
- •
- global unrest, terrorist activities, and economic and other external factors; and
- •
- sales or other transactions involving common stock.
The stock market in general has recently experienced relatively large price and volume fluctuations. In particular, market prices of securities of biotechnology companies have experienced fluctuations that often have been unrelated or disproportionate to the operating results of these companies. Continued market fluctuations could result in extreme volatility in the price of our common stock, which could cause a decline in the value of our common stock. Investors should also be aware that price volatility may be worse if the trading volume of our common stock is low.
We do not expect to pay cash dividends in the foreseeable future.
We currently intend to retain any future earnings to finance the growth and development of our business; therefore, we do not expect to pay any cash dividends in the foreseeable future. Any future dividends will depend on our earnings, if any, and our financial requirements.
Because Redpoint became a public company as a result of the Reverse Merger and Reincorporation Merger and not through a traditional underwritten initial public offering of securities, the Company may not attract the attention of major brokerage firms. Additionally, as a public company, we incur substantial expenses.
As a result of the Reverse Merger and Reincorporation Merger, Redpoint became a publicly-traded company and, accordingly, will be subject to the information and reporting requirements of the United States securities laws. The costs to public companies of preparing and filing annual and quarterly reports, proxy statements and other information with the SEC and furnishing audited reports to stockholders have caused the Company's expenses to be higher than they would be if it were a privately-held company. In addition, the Company incurred substantial expenses in connection with the preparation of the registration statement and related documents with respect to the registration of the common stock issued in the Private Placement and the Reverse Merger. Security analysts of major brokerage firms may not provide coverage of the Company. No assurance can be given that brokerage firms will want to conduct any secondary offerings on behalf of the Company in the future.
We have discretion on how we use any proceeds we receive from the exercise of warrants and options.
Our management has broad discretion on how to use and spend any proceeds we receive as a result of our security holders exercising their warrants and options. Our stockholders may not agree with our decision on how to use such proceeds. If we fail to spend the proceeds effectively, our business and financial condition could be harmed and we may need to seek additional financing sooner than expected.
32
It is not anticipated that there will be an active public market for shares of our common stock in the near term and stockholders may have to hold their shares of common stock for an indefinite period of time. Stockholders may be unable to resell a large number of their shares of common stock within a short time frame or at or above their purchase price.
To have purchased shares of our common stock and warrants to purchase common stock in the Private Placement, an investor must have represented that it was acquiring such shares and warrants for investment and not with a view to distribution or resale, that the investor understood that neither the common stock nor the warrants are readily transferable and, in any event, that it must bear the economic risk of an investment in the common stock for an indefinite period of time.
If we do not comply with registration rights granted to certain holders of our restricted securities, we may be required to pay damages to such holders.
We filed a "resale" registration statement with the SEC covering all shares of common stock issued in connection with the Private Placement, including shares of common stock into which any warrants are exercisable, within 60 days after the final closing of the Private Placement. Such registration statement has been declared effective by the SEC. We will use our best efforts to have such "resale" registration statement maintain its effectiveness until such time as all securities registered under the registration statement have been sold or are otherwise able to be sold under Rule 144 of the Securities Act without regard to volume limitations, whichever is earlier. We cannot assure you that we will be able to obtain or maintain such effective registration statement.
Our common stock may be considered "a penny stock" and may be difficult to sell.
The SEC has adopted regulations that generally define "penny stock" to be an equity security that has a market or exercise price of less than $5 per share, subject to specific exemptions. The market price of our common stock has been and may continue to be less than $5 per share and therefore may be designated a "penny stock" under SEC rules. This designation requires any broker or dealer selling our common stock to disclose certain information about the transaction, obtain a written agreement from the investor and determine that the investment in our common stock by the investor is a reasonably suitable investment for such investor. These rules may restrict the ability of brokers or dealers to sell our common stock and, as a result, may affect the ability of investors to sell their shares. In addition, unless and until our common stock is listed for trading on the American Stock Exchange or NASDAQ Capital Market or similar market, investors may experience a lack of buyers to purchase such stock or a lack of market makers to support the stock price. Resale restrictions on transferring "penny stocks" are sometimes imposed by some states, which may make transactions in our stock more difficult and may reduce the value of your investment.
Our stockholders may experience additional dilution upon the exercise of warrants or options.
As of December 31, 2010, there were 16.6 million shares of common stock underlying options that have been or may be granted pursuant to the Redpoint Bio Corporation 2007 Omnibus Equity Compensation Plan. As of December 31, 2010, there were 3.9 million warrants outstanding to purchase shares of common stock. If the remaining warrants are exercised or converted, or if the options are exercised, you may experience dilution in the net tangible book value of our common stock.
Directors and officers of the Company have a high concentration of our common stock ownership.
Based on the aggregate number of shares of our common stock that are outstanding as of December 31, 2010, our officers and directors beneficially own approximately 12.6% of our outstanding common stock and our 5% stockholders beneficially own approximately 36.4% of our outstanding common stock. Such a high level of ownership by such persons may have a significant effect in
33
delaying, deferring or preventing any potential change in control of the Company. Additionally, as a result of their high level of ownership, our officers and directors might be able to strongly influence the actions of the Company's board of directors, and the outcome of actions brought to our stockholders for approval. Such a high level of ownership may adversely affect the voting and other rights of our stockholders.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
Lease Agreement for Office and Laboratory Space in Ewing, New Jersey
On November 28, 2005, we entered into a lease agreement with BMR-7 Graphics Drive LLC to lease office and laboratory space at 7 Graphics Drive in Ewing, New Jersey. The leased premises include approximately 18,000 square feet of office and laboratory space, and the annual base rent is $818,032, subject to annual increases of approximately 1%. Under the lease agreement, we will also have to pay certain operating expenses associated with the leased premises. The term of the lease is ten years and commenced on May 7, 2007. We began to occupy the space at 7 Graphics Drive on May 21, 2007.
Lease Agreement for Office and Laboratory Space in Philadelphia, Pennsylvania
On September 30, 2005, we entered into an agreement with Albert Einstein Healthcare Network, or AEHN, to use AEHN's laboratory space located at 5501 Old York Road, Philadelphia, Pennsylvania. The term expired in September 2009. We currently occupy the approximately 1,000 square feet of office and laboratory space on a month-to-month basis for approximately $1,500 per month. The lease may be amended in writing by mutual agreement.
From time to time, the Company may become involved in various investigations, claims and legal proceedings that arise in the ordinary course of the Company's business. These matters may relate to intellectual property, employment, tax, regulation, contract or other matters. The resolution of these matters as they arise will be subject to various uncertainties. As of the date of this Annual Report on Form 10-K, Redpoint Bio is not a party to any material pending legal proceeding.
34
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Our common stock currently trades on the OTCQB under the symbol "RPBC". However, for the years ended December 31, 2009 and December 31, 2010, our common stock traded on the OTC Bulletin Board under the symbol "RPBC".
The following table describes the per share range of high and low sale prices for shares of our common stock, as listed for quotation on the OTC Bulletin Board, for the periods indicated. Please note that this information reflects inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
2010:
|
High | Low | |||||
|---|---|---|---|---|---|---|---|
Fourth quarter, ended December 31, 2010 |
$ | 0.18 | $ | 0.08 | |||
Third quarter, ended September 30, 2010 |
0.19 | 0.09 | |||||
Second quarter, ended June 30, 2010 |
0.25 | 0.12 | |||||
First quarter, ended March 31, 2010 |
0.18 | 0.07 | |||||
2009:
|
High | Low | |||||
|---|---|---|---|---|---|---|---|
Fourth quarter, ended December 31, 2009 |
$ | 0.22 | $ | 0.10 | |||
Third quarter, ended September 30, 2009 |
0.30 | 0.13 | |||||
Second quarter, ended June 30, 2009 |
0.19 | 0.04 | |||||
First quarter, ended March 31, 2009 |
0.14 | 0.02 | |||||
As of December 31, 2010, the closing price for our common stock was $0.13 per share. As of February 28, 2011, there were approximately 246 holders of record of our common stock. Our independent stock transfer agent is American Stock Transfer & Trust Company, located at 59 Maiden Lane, New York, New York 10038. Their telephone number is (800) 937-5449.
Dividend Policy
We have not declared or paid any cash dividends on common stock since our inception, and our Board of Directors currently intends to retain all earnings for use in the business for the foreseeable future. Any future payment of dividends will depend upon our results of operations, financial condition, cash requirements, and other factors deemed relevant by our Board of Directors. We are under no contractual obligations or restrictions to declare or pay dividends to our stockholders.
35
Equity Compensation Plan Information
The following table provides information as of December 31, 2010 with respect to the shares of our common stock that may be issued under our existing equity compensation plan. Prior to the Reverse Merger, Original Redpoint maintained the Linguagen Corp. 2003 Stock Incentive Plan, which we refer to as the "2003 Stock Plan." In connection with the adoption of the Redpoint Bio Corporation 2007 Omnibus Equity Compensation Plan, which we refer to as the "2007 Plan," the 2003 Stock Plan merged with and into the 2007 Plan and no additional grants may be made under the 2003 Stock Plan. Outstanding grants under the 2003 Stock Plan continue in effect in accordance with their terms as in effect before March 12, 2007 (subject to such amendments as the Compensation Committee determines, consistent with the 2003 Stock Plan, as applicable).
Plan Category
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Equity compensation plans that have been approved by security holders(1) |
9,569,260 | $ | 0.45 | 6,999,989 | ||||||
Equity compensation plans not approved by security holders |
— | — | ||||||||
Total |
9,569,260 | 6,999,989 | ||||||||
- (1)
- Consists of the 2007 Plan.
Recent Sales of Unregistered Securities
We did not sell any unregistered equity securities during the fiscal year ended December 31, 2010.
Issuer Purchases of Equity Securities
We did not repurchase any shares of our equity securities during the fiscal year ended December 31, 2010.
36
Performance Graph
The following graph compares the cumulative total stockholder return on our common stock with the cumulative total return on the NASDAQ Composite Index, NASDAQ Biotechnology Index and the AMEX Biotechnology Index for the period beginning October 31, 2006 and ending on the last day of our last completed fiscal year. The stock performance shown on the graph below is not indicative of future price performance.
COMPARISON OF CUMULATIVE TOTAL RETURN(1)(2)(3)
Among Redpoint, the NASDAQ Composite Index, the NASDAQ Biotechnology Index and
the AMEX Biotechnology Index
Comparison of 3 Year Cumulative Total Return
Assumes Initial Investment of $100
December 2010
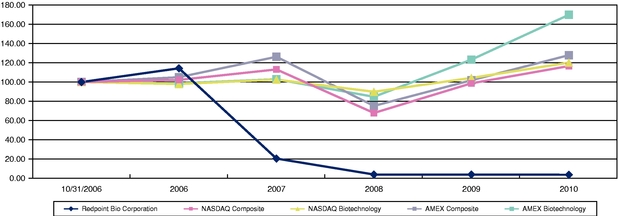
Company/Index
|
Base Period 10/06 |
12/06 | 12/07 | 12/08 | 12/09 | 12/10 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
REDPOINT BIO CORPORATION |
100 | 114.29 | 20.57 | 4.00 | 4.00 | 3.71 | |||||||||||||
NASDAQ COMPOSITE INDEX |
100 | 102.22 | 113.11 | 67.89 | 98.69 | 116.61 | |||||||||||||
NASDAQ BIOTECHNOLOGY INDEX |
100 | 97.99 | 102.54 | 89.93 | 104.28 | 120.15 | |||||||||||||
AMEX COMPOSITE INDEX |
100 | 105.03 | 126.24 | 75.21 | 101.85 | 127.91 | |||||||||||||
AMEX BIOTECHNOLOGY INDEX |
100 | 98.70 | 102.92 | 84.68 | 123.28 | 169.80 | |||||||||||||
- (1)
- Graph
assumes $100 invested on October 31, 2006 in our common stock, the NASDAQ Composite Index, the NASDAQ Biotechnology Index, the AMEX Composite
Index and the AMEX Biotechnology Index.
- (2)
- Cumulative
total return assumes reinvestment of dividends.
- (3)
- As stated earlier in the Explanatory Note, Redpoint consummated the Reverse Merger in March 2007 and the Reincorporation Merger in June 2007. Prior to such transactions, the common stock of our predecessor registrant, Robcor, was traded on the OTC Bulletin Board (trading began in October 2006) under the symbol "RBCR" and was traded with little or no volume at a trading price at or around $3.50 to $4.00 per share from October 2006 until the consummation of the Reincorporation Merger. After the completion of the Reverse Merger and the Reincorporation Merger, our stock price began trading under the symbol "RPBC". We believe that given the lack of liquidity in the trading of our common stock prior to July 20, 2007 (the declaration of effectiveness of our Registration Statement on Form S-1 registering the shares of common stock and the shares of common stock issuable upon exercise of the warrants issued in the private placement in connection with the Reverse Merger) that trading in shares of our common stock began to reflect the stock price comparable to the $0.81 purchase price of one unit in the private placement, which consisted of one share of common stock together with 0.25 warrants, at or around July 20, 2007.
37
ITEM 6. SELECTED FINANCIAL DATA.
The following table sets forth our selected historical financial data as of the dates and for the periods indicated. Our selected financial data set forth below as of December 31, 2009 and 2010 and for each of the years in the three-year period ended December 31, 2010, and for the period August 16, 1995 (inception) to December 31, 2010, has been derived from our audited financial statements included elsewhere herein. Our selected financial data should be read in conjunction with the Financial Statements and the Notes and "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations" which are included elsewhere in this Annual Report on Form 10-K.
| |
|
|
|
|
|
August 16, 1995 (Inception) to December 31, 2010 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years Ended December 31, | ||||||||||||||||||||
| |
2006 | 2007 | 2008 | 2009 | 2010 | ||||||||||||||||
| |
(in thousands, except per share data) |
||||||||||||||||||||
Statements of Operations Data: |
|||||||||||||||||||||
Research and grant revenue |
$ | — | $ | 2,229 | $ | 3,999 | $ | 1,868 | $ | 1,244 | $ | 13,574 | |||||||||
Operating expenses: |
|||||||||||||||||||||
Research and development |
3,631 | 7,266 | 8,647 | 5,630 | 3,041 | 37,110 | |||||||||||||||
General and administrative |
2,811 | 4,511 | 5,608 | 4,961 | 3,644 | 28,807 | |||||||||||||||
Total operating expenses |
6,442 | 11,777 | 14,255 | 10,591 | 6,685 | 65,917 | |||||||||||||||
Operating loss |
(6,442 | ) | (9,548 | ) | (10,256 | ) | (8,723 | ) | (5,441 | ) | (52,343 | ) | |||||||||
Interest income |
53 | 768 | 565 | 142 | 11 | 1,673 | |||||||||||||||
Interest expense |
(929 | ) | (1,696 | ) | (97 | ) | (235 | ) | (141 | ) | (3,807 | ) | |||||||||
Loss before income taxes |
(7,318 | ) | (10,476 | ) | (9,788 | ) | (8,816 | ) | (5,571 | ) | (54,477 | ) | |||||||||
Income tax benefit |
466 | — | 267 | — | 101 | 1,087 | |||||||||||||||
Net loss |
(6,852 | ) | (10,476 | ) | (9,521 | ) | (8,816 | ) | (5,470 | ) | $ | (53,390 | ) | ||||||||
Accretion of redeemable convertible preferred stock |
(1,159 | ) | (225 | ) | — | — | — | ||||||||||||||
Net loss applicable to common stockholders |
$ | (8,011 | ) | $ | (10,701 | ) | $ | (9,521 | ) | $ | (8,816 | ) | $ | (5,470 | ) | ||||||
Basic and diluted net loss per common share |
$ | (1.81 | ) | $ | (0.17 | ) | $ | (0.12 | ) | $ | (0.11 | ) | $ | (0.07 | ) | ||||||
Weighted average number of shares outstanding |
4,429 | 63,315 | 79,444 | 79,651 | 79,842 | ||||||||||||||||
| |
As of December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | 2009 | 2010 | |||||||||||
| |
(in thousands) |
|||||||||||||||
Balance Sheet Data: |
||||||||||||||||
Cash, cash equivalents and marketable securities |
$ | 974 | $ | 22,701 | $ | 14,428 | $ | 5,557 | $ | 1,083 | ||||||
Working capital (deficiency) |
(4,880 | ) | 20,810 | 12,791 | 4,512 | (392 | ) | |||||||||
Total assets |
2,552 | 24,823 | 16,443 | 6,775 | 1,792 | |||||||||||
Long-term debt, net of current portion |
635 | 188 | 1,148 | 774 | 352 | |||||||||||
Deficit accumulated during the development stage |
(22,295 | ) | (32,996 | ) | (42,517 | ) | (51,333 | ) | (56,803 | ) | ||||||
Total stockholders' equity (deficit) |
(20,726 | ) | 21,539 | 12,927 | 4,778 | (107 | ) | |||||||||
38
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
Forward Looking Statements
The statements contained in this Annual Report on Form 10-K that are not historical facts are forward-looking statements (within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended) that involve risks and uncertainties. Such forward-looking statements may be identified by, among other things, the use of forward-looking terminology such as "believes", "expects", "may", "will", "should" or "anticipates" or the negative thereof or other variations thereon or comparable terminology, or by discussions of strategy that involve risks and uncertainties. From time to time, we or our representatives have made or may make forward-looking statements, orally or in writing. Such forward-looking statements may be included in various filings made by us with the SEC, or press releases or oral statements made by or with the approval of one of our authorized executive officers. These forward-looking statements, such as statements regarding anticipated future revenues or operating margins, contract percentage completions, capital expenditures, and other statements regarding matters that are not historical facts, involve predictions. Our actual results, performance or achievements could differ materially from the results expressed in, or implied by, these forward-looking statements. There are a number of important factors that could cause our results to differ materially from those indicated by such forward-looking statements. These factors include those set forth in the section entitled "Item 1A. Risk Factors".
Overview
Redpoint Bio is a development stage biotechnology company focused on the development of healthier foods and beverages and new approaches to the treatment of diabetes and obesity.
Since its inception in 1995, Redpoint has incurred losses and negative cash flows from operations, and such losses have continued subsequent to December 31, 2010. As of December 31, 2010, Redpoint had an accumulated deficit of $56.8 million and anticipates incurring additional losses for the foreseeable future. We will need to spend significant resources over the next several years to enhance our technologies and to fund research and development of our pipeline of potential products. We have not yet developed any products that are commercially available. We had approximately $1.1 million in cash and cash equivalents at December 31, 2010. In addition, in January 2011, Redpoint sold a portion of its New Jersey State net operating loss carryforwards and received cash proceeds of approximately $0.4 million. As of the date of filing this annual report on Form 10-K, we have approximately $0.7 million in cash and cash equivalents. We believe that our current capital resources are only sufficient to meet our operating and capital requirements through April 2011, which raises substantial doubt about our ability to continue as a going concern. Without additional funding, we will be unable to satisfy our obligations and will cease operations.
Redpoint is leveraging recent discoveries in the molecular biology of taste to discover and develop novel taste modulators for the food and beverage industries. Our food and beverage program has been focused on identifying novel flavor modifiers that improve the taste of existing ingredients, enabling the development of better-tasting and more healthful foods and beverages. In June 2009, we announced that we had identified an all-natural sweetness enhancer, RP44. In June 2010, we entered into a license and commercialization agreement with IFF, a global leader in the food and beverage industry, covering the commercialization of RP44. In October 2010, IFF received notification by the Flavor and Extract Manufacturers Association (FEMA) that RP44 had been determined to be Generally Recognized As Safe (GRAS). We believe that our unique technology platform could lead to additional opportunities for discovering novel natural flavorings and flavor enhancers, to help meet increasing consumer demand for more healthful foods and beverages.
39
We are also pursuing a pharmaceutical discovery program for the treatment of diabetes and obesity. Our research in diabetes and obesity stems from the observation that taste signaling circuits responsible for sensing sweet, savory, and bitter compounds on the tongue are also found in the pancreas and gastrointestinal tract, suggesting a potential role in the regulation of metabolism and satiety. We are actively seeking a research and development collaboration with major pharmaceutical companies for the further development of this discovery program. However, there can be no assurance that we will enter into any collaboration or license agreement.
Through December 31, 2010, substantially all of our revenue has been derived from corporate collaborations, license agreements, and government grants. In order to achieve profitability, we must continue to develop products and technologies that can be commercialized by us or through future collaborations.
On March 12, 2007, we completed the first closing of our private placement of shares of common stock and warrants to purchase shares of common stock raising approximately $17.2 million of net proceeds. On April 6, 2007, we completed the final closing of the private placement raising an additional $11.4 million of net proceeds, bringing the total private placement net proceeds to $28.6 million.
In March 2007, we entered into a Joint Research and Development and License Agreement with Givaudan Schweiz AG, a Swiss company, or Givaudan, for the development and commercialization of compounds that act primarily through the modulation of the TRPm5 ion channel and that enhance sweetness or savory sensation, as well as compounds that block or desensitize bitter taste for use in the food and beverage industry. In consideration of the Company's agreement to conduct research and develop compounds and grant exclusive licenses and other rights to Givaudan, Redpoint received an upfront payment of $1.3 million and Givaudan provided research funding to the Company over the term of the agreement. Givaudan terminated the agreement, effective as of May 1, 2009, and Givaudan reserved its rights under the agreement with respect to certain collaboration compounds. Givaudan cited the fact that certain collaboration objectives had not been achieved as its reasoning for terminating the agreement. Through the termination date of the agreement, including the upfront fee, we received $6.9 million in research and development funding in connection with the agreement.
Recognizing the difficult financing environment that has significantly impacted the biotechnology sector and seeking ways to conserve cash, during the year ended December 31, 2009, we announced two restructurings which reduced our workforce by approximately 60%. As of December 31, 2010, we had 10 full time employees. We may need to make further reductions in our expenses.
We first announced that we had identified RP44, an all-natural sweetness enhancer, in June 2009. RP44 is Reb-C (rebaudioside C), a component of the stevia plant. Native to South America, extracts of the leaves of the stevia plant have been used as sweeteners around the world for hundreds of years. More recently, a purified component of stevia known as Reb A has received regulatory approval in the United States and elsewhere and Redpoint believes its popularity as an all-natural non-caloric sweetener is increasing.
In June 2010, we entered into a License and Commercialization Agreement with IFF for the development, manufacture, use and commercialization of RP44. IFF has exclusive rights, for five years, to develop, manufacture and commercialize RP44 in virtually all food and beverage product categories. In return, we received an upfront payment of a $0.5 million and we became eligible to receive two milestone payments of $0.5 million each based upon certain criteria regarding regulatory approval and supply. In October 2010, IFF received notification by the Flavor and Extract Manufacturers Association (FEMA) that RP44 had been determined to be Generally Recognized As Safe (GRAS), triggering $0.5 million regulatory approval milestone payment. We have not yet achieved the supply milestone. In addition, we are eligible to receive royalties based on the amount of RP44 purchased by IFF for use in
40
products. IFF will continue to be responsible for the regulatory process and for costs associated with prosecuting and maintaining our intellectual property covering the sweetness enhancer.
Unlike Reb A, RP44 has a very low intrinsic level of sweetness and therefore is not useful as a sweetener. Instead, we discovered that RP44 acts as a potent sweetness enhancer. A sweetness enhancer imparts no sweet taste of its own when used in a product. Instead, sweetness enhancers act by amplifying the existing sweet taste of caloric sweeteners such as sugar or high fructose corn syrup. We believe this will enable the development of food and beverage products that require reduced amounts of caloric sweeteners while still retaining the "clean sweet taste" associated with a fully sugared product. Taste tests demonstrate that RP44 enables the reduction of up to 25% of the caloric sweetener content in various product prototypes, while still maintaining the taste quality of the fully sweetened product. These results have been demonstrated by using RP44 in combination with several common sweeteners including sucrose (sugar), fructose, glucose and high-fructose corn syrup (HFCS). The worldwide sweetener market is estimated to be in excess of $50 billion with sugar (including sucrose, HFCS, and fructose) being the second most common ingredient used in food and beverages after water.
In October 2010, IFF informed us that they had received notification by the Flavor and Extract Manufacturers Association (FEMA) that RP44 had been determined to be Generally Recognized As Safe (GRAS) under the provisions of the Federal Food, Drug and Cosmetic Act, administered by the United States Food and Drug Administration (FDA). This GRAS determination allows RP44 to be incorporated into specified products in the United States and potentially aids regulatory acceptance in numerous other countries. Although RP44 is currently derived from material found in the side stream of the Reb A production process there can be no assurance that it can be produced in large scale on an economically viable basis.
We believe there is an important potential role for RP44 in all-natural reduced-calorie products. Numerous scientific studies suggest a compelling link between the high levels of refined sugar found in common food and beverage products and the worsening epidemic of obesity and diabetes worldwide. Health and wellness trends continue to be major market drivers for the food and beverage industry, creating consumer demand for natural solutions that can preserve the clean sweet taste of sugar while reducing calories.
We also recently reported that we had advanced our discovery program for all-natural enhancers of salty taste. The objective of the salt enhancer program is to identify natural flavor ingredients that can provide a significant reduction in the amount of sodium in food and beverage products, yet maintain the salty taste that consumers desire.
We are using our proprietary MOG assay technology for the salt enhancer discovery program. The MOG technology was developed specifically to deal with the complex sensory and experimental issues associated with the discovery of all-natural compounds, which are often derived from plant or fermentation sources containing complex mixtures of taste ingredients. Historically, natural tastants or enhancers have been discovered by trial and error using human tasters. The MOG is an enabling technology that facilitates high throughput screens of tastant libraries and natural product extracts by rodents trained to discriminate specific taste standards. MOG-trained rodents are "expert" taste testers capable of rapidly identifying taste from small samples with a high level of accuracy, providing an effective means of evaluating the taste properties of complex natural product extracts.
We originally developed our MOG technology to discover natural high-potency sweeteners and sweetener enhancers. More recently, Redpoint scientists have developed and optimized a high-throughput salt-taste detection system, in which MOG-trained rodents discriminate salt from other essential tastes (savory, sweet, sour, and bitter). The MOG approach will be used to evaluate the salt-tasting and salt-enhancing potential of natural sources such as fermentation products and edible
41
plant extracts, with a focus on isolating active components from ingredients already used in the food industry.
Our initial programs focused on the modulation of the TRPm5 ion channel, a key signaling element in taste sensation, in order to discover novel compounds that modulate the taste of food and beverage products. The TRPm5 ion channel is a member of a larger class of related TRP (Transient Receptor Potential) ion channels that are involved in the sensation of heat, cold, and spicy compounds. The TRPm5 ion channel was originally identified as an important component of taste signaling circuits responsible for sensing sweet, savory, and bitter compounds on the tongue. Recently, an emerging body of scientific evidence has demonstrated that the TRPm5 channel is also found in the pancreas and gastrointestinal tract, suggesting a potential role in the regulation of metabolism and satiety. Specifically, TRPm5 may be involved in the secretion of important hormones like GLP1 and insulin that control sugar uptake and metabolism. Consequently, modulators of TRPm5 could potentially find application as a new therapy for adult-onset diabetes and obesity. We initiated a program designed to leverage the research we have already conducted on the discovery of modulators of the TRPm5 ion channel to further explore potential opportunities for the discovery of new diabetes or obesity therapeutics. In November 2010, we received a grant relating to our diabetes program in the amount of $0.25 million in connection with the Qualifying Therapeutic Discovery Project Program under section 48D of the Internal Revenue Code.
Diabetes is a chronic disease afflicting over 24 million Americans and its poor control is the principle cause of blindness and kidney failure. Consequently, there is a continuing need for new and improved diabetes therapies, especially in an aging and increasingly obese population, which both correlate with an increased incidence of adult-onset diabetes.
TRPm5 modulators discovered by Redpoint have been shown to elicit the secretion of hormones known to play important roles in metabolism in relevant model systems. We believe this discovery may serve as the foundation for a new method of action in the treatment of obesity and diabetes. Our therapeutic objective is the development of an orally acting incretin and insulin secretogogue for the treatment of Type 2 diabetes. Through an ongoing discovery program involving the synthesis of approximately 1,000 novel small molecules, we have identified a lead series of orally acting hormone secretogogues that have demonstrated positive results in a number of in vitro and in vivo experiments. We are seeking collaborations with major pharmaceutical companies for the further development and optimization of our TRPm5 modulators for therapeutic applications in diabetes and obesity.
Revenue
To date, our revenue has come solely from corporate collaborations, licensing agreements and government grants. Since our inception, we have undertaken research projects for which we were the recipient of several Small Business Innovative Research, or SBIR, Awards. The SBIR Awards were sponsored by the National Institutes of Health. The last SBIR related research project was completed in 2004.
In March 2007, we entered into an agreement with Givaudan where we were collaborating exclusively with each other to discover and develop Enhancer Compounds and Bitter Blocker Compounds that act primarily through the modulation of the TRPm5 channel. The agreement with Givaudan was terminated by Givaudan on May 1, 2009. Also, in December 2007, we entered into a one year research agreement with Coca-Cola for the development of certain technology for use in soft drinks and other non-alcoholic beverages. The agreement with Coca-Cola expired by its terms in December 2008.
In June 2010, we entered into a License and Commercialization Agreement with IFF ("the IFF Agreement") for the development, manufacture, use and commercialization of RP44. IFF has exclusive rights, for five years, to develop, manufacture and commercialize RP44 in virtually all food and
42
beverage product categories. Our strategy also includes partnering with other major ingredient suppliers, or food and beverage companies, to utilize our technology to discuss new all natural sweetness enhancers, sweeteners and salt enhancers.
We are also seeking collaborations with major pharmaceutical companies for the further development and optimization of our compounds for therapeutic applications in diabetes and obesity. Any failure to maintain or establish licensing or collaboration arrangements on favorable terms could adversely affect our business prospects, financial condition or ability to develop and commercialize our product candidates.
Research and Development
Our research and development expenses consist primarily of internal costs associated with our taste modulator and diabetes/obesity research programs as well as amounts paid to third parties to conduct research on our behalf. Our internal research and development costs are comprised of salaries and related benefits, facilities and depreciation on laboratory equipment, compound acquisition costs and research supplies. We charge research and development costs to operations as incurred.
General and Administrative
Our general and administrative expenses consist primarily of salaries and related benefit expenses for business development, financial, legal and other administrative functions. In addition, we incur external costs for professional fees for legal, patent, accounting and investor relations services.
Results of Operations
Year ended December 31, 2010 compared to December 31, 2009
Research and Grant Revenue. For the year ended December 31, 2010, we recorded revenue of $1.2 million which included $1.0 million in connection with the IFF Agreement, which we signed in June 2010 for the development, manufacture, use and commercialization of RP44. IFF has exclusive rights, for five years, to develop, manufacture and commercialize RP44 in virtually all food and beverage product categories. The Company has no future performance obligations under the IFF Agreement. The revenues from IFF include $0.5 million from an upfront payment and $0.5 million from a milestone payment that was triggered when IFF received notification by the Flavor and Extract Manufacturers Association (FEMA) that RP44 had been determined to be Generally Recognized As Safe (GRAS). The remaining $0.2 million of revenue was related to the grant approval in connection with the Qualifying Therapeutic Discovery Project Program under section 48D of the Internal Revenue Code. During the year ended December 31, 2009, we recorded revenue of $1.9 million, which included $1.7 million from the agreement with Givaudan, which began in March 2007, for the development and commercialization of compounds that act primarily through the modulation of the TRPM5 channel and enhance sweetness or savory sensation as well as compounds that block or desensitize bitter taste for use in the food and beverage industry. The revenues from Givaudan include $0.7 million attributable to the upfront fee which was originally being recognized as revenue over the initial 3.5 year term of the agreement and $1.0 million of research funding. Givaudan terminated the agreement, effective as of May 1, 2009. As of May 1, 2009, the remaining unamortized portion of the upfront payment was approximately $0.6 million and was recognized as revenue upon termination. The remaining $0.2 million of revenue in 2009 was from other agreements including a feasibility research program we conducted with Schering-Plough Corporation in the area of taste science research for pharmaceutical applications.
Research and Development Expenses. Our research and development expenses were $3.0 million for the year ended December 31, 2010 compared to $5.6 million for the year ended December 31, 2009. The decrease in expenses was primarily attributable to reductions in workforce as a result of the
43
corporate restructurings the Company undertook in February and May 2009. Our research and development expenses consist primarily of internal costs associated with our taste modulator and diabetes research programs as well as amounts paid to third parties to conduct research on our behalf. Our internal research and development costs are comprised of salaries and related benefits, facilities, and depreciation of laboratory equipment, compound acquisition costs and research supplies.
General and Administrative Expenses. Our general and administrative expenses were $3.6 million for the year ended December 31, 2010 compared to $5.0 million for the year ended December 31, 2009. The decrease in expenses was primarily attributable to reduced expenditures associated with professional fees and reductions in workforce as a result of the corporate restructurings the Company undertook in February and May 2009.
Interest Income. Interest income was a nominal amount for the year ended December 31, 2010 compared to $0.1 million for the year ended December 31, 2009. The decrease was primarily the result of decreasing cash and investment balances.
Interest Expense. Interest expense was $0.1 million for the year ended December 31, 2010 compared to $0.2 million during the year ended December 31, 2009.
Income Tax Benefit. During 2010, we sold $0.1 million of our New Jersey State Research and Development Tax Credits resulting in the recognition of an income tax benefit of $0.1 million. Due to the Company's history of losses, we have not recognized the benefit of any of our other net operating loss carryforwards.
Year ended December 31, 2009 compared to December 31, 2008
Research and Grant Revenue. For the year ended December 31, 2009, we recorded revenue of $1.9 million, which included $1.7 million from the agreement with Givaudan, which began in March 2007, for the development and commercialization of compounds that enhance sweetness or savory sensation as well as compounds that block or desensitize bitter taste for use in the food and beverage industry. In consideration of the Company's agreement to conduct research and develop compounds and grant exclusive licenses and other rights to Givaudan, Redpoint received an upfront payment of $1.3 million and Givaudan provided research funding to the Company over the term of the agreement. The revenues from Givaudan during 2009 include $0.7 million attributable to the upfront payment which was being recognized as revenue on a straight-line basis over the initial 3.5 year term of the agreement and $1.0 million of research funding. Givaudan terminated the agreement, effective as of May 1, 2009. As of May 1, 2009, the remaining unamortized portion of the upfront payment was approximately $0.5 million and was recognized as revenue upon the termination of the agreement. The remaining $0.2 million of revenue in 2009 was from other agreements including a feasibility research program we conducted with Schering-Plough Corporation in the area of taste science research of pharmaceutical applications. During the year ended December 31, 2008, we recorded revenue of $4.0 million, which included $3.1 million from the agreement with Givaudan. The revenue from Givaudan included $0.4 million attributable to the upfront payment and $2.7 million of research funding. The remaining $0.9 million of revenue in 2008 was primarily from our research agreement with Coca-Cola, which began in December 2007, for the development of certain technology for use in soft drinks and other non-alcoholic beverages. The agreement with Coca-Cola expired by its terms in December 2008.
Research and Development Expenses. Our research and development expenses were $5.6 million for the year ended December 31, 2009 compared to $8.6 million for the year ended December 31, 2008. The decrease in expenses was primarily attributable to reductions in workforce as a result of the corporate restructurings the Company undertook in 2009. As a result of the restructurings, we are currently not pursuing our research program aimed at suppressing the bitterness of oral medicines.
44
General and Administrative Expenses. Our general and administrative expenses were $5.0 million for the year ended December 31, 2009 compared to $5.6 million for the year ended December 31, 2008. The decrease in expenses was primarily attributable to reduced expenditures associated with patent and other professional fees, offset by a restructuring charge of approximately $0.5 million recognized in 2009.
Interest Income. Interest income was $0.1 million for the year ended December 31, 2009 compared to $0.6 million for the year ended December 31, 2008. The decrease was primarily the result of decreasing cash and investment balances.
Interest Expense. Interest expense was $0.2 million for the year ended December 31, 2009 compared to $0.1 million for the year ended December 31, 2008.
Income Tax Benefit. During 2008, we sold $2.5 million of our New Jersey State operating net loss carryforwards resulting in the recognition of an income tax benefit of $0.3 million. Due to the Company's history of losses, we have not recognized the benefit of any of our other net operating loss carryforwards.
Liquidity and Capital Resources
We believe that our current capital resources are only sufficient to meet our operating and capital requirements through April 2011, which raises substantial doubt about our ability to continue as a going concern. We are actively seeking additional corporate collaborations, equity investments from third parties and license agreements from various strategic partners to enhance our liquidity position. However, there can be no assurance that we will enter into any future corporate collaborations, equity investments or license agreements. Without additional funding, we will be unable to satisfy our obligations and will cease operations.
At December 31, 2010, we had cash and cash equivalents of $1.1 million. In addition, in January 2011, we sold a portion of our New Jersey State net operating loss carryforwards and received cash proceeds of approximately $0.4 million. In addition, in order to conserve cash, on March 1, 2011, each of our executive officers agreed to indefinitely defer one-third of the cash portions of their salaries, at least until the Company has raised additional capital or the compensation committee of the board of directors determines otherwise. The cash amounts due to the executive officers are being accrued for by the Company. As of the date of the filing of this annual report on Form 10-K, we have approximately $0.7 million of cash and cash equivalents. Since inception, we have used $42.4 million of cash to fund our operating activities and $3.4 million for capital expenditures. Through December 31, 2010, we have funded substantially all of our operations and capital expenditures through private placements of equity and convertible debt securities totaling $45.8 million, cash received from corporate collaborations totaling $11.4 million, government grants totaling $2.0 million, and capital equipment and working capital financing totaling $3.6 million.
In September 2008, we entered into a Loan and Security Agreement with CIT Healthcare LLC in which we are authorized to borrow up to $2.0 million for the purchase of certain equipment, a portion of the proceeds which may be used for general corporate working capital purposes. During 2008, we borrowed approximately $1.6 million pursuant to the Loan and Security Agreement, of which approximately $1.0 million was for equipment previously purchased and approximately $0.6 million was for general working capital. As of December 31, 2010, we had approximately $0.8 million of debt outstanding under such facility. The Loan and Security Agreement contains certain provisions, including a material adverse change clause (as defined in the Loan and Security Agreement) which restricts the Company's ability to borrow additional money and also enables the finance company to request full repayment of the loan if such a change has occurred. As of December 31, 2010, and as of the date of the filing of this annual report on Form 10-K, the Company was in compliance with these
45
provisions. As of December 31, 2010, there is no amount available to the Company for borrowing under this facility.
On March 12, 2007, we completed the first closing of our Private Placement of shares of common stock and warrants to purchase shares of common stock raising approximately $17.2 million of net proceeds. On April 6, 2007, we completed the final closing of our Private Placement raising an additional $11.4 million of net proceeds, bringing the total private placement net proceeds to $28.6 million.
Recognizing the difficult financing environment that has significantly impacted the biotechnology sector and seeking ways to conserve cash, during the year ended December 31, 2009, we announced two restructurings which reduced our workforce by approximately 60%. As of December 31, 2010, we had 10 full time employees. We may need to make further reductions in our expenses.
We expect that substantially all of our cash-flow for the foreseeable future will come from future corporate collaborations, equity financings or license agreements. However, there can be no assurance that we will enter into any future corporate collaborations, equity financings or license agreements. We are subject to those risks associated with any biotechnology company that has substantial expenditures for research and development. There can be no assurance that our research and development projects will be successful, that products developed will obtain necessary regulatory approval, or that any approved product will be commercially viable. In addition, we operate in an environment of rapid technological change and we are largely dependent on the services of our employees and consultants. The stock market in general has recently experienced large price and volume fluctuations and the market price of our common stock has experienced significant volatility. For us to fund our operations and to commercially develop our products, additional corporate collaborations and/or equity and/or debt financing will be required before April 2011. There is no assurance that such financing will be available to us as needed, and as a result, we may need to pursue other strategic alternatives, including the possible cessation of operations.
Summary of Contractual Obligations
The following table summarizes our obligations to make future payments under our current contractual obligations as of December 31, 2010:
| |
Payments Due by Period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Total | 2011 | 2012 - 2013 | 2014 - 2015 | Thereafter | |||||||||||
Operating leases |
$ | 5,435,000 | $ | 840,000 | $ | 1,698,000 | $ | 1,724,000 | $ | 1,173,000 | ||||||
Long-term debt |
774,000 | 422,000 | 352,000 | — | — | |||||||||||
License payments |
225,000 | 25,000 | 50,000 | 50,000 | 100,000 | |||||||||||
|
$ | 6,434,000 | $ | 1,287,000 | $ | 2,100,000 | $ | 1,774,000 | $ | 1,273,000 | ||||||
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and reported amount of revenue and expenses during the reporting period. On an ongoing basis, we evaluate our judgments and estimates, including those related to revenue recognition, long-lived assets, accrued liabilities, share-based payments and income taxes. We base our judgment and estimates on historical experience and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making
46
judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. During 2010, there have been no material changes in our critical accounting policies.
While our significant accounting policies are described in more detail in Note 3 to our financial statements, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our financial statements:
Revenue Recognition
Revenue from corporate collaborations and licensing agreements consist of up-front fees, research and development funding, and milestone payments. Nonrefundable up-front fees are deferred and recognized as revenue over the related performance period. We estimate the performance period based on the specific terms of each collaborative agreement, but the actual performance may vary. We adjust the performance periods based on available facts and circumstances. Revenue from research and development agreements and government grants is recognized pursuant to the related agreements as work is performed and related costs are incurred. Revenue resulting from the achievement of substantive milestone events as defined in the agreements is recognized when the milestone is achieved.
Stock-Based Compensation
The Company recognizes compensation for stock-based awards to employees and non-employee board members based on the grant-date fair value of stock- based awards over the period during which an award holder is required to provide service in exchange for the award. No compensation expense is recognized for awards for which the award holder does not tender the requisite service.
Prior to 2006, we applied the intrinsic-value-based method of accounting prescribed by Accounting Principles Board (APB) Opinion No. 25, Accounting for Stock Issued to Employees, and related interpretations, to account for fixed-plan stock options granted to employees and directors. Under APB Opinion No. 25, we did not record compensation expense for stock options since the current fair value of the underlying stock equaled the exercise price of the options.
In estimating the fair value of stock options, we use the Black-Scholes option-pricing model which requires us to make estimates about certain inputs regarding the volatility of our common stock and the expected term of our options. Until there is significant public trading activity in our common stock, we will use the average historical volatility of a group of pubic company peers. In addition, we have used the "simplified" method as described in SEC Staff Accounting Bulletin No. 107 to determine the expected life of our options.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Our carrying values of cash and cash equivalents, marketable securities, accounts receivable, accounts payable, accrued expenses and debt are a reasonable approximation of their fair value. The estimated fair values of financial instruments have been determined by us using available market information and appropriate valuation methodologies.
We have not entered into and do not expect to enter into, financial instruments for trading or hedging purposes. We do not currently anticipate entering into interest rate swaps and/or similar instruments. We have no material currency exchange risk exposure as of December 31, 2010. Our primary market risk exposure with regard to financial instruments is to changes in interest rates, which would impact interest income earned on such instruments. A one percent change (100 basis points) in interest rates on our investments would have impacted interest income by a nominal amount for the year ended December 31, 2010.
47
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
The financial statements required to be filed pursuant to this Item 8 are appended to this Annual Report on Form 10-K. A list of the financial statements filed herewith is found at "Item 15. Exhibits, Financial Statements and Financial Statement Schedules".
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures and Changes in Internal Control over Financial Reporting
Our management, with the participation of our chief executive officer and chief financial officer, evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act) as of December 31, 2010. In designing and evaluating our disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applied its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on this evaluation, our chief executive officer and chief financial officer concluded that, as of December 31, 2010, our disclosure controls and procedures were (1) effective in that they were designed to ensure that material information relating to us is made known and accumulated and communicated to management, including our chief executive officer and chief financial officer, by others within the Company, as appropriate to allow timely decisions regarding required disclosures, and (2) effective in that they provide that information required to be disclosed by us in our reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms.
No changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) occurred during the fiscal quarter ended December 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management's Responsibility for Financial Statements
Our management is responsible for the integrity and objectivity of all information presented in this annual report. The financial statements were prepared in conformity with accounting principles generally accepted in the United States of America and include amounts based on management's best estimates and judgments. Management believes the financial statements fairly reflect the form and substance of transactions and that the financial statements fairly represent the Company's financial position and results of operations.
The Audit Committee of the Board of Directors meets regularly with the Company's independent registered public accounting firm and representatives of management to review accounting, financial reporting, internal control and audit matters, as well as the nature and extent of the audit effort. The Audit Committee is responsible for the engagement of the independent registered public accounting firm. The independent registered public accounting firm has unrestricted access to the Audit Committee.
48
Management's Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Exchange Act and is a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
- •
- Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and
dispositions of our assets;
- •
- Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in
accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of our management and directors; and
- •
- Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
This annual report does not include an attestation report of the Company's independent registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by the Company's independent registered public accounting firm pursuant to newly adopted rules of the Securities and Exchange Commission that permit the company to provide only management's report in this annual report.
Our management assessed the effectiveness of the Company's internal control over financial reporting as of December 31, 2010. In making this assessment, the Company's management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework.
Based on its evaluation, our management has concluded that, as of December 31, 2010, our internal control over financial reporting was effective.
None.
49
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
The information relating to our directors and nominees for election as directors under the heading "Election of Directors" in our definitive proxy statement for the 2011 Annual Meeting of Stockholders is incorporated herein by reference to such proxy statement. The information relating to our executive officers in response to this item is contained in part under the caption "Our Executive Officers" in Part I of this Annual Report on Form 10-K and the remainder is incorporated herein by reference to our definitive proxy statement for the 2011 Annual Meeting of Stockholders.
We have adopted a written code of conduct that applies to all of our employees, including our principal executive officer, principal financial officer and principal accounting officer, or persons performing similar functions. We make available our code of conduct free of charge through our web site which is located at www.redpointbio.com. We intend to disclose any amendments to, or waivers from, our code of conduct that are required to be publicly disclosed pursuant to rules of the SEC and NASDAQ by filing such amendment or waiver with the SEC and by posting it on our web site.
ITEM 11. EXECUTIVE COMPENSATION.
The discussion under the heading "Executive Compensation" in our definitive proxy statement for the 2011 Annual Meeting of Stockholders is incorporated herein by reference to such proxy statement.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The discussion under the heading "Security Ownership of Certain Beneficial Owners and Management" in our definitive proxy statement for the 2011 Annual Meeting of Stockholders is incorporated herein by reference to such proxy statement.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
The discussion under the heading "Certain Relationships and Related Transactions and Director Independence" in our definitive proxy statement for the 2011 Annual Meeting of Stockholders is incorporated herein by reference to such proxy statement.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
The discussion under the heading "Independent Auditors Fees and Other Matters" in our definitive proxy statement for the 2011 Annual Meeting of Stockholders is incorporated herein by reference to such proxy statement.
50
ITEM 15. EXHIBITS, FINANCIAL STATEMENTS AND FINANCIAL STATEMENT SCHEDULES
| (a) | (1) | Financial Statements. Reference is made to the Index to Financial Statements on Page F-1. |
||
(2) |
Financial Statement Schedule. Not applicable. |
|||
(3) |
Exhibits. Reference is made to the Index to Exhibits on Page 46. |
51
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized this 4th day of March, 2011.
|
REDPOINT BIO CORPORATION | |||
|
By: |
/s/ F. RAYMOND SALEMME, PH.D. |
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ F. RAYMOND SALEMME F. Raymond Salemme |
President, Chief Executive Officer and Director (Principal Executive Officer) | March 4, 2011 | ||
/s/ SCOTT HORVITZ Scott Horvitz |
Chief Financial Officer, Treasurer and Secretary (Principal Financial and Accounting Officer) |
March 4, 2011 |
||
/s/ ALLEN BLOOM Allen Bloom |
Director |
March 4, 2011 |
||
/s/ ROBERT CHEFITZ Robert Chefitz |
Director |
March 4, 2011 |
||
/s/ LEIF KJAERGAARD Leif Kjaergaard |
Director |
March 4, 2011 |
||
/s/ IRWIN SCHER Irwin Scher |
Director |
March 4, 2011 |
||
/s/ RICHARD SHANLEY Richard Shanley |
Director |
March 4, 2011 |
52
| 2.1 | Agreement and Plan of Merger, dated May 3, 2007, between Robcor Properties, Inc. and Redpoint Bio Corporation (Incorporated by reference to Exhibit A of the Registrant's Schedule 14C filed with the Commission on May 23, 2007). | ||
3.1 |
Third Amended and Restated Certificate of Incorporation of Redpoint Bio Corporation (Incorporated by reference to Exhibit 3.1 of the Registrant's Amendment No. 1 to the Registration Statement on Form S-1 (Registration No. 333-143507) filed on July 2, 2007). |
||
3.2 |
Amended and Restated Bylaws of Redpoint Bio Corporation (Incorporated by reference to Exhibit C of the Registrant's Schedule 14C filed with the Commission on May 23, 2007). |
||
4.1 |
Form of Warrant issued by the Company to investors in the private placement on March 12, 2007 and April 6, 2007 (Incorporated by reference to Exhibit 4.1 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
4.2 |
Placement Agent Warrant issued by the Company to each of National Securities Corporation and Brean Murray, Carret & Co., LLC (Incorporated by reference to Exhibit 4.2 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
4.3 |
Form of common stock Certificate (Incorporated by reference to Exhibit 4.3 of the Registrant's Amendment No. 1 to the Registration Statement on Form S-1 (Registration No. 333-143507) filed on July 2, 2007). |
||
10.1 |
Agreement and Plan of Merger, dated as of March 12, 2007, by and among Redpoint Bio Corporation, on the one hand, and Robcor Properties, Inc., Robcor Acquisition Corp., Robcor LLC, Halter Financial Investments, L.P. and Michael Heitz (Incorporated by reference to Exhibit 10.1 of the Company's Current Report on Form 8-K filed on March 13, 2007). |
||
10.2 |
Form of Subscription Agreement, dated March 12, 2007 and April 6, 2007, by and between Redpoint Bio Corporation and the signatories thereto (Incorporated by reference to Exhibit 10.1 of the Registrant's Current Report on Form 8-K filed with the Commission on April 9, 2007). |
||
10.3 |
Lease Agreement, dated as of September 30, 2005, between Albert Einstein Healthcare Network and Linguagen Corp. (Incorporated by reference to Exhibit 10.5 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.4 |
Amendment to Lease Agreement between Albert Einstein Healthcare Network and Linguagen Corp., effective as of June 1, 2006, by and between Albert Einstein Healthcare Network and Linguagen Corp. (Incorporated by reference to Exhibit 10.6 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.5 |
Amendment No. 2 to Lease Agreement between Albert Einstein Healthcare Network and Linguagen Corp., dated as of August 4, 2006, by and between Albert Einstein Healthcare Network and Linguagen Corp. (Incorporated by reference to Exhibit 10.7 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.6 |
Amendment No. 3 to Lease Agreement between Albert Einstein Healthcare Network and Linguagen Corp., dated as of January 2, 2007, by and between Redpoint Bio Corporation and Albert Einstein Healthcare Network (Incorporated by reference to Exhibit 10.8 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.7 |
Lease Agreement, dated as of November 28, 2005, by and between BMR-7 Graphics Drive LLC and Linguagen Corp. (Incorporated by reference to Exhibit 10.9 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
53
| 10.8 | First Amendment to Lease Agreement, dated as of October 25, 2005, by and between BMR-7 Graphics Drive LLC and Linguagen Corp. (Incorporated by reference to Exhibit 10.10 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). | ||
10.9 |
Acknowledgement of Term Commencement Date and Term Expiration Date, dated May 7, 2007, by and between Redpoint Bio Corporation and BMR-7 Graphics Drive LLC (Incorporated by reference to Exhibit 10.11 of the Registrant's Amendment No. 1 to the Registration Statement on Form S-1 (Registration No. 333-143507) filed on July 2, 2007). |
||
10.10 |
Employment Agreement, dated May 25, 2004, between Linguagen Corp. and Dr. Raymond F. Salemme (Incorporated by reference to Exhibit 10.11 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007).** |
||
10.11 |
Employment Agreement, dated June 28, 2004, between Linguagen Corp. and Scott Horvitz (Incorporated by reference to Exhibit 10.13 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007).** |
||
10.12 |
Master Security Agreement, dated as of February 16, 2005, by and between Oxford Finance Corporation and Linguagen Corp. (Incorporated by reference to Exhibit 10.3 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.13 |
Redpoint Bio Corporation 2007 Omnibus Equity Compensation Plan (Incorporated by reference to Exhibit D of the Registrant's Schedule 14C filed with the Commission on May 23, 2007).** |
||
10.14 |
Registration Rights Agreement, dated March 12, 2007, by and among Robcor, National Holdings Corporation, Brean Murray, Carret & Co., LLC and the parties set forth on the signature page and Exhibit A thereto (Incorporated by reference to Exhibit 10.16 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.15 |
Registration Rights Agreement, dated March 12, 2007, by and among Robcor and certain existing stockholders of Redpoint (Incorporated by reference to Exhibit 10.17 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.16 |
Placement Agency Agreement, dated December 4, 2006, by and among Redpoint, National Securities Corporation and Brean Murray, Carret & Co., LLC (Incorporated by reference to Exhibit 10.18 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.17 |
Amendment No. 1 to the Placement Agency Agreement, dated March 6, 2007, by and among Redpoint, National Securities Corporation and Brean Murray, Carret & Co., LLC (Incorporated by reference to Exhibit 10.19 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.18 |
Advisory Agreement, dated March 8, 2007, by and between Halter Financial Group, L.P., and Redpoint Bio Corporation (Incorporated by reference to Exhibit 10.20 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.19 |
Separation Agreement and General Release, dated as of April 25, 2007, by and between Redpoint Bio Corporation and Susan Welsh (Incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed with the Commission on April 26, 2007).** |
||
10.20 |
Joint Research and Development and License Agreement, dated March 27, 2007, by and between Givaudan Schweiz AG and Redpoint Bio Corporation (Incorporated by reference to Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-Q filed with the Commission on May 15, 2007).† |
54
| 10.21 | Amended and Restated License Agreement, effective as of April 2, 2003, between Mount Sinai School of Medicine of New York University and Linguagen Corp. (Incorporated by reference to Exhibit 10.1 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). | ||
10.22 |
First Amendment to Amended and Restated License Agreement, dated as of November 10, 2003, between Mount Sinai School of Medicine of New York University and Linguagen Corp. (Incorporated by reference to Exhibit 10.2 of the Registrant's Current Report on Form 8-K filed with the Commission on March 16, 2007). |
||
10.23 |
Side Letter, dated July 11, 2007, to Placement Agency Agreement, dated December 4, 2006, as amended, by and between National Securities Corporation and Brean Murray, Carret & Co., LLC (Incorporated by reference to Exhibit 10.26 of the Registrant's Amendment No. 3 to the Registration Statement on Form S-1 (Registration No. 333-143507) filed on July 18, 2007). |
||
10.24 |
Research Agreement, dated December 13, 2007, by and between the Coca-Cola Company and Redpoint Bio Corporation (Incorporated by reference to Exhibit 10.28 of the Company's Annual Report on Form 10-K filed on March 14, 2008).† |
||
10.25 |
Loan and Security Agreement, dated September 25, 2008, by and between Redpoint Bio Corporation and CIT Healthcare LLC (Incorporated by reference to Exhibit 10.1 of the Registrant's Quarterly Report on Form 10-Q filed with the Commission on November 7, 2008). |
||
10.26 |
Separation of Employment Agreement and General Release, by and between the Company and Robert W. Bryant, Ph.D., dated January 9, 2009 (Incorporated by reference to Exhibit 10.1 of the Registrant's Current Report on Form 8-K filed with the Commission on January 15, 2009).** |
||
10.27 |
Confidential Separation Agreement and General Release, by and between the Company and Scott Siegel, Ph.D., dated February 26, 2009 (Incorporated by reference to Exhibit 10.1 of the Registrant's Current Report on Form 8-K filed with the Commission on March 4, 2009).** |
||
10.28 |
Amendment to Employment Agreement by, and between the Company and Dr. F. Raymond Salemme, dated December 22, 2008 (Incorporated by reference to Exhibit 10.1 of the Company's Current Report on Form 8-K filed on December 23, 2008).** |
||
10.29 |
Amendment to Employment Agreement by, and between the Company and Scott M. Horvitz, dated December 22, 2008 (Incorporated by reference to Exhibit 10.2 of the Company's Current Report on Form 8-K filed on December 23, 2008).** |
||
10.30 |
License and Commercialization Agreement, by and between the Company and International Flavors & Fragrances Inc., dated June 29, 2010 (Incorporated by reference to Exhibit 10.1 of the Company's Quarterly Report on Form 10-Q filed on August 13, 2010).† |
||
21.1 |
List of Subsidiaries.* |
||
23.1 |
Consent of KPMG LLP.* |
||
31.1 |
Certification Pursuant to Rule 13a-14(a) and 15d-14(a) of Exchange Act, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Chief Executive Officer).* |
55
| 31.2 | Certification Pursuant to Rule 13a-14(a) and 15d-14(a) of the Exchange Act, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Chief Financial Officer).* | ||
32.1 |
Certification Pursuant to 18 U.S.C. Section 1350 (Chief Executive Officer).* |
||
32.2 |
Certification Pursuant to 18 U.S.C. Section 1350 (Chief Financial Officer).* |
- *
- Filed
herewith.
- **
- A
management contract or compensatory plan or arrangement required to be filed as an exhibit pursuant to Item 15(a)(3) of
Form 10-K.
- †
- Confidential treatment has been granted as to certain portions, which portions have been filed separately with the Securities and Exchange Commission.
56
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
F-1
Report of Independent Registered Public Accounting Firm
The
Board of Directors and Stockholders
Redpoint Bio Corporation:
We have audited the accompanying balance sheets of Redpoint Bio Corporation (a development-stage enterprise) (the "Company") as of December 31, 2009 and 2010, and the related statements of operations, redeemable convertible preferred stock and stockholders' equity (deficit), and cash flows for each of the years in the three-year period ended December 31, 2010 and for the period from August 16, 1995 (inception) to December 31, 2010. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Redpoint Bio Corporation as of December 31, 2009 and 2010, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2010 and for the period from August 16, 1995 (inception) to December 31, 2010, in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in note 1 to the financial statements, the Company has incurred losses and negative cash flows from operations since inception, has an accumulated deficit and limited cash resources that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ KPMG LLP
Philadelphia,
Pennsylvania
March 4, 2011
F-2
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Balance Sheets
| |
December 31 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | |||||||
Assets |
|||||||||
Current assets: |
|||||||||
Cash and cash equivalents |
$ | 5,557,367 | $ | 1,082,999 | |||||
Prepaid expenses and other current assets |
176,464 | 72,200 | |||||||
Total current assets |
5,733,831 | 1,155,199 | |||||||
Property and equipment, net |
726,730 | 337,297 | |||||||
Other assets |
314,014 | 299,498 | |||||||
Total assets |
$ | 6,774,575 | $ | 1,791,994 | |||||
Liabilities and Stockholders' Equity (Deficit) |
|||||||||
Current liabilities: |
|||||||||
Current portion of long-term debt |
$ | 374,110 | 422,100 | ||||||
Accounts payable |
658,561 | 954,275 | |||||||
Accrued expenses |
151,893 | 171,124 | |||||||
Accrued compensation |
37,702 | — | |||||||
Total current liabilities |
1,222,266 | 1,547,499 | |||||||
Long-term debt |
773,843 | 351,742 | |||||||
Total liabilities |
1,996,109 | 1,899,241 | |||||||
Commitments (Note 13) |
|||||||||
Stockholders' equity (deficit): |
|||||||||
Preferred Stock; 10,000,000 shares authorized, $0.0001 par value, none issued |
— | — | |||||||
Common stock; authorized 150,000,000 shares; $0.0001 par value, issued and outstanding issued and outstanding 79,838,693 at December 31, 2009 and 79,846,893 shares at December 31, 2010 |
7,984 | 7,985 | |||||||
Additional paid-in capital |
56,103,784 | 56,687,893 | |||||||
Deficit accumulated during the development stage |
(51,333,302 | ) | (56,803,125 | ) | |||||
Total stockholders' equity (deficit) |
4,778,466 | (107,247 | ) | ||||||
Total liabilities and stockholders' equity (deficit) |
$ | 6,774,575 | $ | 1,791,994 | |||||
See accompanying notes to financial statements.
F-3
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Statements of Operations
| |
|
|
|
August 16, 1995 (Inception) to December 31, 2010 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Years ended December 31, | ||||||||||||||
| |
2008 | 2009 | 2010 | ||||||||||||
Research and grant revenue |
$ | 3,999,252 | $ | 1,868,428 | $ | 1,244,479 | $ | 13,574,481 | |||||||
Operating expenses: |
|||||||||||||||
Research and development |
8,647,432 | 5,630,336 | 3,041,684 | 37,109,747 | |||||||||||
General and administrative |
5,607,309 | 4,960,913 | 3,644,206 | 28,807,030 | |||||||||||
Total operating expenses |
14,254,741 | 10,591,249 | 6,685,890 | 65,916,777 | |||||||||||
Operating loss |
(10,255,489 | ) | (8,722,821 | ) | (5,441,411 | ) | 52,342,296 | ||||||||
Interest income |
565,177 | 142,040 | 11,033 | 1,671,651 | |||||||||||
Interest expense |
(97,519 | ) | (235,231 | ) | (140,751 | ) | (3,806,875 | ) | |||||||
Loss before income taxes |
(9,787,831 | ) | (8,816,012 | ) | (5,571,129 | ) | (54,477,520 | ) | |||||||
Income tax benefit |
266,772 | — | 101,306 | 1,087,284 | |||||||||||
Net loss |
$ | (9,521,059 | ) | $ | (8,816,012 | ) | (5,469,823 | ) | $ | (53,390,236 | ) | ||||
Basic and diluted net loss per common share |
$ | (0.12 | ) | $ | (0.11 | ) | $ | (0.07 | ) | ||||||
Weighted average number of shares outstanding |
79,443,622 | 79,651,102 | 79,841,703 | ||||||||||||
See accompanying notes to financial statements.
F-4
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Statements of Redeemable Convertible Preferred Stock and Stockholders' Equity (Deficit)
Period from August 16, 1995 (inception) to December 31, 2010
| |
|
|
|
|
Stockholders' Equity (Deficit) | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Redeemable convertible preferred stock | |||||||||||||||||||||||||||||||||
| |
|
|
|
|
Deficit accumulated during development stage |
|
|
|||||||||||||||||||||||||||
| |
Series A | Junior | Common Stock | |
|
Accumulated other comprehensive income |
|
|||||||||||||||||||||||||||
| |
Additional paid-in capital |
Stock subscription receivable |
|
|||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | Total | |||||||||||||||||||||||||||
Inception, August 16, 1995 |
— | $ | — | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||
Issuance of common stock for services |
— | — | — | — | 2,782,000 | 278 | 722 | — | — | — | 1,000 | |||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (3,785 | ) | — | (3,785 | ) | |||||||||||||||||||||
Balance, December 31, 1995 |
— | — | — | — | 2,782,000 | 278 | 722 | — | (3,785 | ) | — | (2,785 | ) | |||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (7,069 | ) | — | (7,069 | ) | |||||||||||||||||||||
Balance December 31, 1996 |
— | — | — | — | 2,782,000 | 278 | 722 | — | (10,854 | ) | — | (9,854 | ) | |||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (48,185 | ) | — | (48,185 | ) | |||||||||||||||||||||
Balance, December 31, 1997 |
— | — | — | — | 2,782,000 | 278 | 722 | — | (59,039 | ) | — | (58,039 | ) | |||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | 113,785 | — | 113,785 | |||||||||||||||||||||||
Balance, December 31, 1998 |
— | — | — | — | 2,782,000 | 278 | 722 | — | 54,746 | — | 55,746 | |||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (73,505 | ) | — | (73,505 | ) | |||||||||||||||||||||
Balance, December 31, 1999 |
— | — | — | — | 2,782,000 | 278 | 722 | — | (18,759 | ) | — | (17,759 | ) | |||||||||||||||||||||
Issuance of common stock |
— | — | — | — | 282,373 | 28 | 294 | — | — | — | 322 | |||||||||||||||||||||||
Issuance of common stock for dilution provisions |
— | — | — | — | 7,428 | — | — | — | — | — | — | |||||||||||||||||||||||
Net income |
— | — | — | — | — | — | — | — | 42,905 | — | 42,905 | |||||||||||||||||||||||
Balance, December 31, 2000 |
— | — | — | — | 3,071,801 | 306 | 1,016 | — | 24,146 | — | 25,468 | |||||||||||||||||||||||
Common stock reacquired and retired |
— | — | — | — | (1,669,200 | ) | (167 | ) | 107 | — | — | — | (60 | ) | ||||||||||||||||||||
Issuance of common stock |
— | — | — | — | 1,112,800 | 111 | 3,995 | (4,106 | ) | — | — | — | ||||||||||||||||||||||
Issuance of common stock for dilution provisions |
— | — | — | — | 4,237 | — | — | — | — | — | — | |||||||||||||||||||||||
Common stock reacquired and retired |
— | — | — | — | (20,170 | ) | — | — | — | — | — | — | ||||||||||||||||||||||
Net income |
— | — | — | — | — | — | — | — | 116,192 | — | 116,192 | |||||||||||||||||||||||
Balance, December 31, 2001 |
— | — | — | — | 2,499,468 | 250 | 5,118 | (4,106 | ) | 140,338 | — | 141,600 | ||||||||||||||||||||||
Issuance of common stock for services |
— | — | — | — | 515,838 | 52 | 926 | — | — | — | 978 | |||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (864,319 | ) | — | (864,319 | ) | |||||||||||||||||||||
Balance, December 31, 2002 |
— | — | — | — | 3,015,306 | 302 | 6,044 | — | (723,981 | ) | — | (721,741 | ) | |||||||||||||||||||||
Sale of Series A Preferred stock net of $257,240 of expenses |
1,285,714 | 2,442,759 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||
Conversion of principal portion of notes payable |
623,963 | 1,310,320 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||
Conversion of accrued interest on notes payable |
— | — | 236,987 | 497,673 | — | — | — | — | — | — | — | |||||||||||||||||||||||
Accretion of Series A Preferred to redemption value |
— | 48,826 | — | — | — | — | — | — | (48,826 | ) | — | (48,826 | ) | |||||||||||||||||||||
Issuance of common stock for dilution provisions |
— | — | — | — | 673,272 | 67 | 101,577 | — | — | — | 101,644 | |||||||||||||||||||||||
Issuance of common stock options for services |
— | — | — | — | — | — | 3,087 | — | — | — | 3,087 | |||||||||||||||||||||||
Proceeds from stockholder note |
— | — | — | — | — | — | — | 4,106 | — | — | 4,106 | |||||||||||||||||||||||
Common stock reacquired and retired |
— | — | — | — | (678,263 | ) | (68 | ) | (110,900 | ) | — | (164,032 | ) | — | (275,000 | ) | ||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (2,360,483 | ) | — | (2,360,483 | ) | |||||||||||||||||||||
Balance, December 31, 2003 |
1,909,677 | 3,801,905 | 236,987 | 497,673 | 3,010,315 | 301 | (192 | ) | — | (3,297,322 | ) | — | (3,297,213 | ) | ||||||||||||||||||||
See accompanying notes to financial statements.
F-5
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Statements of Redeemable Convertible Preferred Stock and Stockholders' Equity (Deficit) (Continued)
Period from August 16, 1995 (inception) to December 31, 2010
| |
|
|
|
|
Stockholders' Equity (Deficit) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Redeemable convertible preferred stock | ||||||||||||||||||||||||||||||||||
| |
|
|
|
|
Deficit accumulated during development stage |
|
|
||||||||||||||||||||||||||||
| |
Series A | Junior | Common Stock | |
|
Accumulated other comprehensive income |
|
||||||||||||||||||||||||||||
| |
Additional paid-in capital |
Stock subscription receivable |
|
||||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | Total | ||||||||||||||||||||||||||||
Sale of Series A Preferred stock net of $83,144 of expenses |
4,535,158 | 9,440,688 | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||
Accretion of Series A Preferred to redemption value |
— | 669,228 | — | — | — | — | — | — | (669,228 | ) | — | (669,228 | ) | ||||||||||||||||||||||
Issuance of common stock for dilution provisions |
— | — | — | — | 1,391,000 | 139 | 209,861 | — | — | — | 210,000 | ||||||||||||||||||||||||
Issuance of common stock options for services |
— | — | — | — | — | — | 15,391 | — | — | — | 15,391 | ||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (4,803,026 | ) | — | (4,803,026 | ) | ||||||||||||||||||||||
Balance, December 31, 2004 |
6,444,835 | 13,911,821 | 236,987 | 497,673 | 4,401,315 | 440 | 225,060 | — | (8,769,576 | ) | — | (8,544,076 | ) | ||||||||||||||||||||||
Accretion of Series A Preferred to redemption value |
— | — | — | — | — | — | — | — | (1,147,404 | ) | — | (1,147,404 | ) | ||||||||||||||||||||||
Issuance of common stock for dilution provisions |
— | — | — | — | 2,790 | — | 421 | — | — | — | 421 | ||||||||||||||||||||||||
Issuance of common stock |
— | — | — | — | 89,204 | 9 | 13,431 | — | — | — | 13,440 | ||||||||||||||||||||||||
Issuance of warrants and stock options for services |
— | — | — | — | — | — | 34,782 | — | — | — | 34,782 | ||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (4,367,326 | ) | — | (4,367,326 | ) | ||||||||||||||||||||||
Balance, December 31, 2005 |
6,444,835 | 15,059,225 | 236,987 | 497,673 | 4,493,129 | 449 | 273,694 | — | (14,284,306 | ) | — | (14,010,163 | ) | ||||||||||||||||||||||
Accretion of Series A Preferred to redemption value |
— | — | — | — | — | — | — | — | (1,158,553 | ) | — | (1,158,553 | ) | ||||||||||||||||||||||
Issuance of warrants to purchase preferred stock |
— | — | — | — | — | — | 1,228,568 | — | — | — | 1,228,568 | ||||||||||||||||||||||||
Proceeds from warrants |
— | — | — | — | — | — | 23,750 | — | — | — | 23,750 | ||||||||||||||||||||||||
Stock-based compensation |
— | — | — | — | — | — | 50,801 | — | — | — | 50,801 | ||||||||||||||||||||||||
Common stock reacquired and retired |
— | — | — | — | (53,784 | ) | (5 | ) | (8,116 | ) | — | — | — | (8,121 | ) | ||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (6,852,139 | ) | — | (6,852,139 | ) | ||||||||||||||||||||||
Balance, December 31, 2006 |
6,444,835 | 16,217,778 | 236,987 | 497,673 | 4,439,345 | 444 | 1,568,697 | — | (22,294,998 | ) | — | (20,725,857 | ) | ||||||||||||||||||||||
Accretion of Series A Preferred to redemption value |
— | 224,846 | — | — | — | — | — | — | (224,846 | ) | — | (224,846 | ) | ||||||||||||||||||||||
Conversion of preferred stock |
(6,444,835 | ) | (16,442,624 | ) | (236,987 | ) | (497,673 | ) | 22,774,963 | 2,278 | 16,938,019 | — | — | — | 16,940,297 | ||||||||||||||||||||
Conversion of convertible notes payable and warrants |
— | — | — | — | 7,256,697 | 725 | 5,093,074 | — | — | — | 5,093,799 | ||||||||||||||||||||||||
Sale of common stock, net of issuance costs of $4,406,015 |
— | — | — | — | 42,180,263 | 4,218 | 28,578,767 | — | — | — | 28,582,985 | ||||||||||||||||||||||||
Issuance of common stock for dilution provisions |
— | — | — | — | 2,017,407 | 202 | 1,631,507 | — | — | — | 1,631,709 | ||||||||||||||||||||||||
Issuance of common stock upon exercise of options and warrants |
— | — | — | — | 555,312 | 55 | 83,241 | — | — | — | 83,296 | ||||||||||||||||||||||||
Stock-based compensation |
— | — | — | — | — | — | 592,068 | — | — | — | 592,068 | ||||||||||||||||||||||||
Comprehensive loss: |
|||||||||||||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (10,476,387 | ) | — | (10,476,387 | ) | ||||||||||||||||||||||
Unrealized gain on marketable securities |
— | — | — | — | — | — | — | — | — | 41,711 | 41,711 | ||||||||||||||||||||||||
Total comprehensive loss |
(10,434,676 | ) | |||||||||||||||||||||||||||||||||
Balance, December 31, 2007 |
— | — | — | — | 79,223,987 | 7,922 | 54,485,373 | — | (32,996,231 | ) | 41,711 | 21,538,775 | |||||||||||||||||||||||
See accompanying notes to financial statements.
F-6
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Statements of Redeemable Convertible Preferred Stock and Stockholders' Equity (Deficit) (Continued)
Period from August 16, 1995 (inception) to December 31, 2010
| |
|
|
|
|
Stockholders' Equity (Deficit) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Redeemable convertible preferred stock | ||||||||||||||||||||||||||||||||||
| |
|
|
|
|
Deficit accumulated during development stage |
|
|
||||||||||||||||||||||||||||
| |
Series A | Junior | Common Stock | |
|
Accumulated other comprehensive income |
|
||||||||||||||||||||||||||||
| |
Additional paid-in capital |
Stock subscription receivable |
|
||||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | Total | ||||||||||||||||||||||||||||
Issuance of common stock upon exercise of options |
— | — | — | — | 271,883 | 28 | 40,754 | — | — | — | 40,782 | ||||||||||||||||||||||||
Stock-based compensation |
— | — | — | — | — | — | 845,374 | — | — | — | 845,374 | ||||||||||||||||||||||||
Comprehensive loss: |
|||||||||||||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (9,521,059 | ) | — | (9,521,059 | ) | ||||||||||||||||||||||
Net unrealized gain on marketable securities |
— | — | — | — | — | — | — | — | — | 22,843 | 22,843 | ||||||||||||||||||||||||
Total comprehensive loss |
(9,498,216 | ) | |||||||||||||||||||||||||||||||||
Balance, December 31, 2008 |
— | — | — | — | 79,495,870 | 7,950 | 55,371,501 | — | (42,517,290 | ) | 64,554 | 12,926,715 | |||||||||||||||||||||||
Issuance of common stock upon exercise of options |
— | — | — | — | 342,823 | 34 | 51,390 | — | — | — | 51,424 | ||||||||||||||||||||||||
Stock-based compensation |
— | — | — | — | — | — | 680,893 | — | — | — | 680,893 | ||||||||||||||||||||||||
Comprehensive loss: |
|||||||||||||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (8,816,012 | ) | — | (8,816,012 | ) | ||||||||||||||||||||||
Net unrealized loss on marketable securities |
— | — | — | — | — | — | — | — | — | (64,554 | ) | (64,554 | ) | ||||||||||||||||||||||
Total comprehensive loss |
(8,880,566 | ) | |||||||||||||||||||||||||||||||||
Balance, December 31, 2009 |
— | $ | — | — | $ | — | 79,838,693 | $ | 7,984 | $ | 56,103,784 | $ | — | $ | (51,333,302 | ) | $ | — | $ | 4,778,466 | |||||||||||||||
Issuance of common stock upon exercise of options |
— | — | — | — | 8,200 | 1 | 573 | — | — | — | 574 | ||||||||||||||||||||||||
Stock-based compensation |
— | — | — | — | — | — | 583,536 | — | — | — | 583,536 | ||||||||||||||||||||||||
Net Loss |
— | — | — | — | — | — | — | — | (5,469,823 | ) | — | (5,469,823 | ) | ||||||||||||||||||||||
Balance, December 31, 2010 |
— | $ | — | — | $ | — | 79,846,893 | $ | 7,985 | $ | 56,687,893 | $ | — | $ | (56,803,125 | ) | $ | — | $ | (107,247 | ) | ||||||||||||||
See accompanying notes to financial statements.
F-7
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Statements of Cash Flows
| |
Years ended December 31, | |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
August 16, 1995 (Inception) to December 31, 2010 |
||||||||||||||||
| |
2008 | 2009 | 2010 | ||||||||||||||
Cash flows from operating activities: |
|||||||||||||||||
Net loss |
$ | (9,521,059 | ) | $ | (8,816,012 | ) | $ | (5,469,823 | ) | $ | (53,390,236 | ) | |||||
Adjustments to reconcile net loss to net cash used in operating activities: |
|||||||||||||||||
Depreciation and amortization |
624,835 | 519,636 | 389,433 | 3,042,905 | |||||||||||||
Amortization of discounts and premiums on marketable securities |
(290,371 | ) | 11,446 | — | (473,654 | ) | |||||||||||
Options, warrants and stock issued for services and dilution provisions |
845,374 | 680,893 | 583,536 | 4,725,713 | |||||||||||||
Beneficial conversion feature |
— | — | — | 1,228,565 | |||||||||||||
Amortization of discount on convertible notes |
— | — | — | 899,935 | |||||||||||||
Amortization of deferred financing costs |
10,952 | 16,470 | 14,516 | 175,760 | |||||||||||||
Debt inducement charge |
— | — | — | 361,598 | |||||||||||||
Interest expense on convertible notes |
— | — | — | 136,075 | |||||||||||||
Changes in assets and liabilities: |
|||||||||||||||||
Accounts receivable |
(88,981 | ) | 139,095 | — | — | ||||||||||||
Prepaid expenses and other assets |
181,915 | 128,486 | 104,264 | (658,762 | ) | ||||||||||||
Accounts payable |
268,340 | (210,994 | ) | 295,714 | 1,262,731 | ||||||||||||
Accrued expenses and accrued compensation |
(595,692 | ) | (131,595 | ) | (18,471 | ) | 302,734 | ||||||||||
Deferred revenue |
(371,529 | ) | (649,902 | ) | — | — | |||||||||||
Net cash used in operating activities |
(8,936,216 | ) | (8,312,477 | ) | (4,100,831 | ) | (42,386,636 | ) | |||||||||
Cash flows from investing activities: |
|||||||||||||||||
Purchases of marketable securities |
(21,069,320 | ) | (3,019,048 | ) | — | (46,513,341 | ) | ||||||||||
Maturity and sale of marketable securities |
26,996,994 | 14,240,000 | — | 46,986,994 | |||||||||||||
Purchases of property and equipment |
(670,983 | ) | (13,939 | ) | — | (3,380,219 | ) | ||||||||||
Net cash (used in) provided by investing activities |
5,256,691 | 11,207,013 | — | (2,906,566 | ) | ||||||||||||
Cash flows from financing activities: |
|||||||||||||||||
Proceeds from issuance of long-term debt |
1,503,900 | — | — | 3,614,710 | |||||||||||||
Net proceeds from convertible notes |
— | — | — | 5,253,811 | |||||||||||||
Repayments of debt |
(524,593 | ) | (519,154 | ) | (374,111 | ) | (2,893,325 | ) | |||||||||
Net proceeds from issuance of preferred stock |
— | — | — | 11,883,447 | |||||||||||||
Net proceeds from issuance of common stock and warrants |
— | — | — | 28,624,603 | |||||||||||||
Proceeds from exercise of common stock options and warrants |
40,782 | 51,424 | 574 | 176,076 | |||||||||||||
Common stock reacquired |
— | — | — | (283,121 | ) | ||||||||||||
Net cash provided by (used in) financing activities |
1,020,089 | (467,730 | ) | (373,537 | ) | 46,376,201 | |||||||||||
Net increase (decrease) in cash and cash equivalents |
(2,659,436 | ) | 2,426,806 | (4,474,368 | ) | 1,082,999 | |||||||||||
Cash and cash equivalents, beginning of year |
5,789,997 | 3,130,561 | 5,557,367 | — | |||||||||||||
Cash and cash equivalents, end of year |
$ | 3,130,561 | $ | 5,557,367 | $ | 1,082,999 | $ | 1,082,999 | |||||||||
Supplemental cash flow disclosures: |
|||||||||||||||||
Noncash investing and financing activities: |
|||||||||||||||||
Increase (decrease) in property and equipment included in accounts payable |
$ | (101,578 | ) | $ | (8,087 | ) | $ | — | $ | — | |||||||
Conversion of notes payable and accrued interest to preferred stock |
— | — | — | 1,446,395 | |||||||||||||
Conversion of notes payable and accrued interest to common stock |
— | — | — | 3,896,001 | |||||||||||||
Conversion of preferred and junior preferred stock to common stock |
— | — | — | 16,940,297 | |||||||||||||
Accretion of preferred stock |
— | — | — | 3,248,857 | |||||||||||||
Warrants issued in connection with notes payable |
— | — | — | 1,258,642 | |||||||||||||
Cash paid for interest |
87,250 | 169,866 | 118,901 | 788,894 | |||||||||||||
See accompanying notes to financial statements.
F-8
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements
(1) Description of the Business and Liquidity
Redpoint Bio ("Redpoint" or the "Company") is a development stage biotechnology company focused on the development of healthier foods and beverages and new approaches to the treatment of diabetes and obesity.
The Company is leveraging recent discoveries in the molecular biology of taste to discover and develop novel taste modulators for the food and beverage industries. The Company's food and beverage program has been focused on identifying novel flavor modifiers that improve the taste of existing ingredients, enabling the development of better-tasting and more healthful foods and beverages. In June 2009, the Company announced that it had identified an all-natural sweetness enhancer, RP44. In June 2010, the Company entered into a license and commercialization agreement with International Flavors & Fragrances Inc. ("IFF"), a global leader in the food and beverage industry, covering the commercialization of RP44.
The Company is also pursuing a pharmaceutical discovery program for the treatment of diabetes and obesity. Redpoint's research in diabetes and obesity stems from the observation that taste signaling circuits responsible for sensing sweet, savory, and bitter compounds on the tongue are also found in the pancreas and gastrointestinal tract, suggesting a potential role in the regulation of metabolism and satiety. The Company is actively seeking a research and development collaboration with major pharmaceutical companies for the further development of this discovery program.
Since its inception in 1995, the Company has incurred losses and negative cash flows from operations, and such losses have continued subsequent to December 31, 2010. As of December 31, 2010, the Company had an accumulated deficit of $56.8 million and anticipates incurring additional losses for the foreseeable future. The Company will need to spend significant resources over the next several years to enhance its technologies and to fund research and development of its pipeline of potential products. Through December 31, 2010, substantially all of the Company's revenue has been derived from corporate collaborations, license agreements, and government grants. The Company expects that substantially all of its cash-flow for the foreseeable future will come from future corporate collaborations, equity financings and license agreements. However, there can be no assurance that the Company will enter into any future corporate collaborations, equity financings or license agreements. In order to achieve profitability, the Company must continue to develop products and technologies that can be commercialized by the Company or through future collaborations. Redpoint had approximately $1.1 million in cash and cash equivalents at December 31, 2010. In addition, in January 2011, the Company sold a portion of its New Jersey State net operating loss carryforwards and received cash proceeds of approximately $0.4 million. As of the date of this filing, the Company had approximately $0.7 million of cash and cash equivalents. The Company believes that its current capital resources are only sufficient to meet its operating and capital requirements through April 2011, which raises substantial doubt about its ability to continue as a going concern. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty. The Company is actively seeking corporate collaborations and license agreements from various strategic partners as well as debt or equity financings to enhance the Company's liquidity position.
Recognizing the difficult financing environment that has significantly impacted the biotechnology sector and seeking ways to conserve cash, during the year ended December 31, 2009, the Company announced two restructurings which reduced its workforce by approximately 60%. As of December 30,
F-9
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(1) Description of the Business and Liquidity (Continued)
2010, the Company has 10 full time employees. The Company may need to make further reductions in its expenses.
The Company is subject to those risks associated with any biotechnology company that has substantial expenditures for research and development. There can be no assurance that the Company's research and development projects will be successful, that products developed will obtain necessary regulatory approval, or that any approved product will be commercially viable. In addition, the Company operates in an environment of rapid technological change and is largely dependent on the services of its employees and consultants. For the Company to fund its operations and to commercially develop its products, additional corporate collaborations, equity and/or debt financing will be required before April 2011. There is no assurance that such collaborations or financing will be available to the Company as needed, and as a result, it may need to pursue other strategic alternatives. Failure to successfully address ongoing liquidity requirements will have a material adverse effect on the Company's business. If the Company is unable to obtain additional capital on acceptable terms when needed, it will be unable to satisfy its existing obligations and may be required to take actions that harm its business and its ability to achieve cash flow in the future, including possibly the surrender of its rights to some technologies or product opportunities, delaying its clinical trials or curtailing or ceasing operations.
(2) Reverse Triangular Merger, Financing, Reincorporation Merger
Completion of Merger
Robcor Properties, Inc., a Florida corporation ("Robcor"), and its newly-formed subsidiary, Robcor Acquisition Corp., a Delaware corporation ("Merger Sub") entered into an Agreement and Plan of Merger (the "Merger Agreement"), dated March 12, 2007, by and among, Redpoint, formerly a privately-held Delaware corporation, on the one hand, and Robcor, Merger Sub, Robcor LLC, a Kentucky limited liability company and wholly-owned subsidiary of Robcor ("Robcor LLC") and Halter Financial Investments, L.P., a Texas limited partnership ("Halter"), and Michael Heitz ("Heitz"), as stockholders of Robcor, on the other hand. Pursuant to the Merger Agreement, Merger Sub, which Robcor had incorporated in the state of Delaware for the purpose of completing the transaction, merged into Redpoint (the "Reverse Merger") on March 12, 2007 (the "Closing" or the "Closing Date") with Redpoint continuing as the surviving entity in the Reverse Merger. As a result of the Reverse Merger, Redpoint became a wholly-owned subsidiary of Robcor. In connection with the Reverse Merger, each share of capital stock of Redpoint was converted into 2.7820 shares of common stock of Robcor and all of Redpoint's convertible promissory notes were converted into shares of common stock of Robcor.
Redpoint was deemed to have been the accounting acquirer in the Reverse Merger. Accordingly, the financial statements of the Company presented reflect the historical results of Redpoint prior to the Reverse Merger, and of the combined entities following the Reverse Merger, and do not include the historical financial results of Robcor prior to the consummation of the Reverse Merger. In connection with the Reverse Merger, Redpoint issued 1,391,000 shares of common stock to Robcor shareholders which have been treated as issuance costs in connection with the Private Placement.
F-10
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(2) Reverse Triangular Merger, Financing, Reincorporation Merger (Continued)
Private Placement
Concurrently with the completion of the Reverse Merger, Robcor received $17.2 million in net proceeds from the initial closing of a private placement of approximately 24.7 million shares of common stock at a price of $0.81 per share, and warrants to purchase approximately 6.2 million shares of Robcor common stock at an exercise price of $1.35 per share (the "Private Placement"). The initial closing of the Private Placement occurred on March 12, 2007, concurrently with the completion of the Reverse Merger.
In connection with the Private Placement, Redpoint engaged two placement agents which were issued five-year warrants to buy approximately 2.0 million shares of Robcor common stock equal to 10% of the number of shares of common stock sold in the Private Placement at an exercise price of $0.97 per share; provided, however, no warrants were issued to the placement agents with respect to shares and warrants sold to existing Redpoint stockholders.
On April 6, 2007, Robcor sold an additional 16.1 million shares of common stock and warrants to purchase 4.0 million shares of common stock which resulted in net proceeds of approximately $11.3 million. In connection with the April closing, warrants to purchase an additional 1.6 million shares of common stock were issued to the placement agents.
Reincorporation Merger
On June 15, 2007, Robcor was merged with and into Redpoint, its wholly-owned subsidiary, with Redpoint being the surviving corporation pursuant to the Agreement and Plan of Merger dated May 3, 2007. As a result of the merger, the Company's state of incorporation changed from Florida to Delaware.
In connection with the reincorporation merger, the authorized number of shares of common stock was decreased from 1,000,000,000 shares of common stock, no par value per share, to 150,000,000 shares of common stock, $0.0001 par value per share, and the authorized number of shares of preferred stock was decreased from 20,000,000 shares of preferred stock, no par value per share, to 10,000,000 shares of preferred stock, $0.0001 par value per share. The Company's share data and capital stock have been retrospectively adjusted for all periods presented to reflect the reincorporation merger.
In addition, the maximum number of shares of common stock reserved for issuance under the Company's 2007 Omnibus Equity Compensation Plan was increased from 13,511,562 to 17,644,267 shares.
(3) Summary of Significant Accounting Policies
(a) Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Illiquid credit markets, volatile equity, and declines in consumer spending have combined to increase the
F-11
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(3) Summary of Significant Accounting Policies (Continued)
uncertainty inherent in such estimates and assumptions. As future events and their effect cannot be determined with precision, actual results could differ significantly from these estimates. Changes in those estimates resulting from continuing changes in the economic environment will be reflected in the financial statements in those future periods.
(b) Cash and Cash Equivalents
The Company considers all highly liquid investments that have original maturities of three months or less when acquired to be cash equivalents. As of December 31, 2009 and 2010, cash and cash equivalents included amounts invested in money market accounts and government sponsored enterprise debt securities.
(c) Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash equivalents. The Company has established guidelines relating to diversification and maturities that allows the Company to manage risk.
(d) Revenue Recognition
Revenue from corporate collaborations and licensing agreements consists of up-front fees, research and development funding, and milestone payments.
License Fees and Multiple Element Arrangements
Non-refundable upfront fees are recognized as revenue when Redpoint has a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured, and Redpoint has no further performance obligations under the license agreement.
Multiple element arrangements are analyzed to determine whether the deliverables, which often include a license and performance obligations, such as research and development services, can be separated or whether they must be accounted for as a single unit of accounting. If the fair value of the undelivered performance obligations can be determined, such obligations would then be accounted for separately as performed. However, if the elements are considered to either (i) not have stand-alone value or (ii) have standalone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement would then be accounted for as a single unit of accounting and all of the payments are recognized as revenue over the estimated period of when the performance obligations are performed. Whenever Redpoint determines that an arrangement should be accounted for as a single unit of accounting, it must determine the period over which the performance obligations will be performed and revenue will be recognized. Significant management judgment is required in determining the period over which the Company is expected to complete our performance obligations under an arrangement.
F-12
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(3) Summary of Significant Accounting Policies (Continued)
Substantive Milestone Payments
Redpoint's collaboration agreements may also contain substantive milestone payments that are recognized upon achievement of the milestone only if all of the following conditions are met:
- •
- the milestone payments are non-refundable;
- •
- achievement of the milestone involves a degree of risk and was not reasonably assured at the inception of the arrangement;
- •
- substantive effort is involved in achieving the milestone;
- •
- a reasonable amount of time passes between the up-front license payment and the first milestone payment as
well as between each subsequent milestone payment; and
- •
- the amount of the milestone payment is reasonable in relation to the effort expended or the risk associated with achievement of the milestone.
Determination as to whether a payment meets the aforementioned conditions involves management's judgment. If any of these conditions are not met, the resulting payment would not be considered a substantive milestone, and therefore the resulting payment would be considered part of the consideration for the single unit of accounting and be recognized as revenue as such performance obligations are performed.
Reimbursement of Research and Development Costs
Reimbursement of research and development costs is recognized as revenue, provided the amounts are fixed and determinable, and collection of the related receivable is reasonably assured.
(e) Property and Equipment
Property and equipment are recorded at cost and depreciated on a straight-line basis over their estimated useful lives. The Company uses a life of four to five years for laboratory equipment, three to seven years for office equipment and furniture, and the lesser of the useful life or the remaining life of the underlying facility lease for leasehold improvements. Expenditures for repairs and maintenance are charged to expense as incurred, while major renewals and betterments are capitalized. Upon disposition of assets, the cost and related accumulated depreciation are removed from the accounts and any resulting gain or loss is included in the Statements of Operations.
(f) Research and Development
Research and development costs, including those incurred in relation to the Company's collaborative arrangements, are charged to expense as incurred. These expenses include internal research and development as well as research conducted on behalf of the Company by third parties, including sponsored university-based research agreements and clinical study vendors. Costs incurred in obtaining technology licenses are charged immediately to research and development expense if the licensed technology has not reached technological feasibility and has no alternative future uses.
F-13
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(3) Summary of Significant Accounting Policies (Continued)
(g) Impairment of Long-Lived Assets
The Company reviews long-lived assets, such as property and equipment, for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, then an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. The Company has not recorded any impairment charges pursuant to our review of long-lived assets.
(h) Income Taxes
Income taxes are accounted for under the asset-and-liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
(i) Stock-Based Compensation
The Company recognizes compensation cost for awards to employees and nonemployee board members based on the grant-date fair value of stock-based awards over the period during which an award holder is required to provide service in exchange for the award. No compensation cost is recognized for awards for which the award holder does not render the requisite service.
Prior to 2006, the Company applied the intrinsic-value-based method of accounting prescribed by Accounting Principles Board (APB) Opinion No. 25, Accounting for Stock Issued to Employees, and related interpretations, to account for its fixed-plan stock options granted to employees and directors. Under APB Opinion No. 25, the Company did not record compensation expense for its stock options since the current fair value of the underlying stock equaled the exercise price of the options.
The fair value of stock options is determined using the Black Scholes option- pricing model and is recognized as expense over the requisite service period using the straight-line method.
To satisfy the exercise of options, the Company plans to issue new shares rather than purchase shares on the open market.
(j) Net Loss per Common Share
Basic net loss per common share is computed by dividing the net loss allocable to common stockholders by the weighted average number of shares of common stock outstanding for the period. Diluted net loss per common share is computed by dividing net loss allocable to common stockholders by the sum of the weighted- average number of common shares outstanding for the period and the number of additional shares that would have been outstanding if dilutive potential common shares had
F-14
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(3) Summary of Significant Accounting Policies (Continued)
been issued. Stock options and warrants to purchase an aggregate of 24,847,307, 22,857,110 and 13,497,205, common shares were excluded from the computation of diluted net loss per common share for the years ended December 31, 2008, 2009 and 2010, respectively. In all periods presented, Redpoint's diluted net loss per common share is equal to basic net loss per common share because giving effect in the computation of diluted net loss per common share to the exercise of outstanding options and warrants would have been antidilutive.
(k) Fair Value of Financial Instruments
The fair value of our financial instruments is the amount for which the instrument could be exchanged in a current transaction between willing parties. As of December 31, 2009 and 2010, the carrying values of cash and cash equivalents, prepaid expenses and other current assets, accounts payable, accrued expenses, and accrued compensation equaled or approximated their respective fair values because of the short duration of these instruments. In addition, the Company believes the carrying value of its debt instrument, which does not have a readily ascertainable market value, approximates its fair value, given that the interest rate approximates market rates.
(l) Comprehensive Loss
The Company classifies items of other comprehensive income (loss) by their nature and disclosure of the accumulated balance of other comprehensive income (loss) separately from accumulated deficit and additional paid-in capital, in the stockholders' equity section of its balance sheet. Other comprehensive income (loss) consisted of unrealized gains and losses on marketable securities for the years ended December 31, 2008 and 2009 and is presented in the Statements of Redeemable Convertible Preferred Stock and Stockholders' Equity (Deficit). There was no other comprehensive income (loss) recorded during the year ended December 31, 2010.
(m) Recent Accounting Pronouncements
In October 2009, the FASB issued accounting guidance related to revenue recognition for transactions with multiple deliverables, which impacts the determination of when the individual deliverables included in a multiple-element arrangement may be treated as separate units of accounting. This guidance is effective for us beginning on January 1, 2011 and is not expected to have a significant impact on the Company's financial statements.
(4) Research and Collaboration Agreements
Givaudan. In March 2007, the Company entered into a Joint Research and Development and License Agreement (the "Agreement") with Givaudan Schweiz AG, a Swiss company ("Givaudan") for the development and commercialization of compounds that act primarily through the modulation of the TRPM5 channel and that enhance sweetness or savory sensation, as well as compounds that block or desensitize bitter taste for use in the food and beverage industry. In consideration of the Company's agreement to conduct research and develop compounds and grant exclusive licenses and other rights to Givaudan, Redpoint received an upfront payment of $1,300,000 and Givaudan provided research funding to the Company over the term of the Agreement. Givaudan terminated the Agreement, effective as of May 1, 2009, and reserved its rights under the Agreement with respect to certain
F-15
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(4) Research and Collaboration Agreements (Continued)
collaboration compounds. The upfront payment of $1,300,000 was being recognized as revenue on a straight-line basis over the initial 3.5 year term of the Agreement. As of May 1, 2009, the remaining unamortized portion of the upfront payment was approximately $526,000 and, as a result of the termination, was recognized as revenue upon termination of the Agreement. In connection with the Agreement, during 2007, 2008 and 2009, the Company recognized $279,000, $371,000 and $650,000, respectively, of revenue attributable to the amortization of the upfront payment and $1,841,000, $2,688,000, and $1,065,000 of revenue from research funding, respectively.
Coca-Cola. In December 2007, the Company entered into a research agreement (the "Coca-Cola Agreement") with The Coca-Cola Company ("Coca-Cola") for the development of certain technology for use in soft drinks and non-alcoholic beverages. Under the terms of the Coca-Cola Agreement, the Company recognized $20,000 and $898,000 of revenue from research funding in 2007 and 2008, respectively. The agreement expired by its terms in December of 2008.
International Flavor & Fragrances. In June 2010, the Company entered into a License and Commercialization Agreement (the "IFF Agreement") with International Flavors & Fragrances Inc., ("IFF"), for the development, manufacture, use and commercialization of RP44, the Company's all-natural sweetness enhancer. Under the terms of the IFF Agreement, IFF has exclusive rights, for five years, to develop, manufacture and commercialize RP44 in virtually all food and beverage product categories. In return, Redpoint received an upfront payment of $0.5 million and became eligible to receive two milestone payments of $0.5 million each contingent upon certain criteria regarding regulatory approval and supply. In addition, Redpoint will receive royalties based on the amount of RP44 purchased by IFF for use in products. IFF will assume responsibility for the regulatory process and for costs associated with prosecuting and maintaining Redpoint's intellectual property covering RP44. The upfront payment of $0.5 million was recognized as revenue on the effective date (June 29, 2010) of the IFF Agreement as the Company has no future performance obligations under the IFF Agreement. In October 2010, RP44 was determined to be Generally Recognized As Safe (GRAS), triggering the $0.5 million regulatory approval milestone payment which was recognized as revenue in the fourth quarter of 2010. The Company will bill and record the remaining supply milestone payment as revenue when and if such event is achieved.
(5) Grant Revenue
In November 2010, the Company received a grant in the amount of $244,479 in connection with the Qualifying Therapeutic Discovery Project Program under section 48D of the Internal Revenue Code.
F-16
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(6) Property and Equipment
Property and equipment consisted of the following:
| |
December 31 | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | |||||
Laboratory equipment |
$ | 2,960,632 | $ | 2,960,632 | |||
Office equipment and furniture |
240,976 | 240,976 | |||||
Leasehold improvements |
24,397 | 24,397 | |||||
|
3,226,005 | 3,226,005 | |||||
Less accumulated depreciation and amortization |
(2,499,275 | ) | (2,888,708 | ) | |||
|
$ | 726,730 | $ | 337,297 | |||
Depreciation and amortization expense was $624,835, $519,636, $389,433 and $3,042,905 for the years ended December 31, 2008, 2009, 2010 and the period from August 16, 1995 (inception) through December 31, 2010, respectively.
(7) Accrued Expenses
Accrued expenses consisted of the following:
| |
December 31 | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | |||||
Professional fees |
$ | 71,000 | $ | 85,324 | |||
Deferred rent |
60,976 | 73,138 | |||||
Other |
19,917 | 12,662 | |||||
|
$ | 151,893 | $ | 171,124 | |||
(8) Debt
(a) Master Security Agreement and Loan and Security Agreement
In February 2005, the Company entered into a Master Security Agreement with a finance company that provided for borrowings up to $3,200,000, subject to certain conditions, through December 2005. During 2005, the Company borrowed $2,001,810 under the Master Security Agreement in order to finance the purchase of laboratory and office equipment. Amounts borrowed were evidenced with promissory notes and were repayable in equal monthly payments, over 36 to 48 months, and are collateralized by the purchased equipment. The notes bore interest at rates varying between 9.4% and 10.4%. As of December 31, 2008, $187,577 was outstanding under the Master Security Agreement. The notes were repaid in full as of December 31, 2009.
In connection with the borrowings under the Master Security Agreement, the Company issued the finance company warrants to purchase 53,039 shares of the Company's common stock at an exercise price of $0.75 per share. The estimated fair value of the warrants of $30,074, determined using the Black-Scholes option- pricing model, was accounted for as deferred financing costs and were amortized
F-17
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(8) Debt (Continued)
to interest expense through the date in which the notes were repaid in full, which occurred during the fourth quarter of 2009.
In September 2008, the Company entered into a Loan and Security Agreement with another finance company that provided for borrowings up $2,000,000 for the purchase of certain equipment and to be used for working capital purposes. During 2008, the Company borrowed $1,556,357 under the Loan and Security Agreement. As of December 31, 2009 and 2010, $1,147,953 and $773,842, respectively, was outstanding under the Loan and Security Agreement. Amounts borrowed are evidenced with a promissory note, are repayable in equal monthly payments over 48 months, and are collateralized by the purchased equipment. The note bears interest of 12.1%. The Loan and Security Agreement contains certain provisions, including a material adverse change clause (as defined in the Loan Security Agreement) which restricts the Company's ability to borrow additional money and also enables the finance company to request full repayment of the loan if such a change has occurred. As of December 31, 2010, the Company was in compliance with these provisions.
The scheduled maturities of long-term debt outstanding at December 31, 2010 are as follows:
2011 |
$ | 422,100 | ||
2012 |
351,742 | |||
|
773,842 | |||
Less current portion |
(422,100 | ) | ||
|
$ | 351,742 | ||
Interest expense on notes payable recorded during the years ended December 31, 2008, 2009 and 2010 and for the period from August 16, 1995 (inception) to December 31, 2010 was $86,568, $167,616, $117,884 and $697,009, respectively.
(b) Convertible Notes Payable
In May, June and October 2006, the Company entered into a convertible debt financing agreement with certain of its existing investors which provided for $4,116,774 in funding. The Company issued 5% secured promissory notes (the Promissory Notes) with principal amounts totaling $4,093,021 and warrants to purchase shares of a new series of preferred stock that may be designated in the future in a Qualified Financing, as defined in such Promissory Notes. The Promissory Notes were due on the earliest to occur of: (i) demand by holders of a majority of the outstanding principal at any time after the one year anniversary of the initial May closing; (ii) a Liquidation Event, as defined; and (iii) the two-year anniversary of the initial May closing. In conjunction with the Reverse Merger, the Promissory Notes and accrued interest were converted into 5,223,522 shares of common stock and warrants were exercised resulting in the issuance of 2,033,175 shares of common stock.
The Company allocated the proceeds from the financing between the Promissory Notes and warrants based on their relative estimated fair values of $4,093,021 and $1,228,565, respectively. The relative fair value of the warrants was recorded against the carrying value of the Promissory Notes as an original issue discount (OID) which was being amortized as interest expense over the one year
F-18
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(8) Debt (Continued)
anniversary of the initial May issuance date of the Promissory Notes until the closing of the Reverse Merger.
After considering the allocation of the proceeds to the Promissory Notes, the Company determined that the Promissory Notes contained a contingent beneficial conversion feature (Contingent BCF). The Contingent BCF existed at the date of the issuance of the Promissory Notes due to the fact that the original carrying value of Promissory Notes, after allocation of the proceeds, would be less than the purchase price of the new series of preferred stock paid by investors in the new series. During the year ended December 31, 2007, the Contingent BCF of $1,228,565 was recognized as additional interest expense when the Promissory Notes were converted into shares of common stock.
(9) Related-Party Transaction
The Company has licensed the rights to certain patents and pending patents in the area of taste receptors and taste inhibitors from a research institution that is a stockholder of the Company. The license agreement, as amended, required a payment of $130,000. In addition, the Company is required to pay royalties on future sales, if any, of certain products covered by the license agreement. Commencing in July 2004, the Company is obligated to pay a minimum annual fee, creditable against royalties, of $25,000 to the licensor through the expiration of the last-to-expire patent. The Company estimates the term of the license agreement to be through December 2019.
(10) Redeemable Convertible Preferred Stock
During 2003 and 2004, the Company sold an aggregate of 5,820,875 shares of convertible preferred stock (Series A) at $2.10 per share, for gross proceeds of $12,223,832.
In connection with the conversion of a previously outstanding convertible note, the Company issued 623,963 shares of Series A and also issued 236,987 shares of Junior Preferred in connection with the conversion of the accrued interest on the convertible notes in 2003.
Dividends on each outstanding share of Series A and Junior Preferred accrued and compounded annually, and were cumulative at the annual rate of 8%. Dividends were payable only when and if declared by the board of directors.
Each share of Preferred Stock was convertible at any time at the option of the holder into the number of shares of common stock obtained by dividing the original issue price (subject to certain adjustments), plus accrued and unpaid dividends, by the conversion price, as defined. The Preferred Stock was mandatorily convertible in the event of an initial public offering, as defined.
At any time on or after October 31, 2008, the holders of a majority of the Preferred Stock could have required the Company to redeem the Series A at the applicable redemption price. In the case of the Series A, the redemption price was equal to the greater of (i) the original issue price plus any accumulated unpaid dividends, or (ii) the fair value of the shares of common stock then issuable upon conversion of such shares of Series A. In the case of the Junior Preferred, the redemption price was equal to the original issue price of $2.10 per share.
In connection with the Reverse Merger, the Series A and Junior Preferred converted into 22,774,963 shares of common stock.
F-19
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(11) Stockholders' Equity (Deficit)
(a) Common Stock
The Company was party to a number of agreements that provided for dilution protection to certain investors. In connection with the Reverse Merger and closing of the Private Placement, the Company issued approximately 2.0 million shares of common stock to a founder and shareholder of the Company pursuant to certain antidilution protection. During 2007, the Company recorded a charge of $1.6 million to research and development expenses related to the issuance of such shares of common stock, which represented the fair value of those shares. As of December 31, 2010, none of the Company's existing agreements contain dilution protection provisions.
(b) Warrants
As of December 31, 2010, the following warrants to purchase Common Stock were outstanding:
| Number of shares |
Exercise price |
Expiration | ||||
|---|---|---|---|---|---|---|
| 53,039 | $ | 0.75 | February - December 2012 | |||
| 300,000 | $ | 0.13 | November 2015 | |||
| 3,574,906 | $ | 0.97 | April 2012 | |||
| 3,927,945 | ||||||
During the year ended December 31, 2010, warrants to purchase 10,197,346 shares of Common Stock at an exercise price of $1.35 per share expired and were not exercised. As of December 31, 2010, 3,627,945 warrants were vested.
(c) Registration Rights
On June 5, 2007, the Company filed a "resale" registration statement with the SEC covering all shares of common stock issued in the Private Placement and in connection with the Reverse Merger, including shares of common stock into which certain warrants are exercisable. Such registration statement was declared effective on July 20, 2007. The Company will use its best efforts to maintain its effectiveness until such time as all securities registered under the registration statement have been sold or are otherwise able to be sold under Rule 144 of the Securities Act without regard to volume limitations, whichever is earlier.
If the Company fails to maintain the effectiveness of the "resale" registration statement, a 1% penalty will be assessed for each monthly period that the registration statement is not effective, capped at 12%. The penalty will be payable monthly in either cash or additional shares of common stock, as determined by the Company. The Company follows the relevant accounting literature, which specifies that registration payment arrangements should play no part in determining the initial classification of, and subsequent accounting for, securities to which the payments relate. Contingent obligations in a registration payment arrangement are separately analyzed. If we determine a penalty payment is probable and can be reasonably estimated, a liability will be recorded. As of December 31, 2010, we concluded the likelihood of having to make any payments under the arrangements was remote, and therefore did not record any related liability.
F-20
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(12) Stock Options Plans
In November 2003, the Company adopted the 2003 Stock Incentive Plan, as amended (the "2003 Plan"), that authorized the Company to grant up to 6,523,790 shares of common stock to eligible employees, directors, and consultants of the Company in the form of restricted stock and stock options. The amount and terms of grants were determined by the board of directors. The term of the options could have been up to 10 years, and options were exercisable in cash or as otherwise determined by the board of directors. Generally, options vested 25% upon the first anniversary of the date of grant and ratably each month thereafter through the fourth anniversary of the date of grant.
On March 12, 2007, the Company adopted the Redpoint Bio Corporation 2007 Omnibus Equity Compensation Plan (the "2007 Plan"), which provided for the issuance of up to 13,511,562 shares of common stock, subject to adjustment in certain circumstances. In connection with the adoption of the 2007 Plan, the 2003 Plan merged with and into the 2007 Plan and no additional grants were to be made thereafter under the 2003 Plan. Outstanding grants under the 2003 Plan continued in effect in accordance with their terms as in effect before March 12, 2007 and the shares with respect to outstanding grants under the 2003 Plan have been or will be issued or transferred under the 2007 Plan. The 2007 Plan is now the Company's only plan in effect.
On April 20, 2007, the Company approved an amendment to the 2007 Plan, which increased the maximum number of shares of common stock reserved for issuance under the 2007 Plan by an additional 4,132,705 shares from 13,511,562 to 17,644,267 shares of common stock. As of December 31, 2010, 6,999,989 shares were reserved for future issuances under the 2007 Plan.
The following is a summary of stock option activity under the 2007 Plan through December 31, 2010.
| |
Number of shares |
Weighted-average exercise price |
Weighted-average remaining contractual term |
Intrinsic Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Outstanding at December 31, 2007 |
9,603,961 | 0.49 | |||||||||||
Granted |
1,689,938 | 0.65 | |||||||||||
Forfeited |
— | — | |||||||||||
Exercised |
(271,883 | ) | 0.15 | ||||||||||
Outstanding at December 31, 2008 |
11,022,016 | 0.52 | |||||||||||
Granted |
1,230,628 | 0.09 | |||||||||||
Forfeited |
(2,878,002 | ) | 0.50 | ||||||||||
Exercised |
(342,823 | ) | 0.15 | ||||||||||
Outstanding at December 31, 2009 |
9,031,819 | 0.48 | |||||||||||
Granted |
800,000 | 0.12 | |||||||||||
Forfeited |
(254,359 | ) | 0.41 | ||||||||||
Exercised |
(8,200 | ) | 0.07 | ||||||||||
Outstanding at December 31, 2010 |
9,569,260 | $ | 0.45 | 5.64 years | $ | 42,090 | |||||||
Exercisable at December 31, 2010 |
8,204,362 | $ | 0.47 | 5.18 years | $ | 24,240 | |||||||
F-21
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(12) Stock Options Plans (Continued)
The total intrinsic value of stock options exercised during the years ended December 31, 2009 and 2010 was $20,664 and $246, respectively.
All options granted to date have exercise prices equal to the fair value of the underlying common stock on the date of grant. Prior to the effectiveness of the registration statement on Form S-1, which occurred on July 20, 2007, all options were granted with an exercise price equal to the estimated fair value of the underlying common stock as determined by the board of directors. Subsequent to that date, all options have been granted with an exercise price equal to the most recent trading price of the Company's common stock on the date of grant. The Company received proceeds of $40,782, $51,424 and $574, for option exercises in 2008, 2009 and 2010, respectively.
The per-share weighted average fair value of the options granted during the years ended December 31, 2008, 2009 and 2010 was estimated at $0.41, $0.07 and $0.07, respectively, on the date of grant using the Black-Scholes option-pricing model with the following weighted average assumptions:
| |
December 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Expected dividend yield |
— | — | — | |||||||
Expected volatility |
68 | % | 75 | % | 69 | % | ||||
Risk-free interest rate |
3.05 | % | 1.96 | % | 1.81 | % | ||||
Expected life of options |
6 years | 6 years | 6 years | |||||||
The expected term assumption is based on the use of the simplified method. The expected volatility of share options was calculated based on a historical volatility analysis of public company peers that were similar to the Company for a term equivalent to the expected term of the option. The risk-free rate of the option is based on the zero-coupon U.S. Treasury yield at the date of grant for a term equivalent to the expected term of the option.
The Company recognized $845,374, $680,893 and $583,536 of stock- based compensation expense related to stock options for the years ended December 31, 2008, 2009 and 2010, respectively. As of December 31, 2010, there was $256,033 of unrecognized compensation expense related to unvested stock options, which is expected to be recognized over a weighted average period of 0.8 years.
(13) Commitments
(a) Leases
The Company leases office and laboratory space and equipment under operating leases expiring through 2017. Rent expense under these operating leases was $967,000, $968,000 and $912,000 for the years ended December 31, 2008, 2009 and 2010, respectively.
F-22
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(13) Commitments (Continued)
Future minimum lease payments as of December 31, 2010 are as follows:
Year Ending December 31,
|
Amount | |||
|---|---|---|---|---|
2011 |
$ | 839,723 | ||
2012 |
845,915 | |||
2013 |
852,230 | |||
2014 |
858,627 | |||
2015 |
865,243 | |||
Thereafter |
1,172,854 | |||
|
$ | 5,434,592 | ||
In October 2006, the Company paid a $250,000 deposit for its facility which has been recorded in other assets on the accompanying balance sheet.
(b) Employment-Related Agreements
The Company has entered into employment agreements with certain key executives, providing for base salaries plus performance incentive bonuses. In January 2009, the Company entered into a separation agreement with a former officer that required the Company to make aggregate payments of $40,175, which was paid in bimonthly installments through March 31, 2009. In February 2009, the Company entered into a separation agreement with a former officer that required the Company to make aggregate payments of $222,888, which was repaid in bimonthly installments through February 28, 2010.
(14) Restructuring
During 2009, the Company announced two restructurings which reduced its workforce by approximately 60%. As of December 31, 2010, the Company has 10 full time employees. In connection with the restructurings, the Company incurred severance costs of $526,553 which are included in general and administrative expenses on the accompanying Statements of Operations for the year ended December 31, 2009. As of December 31, 2010, all amounts related to these restructurings have been paid.
| |
December 31, 2008 | 2009 Expense | 2009 Payments | 2010 Payments | December 31, 2010 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Severance and benefits |
$ | — | $ | 526,553 | $ | 489,405 | $ | 37,148 | $ | — | ||||||
|
$ | — | $ | 526,553 | $ | 489,405 | $ | 37,148 | $ | — | ||||||
(15) Income Taxes
As of December 31, 2010, the Company had approximately $46,072,000 and $30,022,000 of federal and state net operating loss carryforwards, respectively, and approximately $649,000 and $274,000 of federal and state research and development credit carryforwards, respectively, available to offset future federal and state taxable income. The federal net operating loss carryforward will expire beginning in
F-23
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(15) Income Taxes (Continued)
2022, and the state net operating loss carryforwards began expiring in 2008. The federal research and development credit carryforward will expire beginning in 2020, and the state research and development carryforward will expire beginning in 2018.
The following table summarizes the components of the deferred tax assets and liabilities as of:
| |
December 31 | |||||||
|---|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | ||||||
Net operating losses |
$ | 16,302,000 | $ | 17,452,000 | ||||
Research and development credit |
1,302,000 | 830,000 | ||||||
Other temporary differences |
550,000 | 156,000 | ||||||
Gross deferred tax assets |
18,154,000 | 18,438,000 | ||||||
Valuation allowance |
(18,154,000 | ) | (18,438,000 | ) | ||||
Total deferred tax assets |
— | — | ||||||
Fixed assets |
— | — | ||||||
Total deferred tax liabilities |
— | — | ||||||
Net deferred tax assets |
$ | — | $ | — | ||||
The Company's effective tax rate differs from the statutory rate primarily due to its valuation allowance associated with its net operating losses.
In assessing the realizability of deferred tax assets, the Company considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which the temporary differences representing net future deductible amounts become deductible. Due to the Company's history of losses, the deferred tax assets are fully offset by a valuation allowance at December 31, 2009 and 2010. The valuation allowance in 2010 increased by $284,000 over 2009 related primarily to additional net operating losses incurred by the Company. The valuation allowance in 2009 increased by $3,556,000 over 2008 related primarily to additional net operating losses incurred by the Company.
Under the Tax Reform Act of 1986, the utilization of a corporation's net operating loss and tax credit carryforwards is limited following a greater than 50% change in ownership during a three-year period. Any unused annual limitation may be carried forward to future years for the balance of the net operating loss and tax credit carryforward period. Prior equity financings may significantly limit our ability to utilize net operating loss and tax credit carryforwards against future taxable income under Sections 382 and 383 of the Internal Revenue Code.
During 2005, 2006 and 2008, the Company sold $3,128,427, $5,776,876, $2,462,129 and of New Jersey State operating loss carryforwards resulting in the recognition of a benefit of $253,403, $465,803 and $266,772, respectively. In addition, in January 2011 the Company sold $5,268,454 of New Jersey State net operating loss carryforwards resulting in cash proceeds of $410,726 in 2011.
F-24
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(15) Income Taxes (Continued)
During 2010, the Company sold $112,562 of its New Jersey State Research and Development Tax Credits resulting in the recognition of an income tax benefit of $101,306 recorded in the accompanying Statements of Operations.
The Company applies the provisions of FASB Accounting Standards Codification 740-10, "Accounting for Uncertainty in Income Taxes" (ASC 740-10), which clarifies the accounting and disclosure for uncertainties in income taxes recognized in an enterprise's financial statements. ASC 740-10 prescribes a recognition threshold and measurement attribute for financial statement recognition and measurement of a tax position reported or expected to be reported on a tax return. ASC 740-10 also provides guidance on the recognition and classification of interest and penalties in an entity's financial statements. The Company's policy is to recognize potential accrued interest and penalties related to income tax matters in income tax expense. As of December 31, 2010, the Company determined that it had no liability for uncertain income tax matters as prescribed by ASC 740-10. Net operating loss and credit carryforwards since inception remain open to examination by taxing authorities, and will continue to remain open for a period of time post utilization.
(16) 401(k) Plan
The Company maintains a defined contribution 401(k) plan available to eligible employees. Employee contributions are voluntary and are determined on an individual basis, limited by the maximum amounts allowable under federal tax regulations. During the year ended December 31, 2007, the Company began making matching contributions in the amount of 50% of employee contributions up to 6%. The Company, in its discretion, may also make certain contributions to the plan. The Company contributed $92,000, $18,000 and $21,000 as matching contributions for the years ended December 31, 2008, 2009 and 2010, respectively.
(17) Quarterly Results (unaudited)
The following tables summarize the quarterly results of operations for each of the quarters in 2009 and 2010. These quarterly results are unaudited, but in the opinion of management, have been prepared on the same basis as the Company's audited financial information and include all adjustments (consisting only of normal recurring adjustments) necessary for fair presentation of the information set forth herein.
| |
2009 Results | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quarter Ended | |
||||||||||||||
| |
March 31 | June 30 | September 30 | December 31 | Total Year | |||||||||||
Revenues |
$ | 1,058,741 | $ | 809,687 | $ | — | $ | — | $ | 1,868,428 | ||||||
Net loss(2) |
(2,518,403 | ) | (2,062,723 | ) | (2,406,644 | ) | (1,828,242 | ) | (8,816,012 | ) | ||||||
Basic and diluted net loss per common share(1) |
$ | (0.03 | ) | $ | (0.03 | ) | $ | (0.03 | ) | $ | (0.02 | ) | $ | (0.11 | ) | |
F-25
REDPOINT BIO CORPORATION
(A Development-Stage Enterprise)
Notes to Financial Statements (Continued)
(17) Quarterly Results (unaudited) (Continued)
| |
2010 Results | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quarter Ended | |
||||||||||||||
| |
March 31 | June 30 | September 30 | December 31 | Total Year | |||||||||||
Revenues |
$ | — | $ | 500,000 | $ | — | $ | 744,479 | $ | 1,244,479 | ||||||
Net loss(3) |
(1,713,341 | ) | (1,388,774 | ) | (1,772,558 | ) | (595,150 | ) | (5,469,823 | ) | ||||||
Basic and diluted net loss per common share(1) |
$ | (0.02 | ) | $ | (0.02 | ) | $ | (0.02 | ) | $ | (0.01 | ) | $ | (0.07 | ) | |
- (1)
- Per
common share amounts for the quarters and full years have been calculated separately.
- (2)
- The
net loss for the three months ended December 31, 2009 reflects the reversal of $189,217 of accrued bonuses previously recognized in the first
three quarters of 2009, as a result of the Company's decision not to pay 2009 bonuses.
- (3)
- The net loss for the three months ended December 31, 2010 reflects the reversal of $187,983 of accrued bonuses previously recognized in the first three quarters of 2010, as a result of the Company's decision not to pay 2010 bonuses.
F-26
