Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REVA Medical, Inc. | c13245e8vk.htm |
Exhibit 99.1
| The REVA Tyrosine-Derived Polycarbonate Bioresorbable Scaffold: Long-term Outcomes Using Multimodality Imaging Greg L. Kaluza, MD, PhD, FACC Director of Research, Skirball Center for Cardiovascular Research Cardiovascular Research Foundation New York, NY |
| Disclosure Statement REVA Medical, Inc. Personal: scientific consultant Institutional: SCCR performs a small volume of contract animal research for REVA and provides image core lab services |
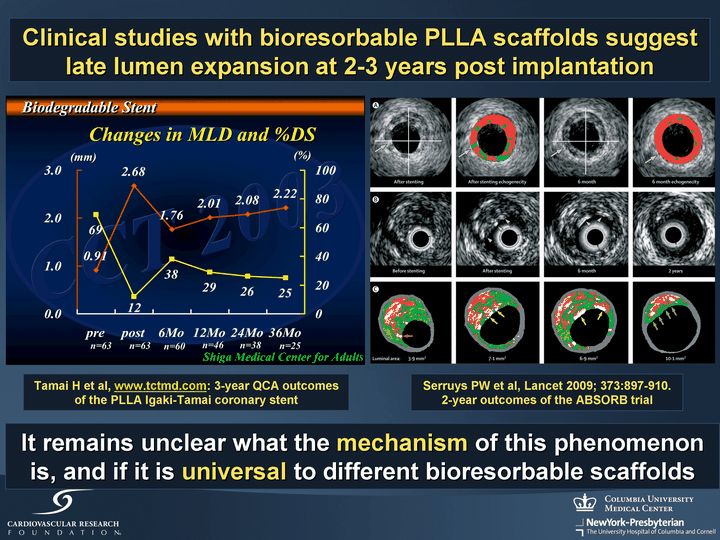
| Tamai H et al, www.tctmd.com: 3-year QCA outcomes of the PLLA Igaki-Tamai coronary stent Serruys PW et al, Lancet 2009; 373:897-910. 2-year outcomes of the ABSORB trial Clinical studies with bioresorbable PLLA scaffolds suggest late lumen expansion at 2-3 years post implantation It remains unclear what the mechanism of this phenomenon is, and if it is universal to different bioresorbable scaffolds |

| REVA 1st Gen Bioresorbable Non-Drug Eluting Scaffold Unique Geometry Unique Material Radiopaque Material Slide and Lock Mechanism |
| Methods Pig (Yucatan miniswine) coronary arteries evaluated (pooled from several studies): 94 REVA 1st Generation ("R100") Bioresorbable Non-Drug Eluting Scaffolds 46 BMS (BSC Express) Evaluated immediately post-implant, 5 days; 3, 6, 12, 18, 24 and 55 months post-treatment In Vivo Multimodality Imaging: Angiography and rotational angiography IVUS OCT RESORB Trial Patients: 3 REVA 1st Generation Bioresorbable Non-Drug Eluting Scaffolds Implant, 4, 12 and 34 months post-treatment |
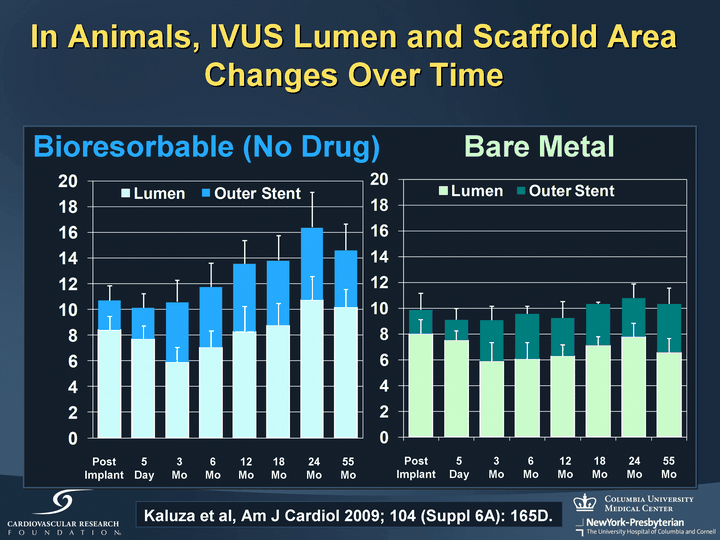
| Bioresorbable (No Drug) Bare Metal Post Implant 5 Day 3 Mo 6 Mo 12 Mo 18 Mo 24 Mo 55 Mo Kaluza et al, Am J Cardiol 2009; 104 (Suppl 6A): 165D. Post Implant 5 Day 3 Mo 6 Mo 12 Mo 18 Mo 24 Mo 55 Mo In Animals, IVUS Lumen and Scaffold Area Changes Over Time |
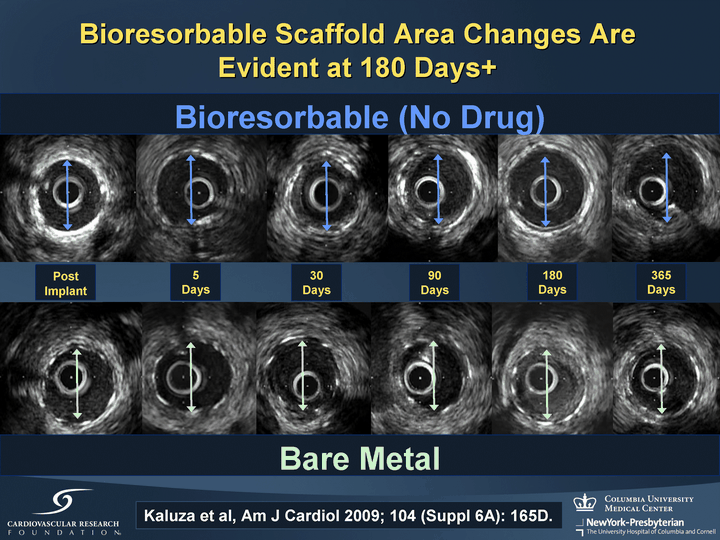
| Post Implant 180 Days 365 Days 90 Days 30 Days 5 Days Bioresorbable (No Drug) Bare Metal Bioresorbable Scaffold Area Changes Are Evident at 180 Days+ Kaluza et al, Am J Cardiol 2009; 104 (Suppl 6A): 165D. |

| Remodeling Ratio The remodeling ratio depicts the patency and tubularity of the scaffold- or stent-treated region vs. the adjacent reference vessel segments. In animals, at 2 years, the lumen diameter of the reference vessel and polymer scaffold stabilized showing no step effect, (p<0.05) in contrast to metal. Follow-Up Lumen Area Follow-Up Reference Vessel Area Remodeling Ratio = Bioresorbable (No Drug) Bare Metal |

| Scaffold Resorbs and Vessel Achieves Tubularity 3 Mos 4.5 Yrs |
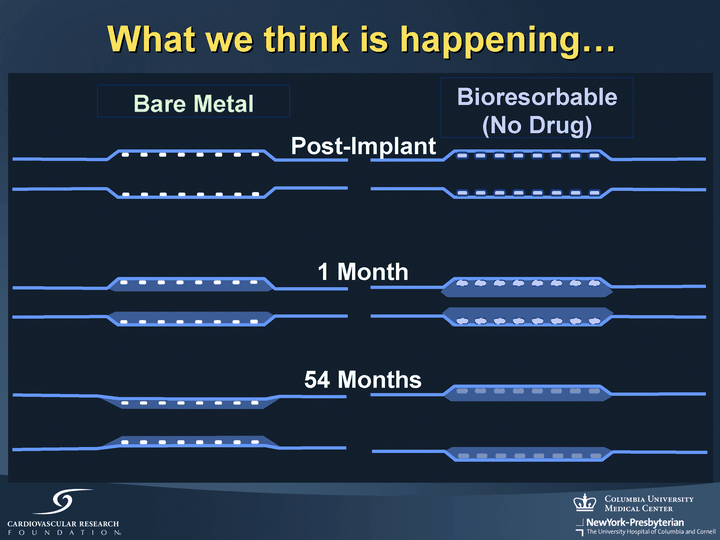
| What we think is happening... Post-Implant 1 Month 54 Months Bioresorbable (No Drug) Bare Metal |
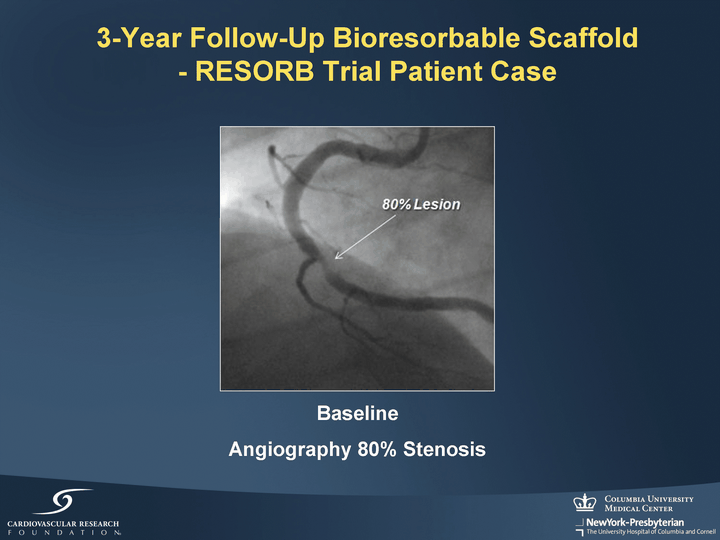
| 3-Year Follow-Up Bioresorbable Scaffold - RESORB Trial Patient Case Baseline Angiography 80% Stenosis |

| 3-Year Follow-Up of Bioresorbable Scaffolds - RESORB Trial Patient Case Scaffold Post-Implant Post-Implant Scaffold 4 Months 12 Months 28% NIH Volume by IVUS Post-Implant 12% NIH Volume by IVUS 15% NIH Volume by IVUS Scaffold Scaffold 34 Months |
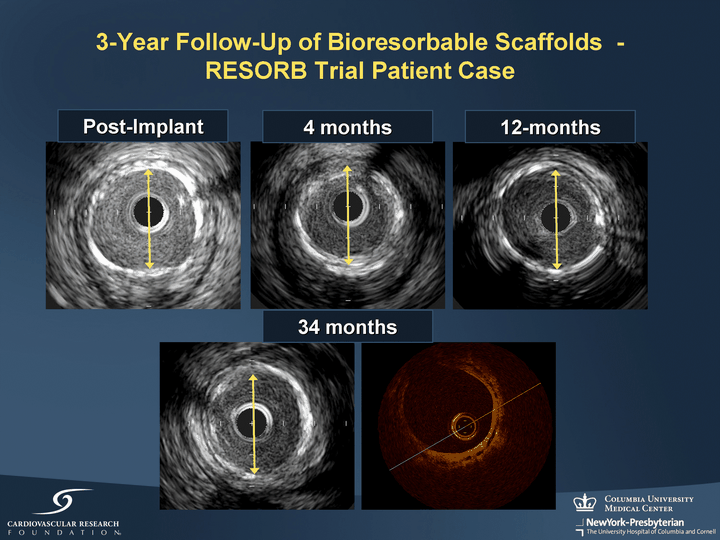
| 4 months 34 months 12-months Post-Implant 3-Year Follow-Up of Bioresorbable Scaffolds - RESORB Trial Patient Case |
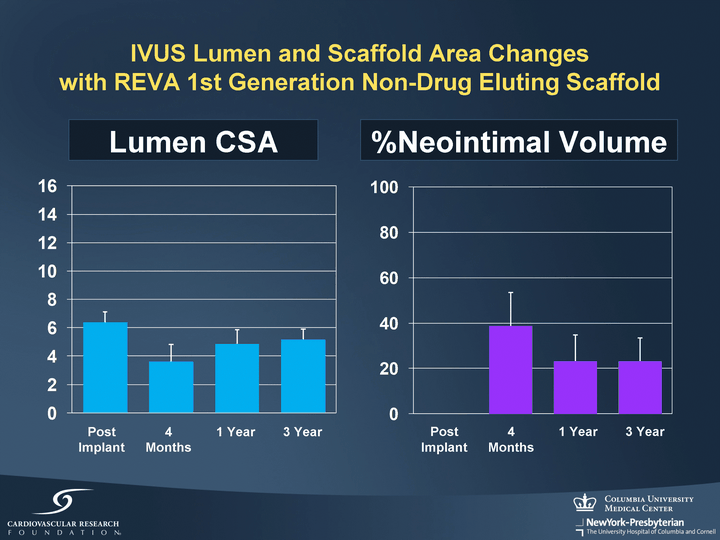
| IVUS Lumen and Scaffold Area Changes with REVA 1st Generation Non-Drug Eluting Scaffold Lumen CSA %Neointimal Volume Post Implant 4 Months 1 Year 3 Year Post Implant 4 Months 1 Year 3 Year |
| Studies confirm that bioresorbable scaffolds: Universally undergo long-term, desirable geometric arterial changes Appear to re-establish vascular homeostasis and patency toward those characterizing a native artery Multimodality Imaging In vivo angiography, rotational angiography, IVUS and OCT provide additional insight in the evaluation of bioresorbable scaffolds Bioresorbable Scaffolds: Unique Long-term Outcomes |
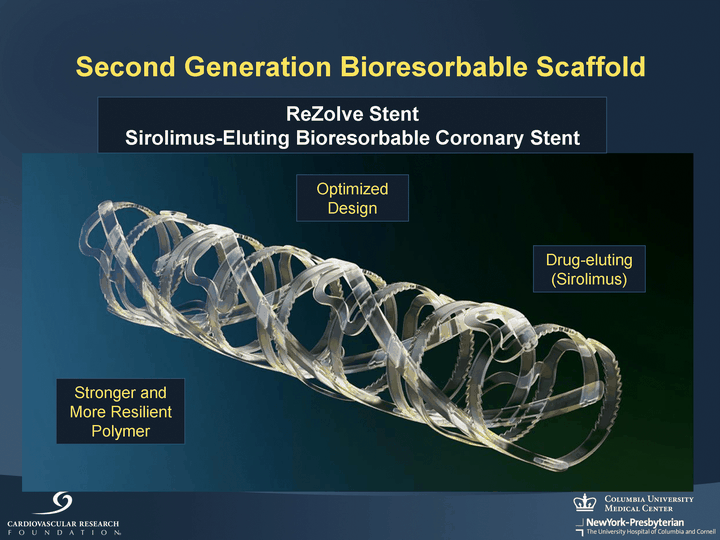
| Second Generation Bioresorbable Scaffold ReZolve Stent Sirolimus-Eluting Bioresorbable Coronary Stent Drug-eluting (Sirolimus) Stronger and More Resilient Polymer Optimized Design |

| Future Clinical Plans RESTORE Pilot Study Start Q2-2011 50 patient pilot safety study Principal Investigator: Alexandre Abizaid Follow up at 1, 6 and 12 months and yearly thereafter No comparator |
