Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - COVANCE INC | Financial_Report.xls |
| EX-21 - EX-21 - COVANCE INC | a2202143zex-21.htm |
| EX-31.2 - EX-31.2 - COVANCE INC | a2202143zex-31_2.htm |
| EX-23.1 - EX-23.1 - COVANCE INC | a2202143zex-23_1.htm |
| EX-31.1 - EX-31.1 - COVANCE INC | a2202143zex-31_1.htm |
| EX-32.2 - EX-32.2 - COVANCE INC | a2202143zex-32_2.htm |
| EX-10.48 - EX-10.48 - COVANCE INC | a2202143zex-10_48.htm |
| EX-32.1 - EX-32.1 - COVANCE INC | a2202143zex-32_1.htm |
QuickLinks -- Click here to rapidly navigate through this document
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2010
Commission File Number: 1-12213
COVANCE INC.
(Exact name of Registrant as specified in its Charter)
| Delaware (State of Incorporation) |
22-3265977 (I.R.S. Employer Identification No.) |
|
210 Carnegie Center, Princeton, New Jersey (Address of Principal Executive Offices) |
08540 (Zip Code) |
Registrant's telephone number, including area code: (609) 452-4440
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class Common Stock, $.01 Par Value |
Name of Each Exchange on Which Registered New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes X No
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Securities Exchange Act (the "Exchange Act") of 1934. Yes No X
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes X No
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Registration S-T during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). Yes X No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. X
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer", "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
Large Accelerated Filer X Accelerated Filer Non-Accelerated Filer Smaller Reporting Company
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes No X
The aggregate market value of the shares of common stock held by non-affiliates of the Registrant was $3,306,438,596 on June 30, 2010, the last business day of Registrant's most recently completed second fiscal quarter.
As of February 18, 2011, the Registrant had 60,410,386 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Those portions of the Company's definitive Proxy Statement which are responsive to Items 10, 11, 12, 13, and 14 of Part III of this Form 10-K are incorporated by reference into this Form 10-K.
General
Covance Inc. is a leading drug development services company providing a wide range of early-stage and late-stage product development services on a worldwide basis primarily to the pharmaceutical, biotechnology and medical device industries. We also provide laboratory testing services to the chemical, agrochemical and food industries. We believe Covance is one of the world's largest drug development services companies, based on annual net revenues, and one of a few that are capable of providing comprehensive global product development services. Covance maintains offices in more than 30 countries.
Business Strategy
Drug development services companies like Covance typically derive substantially all of their revenue from the research, development and marketing expenditures of the pharmaceutical, biotechnology and medical device industries. We believe outsourcing of these services has increased in the past and will increase in the future because of several factors, including: pressures to contain costs, limitations on internal capacity, the need for faster development time for new drugs, simultaneous research in multiple countries, stringent government regulation and expertise that customers lack internally. We believe the investment and amount of time required to develop new drugs has been increasing, and that these trends create opportunities for companies that can help make the process of drug development more efficient.
Our strategy is to provide services that will generate high-quality and timely data in support of new drug approval or use expansion. We do this by developing and delivering innovative high-quality services that apply science, technology and global reach to capture, manage and integrate a vast array of drug development data. An increasing portion of our business is being provided through strategic, long-term arrangements with clients. These strategic arrangements include dedicated laboratory testing services contracts, in which our clients commit to purchasing a specific dollar amount of services in exchange for guaranteed access to a portion of our facilities. This arrangement benefits our clients by guaranteeing them long-term capacity and infrastructure to run their preclinical studies, and benefits Covance by allowing us to more efficiently utilize our capacity and resources. The trend towards dedicated service agreements and strategic collaborations has been moving from preclinical work to broader drug-development contracts. In 2010, Covance acquired sanofi-aventis' facilities in Porcheville, France and Alnwick, United Kingdom and entered into agreements to provide sanofi-aventis with a broad range of early and late stage drug development services over a ten year period with estimated payments ranging from $1.2 billion to $2.2 billion. These agreements include a ten year sole coverage relationship for central laboratory services and the acquisition of sanofi-aventis' CMC (Chemistry, Manufacturing and Controls) services in connection with the acquisition of the Porcheville and Alnwick sites. In 2009, Covance acquired Merck & Co., Inc.'s ("Merck") Seattle, Washington based gene expression laboratory, which performs genomic analysis services, and entered into a five year $145 million contract to supply such services to Merck. Also in 2009, Covance entered into a seven year $42 million agreement with Kellogg Company for the provision of nutritional chemistry services in a facility in Battle Creek, Michigan. In 2008, Covance entered into a strategic research and development collaboration with Eli Lilly and Company ("Lilly"). Under this agreement, Covance acquired Lilly's 450 acre early development campus in Greenfield, Indiana. Covance agreed to provide Lilly with a broad range of drug development services over a ten year period for a minimum agreement value of $1.6 billion. Under this agreement, Lilly transferred responsibility to Covance for its non-GLP (Good Laboratory Practice) toxicology, in vivo pharmacology, quality control laboratory and imaging services. In addition, the agreement includes a committed level of clinical pharmacology, central laboratory, GLP toxicology studies and clinical Phase II-IV services.
Other types of strategic arrangements include multi-year sole source central laboratory services agreements and strategic clinical development alliances. Sole source contracts for central laboratory services benefit our clients by reducing the time and effort spent contracting services on a project-by-project basis. Under strategic clinical development alliances, a pharmaceutical sponsor contracts with one or two trusted
1
clinical development providers to perform all outsourced clinical trial management activities, typically within selected therapeutic classes.
Operational Excellence. Our goal is to consistently deliver outstanding service to our clients on a global scale through our platform focused on people, process and clients. As a scientific services company, people are integral to our success. We work to recruit, develop and retain talented people through our "Compelling Offer" program which is designed to provide and encourage highly qualified people to initiate and build a career at Covance. We aim to enhance the effectiveness of these people with superior processes to efficiently deliver a high level of client service. We use Six Sigma and other proven improvement methodologies to optimize our processes to increase our cost competitiveness, eliminate variability in our client service levels and build competitive advantage. Finally, we seek to leverage consistent outstanding client service by building strategic relationships with our clients that drive growth and help sustain our competitive advantage. Across our people, process and clients platform, we seek to utilize technology to augment the talent of our people, to automate robust processes, and to link us more closely to our clients via proprietary systems such as StudyTracker®, LabLink and Trial Tracker®.
Global Reach. We believe that it is important to provide a broad range of drug research and development services on a global basis. We have offices, regional monitoring sites and laboratories in over 50 locations in more than 30 different countries and conduct field work in many other countries. We believe we are a leader among drug development services companies in our ability to support large, global clinical trial programs.
Acquisitions. In addition to organic development of services, we consider acquisitions that are complementary to our existing services and that expand our ability to serve our clients. While we cannot exclude the possibility that we may opportunistically seek to take advantage of other situations, we generally expect acquisitions to enhance our existing services either qualitatively or geographically or to add new services that can be integrated with our existing services. In 2010, Covance acquired sanofi-aventis' preclinical facilities in Porcheville, France and Alnwick, United Kingdom including its CMC (Chemistry, Manufacturing and Controls) services. In 2009, we purchased Swiss Pharma Contract Ltd., a 50 bed clinical pharmacology company located in Basel, Switzerland as well as Merck's gene expression laboratory in Seattle, Washington. In 2008, we acquired Lilly's 450 acre early development campus in Greenfield, Indiana and we enhanced our biomarker service offerings by purchasing a minority stake in Caprion Proteomics, a leading provider of proteomics-based services to the pharmaceutical industry.
Services
The services we provide constitute two segments for financial reporting purposes: (1) early development services, which includes discovery support services, preclinical services and clinical pharmacology services, and (2) late-stage development services, which includes central laboratory, clinical development, and commercialization services (periapproval and market access service). Although each segment has separate services within it, they can be and increasingly are combined in integrated service offerings.
Early Development
Preclinical Services
Our preclinical services include toxicology services, pharmaceutical chemistry, nutritional chemistry and related services. Our preclinical area has been a source of innovation by introducing new technologies for client access to data such as StudyTracker®, electronic animal identification, multimedia study reports and animal and test tube measures of induced cell proliferation or reproduction. StudyTracker® is an internet-based client access product which allows clients of toxicology, bioanalytical, metabolism and reproductive and developmental toxicology services to review study data and schedules on a near real-time basis. We have laboratories in locations which include Madison, Wisconsin; Chandler, Arizona, Greenfield, Indiana and Vienna, Virginia in the United States and Harrogate, United Kingdom, Alnwick, United Kingdom, Muenster,
2
Germany and Porcheville, France in Europe. In 2010, we opened our preclinical facility near Shanghai, China. We also have bioanalytical laboratories in the United States in Indianapolis, Indiana and Chantilly, Virginia, and an administrative and a sales office in Tokyo, Japan. In 2008, Covance purchased Lilly's 450 acre research campus in Greenfield, Indiana for cash payments totaling $51.6 million and is currently providing a number of services at that location, including non-GLP toxicology, in vivo pharmacology, quality control laboratory and imaging. Covance renovated a facility in Battle Creek, Michigan for nutritional chemistry services, which opened in 2010.
Toxicology. Our preclinical toxicology services include in vivo toxicology studies, which are studies of the effects of drugs in animals; genetic toxicology studies, which include studies of the effects of drugs on chromosomes, as well as on genetically modified mice; and other specialized toxicology services. For example, we provide immunotoxicology services in which we assess the impact of drugs or chemicals on the structure and function of the immune system and reproductive toxicology services which help our clients assess the risk that a potential new medicine may cause birth defects.
Pharmaceutical Chemistry. In our pharmaceutical chemistry services, we determine the metabolic profile and bioavailability of drug candidates. We also provide laboratory testing services to the chemical and agricultural chemical industries. We offer a complete range of services to agricultural chemical manufacturers to determine the potential risk to humans, animals and the environment from plant protection products such as pesticides.
Nutritional Chemistry and Food Safety. In our nutritional chemistry services, we offer a broad range of services to the food, nutriceutical and animal feed industries, including nutritional analysis and equivalency, nutritional content fact labels, microbiological and chemical contaminant safety analysis and stability testing.
Research Products. We provide custom polyclonal and monoclonal antibody services for research purposes and purpose-bred animals for biomedical research. The purpose-bred research animals we provide are required by pharmaceutical and biotechnology companies, university research centers and contract research organizations as part of required preclinical animal safety and efficacy testing. Through a variety of processes, technology and specifically constructed facilities, we provide purpose-bred, pre-acclimated and specific pathogen free animals that meet our clients' rigorous quality control requirements. Covance also has a dedicated animal biosafety level 2 (ABSL-2) containment vivarium to allow us to provide full service vaccine testing.
Discovery and Translational Services. We provide lead optimization services including custom immunology and antibody services, metabolism studies and pharmacokinetic screening as well as non-GLP toxicology, in vivo pharmacology, imaging services and biomarker services. We substantially enhanced our ability to provide discovery services in 2008 with our acquisition of Lilly's Greenfield, Indiana campus and in 2009 with our acquisition of Merck's gene expression laboratory in Seattle, Washington.
Bioanalytical Services. Our bioanalytical testing services, which are conducted in our bioanalytical laboratory in Indianapolis, Indiana and in our immunoanalytical facility in Chantilly, Virginia, as well as in our laboratories in Madison, Wisconsin, Harrogate, United Kingdom and Shanghai, China, help determine the appropriate dose and frequency of drug application from late discovery evaluation through Phase III clinical testing on a full-scale, globally integrated basis.
Clinical Pharmacology Services
We provide clinical pharmacology services, including first-in-human trials, of new pharmaceuticals at our five clinics located throughout the United States and our clinics in Leeds, United Kingdom and Basel, Switzerland. We offer our clients access to specialized patient populations needed for Phase II trials in specific therapeutic areas. We have grown this service offering through acquisitions. In 2009, we acquired Swiss Pharma Contract Ltd.. In 2006, we significantly expanded our capacity and capabilities in the United States with the acquisition of Radiant Research Inc.'s clinical pharmacology sites and its access to specialized patient
3
populations. In 2005, we acquired GFI Clinical Services, and in 2008 we relocated and significantly upgraded this Evansville, Indiana clinical pharmacology research unit.
Late-Stage Development
Central Laboratory Services
We are the world's largest provider of central laboratory services. We have four central laboratories, one in each of the United States, Switzerland, Singapore and China that provide central laboratory services, including biomarker services, to biotechnology and pharmaceutical customers.
Our capabilities provide clients the flexibility to conduct studies on a multinational and simultaneous basis. The data we provide is combinable and results in global clinical trial reference ranges because we use consistent laboratory methods, identical reagents and calibrators, and similar equipment globally. Combinable data eliminates the cumbersome process of statistically correlating results generated using different methods and different laboratories on different equipment.
We also employ a proprietary clinical trials management system that enables us to enter a sponsor's protocol requirements directly into our database. The laboratory data can be audited because all laboratory data can be traced to source documents. In addition, the laboratories are capable of delivering customized data electronically within 24 hours of test completion. Covance also offers pharmacogenomic testing and sample storage technologies in conjunction with our central laboratory services. Central laboratory services also offers LabLink, an internet-based client access program that allows clients to review and query clinical trial lab data on a near real-time basis, and the Covance Local Laboratories service, which uses a proprietary system to harmonize laboratory results from local and regional laboratories to help expand the reach of traditional central laboratory services.
Our central laboratories have an automated kit production line that is located in the United States and supplies kits to investigator sites around the world. This system allows the flexibility to expand kit production volume more quickly and uses consistent methods to reduce supply variation for our clients.
In 2010, Covance opened a state-of-the-art biorepository facility in Greenfield, Indiana dedicated to long-term storage of clinical trial specimens. This facility is able to store a wide range of specimens, including plasma, serum, whole blood, DNA, PBMC and tissue.
In 2009, we purchased Merck's gene expression laboratory for $9.75 million in cash and entered into a contract for the sale of genomic analysis services to Merck for $145 million over five years.
We provide high throughput GLP and non-GLP biomarker services from our central laboratory, bioanalytical and toxicology laboratories, and offer bioimaging capabilities and cardiac related biomarkers for animals and humans. In 2009, Covance formed a biomarker expert team dedicated to the development, validation and testing of biomarkers.
In 2008, we purchased a minority interest in Caprion Proteomics, a leading provider of proteomics-based services to the pharmaceutical industry for $3.1 million in cash. Under the terms of our agreement with Caprion, Covance serves as the exclusive contract research organization distributor of Caprion's proteomic biomarker services.
Clinical Development Services
We offer a comprehensive range of clinical trial services, including the full management of Phase II and III clinical studies. We have extensive experience in a number of therapeutic areas, and we provide the following core services either on an individual or aggregated basis to meet clients' needs: study design and modeling; coordination of study activities; trial logistics; monitoring of study site performance; clinical data management and biostatistical analysis; and medical writing and regulatory services.
4
We have extensive experience in managing clinical trials in North America, Europe, Latin America and Asia Pacific. These trials may be conducted separately or simultaneously as part of a multinational development plan. We can manage every aspect of clinical trials from clinical development plans and protocol design to New Drug Applications, among other supporting services. Over the last several years, clinical development services has continued its expansion into Eastern Europe, the Middle East, Asia Pacific and South America.
Our clinical development services utilize Trial Tracker®, a web-enabled clinical trial project management and tracking tool which allows both our employees and clients to review and manage the various aspects of clinical trial projects.
Periapproval Services. Periapproval trials are studies conducted "around the time of New Drug Application approval," generally after a drug has successfully undergone clinical efficacy and safety testing and the New Drug Application has been submitted to the Food and Drug Administration ("FDA"). We offer a range of periapproval services, including: Treatment Investigational New Drug applications; Phase IIIb clinical studies, which involve studies conducted after New Drug Application submission, but before regulatory approval is obtained; Phase IV clinical studies, which are studies conducted after initial approval of the drug; product withdrawal support services and other types of periapproval studies such as post-marketing surveillance studies, FDA mandated post-marketing commitments generally focusing on characterizing a drug's safety in large, diverse patient groups, and prescription to over-the-counter switch studies.
Clinical Trial Support Services
Cardiac Safety Services. In November 2007, we sold our centralized ECG business to eResearch Technology Inc. for an upfront cash payment of approximately $35 million with the opportunity to receive additional contingent consideration relating to transferred backlog as well as from revenues generated from new contracts secured under a long-term marketing arrangement. We continue to offer this service to our clients through this marketing arrangement.
Interactive Voice and Web Response Services. In 2009, we sold our interactive voice and web response services business to Phase Forward for $10 million in cash. In addition, Covance and Phase Forward entered into a five year marketing agreement with respect to certain of Phase Forward's services which Covance offers to its clinical development clients.
Market Access Services
We offer a wide range of reimbursement and healthcare economics consulting services, including outcomes and pharmacoeconomic studies, reimbursement planning, reimbursement advocacy programs, risk evaluation and mitigation strategy (REMS) services, registry services and specialty pharmacy services. Pharmaceutical, biotechnology and medical device manufacturers purchase these services from us to help optimize their return on research and development investments. We offer InTeleCenter® services that employ state of the art phone, internet and electronic media to manage customer communications. InTeleCenter programs include reimbursement hotlines, patient assistance programs and patient compliance REMS programs.
Customers and Marketing
We provide product development services on a global basis to, among others, the pharmaceutical and biotechnology industries. In 2010, we served in excess of 1,000 biopharmaceutical companies, ranging from the world's largest pharmaceutical companies and biotechnology companies to small and start-up organizations.
While no single customer accounted for more than ten percent of our aggregate net revenue in 2010, we had four customers accounting for more than five but less than ten percent of our net revenues, and our top five customers accounted for approximately 36.1 percent of our net revenues. In our early development segment, one customer accounted for more than ten percent of net revenues and two customers accounted for
5
more than five but less than ten percent of its aggregate net revenues. In our late-stage development segment, one customer accounted for more than ten percent of net revenues and four customers accounted for more than five but less than ten percent of aggregate net revenues.
For net revenues from external customers, assets attributable to each of our business segments, revenues by significant service area and other segment information for each of the last three fiscal years, please review Note 14 to the audited consolidated financial statements included elsewhere in this Annual Report.
For net revenues from external customers and long-lived assets attributable to operations in the United States, United Kingdom, Switzerland and other countries for each of the last three fiscal years, please review Note 14 to the audited consolidated financial statements included elsewhere in this Annual Report.
Our global sales activities are conducted by sales personnel based in our operations in the United States, Europe, Latin America and Asia Pacific.
Contractual Arrangements
Many of our contracts with our clients are either fixed price or fee-for-service with a cap. To a lesser extent, some of our contracts are fee-for-service without a cap. In cases where the contracts are fixed price, we may bear the cost of overruns, or we benefit if the costs are lower than we anticipated. In cases where our contracts are fee-for-service with a cap, the contracts contain an overall budget for the trial based on time and cost estimates. If our costs are lower than anticipated, the client generally keeps the savings, but if our costs are higher than estimated, we may be responsible for the overrun unless the increased cost is a result of a scope change or other factors outside of our control, such as an increase in the number of patients to be enrolled or the type or amount of data to be collected. Contracts may range in duration from a few months to several years or longer depending on the nature of the work performed. Billing schedules and payment terms are generally negotiated on a contract-by-contract basis. In some cases, we bill the client for the total contract value in progress-based installments as we reach certain non-contingent billing milestones over the contract duration. For additional information please refer to Item 7. Critical Accounting Policies—Revenue Recognition.
Most of our contracts may be terminated by the client either immediately or upon notice. These contracts typically require payment to Covance of expenses to wind down a study, payment to Covance of fees earned to date, and, in some cases, a termination fee or payment to Covance of some portion of the fees or profit that could have been earned under the contract if it had not been terminated early.
We also have contracts with minimum volume commitments with certain clients generally ranging in duration from three to ten years. Underlying these arrangements are individual project contracts for the specific services to be provided. These arrangements enable our clients to secure space in our facilities or time of our personnel in exchange for which they agree to provide a guaranteed annual minimum dollar value ("volume") of work. Under these types of arrangements, if the annual minimum volume commitment is not reached, the client is required to pay Covance for the shortfall. Progress towards the achievement of annual minimum volume guarantees is monitored throughout the year. Annual minimum guarantee shortfalls are not included in net revenues until the amount of the shortfall has been determined and agreed to by the client.
Backlog
Some of our studies and projects are performed over an extended period of time, which may exceed several years. We maintain an order backlog to track anticipated net revenues yet to be earned for work that has not yet been performed. However, we do not maintain an order backlog for other services that are performed within a short period of time or where it is not otherwise practical or feasible to maintain an order backlog. Our aggregate backlog at December 31, 2010 and December 31, 2009 was $6.19 billion and $4.87 billion respectively.
6
Backlog generally includes work to be performed under signed agreements (i.e., contracts and letters of intent). Once work under a signed agreement begins, net revenues are recognized over the life of the project. However, in some cases we will begin work on a project once we conclude we have a legally binding agreement, but before executing a signed agreement, and backlog may include the net revenues expected from that project.
We cannot provide any assurance that we will be able to realize all or most of the net revenues included in backlog or estimate the portion expected to be filled in the current year. Although backlog can provide meaningful information to our management with respect to a particular study, we believe that our aggregate backlog as of any date is not necessarily a meaningful indicator of our future results for a variety of reasons. These reasons include the following: studies vary in duration; the scope of studies may change, which may either increase or decrease their value; and studies may be terminated, reduced in scope or delayed at any time by the client or regulatory authorities.
Competition
The contract research organization industry has many participants ranging from hundreds of small, limited-service providers to a limited number of full-service contract research organizations with global capabilities. We primarily compete against in-house departments of pharmaceutical companies, full-service and limited-service contract research organizations and, to a lesser extent, selected universities and teaching hospitals.
In early development services, our most significant competitors include Charles River Laboratories International Inc., PPD, Inc., WIL Research Laboratories, LLC, WuXi PharmaTech and MPI Research, among others. In late-stage development services, our significant competitors include Quintiles Transnational Corp., PPD, Inc., Parexel International Corporation, Kendle International Inc., ICON p.l.c., PRA International, Inc., i3 Research, PharmaNet Development Group, Inc. and Quest Diagnostics Incorporated, among others.
There is competition for customers on the basis of many factors, including the following: reputation for on-time quality performance; expertise and experience in specific areas; scope of service offerings; strengths in various geographic markets; price; technological expertise and efficient drug development processes; ability to acquire, process, analyze and report data in a rapid and accurate manner; historic experience and relationships; ability to manage large-scale clinical trials both domestically and internationally; quality of facilities; expertise and experience in reimbursement and healthcare consulting; and size. We believe that we compete favorably in these areas.
Government Regulation
Our laboratory services are subject to various regulatory requirements designed to ensure the quality and integrity of the testing processes. Covance's standard operating procedures are written in accordance with regulations and guidelines appropriate to the region and the nation where they will be used.
The industry standards for conducting preclinical laboratory testing are embodied in the Good Laboratory Practice ("GLP") and for central laboratory operations in the Clinical Laboratory Improvement Amendments of 1988 ("CLIA"). The standards of GLP are required by the FDA, by the Department of Health in the United Kingdom, by the European Agency for the Evaluation of Medicinal Products ("EMEA") in Europe and by similar regulatory authorities in other parts of the world. To help satisfy its compliance obligations, Covance has established quality assurance controls at its laboratory facilities which monitor ongoing compliance with GLP and CLIA.
Our clinical services are subject to industry standards for the conduct of clinical research and development studies that are embodied in the regulations for Good Clinical Practice ("GCP"). The FDA, EMEA and other regulatory authorities require that test results submitted to such authorities be based on studies conducted in accordance with GCP. As with GLP and Good Manufacturing Practice ("GMP"), noncompliance with GCP can result in the disqualification of data collected during the clinical trial.
7
We strive to perform all clinical research in accordance with the International Conference on Harmonization—Good Clinical Practice Guidelines, and the requirements of the applicable country. Although the United States is a signatory to these guidelines, the FDA has not adopted all of these guidelines as statutory regulations, but has currently adopted them only as guidelines. From an international perspective, when applicable, we have implemented common standard operating procedures across regions to assure consistency whenever it is feasible and appropriate to do so.
Our animal import and breeding facilities and toxicology facilities are also subject to a variety of U.S. federal and state laws and regulations, including The Animal Welfare Act and the rules and regulations promulgated thereunder by the United States Department of Agriculture ("USDA") and corresponding rules and regulations in other jurisdictions. These facilities maintain detailed standard operating procedures and the documentation necessary to comply with applicable regulations for the humane treatment of the animals in their custody. Besides being licensed by the USDA as a dealer and/or research facility, as appropriate, these businesses are also accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and have registered assurance with the United States National Institutes of Health Office of Laboratory Animal Welfare.
The use of controlled substances in testing for drugs with a potential for abuse is regulated in the United States by the U.S. Drug Enforcement Administration and by similar regulatory bodies in other parts of the world. All Covance United States laboratories using controlled substances for testing purposes are licensed by the U.S. Drug Enforcement Administration.
Our United States laboratories are subject to licensing and regulation under federal, state and local laws relating to hazard communication and employee right-to-know regulations, the handling and disposal of medical specimens and hazardous waste and radioactive materials, as well as the safety and health of laboratory employees. All of our laboratories are subject to applicable federal and state laws and regulations relating to the storage and disposal of all laboratory specimens including the regulations of the Environmental Protection Agency, the Nuclear Regulatory Commission, the Department of Transportation, the National Fire Protection Agency and the Resource Conservation and Recovery Act. Although we believe that Covance is currently in compliance in all material respects with such federal, state and local laws, failure to comply could subject Covance to denial of the right to conduct business, fines, criminal penalties and other enforcement actions.
In addition to its comprehensive regulation of safety in the workplace, the Occupational Safety and Health Administration and similar regulatory authorities in foreign countries have established extensive requirements relating to workplace safety for healthcare employers, whose workers may be exposed to blood-borne pathogens such as HIV and the hepatitis B virus. Covance employees receive initial and periodic training focusing on compliance with applicable hazardous materials regulations and health and safety guidelines.
The United States and other national governments are concerned about the disclosure of confidential personal data and have addressed this concern with increased regulation. The European Union, or EU, prohibits certain disclosures of personal confidential information, including medical information, to any entity that does not comply with certain security safeguards. In the United States, various federal and state laws address the security and privacy of health information. We will continue to monitor our compliance with applicable regulations.
The regulations of the U.S. Department of Transportation, the U.S. Public Health Service and the U.S. Postal Service, as well as similar regulations in other countries, apply to the surface and air transportation of laboratory specimens. Covance's laboratories also must comply with the applicable International Air Transport Association regulations, which govern international shipments of laboratory specimens.
Intellectual Property
We have developed certain computer software and technically derived procedures and products intended to maximize the quality and effectiveness of our services. Although our intellectual property rights are important to our results of operations, we believe that such factors as the technical expertise, knowledge, ability and experience of our professionals are more important, and that, overall, these technological capabilities provide significant benefits to our clients.
8
Employees
At December 31, 2010, we had 10,528 employees, approximately 42% of whom were employed outside of the United States and 9,716 of whom were full time employees. Our records indicate that more than 100 of our employees hold M.D. degrees, more than 600 hold Ph.D. degrees, and more than 1,500 hold masters or other postgraduate degrees. We believe that Covance's relations with its employees are good.
Executive Officers
Joseph L. Herring, 55, has been Covance's Chief Executive Officer since January 2005, and Chairman since January 2006. Mr. Herring was President and Chief Operating Officer from November 2001 to December 2004, and was Covance's Corporate Senior Vice President and President—Early Development Services from 1999 to November 2001. From September 1996 to September 1999, Mr. Herring was Corporate Vice President and General Manager of Covance Laboratories North America. Prior to joining Covance, Mr. Herring spent 18 years at the American Hospital Supply/Baxter International/Caremark International family of healthcare service companies where he held a variety of senior leadership positions, culminating in the position of Vice President and General Manager of its oncology business. Mr. Herring has been a member of the Covance Board since 2004.
William E. Klitgaard, 57, has been Covance's Corporate Senior Vice President, Chief Financial Officer and Treasurer since September 2000. From September 1999 to September 2000, Mr. Klitgaard was Covance's Corporate Vice President, Strategy and Corporate Development and Treasurer. From October 1996 to September 1999, Mr. Klitgaard was Covance's Corporate Vice President and Treasurer. Prior to that, Mr. Klitgaard was Treasurer at Kenetech Corporation in San Francisco, and prior to that Mr. Klitgaard spent eleven years in positions of increasing responsibility with Consolidated Freightways Inc.
Richard Cimino, 51, has been Covance's Executive Vice President and Group President, Clinical Development since November 2010. From December 2004 through October 2010, Mr. Cimino was Corporate Senior Vice President and President—Clinical Development. Prior to that, Mr. Cimino was Covance's General Manager of Cardiac Safety Services commencing December 2003. Prior to that, Mr. Cimino was General Manager, America's Health Imaging Group and Corporate Vice President of Eastman Kodak Company. Mr. Cimino serves at Covance's request as a director of BioClinica Inc.
James W. Lovett, 46, has been Covance's Corporate Senior Vice President, General Counsel and Secretary since February 2003, has headed Covance's Nutritional Chemistry and Food Safety Services since January 2008 and has led Covance's Market Access Services since November 2010. From December 2001 to February 2003, Mr. Lovett was Corporate Vice President, General Counsel and Secretary of Covance. From 1997 to 2001, Mr. Lovett was with FMC Corporation in positions of increasing responsibility and, prior to that, was a partner in the law firm of McDermott, Will & Emery.
Michael Lehmann, 48, has been Covance's Corporate Senior Vice President and President—Nonclinical Safety Assessment, since May 2009. From January 2008 to May 2009, Mr. Lehmann was Corporate Vice President and President—Early Development North America. From September 2005 to January 2008, Mr. Lehmann was General Manager—Covance Laboratories North America. Prior to that, Mr. Lehmann held senior management positions for multiple lines of business over a 17-year career at GE Healthcare. Most recently, he served as General Manger, Multi-Vendor Services, GE Healthcare Technologies.
Deborah L. Tanner, 48, has been Covance's Executive Vice President and Group President, Research and Development Laboratories since November 2010. Ms. Tanner was Corporate Senior Vice President and President—Global Central Laboratory Services from February 2006 through October 2010. Prior to that Ms. Tanner was Covance's Global Vice President of Operations in Central Laboratory Services commencing in August 2001 and prior to that, Vice President—Analytical Services for Covance Laboratories—Europe. Ms. Tanner has been with Covance for over 20 years in positions of increasing responsibility.
9
Available Information
Covance makes available free of charge on its website at www.covance.com, its Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 as soon as reasonably practicable after such reports are electronically filed with, or furnished to, the SEC. The charters of the Audit Committee, the Compensation Committee, and the Corporate Governance Committee, as well as the Corporate Governance Guidelines, the Code of Ethics for Financial Professionals and the Company's Business Integrity Program may be accessed through our website at www.covance.com.
This section discusses various risk factors that are attendant with our business and the provision of our services. If the events outlined below were to occur individually or in the aggregate, our business, results of operations, financial condition, and cash flows could be materially adversely affected.
Changes in government regulation or in practices relating to the pharmaceutical industry could decrease the need for the services we provide.
Governmental agencies throughout the world, including in the United States, strictly regulate the drug development process. Our business involves helping pharmaceutical and biotechnology companies navigate the regulatory drug approval process. Changes in regulation, such as a relaxation in regulatory requirements or the introduction of simplified drug approval procedures, or an increase in regulatory requirements that we have difficulty satisfying or that make our services less competitive, could eliminate or substantially reduce the demand for our services. Also, if government efforts contain drug costs and impact pharmaceutical and biotechnology company profits from new drugs, our customers may spend less, or reduce their growth in spending on research and development. If health insurers were to change their practices with respect to reimbursements for pharmaceutical products, our customers may spend less, or reduce their growth in spending on research and development.
Failure to comply with existing regulations could result in a loss of revenue or earnings or in increased costs.
Any failure on our part to comply with applicable regulations could result in the termination of on-going research or the disqualification of data for submission to regulatory authorities. For example, if we were to fail to properly monitor compliance by clinical trial investigators with study protocols, the data collected from that trial could be disqualified. If this were to happen, we could be contractually required to repeat the trial at no further cost to our customer, but at substantial cost to us, or could be exposed to a lawsuit seeking substantial monetary damages.
We may bear financial losses because most of our contracts are of a fixed price nature and may be delayed or terminated or reduced in scope for reasons beyond our control.
Many of our contracts provide for services on a fixed price or fee-for-service with a cap basis and they may be terminated or reduced in scope either immediately or upon notice. Cancellations may occur for a variety of reasons, including:
- •
- the failure of products to satisfy safety requirements;
- •
- unexpected or undesired results of the products;
- •
- insufficient patient enrollment;
- •
- insufficient investigator recruitment;
- •
- the client's decision to terminate the development of a product or to end a particular study; and
- •
- our failure to perform properly our duties under the contract.
The loss, reduction in scope or delay of a large contract or the loss or delay of multiple contracts could materially adversely affect our business, although our contracts frequently entitle us to receive the costs of winding down the terminated projects, as well as all fees earned by us up to the time of termination.
10
We may bear financial risk if we underprice our contracts or overrun cost estimates.
Since our contracts are often structured as fixed price or fee-for-service with a cap, we bear the financial risk if we initially underprice our contracts or otherwise overrun our cost estimates. Such underpricing or significant cost overruns could have a material adverse effect on our business, results of operations, financial condition, and cash flows.
We may not be able to successfully develop and market or acquire new services.
We may seek to develop and market new services that complement or expand our existing business or expand our service offerings through acquisition. If we are unable to develop new services and/or create demand for those newly developed services, or to expand our service offerings through acquisition, our future business, results of operations, financial condition, and cash flows could be adversely affected.
Our quarterly operating results may vary.
Our operating results may vary significantly from quarter to quarter and are influenced by factors over which we have little control such as:
- •
- changes in the general global economy;
- •
- exchange rate fluctuations;
- •
- the commencement, completion, delay or cancellation of large projects or groups of projects;
- •
- the progress of ongoing projects;
- •
- the timing of and charges associated with completed acquisitions or other events; and
- •
- changes in the mix of our services.
We believe that operating results for any particular quarter are not necessarily a meaningful indication of future results. While fluctuations in our quarterly operating results could negatively or positively affect the market price of our common stock, these fluctuations may not be related to our future overall operating performance.
We depend on the pharmaceutical and biotechnology industries.
Our revenues depend greatly on the expenditures made by the pharmaceutical and biotechnology industries in research and development. In some instances, companies in these industries are reliant on their ability to raise capital in order to fund their research and development projects. Accordingly, economic factors and industry trends that affect our clients in these industries also affect our business. If companies in these industries were to reduce the number of research and development projects they conduct or outsource, our business could be materially adversely affected.
We operate in a highly competitive industry.
Competitors in the contract research organization industry range from small, limited-service providers to full service global contract research organizations. Our main competition consists of in-house departments of pharmaceutical companies, full-service and functional contract research organizations, and, to a lesser degree, universities and teaching hospitals. We compete on a variety of factors, including:
- •
- reputation for on-time quality performance and regulatory compliance;
- •
- expertise and experience in specific areas;
- •
- scope of service offerings;
- •
- strengths in various geographic markets;
- •
- price;
- •
- technological expertise and efficient drug development processes;
- •
- quality of facilities;
- •
- ability to acquire, process, analyze and report data in an accurate manner;
- •
- ability to manage large-scale clinical trials both domestically and internationally;
- •
- expertise and experience in market access services; and
- •
- size.
11
For instance, our clinical and other development services have from time-to-time experienced periods of increased price competition which had a material adverse effect on Covance's late-stage development profitability and consolidated net revenues and net income.
There is competition among the larger contract research organizations for both clients and potential acquisition candidates. Additionally, small, limited-service entities considering entering the contract research organization industry will find few barriers to entry, thus further increasing possible competition. These competitive pressures may affect the attractiveness of our services and could adversely affect our financial results.
Unfavorable general economic conditions could negatively impact our operating results and financial condition.
Unfavorable global economic conditions, including the recent recession in the United States and the recent financial crisis affecting the banking system and financial markets, could negatively affect our business. While it is difficult for us to predict the impact of general economic conditions on our business, these conditions could reduce customer demand for some of our services, which could cause our revenue to decline. Also, our customers, particularly smaller biotechnology companies which are especially reliant on the credit and capital markets, may not be able to obtain adequate access to credit or equity funding, which could affect their ability to make timely payments to us. If that were to occur, we could be required to increase our allowance for doubtful accounts, and the number of days outstanding for our accounts receivable could increase. For these reasons, among others, if economic conditions stagnate or decline, our operating results and financial condition could be adversely affected.
We may expand our business through acquisitions.
We review many acquisition candidates and, in addition to acquisitions which we have already made, we are continually evaluating new acquisition opportunities. Factors which may affect our ability to grow successfully through acquisitions include:
- •
- difficulties and expenses in connection with integrating the acquired companies and achieving the expected benefits;
- •
- diversion of management's attention from current operations;
- •
- the possibility that we may be adversely affected by risk factors facing the acquired companies;
- •
- acquisitions could be dilutive to earnings, or in the event of acquisitions made through the issuance of our common stock to the shareholders of the acquired company, dilutive to the percentage of ownership of our existing stockholders;
- •
- potential losses resulting from undiscovered liabilities of acquired companies not covered by the indemnification we may obtain from the seller;
- •
- risks of not being able to overcome differences in foreign business practices, language and other cultural barriers in connection with the acquisition of foreign companies; and
- •
- loss of key employees of the acquired companies.
We may be affected by potential health care reform.
In March 2010, the United States Congress enacted health care reform legislation intended to expand, over time, health insurance coverage and impose health industry cost containment measures. This legislation may significantly impact the pharmaceutical and biotechnology industries. In addition, the U.S. Congress, various state legislatures and European and Asian governments may consider various types of health care reform in order to control growing health care costs. We are presently uncertain as to the effects of the recently enacted legislation on our business and are unable to predict what legislative proposals will be adopted in the future, if any.
Implementation of health care reform legislation that contains costs could limit the profits that can be made from the development of new drugs. This could adversely affect research and development expenditures by pharmaceutical and biotechnology companies which could in turn decrease the business opportunities available to us both in the United States and abroad. In addition, new laws or regulations may create a risk of liability, increase our costs or limit our service offerings.
12
We rely on third parties for important services.
We depend on third parties to provide us with services critical to our business. The failure of any of these third parties to adequately provide the needed services could have a material adverse effect on our business.
Our revenues and earnings are exposed to exchange rate fluctuations.
We derive a large portion of our net revenues from international operations. For the years ended December 31, 2010 and 2009, we derived approximately 44% and 42%, respectively, of our net revenues from operations outside the United States. Since our consolidated financial statements are denominated in U.S. dollars, fluctuations in exchange rates from period to period will have an impact on our reported results. In addition, in certain circumstances, we may incur costs in one currency related to our services or products for which we are paid in a different currency. As a result, factors associated with international operations, including changes in foreign currency exchange rates, could significantly affect our results of operations, financial condition and cash flows.
The loss of our key personnel could adversely affect our business.
Our success depends to a significant extent upon the efforts of our senior management team and other key personnel. The loss of the services of such personnel could adversely affect our business. Also, because of the nature of our business, our success is dependent upon our ability to attract and retain technologically qualified personnel. There is substantial competition for qualified personnel, and an inability to recruit or retain qualified personnel may impact our ability to grow our business and compete effectively in our industry.
Contract research services create a risk of liability.
In contracting to work on drug development trials and studies, we face a range of potential liabilities, for example:
- •
- errors or omissions that create harm during a trial to study volunteers or after a trial to consumers of the drug after regulatory approval of the drug;
- •
- general risks associated with clinical pharmacology facilities, including negative consequences from the administration of drugs to clinical trial participants or the professional malpractice of clinical pharmacology medical care providers;
- •
- errors or omissions from tests conducted for the agrochemical and food industries;
- •
- risks that animals in our breeding facilities may be infected with diseases that may be harmful and even lethal to themselves and humans despite preventive measures contained in our company policies for the quarantine and handling of imported animals; and
- •
- errors and omissions during a trial that may undermine the usefulness of a trial or data from the trial or study.
We also contract with physicians, also referred to as investigators, to conduct the clinical trials to test new drugs on human volunteers. These tests can create a risk of liability for personal injury or death to volunteers, resulting from negative reactions to the drugs administered or from professional malpractice by third party investigators.
While we endeavor to include in our contracts provisions entitling us to be indemnified or entitling us to a limitation of liability, these provisions do not uniformly protect us against liability arising from certain of our own actions, such as negligence or misconduct. We could be materially and adversely affected if we were required to pay damages or bear the costs of defending any claim which is not covered by a contractual indemnification provision or in the event that a party who must indemnify us does not fulfill its indemnification obligations or which is beyond the level of our insurance coverage. There can be no assurance that we will be able to maintain such insurance coverage on terms acceptable to us.
13
Hardware and software failures, delays in the operation of our computer and communications systems or the failure to implement system enhancements may harm our business.
Our success depends on the efficient and uninterrupted operation of our computer and communications systems. A failure of our network or data gathering procedures could impede the processing of data, delivery of databases and services, client orders and day-to-day management of our business and could result in the corruption or loss of data. While certain of our operations have appropriate disaster recovery plans in place, we currently do not have redundant facilities everywhere in the world to provide IT capacity in the event of a system failure. Despite any precautions we may take, damage from fire, floods, hurricanes, power loss, telecommunications failures, computer viruses, break-ins and similar events at our various computer facilities could result in interruptions in the flow of data to our servers and from our servers to our clients. In addition, any failure by our computer environment to provide our required data communications capacity could result in interruptions in our service. In the event of a delay in the delivery of data, we could be required to transfer our data collection operations to an alternative provider of server hosting services. Such a transfer could result in delays in our ability to deliver our products and services to our clients. Additionally, significant delays in the planned delivery of system enhancements, improvements and inadequate performance of the systems once they are completed could damage our reputation and harm our business. Finally, long-term disruptions in the infrastructure caused by events such as natural disasters, the outbreak of war, the escalation of hostilities, and acts of terrorism (particularly involving cities in which we have offices) could adversely affect our business. Although we carry property and business interruption insurance, our coverage may not be adequate to compensate us for all losses that may occur.
Reliance on facilities.
Covance relies on certain of its facilities. In particular, Covance's preclinical and central laboratory facilities are highly specific and would be difficult to replace in a short period of time. Any event that causes a disruption of the operation of these facilities might impact our ability to provide service to our customers and therefore could have a material adverse affect on our financial condition, results of operations and cash flows.
Reliance on air transportation.
Our central laboratories and certain of our other businesses are heavily reliant on air travel for transport of clinical trial kits and other material, products and people, and a significant disruption to the air travel system, or our access to it, could have a material adverse effect on our business.
Certain service offerings and research products are dependent on limited sources of supply of services or products which if interrupted could affect our business.
We depend on a limited number of suppliers for certain services and for certain animal populations. Disruptions to the continued supply of these services or products may arise from export/import restrictions or embargoes, foreign political or economic instability, or otherwise. Disruption of supply could have a material adverse effect on our business.
Actions of animal rights extremists may affect our business.
Our early development services utilize animals in preclinical testing of the safety and efficacy of drugs and also breed and sell animals for biomedical research. Such activities are required for the development of new medicines and medical devices under regulatory regimes in the United States, Europe, Japan and other countries. Acts of vandalism and other acts by animal rights extremists who object to the use of animals in drug development could have a material adverse effect on our business.
14
Our animal populations may suffer diseases that can damage our inventory, harm our reputation, result in decreased sales of research products or result in other liability to us.
It is important that our research products be free of diseases, including infectious diseases. The presence of diseases can distort or compromise the quality of research results, can cause loss of animals in our inventory, can result in harm to humans or outside animal populations if the disease is not contained to animals in inventory, or can result in other losses. Such results could harm our reputation or have a material adverse effect on our financial condition, results of operations, and cash flows.
Item 1B. Unresolved Staff Comments
None.
Covance both owns and leases its facilities. Covance owns substantial facilities in the United States in Madison, Wisconsin, in Chandler, Arizona, in Vienna, Virginia, in Greenfield, Indiana, in Europe in Harrogate, United Kingdom, in Leeds, United Kingdom, in Alnwick, United Kingdom, in Porcheville, France and in Muenster, Germany for its early development services. Covance also owns a newly renovated facility in Battle Creek, Michigan used for nutritional chemistry services. In Asia, Covance owns a preclinical facility near Shanghai, China. Covance owns a substantial facility in Geneva, Switzerland and leases a substantial facility in the United States in Indianapolis, Indiana for its central laboratory services and leases facilities in Indianapolis, Indiana and Chantilly, Virginia for its bioanalytical services. Covance leases substantial facilities for its clinical development services in the United States in Princeton, New Jersey, and in the United Kingdom in Maidenhead. Covance also owns or leases other properties and facilities in the United States, Europe, Latin America and Asia Pacific. Covance believes that its facilities are adequate for its operations and that suitable additional space will be available when needed.
For additional information, please see Note 11 to the audited consolidated financial statements included elsewhere in this Annual Report.
Covance is party to lawsuits and administrative proceedings incidental to the normal course of its business. Covance does not believe that any liabilities related to such lawsuits or proceedings will have a material effect on its financial condition, results of operations or cash flows.
15
Item 5. Market for Registrant's Common Stock and Related Stockholder Matters and Issuer Purchases of Equity Securities
Covance's common stock is traded on the New York Stock Exchange (symbol: CVD). The following table shows the high and low sales prices on the New York Stock Exchange for each of the most recent eight fiscal quarters.
| Quarter | High | Low | |||||
|---|---|---|---|---|---|---|---|
First Quarter 2009 |
$ |
46.68 |
$ |
32.38 |
|||
Second Quarter 2009 |
$ |
49.61 |
$ |
33.72 |
|||
Third Quarter 2009 |
$ |
58.95 |
$ |
45.46 |
|||
Fourth Quarter 2009 |
$ |
57.36 |
$ |
50.09 |
|||
First Quarter 2010 |
$ |
62.39 |
$ |
53.34 |
|||
Second Quarter 2010 |
$ |
63.50 |
$ |
49.91 |
|||
Third Quarter 2010 |
$ |
54.57 |
$ |
37.45 |
|||
Fourth Quarter 2010 |
$ |
52.94 |
$ |
43.06 |
|||
As of February 18, 2011, there were 3.835 holders of record of Covance's common stock.
Covance has not paid any dividends during 2010 or 2009. Covance does not currently intend to pay dividends, but rather, intends to reinvest earnings in its business.
Issuer Purchases of Equity Securities
Repurchases of equity securities as reported on a settlement date basis during the quarter ended December 31, 2010 were as follows:
| Period | Total # of Shares Purchased |
Average Price Paid Per Share |
Total # of Shares Purchased as Part of Currently Authorized Programs |
Estimated Maximum # of Shares that May Yet Be Purchased Under Currently Authorized Programs(a) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
October 1, 2010–October 31, 2010 |
— | — | — | 5,535,889 | |||||||||
November 1, 2010–November 30, 2010 |
4,753,589 | $ | 52.59 | (a) | 4,753,589 | 782,300 | |||||||
December 1, 2010–December 31, 2010 |
— | — | — | 782,300 | |||||||||
- (a)
- In September 2010, the Covance Board of Directors authorized the repurchase of up to an additional $250 million of the Company's outstanding common stock. In November 2010, the Company entered into an agreement with an independent financial institution to execute the repurchase program through an accelerated share repurchase transaction (the "ASR"). Pursuant to the ASR, the Company provided the independent financial institution with an upfront payment totaling $250 million and the independent financial institution delivered to the Company 4.75 million shares of its common stock. The actual number of shares that will be repurchased under the ASR will, in general, be based upon the volume weighted average share price of Covance common stock over the repurchase period, in accordance with the terms of the ASR agreement. Pursuant to the ASR agreement, additional shares may be delivered to Covance at the end of the repurchase period.
16
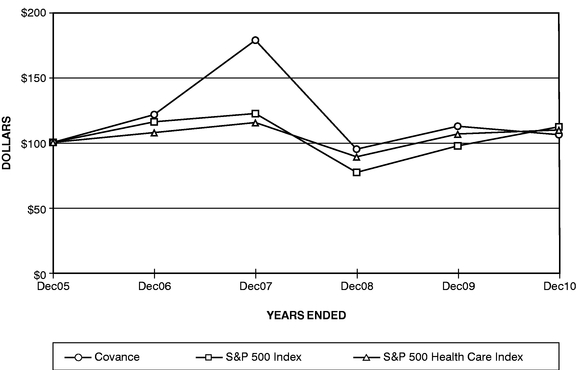
The graph below provides an indicator of cumulative total shareholder returns for Covance as compared with the Standard & Poor's 500 Stock Index® and the Standard & Poor's Health Care Sector Index®. The graph covers the period of time from December 31, 2005 through December 31, 2010 and assumes $100 was invested on December 31, 2005.
Item 6. Selected Financial Data
The following table presents selected historical consolidated financial data of Covance as of and for each of the years ended December 31, 2010, 2009, 2008, 2007 and 2006. This data has been derived from the audited consolidated financial statements of Covance. You should read this selected historical consolidated financial data in conjunction with Covance's audited consolidated financial statements and accompanying notes included elsewhere in this Annual Report. Historical consolidated financial data may not be indicative of Covance's future performance. See also "Management's Discussion and Analysis of Financial Condition and Results of Operations."
17
The information provided in the following table is on an "as reported" basis for all years presented, and includes the results of Covance's interactive voice and web response service offering ("IVR Services") through its divestiture on August 20, 2009 and Covance's cardiac safety service offering ("Cardiac Safety Services") through its divestiture on November 27, 2007. Items affecting comparability between periods have been noted in the following table.
| |
Year Ended December 31 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||||
| |
(Dollars in thousands, except per share data) |
|||||||||||||||||
Income Statement Data: |
||||||||||||||||||
Net revenues |
$ | 1,925,630 | $ | 1,867,634 | $ | 1,728,098 | $ | 1,546,419 | $ | 1,340,203 | ||||||||
Reimbursable out-of-pocket expenses |
112,843 | 94,992 | 98,969 | 85,097 | 65,855 | |||||||||||||
Total revenues |
2,038,473 | 1,962,626 | 1,827,067 | 1,631,516 | 1,406,058 | |||||||||||||
Costs and expenses: |
||||||||||||||||||
Cost of revenue |
1,348,498 | 1,277,142 | 1,142,697 | 1,017,686 | 882,190 | |||||||||||||
Reimbursable out-of-pocket expenses |
112,843 | 94,992 | 98,969 | 85,097 | 65,855 | |||||||||||||
Selling, general and administrative |
307,386 | 270,593 | 250,180 | 233,890 | 207,388 | |||||||||||||
Depreciation and amortization |
103,024 | 91,289 | 71,571 | 66,197 | 57,388 | |||||||||||||
Asset impairment charges |
119,229 | — | — | — | — | |||||||||||||
Total |
1,990,980 | (a) | 1,734,016 | 1,563,417 | 1,402,870 | 1,212,821 | ||||||||||||
Income from operations |
47,493 | (a) | 228,610 | 263,650 | 228,646 | 193,237 | ||||||||||||
Other expense (income), net: |
||||||||||||||||||
Interest expense (income), net |
52 | 201 | (6,461 | ) | (9,801 | ) | (7,564 | ) | ||||||||||
Foreign exchange transaction losses (gains) |
3,649 | 245 | (142 | ) | (1,375 | ) | 212 | |||||||||||
Gain on sale of businesses |
— | (9,681 | ) | (4,070 | ) | (6,590 | ) | — | ||||||||||
Other expense (income), net |
3,701 | (9,235 | )(c) | (10,673 | )(e) | (17,766 | )(f) | (7,352 | ) | |||||||||
Income before taxes and equity investee earnings |
43,792 | (a) | 237,845 | (c) | 274,323 | (e) | 246,412 | (f) | 200,589 | |||||||||
Tax (benefit) expense |
(23,655 | )(a),(b) | 62,870 | (c),(d) | 79,415 | (e) | 72,934 | (f) | 57,179 | (g) | ||||||||
Equity investee earnings |
807 | 907 | 1,852 | 2,451 | 1,588 | |||||||||||||
Net income |
$ | 68,254 | (a),(b) | $ | 175,882 | (c),(d) | $ | 196,760 | (e) | $ | 175,929 | (f) | $ | 144,998 | (g) | |||
Basic earnings per share |
$ | 1.08 | $ | 2.76 | $ | 3.12 | $ | 2.76 | $ | 2.28 | ||||||||
Diluted earnings per share |
$ | 1.06 | (a),(b) | $ | 2.73 | (c),(d) | $ | 3.08 | (e) | $ | 2.71 | (f) | $ | 2.24 | (g) | |||
Balance Sheet Data: |
||||||||||||||||||
Working capital |
$ | 446,637 | $ | 474,928 | $ | 277,895 | $ | 411,897 | $ | 349,862 | ||||||||
Total assets |
$ | 1,965,542 | $ | 1,974,944 | $ | 1,753,088 | $ | 1,560,185 | $ | 1,297,678 | ||||||||
Long term debt |
$ | 97,500 | $ | — | $ | — | $ | — | $ | — | ||||||||
Stockholders' equity |
$ | 1,279,821 | $ | 1,411,004 | $ | 1,194,849 | $ | 1,110,188 | $ | 923,295 | ||||||||
Other Financial Data: |
||||||||||||||||||
Gross margin |
30.0 | % | 31.6 | % | 33.9 | % | 34.2 | % | 34.2 | % | ||||||||
Operating margin |
2.5 | % | 12.2 | % | 15.3 | % | 14.8 | % | 14.4 | % | ||||||||
Net income margin |
3.5 | % | 9.4 | % | 11.4 | % | 11.4 | % | 10.8 | % | ||||||||
Current ratio |
1.89 |
2.18 |
1.60 |
2.15 |
2.21 |
|||||||||||||
Debt to equity |
0.07 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||||||
Book value per share |
21.24 | 22.01 | 18.88 | 17.34 | 14.44 | |||||||||||||
Net days sales outstanding |
31 | 40 | 37 | 36 | 49 | |||||||||||||
- (a)
- Includes
asset impairment charges ($119,229) and restructuring costs ($28,030) totaling $147,259 ($93,604 net of tax or $1.45 per diluted share).
- (b)
- Includes
a $17,298 or $0.27 per diluted share income tax benefit recorded in connection with the favorable resolution of several income tax matters and the
recognition of previously unrecognized benefits.
- (c)
- Includes
a $9,026 gain on 2009 sale of IVR Services ($5,867 net of tax or $0.09 per diluted share) and a $655 gain ($426 net of tax or $0.01 per diluted
share) resulting from contingent consideration received in 2009 associated with the 2007 sale of Cardiac Safety Services related to transferred backlog.
- (d)
- Includes
a $2,072 or $0.03 per diluted share income tax gain associated with the reduction of income tax reserves resulting from the completion of an income
tax audit and the recognition of previously unrecognized tax benefits in jurisdictions where the period of review of filings has expired.
- (e)
- Includes
a $4,070 gain ($2,646 net of tax or $0.05 per diluted share) resulting from contingent consideration received in 2008 associated with the 2007 sale
of Cardiac Safety Services related to transferred backlog.
- (f)
- Includes
a $6,590 gain on the sale of Cardiac Safety Services ($4,152 net of tax or $0.06 per diluted share).
- (g)
- Includes a $2,467 or $0.04 per diluted share income tax gain associated with the reduction of income tax reserves resulting from favorable income tax developments in 2006.
18
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
Overview
Covance is a leading drug development services company providing a wide range of early-stage and late-stage product development services on a worldwide basis primarily to the pharmaceutical, biotechnology and medical device industries. Covance also provides services such as laboratory testing to the chemical, agrochemical and food industries. The foregoing services comprise two reportable segments for financial reporting purposes: early development services, which includes preclinical and clinical pharmacology service offerings; and late-stage development services, which includes central laboratory, clinical development, periapproval and market access services. Although each segment has separate services within it, they can be and increasingly are, combined in integrated service offerings. Covance believes it is one of the largest drug development services companies, based on annual net revenues, and one of a few that is capable of providing comprehensive global product development services. Covance offers its clients high quality services designed to provide data to clients as rapidly as possible and reduce product development time. We believe this enables Covance's customers to introduce their products into the marketplace faster and as a result, maximize the period of market exclusivity and monetary return on their research and development investments. Additionally, Covance's comprehensive services and broad experience provide its customers with a variable cost alternative to fixed cost internal development capabilities.
Critical Accounting Policies
Covance's consolidated financial statements are prepared in accordance with U.S. generally accepted accounting principles ("GAAP"), which require management to make estimates and assumptions about future events that affect the amounts reported in the financial statements and the accompanying notes. Actual results could differ from these estimates. The following discussion highlights what we believe to be the critical accounting policies and judgments made in the preparation of these consolidated financial statements.
Revenue Recognition. Covance recognizes revenue either as services are performed or products are delivered, depending on the nature of the work contracted. Historically, a majority of Covance's net revenues have been earned under contracts which range in duration from a few months to two years, but can extend in duration up to five years or longer. Covance also has committed minimum volume arrangements with certain clients which generally range in duration from three to ten years. Underlying these arrangements are individual project contracts for the specific services to be provided. These arrangements enable our clients to secure our services in exchange for which they commit to purchase an annual minimum dollar value ("volume") of services. Under these types of arrangements, if the annual minimum volume commitment is not reached, the client is required to pay Covance for the shortfall. Progress towards the achievement of annual minimum volume guarantees is monitored throughout the year. Annual minimum guarantee shortfalls are not included in net revenues until the amount has been determined and agreed to by the client.
Covance does not have any individual significant contracts as pertains to revenue recognition. By way of background, at any point in time Covance is working on thousands of active client projects, which are governed by individual contracts. In addition, the Company has not had a single customer who accounted for more than ten percent of aggregate net revenues during any one of the last three years. Covance serves in excess of 1,000 biopharmaceutical companies and has over 14,000 active client projects. Most projects are customized based on the needs of the client, the type of services being provided, therapeutic indication of the drug, geographic locations and other variables. Project specific terms related to pricing, billing milestones and the scope and type of services to be provided are generally negotiated and contracted on a project-by-project basis.
19
Service contracts generally take the form of fee-for-service or fixed-price arrangements. In cases where performance spans multiple accounting periods, revenue is recognized as services are performed, measured on a proportional-performance basis, generally using output measures that are specific to the service provided. Examples of output measures in our early development segment include the number of slides read, dosings performed, or specimens prepared for preclinical laboratory services, or number of dosings or number of volunteers enrolled for clinical pharmacology. Examples of output measures in our late-stage development segment's clinical development service offering include among others, number of investigators enrolled, number of sites initiated, number of patients enrolled and number of monitoring visits completed. Revenue is determined by dividing the actual units of work completed by the total units of work required under the contract and multiplying that percentage by the total contract value. The total contract value, or total contractual payments, represents the aggregate contracted price for each of the agreed upon services to be provided. Covance does not have any contractual arrangements spanning multiple accounting periods where revenue is recognized on a proportional-performance basis under which the Company has earned more than an immaterial amount of performance-based revenue (i.e. potential additional revenue tied to specific deliverables or performance). Changes in the scope of work are common, especially under long-term contracts, and generally result in a change in contract value. Once the client has agreed to the changes in scope and renegotiated pricing terms, the contract value is amended and revenue is recognized, as described above. Estimates of costs to complete are made to provide, where appropriate, for losses expected on contracts. Costs are not deferred in anticipation of contracts being awarded, but instead are expensed as incurred. For the years ended December 31, 2010, 2009 and 2008, Covance did not experience a change in the estimates used to determine the amounts recognized as revenue (i.e. output measures or costs to complete) for any project resulting in a material impact on our financial position, results of operations or cash flows.
Billing schedules and payment terms are generally negotiated on a contract-by-contract basis. In some cases, Covance bills the client for the total contract value in progress-based installments as certain non-contingent billing milestones are reached over the contract duration, such as, but not limited to, contract signing, initial dosing, investigator site initiation, patient enrollment or database lock. The term "billing milestone" relates only to a billing trigger in a contract whereby amounts become billable and payable in accordance with a negotiated predetermined billing schedule throughout the term of a project. These billing milestones are not performance-based (i.e., potential additional arrangement consideration tied to specific deliverables or performance). In other cases, billing and payment terms are tied to the passage of time (e.g., monthly billings). In either case, the total contract value and aggregate amounts billed to the client would be the same at the end of the project. While Covance attempts to negotiate terms that provide for billing and payment of services prior or within close proximity to the provision of services, this is not always the case, as evidenced by fluctuations in the levels of unbilled receivables and unearned revenue from period to period. While a project is ongoing, cash payments are not necessarily representative of aggregate revenue earned at any particular point in time, as revenues are recognized when services are provided, while amounts billed and paid are in accordance with the negotiated billing and payment terms.
In some cases, payments received are in excess of revenue recognized. For example, a contract invoicing schedule may provide for an upfront payment of 10% of the full contract value upon contract signing, but at the time of signing, performance of services has not yet begun, and therefore, no revenue has yet been recognized. Payments received in advance of services being provided, such as in this example, are deferred as unearned revenue on the balance sheet. As the contracted services are subsequently performed and the associated revenue is recognized, the unearned revenue balance is reduced by the amount of revenue recognized during the period.
20
In other cases, services may be provided and revenue is recognized before the client is invoiced. In these cases, revenue recognized will exceed amounts billed, and the difference, representing an unbilled receivable, is recorded for this amount which is currently unbillable to the customer pursuant to contractual terms. Once the client is invoiced, the unbilled receivable is reduced, for the amount billed, and a corresponding account receivable is recorded. All unbilled receivables are billable to customers within one year from the respective balance sheet date.
Most contracts are terminable by the client, either immediately or upon notice. These contracts typically require payment to Covance of expenses to wind down the study, fees earned to date and, in some cases, a termination fee or a payment to Covance of some portion of the fees or profits that could have been earned by Covance under the contract if it had not been terminated early. Termination fees are included in net revenues when realization is assured.
Bad Debts. Covance endeavors to assess and monitor the creditworthiness of its customers to which it grants credit terms in the ordinary course of business. Covance maintains a provision for doubtful accounts relating to amounts due that may not be collected. This bad debt provision is monitored on a monthly basis and adjusted as circumstances warrant. Since the recorded bad debt provision is based upon management's judgment, actual bad debt write-offs may be greater or less than the amount recorded. Historically, bad debt write-offs have not been material.
Taxes. Since Covance conducts operations on a global basis, its effective tax rate has and will continue to depend upon the geographic distribution of its pre-tax earnings among locations with varying tax rates. Covance's profits are further impacted by changes in the tax rates of the various jurisdictions in which Covance operates. In addition, Covance maintains a reserve for unrecognized tax benefits, changes to which could impact Covance's effective tax rate in the period such changes are made.
The Company recognizes a tax benefit from an uncertain tax position only if the Company believes it is more likely than not to be sustained upon examination based on the technical merits of the position. The amount of the accrual for which an exposure exists is measured as the largest amount of benefit determined on a cumulative probability basis that the Company believes is more likely than not to be realized upon ultimate settlement of the position. Components of the reserve are classified as either a current or long-term liability in the consolidated balance sheet based on when the Company expects each of the items to be settled. Covance accrues interest and penalties in relation to unrecognized tax benefits as a component of income tax expense.
Covance maintains a reserve for unrecognized tax benefits for income tax matter exposures such as transfer pricing, nexus, deemed income and research and development credits. As of December 31, 2010, the balance of the reserve for unrecognized tax benefits was $15.0 million, including accrued interest of $1.0 million, which is recorded as a long-term liability in other liabilities on the consolidated balance sheet. As of December 31, 2009, the balance of the reserve for unrecognized tax benefits was $17.2 million, including accrued interest of $1.2 million, of which $0.1 million is recorded as a current liability in accrued expenses and other current liabilities, and $17.1 million is recorded as a long-term liability in other liabilities on the consolidated balance sheet. As of December 31, 2008, the balance of the reserve for unrecognized tax benefits was $11.9 million, including accrued interest of $1.2 million, of which $3.3 million is recorded as a current liability in accrued expenses and other current liabilities, and $8.6 million is recorded as a long-term liability in other liabilities on the consolidated balance sheet. During the year ended December 31, 2010, the reserve for unrecognized tax benefits was reduced by $2.2 million, as tax benefits totaling $7.7 million were recorded relating primarily to the favorable resolution of several income tax audits, partially offset by the accrual of $5.5 million in reserves relating to deemed income, other tax matters and the accrual of interest on matters outstanding. During the year ended December 31, 2009, the reserve for unrecognized tax benefits was increased by $5.3 million as the accrual of additional reserves relating to research and development credits, other income tax positions and the accrual of interest on existing reserves more than offset the recognition of tax benefits due to the settlement of an income tax audit and the expiration of the period of review of filings in certain jurisdictions.
21
Following is a reconciliation of the beginning and ending amount of unrecognized tax benefits, excluding accrued interest for the years ended December 31, 2010, 2009 and 2008:
| (dollars in millions) |
|
|||
|---|---|---|---|---|
Unrecognized tax benefits as of December 31, 2007 |
$ | 7.6 | ||
Additions related to tax positions in the prior year |
2.1 | |||
Additions related to tax positions in the current year |
2.7 | |||
Reductions due to statute expiration |
(1.7 | ) | ||
Unrecognized tax benefits as of December 31, 2008 |
10.7 | |||
Additions related to tax positions in the prior year |
5.3 | |||
Additions related to tax positions in the current year |
2.6 | |||
Reductions due to settlements and payments |
(2.1 | ) | ||
Reductions due to statute expiration |
(0.5 | ) | ||
Unrecognized tax benefits as of December 31, 2009 |
16.0 | |||
Additions related to tax positions in the prior year |
3.8 | |||
Additions related to tax positions in the current year |
1.9 | |||
Reductions due to settlements and payments |
(7.2 | ) | ||
Reductions due to statute expiration |
(0.5 | ) | ||
Unrecognized tax benefits as of December 31, 2010 |
$ | 14.0 | ||
Any future changes in the liability for unrecognized tax benefits, resulting from the recognition of tax benefits, would impact the effective tax rate of Covance. Over the next twelve months, it is reasonably possible that the uncertainty surrounding up to $8.0 million of the reserve for unrecognized tax benefits related to certain income taxes, deemed income, transfer pricing and state tax issues will be resolved as a result of the expiration of the statute of limitations or the conclusion of various federal, state and foreign tax audits.
The following tax years remain open to investigation as of December 31, 2010, for the Company's major jurisdictions:
Tax Jurisdiction
|
Years | |||
|---|---|---|---|---|
US Federal and State |
2005-2010 | |||
United Kingdom |
2008-2010 | |||
Switzerland |
2006-2010 | |||
Germany |
2007-2010 | |||
The Company also maintains a tax reserve related to exposures for non-income tax matters, including value-added tax and state sales and use and other taxes. The balance of this reserve at December 31, 2010 and 2009 was $1.0 million and $0.9 million, respectively, and is recorded as a current liability in accrued expenses and other current liabilities on the consolidated balance sheet.
While Covance believes it has identified all reasonably identifiable exposures and the reserve it has established for identifiable exposures is appropriate under the circumstances, it is possible that additional exposures exist and that exposures will be settled at amounts different than the amounts reserved. It is also possible that changes in facts and circumstances could cause Covance to either materially increase or reduce the carrying amount of its tax reserves.
Covance's policy is to provide income taxes on earnings of foreign subsidiaries only to the extent those earnings are taxable or are expected to be remitted. Covance's historical policy has been to leave its unremitted foreign earnings invested indefinitely outside the United States, except for amounts remitted under the American Jobs Creation Act of 2004. Covance intends to continue to leave its unremitted foreign earnings invested indefinitely outside the United States. As a result, taxes have not been provided on any of the remaining accumulated foreign unremitted earnings totaling approximately $605 million at December 31, 2010.
22
Stock-Based Compensation. The Company sponsors several stock-based compensation plans pursuant to which non-qualified stock options and restricted stock awards are granted to eligible employees.
The grant-date fair value of awards expected to vest is expensed on a straight-line basis over the vesting period of the related awards. The grant-date fair value of stock awards is based upon the underlying price of the stock on the date of grant. The grant-date fair value of stock option awards must be determined using an option pricing model. Option pricing models require the use of estimates and assumptions as to (a) the expected term of the option, (b) the expected volatility of the price of the underlying stock, (c) the risk-free interest rate for the expected term of the option and (d) pre-vesting forfeiture rates. For stock options granted on or subsequent to January 1, 2006, the Company is using the Lattice-Binomial option pricing formula for determining the grant-date fair value of stock option awards, whereas for stock options granted prior to January 1, 2006, the Company used the Black-Scholes-Merton option pricing formula.
The expected term of the option is based upon the contractual term and expected employee exercise and expected post-vesting employment termination behavior. The expected volatility of the price of the underlying stock is based upon the volatility of the Company's stock computed over a period of time equal to the expected term of the option. The risk free interest rate is based upon the implied yields currently available from the U.S. Treasury zero-coupon yield curve for issues with a remaining duration equal to the expected term of the option. Pre-vesting forfeiture rates are estimated based upon past voluntary termination behavior and past option forfeitures.
The following table sets forth the weighted-average assumptions used to calculate the fair value of options granted for the years ended December 31, 2010, 2009 and 2008:
| |
2010 | 2009 | 2008 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Expected stock price volatility |
35% | 36% | 39% | |||||||
Range of risk free interest rates |
0.1% - 3.8% | 0.3% - 2.6% | 1.9% - 3.7% | |||||||
Expected life of options (years) |
4.7 | 4.6 | 4.4 | |||||||
Changes in any of these assumptions could impact, potentially materially, the amount of expense recorded in future periods related to stock-based awards.
As of December 31, 2010, the total unrecognized compensation cost related to non-vested stock options granted was $17.0 million and is expected to be recognized over a weighted average period of 1.9 years, and the total unrecognized compensation cost related to non-vested performance-based shares and restricted stock awards was $31.7 million and is expected to be recognized over a weighted average period of 1.7 years.
Impairment of Assets. Covance reviews its long-lived assets, other than goodwill and other indefinite lived intangible assets, for impairment, when events or changes in circumstances occur that indicate the carrying value of the asset may not be recoverable. The assessment of possible impairment is based upon Covance's judgment of its ability to recover the value of the asset from the expected future undiscounted cash flows of the related operations. Actual future cash flows may be greater or less than estimated. During the third quarter of 2010, Covance determined that long-lived assets used in its North American toxicology operations, located in Chandler, Arizona and Manassas, Virginia with carrying values of $182.7 million and $23.4 million, respectively, were no longer fully recoverable from the cash flows expected from those assets. Accordingly, as of September 30, 2010, Covance recorded an asset impairment charge totaling $119.2 million ($103.0 million of which relates to the Chandler, Arizona assets and $16.2 million relates to the Manassas, Virginia assets), representing the excess of the carrying value of those assets over their respective fair market values.
Covance performs an annual test for impairment of goodwill and other indefinite lived intangible assets during the fourth quarter. This test is performed by comparing, at the reporting unit level, the carrying value of the reporting unit to its fair value. Covance assesses fair value based upon its estimate of the present value of the future cash flows that it expects to be generated by the reporting unit. The test performed for 2010 indicated that no reporting units were at significant risk for impairment. However, changes in expectations as to the present value of a reporting unit's future cash flows might impact subsequent years' assessments of impairment.
23
Defined Benefit Pension Plans. Covance sponsors defined benefit pension plans for the benefit of its employees at three foreign subsidiaries as well as a non-qualified supplemental executive retirement plan and a post-employment retiree health and welfare plan for the benefit of eligible employees at certain U.S. subsidiaries. The measurement of the related benefit obligation and net periodic benefit cost recorded each year is based upon actuarial computations which require the use of judgment as to certain assumptions. The more significant of these assumptions are: (a) the appropriate discount rate to use in computing the present value of the benefit obligation; (b) the expected return on plan assets (for funded plans); and (c) the expected future rate of salary increases (for pay-related plans). Actual results (such as the return on plan assets, future rate of salary increases and plan participation rates) will likely differ from the assumptions used. Those differences, along with changes that may be made in the assumptions used from period to period, will impact the amounts reported in the financial statements and footnote disclosures.
Set forth below is a discussion of the impact that (a) differences between assumed results and actual results and (b) assumption changes have had on our results of operations for the years ended December 31, 2010, 2009 and 2008 and on the financial position of the plans as of December 31, 2010 and 2009 for our United Kingdom defined benefit pension plans (the largest of our defined benefit-type pension plans).
| |
United Kingdom Plans | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (dollars in millions) |
2010 | 2009 | 2008 | 2007 | ||||||||||
Net periodic pension cost |
$ | 1.6 | $ | 2.0 | $ | 3.4 | $ | 4.0 | ||||||
Weighted average assumptions used to determine net periodic pension cost: |
||||||||||||||
Discount rate |
5.75 | % | 6.25 | % | 5.50 | % | 5.25 | % | ||||||
Expected rate of return on assets |
6.75 | % | 6.75 | % | 6.75 | % | 6.75 | % | ||||||
Salary increases |
4.50 | % | 4.25 | % | 4.25 | % | 4.00 | % | ||||||
The decrease in the net periodic benefit cost from period to period is attributable to the following:
| |
United Kingdom Plans | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (dollars in millions) |
2009 to 2010 | 2008 to 2009 | 2007 to 2008 | |||||||
Change in discount rate |
$ | 1.3 | $ | (2.2 | ) | $ | (1.1 | ) | ||
Change in rate of salary increases |
(0.1 | ) | — | (0.1 | ) | |||||
Other, including differences between actual experience and assumptions used |
(1.6 | ) | 1.4 | 0.9 | ||||||
Foreign currency exchange rate changes |
— | (0.6 | ) | (0.3 | ) | |||||
Net change in periodic benefit cost |
$ | (0.4 | ) | $ | (1.4 | ) | $ | (0.6 | ) | |
| |
United Kingdom Plans | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
Weighted average assumptions used to determine benefit obligation: |
|||||||||||
Discount rate |
5.20 | % | 5.75 | % | 6.25 | % | |||||
Salary increases |
4.50 | % | 4.50 | % | 4.25 | % | |||||
24
The change in the projected benefit obligation from period to period is attributable to the following:
| |
United Kingdom Plans | |||||||
|---|---|---|---|---|---|---|---|---|
| (dollars in millions) |
2009 to 2010 | 2008 to 2009 | ||||||
Projected benefit obligation, beginning of year |
$ | 139.9 | $ | 111.9 | ||||
Service/interest cost components of net periodic benefit cost in year |
11.4 | 10.4 | ||||||
Benefits paid |
(2.4 | ) | (4.8 | ) | ||||
Actuarial loss: |
||||||||
Decrease in discount rate |
20.0 | 13.1 | ||||||
Other, including differences between actual experience and assumptions used |
(7.8 | ) | 1.7 | |||||
Foreign currency exchange rate changes |
(4.5 | ) | 7.6 | |||||
Projected benefit obligation, end of year |
$ | 156.6 | $ | 139.9 | ||||
Foreign Currency Risks
Since Covance operates on a global basis, it is exposed to various foreign currency risks. Two specific risks arise from the nature of certain contracts. The first risk can occur when Covance executes contracts with its customers where the contracts are denominated in a currency different than the local currencies of the Covance subsidiaries performing work under the contracts. As a result, revenue recognized for services rendered may be denominated in a currency different from the currencies in which the subsidiaries' expenses are incurred. Fluctuations in exchange rates (from those in effect at the time the contract is executed and pricing is established to the time services are rendered and revenue is recognized) can affect the subsidiary's net revenues and resultant earnings. This risk is generally applicable only to a portion of the contracts executed by Covance's subsidiaries providing clinical services. Historically, fluctuations in exchange rates from those in effect at the time contracts were executed have not had a material effect upon Covance's consolidated financial results. See "Risk Factors".
We also have other cross-currency contracts executed by other Covance subsidiaries where the foreign currency amounts billed are determined by converting local currency revenue amounts to the contract billing currency using the exchange rates in effect at the time services are rendered. These contracts do not give rise to foreign currency denominated revenue and local currency denominated expenses, but they do give rise to a second type of risk. This second type of risk results from the passage of time between the invoicing of customers under both of these types of contracts and the ultimate collection of customer payments against such invoices. Because such invoices are denominated in a currency other than the subsidiary's local currency, Covance recognizes a receivable at the time of invoicing for the local currency equivalent of the foreign currency invoice amount as of the invoice date. Subsequent changes in exchange rates from the time the invoice is prepared to the time payment from the customer is received will result in Covance receiving either more or less in local currency than the local currency equivalent of the invoice amount at the time the invoice was prepared and the receivable was recorded. This difference is recognized by Covance as a foreign currency transaction gain or loss, as applicable, in the consolidated statements of income.
Finally, Covance's consolidated financial statements are denominated in U.S. dollars. Accordingly, changes in exchange rates between the applicable foreign currency and the U.S. dollar will affect the translation of each foreign subsidiary's financial results into U.S. dollars for purposes of reporting Covance's consolidated financial results. The process by which each foreign subsidiary's financial results are translated into U.S. dollars is as follows: income statement accounts are translated at average exchange rates for the period; balance sheet asset and liability accounts are translated at end of period exchange rates; and equity accounts are translated at historical exchange rates. Translation of the balance sheet in this manner affects the stockholders' equity account, referred to as the cumulative translation adjustment account. This account exists only in the foreign subsidiary's U.S. dollar balance sheet and is necessary to keep the foreign balance sheet stated in U.S. dollars in balance. At December 31, 2010, accumulated other comprehensive income on the consolidated balance sheet includes the cumulative translation account balance of $28.7 million.
25
Operating Expenses and Reimbursable Out-of-Pockets
Covance segregates its recurring operating expenses among four categories: cost of revenue; reimbursable out-of-pocket expenses; selling, general and administrative expenses; and depreciation and amortization. Cost of revenue includes direct labor and related benefits, other direct costs, shipping and handling fees, and an allocation of facility charges and information technology costs, and excludes depreciation and amortization. Cost of revenue, as a percentage of net revenues, tends and is expected to fluctuate from one period to another, as a result of changes in labor utilization and the mix of service offerings involving thousands of studies conducted during any period of time. Selling, general and administrative expenses consist primarily of administrative payroll and related benefit charges, advertising and promotional expenses, administrative travel and an allocation of facility charges and information technology costs, and excludes depreciation and amortization.
In connection with the management of multi-site clinical trials, Covance pays on behalf of its customers fees to investigators, volunteers and other out-of-pocket costs (such as for travel, printing, meetings, couriers, etc.), for which it is reimbursed at cost, without mark-up or profit. Investigator fees are not reflected in total revenues or expenses where Covance acts in the capacity of an agent on behalf of the pharmaceutical company sponsor, passing through these costs without risk or reward to Covance. All other out-of-pocket costs are included in total revenues and expenses.
Results of Operations
Year Ended December 31, 2010 Compared with Year Ended December 31, 2009. Net revenues increased 3.1%, or 2.6% excluding the favorable impact of foreign exchange rate variances between both periods, to $1.93 billion for 2010 from $1.87 billion for 2009. Net revenues from Covance's early development segment increased 6.1%, or 6.6% excluding the unfavorable impact of foreign exchange rate variances between both periods. Growth in the early development segment was led by our genomics service offering, which was expanded when we acquired Merck's gene expression laboratory in August 2009, our European toxicology and pharmaceutical chemistry services (due in part to the inclusion of two months of results of the sites acquired in October 2010 from sanofi-aventis), as well as from growth in our nutritional chemistry and early clinical development services. Partially offsetting this growth was lower levels of study activity in our North American toxicology services, as we experienced lower market demand for these services from our biopharmaceutical clients. Net revenues from Covance's late-stage development segment grew 0.9%, but decreased 0.4% excluding the favorable impact of foreign exchange rate variances between both periods. Growth in our clinical development services, resulting from increased study activity, was largely offset by a reduction in net revenues resulting from the sale of our IVR service offering in August 2009, combined with a reduction in periapproval services net revenues from reduced trial activity.
Cost of revenue increased 5.6% to $1.35 billion or 70.0% of net revenues for the year ended December 31, 2010 as compared to $1.28 billion or 68.4% of net revenues for the corresponding 2009 period. Gross margins decreased by 160 basis points to 30.0% for 2010 from 31.6% for 2009 as declines in North American toxicology services and an unfavorable shift in the mix of tests performed in our central laboratory more than offset an expansion in gross margins in other early development and certain late-stage development services resulting from increased revenues, as mentioned above.
Overall, selling, general and administrative expenses increased 13.6% to $307.4 million for 2010 from $270.6 million for 2009. As a percentage of net revenues, selling, general and administrative expenses increased 150 basis points to 16.0% in 2010 from 14.5% in 2009. This increase was driven by the inclusion in selling, general and administrative expenses of $25.1 million (or 1.3% of net revenue) in costs associated with two restructuring actions taken in 2010. During the fourth quarter, $18.4 million in costs were incurred in connection with initiatives to rationalize capacity, reduce the cost of overhead and support functions and to streamline processes ("fourth quarter restructuring initiatives"), $18.1 million (or 0.9% of net revenues) of which have been included in selling, general and administrative expenses. During the second quarter of 2010, the Company announced plans to reduce costs, primarily by closing and transitioning work conducted at its Austin, Texas Phase I clinic and Kalamazoo, Michigan research products facility into other more efficient
26
locations. These actions were completed during 2010 and resulted in costs totaling $9.6 million, of which $7.0 million has been included in selling, general and administrative expense. Selling, general and administrative expenses as a percentage of net revenues can and does vary depending on the timing and nature of various professional fees and other discretionary spending.
Depreciation and amortization increased 12.9% to $103.0 million or 5.4% of net revenues for 2010 from $91.3 million or 4.9% of net revenues for 2009. Depreciation and amortization during the 2010 period includes $2.9 million (or 0.1% of net revenues) in accelerated depreciation associated with the restructuring initiatives described above. The balance of the increase results from depreciation on assets placed in service over the last year.
Income from operations decreased 79.2% to $47.5 million or 2.5% of net revenues for 2010 from $228.6 million or 12.2% of net revenues for the corresponding 2009 period. The 2010 period includes asset impairment charges of $119.2 million (or 6.2% of net revenues) associated with long lived assets in Chandler, Arizona and Manassas, Virginia, which are included in our early development segment results. Additionally, the 2010 period includes costs associated with the restructuring initiatives described above totaling $28.0 million (or 1.5% of net revenues), of which $14.1 million is included in our early development segment results, $7.3 million in our late-stage segment results and $6.6 million in our Corporate expenses.
Results of operations from Covance's early development segment for the year ended December 31, 2010 decreased $131.7 million to a loss of $32.0 million as compared to income of $99.7 million for the corresponding 2009 period. As a percentage of net revenues, early development income (loss) from operations declined from 12.6% of early development net revenues for 2009 to (3.8)% of net revenues in the corresponding 2010 period. Results of operations for the year ended December 31, 2010 includes asset impairment charges totaling $119.2 million (or 14.2% of segment net revenues) and restructuring charges totaling $14.1 million (1.7% of segment revenues). Additionally, early development operating margins were impacted by weakness in North American toxicology and certain chemistry services which more than offset margin expansion in other segment service offerings.
Income from operations from Covance's late-stage development segment for the year ended December 31, 2010 decreased 11.4% or $29.0 million to $225.5 million as compared to $254.5 million for the corresponding 2009 period. As a percentage of net revenues, late-stage development income from operations decreased 290 basis points from 23.7% of late-stage development net revenues in 2009 to 20.8% of net revenues in the corresponding 2010 period driven by a shift in the mix of tests performed in our central laboratory services, the reductions in net revenues in our periapproval services on reduced trial activity, and the sale of our IVR service offering in August 2009, only partially offset by an increase in clinical development net revenues explained above. In addition, income from operations for the year ended December 31, 2010 includes restructuring charges of $7.3 million (or 0.7% of net revenues).
Corporate expense increased $20.4 million to $146.0 million or 7.6% of net revenues for the year ended December 31, 2010, as compared to $125.6 million or 6.7% of net revenues for the corresponding 2009 period. The increase was driven by investments to provide strategic partnering and integrated services, as well as investments in our infrastructure to enhance our ability to manage future growth. In addition, corporate expenses for the year ended December 31, 2010 includes restructuring charges of $6.6 million (or 0.3% of net revenues). Included in corporate expense is stock-based compensation expense of $32.3 million or 1.7% of net revenues for the year ended December 31, 2010, an increase of $5.4 million as compared to $26.9 million or 1.4% of net revenues for the corresponding 2009 period.
Other expense (income) decreased $12.9 million to an expense of $3.7 million for the year ended December 31, 2010 as compared to income of $9.2 million for the corresponding 2009 period. The 2009 period included a $9.0 million pre-tax gain on the sale of Covance's IVR service offering and a $0.7 million pre-tax gain from the receipt of contingent consideration on transferred backlog from the November 2007 sale of Covance's cardiac safety service offering. Net foreign exchange transaction losses increased $3.4 million to $3.6 million for 2010 from $0.2 million for the corresponding 2009 period. Net interest expense decreased $0.2 million for 2010 as compared to the prior year period.
27
Covance's effective tax rate for the year ended December 31, 2010 was a benefit of 54.0% compared to an expense of 26.4% for the corresponding 2009 period. Covance's effective tax rate for the 2010 period includes the tax impact of the asset impairment and restructuring charges totaling $53.7 million. The Company also recorded net income tax benefits totaling $17.3 million, primarily in connection with the favorable resolution of two income tax audits, which resulted in a $7.5 million reduction in the Company's reserve for unrecognized income tax benefits as well as the recognition of $7.1 million in additional unrecognized benefits. The 2009 period included a $2.1 million tax benefit resulting from the completion of an income tax audit and the recognition of tax benefits in jurisdictions where the period of review of filings had expired, as well as the tax impact of the gain on the sale of IVR. The 2010 period also reflects a shift in the mix of our pre-tax earnings across various tax jurisdictions to locations with lower rates and the impact of tax planning initiatives.
Covance has a 47% minority equity position in Noveprim Limited ("Noveprim"), a supplier of research products. During the years ended December 31, 2010 and 2009, Covance recognized income of $0.8 million and $0.9 million, respectively, representing its share of Noveprim's earnings, net of the elimination of profit on inventory purchased from Noveprim and still on hand at Covance at December 31, 2010 and 2009.
Net income of $68.3 million for the year ended December 31, 2010 decreased $107.6 million or 61.2% as compared to $175.9 million for the corresponding 2009 period. Net income for the 2010 period includes the after tax impact of the asset impairment and restructuring charges totaling $93.6 million as well as the favorable tax benefits of $17.3 million discussed above.
Year Ended December 31, 2009 Compared with Year Ended December 31, 2008. Net revenues increased 8.1%, or 11.2% excluding the unfavorable impact of foreign exchange rate variances between both periods, to $1.87 billion for 2009 from $1.73 billion for 2008. Net revenues from Covance's early development segment decreased 6.3%, or 2.7% excluding the unfavorable impact of foreign exchange rate variances between both periods. Softness in the early development segment was driven by a 7.8% decrease in net revenues in our preclinical laboratory services, resulting from lower levels of study activity, delays of scheduled study starts and lower pricing, as we have experienced lower market demand from our biopharmaceutical clients in this and other early development service offerings. Net revenues from Covance's late-stage development segment grew 21.8%, or 24.4% excluding the unfavorable impact of foreign exchange rate variances between both periods. Growth was broad based across the late-stage development segment, led by strong performance in our central laboratory services, where net revenues grew 30.9% on increased investigator and patient enrollment and an overall higher level of study activity. Also driving growth in the late-stage development segment was a strong performance in our clinical development services, where net revenues grew 14.5% on increased study activity, and improved performance in our market access services, where net revenues grew 24.7%.
Cost of revenue increased 11.8% to $1.28 billion or 68.4% of net revenues for the year ended December 31, 2009 as compared to $1.14 billion or 66.1% of net revenues for the corresponding 2008 period. Gross margins decreased by 230 basis points to 31.6% for 2009 from 33.9% for 2008 as the earnings impact from a decline in early development net revenues more than offset strength in late-stage development net revenues.
Overall, selling, general and administrative expenses increased 8.2% to $270.6 million for 2009 from $250.2 million for 2008. Selling, general and administrative expenses were 14.5% of net revenues for each of the years ended December 31, 2009 and 2008.
Depreciation and amortization increased 27.6% to $91.3 million or 4.9% of net revenues for 2009 from $71.6 million or 4.1% of net revenues for 2008 as a result of the commencement of depreciation of assets placed into service during 2009 upon the completion of several large facility construction projects and generally higher levels of capital spending related to other assets placed into service over the last twelve to eighteen months.
Income from operations decreased 13.3% to $228.6 million or 12.2% of net revenues for 2009 from $263.7 million or 15.3% of net revenues for the corresponding 2008 period as a result of the reduction in gross margins from the decline in early development net revenues and the increase in depreciation and amortization, which more than offset the increase in late-stage development net revenues and gross margins.
28
Income from operations from Covance's early development segment for the year ended December 31, 2009 decreased 51.4% or $105.7 million to $99.7 million as compared to $205.4 million for the corresponding 2008 period. As a percentage of net revenues, early development income from operations declined from 24.3% of early development net revenues for 2008 to 12.6% of net revenues in the corresponding 2009 period. During the year ended December 31, 2009, the softness in pre-clinical and other early development net revenues explained above, coupled with start-up losses in our new Chandler, Arizona pre-clinical facility, staffing levels above demand during most of the year, underutilized capacity, and the launch and growth of our service offerings at the Greenfield, Indiana site purchased from Eli Lilly in the fourth quarter of 2008 were the primary drivers of the reduction in operating income.
Income from operations from Covance's late-stage development segment for the year ended December 31, 2009 increased 49.6% or $84.4 million to $254.5 million as compared to $170.1 million for the corresponding 2008 period. As a percentage of net revenues, late-stage development income from operations increased 440 basis points from 19.3% of late-stage development net revenues in 2008 to 23.7% of net revenues in the corresponding 2009 period, driven by the strength in central laboratory, clinical development and market access services net revenues explained above, coupled with increased efficiency gained from leveraging existing support functions across a larger base of revenues.
Corporate expense increased $13.7 million to $125.6 million or 6.7% of net revenues for the year ended December 31, 2009, from $111.9 million or 6.5% of net revenues for the corresponding 2008 period, driven by investments in our infrastructure to enhance our ability to manage future growth. Also included in corporate expense is stock-based compensation expense of $26.9 million or 1.4% of net revenues for the year ended December 31, 2009, an increase of $1.4 million as compared to $25.5 million or 1.5% of net revenues for the corresponding 2008 period.
Other income, net decreased $1.4 million to $9.2 million for the year ended December 31, 2009 as compared to $10.7 million for the corresponding 2008 period. The 2009 period includes a $9.0 million pre-tax gain on the sale of Covance's IVR service offering and a $0.7 million pre-tax gain from the receipt of contingent consideration on transferred backlog from the November 2007 sale of Covance's cardiac safety service offering. The 2008 period includes a $4.1 million pre-tax gain from the receipt of contingent consideration on transferred backlog from the sale of our cardiac safety service offering. Interest income, net, decreased $6.7 million as compared to the prior period, primarily due to lower interest rates on invested cash balances.
Covance's effective tax rate for the year ended December 31, 2009 was 26.4% compared to 28.9% for the corresponding 2008 period. The 2009 period includes a $2.1 million tax benefit resulting from the settlement of an income tax audit and the recognition of previously unrecognized tax benefits in jurisdictions where the period of review of filings has expired. The remainder of the year-over-year decrease in Covance's effective tax rate is attributable to a number of factors, including the mix of our pre-tax earnings across various tax jurisdictions, the tax impact of gains on the sale of businesses, and tax planning initiatives.
Covance has a 47% minority equity position in Noveprim Limited ("Noveprim"), a supplier of research products. During the years ended December 31, 2009 and 2008, Covance recognized $0.9 million and $1.4 million, respectively, representing its share of Noveprim's earnings, less an elimination of profit on inventory purchased from Noveprim and still on hand at Covance at December 31, 2009 and 2008.
Covance has a minority equity position (less than 20%) in BioClinica, Inc. ("BIOC"), formerly known as Bio Imaging Technologies, Inc. BIOC uses proprietary medical imaging technologies to process and analyze medical images, and also provides other services, including the data-basing and regulatory submission of medical images, quantitative data and text. During the year ended December 31, 2008, Covance recognized income of $0.5 million representing its pro-rata share of BIOC's earnings. In the fourth quarter of 2008, Covance suspended the use of the equity method of accounting for its investment in BIOC as its ownership interest fell below 20% and it could no longer exercise significant influence over BIOC's operations. In the fourth quarter of 2008, Covance began accounting for its investment in BIOC as an available-for-sale security. Accordingly, Covance no longer records in equity earnings its pro-rata share of BIOC results, but rather, reflects the fair value of its investment in BIOC on its consolidated balance sheet.
29
Net income of $175.9 million for the year ended December 31, 2009 decreased $20.9 million or 10.6% as compared to $196.8 million for the corresponding 2008 period.
Quarterly Results
Covance's quarterly operating results are subject to variation, and are expected to continue to be subject to variation, as a result of factors such as (1) delays in initiating or completing significant drug development trials, (2) termination or reduction in size of drug development trials, (3) acquisitions and divestitures, (4) changes in the mix of our services, and (5) exchange rate fluctuations. Delays and terminations of trials are often the result of actions taken by Covance's customers or regulatory authorities and are not typically controllable by Covance. Since a large amount of Covance's operating costs are relatively fixed while revenue is subject to fluctuation, moderate variations in the commencement, progress or completion of drug development trials may cause significant variations in quarterly results.
The following table presents unaudited quarterly operating results of Covance for each of the eight most recent fiscal quarters during the period ended December 31, 2010. In the opinion of Covance, the information in the table below has been prepared on the same basis as the audited consolidated financial statements included elsewhere in this Annual Report and reflects all adjustments (consisting only of normal recurring adjustments) necessary for a fair presentation of results of operations for those periods. This quarterly financial data should be read in conjunction with the audited consolidated financial statements included elsewhere in this Annual Report. Operating results for any quarter are not necessarily indicative of the results that may be reported in any future period.
| |
Quarter Ended | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Dec. 31, 2010 |
Sep. 30, 2010 |
June 30, 2010 |
Mar. 31, 2010 |
Dec. 31, 2009 |
Sep. 30, 2009 |
June 30, 2009 |
Mar. 31, 2009 |
|||||||||||||||||||
| |
(Dollars in thousands, except per share data) |
||||||||||||||||||||||||||
Net revenues |
$ | 491,513 | $ | 477,022 | $ | 475,171 | $ | 481,924 | $ | 485,065 | $ | 475,284 | $ | 466,049 | $ | 441,236 | |||||||||||
Reimbursable out-of-pocket expenses |
27,942 | 36,258 | 25,548 | 23,095 | 22,263 | 22,282 | 23,226 | 27,221 | |||||||||||||||||||
Total revenues |
519,455 | 513,280 | 500,719 | 505,019 | 507,328 | 497,566 | 489,275 | 468,457 | |||||||||||||||||||
Costs and expenses: |
|||||||||||||||||||||||||||
Cost of revenue |
346,924 | 337,698 | 331,360 | 332,516 | 337,896 | 324,311 | 313,210 | 301,725 | |||||||||||||||||||
Reimbursable out-of-pocket expenses |
27,942 | 36,258 | 25,548 | 23,095 | 22,263 | 22,282 | 23,226 | 27,221 | |||||||||||||||||||
Selling, general and administrative |
89,810 | 70,731 | 75,045 | 71,800 | 67,544 | 69,526 | 69,569 | 63,954 | |||||||||||||||||||
Depreciation and amortization |
25,919 | 26,105 | 26,256 | 24,744 | 24,753 | 23,649 | 23,273 | 19,614 | |||||||||||||||||||
Asset impairment charges |
— | 119,229 | — | — | — | — | — | — | |||||||||||||||||||
Total |
490,595 | (a) | 590,021 | (c) | 458,209 | (e) | 452,155 | 452,456 | 439,768 | 429,278 | 412,514 | ||||||||||||||||
Income (loss) from operations |
28,860 | (a) | (76,741 | )(c) | 42,510 | (e) | 52,864 | 54,872 | 57,798 | 59,997 | 55,943 | ||||||||||||||||
Other expense (income), net |
1,484 | 445 | 684 | 1,088 | 296 | (9,748 | )(f) | 746 | (h) | (529 | ) | ||||||||||||||||
Income (loss) before taxes and equity investee earnings |
27,376 | (a) | (77,186 | )(c) | 41,826 | (e) | 51,776 | 54,576 | 67,546 | (f) | 59,251 | (h) | 56,472 | ||||||||||||||
Tax expense (benefit) |
(1,121 | )(b) | (46,003 | )(d) | 10,415 | 13,054 | 13,820 | 16,650 | (f),(g) | 16,051 | (h) | 16,349 | |||||||||||||||
Equity investee earnings (loss) |
(119 | ) | 253 | 254 | 419 | 779 | 166 | (210 | ) | 172 | |||||||||||||||||
Net income (loss) |
$ | 28,378 | (a),(b) | $ | (30,930 | )(c),(d) | $ | 31,665 | (e) | $ | 39,141 | $ | 41,535 | $ | 51,062 | (f),(g) | $ | 42,990 | (h) | $ | 40,295 | ||||||
Basic earnings (loss) per share |
$ | 0.46 | $ | (0.49 | ) | $ | 0.50 | $ | 0.62 | $ | 0.65 | $ | 0.80 | $ | 0.67 | $ | 0.63 | ||||||||||
Diluted earnings (loss) per share |
$ | 0.45 | (a),(b) | $ | (0.49 | )(c),(d) | $ | 0.49 | (e) | $ | 0.60 | $ | 0.64 | $ | 0.79 | (f),(g) | $ | 0.67 | (h) | $ | 0.63 | ||||||
- (a)
- Amounts
include restructuring costs of $18,956 ($14,056 net of tax or $0.22/diluted share).
- (b)
- Amounts
include favorable income tax items totaling $6,946 (or $0.11/diluted share) primarily associated with the favorable resolution of an income tax
inquiry.
- (c)
- Amounts
include asset impairment charges and restructuring costs totaling $120,569 ($74,753 net of tax or $1.16/diluted share).
- (d)
- Amounts
include favorable income tax items totaling $10,352 (or $0.16/diluted share) associated with the favorable resolution of an income tax inquiry and
the recognition of previously unrecognized benefits.
- (e)
- Amounts
include restructuring costs of $7,734 ($4,795 net of tax or $0.07/diluted share).
- (f)
- Amounts
include a $9,026 gain ($5,867 or $0.09/diluted share, net of tax totaling $3,159) from the sale of Covance's IVR Services.
- (g)
- Amounts
include a reduction in income tax reserves resulting from favorable income tax developments totaling $2,072 (or $0.03/diluted share).
- (h)
- Amounts include $655 of additional proceeds ($426 or $0.01/diluted share, net of tax totaling $229) from the 2007 sale of Covance's cardiac safety service offering representing contingent consideration related to transferred backlog.
30
Liquidity and Capital Resources
Covance has a centralized cash management function. In the United States, cash received from operations is swept daily to a centrally managed concentration account, while cash disbursements for operations are funded as needed from the concentration account. Outside of the United States, cash balances are generally pooled by currency in order to facilitate cash management and improve investment returns. As in the United States, cash balances are generally maintained in the functional currency of the operating unit.
Cash and cash equivalents at December 31, 2010 and 2009 were $377.2 million and $289.5 million, respectively. Amounts are principally invested in short-term money market funds and bank deposits with major financial institutions which carry a Moody's rating of A1 P1 or better. Covance's expected primary cash needs on both a short and long-term basis are for capital expenditures, expansion of services, possible future acquisitions, geographic expansion, share repurchases, working capital and other general corporate purposes. On October 26, 2010, Covance amended its credit facility which was due to expire in June, 2012. The amended credit agreement (the "Credit Agreement") provides for a revolving credit facility of up to $250 million, which may be increased to $300 million at Covance's election, and a term loan commitment of $100 million. At December 31, 2010, there were $35.0 million of outstanding borrowings and $1.4 million of outstanding letters of credit under the revolving credit facility and $97.5 million of outstanding debt under the term loan facility. Interest on all outstanding borrowings under the Credit Agreement is computed in accordance with the terms of the Credit Agreement and is presently based upon the London Interbank Offered Rate plus a margin of 200 basis points. Costs associated with the Credit Agreement, which expires in October 2015, consisted primarily of bank and legal fees totaling $1.7 million and are being amortized over the five year term. The Credit Agreement contains various financial and other covenants and is collateralized by guarantees of certain of Covance's domestic subsidiaries and a pledge of 65 percent of the capital stock of certain of Covance's foreign subsidiaries. The Company pays a commitment fee of 30 basis points on the undrawn balance of the revolving credit facility, which totaled approximately $0.5 million and $0.2 million during the years ended December 31, 2010 and 2009, respectively. Interest on outstanding borrowings approximated 2.38% per annum during 2010 and 1.63% per annum during 2009. At December 31, 2010, Covance was in compliance with the terms of the Credit Agreement. Covance believes cash on hand plus cash from operations and available borrowings under the Credit Agreement will provide sufficient liquidity for the foreseeable future.
During the year ended December 31, 2010, Covance's operations provided net cash of $334.4 million, an increase of $75.3 million from the corresponding 2009 period. The change in net operating assets, net of businesses acquired, provided $82.6 million in cash during 2010, primarily due to a reduction in accounts receivable coupled with an increase in accrued liabilities, unearned revenue and income taxes payable. The change in net operating assets, net of businesses acquired and sold, used $58.5 million in cash during 2009, primarily due to an increase in accounts receivable and inventory, partially offset by a decrease in unbilled services. Covance's ratio of current assets to current liabilities was 1.89 at December 31, 2010 and 2.18 at December 31, 2009.
Investing activities for the year ended December 31, 2010 used $147.2 million, compared to $148.8 million for the corresponding 2009 period. Capital spending for 2010 totaled $126.3 million and was primarily for significant ongoing information technology projects, expansion of preclinical facilities in China, outfitting of new facilities, upgrade of existing equipment, and the purchase of new equipment, hardware and software. Approximately $55.8 million of capital spending in 2010 represents expenditures associated with assets that have not yet been placed in service at December 31, 2010. Capital spending for the corresponding 2009 period totaled $131.1 million, and was primarily for significant ongoing information technology projects, expansion of preclinical facilities in North America and Europe, outfitting of new facilities, upgrade of existing equipment, and the purchase of new equipment, hardware and software.
31
Investing activities for 2010 also included the acquisition in October 2010 of research and development facilities located in Porcheville, France and Alnwick, UK from sanofi-aventis for a cash payment of $27.9 million ($21.0 million net of cash acquired). Transaction related costs of approximately $2.6 million were included in selling, general and administrative expense in the period incurred. The acquisition of these facilities provides Covance with new capabilities in CMC (Chemistry, Manufacturing and Controls) services, including preformulation, drug formulation, preclinical and early-stage clinical API (Active Pharmaceutical Ingredient) manufacturing, and radiolabeled chemistry. Pursuant to the asset purchase agreement, Covance will provide services to sanofi-aventis at these facilities over a period of 5 years for $350 million. The tangible assets acquired consisted of property and equipment and are included in Covance's consolidated financial statements beginning in October, 2010 based on their estimated fair value of $27.9 million as determined in the preliminary purchase price allocation, partially offset by certain employee related liabilities of $6.9 million assumed in the transaction. The Company has not yet received the final third-party valuation of the assets acquired. As such, this allocation is preliminary pending the outcome of the final valuation. Results of operations for the sites acquired from sanofi-aventis are reported in Covance's early development segment beginning in November 2010. In addition to the acquisition and provision of services at these facilities, Covance and sanofi-aventis also entered into a 10-year strategic alliance, pursuant to which Covance has become sanofi-aventis' R&D partner providing drug development services to sanofi-aventis in amounts ranging from $0.9 billion to $1.9 billion over the term of the agreement. See Note 6 to the audited consolidated financial statements included elsewhere in this Annual Report.
Included in investing activities for 2009 were the acquisition of two businesses totaling $29.1 million ($28.1 million net of cash acquired), and $10.4 million in proceeds from the sale of businesses, consisting of $9.7 million in net proceeds from sale of our IVR service offering and $0.7 million of additional proceeds from contingent consideration related to transferred backlog from the November 2007 sale of our cardiac safety service offering.
In March 2009, Covance acquired 100% of the stock of Swiss Pharma Contract Ltd. ("Swiss Pharma"), and its 50-bed clinical research facility based in Basel, Switzerland, for total consideration of $22.8 million, as to which $19.4 million ($18.3 million net of cash acquired) was paid at closing with the balance contingently payable based upon the achievement of certain performance based milestones through 2011. Transaction related costs of $0.5 million were expensed as incurred and included in selling, general and administrative expense. Net tangible and intangible assets acquired in the acquisition were included in Covance's consolidated financial statements beginning in March 2009 based on their estimated fair values of $3.0 million and $1.8 million, respectively. The remaining purchase price of $18.0 million represents goodwill. Results of operations for Swiss Pharma are reported in Covance's early development segment beginning in March 2009. See Note 6 to the audited consolidated financial statements included elsewhere in this Annual Report.
In August 2009, Covance acquired certain assets and capabilities of Merck & Co., Inc.'s ("Merck") Gene Expression Laboratory ("GEL") located in Seattle, Washington for a cash payment of $9.75 million. Transaction related costs of $0.7 million were expensed as incurred and included in selling, general and administrative expense. This acquisition expanded Covance's footprint in the genomics testing market and added capabilities in genomics testing and personalized medicine. The tangible assets acquired consisted of property and equipment and are included in Covance's consolidated financial statements beginning in August 2009 at their estimated fair value of $5.5 million. The remaining purchase price of $4.2 million represents the fair value of the acquired assembled workforce, which is a component of goodwill. Results of operations for GEL are reported in Covance's early development segment beginning in August 2009. Covance and Merck also entered into an agreement pursuant to which Merck committed to purchase at least $145.0 million of services from Covance over a five year period.
In August 2009, Covance sold its IVR service offering, part of Covance's late-stage development segment, to Phase Forward (which was subsequently acquired by Oracle Corporation) for net cash proceeds totaling $9.7 million and recorded a pre-tax gain of $9.0 million ($5.9 million after tax) on the sale. In addition, Covance and Phase Forward entered into a five-year marketing agreement pursuant to which Covance is entitled to receive a referral fee for providing Phase Forward's IVR services to its clients.
32
Financing activities for the year ended December 31, 2010 used $105.0 million and included the purchase into treasury of 4,753,589 shares of common stock in connection with a $250 million buyback program authorized by Covance's Board of Directors in September 2010, and $6.4 million for the purchase into treasury of 115,224 shares in connection with employee benefit plans, for an aggregate cost of $256.4 million. Partially offsetting these items were $132.5 million of net borrowings under the Credit Agreement used for the repurchase of shares under the Company's share buyback program, $10.0 million in proceeds from the exercise of stock options, $7.2 million from employee contributions to the Company's employee stock purchase plan and $1.6 million in excess tax benefits realized on the exercise of stock options. Financing activities for the year ended December 31, 2009 used $49.6 million and included the repayment of $50.0 million of borrowings under the previous credit facility and the repayment of the entire $5.4 million balance of mortgage debt assumed in connection with the Swiss Pharma acquisition. Additionally, $4.8 million was used to purchase into treasury 106,570 shares of common stock in connection with employee benefit plans. Partially offsetting these items were proceeds of $3.1 million received from the exercise of stock options, $6.8 million received from employee contributions to the Company's employee stock purchase plan, and $0.8 million in excess tax benefits realized on the exercise of stock options.
The effect of exchange rate changes on cash for the year ended December 31, 2010 was an increase of $5.6 million versus $7.4 million for the year ended December 31, 2009. Covance's cash balances increased by $87.8 million during 2010.
The table below sets forth Covance's contractual obligations. A full description of the Company's debt obligations is contained in Note 8 to the audited consolidated financial statements included elsewhere in this Annual Report. Covance is obligated under non-cancelable operating leases, primarily for offices and laboratory facilities. Covance is also obligated under outsourcing agreements related to certain aspects of its information technology and human resources support functions and purchase commitments primarily related to ongoing facility construction projects, both of which are reflected under the caption purchase obligations in the table below. Actual amounts paid under these outsourcing agreements could be higher or lower than the amounts shown below as a result of changes in volume and other variables. In addition, early termination of these outsourcing agreements by Covance could result in the payment of termination fees which are not reflected in the table below. See Note 11 to the audited consolidated financial statements included elsewhere in this Annual Report.
| |
Payments due by period | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contractual Obligations(a) | Total | <1 Year | 1-3 Years | 3-5 Years | > 5 Years | ||||||||||||
| |
(Dollars in thousands) |
||||||||||||||||
Long Term Debt |
$ |
97,500 |
$ |
10,000 |
$ |
20,000 |
$ |
67,500 |
— |
||||||||
Operating Leases |
145,841 | 28,481 | 46,468 | 21,015 | $ | 49,877 | |||||||||||
Purchase Obligations |
100,722 | 35,810 | 47,423 | 15,392 | 2,097 | ||||||||||||
Total |
$ | 344,063 | $ | 74,291 | $ | 113,891 | $ | 103,907 | $ | 51,974 | |||||||
- (a)
- Excludes $15.0 million, including 1.0 million in interest, related to a reserve for unrecognized tax benefits, as the cash settlement date cannot be reasonably estimated.
Off-Balance Sheet Arrangements
At December 31, 2010 and 2009, Covance was not a party to any off-balance sheet arrangements as defined by Regulation S-K Item 303(a)(4)(i), promulgated under the Exchange Act.
33
Inflation
While most of Covance's net revenues are earned under contracts, the long-term contracts (those in excess of one year) generally include an inflation or cost of living adjustment for the portion of the services to be performed beyond one year from the contract date. As a result, Covance believes that the effects of inflation generally do not have a material effect on its operations or financial condition.
Recently Issued Accounting Standards
None.
Forward Looking Statements. Statements in this Management's Discussion and Analysis of Financial Condition and Results of Operations, as well as in certain other parts of this Annual Report on Form 10-K that look forward in time, are forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements concerning plans, objectives, goals, strategies, future events or performance, expectations, predictions, and assumptions and other statements which are other than statements of historical facts. All such forward-looking statements are based on the current expectations of management and are subject to, and are qualified by, risks and uncertainties that could cause actual results to differ materially from those expressed or implied by those statements. These risks and uncertainties include, without limitation, competitive factors, outsourcing trends in the pharmaceutical industry, levels of industry research and development spending, the Company's ability to continue to attract and retain qualified personnel, the fixed price nature of contracts or the loss or delay of large studies, risks associated with acquisitions and investments, the Company's ability to increase order volume, the pace of translation of orders into revenue in late-stage development services, testing mix and geographic mix of kit receipts in central laboratories, fluctuations in currency exchange rates, and other factors described in Covance's filings with the Securities and Exchange Commission, including, without limitation, this Annual Report on Form 10-K.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
For the year ended December 31, 2010, approximately 44% of our net revenues were derived from our operations outside the United States. We do not engage in material or long-term derivative or hedging activities related to our potential foreign exchange exposures. See "Management's Discussion and Analysis of Financial Condition and Results of Operations—Foreign Currency Risks" for a more detailed discussion of our foreign currency risks and exposures.
Covance's short-term investments are with major financial institutions which carry a Moody's rating of A1 P1 or better. These short-term investments are in bank deposits and money market funds which can be readily purchased and sold using established markets. Covance's cash investment policy is to maximize utilization of excess cash according to the following specific criteria (in order of priority): (1) preserve capital (minimize financial market risk); (2) maintain liquidity; (3) manage foreign exchange rate exposure (internal hedging); (4) maximize rate of return; and (5) enhance strategic relationships with select financial institutions. Covance also has strong operating cash flow and ready access to credit available under its Credit Agreement, as evidenced by its ability to secure the amendment to its previous $150 million credit facility, which was due to expire in June, 2012. The Credit Agreement consists of a revolving credit facility of up to $250 million, which may be increased to $300 million at Covance's election, and a term loan commitment of $100 million.
34
Item 8. Financial Statements and Supplementary Data
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| |
Page | |||
|---|---|---|---|---|
Management's Report on Consolidated Financial Statements and Internal Control |
36 | |||
Internal Control Report of Ernst & Young LLP—Independent Registered Public Accounting Firm |
37 | |||
Report of Ernst & Young LLP—Independent Registered Public Accounting Firm |
38 | |||
Consolidated Balance Sheets—December 31, 2010 and 2009 |
39 | |||
Consolidated Statements of Income—Years ended December 31, 2010, 2009 and 2008 |
40 | |||
Consolidated Statements of Cash Flows—Years ended December 31, 2010, 2009 and 2008 |
41 | |||
Consolidated Statements of Stockholders' Equity—Years ended December 31, 2010, 2009 and 2008 |
42 | |||
Notes to Consolidated Financial Statements |
43 | |||
35
Management's Report on Consolidated Financial Statements and Internal Control
The management of Covance Inc. ("Covance") has prepared, and is responsible for, Covance's consolidated financial statements and related footnotes. These consolidated financial statements have been prepared in conformity with U.S. generally accepted accounting principles.
Covance's management is responsible for establishing and maintaining effective internal control over financial reporting and for assessing the effectiveness of internal control over financial reporting. The purpose of this system of internal accounting controls over financial reporting is to provide reasonable assurance that assets are safeguarded, that transactions are executed in accordance with management's authorization and are properly recorded, and that accounting records may be relied upon for the preparation of accurate and complete consolidated financial statements. The design, monitoring and revision of internal accounting control systems involve, among other things, management's judgment with respect to the relative cost and expected benefits of specific control measures. Covance also maintains an internal audit function that evaluates and reports on the adequacy and effectiveness of internal controls, policies and procedures.
Covance's management concluded that its internal control over financial reporting as of December 31, 2010 was effective and adequate to accomplish the objectives described above. Management's assessment was based upon the criteria in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Covance's consolidated financial statements and the effectiveness of control over financial reporting have been audited by an independent registered public accounting firm, Ernst & Young LLP, as stated in their reports which are included elsewhere herein.
| /s/ Joseph L. Herring Joseph L. Herring Chairman of the Board and Chief Executive Officer (Principal Executive Officer) |
/s/ William E. Klitgaard William E. Klitgaard Corporate Senior Vice President and Chief Financial Officer (Principal Financial Officer) |
36
Report of Independent Registered Public Accounting Firm
The
Board of Directors and Stockholders
Covance Inc.
We have audited Covance Inc.'s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Covance Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying report on consolidated financial statements and internal control. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Covance Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Covance Inc. and subsidiaries as of December 31, 2010 and 2009, and the related consolidated statements of income, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2010 and our report dated February 28, 2011 expressed an unqualified opinion thereon.
| /s/ ERNST & YOUNG LLP |
MetroPark,
New Jersey
February 28, 2011
37
Report of Independent Registered Public Accounting Firm
The
Board of Directors and Stockholders
Covance Inc.
We have audited the accompanying consolidated balance sheets of Covance Inc. and subsidiaries as of December 31, 2010 and 2009, and the related consolidated statements of income, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Covance Inc. and subsidiaries at December 31, 2010 and 2009, and the consolidated results of their operations and their cash flows for each of the three years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Covance Inc.'s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 28, 2011 expressed an unqualified opinion thereon.
| /s/ ERNST & YOUNG LLP |
MetroPark,
New Jersey
February 28, 2011
38
COVANCE INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2010 AND 2009
| (Dollars in thousands) |
2010 | 2009 | |||||||
|---|---|---|---|---|---|---|---|---|---|
Assets |
|||||||||
Current Assets: |
|||||||||
Cash and cash equivalents |
$ | 377,223 | $ | 289,469 | |||||
Accounts receivable |
261,160 | 285,119 | |||||||
Unbilled services |
90,729 | 97,279 | |||||||
Inventory |
82,924 | 80,926 | |||||||
Deferred income taxes |
35,648 | 31,512 | |||||||
Prepaid expenses and other current assets |
98,127 | 93,367 | |||||||
Total Current Assets |
945,811 | 877,672 | |||||||
Property and equipment, net |
843,983 | 921,995 | |||||||
Goodwill, net |
127,653 | 127,653 | |||||||
Other assets |
48,095 | 47,624 | |||||||
Total Assets |
$ | 1,965,542 | $ | 1,974,944 | |||||
Liabilities and Stockholders' Equity |
|||||||||
Current Liabilities: |
|||||||||
Accounts payable |
$ | 34,079 | $ | 36,834 | |||||
Accrued payroll and benefits |
107,572 | 111,365 | |||||||
Accrued expenses and other current liabilities |
97,395 | 73,383 | |||||||
Unearned revenue |
186,301 | 166,890 | |||||||
Short-term debt and current portion of long-term debt |
45,000 | — | |||||||
Income taxes payable |
28,827 | 14,272 | |||||||
Total Current Liabilities |
499,174 | 402,744 | |||||||
Long-term debt |
87,500 | — | |||||||
Deferred income taxes |
30,531 | 98,945 | |||||||
Other liabilities |
68,516 | 62,251 | |||||||
Total Liabilities |
685,721 | 563,940 | |||||||
Commitments and Contingencies |
|||||||||
Stockholders' Equity: |
|||||||||
Preferred stock—Par value $1.00 per share; 10,000,000 shares authorized; no shares issued and outstanding at December 31, 2010 and 2009 |
— | — | |||||||
Common stock—Par value $0.01 per share; 140,000,000 shares authorized; 77,391,335 and 76,362,691 shares issued and outstanding, including those held in treasury, at December 31, 2010 and 2009, respectively |
774 | 764 | |||||||
Paid-in capital |
639,341 | 587,995 | |||||||
Retained earnings |
1,373,705 | 1,305,451 | |||||||
Accumulated other comprehensive income (loss) |
277 | (5,281 | ) | ||||||
Treasury stock at cost (17,125,878 and 12,257,065 shares at December 31, 2010 and 2009, respectively) |
(734,276 | ) | (477,925 | ) | |||||
Total Stockholders' Equity |
1,279,821 | 1,411,004 | |||||||
Total Liabilities and Stockholders' Equity |
$ | 1,965,542 | $ | 1,974,944 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
39
COVANCE INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
FOR THE YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
| (Dollars in thousands, except per share data) |
2010 | 2009 | 2008 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net revenues |
$ |
1,925,630 |
$ |
1,867,634 |
$ |
1,728,098 |
||||||
Reimbursable out-of-pocket expenses |
112,843 | 94,992 | 98,969 | |||||||||
Total revenues |
2,038,473 | 1,962,626 | 1,827,067 | |||||||||
Costs and expenses: |
||||||||||||
Cost of revenue (excluding depreciation and amortization) |
1,348,498 | 1,277,142 | 1,142,697 | |||||||||
Reimbursable out-of-pocket expenses |
112,843 | 94,992 | 98,969 | |||||||||
Selling, general and administrative (excluding depreciation and amortization) |
307,386 | 270,593 | 250,180 | |||||||||
Depreciation and amortization |
103,024 | 91,289 | 71,571 | |||||||||
Asset impairment charges |
119,229 | — | — | |||||||||
Total costs and expenses |
1,990,980 | 1,734,016 | 1,563,417 | |||||||||
Income from operations |
47,493 | 228,610 | 263,650 | |||||||||
Other expense (income), net: |
||||||||||||
Interest income |
(1,479 | ) | (1,355 | ) | (8,304 | ) | ||||||
Interest expense |
1,531 | 1,556 | 1,843 | |||||||||
Foreign exchange transaction loss (gain), net |
3,649 | 245 | (142 | ) | ||||||||
Gain on sale of businesses |
— | (9,681 | ) | (4,070 | ) | |||||||
Other expense (income), net |
3,701 | (9,235 | ) | (10,673 | ) | |||||||
Income before taxes and equity investee earnings |
43,792 | 237,845 | 274,323 | |||||||||
Tax (benefit) expense |
(23,655 | ) | 62,870 | 79,415 | ||||||||
Equity investee earnings |
807 | 907 | 1,852 | |||||||||
Net income |
$ | 68,254 | $ | 175,882 | $ | 196,760 | ||||||
Basic earnings per share |
$ |
1.08 |
$ |
2.76 |
$ |
3.12 |
||||||
Weighted average shares outstanding—basic |
63,043,561 | 63,818,717 | 63,096,155 | |||||||||
Diluted earnings per share |
$ |
1.06 |
$ |
2.73 |
$ |
3.08 |
||||||
Weighted average shares outstanding—diluted |
64,472,326 | 64,341,084 | 63,981,505 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
40
COVANCE INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
| (Dollars in thousands) |
2010 | 2009 | 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Cash flows from operating activities: |
|||||||||||
Net income |
$ | 68,254 | $ | 175,882 | $ | 196,760 | |||||
Adjustments to reconcile net income to net cash provided by operating activities: |
|||||||||||
Depreciation and amortization |
103,024 | 91,289 | 71,571 | ||||||||
Asset impairment charges |
119,229 | — | — | ||||||||
Non-cash compensation expense associated with employee benefit and stock compensation plans |
32,289 | 26,949 | 25,389 | ||||||||
Deferred income tax (benefit) provision |
(71,661 | ) | 33,030 | 9,343 | |||||||
Gain on sale of businesses |
— | (9,681 | ) | (4,070 | ) | ||||||
Loss on disposal of property and equipment |
1,487 | 998 | 1,064 | ||||||||
Equity investee earnings |
(807 | ) | (907 | ) | (1,852 | ) | |||||
Changes in operating assets and liabilities, net of businesses acquired and sold: |
|||||||||||
Accounts receivable |
23,959 | (54,937 | ) | (11,294 | ) | ||||||
Unbilled services |
6,550 | 15,724 | (23,884 | ) | |||||||
Inventory |
(1,998 | ) | (12,720 | ) | (13,418 | ) | |||||
Accounts payable |
(2,755 | ) | (5,163 | ) | 9,635 | ||||||
Accrued liabilities |
20,097 | (8,072 | ) | 26,952 | |||||||
Unearned revenue |
19,411 | 4,174 | 17,686 | ||||||||
Income taxes payable |
14,797 | (1,176 | ) | (2,702 | ) | ||||||
Other assets and liabilities, net |
2,547 | 3,699 | (15,023 | ) | |||||||
Net cash provided by operating activities |
334,423 | 259,089 | 286,157 | ||||||||
Cash flows from investing activities: |
|||||||||||
Capital expenditures |
(126,278 | ) | (131,079 | ) | (318,928 | ) | |||||
Acquisition of businesses, net of cash acquired |
(20,994 | ) | (28,096 | ) | — | ||||||
Proceeds from sale of businesses |
— | 10,373 | 4,070 | ||||||||
Minority equity investment |
— | — | (3,136 | ) | |||||||
Other, net |
47 | 29 | 385 | ||||||||
Net cash used in investing activities |
(147,225 | ) | (148,773 | ) | (317,609 | ) | |||||
Cash flows from financing activities: |
|||||||||||
Net borrowings (repayments) under revolving credit facility |
35,000 | (50,000 | ) | 50,000 | |||||||
Borrowings under long-term debt |
100,000 | — | — | ||||||||
Repayments under long-term debt |
(2,500 | ) | — | — | |||||||
Payment of debt assumed upon acquisition of business |
— | (5,431 | ) | — | |||||||
Stock issued under employee stock purchase and option plans |
18,825 | 10,682 | 31,500 | ||||||||
Purchase of treasury stock |
(256,351 | ) | (4,828 | ) | (132,906 | ) | |||||
Net cash used in financing activities |
(105,026 | ) | (49,577 | ) | (51,406 | ) | |||||
Effect of exchange rate changes on cash |
5,582 | 7,396 | (15,293 | ) | |||||||
Net change in cash and cash equivalents |
87,754 | 68,135 | (98,151 | ) | |||||||
Cash and cash equivalents, beginning of year |
289,469 |
221,334 |
319,485 |
||||||||
Cash and cash equivalents, end of year |
$ | 377,223 | $ | 289,469 | $ | 221,334 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
41
COVANCE INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
| (Dollars in thousands) |
Common Stock |
Paid-in Capital |
Retained Earnings |
Accumulated Other Comprehensive Income (Loss) |
Comprehensive Income |
Treasury Stock |
Total Stockholders' Equity |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance, December 31, 2007 |
$ | 746 | $ | 492,373 | $ | 933,106 | $ | 24,154 | $ | (340,191 | ) | $ | 1,110,188 | |||||||||||
Effect of change in pension and post-retirement plan measurement date |
— |
— |
(297 |
) |
— |
— |
(297 |
) |
||||||||||||||||
Comprehensive income: |
||||||||||||||||||||||||
Net income |
— | — | 196,760 | — | $ | 196,760 | — | 196,760 | ||||||||||||||||
Currency translation adjustment |
— | — | — | (40,544 | ) | (40,544 | ) | — | (40,544 | ) | ||||||||||||||
Unrealized gain on securities, net of tax |
— | — | — | 4,359 | 4,359 | — | 4,359 | |||||||||||||||||
Defined benefit pension plans, net of tax: |
||||||||||||||||||||||||
Actuarial loss |
— | — | — | (1,867 | ) | (1,867 | ) | — | (1,867 | ) | ||||||||||||||
Prior service cost |
— | — | — | (77 | ) | (77 | ) | — | (77 | ) | ||||||||||||||
Total comprehensive income |
— | — | — | — | $ | 158,631 | — | — | ||||||||||||||||
Shares issued under various employee benefit and stock compensation plans |
3 | 32,075 | — | — | — | 32,078 | ||||||||||||||||||
Stock option exercises |
5 | 17,884 | — | — | — | 17,889 | ||||||||||||||||||
Tax benefit from stock issued |
— | 9,266 | — | — | — | 9,266 | ||||||||||||||||||
Treasury stock, at cost |
— | — | — | — | (132,906 | ) | (132,906 | ) | ||||||||||||||||
Balance, December 31, 2008 |
754 | 551,598 | 1,129,569 | (13,975 | ) | (473,097 | ) | 1,194,849 | ||||||||||||||||
Comprehensive income: |
||||||||||||||||||||||||
Net income |
— | — | 175,882 | — | $ | 175,882 | — | 175,882 | ||||||||||||||||
Currency translation adjustment |
— | — | — | 14,605 | 14,605 | — | 14,605 | |||||||||||||||||
Unrealized gain on securities, net of tax |
— | — | — | 805 | 805 | — | 805 | |||||||||||||||||
Defined benefit pension plans, net of tax: |
||||||||||||||||||||||||
Actuarial loss |
— | — | — | (6,645 | ) | (6,645 | ) | — | (6,645 | ) | ||||||||||||||
Prior service cost |
— | — | — | (71 | ) | (71 | ) | — | (71 | ) | ||||||||||||||
Total comprehensive income |
— | — | — | — | $ | 184,576 | — | — | ||||||||||||||||
Shares issued under various employee benefit and stock compensation plans |
9 | 33,768 | — | — | — | 33,777 | ||||||||||||||||||
Stock option exercises |
1 | 3,091 | — | — | — | 3,092 | ||||||||||||||||||
Tax benefit from stock issued |
— | (462 | ) | — | — | — | (462 | ) | ||||||||||||||||
Treasury stock, at cost |
— | — | — | — | (4,828 | ) | (4,828 | ) | ||||||||||||||||
Balance, December 31, 2009 |
764 | 587,995 | 1,305,451 | (5,281 | ) | (477,925 | ) | 1,411,004 | ||||||||||||||||
Comprehensive income: |
||||||||||||||||||||||||
Net income |
— | — | 68,254 | — | $ | 68,254 | — | 68,254 | ||||||||||||||||
Currency translation adjustment |
— | — | — | 9,328 | 9,328 | — | 9,328 | |||||||||||||||||
Unrealized gain on securities, net of tax |
— | — | — | 325 | 325 | — | 325 | |||||||||||||||||
Defined benefit pension plans, net of tax: |
||||||||||||||||||||||||
Actuarial loss |
— | — | — | (4,068 | ) | (4,068 | ) | — | (4,068 | ) | ||||||||||||||
Prior service cost |
— | — | — | (27 | ) | (27 | ) | — | (27 | ) | ||||||||||||||
Total comprehensive income |
— | — | — | — | $ | 73,812 | — | — | ||||||||||||||||
Shares issued under various employee benefit and stock compensation plans |
7 | 39,461 | — | — | — | 39,468 | ||||||||||||||||||
Stock option exercises |
3 | 10,026 | — | — | — | 10,029 | ||||||||||||||||||
Tax benefit from stock issued |
— | 1,859 | — | — | — | 1,859 | ||||||||||||||||||
Treasury stock, at cost |
— | — | — | — | (256,351 | ) | (256,351 | ) | ||||||||||||||||
Balance, December 31, 2010 |
$ | 774 | $ | 639,341 | $ | 1,373,705 | $ | 277 | $ | (734,276 | ) | $ | 1,279,821 | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
42
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
1. Organization
Covance Inc. and its subsidiaries ("Covance" or the "Company") is a leading drug development services company providing a wide range of early-stage and late-stage product development services on a worldwide basis primarily to the pharmaceutical, biotechnology and medical device industries. Covance also provides services such as laboratory testing to the chemical, agrochemical and food industries. Covance's operations constitute two segments for financial reporting purposes. The first segment, early development services, includes preclinical and clinical pharmacology service offerings. The second segment, late-stage development services, includes central laboratory, clinical development, periapproval and market access services. Operations are principally focused in the United States and Europe.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of all entities controlled by Covance. All significant intercompany accounts and transactions are eliminated. The equity method of accounting is used for investments in affiliates in which Covance owns between 20 and 50 percent and does not have the ability to exercise control. For investments in which Covance owns less than 20 percent and does not have the ability to exercise significant influence over operating or financial decisions of the investee, the cost method of accounting is applied. Where the fair value of the shares of the cost method investee is based on quoted prices in active markets, Covance accounts for such investment as available-for-sale securities. See Note 5.
Use of Estimates
These consolidated financial statements have been prepared in conformity with U.S. generally accepted accounting principles ("GAAP"), which requires management to make estimates and assumptions about future events that affect the amounts reported in the financial statements and the accompanying notes. Actual results could differ from these estimates.
Foreign Currencies
For subsidiaries outside of the United States that operate in a local currency environment, income and expense items are translated to United States dollars at the monthly average rates of exchange prevailing during the year, assets and liabilities are translated at year-end exchange rates and equity accounts are translated at historical exchange rates. Translation adjustments are accumulated in a separate component of stockholders' equity in the consolidated balance sheets and are included in the determination of comprehensive income in the consolidated statements of stockholders' equity. Transaction gains and losses are included in the determination of net income in the consolidated statements of income.
Cash and Cash Equivalents
Cash and cash equivalents include all highly liquid investments with an original maturity of three months or less at date of purchase and consist principally of amounts invested in money market funds and bank deposits.
43
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
2. Summary of Significant Accounting Policies (Continued)
Financial Instruments
The fair value of cash and cash equivalents, accounts receivable, accounts payable and accrued expenses approximate their carrying amounts as reported at December 31, 2010 and 2009.
Accounts receivable and unbilled services represent amounts due from Covance customers who are concentrated primarily in the pharmaceutical and biotechnology industries. Covance endeavors to monitor the creditworthiness of its customers to which it grants credit terms in the ordinary course of business. Although Covance customers are concentrated primarily within these two industries, management considers the likelihood of material credit risk as remote. In addition, in some cases Covance requires advance payment for a portion of the contract price from its customers upon the signing of a contract for services. These amounts are deferred and recognized as revenue as services are performed. Historically, bad debts have been immaterial.
Inventory
Inventories, which consist principally of finished goods and supplies, are valued at the lower of cost (first-in, first-out method) or market.
Prepaid Expenses and Other Current Assets
In connection with the management of multi-site clinical trials, Covance pays on behalf of its customers fees to investigators, volunteers and other out-of-pocket costs (such as travel, printing, meetings, couriers, etc.), for which the Company is reimbursed at cost, without mark-up or profit. Amounts receivable from customers in connection with billed and unbilled investigator fees, volunteer payments and other out-of-pocket pass-through costs are included in prepaid expenses and other current assets in the accompanying consolidated balance sheets and totaled $49.1 million and $55.6 million at December 31, 2010 and 2009, respectively. See Note 2 "Reimbursable Out-of-Pocket Expenses".
Property and Equipment
Property and equipment are recorded at cost. Depreciation and amortization are provided on the straight-line method at rates adequate to allocate the cost of the applicable assets over their estimated useful lives, which generally range from ten to forty years for buildings and improvements, three to ten years for equipment, furniture and fixtures and three to five years for computer hardware and software, except for certain large enterprise-wide software applications which are depreciated over periods of up to ten years. Leasehold improvements are capitalized and amortized on a straight-line basis over the shorter of the estimated useful life of the improvement or the associated remaining lease term. The cost of computer software developed or obtained for internal use is capitalized and amortized on the straight-line method over the estimated useful life. Costs incurred during the development phase are capitalized, while all other costs are expensed as incurred. Repairs and maintenance are expensed as incurred.
Impairment of Long-Lived Assets
Covance reviews its long-lived assets, other than goodwill and other indefinite lived intangible assets, for impairment when events or changes in circumstances occur that indicate that the carrying value of the asset may not be recoverable. The assessment of possible impairment is based upon Covance's judgment of its ability
44
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
2. Summary of Significant Accounting Policies (Continued)
to recover the value of the asset from the expected future undiscounted cash flows of the related operations. Actual future cash flows may be greater or less than estimated. During the third quarter of 2010, Covance determined that long-lived assets used in its North American toxicology operations, located in Chandler, Arizona and Manassas, Virginia with carrying values of $182.7 million and $23.4 million, respectively, were no longer fully recoverable from the cash flows expected from those assets. Accordingly, as of September 30, 2010, Covance recorded an asset impairment charge totaling $119.2 million ($103.0 million of which relates to the Chandler, Arizona assets and $16.2 million relates to the Manassas, Virginia assets), representing the excess of the carrying value of those assets over their respective fair market values. See Note 13.
Goodwill and Other Intangible Assets and Impairment
Goodwill represents costs in excess of the fair value of net tangible and identifiable net intangible assets acquired in business combinations. Covance performs an annual test for impairment of goodwill and other indefinite lived intangible assets during the fourth quarter. This test is performed by comparing, at the reporting unit level, the carrying value of the reporting unit to its fair value. Covance assesses fair value based upon its estimate of the present value of the future cash flows that it expects to be generated by the reporting unit. The annual test for impairment performed for 2010, 2009 and 2008 indicated that no reporting units were at significant risk for impairment.
Intangible assets with finite lives are amortized on a straight-line basis over their estimated useful lives, which range in term from one to ten years. The Company periodically evaluates the reasonableness of the estimated useful lives of these intangible assets. See Note 4.
Revenue Recognition
Covance recognizes revenue either as services are performed or products are delivered, depending on the nature of the work contracted. Historically, a majority of Covance's net revenues have been earned under contracts which range in duration from a few months to two years, but can extend in duration up to five years or longer. Covance also has committed minimum volume arrangements with certain clients which generally range in duration from three to ten years. Underlying these arrangements are individual project contracts for the specific services to be provided. These arrangements enable our clients to secure our services in exchange for which they commit to purchase an annual minimum dollar value ("volume") of services. Under these types of arrangements, if the annual minimum volume commitment is not reached, the client is required to pay Covance for the shortfall. Progress towards the achievement of annual minimum volume guarantees is monitored throughout the year. Annual minimum guarantee shortfalls are not included in net revenues until the amount has been determined and agreed to by the client.
Service contracts generally take the form of fee-for-service or fixed-price arrangements. In cases where performance spans multiple accounting periods, revenue is recognized as services are performed, measured on a proportional-performance basis, generally using output measures that are specific to the service provided. Examples of output measures in our early development segment include the number of slides read, dosings performed, or specimens prepared for preclinical laboratory services, or number of dosings or number of volunteers enrolled for clinical pharmacology. Examples of output measures in our late-stage development segment's clinical development service offering include among others, number of investigators enrolled, number of sites initiated, number of patients enrolled and number of monitoring visits completed. Revenue is determined by dividing the actual units of work completed by the total units of work required under the
45
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
2. Summary of Significant Accounting Policies (Continued)
contract and multiplying that percentage by the total contract value. The total contract value, or total contractual payments, represents the aggregate contracted price for each of the agreed upon services to be provided. Covance does not have any contractual arrangements spanning multiple accounting periods where revenue is recognized on a proportional-performance basis under which the Company has earned more than an immaterial amount of performance-based revenue (i.e., potential additional revenue tied to specific deliverables or performance). Changes in the scope of work are common, especially under long-term contracts, and generally result in a change in contract value. Once the client has agreed to the changes in scope and renegotiated pricing terms, the contract value is amended and revenue is recognized, as described above. Estimates of costs to complete are made to provide, where appropriate, for losses expected on contracts. Costs are not deferred in anticipation of contracts being awarded, but instead are expensed as incurred.
Billing schedules and payment terms are generally negotiated on a contract-by-contract basis. In some cases, Covance bills the client for the total contract value in progress-based installments as certain non-contingent billing milestones are reached over the contract duration, such as, but not limited to, contract signing, initial dosing, investigator site initiation, patient enrollment or database lock. The term "billing milestone" relates only to a billing trigger in a contract whereby amounts become billable and payable in accordance with a negotiated predetermined billing schedule throughout the term of a project. These billing milestones are not performance-based (i.e., potential additional arrangement consideration tied to specific deliverables or performance). In other cases, billing and payment terms are tied to the passage of time (e.g., monthly billings). In either case, the total contract value and aggregate amounts billed to the client would be the same at the end of the project. While Covance attempts to negotiate terms that provide for billing and payment of services prior or within close proximity to the provision of services, this is not always the case, as evidenced by fluctuations in the levels of unbilled receivables and unearned revenue from period to period. While a project is ongoing, cash payments are not necessarily representative of aggregate revenue earned at any particular point in time, as revenues are recognized when services are provided, while amounts billed and paid are in accordance with the negotiated billing and payment terms.
In some cases, payments received are in excess of revenue recognized. For example, a contract invoicing schedule may provide for an upfront payment of 10% of the full contract value upon contract signing, but at the time of signing, performance of services has not yet begun, and therefore, no revenue has yet been recognized. Payments received in advance of services being provided, such as in this example, are deferred as unearned revenue on the balance sheet. As the contracted services are subsequently performed and the associated revenue is recognized, the unearned revenue balance is reduced by the amount of revenue recognized during the period.
In other cases, services may be provided and revenue is recognized before the client is invoiced. In these cases, revenue recognized will exceed amounts billed, and the difference, representing an unbilled receivable, is recorded for this amount which is currently unbillable to the customer pursuant to contractual terms. Once the client is invoiced, the unbilled receivable is reduced for the amount billed, and a corresponding account receivable is recorded. All unbilled receivables are billable to customers within one year from the respective balance sheet date.
Most contracts are terminable by the client, either immediately or upon notice. These contracts typically require payment to Covance of expenses to wind down the study, fees earned to date and, in some cases, a termination fee or a payment to Covance of some portion of the fees or profits that could have been earned by Covance under the contract if it had not been terminated early. Termination fees are included in net
46
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
2. Summary of Significant Accounting Policies (Continued)
revenues when realization is assured. In connection with the management of multi-site clinical trials, Covance pays on behalf of its customers fees to investigators, volunteers and other out-of-pocket costs (such as for travel, printing, meetings, couriers, etc.), for which it is reimbursed at cost, without mark-up or profit. Investigator fees are not reflected in total revenues or expenses where Covance acts in the capacity of an agent on behalf of the pharmaceutical company sponsor, passing through these costs without risk or reward to Covance. All other out-of-pocket costs are included in total revenues and expenses.
Costs and Expenses
Cost of revenue includes direct labor and related benefit charges, other direct costs, shipping and handling fees, and an allocation of facility charges and information technology costs and excludes depreciation and amortization. Selling, general and administrative expenses consist primarily of administrative payroll and related benefit charges, advertising and promotional expenses, administrative travel and an allocation of facility charges and information technology costs and excludes depreciation and amortization. Cost of advertising is expensed as incurred.
Taxes
Covance uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are recognized for the expected future tax consequences of differences between the carrying amounts of assets and liabilities and their respective tax bases using enacted tax rates in effect for the year in which the temporary differences are expected to reverse. The effect on deferred taxes of a change in enacted tax rates is recognized in income in the period when the change is effective. See Note 7.
The Company recognizes a tax benefit from an uncertain tax position only if the Company believes it is more likely than not to be sustained upon examination based on the technical merits of the position. The amount of the accrual for which an exposure exists is measured as the largest amount of benefit determined on a cumulative probability basis that the Company believes is more likely than not to be realized upon ultimate settlement of the position. Components of the reserve are classified as either a current or long-term liability in the consolidated balance sheet based on when the Company expects each of the items to be settled. Covance accrues interest and penalties in relation to unrecognized tax benefits as a component of income tax expense.
The Company also maintains a tax reserve related to exposures for non-income tax matters, including value-added tax and state sales and use and other taxes. The balance of this reserve was $1.0 million and $0.9 million at December 31, 2010 and 2009, respectively, and is recorded as a current liability in accrued expenses and other current liabilities on the consolidated balance sheet.
While Covance believes it has identified all reasonably identifiable exposures and the reserve it has established for identifiable exposures is appropriate under the circumstances, it is possible that additional exposures exist and that exposures may be settled at amounts different than the amounts reserved. It is also possible that changes in facts and circumstances could cause Covance to either materially increase or reduce the carrying amount of its tax reserve.
47
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
2. Summary of Significant Accounting Policies (Continued)
Covance's historical policy has been to leave its unremitted foreign earnings invested indefinitely outside the United States. Covance intends to continue to leave its unremitted foreign earnings invested indefinitely outside the United States. As a result, taxes have not been provided on any of the remaining accumulated foreign unremitted earnings as of December 31, 2010. See Note 7.
Comprehensive Income
Covance's total comprehensive income for the periods presented is comprised of net income plus the change in the cumulative translation adjustment equity account, the adjustments, net of tax, for the current year actuarial gains (losses) and prior service credits (costs) in connection with its defined benefit pension and other post-retirement plans and the change in the unrealized gain on available-for-sale securities of $0.3 million, $0.8 million and $4.4 million, net of tax, for the years ended December 31, 2010, 2009 and 2008, respectively. In the fourth quarter of 2008, Covance changed the classification of its minority equity investment in BioClinica, Inc. from an equity method investment to an available-for-sale security. See Note 5.
Stock-Based Compensation
The Company sponsors several stock-based compensation plans pursuant to which non-qualified stock options and restricted stock awards are granted to eligible employees. These plans are described more fully in Note 10. The grant-date fair value of awards expected to vest is expensed on a straight-line basis over the vesting period of the related awards.
Defined Benefit Pension Plans
Covance sponsors various pension and other post-retirement benefit plans which are more fully described in Note 9. The measurement of the related benefit obligations and the net periodic benefit costs recorded each year are based upon actuarial computations, which require management's judgment as to certain assumptions. These assumptions include the discount rates to use in computing the present value of the benefit obligations and the net periodic benefit costs, the expected future rate of salary increases (for pay-related plans) and the expected long-term rate of return on plan assets (for funded plans). The discount rates are derived based on a hypothetical yield curve represented by a series of annualized individual discount rates. The expected long-term rate of return on plan assets is based on the target asset allocation and the average expected rate of growth for the asset classes invested. The average expected rate of growth is derived from a combination of historic returns, current market indicators, the expected risk premium for each asset class and the opinion of professional advisors. Liabilities related to all of Covance's pension and other post-retirement benefit plans are measured as of December 31.
Earnings Per Share ("EPS")
Basic EPS is computed by dividing net income available to common stockholders by the weighted average number of shares outstanding during the period. The computation of diluted EPS is similar to the computation of basic EPS, except that the denominator is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common shares had been issued; computed under the treasury stock method.
48
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
2. Summary of Significant Accounting Policies (Continued)
In computing diluted EPS for the years ended December 31, 2010, 2009 and 2008, the denominator was increased by 1,428,765 shares, 522,367 shares and 885,350 shares, respectively, representing the dilutive effect of stock options outstanding at December 31, 2010, 2009 and 2008, with exercise prices less than the average market price of Covance's common stock during each respective period. Excluded from the computation of diluted EPS for the year ended December 31, 2010 were options to purchase 1,639,806 shares of common stock at prices ranging from $51.93 to $94.34 per share because the exercise prices of such options were greater than the average market price of Covance's common stock during 2010. Excluded from the computation of diluted EPS for the year ended December 31, 2009 were options to purchase 825,401 shares of common stock at prices ranging from $47.27 to $94.34 per share because the exercise prices of such options were greater than the average market price of Covance's common stock during 2009. Excluded from the computation of diluted EPS for the year ended December 31, 2008 were options to purchase 251,588 shares of common stock at prices ranging from $77.72 to $94.34 per share because the exercise prices of such options were greater than the average market price of Covance's common stock during 2008.
Reimbursable Out-of-Pocket Expenses
As discussed in Note 2 "Prepaid Expenses and Other Current Assets", Covance pays on behalf of its customers fees to investigators, volunteers and other out-of-pocket costs for which the Company is reimbursed at cost, without mark-up or profit. Amounts paid to volunteers and other out-of-pocket costs are reflected in operating expenses, while the reimbursements received are reflected in revenues in the consolidated statements of income. Covance excludes from revenue and expense in the consolidated statements of income fees paid to investigators and the associated reimbursement since Covance acts as an agent on behalf of the pharmaceutical company sponsors with regard to investigator payments.
Supplemental Cash Flow Information
Cash paid for interest for the years ended December 31, 2010, 2009 and 2008 totaled $0.6 million, $1.2 million and $1.5 million, respectively. Cash paid for income taxes for the years ended December 31, 2010, 2009 and 2008 totaled $33.6 million, $26.8 million and $53.8 million, respectively. The change in income taxes payable in the consolidated statement of cash flows for the years ended December 31, 2010, 2009 and 2008 includes as an operating cash outflow the excess tax benefit received from the exercise of non-qualified stock options of $1.6 million, $0.8 million and $7.3 million, respectively (a corresponding cash inflow of $1.6 million, $0.8 million and $7.3 million, respectively, has been included in financing cash flows).
Subsequent Events
Subsequent events are defined as those events or transactions that occur after the balance sheet date, but before the financial statements are filed with the Securities and Exchange Commission. See Note 15.
49
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
3. Property and Equipment
Property and equipment at December 31, 2010 and 2009 consist of the following:
| |
2010 | 2009 | ||||||
|---|---|---|---|---|---|---|---|---|
Property and equipment at cost: |
||||||||
Land |
$ | 69,715 | $ | 91,800 | ||||
Buildings and improvements |
602,428 | 663,487 | ||||||
Equipment |
294,406 | 288,775 | ||||||
Computer hardware and software |
316,524 | 294,060 | ||||||
Furniture, fixtures & leasehold improvements |
90,265 | 78,884 | ||||||
Construction-in-progress |
93,280 | 73,469 | ||||||
|
1,466,618 | 1,490,475 | ||||||
Less: Accumulated depreciation and amortization |
(622,635 | ) | (568,480 | ) | ||||
Property and equipment, net |
$ | 843,983 | $ | 921,995 | ||||
Depreciation and amortization expense aggregated $101.8 million, $90.3 million and $70.6 million for the years ended December 31, 2010, 2009 and 2008, respectively.
4. Goodwill and Amortizable Intangible Assets
The following table sets forth changes in the carrying amount of goodwill by operating segment, net of accumulated amortization of $15.1 million for each of the years ended December 31, 2010 and 2009, respectively (see Note 6):
| |
Early Development |
Late-Stage Development |
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Balance, December 31, 2008 |
$ | 69,570 | $ | 35,916 | $ | 105,486 | ||||
Goodwill recognized from 2009 acquisition of businesses |
22,167 | — | 22,167 | |||||||
Balance, December 31, 2009 and 2010 |
$ | 91,737 | $ | 35,916 | $ | 127,653 | ||||
The following table summarizes the Company's acquired amortizable intangible assets (see Note 6), which are reflected in other assets on the consolidated balance sheet, as of December 31, 2010 and 2009:
| |
2010 | 2009 | ||||||
|---|---|---|---|---|---|---|---|---|
Intangible assets at cost: |
||||||||
Customer Lists (10 year weighted average useful life) |
$ | 5,753 | $ | 5,753 | ||||
Technology (5 year weighted average useful life) |
2,340 | 2,340 | ||||||
Other—Patient List, Backlog and Non-Compete Agreements (weighted average useful lives ranging from 1 to 4 years) |
1,419 | 1,419 | ||||||
|
9,512 | 9,512 | ||||||
Less: Accumulated amortization |
(5,234 | ) | (4,039 | ) | ||||
Net carrying value |
$ | 4,278 | $ | 5,473 | ||||
50
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
4. Goodwill and Amortizable Intangible Assets (Continued)
Amortization expense for the years ended December 31, 2010, 2009 and 2008 was $1.2 million, $1.0 million and $1.0 million, respectively. Amortization expense expected to be recorded for each of the next five years is as follows:
Year Ending December 31,
|
|
|||
|---|---|---|---|---|
2011 |
$ | 918 | ||
2012 |
$ | 627 | ||
2013 |
$ | 590 | ||
2014 |
$ | 590 | ||
2015 |
$ | 590 | ||
5. Equity Investments
Covance has a minority equity position (less than 20%) in Caprion Proteomics ("Caprion"), a privately held company headquartered in Montreal, Canada, which was acquired in December 2008 for a total cost of $3.1 million. Caprion is a leading provider of proteomics-based services to the pharmaceutical industry. Under the terms of the agreement, Covance serves as the exclusive contract research organization distributor of Caprion's proteomic biomarker services and Caprion serves as Covance's exclusive proteomic discovery provider. As Covance owns less than a 20% interest in Caprion and does not exercise significant influence over the operating or financial decisions of Caprion, the investment is accounted for under the cost method. This investment is included in other assets on the consolidated balance sheet.
Covance has a 47% minority equity position in Noveprim Limited ("Noveprim"), a supplier of research products, which was acquired in March 2004 at a total cost of $20.7 million. The excess of the purchase price over the underlying equity in Noveprim's net assets at the date of acquisition of $13.8 million represents goodwill and is included in the carrying value of Covance's investment. This investment is reflected in other assets on the consolidated balance sheet. During the years ended December 31, 2010, 2009 and 2008, Covance recognized income of $0.8 million, $0.9 million and $1.4 million, respectively, representing its share of Noveprim's earnings, net of the elimination of profit on inventory purchased from Noveprim and still on hand at Covance at December 31, 2010, 2009 and 2008. The carrying value of Covance's investment in Noveprim as of December 31, 2010 and 2009 was $22.0 million and $22.1 million, respectively.
Covance has a minority equity position (less than 20%) in Bio-Clinica, Inc. ("BIOC") (Nasdaq GM:BIOC), formerly known as Bio-Imaging Technologies, Inc. BIOC uses proprietary medical imaging technologies to process and analyze medical images, and also provides other services, including the data-basing and regulatory submission of medical images, quantitative data and text. During the year ended December 31, 2008, Covance recognized income of $0.4 million representing its pro rata share of BIOC's earnings. In the fourth quarter of 2008, Covance suspended the use of the equity method of accounting as its ownership interest in BIOC fell below 20% and it could no longer exercise significant influence over BIOC's operations. In the fourth quarter of 2008, Covance began accounting for its investment in BIOC as an available-for-sale security. The carrying value of Covance's investment in BIOC as of December 31, 2010 and 2009 was $10.5 million and $10.0 million, respectively, as determined based on quoted prices in an active market. This investment is reflected in other assets on the consolidated balance sheet. The $0.5 million increase in the carrying value of the investment results in a $0.3 million increase in the unrealized gain on investment, net of tax, which is included within accumulated other comprehensive income on the consolidated balance sheet. Accordingly, the balance in the unrealized gain on investment at December 31, 2010 and 2009 was $5.5 million and $5.2 million, net of tax, respectively.
51
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
6. Acquisitions and Divestitures
In October 2010, Covance acquired research and development facilities located in Porcheville, France and Alnwick, UK from sanofi-aventis for a cash payment of $27.9 million ($21.0 million net of cash acquired). Transaction related costs of approximately $2.6 million were included in selling, general and administrative expense in the period incurred. The acquisition of these facilities provides Covance with new capabilities in CMC (Chemistry, Manufacturing and Controls) services, including preformulation, drug formulation, preclinical and early-stage clinical API (Active Pharmaceutical Ingredient) manufacturing, and radiolabeled chemistry. Pursuant to the asset purchase agreement, Covance will provide services to sanofi-aventis at these facilities over a period of 5 years for $350 million. The tangible assets acquired consisted of property and equipment and are included in Covance's consolidated financial statements beginning in October 2010 based on their estimated fair value of $27.9 million as determined in the preliminary purchase price allocation, partially offset by certain employee related liabilities of $6.9 million assumed in the transaction. The Company has not yet received the final third-party valuation of the assets acquired. As such, this allocation is preliminary pending the outcome of the final valuation. Results of operations for the sites acquired from sanofi-aventis are reported in Covance's early development segment beginning in November 2010. In addition to the acquisition and provision of services at these facilities, Covance and sanofi-aventis also entered into a 10-year strategic alliance, pursuant to which Covance has become sanofi-aventis' R&D partner providing drug development services to sanofi-aventis in amounts ranging from $0.9 billion to $1.9 billion over the term of the agreement.
In August 2009, Covance acquired certain assets and capabilities of Merck & Co., Inc.'s ("Merck") Gene Expression Laboratory ("GEL") located in Seattle, Washington for $9.75 million in cash. Transaction related costs of $0.7 million were included in selling, general and administrative expense in the period incurred. This acquisition expanded Covance's footprint in the genomics testing market and added capabilities in genomics testing and personalized medicine. The tangible assets acquired consisted of property and equipment and were included in Covance's consolidated financial statements beginning in August 2009 based on their estimated fair value of $5.5 million. The remaining purchase price of $4.2 million represents the fair value of the acquired assembled workforce, which is a component of goodwill. Results of operations for GEL are reported in Covance's early development segment beginning in August 2009. Covance and Merck also entered into an agreement pursuant to which Merck committed to purchase at least $145.0 million of Covance services over a five year period.
In August 2009, Covance sold its interactive voice and web response ("IVR") service offering, part of Covance's late-stage development segment, to Phase Forward (which was subsequently acquired by Oracle Corporation) for net cash proceeds totaling $9.7 million and recorded a pre-tax gain of $9.0 million ($5.9 million after tax) on the sale. In addition, Covance and Phase Forward entered into a five-year marketing agreement pursuant to which Covance is entitled to receive a referral fee for providing Phase Forward's IVR services to its clients.
In March 2009, Covance acquired 100% of the stock of Swiss Pharma Contract Ltd. ("Swiss Pharma"), and its 50-bed clinical research facility based in Basel, Switzerland, for total consideration of $22.8 million, as to which $19.4 million ($18.3 million net of cash acquired) was paid at closing with the balance contingently payable based upon the achievement of certain performance based milestones through 2011. Additionally, Covance repaid the entire $5.4 million balance of mortgage debt assumed in the Swiss Pharma acquisition. Transaction related costs of $0.5 million were included in selling, general & administrative expense in the period incurred. Net tangible and intangible assets acquired in the acquisition were included in the consolidated financial statements beginning in March 2009 based on their estimated fair values of $3.0 million and $1.8 million, respectively. Intangible assets are being amortized over a 7 year weighted average life.
52
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
6. Acquisitions and Divestitures (Continued)
Goodwill of $18.0 million resulting from the acquisition arises largely from the synergies expected from combining the Swiss Pharma operations with our existing European clinical pharmacology operations, as well as from the benefits derived from the assembled Swiss Pharma workforce. None of the goodwill recognized is expected to be deductible for tax purposes. Results of operations for Swiss Pharma are reported in Covance's early development segment beginning in March 2009.
In October 2008, Covance acquired certain assets from Eli Lilly and Company ("Lilly") for cash payments totaling $51.6 million (including transaction related costs of $1.6 million). The acquired assets consisted of 450 acres of Lilly's early drug development campus (land, buildings and equipment) located in Greenfield, Indiana ("Greenfield"). In addition, Covance and Lilly entered into a 10-year agreement with a minimum value of $1.6 billion pursuant to which Covance will provide Lilly a broad range of drug development services. The results of operations for Greenfield and the acquired assets, which are part of Covance's Early Development segment service offering, are included in Covance's consolidated financial statements beginning in October 2008.
7. Taxes on Income
The components of income before taxes and the related provision (benefit) for taxes on income for 2010, 2009 and 2008 are as follows:
| |
2010 | 2009 | 2008 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Income (loss) before taxes and equity investee earnings: |
||||||||||||
Domestic |
$ | (71,012 | ) | $ | 94,166 | $ | 148,636 | |||||
International |
114,804 | 143,679 | 125,687 | |||||||||
Total |
$ | 43,792 | $ | 237,845 | $ | 274,323 | ||||||
Federal income taxes: |
||||||||||||
Current provision |
$ | 36,221 | $ | 15,463 | $ | 42,892 | ||||||
Deferred provision (benefit) |
(57,016 | ) | 18,724 | 9,738 | ||||||||
International income taxes: |
||||||||||||
Current provision |
10,139 | 13,993 | 20,993 | |||||||||
Deferred provision (benefit) |
(8,110 | ) | 10,511 | (391 | ) | |||||||
State and other income taxes: |
||||||||||||
Current provision |
940 | 1,196 | 3,616 | |||||||||
Deferred provision (benefit) |
(5,829 | ) | 2,983 | 2,567 | ||||||||
Income tax provision (benefit) |
$ | (23,655 | ) | $ | 62,870 | $ | 79,415 | |||||
The differences between the provision for income taxes and income taxes computed using the Federal statutory income tax rate for 2010, 2009 and 2008 are as follows:
| |
2010 | 2009 | 2008 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Taxes at statutory rate |
35.0 | % | 35.0 | % | 35.0 | % | ||||
State and local taxes, net of Federal benefit |
1.1 | 1.1 | 1.5 | |||||||
Impact of international operations |
(62.8 | ) | (10.8 | ) | (8.6 | ) | ||||
Previously unrecognized tax benefits |
(27.2 | ) | (0.3 | ) | — | |||||
Other, net |
(0.1 | ) | 1.4 | 1.0 | ||||||
Total |
(54.0 | )% | 26.4 | % | 28.9 | % | ||||
53
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
7. Taxes on Income (Continued)
Previously unrecognized tax benefits in 2010 consists primarily of the tax benefit recorded in connection with the favorable resolution of income tax audits and tax benefits resulting from tax positions taken in returns filed during 2010.
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and liabilities at December 31, 2010 and 2009 are as follows:
| |
2010 | 2009 | |||||||
|---|---|---|---|---|---|---|---|---|---|
Current deferred tax assets: |
|||||||||
Liabilities/expenses not currently deductible |
$ | 34,335 | $ | 28,341 | |||||
Deferred equity compensation |
3,353 | 2,053 | |||||||
Net operating losses |
978 | 1,118 | |||||||
Total current deferred tax assets |
38,666 | 31,512 | |||||||
Current deferred tax liabilities: |
|||||||||
Earnings not currently taxable |
(3,018 | ) | — | ||||||
Net deferred tax assets |
$ | 35,648 | $ | 31,512 | |||||
Non-current deferred taxes: |
|||||||||
Deferred tax assets: |
|||||||||
Net operating losses |
$ | 9,433 | $ | 2,196 | |||||
Deferred equity compensation |
14,872 | 11,685 | |||||||
Liabilities/expenses not currently deductible |
465 | 4,057 | |||||||
Total non-current deferred tax assets |
24,770 | 17,938 | |||||||
Deferred tax liabilities: |
|||||||||
Property and equipment |
(50,222 | ) | (110,919 | ) | |||||
Earnings not currently taxable |
(5,079 | ) | (5,964 | ) | |||||
Total non-current deferred tax liabilities |
(55,301 | ) | (116,883 | ) | |||||
Net non-current deferred tax liabilities |
$ | (30,531 | ) | $ | (98,945 | ) | |||
As of December 31, 2010, Covance has United States and foreign net operating loss carryforwards of $36.9 million. The foreign net operating loss carryforwards of $31.5 million have no expiration, while the United States net operating loss carryforwards of $5.4 million are subject to limitation under Internal Revenue Code §382 and will expire at various dates through 2026. It is expected that all loss carryforwards will be realized, and accordingly, no valuation allowance has been provided.
The net reduction in the deferred tax liability relating to property and equipment results primarily from asset impairment charges, which are not currently deductible for income tax purposes.
Covance currently provides income taxes on the earnings of foreign subsidiaries to the extent those earnings are taxable or are expected to be remitted. Covance's historical policy has been to leave its unremitted foreign earnings invested indefinitely outside the United States. Covance intends to continue to leave its unremitted foreign earnings invested indefinitely outside the United States. It is not practical to estimate the amount of additional tax that might be payable if such accumulated earnings were remitted. Additionally, if such accumulated earnings were remitted, certain countries impose withholding taxes that, subject to certain
54
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
7. Taxes on Income (Continued)
limitations, are available for use as a tax credit against any Federal income tax liability arising from such remittance. As a result, taxes have not been provided on accumulated foreign unremitted earnings totaling approximately $605 million at December 31, 2010.
The Company recognizes a tax benefit from an uncertain tax position only if it is more likely than not to be sustained upon examination based on the technical merits of the position. The amount of the accrual for which an exposure exists is measured as the largest amount of benefit determined on a cumulative probability basis that the Company believes is more likely than not to be realized upon ultimate settlement of the position. Components of the reserve are classified as either a current or long-term liability in the consolidated balance sheet based on when the Company expects each of the items to be settled. Covance accrues interest and penalties in relation to unrecognized tax benefits as a component of income tax expense.
As of December 31, 2010, the balance of the reserve for unrecognized tax benefits was $15.0 million, including accrued interest of $1.0 million, which is recorded as a long-term liability in other liabilities on the consolidated balance sheet. As of December 31, 2009, the balance of the reserve for unrecognized tax benefits was $17.2 million, including accrued interest of $1.2 million, of which $0.1 million was recorded as a current liability in accrued expenses and other current liabilities, and $17.1 million was recorded as a long-term liability in other liabilities on the consolidated balance sheet. This reserve relates to exposures for income tax matters such as transfer pricing, nexus, deemed income and research and development credits. During the year ended December 31, 2010, the reserve for unrecognized tax benefits was reduced by $2.2 million, as tax benefits totaling $7.7 million were recorded, relating primarily to the favorable resolution of several income tax audits, partially offset by the accrual of $5.5 million in reserves relating to deemed income, other tax matters and the accrual of interest on matters outstanding.
Following is a reconciliation of the beginning and ending amount of unrecognized tax benefits, excluding accrued interest for the years ended December 31, 2010, 2009 and 2008:
| (dollars in millions) |
|
|||
|---|---|---|---|---|
Unrecognized tax benefits as of December 31, 2007 |
$ | 7.6 | ||
Additions related to tax positions in the prior year |
2.1 | |||
Additions related to tax positions in the current year |
2.7 | |||
Reductions due to statute expiration |
(1.7 | ) | ||
Unrecognized tax benefits as of December 31, 2008 |
10.7 | |||
Additions related to tax positions in the prior year |
5.3 | |||
Additions related to tax positions in the current year |
2.6 | |||
Reductions due to settlements and payments |
(2.1 | ) | ||
Reductions due to statute expiration |
(0.5 | ) | ||
Unrecognized tax benefits as of December 31, 2009 |
16.0 | |||
Additions related to tax positions in the prior year |
3.8 | |||
Additions related to tax positions in the current year |
1.9 | |||
Reductions due to settlements and payments |
(7.2 | ) | ||
Reductions due to statute expiration |
(0.5 | ) | ||
Unrecognized tax benefits as of December 31, 2010 |
$ | 14.0 | ||
55
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
7. Taxes on Income (Continued)
Any future changes in the liability for unrecognized tax benefits, resulting from the recognition of tax benefits, would impact the effective tax rate. Over the next twelve months, it is reasonably possible that the uncertainty surrounding up to $8.0 million of the reserve for unrecognized tax benefits related to certain income taxes, deemed income, transfer pricing and state tax issues will be resolved as a result of the expiration of the statute of limitations or the conclusion of various federal, state and foreign tax audits.
The following tax years remain open to investigation as of December 31, 2010, for the Company's major jurisdictions:
Tax Jurisdiction
|
Years | |||
|---|---|---|---|---|
US Federal and State |
2005-2010 | |||
United Kingdom |
2008-2010 | |||
Switzerland |
2006-2010 | |||
Germany |
2007-2010 | |||
8. Credit Facilities
On October 26, 2010, Covance amended its credit facility which was due to expire in June 2012. The amended credit agreement (the "Credit Agreement") provides for a revolving credit facility of up to $250 million, which may be expanded to $300 million at Covance's election, and a term loan commitment of $100 million. At December 31, 2010 there were $35.0 million of outstanding borrowings and $1.4 million of outstanding letters of credit under the revolving credit facility and $97.5 million of outstanding debt under the term loan facility. At December 31, 2009 there were no outstanding borrowings and $1.4 million of outstanding letters of credit under the previous credit facility. Interest on all outstanding borrowings under the Credit Agreement is computed in accordance with the terms of the Credit Agreement and is presently based upon the London Interbank Offered Rate plus a margin of 200 basis points. Interest on all outstanding borrowings under the previous credit facility was also based upon the London Interbank Offered Rate plus a margin of 200 basis points. Interest on outstanding borrowings approximated 2.38% per annum during 2010 and 1.63% per annum during 2009. Costs associated with the Credit Agreement, which expires in October 2015, consisted primarily of bank and legal fees totaling $1.7 million, and are being amortized over the five year term.
The Company pays a commitment fee of 30 basis points on the undrawn balance of the revolving credit facility, which totaled approximately $0.5 million and $0.2 million during the years ended December 31, 2010 and 2009, respectively. The Credit Agreement contains various financial and other covenants and is collateralized by guarantees of certain of Covance's domestic subsidiaries and a pledge of 65 percent of the capital stock of certain of Covance's foreign subsidiaries. At December 31, 2010, Covance was in compliance with the terms of the Credit Agreement.
As of December 31, 2010, the contractual maturity of the Company's $97.5 million long term debt is as follows:
Year Ending December 31,
|
Maturities | |||
|---|---|---|---|---|
2011 |
$ | 10,000 | ||
2012 |
$ | 10,000 | ||
2013 |
$ | 10,000 | ||
2014 |
$ | 10,000 | ||
2015 |
$ | 57,500 | ||
56
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
9. Employee Benefit Plans
Covance sponsors various pension and other post-retirement benefit plans. All plans have a measurement date of December 31.
Defined Benefit Pension Plans
Covance sponsors two defined benefit pension plans for the benefit of its employees at two United Kingdom subsidiaries and one defined benefit pension plan for the benefit of its employees at a German subsidiary, all of which are legacy plans of previously acquired companies. Benefit amounts for all three plans are based upon years of service and compensation. The German plan is unfunded while the United Kingdom pension plans are funded. Covance's funding policy has been to contribute annually a fixed percentage of the eligible employee's salary at least equal to the local statutory funding requirements.
The components of net periodic pension cost for these plans for 2010, 2009 and 2008 are as follows:
| |
United Kingdom Plans | German Plan | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | 2010 | 2009 | 2008 | ||||||||||||||
Components of Net Periodic Pension Cost: |
||||||||||||||||||||
Service cost |
$ | 3,682 | $ | 3,150 | $ | 5,226 | $ | 754 | $ | 576 | $ | 597 | ||||||||
Interest cost |
7,718 | 7,200 | 7,825 | 584 | 527 | 486 | ||||||||||||||
Expected return on plan assets |
(8,996 | ) | (7,552 | ) | (8,877 | ) | — | — | — | |||||||||||
Amortization of net actuarial loss |
1,249 | 1,162 | 1,207 | 74 | 7 | 46 | ||||||||||||||
Expected participant contributions |
(2,074 | ) | (1,933 | ) | (1,999 | ) | — | — | — | |||||||||||
Net periodic pension cost |
$ | 1,579 | $ | 2,027 | $ | 3,382 | $ | 1,412 | $ | 1,110 | $ | 1,129 | ||||||||
Weighted Average Assumptions Used to Determine Net Periodic Pension Cost: |
||||||||||||||||||||
Discount rate |
5.75 | % | 6.25 | % | 5.50 | % | 5.50 | % | 6.25 | % | 5.60 | % | ||||||||
Expected rate of return on assets |
6.75 | % | 6.75 | % | 6.75 | % | n/a | n/a | n/a | |||||||||||
Salary increases |
4.50 | % | 4.25 | % | 4.25 | % | 3.00 | % | 3.00 | % | 3.00 | % | ||||||||
The weighted average expected long term rate of return on the assets of the United Kingdom pension plans is based on the target asset allocation and the average rate of growth expected for the asset classes invested. The weighted average rate of expected growth is derived from a combination of historic returns, current market indicators, the expected risk premium for each asset class over the risk-free rate and the opinion of professional advisors.
57
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
9. Employee Benefit Plans (Continued)
The change in the projected benefit obligation and plan assets, the funded status of the plan and a reconciliation of such funded status to the amounts reported in the consolidated balance sheets as of December 31, 2010 and 2009 is as follows:
| |
United Kingdom Plans | German Plan | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2010 | 2009 | ||||||||||
Change in Projected Benefit Obligation: |
||||||||||||||
Benefit obligation, beginning of year |
$ | 139,875 | $ | 111,868 | $ | 11,466 | $ | 8,691 | ||||||
Service cost |
3,682 | 3,150 | 754 | 576 | ||||||||||
Interest cost |
7,718 | 7,200 | 584 | 527 | ||||||||||
Actuarial loss |
12,156 | 14,906 | 850 | 1,740 | ||||||||||
Benefits paid |
(2,374 | ) | (4,823 | ) | (132 | ) | (134 | ) | ||||||
Foreign currency exchange rate changes |
(4,453 | ) | 7,574 | (960 | ) | 66 | ||||||||
Benefit obligation, end of year |
$ | 156,604 | $ | 139,875 | $ | 12,562 | $ | 11,466 | ||||||
Change in Fair Value of Assets: |
||||||||||||||
Fair value of plan assets, beginning of year |
$ | 135,006 | $ | 105,343 | $ | — | $ | — | ||||||
Covance contributions |
6,175 | 9,749 | — | — | ||||||||||
Employee contributions |
2,026 | 1,912 | — | — | ||||||||||
Actual return on plan assets |
17,152 | 15,626 | — | — | ||||||||||
Benefits paid |
(2,374 | ) | (4,823 | ) | — | — | ||||||||
Foreign currency exchange rate changes |
(4,301 | ) | 7,199 | — | — | |||||||||
Fair value of plan assets, end of year |
$ | 153,684 | $ | 135,006 | $ | — | $ | — | ||||||
Funded status at end of year—under funded |
$ | (2,920 | ) | $ | (4,869 | ) | $ | (12,562 | ) | $ | (11,466 | ) | ||
| |
United Kingdom Plans | German Plan | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2010 | 2009 | ||||||||||
Amounts recognized in the consolidated balance sheets: |
||||||||||||||
Current liabilities |
$ | — | $ | — | $ | (172 | ) | $ | (160 | ) | ||||
Non-current liabilities |
(2,920 | ) | (4,869 | ) | (12,390 | ) | (11,306 | ) | ||||||
Total |
$ | (2,920 | ) | $ | (4,869 | ) | $ | (12,562 | ) | $ | (11,466 | ) | ||
Covance contributed $6.2 million in 2010 and $9.7 million (including a discretionary contribution of $4.8 million) in 2009 to its United Kingdom plans and expects to contribute $6.3 million in 2011. No contributions were made during 2010 or 2009 to the German plan, nor are any contributions expected to be made to the German plan in 2011, since that plan is unfunded.
The accumulated benefit obligation for the United Kingdom pension plans was $126.9 million and $111.3 million at December 31, 2010 and 2009, respectively. The accumulated benefit obligation for the German plan was $10.0 million and $8.7 million at December 31, 2010 and 2009, respectively.
58
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
9. Employee Benefit Plans (Continued)
The amounts recognized in accumulated other comprehensive income as of December 31, 2010 and 2009 are as follows:
| |
United Kingdom Plans | German Plan | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2010 | 2009 | ||||||||||
Net actuarial loss |
$ | 40,659 | $ | 37,680 | $ | 2,908 | $ | 2,145 | ||||||
Less: Tax benefit (deferred tax asset) |
(11,385 | ) | (10,850 | ) | (889 | ) | (711 | ) | ||||||
Accumulated other comprehensive income impact |
$ | 29,274 | $ | 26,830 | $ | 2,019 | $ | 1,434 | ||||||
Weighted Average Assumptions Used to Determine Benefit Obligations: |
||||||||||||||
Discount rate |
5.20 | % | 5.75 | % | 4.60 | % | 5.50 | % | ||||||
Salary increases |
4.50 | % | 4.50 | % | 2.50 | % | 3.00 | % | ||||||
The net actuarial loss for the United Kingdom and German pension plans required to be amortized from accumulated other comprehensive income into net periodic pension cost in 2011 is expected to be $1.3 million and $0.1 million, respectively.
The investment policies for the United Kingdom pension plans are set by the plan trustees, based upon the guidance of professional advisors and after consultation with the Company, taking into consideration the plans' liabilities and future funding levels. The trustees have set the long-term investment policy largely in accordance with the asset allocation of a broadly diversified investment portfolio. Assets are generally invested within the target ranges as follows:
Equity securities |
51%–61% | |
Debt securities |
34%–44% | |
Real estate |
4%–9% | |
Other |
0%–5% |
The weighted average asset allocation of the United Kingdom pension plans as of December 31, 2010 and 2009 by asset category is as follows:
| |
2010 | 2009 | ||||||
|---|---|---|---|---|---|---|---|---|
Equity securities |
54% | 53% | ||||||
Debt securities |
37% | 36% | ||||||
Real estate |
5% | 5% | ||||||
Other |
4% | 6% | ||||||
Total |
100% | 100% | ||||||
59
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
9. Employee Benefit Plans (Continued)
Investments are made in pooled investment funds. Pooled investment fund managers are regulated by the Financial Services Authority in the United Kingdom and operate under terms which contain restrictions on the way in which the portfolios are managed and require the managers to ensure that suitable internal operating procedures are in place. The trustees have set performance objectives for each fund manager and routinely monitor and assess the managers' performance against such objectives.
The fair value of the Company's United Kingdom pension plans' assets as of December 31, 2010, by asset category, are as follows:
| |
Total | Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Cash |
$ | 46 | $ | 46 | $ | — | $ | — | |||||
Mutual funds(a) |
153,638 | — | 153,638 | — | |||||||||
Total |
$ | 153,684 | $ | 46 | $ | 153,638 | $ | — | |||||
- (a)
- Mutual funds represent pooled investment vehicles offered by investment managers, which are generally comprised of investments in equities, bonds, property and cash. The plans' trustees hold units in these funds, the value of which is determined by the number of units held multiplied by the unit price calculated by the investment managers. That unit price is derived based on the market value of the securities that comprise the fund, which are determined by quoted prices in active markets. No element of the valuation is based on inputs made by the plans' trustees.
Expected future benefit payments are as follows:
Year Ending December 31,
|
United Kingdom Plans |
German Plan |
|||||
|---|---|---|---|---|---|---|---|
2011 |
$ | 2,089 | $ | 172 | |||
2012 |
$ | 2,233 | $ | 182 | |||
2013 |
$ | 3,093 | $ | 203 | |||
2014 |
$ | 3,127 | $ | 221 | |||
2015 |
$ | 4,013 | $ | 236 | |||
2016-2020 |
$ | 22,655 | $ | 1,257 | |||
Supplemental Executive Retirement Plan
In addition to these foreign defined benefit pension plans, Covance also has a non-qualified Supplemental Executive Retirement Plan ("SERP"). The SERP, which is not funded, is intended to provide retirement benefits for certain executive officers of Covance. Benefit amounts are based upon years of service and compensation of the participating employees.
60
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
9. Employee Benefit Plans (Continued)
The components of net periodic pension cost for the years ended December 31, 2010, 2009 and 2008 are as follows:
| |
2010 | 2009 | 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Components of Net Periodic Pension Cost: |
|||||||||||
Service cost |
$ | 1,391 | $ | 1,056 | $ | 1,234 | |||||
Interest cost |
693 | 594 | 868 | ||||||||
Amortization of prior service credit |
(119 | ) | (119 | ) | (119 | ) | |||||
Amortization of net actuarial loss |
226 | — | 107 | ||||||||
Settlement loss |
— | 643 | — | ||||||||
Net periodic pension cost |
$ | 2,191 | $ | 2,174 | $ | 2,090 | |||||
Weighted Average Assumptions Used to Determine Net Periodic Pension Cost: |
|||||||||||
Discount rate |
5.25 | % | 6.00 | % | 5.75 | % | |||||
Salary increases |
4.00 | % | 4.00 | % | 4.00 | % | |||||
The net periodic pension cost for the year ended December 31, 2009 includes a non-cash charge of $0.6 million as a result of a plan settlement due to total lump-sum distributions from the plan in 2009 exceeding service and interest costs for that year.
The change in the projected benefit obligation, the funded status of the plan and a reconciliation of such funded status to the amounts reported in the consolidated balance sheets as of December 31, 2010 and 2009 is as follows:
| |
|
2010 | 2009 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Change in Projected Benefit Obligation: |
|||||||||||
Benefit obligation, beginning of year |
$ | 12,082 | $ | 14,190 | |||||||
Service cost |
1,391 | 1,056 | |||||||||
Interest cost |
693 | 594 | |||||||||
Actuarial loss |
931 | 2,404 | |||||||||
Benefits paid |
(572 | ) | (6,162 | ) | |||||||
Benefit obligation, end of year |
$ | 14,525 | $ | 12,082 | |||||||
Funded status at end of year—under funded |
$ | (14,525 | ) | $ | (12,082 | ) | |||||
| |
|
2010 | 2009 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Amounts recognized in the consolidated balance sheets: |
|||||||||||
Current liabilities |
$ | — | $ | (568 | ) | ||||||
Non-current liabilities |
(14,525 | ) | (11,514 | ) | |||||||
Total |
$ | (14,525 | ) | $ | (12,082 | ) | |||||
61
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
9. Employee Benefit Plans (Continued)
The accumulated benefit obligation as of December 31, 2010 and 2009 is $12.2 million and $9.5 million, respectively.
The amounts recognized in accumulated other comprehensive income and not yet recognized as a component of net periodic pension cost as of December 31, 2010 and 2009 are as follows:
| |
2010 | 2009 | |||||
|---|---|---|---|---|---|---|---|
Net actuarial loss |
$ | 4,217 | $ | 3,512 | |||
Prior service credit |
(926 | ) | (1,045 | ) | |||
Less: Tax benefit (deferred tax asset) |
(1,163 | ) | (987 | ) | |||
Accumulated other comprehensive income impact |
$ | 2,128 | $ | 1,480 | |||
The net actuarial loss and prior service credit required to be amortized from accumulated other comprehensive income into net periodic pension cost in 2011 are estimated to be $0.3 million and $(0.1) million, respectively.
| |
2010 | 2009 | ||||||
|---|---|---|---|---|---|---|---|---|
Weighted Average Assumptions Used to Determine Benefit Obligation: |
||||||||
Discount rate |
4.40 | % | 5.25 | % | ||||
Salary increases |
3.75 | % | 4.00 | % | ||||
Expected future benefit payments are as follows:
Year Ending December 31,
|
|
|||
|---|---|---|---|---|
2011 |
$ | — | ||
2012 |
$ | — | ||
2013 |
$ | 1,976 | ||
2014 |
$ | 1,117 | ||
2015 |
$ | 6,518 | ||
2016-2020 |
$ | 2,882 | ||
Post-Employment Retiree Health and Welfare Plan
Covance also sponsors a post-employment retiree health and welfare plan for the benefit of eligible employees at certain U.S. subsidiaries who retire after satisfying service and age requirements. This plan is funded on a pay-as-you-go basis and the cost of providing these benefits is shared with the retirees.
62
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
9. Employee Benefit Plans (Continued)
The components of net periodic post-retirement benefit cost for 2010, 2009 and 2008 are as follows:
| |
2010 | 2009 | 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Components of Net Periodic Post-retirement Benefit Cost: |
|||||||||||
Service cost |
$ | 108 | $ | 96 | $ | 119 | |||||
Interest cost |
314 | 310 | 313 | ||||||||
Amortization of net actuarial loss |
33 | — | 5 | ||||||||
Net periodic post-retirement benefit cost |
$ | 455 | $ | 406 | $ | 437 | |||||
Weighted Average Assumptions Used to Determine Net Periodic |
|||||||||||
Post-retirement Benefit Cost: |
|||||||||||
Weighted average discount rate |
5.25 | % | 6.00 | % | 5.75 | % | |||||
Health care cost trend rate |
7.50 | %(a) | 8.00 | %(a) | 8.50 | %(a) | |||||
- (a)
- decreasing to ultimate trend of 5.00% in 2015
The change in the projected post-retirement benefit obligation, the funded status of the plan and the reconciliation of such funded status to the amounts reported in the consolidated balance sheets as of December 31, 2010 and 2009 is as follows:
| |
|
2010 | 2009 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Change in Projected Benefit Obligation: |
|||||||||||
Benefit obligation, beginning of year |
$ | 5,537 | $ | 5,637 | |||||||
Service cost |
108 | 96 | |||||||||
Interest cost |
314 | 310 | |||||||||
Participant contributions |
582 | 553 | |||||||||
Actuarial loss (gain) |
666 | (295 | ) | ||||||||
Benefits paid |
(1,068 | ) | (938 | ) | |||||||
Federal subsidy on benefits paid |
85 | 174 | |||||||||
Benefit obligation, end of year |
$ | 6,224 | $ | 5,537 | |||||||
Funded status at end of year—under funded |
$ | (6,224 | ) | $ | (5,537 | ) | |||||
| |
|
2010 | 2009 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Amounts recognized in the consolidated balance sheets: |
|||||||||||
Current liabilities |
$ | (588 | ) | $ | (553) | ||||||
Non-current liabilities |
(5,636 | ) | (4,984 | ) | |||||||
Total |
$ | (6,224 | ) | $ | (5,537 | ) | |||||
63
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
9. Employee Benefit Plans (Continued)
The amounts recognized in accumulated other comprehensive income as of December 31, 2010 and 2009 are as follows:
| |
2010 | 2009 | |||||
|---|---|---|---|---|---|---|---|
Net actuarial loss |
$ | 786 | $ | 152 | |||
Less: Tax benefit (deferred tax asset) |
(278 | ) | (61 | ) | |||
Accumulated other comprehensive income impact |
$ | 508 | $ | 91 | |||
The net actuarial loss required to be amortized from accumulated other comprehensive income into net periodic post-retirement benefit cost in 2011 is estimated to be $30 thousand.
| |
2010 | 2009 | ||||||
|---|---|---|---|---|---|---|---|---|
Assumptions Used to Determine Benefit Obligation: |
||||||||
Weighted average discount rate |
4.70 | % | 5.25 | % | ||||
Health care cost trend rate |
8.50% | (a) | 7.50 | % | ||||
- (a)
- decreasing to ultimate trend of 5.00% in 2017
A one-percentage-point increase or decrease in the assumed health care cost trend rate would not impact the net service and interest cost components of the net periodic post-retirement benefit cost or the post-retirement benefit obligation since future increases in plan costs are paid by participant contributions. Covance expects to contribute $0.6 million to the post-employment retiree health and welfare plan in 2011.
Expected future gross benefit payments, Federal subsidies and net benefit payments are as follows:
Year Ending December 31,
|
Gross Benefit Payments |
Federal Subsidies |
Net Benefit Payments |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
2011 |
$ | 1,277 | $ | (128 | ) | $ | 1,149 | |||
2012 |
$ | 1,297 | $ | (140 | ) | $ | 1,157 | |||
2013 |
$ | 1,359 | $ | (148 | ) | $ | 1,211 | |||
2014 |
$ | 1,393 | $ | (156 | ) | $ | 1,237 | |||
2015 |
$ | 1,577 | $ | — | $ | 1,577 | ||||
2016-2020 |
$ | 7,798 | $ | — | $ | 7,798 | ||||
Defined Contribution Plans
U.S. employees are eligible to participate in Covance's 401(k) plan, while employees in international locations are eligible to participate in either defined benefit or defined contribution plans, depending on the plan offered at their location. Aggregate Covance contributions to its various defined contribution plans totaled $33.1 million, $26.8 million and $26.6 million for 2010, 2009 and 2008, respectively.
64
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
10. Stockholders' Equity
Preferred Stock
Covance is authorized to issue up to 10.0 million shares of Series Preferred Stock, par value $1.00 per share (the "Covance Series Preferred Stock"). The Covance Board of Directors has the authority to issue such shares from time to time, without stockholder approval, and to determine the designations, preferences, rights, including voting rights, and restrictions of such shares, subject to the Delaware General Corporate Laws. Pursuant to this authority, the Covance Board of Directors has designated 1.0 million shares of the Covance Series Preferred Stock as Covance Series A Preferred Stock. No other class of Covance Series Preferred Stock has been designated by the Board. As of December 31, 2010, no Covance Series Preferred Stock has been issued or is outstanding.
Dividends—Common Stock
Covance's Board of Directors may declare dividends on the shares of Covance common stock out of legally available funds (subject to any preferential rights of any outstanding Covance Series Preferred Stock). However, Covance has no present intention to declare dividends, but instead intends to retain earnings to provide funds for the operation and expansion of its business.
Treasury Stock
The Board of Directors has, from time to time, approved stock repurchase programs enabling Covance to repurchase shares of its common stock. In September 2010, the Covance Board of Directors authorized the repurchase of up to $250 million of the Company's outstanding common stock (the "2010 Repurchase Program"). This was in addition to a 3.0 million share buyback authorization approved by the Covance Board of Directors in 2007 (the "2007 Repurchase Program"). In November 2010, the Company entered into an agreement with an independent financial institution to execute the 2010 Repurchase Program through an accelerated share repurchase transaction (the "ASR"). Pursuant to the ASR, the Company provided the independent financial institution with an upfront payment totaling $250 million and the independent financial institution delivered to the Company 4.75 million shares of its common stock. The number of shares delivered was determined based upon the volume weighted-average price ("VWAP") during the initial averaging period (as defined in the ASR). The actual number of shares that will be repurchased under the ASR will be determined based upon the VWAP during the repurchase period. To the extent that the actual VWAP is lower than the initial VWAP used to determine the 4.75 million shares already delivered, additional shares will be repurchased by the independent financial institution and those shares will be delivered to Covance at the end of the repurchase period. At December 31, 2010, there were 0.8 million shares remaining for purchase under the 2007 Repurchase Program. In addition to the Board approved share repurchase programs, Covance also reacquires shares of its common stock when employees tender shares to satisfy income tax withholdings associated with the vesting of stock awards.
65
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
10. Stockholders' Equity (Continued)
The following table sets forth the treasury stock activity during 2010, 2009 and 2008:
| |
2010 | 2009 | 2008 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (amounts in thousands) |
$ | # shares | $ | # shares | $ | # shares | ||||||||||||||
Shares repurchased in connection with: |
||||||||||||||||||||
Board approved buyback programs |
$ | 250,000 | 4,753.6 | $ | — | — | $ | 126,710 | 1,500.0 | |||||||||||
Employee benefit plans |
6,351 | 115.2 | 4,828 | 106.6 | 6,196 | 102.2 | ||||||||||||||
Total |
$ | 256,351 | 4,868.8 | $ | 4,828 | 106.6 | $ | 132,906 | 1,602.2 | |||||||||||
Stock-Based Compensation Plans
In May 2010, Covance's shareholders approved the 2010 Employee Equity Participation Plan (the "2010 EEPP") in replacement of the 2007 Employee Equity Participation Plan (the "2007 EEPP"). Effective upon approval of the 2010 EEPP, no further grants or awards were permitted under the 2007 EEPP. Shares remaining available for grant under the 2007 EEPP are available for grant under the 2010 EEPP. The 2010 EEPP became effective on May 6, 2010 and will expire on May 5, 2020. The 2010 EEPP authorizes the Compensation and Organization Committee of the Board of Directors (the "Compensation Committee"), or such committee as is appointed by the Covance Board of Directors, to administer the 2010 EEPP and to grant awards to employees of Covance. The 2010 EEPP authorizes the Compensation Committee to grant the following awards: options to purchase common stock; stock appreciation rights; and other stock awards either singly or in combination. Shares granted, other than options or SARs, shall be counted against the shares available for grant based upon the ratio of 1.74 for every 1 share granted. The exercise period for stock options granted under the 2010 EEPP is determined by the Compensation Committee at the time of grant, and is generally ten years from the date of grant. The vesting period for stock options and stock awards granted under the 2010 EEPP is determined by the Compensation Committee at the time of grant. Beginning in 2009, options are generally granted with a pro rata three year vesting period for all employees. Prior to 2009, options were generally granted with a pro rata three year vesting period for senior executives and a pro rata two year vesting period for all other optionees. Stock awards generally vest over a three year period for all employees. The number of shares of Covance common stock initially available for grant under the 2010 EEPP totaled approximately 4.3 million plus approximately 1.3 million shares remaining available under the 2007 EEPP at the time the 2010 EEPP was approved. All grants and awards under the 2007 EEPP remaining outstanding are now administered in accordance with the provisions of the 2007 EEPP out of shares issuable under the 2010 EEPP. The Company may issue authorized but previously unissued shares or treasury shares when options are exercised or for stock awards. There have been no grants of stock appreciation rights under the 2007 EEPP or the 2010 EEPP. At December 31, 2010 there were approximately 5.8 million shares remaining available for grants under the 2010 EEPP.
The Company recognizes stock-based compensation expense on a straight-line basis over the vesting period of the related awards based upon the grant-date fair value of awards expected to vest. Results of operations for the year ended December 31, 2010 include $32.3 million ($22.0 million net of tax benefit of $10.3 million) of total stock-based compensation expense, $17.2 million of which has been included in cost of revenue and $15.1 million of which has been included in selling, general and administrative expenses. Results of operations for the year ended December 31, 2009 include $26.9 million ($18.3 million net of tax benefit of $8.6 million) of total stock-based compensation expense, $11.5 million of which has been included in cost of revenue and $15.4 million of which has been included in selling, general and administrative expenses. Results of
66
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
10. Stockholders' Equity (Continued)
operations for the year ended December 31, 2008 include $25.5 million ($17.2 million net of tax benefit of $8.3 million) of total stock-based compensation expense, $9.9 million of which has been included in cost of revenue and $15.6 million of which has been included in selling, general and administrative expenses.
Options—The grant-date fair value of stock option awards is estimated using an option pricing model. For stock options granted on or subsequent to January 1, 2006, the Company uses the Lattice-Binomial option pricing formula to estimate the grant-date fair value of stock option awards, whereas for stock options granted prior to January 1, 2006, the Company used the Black-Scholes-Merton option pricing formula. In order to estimate the grant-date fair value, option pricing models require the use of estimates and assumptions as to (a) the expected term of the option, (b) the expected volatility of the price of the underlying stock, (c) the risk-free interest rate for the expected term of the option and (d) pre-vesting forfeiture rates. The expected term of the option is based upon the contractual term, taking into account expected employee exercise and expected post-vesting employment termination behavior. The expected volatility of the price of the underlying stock is based upon the volatility of the Company's stock computed over a period of time equal to the expected term of the option. The risk free interest rate is based upon the implied yields currently available from the U.S. Treasury zero-coupon yield curve for issues with a remaining duration equal to the expected term of the option. Pre-vesting forfeiture rates are estimated based upon past voluntary termination behavior and past option forfeitures.
The following table sets forth the weighted-average assumptions used to calculate the fair value of options granted for the years ended December 31, 2010, 2009 and 2008:
| |
2010 | 2009 | 2008 | ||||||
|---|---|---|---|---|---|---|---|---|---|
Expected stock price volatility |
35% | 36% | 39% | ||||||
Range of risk free interest rates |
0.1% - 3.8% | 0.3% - 2.6% | 1.9% - 3.7% | ||||||
Expected life of options (years) |
4.7 | 4.6 | 4.4 | ||||||
The following table sets forth Covance's stock option activity as of and for the year ended December 31, 2010:
| |
Number of Shares (in thousands) |
Weighted Average Price |
Weighted Average Remaining Contractual Life |
Aggregate Intrinsic Value (in millions) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Options outstanding, December 31, 2009 |
2,959.8 | $ | 43.24 | ||||||||||
Granted |
1,007.9 | $ | 56.61 | ||||||||||
Exercised |
(367.3 | ) | $ | 27.31 | |||||||||
Forfeited |
(170.3 | ) | $ | 49.06 | |||||||||
Options outstanding, December 31, 2010 |
3,430.1 | $ | 48.59 | 6.7 years | $ | 26.9 | |||||||
Vested & unvested expected to vest, December 31, 2010 |
3,362.4 |
$ |
48.49 |
6.7 years |
$ |
26.7 |
|||||||
Exercisable at December 31, 2010 |
1,852.9 |
$ |
46.12 |
4.9 years |
$ |
20.9 |
|||||||
67
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
10. Stockholders' Equity (Continued)
The weighted-average grant-date fair value per share of options granted during 2010, 2009 and 2008 was $18.55, $12.73 and $28.34, respectively. As of December 31, 2010, the total unrecognized compensation cost related to non-vested stock options granted was $17.0 million and is expected to be recognized over a weighted average period of 1.9 years.
The following table sets forth the aggregate intrinsic value of options exercised and the aggregate grant-date fair value of shares which vested during 2010, 2009 and 2008:
| |
2010
|
2009
|
2008
|
||||||
|---|---|---|---|---|---|---|---|---|---|
(in millions) |
|||||||||
Aggregate intrinsic value of options exercised |
$ | 9.7 | $ | 3.7 | $ | 30.3 | |||
Aggregate grant-date fair value of shares vested |
$ | 8.3 | $ | 7.3 | $ | 7.1 | |||
Cash proceeds from stock options exercised during the years ended December 31, 2010, 2009 and 2008 totaled $10.0 million, $3.1 million and $17.9 million, respectively. The cash flows resulting from tax benefits realized on tax deductions in excess of the compensation expense recognized for stock options exercised in the period are classified as a financing cash flow. The excess tax benefit classified as a financing cash inflow during the years ended December 31, 2010, 2009 and 2008 was $1.6 million, $0.8 million and $7.3 million, respectively. The actual tax benefit realized on stock options exercised during the years ended December 31, 2010, 2009 and 2008 was $2.9 million, $1.3 million and $8.9 million, respectively. The difference between the actual tax benefit received and the excess tax benefit for the years ended December 31, 2010, 2009 and 2008, of $1.3 million, $0.5 million and $1.6 million, respectively, is classified as an operating cash inflow.
Restricted Stock Awards—Restricted stock awards are granted subject to either service conditions (restricted stock) or service and performance conditions (performance-based shares). The grant-date fair value of restricted stock and performance-based share awards, which has been determined based upon the market value of Covance's shares on the grant date, is expensed over the vesting period.
The following table sets forth Covance's performance-based shares and restricted stock activity as of and for the year ended December 31, 2010:
| |
Performance-based Shares | Restricted Stock | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of Shares (in thousands) |
Weighted Average Grant Date Fair Value |
Number of Shares (in thousands) |
Weighted Average Grant Date Fair Value |
||||||||||
Nonvested at December 31, 2009 |
203.1 | $ | 55.24 | 646.7 | $ | 48.25 | ||||||||
Granted |
74.0 | $ | 56.93 | 567.8 | $ | 55.99 | ||||||||
Vested |
(38.6 | ) | $ | 80.34 | (263.3 | ) | $ | 51.99 | ||||||
Forfeited |
(52.7 | ) | $ | 44.62 | (91.7 | ) | $ | 49.91 | ||||||
Nonvested at December 31, 2010 |
185.8 | $ | 53.70 | 859.5 | $ | 52.05 | ||||||||
The blended weighted average grant-date fair value of performance-based shares and restricted stock awards granted during the year ended December 31, 2010, 2009 and 2008 was $56.10, $41.70 and $78.58, respectively. As of December 31, 2010, the total unrecognized compensation cost related to non-vested performance-based shares and restricted stock awards was $31.7 million. This cost is expected to be recognized over a weighted average period of 1.7 years. The total fair value of performance-based shares and restricted stock which vested during 2010, 2009 and 2008 was $16.8 million, $17.1 million and $14.7 million, respectively.
68
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
10. Stockholders' Equity (Continued)
Employee Stock Purchase Plan—Covance had an employee stock purchase plan (the "ESPP"), pursuant to which Covance made available for sale to employees shares of its common stock at a price equal to 85% of the lower of the market value on the first or last day of each calendar quarter. The ESPP, administered by the Compensation Committee, was intended to give Covance employees the opportunity to purchase shares of Covance common stock through payroll deductions. A maximum of 3.0 million shares were available for purchase by Covance employees under the ESPP. During 2010, 2009 and 2008, a total of 163,232 shares, 200,054 shares and 89,760 shares of common stock, respectively, were issued under the ESPP. At December 31, 2010, there were approximately 0.3 million shares remaining for purchase under the ESPP. Effective January 1, 2011, the ESPP was terminated.
11. Commitments and Contingencies
Minimum annual rental commitments under non-cancelable operating leases, primarily for offices and laboratory facilities, in effect at December 31, 2010 are as follows:
Year Ending December 31,
|
|
|||
|---|---|---|---|---|
2011 |
$ | 28,481 | ||
2012 |
$ | 24,973 | ||
2013 |
$ | 21,495 | ||
2014 |
$ | 11,550 | ||
2015 |
$ | 9,465 | ||
2016 and beyond |
$ | 49,877 | ||
Operating lease rental expense aggregated $35.6 million, $31.8 million and $29.2 million for 2010, 2009 and 2008, respectively.
12. Facility Consolidation and Other Cost Reduction Actions
During the year ended December 31, 2010, Covance incurred costs totaling $28.0 million ($25.1 million of which has been included in selling, general and administrative expenses and $2.9 million of which has been included in depreciation and amortization) in connection with two restructuring actions. First, during the second quarter of 2010, the Company announced plans to reduce costs, primarily by closing and transitioning work conducted at its Austin, Texas Phase I clinic and Kalamazoo, Michigan research products facility into other more efficient locations. These actions were completed during 2010 and resulted in costs totaling $9.6 million. Second, during the fourth quarter, the Company announced plans to further rationalize capacity, reduce the cost of overhead and support functions and to streamline processes. In connection with the fourth quarter actions, the Company expects to incur costs totaling approximately $37 million, of which $18.4 million was incurred during the fourth quarter and the remaining costs are expected to be incurred during 2011.
Costs incurred or expected to be incurred in connection with these restructuring activities include employee separation costs, including severance, benefits and outplacement, lease and facility exit costs, accelerated depreciation associated with assets to be disposed of in connection with the exit activities and other expenses.
69
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
12. Facility Consolidation and Other Cost Reduction Actions (Continued)
The following table sets forth the rollforward of the restructuring activity for the year ended December 31, 2010:
| Description | Balance, Dec. 31, 2009 |
Total Charges |
Cash Payments |
Other | Balance, Dec. 31, 2010 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Employee separation costs |
$ | — | $ | 18,051 | $ | (6,327 | ) | $ | 14 | $ | 11,738 | |||||
Lease and facility exit costs |
— | 4,753 | (1,006 | ) | — | 3,747 | ||||||||||
Accelerated depreciation |
— | 2,873 | — | (2,873 | ) | — | ||||||||||
Other costs |
— | 2,353 | (850 | ) | (657 | ) | 846 | |||||||||
Total |
$ | — | $ | 28,030 | $ | (8,183 | ) | $ | (3,516 | ) | $ | 16,331 | ||||
Costs expected to be incurred by category for both actions total $24 million in employee separation costs, $12 million in lease and facility exit costs, $4 million in accelerated depreciation and $7 million in other costs. Costs by segment are expected to total $22 million in our early development segment, $13 million in our late-stage development segment and $12 million in corporate expenses. Costs incurred through December 31, 2010 totaled $14.1 million in our early development segment, $7.3 million in our late-stage development segment and $6.6 million in corporate expenses.
Other expenses include legal and professional fees primarily associated with employee related matters and the outsourcing of certain functions as well as the loss on disposal of assets. Other activity in the reserve rollforward reflects accelerated depreciation and the loss on disposal of assets.
13. Asset Impairment
Covance reviews its long-lived assets, other than goodwill and other indefinite lived intangible assets, for impairment when events or changes in circumstances occur that indicate that the carrying value of the asset may not be recoverable. The assessment of possible impairment is based upon Covance's judgment of its ability to recover the value of the asset from the expected future undiscounted cash flows of the related operations.
The significant weakening of the market for outsourced toxicology services during the three-month period ended September 30, 2010, which represented a reversal in trend from the recovering demand for these services during the first half of 2010, caused Covance to reassess its North American toxicology services. This assessment included an evaluation of the ongoing value of the long-lived assets associated with those services. Based on that evaluation, Covance determined that long-lived assets located in Chandler, Arizona and Manassas, Virginia with carrying values of $182.7 million and $23.4 million, respectively, were no longer fully recoverable from the cash flows expected from those assets. Accordingly, as of September 30, 2010, Covance recorded an asset impairment charge totaling $119.2 million ($103.0 million of which relates to the Chandler, Arizona assets and $16.2 million relates to the Manassas, Virginia assets), representing the excess of the carrying value of those assets over their respective fair market values.
The fair value of these assets was determined with the assistance of an independent third party appraiser. Covance's Chandler, Arizona facility, which will continue to be held and used, was valued at $79.7 million based upon both the future cash flows expected to be generated from the facility, discounted at the risk-free rate of interest, and an estimated market value of the facility. These assets are included in property and equipment on the consolidated balance sheet as of December 31, 2010. The property in Manassas, Virginia
70
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
13. Asset Impairment (Continued)
consists primarily of land that was intended to be utilized for future expansion projects in the Early Development segment. Covance now intends to sell this property and its carrying value has been reduced to a fair value of $7.2 million, which value is based upon market rates for similar properties sold in the region, net of selling costs. As a result, the Manassas property has been reclassified from property and equipment to other current assets on the consolidated balance sheet as of December 31, 2010.
The following table presents the above non-financial assets measured at estimated fair value as of September 30, 2010 and the resulting impairment losses included in earnings for the year ended December 31, 2010:
| |
|
Assets at Fair Value as of September 30, 2010 | |
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | Previous carrying value |
Quoted prices in active markets for identical assets (Level 1) |
Significant other observable inputs (Level 2) |
Significant unobservable inputs (Level 3) |
Impairment loss |
Adjusted carrying value |
|||||||||||||
Long-lived assets held and used (Chandler, Az.) |
$ | 182,694 | — | — | $ | 79,674 | $ | (103,020 | ) | $ | 79,674 | ||||||||
Long-lived assets held for sale (Manassas, Va.) |
23,371 | — | $ | 7,162 | — | (16,209 | ) | 7,162 | |||||||||||
Total |
$ | 206,065 | $ | — | $ | 7,162 | $ | 79,674 | $ | (119,229 | ) | $ | 86,836 | ||||||
14. Segment Information
Covance has two reportable segments: early development and late-stage development. Early development services, which includes Covance's preclinical and clinical pharmacology service capabilities, involve evaluating a new compound for safety and early effectiveness as well as evaluating the absorption, distribution, metabolism and excretion of the compound in the human body. It is at this stage that a pharmaceutical company, based on available data, will generally decide whether to continue further development of a drug. Late-stage development services, which include Covance's central laboratory, clinical development, periapproval and market access services, are geared toward demonstrating the clinical effectiveness of a compound in treating certain diseases or conditions, obtaining regulatory approval and maximizing the drug's commercial potential. The accounting policies of the reportable segments are the same as those described in Note 2.
71
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
14. Segment Information (Continued)
| |
Early Development |
Late-Stage Development |
Other Reconciling Items |
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Total revenues from external customers: |
||||||||||||||
2010 |
$ | 840,309 | $ | 1,085,321 | $ | 112,843 | (a) | $ | 2,038,473 | |||||
2009 |
$ | 791,801 | $ | 1,075,833 | $ | 94,992 | (a) | $ | 1,962,626 | |||||
2008 |
$ | 844,782 | $ | 883,316 | $ | 98,969 | (a) | $ | 1,827,067 | |||||
Depreciation and amortization: |
||||||||||||||
2010 |
$ | 68,216 | $ | 18,887 | $ | 15,921 | (b) | $ | 103,024 | |||||
2009 |
$ | 62,108 | $ | 16,221 | $ | 12,960 | (b) | $ | 91,289 | |||||
2008 |
$ | 48,010 | $ | 16,808 | $ | 6,753 | (b) | $ | 71,571 | |||||
Operating income: |
||||||||||||||
2010 |
$ | (31,989 | )(g) | $ | 225,482 | (h) | $ | (146,000 | )(c) | $ | 47,493 | |||
2009 |
$ | 99,728 | $ | 254,510 | $ | (125,628 | )(c) | $ | 228,610 | |||||
2008 |
$ | 205,395 | $ | 170,141 | $ | (111,886 | )(c) | $ | 263,650 | |||||
Segment assets: |
||||||||||||||
2010 |
$ | 1,110,862 | (i) | $ | 706,395 | $ | 148,285 | (d) | $ | 1,965,542 | ||||
2009 |
$ | 1,184,401 | $ | 612,572 | $ | 177,971 | (d) | $ | 1,974,944 | |||||
2008 |
$ | 1,136,928 | $ | 453,448 | $ | 162,712 | (d) | $ | 1,753,088 | |||||
Investment in equity method investees: |
||||||||||||||
2010 |
$ | 22,032 | (e) | $ | — | $ | — | $ | 22,032 | |||||
2009 |
$ | 22,062 | (e) | $ | — | $ | — | $ | 22,062 | |||||
2008 |
$ | 23,427 | (e) | $ | — | $ | — | $ | 23,427 | |||||
Capital expenditures: |
||||||||||||||
2010 |
$ | 69,392 | $ | 48,385 | $ | 8,501 | (f) | $ | 126,278 | |||||
2009 |
$ | 69,554 | $ | 40,815 | $ | 20,710 | (f) | $ | 131,079 | |||||
2008 |
$ | 253,652 | $ | 23,539 | $ | 41,737 | (f) | $ | 318,928 | |||||
- (a)
- Represents
revenues associated with reimbursable out-of-pocket expenses.
- (b)
- Represents
depreciation and amortization on corporate fixed assets.
- (c)
- Represents
corporate expenses (primarily information technology, marketing, communications, human resources, finance, legal and stock-based compensation
expense). Corporate expenses include restructuring costs of $6,632 in 2010.
- (d)
- Represents
corporate assets.
- (e)
- Represents
equity investment in Noveprim Limited.
- (f)
- Represents
corporate capital expenditures.
- (g)
- Early
Development operating income includes asset impairment charges of $119,229 and restructuring costs of $14,069 in 2010.
- (h)
- Late-Stage
Development operating income includes restructuring charges of $7,329 in 2010.
- (i)
- Early Development assets reflect impact of asset impairment charges of $119,229 in 2010.
72
COVANCE INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2010, 2009 AND 2008
(Dollars in thousands, unless otherwise indicated)
14. Segment Information (Continued)
Enterprise-Wide Disclosures
Net revenues from external customers for each significant service area for the years ended December 31, 2010, 2009 and 2008 are as follows:
| |
Preclinical Laboratory Services |
Central (Clinical) Laboratory Services |
Clinical Development Services |
All Other Services |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
2010 |
$ | 623,742 | $ | 609,656 | $ | 376,401 | $ | 315,831 | $ | 1,925,630 | ||||||
2009 |
$ | 606,964 | $ | 608,378 | $ | 346,748 | $ | 305,544 | $ | 1,867,634 | ||||||
2008 |
$ | 667,122 | $ | 464,715 | $ | 302,894 | $ | 293,367 | $ | 1,728,098 | ||||||
Net revenues from external customers and long-lived assets for each significant geographic location for the years ended December 31, 2010, 2009 and 2008 are as follows:
| |
United States |
United Kingdom |
Switzerland | Other | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net revenues from external customers(1) |
|||||||||||||||||
2010 |
$ | 1,080,682 | $ | 220,057 | $ | 278,625 | $ | 346,266 | $ | 1,925,630 | |||||||
2009 |
$ | 1,075,611 | $ | 203,973 | $ | 282,152 | $ | 305,898 | $ | 1,867,634 | |||||||
2008 |
$ | 1,038,808 | $ | 227,083 | $ | 215,478 | $ | 246,729 | $ | 1,728,098 | |||||||
Long-lived assets(2) |
|||||||||||||||||
2010 |
$ | 609,237 | (3) | $ | 114,656 | $ | 46,847 | $ | 73,243 | $ | 843,983 | ||||||
2009 |
$ | 722,468 | $ | 111,393 | $ | 42,641 | $ | 45,493 | $ | 921,995 | |||||||
2008 |
$ | 683,897 | $ | 102,851 | $ | 29,447 | $ | 44,762 | $ | 860,957 | |||||||
- (1)
- Net
revenues are attributable to geographic locations based on the physical location where the services are performed.
- (2)
- Long-lived
assets represents the net book value of property and equipment.
- (3)
- Reflects impact of asset impairment charges of $119,229 in 2010.
15. Subsequent Events
Covance completed an evaluation of the impact of any subsequent events through the date these financial statements were issued, and determined there were no subsequent events requiring disclosure in or adjustment to these financial statements.
73
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
(a) Evaluation of Disclosure Controls and Procedures. The Company's Principal Executive Officer and Principal Financial Officer have reviewed and evaluated the effectiveness of the Company's disclosure controls and procedures as of the end of the period covered in this report. Based on that evaluation, the Principal Executive Officer and the Principal Financial Officer have concluded that the Company's current disclosure controls and procedures are effective.
(b) Management's Report on Internal Control over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Under the supervision and with the participation of our management, including our Principal Executive Officer and Principal Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2010. See Management's Report on Consolidated Financial Statements and Internal Control, which is included herein.
For additional information, please see "Management's Report on Consolidated Financial Statements and Internal Control" included in this Annual Report.
(c) Attestation Report of Independent Registered Public Accounting Firm. The attestation report of the Company's independent registered public accounting firm regarding internal control over financial reporting is included in Item 8 of this Annual Report under the caption "Report of Independent Registered Accounting Firm" which is included herein.
(d) Changes in Internal Control over Financial Reporting. There were no changes in the Company's internal control over financial reporting during the fourth quarter of 2010 that have materially affected, or are reasonably likely to materially affect, the Company's internal control over financial reporting.
None.
74
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this item for executive officers is set forth under the heading "Executive Officers" in Part I, Item 1 of this report.
Directors
Robert Barchi, M.D., Ph.D., 64, has been President of Thomas Jefferson University since September 2004. Prior to that, Dr. Barchi was Provost of the University of Pennsylvania since 1999. Previously, he served as Chair of the University of Pennsylvania's Department of Neurology and as founding Chair of the University's Department of Neuroscience. Dr. Barchi was also Director of the Mahoney Institute of Neurological Sciences for more than 12 years and was the Director of the Dana Fellowship Program in Neuroscience and Director of the Clinical Neuroscience Track. He was the founder and President of Penn Neurocare, a regional specialty network. Dr. Barchi has been a member of the Covance Board since October 2003.
Gary E. Costley, Ph.D., 67, is a co-founder and managing director of C&G Capital and Management, LLC, which provides capital and management to health, medical and nutritional products and services companies. He was Chairman and Chief Executive Officer of International Multifoods Corporation, a manufacturer and marketer of branded consumer food and food service products from November 2001 until June 2004, and Chairman, President and Chief Executive Officer from 1997 through 2001. Dr. Costley has been a member of the Covance Board since September 2007. Dr. Costley is also a Director of The Principal Financial Group, a global financial institution, Tiffany & Co., a jewelry company, and Prestige Brand Holdings, Inc., a consumer products company.
Sandra L. Helton, 61, was Executive Vice President and Chief Financial Officer of Telephone & Data Systems, Inc., a telecommunications service company, ("TDS") from October 2000 through December 2006. She joined TDS as Executive Vice President—Finance and Chief Financial Officer in August 1998. Prior to joining TDS, Ms. Helton was the Vice President and Corporate Controller of Compaq Computer Corporation between 1997 and 1998. Prior to that time, Ms. Helton was employed by Corning Incorporated. At Corning, Ms. Helton was Senior Vice President and Treasurer between 1994 and 1997 and was Vice President and Treasurer between 1991 and 1994. Ms. Helton has been a member of the Covance Board since September 2003. Ms. Helton is also a Director of The Principal Financial Group, a global financial institution, and was elected a Director of Lexmark International, Inc., a printing and imaging solutions company, on February 24, 2011. Ms. Helton was a Director of TDS and US Cellular Corporation through December 31, 2006.
Joseph L. Herring, 55, has been Covance's Chief Executive Officer since January 2005, and Chairman since January 2006. Mr. Herring was President and Chief Operating Officer from November 2001 to December 2004, and was Covance's Corporate Senior Vice President and President—Early Development Services from October 1999 to November 2001. From September 1996 to September 1999, Mr. Herring was Corporate Vice President and General Manager of Covance Laboratories North America. Prior to joining Covance, Mr. Herring spent 18 years at the American Hospital Supply/Baxter International/Caremark International family of healthcare service companies where he held a variety of senior leadership positions, culminating in the position of Vice President and General Manager of its oncology business. Mr. Herring has been a member of the Covance Board since 2004.
John McCartney, 58, has been Chairman of Huron Consulting Group Inc., a healthcare and educational consulting company since May 2, 2010. Mr. McCartney served as Chairman of A.M. Castle & Co., a specialty metals and plastics distributor from May 2007 to may 2010 and remains on its Board of Directors. Mr. McCartney served as the Chairman of Westcon Group, Inc., a specialty distributor of networking and communications equipment until March 2009 and remains on its Board of Directors. Mr. McCartney was the Vice-Chairman of Datatec Limited, a technology holding company, from 1998 to 2004 and currently serves on its Board of Directors. Mr. McCartney was formerly President and Chief Operating Officer of U.S. Robotics. Mr. McCartney also served on the Board of Federal Signal Corporation, an environmental, safety and transportation solutions company, until April 24, 2010. Mr. McCartney has been a member of the Covance Board since May 2009.
75
Joseph C. Scodari, 58, was Worldwide Chairman, Pharmaceuticals Group, of Johnson & Johnson, a diversified healthcare company, ("J&J") and a member of J&J's Executive Committee from March 2005 until March 2008. From 2003 to March 2005, Mr. Scodari was Company Group Chairman of J&J's Biopharmaceutical Business. Mr. Scodari joined J&J in 1999 as President and Chief Operating Officer of Centocor, Inc., when J&J acquired the company. Mr. Scodari has been a member of the Covance Board since May 2008. Mr. Scodari is a Director of Actelion Pharmaceuticals, Ltd., a pharmaceuticals company, and Endo Pharmaceuticals, Inc., a pharmaceuticals company.
Bradley T. Sheares, 54, served as Chief Executive Officer of Reliant Pharmaceuticals, Inc., a pharmaceutical company with integrated sales, marketing and development expertise that marketed a portfolio of branded cardiovascular pharmaceutical products, from January 2007 through its acquisition by GlaxoSmithKline plc in December 2007. Prior to joining Reliant, Dr. Sheares served as President of U.S. Human Health, Merck & Co., Inc. from March 2001 until July 2006. Prior to that time, he served as Vice President, Hospital Marketing and Sales for Merck's U.S. Human Health business. Dr. Sheares joined Merck in 1987 as a research fellow in the Merck Research Laboratories and held a wide range of positions within Merck, in business development, sales, and marketing, before becoming Vice President in 1996. Dr. Sheares has been a member of the Covance Board since February 2009. Dr. Sheares is also a Director of The Progressive Corporation, an insurance and related services company, Honeywell International, Inc., a diversified technology and manufacturing company, and Henry Schein, Inc., a healthcare products and services company. Dr. Sheares was a Director of IMS Health, a healthcare services company, until February 2010.
Information under the headings "Proposal 1—Election of Directors," "The Board of Directors and its Committees," "Committees of the Board," "Board Nomination Process" and "Section 16(a) Beneficial Ownership Reporting Compliance" in the Company's definitive Proxy Statement in connection with the 2011 Annual Meeting of Shareholders to be held May 12, 2011, which Proxy Statement is intended to be filed not later than 120 days after December 31, 2010, pursuant to Regulation 14A under the Securities Exchange Act of 1934, as amended, is incorporated herein by reference.
The Company has adopted a Code of Ethics for Finance Professionals in compliance with applicable rules of the Securities and Exchange Commission ("SEC") that applies to its principal executive officer, its principal financial officer, and its principal accounting officer or controller, or persons performing similar functions. A copy of the Code of Ethics for Finance Professionals is available on the Company's web site at www.covance.com, free of charge, under the caption, "Investor Relations—Corporate Governance." The Company intends to satisfy any disclosure requirement under Item 5.05 of Form 8-K regarding an amendment to, or waiver from, a provision of this Code of Ethics for Finance Professionals by posting such information on the Company's web site at the address and location specified above.
Item 11. Executive Compensation
Information on Director and executive compensation is incorporated by reference to the headings "Directors' Compensation" and "Executive Compensation" in the Company's definitive Proxy Statement in connection with its 2011 Annual Meeting of Shareholders to be held on May 12, 2011, which Proxy Statement is intended to be filed not later than 120 days after December 31, 2010, pursuant to Regulation 14A under the Securities Exchange Act of 1934, as amended.
Item 12. Security Ownership by Certain Beneficial Owners and Management of Covance
Information on security ownership by certain beneficial owners and management of Covance is incorporated by reference to the headings "Stock Ownership of Directors, Executive Officers and Certain Shareholders" in the Company's definitive Proxy Statement in connection with its 2011 Annual Meeting of Shareholders to be held on May 12, 2011, which Proxy Statement is intended to be filed not later than 120 days after December 31, 2010 pursuant to Regulation 14A under the Securities Exchange Act of 1934, as amended.
76
Covance maintains the Covance Inc. 2010 Employee Equity Participation Plan, the Covance Inc. 2002 Employee Stock Option Plan, the 2008 Stock Option Plan for Non-Employee Directors, the 1998 Stock Option Plan for Non-Employee Directors, the Deferred Stock Unit Plan for Non-Employee Directors and the Restricted Unit Plan for Non-Employee Members of the Board of Directors, pursuant to which it has granted or may grant equity awards to eligible persons. Covance also maintained an Employee Stock Purchase Plan which was terminated effective January 1, 2011.
The following table gives information about equity awards under Covance's above mentioned plans at December 31, 2010. The only plan mentioned above pursuant to which equity securities are authorized to be issued which has not received shareholder approval is the Covance Inc. 2002 Employee Stock Option Plan. For a description of the material features of these plans, please see Note 10 to the audited consolidated financial statements included elsewhere in this Annual Report.
Equity Compensation Plan Information
|
|
Plan Category |
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) |
|
Weighted-average exercise price of outstanding options, warrants and rights (b) |
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Equity compensation plans approved by security holders |
2,579,702 | $ | 50.92 | 5,966,013 | ||||||||||||||
|
Equity compensation plans not approved by security holders |
850,366 | $ | 41.51 | 318,931 | (1) | |||||||||||||
|
TOTAL | 3,430,068 | $ | 48.59 | 6,284,944 | (1) | |||||||||||||
- (1)
- Includes 318,931 securities available for issuance under Covance's Employee Stock Purchase Plan pursuant to which Covance made available for sale to its employees shares of Common Stock at a price equal to 85% of the lower of fair market value on the first or last day of each calendar quarter. This plan was terminated effective January 1, 2011.
Item 13. Certain Relationships and Related Transactions
Incorporated by reference to the heading "The Board of Directors and its Committees" in the Company's definitive Proxy Statement in connection with its 2011 Annual Meeting of Shareholders to be held on May 12, 2011, which Proxy Statement is intended to be filed not later than 120 days after December 31, 2010, pursuant to Regulation 14A under the Securities Exchange Act of 1934, as amended.
Item 14. Principal Accountant Fees and Services
Incorporated by reference to the heading "Principal Accountant Fees and Services" in the Company's definitive Proxy Statement in connection with its 2011 Annual Meeting of Shareholders to be held on May 12, 2011, which Proxy Statement is intended to be filed not later than 120 days after December 31, 2010, pursuant to Regulation 14A under the Securities Exchange Act of 1934, as amended.
77
Item 15. Exhibits and Financial Statement Schedules
(a) Documents filed as part of this report.
| 1. | Financial Statements. The financial statements filed as part of this report are listed on the Index to Consolidated Financial Statements on page 35. | ||
| 2. | Financial Statement Schedules. Schedules are omitted because they are not applicable or the required information is shown in the consolidated financial statements or notes thereto. | ||
| 3. | Exhibits. The exhibits required by Item 601 of Regulation S-K filed as part of, or incorporated by reference in, this report are listed in (b) below and in the accompanying Exhibit Index. |
(b) Item 601 Exhibits.
| Exhibit Number |
Description | ||
|---|---|---|---|
| 3.1 | Certificate of Incorporation. Incorporated by reference to Covance's filing on Amendment No. 2 on Form 10, filed with the SEC on November 19, 1996. | ||
| 3.2 | By-Laws. Incorporated by reference to Covance's filing on Form 8-K, filed with the SEC on December 16, 2008. | ||
| 4.1 | Form of Common Stock Certificate. Incorporated by reference to Covance's filing on Amendment No. 3 on Form 10, filed with the SEC on December 16, 2008. | ||
| 10.1 | Employee Stock Ownership Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1996. | ||
| 10.2 | Stock Purchase Savings Plan, as amended. Incorporated by reference to Covance's Registration Statement on Form S-8 filed with the SEC on March 5, 2002. | ||
| 10.3 | Restricted Share Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1996. | ||
| 10.4 | Non-Employee Directors' Amended and Restated Restricted Stock Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1998. | ||
| 10.5 | Directors' Deferred Compensation Plan, as amended. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1997. | ||
| 10.6 | Conversion Equity Plan. Incorporated by reference to Covance's filing on a Registration Statement on Form S-8, Registration No. 333-29467, filed with the SEC on June 18, 1997. | ||
| 10.7 | Non-Employee Directors' Stock Option Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1998. | ||
| 10.8 | Deferred Stock Unit Plan for Non-Employee Members of the Board of Directors. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1998. | ||
| 10.9 | 2002 Employee Equity Participation Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 2001. | ||
| 10.10 | 2002 Employee Stock Option Plan. Incorporated by reference to Covance's Registration Statement on Form S-8 filed with the SEC on July 31, 2002. | ||
| 10.11 | Employee Stock Purchase Plan, as amended. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 2002. | ||
| 10.12 | Restricted Unit Plan for Non-Employee Members of the Board of Directors. Incorporated by reference to Covance's Quarterly Report on Form 10-Q for the period ended June 30, 2003. | ||
| 10.13 | Covance Inc. Variable Compensation Plan effective January 1, 2004. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 2003. | ||
| 10.14 | Form of Executive Officer Restricted Stock Agreement. Incorporated by reference to Covance's Quarterly Report on Form 10-Q for the period ended September 30, 2004. | ||
78
| Exhibit Number |
Description | ||
|---|---|---|---|
| 10.15 | Form of Non-Employee Director Stock Option Agreement. Incorporated by reference to Covance's Quarterly Report on Form 10-Q for the period ended September 30, 2004. | ||
| 10.16 | Restricted Share Agreement between Covance Inc. and Richard Cimino dated as of December 17, 2004. Incorporated by reference to Covance's Form 8-K dated December 17, 2004. | ||
| 10.17 | Trust Deed Governing the Covance Laboratories Pension Scheme. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 2004. | ||
| 10.18 | Agreement between Covance Inc. and Deborah Tanner dated February 23, 2006. Incorporated by reference to Covance's Form 8-K dated February 23, 2006. | ||
| 10.19 | Form of Restricted Share Agreement between Covance Inc. and each of Wendel Barr and Richard F. Cimino dated February 23, 2006. Incorporated by reference to Covance's Form 8-K dated February 28, 2006. | ||
| 10.20 | Agreement and Plan of Merger dated April 20, 2006 between Covance Clinical Research Unit Inc., TYD Inc., Radiant Research Inc., and James Stevenson and Christopher Grant, Jr. Incorporated by reference to Covance's Form 8-K dated April 26, 2006. | ||
| 10.21 | Amendment No.1 to the Restricted Unit Plan for Non-Employee Members of the Board of Directors of Covance Inc. Incorporated by reference to Covance's Form 8-K dated May 16, 2006. | ||
| 10.22 | Amendment No.1 to the 1998 Non-Employee Director Stock Option Plan. Incorporated by reference to Covance's Form 8-K dated December 12, 2006. | ||
| 10.23 | Restricted Share Agreement between Covance Inc. and Joseph Herring dated February 22, 2007. Incorporated by reference to Covance's Form 8-K dated February 28, 2007. | ||
| 10.24 | Covance Inc. 2007 Employee Equity Participation Plan. Incorporated by reference to Covance's Form 10-Q dated May 8, 2007. | ||
| 10.25 | Covance Inc. Management Deferral Plan. Incorporated by reference to Covance's Form 8-K dated October 1, 2007. | ||
| 10.26 | Amended and Restated Supplemental Executive Retirement Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 2007. | ||
| 10.27 | Restricted Stock Agreement between Covance Inc. and James Lovett dated March 31, 2008. Incorporated by reference to Covance's Form 8-K dated April 2, 2008. | ||
| 10.28 | Covance Inc. 2008 Non-Employee Director Stock Option Plan. Incorporated by reference to Covance's Form 8-K dated May 12, 2008. | ||
| 10.29 | Amended and Restated Restricted Unit Plan for Non-Employee Members of the Board of Directors of Covance Inc. Incorporated by reference to Covance's Form 8-K dated May 12, 2008. | ||
| 10.30 | Letter agreement between Covance Inc. and Joseph Herring dated as of December 31, 2008. Incorporated by reference to Covance's Form 8-K dated December 16, 2008. | ||
| 10.31 | Form of letter agreement between Covance Inc. and each of Wendel Barr, Richard Cimino, Donald Kraft, William Klitgaard, James Lovett, Michael Lehmann and Deborah Tanner. Incorporated by reference to Covance's Form 8-K dated December 16, 2008. | ||
| 10.32 | Form of Executive Officer Stock Option Agreement. Incorporated by reference to Covance's Form 8-K dated February 25, 2009. | ||
| 10.33 | Letter Agreement between Covance Inc. and Anthony Cork dated as of February 28, 2009. Incorporated by reference to Covance's Form 8-K dated February 25, 2009. | ||
| 10.34 | Form of Indemnification Agreement between Covance Inc. and each member of the Board of Directors dated as of February 19, 2009. Incorporated by reference to Covance's Form 8-K dated February 25, 2009. | ||
| 10.35 | Form of Executive Officer Indemnification Agreement. Incorporated by reference to Covance's Form 8-K dated April 24, 2009. | ||
| 10.36 | Covance Inc. 2010 Employee Equity Participation Plan. Incorporated by reference to Covance's Form 8-K dated May 11, 2010. | ||
79
| Exhibit Number |
Description | ||
|---|---|---|---|
| 10.37 | Credit Agreement dated June 16, 2009 with PNC Bank National Association, as agent and the banks named therein. Incorporated by reference to Covance's Form 8-K dated June 22, 2009. | ||
| 10.38 | Letter Agreement between Covance Inc. and Donald Kraft dated as of June 18, 2010. Incorporated by reference to Covance's Form 8-K dated June 18, 2010. | ||
| 10.39 | Credit Agreement dated October 26, 2010 with PNC Bank National Association, as agent and the banks named therein. Incorporated by reference to Covance's Form 8-K dated October 26, 2010. | ||
| 10.40 | Accelerated Share Repurchase Agreement with JPMorgan Chase Bank, National Association, London Branch dated November 8, 2010. Incorporated by reference to Covance's Form 8-K dated November 8, 2010. | ||
| 10.41 | Letter Agreement between Covance Inc. and Wendel Barr dated as of December 20, 2010. Incorporated by reference to Covance's Form 8-K dated December 23, 2010. | ||
| 10.42 | Letter Agreement Amendment between Covance Inc. and Joseph Herring dated as of December 31, 2010. Incorporated by reference to Covance's Form 8-K dated December 31, 2010. | ||
| 10.43 | Form of Letter Agreement Amendment between Covance Inc. and each of its executive officers other than its Chief Executive Officer (Richard Cimino, William Klitgaard, Michael Lehmann, James Lovett and Deborah Tanner). Incorporated by reference to Covance's Form 8-K dated December 31, 2010. | ||
| 10.44 | Form of Restricted Share Agreement between Covance Inc. and each of Richard F. Cimino and Deborah L. Tanner dated February 17, 2011. Incorporated by reference to Covance's Form 8-K dated February 23, 2011. | ||
| 10.45 | Restricted Share Agreement between Covance Inc. and James W. Lovett dated February 17, 2011. Incorporated by reference to Covance's Form 8-K dated February 23, 2011. | ||
| 10.46 | Form of Performance-related Executive Officer Restricted Share Agreement. Incorporated by reference to Covance's Form 8-K dated February 23, 2011. | ||
| 10.47 | Form of Executive Officer Stock Option Agreement. Incorporated by reference to Covance's Form 8-K dated February 23, 2011. | ||
| 10.48 | Amendment to Supplemental Executive Retirement Plan dated February 24, 2011. Filed herewith. | ||
| 21 | Subsidiaries. Filed herewith. | ||
| 23.1 | Consent of Ernst & Young LLP. Filed herewith. | ||
| 31.1 | Certification of Chief Executive Officer pursuant to SEC Rule 13(a)-14(a). Filed herewith. | ||
| 31.2 | Certification of Chief Financial Officer pursuant to SEC Rule 13(a)-14(a). Filed herewith. | ||
| 32.1 | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350. Filed herewith. | ||
| 32.2 | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350. Filed herewith. | ||
| 101 | The following financial information from Covance's Annual Report on Form 10-K for the year ended December 31, 2010, filed on February 28, 2011, formatted in XBRL (Extensible Business Reporting Language) includes (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Income, (iii) Consolidated Statements of Cash Flows, (iv) Consolidated Statements of Stockholders' Equity, and (v) Notes to Consolidated Financial Statements, tagged as blocks of text. Furnished electronically herewith. | ||
(c) Financial Statement Schedules.
None.
80
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, Covance has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| COVANCE INC. | ||||
Dated: February 28, 2011 |
By: |
/s/ Joseph L. Herring Joseph L. Herring Chairman of the Board and Chief Executive Officer |
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of Covance and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
|---|---|---|---|---|
| /s/ Joseph L. Herring Joseph L. Herring |
Chairman of the Board, Chief Executive Officer and Director (Principal Executive Officer) |
February 28, 2011 | ||
/s/ William E. Klitgaard William E. Klitgaard |
Corporate Senior Vice President and Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
February 28, 2011 |
||
/s/ Robert Barchi Robert Barchi |
Director |
February 28, 2011 |
||
/s/ Gary E. Costley Gary E. Costley |
Director |
February 28, 2011 |
||
/s/ Sandra L. Helton Sandra L. Helton |
Director |
February 28, 2011 |
||
/s/ John McCartney John McCartney |
Director |
February 28, 2011 |
||
/s/ Joseph C. Scodari Joseph C. Scodari |
Director |
February 28, 2011 |
||
/s/ Bradley T. Sheares Bradley T. Sheares |
Director |
February 28, 2011 |
81
| Exhibit Number | Description | ||
|---|---|---|---|
| 3.1 | Certificate of Incorporation. Incorporated by reference to Covance's filing on Amendment No. 2 on Form 10, filed with the SEC on November 19, 1996. | ||
| 3.2 | By-Laws. Incorporated by reference to Covance's filing on Form 8-K, filed with the SEC on December 16, 2008. | ||
| 4.1 | Form of Common Stock Certificate. Incorporated by reference to Covance's filing on Amendment No. 3 on Form 10, filed with the SEC on December 16, 2008. | ||
| 10.1 | Employee Stock Ownership Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1996. | ||
| 10.2 | Stock Purchase Savings Plan, as amended. Incorporated by reference to Covance's Registration Statement on Form S-8 filed with the SEC on March 5, 2002. | ||
| 10.3 | Restricted Share Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1996. | ||
| 10.4 | Non-Employee Directors' Amended and Restated Restricted Stock Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1998. | ||
| 10.5 | Directors' Deferred Compensation Plan, as amended. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1997. | ||
| 10.6 | Conversion Equity Plan. Incorporated by reference to Covance's filing on a Registration Statement on Form S-8, Registration No. 333-29467, filed with the SEC on June 18, 1997. | ||
| 10.7 | Non-Employee Directors' Stock Option Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1998. | ||
| 10.8 | Deferred Stock Unit Plan for Non-Employee Members of the Board of Directors. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 1998. | ||
| 10.9 | 2002 Employee Equity Participation Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 2001. | ||
| 10.10 | 2002 Employee Stock Option Plan. Incorporated by reference to Covance's Registration Statement on Form S-8 filed with the SEC on July 31, 2002. | ||
| 10.11 | Employee Stock Purchase Plan, as amended. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 2002. | ||
| 10.12 | Restricted Unit Plan for Non-Employee Members of the Board of Directors. Incorporated by reference to Covance's Quarterly Report on Form 10-Q for the period ended June 30, 2003. | ||
| 10.13 | Covance Inc. Variable Compensation Plan effective January 1, 2004. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 2003. | ||
| 10.14 | Form of Executive Officer Restricted Stock Agreement. Incorporated by reference to Covance's Quarterly Report on Form 10-Q for the period ended September 30, 2004. | ||
| 10.15 | Form of Non-Employee Director Stock Option Agreement. Incorporated by reference to Covance's Quarterly Report on Form 10-Q for the period ended September 30, 2004. | ||
| 10.16 | Restricted Share Agreement between Covance Inc. and Richard Cimino dated as of December 17, 2004. Incorporated by reference to Covance's Form 8-K dated December 17, 2004. | ||
| 10.17 | Trust Deed Governing the Covance Laboratories Pension Scheme. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 2004. | ||
| 10.18 | Agreement between Covance Inc. and Deborah Tanner dated February 23, 2006. Incorporated by reference to Covance's Form 8-K dated February 23, 2006. | ||
| 10.19 | Form of Restricted Share Agreement between Covance Inc. and each of Wendel Barr and Richard F. Cimino dated February 23, 2006. Incorporated by reference to Covance's Form 8-K dated February 28, 2006. | ||
| 10.20 | Agreement and Plan of Merger dated April 20, 2006 between Covance Clinical Research Unit Inc., TYD Inc., Radiant Research Inc., and James Stevenson and Christopher Grant, Jr. Incorporated by reference to Covance's Form 8-K dated April 26, 2006. | ||
| 10.21 | Amendment No. 1 to the Restricted Unit Plan for Non-Employee Members of the Board of Directors of Covance Inc. Incorporated by reference to Covance's Form 8-K dated May 16, 2006. | ||
| Exhibit Number | Description | ||
|---|---|---|---|
| 10.22 | Amendment No. 1 to the 1998 Non-Employee Director Stock Option Plan. Incorporated by reference to Covance's Form 8-K dated December 12, 2006. | ||
| 10.23 | Restricted Share Agreement between Covance Inc. and Joseph Herring dated February 22, 2007. Incorporated by reference to Covance's Form 8-K dated February 28, 2007. | ||
| 10.24 | Covance Inc. 2007 Employee Equity Participation Plan. Incorporated by reference to Covance's Form 10-Q dated May 8, 2007. | ||
| 10.25 | Covance Inc. Management Deferral Plan. Incorporated by reference to Covance's Form 8-K dated October 1, 2007. | ||
| 10.26 | Amended and Restated Supplemental Executive Retirement Plan. Incorporated by reference to Covance's Annual Report on Form 10-K for the fiscal year ended December 31, 2007. | ||
| 10.27 | Restricted Stock Agreement between Covance Inc. and James Lovett dated March 31, 2008. Incorporated by reference to Covance's Form 8-K dated April 2, 2008. | ||
| 10.28 | Covance Inc. 2008 Non-Employee Director Stock Option Plan. Incorporated by reference to Covance's Form 8-K dated May 12, 2008. | ||
| 10.29 | Amended and Restated Restricted Unit Plan for Non-Employee Members of the Board of Directors of Covance Inc. Incorporated by reference to Covance's Form 8-K dated May 12, 2008. | ||
| 10.30 | Letter agreement between Covance Inc. and Joseph Herring dated as of December 31, 2008. Incorporated by reference to Covance's Form 8-K dated December 16, 2008. | ||
| 10.31 | Form of letter agreement between Covance Inc. and each of Wendel Barr, Richard Cimino, Donald Kraft, William Klitgaard, James Lovett, Michael Lehmann and Deborah Tanner. Incorporated by reference to Covance's Form 8-K dated December 16, 2008. | ||
| 10.32 | Form of Executive Officer Stock Option Agreement. Incorporated by reference to Covance's Form 8-K dated February 25, 2009. | ||
| 10.33 | Letter Agreement between Covance Inc. and Anthony Cork dated as of February 28, 2009. Incorporated by reference to Covance's Form 8-K dated February 25, 2009. | ||
| 10.34 | Form of Indemnification Agreement between Covance Inc. and each member of the Board of Directors dated as of February 19, 2009. Incorporated by reference to Covance's Form 8-K dated February 25, 2009. | ||
| 10.35 | Form of Executive Officer Indemnification Agreement. Incorporated by reference to Covance's Form 8-K dated April 24, 2009. | ||
| 10.36 | Covance Inc. 2010 Employee Equity Participation Plan. Incorporated by reference to Covance's Form 8-K dated May 11, 2010. | ||
| 10.37 | Credit Agreement dated June 16, 2009 with PNC Bank National Association, as agent and the banks named therein. Incorporated by reference to Covance's Form 8-K dated June 22, 2009. | ||
| 10.38 | Letter Agreement between Covance Inc. and Donald Kraft dated as of June 18, 2010. Incorporated by reference to Covance's Form 8-K dated June 18, 2010. | ||
| 10.39 | Credit Agreement dated October 26, 2010 with PNC Bank National Association, as agent and the banks named therein. Incorporated by reference to Covance's Form 8-K dated October 26, 2010. | ||
| 10.40 | Accelerated Share Repurchase Agreement with JPMorgan Chase Bank, National Association, London Branch dated November 8, 2010. Incorporated by reference to Covance's Form 8-K dated November8, 2010. | ||
| 10.41 | Letter Agreement between Covance Inc. and Wendel Barr dated as of December 20, 2010. Incorporated by reference to Covance's Form 8-K dated December 23, 2010. | ||
| 10.42 | Letter Agreement Amendment between Covance Inc. and Joseph Herring dated as of December 31, 2010. Incorporated by reference to Covance's Form 8-K dated December 31, 2010. | ||
| 10.43 | Form of Letter Agreement Amendment between Covance Inc. and each of its executive officers other than its Chief Executive Officer (Richard Cimino, William Klitgaard, Michael Lehmann, James Lovett and Deborah Tanner). Incorporated by reference to Covance's Form 8-K dated December 31, 2010. | ||
| 10.44 | Form of Restricted Share Agreement between Covance Inc. and each of Richard F. Cimino and Deborah L. Tanner dated February 17, 2011. Incorporated by reference to Covance's Form 8-K dated February 23, 2011. | ||
| 10.45 | Restricted Share Agreement between Covance Inc. and James W. Lovett dated February 17, 2011. Incorporated by reference to Covance's Form 8-K dated February 23, 2011. | ||
| Exhibit Number | Description | ||
|---|---|---|---|
| 10.46 | Form of Performance-related Executive Officer Restricted Share Agreement. Incorporated by reference to Covance's Form 8-K dated February 23, 2011. | ||
| 10.47 | Form of Executive Officer Stock Option Agreement. Incorporated by reference to Covance's Form 8-K dated February 23, 2011. | ||
| 10.48 | Amendment to Supplemental Executive Retirement Plan dated February 24, 2011. Filed herewith. | ||
| 21 | Subsidiaries. Filed herewith. | ||
| 23.1 | Consent of Ernst & Young LLP. Filed herewith. | ||
| 31.1 | Certification of Chief Executive Officer pursuant to SEC Rule 13(a)-14(a). Filed herewith. | ||
| 31.2 | Certification of Chief Financial Officer pursuant to SEC Rule 13(a)-14(a). Filed herewith. | ||
| 32.1 | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350. Filed herewith. | ||
| 32.2 | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350. Filed herewith. | ||
| 101 | The following financial information from Covance's Annual Report on Form 10-K for the year ended December 31, 2010, filed on February 28, 2011, formatted in XBRL (Extensible Business Reporting Language) includes (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Income, (iii) Consolidated Statements of Cash Flows, (iv) Consolidated Statements of Stockholders' Equity, and (v) Notes to Consolidated Financial Statements, tagged as blocks of text. Furnished electronically herewith. | ||
PART I
-
Item 1A. Risk Factors
Item 1B. Unresolved Staff Comments
Item 2. Properties
Item 3. Legal Proceedings
Item 4. Reserved
-
Item 5. Market for Registrant's Common Stock and Related Stockholder Matters and Issuer Purchases of Equity Securities
Item 5a. Performance Graph
Item 6. Selected Financial Data
Report of Independent Registered Public Accounting Firm
Report of Independent Registered Public Accounting Firm
-
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Item 9A. Controls and Procedures
Item 9B. Other Information
-
Item 10. Directors, Executive Officers and Corporate Governance
Item 11. Executive Compensation
Item 12. Security Ownership by Certain Beneficial Owners and Management of Covance
Item 13. Certain Relationships and Related Transactions
Item 14. Principal Accountant Fees and Services
SIGNATURES
EXHIBIT INDEX
