Attached files
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
[X]
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE FISCAL YEAR ENDED DECEMBER 31, 2010
|
|
[ ]
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM _________ TO ___________
|
| ALTAIR NANOTECHNOLOGIES INC. |
| (Exact name of registrant as specified in its charter) |
|
Canada
|
1-12497
|
33-1084375
|
||
|
(State or other jurisdiction
of incorporation)
|
(Commission File No.)
|
(IRS Employer
Identification No.)
|
||
|
204 Edison Way
Reno, Nevada 89502-2306
|
||||
| (Address of principal executive offices, including zip code) | ||||
Registrant's telephone number, including area code: (775) 856-2500
Securities registered pursuant to Section 12(b) of the Act:
|
Common Shares, no par value
|
NASDAQ Capital Market
|
|
| (Title of Class) |
(Name of each exchange on which registered)
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark whether the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES [ ] NO [X ]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES [ ] NO [X ]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES [X] NO [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES [ ] NO [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Report or any amendment to this Report. [ ]
i
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definition of “accelerated filer”, “large accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
|
[ ] Large Accelerated Filer
|
[ ] Accelerated Filer
|
|
|
[ ] Non-accelerated Filer
(Do not check if a smaller reporting company)
|
[X] Smaller reporting Company
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act): YES [ ] NO [X]
The aggregate market value of the common shares held by non-affiliates of the Registrant on June 30, 2010, based upon the closing stock price of the common shares on the NASDAQ Capital Market of $1.28 per share on June 30, 2010, was approximately $27.7 million. Common Shares held by each officer and director and by each other person who may be deemed to be an affiliate of the Registrant have been excluded.
As of February 23, 2011, the Registrant had 27,015,680 common shares outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement on Schedule 14A for the Registrant’s 2011 Annual Meeting of Shareholders are incorporated by reference in Part III as specified.
ii
INDEX TO FORM 10-K
|
PART I
|
|
1
|
|
Item 1.
|
Business
|
1
|
|
Item 1A.
|
Risk Factors | 18 |
|
Item 1B.
|
Unresolved Staff Comments
|
32
|
|
Item 2.
|
Properties
|
32
|
|
Item 3.
|
Legal Proceedings
|
32
|
|
Item 4.
|
Reserved
|
32
|
|
PART II
|
33 | |
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
33
|
|
Item 6.
|
Selected Financial Data
|
35
|
|
Item 7.
|
Management's Discussion and Analysis of Financial Condition and Results of Operations.
|
35
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
48
|
|
Item 8.
|
Financial Statements and Supplementary Data.
|
49
|
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
|
49
|
|
Item 9A.
|
Controls and Procedures
|
49
|
|
Item 9B.
|
Other Information
|
50
|
|
PART III
|
|
51
|
|
Item 10.
|
Directors and Executive Officers of the Registrant
|
51
|
|
Item 11.
|
Executive Compensation
|
51
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters………
|
51
|
|
Item 13.
|
Certain Relationships and Related Transactions
|
51
|
|
Item 14.
|
Principal Accountant Fees and Services
|
51
|
|
PART IV
|
|
52
|
|
Item 15.
|
Exhibits and Financial Statement Schedules
|
52
|
iii
PART I
This Annual Report on Form 10-K for the year ended December 31, 2010 (this “Report”) contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that involve risks and uncertainties. Purchasers of any of the common shares (the “common shares”) of Altair Nanotechnologies Inc. are cautioned that our actual results will differ (and may differ significantly) from the results discussed in the forward-looking statements. Factors that could cause or contribute to such differences include those factors discussed herein under “Item 1A. Risk Factors” and elsewhere in this Report generally. The reader is also encouraged to review other filings made by us with the Securities and Exchange Commission (the “SEC”) describing other factors that may affect future results.
Unless the context requires otherwise, all references to “Altair,” “we,” “Altair Nanotechnologies Inc,” or the “Company” in this Report refer to Altair Nanotechnologies Inc. and all of its consolidated subsidiaries. Altair currently has one wholly owned subsidiary, Altair US Holdings, Inc., a Nevada corporation. Altair US Holdings, Inc. directly or indirectly wholly owns Altairnano, Inc., a Nevada corporation, Mineral Recovery Systems, Inc., a Nevada corporation and Fine Gold Recovery Systems, Inc., a Nevada corporation which was dissolved on December 30, 2008. AlSher Titania LLC, a Delaware limited liability company, was 70% owned by Altairnano, Inc. until we sold our interest to Sherwin-Williams on April 30, 2010. We have registered the following trademarks: Altair Nanotechnologies Inc® and Altairnano®. Any other trademarks and service marks used in this Report are the property of their respective holders.
We completed a four-for-one reverse stock split during November 2010. All share and per share amounts included in this filing have been restated for the effects of this reverse stock split.
Item 1. Business
We are a Canadian corporation, with principal assets and operations in the United States, whose primary business is developing, manufacturing and selling our nano lithium titanate battery products. Our primary focus is marketing our large-scale energy storage solutions to power companies and electric grid operators throughout the world. In addition, we market our battery products to electric and hybrid-electric mass-transit vehicle manufacturers. During 2010 we also started to expand our market focus to include use of our battery technology in additional industrial markets with applications requiring batteries that can provide high power quickly, a fast recharge, have a long cycle life, operate at a wide temperature range and are safe.
We also provide contract research services on select projects where we can utilize our resources to develop intellectual property and/or new products and technology. Although contract services revenue comprised a significant portion of our total revenues in recent years accounting for 50%, 65%, and 87%, respectively in 2010, 2009 and 2008, we expect a major decline in this percentage as our battery product sales increase.
On September 20, 2010 we entered into a Share Subscription Agreement (the “Share Subscription Agreement” and related agreements with Canon Investment Holdings Limited (“Canon”) pursuant to which Canon has agreed to acquire, subject to certain conditions precedent to closing and events of termination, a number of common shares such that, following closing, it will own 51% of our outstanding common shares on a fully diluted basis. A summary of the proposed Canon transaction is provided beginning on page 13.
Our Power and Energy Group
Our Primary Products
We are developing, marketing, producing and selling our proprietary rechargeable lithium ion batteries, which we refer to as our nano lithium titanate batteries. As explained in greater detail below, the principal features used to compare rechargeable batteries include charge and discharge rates, power and energy density, cycle and calendar life, operational safety and cleanliness, operating temperature range, and round trip efficiency. In laboratory and field tests, our nano lithium titanate batteries have performed extremely well in nearly all of these categories. In particular, our nano lithium titanate batteries show remarkable power, charge and discharge rates and cycle life, together with high functionality at both high and low temperatures. In some categories our batteries perform as much as an order of magnitude (a factor of 10) better than those of rechargeable batteries currently being used for our targeted applications. Battery uses requiring these strengths include electric utility services for frequency regulation, integration of renewable energy generation sources into the grid, uninterruptible power supplies, and hybrid-electric and full-electric vehicles particularly in the mass-transit market.
1
Our Target Markets
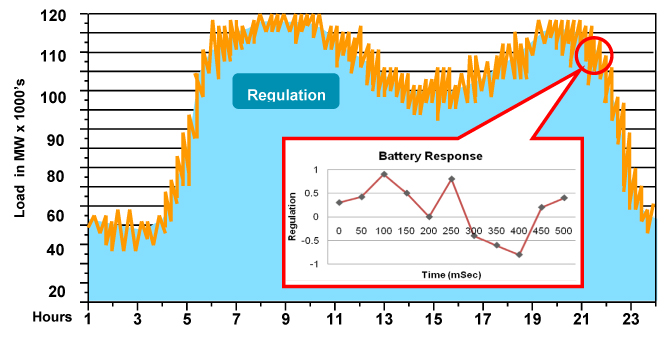
Power and Grid Operators. Power companies and grid operators are seeking cost effective ways to ensure that electric power supply matches electric power demand. There is essentially no inventory of electricity. Power and grid operators are constantly trying to match the electricity generated with the load demanded. They are very good at forecasting from hour to hour the load expected, but they cannot project from minute to minute the exact load anticipated. To maintain proper frequency of the grid (60Hz in the U.S.), the generation and load must be balanced within very tight tolerances. Maintaining these tolerances is typically achieved through the use of auxiliary generators. If the load is either higher or lower than the power being generated, an auxiliary generator is either started or stopped. However, it takes these generators from generally seven to 15 minutes to ramp up to full efficient operation or to shut down. During that period the load may change directions and the grid operator then must direct another auxiliary generator to shut down or ramp up. This is a very inefficient process with the grid operators constantly chasing a variable load. The process of managing these very short-term changes in energy demand is referred to as “frequency regulation.” The chart below depicts what a typical workday in the PJM Regional Transmission Organization that manages the electric grid in the Mid-Atlantic states region looks like and how our battery can help smooth out the fluctuations.
Electricity demand on a typical workday in the PJM electric grid covering the Mid-Atlantic states and District of Columbia
Utilities can address frequency regulation issues by maintaining on-line generating capacity at a level that is always higher than expected peak demand. However this is an expensive solution. Most U.S. utilities are required to maintain between 1% - 1.5% of their peak load capacity to provide frequency regulation. As an example, for the PJM Regional Transmission Organization, this requirement translates into a 900 megawatt daily requirement. In many foreign countries where the electric grid is not as well developed as it is in the U.S., utilities need to reserve up to 5% or more of their capacity strictly to provide frequency regulation. The Cleantech Group estimated in a November 2010 report that the current market for ancillary services is 6.5 gigawatts in the United States annually, or an estimated value in the range of $3-$10 billion depending on power prices and specific ancillary services applications. Globally, they estimate the market to be 33 gigawatts, or a dollar value of between $16 - $45 billion. While these estimates represent a “ballpark” figure and have a highly variable dollar range, the significant magnitude of the market is significant. To reduce the costs of providing frequency regulation, utilities and grid operators are seeking “fast response” energy storage systems. When supply exceeds demand for a short period, these systems accept a charge from the grid until operators reduce output; then when demand exceeds supply for a short period, these fast response storage systems deliver electric energy back to the grid for a short period to give operators time to reroute energy from another power generator or power-up a new power source. Our large-scale nano lithium titanate battery systems are a fast response energy storage system designed to respond in milliseconds and meet this need.
2
The need for a fast response energy storage technology like our large-scale nano lithium titanate battery is increased by the accelerated use of renewable energy sources. Photo Voltaic (PV) solar and wind power generation by nature are intermittent and unpredictable sources of energy that can fluctuate widely in a very short period of time. For example, it is not uncommon for a PV array to fluctuate +/- 50% in less than 90 seconds. With a small rooftop array, it isn’t an issue, because the size of the generator is too small to matter. However, with a 50+ megawatt array, problems arise as the electric grid isn’t currently built to handle this kind of a fluctuation. According to the Federal Energy Regulatory Commission as of August 2010, 29 states and the District of Columbia currently require the integration of renewables into the grid through legislated renewable portfolio standards as shown in the following table.
|
Final Target
|
Number
|
States with Renewable Mandates (RPS)
|
|
10% - 14%
|
6
|
Iowa, Mich., N.C., Ohio, Texas, Wis.
|
|
15% - 19%
|
7
|
Ariz., Mass., Mo., Mont., Pa., R.I., Wash.
|
|
20%
|
4
|
D.C., Kansas, Md., N.M.
|
|
23% - 24%
|
2
|
N.H., N.J.
|
|
25% - 29%
|
6
|
Conn., Del., Ill., Minn., Nev., Ore
|
|
30% - 39%
|
3
|
Calif., Colo., N.Y.
|
|
40%
|
2
|
Hawaii, Maine
|
Many of these states have established targets requiring the integration of renewable generation sources equal to or exceeding 25% of total generation within the next decade. For example, California has a mandate to generate 33% of its electricity from renewable sources by 2020. According to the August 2010 Pacific Gas and Electric Company, Long Term Procurement Plan Proceeding, the 2009 California regulation requirement was 419 megawatt and the California Independent System Operator (CAISO) predicts that to meet the 33% renewable portfolio standard by 2020, California will require 1,114 megawatt of regulation. These levels are substantially higher than what is available today. The mandated adoption of these renewable energy generation systems is likely to increase the need for effective, efficient, clean energy storage technologies to provide frequency regulation services and maintain the reliability and stability of the associated electric grid systems.
Electric and Hybrid Electric Buses. Large cities, counties and transit authorities are increasingly turning to electric and hybrid electric buses to reduce pollution and reliance on diesel fuel for their transportation systems. At this stage of the market development, electric and hybrid electric vehicles generally cost more than their conventional counterparts, although the upfront cost is partially offset by lower operating costs and a potentially longer operating life. Proterra LLC had one of its all electric buses using our batteries tested at the Altoona Test Track by Penn State University and demonstrated a 17.5 to 29.5 miles per gallon (mpg) fuel equivalent vs. a normal diesel bus that gets under 4 mpg. This difference translates into a fuel savings of about $350,000 over the life of the bus assuming fuel cost of $3.50 per gallon. This is in addition to the savings in maintenance costs over the life of the bus as a result of fewer mechanical systems and moving parts to maintain. We believe that cities, counties and mass transit operators are willing to accept the higher upfront costs in order to benefit from the expected savings in long-term operating costs and potentially longer operating life, as well as the environmental benefits.
3
Electric and hybrid electric buses require a significant amount of power, operate throughout the day, have a long expected life and run in all temperatures. The relative strengths of our nano lithium titanate batteries, including the high levels of power, rapid charge and discharge rates, long cycle life and ability to function at temperature extremes, are particularly well suited for electric and hybrid electric buses, giving us what we believe is a compelling competitive advantage in this market.
According to an October 2010 research report from the Freedonia Group Inc., the global market for buses is expanding at a 4.3% annual rate and is expected to hit 423,300 units in 2014. In a separate report published by Pike Research in November 2010, it is projected that the global demand for hybrid-electric buses will grow at a compound annual growth rate of 19.8% between 2010 and 2016. With the growing concern regarding the release of pollutants associated with burning fossil fuels, the attractiveness of all electric and hybrid electric buses is rapidly growing. Working with Proterra and other potential partners, we are attempting to establish our nano lithium titanate batteries as the power source of choice in this emerging market.
Military Uses. As a condition to close our pending funding transaction with Canon planned for May 2011, we ceased all operations in the military market effective December 31, 2010.
Key Features of Our Nano Lithium Titanate Batteries
One of the principal advantages of our nano lithium titanate battery is its rapid charge and discharge rate. The charge rate is the rate at which a battery’s energy is replenished, and the discharge rate is the rate at which the energy stored in a battery is transferred (or, in the case of self-discharge, leaked) out. Through the optimization of materials used in our nano lithium titanate battery cells, our current cells are capable of recharge times of 10 minutes to 95% or more of initial battery capacity. The rapid recharge ability is important in our target markets of frequency regulation and mass-transit buses.
Our nano lithium titanate batteries also discharge rapidly, symmetrical with their charging ability. This balanced charge and discharge capability can be important in frequency regulation. If a battery cannot be charged at the same rate at which it discharges, then over time, with random high rate up and down regulation, a less capable battery system may ultimately be fully discharged and therefore incapable of further regulation.
Our nano lithium titanate batteries have both a longer cycle life and calendar life than commercially available rechargeable battery technologies such as conventional lithium ion, nickel-metal hydride (NiMH) batteries and nickel cadmium (NiCd) batteries. The ability of any rechargeable battery to store energy will diminish as a result of repeated charge/discharge cycles. A battery’s “cycle life” is the number of times it can be charged and discharged without a significant reduction in its energy storage capacity. Our nano lithium titanate is termed a zero strain material, meaning that the material essentially does not change shape upon the entry and exit of a lithium ion in the material. Graphite, the most common material in conventional lithium ion batteries, will expand and contract as much as 8% with each charge/discharge cycle. This constant change in volume rapidly breaks down the battery resulting in significantly shorter calendar and cycle life than with our nano lithium titanate anodes. In a January 2007 test, we completed 25,000 deep charge/discharge cycles of our innovative cells. Even after 25,000 cycles, the cells still retained over 80% of their original charge capacity. This performance represents a significant improvement over conventional batteries, which typically retain that level of charge capacity only through approximately 1,000 to 3,000 deep charge/discharge cycles.
Our nano lithium titanate also represents a breakthrough in low and high-temperature performance. Nearly 90% of room temperature charge retention is realized at -30°C from our nano lithium titanate battery cells. In contrast, common lithium ion technology possesses virtually no charging capabilities at this low temperature, and the other rechargeable battery types such as lead acid, NiMH and NiCd take 10 to 20 times longer to charge at this low temperature. This breakthrough performance at extreme temperatures is important in our target markets, in which large vehicles and large-scale fast storage batteries are expected to function in a wide range of temperature conditions. Transit buses, for example, need to function equally well in the cold New England winters and the hot summers of the Southwest.
4
We also believe that relative safety is one of the strengths of our nano lithium titanate batteries. Any battery cell or large battery unit with lithium ion cell technology must take into account safety considerations, the most important of which is thermal runaway. Thermal runaway is the temperature at which the battery chemistry will break down causing the battery to overheat and potentially explode or catch fire. This temperature is often referred to as the critical temperature. Critical temperature for lithium ion battery cells using conventional graphite anodes is around 130° C, a direct result of chemical reaction between the graphite and the electrolyte. With our current nano lithium titanate anode in place of graphite and an appropriate cathode material, that critical temperature will be close to 200° C, an increase in safety margin of approximately 70° C. Materials we are using in our lab operate at 250oC before the critical temperature is reached. The batteries we and our partners are developing for high power applications often consist of dozens or even thousands of battery cells working together as part of a single modular battery unit. When a large number of cells are aggregated into a single battery unit, the likelihood of, and risks associated with, thermal runaway increases. In this context, we believe that the additional temperature margin our individual battery cells experience before reaching the critical temperature makes our battery cells better suited than competing lithium ion batteries for the high-power applications we are targeting.
The current generation of batteries made with our nano lithium titanate exhibit lower energy density at room temperatures than conventional lithium ion systems. Energy density is normally described as watt-hours per kilogram or watt-hours per liter and refers to the available energy per unit weight or per unit volume. A battery with high energy density will deliver more energy per unit weight or volume than a battery with lower energy density. Our batteries made with our nano lithium titanate have energy densities, watt-hours per kilogram, that are better than lead acid, NiCd and NiMH batteries and approximately 50-70% of conventional lithium ion batteries when operated at room temperature. However, this energy density disadvantage is significantly less compared to conventional lithium ion batteries as the operating temperature moves away from room temperature, particularly to colder environments, and less significant in environments such as large vehicles and utilities in which battery volume is not a significant issue. When the end use of the battery requires constant performance across a wide range of temperatures, such as the need for a hybrid bus to function comparably in both winter and summer, our nano lithium titanate cells may be the preferred solution. Also, conventional lithium ion batteries prefer to cycle between approximately 30% and 80% state of charge to achieve optimum cycle life. As a result, they only use about 50% of their nominal available energy. Our nano lithium titanate batteries, on the other hand, are not so limited and as a result can use approximately 90% of their nominal available energy.
Sources of Supply and Raw Materials
An important consideration as we begin to grow our revenue stream is to ensure that we have access to the various components and raw material we need to manufacture and assemble our various products. With a small product volume having multiple suppliers for each component is not practical. As we anticipate larger orders, establishing multiple sources for key components is becoming much more important to us.
Two raw materials are key components in the manufacture of our nano lithium titanate powder that is the basic building block of our battery products, namely compounds of lithium and of titanium. We currently source our lithium compound from two of the largest producers in the world and do not foresee any problems in scaling up our purchases as our volume of business increases. We source our titanium compound from a single provider who is a global leader in the field, and we are in the process of identifying and qualifying a second supplier for this key material. At this point we are not anticipating any problems or disruptions to our supply of these raw material compounds.
As of Q4 2010, we have two contract manufacturing sources for our nano lithium titanate cells. In 2009 we initiated the establishment of the second contract manufacturing supplier for our cells. In 2010 we completed validation for production readiness and released this supplier in Q4 to begin production for us. We are now receiving volume shipments of high quality battery cells from this second supplier. Once the final documentation steps are completed with this validation process, and all conditions are satisfied under the development contract and initial consignment and master supply agreement, we will initiate the long term contract with this second contract manufacturer. At that point, we will be required to purchase at least $15 million in product from such contract manufacturer over the next several years. During 2010 we continued to experience product quality issues with our first contract manufacturing supplier which limited our supply of new cells during 2010. These quality issues were identified through our quality control process before the cells were sold. We continue to be actively engaged with this supplier to rectify the quality problems. Altairnano is committed to a long-term strategy of a dual sourcing strategy of sourced products of comparable quality and performance and will continue building the maturity of our cell supply chain in line with this objective.
5
All of the other components and materials used in the manufacture of our nano lithium titanate battery products are readily available from multiple suppliers.
Key Business Developments in Power and Energy
Frequency Regulation. As part of a multi-year development program with AES Energy Storage, LLC (“AES”), a subsidiary of global power leader The AES Corporation, we delivered a 2 megawatt battery system, consisting of two 53-foot container-sized 1 megawatt units, to AES in late 2007. AES successfully completed testing of this 2 megawatt battery system in May 2008. The test consisted of AES connecting the battery to the electrical grid at a substation in Indiana and then performing a number of stringent tests to determine if it was capable of providing the services required. These tests were designed and overseen by KEMA, Inc., an independent outside engineering company, and demonstrated that the battery performed well in every respect, meeting or exceeding all expectations. Since then, one of the 1megawatt units has been put into commercial operation in Pennsylvania and has performed flawlessly. The second 1 megawatt unit was recently moved to a location in Texas to provide the same kind of service in that location.
Since May 2008, we have been refining our energy storage solution for the electrical power industry and meeting with potential customers. Because of the significant cost and customization involved in the purchase and sale of a multi-megawatt battery storage system, lead times are long in this industry. However, we are in active negotiations with a number of potential purchasers and have begun building and storing inventory in anticipation of 2011 orders. On February 4, 2011 we accepted a $1.6 million purchase order to supply the University of Hawaii - Hawaii Natural Energy Institute (“HNEI”) with a one-megawatt ALTI-ESS energy storage system for a test of wind energy integration. We anticipate shipping this system to HNEI during the third quarter of 2011. We were also selected by Inversiones Energéticas, S.A. de C.V. (INE), one of El Salvador’s largest electric utilities, to provide a turn-key 10 Megawatt ALTI-ESS advanced battery system for frequency control at its Talnique Power Station.
Hybrid Electric and All Electric Buses. We have been supplying Proterra Inc., a leading designer and manufacturer of heavy-duty drive systems, vehicle control systems, transit buses, and fast charging stations, with battery modules since 2009. In June of 2010 we formalized this relationship with the signing of a long-term supply agreement to provide our advanced lithium-ion battery modules for incorporation into Proterra’s all-electric and hybrid-electric buses. Proterra’s flagship EcoRideTM, BE-35 is a 35-foot all-electric transit bus designed from the ground up to enable transit agencies to replace conventional diesel buses on a one-for-one basis with the world’s first all-electric buses. This is accomplished by combining Proterra’s light-weight composite body, highly efficient ProDriveTM, advanced TerraVoltTM energy storage system and on-route rooftop FastFillTM station to provide the first full size transit vehicle that meets California’s Zero Emission Bus (Zbus) Rules. Proterra’s FastFillTM charge system is comprised of the software and hardware to rapidly charge the TerraVoltTM Energy Storage System (powered by our battery modules) from 0% to 95% with >92% energy charge efficiency in as little as six minutes. The combination of these systems provides a potentially disruptive solution to fleet vehicle operators offering fuel efficiencies between 17.5 and 29 miles per gallon (diesel equivalent range) which is on average more than 500% better than competing solutions.
Proterra is currently working on projects in California, Texas and Washington and recently announced that five major urban transit agencies received more than $25 million in grants from the Federal Transit Administration (FTA) to purchase 20 fast charge battery electric buses and 4 EV charging stations. To our knowledge, the only vehicles that can meet the specifications in the grants are Proterra's EcoRide BE-35™ buses and FastFill™ Charging Stations. Proterra expects to produce 81 transit buses in 2011 and has indicated it is close to closing large contracts in Europe and South America. To meet this increase in demand, Proterra is in the process of expanding its production capacity to a goal of more than 1,500 buses per year with the ability to expand further if necessary.
6
As Proterra continues to grow we look forward to expanding our relationship and working together to enhance the value of their transit solutions. Currently, Proterra is working on resolving investment funding issues. On January 14, 2011 the Securities and Exchange Commission obtained a court order freezing the assets of the Michael Kenwood Group and its related entity MK Energy + Infrastructure, which is Proterra’s lead equity investor. Without further funding from this investor, or from an alternative source,Proterra may not have funds sufficient to continue to purchase battery modules from us. Proterra is currently working with a prominent Silicon Valley investment firm that they anticipate will fund them $20 million by the end of March 2011.
Our Relationship with YTE. In addition, we, Altairnano and Zhuhai Yintong Energy Company Ltd. (“YTE”) entered into a Conditional Supply and Technology Licensing Agreement (the “Supply Agreement”) on September 20, 2010. Pursuant to the Supply Agreement, YTE has agreed to purchase nano lithium titanate, 11 Ahr battery cells and a 1 megawatt ALTI-ESS system from us for an aggregate purchase price of $6.6 million for delivery over the coming years. A portion of nano lithium titanate and the battery cells and ALTI-ESS have already shipped. Pursuant to the First Amendment to Subscription Agreement (the “SSA Amendment”) dated February 16, 2011 between Altair and Canon, YTE’s obligation to purchase the remainder of the nano lithium titanate has been deferred until the parties reach mutually satisfactory resolution on the technical issues relating to the transfer of technology. The Supply Agreement also includes an agreement to license our nano lithium titanate manufacturing technology at no cost to the owner of a manufacturing facility in China, as long as we own a majority of the owner of such facility. In addition, under the Supply Agreement, we grant to YTE a license to use our battery technology to manufacture batteries during a term commencing on the effective date of the Supply Agreement and continuing as long as YTE purchases at least 60 tons of nano lithium titanate annually. If the share purchase closes, the battery technology license will be exclusive in China (including Taiwan, Hong Kong and Macau) as long as YTE purchases at least 1,000 tons of nano lithium titanate per year after 2010 and is non-exclusive in the remainder of Asia (excluding the Middle East), Australia and New Zealand.
Military Relationships. In January 2008, we entered into a development agreement with the Office of Naval Research for $2,490,000. This was a cost reimbursement agreement whereby we developed a proof of concept battery system consisting of two 50-80 kilowatt hour batteries. All testing associated with ONR Phase I was successfully completed in November 2008. We entered into Phase II in May of 2009 and successfully completed all work in this final phase as of December 31, 2010. As a condition to closing our pending funding transaction with Canon planned for May 2011, we ceased all operations in the military market as of December 31, 2010.
Proprietary Rights
We have been awarded a total of 12 U.S. and 42 foreign patents. We have a total of 7 U.S. and 37 foreign patent applications pending. The granted patents cover our nano lithium titanate technology include: 1) Method for producing catalyst structures, 2) Method for producing mixed metal oxides and metal oxide compounds, 3) Process for making lithium titanate, and 4) Process for making nano-sized and sub-micron-sized lithium-transition metal oxides, 5) High performance Lithium Titanium spinel Li4Ti5o12 for electrode material. The U.S. patents expire beginning in 2020.
Pending patent applications are directed to a variety of inventions related to aspects of our electrochemical cells including: ”Lithium-Ion Batteries and the Methods of Operating the Same”; “Method for Preparing a Lithium-Ion Cell”; “Method for Preparing a Lithium-Ion Battery.”
Competition
Frequency Regulation and Fast Energy Storage. A number of battery producers have stated an intent to compete in the frequency regulation and fast energy storage markets; however, to date there are only two that we have directly competed against in customer frequency regulation opportunities and renewable energy integration projects. They are A123 Systems, Inc. (“A123”) and Beacon Power Corporation (“Beacon”). As we or others begin to demonstrate traction in this market we expect to see increasing levels of competition from other credible suppliers. A123 has installed a 2 megawatt battery system in Southern California working with The AES Corporation to demonstrate its ability to provide a frequency regulation service. Unlike the independently conducted stress and performance tests that our 2 megawatt battery system was subjected to in Indianapolis in 2008 where the conclusions of the tests were made publicly available, the performance results of the A123 battery system have not been made public. We are not aware of any direct sales of Beacon’s frequency regulation product to end customers. However, Beacon is constructing a 20 megawatt facility in Stephentown, New York that they will own and operate themselves to provide frequency regulation in the New York market. Unlike A123 or Altair, Beacon employs a flywheel technology to provide frequency regulation.
7
Our products typically compete with existing or alternative technologies for providing frequency regulation and renewables integration rather than a competitor battery manufacturer. However, we expect this situation to change as the market accepts this storage technology to a greater degree. Today most utilities and regional transmission organizations use existing coal, gas and diesel generating sources to provide frequency regulation. Although these sources are inefficient and highly polluting compared to our solution, they are known quantities and accepted by the various regulators and utilities. In many instances, particularly in the U.S., we are attempting to displace this accepted way of doing things. Consequently, there is a longer education and justification period required to help the customer understand the total costs of their current approach and the benefits, both financial and environmental, of switching to our solution. Another major challenge is the significantly lower cost of natural gas in the U.S. Much of the existing frequency regulation in the U.S. is provided by natural gas powered generators. As a result, there is less of a financial incentive for utilities to implement our solution. This cost environment, however, is not the case in many foreign countries. As a result we see greater immediate opportunities for our frequency regulation products outside of the U.S. Once this new energy storage capability starts to get market traction, we expect the rate of acceptance to accelerate. Until then, however, we are experiencing a long sales cycle and don’t expect that to materially change in the near future. We believe that once we demonstrate revenue traction and establish the fact that the market does exist and is very large, other larger suppliers may also target this market.
Electric and Hybrid Electric Bus Applications. In the automotive area there are a large number of battery manufacturers and systems integrators currently serving the market. Many of them are larger companies with substantially stronger financial resources than we have. We believe this market will be driven by low margins and volume. As a result we believe that only larger, well-capitalized companies will ultimately be successful in this market. The mass-transit market, on the other hand, presents a different set of dynamics. The characteristics of our batteries are an excellent fit to satisfy the requirements of this market, and the needs here are different than in the general consumer automotive market. We believe that we can be a successful competitor in this segment of the overall automotive market.
With respect to the electric and hybrid electric mass-transit markets, we are not aware of any commercially available products that have similar performance attributes as our nano lithium titanate batteries. Nonetheless, competitors have announced advanced lithium ion batteries and battery products aimed at these markets. Some may have greater energy density than our nano lithium titanate batteries. However, we believe that these batteries do not match the cycle life, rapid charge and discharge rates and performance at temperature extremes of our nano lithium titanate batteries.
Currently, NiMH batteries dominate the hybrid electric vehicle market, including the mass-transit market. NiMH batteries improve upon the energy capacity and power capabilities of older alternatives, such as NiCd (for the same size cell) by 30% to 40%. Since they contain fewer toxins than NiCd batteries, NiMH batteries are more environmentally friendly than NiCd batteries, although they are not as environmentally friendly as our nano lithium titanate battery. Like NiCd batteries, NiMH batteries can be charged in about 3 hours. Charging rates must be reduced by a factor of 5 to 10 at temperatures below 0°C (32°F) and above 40°C (104°F). NiMH batteries suffer from poor deep cycle ability (i.e. the ability to be discharged to 10% or less of their capacity), possessing a recharge capability following deep discharge on the order of 200 to 300 cycles. While NiMH batteries are capable of high power discharge, dedicated usage in high power applications limits cycle life even further. NiMH batteries also possess high self-discharge rates, which is unintentional leaking of a battery’s charge. NiMH batteries are intolerant to elevated temperature and, as a result, performance and capacity degrade sharply above room temperature. The most serious issue with NiMH, though, involves safety accompanying recharge. The temperature and internal pressure of a NiMH battery cell rises sharply as the cell nears 100% state of charge, necessitating the inclusion of complex cell monitoring electronics and sophisticated charging algorithms in order to prevent thermal runaway, and ultimately fire. A potential limiting factor for the widespread use of NiMH batteries may be the supply of nickel, potentially rendering the technology economically infeasible for these applications as demand continues to rise.
8
Producers of electric and hybrid electric vehicles are seeking to replace NiMH batteries with lithium ion batteries for several reasons. The demand for these vehicles is placing pressures on the limited supply of nickel, potentially rendering the technology economically infeasible for these applications as the demand continues to rise. Compared to NiMH batteries, conventional lithium ion batteries are stable, charge more rapidly (in hours), exhibit low self-discharge, and require very little maintenance. Except as explained below, the safety, cycle life, calendar life, environmental impact and power of lithium ion batteries is comparable to those of NiMH and NiCd batteries.
Conventional lithium ion batteries are the batteries of choice in small electronics, such as cell phones and portable computers, where high energy density and light weight are important. These same attributes are desired for electric vehicle, hybrid electric vehicle, fast energy storage and other markets. However, these applications are principally high power demand applications and/or pose other demands on usage, such as extremes of temperature, need for extremely short recharge times, and even longer extended lifetimes. Because of safety concerns related principally to the presence of graphite in conventional lithium ion batteries, conventional graphite-based lithium ion batteries sufficiently large for such power uses may raise safety concerns. In addition, current lithium ion technology is capable of about 1,000 to 3,000 cycles and has a life of about 3 years, whereas the vehicles in which they are used may have lifetimes as long as 10 to 15 years and require much larger cycle life. Conventional lithium ion batteries also do not function well at extremely hot or cold temperatures. Our batteries --which are safer, have a longer cycle life, rapid charge and discharge rates and function well at extreme temperatures -- are designed to address the power market by providing the key benefits of lithium ion batteries without the shortcomings relative to the power market.
Our All Other Division
Background
During 2008, we operated as three separate divisions – A Power and Energy Group, a Performance Materials Division and a Life Sciences Division. For all of 2010, we were organized into two divisions; a Power and Energy Group and an All Other division. Our All Other division includes the remaining activities of our Performance Materials and Life Sciences divisions.
Based on the results of a review of all our activities, strengths, weaknesses, competitive opportunities and the overall market that was conducted during 2008, we determined to focus our future efforts exclusively in the Power and Energy arena. As a result, we began in late 2008 and early 2009 to eliminate or sell our assets and efforts in the Life Sciences and Performance Materials divisions. As of December 31, 2009, all new efforts in the Life Sciences area had been stopped and the intellectual property rights associated with that division were assigned to Spectrum Pharmaceuticals, Inc. pursuant to an amendment to our existing license agreement. During 2010, the residual work done in the Performance Materials market to fulfill commitments with existing customers totaled $1.7 million in revenue. As of December 31, 2010 we have stopped all ongoing and new efforts in the Performance Materials market with the exception of the nanosensor initiative that we are working on with Western Michigan University (WMU). Although we have completed all of our work on this grant, WMU won’t complete its portion until mid-2011. At that point we will have no further efforts in the Performance Materials market.
9
AlSher Titania LLC
On April 30, 2010 we sold our 70% share in the AlSher Titania, LLC Joint Venture (AlSher) to Sherwin-Williams. Sherwin-Williams now owns 100% of AlSher.
Under terms of the agreement, certain intellectual property relating to the Altairnano Hydrochloride Process (AHP), along with certain other intellectual property owned by us, was licensed to AlSher. We may receive future payments from AlSher based upon future revenues generated from the AHP, or from royalty payments relating to the licensed intellectual property. The amount of future payments from AlSher to us is based on AlSher revenue. All payments are capped at $3,000,000. Payments to us and continuation of the intellectual property licenses are conditional upon certain milestones being achieved and payments being made to us. AlSher also has an option to purchase the licensed intellectual property for $2,000,000.
Life Sciences
Our Life Sciences division was focused on the development and marketing of RenazorbTM products, which were designed to support phosphate control in patients with Chronic Kidney Disease, hyperphosphatemia, and high phosphate levels in blood, associated with End Stage Renal Disease. Based on a comprehensive review of the Life Sciences division, its existing and potential products, the resources available, market opportunity and competition, among other considerations, a decision was made in late 2008 to exit the life sciences arena. Consistent with this decision, in August 2009 we announced an agreement in which we assigned ownership of all patent rights associated with Renazorb™ and Renalan™ to Spectrum Pharmaceuticals, Inc. (Nasdaq: SPPI). The patent assignment amends and restates an existing, limited licensing agreement for Renazorb™ and Renalan™ compounds to Spectrum Pharmaceuticals, which was announced in January 2005. Spectrum Pharmaceuticals now has exclusive worldwide rights to Renazorb™, Renalan™, and any related compounds in any field of use.
Under terms of the agreement, Altairnano received $750,000 in Spectrum Pharmaceuticals common stock, restricted until February 2010. We sold this stock in December 2010 for $649,000 in net proceeds. In addition to the royalty and other payments we were to receive under the prior license agreement, we will now receive 10% of any fees Spectrum Pharmaceuticals may receive from the sublicensing of Renazorb™, Renalan™, and any related compounds. With the execution of this contract with Spectrum Pharmaceuticals, we have completed our efforts to exit the life sciences market and are no longer devoting resources to this area.
Research and Development Expenses
Total research and development expenses were $8.2 million, $9.4 million and $13.0 million for the years ended December 31, 2010, 2009 and 2008, respectively, while research and development costs funded by customers were $4.3 million, $2.9 million and $5.0 million, for the years ended December 31, 2010, 2009 and 2008, respectively.
Dependence on Significant Customers
During the year ended December 31, 2010, we recorded revenues from two major customers in the Power and Energy Group who accounted for 34% and 33% of revenues as follows: Proterra Corporation revenues of $2.7 million and Office of Naval Research revenues of $2.6 million. Our largest customer in the All Other Division, the U.S. Army, had revenue of $1.3 million, or 17% of total revenues.
Government Regulation
Most of our current and proposed activities are subject to numerous federal, state, and local laws and regulations concerning machine and chemical safety and environmental protection. Such laws include, without limitation, the Clean Air Act, the Clean Water Act, the Resource Conservation and Recovery Act, and the Comprehensive Environmental Response Compensation Liability Act. We are also subject to laws governing the packaging and shipment of some of our products, including our nano lithium titanate batteries. Such laws require that we take steps to, among other things, maintain air and water quality standards, protect threatened, endangered and other species of wildlife and vegetation, preserve certain cultural resources, reclaim processing sites and package potentially flammable materials in appropriate ways and pass stringent government mandated testing standards before shipping our battery products.
10
Compliance with federal, state, or local laws or regulations represents a small part of our present budget. If we fail to comply with any such laws or regulations, however, a government entity may levy a fine on us or require us to take costly measures to ensure compliance. Any such fine or expenditure may adversely affect our development.
We are committed to complying with and, to our knowledge, are presently in compliance with, all governmental regulations. In the course of completing our due diligence reviews associated with the Canon investment we discovered several instances in which we had not complied completely with certain International Traffic in Arms Regulations (ITAR). Those instances were reported to the appropriate government agency and have since been corrected. We were directed to comply with all appropriate regulations on a going forward basis, but did not receive any fines or other penalties as a result of those infractions. We cannot predict the extent to which future legislation and regulation could cause us to incur additional operating expenses, capital expenditures, and/or restrictions and delays in the development of our products and properties.
Government Contracts
A substantial portion of our current revenue has been derived from government grants and contracts. The government grants and contracts we enter into are subject to termination or delay of funding at the election of the government. As a result, any termination of such agreements would significantly reduce revenue and the capital to sustain operations and research. In order to comply with ITAR, one of the requirements for us to close the investment from Canon is that we abandon all of our military business, which we have done as of December 31, 2010. We may enter into future non-military government business, but we do not anticipate that this will be a significant portion of our future revenues.
Environmental Regulation and Liability
Any proposed processing operation at our main operating facilities in Reno, Nevada and Anderson, Indiana and any other property we use will be subject to federal, state, and local environmental laws. Under such laws, we may be jointly and severally liable with prior property owners for the treatment, cleanup, remediation, and/or removal of substances discovered at any other property used by us; to the extent the substances are deemed by the federal and/or state government to be toxic or hazardous. Courts or government agencies may impose liability for, among other things, the improper release, discharge, storage, use, disposal, or transportation of hazardous substances. We use hazardous substances in our testing and operations and, although we employ reasonable practicable safeguards to prevent any liability under applicable laws relating to hazardous substances, companies engaged in materials production are inherently subject to substantial risk that environmental remediation will be required.
Financial Information about Segments and Foreign Sales
Information with respect to assets, net sales, loss from operations and depreciation and amortization for the Power and Energy Group, and All Other Division is presented in Note 18, Business Segment Information, of Notes to Consolidated Financial Statements in Part IV.
Information with respect to foreign and domestic sales and related information is also presented in Note 18, Business Segment Information, of Notes to Consolidated Financial Statements in Part IV.
11
Subsidiaries
Altair Nanotechnologies Inc. was incorporated under the laws of the province of Ontario, Canada in April 1973 under the name Diversified Mines Limited, which was subsequently changed to Tex-U.S. Oil & Gas Inc. in February 1981, then to Orex Resources Ltd. in November 1986, then to Carlin Gold Company Inc. in July 1988, then to Altair International Gold Inc. in March 1994, then to Altair International Inc. in November 1996 and then to Altair Nanotechnologies Inc. in July 2002. In July 2002, Altair Nanotechnologies Inc. redomesticated from the Ontario Business Corporations Act to Canada’s federal corporate statute, the Canada Business Corporations Act.
Altair US Holdings, Inc. was incorporated by Altair in December 2003 for the purpose of facilitating a corporate restructuring and consolidation of all U.S. subsidiaries under a U.S. holding company. At the completion of the corporate restructuring, Fine Gold, MRS, and Altairnano, Inc. (f/k/a Altair Nanomaterials, Inc.) were direct wholly-owned subsidiaries of Altair US Holdings, Inc., while Tennessee Valley Titanium, Inc. previously a wholly-owned subsidiary of MRS, was dissolved on July 7, 2006.
Altair acquired Fine Gold in April 1994. Fine Gold has earned no operating revenues to date. Fine Gold acquired the intellectual property associated with the now defunct Altair jig, a fine particle separation device for use in minerals processing, in 1996. Fine Gold was formally dissolved on December 30, 2008.
Mineral Recovery Systems, Inc., or MRS, was incorporated in April, 1987 and was formerly known as Carlin Gold Company. MRS previously has been involved in the exploration for minerals on unpatented mining claims in Nevada, Oregon and California and the holding of mineral leases in Tennessee. MRS currently does not hold any properties or leases.
Altair Nanomaterials, Inc. was incorporated in 1998 as a wholly-owned subsidiary of MRS and holds all of our interest in our nanomaterials and titanium dioxide pigment technology and related assets. Altair Nanomaterials Inc. was subsequently renamed Altairnano, Inc. on July 6, 2006.
AlSher Titania LLC was incorporated in April 2007 as a joint venture company which was 70% owned by Altairnano, Inc. until this interest was sold to Sherwin-Williams on April 30, 2010. This company was formed to combine certain technologies of Altairnano, Inc. with the Sherwin-Williams Company in order to develop, market, and produce titanium dioxide pigment for use in a variety of applications.
Corporate History
Altair Nanotechnologies Inc. was incorporated under the laws of the Province of Ontario, Canada in April 1973 for the purpose of acquiring and exploring mineral properties. It was redomesticated in July 2002 from the Business Corporations Act (Ontario) to the Canada Business Corporations Act, a change that causes Altair to be governed by Canada's federal corporate statute. The change reduced the requirement for resident Canadian directors from 50% to 25% of the board of directors, which gave us greater flexibility in selecting qualified nominees to our board.
During the period from inception through 1994, we acquired and explored multiple mineral properties. In each case, sub-economic mineralization was encountered and the exploration was abandoned.
Beginning in 1996, we entered into leases for mineral property near Camden, Tennessee and owned the rights to the Altair jig. However, we have terminated our leases on all of the Tennessee mineral properties and during 2009 disposed of the remaining centrifugal jigs and abandoned the applicable patents since we were unable to identify an interested party to purchase them.
In November 1999, we acquired all the rights of BHP Minerals International, Inc., or BHP, in the nanomaterials and titanium dioxide pigment technologies and the nanomaterials and titanium dioxide pigment assets from BHP. We are employing the nanomaterials technology as a platform for the production and sale of metal oxide nanoparticles in our nano lithium titanate batteries.
12
During 2010 the Company investigated domesticating from Canada to the state of Nevada and secured shareholder approval to do so. However, in conjunction with the pending Canon investment, the Board determined that it was not in the best interests of shareholders to do so at the present time.
We have experienced an operating loss in every year of operation. In the fiscal year ended December 31, 2010, we experienced a net loss of $22.3 million.
We completed a four-for-one reverse stock split during November 2010. All share and per share amounts included in this filing have been restated for the effects of this reverse stock split.
Our Proposed Transaction With Canon
Share Subscription Agreement. We entered into the Share Subscription Agreement with Canon on September 20, 2010. Pursuant to the terms of the Share Subscription Agreement, Canon has agreed to acquire the number of common shares such that immediately following closing it will own 51% of our outstanding common shares on a fully diluted basis. The purchase price will be $1.5528 per share. Based upon the number of common shares and the rights to acquire common shares outstanding as of December 31, 2010, we estimate that the number of shares to be purchased will be 31,523,017, at an aggregate purchase price of $48,948,799.16. If we issue additional common shares prior to closing, as permitted by the SSA Amendment, the number of shares to be purchased, and the aggregate purchase price, will increase.
We entered into the SSA Amendment with Canon on February 16, 2011. The SSA Amendment (a) extended of the closing deadline and closing date under the Agreement to May 17, 2011 in order to permit the resolution of certain technology transfer issues and other matters, (b) authorized us to raise additional capital from third parties prior to May 1, 2011, subject to a dilution limit of less than 20% of outstanding common shares, and a further limit of US$7,500,000 in aggregate offerings if any issuance will be made at per share price, taking into account the implied value of any warrants issued in connection with such issuance, lower than $1.5528; (c) authorized Canon to terminate the Share Subscription Agreement if we issue our common shares prior to closing at a per share price, taking into account the implied value of any warrants issued in connection with such issuance, lower than $1.5528; and (d) defers the purchase and sale of nano lithium titanate under the existing Supply Agreement.
The obligations of Altair and Canon to close the transactions contemplated by the Share Subscription Agreement are subject to the satisfaction or waiver (where permissible under applicable law) of various closing conditions. Altair has satisfied all conditions to Canon’s obligation to close, other than deliveries to be made at closing; however, such conditions would no longer be satisfied if there were a material adverse change prior to the closing date.
Prior to the earlier of closing of the share purchase and the termination of the Share Subscription Agreement, Altair continues to be subject to certain operational limitations, including agreements that it will not, without Canon’s consent:
| · |
amend its articles or bylaws or other similar organizational documents (whether by merger, consolidation or otherwise);
|
| · |
change its jurisdiction of incorporation from the federal jurisdiction of Canada;
|
| · |
(i) split, combine or reclassify any shares of its capital stock, (ii) declare, set aside or pay any dividend or other distribution (whether in cash, stock or property or any combination thereof) in respect of its capital stock, or (iii) redeem, repurchase or otherwise acquire or offer to redeem, repurchase, or otherwise acquire any of its securities or those of its subsidiaries;
|
13
| · |
(i) issue, deliver or sell, or authorize the issuance, delivery or sale of, any of its securities or those of its subsidiaries, other than the issuance of (A) any common shares upon the exercise of stock options or warrants that are outstanding on the date of the Share Subscription Agreement in accordance with the terms of those options or warrants on the date of the Share Subscription Agreement and (B) any securities of its subsidiaries to it or any other subsidiary; however, as a result of the SSA Amendment, Altair may raise additional capital from third parties prior to May 1, 2011, subject to a dilution limit of less than 20% of outstanding common shares and a further limit of $7,500,000 in aggregate offerings if any issuance will be made at per share price, taking into account the implied value of any warrants issued in connection with such issuance, lower than $1.5528; or (ii) amend any term of any security or its subsidiaries (in each case, whether by merger, consolidation or otherwise);
|
| · |
incur any capital expenditures or any obligations or liabilities in respect thereof, except for (i) those contemplated by the capital expenditure budget made available to Canon and (ii) any unbudgeted capital expenditures not to exceed $75,000 individually;
|
| · |
acquire (by merger, consolidation, acquisition of stock or assets or otherwise), directly or indirectly, any assets, securities, properties, interests or businesses, other than (i) supplies in the ordinary course of the business of Altair and its subsidiaries in a manner that is consistent with past practice and (ii) acquisitions with a purchase price (including assumed indebtedness) that does not exceed $50,000 individually;
|
| · |
sell, lease or otherwise transfer, or create or incur any lien on, any of its assets, securities, properties, interests or businesses, other than (i) sales of its product, inventory or obsolete equipment in the ordinary course of business consistent with past practice, and (ii) sales of assets, securities, properties, interests or businesses with a sale price (including any related assumed indebtedness) that does not exceed $50,000 individually;
|
| · |
other than in connection with certain actions permitted by the Share Subscription Agreement, make any loans, advances or capital contributions to, or investments in, any other person, other than in the ordinary course of business consistent with past practice;
|
| · |
create, incur, assume, or otherwise be liable with respect to any indebtedness for borrowed money or guarantees thereof;
|
| · |
(i) enter into any material contract, agreement, arrangement or understanding or (ii) enter into, amend or modify in any material respect or terminate any material contract or otherwise waive, release or assign any material rights, claims or benefits;
|
| · |
enter into, amend or modify in any respect any government contract (including to extend the terms thereof, but excluding termination or assignment of the same to a third party) if such contract involves the manufacture or export of certain materials subject to the ITAR;
|
| · |
(i) with respect to any director, officer, employee or independent contractor or any of its subsidiaries whose annual base salary exceeds $100,000, (A) grant or increase any severance or termination pay to (or amend any existing severance pay or termination arrangement) or (B) enter into any employment, deferred compensation or other similar agreement (or amend any such existing agreement), (ii) increase benefits payable under any existing severance or termination pay policies, (iii) establish, adopt or amend (except as required by applicable law) any collective bargaining, bonus, profit-sharing, thrift, pension, retirement, deferred compensation, stock option, restricted stock or other benefit plan or arrangement or (iv) increase compensation, bonus or other benefits payable to any employee except, with respect to any director, officer, employee or independent contractor or any of its subsidiaries whose annual base salary does not exceed $100,000, for increases in the ordinary course of business consistent with past practice;
|
| · |
change its methods of accounting, except as required by concurrent changes in Generally Accepted Accounting Principles or in Regulation S-X of the Securities Exchange Act of 1934, as agreed to by its independent public accountants;
|
14
| · |
settle, or offer or propose to settle, (i) any material litigation, investigation, arbitration, proceeding or other claim involving or against it or any of its subsidiaries, (ii) any shareholder litigation or dispute against it or any of its officers or directors or (iii) any litigation, arbitration, proceeding or dispute that relates to the transactions contemplated hereby;
|
| · |
take any action that would make any representation or warranty of it under the Share Subscription Agreement, or omit to take any action necessary to prevent any representation or warranty of it hereunder from being, inaccurate in any respect at, or as of any time before, the closing date;
|
| · |
sell, lease, license, assign, transfer, abandon, allow to lapse or otherwise dispose of, encumber or subject to any lien, any intellectual property it owns other than in accordance with non-exclusive licenses to customers entered into in the ordinary course of business consistent with past practice; or
|
| · |
agree, resolve or commit to do any of the foregoing.
|
Under the Share Subscription Agreement, we have agreed not to solicit, engage in any negotiations with respect to or sign any agreements with respect to an alternative significant investment transactions, a sale of the company or a similar transaction, except that we may raise capital prior to May 1, 2011 as permitted by the SSA Amendment. Our right to accept superior proposals under certain conditions expired following shareholder approval of the transaction.
The Share Subscription Agreement may be terminated by mutual written agreement of Canon and Altair. The agreement may also be terminated by either us or Canon if:
|
|
·
|
the closing has not occurred on or before May 17, 2011 (but only by a party to the agreement whose breach of any provision of the agreement has not resulted in the closing not occurring by such date);
|
|
|
·
|
any applicable law makes the transactions contemplated by the Share Subscription Agreement illegal or enjoins either party from consummating such transactions;
|
|
|
·
|
the U.S. government shall have taken any action to suspend or prohibit the transactions contemplated by the Share Subscription Agreement or the other transaction documents or to impose a condition on Canon, Altair or any of its subsidiaries that would have an adverse effect on our business or that would limit Canon’s ability to exercise its ownership rights with respect to Altair.
|
Canon may terminate the Share Subscription Agreement if:
|
|
·
|
we have breached any of our obligations, representations or warranties set forth in the Share Subscription Agreement that would cause the closing conditions related to representations, warranties or pre-closing covenants not to be satisfied and such condition is incapable of being satisfied by May 17, 2011;
|
|
|
·
|
we have intentionally and materially breached any prohibitions related to alternative transactions;
|
|
|
·
|
we are required to divest any products, technology, or related services that Canon, in its reasonable discretion, deems essential to the transactions contemplated by the Share Subscription Agreement or the other contemplated transaction documents or to our business as a result of our obligation to divest itself of ITAR-controlled assets and technology and to cease manufacturing and exporting “defense articles” and providing “defense services”;
|
|
|
·
|
we are or will be prohibited from exporting or reexporting products, technology or related services possessed, produced, sold by, or under development to countries other than certain embargoed countries; or
|
|
|
·
|
we are or will be prohibited from manufacturing outside the United States products produced, sold by, or under development by us in countries other than certain embargoed countries.
|
15
We may terminate the Share Subscription Agreement if Canon shall have breached any of its obligations, representations or warranties set forth in the Share Subscription Agreement that would cause the closing conditions related to representations, warranties or pre-closing covenants not to be satisfied and such condition is incapable of being satisfied by May 17, 2011.
We will be required to pay to Canon a termination fee in the amount of $2,000,000 (the “Termination Fee”) if (1) the Share Subscription Agreement is terminated by Canon following our breach of any of our obligations, representations or warranties set forth in the Share Subscription Agreement that would cause the closing conditions related to representations, warranties or pre-closing covenants not to be satisfied and such condition is incapable of being satisfied by May 17, 2011; or (2) we intentionally and materially breach the prohibitions related to alternative transactions.
We will also be required to pay the Termination Fee if (1) the Share Subscription Agreement is terminated by either Altair or Canon based on the failure to close by May 17, 2011, we are required to divest essential products, technology or related services pursuant to the closing conditions related to ITAR, we are subject to certain exporting or reexporting restrictions, or we are prohibited from manufacturing our products outside the United States in countries other than certain embargoed countries, (2) a proposal for an alternative transaction is publicly announced or otherwise communicated to our Board or shareholders and (3) we consummate, enter into a definitive agreement with respect to, or recommend to our shareholders an alternative transaction within eighteen months of the Share Subscription Agreement being terminated. Upon termination of the Share Subscription Agreement in connection with the events identified in subsection (1) of this paragraph, we will also be required to reimburse Canon’s out-of-pocket fees and expenses (including attorney fees) in connection with the Share Subscription Agreement, up to an aggregate amount of $500,000.
Investor Rights Agreement. Simultaneous to our execution of the Share Subscription Agreement, we and Canon also entered into an Investor Rights Agreement, pursuant to which we granted certain rights to Canon following closing, including (i) rights to proportional representation on our Board of Directors, rounded up to the nearest director, (ii) the right to cause us to file a shelf registration statement two years after closing, together with certain demand and piggy-back registration rights, (iii) certain indemnification rights related to the registration rights, and (iv) an option to purchase our common shares at market price in an amount sufficient to maintain proportionate ownership in connection with future dilutive issuances.
Supply Agreement. In addition, we, Altairnano and YTE entered into the Supply Agreement. Pursuant to the Supply Agreement, YTE has agreed to purchase nano lithium titanate, 11 Ahr battery cells and a 1 megawatt ALTI-ESS system from us for an aggregate purchase price of $6.6 million for delivery over the coming years. A portion of nano lithium titanate and the battery cells and ALTI-ESS have already shipped. Pursuant to the SSA Amendment, YTE’s obligation to purchase the remainder of the nano lithium titanate has been deferred until the parties reach mutually satisfactory resolution on the technical issues relating to the transfer of technology. The Supply Agreement also includes an agreement to license our nano lithium titanate manufacturing technology at no cost to the owner of a manufacturing facility in China, as long as we own a majority of the owner of such facility. In addition, under the Supply Agreement, we grant to YTE a license to use our battery technology to manufacture batteries during a term commencing on the effective date of the Supply Agreement and continuing as long as YTE purchases at least 60 tons of nano lithium titanate annually. If the share purchase closes, the battery technology license will be exclusive in China (including Taiwan, Hong Kong and Macau) as long as YTE purchases at least 1,000 tons of nano lithium titanate per year after 2010 and is non-exclusive in the remainder of Asia (excluding the Middle East), Australia and New Zealand.
Waiver and Rights Agreement. We entered into a Waiver and Rights Agreement with Al Yousuf LLC on September 20, 2010. Under the Waiver and Rights Agreement, Al Yousuf LLC has waived its right of first offer with respect to the Share Subscription Agreement. Al Yousuf LLC has also agreed that, with respect to any underwritten demand registration under its pre-existing registration rights agreement with us, to the extent Canon exercises piggyback registration rights under the Investor Rights Agreement and there is an underwriter cutback, Canon and Al Yousuf LLC will participate on a pro rata basis proportionate to their share ownership.
16
We have agreed that, following the closing of the share issuance, Al Yousuf LLC will have the right to designate one director until such time as Al Yousuf LLC holds less than 5% of our outstanding common shares on a fully-diluted basis. During the period we have only nine directors, the director appointed by Al Yousuf LLC will be one of the independent directors and serve as a member of the audit committee of our Board of Directors.
We have further agreed that, at our next annual shareholder meeting following the closing of the transactions contemplated by the Share Subscription Agreement or if the Board decides to call a special shareholder meeting, at such shareholder meeting, we will propose to amend our articles to increase the size of the Board to no less than eleven directors and to nominate two new directors to the Board, one of whom to be designated by Canon and the other to be an independent director nominated by the Board pursuant our then-existing director nomination practice. Canon and Al Yousuf LLC have agreed to vote their common shares in favor of such proposal and the election of the two new directors. Under the Waiver and Rights Agreement, the parties have agreed that, upon closing of the share issuance, the lock up provisions applicable to the shares Al Yousuf LLC acquired from us will terminate.
Employees
Our business is currently managed by Dr. Terry Copeland, President and Chief Executive Officer, Mr. John Fallini, Chief Financial Officer, Dr. Bruce Sabacky, Chief Technology Officer, Mr. Tom Kieffer, Vice President Marketing & Sales, Mr. Steven Balogh, Vice President Human Resources, Mr. Dan Voelker, Vice President Operations, and Mr. C. Robert Pedraza, Vice President Corporate Strategy and Business Development. We have 99 additional regular employees. As of December 31, 2010, we have employment agreements with Messrs. Copeland, Fallini, Kieffer, Balogh, Pedraza, Sabacky and Voelker, as well as various technical employees.
During 2011, we anticipate hiring additional employees, primarily in operations, engineering and sales. Such additional hiring, if it occurs, will be dependent upon business volume growth.
Available Information
We file annual, quarterly and current reports and other information with the SEC. These materials can be inspected and copied at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Copies of these materials may also be obtained by mail at prescribed rates from the SEC’s Public Reference Room at the above address. Information about the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet site that contains reports, proxy information statements, and other information regarding issuers that file electronically with the SEC. The address of the SEC’s Internet site is www.sec.gov.
We make available, free of charge on our Internet website located at www.altairnano.com behind the “Investors” tab under “SEC Filings,” our most recent Annual Report on Form 10-K, our most recent Quarterly Report on Form 10-Q, any current reports on Form 8-K filed since our most recent Annual Report on Form 10-K and any amendments to such reports as soon as reasonably practicable following the electronic filing of such report with the SEC. In addition, we provide electronic or paper copies of our filings free of charge upon request.
Enforceability of Civil Liabilities against Foreign Persons
We are a Canadian corporation, and two of our directors and our Canadian legal counsel are residents of Canada. Two directors are residents of Dubai. As a result, investors may be unable to effect service of process upon such persons within the United States and may be unable to enforce court judgments against such persons predicated upon civil liability provisions of the U.S. securities laws. It is uncertain whether Canadian or Dubai courts would enforce judgments of U.S. courts obtained against us or such directors, officers or experts predicated upon the civil liability provisions of U.S. securities laws or impose liability in original actions against us or our directors, officers or experts predicated upon U.S. securities laws.
17
Forward-Looking Statements
This Report contains various forward-looking statements. Such statements can be identified by the use of the forward-looking words “anticipate,” “estimate,” “project,” “likely,” “believe,” “intend,” “expect,” or similar words. These statements discuss future expectations, contain projections regarding future developments, operations, or financial conditions, or state other forward-looking information. When considering such forward-looking statements, you should keep in mind the risk factors noted in Item 1A and other cautionary statements throughout this Report and our other filings with the SEC. You should also keep in mind that all forward-looking statements are based on management’s existing beliefs about present and future events outside of management’s control and on assumptions that may prove to be incorrect. If one or more risks identified in this Report or any other applicable filings materializes, or any other underlying assumptions prove incorrect, our actual results may vary materially from those anticipated, estimated, projected, or intended.
Item 1A. Risk Factors
An investment in our common shares and related derivative securities involves significant risks. You should carefully consider the risks described in this Report before making an investment decision. Any of these risks could materially and adversely affect our business, financial condition or results of operations. In such case, you may lose all or part of your investment. Some factors in this section are forward-looking statements.
Our significant losses and negative cash flow raise questions about our ability to continue as a going concern.
We have suffered recurring losses from operations resulting in an accumulated deficit of $184,490,000, and a cash balance of $4,695,000 at December 31, 2010. Additionally, we experienced $15,172,000 in negative cash flows from operations during the year ended December 31, 2010. We cannot assure you that we will be able to achieve or sustain revenue growth, profitability, or positive cash flow on either a quarterly or annual basis or that profitability, if achieved, will be sustained. No adjustments have been made to the financial statements that might result from the outcome of this uncertainty. If we are unable to achieve or sustain profitability, we may not be financially viable in the future and may have to curtail, suspend, or cease operations, restructure existing operations to attempt to ensure future viability, or pursue other alternatives such as filing for bankruptcy, pursuing dissolution and liquidation or seeking to merge with another company or sell all or substantially all of our assets. Because of our recurring losses and negative cash flows from operations, the audit report of our independent registered public accounting firm on our financial statements for the fiscal years ended December 31, 2010 contains an explanatory paragraph stating that the independent auditor has substantial doubt about our ability to continue as a going concern.
We may not be able to raise sufficient capital to finance our operations.
As of December 31, 2010, we had approximately $4.7 million in cash and cash equivalents. As a result of our deferral of the end date under our Share Subscription Agreement with Canon until May 17, 2011, we will need to raise additional capital in the next few months. Even if we are able to raise capital in the immediate future, if our transaction with Canon does not close prior to the end date, or at all, we will again need to raise capital. Even if our transaction with Canon closes, we will likely need to raise capital to fund our expanding operations and planned nano lithium titanate plant in China. With respect to any such capital raise, we may be unable to raise the amount of capital needed and may be forced to pay an extremely high price for capital. Factors affecting the availability and price of capital may include the following:
|
|
·
|
market factors affecting the availability and cost of capital generally, including increases or decreases in major stock market indexes, the stability of the banking and investment banking systems and general economic stability or instability;
|
|
|
·
|
the price, volatility and trading volume of our common shares;
|
|
|
·
|
our financial results, particularly the amount of revenue we are generating from product sales;
|
|
|
·
|
the market's perception of our ability to execute our business plan and any specific projects identified as uses of proceeds;
|
|
|
·
|
our ownership structure and recent or anticipated dilution;
|
|
|
·
|
the amount of our capital needs;
|
|
|
·
|
the market's perception of our company and companies in our line of business; and
|
|
|
·
|
the economics of projects being pursued.
|
If we are unable to raise required capital we may be forced to discontinue operations.
We may continue to experience significant losses from operations.
We have experienced a net loss in every fiscal year since our inception. Our loss from operations was $20.5 million for the twelve months ended December 31, 2010. We may never be profitable in the future. Even if we are profitable in one or more future years, subsequent developments in the economy, our industry, customer base, business or cost structure, or an event such as significant litigation or a significant transaction, may cause us to again experience losses.
18
If we are unable to raise required capital we may be forced to discontinue operations.
The amendment to the Share Subscription Agreement with Canon includes limits on our ability to raise capital.
We entered into an amendment to the Share Subscription Agreement with Canon, or the SSA Amendment, extending the end date until May 17, 2011. The end date is the date after which either party can terminate the Share Subscription Agreement without cause. It is likely that the share issuance to Canon will not close until the end date, if at all. As a result of the expected delay in closing, we must raise additional working capital to fund our operations. Our ability to raise additional capital is limited by the terms of the SSA Amendment, in addition to market factors. Under the terms of the SSA Amendment, we are able to raise additional capital from third parties prior to May 1, 2011 through issuances of our common shares and warrants to purchase common shares subject to the following limitations:
|
|
·
|
the aggregate number of common shares, together with the number of common shares issuable upon the exercise of any warrants, must be less than 20% of our outstanding common shares; and
|
|
|
·
|
if any issuance is made at a price (taking into account the implied value of any warrants issued in connection with such issuance) lower than $1.5528, we may not raise more than $7.5 million in capital.
|
Such limitations may harm our ability to raise capital, which may limit our ability to implement our business plan or continue operations. If the price floor limitation is exceeded, Canon will have the right to terminate the Share Subscription Agreement at any time, which may decrease the likelihood that the Canon share issuance will close.
The share issuance to Canon may not close.
The amended Share Subscription Agreement gives Canon the right to terminate the Share Subscription Agreement upon certain conditions, including if we make any issuance at a price (taking into account the implied value of any warrants issued in connection with such issuance) lower than $1.55. In addition, Canon has the right to not close if certain condition precedents under the agreement are not satisfied or if we are otherwise in breach of the Share Subscription Agreement. Even if all conditions to close are satisfied and Canon has no right to terminate the Share Subscription Agreement or to decline to close, Canon may fail to close in breach of the Share Subscription Agreement. In such event, although we could pursue legal remedies, including damages, it is unlikely we could compel Canon to close. If Canon fails to close under the Share Subscription Agreement for any reason, we will have a near term need to raise capital in order to continue our operations.
Even if we close the transaction with Canon, we may not realize the anticipated benefits of such transaction.
We have identified various potential benefits, in addition to the receipt of capital, from the share issuance to Canon. Examples include the possibility that YTE would be a significant long-term customer for us and that, together with or as a result of our relationship with Canon or YTE, we would have better access to the Chinese markets than we do today. We may not realize benefits for various reasons, including without limitation, the following:
|
|
·
|
After the close of the Canon transaction, YTE may be unable to use our nano lithium titanate or battery technology in its products and, as a result, may not purchase products from us long term;
|
|
|
·
|
Even if YTE is able to integrate our nano lithium titanate and/or technology into its products, it may not be able to achieve significant sales with its products;
|
|
|
·
|
We may be unable to continue to reach agreement with YTE on the pricing of any products or services we supply them or may, as a result of market or other circumstances, be compelled to agree to prices that are not consistent with profitability;
|
19
|
|
·
|
As a result of the terms of the Conditional Supply and Technology Licensing Agreement, subsequent agreements or gaps in our intellectual property protection, Canon or YTE may be able to exploit our technology under circumstances in which we do not receive significant economic benefits;
|
|
|
·
|
Canon may not be able to, or exert significant efforts to, provide us access to the Chinese markets, particularly if our products could compete with products produced by YTE.
|
If one or more of these risks materializes in a significant manner, we may not experience the anticipated benefits from our relationship with Canon or YTE, which may harm our business and operations.
If Canon acquires a 51% ownership interest, we will face risks associated with having a majority shareholder.
If the Canon funding occurs they will own a majority of our outstanding common shares. This presents certain risks to us, including the following:
|
|
·
|
Certain of our existing or potential customers or suppliers may be reluctant to do business with a company controlled by a single shareholder, or a China-based affiliate of a battery manufacturer, and, as a result, may cancel or choose not to make, orders.
|
|
|
·
|
We may experience significant turnover in key management, technical or other employees;
|
|
|
·
|
Because of the physical distance, cultural differences and language difference between the United States and China, we may experience conflicts or inefficiencies in Board-management communication, management-employee communication, strategy formation and other parts of our business; this risk may be exacerbated by the fact that most of the nominees proposed by Canon do not speak English, and none of our current management speaks Mandarin Chinese;
|
|
|
·
|
We have agreed to potentially spend a portion, which may be substantial, of the proceeds from the Canon transaction on a nano lithium titanate manufacturing plant in China, this project may divert management attention and consume a significant amount of capital; and
|
|
|
·
|
As a majority shareholder, Canon may be able to influence our Board of Directors to enter into transactions with Canon affiliates, or third parties, that are more favorable to such Canon affiliate or third party than would be negotiated by an independent Board of Directors.
|
If one or more of these risks, or other risks, materializes, our business will be harmed, and it may be harmed materially.
If the share issuance to Canon closes, we would lose certain net operating loss carryforwards, which may increase our tax burden if we become profitable in the future.
We currently have approximately $U.S.144.1 million in U.S. net operating loss carryforwards and approximately $Cdn.6.5 million in Canadian equivalents to net operating loss carryforwards. Our stock issuance to Canon would constitute a change of control which would result in a substantial reduction in the value of, and limits on the availability of any net operating loss carryforwards. We anticipate that the available U.S. net operating loss carryforwards may be significantly limited and the Canadian equivalents will be forfeited in total. If we are profitable in the future, the loss of such net operating loss carryforwards will lead to higher income taxes than we would have paid absent the change of control.
Proterra, Inc. may have investment funding issues that might prohibit them from purchasing additional battery modules from us on a timely basis or at all.
Proterra Inc. is currently our sole significant customer in the mass transit market. On January 14, 2011 the Securities and Exchange Commission obtained a court order freezing the assets of the Michael Kenwood Group and its related entity MK Energy + Infrastructure, which is Proterra’s lead equity investor. Without further funding from this investor, Proterra may not have funds sufficient to continue to purchase battery modules from us in the near term. Proterra is currently seeking capital from other sources. If Proterra is unable to obtain capital from MK Energy or another source, we will not be able to continue to ship Proterra battery modules and may lose a significant source of revenue and opportunity to establish market credibility.
20
Sherwin-Williams may elect not to, or be unable to, finance and continue AlSher Titania LLC or a related enterprise using our pigment production technology; in this case, we would not receive any revenues or royalties related to such technology.
We transferred to Sherwin-Williams our 70% interest in AlSher Titania LLC, which holds an exclusive license to use our intellectual property relating to the Altairnano Hydrochloride Process for the production of pigments and similar powders or materials. Under agreements related to the transfer, we received no upfront consideration, and our right to receive a percentage of revenue over time is capped at $3,000,000 in the aggregate. Our receipt of any revenue under our agreement is tied to Sherwin-Williams or AlSher continuing to develop and exploit the technology, over which we have no control or influence. It is uncertain that we will receive any proceeds related to our pigment technology, and it is unlikely that total revenues will be significant in the long term.
We may not realize anticipated benefits from our agreement with Inversiones Energeticas.
We recently entered into a purchase contract with Inversiones Energeticas, S.A. de C.V., or INE, related to the purchase of a turn-key 10 Megawatt ALTI-ESS advanced battery system for $18 million. Projected revenue under this agreement represents a substantial portion of our expected revenue in 2011. It is possible that the transactions contemplated by such agreement will not be completed, or that we will not realize the anticipated benefits, for various reasons, including the following:
|
|
·
|
INE is not obligated to issue the initiation order if the conditions precedent to the initiation order including performance bonds, are not met;
|
|
|
·
|
the costs of supplying the ALTI-ESS on a turn-key basis may exceed our projections, and in such event, imposing such increased costs on INE is precluded by the contract;
|
|
|
·
|
INE may terminate the contract for breach, including a failure to maintain performance and warranty bonds, failure to deliver products and services in conformance with a strict timeline and failure to meet specific performance standards;
|
|
|
·
|
INE may terminate the contract without cause upon thirty days notice, and we will be entitled only to equitable compensation for our efforts (rather than the full benefits of the contract); and
|
|
|
·
|
we have provide warranties and indemnified INE with respect to certain matters, which provisions may lead to substantial post-completion expenses related to the agreement.
|
If any of these risks, or other risks, materializes, we may not realize expected benefits associated with the INE contract and may experience substantial net costs and liabilities.
We may be obligated to pay a royalty on sales into the stationary power market.
In a joint development agreement we entered into in 2007 to develop a collection of advanced lithium based battery systems to provide frequency regulation and other services to the electricity generations and transmission markets, we granted a royalty of 5% of the gross revenue we realize from the sale of certain battery systems through July 20, 2012. It is uncertain whether the battery systems we are marketing are within the scope of the royalty provisions. As we begin generating revenue from the sale of large scale stationary batteries for use in connection with electrical transmission and regulation, there is a reasonable likelihood that we will be required to pay this royalty. This would harm our gross margins on such sales. We may also incur litigation expenses, and management attention may be diverted from the operation of our business.
21
We depend upon several sole-source and limited-source third-party suppliers.
We rely on certain suppliers as the sole-source, or as a primary source, of certain services, raw materials and other components of our products. We do not yet have long-term supply or service agreements engaged with any such suppliers. As a result, the providers of such services and components could terminate or alter the terms of service or supply with little or no advance notice. If our arrangements with any sole-source supplier were terminated, or if such a supplier failed to provide essential services or deliver essential components on a timely basis, failed to meet our product specifications and/or quality standards, or introduced unacceptable price increases, our production schedule would be delayed, possibly by as long as six months. Any such delay in our production schedule would result in delayed product delivery and may also result in additional production costs, customer losses and litigation.
An area in which our dependence upon a limited number of sources creates significant vulnerability is the manufacturing of our nano lithium titanate cells. Prior to the fourth quarter of 2010, we relied upon a single supplier of nano lithium titanate cells. We experienced significant quality issues with this supplier in early 2010 and continue to have quality issues with this supplier. In late 2010, we completed validation for a second supplier and began receiving shipments from that second supplier without any quality issues to date. Our nano lithium titanate battery cells are the building blocks of all of our products (other than our nano lithium titanate powder). If we continue to experience quality issues with our initial supplier, or begin to experience them with our second supplier, we may be unable to meet our deadlines, or quality specifications, with respect to existing or future orders. This would harm our reputation and our ability to grow our business.
Our operating results have fluctuated significantly in the past, and we believe that they will continue to fluctuate in the future, due to a number of factors, many of which are beyond our control. If in future periods our operating results do not meet the expectations of investors or analysts who choose to follow our company, the price of our common shares may fall. Factors that may affect our operating results include the following:
|
|
·
|
fluctuations in the size, quantity and timing of customer orders;
|
|
|
·
|
timing of delivery of our services and products;
|
|
|
·
|
additions of new customers or losses of existing customers;
|
|
|
·
|
positive or negative business or financial developments announced by us or our key customers;
|
|
|
·
|
our ability to commercialize and obtain orders for products we are developing;
|
|
|
·
|
costs associated with developing our manufacturing capabilities;
|
|
|
·
|
the retention of our key employees;
|
|
|
·
|
new product announcements or introductions by our competitors or potential competitors;
|
|
|
·
|
the effect of variations in the market price of our common shares on our equity-based compensation expenses;
|
|
|
·
|
disruptions in the supply of raw materials or components used in the manufacture of our products;
|
|
|
·
|
the pace of adoption of regulation facilitating our ability to sell our products in our target markets;
|
|
|
·
|
technology and intellectual property issues associated with our products;
|
|
|
·
|
general political, social, geopolitical and economic trends and events; and
|
|
|
·
|
availability of components sourced from Korea if tensions between North Korea and South Korea erupt into a greater military conflict.
|
22
Our patents and other protective measures may not adequately protect our proprietary intellectual property.
We regard our intellectual property, particularly our proprietary rights in our nano lithium titanate technology, as critical to our success. We have received various patents, and filed other patent applications, for various applications and aspects of our nano lithium titanate technology and other intellectual property. Such patents and agreements and various other measures we take to protect our intellectual property from use by others may not be effective for various reasons, including the following:
|
|
·
|
Our pending patent applications may not be granted for various reasons, including the existence of conflicting patents or defects in our applications, if there was in existence relevant prior art or the invention was deemed by the examiner to be obvious to a person skilled in the art whether or not there were other existing patents. Risks associated with patent applications are enhanced because patent applications of others remain confidential for a period of approximately 18 months after filing; as a result, our belief that we are the first creator of an invention or the first to patent it may prove incorrect, as information related to conflicting patents is first published or first brought to our attention;
|
|
|
·
|
The patents we have been granted may be challenged, invalidated, narrowed or circumvented because of the pre-existence of similar patented or unpatented intellectual property rights or for other reasons;
|
|
|
·
|
The costs associated with enforcing patents, confidentiality and invention agreements or other intellectual property rights may make aggressive enforcement cost prohibitive;
|
|
|
·
|
We have not filed for patent protection in many countries in which we are currently selling produce or seek to sell product; as a result, we may be unable to prevent competitors in such markets from selling infringing products;
|
|
|
·
|
Even if we enforce our rights aggressively, injunctions, fines and other penalties may be insufficient to deter violations of our intellectual property rights; and
|
|
|
·
|
Other persons may independently develop proprietary information and techniques that, although functionally equivalent or superior to our intellectual proprietary information and techniques, do not breach proprietary rights.
|
Our inability to protect our proprietary intellectual property rights or gain a competitive advantage from such rights could harm our ability to generate revenues and, as a result, our business and operations.
We may be involved in lawsuits to protect or enforce our patents, which could be expensive, time consuming and involve adverse publicity and adverse results.
Competitors or others may infringe our patents. To counter infringement or unauthorized use, we may be required to file patent infringement claims, which can be expensive and time-consuming. Interference proceedings brought by the United States Patent and Trademark Office may be necessary to determine the priority of inventions with respect to our patent applications. Litigation or interference proceedings may result in substantial costs and be a distraction to our management.
Because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure. In addition, during the course of this litigation (even if ultimately successful), there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common shares.
In addition, in an infringement proceeding, a court may decide that a patent of ours is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover that technology. An adverse determination of any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
23
We may not prevail in any litigation or interference proceeding in which we are involved. Even if we do prevail, these proceedings can be expensive, result in adverse publicity and distract our management.
Other parties may bring intellectual property infringement claims against us, which would be time-consuming and expensive to defend, and if any of our products or processes is found to be infringing, we may not be able to procure licenses to use patents necessary to our business at reasonable terms, if at all.
Our success depends in part on avoiding the infringement of other parties’ patents and proprietary rights. We may inadvertently infringe existing third-party patents or third-party patents issued on existing patent applications. Third party holders of such patents or patent applications could bring claims against us that, even if resolved in our favor, could cause us to incur substantial expenses and, if resolved against us, could cause us to pay substantial damages. Under some circumstances in the United States, these damages could be triple the actual damages the patent holder incurs.
If we have supplied infringing products to third parties for marketing or licensed third parties to manufacture, use or market infringing products, we may be obligated to indemnify these third parties for any damages they may be required to pay to the patent holder and for any losses the third parties may sustain themselves as the result of lost sales or damages paid to the patent holder. In addition, we have, and may be required to, make representations as to our right to supply and/or license intellectual property and to our compliance with laws. Such representations are usually supported by indemnification provisions requiring us to defend our customers and otherwise make them whole if we license or supply products that infringe on third party technologies or violate government regulations. Further, if a patent infringement suit were brought against us, we and our customers, development partners and licensees could be forced to stop or delay research, development, manufacturing or sales of products based on our technologies in the country or countries covered by the patent we infringe, unless we can obtain a license from the patent holder. Such a license may not be available on acceptable terms, or at all, particularly if the third party is developing or marketing a product competitive with products based on our technologies. Even if we were able to obtain a license, the rights may be nonexclusive, which would give our competitors access to the same intellectual property.
Any successful infringement action brought against us may also adversely affect marketing of products based on our technologies in other markets not covered by the infringement action. Furthermore, we may suffer adverse consequences from a successful infringement action against us even if the action is subsequently reversed on appeal, nullified through another action or resolved by settlement with the patent holder. As a result, any infringement action against us would likely harm our competitive position, be costly and require significant time and attention of our key management and technical personnel.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
We rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. Trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, contractors, consultants, outside scientific collaborators and other advisors to protect our trade secrets and other proprietary information. Parties to the confidentiality agreements may have such agreements declared unenforceable or, even if the agreements are enforceable, may breach such agreements. Remedies available in connection with the breach of such agreements may not be adequate, or enforcing such agreement may be cost prohibitive. Courts outside the United States may be less willing to protect trade secrets. In addition, others may independently discover our trade secrets or independently develop processes or products that are similar or identical to our trade secrets. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection would harm our competitive business position.
24
If we are sued on a product liability claim, our insurance policies may not be sufficient.
Although we maintain general liability insurance and product liability insurance, our insurance may not cover all potential types of product liability claims to which manufacturers are exposed or may not be adequate to indemnify us for all liability that may be imposed. Any imposition of liability that is not covered by insurance or is in excess of our insurance coverage could harm our business, including our relationships with current customers and our ability to attract and retain new customers. In addition, if the liability were substantial relative to the size of our business, any uncovered liability could harm our liquidity and ability to continue as a going concern.
Laws regulating the manufacture or transportation of batteries may be enacted which could result in a delay in the production of our batteries or the imposition of additional costs that could harm our ability to be profitable.
At the present time, international, federal, state and local laws do not directly regulate the storage, use and disposal of the component parts of our batteries. However, laws and regulations may be enacted in the future which could impose environmental, health and safety controls on the storage, use and disposal of certain chemicals and metals used in the manufacture of lithium and lithium-ion batteries. Satisfying any future laws or regulations could require significant time and resources from our technical staff, including those related to possible redesign which may result in substantial expenditures and delays in the production of our product, all of which could harm our business and reduce our future profitability.
The transportation of lithium and lithium-ion batteries is regulated both domestically and internationally. Under recently revised United Nations recommendations and as adopted by the International Air Transport Association, our batteries and battery systems currently fall within the level such that they are not exempt and require a Class 9 designation for transportation. The revised United Nations recommendations and other recommendations are not U.S. law until such time as they are incorporated into the Hazardous Material Regulations of the U.S. Department of Transportation, or DOT. However, DOT has proposed new regulations harmonizing with the U.N. guidelines and is reviewing other proposed changes under consideration for inclusion. At present it is not known if or when the proposed regulations would be adopted by the United States. Although we fall under the equivalency levels for the United States and comply with all safety packaging requirements worldwide, future DOT or IATA approval process could require significant time and resources from out technical staff and, if redesign were necessary, could delay the introduction of new products.
If our warranty expense estimates differ materially from our actual claims, or if we are unable to estimate future warranty expense for new products, our business and financial results could be harmed.
Our warranty for our products ranges from one to three years from the date of sale, depending on the type of product and its application. We expect that in the future some of our warranties may extend for longer periods. Because our supply arrangements are negotiated, the scope of our product warranties differ substantially depending upon the product, the purchaser and the intended use; however, we have granted and may grant broad warranties, addressing such issues as leakage, cycle life and decline in power. We have a limited product history on which to base our warranty estimates. Because of the limited operating history of our batteries and battery systems, our management is required to make assumptions and to apply judgment regarding a number of factors, including anticipated rate of warranty claims, the durability and reliability of our products, and service delivery costs. Our assumptions could prove to be materially different from the actual performance of our batteries and battery systems, which could cause us to incur substantial expense to repair or replace defective products in the future and may exceed expected levels against which we have reserved. If our estimates prove incorrect, we could be required to accrue additional expenses from the time we realize our estimates are incorrect and also face a significant unplanned cash burden at the time our customers make a warranty claim, which could harm our operating results.
25
In addition, with our new products and products that remain under development, we will be required to base our warranty estimates on historical experience of similar products, testing of our batteries under laboratory conditions and limited performance information learned during our development activities with the customer. As a result, actual warranty claims may be significantly different from our estimates and our financial results could vary significantly from period-to-period.
Product liability or other claims could cause us to incur losses or damage our reputation.
The risk of product liability claims and associated adverse publicity is inherent in the development, manufacturing and sale of batteries and battery system. Certain materials we use in our batteries, as well as our battery systems, could, if used improperly, cause injuries to others. Improperly charging or discharging our batteries could cause fires. Any accident involving our batteries or other products could decrease or even eliminate demand for our products. Because some of our batteries are designed to be used in electric and hybrid electric buses, and because vehicle accidents can cause injury to persons and damage to property, we are subject to a risk of claims for such injuries and damages. In addition, we could be harmed by adverse publicity resulting from problems or accidents caused by third party products that incorporate our batteries. We could even be harmed by problems or accidents involving competing battery systems, if the market viewed our batteries as being vulnerable to similar problems. Any such claims, loss of customers or reputation harm would harm our financial results and ability to continue as a going concern.
A majority of our revenue has historically been generated from low-margin contract research and development services; if we cannot expand revenues from other products and services, our business will fail.
Historically, a majority of our revenue has come from contract research and development services for businesses and government agencies. During the years ended December 31, 2010, 2009 and 2008, contract service revenues comprised 50%, 65% and 87% respectively, of our operating revenues. Contract services revenue is low margin, and is unlikely to grow at a rapid pace. In addition, a majority of our contract services revenue has historically been under contracts with, or related to, the U.S. military, which contracts we terminated and stopped bidding for in connection with the Share Subscription Agreement. Our business plan anticipates revenues from product sales and licensing, both of which have potential for higher margins than contract services and have potential for rapid growth, increasing in coming years. If we are not successful in significantly expanding our revenues from licensing and product sales, or if we are forced to continue to accept low or negative margins in order to achieve revenue growth, we may fail to reach profitability in the future.
Continuing adverse economic conditions could reduce, or delay demand for our products.
Although improving compared to recent years, the financial markets and general economic conditions are still relatively weak in certain geographic markets worldwide. Our products are targeted primarily at large power producers worldwide bus manufacturers and other industrial parties. Due to declining revenues and concerns about liquidity, companies and government agencies in some of our target markets have reduced, delayed or eliminated many research and development initiatives, including those related to energy storage. This reduction or delay in development spending by targeted key customers is hindering our development and production efforts and will continue to do so until development spending increases from current depressed levels.
The commercialization of many of our products is dependent upon the efforts of commercial partners and other third parties over which we have no or little control.
The commercialization of our principal products requires the cooperation and efforts of commercial partners and customers. For example, because completion and testing of our large-scale stationary batteries for power suppliers requires input from utilities and connection to a power network, commercialization of such batteries can only be done in conjunction with a power or utility company. The commercialization of transportation and other applications of our technology are also dependent, in part, upon the expertise, resources and efforts of our commercial partners. This presents certain risks, including the following:
26
|
|
·
|
we may not be able to enter into development, licensing, supply and other agreements with commercial partners with appropriate resources, technology and expertise on reasonable terms or at all;
|
|
|
·
|
our commercial partners may not place the same priority on a project as we do, may fail to honor contractual commitments, may not have the level of resources, expertise, market strength or other characteristics necessary for the success of the project, may dedicate only limited resources to, and/or may abandon, a development project for reasons, including reasons such as a shift in corporate focus, unrelated to its merits;
|
|
|
·
|
our commercial partners may be in the early stages of development and may not have sufficient liquidity to invest in joint development projects, expand their businesses and purchase our products as expected or honor contractual commitments;
|
|
|
·
|
our commercial partners may terminate joint testing, development or marketing projects on the merits of the projects for various reasons, including determinations that a project is not feasible, cost-effective or likely to lead to a marketable end product;
|
|
|
·
|
at various stages in the testing, development, marketing or production process, we may have disputes with our commercial partners, which may inhibit development, lead to an abandonment of the project or have other negative consequences; and
|
|
|
·
|
even if the commercialization and marketing of jointly developed products is successful, our revenue share may be limited and may not exceed our associated development and operating costs.
|
As a result of the actions or omissions of our commercial partners, or our inability to identify and enter into suitable arrangements with qualified commercial partners, we may be unable to commercialize apparently viable products on a timely and cost-effective basis, or at all.
Interest in our nano lithium titanate batteries is affected by energy supply and pricing, political events, popular consciousness and other factors over which we have no control.
Currently, our marketing and development efforts for our batteries and battery materials are focused primarily on stationary power and transportation applications. In the transportation market, batteries containing our nano lithium titanate materials are designed to replace or supplement gasoline and diesel engines. In the stationary power applications, our batteries are designed to conserve and regulate the stable supply of electricity, including from renewable sources. The interest of our potential customers and business partners in our products and services is affected by a number of factors beyond our control, including:
|
|
·
|
economic conditions and capital financing and liquidity constraints;
|
|
|
·
|
short-term and long-term trends in the supply and price of natural gas, gasoline, diesel, coal and other fuels;
|
|
|
·
|
the anticipated or actual granting or elimination by governments of tax and other financial incentives favoring electric or hybrid electric vehicles and renewable energy production;
|
|
|
·
|
the ability of the various regulatory bodies to define the rules and procedures under which this new technology can be deployed into the electric grid;
|
|
|
·
|
the anticipated or actual funding, or elimination of funding, for programs that support renewable energy programs and electric grid improvements;
|
|
|
·
|
changes in public and investor interest for financial and/or environmental reasons, in supporting or adopting alternatives to gasoline and diesel for transportation and other purposes;
|
|
|
·
|
the overall economic environment and the availability of credit to assist customers in purchasing our large battery systems;
|
|
|
·
|
the expansion or contraction of private and public research and development budgets as a result of global and U.S. economic trends; and
|
|
|
·
|
the speed of incorporation of renewable energy generating sources into the electric grid.
|
27
Adverse trends in one or more of these factors may inhibit our ability to commercialize our products and expand revenues from our battery materials and batteries.
Our nano lithium titanate battery materials and battery business is currently dependent upon a few customers and potential customers, which presents various risks.
Our nano lithium titanate battery materials and battery business is dependent upon a few current or potential customers, including a small number of power producers, an affiliate of Canon and smaller companies developing electric or hybrid electric buses. In addition, many of these customers are, or are expected to be, development partners who are subsidizing the research and development of products for which they may be the sole, or one of a few, potential purchasers. As a result of the small number of potential customers and partners, our existing or potential customers and partners may have significant leverage on pricing terms, exclusivity terms and other economic and noneconomic terms. This may harm our attempts to sell products at prices that reflect desired gross margins. In addition, the decision by a single customer to abandon use or development of a product, budget cutbacks, funding reductions, liquidity shortages and other events may harm the ability of a single customer to continue to purchase products or continue development and may significantly harm both our financial results and the development track of one or more products.
If we combine with other companies, we may be unable to successfully integrate our business, technology, management or other aspects of our business with the other party to the transaction.
As evidenced by our signing the Share Subscription Agreement with Canon and related agreements with YTE, we routinely consider entering into acquisition, strategic or combination transactions with other companies for strategic and/or financial reasons. We do not have extensive experience in conducting diligence on, evaluating, purchasing, merging with, selling to or integrating new businesses or technologies with other entities. If we do succeed in closing a combination with another company, we will be exposed to a number of risks, including:
|
|
·
|
we may have difficulty integrating our assets, technologies, operations and personnel in connection with a business combination;
|
|
|
·
|
our ongoing business and management's attention may be disrupted or diverted by transition or integration issues and the complexity of managing, or being a part of, a geographically or culturally diverse enterprises;
|
|
|
·
|
we may find that the transaction does not further our business strategy or that the economic and strategic assumptions underlying the transaction have proved inaccurate;
|
|
|
·
|
we may encounter difficulty entering and competing in new product or geographic markets;
|
|
|
·
|
we may face business, product, structural or other limitations or prohibitions as our business becomes subject to the laws or customs of other jurisdictions; and
|
|
|
·
|
we may experience significant problems or liabilities associated with product quality, technology and legal contingencies relating to the integrated business or technology, such as intellectual property or employment matters.
|
In addition, from time to time we may enter into negotiations for acquisitions, dispositions, mergers or other transactions that are not ultimately consummated. These negotiations could result in significant diversion of management time, substantial out-of-pocket costs and, while such transactions are pending, limitations on the operation of our business (including negotiation of alternative business combinations and capital raising transactions). To the extent we issue shares of capital stock or other rights to purchase capital stock in any such transactions, including options and warrants, existing stockholders would be diluted. Any of these issues will harm our business and financial condition.
28
We intend to expand our operations and increase our expenditures in an effort to grow our business. If we are unable to achieve or manage significant growth and expansion, or if our business does not grow as we expect, our operating results may suffer.
During the past several years, we have increased our research and development expenditures in an attempt to accelerate the commercialization of certain products, particularly our nano lithium titanate batteries. Our business plan anticipates continued expenditure on development, manufacturing and other growth initiatives. We may fail to achieve significant growth despite such expenditures.
If achieved, significant growth would place increased demands on our management, accounting systems, quality control and internal controls. We may be unable to expand associated resources and refine associated systems fast enough to keep pace with expansion, especially as we expand into multiple facilities at distant locations. If we fail to ensure that our management, control and other systems keep pace with growth, we may experience a decline in the effectiveness and focus of our management team, problems with timely or accurate reporting, issues with costs and quality controls and other problems associated with a failure to manage rapid growth, all of which would harm our results of operations.
Our competitors have more resources than we do, and may be supported by more prominent partners, which may give them a competitive advantage.
We have limited financial, personnel and other resources and, because of our early stage of development, have limited access to capital. We compete or may compete against entities that are much larger than we are, have more extensive resources than we do and have an established reputation and operating history. In addition, certain of our early stage competitors, including A123 Systems, are partnered with, associated with or supported by larger business or financial partners. This may increase their ability to raise capital, attract media attention, develop products and attract customers. Because of their size, resources, reputation and history (or that of their business and financial partners), certain of our competitors may be able to exploit acquisition, development and joint venture opportunities more rapidly, easily or thoroughly than we can. In addition, potential customers may choose to do business with our more established competitors, without regard to the comparative quality of our products, because of their perception that our competitors are more stable, are more likely to complete various projects, are more likely to continue as a going concern and lend greater credibility to any joint venture.
As manufacturing becomes a larger part of our operations, we will become exposed to accompanying risks and liabilities.
In-house and outsourced manufacturing is becoming an increasingly significant part of our business. As a result, we expect to become increasingly subject to various risks associated with the manufacturing and supply of products, including the following:
|
|
·
|
If we fail to supply products in accordance with contractual terms, including terms related to time of delivery and performance specifications, we may be required to repair or replace defective products and may become liable for direct, special, consequential and other damages, even if manufacturing or delivery was outsourced;
|
|
|
·
|
Raw materials used in the manufacturing process, labor and other key inputs may become scarce and expensive, causing our costs to exceed cost projections and associated revenues;
|
|
|
·
|
Manufacturing processes typically involve large machinery, fuels and chemicals, any or all of which may lead to accidents involving bodily harm, destruction of facilities and environmental contamination and associated liabilities;
|
|
|
·
|
As our manufacturing operations expand, we expect that a significant portion of our manufacturing will be done overseas, either by third-party contractors or in a plant owned by the company. Any manufacturing done overseas presents risks associated with quality control, currency exchange rates, foreign laws and customs, timing and loss risks associated with overseas transportation and potential adverse changes in the political, legal and social environment in the host county; and
|
|
|
·
|
We have made, and may be required to make, representations as to our right to supply and/or license intellectual property and to our compliance with laws. Such representations are usually supported by indemnification provisions requiring us to defend our customers and otherwise make them whole if we license or supply products that infringe on third-party technologies or violate government regulations.
|
29
Any failure to adequately manage risks associated with the manufacture and supply of materials and products could lead to losses (or small gross profits) from that segment of our business and/or significant liabilities, which would harm our business, operations and financial condition.
Our past and future operations may lead to substantial environmental liability.
Virtually any prior or future use of our nanomaterials and titanium dioxide pigment technology is subject to federal, state and local environmental laws. Under such laws, we may be jointly and severally liable with prior property owners for the treatment, cleanup, remediation and/or removal of any hazardous substances discovered at any property we use. In addition, courts or government agencies may impose liability for, among other things, the improper release, discharge, storage, use, disposal or transportation of hazardous substances. If we incur any significant environmental liabilities, our ability to execute our business plan and our financial condition would be harmed.
Certain of our experts and directors reside in Canada or Dubai and may be able to avoid civil liability.
We are a Canadian corporation, and a majority of our directors reside outside the United States in Canada or the United Arab Emirates. As a result, investors may be unable to effect service of process upon such persons within the United States and may be unable to enforce court judgments against such persons predicated upon civil liability provisions of the U.S. securities laws. It is uncertain whether Canadian or Dubai courts would enforce judgments of U.S. courts obtained against us or such directors, officers or experts predicated upon the civil liability provisions of U.S. securities laws or impose liability in original actions against us or our directors, officers or experts predicated upon U.S. securities laws.
We are dependent on key personnel.
Our continued success will depend, to a significant extent, on the services of our executive management team and certain key scientists and engineers. We do not have key man insurance on any of these individuals. Nor do we have agreements requiring any of our key personnel to remain with our company. The loss or unavailability of any or all of these individuals could harm our ability to execute our business plan, maintain important business relationships and complete certain product development initiatives, which would harm our business.
We may issue substantial amounts of additional shares without stockholder approval.
Our articles of incorporation authorize the issuance of an unlimited number of common shares that may be issued without any action or approval by our stockholders. In addition, we have various stock option plans that have potential for diluting the ownership interests of our stockholders. The issuance of any additional common shares would further dilute the percentage ownership of our company held by existing stockholders.
The market price of our common shares is highly volatile and may increase or decrease dramatically at any time.
The market price of our common shares is highly volatile. Our stock price may change dramatically as the result of announcements of product developments, new products or innovations by us or our competitors, uncertainty regarding the viability of our technology or our product initiatives, significant customer contracts, significant litigation, our liquidity situation, revenues or losses, or other factors or events that would be expected to affect our business, financial condition, results of operations and future prospects.
30
The market price for our common shares may be affected by various factors not directly related to our business or future prospects, including the following:
|
|
·
|
intentional manipulation of our stock price by existing or future shareholders or a reaction by investors to trends in our stock rather than the fundamentals of our business;
|
|
|
·
|
a single acquisition or disposition, or several related acquisitions or dispositions, of a large number of our shares, including by short sellers covering their position;
|
|
|
·
|
the interest of the market in our business sector, without regard to our financial condition, results of operations or business prospects;
|
|
|
·
|
positive or negative statements or projections about our company or our industry, by analysts, stock gurus and other persons;
|
|
|
·
|
the adoption of governmental regulations or government grant programs and similar developments in the United States or abroad that may enhance or detract from our ability to offer our products and services or affect our cost structure; and
|
|
|
·
|
economic and other external market factors, such as a general decline in market prices due to poor economic conditions, investor distrust or a financial crisis.
|
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they change their recommendations regarding our common shares, our stock price and trading volume could decline.
The trading market for our common shares is influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. If any of the analysts who may cover us change their recommendation regarding our common shares adversely, or provide more favorable relative recommendations about our competitors, the price of our common shares would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial market, which in turn could cause the price or trading volume of our common shares to decline.
We have never declared a cash dividend and do not intend to declare a cash dividend in the foreseeable future.
We have never declared or paid cash dividends on our common shares. We currently intend to retain any future earnings, if any, for use in our business and, therefore, do not anticipate paying dividends on our common shares in the foreseeable future.
We are subject to various regulatory regimes, and may be adversely affected by inquiries, investigations and allegations that we have not complied with governing rules and laws.
In light of our status as a public company and our lines of business, we are subject to a variety of laws and regulatory regimes in addition to those applicable to all businesses generally. For example, we are subject to the reporting requirements applicable to Canadian and United States reporting issuers, such as the Sarbanes-Oxley Act of 2002, the rules of the NASDAQ Capital Market and certain state and provincial securities laws. We are also subject to state and federal environmental, health and safety laws, and rules governing department of defense contracts. Such laws and rules change frequently and are often complex. In connection with such laws, we are subject to periodic audits, inquiries and investigations. Any such audits, inquiries and investigations may divert considerable financial and human resources and adversely affect the execution of our business plan.
Through such audits, inquiries and investigations, we or a regulator may determine that we are out of compliance with one or more governing rules or laws. Remedying such non-compliance diverts additional financial and human resources. In addition, in the future, we may be subject to a formal charge or determination that we have materially violated a governing law, rule or regulation. We may also be subject to lawsuits as a result of alleged violation of the securities laws or governing corporate laws. Any charge or allegation, and particularly any determination, that we had materially violated a governing law would harm our ability to enter into business relationships, recruit qualified officers and employees and raise capital.
31
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Our corporate headquarters is located at 204 Edison Way, Reno, Nevada 89502 in a building we purchased in August 2002. Our nanomaterials and titanium dioxide pigment assets are located in this building, which contains approximately 85,000 square feet of production, laboratory, testing and office space.
We are party to a lease agreement effective as of July 1, 2007, for 30,000 square feet of space in the Flagship Business Accelerator Building located at 3019 Enterprise Drive, Anderson, Indiana. The space is used for the production of prototype batteries and battery systems. The lease is for an initial term of five years with a single one-year renewal term. On March 1, 2008, we signed an addendum to this lease that increased the space leased by 40,000 square feet and set forth corresponding adjustments in our rent. Total rent to be paid over the five year term including real estate taxes is $1.3 million. In addition to the Flagship lease, we rent another 2,210 square feet of space at 1305 W. 29th Street, Anderson, Indiana, on a month to month basis.
We also maintain a registered office at 360 Bay Street, Suite 500, Toronto, Ontario M5H 2V6. We do not lease any space for, or conduct any operations out of, the Toronto, Ontario registered office.
Item 3. Legal Proceedings
In 2009 we filed a collection action against DesignLine International Holdings, LLC in the Wake County District Court of North Carolina under Case Number 09-CVD-17792 for collection of $354,000 for payment of product and services previously provided by us. The matter was subsequently transferred to the State of North Carolina, County of Mecklenburg, General Court of Justice, Superior Court Division, under Case Number 09 CvS 30107. On January 15, 2010, DesignLine International Holdings, LLC filed its answer denying our allegations and filing counterclaims alleging breach of contract, breach of implied warranty of merchantability and breach of implied warranty of fitness for a particular purpose in response to our complaint. The lawsuit with Design Line International Holdings, LLC was resolved in November 2010.
Beside the matter stated above we are not a party to any pending or threatened litigation, the outcome of which could be expected to have a material adverse effect upon our financial condition, our results of operations or cash flows.
Item 4. Reserved.
32
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Price
Our common shares are traded on the NASDAQ Capital Market under the symbol "ALTI." The following table sets forth, during the periods indicated, the high and low sales prices for our common shares, as reported on our principal trading market. All market prices have been adjusted to reflect the reverse stock split completed in November 2010.
|
Fiscal Year Ended December 31, 2010
|
Low
|
High
|
||||||
|
1st Quarter
|
$ | 2.80 | $ | 3.84 | ||||
|
2nd Quarter
|
$ | 1.20 | $ | 3.12 | ||||
|
3rd Quarter
|
$ | 1.32 | $ | 3.24 | ||||
|
4th Quarter
|
$ | 1.72 | $ | 2.89 | ||||
|
Fiscal Year Ended December 31, 2009
|
Low
|
High
|
||||||
|
1st Quarter
|
$ | 2.40 | $ | 5.12 | ||||
|
2nd Quarter
|
$ | 3.44 | $ | 6.20 | ||||
|
3rd Quarter
|
$ | 3.16 | $ | 5.80 | ||||
|
4th Quarter
|
$ | 3.20 | $ | 4.72 | ||||
The last sale price of our common shares, as reported on the NASDAQ Capital Market on February 23, 2011, was $2.51 per share.
Outstanding Shares and Number of Shareholders
As of February 23, 2011, the number of common shares outstanding was 27,015,680 held by approximately 418 holders of record. In addition, as of the same date, we have reserved 1,500,616 common shares for issuance upon exercise of options that have been, or may be, granted under our employee stock option plans and 1,757,115 common shares for issuance upon exercise of outstanding warrants. As described under the heading “Business” in Part I, Item 1, we have entered into the Share Subscription Agreement with Canon with respect to the purchase by Canon of a number of common shares such that, after closing, Canon will own 51% of the outstanding common shares on a fully diluted basis.
Dividends
We have never declared or paid cash dividends on our common shares. Moreover, we currently intend to retain any future earnings for use in our business and, therefore, do not anticipate paying any dividends on our common shares in the foreseeable future.
Securities Authorized for Issuance under Equity Compensation Plans
We have stock option plans administered by the Compensation Committee of our Board of Directors that provide for the granting of options to employees, officers, directors and other service providers of the Company. Security holders have approved all option plans. The following table sets forth certain information with respect to compensation plans under which equity securities are authorized for issuance at December 31, 2010:
33
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights
|
Weighted-average exercise price of outstanding options, warrants and rights
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a))
|
|||||||
|
Plan Category
|
(a)
|
(b)
|
(c)
|
||||||
|
Equity compensation plans approved by security holders
|
1,514,025 | 7.93 | 724,740 | ||||||
|
Equity compensation plans not approved by security holders
|
None
|
N/A |
None
|
||||||
|
Total
|
1,514,025 | 7.93 | 724,740 |
Recent Sales of Unregistered Securities
We did not sell any securities in transactions that were not registered under the Securities Act in the quarter ended December 31, 2010.
Authorization of Employee Option Exchange
On December 27, 2010 the shareholders authorized our Board of Directors in its discretion to implement a one-time fair value stock option exchange program for eligible employees. Pursuant to this stock option exchange program, employees would be offered stock options with an exercise price equal to the market price on the date of the closing of the offer in exchange for their existing out-of-the-money stock options. The exchange ratio would not be one-for-one; rather, the employees would be offered a number of new options with a value that approximately equals the value of the out-of-the-money options they are tendering.
As of February 24, 2011 no implementation decision of the stock option exchange program has been made primarily due to the delay of the pending Canon funding transaction.
Transfer Agent and Registrar
The Transfer Agent and Registrar for our common shares is Equity Transfer Services, Inc., 200 University Ave, Suite 400, Toronto, Ontario, M5H 4H2.
Dividends paid on our common shares which are owned by non-residents of Canada (for purposes of the Income Tax Act (Canada)(the “Tax Act”)(a “Non-Resident”) will be subject to Canadian withholding tax generally at the rate of 25%. However, Article X of the Canada -United States Income Tax Convention (1980), as amended, (the “Treaty”) generally limits the rate of withholding tax on dividends paid to United States residents to 15%. The Treaty further limits the rate of withholding tax to 5% if the beneficial owner of the dividends is a U.S. company that owns at least 10% of the voting shares of the Company.
A capital gain realized on the disposition of our common shares by a Non-Resident will generally not be subject to tax under the Tax Act provided the shares are not “taxable Canadian property” of the Non-Resident. In general, our common shares will not be taxable Canadian property of a Non-Resident at a particular time provided that: (i) such shares are listed on a “designated stock exchange” (which currently includes NASDAQ) for the purposes of the Tax Act at the time of disposition; and (ii) at no time during the 60 month period immediately preceding the disposition of such shares were 25% or more of the issued shares of any class or series of the our capital stock owned by the Non-Resident, by persons with whom the Non-Resident did not deal at arm’s length, or by the Non-Resident together with such persons.
This summary is of a general nature only and is not intended to be, nor shall be construed to be, legal or tax advice to any particular Non-Resident. Accordingly, Non-Resident holders of our common shares are urged to consult their own tax advisors for advice with regard to their particular circumstances.
34
Item 6. Selected Financial Data
The following table sets forth selected consolidated financial information with respect to the Company and its subsidiaries for the periods indicated. The data is derived from financial statements prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The selected financial data should be read in conjunction with the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and accompanying notes included herein. All amounts are stated in thousands of U.S. dollars.
|
For the Year Ended December 31,
|
2010
|
2009
|
2008
|
2007
|
2006
|
|||||||||||||||
|
STATEMENTS OF OPERATIONS
|
||||||||||||||||||||
|
Revenues
|
$ | 7,830 | $ | 4,371 | $ | 5,726 | $ | 9,108 | $ | 4,324 | ||||||||||
|
Operating expenses
|
(22,481 | ) | (22,114 | ) | (33,202 | ) | (42,176 | ) | (22,005 | ) | ||||||||||
|
Interest expense
|
(19 | ) | (107 | ) | (97 | ) | (134 | ) | (172 | ) | ||||||||||
|
Interest income
|
101 | 188 | 982 | 1,101 | 655 | |||||||||||||||
|
Loss on foreign exchange
|
(1 | ) | (2 | ) | (10 | ) | (1 | ) | (2 | ) | ||||||||||
|
Realized (loss)/gain on investment
|
(2,045 | ) | 851 | (89 | ) | - | - | |||||||||||||
|
Loss from continuing operations before non-controlling interest’s share
|
(22,291 | ) | (21,931 | ) | (29,340 | ) | (32,102 | ) | (17,200 | ) | ||||||||||
|
Non-controlling interest’s share
|
5 | 619 | 272 | 631 | - | |||||||||||||||
|
Net loss
|
(22,286 | ) | (21,312 | ) | (29,068 | ) | (31,471 | ) | (17,200 | ) | ||||||||||
|
Basic and diluted net loss per common share
|
(0.84 | ) | (0.85 | ) | (1.35 | ) | (1.80 | ) | (1.16 | ) | ||||||||||
|
Cash dividends declared per common share
|
- | - | - | - | - | |||||||||||||||
|
BALANCE SHEET DATA
|
||||||||||||||||||||
|
Working capital
|
8,161 | 22,118 | 26,067 | 39,573 | 25,928 | |||||||||||||||
|
Total assets
|
24,260 | 40,317 | 48,071 | 73,859 | 43,121 | |||||||||||||||
|
Current liabilities
|
(6,946 | ) | (4,055 | ) | (3,647 | ) | (14,329 | ) | (3,500 | ) | ||||||||||
|
Long-term obligations
|
(16 | ) | (37 | ) | (608 | ) | (1,200 | ) | (1,800 | ) | ||||||||||
|
Non-controlling interest in subsidiary
|
- | (541 | ) | (1,098 | ) | (1,369 | ) | - | ||||||||||||
|
Net shareholders' equity
|
$ | (17,298 | ) | $ | (35,684 | ) | $ | (42,718 | ) | $ | (56,961 | ) | $ | (37,821 | ) | |||||
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion should be read in conjunction with the consolidated financial statements and notes thereto.
Overview
We are a Canadian corporation, with principal assets and operations in the United States, whose primary business is developing, manufacturing and selling our nano-lithium titanate battery cells, batteries and battery packs and providing related design, installation and test services. Our primary focus is marketing our large-scale energy storage solutions to power companies and electric grid operators throughout the world. In addition, we market our batteries to electric and hybrid-electric bus manufacturers, and are working to expand into new industrial markets.
Starting in 2010 we began looking at additional opportunities to expand the application of our battery technology into various industrial markets that have a need for the attributes of our battery technology. We believe that in the aggregate, our target markets are multi-billion dollar emerging markets with room for a number of successful suppliers. At the present time, we perceive no dominant provider and we believe that as a result of our significant differentiated product attributes, the overall strength of our management team, and the recognition we are receiving in the marketplace, that we have a very good chance of becoming one of the successful suppliers. Our proprietary technology platform gives our products a number of unique, highly sought after attributes that clearly differentiate our products from their alternatives. Included in these attributes are substantially longer cycle and calendar lives, a rapid recharge time, the ability to provide instantaneous high power, a wide operating temperature range and increased operational safety.
35
2010 has been a transition year as we have now completely exited the life sciences and performance materials markets to focus on selling products into the power and energy systems market. We expect 2011 to be the year that we gain traction in the sale of our various battery products.
Historically, we have provided contract research services on select projects where we can utilize our resources to develop intellectual property and/or new products and technology. Although contract services revenue comprised a significant portion of our total revenues in recent years accounting for 50%, 65%, and 87%, respectively in 2010, 2009 and 2008, we expect this percentage to immediately diminish as we cease provided services under military contracts and as our battery sales expand.
Our revenues have been generated by license fees, product sales, commercial collaborations, and government contracts and grants. We expect future revenues to consist primarily of product sales. We currently have agreements in place to (1) develop a suite of energy storage solutions for the stationary power market, (2) provide battery modules to a U.S. based bus manufacturer and are negotiating agreements to develop battery modules for various other industrial applications, (3) supply a one-megawatt ALTI-ESS energy storage system for a test of wind energy integration and (4) supply our nano lithium titanate powder to an early stage Chinese battery manufacturer affiliated with Canon (although deliveries under such agreement are suspended); we have also sold a battery system and battery cells under this agreement.
General Outlook
We have generated net losses in each fiscal year since incorporation. Revenues from product sales increased from $945,000 in 2009 to $3.5 million in 2010 from our increasing battery module sales to Proterra for their all-electric and hybrid commercial buses. Commercial collaborations, contracts and grants increased $1.4 million from $2.9 million in 2009 to $4.3 million in 2010 primarily as a result of our work with the Office of Naval Research. However, as a condition to closing the Canon investment we have had to abandon all future military business. We completed our contract with the U.S. Office of Naval research and most of our nanosensor grant during 2010.
Our current focus is on the development of products in energy storage that we anticipate will eventually bring a substantial amount of higher-margin revenues from product sales. We expect our nano lithium titanate batteries and battery systems to be the source of such higher-margin revenues.
As we attempt to significantly expand our revenues from licensing, manufacturing and other sources, some of the key near-term events that will affect our long-term success prospects include the following:
|
|
·
|
Based on the success of the 2008 AES 2 megawatt frequency regulation trial, as validated in the KEMA, Inc. analysis and report, we have experienced a substantial amount of interest in our large scale battery systems from other entities and are in active sales development discussions with a number of them. On February 4, 2011, we accepted a $1.6 million purchase order to supply the University of Hawaii - Hawaii Natural Energy Institute (“HNEI”) a 1 megawatt ALTI-ESS energy storage system for a test of wind energy integration. We anticipate shipping this system to HNEI during the third quarter of 2011. On February 9, 2011, we also signed a contract with Inversiones Energéticas, S.A. de C.V. (“INE”) for the supply and installation of a ten megawatt ALTI-ESS advanced battery system in El Salvador. Total revenues under the Contract are $18 million to be recognized over an expected 14-month period following Altair’s receipt of the notice to proceed, which is expected in the next 30 days. Under the Contract, we are responsible for virtually all of the design, supply, labor, installation and commission of the advanced battery system. We will provide a one-year warranty for the entire system and an additional two-year warranty for the batteries and control system.
|
36
|
|
·
|
In June 2010, we signed a contract with Proterra, LLC, a Golden, Colorado based leading designer and manufacturer of heavy-duty drive systems, energy storage systems, vehicle control systems and transit buses to sell them battery modules for their all-electric and hybrid-electric buses. Proterra’s systems are scalable to all forms of commercial buses and Class 6-8 trucks. During 2010 we sold $2.4 million of battery modules to Proterra. We anticipate battery module sales to significantly expand to Proterra in 2011 and future years as they continue to scale up their business.
|
|
|
·
|
Based on the demonstrated success of our battery in the Proterra bus application, we have also entered into discussions with a number of other bus manufacturers or systems integrators regarding the purchase of our battery products for their respective applications.
|
|
|
·
|
We are in discussions with a number of industrial manufacturers of fork lifts, elevators and other electric equipment whose use requires the long-life, rapid recharge, extreme operating temperature range or other differentiating attributes of our battery technology.
|
|
|
·
|
We signed a supply agreement with YTE, an affiliate of Canon, to provide them with 122 metric tons of nano lithium titanate by the end of 2011 (which agreement was suspended in February 2011). As specified in the Share Subscription Agreement with Canon, if such transaction closes, we will explore the establishment of an nano lithium titanate manufacturing plant in China to supply YTE with over 1,000 metric tons of nano lithium titanate per year.
|
Although it is not essential that all of these markets become successful for our battery technology in order to permit substantial long-term revenue growth, we believe that full commercialization of several of our battery applications will be necessary in order to expand our revenues enough to create a likelihood of our becoming profitable in the long-term. We remain optimistic with respect to our current key projects, as well as others we are pursuing, but recognize that, with respect to each, there are development, marketing, partnering and other risks to be overcome.
Our Operating Divisions
Since early 2009 we have been organized into two divisions: The Power and Energy Group and an All Other division. During 2008, we operated as three separate divisions, including a Performance Materials Division and a Life Sciences Division in addition to the Power and Energy Group. Based on the results of a comprehensive review of all our activities, strengths, weaknesses, competitive opportunities and the overall market that was conducted during 2008, we determined to focus our future efforts exclusively in the Power and Energy arena. As a result, we began in late 2008 and early 2009 to eliminate or sell our assets and efforts in the Life Sciences and Performance Materials divisions. As of December 31, 2009, all new efforts in the Life Sciences area had been stopped and the intellectual property rights associated with that division were assigned to Spectrum Pharmaceuticals, Inc. pursuant to an amendment to our existing license agreement. During 2010, the residual work done in the Performance Materials market to fulfill commitments with existing customers totaling $1.7 million in revenue was completed, our interest in the AlSher Titania joint venture with Sherwin-Williams was transferred to Sherwin-Williams, and all other Performance Materials related work was stopped.
Liquidity and Capital Resources
Current and Expected Liquidity
As of December 31, 2010, we had cash totaling $4.7 million. Net cash used in operating activities for the year ended December 31, 2010 totaled $15.2 million compared to $23.6 million for the year ended December 31, 2009. The decrease in cash used in operating activities for 2010 compared to 2009 was due primarily to less inventory purchases ($3.3 million), a swing from a gain on sale of our Spectrum stock in 2009 to a loss on the sale of our marketable securities in 2010, primarily our auction rate securities ($2.9 million), and an increase in deferred revenues collected from Proterra of $922,000 and YTE of $1.6 million.
37
Investing activities for the year ended December 31, 2010 reflect the sale of our Auction Rate Securities and Spectrum Stock totaling $2.6 million in net cash proceeds. Additionally, we purchased property and equipment totaling $965,000 compared to property and equipment purchases of $768,000 made for the year ended December 31, 2009.
Our cash and short-term investments decreased by $13.9 million, from $18.6 million at December 31, 2009 to $4.7 million at December 31, 2010, due primarily to net cash used in operations of $15.2 million
The net source of cash from financing activities of $78,000 for the year ended December 31, 2010 primarily reflects the raise of $692,000 net of expenses from the At the Market sale of common stock less our last payment on the note payable on our Reno, Nevada building of $600,000 plus interest. We had a single note payable in the original principal amount of $3.0 million secured by a first lien on our building. The final payment of principal and interest was due on February 8, 2010 and paid on January 29, 2010.
In September of 2010 we entered into the Share Subscription Agreement (the “Share Subscription Agreement” with Canon Investment Holdings Limited (“Canon”) under which they have agreed, subject to conditions to closing and the risk of termination, to purchase a number a number of common shares such that, following closing, Canon will own 51% of the outstanding common shares on a fully diluted basis. Based upon the number of common shares and the rights to acquire common shares outstanding as of December 31, 2010, we estimate that the number of shares to be purchased will be 31,523,017, at an aggregate purchase price of $48,948,799.. If we issue additional common shares prior to closing, as permitted by recent amendment, the number of shares to be purchased, and the aggregate purchase price, will increase.
As a result of restrictive conditions associated with the Canon negotiations and then contained in the Share Subscription Agreement, we have been precluded from raising any additional external equity or debt capital since July of 2010. As a result of delays in filing of the joint request with the Committee for Foreign Investment in the United States (“CFIUS”) to review the Canon transaction and the decision by CFIUS to extend their initial 30-day review into an additional 45-day review, the transaction did not close in 2010.
As we were anticipating closing in early February 2011, Canon indicated that it was not in a position to close. The rights under the Conditional Supply and Licensing Agreement to purchase our nano lithium titanate, and manufacture batteries using our design principles, were significant motivations for Canon to enter into the share purchase transaction, and Canon indicated that its operating subsidiary was having difficulty implementing the battery manufacturing technology called for in our Conditional Supply and Licensing Agreement. Canon also indicated, although we have not verified, that it had permitted certain credit facilities required to close to expire near the original end date of the Share Subscription Agreement and needed time to re-establish such facilities.
On February 16, 2011, we signed the First Amendment to Share Subscription Agreement (the “SSA Amendment”) which, among other things, extended the end date under the Share Subscription Agreement to May 17, 2011. The SSA Amendment also authorized us, subject to certain limitations, to sell equity securities in order to raise interim capital. We have since engaged JMP Securities with respect to a possible interim financing. If we are unable to raise interim capital, we will face a liquidity shortage prior to the anticipate closing of the Canon transaction in May 2011.
Assuming we raise interim capital and we close the pending Canon equity investment in May 2011, we believe we will have enough cash on hand to fund our operations through 2012 and to provide a portion of the capital needed to construct a China-based nano lithium titanate manufacturing facility. If such facility is constructed as anticipated by the Share Subscription Agreement, we will likely need to either seek project financing or other capital specifically for the construction of the facility or use additional capital toward construction of the facility and seek additional capital for operations. If the Canon transaction does not close in May 2011, as expected, we will need to promptly raise additional capital, through a strategic investor or from the equity markets, in order to fund our operations.
38
We evaluate our capital needs and the availability of capital on an ongoing basis and, consistent with past practice, expect to seek capital when and on such terms as we deem appropriate based upon our assessment of our current liquidity, capital needs and the availability of capital. Given that we are not yet in a positive cash flow or earnings position, the options available to us are fewer than to a positive cash flow company. Specifically, we would not generally qualify for long-term institutional debt financing. Other than the Share Subscription Agreement, we do not have any commitments from any party to provide required capital and may or may not, be able to obtain such capital on reasonable terms. Consistent with past practice we expect to raise additional capital through the sale of common shares, convertible notes, stock options, and warrants. We do not expect the current economic environment to preclude our ability to raise capital, but the overall cost to the Company of doing so will most likely be high.
To the extent we expect shortages in capital and do not have significant order volume, purchases of raw materials would be discontinued and other measures to conserve cash would be implemented as necessary to extend cash availability. Other measures to preserve cash on hand as required would include the following:
|
|
§
|
reducing production levels;
|
|
|
§
|
deferring discretionary expenditures such as capital purchases, internal research costs, training, and routine equipment and building maintenance;
|
|
|
§
|
eliminating or deferring filling non-critical positions through attrition; and
|
|
|
§
|
reductions in workforce.
|
Over the long-term, we anticipate substantially increasing revenues by entering into new contracts and increasing product sales in the stationary power, electric bus and selected other industrial markets. However, this increase in revenues will be dependent on our ability to transition our stationary power products from prototypes into commercial grade products. During 2010, we continued making significant expenditures for our battery initiative, with our existing staff and some new equipment for the manufacture of nano lithium titanate products and increased our sales and marketing efforts. In 2011, we intend to increase the 2010 level of spending on our battery initiatives continuing the enhancement of our products and their conversion into commercial grade products.
As we move into 2011, we do not expect to build up our inventory for anticipated sales more than its current level. Because of the size of the INE contract we do expect our inventory of finished goods to be larger than our current inventory level. With regard to inventory decisions, we also consider the lengthy manufacturing cycle of four to six months required to produce our large battery systems. Depending on the time lag between the initial inventory buildup and the actual sales, our cash balance will be negatively impacted. Since actual sales and production volumes for the full year of 2011 are unknown at this time, we are not able to currently estimate our anticipated inventory purchases through December 31, 2011. We expect that we will end 2011, however, with an inventory level, excluding inventory specifically tied to identified customer sales, in the same range or lower than we ended 2010. Liquidity will be a consideration in our final determination of production volumes. Our objective is to manage cash expenditures in a manner consistent with rapid product development that leads to the generation of revenues in the shortest possible time.
39
Capital Commitments and Expenditures
The following table discloses aggregate information about our contractual obligations and the periods in which payments are due as of December 31, 2010:
|
In thousands of dollars
|
Less Than
|
After
|
||||||||||||||||||
|
Contractual Obligations
|
Total
|
1 Year
|
1-3 Years
|
3-5 Years
|
5 Years
|
|||||||||||||||
|
Notes Payable
|
$ | 196 | $ | 196 | $ | - | $ | - | $ | - | ||||||||||
|
Interest on Notes Payable
|
- | - | - | - | - | |||||||||||||||
|
Contractual Service Agreements
|
687 | 687 | - | - | - | |||||||||||||||
|
Capital Leases
|
36 | 20 | 16 | - | - | |||||||||||||||
|
Operating Leases
|
513 | 365 | 149 | - | - | |||||||||||||||
|
Unfulfilled Purchase Orders
|
2,905 | 2,905 | - | - | - | |||||||||||||||
|
Total Contractual Obligations
|
$ | 4,338 | $ | 4,173 | $ | 165 | $ | - | $ | - | ||||||||||
Depending upon the speed of our revenue growth in 2011, we plan to spend approximately $4.9 million on production and tooling equipment associated with our Power and Energy Group capacity expansion (excluding the construction of a China based nano lithium titanate manufacturing plant). This level of expenditures assumes that we will continue to rely on contract manufacturers to manufacture our cells. Should we change this model and decide to manufacture the cells ourselves, a much higher level of capital investment would be required to build a cell manufacturing capability here in the U.S. The size of this investment would be dependent upon the size of the facility required and to what extent we could use existing available space in our current locations.
In addition, assuming the Canon transaction closes and based upon projected nano lithium titanate order volume, in 2011 or 2012, we anticipate significant capital expenditures related to the construction of a nano lithium titanate manufacturing plant in China in accordance with the use of proceeds provisions of the Share Subscription Agreement. Such plant is at the early stage of planning, and we cannot reasonably estimate the related capital expenditures.
Off-Balance Sheet Arrangements
The company did not have any off-balance sheet transactions during 2010.
40
Results of Operations
The following table sets forth certain selected, unaudited, condensed consolidated financial data for the periods indicated.
|
ALTAIR NANOTECHNOLOGIES INC. AND SUBSIDIARIES
|
|
CONSOLIDATED STATEMENTS OF OPERATIONS
|
|
(Expressed in thousands of United States Dollars)
|
|
(Unaudited)
|
|
Power and Energy Group
|
All Other
|
Corporate
|
Consolidated
|
||||||||||||||||||||||||||||||||||||||||||||
|
Twelve Months Ended
|
Twelve Months Ended
|
Twelve Months Ended
|
Twelve Months Ended
|
||||||||||||||||||||||||||||||||||||||||||||
|
December 31,
|
December 31,
|
December 31,
|
December 31,
|
||||||||||||||||||||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2010
|
2009
|
2008
|
2010
|
2009
|
2008
|
2010
|
2009
|
2008
|
||||||||||||||||||||||||||||||||||||
|
Revenues
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Product sales
|
$ | 3,232 | $ | 636 | $ | 428 | $ | 311 | $ | 309 | $ | 329 | $ | - | $ | - | $ | - | $ | 3,543 | $ | 945 | $ | 757 | |||||||||||||||||||||||
|
Less: Sales returns
|
- | (113 | ) | - | - | (71 | ) | - | - | - | - | - | (184 | ) | - | ||||||||||||||||||||||||||||||||
|
License fees
|
- | - | - | - | 750 | - | - | - | - | - | 750 | - | |||||||||||||||||||||||||||||||||||
|
Commercial collaborations
|
322 | 1,405 | 917 | 42 | 5 | 1,090 | - | - | - | 364 | 1,410 | 2,007 | |||||||||||||||||||||||||||||||||||
|
Contracts and grants
|
2,602 | 1,321 | 2,730 | 1,321 | 129 | 232 | - | - | - | 3,923 | 1,450 | 2,962 | |||||||||||||||||||||||||||||||||||
|
Total revenues
|
6,156 | 3,249 | 4,075 | 1,674 | 1,122 | 1,651 | - | - | - | 7,830 | 4,371 | 5,726 | |||||||||||||||||||||||||||||||||||
|
Cost of goods sold
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Product
|
2,589 | 915 | 105 | 73 | 39 | 78 | - | - | - | 2,663 | 954 | 183 | |||||||||||||||||||||||||||||||||||
|
Commercial collaborations
|
180 | 781 | 1,418 | 14 | - | 1,031 | - | - | - | 194 | 781 | 2,449 | |||||||||||||||||||||||||||||||||||
|
Contracts and grants
|
1,504 | 1,039 | 1,768 | 1,031 | 81 | 210 | - | - | - | 2,534 | 1,120 | 1,978 | |||||||||||||||||||||||||||||||||||
|
Warranty and inventory reserves
|
409 | 198 | (2,865 | ) | - | - | - | - | - | - | 409 | 198 | (2,865 | ) | |||||||||||||||||||||||||||||||||
|
Total cost of goods sold
|
4,682 | 2,933 | 426 | 1,118 | 120 | 1,319 | - | - | - | 5,800 | 3,053 | 1,745 | |||||||||||||||||||||||||||||||||||
|
Gross profit
|
1,474 | 316 | 3,649 | 556 | 1,002 | 332 | - | - | - | 2,030 | 1,318 | 3,981 | |||||||||||||||||||||||||||||||||||
|
Operating expenses
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Research and development
|
5,076 | 6,210 | 8,096 | 353 | 94 | 2,140 | 2,783 | 3,085 | 2,757 | 8,212 | 9,389 | 12,993 | |||||||||||||||||||||||||||||||||||
|
Sales and marketing
|
- | - | - | - | - | - | 4,051 | 2,894 | 2,969 | 4,051 | 2,894 | 2,969 | |||||||||||||||||||||||||||||||||||
|
General and administrative
|
346 | 168 | 230 | 97 | 2 | 368 | 7,109 | 7,626 | 9,239 | 7,552 | 7,796 | 9,837 | |||||||||||||||||||||||||||||||||||
|
Depreciation and amortization
|
1,349 | 1,320 | 1,281 | 216 | 531 | 628 | 331 | 184 | 167 | 1,896 | 2,035 | 2,076 | |||||||||||||||||||||||||||||||||||
|
Notes receivable extinguishment
|
- | - | - | - | - | - | - | - | 1,722 | - | - | 1,722 | |||||||||||||||||||||||||||||||||||
|
Settlement and release
|
- | - | - | - | - | - | - | - | 3,605 | - | - | 3,605 | |||||||||||||||||||||||||||||||||||
|
Loss on diposal of assets
|
770 | - | - | - | - | - | - | - | - | 770 | - | ||||||||||||||||||||||||||||||||||||
|
Total operating expenses
|
7,541 | 7,698 | 9,607 | 666 | 627 | 3,136 | 14,274 | 13,789 | 20,459 | 22,481 | 22,114 | 33,202 | |||||||||||||||||||||||||||||||||||
|
Loss from operations
|
(6,067 | ) | (7,382 | ) | (5,958 | ) | (110 | ) | 375 | (2,804 | ) | (14,274 | ) | (13,789 | ) | (20,459 | ) | (20,451 | ) | (20,796 | ) | (29,221 | ) | ||||||||||||||||||||||||
|
Other income (expense)
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Interest expense
|
(6 | ) | (5 | ) | - | - | - | - | (13 | ) | (102 | ) | (97 | ) | (19 | ) | (107 | ) | (97 | ) | |||||||||||||||||||||||||||
|
Interest income
|
- | - | - | - | - | - | 101 | 188 | 982 | 101 | 188 | 982 | |||||||||||||||||||||||||||||||||||
|
Realized (loss)/gain on investment
|
- | - | - | (95 | ) | 869 | - | (1,950 | ) | (18 | ) | (89 | ) | (2,045 | ) | 851 | (89 | ) | |||||||||||||||||||||||||||||
|
Loss on foreign exchange
|
- | - | - | - | - | - | (1 | ) | (2 | ) | (10 | ) | (1 | ) | (2 | ) | (10 | ) | |||||||||||||||||||||||||||||
|
Total other (expense) income, net
|
(6 | ) | (5 | ) | - | (95 | ) | 869 | - | (1,863 | ) | 66 | 786 | (1,964 | ) | 930 | 786 | ||||||||||||||||||||||||||||||
|
Loss from continuing operations
|
(6,073 | ) | (7,387 | ) | (5,958 | ) | (205 | ) | 1,244 | (2,804 | ) | (16,137 | ) | (13,723 | ) | (19,673 | ) | (22,415 | ) | (19,866 | ) | (28,435 | ) | ||||||||||||||||||||||||
|
Gain from discontinued operations
|
- | - | - | 124 | (2,065 | ) | (905 | ) | - | - | - | 124 | (2,065 | ) | (905 | ) | |||||||||||||||||||||||||||||||
|
Net loss
|
(6,073 | ) | (7,387 | ) | (5,958 | ) | (81 | ) | (821 | ) | (3,709 | ) | (16,137 | ) | (13,723 | ) | (19,673 | ) | (22,291 | ) | (21,931 | ) | (29,340 | ) | |||||||||||||||||||||||
|
Less: Net loss attributable to noncontrolling interests
|
- | - | - | 5 | 619 | 272 | - | - | - | 5 | 619 | 272 | |||||||||||||||||||||||||||||||||||
|
Net loss attributable to Altair Nanotechnologies Inc.
|
$ | (6,073 | ) | $ | (7,387 | ) | $ | (5,958 | ) | $ | (76 | ) | $ | (202 | ) | $ | (3,437 | ) | $ | (16,137 | ) | $ | (13,723 | ) | $ | (19,673 | ) | $ | (22,286 | ) | $ | (21,312 | ) | $ | (29,068 | ) | |||||||||||
|
Amounts attributable to Altair Nanotechnologies Inc. shareholders:
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Loss from continuing operations
|
$ | (6,073 | ) | $ | (7,387 | ) | $ | (5,958 | ) | $ | (205 | ) | $ | 1,244 | $ | (2,804 | ) | $ | (16,137 | ) | $ | (13,723 | ) | $ | (19,673 | ) | $ | (22,415 | ) | $ | (19,866 | ) | $ | (28,435 | ) | ||||||||||||
|
Gain from discontinued operations
|
- | - | - | 129 | (1,446 | ) | (633 | ) | - | - | - | 129 | (1,446 | ) | (633 | ) | |||||||||||||||||||||||||||||||
|
Net loss
|
$ | (6,073 | ) | $ | (7,387 | ) | $ | (5,958 | ) | $ | (76 | ) | $ | (202 | ) | $ | (3,437 | ) | $ | (16,137 | ) | $ | (13,723 | ) | $ | (19,673 | ) | $ | (22,286 | ) | $ | (21,312 | ) | $ | (29,068 | ) | |||||||||||
Fiscal Year 2010 vs. 2009
Revenues
Total revenues for the year ended December 31, 2010 were $7.8 million, up 79% from $4.4 million for 2009. Power and Energy Group revenue increased $3.0 million from $3.2 million in 2009 to 6.2 million in 2010. This was due primarily to $2.0 million of increased battery module sales to Proterra and a $1.4 million increase in revenue from our Office of Naval Research (ONR) contract, offset by $744,000 in non-recurring revenues in 2009 with BAE Systems. The ONR contract was completed as of December 31, 2010.
41
All Other contracts and grants revenue increased $1.2 million from $129,000 in 2009 to $1.3 million in 2010 due to our U.S. Army nanosensor contract.
Cost of Goods Sold (“COGS”)
Product COGS in the Power and Energy Group increased from $915,000 in 2009 to $2.6 million in 2010 primarily from increased battery module sales to Proterra. Commercial collaborations COGS in the Power and Energy Group dropped from $781,000 in 2009 to $180,000 in 2010 due primarily to the nonrecurring contract with BAE Systems during 2009. Contract and grants COGS in the Power and Energy Group increased from $1 million in 2009 to $1.5 million in 2010 related primarily to the $1.4 million increase in revenue from our Office of Naval Research contract.
All Other contracts and grants COGS increased from $81,000 in 2009 to $1 million in 2010 due to our U.S. Army nanosensor contract.
Operating Expenses
Total research and development expenses decreased $1.2 million or 13% from $9.4 million in 2009 to $8.2 million in 2010. Power and Energy Group research and development expenses decreased $1.1 million from $6.2 million in 2009 to $5.1 million in 2010. Both trends were due to the transfer of more costs to cost of good sold that directly related to our Office of Naval Research and U.S. Army nanosensor contracts.
Total sales and marketing expenses increased $1.2 million or 40% from 2009 to 2010 as the result of our effort to enhance our sales penetration into our target markets of electricity generation and distribution and electric buses.
Total general and administrative costs were down $244,000 or 3% from 2009 to 2010 as the result of our focus on company-wide cost controls.
Loss on disposal of assets of $770,000 during 2010 resulted from the early retirement of various production and research and development-related assets no longer used in our Power and Energy Group.
Other Income and Expense
The $2 million realized loss on investments during 2010 resulted primarily from the sale of our auction rate securities ($1.95 million).
Net Loss
Overall net loss of $22.3 million in 2010 versus the overall net loss of $21.3 million during 2009 was the result of our transitions in 2010 described above. Overall this transition consisted of eliminating our defense and performance materials businesses as we focused on growing our Power and Energy Group business.
Fiscal Year 2009 vs. 2008
Revenues
Total revenues for the year ended December 31, 2009 were $4.4 million compared to $5.7 million for 2008.
42
Power and Energy Group revenue for the year ended December 31, 2009 was $3.2 million compared to $4.1 million for 2008. This decrease is attributable to our shift from lower margin contracts and grants revenues partially offset by higher product sales and commercial collaboration revenues, primarily with electric bus manufacturer Proterra, LLC. Contracts and grants revenue was down $1.4 million from 2008 to 2009. This was due primarily to the completion of the ONR I contract in November 2008 and the ramp up of the ONR II contract starting in June 2009.
All Other revenues for the year ended December 31, 2009 was $1.1 million compared to $1.7 million in 2008. This net decrease was primarily from $1.1 million in reduced commercial collaboration revenue from several customers, netted with a $750,000 one-time license fee revenue from Spectrum Pharmaceuticals in 2009.
Operating Expenses
Power and Energy Group cost of goods sold increased $2.5 million in the Power and Energy Group from $0.4 million in 2008 to $2.9 million in 2009. The warranty and inventory reserves increased from a credit of $2.9 million in 2008 to $198,000 in expense in 2009. The $2.9 million credit in 2008 resulted from an agreement effective July 2008 with Phoenix Motor Cars, whereby the 2007 purchase and supply agreement was terminated and the parties resolved all outstanding issues with respect to the warranty associated with the 47 battery packs sold in 2007. The $198,000 expense in 2009 includes a $71,000 inventory valuation allowance primarily associated with the cell manufacture issue previously described.
Total research and development expense decreased by $3.6 million, or 28%, from $13.0 million in 2008 to $9.4 million 2009. Power and Energy Group research and development costs decreased $1.9 million from $8.1 million in 2008 to $6.2 million in 2009 associated with the completion of grant work in 2008. Research and development costs for All Other operations decreased by $2.0 million attributable to a shift in focus and realignment of resources from Life Sciences and Performance Materials to the battery production arena. The Corporate segment research and development expenses primarily reflect the personnel and operating costs associated with our science and technology group which supports the Company’s overall research and development efforts.
Notes receivable extinguishment expense of $1.7 million in 2008 relates to an agreement effective July 2008 with Phoenix Motorcars, whereby all accounts receivable and notes receivable relating to the 2007 Purchase and Supply Agreement were cancelled.
Settlement and release expense of $3.6 million in 2008 resulted from a settlement agreement with Al Yousuf LLC related to claims associated with their November 2007 investment in the Company.
Total sales and marketing remained relatively flat at $2.9 million in 2009 compared to $3.0 million in 2008.
Total general and administrative expenses decreased by $2.0 million, from $9.8 million in 2008 to $7.8 million in 2009. This decrease is related to the separation expenses related to our former President and Chief Executive Officer that were incurred in February 2008, and due to strict cost containment in this area of the business during 2009.
Interest income decreased by $794,000, or 81%, from 2008 to 2009. On average, a higher average level of cash during 2008 than in 2009 and lower interest rates in 2009 drove this reduction.
Interest expense remained flat at approximately $100,000 for 2008 and 2009.
43
Realized gain (loss) on investments increased from an $89,000 loss in 2008 to an $851,000 gain in 2009. This $851,000 gain in 2009 arose primarily from the sale of 240,000 shares of Spectrum common stock at a higher market price than recorded on our books when the stock was originally received as payment from Spectrum for the achievement of certain contract milestones.
Net Loss
The net loss for the year ended December 31, 2009 totaled $21.3 million compared to a net loss of $29.1 million for the year ended December 31, 2008.
Power and Energy Group net loss for the year ended December 31, 2009 was $7.4 million compared to a net loss of $6.0 million in 2008. This increased loss was due to a shift away from low margin contracts and grants revenue in 2009 and only partially offset by increased product sales and commercial collaborations revenues, and the $2.9 million product warranty credit in 2008.
The net loss of Corporate for the year ended December 31, 2009 was $13.7 million compared to a net loss of $19.7 million in 2008. This decrease is primarily related to a number of one-time expenses reflected in the 2008 results including the costs associated with the Al Yousuf LLC settlement, the Phoenix notes receivable extinguishment and the separation expenses related to our former President and Chief Executive Officer, netted with the 2009 AlSher Titania asset impairment as described above.
Critical Accounting Policies and Estimates
Management based the preceding discussion and analysis of our financial condition and results of operations on our consolidated financial statements. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our critical accounting policies and estimates, including those related to long-lived assets, share-based compensation, revenue recognition, overhead allocation, allowance for doubtful accounts, inventory, and deferred income tax. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies affect the more significant judgments and estimates used in the preparation of our consolidated financial statements. These judgments and estimates affect the reported amounts of assets and liabilities and the reported amounts of revenues and expenses during the reporting periods. Changes to these judgments and estimates could adversely affect the Company’s future results of operations and cash flows.
Use of Estimates — The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires that we make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents — Cash and cash equivalents consist principally of bank deposits and institutional money market funds. Short-term investments that are highly liquid have insignificant interest rate risk and original maturities of 90 days or less are classified as cash and cash equivalents. Investments that do not meet the definition of cash equivalents are classified as held-to-maturity or available-for-sale.
Our cash balances are maintained in bank accounts that are insured by the Federal Deposit Insurance Corporation (“FDIC”) and Canada Deposit Insurance Corporation (“CDIC”) up to a maximum of US $250,000 and CN $100,000, respectively, per depositor. At December 31, 2010 and December 31, 2009 we had $2.2 million and $1.2 million, respectively, in excess of insurance limits in bank accounts insured by the FDIC or CDIC.
44
Investment in Available for Sale Securities — The investment in short-term available for sale securities consisted of 113,809 Shares of Spectrum Pharmaceuticals, Inc. common stock valued at $505,000 at December 31, 2009. This stock was received in exchange for ownership assignment of all patent rights associated with RenazorbTM and RenalanTM compounds to Spectrum. Spectrum must also pay us future milestone and royalty payments as they develop revenues for these compounds. On December 10, 2010, we sold all of these shares and realized a loss on sale of investment of $95,000.
Accounts Receivable — Accounts receivable consists of amounts due from customers for services and product sales, net of an allowance for losses. We determine the allowance for doubtful accounts by reviewing each customer account and specifically identifying any potential for loss.
The allowance for doubtful accounts is as follows:
|
In thousands of dollars
|
||||||||
|
2010
|
2009
|
|||||||
| Beginning Balance, January 1, | $ | 161 | $ | 84 | ||||
| Additions charged to costs and expenses | - | 177 | ||||||
| Net deductions (write-offs, net of collections) | (161 | ) | (100 | ) | ||||
| Ending Balance, December 31, | $ | - | $ | 161 | ||||
Inventory – We value our inventories generally at the lower of cost (first-in, first-out method) or market. We employ a full absorption procedure using standard cost techniques. The standards are customarily reviewed and adjusted every three months. Overhead rates are recorded to inventory based on normal capacity. Any idle facility costs or excessive spoilage are recorded as current period charges. As of December 31, 2010, we recorded a $623,000 inventory valuation allowance due to quality issues with our cell supplier, of which $541,000 is recorded as a receivable from our vendor covered under their product warranty. As of December 31, 2009, we had $71,000 of inventory valuation allowance recorded.
Research and Development Expenditures — The costs of materials, equipment, or facilities that are acquired or constructed for a particular research and development project and that have no alternative future uses (in other research and development projects or otherwise) are expensed as research and development costs at the time the costs are incurred. Research and development expenditures related to materials and equipment or facilities that are acquired or constructed for research and development activities and that have alternative future uses (in research and development projects or otherwise) are capitalized when acquired or constructed. Research and development expenditures, which include the cost of materials consumed in research and development activities, salaries, wages and other costs of personnel engaged in research and development, costs of services performed by others for research and development on our behalf and indirect costs are expensed as research and development costs when incurred.
Foreign Currency Translation — Asset and liability accounts, which are originally recorded in the appropriate local currencies, are translated into U.S. dollars at year-end exchange rates. Revenue and expense accounts are translated at the average exchange rates for the period. Transaction gains and losses are included in the accompanying consolidated statements of operations. Substantially all of our assets are located in the United States of America.
Stock-Based Compensation — We measure the cost of services received in exchange for an award of equity instruments based on the grant-date fair value of the award. That cost is recognized over the period during which services are provided in exchange for the award, known as the requisite service period (usually the vesting period).
45
Long-Lived Assets — We evaluate the carrying value of long-lived assets whenever events or changes in business circumstances indicate that the carrying value of the assets may not be recoverable. The carrying value of a long-lived asset is considered impaired when the total projected undiscounted cash flows expected to be generated by the asset are less than the carrying value. Our estimate of the cash flows is based on the information available at the time including the following: internal budgets; sales forecasts; customer trends; anticipated production volumes; and market conditions over an estimate of the remaining useful life of the asset which may range from 3 to 10 years for most equipment and up to 22 years for our building and related building improvements. If an impairment is indicated, the asset value is written down to its fair value based upon market prices, or if not available, upon discounted cash flow value, at an appropriate discount determined by us to be commensurate with the risk inherent in the business model. The determination of both undiscounted and discounted cash flows requires us to make significant estimates and consider the expected course of action at the balance sheet date. Our assumptions about future sales and production volumes require significant judgment because actual sales prices and volumes have fluctuated significantly in the past and are expected to continue to do so. Until the Company’s products reach commercialization, the demand for our products is difficult to estimate. Subsequent changes in estimated undiscounted and discounted cash flows arising from changes in anticipated actions could impact the determination of whether an impairment exists, the amount of the impairment charge recorded and whether the effects could materially impact our consolidated financial statements. Events or circumstances that could indicate the existence of a possible impairment include obsolescence of the technology, an absence of market demand for the product or the assets used to produce it, a history of operating or cash flow losses and/or the partial or complete lapse of technology rights protection.
As of December 31, 2010, we estimate that our future cash flows, on an undiscounted basis, are greater than our $9.1 million investment in long-lived assets. Our estimated future cash flows include anticipated product sales, commercial collaborations, and contracts and grant revenue, since our long-lived asset base, which is primarily composed of production, laboratory and testing equipment is utilized to fulfill contracts in all revenue categories.
As a result of management’s determination to focus on the Power and Energy segment of the business and reduce resources committed to Performance Materials and Life Sciences, in combination with the delays experienced in commercializing our products, the following qualitative reviews were performed regarding our patents and fixed assets in addition to an undiscounted cash flow analysis to determine if our long-lived assets are impaired:
In the first quarter of 2010, we reviewed our four capitalized patents and determined that three of these patents had value in excess of their net book value of $483,000 at that time. In the first quarter, we determined that the fourth patent no longer had value. The fourth patent had an original cost of $152,000, accumulated depreciation of $105,000 and a net value of $47,000. Accordingly, an impairment charge of $47,000 was recorded, and is reflected for the twelve months ended December 31, 2010.
During the third quarter of 2010, we reviewed the remaining three capitalized patents and determined that these patents had value in excess of their net book value of $426,000 as of December 31, 2010. AlSher currently has an exclusive license to use this technology from Altair.
Based on our assessment, which represents no change from the prior year in our approach to valuing long-lived assets, after recording the impairment described above, we believe that our long-lived assets are not impaired.
Property, plant and equipment held and used are stated at cost less accumulated depreciation. Depreciation is recorded using the straight-line method over the following useful lives:
|
Furniture and office equipment
|
3–7 years
|
|
|
Vehicles
|
5 years
|
|
|
Nanoparticle production equipment
|
5–10 years
|
|
|
Building and improvements
|
30 years
|
We have property, plant and equipment that is held and not used stated at cost less accumulated depreciation. Depreciation is recorded using the straight-line method over the useful lives established for property, plant and equipment held and used.
46
Property Plant and Equipment, net – held and not used included assets being redeployed from our Life Sciences and Performance Materials Divisions, into our Power and Energy Group. We redeployed these assets during the second quarter of 2010 into our Power and Energy Group or transferred to Sherwin Williams through our transfer of AlSher Titania, LLC on April 30, 2010.
Patents related to the nanoparticle production technology are carried at cost and amortized on a straight-line basis over their estimated useful lives, which range from 14 to 17 years.
Revenue Recognition — We recognize revenue when persuasive evidence of an arrangement exists, delivery has occurred or service has been performed, the fee is fixed and determinable, and collectability is reasonably assured. Our revenues were derived from product sales, commercial collaborations and contracts and grants. Revenue from product sales is recognized upon delivery of the product, unless specific contractual terms dictate otherwise. Based on the specific terms and conditions of each contract/grant, revenues are recognized on a time and materials basis, a percentage of completion basis and/or a completed contract basis. Revenue under contracts based on time and materials is recognized at contractually billable rates as labor hours and expenses are incurred. Revenue under contracts based on a fixed fee arrangement is recognized based on various performance measures, such as stipulated milestones. As these milestones are achieved, revenue is recognized. From time to time, facts develop that may require us to revise our estimated total costs or revenues expected. The cumulative effect of revised estimates is recorded in the period in which the facts requiring revisions become known. The full amount of anticipated losses on any type of contract is recognized in the period in which it becomes known. Payments received in advance relating to the future performance of services or deliveries of products are deferred until the performance of the service is complete or the product is shipped. Upfront payments received in connection with certain rights granted in contractual arrangements are deferred and revenue is recognized over the related time period which the benefits are received. Based on specific customer bill and hold agreements, revenue is recognized when the inventory is shipped to a third party storage warehouse, the inventory is segregated and marked as sold, the customer takes the full rights of ownership and title to the inventory upon shipment to the warehouse per the bill and hold agreement. When contract terms include multiple components that are considered separate units of accounting, the revenue is attributed to each component and revenue recognition may occur at different points in time for product shipment, installation, and service contracts based on substantial completion of the earnings process.
Accrued Warranty — We provide a limited warranty for battery packs and energy storage systems. A liability is recorded for estimated warranty obligations at the date products are sold. Since these are new products, the estimated cost of warranty coverage is based on cell and module life cycle testing and compared for reasonableness to warranty rates on competing battery products. As sufficient actual historical data is collected on the new product, the estimated cost of warranty coverage will be adjusted accordingly. The liability for estimated warranty obligations may also be adjusted based on specific warranty issues identified.
Non-controlling Interest — In April 2007, The Sherwin-Williams Company (“Sherwin”) entered into an agreement with us to form AlSher Titania LLC (“AlSher”), a Delaware limited liability company. AlSher is a joint venture combining certain technologies of ours and Sherwin in order to develop and produce titanium dioxide pigment for use in paint and coatings and nano titanium dioxide materials for use in a variety of applications, including those related to removing contaminants from air and water. Pursuant to a Contribution Agreement dated April 24, 2007 among Sherwin, AlSher, and us, we contributed to AlSher an exclusive license to use our technology (including our hydrochloride pigment process) for the production of titanium dioxide pigment and other titanium containing materials (other than battery or nanoelectrode materials) and certain pilot plant assets with a net book value of $3,110,000. We received no consideration for the license granted to AlSher other than our ownership interest in AlSher. Sherwin agreed to contribute to AlSher cash and a license agreement related to a technology for the manufacture of titanium dioxide using the digestion of ilmenite in hydrochloric acid. As a condition to enter into the second phase of the joint venture, we agreed to complete the pigment pilot processing plant and related development activities by January 2008. The 100 ton pigment pilot processing plant was commissioned in February 2008 and the costs associated with this effort were partially reimbursed by AlSher. We contribute any work in process and fixed assets associated with completion of the pigment pilot processing plant to the AlSher joint venture. For each reporting period, AlSher is consolidated with our subsidiaries because we have a controlling interest in AlSher and any inter-company transactions are eliminated (refer to Note 1 – Basis of Preparation of Consolidated Financial Statements). The non-controlling shareholder’s interest in the net assets and net income or loss of AlSher are reported as non-controlling interest in subsidiary on the condensed consolidated balance sheet and as non-controlling interest share in the condensed consolidated statement of operations, respectively.
47
Asset impairment of $1.3 million in 2009 relates to the expense of adjusting AlSher Titania, LLC assets to fair market value as of December 31, 2009. These assets were temporarily idled throughout 2009 as we searched for an interested party to acquire our interests in AlSher Titania. On April 30, 2010, we sold our 70% share in the AlSher Titania Joint Venture to Sherwin-Williams. We recorded a gain of $124,000 on discontinued operations that was comprised of $400,000 loss on disposal of fixed assets and $524,000 remaining equity in noncontrolling interest.
Overhead Allocation — Facilities overhead, which is comprised primarily of occupancy and related expenses, are initially recorded in general and administrative expenses and then allocated to research and development and product inventories based on relative labor costs.
Net Loss per Common Share — Basic loss per share is computed using the weighted average number of common shares outstanding during the period. Diluted loss per share is computed using the weighted average number of common and potentially dilutive shares outstanding during the period. Potentially dilutive shares consist of the incremental common shares issuable upon the exercise of stock options and warrants. Potentially dilutive shares are excluded from the computation if their effect is anti-dilutive. We had a net loss for all periods presented herein; therefore, none of the stock options and warrants outstanding during each of the periods presented were included in the computation of diluted loss per share as they were anti-dilutive. Stock options and warrants to purchase a total of 3,271,138 shares as of December 31, 2010, 2,987,162 shares as of December 31, 2009 and 1,159,497 shares as of December 31, 2008 were excluded from the calculations of diluted loss per share for the years ended December 31, 2010, 2009 and 2008, respectively.
Accumulated Other Comprehensive Loss — Accumulated other comprehensive loss consists entirely of unrealized loss on the investment in available for sale securities.
Deferred Income Taxes — Income taxes are accounted for using the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry forwards. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Future tax benefits are subject to a valuation allowance when management is unable to conclude that its deferred income tax assets will more likely than not be realized from the results of operations. We have recorded a valuation allowance to reflect the estimated amount of deferred income tax assets that may not be realized. The ultimate realization of deferred income tax assets is dependent upon generation of future taxable income during the periods in which those temporary differences become deductible. Management considers projected future taxable income and tax planning strategies in making this assessment.
Fair Value of Financial Instruments — Our financial instruments such as cash and cash equivalents and long-term debt, when valued using market interest rates, would not be materially different from the amounts presented in the consolidated financial statements.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
None.
48
Item 8. Financial Statements and Supplementary Data.
Supplementary Data
The following Supplementary Financial Information for the fiscal quarters ended March 31, June 30, September 30 and December 31 in each of the years 2010 and 2009 was derived from our unaudited quarterly consolidated financial statements filed by us with the SEC in our Quarterly Reports on Form 10-Q with respect to such periods (except for 4th quarter data).
|
Supplementary Financial Information by Quarter, 2010 and 2009
|
||||||||||||||||
|
(Unaudited – in 000s)
|
|
Quarter Ended
|
Quarter Ended
|
Quarter Ended
|
Quarter Ended
|
|||||||||||||
| 3/31 | 6/30 | 9/30 | 12/31(2) | |||||||||||||
|
Year Ended December 31, 2010:
|
||||||||||||||||
|
Revenues
|
$ | 1,192 | $ | 1,500 | $ | 2,029 | $ | 3,109 | ||||||||
|
Gross profit
|
297 | 422 | 545 | 766 | ||||||||||||
|
Operating expenses
|
6,386 | 5,493 | 5,847 | 4,755 | ||||||||||||
|
Net loss
|
(6,067 | ) | (4,925 | ) | (5,281 | ) | (6,013 | ) | ||||||||
|
Loss per common share: (1)
|
||||||||||||||||
|
Basic and diluted
|
(0.24 | ) | (0.20 | ) | (0.20 | ) | (0.20 | ) | ||||||||
|
Year Ended December 31, 2009:
|
||||||||||||||||
|
Revenues
|
$ | 902 | $ | (3 | ) | $ | 1,667 | $ | 1,805 | |||||||
|
Gross profit
|
466 | (253 | ) | 1,070 | 35 | |||||||||||
|
Operating expenses (3)
|
6,938 | 6,232 | 5,309 | 3,635 | ||||||||||||
|
Net loss
|
(6,385 | ) | (6,393 | ) | (3,316 | ) | (5,218 | ) | ||||||||
|
Loss per common share: (1)
|
||||||||||||||||
|
Basic and diluted
|
(0.28 | ) | (0.28 | ) | (0.12 | ) | (0.17 | ) | ||||||||
|
(1) Loss per common share is computed independently for each of the quarters presented. Therefore, the sum of the quarterly loss per common share amounts does not necessarily equal the total for the year.
|
|
(2) Net loss increased due to the loss realized from the sale of our Auction Rate Securities of $1.9 million and Spectrum stock of $95,000.
|
|
(3) Adjusted for a reclassification for the AlSher sale that occurred in April 2010. Activity that was expensed to operating expenses has been reclassed to the gain/(loss) on discontinued operations for consistent reporting for the three years presented in the income statement.
|
Financial Statements
The financial statements required by this Item appear on pages F-4 through F-34 of this Report.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures. Based on an evaluation required by paragraph (b) of Rule 13a-15 or Rule 15d-15 of the Securities Exchange Act of 1934, as amended, as of December 31, 2010, which is the end of the period covered by this annual report on Form 10-K, our principal executive officer and principal financial officer have concluded that our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Securities Exchange Act of 1934, as amended) are effective.
49
Internal Control Over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) under the Securities Exchange Act of 1934, as amended. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes those written policies and procedures that:
|
|
·
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
|
|
|
·
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America;
|
|
|
·
|
provide reasonable assurance that our receipts and expenditures are being made only in accordance with authorization of our management; and
|
|
|
·
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on our consolidated financial statements.
|
Internal control over financial reporting includes the controls themselves, monitoring and internal auditing practices and actions taken to correct deficiencies as identified.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2010. Our management’s assessment was based on criteria for effective internal control over financial reporting described in “Internal Control – Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Our management’s assessment included an evaluation of the design of our internal control over financial reporting and testing of the operational effectiveness of our internal control over financial reporting. Our management reviewed the results of its assessment with the Audit Committee of our Board of Directors. Based on this assessment, our management determined that, as of December 31, 2010, we maintained effective internal control over financial reporting.
The effectiveness of our internal controls over financial reporting as of December 31, 2010 has been audited by Perry-Smith LLP, independent registered public accounting firm, as stated in their report, which is included in Part II, Item 8 of this Form 10-K on pages F-2 and F-3.
Changes in Internal Control Over Financial Reporting. There were no significant changes (including corrective actions with regard to significant deficiencies or material weaknesses) in our internal controls over financial reporting that occurred during the fourth quarter of fiscal 2010 that materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
50
PART III
Item 10. Directors and Executive Officers of the Registrant
The information required by this Item is incorporated by reference to the Company’s definitive proxy statement to be filed with the SEC.
The Company has adopted a Code of Ethics that applies to the Company’s Senior Executive Officers and Financial Officers. A copy of the Company’s Code of Ethics is available on the Company’s website at www.altairnano.com.
Item 11. Executive Compensation
The information required by this Item is incorporated by reference to the Company’s definitive proxy statement to be filed with the SEC.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item is incorporated by reference to the Company’s definitive proxy statement to be filed with the SEC.
Item 13. Certain Relationships and Related Transactions
The information required by this Item is incorporated by reference to the Company’s definitive proxy statement to be filed with the SEC.
Item 14. Principal Accountant Fees and Services
The information required by this Item is incorporated by reference to the Company’s definitive proxy statement to be filed with the SEC.
51
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a) Documents Filed
|
1.
|
Financial Statements. The following Consolidated Financial Statements of the Company and Auditors’ Report are filed as part of this Annual Report on Form 10-K:
|
|
|
·
|
Report of Independent Registered Public Accounting Firm
|
|
|
·
|
Report of Independent Registered Public Accounting Firm on Internal Control over Financial Reporting
|
|
|
·
|
Consolidated Balance Sheets, December 31, 2010 and 2009
|
|
|
·
|
Consolidated Statements of Operations for Each of the Three Years
|
|
|
in the Period Ended December 31, 2010
|
|
|
·
|
Consolidated Statements of Shareholders’ Equity and Comprehensive Loss for Each of the Three Years in the Period Ended December 31, 2010
|
|
|
·
|
Consolidated Statements of Cash Flows for Each of the Three Years in the Period Ended December 31, 2010
|
|
|
·
|
Notes to Consolidated Financial Statements
|
|
|
2.
|
Financial Statement Schedule. Not applicable.
|
|
|
3.
|
Exhibits. The information required by this item is set forth on the exhibit index that follows the signature page of this report.
|
52
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
ALTAIR NANOTECHNOLOGIES INC.
|
|||
|
By:
|
/s/ Terry Copeland | ||
|
Terry Copeland,
|
|||
|
President and Chief Executive Officer
|
|||
|
Date: February 25, 2011
|
|||
53
POWER OF ATTORNEY AND ADDITIONAL SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the following persons in the capacities and on the dates indicated have signed this Report. Each person whose signature to this Report appears below hereby constitutes and appoints Terry Copeland and John Fallini, and each of them, as his true and lawful attorney-in-fact and agent, with full power of substitution, to sign on his behalf individually and in the capacity stated below and to perform any acts necessary to be done in order to file all amendments to this Report, and any and all instruments or documents filed as part of or in connection with this Report or the amendments thereto and each of the undersigned does hereby ratify and confirm all that said attorney-in-fact and agent, or his substitutes, shall do or cause to be done by virtue hereof.
|
Signature
|
Title
|
Date
|
||
|
/s/ Terry Copeland
|
President, Chief Executive Officer (Principal Executive Officer) and Director
|
February 25, 2011
|
||
|
Terry Copeland
|
||||
|
/s/ John Fallini
|
Chief Financial Officer and Corporate Secretary (Principal Financial and Accounting Officer)
|
February 25, 2011
|
||
|
John Fallini
|
||||
|
/s/ Jon N. Bengtson
|
Director
|
February 25, 2011
|
||
|
Jon N. Bengtson
|
||||
|
/s/ George E. Hartman
|
Director
|
February 25, 2011
|
||
|
George E. Hartman
|
||||
|
/s/ Hossein Asrar Haghighi
|
Director
|
February 25, 2011
|
||
|
Hossein Asrar Haghighi
|
||||
|
/s/ Pierre Lortie
|
Director
|
February 25, 2011
|
||
|
Pierre Lortie
|
||||
|
/s/ Robert G. van Schoonenberg
|
Director
|
February 25, 2011
|
||
|
Robert G. van Schoonenberg
|
||||
|
/s/ Alexander Lee
|
Director
|
February 25, 2011
|
||
|
Alexander Lee
|
54
Exhibit Index
|
Exhibit No.
|
Description
|
Incorporated by Reference/
Filed Herewith (and Sequential Page #)
|
|
3.1
|
Articles of Continuance
|
Incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 4, 2010.**
|
||
|
3.2
|
Bylaws
|
Incorporated by reference to the Amendment No. 1 to Annual Report on Form 10-K/A filed with the SEC on March 10, 2005. **
|
||
|
4.1
|
Form of Common Stock Certificate
|
Filed herewith
|
||
|
4.2
|
Amended and Restated Shareholder Rights Plan dated October 15, 1999, with Equity Transfer Services, Inc.
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 1999. **
|
||
|
4.2.1
|
Amendment No. 1 to Shareholders Rights Plan Agreement dated October 6, 2008, with Equity Transfer Services, Inc.
|
Incorporated by reference to the Company's Current Report on Form 8-K filed with the SEC on October 6, 2008. **
|
||
|
4.2.2
|
Amendment No. 2 to Amended and Restated Shareholder Rights Plan Agreement with Equity Transfer Services
|
Incorporated by reference to the Company's Current Report on Form 8-K filed with the SEC on September 20, 2010. **
|
||
|
4.3
|
Form of Common Share Purchase Warrant re May 2009 Offering
|
Incorporated by reference to the Company's Current Report on Form 8-K filed with the SEC on May 22, 2009. **
|
||
|
10.1
|
Altair International Inc. Stock Option Plan (1996)***
|
Incorporated by reference to the Company’s Registration Statement on Form S-8, File No. 333-33481 filed with the SEC on July 11, 1997
|
||
|
10.2
|
1998 Altair International Inc. Stock Option Plan***
|
Incorporated by reference to the Company’s Definitive Proxy Statement on Form 14A filed with the SEC on May 12, 1998. **
|
||
|
10.3
|
Altair Nanotechnologies Inc. 2005 Stock Incentive Plan (Amended and Restated)***
|
Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 13, 2007. **
|
||
|
10.4
|
Standard Form of Stock Option Agreement under 2005 Stock Incentive Plan***
|
Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 13, 2007.**
|
||
|
10.5
|
Standard Form of Stock Option Agreement for Executives under 2005 Stock incentive Plan ***
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed with the SEC on May 8, 2008. **
|
||
|
10.6
|
Standard Form of Restricted Stock Agreement under 2005 Stock Incentive Plan***
|
Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 13, 2007. **
|
55
|
Exhibit No.
|
Description
|
Incorporated by Reference/
Filed Herewith (and Sequential Page #)
|
|
10.7
|
Standard Form of Director’s Indemnification Agreement***
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on June 20, 2008. **
|
||
|
10.8
|
Flagship Business Accelerator Tenant Lease dated July 1, 2007 with the Flagship Enterprise Center, Inc.
|
Incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed with the SEC August 9, 2007. **
|
||
|
10.8.1
|
Amendment to the Flagship Business Accelerator Tenant Lease dated March 1, 2008 with the Flagship Enterprise Center, Inc.
|
Incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed with the SEC on May 8, 2008. **
|
||
|
10.9
|
Letter agreement dated July 20, 2008 with Phoenix Motorcars, Inc.
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on July 24, 2008. **
|
||
|
10.10
|
License Agreement dated April 24, 2007 with the Sherwin-Williams Company and AlSher Titania LLC
|
Incorporated by reference to the Current Report on Form 8-K filed with the SEC on April 30, 2007. **
|
||
|
10.11
|
Contract dated January 29, 2008 with the office of Naval Research
|
Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 14, 2008**
|
||
|
10.12
|
Service Agreement dated February 11, 2008 with Melpar BVBP
|
Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 14, 2008.**
|
||
|
10.13
|
Mandate & Contractor ship Agreement dated February 11, 2008 with Rik Dobbelaere***
|
Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 14, 2008.**
|
||
|
10.14
|
Separation Agreement and Release of All Claims dated April 18, 2008 with Alan Gotcher***
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on April 23, 2008. **
|
||
|
10.15
|
Employment Agreement dated March 10, 2008 with Jeffrey A. McKinney***
|
Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 14, 2008. **
|
||
|
10.16
|
Separation Agreement and Release of All Claims dated September 5, 2008 with Jeffrey McKinney***
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on September 5, 2008. **
|
||
|
10.17
|
Employment Agreement dated April 7, 2008 with John Fallini***
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on April 9, 2008. **
|
||
|
10.18
|
Employment Agreement dated September 9, 2010 with Robert Pedraza***
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on September 13, 2010. **
|
56
|
Exhibit No.
|
Description
|
Incorporated by Reference/
Filed Herewith (and Sequential Page #)
|
|
10.19
|
2008 Annual Incentive Bonus Plan***
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on July 2, 2008. **
|
||
|
10.20
|
Registration Rights Agreement dated November 29, 2007 with Al Yousuf LLC
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on November 30, 2007. **
|
||
|
10.20.1
|
Amendment No. 1 to Registration Rights Agreement with Al Yousuf, LLC dated as of September 30, 2008
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on October 6, 2008. **
|
||
|
10.20.2
|
Amendment No. 2 to Registration Rights Agreement with Al Yousuf, LLC dated August 14, 2009
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on September 4, 2009. **
|
||
|
10.21
|
Stock Purchase and Settlement Agreement with Al Yousuf, LLC dated as of September 30, 2008
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on October 6, 2008. **
|
||
|
10.22
|
2009 Annual Incentive Bonus Plan* ***
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed with the SEC on May 8, 2008. **
|
||
|
10.23
|
Amended and Restated Agreement dated August 4, 2009 with Spectrum Pharmaceuticals, Inc. *
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed with the SEC on August 7, 2009. **
|
||
|
10.24
|
Product Purchase Agreement dated August 4, 2009 with Proterra LLC*
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed with the SEC on August 7, 2009. **
|
||
|
10.25
|
Employment Agreement dated December 9, 2009 with Stephen Balogh***
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on December 14, 2009. **
|
||
|
10.26
|
Contract with the U.S. Army RDECOM Acquisition Center dated September 3, 2009.
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on October 13, 2009. **
|
||
|
10.27
|
Employment Agreement dated September 4, 2009 with Bruce Sabacky***
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on September 10, 2009. **
|
||
|
10.28
|
Placement Agent Agreement with Lazard Capital Markets, LLC dated May 22, 2009
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on May 22, 2009. **
|
||
|
10.29
|
Form of Subscription Agreement re May 2009 Offering
|
Incorporated by reference to the Company's Current Report on Form 8-K filed with the SEC on May 22, 2009. **
|
||
|
10.30
|
Contract dated May 22, 2009 with the Office of Naval Research
|
Incorporated by reference to the Company's Current Report on Form 8-K filed with the SEC on May 29, 2009. **
|
57
|
Exhibit No.
|
Description
|
Incorporated by Reference/
Filed Herewith (and Sequential Page #)
|
|
10.31
|
Employment Agreement dated April 7, 2010 with Terry Copeland***
|
Incorporated by reference to the Current Report on Form 8-K filed with the SEC on April 9, 2010, File No. 001-12497
|
||
|
10.32
|
Product Purchase Agreement dated May 4, 2010 with Proterra Inc.
|
Incorporated by reference to the Current Report on Form 8-K filed with the SEC on May 6, 2010, File No. 001-12497
|
||
|
10.33
|
Redemption Agreement dated April 30, 2010 with The Sherwin-Williams Company and AlSher Titania LLC
|
Incorporated by reference to the Current Report on Form 8-K filed with the SEC on May 5, 2010, File No. 001-12497
|
||
|
10.34
|
Definitive Agreement dated April 30, 2010 with The Sherwin-Williams Company and AlSher Titania LLC
|
Incorporated by reference to the Current Report on Form 8-K filed with the SEC on May 5, 2010, File No. 001-12497
|
||
|
10.35
|
License Agreement dated April 30, 2010 with AlSher Titania LLC
|
Incorporated by reference to the Current Report on Form 8-K filed with the SEC on May 5, 2010, File No. 001-12497
|
||
|
10.36
|
At Market Issuance Sales Agreement dated June 9, 2010 with Thomas Weisel Partners LLC
|
Incorporated by reference to the Current Report on Form 8-K filed with the SEC on June 9, 2010, File No. 001-12497
|
||
|
10.37
|
Amended and Restated Master Product Purchase Agreement dated June 22, 2010 with Proterra, Inc.
|
Incorporated by reference to the Current Report on Form 8-K filed with the SEC on June 25, 2010, File No. 001-12497
|
||
|
10.38
|
Purchase Order No. 3 and Security Agreement dated June 22, 2010 with Proterra, Inc.
|
Incorporated by reference to the Current Report on Form 8-K filed with the SEC on June 25, 2010, File No. 001-12497
|
||
|
10.39
|
Share Subscription Agreement dated September 20, 2010 with Canon Investment Holdings Limited
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed with the SEC on November 4, 2010. **
|
||
|
10.39.1
|
First Amendment to Share Subscription Agreement dated February 16, 2011 with Canon Investment Holdings Limited
|
Incorporated by reference to the Current Report on Form 8-K filed with the SEC on February 16, 2011. **
|
||
|
10.40
|
Conditional Supply and Technology Licensing Agreement dated September 20, 2010 with Zhuhai Yintong Energy Co. Ltd., a wholly-owned subsidiary of Canon.
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed with the SEC on November 4, 2010. **
|
||
|
10.41
|
Investor Rights Agreement dated September 20, 2010 with Canon Investment Holdings Limited
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed with the SEC on November 4, 2010. **
|
||
|
10.42
|
Waiver and Rights Agreement dated September 20, 2010 with Al Yousuf LLC and Canon Investment Holdings Limited
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed with the SEC on November 4, 2010. **
|
58
|
Exhibit No.
|
Description
|
Incorporated by Reference/
Filed Herewith (and Sequential Page #)
|
|
10.43
|
Employment Agreement dated August 25, 2010 with Dan Voelker***
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed with the SEC on November 4, 2010. **
|
||
|
10.44
|
Employment Agreement dated August 25, 2010 with Tom Kieffer***
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed with the SEC on November 4, 2010. **
|
||
|
10.45
|
2010 Annual Incentive Bonus Plan***
|
Filed herewith.
|
||
|
10.46
|
Sales Agreement dated February 9, 2011 with Inversiones Energeticas, S.A de C.V.*
|
Filed herewith.
|
||
|
21
|
List of Subsidiaries*
|
Incorporated by reference from Item 1 of this report.
|
||
|
23.1
|
Consent of Perry-Smith LLP
|
Filed herewith.
|
||
|
24
|
Powers of Attorney
|
Included in the Signature Page hereof.
|
||
|
31.1
|
Rule 13-14(a)/15d-14a Certification of Chief Executive Officer
|
Filed herewith
|
||
|
31.2
|
Rule 13-14(a)/15d-15a Certification of Chief Financial Officer
|
Filed herewith
|
||
|
32.1
|
Section 1350 Certification of Chief Executive Officer
|
Filed herewith
|
||
|
32.2
|
Section 1350 Certification of Chief Financial Officer
|
Filed herewith
|
*Portions of this Exhibit have been omitted pursuant to Rule 24b-2, are filed separately with the SEC and are subject to a confidential treatment request.
** SEC File No. 1-12497.
*** Indicates management contract or compensatory plan or arrangement.
59
|
Altair Nanotechnologies Inc.
and Subsidiaries
Consolidated Financial Statements as of December 31, 2010 and 2009 and for Each of the Three Years in the Period Ended December 31, 2010 and Reports of the Independent Registered Public Accounting Firm
|
60
ALTAIR NANOTECHNOLOGIES INC. AND SUBSIDIARIES
|
TABLE OF CONTENTS
|
||
|
|
Page
|
|
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
|
F-1
|
|
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM ON INTERNAL CONTROL OVER FINANCIAL REPORTING
|
F-2 | |
|
FINANCIAL STATEMENTS:
|
||
|
Consolidated Balance Sheets, December 31, 2010 and 2009
|
F-4
|
|
|
Consolidated Statements of Operations for Each of the Three Years in the Period Ended December 31, 2010
|
F-5
|
|
|
Consolidated Statements of Stockholders’ Equity and Comprehensive Loss for Each of the Three Years in the Period Ended December 31, 2010
|
F-6
|
|
|
|
||
|
Consolidated Statements of Cash Flows for Each of the Three Years in the Period Ended December 31, 2010
|
F-7
|
|
|
Notes to Consolidated Financial Statements
|
F-8
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Altair Nanotechnologies Inc.
We have audited the accompanying consolidated balance sheets of Altair Nanotechnologies Inc. and subsidiaries (the "Company") as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders' equity and comprehensive loss, and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Altair Nanotechnologies Inc. and subsidiaries as of December 31, 2010 and 2009, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2010, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred recurring losses from operations resulting in an accumulated deficit of $184,490,000 at December 31, 2010. Additionally, the Company experienced $15,172,000 in negative cash flows from operations during the year ended December 31, 2010, resulting in a cash balance of $4,695,000 at December 31, 2010. This raises substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Altair Nanotechnologies Inc. and subsidiaries' internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 25, 2011 expressed an unqualified opinion on the effectiveness of Altair Nanotechnologies Inc.'s internal control over financial reporting.
| /s/ Perry-Smith LLP |
Sacramento, California
February 25, 2011
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
ON INTERNAL CONTROL OVER FINANCIAL REPORTING
To the Board of Directors and Stockholders
Altair Nanotechnologies Inc.
We have audited Altair Nanotechnologies Inc.'s (the "Company") internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Altair Nanotechnologies Inc.'s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (a) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (b) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (c) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
F-2
ON INTERNAL CONTROL OVER FINANCIAL REPORTING
(Continued)
In our opinion, Altair Nanotechnologies Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the accompanying consolidated balance sheets as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders' equity and comprehensive loss, and cash flows for each of the three years in the period ended December 31, 2010 of Altair Nanotechnologies Inc. and our report dated February 25, 2011 expressed an unqualified opinion.
| /s/ Perry-Smith LLP |
Sacramento, California
February 25, 2011
F-3
|
PART I - FINANCIAL INFORMATION
|
||||||
|
Item 1. Financial Statements
|
||||||
|
ALTAIR NANOTECHNOLOGIES INC. AND SUBSIDIARIES
|
||||||
|
CONSOLIDATED BALANCE SHEETS
|
||||||
|
(Expressed in thousands of United States Dollars, except shares)
|
||||||
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$ | 4,695 | $ | 18,122 | ||||
|
Investment in available for sale securities
|
- | 505 | ||||||
|
Accounts receivable, net
|
1,318 | 683 | ||||||
|
Product inventories
|
6,825 | 5,043 | ||||||
|
Prepaid expenses and other current assets
|
2,269 | 1,820 | ||||||
|
Total current assets
|
15,107 | 26,173 | ||||||
|
Investment in available for sale securities, non-current
|
- | 2,587 | ||||||
|
Property, plant and equipment, net
|
8,727 | 8,670 | ||||||
|
Property, plant and equipment, net held and not used
|
- | 2,211 | ||||||
|
Patents, net
|
426 | 551 | ||||||
|
Other assets
|
- | 125 | ||||||
|
Total Assets
|
$ | 24,260 | $ | 40,317 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current Liabilities
|
||||||||
|
Trade accounts payable
|
$ | 2,873 | $ | 1,783 | ||||
|
Accrued salaries and benefits
|
743 | 625 | ||||||
|
Accrued warranty
|
211 | 79 | ||||||
|
Accrued liabilities
|
387 | 447 | ||||||
|
Deferred revenues
|
2,516 | 311 | ||||||
|
Current portion of long-term debt
|
216 | 810 | ||||||
|
Total current liabilities
|
6,946 | 4,055 | ||||||
|
Long-term debt, less current portion
|
16 | 37 | ||||||
|
Total liabilities
|
6,962 | 4,092 | ||||||
|
|
||||||||
|
Stockholders' equity
|
||||||||
|
Common stock, no par value, unlimited shares authorized;
|
||||||||
|
27,015,680 and 26,350,282 shares issued and
|
||||||||
|
outstanding at December 31, 2010 and December 31, 2009
|
189,491 | 188,515 | ||||||
|
Additional paid in capital
|
12,297 | 10,933 | ||||||
|
Accumulated deficit
|
(184,490 | ) | (162,204 | ) | ||||
|
Accumulated other comprehensive loss
|
- | (1,560 | ) | |||||
|
Total Altair Nanotechnologies Inc.'s stockholders' equity
|
17,298 | 35,684 | ||||||
|
Noncontrolling interest in Subsidiary
|
- | 541 | ||||||
|
Total stockholders' equity
|
17,298 | 36,225 | ||||||
|
Total Liabilities and Stockholders' Equity
|
$ | 24,260 | $ | 40,317 | ||||
See notes to the consolidated financial statements.
F-4
|
ALTAIR NANOTECHNOLOGIES INC. AND SUBSIDIARIES
|
||||||||||||
|
CONSOLIDATED STATEMENTS OF OPERATIONS
|
||||||||||||
|
(Expressed in thousands of United States Dollars, except shares and per share amounts)
|
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Revenues
|
||||||||||||
|
Product sales
|
$ | 3,543 | $ | 945 | $ | 757 | ||||||
|
Less: Sales returns
|
- | (184 | ) | - | ||||||||
|
License fees
|
- | 750 | - | |||||||||
|
Commercial collaborations
|
364 | 1,410 | 2,007 | |||||||||
|
Contracts and grants
|
3,923 | 1,450 | 2,962 | |||||||||
|
Total revenues
|
7,830 | 4,371 | 5,726 | |||||||||
|
Cost of goods sold
|
||||||||||||
|
Product
|
2,663 | 954 | 183 | |||||||||
|
Commercial collaborations
|
194 | 781 | 2,449 | |||||||||
|
Contracts and grants
|
2,534 | 1,120 | 1,978 | |||||||||
|
Warranty and inventory reserves
|
409 | 198 | (2,865 | ) | ||||||||
|
Total cost of goods sold
|
5,800 | 3,053 | 1,745 | |||||||||
|
Gross profit
|
2,030 | 1,318 | 3,981 | |||||||||
|
Operating expenses
|
||||||||||||
|
Research and development
|
8,212 | 9,389 | 12,993 | |||||||||
|
Sales and marketing
|
4,051 | 2,894 | 2,969 | |||||||||
|
General and administrative
|
7,552 | 7,796 | 9,837 | |||||||||
|
Depreciation and amortization
|
1,896 | 2,035 | 2,076 | |||||||||
|
Notes receivable extinguishment
|
- | - | 1,722 | |||||||||
|
Settlement and release
|
- | - | 3,605 | |||||||||
|
Loss on disposal of assets
|
770 | - | - | |||||||||
|
Total operating expenses
|
22,481 | 22,114 | 33,202 | |||||||||
|
Loss from operations
|
(20,451 | ) | (20,796 | ) | (29,221 | ) | ||||||
|
Other income (expense)
|
||||||||||||
|
Interest expense
|
(19 | ) | (107 | ) | (97 | ) | ||||||
|
Interest income
|
101 | 188 | 982 | |||||||||
|
Realized (loss)/gain on investment
|
(2,045 | ) | 851 | (89 | ) | |||||||
|
Loss on foreign exchange
|
(1 | ) | (2 | ) | (10 | ) | ||||||
|
Total other (expense) income, net
|
(1,964 | ) | 930 | 786 | ||||||||
|
Loss from continuing operations
|
(22,415 | ) | (19,866 | ) | (28,435 | ) | ||||||
|
Gain/(loss) from discontinued operations
|
124 | (2,065 | ) | (905 | ) | |||||||
|
Net loss
|
(22,291 | ) | (21,931 | ) | (29,340 | ) | ||||||
|
Less: Net loss attributable to non-controlling interest
|
5 | 619 | 272 | |||||||||
|
Net loss attributable to Altair Nanotechnologies Inc.
|
$ | (22,286 | ) | $ | (21,312 | ) | $ | (29,068 | ) | |||
|
Net loss attributable to Altair Nanotechnologies Inc. shareholders:
|
||||||||||||
|
Loss from continuing operations
|
$ | (22,415 | ) | $ | (19,866 | ) | $ | (28,435 | ) | |||
|
Gain/(loss) from discontinued operations
|
129 | (1,446 | ) | (633 | ) | |||||||
|
Net loss
|
$ | (22,286 | ) | $ | (21,312 | ) | $ | (29,068 | ) | |||
|
Earnings per share attributable to Altair Nanotechnologies Inc. shareholders:
|
||||||||||||
|
Basic and diluted:
|
||||||||||||
|
Loss from continuing operations
|
$ | (0.84 | ) | $ | (0.79 | ) | $ | (1.32 | ) | |||
|
Gain/(loss) from discontinued operations
|
$ | - | $ | (0.06 | ) | $ | (0.03 | ) | ||||
|
Loss per common share - Basic and diluted
|
$ | (0.84 | ) | $ | (0.85 | ) | $ | (1.35 | ) | |||
|
Weighted average shares - basic and diluted
|
26,550,288 | 25,044,432 | 21,475,928 | |||||||||
See notes to the consolidated financial statements.
F-5
ALTAIR NANOTECHNOLOGIES INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY AND COMPREHENSIVE LOSS
(Expressed in thousands of United States Dollars, except shares and per share amounts)
|
Altair Nanotechnologies Inc. Shareholders
|
Non-controlling Interest in Subsidiary
|
|||||||||||||||||||||||||||||||||||||||
|
Accumulated
|
Accumulated
|
|||||||||||||||||||||||||||||||||||||||
|
Other
|
Other
|
|||||||||||||||||||||||||||||||||||||||
|
Additional
|
Compre-
|
Interest
|
Compre-
|
|||||||||||||||||||||||||||||||||||||
|
Common Stock
|
Paid In
|
Accumulated
|
hensive
|
In
|
hensive
|
|||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Deficit
|
(Loss) Gain
|
Subtotal
|
Subsidiary
|
Gain (Loss)
|
Subtotal
|
Total
|
|||||||||||||||||||||||||||||||
|
BALANCE, JANUARY 1, 2008
|
21,017,194 | $ | 163,780 | $ | 5,490 | $ | (111,824 | ) | $ | (485 | ) | $ | 56,961 | $ | 1,370 | $ | - | $ | 1,370 | $ | 58,331 | |||||||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||||||||||||
|
Net loss
|
- | - | - | (29,068 | ) | - | (29,068 | ) | (272 | ) | - | (272 | ) | (29,340 | ) | |||||||||||||||||||||||||
|
Other comprehensive loss,
|
||||||||||||||||||||||||||||||||||||||||
|
net of taxes of $0
|
- | - | - | - | (1,388 | ) | (1,388 | ) | - | - | - | (1,388 | ) | |||||||||||||||||||||||||||
|
Comprehensive loss
|
- | - | - | - | - | (30,456 | ) | - | - | (272 | ) | (30,728 | ) | |||||||||||||||||||||||||||
|
Share-based compensation
|
- | 1,263 | (112 | ) | - | - | 1,151 | - | - | - | 1,151 | |||||||||||||||||||||||||||||
|
Exercise of stock options
|
84,803 | 528 | - | - | - | 528 | - | - | - | 528 | ||||||||||||||||||||||||||||||
|
Exercise of warrants
|
100,056 | 752 | - | - | - | 752 | - | - | - | 752 | ||||||||||||||||||||||||||||||
|
Issuance of restricted stock
|
35,437 | - | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||
|
Recovery of short swing profits
|
- | 177 | - | - | - | 177 | - | - | - | 177 | ||||||||||||||||||||||||||||||
|
Common stock issued, net of $0 issuance costs
|
2,048,428 | 13,605 | - | - | - | 13,605 | - | - | - | 13,605 | ||||||||||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2008
|
23,285,918 | $ | 180,105 | $ | 5,378 | $ | (140,892 | ) | $ | (1,873 | ) | $ | 42,718 | $ | 1,097 | $ | - | $ | 1,097 | $ | 43,816 | |||||||||||||||||||
|
Investment from non-controlling interest
|
- | - | - | - | - | 62 | - | 62 | 62 | |||||||||||||||||||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||||||||||||
|
Net loss
|
- | - | - | (21,312 | ) | - | (21,312 | ) | (619 | ) | - | (619 | ) | (21,931 | ) | |||||||||||||||||||||||||
|
Other comprehensive gain,
|
||||||||||||||||||||||||||||||||||||||||
|
net of taxes of $0
|
- | - | - | - | 313 | 313 | - | - | - | 313 | ||||||||||||||||||||||||||||||
|
Comprehensive loss
|
- | - | - | - | - | (20,999 | ) | - | - | (619 | ) | (21,618 | ) | |||||||||||||||||||||||||||
|
Share-based compensation
|
- | 221 | 931 | - | - | 1,152 | - | - | - | 1,152 | ||||||||||||||||||||||||||||||
|
Issuance of restricted stock
|
65,747 | - | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||
|
Common stock issued, net of $1,220,735 issuance costs
|
2,998,617 | 8,189 | 4,624 | - | - | 12,813 | - | - | - | 12,813 | ||||||||||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2009
|
26,350,282 | $ | 188,515 | $ | 10,933 | $ | (162,204 | ) | $ | (1,560 | ) | $ | 35,684 | $ | 541 | $ | - | $ | 541 | $ | 36,225 | |||||||||||||||||||
|
Disposal from non-controlling interest
|
- | - | - | - | - | - | (536 | ) | - | (536 | ) | (536 | ) | |||||||||||||||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||||||||||||
|
Net loss
|
- | - | - | (22,286 | ) | - | (22,286 | ) | (5 | ) | - | (5 | ) | (22,291 | ) | |||||||||||||||||||||||||
|
Reclassification adjustment for realized loss on securities included in net loss
|
- | - | - | - | 1,560 | 1,560 | - | - | 1,560 | |||||||||||||||||||||||||||||||
|
Comprehensive loss
|
- | - | - | - | - | (20,726 | ) | - | - | (5 | ) | (20,731 | ) | |||||||||||||||||||||||||||
|
Share-based compensation
|
- | 283 | 1,364 | - | - | 1,647 | - | - | - | 1,647 | ||||||||||||||||||||||||||||||
|
Issuance of restricted stock
|
177,744 | - | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||
|
Common stock issued, net of $212,737 issuance costs
|
487,654 | 692 | - | - | - | 692 | - | - | - | 692 | ||||||||||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2010
|
27,015,680 | $ | 189,491 | $ | 12,297 | $ | (184,490 | ) | $ | - | $ | 17,298 | $ | - | $ | - | $ | - | $ | 17,298 | ||||||||||||||||||||
See notes to the consolidated financial statements.
F-6
|
ALTAIR NANOTECHNOLOGIES INC. AND SUBSIDIARIES
|
||||||||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
||||||||||||
|
(Expressed in thousands of United States Dollars)
|
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
OPERATING ACTIVITIES:
|
||||||||||||
|
Net loss
|
$ | (22,291 | ) | $ | (21,931 | ) | $ | (29,340 | ) | |||
|
Adjustments to reconcile net loss to net cash
|
||||||||||||
|
used in operating activities:
|
||||||||||||
|
Depreciation and amortization
|
1,896 | 2,035 | 2,076 | |||||||||
|
Securities received in payment of license fees
|
- | (750 | ) | - | ||||||||
|
(Gain)/loss on discontinued operations
|
(129 | ) | 1,446 | 633 | ||||||||
|
Share-based compensation
|
1,647 | 1,152 | 1,151 | |||||||||
|
Loss on disposal of fixed assets
|
710 | 17 | 382 | |||||||||
|
Impairment of patents
|
47 | - | - | |||||||||
|
Loss/(gain) on sale of available for sale securities
|
2,045 | (868 | ) | - | ||||||||
|
Settlement and release
|
- | - | 3,605 | |||||||||
|
Impairment of investment
|
- | - | 89 | |||||||||
|
Asset deposit
|
- | 375 | - | |||||||||
|
Accrued interest on notes receivable
|
- | - | (83 | ) | ||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Accounts receivable, net
|
(635 | ) | 276 | 363 | ||||||||
|
Accounts receivable from related party, net
|
- | (4 | ) | - | ||||||||
|
Notes receivable from related party, net
|
- | - | 1,722 | |||||||||
|
Product inventories
|
(1,599 | ) | (4,896 | ) | (98 | ) | ||||||
|
Prepaid expenses and other current assets
|
(449 | ) | (1,352 | ) | 226 | |||||||
|
Other assets
|
125 | 33 | - | |||||||||
|
Trade accounts payable
|
1,064 | 958 | (7,297 | ) | ||||||||
|
Accrued salaries and benefits
|
118 | (736 | ) | (878 | ) | |||||||
|
Accrued warranty
|
132 | 43 | (2,880 | ) | ||||||||
|
Deferred revenues
|
2,205 | - | - | |||||||||
|
Accrued liabilities
|
(59 | ) | (6 | ) | 5 | |||||||
|
Net cash used in operating activities
|
(15,172 | ) | (24,208 | ) | (30,324 | ) | ||||||
|
INVESTING ACTIVITIES
|
||||||||||||
|
Sale of available for sale securities
|
2,599 | 2,006 | - | |||||||||
|
Interest on available for sale securities
|
8 | 6 | 4 | |||||||||
|
Purchase of property, plant and equipment
|
(953 | ) | (149 | ) | (2,773 | ) | ||||||
|
Proceeds from sale of assets
|
13 | - | 35 | |||||||||
|
Net cash provided by (used in) investing activities
|
1,667 | 1,863 | (2,734 | ) | ||||||||
|
FINANCING ACTIVITIES:
|
||||||||||||
|
Issuance of common shares for cash, net of issuance costs
|
692 | 12,813 | 10,000 | |||||||||
|
Proceeds from exercise of stock options
|
- | - | 528 | |||||||||
|
Proceeds from exercise of warrants
|
- | - | 752 | |||||||||
|
Proceeds from recovery of short swing profits
|
- | - | 177 | |||||||||
|
Proceeds from notes payable
|
6 | 387 | 345 | |||||||||
|
Payment of notes payable
|
(600 | ) | (926 | ) | (813 | ) | ||||||
|
Proceeds from long-term debt
|
- | 58 | 12 | |||||||||
|
Repayment of long-term debt
|
(20 | ) | (15 | ) | - | |||||||
|
Contributions from minority interest
|
- | 62 | - | |||||||||
|
Net cash provided by financing activities
|
78 | 12,379 | 11,001 | |||||||||
|
Net decrease in cash and cash equivalents
|
(13,427 | ) | (9,966 | ) | (22,057 | ) | ||||||
|
CASH AND CASH EQUIVALENTS:
|
||||||||||||
|
Beginning of period
|
18,122 | 28,088 | 50,145 | |||||||||
|
End of period
|
$ | 4,695 | $ | 18,122 | $ | 28,088 | ||||||
|
SUPPLEMENTAL DISCLOSURE:
|
||||||||||||
|
Cash paid for interest
|
$ | 49 | $ | 97 | $ | 133 | ||||||
|
Cash paid for income taxes
|
None
|
None
|
None
|
|||||||||
|
NON-CASH TRANSACTIONS:
|
||||||||||||
|
Acquisition of assets included in accounts payable
|
$ | 26 | $ | 75 | $ | 10 | ||||||
|
Unrealized gain/(loss) on available for sale securities
|
$ | 1,560 | $ | 313 | $ | (1,388 | ) | |||||
|
Issuance of restricted stock to directors
|
$ | 320 | $ | 397 | $ | 303 | ||||||
|
Settlement of all known claims to Al Yousuf, LLC
|
$ | - | $ | - | $ | 3,605 | ||||||
|
Impairment of AlSher Titania fixed assets
|
$ | - | $ | 1,308 | $ | - | ||||||
|
Payment of license with stock
|
$ | - | $ | 750 | $ | - | ||||||
See notes to the consolidated financial statements.
F-7
ALTAIR NANOTECHNOLOGIES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2010, 2009, AND 2008
|
(Expressed in United States Dollars)
|
|
1.
|
DESCRIPTION OF BUSINESS AND BASIS OF PRESENTATION
|
Description of Business — We are a Canadian corporation, with principal assets and operations in the United States, whose primary business is developing, manufacturing and selling our nano lithium titanate battery products. Our primary focus is marketing our large-scale energy storage solutions to power companies and electric grid operators throughout the world. In addition, we market our battery products to electric and hybrid-electric mass-transit vehicle manufacturers. During 2010 we also started to expand our market focus to include use of our battery technology in additional industrial markets with applications requiring batteries that can provide high power quickly, a fast recharge, have a long cycle life, operate at a wide temperature range and are extremely safe.
We also provide contract research services on select projects where we can utilize our resources to develop intellectual property and/or new products and technology. Although contract services revenue comprised a significant portion of our total revenues in recent years accounting for 50%, 65%, and 87%, respectively in 2010, 2009 and 2008, we expect a major decline in this percentage as our battery product sales increase.
Principles of Consolidation — The consolidated financial statements include the accounts of Altair Nanotechnologies Inc. and our subsidiaries which include (1) Altair US Holdings, Inc., (2) Mineral Recovery Systems, Inc. (“MRS”), (3) Fine Gold Recovery Systems, Inc. (“FGRS”) dissolved on December 30, 2008, and (4) Altairnano, Inc. (“ANI”), (collectively referred to as the “Company”), all of which are 100% owned and (5) AlSher Titania LLC, which was 70% owned by ANI but was sold April 30, 2010. All of the subsidiaries are incorporated in the United States of America. Inter-company transactions and balances have been eliminated in consolidation.
Basis of Presentation and Going Concern — The accompanying consolidated financial statements have been prepared on a going concern basis which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. (The Company has incurred recurring losses from operations resulting in an accumulated deficit of $184,490,000. Additionally, the Company experienced $15,172,000 in negative cash flows from operations during the year ended December 31, 2010, resulting in a cash balance of $4,695,000 at December 31, 2010. This raises substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.)
In September of 2010 the Company entered into a Share Subscription Agreement (the “Share Subscription Agreement” with Canon Investment Holdings Limited (“Canon”) under which they have agreed, subject to conditions to closing and the risk of termination, to purchase a number of shares of common stock such that, following closing, Canon will own 51% of the outstanding shares of common stock of the Company on a fully diluted basis. Based upon the number of shares of common stock and the rights to acquire shares of common stock outstanding as of December 31, 2010, the Company estimates that the number of shares to be purchased will be 31,523,017, at an aggregate purchase price of $48,948,799. If the Company issues additional shares of common stock prior to closing, as permitted by a recent amendment, the number of shares to be purchased, and the aggregate purchase price, will increase. As a result of delays in filing of the joint request with the Committee for Foreign Investment in the United States (“CFIUS”) to review the Canon transaction and the decision by CFIUS to extend their initial 30-day review into an additional 45-day review, the transaction did not close in 2010. Management began taking steps in the fourth quarter to reduce its cash burn rate and extend its runway until the Canon transaction closes. Those steps included:
F-8
|
|
§
|
a freeze on all hiring,
|
|
|
§
|
a deferral of all inventory purchases not tied to a specific customer contract,
|
|
|
§
|
a delay in all development efforts not needed in the immediate timeframe,
|
|
|
§
|
a cancellation of a number of non-critical consulting contracts,
|
|
|
§
|
sale of the auction rate securities that the Company was holding, and
|
|
|
§
|
sale of the remaining Spectrum Pharmaceutical common stock that the Company held.
|
As a result of restrictive conditions associated with the Canon negotiations and then contained in the Share Subscription Agreement, the Company has been precluded from raising any additional external equity or debt capital since July of 2010.
The Company anticipated closing in early February 2011, however, Canon indicated that it was not in a position to close. The rights under the Conditional Supply and Licensing Agreement to purchase the Company’s nano lithium titanate, and manufacture batteries using the Company’s design principles, were significant motivations for Canon to enter into the share purchase transaction, and Canon indicated that its operating subsidiary was having difficulty implementing the battery manufacturing technology called for in the Conditional Supply and Licensing Agreement. Canon also stated, although the Company has not verified, that it had permitted certain credit facilities required to close to expire near the original end date of the Share Subscription Agreement and needed time to re-establish these credit facilities.
On February 16, 2011, the Company signed the First Amendment to the Share Subscription Agreement (the “SSA Amendment”) which, among other things, extends the end date under the Share Subscription Agreement to May 17, 2011. The SSA Amendment also authorized the Company, subject to certain limitations, to sell equity securities in order to raise interim capital. The Company has since engaged JMP Securities with respect to a possible interim financing. If we are unable to raise interim capital, we will face a liquidity shortage prior to the anticipated closing of the Canon transaction in May 2011.
In the meantime the Company has implemented additional steps to further extend our runway and lower our cash burn including:
|
|
§
|
an immediate reduction in the manufacture of nano lithium titanate that was to be shipped to YTE and termination of employment of the individuals performing this work,
|
|
|
§
|
elimination of all contract personnel not working directly on the Proterra customer contract,
|
|
|
§
|
renegotiation of payment terms with our suppliers and vendors,
|
|
|
§
|
elimination of all but essential travel related to customer contracts or this immediate financing, and
|
|
|
§
|
deferral of all activities requiring the purchase of supplies or material not tied directly to an existing customer contract.
|
At this point, it is the Company’s expectation that the Canon transaction will close. If the Canon transaction were not to close, however, the Company would face an immediate liquidity shortage and need to find an alternative source of financing.
F-9
On October 21, 2010, the Board of Directors of the Company authorized a reverse split of the Company’s common stock at a ratio of one-for-four, effective close of business on November 15, 2010. The Company’s stockholders previously approved the reverse split in May 2010. As a result of the reverse split, every four shares of common stock outstanding were combined into one share of common stock. The reverse split did not affect the amount of equity the Company has nor did it affect the Company’s market capitalization. All previously reported share and per share amounts have been restated in the accompanying consolidated financial statements and related notes to reflect the reverse stock split.
The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern. Our continuation as a going concern is dependent upon our ability to generate sufficient cash flow to meet our obligations on a timely basis, to obtain additional financing or refinancing as may be required, to develop commercially viable products and processes, and ultimately to establish profitable operations. We have financed operations through operating revenues and through the issuance of equity securities (common shares, convertible debentures, stock options and warrants), and debt (term notes). Until we are able to generate positive operating cash flows, additional funds will be required to support operations.
|
2.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
Use of Estimates — The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires that we make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents — Cash and cash equivalents consist principally of bank deposits and institutional money market funds. Short-term investments that are highly liquid have insignificant interest rate risk and original maturities of 90 days or less are classified as cash and cash equivalents. Investments that do not meet the definition of cash equivalents are classified as held-to-maturity or available-for-sale.
Our cash balances are maintained in bank accounts that are insured by the Federal Deposit Insurance Corporation (“FDIC”) and Canada Deposit Insurance Corporation (“CDIC”) up to a maximum of US $250,000 and CN $100,000, respectively, per depositor. At December 31, 2010 and December 31, 2009 we had $2.6 million and $1.2 million, respectively, in excess of insurance limits in bank accounts insured by the FDIC or CDIC. The FDIC adopted a final rule amending its deposit insurance regulations on November 15, 2010 to implement Section 343 of the Dodd-Frank Wall Street Reform and Consumer Protection Act providing for unlimited deposit insurance for non-interest bearing transaction accounts for two years starting December 31, 2010.
Investment in Available for Sale Securities — The investment in short-term available for sale securities consisted of 113,809 Shares of Spectrum Pharmaceuticals, Inc. common stock valued at $505,000 at December 31, 2009. This stock was received in exchange for ownership assignment of all patent rights associated with RenazorbTM and RenalanTM compounds to Spectrum. Spectrum must also pay us future milestone and royalty payments as they develop revenues for these compounds. On December 10, 2010, we sold all of these shares and realized a loss on sale of investment of $95,000.
Accounts Receivable — Accounts receivable consists of amounts due from customers for services and product sales, net of an allowance for doubtful accounts. We determine the allowance for doubtful accounts by reviewing each customer account and specifically identifying any potential for loss.
F-10
The allowance for doubtful accounts is as follows:
|
In thousands of dollars
|
||||||||
|
2010
|
2009
|
|||||||
|
Beginning Balance, January 1,
|
$ | 161 | $ | 84 | ||||
|
Additions charged to costs and expenses
|
- | 177 | ||||||
|
Net deductions (write-offs, net of collections)
|
(161 | ) | (100 | ) | ||||
|
Ending Balance, December 31,
|
$ | - | $ | 161 | ||||
Inventory – We value our inventories generally at the lower of cost (first-in, first-out method) or market. We employ a full absorption procedure using standard cost techniques. The standards are customarily reviewed and adjusted every three months. Overhead rates are recorded to inventory based on normal capacity. Any idle facility costs or excessive spoilage are recorded as current period charges. As of December 31, 2010, we recorded a $623,000 inventory valuation allowance due to quality issues with our cell supplier, of which $541,000 is recorded as a receivable from our vendor covered under their product warranty. As of December 31, 2009, we had $71,000 of inventory valuation allowance recorded.
Research and Development Expenditures — The costs of materials, equipment, or facilities that are acquired or constructed for a particular research and development project and that have no alternative future uses (in other research and development projects or otherwise) are expensed as research and development costs at the time the costs are incurred. Research and development expenditures related to materials and equipment or facilities that are acquired or constructed for research and development activities and that have alternative future uses (in research and development projects or otherwise) are capitalized when acquired or constructed. Research and development expenditures, which include the cost of materials consumed in research and development activities, salaries, wages and other costs of personnel engaged in research and development, costs of services performed by others for research and development on our behalf and indirect costs are expensed as research and development costs when incurred.
Foreign Currency Translation — Asset and liability accounts, which are originally recorded in the appropriate local currencies, are translated into U.S. dollars at year-end exchange rates. Revenue and expense accounts are translated at the average exchange rates for the period. Transaction gains and losses are included in the accompanying consolidated statements of operations. Substantially all of our assets are located in the United States of America.
Stock-Based Compensation — We measure the cost of services received in exchange for an award of equity instruments based on the grant-date fair value of the award. That cost is recognized over the period during which services are provided in exchange for the award, known as the requisite service period (usually the vesting period).
Long-Lived Assets — We evaluate the carrying value of long-lived assets whenever events or changes in business circumstances indicate that the carrying value of the assets may not be recoverable. The carrying value of a long-lived asset is considered impaired when the total projected undiscounted cash flows expected to be generated by the asset are less than the carrying value. Our estimate of the cash flows is based on the information available at the time including the following: internal budgets; sales forecasts; customer trends; anticipated production volumes; and market conditions over an estimate of the remaining useful life of the asset which may range from 3 to 10 years for most equipment and up to 22 years for our building and related building improvements. If an impairment is indicated, the asset value is written down to its fair value based upon market prices, or if not available, upon discounted cash flow value, at an appropriate discount determined by us to be commensurate with the risk inherent in the business model. The determination of both undiscounted and discounted cash flows requires us to make significant estimates and consider the expected course of action at the balance sheet date. Our assumptions about future sales and production volumes require significant judgment because actual sales prices and volumes have fluctuated significantly in the past and are expected to continue to do so. Until the Company’s products reach commercialization, the demand for our products is difficult to estimate. Subsequent changes in estimated undiscounted and discounted cash flows arising from changes in anticipated actions could impact the determination of whether an impairment exists, the amount of the impairment charge recorded and whether the effects could materially impact our consolidated financial statements. Events or circumstances that could indicate the existence of a possible impairment include obsolescence of the technology, an absence of market demand for the product or the assets used to produce it, a history of operating or cash flow losses and/or the partial or complete lapse of technology rights protection.
F-11
As of December 31, 2010, we estimate that our future cash flows, on an undiscounted basis, are greater than our $9.1 million investment in long-lived assets. Our estimated future cash flows include anticipated product sales, commercial collaborations, and contracts and grant revenue, since our long-lived asset base, which is primarily composed of production, laboratory and testing equipment is utilized to fulfill contracts in all revenue categories.
In the first quarter of 2010, we reviewed our four capitalized patents and determined that three of these patents had value in excess of their net book value of $483,000 at that time. In the first quarter, we determined that the fourth patent no longer had value. The fourth patent had an original cost of $152,000, accumulated depreciation of $105,000 and a net value of $47,000. Accordingly, an impairment charge of $47,000 was recorded, and is reflected for the twelve months ended December 31, 2010.
During the third quarter of 2010, we reviewed the remaining three capitalized patents and determined that these patents had value in excess of their net book value of $426,000 as of December 31, 2010. AlSher currently has an exclusive license to use this technology from Altair.
Based on our assessment, which represents no change from the prior year in our approach to valuing long-lived assets, after recording the impairment described above, we believe that our long-lived assets are not impaired.
Property, plant and equipment held and used are stated at cost less accumulated depreciation. Depreciation is recorded using the straight-line method over the following useful lives:
|
Furniture and office equipment
|
3–7 years
|
|
|
Vehicles
|
5 years
|
|
|
Nanoparticle production equipment
|
5–10 years
|
|
|
Building and improvements
|
30 years
|
We have property, plant and equipment that is held and not used stated at cost less accumulated depreciation. Depreciation is recorded using the straight-line method over the useful lives established for property, plant and equipment held and used.
Property Plant and Equipment, net – held and not used included assets being redeployed from our Life Sciences and Performance Materials Divisions, into our Power and Energy Group. We redeployed these assets during the second quarter of 2010 into our Power and Energy Group or transferred to Sherwin Williams through our transfer of AlSher Titania, LLC on April 30, 2010.
F-12
Patents related to the nanoparticle production technology are carried at cost and amortized on a straight-line basis over their estimated useful lives, which range from 14 to 17 years.
Revenue Recognition — We recognize revenue when persuasive evidence of an arrangement exists, delivery has occurred or service has been performed, the fee is fixed and determinable, and collectability is reasonably assured. Our revenues were derived from product sales, commercial collaborations and contracts and grants. Revenue from product sales is recognized upon delivery of the product, unless specific contractual terms dictate otherwise. Based on the specific terms and conditions of each contract/grant, revenues are recognized on a time and materials basis, a percentage of completion basis and/or a completed contract basis. Revenue under contracts based on time and materials is recognized at contractually billable rates as labor hours and expenses are incurred. Revenue under contracts based on a fixed fee arrangement is recognized based on various performance measures, such as stipulated milestones. As these milestones are achieved, revenue is recognized. From time to time, facts develop that may require us to revise our estimated total costs or revenues expected. The cumulative effect of revised estimates is recorded in the period in which the facts requiring revisions become known. The full amount of anticipated losses on any type of contract is recognized in the period in which it becomes known. Payments received in advance relating to the future performance of services or deliveries of products are deferred until the performance of the service is complete or the product is shipped. Upfront payments received in connection with certain rights granted in contractual arrangements are deferred and revenue is recognized over the related time period which the benefits are received. Based on specific customer bill and hold agreements, revenue is recognized when the inventory is shipped to a third party storage warehouse, the inventory is segregated and marked as sold, the customer takes the full rights of ownership and title to the inventory upon shipment to the warehouse per the bill and hold agreement. When contract terms include multiple components that are considered separate units of accounting, the revenue is attributed to each component and revenue recognition may occur at different points in time for product shipment, installation, and service contracts based on substantial completion of the earnings process.
Accrued Warranty — We provide a limited warranty for battery packs and energy storage systems. A liability is recorded for estimated warranty obligations at the date products are sold. Since these are new products, the estimated cost of warranty coverage is based on cell and module life cycle testing and compared for reasonableness to warranty rates on competing battery products. As sufficient actual historical data is collected on the new product, the estimated cost of warranty coverage will be adjusted accordingly. The liability for estimated warranty obligations may also be adjusted based on specific warranty issues identified.
Non-controlling Interest — In April 2007, The Sherwin-Williams Company (“Sherwin”) entered into an agreement with us to form AlSher Titania LLC (“AlSher”), a Delaware limited liability company. AlSher is a joint venture combining certain technologies of ours and Sherwin in order to develop and produce titanium dioxide pigment for use in paint and coatings and nano titanium dioxide materials for use in a variety of applications, including those related to removing contaminants from air and water. Pursuant to a Contribution Agreement dated April 24, 2007 among Sherwin, AlSher, and us, we contributed to AlSher an exclusive license to use our technology (including our hydrochloride pigment process) for the production of titanium dioxide pigment and other titanium containing materials (other than battery or nanoelectrode materials) and certain pilot plant assets with a net book value of $3,110,000. We received no consideration for the license granted to AlSher other than our ownership interest in AlSher. Sherwin agreed to contribute to AlSher cash and a license agreement related to a technology for the manufacture of titanium dioxide using the digestion of ilmenite in hydrochloric acid. As a condition to enter into the second phase of the joint venture, we agreed to complete the pigment pilot processing plant and related development activities by January 2008. The 100 ton pigment pilot processing plant was commissioned in February 2008 and the costs associated with this effort were partially reimbursed by AlSher. We contribute any work in process and fixed assets associated with completion of the pigment pilot processing plant to the AlSher joint venture. For each reporting period, AlSher is consolidated with our subsidiaries because we have a controlling interest in AlSher and any inter-company transactions are eliminated (refer to Note 1 – Basis of Preparation of Consolidated Financial Statements). The non-controlling shareholder’s interest in the net assets and net income or loss of AlSher are reported as non-controlling interest in subsidiary on the condensed consolidated balance sheet and as non-controlling interest share in the condensed consolidated statement of operations, respectively.
F-13
Asset impairment of $1.3 million in 2009 relates to the expense of adjusting AlSher Titania, LLC assets to fair market value as of December 31, 2009. These assets were temporarily idled throughout 2009 as we searched for an interested party to acquire our interests in AlSher Titania. On April 30, 2010, we sold our 70% share in the AlSher Titania Joint Venture to Sherwin-Williams. We recorded a gain of $124,000 on discontinued operations that was comprised of $400,000 loss on disposal of fixed assets and $524,000 remaining equity in noncontrolling interest.
Overhead Allocation — Facilities overhead, which is comprised primarily of occupancy and related expenses, are initially recorded in general and administrative expenses and then allocated to research and development and product inventories based on relative labor costs.
Net Loss per Common Share — Basic loss per share is computed using the weighted average number of common shares outstanding during the period. Diluted loss per share is computed using the weighted average number of common and potentially dilutive shares outstanding during the period. Potentially dilutive shares consist of the incremental common shares issuable upon the exercise of stock options and warrants. Potentially dilutive shares are excluded from the computation if their effect is anti-dilutive. We had a net loss for all periods presented herein; therefore, none of the stock options and warrants outstanding during each of the periods presented, as discussed in Notes 11 and 12, were included in the computation of diluted loss per share as they were anti-dilutive. Stock options and warrants to purchase a total of 3,271,138 shares as of December 31, 2010, 2,987,162 shares as of December 31, 2009 and 1,159,497 shares as of December 31, 2008 were excluded from the calculations of diluted loss per share for the years ended December 31, 2010, 2009 and 2008, respectively.
Accumulated Other Comprehensive Loss — Accumulated other comprehensive loss consists entirely of unrealized loss on the investment in available for sale securities.
Deferred Income Taxes — Income taxes are accounted for using the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry forwards. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Future tax benefits are subject to a valuation allowance when management is unable to conclude that its deferred income tax assets will more likely than not be realized from the results of operations. We have recorded a valuation allowance to reflect the estimated amount of deferred income tax assets that may not be realized. The ultimate realization of deferred income tax assets is dependent upon generation of future taxable income during the periods in which those temporary differences become deductible. Management considers projected future taxable income and tax planning strategies in making this assessment.
Fair Value of Financial Instruments — Our financial instruments such as cash and cash equivalents and long-term debt, when valued using market interest rates, would not be materially different from the amounts presented in the consolidated financial statements.
F-14
Recent Accounting Pronouncements —
Issued and adopted:
In January 2010, the FASB revised two disclosure requirements concerning fair value measurements and clarified two others. These requirements mandate separate presentation of significant transfers into and out of Levels 1 and 2 of the fair value hierarchy and disclosure of the reasons for such transfers. They will also require the presentation of purchases, sales, issuances and settlements within Level 3 on a gross basis rather than a net basis. The amendments also clarify that disclosures should be disaggregated by class of asset or liability and that disclosures about inputs and valuation techniques should be provided for both recurring and non-recurring fair value measurements. Our disclosures about fair value measurements are presented in Note 4 – Fair Value Measurements. We have adopted the changes required except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements which will become effective for the Company on January 1, 2011. Management has determined that the adoption of these changes will not have a material impact on the Financial Statements.
Issued and not yet adopted:
In October 2009, the FASB issued changes to Multiple-Deliverable Revenue Arrangements. This guidance establishes a selling price hierarchy for determining the selling price of a deliverable and expands the disclosures required for multiple-deliverable revenue arrangements. This guidance is effective for revenue arrangements that are entered into or are materially modified in fiscal years beginning on or after June 15, 2010, with early adoption permitted. Management has determined that the adoption of these changes will not have an impact on the Financial Statements.
In April 2010, the FASB issued guidance on the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. It is effective for fiscal years beginning on or after June 15, 2010. Management has determined that the adoption of these changes will not have an impact on the Financial Statements.
Reclassifications — Certain reclassifications have been made to prior period amounts to conform to classifications adopted in the current year.
3. INVESTMENT IN AVAILABLE FOR SALE SECURITIES
The auction rate corporate notes are long-term instruments with expiration dates through 2017. Through the third quarter of 2007, the interest was settled and the rate reset every 7 to 28 days and historically these investments were classified as short-term investments. However, in the fourth quarter of 2007 due to the reduction of liquidity in the auction rate market, sell orders exceeded bid orders in that market, and the interest relating to these investments was reset to a contractual rate of London Interbank Offering Rate plus 50 basis points, which is not a market rate. Based on this change in the liquidity, these investments were evaluated to determine if there was impairment during 2009 and 2010. Our evaluation included consultation with our investment advisors, assessment of the strength of the financial institution paying the interest on these investments, ratings of the underlying collateral, and a probability-weighted discounted cash flow analysis. We continued this analysis for the first three quarters of 2010 and then sold all auction rate corporate notes in the fourth quarter of 2010. The sale resulted in $1.95 million in cash received and a $1.95 million realized loss on investments.
As of December 31, 2009, investment in available for sale securities (short-term) consisted of 113,809 shares of Spectrum Pharmaceuticals, Inc. (“Spectrum”) common stock. The shares were received as partial compensation for the assignment of all rights and title to RenaZorbTM and RenalanTM. Upon receipt, the shares were recorded at their market value as measured by their closing price on the NASDAQ Capital Market, resulting in a recorded basis of $750,000. At December 31, 2009, their fair value was $505,000 representing an unrealized holding loss of $245,000. On December 10, 2010, the 113,809 shares of Spectrum stock were sold at $5.752 per share for a gross amount of $655,000. After charges and fees, net proceeds were $649,000. Altair realized a loss on sale of investment of $95,000.
F-15
|
4.
|
FAIR VALUE MEASUREMENTS
|
The following are the methods and assumptions we use to estimate the fair value of our financial instruments.
Cash and cash equivalents
Due to their short term nature, carrying amount approximates fair value.
Accounts receivable
Due to their short term nature, carrying amount approximates fair value.
Investment in available for sale securities
For investment in available for sale securities, fair values are based on quoted market prices. For auction rate securities, fair values are estimated using discounted cash flow analyses incorporating significant unobservable inputs.
Trade accounts payable
Due to their short term nature, carrying amount approximates fair value.
Long-term debt
Due to the short term nature of the current portion of long-term debt, the carrying amount approximates fair value. The non current portion of long-term debt is not material and the carrying amount approximates fair value.
Our financial instruments are accounted for at fair value on a recurring basis. We have no financial instruments accounted for on a non-recurring basis as of December 31, 2010 or 2009. Fair value is determined based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. A market or observable inputs is the preferred source of values, followed by assumptions based on hypothetical transactions in the absence of market inputs.
The valuation techniques are based upon observable and unobservable inputs. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect our market assumptions. These two types of inputs create the following fair value hierarchy:
|
Level 1 -
|
Quoted prices for identical instruments in active markets.
|
|
Level 2 -
|
Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations whose inputs are observable or whose significant value drivers are observable.
|
|
Level 3 -
|
Significant inputs to the valuation model are unobservable.
|
No assets or liabilities were recorded at fair value on a recurring basis at December 31, 2010.
F-16
The following table summarizes the valuation of our assets by the fair value hierarchy at December 31, 2009:
|
In thousands of dollars
|
||||||||||||||||
|
Assets at fair value:
|
Total
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Auction rate corporate notes
|
$ | 2,587 | $ | - | $ | - | $ | 2,587 | ||||||||
|
Spectrum Pharmaceuticals, Inc.
|
505 | 505 | - | - | ||||||||||||
|
Investment in available for sale securities
|
$ | 3,092 | $ | 505 | $ | - | $ | 2,587 | ||||||||
The Spectrum Pharmaceuticals shares listed above at December 31, 2009 were acquired from Spectrum on August 4, 2009 when we entered into an amended agreement with Spectrum in which we transferred them the rights to RenalanTM in addition to RenaZorbTM. A component of this agreement was the payment to us of an additional 113,809 shares of Spectrum common stock. On December 10, 2010, Altair sold 113,809 shares of Spectrum stock at $5.752 per share for a gross amount of $655,000. After charges and fees, net proceeds were $649,000. Altair realized a loss on sale of investment of $95,000.
The following table summarizes current and non-current marketable securities, accounted for as "available for sale" securities at December 31, 2010 and 2009:
In thousands of dollars
|
2010
|
2009
|
|||||||||||||||||||||||||||||||
|
|
Unrealized
|
|
Unrealized
|
|||||||||||||||||||||||||||||
|
Fair
|
Carrying
|
(loss)/gain
|
Fair
|
Carrying
|
(loss)/gain
|
|||||||||||||||||||||||||||
|
Cost
|
Value
|
Value
|
accumulated
|
Cost
|
Value
|
Value
|
accumulated
|
|||||||||||||||||||||||||
|
Current marketable securities:
|
||||||||||||||||||||||||||||||||
|
Spectrum Pharmaceuticals, Inc.
|
- | - | - | - | 752 | 505 | 505 | (247 | ) | |||||||||||||||||||||||
|
Total current
|
$ | - | $ | - | $ | - | $ | - | $ | 752 | $ | 505 | $ | 505 | $ | (247 | ) | |||||||||||||||
|
Non-current marketable securities:
|
||||||||||||||||||||||||||||||||
|
Auction rate corporate notes
|
- | - | - | - | 3,900 | 2,587 | 2,587 | (1,313 | ) | |||||||||||||||||||||||
|
Total non-current
|
$ | - | $ | - | $ | - | $ | - | $ | 3,900 | $ | 2,587 | $ | 2,587 | $ | (1,313 | ) | |||||||||||||||
The activity relating to assets valued on a recurring basis utilizing Level 3 inputs for the twelve months ended December 31, 2010 and December 31, 2009 is summarized below:
|
In thousands of dollars
|
||||||||
|
Auction rate
|
Auction rate
|
|||||||
|
corporate notes
|
corporate notes
|
|||||||
|
2010
|
2009
|
|||||||
|
Beginning Balance, January 1
|
$ | 2,587 | $ | 2,816 | ||||
|
Sale or settlements
|
(1,950 | ) | - | |||||
|
Realized losses
|
(1,950 | ) | - | |||||
|
Unrealized gains/(losses)
|
- | (223 | ) | |||||
|
Other adjustments
|
- | (6 | ) | |||||
|
Reclassification adjustment for realized loss on securities included in net loss
|
1,313 | - | ||||||
|
Ending Balance, December 31
|
$ | - | $ | 2,587 | ||||
The amount of total gains or losses for the twelve months ended December 31, 2010 was a realized loss of $1,950,000 associated with the sale of the auction rate securities. For the twelve months ended December 31, 2009 included in other comprehensive income in Stockholder’s Equity attributable to the change in unrealized gain (loss) relating to assets still held at the reporting date was $(223,000).
F-17
Financial instruments that trade in less liquid markets with limited pricing information generally include both observable and unobservable inputs. In instances where observable data is unavailable, we consider the assumptions that market participants would use in valuing the asset. Such investments are categorized in Level 3 as the inputs generally are not observable. Our evaluation included consultation with our investment advisors, assessment of the strength of the financial institution paying the interest on these investments, ratings of the underlying collateral, and a probability-weighted discounted cash flow analysis.
|
5.
|
PRODUCT INVENTORIES
|
Product Inventories consisted of the following at December 31, 2010 and 2009:
|
In thousands of dollars
|
||||||||
|
2010
|
2009
|
|||||||
|
Raw Materials
|
$ | 2,979 | $ | 3,933 | ||||
|
Work in process
|
920 | 908 | ||||||
|
Finished goods
|
2,926 | 202 | ||||||
|
Total product inventories
|
$ | 6,825 | $ | 5,043 | ||||
Once products reach the commercialization stage, the related inventory is recorded. The costs associated with products undergoing research and development are expensed as incurred. As of December 31, 2009 and 2010, inventory relates to the production of batteries targeted at the stationary power and electric bus markets.
We recorded an inventory valuation allowance on finished goods of $623,000 and $71,000 at December 31, 2010 and 2009, respectively.
|
6.
|
PROPERTY, PLANT AND EQUIPMENT
|
Property, plant and equipment used in operations consisted of the following as of December 31, 2010 and 2009:
|
In thousands of dollars
|
||||||||
|
2010
|
2009
|
|||||||
|
Machinery and equipment
|
$ | 11,035 | $ | 9,116 | ||||
|
Building and improvements
|
5,155 | 4,288 | ||||||
|
Furniture, office equipment & other
|
1,200 | 1,251 | ||||||
|
Total
|
17,390 | 14,655 | ||||||
|
Less accumulated depreciation
|
(8,663 | ) | (5,985 | ) | ||||
|
Total property, plant and equipment
|
$ | 8,727 | $ | 8,670 | ||||
F-18
Property, plant and equipment held and not used in operations consisted of the following as of December 31, 2010 and 2009:
|
In thousands of dollars
|
||||||||
|
2010
|
2009
|
|||||||
|
Machinery and equipment
|
$ | - | $ | 5,642 | ||||
|
Building and improvements
|
- | 849 | ||||||
|
Furniture, office equipment & other
|
- | 49 | ||||||
|
Asset impairment
|
- | (1,308 | ) | |||||
|
Total
|
- | 5,232 | ||||||
|
Less accumulated depreciation
|
- | (3,021 | ) | |||||
|
Total property, plant and equipment
|
$ | - | $ | 2,211 | ||||
Depreciation expense for the years ended December 31, 2010, 2009, and 2008 totaled $1.9 million, $2.6 million and $2.7 million, respectively.
Asset impairment of $1.3 million in 2009 relates to the expense of adjusting AlSher Titania, LLC assets to fair market value as of December 31, 2009. These assets were temporarily idled throughout 2009 as we searched for an interested party to acquire our interests in AlSher Titania. On April 30, 2010, we sold our 70% share in the AlSher Titania Joint Venture to Sherwin-Williams. We recorded a gain of $124,000 on discontinued operations that was comprised of $400,000 loss on disposal of fixed assets and $524,000 remaining equity in noncontrolling interest.
The remaining Performance Materials fixed assets of $609,000 at December 31, 2009 consist primarily of production assets such as mills, furnaces and laboratory equipment suited for general use in our business. These assets were either re-purposed to the Power and Energy segment to support the anticipated growth in sales volume, sold or scrapped during 2010. These assets are expected to have in-service lives at least equal to their depreciation lives and with reasonable ongoing maintenance are expected to continue functioning throughout that period. If we are unable to commercialize our battery products, the value of these assets could be impaired, but we believe this outcome is unlikely. These assets were classified as held and not used as of December 31, 2009.
Life Sciences fixed assets with a net book value of $1.2 million as of December 31, 2009 are primarily building improvements that expand production and lab areas. In 2009, these assets were reported as held but not used. It was determined that these improvements do add to the value of our Reno, Nevada building and the space and will be required for the expansion of Power and Energy operations based on anticipated growth in sales volume within the next two years. During 2010, these fixed assets were either transferred to the Power and Energy segment, sold or scrapped. Failure to commercialize our battery products and a significant drop in real estate values could lead to impairment of these assets. We believe that the occurrence of such events is unlikely.
F-19
|
7.
|
PATENTS
|
Patents consisted of the following at December 31, 2010 and 2009:
|
In thousands of dollars
|
||||||||
|
2010
|
2009
|
|||||||
|
Patents and patent applications
|
$ | 1,366 | $ | 1,518 | ||||
|
Less accumulated amortization
|
(940 | ) | (967 | ) | ||||
|
Total patents and patent applications
|
$ | 426 | $ | 551 | ||||
All patents are being amortized on a straight-line basis over their useful lives with a weighted average amortization period of approximately 16.7 years. Amortization expense was $78,000, $84,000 and $84,000 for the years ended December 31, 2010, 2009 and 2008, respectively. For each of the next five years, amortization expense relating to intangibles is expected to be approximately $76,000 per year. We expense all costs, as incurred, associated with renewing or extending our patents.
|
8.
|
ACCRUED WARRANTY
|
Accrued warranty consisted of the following at December 31, 2010 and 2009:
|
In thousands of dollars
|
||||||||
|
2010
|
2009
|
|||||||
|
Beginning Balance – January 1,
|
$ | 79 | $ | 36 | ||||
|
Increases to reserve based on sales
|
152 | 47 | ||||||
|
Charges against warranty reserve
|
(20 | ) | (4 | ) | ||||
|
Ending Balance – December 31,
|
$ | 211 | $ | 79 | ||||
The $47,000 added to the warranty reserve during 2009 is associated with battery packs sold during 2009. During 2010, the warranty provision increased $152,000 based on sales. The charges against the provision of $20,000 primarily reflects activity in connection with the AES prototype battery pack purchased in 2007.
|
9.
|
ACCRUED LIABILITIES
|
Accrued liabilities consisted of the following at December 31, 2010 and 2009:
|
In thousands of dollars
|
||||||||
|
2010
|
2009
|
|||||||
|
Accrued interest
|
$ | - | $ | 38 | ||||
|
Accrued use tax
|
7 | 6 | ||||||
|
Accrued property tax
|
21 | - | ||||||
|
Accrued mineral lease payments
|
67 | 67 | ||||||
|
Accrued reclamation costs
|
6 | 6 | ||||||
|
Accrued straight line rent
|
17 | 54 | ||||||
|
Accrued fees to vendors
|
269 | 276 | ||||||
|
Total accrued liabilities
|
$ | 387 | $ | 447 | ||||
F-20
|
10.
|
NOTES PAYABLE
|
Notes payable consisted of the following at December 31, 2010 and 2009:
|
In thousands of dollars
|
||||||||
|
2010
|
2009
|
|||||||
|
Note payable to BHP Minerals International, Inc.
|
$ | - | $ | 600 | ||||
|
Note payable to AICCO, Inc.
|
- | 194 | ||||||
|
Note payable to Imperial Credit Corporation
|
196 | - | ||||||
|
Capital Leases
|
36 | 53 | ||||||
|
Subtotal
|
232 | 847 | ||||||
|
Less current portion
|
(216 | ) | (810 | ) | ||||
|
Long-term portion
|
$ | 16 | $ | 37 | ||||
On August 8, 2002, we entered into a purchase and sale agreement with BHP Minerals International, Inc. (“BHP”), wherein we purchased the land, building and fixtures in Reno, Nevada where our titanium processing assets are located. In connection with this transaction, BHP also agreed to terminate our obligation to pay royalties associated with the sale or use of the titanium processing technology. In return, we issued to BHP a note in the amount of $3.0 million, at an interest rate of 7%, secured by the property we acquired. Interest did not begin to accrue until August 8, 2005. As a result, we imputed interest and reduced the face amount of the note payable by $567,000, which was then amortized to interest expense from inception of the note through August 8, 2005. Payments were due in February of each year beginning in 2006. The note and all accrued interest were paid in full in January 2010.
|
11.
|
STOCK BASED COMPENSATION
|
At December 31, 2010, we have a stock incentive plan, administered by the Board of Directors, which provides for the granting of options and restricted shares to employees, officers, directors and other service providers. This Plan is described in more detail below. The compensation cost that has been charged against income for this Plan was $1.6 million, $1.1 million, and $1.2 million for the years ended 2010, 2009 and 2008, respectively. Of this amount, $303,000, $221,000 and $168,000 was recognized in connection with restricted stock and options granted to non-employees for the years ended 2010, 2009 and 2008, respectively.
Stock Options
The total number of shares authorized to be granted under the 2005 stock plan was increased from 750,000 to an aggregate of 2,250,000 based on the proposal approved at the annual and special meeting of shareholders on May 30, 2007. Prior stock option plans, under which we may not make future grants, authorized a total of 1,650,000 shares, of which options for 1,058,725 were granted (net of expirations) and options for 45,375 are outstanding and unexercised at December 31, 2010. Options granted under the plans generally are granted with an exercise price equal to the market value of a common share at the date of grant, have five- or ten-year terms and typically vest over periods ranging from immediately to three years from the date of grant. The estimated fair value of equity-based awards, less expected forfeitures, is amortized over the awards’ vesting period utilizing the graded vesting method. Under this method, unvested amounts begin amortizing at the beginning of the month in which the options are granted.
In calculating compensation recorded related to stock option grants for the years ended December 31, 2010, 2009 and 2008, the fair value of each stock option is estimated on the date of grant using the Black-Scholes-Merton option-pricing model and the following weighted average assumptions:
F-21
|
2010
|
2009
|
2008
|
||||||||||
|
Dividend yield
|
None
|
None
|
None
|
|||||||||
|
Expected volatility
|
84% | 82% | 76% | |||||||||
|
Risk-free interest rate
|
1.83% | 1.50% | 3.00% | |||||||||
|
Expected life (years)
|
5.75 | 5.72 | 4.92 | |||||||||
The computation of expected volatility used in the Black-Scholes Merton option-pricing model is based on the historical volatility of our share price. The expected term is estimated based on a review of historical and future expectations of employee exercise behavior.
A summary of option activity under our equity-based compensation plans as of December 31, 2010, 2009 and 2008, and changes during the year then ended is presented below:
|
2010
|
2009
|
2008
|
||||||||||||||||||||||||||||||||||||||||||||||
|
Shares
|
Weighted
Average |
Weighted
Average |
Aggregate
Intrinsic |
Shares
|
Weighted
Average |
Weighted
Average |
Aggregate
Intrinsic |
Shares
|
Weighted
Average |
Weighted
Average |
Aggregate
Intrinsic |
|||||||||||||||||||||||||||||||||||||
|
Outstanding at January 1,
|
1,230,034 | $ | 9.60 | 7.8 | $ | 2,000 | 989,108 | $ | 12.12 | 7.4 | $ | 11,000 | 1,041,533 | $ | 11.24 | 7.0 | $ | 6,024,389 | ||||||||||||||||||||||||||||||
|
Granted
|
444,312 | $ | 3.94 | 401,188 | $ | 4.64 | 532,979 | $ | 12.16 | |||||||||||||||||||||||||||||||||||||||
|
Exercised
|
- | - | - | - | (84,803 | ) | $ | 6.24 | ||||||||||||||||||||||||||||||||||||||||
|
Forfeited/expired
|
(160,321 | ) | $ | 9.57 | (160,262 | ) | $ | 12.80 | (500,602 | ) | $ | 11.32 | ||||||||||||||||||||||||||||||||||||
|
Outstanding at December 31,
|
1,514,025 | $ | 7.93 | 7.5 | - | 1,230,034 | $ | 9.60 | 7.8 | $ | 2,000 | 989,108 | $ | 12.12 | 7.4 | $ | 10,661 | |||||||||||||||||||||||||||||||
|
Exercisable at December 31,
|
669,872 | $ | 10.96 | 6.3 | - | 554,854 | $ | 12.12 | 6.6 | - | 476,401 | $ | 12.56 | 5.8 | $ | 900 | ||||||||||||||||||||||||||||||||
Shares issued to non-employees reflected in the table above include 133,416 shares outstanding at January 1, 2010, with no shares granted, no shares exercised, and 12,396 shares forfeited or expired during the year ended December 31, 2010, resulting in 93,416 shares outstanding of which 76,229 shares were exercisable as of December 31, 2010. Shares issued to non-employees reflected in the table above include 176,917 shares outstanding at January 1, 2009, 6,250 shares granted, no shares exercised, and 49,750 shares forfeited or expired during the year ended December 31, 2009, resulting in 133,417 shares outstanding of which 103,833 shares were exercisable as of December 31, 2009. Shares issued to non-employees reflected in the table above include 646,000 shares outstanding at January 1, 2008, 136,667 shares granted, and 75,000 shares exercised during the year ended December 31, 2008, resulting in 707,667 shares outstanding of which 508,166 shares were exercisable as of December 31, 2008.
The weighted-average grant-date fair value of options granted during 2010, 2009 and 2008 was $2.27, $2.96 and $7.56, respectively. The total intrinsic value of options exercised during the years ended December 31, 2010 and 2009 was $0 and was $408,000 for 2008.
A summary of the status of non-vested shares at December 31, 2010, 2009 and 2008 and changes during the year then ended, is presented below:
|
2010
|
2009
|
2008
|
||||||||||||||||||||||
|
Shares
|
Weighted
Average |
Shares
|
Weighted
Average |
Shares
|
Weighted
Average |
|||||||||||||||||||
|
Non-vested shares at January 1,
|
675,180 | $ | 7.52 | 512,707 | $ | 11.68 | 392,161 | $ | 11.84 | |||||||||||||||
|
Granted
|
444,312 | 3.94 | 401,188 | 4.64 | 532,979 | 12.16 | ||||||||||||||||||
|
Vested
|
(201,660 | ) | 8.52 | (199,537 | ) | 11.84 | (176,900 | ) | 12.16 | |||||||||||||||
|
Forfeited/Expired
|
(73,679 | ) | 5.96 | (39,177 | ) | 10.60 | (235,534 | ) | 12.72 | |||||||||||||||
|
Non-vested shares at December 31,
|
844,153 | $ | 5.17 | 675,180 | $ | 7.52 | 512,707 | $ | 11.68 | |||||||||||||||
F-22
Non-vested shares relating to non-employees reflected in the table above include 29,583 shares outstanding at January 1, 2010, no shares granted, no shares exercised, and 12,396 shares vested during the year ended December 31, 2010, resulting in 17,187 non-vested shares outstanding at December 31, 2010. Non-vested shares relating to non-employees reflected in the table above include 49,875 shares outstanding at January 1, 2009, 6,250 shares granted, no shares exercised, and 26,542 shares vested during the year ended December 31, 2009, resulting in 29,584 non-vested shares outstanding at December 31, 2009. Non-vested shares relating to non-employees reflected in the table above include 155,666 shares outstanding at January 1, 2008, 136,667 shares granted and 92,832 shares exercised during the year ended December 31, 2008, resulting in 199,501 non-vested shares outstanding at December 31, 2008.
As of December 31, 2010, 2009, and 2008 there was $1.2 million, $902,000, and $869,455, respectively, of total unrecognized compensation cost related to non-vested options granted under the plans. That cost is expected to be recognized over a weighted average period of one year for 2010, 2009, and 2008. The total fair value of options vested during the year ended December 31, 2010, 2009, and 2008 was $1.1 million, $1.5 million, and $1.5 million, respectively.
Cash received from warrant and stock option exercises for the years ended December 31, 2010 and 2009 was $0 and for 2008 was $1.3 million.
Restricted Stock
Our stock incentive plan provides for the granting of other incentive awards in addition to stock options. During the year ended December 31, 2010, the Board of Directors approved grants of 177,744 shares of restricted stock under the plan with a weighted average fair value of $1.80 per share. Restricted shares have the same voting and dividend rights as our unrestricted common shares, vest over a two-year period and are subject to the employee’s or director’s continued service. Compensation cost for restricted stock is recognized in the financial statements on a pro rata basis over the vesting period.
A summary of the changes in restricted stock outstanding during the year ended December 31, 2010, 2009 and 2008, is presented below:
|
2010
|
2009
|
2008
|
||||||||||||||||||||||
|
Shares
|
Weighted
Average |
Shares
|
Weighted
Average |
Shares
|
Weighted
Average |
|||||||||||||||||||
|
Non-vested shares at January 1,
|
76,624 | $ | 4.64 | 41,077 | $ | 9.08 | 21,964 | $ | 12.28 | |||||||||||||||
|
Granted
|
177,744 | 1.80 | 95,529 | 4.16 | 35,770 | 8.44 | ||||||||||||||||||
|
Vested
|
(43,372 | ) | 5.10 | (30,200 | ) | 9.20 | (16,324 | ) | 11.72 | |||||||||||||||
|
Forfeited/Expired
|
0 | 0.00 | (29,782 | ) | 4.00 | (333 | ) | 9.08 | ||||||||||||||||
|
Non-vested shares at December 31,
|
210,996 | $ | 2.16 | 76,624 | $ | 4.64 | 41,077 | $ | 9.08 | |||||||||||||||
As of December 31, 2010, 2009, and 2008 we had total unrecognized compensation expense of $228,000, $225,000, and $373,000, respectively, net of estimated forfeitures, related to restricted stock which will be recognized over the weighted average period for December 31, 2010, 2009 and 2008 of 0.92 years, 1.6 years, and 1.7 years, respectively.
F-23
|
12.
|
WARRANTS
|
Warrants — Warrant activity for the years ended December 31, 2010, 2009 and 2008 is summarized as follows:
In thousands of dollars
|
2010
|
2009
|
2008
|
||||||||||||||||||||||
|
Weighted
|
Weighted
|
Weighted
|
||||||||||||||||||||||
|
Average
|
Average
|
Average
|
||||||||||||||||||||||
|
Exercise
|
Exercise
|
Exercise
|
||||||||||||||||||||||
|
Warrants
|
Price
|
Warrants
|
Price
|
Warrants
|
Price
|
|||||||||||||||||||
|
Outstanding at beginning of year
|
1,757,115 | $ | 4.61 | 170,371 | $ | 16.60 | 285,427 | $ | 13.04 | |||||||||||||||
|
Issued
|
- | - | 1,649,245 | 4.00 | - | - | ||||||||||||||||||
|
Expired
|
- | - | (62,500 | ) | 21.06 | (15,000 | ) | 10.00 | ||||||||||||||||
|
Exercised
|
- | - | - | (100,056 | ) | 7.52 | ||||||||||||||||||
|
Outstanding at end of year
|
1,757,115 | $ | 4.61 | 1,757,115 | $ | 4.60 | 170,371 | $ | 16.60 | |||||||||||||||
|
Currently exercisable
|
1,757,115 | $ | 4.61 | 1,757,115 | $ | 4.60 | 170,371 | $ | 16.60 | |||||||||||||||
The following table summarizes information about warrants outstanding at December 31, 2010:
|
Warrants Outstanding and Exercisable
|
||||||||||||
|
Weighted
|
||||||||||||
|
Average
|
Weighted
|
|||||||||||
|
Remaining
|
Average
|
|||||||||||
|
Range of
|
Contractual
|
Exercise
|
||||||||||
|
Exercise Prices
|
Warrants
|
Life (Years)
|
Price
|
|||||||||
|
$1.00 to $4.00
|
1,649,244 | 5.4 | $ | 4.00 | ||||||||
|
$4.01 to $13.50
|
57,871 | 1.0 | 13.50 | |||||||||
|
$13.50 to $14.56
|
50,000 | 0.6 | 14.56 | |||||||||
| 1,757,115 | 5.1 | $ | 4.61 | |||||||||
Except as noted below, the warrants were issued in conjunction with debt and equity offerings. The warrants expire on various dates ranging to May 2016.
Warrants Issued to Investors
During the year ending December 31, 2009, 1,649,240 warrants were issued in connection with the May 28, 2009 common stock offering at a strike price of $4.00 per common share. As a result, no intrinsic value existed at the issuance date. The following assumptions were used to value the warrant cost of $4.6 million, recorded as additional paid in capital within the consolidated statement of stockholders' equity and comprehensive loss: expected life of 7 years, volatility of 89.8%, annual rate of quarterly dividends of $0 and risk free interest rate of 1.86%. All of these warrants are outstanding at December 31, 2010.
Warrants Issued in Payment of Services
The cost associated with warrants issued as payment for outside services is estimated on the date of issuance using the Black-Scholes-Merton option-pricing model.
During the year ending December 31, 2007, 200,000 warrants were issued in connection with the Joint Development and Equipment Purchase Agreement with AES Energy Storage, LLC and the related Warrant Issuance Agreement signed on July 20, 2007. Pursuant to this agreement, an initial warrant to purchase 50,000 common shares of ours at $14.56 per share was issued. Since the Initial Warrant did not become exercisable until December 31, 2007, the fair value of the warrants was estimated at the issuance date and adjusted using variable accounting until the final vesting date occurred. Based on the following assumptions at the vesting date of expected life of 1.83 years, volatility of 43.7 %, annual rate of quarterly dividends of $0 and the risk free interest rate of 3.5%, a total of $261,000 was recorded in stock compensation expense. All of these warrants are outstanding at December 31, 2010.
F-24
|
13.
|
OTHER TRANSACTIONS
|
On October 21, 2010, our Board of Directors authorized a reverse split of our common stock at a ratio of one for four, effective the end of business November 15, 2010. Our stockholders previously approved the reverse split in May 2010. As a result of the reverse split, every four shares of common stock outstanding were combined into one share of common stock. The reverse split did not affect the amount of equity the Company has nor did it affect the Company’s market capitalization.
On September 20, 2010, we reached a definitive agreement to sell additional common shares to Canon, a Hong Kong-based company. This transaction will result in Canon owning 51% of Altair Nanotechnologies, Inc. on a fully-diluted basis (approximately 53.8% of shares outstanding following the transaction). At the same time we signed the Share Subscription Agreement with Canon we also signed a supply and technology licensing agreement with a Canon affiliate, China-based Zhuhai Yintong Energy Company (“YTE”). The agreement calls for the Company to supply YTE with nano-lithium titanate powder (“LTO”), 11 AHr cells and an ALTI-ESS 1 MW battery system totaling $6.6 million. We shipped these products to YTE during the fourth quarter of 2010. As a result of the SSA Amendment, purchases under this Agreement have been indefinitely suspended. During September 2010 we received a $2.0 million prepayment from YTE for these goods, of which $437,000 was recognized in the fourth quarter, leaving $1.6 million in deferred revenue as of December 31, 2010. Deferred issuance costs related to this stock sale totaled $831,000 for the year ending December 31, 2010 and are included in current assets.
On June 9, 2010, we entered into an At the Market Issuance Sales Agreement with Thomas Weisel Partners LLC pursuant to which we may issue and sell our common shares having an aggregate offering price of up to $15.0 million from time to time through Thomas Weisel, acting as agent. The sales, if any, of shares made under the Sales Agreement will be made on the NASDAQ Capital Market by means of ordinary brokers' transactions at market prices, in privately negotiated transactions or as otherwise agreed by Thomas Weisel and Altair. Thomas Weisel will use commercially reasonable efforts to sell the common shares from time to time, based upon our instructions (including any price, time or size limits or other customary parameters or conditions we may impose). We will pay Thomas Weisel commissions at a fixed commission rate of 5% of the gross sales price per share for any shares sold under the Sales Agreement. We have also agreed to reimburse Thomas Weisel for certain specified expenses and have provided Thomas Weisel with customary indemnification rights.
As of December 31, 2010, we have sold 487,653 common shares under the At the Market Issuance Sales Agreement. The sales were made at an average weighted price of $1.84 per share, generating gross proceeds of $905,000. Issuance costs totaled $213,000 resulting in net proceeds to the Company of $692,000.
Based on the Share Subscription Agreement signed with Canon Investment Holdings Limited (“Canon”) on September 20, 2010, sales of shares under the At The Market Issuance Sales Agreement have been suspended.
For the year ending December 31, 2009, 1,649,240 warrants were issued in connection with the May 28, 2009 common stock offering at a strike price of $4.00 per common share. As a result, no intrinsic value existed at the issuance date. The following assumptions were used to value the warrant cost of $4.6 million, recorded as common stock issuance cost: expected life of 7 years, volatility of 89.8%, annual rate of quarterly dividends of $0 and risk free interest rate of 1.86%. All of these warrants are outstanding at December 31, 2009.
F-25
On October 6, 2008, we entered into a Stock Purchase and Settlement Agreement with Al Yousuf, LLC. 529,412 shares of common stock were issued at a fair value of $6.80 that were agreed upon as part of arms length negotiations, and were recorded as settlement expense in Operating Expense for the twelve months ended December 31, 2008. Additionally, 1,470,588 shares were acquired by Al Yousuf, LLC at a purchase price of $6.80 per share for an aggregate purchase price of $10.0 million (refer to Note 17).
|
14.
|
LEASES
|
Operating Leases — We lease certain premises for office space and other corporate purposes. Operating lease commitments at December 31, 2010 were:
|
In thousands of dollars:
|
||||
|
2011
|
$ | 319 | ||
|
2012
|
166 | |||
|
Thereafter
|
- | |||
|
Total
|
$ | 485 | ||
Lease expense for the years ended December 31, 2010, 2009 and 2008 totaled $319,000, $263,000 and $263,000, respectively.
Future minimum payments on capitalized leases are as follows:
|
In thousands of dollars:
|
||||
|
Year ending December 31:
|
||||
|
2011
|
$ | 22 | ||
|
2012
|
17 | |||
|
2013
|
- | |||
| 39 | ||||
|
Less amount representing interest
|
(3 | ) | ||
|
Present value of net minimum lease payments
|
36 | |||
|
Less current maturity
|
(20 | ) | ||
|
Present value of net minimum leases included in long-term debt
|
$ | 16 | ||
|
15.
|
INCOME TAXES
|
Losses before income taxes include losses relating to non-U.S. operations of $2.7 million, $361,000 and $3.1 million in the years ended December 31, 2010, 2009 and 2008, respectively.
Because of the net operating losses and a valuation allowance on deferred tax assets, there was no provision for income taxes recorded in the accompanying consolidated financial statements for each of the three years ended December 31, 2010, 2009, and 2008.
F-26
A reconciliation of the federal statutory income tax rate 35% and our effective income tax rates is as follows:
|
In thousands of dollars:
|
||||||||||||
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Federal statutory income tax benefit
|
$ | (7,802 | ) | $ | (7,459 | ) | $ | (10,174 | ) | |||
|
Expiration of net operating loss carry forwards
|
942 | 1,509 | 517 | |||||||||
|
Other, net
|
(60 | ) | (17 | ) | 29 | |||||||
|
True up to prior tax returns
|
(607 | ) | (682 | ) | (3,481 | ) | ||||||
|
Exercise of incentive stock options
|
478 | 318 | 390 | |||||||||
|
Valuation allowance
|
7,049 | 6,331 | 12,719 | |||||||||
|
Total
|
$ | - | $ | - | $ | - | ||||||
The components of the deferred tax assets consisted of the following as of December 31, 2010 and 2009:
|
In thousands of dollars:
|
||||||||
|
2010
|
2009
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Net operating loss carry forwards
|
$ | 52,706 | $ | 46,938 | ||||
|
Basis difference in intangible assets
|
724 | 709 | ||||||
|
Accruals
|
640 | 395 | ||||||
|
Tax credits
|
465 | 465 | ||||||
|
Basic difference in property, plant, and equipment
|
4 | - | ||||||
|
Other, net
|
504 | 724 | ||||||
|
Total deferred tax assets
|
55,043 | 49,231 | ||||||
|
Deferred tax liabilities:
|
||||||||
|
Basis difference in property, plant, and equipment
|
- | (896 | ) | |||||
|
Total deferred tax liabilities
|
- | (896 | ) | |||||
|
Valuation allowance
|
(55,043 | ) | (48,335 | ) | ||||
|
Net deferred tax assets
|
$ | - | $ | - | ||||
As a result of certain realization requirements, the table of deferred tax assets shown above does not include certain deferred tax assets at December 31, 2010 and 2009 that arose directly from tax deductions related to equity compensation in excess of compensation recognized for financial reporting. Equity will be increased by approximately $27,000 if and when such deferred tax assets are ultimately realized. We use tax law ordering for purposes of determining when excess tax benefits have been realized.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
|
In thousands of dollars:
|
||||||||
|
2010
|
2009
|
|||||||
|
Balance at January 1
|
$ | 27 | $ | 27 | ||||
|
Reductions based on tax positions related to the current year
|
- | - | ||||||
|
Balance at December 31
|
$ | 27 | $ | 27 | ||||
The Company does not recognize interest or penalties related to unrecognized tax benefits.
The Company has no material uncertain tax positions.
Our operating loss carry-forwards include losses generated in the United States and in Canada. The net operating loss carry-forwards total approximately $150.6 million as of December 31, 2010, excluding any potential Section 382 limitations described below, and will expire at various dates as follows:
F-27
|
2011 - 2014
|
$ | 2,300,000 | ||
|
2015 - 2019
|
$ | 2,046,000 | ||
|
2020 - 2024
|
$ | 22,558,000 | ||
|
2025 - 2030
|
$ | 123,685,000 |
Due to the significant increase in common stock issued and outstanding from 2005 through 2010, Section 382 of the Internal Revenue Code may provide significant limitations on the utilization of net operating loss carryforwards. As a result of these limitations, it is estimated that as of December 31, 2010, approximately $25.0 million of these operating loss carryforwards have expired without being utilized. The company has not performed a formal section 382 study, but will do so in future years as needed.
Based upon the significant increase in common stock expected to be issued in connection with the pending Share Subscription agreement with Canon, Section 382 of the Internal Revenue Code may require significant additional limitations on the utilization of net operating loss carry-forwards than those disclosed above.
Based on the historical taxable income and projections for future taxable income over the periods in which the deferred income tax assets become deductible, management believes it more likely than not that the Company will not realize benefits of these deductible differences as of December 31, 2010. Management has, therefore, established a full valuation allowance against its net deferred income tax assets as of December 31, 2010.
We are subject to taxation in the U.S., Canada and various states. We record liabilities for income tax contingencies based on our best estimate of the underlying exposures. We have not been audited by any jurisdiction since our inception in 1998. We are open for audit by the U.S. Internal Revenue Service, the Canada Revenue Agency and U.S. state tax jurisdictions from our inception in 1998 to 2010.
|
16.
|
COMMITMENTS AND CONTINGENCIES
|
Contingencies — We are subject to claims in the normal course of business. Management, after consultation with legal counsel, believes that liabilities, if any, resulting from such claims will not materially effect our financial position or results of operations.
Litigation — We are currently not aware of any investigations, claims, or lawsuits that we believe could have a material adverse effect on our consolidated financial position or on our consolidated results of operations.
|
17.
|
RELATED PARTY TRANSACTIONS
|
In connection with the Share Subscription Agreement with Canon, we entered into a Waiver and Rights Agreement with Al Yousuf LLC on September 20, 2010. Under the Waiver and Rights Agreement, Al Yousuf LLC has waived its right of first offer with respect to the Share Subscription Agreement. Al Yousuf LLC has also agreed that, with respect to any underwritten demand registration under its pre-existing registration rights agreement with us, to the extent Canon exercises piggyback registration rights under the Investor Rights Agreement and there is an underwriter cutback, Canon and Al Yousuf LLC will participate on a pro rata basis proportionate to their share ownership.
We have agreed that, following the closing of the share issuance, Al Yousuf LLC will have the right to designate one director until such time as Al Yousuf LLC holds less than 5% of our outstanding common shares on a fully-diluted basis. During the period we have only nine directors, the director appointed by Al Yousuf LLC will be one of the independent directors and serve as a member of the audit committee of our Board of Directors.
F-28
We have further agreed that, at our next annual shareholder meeting following the closing of the transactions contemplated by the Share Subscription Agreement or if the Board decides to call a special shareholder meeting, at such shareholder meeting, we will propose to amend our articles to increase the size of the Board to no less than eleven directors and to nominate two new directors to the Board, one of whom to be designated by Canon and the other to be an independent director nominated by the Board pursuant our then-existing director nomination practice. Canon and Al Yousuf LLC have agreed to vote their common shares in favor of such proposal and the election of the two new directors. Under the Waiver and Rights Agreement, the parties have agreed that, upon closing of the share issuance, the lock up provisions applicable to the shares Al Yousuf LLC acquired from us will terminate.
As part of a 2007 stock purchase by Al Yousuf, LLC, we entered into a Registration Rights Agreement (the “Registration Rights Agreement”) pursuant to which we are required to cause a registration statement registering the re-sale of the purchased shares to be effective on the two-year anniversary of closing, to the extent the purchased shares are not at such time eligible for resale without restriction under Rule 144 under the Securities Act of 1933, as amended. This period was extended to thirty-months pursuant to an amendment dated August 14, 2009 and further amended to permit delay of the filing of such registration statement until May 29, 2010. A registration statement satisfying this initial registration obligation was declared effective on July 26, 2010. The Registration Rights agreement also includes demand registration rights that extend until November 29, 2015.
On October 6, 2008, we entered into a Stock Purchase and Settlement Agreement dated as of September 30, 2008 with Al Yousuf, LLC. Pursuant to the agreement, we agreed to issue an aggregate of 2,000,000 common shares to Al Yousuf LLC. Of such shares, 1,470,588 shares were acquired on October 14, 2008 by Al Yousuf LLC at a purchase price of $6.80 per share, for an aggregate purchase price of $10.0 million. The remaining 529,412 shares were issued upon execution of the agreement in exchange for a release by Al Yousuf LLC of all potential claims arising from design concerns related to battery packs delivered to Phoenix Motorcars, Inc. in 2007, our related offer of a warranty replacement and inventory write-off, and any other known claims existing as of the date of the agreement. Under the Purchase Agreement dated November 29, 2007 between us and Al Yousuf LLC, pursuant to which Al Yousuf LLC purchased $40.0 million in common shares, we made certain representations and warranties related to our inventory, warranty reserve and similar matters that were affected by the write-off of battery inventories and warranty offer announced in March 2008. (Also refer to Note 13).
On April 20, 2008, we executed an Amended and Restated Agreement to recover Short-Swing Profits with Al Yousuf LLC. Section 16 of the Securities and Exchange Act of 1934 requires directors, officers and 10% beneficial owners of ours to disgorge any short-swing profits realized on a non-exempt purchase and sale of our securities within any six-month period. Consistent with the terms of the Recovery Agreement, we received payment in the amount of $177,000.
In March 2008, Phoenix Motorcars, Inc. completed a merger, wherein the surviving corporation, Phoenix MC, Inc. became a wholly owned subsidiary of All Electric, LLC (“AELLC”). On March 19, 2008, Phoenix MC, Inc. announced receipt of their next round of funding provided by Al Yousuf, LLC and The AES Corporation. These changes resulted in conversion of our 2,500,000 common share investment in Phoenix Motorcars, Inc. to ownership of 2,000 units in AELLC and diluted our ownership percentage in Phoenix to 1.56%. At December 31, 2008, there was no deferred revenue relating to the unamortized investment. We have concluded the investment is other-than-temporarily-impaired. A realized loss of the investment of $88,701 was recognized in December 2008. The remaining investment of $17,817 was recognized as a loss in March 2009.
F-29
|
18.
|
BUSINESS SEGMENT INFORMATION
|
Management views the Company as operating in two major business segments: Power and Energy Group, and All Other operations.
The Power and Energy Group develops, produces, and sells nano-structured lithium titanate spinel, battery cells, battery packs, multi-megawatt battery systems and provides related design and test services. The All Others group consists of the remaining portions of the previous Life Sciences and Performance Materials groups. Management completed a thorough review of operations and strategies and determined that it was in the best interests of the shareholders for the Company to focus primarily on the Power and Energy Group. As a result of this assessment resources devoted to the Performance Materials Group and Life Sciences Group were considerably reduced and no new significant development is being pursued in those areas by the Company. For all years presented, the activity relating to the Performance Materials and Life Sciences divisions have been reclassified into All Other.
Corporate assets consist primarily of cash, short term investments, and long-lived assets. Since none of the business units has reached cash flow break-even, cash funding is provided at the corporate level to the business units. The long-lived assets primarily consist of the corporate headquarters building, building improvements, and land. As such, these assets are reported at the corporate level and are not allocated to the business segments.
Corporate expenses include overall company support costs as follows: research and development expenses; sales and marketing expense; general and administrative expenses; and depreciation & amortization of the Reno headquarters building improvements.
The accounting policies of these business segments are the same as described in Note 2 to the consolidated financial statements. Reportable segment data reconciled to the consolidated financial statements as of and for the fiscal years ended December 31, 2010, 2009 and 2008 is as follows:
|
In thousands of dollars:
|
||||||||||||||||
|
|
||||||||||||||||
|
Loss
|
Depreciation
and |
|||||||||||||||
|
Net Sales
|
From Operations
|
Amortization
|
Assets
|
|||||||||||||
|
2010:
|
||||||||||||||||
|
Power & Energy Group
|
$ | 6,156 | $ | 6,067 | $ | 1,349 | $ | 19,979 | ||||||||
|
All Other
|
1,674 | 110 | 216 | 718 | ||||||||||||
|
Corporate
|
- | 14,274 | 331 | 3,564 | ||||||||||||
|
Consolidated Total
|
$ | 7,830 | $ | 20,451 | $ | 1,896 | $ | 24,260 | ||||||||
|
2009:
|
||||||||||||||||
|
Power & Energy Group
|
$ | 3,249 | $ | 7,382 | $ | 1,320 | $ | 11,574 | ||||||||
|
All Other
|
1,122 | (375 | ) | 531 | 3,269 | |||||||||||
|
Corporate
|
- | 13,789 | 184 | 25,474 | ||||||||||||
|
Consolidated Total
|
$ | 4,371 | $ | 20,796 | $ | 2,035 | $ | 40,317 | ||||||||
|
2008:
|
||||||||||||||||
|
Power & Energy Group
|
$ | 4,075 | $ | 5,958 | $ | 1,281 | $ | 4,207 | ||||||||
|
All Other
|
1,651 | 2,804 | 628 | 9,728 | ||||||||||||
|
Corporate
|
- | 20,459 | 167 | 34,136 | ||||||||||||
|
Consolidated Total
|
$ | 5,726 | $ | 29,221 | $ | 2,076 | $ | 48,071 | ||||||||
F-30
In the table above, corporate expense in the Loss from Operations column includes such expenses as business consulting, general legal expense, accounting and audit, general insurance expense, stock-based compensation expense, shareholder information expense, investor relations, and general office expense.
Additions to long-lived assets in 2010 consisted of $138,000 for Corporate and $1,007,000 for the Power and Energy Group. In 2009 long-lived asset additions consisted of $211,000 for Corporate and $579,000 for the Power and Energy Group.
For the year ended December 31, 2010, we had sales to 3 major customers, each of which accounted for 10% or more of revenues. Total sales to these customers for the year ended December 31, 2010 and the balance of their accounts receivable at December 31, 2010 were as follows:
|
In thousands of dollars:
|
||||||||
|
Sales - Year Ended
|
Accounts Receivable at
|
|||||||
|
Customer
|
December 31, 2010 | December 31, 2010 | ||||||
|
Power and Energy Group:
|
||||||||
|
Office of Naval Research
|
$ | 2,559 | $ | 44 | ||||
|
Proterra, LLC
|
$ | 2,668 | $ | 359 | ||||
|
All Other Division:
|
||||||||
|
US Army RDECOM
|
$ | 1,320 | $ | 93 | ||||
For the year ended December 31, 2009, we had sales to 4 major customers, each of which accounted for 10% or more of revenues. Total sales to these customers for the year ended December 31, 2009 and the balance of their accounts receivable at December 31, 2009 were as follows:
|
In thousands of dollars:
|
||||||||
|
Sales - Year Ended
|
Accounts Receivable at
|
|||||||
|
Customer
|
December 31, 2009 | December 31, 2009 | ||||||
|
Power and Energy Group:
|
||||||||
|
Office of Naval Research
|
$ | 1,198 | $ | 382 | ||||
|
Proterra, LLC
|
$ | 635 | $ | 117 | ||||
|
BAE Systems
|
$ | 482 | $ | - | ||||
|
All Other Division:
|
||||||||
|
Spectrum Pharmaceuticals
|
$ | 751 | $ | - | ||||
For the year ended December 31, 2008, we had sales to 2 major customers, each of which accounted for 10% or more of revenues. Total sales to these customers for the year ended December 31, 2008 and the balance of their accounts receivable at December 31, 2008 were as follows:
|
In thousands of dollars:
|
||||||||
|
Sales - Year Ended
|
Accounts Receivable at
|
|||||||
|
Customer
|
December 31, 2008 | December 31, 2008 | ||||||
|
P&ES Division
|
||||||||
|
Office of Naval Research
|
$ | 2,493 | $ | 301 | ||||
|
Performance Materials & Life Sciences
|
||||||||
|
Eli Lilly
|
$ | 623 | $ | - | ||||
F-31
Revenues for the years ended December 31, 2010, 2009 and 2008 by geographic area were as follows:
|
In thousands of dollars:
|
||||||||||||
|
Geographic information (a):
|
2010
|
2009
|
2008
|
|||||||||
|
United States
|
$ | 7,382 | $ | 3,843 | $ | 5,261 | ||||||
|
Canada
|
- | 2 | 245 | |||||||||
|
Other foreign countries
|
448 | 526 | 220 | |||||||||
|
Total
|
$ | 7,830 | $ | 4,371 | $ | 5,726 | ||||||
|
(a) Revenues are attributed to countries based on location of customer.
|
||||||||||||
All assets are held within the United States with the exception of a Canadian cash account having a balance of $21,000 and $39,000 in raw material inventory located in South Korea at our cell contract manufacturers.
|
19.
|
SUBSEQUENT EVENTS
|
HNEI Customer Purchase Order
On February 4, 2011 we accepted a $1.6 million purchase order to supply the University of Hawaii - Hawaii Natural Energy Institute (“HNEI”) a one-megawatt ALTI-ESS energy storage system for a test of wind energy integration. We anticipate shipping this system to HNEI during the third quarter of 2011.
INE Customer Contract
On February 9, 2011, we signed a contract with Inversiones Energéticas, S.A. de C.V. (“INE”) for the supply and installation of a ten megawatt ALTI-ESS advanced battery system in El Salvador. Total revenues under the Contract are $18 million to be recognized over an expected 14-month period following Altair’s receipt of the notice to proceed, which is expected in the next 30 days. Under the Contract, we are responsible for virtually all of the design, supply, labor, installation and commission of the advanced battery system. We will provide a one-year warranty for the entire system and an additional two-year warranty for the batteries and control system.
Delay in Canon Deal
On February 16, 2011, the Company and Canon entered into a First Amendment to the Share Subscription Agreement dated September 20, 2010 between the Company and Canon. Key amendments to the Agreement effected by the Amendment include the following:
|
|
·
|
extension of the closing deadline and closing date under the Agreement to May 17, 2011;
|
|
|
·
|
authorization for the Company to raise additional capital from third parties prior to May 1, 2011, subject to a dilution limit of less than 20% of outstanding common shares, and a further limit of $7.5 million in aggregate offerings if any issuance will be made at per share price, taking into account the implied value of any warrants issued in connection with such issuance (the “Per Share Issuance Price”), lower than $1.55;
|
|
|
·
|
if the Company makes any issuance at a Per Share Issuance Price lower than $1.55, Canon is able to terminate the Agreement in its sole discretion without any liability; and
|
|
|
·
|
deferral of the purchase and sale of nano lithium titanate under the existing Conditional Supply and Technology Licensing Agreement supply agreement between the Company, its subsidiary Altairnano, Inc. and Canon’s subsidiary Zhuhai Yingtong Energy Co. Ltd. for a period of up to six months or, if later, until the parties have resolved issues related to the transfer of technology.
|
F-32
